UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2023
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 001-38247

AYTU BIOPHARMA, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
|
|
Delaware |
|
47-0883144 |
(State or other jurisdiction of incorporation |
|
(I.R.S. Employer Identification Number) |
|
|
|
7900 East Union Avenue |
|
80237 |
(Address of principal executive offices) |
|
(Zip Code) |
(720) 437-6580
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Trading Symbol |
Name of each exchange on which |
Common Stock, par value $0.0001 per share |
AYTU |
The NASDAQ Capital Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ◻ No ⌧
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ◻ No ⌧
Indicate by a check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ⌧ No ◻
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ⌧ No ◻
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (check one):
Large accelerated filer |
◻ |
|
Accelerated filer |
☐ |
Non-accelerated filer |
⌧ |
|
Smaller reporting company |
☒ |
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of common stock held by non-affiliates of the Registrant as of December 30, 2022 was $12.3 million based on the closing price of $3.80 as of that date.
As of September 20, 2023, there were 5,530,027 shares of common stock issued and outstanding.
TABLE OF CONTENTS
|
|
|
PAGE |
|
|||
|
|
|
|
|
7 |
||
|
22 |
||
|
53 |
||
|
53 |
||
|
53 |
||
|
54 |
||
|
|
|
|
|
|||
|
|
|
|
|
55 |
||
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
57 |
|
|
69 |
||
|
69 |
||
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
69 |
|
|
69 |
||
|
71 |
||
|
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
71 |
|
|
|
|
|
|
|||
|
|
|
|
|
72 |
||
|
76 |
||
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
80 |
|
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
81 |
|
|
83 |
||
|
|
|
|
|
|||
|
|
|
|
|
85 |
||
|
90 |
||
|
|
|
|
91 |
|||
2
Forward-Looking Statements
This Annual Report on Form 10-K, or Annual Report, includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act. All statements other than statements of historical facts contained in this Annual Report, including statements regarding our anticipated future clinical and regulatory events, future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. Forward-looking statements are generally written in the future tense and/or are preceded by words such as “may,” “will,” “should,” “forecast,” “could,” “expect,” “suggest,” “believe,” “estimate,” “continue,” “anticipate,” “intend,” “plan,” or similar words, or the negatives of such terms or other variations on such terms or comparable terminology. Such forward-looking statements include, without limitation, statements regarding the markets for our approved products and our plans for our approved products, the anticipated start dates, durations and completion dates, as well as the potential future results, of our ongoing and future clinical trials, the anticipated designs of our future clinical trials, anticipated future regulatory submissions and events, the potential future commercialization of our product candidates, our anticipated future cash position and future events under our current and potential future collaborations. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including without limitation the risks described in “Risk Factors” in Part I, Item 1A of this Annual Report. These risks are not exhaustive. Other sections of this Annual Report include additional factors that could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. We assume no obligation to update or supplement forward-looking statements.
Unless otherwise indicated or unless the context otherwise requires, references in this Form 10-K to the “Company,” “Aytu,” “we,” “us,” or “our” are to Aytu BioPharma, Inc. and its wholly owned subsidiaries.
This Annual Report on Form 10-K refers to trademarks, such as Aytu, Adzenys XR-ODT, Cotempla XR-ODT, FlutiCare, Innovus Pharma, Neos, OmepraCare, Poly-Vi-Flor, Regoxidine, and Tri-Vi-Flor which are protected under applicable intellectual property laws and are our property or the property of our subsidiaries. This Form 10-K also contains trademarks, service marks, copyrights and trade names of other companies which are the property of their respective owners. Solely for convenience, our trademarks and tradenames referred to in this Form 10-K may appear without the ® or ™ symbols, but such references are not intended to indicate in any way that we will not assert, to the fullest extent under applicable law, our rights to these trademarks and tradenames.
We obtained statistical data, market and product data, and forecasts used throughout this Form 10-K from market research, publicly available information and industry publications. While we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information.
3
Summary of Risk Factors
The following list summarizes what we believe to be the principal risks relevant to our company. The following summary is further elaborated on by the full text of the risk factors provided in the “Risk Factors” section of this Annual Report on Form 10-K for the year ended June 30, 2023. All capitalized terms in this section not defined herein shall have the meanings given to them elsewhere in this Annual Report. Material risks that may affect our business, operating results and financial condition include, but are not necessarily limited to, the following:
Risks Related to Our Business and Financial Position
● |
We have incurred losses since our inception and may incur continued losses in the future. We may never achieve or maintain profitability, and we may require additional capital to fund our operations. |
● |
Our failure to comply with the covenants or other terms of the loan and security agreement with Avenue Capital and our secured revolving loans with Eclipse could result in a default under those agreements that could materially and adversely affect the ongoing viability of our business. |
●Our credit facility agreements contain restrictions that limit our flexibility in operating our business.
● |
We have indefinitely suspended development of our AR101 (enzastaurin) clinical development program and shifted our strategic focus towards accelerating the growth of our commercial business. |
Risks Related to Commercialization
● |
If we are unable to successfully commercialize our commercial prescription products, our business, financial condition and results of operations may be materially adversely affected, and the price of our common stock may decline. |
● |
The commercial success of our commercial prescription products will depend upon their acceptance by multiple stakeholders, including physicians, patients, and healthcare payors. |
● |
If we are unable to differentiate our commercial prescription products from current and future products or existing methods of treatments or if the market opportunities for our commercial prescription products are smaller than we believe, our ability to successfully commercialize our commercial prescription products would be adversely affected and our revenue may be adversely affected. |
● |
If we or our contract manufacturing organizations (“CMOs”) fail to manufacture sufficient quantities of our attention deficit/hyperactivity disorder (“ADHD”) prescription products, we may be unable to meet market demand and our ability to generate revenues could be affected. |
● |
We may encounter manufacturing problems resulting in insufficient quantities being produced or not having access to the requisite supplies. |
● |
If we do not secure collaborations with strategic partners to test, commercialize and manufacture product candidates, we may not be able to successfully develop products and generate meaningful revenues. |
● |
If third-party payors do not reimburse pharmacies or patients for our commercial prescription products or if reimbursement levels are set too low for us to sell our commercial prescription products at a profit, our ability to successfully commercialize our commercial prescription products and our results of operations will be harmed. |
● |
If we cannot implement and maintain effective patient affordability programs or improve formulary access for our commercial prescription products in the face of increasing payor pressures, the adoption of our commercial prescription products by physicians and patients may decline. |
4
● |
If the U.S. Food and Drug Administration (“FDA”) or other applicable regulatory authorities approve generic or similar products that compete with our commercial prescription products, or if the FDA or other applicable regulatory authorities change or create new pathways that may expedite approval of such products, it could decrease our expected sales of our commercial prescription products. |
● |
Even though we have obtained regulatory approval for our commercial prescription products, we still face extensive FDA regulatory requirements and may face future regulatory difficulties. |
● |
Our relationships with physicians, patients, payors, and pharmacies in the U.S. are subject to applicable anti-kickback, fraud and abuse laws and regulations. Our failure to comply with these laws could expose us to criminal, civil and administrative sanctions, reputational harm, and could harm our results of operations and financial conditions. |
Risks Related to Our Intellectual Property
● |
If we are unable to protect our intellectual property rights or if our intellectual property rights are inadequate to protect our technology, our commercial prescription products or our other product candidates, our competitors could develop and commercialize technology similar to ours, and our competitive position could be harmed. |
● |
We may become involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful. |
● |
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which could be uncertain and could harm our business. |
Risks Related to Our Organization, Structure and Operations
● |
Our efforts to expand and transform our business may require significant investments and may be unsuccessful. |
● |
We may have difficulties integrating acquired businesses and as a result, our business, results of operations and/or financial condition may be materially adversely affected. |
● |
Product liability and other lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our product candidates. |
Risks Related to Securities Markets and Investment in Our Securities
● |
Our failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a delisting of our common stock. |
●The price of our common stock may be volatile, and you may lose all or part of your investment.
● |
Future issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall. |
● |
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others. |
General Risk Factors
● |
Our business and operations would suffer in the event of system failures or security breaches whether such failure or breach was physically affected or affected via a cybersecurity failure. |
5
● |
Our sales force and other employees, third party logistics partners, CMOs, contract research organizations (“CROs”), principal investigators, collaborators, independent contractors, consultants and other vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. |
● |
Investing in our securities includes a high degree of risk. You should consider carefully the specific factors discussed below, together with all of the other information contained in this Annual Report on Form 10-K. If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects would likely be materially and adversely affected. This could cause the market price of our securities to decline and could cause you to lose all or part of your investment. |
6
AYTU BIOPHARMA, INC.
PART I
ITEM 1. BUSINESS
COMPANY OVERVIEW
Aytu BioPharma, Inc. (“Aytu,” the “Company”, “we”) is a pharmaceutical company focused on commercializing novel therapeutics and consumer healthcare products. The Company operates through two business segments (i) the Rx Segment, consisting of prescription pharmaceutical products and (ii) the Consumer Health Segment, which consists of various consumer healthcare products (the “Consumer Health Portfolio”). We were originally incorporated as Rosewind Corporation on August 9, 2002 in the State of Colorado and were re-incorporated as Aytu BioScience, Inc in the state of Delaware on June 8, 2015. Following the acquisition of Neos Therapeutics, Inc. (“Neos”) in March 2021 (the “Neos Acquisition”), we changed our name to Aytu BioPharma, Inc.
We have incurred significant losses in each year since inception. Our net loss was $17.1 million for the year ended June 30, 2023, and as of June 30, 2023, we had an accumulated deficit of $304.1 million. We expect to continue to incur significant expenses in connection with our ongoing activities, including the integration of our acquisitions, although we do expect to become profitable following that integration and through continued growth of our commercial business.
Effective January 6, 2023, we effected a 1-for-20 reverse stock split of our outstanding shares of common stock. Unless specifically provided otherwise herein, the share and per share information that follows in this Annual Report, other than in the historical financial statements and related notes included elsewhere in this Form 10-K, assumes the effect of the reverse stock split.
RECENT BUSINESS DEVELOPMENT
As part of our ongoing strategic evaluation and go-forward operating plan, we are prioritizing growing our Rx Segment given the encouraging prescription trends for both our attention deficit hyperactivity disorder (“ADHD”) Portfolio and Pediatric Portfolio, and the current market trends supporting our products’ growth. We believe focusing resources on our most profitable, rapidly growing products and business segments provides the most effective pathway to achieve near-term companywide profitability and continued growth. As part of our plan, we expect to monetize, divest, or otherwise discontinue the Consumer Health Segment in order to maximize profitability and, if a divestiture is made, provide us with non-dilutive capital.
In fiscal year 2023, we recorded net revenue of $73.8 million in our Rx Segment, the highest revenue achieved in our history. During the year, the ADHD market encountered several supply chain interruptions, causing a shortage of medications for these patients. We were able to increase the production of our ADHD medications, Adzenys XR-ODT (“Adzenys”), and Cotempla XR-ODT (“Cotempla”) to provide patients with alternative solutions to products that have experienced supply chain interruptions. As a result, we recorded the highest prescription levels for both Adzenys and Cotempla in 2023. Our Pediatric Portfolio products, Poly-VI-Flor, Tri-Vi-Flor and Karbinal, also recorded record prescriptions in our fiscal 2023, which was largely attributable to sales force execution and our Aytu Rx Connect program.
We currently manufacture both Adzenys and Cotempla in our facility in Grand Prairie, Texas. In an effort to reduce costs, we are in the process of transferring the manufacture of these products to a third-party manufacturer. In April 2023, we received approval from the U.S. Food & Drug Administration (“FDA”) of the Adzenys Prior Approval Supplement (“PAS”), which enables the transfer of manufacturing of Adzenys to a third-party manufacturer. In June 2023, we submitted the Cotempla PAS to the FDA. We expect to have a six-month review process for the Cotempla PAS.
7
AR101 (enzastaurin) is a development-stage asset we had been developing as an investigational treatment for Vascular Ehlers-Danlos Syndrome (“VEDS”), a rare connective tissue disorder for which there are no approved treatments. AR101 has received Orphan Drug Designation from both the FDA and from the European Commission, thus making AR101 eligible for market exclusivity upon product approval. AR101 also received Fast Track Designation from the FDA given the urgent, unmet need in VEDS. We do not expect the development of AR101 to advance until we are able to either fund development through operating cash flows, or through an out-license or sale to a strategic partner as we focus our resources to commercial operations.
In April 2020, we entered into a licensing agreement with Cedars-Sinai Medical Center (“Cedars-Sinai”) to secure worldwide rights to various potential esophageal and nasopharyngeal uses of Healight, an investigational ultraviolet light-based medical device platform being investigated as a prospective treatment for severe respiratory infections. The licensing agreement with Cedars-Sinai grants us a license to all patent and development related technology rights for the intra-corporeal therapeutic use of ultraviolet light in the field of endotracheal and nasopharyngeal applications. We terminated the Healight license on May 9, 2023 and are in the process of returning materials and transferring all intellectual property to Cedars-Sinai as we shift our resources to commercial purposes.
In October 2018, we entered into an Exclusive License Agreement (“NeuRx License”) with NeuRx Pharmaceuticals LLC (“NeuRx”), pursuant to which NeuRx granted Neos an exclusive, worldwide, royalty-bearing license to research, develop, manufacture, and commercialize certain pharmaceutical products containing NeuRx’s proprietary compound designated as NRX 101, subsequently referred to as NT0502. NT0502 is a new chemical entity that was being developed for the treatment of sialorrhea, which is excessive salivation or drooling. In April 2023, and in order to focus our resources on commercial operations, we returned the NT0502 rights to NeuRx in exchange for royalties and milestone payments on monies received by NeuRx from future licensing agreements, asset sales or revenue generated on NT0502.
Debt and Equity Financings
Avenue Capital Agreement
On January 26, 2022, we entered into a Loan and Security Agreement (the “Avenue Capital Agreement”) with Avenue Venture Opportunities Fund II, L.P. and Avenue Venture Opportunities Fund II, L.P. (the “Avenue Capital Lenders”), collectively (“Avenue Capital”), pursuant to which the Avenue Capital Lenders provided the Company and certain of its subsidiaries with a secured $15.0 million loan. The interest rate on the loan is the greater of the prime rate and 3.25%, plus 7.4%, payable monthly in arrears. The maturity date of the loan is January 26, 2025. The proceeds from the Avenue Capital Agreement were used towards the repayment of existing debt, which was assumed through the acquisition of Neos Therapeutics.
On June 13, 2023, in conjunction with the equity financing described below, we announced that the interest-only period of the Avenue Capital Agreement was extended further upon the achievement of both the revenue-based milestone and equity raise-based milestone stipulated in the Avenue Capital Agreement. The interest-only period now extends to the January 26, 2025 maturity date.
Eclipse Loan Agreement
In connection with the Avenue Capital Agreement, we entered into a Consent, Waiver and Second Amendment to Eclipse Loan Agreement with Eclipse Business Capital LLC (f/k/a Encina Business Credit, LLC) (“Eclipse”), dated as of January 26, 2022 (the “Eclipse Loan Agreement”). Pursuant to the Eclipse Loan Agreement, we, among other things, extended the maturity date of the Eclipse Loan Agreement to January 26, 2025 and reduced the maximum availability under the Eclipse Loan Agreement from $25.0 million to $12.5 million minus a $3.5 million availability block.
On March 24, 2023, the Company and certain of its subsidiaries entered into Amendment No. 4 (the Eclipse Amendment”) to the Loan and Security Agreement dated October 2, 2019. The Eclipse Amendment, among other things, increased the maximum amount available under the revolving credit facility provided under the Eclipse Loan Agreement to $14.5 million.
8
The ability to make borrowings and obtain advances of revolving loans under the Eclipse Loan Agreement remains subject to a borrowing base and reserve, and availability blockage requirements.
Equity Financings
In August 2022, we raised gross proceeds of $10.0 million from the issuance of (i) 1,075,290 shares of our common stock, and in lieu of common stock to certain investors that so chose, pre-funded warrants to purchase 87,500 shares of its common stock (the “Pre-Funded Warrants”), and (ii) accompanying warrants (the "Common Warrants") to purchase 1,265,547 shares, as adjusted, of our common stock. We received $9.1 million in proceeds net of underwriting fees and other expenses. In August 2022, the Pre-Funded Warrants were exercised in full.
In June 2023, we raised gross proceeds of $4.0 million from the issuance of (i) 1,743,695 shares of our common stock, and (ii) in lieu of common stock to certain investors that so chose, pre-funded warrants to purchase 430,217 shares of common stock and (iii), accompanying Tranche A warrants to purchase 2,173,912 shares of common stock, (iv) and accompanying Tranche B warrants to purchase 2,173,912 shares of common stock. We received approximately $3.4 million in proceeds net of underwriting fees and other expenses.
COMMERCIAL BUSINESS OVERVIEW
We operate through two business segments (i) the Rx Segment, consisting of various prescription pharmaceutical products sold through third parties, and (ii) the Consumer Health Segment, which consists of various consumer health products sold directly to consumers. We generate revenue by selling our products through third party intermediaries in our marketing channels as well as directly to our customers. We currently manufacture our ADHD products at our facility in Grand Prairie, Texas, and use third party manufacturers for our other prescription and consumer health products.
Rx Segment
Our Rx Segment consists of our ADHD Portfolio and our Pediatric Portfolio. Our prescription products are sold solely in the United States and are distributed through multiple channels, including sales to pharmaceutical wholesalers and pharmacies, using third-party logistics enterprises.
We acquired our ADHD Portfolio in March 2021 with the acquisition of Neos Therapeutics. These commercial ADHD products are extended-release (“XR”) medications formulated in patient-friendly, orally disintegrating tablets (“ODT”) that utilize the Neos-developed microparticle modified-release drug delivery technology platform. Products containing amphetamine or methylphenidate are the most commonly prescribed medications in the United States for the treatment of ADHD. Adzenys (for patients six years of age and above) and Cotempla (for patients six to seventeen years of age) are the first and only FDA-approved amphetamine and methylphenidate extended-release, orally disintegrating tablets, respectively, for the treatment of ADHD.
Our prescription Pediatric Portfolio includes Karbinal® ER, an extended-release carbinoxamine (antihistamine) suspension indicated to treat numerous allergic conditions for patients two years and above and Poly-Vi-Flor® and Tri-Vi-Flor®, two complementary prescription fluoride-based multi-vitamin product lines containing combinations of fluoride and vitamins in liquid and chewable tablet form for infants and children with fluoride deficiency (Karbinal ER, Poly-Vi-Flor and Tri-Vi-Flor are collectively the “Pediatric Portfolio”). These products serve established pediatric markets and offer distinct clinical features and patient benefits.
We commercialize our Rx Portfolio through our internal commercial organization that includes approximately forty sales territories for our ADHD Portfolio and approximately six sales territories for our Pediatric Portfolio.
Our Aytu RxConnect™ patient support program operates through a network of approximately 1,000 pharmacies to offer affordable, predictable copays and hassle-free availability to all commercially insured patients, regardless of their individual insurance plan. In addition, RxConnect seeks to significantly reduce the challenges and frustrations that health care professionals and their office staff can face when prescribing branded medications, including our medications, for their patients.
9
In July 2023, we entered into an exclusive collaboration, distribution and supply agreement with Medomie Pharma Ltd, (“Medomie”) a privately owned pharmaceutical company, for Medomie to sell Adzenys and Cotempla in Israel and the Palestinian Authority. We will supply Adzenys and Cotempla to Medomie and Medomie will seek local regulatory approvals and marketing authorizations for each. This agreement represents Aytu’s first international commercial agreement for Adzenys and Cotempla.
Consumer Health Segment
Our Consumer Health Segment is dedicated to commercializing safe and effective “over-the-counter” (“OTC”) medicines, personal care products, and dietary supplements to improve health and vitality. Our core products compete in categories such as hair loss, digestive health, urological health, diabetes management, and allergy. All products are intended to be used by consumers on a regular basis, and as such, we offer a monthly subscription program to allow for ongoing use and to simplify product ordering and use by patients. We acquired our Consumer Health Segment, previously known as Innovus Pharmaceuticals, Inc., in February 2020 (the “Innovus Acquisition”).
The Consumer Health Segment currently sells directly to consumers primarily in the United States through e-commerce platforms, including branded websites and Amazon.com which utilize marketing strategies focused on search engine optimization, search marketing and affiliate marketing. Additionally, the segment sells products through direct mail solicitations and advertisements, allowing consumers to purchase directly through business reply mail, through call centers, or online with shipment directly to their homes.
We expect to monetize, divest, or otherwise discontinue the Consumer Health Segment in order to maximize profitability and, if a divestiture is made, provide us with non-dilutive capital.
Development Portfolio – AR101
On April 12, 2021, we entered into an asset purchase agreement with Rumpus VEDS, LLC, Rumpus Therapeutics, LLC, and Rumpus Vascular, LLC (together “Rumpus”) pursuant to which we acquired commercial global licenses, relating primarily to the pediatric-onset rare disease development asset enzastaurin, or AR101. AR101 is initially being studied for the treatment of VEDS.
AR101 is an orally available investigational first-in-class small molecule, serine/threonine kinase inhibitor of the PKC beta, PI3K and AKT pathways. AR101 has been studied in more than 3,300 patients across a range of solid and hematological tumor types in trials previously conducted by Eli Lilly & Company. Harry “Hal” C. Dietz III, M.D. developed the first preclinical model that mimics the human condition and recapitulates VEDS, and this model serves as the basis for the plausible clinical benefit and rationale for conducting a clinical trial with AR101 in VEDS. This novel knock-in mouse model has the same genetic mutation most prevalent in VEDS patients and is representative of the human condition in both the timing and location of VEDS-related vascular events. The model has generated identical structural histology and mechanical characteristics, and unbiased findings demonstrated that vascular structure alone does not lead to vascular events. Objective comparative transcriptional profiling by high-throughput RNA sequencing of the aorta displayed a molecular signature for excessive PKC/ERK cell signaling that is the purported driver of disease. PKC inhibitors proved efficacious in multiple pre-clinical and murine (mice) models and indeed prevented death due to vascular rupture.
We have secured exclusive global rights to AR101 in the fields of rare genetic pediatric onset or congenital disorders outside of oncology. AR101 is protected by a suite of pending patents being pursued in major markets globally which have been licensed from The Johns Hopkins University (“Johns Hopkins”) and have an earliest priority date of March 2017. In December 2021, the FDA granted Orphan Drug Designation (“ODD”) to AR101 for the treatment of EDS, inclusive of VEDS, allowing for seven years of marketing exclusivity in the United States. The FDA has cleared the IND application for AR101, although, we do not expect to advance development of AR101 until we are able to either fund development through operating cash flows or through an out-license or sale to a strategic partner.
10
OUR STRATEGY
Our goal is to become a leading pharmaceutical company that improves the lives of patients and healthcare consumers. We will do this by employing a focused approach of in-licensing, acquiring, developing, and commercializing novel prescription therapeutics and consumer health products. Our primary focus is on commercializing innovative prescription products that address conditions frequently developed or diagnosed in childhood, including ADHD. We also commercialize consumer healthcare products through efficient e-commerce and direct-to-patient platforms, although we expect to monetize, divest, or discontinue the Consumer Health Segment in favor of focusing on the Rx Segment and attaining profitability.
Our strategic priorities are to continue to increase revenues from our Rx Segment and enhance our financial performance through operational and manufacturing efficiencies and portfolio prioritization. Specifically, we intend to:
| ● | continue to grow our commercial branded, revenue-generating products, by increasing product sales and improving patient access. Our primary commercial objective is to drive revenue growth of our ADHD and pediatric brands, which consists of Adzenys, Cotempla, Poly-Vi-Flor, Tri-Vi-Flor, and Karbinal ER. We expect to increase market share using our internal commercial organization and leveraging our advanced analytics platform to optimize sales force performance and increase both the breadth, or number of healthcare professionals (“HCPs”) prescribing our medicines, and the depth, or the number of appropriate patients per HCP for our products; |
| ● | leverage our novel Aytu RxConnect patient support platform, which is designed to reduce access barriers to medicines facing patients and HCPs by providing coverage for all commercially insured patients, regardless of their individual insurance plan, thus establishing an affordable and predictable monthly co-pay for patients, and eliminating many of the hassles facing HCPs and their staffs by improving availability of Aytu products at participating pharmacies; |
| ● | improve gross margins for our ADHD product franchise through the manufacturing transfer of Adzenys and Cotempla to a contract manufacturing organization, a transition that is expected to occur in early calendar 2024; |
We believe our history of acquiring companies and in-licensing and acquiring products and pipeline assets, along with our success in building out commercial organizations and executing product launch and growth strategies, is a distinct competitive advantage. Our transactional adeptness and execution orientation enable us to continue to seek growth opportunities through both organic growth and opportunistic in-licensing or strategic acquisitions. Further, our commercial infrastructure and distribution capability is scalable and lends itself to additional on-market assets and future product candidates that fit within our core therapeutic focus or within our commercial capabilities and infrastructure. As such, in the near term, we may seek to leverage our commercial model and infrastructure by expanding our commercial portfolio with external product opportunities as we have done since our inception.
OUR PRODUCTS AND MARKETS
Prescription Products
ADHD Portfolio
ADHD Market and Treatment Options
ADHD is a neurobehavioral disorder characterized by a persistent pattern of inattention and/or hyperactivity/impulsivity that interferes with functioning and/or development. ADHD can have a profound impact on an individual’s life, causing disruption at school, work, home and in relationships. It is one of the most common developmental disorders in children and often persists into adulthood. The Centers for Disease Control and Prevention (“CDC”) reported that six million children in the United States ages 3 to 17 had previously received an ADHD diagnosis between 2016-2019, up 36% since 2003.
11
Current ADHD treatment guidelines recommend a multi-faceted approach that uses medications in conjunction with behavioral interventions.
In 2022, approximately 83.5 million prescriptions for medications with ADHD labeling were written in the United States generating $21.2 billion in sales. Approximately 91% of these prescriptions were for stimulant medications, such as amphetamine and methylphenidate, which are and have remained the standard of care for several decades. The market for ADHD medications outside of the United States is less developed, but we believe it will continue to grow as recognition and awareness of the disorder increase.
Extended-release, or long-acting, dosage forms of stimulant medications are the standard of care for treating ADHD, making up approximately 43% of ADHD prescriptions. The most prescribed extended-release medications for ADHD, Adderall XR® and Concerta® (and each of their generic equivalents), are long-acting versions of previously short-acting amphetamine and methylphenidate medications, respectively. Most of these extended-release dosage forms allow for once-daily dosing in the morning, which eliminates the need to re-dose during the day. Our products, Adzenys XR-ODT and Cotempla XR-ODT, are extended-release orally disintegrating tablets that allow for once-daily dosing based upon our internally developed proprietary microparticle delivery technology and are the only approved extended-release orally disintegrating tablet formulations of amphetamine and methylphenidate for the treatment of ADHD.
There is significant competition in the ADHD market, including from well-established companies, many of whom have substantially greater financial, technical and commercial resources than we do, and entrenched existing ADHD products. For example:
| ● | Extended-release amphetamine products are currently marketed in the United States by (i) Takeda Pharmaceutical Company Limited under the brand names Adderall XR®, Vyvanse® and Mydayis® and (ii) Tris Pharma, Inc. (“Tris”), under the brand names Dyanavel® XR, Dyanavel® XR tablets; |
| ● | Extended-release methylphenidate products are marketed in the United States by (i) Janssen Pharmaceuticals, Inc. under the brand name Concerta®, (ii) Tris under the brand names Quillivant XR® and QuilliChew ER®, (iii) Rhodes Pharmaceuticals LP under the brand name Aptensio XR®, (iv) Ironshore Pharmaceuticals Inc. under the brand name Jornay PM®, (v) Alora Pharmaceuticals under the name Methylphenidate HCl ER 72 mg Tablets, (vi) Novartis under the brand names Focalin XR® and Ritalin LA® and (vii) Azstarys®, a product developed by KemPharm (now Zevra Therapeutics) and sold by Corium; and |
| ● | a non-stimulant treatment for ADHD was approved by the FDA and commercially launched by Supernus in the U.S in 2021 is being sold under the brand name Qelbree®. |
Further, makers of branded drugs could also enhance their own formulations in a manner that competes with our enhancements of these drugs. We are also aware of efforts by several pharmaceutical companies with ADHD medications in clinical development, including Cingulate Therapeutics, NLS Pharma and Neurovance, a subsidiary of Otsuka Pharmaceutical Co., Ltd.
Our ADHD Product Portfolio
Our modified-release drug delivery technology platform has enabled us to create extended-release ODT formulations of amphetamine and methylphenidate. This was achieved by developing an extended-release profile that allows for once daily dosing and an ODT formulation that allows for easier administration and ingestion and twelve-hour duration of action.
Adzenys XR-ODT and Cotempla XR-ODT are the first and only XR-ODT products for the treatment of ADHD. These XR-ODT products offer unique attributes to ADHD patients and caregivers, including:
| ● | ease of administration and ingestion because they disintegrate rapidly in the mouth and may be taken without water; |
12
| ● | taste-masking of bitter ADHD medications, with pleasant-tasting flavor; |
| ● | prevention of “cheeking,” the practice of hiding medication in the mouth and later spitting it out rather than swallowing it; and |
Adzenys XR-ODT: Amphetamine XR-ODT for the treatment of ADHD
Adzenys XR-ODT is approved by the FDA for the treatment of ADHD in patients six years and older and is the first FDA-approved amphetamine XR-ODT for the treatment of ADHD. The New Drug Application (“NDA”) for Adzenys XR-ODT relies on the efficacy and safety data that formed the basis of FDA approval for the reference listed drug, Adderall XR, 30 mg, together with bioequivalence, bioavailability, and aggregate safety data from the Adzenys XR-ODT clinical program. Adzenys XR-ODT contains amphetamine loaded onto a mixture of immediate-release and polymer-coated delayed-release resin particles, which are formulated and compressed into an ODT along with other tableting excipients using our patented Rapidly Disintegrating Ionic Masking (“RDIM”) technology. The result is amphetamine with an in vivo extended-release profile delivered through a tablet that quickly disintegrates in the mouth without the need for water. Adzenys XR-ODT is available in 30-day supply, child-resistant blister packs.
The suite of composition-of-matter patents for Adzenys XR-ODT are scheduled to expire in 2026 and 2032. These patents are listed in the FDA’s publication of approved drug products with therapeutic equivalence evaluations (the “Orange Book”). In addition, we entered into a settlement agreement with Actavis Laboratories FL, Inc. (“Actavis”) (acquired by Teva Pharmaceutical Industries), which resolved all ongoing litigation involving Adzenys XR-ODT patents and Actavis’ ANDA with the FDA for a generic version of Adzenys XR-ODT. Under the agreement with Actavis, Actavis has the right to manufacture and market its approved generic version of Adzenys XR-ODT under the ANDA beginning on September 1, 2025, or earlier under certain circumstances.
In conjunction with the approval of the Adzenys XR-ODT NDA, the FDA has required us to conduct certain clinical studies in preschool (age four to five years) children with ADHD as a post-marketing requirement. A pharmacokinetic study in this population was completed in 2018, and we are in discussions with the FDA to further clarify the design protocols required to conduct the remaining studies.
Cotempla XR-ODT: Methylphenidate XR-ODT for the treatment of ADHD
The FDA approved Cotempla XR-ODT for the treatment of ADHD in patients six to seventeen years old. The Cotempla XR-ODT NDA relies on the efficacy and safety data that formed the basis of FDA approval for the reference listed drug, Metadate CD®, together with bioavailability/bioequivalence data and efficacy/safety data from the Cotempla XR-ODT clinical program. The results of the Cotempla XR-ODT Phase 3 clinical efficacy and safety trial showed a statistically significant improvement in ADHD symptom control compared to placebo across the school day. Onset of effect was observed within one hour post-dose and persisted through 12 hours. No serious adverse events were reported during the study, and the adverse event profile was consistent with the drug’s mechanism of action.
Cotempla XR-ODT contains methylphenidate loaded onto a mixture of immediate-release and polymer-coated delayed-release resin particles, which are formulated and compressed into an ODT along with other tableting excipients using our RDIM technology. The result is methylphenidate with an in vivo extended-release profile delivered through a tablet that quickly disintegrates in the mouth. Cotempla XR-ODT is available in 30-day supply, child-resistant blister packs. Cotempla XR-ODT is the first FDA-approved methylphenidate XR-ODT for the treatment of ADHD.
We hold composition-of-matter patents in the U.S. which we expect will provide Cotempla XR-ODT intellectual property protection until 2032, and a method-of-use patent was issued which will extend protection until 2038. These patents are listed in the Orange Book. In addition, Neos entered into a settlement agreement with Teva Pharmaceuticals USA, Inc. (“Teva”), which resolved all ongoing litigation involving the Cotempla XR-ODT patents and Teva’s ANDA with the FDA for a generic version of Cotempla XR-ODT. Under the agreement with Teva, Neos granted Teva the right to manufacture and market its approved generic version of Cotempla XR-ODT under the ANDA beginning on July 1, 2026, or earlier under certain circumstances.
13
In conjunction with the approval of the Cotempla XR-ODT NDA, the FDA required us to perform additional clinical studies in preschool (age four to five years) children with ADHD as a post-marketing requirement. A pharmacokinetic study in this population was completed in 2019. In light of a new draft guidance for industry that was published in May 2019, “Attention Deficit Hyperactivity Disorder: Developing Stimulant Drugs for Treatment Guidance for Industry,” we remain in discussions with the FDA to gain concurrence on the design of the protocols required to meet the remaining post-marketing requirements.
Pediatric Portfolio
Poly-Vi-Flor and Tri-Vi-Flor: Our fluoride-based multivitamin prescription supplement product line for infants and children
Poly-Vi-Flor and Tri-Vi-Flor are two complementary prescription fluoride-based supplement product lines containing combinations of vitamins and sodium fluoride in various oral formulations. These prescription supplements are prescribed for infants and children to treat or prevent fluoride deficiency due to poor diet or low levels of fluoride in drinking water and other sources while also providing multi-vitamin support and folic acid supplementation. Because these products contain at least .25 mg of sodium fluoride, Poly-Vi-Flor and Tri-Vi-Flor are classified as products that should be administered under the supervision of a licensed prescriber.
Fluoride supplementation has been proven to protect teeth from decay. Community water fluoridation prevents tooth decay by providing frequent and consistent contact with low levels of fluoride. By keeping the teeth strong and solid, fluoride stops cavities from forming and can rebuild the tooth’s surface. Community water fluoridation began in the United States in 1945 and is the process of adjusting the amount of fluoride in drinking water to a level recommended for preventing tooth decay. As of 2016, more than 200 million people, or nearly 3 in 4 Americans who use public water supplies, drank water with enough fluoride to prevent tooth decay. However, Americans living in municipalities that do not fluoridate the water supply or in rural areas that rely on well water supplies do not receive recommended levels of fluoride through fluoridation. Therefore, many children living in these areas often require daily fluoride supplementation as part of their mineral and vitamin intake. In many instances, physicians prescribe fluoride-based multi-vitamins (Vitamins A, B, C, D and folic acid) regularly to supplement their fluoride intake and enable convenient supplementation. Infants are prescribed easier-to-take multi-vitamin drops while older children are prescribed tablet formulations.
In 2022, 8 million multi-vitamin prescriptions were written in the U.S. Of those prescriptions, multi-vitamins containing sodium fluoride accounted for 1.1 million total prescriptions. Common multi-vitamin combinations contain vitamins A, B, C, D and E, but no other prescription pediatric multi-vitamin products contain Metafolin, which makes the Poly-Vi-Flor and Tri-Vi-Flor product lines distinct, single-source brands. Other brands include Tri-Vite (marketed by Method Pharmaceuticals), Floriva (marketed by BonGeo Pharmaceuticals) and Quflora (marketed by Carwin Pharmaceutical Associates).
Poly-Vi-Flor is available in both chewable tablet and oral liquid suspension multivitamin formulations in six different product presentations: Poly-Vi-Flor Chewable Tablets .25 mg, .50 mg, and 1 mg tablets, Poly-Vi-Flor Chewable Tablets with Iron, Poly-Vi-Flor Oral Suspension and Poly-Vi-Flor Oral Suspension with Iron. Poly-Vi-Flor contains Vitamin A, Vitamins B1, B2, B3, and B6, Vitamin C, Sodium Fluoride in various doses and Metafolin, a proprietary, trademarked L-methylfolate form of folic acid developed by and licensed from Merck & Cie (“Merck”). Beginning in the second half of fiscal 2023, we introduced Poly-Vi-Flor and Tri-Vi-Flor containing Arcofolin, Arcofolin offers an improved profile over Metafolin as a body ready L-methylfolate. Arcofolin’s low water content and low molecular weight of the counterion yield higher levels of assayed folate than other forms of L-methylfolate currently available on the market. It also has an improved purity profile, enhanced water solubility and an excellent overall stability profile. The addition of Arcofolin also broadens the brands’ IP protection and extends the patent life and provides further differentiation with this novel ingredient.
Tri-Vi-Flor is available as an oral liquid suspension in two different strengths (.25 mg and .50 mg fluoride) containing Vitamin A, Vitamin C, Vitamin D3, Sodium Fluoride, Sodium Benzoate and L-methylfolate. By virtue of its L-methylfolate content, Tri-Vi-Flor offers a similar clinical profile: a fluoride-based multivitamin containing a proprietary, body-ready L-methylfolate.
14
Arcofolin®, which we also licensed exclusively in our field of use, is Merck’s manufactured calcium salt of L-5-methyltetrahydrofolic or L-methylfolate. It is a ‘body ready’ alternative to folic acid and offers good stability, solubility, and bioavailability. Folic acid supplementation is recommended in various patient groups, but a significant number of patients have difficulty metabolizing folate due to an enzymatic deficiency caused by a genetic mutation affecting the enzyme methylenetetrahydrofolate reductase, or MTHFR. MTHFR converts ingested folate (such as supplemented folic acid) into L-methylfolate, the body’s usable form. Clinical studies have demonstrated that 75% of patients may have at least one MTHFR genetic mutation while 40% may have two mutations. These mutations lead to impaired function of the enzyme and result in folate deficiency. Both Arcofolin and Metafolin are unaffected by the MTHFR mutation, thereby directly delivering bioavailable L-methylfolate, and offering a distinct clinical advantage over other folic acid supplements.
The core family of patent covering Arcofolin has a priority date of March 31, 2017 and describes a crystalline sodium salt of 5-methyl-(6S)- tetrahydrofolic acid wherein the molar ratio of 5-methyl-(6S)-tetrahydrofolic acid to sodium is from 1:0.5 to 1:1.5 (in mol/mol) and/or hydrates and/or solvates thereof, as well as a process of obtaining the same. Upon issuance, the standard 20-year exclusivity for this patent would expire in 2037.
The prescription multi-vitamin market is dominated by generic products, with brands accounting for 9.5% of the multivitamin plus fluoride market for the year ending December 31, 2022. Poly-Vi-Flor and Tri-Vi-Flor primarily compete in the generic prescription multi-vitamin fluoride market and with the branded products FLORIVA and QFLORA.
Karbinal ER: Extended release carbinoxamine oral suspension for the treatment of seasonal and perennial allergies
Karbinal® ER (carbinoxamine maleate extended-release oral suspension) is an H1 receptor antagonist (antihistamine) indicated to treat seasonal and perennial allergic rhinitis, vasomotor rhinitis, allergic conjunctivitis due to inhalant allergens and food, mild, uncomplicated allergic skin manifestations of urticaria and angioedema, dermatographism, as therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled, and amelioration of the severity of allergic reactions to blood or plasma for patients two years of age and above.
Over 50 million Americans suffer from allergies in any given year, and allergies are the sixth leading cause of chronic illness in the U.S. Numerous allergy treatments exist to address allergies and allergic symptoms depending upon the symptom(s). Oral antihistamines are considered a mainstay of allergy treatment, and the prescription antihistamine market is a large category with approximately 52 million prescriptions written in 2021. The prescription antihistamine category is dominated by generic products and consists of first generation and second-generation molecules. Generally, first-generation antihistamines block both histaminic and muscarinic receptors and pass the blood-brain barrier. Second-generation antihistamines mainly block histaminic receptors, but they do not pass the blood-brain barrier. First generation antihistamines, which are generally characterized as more sedating, accounted for 6% of 2021 total prescriptions, while non-sedating, second generation antihistamines accounted for 94% of total prescriptions. The most widely prescribed oral, second-generation antihistamines are cetirizine (brand name Zyrtec®) and loratadine (brand name Claritin®). Diphenhydramine (brand name Benadryl®) is the most widely prescribed first-generation molecule.
Karbinal ER is the only FDA-approved, 12-hour carbinoxamine oral suspension and is an effective antihistamine with a broad range of indications. Karbinal ER is positioned as a second-line allergy treatment for patients who continue to suffer from allergic symptoms following initial treatment with a second-generation, non-sedating antihistamine. Further, as Karbinal ER is an oral suspension formulation, children are the primary target patient given their preference for liquid treatments and, in many cases, their inability to swallow tablets or capsules. Karbinal ER is indicated for children as young as two years of age. Karbinal has a pleasant strawberry-banana taste and is available in 480 mL bottles.
15
Through a supply and distribution agreement with Tris, we own exclusive rights to distribute Karbinal ER in the U.S. through August 2032, unless the agreement is terminated earlier pursuant to the termination provisions in the agreement. As part of the agreement, we pay sales-based royalties based on net revenue. Additionally, we are committed to make annual minimum payments to Tris through 2025.
Two core patents protect Karbinal ER in the U.S., and both parents are listed in the FDA’s Orange Book. The first patent describes a coated drug-ion exchange resin complex comprising a core composed of a drug complexed with a pharmaceutically acceptable ion-exchange resin. The priority date for this family is March 29, 2009, so the standard 20-year exclusivity for this patent will expire in 2029. The second patent describes an aqueous liquid suspension containing a coated drug-ion exchange resin complex comprising a core molecule complexed with a pharmaceutically acceptable ion-exchange resin and an uncoated ion exchange resin complex. The priority date for this family is June 15, 2007, so the standard 20-year exclusivity for this patent will expire in 2027.
Karbinal ER faces competition from OTC products such as non-sedating antihistamines, sedating antihistamines as well as nasal steroids, nasal antihistamines, and anticholinergics.
Consumer Health Segment
We acquired our consumer health business through the acquisition of Innovus Pharmaceuticals, Inc. in February 2020. The consumer health business is focused on OTC medicines and consumer health products designed to address common conditions. Now doing business as Aytu Consumer Health, we commercialize numerous products in the U.S. and Canada through two distinct marketing channels: e-commerce platforms including our websites and Amazon.com and via direct mail campaigns.
We classify our products into three categories:
| ● | ANDA/Medical Device OTC products, which compete in large consumer health categories and are marketed primarily through Amazon.com; |
| ● | OTC monograph products, which compete in large consumer health categories; and |
| ● | Dietary supplements and personal care products, which are proprietary products with strong scientific evidence and clinical support. |
The following represents the core Aytu Consumer Health OTC medicines, which are expected to be the Consumer Health Segment’s primary profit drivers:
| ● | Regoxidine® - for Men & Women – proprietary over-the-counter aerosol foam that works to treat hair loss in both men and women. |
| ● | OmepraCareDR® - acid reducer to treat frequent heartburn. |
| ● | EsomepraCareDR® - acid reducer to treat frequent heartburn. |
Given the company’s shift in focus and objective of generating near-term profitability, we expect to divest, monetize, or discontinue the Consumer Health operations by the end of fiscal 2024 or shortly thereafter.
We own over 200 trademarks for products in our Consumer Health Portfolio and own or license patents covering 9 of these products, some of which we plan to either license, sell, or discontinue as part of the planned divestiture, sale, or discontinuation of the Consumer Health Segment.
16
MANUFACTURING
ADHD Product Portfolio
For the production of our ADHD products, we lease a manufacturing site in Grand Prairie, Texas. This facility has 77,112 square feet of manufacturing and laboratory space and contains dedicated current Good Manufacturing Practices (“cGMP”) manufacturing suites for both Adzenys XR-ODT and Cotempla XR-ODT. We hold U.S. Drug Enforcement Administration (“DEA”) manufacturing and analytical licenses and maintain storage and use of Schedule II through IV controlled substances. The manufacture of our products is subject to extensive cGMP regulations, which impose various procedural and documentation requirements and govern all areas of record keeping, production processes and controls, personnel and quality control.
We are in the process of transferring the manufacturing of our ADHD products to a contract manufacturing organization (“CMO”). The transfer of the manufacturing of pharmaceutical products requires several steps including knowledge and method transfer, manufacturing of materials for feasibility studies and confirmation batch materials, bioequivalence studies, inspections from regulatory agencies, and regulatory filings. We have completed the required activities, including the successful completion of bioequivalence studies, which are required in order to enable the transfer of both Adzenys XR-ODT and Cotempla XR-ODT. The Adzenys XR-ODT Prior Approval Supplement (“PAS”) was approved by the FDA in April 2023, and the Cotempla XR-ODT PAS was submitted to the FDA in June 2023. We expect to receive approval for the Cotempla XR-ODT PAS by early calendar 2024. Thus, we expect the CMO to begin manufacturing both ADHD products in early calendar 2024.
In conjunction with transferring the manufacturing of our ADHD products to a CMO, we entered into an agreement with AMT Manufacturing Solutions, LLC, a newly established, full service CMO, to sublease 22,909 square feet of our Grand Prairie, Texas manufacturing facility. This sublease represents over 30% of our facility. In addition, commencing as early as April 1, 2024, but no later than December 31, 2024, the sublease will be expanded to include the remaining portion of the manufacturing facility. This agreement enables us to reduce costs associated with exiting the facility and allows for increased supply chain flexibility.
Pediatric Product Portfolio
We contract with CMOs for the manufacture and testing of our Pediatric Portfolio products. We have entered into the following key supply agreements for the commercial manufacture and supply of certain of these products:
| ● | Poly-Vi-Flor and Tri-Vi-Flor drops are purchased through a supply agreement with a CMO based in the U.S., and we expect to add our multivitamin chewable tables to this supply agreement. Until that time, the chewable tablets are being produced and purchased without a supply agreement specifically covering those purchases. Merck & Cie is responsible for providing Metafolin and Arcofolin to our designated CMO. |
| ● | A supply agreement with Tris Pharma for the supply of Karbinal. This agreement terminates in August 2033, subject to earlier termination or extension in accordance with the terms of the agreement. |
We believe the third-party manufacturers of our Pediatric Portfolio products have adequate capacity to manufacture sufficient quantities of these products to meet anticipated commercial demands. As we rely on CMOs, we employ personnel with extensive technical, manufacturing, supply chain management, and analytical and quality experience to oversee contract manufacturing and testing activities, and to compile manufacturing and quality information for our regulatory submissions. Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, and which govern record-keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among other activities. Our systems and our contractors are required to comply with these regulations, and we assess this compliance regularly through monitoring of performance and a formal audit program.
17
Consumer Health Segment
The Consumer Health Segment maintains relationships with a number of manufacturers and brokers from which it obtains its products. We attempt to work with a variety of manufacturers to broaden our supplier base and to optimize product acquisition costs and delivery schedules. For our OTC medicines we have relationships with three primary suppliers and one broker through which we source our consumer health products.
RESEARCH AND DEVELOPMENT
We have indefinitely suspended product candidate research and development activities in favor of focusing our resources on our commercialization efforts. With this re-focusing on commercial operations, development of our lead product candidate, AR101, is on indefinite hold. We are pursuing strategic partnerships in order to advance this program but have no assurance that a partnership will be consummated.
Our Development Pipeline: AR101 (enzastaurin for the treatment of Vascular Ehlers-Danlos Syndrome (VEDS))
AR101 (enzastaurin) is an orally available investigational first-in-class small molecule, serine/threonine kinase inhibitor of the protein kinase C (“PKC”) beta, PI3K and AKT pathways. AR101 has been studied in more than 3,300 patients across a range of solid and hematological tumor types. AR101 was originally developed by Eli Lilly and Company (“Lilly”), and worldwide rights were acquired by Denovo Biopharma in September 2014 following Lilly’s discontinuation of the enzastaurin development program.
VEDS is a rare genetic disorder typically diagnosed in childhood and characterized by arterial aneurysm, dissection and rupture, bowel rupture and rupture of the gravid uterus. VEDS is the severe subtype of Ehlers-Danlos Syndrome, affecting 1 in 50,000 people worldwide. VEDS results from pathogenic variants in the COL3A1 gene, which encodes the chains of type III procollagen, a major protein in vessel walls and hollow organs. Twenty-five percent of VEDS patients have a first complication by the age of 20 years, and more than 80 percent have at least one complication by the age of 40. VEDS patients have a median lifespan of 51 years. There are currently no FDA approved treatments for VEDS.
The research underpinning the application of enzastaurin for the treatment of VEDS has been conducted by Dr. Harry (Hal) Dietz and his research colleagues. Dr. Dietz is the Victor A. McKusick Professor of Genetics in the departments of medicine, pediatrics, and molecular biology and genetics at The Johns Hopkins University School of Medicine and director of the William S. Smilow Center for Marfan Syndrome Research. He has also been an investigator at Howard Hughes Medical Institute since 1997. Dr. Dietz is a leading scientist in the field of genetic connective tissue disorders and developed the first preclinical model that mimics the human condition and recapitulates VEDS. His group’s research findings were published in the Journal of Clinical Investigation in February 2020. The VEDS knock-in murine (mouse) preclinical model from Dr. Dietz has the same genetic mutation most prevalent in VEDS patients and is representative of the human condition in both the timing and location of vascular events. The model has generated identical structural histology and mechanical characteristics, and unbiased findings demonstrated that structure alone does not lead to vascular events. Objective comparative transcriptional profiling by high-throughput RNA sequencing of the aorta displayed a consistent molecular signature for excessive PKC/ERK cell signaling that is now known to be the driver of disease. Based on the scientific rationale for intervention along the PKC/ERK pathway, PKC inhibition and treatment with PKCβ inhibitors proved efficacious in multiple pre-clinical and murine studies and indeed prevented death due to vascular rupture.
In fiscal 2022 we received Orphan Drug Designation for AR101 in Ehlers-Danlos Syndrome including VEDS and in Europe, allowing for seven years’ marketing exclusivity in the United States and ten years in Europe. We also received Fast Track designation for AR101 in VEDS by the FDA, allowing for an accelerated review timeline upon submission of the New Drug Application (“NDA”) and more frequent interaction with the FDA during the development process.
AR101 is protected by a suite of five pending patents being pursued in major markets globally which have been licensed from Johns Hopkins and have an earliest priority date of March 2017.
18
The cornerstone of the intellectual property family surrounds enzastaurin initially targeting the treatment of VEDS focused on the U.S. and certain foreign jurisdictions which include Europe, Japan, China, Brazil, Mexico, Canada, Israel, Australia, New Zealand, and South Korea. This pending patent provides compositions and methods for treating VEDS and associated connective tissue disorders and has a priority date of October 2018. The second pending patent provides methods and compositions for the diagnosis, treatment, and prevention of Marfan syndrome and related diseases, disorders and conditions and has a priority date of March 2017, in select geographies. The third pending patent, titled “Targeted Epigenetic Therapy for Inherited Aortic Aneurysm Conditions,” broadens the coverage of the potential therapeutic application of AR101/Enzastaurin and has a priority date of September 2017. The fourth pending patent, titled “Pathway Targets for the Treatment of Vascular Ehlers-Danlos Syndrome”, and the fifth pending patent, titled “Endothelin-1 Signaling Contributes to Vascular Rupture Risk”, deepens the scientific evidence of the pathophysiology of Vascular Ehlers-Danlos Syndrome and are highly confirmatory of the therapeutic approach for AR101/Enzastaurin. These pending patents have priority dates of September 2020 and February 2022 respectively. Additional molecule intellectual property is afforded through the license with Denovo whose pending patent provides methods and compositions for the prediction of the activity of enzastaurin and has a priority date of September 1, 2016.
INTELLECTUAL PROPERTY
We seek trademark protection in the United States when appropriate. We currently own or license registered trademarks for Aytu, Aytu Biopharma, Neos Therapeutics, Innovus Pharma, Healight, Poly-Vi-Flor, Adzenys, Adzenys XR-ODT, Adzenys ER and Cotempla XR-ODT in the United States, as well as trademarks related to our DTRS technology.
From time to time, we may find it necessary or prudent to obtain licenses from third party intellectual property holders.
GOVERNMENT REGULATION
We are subject to extensive regulation by the FDA and other federal, state, and local regulatory agencies. The FDCA and the FDA's implementing regulations set forth, among other things, requirements for the testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record-keeping, reporting, distribution, import, export, sale, advertising and promotion of our products and product candidates. We may seek approval for, and market, our products in other countries in the future. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the U.S., although there can be important differences.
Development and Approval
Under the FDCA, FDA approval of an NDA is required before any new drug can be marketed in the U.S. NDAs in the case of new drugs, or PMAs or 510(k)s in the case of medical devices, may require extensive studies and submission of a large amount of data by the applicant, including the following:
Preclinical Testing. Preclinical testing generally includes laboratory evaluation of product chemistry and formulation, as well as toxicological and pharmacological studies in several animal species to assess the toxicity and dosing of the product.
Clinical Trials. Clinical trials involve the administration of a drug to healthy human volunteers or to patients, under the supervision of a qualified investigator.
| ● | Phase 1 clinical trials involve the initial administration of the investigational drug to humans, typically to a small group of healthy human subjects, but occasionally to a group of patients with the targeted disease or disorder. Phase 1 clinical trials generally are intended to evaluate the safety, metabolism and pharmacologic actions of the drug, the side effects associated with increasing doses, and, if possible, to gain early evidence of effectiveness. |
19
| ● | Phase 2 clinical trials generally are controlled studies that involve a relatively small sample of the intended patient population and are designed to develop initial data regarding the product's effectiveness, to determine dose response and the optimal dose range, and to gather additional information relating to safety and potential AEs. |
| ● | Phase 3 clinical trials are conducted after preliminary evidence of effectiveness has been obtained and are intended to gather the additional information about safety and effectiveness necessary to evaluate the drug's overall risk-benefit profile, and to provide a basis for physician labeling. Generally, Phase 3 clinical development programs consist of expanded, multi-site, large-scale studies of patients with the target disease or disorder to obtain statistical evidence of the efficacy and safety of the drug at the proposed dosing regimen. Phase 3 data often form the core basis on which the FDA evaluates a drug’s safety and effectiveness when considering the product application. |
Post-Approval Regulation
Once approved, drug products are subject to continuing regulation by the FDA. If ongoing regulatory requirements are not met or if safety or manufacturing problems occur after the product reaches the market, the FDA may at any time withdraw product approval or take actions that would limit or suspend marketing. Additionally, the FDA may require post-marketing studies or clinical trials, changes to a product’s approved labeling, including the addition of new warnings and contraindications, or the implementation of other risk management measures, including distribution-related restrictions, if there are new safety information developments.
DEA Regulation
Our ADHD products are considered a “controlled substance” as defined in the Controlled Substances Act of 1970, or CSA, because Adzenys XR-ODT contains amphetamine and Cotempla XR-ODT contains methylphenidate. Because amphetamine and methylphenidate are Schedule II controlled substances, the DEA has Adzenys XR-ODT and Cotempla XR-ODT listed and regulated as Schedule II controlled substances. None of our pediatric products (Poly-Vi-Flor, Tri-Vi-Flor and Karbinal ER) are considered “controlled substances.”
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule.
The DEA establishes annually an aggregate quota for how much of a controlled substance may be produced in and/or imported into the U.S. based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments. Our or our manufacturers’ quotas of an active ingredient may not be sufficient to meet commercial demand or complete clinical trials. Any delay, limitation or refusal by the DEA in establishing our or our manufacturers’ quota for controlled substances could delay or stop our clinical trials or product launches, which could have a material adverse effect on our business, financial position and results of operations.
Individual states also independently regulate controlled substances. We and our manufacturers will be subject to state regulation on distribution of these products, including, for example, state requirements for licensures or registration. Additionally, we use third-party logistics firms to inventory and fill sales orders for our commercial portfolio.
We contract with third parties for the manufacture and testing of Karbinal, Poly-Vi-Flor and Tri-Vi-Flor. Poly-Vi-Flor and Tri-Vi-Flor are not supplied under any contract. We have entered into the following key supply agreements for the commercial manufacture and supply of certain of these products:
| ● | A supply agreement with Tris for the supply of Karbinal. This agreement terminates in August 2033, subject to earlier termination or extension in accordance with the terms of the agreement. |
20
| ● | Poly-Vi-Flor and Tri-Vi-Flor drops are produced under a supply agreement with a CMO based in the U.S., and we expect to expand that agreement to include the chewable tablet formations. Until that time, the Ploy-Vi-Flor chewable tablets are produced by the same CMO on a purchase order-to-purchase order basis, Merck & Cie is responsible for providing Metafolin and Arcofolin to our designated CMO. |
We believe our third-party manufacturers have adequate capacity to manufacture sufficient quantities of these products to meet anticipated commercial demands. Because we rely on CMOs, we employ personnel with extensive technical, manufacturing, supply chain management, and analytical and quality experience to oversee contract manufacturing and testing activities, and to compile manufacturing and quality information for our regulatory submissions. Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, and which govern record-keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among other activities. Our systems and our contractors are required to comply with these regulations, and we assess this compliance regularly through monitoring of performance and a formal audit program.
For the production of our ADHD products, we lease one manufacturing site in Grand Prairie, Texas. This facility has 77,112 square feet of manufacturing and laboratory space, and contains dedicated cGMP manufacturing suites for both Adzenys XR-ODT and Cotempla XR-ODT. We hold DEA manufacturing and analytical licenses, and maintain storage and use of Schedule II through IV controlled substances. The manufacture of our products is subject to extensive cGMP regulations, which impose various procedural and documentation requirements and govern all areas of record keeping, production processes and controls, personnel, and quality control.
We are in the process of a technology transfer to outsource the manufacturing of our ADHD products to a CMO. The transfer of the manufacturing of pharmaceutical products requires several steps including knowledge and method transfer, manufacturing of materials for feasibility study and confirmation batch materials, bioequivalence studies and regulatory filings. We have completed the required activities, including the successful completion of bioequivalence studies, which are required in order to enable the transfer of both Adzenys XR-ODT and Cotempla XR-ODT. The Adzenys XR-ODT Prior Approval Supplement (“PAS”) was approved by the FDA in April 2023, and the Cotempla XR-ODT PAS was submitted to the FDA in June 2023. We expect to receive approval for the Cotempla XR-ODT PAS by early calendar 2024. We expect the CMO to begin manufacturing both ADHD products in early calendar 2024.
HUMAN CAPITAL
As of June 30, 2023, we employed 150 full-time employees, including 53 who are involved in operations, 5 who are directly involved in research and development, 60 who are involved in commercialization and 32 who are involved in general and administrative activities. All of our colleagues are located in the U.S. Of these colleagues, 45% are female and 55% are male. Our colleagues are not represented by a labor union.
Our values – team-oriented, hard-working, relentlessly determined, integrity, visionary, entrepreneurial, and servant-minded - are built on the foundation that the colleagues we hire and the way we treat one another promote creativity, innovation, and productivity, which spur our success. This culture depends in large part on our ability to attract, retain and develop a diverse population of talents and high-performing employees at all levels of our organization. Providing market competitive pay and benefit programs, opportunities to participate in the success they help create, while engaging colleagues in important dialogue regarding organization performance, we create a culture of inclusion in which all colleagues have the opportunity to thrive.
AVAILABLE INFORMATION
Our principal executive offices are located at 7900 East Union Avenue, Suite 920, Denver, Colorado 80237 USA, and our phone number is (720) 437-6580.
We maintain a website on the internet at http://aytubio.com. We make available, free of charge, through our website, by way of a hyperlink to a third-party site that includes filings we make with the SEC website (www.sec.gov), our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports electronically filed or furnished pursuant to Section 15(d) of the Exchange Act.
21
The information on our website is not, and shall not be deemed to be, a part of this Annual Report on Form 10-K or incorporated into any other filings we make with the SEC. In addition, the public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington D.C., 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330.
CODE OF ETHICS
We have adopted a written code of ethics that applies to our officers, directors, and employees, including our principal executive officer and principal accounting officer. We intend to disclose any amendments to, or waivers from, our code of ethics that are required to be publicly disclosed pursuant to rules of the SEC by filing such amendment or waiver with the SEC. This code of ethics and business conduct can be found in the corporate governance section of our website, https://investors.aytubio.com/corporate-governance#CorporateGovernance.
ITEM 1A. RISK FACTORS
Investing in our securities includes a high degree of risk. You should consider carefully the specific factors discussed below, together with all of the other information contained in this Annual Report on Form 10-K. If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects would likely be materially and adversely affected. This could cause the market price of our securities to decline and could cause you to lose all or part of your investment.
RISKS RELATED TO OUR BUSINESS AND FINANCIAL POSITION
We have incurred losses to date and can give no assurance of profitability.
We have incurred losses in each year since our inception. As of the filing of this Annual Report on Form 10-K, there is a substantial doubt regarding our ability to continue as a going concern. Our net loss for the years ended June 30, 2023 and 2022 was $17.1 million and $108.8 million, respectively. We have not demonstrated the ability to be a profit-generating enterprise to date. Even though we expect to have revenue growth in the next several fiscal years, it is uncertain that the revenue growth will be significant enough to offset our expenses and generate a profit in the future. Potential investors should evaluate us in light of the expenses, delays, uncertainties, and complications typically encountered by healthcare businesses, many of which will be beyond our control. These risks include the following:
●uncertain market acceptance of our products;
●difficulties in maintaining coverage and reimbursement for our products;
●lack of sufficient capital;
●U.S. and foreign regulatory approval of our products;
●unanticipated problems, delays, and expense relating to product development and implementation;
●lack of sufficient intellectual property;
●the ability to attract and retain qualified employees;
●competition; and
●technological changes.
As a result of the increasingly competitive nature of the markets in which we compete, our historical financial data is of limited value in anticipating future operating expenses. Our planned expense levels will be based in part on our expectations concerning future operations, which is difficult to forecast accurately based on our historical strategy of product and/or business acquisition to develop our product and business portfolio.
22
We may be unable to adjust spending in a timely manner to compensate for any unexpected budgetary shortfall.
To obtain revenues from our products, we must succeed, either alone or with others, in a range of challenging activities, including expanding markets for our existing products, manufacturing, marketing and selling our existing products, satisfying any post-marketing requirements, and obtaining reimbursement for our products from private insurance or government payors. We, and our collaborators, as applicable, may not be successful in these activities and, even if we or our collaborators do, we may never generate revenues that are sufficient to achieve profitability.
We have not established sources of ongoing revenue sufficient to cover operating costs and allow us to continue as a going concern.
Since our inception, we have had significant operating losses. As of June 30, 2023, we had accumulated deficit of $304.1 million. Even though we plan to mitigate the conditions that raise substantial doubt about our ability to continue as a going concern, we may continue to incur net losses, and our ability to generate positive cash flows from operating activities is uncertain for the foreseeable future. We have not established an ongoing source of revenue sufficient to cover operating costs. Our ability to continue as a going concern is dependent on our continued operational improvements, refinancing, or obtaining adequate capital to fund operating losses until we become profitable. If we are unable to generate sufficient cash flows or obtain adequate capital, we may be unable to develop and commercialize our product offerings and we could be forced to cease operations.
We may need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain necessary capital when needed may force us to delay, limit or terminate our product expansion efforts or other operations. Further, future sales and issuances of our common stock or rights to purchase common stock will result in dilution of the percentage ownership of our existing stockholders and could cause our stock price to fall.
We are expending resources to commercialize our prescription products and to service our debt obligations. We may require additional funding through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements, or a combination of these approaches. As of June 30, 2023, our cash and cash equivalents totaled $23.0 million. During the year ended June 30, 2023, we raised approximately $15.6 million, net of fees, from a combination of common stock offerings.
Our operating plans may change as a result of many factors currently unknown to us, and we could need additional capital in the future to continue our operations and may need to seek additional funds sooner than planned. Raising funds in the current economic environment may present additional challenges. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
If we sell common stock, convertible securities or other equity securities in more than one transaction, any such sales may result in material dilution to our existing stockholders, and new investors could gain rights, preferences, and privileges senior to those of our existing common stockholders. Further, any future sales of our common stock by us or resales of our common stock by our existing stockholders could cause the market price of our common stock to decline. Any future grants of securities exercisable or convertible into our common stock, or the exercise or conversion of such shares, and any sales of such shares in the market, could also have an adverse effect on the market price of our common stock.
In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. The incurrence of additional indebtedness would result in increased fixed payment obligations and we may be required to agree to additional restrictive covenants, such as further limitations on our ability to incur additional debt, additional limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business.
23
We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or products or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain funding on a timely basis, we may be unable to expand the market for our products or expand our operations generally or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
We may not have cash available to us in an amount sufficient to enable us to make interest or principal payments on our indebtedness when due.
We have a $15.0 million term loan with Avenue Capital and up to $14.5 million of secured revolving loans with Eclipse. As of June 30, 2023, $1.6 million was outstanding under the secured revolving loan. All obligations under our loans are secured by substantially all of our existing property and assets subject to certain exceptions. These debt financings and any future debt financings may create additional financial risk for us, particularly if our business or prevailing financial market conditions are not conducive to paying off or refinancing our outstanding debt obligations at maturity.
As a result, we may not have sufficient funds, or may be unable to arrange for additional financing, to pay the amounts due on our outstanding indebtedness under our debt agreements. Further, funds from external sources may not be available on economically acceptable terms, if at all. For example, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our products or technologies, or to grant licenses on terms that are not favorable to us. If adequate funds are not available when and if needed, our ability to make interest or principal payments on our debt obligations, and finance our operations and other general corporate activities would be significantly limited and we may be required to delay, significantly curtail, or eliminate one or more of our programs.
Failure to satisfy our current and future debt obligations under our loan agreements with Avenue Capital or Eclipse could result in an event of default and, as a result, our lenders could accelerate all of the amounts due. In the event of an acceleration of amounts due under one or both of our debt agreements as a result of an event of default, we may not have sufficient funds or may be unable to arrange for additional financing to repay our indebtedness. In addition, our lenders could seek to enforce their security interests in any collateral securing such indebtedness.
The terms of our loan agreement place restrictions on our operating and financial flexibility. If we raise additional capital through debt financing, the terms of any new debt could further restrict our operating and financial flexibility.
The loan agreements with Avenue Capital and Eclipse subject us to financial covenants and restrictions on our ability to incur liens, incur additional indebtedness, make certain dividends and distributions with respect to equity securities, engage in mergers and acquisitions or make asset sales without the prior written consent of the lender. Failure to comply with such covenants could permit the lenders to declare our obligations under the loan agreements, together with accrued interest and fees, to be immediately due and payable, plus any applicable additional amounts relating to a prepayment or termination.
These restrictive covenants could limit our flexibility in operating our business and our ability to pursue business opportunities that we or our stockholders may consider beneficial. Any declaration by the lender of an event of default could significantly harm our business and prospects and could cause the price of our common stock to decline. We may not have enough available cash or be able to raise additional funds through equity or debt financings to repay these outstanding obligations at the time any event of default occurs. Further, if we raise any additional capital through debt financing, the terms of such additional debt could further restrict our operating and financial flexibility.
24
We recently announced that we have been engaged in discussions with various parties regarding potential strategic transactions and potential financing options. There can be no assurance that this process will result in the pursuit or consummation of any potential transaction, or that any such potential transaction, if implemented, will provide sufficient funding to continue our operations.
We recently announced that we are engaged in discussions with various parties regarding potential strategic transactions and potential financing, which could include a financing, sale or licensing of assets, acquisition, merger, business combination, and/or other strategic transaction or series of related transactions. This process, including any uncertainty created by this process, involves a number of risks which could impact our business and our stockholders, including the following:
| ● | significant fluctuations in our stock price could occur in response to developments relating to the process or market speculation regarding any such developments; |
| ● | we may encounter difficulties in hiring, retaining and motivating key personnel during this process or as a result of uncertainties generated by this process or any developments or actions relating to it; |
| ● | we may incur substantial increases in general and administrative expense associated with increased legal fees and the need to retain and compensate third-party advisors; and |
| ● | we may experience difficulties in preserving the commercially sensitive information that may need to be disclosed to third parties during this process or in connection with an assessment of our strategic options. |
The review process also requires significant time and attention from management, which could distract them from other tasks in operating our business or otherwise disrupt our business. Such disruptions could cause concern to our suppliers, strategic partners or other constituencies and may have a material impact on our business and operating results and volatility in our share price.
There can be no assurance that this process will result in the pursuit or consummation of any potential transaction or strategy, or that any such potential transaction or strategy, if implemented, will provide sufficient funding to conduct our operations. Any outcome of this process would be dependent upon a number of factors that may be beyond our control, including, among other things, market conditions, industry trends, regulatory approvals, and the availability of financing on reasonable terms. The occurrence of any one or more of the above risks could have a material adverse impact on our business, financial condition, results of operations and cash flows.
We have indefinitely suspended development of our AR101 (enzastaurin) clinical development program and shifted our strategic focus towards accelerating the growth of our commercial business. If we fail to execute successfully on this reprioritized strategic focus, our business, results of operations and financial condition could be materially and adversely affected.
We have indefinitely suspended our AR101 (enzastaurin) clinical development program and shifted our focus towards accelerating the growth of our commercial business and achieving operating cash flows. Though we expect that the suspension of this program will save over $20 million in projected future study costs over the next three fiscal years, the process of reorienting our business strategy may be costly, time consuming and complex, and we have incurred, and may in the future incur, costs related to this strategic shift. Our strategic reprioritization may result in unexpected expenses or liabilities and/or write-offs. There is no assurance that we will be successful at executing on our revised strategy or that any particular course of action, business arrangement or transaction, or series of transactions, will be pursued, successfully consummated, lead to increased stockholder value, or achieve the anticipated results.
If we are unable to execute successfully on our reprioritized strategic focus, our cash resources may not last as long as estimated and our business, results of operations and financial condition could be materially and adversely affected.
25
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
As of June 30, 2023, we had federal net operating loss carryforwards of approximately $504.0 million. The available net operating losses, if not utilized to offset taxable income in future periods, will begin to expire in 2024 and, except for certain indefinite-lived net operating loss carryforwards, will completely expire in 2037. Under the Internal Revenue Code of 1986, as amended (the “Code”) and the regulations promulgated thereunder, including, without limitation, the consolidated income tax return regulations, various corporate ownership changes could limit our ability to use our net operating loss carryforwards and other tax attributes to offset our income.
An “ownership change” (generally a 50% change in equity ownership over a three-year period) under Section 382 of the Code could limit our ability to offset, post-change, our U.S. federal taxable income. Section 382 of the Code imposes an annual limitation on the amount of post-ownership change taxable income a corporation may offset with pre-ownership change net operating loss carryforwards and certain recognized built-in losses. We believe that the June 2021 acquisition of Neos caused an ownership change of Neos, resulting in a limitation in our ability to use their pre-acquisition net operating loss carryovers. We also believe that the financing transactions in fiscal 2022 and 2023 may have caused, together with equity ownership changes in the past three years, an ownership change resulting in a limitation of our ability to use our pre-acquisition net operating loss carryovers. The ownership change scenario could result in an increased future tax liability to us.
If we fail to establish and maintain proper internal controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Pursuant to Section 404 of the Sarbanes-Oxley Act, our management conducted an assessment of the effectiveness of our internal controls over financial reporting for the quarter ended September 30, 2022, and concluded that a certain control was not effective. We concluded that we had a material weakness in internal control over financial reporting related to accounting for complex warrant issuances and the classification of these issued warrants. In addition, we concluded that we had a material weakness in internal control over financial reporting for the year ended June 30, 2023 related to our analysis for the accounting for valuation of our inventory. Our Audit Committee conducted an internal investigation to identify and determine plans to remediate the material weaknesses and to enhance our overall control environment. We will not consider the material weaknesses remediated until our enhanced control is operational for a sufficient period of time and tested, enabling management to conclude that the enhanced controls are operating effectively. Our remediation plan includes the implementation of controls over the process of reviewing significant and complex contracts and agreements and we believe that the issues have been remediated.
If in the future we were to conclude that our internal controls over financial reporting were not effective, we cannot be certain as to the timing of completion of our evaluation, testing and remediation actions or their effect on our operations because there is presently no precedent available by which to measure compliance adequacy. As a consequence, we may not be able to complete any necessary remediation process in time to meet our deadline for compliance with Section 404 of the Sarbanes-Oxley Act. Also, there can be no assurance that we will not identify one or more material weaknesses in our internal controls in connection with evaluating our compliance with Section 404 of the Sarbanes-Oxley Act. The presence of material weaknesses could result in financial statement errors which, in turn, could require us to restate our operating results.
If we are unable to conclude that we have effective internal controls over financial reporting or if our independent auditors are unwilling or unable to provide us, when required, with an attestation report on the effectiveness of internal controls over financial reporting as required by Section 404 of the Sarbanes-Oxley Act, investors may lose confidence in our operating results, our stock price could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, we may not be able to maintain listing on the NASDAQ Capital Market. Due to our current filing status, we are not required to have our independent registered public accounting firm deliver an attestation report on the effectiveness of our internal control over financial reporting.
26
We have been and in the future may become a defendant in one or more stockholder derivative, class-action, and other litigation, and any such lawsuits may adversely affect our business, financial condition, results of operations and cash flows.
We and certain of our officers and directors have been and may in the future become defendants in one or more stockholder derivative actions or other class-action lawsuits. For example:
| ● | Two putative class action lawsuits were filed on February 9, 2022 and March 7, 2022 derivatively and on behalf of all Aytu stockholders, challenging the grant in 2021 of certain stock option awards to directors and officers, and seeking rescission of the awards, unspecified damages to stockholders as a result of the awards, and attorneys’ fees. |
| ● | A shareholder derivative suit was filed on September 12, 2022, derivatively and on behalf of all Aytu stockholders, against certain of our current and former directors and stockholders, alleging breaches of fiduciary duties in connection with certain acquisitions, and seeking unspecified damages, equitable relief, restitution, disgorgement of profits, enhanced governance and internal procedures, and attorneys’ fees. |
See Part I, Item 3. Legal Proceedings for more information on these lawsuits.
These lawsuits can divert our management’s attention and resources from our ordinary business operations, and we would likely incur significant expenses associated with their defense (including, without limitation, substantial attorneys’ fees and other fees of professional advisors and potential obligations to indemnify current and former officers and directors who are or may become parties to such actions). In connection with these lawsuits, we may be required to pay material damages, consent to injunctions on future conduct and/or suffer other penalties, remedies or sanctions, or issue additional shares upon the exercise of certain warrants, which may cause additional dilution. In addition, any such future lawsuits could adversely impact our reputation and/or ability to launch and commercialize our products, thereby harming our ability to generate revenue. Accordingly, the ultimate resolution of these matters and any future matters could have a material adverse effect on our business, financial condition, results of operation and cash flow and, consequently, could negatively impact the trading price of our common stock.
RISKS RELATED TO COMMERCIALIZATION
We are heavily dependent on the commercial success of our commercial products. To date, we have not generated sufficient revenues from the sales of these products to achieve profitability and we may never achieve or maintain profitability.
Our ability to become profitable depends upon our ability to generate increased revenues from sales of our prescription and consumer health product portfolios. While we have been selling pharmaceutical products for several years, we have limited commercial experience selling our current lineup of pharmaceutical products, having only generated revenues from the sale of our pediatric products since acquiring that portfolio in November 2019 and from our ADHD products since acquiring that portfolio in March 2021. None of our marketed prescription or consumer health products have thus far generated product sales revenues at levels sufficient for us to attain profitability. We have not generated any revenues from product sales of any other product candidates and, to date, have incurred significant operating losses.
We have incurred, and anticipate continuing to incur, significant costs associated with commercialization of our approved products and, if approved, any other product candidates that we may develop. It is possible that we will never attain sufficient product sales revenues to achieve profitability.
27
If we are unable to differentiate our products from branded drugs or existing generic therapies for similar treatments, or if the FDA or other applicable regulatory authorities approve additional generic products that compete with any of our products, our ability to successfully commercialize such products would be adversely affected.
We expect to compete against branded drugs with distinct clinical attributes and to compete with their generic counterparts that will be sold for a lower price. Although we believe that our Rx Portfolio is or will be differentiated from branded drugs and their generic counterparts, if any, including through clinical efficacy or through improved patient compliance, ease of administration, and our patient support programs, it is possible that such differentiation will not impact our market position. If we are unable to achieve significant differentiation for our products and accompanying support services against other drugs, the opportunity for our products to achieve premium pricing and be commercialized successfully would be adversely affected.
After a New Drug Application (“NDA”), including a 505(b)(2) application, is approved, the covered product becomes a “listed drug” that, in turn, can be cited by potential competitors in support of approval of an abbreviated new drug application, or ANDA. The FDCA, implementing regulations and other applicable laws provide incentives to manufacturers to create modified, non-infringing versions of a drug to facilitate the approval of an ANDA or other application for generic substitutes. These manufacturers might only be required to conduct a relatively inexpensive study to show that their product has the same active ingredient(s), dosage form, strength, route of administration, and conditions of use, or labeling as our product candidate and that the generic product is bioequivalent to ours, meaning it is absorbed in the body at the same rate and to the same extent as our product candidate. These generic equivalents, which must meet the same quality standards as the listed drugs, would be significantly less costly than ours to bring to market and companies that produce generic equivalents are generally able to offer their products at lower prices.
Thus, after the introduction of a generic competitor, a significant percentage of the sales of any branded product, such as our Rx Portfolio products, can be lost to the generic version. Accordingly, competition from generic equivalents to our products could materially adversely impact our revenues, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in our products. For example, on July 25, 2016, Neos received a paragraph IV certification from Actavis advising them that Actavis filed an ANDA with the FDA for a generic version of Adzenys XR-ODT. On October 17, 2017, Neos entered into a Settlement Agreement and a Licensing Agreement with Actavis (which is now owned by Teva), pursuant to which Neos granted Actavis the right to manufacture and market its now approved generic version of Adzenys XR-ODT under the ANDA beginning on September 1, 2025, or earlier under certain circumstances. On October 31, 2017, Neos received a paragraph IV certification from Teva advising them that Teva filed an ANDA with the FDA for a generic version of Cotempla XR-ODT. On December 21, 2018, Neos entered into a Settlement Agreement and a Licensing Agreement with Teva, pursuant to which we have granted Teva the right to manufacture and market its now approved generic version of Cotempla XR-ODT under the ANDA beginning on July 1, 2026, or earlier under certain circumstances.
While we expect to wind down or monetize our Consumer Health Segment, the Consumer Health Segment relies heavily on obtaining products that change from a prescription to over the counter through an FDA approval process. Any delays in this process might impact the financial performance of our consumer Health Segment.
Our Consumer Health Segment has pursued opportunities where existing prescription drugs have recently, or are expected to, change from a prescription to over-the-counter. Historically the FDA has highly scrutinized any product application submitted to switch a product from prescription to unsupervised over-the-counter use by the general public. The continued expansion of Rx-to-OTC switches is important to our Consumer Health Segment’s future growth. Reluctance of FDA to approve Rx-to-OTC switches in new product categories could impact that growth and could impact the financial performance of our Consumer Health Segment.
Our pharmaceutical and consumer health products may prove to be difficult to effectively commercialize as planned or on the timeframes we announce and expect.
Various commercial, regulatory, and manufacturing factors may impact our ability to maintain or grow revenues from sales of our pharmaceutical and consumer health product offerings. Moreover, we have limited experience selling some of our current products given their acquisition from other companies or their recent approval.
28
We sometimes estimate for planning purposes the timing of the accomplishment of various scientific, clinical, regulatory, and other product development objectives and, from time to time, we may publicly announce the expected timing of some of these milestones. The achievement of many of these milestones may be outside of our control and if we fail to achieve announced milestones in the timeframes we announce and expect, the commercialization of our products may be delayed and our business, prospects and results of operations may be harmed. Specifically, we may encounter difficulty by virtue of the following, each of which could be negatively impacted if expected timeframe goals are not achieved:
●our available capital resources;
●our inability to have clear proprietary rights to the products;
●our inability to manufacture or cost-effectively manufacture the products;
●our inability to adequately market and increase sales of any of these products;
●existence of adverse side effects that make using the products less desirable;
● |
our inability to attract and retain a skilled support team, marketing staff and sales force necessary to increase the market for our approved products and to maintain market acceptance for our products; |
● |
our inability to secure continuing prescribing of any of these products by current or previous users of the product; |
● |
our inability to effectively transfer and scale manufacturing as needed to maintain an adequate commercial supply of these products; |
● |
reimbursement and medical policy changes that may adversely affect the pricing, profitability or commercial appeal of pharmaceutical products; and |
● |
our inability to effectively identify and align with commercial partners outside the U.S., or the inability of those selected partners to gain the required regulatory, reimbursement, and other approvals needed to enable commercial success of our products. |
We rely on limited sources of supply for our products, and any disruption in the chain of supply may impact production and sales of our products, and cause delays in developing and commercializing our currently manufactured and commercialized products.
Some of our products are produced in single annual production lots by single-source suppliers. Due to the limited production quantities, production of these lots may not be prioritized by the third-party manufacturer, and may not be scheduled and produced at all. We are reliant on a limited number of suppliers for resin, drug compounds, coating and other component substances of our final products. If any of these single source suppliers were to breach or terminate its supply agreement, if any, with us or otherwise not supply us, we would need to identify an alternative source for the supply of component substances for our products. If we fail to procure supply of our products, we could lose potential revenue and our business, financial condition, results of operation and reputation could be adversely affected.
Identifying an appropriately qualified source of alternative supply for any one or more of the component substances for our products could be time consuming, and we may not be able to do so without incurring material delays in the development and commercialization of our approved products or a decrease in sales of our approved products, which could harm our financial position and commercial potential for our products. Any alternative vendor would also need to be qualified through an FDA Prior Approval Supplement process which could result in further delay. The FDA, DEA, or other regulatory agencies outside of the United States may also require additional studies if we enter into agreements with new suppliers for the manufacture of our ADHD products that differ from the suppliers used for clinical development of such products.
29
These factors could cause the delay of commercialization of our products, cause us to incur higher costs and prevent us from commercializing them successfully. Furthermore, if our suppliers fail to deliver the required commercial quantities of components and APIs on a timely basis and at commercially reasonable prices, including if our suppliers did not receive adequate DEA quotas for the supply of certain scheduled components, and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, commercialization of our ADHD products may be delayed or we could lose potential revenue and our business, financial condition, results of operation and reputation could be adversely affected.
We rely on third parties manufacture certain products, and third-party manufacturing risks and inefficiencies may result in costs and delays that prevent us from successfully commercializing products and adversely affect our ability to produce our products.
Our ADHD products are currently manufactured in our own production facility in Grand Prairie, Texas. We are in the process of outsourcing the manufacturing of our ADHD products to a third-party manufacturer to produce commercial quantities of our ADHD products beginning in late calendar 2023 or early calendar 2024. If the third party is not successful or does not meet our expectations (for example, timeliness of production, quantity of production, maintenance of needed documentation or regulatory compliance), we may have to find a different manufacturer and incur expenses and delays in the process. Manufacturers of our ADHD products must comply with good manufacturing practice ("GMP") requirements enforced by the FDA, NMPA, EMA and other comparable foreign health authorities through facilities inspection programs. These requirements include quality control, quality assurance, and the maintenance of records and documentation. Manufacturers of our FDA regulated products may be unable to comply with these GMP requirements and with other FDA, NMPA, EMA, DEA, state, and foreign regulatory requirements. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any quantities supplied is compromised due to a manufacturer’s failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our drugs, which would seriously harm our business.
For all other products and any future product, we expect to use third-party manufacturers because we do not expect to have our own manufacturing capabilities. In determining the required quantities of any product and the manufacturing schedule, we must make significant judgments and estimates based on inventory levels, current market trends and other related factors. Because of the inherent nature of estimates and our limited experience in marketing our current products, there could be significant differences between our estimates and the actual amounts of product we require. If we do not effectively maintain our supply agreements, we will face difficulty finding replacement suppliers, which could harm sales of those products. If we fail in similar endeavors for future products, we may not be successful in establishing or continuing the commercialization of our products.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured these components ourselves, including:
●reliance on third parties for regulatory compliance and quality assurance;
● |
possible breaches of manufacturing agreements by the third parties because of factors beyond our control; |
●possible regulatory violations or manufacturing problems experienced by our suppliers; and
● |
possible termination or non-renewal of agreements by third parties, based on their own business priorities, at times that are costly or inconvenient for us. |
Further, if we are unable to secure the needed financing to fund our internal operations, we may not have adequate resources required to effectively and rapidly transition to a third-party CMO for our ADHD products. We may not be able to meet the demand for our products if one or more of any third-party manufacturers is unable to supply us with the necessary components that meet our specifications.
30
It may be difficult to find alternate suppliers for any of our products in a timely manner and on terms acceptable to us.
The manufacturing processes and facilities of third-party manufacturers we have engaged for our current approved products are, and any future third-party manufacturer will be, required to comply with the federal Quality System Regulation, or QSR, which covers procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of devices. The FDA enforces the QSR through periodic unannounced inspections of manufacturing facilities. Any inspection by the FDA could lead to additional compliance requests that could cause delays in our product commercialization. Failure to comply with applicable FDA requirements, or later discovery of previously unknown problems with the manufacturing processes and facilities of third-party manufacturers we engage, including the failure to take satisfactory corrective actions in response to an adverse QSR inspection, can result in, among other things:
●administrative or judicially imposed sanctions;
●injunctions or the imposition of civil penalties;
●recall or seizure of the product in question;
●total or partial suspension of production or distribution;
●the FDA’s refusal to grant pending future clearance or pre-market approval;
●withdrawal or suspension of marketing clearances or approvals;
●clinical holds;
●warning letters;
●refusal to permit the export of the product in question; and
●criminal prosecution.
Any of these actions, in combination or alone, could prevent us from marketing, distributing or selling our products, and would likely harm our business.
In addition, a product defect or regulatory violation could lead to a government-mandated or voluntary recall by us. We believe the FDA would request that we initiate a voluntary recall if a product was defective or presented a risk of injury or gross deception. Regulatory agencies in other countries have similar authority to recall drugs or devices because of material deficiencies or defects in design or manufacture that could endanger health. Any recall would divert our management attention and financial resources, expose us to product liability or other claims, and harm our reputation with customers.
Third party performance failures may increase our development costs, delay our ability to obtain regulatory approval, and delay or prevent the commercialization of our products. While we believe that there are numerous alternative sources to provide these services, in the event that we seek such alternative sources, we may not be able to enter into replacement arrangements without incurring delays or additional costs.
31
If we or our contract manufacturer fail to manufacture our ADHD products in sufficient quantities and at acceptable quality and pricing levels, or fail to obtain adequate DEA quotas for controlled substances, or to fully comply with cGMP regulations, we may face delays in the commercialization of these products, or be unable to meet market demand, and may be unable to generate potential revenues.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls, and the use of specialized processing equipment. Pharmaceutical companies often encounter difficulties in manufacturing, particularly in scaling up production of their products. These problems include manufacturing difficulties relating to production costs and yields, quality control, including stability of the product and quality assurance testing, shortages of qualified personnel, as well as compliance with federal, state, and foreign regulations. If we are unable to demonstrate stability in accordance with commercial requirements, or if our raw material manufacturers were to encounter difficulties or otherwise fail to comply with their obligations to us, our ability to obtain FDA approval and market our products would be jeopardized. We purchase raw materials and components from various suppliers in order to manufacture our ADHD products. If we are unable to source the required raw materials from our suppliers, or if we do not obtain DEA quotas or receive inadequate DEA quotas, we may experience delays in manufacturing our ADHD products, and may not be able to meet customer demand for our products.
In addition, we and our contract manufacturer must comply with federal, state, and foreign regulations, including cGMP requirements enforced by the FDA through its facilities inspection program. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. We may be unable to comply with these cGMP requirements and with other FDA and foreign regulatory requirements. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or voluntary recall, or withdrawal of product approval. If the safety of any of our products is compromised due to failure to adhere to applicable laws or for other reasons, we may not be able to obtain, or to maintain once obtained, regulatory approval for such products or successfully commercialize such products, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay in commercialization of our products, entail higher costs or adversely impact our commercialization of our products. Any manufacturing defect or error discovered after products have been produced and distributed could result in even more significant consequences, including costly recall procedures, re-stocking costs, damage to our reputation and potential for product liability claims.
If our manufacturing facility becomes damaged or inoperable or we decide to or are required to vacate our facility, our ability to manufacture our ADHD products may be jeopardized. Our inability to continue manufacturing adequate supplies of our products could adversely affect our ability to generate revenues.
While we are in the process of transferring manufacturing at our Grand Prairie, Texas facility to a third-party manufacturer, all of our ADHD products manufacturing capabilities are currently housed in our sole manufacturing facility located in Grand Prairie, Texas. Our facility and equipment could be harmed or rendered inoperable by natural or manmade disasters, including war, fire, tornado, power loss, communications failure or terrorism, any of which may render it difficult or impossible for us to operate our drug delivery technology platform and manufacture our products for some period of time. While we seek to maintain finished goods inventory of our products outside of this facility, it is unlikely that the level of such inventory would be sufficient if we were to sustain anything other than a short-term disruption in our ability to manufacture our products at our Grand Prairie, Texas facility. The inability to manufacture our products if our facility or our equipment is inoperable, for even a short period of time, may result in the loss of customers or harm to our reputation, and we may be unable to regain those customers or repair our reputation in the future. Furthermore, our facility and the equipment we use to manufacture our products could become damaged and time consuming to repair or replace. It would be difficult, time consuming and expensive to rebuild our facility or repair or replace our equipment or to complete the transfer of our proprietary technology to a third party, particularly in light of the requirements for a DEA registered manufacturing and storage facility like ours and FDA site change requirements.
We carry insurance for damage to our property and the disruption of our business, but this insurance may not cover all of the risks associated with damage or disruption to our business, may not provide coverage in amounts sufficient to cover our potential losses and may not continue to be available to us on acceptable terms, if at all. An inability to continue manufacturing adequate supplies of our ADHD products at our Grand Prairie, Texas facility could result in a disruption in the supply of our products to physicians and pharmacies, which would adversely affect our ability to generate revenues.
