UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2023
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___ to ___
Commission File Number 001-35023
iBio, Inc.
(Exact name of registrant as specified in its charter)
Delaware |
|
26-2797813 |
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
8800 HSC Parkway, Bryan, TX |
|
77807-1107 |
(Address of principal executive offices) |
|
(Zip Code) |
(979) 446-0027
(Registrant’s telephone number, including area code)
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading symbol(s) |
|
Name of each exchange on which registered |
Common Stock |
|
IBIO |
|
NYSE American |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ⌧ No ◻
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ⌧ No ◻
Indicate by check mark whether the registrant is a large, accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large, accelerated filer,” “accelerated filer” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated Filer ☐ |
|
|
|
|
|
Accelerated Filer ☐ |
Non-accelerated Filer ☒ |
|
|
|
|
|
Smaller reporting company ☒ |
|
|
|
|
|
|
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
Shares of Common Stock outstanding as of May 10, 2023: 16,798,014
iBio, Inc.
TABLE OF CONTENTS
3 |
||
|
|
|
Consolidated Financial Statements (Unaudited) |
3 |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
37 |
|
45 |
||
45 |
||
|
|
|
46 |
||
|
|
|
46 |
||
46 |
||
51 |
||
51 |
||
|
|
|
52 |
||
2
PART I - FINANCIAL INFORMATION
Item 1. Consolidated Financial Statements (Unaudited).
iBio, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
(In thousands, except share and per share amounts)
|
|
March 31, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
|
|
(Unaudited) |
|
(See Note 2) |
||
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
6,562 |
|
$ |
22,676 |
Investments in debt securities |
|
|
— |
|
|
10,845 |
Accounts receivable - trade |
|
|
70 |
|
|
1,000 |
Subscription receivable |
|
|
260 |
|
|
— |
Settlement receivable - current portion |
|
|
— |
|
|
5,100 |
Convertible promissory note receivable and accrued interest |
|
|
912 |
|
|
— |
Prepaid expenses and other current assets |
|
|
921 |
|
|
1,549 |
Current assets held for sale |
|
|
18,368 |
|
|
3,900 |
Total Current Assets |
|
|
27,093 |
|
|
45,070 |
|
|
|
|
|
|
|
Restricted cash |
|
|
3,253 |
|
|
5,996 |
Convertible promissory note receivable and accrued interest |
|
|
775 |
|
|
1,631 |
Finance lease right-of-use assets, net of accumulated amortization |
|
|
678 |
|
|
— |
Operating lease right-of-use asset |
|
|
2,786 |
|
|
3,068 |
Fixed assets, net of accumulated depreciation |
|
|
4,358 |
|
|
1,373 |
Intangible assets, net of accumulated amortization |
|
|
5,393 |
|
|
4,851 |
Security deposits |
|
|
50 |
|
|
29 |
Prepaid expenses - noncurrent |
|
|
— |
|
|
74 |
Noncurrent assets held for sale |
|
|
— |
|
|
37,314 |
Total Assets |
|
$ |
44,386 |
|
$ |
99,406 |
|
|
|
|
|
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
2,013 |
|
$ |
4,264 |
Accrued expenses |
|
|
3,821 |
|
|
3,764 |
Finance lease obligations - current portion |
|
|
265 |
|
|
— |
Operating lease obligation - current portion |
|
|
377 |
|
|
91 |
Equipment financing payable - current portion |
|
|
156 |
|
|
— |
Term note payable - net of deferred financing costs |
|
|
13,700 |
|
|
22,161 |
Contract liabilities |
|
|
— |
|
|
100 |
Current liabilities related to assets held for sale |
|
|
1,944 |
|
|
56 |
Total Current Liabilities |
|
|
22,276 |
|
|
30,436 |
|
|
|
|
|
|
|
Finance lease obligations - net of current portion |
|
|
422 |
|
|
— |
Operating lease obligation - net of current portion |
|
|
3,225 |
|
|
3,514 |
Equipment financing payable - net of current portion |
|
|
283 |
|
|
— |
Accrued expenses - noncurrent |
|
|
791 |
|
|
— |
Noncurrent liabilities related to assets held for sale |
|
|
— |
|
|
1,971 |
|
|
|
|
|
|
|
Total Liabilities |
|
|
26,997 |
|
|
35,921 |
|
|
|
|
|
|
|
Stockholders' Equity |
|
|
|
|
|
|
Series 2022 Convertible Preferred Stock - $0.001 par value; 1,000,000 shares authorized; 0 and 1,000 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively |
|
|
— |
|
|
— |
Common Stock - $0.001 par value; 275,000,000 shares authorized at March 31, 2023 and June 30, 2022; 15,818,149 and 8,727,158 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively |
|
|
16 |
|
|
9 |
Additional paid-in capital |
|
|
300,280 |
|
|
287,619 |
Accumulated other comprehensive loss |
|
|
— |
|
|
(213) |
Accumulated deficit |
|
|
(282,907) |
|
|
(223,930) |
Total Stockholders’ Equity |
|
|
17,389 |
|
|
63,485 |
Total Liabilities and Stockholders' Equity |
|
$ |
44,386 |
|
$ |
99,406 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
3
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited; in thousands, except per share amounts)
|
|
Three Months Ended |
|
Nine Months Ended |
||||||||
|
|
March 31, |
|
March 31, |
||||||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
— |
|
$ |
1,800 |
|
$ |
— |
|
$ |
1,884 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
2,644 |
|
|
3,272 |
|
|
7,971 |
|
|
6,265 |
General and administrative |
|
|
3,525 |
|
|
5,316 |
|
|
16,407 |
|
|
14,863 |
Total operating expenses |
|
|
6,169 |
|
|
8,588 |
|
|
24,378 |
|
|
21,128 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss |
|
|
(6,169) |
|
|
(6,788) |
|
|
(24,378) |
|
|
(19,244) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
(35) |
|
|
— |
|
|
(66) |
|
|
— |
Interest income |
|
|
23 |
|
|
40 |
|
|
163 |
|
|
111 |
Loss on sale of debt securities |
|
|
(98) |
|
|
— |
|
|
(98) |
|
|
— |
Royalty income |
|
|
— |
|
|
2 |
|
|
— |
|
|
7 |
Total other income (expense) |
|
|
(110) |
|
|
42 |
|
|
(1) |
|
|
118 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated net loss from continuing operations |
|
|
(6,279) |
|
|
(6,746) |
|
|
(24,379) |
|
|
(19,126) |
Net loss attributable to noncontrolling interest |
|
|
— |
|
|
— |
|
|
— |
|
|
1 |
Net loss attributable to iBio, Inc. from continuing operations |
|
|
(6,279) |
|
|
(6,746) |
|
|
(24,379) |
|
|
(19,125) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock dividends - iBio CDMO Tracking Stock |
|
|
— |
|
|
— |
|
|
— |
|
|
(88) |
Net loss available to iBio, Inc. stockholders from continuing operations |
|
|
(6,279) |
|
|
(6,746) |
|
|
(24,379) |
|
|
(19,213) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from discontinued operations |
|
|
(1,015) |
|
|
(5,644) |
|
|
(34,598) |
|
|
(14,124) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss available to iBio, Inc. stockholders |
|
$ |
(7,294) |
|
$ |
(12,390) |
|
$ |
(58,977) |
|
$ |
(33,337) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated net loss |
|
$ |
(7,294) |
|
$ |
(12,390) |
|
$ |
(58,977) |
|
$ |
(33,250) |
Other comprehensive income (loss) - unrealized gain (loss) on debt securities |
|
|
134 |
|
|
(103) |
|
|
180 |
|
|
(131) |
Other comprehensive income - foreign currency adjustment |
|
|
33 |
|
|
— |
|
|
33 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss |
|
$ |
(7,127) |
|
$ |
(12,493) |
|
$ |
(58,764) |
|
$ |
(33,381) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss per common share attributable to iBio, Inc. stockholders - basic and diluted - continuing operations |
|
$ |
(0.47) |
|
$ |
(0.77) |
|
$ |
(2.30) |
|
$ |
(2.20) |
Loss per common share attributable to iBio, Inc. stockholders - basic and diluted - discontinued operations |
|
$ |
(0.08) |
|
$ |
(0.65) |
|
$ |
(3.27) |
|
$ |
(1.62) |
Loss per common share attributable to iBio, Inc. stockholders - basic and diluted - total |
|
$ |
(0.55) |
|
$ |
(1.42) |
|
$ |
(5.57) |
|
$ |
(3.82) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding - basic and diluted |
|
|
13,184 |
|
|
8,719 |
|
|
10,592 |
|
|
8,719 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
4
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Equity
(Unaudited; in thousands)
Nine Months Ended March 31, 2023
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
Other |
|
|
|
|
|
|
||
|
|
Preferred Stock |
|
Common Stock |
|
Paid-In |
|
Comprehensive |
|
Accumulated |
|
|
|
||||||||||
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Loss |
|
Deficit |
|
Total |
|||||||
Balance as of July 1, 2022 |
|
|
1 |
|
$ |
— |
|
8,727 |
|
$ |
9 |
|
$ |
287,619 |
|
$ |
(213) |
|
$ |
(223,930) |
|
$ |
63,485 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital raise |
|
|
— |
|
|
— |
|
176 |
|
|
— |
|
|
1,151 |
|
|
— |
|
|
— |
|
|
1,151 |
Conversion of preferred stock to common stock |
|
|
(1) |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Common stock issued - RubrYc transaction |
|
|
— |
|
|
— |
|
102 |
|
|
— |
|
|
650 |
|
|
— |
|
|
— |
|
|
650 |
Vesting of RSUs |
|
|
— |
|
|
— |
|
1 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Share-based compensation |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,222 |
|
|
— |
|
|
— |
|
|
1,222 |
Unrealized loss on available-for-sale debt securities |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(10) |
|
|
— |
|
|
(10) |
Net loss |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(18,130) |
|
|
(18,130) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of September 30, 2022 |
|
|
— |
|
$ |
— |
|
9,006 |
|
$ |
9 |
|
$ |
290,642 |
|
$ |
(223) |
|
$ |
(242,060) |
|
$ |
48,368 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital raise |
|
|
— |
|
|
— |
|
3,366 |
|
|
3 |
|
|
3,497 |
|
|
— |
|
|
— |
|
|
3,500 |
Cost to raise capital |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
(636) |
|
|
— |
|
|
— |
|
|
(636) |
Payment for fractional shares after reverse stock split |
|
|
— |
|
|
— |
|
(8) |
|
|
— |
|
|
(39) |
|
|
— |
|
|
— |
|
|
(39) |
Vesting of RSUs |
|
|
— |
|
|
— |
|
4 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Share-based compensation |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,127 |
|
|
— |
|
|
— |
|
|
1,127 |
Unrealized gain on available-for-sale debt securities |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
56 |
|
|
— |
|
|
56 |
Net loss |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(33,553) |
|
|
(33,553) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2022 |
|
|
— |
|
$ |
— |
|
12,368 |
|
$ |
12 |
|
$ |
294,591 |
|
$ |
(167) |
|
$ |
(275,613) |
|
$ |
18,823 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vesting of RSUs |
|
|
— |
|
|
— |
|
28 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Capital raise |
|
|
— |
|
|
— |
|
3,422 |
|
|
4 |
|
|
4,093 |
|
|
— |
|
|
— |
|
|
4,097 |
Share-based compensation |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,596 |
|
|
— |
|
|
— |
|
|
1,596 |
Unrealized gain on debt securities |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
113 |
|
|
— |
|
|
113 |
Reclassification adjustment for loss on available-for-sale debt securities realized in net income |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
21 |
|
|
— |
|
|
21 |
Foreign currency translation adjustment |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
33 |
|
|
— |
|
|
33 |
Net loss |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(7,294) |
|
|
(7,294) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of March 31, 2023 |
|
|
— |
|
$ |
— |
|
15,818 |
|
$ |
16 |
|
$ |
300,280 |
|
$ |
— |
|
$ |
(282,907) |
|
$ |
17,389 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
5
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Equity
(Unaudited; in thousands)
Nine Months Ended March 31, 2022
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
Other |
|
|
|
|
|
|
|
|
|
||
|
|
Preferred Stock |
|
Common Stock |
|
Paid-In |
|
Comprehensive |
|
Accumulated |
|
Noncontrolling |
|
|
|
|||||||||||
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Loss |
|
Deficit |
|
Interest |
|
Total |
||||||||
Balance as of July 1, 2021 |
|
|
— |
|
$ |
— |
|
8,715 |
|
$ |
9 |
|
$ |
282,266 |
|
$ |
(63) |
|
$ |
(173,627) |
|
$ |
(17) |
|
$ |
108,568 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock options |
|
|
— |
|
|
— |
|
3 |
|
|
— |
|
|
77 |
|
|
— |
|
|
— |
|
|
— |
|
|
77 |
Share-based compensation |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
821 |
|
|
— |
|
|
— |
|
|
— |
|
|
821 |
Unrealized loss on debt securities |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(1) |
|
|
— |
|
|
— |
|
|
(1) |
Net loss |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(8,939) |
|
|
(1) |
|
|
(8,940) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of September 30, 2021 |
|
|
— |
|
$ |
— |
|
8,718 |
|
$ |
9 |
|
$ |
283,164 |
|
$ |
(64) |
|
$ |
(182,566) |
|
$ |
(18) |
|
$ |
100,525 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vesting of RSUs |
|
|
— |
|
|
— |
|
4 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Warrant issued for Transaction |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
967 |
|
|
— |
|
|
— |
|
|
— |
|
|
967 |
Acquisition of remaining portion of iBio CDMO |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
(68) |
|
|
— |
|
|
— |
|
|
18 |
|
|
(50) |
Share-based compensation |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,103 |
|
|
— |
|
|
— |
|
|
— |
|
|
1,103 |
Unrealized loss on debt securities |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(27) |
|
|
— |
|
|
— |
|
|
(27) |
Net loss |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(11,920) |
|
|
— |
|
|
(11,920) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2021 |
|
|
— |
|
$ |
— |
|
8,722 |
|
$ |
9 |
|
$ |
285,166 |
|
$ |
(91) |
|
$ |
(194,486) |
|
$ |
— |
|
$ |
90,598 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vesting of RSUs |
|
|
— |
|
|
— |
|
4 |
|
|
— |
|
|
1 |
|
|
— |
|
|
— |
|
|
— |
|
|
1 |
Cost to raise capital |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Exercise of stock options |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Share-based compensation |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,274 |
|
|
— |
|
|
— |
|
|
— |
|
|
1,274 |
Foreign currency translation adjustment |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Unrealized loss on debt securities |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(103) |
|
|
— |
|
|
— |
|
|
(103) |
Net loss |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(12,390) |
|
|
— |
|
|
(12,390) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of March 31, 2022 |
|
|
— |
|
$ |
— |
|
8,726 |
|
$ |
9 |
|
$ |
286,441 |
|
$ |
(194) |
|
$ |
(206,876) |
|
$ |
— |
|
$ |
79,380 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
6
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(Unaudited; in Thousands)
|
|
Nine Months Ended |
|
||||
|
|
March 31, |
|
||||
|
|
2023 |
|
2022 |
|
||
|
|
|
|
|
|
|
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
Consolidated net loss |
|
$ |
(58,977) |
|
$ |
(33,250) |
|
Adjustments to reconcile consolidated net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
Share-based compensation |
|
|
3,945 |
|
|
3,198 |
|
Amortization of intangible assets |
|
|
121 |
|
|
333 |
|
Amortization of finance lease right-of-use assets |
|
|
156 |
|
|
587 |
|
Amortization of operating lease right-of-use assets |
|
|
290 |
|
|
386 |
|
Depreciation of fixed assets |
|
|
508 |
|
|
1,532 |
|
Gain on sale of fixed assets |
|
|
(732) |
|
|
— |
|
Accrued interest receivable on convertible promissory note receivable |
|
|
(56) |
|
|
(56) |
|
Amortization of premiums on debt securities |
|
|
67 |
|
|
269 |
|
Loss on sale of debt securities |
|
|
98 |
|
|
— |
|
Amortization of deferred financing costs |
|
|
160 |
|
|
67 |
|
Inventory reserve |
|
|
4,915 |
|
|
— |
|
Impairment of fixed assets |
|
|
17,600 |
|
|
— |
|
Impairment of intangible assets |
|
|
565 |
|
|
— |
|
Gain on disposition of finance lease ROU assets |
|
|
(5) |
|
|
— |
|
Forgiveness of note payable and accrued interest - SBA loan |
|
|
— |
|
|
(607) |
|
Settlement of revenue contract |
|
|
— |
|
|
(84) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
Accounts receivable - trade |
|
|
931 |
|
|
(890) |
|
Settlement receivable |
|
|
5,100 |
|
|
5,100 |
|
Inventory |
|
|
(1,015) |
|
|
(3,257) |
|
Prepaid expenses and other current assets |
|
|
627 |
|
|
(494) |
|
Prepaid expenses - noncurrent |
|
|
74 |
|
|
(975) |
|
Security deposit |
|
|
(21) |
|
|
(5) |
|
Accounts payable |
|
|
(481) |
|
|
1,649 |
|
Accrued expenses |
|
|
(18) |
|
|
618 |
|
Accrued expenses - noncurrent |
|
|
791 |
|
|
— |
|
Operating lease obligations |
|
|
(9) |
|
|
(12) |
|
Contract liabilities |
|
|
(100) |
|
|
(86) |
|
Net cash used in operating activities |
|
|
(25,466) |
|
|
(25,977) |
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
Purchases of debt securities |
|
|
— |
|
|
(5,355) |
|
Redemption of debt securities |
|
|
4,100 |
|
|
9,711 |
|
Sale of debt securities |
|
|
6,739 |
|
|
— |
|
Purchase of equity security |
|
|
— |
|
|
(1,760) |
|
Additions to intangible assets |
|
|
— |
|
|
(4,300) |
|
Purchases of fixed assets |
|
|
(5,232) |
|
|
(3,900) |
|
Sales proceeds for fixed assets |
|
|
2,100 |
|
|
— |
|
Payment for RubrYc asset acquisition |
|
|
(692) |
|
|
— |
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities |
|
|
7,015 |
|
|
(5,604) |
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
Proceeds from sales of common stock |
|
|
7,851 |
|
|
— |
|
Payments for fractional shares after reverse stock split |
|
|
(39) |
|
|
— |
|
Acquisition of noncontrolling interest |
|
|
— |
|
|
(50) |
|
Proceeds from equipment financing loan |
|
|
500 |
|
|
— |
|
Payment of equipment financing loan |
|
|
(62) |
|
|
— |
|
Payment of term note payable |
|
|
(8,523) |
|
|
— |
|
Proceeds from exercise of stock options |
|
|
— |
|
|
77 |
|
Costs to attain term note |
|
|
(22) |
|
|
(322) |
|
Payment of finance lease obligation |
|
|
(144) |
|
|
(5,820) |
|
Net cash used in financing activities |
|
|
(439) |
|
|
(6,115) |
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes |
|
|
33 |
|
|
— |
|
|
|
|
|
|
|
|
|
Net decrease in cash, cash equivalents and restricted cash |
|
|
(18,857) |
|
|
(37,696) |
|
Cash, cash equivalents and restricted cash - beginning |
|
|
28,672 |
|
|
77,404 |
|
Cash, cash equivalents and restricted cash - end |
|
$ |
9,815 |
|
$ |
39,708 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
7
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(Unaudited; in Thousands)
|
|
Nine Months Ended |
|
||||
|
|
March 31, |
|
||||
|
|
2023 |
|
2022 |
|
||
Schedule of non-cash activities: |
|
|
|
|
|
|
|
Fixed assets included in accounts payable in prior period, paid in current period |
|
$ |
1,769 |
|
$ |
791 |
|
Increase in finance lease ROU assets for new leases |
|
$ |
814 |
|
$ |
— |
|
Increase in finance lease obligation for new leases |
|
$ |
814 |
|
$ |
— |
|
RubrYc asset acquisition by issuance of common stock |
|
$ |
650 |
|
$ |
— |
|
Costs to raise capital paid directly from gross proceeds |
|
$ |
636 |
|
$ |
— |
|
Sales of fixed assets receivable |
|
$ |
460 |
|
$ |
— |
|
Subscription receivable |
|
$ |
260 |
|
$ |
— |
|
Cost accrued to amend term note payable |
|
$ |
75 |
|
$ |
— |
|
Unpaid fixed assets included in accounts payable |
|
$ |
21 |
|
$ |
2,193 |
|
Termination of finance ROU assets including issuance of warrant |
|
$ |
— |
|
$ |
25,386 |
|
Note payable to acquire Facility |
|
$ |
— |
|
$ |
22,375 |
|
Increase in operating lease ROU assets for new lease - net of lease incentive |
|
$ |
— |
|
$ |
5,570 |
|
Settlement of revenue contract |
|
$ |
— |
|
$ |
580 |
|
Issuance of warrant for final finance lease obligation payment |
|
$ |
— |
|
$ |
217 |
|
Lease incentive for construction in progress |
|
$ |
— |
|
$ |
82 |
|
Acquisition of noncontrolling interest |
|
$ |
— |
|
$ |
18 |
|
Unrealized (gain) loss on available-for-sale debt securities |
|
$ |
(159) |
|
$ |
131 |
|
Supplemental cash flow information: |
|
|
|
|
|
|
|
Cash paid during the period for interest |
|
$ |
523 |
|
$ |
860 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
8
iBio, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Nature of Business
iBio, Inc. (“we”, “us”, “our”, “iBio”, “iBio, Inc” or the “Company”) is an Artificial Intelligence (“AI”)-driven innovator of precision antibody immunotherapies. The Company has a pipeline of innovative primarily immuno-oncology antibodies against hard-to-drug targets where it may face reduced competition and with antibodies that may be more selective. The Company plans to use its AI-driven discovery platform to continue adding antibodies against hard-to-drug targets or to work with partners on AI-driven drug development.
Therapeutics Pipeline
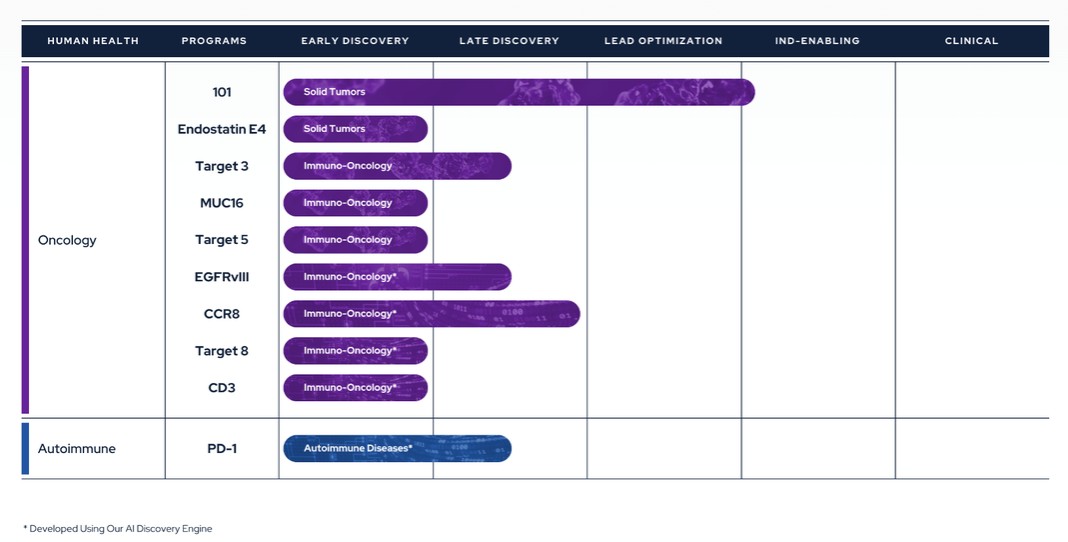
IBIO-101: an anti-CD25 molecule that works by depletion of immunosuppressive T-regulatory cells (“Tregs”) via antibody-dependent cellular cytotoxicity (“ADCC”), without disrupting activation of effector T-cells (“Teffs”) in the tumor microenvironment. IBIO-101 could potentially be used to treat solid tumors, hairy cell leukemia, relapsed multiple myeloma, lymphoma, or head and neck cancer. IBIO-101 is currently in the Investigational New Drug (“IND”) enabling stage. We have contracted with a contract research organization (“CRO”) to assist with the development of the manufacturing process, which includes but is not limited to process and cell line development for the production of the drug substance and drug product. IBIO-101 is strategically positioned as a fast follower to Hoffman-La Roche’s RG6292 molecule that recently released Phase 1 clinical data. While RG6292 showed signs of efficacy, especially in combination with PD-L1 monoclonal antibody, and was well tolerated, we anticipate additional clinical research will be needed to determine whether different cancer types are more efficacious than others. As a result, we have decided to pause the IND enabling studies until additional data is released on RG6292. This approach will allow us to gather more information, thoroughly evaluate the market potential and optimize our financial resources and the development plan for IBIO-101 to maximize its potential for success.
CCR8: targets depletion of highly immunosuppressive CCR8+ Tregs in the tumor microenvironment via an ADCC mechanism with selective binding to CCR8 over its closely related cousin, CCR4, to avoid off-target effects. A CCR8 program could potentially be broadly applicable in solid tumors and/or as a prospective combination therapy.
EGFRvIII: binds a tumor-specific mutation of EGFR variant III with an afucosylated antibody for high ADCC. Because of its specificity binding to the tumor-specific mutation, it could potentially reduce toxicity and/or expand the therapeutic window compared to simple broad EGFR-targeted alternatives. EGFRvIII is constantly “switched on” which can lead to the development of a range of different cancers. An EGFRvIII antibody could potentially be used to treat glioblastoma, head and neck cancer or non-small cell lung cancer.
9
CD3 antibody panel: provides a range of CD3 affinities with cross-reactivity to non-human primates and increased the humanness of the antibody sequences. The antibody panel is intended to serve as one arm of T-cell-redirecting bispecific antibodies, a new class of therapeutic antibodies designed to simultaneously bind to T-cells via CD3 and to tumor cells via tumor-specific antigens or tumor-associated antigens, inducing T-cell-mediated killing of tumor cells.
MUC16: a highly expressed target on ovarian cancer cells and an attractive tumor associated target for therapeutic antibodies. However, antibodies targeting MUC16 are prone to tumor resistance via epitope shedding and dysregulated glycosylation. Epitope-steered antibodies that bind to an epitope that avoids both of these tumor resistance mechanisms could potentially be used to treat MUC16 positive tumors, particularly those tumors that are resistant to other MUC16 antibodies.
PD-1 Agonist: selectively binds PD-1 to suppress auto-reactive T-cells without PD-L1/PD-L2 blocking. A PD-1 agonist could potentially be used to treat inflammatory bowel disease, systemic lupus erythematosus, multiple sclerosis or other inflammatory diseases.
In addition to the programs described above, the Company also has three additional early discovery programs that have the potential to advance into later stages of preclinical development and are designed to tackle hard-to-drug targets.
IBIO-100 and Endostatin E4
Our preclinical anti-fibrotic program, IBIO-100, has been undergoing a review process as part of our ongoing effort to prioritize our resources and focus on the most promising opportunities. The IBIO-100 program design is based upon work by Dr. Carol Feghali-Bostwick, Professor of Medicine at the Medical University of South Carolina and Vice-Chair of the Scleroderma Foundation. Her initial work was conducted at the University of Pittsburgh, and we have licensed the patents relevant for the continued development of the molecule from the university. After careful consideration, in February 2023, the Company terminated all efforts on IBIO-100 anti-fibrotic program and provided a six (6) month notice of termination of the license agreement to the University of Pittsburgh, as required by the license agreement. Pursuant to the license agreement with the University of Pittsburgh, the Company’s financial obligations for the management of the patents under the license will cease on August 14, 2023, and at such time, will transition back to the University of Pittsburgh.
As part of this decision, we are intending to complete the pre-clinical cancer studies we are conducting in collaboration with University of Texas Southwestern using E4 endostatin peptide, which is derived from IBIO-100. After the pre-clinical studies are completed, we will re-assess whether to further pursue the oncology program and have further discussions with the University of Pittsburgh. This approach allows us to gather valuable data and insights that will inform our future decisions regarding the potential of E4 endostatin peptide as an oncology program.
AI Drug Discovery Platform
In September 2022, the Company purchased substantially all of the assets of RubrYc Therapeutics (for a complete description of the transaction please see Note 6 – Significant Transactions). The AI Drug Discovery platform technology is designed to be used to discover antibodies that bind to hard-to-target subdominant and conformational epitopes for further development within our existing portfolio or in partnership with outside entities. The RubrYc AI platform is built upon three key technologies.
| 1. | Epitope Targeting Engine: A patented machine-learning platform that combines computational biology and 3D-modeling to identify molecules that mimic hard-to-target binding sites on target proteins, specifically, subdominant and conformational epitopes. The creation of these small mimics enables the engineering of therapeutic antibody candidates that can selectively bind immune and cancer cells better than ”trial and error” antibody engineering and screening methods that are traditionally focused on dominant epitopes. |
| 2. | RubrYcHuTM Library: An AI-generated human antibody library free of significant sequence liabilities that provides a unique pool of antibodies to screen. The combination of the Epitope Targeting Engine and screening with the RubrYcHu Library has been shown to reduce the discovery time from ideation to in vivo proof-of-concept (“PoC’) by up to four months. This has the potential to enable more, and better, therapeutic candidates to reach the clinic faster. |
| 3. | StableHuTM Library: An AI-powered sequence optimization library used to improve antibody performance. Once an antibody has been advanced to the lead optimization stage, StableHu allows precise and rapid optimization of the antibody binding regions to rapidly move a candidate molecule into the IND-enabling stage. |
On January 3, 2023, the United States Patent and Trademark Office issued U.S. Patent No. 11,545,238, entitled “Machine Learning Method for Protein Modelling to Design Engineered Peptides,” which, among other claims, covers a machine learning model for engineering peptides, including antibody epitope therapeutics.
10
Subject to any potential patent term extensions, the patent will expire on May 13, 2040.
2. Basis of Presentation
Interim Consolidated Financial Statements
The accompanying unaudited condensed consolidated financial statements have been prepared from the books and records of the Company and include all normal and recurring adjustments which, in the opinion of management, are necessary for a fair presentation in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) for interim consolidated financial information and Rule 8-03 of Regulation S-X promulgated by the U.S. Securities and Exchange Commission (the “SEC”). Accordingly, these interim financial statements do not include all of the information and footnotes required for complete annual consolidated financial statements. Interim results are not necessarily indicative of the results that may be expected for the full year. Interim unaudited condensed consolidated financial statements should be read in conjunction with the audited financial statements and the notes thereto included in the Company’s Annual Report on Form 10-K for the prior year ended June 30, 2022, filed with the SEC on October 11, 2022 (the “Annual Report”), from which the accompanying condensed consolidated balance sheet dated June 30, 2022 was derived.
Principles of Consolidation
The condensed consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated as part of the consolidation. Non-controlling interest in the consolidated financial statements represented the share of the loss in iBio CDMO, LLC (“iBio CDMO”) for an affiliate of Eastern Capital Limited (“Eastern Capital”) through November 1, 2021, the date the Company acquired the remaining interest in iBio CDMO. See Note 6 – Significant Transactions.
Going Concern
The history of significant losses, the negative cash flow from operations, the limited cash resources on hand and the dependence by the Company on its ability to obtain additional financing to fund its operations after the current cash resources are exhausted raise substantial doubt about the Company's ability to continue as a going concern. In an effort to remain a going concern and increase cash reserves, the Company completed a public equity offering, reduced its work force by approximately 60% (a reduction of approximately 69 positions) in November 2022, and ceased operations of its CDMO facility thereby reducing annual spend on expenses by approximately 50%. Additionally, in July 2022, the Company initiated the selling of the CDMO assets and facility, and since then has sold a substantial portion of the CDMO assets. (See Note 3 – Discontinued Operations for more information.) Additional potential options being considered to further increase liquidity include lowering the Company’s expenses further, focusing product development on a select number of product candidates, the sale of the CDMO, the sale or out-licensing of certain product candidates, additional equipment sales, raising money from capital markets, grant revenue or collaborations, or a combination thereof.
The Company’s cash, cash equivalents, and restricted cash of $9.8 million as of March 31, 2023, is not anticipated to be sufficient to support operations through the first quarter of Fiscal 2024 unless the Company reduces its burn rate further, sells the CDMO facility for amounts above its term note payable, or increases its capital as described above. Regardless of whether the Company is able to reduce its burn rate or sell or out-license certain assets or parts of the business, the Company will need to raise additional capital in order to fully execute its near and long-term business plan. It is the Company’s goal to implement one or more potential options described above to allow the Company to have a cash runway for at least 12 months from the date of the filing of this Quarterly Report on Form 10-Q. However, there can be no assurance that the Company will be successful in implementing any of the options that it is evaluating.
The accompanying consolidated financial statements do not include any adjustments related to the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
Reverse Stock Split
On September 22, 2022, the Company's Board of Directors approved the implementation of a reverse stock split (the “Reverse Split”) at a ratio of one-for-twenty five (1:25) shares of the Company's common stock, par value $0.001 (the “Common Stock”). The Reverse Split was effective as of October 7, 2022. All share and per share amounts of the Common Stock presented have been retroactively adjusted to reflect the Reverse Split. See Note 16 – Stockholders’ Equity for more information.
11
3. Discontinued Operations
On November 3, 2022, the Company announced it is seeking to divest its contract development and manufacturing organization (iBio CDMO, LLC) in order to complete its transformation into an antibody discovery and development company. In conjunction with the divestment, the Company commenced a workforce reduction of approximately 60% of the current Company staffing levels (a reduction of approximately 69 positions). The Company substantially completed the employee reduction by January 2, 2023. Through the process of seeking to divest its contract development and manufacturing organization, the Company continues to market for sale the 130,000-square-foot cGMP facility location in Bryan, Texas (the “Facility”). Additionally, on February 10, 2021, the Company, entered into an Auction Sale Agreement (the “Auction Sale Agreement”) with Holland Industrial Group, together with Federal Equipment Company and Capital Recovery Group LLC (collectively, the “Auctioneers”) for the sale at public auction of equipment and other tangible personal property (the “Equipment”) located at the Facility. The Auctioneer guaranteed an amount of gross proceeds from the sale of the equipment of $2.1 million, which was paid to the Company on February 17, 2023. The auction, which commenced on March 24, 2023 and concluded on March 30, 2023, resulted in total proceeds of approximately $2.9 million. In accordance with the Auction Sale Agreement, the Company received 80% of the excess proceeds, after Holland Industrial Group’s $0.2 million fee, which approximated $0.5 million during the fourth quarter of Fiscal 2023 and is included in prepaid and other assets as of March 31, 2023.
The Company incurred pre-tax charges of approximately $1.9 million for the employee reduction which consisted of severance obligations, continuation of salaries and benefits over a 60-day transitional period during which impacted employees remain employed but were not expected to provide active service, and other customary employee benefit payments in connection with an employee reduction. At March 31, 2023, $0.1 million remains in accrued expenses as a current liability. The Company recorded a charge in discontinued operations for $34.6 million for the nine months ended March 31, 2023, of which approximately $17.6 million was the result of a fixed asset impairment charge (see Note 11 – Fixed Assets for more information), approximately $4.9 million to write down inventory to its net realizable value, approximately $7.5 million of personnel costs including severance and the balance related to operational costs related to winding down the CDMO business.
As such, the results of iBio CDMO's operations are reported as discontinued operations for the three and nine months ended March 31, 2023. The Company has retrospectively recast its condensed consolidated statement of operations for the three and nine months ended March 31, 2022 presented. In addition, those assets and liabilities associated with the discontinued operations of the CDMO that the Company intends to sell have been classified as “held for sale” as of March 31, 2023. The Company has retrospectively recast its consolidated balance sheet as of June 30, 2022 for assets and liabilities held for sale. The Company has chosen not to segregate the cash flows of iBio CDMO in the consolidated statement of cash flows. Supplemental disclosures related to discontinued operations for the statements of cash flows have been provided below. Unless noted otherwise, discussion in the Notes to the Condensed Consolidated Financial Statements refers to the Company's continuing operations.
The following table presents a reconciliation of the major financial lines constituting the results of operations for discontinued operations to the loss from discontinued operations presented separately in the condensed consolidated statements of operations (in thousands):
|
|
Three Months Ended |
|
Three Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Revenues |
|
$ |
205 |
|
$ |
143 |
Cost of goods sold |
|
|
25 |
|
|
48 |
Gross profit |
|
|
180 |
|
|
95 |
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
837 |
|
|
2,278 |
General and administrative |
|
|
929 |
|
|
3,211 |
Gain on sale of fixed assets |
|
|
(732) |
|
|
— |
Total operating expenses |
|
|
1,034 |
|
|
5,489 |
|
|
|
|
|
|
|
Other expenses: |
|
|
|
|
|
|
Interest expense - term note payable |
|
|
(158) |
|
|
(249) |
Other |
|
|
(3) |
|
|
(1) |
Total other expenses |
|
|
(161) |
|
|
(250) |
|
|
|
|
|
|
|
Loss from discontinued operations |
|
$ |
(1,015) |
|
$ |
(5,644) |
12
|
|
Nine Months Ended |
|
Nine Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Revenues |
|
$ |
391 |
|
$ |
438 |
Cost of goods sold |
|
|
52 |
|
|
201 |
Gross profit |
|
|
339 |
|
|
237 |
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
6,361 |
|
|
5,128 |
General and administrative |
|
|
6,165 |
|
|
8,659 |
Fixed asset impairments |
|
|
17,600 |
|
|
— |
Gain on sale of fixed assets |
|
|
(732) |
|
|
— |
Inventory reserve |
|
|
4,933 |
|
|
— |
Total operating expenses |
|
|
34,327 |
|
|
13,787 |
|
|
|
|
|
|
|
Other income (expenses): |
|
|
|
|
|
|
Interest expense - term note payable |
|
|
(606) |
|
|
(372) |
Interest expense - related party |
|
|
— |
|
|
(810) |
Forgiveness of note payable and accrued interest - SBA loan |
|
|
— |
|
|
607 |
Other |
|
|
(4) |
|
|
1 |
Total other income (expenses) |
|
|
(610) |
|
|
(574) |
|
|
|
|
|
|
|
Loss from discontinued operations |
|
$ |
(34,598) |
|
$ |
(14,124) |
The following table presents net carrying values related to the major classes of assets that were classified as held for sale at March 31, 2023 and June 30, 2022 (in thousands):
|
|
March 31, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
Current assets: |
|
|
|
|
|
|
Inventory |
|
$ |
— |
|
$ |
3,900 |
Operating lease right-of-use assets |
|
|
1,944 |
|
|
— |
Property and equipment, net |
|
|
16,424 |
|
|
— |
Total current assets |
|
$ |
18,368 |
|
$ |
3,900 |
|
|
|
|
|
|
|
Other assets: |
|
|
|
|
|
|
Property and equipment, net |
|
$ |
— |
|
$ |
35,289 |
Finance lease right-of-use assets |
|
|
— |
|
|
74 |
Operating lease right-of-use assets |
|
|
— |
|
|
1,951 |
Total other assets |
|
$ |
— |
|
$ |
37,314 |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Finance lease obligation |
|
$ |
— |
|
$ |
46 |
Operating lease obligation |
|
|
1,944 |
|
|
10 |
Total current liabilities |
|
$ |
1,944 |
|
$ |
56 |
|
|
|
|
|
|
|
Long-term liabilities: |
|
|
|
|
|
|
Finance lease obligation |
|
$ |
— |
|
$ |
30 |
Operating lease obligation |
|
|
— |
|
|
1,941 |
Total long-term liabilities |
|
$ |
— |
|
$ |
1,971 |
13
The following table presents the supplemental disclosures related to discontinued operations for the statements of cash flows (in thousands):
|
|
Nine Months Ended |
|
Nine Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Depreciation expense |
|
$ |
273 |
|
$ |
1,532 |
Amortization of finance lease right-of-use assets |
|
|
20 |
|
|
587 |
Purchase of fixed assets |
|
|
1,041 |
|
|
28,384 |
Fixed asset impairments |
|
|
17,600 |
|
|
— |
Inventory reserve |
|
|
4,933 |
|
|
— |
Sales proceeds of fixed assets |
|
|
2,100 |
|
|
— |
Investing non-cash transactions: |
|
|
|
|
|
|
Fixed assets included in accounts payable in prior period, paid in current period |
|
|
1,542 |
|
|
791 |
Unpaid fixed assets included in accounts payable |
|
|
— |
|
|
2,193 |
Sales of fixed assets receivable |
|
|
460 |
|
|
— |
4. Summary of Significant Accounting Policies
The Company’s significant accounting policies are described in Note 3 of the Notes to Consolidated Financial Statements in the Annual Report.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect reported amounts of assets and liabilities, disclosures of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. These estimates include liquidity assertions, the valuation of intellectual property and fixed assets held for sale, the incremental borrowing rate utilized in the finance and operating lease calculations, legal and contractual contingencies and share-based compensation. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
Accounts Receivable
Accounts receivable are reported at their outstanding unpaid principal balances net of allowances for uncollectible accounts. The Company provides for allowances for uncollectible receivables based on its estimate of uncollectible amounts considering age, collection history, and other factors considered appropriate. Management’s policy is to write off accounts receivable against the allowance for doubtful accounts when a balance is determined to be uncollectible. At March 31, 2023 and June 30, 2022, the Company determined that an allowance for doubtful accounts was not needed. The Company had accounts receivable of $426,000 at June 30, 2021.
Revenue Recognition
The Company accounts for its revenue recognition under Accounting Standards Codification ("ASC") 606, Revenue from Contracts with Customers. Under this standard, the Company recognizes revenue when a customer obtains control of promised services or goods in an amount that reflects the consideration to which the Company expects to receive in exchange for those goods or services. In addition, the standard requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer contracts.
The Company’s contract revenue consists primarily of amounts earned under contracts with third-party customers and reimbursed expenses under such contracts. The Company analyzes its agreements to determine whether the elements can be separated and accounted for individually or as a single unit of accounting. Allocation of revenue to individual elements that qualify for separate accounting is based on the separate selling prices determined for each component, and total contract consideration is then allocated pro rata across the components of the arrangement. If separate selling prices are not available, the Company will use its best estimate of such selling prices, consistent with the overall pricing strategy and after consideration of relevant market factors.
In general, the Company applies the following steps when recognizing revenue from contracts with customers: (i) identify the contract, (ii) identify the performance obligations, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations and (v) recognize revenue when a performance obligation is satisfied.
14
Recognition of revenue is driven by satisfaction of the performance obligations using one of two methods: revenue is either recognized over time or at a point in time. Contracts containing multiple performance obligations classify those performance obligations into separate units of accounting either as standalone or combined units of accounting. For those performance obligations treated as a standalone unit of accounting, revenue is generally recognized based on the method appropriate for each standalone unit. For those performance obligations treated as a combined unit of accounting, revenue is generally recognized as the performance obligations are satisfied, which generally occurs when control of the goods or services have been transferred to the customer or client or once the client or customer is able to direct the use of those goods and/or services as well as obtaining substantially all of its benefits. As such, revenue for a combined unit of accounting is generally recognized based on the method appropriate for the last delivered item but due to the specific nature of certain project and contract items, management may determine an alternative revenue recognition method as appropriate, such as a contract whereby one deliverable in the arrangement clearly comprises the overwhelming majority of the value of the overall combined unit of accounting. Under this circumstance, management may determine revenue recognition for the combined unit of accounting based on the revenue recognition guidance otherwise applicable to the predominant deliverable.
If a loss on a contract is anticipated, such loss is recognized in its entirety when the loss becomes evident. When the current estimates of the amount of consideration that is expected to be received in exchange for transferring promised goods or services to the customer indicates a loss will be incurred, a provision for the entire loss on the contract is made. At March 31, 2023 and June 30, 2022, the Company had no contract loss provisions.
Fixed-Fee
Under a fixed-fee contract, the Company charges a fixed agreed upon amount for a deliverable. Fixed-fee contracts have fixed deliverables upon completion of the project. Typically, the Company recognizes revenue for fixed-fee contracts after projects are completed, delivery is made and title transfers to the customer, and collection is reasonably assured.
Revenue can be recognized either 1) over time or 2) at a point in time. All revenue was recognized at a point in time for all periods presented.
For the three months ended March 31, 2022, revenue was recognized from a license agreement and for the nine months ended March 31, 2022, revenue was recognized from a license agreement and the settlement of a revenue contract. No revenue was recognized for all other periods presented.
Time and Materials
Under a time and materials contract, the Company charges customers an hourly rate plus reimbursement for other project specific costs. The Company recognizes revenue for time and material contracts based on the number of hours devoted to the project multiplied by the customer’s billing rate plus other project specific costs incurred.
Contract Assets
A contract asset is an entity’s right to payment for goods and services already transferred to a customer if that right to payment is conditional on something other than the passage of time. Generally, an entity will recognize a contract asset when it has fulfilled a contract obligation but must perform other obligations before being entitled to payment.
Contract assets consist primarily of the cost of project contract work performed by third parties for which the Company expects to recognize any related revenue at a later date, upon satisfaction of the contract obligations. At March 31, 2023 and June 30, 2022, contract assets were $0.
Contract Liabilities
A contract liability is an entity’s obligation to transfer goods or services to a customer at the earlier of (1) when the customer prepays consideration or (2) the time that the customer’s consideration is due for goods and services the entity will yet provide. Generally, an entity will recognize a contract liability when it receives a prepayment.
Contract liabilities consist primarily of consideration received, usually in the form of payment, on project work to be performed whereby the Company expects to recognize any related revenue at a later date, upon satisfaction of the contract obligations. At March 31, 2023, June 30, 2022, and June 30, 2021, contract liabilities were $0, $100,000 and $423,000, respectively. The Company recognized revenue of $53,000 and $100,000 during the three and nine months ended March 31, 2023, respectively, that was included in the contract liabilities balance as of June 30, 2022 and was reported in discontinued operations. The Company recognized revenue of $52,000 and $178,000 during the three and nine months ended March 31, 2022, respectively, that was included in the contract liabilities balance as of June 30, 2021 and was reported in discontinued operations. The Company recognized revenue of $0 and $84,000 during the three and nine months ended March 31, 2022 that was included in the contract liabilities balance as of June 30, 2021 and reported as part of continuing operations.
15
Leases
The Company accounts for leases under the guidance of Accounting Standards Codification ("ASC") 842, Leases ("ASC 842"). The standard established a right-of-use (“ROU”) model requiring a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months and classified as either an operating or finance lease. The adoption of ASC 842 had a significant effect on the Company’s balance sheet, resulting in an increase in non-current assets and both current and non-current liabilities.
In accordance with ASC 842, at the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present and the classification of the lease including whether the contract involves the use of a distinct identified asset, whether the Company obtains the right to substantially all the economic benefit from the use of the asset, and whether the Company has the right to direct the use of the asset. Leases with a term greater than one year are recognized on the balance sheet as ROU assets, lease liabilities and, if applicable, long-term lease liabilities. The Company has elected not to recognize on the balance sheet leases with terms of one year or less under practical expedient in paragraph ASC 842-20-25-2. For contracts with lease and non-lease components, the Company has elected not to allocate the contract consideration and to account for the lease and non-lease components as a single lease component.
The lease liabilities and the corresponding ROU assets are recorded based on the present value of lease payments over the expected remaining lease term. The implicit rate within the Company’s existing finance (capital) lease was determinable and, therefore, used at the adoption date of ASC 842 to determine the present value of lease payments under the finance lease. The implicit rate within the Company’s operating lease was not determinable and, therefore, the Company used the incremental borrowing rate at the lease commencement date to determine the present value of lease payments. The determination of the Company’s incremental borrowing rate requires judgment. The Company will determine the incremental borrowing rate for each new lease using its estimated borrowing rate.
An option to extend the lease is considered in connection with determining the ROU asset and lease liability when it is reasonably certain the Company will exercise that option. An option to terminate is considered unless it is reasonably certain the Company will not exercise the option.
Cash, Cash Equivalents and Restricted Cash
The Company considers all highly-liquid instruments purchased with an original maturity of three months or less to be cash equivalents. Cash equivalents at March 31, 2023 and June 30, 2022 consisted of money market accounts. Restricted cash consisted of $3.0 million held within a Company account at Woodforest Bank for the term note payable (see Note 6 – Significant Transactions and Note 13 - Debt), collateral for a letter of credit obtained related to the San Diego operating lease (see Note 15 – Operating Lease Obligations) and collateral for a Company purchasing card. The Company’s bank required an additional 5% collateral held above the actual letters of credit issued for the San Diego lease and Company purchasing card. Restricted cash was approximately $3.3 million at March 31, 2023 and $6.0 million on June 30, 2022. The reduction to the restricted cash occurred because on October 11, 2022, the Company, as part of the First Amendment to the Credit Agreement with Woodforest National Bank (“Woodforest”), paid down $5.5 million of the term loan and subsequently Woodforest cancelled the irrevocable letter of credit issued by JPMorgan Chase Bank upon closing of the amendment. In accordance with the Fourth Amendment with Woodforest, the Company deposited $3 million in a restricted account at Woodforest. (For a complete description of the transaction please see Note 6 – Significant Transactions and Note 13 - Debt).
The following table summarizes the components of total cash, cash equivalents and restricted cash in the condensed consolidated statements of cash flows (in thousands):
|
|
March 31, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
Cash and equivalents |
|
$ |
6,562 |
|
$ |
22,676 |
Collateral held for letter of credit - term note payable |
|
|
3,000 |
|
|
5,743 |
Collateral held for letter of credit - San Diego lease |
|
|
198 |
|
|
198 |
Collateral held for Company purchasing card |
|
|
55 |
|
|
55 |
Total cash, cash equivalents and restricted cash |
|
$ |
9,815 |
|
$ |
28,672 |
16
Investments in Debt Securities
Debt investments were classified as available-for-sale. Changes in fair value are recorded in other comprehensive income (loss). Fair value was calculated based on publicly available market information. Discounts and/or premiums paid when the debt securities were acquired are amortized to interest income over the terms of the debt securities. See Note 8 – Investments in Debt Securities.
Inventory
Inventory is stated at the lower of cost or net realizable value on the first-in, first-out basis. Inventory held is related to the CDMO business and has been classified as held for sale. See Note 3 – Discontinued Operations for information on inventory reserved reflected in the period ended March 31, 2023.
Research and Development
The Company accounts for research and development costs in accordance with the Financial Accounting Standards Board (“FASB”) ASC 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and development costs are expensed when the contracted work has been performed or as milestone results have been achieved. For the nine months ended March 31, 2023 and 2022, research and development expense was reported in both continuing and discontinued operations.
Right-of-Use Assets
Assets held under the terms of finance (capital) leases are amortized on a straight-line basis over the terms of the leases or the economic lives of the assets. Obligations for future lease payments under finance (capital) leases are shown within liabilities and are analyzed between amounts falling due within and after one year. See Note 9 – Finance Lease ROU Assets and Note 14 – Finance Lease Obligations for additional information.
Fixed Assets
Fixed assets are stated at cost net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets ranging from three to 39 years.
The Company monitors fixed assets for impairment indicators throughout the year. When necessary, charges for impairments of long-lived assets are recorded for the amount by which the fair value is less than the carrying value of these assets. Changes in the Company’s business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
See Note 11 – Fixed Assets for additional information.
Intangible Assets
Identifiable intangible assets are comprised of definite life intangible assets and indefinite life intangible assets.
The Company accounts for definite life intangible assets at either their historical cost or allocated purchase price at asset acquisition and records amortization utilizing the straight-line method based upon their estimated useful lives. Intellectual property is amortized over 20 years. The Company reviews the carrying value of its definite life intangible assets for impairment whenever events or changes in business circumstances indicate the carrying amount of such assets may not be fully recoverable. The carrying value is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. An impairment loss is measured as the amount by which the carrying amount exceeds it fair value.
For indefinite life intangible assets, the Company performs an impairment test annually and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. The Company determines the fair value of the asset quarterly or when triggering events are present, based on discounted cash flows and records an impairment loss if book value exceeds fair value.
Evaluating for impairment requires judgment, including the estimation of future cash flows, future growth rates and profitability and the expected life over which cash flows will occur. Changes in the Company’s business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
17
See Note 12 – Intangible Assets for additional information.
Share-based Compensation
The Company recognizes the cost of all share-based payment transactions at fair value. Compensation cost, measured by the fair value of the equity instruments issued, adjusted for estimated forfeitures, is recognized in the financial statements as the respective awards are earned over the performance or service period. The Company uses historical data to estimate forfeiture rates.
The impact that share-based payment awards will have on the Company’s results of operations is a function of the number of shares awarded, the trading price of the Company’s stock at the date of grant or modification, the vesting schedule and forfeitures. Furthermore, the application of the Black-Scholes option pricing model employs weighted-average assumptions for expected volatility of the Company’s stock, expected term until exercise of the options, the risk-free interest rate, and dividends, if any, to determine fair value.
Expected volatility is based on historical volatility of the Common Stock; the expected term until exercise represents the weighted-average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and the Company’s historical exercise patterns; and the risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected life of the option. The Company has not paid any dividends since its inception and does not anticipate paying any dividends for the foreseeable future, so the dividend yield is assumed to be zero. In addition, the Company estimates forfeitures at each reporting period, rather than electing to record the impact of such forfeitures as they occur. See Note 18 – Share-Based Compensation for additional information.
Concentrations of Credit Risk
Cash
The Company maintains principally all cash balances in two financial institutions which, at times, may exceed the insured amounts. The exposure to the Company is solely dependent upon daily balances and the strength of the financial institutions. The Company has not incurred any losses on these accounts. At March 31, 2023 and June 30, 2022, amounts in excess of insured limits were approximately $6,300,000 and $18,200,000, respectively.
Revenue
During the three months ended March 31, 2023, the Company reported no revenue from continuing operations and generated 100% of its revenue reported in discontinued operations from two customers. During the three months ended March 31, 2022, the Company reported $1.8 million of license revenue from continuing operations and generated $144,000 of revenue reported in discontinued operations from two customers.
During the nine months ended March 31, 2023, the Company reported no revenue from continuing operations and generated 100% of its revenue reported in discontinued operations from two customers. During the nine months ended March 31, 2022, the Company reported revenue from continuing operations related to a license agreement and a settlement of a revenue contract. The Company also generated $438,000 of revenue reported in discontinued operations from six customers.
Recently Issued Accounting Pronouncements
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires an entity to assess impairment of its financial instruments based on its estimate of expected credit losses. Since the issuance of ASU 2016-13, the FASB released several amendments to improve and clarify the implementation guidance. In November 2019, the FASB issued ASU 2019-10, Financial Instruments - Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842): Effective Dates, which amended the effective date of the various topics. As the Company is a smaller reporting company, the provisions of ASU 2016-13 and the related amendments are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2022 (quarter ending September 30, 2023, for the Company). Entities are required to apply these changes through a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is effective. The Company does not expect the adoption of ASU 2016-13 to have a significant impact on the Company’s consolidated financial statements.
Management does not believe that any other recently issued, but not yet effective, accounting standard if currently adopted would have a material effect on the accompanying condensed consolidated financial statements. Most of the newer standards issued represent technical corrections to the accounting literature or application to specific industries which have no effect on the Company’s condensed consolidated financial statements.
18
5. Financial Instruments and Fair Value Measurement
The carrying values of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and term note payable in the Company’s condensed consolidated balance sheets approximated their fair values as of March 31, 2023 and June 30, 2022 due to their short-term nature. The carrying value of the convertible promissory note receivable, the term note payable and finance lease obligation approximated to fair value as of March 31, 2023 and June 30, 2022 as the interest rates related to the financial instruments approximated to market value.
The Company accounts for its investments in debt securities at fair value. The following provides a description of the three levels of inputs that may be used to measure fair value under the standard, the types of investments that fall under each category, and the valuation methodologies used to measure these investments at fair value.
| • | Level 1 – Inputs are based upon unadjusted quoted prices for identical instruments in active markets. |
| • | Level 2 – Inputs to the valuation include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in inactive markets, inputs other than quoted prices that are observable for the asset or liability, and inputs that are derived principally from or corroborated by observable market data by correlation or other means. If the asset or liability has a specified (contractual) term, the Level 2 input must be observable for substantially the full term of the asset or liability. All debt securities were valued using Level 2 inputs. |
| • | Level 3 – Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company’s fixed assets and amortizable intangible assets are measured at fair value on a nonrecurring basis; that is, these assets are not measured at fair value on an ongoing basis, but are subject to fair value adjustments in certain circumstances, such as when there is evidence of impairment.
The Company initially marketed the CDMO business and during the second quarter of Fiscal 2023, changed its strategy to selling the stand-alone CDMO assets. These assets were assessed for impairment and the analysis resulted in the expected future cash flows from the sale of the property and equipment falling below its current carrying value. The Company utilized a market approach, using independent third-party appraisals, including comparable assets, in addition to bids received from prospective buyers, to estimate the fair value of the property and equipment. As a result, the carrying value of the building and equipment was reduced to their estimated fair values of $16,350,000 and $2,100,000, respectively. In the second quarter of Fiscal 2023, impairment charges were recorded in discontinued operations under general and administrative expense of $6,300,000 and $11,300,000 for the building and machinery and equipment, respectively. The machinery and equipment were sold during the third quarter of Fiscal 2023.
The following table shows the fair value of the Company's fixed assets included in Current Assets Held For Sale measured at fair value on a non-recurring basis as of March 31, 2023 (amounts in thousands):
|
|
|
March 31, 2023 |
||||||||||||
|
|
|
Fair Value Hierarchy |
||||||||||||
|
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Total Fair Value |
|
|
Total Impairments |
Building in Bryan, Texas |
|
$ |
— |
|
$ |
— |
|
$ |
16,364 |
|
$ |
16,364 |
|
$ |
6,300 |
During the second quarter of Fiscal 2023, the Company re-evaluated its business strategy and reviewed its product portfolio. After such review, the Company identified intellectual property, patent and licenses that would no longer be utilized and therefore were fully impaired (Level 3). See Note 12 – Intangible Assets for additional information.
19
6. Significant Transactions
Affiliates of Eastern Capital Limited
On November 1, 2021, the Company and its subsidiary, iBio CDMO (“iBio CDMO”, and collectively with the Company, the “Purchaser”) entered into a series of agreements (the “Transaction”) with College Station Investors LLC (“College Station”), and Bryan Capital Investors LLC (“Bryan Capital” and, collectively with College Station, “Seller”), each affiliates of Eastern Capital Limited (“Eastern,” a former significant stockholder of the Company) described in more detail below whereby in exchange for a certain cash payment and a warrant the Company:
| (i) | acquired both the Facility where iBio CDMO at that time and currently conducts business and also the rights as the tenant in the Facility’s ground lease; |
| (ii) | acquired all of the equity owned by one of the affiliates of Eastern in the Company and iBio CDMO; and |
| (iii) | otherwise terminated all agreements between the Company and the affiliates of Eastern. |
The Facility is a life sciences building located on land owned by the Board of Regents of the Texas A&M University System (“Texas A&M”) and is designed and equipped for the manufacture of plant-made biopharmaceuticals. iBio CDMO had held a sublease for the Facility through 2050, subject to extension until 2060 (the “Sublease”) until the purchase of the Facility described below.
The Purchase and Sale Agreement
On November 1, 2021, the Purchaser entered into a Purchase and Sale Agreement (the “Purchase and Sale Agreement”) with the Seller pursuant to which: (i) the Seller sold to Purchaser all of its rights, title and interest as the tenant in the Ground Lease Agreement (the “Ground Lease Agreement”) that it entered into with Texas A&M (the “Landlord’’) related to the property at which the Facility is located together with all improvements pertaining thereto (the “Property”), which previously had been the subject of the Sublease; (ii) the Seller sold to Purchaser all of its rights, title and interest to any tangible personal property owned by Seller and located on the Property including the Facility; (iii) the Seller sold to Purchaser all of its rights, title and interest to all licensed, permits and authorization for use of the Property; and (iv) College Station and iBio CDMO terminated the Sublease. The total purchase price for the Property, the termination of the Sublease and other agreements among the parties, and the equity described below is $28,750,000, which was paid $28,000,000 in cash and by the issuance to Seller of warrants (the “Warrant”) described below. As part of the transaction, iBio CDMO became the tenant under the Ground Lease Agreement for the Property until 2060 upon exercise of available extensions. The base rent payable under the Ground Lease Agreement, which was $151,450 for the current year, is 6.5% of the Fair Market Value (as defined in the Ground Lease Agreement) of the Property. The Ground Lease Agreement includes various covenants, indemnities, defaults, termination rights, and other provisions customary for lease transactions of this nature.
As discussed above, iBio CDMO is being accounted for as a discontinued operation. As such, the assets acquired and/or leased are now classified as assets held for sale on the March 31, 2023 and June 30, 2022 condensed consolidated balance sheets.
The Equity Purchase Agreement
The Company also entered into an Equity Purchase Agreement with Bryan Capital on November 1, 2021 (the “Equity Purchase Agreement”) pursuant to which the Company acquired for $50,000 cash, plus the Warrant, the one (1) share of iBio CMO Preferred Tracking Stock and the 0.01% interest in iBio CDMO owned by Bryan Capital. As a result, iBio CDMO is now a wholly-owned subsidiary of the Company.
The Credit Agreement
In connection with the Purchase and Sale Agreement, iBio CDMO entered into a Credit Agreement, dated November 1, 2021, with Woodforest pursuant to which Woodforest provided iBio CDMO a $22,375,000 secured term loan to purchase the Facility, which Term Loan is evidenced by a term note. The term loan was advanced in full on the closing date.
See Note 13 – Debt for further information of the Term Loan.
20
The Warrant
As part of the consideration for the purchase and sale of the rights set forth above, the Company issued to Bryan Capital a Warrant to purchase 51,583 shares of the Common Stock at an exercise price of $33.25 per share. The Warrant expires October 10, 2026, is exercisable immediately, provides for a cashless exercise at any time and automatic cashless exercise on the expiration date if on such date the exercise price of the Warrant exceeds its fair market value as determined in accordance with the terms of the Warrant and adjustments in the case of stock dividends and stock splits. Of the shares issued under the Warrant, 11,583, which were originally valued at $217,255, reflected the final payment of rent due under the Sublease. The Warrant, as shown on the condensed consolidated statements of equity, was recorded in additional paid-in capital with the corresponding activity included in the basis of the purchase price allocation of the Property acquired. See Note 16 – Stockholders’ Equity for additional information.
RubrYc
On August 23, 2021, the Company entered into a series of agreements with RubrYc Therapeutics, Inc. (“RubrYc”) described in more detail below:
Collaboration and License Agreement
The Company entered into a collaboration and licensing agreement (the “RTX-003 License Agreement”) with RubrYc to further develop RubrYc’s immune-oncology antibodies in its RTX-003 campaign. Under the terms of the agreement, the Company is solely responsible for worldwide research and development activities for development of the RTX-003 antibodies for use in pharmaceutical products in all fields. RubrYc was also entitled to receive royalties in the mid-single digits on net sales of RTX-003 antibodies, subject to adjustment under certain circumstances. The RTX-003 License Agreement was terminated when the Company acquired substantially all of the assets of RubrYc in September 2022.
Collaboration, Option and License Agreement
The Company entered into an agreement with RubrYc (the “Collaboration, Option and License Agreement”) to collaborate for up to five years to discover and develop novel antibody therapeutics using RubrYc’s artificial intelligence discovery platform. The Company agreed to pay RubrYc for each Selected Compound as it achieves various milestones in addition to royalties if the Selected Compounds are commercialized. RubrYc was also entitled to receive tiered royalties ranging from low- to mid-single digits on net sales of Collaboration Products, subject to adjustment under certain circumstances. Royalties are payable on a country-by-country and collaboration product-by-collaboration product basis until the latest to occur of: (i) the last-to-expire of specified patent rights in such country; (ii) expiration of marketing or regulatory exclusivity in such country; or (iii) ten (10) years after the first commercial sale of a product in such country, provided that no biosimilar product has been approved in such country. With the exception of any obligations that survive the termination, the Collaboration, Option and License Agreement was terminated when the Company acquired substantially all of the assets of RubrYc in September 2022.
Stock Purchase Agreement
In connection with the entry into the Collaboration, Option and License Agreement and RTX-003 License Agreement, the Company also entered into a Stock Purchase Agreement (“Stock Purchase Agreement”) with RubrYc whereby the Company purchased a total of 2,864,345 shares of RubrYc’s Series A-2 preferred stock (“Series A-2 Preferred”) for $7,500,000.
The Company accounted for the agreements as an asset purchase and allocated the purchase price of $7,500,000 as follows:
Preferred stock |
|
$ |
1,760,000 |
Intangible assets |
|
|
4,300,000 |
Prepaid expenses |
|
|
1,440,000 |
|
|
$ |
7,500,000 |
Subsequently after the Company acquired substantially all of the assets of RubrYc in September 2022, RubrYc ceased its operations, and accordingly, the Company recorded an impairment of the investment in the amount of $1,760,000 during the year ended June 30, 2022. The amount was recorded in the condensed consolidated statement of operations and comprehensive loss under general and administrative expense. The Company also recorded an impairment of current and non-current prepaid expense of $288,000 and $864,000, respectively, during the year ended June 30, 2022. The amount was recorded in the condensed consolidated statement of operations and comprehensive loss under research and development expense.
21
On September 16, 2022, the Company entered an Asset Purchase Agreement with RubrYc pursuant to which it acquired substantially all of the assets of RubrYc. The Company issued 102,354 shares of the Common Stock to RubrYc with an approximate market value of $1,000,000 (the “Closing Shares”). Pursuant to the Asset Purchase Agreement, the shares are subject to an initial lockup period and the estimated fair value was calculated as $650,000. The Company also agreed to make potential additional payments of up to $5,000,000 upon the achievement of specified developmental milestones on or before the fifth anniversary of the closing date, payable in cash or shares of the Common Stock, at the Company’s option. In addition, the Company had advanced RubrYc $484,000 to support their operation costs during the negotiation period and incurred transaction costs totaling $208,000, which were also capitalized as part of the assets acquired. The assets acquired include the patented AI drug discovery platform, all rights with no future milestone payments or royalty obligations, to IBIO-101, in addition to CCR8, EGFRvIII, MUC16, CD3 and one additional immuno-oncology candidate plus a PD-1 agonist. The Purchase Agreement contained representations, warranties and covenants of RubrYc and the Company. The acquisition closed on September 19, 2022 after receipt of approval of the NYSE American.
The Company accounted for the agreements as an asset purchase and allocated the purchase price of approximately $1,342,000 as follows:
Intangible assets |
|
$ |
1,228,000 |
Fixed assets |
|
|
114,000 |
|
|
$ |
1,342,000 |
In addition, the Company assumed three equipment leases that were accounted for as finance leases totaling approximately $814,000. See Note 9 – Finance Lease ROU Assets and Note 14 – Finance Lease Obligations.
Former CEO Departure
Effective December 1, 2022, the Company and Mr. Thomas F. Isett, the former Chief Executive Officer (the “CEO) and former member of the Board of Directors (the “Board”), agreed for Mr. Isett to resign as a member of the Board and relinquish his duties, rights and obligations as the CEO of the Company.
Separation Agreement and General Release
In connection with Mr. Isett’s resignation, the Company entered into a separation agreement and general release with Mr. Isett effective December 1, 2022 (the “Agreement”). Pursuant to the Agreement, Mr. Isett resigned as CEO of the Company effective December 1, 2022, and will remain an employee of the Company until December 31, 2022, on which date his employment with the Company will automatically terminate. Following Mr. Isett’s termination of employment with the Company, pursuant to the Agreement, Mr. Isett will receive the severance benefits set forth in his employment agreement, as previously disclosed by the Company, including (i) an amount equal to his current base salary in equal bi-monthly installments for twenty-four (24) months; (ii) an amount equal to a pro rata share of his target bonus for the current fiscal year; (iii) an amount equal to the target bonus in equal bi-monthly installments for the twenty-four (24) month severance period and (iv) provided that he elects continuation coverage for health insurance under the Consolidated Omnibus Budget Reconciliation Act of 1985 (“COBRA”), the Company will pay the full cost of this benefit for up to eighteen (18) months, or if he has not obtained alternative employer-provided health coverage by the end of the eighteen (18) month COBRA subsidy period, the Company will provide him with a lump-sum cash payment equal to six (6) times the monthly amount paid by the Company for the COBRA subsidy. The Agreement includes a general release of claims by Mr. Isett. The Company accrued approximately $2.13 million to general and administrative expenses in the second quarter of Fiscal 2023. As of March 31, 2023, approximately $1.1 million is recorded in accrued expenses and $791,000 in accrued expenses – noncurrent.
7. Convertible Promissory Note Receivable
On October 1, 2020, the Company entered into a master services agreement with Safi Biosolutions, Inc. (“Safi”). In addition, the Company invested $1.5 million in Safi in the form of a convertible promissory note (the "Note"). The Note bears interest at the rate of 5% per annum and is convertible into shares of Safi’s common stock (as defined). Principal and accrued interest mature on October 1, 2023. For the three months ended March 31, 2023 and 2022, interest income amounted to $18,000. For the nine months ended March 31, 2023 and 2022, interest income amounted to $56,000. As of March 31, 2023 and June 30, 2022, the Note balance and accrued interest totaled $1,687,000 and $1,631,000, respectively.
The Company is currently renegotiating the terms of the Note with Safi, which are expected to be finalized in the fourth quarter of Fiscal 2023. Proceeds expected to be received in the next twelve months are approximately $912,000 and accordingly, $775,000 has been classified to long term.
22
8. Investments in Debt Securities
The Company did not hold any investments in debt securities at March 31, 2023. The components of investments in debt securities are as follows (in thousands):
|
|
March 31, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
Adjusted cost |
|
$ |
— |
|
$ |
11,029 |
Gross unrealized losses |
|
|
— |
|
|
(184) |
Fair value |
|
$ |
— |
|
$ |
10,845 |
The fair value of available-for-sale debt securities, by contractual maturity, was as follows (in thousands):
|
|
March 31, |
|
June 30, |
||
Fiscal period ending: |
|
2023 |
|
2022 |
||
2023 |
|
$ |
— |
|
$ |
8,054 |
2024 |
|
|
— |
|
|
2,791 |
|
|
$ |
— |
|
$ |
10,845 |
Amortization of premiums paid on the debt securities amounted to $7,000 and $74,000 for the three months ended March 31, 2023 and 2022, respectively, and $67,000 and $269,000 for the nine months ended March 31, 2023 and 2022, respectively.
Realized gains on available-for-sale debt securities are as follows (in thousands):
|
|
Three Months Ended |
|
Three Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Proceeds from sale of debt securities |
|
$ |
5,943 |
|
$ |
— |
Cost of debt securities |
|
|
6,036 |
|
|
— |
Realized loss on sale of debt securities |
|
$ |
(93) |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended |
|
Nine Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Proceeds from sale of debt securities |
|
$ |
6,739 |
|
$ |
— |
Cost of debt securities |
|
|
6,837 |
|
|
— |
Realized loss on sale of debt securities |
|
$ |
(98) |
|
$ |
— |
9. Finance Lease ROU Assets
As discussed above, the Company assumed three equipment leases as part of the RubrYc asset acquisition. In addition, the Company leased a mobile office trailer which is classified as part of assets held for sale. The lease was terminated in December 2022.
See Note 14 – Finance Lease Obligations for more details of the terms of the leases.
23
The following table summarizes by category the gross carrying value and accumulated amortization of finance lease ROU (in thousands):
|
|
March 31, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
ROU - Equipment |
|
$ |
814 |
|
$ |
— |
Accumulated amortization |
|
|
(136) |
|
|
— |
Net finance lease ROU |
|
$ |
678 |
|
$ |
— |
Amortization of finance lease ROU assets was approximately $68,000 and $0 for the three months ended March 31, 2023 and 2022, respectively, and $136,000 and $0 for the nine months ended March 31, 2023 and 2022, respectively.
10. Operating Lease ROU Assets
San Diego, California
On September 10, 2021, the Company entered into a lease for approximately 11,383 square feet of space in San Diego, California. Based on the terms of the lease payments, the Company recorded an operating lease ROU asset of $3,603,000. The net carrying amount of this ROU operating lease asset was $2,798,000 and $3,068,000 at March 31, 2023 and June 30, 2022, respectively.
Bryan, Texas
On November 1, 2021, as discussed above, iBio CDMO acquired the Facility and became the tenant under the ground lease for the Property upon which the Facility is located. Based on the terms of the lease payments, the Company recorded an operating lease ROU asset of $1,967,000. The net amount of this ROU operating lease asset is included in assets held for sale.
See Note 15 - Operating Lease Obligation for additional information.
11. Fixed Assets
The following table summarizes by category the gross carrying value and accumulated depreciation of fixed assets (in thousands):
|
|
March 31, |
|
June 30, |
||
|
|
2023 |
|
2022 |
||
|
|
|
|
|
|
|
Building and improvements |
|
$ |
695 |
|
$ |
— |
Machinery and equipment |
|
|
3,461 |
|
|
— |
Office equipment and software |
|
|
403 |
|
|
— |
Construction in progress |
|
|
34 |
|
|
1,373 |
|
|
|
4,593 |
|
|
1,373 |
Accumulated depreciation |
|
|
(235) |
|
|
— |
Net fixed assets |
|
$ |
4,358 |
|
$ |
1,373 |
Depreciation expense reported in continuing operations was approximately $120,000 and $235,000 for the three and nine months ended March 31, 2023, respectively, and $0 for both the three and nine months ended March 31, 2022.
Fixed assets held for sale at March 31, 2023 and June 30, 2022 in the amount of $16,424,000 and $35,289,000, respectively, are included in assets held for sale. The depreciation expense for the three months ended March 31, 2023 and 2022 and the nine months ended March 31, 2023 and 2022 is classified as part of loss from discontinued operations.
During the third quarter of Fiscal 2023, the Company re-evaluated its business strategy and reviewed its product portfolio. After such review, the Company recorded an impairment charge of approximately $17.6 million for the three and nine months ended March 31, 2023, respectively.
See Note 5 - Financial Instruments and Fair Value Measurement for more information.
24
12. Intangible Assets
The Company has two categories of intangible assets – intellectual property and patents. Intellectual property consists of all technology, know-how, data, and protocols for producing targeted proteins in plants and related to any products and product formulations for pharmaceutical uses and for other applications. Intellectual property includes, but is not limited to, certain technology for the development and manufacture of novel vaccines and therapeutics for humans and certain veterinary applications acquired in December 2003 from Fraunhofer USA Inc., acting through its Center for Molecular Biotechnology (“Fraunhofer”), pursuant to a Technology Transfer Agreement, as amended (the “TTA”). The Company designates such technology further developed and acquired from Fraunhofer as iBioLaunchTM or LicKMTM or FastPharming Technology. The value on the Company’s books attributed to patents owned or controlled by the Company is based only on payments for services and fees related to the protection of the Company’s patent portfolio. The intellectual property also includes certain trademarks.
On August 23, 2021, the Company entered into a series of agreements with RubrYc described in more detail above (see Note 6 – Significant Transactions) whereby in exchange for a $7.5 million investment in RubrYc, the Company acquired a worldwide exclusive license to certain antibodies that RubrYc develops under what it calls its RTX-003 campaign, which are promising immuno-oncology antibodies that bind to the CD25 protein without interfering with the IL-2 signaling pathway thereby potentially depleting T-regulatory (Tregs) cells while enhancing T effector (Teffs) cells and encouraging the immune system to attack cancer cells. The Company accounted for this license as an indefinite-lived intangible asset until the completion or abandonment of the associated research and development efforts. In addition, the Company also received preferred shares and an option for future collaboration licenses.
On September 16, 2022, the Company entered into an Asset Purchase Agreement with RubrYc described in more detail above (see Note 6 – Significant Transactions) pursuant to which it acquired substantially all of the assets of RubrYc. The assets acquired include the patented AI drug discovery platform, all rights with no future milestone payments or royalty obligations, to IBIO-101, in addition to CCR8, EGFRvIII, MUC16, CD3, and one additional immuno-oncology candidate, plus a PD-1 agonist.
In January 2014, the Company entered into a license agreement with the University of Pittsburgh whereby the Company acquired exclusive worldwide rights to certain issued and pending patents covering specific candidate products for the treatment of fibrosis (the "Licensed Technology") which license agreement was amended in August 2016 and again in December 2020 and February 2022. The license agreement provides for payment by the Company of a license issue fee, annual license maintenance fees, reimbursement of prior patent costs incurred by the university, payment of a milestone payment upon regulatory approval for sale of a first product, and annual royalties on product sales. In addition, the Company has agreed to meet certain diligence milestones related to product development benchmarks. As part of its commitment to the diligence milestones, the Company successfully commenced production of a plant-made peptide comprising the Licensed Technology before March 31, 2014. The next milestone – filing an Investigational New Drug Application with the FDA or foreign equivalent covering the Licensed Technology ("IND") – initially was required to be met by December 1, 2015, and on November 2, 2020, was extended to be required to be met by December 31, 2021 and on February 8, 2022, was further extended to December 31, 2023. In addition, the amounts of the annual license maintenance fee and payment upon completion of various regulatory milestones were amended. On February 14, 2023, the Company provided notification to the University of Pittsburgh terminating the license agreement. Pursuant to the termination of the license agreement with the University of Pittsburgh, the Company’s financial obligations for the management of the patents under the license will cease on August 14, 2023, and at such time, will transition back to the University of Pittsburgh. As a result of the termination of the license agreement, the Company recorded a full impairment of the related intangible asset associated with IBIO-100 in the amount of $25,000.
See Note 4 - Summary of Significant Accounting Policies for more information.
The following table reflects changes in the carrying amount of intangible assets (in thousands):
|
|
June 30, |
|
|
|
|
|
|
|
March 31, |
|||||
|
|
2022 |
|
Amortization |
|
Additions |
|
Impairments |
|
2023 |
|||||
Intellectual property – gross carrying value |
|
$ |
3,100 |
|
$ |
— |
|
$ |
400 |
|
$ |
(3,100) |
|
$ |
400 |
Patents and licenses – gross carrying value |
|
|
2,846 |
|
|
— |
|
|
— |
|
|
(2,846) |
|
|
— |
|
|
|
5,946 |
|
|
— |
|
|
400 |
|
|
(5,946) |
|
|
400 |
Intellectual property – accumulated amortization |
|
|
(2,867) |
|
|
(74) |
|
|
— |
|
|
2,931 |
|
|
(10) |
Patents and licenses – accumulated amortization |
|
|
(2,403) |
|
|
(47) |
|
|
— |
|
|
2,450 |
|
|
— |
|
|
|
(5,270) |
|
|
(121) |
|
|
— |
|
|
5,381 |
|
|
(10) |
Total definite lived intangible assets |
|
$ |
676 |
|
$ |
(121) |
|
$ |
400 |
|
$ |
(565) |
|
$ |
390 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License - indefinite lived |
|
$ |
4,175 |
|
|
— |
|
$ |
828 |
|
|
— |
|
$ |
5,003 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net intangible |
|
$ |
4,851 |
|
|
|
|
$ |
1,228 |
|
|
|
|
$ |
5,393 |
25
Amortization expense was approximately $5,000 and $122,000 for the three months ended March 31, 2023 and 2022, respectively. Amortization expense was approximately $121,000 and $333,000 for the nine months ended March 31, 2023 and 2022, respectively.
During the second quarter of Fiscal 2023, the Company re-evaluated its business strategy and reviewed its product portfolio. After such review, the Company identified intellectual property, patent and licenses that will no longer be utilized and therefore were fully impaired. The Company recorded an impairment charge in general and administrative expenses of approximately $565,000 for the nine months ended March 31, 2023.
See Note 4 - Summary of Significant Accounting Policies and Note 5 – Financial Instruments and Fair Value Measurement for more information.
13. Debt
The Credit Agreement
In connection with the Purchase and Sale Agreement, iBio CDMO entered into a Credit Agreement, dated November 1, 2021, with Woodforest pursuant to which Woodforest provided iBio CDMO a $22,375,000 secured term loan (the “Term Loan”) to purchase the Facility, which Term Loan is evidenced by a term note (the “Term Note”) (for a complete description of the transaction please see Note 6 – Significant Transactions). The Term Loan was advanced in full on the closing date. The Term Loan bears interest at a rate of 3.25%, with higher interest rates upon an event of default, which interest is payable monthly beginning November 5, 2021. Principal on the Term Loan was originally payable on November 1, 2023, subject to early termination upon events of default. The Term Loan provides that it may be prepaid by iBio CDMO at any time and provides for mandatory prepayment upon certain circumstances.
On October 11, 2022, iBio CDMO and Woodforest amended the Credit Agreement to: (i) include a payment of $5,500,000 of the outstanding principal balance owed under the Credit Agreement on the date of the amendment, (ii) include a payment of $5,100,000 of the outstanding principal balance owed under the Credit Agreement within two (2) business days upon our receipt of such amount owed to us by Fraunhofer as part of our legal settlement with them (the “Fraunhofer Settlement Funds”) (see Note 19 – Fraunhofer Settlement for more information), (iii) include principal payments of $250,000 per month in debt amortization for a six-month period commencing the date of the amendment through March 2023, (iv) include an amendment fee of $22,375 and all costs and expenses, (v) require delivery of a report detailing cash flow expenditures every two (2) weeks for the period prior to the delivery of the last report and a monthly 12-month forecast, (vi) reduce the liquidity covenant (the “Liquidity Covenant”) in the Guaranty (as defined in the Credit Agreement) from $10 million to $7.5 million with the ability to lower the liquidity covenant to $5.0 million upon the occurrence of a specific milestone in the Credit Agreement, and (vii) change the annual filing requirement solely for the fiscal year ended June 30, 2022, such that the filing is acceptable with or without a “going concern” designation. In addition, Woodforest cancelled the irrevocable letter of credit issued by JPMorgan Chase Bank upon closing of the amendment.
In January 2023, the Company’s unrestricted cash decreased below the required $7,500,000, which created an event of default under the Credit Agreement and Guaranty as a result of not complying with the Liquidity Covenant. As a result, on February 9, 2023, iBio CDMO and Woodforest entered into a second amendment to the Credit Agreement (the “Second Amendment”), which amended, among other things, added a milestone that had to be met by a specified date, the failure of which would be an event of default. In addition, on February 9, 2023, the Company, as guarantor, entered into a second amendment to the Guaranty, which amended, among other things, allowed the Company to account for the Fraunhofer Settlement Funds in determining whether the Company is in compliance with the Liquidity Covenant until a specified period dependent upon the occurrence of a specific milestone in the Credit Agreement.
On February 20, 2023, iBio CDMO entered into a third amendment to the Credit Agreement (the “Third Amendment”), which removed the added milestone specified in the Second Amendment, the failure of which would be an event of default. In addition, the Guaranty was amended to allow the Company until February 28, 2023, to account for the Fraunhofer Settlement Funds in determining whether the Company is in compliance with the Liquidity Covenant without being dependent upon a specified milestone. In addition, the Company agreed that each time it consummates an at-the-market issuance of Equity Interests (as defined within the Credit Agreement), no later than five (5) days following such issuance of Equity Interests, it will (i) pay to Woodforest in immediately available cash funds, without setoff or counterclaim of any kind, forty percent (40%) of the Net Proceeds (as defined within the Credit Agreement) received by the Company for such issuance of Equity Interests; provided, any such payment would cease upon payment obligations in full and (ii) provide Woodforest with a detailed accounting of each such issuance of Equity Interests.
On March 24, 2023, iBio CDMO and Woodforest entered into a fourth amendment to the Credit Agreement (the “Fourth Amendment”), which within the Fourth Amendment Woodforest agreed to (i) reduce the percentage of any payment to Woodforest the Company is required to make from the proceeds of sales of its common stock under its at-the-market facility from 40% to 20%, (ii) reduce the percentage of any payment to Woodforest the Company is required to make from the proceeds of sales of its equipment from 40% to 20%, and (iii) allowed the Company to retain $2,000,000 million of the $5,100,000 million that the Company received from the Fraunhofer Settlement Funds, with the remaining $3,000,000 million being held in a Company account at Woodforest.
26
In addition, the Company is obligated to (y) deliver to Woodforest an executed copy of a purchase agreement (the “Purchase Agreement”) for the sale of the Facility, no later than April 14, 2023, and (z) pay to Woodforest a fee in the amount of $75,000 on the earlier of the date of the closing of the Purchase Agreement, or the Maturity Date (as defined in the Credit Agreement). In addition, on March 24, 2023, the Company, as guarantor, entered into a fourth amendment to the Guaranty, which reduced the Liquidity Covenant from $7,500,000 to $1,000,000.
On May 10, 2023, iBio CDMO and Woodforest entered into a fifth amendment to the Credit Agreement (the “Fifth Amendment”), which within the Fifth Amendment Woodforest agreed to: (i) waive our obligation to deliver to Woodforest an executed copy of a Purchase Agreement for the sale of the Facility no later than April 14, 2023 and, (ii) release $500,000 of the $3.0 million being held in a Company account at Woodforest when the outstanding principal amount is reduced to $10.0 million and for each additional $2.5 million reduction of the outstanding principal amount, an additional $750,00 will be released from the Company account at Woodforest. In addition, starting on the effective date of the Fifth Amendment, the interest on the Term Loan increased to 5.25%, and the Term Loan shall further accrue interest, payable in kind and added to the balance of the outstanding principal amount at a fixed rate per annum equal to (a) 1.00%, if the Facility is sold on or before June 30, 2023, (b) 2.00% if the Facility is sold after June 2023, but on or before September 30, 2023, or (c) 3:00%, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. The Company also agreed to pay Woodforest a fee in the amount of (x) $75,000 if the Facility is sold on or before June 30, 2023, (y) $100,000 if the Facility is sold after June 2023, but on or before September 30, 2023, or (x) $125,000, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. As of the date of the filing of this Quarterly Report on Form 10-Q, the Company is not in negotiations on a Purchase Agreement with a potential buyer of the Facility.
At March 31, 2023, the balance of the Term Loan was $13,700,000 which consisted of the Term Note of $13,852,000, net of approximately $152,000 of deferred finance costs. At June 30, 2022, the balance was $22,161,000 which consisted of the Term Note of $22,375,000, net of approximately $214,000 of deferred finance costs.
Equipment Financing
On October 12, 2022, the Company entered into an equipment financing master lease agreement and a lease supplement whereby $500,000 was borrowed over 36 months at an imputed interest rate of 10.62% and securitized by certain assets purchased for the San Diego research site. The financing is payable in monthly installments of $16,230 through October 2025. At March 31, 2023, the balance owed under the financing was $438,000. Interest incurred under the financing for the three and nine months ended March 31, 2023 totaled approximately $12,000 and $19,000, respectively.
Future minimum payments under the finance lease obligation are due as follows (in thousands):
Fiscal period ending on March 31: |
|
Principal |
|
Interest |
|
Total |
|||
2024 |
|
$ |
156 |
|
$ |
39 |
|
$ |
195 |
2025 |
|
|
173 |
|
|
22 |
|
|
195 |
2026 |
|
|
110 |
|
|
4 |
|
|
114 |
|
|
|
|
|
|
|
|
|
|
Total minimum equipment financing payments |
|
|
439 |
|
$ |
65 |
|
$ |
504 |
Less: current portion |
|
|
(156) |
|
|
|
|
|
|
Long-term portion of minimum equipment financing obligation |
|
$ |
283 |
|
|
|
|
|
|
Note Payable – PPP Loan
On April 16, 2020, the Company received $600,000 related to its filing under the Paycheck Protection Program (“PPP”) and Coronavirus Aid, Relief, and Economic Security Act (the "CARES Act"). The Company elected to treat the Small Business Administration (“SBA”) loans as debt under ASC 470, Debt.
On July 21, 2021, iBio was granted forgiveness in repaying the loan. In accordance with ASC 405-20-40, Liabilities - Extinguishments of Liabilities – Derecognition, the Company derecognized the liability and accrued interest of approximately $7,000 in the first quarter of Fiscal 2022. The forgiveness is included in loss from discontinued operations.
See Note 3 – Discontinued Operations.
27
14. Finance Lease Obligations
Sublease
As discussed above, until November 1, 2021, iBio CDMO leased the Facility as well as certain equipment from College Station under the Sublease.
The Sublease was terminated on November 1, 2021, when iBio CDMO acquired the Facility and became the tenant under the ground lease for the property upon which the Facility is located. See Note 15 – Operating Lease Obligations for additional information related to the ground lease.
General and administrative expenses related to College Station, including rent related to the increases in the Consumer Price Index (“CPI”) and real estate taxes, were approximately $0 and $250,000 for the three and nine months ended March 31, 2022, respectively. Interest expense related to College Station was approximately $0 and $810,000 for the three and nine months ended March 31, 2022, respectively. Such expenses were classified as part of loss from discontinued operations.
Equipment
As discussed above, the Company assumed three equipment leases that were accounted for as finance leases totaling $813,822 as part of the RubrYc Asset Purchase Agreement. The monthly rental for the three leases is approximately $14,000 per month and all three expire on August 1, 2025.
Mobile Office Trailer
Commencing April 1, 2021, the Company leased a mobile office trailer that was located at the Facility in Bryan, Texas, at a monthly rental of $3,819 through March 31, 2024. In December 2022, the Company terminated the lease and returned the mobile office trailer. Expenses related to the lease prior to its termination are included in discontinued operations.
The following tables present the components of lease expense and supplemental balance sheet information related to the finance lease obligation (in thousands).
|
|
Three Months Ended |
|
Three Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Finance lease cost: |
|
|
|
|
|
|
Amortization of ROU assets |
|
$ |
68 |
|
$ |
— |
Interest on lease liabilities |
|
|
16 |
|
|
— |
Total lease cost |
|
$ |
84 |
|
$ |
— |
|
|
|
|
|
|
|
Other information: |
|
|
|
|
|
|
Cash paid for amounts included in the measurement lease liabilities: |
|
|
|
|
|
|
Operating cash flows from finance lease |
|
$ |
— |
|
$ |
— |
Financing cash flows from finance lease obligations |
|
$ |
62 |
|
$ |
— |
|
|
Nine Months Ended |
|
Nine Months Ended |
||
|
|
March 31, |
|
March 31, |
||
|
|
2023 |
|
2022 |
||
Finance lease cost: |
|
|
|
|
|
|
Amortization of ROU assets |
|
$ |
156 |
|
$ |
— |
Interest on lease liabilities |
|
|
33 |
|
|
— |
Total lease cost |
|
$ |
189 |
|
$ |
— |
|
|
|
|
|
|
|
Other information: |
|
|
|
|
|
|
Cash paid for amounts included in the measurement lease liabilities: |
|
|
|
|
|
|
Operating cash flows from finance lease |
|
$ |
— |
|
$ |
— |
Financing cash flows from finance lease obligations |
|
$ |
144 |
|
$ |
— |
28
|
|
March 31, |
|
June 30, |
||||
|
|
2023 |
|
2023 |
||||
Finance lease ROU assets |
|
$ |
678 |
|
|
$ |
— |
|
Finance lease obligation - current portion |
|
$ |
265 |
|
|
$ |
— |
|
Finance lease obligation - noncurrent portion |
|
$ |
421 |
|
|
$ |
— |
|
Weighted average remaining lease term - finance lease |
|
|
2.34 |
years |
|
|
— |
years |
Weighted average discount rate - finance lease obligation |
|
|
9.50 |
% |
|
|
— |
% |
Future minimum payments under the finance lease obligation are as follows (in thousands):
Fiscal period ending on March 31: |
|
Principal |
|
Interest |
|
Total |
|||
2024 |
|
$ |
265 |
|
$ |
54 |
|
$ |
319 |
2025 |
|
|
292 |
|
|
28 |
|
|
320 |
2026 |
|
|
130 |
|
|
3 |
|
|
133 |
|
|
|
|
|
|
|
|
|
|
Total minimum lease payments |
|
|
687 |
|
$ |
85 |
|
$ |
772 |
Less: current portion |
|
|
(265) |
|
|
|
|
|
|
Long-term portion of minimum lease obligations |
|
$ |
422 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15. Operating Lease Obligations
Texas Ground Lease
As discussed above, as part of the Transaction, iBio CDMO became the tenant under the Ground Lease Agreement for the Property until 2060 upon exercise of available extensions. The base rent payable under the Ground Lease Agreement, which was $151,450 for the prior year, is 6.5% of the Fair Market Value (as defined in the Ground Lease Agreement) of the Property. The Ground Lease Agreement includes various covenants, indemnities, defaults, termination rights, and other provisions customary for lease transactions of this nature.
San Diego
On September 10, 2021, the Company entered into a lease for approximately 11,383 square feet of space in San Diego, California. Terms of the lease include the following:
| ● | The length of term of the lease is 88 months from the lease commencement date (as defined). |
| ● | The lease commencement date was estimated to be on or around January 1, 2022. |
| ● | The monthly rent for the first year of the lease is $51,223 and increases approximately 3% per year. |
| ● | The lease provides for a base rent abatement for months two through five in the first year of the lease. |
| ● | The landlord is providing a tenant improvement allowance of $81,860 to be used for improvements as specified in the lease. |
| ● | The Company is responsible for other expenses such as electric, janitorial, etc. |
| ● | The Company opened an irrevocable letter of credit in the amount of $188,844 in favor of the landlord. The letter of credit expires on October 8, 2023 and renews annually as required. |
As discussed above, the lease provides for scheduled increases in base rent and scheduled rent abatements. Rent expense is charged to operations using the straight-line method over the term of the lease which results in rent expense being charged to operations at inception of the lease in excess of required lease payments. This excess (formerly classified as deferred rent) is shown as a reduction of the operating lease ROU asset in the accompanying balance sheet. As the Company has already started making improvements to the facility, the rent expense will be recognized.
29
The following tables present the components of lease expense and supplemental balance sheet information related to the operating lease obligation (in thousands).
|
Three Months Ended |
|
Three Months Ended |
||
|
March 31, |
|
March 31, |
||
|
2023 |
|
2022 |
||
Operating lease cost: |
$ |
141 |
|
$ |
141 |
Total lease cost |
$ |
141 |
|
$ |
141 |
|
|
|
|
|
|
Other information: |
|
|
|
|
|
Cash paid for amounts included in the measurement lease liability: |
|
|
|
|
|
Operating cash flows from operating lease |
$ |
141 |
|
$ |
141 |
Operating cash flows from operating lease obligation |
$ |
103 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended |
|
Nine Months Ended |
||
|
March 31, |
|
March 31, |
||
|
2023 |
|
2022 |
||
Operating lease cost: |
$ |
422 |
|
$ |
313 |
Total lease cost |
$ |
422 |
|
$ |
313 |
|
|
|
|
|
|
Other information: |
|
|
|
|
|
Cash paid for amounts included in the measurement lease liability: |
|
|
|
|
|
Operating cash flows from operating lease |
$ |
422 |
|
$ |
313 |
Operating cash flows from operating lease obligation |
$ |
154 |
|
$ |
— |
Future minimum payments under the operating lease obligation are as follows (in thousands):
Fiscal period ending on December 31: |
|
Principal |
|
Imputed Interest |
|
Total |
|||
2024 |
|
$ |
377 |
|
$ |
249 |
|
$ |
626 |
2025 |
|
|
424 |
|
|
220 |
|
|
644 |
2026 |
|
|
476 |
|
|
188 |
|
|
664 |
2027 |
|
|
532 |
|
|
151 |
|
|
683 |
2028 |
|
|
593 |
|
|
111 |
|
|
704 |
Thereafter |
|
|
1,200 |
|
|
82 |
|
|
1,282 |
|
|
|
|
|
|
|
|
|
|
Total minimum lease payments |
|
|
3,602 |
|
$ |
1,001 |
|
$ |
4,603 |
Less: current portion |
|
|
(377) |
|
|
|
|
|
|
Long-term portion of minimum lease obligation |
|
$ |
3,225 |
|
|
|
|
|
|
16. Stockholders’ Equity
Preferred Stock
The Company’s Board is authorized to issue, at any time, without further stockholder approval, up to 1 million shares of preferred stock. The Board has the authority to fix and determine the voting rights, rights of redemption and other rights and preferences of preferred stock.
30
Series 2022 Convertible Preferred Stock (“Series 2022 Preferred”)
On May 9, 2022, the Board of the Company created the Series 2022 Preferred, par value $0.001 per share, out of the Company’s 1 million authorized shares of preferred stock. Each share of Series 2022 Preferred was convertible at a ratio of one-for-one (1:1) shares of the Common Stock on a pre-split basis.
The Company issued 1,000 shares of Series 2022 Preferred and received proceeds of $270. Pursuant to the terms of the preferred stock, the Company’s Board converted the Preferred Stock to 40 shares of Common Stock on July 19, 2022, on a post-split basis.
iBio CMO Preferred Tracking Stock
On February 23, 2017, the Company entered into an exchange agreement with Bryan Capital pursuant to which the Company acquired substantially all of the interest in iBio CDMO held by Bryan Capital and issued one share of a newly created Preferred Tracking Stock, in exchange for 29,990,000 units of limited liability company interests of iBio CDMO held by Bryan Capital at an original issue price of $13 million. After giving effect to the transaction, the Company owned 99.99% and Bryan Capital owned 0.01% of iBio CDMO.
On February 23, 2017, the Board of the Company created the Preferred Tracking Stock out of the Company’s 1 million authorized shares of preferred stock. The Preferred Tracking Stock accrued dividends at the rate of 2% per annum on the original issue price. Accrued dividends were cumulative and were payable if and when declared by the Board, upon an exchange of the shares of Preferred Tracking Stock and upon a liquidation, winding up or deemed liquidation (such as a merger) of the Company. No dividends were declared through October 31, 2021.
On November 1, 2021, iBio purchased the iBio CMO Preferred Tracking Stock held by Bryan Capital. No iBio CMO Preferred Tracking Stock remains outstanding. As a result, the iBio CDMO subsidiary and its intellectual property are now wholly owned by iBio.
Common Stock
The number of authorized shares of the Common Stock is 275 million. In addition, the Company has reserved 1,280,000 shares of Common Stock for issuance pursuant to the grant of new awards under the Company’s 2020 Omnibus Incentive Plan (the “2020 Plan”).
Reverse Stock Split
On June 30, 2022, the Company held a special meeting of its stockholders at which the stockholders approved a proposal to affect an amendment to the Company's certificate of incorporation, as amended, to implement a reverse stock split at a ratio of one-for-twenty-five (1:25). On September 22, 2022, the Company's Board approved the implementation of the reverse stock split of the Common Stock. As a result of the reverse stock split, every twenty-five (25) shares of the Common Stock either issued and outstanding or held by the Company in its treasury immediately prior to the effective time was, automatically and without any action on the part of the respective holders thereof, combined and converted into one (1) share of the Common Stock. No fractional shares were issued in connection with the reverse stock split. Stockholders who otherwise were entitled to receive a fractional share in connection with the reverse stock split instead were eligible to receive a cash payment, which was not material in the aggregate, instead of shares. On October 7, 2022, the Company filed a Certificate of Amendment of its Certificate of Incorporation, as amended with the Secretary of State of Delaware effecting a one-for-twenty-five (1:25) reverse stock split of the shares of the Common Stock, either issued or outstanding, effective October 7, 2022. The Common Stock began trading on a reverse split adjusted basis when the market opened Monday, October 10, 2022.
Recent issuances of Common Stock include the following:
Cantor Fitzgerald Underwriting
On November 25, 2020, the Company entered into a Controlled Equity Offering SM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. ("Cantor Fitzgerald") to sell shares of Common Stock, from time to time, through an “at the market offering” program having an aggregate offering price of up to $100,000,000 through which Cantor Fitzgerald would act as sales agent. Between July 25, 2022, and March 31, 2023, Cantor Fitzgerald sold as sales agent pursuant to the Sales Agreement 1,551,879 shares of Common Stock. The Company received net proceeds of approximately $2.9 million during the nine months ended March 31, 2023 and holds a subscription receivable for $260,000 at March 31, 2023 for proceeds received on April 4, 2023.
31
RubrYc Transaction
On September 19, 2022, the Company issued 102,354 shares valued at approximately $1,000,000 to RubrYc as part of the payment for purchasing the assets of RubrYc.
Wainwright Underwriting
On December 6, 2022, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”). Pursuant to the Underwriting Agreement, the Company agreed to sell to Wainwright, in a firm commitment underwritten offering (the “Offering”) (i) 1,530,769 shares of the Company’s Common Stock, (ii) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,834,616 shares of Common Stock, (iii) Series A Common Stock purchase warrants (the “Series A Warrants”) to purchase up to 3,365,385 shares of Common Stock and (iv) Series B Common Stock purchase warrants (the “Series B Warrants” and together with the Series A Warrants, the “Common Warrants”) to purchase up to 3,365,385 shares of Common Stock. The offering closed on December 9, 2022.
Wainwright acted as the sole book-running manager for the Offering. The Company paid Wainwright an underwriting discount equal to 7.0% of the gross proceeds of the offering, and reimbursed Wainwright for the legal fees and certain expenses of the underwriter. Pursuant to the Underwriting Agreement, the Company has granted Wainwright a 30-day option to purchase up to an additional 504,807 shares of Common Stock and/or Common Warrants to purchase up to an additional 1,009,614 shares of Common Stock at the public offering price, less the underwriting discounts and commissions, solely to cover over-allotments. Wainwright elected to purchase 504,807 Series A Warrants and 504,807 Series B Warrants.
The Company has also agreed to issue to Wainwright, as the representative of the underwriters, warrants (the “Representative’s Warrants”) to purchase a number of shares of Common Stock equal to 6.0% of the aggregate number of shares of Common Stock and Pre-Funded Warrants being offered in the offering. Wainwright received warrants to purchase up to 201,923 shares of Common Stock.
The Company received net proceeds of approximately $2,864,000 after deducting underwriting discounts, commissions and other issuance costs.
Vesting of Restricted Stock Units “RSUs”
On August 23, 2022, RSUs for 1,057 shares of Common Stock were vested. On December 1, 2022, RSUs for 4,120 shares of Common Stock were vested. In addition, RSUs for 27,740 of Common Stock vested during the third quarter of Fiscal 2023.
Warrants
Bryan Capital
As discussed above, the Company issued to Bryan Capital a Warrant to purchase 51,583 shares of the Common Stock of the Company at an exercise price of $33.25 per share. The Warrant expires October 10, 2026, is exercisable immediately, provides for a cashless exercise at any time and automatic cashless exercise on the expiration date if on such date the exercise price of the Warrant exceeds its fair market value as determined in accordance with the terms of the Warrant and adjustments in the case of stock dividends and stock splits.
Wainwright
As discussed above, the Company issued various warrants with the following terms:
| 1. | Pre-Funded Warrants – Immediately exercisable at an exercise price of $0.001 per share. All of the Pre-Funded Warrants were exercised in December 2022. |
| 2. | Class A Warrants – Immediately exercisable at an exercise price of $1.04 per share for a term of five years. |
| 3. | Class B Warrants – Immediately exercisable at an exercise price of $1.04 per share for a term of two years. |
| 4. | Representative Warrants – Immediately exercisable at an exercise price of $1.30 per share for a term of five years. |
During the third quarter of Fiscal 2023, 341,300 Class A Warrants and 1,704,916 Class B Warrants were exercised. On April 3, 2023, an additional 76,300 Class B Warrants were exercised. The total proceeds from Class A and B Warrants exercised during the third quarter of Fiscal 2023 and on April 3, 2023 were $2,207,000.
32
17. Earnings (Loss) Per Common Share
Basic earnings (loss) per common share is computed by dividing the net income (loss) allocated to common stockholders by the weighted-average number of shares of Common Stock outstanding during the period. For purposes of calculating diluted earnings (loss) per common share, the denominator includes both the weighted-average number of shares of Common Stock outstanding during the period and the number of common stock equivalents if the inclusion of such common stock equivalents is dilutive. Dilutive common stock equivalents potentially include stock options and warrants using the treasury stock method. The following table summarizes the components of the earnings (loss) per common share calculation (in thousands, except per share amounts):
|
|
Three Months Ended |
|
Nine Months Ended |
|
||||||||
|
|
March 31, |
|
March 31, |
|
||||||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
||||
Basic and diluted numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to iBio, Inc. from continuing operations |
|
$ |
(6,279) |
|
$ |
(6,746) |
|
$ |
(24,379) |
|
$ |
(19,125) |
|
Preferred stock dividends – iBio CMO Preferred Tracking Stock |
|
|
— |
|
|
— |
|
|
— |
|
|
(88) |
|
Net loss available to iBio, Inc. stockholders from continuing operations |
|
$ |
(6,279) |
|
$ |
(6,746) |
|
$ |
(24,379) |
|
$ |
(19,213) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss available to iBio, Inc. stockholders from discontinued operations |
|
$ |
(1,015) |
|
$ |
(5,644) |
|
$ |
(34,598) |
|
$ |
(14,124) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss available to iBio, Inc. stockholders - total |
|
$ |
(7,294) |
|
$ |
(12,390) |
|
$ |
(58,977) |
|
$ |
(33,337) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding |
|
|
13,184 |
|
|
8,719 |
|
|
10,592 |
|
|
8,719 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per share amount - continuing operations |
|
$ |
(0.47) |
|
$ |
(0.77) |
|
$ |
(2.30) |
|
$ |
(2.20) |
|
Per share amount - discontinued operations |
|
$ |
(0.08) |
|
$ |
(0.65) |
|
$ |
(3.27) |
|
$ |
(1.62) |
|
Per share amount - total |
|
$ |
(0.55) |
|
$ |
(1.42) |
|
$ |
(5.57) |
|
$ |
(3.82) |
|
In Fiscal year 2023 and Fiscal year 2022, the Company incurred net losses which cannot be diluted; therefore, basic and diluted loss per common share is the same. As of March 31, 2023 and 2022, shares issuable which could potentially dilute future earnings were as follows:
|
|
March 31, |
||
|
|
2023 |
|
2022 |
|
|
(in thousands) |
||
Stock options |
|
457 |
|
625 |
Restricted stock units |
|
443 |
|
22 |
Warrants |
|
5,948 |
|
52 |
Shares excluded from the calculation of diluted loss per share |
|
6,848 |
|
699 |
18. Share-Based Compensation
The following table summarizes the components of share-based compensation expense in the condensed consolidated statements of operations (in thousands):
|
|
Three Months Ended |
||||
|
|
March 31, |
||||
|
|
2023 |
|
2022 |
||
Research and development |
|
$ |
40 |
|
$ |
101 |
General and administrative |
|
|
345 |
|
|
1,112 |
Total |
|
$ |
385 |
|
$ |
1,213 |
|
|
Nine Months Ended |
||||
|
|
March 31, |
||||
|
|
2023 |
|
2022 |
||
Research and development |
|
$ |
96 |
|
$ |
132 |
General and administrative |
|
|
2,330 |
|
|
2,761 |
Total |
|
$ |
2,426 |
|
$ |
2,893 |
33
In addition, share-based compensation expense included in loss from discontinued operations totaled approximately $1,211,000 and $62,000 for the three months ended March 31, 2023 and 2022, respectively, and $1,519,000 and $305,000 for the nine months ended March 31, 2023 and 2022, respectively.
Stock Options
iBio, Inc. 2020 Omnibus Equity Incentive Plan (the “2020 Plan”)
On December 9, 2020, the Company adopted the 2020 Plan for employees, officers, directors and external service providers. The total number of shares of Common Stock reserved under the 2020 Plan is 1,280,000 shares of Common Stock for issuance pursuant to the grant of new awards under the 2020 Plan. The 2020 Plan allows for the award of stock options, stock appreciation rights, restricted stock, restricted stock units, unrestricted stock, cash-based awards, and dividend equivalent rights. The value of all awards awarded under the 2020 Plan and all other cash compensation paid by the Company to any non-employee director in any calendar year may not exceed $500,000; provided, however, that such amount shall be $750,000 for the calendar year in which the applicable non-employee director is initially elected or appointed to the Board and $1,500,000 for any non-executive chair of our Board should one be appointed. Notwithstanding the foregoing, the independent members of the Board may make exceptions to such limits in extraordinary circumstances. The term of the 2020 Plan will expire on the tenth anniversary of the date the Plan is approved by the stockholders.
Vesting of service awards are determined by the Board and stated in the award agreements. In general, vesting occurs ratably on the anniversary of the grant date over the service period, generally three or five years, as determined at the time of grant. Vesting of performance awards occurs when the performance criteria is satisfied. The Company uses historical data to estimate forfeiture rates.
Stock options issued
During the first quarter of Fiscal year 2023, the Company granted stock option agreements to various employees to purchase 303,869 shares of the Common Stock at exercise prices between $6.75 and $9.50 per share. The options vest 25% after one year and then in equal quarterly installments over a 36-month period and expire on the tenth anniversary of the grant date.
During the first quarter of Fiscal year 2023, the Company granted a stock option agreement to a consultant to purchase 4,000 shares of the Common Stock at an exercise price of $6.75 per share. The options vest in equal monthly installments, over a period of twelve months, starting after the second month and expire on the tenth anniversary of the grant date. During the third quarter of Fiscal year 2023, the Company terminated the consultant’s services. As a result, none of the 4,000 shares pursuant to the stock option agreement were exercised and as such, all 4,000 shares will be forfeited.
No stock options were granted in the second or third quarter of Fiscal year 2023.
The Company estimated the fair value of options granted using the Black-Scholes option pricing model with the following assumptions:
|
|
|
|
|
Weighted average risk-free interest rate |
|
|
3.21% - 3.61 |
% |
Dividend yield |
|
|
0 |
% |
Volatility |
|
|
115.52 - 116.93 |
% |
Expected term (in years) |
|
|
7 |
|
RSUs
On August 29, 2022, the Company issued RSUs to acquire 6,954 shares of common stock to various employees at a market value of $7.06 per share. The RSUs vest over a four-year period. The grant date fair value of the RSUs totaled approximately $49,000.
On November 10, 2022, as previously disclosed in relation to the Employment Agreement with Mr. Isett, the Company’s former CEO, dated April 30, 2021, the Company granted Mr. Isett RSUs to acquire 200,000 shares of Common Stock, on a post-split basis. The RSUs vest over a three-year period commencing April 30, 2021 provided the vesting is subject to the following performance conditions: (i) submission to the U.S. Food and Drug Administration (FDA) of an Investigational New Drug (IND) application, or alternatively, if the Board approves not to file an IND, (ii) consummation of a disposition of iBio CDMO, LLC, or (iii) out-licensing, with full global rights, any of its investigational product candidates prior to the submission to the FDA an IND application. The grant-date fair value of the RSUs totaled approximately $296,000. The performance conditions were not met and the RSUs did not vest.
34
On November 11, 2022, the Company granted Mr. Robert Lutz, the Company’s Chief Financial and Business Officer at the time, RSUs to acquire 100,057 shares of the Company’s Common Stock in exchange for Mr. Lutz’s agreement to continue employment with the Company through July 1, 2023. The RSUs vest the earlier of: (i) July 1, 2023, or (ii) the successful achievement of the Company’s 2023 objectives, as defined by the Board. The grant-date fair value of the RSUs totaled approximately $175,100. Mr. Lutz resigned from the Company and was no longer an employee of the Company effective February 10, 2023; accordingly, the RSUs did not vest.
On November 11, 2022, the Company granted Dr. Martin Brenner, the Company’s Chief Scientific Officer, RSUs to acquire 95,348 shares of the Company’s Common Stock in exchange for Mr. Brenner’s agreement to continue employment with the Company through July 1, 2023. The RSUs vest the earlier of: (i) July 1, 2023, or (ii) the successful achievement of the Company’s 2023 objectives, as defined by the Board. The grant-date fair value of the RSUs totaled approximately $167,000.
On January 20, 2023, the Board of the Company appointed Dr. Brenner to the position of Interim Chief Executive Officer, effective immediately. Dr. Brenner was granted RSUs to acquire 130,000 shares of the Company’s Common Stock, which RSUs shall vest pro rata over a twelve-month period, such vesting to terminate if Dr. Brenner is no longer the Company’s Interim Chief Executive Officer. The grant-date fair value of the RSUs totaled approximately $91,000.
On March 31, 2023, the Compensation Committee (the “Committee”) of the Board of the Company approved a special equity award program pursuant to which it awarded to its employees an aggregate of 225,000 RSUs under the Company’s 2020 Omnibus Equity Incentive Plan, as amended (the “Plan”), which awards included a grant of 50,000 and 37,500 restricted stock units to each of Dr. Brenner, and Felipe Duran, the Company’s Interim, Chief Financial Officer, respectively, vesting quarterly over 12 months commencing April 1, 2023. The grant-date fair value of the RSUs totaled approximately $468,000.
19. Fraunhofer Settlement
On May 4, 2021, the Company and Fraunhofer USA, Inc. (“FhUSA”) entered into a Confidential Settlement Agreement and Mutual Release (the “Settlement Agreement”) to settle all claims and counterclaims in the litigation captioned iBio, Inc. v. Fraunhofer USA, Inc. (Case No. 10256-VCF) in Delaware Chancery Court (the “Lawsuit”). The Settlement Agreement, among other things, resolves the Company’s claims to ownership of certain plant-based technology developed by FhUSA from 2003 through 2014, and sets forth the terms of a license of intellectual property. The Lawsuit was commenced against FhUSA by the Company in March 2015 in the Court of Chancery of the State of Delaware and is described in more detail in the Company’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2020. The Settlement Agreement is not an admission of liability or fault of the parties.
The terms of the Settlement Agreement provide for cash payments to the Company of $28,000,000 as follows: (i) $16,000,000 to be paid no later than May 14, 2021 (which is expected to be paid 100% to cover legal fees and expenses); (ii) two payments of $5,100,000 payable by March 31, 2022 and 2023 and (iii) as additional consideration for a license agreement, two payments of $900,000 due on March 1, 2022 and 2023. The license provides for a nonexclusive, nontransferable, worldwide, fully paid-up license to all intellectual property rights in and to certain plant-based technology developed by FhUSA from 2003 through 2014 that were the subject of the Lawsuit. After payment of the fees and expenses of its attorneys and others retained by the Company, including the litigation funding company, the Company’s estimated aggregate net cash recovery as a result of the Settlement Agreement will be approximately $10,200,000.
As of June 30, 2021, the Company held receivables related to the settlement in the amount of $10,200,000. This amount was recorded in the consolidated statement of operations and comprehensive loss as settlement income in Fiscal 2021. During the quarter ended March 31, 2022, the Company received the first payment of $5,100,000.
On March 17, 2023, the Company received a payment of $5,100,000 from Fraunhofer related to the Fraunhofer Settlement Funds and in accordance with the Fourth Amendment to the Credit Agreement with Woodforest, transferred $3,000,0000 to a Company account at Woodforest on March 24, 2023.
The Company would recognize the $1.8 million of license revenue when it determines the collection of the license fees was reasonably assured in accordance with ASC 606. On February 9, 2022, the Company received the first $900,000 payment under the license agreement. As such, the Company determined that the collection of the license fees was reasonably assured, and the Company recognized license revenue related to the license fees and recorded a receivable for the second payment in the third quarter of 2022. The second $900,000 payment was received on February 17, 2023.
35
20. Income Taxes
The Company recorded no income tax expense for the three months ended March 31, 2023 because the estimated annual effective tax rate was zero. As of March 31, 2023, the Company continues to provide a valuation allowance against its net deferred tax assets since the Company believes it is more likely than not that its deferred tax assets will not be realized.
21. Commitments and Contingencies
CRO Agreement
On October 10, 2022, the Company entered into an agreement with a CRO for cell line development and master cell banking to produce iBio-101 in addition to process development and GMP manufacturing of iBio-101 drug substance and drug product to support GLP toxicology and Phase 1 clinical studies. The Company has incurred costs totaling approximately $422,000 through March 31, 2023. The Company is committed to additional costs totaling approximately $958,000 as of the date of the filing of this report.
Inflation
Although the Company has not experienced any material adverse effects on our business due to increasing inflation, it has raised operating costs for many businesses and, in the future, could impact demand or pricing of manufacturing services, foreign exchange rates or employee wages. We are actively monitoring the effects these disruptions and increasing inflation could have on the Company’s operations.
22. Employee 401(K) Plan
Commencing January 1, 2018, the Company established the iBio, Inc. 401(K) Plan (the “Plan”). Eligible employees of the Company may participate in the Plan, whereby they may elect to make elective deferral contributions pursuant to a salary deduction agreement and receive matching contributions upon meeting age and length-of-service requirements. The Company will make a 100% matching contribution that is not in excess of 5% of an eligible employee’s compensation. In addition, the Company may make qualified non-elective contributions at its discretion. For the three months ended March 31, 2023 and 2022, employer contributions made to the Plan totaled approximately $56,000 and $54,000, respectively. For the nine months ended March 31, 2023 and 2022, employer contributions made to the Plan totaled approximately $200,000 and $116,000, respectively. In addition, employer contributions included in loss from discontinued operations totaled approximately $17,000 and $41,000 for the three months ended March 31, 2023 and 2022, respectively, and approximately $129,000 and $110,000 for the nine months ended March 31, 2023 and 2022, respectively.
23. Subsequent Events
On April 3, 2023, 76,300 Class B Warrants were exercised, which resulted in proceeds of $79,352.
Between April 3 and April 17, 2023, Cantor Fitzgerald sold as sales agent pursuant to the Sales Agreement 892,194 shares of Common Stock. The Company received net proceeds of approximately $1.1 million during April 2023.
On May 10, 2023, iBio CDMO and Woodforest entered into a fifth amendment to the Credit Agreement (the “Fifth Amendment”), which within the Fifth Amendment Woodforest agreed to: (i) waive the obligation to deliver to Woodforest an executed copy of a Purchase Agreement for the sale of the 130,000 square foot cGMP manufacturing facility in Bryan, Texas, no later than April 14, 2023 and, (ii) release $500,000 of the $3.0 million being held in a Company account at Woodforest when the outstanding principal amount is reduced to $10.0 million and for each additional $2.5 million reduction of the outstanding principal amount, an additional $750,000 will be released from the Company account at Woodforest. In addition, starting on the effective date of the Fifth Amendment, the interest on the Term Loan increased to 5.25%, and the Term Loan shall further accrue interest, payable in kind and added to the balance of the outstanding principal amount at a fixed rate per annum equal to (a) 1.00%, if the Facility is sold on or before June 30, 2023, (b) 2.00% if the Facility is sold after June 2023, but on or before September 30, 2023, or (c) 3:00%, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. The Company also agreed to pay Woodforest a fee in the amount of (x) $75,000 if the Facility is sold on or before June 30, 2023, (y) $100,000 if the Facility is sold after June 2023, but on or before September 30, 2023, or (x) $125,000, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. After marketing the Facility with a real estate firm for approximately six months, in May 2023, the Company entered into a new agreement with a new global commercial real estate firm to remarket the Facility. As of the date of the filing of this Quarterly Report on Form 10-Q, the Company is not in negotiations on a Purchase Agreement with a potential buyer of the Facility.
36
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following information should be read together with the consolidated financial statements and the notes thereto and other information included elsewhere in this Quarterly Report on Form 10-Q (this “Report”) and in our Annual Report on Form 10-K for the year ended June 30, 2022, as filed with the SEC on October 11, 2022 (the “Annual Report”). Unless the context requires otherwise, references in this Report to “iBio,” the “Company,” “we,” “us,” or “our” and similar terms mean iBio, Inc.
Forward-Looking Statements
This Report contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). For this purpose, any statements contained herein regarding our strategy, future operations, financial position, future revenues, projected costs and expenses, prospects, plans and objectives of management, other than statements of historical facts, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “may,” “plan,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such statements reflect our current views with respect to future events. Because these forward-looking statements involve known and unknown risks and uncertainties, actual results, performance or achievements could differ materially from those expressed or implied by these forward-looking statements for a number of important reasons, including those discussed in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Report, as well as in the section titled “Risk Factors” in the Company’s Annual Report. We cannot guarantee any future results, levels of activity, performance or achievements. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this Report as anticipated, believed, estimated or expected. The forward-looking statements contained in this Report represent our estimates as of the date of this Report (unless another date is indicated) and should not be relied upon as representing our expectations as of any other date. While we may elect to update these forward-looking statements, we specifically disclaim any obligation to do so unless otherwise required by securities laws.
Overview
We are an AI-driven innovator of precision antibody immunotherapies. We have a pipeline of innovative primarily immuno-oncology antibodies against hard-to-drug targets where we may face reduced competition and with antibodies that may be more selective. We plan to use our AI-driven discovery platform to continue adding antibodies against hard-to-drug targets or to work with partners on AI-driven drug development.
37
Therapeutics Pipeline
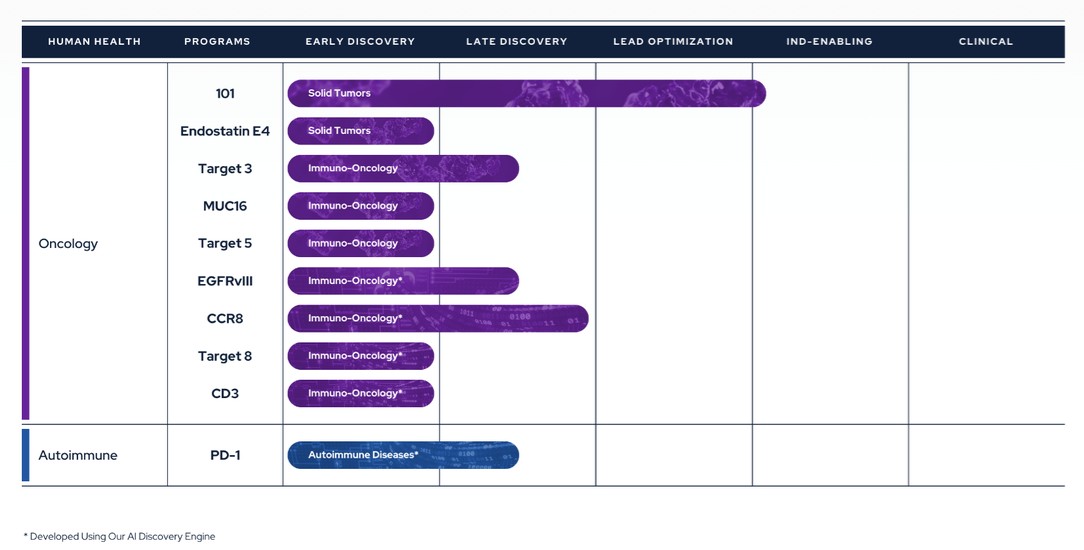
IBIO-101: an anti-CD25 molecule that works by depletion of immunosuppressive T-regulatory cells (“Tregs”) via antibody-dependent cellular cytotoxicity (“ADCC”), without disrupting activation of effector T-cells (“Teffs”) in the tumor microenvironment. IBIO-101 could potentially be used to treat solid tumors, hairy cell leukemia, relapsed multiple myeloma, lymphoma, or head and neck cancer. IBIO-101 is currently in the Investigational New Drug (“IND”) enabling stage. We have contracted with a contract research organization (“CRO”) to assist with the development of the manufacturing process, which includes but is not limited to process and cell line development for the production of the drug substance and drug product. IBIO-101 is strategically positioned as a fast follower to Hoffman-La Roche’s RG6292 molecule that recently released Phase 1 clinical data. While RG6292 showed signs of efficacy, especially in combination with PD-L1 monoclonal antibody, and was well tolerated, we anticipate additional clinical research will be needed to determine whether different cancer types are more efficacious than others. As a result, we have decided to pause the IND enabling studies until additional data is released on RG6292. This approach will allow us to gather more information, thoroughly evaluate the market potential and optimize our financial resources and the development plan for IBIO-101 to maximize its potential for success.
CCR8: targets depletion of highly immunosuppressive CCR8+ Tregs in the tumor microenvironment via an ADCC mechanism with selective binding to CCR8 over its closely related cousin, CCR4, to avoid off-target effects. A CCR8 program could potentially be broadly applicable in solid tumors and/or as a prospective combination therapy.
EGFRvIII: binds a tumor-specific mutation of EGFR variant III with an afucosylated antibody for high ADCC. Because of its specificity binding to the tumor-specific mutation, it could potentially reduce toxicity and/or expand the therapeutic window compared to simple broad EGFR-targeted alternatives. EGFRvIII is constantly “switched on” which can lead to the development of a range of different cancers. An EGFRvIII antibody could potentially be used to treat glioblastoma, head and neck cancer or non-small cell lung cancer.
CD3 antibody panel: provides a range of CD3 affinities with cross-reactivity to non-human primates and increased the humanness of the antibody sequences. The antibody panel is intended to serve as one arm of T-cell-redirecting bispecific antibodies, a new class of therapeutic antibodies designed to simultaneously bind to T-cells via CD3 and to tumor cells via tumor-specific antigens or tumor-associated antigens, inducing T-cell-mediated killing of tumor cells.
MUC16: a highly expressed target on ovarian cancer cells and an attractive tumor associated target for therapeutic antibodies. However, antibodies targeting MUC16 are prone to tumor resistance via epitope shedding and dysregulated glycosylation.
38
Epitope-steered antibodies that bind to an epitope that avoids both of these tumor resistance mechanisms could potentially be used to treat MUC16 positive tumors, particularly those tumors that are resistant to other MUC16 antibodies.
PD-1 Agonist: selectively binds PD-1 to suppress auto-reactive T-cells without PD-L1/PD-L2 blocking. A PD-1 agonist could potentially be used to treat inflammatory bowel disease, systemic lupus erythematosus, multiple sclerosis or other inflammatory diseases.
In addition to the programs described above, the Company also has three additional early discovery programs that have the potential to advance into later stages of preclinical development and are designed to tackle hard-to-drug targets.
IBIO-100 and Endostatin E4
Our preclinical anti-fibrotic program, IBIO-100, has been undergoing a review process as part of our ongoing effort to prioritize our resources and focus on the most promising opportunities. The IBIO-100 program design is based upon work by Dr. Carol Feghali-Bostwick, Professor of Medicine at the Medical University of South Carolina and Vice-Chair of the Scleroderma Foundation. Her initial work was conducted at the University of Pittsburgh, and we have licensed the patents relevant for the continued development of the molecule from the university. After careful consideration, in February 2023, we terminated all efforts on IBIO-100 anti-fibrotic program and provided a six (6) month notice of termination of the license agreement to the University of Pittsburgh, as required by the license agreement. Pursuant to termination of the license agreement with the University of Pittsburgh, our financial obligations for the management of the patents under the license will cease on August 14, 2023, and at such time, will transition back to the University of Pittsburgh.
As part of this decision, we are intending to complete the pre-clinical cancer studies we are conducting in collaboration with University of Texas Southwestern using E4 endostatin peptide, which is derived from IBIO-100. After the pre-clinical studies are completed, we will re-assess whether to further pursue the oncology program and have further discussions with the University of Pittsburgh. This approach allows us to gather valuable data and insights that will inform our future decisions regarding the potential of E4 endostatin peptide as an oncology program.
AI Drug Discovery Platform
In September 2022, we purchased substantially all of the assets of RubrYc Therapeutics (for a complete description of the transaction please see Note 6 – Significant Transactions). The AI Drug Discovery platform technology is designed to be used to discover antibodies that bind to hard-to-target subdominant and conformational epitopes for further development within our existing portfolio or in partnership with outside entities. The RubrYc AI platform is built upon three key technologies.
| 1. | Epitope Targeting Engine: A proprietary machine-learning platform that combines computational biology and 3D-modeling to identify molecules that mimic hard-to-target binding sites on target proteins, specifically, subdominant and conformational epitopes. The creation of these small mimics enables the engineering of therapeutic antibody candidates that can selectively bind immune and cancer cells better than ”trial and error” antibody engineering and screening methods that are traditionally focused on dominant epitopes. |
| 2. | RubrYcHuTM Library: An AI-generated human antibody library free of significant sequence liabilities that provides a unique pool of antibodies to screen. The combination of the Epitope Targeting Engine and screening with the RubrYcHu Library has been shown to reduce the discovery time from ideation to in vivo proof-of-concept (PoC) by up to four months. This has the potential to enable more, and better, therapeutic candidates to reach the clinic faster. |
| 3. | StableHuTM Library: An AI-powered sequence optimization library used to improve antibody performance. Once an antibody has been advanced to the lead optimization stage, StableHu allows precise and rapid optimization of the antibody binding regions to rapidly move a candidate molecule into the IND-enabling stage. |
On January 3, 2023, the United States Patent and Trademark Office issued U.S. Patent No. 11,545,238, entitled “Machine Learning Method for Protein Modelling to Design Engineered Peptides,” which, among other claims, covers a machine learning model for engineering peptides, including antibody epitope therapeutics. Subject to any potential patent term extensions, the patent will expire on May 13, 2040.
39
Recent Developments
On January 17, 2023, we announced the resignation of Mr. Robert Lutz, the Chief Financial and Business Officer, effective February 10, 2023. On January 25, 2023, we announced the Board of Directors appointed Dr. Martin Brenner to the position of Interim Chief Executive Officer, effective January 20, 2023, and Mr. Felipe Duran, to the position of Interim Chief Financial Officer, effective as of February 13, 2023. We are continuing the search for a successor Chief Executive Officer and as such, Dr. Brenner’s position of Interim Chief Executive Officer will end when the Company appoints a successor.
On February 9, 2023, we and Woodforest entered into the Second Amendment to the Credit Agreement and amended the Credit Agreement to: (i) waive any current or prior default of the Liquidity Covenant until a period specified in such amendment which is dependent upon the occurrence of a specific milestone in the Credit Agreement, (ii) in addition to our unrestricted cash, until such period dependent upon the occurrence of a specific milestone in the Credit Agreement, we can account for all amounts owed to us by Fraunhofer as part of our legal settlement with them (see Note 19 – Fraunhofer Settlement for more information) in determining whether we are in compliance with the Liquidity Covenant, (iii) permit us to sell certain equipment located at the Facility, whereby forty percent (40%) of the net proceeds will be paid to Woodforest within ten (10) days following the end of the month of when the sales occurred, and (iv) remove any affirmative obligation on the part of the iBio CDMO to continue conducting its primary business.
On February 20, 2023, we and Woodforest entered into a third amendment to the Credit Agreement (the “Third Amendment”), which removed the added milestone specified in the Second Amendment, the failure of which would be an event of default. In addition, the Guaranty was amended to allow us until February 28, 2023, to account for the Fraunhofer Settlement Funds in determining whether we are in compliance with the Liquidity Covenant without being dependent upon a specified milestone. In addition, we agreed that each time we consummate an at-the-market issuance of Equity Interests (as defined within the Credit Agreement), no later than five (5) days following such issuance of Equity Interests, we will (i) pay to Woodforest in immediately available cash funds, without setoff or counterclaim of any kind, forty percent (40%) of the Net Proceeds (as defined within the Credit Agreement) received by us for such issuance of Equity Interests; provided, any such payment would cease upon payment obligations in full and (ii) provide Woodforest with a detailed accounting of each such issuance of Equity Interests.
On March 24, 2023, we and Woodforest entered into a fourth amendment to the Credit Agreement (the “Fourth Amendment”), which within the Fourth Amendment Woodforest agreed to (i) reduce the percentage of any payment to Woodforest we are required to make from the proceeds of sales of its common stock under its at-the-market facility from 40% to 20%, (ii) reduce the percentage of any payment to Woodforest we are required to make from the proceeds of sales of its equipment from 40% to 20%, and (iii) allow us to retain $2,000,000 million of the $5,100,000 million that the Company received from the Fraunhofer Settlement Funds, with the remaining $3,000,000 million being held in a Company account at Woodforest. In addition, we are obligated to (y) deliver to Woodforest an executed copy of a purchase agreement (the “Purchase Agreement”) for the sale of the 130,000 square foot cGMP manufacturing facility in Bryan, Texas (“Facility”), no later than April 14, 2023, and (z) pay to Woodforest a fee in the amount of $75,000 on the earlier of the date of the closing of the Purchase Agreement, or the Maturity Date (as defined in the Credit Agreement). In addition, on March 24, 2023, we entered into a fourth amendment to the Guaranty, which reduced the Liquidity Covenant from $7,500,000 to $1,000,000.
On May 10, 2023, we and Woodforest entered into a fifth amendment to the Credit Agreement (the “Fifth Amendment”), which within the Fifth Amendment Woodforest agreed to: (i) waive our obligation to deliver to Woodforest an executed copy of a Purchase Agreement for the sale of the Facility no later than April 14, 2023 and, (ii) release $500,000 of the $3.0 million being held in a Company account at Woodforest when the outstanding principal amount is reduced to $10.0 million and for each additional $2.5 million reduction of the outstanding principal amount, an additional $750,00 will be released from the Company account at Woodforest. In addition, starting on the effective date of the Fifth Amendment, the interest on the Term Loan increased to 5.25%, and the Term Loan shall further accrue interest, payable in kind and added to the balance of the outstanding principal amount at a fixed rate per annum equal to (a) 1.00%, if the Facility is sold on or before June 30, 2023, (b) 2.00% if the Facility is sold after June 2023, but on or before September 30, 2023, or (c) 3:00%, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. The Company also agreed to pay Woodforest a fee in the amount of (x) $75,000 if the Facility is sold on or before June 30, 2023, (y) $100,000 if the Facility is sold after June 2023, but on or before September 30, 2023, or (x) $125,000, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. After marketing the Facility with a real estate firm for approximately six months, in May 2023, we entered into an agreement with a global commercial real estate firm to market the Facility. As of the date of the filing of this Quarterly Report on Form 10-Q, we are not in negotiations on a Purchase Agreement with a potential buyer of the Facility.
Liquidity and Capital Resources
The history of significant losses, the negative cash flow from operations, the limited cash resources on hand and the dependence by the Company on its ability to obtain additional financing to fund its operations after the current cash resources are exhausted raises substantial doubt about the Company's ability to continue as a going concern. Our management concluded that our recurring losses from operations and the fact that we have not generated significant revenue or positive cash flows from operations raise substantial doubt about our ability to continue as a going concern for the next 12 months after issuance of our financial statements.
40
Our auditors also included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended June 30, 2022 with respect to this uncertainty.
In an effort to remain a going concern and increase cash reserves, we completed a public offering in December 2022, reduced our work force by approximately 60% (a reduction of approximately 69 positions) in November 2022, and ceased operations of our CDMO facility thereby reducing annual spend on expenses by approximately 50%. Additionally, we continue our efforts to sell our CDMO assets and facility that were initiated by management in July 2022; however, there can be no assurance that we will be successful in selling the CDMO assets and facility at favorable prices, if at all. (See Note 3 – Discontinued Operations for more information.) Additional potential options being considered to further increase liquidity include lowering our expenses further, focusing product development on a select number of product candidates, the sale or out-licensing of certain product candidates, sell the CDMO, equipment sales, raising money from capital markets, grant revenue or collaborations, or a combination thereof.
During the first quarter of Fiscal year 2023, we completed at-the-market offerings and sold 175,973 shares of Common Stock. We received net proceeds of approximately $1.2 million. During the second quarter of Fiscal year 2023, we completed a public offering and raised gross proceeds of approximately $3.5 million selling an aggregate of 3,365,385 shares of its common stock (or pre-funded warrants in lieu thereof), Series A warrants to purchase up to 3,870,192 shares of common stock and Series B warrants to purchase up to 3,870,192 shares of common stock. Subsequently after the public offering, during the third quarter of Fiscal year 2023, 341,300 Class A Warrants and 1,704,916 Class B Warrants were exercised. On April 3, 2023, an additional 76,300 Class B Warrants were exercised. The total proceeds from Class A and B Warrants exercised during the third quarter of Fiscal 2023 and on April 3, 2023 were $2,207,000. Also, during the third quarter of Fiscal year 2023, we completed at-the-market offerings and sold 1,375,906 shares for which we received approximately $2.0 million. Additionally, between April 3 and April 17, 2023, we completed at-the-market offerings and sold 892,194 shares of which we received approximately $1.1 million. (See Note 16 – Stockholder’s Equity for more information.)
Our cash, cash equivalents and restricted cash of $9.8 million as of March 31, 2023, is not anticipated to be sufficient to support operations through the first quarter of fiscal year 2024, unless we further reduce our burn rate, sell the CDMO assets or the facility for amounts above the debt on the facility, or increase our capital as described above. Regardless of whether we are able to reduce our burn rate or sell or out-license certain assets or parts of the business, we will need to raise additional capital in order to fully execute our longer-term business plan. It is our goal to implement one or more potential options described above to allow us to have a cash runway for at least 12 months from the date of the filing of this Quarterly Report on Form 10-Q. However, there can be no assurance that we will be successful in implementing any of the options that we are evaluating.
Our liquidity and operations could also be impacted by our obligations under the Woodforest Credit Agreement. As described in detail in Section 6. Significant Transactions, to avoid violating the Liquidity Covenant associated with the parent guarantee of the debt we need to pay off the debt through the sale of the facility or we need to raise additional capital.
Results of Operations - Comparison of the three months ended March 31, 2023 and 2022
Revenue
Revenue from the CDMO operations is now included in discontinued operations and not broken out separately on the financial statements. Our ongoing business is primarily focused on i) development of our pipeline for which we do not expect revenue and ii) on our AI-driven discovery platform. We may have revenue with the AI-driven discovery platform in the future. Revenue for the three months ended March 31, 2022 was related to a licensing agreement.
Research and Development Expenses (“R&D”)
R&D expenses for the three months ended March 31, 2023 and 2022 were $2.6 million and $3.3 million, respectively, a reduction of approximately ($0.7) million. The decrease in R&D expenses is mainly due to certain tasks and assays being performed in-house which were previously outsourced, and a decrease in spending for consultants or outside services.
General and Administrative Expenses (“G&A”)
G&A expenses for the three months ended March 31, 2023 and 2022 were approximately $3.5 million and $5.3 million, respectively, a decrease of ($1.8) million. The decrease in expenses is primarily attributable to the reduction in personnel costs, a decrease in spending for consultants or outside services, and reduced amortization of intangible assets impaired during Q2 of Fiscal year 2023.
41
Total Operating Expenses
Total operating expenses, consisting primarily of R&D and G&A expenses, for the three months ended March 31, 2023 were approximately $6.2 million, compared to approximately $8.6 million in Fiscal year 2022.
Discontinued Operations
On November 2, 2022, we announced our plans to divest our contract development and manufacturing organization (iBio CDMO, LLC) in order to complete its transformation into an AI-driven precision antibody discovery and development company. In conjunction with the divestment, we completed a workforce reduction of approximately 60% and discontinued CDMO operations. CDMO operations remain treated as a discontinued operation on our financial statements. Losses for Discontinued Operations for the three months ended March 31, 2023 and 2022 were approximately ($1.0) million and ($5.6) million, respectively. In the three months ended March 31, 2023, we had a reduction in consumable supplies expenses of approximately ($1.1) million, decrease of approximately ($1.1) million for consultants and outside services, decrease in depreciation expense of ($0.7) million, decrease in personnel costs ($0.6) million and a net gain of approximately $0.5 million from auction of machinery and equipment.
Net Loss Available to iBio, Inc. Stockholders
Net loss available to iBio, Inc. stockholders for the three months ended March 31, 2023 was ($7.3) million, or ($0.55) per share. Net loss available to iBio, Inc. stockholders for the three months ended March 31, 2022 was approximately ($12.4) million or ($1.42) per share.
Results of Operations - Comparison of the nine months ended March 31, 2023 and 2022
Revenue
Revenue from the CDMO operations is now included in discontinued operations and not broken out separately on the financial statements. Revenue was otherwise immaterial. Our ongoing business is primarily focused on i) development of our pipeline for which we do not expect revenue and ii) on our AI-driven discovery platform. We may have revenue with the AI-driven discovery platform in the future. Revenue for the nine months ended March 31, 2022 was mainly related to a licensing agreement.
Research and Development Expenses (“R&D”)
R&D expenses for the nine months ended March 31, 2023 and 2022 were $8.0 million and $6.3 million, respectively, an increase of approximately $1.7 million. The increase was primarily driven by the investments into our pipeline including IBIO-101, CCR8, and EGFRvIII, and the expansion of our AI-driven discovery platform to include increases in R&D personnel at the San Diego facility, increased spend on lab supplies, lab equipment/maintenance, and facility rent for the San Diego team. This increase was partially offset by a reduction in spending on consultants and outside services.
General and Administrative Expenses (“G&A”)
G&A expenses for the nine months ended March 31, 2023 and 2022 were approximately $16.4 million and $14.9 million, respectively, an increase of $1.5 million. The increase is primarily from the increase in personnel costs for severance and retention expenses offset by a reduction in spending for legal and consulting fees.
Total Operating Expenses
Total operating expenses, consisting primarily of R&D and G&A expenses, for the nine months ended March 31, 2023, were approximately $24.4 million, compared to approximately $21.1 million in the same period of 2022.
Discontinued Operations
On November 2, 2022, we announced our plans to divest its contract development and manufacturing organization (iBio CDMO, LLC) in order to complete its transformation into an AI-driven precision antibody discovery and development company. In conjunction with the divestment, we completed a workforce reduction of approximately 60% and discontinued CDMO operations. CDMO operations are now treated as a discontinued operation on our financial statements. Losses for Discontinued Operations for the nine months ended March 31, 2023 and 2022 were approximately ($34.6) million and ($14.1) million, respectively. For the nine months ended March 31, 2023, the loss included impairments of fixed assets of approximately ($17.6) million, consumables and inventory of approximately ($4.9) million, personnel related costs of ($7.5) million including severance, and the remaining costs were operational.
42
Net Loss Available to iBio, Inc. Stockholders from Continuing Operations
Net loss available to iBio, Inc. stockholders for the nine months ended March 31, 2023 was ($59.0) million, or ($5.57) per share. Net loss available to iBio, Inc. stockholders for the nine months ended March 31, 2022 was approximately ($33.3) million or ($3.82) per share.
Uses of Cash and Funding Requirements
Net Cash Used in Operating Activities
Net cash used in operating activities was approximately ($25.5) million for the nine months ended March 31, 2023. The use of cash was primarily attributable to funding our net loss for the period.
Net Cash Provided by Investing Activities
Net cash provided by investing activities of approximately $7.0 million for the nine months ended March 31, 2023 was primarily attributable to the redemption and sale of debt securities of $11.0 million and to a lesser extent $2.1 million from the sale of fixed assets in an auction process which was offset by the purchase of fixed assets of ($5.2) million. (Refer to Note 6 – Significant Transactions for details.)
Net Cash Used in Financing Activities
Net cash used in financing activities during the nine months ended March 31, 2023, was approximately ($0.4) million and was mainly attributable to the proceeds from the sale of common stock, warrants exercised, offset by payments towards the term note made to Woodforest (see Note 13 – Debt for further details).
Funding Requirements
We have incurred significant losses and negative cash flows from operations since our spin-off from Integrated BioPharma in August 2008. As of March 31, 2023, our accumulated deficit was approximately $(282.9) million and we used approximately ($24.8) million of cash for operating activities during the nine months ended March 31, 2023.
We plan to fund our future business operations using cash on hand, through proceeds realized in connection with the commercialization of our technologies, through proceeds from the sale of the CDMO entity or the facility, through the proceeds from our license agreement with Fraunhofer, through potential proceeds from the sale or out-licensing of assets, and through proceeds from the sale of additional equity or other securities. However, there can be no assurance that we will be successful in implementing these plans, many of which will take several years before we will realize proceeds. The Term Loan with Woodforest and the Guaranty requires we maintain an unrestricted cash balance of $1.0 million, which limits our ability to use our funds for operations. If we should default on the Credit Agreement and Woodforest does not waive the default, and if Woodforest makes a demand for the acceleration of all payments due to this default, it could result in all obligations that are guaranteed being due and payable immediately without further notice. We cannot be certain that such funding will be available on favorable terms or available at all. If we are unable to raise funds when required or on favorable terms, this assumption may no longer be operative, and we may have to: a) significantly delay, scale back, or discontinue the product application and/or commercialization of our proprietary technologies; b) seek collaborators for our technology and product candidates on terms that are less favorable than might otherwise be available; c) relinquish or otherwise dispose of rights to technologies, product candidates, or products that we would otherwise seek to develop or commercialize; or d) possibly cease operations.
See Liquidity and Capital Resources above for further information.
Off-Balance Sheet Arrangements
As part of our ongoing business, we do not participate in transactions that generate relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities (“SPE”s), which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually limited purposes. As of March 31, 2023, we were not involved in any SPE transactions.
Critical Accounting Estimates
Our condensed consolidated financial statements are presented in accordance with U.S. GAAP, and all applicable U.S. GAAP accounting standards effective as of March 31, 2023, have been taken into consideration in preparing the condensed consolidated financial statements.
43
The preparation of condensed consolidated financial statements requires estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. Some of those estimates are subjective and complex, and, consequently, actual results could differ from those estimates. We base our estimates, to the extent possible, on historical experience. Historical information is modified as appropriate based on current business factors and various assumptions that we believe are necessary to form a basis for making judgments about the carrying value of assets and liabilities. We evaluate our estimates on an ongoing basis and make changes when necessary. Actual results could differ from our estimates.
Critical accounting estimates are those estimates made in accordance with U.S. GAAP that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on the financial condition or results of operations of the Company. The following accounting estimate had a material impact on the results of operations of the Company for the three and nine months ended March 31, 2023.
Impairment of Fixed Assets
We monitor fixed assets for impairment indicators throughout the year. When necessary, charges for impairments of long-lived assets are recorded for the amount by which the fair value is less than the carrying value of these assets. Changes in the Company’s business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although we base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
On November 3, 2022, we announced we are seeking to divest our contract development and manufacturing organization (iBio CDMO) in order to complete our transformation into an antibody discovery and development company. Through the process of seeking to divest its contract development and manufacturing organization, we continue to market for sale the 130,000-square-foot cGMP facility location in Bryan, Texas (the “Facility”). The decision to divest triggered a quantitative impairment analysis of our CDMO fixed assets of the building in Bryan, Texas totaling $22.65 million and machinery and equipment totaling $13.4 million.
We utilized a market approach, using independent third-party appraisals, including comparable assets, in addition to bids received from prospective buyers, to estimate the fair value of the property and equipment. We recorded an impairment charge of $6.3 million for the building and $11.3 million for the machinery and equipment in the quarter ended December 31, 2022. The key assumption in the valuation analysis is the expected sale price of $21.1 million for the Bryan, Texas facility and the associated machinery and equipment less approximate costs to sell of $2.7 million. The carrying amount of the CDMO fixed assets after impairment at December 31, 2022 was $18.5 million.
On February 10, 2021, the “Company, entered into an Auction Sale Agreement (the “Auction Sale Agreement”) with Holland Industrial Group, together with Federal Equipment Company and Capital Recovery Group LLC (collectively, the “Auctioneers”) for the sale at public auction of equipment and other tangible personal property (the “Equipment”) located at the Facility. The Auctioneer guaranteed an amount of gross proceeds from the sale of the equipment of $2.1 million, which was paid to the Company on February 17, 2023. The auction, which commenced on March 24, 2023 and concluded on March 30, 2023, resulted in total proceeds of approximately $2.9 million. In accordance with the Auction Sale Agreement, the Company received 80% of the excess proceeds, after Holland was paid a fixed amount of $0.2 million for expenses, which was approximately $0.5 million and was received on May 2, 2023.
We may have to record a further, potentially material, impairment to the fair value of this asset group if we do not realize a sale transaction for the expected amount of $19 million less costs to sell in the near term.
Impairment of Indefinite-Lived Intangible Assets
For indefinite life intangible assets, we perform an impairment test annually and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable.
Evaluating for impairment requires judgment, including the estimation of future cash flows, future growth rates and profitability and the expected life over which cash flows will occur. Changes in the Company’s business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although we base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
We tested for impairment of the IBIO-101 therapeutic technology (or “IP”), classified as an indefinite-lived intangible asset, which had a carrying amount of $5 million at December 31, 2022. The key impairment trigger was the decline in the Common Stock price over the month of December 2022. We did not record an impairment charge to the IP as of December 31, 2022. No triggering events were identified during the third quarter of Fiscal 2023 requiring additional testing. We will continue to monitor the value of the IP, since we believe it is at risk for impairment, and will perform our annual impairment testing in the fourth quarter of Fiscal 2023.
44
The primary impairment indicators that may arise in the near future are (1) any sustained decline in our common stock market price and (2) FDA decisions on similar competing technologies that are applying for Phase 1 approval.
The December 31, 2022 impairment analysis on the IP is considered a critical accounting estimate because it entailed management preparing highly uncertain cash flow projections and valuation assumptions of significant complexity and subjectivity. One fair value indicator was developed using a discounted cash flow (“DCF”) analysis, where the most subjective assumptions were the estimated probability of obtaining Phase 1 approval of the IBIO-101 technology from the FDA of 65% and a discount rate of 12%. These two key assumptions remained the same with those we included in our June 30, 2022 impairment analysis for the IP. We provide the following quantitative sensitivity analysis only to allow our investors to obtain a better understanding of the degree and direction of potential material change in the fair value estimate developed using the DCF approach:
| ● | A hypothetical increase in the discount rate to 15% alone (all other assumptions kept constant) would not lead to an impairment of the intangible. However, a hypothetical increase in the discount rate to 16% would lead to an impairment of $1 million to the intangible. |
| ● | A decrease in the estimated probability of clearing Phase 1 FDA approval to 45% (all other assumptions kept constant) would not lead to an impairment of the intangible. However, a hypothetical decrease in the estimated probability of clearing Phase 1 FDA approval to 35% would lead to an impairment charge of $1.4 million to the intangible. |
A second fair value indicator was developed using a market approach. We reviewed public pharma/biotech company acquisitions & mergers completed from 2018-2023 with deal value less than $1 billion from the GlobalData database. The equity premium observed in merger and acquisition transactions that closed during 2022 for similar therapeutic technologies was in the 50% to 125% range. We selected an equity market premium of 100% as the most appropriate assumption at December 31, 2022, which was consistent with the 20-day median premium of the comparable pulled. A hypothetical decrease in the observable equity market premiums of 5% would lead to the estimated fair value attributable to the IP under the market approach to decline materially and result in an approximate $1 million impairment amount.
We continue to operate in a highly competitive environment, rising interest rates (and cost of capital) and experiences liquidity challenges. Accordingly, we may have to adjust our cash flow projections and valuation assumptions in the near future to account for market trends and any changes to our research and development plans. Any such future adjustments may lead to material future impairments in the IP and other related assets.
Our remaining critical accounting estimates remain consistent with the information disclosed in the same section in our last annual report on Form 10-K for the year ended June 30, 2022.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As a smaller reporting company, we are not required to provide the information required by this Item 3.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, under the direction of our interim Chief Executive Officer (our Principal Executive Officer) and interim Chief Financial Officer (our Principal Financial Officer) have evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as amended, as of March 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. The Company’s disclosure controls and procedures are also designed to ensure that such information is accumulated and communicated to management to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
45
Based on our evaluation, our Principal Executive Officer and Principal Financial Officer concluded that our disclosure controls and procedures were ineffective as of March 31,2023 due to the material weakness identified below.
During the preparation of the Quarterly Report for the quarter ended March 31, 2023, we identified a material weakness in our controls relating to accounting for stock-based compensation expense relating to the vesting of severed employees’ awards.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, during the quarter ended March 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Plans to Remediate Internal Control over Financial Reporting
Management is currently assessing the actions that need to be taken to remediate the material weakness identified above. The material weakness will only be deemed to have been remediated after the new controls and procedures have been in place and management has concluded through appropriate testing that the controls are operating effectively.
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
We are not subject to any material legal proceedings. From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 1A. Risk Factors
Our business is subject to risks and events that, if they occur, could adversely affect our financial condition and results of operations and the trading price of our securities. The following information updates, and should be read in conjunction with, the information disclosed in Part I, Item 1A, “Risk Factors,” contained in the Annual Report. Except as described below, our risk factors as of the date of this Report have not changed materially from those described in “Part I, Item 1A. Risk Factors” of our Annual Report.
We are reviewing potential options to extend our cash runway. This review could impact our future operations and financial position.
We are currently evaluating a number of potential options to expand our cash runway, the implementation of which will impact the Company’s liquidity. In an effort to improve liquidity and runway, we recently announced that we were selling our CDMO business and Facility, reducing our work force by approximately 60%, and ceasing operations of our CDMO, thereby reducing annual spend on expenses by approximately 50%. Potential options being considered to further increase liquidity include lowering our expenses further, focusing product development on a select number of product candidates, the sale or out-licensing of certain product candidates, raising money from capital markets, grant revenue or collaborations, or a combination thereof.
Our cash, cash equivalents and restricted cash of $9.8 million as of March 31, 2023, is not anticipated to be sufficient to support our operations for at least 12 months from the date of the filing of this Quarterly Report on Form 10-Q unless we reduce our burn rate further, sell the CDMO facility for amounts above its term note payable, or increase our capital. Regardless of whether we are able to reduce our burn rate or sell or out-licensing certain assets or parts of the business, we will need to raise additional capital in order to fully execute our near and long-term business plans. In fact, we do not believe that our current cash, cash equivalents and restricted cash as of March 31, 2023 will be sufficient to support our operations through first quarter of Fiscal 2024.
There can be no assurance that we will be able to sell the CDMO assets or that if we are able to do so that we do so on favorable terms or that he exploration of potential options will result in any agreements or transactions, or that, if completed, any agreements or transactions will be successful or on attractive terms. Although we expect to be able to sell the CDMO assets in 2023, no guaranteed timetable has been established for the completion of this process, and we do not expect to disclose developments unless and until we have a material update to provide or the Board of Directors has concluded that disclosure is appropriate or required. If we determine to change our business strategy, our future business, prospects, financial position and operating results could be significantly different than those in historical periods or projected by our management. Because of the significant uncertainty regarding our future plans, we are not able to accurately predict the impact of a potential change in our business strategy and future funding requirements.
46
Our historical operating results indicate substantial doubt exists related to our ability to operate as a going concern.
We have incurred net losses and used significant cash in operating activities since inception, and we expect to continue to generate operating losses for the foreseeable future. As of March 31, 2023, we have an accumulated deficit of ($282.9) million. In addition, our projections regarding our cash runway are based upon certain assumptions, including that payments owed to us under outstanding notes receivable are paid at maturity. Accordingly, these assumptions are based upon the financial positions of the parties from which we are owed payments, for which there can be no guarantee.
We held cash, cash equivalents and restricted cash of $9.8 million as of March 31, 2023. Based on current trends and activities, there is significant doubt that we can continue as a going concern through the first quarter of fiscal year 2024. We have announced that we have implemented and are continuing to implement cost savings measures to expand our cash runway, the implementation of which will impact our liquidity, but these measures alone will not be sufficient to provide the financing needed to meet our near and long-term goals. Potential options being considered to increase liquidity include further lowering our expenses through decreasing spending and focusing product development on a select number of product candidates, the sale or out-licensing of certain product candidates or parts of the business, raising money from capital markets, grant revenue or collaborations, or a combination thereof. Regardless of whether we are able to reduce our burn rate or sell or out-licensing certain assets or parts of the business, we will need to raise additional capital in order to fully execute our longer-term business plan. We believe based on input from expert advisors, that it is likely we will be able to implement one or more options that will extend our cash runway for 12 months or more from the date of the filing of this Quarterly Report on Form 10-Q. However, there can be no assurance that we will be successful in implementing any of the options that we are evaluating.
Our condensed consolidated financial statements as of and for the period ended March 31, 2023, have been prepared under the assumption that we will continue as a going concern for the next 12 months. Our management concluded that our recurring losses from operations and the fact that we have not generated significant revenue or positive cash flows from operations raise substantial doubt about our ability to continue as a going concern for the next 12 months after issuance of our financial statements. Our auditors also included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended June 30, 2022 with respect to this uncertainty. If we continue to experience operating losses, and we are not able to generate additional liquidity through a capital raise or other cash infusion, we might need to secure additional sources of funds, which may or may not be available to us. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to further scale back or discontinue the development of our product candidates or other research and development initiatives or initiate steps to cease operations.
We need additional funding to fully execute our business plan, which funding may not be available on commercially acceptable terms or at all. If we are unable to raise capital when needed, we may be forced to delay, reduce or eliminate the commercialization of our development and manufacturing services and efforts for our product development programs.
Even if we are able to sell the CDMO assets or the facility upon favorable terms, we will need additional capital to fully implement our near and long-term business, operating and development plans as we do not anticipate that any of our product candidates will generate revenue in the next few years, if at all. To the extent that we initiate or continue clinical development without securing collaborator or licensee funding, our research and development expenses could increase substantially.
When we elect to raise additional funds or additional funds are required, we may raise such funds from time to time through public or private equity offerings, debt financings, corporate collaboration and licensing arrangements or other financing alternatives. Additional equity or debt financing or corporate collaboration and licensing arrangements may not be available on acceptable terms, if at all. We currently have no committed sources of funding. On November 25, 2020, we entered into a Controlled Equity Offering SM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. ("Cantor Fitzgerald") to sell shares of common stock, from time to time, through an “at the market offering” program having an aggregate offering price of up to $100,000,000 through which Cantor Fitzgerald would act as sales agent (the “Sales Agent”). There can be no assurance that we will meet the requirements to be able to sell securities pursuant to the Sales Agreement, of if we meet the requirements that we will be able to raise sufficient funds on favorable terms. If we are unable to raise capital in sufficient amounts when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or commercialization efforts and our ability to generate revenues and achieve or sustain profitability will be substantially harmed.
If we are unable to raise funds when required or on favorable terms, this assumption may no longer be operative, and we may have to: a) significantly delay, scale back, or discontinue the product application and/or commercialization of our proprietary technologies; b) seek collaborators for our technology and product candidates on terms that are less favorable than might otherwise be available; c) relinquish or otherwise dispose of rights to technologies, product candidates, or products that we would otherwise seek to develop or commercialize; or d) possibly cease operations.
47
The failure to comply with the terms of the Credit Agreement, as amended, could result in a default under the terms of the Credit Agreement, as amended, and, if uncured, it could potentially result in action against our pledged assets.
There is no assurance that iBio CDMO or we will generate sufficient revenue or raise sufficient capital to be able to make the required principal payment under the Term Loan that iBio CDMO entered into with Woodforest National Bank (“Woodforest”). The Term Loan with Woodforest is secured by (a) a leasehold deed of trust on our sole manufacturing facility (the “Facility”), (b) a letter of credit issued by JPMorgan Chase Bank and (c) a first lien on all assets of iBio CDMO including the Facility. We have also guaranteed the payment of all iBio CDMO’s obligations under the Credit Agreement. On March 24, 2023, we and Woodforest entered into a fourth amendment to the Credit Agreement (the “Fourth Amendment”), which within the Fourth Amendment Woodforest agreed to (i) reduce the percentage of any payment to Woodforest we are required to make from the proceeds of sales of its common stock under its at-the-market facility from 40% to 20%, (ii) reduce the percentage of any payment to Woodforest we are required to make from the proceeds of sales of its equipment from 40% to 20%, and (iii) allow us to retain $2,000,000 million of the $5,100,000 million that the Company received from the Fraunhofer Settlement Funds, with the remaining $3,000,000 million being held in a Company account at Woodforest. In addition, we are obligated to (y) deliver to Woodforest an executed copy of a purchase agreement (the “Purchase Agreement”) for the sale of the 130,000 square foot cGMP manufacturing facility in Bryan, Texas, no later than April 14, 2023, and (z) pay to Woodforest a fee in the amount of $75,000 on the earlier of the date of the closing of the Purchase Agreement, or the Maturity Date (as defined in the Credit Agreement). In addition, on March 24, 2023, we entered into a fourth amendment to the Guaranty, which reduced the Liquidity Covenant from $7,500,000 to $1,000,000.
On May 10, 2023, iBio CDMO and Woodforest entered into a fifth amendment to the Credit Agreement (the “Fifth Amendment”), which within the Fifth Amendment Woodforest agreed to: (i) waive the obligation to deliver to Woodforest an executed copy of a Purchase Agreement for the sale of the 130,000 square foot cGMP manufacturing facility in Bryan, Texas, no later than April 14, 2023 and, (ii) release $500,000 of the $3.0 million being held in a Company account at Woodforest when the outstanding principal amount is reduced to $10.0 million and for each additional $2.5 million reduction of the outstanding principal amount, an additional $750,00 will be released from the Company account at Woodforest. In addition, starting on the effective date of the Fifth Amendment, the interest on the Term Loan increased to 5.25%, and the Term Loan shall further accrue interest, payable in kind and added to the balance of the outstanding principal amount at a fixed rate per annum equal to (a) 1.00%, if the Facility is sold on or before June 30, 2023, (b) 2.00% if the Facility is sold after June 2023, but on or before September 30, 2023, or (c) 3:00%, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. The Company also agreed to pay Woodforest a fee in the amount of (x) $75,000 if the Facility is sold on or before June 30, 2023, (y) $100,000 if the Facility is sold after June 2023, but on or before September 30, 2023, or (x) $125,000, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date.
If we fail to successfully extend our cash runway via strategic options, not sell the Facility by the end of the first quarter of Fiscal 2024, or other alternatives as described, we would be in violation of the Liquidity Covenant in the first quarter of Fiscal 2024. If we or iBio CDMO fails to comply with the terms of the Term Loans and/or the related agreements, including the affirmative and negative covenants contained therein and fails to meet the Liquidity Covenant, Woodforest could declare a default, accelerate the payment of all amounts owed by us to Woodforest and if the default were to remain uncured, Woodforest would have the right to proceed against any or all of the collateral securing their Term Loan. Our failure to make such payments when due could result in our loss of the Facility.
The Credit Agreement with Woodforest originally included an affirmative covenant that required us to provide to Woodforest within 120 days of our fiscal year end, our consolidated financial statements, audited by independent certified public accountants without a “going concern” qualification. The consolidated financial statements for the year ended June 30, 2022 include a qualification that raises substantial doubt about our ability to continue as a going concern. As a result, without the amendment to the Credit Agreement, we would have been in violation of the covenant after the expiration of the cure period.
Covenant restrictions in the Credit Agreement, as amended, may limit our ability to operate our business.
The Credit Agreement with Woodforest contains, and our future indebtedness agreements may contain covenants that restrict our ability to finance future operations or capital needs or to engage in other business activities. The Credit Agreement, as amended, currently requires maintaining $1,000,000 of unrestricted cash and cash equivalents and restricts iBio CDMO’s ability to:
● |
incur, assume or guarantee additional Debt (as defined in the Credit Agreement); |
● |
repurchase capital stock; |
● |
make other restricted payments including, without limitation, paying dividends and making investments; |
● |
sell or otherwise dispose of assets other than as specified in the Credit Agreement, as amended. |
If our acquired intangible assets become impaired, we may be required to record a significant charge to earnings.
48
We regularly review acquired intangible assets for impairment when events or changes in circumstances indicate that the carrying value may not be recoverable. We test indefinite-lived intangible assets for impairment at least annually. Factors that may be considered a change in circumstances, indicating that the carrying value of the intangible assets may not be recoverable, include: macroeconomic conditions, such as deterioration in general economic conditions; industry and market considerations, such as deterioration in the environment in which we operate; cost factors, such as increases in labor or other costs that have a negative effect on earnings and cash flows; our financial performance, such as negative or declining cash flows or a decline in actual or planned revenue or earnings compared with actual and projected results of relevant prior periods; the decision not to further develop certain pipeline assets; other relevant entity-specific events, such as changes in management, key personnel, strategy, or customers; and sustained decreases in share price.
We have identified material weaknesses in our internal controls, and we cannot provide assurances that these material weaknesses will be effectively remediated or that additional weaknesses will not occur in the future.
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rule 13a- 15(f) under the Exchange Act. During the preparation of the Quarterly Report for the quarter ended March 31, 2023, we identified a material weakness in our controls relating to accounting for stock-based compensation expense. Specifically, stock-based compensation expense was overstated by $1.2 million in the fiscal quarter ended March 31, 2023 and was understated by the same amount in the fiscal quarter ended December 31, 2022, as a result of an equity awards expense of approximately $1.2 million being incorrectly recorded in the fiscal quarter ended March 31, 2023 upon the immediate vesting of severed employees’ awards on January 2, 2023, instead of accelerating the stock-based compensation expense in accordance with ASC 718-10-55-77 - Share-Based Compensation over the period between giving the employees their notice and their last day of service (i.e., November 3, 2022 – January 2, 2023).
While we plan to take remedial action to address the material weakness in our internal controls, we cannot provide any assurance that such remedial measures, or any other remedial measures we take, will be effective. In addition, a material weakness will not be considered remediated until the applicable controls operate for a sufficient period of time and management has concluded, through testing, that these controls are designed and operate effectively. Although management believes that the material weakness in our internal controls will be remediated, there can be no assurance that the deficiencies will be remediated in the near future or that the internal control over financial reporting, as modified, will enable us to identify or avoid material weaknesses in our internal controls in the future.
As a result of our failure to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, security holders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, is designed to prevent fraud. Our failure to maintain an effective system of internal controls, and any failure by us to implement required new or improved internal controls or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. In addition, any testing by us, as and when required, conducted in connection with Section 404 of the Sarbanes-Oxley Act, or Section 404, or any subsequent testing by our independent registered public accounting firm, as and when required, may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. As a growing company, implementing and maintaining effective controls may require more resources, and we may encounter internal control integration difficulties. Our failure to maintain effective internal controls over financial reporting, may result in us not being able to accurately report our financial results, detect or prevent fraud, or file our periodic reports in a timely manner, which may, among other adverse consequences, cause investors to lose confidence in our reported financial information and lead to a decline in the trading price of our common stock.
Pursuant to Section 404, we will be required to furnish a report by our management on our internal control over financial reporting. However, as a smaller reporting company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm until we are no longer a smaller reporting company. To achieve compliance with Section 404 within the prescribed period, we are engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
49
Changes in general economic conditions, geopolitical conditions, domestic and foreign trade policies, monetary policies and other factors beyond our control may adversely impact our business and operating results.
The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values could impact our business in the future. We and our third-party contract manufacturers, contract research organizations, and clinical sites may also face disruptions in procuring items that are essential to our research and development activities, including, for example, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak. These minor disruptions have had an immaterial effect on business, which we have been able to address with minimal impact to our business operations to date. Further, although we have not experienced any material adverse effects on our business due to increasing inflation, it has raised operating costs for many businesses and, in the future, could impact demand or pricing manufacturing of our drug candidates or services providers, foreign exchange rates or employee wages. We are actively monitoring the effects these disruptions and increasing inflation could have on our operations.
Our operations and performance depend on global, regional and U.S. economic and geopolitical conditions. Russia’s invasion and military attacks on Ukraine have triggered significant sanctions from U.S. and European leaders. These events are currently escalating and creating increasingly volatile global economic conditions. Resulting changes in U.S. trade policy could trigger retaliatory actions by Russia, its allies and other affected countries, including China, resulting in a “trade war.” Furthermore, if the conflict between Russia and Ukraine continues for a long period of time, or if other countries, including the U.S., become further involved in the conflict, we could face significant adverse effects to our business and financial condition.
The above factors, including a number of other economic and geopolitical factors both in the U.S. and abroad, could ultimately have material adverse effects on our business, financial condition, results of operations or cash flows, including the following:
| ● | effects of significant changes in economic, monetary and fiscal policies in the U.S. and abroad including currency fluctuations, inflationary pressures and significant income tax changes; |
| ● | supply chain disruptions; |
| ● | industrial policies in various countries that favor domestic industries over multinationals or that restrict foreign companies altogether; |
| ● | new or stricter trade policies and tariffs enacted by countries, such as China, in response to changes in U.S. trade policies and tariffs; |
We may maintain our cash assets at certain financial institutions in the U.S. in amounts that may be in excess of the Federal Deposit Insurance Corporation (“FDIC”) insurance limit of $250,000.
We may maintain our cash assets at certain financial institutions in the U.S. in amounts that may be in excess of the Federal Deposit Insurance Corporation (“FDIC”) insurance limit of $250,000. In the event of a failure of any financial institutions where we maintain our deposits or other assets, we may incur a loss to the extent such loss exceeds the FDIC insurance limitation, which could have a material adverse effect upon our liquidity, financial condition and our results of operations.
Due to the discontinuance of the CDMO business, we will not be generating material revenue from CDMO operations going forward.
As a result of the discontinuance of the CDMO business, we will not generate material revenue from the CDMO operations any longer.
We have experienced turnover in our senior management team, and the loss of one or more of our executive officers or key employees or an inability to attract and retain highly skilled employees could adversely affect our business.
Our success depends largely upon the continued services of our key executive officers. We have in the past and may in the future experience changes in our executive management team resulting from the departure of executives, which may be disruptive to our business.
50
To continue to develop our pipeline and execute our strategy, we also must attract and retain highly skilled personnel in our industry.
Item 5. Other Information
On May 10, 2023, iBio CDMO and Woodforest entered into a fifth amendment to the Credit Agreement (the “Fifth Amendment”), which within the Fifth Amendment Woodforest agreed to: (i) waive the obligation to deliver to Woodforest an executed copy of a Purchase Agreement for the sale of the Facility no later than April 14, 2023 and, (ii) release $500,000 of the $3.0 million being held in a Company account at Woodforest when the outstanding principal amount is reduced to $10.0 million and for each additional $2.5 million reduction of the outstanding principal amount, an additional $750,00 will be released from the Company account at Woodforest. In addition, starting on the effective date of the Fifth Amendment, the interest on the Term Loan increased to 5.25%, and the Term Loan shall further accrue interest, payable in kind and added to the balance of the outstanding principal amount at a fixed rate per annum equal to (a) 1.00%, if the Facility is sold on or before June 30, 2023, (b) 2.00% if the Facility is sold after June 2023, but on or before September 30, 2023, or (c) 3:00%, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. The Company also agreed to pay Woodforest a fee in the amount of (x) $75,000 if the Facility is sold on or before June 30, 2023, (y) $100,000 if the Facility is sold after June 2023, but on or before September 30, 2023, or (x) $125,000, if the Facility is sold after September 30, 2023, or not sold prior to the maturity date. After marketing the Facility with a real estate firm for approximately six months, in May 2023, the Company entered into a new agreement with a new global commercial real estate firm to remarket the Facility. As of the date of the filing of this Quarterly Report on Form 10-Q, the Company is not in negotiations on a Purchase Agreement with a potential buyer of the Facility.
Item 6. Exhibits.
Exhibit No. |
|
Description |
|
|
|
3.1 |
||
|
|
|
3.2 |
|
|
|
|
|
3.3 |
|
|
|
|
|
3.4 |
|
|
|
|
|
3.5 |
|
|
|
|
|
10.1 |
|
|
|
|
|
10.2 |
|
|
|
|
|
10.3+ |
|
|
|
|
|
10.4+ |
|
|
|
|
|
51
10.5 |
|
|
|
|
|
10.6 |
|
|
|
|
|
10.7* |
|
|
|
|
|
10.8* |
|
|
|
|
|
31.1* |
|
|
|
|
|
31.2* |
|
|
|
|
|
32.1* |
|
|
|
|
|
32.2* |
|
|
|
|
|
101.INS |
|
Inline XBRL Instance* |
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema* |
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation* |
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition* |
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Labeled* |
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation* |
|
|
|
104 |
|
Cover page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
+ Certain portions of this exhibit indicated therein by [**] have been omitted in accordance with Item 601(b)(10) of Regulation 8-K.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
iBio, Inc. |
|
(Registrant) |
|
|
Date: May 15, 2023 |
/s/ Martin Brenner |
|
Martin Brenner |
|
Interim Chief Executive Officer and Chief Scientific Officer |
|
|
Date: May 15, 2023 |
/s/ Felipe Duran |
|
Felipe Duran |
|
Interim Chief Financial Officer |
52
Exhibit 10.7