32
In conjunction with transferring the manufacturing of our ADHD products to a CMO, we entered into an agreement with AMT Manufacturing Solutions, LLC to sublease approximately 30% of our Grand Prairie, Texas manufacturing facility. Commencing as early as April 1, 2024, but no later than December 31, 2024, the sublease will be expanded to include the remaining portion of the manufacturing facility.
If we do not secure collaborations with strategic partners to test, commercialize and manufacture products, we may not be able to successfully develop products and generate meaningful revenues.
We may enter into collaborations with third parties to commercialize and manufacture our products. If we are able to identify and reach an agreement with one or more collaborators, our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. Collaboration agreements typically call for milestone payments that depend on successful demonstration of efficacy and safety, obtaining regulatory approvals, and clinical trial results. Collaboration revenues are not guaranteed, even when efficacy and safety are demonstrated. Further, the economic environment at any given time may result in potential collaborators electing to reduce their external spending, which may prevent us from developing our products.
Collaboration agreements typically provide for the ownership of intellectual property. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration and we may be limited in our ability to use, make or sell these inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property.
Even if we succeed in securing collaborators, the collaborators may fail to develop or effectively commercialize our products. Collaborations involving our products pose a number of risks, including the following:
● |
collaborators may not have sufficient resources or may decide not to devote the necessary resources due to internal constraints such as budget limitations, lack of human resources, or a change in strategic focus; |
● |
collaborators may believe our intellectual property is not valid or is unenforceable or the product candidate infringes on the intellectual property rights of others; |
● |
collaborators may dispute their responsibility to conduct development and commercialization activities pursuant to the applicable collaboration, including the payment of related costs or the division of any revenues; |
● |
collaborators may decide to pursue a competitive product developed outside of the collaboration arrangement; |
● |
collaborators may not be able to obtain, or believe they cannot obtain, the necessary regulatory approvals; |
● |
collaborators may delay the development or commercialization of our products in favor of developing or commercializing their own or another party’s products; or |
●collaborators may decide to terminate or not to renew the collaboration for these or other reasons.
As a result, collaboration agreements may not lead to development or commercialization of our products in the most efficient manner or at all.
33
Collaboration agreements are generally terminable without cause on short notice. Once a collaboration agreement is signed, it may not lead to commercialization of a product. We also face competition in seeking out collaborators. If we are unable to secure collaborations that achieve the collaborator’s objectives and meet our expectations, we may be unable to advance our products and may not generate meaningful revenues.
We face substantial competition from companies with considerably more resources and experience than we have, which may result in others discovering, developing, receiving approval for, or commercializing products before or more successfully than us.
The biopharmaceutical industries are intensely competitive and subject to rapid and significant technological change. We compete with companies that design, manufacture and market already-existing and new products. We anticipate that we will face increased competition in the future as new companies enter the market with new technologies and/or our competitors improve their current products. One or more of our competitors may offer technology superior to ours and render our technology obsolete or uneconomical. Most of our current competitors, as well as many of our potential competitors, have greater name recognition, more substantial intellectual property portfolios, longer operating histories, significantly greater resources to invest in new technologies, more substantial experience in product marketing and new product development, greater regulatory expertise, more extensive manufacturing capabilities and the distribution channels to deliver products to customers. Our competitors may be more successful in acquiring new products than we are. If we fail to acquire new products, implementation of our business plan would be delayed, which could have a negative adverse effect on our business and prospects. If we are not able to compete successfully, we may not generate sufficient revenue to become profitable. Our ability to compete successfully will depend largely on our ability to:
● |
expand the market for our approved products, especially our pharmaceutical and devices regulated by the FDA; |
●successfully commercialize our products alone or with commercial partners;
●discover and develop products that are superior to other products in the market;
●obtain required regulatory approvals;
●attract and retain qualified personnel; and
●obtain patent and/or other proprietary protection for our products.
Established pharmaceutical companies devote significant financial resources to discovering, developing or licensing novel compounds that could make our products obsolete. Our competitors may obtain patent protection, receive FDA approval, and commercialize medicines before us. Other companies are or may become engaged in the discovery of compounds that may compete with the products we are developing.
We compete with companies that design, manufacture and market treatments that compete with our products. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and more experienced marketing and manufacturing organizations. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. As a result, these companies may obtain regulatory approval more rapidly than we are able and may be more effective in selling and marketing their products as well. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis drug products or drug delivery technologies that are more effective or less costly than that of our products or any product candidate that we are currently developing or that we may develop.
34
We anticipate that we will face increased competition in the future as new companies enter the market with new technologies and our competitors improve their current products. One or more of our competitors may offer technology superior to ours and render our technology obsolete or uneconomical. Most of our current competitors, as well as many of our potential competitors, have greater name recognition, more substantial intellectual property portfolios, longer operating histories, significantly greater resources to invest in new technologies, more substantial experience in new product development, greater regulatory expertise, more extensive manufacturing capabilities and the distribution channels to deliver products to customers. If we are not able to compete successfully, we may not generate sufficient revenue to become profitable. If we are not able to compete effectively against our current and future competitors, our business will not grow, and our financial condition and operations will suffer.
Government restrictions on pricing and reimbursement, as well as other healthcare payor cost-containment initiatives, may negatively impact our ability to generate revenues.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of health care costs to contain or reduce costs of health care may adversely affect one or more of the following:
●our or our collaborators’ ability to set a price we believe is fair for our approved products;
●our ability to generate revenue from our approved products and achieve profitability; and
●the availability of capital.
The Patient Protection and Affordable Care Act, or PPACA, and the Health Care and Education Reconciliation Act, or the Health Care Reconciliation Act, significantly impacted the provision of, and payment for, health care in the U.S. Various provisions of these laws are designed to expand Medicaid eligibility, subsidize insurance premiums, provide incentives for businesses to provide health care benefits, prohibit denials of coverage due to pre-existing conditions, establish health insurance exchanges, and provide additional support for medical research. Amendments to the PPACA and/or the Health Care Reconciliation Act, as well as new legislative proposals to reform healthcare and government insurance programs, along with the trend toward managed healthcare in the U.S., could influence the purchase of medicines and medical devices and reduce demand and prices for our products, if approved. This could harm our or our collaborators’ ability to market any approved products and generate revenues. As we expect to receive significant revenues from reimbursement of our Rx Portfolio products by commercial third-party payors and government payors, cost containment measures that health care payors and providers are instituting and the effect of further health care reform could significantly reduce potential revenues from the sale of any of our products approved in the future, and could cause an increase in our compliance, manufacturing or other operating expenses. In addition, in certain foreign markets, the pricing of prescription drugs and devices is subject to government control and reimbursement may in some cases be unavailable. We believe that pricing pressures at the federal and state level, as well as internationally, will continue and may increase, which may make it difficult for us to sell any approved product at a price acceptable to us or any of our future collaborators.
In addition, in some foreign countries, the proposed pricing for a drug or medical device must be approved before it may be lawfully marketed. The requirements governing pricing vary widely from country to country. For example, the EU provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product, or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. A member state may require that physicians prescribe the generic version of a drug instead of our approved branded product. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products or product candidates. Historically, pharmaceutical products launched in the EU do not follow price structures of the U.S. and generally tend to have significantly lower prices.
35
Our financial results will depend on the acceptance among clinicians, third-party payors and the medical community of our products.
Physicians may not choose to prescribe our products if we or any collaborator is unable to demonstrate that, based on experience, clinical data, side-effect profiles and other factors, our product is preferable to existing medicines or treatments. Our future success depends on the acceptance by our target customers, third-party payors, and the medical community that our products are reliable, safe, and cost-effective. We cannot predict the degree of market acceptance of any of our approved products. Many factors may affect the market acceptance and commercial success of our products, including:
● |
our ability to convince our potential customers of the advantages, safety and economic value our products and product candidates over existing technologies and products; |
●the approved labeling for the product and any required warnings;
●the prevalence and severity of adverse events or publicity;
●potential product liability claims
● |
the relative convenience and ease of our products over existing technologies and products; |
● |
the introduction of new technologies and competing products that may make our products less attractive for our target customers; |
●our success in training medical personnel on the proper use of our products;
● |
the willingness of third-party payors to reimburse our target customers that adopt our products; |
●increases in rebate payments with payors;
●the acceptance in the medical community of our products;
●the extent and success of our manufacturing, marketing, and sales efforts; and
●general economic conditions.
If our future products fail to gain market access and acceptance, this will have a material adverse impact on our ability to generate revenue to provide a satisfactory, or any, return on our investments. Even if some therapies achieve market access and acceptance, the market may prove not to be large enough to allow us to generate significant revenue.
If third-party payors do not reimburse our customers for the products we sell or if reimbursement levels are set too low for us to sell one or more of our products at a profit, our ability to sell those products and our results of operations will be harmed.
While our pharmaceutical products are approved and generating revenues in the U.S., they may not receive, or continue to receive, clinician or patient acceptance, or they may not maintain adequate reimbursement from third party payors. In the future, we might possibly sell other products to target customers substantially all of whom receive reimbursement for the health care services they provide to their patients from third-party payors, such as Medicare, Medicaid, other domestic and foreign government programs, private insurance plans and managed care programs. Reimbursement decisions by particular third-party payors depend upon a number of factors, including each third-party payor’s determination that use of a product is:
●a covered benefit under its health plan; ●appropriate and medically necessary for the specific indication;
36
●cost effective; and
●neither experimental nor investigational.
Third-party payors may deny reimbursement for covered products if they determine that a medical product was not used in accordance with cost-effective diagnosis methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors also may refuse to reimburse for procedures and devices deemed to be experimental.
Obtaining coverage and reimbursement approval for a product from each government or third-party payor is a time consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our potential product to each government or third-party payor. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. In addition, eligibility for coverage does not imply that any product will be covered and reimbursed in all cases or reimbursed at a rate that allows our potential customers to make a profit or even cover their costs.
Third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for medical products and services. Levels of reimbursement may decrease in the future, and future legislation, regulation or reimbursement policies of third-party payors may adversely affect the demand for and reimbursement available for any product or product candidate, which in turn, could negatively impact pricing. If our customers are not adequately reimbursed for our products, they may reduce or discontinue purchases of our products, which would result in a significant shortfall in achieving revenue expectations and negatively impact our business, prospects and financial condition.
Reporting and payment obligations under the Medicaid Drug Rebate Program and other governmental drug pricing programs are complex and may involve subjective decisions. Any failure to comply with those obligations could subject us to penalties and sanctions.
As a condition of reimbursement by various federal and state health insurance programs, pharmaceutical companies are required to calculate and report certain pricing information to federal and state agencies. The regulations governing the calculations, price reporting and payment obligations are complex and subject to interpretation by various government and regulatory agencies, as well as the courts. Reasonable assumptions have been made where there is a lack of regulations or clear guidance and such assumptions involve subjective decisions and estimates. Pharmaceutical companies are required to report any revisions to their calculations, price reporting and payment obligations previously reported or paid. Such revisions could affect liability to federal and state payers and also adversely impact reported financial results of operations in the period of such restatement.
Uncertainty exists as new laws, regulations, judicial decisions, or new interpretations of existing laws, or regulations related to our calculations, price reporting or payments obligations increases the chances of a legal challenge, restatement or investigation. If a company becomes subject to investigations, restatements, or other inquiries concerning compliance with price reporting laws and regulations, it could be required to pay or be subject to additional reimbursements, penalties, sanctions or fines, which could have a material adverse effect on the business, financial condition and results of operations. In addition, it is possible that future healthcare reform measures could be adopted, which could result in increased pressure on pricing and reimbursement of products and thus have an adverse impact on financial position or business operations.
Further, state Medicaid programs may be slow to invoice pharmaceutical companies for calculated rebates resulting in a lag between the time a sale is recorded and the time the rebate is paid. This results in a company having to carry a liability on its consolidated balance sheets for the estimate of rebate claims expected for Medicaid patients. If actual claims are higher than current estimates, the company’s financial position and results of operations could be adversely affected.
37
In addition to retroactive rebates and the potential for 340B Program refunds, if a pharmaceutical firm is found to have knowingly submitted any false price information related to the Medicaid Drug Rebate Program to the Centers for Medicare & Medicaid Services (“CMS”), it may be liable for civil monetary penalties. Such failure could also be grounds for CMS to terminate the Medicaid drug rebate agreement, pursuant to which companies participate in the Medicaid program. In the event that CMS terminates a rebate agreement, federal payments may not be available under government programs, including Medicaid or Medicare Part B, for covered outpatient drugs.
Additionally, if a pharmaceutical company overcharges the government in connection with the FSS program or Tricare Retail Pharmacy Program, whether due to a misstated Federal Ceiling Price or otherwise, it is required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges can result in allegations against a company under the FCA and other laws and regulations. Unexpected refunds to the government, and responding to a government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Our collaborators are also subject to similar requirements outside of the U.S. and thus the attendant risks and uncertainties. If our collaborators suffer material and adverse effects from such risks and uncertainties, our rights and benefits for our licensed products could be negatively impacted, which could have a material and adverse impact on our revenues.
Our future growth may depend, in part, on our ability to penetrate foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability may depend, in part, on our ability to commercialize our products in foreign markets for which we intend to primarily rely on collaboration with third parties such as the agreement we entered into with Medomie Pharma Ltd. in July 2023 to sell Adzenys and Cotempla in Israel and the Palestinian Authority. If we commercialize our products in foreign markets, we would be subject to additional risks and uncertainties, including:
●our inability to directly control commercial activities because we are relying on third parties;
● |
the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements; |
●different medical practices and customs in foreign countries affecting acceptance in the marketplace;
●import or export licensing requirements;
●longer accounts receivable collection times;
●longer lead times for shipping;
●language barriers for technical training;
● |
reduced protection of intellectual property rights in some foreign countries, and related prevalence of generic alternatives to our products; |
●foreign currency exchange rate fluctuations;
●our customers’ ability to obtain reimbursement for our products in foreign markets; and
●the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute.
Foreign sales of our products could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs.
38
We are subject to U.S. and foreign anti-corruption and anti-money laundering laws with respect to our operations and non-compliance with such laws can subject us to criminal and/or civil liability and harm our business.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, third-party intermediaries, joint venture partners and collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. We may have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. In addition, we may engage third party intermediaries to obtain necessary permits, licenses, and other regulatory approvals. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize or have actual knowledge of such activities.
We have adopted a Code of Business Conduct and Ethics that mandates compliance with the FCPA and other anti-corruption laws applicable to our business throughout the world. We cannot ensure, however, that our employees and third party intermediaries will comply with this code or such anti-corruption laws. Noncompliance with anti-corruption and anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension and/or debarment from contracting with certain persons, the loss of export privileges, reputational harm, adverse media coverage, and other collateral consequences. If any subpoenas, investigations, or other enforcement actions are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations and financial condition could be materially harmed. In addition, responding to any such action will likely result in a materially significant diversion of management's attention and resources and significant defense and compliance costs and other professional fees. In certain cases, enforcement authorities may even cause us to appoint an independent compliance monitor which can result in added costs and administrative burdens.
We are subject to various health care fraud and abuse and reimbursement laws pertaining to the marketing of our approved products.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including prohibitions on the offer of payment or acceptance of kickbacks or other remuneration for the purchase of our products, including inducements to potential patients to request our products and services. Additionally, any product promotion educational activities, support of continuing medical education programs, and other interactions with health-care professionals must be conducted in a manner consistent with the FDA regulations, Physician Payments Sunshine Act, and the Anti-Kickback Statute. The Anti-Kickback Statute prohibits persons or entities from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Violations of the Anti-Kickback Statute can also carry potential federal False Claims Act liability. Additionally, many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any third-party payer, not only the Medicare and Medicaid programs, and do not contain identical safe harbors. These and any new regulations or requirements may be difficult and expensive for us to comply with, may adversely impact the marketing of our existing products or delay introduction of our products, which may have a material adverse effect on our business, operating results and financial condition.
Adzenys XR-ODT and Cotempla XR-ODT contain controlled substances, and their manufacture, use, sale, importation, exportation, prescribing and distribution are subject to regulation by the DEA.
Adzenys XR-ODT and Cotempla XR-ODT, (collectively, our “Controlled Substance Products”), which are approved by the FDA, are regulated by the DEA as Schedule II controlled substances.
39
Before any commercialization of any product candidate that contains a controlled substance, the DEA determines the controlled substance schedule of a drug, taking into account the recommendation of the FDA. Our Controlled Substance Products are, and our other future products may, if approved, be regulated as “controlled substances” as defined in the Controlled Substances Act of 1970, or CSA, and the implementing regulations of the DEA, which establish registration, security, recordkeeping, reporting, storage, distribution, importation, exportation, inventory, quota and other requirements administered by the DEA. These requirements are applicable to us, to our third-party manufacturers and to distributors, prescribers, and dispensers of our products. For example, Schedule II controlled substances are subject to various restrictions, including, but not limited to, mandatory written prescriptions and the prohibition of refills. The DEA regulates the handling of controlled substances through a closed chain of distribution. This control extends to the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce. A number of states and foreign countries also independently regulate these drugs as controlled substances. State-controlled substance laws and regulations may have more extensive requirements than those determined by the DEA and FDA. Though state-controlled substances laws often mirror federal law because the states are separate jurisdictions, they may schedule products separately. While some states automatically schedule a drug when the DEA does so, other states require additional state rulemaking or legislative action, which could delay commercialization. Some state and local governments also require manufacturers to operate a drug stewardship program that collects, secures, transports, and safely disposes of unwanted drugs. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use, and may not be marketed or sold in the U.S. A pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances are considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances.
Amphetamine and methylphenidate, which are the active ingredients in our Adzenys XR-ODT and Cotempla XR-ODT products, respectively, are listed by the DEA as a Schedule II controlled substance under the CSA. Scheduled controlled substances are subject to DEA regulations relating to supply, procurement, manufacturing, storage, distribution, and physician prescription procedures. We currently manufacture these products in our own facilities, which are registered with and inspected by the DEA. Our planned contract manufacturer is also registered with and inspected by the DEA.
Registered entities are subject to DEA inspection and also must follow specific labeling and packaging requirements, and provide appropriate security measures to control against diversion of controlled substances. Security requirements vary by controlled substance schedule with the most stringent requirements applying to Schedule I and Schedule II controlled substances. Required security measures include background checks on employees and physical control of inventory through measures such as vaults and inventory reconciliations. Failure to follow these requirements can lead to significant civil and/or criminal penalties and possibly even lead to a revocation of a DEA registration. The DEA also has a production and procurement quota system that controls and limits the availability and production of Schedule I or II controlled substances. If we or any of our suppliers of raw materials that are DEA classified as Schedule I or II controlled substances are unable to receive any quota or a sufficient quota to meet demand for our products, if any, our business would be negatively impacted.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule.
Because of their restrictive nature, these laws and regulations could limit commercialization of our products containing controlled substances. Failure to comply with these laws and regulations could also result in withdrawal of our DEA registrations, disruption in manufacturing and distribution activities, consent decrees, criminal and civil penalties, and state actions, among other consequences.
The design, development, manufacture, supply and distribution of our products are highly regulated processes and technically complex.
We are subject to extensive regulation of the preparation and manufacture of our products for commercial sale. Components of a finished therapeutic product approved for commercial sale or used in late stage clinical trials must be manufactured in accordance with cGMPs and equivalent foreign standards. These regulations govern manufacturing processes and procedures, including record keeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of our products that may not be detectable in final product testing.
40
The development, manufacture, supply, and distribution of our approved products as well as any of our future potential products, are highly regulated processes and technically complex. We, along with our third-party suppliers, must comply with all applicable regulatory requirements of the FDA and foreign authorities. For instance, because each of our ADHD products is a regulated drug product and subject to the DEA and state-level regulations, we have had to, and will continue to, need to secure state licenses from each required state in which we intend to sell such product allowing us to distribute a regulated drug product in such state.
Regulatory authorities also may audit our manufacturing facilities. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we may be required to take remedial measures that may be costly and/or time consuming for us to implement and that may include the temporary or permanent suspension of commercial sales or the temporary or permanent closure of our facility. Any such remedial measures imposed upon us could materially harm our business. If we fail to maintain regulatory compliance, the FDA can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new drug product or revocation of a pre-existing approval, or civil or criminal penalties. As a result, our business, financial condition and results of operations may be materially harmed.
There is a risk we may be unable to sell and distribute certain of our products if we cannot continue to comply with the serialization requirements of the Drug Quality and Security Act within the necessary time frames.
Title II of the Drug Quality and Security Act of 2013 provided increased FDA oversight over tracking and monitoring of the sale and distribution of prescription drugs. We are required to provide product identification information, or serialization, at the manufacturing batch, or lot level. In addition, we are required to track and verify wholesaler and pharmacy authentication and verification. By the end of 2023 we will be required to conduct unit level tracking throughout the entire supply chain. We are now serializing our products and are compliant with the Drug Quality and Security Act, but there is no guarantee that we will be able to continue to satisfy each ever-stringent product identification requirements. Failing to do so could result in a delay or inability to sell our products within the United States.
Failure to comply with health and data protection laws and regulations could lead to U.S. federal and state government enforcement actions, including civil or criminal penalties, private litigation, and adverse publicity and could negatively affect our operating results and business.
We and any potential collaborators may be subject to U.S. federal and state data protection laws and regulations, such as laws and regulations that address privacy and data security. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws, govern the collection, use, disclosure, and protection of health-related and other personal information. In addition, we may obtain health information from third parties, including research institutions which are subject to privacy and security requirements under HIPAA, as amended by Health Information Technology for Economic and Clinical Health (“HITECH”). To the extent that we act as a business associate to a healthcare provider engaging in electronic transactions, we may also be subject to the privacy and security provisions of HIPAA, as amended by HITECH, which restricts the use and disclosure of patient-identifiable health information, mandates the adoption of standards relating to the privacy and security of patient-identifiable health information, and requires the reporting of certain security breaches to healthcare provider customers, the federal government, and media outlets with respect to such information. Additionally, many states have enacted similar laws that may impose more stringent requirements on entities like ours. Depending on the facts and circumstances, we could be subject to significant civil, criminal, and administrative penalties if we obtain, use, or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
Compliance with U.S. and foreign privacy and data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Failure to comply with these laws and regulations could result in government enforcement actions (which could include civil, criminal, and administrative penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business.
41
Moreover, employees and other individuals about whom we or our potential collaborators obtain personal information, as well as the providers who share this information with us, may limit our ability to collect, use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.
We may use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time-consuming and costly.
Our research and development processes may involve the controlled use of hazardous materials, including chemicals and biological materials. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed any insurance coverage and our total assets. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these hazardous materials and specified waste products, as well as the discharge of pollutants into the environment and human health and safety matters. Compliance with environmental laws and regulations may be expensive and may impair our research and development efforts. If we fail to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
We are dependent on our relationships and license agreements, and we rely on the intellectual property rights granted to us pursuant to the license agreements.
A number of our patent and trademark rights are derived from our license agreements with third parties. Pursuant to these license agreements, we have licensed rights to various patents, patent applications, trademarks and trademark applications within and outside of the United States. We may lose our rights to this intellectual property if we breach our obligations under such license agreements, including, without limitation, our financial obligations to the licensors. If we violate or fail to perform any term or covenant of the license agreements, the licensors may terminate the license agreements upon satisfaction of applicable notice requirements and expiration of any applicable cure periods. Additionally, any termination of license agreements, whether by us or the licensors may not relieve us of our obligation to pay any license fees owing at the time of such termination. If we fail to retain our rights under these license agreements, we will not be able to commercialize certain products subject to patent or patent application or trademark or trademark application, and our business, results of operations, financial condition and prospects would be materially adversely affected. In addition, the licensor may not be able to obtain valid and enforceable patents that protect the licensed products and may not be able to prevent third parties from infringing on those rights.
From time to time we may renegotiate the terms of our existing licensing agreements or other material contracts. There can be no guarantee that the terms of the renegotiated license agreement will be viewed favorably by the market although the renegotiated terms might be advantageous to our business or that the other party would agree to material changes to benefit the Company. For example, in May 2022, we negotiated to terminate the License, Development, Manufacturing and Supply agreement with Tris. The negotiations resulted in reducing the future minimum payments we owed to Tris by approximately $8 million. If we were unable to renegotiate the terms of the agreement, it would have had a material negative impact on our cash flows and financial position.
42
The expiration or loss of patent protection may adversely affect our future revenues and operating results.
The suite of composition-of-matter patents for Adzenys XR-ODT are scheduled to expire in 2026 and 2032. The composition-of-matter patents in the U.S. for Cotempla XR-ODT expire in 2032, and the method-of-use patent expires in 2038. There is no guarantee that we will be able to extend the life of these patents or to obtain additional patents, licenses, or other instruments that can provide us with a comparable level of exclusivity to the intellectual property underlying the expiring patents.
We rely on patent, trademark and other intellectual property protection in the discovery, development, manufacturing and sale of our products. In particular, patent protection is, in the aggregate, important in our marketing of products in the United States. Patents covering our products normally provide market exclusivity, which is important for the profitability of many of our products.
As patents for certain of our products expire, we may face competition from lower priced generic or bioequivalent products. In general, the expiration or loss of patent protection for a product may allow market entry by substitute products that could significantly reduce sales for the original product in a short amount of time. If our competitive position is compromised because of generic or bioequivalent products or otherwise, it could have a material adverse effect on our business and results of operations. In addition, proposals emerge from time to time for legislation to further encourage the early and rapid approval of generic or bioequivalent products. Any such proposals that are enacted into law could increase the negative effect of generic competition.
Our ability to compete may decline if we do not adequately protect or enforce our intellectual property rights.
Our success depends in part on our ability to manufacture, use, sell and offer to sell our products and in obtaining and maintaining intellectual property rights in our products, proprietary know-how and technology advances. We rely on patent protection, as well as a combination of trademark and trade secret laws to protect and prevent others from making, using and/or selling our compounds, processes, apparatuses and technology. While a presumption of validity exists with respect to patents issued to us in the U.S., there can be no assurance that any of our patents will not be challenged, invalidated, circumvented or rendered unenforceable. Such means may afford only limited protection of our intellectual property and may not (i) prevent our competitors from duplicating our inventions; (ii) prevent our competitors from gaining access to our proprietary information and technology; or (iii) permit us to gain or maintain a competitive advantage. In addition, our competitors or other third parties may obtain patents that restrict or preclude our ability to lawfully practice, produce or sell our products in a competitive manner.
Obtaining and maintaining a patent portfolio entails significant expense and resources. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer. In addition, the patent scope can be limited in prosecution or by the courts after issuance.
In addition, we may face claims by third parties that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have an adverse impact on our business.
Legal actions to enforce our patent rights and administrative challenges at the U.S. Patent and Trademark Office can be expensive and may involve the diversion of significant management time. In addition, these actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or other actions against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our business, prospects, financial condition and results of operations.
43
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, because we operate in the highly technical field of development of therapies and medical devices, we rely in part on trade secret protection in order to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We expect to enter into confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific and commercial collaborators, sponsored researchers, and other advisors. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements also generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, courts outside the U.S. may be less willing to protect trade secrets. Trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the U.S. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to pharmaceuticals and medical devices. This could make it difficult for us to stop the infringement of some of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. In addition, some countries allow patents to be challenged by third parties in administrative proceedings, which may result in a reduction in scope or cancelation of some or all of the claims. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the U.S. and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
There is significant litigation in the pharmaceutical industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, and are not aware of any such threatened litigation, we may be exposed to future litigation by third parties based on claims that our products infringe the intellectual property rights of others.
44
If our development and commercialization activities are found to infringe any such patents, we may have to pay significant damages or seek licenses to such patents. A patentee could prevent us from using the patented drugs, compositions or devices that relate to our prescription and consumer health business. We may need to resort to litigation to enforce a patent issued to us, to protect our trade secrets, or to determine the scope and validity of third-party proprietary rights. From time to time, we may hire scientific personnel or consultants formerly employed by other companies or universities involved in one or more areas similar to the activities conducted by us. Either we or these individuals may be subject to allegations of trade secret misappropriation, wrongful disclosure of confidential information, or other similar claims as a result of prior affiliations. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. We may not be able to afford the costs of litigation. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our cash position and stock price. Any legal action against us or our collaborators could lead to:
● |
payment of damages, potentially treble damages, if we are found to have willfully infringed a party’s intellectual property rights; |
● |
injunctive or other equitable relief that may effectively block our ability to further develop, commercialize, and sell products; or |
● |
we or our collaborators having to enter into license arrangements that may not be available on commercially reasonable or acceptable terms, if at all, all of which could have a material adverse impact on our cash position and business, prospects and financial condition. As a result, we could be prevented from commercializing our products. |
RISKS RELATED TO OUR ORGANIZATION, STRUCTURE AND OPERATION
Our efforts to expand and transform our businesses may require significant investments; if our strategies are unsuccessful, our business, results of operations and/or financial condition may be materially adversely affected.
We continuously evaluate opportunities for expansion and change. These initiatives may involve making acquisitions, entering into partnerships and joint ventures, divesting assets, restructuring our existing operations and assets, creating new financial structures and building new facilities—any of which could require a significant investment and subject us to new kinds of risks. We may incur additional indebtedness to finance these opportunities. If our strategies for growth and change are not successful, we could face increased financial pressure, such as increased cash flow demands, reduced liquidity and diminished access to financial markets, and the equity value of our businesses could be diluted.
The implementation of strategies for growth and change may create additional risks, including:
●diversion of management time and attention away from existing operations;
● |
requiring capital investment that could otherwise be used for the operation and growth of our existing businesses; |
●disruptions to important business relationships;
●increased operating costs;
●limitations imposed by various governmental entities; and
●difficulties due to lack of or limited prior experience in any new markets we may enter.
45
Our inability to mitigate these risks or other problems encountered in connection with our strategies for growth and change could have a material adverse effect on our business, results of operations and financial condition. In addition, we may fail to fully achieve the savings or growth projected for current or future initiatives notwithstanding the expenditure of substantial resources in pursuit thereof.
We may have difficulties integrating acquired products and businesses and as a result, our business, results of operations and/or financial condition may be materially adversely affected.
We have completed a number of acquisitions, and we intend to continue to acquire additional products and businesses through mergers, asset purchases or in-licensing, businesses or products, or form strategic alliances as part of our business strategy. Such growth strategies involve risks, including:
●inability to efficiently operate new businesses or to integrate acquired products and businesses;
● |
inability to accurately predict delays in realizing the costs and benefits of acquisitions, partnerships, or joint ventures; |
●unexpected losses of customers or suppliers of an acquired or existing business;
●difficulties in retaining key employees of acquired businesses;
●difficulties in realizing projected synergies;
●failure of the acquired business to produce the expected value;
● |
exposure to unanticipated liabilities, including unexpected environmental exposures, litigation challenging a merger, product liability or illegal activities conducted by an acquired company or a joint venture partner. |
Our inability to address these risks in a timely manner or at all could cause us to fail to realize the anticipated benefits of such acquisitions or joint ventures and could have a material adverse effect on our business, results of operations and financial condition.
In fiscal 2023, the great majority of our gross revenue and gross accounts receivable were due to three significant customers, the loss of which could materially and adversely affect our results of operations.
Three customers contributed greater than 10% of our gross revenue during the years ended June 30, 2023 and 2022. During the years ended June 30, 2023 and 2022, three customers accounted for 78% of gross revenue, respectively. The loss of one or more of our significant customers could have a material adverse effect on our business, operating results or financial condition. Any reduction, delay or cancellation of an order from these customers or the loss of any of these customers could cause our revenue to decline. If we are unable to diversify our customer base, we will continue to be susceptible to risks associated with customer concentration.
Our accounts receivable subjects us to credit risk.
We are also subject to credit risk from our accounts receivable related to our product sales. As of June 30, 2023, three customers accounted for 83% of gross accounts receivable. Our profitability and cash flow are dependent on receipt of timely payments from customers. Any delay in payment by our customers may have an adverse effect on our profitability, working capital and cash flow. There is no assurance that we will be able to collect all or any of its trade receivables in a timely matter. If any of our customers face unexpected situations such as financial difficulties, we may not be able to receive full or any payment of the uncollected sums or enforce any judgment debts against such clients, and our business, results of operations and financial condition could be materially and adversely affected.
46
We depend on key personnel and attracting qualified management personnel and our business could be harmed if we lose personnel and cannot attract new personnel.
Our success depends to a significant degree upon the technical and management skills of our directors, officers, and key personnel. Any of our directors could resign from our board at any time and for any reason. Although our named executive officers Joshua Disbrow and Mark Oki have employment agreements, the existence of an employment agreement does not guarantee the retention of the executive officer for any period of time, and each agreement obligates us to pay the officer lump sum severance of two and a half years and one year, respectively, of salary if we terminate him without cause, as defined in the agreement, which could hurt our liquidity. The loss of the services of either of these individuals would likely have a material adverse effect on us. Our success also will depend upon our ability to attract and retain additional qualified management, marketing, technical, and sales executives and personnel. We do not maintain key person life insurance for any of our officers or key personnel. The loss of any of our directors or key executives, or the failure to attract, integrate, motivate, and retain additional key personnel could have a material adverse effect on our business.
We compete for such personnel, including directors, against numerous companies, including larger, more established companies with significantly greater financial resources than we possess. There can be no assurance that we will be successful in attracting or retaining such personnel, and the failure to do so could have a material adverse effect on our business, prospects, financial condition, and results of operations.
Product liability and other lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
We will be exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing, and use of therapeutic candidates. Any failure of future therapeutic candidates by us and our corporate collaborators may expose us to liability claims as may the potential sale of any therapies approved in the future. These claims might be made by patients who use our therapies, healthcare providers, pharmaceutical companies, our corporate collaborators or other third parties that research or sell our therapies. Any claims against us, regardless of their merit, could be difficult and costly to defend and could materially adversely affect the market for our future therapeutic candidates or any prospects for commercialization of our future therapeutic candidates.
The risk that we may be sued on product liability claims is inherent in the development and commercialization of pharmaceutical, medical device, dietary supplement and personal care products. Side effects of, or manufacturing defects in, products that we develop and commercialized could result in the deterioration of a patient’s condition, injury or even death. Once a product is approved for sale and commercialized, the likelihood of product liability lawsuits increases. Claims may be brought by individuals seeking relief for themselves or by individuals or groups seeking to represent a class. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forgo further commercialization of the affected products.
We may be subject to legal or administrative proceedings and litigation other than product liability lawsuits which may be costly to defend and could materially harm our business, financial condition and operations.
Although we maintain general liability and product liability insurance, this insurance may not fully cover potential liabilities. In addition, insurance coverage is increasingly expensive and difficult to obtain. For example, we have experienced increasing difficulty in procuring insurance coverage for our products, in particular, our ADHD products, due to their status as controlled substances. Inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product or other legal or administrative liability claims could prevent or inhibit the commercial production and sale of any of our products that receive regulatory approval, which could adversely affect our business. Product liability claims could also harm our reputation, which may adversely affect our collaborators’ ability to commercialize our products successfully. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
47
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to the Company.
Our certificate of incorporation provides that we will indemnify our directors to the fullest extent permitted by Delaware law.
In addition, as permitted by Section 145 of the Delaware General Corporation Law, our bylaws provide that:
|
● |
we may, in our discretion, indemnify other officers, employees and agents in those circumstances where indemnification is permitted by applicable law; |
|
● |
we are required to advance expenses, as incurred, to our directors and executive officers in connection with defending a proceeding, except that such directors or executive officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification; |
|
● |
we will not be obligated pursuant to our bylaws to indemnify any director or executive officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law, (ii) the proceeding was authorized by our Board of Directors, (iii) such indemnification is provided by us, in our sole discretion, pursuant to the powers vested in the corporation under applicable law or (iv) such indemnification is required to be made pursuant to our amended and restated bylaws; |
|
● |
the rights conferred in our bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons; and |
|
● |
we may not retroactively amend our bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents. |
As a result, if we are required to indemnify one or more of our directors or executive officers, it may reduce our available funds to satisfy successful third-party claims against us, may reduce the amount of money available to us and may have a material adverse effect on our business and financial condition.
Public concern over the abuse of medications that are controlled substances, including increased legislative, legal and regulatory action, could negatively affect our business.
Products containing controlled substances may generate public controversy. Certain governmental and regulatory agencies, as well as state and local jurisdictions, are focused on the abuse of controlled substances such as opioids in the United States. State and local governmental agencies have commenced investigations into pharmaceutical companies and others in the supply chain in connection with the distribution of opioid medications. For example, on March 7, 2018 and April 18, 2019, Neos Therapeutics, which we now own, received citations advising Neos that the County of Harris Texas and the County of Walker Texas filed lawsuits on December 13, 2017 and January 11, 2019, respectively, against Neos and various other alleged manufacturers, promoters, sellers and distributors of opioid pharmaceutical products. Through these lawsuits, each of Harris County and Walker County seek to recoup as damages some of the expenses they allegedly have incurred to combat opioid use and addiction. Each of Harris County and Walker County also seeks punitive damages, disgorgement of profits and attorneys’ fees. In addition, multiple lawsuits have been filed against pharmaceutical companies alleging, among other claims, failures to provide effective controls and procedures to guard against the diversion of controlled substances, negligence by distributing controlled substances to pharmacies that serve individuals who abuse controlled substances, and failures to report suspicious orders of controlled substances in accordance with regulations. Certain cases noted above have recently been settled, some for hundreds of millions of dollars. In the future, political pressures and adverse publicity could lead to delays in, and increased expenses for, and limit or restrict, the introduction and marketing of our products, the withdrawal of currently approved products from the market, or result in other legal action.
48
In addition, we are aware of other legislative, regulatory or industry measures to address the misuse of prescription opioid medications which could affect our business in ways that we may not be able to predict. Liabilities for taxes or assessments under any such laws will likely have an adverse impact on our results of operations, unless we are able to mitigate them through operational changes or commercial arrangements where permitted and may result in us ceasing to continue to sell our products in these jurisdictions.
Certain of our stockholders own a significant percentage of our stock and may and their interests may conflict with yours.
As of June 30, 2023, one stockholder holds approximately 20% of our outstanding common stock and holds warrants which can be exercised to purchase additional shares of our common stock resulting in ownership of approximately 40% of our currently outstanding common stock. Accordingly, this stockholder will be able to exert a significant degree of influence over our management and affairs and over matters requiring security holder approval.
In addition, in connection with our recent public offering of securities in June 2023, this stockholder has been granted the right to designate an individual to join our board of directors, who has since joined the board of directors, and to nominate an additional candidate who is acceptable to us to be elected to the Board, subject to Nasdaq regulations. The interests of this stockholder could conflict with the interests of our other stockholders.
Our business could be negatively affected as a result of the actions of activist stockholders.
Proxy contests have been waged against many companies in the pharmaceutical industry over the last several years. It is possible that one or more of our stockholders may publicly voice opposition to certain aspects of our corporate governance and strategy, or undertake a proxy contest to reconstitute our board. If faced with a proxy contest or other type of stockholder activism, we may not be able to respond successfully to the contest or other type of activism which would be disruptive to our business. Even if we are successful, our reputation and/or business could be adversely affected by a proxy contest or other form of stockholder activism because:
| ● | responding to proxy contests and other actions by activist stockholders can be costly and time-consuming, disrupting operations and diverting the attention of management and employees; |
| ● | perceived uncertainties as to our company and future strategic direction may result in the loss of potential financing, acquisitions, collaboration, in-licensing or other business opportunities, and may make it more difficult to attract and retain qualified personnel and business partners; and |
| ● | if individuals are elected to our board of directors with a specific agenda, it may adversely affect our ability to effectively and timely implement our strategic plan and create additional value for our stockholders. |
Any or all of these activities could cause our stock price to decline or experience periods of volatility, and could be particularly problematic as our company seeks to transition to a commercial enterprise in a challenging environment.
RISK RELATED TO SECURITIES MARKETS AND INVESTMENT IN OUR SECURITIES
Our failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a delisting of our common stock.
If we fail to satisfy the continued listing requirements of the Nasdaq Capital Market, such as the corporate governance requirements or the minimum closing bid price requirement, the exchange may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting notification, we anticipate that we would take actions to restore our compliance with applicable exchange requirements, such as stabilize our market price, improve the liquidity of our common stock, prevent our common stock from dropping below such exchange’s minimum bid price requirement, or prevent future non-compliance with such exchange’s listing requirements.
49
Effecting a reverse stock split, if determined by the Board in its discretion, may not achieve one or more of our objectives.
We have effected five reverse stock splits since June 8, 2015, each of which has impacted the trading liquidity of the shares of our common stock. There can be no assurance that the market price per share of our common stock after a reverse stock split will remain unchanged or increase in proportion to the reduction in the number of shares of our common stock outstanding before the reverse stock split. The market price of our shares may fluctuate and potentially decline after a reverse stock split. Accordingly, the total market capitalization of our common stock after a reverse stock split may be lower than the total market capitalization before the reverse stock split. Moreover, the market price of our common stock following a reverse stock split may not exceed or remain higher than the market price prior to the reverse stock split.
Additionally, there can be no assurance that a reverse stock split will result in a per-share market price that will attract institutional investors or investment funds or that such share price will satisfy investing guidelines of institutional investors or investment funds. As a result, the trading liquidity of our common stock may not necessarily improve. Further, if a reverse stock split is effected and the market price of our common stock declines, the percentage decline may be greater than would occur in the absence of a reverse stock split.
Our share price is volatile and may be influenced by numerous factors, some of which are beyond our control.
The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this prospectus, these factors include:
● |
the success of products we acquire for development or commercialization relative to the success of our competitors; |
●product safety;
● |
conditions or trends in the healthcare, biotechnology and pharmaceutical industries, including healthcare payment systems; |
● |
our ability to effectively manage operations, financial decisions, internal controls over financial reporting or disclosure controls, performance relative to projections, and attract and retain employees; |
●our dependence on third parties, including CROs and scientific and medical advisors;
●adverse regulatory decisions or changes in laws or regulations;
● |
disputes or other developments relating to patents and other proprietary rights and our ability to obtain patent protection for our products; |
●general political and economic conditions and effects of natural or man-made catastrophic events; and
●other events or factors, many of which are beyond our control.
In addition, the stock market in general, and the stocks of small-cap healthcare, biotechnology, and pharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks, including those described in these “Risk Factors,” could have a dramatic and material adverse impact on the market price of our common stock. You might not be able to resell your shares at or above the price you paid for them.
50
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and any trading volume could decline.
Any trading market for our common stock that may develop will depend in part on the research and reports that securities or industry analysts publish about us or our business. We cannot control the number of securities and industry analysts who publish research on us, the extent of their coverage or the content of their reports. Downgrades of our stock or publishing inaccurate or unfavorable research about our business, would likely lead to a decline in our stock price. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose market visibility and demand for our stock could decrease, which might cause our stock price and any trading volume to decline.
Some provisions of our charter documents and applicable Delaware law may discourage an acquisition of us by others, even if the acquisition may be beneficial to some of our stockholders.
Provisions in our Certificate of Incorporation and Amended and Restated Bylaws, as well as certain provisions of Delaware law, could make it more difficult for a third-party to acquire us, even if doing so may benefit some of our stockholders. These provisions include:
● |
the authorization of 50.0 million shares of “blank check” preferred stock, the rights, preferences and privileges of which may be established and shares of which may be issued by our Board of Directors at its discretion from time to time and without stockholder approval; |
●limiting the removal of directors by the stockholders;
●allowing for the creation of a staggered board of directors;
●eliminating the ability of stockholders to call a special meeting of stockholders; and
● |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by the board of directors. This provision could have the effect of discouraging, delaying or preventing someone from acquiring us or merging with us, whether or not it is desired by or beneficial to our stockholders.
Any provision of our Certificate of Incorporation or Bylaws or of Delaware law that is applicable to us that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock in the event that a potentially beneficial acquisition is discouraged, and could also affect the price that some investors are willing to pay for our common stock.
We do not intend to pay cash dividends on our capital stock in the foreseeable future.
We have never declared or paid any dividends on our common stock and do not anticipate paying any dividends in the foreseeable future. Any payment of cash dividends in the future would depend on our financial condition, contractual restrictions, solvency tests imposed by applicable corporate laws, results of operations, anticipated cash requirements and other factors and will be at the discretion of our Board of Directors. Our stockholders should not expect that we will ever pay cash or other dividends on our outstanding capital stock.
51
We are and may continue to be subject to short selling strategies.
Short sellers of our stock may be manipulative and may attempt to drive down the market price of shares of our Common Stock. Short selling is the practice of selling securities that the seller does not own but rather has borrowed from a third party with the intention of buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects to pay less in that purchase than it received in the sale. As it is therefore in the short seller’s best interests for the price of the stock to decline, many short sellers (sometime known as “disclosed shorts”) publish, or arrange for the publication of, negative opinions regarding the relevant issuer and its business prospects to create negative market momentum and generate profits for themselves after selling a stock short. Although traditionally these disclosed shorts were limited in their ability to access mainstream business media or to otherwise create negative market rumors, the rise of the Internet and technological advancements regarding document creation, videotaping and publication by blogging have allowed many disclosed shorts to publicly attack a company’s credibility, strategy and veracity by means of so-called “research reports” that mimic the type of investment analysis performed by large Wall Street firms and independent research analysts. These short attacks have, in the past, led to selling of shares in the market, on occasion in large scale and broad base. Issuers who have limited trading volumes and are susceptible to higher volatility levels than large-cap stocks, can be particularly vulnerable to such short seller attacks. These short seller publications are not regulated by any governmental, self-regulatory organization or other official authority in the United States, are not subject to certification requirements imposed by the SEC and, accordingly, the opinions they express may be based on distortions or omissions of actual facts or, in some cases, fabrications of facts. In light of the limited risks involved in publishing such information, and the enormous profit that can be made from running a successful short attack, unless the short sellers become subject to significant penalties, it is more likely than not that disclosed short sellers will continue to issue such reports.
Significant short selling of a company’s stock creates an incentive for market participants to reduce the value of that company’s common stock. Short selling may lead to the placement of sell orders by short sellers without commensurate buy orders because the shares borrowed by short sellers do not have to be returned by any fixed period of time. If a significant market for short selling our common stock develops, the market price of our common stock could be significantly depressed.
The Sabby litigation may result in the issuance of additional shares on the exercise of certain of our warrants and cause dilution to existing shareholders.
A complaint was filed on February 22, 2023 by holders of certain warrants to purchase common stock, against the Company. The complaint alleges that the Company improperly adjusted the exercise price of the warrants and miscalculated the number of shares the warrantholders may receive, and that the Company failed to provide prompt notice to the warrantholders of such adjustment. The complaint seeks, among other things, a declaratory judgment of the warrant share calculation such that 2,325,581 warrant shares be due to the warrantholders on the exercise of the warrants rather than 1,265,547 shares. While we believe that this lawsuit is without merit and we intend to vigorously defend against it, we are not able to predict at this time whether this proceeding will have a material impact on our financial condition or results of operations. If this lawsuit is successful and the warrantholders exercise their warrants, it will result in significant dilution of the percentage ownership of our existing stockholders and could cause our stock price to fall. See Part I, Item 3. Legal Proceedings for more information on this lawsuit.
GENERAL RISK FACTORS
Our business and operations would suffer in the event of system failures, cybersecurity attacks or other security breaches.
We utilize information technology, or IT, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate cybersecurity attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks and the confidentiality, availability, and integrity of our data.
52
There can be no assurance that we will be successful in preventing cyber attacks or successfully mitigating their effects.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from such cybersecurity attacks, including computer viruses, unauthorized access, ransomware attacks, phishing expeditions, natural disasters, terrorism, war and telecommunication and electrical failures. Such an event could cause interruption of our operations. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could suffer reputational harm or face litigation or adverse regulatory action and the development of our products could be delayed.
Our sales force and other employees, third party logistics partners, CMOs, CROs, principal investigators, collaborators, independent contractors, consultants and other vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
Major bank failure or sustained financial market illiquidity, could adversely affect our business, financial condition and results of operations.
We face certain risks in the event of a sustained deterioration of domestic or international financial market liquidity. In particular:
| ● | We may be unable to access funds in our deposit accounts on a timely basis. Any resulting need to access other sources of liquidity or short-term borrowing would increase our costs. |
| ● | In the event of a major bank failure, we could face major risks to the recovery of our bank deposits. A substantial portion of our cash and cash equivalents are either held at banks that are not subject to insurance protection against loss or exceed the deposit insurance limit. While we are not currently aware of any liquidity issues directly impacting the financial institutions where we hold cash deposits or securities, if financial liquidity deteriorates, there can be no assurance we will not experience an adverse effect, which may be material, on our ability to access capital and on our business, financial condition and results of operations. |
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We lease or sublease various properties, including office buildings, manufacturing, research and development facilities and sales offices within the U.S. We continuously review and evaluate our facilities as a part of our strategy to optimize our business operations. The following table sets forth a list of our properties as of June 30, 2023.
Location |
|
Leased/Owned |
|
Purpose |
Englewood, CO |
|
Leased |
|
Corporate headquarters |
Grand Prairie, TX |
|
Leased |
|
Administrative offices, Laboratory and Manufacturing facilities |
Berwyn, PA |
|
Leased |
|
Office |
Oceanside, CA |
|
Leased |
|
Warehouse |
ITEM 3. LEGAL PROCEEDINGS
Witmer Class-Action Securities Litigation.
53
A shareholder derivative suit was filed on September 12, 2022 in the Delaware Chancery Court by Paul Witmer, derivatively and on behalf of all Aytu stockholders, against Armistice Capital, LLC, Armistice Capital Master Fund, Ltd., Steve Boyd (Armistice’s Chief Investment Officer and Managing Partner, and a former director of Aytu), and certain other current and former directors of Aytu, Joshua Disbrow, Gary Cantrell, John Donofrio, Jr., Michael Macaluso, Carl Dockery and Ketan B. Mehta. Plaintiff amended the complaint on April 5, 2023. The Amended Complaint drops Mr. Macaluso as a defendant and alleges that (i) Armistice facilitated the sale of assets of Cerecor in 2019 and Innovus in 2020 to Aytu in exchange for convertible securities which it subsequently converted and sold at a profit on the open market; (ii) the Armistice defendants breached their fiduciary duties, were unjustly enrichment and wasted corporate assets in connection with these acquisitions; (iii) the Armistice defendants breached their fiduciary duties by engaging in as insider trading; and (iv) the other directors breached their fiduciary duties, and aided and abetted the Armistice defendants breaches of fiduciary duties, in connection with these acquisitions. The Amended Complaint seeks unspecified damages, equitable relief, restitution, disgorgement of profits, enhanced governance and internal procedures, and attorneys’ fees. While we believe that this lawsuit is without merit and have vigorously defended against it, we have agreed to settle the matter for various corporate governance modifications and the payment of plaintiff’s attorneys’ fees.
Sabby Litigation. A complaint was filed on February 22, 2023 in the Supreme Court of the State of New York by Sabby Volatility Warrant Master Fund LTD (“Sabby”) and Walleye Opportunities Master Fund Ltd (“Walleye”), holders of certain warrants to purchase common stock, against the Company. The complaint alleges that the Company improperly adjusted the exercise price of the warrants and miscalculated the number of shares the warrantholders may receive, and that the Company failed to provide prompt notice to the warrantholders of such adjustment. The complaint seeks a declaratory judgment of the warrant share calculation, that 575,000 warrant shares be due to Sabby on exercise of its warrants rather than 312,908 shares, and that 100,000 warrant shares be due to Walleye on exercise of its warrants rather than 54,146 shares. While we believe that this lawsuit is without merit and we intend to vigorously defend against it, we are not able to predict at this time whether this proceeding will have a material impact on our financial condition or results of operations.
Stein Litigation. Cielo Stein (“Stein”), a former sales specialist, filed a complaint on February 1, 2023 in Jefferson County Circuit Court in Kentucky against the Company and its wholly-owned subsidiary Neos Therapeutics. The complaint alleges that Aytu retaliated against Stein in violation of the Kentucky Civil Rights Act after she opposed what she contends was unwelcome behavior by her supervisor. The complaint also alleges that the Company’s response to Stein’s subsequent complaint to human resources was inadequate. The complaint seeks an award of unspecified compensatory damages, emotional-distress damages, and attorneys’ fees and costs. The Company removed the lawsuit to the United States District Court for the Western District of Kentucky and filed a motion to dismiss the complaint, which is pending. Due to the early stage of litigation, we are not able to predict at this time whether this proceeding will have a material impact on our financial condition or results of operations, and intend to vigorously defend this case in the event it is not dismissed.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
54
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock has been listed on the NASDAQ Capital Market under the symbol “AYTU” since October 20, 2017.
On January 6, 2023, we effected a 1-for-20 reverse stock split of our outstanding shares of common stock. Unless specifically provided otherwise herein, the share and per share information that follows in this Annual Report on Form 10-K other than in the historical financial statements and related notes included elsewhere in this Form 10-K, assumes the effect of the reverse stock split.
On September 20, 2023, the closing price as reported on the Nasdaq of our common stock was $1.585, and there were 190 holders of record of our common stock.
55
Equity Compensation Plan Information
On May 18, 2023, our stockholders approved the adoption of the Aytu BioPharma, Inc. 2023 Equity Incentive Plan (the "2023 Equity Incentive Plan”). Prior to our adoption of the 2023 Equity Incentive Plan, we awarded equity incentive grants to our directors and employees under the Aytu BioScience, Inc. 2015 Stock Option and Incentive Plan (“Aytu 2015 Plan”) and the Neos Therapeutics, Inc. 2015 Stock Options and Incentive Plan (“the Neos 2015 Plan”) (collectively the “2015 Plans”). For the 2023 Equity Incentive Plan, the stockholders approved (a) 200,000 new shares, (b) 87,155 shares available for grant under the 2015 Plans be “rolled over” to the 2023 Equity Incentive Plan and (c) any shares that are returned to the company under the 2015 Plans be added to the 2023 Equity Incentive Plan.
The following table displays equity compensation plan information as of June 30, 2023 relating to securities reserved for future issuance upon exercise.
|
|
|
|
|
|
|
Number of |
|
|
|
|
|
|
|
Securities |
|
|
|
|
|
|
|
Remaining |
|
|
Number of |
|
|
|
|
Available for |
|
|
Securities to |
|
Weighted- |
|
Issuance under |
|
|
|
be Issued |
|
Average |
|
Equity |
|
|
|
upon |
|
Exercise |
|
Compensation |
|
|
|
Exercise of |
|
Price of |
|
Plans |
|
|
|
Outstanding |
|
Outstanding |
|
(Column C - |
|
|
|
Options, |
|
Options, |
|
Excluding |
|
|
|
Warrants |
|
Warrants |
|
Securities |
|
|
|
and Rights |
|
and Rights |
|
Reflected in |
|
Plan Category |
|
(Column A) |
|
(Column B)(1) |
|
(Column (A)) |
|
Equity compensation plans approved by security holders |
|
94,302 |
|
$ |
15.17 |
|
84,560 |
Equity compensation plans not approved by security holders(2) |
|
4,419 |
|
$ |
127.77 |
|
2,595 |
Total |
|
98,721 |
|
$ |
18.37 |
|
87,155 |
| (1) | It reflects the weighted-average exercise prices of options outstanding. Restricted stocks and restricted stock units (RSUs) do not have exercise prices (see Note 15 - Equity Incentive Plan). |
| (2) | It reflects the equity plan we assumed pursuant to the Neos Acquisition and restricted stock previously issued outside of the Aytu 2015 Plan (see Note 15 - Equity Incentive Plan). |
Dividend Policy
We have never declared or paid any dividends on our capital stock. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors. Our ability to pay dividends on our common stock is limited by restrictions under the terms of our credit facility with Avenue Capital. In addition, any future indebtedness that we may incur could preclude us from paying dividends. Investors should not purchase our common stock with the expectation of receiving cash dividends.
56
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes appearing elsewhere in this Annual Report. Some of the information contained in this discussion and analysis, including information with respect to our plans and strategy for our business and related financing strategy, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” section of this Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
OBJECTIVE
The purpose of the Management Discussion and Analysis (the “MD&A”) is to present information that management believes is relevant to an assessment and understanding of our results of operations and cash flows for the fiscal year ended June 30, 2023 and our financial condition as of June 30, 2023. The MD&A is provided as a supplement to, and should be read in conjunction with, our financial statements and notes.
OVERVIEW
We are a commercial-stage pharmaceutical company focused on commercializing novel therapeutics and consumer healthcare products. We operate through two business segments (i) the Rx Segment, consisting of various prescription pharmaceutical products sold through third party wholesalers (the Rx Portfolio”), and (ii) the Consumer Health Segment, which consists of various consumer health products sold directly to consumers. We generate revenue by selling our products through third party intermediaries in our marketing channels as well as directly to our customers. We currently manufacture our products for the treatment of ADHD at our manufacturing facility in Grand Prairie, Texas and use third party manufacturers for our other prescription and consumer health products. We also have a product candidate in development, AR101 (enzastaurin) for the treatment of VEDS, for which the development has been indefinitely suspended.
We have incurred significant losses in each year since inception. Our net losses were $17.1 million and $108.8 million for the years ended June 30, 2023 and 2022, respectively. As of June 30, 2023 and 2022, we had an accumulated deficit of approximately $304.1 million and $287.1 million, respectively. We expect to continue to incur significant expenses in connection with our ongoing activities, including the integration of our acquisitions and the commercialization of our product pipeline.
SIGNIFICANT DEVELOPMENTS
Business Environment
We have continued to experience significant inflationary pressure and supply chain disruptions related to the sourcing of raw materials, energy, logistics and labor during fiscal 2023. While we do not have sales or operations in Russia or Ukraine, it is possible that the conflict or actions taken in response, could adversely affect some of our markets and suppliers, economic and financial markets, costs and availability of energy and materials, or cause further supply chain disruptions. We expect that inflationary pressures and supply chain disruptions could continue to be significant across the business throughout the year.
Commercial Products
On March 23, 2022, our newly issued US patent No. 11,166,947 for Cotempla XR-ODT was listed in the U.S. FDA publication "Approved Drug Products with Therapeutic Equivalence Evaluations", commonly known as the "Orange Book." The Cotempla XR-ODT patent covers methods of use for the effective pediatric dosing of methylphenidate for the treatment of attention deficit hyperactivity disorder. The Orange Book listing extends the exclusivity period for Cotempla XR-ODT to 2038. Teva Pharmaceuticals USA, Inc. has the right to manufacture and market its generic version of Cotempla XR-ODT under its ANDA beginning on July 1, 2026, or earlier under certain circumstances.
57
Development Products
AR101
On December 7, 2021, the FDA granted Orphan Drug Designation (“ODD”) to AR101 for the treatment of Ehlers-Danlos Syndrome, a group of rare inherited connective tissue disorders that includes the severe subtype VEDS. The FDA grants ODD status to drugs and biologics that are intended for the safe and effective treatment, diagnosis or prevention of rare diseases, or conditions that affect fewer than 200,000 people in the U.S. ODD affords us with certain financial incentives to support clinical development and the potential for up to seven years of market exclusivity in the U.S. upon regulatory approval.
On December 13, 2021, the FDA cleared the IND application for AR101 in VEDS to enable the initiation of the AR101 PREVEnt Trial in VEDS.
On March 2, 2022, the European Commission granted orphan designation to AR101 (enzastaurin) for the treatment of Ehlers-Danlos Syndrome. The European Medicines Agency orphan designation affords us with certain benefits and incentives, including clinical protocol assistance, differentiated evaluation procedures for Health Technology Assessments in certain countries, access to a centralized marketing authorization procedure valid in all EU member states, reduced regulatory fees and 10 years of market exclusivity.
On April 19, 2022, we were notified by the FDA that AR101 received Fast Track designation. Fast Track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious conditions and fill an unmet medical need. Fast Track addresses a broad range of serious conditions, and the request can be initiated by a pharmaceutical company at any time during the development process. FDA reviews the request and decides based on whether or not the drug fills an unmet medical need in a serious condition. Once a drug receives Fast Track designation, early and frequent communication between the FDA and the sponsor is encouraged throughout the entire drug development and review process.
In October 2022, we announced the indefinite suspension of the development of AR101 to focus on our commercial operations.
Healight
In November 2021, we received U.S. Patent Number 11,179,575, titled "Internal Ultraviolet Therapy," which is the first issued patent protecting the Healight investigational device and covers methods of treating a patient for an infectious condition inside the patient's body through the insertion of a UV-light-emitting delivery tube inside a respiratory cavity of the patient at specific UV-A light wavelengths. The term of this patent extends to August of 2040.
In April 2022, our preclinical pilot study showed that administration of Healight delayed the time to development of VAP in a novel porcine model. The proof-of-concept study was conducted at Hospital Clinic de Barcelona under the supervision of principal investigator Antonio Torres, M.D., Ph.D., FERS, FCCP, ATSF, Senior Consultant, Pulmonology Department - one of the only centers in the world with access to this well-characterized porcine model of VAP caused by oropharyngeal secretions colonized by Pseudomonas aeruginosa. In the study, administration of the Healight UV-A endotracheal catheter resulted in a 46% reduction in multidrug-resistant Pseudomonas aeruginosa (“PA C1-17”) versus controls following two separate 20-minute treatments. Based on these positive data, Hospital Clinic de Barcelona and we conducted a second, larger porcine VAP study to guide the future development of Healight for patients with VAP. We have since terminated the license agreement with Cedars-Sinai Medical Center (“CSMC”) and have discontinued development of Healight.
58
Debt and Equity Financings
On January 26, 2022, we entered into the Avenue Capital Agreement with the Avenue Capital, pursuant to which Avenue Capital provided the Company and certain of its subsidiaries with a secured $15.0 million loan. The interest rate on the loan is the greater of the prime rate and 3.25%, plus 7.4%, payable monthly in arrears. The maturity date of the loan is January 26, 2025. The proceeds from the Avenue Capital Agreement were used towards the repayment of the Deerfield Facility, which was otherwise due and payable on May 11, 2022.
In connection with the Avenue Capital Agreement, we entered into an amendment to the Eclipse Loan Agreement. Pursuant to the amendment, the Company, among other things, extended the maturity date of the Eclipse Loan Agreement to January 26, 2025 and reduced the maximum availability under the Eclipse Loan Agreement from $25.0 million to $12.5 million minus a $3.5 million availability block.
On March 24, 2023, we entered into an Amendment No. 4 (the “Eclipse Amendment”) to the Loan and Security Agreement dated October 2, 2019. The Eclipse Amendment, among other things, provided for an aggregate increase of $2.0 million to the Eclipse Lender’s commitment to make revolving loans from time to time under the Eclipse Agreement and increased the maximum amount available under the revolving credit facility provided under the Eclipse Agreement to $14.5 million. The ability to make borrowings and obtain advances of revolving loans under the Eclipse Agreement remains subject to a borrowing base and reserve, and availability blockage requirements.
On March 7, 2022, upon closing of an underwritten public offering, we raised gross proceeds of $7.6 million from the issuance of (i) 151,500 shares of our common stock, (ii) pre-funded warrants to purchase up to 151,500 shares of common stock, and (iii) common stock purchase warrants to purchase up to 333,300 shares of common stock. We received $6.8 million in proceeds net of underwriting fees and other expenses. In April 2022, the pre-funded warrants were exercised in full.
On August 11, 2022, upon the closing of an underwritten public offering, we raised proceeds of $10.0 million from the issuance of (i) 1,075,290 shares of our common stock, and, in lieu of common stock to certain investors that so chose, pre-funded warrants to purchase 87,500 shares of our common stock, and (ii) accompanying warrants to purchase 1,265,547 shares of our common stock. We received $9.1 million in proceeds net of underwriting fees and other expenses. In August 2022, the pre-funded warrants were exercised in full.
On January 6, 2023, we effected a 1-for-20 reverse stock split of our common stock. All share and per share amounts in this quarterly report have been adjusted to reflect the effect of the Reverse Stock Split. Aytu’s Board of Directors implemented the reverse stock split with the objective of regaining compliance with the $1.00 minimum bid price requirement of the Nasdaq Capital Market. On January 23, 2023, Nasdaq confirmed we regained compliance with this listing rule.
A cash payment was made to each stockholder in lieu of any fractional interest in a share to which each stockholder would otherwise be entitled as a result of the reverse stock split. The reverse stock split reduced the number of shares of outstanding common stock from approximately 68.8 million shares to approximately 3.4 million shares. As a result of the reverse stock split, proportional adjustments were also made to outstanding warrants and options, and to the shares available for grant in our equity incentive plan.
On June 8, 2023, we entered into a securities purchase agreement with certain institutional investors named therein and a placement agency agreement with Maxim Group LLC, pursuant to which the Company agreed to issue and sell to investors in the offering an aggregate of 1,743,695 shares of the Company’s common stock, pre-funded warrants in lieu of shares to purchase 430,217 shares of common stock, accompanying Tranche A warrants to purchase 2,173,912 shares of common stock, and accompanying Tranche B warrants to purchase 2,173,912 shares of common stock in a best-efforts offering. The common warrants may be exercised for either shares of common stock or pre-funded warrants to purchase common stock at a future exercise price of $0.0001 per share in the same form as the pre-funded warrant. The gross proceeds were $4.0 million and net proceeds were approximately $3.4 million after deducting offering expenses. The offering closed on June 13, 2023.
59
During the year ended June 30, 2023, we issued 699,929 shares of common stock under the ATM Sales Agreement (as defined below) with total gross proceeds of approximately $3.0 million before deducting commissions of 3% and other offering expenses including legal and audit fees.
Discontinued Products
As part of our realization of post-acquisition synergies and product prioritization, we have implemented a portfolio rationalization plan whereby we will discontinue or divest five non-core products in our Rx Segment: Cefaclor Oral Suspension, Flexichamber, Tussionex, Tuzistra XR, and ZolpiMist. These products, collectively, contributed $1.6 million and $2.1 million in net revenue during the years ended June 30, 2023 and 2022, respectively.
RESULTS OF OPERATIONS
Comparison of the years ended June 30, 2023 and 2022
|
|
Year Ended |
|||||||
|
|
June 30, |
|||||||
|
|
2023 |
|
2022 |
|
Change |
|||
|
|
(In thousands) |
|||||||
Product revenue, net |
|
$ |
107,399 |
|
$ |
96,669 |
|
$ |
10,730 |
Cost of sales |
|
|
40,767 |
|
|
44,386 |
|
|
(3,619) |
Gross profit |
|
|
66,632 |
|
|
52,283 |
|
|
14,349 |
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Advertising and direct marketing |
|
|
17,217 |
|
|
19,589 |
|
|
(2,372) |
Other selling and marketing |
|
|
24,231 |
|
|
19,124 |
|
|
5,107 |
General and administrative |
|
|
28,630 |
|
|
31,167 |
|
|
(2,537) |
Research and development |
|
|
4,095 |
|
|
12,662 |
|
|
(8,567) |
Goodwill impairment expense |
|
|
— |
|
|
65,802 |
|
|
(65,802) |
Other impairment expense |
|
|
5,705 |
|
|
9,656 |
|
|
(3,951) |
Amortization of intangible assets |
|
|
4,788 |
|
|
5,844 |
|
|
(1,056) |
Gain from contingent consideration |
|
|
(969) |
|
|
(1,655) |
|
|
686 |
Total operating expenses |
|
|
83,697 |
|
|
162,189 |
|
|
(78,492) |
Loss from operations |
|
|
(17,065) |
|
|
(109,906) |
|
|
92,841 |
Other income (expense) |
|
|
|
|
|
|
|
|
|
Other expense, net |
|
|
(4,779) |
|
|
(757) |
|
|
(4,022) |
Gain on extinguishment of debt |
|
|
— |
|
|
169 |
|
|
(169) |
Gain on derivative warrant liability |
|
|
4,793 |
|
|
1,605 |
|
|
3,188 |
Total other income, net |
|
|
14 |
|
|
1,017 |
|
|
(1,003) |
Loss before income tax |
|
|
(17,051) |
|
|
(108,889) |
|
|
91,838 |
Income tax (benefit) expense |
|
|
— |
|
|
(110) |
|
|
110 |
Net loss |
|
$ |
(17,051) |
|
$ |
(108,779) |
|
$ |
91,728 |
Revenue by segment
|
|
Year Ended |
|||||||
|
|
June 30, |
|||||||
|
|
2023 |
|
2022 |
|
Change |
|||
|
|
(In thousands) |
|||||||
Net revenue by segment: |
|
|
|
|
|
|
|
|
|
Rx Segment |
|
$ |
73,799 |
|
$ |
61,121 |
|
$ |
12,678 |
Consumer Health Segment |
|
|
33,600 |
|
|
35,548 |
|
|
(1,948) |
Total net revenue |
|
$ |
107,399 |
|
$ |
96,669 |
|
$ |
10,730 |
60
During the year ended June 30, 2023, net product revenue increased by $10.7 million, or 11% compared to the year ended June 30, 2022. The increase in our Rx Segment product lines was primarily due to higher volume due to shortages of competing ADHD products, the effectiveness of our Aytu Rx Connect program, and the effectiveness of our sales and marketing programs. The decrease in the Consumer Health Segment’s net revenue was due to the reduction of our direct mailing business to focus our efforts on the higher profitability of our e-commerce business. We expect the revenue from our Consumer Health Segment to continue to decrease in fiscal 2024 as we look to monetize or discontinue this segment.
Gross margin by segment
|
|
Year Ended |
|||||||
|
|
June 30, |
|||||||
|
|
2023 |
|
2022 |
|
Change |
|||
|
|
|
|||||||
Gross margin by segment: |
|
|
|
|
|
|
|
|
|
Rx Segment |
|
|
71% |
|
|
56% |
|
|
15% |
Consumer Health Segment |
|
|
43% |
|
|
51% |
|
|
(8)% |
Total gross margin by segment |
|
|
62% |
|
|
54% |
|
|
8% |
During the year ended June 30, 2023, gross margins increased by 8% compared to the year ended June 30, 2022. The improvement in Rx Segment gross margin percentage was primarily due to the greater volumes resulting in greater utilization of our manufacturing facilities. The lower gross margin in our Consumer Health Segment was due to the focus on our e-commerce business, which has lower gross margins, but higher contribution margins than our direct mailing business. In addition, we recorded an inventory impairment write-off of $2.1 million in the Consumer Health Segment.
Advertising and direct marketing (Consumer Health Segment)
During the year ended June 30, 2023, advertising and direct marketing expenses decreased by $2.4 million, or 12%, compared to the year ended June 30, 2022. Advertising and direct marketing expense include direct-to-consumer marketing, advertising, sales, and customer support and processing fees related to our Consumer Health Segment. The reduction in advertising and direct marketing costs were due to our focus on our e-commerce business during fiscal 2023. We expect advertising and direct marketing expenses to decrease from 2023 levels as we continue to reduce our direct mailing business and monetize or discontinue our Consumer Health Segment.
Other selling and marketing
During the year ended June 30, 2023, other selling and marketing expense increased $5.1 million, or 27%, compared to the year ended June 30, 2022. The increase was primarily driven by higher commission expense, a result of the higher subscriptions generated by our sales force. In addition, we incurred increased commercial marketing program fees due to the higher volumes generated.
General and administrative
During the year ended June 30, 2023, general and administrative expense decreased by $2.5 million or 8%, compared to the year ended June 30, 2022. The decrease was primarily due to reductions of redundancies from the Neos and Innovus acquisitions.
61
Research and development
|
|
Year Ended |
|||||||
|
|
June 30, |
|||||||
|
|
2023 |
|
2022 |
|
Change |
|||
|
|
(In thousands) |
|||||||
Research and development: |
|
|
|
|
|
|
|
|
|
AR101 |
|
$ |
1,880 |
|
$ |
10,673 |
|
$ |
(8,793) |
ADHD |
|
|
1,803 |
|
|
702 |
|
|
1,101 |
Healight |
|
|
250 |
|
|
926 |
|
|
(676) |
Others |
|
|
162 |
|
|
361 |
|
|
(199) |
Total Research and development |
|
$ |
4,095 |
|
$ |
12,662 |
|
$ |
(8,567) |
During the year ended June 30, 2023, research and development expense decreased by $8.6 million, or 68%, compared to the year ended June 30, 2022. Our research and development costs were primarily associated with our AR101 product candidate and support of our ADHD products, Adzenys and Contempla, and to a lesser extent, the development of our Healight product candidate, and support for our commercialized products. In October 2022, we announced the suspension of the development of AR101 and Healight to focus on our commercial operations, resulting in the decrease in expenses in fiscal 2023.
Impairment expense
During the year ended June 30, 2023, we recognized total impairment expense of $5.7 million, consisting of (i) $5.6 million intangible assets, and (ii) $0.1 million other assets. The impairments were due to increased focus on our commercial efforts in the Rx Segment and discontinued product distributions in the Consumer Health Segment. See Note 7 – Goodwill and Other Intangible Assets in the accompanying consolidated financial statements for further information.
During the year ended June 30, 2022, we recognized total impairment expense of $75.5 million, consisting of (i) $65.8 million in goodwill, (ii) $7.1 million intangible assets, (iii) $2.0 million inventory, (iv) $0.4 million other assets and (v) $0.2 million property and equipment. The impairment expense related to write-down of assets was due to the discontinuation of commercializing certain products and products not marketed. See Note 7 – Goodwill and Other Intangible Assets in the accompanying consolidated financial statements for further information.
Amortization of intangible assets
During the year ended June 30, 2023, amortization expense of intangible assets, excluding amounts included in cost of sales, decreased by $1.1 million, or 18%, compared to the year ended June 30, 2022. The decreases were primarily related to the smaller intangible asset base due to the impairments of certain intangible assets during fiscal year 2022.
Gain or loss from contingent consideration
We fair value our acquisition-related contingent considerations based on our projected results, any changes are reflected through income or expense. During the year ended June 30, 2023, the gain from contingent considerations decreased by $0.7 million, or 41%, compared to the year ended June 30, 2022. The decrease was primarily due to the contingent considerations (including CVRs) expiring or winding down during the fiscal year of 2023.
Other (expense) income, net
During the year ended June 30, 2023, other expense, net increased by $4.0 million compared to the year ended June 30, 2022. Other expense, net, includes interest expense, accretion from fixed payment arrangements, and other income. In the fiscal year ended June 30, 2022, we received payments related to the divestiture of Natesto, which was recorded as other income. Starting the third quarter of fiscal 2023, we did not receive such payments.
62
Unrealized gain or loss on derivative warrant liabilities
We fair value our derivative warrant liabilities using either the Monte Carlo simulation model or the Black-Scholes option pricing model. Derivative warrant liabilities are revalued at each reporting period and changes are reflected through income or expense. During the year ended June 30, 2023, the net gain from derivative warrant liability increased by $3.2 million when compared to the year ended June 30, 2022. The net increase was primarily due to higher fair values of derivative liabilities from warrants issued during fiscal year 2023. See Note 14 – Stockholders’ Equity and Note 12 – Fair Value Considerations in the accompanying consolidated financial statements for further details.
Income tax benefit
For the fiscal year ended 2023, there was no income tax benefit, primarily driven by the Internal Revenue Code Section 382 limitation on net operating loss utilization.
For the fiscal year ended 2022, the impairment of the Rx Segment book goodwill decreased the net deferred tax liability by $0.1 million resulting in an income tax benefit of $0.1 million.
LIQUIDITY AND CAPITAL RESOURCES
Cash Flows
The following table sets forth the primary sources and uses of cash for the periods indicated:
|
|
Year Ended June 30, |
|
|
|||||
|
|
2023 |
|
2022 |
|
Change |
|||
|
|
(In thousands) |
|||||||
Net cash used in operating activities |
|
$ |
(5,129) |
|
$ |
(28,823) |
|
$ |
23,694 |
Net cash used in investing activities |
|
$ |
(117) |
|
$ |
(3,248) |
|
$ |
3,131 |
Net cash provided by financing activities |
|
$ |
8,871 |
|
$ |
1,530 |
|
$ |
7,341 |
Net Cash Used in Operating Activities
Net cash used in operating activities during these periods primarily reflected our net losses, partially offset by changes in working capital and non-cash charges including goodwill and intangible asset impairment, inventory write-down, changes in fair values of various liabilities, stock-based compensation expense, depreciation, amortization and accretion, and other charges.
During the fiscal year ended June 30, 2023, net cash used in operating activities totaled $5.1 million. The decrease in net cash used was primarily the result of the decrease in operating loss, and increases in accounts payable and accrued liabilities, partially offset by an increase in accounts receivable, inventory, and prepaid expenses.
During the fiscal year ended June 30, 2022, net cash used in operating activities totaled $28.8 million. The use of cash was approximately $81.4 million less than the net loss primarily due to non-cash charges of depreciation, amortization and accretion, impairment of goodwill and intangible assets, stock-based compensation, inventory and other assets write-downs and loss on debt extinguishment. These charges were offset by gains from change in fair values of contingent consideration and contingent value rights. In addition, our use of cash decreased due to changes in working capital including decreases in accounts receivable, inventory and prepaids, offset by a decrease in accrued liabilities and accounts payable.
Net Cash Used in Investing Activities
Net cash used in investing activities is generally related to our merger and acquisitions as well as purchase of assets to support our operations.
63
Net cash used in investing activities was $0.1 million during the year ended June 30, 2023.
Net cash used in investing activities of $3.2 million during the year ended June 30, 2022, was primarily due to $3.2 million payment of contingent consideration.
Net Cash from Financing Activities
Net cash provided by financing activities of $8.9 million during the year ended June 30, 2023, was primarily from $3.4 million of net proceeds from our securities purchase agreement in June 2023, $9.1 million of net proceeds from our August 2022 equity raise, and $2.9 million net proceeds from our sales under the ATM Sales Agreement; partially offset by $2.3 million of net payments made under our short-term line of credit, and fixed payment arrangements totaling $4.3 million.
Net cash provided by financing activities of $1.5 million during the year ended June 30, 2022, was primarily from $15.0 million proceeds from long-term debt and $11.7 million net proceeds from issuance of our common stock, partially offset by $16.1 million full repayment of long-term debt, $4.1 million net reduction in our revolving loan, $4.4 million in payments of fixed payment arrangements and $0.5 million payment of debt issuance costs.
Capital Resources
Sources of Liquidity
We have obligations related to our loan agreements, contingent considerations related to our acquisitions, milestone payments for licensed products and manufacturing purchase commitments.
We finance our operations through a combination of sales of our common stock and warrants, borrowings under our line of credit facility and cash generated from operations.
Shelf Registrations
On September 28, 2021, we filed a shelf registration statement on Form S-3, which was declared effective by the SEC on October 7, 2021. This shelf registration statement covered the offering, issuance and sale by the Company of up to an aggregate of $100.0 million of its common stock, preferred stock, debt securities, warrants, rights and units (the “2021 Shelf”). As of June 30, 2023, approximately $82.4 million remains available under the 2021 Shelf. This availability is subject to SEC 1.B.6 limitations of Form S-3.
On June 8, 2020, the Company filed a shelf registration statement (the “2020 Shelf”), which was declared effective by the SEC on June 17, 2020, covering up to $100.0 million of its common stock, preferred stock, debt securities, warrants, rights, and units. The 2020 Shelf expired in June 2023.
In June 2020, under the 2020 Shelf, we initiated an at-the-market offering program ("ATM"), which allows us to sell and issue shares of our common stock from time-to-time. On June 2, 2021, we terminated our “at-the-market” sales agreement with a sales agent, and on June 4, 2021, we entered into a Controlled Equity OfferingSM Sales Agreement (the “ATM Sales Agreement”) with a sales agent, pursuant to which we agreed to sell up to $30.0 million of our common stock from time to time in “at-the-market” offerings under the 2020 Shelf. During the year ended June 30, 2023, we issued 699,929 shares of common stock under the ATM Sales Agreement, with total net proceeds of approximately $2.9 million. As of June 30, 2023, we had approximately $3.0 million of capacity under the ATM Sales Agreement due to baby self-limitations. As our market capitalization increases, these limitations will be adjusted and we will be able to issue additional ATM sales. We terminated the Controlled Equity Offering in July 2023.
Underwriting & Placement Agency Agreements
On June 8, 2023, we entered into a securities purchase agreement with certain institutional investors named therein and a placement agency agreement with Maxim Group LLC, pursuant to which the Company agreed to issue and sell to investors in the offering an aggregate of 1,743,695 shares of the Company’s common stock, pre-funded warrants in lieu of shares to purchase 430,217 shares of common stock, accompanying Tranche A warrants to purchase 2,173,912 shares of common stock, and accompanying Tranche B warrants to purchase 2,173,912 shares of common stock in a best-efforts offering.
64
The common warrants may be exercised for either shares of common stock or pre-funded warrants to purchase common stock at a future exercise price of $0.0001 per share in the same form as the pre-funded warrant. The gross proceeds were $4.0 million, and net proceeds were approximately $3.4 million after deducting offering expenses. The offering closed on June 13, 2023.
On August 11, 2022, we closed an underwritten public offering, pursuant to which we sold an aggregate of (i) 1,075,290 shares of its common stock, (ii), pre-funded warrants to purchase 87,500 shares of its common stock, and (ii) accompanying warrants to purchase 1,265,547 shares of our common stock. The shares of common stock (or pre-funded warrants) and the accompanying common warrants were issued separately but could only be purchased together. The combined public offering price for each share of common stock and accompanying common warrant was $8.60, and the combined offering price for each pre-funded warrant and accompanying common warrant is $8.58, which equals the public offering price per share of the common stock and accompanying common warrant, less the $0.001 per share exercise price of each pre-funded warrant. The pre-funded warrants were exercised in full in August 2022. The common warrants are exercisable at any time after the date of issuance for a period of five years from the date such common warrants are first exercisable. The number of shares of common stock issuable upon exercise of the common warrants is subject to adjustment in certain circumstances, including a stock split of, stock dividend on, or a subdivision, combination or recapitalization of the common stock. The Company received gross proceeds of $10.0 million and net proceeds were approximately $9.1 million, after deducting underwriting discounts and commissions and estimated offering expenses.
On March 7, 2022, we closed on an underwritten public offering, pursuant to which, we sold (i) 151,500 shares of our common stock, (ii) pre-funded warrants to purchase up to 151,500 shares of common stock, and (iii) common warrants to purchase up to 333,300 shares of common stock. The shares of common stock and the pre-funded warrants were each sold in combination with corresponding common warrants, with one common warrant to purchase 1.1 shares of common stock for each share of common stock or each pre-funded warrant sold. The pre-funded warrants have an exercise price of $0.002 per share of common stock and were exercised in full in April 2022. The common warrants have an exercise price of $26.00 per share of common stock and are exercisable six months after the date of issuance and have a term of five years from the date of exercisability. We raised gross proceeds of $7.6 million before commission and other costs of $0.8 million.
Avenue Capital Agreement
On January 26, 2022, we entered into the Avenue Capital Agreement, pursuant to which the Company received $15.0 million loan. The interest rate on the loan is the greater of the prime rate and 3.25%, plus 7.4%, payable monthly in arrears. We met certain milestones which resulted in monthly payments consisting of interest only. The principal amount will become due on the maturity date of the loan is January 26, 2025. The proceeds from the Avenue Capital Agreement were used towards the repayment of outstanding debt.
In the event we prepay the outstanding principal prior to the maturity date, we will pay Avenue Capital a fee equal to (i) 3.0% of the loan if such event occurs on or before January 26 2023, (ii) 2.0% of the loan if such event occurs after January 26, 2023 but on or before January 26, 2024, and (iii) 1.0% of the loan if such event occurs after January 26, 2024 but before January 26, 2025. In addition, upon the payment in full of the obligations, we shall pay to Avenue Capital a non-refundable fee in the amount of $0.6 million (“Final Payment”). See Note 11 – Long-term Debt in the accompanying consolidated financial statements for further information.
Eclipse Loan Agreement
The Eclipse Loan Agreement, as amended, provides us with up to $14.5 million in Revolving Loans, of which up to $2.5 million may be available for short-term swingline loans, against 85% of eligible accounts receivable. The Revolving Loans bore interest at Secure Overnight Financing Rate (“SOFR”), plus 4.50% through April 2022. Beginning in May 2022 through maturity, the Revolving Loans bear interest at the SOFR plus 4.50%. In addition, we are required to pay an unused line fee of 0.50% of the average unused portion of the maximum Revolving Loans amount during the immediately preceding month.
65
Interest is payable monthly in arrears. The maturity date under the Eclipse Loan Agreement, as amended, is January 26, 2025.
In the event that, for any reason, all or any portion of the Eclipse Loan Agreement is terminated prior to the scheduled maturity date, in addition to the payment of all outstanding principal and unpaid accrued interest, we are required to pay a fee equal to (i) 2.0% of the Revolving Loans commitment if such event occurs on or before January 26, 2023, (ii) 1.0% of the Revolving Loans commitment if such event occurs after January 26, 2023 but on or before January 26, 2024, and (iii) 0.5% of the Revolving Loans commitment if such event occurs after January 26, 2024 but on or before January 26, 2025. We may permanently terminate the Eclipse Loan Agreement with at least five business days prior notice. See Note 10 – Line of Credit in the accompanying consolidated financial statements for further information.
Contractual Obligations, Commitments and Contingencies
As a result of our acquisitions and licensing agreements, we are contractually and contingently obliged to pay, when due, various fixed and contingent milestone payments. See Note 18 – Commitments and Contingencies in the accompanying consolidated financial statements for further information.
On May 12, 2022, the Company entered into an agreement with Tris to terminate the License, Development, Manufacturing and Supply Agreement dated November 2, 2018 related to Tuzistra (the “Tuzistra License Agreement”). Pursuant to such termination, the Company agreed to pay Tris a total of approximately $6 million to $9 million, which reduced our total liability for minimum payments by approximately $8.0 million from the original License Agreement. The settlement payment will be paid in three installments from December 2022 through July 2024, with a provision that allows for the Company to pay interest on any principal amounts due but remaining unpaid past the scheduled payment date.
Upon closing of the acquisition of a line of prescription pediatric products from Cerecor, Inc. in October 2019, we assumed payment obligations that require us to make fixed and product milestone payments. As of June 30, 2023, up to $3.8 million of fixed and product milestone payments remain through 2026 and are expected to be paid from the revenue generated by Karbinal ER.
In connection with the February 2020 acquisition of Innovus Pharmaceuticals, Inc. (the “Innovus Acquisition”), all of Innovus’s shares were converted to our common stock and contingent value rights (“CVRs”), which represents contingent additional consideration of up to $16.0 million payable to satisfy future performance milestones. As of June 30, 2023, up to $5.0 million of potential CVR milestone payments remain, which we do not expect to pay. These CVR milestone payments expire on December 31, 2023.
In connection with our Innovus Acquisition, we assumed a contingent obligation which required us to make milestone payment of $0.5 million, between fiscal year 2026 through fiscal year 2033 to Novalere, if and when certain levels of FlutiCare sales are achieved.
In connection with our acquisition of the Rumpus assets, upon satisfaction of milestones, we may be required to pay up to $67.5 million in regulatory and commercial-based earn-out payments to Rumpus. Under the licensing agreement with Denovo Biopharma LLC (“Denovo”), we are required to make a payment of $0.6 million for a license fee in April 2022 and upon achievement of regulatory and commercial milestones, up to $101.7 million. Under the licensing agreement with Johns Hopkins University (“JHU”), upon achievement of regulatory and commercial milestone, we may be required to pay up to $1.6 million to JHU. In fiscal 2022, two milestones payable to Rumpus were achieved totaling $4.0 million, which were paid in 109,447 shares of common stock and $2.6 million in cash.
CRITICAL ACCOUNTING ESTIMATES
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of any contingent assets and liabilities at the date of the financial statements, as well as reported revenue and expenses during the reporting periods.
66
On an ongoing basis, we evaluate our estimates and judgments. We base our estimates on our historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Our actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2 – Summary of Significant Accounting policies to the notes to our audited financial statements included elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Revenue recognition
We generate revenue from product sales through our Rx Segment and Consumer Health Segment. We evaluate our contracts with customers to determine revenue recognition using the following five-step model: (1) identify the contract with the customer; (2) identify the performance obligations and if they are distinct; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations; and (5) recognize revenue when (or as) a performance obligation is satisfied.
Net product sales in the Rx Segment consist of sales of prescription pharmaceutical products under the Rx Portfolio, principally to a limited number of wholesale distributors and pharmacies in the United States. Rx product revenue is recognized at the point in time that control of the product transfers to the customer in accordance with shipping terms (i.e., upon delivery), which is generally “free-on-board” destination when shipped domestically within the United States and “free-on-board” shipping point when shipped internationally consistent with the contractual terms.
The Company makes estimates of the net sales price, including estimates of variable consideration to be incurred on the respective product sales (known as “Gross to Net” adjustments). Estimating gross to net adjustments and applying the constraint on variable consideration requires the use of significant management judgment and other market data.
The Gross to Net adjustments include:
| ● | Savings offers The Company offers savings programs for its patients covered under commercial payor plans in which the cost of a prescription to such patients is discounted. |
| ● | Prompt payment discounts Prompt payment discounts are based on standard provisions of wholesalers’ services. |
| ● | Wholesale distribution fees Wholesale distribution fees are based on definitive contractual agreements for the management of the Company’s products by wholesalers. |
| ● | Rebates The Rx Portfolio products are subject to commercial managed care and government (i.e. Medicaid) programs whereby discounts and rebates are provided to participating managed care organizations and federal and/or state governments. Calculations related to rebate accruals are estimated based on historical information from third-party providers. |
| ● | Wholesaler chargebacks The Rx Portfolio products are subject to certain programs with wholesalers whereby pricing on products is discounted below wholesaler list price to participating entities. These entities purchase products through wholesalers at the discounted price, and the wholesalers charge the difference between their acquisition cost and the discounted price back to the Company following the product purchases of the wholesalers’ end customers. |
67
| ● | Returns Wholesalers’ contractual return rights are limited to defective product, product that was shipped in error, product ordered by customer in error, product returned due to overstock, product returned due to dating or product returned due to recall or other changes in regulatory guidelines. The return policy for expired product allows the wholesaler to return such product starting six months prior to expiry date to twelve months post expiry date. The Company analyzes return data available from sales since inception date to determine a reliable return rate. |
Savings offers, rebates and wholesaler chargebacks reflect the terms of underlying agreements, which may vary. Accordingly, actual amounts will depend on the mix of sales by product and contracting entity. Future returns may not follow historical trends. Our periodic adjustments of our estimates are subject to time delays between the initial product sale and ultimate reporting and settlement of deductions. We continually monitor these provisions and do not believe variances between actual and estimated amounts have been material.
A 10% increase or decrease in these estimates impacts net sales by a corresponding increase or decrease of approximately $3.3 million.
We generate Consumer Health Segment product revenue from sales of various consumer health products through e-commerce platforms and direct mail. Revenue is generally recognized “free-on-board” shipping point, as those are the agreed-upon contractual terms. Taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction that are collected by us from a customer are excluded from revenue. Shipping and handling costs associated with outbound freight after control over a product has transferred to a customer are accounted for as a fulfillment cost and are included in cost of sales.
Impairment of Long-lived Assets
We assess impairment of long-lived assets annually and when events or changes in circumstances indicates that their carrying value amount may not be recoverable. Long-lived assets consist of property and equipment, net and goodwill and other intangible assets, net. Circumstances which could trigger a review include but are not limited to: (i) significant decreases in the market price of the asset; (ii) significant adverse changes in the business climate or legal or regulatory factors; (iii) changes in business plans or (iv) expectations that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. If the estimated future undiscounted cash flows, excluding interest charges, from the use of an asset are less than the carrying value, a write-down would be recorded to reduce the related asset to its estimated fair value. Such estimates involve projections of future sales and costs, which may vary from actual results. Declines in the outlook for the related products, particularly soon after fair-value measurement upon acquisition or prior impairment, can negatively impact our ability to recover the carrying value and can result in an impairment charge.
Our strategy is to continue building our portfolio of revenue-generating products by leveraging our commercial team’s expertise to build leading brands within large therapeutic markets. As a result of focusing on building the portfolio of revenue-generating prescription products, we have decided to abandon active development of its NT0502 (N-desethyloxybutynin), a new chemical entity that is for the treatment of sialorrhea, which is excessive salivation or drooling. During the year ended June 30, 2023, we incurred an impairment charge of $2.6 million related to NT0502 and have terminated the licensing agreement. We also terminated the license agreement with Cedars-Sinai Medical Center surrounding the Healight technology platform as an additional result of terminating the development of the Healight program. Further, the acquired product distribution rights from Innovus was impaired by $3.0 million due to discontinuance of products in the Consumer Health Segment.
During the year ended June 30, 2022, in connection with the decision to discontinue commercializing or divesting certain products within the Rx Segment that have minimal revenue and gross margin contribution, the Company recorded $4.9 million impairment expense for the write-down of intangible assets consisting of (i) $2.6 million for AcipHex Sprinkle, (ii) $1.4 million for ZolpiMist, (iii) $0.5 million for Tussionex, (iv) $0.2 million for Cefaclor and (v) $0.2 million for the Neos tradename. Additionally, our Consumer Health Segment recorded an impairment of $2.2 million related to products no longer being marketed and products that have been underperforming.
68
Goodwill
Goodwill is recorded as the difference between the fair value of the purchase consideration and the fair value of the net identifiable tangible and intangible assets acquired. As described in Note 2 – Summary of Significant Accounting Policies to our financial statements, Goodwill is reviewed for impairment at least annually or whenever events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable. If, after assessing events or circumstances, we determine it is more likely than not that the fair value of a reporting unit is less than its carrying amount, we perform a quantitative impairment test by comparing the fair value of the reporting unit with the carrying value. If the fair value of a reporting unit is less than the carrying amount, an impairment charge is recorded in the amount of the difference. The fair value of a reporting unit is estimated using a combination of a market multiple and a discounted cash flow approach. Determining the fair value of a reporting unit requires the use of estimates, assumptions and judgment. The principal estimates and assumptions that we use include prospective financial information (revenue growth, operating margins, and capital expenditures), future market conditions, weighted average costs of capital, a terminal growth rate, comparable multiples of publicly traded companies in our industry, and the earnings metrics and multiples utilized. We believe that the estimates and assumptions used in impairment assessments are reasonable. We have determined that we have two reporting units that require periodic review for goodwill impairment, the Rx Segment, and the Consumer Health Segment.
During the fiscal year 2022, our market capitalization significantly declined. The decline was considered a qualitative factor that led us to assess whether an impairment had occurred. The evaluation indicated that the goodwill related to one of the reporting units within the Rx Segment and Consumer Health Segment was potentially impaired. We performed a quantitative impairment test. As a result, we recorded an impairment charge of $65.8 million for the year ended June 30, 2022. At June 30, 2022, we had no goodwill recorded on our balance sheet.
Warrants
Equity classified warrants are valued using a Black-Scholes options pricing model at issuance and are not remeasured. Liability classified warrants are carried at fair value using either the Black-Scholes option pricing or Monte Carlo simulation model. Changes in the fair value of liability classified warrants in subsequent periods are recorded as a gain or loss on remeasurement and reported as a component of cash flows from operations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide information under this item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this item are identified in Item (a)(1) of Part IV and begin at page F-1 of this Annual Report on Form 10-K and are incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining adequate “disclosure controls and procedures,” as such term is defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934 (the “Exchange Act”), that are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
69
Based on the evaluation of our disclosure controls and procedures as of June 30, 2023, our principal executive officer and principal financial officer concluded that our controls were not effective as of the end of the period covered by this report. Notwithstanding the material weakness, our management believes that the financial statements included elsewhere in this report present fairly, in all material respects, our financial position, results of operations, changes in stockholders’ equity and cash flows in conformity with GAAP.
In connection with the preparation of our financial statements for the period ended June 30, 2023, we concluded that we had a material weakness in internal control over financial reporting related to our analysis for the accounting for valuation of our inventory. At year end, it was determined that the analysis of over/under absorbed manufacturing costs was not performed, which could have led to material misstatement of our financial statement. If not addressed, the deficiency could result in a material misstatement in the future. In response, we have incorporated the process for quantifying any over or under absorbed manufacturing costs, and having the appropriate level of management evaluate the analysis and materiality of any over or under absorption.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rules 13a-15(f) under the Exchange Act). Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2023. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Our management has concluded that, as of June 30, 2023, our internal control over financial reporting is effective based on these criteria.
Grant Thornton, LLP, the independent registered public accounting firm that audited our financial statements included in this Annual Report on Form 10-K, was not required to issue an attestation report on our internal control over financial reporting.
Changes in Internal Control over Financial Reporting
Previous Disclosure of Material Weakness in Internal Controls Over Financial Reporting
As disclosed in our September 30, 2022 Form 10-Q/A, we identified a material weakness in controls over the accounting for complex warrant issuances and the classification of these issued warrants. This material weakness resulted in the failure to prevent material adjustments in accounting for the warrants as equity classification when the warrants should have been classified as liabilities and marked to market each reporting period. While we have processes to properly identify and evaluate the appropriate accounting technical pronouncements, other literature, and consultation with third-party experts, we did not classify the warrants correctly.
Remediation Plan
Our Audit Committee conducted an internal investigation to identify and determine a plan to remediate the material weakness described above and to enhance our overall control environment. We will not consider the material weakness remediated until our enhanced control is operational for a sufficient period of time and tested, enabling management to conclude that the enhanced controls are operating effectively. Our remediation plan includes the implementation of controls over the process of reviewing significant and complex contracts and agreements.
Changes in Internal Control Over Financial Reporting
Except for the material weakness noted above, there have been no changes in the Company’s internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarter ended June 30, 2023, that have material effect, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
70
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTION THAT PREVENT INSPECTIONS
Not applicable
71
PART III
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
The following table sets forth the names and ages of all of our directors and executive officers. Our Board of Directors is currently comprised of five members, who are elected annually to serve for one year or until their successor is duly elected and qualified, or until their earlier resignation or removal. We have two executive officers that serve at the discretion of the Board of Directors and are appointed by the Board of Directors.
Name |
|
Age |
|
Position |
Joshua R. Disbrow |
|
48 |
|
Chairman and Chief Executive Officer |
Mark Oki |
|
54 |
|
Chief Financial Officer, Secretary, and Treasurer |
Greg Pyszczymuka |
|
44 |
|
Chief Commercial Officer |
Carl C. Dockery |
|
60 |
|
Director |
John A. Donofrio, Jr. |
|
55 |
|
Director |
Abhinav Jian |
|
32 |
|
Director |
Vivian H. Liu |
|
61 |
|
Director |
The following is a biographical summary of the experience of our executive officers and directors during the past five years, and an indication of directorships held by the directors in other companies subject to the reporting requirements under the federal securities law.
Joshua R. Disbrow – Chairman and Chief Executive Officer
Mr. Disbrow has been employed by us since April 16, 2015 and a member of our Board of Directors since January 2016. Prior to the closing of the merger between Luoxis Diagnostics, Inc. and Vyrix Pharmaceuticals, Inc. that formed Aytu BioPharma, Mr. Disbrow was the Chief Executive Officer of Luoxis since January 2013. Mr. Disbrow jointly served as the Chief Operating Officer of Ampio Pharmaceuticals, Inc. (“Ampio”) from December 2012 until April 2015. Prior to joining Ampio, he served as the Vice President of Commercial Operations at Arbor Pharmaceuticals, LLC (“Arbor”), a specialty pharmaceutical company, from May 2007 through October 2012. He joined Arbor as that company’s second full-time employee. Mr. Disbrow led the company’s commercial efforts from inception to the company’s acquisition in 2010 and growth to over $127 million in net sales in 2011 and to over $250 million in net sales in 2012. By the time Mr. Disbrow departed Arbor in late 2012, he handled the growth of the commercial organization to comprise over 150 people in sales, marketing sales training, managed care, national accounts, and other commercial functions. Mr. Disbrow has spent over 26 years in the pharmaceutical, diagnostic, and medical device industries and has held positions of increasing responsibility in sales, sales management, marketing, commercial operations, commercial strategy, and business development. Prior to joining Arbor, Mr. Disbrow served as Regional Sales Manager with Cyberonics, Inc., a medical device company focused on neuromodulation therapies from June 2005 through April 2007. Prior to joining Cyberonics he was the Director of Marketing at LipoScience Inc., an in vitro diagnostics company. Mr. Disbrow holds an MBA from Wake Forest University School of Business and BS in Management from North Carolina State University. Mr. Disbrow’s experience in executive management and commercialization within the pharmaceutical industry, monetizing company opportunities, and corporate finance led to the conclusion that he should serve as a member of our Board of Directors.
Mark K. Oki – Chief Financial Officer, Secretary, and Treasurer
Mr. Oki has served as our Chief Financial Officer since January 2022 and as our Secretary and Treasurer since May 5, 2022. From October 2015 through January 2022, Mr. Oki served as Chief Financial Officer of Vivus LLC, (formerly Vivus Inc.) a commercial-stage pharmaceutical company. Vivus was a Nasdaq listed company up to December 2020. From April 2006 to October 2015, Mr. Oki held several positions at Alexza Pharmaceuticals, Inc., a publicly listed specialty pharmaceutical company, most recently as Senior Vice President, Finance, Chief Financial Officer and Secretary. Before Alexza, Mr. Oki held roles of increasing responsibility at life science companies, Pharmacyclics, Inc. and Incyte Genomics, Inc. (now Incyte Corporation). Mr. Oki began his career in public accounting at Deloitte & Touche, LLP (now Deloitte).
72
Mr. Oki received his degree in Business Administration – Accounting and graduated with honors from San Jose State University and is a Certified Public Accountant (inactive).
Greg Pyszczymuka – Chief Commercial Officer
Mr. Pyszczymuka has served as our Chief Commercial Officer since January 2022. Prior to joining the Company, at the closing of the company’s merger with Neos Therapeutics in March 2021, Greg Pyszczymuka served as Vice President, Commercial at Neos Therapeutics since June 2020. He previously served as Vice President, Commercial Strategy & Market Access at Neos from November 2018 to June 2020, and as Executive Director of Channel Strategy & Access Programs. Prior to joining Neos, Greg had served in roles of increasing responsibility over a 15-year career including sales management, brand management, channel strategy, managed markets and new products planning. Greg joined Neos most recently from Aqua Pharmaceuticals (an Almirall company), and previously was with Iroko Pharmaceuticals, Zogenix, and Endo Pharmaceuticals. He holds a B.S. from Rutgers University and an M.B.A. from Argosy University.
John A. Donofrio, Jr. - Director
Mr. Donofrio joined our Board of Directors in July 2016. He is a senior pharmaceutical executive with over 30 years of experience in the industry across a broad range of areas, including President, Chief Financial Officer, and Chief Operating Officer positions. Mr. Donofrio has significant finance experience in consolidated financial reporting, international accounting and internal controls, financial systems development and implementation, cost accounting, inventory management, supply chain, transfer pricing, budget and forecast planning, integration of mergers and acquisitions and business development. Since March 2022 Mr. Donofrio has served as Executive Vice President, Chief Operating Officer of Novan Inc., a publicly held specialty dermatology company, and as President of Novan Inc.’s wholly owned subsidiary EPI Health, a specialty pharmaceutical company commercializing products in the dermatology market. From March 2019 until its acquisition by Novan, Inc in March 2022, Mr. Donofrio served as EPI Health’s President. Mr. Donofrio previously served as Chief Financial Officer and Head of Business Development at TrialCard from March of 2018 to March 2019. TrialCard is a technology-driven pharmaceutical services company providing patient access and support programs to the pharmaceutical and biotechnology industries. Prior to joining TrialCard, Mr. Donofrio was the Chief Financial Officer and Head of North American Business Development for Merz North America, or Merz, from August 2013 to March 2018. Merz is a specialty healthcare company that develops and commercializes innovative treatment solutions in aesthetics, dermatology and neurosciences in the United States and Canada. At Merz, Mr. Donofrio was accountable for financial performance, cost management, business development and strategic business planning and analysis for the finance organization in North America. Prior to joining Merz, Mr. Donofrio served as Vice President, Stiefel Global Finance, U.S. Specialty Business and Puerto Rico for Stiefel, a GlaxoSmithKline plc company from July 2009 to July 2013. In that role, Mr. Donofrio was responsible for the financial strategy, management reporting, and overall control framework for the Global Dermatology Business Unit. Mr. Donofrio served as a director of Vyrix from February 2014 to April 2015. Mr. Donofrio holds a degree in Accounting from North Carolina State University. Mr. Donofrio’s broad executive leadership experience and financial expertise along with experience in the pharmaceutical industry led to the conclusion that he should serve as a member of our Board of Directors.
Carl C. Dockery – Director
Mr. Dockery joined our Board of Directors in April 2016. Mr. Dockery is a financial executive with 30 years of experience as an executive in the insurance and reinsurance industry and more recently since 2006 as the founder and president of a registered investment advisory firm, Alpha Advisors, LLC. Mr. Dockery’s career as an insurance executive began in 1988 as an officer and director of two related and closely held insurance companies, including serving as secretary of Crossroads Insurance Co. Ltd. of Bermuda and as vice president of Gulf Insurance Co. Ltd. of Grand Cayman. Familiar with the London reinsurance market, in the 1990s, Mr. Dockery worked at Lloyd’s and the London Underwriting Centre brokering various types of reinsurance placements. From September 2014 through September 2019, Mr. Dockery served as a director of CytoDyn Inc. (OTCQB: CYDY), and a publicly-traded biotechnology company focused on the development and potential commercialization of humanized monoclonal antibodies for the treatment and prevention of HIV and cancers. Mr. Dockery graduated from Southeastern University with a Bachelor of Arts in Humanities. Mr. Dockery’s financial expertise and experience, as well as his experience as a director of a publicly traded biopharmaceutical company led to the conclusion that he should serve as a member of our Board of Directors.
73
Abhinav “Abi” Jain - Director
Mr. Jain joined our Board of Directors in June 2023. Since July 2019, Mr. Jain has served as an Analyst at Nantahala Capital Management and is focused on investments in various sectors, including specialty and generic pharmaceuticals. From 2015-2017, Mr. Jain was an Associate at Angelo, Gordon & Co., an alternative asset manager. At Angelo, Gordon & Co., Mr. Jain focused on private equity and structured credit investments. He graduated from Massachusetts Institute of Technology in 2012 with an S.B. in Chemical-Biological Engineering and from The Wharton School of the University of Pennsylvania in 2019 with an M.B.A. with honors in Finance and Entrepreneurial Management. Mr. Jain’s financial expertise and experience led to the conclusion that he should serve as a member of our Board of Directors. Mr. Jain was appointed pursuant to a board designation right granted to Nantahala Capital Management, LLC to appoint one director to our Board of Directors, pursuant to the Securities Purchase Agreement dated June 8, 2023 with Nantahala and other investors.
Vivian H. Liu - Director
Ms. Liu joined our Board of Directors in July 2022. Ms. Liu currently serves as Head of Corporate Affairs for PREMIA Holdings (HK) Limited, a developer of clinical-genomic oncology databases and service provider to pharmaceutical companies seeking to operate clinical trials throughout Asia. Prior to joining PREMIA, Ms. Liu served in various roles, including as a member of Board of Directors and President, Chief Executive Officer and Chief Financial Officer for Innovus Pharmaceuticals, Inc., a publicly listed consumer healthcare company acquired by Aytu BioPharma in February 2020. Prior to Innovus, she served as the President and Chief Executive Officer of FasTrack Pharmaceuticals, Inc. From 2017-2018, she served as the Chief Operating Officer and a member of the Board of Directors of Cesca Therapeutics, Inc. Previously, Vivian served as Managing Director of OxOnc Services Company, an oncology development company, and prior to that, Ms. Liu co-founded and served as President, Chief Executive Officer, and board director of NexMed, Inc., a drug development company which was later renamed Apricus BioSciences. Prior to her appointment as President of NexMed, Ms. Liu served in several executive capacities, including as Executive Vice President, Chief Operating Officer, Chief Financial Officer and Vice President of Corporate Affairs. Ms. Liu has an M.P.A. from the University of Southern California and a B.A. from the University of California, Berkeley. Ms. Liu’s experience in executive management within the pharmaceutical industry, as a director of a publicly traded biotech company and in corporate finance led to the conclusion that she should serve as a member of our Board of Directors.
Family Relationships
Jarrett T. Disbrow, our Chief Business Officer is the brother of Joshua R. Disbrow, our Chairman and Chief Executive Officer. There are no other family relationships among or between any of our current or former executive officers and directors.
Involvement in Certain Legal Proceedings
Mr. Oki was the Chief Financial Officer of Vivus, Inc. at the time a Chapter 11 petition was filed under the Federal bankruptcy laws in July 2020. Mr. Donofrio was Executive Vice President, Chief Operating Officer of Novan, Inc. at the time a Chapter 11 petition was filed under the Federal bankruptcy laws in July 2023.
Our directors or executive officers have not been involved in any legal proceedings in the past 10 years that would require disclosure under Item 401(f) of Regulation S-K promulgated under the Securities Act.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act requires our officers and directors and persons who own more than 10% of our outstanding common stock to file reports of ownership and changes in ownership with the Securities and Exchange Commission.
74
These officers, directors and stockholders are required by regulations under the Securities Exchange Act to furnish us with copies of all forms they file under Section 16(a).
Based solely on our review of the copies of forms we have received, we believe that all such required reports have been timely filed, except for one late filing of a Form 4 by Josh Disbrow relating to a rescission of 80,000 shares of restricted common stock on December 20, 2022, which was inadvertently filed one day late on December 23, 2022, and two late filings of Form 4s by Greg Pyszczymuka, one of which related to the conversion of 833 restricted stock units to shares of common stock on April 25, 2023, which was inadvertently filed late on June 20, 2023, and the second of which related to the conversion of 208 restricted stock units to shares of common stock on June 30, 2023, which was inadvertently filed late on July 6, 2023.
Code of Ethics
The information required by this Item regarding our Code of Ethics is found in Part I, Item 1, under the caption “Code of Ethics.”
Board Committees
Our Board has established an Audit Committee, Compensation Committee and a Nominating and Governance Committee. Our Audit Committee consists of Mr. Donofrio (Chair), Mr. Dockery, Mr. Jain, and Ms. Liu. Our Compensation Committee consists of Ms. Liu (Chair), Mr. Dockery, Mr. Jain, and Mr. Donofrio. Our Nominating and Governance Committee consists of Mr. Dockery (Chair), Mr. Donofrio, Mr. Jain, and Ms. Liu. The independence of our directors is discussed in Part III, Item 13 under the caption “Director Independence.”
Each of the above-referenced committees operates pursuant to a formal written charter. The charters for these committees, which have been adopted by our Board, contain a detailed description of the respective committee’s duties and responsibilities and are available on our website at http://www.aytubio.com under the “Investor Relations—Corporate Governance” tab.
Our Board has determined Mr. Donofrio qualifies as an audit committee financial expert, as defined in Item 407(d)(5) of Regulation S-K promulgated by the SEC.
Stockholder Proposals
Our bylaws establish procedures for stockholder nominations for elections of directors and bringing business before any annual meeting or special meeting of stockholders. A stockholder entitled to vote in the election of directors may nominate one or more persons for election as directors at a meeting only if written notice of such stockholder’s intent to make such nomination or nominations has been delivered to our Corporate Secretary at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary of the prior year’s annual meeting. In the event that the date of the annual meeting is more than 30 days before or more than 60 days after the anniversary date of the prior year’s annual meeting, the stockholder notice must be given not more than 120 days nor less than the later of 90 days prior to the date of the annual meeting or, if it is later, the 10th day following the date on which the date of the annual meeting is first publicly announced or disclosed by us. These notice deadlines are the same as those required by the SEC’s Rule 14a-8.
Pursuant to the bylaws, a stockholder’s notice must set forth among other things: (a) as to each person whom the stockholder proposes to nominate for election or reelection as a director all information relating to such person that is required to be disclosed in solicitations of proxies for election of directors in an election contest, or is otherwise required, in each case pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder; and (b) as to any other business that the stockholder proposes to bring before the meeting, a brief description of the business desired to be brought before the meeting, the reasons for conducting such business at the meeting and any material interest in such business of such stockholder and the beneficial owner, if any, on whose behalf the proposal is made.
75
There have been no changes to these nominating procedures since the adoption of the bylaws.
ITEM 11. EXECUTIVE COMPENSATION
Executive Compensation
In accordance with Item 402 of Regulation S-K promulgated by the SEC, we are required to disclose certain information regarding the makeup of and compensation of our Company’s named executive officers.
In establishing executive compensation, our Board is guided by the following goals:
| ● | compensation should consist of a combination of cash and equity awards that are designed to fairly pay the executive officers for work required for a company of our size and scope; |
| ● | compensation should align the executive officers’ interests with the long-term interests of stockholders; and |
| ● | compensation should assist with attracting and retaining qualified executive officers and directors. |
Compensation of Directors
Our current compensation package for non-employee directors, effective July 1, 2020, consists of: an annual cash retainer of $70,000 for the non-executive Board chair, $40,000 for each other director, $20,000 for each audit committee and compensation committee chair, $10,000 for nominating and governance committee chair, and $10,000 for each other committee member of the audit and compensation committees and $5,000 for each other committee member of the nominating and governance committee; a grant of 6,500 restricted shares of stock or restricted stock units upon appointment to the Board; and an annual stock option grant of 1,500 shares thereafter.
The following table provides information regarding all compensation paid to non-employee directors of Aytu during the fiscal year ended June 30, 2023.
|
|
Fees Earned |
|
|
|
|
|
||
|
|
or Paid in |
|
Stock |
|
|
|
||
Name |
|
Cash |
|
Awards |
|
Total |
|||
Carl C. Dockery (1)(2) |
|
$ |
70,000 |
|
$ |
— |
|
$ |
70,000 |
John A. Donofrio Jr. (1)(2) |
|
$ |
90,000 |
|
$ |
— |
|
$ |
90,000 |
Abhinav Jian (1)(2) |
|
$ |
3,306 |
|
$ |
— |
|
$ |
3,306 |
Vivian H. Liu (1)(2) |
|
$ |
63,750 |
|
$ |
25,870 |
|
$ |
89,620 |
(1) |
As of June 30, 2023, the number of restricted shares held by each non-employee director was as follows: 3,893 restricted shares for Mr. Dockery and 762 restricted shares for Mr. Donofrio, both adjusted for the recission of shares from the Aponowicz and Paguia settlement (for more information, see Stipulation of Compromise and Settlement in Note 15 to the Consolidated Financial Statements included in Aytu’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023). Ms. Liu held 6,825 restricted shares. |
(2) |
As of June 30, 2023, the number of stock options held by each non-employee director was as follows: (i) 200 shares for Mr. Dockery; (ii) 200 shares for Mr. Donofrio. |
76
Executive Officer Compensation
The following table sets forth all cash compensation earned, as well as certain other compensation paid or accrued for the years ended June 30, 2023 and 2022 to each of the following named executive officers.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Value and |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity |
|
Nonqualified |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incentive |
|
Deferred |
|
|
|
|
|
|
||
Name and |
|
|
|
|
|
|
|
|
|
Stock |
|
Option |
|
Plan |
|
Compensation |
|
|
All Other |
|
|
|
||||
Principal |
|
|
|
Salary |
|
Bonus |
|
Award |
|
Award |
|
Compensation |
|
Earnings |
|
Compensation |
|
|
|
|||||||
Position |
|
Year |
|
($) |
|
($) |
|
($) |
|
($)(1) |
|
($) |
|
($) |
|
($) |
|
Total ($) |
||||||||
(a) |
|
(b) |
|
(c) |
|
(d) |
|
(e) |
|
(f) |
|
(g) |
|
(h) |
|
(i) |
|
(j) |
||||||||
Named Executive Officers: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joshua R. Disbrow |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Executive Officer |
|
2023 |
|
$ |
590,000 |
|
$ |
118,000 |
|
$ |
— |
|
$ |
— |
|
|
— |
|
|
— |
|
|
— |
|
$ |
708,000 |
since December 2012 |
|
2022 |
|
$ |
590,000 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
— |
|
|
— |
|
|
— |
|
$ |
590,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark K. Oki |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Financial Officer, Secretary |
|
2023 |
|
$ |
415,000 |
|
$ |
83,000 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
24,840 |
|
$ |
522,840 |
and Treasurer since January 2022 |
|
2022 |
|
$ |
183,558 |
|
$ |
50,000 |
|
$ |
135,000 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
368,558 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Greg Pyszczymuka |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Commercial Officer |
|
2023 |
|
$ |
375,000 |
|
$ |
150,000 |
|
$ |
15,046 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
540,046 |
(1) |
Option awards are reported at fair value at the date of grant. |
Our executive officers are reimbursed by us for any out-of-pocket expenses incurred in connection with activities conducted on our behalf. Executives are reimbursed for business expenses directly related to our business activities, such as travel, primarily for business development as we grow and expand our product lines. On average, each executive incurs between $1,000 to $3,000 of out-of-pocket business expenses each month. The executive management team meets weekly and determines which activities they will work on based upon what we determine will be most beneficial to the Company and our stockholders. No interest is paid on amounts reimbursed to the executives.
77
Outstanding Equity Awards at Fiscal Year-End 2023
The following table contains certain information concerning unexercised options for the Named Executive Officers as of June 30, 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards |
|
Stock Awards |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
Incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incentive |
|
Plan |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan |
|
Awards: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Awards: |
|
Market |
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
Number |
|
or Payout |
|
|
|
|
|
|
|
Incentive |
|
|
|
|
|
|
Number |
|
|
|
|
of |
|
Value of |
|
|
|
|
|
|
|
Plan |
|
|
|
|
|
|
of |
|
Market |
|
Unearned |
|
Unearned |
||
|
|
|
|
|
|
Awards: |
|
|
|
|
|
|
Shares |
|
Value of |
|
Shares, |
|
Shares, |
||
|
|
Number of |
|
Number of |
|
Number |
|
|
|
|
|
|
or Units |
|
Shares or |
|
Units or |
|
Units or |
||
|
|
Securities |
|
Securities |
|
of |
|
|
|
|
|
|
of Stock |
|
Units of |
|
Other |
|
Other |
||
|
|
Underlying |
|
Underlying |
|
Securities |
|
|
|
|
|
|
That |
|
Stock |
|
Rights |
|
Rights |
||
|
|
Unexercised |
|
Unexercised |
|
Underlying |
|
|
|
|
|
|
Have |
|
That |
|
That |
|
That |
||
|
|
Options |
|
Options |
|
Unexercised |
|
Option |
|
Option |
|
Not |
|
Have Not |
|
Have Not |
|
Have Not |
|||
|
|
Exercisable |
|
Unexercisable |
|
Unearned Options |
|
Exercise |
|
Expiration |
|
Vested |
|
Vested ($) |
|
Vested |
|
Vested |
|||
Name |
|
(#) |
|
(#) |
|
(#) |
|
Price ($) |
|
Date |
|
(#) |
|
(1) |
|
(#) |
|
($) |
|||
Named Executive Officers: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joshua R. Disbrow |
|
375 |
|
125 |
|
— |
|
$ |
290.00 |
|
6/8/2030 |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
Chief Executive Officer |
|
— |
|
— |
|
— |
|
|
— |
|
— |
|
37 |
|
|
59 |
|
— |
|
|
— |
|
|
— |
|
— |
|
— |
|
|
— |
|
— |
|
2,227 |
|
|
3,563 |
|
— |
|
|
— |
|
|
— |
|
— |
|
— |
|
|
— |
|
— |
|
13,334 |
|
|
21,334 |
|
— |
|
|
— |
|
|
— |
|
— |
|
— |
|
|
— |
|
— |
|
563 |
|
|
901 |
|
— |
|
|
— |
|
|
— |
|
— |
|
— |
|
|
— |
|
— |
|
2 |
|
|
3 |
|
— |
|
|
— |
Total |
|
375 |
|
125 |
|
— |
|
$ |
|
|
|
|
16,163 |
|
$ |
25,861 |
|
— |
|
$ |
— |
Mark K. Oki |
|
— |
|
— |
|
— |
|
$ |
— |
|
— |
|
2,917 |
|
$ |
4,667 |
|
— |
|
$ |
— |
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
— |
|
— |
|
— |
|
$ |
|
|
|
|
2,917 |
|
$ |
4,667 |
|
— |
|
$ |
— |
Greg Pyszczymuka |
|
— |
|
7,031 |
|
— |
|
$ |
4.00 |
|
10/1/2032 |
|
3,335 |
|
$ |
5,336 |
|
— |
|
$ |
— |
Chief Commercial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
— |
|
7,031 |
|
— |
|
$ |
|
|
|
|
3,335 |
|
$ |
5,336 |
|
— |
|
$ |
— |
(1) |
Based on $1.60 per share which was the closing price of our common stock on NASDAQ on June 30, 2023, the last trading day of that fiscal year. |
Employment Agreements
Joshua R. Disbrow Agreement
On February 13, 2023, we entered into an amended and restated employment agreement with Mr. Disbrow. The agreement supersedes any prior employment agreements or amendments with the Company. The agreement was amended to: (i) provide for one-year employment terms with auto-renewal; (ii) modify the acceleration provision in connection with a change of control such that he would need to be terminated within 12 months following a change of control for “Cause” or resign for “Good Reason”; and (iii) provide associated changes to the “Cause” definition to (a) change material misconduct in connection with his employment to willful malfeasance or willful misconduct; and (b) change material breach of the employment agreement to willful and deliberate breach.
Mark K. Oki Agreement
On February 13, 2023, we entered into an amended and restated employment agreement with Mr. Oki. The agreement supersedes any prior employment agreements with the Company. The agreement was amended to: (i) modify the equity acceleration provision to conform to Mr. Disbrow’s agreement relating to the equity awards referenced and acceleration language; and (ii) provide associated changes to the “Cause” definition to (a) change material misconduct in connection with his other agreements with the Company to willful malfeasance or willful misconduct; (b) make conforming changes related to Mr. Oki’s unintended but material breach of the agreement instead of a material and repeated breach; and (c) change gross negligence in connection with his employment to willful malfeasance.
78
Greg Pyszczymuka Agreement
On March 21, 2023, we entered into an amended and restated employment agreement with Mr. Pyszczymuka. The agreement supersedes any prior employment agreements with the Company. The agreement was amended to: (i) modify the equity acceleration provision to conform to Mr. Disbrow’s agreement relating to the equity awards referenced and acceleration language; and (ii) provide associated changes to the “Cause” definition to (a) change material misconduct in connection with his other agreements with the Company, to willful malfeasance or willful misconduct; (b) make conforming changes related to Mr. Pyszczymuka’s unintended but material breach of the agreement, instead of a material and repeated breach; and (c) change gross negligence in connection with his employment to willful malfeasance.
Payments Provided Upon Termination for Good Reason or Without Cause
Pursuant to the employment agreements, in the event employment is terminated without Cause by us or the officer terminates his employment with Good Reason, we will be obligated to pay him any accrued compensation and, in the case of Mr. Disbrow, (i) a lump sum payment equal to two and one half (2.5) times his base salary in effect at the date of termination; (ii) continued participation in the health and welfare plans for up to two years; and (iii) a pro-rata amount of the target bonus determined by the percentage of time he was employed during the fiscal year. Messrs. Oki and Pyszczymuka shall receive, (i) a payment equal to his base salary in effect at the date of termination; (ii) immediate vesting of all stock-based awards; (iii) continued participation in the health and welfare plans for up to 12 months; and (iv) a pro-rata amount of the target bonus determined by the percentage of time he was employed during the fiscal year.
“Good Reason” means (i) there is a material reduction of the level compensation (excluding any bonuses) except where there is a general reduction applicable to the management team generally; (ii) there is a material reduction in overall responsibilities or authority, or scope of duties; or (iii) without the officer’s written consent, a material change in the principal geographic location at which the officer must perform his services (it being understood that the relocation of the officer to a facility or a location within forty (40) miles of the State Capitol Building in Denver, Colorado shall not be deemed material for purposes of the employment agreements).
“Cause” means (i) willful malfeasance or willful misconduct in connection with his employment; (ii) conviction of, or entry of a plea of guilty to, or entry of a plea of nolo contendere with respect to, any crime other than a traffic violation or infraction which is a misdemeanor; (iii) willful and deliberate violation of a Company policy, (iv) unintended but material breach of any written policy applicable to all employees adopted by the Company which is not cured to the reasonable satisfaction of the Board of Directors within thirty (30) business days after notice thereof; (v) the unauthorized use or disclosure of any proprietary information or trade secrets of the Company or any other party as to which the officer owes an obligation of nondisclosure as a result of the officer’s relationship with the Company, or (vi) the willful and deliberate breach of the employment agreement.
Payments Provided Upon a Change in Control
In the event the officer’s employment is terminated within 12 months of a Change in Control of us, all stock options, restricted stock, and other stock-based grants granted or may be granted in the future by us to the officers will immediately vest and become exercisable. In addition, Mr. Disbrow shall be paid a pro-rata amount of the target bonus determined by the percentage of time he was employed during the fiscal year. In addition, Mr. Oki shall receive (i) a payment equal to his base salary in effect at the date of the Change in Control; (ii) continued participation in the health and welfare plans for up to 12 months; and (iii) a pro-rata amount of the target bonus determined by the percentage of time he was employed during the fiscal year.
“Change in Control” means: the occurrence of any of the following events:
| ● | the sale of all or substantially all of the assets of the Company on a consolidated basis to an unrelated person or entity; or |
79
| ● | a merger, reorganization or consolidation pursuant to which the holders of the Company’s outstanding voting power and outstanding stock immediately prior to such transaction do not own a majority of the outstanding voting power and outstanding stock or other equity interests of the resulting or successor entity (or its ultimate parent, if applicable) immediately upon completion of such transaction |
| ● | the sale of all of the stock of the Company to an unrelated person, entity or group thereof acting in concert; or |
| ● | any other transaction in which the owners of the Company’s outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the Company or any successor entity immediately upon completion of the transaction other than as a result of the acquisition of securities directly from the Company. |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth information with respect to the beneficial ownership of our common stock as of August 31, 2023 for:
| ● | each beneficial owner of more than 10% of our outstanding common stock; |
| ● | each of our director and named executive officers; and |
| ● | all of our directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include common stock that can be acquired within 60 days of August 31, 2023. The percentage ownership information shown in the table is based upon 5,530,027 shares of common stock outstanding as of August 31, 2023.
Except as otherwise indicated, all of the shares reflected in the table are shares of common stock and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares of common stock subject to options and warrants held by that person that are immediately exercisable or exercisable within 60 days of August 31, 2023. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. Beneficial ownership representing less than 1% is denoted with an asterisk (*). The information in the tables below are based on information known to us or ascertained by us from public filings made by the stockholders. Except as otherwise indicated in the table below, addresses of the director, executive officers and named beneficial owners are in care of Aytu BioPharma, Inc., 7900 East Union Avenue, Suite 920, Denver, Colorado 80237.
80
|
|
Number of |
|
Percentage of |
|
|
|
Shares |
|
Shares |
|
|
|
Beneficially |
|
Beneficially |
|
|
|
Owned |
|
Owned |
|
5% or more Beneficial Owners |
|
|
|
|
|
Nantahala Capital Management, LLC (1) |
|
1,086,812 |
|
19.7 |
% |
|
|
|
|
|
|
Non-employee Directors |
|
|
|
|
|
Carl C. Dockery(2) |
|
8,402 |
|
* |
|
John A. Donofrio Jr.(3) |
|
962 |
|
* |
|
Vivian H. Liu(4) |
|
6,825 |
|
* |
|
Named Officers |
|
|
|
|
|
Joshua R. Disbrow(5) |
|
71,966 |
|
1.30 |
% |
Mark K. Oki (6) |
|
9,500 |
|
* |
|
Greg Pyszczymuka (7) |
|
22,143 |
|
* |
|
All directors and executive officers as a group, including those named above (eight persons) (8) |
|
190,951 |
|
3.45 |
% |
* |
Represents beneficial ownership of less than 1%. |
(1) |
The number of shares is from a schedule 13D/A filed by Nantahala Capital Management with the SEC on June 16, 2023. Based on such filing, Nantahala Capital Management are deemed to have the voting and dispositive power with respect to 1,086,812 shares of common stock. Nantahala Capital have their principal business office at 130 Main St. 2nd Floor, Nan Canaan, CT 06840. |
(2) |
Consists of (i) 4,259 shares of common stock, (ii) 3,893 unvested restricted shares, and (iii) 200 shares of common stock issuable upon the exercise of vested options, (iv) 50 shares of common stock held by Alpha Venture Capital Partners, L.P Mr. Dockery is the President of the general partner of Alpha Venture Capital Partners, L.P. and therefore may be deemed to beneficially own the shares beneficially owned by Alpha Venture Capital Partners, L.P. |
(3) |
Consists of (i) 762 unvested restricted shares, (ii) 200 shares of common stock issuable upon the exercised of vested options. |
(4) Consists of 6,825 unvested restricted shares.
(5) |
Consists of (i) 55,428 shares of common stock, (ii) 16,163 unvested restricted shares, (iii) 375 shares of common stock issuable upon the exercise of vested options. Does not include 116 shares of common stock held by an irrevocable trust for estate planning in which Mr. Disbrow is a beneficiary. Mr. Disbrow does not have or share investment control over the shares held by the trust, Mr. Disbrow is not the trustee of the trust (nor is any member of Mr. Disbrow’s immediate family) and Mr. Disbrow does not have or share the power to revoke the trust. As such, under Rule 16a 8(b) and related rules, Mr. Disbrow does not have beneficial ownership over the shares purchased and held by the trust. |
(6) |
Consists of (i) 6,583 shares of common stock, (ii) 2,917 shares of unvested restricted shares. |
(7) Consists of (i) 20,267 shares of common stock, (ii) 1,876 shares of unvested restricted shares.
(8) |
In addition to the above stated for directors and officers, includes (i) 63,941 shares of common stock, and (ii) 7,212 shares of unvested restricted shares. |
ITEM 13. CERTAIN RELATIONSHIPS, RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Related Party Transactions
We describe below all transactions and series of similar transactions, other than compensation arrangements, during the last three fiscal years, to which we were a party or will be a party, in which:
| ● | the amounts involved exceeded or will exceed $120,000; and |
| ● | any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
81
Jarrett T. Disbrow, the brother of Joshua R. Disbrow, our Chief Executive Officer, is employed by us as Chief Business Officer and President, Consumer Health. His total annual salary and other cash compensation was approximately $427,000, which consists of $365,000 base salary plus $62,000 cash bonus during the year ended June 30, 2023, and he receives benefits consistent with other employees serving in the same capacity.
Review, Approval or Ratification of Transactions with Related Persons
Effective upon its adoption in July 2016, pursuant to the Audit Committee Charter, the Audit Committee is responsible for reviewing and approving all related party transactions as defined under Item 404 of Regulation S-K, after reviewing each such transaction for potential conflicts of interests and other improprieties. Our policies and procedures for review and approval of transactions with related persons are in writing in our Code of Conduct and Ethics available on our website at http://www.aytubio.com under the “Investor Relations—Corporate Governance” tab.
Prior to the adoption of the Audit Committee Charter, and due to the small size of our company, we did not have a formal written policy regarding the review of related party transactions, and relied on our Board of Directors to review, approve or ratify such transactions and identify and prevent conflicts of interest. Our Board of Directors reviewed any such transaction in light of the particular affiliation and interest of any involved director, officer or other employee or stockholder and, if applicable, any such person’s affiliates or immediate family members.
Director Independence
Our common stock is listed on the NASDAQ Capital Market. Therefore, we must comply with the exchange rules regarding director independence. Audit Committee members must satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, for listed companies. In order to be considered to be independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors or any other board committee: (1) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
Four of our five directors are independent under the definition of NASDAQ. Josh Disbrow is not independent under either definition due to being an executive officer of our Company.
82
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Grant Thornton, LLP, or Grant Thornton has served as our independent auditor since December 2022 and has been appointed by our Audit Committee to continue as our independent auditor for the fiscal year ending June 30, 2023.
Plante & Moran, PLLC, or Plante Moran has served as our independent auditor until December 2022.
The following table presents aggregate fees for professional services rendered by our principal independent registered public accounting firms, Grant Thornton for the fiscal year ended June 30, 2023, and Plante Moran for the year ended June 30, 2022, for the audit of our annual financial statements.
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Audit fees |
|
$ |
940 |
|
$ |
547 |
Audit related fees* |
|
|
— |
|
|
32 |
Total fees |
|
$ |
940 |
|
$ |
579 |
* |
Audit-related fees for both fiscal years 2023 and 2022 were comprised of fees related to registration statements, including S-1, S-3 and S-8 filings, our registered offerings, and at-the-market (ATM) offerings. |
In addition to the amounts above, $0.1 million in professional services was rendered by Plante Moran as our principal independent auditor for the financial statements included in our Form 10–Q and 10-Q/A during the fiscal year ended June 30, 2023.
Dismissal of Independent Registered Public Accountants.
On December 12, 2022, the Audit Committee of the Board of Directors of Aytu BioPharma, Inc. (the “Company”) dismissed Plante & Moran, PLLC (“Plante Moran”), as the Company’s independent registered public accounting firm.
The reports of Plante Moran on the Company’s consolidated financial statements for the fiscal years ended June 30, 2022 and 2021 did not contain any adverse opinion or disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope or accounting principles, except for Plante Moran’s report on the financial statements for the year ended June 30, 2022, which contained an explanatory paragraph expressing substantial doubt about the Company’s ability to continue as a going concern.
During the fiscal years ended June 30, 2022 and 2021, and through the date of Plante Moran’s dismissal, there were (i) no “disagreements” (as that term is defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions) between the Company and Plante Moran on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to the satisfaction of Plante Moran would have caused Plante Moran to make reference to the subject matter of the disagreement in connection with its reports on the Company’s consolidated financial statements for such years and (ii) no “reportable events” as that term is defined in Item 304(a)(1)(v) of Regulation S-K, except for the material weakness in the Company’s internal control over financial reporting previously reported in Part II, Item 9A “Controls and Procedures” in the Company’s Annual Report on Form 10-K for the year ended June 30, 2021, as amended.
The Company concluded that it had a material weakness in its internal control over financial reporting related to the analysis for the accounting for the impairment of long-lived assets, including goodwill and other intangible assets. The Company performs an assessment to determine if an impairment of long-lived assets has occurred annually or when circumstances indicate an impairment may have occurred. This assessment was prepared by internal staffing and reviewed by the Chief Financial Officer. At the June 30, 2021 fiscal year end, it was determined that the Company improperly aggregated certain assets when performing this assessment. This resulted in an incorrect conclusion that no impairment had occurred. This deficiency did not result in a revision of any of the Company’s previously issued financial statements. However, if not addressed, the deficiency could have resulted in a material misstatement in the future. In response, the Company incorporated utilization of third-party providers to review its assumptions and computations in the Company’s impairment analysis for completeness and accuracy. The Company believes that its controls are now designed properly and operating effectively.
83
The material weakness was discussed with the Audit Committee. The Company has authorized Plante Moran to respond fully to inquiries of Grant Thornton LLP (“Grant Thornton”), the Company’s successor accountant as described below, concerning the material weaknesses.
Engagement of New Independent Registered Public Accountants.
On December 12, 2022, the Audit Committee appointed Grant Thornton LLP as the Company’s independent registered public accounting firm for the fiscal year ended June 30, 2023.
During the fiscal years ended June 30, 2021 and 2022 and the subsequent interim period through December 12, 2022, neither the Company nor anyone on its behalf has consulted with Grant Thornton with respect to either (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s consolidated financial statements, and neither a written report nor oral advice was provided to the Company that Grant Thornton concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a disagreement (as defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions to Item 304 of Regulation S-K) or a reportable event (as defined in Item 304(a)(1)(v) of Regulation S-K).
84
PART IV
ITEM 15. EXHIBITS AND CONSOLIDATED FINANCIAL STATEMENT SCHEDULES
(a)(1)Financial Statements
The following documents are filed as part of this Form 10-K, as set forth on the Index to the Consolidated Financial Statements found on page F-1.
| ● | Reports of Independent Registered Public Accounting Firms |
| ● | Consolidated Balance Sheets as of June 30, 2023 and 2022 |
| ● | Consolidated Statements of Operations for the years ended June 30, 2023 and 2022 |
| ● | Consolidated Statements of Stockholders’ Equity for the years ended June 30, 2023 and 2022 |
| ● | Consolidated Statements of Cash Flows for the years ended June 30, 2023 and 2022 |
| ● | Notes to Consolidated Financial Statements |
(a)(2)Financial Statement Schedules
Not Applicable.
85
(a)(3)Exhibits
Exhibit |
|
Description |
|
Registrant’s |
|
Date Filed |
|
Exhibit |
|
Filed |
|
|
|
|
|
|
|
|
|
|
|
2.1 |
|
|
8-K |
|
09/18/19 |
|
2.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2 |
|
|
8-K |
|
10/15/19 |
|
2.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3 |
|
|
8-K |
|
12/10/20 |
|
2.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.4 |
|
|
10-Q |
|
05/17/21 |
|
2.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
|
8-K |
|
06/09/15 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
Certificate of Amendment of Certificate of Incorporation, effective June 1, 2016. |
|
8-K |
|
06/02/16 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.3 |
|
Certificate of Amendment of Certificate of Incorporation, effective June 30, 2016. |
|
8-K |
|
07/01/16 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.4 |
|
Certificate of Amendment of Certificate of Incorporation, effective August 25, 2017. |
|
8-K |
|
08/29/17 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.5 |
|
Certificate of Amendment to the Restated of Certificate of Incorporation, effective August 10, 2018. |
|
8-K |
|
08/10/18 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.6 |
|
Certificate of Amendment to the Restated Certificate of Incorporation, effective December 8, 2020. |
|
8-K |
|
12/08/20 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.7 |
|
Certificate of Amendment of Certificate of Incorporation, effective March 22, 2021. |
|
8-K |
|
03/22/21 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.8 |
|
Certificate of Amendment of Certificate of Incorporation, effective January 6, 2023. |
|
8-K |
|
01/25/23 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.9 |
|
|
8-K |
|
05/09/22 |
|
3.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
|
8-K |
|
03/13/20 |
|
4.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.2 |
|
|
8-K |
|
03/13/20 |
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.3 |
|
|
8-K |
|
03/20/20 |
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.4 |
|
|
8-K |
|
03/20/20 |
|
4.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.5 |
|
|
8-K |
|
07/02/20 |
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.6 |
|
|
8-K |
|
03/04/22 |
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.7 |
|
|
8-K |
|
03/04/22 |
|
4.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.7 |
|
|
8-K |
|
08/10/22 |
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.7 |
|
|
8-K |
|
08/10/22 |
|
4.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.7 |
|
|
S-1/A |
|
06/05/23 |
|
4.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
86
4.7 |
|
|
S-1/A |
|
06/05/23 |
|
4.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.7 |
|
|
S-1/A |
|
06/05/23 |
|
4.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.8 |
|
|
10-K |
|
09/27/22 |
|
4.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
|
|
8-K |
|
07/28/16 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2 |
|
2015 Stock Option and Incentive Plan, as amended on July 26, 2017. |
|
8-K |
|
07/27/17 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3 |
|
|
8-K |
|
08/16/17 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5 |
|
|
10-K |
|
09/06/18 |
|
10.31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6 |
|
|
10-Q |
|
02/07/19 |
|
10.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7 |
|
License, Development, Manufacturing and Supply Agreement, dated November 2, 2018. |
|
10-Q |
|
02/07/19 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.8 |
|
|
10-Q |
|
05/14/19 |
|
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9 |
|
Employment Agreement with Joshua R. Disbrow, dated April 16, 2019. |
|
10-Q |
|
05/14/19 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10 |
|
Amended and restated License and Supply Agreement with Acerus Pharmaceuticals, dated July 29, 2019. |
|
8-K |
|
08/02/19 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.11 |
|
|
8-K |
|
09/18/19 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12 |
|
|
8-K |
|
10/3/2019 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.13 |
|
|
8-K |
|
10/15/19 |
|
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.14 |
|
First Amendment to Asset Purchase Agreement with Cerecor, Inc., dated November 1, 2019. |
|
8-K |
|
11/04/19 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15 |
|
Registration Rights Agreement with Cerecor, Inc., dated November 1, 2019. |
|
8-K |
|
11/04/19 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.16 |
|
|
8-K |
|
11/04/19 |
|
10.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.17 |
|
Consent and Limited Waiver Agreement, dated November 1, 2019. |
|
8-K/A |
|
11/04/19 |
|
10.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.18 |
|
Consent and Limited Waiver Agreement, dated November 1, 2019. |
|
8-K/A |
|
11/07/19 |
|
10.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.19 |
|
|
8-K |
|
12/02/19 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.20 |
|
Form of Restricted Stock Cancelation and Exchange Agreement. |
|
8-K |
|
07/02/20 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.22 |
|
|
8-K |
|
12/10/20 |
|
10.3 |
|
|
87
|
|
|
|
|
|
|
|
|
|
|
10.23 |
|
|
8-K |
|
03/22/21 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.24 |
|
|
10-Q |
|
05/17/21 |
|
10.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.26 |
|
Employment Agreement between Aytu BioPharma, Inc. and Christopher Brooke, dated April 12, 2021 |
|
10-Q |
|
05/17/21 |
|
10.13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.27 |
|
Option Agreement between Rumpus VEDS, LLC and Denovo Biopharma LLC, dated December 21, 2019. |
|
10-Q |
|
05/17/21 |
|
10.14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.28 |
|
|
10-Q |
|
05/17/21 |
|
10.15 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.29 |
|
|
8-K |
|
06/04/21 |
|
1.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.30 |
|
|
10-K |
|
9/28/2021 |
|
10.79 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.31 |
|
|
10-K |
|
9/28/2021 |
|
10.80 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.33† |
|
|
10-Q |
|
02/14/22 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.34& |
|
|
10-Q |
|
02/14/22 |
|
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.35& |
|
|
10-Q |
|
02/14/22 |
|
10.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.36 |
|
Registration Rights Agreement dated January 26, 2022 between Aytu and each of the warrant holders. |
|
10-Q |
|
02/14/22 |
|
10.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.37& |
|
|
10-Q |
|
02/14/22 |
|
10.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38#& |
|
|
10-Q |
|
05/16/22 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38 |
|
|
8-K |
|
07/01/22 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38 |
|
|
10-Q |
|
05/11/23 |
|
10.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38 |
|
|
10-Q |
|
05/11/23 |
|
10.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38† |
|
|
S-1/A |
|
06/05/23 |
|
10.42 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38† |
|
|
S-1/A |
|
06/05/23 |
|
10.43 |
|
|
88
|
|
|
|
|
|
|
|
|
|
|
10.45† |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
10.46† |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
10.47 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
10.48 |
|
Commercial Lease Agreement dated June 10, 1999, between Walstib, L.P. and Pharmafab, Inc. |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
10.49 |
|
First Amendment to Lease dated September 1, 2002, between Walstib, L.P. and PFAB, LP |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
10.50 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
10.51 |
|
Third Amendment to Lease dated October 1, 2003, Between TIAA and PFAB, LP |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
10.52 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
10.53 |
|
Fifth Amendment to Lease dated April 5, 2010, between TIAA and Neos Therapeutics, LP |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
10.54 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
10.55† |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
21.1 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
23.1 |
|
Consent of Plante & Moran, PLLC, Independent Registered Public Accounting Firm |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
23.2 |
|
Consent of Grant Thornton, LLP, Independent Registered Public Accounting Firm |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
24.1 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
32.1 |
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
101 INS |
|
XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
101 SCH |
|
Inline XBRL Taxonomy Schema Linkbase Document |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
101 CAL |
|
Inline XBRL Taxonomy Calculation Linkbase Document |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
101 DEF |
|
Inline XBRL Taxonomy Definition Linkbase Document |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
101 LAB |
|
Inline XBRL Taxonomy Labels Linkbase Document |
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
101 PRE |
|
Inline XBRL Taxonomy Presentation Linkbase Document |
|
|
|
|
|
|
|
X |
89
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
|
|
X |
† |
Indicates is a management contract or compensatory plan or arrangement. |
# |
The company has received confidential treatment of certain portions of this agreement. These portions have been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request. |
& |
Pursuant to Item 601(b)(10) of Regulation S-K, portions of this exhibit (indicated by asterisks) have been omitted as the registrant has determined that (1) the omitted information is not material and (2) the omitted information would likely cause competitive harm to the registrant if publicly disclosed. |
ITEM 16. FORM 10-K SUMMARY Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
None
90
SIGNATURES
|
|
|
AYTU BIOPHARMA, INC. |
|
|
|
|
Date: October 12, 2023 |
|
By: |
/s/ Joshua R. Disbrow |
|
|
|
Joshua R. Disbrow |
|
|
|
Chairman and Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
POWER OF ATTORNEY
We the undersigned directors and officers of Aytu BioPharma, Inc. (the “Company”), hereby severally constitute and appoint Joshua R. Disbrow and Mark Oki, and each of them singly, our true and lawful attorneys, with full power to them, and to each of them singly, to sign for us and in our names in the capacities indicated below, to file any and all amendments to this Annual Report on Form 10‑K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys‑in‑fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys‑in‑fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities indicated, on October 12, 2023.
Signature |
|
Title |
|
|
|
/s/ Joshua R. Disbrow |
|
Chairman and Chief Executive Officer |
Joshua R. Disbrow |
|
(Principal Executive Officer) |
|
|
|
/s/ Mark K. Oki |
|
Chief Financial Officer |
Mark K. Oki |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
/s/ John A. Donofrio, Jr. |
|
Lead Independent Director |
John A. Donofrio, Jr. |
|
|
|
|
|
/s/ Carl C. Dockery |
|
Director |
Carl C. Dockery |
|
|
|
|
|
/s/ Abhinav Jain |
|
Director |
Abhinav Jain |
|
|
|
|
|
/s/ Vivian H. Liu |
|
Director |
Vivian H. Liu |
|
|
|
|
|
|
|
|
|
|
|
91
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
AYTU BIOPHARMA, INC.
|
Page |
Reports of Independent Registered Public Accounting Firms (PCAOB ID 248 & 166) |
F-1 |
F-4 |
|
F-5 |
|
F-6 |
|
F-7 |
|
F-9 |
92
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Aytu BioPharma, Inc.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheet of Aytu BioPharma, Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of June 30, 2023, the related consolidated statements of operations, stockholders’ equity, and cash flows for the year then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2023, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Going concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s net loss was $17.1 million and cash used in operating activities was $5.1 million for the year ended June 30, 2023, and as of that date, the Company’s accumulated deficit was $304.1 million. These conditions, along with other matters as set forth in Note 1, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical audit matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing an opinion on the critical audit matter or on the accounts or disclosures to which it relates.
F-1
Variable consideration in contracts with customers
As described in Note 2 to the financial statements, the Company makes estimates of the net sales price, including estimates of variable consideration to be incurred on the respective product sales. A key source of information used by management to develop the estimate for the ADHD portfolio savings offerings and commercial rebates (collectively the “GtN adjustments”) is inventory levels in the distribution channel as of the balance sheet date. We identified this key source of information as a critical audit matter.
The principal considerations for our determination that those inventory levels in the distribution channel as of the balance sheet date are a critical audit matter are (a) the inherent limitations over management’s visibility and insight into the underlying details of the source data, which requires management to depend and rely on external data from multiple sources and (b) the extent to which the external data is used by management to develop the estimate of GtN adjustments.
Our audit procedures related to this critical audit matter included the following, among others.
| (i) | We evaluated the relevance and reliability of the external data used by management to develop the estimate of inventory levels in the distribution channel as of the balance sheet date. |
| (ii) | We tested management’s process of reconciling the external data from multiple sources used to develop the estimate of inventory levels in the distribution channel as of the balance sheet date. |
(iii) |
We evaluated the appropriateness and consistency in the application of the inventory levels in the distribution channel as of the balance sheet date as it relates to management’s methods and assumptions used in developing the estimate of GtN adjustments. |
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2022.
Denver, Colorado
October 12, 2023
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Aytu BioPharma, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Aytu BioPharma, Inc. (the “Company”) as of June 30, 2022; the related consolidated statements of operations, stockholders' equity, and cash flows for the year then ended; and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2022, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
The Company's management is responsible for these financial statements. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Plante & Moran, PLLC
Denver, Colorado
September 27, 2022, except for Note 2, as to which the date is October 12, 2023
We served as the Company’s auditor from 2015 to 2022.
F-3
AYTU BIOPHARMA, INC.
Consolidated Balance Sheets
(In thousands, except shares and per-share amounts)
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
Assets |
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
22,985 |
|
$ |
19,360 |
Accounts receivable, net |
|
|
28,937 |
|
|
21,712 |
Inventories |
|
|
11,995 |
|
|
10,849 |
Prepaid expenses |
|
|
8,047 |
|
|
7,375 |
Other current assets |
|
|
868 |
|
|
633 |
Total current assets |
|
|
72,832 |
|
|
59,929 |
Property and equipment, net |
|
|
1,815 |
|
|
3,025 |
Operating lease right-of-use asset |
|
|
2,054 |
|
|
3,271 |
Intangible assets, net |
|
|
58,970 |
|
|
70,632 |
Other non-current assets |
|
|
792 |
|
|
766 |
Total non-current assets |
|
|
63,631 |
|
|
77,694 |
Total assets |
|
$ |
136,463 |
|
$ |
137,623 |
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
Accounts payable and other |
|
$ |
13,478 |
|
$ |
10,987 |
Accrued liabilities |
|
|
46,799 |
|
|
44,187 |
Short-term line of credit |
|
|
1,563 |
|
|
3,813 |
Current portion of debt |
|
|
85 |
|
|
96 |
Other current liabilities |
|
|
7,090 |
|
|
5,359 |
Total current liabilities |
|
|
69,015 |
|
|
64,442 |
|
|
|
|
|
|
|
Debt, net of current portion |
|
|
14,713 |
|
|
14,279 |
Derivative warrant liabilities |
|
|
6,403 |
|
|
1,796 |
Other non-current liabilities |
|
|
6,975 |
|
|
12,798 |
Total liabilities |
|
|
97,106 |
|
|
93,315 |
|
|
|
|
|
|
|
Commitments and contingencies (Note 18) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity |
|
|
|
|
|
|
Preferred Stock, par value $.0001; 50,000,000 shares authorized; no shares issued or outstanding as of June 30, 2023 and June 30, 2022 |
|
|
— |
|
|
— |
Common Stock, par value $.0001; 200,000,000 shares authorized; shares issued and outstanding 5,517,174 and 1,928,941, respectively, as of June 30, 2023 and June 30, 2022 |
|
|
1 |
|
|
— |
Additional paid-in capital |
|
|
343,485 |
|
|
331,386 |
Accumulated deficit |
|
|
(304,129) |
|
|
(287,078) |
Total stockholders’ equity |
|
|
39,357 |
|
|
44,308 |
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity |
|
$ |
136,463 |
|
$ |
137,623 |
See the accompanying Notes to the Consolidated Financial Statements.
F-4
AYTU BIOPHARMA, INC.
Consolidated Statements of Operations
(In thousands, except share and per-share amounts)
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
Product revenue, net |
|
$ |
107,399 |
|
$ |
96,669 |
Cost of sales |
|
|
40,767 |
|
|
44,386 |
Gross profit |
|
|
66,632 |
|
|
52,283 |
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
Selling and marketing |
|
|
41,448 |
|
|
38,713 |
General and administrative |
|
|
28,630 |
|
|
31,167 |
Research and development |
|
|
4,095 |
|
|
12,662 |
Impairment of goodwill |
|
|
— |
|
|
65,802 |
Impairment of other assets |
|
|
5,705 |
|
|
9,656 |
Amortization of intangible assets |
|
|
4,788 |
|
|
5,844 |
Gain from contingent consideration |
|
|
(969) |
|
|
(1,655) |
Total operating expenses |
|
|
83,697 |
|
|
162,189 |
|
|
|
|
|
|
|
Loss from operations |
|
|
(17,065) |
|
|
(109,906) |
|
|
|
|
|
|
|
Other income (expense) |
|
|
|
|
|
|
Other expense, net |
|
|
(4,779) |
|
|
(757) |
Gain on extinguishment of debt |
|
|
— |
|
|
169 |
Gain on derivative warrant liability |
|
|
4,793 |
|
|
1,605 |
Total other income, net |
|
|
14 |
|
|
1,017 |
Loss before income tax |
|
|
(17,051) |
|
|
(108,889) |
Income tax benefit |
|
|
— |
|
|
(110) |
Net loss |
|
$ |
(17,051) |
|
$ |
(108,779) |
|
|
|
|
|
|
|
Weighted average number of common shares outstanding |
|
|
3,339,906 |
|
|
1,469,875 |
|
|
|
|
|
|
|
Basic and diluted net loss per common share |
|
$ |
(5.11) |
|
$ |
(74.01) |
See the accompanying Notes to the Consolidated Financial Statements.
F-5
AYTU BIOPHARMA, INC.
Consolidated Statements of Stockholders’ Equity
(In thousands, except shares)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
Total |
||
|
|
Preferred Stock |
|
Common Stock |
|
Paid-in |
|
Accumulated |
|
Stockholders’ |
|||||||||
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
Equity |
|||||
Balance, June 30, 2022 |
|
— |
|
$ |
— |
|
1,928,941 |
|
$ |
— |
|
$ |
331,386 |
|
$ |
(287,078) |
|
$ |
44,308 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
— |
|
|
— |
|
(18,180) |
|
|
— |
|
|
6,046 |
|
|
— |
|
|
6,046 |
Issuance of common stock, net of $1,004 issuance cost |
|
— |
|
|
— |
|
3,606,413 |
|
|
1 |
|
|
6,053 |
|
|
— |
|
|
6,054 |
Net loss |
|
— |
|
|
— |
|
- |
|
|
— |
|
|
— |
|
|
(17,051) |
|
|
(17,051) |
Balance, June 30, 2023 |
|
— |
|
$ |
— |
|
5,517,174 |
|
$ |
1 |
|
$ |
343,485 |
|
$ |
(304,129) |
|
$ |
39,357 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
Total |
||
|
|
Preferred Stock |
|
Common Stock |
|
Paid-in |
|
Accumulated |
|
Stockholders’ |
|||||||||
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
Equity |
|||||
Balance, June 30, 2021 |
|
— |
|
$ |
— |
|
1,374,521 |
|
$ |
— |
|
$ |
315,867 |
|
$ |
(178,299) |
|
$ |
137,568 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
— |
|
|
— |
|
20,434 |
|
|
— |
|
|
5,248 |
|
|
— |
|
|
5,248 |
Issuance of common stock, net of $1,048 issuance cost |
|
— |
|
|
— |
|
424,539 |
|
|
— |
|
|
8,854 |
|
|
— |
|
|
8,854 |
Issuance of common stock related to milestone payment |
|
— |
|
|
— |
|
109,447 |
|
|
— |
|
|
1,425 |
|
|
— |
|
|
1,425 |
Tax withholding for stock-based compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
(8) |
|
|
— |
|
|
(8) |
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(108,779) |
|
|
(108,779) |
Balance, June 30, 2022 |
|
— |
|
$ |
— |
|
1,928,941 |
|
$ |
— |
|
$ |
331,386 |
|
$ |
(287,078) |
|
$ |
44,308 |
See the accompanying Notes to the Consolidated Financial Statements
F-6
AYTU BIOPHARMA, INC.
Consolidated Statements of Cash Flows
(In thousands)
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
Operating Activities |
|
|
|
|
|
|
Net loss |
|
$ |
(17,051) |
|
$ |
(108,779) |
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
|
Depreciation, amortization and accretion |
|
|
8,815 |
|
|
10,146 |
Impairment expense |
|
|
5,705 |
|
|
75,458 |
Inventory write-down |
|
|
2,351 |
|
|
2,186 |
Stock-based compensation expense |
|
|
6,046 |
|
|
5,248 |
Gain on derivative warrant liability |
|
|
(4,793) |
|
|
(1,605) |
Gain from contingent considerations |
|
|
(969) |
|
|
(1,655) |
Amortization of senior debt (premium) discount |
|
|
559 |
|
|
(126) |
Shares issuance related to milestone payment |
|
|
— |
|
|
1,425 |
Gain on debt extinguishment |
|
|
— |
|
|
(193) |
Other noncash adjustments |
|
|
7 |
|
|
(65) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Accounts receivable |
|
|
(7,153) |
|
|
6,533 |
Inventory |
|
|
(3,609) |
|
|
1,299 |
Prepaid expenses and other current assets |
|
|
(914) |
|
|
2,228 |
Accounts payable and other |
|
|
2,384 |
|
|
(7,681) |
Accrued liabilities |
|
|
3,605 |
|
|
(13,292) |
Other operating assets and liabilities, net |
|
|
(111) |
|
|
50 |
Net cash used in operating activities |
|
|
(5,129) |
|
|
(28,823) |
Investing Activities |
|
|
|
|
|
|
Contingent consideration payment |
|
|
(5) |
|
|
(3,178) |
Other investing activities |
|
|
(112) |
|
|
(70) |
Net cash used in investing activities |
|
|
(117) |
|
|
(3,248) |
Financing Activities |
|
|
|
|
|
|
Net proceeds from issuance of stock |
|
|
15,575 |
|
|
11,694 |
Payment made to fixed payment arrangement |
|
|
(4,266) |
|
|
(4,409) |
Net payments made on short-term line of credit |
|
|
(2,250) |
|
|
(4,121) |
Payments made to borrowings |
|
|
(96) |
|
|
(16,101) |
Proceeds from borrowings |
|
|
— |
|
|
15,000 |
Payment for debt issuance costs |
|
|
(92) |
|
|
(526) |
Other financing activities |
|
|
— |
|
|
(7) |
Net cash provided by financing activities |
|
|
8,871 |
|
|
1,530 |
Net change in cash and cash equivalents |
|
|
3,625 |
|
|
(30,541) |
Cash and cash equivalents at beginning of period |
|
|
19,360 |
|
|
49,901 |
Cash and cash equivalents at end of period |
|
$ |
22,985 |
|
$ |
19,360 |
See accompanying Notes to Consolidated Financial Statements
F-7
AYTU BIOPHARMA, INC.
Consolidated Statements of Cash Flows, Cont’d
(In thousands)
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
Supplemental cash flow data |
|
|
|
|
|
|
Cash paid for interest |
|
$ |
3,812 |
|
$ |
3,148 |
Non-cash investing and financing activities: |
|
|
|
|
|
|
Other noncash investing and financing activities |
|
$ |
147 |
|
$ |
54 |
See accompanying Notes to Consolidated Financial Statements
F-8
AYTU BIOPHARMA, INC.
Notes to the Consolidated Financial Statements
1. Nature of Business and Financial Condition
Aytu BioPharma, Inc. (“Aytu,” or the “Company”), is a pharmaceutical company focused on commercializing novel therapeutics and consumer health products. The Company operates through two business segments (i) the Rx Segment, consisting of prescription pharmaceutical products and (ii) the Consumer Health Segment, which consists of various consumer healthcare products (the “Consumer Health Portfolio”). The Company was originally incorporated as Rosewind Corporation on August 9, 2002 in the State of Colorado and was re-incorporated as Aytu BioScience, Inc in the state of Delaware on June 8, 2015. Following the acquisition of Neos Therapeutics, Inc. (“Neos”) in March 2021, (the “Neos Acquisition”) the Company changed its name to Aytu BioPharma, Inc.
On January 6, 2023, the Company effected a reverse stock split in which each common stockholder received one share of common stock for every twenty shares held (“Reverse Stock Split”). Where applicable, all share and per share amounts in this annual report have been adjusted to reflect the effect of the Reverse Stock Split.
The Rx Segment primarily consists of two product portfolios: Adzenys XR-ODT (amphetamine) extended-release orally disintegrating tablets and Cotempla XR-ODT (methylphenidate) extended-release orally disintegrating tablets for the treatment of attention deficit hyperactivity disorder (“ADHD”) together the “ADHD Portfolio”, and the “Pediatric Portfolio” consisting of Poly-Vi-Flor and Tri-Vi-Flor, two complementary prescription fluoride-based supplement product lines containing combinations of fluoride and vitamins in various formulations for infants and children with fluoride deficiency, and Karbinal ER, an extended-release antihistamine suspension containing carbinoxamine indicated to treat numerous allergic conditions.
The Consumer Health Portfolio consists of multiple consumer health products competing in large healthcare categories, including allergy, hair regrowth, diabetes support, digestive health, urological health and general wellness, commercialized through direct mail and e-commerce marketing channels.
The Company’s strategy is to continue building its portfolio of revenue-generating products, leveraging its commercial team’s expertise to build leading brands within large therapeutic and consumer markets. As a result of focusing on building the portfolio of revenue-generating products, the Company has indefinitely suspended active development of its clinical development program AR101 (enzastaurin), and has terminated the license agreements relating to Healight and NT0502 (N-desethyloxybutynin).
As of June 30, 2023, the Company had $23.0 million of cash and cash equivalents and $28.9 million in accounts receivable. The Company incurred a net loss of $17.1 million and $108.8 million during the years ended June 30, 2023 and 2022, respectively. The Company had an accumulated deficit of $304.1 million and $287.1 million as of June 30, 2023 and 2022, respectively. Cash used in operations was $5.1 million and $28.8 million during the years ended June 30, 2023 and 2022, respectively.
In addition, the Company has non-operating liabilities that are scheduled to, or may become current in the eighteen months following the filing of this 10-K, most notably the maturity of the $15 million Avenue Capital term note (the “Avenue Note”). The Company expects to refinance the Avenue Note in the event it does not have sufficient cash on hand to retire it. As a result, there exists substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include adjustments that might be necessary if the Company is unable to continue as a going concern.
Management plans to mitigate the conditions that raise substantial doubt about its ability to continue as a going concern are primarily focused on i) eliminating expenses for clinical development, ii) winding down or monetizing the Consumer Health Segment, which has generated negative cash flows since its acquisition in 2021, iii) refinancing its $15 million Avenue Note (see Note 11 – Long-term Debt) to extend its maturity date, and, if necessary iv) raising additional capital through public or private equity, debt offerings, or monetizing additional assets in order to meet its obligations.
F-9
Management believes that the Company has access to capital resources, however, the Company cannot provide any assurance that it will be able to raise additional capital, monetize assets, or obtain new financing on commercially acceptable terms. If the Company is unable to support its operations and obligations, it may be required to curtail its operations, or delay the execution of its business plan. Alternatively, any efforts by the Company to reduce its expenses may adversely impact its ability to sustain revenue-generating activities or otherwise operate its business. As a result, there can be no assurance that the Company will be successful in implementing its plans to alleviate this substantial doubt about its ability to continue as a going concern.
2. Summary of Significant Accounting Policies
Principals of Consolidation. The Company’s consolidated financial statements include the accounts of: Aytu Therapeutics, LLC, Innovus Pharmaceuticals, Inc. and Neos Therapeutics, Inc. and their respective wholly owned subsidiaries. All significant inter-company balances and transactions have been eliminated in consolidation.
Basis of Presentation. The Company’s consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”).
Use of estimates. The preparation of financial statements and footnotes requires the use of management estimates, judgments and assumptions. Actual results may differ from estimates. In the accompanying consolidated financial statements, estimates are used for, but not limited to, stock-based compensation; revenue recognition, determination of variable consideration for accruals of chargebacks, administrative fees and rebates, government rebates, returns and other allowances; allowance for doubtful accounts; inventory impairment; determination of right-of-use assets and lease liabilities; valuation of financial instruments, derivative warrant liabilities, intangible assets, long-lived assets, and goodwill; purchase price allocations, and the depreciable lives of long-lived assets; accruals for contingent liabilities; and determination of the income tax provision, deferred taxes and valuation allowance.
Prior Period Reclassification. Certain prior year amounts in the consolidated statements of operations and statements of cash flows have been reclassified to conform to the current year presentation, including a reclassification made in the presentation of amortization of intellectual property, and a reclassification of fair value adjustment from contingent consideration. Amortization of intellectual property was previously included in research and development expenses and is currently recorded in amortization of intangible assets expenses on the consolidated statements of operations. Gain or loss from the fair value of contingent consideration was previously included in Other expense, net, and is currently recorded in operating expenses on the consolidated statements of operations. These reclassifications did not impact operating results or cash flows for the fiscal years ended June 30, 2023 and 2022 or its financial position as of June 30, 2023 or June 30, 2022.
Previously Reported Financial Statements. The classification of certain of the Company’s warrants was previously recorded as equity. These warrants according to U.S. GAAP should have been classified as derivative warrant liabilities at fair value and marked to market at each reporting period, with changes in fair value recorded in earnings. The affected filing periods include the audited financial statements as of June 30, 2022.
SEC Staff Accounting Bulletin No. 99, “Materiality,” and the Financial Accounting Standards Board (“FASB”), Statement of Financial Accounting Concepts No. 2 “Qualitative Characteristics of Accounting Information” indicate that quantifying and aggregating adjustments is only the beginning of an analysis of materiality and that both quantitative and qualitative factors must be considered in determining whether individual adjustments are material. The Company evaluated the adjustments and determined that the impact was not material to the consolidated financial statements as of and for the fiscal year ended June 30, 2022. As a result, adjustments for the immaterial adjustments were applied to this period for comparative purposes. The adjustments did not change the Company’s reported total assets, cash and cash equivalents, operating expenses, operating losses or cash flows from operations.
F-10
The consolidated financial statements as of and for the fiscal year ended June 30, 2022 have been adjusted as shown in the following tables.
|
|
As of June 30, 2022 |
|||||||
|
|
As Previously |
|
|
|
|
|
|
|
|
|
Reported |
|
Adjustment |
|
As Adjusted |
|||
|
|
(in thousands) |
|||||||
Balance Sheet data |
|
|
|
|
|
|
|
|
|
Derivative warrant liabilities |
|
$ |
- |
|
$ |
1,796 |
|
$ |
1,796 |
Total liabilities |
|
$ |
91,531 |
|
$ |
1,784 |
|
$ |
93,315 |
Additional paid-in capital |
|
$ |
334,560 |
|
$ |
(3,174) |
|
$ |
331,386 |
Accumulated deficit |
|
$ |
(288,472) |
|
$ |
1,394 |
|
$ |
(287,078) |
Total stockholders’ equity |
|
$ |
46,092 |
|
$ |
(1,784) |
|
$ |
44,308 |
|
|
Twelve Months Ended |
|||||||
|
|
June 30, 2022 |
|||||||
|
|
As Previously |
|
|
|
|
|
|
|
|
|
Reported |
|
Adjustment |
|
As Adjusted |
|||
|
|
(in thousands) |
|||||||
Statement of Operation data |
|
|
|
|
|
|
|
|
|
Gain on derivative warrant liability |
|
$ |
211 |
|
$ |
1,394 |
|
$ |
1,605 |
Total other income, net (1) |
|
$ |
1,278 |
|
$ |
(261) |
|
$ |
1,017 |
Loss before income tax |
|
$ |
(110,283) |
|
$ |
1,394 |
|
$ |
(108,889) |
Net loss |
|
$ |
(110,173) |
|
$ |
1,394 |
|
$ |
(108,779) |
Basic and diluted net loss per common share |
|
$ |
(75.00) |
|
$ |
0.99 |
|
$ |
(74.01) |
|
|
|
|
|
|
|
|
|
|
Statement of Stockholders' Equity data |
|
|
|
|
|
|
|
|
|
Issuance of common stock, net of issuance cost |
|
$ |
11,652 |
|
$ |
(2,798) |
|
$ |
8,854 |
|
|
|
|
|
|
|
|
|
|
Statement of Cash Flow data |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(110,173) |
|
$ |
1,394 |
|
$ |
(108,779) |
Gain on derivative warrant liability |
|
$ |
(211) |
|
$ |
(1,394) |
|
$ |
(1,605) |
|
|
|
|
|
|
|
|
|
|
| 1) | Includes reclassification of gain or loss from the fair value of contingent consideration. See Prior Period Reclassification in Note 2 – Summary of Significant Accounting Policies. |
Previously Reported Segment Information. During the year ended June 30, 2023, the Company identified an omission regarding the disclosure of certain key metrics of its reportable segments under ASC 280 related to the year ended June 30, 2022. During the year ended June 30, 2022, the Company inappropriately reported the net income of each of its two segments as opposed to operating income which more closely aligns with the adjusted EBITDA metric that is utilized by its chief operating decision maker. The impact on June 30, 2022 was that $92.4 million and $17.5 million of operating loss relating to the Rx Segment and Consumer Health segment, respectively, should have been reported as a separate line. The Company assessed the materiality of this omission on the previously issued interim and annual consolidated financial statements in accordance with SEC Staff Accounting Bulletin No. 99. The Company concluded that the omission was not material to any of the previously issued consolidated financial statements and began reporting operating results by segment in accordance with ASC 280 on a prospective basis starting with the year ended June 30, 2023
Cash and Cash Equivalents. The Company’s primary objectives for investment of available cash are the preservation of capital and the maintenance of liquidity. The Company invests its available cash balances in bank deposits and money market funds. The cash balances in bank deposits are subject to FDIC (the “Federal Deposit Insurance Corporation”) insurance limits, and cash balances in the money market funds are not FDIC insured. The Company considers all highly liquid instruments purchased with an original maturity of three months or less to be cash equivalents.
F-11
Accounts Receivable, net. Accounts receivable represent amounts due from customers less allowances for doubtful accounts, discounts and pricing chargebacks. An allowance for doubtful accounts, when needed, is based upon the financial condition and payment history of customers; collections experience on other accounts; and economic factors or events expected to affect future collections. The allowance for doubtful accounts was zero for both years ended June 30, 2023 and 2022. The allowance for discounts was $1.8 million and $1.3 million for the years ended June 30, 2023 and 2022, respectively. The allowance for chargebacks was $1.2 million for both years ended June 30, 2023 and 2022.
The table below presents the opening and closing balances of receivables from customers.
|
|
|
Accounts Receivable, gross |
|
|
|
(in thousands) |
Opening balance, June 30, 2022 |
|
$ |
24,219 |
Closing balance, June 30, 2023 |
|
|
31,927 |
Increase |
|
$ |
7,708 |
|
|
|
|
Opening balance, June 30, 2021 |
|
$ |
30,325 |
Closing balance, June 30, 2022 |
|
|
24,219 |
Decrease |
|
$ |
(6,106) |
|
|
|
|
The table below details the change in allowance for discount, and allowance for chargeback for the periods presented.
|
|
|
Allowance for Discount |
|
|
Allowance for Chargeback |
|
|
Total Allowance |
|
|
|
(in thousands) |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2021 |
|
$ |
1,133 |
|
$ |
1,016 |
|
$ |
2,149 |
Charges to expense |
|
|
6,760 |
|
|
4,598 |
|
|
11,358 |
Payments |
|
|
(6,592) |
|
|
(4,408) |
|
|
(11,000) |
Balance, June 30, 2022 |
|
$ |
1,301 |
|
$ |
1,206 |
|
$ |
2,507 |
Charges to expense |
|
|
9,074 |
|
|
4,554 |
|
|
13,628 |
Payments |
|
|
(8,597) |
|
|
(4,548) |
|
|
(13,145) |
Balance, June 30, 2023 |
|
$ |
1,778 |
|
$ |
1,212 |
|
$ |
2,990 |
Inventories. Inventories consist of raw materials, work in process and finished goods and are recorded at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. Prior to regulatory approval, before economic benefit is probable, pre-launch inventories are expensed as research and development.
The Company periodically reviews the composition of its inventories in order to identify obsolete, slow-moving or otherwise unsaleable items. If evidence exists that the net realizable value of inventory is lower than its cost, the difference is recognized as a loss in the period the impairment is identified.
Going Concern Determination. In connection with the preparation for each annual and interim financial reporting period, management evaluates whether there are events that, in the aggregate, raise substantial doubt about the Company’s ability to continue as a going concern within one year after the financial statements are issued. The evaluation is based on relevant conditions and events that are known and reasonably knowable within one year after the date that the financial statements are issued. Recurring operating losses or year over year negative cash flows from operating activities are considered negative trends.
F-12
Property and equipment, net. Property and equipment are recorded at cost less accumulated depreciation. Furniture and equipment are depreciated on a straight-line basis over their estimated useful lives which are generally two to seven years. Leasehold improvements are amortized over the shorter of the estimated useful life or remaining lease term. The Company begins depreciating assets when they are placed into service. Maintenance and repairs are expensed as incurred.
Leases. At the inception of an arrangement, the Company determines if an arrangement is, or contains, a lease. Lease classification, recognition and measurement are determined at the lease commencement date. Lease liabilities and right-of-use (“ROU”) assets are recorded based on the present value of lease payments over the expected lease term, including options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. In determining the present value of the lease payments, the Company uses the implicit interest rate when readily determinable and uses the Company’s incremental borrowing rate when the implicit rate is not readily determinable based upon the information available at the lease commencement date.
Fixed lease payments, or in substance fixed, are recognized over the expected term of the lease using the effective interest method. Variable lease payments are expensed as incurred. Fixed and variable lease expenses on operating leases are recognized within cost of sales and operating expenses in the Company’s consolidated statements of operations. ROU asset amortization and interest costs on financing leases are recorded within cost of sales and interest expense, respectively, in the Company’s consolidated statements of operations. The Company has elected to account for payments on short-term leases as lease expense on a straight-line basis over lease terms of 12 months or less.
Operating leases are included in other liabilities in the Company’s consolidated balance sheets. Financing leases are included in property and equipment, net, current portion of long-term debt and long-term debt, net of current portion in the Company’s consolidated balance sheets.
Income from subleasing is recognized on a straight-line basis over the sublease term, subject to collectability issues which will limit the income recognized to payment received until collectability is no longer an issue. Any variable payments are recognized as incurred.
Fair Value of Financial Instruments.
Acquisitions. In an acquisition of a business or a group of assets, the Company uses the acquisition method of accounting which identifies, recognizes, and measures the identifiable assets acquired, liabilities assumed and any non-controlling interest at their acquisition date fair values. Any excess of the purchase consideration over the fair values of the net identifiable assets acquired is recorded as goodwill. If the Company determines the assets acquired do not meet the definition of a business, the transaction is accounted for as an acquisition of assets, which records the assets acquired at the purchase price and does not result in goodwill. Contingent consideration is accounted for Acquired in-process research and development with no alternative future use is charged to expense.
Warrants. The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB Accounting Standards Codification (“ASC”) 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC480, and whether the warrants meet all of the requirements for equity classification under ASC 815,including whether the warrants are indexed to the Company’s own common shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding. Liability classified warrants are valued using the Monte Carlo simulation model or the Black-Scholes option pricing model at issuance, and for each reporting period. Equity classified warrants are valued using the Black-Scholes model.
Revenue Recognition. The Company generates revenue from product sales through its prescription pharmaceutical products segment (“Rx Segment”) and its consumer healthcare products segment (“Consumer Health Segment”).
F-13
The Company evaluates its contracts with customers to determine revenue recognition using the following five-step model: (1) identify the contract with the customer; (2) identify the performance obligations; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations; and (5) recognize revenue when (or as) a performance obligation is satisfied. There is not a recognized financing component related to product sales.
Rx Segment
Net product sales for the Rx Segment (which includes the ADHD Portfolio and the Pediatric Portfolio) consist of sales of prescription pharmaceutical products, principally to a limited number of wholesale distributors and pharmacies in the United States. Rx product revenue is recognized at the point in time that control of the product transfers to the customer in accordance with shipping terms (i.e., upon delivery), which is generally “free-on-board” destination when shipped domestically within the United States and “free-on-board” shipping point when shipped internationally consistent with the contractual terms.
Rx product revenue is recognized net of consideration paid to the Company’s customers and other adjustments to the transaction price (known as “Gross to Net” adjustments). Estimating adjustments to the transaction price and applying the constraint on variable consideration requires the use of significant management judgment and other market data. Gross to Net adjustments include provisions for product returns, wholesaler distribution fees and chargebacks for discounted pricing to participating entities, managed care rebate programs, savings programs for patients covered under commercial payor plans and other deductions.
The Company makes estimates of the net sales price, including estimates of variable consideration to be incurred on the respective product sales (known as “Gross to Net” adjustments). Estimating gross to net adjustments and applying the constraint on variable consideration requires the use of significant management judgment and other market data.
The Gross to Net adjustments include:
| ● | Savings offers The Company offers savings programs for its patients covered under commercial payor plans in which the cost of a prescription to such patients is discounted. |
| ● | Prompt payment discounts Prompt payment discounts are based on standard provisions of wholesalers’ services. |
| ● | Wholesale distribution fees Wholesale distribution fees are based on definitive contractual agreements for the management of the Company’s products by wholesalers. |
| ● | Rebates The Rx Portfolio products are subject to commercial managed care and government (i.e. Medicaid) programs whereby discounts and rebates are provided to participating managed care organizations and federal and/or state governments. Calculations related to rebate accruals are estimated based on historical information from third-party providers. |
| ● | Wholesaler chargebacks The Rx Portfolio products are subject to certain programs with wholesalers whereby pricing on products is discounted below wholesaler list price to participating entities. These entities purchase products through wholesalers at the discounted price, and the wholesalers charge the difference between their acquisition cost and the discounted price back to the Company following the product purchases of the wholesalers’ end customers. |
| ● | Returns Wholesalers’ contractual return rights are limited to defective product, product that was shipped in error, product ordered by customer in error, product returned due to overstock, product returned due to dating or product returned due to recall or other changes in regulatory guidelines. The return policy for expired product allows the wholesaler to return such product starting six months prior |
F-14
| to expiry date to twelve months post expiry date. The Company analyzes return data available from sales since inception date to determine a reliable return rate. |
Savings offers, rebates and wholesaler chargebacks reflect the terms of underlying agreements, which may vary. Accordingly, actual amounts will depend on the mix of sales by product and contracting entity. Future returns may not follow historical trends. The Company’s periodic adjustments of its estimates are subject to time delays between the initial product sale and ultimate reporting and settlement of deductions. The Company continually monitors these provisions and do not believe variances between actual and estimated amounts have been material.
Consumer Health Segment
The Consumer Health Segment revenue (consisting of the Consumer Health Portfolio) is from sales of various consumer health products through e-commerce platforms and direct-to-consumer marketing channels. Revenue is generally recognized “free-on-board” shipping point, as those are the agreed-upon contractual terms. Taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction that are collected by the Company from a customer are excluded from revenue. Shipping and handling costs associated with outbound freight after control over a product has transferred to a customer are accounted for as a fulfillment cost and are included in cost of sales.
Customer Contract Costs. The Company expenses the incremental costs to obtain a contract as incurred, since they are satisfied within one year.
Concentration of Credit Risk. Financial instruments that potentially subject the Company to credit risk concentrations consist of cash, cash equivalents and accounts receivable.
The Company maintains deposits in financial institutions in excess of federally insured limits. The Company periodically monitors the credit quality of the financial institutions with which it invests and believes that the Company is not exposed to significant credit risk due to the financial position of those institutions.
The Company is also subject to credit risk from accounts receivable related to product sales. The Company’s customers, sometimes referred to as partners or customers, are primarily large wholesale distributors that resell the Company’s products to retailers. The loss of one or more of these large customers could have a material adverse effect on the Company’s business, operating results or financial condition. The Company does not charge interest or require collateral related to its accounts receivable. Credit terms are generally forty to sixty days.
The following table presents customers that contributed more than 10% of gross revenue and accounts receivable:
|
|
Percentage of gross revenue |
|
Percentage of accounts receivable |
||||||||
|
|
June 30, |
||||||||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
||||
Customer A |
|
43 |
% |
|
41 |
% |
|
50 |
% |
|
52 |
% |
Customer B |
|
18 |
% |
|
20 |
% |
|
19 |
% |
|
25 |
% |
Customer C |
|
17 |
% |
|
18 |
% |
|
14 |
% |
|
18 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Costs of Sales. Costs of sales consists primarily of manufactured product cost, products acquired from third-party manufacturers, freight, production, and indirect manufacturing overhead costs and FDA fees for commercialized products. Certain of the Company’s sales activities depend on licensing arrangements that may require periodic milestone payments or royalty payments, which are also included in costs of sales. In addition, distribution, shipping and handling costs invoiced by the Company's third-party logistics companies are included in costs of sales.
Stock-Based Compensation. The Company accounts for share-based payments compensation expense using a fair value based model.
F-15
Restricted stock and restricted stock unit grants are valued based on the estimated grant date fair value of the Company’s common stock and recognized ratably over the requisite service period.
Stock option grants are valued using the Black-Scholes option pricing model and compensation costs are recognized ratably over the period of service using the graded method. The Black-Scholes option pricing model requires the Company to estimate the expected term of the award, the expected volatility, the risk-free interest rate, and the expected dividends. The expected term is determined using the “simplified method,” which is the midpoint between the vesting date and the end of the contractual term. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant for the expected term of the award. The Company doesn’t anticipate paying any dividends in the near future. Forfeitures are recognized as they occur.
Research and Development. Research and development costs are expensed as incurred and include salaries and benefits, facilities costs, overhead costs, raw materials, laboratory and clinical supplies, clinical trial costs, contract services, milestone payments and fees paid to regulatory authorities for review and approval of the Company’s product candidates and other related costs.