AUCTION SALE AGREEMENT
HOLLAND INDUSTRIAL GROUP LLC, together with FEDERAL EQUIPMENT COMPANY, and CAPITAL RECOVERY GROUP LLC (collectively, the “Auctioneers”) which will act jointly and severally as the exclusive agents on behalf of IBIO INC (“IBIO”) pursuant to this agreement (the “Agreement”), dated February 10, 2023 (the “Effective Date”) for the sale at public auction (the “Auction”) or by negotiated sales (“Negotiated Sales”) of equipment and other tangible personal property (collectively, the “Equipment”) located at IBIO’s facility at 8800 Health Science Center Pkwy, Bryan TX 77807 (the “Facility”) upon the following terms and conditions:
1. |
AUCTION & AUCTION DATES. |
The Auction will be conducted as an online Auction on the Internet through an Internet provider selected by Auctioneers (the “Internet Provider”) commencing on a mutually agreed upon date to be scheduled following the Effective Date of this Agreement.
2.EQUIPMENT TO BE SOLD.
The Equipment to be sold shall include all of the Equipment at the Facility designated by IBIO for sale as per Exhibit A. Once an item of Equipment is advertised for sale by Auctioneers it shall remain available for sale unless otherwise agreed in writing by Auctioneers, which shall not be unreasonably withheld, conditioned or delayed.
3. |
AUCTION PROMOTION & ADVERTISING. |
Prior to the Auction, Auctioneers will conduct a promotional marketing campaign for the sale of the Equipment utilizing digital publication on Industry websites, Internet publication on Auctioneers’ Websites and the Internet Provider’s Website, emails to Auctioneers’ subscriber lists and by such other means as Auctioneers deem appropriate in their professional judgment.
Auctioneers’ customer lists, which have been developed over years of auction selling to customers throughout the world will be used for marketing the sale of the Equipment.
A detailed catalog listing each item of Equipment with photographs and a description will be prepared by Auctioneers, posted on Auctioneers’ Websites and the Internet Provider’s Website.
4.SALE PREPARATION; LABOR & ACCESS TO FACILITY.
Auctioneers will supervise and direct the preparation of the Equipment for sale no later than [DATE]. IBIO, at its expense, shall provide at least one (1) person, upon a mutually agreed upon date for a period not to exceed two (2) business days, reasonably familiar with the Equipment at the Facility to answer questions from Auctioneers relating to all matters relating to the preparation, inspection, sale and removal of the Equipment.