Intangible Assets. The Company records acquired intangible assets based on fair value on the date of acquisition. Finite-lived intangible assets are recorded at cost and amortized on a straight-line basis over the estimated lives of the assets. Indefinite-lived intangible assets are not subject to amortization.
Impairment of Long-lived Assets and Goodwill. The Company assesses impairment of asset groups, including intangible assets, when events or changes in circumstances indicate that their carrying amount may not be recoverable. Long-lived assets consist of property and equipment, net, right of use assets and other intangible assets, net. Circumstances which could trigger a review include, but are not limited to: (i) changes in Company plans; (ii) competition; (iii) significant adverse changes in the business climate or legal or regulatory factors; (iv) or, expectations that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. If the estimated future undiscounted cash flows, excluding interest charges, from the use of an asset are less than its carrying value, a write-down would be recorded to reduce the related asset to its estimated fair value.
Goodwill is reviewed for impairment at least annually or whenever events or changes in circumstances, including a decline in the Company’s stock price, indicate that its carrying amount is less than its fair value. If qualitative factors, such as general economic conditions, the Company’s outlook and market performance of the Company’s industry forecasted financial performance indicate that it is more likely than not that a reporting unit’s fair value is less than its carrying amount, the Company performs a quantitative analysis of fair value. The Company determines the fair value of a reporting unit utilizing a discounted cash flow model. Significant assumptions inherent in the valuation methodologies include, but are not limited to, prospective financial information, growth rates, terminal value, discount rates and comparable multiples from publicly traded companies in the Company’s industry.
Contingent consideration. The consideration for our acquired businesses and licenses often includes future payments that are contingent upon the occurrence of a particular event or events. The Company records an obligation for such contingent payments at fair value on the acquisition date. Changes in the fair value of contingent consideration obligations are recognized in the consolidated statements of income.
Advertising Costs. Advertising costs consist of the direct marketing activities related to the Consumer Health Segment. The Company expenses all advertising costs as incurred. The Company incurred $11.1 million and $13.6 million of advertising costs for the years ended June 30, 2023 and 2022, respectively.
Income Taxes. The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes and net operating loss and tax credit carryforwards. The amount of deferred taxes on these temporary differences is determined using the tax rates that are expected to apply to the period when the asset is realized or the liability is settled, as applicable, based on tax rates and laws in the respective tax jurisdiction enacted as of the balance sheet date.
F-16
A valuation allowance is recorded to reduce the net deferred tax asset when it is more likely than not that some portion or all of its deferred tax asset will not be utilized.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of to be sustained upon an examination.
The Company recognizes interest and penalties related to uncertain tax positions in Income tax (provision) benefit in the consolidated statements of operations.
Debt issuance costs, discounts (premiums). Debt issuance costs reflect fees paid to lenders and third parties directly related to issuing debt. Debt issuance costs and discounts (premiums) related to term loans are reported as direct deductions (increases) to the outstanding debt and amortized over the term of the debt using the effective interest method as an addition (reduction) to interest expense. Debt issuance costs related to a line of credit facility are classified as assets and subsequently amortized over the term of the line of credit as additional interest expense.
Segment information. The Company’s operating segments engage in business activities from which it may earn revenues and incur expenses and for which discrete information is available and regularly reviewed by the Company’s chief operating decision maker, who is the Company’s Chief Executive Officer, to make decisions about resources to be allocated to the segment and to assess performance. Operating segments are aggregated for reporting purposes when the operating segments are identified as similar in accordance with the basic principles and aggregation criteria in the accounting standards. The Company’s reporting segments are based on product lines, which have different lines of management responsibility and marketing strategies. The Company has two reportable segments: the Rx Segment and the Consumer Health Segment.
Paragraph IV litigation costs. Legal costs incurred by the Company in the enforcement of the Company’s intellectual property rights are charged to expense.
Business Combination and Contingent considerations. The Company recognizes the identifiable tangible and intangible assets acquired and liabilities assumed based on their estimated fair values as of the acquisition date. The excess of purchase price over the aggregate fair values is recorded as goodwill. The Company calculates the fair value of the identifiable tangible and intangible assets acquired and liabilities assumed to allocate the purchase price at the acquisition date.
The consideration for our acquisitions and certain licensing agreements often includes future payments that are contingent upon the occurrence of a particular event or events. The Company records an obligation for such contingent payments at fair value on the acquisition date. Management estimates the fair value of contingent consideration obligations through valuation models that incorporate probability-adjusted assumptions related to the achievement of the milestones and thus likelihood of making related payments. The Company revalues its contingent consideration obligations each reporting period using Monte Carlo simulation. Changes in the fair value of contingent consideration obligations are recognized in the consolidated statements of income.
Net Loss Per Common Share. Basic income (loss) per common share is calculated by dividing the net income (loss) available to the common shareholders by the weighted average number of common shares outstanding during that period. Diluted net loss per share reflects the potential of securities that could share in the net loss of the Company. For the years ended June 30, 2023 and 2022, the Company incurred a net loss and did not include common equivalent shares in the computation of diluted net loss per share because the effect would have been anti-dilutive.
F-17
The following table sets-forth securities excluded from the calculation of diluted earnings per share.
|
|
|
|
June 30, |
||
|
|
|
|
2023 |
|
2022 |
Warrant to purchase common stock |
|
(Note 16) |
|
6,538,052 |
|
434,328 |
Employee stock options |
|
(Note 15) |
|
52,762 |
|
3,899 |
Employee unvested restricted stock |
|
(Note 15) |
|
40,996 |
|
85,377 |
Employee unvested restricted stock units |
|
(Note 15) |
|
4,963 |
|
8,500 |
Total |
|
|
|
6,636,773 |
|
532,104 |
Recently Adopted Accounting Pronouncements
Reference Rate Reform. In March 2020, the Financial Accounting Standards Board ("FASB") issued Accounting Standard Update (“ASU”) 2020-04, Reference Rate Reform (Topic 848): “Facilitation of the Effects of Reference Rate Reform on Financial Reporting”, which provide optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate expected to be discontinued if contract modifications are made on or before December 31, 2022. The Company adopted the guidance effective July 1, 2022 for the accounting of its LIBOR indexed revolving loans by prospectively applying the interest rate. The Company elected not to reassess the discount rate of its leases. The adoption of this standard did not have a material impact on the Company’s consolidated financial position and results of operations.
Earnings Per Share. In May 2021, the FASB issued ASU 2021-04, “Earnings Per Share (Topic260), Debt – Modifications and Extinguishments (Subtopic 470-50), Compensation – Stock Compensation (Topic 718), and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options”. The amendments in ASU 2021-04 provide guidance to clarify and reduce diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options (for example, warrants) that remain equity classified after modification or exchange. ASU 2021-04 is effective for all entities for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years, with early adoption permitted. The adoption of ASU 2021-04 and related updates did not have a material impact on the Company’s consolidated financial statements.
Recent Accounting Pronouncements Not Yet Adopted
Debt—Debt with Conversion and Other Options. In August 2020, the FASB issued ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging— Contracts in Entity’s Own Equity (Subtopic 815-40)— “Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity”, which simplifies the accounting for convertible instruments by removing major separation models currently required. Consequently, more convertible debt instruments will be reported as a single liability instrument with no separate accounting for embedded conversion features. ASU 2020-06 removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception, which will permit more equity contracts to qualify for it. The standard also simplifies the diluted net income per share calculation in certain areas. The amendments in this update are effective for public entities that are smaller reporting companies, as defined by the Securities and Exchange Commission (”SEC”), for the fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption is permitted through a modified retrospective or full retrospective method. The Company will adopt the guidance on July 1, 2024 and does not expect the adoption of the standard to have any material impact on the Company’s consolidated financial statements.
Financial Instruments – Credit Losses. In June 2016, the FASB issued ASU 2016-13, “Financial Instruments – Credit Losses” requiring the measurement of expected credit losses for financial instruments held at the reporting date based on historical experience, current conditions and reasonable forecasts. The main objective of ASU 2016-13 is to provide additional information about the expected credit losses on financial instruments and other commitments to extend credit. The standard is effective for smaller reporting companies for fiscal periods beginning after December 15, 2022. In May 2019, the FASB issued ASU 2019-05, “Financial Instruments – Credit Losses”, to allow entities to irrevocably elect the fair value option for certain financial assets previously measured at amortized cost upon adoption of the new credit losses standard.
F-18
The effective dates and transition for ASU 2019-05 aligns with those of ASU 2016-13. In March 2022, the FASB issued ASU 2022-02, “Financial Instruments – Credit Losses (topic 326) Troubled Debt Restructurings and Vintage Disclosures” which eliminates the accounting guidance for troubled debt restructurings by creditors and adds disclosure requirements for current period gross write-offs by year of origination for financing receivables and net investments in leases. The Company had adopted ASU 2016-13 and ASU 2019-05 for the fiscal year ended June 30, 2024. The effective dates for the amendments in ASU 2022-02 align with those of ASU 2016-13. The Company had evaluated the impact of adoption of ASUs 2016-13, 2019-05, and 2022-02 and concluded that the application of the new standards did not have a material impact on the Company’s consolidated financial statements.
Management has evaluated other recently issued accounting pronouncements and does not believe that any of these pronouncements will have a significant impact on our consolidated financial statements and related disclosures.
3. Revenues from Contracts with Customers
The Company disaggregates its revenue into two segments, the Rx Segment and the Consumer Health Segment. The Rx Segment includes the ADHD Portfolio, comprised of Adzenys XR-ODT and Cotempla XR-ODT; and the Pediatric Portfolio, comprised of Poly-Vi-Flor, Tri-Vi-Flor, and Karbinal ER. The Consumer Health portfolio is comprised of over ten consumer health products competing in large healthcare categories.
Revenues by Segment: Net revenue disaggregated by segment for the years ended June 30, 2023 and 2022 were as follows.
|
|
|
|
|
|
|
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Rx Segment |
|
$ |
73,799 |
|
$ |
61,121 |
Consumer Health Segment |
|
|
33,600 |
|
|
35,548 |
Consolidated revenue |
|
$ |
107,399 |
|
$ |
96,669 |
Revenues by Product Portfolio: Net revenue disaggregated by significant product portfolios in the Rx Segment for the years ended June 30, 2023 and 2022 were as follows.
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
Rx Segment |
|
(In thousands) |
||||
ADHD |
|
$ |
46,855 |
|
$ |
42,855 |
Pediatric |
|
|
25,377 |
|
|
16,084 |
Other |
|
|
1,567 |
|
|
2,182 |
|
|
$ |
73,799 |
|
$ |
61,121 |
Other includes discontinued and deprioritized products in the Rx Segment. The Consumer Health Segment is comprised of one product portfolio, the Consumer Health Portfolio.
F-19
Revenues by Geographic location. The following table reflects product revenues by geographic location as determined by the billing address of the Company’s customers:
|
|
|
|
|
|
|
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
U.S. |
|
$ |
106,918 |
|
$ |
94,606 |
International |
|
|
481 |
|
|
2,063 |
Total net revenue |
|
$ |
107,399 |
|
$ |
96,669 |
4. Inventories
Inventories consist of the following:
|
|
June 30, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
|
(In thousands) |
|||||
Raw materials |
|
$ |
1,301 |
|
$ |
1,814 |
Work in process |
|
|
2,956 |
|
|
1,838 |
Finished goods |
|
|
7,738 |
|
|
7,197 |
Inventories |
|
$ |
11,995 |
|
$ |
10,849 |
The Company incurred charges of $2.4 million and $4.2 million to reduce the carrying value of inventory to net realizable value during the years ended June 30, 2023 and 2022, respectively, primarily as a result of unsalable and slow-moving products.
5. Property and Equipment
Property and equipment, net consist of the following:
|
|
June 30, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Manufacturing equipment |
|
$ |
2,433 |
|
$ |
2,487 |
Leasehold improvements |
|
|
999 |
|
|
999 |
Office equipment, furniture and other |
|
|
1,125 |
|
|
1,128 |
Lab equipment |
|
|
832 |
|
|
832 |
Assets under construction |
|
|
107 |
|
|
— |
Property and equipment, gross |
|
|
5,496 |
|
|
5,446 |
Less accumulated depreciation and amortization |
|
|
(3,681) |
|
|
(2,421) |
Property and equipment, net |
|
$ |
1,815 |
|
$ |
3,025 |
Depreciation expense was $1.3 million and $1.6 million for the years ended June 30, 2023 and 2022, respectively. During the year ended June 30, 2022, the Company recognized a gain of $0.1 million on the disposal of equipment.
During the year ended June 30, 2022, in connection with the decision to divest Tussionex, the Company recorded a $0.2 million impairment charge related to manufacturing equipment associated with this product.
6. Leases
The Company’s operating leases are for its offices, manufacturing facilities and equipment, and its finance leases are for equipment. These leases have original lease periods expiring between 2022 and 2027. Most leases include option provisions under which the parties may extend the lease term.
F-20
Certain non-real estate leases also include options to purchase the leased property. The Company’s lease agreements generally do not contain any material residual value guarantees or material restrictive covenants.
In connection with the Neos Acquisition, Aytu assumed an operating lease ROU asset and lease liability of $3.5 million, which represented the present value of the remaining lease payments as of the acquisition date, for the office space and manufacturing facilities at Grand Prairie, Texas. As the lease agreement does not provide an implicit rate, a borrowing rate of 6.7% was used to determine the present value of future lease payments. The finance leases are related to equipment finance leases with fixed contract terms and an implicit interest rate of approximately 5.9%.
In April 2023, the Company entered into an agreement with a manufacturing company to sublease 22,909 square feet of the Company’s manufacturing facility in Grand Prairie, Texas (the “Sublease Agreement”). The sublease commenced in May 2023 and will terminate on December 31, 2024. The Sublease Agreement provides the sublessee an option to expand the subleased property to include the remaining 54,203 square feet of the Company’s manufacturing facility. The expansion date may commence as early as April 1, 2024 but no later than December 31, 2024 (the “Expansion Date”). Under the terms of the Sublease Agreement, the sublessee will pay base rent of approximately $20,500 per month through the Expansion Date. Beginning on the Expansion Date, base rent will be $70,686 per month through the expiration of the sublease. In addition to the base rent, the sublessee will pay the Company certain operating expenses incurred by the Company.
During the fiscal year ended June 30, 2023, in addition to the sublease mentioned above, the Company entered into an operating lease agreement to relocate its principal office (See Note 18 – Commitments and Contingencies). During the fiscal year ended June 30, 2022, the Company commenced a five-year operating lease and recorded an ROU of $0.3 million.
The components of lease expenses are as follows;
|
|
Year Ended |
|
|
||||
|
|
June 30, |
|
|
||||
|
|
2023 |
|
2022 |
|
Statement of Operations Classification |
||
|
|
(In thousands) |
|
|
||||
Lease cost: |
|
|
|
|
|
|
|
|
Operating lease cost |
|
$ |
1,402 |
|
$ |
1,299 |
|
Operating expenses |
Short-term lease cost |
|
|
97 |
|
|
152 |
|
Operating expenses |
Finance lease cost: |
|
|
|
|
|
|
|
|
Amortization of leased assets |
|
|
66 |
|
|
73 |
|
Cost of sales |
Interest on lease liabilities |
|
|
9 |
|
|
14 |
|
Other (expense), net |
Total net lease cost |
|
$ |
1,574 |
|
$ |
1,538 |
|
|
F-21
Supplemental balance sheet information related to leases is as follows:
|
|
June 30, |
|
June 30, |
|
Balance Sheet Classification |
||
|
|
2023 |
|
2022 |
|
|
||
|
|
(In thousands) |
|
|
||||
Assets: |
|
|
|
|
|
|
|
|
Operating lease assets |
|
$ |
2,054 |
|
$ |
3,271 |
|
Operating lease right-of-use asset |
Finance lease assets |
|
|
159 |
|
|
256 |
|
Property and equipment, net |
Total leased assets |
|
$ |
2,213 |
|
$ |
3,527 |
|
|
Liabilities: |
|
|
|
|
|
|
|
|
Current: |
|
|
|
|
|
|
|
|
Operating leases |
|
$ |
1,258 |
|
$ |
1,227 |
|
Other current liabilities |
Finance leases |
|
|
85 |
|
|
96 |
|
Current portion of debt |
Non-current |
|
|
|
|
|
|
|
|
Operating leases |
|
|
832 |
|
|
2,090 |
|
Other liabilities |
Finance leases |
|
|
— |
|
|
84 |
|
Debt, net of current portion |
Total lease liabilities |
|
$ |
2,175 |
|
$ |
3,497 |
|
|
Remaining lease terms and discount rates used are as follows;
|
|
June 30, |
|
June 30, |
|
||
|
|
2023 |
|
2022 |
|
||
Weighted-Average Remaining Lease Term (years) |
|
|
|
|
|
|
|
Operating lease assets |
|
|
1.72 |
|
|
2.63 |
|
Finance lease assets |
|
|
0.87 |
|
|
1.73 |
|
Weighted-Average Discount Rate |
|
|
|
|
|
|
|
Operating lease assets |
|
|
7.78 |
% |
|
7.48 |
% |
Finance lease assets |
|
|
6.54 |
% |
|
6.43 |
% |
Supplemental cash flow information related to leases is as follows:
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Cash flow classification of lease payments: |
|
|
|
|
|
|
Operating cash flows - operating leases |
|
$ |
1,436 |
|
$ |
1,016 |
Operating cash flows - finance leases |
|
$ |
9 |
|
$ |
15 |
Financing cash flows - finance leases |
|
$ |
96 |
|
$ |
102 |
As of June 30, 2023, the maturities of the Company’s future minimum lease payments were as follows:
|
|
Operating |
|
Finance |
||
|
|
(In thousands) |
||||
2024 |
|
$ |
1,378 |
|
$ |
88 |
2025 |
|
|
749 |
|
|
— |
2026 |
|
|
90 |
|
|
— |
2027 |
|
|
46 |
|
|
— |
Total lease payments |
|
|
2,263 |
|
|
88 |
Less: Imputed interest |
|
|
(173) |
|
|
(3) |
Lease liabilities |
|
$ |
2,090 |
|
$ |
85 |
F-22
7. Goodwill and Other Intangible Assets
Goodwill
There were no goodwill carrying amounts in the consolidated balance sheets as of June 30, 2023 and 2022. The carrying amount of goodwill by reportable segment and changes during the year ended June 30, 2022 are as follows:
|
|
Rx Segment |
|
Consumer Health Segment |
|
Consolidated |
|||
|
|
(In thousands) |
|||||||
|
|
|
|
|
|
|
|
|
|
Balance as of June 30, 2021 |
|
$ |
57,165 |
|
$ |
8,637 |
|
$ |
65,802 |
Goodwill impairment |
|
|
(57,165) |
|
|
(8,637) |
|
|
(65,802) |
Balance as of June 30, 2022 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
During the year ended June 30, 2022, the Company’s market capitalization significantly declined. The decline was considered a qualitative factor that led management to reassess whether an impairment had occurred. Management’s evaluation indicated that the goodwill related to its reporting units in both the Rx and Consumer Health segments were potentially impaired. The Company then performed a quantitative impairment test by calculating the fair value of the reporting unit and compared that amount to its carrying value. Significant assumptions inherent in the valuation methodologies include, but were not limited to prospective financial information, growth rates, terminal value, discount rates and comparable multiples from publicly traded companies in our industry. The decline in market capitalization was an indicator of increased risk thereby increasing the discount rates in the valuation models. The Company determined the fair value of the reporting unit utilizing the discounted cash flow model. Using a risk adjusted weighted-average discount rate, the fair value of the reporting units was less than its carrying value. The Company recognized an impairment charge of $57.2 million in the Rx Segment, associated with the Cerecor and Neos acquisition and a $8.6 million impairment charge in the Consumer Health Segment related to the goodwill associated with the Innovus Acquisition.
Other Intangible Assets
The tables below provide the summary of the Company’s intangible assets as of June 30, 2023 and June 30, 2022, respectively. Carrying amounts are net of any impairment charges from prior periods. Intangible asset with zero net carrying amount at the end of a reporting period is not presented in the table of a future reporting period.
|
|
June 30, 2023 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted- |
|
|
|
|
|
|
|
|
|
|
Net |
|
Average |
||
|
|
Carrying |
|
Accumulated |
|
|
|
|
Carrying |
|
Remaining |
|||
|
|
Amount |
|
Amortization |
|
Impairment |
|
Amount |
|
Life (in years) |
||||
|
|
(In thousands) |
||||||||||||
Definite-lived intangibles: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired product technology right |
|
$ |
42,176 |
|
$ |
(10,881) |
|
$ |
— |
|
$ |
31,295 |
|
11.49 |
Acquired technology right |
|
|
30,200 |
|
|
(4,054) |
|
|
— |
|
|
26,146 |
|
14.75 |
Acquired product distribution rights |
|
|
9,182 |
|
|
(4,678) |
|
|
(2,975) |
|
|
1,529 |
|
1.00 |
|
|
|
81,558 |
|
|
(19,613) |
|
|
(2,975) |
|
|
58,970 |
|
12.67 |
Indefinite-lived intangibles: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired in-process R&D |
|
|
2,600 |
|
|
— |
|
|
(2,600) |
|
|
— |
|
Indefinite-lived |
|
|
|
2,600 |
|
|
— |
|
|
(2,600) |
|
|
— |
|
|
Total |
|
$ |
84,158 |
|
$ |
(19,613) |
|
$ |
(5,575) |
|
$ |
58,970 |
|
12.67 |
F-23
|
|
June 30, 2022 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted- |
|
|
|
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
Carrying |
|
Accumulated |
|
|
|
|
|
Net Carrying |
|
Remaining |
||
|
|
Amount |
|
Amortization |
|
Impairment |
|
Amount |
|
Life (in years) |
||||
|
|
(In thousands) |
||||||||||||
Definite-lived intangibles: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired product technology right |
|
|
45,400 |
|
|
(7,667) |
|
|
(3,224) |
|
|
34,509 |
|
12.33 |
Acquired technology right |
|
|
30,200 |
|
|
(2,278) |
|
|
— |
|
|
27,922 |
|
15.75 |
Acquired product distribution rights |
|
|
11,354 |
|
|
(3,581) |
|
|
(2,172) |
|
|
5,601 |
|
7.60 |
Other intangible assets |
|
|
4,666 |
|
|
(3,004) |
|
|
(1,662) |
|
|
— |
|
— |
|
|
|
91,620 |
|
|
(16,530) |
|
|
(7,058) |
|
|
68,032 |
|
13.35 |
Indefinite-lived intangibles: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired in-process R&D |
|
|
2,600 |
|
|
— |
|
|
— |
|
|
2,600 |
|
Indefinite-lived |
|
|
|
2,600 |
|
|
— |
|
|
— |
|
|
2,600 |
|
|
Total |
|
$ |
94,220 |
|
$ |
(16,530) |
|
$ |
(7,058) |
|
$ |
70,632 |
|
13.35 |
The following table summarizes the estimated future amortization expense to be recognized over the next five years and periods thereafter:
|
|
June 30, |
|
|
|
(In thousands) |
|
2024 |
|
$ |
6,518 |
2025 |
|
|
4,989 |
2026 |
|
|
4,989 |
2027 |
|
|
4,989 |
2028 |
|
|
4,989 |
Thereafter |
|
|
32,496 |
Total future amortization expense |
|
$ |
58,970 |
Acquired Product Technology Rights
The acquired Product technology rights are related to the rights to production, supply and distribution agreements of various products pursuant to the acquisitions of Pediatric Portfolio in November 2019 and the Neos Acquisition in March 2021.
Karbinal® ER. The Company acquired and assumed all rights and obligations pursuant to the Supply and Distribution Agreement, as Amended, with Tris for the exclusive rights to commercialize Karbinal® ER in the United States (the “Tris Karbinal Agreement”). The Tris Karbinal Agreement’s initial term terminates in August of 2033, with an optional initial 20-year extension.
Poly-Vi-Flor and Tri-Vi-Flor. The Company acquired and assumed all rights and obligations pursuant to a Supply and License Agreement and various assignment and release agreements, including a previously agreed to Settlement and License Agreements (the “Poly-Tri Agreements”) for the exclusive rights to commercialize Poly-Vi-Flor and Tri-Vi-Flor in the United States.
ADHD Portfolio. As part of the Neos Acquisition, the Company acquired developed product technology for the production and sale of Adzenys XR-ODT and Cotempla XR-ODT. The formulations for the ADHD products are protected by patented technology. The estimated economic life of these proprietary technologies is 17 years.
Acquired Technology Right
TRRP Technology. As part of the Neos Acquisition, the Company acquired Time Release Resin Particle (“TRRP”) proprietary technology, which is a proprietary drug delivery technology protected by the Company as a trade secret that allows the Company to modify the drug release characteristics of each of its respective products. The TRRP technology underlines each of Neos’ core products and can potentially be used in future product development initiatives as well.
F-24
Acquired Product Distribution Rights (and customer list)
In connection with the Innovus Acquisition, the Company obtained 35 products with a combination of over 300 registered trademarks and/or patent rights and customer lists. As of June 30, 2022, the customer list intangible asset was fully amortized. During the fiscal year ended June 30, 2023, this intangible asset was impaired by $3.0 million due to the discontinuance of products in the Consumer Health Segment.
Acquired In-Process R&D
IPR&D – NT0502. As part of the Neos Acquisition, the Company acquired in-process research and development associated with NT0502, a new chemical entity that is for the treatment of sialorrhea, which is excessive salivation or drooling. As this is an indefinite-lived intangible asset, this acquired asset remains an indefinite-lived asset until the completion or abandonment of the associated research and development efforts. If a product using this technology is eventually approved for commercial sale, at that time, the IPR&D will begin amortizing on a straight-line over the life of the product. During the fiscal year ended June 30, 2023, the Company fully impaired the IPR&D of NT0502 due to the termination of its development program.
Other
Other intangible assets consist of customer lists, trade names and other technology and licenses.
Certain of the Company’s amortizable intangible assets include renewal options, extending the expected life of the asset. The renewal periods range between approximately 1 to 20 years depending on the license, patent or other agreement. Renewals are accounted for when they are reasonably assured. Intangible assets are amortized using the straight-line method over the estimated useful lives. Amortization expense of intangible assets was $6.1 million and $7.8 million during the years ended June 30, 2023 and 2022, respectively.
The Company’s strategy is to continue building its portfolio of revenue-generating products by leveraging its commercial team’s expertise to build leading brands within large therapeutic and consumer health markets. As a result of focusing on building the portfolio of revenue-generating products, the Company decided to abandon active development of its NT0502 (N-desethyloxybutynin), a new chemical entity that is for the treatment of sialorrhea, which is excessive salivation or drooling. During the year ended June 30, 2023, the Company incurred an impairment charge of $2.6 million related to NT0502 and terminated the licensing agreement. The Company also terminated the license agreement with Cedars-Sinai Medical Center surrounding the Healight technology platform as an additional result of terminating the development of the Healight program. Further, the acquired product distribution rights from Innovus was impaired by $3.0 million due to discontinuance of products in the Consumer Health Segment.
During the year ended June 30, 2022, in connection with the decision to discontinue commercializing or divesting certain products within the Rx Segment that have minimal revenue and gross margin contribution, the Company recorded $4.9 million impairment expense for the write-down of intangible assets consisting of (i) $2.6 million for AcipHex, (ii) $1.4 million for ZolpiMist, (iii) $0.5 million for Tussionex, (iv) $0.2 million for Cefaclor and (v) $0.2 million for the Neos tradename. Additionally, the Company’s Consumer Health Segment recorded an impairment of $2.2 million related to products no longer being marketed and products that have been underperforming.
F-25
8. Accrued liabilities
Accrued liabilities consist of the following:
|
|
June 30, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
|
|
|
|
|
|
|
Accrued savings offers |
|
$ |
15,739 |
|
$ |
12,711 |
Accrued program liabilities |
|
|
11,012 |
|
|
9,468 |
Accrued compensation |
|
|
5,675 |
|
|
4,765 |
Accrued customer and product related fees |
|
|
6,579 |
|
|
7,817 |
Return reserve |
|
|
5,777 |
|
|
5,770 |
Other accrued liabilities |
|
|
2,017 |
|
|
3,656 |
Total accrued liabilities |
|
$ |
46,799 |
|
$ |
44,187 |
The following table details the change in return reserve for the periods presented:
|
|
|
Return Reserve |
|
|
|
(In thousands) |
|
|
|
|
Balance, June 30, 2021 |
|
$ |
6,367 |
Charges to expense |
|
|
8,568 |
Payments |
|
|
(9,165) |
Balance, June 30, 2022 |
|
$ |
5,770 |
Charges to expense |
|
|
8,353 |
Payments |
|
|
(8,346) |
Balance, June 30, 2023 |
|
$ |
5,777 |
Savings offers represent programs for the Company’s patients covered under commercial payor plans in which the cost of a prescription to such patients is discounted.
Program liabilities include government and commercial rebates.
Accrued customer and product related fees include accrued expenses and deductions for rebates, wholesaler chargebacks and fees, and other product-related fees and deductions.
Accrued employee compensation includes sales commissions, vacation earned, and accrued payroll.
Other accrued liabilities consist of accrued license fees, professional fees, credit card liabilities, taxes payable, legal settlements, and samples expense, none of which individually represent greater than five percent.
F-26
9. Other Liabilities
|
|
June 30, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
|
|
|
|
|
|
|
Fixed payment arrangement |
|
$ |
10,420 |
|
$ |
13,051 |
Operating lease liabilities |
|
|
2,090 |
|
|
3,317 |
Contingent value rights |
|
|
— |
|
|
578 |
Contingent consideration |
|
|
— |
|
|
396 |
Other |
|
|
1,555 |
|
|
815 |
Total other liabilities |
|
|
14,065 |
|
|
18,157 |
Less: current portion |
|
|
(7,090) |
|
|
(5,359) |
Total other liabilities, noncurrent |
|
$ |
6,975 |
|
$ |
12,798 |
Fixed payment arrangements. Fixed payment arrangements represent obligations to an investor assumed as part of the acquisition of products from Cerecor, Inc. in 2019, including fixed and variable payments. These obligations included fixed monthly payments equal to $0.1 million from November 2019 through January 2021 plus $15.0 million due in January 2021, of which $15.0 million was paid down early in March 2020. Monthly variable payments due to the same investor are equal to 15.0% of net revenue generated from a subset of the Pediatric Portfolio, subject to an aggregate monthly minimum of $0.1 million, except for January 2021, when a one-time payment of $0.2 million was due and paid. The variable payment obligation was to continue until the earlier of (i) aggregate variable payments of approximately $9.3 million have been made or (ii) February 12, 2026.
On June 21, 2021, the Company entered into a Waiver, Release and Consent pursuant to which the Company paid $2.8 million to the investor in early satisfaction of the fixed obligation. The Company agreed to pay the remaining fixed obligation of $3.0 million in six equal quarterly payments of $0.5 million each over six quarters beginning September 30, 2021. The Company accounted the Waiver, Release and Consent as a debt and remeasured the related liabilities using a discounted cash flow model. This fixed payment arrangement was paid in full by January 2023.
The Tris Karbinal Agreement grants the Company exclusive right to distribute and sell the product in the United States. The initial term of the agreement was 20 years. The Company will pay Tris a royalty equal to 23.5% of net sales. The Tris Karbinal Agreement also contains minimum unit sales commitments, which is based on a commercial year that spans from August 1 through July 31, of 70,000 units annually through 2025. The Company is required to pay Tris a royalty make whole payment of $30 for each unit under the 70,000-unit annual minimum sales commitment through 2025. The Tris Karbinal Agreement make-whole payment is capped at $2.1 million each year. The annual payment is due in August of each year. The Tris Karbinal Agreement also has multiple commercial milestone obligations that aggregate up to $3.0 million based on cumulative net sales, the first of which is triggered at $40.0 million of net revenues. As of June 30, 2023, the fixed payment arrangement balance was $1.7 million in other current liabilities and $2.1 million in other non-current liabilities on the consolidated balance sheet.
On May 12, 2022, the Company entered into an agreement with Tris to terminate the License, Development, Manufacturing and Supply Agreement dated November 2, 2018 (the “License Agreement”). Pursuant to such termination, the Company agreed to pay Tris a total of approximately $6.0 million to $9.0 million, which reduced our total liability for minimum payments by approximately $8.0 million from the original License Agreement. The settlement payment will be paid in three installments from December 2022 through July 2024. As of June 30, 2023, the balance was $6.6 million.
Contingent value rights. Contingent value rights (“CVRs”) represent contingent consideration related to the Company’s 2020 acquisition of Innovus of up to $16.0 million payable upon attainment of future performance milestones. Consideration can be satisfied in up to 470,000 shares of the Company’s common stock, or cash either upon the option of the Company or in the event there are insufficient shares available to satisfy such obligations. In the fiscal years ended June 30, 2020 and 2021, the Company issued to the CVR holders 6,191 and 5,160 shares of common stock, respectively, upon achievement of specified revenues. No milestones were met during the fiscal years ended June 30, 2022 and 2023.
F-27
As of June 30, 2023, up to $5.0 million of future milestone payments potentially remain. During the years ended June 30, 2023 and 2022, the Company recognized a gain of $0.6 million and $0.8 million, respectively, in the consolidated statements of operations related to the changes in fair values of CVRs. As of June 30, 2023 and 2022, the CVRs balance was zero and $0.6 million, respectively.
Contingent consideration. Contingent consideration represents the fair value of potential future payments in connection with acquisitions that are contingent upon the occurrence of a particular event or events. The Company records an obligation for such contingent payments at fair value on the acquisition date. Subsequent changes in the fair value of contingent consideration obligations are recognized in the consolidated statements of income.
In connection with the Company’s 2020 acquisition of Innovus, the Company recognized approximately $0.2 million in product related contingent consideration. The fair value was based on a discounted value of the future contingent payment using a 30% discount rate based on the estimated risk that the milestones are achieved.
Prior to June 30, 2022, the Company’s contingent consideration liabilities included obligations under licensing arrangements for Tuzistra XR. The royalty and make-whole milestone payments related to licensing agreements with TRIS Pharma, Inc. (“Tris”) for Tuzistra XR were being accounted for as contingent consideration and revalued at each reporting period. As a result of the discontinuation of commercializing Tuzistra (see Note 3 – Revenue from Contracts with Customers) and a settlement agreement with Tris, the Company concluded that the product milestone payments underlying the contingent consideration liability ceased to exist. The Company reversed the remaining contingent consideration liabilities of $8.5 million and recorded a liability of $7.6 million related to the settlement payments payable to Tris for termination of the Tuzistra licensing agreement. The settlement payments are included in fixed payment arrangements at their present value using the Company’s estimated borrowing rate. The Company recognized $0.9 million gain on settlement of the Tris contingent consideration liabilities in the consolidated statements of operations for the year ended June 30, 2022.
Prior to June 30, 2022, the royalty payments related to licensing agreements with Magna Pharmaceuticals, Inc. (“Magna”) for ZolpiMist were being accounted for as contingent consideration and revalued at each reporting period. As a result of the discontinuation of commercializing ZolpiMist, the Company concluded that the royalty-based product milestone payments underlying the contingent consideration liability ceased to exist. In 2022, the Company reversed the remaining contingent consideration liabilities of $0.6 million and recorded the $50,000 payment due for termination of the Manga licensing agreements in other current liabilities. The Company recognized a $0.6 million gain from termination of the contingent consideration liability in the consolidated statements of operations for the year ended June 30, 2022.
During the year ended June 30, 2023 and 2022, the Company recognized a gain of $0.4 million and a loss of $0.5 million, respectively, from the changes in fair values of contingent considerations. As of June 30, 2023 and 2022, the contingent consideration balance was zero and $0.4 million, respectively.
Other. Consist of taxes payable and deferred cost related to our technology transfer.
10. Line of Credit
Upon closing of the Neos Acquisition in March 2021, the Company assumed obligations under the secured credit agreement that Neos had entered into with Eclipse Business Capital LLC (f/k/a Encina Business Credit, LLC) (“Eclipse”) as agent for the lenders (the “Eclipse Loan Agreement”). Under the Eclipse Loan Agreement, Eclipse extended up to $25.0 million in secured revolving loans to Neos (the “Revolving Loans”), of which up to $2.5 million was available for short-term swingline loans, against 85% of eligible accounts receivable. The Revolving Loans thereunder accrued variable interest through maturity at the one-month Secure Overnight Financing Rate (“SOFR), plus 4.50%. The Eclipse Loan Agreement included an unused line fee of 0.50% of the average unused portion of the maximum revolving facility amount during the immediately preceding month. Interest is payable monthly in arrears. The original maturity date under the Eclipse Loan Agreement was May 11, 2022.
F-28
In connection with the Avenue Capital Agreement, described in Note 12 – Long Term Debt, the Company entered into a Consent, Waiver and Second Amendment to Eclipse Loan Agreement, dated as of January 26, 2022 (together, the “Eclipse Second Amendment”). Pursuant to the Eclipse Second Amendment, Eclipse (i) consented to Aytu and certain of its subsidiaries joining as obligors to the Revolving Loans provided by the Eclipse Loan Agreement, (ii) consented to the Company entering into the Avenue Capital Agreement, (iii) extended the maturity date of the Eclipse Loan Agreement to January 26, 2025, (iv) removed the requirement for the Company to comply with the ongoing fixed charge coverage ratio financial covenant applicable to the borrowers under the Eclipse Loan Agreement, (v) consented to the first priority lien granted by Aytu in favor of the Avenue Capital Agent, (vi) reduced the maximum availability under the Revolving Loans from $25.0 million to $12.5 million minus a $3.5 million availability block, (vii) increased the availability block from $1.0 million to $3.5 million, (viii) consented to the full repayment under the Deerfield Facility, defined below, and (ix) made certain other modifications to conform to the Avenue Capital Agreement and to reflect the consummation of the transactions thereof, in each case subject to the terms and conditions of the Eclipse Second Amendment.
The Company incurred $0.1 million in legal and other fees related to the Eclipse Second Amendment, all of which were recorded as deferred financing costs and are being amortized on a straight-line basis over the remaining term of the Eclipse Loan Agreement as interest expense. The unamortized cost of $0.1 million as of June 30, 2022 was included in other noncurrent assets in the consolidated balance sheets.
On March 24, 2023, the Company and certain of its subsidiaries entered into an Amendment No. 4 (the Eclipse Amendment”) to the Loan and Security Agreement dated October 2, 2019 (as amended by Amendment No. 1, dated March 19, 2021, Amendment No. 2, dated January 26, 2022, Amendment No. 3, dated June 1, 2022, and the Eclipse Amendment (the “Eclipse Agreement”). The Eclipse Amendment, among other things, provided for an aggregate increase of $2.0 million to the Eclipse Lender’s commitment to make revolving loans from time to time under the Eclipse Agreement and increased the maximum amount available under the revolving credit facility provided under the Eclipse Agreement to $14.5 million. The ability to make borrowings and obtain advances of revolving loans under the Eclipse Agreement remains subject to a borrowing base and reserve, and availability blockage requirements.
In the event that, for any reason, all or any portion of the Eclipse Loan Agreement is terminated prior to the scheduled maturity date, in addition to the payment of all outstanding principal and unpaid accrued interest, the Company is required to pay a fee equal to (i) 2.0% of the Revolving Loans commitment if such event occurs on or before January 26, 2023, (ii) 1.0% of the Revolving Loans commitment if such event occurs after January 26, 2023 but on or before January 26, 2024, and (iii) 0.5% of the Revolving Loans commitment if such event occurs after January 26, 2024 but on or before January 26, 2025. The Company may permanently terminate the Eclipse Loan Agreement with at least five business days prior notice to Eclipse.
The Eclipse Loan Agreement contains customary affirmative covenants, negative covenants and events of default, as defined in the agreement, including covenants and restrictions that, among other things, require the Company to satisfy certain capital expenditure limitations and other financial covenants, and restrict the Company’s ability to incur liens, incur additional indebtedness, make certain dividends and distributions with respect to equity securities, engage in mergers and acquisitions or make asset sales without the prior written consent of Eclipse. A failure to comply with these covenants could permit Eclipse to declare the Company’s obligations under the Eclipse Loan Agreement, together with accrued interest and fees, to be immediately due and payable, plus any applicable additional amounts relating to a prepayment or termination, as described above. As of June 30, 2023, the Company was in compliance with the covenants under the Eclipse Loan Agreement as amended.
The Company’s obligations under the Eclipse Loan Agreement are secured by substantially all of the Company’s assets, with a first priority lien in favor of Eclipse on the ABL Priority Collateral, and a second priority lien in favor of Eclipse on the Term Loan Priority Collateral, as each is defined in the Replacement Term Loan Intercreditor Agreement, as defined in the Eclipse Loan Agreement, as amended by the Eclipse Second Amendment.
Total interest expense on the Revolving Loans, including amortization of deferred financing costs, were $0.7 million and $0.4 million for the years ended June 30, 2023 and 2022. As of June 30, 2023 and 2022, the outstanding Revolving Loans under the Eclipse Loan Agreement, as amended, were $1.6 million and $3.8 million, respectively.
F-29
Unused line of credit amount as of June 30, 2023 was $9.3 million.
11. Long-term Debt
Deerfield Debt. Upon closing of the Neos Acquisition, the Company assumed a senior secured term credit facility (the “Deerfield Facility”) with Deerfield Private Design Fund III, L.P. and Deerfield Partners, L.P. (collectively, “Deerfield”) with an outstanding balance of $16.6 million.
The Company evaluated and determined that the fair value of the remaining outstanding debt was $17.4 million as of the March 19, 2021 acquisition date. Accordingly, the Company recorded a premium of $0.8 million, which was the difference between carrying amount and the fair value of the debt and was being amortized into interest expense using the effective interest method over the remaining term of the debt.
On January 26, 2022, the Company repaid the remaining principal outstanding in full, plus exit fees and accrued interest under the Deerfield Facility. The Company recognized a gain of $0.2 million during the year ended June 30, 2022 related to the extinguishment of the Deerfield Facility. Total interest expense on the facility, net of premium amortization, was $0.8 million for the period from July 1, 2021 through full repayment on January 26, 2022.
Avenue Capital Loan. On January 26, 2022 (“Closing Date”), the Company entered into a Loan and Security Agreement (the “Avenue Capital Agreement”) with Avenue Venture Opportunities Fund II, L.P. and Avenue Venture Opportunities Fund II, L.P. as lenders (the “Avenue Capital Lenders”), and Avenue Capital Management II, L.P. as administrative agent (the “Avenue Capital Agent”), collectively (“Avenue Capital”), pursuant to which the Avenue Capital Lenders provided the Company and certain of its subsidiaries with a secured $15.0 million loan. The interest rate on the loan is the greater of the prime rate and 3.25%, plus 7.4%, payable monthly in arrears. The maturity date of the loan is January 26, 2025. The proceeds from the Avenue Capital Agreement were used towards the repayment of the Deerfield Facility.
Pursuant to the Avenue Capital Agreement, the Company will make interest only payments for the first 18 months following the Closing Date (“Interest-only Period”). The Interest-only Period could be extended automatically without any action by any party for six months provided as of the last day of the Interest-only Period then in effect, the Company received, prior to June 15, 2023, a specified amount of net proceeds from the sale and issuance of its equity securities (“Interest-only Milestone 1”). The Interest-only Period could further be extended automatically without any action by any party for an additional six months provided, the Company has achieved, prior to December 31, 2023, (i) Interest-only Milestone 1 and (ii) a specified amount of trailing 12 months revenue as of the date of determination.
In the event the Company prepays the outstanding principal prior to the maturity date, the Company will pay Avenue Capital a fee equal to (i) 3.0% of the loan if such event occurs on or before January 26, 2023, (ii) 2.0% of the loan if such event occurs after January 26, 2023 but on or before January 26, 2024, and (iii) 1.0% of the loan if such event occurs after January 26, 2024 but before January 26, 2025. In addition, upon the payment in full of the obligations, the Company shall pay to Avenue Capital a fee in the amount of $0.6 million (“Final Payment”). The Company accounted for the Final Payment as additional obligations on the debt, with the corresponding charge being recorded as debt discount.
The Company’s obligations under Avenue Capital Agreement are secured by substantially all of the Company’s assets, with a first priority lien in favor of the Avenue Capital Agent on the Term Loan Priority Collateral, and a second priority lien in favor of the Avenue Capital Agent on the ABL Priority Collateral, as each is defined in the Intercreditor Agreement, as defined in the Avenue Capital Agreement.
The Avenue Capital Agreement contains customary affirmative covenants, negative covenants and events of default, as defined in the agreement, including covenants and restrictions that, among other things, require the Company to satisfy certain capital expenditure limitations and other financial covenants, and restricts the Company’s ability to incur liens, incur additional indebtedness, make certain dividends and distributions with respect to equity securities, engage in mergers and acquisitions or make asset sales without the prior written consent of the Avenue Capital Lenders.
F-30
A failure to comply with these covenants could permit the Avenue Capital Lenders to declare the Company’s obligations under the agreement, together with accrued interest and fees, to be immediately due and payable, plus any applicable additional amounts relating to a prepayment or termination, as described above. As of June 30, 2023, the Company was in compliance with the covenants under the Avenue Capital Agreement.
On January 26, 2022 (“Issuance Date”), as consideration for entering into the Avenue Capital Agreement, the Company issued warrants to the Avenue Capital Lenders to purchase shares of common stock at an exercise price equal to $24.20 per share (the “Avenue Capital Warrants”). The Avenue Capital Warrants provided that in the event the Company were to engage in an equity offering at a price lower than $24.20 prior to June 30, 2022, the exercise price would be adjusted to the effective price of such equity offering and the number of shares of common stock to be issued under the Avenue Capital Warrants would be adjusted as set forth in the agreement. The Avenue Capital Warrants were immediately exercisable and expire on January 31, 2027. At inception, the Company accounted for the Avenue Capital Warrants as a derivative warrant liability as the number of warrants was not fixed at the Issuance Date. The fair value of the Avenue Capital Warrants at issuance was approximately $0.6 million.
On March 7, 2022, the Company closed on an equity offering of shares of common stock and warrants, as described in Note 15 – Stockholders Equity, at an offering price of $25.00 per share. As this offering precluded the Company from pursuing any equity financing prior to July 7, 2022 and the effective price of the March 7, 2022 offering was more than the exercise price of the Avenue Capital Warrants, the shares of common stock issuable upon exercise of the Avenue Capital Warrants were set at an exercise price of $24.20.
On October 25, 2022, the Company entered into an agreement with Avenue Venture Opportunities Fund, L.P (“Avenue”) to extend the interest-only period of its existing senior secure loan facility held with Avenue. The amendment to the original loan agreement, which was executed in January 2022, extends the interest-only period to January of 2024. In exchange for this extension of the interest-only period, the Company and Avenue agreed to reset the exercise price of the warrants issued in conjunction with the original loan agreement to $8.60, corresponding to the warrant exercise price associated with the Company’s August 2022 equity financing.
On June 13, 2023, in conjunction with the Securities Purchase Agreement described in Note 16 – Warrants, the interest-only period of the Avenue Capital Agreement was extended further upon the achievement of both the revenue-based milestone and equity raise-based milestone stipulated in the Avenue Capital Agreement. The interest-only period now extends to the January 26, 2025 maturity date.
In addition to the debt discounts discussed above, the Company also incurred $0.4 million loan origination, legal and other fees. The debt discount and issuance costs are being amortized over the term of the loan, using the effective interest method resulting in an effective rate of 16.59%. Total interest expense on the Avenue Capital loan including debt discount amortization, were $2.7 million and $0.9 million for the years ended June 30, 2023 and 2022.
Long-term debt consists of the following;
|
|
June 30, |
|
|
|
2023 |
|
|
|
(In thousands) |
|
Long-term debt, due on January 26, 2025 |
|
$ |
15,000 |
Long-term, final payment fee |
|
|
638 |
Unamortized discount and issuance costs |
|
|
(925) |
Financing leases, maturing through May 2024 |
|
|
85 |
Total debt |
|
|
14,798 |
Less: current portion |
|
|
(85) |
Non-current portion of debt |
|
$ |
14,713 |
F-31
Future principal payments of long-term debt, including financing leases, are as follows;
|
|
June 30, |
|
|
|
(In thousands) |
|
2024 |
|
$ |
85 |
2025 |
|
|
15,638 |
Future principal payments |
|
|
15,723 |
Less unamortized discount and issuance costs |
|
|
(925) |
Less current portion |
|
|
(85) |
Non-current portion of debt |
|
$ |
14,713 |
12. Fair Value Measurements
We determine the fair value of financial and non-financial assets using the fair value hierarchy, which establishes three levels of inputs that may be used to measure fair value as follows:
Level 1: |
Inputs that reflect unadjusted quoted prices in active markets that are accessible to Aytu for identical assets or liabilities; |
Level 2: |
Inputs include quoted prices for similar assets and liabilities in active or inactive markets or that are observable for the asset or liability either directly or indirectly; and |
Level 3: |
Unobservable inputs that are supported by little or no market activity. |
The Company’s financial instruments include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, derivative warrant liabilities, contingent consideration liabilities, fixed payment arrangements, and short-term and long-term debt. The carrying amounts of certain short-term financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximate their fair value due to their short maturities. Short-term and long-term debt are reported at their amortized costs on our consolidated balance sheets. The remaining financial instruments are reported on our consolidated balance sheets at amounts that approximate current fair values. The Company’s policy is to recognize transfers in and/or out of fair value hierarchy as of the date in which the event or change in circumstances caused the transfer. There were no transfers between Level 1, Level 2 and Level 3 in the periods presented.
F-32
Recurring Fair Value Measurement
The following table presents the Company’s financial assets and liabilities that were accounted for at fair value on a recurring basis as of June 30, 2023 and 2022, by level within the fair value hierarchy:
|
|
|
|
|
Fair Value Measurements at June 30, 2023 |
|||||||
|
|
Fair Value at June 30, |
|
|
|
|
|
|
||||
|
|
2023 |
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
||||
|
|
(In thousands) |
||||||||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant liabilities |
|
$ |
6,403 |
|
$ |
— |
|
$ |
— |
|
$ |
6,403 |
Total |
|
$ |
6,403 |
|
$ |
— |
|
$ |
— |
|
$ |
6,403 |
|
|
|
|
|
Fair Value Measurements at June 30, 2022 |
|||||||
|
|
Fair Value at June 30, |
|
|
|
|
|
|
||||
|
|
2022 |
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
||||
|
|
(In thousands) |
||||||||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
396 |
|
$ |
— |
|
$ |
— |
|
$ |
396 |
CVR liability |
|
|
578 |
|
|
— |
|
|
— |
|
|
578 |
Derivative warrant liabilities |
|
|
1,796 |
|
|
— |
|
|
— |
|
|
1,796 |
Total |
|
$ |
2,770 |
|
$ |
— |
|
$ |
— |
|
$ |
2,770 |
Cash and cash equivalents in the consolidated balance sheets include bank deposits and money market funds, and reflect their fair value at Level 1 in the fair value hierarchy.
Non-Recurring Fair Value Measurement
The Company’s financial assets and liabilities that were accounted for at fair value on a non-recurring basis during the years ended June 30, 2023 and 2022, were fixed payment arrangements, goodwill and intangible assets.
Fixed payment arrangements are recognized at their amortized cost basis using market appropriate discount rates and are accreted up to their notional face value over time. Significant assumptions used in valuing the fixed payment arrangements were discount rates from 10.0% to 15.4%, and are classified as Level 3 inputs in the fair value hierarchy. In May 2022, the Company recognized a fixed payment arrangement liability of $7.6 million relating to the termination of the License, Development, Manufacturing and Supply Agreement with Tris. See Note 9 – Other Liabilities for further information on fixed payment arrangements.
Based on the Company’s impairment analyses for fiscal years 2023 and 2022, the Company recorded an impairment charge of $5.6 million on intangible assets during the year ended June 30, 2023; and an impairment charge of $7.1 million on intangible assets and $65.8 million on goodwill for the year ended June 30, 2022. Valuation of goodwill and intangible assets involves significant Level 3 inputs in estimating their fair values. These input assumptions included revenue growth rates, forecasted EBITDA margins, and the selection of a discount rate. These assumptions may be affected by expectations about future market or economic conditions. See Note 7 - Goodwill and Other Intangible Assets and Note 2 - Summary of Significant Accounting Policies, for further discussion on the fair value measurement of goodwill and other intangible assets.
F-33
Summary of Level 3 Input Changes
The following table sets forth a summary of changes to those fair value measures using Level 3 inputs for the year ended June 30, 2023:
|
|
CVR |
|
Contingent |
|
|
Warrant |
||
|
|
Liability |
|
Consideration |
|
|
Liability |
||
|
|
(In thousands) |
|||||||
Balance as of June 30, 2022 |
|
$ |
578 |
|
$ |
396 |
|
$ |
1,796 |
Included in earnings |
|
|
(578) |
|
|
(391) |
|
|
(6,391) |
Purchases, issues, sales and settlements: |
|
|
|
|
|
|
|
|
|
Issues |
|
|
— |
|
|
— |
|
|
10,998 |
Settlements |
|
|
— |
|
|
(5) |
|
|
— |
Balance as of June 30, 2023 |
|
$ |
— |
|
$ |
— |
|
$ |
6,403 |
Level 3 Inputs
Changes in the fair value of contingent liabilities in subsequent periods are recorded as a gain or loss in the consolidated statements of operations.
Significant assumptions used in valuing the CVRs were as follows:
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
Leveraged Beta |
|
0.84 |
|
|
0.85 |
|
Market risk premium |
|
6.35 |
% |
|
6.22 |
% |
Risk-free interest rate |
|
5.47 |
% |
|
2.86 |
% |
Discount |
|
22.00 |
% |
|
20.50 |
% |
Company specific discount |
|
10.00 |
% |
|
10.00 |
% |
Significant assumptions used in valuing the derivative warrant liabilities at issuance date were as follows:
|
|
August 9, |
|
|
|
2022 |
|
Expected volatility |
|
89.89 |
% |
Equivalent term (years) |
|
4.11 |
|
Risk-free rate |
|
3.09 |
% |
Dividend yield |
|
0.00 |
% |
|
|
June 8, |
|
|
|
2023 |
|
Expected volatility |
|
83.26 |
% |
Equivalent term (years) |
|
5.01 |
|
Risk-free rate |
|
3.87 |
% |
Dividend yield |
|
0.00 |
% |
F-34
Significant assumptions used in valuing the derivative warrant liabilities, marked to market, were as follows:
|
|
June 30, |
|
|
|
2023 |
|
Expected volatility |
|
83.42 |
% |
Equivalent term (years) |
|
3.59-4.95 |
|
Risk-free rate |
|
4.13-4.40 |
% |
Dividend yield |
|
0.00 |
% |
|
|
|
|
Expected volatility was based primarily on historical volatility. The Company chose to use a two-year lookback on historical volatility to avoid the effects of COVID-19 and the Innovus acquisition. The Company believes this method produced an estimate that was representative of the Company’s expectations of future volatility over the expected term of these warrants, and will not differ materially. If expected volatility by the active market is higher than estimated, the derivative may result in a greater fair value. The expected life was based on the remaining contractual term of the warrants. The risk-free rate was based on the U.S. Treasury rate that corresponded to the expected term of the warrants.
13. Income Taxes
For the fiscal year of 2023, there was no income tax benefit, primarily driven by Section 382 limitation on post-TCJA (“Tax Cuts and Jobs Act”) net operating loss (“NOL”) utilization, further described below. As of June 30, 2023, the Company had $0.1 million deferred tax asset (DTA) included in other non-current assets, $0.1 million deferred tax liability (DTL) included in other long-term liabilities, and $0.1 million income tax payable in accrued liabilities in the consolidated balance sheet.
Section 382 Limitation
Under the provisions of the Internal Revenue Code, substantial changes in the Company’s ownership may result in limitations on the amount of NOL carryforwards that can be utilized in future years. NOL carryforwards are subject to examination in the year they are utilized regardless of whether the tax year in which they are generated has been closed by statute. The amount subject to disallowance is limited to the NOL utilized. Accordingly, the Company may be subject to examination for prior NOLs generated as such NOLs are utilized.
As part of the Company’s Section 382 analysis, an ownership change was determined to have occurred in March 2022 at a point in time when the Company had a net unrealized built-in gain. As such, the NOL generated during that period has been allocated and the post-change NOL (approximately $12 million) is determined to be fully available to offset fiscal 2023 pre-change income subject to the 80% limitation. The Company also determined that ownership change occurred in June 2023 at a time that the Company was in a net unrealized loss position. As a result of the Section 382 analysis, the Company had estimated $0.3 million of disallowed recognized built-in loss and had carried forward as an operating loss as of June 30, 2023.
The Company had federal net operating losses of approximately $504.0 million as of June 30, 2023, that subject to limitation (as described above), may be available in future tax years to offset taxable income. Of the available federal net operating losses, approximately $172.0 million can be carried forward indefinitely, while the remaining balance will begin to expire in 2024 and completely expire in 2027. As of June 30, 2023, the Company had research and development credits of $3.0 million, which begin to expire in 2024. The available state net operating losses, if not utilized to offset taxable income in future periods, will begin to expire in 2025 through 2039.
As of June 30, 2023, the Company had various state NOL carryforwards. The determination of the state NOL carryforwards is dependent on apportionment percentages and state laws that can change from year to year and impact the amount of such carryforwards.
F-35
The Company notes there is diversity in practice regarding the treatment of deductions or loss carryforwards that are expected to expire unutilized. Generally, it is not appropriate to use zero as an applicable tax rate and rather, a deferred tax asset should be recorded at the applicable tax rate and a valuation of an equal amount would be provided. However, under certain circumstances it may be appropriate to follow an alternative approach and use a zero rate to write off the asset against the valuation allowance, reducing the valuation allowance and gross deferred tax assets disclosed. The Company considered both accounting viewpoints and determined it would present its NOL carryforwards gross with a full valuation allowance and not apply a zero rate to NOL carryforwards expected to expire unutilized.
In review of the Company’s consolidated deferred position excluding NOLs and other tax attributes, the Company is in a net DTA position and therefore all NOLs are being fully valued and not utilized against a net DTL.
The provision for income taxes consisted of the following:
|
|
Year Ended June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Current: |
|
|
|
|
|
|
Federal |
|
$ |
80 |
|
$ |
— |
State |
|
|
46 |
|
|
7 |
Total current tax expense |
|
|
126 |
|
|
7 |
Deferred: |
|
|
|
|
|
|
Federal |
|
|
(109) |
|
|
(91) |
State |
|
|
(17) |
|
|
(26) |
Total deferred tax expense |
|
|
(126) |
|
|
(117) |
Provision for income taxes |
|
$ |
— |
|
$ |
(110) |
Income tax benefit resulting from applying statutory rates in jurisdictions in which the Company is taxed (Federal and various states) differs from the income tax provision (benefit) in the financial statements. Reconciliation of the U.S. federal statutory income tax rates to our effective tax rate is as follows.
|
|
Year Ended June 30, |
||||||||||
|
|
2023 |
|
|
2022 |
|||||||
|
|
(In thousands) |
||||||||||
Tax at statutory rate |
|
$ |
(3,581) |
|
(22.30) |
% |
|
$ |
(23,159) |
|
(21.00) |
% |
State income taxes, net of federal benefit |
|
|
16 |
|
0.10 |
% |
|
|
601 |
|
0.55 |
% |
Permanent difference |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Stock based compensation |
|
|
— |
|
— |
% |
|
|
273 |
|
0.27 |
% |
Contingent consideration |
|
|
(193) |
|
(1.20) |
% |
|
|
(155) |
|
(0.14) |
% |
162(m) limitation |
|
|
— |
|
— |
% |
|
|
76 |
|
0.08 |
% |
Goodwill impairment |
|
|
— |
|
— |
% |
|
|
9,733 |
|
8.83 |
% |
Transaction costs |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Change in tax rate |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Remeasurement of deferred taxes |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Effect of phased-in tax rate |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Loss on debt extinguishment and interest expense |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Change in valuation allowance |
|
|
3,641 |
|
22.68 |
% |
|
|
12,472 |
|
11.31 |
% |
Derivative income |
|
|
— |
|
— |
% |
|
|
— |
|
— |
% |
Other |
|
|
117 |
|
0.72 |
% |
|
|
49 |
|
0.01 |
% |
Net income tax provision (benefit) |
|
$ |
— |
|
0.00 |
% |
|
$ |
(110) |
|
(0.09) |
% |
F-36
Deferred income taxes arise from temporary differences in the recognition of certain items for income tax and financial reporting purposes. The approximate tax effects of significant temporary differences which comprise the deferred tax assets and liabilities are as follows for the respective periods:
|
|
Year Ended June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Deferred tax assets: |
|
|
|
|
|
|
Net operating loss carry forward |
|
$ |
114,265 |
|
$ |
114,443 |
Accrued Rebates |
|
|
6,994 |
|
|
5,944 |
Share-based compensation |
|
|
4,250 |
|
|
2,773 |
Accrued expenses |
|
|
758 |
|
|
817 |
R&D credits |
|
|
2,416 |
|
|
2,423 |
Interest |
|
|
4,188 |
|
|
2,975 |
Warrant Derivatives |
|
|
1,504 |
|
|
— |
Section 174 Capitalization |
|
|
836 |
|
|
— |
Inventory |
|
|
743 |
|
|
1,177 |
Lease liability |
|
|
492 |
|
|
799 |
Other |
|
|
1,332 |
|
|
1,301 |
Total deferred tax assets |
|
|
137,778 |
|
|
132,652 |
Less: valuation allowance |
|
|
(136,400) |
|
|
(128,966) |
Deferred tax assets, net of valuation allowance |
|
|
1,378 |
|
|
3,686 |
Deferred tax liabilities: |
|
|
|
|
|
|
Intangibles |
|
|
(845) |
|
|
(2,717) |
Fixed Assets |
|
|
(50) |
|
|
(308) |
ROU asset |
|
|
(483) |
|
|
(788) |
Total deferred tax liabilities |
|
|
(1,378) |
|
|
(3,813) |
Net deferred tax liabilities |
|
$ |
— |
|
$ |
(127) |
In fiscal year 2022, the impairment of goodwill decreased net deferred tax liabilities by $0.1 million resulting in an income tax benefit of $0.1 million. As of June 30, 2022, the Company had $0.1 million deferred tax liabilities included in other long-term liabilities in the consolidated balance sheet. The Company had federal net operating losses of approximately $503.2 million as of June 30, 2022, subject to Section 382 limitation.
The Company has recorded a valuation allowance of $136.4 million and $129.0 million at June 30, 2023 and 2022, respectively, to reserve its net deferred tax assets. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, carry back opportunities and tax planning strategies in making the assessment. The Company believes it is more likely than not, that it will realize the benefits of these deductible differences, net of the valuation allowance provided.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. The Company has no accrued interest related to its uncertain tax positions as they all relate to timing differences that would adjust the Company’s net operating loss carryforward, interest expense carryover or research and development credit carryover and therefore do not require recognition. As a result of these timing differences, at June 30, 2023 and 2022, the Company had gross unrecognized tax benefits related to uncertain tax positions of $2.9 million and $2.8 million, respectively. Changes in unrecognized benefits in any given year are recorded as a component of deferred tax expense.
F-37
A tabular roll-forward of the Company’s gross unrecognized tax benefits is below.
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Beginning balance |
|
$ |
2,822 |
|
$ |
3,435 |
Increase resulting from prior period tax positions |
|
|
— |
|
|
— |
Increase resulting from current period tax positions |
|
|
246 |
|
|
34 |
Decrease resulting from current period tax positions |
|
|
(120) |
|
|
(647) |
Ending balance |
|
$ |
2,948 |
|
$ |
2,822 |
The change in the Company’s gross unrecognized tax benefits relates to the acquisition of Neos, whereby historic tax positions of Neos were inherited in the acquisition.
Additionally, Neos pre-acquisition tax years are subject to the same general statute of limitations, resulting in its tax years back to 2004 being subject to examination.
14. Stockholders’ Equity
The Company has 200.0 million shares of common stock authorized with a par value of $0.0001 per share and 50.0 million shares of preferred stock authorized with a par value of $0.0001 per share. As of June 30, 2023 and 2022, the Company had 5,517,174 and 1,928,941 common shares issued and outstanding, respectively, and no preferred shares issued and outstanding.
Included in the common stock outstanding are 40,996 shares of unvested restricted stock issued to executives, directors, and employees.
On June 8, 2020, the Company filed a shelf registration statement (the “2020 Shelf”), which was declared effective by the SEC on June 17, 2020, covering up to $100.0 million of its common stock, preferred stock, debt securities, warrants, rights, and units. On June 4, 2021, the Company entered into an agreement with an agent for the sale of up to $30.0 million of its common stock from time to time in “at-the-market” offerings under the 2020 Shelf (the “ATM Sales Agreement”). During the year ended June 30, 2023, the Company issued 699,929 shares of common stock under the ATM Sales Agreement, with total gross proceeds of approximately $3.0 million before deducting underwriting discounts, commissions, and other offering expenses of $0.1 million. The 2020 Shelf expired in June 2023.
On September 28, 2021, the Company filed a shelf registration statement (the “2021 Shelf”), which was declared effective by the SEC on October 7, 2021, covering up to $100.0 million of its common stock, preferred stock, debt securities, warrants, rights, and units. As of June 30, 2023, approximately $82.4 million remain available under the 2021 Shelf. This availability is subject to SEC 1.B.6 limitation to the Form S-3. The 2021 Shelf expires in October 2024.
On March 7, 2022, the Company closed on an underwritten public offering utilizing the 2021 Shelf, pursuant to which, the Company sold, (i) 151,500 shares of the Company’s common stock, (ii) pre-funded warrants to purchase up to 151,500 shares of common stock, and (iii) common stock purchase warrants to purchase up to 333,300 shares of common stock (the “March 2022 Offering”). The shares of common stock and the pre-funded warrants were each sold in combination with corresponding common warrants, with one common warrant to purchase 1.1 shares of common stock for each share of common stock or each pre-funded warrant sold. The pre-funded warrants have an exercise price of $0.002 per share of common stock and were exercised in full in April 2022. The common warrants have an exercise price of $26.00 per share of common stock and are exercisable six months after the date of issuance and have a term of five years from the date of exercisability. The Company raised gross proceeds of $7.6 million through the March 2022 Offering before commission and other costs of $0.8 million. The pre-funded and common warrants have a combined fair value of approximately $2.8 million at issuance, and are classified as a derivative warrant liabilities with the offset in additional paid in capital in stockholders’ equity in the Company’s consolidated financial statements (see Note 16 - Warrants).
F-38
On August 11, 2022, the Company closed on an underwritten public offering (the “August 2022 Offering”) utilizing the 2021 Shelf, pursuant to which it sold an aggregate of (i) 1,075,290 shares of its common stock, (ii) and, in lieu of common stock to certain investors that so chose, pre-funded warrants to purchase 87,500 shares of its common stock, and (iii) accompanying warrants to purchase 1,265,547 shares of its common stock. The shares of common stock and the pre-funded warrants were each sold in combination with corresponding common warrants, with one common warrant to purchase one share of common stock for each share of common stock or each pre-funded warrant sold. The combined public offering price for each share of common stock and accompanying common warrant was $8.60, and the combined offering price for each pre-funded warrant and accompanying common warrant was $8.58, which equated to the public offering price per share of the common stock and accompanying common warrant, less the $0.001 per share exercise price of each pre-funded warrant. The pre-funded warrants were exercised in full in August 2022. The common warrants have an exercise price of $8.60 per share of common stock and are exercisable for a period of five years from issuance. The Company raised $10.0 million in gross proceeds through the August 2022 Offering before underwriting fees and other expenses of $0.9 million. The pre-funded and common warrants have a combined fair value of approximately $6.0 million at issuance, and are classified as derivative warrant liabilities with the offset in additional paid in capital in stockholders’ equity in the Company’s consolidated financial statements (See Note 16 – Warrants).
On June 8, 2023, the Company entered into a securities purchase agreement (the “Securities Purchase Agreement”) pursuant to which the Company agreed to issue and sell an aggregate of (i) 1,743,695 shares of the Company’s common stock, (ii) pre-funded warrants in lieu of shares to purchase 430,217 shares of common stock (the “Pre-Funded Warrants”), (iii) accompanying Tranche A Warrants to purchase 2,173,912 shares of common stock, (iv) and accompanying Tranche B Warrants to purchase 2,173,912 shares of common stock in a best-efforts offering (the Tranche B Warrants together with the Tranche A Warrants, the “Common Warrants”). The Common Warrants may be exercised for either shares of common stock or pre-funded warrants to purchase common stock at a future exercise price of $0.0001 per share in the same form as the Pre-Funded Warrant (the “Exchange Warrants”). Each Pre-Funded Warrant will be exercisable for one share of common stock at an exercise price of $0.0001 per share. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The Common Warrants will be immediately exercisable at a price of $1.59 per share (or $1.5899 per Exchange Warrant). The Tranche A Warrants will expire upon the earlier of (i) five years after the date of issuance, and (ii) 30 days following the closing price of the Company’s common stock equaling 200% of the exercise price for at least 40 consecutive trading days. The Tranche B Warrants will expire upon the earlier of (x) five years after the date of issuance, and (y) 30 days following the Company’s achievement of consolidated trailing twelve-month adjusted EBITDA (as defined in the Securities Purchase Agreement) of $12 million. The Company raised $4.0 million in gross proceeds and net proceeds were approximately $3.4 million after deducting offering expenses. The warrants have a combined fair value of approximately $5.0 million at issuance and are classified as derivative warrant liabilities. The resulting offset is recorded in other expense along with the issuance costs of $0.6 million in the consolidated financial statement of operations (See Note 16 – Warrants).
15. Equity Incentive Plans
2023 Equity Incentive Plan. On May 18, 2023, the Company’s stockholders approved the Aytu BioPharma, Inc. 2023 Equity Incentive Plan (the “2023 Equity Incentive Plan”). Prior to the Company’s adoption of the 2023 Equity Incentive Plan, the Company awarded equity incentive grants to its directors and employees under the Aytu BioScience, Inc. 2015 Stock Option and Incentive Plan (“Aytu 2015 Plan”) and the Neos Therapeutics, Inc. 2015 Stock Options and Incentive Plan (“the Neos 2015 Plan”) (collectively the “2015 Plans”). For the 2023 Equity Incentive Plan, the stockholders approved (a) 200,000 new shares, (b) 87,155 shares available for grant under the 2015 Plans be “rolled over” to the 2023 Equity Incentive Plan and (c) any shares that are returned to the company under the 2015 Plans be added to the 2023 Equity Incentive Plan. With the approval of the 2023 Equity Incentive Plan, no additional awards will be granted under the 2015 Plans. All outstanding awards previously granted under previous stock incentive plans will remain outstanding and subject to the terms of the plans. As of June 30, 2023 the Company had 287,155 shares that are available for grant under the 2023 Equity Incentive Plan.
Aytu 2015 Plan. On June 1, 2015, the Company’s stockholders approved the Aytu 2015 Plan, which, as amended in July 2017, provides for the award of stock options, stock appreciation rights, restricted stock, and other
F-39
equity awards. On February 13, 2020, the Company’s stockholders approved an increase to 250,000 total shares of common stock in the Aytu 2015 Plan. The shares of common stock underlying any awards that are forfeited, canceled, reacquired by Aytu prior to vesting, satisfied without any issuance of stock, expire or are otherwise terminated (other than by exercise) under the Aytu 2015 Plan will be added back to the shares of common stock available for issuance under the 2023 Equity Incentive Plan. Stock options granted under this plan have contractual terms of 10 years from the grant date and a vesting period ranging from 3 to 4 years. The restricted stock awards have a vesting period ranging from 4 to 10 years, and the restricted stock units have a vesting period of 4 years.
Neos 2015 Plan. Pursuant to the Neos Acquisition, the Company assumed 3,486 stock options and 1,786 restricted stock units (RSUs) previously granted under Neos plan. Accordingly, on April 19, 2021, the Company registered 5,272 shares of its common stock under the Neos 2015 Plan with the SEC. The terms and conditions of the assumed equity securities will stay the same as they were under the previous Neos plan. The Company allocated costs of the replacement awards attributable to pre- and post-combination service periods. The pre-combination service costs were included in the considerations transferred. The remaining costs attributable to the post-combination service period are being recognized as stock-based compensation expense over the remaining terms of the replacement awards. Stock options granted under this plan have contractual terms of 10 years from the grant date and a vesting period ranging from 1 to 4 years.
Stock Options
During the fiscal year ended June 30, 2023, 49,212 stock options were granted. The weighted-average grant date fair value of options granted during the year ended June 30, 2023 was $4.00. As of June 30, 2023, there was $0.1 million of total unrecognized compensation cost adjusted for estimated forfeitures, related to non-vested stock options granted under the Company’s equity incentive plan. The unrecognized compensation cost is expected to be recognized over a weighted average period of 2.2 years. No options were granted during the fiscal year 2022.
Stock option activity is as follows:
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
Average |
|
|
|
|
Weighted |
|
Remaining |
|
|
|
Number of |
|
Average |
|
Contractual |
|
|
|
Options |
|
Exercise Price |
|
Life in Years |
|
Outstanding June 30, 2022 |
|
3,899 |
|
$ |
209.70 |
|
7.77 |
Granted |
|
49,212 |
|
|
4.00 |
|
|
Forfeited/Cancelled |
|
(172) |
|
|
128.99 |
|
|
Expired |
|
(177) |
|
|
131.39 |
|
|
Outstanding at June 30, 2023 |
|
52,762 |
|
$ |
18.37 |
|
9.06 |
|
|
|
|
|
|
|
|
Exercisable at June 30, 2023 |
|
3,022 |
|
$ |
225.74 |
|
6.17 |
The following table details the options outstanding at June 30, 2023 by range of exercise prices:
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
|
|
|
|
|
Remaining |
|
|
|
|
|
|
|
|
|
|
Weighted |
|
Contractual |
|
|
|
|
|
|
Range of |
|
Number of |
|
Average |
|
Life of |
|
Number of |
|
Weighted |
|||
Exercise |
|
Options |
|
Exercise |
|
Options |
|
Options |
|
Average |
|||
Prices |
|
Outstanding |
|
Price |
|
Outstanding |
|
Exercisable |
|
Exercise Price |
|||
$ |
4.00 |
|
49,212 |
|
$ |
4.00 |
|
9.26 |
|
— |
|
$ |
— |
$ |
123.16 - 290.00 |
|
3,550 |
|
$ |
217.52 |
|
6.26 |
|
3,022 |
|
$ |
225.74 |
|
|
|
52,762 |
|
$ |
18.37 |
|
9.06 |
|
3,022 |
|
$ |
225.74 |
F-40
Restricted Stock
During the year ended June 30, 2023, as a result of the change in members of the Company’s board, the Company accelerated unvested shares for two former members and recorded $1.5 million of non-cash equity compensation expense.
On December 19, 2022, the Company entered into a Stipulation of Compromise and Settlement (the “Stipulation”). As a part of the terms of the Stipulation, the Company agreed to rescind 25% of the aggregate 2021 grants to board members. As a result of the recission of the shares, the Company recorded $0.6 million in non-cash compensation during the year ended June 30, 2023.
During the year ended June 30, 2023, the Company granted a total of 6,825 shares of restricted stock, with certain accelerated vesting conditions, to members of its management team pursuant to the Aytu 2015 Plan, of which 1/3 vest on the grant date and 1/12 on the first day of each quarter thereafter, subject to continuing employment with the Company through each vesting date. These restricted stock grants have a grant date fair value ranging from $3.31 per-share to $13.4 per-share.
Restricted stock activity under the Aytu 2015 Plan is as follows:
|
|
|
|
Weighted |
|
|
|
|
|
Average Grant |
|
|
|
Number of |
|
Date Fair |
|
|
|
Shares |
|
Value |
|
Unvested at June 30, 2022 |
|
80,373 |
|
$ |
148.91 |
Granted |
|
6,825 |
|
|
3.79 |
Vested |
|
(42,434) |
|
|
126.98 |
Forfeited/Cancelled |
|
(6,689) |
|
|
135.66 |
Unvested at June 30, 2023 |
|
38,075 |
|
$ |
142.20 |
As of June 30, 2023, there was $3.6 million of total unrecognized compensation costs adjusted for estimated forfeitures, related to non-vested restricted stock granted under the Company’s equity incentive plan. The unrecognized compensation cost is expected to be recognized over a weighted average period of 2.0 years. The total fair value of restricted stock vested during the year ended June 30, 2023 was $0.2 million.
The Company previously issued 4 shares of restricted stock outside of the Aytu 2015 Plan, which vest in July 2026. On January 17, 2022, the Company granted 5,000 shares of restricted stock to a member of its management team outside of the Aytu 2015 Plan, of which 1/3 vest on January 17, 2023 and 1/12 each quarter thereafter, subject to continuing employment with the Company through each vesting date until January 17, 2025. This restricted stock grant has a grant date fair value of $27.00 per-share. As of June 30, 2023, there was $0.4 million total unrecognized costs adjusted for estimated forfeitures, related to non-vested restricted stock outside of the Company’s equity incentive plan. The unrecognized compensation cost is expected to be recognized over a weighted average period of 1.56 years.
Restricted Stock Units
For the year ended June 30, 2023, the Company did not grant restricted stock units (“RSU”). RSU activity is as follows:
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
Average Grant |
|
|
|
Number of |
|
Date Fair |
|
|
|
Shares |
|
Value |
|
Unvested at June 30, 2022 |
|
8,500 |
|
$ |
25.88 |
Vested |
|
(3,537) |
|
|
26.26 |
Unvested at June 30, 2023 |
|
4,963 |
|
$ |
25.62 |
F-41
As of June 30, 2023, there was $0.1 million of total unrecognized compensation costs adjusted for estimated forfeitures, related to non-vested RSUs granted under the Company’s equity incentive plans. The unrecognized compensation cost is expected to be recognized over a weighted average period of 1.6 years. The total fair value of RSUs vested during the year ended June 30, 2023 was immaterial.
Stock-based compensation expense related to the fair value of stock options, restricted stock and RSUs was included in the consolidated statements of operations as set forth in the below table:
|
|
Year Ended |
||||
|
|
June 30, |
||||
|
|
2023 |
|
2022 |
||
|
|
(In thousands) |
||||
Cost of sales |
|
$ |
28 |
|
$ |
31 |
Research and development |
|
|
30 |
|
|
536 |
Selling and marketing |
|
|
23 |
|
|
24 |
General and Administrative |
|
|
5,965 |
|
|
4,657 |
Total stock-based compensation expense |
|
$ |
6,046 |
|
$ |
5,248 |
16. Warrants
Liability Classified Warrants
The Company accounts for liability classified warrants by recording the fair value of each instrument in its entirety and recording the fair value of the warrant derivative liability. The fair value of liability classified derivative financial instruments was calculated using either the Black-Scholes option pricing model or the Monte Carlo simulation valuation model, and is revalued every quarter. Changes in the fair value of liability classified derivative financial instruments in subsequent periods are recorded as unrealized derivative gain or loss in the consolidated statements of operations.
On June 8, 2023, the Company entered into a securities purchase agreement (the “Security Purchase Agreement”) pursuant to which the Company agreed to issue and sell an aggregate of (i) 1,743,695 shares of the Company’s common stock, (ii) pre-funded warrants in lieu of shares to purchase 430,217 shares of common stock (the “Pre-Funded Warrants”), (iii) accompanying Tranche A Warrants to purchase 2,173,912 shares of common stock, (iv) and accompanying Tranche B Warrants to purchase 2,173,912 shares of common stock in a best-efforts offering (the Tranche B Warrants together with the Tranche A Warrants, the “Common Warrants”). The Common Warrants may be exercised for either shares of common stock or pre-funded warrants to purchase common stock at a future exercise price of $0.0001 per share in the same form as the Pre-Funded Warrant (the “Exchange Warrants”). Each Pre-Funded Warrant will be exercisable for one share of common stock at an exercise price of $0.0001 per share. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The Common Warrants will be immediately exercisable at a price of $1.59 per share (or $1.5899 per Exchange Warrant). The Tranche A Warrants will expire upon the earlier of (i) five years after the date of issuance, and (ii) 30 days following the closing price of the Company’s common stock equaling 200% of the exercise price for at least 40 consecutive trading days. The Tranche B Warrants will expire upon the earlier of (x) five years after the date of issuance, and (y) 30 days following the Company’s achievement of consolidated trailing twelve-month adjusted EBITDA (as defined in the Security Purchase Agreement) of $12 million (see Note 14 – Stockholders’ Equity).
On August 11, 2022, the Company closed on the August 2022 Offering, pursuant to which, the Company issued pre-funded warrants to purchase 87,500 shares of its common stock and common warrants to purchase 1,265,547 shares of its common stock. The shares of common stock and the pre-funded warrants were each sold in combination with corresponding common warrants, which one common warrant to purchase one share of common stock for each share of common stock or each pre-funded warrant sold. The pre-funded warrants had an exercise price of $0.02 per share of common stock and were exercised in full in August 2022. The common warrants have an exercise price of $8.60 per share of common stock and are exercisable for a period of five years from issuance.
F-42
The common warrants provide that if there occurs any a stock split, stock dividend stock recapitalization, or similar event (a “Stock Combination Event”), then the warrant exercise price will be adjusted to the greater of the quotient determined by dividing (x) the sum of the VWAP of the common stock for each of the five lowest trading days during the 20 consecutive trading day period ending immediately preceding the 16th trading day after such Stock Combination Event, divided by (y) five; or $2.32 and the number of shares of common stock to be issued would be adjusted proportionately as set forth in the agreement limited to a maximum of 2,325,581 shares. The common warrants also provide that in the event the Company were to engage in an equity offering at a common stock price lower than the warrant exercise price prior to the second anniversary of a Stock Combination Event, the exercise price would be adjusted to the greater of the effective price of such equity offering or $2.32 (see Note 14 – Stockholders’ Equity).
In November 2022 and throughout the quarter ended December 31, 2022, the Company sold shares through its ATM Sales Agreement. Per the warrant agreement in the August 2022 Offering, these sales qualified as an equity offering and the sales price was less than the current exercise price of $8.60. As a result, the associated common warrants exercise price was adjusted to $3.30. On January 6, 2023, the Company consummated a 20 to 1 reverse stock split. Pursuant to the aforementioned warrant agreement, the Company triggered a Stock Combination Event and the warrant exercise price and number to be issued was adjusted based on the average of each of the lowest five trading days during the twenty-day consecutive trading day period beginning on December 30, 2022. Subsequently, as a result of the Securities Purchase Agreement in June 2023, the common warrants from the August 2022 Offering had an adjusted exercise price of $2.32.
On March 7, 2022, the Company closed on an underwriting agreement, pursuant to which, the Company sold, (i) 151,500 shares of the Company’s common stock, (ii) pre-funded warrants to purchase up to 151,500 shares of common stock, and (iii) common warrants to purchase up to 333,300 shares of common stock. The shares of common stock and the pre-funded warrants were each sold in combination with corresponding common warrants, with one common warrant to purchase 1.1 shares of common stock for each share of common stock or each pre-funded warrant sold. The pre-funded warrants have an exercise price of $0.002 per share of common stock and were exercised in full in April 2022. The common warrants have an exercise price of $26.00 per share of common stock and are exercisable six months after the date of issuance and have a term of five years from the date of exercisability (see Note 14 – Stockholders’ Equity).
On January 26, 2022, as consideration for entering into the Avenue Capital Agreement as described in Note 11 – Long-term Debt, the Company issued warrants to the Avenue Capital Lenders to purchase shares of common stock at an exercise price equal to $24.20 per share (the “Avenue Capital Warrants”). The Avenue Capital Warrants provided that in the event the Company were to engage in an equity offering at a price lower than $24.20 prior to June 30, 2022, the exercise price would be adjusted to the effective price of such equity offering and the number of shares of common stock to be issued under the Avenue Capital Warrants would be adjusted as set forth in the agreement. The Avenue Capital Warrants were immediately exercisable and expire on January 31, 2027. At inception, the Company accounted for the Avenue Capital Warrants as a derivative warrant liability as the number of warrants was not fixed at the issuance (see Note 11 – Long-term Debt for further details).
Outstanding warrants are classified as derivative warrant liabilities in the consolidated balance sheets and are marked to market at each reporting period (see Note 12 – Fair Value Considerations).
F-43
A summary of warrants is as follows:
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
Average |
|
|
|
|
Weighted |
|
Remaining |
|
|
|
Number of |
|
Average |
|
Contractual |
|
|
|
Warrants |
|
Exercise Price |
|
Life in Years |
|
Outstanding June 30, 2022 |
|
434,328 |
|
$ |
92.60 |
|
4.8 |
Warrants issued |
|
6,028,331 |
|
|
1.61 |
|
5.0 |
Warrants exercised |
|
(87,500) |
|
|
0.02 |
|
5.0 |
Warrant adjusted |
|
181,461 |
|
|
5.04 |
|
3.9 |
Warrants expired |
|
(18,568) |
|
|
2,011.56 |
|
— |
Outstanding June 30, 2023 |
|
6,538,052 |
|
$ |
4.42 |
|
4.71 |
17. Employee Benefit Plan
Subsequent to the merger with Neos, Aytu had two 401(k) plans the (“Neos Plan”) and the (“Aytu Plan’) both plans allow participants to contribute a portion of their salary, subject to eligibility requirements and annual IRS limits. The Neos Plan matched 100% of the first 3% contributed by employees and matched 50% on the next 4% and 5% contributed by the employees. The Company’s match for the Neos Plan was approximately $0.4 million for the year ended June 30, 2022. The Aytu Plan matched 50% of the first 6% contributed to the plan by employees. The Company’s match for the Aytu Plan was approximately $0.2 million for both years ended June 30, 2023 and 2022. In July 2022, the Company transferred the Neos Plan into the Aytu BioPharma Employee Retirement Plan and in February 2023 the Company transferred the Aytu Plan into the Aytu BioPharma Employee Retirement Plan. The Aytu BioPharma Employee Retirement Plan matches 100% of the first 3% contributed by employees and matches 50% of the next 4% and 5% contributed by the employees. The Company’s match for the Aytu BioPharma Employee Retirement Plan was approximately $0.7 million during the year ended June 30, 2023.
18. Commitments and Contingencies
Pediatric Portfolio Fixed Payments and Product Milestone
The Company assumed two fixed, periodic payment obligations to an investor (the “Fixed Obligation”). Under the first fixed obligation, the Company was to pay monthly payment of $0.1 million beginning November 1, 2019 through January 2021, with a balloon payment of $15.0 million that was to be due in January 2021 (“Balloon Payment Obligation”). A second fixed obligation requires the Company pay a minimum of $0.1 million monthly through February 2026, except for $0.2 million paid in January 2020.
On May 29, 2020, the Company entered into an Early Payment Agreement and Escrow Instruction (the “Early Payment Agreement”) pursuant to which the Company agreed to pay $15.0 million to the investor in satisfaction of the Balloon Payment Obligation. The parties to the Early Payment Agreement acknowledged and agreed that the remaining fixed payments other than the Balloon Payment Obligation remained due and payable pursuant to the terms of the Agreement, and that nothing in the Early Payment Agreement alters, amends, or waives any provisions or obligations in the Waiver or the Investor agreement other than as expressly set forth therein. The first fixed obligation was fully paid as of January 2021.
On June 21, 2021, the Company entered into a Waiver, Release and Consent pursuant to which the Company paid $2.8 million to the investor in satisfaction of the second fixed obligation. The company agreed to pay the remaining fixed obligation of $3.0 million in six equal quarterly payments of $0.5 million over the next six quarters commencing September 30, 2021. The Company accounted for the Waiver, Release and Consent as a debt and remeasured the related liabilities using a discounted cash flow model. This fixed payment arrangement was paid in full by January 2023.
F-44
The Company acquired a Supply and Distribution Agreement with Tris (the “Tris Karbinal Agreement”), under which the Company is granted the exclusive right to distribute and sell the product in the United States. The initial term of the Tris Karbinal Agreement was 20 years. The Company will pay Tris a royalty equal to 23.5% of net sales.
The Tris Karbinal Agreement also contains minimum unit sales commitments, which is based on a commercial year that spans from August 1 through July 31, of 70,000 units annually through 2025. The Company is required to pay Tris a royalty make whole payment of $30 for each unit under the 70,000-unit annual minimum sales commitment through 2025. The Karbinal Agreement make-whole payment is capped at $2.1 million each year. The annual payment is due in August of each year. The Karbinal Agreement also has multiple commercial milestone obligations that aggregate up to $3.0 million based on cumulative net sales, the first of which is triggered at $40.0 million of net revenues.
Prior to June 30, 2022, the Company’s contingent consideration liabilities included obligations under licensing arrangements for Tuzistra XR. The royalty and make-whole milestone payments related to licensing agreements with TRIS Pharma, Inc. (“Tris”) for Tuzistra XR were being accounted for as contingent consideration and revalued at each reporting period. As a result of the discontinuation of commercializing Tuzistra (see Note 3 – Revenue from Contracts with Customers) and a settlement agreement with Tris, the Company concluded that the product milestone payments underlying the contingent consideration liability ceased to exist. The Company reversed the remaining contingent consideration liabilities of $8.5 million and recorded a liability of $7.6 million related to the settlement payments payable to Tris for termination of the Tuzistra licensing agreement. The settlement payments are included in fixed payment arrangements at their present value using the Company’s estimated borrowing rate. The Company recognized $0.9 million gain on settlement of the Tris contingent consideration liabilities in the consolidated statements of operations for the year ended June 30, 2022.
Product Contingent Liability
In February 2015, Innovus acquired Novalere, which included the rights associated with distributing FlutiCare. As part of the Merger, Innovus is obligated to make five additional payments of $0.5 million when certain levels of FlutiCare sales are achieved. In fiscal year 2023, the manufacturer associated with this contingent liability filed for bankruptcy. There were no payments required in fiscal 2023.
Rumpus Earn Out Payments
On April 12, 2021, the Company acquired substantially all of the assets of Rumpus, pursuant to which the Company acquired certain rights and other assets, including key commercial global licenses with Denovo Biopharma LLC (“Denovo”) and Johns Hopkins University (“JHU”), relating to AR101. Upon the achievement of certain regulatory and commercial milestones, up to $67.5 million in earn-out payments, which are payable in cash or shares of common stock, generally at the Company’s option, are payable to Rumpus. Under the license agreement with Denovo, the Company assumed the responsibility for paying annual maintenance fees of $25,000, a license option fee of $0.6 million payable in April 2022, and upon the achievement of certain regulatory and commercial milestones, up to $101.7 million, and escalating royalties based on net product sales ranging in percentage from the low teens to the high teens. Finally, under the license agreement with Johns Hopkins, the Company assumed the responsibility for paying minimum annual royalties escalating from $5,000 to $20,000 beginning in calendar year 2022, royalties of 3.0% of net product sales, and upon the achievement of certain regulatory and commercial milestones, up to $1.6 million.
During the year ended June 30, 2022, AR101 received Orphan Drug Designation (“ODD”) and Fast Track designation from the FDA, resulting in total milestone payments of $4.0 million, which were paid in 109,447 shares of common stock and $2.6 million in cash.
Operating Lease
In May 2023, the Company entered into an operating lease agreement to relocate its principal office within Denver, Colorado. The lease has a commencement date of October 1, 2023 with an initial term of five and a half years. Undiscounted minimum monthly rent payments average approximately $15,500 over the initial term of the lease.
F-45
Variable lease payments will be expensed as incurred. Under the lease agreement, the Company has one five-year renewal option through March 2034.
Legal Matters
Witmer Class-Action Securities Litigation. A shareholder derivative suit was filed on September12, 2022 in the Delaware Chancery Court by Paul Witmer, derivatively and on behalf of all Aytu stockholders, against Armistice Capital, LLC, Armistice Capital Master Fund, Ltd., Steve Boyd (Armistice’s Chief Investment Officer and Managing Partner, and a former director of Aytu), and certain other current and former directors of Aytu, Joshua Disbrow, Gary Cantrell, John Donofrio, Jr., Michael Macaluso, Carl Dockery and Ketan B. Mehta. Plaintiff amended the complaint on April 5, 2023. The Amended Complaint drops Mr. Macaluso as a defendant and alleges that (i) Armistice facilitated the sale of assets of Cerecor in 2019 and Innovus in 2020 to Aytu in exchange for convertible securities which it subsequently converted and sold at a profit on the open market; (ii) the Armistice defendants breached their fiduciary duties, were unjustly enrichment and wasted corporate assets in connection with these acquisitions; (iii) the Armistice defendants breached their fiduciary duties by engaging in as insider trading; and (iv) the other directors breached their fiduciary duties, and aided and abetted the Armistice defendants breaches of fiduciary duties, in connection with these acquisitions. The Amended Complaint seeks unspecified damages, equitable relief, restitution, disgorgement of profits, enhanced governance and internal procedures, and attorneys’ fees. While we believe that this lawsuit is without merit and have vigorously defended against it, we have agreed to settle the matter for various corporate governance modifications and the payment of plaintiff’s attorneys’ fees.
Sabby Litigation. A complaint was filed on February 22, 2023 in the Supreme Court of the State of New York by Sabby Volatility Warrant Master Fund LTD (“Sabby”) and Walleye Opportunities Master Fund Ltd (“Walleye”), holders of certain warrants to purchase common stock, against the Company. The complaint alleges that the Company improperly adjusted the exercise price of the warrants and miscalculated the number of shares the warrantholders may receive, and that the Company failed to provide prompt notice to the warrantholders of such adjustment. The complaint seeks a declaratory judgment of the warrant share calculation, that 575,000 warrant shares be due to Sabby on exercise of its warrants rather than 312,908 shares, and that 100,000 warrant shares be due to Walleye on exercise of its warrants rather than 54,146 shares. While we believe that this lawsuit is without merit and we intend to vigorously defend against it, we are not able to predict at this time whether this proceeding will have a material impact on our financial condition or results of operations.
Stein Litigation. Cielo Stein (“Stein”), a former sales specialist, filed a complaint on February 1, 2023 in Jefferson County Circuit Court in Kentucky against the Company and its wholly-owned subsidiary Neos Therapeutics. The complaint alleges that Aytu retaliated against Stein in violation of the Kentucky Civil Rights Act after she opposed what she contends was unwelcome behavior by her supervisor. The complaint also alleges that the Company’s response to Stein’s subsequent complaint to human resources was inadequate. The complaint seeks an award of unspecified compensatory damages, emotional-distress damages, and attorneys’ fees and costs. The Company removed the lawsuit to the United States District Court for the Western District of Kentucky and filed a motion to dismiss the complaint, which is pending. Due to the early stage of litigation, we are not able to predict at this time whether this proceeding will have a material impact on our financial condition or results of operations, and intend to vigorously defend this case in the event it is not dismissed.
19. License Agreements
Healight
In April 2020, the Company entered into a licensing agreement with Cedars-Sinai Medical Center to secure worldwide rights to various potential esophageal and nasopharyngeal uses of Healight, an investigational medical device platform technology. The agreement with Cedars-Sinai grants the Company a license to all patent and development related technology rights for the intra-corporeal therapeutic use of ultraviolet light in the field of endotracheal and nasopharyngeal applications. The term of the agreement is on a country-by-country basis and will expire on the latest of the date upon which the last to expire valid claim shall expire, ten years after the first bona fide commercial sale of such licensed product in a country, or the expiration of any market exclusivity period granted by a regulatory agency.
F-46
Pursuant to the terms of the agreement, the Company paid an initial $0.3 million license fee and approximately $0.1 million in earlier patent prosecution fees.
As a result of the Company’s focus on the revenue growth of its commercial business, the Company had terminated the licensing agreement with Cedars-Sinai Medical Center, effective May 9, 2023.
NeuRx
In October 2018, Neos entered into an Exclusive License Agreement (“NeuRx License”) with NeuRx Pharmaceuticals LLC (“NeuRx”), pursuant to which NeuRx granted Neos an exclusive, worldwide, royalty-bearing license to research, develop, manufacture, and commercialize certain pharmaceutical products containing NeuRx’s proprietary compound designated as NRX-101, referred to by Neos as NT0502. NT0502 is a new chemical entity that is being developed by Neos for the treatment of sialorrhea, which is excessive salivation or drooling. The Company may be required to make certain development and milestone payments and royalties based on annual net sales, as defined in the NeuRx License. Royalties are to be paid on a country-by-country and licensed product-by-licensed product basis, during the period of time beginning on the first commercial sale of such licensed product in such country and continuing until the later of: (i) the expiration of the last-to-expire valid claim in any licensed patent in such country that covers such licensed product in such country; and/or (ii) expiration of regulatory exclusivity of such licensed product in such country.
In April 2023, the Company returned the NT0502 rights to NeuRx in exchange for, and to receive a royalty and potential milestone payments on amounts received for future revenue generated by NeuRx (or a future licensee) on NT0502.
Teva
On December 21, 2018, Neos and Teva Pharmaceuticals USA, Inc. (“Teva”) entered into an agreement granting Teva a non-exclusive license to certain patents owned by Neos by which Teva has the right to manufacture and market its generic version of Cotempla XR-ODT under an Abbreviated New Drug Application (“ANDA”) filed by Teva beginning on July 1, 2026, or earlier under certain circumstances.
Actavis
On October 17, 2017, Neos entered into an agreement granting Actavis a non-exclusive license to certain patents owned by Neos by which Actavis has the right to manufacture and market its generic version of Adzenys XR-ODT under its ANDA beginning on September 1, 2025, or earlier under certain circumstances.
Shire
In July 2014, Neos entered into a Settlement Agreement and an associated License Agreement (the “2014 License Agreement”) with Shire LLC (“Shire”) for a non-exclusive license to certain patents for certain activities with respect to Neos’ New Drug Application (the “NDA”) No. 204326 for an extended-release orally disintegrating amphetamine polistirex tablet. In accordance with the terms of the 2014 License Agreement, following the receipt of the approval from the FDA for Adzenys XR-ODT, Neos paid a lump sum, non-refundable license fee of an amount less than $1.0 million in February 2016. Neos is paying a single digit royalty on net sales of Adzenys XR-ODT during the life of the patents. The settlement agreement expires May of 2023.
In March 2017, Neos entered into a License Agreement (the “2017 License Agreement”) with Shire, pursuant to which Shire granted Neos a non-exclusive license to certain patents owned by Shire for certain activities with respect to Neos’ NDA No. 204325 for an extended-release amphetamine oral suspension. In accordance with the terms of the 2017 License Agreement, following the receipt of the approval from the FDA for Adzenys ER, Neos paid an up-front, non-refundable license fee of an amount less than $1.0 million in October 2017. Neos is paying a single digit royalty on net sales of Adzenys ER during the life of the patents. Adzenys ER was discontinued as of September 30, 2021.
F-47
The royalties are recorded as cost of sales in the same period as the net sales upon which they are calculated.
Additionally, each of the 2014 and 2017 License Agreements contains a covenant from Shire not to file a patent infringement suit against Neos alleging that Adzenys XR-ODT or Adzenys ER, respectively, infringes the Shire patents.
20. Segment Information
The Company’s chief operating decision maker (“CODM”), who is the Company’s Chief Executive Officer, allocates resources and assesses performance based on financial information of the Company. The CODM reviews financial information presented for each reportable segment for purposes of making operating decisions and assessing financial performance.
The Company manages and aggregates its operational and financial information in accordance with two reportable segments: Rx and Consumer Health. The Rx Segment consists of the Company’s prescription products. The Consumer Health Segment contains the Company’s consumer healthcare products. For purposes of determining operating income or loss by segment, the Company allocates common expenses such as corporate administration, executive and board compensation, insurance, and fees associated with being a publicly traded entity, among others, to the Rx Segment. The Rx Segment also includes pipeline research and development. The CODM does not regularly review asset information by segment, accordingly, asset information is not provided by segment.
During the year ended June 30, 2023, the Rx Segment recognized an impairment loss of $2.6 million due to ceasing active development of the NT0502 product candidate as a result of the Company’s increased focus on commercial efforts. The Consumer Health Segment recognized an impairment loss of $3.0 million from intangible assets (see Note 7 — Goodwill and Other Intangible Assets) and an inventory write-off of $2.1 million due to the discontinuance of its products.
During the year ended June 30, 2022, the Rx Segment recognized a total impairment loss of $64.6 million related to impairment of goodwill and write-down of assets due to the discontinuance of five non-core products, the Consumer Health Segment recognized $10.8 million of goodwill and intangible assets write downs (see Note 7 — Goodwill and Other Intangible Assets).
Select financial information for these segments is as follows:
(In thousands) |
|
Rx |
|
|
Consumer Health |
|
|
Consolidated |
|
|
|
|
|
|
|
|
|
Year Ended June 30, 2023: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenue, net |
$ |
73,799 |
|
$ |
33,600 |
|
$ |
107,399 |
Loss from operations |
$ |
(7,358) |
|
$ |
(9,707) |
|
$ |
(17,065) |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
$ |
6,271 |
|
$ |
1,116 |
|
$ |
7,387 |
Impairment and write-off expense |
$ |
2,730 |
|
$ |
5,094 |
|
$ |
7,824 |
Stock based compensation |
$ |
5,722 |
|
$ |
324 |
|
$ |
6,046 |
|
|
|
|
|
|
|
|
|
Year Ended June 30, 2022: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenue, net |
$ |
61,121 |
|
$ |
35,548 |
|
$ |
96,669 |
Loss from operations |
$ |
(92,441) |
|
$ |
(17,465) |
|
$ |
(109,906) |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
$ |
7,821 |
|
$ |
1,557 |
|
$ |
9,378 |
Impairment expense |
$ |
64,649 |
|
$ |
10,809 |
|
$ |
75,458 |
Stock based compensation |
$ |
5,190 |
|
$ |
58 |
|
$ |
5,248 |
F-48
21. Subsequent Events
Distribution and Supply Agreement
In July 2023, the Company entered into an exclusive collaboration, distribution and supply agreement with a privately-owned pharmaceutical company for Adzenys XR-ODT and Cotempla XR-ODT product lines. The pharmaceutical company will seek local regulatory approvals and marketing authorizations for both Adzenys XR-ODT and Cotempla XR-ODT; and will focus on distributing and selling these products for patients in Israel and the Palestinian Authority. The Company will commit to product supply based on forecasts and provide product training. Due to the nascency of the collaboration, estimates of its financial effect cannot be made. This agreement represents the Company’s first international commercial agreement for Adzenys and Cotempla.
F-49
Exhibit 10.45
AMENDED AND RE-STATED EMPLOYMENT AGREEMENT
This Amended and Re-Stated Employment Agreement (the "Agreement"), is effective as of February 13, 2023 (the “Effective Date”), between Aytu BioPharma, Inc., a Delaware corporation headquartered at 373 Inverness Parkway, Suite 206, Englewood, CO 80112 USA, hereinafter referred to as the "Company"), and Joshua R. Disbrow (“Employee").
RECITALS
WHEREAS, the Company is a duly organized Delaware corporation, with its principal place of business within the State of Colorado, and is in the business of developing and marketing pharmaceutical products; and
WHEREAS, the Company and Employee originally entered into an Employment Agreement on April 16, 2019, which was amended on July 1, 2020, and again on April 7, 2021; and
WHEREAS, the Company and Employee desire to amend and re-state the Employment Agreement; and
WHEREAS, the Company desires assurance of the continued association and services of the Employee in order to continue to retain the Employee’s experience, skills, abilities, background and knowledge, and is willing to continue to engage the Employee’s services on the terms and conditions set forth in this Agreement; and
WHEREAS, Employee desires to be in the continued employ of the Company, and is willing to accept such continued employment on the terms and conditions set forth in this Agreement.
NOW, THEREFORE, the parties hereto agree to the terms and conditions of this Agreement as follows:
1. Employment Term. The Company hereby agrees to continue to employ Employee and Employee hereby accepts such continued employment with the Company for a period of 12 months beginning on the Effective Date (the “Continued Term”). Upon the expiration of the Continued Term, the Agreement shall automatically renew for successive terms of 12 months each (with each such successive term constituting a “Renewal Term,” together with the Continued Term, the “Term”), unless terminated in accordance with the provisions of the Agreement. The termination of Employee's employment under the Agreement shall end the Term but shall not terminate Employee’s or the Company’s other obligations that are intended to survive the termination of this Agreement (including without limitation, the payments under Section 7 and 8 and Employee’s obligations under Section 9).
2. Position and Duties. During the Term, Employee shall serve as Chairman of the Board (Chairman) and Chief Executive Officer (CEO) of the Company, and perform such duties as are consistent with this position. The Employee shall report to the Board of Directors of the Company. During the Term, Employee shall also hold such additional positions and titles as the Board of Directors of the Company (the "Board") may determine from time to time. During the Term, Employee shall devote as much time as is necessary to satisfactorily perform his duties as CEO of the Company. Employee may engage in any civic and not-for-profit activities so long as such activities do not materially interfere with the performance of his duties hereunder or present a conflict of interest with the Company During the Term of this Agreement, Employee agrees not to acquire, assume or participate in, directly or indirectly, any position, investment or interest known by the Employee to be adverse or antagonistic to the Company, its business or prospects, its financial position, or otherwise or in any company, person or entity that is, directly or indirectly, in competition with the business of the Company or any of its affiliates. This provision shall encompass any advisory boards of which Employee is or becomes a member of during the term hereof.
1
Employee shall provide written disclosure to the Compensation Committee of the Company’s Board of Directors as to all advisory boards on which Employee sits, and will provide the Company with written notice within 10 business days of Employee agreeing to sit on any additional advisory boards. On termination of Employee’s employment, regardless of the reason for such termination, Employee shall immediately (and with contemporaneous effect) resign any directorships, offices or other positions that Employee may hold in the Company or any affiliate, unless otherwise agreed in writing by the parties.
3. Compensation.
(a) Base Salary. The Company shall pay Employee a base salary of $590,000 per annum, payable at least monthly on the Company's regular pay cycle for professional employees (the “Base Salary”). Except as specifically otherwise provided herein, the Base Salary may be increased only by recommendation of the Compensation Committee of the Board and ratified by the Compensation Committee or a majority of the independent members of the Board.
(b) Annual Review. The Base Salary shall be reviewed at the end of each fiscal year (the first such review to occur at the end of fiscal year 2020).
(c) Equity Compensation. Employee acknowledges that, pursuant to his original Employment Agreement dated April 16, 2019, the First Amendment to the Employment Agreement dated July 1, 2020, and the Second Amendment to the Employment Agreement dated April 7, 2021, Employee received equity and restricted shares, as well as a one-time cash payment in lieu of additional equity. Employee continues to participate in the Stock Incentive Plan and his Awards under the Stock Incentive Plan are governed by the terms thereof.
(d) Other and Additional Compensation. Subsections (a) and (c) above establish Employee’s compensation during the Term which shall not preclude the Board from awarding Employee a higher salary or any bonuses or stock options, restricted stock or other forms of additional equity awards in the discretion of the Board during the Term at any time. The Employee shall be eligible for an annual discretionary bonus (hereinafter referred to as the “Bonus”) with a target amount of sixty percent (60%) of the Base Salary, subject to standard deductions and withholdings, based on the Compensation Committee’s determination, in good faith, and based upon the Employee’s individual achievement and company performance objectives as set by the Board or the Compensation Committee, of whether the Employee has met such performance milestones as are established for the Employee by the Board or the Compensation Committee, in good faith, in consultation with the Employee (hereinafter referred to as the “Performance Milestones”). The Performance Milestones will be based on certain factors including, but not limited to, the Employee’s performance and the Company’s financial and operational performance. The Employee’s Bonus target will be reviewed annually and may be adjusted by the Board or the Compensation Committee in its discretion, provided however, that the Bonus target may only be reduced upon Employee’s written consent. The Employee must be employed on the date the Bonus is awarded to be eligible for the Bonus, subject to the termination provisions hereof. Bonuses shall be paid during the calendar quarter following the calendar quarter for which such Bonus was earned when Performance Milestones are met during a calendar quarter. Fourth quarter Bonuses and Bonuses calculated on the basis of partial Performance Milestone satisfaction shall be paid within 75 days of fiscal year-end.
4. Employee Benefits. During the Term, Employee shall be entitled to participate at the same level as other senior executive officers of the Company in any group insurance, hospitalization, medical, health and accident, disability, fringe benefit and tax-qualified retirement plans or programs of the Company now existing or hereafter established to the extent that he is eligible under the general provisions thereof.
2
For the term of this Agreement, Employee shall be entitled to paid time off at the rate of (5) weeks per annum. In accordance with Company policy, unused paid time off may not be carried over from year to year.
5. Expenses. The Company shall reimburse Employee for actual, reasonable out-of-pocket expenses incurred by him in the performance of his services for the Company upon the receipt of appropriate documentation of such expenses which shall be submitted in such form, and with such supporting documentation, as called for or required by Company policy.
6. Termination.
(a) General. The Term shall end immediately upon Employee's death. Employee’s employment may also be terminated by the Company with or without Cause or as a result of Employee’s Disability, as defined in Section 7 or by Employee with or without Good Reason (as such terms are defined below).
(b) Notice of Termination. Either party shall give written notice of termination to the other party.
(c) Notification of New Employer. In the event that Employee leaves the employ of the Company, Employee grants consent to notification by the Company to Employee’s new employer about his rights and obligations under this Agreement and the PIA (hereinafter defined).
7. Severance Benefits.
(a) Cause Defined. “Cause” means (i) willful malfeasance or willful misconduct by Employee in connection with his employment; (ii) Employee’s conviction of, or entry of a plea of guilty to, or entry of a plea of nolo contendere with respect to, any crime other than a traffic violation or infraction which is a misdemeanor; (iii) Employee’s willful and deliberate violation of a Company policy, (iv) Employee's unintended but material breach of any written policy applicable to all employees adopted by the Company which is not cured to the reasonable satisfaction of the Board of Directors within thirty (30) business days after notice thereof; (v) the Employee’s unauthorized use or disclosure of any proprietary information or trade secrets of the Company or any other party as to which the Employee owes an obligation of nondisclosure as a result of the Employee’s relationship with the Company, or (vi) the Employee’s willful and deliberate breach of his obligations under this Agreement..
(b) Disability Defined. "Disability" shall mean (i) Employee's incapacity due to a physical or mental condition and, if reasonable accommodation is required by law, after any reasonable accommodation, that results in Employee being substantially unable to perform his duties hereunder for six consecutive months (or for six months out of any nine month period) or (ii) a qualified independent physician mutually acceptable to the Company and Employee determines that Employee is incapacitated due to a physical or mental condition and, if reasonable accommodation is required by law, after any reasonable accommodation so as to be unable to regularly perform the duties of his position and such condition is expected to be of a permanent or near-permanent duration. Until such time as Employee is terminated for Disability under this paragraph (b), Employee shall continue to receive his Base Salary hereunder, provided that if the Company provides Employee with disability insurance coverage, payments of Employee's Base Salary shall be reduced by the amount of any disability insurance payments received by Employee due to such coverage. The Company shall give Employee written notice of termination due to Disability which shall take effect sixty (60) days after the date it is sent to Employee unless Employee shall have returned to the performance of his duties hereunder during such sixty (60) day period (whereupon such notice shall become void).
3
In the event that the Company terminates Employee’s employment as a result of his Disability, Employee shall be entitled to the same benefits as if his employment had been terminated by the Company without Cause.
(c) Good Reason Defined. For purposes of this Agreement. “Good Reason” shall mean: : (i) there is a material reduction of the level of Employee’s compensation (excluding any bonuses) (except where there is a general reduction applicable to the management team generally, provided, however, that in no case may the Base Salary be reduced below the amount stated in Section 3(a)), (ii) there is a material reduction in Employee’s overall responsibilities or authority, or scope of duties (it being understood that the occurrence of a Change in Control shall not, by itself, necessarily constitute a reduction in Employee’s responsibilities or authority); or (iii) without Employee’s written consent, a material change in the principal geographic location at which Employee must perform his services (it being understood that the relocation of Employee to a facility or a location within forty (40) miles of the State Capitol Building in Denver, Colorado shall not be deemed material for purposes of this Agreement). No event shall be deemed to be “Good Reason” if the Company has cured the event (if susceptible to cure) within 30 days of receipt of written notice from Employee specifying the event or events which, absent cure, would constitute “Good Cause.”
(d) Accrued Compensation Defined. Accrued Compensation shall mean an amount which shall include all amounts earned or accrued by Employee through the date of termination of this Agreement but not paid as of such date, including (i) Base Salary, (ii) reimbursement for business expenses incurred by the Employee on behalf of the Company, pursuant to the Company’s expense reimbursement policy in effect at such time, (iii) any expense allowance pursuant to Company policy, (iv) accrued but unused vacation pay per Company policy, and (v) bonuses and incentive compensation earned and awarded prior to the date of termination. Accrued Compensation shall be paid on the first regular pay date after the date of termination (or earlier, if required by applicable law).
(e) Termination.
(i) Cause; Without Good Reason; Death; Disability. If the Company ends the Term for Cause, if Employee resigns as an employee of the Company for reasons other than an event of Good Reason, the Employee dies or Disability occurs , then the Company shall pay to Employee the Accrued Compensation but shall have no obligation to pay Employee any amount, whether for salary, benefits, bonuses, or other compensation or expense reimbursements of any kind, accruing after the end of the Term, and such rights shall, except as otherwise required by law or pursuant to the applicable award agreement or plan, be forfeited immediately upon the end of the Term. For the sake of clarity, any stock options, restricted stock or other equity compensation shall, to the extent vested on the date of resignation without Good Reason, the date the Company ends the Term for Cause, or the date of Employee’s death, remain outstanding and exercisable to the extent provided in the applicable award agreement or plan, by the Employee or his personal representative or executor. For the avoidance of doubt, Employee shall receive the payments specified in this Section 7(e)(i) and shall not be entitled to any other compensation that would have been made for the remainder of a Continued Term or Renewal Term or payments under any other severance plan. For example, if the Employment Period has been automatically renewed for twelve (12) months starting April 7, 2025 and Employee resigns as an employee of the Company for reasons other than an event of Good Reason on July 7, 2025, Employee shall not be entitled to receive payments for the period of July 7, 2025 through April 7, 2026; Employee will receive only the payments noted in this Section 7(e)(i). In no case shall Employee be entitled to payment for any period Employee is not performing services under this Agreement beyond the payments provided for under this Section 7(e)(i).
4
(ii) Without Cause; Good Reason. In the event that the Company terminates Employee’s employment hereunder without Cause, or the Employee terminates his employment with Good Reason, he shall be entitled to the Accrued Compensation and, subject to Section 21 and 22 below,
(A) A lump sum payment equal to two and one half (2.5) times his Base Salary in effect at the date of termination, less applicable withholding.
(B) Continued participation (via state or federal insurance continuation laws such as COBRA, to the extent available) in the health and welfare plans (or comparable plans, if continued participation in the Company’s plans is not available) provided by the Company to Employee at the time of termination for a period of two years from the date of termination or, if earlier, until he is eligible for comparable coverage with a subsequent employer. The Company agrees to reimburse the payments Employee makes for such coverage, whether via continuation or separate comparable policy. Premium reimbursements shall be made by the Company to Employee consistent with the Company’s normal expense reimbursement policy, provided that Employee submits documentation to the Company substantiating his payments for insurance coverage. Employee shall give the Company prompt notice of his eligibility for comparable coverage.
(C) Any severance payments and/or other separation benefits contemplated by this Agreement are conditional on Employee: (i) continuing to comply with the terms of this Agreement and the PIA (as defined herein); (ii) delivering prior to or contemporaneously with any such severance payments, and not revoking, (x) a customary general release of claims relating to Employee’s employment and/or this Agreement against the Company or its successor, its subsidiaries and their respective directors, officers and stockholders and (y) a customary affirmation of Employee’s continuing obligations hereunder and under the PIA.
(D) In the event of a termination Without Cause or Change in Control, Employee shall be paid a pro-rata amount of the target bonus determined by the percentage of time Employee was employed during the fiscal year.
(E) For the avoidance of doubt, Employee shall receive the payments specified in this Section 7(e)(ii) and shall not be entitled to any other compensation that would have been made for the remainder of a Continued Term or Renewal Term or payments under any other severance plan. For example, if the Employment Period has been automatically renewed for twelve (12) months starting April 7, 2025 and Employee resigns as an employee of the Company for an event of Good Reason on July 7, 2025, Employee shall not be entitled to receive payments for the period of July 7, 2025 through April 7, 2026; Employee will receive only the payments noted in this Section 7(e)(ii). In no case shall Employee be entitled to payment for any period Employee is not performing services under this Agreement beyond the payments provided for under this Section 7(e)(ii).
Unless otherwise required by law, no severance payments and/or benefits under this Agreement will be paid and/or provided until after the expiration of any relevant revocation period. Subject to the effectiveness of the release, the severance payments shall be paid on the first payroll date that begins 30 days after Employee’s termination of employment.
5
8. Change in Control Payments. The provisions of this paragraph 8 set forth the terms of an agreement reached between Employee and the Company regarding Employee's rights and obligations upon the occurrence of a "Change in Control" (as hereinafter defined) of the Company during the Term. These provisions are intended to assure and encourage in advance Employee's continued attention and dedication to his assigned duties and his objectivity during the pendency and after the occurrence of any such Change in Control. The following provisions shall apply in the event of a Change in Control, in addition to any payment or benefit that may be required pursuant to Section 7.
(a) Equity. If within 12 months after a Change in Control, Employee’s employment is terminated by the Company without Cause or by the Employee for Good Reason, all stock options, restricted stock and other stock-based grants to Employee by the Company or that may be granted in the future shall, irrespective of any provisions of his award agreements, immediately and irrevocably vest and become exercisable and any restrictions thereon shall lapse. All stock options shall remain exercisable from the date of such employment termination until the expiration of the term of such stock options.
(b) Definitions. For purposes of this paragraph 8, the following terms shall have the following meanings:
“Change in Control,” also referred to as a “Sale Event” in the Company’s Restricted Stock Award Agreement, shall mean any of the following:
(i) the sale of all or substantially all of the assets of the Company on a consolidated basis to an unrelated person or entity; or
(ii) a merger, reorganization or consolidation pursuant to which the holders of the Company’s outstanding voting power and outstanding stock immediately prior to such transaction do not own a majority of the outstanding voting power and outstanding stock or other equity interests of the resulting or successor entity (or its ultimate parent, if applicable) immediately upon completion of such transaction; or
(iii) the sale of all of the Stock of the Company to an unrelated person, entity or group thereof acting in concert; or
(iv) any other transaction in which the owners of the Company’s outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the Company or any successor entity immediately upon completion of the transaction other than as a result of a the acquisition of securities directly from the Company.
9. Proprietary Information and Inventions Agreement. As a condition of Employee’s employment with the Company, Employee agrees to sign the Company’s standard form of Proprietary Information and Inventions Agreement (“PIA”).
10. Successors and Assigns.
(a) Employee. This Agreement is a personal contract, and the rights and interests that the Agreement accords to Employee may not be sold, transferred, assigned, pledged, encumbered, or hypothecated by him. All rights and benefits of Employee shall be for the sole personal benefit of Employee, and no other person shall acquire any right, title or interest under this Agreement by reason of any sale, assignment, transfer, claim or judgment or bankruptcy proceedings against Employee.
6
Except as so provided, this Agreement shall inure to the benefit of and be binding upon Employee and his personal representatives, distributees and legatees.
(b) The Company. This Agreement shall be binding upon the Company and inure to the benefit of the Company and of its successors and assigns, including (but not limited to) any Company that may acquire all or substantially all of the Company's assets or business or into or with which the Company may be consolidated or merged. Any such successor of the Company will be deemed substituted for the Company under the terms of this Agreement for all purposes. For this purpose, “successor” means any person, firm, corporation or other business entity which at any time, whether by purchase, merger or otherwise, directly or indirectly acquires all or substantially all of the assets or business of the Company.
11. Entire Agreement. This Agreement (together with the equity award agreements referred to herein) represents the entire agreement between the parties concerning Employee's employment with the Company and supersedes all prior negotiations, discussions, understanding and agreements, whether written or oral, between Employee and the Company relating to the subject matter of this Agreement.
12. Amendment or Modification, Waiver. No provision of this Agreement may be amended or waived unless such amendment or waiver is agreed to in writing signed by Employee and by a duly authorized officer of the Company. No waiver by any party to this Agreement or any breach by another party of any condition or provision of this Agreement to be performed by such other party shall be deemed a waiver of a similar or dissimilar condition or provision at the same time, any prior time or any subsequent time.
13. Notices. Any notice to be given under this Agreement shall be in writing and delivered personally or sent by overnight courier or registered or certified mail, postage prepaid, return receipt requested, addressed to the party concerned at the address indicated below, or to such other address of which such party subsequently may give notice in writing:
If to Employee: 3631 East 7th Avenue Parkway
Denver, CO 80206
If to the Company:Aytu BioPharma, Inc.
373 Inverness Parkway
Suite 206
Englewood, Colorado 80112
Any notice delivered personally or by overnight courier shall be deemed given on the date delivered and any notice sent by registered or certified mail, postage prepaid, return receipt requested, shall be deemed given on the date mailed.
14. Severability. If any provision of this Agreement or the application of any such provision to any party or circumstances shall be determined by any court of competent jurisdiction or arbitrator acting pursuant to Section 19 below to be invalid and unenforceable to any extent, the remainder of this Agreement or the application of such provision to such person or circumstances other than those to which it is so determined to be invalid and unenforceable shall not be affected, and each provision of this Agreement shall be validated and shall be enforced to the fullest extent permitted by law.
7
If for any reason any provision of this Agreement containing restrictions is held to cover an area or to be for a length of time that is unreasonable or in any other way is construed to be too broad or to any extent invalid, such provision shall not be determined to be entirely null, void and of no effect; instead, it is the intention and desire of both the Company and Employee that, to the extent that the provision is or would be valid or enforceable under applicable law, any court of competent jurisdiction or arbitrator acting pursuant to Section 19 below shall construe and interpret or reform this Agreement to provide for a restriction having the maximum enforceable area, time period and such other constraints or conditions (although not greater than those contained currently contained in this Agreement) as shall be valid and enforceable under the applicable law.
15. Survivorship. The respective rights and obligations of the parties hereunder shall survive any termination of this Agreement to the extent necessary to the intended preservation of such rights and obligations.
16. Headings. All descriptive headings of sections and paragraphs in this Agreement are intended solely for convenience of reference, and no provision of this Agreement is to be construed by reference to the heading of any section or paragraph.
17. Withholding Taxes. All salary, benefits, reimbursements and any other payments to Employee under this Agreement shall be subject to all applicable payroll and withholding taxes and deductions required by any law, rule or regulation of and federal, state or local authority.
18. Counterparts. This Agreement may be executed in one or more counterparts, each of which shall be deemed to be an original but all of which together constitute one and same instrument. The parties agree that facsimile signatures shall have the same force and effect as original signatures.
19. Applicable Law; Arbitration. The validity, interpretation and enforcement of this Agreement and any amendments or modifications hereto shall be governed by the laws of the State of Colorado, as applied to a contract executed within and to be performed in such State. The parties agree that any disputes shall be definitively resolved by binding arbitration before the American Arbitration Association in Denver, Colorado in accordance with its rules of arbitration procedure then in effect. The parties consent to the jurisdiction to the federal courts of the District of Colorado or, if there shall be no jurisdiction, to the state courts located in Arapahoe County, Colorado, to enforce any arbitration award rendered with respect thereto. Each party shall choose one arbitrator and the two arbitrators shall choose a third arbitrator. All costs and fees related to such arbitration (and judicial enforcement proceedings, if any) shall be borne by the Company unless Employee’s claim is deemed to be frivolous by the arbitrator(s) or judge.
20. Legal Fees. The Company shall pay the reasonable expenses of Employee’s counsel in negotiating this Agreement.
21. Section 409A.
(a) Anything in this Agreement to the contrary notwithstanding, if at the time of Employee’s separation from service within the meaning of Section 409A of the Internal Revenue Code of 1986, as amended (the “Code”), the Company determines that Employee is a “specified employee” within the meaning of Section 409A(a)(2)(B)(i) of the Code, then to the extent any payment or benefit that Employee becomes entitled to under this Agreement on account of Employee’s separation from service would be considered deferred compensation otherwise subject to the 20 percent additional tax imposed pursuant to Section 409A(a) of the Code as a result of the application of Section 409A(a)(2)(B)(i) of the Code, such payment shall not be payable and such benefit shall not be provided until the date that is the earlier of (A) six months and one day after Employee’s separation from service, or (B) Employee’s death.
8
If any such delayed cash payment is otherwise payable on an installment basis, the first payment shall include a catch-up payment covering amounts that would otherwise have been paid during the six-month period but for the application of this provision, and the balance of the installments shall be payable in accordance with their original schedule.
(b) All in-kind benefits provided and expenses eligible for reimbursement under this Agreement shall be provided by the Company or incurred by Employee during the time periods set forth in this Agreement. All reimbursements shall be paid as soon as administratively practicable, but in no event shall any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year shall not affect the in-kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year (except for any lifetime or other aggregate limitation applicable to medical expenses). Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit.
(c) To the extent that any payment or benefit described in this Agreement constitutes “non-qualified deferred compensation” under Section 409A of the Code, and to the extent that such payment or benefit is payable upon Employee’s termination of employment, then such payments or benefits shall be payable only upon Employee’s “separation from service.” The determination of whether and when a separation from service has occurred shall be made in accordance with the presumptions set forth in Treasury Regulation Section 1.409A 1(h).
(d) The parties intend that this Agreement will be administered in accordance with Section 409A of the Code. To the extent that any provision of this Agreement is ambiguous as to its compliance with Section 409A of the Code, the provision shall be read in such a manner so that all payments hereunder comply with Section 409A of the Code. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A 2(b)(2). The parties agree that this Agreement may be amended, as reasonably requested by either party, and as may be necessary to fully comply with Section 409A of the Code and all related rules and regulations in order to preserve the payments and benefits provided hereunder without additional cost to either party.
22. Application of Internal Revenue Code Section 280G. If any payment or benefit Employee would receive pursuant to a Change in Control from the Company or otherwise (“Payment”) would (i) constitute a “parachute payment” within the meaning of Section 280G of the Code, and (ii) but for this sentence, be subject to the excise tax imposed by Section 4999 of the Code (the “Excise Tax”), then such Payment shall be equal to the Reduced Amount. The “Reduced Amount” shall be either (x) the largest portion of the Payment that would result in no portion of the Payment being subject to the Excise Tax or (y) the largest portion, up to and including the total, of the Payment, whichever amount, after taking into account all applicable federal, state and local employment taxes, income taxes, and the Excise Tax (all computed at the highest applicable marginal rate), results in Employee’s receipt, on an after-tax basis, of the greater economic benefit notwithstanding that all or some portion of the Payment may be subject to the Excise Tax. If a reduction in payments or benefits constituting “parachute payments” is necessary so that the Payment equals the Reduced Amount, reduction shall occur in the manner that results in the greatest economic benefit for Employee. If more than one method of reduction will result in the same economic benefit, the items so reduced will be reduced pro rata.
In the event it is subsequently determined by the Internal Revenue Service that some portion of the Reduced Amount as determined pursuant to clause (x) in the preceding paragraph is subject to the Excise Tax, Employee agrees to promptly return to the Company a sufficient amount of the Payment so that no portion of the Reduced Amount is subject to the Excise Tax.
9
For the avoidance of doubt, if the Reduced Amount is determined pursuant to clause (y) in the preceding paragraph, Employee will have no obligation to return any portion of the Payment pursuant to the preceding sentence.
Unless Employee and the Company agree on an alternative accounting firm, the accounting firm engaged by the Company for general tax compliance purposes as of the day prior to the effective date of the Change in Control shall perform the foregoing calculations. If the accounting firm so engaged by the Company is serving as accountant or auditor for the individual, entity or group effecting the Change in Control, the Company shall appoint a nationally recognized accounting firm to make the determinations required hereunder. The Company shall bear all expenses with respect to the determinations by such accounting firm required to be made hereunder.
The Company shall use commercially reasonable efforts to cause the accounting firm engaged to make the determinations hereunder to provide its calculations, together with detailed supporting documentation, to the Employee and the Company within fifteen (15) calendar days after the date on which Employee’s right to a Payment is triggered (if requested at that time by the Employee or the Company) or such other time as requested by Employee or the Company.
23.Indemnification. As a condition to the effectiveness of this Agreement, the Company and Employee shall enter into a mutually acceptable indemnification agreement.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above.
AYTU BIOPHARMA, INC.EMPLOYEE
By: __________________________ ______________________________
Name: VIVIAN LIUName: JOSHUA R.
Chairwoman of the Compensation CommitteeChairman and Chief Executive Officer
Board of Directors
10
Exhibit 10.46
AMENDED AND RE-STATED EMPLOYMENT AGREEMENT
DISBROW This Amended and Re-stated Employment Agreement (the "Agreement"), is effective as of February 13, 2023 (the “Effective Date”), between Aytu BioPharma, Inc., a Delaware corporation headquartered at 373 Inverness Parkway, Suite 206, Englewood, CO 80112 USA, hereinafter referred to as the "Company"), and Mark Oki (“Executive").
RECITALS
WHEREAS, the Company is a duly organized Delaware corporation, with its principal place of business within the State of Colorado, and is in the business of developing and marketing pharmaceuticals, medical devices, and other healthcare products; and
WHEREAS, the Company desires Executive’s continued experience, skills, abilities, background and knowledge, and is willing to engage Executive’s services on the terms and conditions set forth in this Agreement; and
WHEREAS, Executive desires to continue to be in the employ of the Company, and is willing to accept such employment on the terms and conditions set forth in this Agreement.
NOW, THEREFORE, in consideration of the mutual covenants and agreements herein contained and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties agree as follows:
1.Employment.
(a)Term. The term of this Agreement shall commence on the Effective Date and continue until terminated in accordance with the provisions hereof (the “Term”).
(b)Position and Duties. During the Term, the Executive shall serve as the Chief Financial Officer of the Company, and shall have supervision and control over and responsibility for the day-to-day business and affairs of the Company and shall have such other powers and duties as may from time to time be prescribed by the Chief Executive Officer (“CEO”) of the Company, provided that such duties are consistent with the Executive’s position or other positions that he may hold from time to time. The Executive shall devote his full working time and efforts to the business and affairs of the Company. Notwithstanding the foregoing, the Executive may serve on other boards of directors, with the approval of the CEO, or engage in religious, charitable or other community activities as long as such services and activities are disclosed to the Board and do not interfere with the Executive’s performance of his duties to the Company as provided in this Agreement. During the Term, Executive agrees not to acquire, assume or participate in, directly or indirectly, any position, investment or interest known by the Executive to be adverse or antagonistic to the Company, its business or prospects, its financial position, or otherwise or in any company, person or entity that is, directly or indirectly, in competition with the business of the Company or any of its affiliates. This provision shall encompass any advisory boards of which Executive is or becomes a member of during the term hereof. Executive shall provide written disclosure to the Compensation Committee (“Compensation Committee”) of the Company’s Board of Directors (the “Board”) as to all advisory boards on which Executive sits, and will provide the Company with written notice within 10 business days of Executive agreeing to sit on any additional advisory boards. On termination of Executive’s employment, regardless of the reason for such termination, Executive shall immediately (and with contemporaneous effect) resign any directorships, offices or other positions that Executive may hold in the Company or any affiliate, unless otherwise agreed in writing by the parties.
1
2.Compensation and Related Matters.
(a)Base Salary. During the Term, the Executive’s initial annual base salary shall be four hundred fifteen thousand dollars ($415,000.00), less applicable deductions and withholdings. The Executive’s base salary shall be reviewed at least annually by the Compensation Committee or a majority of the independent members of the Board, and the base salary may be increased only by the Compensation Committee or a majority of the independent members of the Board. The base salary in effect at any given time is referred to herein as “Base Salary.” The Base Salary shall be payable in a manner that is consistent with the Company’s usual payroll practices for senior executives.
(b)Bonus Compensation. The Executive shall be eligible for an annual discretionary bonus (hereinafter referred to as the “Bonus”) with a target amount of forty percent (40%) of the Base Salary, subject to standard deductions and withholdings, based on the Compensation Committee’s determination, in good faith, and based upon the Executive’s individual achievement and company performance objectives as set by the Board or the Compensation Committee, of whether the Executive has met such performance milestones as are established for the Executive by the Board or the Compensation Committee, in good faith, in consultation with the Executive (hereinafter referred to as the “Performance Milestones”). The Performance Milestones will be based on certain factors including, but not limited to, the Executive’s performance and the Company’s financial and operational performance. The Executive’s Bonus target will be reviewed annually and may be adjusted by the Board or the Compensation Committee in its discretion, provided however, that the Bonus target may only be reduced upon Executive’s written consent. The Executive must be employed on the date the Bonus is awarded to be eligible for the Bonus, subject to the termination provisions hereof. Bonuses shall be paid during the calendar quarter following the calendar quarter for which such Bonus was earned when Performance Milestones are met during a calendar quarter. Fourth quarter Bonuses and Bonuses calculated on the basis of partial Performance Milestone satisfaction shall be paid within 75 days of fiscal year-end.
(c)Signing Bonus. The Executive shall receive a bonus upon signing this agreement in the amount of fifty thousand dollars and zero cents ($50,000.00) less applicable deductions and withholdings (hereinafter referred to as the “Signing Bonus”).
(d)Stock Grant. The Company shall grant to Executive a stock grant of 100,000 shares, which will vest over a (3) year period of employment with the Company beginning with one third (1/3) of the stock vesting on the one-year anniversary of the Effective Date of this Agreement, and the remaining stock vesting in equal quarterly tranches for two years.
(e)Expenses. The Executive shall be entitled to receive prompt reimbursement for all reasonable expenses incurred by him during the Term in performing services hereunder, in accordance with the policies and procedures then in effect and established by the Company for its senior executive officers.
(f)Relocation Expenses. The Executive shall be entitled to receive prompt reimbursement for all reasonable expenses associated with relocating to the Denver, Colorado area inclusive of travel, hotel and other lodging, expenses associated with travel to and from Denver for home searches and moving and packing of household items.
2
(g)Other Benefits. During the Term, the Executive shall be eligible to participate in or receive benefits under the Company’s employee benefit plans in effect from time to time, subject to the terms of such plans.
(h)Vacations. For the term of this Agreement, Executive shall be entitled to paid time off at the rate of twenty-one (21) days per annum. In accordance with Company policy, unused paid time off may not be carried over from year to year. The Executive shall also be entitled to all paid holidays given by the Company to its executives.
3.Termination. During the Term, the Executive’s employment hereunder may be terminated without any breach of this Agreement under the following circumstances:
(a)Death. The Executive’s employment hereunder shall terminate upon his death.
(b)Disability. The Company may terminate the Executive’s employment if he is disabled and unable to perform the essential functions of the Executive’s then existing position or positions under this Agreement with or without reasonable accommodation for a period of 180 days (which need not be consecutive) in any 12-month period. If any question shall arise as to whether during any period the Executive is disabled so as to be unable to perform the essential functions of the Executive’s then existing position or positions with or without reasonable accommodation, the Executive may, and at the request of the Company shall, submit to the Company a certification in reasonable detail by a physician selected by the Company to whom the Executive or the Executive’s guardian has no reasonable objection as to whether the Executive is so disabled or how long such disability is expected to continue, and such certification shall for the purposes of this Agreement be conclusive of the issue. The Executive shall cooperate with any reasonable request of the physician in connection with such certification. If such question shall arise and the Executive shall fail to submit such certification, the Company’s determination of such issue shall be binding on the Executive. Nothing in this Section 3(b) shall be construed to waive the Executive’s rights, if any, under existing law including, without limitation, the Family and Medical Leave Act of 1993, 29 U.S.C. §2601 et seq. and the Americans with Disabilities Act, 42 U.S.C. §12101 et seq.
(c)Termination by Company for Cause(d). The Company may terminate the Executive’s employment hereunder for Cause. For purposes of this Agreement, “Cause” shall mean any of the following: (i) the Executive’s willful and deliberate breach of any agreement with the Company, including the Confidentiality and Intellectual Property Agreement, dated November 28, 2021 (the “Confidentiality Agreement”), the provisions of Section 8 of this Agreement, the Code of Conduct or any other material policy that may result in material injury to the Company, including Executive’s unauthorized use or disclosure of any proprietary information or trade secrets of the Company or any other party as to which the Employee owes an obligation of nondisclosure as a result of the Employee’s relationship with the Company; (ii) the Executive’s conviction of, or entry of a plea of guilty to, or entry of a plea of nolo contendre with respect to, any crime other than a traffic violation or infraction which is a misdemeanor; (iii) the Executive’s act of fraud or intentional misrepresentation in connection with the Executive’s duties or otherwise in connection with the business of the Company, that may result in material injury to the Company; (iv) the Executive’s unintended but material breach in the performance of duties under this Agreement, including insubordination or failure to implement or follow a lawful policy or directive of the Company, provided that if such failure is curable, it is not cured within 30 days following written notice thereof from the Board; or (v) the Executive’s willful malfeasance or willful misconduct in the performance of the Executive’s employment.
(d)Termination Without Cause. The Company may terminate the Executive’s employment hereunder at any time without Cause. Any termination by the Company of the Executive’s employment under this Agreement which does not constitute a termination for Cause under Section 3(c) and does not result from the death or disability of the Executive under Section 3(a) or (b) shall be deemed a termination without Cause.
3
(e)Termination by the Executive(q). The Executive may terminate his employment hereunder at any time for any reason, including but not limited to Good Reason. For purposes of this Agreement, “Good Reason” shall mean, without the Executive’s consent, the occurrence of any of the following: (i) the Company materially breaches any term of this Agreement, and such breach causes or is likely to cause material harm to the Executive; (ii) there is a change in the Executive’s responsibilities that represents a material and adverse change from the Executive’s overall responsibilities, taken as a whole; (iii) there is a Change in Control that results in a change in the Executive’s responsibilities that represents a material and adverse change from the Executive’s overall responsibilities, taken as a whole; (iv) the Executive’s Base Salary is substantially reduced or diminished; or (v) the Executive’s place of employment is relocated by the Company more than a 50-mile radius from Englewood, CO (it being understood and agreed that the Executive may be required to travel in connection with Company business and none of such travel shall constitute or give rise to “Good Reason”). The Executive’s voluntary termination shall be deemed to have occurred for Good Reason for purposes of this Agreement only if (x) the Executive provides written notice to the Company within 30 days after the Executive becomes aware of circumstances giving rise to Good Reason, (y) the Company fails to correct the circumstances giving rise to Good Reason within 30 days following the receipt of such notice (the “Cure Period”) and (z) the Executive resigns within 30 days following the end of the Cure Period. If the Company cures the Good Reason condition during the Cure Period, Good Reason shall be deemed not to have occurred.
(r)Notice of Termination. Except for termination as specified in Section 3(a), any termination of the Executive’s employment by the Company or any such termination by the Executive shall be communicated by written Notice of Termination to the other party hereto. For purposes of this Agreement, a “Notice of Termination” shall mean a notice which shall indicate the specific termination provision in this Agreement relied upon.
(s)Date of Termination. “Date of Termination” shall mean: (i) if the Executive’s employment is terminated by his death, the date of his death; (ii) if the Executive’s employment is terminated on account of disability under Section 3(b) or by the Company for Cause under Section 3(c), the date on which Notice of Termination is given; (iii) if the Executive’s employment is terminated by the Company under Section 3(d), the date on which a Notice of Termination is given; (iv) if the Executive’s employment is terminated by the Executive under Section 3(e) without Good Reason, 30 days after the date on which a Notice of Termination is given, (v) if the Executive’s employment is terminated by the Executive under Section 3(e) with Good Reason, the date on which a Notice of Termination is given after the end of the Cure Period, and (vi) if the Executive’s employment is terminated by Executive for a Bona Fide Retirement, 30 days after the date on which a Notice of Termination is given. Notwithstanding the foregoing, in the event that the Executive gives a Notice of Termination to the Company, the Company may unilaterally accelerate the Date of Termination and such acceleration shall not result in a termination by the Company for purposes of this Agreement.
4.Compensation Upon Termination.
(a)Termination Generally. If the Executive’s employment with the Company is terminated for any reason, the Company shall pay or provide to the Executive (or to his authorized representative or estate) (i) any Base Salary earned through the Date of Termination, unpaid expense reimbursements (subject to, and in accordance with, Section 2(c) of this Agreement) and unused vacation that accrued through the Date of Termination on or before the time required by law but in no event more than 30 days after the Executive’s Date of Termination; and (ii) any vested benefits the Executive may have under any employee benefit plan of the Company through the Date of Termination, which vested benefits shall be paid and/or provided in accordance with the terms of such employee benefit plans (collectively, the “Accrued Benefit”).
4
(b)Termination by the Company Without Cause, by the Executive with Good Reason. During the Term, if the Executive’s employment is terminated by the Company without Cause as provided in Section 3(d), or the Executive terminates his employment for Good Reason as provided in Section 3(e), then the Company shall pay the Executive his Accrued Benefit. In addition, subject to the Executive signing a separation agreement containing, among other provisions, a general release of claims in favor of the Company and related persons and entities, confidentiality, return of property and non-disparagement, in a form and manner satisfactory to the Company (the “Separation Agreement and Release”) and the Separation Agreement and Release becoming fully effective, all within the time frame set forth in the Separation Agreement and Release:
(i)the Company shall pay the Executive an amount equal to the Executive’s annual Base Salary plus any pro-rated incentive compensation earned (as determined by the Board or the Compensation Committee) but unpaid as of the Date of Termination (the “Severance Amount”). Notwithstanding the foregoing, if the Executive breaches any of the provisions of the Confidentiality Agreement, or Section 8 of this Agreement, all payments of the Severance Amount shall immediately cease; and
(ii)Notwithstanding anything to the contrary in the applicable stock-based award agreement, the underlying shares of the stock-based award will immediately vest following the expiration of the revocation period as set forth in Separation Agreement and Release; and
(iii)if the Executive was participating in the Company’s group health plan immediately prior to the Date of Termination and elects COBRA health continuation, then the Company shall pay to the Executive a monthly cash payment for 12 months or the Executive’s COBRA health continuation period, whichever ends earlier, in an amount equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company; and
(iv)the amounts payable under this Section 4(b) shall be paid out in substantially equal installments in accordance with the Company’s payroll practice over 12 months commencing within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, the Severance Amount shall begin to be paid in the second calendar year by the last day of such 60-day period; provided, further, that the initial payment shall include a catch-up payment to cover amounts retroactive to the day immediately following the Date of Termination. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A-2(b)(2); and
5.Change in Control Payment. The provisions of this Section 5 set forth certain terms of an agreement reached between the Executive and the Company regarding the Executive’s rights and obligations upon the occurrence of a Change in Control of the Company. These provisions are intended to assure and encourage in advance the Executive’s continued attention and dedication to his assigned duties and his objectivity during the pendency and after the occurrence of any such event. These provisions shall apply in lieu of, and expressly supersede, the provisions of Section 4(b) regarding severance pay and benefits upon a termination of employment, if such termination of employment occurs within 12 months after the occurrence of the first event constituting a Change in Control.
5
These provisions shall terminate and be of no further force or effect beginning 12 months after the occurrence of a Change in Control.
(a)Change in Control. During the Term, if within 12 months after a Change in Control, the Executive’s employment is terminated by the Company without Cause as provided in Section 3(d) or the Executive terminates his employment for Good Reason as provided in Section 3(e), then, subject to the signing of the Separation Agreement and Release by the Executive and the Separation Agreement and Release effective all within the time frame set forth in the Separation Agreement and Release,
(i)the Company shall pay the Executive a lump sum in cash in an amount equal to one times the sum of (A) the Executive’s then current Base Salary (or the Executive’s Base Salary in effect immediately prior to the Change in Control, if higher) plus (B) the Executive’s target annual incentive compensation for the then- current year; and
(ii)notwithstanding anything to the contrary in any applicable option agreement or stock-based award agreement, all stock options, restricted stock and other stock-based grants to Executive by the Company or that may be granted in the future shall, irrespective of any provisions of his award agreements, immediately and irrevocably vest and become exercisable and any restrictions thereon shall lapse. All stock options shall remain exercisable from the date of such employment termination until the expiration of the term of such stock options; and
(iii)if the Executive was participating in the Company’s group health plan immediately prior to the Date of Termination and elects COBRA health continuation, then the Company shall pay to the Executive a monthly cash payment for 12 months or the Executive’s COBRA health continuation period, whichever ends earlier, in an amount equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company; and
(iv)The amounts payable under this Section 5(a) shall be paid or commence to be paid within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, such payment shall be paid or commence to be paid in the second calendar year by the last day of such 60-day period.
For the avoidance of doubt, all stock options and other stock-based awards held by the Executive as of the Effective Date shall be treated as indicated in the applicable award agreements.
(b)Additional Limitation.
(i)Anything in this Agreement to the contrary notwithstanding, in the event that the amount of any compensation, payment or distribution by the Company to or for the benefit of the Executive, whether paid or payable or distributed or distributable pursuant to the terms of this Agreement or otherwise, calculated in a manner consistent with Section 280G of the Code and the applicable regulations thereunder (the “Aggregate Payments”), would be subject to the excise tax imposed by Section 4999 of the Code, then the Aggregate Payments shall be reduced (but not below zero) so that the sum of all of the Aggregate Payments shall be $1.00 less than the amount at which the Executive becomes subject to the excise tax imposed by Section 4999 of the Code; provided that such reduction shall only occur if it would result in the Executive receiving a higher After Tax Amount (as defined below) than the Executive would receive if the Aggregate Payments were not subject to such reduction. In such event, the Aggregate Payments shall be reduced in the following order, in each case, in reverse chronological order beginning with the Aggregate Payments that are to be paid the furthest in time from consummation of the transaction that is subject to Section 280G of the Code: (1) cash payments not subject to Section 409A of the Code; (2) cash payments subject to Section 409A of the Code; (3) equity-based payments and acceleration; and (4) non-cash forms of benefits; provided that in the case of all the foregoing Aggregate Payments all amounts or payments that are not subject to calculation under Treas. Reg. §1.280G-1, Q&A-24(b) or (c) shall be reduced before any amounts that are subject to calculation under Treas. Reg. §1.280G-1, Q&A-24(b) or (c).
6
(ii)For purposes of this Section 5(b), the “After Tax Amount” means the amount of the Aggregate Payments less all federal, state, and local income, excise and employment taxes imposed on the Executive as a result of the Executive’s receipt of the Aggregate Payments. For purposes of determining the After Tax Amount, the Executive shall be deemed to pay federal income taxes at the highest marginal rate of federal income taxation applicable to individuals for the calendar year in which the determination is to be made, and state and local income taxes at the highest marginal rates of individual taxation in each applicable state and locality, net of the maximum reduction in federal income taxes which could be obtained from deduction of such state and local taxes.
(iii)The determination as to whether a reduction in the Aggregate Payments shall be made pursuant to Section 5(b)(i) shall be made by a nationally recognized accounting firm selected by the Company (the “Accounting Firm”), which shall provide detailed supporting calculations both to the Company and the Executive within 15 business days of the Date of Termination, if applicable, or at such earlier time as is reasonably requested by the Company or the Executive. Any determination by the Accounting Firm shall be binding upon the Company and the Executive.
(c)Definitions. For purposes of this Section 5, the following terms shall have the following meanings:
“Change in Control” shall mean the consummation of any of the following:
(i)A sale of all or substantially all of the assets of the Company on a consolidated basis to an unrelated person or entity; or
(ii)A merger, reorganization or consolidation in which the outstanding shares of common stock of the Company are converted into or exchanged for shares of the successor entity and the holders of the Company’s outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the surviving entity immediately upon the completion of such transaction; or
(iii)The sale of all or a majority of the common stock of the Company to an unrelated person or entity; or
(iv)Any other transaction in which the holders of the Company’s outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the surviving entity in the transaction immediately upon the completion of such transaction.
7
6.Section 409A.
(a)Anything in this Agreement to the contrary notwithstanding, if at the time of the Executive’s separation from service within the meaning of Section 409A of the Code, the Company determines that the Executive is a “specified employee” within the meaning of Section 409A(a)(2)(B)(i) of the Code, then to the extent any payment or benefit that the Executive becomes entitled to under this Agreement on account of the Executive’s separation from service would be considered deferred compensation otherwise subject to the 20 percent additional tax imposed pursuant to Section 409A(a) of the Code as a result of the application of Section 409A(a)(2)(B)(i) of the Code, such payment shall not be payable and such benefit shall not be provided until the date that is the earlier of (A) six months and one day after the Executive’s separation from service, or (B) the Executive’s death. If any such delayed cash payment is otherwise payable on an installment basis, the first payment shall include a catch-up payment covering amounts that would otherwise have been paid during the six-month period but for the application of this provision, and the balance of the installments shall be payable in accordance with their original schedule.
(b)All in-kind benefits provided and expenses eligible for reimbursement under this Agreement shall be provided by the Company or incurred by the Executive during the time periods set forth in this Agreement. All reimbursements shall be paid as soon as administratively practicable, but in no event shall any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year shall not affect the in-kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year (except for any lifetime or other aggregate limitation applicable to medical expenses). Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit.
(c)To the extent that any payment or benefit described in this Agreement constitutes “non-qualified deferred compensation” under Section 409A of the Code, and to the extent that such payment or benefit is payable upon the Executive’s termination of employment, then such payments or benefits shall be payable only upon the Executive’s “separation from service.” The determination of whether and when a separation from service has occurred shall be made in accordance with the presumptions set forth in Treasury Regulation Section 1.409A-1(h).
(d)The parties intend that this Agreement will be administered in accordance with Section 409A of the Code. To the extent that any provision of this Agreement is ambiguous as to its compliance with Section 409A of the Code, the provision shall be read in such a manner so that all payments hereunder comply with Section 409A of the Code. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A-2(b)(2). The parties agree that this Agreement may be amended, as reasonably requested by either party, and as may be necessary to fully comply with Section 409A of the Code and all related rules and regulations in order to preserve the payments and benefits provided hereunder without additional cost to either party.
(e)The Company makes no representation or warranty and shall have no liability to the Executive or any other person if any provisions of this Agreement are determined to constitute deferred compensation subject to Section 409A of the Code but do not satisfy an exemption from, or the conditions of, such Section.
7.Intellectual Property.
8
The Executive acknowledges that all discoveries, concepts, ideas, inventions, innovations, improvements, developments, methods, designs, analyses, drawings, reports, patent applications, copyrightable work and mask work (whether or not including any confidential information) and all registrations or applications related thereto, all other proprietary information and all similar or related information (whether or not patentable) which relate to the Company’s or any of its affiliates’ actual or anticipated business, research and development or existing or future products or services and which are conceived, developed or made by the Executive (whether alone or jointly with others) while employed by the Company and its affiliates, whether before or after the date of this Agreement (collectively referred to as “Work Product”), are the property of the Company or such affiliated companies. The Executive shall promptly disclose such Work Product to the Board and, at the Company’s expense, perform all actions reasonably requested by the Board (whether during or after the period of employment) to establish and confirm such ownership (including, without limitation, executing and delivering assignments, consents, powers of attorney and other instruments). The Executive acknowledges that all Work Product shall be deemed to constitute “works made for hire” under the U.S. Copyright Act of 1976, as amended.
8.Confidential Information, Noncompetition and Cooperation. The Executive agrees that he continues to be bound by the terms of the Confidentiality Agreement.
(a)The Executive agrees that all property (including, without limitation, all equipment, tangible proprietary information, documents, records, notes, contracts and computer- generated materials) furnished to or created or prepared by the Executive incident to the Executive’s employment belongs to the Company and shall be promptly returned to the Company upon termination of the Executive’s employment.
(b)Upon termination of the Executive’s employment, the Executive shall be deemed to have resigned from any and all offices and directorships then held with the Company and its affiliates. Following any termination of employment, the Executive shall reasonably cooperate with the Company (i) in the winding up of pending work on behalf of the Company and the orderly transfer of work to other employees, and (ii) in the defense of any action brought by any third party against the Company that relates to the Executive’s employment by the Company; provided, that in each case the Company shall reimburse the Executive for any reasonable and documented out-of-pocket fees and expenses incurred by the Executive in connection with such cooperation.
(c)The Executive acknowledges that in the course of the Executive’s employment with the Company, the Executive will become familiar with the Company’s and its affiliates’ trade secrets and with other confidential and proprietary information and that the Executive’s services will be of special, unique and extraordinary value to the Company and its affiliates. Therefore, the Executive agrees that the Executive shall not, during the Term and for a period of one (1) year thereafter, directly or indirectly, either for himself or for any other person or entity or otherwise, (i) participate in any business or enterprise (including, without limitation, any division, group or franchise of a larger organization), engaged, anywhere within North America at the time of termination (the “Restricted Territory”), in the business of developing or commercializing controlled release, ion exchange resin based pharmaceutical products or any therapeutic agent being studied for or approved for the treatment of vascular Ehlers-Danlos Syndrome (VEDS) or an associated connective tissue disorder, or any other business in which the Executive would be required to employ, reveal or otherwise utilize trade secrets of the Company and its affiliates used prior to termination that may result in a material injury to the Company (with it being understood that the term “participate in” shall include, without limitation, having any direct or indirect interest in any person or entity, whether as a sole proprietor, owner, stockholder, partner, joint venturer, creditor or otherwise, or rendering any direct or indirect service or assistance to any person or entity -whether as a director, officer, manager, supervisor, employee, agent, consultant, advisor or otherwise); provided that, nothing herein shall prohibit the Executive from being a passive owner of not more than two percent (2%) of the outstanding stock of any class of an entity which is publicly traded so long as the Executive has no active participation in the business of such corporation; (ii) induce or attempt to induce any customer, supplier, licensee or other business relation of the Company or any of its subsidiaries to cease doing business with the Company or any such subsidiary or in any way interfere with the relationship between any such customer, supplier, licensee or business relation and the Company and any such subsidiary; or (iii) induce or attempt to induce any employee of the Company or its affiliates to leave the employ of the Company or any such affiliated company, or in any way interfere with the relationship between the Company and any of its affiliates and any employee thereof, or hire or otherwise engage any person who was an employee of the Company or any of its affiliated companies within one year before any such hiring would take place.
9
(d)The Executive agrees that he will not directly or indirectly, individually or in concert with others, make any statement calculated or likely to have the effect of undermining or disparaging the business or the business reputation of the Company or its affiliates or their respective employees, officers, directors, customers, suppliers, successors and assigns, including, without limitation, negative comments about any such person or company, its management methods, policies and/or practices. Notwithstanding the foregoing, nothing herein shall prohibit the Executive from responding accurately and fully to any question, inquiry or request made in connection with any governmental inquiry, investigation, review, audit or proceeding, any legal proceeding or claim (whether in court, arbitration or otherwise) of any nature, or as otherwise required by law.
(e)If, at the time of enforcement of this Section 8, a court holds that the restrictions stated herein are unreasonable under circumstances then existing, the parties hereto agree that the maximum duration, scope or geographical area reasonable under such circumstances shall be substituted for the stated period, scope or area and that the court shall be allowed to revise the restrictions contained herein to cover the maximum duration, scope and area permitted by law. Because the Executive’s services are unique and because the Executive has access to confidential and proprietary information of the Company and its business, the parties hereto agree that money damages would not be an adequate remedy for any breach of Section 8 of this Agreement. Therefore, in the event of a breach or threatened breach of Section 8 of this Agreement, the Company or its successors or assigns may, in addition to other rights and remedies existing in their favor and notwithstanding anything herein to the contrary, apply to any court of competent jurisdiction for specific performance and/or injunctive or other equitable relief in order to enforce or prevent any violations of, the provisions hereof (without posting a bond or other security).
(f)The Executive acknowledges that the provisions of this Section 8 are in consideration of the Executive’s employment with the Company and additional good and valuable consideration as set forth in this Agreement. The Executive agrees and acknowledges that the restrictions contained in Section 8 do not preclude the Executive from earning a livelihood, nor do they unreasonably impose limitations on the Executive’s ability to earn a living. The Executive acknowledges (i) that the business of the Company and its affiliates will be conducted throughout the Restricted Territory, (ii) notwithstanding the state of formation or principal office of the Company and its affiliates, or any of their respective executives or employees (including the Executive), it is expected that the Company will have business activities and have valuable business relationships within its industry throughout the Restricted Territory, and (iii) as part of the Executive’s responsibilities, the Executive may be traveling throughout the Restricted Territory in furtherance of the Company’s and its affiliates’ business and its relationships. The Executive acknowledges that the potential harm to the Company of the non-enforcement of Section 8 outweighs any potential harm to the Executive of its enforcement by injunction or otherwise. The Executive acknowledges that the Executive has carefully read this Agreement and has given careful consideration to the restraints imposed upon the Executive by this Agreement and is in full accord as to their necessity for the reasonable and proper protection of confidential and proprietary information of the Company and its subsidiaries now existing or to be developed in the future.
10
The Executive acknowledges that each and every restraint imposed by this Agreement is reasonable with respect to scope, duration, and geographical area.
9.Arbitration of Disputes. Any controversy or claim arising out of or relating to this Agreement or the breach thereof or otherwise arising out of the Executive’s employment or the termination of that employment (including, without limitation, any claims of unlawful employment discrimination whether based on age or otherwise) shall, to the fullest extent permitted by law, be settled by arbitration in any forum and form agreed upon by the parties or, in the absence of such an agreement, under the auspices of the American Arbitration Association (“AAA”) in accordance with the Employment Dispute Resolution Rules of the AAA, including, but not limited to, the rules and procedures applicable to the selection of arbitrators. In the event that any person or entity other than the Executive or the Company may be a party with regard to any such controversy or claim, such controversy or claim shall be submitted to arbitration subject to such other person or entity’s agreement. Judgment upon the award rendered by the arbitrator may be entered in any court having jurisdiction thereof. This Section 8 shall be specifically enforceable. Notwithstanding the foregoing, this Section 8 shall not preclude either party from pursuing a court action for the sole purpose of obtaining a temporary restraining order or a preliminary injunction in circumstances in which such relief is appropriate; provided that any other relief shall be pursued through an arbitration proceeding pursuant to this Section 9.
10.Consent to Jurisdiction. To the extent that any court action is permitted consistent with or to enforce Section 9 of this Agreement, the parties hereby consent to the jurisdiction of the District Court of Douglas County, Colorado and the United States District Court for the District of Colorado. Accordingly, with respect to any such court action, the Executive (a) submits to the personal jurisdiction of such courts; (b) consents to service of process; and (c) waives any other requirement (whether imposed by statute, rule of court, or otherwise) with respect to personal jurisdiction or service of process.
11.Integration. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements between the parties concerning such subject matter, provided that the Confidentiality Agreement remains in full force and effect.
12.Withholding. All payments made by the Company to the Executive under this Agreement shall be net of any tax or other amounts required to be withheld by the Company under applicable law.
13.Successor to the Executive. This Agreement shall inure to the benefit of and be enforceable by the Executive’s personal representatives, executors, administrators, heirs, distributees, devisees and legatees. In the event of the Executive’s death after his termination of employment but prior to the completion by the Company of all payments due him under this Agreement, the Company shall continue such payments to the Executive’s beneficiary designated in writing to the Company prior to his death (or to his estate, if the Executive fails to make such designation).
14.Enforceability. If any portion or provision of this Agreement (including, without limitation, any portion or provision of any section of this Agreement) shall to any extent be declared illegal or unenforceable by a court of competent jurisdiction, then the remainder of this Agreement, or the application of such portion or provision in circumstances other than those as to which it is so declared illegal or unenforceable, shall not be affected thereby, and each portion and provision of this Agreement shall be valid and enforceable to the fullest extent permitted by law.
11
15.Survival. The provisions of this Agreement shall survive the termination of this Agreement and/or the termination of the Executive’s employment to the extent necessary to effectuate the terms contained herein.
16.Waiver. No waiver of any provision hereof shall be effective unless made in writing and signed by the waiving party. The failure of any party to require the performance of any term or obligation of this Agreement, or the waiver by any party of any breach of this Agreement, shall not prevent any subsequent enforcement of such term or obligation or be deemed a waiver of any subsequent breach.
17.Notices. Any notice to be given under this Agreement shall be in writing and delivered personally or sent by overnight courier or registered or certified mail, postage prepaid, return receipt requested, addressed to the party concerned at the address indicated below, or to such other address of which such party subsequently may give notice in writing:
If to Executive: To the address specified in the payroll records of the Company.
If to the Company: |
Aytu BioPharma, Inc., 373 Inverness Parkway, Suite 206, Englewood, Colorado 80112 |
Any notice delivered personally or by overnight courier shall be deemed given on the date delivered and any notice sent by registered or certified mail, postage prepaid, return receipt requested, shall be deemed given on the date mailed.
18.Amendment. This Agreement may be amended or modified only by a written instrument signed by the Executive and by a duly authorized representative of the Company.
19.Governing Law. This is a Colorado contract and shall be construed under and be governed in all respects by the laws of the State of Colorado, without giving effect to the conflict of laws principles of such State. With respect to any disputes concerning federal law, such disputes shall be determined in accordance with the law as it would be interpreted and applied by the United States Court of Appeals for the District of Colorado.
20.Counterparts. This Agreement may be executed in any number of counterparts, each of which when so executed and delivered shall be taken to be an original; but such counterparts shall together constitute one and the same document.
21.Successor to Company. The Company shall require any successor (whether direct or indirect, by purchase, merger, consolidation or otherwise) to all or substantially all of the business or assets of the Company expressly to assume and agree to perform this Agreement to the same extent that the Company would be required to perform it if no succession had taken place. Failure of the Company to obtain an assumption of this Agreement at or prior to the effectiveness of any succession shall be a material breach of this Agreement.
12
22.Gender Neutral. Wherever used herein, a pronoun in the masculine gender shall be considered as including the feminine gender unless the context clearly indicates otherwise.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above.
AYTU BIOPHARMA, INC.EXECUTIVE
By: __________________________ ______________________________
Chairwoman of the Compensation CommitteeChairman and Chief Executive Officer
Board of Directors
13
Exhibit 10.47
SUBLEASE
Name: VIVIAN LIUName: MARK OKI THIS SUBLEASE (this “Sublease”) is dated for reference purposes as of May 15, 2023, and is made by and between Neos Therapeutics, LP, a Texas limited partnership (“Sublessor”), and AMT Manufacturing Solutions, LLC, a Delaware limited liability company (“Sublessee”). Sublessor and Sublessee hereby agree as follows:
1.Recitals: This Sublease is made with reference to the fact that Riverside Business Green, LP, a Delaware limited partnership, as Landlord, as successor-in-interest to Walstib, L.P. (“Master Lessor”), and Sublessor, as successor-in-interest to PharmaFab, Inc., as tenant, entered into that certain Commercial Lease Agreement, dated as of June 29, 1999, as amended by that certain First Amendment to Lease dated as of September 1, 2002 (the “First Amendment”), that certain Interim Amendment to Lease dated as of September 4, 2003 (the “Second Amendment”), that certain Third Amendment to Lease dated as of October 1, 2003 (the “Third Amendment”), that certain Fourth Amendment to Lease effective as of May 1, 2009 (the “Fourth Amendment”), that certain Fifth Amendment to Lease dated as of April 5, 2010 (the “Fifth Amendment”), and that certain Sixth Amendment to Lease dated as of August 14, 2013 (the “Sixth Amendment”; together with the Original Lease, First Amendment, Second Amendment, Third Amendment, Fourth Amendment, and Fifth Amendment, the “Master Lease”), with respect to premises consisting of approximately 97,282 rentable square feet of space, located at 2490 N. Highway 360, Grand Prairie, Texas (the “Premises”) consisting of approximately 77,112 rentable square feet in Suites 100 and 200 (the “Suites 100 and 200 Premises”) and approximately 20,170 rentable square feet located in Suite 400). A copy of the Master Lease is attached hereto as Exhibit A.
2.Premises:
A.Commencing on the Commencement Date, Sublessor hereby subleases to Sublessee, and Sublessee hereby subleases from Sublessor, a portion of the Premises consisting of approximately 22,909 rentable square feet of space located in Suite 200 of the Premises (hereinafter, the “Subleased Premises”). The Subleased Premises are more particularly described as the area outlined in red on Exhibit B attached hereto. Commencing as early as April 1, 2024 but no later than December 31, 2024, which date shall be designated by Sublessor by written notice to Sublessee not less than thirty (30) days prior to the date of expansion (such date, the “Expansion Date”), the Subleased Premises shall be expanded to include the remainder of the Suites 100 and 200 Premises (other than Sublessor’s server room therein) outlined in green on Exhibit B attached hereto (the “Expansion Space”). If Sublessee needs additional space during the period of time preceding the Expansion Date, Sublessor and Sublessee shall work in good faith to accommodate such request.
B.Notwithstanding the foregoing, within thirty (30) days of the Commencement Date, subject to receipt of Master Lessor’s consent, Sublessee, at its sole cost and expense, shall construct a wall or fence as reasonably determined by Sublessor in the location shown in yellow-green dividing the initial Subleased Premises from the Expansion Space on Exhibit B attached hereto in accordance with the terms of Paragraph 13 below and the terms of the Master Lease. Sublessee shall only be entitled to access the office area within the Subleased Premises until the date upon which the wall or fence is fully constructed.
C.Sublessor shall have the exclusive right to use, and Sublessor’s security and information technology personnel may enter the Subleased Premises at any time to access the server room which is accessible from within the Subleased Premises. Sublessor shall make commercially reasonable effort to notify Sublessee of such access.
3.Term: The term (the “Term”) of this Sublease shall be for the period commencing on the date which is one (1) business day after the date by which Sublessor has received consent of the Sublease from both Master Lessor and Sublessor’s lender (the “Lender”) in a form acceptable to Sublessor (the “Commencement Date”) and ending on December 31, 2024 (the “Expiration Date”), unless this Sublease is sooner terminated pursuant to its terms or the Master Lease sooner expires pursuant to its terms. For the avoidance of doubt, the Subleased Premises shall be deemed delivered when Sublessor vacates the Subleased Premises and provides Sublessee keys or other means of access thereto.
4.Rent:
A.Base Rent. Sublessee shall pay to Sublessor base rent for the Subleased Premises for each month during the period commencing on the Commencement Date and expiring on December 31, 2023 the amount of Twenty Thousand Forty-Five Dollars ($20,045) per month, and during the period commencing on January 1, 2024 until the day preceding the Expansion Date the amount of Twenty-One Thousand Dollars ($21,000) per month and during the period commencing on the Expansion Date until the Expiration Date the amount of Seventy Thousand Six Hundred Eighty-Six Dollars ($70,686) per month (“Base Rent”). Base Rent and Additional Rent, as defined in Paragraph 4.B below, shall be paid on or before the first (1st) day of each month. Base Rent and Additional Rent for any period during the Term hereof which is for less than one (1) month of the Term shall be a pro rata portion of the monthly installment based on a thirty (30) day month. Base Rent and Additional Rent shall be payable without notice or demand and without any deduction, offset, or abatement, in lawful money of the United States of America. Base Rent and Additional Rent shall be paid directly to Sublessor by ACH pursuant to instructions provided by Sublessor, or by such other means as may be designated in writing by Sublessor.
B.Additional Rent. All monies other than Base Rent required to be paid by Sublessor under the Master Lease as to the Subleased Premises, including, without limitation, any amounts payable by Sublessor to Master Lessor as “Operating Expenses” (as defined in Section 4.2 of the Master Lease), shall be paid by Sublessee hereunder as and when such amounts are due under the Master Lease, as incorporated herein. Sublessee shall also pay to Sublessor (i) any gross receipts or rent tax payable with respect to this Sublease, (ii) all costs directly incurred by or at the request of Sublessee with respect to its use of the Subleased Premises and (iii) the share allocable to the Subleased Premises as reasonably determined by Sublessor of the actual, reasonable costs incurred by Sublessor with respect to the Premises, including to perform its obligations under the Master Lease, including with respect to utilities and roof and Building system maintenance, or to provide the services, including security, described herein to the extent not included in Operating Expenses. Sublessee shall pay to Sublessor all such amounts within thirty (30) days of invoice therefor. All such amounts shall be deemed additional rent (“Additional Rent”). Base Rent and Additional Rent hereinafter collectively shall be referred to as “Rent”. Sublessee and Sublessor agree, as a material part of the consideration given by Sublessee to Sublessor for this Sublease, that Sublessee shall pay all costs, expenses, taxes, insurance, maintenance and other charges of every kind and nature arising in connection with this Sublease, the Master Lease as to the Subleased Premises or the Subleased Premises, such that Sublessor shall receive, as a net consideration for this Sublease, the Base Rent payable under Paragraph 4.A hereof.
5.Security Deposit: Upon execution hereof by Sublessee, Sublessee shall deposit with Sublessor the sum of Ninety Thousand Seven Hundred Thirty-One Dollars ($90,731) (the “Security Deposit”), in cash, as security for the performance by Sublessee of the terms and conditions of this Sublease. Upon the full execution and delivery by Sublessee and Master Lessor of a lease of all of Suites 100 and 200 of the Building commencing upon the termination of this Sublease and Master Lessor’s agreement that the Premises does not need to be restored by Sublessor, and Sublessee’s delivery of a copy thereof to Sublessor, if Sublessee is not then in default under this Sublease, Sublessor shall apply (a) an amount equal to $20,045 to Base Rent next coming due under this Sublease and (b) the remainder of the Security Deposit to Base Rent due in December 2024.
-2-
6.Holdover: The parties hereby acknowledge that the expiration date of the Master Lease is December 31, 2024 and that it is therefore critical that Sublessee surrender the Subleased Premises to Sublessor no later than the Expiration Date in accordance with the terms of this Sublease. In the event that Sublessee does not surrender the Subleased Premises by the Expiration Date in accordance with the terms of this Sublease, Sublessee shall indemnify, defend, protect and hold harmless Sublessor from and against all loss and liability resulting from Sublessee’s delay in surrendering the Subleased Premises and pay Sublessor holdover rent as provided in Section 16.9 of the Master Lease.
7.Repairs: The parties acknowledge and agree that Sublessee is subleasing the Subleased Premises on an “as is” basis, and that Sublessor has made no representations or warranties with respect to the condition of the Subleased Premises. Sublessor shall have no obligation whatsoever to make or pay the cost of any alterations, improvements or repairs to the Subleased Premises, including, without limitation, any improvement or repair required to comply with any law; provided, however, subject to reimbursement by Sublessee under Paragraph 4.B, Sublessor shall maintain the roof and certain systems serving the Subleased Premises. Master Lessor shall be solely responsible for performance of any repairs required to be performed by Master Lessor under the terms of the Master Lease.
8.Right to Cure Defaults: If Sublessee fails to pay any sum of money under this Sublease, or fails to perform any other act on its part to be performed hereunder, then Sublessor may, but shall not be obligated to, after passage of any applicable notice and cure periods, make such payment or perform such act. All such sums paid, and all reasonable costs and expenses of performing any such act, shall be deemed Additional Rent payable by Sublessee to Sublessor upon demand, together with interest thereon at the interest rate set forth in Section 16.2 of the Master Lease (the “Interest Rate”) from the date of the expenditure until repaid.
9.Assignment and Subletting: Sublessee may not assign this Sublease, sublet the Subleased Premises, transfer any interest of Sublessee therein or permit any use of the Subleased Premises by another party (collectively, “Transfer”), without the prior written consent of Sublessor and Master Lessor. Sublessee acknowledges that the Master Lease contains a “recapture” right in Section 12.2, and that Sublessor may withhold consent to a proposed Transfer in its sole discretion unless Master Lessor confirms in writing that the recapture right does not apply to the Subleased Premises or otherwise waives such right. A consent to one Transfer shall not be deemed to be a consent to any subsequent Transfer. Any Transfer without such consent shall be void and, at the option of Sublessor, shall terminate this Sublease. Sublessor’s waiver or consent to any assignment or subletting shall be ineffective unless set forth in writing. Any Transfer shall be subject to the terms of Section 12 of the Master Lease.
10.Use:
A.Sublessee may use the Subleased Premises only for the uses identified in Section 1.8 of the Master Lease, as modified by Section 7 of the Fifth Amendment. All use of the Subleased Premises by Sublessee shall comply with all applicable laws and regulations and shall not violate any laws administered by the U.S. Drug Enforcement Administration or the U.S. Food and Drug Administration.
B.Sublessee shall not do or permit anything to be done in or about the Subleased Premises that would (i) injure the Subleased Premises; or (ii) vibrate, shake, overload, or impair the efficient operation of the Subleased Premises or the sprinkler systems, heating, ventilating or air conditioning equipment, or utilities systems located therein. Sublessee shall not store any materials, supplies, finished or unfinished products or articles of any nature outside of the Subleased Premises. For purposes of this Sublease and Sections 2.4 and 16.20 of the Master Lease, Sublessee shall comply with all reasonable rules and regulations promulgated from time to time by Sublessor and Master Lessor, including with respect to security measures imposed by Sublessor.
-3-
11.Effect of Conveyance: As used in this Sublease, the term “Sublessor” means the holder of the tenant’s interest under the Master Lease. In the event of any assignment, transfer or termination of the tenant’s interest under the Master Lease, which assignment, transfer or termination may occur at any time during the Term hereof in Sublessor’s sole discretion, Sublessor shall be and hereby is entirely relieved of all covenants and obligations of Sublessor hereunder, and it shall be deemed and construed, without further agreement between the parties, that any transferee has assumed and shall carry out all covenants and obligations thereafter to be performed by Sublessor hereunder. Sublessor may transfer and deliver any security of Sublessee to the transferee of the tenant’s interest under the Master Lease, and thereupon Sublessor shall be discharged from any further liability with respect thereto.
12.Delivery and Acceptance: If Sublessor fails to deliver possession of the Subleased Premises to Sublessee on or before the Commencement Date for any reason whatsoever, then this Sublease shall not be void or voidable; provided, however, that in such event, Rent shall abate until Sublessor delivers possession of the Subleased Premises to Sublessee. By taking possession of the Subleased Premises, Sublessee conclusively shall be deemed to have accepted the Subleased Premises in their as-is, then-existing condition, without any warranty whatsoever of Sublessor with respect thereto.
13.Improvements: No alteration or improvements shall be made to the Subleased Premises, except in accordance with the Master Lease, and with the prior written consent of both Master Lessor and Sublessor, provided Sublessor has conceptually approved of the alterations described under Paragraph 2.B. above, subject to Master Lessor’s consent and its review of more detailed plans therefor, including the design and materials for such alterations.
14.Insurance: Sublessee shall obtain and keep in full force and effect, at Sublessee’s sole cost and expense, during the Term the insurance required under Section 8.2 of the Master Lease. Sublessee shall name Master Lessor and Sublessor as additional insureds under its liability insurance policy. The release and waiver of subrogation set forth in Section 8.4 of the Master Lease, as incorporated herein, shall be binding on the parties.
15.Default: Sublessee shall be in default under this Sublease if Sublessee commits any act or omission which constitutes a default under the Master Lease, which has not been cured after delivery of written notice and passage of the applicable grace period provided in the Master Lease as modified, if at all, by the provisions of this Sublease. In the event of any default by Sublessee, Sublessor shall have all remedies provided pursuant to the Landlord’s Remedies Addendum attached to the Master Lease and by applicable law.
16.Surrender: Prior to expiration of this Sublease, Sublessee shall remove all of its trade fixtures and shall surrender the Subleased Premises to Sublessor in the condition required under the Master Lease, provided Sublessor shall remain responsible for the removal of its signs from the Subleased Premises prior to the expiration of the Master Lease. If the Subleased Premises are not so surrendered, then Sublessee shall be liable to Sublessor for all liabilities Sublessor incurs as a result thereof, including costs incurred by Sublessor in returning the Subleased Premises to the required condition, plus interest thereon at Interest Rate.
17.Broker: Sublessor and Sublessee each represents to the other that it has dealt with no real estate brokers, finders, agents or salesmen in connection with this transaction. Each party agrees to hold the other party harmless from and against all claims for brokerage commissions, finder’s fees or other compensation made by any other agent, broker, salesman or finder as a consequence of such party’s actions or dealings with such agent, broker, salesman, or finder.
18.Notices: Unless at least five (5) days’ prior written notice is given in the manner set forth in this paragraph, the address of each party for all purposes connected with this Sublease shall be that address set forth below its signature at the end of this Sublease. All notices, demands or communications in connection with this Sublease shall be (a) personally delivered; or (b) properly addressed and (i) submitted to an overnight courier service, charges prepaid, or (ii) deposited in the mail (certified, return receipt requested, and postage prepaid).
-4-
Notices shall be deemed delivered upon receipt, if personally delivered, one (1) business day after being submitted to an overnight courier service and three (3) business days after mailing, if mailed as set forth above. All notices given to Master Lessor under the Master Lease shall be considered received only when delivered in accordance with the Master Lease.
19.Miscellaneous: Sublessee and Sublessor each represent and warrant to the other that each person executing this Sublease on behalf of such party is duly authorized to execute and deliver this Sublease on behalf of that party. Capitalized terms used but not defined in this Sublease shall have the meanings ascribed to such terms in the Master Lease.
20.Other Sublease Terms:
A.Incorporation by Reference. Except as set forth below, the terms and conditions of this Sublease shall include all of the terms of the Master Lease and such terms are incorporated into this Sublease as if fully set forth herein, except that: (i) each reference in such incorporated sections to “Lease” shall be deemed a reference to “Sublease”; (ii) each reference to the “Premises” shall be deemed a reference to the “Subleased Premises”; (iii) each reference to “Landlord” and “Tenant” shall be deemed a reference to “Sublessor” and “Sublessee”, respectively, except as otherwise expressly set forth herein; (iv) with respect to work, services, repairs, restoration, insurance, indemnities, representations, warranties or the performance of any other obligation of Master Lessor under the Master Lease, the sole obligation of Sublessor shall be to request the same in writing from Master Lessor as and when requested to do so by Sublessee, and to use Sublessor’s reasonable efforts (without requiring Sublessor to spend more than a nominal sum) to obtain Master Lessor’s performance; (v) with respect to any obligation of Sublessee to be performed under this Sublease, wherever the Master Lease grants to Sublessor a specified number of days to perform its obligations under the Master Lease, except as otherwise provided herein, Sublessee shall have three (3) fewer days to perform the obligation, including, without limitation, curing any defaults; (vi) with respect to any approval required to be obtained from the “Landlord” under the Master Lease, such consent must be obtained from both Master Lessor and Sublessor, and the approval of Sublessor may be withheld if Master Lessor’s consent is not obtained; (vii) in any case where the “Landlord” reserves or is granted the right to manage, supervise, control, repair, alter, regulate the use of, enter or use the Premises or any areas beneath, above or adjacent thereto, perform any actions or cure any failures, such reservation or right shall be deemed to be for the benefit of both Master Lessor and Sublessor; (viii) in any case where “Tenant” is to indemnify, release or waive claims against “Landlord”, such indemnity, release or waiver shall be deemed to cover, and run from Sublessee to, both Master Lessor and Sublessor; (ix) in any case where “Tenant” is to execute and/or deliver certain documents or notices to “Landlord”, such obligation shall be deemed to run from Sublessee to both Master Lessor and Sublessor; (x) all payments shall be made to Sublessor; (xi) Sublessee shall pay all consent and review fees set forth in the Master Lease to each of Master Lessor and Sublessor; (xii) Sublessee shall not have the right to terminate this Sublease due to casualty or condemnation unless Sublessor has such right under the Master Lease; (xiii) Sublessor’s obligations under Section 4.2 are limited to forwarding statements and refunds provided by Master Lessor, and Sublessee shall have no right to dispute or audit such statements; and (xiv) “Sublessee’s Share” shall mean, initially, 23.55% of the Premises, 20.17% of the Building and 10.68% of the Industrial Park, and such amounts shall be increased pro rata based on the increased square footage of the Subleased Premises on the Expansion Date to 79.27% of the Premises, 67.88% of the Building and 35.93% of the Industrial Park. Under no circumstances shall rent abate under this Sublease except to the extent that rent correspondingly abates under the Master Lease as to the Subleased Premises.
Notwithstanding the foregoing, the following provisions of the Master Lease shall not be incorporated herein: Sections 1.1, 1.2 (the first sentence only), 1.3-1.7, 1.10, 1.11, 3.1, 3.2, 4.2(b) (first two sentences), 4.2(d) (the reference to Paragraph 1.6 only), 4.2(e), 4.2(f), 5 (the reference to Paragraph 1.7 only), 6.2 (the third and fourth sentences and the first clause in the fifth sentence only), 6.5, 7.3 (the second sentence only), 11 (reference to paying utilities directly instead of to Sublessor), 12.1(c), 16.6, 16.7, 16.18(c) and 16.27; Guaranty of Lease; Tenant Improvements Addendum; Option to Extend Addendum; Additional Security Deposit Addendum; Exhibit A; First Amendment (except the first sentence of Section 5); Second Amendment; Third Amendment; Fourth Amendment (except Sections 5(b), 6 (except that the reference to “July 1, 2009” in Section 6(b) shall mean the Commencement Date), 7, 8 and 9); Fifth Amendment (except Section 7); Sixth Amendment (except Section 7).