Auctioneers and their representatives shall, at no additional cost and expense be permitted reasonable access to the Facility during IBIO’s standard business hours starting upon a mutually agreed upon date through the Removal Period (as defined in Section 7 below) and provided with normal utility service for water, sewer, heat and electricity (collectively, the “Utilities”) to prepare for and conduct the Auction and for the removal of the Equipment by the purchasers (the “Purchaser”) and their riggers following its sale. Prospective purchasers are to be permitted to inspect the Equipment at the Facility on the day before commencement of the Auction and at other times by appointment made in advance with IBIO.
Prior to the sale of any of the Equipment, IBIO, at its sole cost and expense, shall remove all chemicals, hazardous materials and toxic substances as defined in any applicable environmental laws, rules and regulations (“Hazardous Materials”) contained in the Equipment and in any of the lines running to and from the Equipment, with the exception for any lines, lubricants or other materials reasonable required for the storage, operation and maintenance of the Equipment, as reasonably determined by IBIO.
5.EXPENSES.
All marketing expenses for promoting the sale and all labor, travel, lodging and other necessary expenses for Auctioneers’ personnel to prepare for and conduct the Auction and conclude the sale of the Equipment have been budgeted not to exceed Two Hundred Thousand Dollars ($200,000) (the “Expenses”). The Expenses will be advanced by Auctioneers and reimbursed out of the proceeds from the sale of the Equipment as provided in Section 10 below.
6. |
SALE PROCEDURES & TERMS. |
The Equipment to be sold at the Auction will be sold to the highest bidders for cash. At the time of the Auction, IBIO represents, warrants and covenants that IBIO is authorized to execute and perform its obligations under this Agreement, has the right to sell the Equipment, has good and marketable title to the Equipment, and, to its knowledge, all Equipment will be free and clear of all liens, claims and encumbrances of any kind or nature. Auctioneers will be auctioning the Equipment on an “as is,” “where is” and “with all faults” basis. Auctioneers will consult with IBIO regarding the manner and method of Auction but shall have the right to present the Equipment and conduct the Auction using their best efforts provided, however in the manner, and utilizing the methods, that they deem in their professional judgment to be appropriate.
All bidders will be required to register with Auctioneers and the Internet Provider prior to bidding and agree to be bound by the Terms of Sale established by Auctioneers and the Internet Provider; provided, however, such Terms of Sale shall not provide any representations, warrants or covenants from IBIO without its express prior consent.
With the prior written approval of IBIO, Equipment may be sold by Negotiated Sales before the Auction.
The sale of an item of will be considered final once the item has been paid for at the Auction (or in a Negotiated Sale). In the event payment is not made for an item bid upon at the Auction, the item shall be considered a “non-sale.” Auctioneers in conjunction with IBIO shall use their best efforts to resell any non-sale item following the Auction by Negotiated Sales.

7.REMOVAL.
Removal of the Equipment shall be done by qualified riggers at the expense, risk and liability of the Purchasers, including, without limitation, liability for any damages to the Facility or for any environmental conditions that may be caused or exacerbated in connection with the removal of the Equipment. Auctioneers will assist in identifying qualified riggers with the understanding that the riggers will not be employees or agents of Auctioneers, and will be engaged by the Purchasers, perhaps as independent contractors. Removal parameters for the removal by the selected riggers will be pursuant to terms and conditions mutually agreed to in writing by Auctioneer and IBIO. Neither Auctioneers nor IBIO shall have any liability for any damages or injuries to persons or property in connection with removal of the Equipment. Purchasers will be required to remove all Equipment following the Auction within a period no later than April 28th 2023 (the “Removal Period”). All riggers prior to commencement of removal will be required to furnish a certificate of insurance for general liability insurance with limits of no less than Two Million Dollars ($2,000,000) per occurrence with financially sound, duly licensed and reputable insurers on account of injury or damage to persons or property at the Facility, naming IBIO and Auctioneers as additional insureds. During the Removal Period, the Purchasers and their riggers, while in coordination with Auctioneers, shall be given access to the Facility by IBIO during IBIO’s normal business hours with 48 hours in advance notice and provided with the utilities at no cost to remove their purchases. If the Purchaser or their riggers do not show after the confirmation by IBIO of a pick-up date, IBIO may reschedule an alternative date in their reasonable discretion. If a Purchaser, or their riggers, never picks up their items from the Auction after reasonable efforts by IBIO to make these arrangements (a “No-Show”), the proceeds from any sale of a No Show shall be credited towards the Sales Proceeds regardless of whether the Purchaser ever takes possession of those items or not. The Auctioneers will supervise the Purchasers are removing only the Equipment they purchased, and be present at the Facility when the Purchasers and their riggers remove their purchases. Notwithstanding the foregoing, if any Purchaser does not remove their purchases prior to the end of the Removal Period, the Auctioneers will remove such purchases from the facility no later than May 1, 2023.
8. |
SECURITY & RISK OF LOSS. |
From the Effective Date until the Removal Period, IBIO shall be responsible, at its sole cost and expense, for all reasonable security at the Facility before, during and through the Removal Period, and shall be responsible for all risk of loss or damage to the Equipment until it is paid for by the Purchasers and removed by the Purchasers, except for any loss or damage to either: (i) the Equipment caused by Auctioneers or their employees, agents or representatives, or (ii) the Facility or the Equipment caused by the Purchasers or their employees, agents, or representatives, including but not limited to the rigger removing items on behalf of any Purchaser.
9.AUCTIONEERS’ COMPENSATION.
a.Guaranteed Amount. Auctioneer hereby guarantees that the aggregate amount of the gross proceeds from the sale of the Equipment (“Sale Proceeds”) shall be an amount at least equal to Two Million One Hundred Thousand Dollars ($2,100,000) (the “Guaranteed Amount”). As used herein, “Sale Proceeds” excludes any and all Buyer’s Premium (as that term is defined in Section 9c below).