-5-
In addition, notwithstanding subpart (iii) above, (a) references in the following provisions to “Landlord” shall mean Master Lessor only: Sections 2.2-2.5, 4.2(a), 6.4 (the first reference only), 7.1 (the first instance only), 7.2, 8.1, 8.3, 9.1, 10.1-10.4, 12.1(a) (the penultimate sentence), 14, and 16.20 (the last instance only); Fourth Amendment (Section 5(a) only); (b) references in the following provisions to “Landlord” shall mean Master Lessor and Sublessor: Sections 6.1 and 6.18(a) and Fourth Amendment (Section 6(c) only); and (c) the terms of the second sentence of Section 10.3 shall also apply to any improvements currently located in the Subleased Premises.
B.Assumption of Obligations. This Sublease is and at all times shall be subject and subordinate to the Master Lease and the rights of Master Lessor thereunder. Sublessee hereby expressly assumes and agrees: (i) to comply with all provisions of the Master Lease which are incorporated hereunder; and (ii) to perform all the obligations on the part of the “Tenant” to be performed under the terms of the Master Lease during the Term of this Sublease that are incorporated hereunder. In the event the Master Lease is terminated for any reason whatsoever, this Sublease shall terminate simultaneously with such termination (unless Master Lessor or a successor tenant agrees to permit Sublessee to continue to occupy the Subleased Premises on the terms of this Sublease for the remainder of the Term), without any liability of Sublessor to Sublessee except to the extent such termination is due to the violation by a party of the terms of this Sublease. In the event of a conflict between the provisions of this Sublease and the Master Lease, as between Sublessor and Sublessee, the provisions of this Sublease shall control. In the event of a conflict between the express provisions of this Sublease and the provisions of the Master Lease, as incorporated herein, the express provisions of this Sublease shall prevail.
21.Conditions Precedent: This Sublease and Sublessor’s and Sublessee’s obligations hereunder are conditioned upon the written consent of Master Lessor and the Lender. Each party shall use commercially reasonable efforts to obtain such consent, including by promptly signing Master Lessor’s and Lender’s commercially reasonable the consent forms. If Sublessor fails to obtain Master Lessor’s or Lender’s consent within thirty (30) days after execution of this Sublease by Sublessor, then Sublessor or Sublessee may terminate this Sublease by giving the other party written notice thereof prior to the date such consent is received, and Sublessor shall return to Sublessee its payment of the first month’s Rent paid by Sublessee pursuant to Paragraph 4 hereof and the Security Deposit.
22.Termination; Recapture: Notwithstanding anything to the contrary herein, Sublessee acknowledges that, under the Master Lease, both Master Lessor and Sublessor have certain termination and recapture rights, including, without limitation, in Sections 9, 12.2 and 14. Nothing herein shall prohibit Master Lessor or Sublessor from exercising any such rights and neither Master Lessor nor Sublessor shall have any liability to Sublessee as a result thereof. In the event Master Lessor or Sublessor exercise any such termination or recapture rights as to the Subleased Premises, this Sublease shall terminate without any liability to Master Lessor or Sublessor.
23.Furniture, Fixtures and Equipment: Upon execution hereof by Sublessee, Sublessee shall pay to Sublessor the sum of One Hundred Thousand Dollars ($100,000) (the “Purchase Price”). Upon receipt of the Purchase Price, Sublessor shall transfer all of its right, title and interest in those assets identified on Exhibit C attached hereto (the “FF&E”), and Sublessee shall accept the FF&E in their “AS IS, WHERE IS” condition, without representation or warranty whatsoever, pursuant to and in accordance with the form bill of sale attached hereto as Exhibit D. Sublessee shall be responsible for payment of all sales tax for the FF&E.
-6-
IN WITNESS WHEREOF, the parties have executed this Sublease as of the day and year first above written.
SUBLESSOR:SUBLESSEE:
NEOS THERAPEUTICS, LP, AMT MANUFACTURING SOLUTIONS, LLC,
a Texas limited partnership a Delaware limited liability company
f/k/a PFAB LP
By: ___________________________By: ___________________________
Name:_________________________Name: _________________________
Its: ___________________________Its: ____________________________
Address: c/o Aytu BioPharma, Inc.Address: 2490 N. Highway 360, Suite 200
373 Inverness Parkway, Suite 206Grand Prairie, TX 75052
Englewood, CO 80112
Attn: Chief Financial Officer
Email: CFO@aytubio.com
-7-
EXHIBIT A
MASTER LEASE
Exhibit A
EXHIBIT B
SUBLEASED PREMISES, EXPANSION SPACE
AND LOCATION OF DEMISING WALL
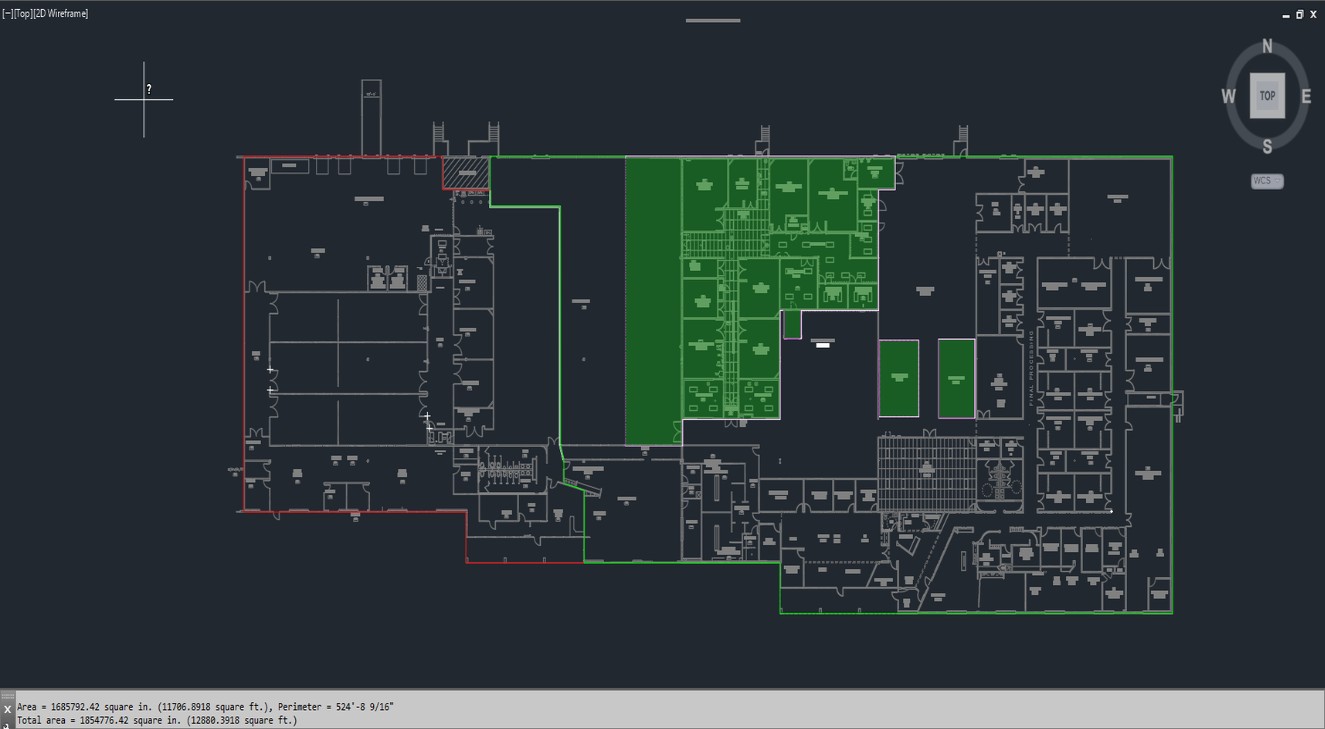
Exhibit B
EXHIBIT C
Furniture, Fixtures and Equipment Listing
Major Type |
Fixed asset group |
Fixed asset number |
Name |
Area |
Locaton/Comments |
MfgEquip |
ME_COG_42M |
1923 |
Sanimatic (Liquids CIP SYSTEM) |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
1982 |
Type (316) Air-Powe (diaphragm pump) |
Suite200 |
48 |
MfgEquip |
ME_COG_42M |
55042 |
Purified Water Syst |
Suite200 |
47 |
MfgEquip |
ME_COG_42M |
55046 |
Kugler Linofill Mac |
Suite200 |
56 |
MfgEquip |
ME_COG_42M |
55047 |
Kugler Reservoir - 100 Liter Hopper |
Suite200 |
56 |
MfgEquip |
ME_COG_42M |
55048 |
Level Regulation Sy - part of the kugler |
Suite200 |
56 |
MfgEquip |
ME_COG_42M |
55049 |
Addl Rotory Piston - Kugler Pumps |
Suite200 |
56 |
MfgEquip |
ME_COG_42M |
55060 |
McBrady- Orbit Bottle Rinser |
Suite200 |
56 |
MfgEquip |
ME_COG_42M |
55145 |
Kaps All Surge Tabl |
Suite200 |
56 |
MfgEquip |
ME_COG_42M |
55154 |
Load cells - for Tank 55213 |
Suite200 |
48 |
MfgEquip |
ME_COG_42M |
55126 |
SP 500 VFD |
Suite200 |
48 |
MfgEquip |
ME_COG_42M |
55204 |
Joni Electric Tilting Kettle |
Suite200 |
48 |
MfgEquip |
ME_COG_42M |
55213 |
Atmos Open Top Tank 2000L |
Suite200 |
48 |
MfgEquip |
ME_COG_42M |
55215 |
Brawn Mixer BGMF300 - installed on Tank 55213 |
Suite200 |
48 |
MfgEquip |
ME_COG_7Yr |
1640 |
Heavy Duty floor scale |
Suite200 |
54 |
MfgEquip |
ME_COG_7Yr |
7008 |
Heavy Duty floor scale |
Suite200 |
54 |
MfgEquip |
ME_COG_7Yr |
55223 |
B-Tek Heavy Duty floor scale |
Suite200 |
54 |
MfgEquip |
ME_COG_7Yr |
55283 |
Diaphragm pump |
Suite200 |
48 |
PkgEquip |
ME_COG_7Yr |
55447 |
Kugler Pump Svc Cart |
Suite200 |
56 |
PkgEquip |
ME_COG_7Yr |
55448 |
Kugler Pump Svc Cart |
Suite200 |
56 |
MfgEquip |
ME_COG_7Yr |
55526 |
Positive Displacement Pump |
Suite200 |
48 |
MfgEquip |
ME_COG_7Yr |
55540 |
Handheld Oxygen Analyzer |
Suite200 |
54 |
PkgEquip |
PE_COG_42M |
55206 |
Bottle turntable |
Suite200 |
56 |
PkgEquip |
PE_COG_42M |
55211 |
Surge table |
Suite200 |
56 |
PkgEquip |
PE_COG_42M |
55417-1 |
Upack Conveyor |
Suite200 |
56 |
PkgEquip |
PE_COG_42M |
55210 |
Pack Off Table |
Suite200 |
54 |
PkgEquip |
PE_COG_42M |
1639 |
Liquid packoff table & conveyor |
Suite200 |
54 |
PkgEquip |
PE_COG_42M |
55040 |
12' Conveyor - new |
Suite200 |
56 |
PkgEquip |
PE_COG_7Yr |
55408 |
Sneeze Guards for Kugler |
Suite200 |
56 |
PkgEquip |
PE_COG_7Yr |
55409 |
Sliding Cover for Kugler |
Suite200 |
56 |
PkgEquip |
PE_COG_7Yr |
55441 |
BellatRx Chuck Capper |
Suite200 |
56 |
PkgEquip |
PE_COG_7Yr |
55513 |
NJM Labeler Trotter W127 |
Suite200 |
56 |
PkgEquip |
PE_COG_7Yr |
55542 |
Nitrogen Purge System |
Suite200 |
56 |
PkgEquip |
PE_COG_5Yr |
55417 |
Liquid Line U-Pack |
Suite200 |
56 |
PkgEquip |
PE_COG_5Yr |
55493 |
Liquid Line U-Track |
Suite200 |
56 |
WhseEquip |
WE_COG_42M |
C06H07318 |
ACH-1 10-60 ton trane Chiller |
WhseEquip |
outside |
OfficeEquip |
WE_COG_42M |
N/A |
Warehouse racking (S/R and R1 - R4) |
WhseEquip |
200 warehouse |
OfficeEquip |
WE_COG_42M |
N/A |
Conference Table |
WhseEquip |
office area |
OfficeEquip |
WE_COG_42M |
N/A |
Office desk sets (5 total) |
WhseEquip |
office area |
OfficeEquip |
WE_COG_42M |
N/A |
Cubicles (13 Total) |
WhseEquip |
office area |
PkgEquip |
PE_COG_5Yr |
55407 |
Conveyor |
Suite200 |
50 |
MfgEquip |
ME_COG_42M |
55123 |
SP500 VFD |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
55124 |
VFD |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
1652 |
Diaphragm pump |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
1653 |
Load cells - for Tank 1840 |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
1840 |
500 Gallon tank |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
55196-1 |
Dayton Mixer on tank 1840 |
Suite200 |
49 |
Exhibit C
MfgEquip |
ME_COG_42M |
2018 |
Diaphram Pump (Liquids) |
Suite200 |
54 - In blue cabinet |
MfgEquip |
ME_COG_42M |
7006 |
Diaphragm Pump - 1/2" PTFE |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55036 |
Diaphragm Pump - 1/2" PTFE |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55541 |
Drum Pump - Finish Thompson STTS440 |
Suite200 |
55 |
MfgEquip |
ME_COG_42M |
55261 |
100L mixing tank and SPX Air mixer |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55043 |
Drum Heating Oven |
Suite200 |
Shipping warehouse |
MfgEquip |
ME_COG_42M |
55144 |
Moyno Cavity Pump |
Suite200 |
55 |
MfgEquip |
ME_COG_42M |
55146 |
MPS Pack-Off Table and Conveyor |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55154 |
Lynx Batch - old Controller for load cells |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55180 |
SP 500 VFD |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
55127 |
SP 500 VFD |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
55198 |
SP 500 VFD |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
55128 |
SP 500 VFD |
Suite200 |
49 |
MfgEquip |
ME_COG_42M |
55182 |
Portable Mixer 2hp |
Suite200 |
Sold with Groen Kettle - June 22 |
MfgEquip |
ME_COG_42M |
55186 |
30G Stainless Steel |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55189 |
Portable Mixer 1/2 HP |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55190 |
Portable Mixer 1/2 HP |
Suite200 |
54 |
MfgEquip |
ME_COG_42M |
55200 |
Moyno Cavity Pump |
Suite200 |
54 |
MfgEquip |
ME_COG_7Yr |
55060-02 |
Chg Prts 125ML&90ML |
Suite200 |
55 |
MfgEquip |
ME_COG_7Yr |
55226-01 |
AC Tech Inverter - LenzeVFD |
Suite200 |
49 |
MfgEquip |
ME_COG_7Yr |
55243 |
Brawn Mixer BGMF150 |
Suite200 |
On a shelf in shipping dock. |
MfgEquip |
ME_COG_7Yr |
55243.02 |
17 AF# Impeller |
Suite200 |
On a shelf in shipping dock. |
MfgEquip |
ME_COG_7Yr |
55243.03 |
15# A35 Impeller |
Suite200 |
On a shelf in shipping dock. |
MfgEquip |
ME_COG_7Yr |
55243.04 |
SS Polished Shaft |
Suite200 |
On a shelf in shipping dock. |
MfgEquip |
ME_COG_7Yr |
55266 |
"2"" Diaphragm Pump" NT Mfg |
Suite200 |
54 - in blue cabinet |
PkgEquip |
ME_COG_7Yr |
N/A |
Presuure Tank PC-37 |
Suite200 |
54 |
PkgEquip |
PE_COG_42M |
N/A |
Stainless Steel Lidding & Foil Racks |
Suite200 |
54 |
PkgEquip |
PE_COG_42M |
55224 |
Eastey - Tape Machine |
Suite200 |
54 |
LAB |
LAB |
2473 |
Stability Chamber |
Suite200 |
54 |
LAB |
LAB |
2474 |
Stability Chamber |
Suite200 |
54 |
LAB |
LAB |
2475 |
Stability Chamber |
Suite200 |
54 |
LAB |
LAB |
2308 |
Incubator |
Suite200 |
54 |
PkgEquip |
PE_COG_42M |
N/A |
Scate Conveyors (3 Total) |
Suite200 |
55 |
PkgEquip |
PE_COG_42M |
55026 |
VideoJet Data Flex Controller |
Suite200 |
54 |
WhseEquip |
WE_COG_42M |
QA Label /Rejection |
Cage Material |
WhseEquip |
200 warehouse |
Exhibit C
EXHIBIT D
FORM BILL OF SALE
BILL OF SALE
FOR VALUE RECEIVED, the sufficiency of which is hereby acknowledged, Neos Therapeutics, LP, a Texas limited partnership (“Seller”), does hereby transfer, assign, sell and convey to AMT Manufacturing Solutions, LLC, a Delaware limited liability company (“Purchaser”), all of the furniture, equipment and other personal property described on Exhibit C attached hereto (the “Assets”), which are currently located a portion of Suite 200 of in those certain premises at 2490 N. Highway 360, Grand Prairie, Texas.
Seller warrants and represents that it currently holds title to the Assets free and clear of any liens or encumbrances. EXCEPT AS STATED IN THE PRECEDING SENTENCE, SELLER MAKES NO WARRANTY OR REPRESENTATION WHATSOEVER REGARDING THE ASSETS AND EXPRESSLY EXCLUDES ANY SUCH WARRANTY OR REPRESENTATION, EITHER EXPRESS OR IMPLIED, AS TO THE MANUFACTURE, FITNESS, MERCHANTABILITY, QUALITY, CONDITION, CAPACITY, SUITABILITY, OR FITNESS FOR A PARTICULAR PURPOSE OF THE ASSETS. THE ASSETS ARE SOLD TO PURCHASER AS IS, WHERE IS, AND WITH ALL FAULTS AND DEFECTS.
WHEREFORE, Seller has executed this Bill of Sale as of the ___ day of ________, 2023.
NEOS THERAPEUTICS, LP,
a Texas limited partnership
By:
Print Name:
Title:
Exhibit C
Exhibit 10.48
TRAMMELL CROW COMPANY
COMMERCIAL LEASE AGREEMENT
WALSTIB, L.P., A DELAWARE LIMITED PARTNERSHIP
Landlord
AND
PHARMAFAB, INC.
Tenant
TABLE OF CONTENTS
|
|
Page |
1. BASIC PROVISIONS |
1 |
|
1.1.Parties |
1 |
|
1.2.Premises |
1 |
|
1.3.Term |
1 |
|
1.4.Base Rent |
1 |
|
1.5.Tenant’s Share of Operating Expenses |
1 |
|
1.6.Tenant’s Estimated Monthly Rent Payment |
1 |
|
1.7.Security Deposit |
2 |
|
1.8.Permitted Use |
2 |
|
1.9.Guarantor |
2 |
|
1.10.Addenda and Exhibits |
2 |
|
1.11.Address for Rent Payments |
2 |
|
|
|
|
2. PREMISES, PARKING AND COMMON AREAS |
2 |
|
2.1.Letting |
2 |
|
2.2.Common Areas - Definition |
2 |
|
2.3.Common Areas - Tenant’s Rights |
3 |
|
2.4.Common Areas - Rules and Regulations |
3 |
|
2.5.Common Area Changes |
3 |
|
|
|
|
3. TERM |
3 |
|
3.1.Term |
3 |
|
3.2.Delay in Possession |
3 |
|
3.3.Commencement Date Certificate |
4 |
|
|
|
|
4. RENT |
4 |
|
4.1.Base Rent |
4 |
|
4.2.Operating Expenses |
5 |
|
|
|
|
5. SECURITY DEPOSIT |
7 |
|
|
|
|
6. USE |
7 |
|
6.1.Permitted Use |
7 |
|
6.2.Hazardous Substances |
7 |
|
6.3.Tenant’s Compliance with Requirements |
8 |
|
6.4.Inspection; Compliance with Law |
9 |
|
6.5.Existing Environmental Conditions |
9 |
|
|
|
|
7. MAINTENANCE, REPAIRS, TRADE FIXTURES AND ALTERATIONS |
9 |
|
7.1.Tenant’s Obligations |
9 |
|
7.2.Landlord’s Obligations |
10 |
|
7.3.Alterations |
10 |
|
7.4.Surrender/Restoration |
10 |
|
i
8. INSURANCE; INDEMNITY |
10 |
|
8.1.Payment of Premiums |
10 |
|
8.2.Tenant’s Insurance |
10 |
|
8.3.Landlord’s Insurance |
11 |
|
8.4.Waiver of Subrogation |
11 |
|
8.5.Indemnity |
11 |
|
8.6.Exemption of Landlord from Liability |
12 |
|
8.7.Breach of Lease by Landlord |
12 |
|
|
|
|
9. DAMAGE OR DESTRUCTION |
12 |
|
9.1.Termination Right |
12 |
|
9.2.Damage Caused by Tenant |
13 |
|
|
|
|
10. REAL PROPERTY TAXES |
13 |
|
10.1.Payment of Real Property Taxes |
13 |
|
10.2.Real Property Tax Definition |
13 |
|
10.3.Additional Improvements |
13 |
|
10.4.Joint Assessment |
13 |
|
10.5.Tenant’s Property Taxes |
14 |
|
|
|
|
11. UTILITIES |
14 |
|
|
|
|
12. ASSIGNMENT AND SUBLETTING |
14 |
|
12.1.Landlord’s Consent Required |
14 |
|
12.2.Rent Adjustment |
15 |
|
|
|
|
13. DEFAULT; REMEDIES |
15 |
|
13.1.Default |
15 |
|
13.2.Remedies |
16 |
|
13.3.Late Charges |
16 |
|
|
|
|
14. CONDEMNATION |
16 |
|
|
|
|
15. ESTOPPEL CERTIFICATE AND FINANCIAL STATEMENTS |
16 |
|
15.1.Estoppel Certificate |
16 |
|
15.2.Financial Statement |
17 |
|
|
|
|
16. ADDITIONAL COVENANTS AND PROVISIONS |
17 |
|
16.1.Severability |
17 |
|
16.2.Interest on Past-Due Obligations |
17 |
|
16.3.Time of Essence |
17 |
|
16.4.Landlord Liability |
17 |
|
16.5.No Prior or Other Agreements |
17 |
|
16.6.Notice Requirements |
17 |
|
16.7.Date of Notice |
18 |
|
16.8.Waivers |
18 |
|
16.9.Holdover |
18 |
|
16.10.Cumulative Remedies |
18 |
|
ii
16.11.Binding Effect; Choice of Law |
18 |
|
16.12.Landlord |
18 |
|
16.13.Attorneys’ Fees and Other Costs |
19 |
|
16.14.Landlord’s Access; Showing Premises; Repairs |
19 |
|
16.15.Signs |
19 |
|
16.16.Termination; Merger |
19 |
|
16.17.Quiet Possession |
19 |
|
16.18.Subordination; Attornment; Non-Disturbance |
20 |
|
16.19.Financing by Tenant |
20 |
|
16.20.Rules and Regulations |
21 |
|
16.21.Security Measures |
21 |
|
16.22.Reservations |
21 |
|
16.23.Conflict |
21 |
|
16.24.Offer |
21 |
|
16.25.Amendments |
21 |
|
16.26.Multiple Parties |
21 |
|
16.27.Authority |
21 |
|
|
|
|
SIGNATURES |
22 |
|
EXHIBIT A |
|
|
EXHIBIT B |
|
|
EXHIBIT C |
|
|
EXHIBIT D |
|
|
iii
GLOSSARY
The following terms in the Lease are defined in the paragraphs opposite the terms.
TERM |
DEFINED IN PARAGRAPH |
Additional Rent |
4.1 |
Applicable Requirements |
6.3 |
Assign |
12.1 |
Base Rent |
1.4 |
Basic Provisions |
1.1 |
Building |
1.2 |
Building Operating Expenses |
4.2(b) |
Code |
12.1 |
Commencement Date |
1.3 |
Commencement Date Certified |
3.3 |
Common Areas |
2.2 |
Common Area Operating Expenses |
4.2(b) |
Condemnation |
14 |
Default |
13.1 |
Expiration Date |
1.3 |
HVAC |
4.2(a) |
Hazardous Substance |
6.2 |
Indemnity |
8.5 |
Industrial Center |
1.2 |
Landlord |
1.1 |
Landlord Entities |
6.2(c) |
Lease |
1.1 |
Lenders |
6.4 |
Mortgage |
16.18 |
Operating Expenses |
4.2 |
Party/Parties |
1.1 |
Permitted Use |
1.8 |
Premises |
1.2 |
Prevailing Party |
16.13 |
Real Property Taxes |
10.2 |
Rent |
4.1 |
Reportable Use |
6.2 |
Requesting Party |
15 |
Responding Party |
15 |
Rules and Regulations |
2.4 |
Security Deposit |
1.7 |
Taxes |
10.2 |
Tenant |
1.1 |
Tenant Acts |
9.2 |
Tenant’s Share |
1.5 |
Term |
1.3 |
Use |
6.1 |
iv
WALSTIB, L.P.,
A DELAWARE LIMITED PARTNERSHIP
INDUSTRIAL MULTI-TENANT LEASE
1.Basic Provisions (“Basic Provisions”).
1.1.Parties: This Lease (“Lease”) dated June ____, 1999, is made by and between WALSTIB, L.P., a Delaware limited partnership (“Landlord”) and PHARMAFAB, INC., a Texas corporation (“Tenant”) (collectively the “Parties,” or individually a “Party”).
1.2.Premises: A portion, outlined on Exhibit A attached hereto (“Premises”), of the building (“Building”) located at 360 Riverside Business Center (Building B) in the City of Grand Prairie, State of Texas. The Building is located in the industrial center commonly known as 360 Riverside Business Center (the “Industrial Center”). Tenant shall have non-exclusive rights to the Common Areas (as defined in Paragraph 2.3 below), but shall not have any rights to the roof exterior walls or utility raceways of the Building or to any other buildings in the Industrial Center. The Premises, the Building, the Common Areas, the land upon which they are located and all other buildings and improvements thereon are herein collectively referred to as the “Industrial Center.”
1.3.Term: The period (“Term”) commencing on July 15, 1999, subject to the provisions of Section 3.2 below (“Commencement Date”), and ending July 31, 2006 (“Expiration Date”).
1.4.Base Rent: Initially, the sum of $28,930.00 per month, subject to adjustment as set forth below (“Base Rent”). The first installment of Base Rent in the amount of $28,930.00 shall be payable on execution of this Lease. The Base Rent shall be as follows:
Months |
Net PSF |
Monthly |
1-12 |
$7.89 |
$28,930.00 |
13-60 |
$8.70 |
$31,900.00 |
61-72 |
$9.58 |
$35,126.67 |
73-84 |
$9.98 |
$36,593.33 |
1.5.Tenant’s Share of Operating Expenses (“Tenant’s Share”):
(a) |
Industrial Park |
20.5 |
% |
(b) |
Building |
38.7 |
% |
1.6.Tenant’s Estimated Monthly Rent Payment: Following is the estimated monthly Rent payment to Landlord pursuant to the provisions of this Lease. This estimate is made at the inception of the Lease and is subject to adjustment pursuant to the provisions of this Lease:
(a) |
Base Rent (Paragraph 4.1) |
$ |
28,930.00 |
(b) |
Operating Expenses (Paragraph 4.2; |
$ |
1,283.33 |
(c) |
Landlord Insurance (Paragraph 8.3) |
$ |
183.33 |
1
(d) |
Real Property Taxes (Paragraph 10) |
$ |
3,300.00 |
|
|
|
|
|
Estimated Monthly Payment |
$ |
33,696.66 |
1.7.Security Deposit: $38,000.00 (“Security Deposit”).
1.8.Permitted Use: Manufacturing, storage and distribution of pharmaceutical products (“Permitted Use”).
1.9.Guarantor: Bruce K. Montgomery and Darlene Ryan.
1.10.Addenda and Exhibits: Attached hereto are the following Addenda and Exhibits, all of which constitute a part of this Lease:
(a) |
Addenda: |
Remedies Addendum |
|
|
|
Tenant Improvements Addendum |
|
|
|
Option To Extend Addendum |
|
|
|
Additional Security Deposit Addendum |
|
(b) |
Exhibits: |
Exhibit A: |
Diagram of Premises. |
|
|
Exhibit B: |
Commencement Date Certificate. |
|
|
Exhibit C: |
Signage Criteria |
|
|
Exhibit D: |
Subordination Agreement |
1.11.Address for Rent Payments: All amounts payable by Tenant to Landlord shall until further notice from Landlord be paid to WALSTIB, L.P., a Delaware limited partnership at the following address:
c/o Trammell Crow Dallas/Fort Worth
801 Avenue H East, Suite 101
Arlington, Texas 76011
2.Premises, Parking and Common Areas.
2.1.Letting. Landlord hereby leases to Tenant and Tenant hereby leases from Landlord the Premises upon all of the terms, covenants and conditions set forth in this Lease. Any statement of square footage set forth in this Lease or that may have been used in calculating Base Rent and/or Operating Expenses is an approximation which Landlord and Tenant agree is reasonable and the Base Rent and Tenant’s Share based thereon is not subject to revision whether or not the actual square footage is more or less.
2.2.Common Areas - Definition. “Common Areas” are all areas and facilities outside the Premises and within the exterior boundary line of the Industrial Center and interior utility raceways within the Premises that are provided and designated by the Landlord from time to time for the general non-exclusive use of Landlord, Tenant and other tenants of the Industrial Center and their respective employees, suppliers, shippers, tenants, contractors and invitees.
2
2.3.Common Areas - Tenant’s Rights. Landlord hereby grants to Tenant, for the benefit of Tenant and its employees, suppliers, shippers, contractors, customers and invitees, during the Term of this Lease, the non-exclusive right to use, in common with others entitled to such use, the Common Areas as they exist from time to time, subject to any rights, powers, and privileges reserved by Landlord under the terms hereof or under the terms of any rules and regulations or covenants, conditions and restrictions governing the use of the Industrial Center.
2.4.Common Areas - Rules and Regulations. Landlord shall have the exclusive control and management of the Common Areas and shall have the right, from time to time, to establish, modify, amend and enforce reasonable Rules and Regulations with respect thereto in accordance with Paragraph 16.19.
2.5.Common Area Changes. Landlord shall have the right, in Landlord’s sole discretion, from time to time:
(a)To make such changes to the Common Areas as Landlord, in the exercise of sound business judgment, may deem to be appropriate, including, without limitation, changes in the locations, size, shape and number of driveways, entrances, parking spaces, parking areas, loading and unloading areas, ingress, egress, direction of traffic, landscaped areas, walkways and utility raceways; provided, however, that no such changes shall result in access to the Premises or the parking areas or loading areas adjacent to the Premises being denied to Tenant, and in any event Landlord shall use reasonable efforts to minimize the extent to which any such changes in the Common Areas will impede or interfere with access to the Premises, including the use of parking spaces and loading areas adjacent to the Premises and the use of driveways providing ingress and egress to and from the Premises.
(b)To close temporarily any of the Common Areas for maintenance purposes so long as reasonable access to the Premises remains available;
(c)To designate other land outside the boundaries of the Industrial Center to be a part of the Common Areas;
(d)To add additional buildings and improvements to the Common Areas;
(e)To use the Common Areas while engaged in making additional improvements, repairs or alterations to the Industrial Center, or any portion thereof; and
(f)To do and perform such other acts and make such other changes in, to or with respect to the Common Areas and Industrial Center as Landlord may, in the exercise of sound business judgment, deem to be appropriate.
3.Term.
3.1.Term. The Commencement Date, Expiration Date and Term of this Lease are as specified in Paragraph 1.3.
3.2.Delay in Possession. If for any reason Landlord cannot deliver possession of the Premises to Tenant by the Commencement Date, Landlord shall not be subject to any liability
3
therefor, nor shall such failure affect the validity of this Lease or the obligations of Tenant hereunder. In such case, Tenant shall not, except as otherwise provided herein, be obligated to pay Rent or perform any other obligation of Tenant under the terms of this Lease until Landlord delivers possession of the Premises to Tenant. The term of the Lease shall commence on the earlier of (a) the date upon which Tenant takes possession of the Premises, or (b) ten (10) days following notice to Tenant that the Leasehold Improvements (as defined in the Tenant Improvements Addendum) are substantially complete (as such term is defined in the Tenant Improvements Addendum) and Landlord is prepared to tender possession of the Premises to Tenant. If possession of the Premises is not delivered to Tenant within sixty (60) days after the receipt of a building permit in respect of the Premises from the City of Grand Prairie and such delay is not due to Tenant’s acts, failure to act or omissions, then Tenant shall be entitled, as its sole remedy, to receive one (1) day’s rental abatement (effective as of the Commencement Date) for each day of delay beyond such sixty (60) day period. If possession of the Premises is not delivered to Tenant within ninety (90) days after the receipt of a building permit in respect of the Premises from the City of Grand Prairie and such delay is not due to Tenant’s acts, failure to act or omissions, then Tenant shall be entitled, as its sole remedy, to receive two (2) days’ rental abatement (effective as of the Commencement Date) for each day of delay beyond such ninety (90) day period. If possession of the Premises is not delivered to Tenant within one hundred twenty (120) days after the receipt of a building permit in respect of the Premises from the City of Grand Prairie and such delay is not due to Tenant’s acts, failure to act or omissions, then Tenant may, as its sole remedy, cancel this Lease by notice in writing to Landlord within ten (10) days after the end of said one hundred twenty (120) day period, and in such event the parties shall be discharged from all obligations hereunder. If such written notice from Tenant is not received by Landlord within said ten (10) day period, Tenant’s right to cancel this Lease shall terminate. If the Commencement Date is after August 1, 1999, and is not the first day in a calendar month, then the Term shall end, and the Expiration Date shall be, the last day of the eighty-four (84) month period that begins on the first day of the first full calendar month of the Term.
3.3.Commencement Date Certificate. At the request of Landlord, Tenant shall execute and deliver to Landlord a completed certificate (“Commencement Date Certificate”) in the form attached hereto as Exhibit B.
4.Rent.
4.1.Base Rent. Tenant shall pay to Landlord Base Rent and other monetary obligations of Tenant to Landlord under the terms of this Lease (such other monetary obligations are herein referred to as “Additional Rent”) in lawful money of the United States, without offset or deduction, in advance on or before the first day of each month. Base Rent and Additional Rent for any period during the term hereof which is for less than one full month shall be prorated based upon the actual number of days of the month involved. Payment of Base Rent and Additional Rent shall be made to Landlord at its address stated herein or to such other persons or at such other addresses as Landlord may from time to time designate in writing to Tenant. Base Rent and Additional Rent are collectively referred to as “Rent”. All monetary obligations of Tenant to Landlord under the terms of this Lease are deemed to be rent.
4
4.2.Operating Expenses. Tenant shall pay to Landlord on the first day of each month during the term hereof, in addition to the Base Rent, Tenant’s Share of all Operating Expenses in accordance with the following provisions:
(a)“Operating Expenses” are all costs incurred by Landlord relating to the ownership and operation of the Industrial Center, Building and Premises including, but not limited to, the following:
(i)The operation, repair, maintenance and replacement in neat, clean, good order and condition of the Common Areas, including parking areas, loading and unloading areas, trash areas, roadways, sidewalks, walkways, parkways, driveways, landscaped areas, striping, bumpers, irrigation systems, drainage systems, lighting facilities, fences and gates, exterior signs and tenant directories.
(ii)Water, gas, electricity, telephone and other utilities servicing the Common Areas.
(iii)Trash disposal, janitorial services, snow removal, property management and security services.
(iv)Reasonable reserves set aside for maintenance, repair and replacement of the Common Areas and Building.
(v)Real Property Taxes.
(vi)Premiums for the insurance policies maintained by Landlord under Paragraph 8 hereof.
(vii)Environmental monitoring and insurance programs.
(viii)Monthly amortization of capital improvements to the Common Areas and the Building, it being agreed that the monthly amortization of any given capital improvement shall be equal to the quotient obtained by dividing the cost of the capital improvement by Landlord’s estimate of the number of months of useful life of such improvement.
(ix)Maintenance of the Building including, but not limited to, painting, caulking and repair and replacement of Building components, including, but not limited to, roof, elevators, mechanical, systems, and fire detection and sprinkler systems.
(x)If Tenant fails to maintain the Premises, any expense incurred by Landlord for such maintenance.
(b)Tenant’s Share of Operating Expenses that are not specifically attributed to the Premises or Building (“Common Area Operating Expenses”) shall be that percentage shown in Paragraph 1.5(a). Tenant’s Share of Operating Expenses that are attributable to the Building (“Building Operating Expenses”) shall be that percentage shown in Paragraph 1.5(b). Landlord in its reasonable discretion shall determine which Operating Expenses are Common Area Operating Expenses, Building Operating Expenses or expenses to be entirely borne by Tenant.
5
(c)The inclusion of the improvements, facilities and services set forth in Subparagraph 4.2(a) shall not be deemed to impose any obligation upon Landlord to either have said improvements or facilities or to provide those services.
(d)Tenant shall pay monthly in advance on the same day as the Base Rent is due Tenant’s Share of estimated Operating Expenses in the amount set forth in Paragraph 1.6. Landlord shall deliver to Tenant within ninety (90) days after the expiration of each calendar year a reasonably detailed statement showing Tenant’s Share of the actual Operating Expenses incurred during the preceding year. If Tenant’s estimated payments under this Paragraph 4(d) during the preceding year exceed Tenant’s Share as indicated on said statement, Tenant shall be credited the amount of such overpayment against Tenant’s Share of Operating Expenses next becoming due. If Tenant’s estimated payments under this Paragraph 4.2(d) during said preceding year were less than Tenant’s Share as indicated on said statement, Tenant shall pay to Landlord the amount of the deficiency within ten (10) days after delivery by Landlord to tenant of said statement. At any time Landlord may adjust the amount of the estimated Tenant’s Share of Operating Expenses to reflect Landlord’s estimate of such expenses for the year.
(e)Notwithstanding anything contained herein to the contrary, the Controllable Operating Expenses (as hereinafter defined) payable by Tenant for each calendar year after 2000 shall not be more than the sum of (i) the aggregate amount of Controllable Operating Expenses for the year 2000 and (ii) the product obtained by multiplying (A) .15, times (B) the number of complete calendar years that have elapsed between January 1 of the year 2000 and January 1 of the year for which such calculation is being made, times (C) the aggregate amount of Controllable Operating Expenses for the year 2000. For purposes of this Lease, the term “Controllable Operating Expenses” shall mean all items of Operating Expenses which are within the reasonable control of Landlord; thus, excluding Real Property Taxes, insurance, utilities, and other costs beyond the reasonable control of Landlord. The limit on the increases in Controllable Operating Expenses shall continue during any renewal or extended Term, using the year 2000 as the base year to calculate the applicable limit.
(f)After giving Landlord thirty (30) days’ prior written notice thereof, Tenant may inspect or audit Landlord’s records relating to Operating Expenses for any periods of time within one year before the audit or inspection; however, no audit or inspection shall extend to periods of time before the Commencement Date. If Tenant fails to object to the calculation of Operating Expenses on an annual Operating Expense statement within thirty (30) days after the statement has been delivered to Tenant, then Tenant shall have waived its right to object to the calculation of Operating Expenses for the year in question and the calculation of Operating Expenses set forth on such statement shall be final. Tenant’s audit or inspection shall be conducted only during business hours reasonably designated by Landlord. Tenant shall pay the cost of such audit or inspection, including $100 per hour of Landlord’s or the building manager’s employee time devoted to such inspection or audit, to reimburse Landlord for its overhead costs allocable to the inspection or audit, unless the total Operating Expenses charged to Tenant for the time period in question is determined to be in error by more than five percent (5 %) in the aggregate, in which case Landlord shall pay the audit cost. Tenant may not conduct an inspection or have an audit performed more than once during any calendar year. If such inspection or audit reveals that an error was made in the Operating Expenses previously charged to Tenant, then Landlord shall refund to Tenant any overpayment of any such costs, or Tenant shall pay to Landlord any underpayment of any such costs, as the case may be, within thirty (30) days after notification thereof.
6
Tenant shall maintain the results of each such audit or inspection confidential and shall not be permitted to use any third party to perform such audit or inspection, other than an independent firm of certified public accountants reasonably acceptable to Landlord which agrees with Landlord in writing to maintain the results of such audit or inspection confidential.
5.Security Deposit. Tenant shall deposit with Landlord upon Tenant’s execution hereof the Security Deposit set forth in Paragraph 1.7 as security for Tenant’s faithful performance of Tenants obligations under this Lease. If Tenant fails to pay Base Rent or Additional Rent or otherwise defaults under this Lease (as defined in Paragraph 13.1), Landlord may use the Security Deposit for the payment of any amount due Landlord or to reimburse or compensate Landlord for any liability, cost, expense, loss or damage (including attorney’s fees) which Landlord may suffer or incur by reason thereof. Tenant shall on demand pay Landlord the amount so used or applied so as to restore the Security Deposit to the amount set forth in Paragraph 1.7. Landlord shall not be required to keep all or any part of the Security Deposit separate from its general accounts. Landlord shall, at the expiration or earlier termination of the term hereof and after Tenant has vacated the Premises, return to Tenant that portion of the Security Deposit not used or applied by Landlord. No part of the Security Deposit shall be considered to be held in trust, to bear interest, or to be prepayment for any monies to be paid by Tenant under this Lease.
6.Use.
6.1.Permitted Use. Tenant shall use and occupy the Premises only for the Permitted Use set forth in Paragraph 1.8. Tenant shall not commit any nuisance, permit the emission of any objectionable noise or odor, suffer any waste, or make any use of the Premises which is contrary to any law or ordinance, or which would invalidate any of Landlord’s insurance, or which would increase the premiums for any insurance carried by Landlord which is typically carried by landlords of properties comparable to the Industrial Center. Tenant shall not service, maintain or repair vehicles on the Premises, Building or Common Areas. Tenant shall not store foods, pallets, drums or any other materials outside the Premises.
6.2.Hazardous Substances.
(a)Reportable Uses Require Consent. The term “Hazardous Substance” as used in this Lease shall mean any product, substance, chemical, material or waste whose presence, nature, quantity and/or intensity of existence, use, manufacture, disposal, transportation, spill, release or effect, either by itself or in combination with other materials expected to be on the Premises, is either: (i) potentially injurious to the public health, safety or welfare, the environment, or the Premises; (ii) regulated or monitored by any governmental authority; or (iii) a basis for potential liability of Landlord to any governmental agency or third party under any applicable statute or common law theory. Hazardous Substance shall include, but not be limited to, hydrocarbons, petroleum, gasoline, crude oil or any products or by-products thereof. Landlord acknowledges and agrees that Tenant will be using the Premises for the manufacture, storing and distribution of pharmaceutical and related materials (some of which may contain Hazardous Substances) in the ordinary course of Tenant’s business (collectively, “Tenant’s Products”). Nothing contained in this Lease shall be construed to prohibit Tenant from bringing Tenant’s Products upon the Premises; provided, however, that Tenant must comply with all Applicable Requirements in respect of Tenant’s Products.
7
With the exception of Tenant’s Products, Tenant shall not, without the prior written consent of Landlord, generate, possess, store, use, transport, or dispose of a Hazardous Substance (i) that requires a permit from, or with respect to which a report, notice, registration or business plan is required to be filed with, any governmental authority, or (ii) with respect to which any Applicable Requirements require that a notice be given to persons entering or occupying the Premises or neighboring properties. Furthermore, Tenant shall not install or use any above or below ground storage tank in the Industrial Center.
(b)Duty to Inform Landlord. If Tenant knows that a Hazardous Substance is located in, under or about the Premises or the Building in violation of Applicable Requirements, Tenant shall immediately give Landlord written notice thereof, together with a copy of any statement, report, notice, registration, application, permit, business plan, license, claim, action, or proceeding given to, or received from, any governmental authority or private party concerning the presence, spill, release, discharge of, or exposure to, such Hazardous Substance. Tenant shall not cause or permit any Hazardous Substance to be spilled or released in, on, under or about the Premises (including, without limitation, through the plumbing or sanitary sewer system) in violation of Applicable Requirements.
(c)Indemnification. Tenant shall indemnify, protect, defend and hold Landlord, Landlord’s affiliates, Lenders, and the officers, directors, shareholders, partners, employees, managers, independent contractors, attorneys and agents of the foregoing (“Landlord Entities”) and the Premises, harmless from and against any and all damages, liabilities, judgments, costs, claims, liens, expenses, penalties, loss of permits and attorneys’ and consultants’ fees arising out of or involving any Hazardous Substance brought onto the Premises by or for Tenant or by any of Tenant’s employees, agents, contractors or invitees. Tenant’s obligations under this Paragraph 6.2(c) shall include, but not be limited to, the effects of any contamination or injury to person, property or the environment created or suffered by Tenant, and the cost of investigation (including consultants’ and attorneys’ fees and testing), removal, remediation, restoration and/or abatement thereof, or of any contamination therein involved. Tenant’s obligations under this Paragraph 6.2(c) shall survive the expiration or earlier termination of this Lease.
6.3.Tenant’s Compliance with Requirements. Tenant shall, at Tenant’s sole cost and expense, fully, diligently and in a timely manner, comply with all “Applicable Requirements,” which term is used in this Lease to mean all laws, rules, regulations, ordinances, directives, covenants, easements and restrictions of record, permits, the requirements of any applicable fire insurance underwriter or rating bureau, and the recommendations of Landlord’s engineers and/or consultants, relating in any manner to the Premises (including but not limited to matters pertaining to (i) industrial hygiene, (ii) environmental conditions on, in, under or about the Premises, including soil and groundwater conditions, and (iii) the use, generation, manufacture, production, installation, maintenance, removal, transportation, storage, spill or release of any Hazardous Substance), now in effect or which may hereafter come into effect. Tenant shall, within five (5) days after receipt of Landlord’s written request, provide Landlord with copies of all documents and information evidencing Tenant’s compliance with any Applicable Requirements and shall immediately upon receipt, notify Landlord in writing (with copies of any documents involved) of any threatened or actual claim, notice, citation, warning, complaint or report pertaining to or involving failure by Tenant or the Premises to comply with any Applicable Requirements.
8
6.4.Inspection; Compliance with Law. In addition to Landlord’s environmental monitoring and insurance program, the cost of which is included in Operating Expenses, Landlord and the holders of any mortgages, deeds of trust or ground leases on the Premises (“Lenders”) shall have the right to enter the Premises at any time in the case of an emergency, and otherwise at reasonable times, for the purpose of inspecting the condition of the Premises and for verifying compliance by Tenant with this Lease and all Applicable Requirements. Landlord shall be entitled to employ experts and/or consultants in connection therewith to advise Landlord with respect to Tenant’s installation, operation, use, monitoring, maintenance, or removal of any Hazardous Substance on or from the Premises. Any inspection of the Premises conducted by Landlord or Lenders (other than in the event of an emergency) shall be done in compliance with any applicable requirements of the federal Food and Drug Administration (the “FDA”) or any requirements imposed by Tenant in order to comply with requirements of the FDA. The cost and expenses of any such inspections shall be paid by the party requesting same unless a violation of Applicable Requirements exists or is imminent or the inspection is requested or ordered by a governmental authority. In such case, Tenant shall upon request reimburse Landlord or Landlord’s Lender, as the case may be, for the costs and expenses of such inspections.
6.5.Existing Environmental Conditions. Prior to the execution of this Lease, Landlord has delivered to Tenant an environmental report (the “Environmental Report”) in respect of the Building commissioned by Landlord. Landlord represents to Tenant that as of the date hereof Landlord has no actual knowledge of any environmental matters affecting the Property other than those disclosed in the Environmental Report.
7.Maintenance, Repairs, Trade Fixtures and Alterations.
7.1.Tenant’s Obligations. Subject to the provisions of Paragraph 7.2 (Landlord’s Obligations), Paragraph 9 (Damage or Destruction) and Paragraph 14 (Condemnation), Tenant shall, at Tenant’s sole cost and expense and at all times, keep the Premises and every part thereof in good order, condition and repair (whether or not such portion of the Premises requiring repair, or the means of repairing the same, are reasonable or readily accessible to Tenant and whether or not the need for such repairs occurs as a result of Tenant’s use, the elements or the age of such portion of the Premises) including, without limiting the generality of the foregoing, all equipment or facilities specifically serving the Premises, such as heating, ventilating and air conditioning (“HVAC”), plumbing, electrical, lighting facilities, boilers, fired or unfired pressure vessels, fire hose connectors if within the Premises, fixtures, interior walls, interior surfaces of exterior walls, ceilings, floors, windows, doors, plate glass, and skylights, but excluding any items which are the responsibility of Landlord pursuant to Paragraph 7.2 below. Heating, ventilation and air conditioning systems serving the Premises shall be operated at Tenant’s sole expense and shall be maintained, at Tenant’s sole expense, pursuant to maintenance service contracts entered into by Tenant; provided, however, that the scope of services and contractors under such maintenance contracts shall be reasonably approved by Landlord. Tenant shall be responsible for removal of snow and ice from the sidewalks adjacent to the Premises. Tenant’s obligations shall include restorations, replacements or renewals when necessary to keep the Premises and all improvements thereon or a part thereof in good order, condition and state of repair. Landlord shall grant Tenant the benefit of any assignable warranty covering the equipment serving the Premises for which Tenant is responsible hereunder.
9
7.2.Landlord’s Obligations. Subject to the provisions of Paragraph 6 (Use), Paragraph 7.1 (Tenant’s Obligations), Paragraph 9 (Damage or Destruction) and Paragraph 14 (Condemnation), Landlord at its expense and not subject to reimbursement pursuant to Paragraph 4.2, shall keep in good order, condition and repair the foundations and exterior walls of the Building and utility systems outside the Building. Landlord, subject to reimbursement pursuant to Paragraph 4.2, shall keep in good order, condition and repair the Common Areas and the roof of the Building. Landlord’s obligation to keep the Common Areas in good order, condition and repair shall include, without limitation, the obligation to keep the driveways and parking areas included in the Common Areas adequately paved, to keep such parking areas adequately striped, and to provide adequate drainage to the Common Areas.
7.3.Alterations. Tenant shall not make nor cause to be made any alterations or installations in, on, under or about the Premises without the prior written consent of Landlord, which consent shall not be unreasonably withheld. Notwithstanding the foregoing, Tenant shall not be required to obtain Landlord’s consent for alterations totaling less than $20,000 in any single instance or series of related alterations performed within a six (6) month period, provided that such alterations do not (a) involve penetration of the roof of the Building or any load-bearing walls or exterior glass panes in the Building, (b) affect any structural elements of the Building, (c) affect the configuration or location of any exterior or load-bearing interior walls of the Building, or (d) affect any mechanical systems in the Building (including, without limitation, the electrical and plumbing systems in the Building).
7.4.Surrender/Restoration. Tenant shall surrender the Premises by the end of the last day of the Lease term or any earlier termination date, clean and free of debris and in good operating order, condition and state of repair ordinary wear and tear excepted. Without limiting the generality of the above, Tenant shall remove all personal property, trade fixtures and floor bolts, patch all floors and cause all lights to be in good operating condition.
8.Insurance; Indemnity.
8.1.Payment of Premiums. The cost of the premiums for the insurance policies maintained by Landlord under this Paragraph 8 shall be a Common Area Operating Expense pursuant to Paragraph 4.2 hereof. Premiums for policy periods commencing prior to, or extending beyond, the term of this Lease shall be prorated to coincide with the corresponding Commencement Date or Expiration Date.
8.2.Tenant’s Insurance.
(a)At its sole cost and expense, Tenant shall maintain in full force and effect during the Term of the lease the following insurance coverages insuring against claims which may arise from or in connection with the Tenant’s operation and use of the leased premises.
(i)Commercial General Liability with minimum limits of $1,000,000 per occurrence; $2,000,000 general aggregate for bodily injury, personal injury and property damage. If required by Landlord, liquor liability coverage will be included.
(ii)Workers’ Compensation insurance with statutory limits and Employers Liability with a $1,000,000 per accident limit for bodily injury or disease.
10
(iii)Automobile Liability covering all owned, non-owned and hired vehicles with a $1,000,000 per accident limit for bodily injury and property damage.
(iv)Property insurance against all risks of loss to any tenant improvements or betterments and business personal property on a full replacement cost basis with no coinsurance penalty provision; and Business Interruption Insurance with a limit of liability representing loss of at least approximately six (6) months of income.
(b)Tenant shall deliver to Landlord certificates of all insurance reflecting evidence of required coverages prior to initial occupancy; and annually thereafter.
(c)All insurance required under this Paragraph 8.2: (i) shall be primary and non-contributory, (ii) shall provide for severability of interests, (iii) shall be issued by insurers licensed to do business in the state in which the Premises are located and rated A:VII or better by Best’s Key Rating Guide, (iv) shall be endorsed to include Landlord and such other persons or entities as Landlord may from time to time designate, as additional insureds (Commercial General Liability only), and (v) shall be endorsed to provide at least thirty (30)days prior notification of cancellation or material change in coverage to said additional insureds.
8.3.Landlord’s Insurance. Landlord may, but shall not be obligated to, maintain all risk, including earthquake and flood, insurance covering the buildings within the Industrial Center, Commercial General Liability and such other insurance in such amounts and covering such other liability or hazards as deemed appropriate by Landlord. The amount and scope of coverage of Landlord’s insurance shall be determined by Landlord from time to time in its sole discretion and shall be subject to such deductible amounts as Landlord may elect. Landlord shall have the right to reduce or terminate any insurance or coverage. Premiums for any such insurance shall be a Common Area Operating Expense.
8.4.Waiver of Subrogation. To the extent permitted by law and without affecting the coverage provided by insurance required to be maintained hereunder, Landlord and Tenant each waive any right to recover against the other on account of any and all claims Landlord or Tenant may have against the other with respect to property insurance actually carried, or required to be carried hereunder, to the extent of the proceeds realized from such insurance coverage.
8.5.Indemnity. Subject to Section 8.6, Tenant shall indemnify, defend, and hold harmless Landlord, its successors, assigns, agents, employees, contractors, partners, directors, officers and affiliates (collectively, the “Indemnified Parties) from and against all fines, suits, losses, costs, liabilities, claims, demands, actions and judgments of every kind or character (a) arising from Tenant’s failure to perform its covenants hereunder, (b) recovered from or asserted against any of the Indemnified Parties on account of any Loss (defined below) to the extent that any such Loss is caused by a Tenant Party or any other person entering upon the Premises under or with a Tenant Party’s express or implied invitation or permission, (c) arising from or out of the occupancy or use by a Tenant Party or arising from or out of any occurrence in the Premises, or (d) suffered by, recovered from or asserted against any of the Indemnified Parties by a Tenant Party, regardless of whether Landlord’s negligence caused such loss or damage. However, such indemnification of the Indemnified Parties by Tenant shall not be applicable if such loss, damage, or injury is caused by the gross negligence or willful misconduct of Landlord or any of its duly authorized agents or employees.
11
The provisions of this Paragraph 8.5 shall survive the termination of this Lease with respect to any claims or liability accruing prior to such termination.
8.6.Exemption of Landlord from Liability. Landlord shall not be liable to Tenant or those claiming by, through, or under Tenant for any injury to or death of any person or persons or the damage to or theft, destruction, loss or loss of use of any property or inconvenience (a “Loss”) caused by casualty, theft, fire, third parties, or any other matter (including Losses arising through repair or alteration of any part of the Building, or failure to make repairs, or from any other cause), regardless of whether the negligence (other than gross negligence) of either party caused such Loss in whole or in part, Landlord and Tenant each waives any claim it might have against the other for any damage to or theft, destruction, loss, or loss of use of any property, to the extent the same is insured against under any insurance policy maintained by it that covers the Building, the Premises, Landlord’s or Tenant’s fixtures, personal property, leasehold improvements, or business, or is required to be insured against by the waiving party under the terms hereof, regardless of whether the negligence or fault of the other party caused such loss; however, Landlord’s waiver shall not apply to any deductible amounts maintained by Landlord under its insurance. Each party shall cause its insurance carrier to endorse all applicable policies waiving the carrier’s rights of recovery under subrogation or otherwise against the other party.
8.7.Breach of Lease by Landlord. Nothing contained in Section 8.5 or Section 8.6 above shall be construed to limit the remedies for breach of contract which Tenant may have for breach of this Lease by Landlord (it being agreed, however, that such remedies shall in all events be subject to other applicable provisions of this Lease, including, without limitation, Section 16.4 hereof).
9.Damage or Destruction.
9.1.Termination Right. Tenant shall give Landlord immediate written notice of any damage to the Premises. Thereafter, Landlord shall send Tenant written notice (the “Estimated Time Notice”) of the estimated period of time, as determined by Landlord’s architect, that repair of such damage will substantially interfere with the conduct of Tenant’s business at the Premises. Subject to the provisions of Paragraph 9.2, if the damage to the Premises is such that, in the opinion of Landlord’s architect as set forth in the Estimated Time Notice, there will be substantial interference with the conduct by Tenant of its business at the Premises for a period exceeding ninety (90) consecutive days, then Tenant may terminate this Lease by sending Landlord written notice thereof within ten (10) days after Tenant receives the Estimated Time Notice (time being of the essence), which termination shall be effective thirty (30) days after delivery of such notice of termination to Landlord. If the Estimated Time Notice states that repair of damage will not interfere with the conduct of Tenant’s business at the Premises for more than ninety (90) consecutive days, but such repair does in fact interfere with the conduct of Tenant’s business at the Premises for a period in excess of ninety (90) days (excluding delays caused by events of force majeure), then Tenant may terminate this Lease by sending Landlord written notice thereof within ten (10) days after the expiration of such ninety (90) day period (time being of the essence), which termination shall be effective thirty (30) days after delivery of such notice of termination to Landlord. No such termination shall excuse the performance by Tenant of those covenants which under the terms hereof survive termination. Rent shall be abated in proportion to the degree of interference during the period that there is such substantial interference with the conduct of Tenant’s business at the Premises.
12
Abatement of rent and Tenant’s right of termination pursuant to this provision shall be Tenant’s only remedies for failure of Landlord to keep in good order, condition and repair the foundations and exterior walls of the Building, Building roof, utility systems outside the Building, the Common Areas and HVAC.
9.2.Damage Caused by Tenant. Tenant’s termination rights under Paragraph 9.1 shall not apply if the damage to the Premises or Building is the result of any act or omission of Tenant or of any of Tenant’s agents, employees, customers, invitees or contractors (“Tenant Acts”). Any damage resulting from a Tenant Act shall be promptly repaired by Tenant. Landlord at its option may at Tenant’s expense repair any damage caused by Tenant Acts. Tenant shall continue to pay all rent and other sums due hereunder and shall be liable to Landlord for all damages that Landlord may sustain resulting from a Tenant Act.
10.Real Property Taxes.
10.1.Payment of Real Property Taxes. Landlord shall pay the Real Property Taxes due and payable during the term of this Lease and, except as otherwise provided in Paragraph 10.3, any such amounts shall be included in the calculation of Operating Expenses in accordance with the provisions of Paragraph 4.2. The amount of Real Property Taxes included in Operating Expenses shall take into account fully any tax abatement in effect with respect to the Industrial Center, such that Tenant derives its proportionate share of the benefit of any such tax abatement.
10.2.Real Property Tax Definition. As used herein, the term “Real Property Taxes” is any form of tax or assessment, general, special, ordinary or extraordinary, imposed or levied by any governmental authority or by any owners’ association upon the Industrial Center or any interest of Landlord in the Industrial Center. Real Property Taxes include (a) any tax or charge which replaces or is in addition to any of such above-described “Real Property Taxes,” (b) any charge or assessment imposed upon the Industrial Center by an owners’ association or similar entity, and (c) any fees, expenses or costs (including attorney’s fees, expert fees and the like) incurred by Landlord in protesting or contesting any assessments levied or any tax rate. Real Property Taxes for tax years commencing prior to, or extending beyond, the term of this Lease shall be prorated to coincide with the corresponding Commencement Date or Expiration Date.
10.3.Additional Improvements. Operating Expenses shall not include Real Property Taxes attributable to improvements placed upon the Industrial Center by other tenants or by Landlord for the exclusive enjoyment of such other tenants. Notwithstanding Paragraph 10.1 hereof, Tenant shall, however, pay to Landlord at the time Operating Expenses are payable under Paragraph 4.2, the entirety of any increase in Real Property Taxes if assessed by reason of improvements placed upon the Premises by Tenant or at Tenant’s request.
10.4.Joint Assessment. If the Building is not separately assessed, Real Property Taxes allocated to the Building shall be based upon the assessed value of the Building and the land associated with the Building relative to the total assessed value of all of the land and improvements included within the tax parcel assessed (it being agreed that Tenant shall be responsible for payment of Tenant’s Share of taxes allocated to the Building, as set forth in Section 1.5(b) of the Basic Provisions). In the absence of manifest error, Landlord’s allocation of Real Property Taxes to the Building shall be binding upon Landlord and Tenant.
13
10.5.Tenant’s Property Taxes. Tenant shall pay prior to delinquency all taxes assessed against and levied upon Tenant’s improvements, fixtures, furnishings, equipment and all personal property of Tenant contained in the Premises or stored within the Industrial Center.
11.Utilities. Tenant shall pay directly for all utilities and services supplied to the Premises, including but not limited to electricity, HVAC, telephone, security, gas and cleaning of the Premises, together with any taxes thereon.
12.Assignment and Subletting.
12.1.Landlord’s Consent Required.
(a)Except as otherwise provided in Paragraph 12.1(c) below, Tenant shall not assign, transfer, mortgage or otherwise transfer or encumber (collectively, “assign”) or sublet all or any part of Tenant’s interest in this Lease or in the Premises without Landlord’s prior written consent, which consent shall not be unreasonably withheld. Relevant criteria in determining reasonableness of consent include, but are not limited to, credit history of a proposed assignee or sublessee, references from prior landlords, any change or intensification of use of the Premises or the Common Areas and any limitations imposed by the Internal Revenue Code and the Regulations promulgated thereunder relating to Real Estate Investment Trusts. Any assignment or subletting shall not release Tenant from its obligations hereunder. Tenant shall not (i) sublet or assign or enter into other arrangements such that the amounts to be paid by the sublessee or assignee thereunder would be based, in whole or in part, on the income or profits derived by the business activities of the sublessee or assignee; (ii) sublet the Premises or assign this Lease to any person in which Landlord owns an interest, directly or indirectly (by applying constructive ownership rules set forth in Section 856(d)(5) of the Internal Revenue Code (the “Code”); or (iii) sublet the Premises or assign this Lease in any other manner which could cause any portion of the amounts received by Landlord pursuant to this Lease or any sublease to fail to qualify as “rents from real property” within the meaning of Section 856(d) of the Code, or which could cause any other income received by Landlord to fail to qualify as income described in Section 856(c)(2) of the Code. The requirements of this Section 12.1 shall apply to any further subleasing by any subtenant.
(b)A change in the control of Tenant shall constitute an assignment requiring Landlord’s consent. The transfer, on a cumulative basis, of twenty-five percent (25%) or more of the voting or management control of Tenant shall constitute a change in control for this purpose.
(c)Notwithstanding the provisions of Paragraph 12.1(a), Landlord agrees that during the twelve (12) month period after the Commencement Date, Tenant may assign this Lease, without obtaining the consent of Landlord, to a limited partnership (to be named PFab LP) in which the partners (general and limited) are entities owned or controlled by PharmaFab, Inc., Bruce K. Montgomery or Darlene Ryan (or a combination thereof). However, Tenant shall promptly notify Landlord of such assignment. No such assignment shall relieve Tenant of its obligations under this Lease or relieve Bruce K. Montgomery or Darlene Ryan of their obligations as guarantors of the obligations of Tenant under this Lease. Upon the request of Landlord, Tenant shall (i) cause the assignee to execute an instrument reasonably satisfactory to Landlord evidencing the assumption by such assignee of all of Tenant’s obligations under this Lease, and (ii) cause each guarantor of this Lease to execute a ratification of his or her guaranty.
14
12.2.Rent Adjustment. If, as of the effective date of any permitted assignment or subletting the then remaining term of this Lease is less than three (3) years, Landlord may terminate this Lease as of the date of assignment or subletting subject to the performance by Tenant of those covenants which under the terms hereof survive termination.
13.Default; Remedies.
13.1.Default. The occurrence of any one of the following events shall constitute an event of default on the part of Tenant (“Default”):
(a)The abandonment of the Premises by Tenant;
(b)Failure to pay any installment of Base Rent, Additional Rent or any other monies due and payable hereunder for a period of three (3) days after Landlord has delivered to Tenant written notice thereof; provided, however, that a Default shall immediately occur hereunder, without Landlord first having to give Tenant written notice, if Tenant is more than three (3) days delinquent in paying any Base Rent, Additional Rent or any other monies due under this Lease and Landlord has given Tenant written notice under this Paragraph 13.1(b) on more than one (1) occasion during the Term.
(c)A general assignment by Tenant or any guarantor for the benefit of creditors;
(d)The filing of a voluntary petition in bankruptcy by Tenant or any guarantor, the filing of a voluntary petition for an arrangement, the filing of a petition, voluntary or involuntary, for reorganization, or the filing of an involuntary petition by Tenant’s creditors or guarantors;
(e)Receivership, attachment, of other judicial seizure of the Premises or all or substantially all of Tenant’s assets on the Premises;
(f)Failure of Tenant to maintain insurance as required by Paragraph 8.2;
(g)Any breach by Tenant of its covenants under Paragraph 6.2;
(h)Failure in the performance of any of Tenant’s covenants, agreements or obligations hereunder (except those failures specified as events of Default in other Paragraphs of this Paragraph 13.1 which shall be governed by such other Paragraphs), which failure continues for 10 days after written notice thereof from Landlord to Tenant provided that, if Tenant has exercised reasonable diligence to cure such failure and such failure cannot be cured within such ten (10) day period despite reasonable diligence, Tenant shall not be in default under this subparagraph unless Tenant fails thereafter diligently and continuously to prosecute the cure to completion; and (i)The default of any guarantors of Tenant’s obligations hereunder under any guaranty of this Lease, or the attempted repudiation or revocation of any such guaranty.
15
13.2.Remedies. In the event of any Default by Tenant, Landlord shall have the remedies set forth in the Addendum attached hereto entitled “Landlord’s Remedies in Event of Tenant Default”.
13.3.Late Charges. Tenant hereby acknowledges that late payment by Tenant to Landlord of rent and other sums due hereunder will cause Landlord to incur costs not contemplated by this Lease, the exact amount of which will be extremely difficult to ascertain. Such costs include, but are not limited to, processing and accounting charges. Accordingly, if any installment of rent or other sum due from Tenant shall not be received by Landlord or Landlord’s designee within 10 days after such amount shall be due, then, without any requirement for notice to Tenant, Tenant shall pay to Landlord a late charge equal to 5% of such overdue amount. The parties hereby agree that such late charge represents a fair and reasonable estimate of the costs Landlord will incur by reason of late payment by Tenant. Acceptance of such late charge by Landlord shall in no event constitute a waiver of Tenant’s Default with respect to such overdue amount, nor prevent Landlord from exercising any of the other rights and remedies granted hereunder.
14.Condemnation. If the Premises or any portion thereof are taken under the power of eminent domain or sold under the threat of exercise of said power (all of which are herein called “condemnation”), this Lease shall terminate as to the part so taken as of the date the condemning authority takes title or possession, whichever first occurs. If more than ten percent (10%) of the floor area of the Premises, or more than twenty-five (25%) of the portion of the Common Areas designated for Tenant’s parking, is taken by condemnation, Tenant may, at Tenant’s option, to be exercised in writing within ten (10) days after Landlord shall have given Tenant written notice of such taking (or in the absence of such notice, within ten (10) days after the condemning authority shall have taken possession) terminate this Lease as of the date the condemning authority takes such possession. If Tenant does not terminate this Lease in accordance with the foregoing, this Lease shall remain in full force and effect as to the portion of the Premises remaining, except that the Base Rent shall be reduced in the same proportion as the rentable floor area of the Premises taken bears to the total rentable floor area of the Premises. No reduction of Base Rent shall occur if the condemnation does not apply to any portion of the Premises. Any award for the taking of all or any part of the Premises under the power of eminent domain or any payment made under threat of the exercise of such power shall be the property of Landlord, provided, however, that Tenant shall be entitled to any compensation, separately awarded to Tenant for Tenant’s relocation expenses and/or loss of Tenants trade fixtures. In the event that this Lease is not terminated by reason of such condemnation, Landlord shall to the extent of its net severance damages in the condemnation matter, repair any damage to the Premises caused by such condemnation authority. Tenant shall be responsible for the payment of any amount in excess of such net severance damages required to complete such repair.
15.Estoppel Certificate and Financial Statements.
15.1.Estoppel Certificate. Each party (herein referred to as “Responding Party”) shall within 10 days after written notice from the other Party (the “Requesting Party”) execute, acknowledge and deliver to the Requesting Party, to the extent it can truthfully do so, an estoppel certificate in the form attached hereto, plus such additional information, confirmation a/or statements as be reasonably requested by the Requesting Party.
16
15.2.Financial Statement. If Landlord desires to finance, refinance, or sell the Building, Industrial Center or any part thereof, Tenant and all Guarantors shall deliver to any potential lender or purchaser designated by Landlord such financial statements of Tenant and such Guarantors as may be reasonably required by such lender or purchaser, including but not limited to Tenant’s financial statements for the past 3 years. All such financial statements shall be received by Landlord and such lender or purchaser in confidence and shall be used only for the purposes herein set forth.
16.Additional Covenants and Provisions.
16.1.Severability. The invalidity of any provision of this Lease, as determined by a court of competent jurisdiction, shall not affect the validity of any other provision hereof.
16.2.Interest on Past-Due Obligations. Any monetary payment due Landlord hereunder not received by Landlord within 10 days following the date on which it was due shall bear interest from the date due at twelve percent (12%) per annum, but not exceeding the maximum rate allowed by law in addition to the late charge provided for in Paragraph 13.3.
16.3.Time of Essence. Time is of the essence with respect to the performance of all obligations to be performed or observed by the Parties under this Lease.
16.4.Landlord Liability. Tenant, its successors and assigns, shall not assert nor seek to enforce any claim for breach of this Lease against any of Landlord’s assets other than Landlord’s interest in the Industrial Center. Tenant agrees to look solely to such interest for the satisfaction of any liability or claim against Landlord under this Lease. In no event whatsoever shall Landlord (which term shall include, without limitation, any general or limited partner, trustees, beneficiaries, officers, directors, or stockholders of Landlord) ever be personally liable for any such liability.
16.5.No Prior or Other Agreements. This Lease contains all agreements between the Parties with respect to any matter mentioned herein, and supersedes all oral, written prior or contemporaneous agreements or understandings.
16.6.Notice Requirements. All notices required or permitted by this Lease shall be in writing and may be delivered in person (by hand or by messenger or courier service) or may be sent by regular, certified or registered mail or U.S. Postal Service Express Mail, with postage prepaid, or by facsimile transmission during normal business hours, and shall be deemed sufficiently given if served in a manner specified in the Paragraph 16.6. The addresses noted adjacent to a Party’s signature on this Lease shall be that Party’s address for delivery or mailing of notice purposes. Either Party may by written notice to the other specify a different address for notice purposes, except that upon Tenant’s taking possessing of the Premises, the Premises shall constitute Tenant’s address for the purpose of mailing or delivering notices to Tenant. A copy of all notices required or permitted to be given to Landlord hereunder shall be concurrently transmitted to such party or parties at such addresses as Landlord may from time to time hereafter designate by written notice to Tenant.
17
16.7.Date of Notice. Any notice sent by registered or certified mail, return receipt requested, shall be deemed given on the date of delivery shown on the receipt card, or if no delivery date is shown, the postmark thereon. If sent by regular mail, the notice shall be deemed given 48 hours after the same is addressed as required herein and mailed with postage prepaid. Notices delivered by United States Express Mail or overnight courier that guarantees next day delivery shall be deemed given 24 hours after delivery of the same to the United States Postal Service or courier. If any notice is transmitted by facsimile transmission or similar means, the same shall be deemed served or delivered upon telephone or facsimile confirmation of receipt of the transmission thereof, provided a copy is also delivered via hand or overnight delivery or certified mail. If notice is received on a Saturday or a Sunday or a legal holiday, it shall be deemed received on the next business day.
16.8.Waivers. No waiver by Landlord or Tenant of a default by the other party shall be deemed a waiver of any subsequent default by such defaulting party or a waiver of any other term, covenant or condition hereof.
16.9.Holdover. Tenant has no right to retain possession of the Premises or any part thereof beyond the expiration or earlier termination of this Lease. If Tenant holds over with the consent of Landlord: (a) the Base Rent payable shall be increased to one hundred fifty percent (150%) of the Base Rent applicable during the month immediately preceding such expiration or earlier termination; (b) Tenant’s right to possession shall terminate on thirty (30) days notice from Landlord and (c) all other terms and conditions of this Lease shall continue to apply. Nothing contained herein shall be construed as a consent by Landlord to any holding over by Tenant. Tenant shall indemnify, defend and hold Landlord harmless from and against any and all claims, demands, actions, losses, damages, obligations, costs and expenses, including, without limitation, attorneys’ fees incurred or suffered by Landlord by reason of Tenant’s failure to surrender the Premises on the expiration or earlier termination of this Lease in accordance with the provisions of this Lease.
16.10.Cumulative Remedies. No remedy or election hereunder shall be deemed exclusive but shall, wherever possible, be cumulative with all other remedies in law or in equity.
16.11.Binding Effect; Choice of Law. This Lease shall be binding upon the Parties, their personal representatives, successors and assigns and be governed by the laws of the State in which the Premises are located. Any litigation between the Parties hereto concerning this Lease shall be initiated in the county in which the Premises are located.
16.12.Landlord. The covenants and obligations contained in this Lease on the part of Landlord are binding on Landlord, its successors and assigns, only during and in respect of their respective period of ownership of such interest in the Industrial Center. In the event of any transfer or transfers of such title to the Industrial Center, Landlord (and in case of any subsequent transfers or conveyances, the then grantor) shall be concurrently freed and relieved from and after the date of such transfer or conveyance, without any further instrument or agreement, of all liability with respect to the performance of any covenants or obligations on the part of Landlord contained in this Lease thereafter to be performed.
18
16.13.Attorneys’ Fees and Other Costs. If any Party brings an action or proceeding to enforce the terms hereof or declare rights hereunder, the Prevailing Party (as hereafter defined) in any such proceeding shall be entitled to reasonable attorneys’ fees. The term “Prevailing Party” shall include, without limitation, a Party who substantially obtains or defeats the relief sought. Landlord shall be entitled to attorneys’ fees, costs and expenses incurred in preparation and service of notices of Default and consultations in connection therewith, whether or not a legal action is subsequently commenced in connection with such Default or resulting breach. Tenant shall reimburse Landlord on demand for all reasonable legal, engineering and other professional services expenses incurred by Landlord in connection with all requests by Tenant for consent or approval hereunder.
16.14.Landlord’s Access; Showing Premises; Repairs. Landlord and Landlord’s agents shall have the right to enter the Premises at any time, in the case of an emergency, and otherwise at reasonable times upon reasonable notice for the purpose of showing the same to prospective purchasers, lenders, or tenants, and making such alterations, repairs, improvements or additions to the Premises or to the Building, as Landlord may reasonably deem necessary. Any inspection of the Premises conducted by Landlord or Landlord’s agents (other than in the event of an emergency) shall be done in compliance with any applicable requirements of the FDA or any requirements imposed by Tenant in order to comply with requirements of the FDA. Landlord may at any time place on or about the Premises or Building any ordinary “For Sale” signs and Landlord may at any time during the last one hundred eighty (180) days of the term hereof place on or about the Premises any ordinary “For Lease” signs. All such activities of Landlord shall be without abatement of rent or liability to Tenant.
16.15.Signs. Tenant shall not place any signs at or upon the exterior of the Premises or the Building, except that Tenant may, with Landlord’s prior written consent, install (but not on the roof) such signs as are reasonably required to advertise Tenant’s own business so long as such signs are in a location designated by Landlord and comply with sign ordinances and the sign age criteria established for the Industrial Center by Landlord. The sign age criteria of Landlord is attached hereto as Exhibit C.
16.16.Termination; Merger. Unless specifically stated otherwise in writing by Landlord, the voluntary or other surrender of this Lease by Tenant, the mutual termination or cancellation hereof, or a termination hereof by Landlord for Default by Tenant, shall automatically terminate any sublease or lesser estate in the Premises; provided, however, Landlord shall, in the event of any such surrender, termination or cancellation, have the option to continue any one or all of any existing subtenancies. Landlord’s failure within ten (10) days following any such event to make a written election to the contrary by written notice to the holder of any such lesser interest, shall constitute Landlord’s election to have such event constitute the termination of such interest.
16.17.Quiet Possession. Upon payment by Tenant of the Base Rent and Additional Rent for the Premises and the performance of all of the covenants, conditions and provisions on Tenant’s part to be observed and performed under this Lease, Tenant shall have quiet possession of the Premises for the entire term hereof subject to all of the provisions of this Lease.
19
16.18.Subordination; Attornment; Non-Disturbance.
(a)Subordination. This Lease shall be subject and subordinate to any ground lease, mortgage, deed of trust, or other hypothecation or mortgage (collectively, “Mortgage”) now or hereafter placed by Landlord upon the real property of which the Premises are a part, to any and all advances made on the security thereof and to all renewals, modifications, consolidations, replacements and extensions thereof. Tenant agrees that any person holding any Mortgage shall have no duty, liability or obligation to perform any of the obligations of Landlord under this Lease. In the event of Landlord’s default with respect to any such obligation, Tenant will give any Lender, whose name and address have previously in writing been furnished Tenant, notice of a default by Landlord. Tenant may not exercise any remedies for default by Landlord unless and until Landlord and the Lender shall have received written notice of such default and a reasonable time (not less than sixty (60) days) shall thereafter have elapsed without the default having been cured. If any Lender shall elect to have this Lease superior to the lien of its Mortgage and shall give written notice thereof to Tenant, this Lease shall be deemed prior to such Mortgage. The provisions of a Mortgage relating to the disposition of condemnation and insurance proceeds shall prevail over any contrary provisions contained in this Lease.
(b)Attornment. Subject to the non-disturbance provisions of subparagraph C of this Paragraph 16.18, Tenant agrees to attorn to a Lender or any other party who acquires ownership of the Premises by reason of a foreclosure of a Mortgage. In the event of such foreclosure, such new owner shall not: (i) be liable for any act or omission of any prior landlord or with respect to events occurring prior to acquisition of ownership, (ii) be subject to any offsets or defenses which Tenant might have against any prior Landlord, or (iii) be liable for security deposits or be bound by prepayment of more than one month’s rent.
(c)Non-Disturbance. With respect to Mortgage entered into by Landlord after the execution of this Lease, Tenant’s subordination of this Lease shall be subject to receiving assurance (a “non-disturbance agreement”) from the Mortgage holder not disturbing Tenant’s possession of the Premises so long as this Lease is in full force and effect and Tenant is not in default under this Lease.
(d)Self-Executing. The agreements contained in this Paragraph 16.18 shall be effective without the execution of any further documents; provided, however, that upon written request from Landlord or a Lender in connection with a sale, financing or refinancing of Premises, Tenant and Landlord shall execute such further writings as may be reasonably required to separately document any such subordination or non-subordination, attornment and/or non-disturbance agreement as is provided for herein. Landlord is hereby irrevocably vested with full power to subordinate this Lease to a Mortgage.
16.19.Financing by Tenant. Landlord acknowledges and agrees that Tenant may grant a security interest in the personally, furniture, trade fixtures and inventory owned by Tenant in the Premises to an institutional lender. Upon the grant of such security interest, Landlord shall, upon the request of Tenant, execute and deliver to Tenant a subordination agreement in the form of Exhibit D attached hereto.
20
16.20.Rules and Regulations. Tenant agrees that it will abide by, and to cause its employees, suppliers, shippers, customers, tenants, contractors and invitees to abide by all reasonable rules and regulations (“Rules and Regulations”) which Landlord may make from time to time for the management, safety, care, and cleanliness of the Common Areas, the parking and unloading of vehicles and the preservation of good order, as well as for the convenience of other occupants or tenants of the Building and the Industrial Center and their invitees. Landlord shall not be responsible to Tenant for the non-compliance with said Rules and Regulations by other tenants of the Industrial Center; provided, however, that Landlord agrees to use reasonable efforts to attempt to enforce the Rules and Regulations on a uniform basis.
16.21.Security Measures. Tenant acknowledges that the rental payable to Landlord hereunder does not include the cost of guard service or other security measures. Landlord has no obligations to provide same. Tenant assumes all responsibility for the protection of the Premises, Tenant, its agents and invitees and their property from the acts of third parties.
16.22.Reservations. Landlord reserves the right to grant such easements that Landlord deems necessary and to cause the recordation of parcel maps, so long as such easements and maps do not reasonably interfere with the use of the Premises by Tenant. Tenant agrees to sign any documents reasonably requested by Landlord to effectuate any such easements or maps.
16.23.Conflict. Any conflict between the printed provisions of this Lease and the typewritten or handwritten provisions shall be controlled by the typewritten or handwritten provisions.
16.24.Offer. Preparation of this Lease by either Landlord or Tenant or Landlord’s agent or Tenant’s agent and submission of same to Tenant or Landlord shall not be deemed an offer to lease. This Lease is not intended to be binding until executed and delivered by all Parties hereto.
16.25.Amendments. This Lease may be modified only in writing, signed by the parties in interest at the time of the modification.
16.26.Multiple Parties. Except as otherwise expressly provided herein, if more than one person or entity is named herein as Tenant, the obligations of such persons shall be the joint and several responsibility of all persons or entities named herein as such Tenant.
16.27.Authority. Each person signing on behalf of Landlord or Tenant warrants and represents that she or is authorized to execute and deliver this Lease and to make it a binding obligation of Landlord or Tenant.
[Signatures appear on following page]
21
The parties hereto have executed this Lease at the place and on the dates specified above their respective signatures.
Landlord: |
|
Tenant: |
||||||||
|
|
|
|
|||||||
WALSTIB, L.P., |
|
PharmaFab, Inc. |
||||||||
a Delaware limited partnership |
|
|
||||||||
|
|
|
|
|||||||
By: |
WALSTIB Venture, L.L.C., |
|
By: |
|
||||||
|
a Delaware limited liability company |
|
Its: |
|
||||||
|
Its sole general partner |
|
Telephone: |
|
||||||
|
|
|
Facsimile: |
|
||||||
By: |
TCDFW Development, Ltd., |
|
Executed at: |
|
||||||
|
a Texas limited partnership |
|
on: |
|
||||||
|
Its Administrative Member |
|
|
|
||||||
|
|
|
|
|||||||
By: |
Trammell Crow DFW Development, Inc., |
|
|
|||||||
|
a Delaware corporation |
|
|
|||||||
|
Its sole general partner |
|
|
|||||||
|
|
|
|
|||||||
|
|
|
||||||||
By: |
|
|
|
|||||||
Its: |
|
|
|
|||||||
Telephone: |
|
|
|
|||||||
Facsimile: |
|
|
|
|||||||
Executed at: |
|
|
|
|||||||
on: |
|
|
|
|||||||
22
Landlord’s Remedies Addendum In Event
of Tenant Default
(State of Texas)
(a)Upon any Default, Landlord may, in addition to all other rights and remedies afforded Landlord hereunder or by Applicable Requirements, take any of the follow actions:
(i)Terminate this Lease by giving Tenant written notice thereof, in which event, Tenant shall pay to Landlord the sum of (A) all unpaid past due rent accrued hereunder through the date of termination, (B) all amounts due under paragraph (b) below, and (C) an amount equal to (1) the total rent that Tenant would have been required to pay for the remainder of the Term discounted to a present value at a per annum rate equal to the “Prime Rate” as published on the date this Lease is terminated by The Wall Street Journal, Southwest Edition, in its listing of “Money Rents”, minus (2) the then present fair rental value of the Premises for such period, as determined by Landlord in good faith, similarly discounted; or
(ii)Terminate Tenant’s right to possess the Premises and change the door locks to the Premises without terminating this Lease, with or without notice thereof to Tenant, and without judicial proceedings, in which event Tenant shall pay to Landlord (A) all unpaid past due rent and other amounts accrued hereunder to the date of termination of possession, (B) all amounts due from time to time under paragraph (b) below, and (C) all rent and other sums required hereunder to be paid by Tenant during the remainder of the Term, diminished by any net sums thereafter received by Landlord through reletting the Premises during such period. Tenant shall not be entitled to the excess of any consideration obtained by reletting over the rent due hereunder. Reentry to Landlord in the Premises shall not affect Tenant’s obligations hereunder for the unexpired Term; rather, Landlord may, from time to time, bring action against Tenant to collect amounts due by Tenant, without the necessity of Landlord’s waiting until the expiration of the Term. Unless Landlord delivers written notice to Tenant expressly stating that it has elected to terminate this Lease, all actions taken by Landlord to exclude or dispossess Tenant of the Premises shall be deemed to be taken under this paragraph (a)(ii). If Landlord elects to proceed under this paragraph (a)(ii), it may at any time elect to terminate this Lease under paragraph (a)(i) above. Landlord and Tenant hereby confirm that the terms and provisions of this addendum supersedes Section 93.002 of the Texas Property Code to the extent of any conflict.
(b)Tenant shall pay to Landlord all reasonable costs and expenses incurred by Landlord (including court costs and reasonable attorney’s fees and expenses) in (i) obtaining possession of the Premises, (ii) removing and storing Tenant’s or any other occupant’s property, (iii) renovating, repairing and altering the Premises for a new tenant or tenants, (iv) if Tenant is dispossessed of the Premises and this Lease is not terminated, reletting all or any part of the Premises (including brokerage commissions, reasonable cost of tenant finish work, and other costs incidental to such reletting), (v) performing Tenant’s obligations which Tenant failed to perform, and (vi) enforcing, or advising Landlord of its rights, remedies, and recourses. Landlord’s acceptance of rent following the occurrence of a Default shall not waive Landlord’s rights regarding such Default. Landlord’s receipt of rent with knowledge of any Default by Tenant hereunder shall not be a waiver of such Default, and no waiver by Landlord of any provision of this Lease shall be deemed to have been made unless set forth in writing and signed by Landlord.
23
No waiver by Landlord of any violation or breach of any of the terms contained herein shall waive Landlord’s rights regarding any future violation of such term or violation of any other term. If Landlord repossesses the Premises pursuant to the authority herein granted, then Landlord shall have the right to (A) keep in place and use or (B) remove and store, at Tenant’s expense, all of the furniture, fixtures, equipment or other property, deemed abandoned by Tenant in the Premises, including that which is owned by or leased to Tenant at all times before any foreclosure thereon by Landlord or repossession thereof by any lessor thereof or third party having a lien thereon. Landlord may relinquish possession of all or any portion of such furniture, fixtures, equipment and other property to any person (a “Claimant”) who presents to Landlord a copy of any instrument represented by Claimant to have been executed by Tenant (or any predecessor of Tenant) granting Claimant the right under various circumstances to take possession of such furniture, fixtures, equipment or other property, without the necessity on the part of Landlord to inquire into the authenticity or legality of the instrument. The rights of Landlord herein stated are in addition to any and all other rights that Landlord has or may hereafter have at law or in equity, and Tenant agrees that the rights herein granted Landlord are commercially reasonable.
24
WALSTIB, L.P.,
A DELAWARE LIMITED PARTNERSHIP
INDUSTRIAL MULTI-TENANT LEASE
Tenant Improvements Addendum
1.Landlord shall construct the leasehold improvements in the Premises (the “Leasehold Improvements”) in substantial accordance with plans and specifications (the “Plans”) which have been mutually approved in writing by Landlord and Tenant. Landlord and Tenant will endeavor to agree upon the Final Plans not later than June 10, 1999. Tenant shall be responsible for the costs of preparing the Final Plans and any other architectural and design costs in respect of the Leasehold Improvements (which costs may be paid from the Tenant Allowance, as hereinafter defined).
2.After the Plans have been approved by Landlord and Tenant, Landlord shall competitively bid the job to a minimum of three (3) qualified general contractors reasonably acceptable to Landlord and Tenant. Landlord shall contract with the lowest qualified bidder, or such other bidder as is agreed upon by Landlord and Tenant (the “Contractor”), to construct the Leasehold Improvements in the Premises; provided, however, that if the lowest bid (or such other bid agreed upon by Landlord and Tenant) is more than Nine Hundred Thousand Dollars ($900,000), Landlord and Tenant shall revise the Plans in such a manner that the cost of constructing the Leasehold Improvements reflected in the Plans, as revised, is not more than Nine Hundred Thousand Dollars ($900,000). Landlord shall cause the Contractor to construct the Leasehold Improvements in substantial accordance with the approved Plans and in a good and workmanlike manner utilizing all new materials. The contract with the Contractor shall obligate the Contractor to obtain necessary permits and approvals from local governmental authorities in respect of the construction of the Leasehold Improvements and to construct the Leasehold Improvements in accordance with all applicable laws, codes and regulations (it being expressly agreed that it shall be the responsibility of Tenant to cause any requirements of the FDA to be incorporated into the approved Plans). During the construction period, Tenant shall have access to the Premises during reasonable times to observe the progress and quality of the work; provided, however, that Tenant shall at all time when visiting the Premises comply with Contractor’s safety requirements. Any changes which the Tenant may request during the construction of Leasehold Improvements shall be submitted to the Landlord in written form, and change orders shall be subject to the written approval of both Landlord and Tenant. Thereupon, Landlord shall prepare a written change order for Tenant’s review and approval, which, when signed by Tenant and Landlord, shall authorize Landlord to make such change in the Leasehold Improvements.
3.The Leasehold Improvements shall be deemed to be “substantially complete” and ready for delivery to Tenant at such time as a certificate of occupancy for the Premises is secured from the City of Grand Prairie. At such time as the Leasehold Improvements are substantially complete, Landlord, Tenant and Contractor shall walk the Premises for the purpose of preparing a “punch list” of items needing correction. Thereafter, the architect for construction of the Leasehold Improvements shall issue a certificate of substantial completion to which the “punch list” shall be attached. Landlord shall cause the Contractor to correct or complete the items on the “punch list”
25
as soon as reasonably practicable, but in no event later than thirty (30) days after the date upon which the Leasehold Improvements are substantially complete.