b.Initial Deposit; Balance of Remaining Guaranteed Amount. Within five (5) business days after IBIO provides Auctioneer with evidence reasonably satisfactory to Auctioneer that IBIO will deliver free and clear title to the Equipment, including the payment of any Personal Property Taxes as set forth in Section 9e below, if any, Auctioneer shall pay to IBIO as an advance the sum of by certified check or wire transfer of immediately available funds, representing one hundred percent (100 %) of the Guaranteed Amount (“Initial Deposit”). IBIO shall have the right to terminate this Agreement effective immediately if Auctioneer fails to pay IBIO in accordance with this Section 9b.
c.Auctioneer's Compensation. Provided that Auctioneer has paid the Initial Deposit to IBIO pursuant to the terms of the immediately preceding Section 9b, IBIO hereby agrees that: (i) Auctioneer shall retain the portion of the Sale Proceeds in an amount not to exceed the Guaranteed Amount; and (ii) Auctioneer shall retain the portion of Sale Proceeds, if any, after subtracting the Guaranteed Amount there from, in the amount not to exceed the Expenses, as defined in Section 5. Auctioneer shall also charge Purchasers and retain a buyer’s premium (the “Buyer’s Premium”) equal to a percent to be determined by Auctioneer of the gross purchase price as compensation to Auctioneer for all Equipment sold prior to, during and after the Auction (including any Negotiated Sales), which shall be payable by each Purchaser in addition to the purchase price bid. Auctioneer intends to charge a Buyer’s Premium of eighteen percent (18%), which shall not be increased without the prior written consent of IBIO.
d.Sale Expenses. Subject to Section 5 above and in accordance with Section 9a-c above, Auctioneer shall be entitled to be reimbursed for Expenses. Notwithstanding the foregoing, expenses related to the use of the Facility (for example, utilities, trash removal, internet service, rent, insurance for the Subject Premises, etc.), shall be paid for solely by IBIO and not be part of the Expenses reimbursed to Auctioneer. For purposes of clarification, IBIO shall be entitled to charge any costs or expense related to the disposal of any Equipment purchased by the Purchaser and not removed prior to the Removal Period and which are incurred after the Removal Period to the Purchaser as a condition of the removal of any Equipment purchased.
e.Personal Property Taxes. If applicable, IBIO is responsible for paying all Personal Property Taxes on the Equipment for taxes due and owing but unpaid and taxes for the entire calendar year 2022/2023 which have been assessed against IBIO for the Equipment for the year 2022/2023 but may not yet be due and owing (collectively the “Personal Property Taxes”). Before Auctioneer directs final payment of the Sales Proceeds, as provided in this Agreement, IBIO will provide Auctioneer paid receipts from the taxing municipality evidencing IBIO’s payment of all Personal Property Taxes. In the event that Auctioneer receives evidence of IBIO not paying all Personal Property Taxes on the Equipment for taxes due and owing pursuant to this Section 9e, Auctioneer shall provide such evidence promptly to IBIO, and Auctioneer may deduct from the Sales Proceeds the amount of such Personal Property Taxes and pay them to the taxing authority directly within 10 business days after the Auction has ended.
f.Upside Sharing. In addition to Auctioneer’s obligation to deliver the Guaranteed Amount pursuant to Section 9a hereof, if applicable, Auctioneer agrees to deliver to IBIO per the ii.Any Sale Proceeds over $2,300,000 will be split 80:20 in favor of IBIO (with Sales Proceeds between $2,100,000 and $2,300,000 remitted to Auctioneer for Expenses); and

following formula:
i.the Guaranteed Amount;
iii.Auctioneer shall be entitled to the Buyer’s Premium on the sale of all items.
10. |
ACCOUNTING & SETTLEMENT. |
Auctioneers will maintain accurate records of the bidding during the Auction and accurate records of all Negotiated Sales. A preliminary accounting for all items sold at the Auction will be provided to IBIO immediately following the Auction.
All Sales Proceeds from the Auction of the Equipment will be collected by Auctioneers. Auctioneers will deliver to IBIO an itemized accounting setting forth the purchase price for each item sold together with an itemization of Sale Expenses within Fourteen (14) calendar days of the Auction. At the time of the delivery of the accounting, remittance will be made to IBIO of the Sales Proceeds, less the Guaranteed Amount, Expenses, and the portion of Sales Proceeds due to Auctioneer (which are over $2,300,000) pursuant to Section 9f (iii). All amounts in this Agreement are to be paid in U.S. Dollars.
Copies of all records of the sale of the Equipment shall be maintained at Auctioneers’ offices for a period of one (1) year following completion of the Auction and at all times during that one (1) year period shall be available to IBIO upon reasonable advance notice.
11.UTILITY DISCONNECTION AND EQUIPMENT REMOVAL.
The Purchasers at the Auction shall be solely responsible for disconnecting any utilities from the Equipment and rigging and shipping the purchased Equipment. Under no circumstances shall IBIO be responsible for Equipment removal or utility disconnection unless IBIO specifically is requested and agrees to do so. All Equipment purchased must be removed from the Facility by the end of the Removal Period. The Equipment shall be removed in a workmanlike manner under Auctioneers’ direct supervision, provided however, Auctioneer has no responsibility for supervising the Facility or providing security to protect the Facility or any of its contents, including the Equipment, from theft, vandalism, casualty or other damage at any time before, during or after the Removal Period. Subject to the limitations of liability set forth in Section 19, Auctioneer will instruct all Purchasers to remove oils or other fluids that any such Purchaser drained or caused to be drained from any of the Equipment before such Purchaser removes such Equipment from the Facility. Any oils or other fluids taken from any Equipment, if not removed by the Purchaser, will remain the responsibility of Auctioneer to store and/or remove from the Facility. Unless included in Exhibit A, under no circumstances will Auctioneer ever be responsible for draining, collecting, cleaning, storing or removing any oils, fluids or any other Hazardous Materials which is located on the Facility, whether as part of Equipment or separately contained or occurring.
12.USE OF PREMISES.

IBIO authorizes Auctioneer and its representatives to enter upon and use the Facility for the purposes of a) storing the Equipment thereupon; b) preparing for and conducting the Auction (or Negotiated Sales); c) otherwise exhibiting the Equipment to prospective purchasers; and d) with IBIO’s prior consent, for such other purposes as are reasonably and necessary to conduct the Auction and all manner of sales public or private. IBIO agrees that Auctioneer will have rent-free use of the Facility until completion of the project but in no event beyond the Removal Period. IBIO further agrees that it will furnish utilities, heating and proper lighting necessary to prepare for and conduct the Auction at IBIO’s sole expense. The Auctioneer will leave the Facility in the same general condition that the Facility is in currently (wear and tear excepted). Notwithstanding the foregoing, IBIO acknowledges that Auctioneer shall remove all items in EXHIBIT A on or before May 1, 2023 .
13.ENVIRONMENTAL MATTERS.
Auctioneers shall have no liability or responsibility with respect to any environmental conditions or matters or Hazardous Materials with respect to the Equipment and the Facility, including, without limitation, any responsibility for any Hazardous Materials contained in any of the Equipment or for any environmental conditions that may be caused or exacerbated in connection with the removal of the Equipment. IBIO will undertake all reasonable precautions to ensure that Hazardous Materials, if any, at the Facility and in the Equipment do not become exposed to the Purchasers or Auctioneers’ personnel.
14. |
TITLE TO EQUIPMENT; CONSENTS. |
If requested by the Purchaser, IBIO will provide a bill of sale in order to warrant to the Purchasers of the Equipment that IBIO has the right to sell the Equipment and that to its knowledge, the Equipment will be sold with good and marketable title to the Purchasers free and clear of all liens, claims, restrictions and encumbrances of any kind or nature.
15.INSURANCE.
Auctioneers shall carry general liability insurance with limits of no less than Two Million Dollars ($2,000,000) in the aggregate with financially sound, duly licensed and reputable insurers on account of injury or damage to persons or property at the Facility, naming IBIO as an additional insured. Prior to entering the Facility to prepare for the sale of the Equipment, Auctioneers shall furnish IBIO with a certificate evidencing such coverage. Throughout the duration of this Agreement, IBIO shall maintain property and casualty insurance on the Equipment and any insurance required on the Facility, naming Auctioneer as additional insured. Should an insurance claim be required due to damage to any of the Equipment (sold pursuant to Section 6) because of fire, theft, flood, etc., IBIO will be solely responsible for filing any such claim and any insurance proceeds due shall be deemed part of the Sales Proceeds of the Auction and distributed in accordance with Section 9 above. Risk of loss to the Equipment due to theft or vandalism remains with IBIO until the Equipment is paid for at the Auction by a Purchaser.
16. INDEMNIFICATION.

IBIO agrees to indemnity and hold Auctioneer, their members, directors, officers, shareholders, agents and employees (each an “Auctioneer Indemnitee”) harmless from any third party claims, causes of action, damages and liabilities of any kind (each a “Claim”) arising from or in connection with: (i) any breach of the warranties contained in Section 6 directly relating to the Equipment made by IBIO to Auctioneer, or (ii) any material breach of the remaining provisions of this Agreement by IBIO, except if and to the extent that any such Claim has resulted from any fraud, intentional misconduct, willful misconduct, or failure to comply with applicable laws by any Auctioneer Indemnitees in connection with the performance of this Agreement.
Auctioneer agrees to indemnify and hold IBIO, its subsidiary iBio CDMO LLC, and their respective directors, officers, employees, shareholders, agents and advisors (each an “IBIO Indemnitee”) harmless from any Claims arising from or in connection with (i) any material breach of the Agreement by Auctioneer, or (ii) any fraud, intentional misconduct, willful misconduct, or failure to comply with applicable laws by any Auctioneer Indemnitees in connection with the performance of this Agreement.
In the event an Indemnitee seeks indemnification hereunder, it shall (a) inform the indemnifying party of the Claim as soon as reasonably practicable after it receives notice of the same, (b) permit the indemnifying party to assume direction and control of the defense or investigation of the Claim at the expense of the indemnifying party (including the right to settle the claim solely for monetary consideration provided that the Indemnitee receives a full and unconditional release of the Claim in connection therewith) and (c) cooperate as requested (at the expense of the indemnifying party) in the defense of the Claim. The failure of an Indemnitee to perform any obligations under this Section shall not relieve the indemnifying party of its obligations under this Section, except to the extent that the indemnifying party can demonstrate that defense of the Claim has been materially affectedy as a result of such failure.
17.GOVERNING LAW.
This Agreement is executed in and shall be governed by and construed under, the laws of the State of Delaware.
18. BINDING ARBITRATION CLAUSE
THE PARTIES HEREBY WAIVE THEIR RIGHT TO A JURY TRIAL. ANY CONTROVERSY OR CLAIM ARISING OUT OF CONTRACT, TORT, STATUTE OR OTHERWISE (INCLUDING THE INTERPRETATION OF THIS ARBITRATION CLAUSE, AND THE ARBITRABILITY OF THIS CLAIM OR DISPUTE BETWEEN THE PARTIES HERETO AND/OR ANY OF THEIR RESPECTIVE EMPLOYEES, AGENTS, SUCCESSORS, ASSIGNS, OR CONSIGNORS, SHALL BE SETTLED BY ARBITRATION ADMINISTERED BY JUDICIAL ARBITRATION AND MEDIATION SERVICES, INC., UNDER JAMS COMPREHENSIVE ARBITRATION RULES & PROCEDURES. THE NUMBER OF ARBITRATORS SHALL BE ONE (1). THE PARTIES AGREE THAT THEY SHALL EACH BE RESPONSIBLE FOR THEIR OWN ARBITRATION FEES AND COSTS UNTIL SUCH TIME AS THE ARBITRATOR AWARDS ARBITRATION COSTS TO THE PREVAILING PARTY. THE ARBITRATION SHALL BE HELD NEW CASTLE COUNTY, DELAWARE. JUDGMENT ON THE AWARD RENDERED BY THE ARBITRATOR MAY BE ENTERED IN ANY COURT HAVING JURISDICTION THEREOF. THIS AGREEMENT SHALL BE CONSTRUED AND INTERPRETED ACCORDING TO THE LAWS OF THE STATE OF DELAWARE. IF ANY ACTION BASED ON THE PERFORMANCE, BREACH OR INTERPRETATION OF THIS CONTRACT IS BROUGHT, THE PREVAILING PARTY IN SUCH ACTION AS DETERMINED BY THE ARBITRATOR SHALL BE ENTITLED TO RECOVER FROM THE LOSING PARTY ALL ACTUAL AND DOCUMENTED COSTS, EXPENSES OF ARBITRATION, AND REASONABLE ATTORNEY'S FEES.

ANY AWARD OF THE ARBITRATOR SHALL BE IN WRITING AND WILL BE FINAL AND BINDING ON ALL PARTIES, SUBJECT TO ANY LIMITED RIGHT OF APPEAL UNDER THE FEDERAL ARBITRATION ACT.
19.LIMITATION OF LIABILITY.
Auctioneer has the obligations set forth in this Agreement but in no event, and regardless of any undertaking by Auctioneer in this Agreement, in any other document, or made without a writing, shall Auctioneer have any liability to IBIO which exceeds the amount equal to the total Sales Proceeds retained by Auctioneer plus the amount of Buyer’s Premium collected by the Auctioneer.
TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, IN NO EVENT SHALL EITHER PARTY BE RESPONSIBLE OR HAVE ANY LIABILITY UNDER THIS AGREEMENT FOR ANY INDIRECT, SPECIAL, EXEMPLARY, INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES OR ANY LOST PROFITS OR LOSS OF REVENUE ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT EVEN IF ADVISED OF THE POSSIBILITY THEREOF.
20.DISCLAIMER OF WARRANTIES.
WITH THE EXCEPTION OF THE EXPRESS WARRANTIES OR REPRESENTATIONS CONTAINED IN SECTION 6 OF THIS AGREEMENT, IBIO HAS NOT HERETOFORE MADE AND DOES NOT HEREBY MAKE ANY WARRANTIES OR REPRESENTATIONS OF ANY KIND, EXPRESS OR IMPLIED, WITH RESPECT TO THE EQUIPMENT, INCLUDING, WITHOUT LIMITATION, WITH RESPECT TO THE QUALITY, QUANTITY, CONDITION, VALUE, QUIET ENJOYMENT, MERCHANTABILITY, OR FITNESS FOR ANY PURPOSE WHATSOEVER. THE EQUIPMENT IS SOLD AND ASSIGNED “AS IS,” “WHERE IS”.
21.COUNTERPARTS; FACSIMILE SIGNATURES.
This Agreement may be executed in any number of counterparts, each of which, when executed, will be deemed to be an original and all of which, when taken together, will be deemed to be but one and the same legally enforceable and binding instrument. Delivering signatures via facsimile or electronic transmission in .pdf format shall be an acceptable means of executing this Agreement and signatures so delivered shall be treated as originals and be fully binding on the signing party.
22. SEVERABILITY.
The provisions of this Agreement shall be severable. Should any part, term or provision of this Agreement be construed by any court of competent jurisdiction to be illegal, invalid or unenforceable for any reason, the legality, validity and enforceability of the remaining parts, terms and provisions shall not be affected thereby.
23. INDEPENDENT CONTRACTOR.