4.Tenant shall be responsible for the payment of all costs associated with the construction of the Leasehold Improvements, including, but not limited to, the following: (a) all costs, including professional fees, of the architect and all other design and planning costs; (b) the costs of labor and material associated with the construction of the Leasehold Improvements; and (c) a fee to Trammell Crow Dallas Fort Worth equal to four percent (4%) of the sum of all design, planning and construction costs associated with the construction of the Leasehold Improvements. It is understood and agreed that the Tenant Allowance, as hereinafter defined, may be used to pay the costs described in clauses (a), (b) and (c) of the preceding sentence.
5.Notwithstanding anything to the contrary contained herein, Landlord grants to Tenant an allowance (the “Tenant Allowance”) in the amount of Seven Hundred Ninety-Two Thousand Dollars ($792,000) to be applied to the cost of the Leasehold Improvements and other costs described in Paragraph 4 above. In the event that the bid for the Leasehold Improvements exceeds the Tenant Allowance, Tenant shall prepay to Landlord the amount of such excess prior to the time Landlord enters into the construction contract with Contractor, it being agreed that the Tenant Allowance shall be advanced before such funds deposited by Tenant are used to pay for construction of the Leasehold Improvements. If the cost of constructing the Leasehold Improvements is less than the Tenant Allowance, Tenant may, within the twelve (12) month period after the Commencement Date, apply the unused portion of the Tenant Allowance towards the cost of additional construction in the Premises or an upgrade of the tenant finish work in the Premises. In the event that any portion of the Tenant Allowance remains unused upon the expiration of the twelve (12) month period after the Commencement Date, Landlord shall have no obligation to pay such unused portion to Tenant or otherwise allow Tenant the benefit thereof.
6.Tenant shall cooperate with the Contractor to promote the efficient and expeditious completion of the Leasehold Improvements. Tenant agrees that in the event of default in payment hereunder, including, but not limited to, those payments due under Paragraph 5 above, Landlord, in addition to any and all other remedies at law or in equity, shall have the same rights and remedies against Tenant as in the event of default in payment of rent under the Lease.
7.It is understood and agreed that any delay in construction of the Leasehold Improvements caused by Tenant shall not operate to delay the Commencement Date. Any of the following shall constitute a delay caused by Tenant: (a) Tenant’s failure to have preliminary plans prepared or Tenant’s failure to approve the plans in a timely manner; (b) any delay resulting from Tenant’s changes in the Plans; (c) any delay resulting from Tenant’s request for materials, finishes or installations which are not readily available; or (d) any delay in the construction of the Leasehold Improvements otherwise caused by Tenant.
Landlord: |
|
Tenant: |
||
|
|
|
|
|
WALSTIB, L.P., |
|
PharmaFab, Inc. |
||
a Delaware limited partnership |
|
|
||
|
|
|
|
|
By: |
WALSTIB Venture, L.L.C., |
|
By: |
|
|
|
|
Its: |
|
26
|
a Delaware limited liability company |
|
Telephone: |
|
||||||
|
Its sole general partner |
|
Facsimile: |
|
||||||
|
|
|
Executed at: |
|
||||||
By: |
TCDFW Development, Ltd., |
|
on: |
|
||||||
|
a Texas limited partnership |
|
|
|
||||||
|
Its Administrative Member |
|
|
|||||||
|
|
|
|
|||||||
By: |
Trammell Crow DFW Development, Inc., |
|
|
|||||||
|
a Delaware corporation |
|
|
|||||||
|
Its sole general partner |
|
|
|||||||
|
|
|
|
|||||||
|
|
|
|
|||||||
|
|
|
||||||||
By: |
|
|
|
|||||||
Its: |
|
|
|
|||||||
Telephone: |
|
|
|
|||||||
Facsimile: |
|
|
|
|||||||
Executed at: |
|
|
|
|||||||
on: |
|
|
|
|||||||
27
WALSTIB, L.P.,
A DELAWARE LIMITED PARTNERSHIP
INDUSTRIAL MULTI-TENANT LEASE
Option To Extend Addendum
This Option To Extend Addendum is a part of the Lease dated __________, 1999 by and between WALSTIB, L.P., a Delaware limited partnership (“Landlord”) and PharmaFab, Inc., a Texas corporation (“Tenant”) for the premises commonly known as 360 Riverside Business Center (Building B).
1.Option to Extend. Landlord hereby grants to Tenant the option to extend the term of this Lease for the following periods (“Option Periods”) commencing when the prior term expires:
Months 85 – 145 “Period One”
Months 145 – 205 “Period Two”
2.Exercise Dates. For purposes of Paragraph 5 of this Addendum:
(a)the Earliest Exercise Date is12 months prior to the date that the applicable Option Period would commence; and
(b)the Last Exercise Date is 9 months prior to the date that the applicable Option Period would commence.
3.Monthly Base Rent. The monthly Base Rent for each month of an Option Period shall be the amount calculated in accordance with the alternative selected below (“Rent Adjustment Alternative”).
[X] Market rent (“Market Rent Adjustment”)
4.Conditions to Exercise of Option. Tenant’s right to extend is conditioned upon and subject to each of the following:
(a)In order to exercise an option to extend, Tenant must give written notice of such election to Landlord and Landlord must receive the same by the Last Exercise Date but not prior to the Earliest Exercise Date. If proper notification of the exercise of an option is not given and/or received, such option shall automatically expire. Options (if there are more than one) may only be exercised consecutively. Failure to exercise an option terminates that option and all subsequent options. Tenant acknowledges that because of the importance to Landlord of knowing no later than the Last Exercise Date whether or not Tenant will exercise the option, the failure of Tenant to notify Landlord by the Last Exercise Date will conclusively be presumed an election by Tenant not to exercise the option.
28
(b)Tenant shall have no right to exercise an option (i) if Tenant is in Default. or (ii) if during the twelve (12) month period immediately preceding the exercise of the option, three (3) or more Defaults have occurred which were not cured within any applicable notice or grace period. The period of time within which an option may be exercised shall not be extended or enlarged by reason of Tenant’s inability to exercise an option because of the provisions of this paragraph.
(c)All of the terms and conditions of this Lease except where specifically modified by this Addendum shall apply.
(d)The options are personal to Tenant, cannot be assigned or exercised by anyone other than Tenant and only while Tenant is in full possession of the Premises and without the intention of thereafter assigning or subletting. For purposes of the preceding sentence only, the term “Tenant” shall include any entity to which Tenant assigns the Lease pursuant to Section 12.1(c) of the Lease.
5.Calculation of Rent Adjustment
(a)Market Rent Adjustment. Four months prior to the commencement of each Option Period, if the selected Rent Adjustment Alternative is the Market Rent Adjustment, the Parties shall negotiate in good faith to determine the Base Rent for the Option Period. If agreement cannot be reached within 30 days, then Landlord and Tenant shall each, no later then 90 days prior to the commencement of the Option Period, make a reasonable determination of the fair market rental for the Premises for the Option Period and submit such determination, in writing, to arbitration in accordance with the following provisions:
(i)No later than 90 days prior to the commencement of the Option Period, Landlord and Tenant shall each select an industrial leasing broker to act as an arbitrator. The two arbitrators so appointed shall, no later then 75 days prior to the commencement of the Option Period, select a third mutually acceptable industrial leasing broker to act as a third arbitrator.
(ii)The three arbitrators, acting by a majority, shall no later then 75 days prior to the commencement of the Option Period, determine the actual fair market rental for the Premises for the Option Period. The decision of a majority of the arbitrators shall be binding on the parties. The fair market rental determination of Landlord or Tenant which is closest to the fair market rental as determined by the arbitrators shall be the Base Rent for the Option Period.
(iii)If either of the parties fails to appoint an arbitrator within the period required by this Addendum, the arbitrator timely appointed shall determine the Base Rent for the Option Period.
(iv)The entire cost of such arbitration shall be paid by the party whose fair market rental submission is not selected.
[Signatures appear on following page]
29
Landlord: |
|
Tenant: |
||||||||
|
|
|
|
|||||||
WALSTIB, L.P. |
|
PharmaFab, Inc. |
||||||||
a Delaware limited partnership |
|
|
||||||||
|
|
|
|
|||||||
By: |
WALSTIB Venture, L.L.C., |
|
By: |
|
||||||
|
a Delaware limited liability company |
|
Its: |
|
||||||
|
Its sole general partner |
|
Telephone: |
|
||||||
|
|
|
Facsimile: |
|
||||||
By: |
TCDFW Development, Ltd., |
|
Executed at: |
|
||||||
|
a Texas limited partnership |
|
on: |
|
||||||
|
Its Administrative Member |
|
|
|
||||||
|
|
|
|
|||||||
By: |
Trammell Crow DFW Development, Inc., |
|
|
|||||||
|
a Delaware corporation |
|
|
|||||||
|
Its sole general partner |
|
|
|||||||
|
|
|
|
|||||||
|
|
|
||||||||
By: |
|
|
|
|||||||
Its: |
|
|
|
|||||||
Telephone: |
|
|
|
|||||||
Facsimile: |
|
|
|
|||||||
Executed at: |
|
|
|
|||||||
on: |
|
|
|
|||||||
30
WALSTIB, L.P.,
A DELAWARE LIMITED PARTNERSHIP
INDUSTRIAL MULTI-TENANT LEASE
Additional Security Deposit Addendum
This Additional Security Deposit Addendum is a part of the Lease dated ______________, 1999 by and between WALSTIB, L.P., a Delaware limited partnership (“Landlord”) and PharmaFab, Inc., a Texas corporation (“Tenant”) for the premises commonly known as 360 Riverside Business Center (Building B).
Tenant agrees to pay Landlord an Additional Security Deposit in the amount of $80,000.00 that can be held in the form of a Certificate Of Deposit or Letter Of Credit. Such Additional Security Deposit shall be refunded after five (5) years, unless during the first five (5) years of occupancy there have occurred three (3) or more Defaults which were not cured within any applicable notice or grace period. If the Additonal Security Deposit is in the form of a Certificate Of Deposit or Letter Of Credit and the Premises are sold, Tenant shall, at its expense, cause the Certificate Of Deposit or Letter Of Credit, as the case may be, to be reissued in the name of the purchaser.
Landlord: |
|
Tenant: |
||||||||
|
|
|
|
|||||||
WALSTIB, L.P., |
|
PharmaFab, Inc. |
||||||||
a Delaware limited partnership |
|
|
||||||||
|
|
|
|
|||||||
By: |
WALSTIB Venture, L.L.C., |
|
By: |
|
||||||
|
a Delaware limited liability company |
|
Its: |
|
||||||
|
Its sole general partner |
|
Telephone: |
|
||||||
|
|
|
Facsimile: |
|
||||||
By: |
TCDFW Development, Ltd., |
|
Executed at: |
|
||||||
|
a Texas limited partnership |
|
on: |
|
||||||
|
Its Administrative Member |
|
|
|
||||||
|
|
|
|
|||||||
By: |
Trammell Crow DFW Development, Inc., |
|
|
|||||||
|
a Delaware corporation |
|
|
|||||||
|
Its sole general partner |
|
|
|||||||
|
|
|
|
|||||||
|
|
|
||||||||
By: |
|
|
|
|||||||
Its: |
|
|
|
|||||||
Telephone: |
|
|
|
|||||||
Facsimile: |
|
|
|
|||||||
Executed at: |
|
|
|
|||||||
on: |
|
|
|
|||||||
31
GUARANTY OF LEASE
WHEREAS, WALSTIB, L.P., a Delaware limited partnership (“Landlord”), and PharmaFab, Inc., a Texas corporation (“Tenant”) are about to execute a lease (“Lease”) dated ___________________ 1999, for the premises commonly known as 360 Riverside Business Center (Building B).
WHEREAS, Bruce K. Montgomery and Darlene Ryan (each a “Guarantor”) have a financial interest in Tenant;
WHEREAS, Landlord would not execute the Lease if Guarantor did not execute and deliver to Landlord this Guaranty of Lease.
NOW THEREFORE, in consideration of the execution of the foregoing Lease by Landlord and as a material inducement to Landlord to execute the Lease:
1.Guarantor hereby jointly, severally, unconditionally and irrevocably guarantee the prompt payment by Tenant of all rents and all other sums payable by Tenant under the Lease and the faithful and prompt performance by Tenant of each and every one of the terms, conditions and covenants of the Lease to be kept and performed by Tenant.
2.The terms of the Lease may, without the consent of or notice to Guarantor, be modified by Landlord and Tenant or by a course of conduct and this Guaranty shall guarantee the performance of said Lease as so modified. The Lease may be assigned by Landlord or any assignee of Landlord without consent or notice to Guarantor.
3.This Guaranty shall not be released, modified or affected by the failure or delay on the part of Landlord to enforce any of the rights or remedies of the Landlord under the Lease, whether pursuant to the terms thereof or at law or in equity.
4.No notice of default need be given to Guarantor. The guaranty of the undersigned is a continuing guaranty under which Landlord may proceed immediately against Tenant and/or against any Guarantor (or each Guarantor) following any breach or default by Tenant or for the enforcement of any rights which Landlord may have against Tenant under the terms of the Lease or at law or in equity.
5.Landlord shall have the right to proceed against any Guarantor (or each Guarantor) hereunder following any breach or default by Tenant without first proceeding against Tenant and without previous notice to or demand upon either Tenant or any Guarantor.
6.Each Guarantor hereby waives (a) notice of acceptance of this Guaranty, (b) demand of payment, presentation and protest, (c) any right to require the Landlord to proceed against the Tenant or any other Guarantor or any other person or entity liable to Landlord, (d) any right to require Landlord to apply to any default any security deposit or other security it may hold under the Lease, (e) any right to require Landlord to proceed under any other remedy Landlord may have before proceeding against Guarantor and (f) any right of subrogation.
32
7.Each Guarantor does hereby subrogate all existing or future indebtedness of Tenant to such Guarantor to the obligations owed to Landlord under the Lease and this Guaranty.
8.If a Guarantor is married, such Guarantor expressly agrees that recourse may be had against his or her separate property for all of the obligations hereunder. If there is more than one Guarantor, the obligations of each Guarantor hereunder shall be joint and several.
9.The obligations of Tenant under the Lease to execute and deliver estoppel certificates and financial statements shall be deemed to also require each Guarantor hereunder to do and provide the same.
10.The term “Landlord” refers to and means the Landlord named in the Lease and also Landlord’s successors and assigns. So long as Landlord’s interest in the Lease, the leased premises or the rents, issues and profits therefrom, are subject to any mortgage or deed of trust or assignment for security, no acquisition by Guarantor of the Landlord’s interest shall affect the continuing obligation of Guarantor under this Guaranty which shall nevertheless continue in full force and effect for the benefit of the mortgagee, beneficiary, trustee or assignee under such mortgage, deed of trust or assignment and their successors and assigns.
11.The term “Tenant” refers to and means the Tenant named in the Lease and also Tenant’s successors and assigns.
12.In the event any action be brought by said Landlord against Guarantor hereunder to enforce the obligation of Guarantor hereunder, the unsuccessful party in such action shall pay to the prevailing party therein a reasonable attorney’s fee which shall be fixed by the court.
13.After three (3) years, provided Tenant has not had an uncured Default as described in the Lease, the aggregate liability of the Guarantors hereunder shall be limited to the sum of $380,000.00 on a joint and several basis (i.e. the liability of Bruce K. Montgomery hereunder shall be limited to $380,000, and the liablity of Darlene Ryan hereunder shall be limited to $380,000, but the maximum aggregate amount Landlord can recover from the Guarantors under this Guranty shall be limited to a total of $380,000). If no uncured Default has occurred under the Lease at the expiration of the fifth (5th) year of Tenant’s occupancy, this Guaranty shall terminate. It is agreed that if a Default occurs under the Lease which continues beyond the expiration of any applicable grace or cure period, Landlord may condition its acceptance of any cure of such Default upon keeping this Guaranty in effect.
Executed on the ______ day of June, 1999.
“GUARANTOR” | |||
| |||
| |||
Bruce K. Montgomery | |||
Executed at: |
|
||
on: |
|
||
Address: |
|
||
33
|
|
|||
Darlene Ryan | ||||
Executed at: |
|
|||
on: |
|
|||
Address: |
|
|||
|
|
|||
34
Exhibit A
Diagram of Premises
35
Exhibit B
COMMENCEMENT DATE MEMORANDUM
LANDLORD: |
WALSTIB, L.P., DELAWARE UNITED PARTNERSHIP |
TENANT: |
PHARMAFAB, INC. |
LEASE DATE: |
_____________, 1999 |
PREMISES: |
360 Riverside Business Center (Building B) |
Tenant hereby accepts the Premises as being in the condition required under the Lease.
The Commencement Date of the Lease is ______________________.
The Expiration Date of the Lease is ______________________.
Landlord: |
|
Tenant: |
||||||||
|
|
|
|
|||||||
WALSTIB, L.P., |
|
PharmaFab, Inc. |
||||||||
a Delaware limited partnership |
|
|
||||||||
|
|
|
|
|||||||
By: |
WALSTIB Venture, L.L.C., |
|
By: |
|
||||||
|
a Delaware limited liability company |
|
Its: |
|
||||||
|
Its sole general partner |
|
Telephone: |
|
||||||
|
|
|
Facsimile: |
|
||||||
By: |
TCDFW Development, Ltd., |
|
Executed at: |
|
||||||
|
a Texas limited partnership |
|
on: |
|
||||||
|
Its Administrative Member |
|
|
|
||||||
|
|
|
|
|||||||
By: |
Trammell Crow DFW Development, Inc., |
|
|
|||||||
|
a Delaware corporation |
|
|
|||||||
|
Its sole general partner |
|
|
|||||||
|
|
|
|
|||||||
|
|
|
||||||||
By: |
|
|
|
|||||||
Its: |
|
|
|
|||||||
Telephone: |
|
|
|
|||||||
Facsimile: |
|
|
|
|||||||
Executed at: |
|
|
|
|||||||
on: |
|
|
|
|||||||
36
Exhibit C
Signage Criteria
Per Paragraph 16.15, Tenant can install the sign described hereunder, provided that upon removal of such sign, Tenant shall make all repairs and maintenance to the building (i.e., repairing holes, texture, paint, etc.).
Sign criteria for the project shall consist of internally illuminated channel letters attached to a single raceway centered on each building entrance. The raceway will be located on the top facade band directly above the reveal, and the raceway shall be confined to a 40 foot maximum length with an 8 inch height. The copy shall be limited to 30 inches in height with 5 inch painted returns. Raceway and return colors will be specified by Landlord along with electrical specifications. Tenant shall replace the raceway face to its original condition upon vacating the Premises.
37
Exhibit D
Subordination Agreement
LANDLORD’S SUBORDINATION AGREEMENT
WHEREAS, WALSTIB, L.P. (hereinafter referred to as “Landlord”) is the owner and/or lessor of the following premises (hereinafter referred to as “Premises”) which are leased to the following tenant (hereinafter referred to as “Tenant”) pursuant to that certain lease executed by and between Landlord and Tenant on or about the ___day of _________________, 1999, as same may have been amended from time to time (hereinafter referred to as the “Lease”);
Premises: |
|
|
|
Address: |
|
|
|
|
|
Tenant: |
|
|
|
WHEREAS, Summit National Bank, N.A. (“Lender”) has or is about to loan funds to Tenant, and to secure such loan Tenant has or will grant a security interest to Lender on personalty, furniture, trade fixtures and inventory owned by Tenant and located in the Premises, as described on Exhibit “A” attached hereto (the “Property”); and
WHEREAS, all or a portion of the Property may from time to time be located at the Premises or may become wholly or partially affixed to the Premises.
NOW, THEREFORE, for and in consideration of the covenants contained herein, it is hereby agreed as follows:
1.Except as limited in this Agreement, Landlord subordinates to the interest of Lender any and all liens, claims or other rights which Landlord may have in or to the Property now or hereafter located in or on the Premises.
2.Except if Tenant is a debtor in a proceeding under Title 11 of the United States Code, to the extent of the subordination provided for herein, Landlord agrees that upon prior written notice to Landlord, Lender, through its authorized representatives or agents, may enter upon the Premises at any time mutually agreeable to Lender and Landlord after three (3) days written notice for the purpose of inspecting, repairing, or removing the Property, and Landlord agrees not to hinder or prevent Lender from taking any such action; provided, however, that nothing herein shall release Lender from, and Lender agrees to be responsible for, any and all damages resulting to the Premises as a result of any such entry or removal. Lender agrees to indemnify and hold Landlord harmless from any and all claims arising from or related to the removal of the Property from the Premises.
38
3.Within ten (10) business days after Landlord notifies Lender that Tenant is in default under the Lease and Landlord has notified Lender of which remedy Landlord has pursued under the lease between Landlord and Tenant, Lender must notify Landlord of Lender’s election to:
(a)Remove the Property from the Premises within ten (10) business days of such election; or
(b)With Landlord’s approval, retain the Property at the Premises for a period of time to be mutually determined by Landlord and Lender and pay to Landlord rental for such use of the Premises, as set forth in the Lease.
4.Any notice pursuant to this Agreement shall be deemed to have been given, whether or not received, when deposited in the United States mail, postage prepaid, certified mail, return receipt requested, at the following addresses:
Lender: |
|
|
|
|
|
|
|
|
|
Landlord: |
|
|
|
|
|
|
|
|
|
5.This Agreement shall be binding upon and inure to the benefit of the heirs, representatives, successors and assigns of Landlord and Lender.
6.Governing Law: THIS LANDLORD’S SUBORDINATION AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF TEXAS.
[Signatures and acknowledgments appear on following pages]
39
SIGNED this ____ day of ________________, 19___.
Landlord: |
|
Lender: |
||||||||
|
|
|
|
|||||||
WALSTIB, L.P., |
|
SUMMIT NATIONAL BANK, N.A. |
||||||||
a Delaware limited partnership |
|
|
||||||||
|
|
|
|
|||||||
By: |
WALSTIB Venture, L.L.C., |
|
By: |
|
||||||
|
a Delaware limited liability company |
|
Its: |
|
||||||
|
Its sole general partner |
|
Telephone: |
|
||||||
|
|
|
Facsimile: |
|
||||||
By: |
TCDFW Development, Ltd., |
|
Executed at: |
|
||||||
|
a Texas limited partnership |
|
on: |
|
||||||
|
Its Administrative Member |
|
|
|
||||||
|
|
|
|
|||||||
By: |
Trammell Crow DFW Development, Inc., |
|
|
|||||||
|
a Delaware corporation |
|
|
|||||||
|
Its sole general partner |
|
|
|||||||
|
|
|
|
|||||||
|
|
|
||||||||
By: |
|
|
|
|||||||
Its: |
|
|
|
|||||||
Telephone: |
|
|
|
|||||||
Facsimile: |
|
|
|
|||||||
Executed at: |
|
|
|
|||||||
on: |
|
|
|
|||||||
ACKNOWLEDGMENTS
STATE OF TEXAS |
§ |
COUNTY OF _____ |
§ |
This instrument was acknowledged before me on the ______ day of __________, 19___, by ____________________, the _______________ of _________________________________.
|
|
||
|
Notary Public |
||
|
Commission Expires: |
|
|
|
Printed Name: |
|
|
40
Exhibit 10.49
FIRST AMENDMENT TO LEASE
This First Amendment to Lease (this “Amendment”) is made to be effective as of September 1, 2002, by and between WALSTIB, L.P., a Delaware limited partnership (“Landlord”), and PFAB, LP, a Texas limited partnership (“Tenant”).
RECITALS
A.Landlord and PharmaFab, Inc., a Texas corporation (“PharmaFab”), entered into that certain Commercial Lease Agreement dated on or about June 29, 1999, and having a Commencement Date as of October 25, 1999 (as same may have been amended and assigned, the “Lease”) regarding certain premises (the “Premises”) located at 360 Riverside Business Center in the City of Grand Prairie, Tarrant County, Texas, as more particularly described in the Lease.
B.Pursuant to that certain Assignment of Lease dated on or about June 29, 1999, to be effective as of July 1, 1999, PharmaFab assigned its right, title and interest in the Lease to Tenant.
C.Pursuant to that certain Guaranty of Lease (the “Original Guaranty”), Bruce K. Montgomery and Darlene M. Ryan guaranteed the obligations of Tenant under the Lease, subject to certain limitations set forth in said Original Guaranty.
D.Landlord and Tenant have agreed to amend the Lease as hereinafter set forth, and Darlene M. Ryan has agreed to execute a new guaranty of Tenant’s obligations under the Lease as hereinafter set forth.
NOW, THEREFORE, in consideration of the premises, Landlord and Tenant agree as follows:
1.Defined Terms. All capitalized terms not defined herein shall have the meanings set forth for such terms in the Lease.
2.Expansion of Premises. Landlord and Tenant agree that the Premises shall be expanded to include the space comprised of approximately 50,000 rentable square feet in Building A of the Industrial Center that is denoted on Exhibit A attached hereto and made a part hereof (and herein referred to) as the “Expansion Space”. Tenant acknowledges that Tenant has inspected the Expansion Space and agrees to accept the Expansion Space in “as is” condition.
3.Term. Landlord and Tenant agree that the Expiration Date of the term of the Lease shall be November 30, 2010 (rather than October 31, 2006).
4.Base Rent: Landlord and Tenant agree that the Base Rent shall be as follows:
|
|
Monthly |
Months |
|
Base Rent |
September 1, 2002 —March 31, 2003 |
|
$31,900.60 |
April 1, 2003 — April 30, 2003 |
|
$45,962.50 |
May 1, 2003 — October 31, 2004 |
|
$49,608.33 |
November 1, 2004 — October 31, 2005 |
|
$52,835.00 |
November 1, 2005 — March 31, 2008 |
|
$54,301.66 |
April 1, 2008 — November 30, 2010 |
|
$56,697.50 |
5.Adjustment in Tenant’s Share. As used in the Lease, the term “Building” shall refer to Building A and Building B. Landlord and Tenant agree that, as a result of adding the Expansion Space to the Premises, Tenant’s Share is as follows:
(a) |
|
Industrial Park |
|
43.8% |
(b) |
|
Building A |
|
49.5% |
(c) |
|
Building B |
|
38.7% |
6.Refurbishment Allowance. On December 1, 2005, Landlord shall provide to Tenant an allowance (the “Refurbishment Allowance”) of Two Hundred Fifty Thousand Dollars ($250,000) to be used by Tenant to design and construct improvements in the Premises; provided, however, that Landlord shall have no obligation to provide the Refurbishment Allowance if at such time (a) Tenant is the subject of a bankruptcy proceeding or any other insolvency proceeding, or (b) Tenant is in Default, or any circumstance exists which, with the giving of notice or the passage of time, or both, would constitute a Default. All improvements constructed with the Refurbishment Allowance shall be subject to the provisions of the Lease governing alterations to the Premises, and, unless waived in writing by Landlord, Trammell Crow Dallas Fort Worth (or its designee) shall serve as the construction manager for such improvements. The Refurbishment Allowance shall be used only for payment of the following: (i) costs, including professional fees, of the architect and other design and planning costs in connection with tenant improvements constructed in the Premises; (ii) the costs of labor and material associated with the construction of the tenant improvements in the Premises; and (iii) a fee equal to four percent (4%) of the sum of all design, planning and construction costs associated with the construction of the tenant improvements in the Premises, unless Landlord has waived in writing the requirement that Trammell Crow Dallas Fort Worth (or its designee) serve as construction manager, in which event no fee shall be owed. Landlord shall have the right to require, as a condition to any disbursement of any portion of the Refurbishment Allowance requested by Tenant, that Tenant provide Landlord copies of invoices and/or similar evidence that the requested disbursement is for one of the purposes set forth in the foregoing clauses (i), (ii) or (iii). In no event shall Landlord have any obligation to make any disbursement of the Refurbishment Allowance requested after May 1, 2006, it being agreed that after such date any unused portion of the Refurbishment Allowance shall be deemed forfeited by Tenant. Each payment of the Refurbishment Allowance shall be made within thirty (30) days after Landlord has received a request therefor together with all other documents reasonably required by Landlord.
7.Right of First Offer. Tenant shall have a right of first offer with respect to the space in Building A shown on Exhibit B attached hereto and made a part hereof (the “Offered Space”), under the following terms and conditions:
(a)Subject to the provisions of Section 7(d) below, if at any time during the term of the Lease any lease for any portion of the Offered Space shall expire and if Landlord intends to market the Offered Space to prospects for lease with third parties (a
First Amendment to Lease – Page 2
“Proposed Tenant”) other than the tenant then occupying such space (or its affiliates), Landlord shall first allow Tenant the right to include the Offered Space within the Premises.
(b)Such offer shall be made by Landlord to Tenant in a written notice (hereinafter called the “Offer Notice”) which notice shall designate the space being offered and shall specify the terms for such Offered Space that Landlord intends to submit to prospective tenants in an effort to market the Offered Space. Tenant may accept the offer set forth in the Offer Notice by delivering to Landlord an unconditional acceptance (hereinafter called “Tenant’s Notice”) of such offer within five (5) business days after delivery by Landlord of the Offer Notice to Tenant. Time shall be of the essence with respect to the giving of Tenant’s Notice. If Tenant does not accept (or fails to timely accept) an offer made by Landlord pursuant to the provisions hereof with respect to the Offered Space designated in the Offer Notice, Landlord shall be under no further obligation whatsoever respect to such space. In order to send the Offer Notice, Landlord does not need to have negotiated a lease with any particular Proposed Tenant but may merely have determined on what basis it will market the Offered Space to Proposed Tenants. Tenant must make its decision with respect to the Offered Space as long as it has received a description of such material economic terms.
(c)Tenant must accept all Offered Space offered by Landlord at any one time if it desires to accept any of such Offered Space and may not exercise its right with respect to only part of such space. In addition, if Landlord desires to lease more than just the Offered Space to one tenant. Landlord may offer to Tenant pursuant to the terms hereof all such space which Landlord desires to lease, and Tenant must exercise its rights hereunder with respect to all such space and may not insist on receiving an offer for just the Offered Space.
(d)If Tenant at any time declines any Offered Space offered by Landlord, Tenant shall be deemed to have irrevocably waived all further rights under this Addendum, and Landlord shall be free to lease the Offered Space to any Proposed Tenant including on terms which may be less favorable to Landlord than those set forth in the Offer Notice.
(e)Tenant shall not have the benefit of the foregoing right of first offer if Tenant is in Default at the time the Offer Notice is to be sent, or if at such time any circumstance exists which, with the giving of notice or the passage of time, or both, would constitute a Default. The period of time Tenant’s right of first offer may be exercised shall not be extended or enlarged by reason of Tenant’s inability to exercise such right because of the provisions of this paragraph.
8.Option to Extend. The addendum to the Lease captioned “Option to Extend’ Addendum” is hereby deleted in its entirety. Landlord hereby grants to Tenant the options to extend the term of this Lease for the periods (the “Option Periods”) (1) from December 1, 2010 to November 30, 2015, and (2) from December 1, 2015 to November 30, 2020, under the following terms and conditions.
(a)In order to exercise an option to extend, Tenant must give written notice of such election to Landlord and Landlord must receive the same by no later than twenty four
First Amendment to Lease – Page 3
(24) months prior to the date (the “Last Exercise Date”) that the applicable Option Period would commence, but not earlier than thirty (30) months prior to the date that the applicable Option Period would commence. If proper notification of the exercise of an option is not given and/or received, such option shall automatically expire. The renewal options may only be exercised consecutively. Failure to exercise an option terminates that option and all subsequent options. Tenant acknowledges that because of the importance to Landlord of knowing no later than the Last Exercise Date whether or not Tenant will exercise the option, the failure of Tenant to notify Landlord by the Last Exercise Date will conclusively be presumed an election by Tenant not to exercise the option. Upon the exercise of an option to extend, both parties shall execute an amendment to the Lease evidencing the renewal thereof and setting forth the new Base Rent (as determined pursuant to Section 8(c) below) no later than eighteen (18) months prior to the date that the applicable Option Period would commence.
(b)Tenant shall have no right to exercise an option (i) if Tenant is in Default. or (ii) if during the twelve (12) month period immediately preceding the exercise of the option, three (3) or more Defaults have occurred which were not cured within any applicable notice or grace period. The period of time within which an option may be exercised shall not be extended or enlarged by reason of Tenant’s inability to exercise an option because of the provisions of this paragraph.
(c)If an option to extend is exercised, all of the terms and conditions of this Lease shall apply, except that (i) Landlord shall not be required to grant any allowance or perform any tenant improvement work in the Premises, and (ii) for each Option Period, Base Rent shall be adjusted to equal fair market rent. After an option to renew is exercised, Landlord and Tenant shall negotiate in good faith to determine the Base Rent for the applicable Option Period. If agreement cannot be reached within thirty (30) days, then Landlord and Tenant shall each, no later than sixty (60) days after the date of exercise of the option, make a reasonable determination of the fair market rental for the Premises for the applicable Option Period and submit such determination, in writing, to arbitration in accordance with the following provisions:
(i)No later than ninety (90) days after the option to renew is exercised, Landlord and Tenant shall each select an industrial leasing broker to act as an arbitrator. The two arbitrators so appointed shall, no later then one hundred twenty (120) days after the option to renew is exercised, select a third mutually acceptable industrial leasing broker to act as a third arbitrator.
(ii)The three arbitrators, acting by a majority, shall no later then one hundred fifty (150) days after the option to renew is exercised, determine the actual fair market rental for the Premises for the Option Period. The decision of a majority of the arbitrators shall be binding on the parties. The fair market rental determination of Landlord or Tenant which is closest to the fair market rental as determined by the arbitrators shall be the Base Rent for the Option Period.
First Amendment to Lease – Page 4
(iii)If either of the parties fails to appoint an arbitrator within the period required by this Addendum, the arbitrator timely appointed shall determine the Base Rent for the Option Period.
(iv)The entire cost of such arbitration shall be paid by the party whose fair market rental submission is not selected.
(d)The renewal options set forth herein are personal to Tenant, cannot be assigned or exercised by anyone other than Tenant and only while Tenant is in full possession of the Premises and without the intention of thereafter assigning or subletting.
9.Guaranty. Concurrently with the execution hereof, Tenant shall cause Darlene M. Ryan to execute and deliver to Landlord a guaranty in the form of Exhibit C attached hereto and made a part hereof, which shall supersede the Original Guaranty.
10.Commission Agreement. Attached hereto as Exhibit D is a copy of the Commission Agreement (herein so called) executed between Landlord, as “Owner,” and Fobare Commercial, as “Agent,” in connection with the Lease. The Commission Agreement shall remain in full force and effect, except that in connection with this Amendment (and this Amendment only) the compensation paid to Agent shall be as set forth on Exhibit E attached hereto. The Commission Agreement, as modified with respect to this Amendment only in the manner described on Exhibit E, shall be deemed included as a provision of this Lease in compliance with Section 62.022 of the Texas Property Code.
11.Counterparts. The parties may execute this Amendment in any number of counterparts with the same effect as if all parties to this Amendment had signed the same document.
12.Governing Law. The Lease, as amended hereby, shall be governed and construed in accordance with the laws of the State of Texas.
13.Continued Effect. The Lease, as amended hereby, is ratified and confirmed and shall continue in full force and effect.
First Amendment to Lease – Page 5
IN WITNESS WHEREOF, Landlord and Tenant have duly executed this Amendment to be effective as of the day and year first above written.
Landlord: |
|
Tenant: |
||||||
|
|
|
||||||
WALSTIB, L.P. |
|
PFAB, L.P. |
||||||
a Delaware limited partnership |
|
a Texas limited partnership |
||||||
|
|
|
||||||
By: |
AMB Property, L.P. |
|
By: |
PharmaFab Texas, LLC, |
||||
|
a Delaware limited partnership |
|
|
a Texas limited liability company |
||||
|
Its sole general partner |
|
|
Its General Partner |
||||
|
|
|
|
|
||||
|
By: |
|
|
|
By: |
|
||
|
Name: Doug McGregor |
|
|
|
Darlene M. Ryan, Manager |
|||
|
Its Vice President |
|
|
|
||||
|
|
|
||||||
Date of Execution: |
|
|
Date of Execution: |
|
||||
First Amendment to Lease – Page 6
Exhibit A
Expansion Space
First Amendment to Lease – Page 7
Exhibit B
Offered Space
First Amendment to Lease – Page 8
Exhibit C
GUARANTY OF LEASE
This Guaranty of Lease (this “Guaranty”) is made to be effective as of September 1, 2002, by DARLENE M. RYAN (“Guarantor”) for the benefit of WALSTIB, L.P., a Delaware limited partnership (“Landlord”).
A.Landlord and PharmaFab, Inc., a Texas corporation (“PharmaFab”), entered into that certain Commercial Lease Agreement dated on or about June 29, 1999, and having a Commencement Date as of October 25, 1999 (as same may have been amended and assigned, the “Original Lease”) regarding certain premises located at 360 Riverside Business Center in the City of Grand Prairie, Tarrant County, Texas, as more particularly described in the Original Lease.
B.Pursuant to that certain Assignment of Lease dated on or about June 29, 1999, to be effective as of July 1, 1999, PharmaFab assigned its right, title and interest in the Original Lease to PFab, LP, a Texas limited partnership (“Tenant”).
C.Pursuant to that certain Guaranty of Lease (the “Original Guaranty”), Bruce K. Montgomery (“Montgomery”) and Guarantor guaranteed the obligations of Tenant under the Original Lease, subject to certain limitations set forth in said Original Guaranty.
D.Landlord would not have executed the Original Lease if Montgomery and Guarantor had not executed and delivered the Original Guaranty to Landlord.
E.Landlord and Tenant have entered into that certain First Amendment to Lease of even date herewith (the “First Amendment”) providing for, among other things, the expansion of the premises demised by Landlord to Tenant (the Original Lease, as amended by the First Amendment, being herein referred to as the “Lease”).
F.In connection with the First Amendment, Guarantor (who owns an interest in Tenant) has agreed to execute and deliver to Landlord, and Landlord has agreed to accept, this Guaranty as a replacement of the Original Guaranty, it being understood that Landlord is not willing to execute the First Amendment if Guarantor does not execute and deliver this Guaranty to Landlord.
NOW THEREFORE, in consideration of the execution of the First Amendment by Landlord and as a material inducement to Landlord to execute the First Amendment:
1.Guarantor hereby unconditionally and irrevocably guarantees the prompt payment by Tenant of all rents and all other sums payable by Tenant under the Lease and the faithful and prompt performance by Tenant of each and every one of the terms, conditions and covenants of the Lease to be kept and performed by Tenant.
2.The terms of the Lease may, without the consent of or notice to Guarantor, be modified by Landlord and Tenant or by a course of conduct and this Guaranty shall guarantee the performance of said Lease as so modified. The Lease may be assigned by Landlord or any assignee of Landlord without consent or notice to Guarantor.
First Amendment to Lease – Page 9
3.This Guaranty shall not be released, modified or affected by the failure or delay on the part of Landlord to enforce any of the rights or remedies of the Landlord under the Lease, whether pursuant to the terms thereof or at law or in equity.
4.No notice of default need be given to Guarantor. The guaranty of the undersigned is a continuing guaranty under which Landlord may proceed immediately against Tenant and/or against Guarantor following any breach or default by Tenant or for the enforcement of any rights which Landlord may have against Tenant under the terms of the Lease or at law or in equity.
5.Landlord shall have the right to proceed against Guarantor hereunder following any breach or default by Tenant without first proceeding against Tenant and without previous notice to or demand upon either Tenant or Guarantor.
6.Guarantor hereby waives (a) notice of acceptance of this Guaranty, (b) demand of payment, presentation and protest, (c) any right to require Landlord to proceed against Tenant or any other person or entity liable to Landlord, (d) any right to require Landlord to apply to any default any security deposit or other security it may hold under the Lease, (e) any right to require Landlord to proceed under any other remedy Landlord may have before proceeding against Guarantor, and (f) any right of subrogation.
7.Guarantor does hereby subrogate all existing or future indebtedness of Tenant to such Guarantor to the obligations owed to Landlord under the Lease and this Guaranty.
8.If a Guarantor is married, such Guarantor expressly agrees that recourse may be had against his or her separate property for all of the obligations hereunder.
9.The obligations of Tenant under the Lease to execute and deliver estoppel certificates and financial statements shall be deemed to also require Guarantor to do and provide the same.
10.The term “Landlord” refers to and means the Landlord named in the Lease and also Landlord’s successors and assigns. So long as Landlord’s interest in the Lease, the leased premises or the rents, issues and profits therefrom, are subject to any mortgage or deed of trust or assignment for security, no acquisition by Guarantor of the Landlord’s interest shall affect the continuing obligation of Guarantor under this Guaranty which shall nevertheless continue in full force and effect for the benefit of the mortgagee, beneficiary, trustee or assignee under such mortgage, deed of trust or assignment and their successors and assigns.
11.The term “Tenant” refers to and means the Tenant named in the Lease and also Tenant’s successors and assigns.
12.In the event any action be brought by said Landlord against Guarantor hereunder to enforce the obligation of Guarantor hereunder, the unsuccessful party in such action shall pay to the prevailing party therein a reasonable attorney’s fee which shall be fixed by the court.
13.Provided Tenant has not had an uncured Default as described in the Lease, as of October 25, 2002, the liability of Guarantor hereunder shall be limited to the sum of $760,000.00. If no uncured Default has occurred under the Lease as of October 25, 2004, then this Guaranty
First Amendment to Lease – Page 10
shall terminate. It is agreed that if a Default occurs under the Lease which continues beyond the expiration of any applicable grace or cure period, Landlord may condition its acceptance of any cure of such Defauit upon keeping this Guaranty in effect.
14.This Guaranty shall supersede the Original Guaranty in its entirety, it being specifically agreed that Montgomery shall no longer have any liability as a guarantor of the Lease.
Executed to be effective as of the date and year first above written.
“GUARANTOR” |
|
|||
|
|
|||
|
|
|||
Darlene M. Ryan |
|
|||
Executed at: |
|
|
||
Address: |
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
STATE OF TEXAS |
§ |
|
||
|
§ |
|
||
COUNTY OF TARRANT |
§ |
|
||
This instrument was acknowledged before me on August __ 2002, by DARLENE M. RYAN.
|
|
|
|
Notary Public - State of Texas |
|
|
|
|
[Seal] |
My Commission Expires: |
|
First Amendment to Lease – Page 11
Exhibit D
Copy of Commission Agreement
COMMISSION AGREEMENT BETWEEN
WALSTIB, L.P., A DELAWARE LIMITED PARTNERSHIP
AND
FOBARE COMMERCIAL
THIS CONTRACT OF AGREEMENT, entered into by and between Walstib, L.P., a Delaware limited partnership hereinafter referred to as “Owner,” and Fobare Commercial hereinafter referred to as “Agent.”
W I T N E S S E T H:
Agent has assisted or is assisting Owner in negotiating and consummating a Lease Agreement between Owner and PharmaFab, Inc., hereinafter referred to as “Prospect,” covering an approximate 44,000 square foot facility located at (in) 360 Riverside Business Park (Building B) (the “Project”) for a term of 84 months beginning June 1, 1999 with rentals being due to Owner as therein provided, to which lease reference is hereby made, and the same is hereby incorporated herein for all purposes; and
Agent and Owner hereby and herein desire to agree upon a commission to which Agent shall be entitled for such services based on rentals actually received by Owner, as hereinafter provided.
It is mutually agreed as follows:
1. REGISTRATION
a.Registration of the Prospect requires either (a) this Registration Letter from Agent naming the Prospect and a face-to-face meeting with a decision maker representing the Prospect or (b) a duly executed and authorized letter from the Prospect designating Agent as its exclusive Agent.
b.This Registration and Agreement is effective for sixty (60) days after the date hereof and may be extended for another sixty (60) days if the Agent shows evidence satisfactory to Owner that Agent is actively pursuing the execution of a Lease Agreement between Owner and the Prospect.
c.If at any time following the registration of the Prospect pursuant to (a) or (b) above, Agent loses control of the Prospect as evidenced by a letter from the Prospect designating some other Agent as the Prospect’s Agent, then upon notification of such event to Agent by Owner, this Registration Letter and Commission Agreement shall become null and void.
d.Agent has satisfied the aforesaid initial registration requirements of Owner and accordingly Owner agrees to recognize PharmaFab, Inc. as Agent’s Prospect and agrees to pay
First Amendment to Lease – Page 12
a commission to Agent if a lease of space of the Project is consummated between the Prospect and Owner during the term of this Agreement.
2. CASH COMMISSION
a.Primary Term. (1) If Agent requests a front-end cash-out of commission obligation hereunder, and if Prospect in Owner’s opinion has sufficient credit to meet all of Prospect’s obligations under the lease, Owner shall, as set forth in d. below, pay Agent as the Commission hereunder four and one-half percent (4-1/2%) of the total Rentals as hereafter payable for the entire initial term of the lease up to ten (10) years. Financial statements and other information necessary to determine credit risk are to be provided by Agent.
b.Renewal. If Agent requests a front-end cash-out of the commission obligation hereunder with respect to a renewal option contained in the lease which has been duly exercised, and if Prospect in Owner’s opinion then has sufficient credit to meet all of Prospect’s obligations under the lease during the renewal term, Owner shall pay Agent the then prevailing market commission for a renewal, based upon the total Rentals payable for the renewal term, up to ten (10) years, providing that Agent is instrumental in assisting Owner in obtaining such renewal.
c.Expansion. If Agent requests a front-end cash-out of the commission obligation hereunder with respect to an Option to Expand contained in the Lease Agreement which has been duly exercised prior to the termination of the Lease Agreement and if Prospect in Owner’s opinion then has sufficient credit to meet all of Prospect’s obligations under the lease as so expanded, Owner may pay Agent a commission of four and one-half percent (4-1/2%) of the total Rentals payable for the expansion space providing that Agent is instrumental in assisting Owner in obtaining such expansion.
d.Time of Payment. (1) Initial Term. One-half (1/2) upon execution of the lease by Owner and Prospect, delivery thereof to both parties and receipt by Owner of first full month’s rent and any security deposit; and the balance upon occupancy by Prospect, delivery to Owner of satisfactory commencement letter executed by Prospect. In addition, no payment shall be payable until contingencies relating to the actual consummation or cancellation of the agreement by Prospect (including but not limited to contingencies regarding zoning, restrictive covenants, permitting, plan approvals and finish cost limits) are satisfactorily eliminated by the parties to the Lease Agreement. In addition for Build-to-Suit facilities no commission will be payable until Owner has received interim financing for the project.
(2)Renewal Term. If a renewal commission is due pursuant to Paragraph 2.b. above, the commission shall be payable upon written exercise of the renewal option and payment of the rental for the first full month of the renewal term.
e.Cancellation. Notwithstanding the above provisions, where Prospect individually or Prospect and Owner, jointly, have the right to cancel the lease, a commission shall only be paid to Agent for the period up to the date on which the lease may be canceled. If the lease is not then canceled, Owner shall pay the balance of the commission due for the remainder of the period covered under the lease at the time the Prospect’s right to cancel expires, provided Prospect remains in occupancy of the space and is not in default under the Lease.
First Amendment to Lease – Page 13
f.New Lease. In the event Agent furnishes Owner written authorization to represent Prospect and negotiates and consummates a new Lease Agreement between Owner and Prospect covering an expansion or relocation facility, Owner shall pay Agent a commission based on the above provisions for the expansion or relocation premises, but Agent’s commission under the original Lease Agreement shall be terminated as of the commencement date of the new Lease Agreement and any unearned commission paid to the Agent with respect to the original Lease Agreement shall be netted out of commission payable for the new Lease Agreement.
3. GENERAL
a.Sale of Premises. In the event of sale of the premises and assignment of the Lease Agreement by Owner, Owner agrees to provide a copy of this Commission Agreement to the Purchaser and to use its reasonable efforts to obtain from the purchaser or assignee an agreement whereby such purchaser or assignee assumes the obligation to pay to Agent under the terms and provisions hereof, and Owner shall be released from all liability for all future payments of commissions as may thereafter become due under this agreement.
b.Definitions. “Rentals” shall mean the base monthly cash rent, and shall not include any amounts payable by Prospect to Owner for reimbursement of costs and expenses passed through to Prospect, such as real estate taxes, insurance premiums, utilities, janitorial and common area maintenance, or any operating expense escalations or amortization of tenant finish costs above Owner’s building standard allowance.
In addition rentals shall be reduced by the cost of any lease takeover obligations to Owner, free rent or other considerations by Owner to the benefit of Prospect, whether agreed to in the Lease Agreement or other written instrument. Rentals for the purposes of this Commission Agreement, does not include that portion of Prospect’s payments that relate to costs to Owner that are separately amortized because the cost of such improvements are above Owner’s building standard allowance, relate to extraordinary items or otherwise would not be considered within the ordinary course of rental payments in an industrial facility.
c.Binding Effect. Except as otherwise herein provided, all rights and obligations hereunder shall be binding upon and inure to the benefit of the successors, assigns, heirs, administrators and personal representatives of the Owner and Agent.
d.Entire Agreement. Except as expressly herein provided, Owner shall have no obligation to pay, and Agent shall have no right to receive, any commission or other payment with respect to the Lease or any transactions between Owner and Prospect, unless such right or obligation is set forth in writing and signed by Owner and Prospect after the date hereof.
e.Acceptance. This agreement is intended to be an offer, and if not accepted and returned to Owner in writing within three business days from the date hereon, it is hereby withdrawn. If not received by said date, this offer is null and void. This Agreement is not valid unless fully signed by both Owner and Agent.
f.Owner’s Interest. Broker shall look solely to Owner’s interest in the Project for the recovery of any judgement against Owner for failure to perform under this Agreement, and Owner
First Amendment to Lease – Page 14
(and Its partners, shareholders and agents) shall not be personally liable for any such judgement therefore.
EXECUTED BY OWNER this ___________ day of _______________, 1999.
WALSTIB, L.P., A DELAWARE LIMITED PARTNERSHIP | |||||
| |||||
By: |
WALSTIB Venture, L.L.C., a Delaware limited liability company |
||||
|
Its sole general partner |
||||
|
|
||||
By: |
TCDFW Development, Ltd., a Texas limited partnership |
||||
|
Its Administrative Member |
||||
|
|
||||
By: |
Trammell Crow DFW Development, Inc., a Delaware corporation |
||||
|
Its sole general partner |
||||
|
|
||||
|
|
||||
By: |
|
|
|||
|
|
||||
Its: |
Executive Vice President |
|
|||
|
|
||||
Telephone: |
(214) 979-6180 |
|
|||
|
|
||||
Facsimile: |
(214) 979-6355 |
|
|||
|
|
||||
Executed At: |
2200 Ross Avenue, Suite 3700 |
|
|||
| |||||
On: |
|
|
|||
|
|
||||
|
EXECUTED BY AGENT this _________ day of _________, 1999. |
||||
FOBARE COMMERCIAL | ||
| ||
| ||
By: |
|
|
Title: |
|
|
First Amendment to Lease – Page 15
Exhibit E
Payment of Commission with Respect to this Amendment Only
1.Capitalized terms used in this exhibit which are not defined in the Amendment to which this exhibit is attached shall have the same meanings attributed to such terms in the Commission Agreement. Upon the execution of the Amendment by Landlord and Tenant, Agent shall be paid, in full, the commission due to Agent by reason of the existence of the Amendment.
2.The foregoing timing of the payment due to Agent applies only with respect to the Amendment to which this exhibit is attached and Landlord reserves the right to insist hereafter upon strict compliance with the terms of the Commission Agreement. The terms of the Commission Agreement shall remain in full force and effect.
First Amendment to Lease – Page 16
COMMISSION AGREEMENT BETWEEN
WALSTIB, L.P., A DELAWARE LIMITED PARTNERSHIP
AND
FOBARE COMMERCIAL
THIS CONTRACT OF AGREEMENT, entered into by and between Walstib, L.P., a Delaware limited partnership hereinafter referred to as “Owner,” and Fobare Commercial hereinafter referred to as “Agent.”
W I T N E S S E T H:
Agent has assisted or is assisting Owner in negotiating and consummating a Lease Agreement between Owner and PharmaFab, Inc., hereinafter referred to as “Prospect,” covering an approximate 44,000 square foot facility located at (in) 360 Riverside Business Park (Building B) (the “Project”) for a term of 84 months beginning June 1, 1999 with rentals being due to Owner as therein provided, to which lease reference is hereby made, and the same is hereby incorporated herein for all purposes; and
Agent and Owner hereby and herein desire to agree upon a commission to which Agent shall be entitled for such services based on rentals actually received by Owner, as hereinafter provided.
It is mutually agreed as follows:
1. REGISTRATION
a.Registration of the Prospect requires either (a) this Registration Letter from Agent naming the Prospect and a face-to-face meeting with a decision maker representing the Prospect or (b) a duly executed and authorized letter from the Prospect designating Agent as its exclusive Agent.
b.This Registration and Agreement is effective for sixty (60) days after the date hereof and may be extended for another sixty (60) days if the Agent shows evidence satisfactory to Owner that Agent is actively pursuing the execution of a Lease Agreement between Owner and the Prospect.
c.If at any time following the registration of the Prospect pursuant to (a) or (b) above, Agent loses control of the Prospect as evidenced by a letter from the Prospect designating some other Agent as the Prospect’s Agent, then upon notification of such event to Agent by Owner, this Registration Letter and Commission Agreement shall become null and void.
d.Agent has satisfied the aforesaid initial registration requirements of Owner and accordingly Owner agrees to recognize PharmaFab, Inc. as Agent’s Prospect and agrees to pay a commission to Agent if a lease of space of the Project is consummated between the Prospect and Owner during the term of this Agreement.
2. CASH COMMISSION
a.Primary Term. (1) If Agent requests a front-end cash-out of commission obligation hereunder, and if Prospect in Owner’s opinion has sufficient credit to meet all of Prospect’s obligations under the lease, Owner shall, as set forth in d. below, pay Agent as the Commission hereunder four and one-half percent (4-1/2%) of the total Rentals as hereafter payable for the entire initial term of the lease up to ten (10) years. Financial statements and other information necessary to determine credit risk are to be provided by Agent.
b.Renewal. If Agent requests a front-end cash-out of the commission obligation hereunder with respect to a renewal option contained in the lease which has been duly exercised, and if Prospect in Owner’s opinion then has sufficient credit to meet all of Prospect’s obligations under the lease during the renewal term, Owner shall pay Agent the then prevailing market commission for a renewal, based upon the total Rentals payable for the renewal term, up to ten (10) years, providing that Agent is instrumental in assisting Owner in obtaining such renewal.
c.Expansion. If Agent requests a front-end cash-out of the commission obligation hereunder with respect to an Option to Expand contained in the Lease Agreement which has been duly exercised prior to the termination of the Lease Agreement and if Prospect in Owner’s opinion then has sufficient credit to meet all of Prospect’s obligations under the lease as so expanded, Owner may pay Agent a commission of four and one-half percent (4-1/2%) of the total Rentals payable for the expansion space providing that Agent is instrumental in assisting Owner in obtaining such expansion.
d.Time of Payment. (1) Initial Term. One-half (1/2) upon execution of the lease by Owner and Prospect, delivery thereof to both parties and receipt by Owner of first full month’s rent and any security deposit; and the balance upon occupancy by Prospect, delivery to Owner of satisfactory commencement letter executed by Prospect. In addition, no payment shall be payable until contingencies relating to the actual consummation or cancellation of the agreement by Prospect (including but not limited to contingencies regarding zoning, restrictive covenants, permitting, plan approvals and finish cost limits) are satisfactorily eliminated by the parties to the Lease Agreement. In addition for Build-to-Suit facilities no commission will be payable until Owner has received interim financing for the project.
(2)Renewal Term. If a renewal commission is due pursuant to Paragraph 2.b. above, the commission shall be payable upon written exercise of the renewal option and payment of the rental for the first full month of the renewal term.
e.Cancellation. Notwithstanding the above provisions, where Prospect individually or Prospect and Owner, jointly, have the right to cancel the lease, a commission shall only be paid to Agent for the period up to the date on which the lease may be canceled. If the lease is not then canceled, Owner shall pay the balance of the commission due for the remainder of the period covered under the lease at the time the Prospect’s right to cancel expires, provided Prospect remains in occupancy of the space and is not in default under the Lease.
f.New Lease. In the event Agent furnishes Owner written authorization to represent Prospect and negotiates and consummates a new Lease Agreement between Owner and Prospect
covering an expansion or relocation facility, Owner shall pay Agent a commission based on the above provisions for the expansion or relocation premises, but Agent’s commission under the original Lease Agreement shall be terminated as of the commencement date of the new Lease Agreement and any unearned commission paid to the Agent with respect to the original Lease Agreement shall be netted out of commission payable for the new Lease Agreement.
3. GENERAL
a.Sale of Premises. In the event of sale of the premises and assignment of the Lease Agreement by Owner, Owner agrees to provide a copy of this Commission Agreement to the Purchaser and to use its reasonable efforts to obtain from the purchaser or assignee an agreement whereby such purchaser or assignee assumes the obligation to pay to Agent under the terms and provisions hereof, and Owner shall be released from all liability for all future payments of commissions as may thereafter become due under this agreement.
b.Definitions. “Rentals” shall mean the base monthly cash rent, and shall not include any amounts payable by Prospect to Owner for reimbursement of costs and expenses passed through to Prospect, such as real estate taxes, insurance premiums, utilities, janitorial and common area maintenance, or any operating expense escalations or amortization of tenant finish costs above Owner’s building standard allowance.
In addition rentals shall be reduced by the cost of any lease takeover obligations to Owner, free rent or other considerations by Owner to the benefit of Prospect, whether agreed to in the Lease Agreement or other written instrument. Rentals for the purposes of this Commission Agreement, does not include that portion of Prospect’s payments that relate to costs to Owner that are separately amortized because the cost of such improvements are above Owner’s building standard allowance, relate to extraordinary items or otherwise would not be considered within the ordinary course of rental payments in an industrial facility.
c.Binding Effect. Except as otherwise herein provided, all rights and obligations hereunder shall be binding upon and inure to the benefit of the successors, assigns, heirs, administrators and personal representatives of the Owner and Agent.
d.Entire Agreement. Except as expressly herein provided, Owner shall have no obligation to pay, and Agent shall have no right to receive, any commission or other payment with respect to the Lease or any transactions between Owner and Prospect, unless such right or obligation is set forth in writing and signed by Owner and Prospect after the date hereof.
e.Acceptance. This agreement is intended to be an offer, and if not accepted and returned to Owner in writing within three business days from the date hereon, it is hereby withdrawn. If not received by said date, this offer is null and void. This Agreement is not valid unless fully signed by both Owner and Agent.
f.Owner’s Interest. Broker shall look solely to Owner’s interest in the Project for the recovery of any judgement against Owner for failure to perform under this Agreement, and Owner (and Its partners, shareholders and agents) shall not be personally liable for any such judgement therefore.
EXECUTED BY OWNER this _________ day of ___________, 1999.
WALSTIB, L.P., A DELAWARE LIMITED PARTNERSHIP | |||||
| |||||
By: |
WALSTIB Venture, L.L.C., a Delaware limited liability company |
||||
|
Its sole general partner |
||||
|
|
||||
By: |
TCDFW Development, Ltd., a Texas limited partnership |
||||
|
Its Administrative Member |
||||
|
|
||||
By: |
Trammell Crow DFW Development, Inc., a Delaware corporation |
||||
|
Its sole general partner |
||||
|
|
||||
|
|
||||
By: |
|
|
|||
|
|
||||
Its: |
Executive Vice President |
|
|||
|
|
||||
Telephone: |
(214) 979-6180 |
|
|||
|
|
||||
Facsimile: |
(214) 979-6355 |
|
|||
|
|
||||
Executed At: |
2200 Ross Avenue, Suite 3700 |
|
|||
| |||||
On: |
|
|
|||
|
|
||||
|
EXECUTED BY AGENT this _________ day of _________, 1999. |
||||
FOBARE COMMERCIAL | ||
| ||
| ||
By: |
|
|
Title: |
|
|
Exhibit 10.50
INTERIM AMENDMENT TO LEASE
This INTERIM AMENDMENT TO LEASE (this “Amendment”) is made and entered into as of September 4, 2003, by and between TEACHERS INSURANCE AND ANNUITY ASSOCIATION OF AMERICA, a New York corporation (hereinafter referred to as “Landlord”), and PFAB LP, a Texas limited partnership (hereinafter referred to as “Tenant”).
BACKGROUND:
A. |
Walstib, L.P. (“Walstib”) and PharmaFab, Inc., a Texas corporation (“PharmaFab”), entered into that certain Commercial Lease Agreement dated on or about June 29, 1999, and having a Commencement Date as of October 25, 1999 (as same may have been amended and assigned, the “Lease”) regarding certain premises (the “Premises”) located at 360 Riverside Business Center in the City of Grand Prairie, Tarrant County, Texas, as more particularly described in the Lease. |
B. |
Pursuant to that certain Assignment of Lease dated on or about June 29, 1999, to be effective as of July 1, 1999, PharmaFab assigned its right, title and interest in the Lease to Tenant. |
C. |
Walstib and Tenant amended the Lease pursuant to that certain First Amendment to Lease dated effective as of September 1, 2002 (the “First Amendment”) pursuant to which the Premises were expended from approximately 44,000 square feet of space in Suite 100 of Building B of the industrial Center (the “Suite 100 Space”) to include approximately 50,000 additional square feet in Suite 100 of Building A of the Industrial Canter (the “Building A Space”) and Bruce K. Montgomery was released from his Guaranty of Lease. |
D. |
Landlord succeeded to the interest of Walstib under the Lease. |
E. |
Landlord and Tenant are currently negotiating a Second Amendment to Lease (the “Second Amendment”) relocating a portion of the Premises. |
F. |
Landlord and Tenant desire to amend the Lease on an interim basis, to allow entry by Tenant into the space located at 2940 North Highway 360, Grand Prairie, Texas (the “Expansion Premises”) for the purpose of installing Tenant’s equipment and telecommunications equipment. |
AGREEMENT
NOW, THEREFORE, in consideration of the foregoing, and for other good and valuable consideration, the receipt and sufficiency of which are acknowledged, the parties agree as follows:
1. |
Capitalized Terms. All capitalized terms which are not otherwise defined herein shall have the meaning set forth in the Lease, as amended hereby. |
2. |
Expansion. As of September 4, 2003 (the “Effective Date’’, the term “Premises” as used In the Lease shall include the Expansion Premises, Tenant’s occupancy of the Expansion Premises is under all terms and conditions of the Lease, as modified hereby. |
3. |
Term - Expansion Premises. The term of the Lease with regard to the Expansion Premises is month to month (‘Temporary Expansion Term”). The Temporary Expansion Term shall expire upon the earlier of (i) full execution of the Second Amendment and fulfillment of the contingency set forth therein, or (ii) 15 days after written notice of termination by either party to the other party. |
4. |
Rental. The Rent for the Expansion Premises is $1,000 per month, payable as provided in the Lease. Tenant shall not be responsible for payment of Tenant’s Share of Operating Expenses with regard to the Expansion Premises during the Temporary Expansion Term. Rent for any partial calendar month shall be prorated on a per diem basis. |
5. |
Acceptance of Premises. Tenant accepts the Expansion Premises in their “AS IS, WHERE IS” condition, and Landlord has no obligation to Improve, repair, restore, or refurbish the Premises. Tenant’s occupancy of any portion of the Premises is conclusive evidence that Tenant: (A) accepts such portion of the Premises as suitable for the purposes for which they are leased; (B) accepts such portion of the Premises as being in a good and satisfactory condition; (C) waives any defects in the Premises; and (D) having been provided an opportunity to inspect and measure each portion of the Premises, agrees that the square footage numbers specified in this Amendment are accurate, binding, and conclusive for all purposes. Neither Landlord nor any other Landlord Entity has made, and Tenant waives, any express or implied representation or warranty with respect to the Premises or any other portion of the Industrial Center including, without limitation, any representation or warranty with respect to the suitability or fitness of the Premises or any other portion of the Industrial Center for the conduct of Tenant’s business. Landlord expressly recognizes, covenants and agrees that notwithstanding the terms of this Paragraph 4 or Paragraph 7, to the contrary, nothing in this Paragraph 4 relieves Landlord of any of its obligations pursuant to the Lease. Including, without limitation, Paragraph 7.2 of the Lease and Paragraph 16.17 of the Lease. |
6. |
Brokerage Mutual Indemnities. |
a.Tenant warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than Trammel Crow Company and CB Richard Ellis (collectively, “Brokers”). Tenant shall indemnify, defend, and hold Landlord harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent other than Brokers claiming by, through, or under Tenant with respect to this Amendment.
b.Landlord Warrants that it has had no dealing; with any broker or agent in connection with the negotiation or execution of this Amendment other than Brokers.
2
Landlord shall indemnify, defend, and hold Tenant harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent, including Brokers, claiming by, through or under Landlord with respect to this Amendment.
c.Any brokerage commissions payable to Brokers are payable by Landlord pursuant to the terms of separate agreements between Landlord and Broker.
7. |
No Offsets. Tenant hereby represents to Landlord that to the best of Tenant’s knowledge, as of the date of this Amendment. Tenant has no defenses to or offsets against the full and timely payment and performance of each and every covenant and obligation required to be performed by Tenant under the terms of the Lease. |
8. |
Conflicts. The terms of this Amendment prevail if there is a conflict with the terms of the Lease. |
9. |
Headings. The headings or captions of the paragraphs in this Amendment are for convenience only and shall not act and shall not be implied to act to limit or expand the construction and intent of the contents of the respective Paragraph. |
10. |
Binding Effect. This Amendment is binding upon and shall inure to the benefit of the parties and their respective successors and assigns (but this reference to assigns shall not be deemed to act as a consent to an assignment by Tenant). |
11. |
Ratification. The Lease, as amended and modified hereby, is ratified and confirmed by the parties as being in full force and effect. |
3
EXECUTED as of the date first above written.
|
|
|
||||
|
|
LANDLORD: |
||||
|
|
|
||||
|
|
TEACHERS INSURANCE AND ANNUITY| |
||||
|
|
ASSOCIATION OF AMERICA, |
||||
|
|
a New York Corporation |
||||
|
|
|
||||
|
|
By: |
|
|||
|
|
|
Print Name: |
|
||
|
|
|
As Its: |
|
||
|
|
|
||||
|
|
TENANT: |
||||
|
|
|
||||
|
|
PFAB LP, |
||||
|
|
|
||||
|
|
a Texas limited partnership |
||||
|
|
|
||||
|
|
By: |
PharmaFab Texas, LLC, |
|||
|
|
|
its general partner |
|||
|
|
|
|
|||
|
|
|
By: |
|
||
|
|
|
Print Name: Darlene M. Ryan |
|||
|
|
|
As Its: Sole Manager |
|||
ATTACHMENT “A”
to Interim Amendment to Lease by and between
and
PFAB LP, as Tenant
FLOOR PLAN OF THE EXPANSION PREMISES
Exhibit 10.51
THIRD AMENDMENT TO LEASE
Teachers Insurance and Annuity Association of America, as Landlord This THIRD AMENDMENT TO LEASE (this “Amendment”) is made and entered into as of _______________, 2003, by and between TEACHERS INSURANCE AND ANNUITY ASSOCIATION OF AMERICA, a New York corporation (hereinafter referred to as “Landlord”), and PFAB LP, a Texas limited partnership (hereinafter referred to as “Tenant”).
BACKGROUND:
| A. | Walstib, L.P. (“Walstib”) and PharmaFab, Inc., a Texas corporation (“PharmaFab”), entered into that certain Commercial Lease Agreement dated on or about June 29, 1999, and having a Commencement Date as of October 25, 1999 (as same may have been amended and assigned, the “Lease”) regarding certain premises (the “Premises”) located at 360 Riverside Business Center in the City of Grand Prairie, Tarrant County, Texas, as more particularly described in the Lease. |
| B. | Pursuant to that certain Assignment of Lease dated on or about June 29, 1999, to be effective as of July 1, 1999, PharmaFab assigned its right, title and interest in the Lease to Tenant. |
| C. | Walstib and Tenant amended the Lease pursuant to that certain First Amendment to Lease dated effective as of September 1, 2002 (the “First Amendment”) pursuant to which the Premises were expanded from approximately 44,000 square feet of space in Suite 100 of Building B of the Industrial Center (the “Suite 100 Space”) to include approximately 50,000 additional square feet in Suite 100 of Building A of the Industrial Center (the “Building A Space”) and Bruce K. Montgomery was released from his Guaranty of Lease. |
| D. | Landlord succeeded to the interest of Walstib under the Lease. |
| E. | Landlord and Tenant amended the Lease on a short term basis pursuant to an Interim Amendment to Lease, dated September 4, 2003. |
| F. | Landlord and Tenant desire to further amend the Lease as hereinafter set forth to include, without limitation, the release of Darlene M. Ryan from any guaranty of the Lease. |
AGREEMENT:
NOW, THEREFORE, in consideration of the foregoing, and for other good and valuable consideration, the receipt and sufficiency of which are acknowledged, the parties agree as follows:
| 1. | Capitalized Terms. All capitalized terms which are not otherwise defined herein shall have the meaning set forth in the Lease, as amended hereby. |
| 2. | Relocation. As of the respective Effective Dates set forth below, the Building A Space will be relocated to 2940 N. Highway 360, Building B, Suite 400 (“Suite 400 Space”) consisting of approximately 20,170 square feet of space and 2940 N. Highway 360, Building B, Suite 200 consisting of approximately 33,112 square feet of space (“Suite 200 Space”) for a total of approximately 53,282 square feet of space. |
The Lease will terminate as to the Building A Space [except with regard to liabilities due and outstanding as of the Suite 400 Effective Date (hereinafter defined)] and Tenant will relocate from the Building A Space to the Suite 400 Space on September 9, 2003 (the “Suite 400 Effective Date”).
Upon vacating the Building A Space, Tenant shall surrender such space in accordance with the terms of the Lease, as though the Lease had expired with respect to the Building A Space. Tenant is solely responsible for all costs of relocating from the Building A Space.
Subject to the satisfaction of the contengency set forth in Paragraph 10 of this Amendment, effective 5 days after the date SmartMail, LLC (“SmartMail”), the current tenant of Suite 200 of Building B, vacates Suite 200 (the “Suite 200 Effective Date”), the Premises shall be deemed to include the Suite 200 Space. From and after the Suite 200 Effective Date, the term “Premises” shall be deemed to refer to the Suite 100 Space, the Suite 200 Space and the Suite 400 Space for a total of approximately 97,282 square feet of space, all as more fully described on Attachment A hereto.
| 3. | Term. The Lease, as amended hereby shall expire on November 30, 2010 (the “Expiration Date”). |
| 4. | Base Rental. Upon the Suite 400 Effective Date, monthly Base Rent is payable as follows: |
| a. | Suite 400 Space. From the Suite 400 Effective Date through the date prior to the Suite 200 Effective Date, Monthly Base Rent for the Suite 400 Space is $19,867.45. From the Suite 200 Effective Date until May 9, 2004, Base Rental for the Suite 400 Space is abated. Thereafter, Monthly Base Rental for the Suite 400 Space is as follows: |
5/9/04 - 4/30/09$16,505.78
5/1/03 - 11/30/10$18,774.91
| b. | Suite 200 Space. From the Suite 200 Effective Date through the date prior to the fifth anniversary thereof, Monthly Base Rental for the Suite 200 Space is $21,633.17. From and after the fifth anniversary of the Suite 200 Effective Date through the Expiration Date, the Monthly Base Rental for the Suite 200 Space is $23,702.67. |
| c. | Suite 100 Space. Monthly Base Rental for the Suite 100 Space is as follows: |
2
9/1/03 - 10/31/04 |
$28,930.00 |
11/1/04 - 10/31/05 |
$35,126.67 |
11/1/05 - 11/30/10 |
$36,593.33 |
Landlord and Tenant agree to execute and deliver a memorandum setting forth the actual dates and rental rates once such dates are determined.
| 5. | Operating Expenses. Tenant shall be liable for Tenant’s Share of Operating Expenses for each portion of the Premises on the applicable Effective Date for such portion, payable as provided in the Lease. Tenant’s Share of Operating Expenses for the Industrial Park and Building is determined on a per square foot basis by dividing the number of square feet in the Premises (or applicable portion thereof) by the total number of square feet in the Industrial Park or Building, as applicable. From the Suite 400 Effective Date through the date prior to the Suite 200 Effective Date, Landlord estimates Tenant’s Share of Operating Expenses as follows: |
(a)Industrial Park29.90%
(b)Building56.49%
Upon the Suite 200 Effective Date, Landlord estimates Tenant’s Share of Operating Expenses as follows:
(a)Industrial Park45.33%
(b)Building85.64%
| 6. | Right of First Refusal. |
| a. | If during the Lease Term, Suite 300 of Building B is available for lease and Landlord enters into a letter of intent with a third party covering all of the essential terms (collectively, the “Third Party Terms”) for any of such space (the “First Refusal Space”), then Landlord shall deliver a notice to Tenant (the “First Refusal Notice”) offering to lease the First Refusal Space to Tenant under the Third Party Terms (the “ROFR Terms”). As used in this Paragraph 6 only, the term available for lease means that the First Refusal Space is neither: (i) subject to any rights of third parties existing as of the date of this Amendment, including, without limitation, previously granted rights of first notice, expansion rights, extension rights, options to lease, or other previously granted rights, nor (ii) subject to renewal by its current tenant, Statewide, whether or not such renewal is pursuant to an option to renew set forth in its lease as of the date of this Amendment. |
| b. | Tenant may elect to lease the First Refusal Space under the ROFR Terms by delivering a notice (the “Response Notice”) to Landlord within 5 business days after the date Tenant receives the First Refusal Notice. If (i) Landlord does not receive the Response Notice within the 5 business day period or (ii) in the Response Notice Tenant elects not to lease the First Refusal Space under the ROFR Terms, then Tenant is deemed to waive its right to lease the First Refusal Space and Tenant has no further rights under this Paragraph 6. Notwithstanding the foregoing, however, if Landlord does not then execute a lease for the First Refusal Space with |
3
any third party, under economic terms no more than 5% different from those set forth in the Third Party Terms, then this Paragraph 6, and the parties’ rights and obligations hereunder, will be reinstated in their entirety.
| c. | If Tenant timely delivers a Response Notice electing to lease First Refusal Space under the ROFR Terms, then Landlord shall promptly prepare, and deliver to Tenant an amendment to the Lease adding the First Refusal Space to the Premises upon the ROFR Terms, which amendment will be in a form substantially similar to this Amendment. Landlord and Tenant shall execute and deliver such amendment within 5 business days thereafter. |
| d. | Landlord is not obligated to offer the First Refusal Space to Tenant, and Tenant may not exercise its option to lease the First Refusal Space, if Tenant is in default under the Lease at the time Landlord would otherwise be obligated to give notice to Tenant under this Paragraph. |
The Right of First Offer set forth in Section 7 of the First Amendment is hereby deleted and shall be of no further force and effect.
4
| 7. | Security Deposit; Release of Guaranties. Upon execution of this Amendment, Tenant shall deposit the amount of $48,706.56 as in increase in the Security Deposit currently held by Landlord under Section 5 of the Lease for a total Security Deposit of $86,706.57. The Security Deposit shall be held and applied by Landlord as provided in the Lease. Upon execution of this Amendment by Landlord, Darlene M. Ryan is and shall automatically without any further action be deemed released from and shall have no further liability pursuant to the guaranties previously executed by her in connection with the Lease, including without limitation, that certain Guaranty of Lease dated June 29, 1999 and that certain Guaranty of Lease dated effective September 1, 2002. It is further recognized and agreed that pursuant to the terms of the First Amendment, Bruce K. Montgomery, was previously released from any and all guaranties previously executed by him in connection with the Lease, including, without limitation, that certain Guaranty of Lease Dated June 29, 1999. |
| 8. | Acceptance of Premises. Tenant accepts the Premises including Suites 200 and 400 in their “AS IS, WHERE IS” condition, and Landlord, subject to its obligations under Attachment B, has no obligation to improve, repair, restore, or refurbish the Premises. Tenant’s occupancy of any portion of the Premises is conclusive evidence that Tenant: (A) accepts such portion of the Premises as suitable for the purposes for which they are leased; (B) accepts such portion of the Premises as being in a good and satisfactory condition; (C) waives any defects in the Premises; and (D) having been provided an opportunity to inspect and measure each portion of the Premises, agrees that the square footage numbers specified in this Amendment are accurate, binding, and conclusive for all purposes. Neither Landlord nor any other Landlord Entity has made, and Tenant waives, any express or implied representation or warranty with respect to the Premises or any other portion of the Industrial Center including, without limitation, any representation or warranty with respect to the suitability or fitness of the Premises or any other portion of the Industrial Center for the conduct of Tenant’s business. Landlord expressly recognizes, covenants and agrees that notwithstanding the terms of this Paragraph 8 or Paragraph 13, to the contrary, nothing in this Paragraph 8 relieves Landlord of any of its obligations pursuant to the Lease, including, without limitation, Paragraph 7.2 of the Lease and Paragraph 16.17 of the Lease. |
| 9. | Finish-Out of Premises. Landlord agrees to provide Tenant with a finish-out allowance of $301,036 for costs incurred in connection with renovations to the Premises. Tenant hereby waives any rights it may have to approximately $250,000 in additional tenant improvements to be made to the Building A Space. Tenant must perform all Tenant Finish Work in accordance with the terms of the Lease and Attachment B to this Amendment. |
| 10. | Contingency. The Suite 200 Space is currently subject to a lease dated February 25, 2000 by and between Landlord, as successor in interest to Walstib and SmartMail (the “SmartMail Lease”) The effectiveness of this Amendment is contingent on Landlord executing an amendment to the SmartMail Lease in form and substance satisfactory to Landlord, relocating SmartMail to the Building A Space. If Landlord and SmartMail fail to so amend the SmartMail Lease on or before October 2, 2003, Landlord shall notify Tenant in writing and this Amendment shall be terminated and of no further force and effect. |
5
| 11. | Brokerage; Mutual Indemnities. |
| a. | Tenant warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than Trammell Crow Company and CB Richard Ellis (collectively, “Brokers”). Tenant shall indemnify, defend, and hold Landlord harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent other than Brokers claiming by, through, or under Tenant with respect to this Amendment. |
| b. | Landlord warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than Brokers. Landlord shall indemnify, defend, and hold Tenant harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent, including Brokers, claiming by, through or under Landlord with respect to this Amendment. |
| c. | Any brokerage commissions payable to Brokers are payable by Landlord pursuant to the terms of separate agreements between Landlord and Broker. |
| 12. | No Offsets. Tenant hereby represents to Landlord that to the best of Tenant’s knowledge, as of the date of this Amendment, Tenant has no defenses to or offsets against the full and timely payment and performance of each and every covenant and obligation required to be performed by Tenant under the terms of the Lease. |
| 13. | Conflicts. The terms of this Amendment prevail if there is a conflict with the terms of the Lease. |
| 14. | Headings. The headings or captions of the paragraphs in this Amendment are for convenience only and shall not act and shall not be implied to act to limit or expand the construction and intent of the contents of the respective paragraph. |
| 15. | Binding Effect. This Amendment is binding upon and shall inure to the benefit of the parties and their respective successors and assigns (but this reference to assigns shall not be deemed to act as a consent to an assignment by Tenant). |
| 16. | Ratification. The Lease, as amended and modified hereby, is ratified and confirmed by the parties as being in full force and effect. |
| 17. | Acknowledgement of Option to Extend. Landlord recognizes, covenants and agrees that the Option to Extend set forth in Paragraph 8 of the First Amendment remains in full force and effect and applies and shall continue to apply to the Premises as defined herein. |
EXECUTED as of the date first above written.
LANDLORD:
6
|
TEACHERS INSURANCE AND ANNUITY ASSOCIATION OF AMERICA, |
||||
|
a New York corporation |
||||
|
|
||||
|
By: |
|
|||
|
|
Print Name: |
|
||
|
|
As Its: |
|
||
|
|
|
|||
|
TENANT: |
|
|||
|
|
|
|||
|
PFAB LP, |
||||
|
a Texas limited partnership |
||||
|
|
||||
|
By: |
PharmaFab Texas, LLC, |
|||
|
|
its general partner |
|||
|
|
|
|||
|
|
By: |
|
||
|
|
Print Name: |
Darlene M. Ryan |
||
|
|
As Its: |
Sole Manager |
||
7
ATTACHMENT “A”
to Third Amendment to Lease by and between
Teachers Insurance and Annuity Association of America, as Landlord,
and
PFAB LP, as Tenant
FLOOR PLAN OF THE PREMISES
8
ATTACHMENT B
to Third Amendment to Lease by and between
Teachers Insurance and Annuity Association of America, as Landlord,
and
PFAB LP, as Tenant
TENANT FINISH CONSTRUCTION
| A. | Plans and Specifications: Tenant shall submit to Landlord at least 30 days prior to commencement of any remodeling in the Premises complete initial plans and specifications (the “Initial Construction Documents”) for the remodeling of the Premises. The Initial Construction Documents must include, without limitation: |
| 1. | General Notes Sheet |
| 2. | Demolition Plan |
| 3. | New Construction Plan with details of all new improvements |
| 4. | Finishes Plan |
| 5. | Electrical, Mechanical and Plumbing Plan |
Within 15 days after receipt of the Initial Construction Documents, Landlord shall deliver to Tenant a notice either approving or disapproving them. Any disapproval must specify in reasonable detail the reasons for the disapproval. If Tenant does not receive a notice from Landlord disapproving the Initial Construction Documents within the 15-day period, Landlord is deemed to approve the Initial Construction Documents. If Landlord disapproves the Initial Construction Documents, Tenant shall revise them to conform to Landlord’s objections and deliver complete copies of the revised Initial Construction Documents to Landlord.
The approved Initial Construction Documents are referred to as the “Construction Documents” and all work to be performed by Tenant pursuant to the Construction Documents is referred to as the “Tenant Finish Work”. Landlord’s approval of the Construction Documents is not a warranty that the Construction Documents comply with Applicable Laws.
| B. | Tenant Finish Work. Tenant shall pay the Actual Cost (defined below) of all Tenant Finish Work. Within 45 days of completion of the Tenant Finish Work, and presentation to Landlord of (i) receipts and invoices marked “paid” (ii) lien releases executed by all parties performing Tenant Finish Work, including without limitation, the general contractors, |
9
materialmen and other vendors performing any portion of the Tenant Finish Work or supplying any materials used in connection therewith, and (iii) the Architect’s Certificate of Substantial Completion, Landlord will reimburse Tenant for a portion of the Tenant Finish Work, up to the amount of $301,036.00 (the “Work Allowance”). The term “Actual Cost” means the cost of all labor and materials and all hard and soft costs relating to the Tenant Finish Work, together with the Building Service Fee of 4% of all hard costs of the Tenant Finish Work.
Tenant, at its cost and risk (subject to reimbursement of the Work Allowance by Landlord), shall construct or cause to be constructed the Tenant Finish Work in substantial accordance with the Construction Documents. Tenant shall allow Landlord access to the Premises at all times to inspect the Tenant Finish Work. Landlord has no obligation to inspect the Tenant Finish Work. No inspection by Landlord of the Tenant Finish Work is a warranty that the Tenant Finish Work complies with the Construction Documents or any Applicable Laws.
| C. | General. |
| 1. | Any changes to the Construction Documents must first be submitted to Landlord for review and approval prior to the work reflected in such amended Plans being undertaken by Tenant. |
| 2. | Workmanship and materials to be used in the Tenant Finish Work shall be of best quality. Any approval by Landlord of the Plans shall not in any way constitute a representation or warranty of Landlord as to the adequacy of sufficiency of the Plans; such approval shall merely be the consent of Landlord as may be required hereunder in connection with the Tenant Finish Work in accordance with the Plans under the terms of the Lease. |
| 3. | Tenant shall perform the Tenant Finish Work consistent with Building Rules and Regulations, in a manner to minimize noise and other interference with tenants of the Industrial Center and shall remove all trash and debris from the Premises on a daily basis. |
| 4. | Upon completion of the construction of Tenant Finish Work, Tenant shall promptly restore any area of the Industrial Center damaged as a result of Tenant’s construction of the Tenant Finish Work to the condition existing prior to the commencement of such construction. |
| 5. | All contractors, subcontractors, suppliers, service providers, moving companies, and others performing work of any type for Tenant in the Industrial Center shall carry the insurance listed below with companies acceptable to Landlord: |
| a. | Commercial General Liability Insurance (ISO Form CG00010798 or its equivalent), written on an “occurrence” basis, with minimum limits of $1,000,000 per occurrence; $3,000,000 general aggregate for bodily injury, personal injury and property damage. If required by Landlord, liquor liability coverage will be included. |
10
| b. | Workers’ Compensation insurance with statutory limits and Employers Liability with a $1,000,000 per accident limit for bodily injury or disease. |
| c. | Automobile Liability covering all owned, non-owned and hired vehicles with a $1,000,000 per accident limit for bodily injury and property damage. |
Tenant shall deliver to Landlord, do Trammell Crow Company (“Manager”), 2200 Ross Avenue, Suite 3700, Dallas, Texas 75201, Attention: Betty Venator, duly executed certificates of all insurance (Acord Form 27, modified as necessary to cover liability insurance) and additional insured endorsements reasonably satisfactory to Landlord (on ISO Form 2026 or its equivalent, without modification) prior to entering the Premises and annually thereafter, reflecting evidence of required coverages.
All insurance required under hereunder (i) shall be primary and non-contributory (ii) shall provide for severability of interests, (iii) shall be issued by insurers, licensed to do business in the state in which the Premises are located and which are rated A:IX or better by Best’s Key Rating Guide, (iv) shall be endorsed to include Landlord and such other persons or entities as Landlord may from time to time designate, as additional insureds without restriction (Commercial General Liability only), and (v) shall be endorsed to provide at least 30-days prior notification of cancellation or material change in coverage to said additional insureds. Each policy must be endorsed to waive any rights of subrogation against Landlord, its officers, directors, employees, agents, partners and assigns.
11
Exhibit 10.52
FOURTH AMENDMENT TO LEASE
This FOURTH AMENDMENT TO LEASE (this Amendment) is made and entered into as of June ___, 2009 (the Execution Date), to be effective as of May 1, 2009 (the Effective Date), by and between TEACHERS INSURANCE AND ANNUITY ASSOCIATION OF AMERICA, a New York corporation (Landlord), and NEOS THERAPEUTICS, LP, a Texas limited partnership, formerly known as PFAB LP (Tenant).
BACKGROUND:
| A. | Walstib, L.P., a Delaware limited partnership (Walstib), and PharmaFab, Inc., a Texas corporation (PharmaFab), entered into that certain Commercial Lease Agreement dated on or about June 29, 1999, and having a Commencement Date as of October 25, 1999 (the Original Lease) regarding certain premises containing approximately 44,000 square feet in Suite 100 (the Original Premises) of Building B (the Building) in the industrial complex commonly known as 360 Riverside Business Center located at 2940 N. Highway 360 in the City of Grand Prairie, Tarrant County, Texas, as more particularly described in the Original Lease (the Industrial Center). |
| B. | Pursuant to that certain Assignment of Lease dated on or about June 29, 1999, to be effective as of July 1, 1999, PharmaFab assigned its right, title and interest in the Original Lease to PFAB LP, a Texas limited partnership (PLP). |
| C. | Walstib and PLP amended the Original Lease pursuant to that certain First Amendment to Lease dated effective as of September 1, 2002 (the First Amendment) pursuant to which (i) the Original Premises were expanded to include approximately 50,000 additional square feet in Suite 100 of Building A of the Industrial Center (the Building A Premises) and (ii) Bruce K. Montgomery was released from his Guaranty of Lease. |
| D. | Landlord succeeded to the interest of Walstib under the Lease. |
| E. | Landlord and PLP further amended the Original Lease on a short term basis pursuant to an Interim Amendment to Lease dated September 4, 2003 (the Second Amendment). |
| F. | Landlord and PLP further amended the Original Lease pursuant to that certain Third Amendment to Lease dated October 1, 2003 (the Third Amendment) pursuant to which (i) the Building A Premises were relocated to Suites 200 and 400 of the Building such that the Premises (as defined in the Original Lease) consist of (1) approximately 77,112 square feet of space in Suites 100 and 200 of the Building (collectively, the Renewal Premises) and (2) approximately 20,170 square feet of space in Suite 400 of the Building (the Suite 400 Space) and (ii) Darlene M. Ryan was released from her Guaranty of Lease. The Original Lease, as amended by the First Amendment, Second Amendment and Third Amendment, is referred to herein as the Lease. |
| G. | On June 22, 2007, Tenant changed its name to Neos Therapeutics, LP. |
FOURTH AMENDMENT TO LEASE |
PAGE 1 |
| H. | Landlord and Tenant desire to further amend the Lease to extend the Term of the Lease for the Renewal Premises and modify certain provisions of the Lease as hereinafter set forth, but not otherwise. |
AGREEMENT:
NOW, THEREFORE, in consideration of the foregoing, and for other good and valuable consideration, the receipt and sufficiency of which are acknowledged, the parties agree as follows:
| 1. | Capitalized Terms. All capitalized terms which are not otherwise defined herein shall have the meaning set forth in the Lease, as amended hereby. |
| 2. | Term. The Term for the Renewal Premises is extended to expire on December 31, 2019 (the Expiration Date). The Term for the Suite 400 Space shall expire on November 30, 2010. Commencing December 1, 2010, the term “Premises” shall refer to the Renewal Premises only. |
| 3. | Base Rent. From the Effective Date through the Expiration Date, the Base Rent payable with respect to the Renewal Premises and the Suite 400 Space, as applicable, is as follows: |
Period |
Base Rent/SF (Renewal Premises – |
Base Rent/SF (Suite 400 Space – |
Monthly Base |
5/1/09 - 9/30/09 |
$0.00 |
$11.17 |
$18,774.91 |
10/1/09-11/30/10 |
$7.50 |
$11.17 |
$66,969.91 |
12/1/10-4/30/11 |
$7.50 |
- |
$48,195.00 |
5/1/11 -12/31/13 |
$8.50 |
- |
$54,621.00 |
1/1/14-12/31/16 |
$9.00 |
- |
$57,834.00 |
1/1/17 -12/31/19 |
$9.50 |
- |
$61,047.00 |
All other terms of the Lease regarding the payment of Base Rent remain unchanged. Tenant shall continue to pay all other amounts payable under the Lease; provided, however, Tenant’s Share shall be amended in accordance with Section 5 of this Amendment.
| 4. | Acceptance of Premises. Tenant accepts the Premises in their “AS-IS” condition and Landlord shall have no obligation to improve, repair, restore or refurbish the Renewal Premises except as otherwise specifically provided in this Amendment. Tenant acknowledges that neither Landlord nor any agent of Landlord has made any representation or warranty, except as otherwise expressly provided in this Amendment, with respect to the Renewal Premises or any other portion of the Industrial Center including, without limitation, any representation or warranty with respect to the suitability or fitness of the Renewal Premises or any other portion of the Industrial Center for the conduct of Tenant’s business. Nothing in this Paragraph 4 shall negate or diminish Landlord’s repair or restoration obligations under the Lease. |
| 5. | Operating Expenses. |
FOURTH AMENDMENT TO LEASE |
PAGE 2 |
a. |
Tenant shall be liable for Tenant’s Share of Operating Expenses for the Premises, payable as provided in the Lease. Tenant’s Share for the Industrial Center and the Building is determined on a per square foot basis by dividing the number of square feet in the Premises (or applicable portion thereof) by the total number of square feet in the Industrial Center or the Building, as applicable. |
From the Effective Date through November 30, 2010, Tenant’s Share of Operating Expenses is as follows:
(a)Industrial Center45.33%
(b)Building85.64%
From December 1, 2010 through the Expiration Date, Tenant’s Share of Operating Expenses is as follows:
(a)Industrial Center35.93%
(b)Building67.88%
b. |
Upon the Effective Date, Section 4.2 of the Lease is amended by adding the following: |
(g) |
For purposes of determining Tenant’s Share of Operating Expenses, Controllable Operating Expenses (defined below) for any calendar year will not increase over the amount of Controllable Operating Expenses during the previous year (calculated upon a base equal to the actual expenses incurred for calendar year 2009) by more than 8% per year on a cumulative basis, compounded annually. For example, the maximum amount of Controllable Operating Expenses that may be included in the calculation of such Additional Rent for each calendar year after 2009 shall equal the product of the Controllable Operating Expenses incurred for calendar year 2009 and the following percentages for the following calendar years: 108% for 2010, 116.64% for 2011, 125.97% for 2012, etc. The term Controllable Operating Expenses means all Operating Expenses except costs and expenses for taxes, insurance, property management fees (except for property management fees paid to affiliates of Landlord), utilities, costs to Landlord resulting from compliance with Applicable Requirements, and any increases in service contract fees and expenses resulting from government-mandated wage increases. |
| 6. | Tenant’s Insurance. Upon the Effective Date, Section 8.2 of the Lease is deleted and the following substituted therefor: |
(a)At its sole cost and expense, Tenant shall maintain in full force and effect during the Term of this Lease the following insurance coverages insuring against claims which may arise from or in connection with the Tenant’s operation and use of the Premises.