Nothing contained in this Agreement will be construed as creating any agency, partnership, joint enterprise or other similar relationship between the parties. The relationship between the parties will at all times be that of independent contractors. Neither party will have authority to contract for or bind the other in any manner whatsoever. This Agreement confers no rights upon either party except those expressly granted herein and does not confer any right upon either party to make any representations or commitment on behalf of the other.
24.FORCE MAJEURE.
Neither party will be liable or responsible to the other party, nor be deemed to have defaulted or breached this Agreement, for any failure or delay in fulfilling or performing any term of this Agreement when and to the extent such failure or delay is caused by or results from acts of God, flood, fire, earthquake, explosion, governmental actions, war, invasion or hostilities (whether war is declared or not), terrorist threats or acts, riot, or other civil unrest, national emergency, revolution, insurrection, epidemic, pandemic, lock-outs, strikes or other labor disputes (whether or not relating to either party’s workforce), or restraints or delays affecting carriers or inability or delay or telecommunication breakdown or power outage, provided that, if the event in question continues for a continuous period in excess of thirty (30) days, either party shall be entitled to give notice in writing to the other to terminate this Agreement. Neither party will be liable for any loss, injury, delays or damages suffered or incurred by the other party due to the above causes or to the termination of the Agreement pursuant to this Section.
25.COMPLETE AGREEMENT; ASSIGNMENT.
This Agreement constitutes the entire understanding between the parties and replaces any and all prior agreements related to the Auction. This Agreement may not be modified or amended except in writing signed by the parties. This Agreement may not be assigned to any party without the written consent of the other parties.
26.AUCTIONEER’S TERMS AND CONDITIONS.
Attached here as Exhibit B are the terms and conditions Auctioneers provide Purchasers and other participants to its auctions and sales. These terms and conditions will be used for the Auction. The terms and conditions include terms which give to the Purchasers the same responsibilities as those described in Sections 7 and 11 of this Agreement as being performed by Purchasers, and not IBIO or Auctioneers.

We thank you for the opportunity to present this Agreement. Please indicate your acceptance by signing and returning a copy of this Agreement to us and we will schedule the Auction and commence our promotional campaign.
|
HOLLAND INDUSTRIAL GROUP LLC |
||||
|
|
||||
|
|
||||
DATED: February 9, 2023 |
|
By: |
/s/ Brian Holland |
||
|
|
Brian Holland, Authorized Signatory |
|||
|
|
||||
|
|
||||
|
FEDERAL EQUIPMENT COMPANY |
||||
|
|
||||
DATED: February 9, 2023 |
By: |
/s/ Adam Covitt |
|||
|
Title: |
President |
|||
|
Print Name: |
Adam Covitt |
|||
|
|
||||
|
|
||||
|
CAPITAL RECOVERY GROUP LLC |
||||
|
|
||||
DATED: February 9, 2023 |
By: |
/s/ William Firestone |
|||
|
Title: |
Chief Executive Officer |
|||
|
Print Name: |
William Firestone |
|||
|
|
||||
|
|
||||
Agreed and accepted this 10th day of |
|
||||
February, 2023 |
|
||||
|
|
||||
IBIO, Inc. |
|
||||
|
|
||||
By: |
/s/ Marc Banjak |
|
|||
Title: |
General Counsel |
|
|||
Print Name: |
Marc Banjak |
|
|||
|
|
||||

EXHIBIT A
[List of Equipment]

iBio Tag # |
Equipment |
Equipment |
Vendor / |
Model # |
Serial # |
A-501 |
Part; Motor |
Homogenizer Motor |
|
108705200260 |
61738H001-4 |
ALIC-25 |
Other |
Seeder Emergency Stop |
|
PWS4PDRC5000 |
JJPWS4PDRCP50003 |
ALID-30-32 |
Other |
Wyckoma UV Water Purifier |
|
N/A |
N/A |
AM-01 |
Benchtop pH/Conductivity Meter |
Orion Versa Star Pro pH/Conductivity Benchtop Meter |
Thermo Scientific |
Orion VeraStar Pro |
V10865 |
AM-01_PR-01 |
Benchtop pH/Conductivity Meter; Printer |
Orion versa printer |
SNBC |
BTP-M300 |
1912610956 |
AM-02 |
Benchtop pH/Conductivity Meter |
PH/conductivity meter (Thermo VSTAR50) |
Thermo Scientific |
Orion VeraStar Pro |
V10372 |
AM-03 |
Handheld pH/Conductivity Meter |
Benchtop pH/conductivity meter |
Thermo Scientific |
Orion Star A329 |
G08221 |
AM-04 |
Handheld pH/Conductivity Meter |
Benchtop pH/conductivity meter |
Thermo Scientific |
Orion Star A329 |
G08479 |
AM-05 |
Handheld pH/Conductivity Meter |
Benchtop pH/conductivity meter |
Thermo Scientific |
Orion Star A329 |
G07509 |
AM-06 |
Handheld pH/Conductivity Meter |
Benchtop pH/conductivity meter |
Thermo Scientific |
Orion Star A329 |
G08220 |
AM-07 |
Handheld pH/Conductivity Meter |
pH/EC Meter |
Thermo Scientific |
Orion Star A329 |
G08790 |
AM-08 |
Benchtop pH/Conductivity Meter |
PH/Conductivity meter (Thermo VSTAR50) |
Thermo Scientific |
Orion VeraStar Pro |
V13929 |

AM-10 |
Benchtop pH/Conductivity Meter |
PH Meter |
THERMO FISHER |
Orion VeraStar Pro (VSTAR90) |
V16639 |
AM-11 |
Benchtop pH/Conductivity Meter |
Benchtop pH/conductivity meter |
Thermo Scientific |
Orion VeraStar Pro |
V16857 |
AM-12 |
Benchtop pH/Conductivity Meter |
Benchtop pH/conductivity meter |
VWR |
MU6100L |
21230692 |
BC-01 |
BSC |
Bio Safety Cabinet (SterilGARD SC403A-HE) |
Baker |
SterilGARD SC403A-HE |
97741 |
BC-02 |
BSC |
Biosafety Cabinet |
THE BAKER COMPANY |
SG403A-HE |
98461 |
BC-03 |
BSC |
Biological Safety Cabinet |
Baker Company |
SG403A-HE |
103026 |
BC-04 |
BSC |
Biosafety Cabinet |
BAKER SterilGard |
SG603A-HE |
103270 |
BC-05 |
BSC |
Bio Safety Cabinet (SterilGARD SG404) |
THE BAKER COMPANY |
SG404 |
121145 |
BC-06 |
BSC |
Biosafety Cabinet |
BAKER SterilGard |
SG604 |
121144 |
BC-07 |
BSC |
Biosafety Cabinet |
BAKER SterilGard |
SG404 |
125223 |
BC-08 |
BSC |
Biosafety Cabinet |
Baker |
SterilGARD SC-404 |
|
BIO-01 |
Analyzer |
Agilent 2100 Bioanalyzer; Computer: IBIOAGILENT |
Agilent |
G2939B |
DEDAE01028 |
BL-01 |
Other |
Compactor; Bailer |
Marathon |
4224 |
2091883 |
BLI-01 |
Analyzer |
Biolayer Interferometer (BLItz) |
Pall Forte-bio |
Blitz |
FB-60066 |
BLOT-01 |
Gel Box |
Blotter |
Bio-Rad |
Criterion Blotter |
560BR 00926 |

BLOT-02 |
Gel Box |
Blotter |
Invitrogen by Thermo Scientific |
iBlot |
10069096 |
BLOT-03 |
Gel Box |
Blotter |
Invitrogen by Thermo Scientific |
iBlot |
10060140 |
BR-04 |
Bioreactor |
Bio Flo Controller; Computer: DT-72a |
Eppendorf |
BioFlo 320 |
B320HP001718 |
BR-05 |
Bioreactor |
Bio Flo Controller; Computer: DT-72a |
Eppendorf |
BioFlo 320 |
B320HP001719 |
BR-06 |
Bioreactor |
BioFlo 320, configured controller |
Eppendorf |
BIOFLO_320_CS |
|
BR-06 & BR-07_N/A |
Bioreactor |
DO Cable, with T-82 connector for BioFlo 320 |
Eppendorf |
M1379-8106 |
|
BR-06 & BR-07_N/A |
Bioreactor |
DO Sensor, Mettler Toledo InPro 6820, 225mm, straight T-82 connector |
Eppendorf |
P0720-6526 |
|
BR-06 & BR-07_N/A |
Bioreactor |
Single-Use Vessel Bundle for BioFlo 320 |
Eppendorf |
M1379-0322 |
|
BR-07 |
Bioreactor |
BioFlo 320, configured controller |
Eppendorf |
BIOFLO_320_CS |
|
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick Power Controller and System |
Eppendorf |
BIOFLO-110 |
301062575 |
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick Controller |
Eppendorf |
BIOFLO-110 |
301048357 |
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick |
Eppendorf |
BIOFLO-110 |
301062554 |

|
|
Liquid Addition Pump |
|
|
|
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick Heat Jacket |
|
DO302 |
R0723681 |
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick Vessel (5L) |
|
N/A |
N/A |
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick DO/PH Controller |
Eppendorf |
BIOFLO-110 |
300847316 |
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick Foam Level Controller |
Eppendorf |
BIOFLO-110 |
301162598 |
BR-N/A (BIOFLO-110) |
Bioreactor |
New Brunswick Gas Mix Controller Bus |
Eppendorf |
BIOFLO-110 |
301062566 |
BR-N/A (Holloway) |
|
Holloway America Bioreactor (200L) |
|
609458 |
30527 |
BR-N/A (Holloway) |
|
Holloway America Bioreactor (50L) |
|
608719 |
30129 |
BR-N/A (Holloway) |
|
Holloway America Heat Jacket |
|
30437 |
10453C37 |
C-01 |
Other |
Hoopman Seed Coater and Power Switch (pan coater) |
HOOPMAN EQUIPMENT AND ENGINEERING |
PC-S |
20E60-0275 |
CAP-01 |
Capillary Electrophoresis |
ESI Separation System; Capillary Electrophoresis |
deltaDOT |
Peregrine 1 |
PER00128 |

CAP-02 |
Capillary Electrophoresis |
CESI 8000 plus; HIGH PERFORMANCE SEPARATION ESI MODULE; Computer: ABSCIEX-TTOATVR |
SCIEX |
CESI 8000 PLUS |
B038775072 |
CBTK-01 |
CO2 FYRITE Gas Analyzer |
Combustion Test Kit |
BACHARACH |
0011-7032 |
20100735 |
CC-01 |
Analyzer |
Countess Automated Cell Counter |
Invitrogen by Thermo Scientific |
C10281 |
13011-032 |
CC-101 |
Part; Column |
Axichrom Chromatography Column 70/300; B1 |
CYTIVA |
28901840 |
2851549 |
CC-102 |
Part; Column |
Axichrom Chromatography Column 70/300; B1 |
CYTIVA |
28901840 |
2865490 |
CE-01 |
Handheld Conductivity Meter |
Handheld Conductivity Meter |
Fisher Scientific |
15-077-977 |
170038251 |
CF-01 |
Centrifuge |
Centrifuge - Roto evaporator/concentrator |
Labconco |
7810010 |
120559385 C |
CF-02 |
Centrifuge |
Eppendorf 5417R Centrifuge |
Eppendorf |
5417R |
5407ZO031839 |
CF-03 |
Centrifuge |
Centrifuge (Avanti J-265 XPI) |
Beckman Coulter |
AVANTI J-26S XPI |
JST12G01 |
CF-04 |
Centrifuge |
Table Top Centrifuge 5804R, 15amp version |
Eppendorf |
5804R |
5805CP064983 |
CF-05 |
Centrifuge |
Centrifuge (benchtop) - Microcentrifuge C1603 |
Eppendorf |
5427R |
5409FL804956 |

CF-06 |
Centrifuge |
Microcentrifuge 210A |
DENVILL SCIENTIFIC |
C0210 |
4097 |
CF-07 |
Centrifuge |
Mini Centrifuge |
VWR |
10067-588 |
2015120240 |
CF-08 |
Centrifuge |
Galaxy Mini Centrifuge |
VWR |
C1413 |
10060863 |
CF-09 |
Centrifuge |
Galaxy Mini Centrifuge |
VWR |
C1413 |
1008 0515 |
CF-10 |
Centrifuge |
Allegra X-30 Centrifuge |
Beckman Coulter |
Allegra X-30 |
ALT12K047 |
CF-11 |
Centrifuge |
Mini Centrifuge |
VWR |
10067-588 |
2015120239 |
CF-13 |
Centrifuge |
Mini Centrifuge (MySPIN 6) |
THERMO SCIENTIFIC |
75004061 |
HSF6029 (or HSF56029) |
CF-14 |
Centrifuge |
Mini Centrifuge |
VWR |
C0803 |
179-16031-19120149 |
CF-15 |
Centrifuge |
Benchtop Centrifuge |
Fisherbrand |
14955300 |
14955300-1659 |
CF-16 |
Centrifuge |
Centrifuge (swing bucket) |
Eppendorf |
5810 R |
5811IN188964 |
CF-17 |
Centrifuge |
Centrifuge 5420 |
Eppendorf |
5420 |
5420KH204129 |
CF-18 |
Centrifuge |
Centrifuge (benchtop) |
Beckman Coulter |
Microfuge 20R |
MRB20L15 |
CF-N/A |
Centrifuge |
Digital High Speed Microcentrifuge |
VWR |
C1603 |
110-18031-19110088 |
CF-N/A (Grundfos Centrifuge) |
|
Grundfos Centrifuge Pump |
|
A96523265-P11101546 |
N/A |
CF-N/A (Grundfos Centrifuge) |
|
Grundfos Centrifuge Pump |
|
A96523265-P11101547 |
N/A |
CF-N/A (New) |
Centrifuge |
Mini Centrifuge |
VWR |
C0803 |
179-16031-21080274 |
CF-N/A (New) |
Centrifuge |
Mini Centrifuge |
VWR |
C0803 |
179-16031-21120277 |

CF-N/A (New) |
Centrifuge |
Mini Centrifuge |
VWR |
C0803 |
179-16031-21120280 |
CF-N/A (New) |
Centrifuge |
Mini Centrifuge |
VWR |
C0803 |
179-16031-21120281 |
CIP-01_CE-104 |
|
CIP SKID WASH CONDUCTIVITY SENSOR |
Mettler Thornton |
244-634 |
10060571 |
CM-01 |
Handheld Colorimeter |
Handheld Colorimeter |
Hach |
DR/890 |
Unknown |
CMR-01 |
Camera/Microscope |
FLUORESCENCE IMAGING CAMERA; Computer: DT-75 |
Tucsen |
FL-20BW |
KBLF06520003 |
CMR-01_DT-75 |
|
Desktop; Instrument: CMR-01 |
Dell |
|
|
CRSY-01 |
Condensate Recirculator |
Condensate Recirculator |
Caron |
CRSY102-1 |
CRSY102-1-1799 |
CRYO-01 |
CryoPod Carrier |
CryoPod Carrier |
biocision |
CP3L |
CP001109 |
CWT-01 |
Weight Set |
1 mg to 10 kg ASTM Class 1 Weight Set |
Troemner |
30391383 |
4000020171 |
CWT-02 |
Weight Set |
20 kg ASTM Class 1 Weight |
Troemner |
30391454 |
1000145468 |
CWT-03 |
Weight Set |
1 mg Class 1 Weight |
Troemner |
80781102 |
1000145467 |
CWT-04 |
Weight Set |
100 g ASTM Class 1 Weight |
Troemner |
80781142 |
1000179174 |
CWT-05 |
Weight Set |
1 mg to 500 g ASTM Class 1 Weight Set |
Troemner |
30390154 |
4000029090 |
CWT-06 |
Weight Set |
1 kg ASTM Class 1 Weight |
Troemner |
30390261 |
4000029090-1 |
CWT-08 |
Weight Set |
50 g and 1000 g ASTM Class 1 CarePac Weight Set |
Mettler Toledo |
11123108 |
4000029799 |

CWT-09 |
Weight Set |
5 kg ASTM Class 4 Stainless Weight |
Troemner |
30391187 |
1000234156 |
CWT-10 |
Weight Set |
10 kg ASTM Class 4 Stainless Weight |
Troemner |
30391324 |
1000234258 |
CWT-11 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235582 |
CWT-12 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235583 |
CWT-13 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235584 |
CWT-14 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235585 |
CWT-15 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235586 |
CWT-16 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235587 |
CWT-17 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235588 |
CWT-18 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235589 |
CWT-19 |
Weight Set |
25 kg ASTM Class 4 Stainless Weight |
Troemner |
30391193 |
1000235590 |

CWT-N/A (New) |
Weight Set |
10 g and 200 g ASTM Class 1 Weight Set |
Mettler Toledo |
11123101 |
4000026048 |
DA-01 |
Meter |
Digital Anemmometer |
EMD MILLIPORE |
DA-100-NT |
16268 |
DB-01 |
Dry/Heat Block |
ThermoMixer 5350 |
Eppendorf |
5350 |
5350BJ036961 |
DB-02 |
Dry/Heat Block |
Digital Mini Dry Bath |
BioExpress |
BSH200-HL |
AS-BSH200-5643 |
DB-04 |
Dry/Heat Block |
Digital Mini Dry Bath |
BioExpress |
BSH200-HL |
AS-BSH200-5879 |
DB-05 |
Dry/Heat Block |
Digital Drybath (ISOTEMP 145D) |
Fisher Scientific |
1450 |
102N0028 |
DB-06 |
Dry/Heat Block |
Digital Mini Heat Block |
Fisherbrand |
14955218 |
A37-00746 |
DB-07 |
Dry/Heat Block |
Digital Drybath (Drybath Stdrd 1 blck 100-120V) |
Thermo Scientific |
88870001 |
JCBT70001083 |
DB-08 |
Dry/Heat Block |
Digital Mini Heat Block |
Fisherbrand |
14955218 |
137-16031-19120177 |
DL-01-MT/TT-04 |
Data Logger |
TraceableGO Bluetooth Temperature/Humidity Monitor |
VWR |
76214-378 |
200782720 |
DL-01-MT/TT-05 |
Data Logger |
TraceableGO Bluetooth Temperature/Humidity Monitor |
VWR |
76214-378 |
200782721 |
ELPC-02 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Sartorius |
Midi Plus |
4539404876 |
ELPC-03 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Sartorius |
Midi Plus |
4539604192 |
ELPC-04 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Sartorius |
Midi Plus |
4539604190 |

ELPC-05 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Thermo Scientific |
S1 Pipet Filler |
211391 |
ELPC-06 |
Electronic Pipette Aid |
Pipet-Aid XP |
Drummond Scientific |
PIPET AID XP |
236470L |
ELPC-07 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Fisherbrand |
Unknown |
MJ759512 |
ELPC-08 |
Electronic Pipette Aid |
Pipette Controller |
Jencons |
POWERPETTE PLUS |
AD7182 |
ELPC-11 |
Electronic Pipette Aid |
ELECTRONIC PIPETTE CONTROLLER, ELPC-11 |
Cole Parmer |
25300-96 |
J0900072 |
ELPC-12 |
Electronic Pipette Aid |
ELECTRONIC PIPETTE CONTROLLER, ELPC-12 |
Cole Parmer |
25300-96 |
J0900075 |
ELPC-13 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Cole-Parmer |
Omega Zen |
J0900079 |
ELPC-14 |
Electronic Pipette Aid |
FB MOTORIZED PIPET FILLER, ELPC-14 |
Fisherbrand |
FB14955202 |
None |
ELPC-15 |
Electronic Pipette Aid |
FB MOTORIZED PIPET FILLER, ELPC-15 |
Fisherbrand |
FB14955202 |
None |
ELPC-16 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Sartorius |
Midi Plus |
4542404808 |
ELPC-17 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Sartorius |
Midi Plus |
4542404807 |
ELPC-18 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Thermo Scientific |
S1 Pipet Filler |
210637 |
ELPC-N/A |
Electronic Pipette Aid |
Pipet-Aid XP |
Drummond Scientific |
Pipet Aid XP |
255733L |
ENDO-01 |
|
Endotoxin System; Computer: DT-60 |
Charles Rivers |
Nexgen MCS 150 |
21381257 |
ENDO-01_DT-60 |
|
Dell Desktop/Charles Rivers; |
Dell |
|
|

|
|
Instrument: ENDO-01 |
|
|
|
EPLC-12 |
Electronic Pipette Aid |
Electronic Pipette Aid |
Thermo Scientific |
S1 Pipet Filler |
233593 |
EPWR-01 |
Electrophoresis Power Supply |
Electrophoresis Power Supply |
Bio-Rad |
PowerPac HC |
043BR40278 |
EPWR-02 |
Electrophoresis Power Supply |
Electrophoresis Power Supply |
Bio-Rad |
PowerPac Basic |
041BR156066 |
EPWR-03 |
Electrophoresis Power Supply |
Power source, Electrophoresis Power Supply |
BIO-RAD |
PowerPac HC |
043BR38975 |
EPWR-04 |
Electrophoresis Power Supply |
PowerEase Touch 90W, Electrophoresis Power Supply |
Invitrogen by Thermo Scientific |
ZP10001 |
19A31A014 |
EPWR-05 |
Electrophoresis Power Supply |
Electrophoresis Power Supply |
Bio-Rad |
PowerPac HC |
043BR38969 |
EPWR-N/A |
Electrophoresis Power Supply |
Electrophoresis Power Supply |
Invitrogen by Thermo Scientific |
PS0120 |
PS0120200919003 |
EPWR-N/A (New) |
Electrophoresis Power Supply |
Gel Power Supply Adapter; ZOOM IPGRunner |
Invitrogen by Thermo Scientific |
ZM0002 |
202008-0008 |
FBD-01 |
Other |
Hoopman Tank and Power Switch, SEED COATING FLUID BED DRYER, FBD-01 |
HOOPMAN EQUIPMENT AND ENGINEERING |
FBL |
20E63-0287 |
FH-01 |
BSC |
AMS Chemical Fume Hood |
AIR MASTER SYSTEMS |
AIR MASTER SYSTEMS |
N/A |
FIT-01 |
Filter Integrity Tester |
Milipore Integritest 4; B1 |
EMD MILLIPORE |
XIT450001 |
IT40138 |

FIT-N/A (New) |
Filter Integrity Tester |
Filter Integrity Tester |
Millipore |
IT5INS001 |
IT506223115 |
FM-100 |
Fill Machine |
Proface Control Panel (For Flexicon) |
M&O Perry |
P1540 |
P-1052 |
FM-100_HEPA-01 |
Fill Machine |
Magnhelic HEPA Filter Housing |
M&O Perry |
SAM 24 CRF/LI |
36500 [1052 B4 (2x4x6" - 7.03ft2)] |
FM-200 |
Fill Machine |
Flexicon Filling Machine (Pump) |
FLEXICON |
91-060-00A |
190311-213120 |
FM-200_CC-01 |
Fill Machine |
Flexicon (Air Compressor) |
FLEXICON |
93-100-100 |
181214-002255 |
FM-200_N/A |
Fill Machine |
Burket Pilot 80-100 PSI (on fill finish machine) |
|
98124608 |
1000 |
FM-200_N/A |
Fill Machine |
Burket Pilot 80-100 PSI (on fill finish machine) |
|
98124608 |
1001 |
GCEL-01 |
Electrophoresis Gel Box |
Xcell SureLock |
Novex by Life Technologies |
EI0001 |
009436691 |
GCEL-02 |
Electrophoresis Gel Box |
Xcell SureLock |
Novex by Life Technologies |
EI0001 |
009489764 |
GCEL-03 |
Electrophoresis Gel Box |
Electrophoresis Gel Cell; Mini PROTEAN Tetra |
Bio-Rad |
Mini PROTEAN Tetra Cell |
552BR112534 |
GCEL-04 |
Electrophoresis Gel Box |
Electrophoresis Gel Cell; Mini PROTEAN Tetra |
Bio-Rad |
Mini PROTEAN Tetra Cell |
552BR160175 |

GCEL-05 |
Electrophoresis Gel Box |
Electrophoresis Gel Cell; Mini PROTEAN Tetra |
Bio-Rad |
Mini PROTEAN Tetra Cell |
552BR165669 |
GCEL-06 |
Electrophoresis Power Supply |
Mini-Gel Electrophoresis Power Supply |
Life Technologies |
PowerEase 90W PS0090 |
0900532107 |
GCEL-07 |
Electrophoresis Gel Box |
Mini Gel Tank |
Invitrogen by Thermo Scientific |
None |
None |
GCEL-N/A |
|
E-Gel Power Snap Electrophoresis |
Invitrogen |
G8100 |
2848021070066 |
HBLK-01 |
Digital Heatblock |
Digital Heatblock |
VWR |
NO75838-294 |
210616003 |
HBLK-02 |
Digital Heatblock |
Digital Mini Heat Block |
Fisherbrand |
14955218 |
137-16031-19120036 |
HBLK-03 |
Digital Heatblock |
Digital Mini Heat Block |
Fisherbrand |
14955218 |
137-16031-19120035 |
HM-01 |
Mixer |
Homogenator |
WARING LABORATORY |
38BL54 |
120315 |
HM-02 |
Mixer |
Homogenator |
WARING LABORATORY |
38BL54 |
100913 |
HM-03 |
Mixer |
Homogenator; Heavy Duty Blender |
WARING LABORATORY |
CB15 |
101105 |
HM-04 |
Mixer |
High Shear Laboratory Mixer |
Silverson Machines Ltd |
L5M-A |
38022 |
HM-05 |
Mixer |
Disintegrator Motor |
Bepex-Reitz |
Angle Disintegrator RP Series |
2010041824 |
Hoist |
Other |
Pittsburgh Centrifuge Lift |
Pittsburgh |
Heavy Duty 2Ton Folding Engine Crane |
367821634 |

HPLC-03 |
HPLC |
HPLC, ANALYTICAL SCIENCES HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) SYSTEM |
Shimadzu |
LC-20AD |
L20105357031 |
HPLC-04 |
HPLC |
HPLC LC Infinity 1260IIE; Computer: DT-74 |
Agilent |
SYS-LC-1260IIE |
DEAEW06746 |
HPLC-04_ DT-74 |
HPLC |
Dell Desktop; Instrument: HPLC-04 |
Dell |
|
|
HPLC-05 |
HPLC |
HPLC DAD/FLD; Computer: DT-73 |
Agilent |
SYS-LC-1260IIE |
DEAEW06594 |
HPLC-06 |
HPLC |
HPLC 1260 Infinity II Vial Sampler G7129A |
Agilent |
Agilent 1260 Infinity II |
DEAEW06782 |

|
|
|
|
(DEAEJ01869); FS-AC G1364F (DEAGS00935) |
|
HPLC-07 |
HPLC |
HPLC, ANALYTICAL SCIENCES LAB HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY SYSTEM |
SCIEX |
Exion LC |
ABRES5803372 |
HRV-01 |
|
Hedge Trimmer (For Harvest) |
Stihl |
HSE 70 Hedge Trimmer |
N/A |
HRV-01_A-501 |
|
Tank 501 Agitator |
Brawn Mixer, Inc. |
BGMF75 |
110003 |
HRV-01_A-503 |
|
Tank 503 Agitator Blade |
Brawn Mixer, Inc. |
BGMF75 |
110101 |
ICE-01 |
Other |
Ice Maker |
|
|
|
IMGR-01 |
Gel Imager |
Gel Doc XR+ Imager; Computer: WRoom2 |
Bio-Rad |
1708195 |
721BR13736 |
IMGR-02 |
Gel Imager |
Gel Imager, ZOE |
Bio-Rad |
ZOE Fluorescent Cell Imager |
742BR2344 |
INC-01 |
Incubator |
28 C INCUBATOR, INC-01 |
SANYO |
MIR-262 |
10080233 |
INC-02 |
Incubator |
37 C INCUBATOR, INC-02 |
SANYO |
MIR-262 |
11040095 |
INC-03 |
Incubator |
32.5 C IncuCell 404 INCUBATOR (32.5 deg C +/-2.5 deg C) |
BMT |
LSIS-B2V/1C 404 |
D132099 |
INC-03-TE-01 |
Incubator |
32.5 C IncuCell INCUBATOR, |
ENDRESS + HAUSER |
TH12-A8ADX2A1BK1 |
S50407232A1 |

|
|
INC-03, EMS TEMPERATURE SENSOR |
|
|
|
INC-04 |
Incubator |
22.5 C FrioCell 404 INCUBATOR (22.5 deg C +/-2.5 deg C) |
BMT |
FC-B2V-M/FC404 |
E150801 |
INC-04-TE-01 |
Incubator |
22.5 C FrioCell INCUBATOR, INC-04, EMS TEMPERATURE SENSOR |
Endress Hauser |
TH12-A8ADX2A1BK1 |
S50411232A1 |
INC-05 |
Incubator |
Cell Culture CO2 Incubator |
PHCBI |
MCO-170AICUVL-PA |
190160106 |
INC-06 |
Incubator |
Incubator |
Sanyo |
MIR-262 |
10080230 |
INC-07 |
Incubator |
Incubator Max Q 6000 |
THERMO SCIENTIFIC |
4353 |
107548-28 |
INC-08 |
Incubator |
Refrigerated Incubator Shaker |
Eppendorf |
New Brunswick Innova 43R |
110355172 |
INC-09 |
Incubator |
Incubator Shaker |
Labnet |
I5311DS |
10038810 |
INC-10 |
Incubator |
Refrigerated Incubator Shaker |
Eppendorf |
New Brunswick Innova 42R |
SI42HR204111 |
INC-11 |
Incubator |
Biological Indicator Incubator |
Steris |
S3271 |
20012863 |
INC-12 |
Incubator |
Digital Microplate Incubator |
BT LabSystems |
BT1101 |
MU2018010455 |
INC-13 |
Incubator |
Refrigerated Incubator Shaker |
Eppendorf |
New Brunswick Innova 43R |
SI43JH2000928 |
INC-16 |
Incubator |
Refrigerated Incubator Shaker |
Eppendorf |
New Brunswick Innova 43R |
S142KL305802 |
INC-17 (New) |
Incubator |
SRS CO2 Incubator |
PHCBI |
MC0-1708ICUVL |
220160007 |

INC-N/A |
Incubator |
New Brunswick Incubator Galaxy 170S |
Eppendorf |
C0170S-120-1000 |
40233 |
INC-N/A (Lab Companion Incubator) |
|
Lab Companion Incubator |
|
SI600 |
N/A |
INC-N/A (Lab Companion Incubator) |
|
Lab Companion Incubator holder rack |
|
SI600 |
N/A |
INC-N/A (Lab Companion Incubator) |
|
Lab Companion Shaker Rack |
|
SI600 |
N/A |
INC-N/A (TBD) |
Incubator |
Microplate Incubator |
BT LabSystems |
BT1101 |
BL-E28-1032 |
INF-02 |
Other |
Small-Scale Infiltrator |
APF |
None |
None |
LC-01 |
Liquid Chromatography |
Cytiva AKTA Pure 150; FPLC--Pure (AKTA Pure 150 BENCHTOP CHROMATOGRAPHY SYSTEM); Computer: DT-94 |
CYTIVA |
29046665 |
1803232 |
LC-01_DT-94 |
Liquid Chromatography |
Desktop; Instrument: LC-01 |
|
|
|
LC-02 |
Liquid Chromatography |
Cytiva AKTA Process (Gradient |
GE Healthcare |
IQ150FPW |
28980354 |

|
|
System); LC-02; AKTA Chromatography Skid (Gradient System) |
Bio-Sciences |
|
|
LC-03 |
Liquid Chromatography |
Cytiva AKTA Pilot 400; LC-03; AKTA Pilot Chromatography Skid (GE AKTA Pilot 400); Computer: DT-78 |
GE Healthcare Bio-Sciences |
56317181 |
1465844 |
LC-03_DT-78 |
Liquid Chromatography |
Desktop; Instrument: LC-03 |
|
|
|
LC-04 |
Liquid Chromatography |
Cytiva AKTA Process (Isocratic System); LC-04; AKTA Chromatography Skid (Isocratic System) |
GE Healthcare Bio-Sciences |
IQ150FPW |
28980355 |
LC-05 |
Liquid Chromatography |
Cytiva AKTA FPLC - Avant; Computer: DT-93 |
GE Healthcare Bio-Sciences |
28930842 |
1521342 |
LC-05_DT-93 |
Liquid Chromatography |
Desktop; Instrument: LC-05 |
|
|
|
LC-06 |
Liquid Chromatography |
Cytiva AKTA Avant 25 CHROMATOGRAPHY SYSTEM; Computer: DT-61 |
GE Healthcare Bio-Sciences |
28930842 |
2584237 |
LC-06_DT-61 |
Liquid Chromatography |
Desktop; Instrument: LC-06 |
|
|
|

LC-07 |
Liquid Chromatography |
Cytiva AKTA 25 LIQUID CHROMATOGRAPHY, BUILDING 1 MB/PD LAB; Computer: DT-67 |
GE Healthcare Bio-Sciences |
28930842 |
2626667 |
LC-07_DT-67 |
Liquid Chromatography |
Desktop; Instrument: LC-07 |
|
|
|
LC-08 |
Liquid Chromatography |
Cytiva AKTA 25 LIQUID CHROMATOGRAPHY, BUILDING 1 MB/PD LAB; Computer: DT-68 |
GE Healthcare Bio-Sciences |
28930842 |
2628404 |
LC-08_DT-68 |
Liquid Chromatography |
Desktop; Instrument: LC-08 |
|
|
|
LC-09 |
Liquid Chromatography |
Cytiva AKTA Avant 25 FPLC - Avant; Computer: LP-AKTA-124 |
GE Healthcare Bio-Sciences |
28930842 |
2814045 |
LC-10 |
Liquid Chromatography |
Cytiva AKTA FPLC - Avant 150; Computer: DESKTOP-JADJOEO |
CYTIVA |
28976337 |
2825681 |
LC-11 |
Liquid Chromatography |
Cytiva AKTA Pilot 600 R; B1; Computer: LP-Akta-127 |
CYTIVA |
29704661 |
2843929 |
LC-12 |
Liquid Chromatography |
Cytiva AKTA Avant 25 CHROMATOGRAPHY SYSTEM; Computer: LP-AKTA-125 |
CYTIVA |
28930842 |
2881938 |

LSH-503 |
|
Tank 503 Sensor, HIGH LEVEL SWITCH |
ROSEMOUNT |
2120D2RBCSX211 |
MSKK74800K0000 |
LT-501 |
|
Tank 501 Sensor |
OMEGA |
LV01503-LP |
110214 |
LT-503 |
|
Tank 503 Sensor, LEVEL TRANSMITTER |
Omega Engineering |
LVU1505-LP |
11D217 |
MA-01 |
Moisture Analyzer |
BENCHTOP MOISTURE ANALYZER, MA-01 |
Mettler Toledo |
HE73/03 |
C129191478 |
MANF-01 |
Filtration Manifold |
Millipore EZ-Fit Manifold (Vacuum manifolds support simultaneous filtration of three test samples) |
Millipore |
EZFITBASE3 |
BM7AA9924 |
MANF-02 |
Filtration Manifold |
Millipore EZ-Fit Manifold (Vacuum manifolds support simultaneous filtration of six test samples) |
Millipore |
EZFITBASE6 |
None |
MIC-01 |
Microscope |
Microscope |
Olympus |
BX43F |
0M11045 |
MIC-02 |
Microscope |
Microscope |
Jenco |
CP-2A1 |
V206116 |
MPR-01 |
|
BioTek Synergy H1, SYNERGY H1 HYBRID MULTI-MODE PLATEREADER; Computer: DT-65 |
BioTek |
H1M |
253156 |

MPR-01_DT-65 |
|
Dell Desktop; Instrument: MPR-01 (plate reader) |
Dell |
|
|
MPR-02 |
|
Microplate Reader; Computer: DT-39 |
BioTek |
EPOCH 2 |
18041929 |
MPR-02_DT-39 |
|
Desktop; Instrument: MPR-02 |
|
|
|
MPS-01 |
MicroPulser Electroporator |
MicroPulser Electroporator |
Bio-Rad |
165-2100 |
411BR7412 |
MS-01 |
Mass Spec |
QQQ TOF Mass Spec; Computer: Desktop-O0MS0BV |
SCIEX |
Triple TOF 5600 |
AY22791203 |
MS-01_NG-02 |
|
Nitrogen Generator |
Peak Scientific |
Genius 1024 230v |
721110550 |
MS-01-UPS |
Mass Spec |
UPS |
Powervar |
42052-108R |
42052108R-2210082 |
MS-02 |
|
QTOF Mass Spec; Computer: SCIEX-X500B |
SCIEX |
X500 QTOX |
DN230022002 |
MS-02_NG-01 |
|
Nitrogen Generator |
Peak Scientific |
Genius 1024 230v |
771061676 |
MS-02-UPS |
|
UPS |
Powervar |
42080-72R |
4208072R-2040003 |
MX-02 |
Mixer |
Analog Stir Plate |
Thermo Scientific |
SP18425 |
C1725110102729 |
MX-03 |
Mixer |
Digital Magnetic Mixer |
VWR |
12621-054 |
160629002 |
MX-07 |
Mixer |
Magnetic Stirrer Mixer (Midi MR1) |
IKA |
IP21 |
None |
MX-08 |
Mixer |
Analog Magnetic Stirrer |
Thermo Scientific |
88880009 |
K3KT09034 |

MX-10 |
Vortex mixer |
Analog Vortex mixer |
VWR |
58816-121 |
110310030 |
MX-11 |
Mixer |
Magnetic Stirrer Mixer (Midi MR1) |
IKA |
IP21 |
None |
MX-12 |
Vortex mixer |
Analog Vortex Mixer |
VWR |
58816-121 |
101011006 |
MX-N/A |
heater/mixer |
Thermal heater/mixer; ThermoMixer F1.5 |
Eppendorf |
5384 |
5384KO708635 |
MX-N/A |
heater/mixer |
Thermal heater/mixer; ThermoMixer F1.5 |
Eppendorf |
5384 |
5384LI309238 |
MX-N/A (New) |
Mixer |
Compact Digital Mixer System |
Cole-Parmer |
50006-01 |
250CP30717 |
N/A |
|
Stainless Steel Square Table |
|
|
|
N/A |
|
5.5 ft Stainless Steel Bench |
|
|
|
N/A |
Furniture |
3 ft Stainless Steel Bench |
|
|
|
N/A |
Storage |
30 gallon flammable cabinet |
|
|
|
N/A |
|
Diaphragm Pump |
|
3HJW3B |
MF654921 |
N/A |
|
DYMO Label Writer 450 |
DYMO |
1750110 |
1841021750110 |
N/A |
|
Sanyo Incubator |
SANYO |
MIR262 |
10080232 |
N/A |
|
Rheometer (in box) |
LungBio |
|
|
N/A |
|
Filter Cart (used) |
Repligen |
N/A |
N/A |
N/A |
|
Boegthin Flow Monitor Sensor Probe |
|
401565 |
502398-1-004 |

N/A |
|
Cleanrooms International Filter Fan |
|
SAM-24 |
36500 |
N/A |
|
Contec 6.5x2 Roller |
|
284EPJ |
3667 |
N/A |
|
Donaldson Filter (Sterile Tank Filtering complete housing) |
|
P-BE0576 |
73903488 |
N/A |
|
6 ft Desk |
|
|
|
N/A |
|
Gray Desk |
|
|
|
N/A |
|
Envircon Filters |
|
N/A |
N/A |
N/A |
|
20L Carboys |
Thermo Scientific |
|
|
N/A |
Bioreactor |
Whatman Bioreactor Bioreactor Vessel (8L) |
|
N/A |
N/A |
N/A |
|
Contec 10x2 Roller |
|
284EPJ |
3667 |
N/A |
|
0.5mL tube Heatblock, 1 each |
VWR |
13259-000 |
Z10709001 |
N/A |
|
2mL tube Heatblock, 3 each |
VWR |
12985-048 |
210602001 |
N/A |
|
Dry Heat Block |
VWR |
75838-294 |
|
N/A |
|
APC Uniterruptable Power Supply |
|
SRT8KRMXLT |
AS1641272199 |
N/A |
|
Boxes of single-use coveralls, expired |
3M |
|
|
N/A |
|
Grow racks (10 shelves, 1 unit) |
CC Pharming |
|
|

N/A |
|
Durachill (2-3 HP DuraChill™ Chiller, Air-Cooled, Water-Cooled) |
PolyScience |
DCA304D1XC-101 |
1A10D1373 |
N/A |
|
Automatic Paper Towel Dispensors |
Uline |
|
|
N/A |
|
Pallet of single-use coveralls |
Ultraguard |
|
|
N/A |
|
Control Inc Bioreactor Control Panel |
WATSON-MARLOW |
CSD363612SS6R |
A-1958 |
N/A |
|
AAF Air Filter Housing |
|
WE2717 |
SA090154 |
N/A |
|
Agronomy Biohazard Dumpster |
|
N/A |
N/A |
N/A |
|
American Lifts Elevator Platform |
|
36525 |
N/A |
N/A |
|
APC Uniterruptable Power Supply |
|
RW500DR |
AS1029143523 |
N/A |
|
Auto Up Lift |
|
STE30-25-4 |
211108791 |
N/A |
|
Baldor Electrical Pump |
|
N/A |
K070079 |
N/A |
|
Beckman Coulter Rotor |
|
N/A |
12U57022 |
N/A |
|
Beckman Coulter Rotor |
|
N/A |
12U57053 |
N/A |
|
Beckman Coulter Rotor |
|
N/A |
18U41966 |
N/A |
|
Beckman Coulter Rotor |
|
N/A |
18U41972 |
N/A |
|
Beckman Coulter Rotor |
|
N/A |
18U41975 |
N/A |
|
Beckman Coulter Rotor |
|
N/A |
18U41981 |
N/A |
|
Bioractor Rack |
|
N/A |
N/A |

N/A |
|
Black Chemical Locker |
|
N/A |
N/A |
N/A |
|
Burket Flow Switch for centrifuge |
|
N/A |
429136 |
N/A |
|
Centrifuge Base |
|
N/A |
N/A |
N/A |
|
Depth Filter |
|
N/A |
N/A |
N/A |
|
Envirco fan filter |
|
11156-001 |
S08-IQ-01750 |
N/A |
|
Envirco fan filter |
|
11156-001 |
S08-IQ-01753 |
N/A |
|
Envirco fan filter |
|
11156-001 |
S08-IQ-01757 |
N/A |
|
Envirco fan filter |
|
11156-001 |
S08-IQ-01783 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
09022001740001170072 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
0902200174001170107 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
0902205170001173427 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
09022051790001173430 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
09041007270001171051 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
09041607270001171054 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
09041607460001173840 |
N/A |
|
Evoguard Disc Valve |
|
N/A |
09041607460001173840 |
N/A |
|
Evoguard Valve |
|
0-903-355-331 |
9033553310001170000 |
N/A |
|
GE Thermal Motor (on Mixer) |
|
5KH36PNB050T |
D07J170126 |
N/A |
|
Newman Lanelling Power Supply |
|
NV2 |
10450 |
N/A |
|
Parker Door Lift |
|
02.00HB2ALU34A |
CC607668C |

N/A |
|
Polyscience recirculator |
|
5870T87XC751 |
1C1081640 |
N/A |
|
Sartorius Pump (on the bioreactor) |
|
8843415 |
D02101088 |
N/A |
|
Sartorius Servomotor |
|
AKM23D-ANBNC-00 |
80396035 |
N/A |
|
Sartorius Servomotor |
|
AKM23D-ANBNC-00 |
80396036 |
N/A |
|
Scrubbing Tank |
|
N/A |
N/A |
N/A |
|
Stackup Homogenizer and Disintegrator |
|
N/A |
N/A |
N/A |
|
Sump pump (under grate) Post Infiltration |
|
N/A |
N/A |
N/A |
|
Sump pump fuse box |
|
N/A |
N/A |
N/A |
|
Sump Pump Germ |
|
N/A |
N/A |
N/A |
|
Sump Pump Harvest |
|
N/A |
N/A |
N/A |
|
Sump Pump Infiltration |
|
N/A |
N/A |
N/A |
|
Sump pump on/off switch |
|
N/A |
N/A |
N/A |
|
Sump pump on/off switch |
|
N/A |
N/A |
N/A |
|
Tablecraft Kenkut 3 |
|
|
|
N/A |
|
Tray Racks for growing plants |
|
N/A |
N/A |
N/A |
|
Pallet washer (used as tray washer) |
|
N/A |
N/A |
N/A |
|
TRUDO DO Sensor |
|
DOS-OFF-VP-225 |
477 |
N/A |
|
TRUDO DO Sensor |
|
DOS-OFF-VP-225 |
697 |
N/A |
|
TRUDO DO Sensor |
|
DOS-OFF-VP-225 |
698 |

N/A |
|
VWR Recirculator |
|
1177MD |
108800807 |
N/A |
|
VWR Recirculator |
|
1177PD |
108C00855 |
N/A |
|
VWR Recirculator |
|
1177PD |
3F1081196 |
N/A |
|
Wave Tube Sealer |
|
28411704 |
1146852 |
N/A (Column) |
|
BPG Column (BPG 100/500) |
CYTIVA |
TO 80405 |
56116562 |
N/A (Column) |
|
AXI-CHROM 300-1000 |
|
28903963 |
44 |
N/A (Intermec Label Printer A) |
|
Intermec Label Printer; Computer: IBIODT-10 |
Intermec |
PC43t |
116C1730219 |
N/A (Intermec Label Printer A)_DT-10 |
|
Dell Desktop Workstation, Instrument: Intermec Label Printer |
Dell |
|
|
N/A (Intermec Label Printer B) |
|
Intermec Label Printer; Computer: IBIODT55 |
Intermec |
|
|
N/A (Intermec Label Printer B)_DT-55 |
|
Dell Desktop/Intermec; Instrument: Label Printer |
Dell |
|
|
N/A (Lenze Labeler) |
|
Lenze Labeler Motor |
|
13019972 |
1786089 |
N/A (Lenze Labeler) |
|
Lenze Labeler Motor |
|
13020623 |
1785062 |
N/A (Lowara) |
|
Lowara Water Pump (for centrifuge) |
|
20171215 |
7520560967 |
N/A (Lowara) |
|
Lowara water pump motor |
|
20171215 |
13304R002F |

N/A (New) |
|
Fluorescence Test Plate |
BioTek Instruments |
1400501 |
531367 |
N/A (Peristaltic Pump) |
|
Control Inc Bioreactor Control Panel |
WATSON-MARLOW |
010.NS22.210 |
10195787 |
N/A (Proteus) |
|
Glas-Col Temperature Control (On Proteus) |
|
099ARD4512 |
474933 |
N/A (Proteus) |
|
Proteus 2000 |
|
590 |
N/A |
N/A (Rosemont) |
|
Rosemont Heat Jacket |
|
1116C-43 |
20006 |
N/A (Siemens Centrifuge) |
|
Siemens Centrifuge Motor |
|
1705/1933953-001 |
N/A |
N/A (Supply) |
|
Spool Printer (Tape) |
N/A |
PC43t |
116c1730061 |
N/A (Westfalia Centrifuge) |
|
Westfalia Centrifuge |
|
CSA19-06-476 |
1694-995 |
N/A (Wiegmann Mixer) |
|
Wiegmann Mixer |
|
B141206CH |
E6924 |
N/A (Xcellerex Bioreactor System) |
|
Xcellerex Bioreactor System |
|
XD-50 |
X0212010 |
N/A? (PP-10?) |
|
Masterflex L/S (Pump Head) |
MASTER FLEX |
77200-60 |
N/A |
New |
Sterilizer |
Glass Bead Sterilizer |
VWR |
B1205 |
172-16031-21060065 |
New |
Test Plate |
Absorbance Test Plate |
BioTek Instruments |
7260522 |
531361 |
NF-02 |
|
Baby Nutsche Filter (5L) |
Pope Scientific |
C276-1.5FVTR150-FL |
147671-1-3 |

OV-01 |
|
Microwave Oven |
Danby |
DMW111KBLDB |
4720000000000 |
P-303 |
|
Hydrovar Pump System; GERMINATION ROOM IRRIGATION DISTRIBUTION PUMP, P-303 |
Goulds |
5SV5FA30 |
K1609123 |
P-304 |
|
Goulds Hydrovar Pump, Irrigation; (IRR-01-VFD-P-304), VARIABLE FREQUENCY DRIVE |
|
VEM3555 |
N/A |
P-402 |
|
peristaltic pump; INOCULATION SOLUTION TRANSFER PUMP |
WATSON MARLOW |
520DUS/REM |
L020910 |
P-803 |
|
Sump pump (under grate) Post Infiltration |
|
N/A |
N/A |
PC-01 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
161104042 |
PC-02 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
161104043 |
PC-03 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
101204044 |
PC-04 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
200404034 |
PC-05 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
210604006 |

PC-06 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
210604010 |
PC-07 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
210604007 |
PC-08 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
210604009 |
PC-09 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
210604011 |
PC-10 |
|
Laser Particle Counter |
Lighthouse Worldwide Solutions |
Solair 3100 |
210604008 |
PCR-01 |
|
QPCR THERMAL CYCLER, BUILDING 1 MB/PD LAB |
THERMO FISHER |
QUANTSTUDIO 5 REAL-TIME PCR (CATALOG #: A28138) |
272511703 |
PCR-02 |
|
PCR Workstation Cabinet |
VWR |
10783-132 |
31102-02F 00351 |
PCR-03 |
|
Thermocycler |
Eppendorf |
Nexus GX2 Mastercycler |
6336JL526723 |
PCR-N/A (New) |
|
UV Cabinet (PCR) |
Grant Instruments |
UVC/T-M-AR |
04010422010009 |
PGCH-01 |
|
gBrite LED PLANT GROWTH CHAMBER, PGCH-01 |
CARON |
7312-50-2 |
7312-50-2-013 |
PGCH-02 |
|
gBrite LED PLANT GROWTH CHAMBER, PGCH-02 |
CARON |
7312-50-2 |
7312-50-2-014 |
PH-02 |
|
Benchtop pH Meter |
Thermo Scientific |
Orion 4 STAR |
B32770 |
PH-03 |
|
Benchtop pH Meter |
VWR |
SympHony SB70P |
D05492 |

PIP-002 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-02 |
VWR |
Signature Variable 1000uL |
42764417 |
PIP-003 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-03 |
VWR |
Signature 20µL |
42731744 |
PIP-004 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-04 |
VWR |
Signature EHP Variable 200 uL |
42753091 |
PIP-005 |
12-Channel Pipette |
30 to 300 uL 12 CHANNEL PIPETTE, PIP-05 |
Eppendorf |
Research Plus |
M18968B |
PIP-006 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-06 |
Eppendorf |
Research Plus 1000 uL |
156701A |
PIP-007 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-07 |
Eppendorf |
Research Plus 10 uL |
163850A |
PIP-008 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL PIPETTE, PIP-08 |
Eppendorf |
Research Plus |
159502A |
PIP-009 |
8-Channel Pipette |
0.5 to 10 uL 8 CHANNEL PIPETTE, PIP-09 |
Finnpipette |
8ch 4510 |
C70350 |
PIP-010 |
12-Channel Pipette |
20 to 200 uL 12 CHANNEL PIPETTE, PIP-10 |
VWR |
Signature EHP Variable 200 uL |
153870016 |

PIP-011 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-11 |
Gilson |
P1000L |
NK73657 |
PIP-012 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-12 |
VWR |
Signature 1000µL |
242763608 |
PIP-013 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-13 |
VWR |
Signature 1000µL |
42764592 |
PIP-014 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-14 |
VWR |
Signature 20µL |
42732892 |
PIP-015 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-15 |
VWR |
Signature 20µL |
242732436 |
PIP-016 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-16 |
VWR |
Signature 200µL |
242751755 |
PIP-017 |
12-Channel Pipette |
20 to 200 uL 12 CHANNEL PIPETTE, PIP-17 |
Gilson |
LP12X200L |
MC70193 |
PIP-018 |
12-Channel Pipette |
20 to 200 uL 12 CHANNEL PIPETTE, PIP-18 |
Gilson |
P12x200L |
MC71420 |
PIP-019 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-19 |
Gilson |
PIPETMAN P10 |
T69789G |

PIP-020 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL PIPETTE, PIP-20 |
Gilson |
PIPETMAN P100 |
T63429E |
PIP-021 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-21 |
Rainin |
E4 XLS 1000 |
B624604126 |
PIP-022 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-22 |
Rainin |
E4 XLS 200 |
B625647698 |
PIP-023 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-23 |
Rainin |
E4 XLS |
B619464014 |
PIP-024 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-24 |
Rainin |
Pipet-Lite SL-10 XLS |
B615353398 |
PIP-026 |
12-Channel Pipette |
50 to 300 uL 12 CHANNEL PIPETTE, PIP-26 |
VWR |
Signature 300uL x12 |
053880021 |
PIP-027 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-27 |
Gilson |
P200 |
NK71010 |
PIP-028 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-28 |
VWR |
Signature EHP Variable 10uL |
42722247 |
PIP-029 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-29 |
VWR |
Signature EHP Variable 10uL |
242722188 |

PIP-030 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-30 |
Gilson |
P1000 |
NK72771 |
PIP-031 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-31 |
VWR |
Signature EHP Variable 20uL |
242731580 |
PIP-032 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-32 |
VWR |
Signature EHP Variable 20uL |
42732814 |
PIP-033 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-33 |
Gilson |
P200 |
NK71001 |
PIP-034 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-34 |
VWR |
Signature 200µL |
42753009 |
PIP-035 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-36 |
VWR |
Signature 200µL |
242753411 |
PIP-037 |
Single Channel Pipette |
0.1 to 2.5 uL SINGLE CHANNEL PIPETTE, PIP-37 |
Eppendorf |
Reference |
402107A |
PIP-038 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-38 |
Eppendorf |
Reference |
395817A |
PIP-039 |
Single Channel Pipette |
50 to 200 uL SINGLE CHANNEL |
Eppendorf |
Reference |
398159A |

|
|
PIPETTE, PIP-39 |
|
|
|
PIP-040 |
Single Channel Pipette |
0.1 to 2.5 uL SINGLE CHANNEL PIPETTE, PIP-40 |
Eppendorf |
Reference 2 |
G17616F |
PIP-041 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-41 |
Eppendorf |
Reference 2 |
K48791J |
PIP-042 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-42 |
Eppendorf |
Reference 2 |
H12806F |
PIP-043 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-43 |
Eppendorf |
Reference 2 |
I10393F |
PIP-044 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-44 |
Eppendorf |
Research Plus |
N20509B |
PIP-045 |
Single Channel Pipette |
20ul Single Channel Pipette |
Eppendorf |
Research Plus |
N17220B |
PIP-046 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-46 |
Eppendorf |
Research Plus |
N19398B |
PIP-047 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-47 |
VWR |
Signature Variable 10uL |
42722243 |
PIP-048 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL |
VWR |
Signature Variable 10uL |
42721230 |

|
|
PIPETTE, PIP-48 |
|
|
|
PIP-050 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-50 |
VWR |
Signature Variable 10uL |
242722478 |
PIP-051 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-51 |
Gilson |
Pipetman P1000 |
NL71701 |
PIP-052 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-52 |
VWR |
Signature Variable 1000uL |
242764691 |
PIP-053 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-53 |
VWR |
Signature 1000µL |
242764693 |
PIP-054 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-54 |
VWR |
Signature 1000µL |
242764712 |
PIP-055 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-55 |
Gilson |
Pipetman P1000 |
NL71702 |
PIP-057 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-57 |
VWR |
Signature Variable 20uL |
924733690 |
PIP-058 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-58 |
VWR |
Signature Variable 20uL |
242732461 |

PIP-059 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-59 |
VWR |
Signature Variable 20uL |
242732416 |
PIP-060 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-60 |
VWR |
Signature Variable 20uL |
42731683 |
PIP-061 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-61 |
VWR |
Signature Variable 20uL |
42732744 |
PIP-062 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-62 |
VWR |
Signature 200µL |
42751747 |
PIP-063 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-63 |
VWR |
Signature Variable 200uL |
242753406 |
PIP-064 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-64 |
VWR |
Signature Variable 200uL |
242753412 |
PIP-065 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-65 |
VWR |
Signature Variable 200uL |
42750938 |
PIP-066 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-66 |
VWR |
Signature Variable 200uL |
42751732 |
PIP-067 |
12-Channel Pipette |
50 to 300 uL 12 CHANNEL PIPETTE, PIP-67 |
Fisherbrand |
Finnpipette 4510 |
E47292 |

PIP-068 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-68 |
Rainin |
Pipet-Lite XLS |
B648361415 |
PIP-069 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-69 |
Rainin |
Pipet-Lite XL-200 XLS |
B648359909 |
PIP-070 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-70 |
Rainin |
Pipet-Lite XLS |
B646316130 |
PIP-071 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-71 |
Rainin |
Pipet-Lite XLS |
B648362151 |
PIP-072 |
Repeater Pipette |
100 to 500 uL SINGLE CHANNEL PIPETTE, PIP-72 |
VWR |
75836-686 |
127343 |
PIP-073 |
Repeater Pipette |
10mL Repeating Pipette |
Eppendorf |
Repeater Plus |
N11121C |
PIP-074 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-74 |
Eppendorf |
Research Plus |
L48870H |
PIP-075 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-75 |
Eppendorf |
Research Plus |
M29111H |
PIP-076 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-76 |
Eppendorf |
Research Plus |
M29436H |

PIP-077 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-77 |
Eppendorf |
Research Plus |
H427178J |
PIP-078 |
Single Channel Pipette |
0.1 to 2.5 uL SINGLE CHANNEL PIPETTE, PIP-78 |
Eppendorf |
Research Plus |
H26175I |
PIP-079 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-79 |
Eppendorf |
Research Plus |
P40110H |
PIP-080 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-80 |
Eppendorf |
Research Plus |
O22719H |
PIP-083 |
Single Channel Pipette |
500 to 5000 uL SINGLE CHANNEL PIPETTE, PIP-83 |
Eppendorf |
Research Plus |
P58717I |
PIP-084 |
Single Channel Pipette |
0.1 to 2.5 uL SINGLE CHANNEL PIPETTE, PIP-84 |
Eppendorf |
Research Plus |
O24096I |
PIP-085 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-85 |
Eppendorf |
Research Plus |
N44888I |
PIP-086 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-86 |
Eppendorf |
Research Plus |
N17768I |
PIP-087 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL |
Eppendorf |
Research Plus |
H23849I |

|
|
PIPETTE, PIP-87 |
|
|
|
PIP-088 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL PIPETTE, PIP-88 |
Eppendorf |
Research Plus |
I22938I |
PIP-089 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-89 |
Eppendorf |
Research Plus |
I44926I |
PIP-090 |
12-Channel Pipette |
10 to 100 uL 12 CHANNEL PIPETTE, PIP-90 |
Eppendorf |
Research Plus |
M49917I |
PIP-091 |
12-Channel Pipette |
0.5 to 10 uL 12 CHANNEL PIPETTE, PIP-91 |
Eppendorf |
Research Plus |
G54609I |
PIP-092 |
Single Channel Pipette |
0.1 to 2.5 uL SINGLE CHANNEL PIPETTE, PIP-92 |
Eppendorf |
Research Plus |
N28417I |
PIP-093 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-93 |
Eppendorf |
Research Plus |
K46043I |
PIP-094 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-94 |
Eppendorf |
Research Plus |
P36012I |
PIP-095 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-95 |
Eppendorf |
Research Plus |
I48244I |
PIP-096 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL |
Eppendorf |
Research Plus |
H37818I |

|
|
PIPETTE, PIP-96 |
|
|
|
PIP-097 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-97 |
Eppendorf |
Research Plus |
H45953I |
PIP-098 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-98 |
Eppendorf |
Reference 2 |
G29123J |
PIP-099 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-99 |
Eppendorf |
Reference 2 |
H24626J |
PIP-100 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-100 |
Eppendorf |
Reference 2 |
G17609J |
PIP-101 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-101 |
Eppendorf |
Repeater E3x |
O42195I |
PIP-102 |
12-Channel Pipette |
30 to 300 uL 12 CHANNEL PIPETTE, PIP-102 |
Eppendorf |
Xplorer plus |
Q39708I |
PIP-104 |
12-Channel Pipette |
50 to 1200 uL 12 CHANNEL PIPETTE, PIP-104 |
Eppendorf |
Xplorer |
J77942J |
PIP-106 |
Single Channel Pipette |
50 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-106 |
Eppendorf |
Xplorer plus |
N46608I |
PIP-107 |
Repeater Pipette |
100 to 1000 uL SINGLE CHANNEL |
Eppendorf |
Repeater M4 |
H54341J |

|
|
PIPETTE, PIP-107 |
|
|
|
PIP-108 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL PIPETTE, PIP-108 |
Gilson |
P100 |
QN73615 |
PIP-109 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-109 |
Eppendorf |
Reference 2 |
Q11258H |
PIP-110 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-110 |
Eppendorf |
Research Plus |
G29737J |
PIP-111 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-111 |
Eppendorf |
Research Plus |
J43098J |
PIP-112 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-112 |
Eppendorf |
Reference 2 |
P41973I |
PIP-113 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL PIPETTE, PIP-113 |
Eppendorf |
Reference 2 |
N12835I |
PIP-114 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-114 |
Eppendorf |
Reference 2 |
Q32149I |
PIP-115 |
Single Channel Pipette |
0.1 to 2.5 uL SINGLE CHANNEL PIPETTE, PIP-115 |
Eppendorf |
Reference 2 |
R31901I |

PIP-116 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-116 |
Eppendorf |
Reference 2 |
M10785H |
PIP-117 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-117 |
Eppendorf |
Reference 2 |
H13218J |
PIP-118 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-118 |
Rainin |
Pipet-Lite XLS |
C037046735 |
PIP-119 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-119 |
Rainin |
Pipet-Lite XL-200 XLS |
C038113434 |
PIP-120 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-120 |
Rainin |
Pipet-Lite XLS |
C038111669 |
PIP-121 |
12-Channel Pipette |
30 to 300 uL 12 CHANNEL PIPETTE, PIP-121 |
Eppendorf |
Research Plus |
O48187J |
PIP-122 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-122 |
Eppendorf |
Research Plus |
J30097J |
PIP-123 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-123 |
Eppendorf |
Research Plus |
I23667J |
PIP-124 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-124 |
Eppendorf |
Research Plus |
I45909J |

PIP-125 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-125 |
Eppendorf |
Research Plus |
G46301J |
PIP-126 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-126 |
Eppendorf |
Research Plus |
G20345J |
PIP-130 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-130 |
Eppendorf |
Research Plus |
L45794J |
PIP-131 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-131 |
Eppendorf |
Research Plus |
O20588J |
PIP-132 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-132 |
Eppendorf |
Research Plus |
K36581J |
PIP-134 |
Repeater Pipette |
50mL Single Channel Pipette |
Eppendorf |
Repeater E3 |
R37240J |
PIP-135 |
Single Channel Pipette |
0.2 to 2 uL SINGLE CHANNEL PIPETTE, PIP-135 |
Gilson |
Pipetman P2 |
MK71943 |
PIP-136 |
Single Channel Pipette |
0.2 to 2 uL SINGLE CHANNEL PIPETTE, PIP-136 |
Gilson |
Pipetman P2 |
QK71741 |
PIP-137 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-137 |
Gilson |
Pipetman P10 |
QH53528 |

PIP-138 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-138 |
Gilson |
Pipetman P10 |
QC52021 |
PIP-139 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-139 |
Gilson |
Pipetman P10 |
QH53524 |
PIP-140 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-140 |
Gilson |
PIPETMAN P10 |
PK53604 |
PIP-141 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-141 |
Gilson |
PIPETMAN P10 |
QH53534 |
PIP-142 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-142 |
Gilson |
PIPETMAN G P10G |
QH53523 |
PIP-143 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-143 |
Gilson |
Pipetman P20 |
RC72611 |
PIP-144 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-144 |
Gilson |
Pipetman P20 |
RA73937 |
PIP-145 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-145 |
Gilson |
Pipetman P20 |
RA73987 |
PIP-146 |
Single Channel Pipette |
10 to 100 uL SINGLE CHANNEL |
Gilson |
PIPETMAN P100 |
QK20542 |

|
|
PIPETTE, PIP-146 |
|
|
|
PIP-147 |
Single Channel Pipette |
20 to 200 ul SINGLE CHANNEL PIPETTE, PIP-147 |
Gilson |
Pipetman P200 |
PC71724 |
PIP-148 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-148 |
Gilson |
Pipetman P200 |
RC74689 |
PIP-149 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-149 |
Gilson |
Pipetman P200 |
RC74663 |
PIP-150 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-150 |
Gilson |
Pipetman P200 |
RC73454 |
PIP-151 |
Single Channel Pipette |
100 to 1000 ul SINGLE CHANNEL PIPETTE, PIP-151 |
Gilson |
Pipetman P1000 |
RA73605 |
PIP-152 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-152 |
Gilson |
Pipetman P1000 |
NK71861 |
PIP-153 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-153 |
Gilson |
Pipetman P1000 |
RC71364 |
PIP-154 |
12-Channel Pipette |
20 to 200 uL 12 CHANNEL PIPETTE, PIP-154 |
Gilson |
PIPETMAN L P200 |
RK71274 |
PIP-155 |
12-Channel Pipette |
20 to 300 uL 12 CHANNEL |
Gilson |
PIPETMAN L P300 