(i)Commercial General Liability Insurance (ISO Form CG00010798 or its equivalent), written on an “occurrence” basis, with minimum limits of
FOURTH AMENDMENT TO LEASE |
PAGE 3 |
$1,000,000 per occurrence; $2,000,000 general aggregate for bodily injury, personal injury and property damage and excess umbrella liability insurance in the amount of $5,000,000. Tenant’s Commercial General Liability Insurance must cover business carried on, in or from the Premises and Tenant’s use and occupancy of the Premises (including contractual liability and must have no deductible).
(ii)Workers’ compensation insurance complying with statutory requirements of the State of Texas and employers liability insurance in amounts not less than $500,000 bodily injury per accident/$500,000 disease each employee/$500,000 disease policy limit.
(iii)Automobile Liability covering all owned, non-owned and hired vehicles with a $1,000,000 per accident limit for bodily injury and property damage.
(iv)Property insurance against all risks of loss to any tenant improvements or betterments and business personal property on a full replacement cost basis with no coinsurance penalty provision and a deductible not to exceed $25,000; and Business Interruption Insurance with a limit of liability representing loss of at least approximately six months of income.
(b)Tenant shall deliver to Landlord duly executed certificates of all insurance (Acord Form 28, modified as necessary to cover liability insurance) and additional insured endorsements reasonably satisfactory to Landlord (on ISO Form 2026 or its equivalent, without modification) prior to July 1, 2009 and annually thereafter, reflecting evidence of required coverages together with endorsements required under this Section 8.2.
(c)All insurance required under Paragraph 8.2 (i) shall be primary and non- contributory, (ii) shall provide for severability of interests, (iii) shall be issued by insurers, licensed to do business in the state in which the Premises are located and which are rated A-:IX or better by Best’s Key Rating Guide, (iv) shall be endorsed to include Landlord, Landlord’s property manager and such other persons or entities as Landlord may from time to time designate, as additional insureds without restriction (Commercial General Liability and excess umbrella liability insurance only), (v) shall be endorsed to endeavor to provide at least 30-days prior notification of cancellation or material change in coverage to said additional insureds, and (vi) shall be endorsed to waive any rights of subrogation against Landlord, its officers, directors, employees, agents, partners and assigns. All deductibles shall be at Tenant’s sole risk and shall be paid by, assumed by and for the account of Tenant.
(d)If Tenant fails to comply with the insurance requirements, Landlord may obtain the required insurance on behalf of Tenant and Tenant shall pay the cost thereof as Additional Rent.
| 7. | Waiver of Subrogation. Upon the Effective Date, Section 8.4 of the Lease is deleted and the following substituted therefor: |
FOURTH AMENDMENT TO LEASE |
PAGE 4 |
Each party waives all claims that arise or may arise in its favor against the other party, or anyone claiming through or under them, by way of subrogation or otherwise, during the Lease Term or any extension or renewal thereof, for all losses of, or damage to, any of its property (WHETHER OR NOT THE LOSS OR DAMAGE IS CAUSED IN WHOLE OR IN PART BY THE FAULT OR NEGLIGENCE OR STRICT LIABILITY OF THE OTHER PARTY OR ANYONE FOR WHOM THE OTHER PARTY IS RESPONSIBLE), which loss or damage is covered by valid and collectible Special Form Property insurance policies or would have been covered by such insurance policies had the party maintained such insurance. The party incurring the loss or damage will be responsible for any deductible or self-insured retention under its property insurance. These waivers are in addition to, and not in limitation of, any other waiver or release in this Lease with respect to any loss or damage to property of the parties. Each party shall immediately give each insurance company issuing to it policies of Special Form Property insurance written notice of the terms of these mutual waivers, and have the insurance policies properly endorsed, if necessary, to prevent the invalidation of the insurance coverages by reason of these waivers.
| 8. | Indemnity. Upon the Effective Date, Section 8.5 of the Lease is amended by adding the following: |
THE INDEMNITY IN THIS SECTION WILL APPLY EVEN IF THE LOSS OR DAMAGE IS CAUSED OR ALLEGED TO BE CAUSED IN WHOLE OR IN PART BY THE NEGLIGENCE OR STRICT LIABILITY OF A LANDLORD ENTITY BUT WILL NOT APPLY TO THE EXTENT THE DAMAGE OR LOSS IS CAUSED BY THE GROSS NEGLIGENCE OR WILLFUL MISCONDUCT OF SUCH LANDLORD ENTITY.
| 9. | Exemption of Landlord from Liability. Upon the Effective Date, Section 8.6 of the Lease is deleted and the following substituted therefor: |
Landlord Entities shall not be liable for and Tenant waives any claims against Landlord Entities (EVEN IF SUCH CLAIMS ARE CAUSED SOLELY OR IN PART BY THE NEGLIGENCE OF LANDLORD ENTITIES BUT NOT TO THE EXTENT CAUSED BY THE GROSS NEGLIGENCE OR WILLFUL MISCONDUCT OF LANDLORD ENTITIES) for injury or damage to the person or the property of Tenant, Tenant’s employees, contractors, invitees, customers or any other person in or about the Premises, Building or Industrial Center from any cause whatsoever, including, but not limited to, damage or injury which is caused by or results from (i) fire, steam, electricity, gas, water or rain, or from the breakage, leakage, obstruction or other defects of pipes, fire sprinklers, wires, appliances, plumbing, air conditioning or lighting fixtures or (ii) from the condition of the Premises, other portions of the Building or Industrial Center. Landlord shall not be liable for any damages arising from any act or neglect of any other tenant of Landlord nor from the failure by Landlord to enforce the provisions of any other lease in the Industrial Center. NOTWITHSTANDING LANDLORD’S NEGLIGENCE OR BREACH OF THIS LEASE, LANDLORD SHALL UNDER NO CIRCUMSTANCES BE LIABLE FOR INJURY TO TENANT’S BUSINESS, FOR ANY LOSS OF INCOME OR PROFIT THEREFROM OR ANY INDIRECT, CONSEQUENTIAL OR PUNITIVE DAMAGES.
| 10. | Right of First Refusal. The Right of First Refusal covering Suite 300 of the Building as set forth in Section 6 of the Third Amendment, remains in full force and effect during the |
FOURTH AMENDMENT TO LEASE |
PAGE 5 |
Term as extended by this Amendment, and may be exercised by Tenant during such extended Term in accordance with Section 6 of the Third Amendment.
| 11. | Security Deposit. Upon execution of this Amendment, Tenant shall deposit the amount of $48,195.00 (the Security Deposit Increase) which shall be added to and become part of the Security Deposit currently held by Landlord under Section 5 of the Lease for a total Security Deposit of $134,901.57. The Security Deposit, as increased by the Security Deposit Increase, shall be held and applied by Landlord as provided in the Lease. |
| 12. | Contingency. |
a. |
Landlord acknowledges that in anticipation of the execution of this Amendment, Tenant is withholding payment of Base Rent and Operating Expenses for the months of May and June, 2009 (the Deferred Rent). Subject to the terms of this Paragraph 12, such failure to make Base Rent and Operating Expense payments is not a default under the Lease, and Landlord will not assess interest or a late charge as provided under the Lease. |
b. |
Tenant is currently negotiating a Venture Capital Agreement (the VC Agreement) with third parties. Tenant shall notify Landlord in writing on or before July 2, 2009 (the Notification Date) whether the VC Agreement was fully executed (the Tenant’s Notice). If Tenant fails to timely deliver the Tenant’s Notice on or prior to the Notification Date, Tenant shall be deemed to have executed the VC Agreement, and this Amendment shall remain in full force and effect. If Tenant delivers the Tenant’s Notice on or prior to the Notification Date advising Landlord that the VC Agreement was not fully executed on or before July 1, 2009, then (i) this Amendment shall automatically terminate without further action by Landlord or Tenant, (ii) within twenty (20) days of receipt of such Tenant’s Notice, Landlord will return the Security Deposit Increase to Tenant and (iii) within five (5) business days after the date of such Tenant’s Notice, Tenant shall pay the Deferred Rent. Failure to pay such Deferred Rent within such 5-business day period shall constitute a Default under the Lease. |
| 13. | Option to Extend. Tenant may extend the Term subject to all of the following conditions: |
a. |
If Tenant is not in default under the Lease at the time of the exercise of this option or at the commencement of the extended Term, Tenant may extend the Term for 2 extension terms of 5 years each (each, an Extension Term), commencing on the next day after the then-current Expiration Date, by giving Landlord an extension notice at least 9 months, but not more than 12 months, prior to the then-current Expiration Date (the Extension Notice Period). |
b. |
If Tenant gives a valid extension notice, then subject to this Section 13, the Term is extended for 5 years upon the same terms as in the Lease (as amended hereby), except that the Rent and other applicable terms adjust based on the Market Rate (defined and determined below), and Tenant has no further option to extend the Term after both options set forth in this Section 13 are exercised. If Tenant does |
FOURTH AMENDMENT TO LEASE |
PAGE 6 |
not give an extension notice during the applicable Extension Notice Period, then this option expires automatically at the end of the applicable Extension Notice Period.
c. |
Within 30 days after Landlord receives Tenant’s extension notice, Landlord shall deliver a notice to Tenant specifying the Market Rate (the Market Rate Notice). The term Market Rate means the minimum rent, refurbishment allowance, and other economic terms that a non-encumbered, non-equity tenant, having a credit standing substantially similar to that of Tenant as of the date of the Lease and requiring substantially the same space and terms as Tenant, would be able to obtain from a willing landlord comparable to Landlord, in arms-length negotiations for comparable space located within the Industrial Center and other comparable buildings in the Grand Prairie/Arlington markets, and leased at the time in question for a comparable period of time, as determined by Landlord in its reasonable discretion based upon current market conditions for comparable space located within the Industrial Center and other comparable buildings in the Grand Prairie/Arlington markets. In determining Market Rate, Landlord shall consider, among other things and in addition to base rent and refurbishment allowance: (i) Operating Expense treatment, (ii) rental abatement concessions, lease takeover payments, (iv) whether or not brokerage commissions are involved, and (v) other allowance, inducements, and concessions. |
d. |
Tenant shall notify Landlord within 15 days after the date of the Market Rate Notice whether it approves Landlord’s designation of Market Rate (the Response Notice), and if Tenant does not timely give a Response Notice, Tenant is deemed to approve the Market Rate specified in the Market Rate Notice. If Tenant gives a valid Response Notice that it disapproves of Landlord’s designation of Market Rate, then Landlord and Tenant shall negotiate in good faith for a period of 20 days after the date of the Response Notice to reach agreement on the Market Rate. If Landlord and Tenant do not reach agreement on the Market Rate within the 20-day period, then Tenant, as its sole remedy, may either (i) revoke its exercise notice by delivering a Revocation Notice (herein so called) to Landlord within 5 days after the end of the 20-day negotiation period (the Revocation Period), or (ii) deliver an Arbitration Notice (herein so called) to Landlord before the end of the Revocation Period, notifying Landlord of its election to submit the determination of Market Rate to arbitration, to be conducted by the American Arbitration Association office located in Dallas, Texas, in accordance with Section 13(e) below. If Tenant does not deliver a Revocation Notice or an Arbitration Notice before the end of the Revocation Period, then Tenant is deemed to have given a timely Revocation Notice. |
e. |
If Tenant gives a valid Arbitration Notice, then Landlord, or its designated representative, and Tenant shall each place in a separate sealed envelope their final proposal as to Market Rate (as to each party individually, Final Proposal) and shall meet with each other within 10 days after the last day of the Revocation Period (the Meeting Date). At the meeting, Landlord and Tenant shall exchange and then open the envelopes in each other’s presence. If Landlord and Tenant do not mutually |
FOURTH AMENDMENT TO LEASE |
PAGE 7 |
agree upon the Market Rate within 5 days after the Meeting Date, then the Market Rate will be submitted to baseball-style arbitration, and within 15 days after the Meeting Date, Landlord and Tenant shall agree upon and jointly appoint a 3 - member arbitration panel (the Panel) to conduct the arbitration. In appointing the Panel, Landlord and Tenant shall choose real estate professionals (excluding appraisers and attorneys) with knowledge of the Grand Prairie and Arlington markets for industrial space comparable to the Industrial Center. If Landlord and Tenant do not agree upon and appoint a 3- member Panel within 15 days after the Meeting Date, then within 5 days, Landlord and Tenant shall each appoint one panel member, and within 5 days thereafter, the two appointed members shall select the third. The determination of the Panel will be limited solely to the issue whether Landlord’s or Tenant’s Final Proposal is closer to the actual Market Rate for the Premises as determined by the Panel. The Panel may hold hearings and require briefs from Landlord and Tenant as the Panel, in its sole discretion, determines is necessary. In addition, Landlord or Tenant may submit to the Panel and the other party, within 5 days after the appointment thereof, any market data and additional information that the party deems relevant to the determination of Market Rate (MR Data), and the other party may submit a reply in writing within 5 days after receipt of such MR Data. The Panel shall, within 30 days after its appointment, notify Landlord and Tenant whether Landlord’s or Tenant’s Final Proposal is closer to the actual Market Rate for the Premises as determined by the Panel. The Panel’s decision is binding upon Landlord and Tenant. The cost of arbitration shall be paid by Landlord and Tenant equally, except that each party will be responsible for its own legal fees and costs of experts in connection with its presentation of information and evidence to the Panel.
f. |
If the Term is extended under this Section 13, Landlord shall prepare, and Landlord and Tenant shall promptly execute and deliver, an amendment to the Lease to reflect the extension of the Term and, if applicable, the modification of the Premises. |
g. |
The Option to Extend contained in Section 8 of the First Amendment is hereby deleted in its entirety. |
| 14. | Brokerage; Mutual Indemnities. |
a. |
Tenant warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than CB Richard Ellis, Inc. and Jackson & Cooksey, Inc. (collectively, Brokers). Tenant shall indemnify, defend, and hold Landlord harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent other than Brokers claiming by, through, or under Tenant with respect to this Amendment. |
b. |
Landlord warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than Brokers. Landlord shall indemnify, defend, and hold Tenant harmless against all costs, expenses, |
FOURTH AMENDMENT TO LEASE |
PAGE 8 |
attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent, including Brokers, claiming by, through or under Landlord with respect to this Amendment.
c. |
Any brokerage commissions payable to Brokers are payable by Landlord pursuant to the terms of separate agreements between Landlord and Broker. |
| 15. | No Offsets. Tenant hereby represents to Landlord that to the best of Tenant’s knowledge, as of the date of this Amendment, Tenant has no defenses to or offsets against the full and timely payment and performance of each and every covenant and obligation required to be performed by Tenant under the terms of the Lease, as amended hereby. |
| 16. | Conflicts. The terms of this Amendment prevail if there is a conflict with the terms of the Lease. |
| 17. | Headings. The headings or captions of the paragraphs in this Amendment are for convenience only and shall not act and shall not be implied to act to limit or expand the construction and intent of the contents of the respective paragraph. |
| 18. | Binding Effect. This Amendment is binding upon and shall inure to the benefit of the parties and their respective successors and assigns (but this reference to assigns shall not be deemed to act as a consent to an assignment by Tenant). |
| 19. | Ratification. The Lease, as amended and modified hereby, is ratified and confirmed by the parties as being in full force and effect. |
[Remainder of page intentionally left blank.]
FOURTH AMENDMENT TO LEASE |
PAGE 9 |
EXECUTED as of the date first above written.
|
|
LANDLORD: |
||
|
|
|
||
|
|
TEACHERS INSURANCE AND ANNUITY ASSOCIATION OF AMERICA, |
||
|
|
a New York corporation |
||
|
|
|
||
|
|
By: |
|
|
|
|
Print Name: |
|
|
|
|
As Its: |
|
|
|
|
TENANT: |
|||
|
|
|
|||
|
|
NEOS THERAPEUTICS, LP, |
|||
|
|
|
|||
|
|
By: |
PharmaFab Texas, LLC, |
||
|
|
|
a Texas limited liability company, |
||
|
|
|
its manager |
||
|
|
|
|
||
|
|
|
By: |
|
|
|
|
|
Name: |
|
|
|
|
|
As Its: |
|
|
FOURTH AMENDMENT TO LEASE |
PAGE 10 |
Exhibit 10.53
FIFTH AMENDMENT TO LEASE
This FIFTH AMENDMENT TO LEASE (this Amendment) is made and entered into as of April ___., 2010 (the Execution Date), by and between TEACHERS INSURANCE AND ANNUITY ASSOCIATION OF AMERICA, a New York corporation (Landlord), and NEOS THERAPEUTICS, LP, a Texas limited partnership, formerly known as PFAB LP (Tenant).
BACKGROUND:
A. |
Walstib, L.P., a Delaware limited partnership (Walstib), and PharmaFab, Inc., a Texas corporation (PharmaFab), entered into that certain Commercial Lease Agreement dated on or about June 29, 1999, and having a Commencement Date as of October 25, 1999 (the Original Lease), regarding certain premises containing approximately 44,000 square feet in Suite 100 (the Original Premises) of Building B (the Building) in the industrial complex commonly known as 360 Riverside Business Center located at 2940 N. Highway 360 in the City of Grand Prairie, Tarrant County, Texas, as more particularly described in the Original Lease (the Industrial Center). |
B. |
Pursuant to that certain Assignment of Lease dated on or about June 29, 1999, to be effective as of July 1, 1999, PharmaFab assigned its right, title and interest in the Original Lease to PFAB LP, a Texas limited partnership (PLP). |
C. |
Walstib and PLP amended the Original Lease pursuant to that certain First Amendment to Lease dated effective as of September 1, 2002 (the First Amendment) pursuant to which (i) the Original Premises were expanded to include approximately 50,000 additional square feet in Suite 100 of Building A of the Industrial Center (the Building A Premises) and (ii) Bruce K. Montgomery was released from his Guaranty of Lease. |
D. |
Landlord succeeded to the interest of Walstib under the Lease. |
E. |
Landlord and PLP further amended the Original Lease on a short term basis pursuant to an Interim Amendment to Lease dated September 4, 2003 (the Second Amendment). |
F. |
Landlord and PLP further amended the Original Lease pursuant to that certain Third Amendment to Lease dated October 1, 2003 (the Third Amendment) pursuant to which (i) the Building A Premises were relocated to Suites 200 and 400 of the Building such that the definition of the premises (as defined in the Original Lease and as amended by the Third Amendment) consist of (1) approximately 77,112 square feet of space in Suites 100 and 200 of the Building (collectively, the Suite 100 Space) and (2) approximately 20,170 square feet of space in Suite 400 of the Building (the Suite 400 Space) (collectively, the Premises) and (ii) Darlene M. Ryan was released from her Guaranty of Lease. |
G. |
On June 22, 2007, Tenant changed its name to Neos Therapeutics, LP. |
H. |
Landlord and Tenant further amended the Original Lease pursuant to that certain Fourth Amendment to Lease effective as of May 1,,2009 (the Fourth Amendment), whereby the Term was extended for the Suite 100 Space. The Original Lease, as amended by the First |
FIFTH AMENDMENT TO LEASE |
PAGE 1 |
Amendment, Second Amendment, Third Amendment and Fourth Amendment, is referred to herein as the Lease.
I. |
Landlord and Tenant desire to further amend the Lease to extend the Term of the Lease for the Suite 400 Space and modify certain provisions of the Lease as hereinafter set forth, but not otherwise. |
AGREEMENT:
NOW, THEREFORE, in consideration of the foregoing, and for other good and valuable consideration, the receipt and sufficiency of which are acknowledged, the parties agree as follows:
1. |
Capitalized Terms. All capitalized terms which are not otherwise defined herein shall have the meaning set forth in the Lease, as amended hereby. |
2. |
Term. The Term for the Suite 400 Space is hereby extended to expire on December 31, 2019 (the Expiration Date). Therefore, in accordance with the Fourth Amendment and this Amendment, the Term for the entire Premises (consisting of the Suite 100 Space and the Suite 400 Space) expires on the Expiration Date. |
3. |
Base Rent. From March 1, 2010 (the “Effective Date”) through the Expiration Date, the Base Rent payable with respect to the Suite 400 Space only is as follows: |
Period |
Base Rent/SF |
Monthly Base Rent |
3/1/10 - 5/31/11 |
$8.00 |
$13,446.67 |
6/1/11 - 1/31/14 |
$10.05 |
$16,892.38 |
2/1/14 - 1/31/17 |
$10.55 |
$17,732.79 |
2/1/17 - 12/31/19 |
$11.05 |
$18,573.21 |
All other terms of the Lease regarding the payment of Base Rent remain unchanged. Tenant shall continue to pay all other amounts payable under the Lease; provided, however, Tenant’s Share shall be amended in accordance with Section 5 of this Amendment.
4. |
Acceptance of Premises. Tenant accepts the Premises in their “AS-IS” condition and Landlord shall have no obligation to improve, repair, restore or refurbish the Premises except as otherwise specifically provided in this Amendment. Tenant acknowledges that neither Landlord nor any agent of Landlord has made any representation or warranty, except as otherwise expressly provided in this Amendment or the Lease, with respect to the Premises or any other portion of the Industrial Center including, without limitation, any representation or warranty with respect to the suitability or fitness of the Premises or any other portion of the Industrial Center for the conduct of Tenant’s business. Nothing in this Paragraph 4 shall negate or diminish Landlord’s repair or restoration obligations under the Lease, as amended. |
5. |
Operating Expenses. From the Effective Date through Expiration Date, Tenant’s Share of Operating Expenses is as follows: |
FIFTH AMENDMENT TO LEASE |
PAGE 2 |
(a)Industrial Center45.33%
(b)Building85.64%
6. |
Finish-Out of Premises. Landlord agrees to provide Tenant with a finish-out allowance of $500,000.00 for costs incurred in connection with renovations to the Premises. The Tenant Finish Work will be performed and the allowance applied in accordance with Attachment A attached to this Amendment. |
7. |
Permitted Use. Upon the Execution Date, Section 1.8 of the Lease is hereby deleted and the following substituted therefor: |
“1.8Permitted Use: Manufacturing, storage and distribution of pharmaceutical products in compliance with all Applicable Requirements, including use of portions of the Premises as laboratory space for research and manufacturing purposes in connection with Tenant’s business.”
8. |
Right of First Refusal. The Right of First Refusal covering Suite 300 of the Building, as set forth in Section 6 of the Third Amendment, (i) remains in full force and effect during the Term as extended by this Amendment, and (ii) may be exercised by Tenant during such extended Term in accordance with Section 6 of the Third Amendment. |
9. |
Option to Extend. The Option to Extend set forth in Section 13 of the Fourth Amendment (i) remains in full force and effect and (ii) shall include and be applicable to the entire Premises (including the Suite 400 Space). |
10. |
Brokerage; Mutual Indemnities. |
a. |
Tenant warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than CB Richard Ellis, Inc. and Jackson & Cooksey, Inc. (collectively, the Brokers). Tenant shall indemnify, defend, and .hold Landlord harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent other than Brokers claiming by, through, or under Tenant with respect to this Amendment, |
b. |
Landlord warrants that it has had no dealings with any broker or agent in connection with the negotiation or execution of this Amendment other than Brokers. Landlord shall indemnify, defend, and hold Tenant harmless against all costs, expenses, attorneys’ fees, or other liability for commissions or other compensation or charges claimed by any broker or agent, including Brokers, claiming by, through or under Landlord with respect to this Amendment. |
c. |
Any brokerage commissions payable to Brokers are payable by Landlord pursuant to the terms of separate agreements between Landlord and Broker. |
11. |
No Offsets. Tenant hereby represents to Landlord that to the best of Tenant’s knowledge, as of the date of this Amendment, Tenant has no defenses to or offsets against the full and |
FIFTH AMENDMENT TO LEASE |
PAGE 3 |
timely payment and performance of each and every covenant and obligation required to be performed by Tenant under the terms of the Lease, as amended hereby.
12. |
Conflicts. The terms of this Amendment prevail if there is a conflict with the terms of the Lease. |
13. |
Headings. The headings or captions of the paragraphs in this Amendment are for convenience only and shall not act and shall .not be implied to act to limit or expand the construction and intent of the contents of the respective paragraph. |
14. |
Binding Effect. This Amendment is binding upon and shall inure to the benefit of the parties and their respective successors and assigns (but this reference to assigns shall not be deemed to act as a consent to an assignment by Tenant). |
15. |
Ratification. The Lease, as amended and modified hereby, is ratified and confirmed by the parties as being in full force and effect. |
[Remainder of page intentionally left blank]
FIFTH AMENDMENT TO LEASE |
PAGE 4 |
EXECUTED as of the date first above written.
|
|
LANDLORD: |
|||
|
|
|
|||
|
|
TEACHERS INSURANCE AND ANNUITY |
|||
|
|
a New York corporation |
|||
|
|
|
|||
|
|
By: |
|
||
|
|
|
Print Name: |
|
|
|
|
|
As Its: |
|
|
|
|
TENANT: |
||
|
|
|
||
|
|
NEOS THERAPEUTICS, LP |
||
|
|
|
||
|
|
By: |
PharmaFab Texas, LLC, |
|
|
|
|
A Texas limited liability company, |
|
|
|
|
Its manager |
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
Name: |
|
|
|
|
Title: |
|
FIFTH AMENDMENT TO LEASE |
PAGE 5 |
ATTACHMENT A
to Fifth Amendment to Lease by and between
Teachers Insurance and Annuity Association of America, as Landlord,
and
Neos Therapeutics, LP, as Tenant
TENANT FINISH WORK
A. |
Plans and Specifications: Tenant shall submit to Landlord within thirty (30) days following the Execution Date initial plans and specifications (the “Initial Construction Documents”) for the remodeling of the Premises. The Initial Construction Documents must include, without limitation: |
1. |
General Notes Sheet |
2. |
Demolition Plan |
3. |
New Construction Plan with details of all new improvements |
4. |
Finishes Plan |
5. |
Electrical, Mechanical and Plumbing Plan |
Within 15 days after receipt of the Initial Construction Documents, Landlord shall deliver to Tenant a notice either approving or disapproving them. Landlord shall not unreasonably withhold, condition or delay its approval of any plans, changes to such plans, designation of contractors or other matters related to the Tenant Finish Work; provided, if the Tenant Finish Work affects the structural portions of the Building or the Building systems, Landlord’s approval shall be in its commercially reasonable discretion. Any disapproval must specify in reasonable detail the reasons for the disapproval. If Tenant does not receive a notice from Landlord disapproving the Initial Construction Documents within the 15-day period, Landlord is deemed to approve the Initial Construction Documents. If Landlord disapproves the Initial Construction Documents, Tenant shall revise them to conform to Landlord’s reasonable objections and deliver copies of the revised Initial Construction Documents to Landlord.
The approved Initial Construction Documents are referred to as the “Construction Documents” and all work to be performed by Tenant pursuant to the Construction Documents is referred to as the “Tenant Finish Work”. Landlord’s approval of the Construction Documents is not a warranty that the Construction Documents comply with Applicable Requirements.
FIFTH AMENDMENT TO LEASE |
PAGE 6 |
B. |
Tenant Finish Work. Tenant, at its cost and risk (subject to reimbursement of the Work Allowance by Landlord), shall construct or cause to be constructed the Tenant Finish Work in substantial accordance with the Construction Documents. Tenant shall solicit bids from three contractors approved by Landlord for performance of the Tenant Finish Work, and Tenant shall select one of the three contractors. |
Tenant shall pay the Actual Cost (defined below) of all Tenant Finish Work subject to reimbursement by Landlord as specified below of a work allowance not to exceed a maximum of $500,000.00 (the “Work Allowance”).
The term “Actual Cost” means the cost of all labor and materials and all hard and soft costs relating to the Tenant Finish Work (including thirty-party, out-of-pocket costs incurred by Tenant in designing and constructing Tenant Finish Work [i.e. preparation and revisions of Tenant’s space plan, preparation and revisions of the Construction Documents and Initial Construction Documents, Tenant’s working drawings, space planning, interior architect, engineering, all construction and materials costs of Tenant’s contractor and all subcontractors, relocation, and cabling, and any and all other hard and soft costs incurred by Tenant in connection with the Tenant Finish Work]), together with the Building Service Fee of 5% of all hard costs of the Tenant Finish Work
Tenant shall allow Landlord access to the Premises at all times to inspect the Tenant Finish Work. Landlord has no obligation to inspect the Tenant Finish Work. No inspection by Landlord of the Tenant Finish Work is a warranty that the Tenant Finish Work complies with the Construction Documents or any Applicable Requirements.
The Work Allowance is available for Tenant’s use from the date of this Amendment through February 28, 2011, after which Tenant’s right to same will expire and be of no further force and effect.
C. |
DISBURSEMENT OF WORK ALLOWANCE. Landlord shall pay to Tenant the Actual Cost of the Tenant Finish Work, up to the total Work Allowance, as follows: |
1. |
On or about the 15th of each month (each, a “Submittal Date”), Tenant shall deliver to Landlord the following (the “Payment Conditions”): (a) a request for reimbursement to Tenant of a specified portion of the Work Allowance (the “Disbursement”), showing the schedule, by trade, of the percentage of completion of the Tenant Finish Work, describing with specificity the portion of the Tenant Finish Work completed since the previous Submittal Date (the “Completed Work”) (the Completed Work shall be summarized on AIA form G-702 — Contractor’s Application for Payment, or its equivalent), and substantiating, to Landlord’s reasonable satisfaction, the amount of the Disbursement in relation to the Completed Work; (b) executed, unconditional, and recordable lien waivers and releases reasonably satisfactory to Landlord covering all of the Completed Work; (c) paid receipts for all items of the Completed Work in excess of $1,000; and (d) all other information reasonably required by Landlord, including copies of all operating manuals and service manuals that Tenant has received relating to the Tenant Finish Work, if any. |
FIFTH AMENDMENT TO LEASE |
PAGE 7 |
2. |
Within 30 days after each Submittal Date (each, a “Reimbursement Date”), if Tenant has fully complied with Paragraph C(1) above, Landlord shall pay Tenant the lesser of: (a) the applicable Disbursement, less a 10% retainage (all of which are collectively the “Retainage”) (except to the extent Tenant’s payments to its contractor, architect, engineer, etc., already reflect a 10% retainage), and (b) the balance of any remaining available portion of the Work Allowance (not including the Retainage). The final disbursement of the Work Allowance by Landlord (not including the Retainage) will be adjusted, if necessary, so that the total Retainage is 10% of the total available Work Allowance. |
3. |
Within five (5) business days after each Submittal Date, Landlord will give Tenant written notice of any missing or incomplete Payment Conditions, information or documentation reasonably required by Landlord in order to process Tenant’s reimbursement request. The applicable Reimbursement Date shall be automatically extended by the number of days beyond the five (5) business day notice period taken by Tenant to submit the missing or incomplete documentation. Further, upon Tenant’s written request, Landlord may omit payment for costs with incomplete documentation and make immediate payment on only that portion of the reimbursement request that the parties agree is complete, with the balance of such payment request to be paid along with Tenant’s next monthly reimbursement request, subject to Tenant’s submittal of all missing or incomplete documentation. |
4. |
Within 30 days after the completion of the Tenant Finish Work, if Tenant has fully complied with Paragraph C(1) above with respect to all of the Tenant Finish Work, Landlord shall pay the Retainage to Tenant. |
5. |
If Landlord fails to pay all or any portion of the Work Allowance to Tenant when due, and if Tenant has fully complied with this Paragraph C, then Tenant shall notify Landlord in writing of the failure (the “Delinquency Notice”), and Landlord shall have 30 days after it receives the Delinquency Notice to cure the failure. If Landlord does not cure the failure within that 30-day period, then Tenant may, at its option, pay all or any portion of the amounts due and offset those payments, plus interest at 6% per annum (but not to exceed the highest rate allowable under applicable law), against the next due installments of Base Rent. |
D. |
General. |
1. |
Any material changes to the Construction Documents must first be submitted to Landlord for review and approval prior to the work reflected in such amended Construction Documents being undertaken by Tenant. |
2. |
Workmanship and materials to be used in the Tenant Finish Work shall be of best quality. Any approval by Landlord of the Construction Documents shall not in any way constitute a representation or warranty of Landlord as to the adequacy or sufficiency of the Construction Documents; such approval shall merely be the consent of Landlord as may be required hereunder in connection with the Tenant |
FIFTH AMENDMENT TO LEASE |
PAGE 8 |
Finish Work in accordance with the Construction Documents under the terms of the Lease.
3. |
Tenant shall perform the Tenant Finish Work consistent with Building Rules and Regulations, in a manner to minimize noise and other interference with tenants of the Industrial Center and shall remove all trash and debris from the Premises on a daily basis. |
4. |
Upon completion of the construction of Tenant Finish Work, Tenant shall promptly restore any area of the Industrial Center damaged as a result of Tenant’s construction of the Tenant Finish Work to the condition existing prior to the commencement of such construction. |
5. |
Any entry upon the Premises by Tenant and its agents and contractors shall be deemed to be under all of the terms, the covenants provisions and conditions of the Lease. |
6. |
All contractors, subcontractors, suppliers, service providers, moving companies and others (the “Service Providers”) performing work of any type for Tenant in the Industrial Center shall (i) carry the insurance listed below with companies acceptable to Landlord, and (ii) furnish certificates of insurance to Landlord evidencing required coverages at least 10 days prior to entry on the Industrial Center and annually thereafter; |
a. |
Commercial general liability insurance written on the most current form of ISO CG 00 01 (occurrence basis) or its equivalent, have a minimum each occurrence limit of $1,000,000, a minimum general aggregate limit of $2,000,000, and not exclude the Lease from the definition of “Insured Contract” under the contractual liability provisions; |
b. |
Workers’ compensation insurance complying with statutory requirements of the State of Texas and employers liability insurance in amounts not less than $500,000 bodily injury per accident/$500,000 disease each employee/$500,000 disease policy limit; |
c. |
Business automobile insurance for claims arising out of ownership, maintenance, or use of owned, non-owned, and hired motor vehicles at, upon, or away from the Industrial Center. The minimum limits must be $1,000,000 each occurrence; and |
d. |
Excess/umbrella liability insurance, applying on at least a “following form” (or primary) basis, in excess of commercial general liability, employers liability, and business automobile liability, with a minimum limit of $3,000,000 each occurrence and aggregate, where applicable. |
Landlord, Landlord’s designated property management firm, and all Landlord Entities shall be named additional insureds on each of said policies (excluding the worker’s compensation policy) and said policies shall be issued by an insurance
FIFTH AMENDMENT TO LEASE |
PAGE 9 |
company or companies authorized to do business in the State and which have policyholder ratings not lower than “A-” and financial ratings not lower than “IX” in Best’s Insurance Guide (latest edition in effect as of the date of the Lease and subsequently in effect as of the date of renewal of the required policies). EACH OF SAID POLICIES SHALL ALSO INCLUDE A WAIVER OF SUBROGATION PROVISION OR ENDORSEMENT IN FAVOR OF LANDLORD AND THE LANDLORD ENTITIES, AND AN ENDORSEMENT PROVIDING THAT LANDLORD SHALL RECEIVE THIRTY (30) DAYS PRIOR WRITTEN NOTICE OF ANY CANCELLATION OF, NON-RENEWAL OF, REDUCTION OF COVERAGE OR MATERIAL CHANGE IN COVERAGE ON SAID POLICIES. In addition, all policies of the Service Providers shall be endorsed to be primary, with the policies of all Landlord Entities being excess, secondary and non-contributing. Each Service Provider hereby waives its right of recovery against any Landlord indemnitee of any amounts paid by it or on its behalf to satisfy applicable worker’s compensation laws. The policies or duly executed certificates showing the material terms for the same, together with satisfactory evidence of the payment of the premiums therefor, shall be deposited with Landlord on the date each Service Provider first enters the Industrial Center and upon renewals of such policies not less than fifteen (15) days prior to the expiration of the term of such coverage. If certificates are supplied rather than the policies themselves, each Service Provider shall allow Landlord, at all reasonable times, to inspect its policies of insurance required herein.
With respect to insurance coverages, except worker’s compensation, maintained hereunder by each Service Provider and insurance coverages separately obtained by Landlord, all insurance coverages afforded by policies of insurance maintained by each Service Provider shall be primary insurance as such coverages apply to Landlord, and such insurance coverages separately maintained by Landlord shall be excess, and each Service Provider shall have its insurance policies so endorsed. The amount of liability insurance under insurance policies maintained by each Service Provider shall not be reduced by the existence of insurance coverage under policies separately maintained by Landlord. Each Service Provider shall be solely responsible for any premiums, assessments, penalties, deductible assumptions, retentions, audits, retrospective adjustments or any other kind of payment due under its policies.
FIFTH AMENDMENT TO LEASE |
PAGE 10 |
Exhibit 10.54
SIXTH AMENDMENT TO LEASE
This Sixth Amendment to Lease (“Amendment”) is made effective as of August ____, 2013, by and between RIVERSIDE BUSINESS GREEN, LP, a Delaware limited partnership (“Landlord”) and NEOS THERAPEUTICS, LP, a Texas limited partnership (“Tenant”), with reference to the following facts and circumstances.
A. |
Landlord is the owner of that certain building located at 2940 N. Highway 360, Grand Prairie, Texas (the “Building”). |
B. |
Walstib, L.P., predecessor in interest to Landlord, and PharmaFab, Inc. predecessor in interest to Tenant, entered into a certain Commercial Lease Agreement dated June 29, 1999, as amended by that certain First Amendment to Lease dated September 1, 2002, that certain Interim Amendment to Lease dated September 4, 2003, that certain Third Amendment to Lease dated October 1, 2003, that certain Fourth Amendment to Lease dated May 1, 2009 and that certain Fifth Amendment to Lease dated April 5, 2010 (collectively, the “Lease”), for (i) certain premises containing approximately 77,112 rentable square feet located in Suites 100 and 200 of the Building (the “Suite 100 Space”) and certain premises containing approximately 20,170 rentable square feet located in Suite 400 f the Building (the “Suite 400 Space”). The Suite 100 Space and Suite 400 Space shall be known collectively herein as the “Premises”). |
C. |
Landlord and Tenant desire to amend the Lease upon terms and conditions hereinafter set forth. |
NOW, THEREFORE, in consideration of the foregoing facts and circumstances, the mutual covenants and promises contained herein and after good and valuable consideration, the receipt and sufficiency of which is acknowledged by each of the parties, the parties do hereby agree to the following:
1.Definitions. Each capitalized term used in this Amendment shall have the same meaning as is ascribed to such capitalized term in the Lease, unless otherwise provided for herein.
2.Term. The term of the Lease is hereby extended for the period commencing on January 1, 2020, and ending on December 31, 2024 (the “Extended Term”).
3.Base Rent.
a. |
During the Extended Term, monthly installments of Base Rent for the Suite 100 Space shall be as follows: |
Months |
Monthly Installment |
Annual |
January 1, 2020 - December 31, 2020 |
$64,260.00 |
$771,120.00 |
January 1, 2021 - December 31, 2021 |
$64,260.00 |
$771,120.00 |
January 1, 2022 - December 31, 2022 |
$67,473.00 |
$809,676.00 |
- 1 -
January 1, 2023 - December 31, 2023 |
$67,473.00 |
$809,676.00 |
January 1, 2024 - December 31, 2024 |
$70,686.00 |
$848,232.00 |
b. |
During the Extended Term, monthly installments of Base Rent for the Suite 400 Space shall be as follows: |
Months |
Monthly Installment |
Annual |
January 1, 2020 - December 31, 2020 |
$19,497.67 |
$233,972.00 |
January 1, 2021 - December 31, 2021 |
$19,497.67 |
$233,972.00 |
January 1, 2022 - December 31, 2022 |
$20,472.55 |
$245,670.60 |
January 1, 2023 - December 31, 2023 |
$20,472.55 |
$245,670.60 |
January 1, 2024 - December 31, 2024 |
$21,514.67 |
$258,176.00 |
Provided that Tenant has faithfully performed all of the terms and conditions of this Lease, Landlord agrees to abate Tenant’s obligation to pay Base Rent for months of August, September, October, November, and December of 2013 and January of 2014.
4.Landlord Work. Landlord shall, at Landlord’s expense, (i) add seven (7) speed bumps around the Building to be located in the area mutually and reasonably agreed upon by Landlord and Tenant and (ii) install a fence or comparable barrier on the north side of the Building, which shall be mutually and reasonably acceptable to Landlord and Tenant.
5.Option to Extend. The Option to Extend as set forth in Section 13 of that certain Fourth Amendment to Lease dated May 1, 2009, shall remain in full force during the Extended Term and effect and shall be applicable to the entire Premises.
6.Right of First Refusal. The Right of First Refusal covering Suite 300 of the Building, as set forth in Section 6 of that certain Third Amendment to Lease dated October 1, 2003 (the “Third Amendment”) (i) shall remain in full force and effect during the Term and the Extended Term, and (ii) may be exercised by Tenant during the Term or the Extended Term in accordance with Section 6 of the Third Amendment.
7.Assignment and Subletting: Effective as of the full execution of this Amendment, Section 12.1(b) of the Lease is hereby deleted and replaced with the following:
“(b) A change in control of Tenant shall constitute an assignment requiring Landlord’s consent. The transfer, on a cumulative basis of a majority of the voting or management control of Tenant shall constitute a change in control for this purpose. So long as such change in control does not result in a material decline in Tenant’s credit, in the event that Landlord fails to provide written consent to an assignment that arises from a change in control, Landlord’s sole remedy for such assignment shall be to provide Tenant with forty-eight (48) months prior written notice of termination of the Lease.”
8.Confidentiality.
- 2 -
a. |
Landlord acknowledges that Landlord has or may have access to and gain knowledge of confidential and proprietary information of Tenant and its Affiliates and business partners, including, but not limited to, business practices, discoveries, ideas, formulations, costs and pricing data, techniques, programs, marketing plans, strategies and tactics, research and development information, data relating to the approval, administration, use or experience relating to any product of Tenant or any of its Affiliates or business partners (whether marketed or in development), and financial and technical information, all of which information is considered confidential by Tenant (“Confidential Information”). For the purposes of this Lease, the term “Affiliates” shall mean all entities controlling, controlled by or under common control with Tenant. The term “control” shall mean the ability to vote fifty percent (50%) or more of the voting securities of an entity or otherwise having the ability to influence and direct the policies and direction of an entity. Landlord agrees that Landlord will not use or disclose Confidential Information for any reason other than to carry out the purpose of this Lease without the prior written consent of Tenant. Notwithstanding the foregoing, Landlord is expressly permitted to disclose tenant’s financial and other related information to Landlord’s Affiliates, agents, employees, lenders (both current and potential and any potential buyer of the Building. The foregoing restrictions on use and disclosure shall not apply to information which Landlord can prove was or became public knowledge through no fault of Landlord. |
b. |
Landlord may disclose Confidential Information (i) in response to a valid order of a court or any governmental agency or regulatory body or (ii) as otherwise required by law; provided that the Landlord promptly notifies Tenant of such pending order or requirement and lends Tenant all reasonable assistance, so that the Tenant may seek a protective order or other appropriate remedy; and provided further that in the event that no such protective order or other remedy is obtained, the Landlord will furnish only that portion of the Confidential Information which it is legally required to furnish in order to comply. Notwithstanding the foregoing, information required to be disclosed pursuant this Section 8(b) will continue to be considered Confidential Information for all other purposes. |
9.Recover Reconciliation. Except for a $44,738.77 credit due Tenant for the 2012 Recovery Reconciliation as shown in more detail on Exhibit A, attached hereto, Tenant hereby represents to Landlord that, to the best of Tenant’s knowledge, as of the date of this Amendment, that Tenant has no defenses, offsets or counterclaims that could be asserted in an action by Landlord to enforce Landlord’s remedies under the Lease.
10.No Defenses. Tenant affirms that, to the best of its knowledge, as of the date of execution of this Amendment: (a) no default or breach by Landlord exists under the Lease; (b) all tenant improvements to be constructed by Landlord prior to the date of this Amendment, if any, are complete and Tenant has accepted the Premises in “as is, where is” condition as of the date of this Amendment; and (c) Landlord has fully funded or Tenant has waived any unfunded tenant improvement allowances payable under the Lease.
- 3 -
11.Broker. Tenant represents to Landlord that except for CB Richard Ellis, Inc. and Jackson and Cooksey (the “Brokers”), Tenant has not dealt with any real estate broker, salesperson or finder in connection with this Amendment, and no other such person initiated or participated in the negotiation of this Amendment or is entitled to any commission in connection herewith. Tenant hereby agrees to indemnify, defend and hold Landlord, its property manager and their respective employees harmless from and against any and all liabilities, claims, demands, actions, damages, costs and expenses (including attorneys fees) arising from either (a) a claim for a fee or commission made by any broker, other than the Brokers, claiming to have acted by or on behalf of Tenant in connection with this Amendment, or (b) a claim of, or right to lien under the statutes of the state in which the Premises are located relating to real estate broker liens with respect to any such broker retained by Tenant.
12.Submission. Submission of this Amendment by Landlord to Tenant for examination and/or execution shall not in any manner bind Landlord and no obligations on Landlord shall arise under this Amendment unless and until this Amendment is fully signed and delivered by Landlord and Tenant; provided, however, the execution and delivery by Tenant of this Amendment to Landlord shall constitute an irrevocable offer by Tenant of the terms and conditions herein contained, which offer may not be revoked for ten (10) days after such delivery.
13.Miscellaneous.
a. |
Modification. A modification of any provision herein contained, or any other amendment to this Amendment, shall be effective only if the modification or amendment is in writing and signed by both Landlord and Tenant. |
b. |
Successors and Assigns. This Amendment shall be binding upon and inure to the benefit of the parties hereto and their respective successors and permitted assigns. |
c. |
Number and Gender. As used in this Amendment, the neuter includes masculine and feminine, and the singular includes the plural. |
d. |
Construction. Headings at the beginning of each Section and subsection are solely for the convenience of the parties and are not a part of this Amendment. Except as otherwise provided in this Amendment, all exhibits referred to herein are attached hereto and are incorporated herein by this reference. Unless otherwise indicated, all references herein to Articles, Section, subsections, paragraphs, subparagraphs or provisions are to those in this Amendment. Any reference to a paragraph or Section herein includes all subparagraphs or subsections thereof. In the event any portion of this Amendment shall be declared by any court of competent jurisdiction to be invalid, illegal or unenforceable, such portion shall be deemed severed from this Amendment, and the remaining parts hereof shall remain in full force |
- 4 -
and effect, as fully as though such invalid, illegal or unenforceable portion had never been part of this Amendment.
e.Integration of Other Agreements. This Amendment, the Lease and prior amendments set forth the entire agreement and understanding of the parties with respect to the matters set forth herein and supersedes all previous written or oral understandings, agreements, contracts, correspondence and documentation with respect thereto.Any oral representation or modifications concerning this Amendment shall be of no force or effect.
f. |
Duplicate Originals; Counterparts. This Amendment may be executed in any number of duplicate originals, all of which shall be of equal legal force and effect. Additionally, this Amendment may be executed in counterparts, but shall become effective only after a counterpart hereof has been executed by each party; all said counterparts shall, when taken together, constitute the entire single agreement between parties. |
g. |
No Waiver. No failure or delay of either party in the exercise of any right given to such party hereunder shall constitute a waiver thereof unless the time specified herein for exercise of such right has expired, nor shall any single or partial exercise of any right preclude other or further exercise thereof or of any other right. No waiver by any party hereto of any breach or default shall be considered to be a waiver of any other breach or default. The waiver of any condition shall not constitute a waiver of any breach or default with respect to any covenant, representation or warranty. |
h. |
Further Assurances. Landlord and Tenant each agree to execute any and all other documents and to take any further actions reasonably necessary to consummate the transactions contemplated hereby. |
i. |
No Third Party Beneficiaries. Except as otherwise provided herein, no person or entity shall be deemed to be a third party beneficiary hereof, and nothing in this Amendment, (either expressed or implied) is intended to confer upon any person or entity, other than Landlord and/or Tenant (and their respective nominees, successors and assigns), any rights, remedies, obligations or liabilities under or by reason of this Amendment. |
j. |
Full Force and Effect. The Lease, as amended hereby, shall continue in full force and effect, subject to the terms and provisions thereof and hereof. In the event of any conflict between the terms of the Lease and the terms of this Amendment, the terms of this Amendment shall control. |
- 5 -
IN WITNESS WHEREOF, this Amendment is executed as of the day and year aforesaid.
|
|
LANDLORD: |
||
|
|
|
||
|
|
RIVERSIDE BUSINESS GREEN, LP |
||
|
|
|
||
|
|
By: |
|
|
|
|
Printed Name: |
|
|
|
|
Title: |
|
|
|
|
Date: |
|
|
|
|
TENANT: |
||
|
|
|
||
|
|
NEOS THERAPEUTICS, LP |
||
|
|
|
||
|
|
By: |
|
|
|
|
Printed Name: |
|
|
|
|
Title: |
|
|
|
|
Date: |
|
|
- 6 -
Exhibit 10.55
AMENDED AND RE-STATED
EMPLOYMENT AGREEMENT
This Amended and Re-Stated Employment Agreement (the "Agreement"), is effective as of March 21, 2023 (the "Effective Date"), between Aytu BioPharma, Inc., a Delaware corporation headquartered at 373 Inverness Parkway, Suite 206, Englewood, CO 80112 USA, hereinafter referred to as the "Company"), and Greg Pyszczymuka as ("Executive").
RECITALS
WHEREAS, the Company is a duly organized Delaware corporation, with its principal place of business within the State of Colorado, and is in the business of developing and marketing pharmaceuticals, medical devices, and other healthcare products; and
WHEREAS, the Company desires assurance of the continued association and services of the Executive in order to continue to retain the Executive's experience, skills, abilities, background and knowledge, and is willing to engage the Executive's services on the terms and conditions set forth in this Agreement; and
WHEREAS, Executive desires to be in the continued employ of the Company, and is willing to accept such continued employment on the terms and conditions set forth in this Agreement.
NOW, THEREFORE, in consideration of the mutual covenants and agreements herein contained and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties agree as follows:
1.Employment.
(a)Term. The Company hereby agrees to employ Executive and Executive hereby accepts such employment with the Company for the period of 12 months beginning on the Effective Date, with automatic renewal of the employment period every 12 months. . The Term of this Agreement (the “Term”) shall continue until the termination of Executive's employment in accordance with the provisions of this Agreement. The termination of Executive's employment under this Agreement shall end the Term but shall not terminate Executive's or the Company's other obligations that are intended to survive the termination of this Agreement (including without limitation, the payments under Section 4 and 5 and Executive's obligations under Section 8).
(b)Position and Duties. During the Term, the Executive shall serve as the Chief Commercial Officer, and shall have supervision and control over and responsibility for the day-to-day business and affairs of the commercial operational aspects of the Company and shall have such other powers and duties as may from time to time be prescribed by the Chief Executive Officer ("CEO") of the Company, provided that such duties are consistent with the Executive's position or other positions that he may hold from time to time. The Executive shall devote his full working time and efforts to the business and affairs of the Company. Notwithstanding the foregoing, the Executive may serve on other boards of directors, with the approval of the CEO, or engage in religious, charitable or other community activities as long as such services and activities are disclosed to the Board and do not interfere with the Executive's performance of his duties to the Company as provided in this Agreement.
During the Term, Executive agrees not to acquire, assume or participate in, directly or indirectly, any position, investment or interest known by the Executive to be adverse, competitive, or antagonistic to the Company, its business or prospects, its financial position, or otherwise or in any company, person or entity that is, directly or indirectly, in competition with the business of the Company or any of its affiliates. This provision shall encompass any advisory boards of which Executive is or becomes a member of during the term hereof. Executive shall provide written disclosure to the Compensation Committee ("Compensation Committee") of the Company's Board of Directors (the "Board") as to all advisory boards on which Executive sits, and will provide the Company with written notice within 10 business days of Executive agreeing to sit on any additional advisory boards. On termination of Executive's employment, regardless of the reason for such termination, Executive shall immediately (and with contemporaneous effect) resign any directorships, offices or other positions that Executive may hold in the Company or any affiliate, unless otherwise agreed in writing by the parties.
2.Compensation and Related Matters.
(a)Base Salary. During the Term, the Executive's initial annual base salary shall be three hundred seventy-five thousand dollars ($375,000.00), less applicable deductions and withholdings. The Executive's base salary shall be reviewed at least semi-annually by the Compensation Committee or a majority of the independent members of the Board, and the base salary may be increased only by the Compensation Committee or a majority of the independent members of the Board. The base salary in effect at any given time is referred to herein as "Base Salary." The Base Salary shall be payable in a manner that is consistent with the Company’s usual payroll practices for senior executives.
(b)Bonus Compensation. The Executive shall be eligible for an annual discretionary bonus (hereinafter referred to as the "Bonus") with a target amount of forty percent (40%) of the Base Salary, subject to standard deductions and withholdings, based on the Compensation Committee's determination, in good faith, and based upon the Executive's individual achievement and company performance objectives as set by the Board or the Compensation Committee, of whether the Executive has met such performance milestones as are established for the Executive by the Board or the Compensation Committee, in good faith, in consultation with the Executive (hereinafter referred to as the "Performance Milestones"). The Performance Milestones will be based on certain factors including, but not limited to, the Executive's performance and the Company's financial and operational performance. The Executive must be employed on the date the Bonus is awarded to be eligible for the Bonus, subject to the termination provisions hereof. Bonuses shall be paid during the calendar quarter following the calendar quarter for which such Bonus was earned when Performance Milestones are met during a calendar quarter. Fourth quarter Bonuses and Bonuses calculated on the basis of partial Performance Milestone satisfaction shall be paid within 75 days of fiscal year-end.
(c)Transaction Bonus Compensation. In recognition of Executive's significant contributions in securing the Rumpus Therapeutics asset purchase and success in integrating the Company's commercial operations following the Neos Therapeutics merger (the "Transactions"), Executive acknowledges prior receipt of a one-time, discretionary bonus (the "Transaction Bonus") in the amount of one hundred thousand dollars (d)Restricted Stock Grant.
2
($100,000.00).
Executive acknowledges previous receipt of a sign on restricted stock grant of 75,000 shares, scheduled to vest over a (3) year period of employment with the Company beginning on October 1, 2021, and performance-based restricted stock units of 50,000 shares scheduled to vest over a (3) year period of employment with the Company beginning on the grant date of the performance-based award.
(e)Expenses. The Executive shall be entitled to receive prompt reimbursement for all reasonable expenses incurred by him during the Term in performing services hereunder, in accordance with the policies and procedures then in effect and established by the Company for its senior executive officers.
(f)Other Benefits. During the Term, the Executive shall be eligible to participate in or receive benefits under the Company's employee benefit plans in effect from time to time, subject to the terms of such plans.
(g)Vacations. For the term of this Agreement, Executive shall be entitled to paid time off at the rate of five (5) weeks per annum. In accordance with Company policy, unused paid time off will accrue up to a maximum accrual of 240 hours, which is considered the maximum accrual amount. Current accrued PTO of Executive, at the time of this employment agreement execution, shall not be impacted and remain unchanged. The Executive shall also be entitled to all paid holidays given by the Company to its executives.
3.Termination. During the Term, the Executive's employment hereunder may be terminated without any breach of this Agreement under the following circumstances:
(a)Death. The Executive's employment hereunder shall terminate upon his death.
(b)Disability. The Company may terminate the Executive's employment if he is disabled and unable to perform the essential functions of the Executive's then existing position or positions under this Agreement with or without reasonable accommodation for a period of 180 days (which need not be consecutive) in any 12-month period. If any question shall arise as to whether during any period the Executive is disabled so as to be unable to perform the essential functions of the Executive's then existing position or positions with or without reasonable accommodation, the Executive may, and at the request of the Company shall, submit to the Company a certification in reasonable detail by a physician selected by the Company to whom the Executive or the Executive's guardian has no reasonable objection as to whether the Executive is so disabled or how long such disability is expected to continue, and such certification shall for the purposes of this Agreement be conclusive of the issue. The Executive shall cooperate with any reasonable request of the physician in connection with such certification. If such question shall arise and the Executive shall fail to submit such certification, the Company's determination of such issue shall be binding on the Executive. Nothing in this Section 3{b) shall be construed to waive the Executive's rights, if any, under existing law including, without limitation, the Family and Medical Leave Act of 1993, 29 U.S.C. §260 I et seq. and the Americans with Disabilities Act, 42 U.S.C. §12101 et seq.
(c)Termination by Company for Cause. The Company may terminate the Executive's employment hereunder for Cause.
3
For purposes of this Agreement, "Cause" shall mean any of the following: (i) the Executive's material breach of any agreement with the Company, including the Confidentiality and Intellectual Property Agreement, dated 10/04/2021 (the "Confidentiality Agreement"), the provisions of Section 8 of this Agreement, the Code of Conduct or any other material policy that may result in material injury to the Company; (ii) the Executive's conviction of , or entry of a plea of guilty to, or a plea of nolo contender with respect to, any crime other than a traffic violation or infraction which is a misdemeanor; (iii) the Executive's act of fraud or intentional misrepresentation in connection with the Executive's duties or otherwise in connection with the business of the Company, that may result in material injury to the Company; (iv) the Executive's unintended but material breach in the performance of duties under this Agreement, including insubordination or failure to implement or follow a lawful policy or directive of the CEO or Company, provided that if such failure is curable, it is not cured within 30 days following written notice thereof from the CEO or Board; or (v) the Executive's willful malfeasance or willful misconduct in the performance of the Executive's duties for the Company.
(d)Termination Without Cause. The Company may terminate the Executive's employment hereunder at any time without Cause. Any termination by the Company of the Executive's employment under this Agreement which does not constitute a termination for Cause under Section 3(c) and does not result from the death or disability of the Executive under Section 3(a) or (b) shall be deemed a termination without Cause.
(e)Termination by the Executive. The Executive may terminate his employment hereunder at any time for any reason, including but not limited to Good Reason. For purposes of this Agreement, "Good Reason" shall mean, without the Executive's consent, the occurrence of any of the following: (i) the Company materially breaches a material term of this Agreement, and such breach causes or is likely to cause material harm to the Executive; (ii) there is a change in the Executive's responsibilities that represents a material and adverse change from the Executive's overall responsibilities, taken as a whole; (iii) there is a Change in Control that results in a change in the Executive's responsibilities that represents a material and adverse change from the Executive's overall responsibilities, taken as a whole; (iv) the Executive's Base Salary is substantially reduced or diminished; or (v) the Executive's place of employment is relocated by the Company more than a 50-mile radius from Philadelphia, Pennsylvania (it being understood and agreed that the Executive may be required to travel in connection with Company business and none of such travel shall constitute or give rise to "Good Reason"). The Executive's voluntary termination shall be deemed to have occurred for Good Reason for purposes of this Agreement only if (x) the Executive provides written notice to the Company within 30 days after the Executive becomes aware of circumstances giving rise to Good Reason, (y) the Company fails to correct the circumstances giving rise to Good Reason within 30 days following the receipt of such notice (the "Cure Period") and (z) the Executive resigns within 30 days following the end of the Cure Period. If the Company cures the Good Reason condition during the Cure Period, Good Reason shall be deemed not to have occurred.
(f)Notice of Termination. Except for termination as specified in Section 3(a), any termination of the Executive's employment by the Company or any such termination by the Executive shall be communicated by written Notice of Termination to the other party hereto. For purposes of this Agreement, a "Notice of Termination" shall mean a notice, which shall indicate the specific termination provision in this Agreement relied upon.
(g)Date of Termination.
4
"Date of Termination" shall mean: (i) if the Executive's employment is terminated by his death, the date of his death; (ii) if the Executive's employment is terminated on account of disability under Section 3(b) or by the Company for Cause under Section 3(c), the date on which Notice of Termination is given; (iii) if the Executive's employment is terminated by the Company under Section 3(d), the date on which a Notice of Termination is given; (iv) if the Executive's employment is terminated by the Executive under Section 3(e) without Good Reason, 30 days after the date on which a Notice of Termination is given, and (v) if the Executive's employment is terminated by the Executive under Section 3(e) with Good Reason, the date on which a Notice of Termination is given after the end of the Cure Period, 30 days after the date on which a Notice of Termination is given. Notwithstanding the foregoing, in the event that the Executive gives a Notice of Termination to the Company, the Company may unilaterally accelerate the Date of Termination and such acceleration shall not result in a termination by the Company for purposes of this Agreement.
4.Compensation Upon Termination.
(a)Termination Generally. If the Executive's employment with the Company is terminated for any reason, the Company shall pay or provide to the Executive (or to his authorized representative or estate) (i) any Base Salary earned through the Date of Termination, unpaid expense reimbursements (subject to, and in accordance with, Section 2(c) of this Agreement) and unused vacation that accrued through the Date of Termination on or before the time required by law but in no event more than 30 days after the Executive's Date of Termination in a lump sum payment; and (ii) any vested benefits the Executive may have under any employee benefit plan of the Company through the Date of Termination, which vested benefits shall be paid and/or provided in accordance with the terms of such employee benefit plans (collectively, the "Accrued Benefit").
(b)Termination by the Company Without Cause, by the Executive with Good Reason. During the Term, if the Executive's employment is terminated by the Company without Cause as provided in Section 3(d), or the Executive terminates his employment for Good Reason as provided in Section 3(e), then the Company shall pay the Executive his Accrued Benefit. In addition, subject to the Executive signing a separation agreement containing, among other provisions, a general release of claims in favor of the Company and related persons and entities, confidentiality, return of property and non-disparagement, in a form and manner satisfactory to the Company (the "Separation Agreement and Release") and the Separation Agreement and Release becoming fully effective, all within the time frame set forth in the Separation Agreement and Release:
(i)The Company shall pay the Executive an amount equal to the Executive's annual Base Salary plus any pro-rated incentive compensation earned (at target amount of forty percent (40%) of the Executive's Base Salary) but unpaid as of the Date of Termination (the "Severance Amount"). In the event the Company determines that bonus payouts for Company executives will not be paid in full for the fiscal year during which the Executive's termination occurs, at the Company's sole determination, Executive's pro-rated incentive compensation may be reduced below forty percent (40%) commensurate with such other executive bonuses paid for that fiscal year. Notwithstanding the foregoing, if the Executive breaches any of the provisions of the Confidentiality Agreement or Section 8 of this Agreement, all payments of the Severance Amount sha11 immediately cease; and
5
(ii)Notwithstanding anything to the contrary in the applicable Restricted Stock Agreement, the issued restricted stock will immediately vest following the expiration of the revocation period as set forth in Separation Agreement and Release; and
(iii)If the Executive was participating in the Company's group health plan immediately prior to the Date of Termination and elects COBRA health continuation, then the Company shall pay to the Executive a monthly cash payment for 12 months or the Executive's COBRA health continuation period, whichever ends earlier, in an amount equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company; and
(iv)The amounts payable under this Section 4(b) shall be paid out in a substantially equal insta11ments in accordance with the Company's payroll practice over 12 months commencing within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, the Severance Amount shall begin to be paid in the second calendar year by the last day of such 60-day period; provided, further, that the initial payment shall include a catch-up payment to cover amounts retroactive to the day immediately fol1owing the Date of Termination. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section l.409A-2(b)(2)
5.Change in Control Payment. The provisions of this Section 5 set forth certain terms of an agreement reached between the Executive and the Company regarding the Executive's rights and obligations upon the occurrence of a Change in Control of the Company. These provisions are intended to assure and encourage in advance the Executive's continued attention and dedication to his assigned duties and his objectivity during the pendency and after the occurrence of any such event. These provisions shall apply in lieu of, and expressly supersede, the provisions of Section 4(b) regarding severance pay and benefits upon a termination of employment, if such termination of employment occurs within 12 months after the occurrence of the first event constituting a Change in Control. These provisions shall terminate and be of no further force or effect beginning 12 months after the occurrence of a Change in Control. Regardless of change in employment status following Change in Control, all stock options and other stock-based awards held by the Executive and granted after the Effective Date shall immediately accelerate and become fully exercisable or non-forfeitable as of the Date of Change in Control.
(a)Change in Control. During the Term, if within 12 months after a Change in Control, the Executive's employment is terminated by the Company without Cause as provided in Section 3(d) or the Executive terminates his employment for Good Reason as provided in Section 3(e), then, subject to the signing of the Separation Agreement and Release by the Executive and the Separation Agreement and Release effective all within the time frame set forth in the Separation Agreement and Release,
(i)The Company shall pay the Executive a lump sum in cash in an amount equal to one time the sum of (A) the Executive's then current Base Salary (or the Executive's Base Salary in effect immediately prior to the Change in Control, if higher) plus (B) the Executive's target annual incentive compensation for the then-
6
current year; and
(ii)Notwithstanding anything to the contrary in any applicable option agreement or stock-based award agreement, all stock options and other stock-based awards held by the Executive and granted after the Effective Date shall immediately accelerate and become fully exercisable or non-forfeitable as of the Date of Termination; and
(iii)If the Executive was participating in the Company's group health plan immediately prior to the Date of Termination and elects COBRA health continuation, then the Company shall pay to the Executive a monthly cash payment for 12 months or the Executive's COBRA health continuation period, whichever ends earlier, in an amount equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company; and
(iv)The amounts payable under this Section S(a) shall be paid or commence to be paid within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, such payment shall be paid or commence to be paid in the second calendar year by the last day of such 60-day period.
For the avoidance of doubt, all stock options and other stock-based awards held by the Executive as of the Effective Date shall be treated as indicated in the applicable award agreements.
(b)Additional Limitation.
(i)Anything in this Agreement to the contrary notwithstanding, in the event that the amount of any compensation, payment or distribution by the Company to or for the benefit of the Executive, whether paid or payable or distributed or distributable pursuant to the terms of this Agreement or otherwise, calculated in a manner consistent with Section 280G of the Code and the applicable regulations thereunder (the "Aggregate Payments"), would be subject to the excise tax imposed by Section 4999 of the Code, then the Aggregate Payments shall be reduced (but not below zero) so that the sum of all of the Aggregate Payments shall be $1.00 less than the amount at which the Executive becomes subject to the excise tax imposed by Section 4999 of the Code; provided that such reduction shall only occur if it would result in the Executive receiving a higher After Tax Amount (as defined below) than the Executive would receive if the Aggregate Payments were not subject to such reduction. In such event, the Aggregate Payments shall be reduced in the following order, in each case, in reverse chronological order beginning with the Aggregate Payments that are to be paid the furthest in time from consummation of the transaction that is subject to Section 280G of the Code: (1) cash payments not subject to Section 409A of the Code; (2) cash payments subject to Section 409A of the Code; (3) equity-based payments and acceleration; and (4) non-cash forms of benefits; provided that in the case of all the foregoing Aggregate Payments all amounts or payments that are not subject to calculation under Treas. Reg. § I .280G-1, Q&A-24(b) or (c) shall be reduced before any amounts that are subject to calculation under Treas. Reg. §1.2800-1, Q&A-24(b) or(c).
7
(ii)For purposes of this Section 5(b), the "After Tax Amount" means the amount of the Aggregate Payments less all federal, state, and local income, excise and employment taxes imposed on the Executive as a result of the Executive's receipt of the Aggregate Payments. For purposes of determining the After Tax Amount, the Executive shall be deemed to pay federal income taxes at the highest marginal rate of federal income taxation applicable to individuals for the calendar year in which the determination is to be made, and state and local income taxes at the highest marginal rates of individual taxation in each applicable state and locality, net of the maximum reduction in federal income taxes which could be obtained from deduction of such state and local taxes.
(iii)The determination as to whether a reduction in the Aggregate Payments shall be made pursuant to Section 5(b)(i) shall be made by a nationally recognized accounting firm selected by the Company (the "Accounting Finn"), which shall provide detailed supporting calculations both to the Company and the Executive within 15 business days of the Date of Termination, if applicable, or at such earlier time as is reasonably requested by the Company or the Executive. Any determination by the Accounting Firm shall be binding upon the Company and the Executive.
(c)Definitions. For purposes of this Section 5, the following terms shall have the following meanings:
"Change in Control" shall mean the consummation of any of the following:
(i)A sale of all or substantially all of the assets of the Company on a consolidated basis to an unrelated person or entity; or
(ii)A merger, reorganization or consolidation in which the outstanding shares of common stock of the Company are converted into or exchanged for shares of the successor entity and the holders of the Company's outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the surviving entity immediately upon the completion of such transaction; or
(iii)The sale of all or a majority of the common stock of the Company to an unrelated person or entity; or
(iv)Any other transaction in which the holders of the Company's outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the surviving entity in the transaction immediately upon the completion of such transaction.
6.Section 409A.
8
(a)Anything in this Agreement to the contrary notwithstanding, if at the time of the Executive's separation from service within the meaning of Section 409A of the Code, the Company determines that the Executive is a "specified employee" within the meaning of Section 409A(a){2)(B)(i) of the Code, then to the extent any payment or benefit that the Executive becomes entitled to under this Agreement on account of the Executive's separation from service would be considered deferred compensation otherwise subject to the 20 percent additional tax imposed pursuant to Section 409A(a) of the Code as a result of the application of Section 409A(a)(2)(B)(i) of the Code, such payment shall not be payable and such benefit shall not be provided until the date that is the earlier of (A) six months and one day after the Executive's separation from service, or (B) the Executive's death. If any such delayed cash payment is otherwise payable on an installment basis, the first payment shall include a catch-up payment covering amounts that would otherwise have been paid during the six-month period but for the application of this provision, and the balance of the installments shall be payable in accordance with their original schedule.
(b)All in-kind benefits provided and expenses eligible for reimbursement under this Agreement shall be provided by the Company or incurred by the Executive during the time periods set forth in this Agreement. All reimbursements shall be paid as soon as administratively practicable, but in no event shall any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year sha11 not affect the in-kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year (except for any lifetime or other aggregate limitation applicable to medical expenses). Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit.
(c)To the extent that any payment or benefit described in this Agreement constitutes "non-qualified deferred compensation" under Section 409A of the Code, and to the extent that such payment or benefit is payable upon the Executive's termination of employment, then such payments or benefits shall be payable only upon the Executive's "separation from service." The determination of whether and when a separation from service has occurred sha11 be made in accordance with the presumptions set forth in Treasury Regulation Section 1.409A- 1(h).
(d)The parties intend that this Agreement will be administered in accordance with Section 409A of the Code. To the extent that any provision of this Agreement is ambiguous as to its compliance with Section 409A of the Code, the provision shall be read in such a manner so that all payments hereunder comply with Section 409A of the Code. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section l.409A-2(b)(2). The parties agree that this Agreement may be amended, as reasonably requested by either party, and as may be necessary to fully comply with Section 409A of the Code and all related rules and regulations in order to preserve the payments and benefits provided hereunder without additional cost to either party.
(e)The Company makes no representation or warranty and shall have no liability to the Executive or any other person if any provisions of this Agreement are determined to constitute deferred compensation subject to Section 409A of the Code but do not satisfy an exemption from, or the conditions of, such Section.
7.Intellectual Property.
9
The Executive acknowledges that all discoveries, concepts, ideas, inventions, innovations, improvements, developments, methods, designs, analyses, drawings, reports, patent applications, copyrightable work and mask work (whether or not including any confidential information) and all registrations or applications related thereto, all other proprietary information and all similar or related information (whether or not patentable) which relate to the Company's or any of its affiliates' actual or anticipated business, research and development or existing or future products or services and which are conceived, developed or made by the Executive (whether alone or jointly with others) while employed by the Company and its affiliates, whether before or after the date of this Agreement (collectively referred to as "Work Product"), are the property of the Company or such affiliated companies. The Executive shall promptly disclose such Work Product to the Board and, at the Company's expense, perform all actions reasonably requested by the Board (whether during or after the period of employment) to establish and confirm such ownership (including, without limitation, executing and delivering assignments, consents, powers of attorney and other instruments). The Executive acknowledges that all Work Product shall be deemed to constitute "works made for hire" under the U.S. Copyright Act of 1976, as amended.
8.Confidential Information, Noncompetition and Cooperation. The Executive agrees that he continues to be bound by the terms of the Confidentiality Agreement.
(a)The Executive agrees that all property (including, without limitation, all equipment, tangible proprietary information, documents, records, notes, contracts and computer generated materials) furnished to or created or prepared by the Executive incident to the Executive's employment belongs to the Company and shall be promptly returned to the Company upon termination of the Executive's employment.
(b)Upon termination of the Executive's employment, the Executive shall be deemed to have resigned from any and all offices and directorships then held with the Company and its affiliates. Following any termination of employment, the Executive shall reasonably cooperate with the Company (i) in the winding up of pending work on behalf of the Company and the orderly transfer of work to other employees, and (ii) in the defense of any action brought by any third party against the Company that relates to the Executive's employment by the Company; provided, that in each case the Company shall reimburse the Executive for any reasonable and documented out-of-pocket fees and expenses incurred by the Executive in connection with such cooperation.
(c)The Executive acknowledges that in the course of the Executive's employment with the Company, the Executive will become familiar with the Company's and its affiliates' trade secrets and with other confidential and proprietary information and that the Executive's services will be of special, unique and extraordinary value to the Company and its affiliates. Therefore, the Executive agrees that the Executive shall not, during the Term and for a period of one (1) year thereafter, directly or indirectly, either for himself or for any other person or entity or otherwise, (i) participate in any business or enterprise (including, without limitation, any division, group or franchise of a larger organization), engaged, anywhere within North America at the time of termination (the "Restricted Territory"), in the business of developing or commercializing controlled release, ion exchange resin based pharmaceutical products, developing or commercializing ADHD products, or developing or commercializing any products addressing Vascular Ehlers-Danlos Syndrome, or any other business in which the Executive would be required to employ, reveal or otherwise utilize trade secrets of the Company and its affiliates used prior to termination that may result in a material injury to the Company (with it being understood that the term "participate in" shall include, without limitation, having any direct or indirect interest in any person or entity, whether as a sole proprietor, owner, stockholder, partner, joint venturer, creditor or otherwise, or rendering any direct or indirect service or assistance to any person or entity -whether as a director, officer, manager, supervisor, employee, agent, consultant, advisor or otherwise); provided that, nothing herein shall prohibit the Executive from being a passive owner of not more than two percent (2%) of the outstanding stock of any class of an entity which is publicly traded so long as the Executive has no active participation in the business of such corporation; (ii) induce or attempt to induce any customer, supplier, licensee or other business relation of the Company or any of its subsidiaries to cease doing business with the Company or any such subsidiary or in any way interfere with the relationship between any such customer, supplier, licensee or business relation and the Company and any such subsidiary; or (iii) induce or attempt to induce any employee of the Company or its affiliates to leave the employ of the Company or any such affiliated company, or in any way interfere with the relationship between the Company and any of its affiliates and any employee thereof, or hire or otherwise engage any person who was an employee of the Company or any of its affiliated companies within one year before any such hiring would take place.
10
(d)The Executive agrees that he will not directly or indirectly, individually or in concert with others, make any statement calculated or likely to have the effect of undermining or disparaging the business or the business reputation of the Company or its affiliates or their respective employees, officers, directors, customers, suppliers, successors and assigns, including, without limitation, negative comments about any such person or company, its management methods, policies and/or practices. Notwithstanding the foregoing, nothing herein shall prohibit the Executive from responding accurately and fully to any question, inquiry or request made in connection with any governmental inquiry, investigation, review, audit or proceeding, any legal proceeding or claim (whether in court, arbitration or otherwise) of any nature, or as otherwise required by law.
(e)If, at the time of enforcement of this Section 8, a court holds that the restrictions stated herein are unreasonable under circumstances then existing, the parties hereto agree that the maximum duration, scope or geographical area reasonable under such circumstances shall be substituted for the stated period, scope or area and that the court shall be allowed to revise the restrictions contained herein to cover the maximum duration, scope and area permitted by law. Because the Executive's services are unique and because the Executive has access to confidential and proprietary information of the Company and its business, the parties hereto agree that money damages would not be an adequate remedy for any breach of Section 8 of this Agreement. Therefore, in the event of a breach or threatened breach of Section 8 of this Agreement, the Company or its successors or assigns may, in addition to other rights and remedies existing in their favor and notwithstanding anything herein to the contrary, apply to any court of competent jurisdiction for specific performance and/or injunctive or other equitable relief in order to enforce or prevent any violations of, the provisions hereof (without posting a bond or other security).
(f)The Executive acknowledges that the provisions of this Section 8 are in consideration of the Executive's employment with the Company and additional good and valuable consideration as set forth in this Agreement. The Executive agrees and acknowledges that the restrictions contained in Section 8 do not preclude the Executive from earning a livelihood, nor do they unreasonably impose limitations on the Executive's ability to earn a living.
11
The Executive acknowledges (i) that the business of the Company and its affiliates will be conducted throughout the Restricted Territory, (ii) notwithstanding the state of formation or principal office of the Company and its affiliates, or any of their respective executives or employees (including the Executive), it is expected that the Company will have business activities and have valuable business relationships within its industry throughout the Restricted Territory, and (iii) as part of the Executive's responsibilities, the Executive may be traveling throughout the Restricted Territory in furtherance of the Company's and its affiliates' business and its relationships. The Executive acknowledges that the potential harm to the Company of the non-enforcement of Section 8 outweighs any potential harm to the Executive of its enforcement by injunction or otherwise. The Executive acknowledges that the Executive has carefully read this Agreement and has given careful consideration to the restraints imposed upon the Executive by this Agreement and is in full accord as to their necessity for the reasonable and proper protection of confidential and proprietary information of the Company and its subsidiaries now existing or to be developed in the future. The Executive acknowledges that each and every restraint imposed by this Agreement is reasonable with respect to scope, duration, and geographical area.
9.Arbitration of Disputes. Any controversy or claim arising out of or relating to this Agreement or the breach thereof or otherwise arising out of the Executive's employment or the termination of that employment (including, without limitation, any claims of unlawful employment discrimination whether based on age or otherwise) shall, to the fullest extent permitted by law, be settled by arbitration in any forum and form agreed upon by the parties or, in the absence of such an agreement, under the auspices of the American Arbitration Association ("AAA") in accordance with the Employment Dispute Resolution Rules of the AAA, including, but not limited to, the rules and procedures applicable to the selection of arbitrators. In the event that any person or entity other than the Executive or the Company may be a party with regard to any such controversy or claim, such controversy or claim shall be submitted to arbitration subject to such other person or entity's agreement. Judgment upon the award rendered by the arbitrator may be entered in any court having jurisdiction thereof. The parties consent to the jurisdiction to the federal courts of the District of Colorado or, if there shall be no jurisdiction, to the state courts located in Arapahoe County, Colorado, to enforce any arbitration award rendered with respect thereto. This Section 8 shall be specifica1ly enforceable. Notwithstanding the foregoing, this Section 8 shall not preclude either party from pursuing a court action for the sole purpose of obtaining a temporary restraining order or a preliminary injunction in circumstances in which such relief is appropriate; provided that any other relief shall be pursued through an arbitration proceeding pursuant to this Section 9.
10.Consent to Jurisdiction. To the extent that any court action is permitted consistent with or to enforce Section 9 of this Agreement, the parties hereby consent to the jurisdiction of the Superior Court of the State of Colorado and the United States District Court for the District of Colorado. Accordingly, with respect to any such court action, the Executive (a) submits to the personal jurisdiction of such courts; (b) consents to service of process; and (c) waives any other requirement (whether imposed by statute, rule of court, or otherwise) with respect to personal jurisdiction or service of process.
11.Integration. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements between the parties concerning such subject matter, provided that the Confidentiality Agreement remains in full force and effect.
12.Withholding. All payments made by the Company to the Executive under this Agreement shall be net of any tax or other amounts required to be withheld by the Company under applicable law.
12
13.Successor to the Executive. This Agreement shall inure to the benefit of and be enforceable by the Executive's personal representatives, executors, administrators, heirs, distributees, devisees and legatees. In the event of the Executive's death after his termination of employment but prior to the completion by the Company of all payments due him under this Agreement, the Company shall continue such payments to the Executive's beneficiary designated in writing to the Company prior to his death (or to his estate, if the Executive fails to make such designation).
14.Enforceability. If any portion or provision of this Agreement (including, without limitation, any portion or provision of any section of this Agreement) shall to any extent be declared illegal or unenforceable by a court of competent jurisdiction, then the remainder of this Agreement, or the application of such portion or provision in circumstances other than those as to which it is so declared illegal or unenforceable, shall not be affected thereby, and each portion and provision of this Agreement shall be valid and enforceable to the fullest extent permitted by law.
15.Survival. The provisions of this Agreement shall survive the termination of this Agreement and/or the termination of the Executive's employment to the extent necessary to effectuate the terms contained herein.
16.Waiver. No waiver of any provision hereof shall be effective unless made in writing and signed by the waiving party. The failure of any party to require the performance of any term or obligation of this Agreement, or the waiver by any party of any breach of this Agreement, shall not prevent any subsequent enforcement of such term or obligation or be deemed a waiver of any subsequent breach.
17.Notices. Any notice to be given under this Agreement shall be in writing and delivered personally or sent by overnight courier or registered or certified mail, postage prepaid, return receipt requested, addressed to the party concerned at the address indicated below, or to such other address of which such party subsequently may give notice in writing:
(a) |
If to Executive: To the address specified in the payroll |
records of the Company.
(b) |
If to the Company: |
Aytu BioPharma, Inc.
373 Inverness Parkway
Suite 206
Englewood, Colorado 80112
Any notice delivered personally or by overnight courier shall be deemed given on the date delivered and any notice sent by registered or certified mail, postage prepaid, return receipt requested, shall be deemed given on the date mailed.
18.Amendment. This Agreement may be amended or modified only by a written instrument signed by the Executive and by a duly authorized representative of the 19.Governing Law.
13
Company.
This is a Colorado contract and shall be construed under and be governed in all respects by the laws of the State of Colorado, without giving effect to the conflict of laws principles of such State. With respect to any disputes concerning federal law, such disputes shall be determined in accordance with the law as it would be interpreted and applied by the United States Court of Appeals for the Tenth Circuit.
20.Counterparts. This Agreement may be executed in any number of counterparts, each of which when so executed and delivered shall be taken to be an original; but such counterparts shall together constitute one and the same document.
21.Successor to Company. The Company shall require any successor (whether direct or indirect, by purchase, merger, consolidation or otherwise) to all or substantially all of the business or assets of the Company expressly to assume and agree to perform this Agreement to the same extent that the Company would be required to perform it if no succession had taken place. Failure of the Company to obtain an assumption of this Agreement at or prior to the effectiveness of any succession shall be a material breach of this Agreement.
IN WITNESS WHEREOF, the parties have executed this Agreement effective on the date and year first above written.
|
AYTU BIOPHARMA, INC. |
|
|
|
|
By: Vivian Lu |
|
|
Its: Chairwoman of the Compensation Committee Board of Directors |
|
|
|
Executive |
|
|
|
|
|
Greg Pyszczymuka |
14
Exhibit 21.1
SUBSIDIARIES OF AYTU BIOPHARMA, INC.
|
Name of Subsidiary |
State Jurisdiction |
|
|
|
1.
|
Aytu Therapeutics, LLC |
Delaware |
2.
|
Innovus Pharmaceuticals, Inc. |
Nevada |
3.
|
Semprae Laboratories, Inc |
Delaware |
4.
|
Supplement Hunt, Inc. |
Nevada |
5.
|
Delta Prime Savings Club, Inc |
Nevada |
6.
|
Neos Therapeutics, Inc. |
Delaware |
7.
|
Neos Therapeutics Brands, LLC |
Delaware |
8.
|
Neos Therapeutics, LP |
Texas |
9.
|
PharmaFab Texas, LLC |
Texas |
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in Aytu BioPharma, Inc. Registration Statements on Form S-8 (No. 333-255325, 333-205462, 333-236598 and 333-272897), Form S-3 (Nos. 333-259862, 333-235548, 333-236599, 333-239010 and 333-265479) and Form S-1 (File Nos. , 333-212100, 333-213489, 333-222994, 333-223385, 333-227243, 333-227706 and 333-271556) of our report dated September 27, 2022, except for Note 2, as to which the date is October 12, 2023, relating to the consolidated financial statements as of and for the year ended June 30, 2022 that appear in this Annual Report on Form 10-K.
/s/Plante & Moran, PLLC
Denver, Colorado
October 12, 2023
Exhibit 23.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We have issued our report dated October 12, 2023, with respect to the consolidated financial statements included in the Annual Report of Aytu BioPharma, Inc. on Form 10-K for the year ended June 30, 2023. We consent to the incorporation by reference of said report into the Registration Statements of Aytu BioPharma, Inc. on Forms S-1 (File Nos. 333-212100, 333-213489, 333-222994, 333-223385, 333-227243, 333-227706 and 333-271556), on Forms S-3 (File Nos. 333-259862, 333-235548, 333-236599, 333-239010 and 333-265479) and on Forms S-8 (File Nos. 333-255325, 333-205462, 333-236598 and 333-272897).
/s/ GRANT THORNTON LLP
Denver, Colorado
October 12, 2023
Exhibit 31.1
AYTU BIOPHARMA, INC.
Certification by Chief Executive Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Joshua R. Disbrow, certify that:
1. |
I have reviewed this report on Form 10-K for the year ended June 30, 2023 of Aytu BioPharma, Inc.; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. |
The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a—15(e) and 15d—15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a—15(f) and 15d—15(f)) for the registrant and have: |
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant is made known to us by others within those entities particularly during the period in which this report is being prepared; |
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting. |
5. |
The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of registrant’s board of directors (or persons performing the equivalent functions): |
a) |
All significant deficiencies or material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: October 12, 2023 |
|
|
|
|
|
|
|
|
|
By: |
/s/ Joshua R. Disbrow |
|
|
Joshua R. Disbrow |
|
|
Chief Executive Officer (Principal Executive Officer) |
Exhibit 31.2
AYTU BIOPHARMA, INC.
Certification by Chief Financial Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Mark Oki, certify that:
1. |
I have reviewed this report on Form 10-K for the year ended June 30, 2023 of Aytu BioPharma, Inc.; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. |
The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a—15(e) and 15d—15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a—15(f) and 15d—15(f)) for the registrant and have: |
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant is made known to us by others within those entities particularly during the period in which this report is being prepared; |
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting. |
5. |
The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of registrant’s board of directors (or persons performing the equivalent functions): |
a) |
All significant deficiencies or material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: October 12, 2023 |
|
|
|
|
|
|
|
|
|
By: |
/s/ Mark Oki |
|
|
Mark Oki |
|
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
Exhibit 32.1
CERTIFICATION OF CHIEF EXECUTIVE OFFICER AND CHIEF FINANCIAL OFFICER
PURSUANT TO
18 U.S. C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
I Joshua R. Disbrow, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes‑Oxley Act of 2002, that, to my knowledge, the Annual Report on Form 10‑K of Aytu BioPharma, Inc. for the fiscal year ended June 30, 2023 fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and that information contained in such Annual Report on Form 10‑K fairly presents, in all material respects, the financial condition and results of operations of Aytu BioPharma, Inc.
Date: October 12, 2023 |
|
|
|
|
|
|
|
|
|
By: |
/s/ Joshua R. Disbrow |
|
|
Joshua R. Disbrow |
|
|
Chief Executive Officer (Principal Executive Officer) |
I Mark Oki, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes‑Oxley Act of 2002, that, to my knowledge, the Annual Report on Form 10‑K of Aytu BioPharma, Inc. for the fiscal year ended June 30, 2022 fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and that information contained in such Annual Report on Form 10‑K fairly presents, in all material respects, the financial condition and results of operations of Aytu BioPharma, Inc.
Date: October 12, 2023 |
|
|
|
|
|
|
|
|
|
By: |
/s/ Mark Oki |
|
|
Mark Oki |
|
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |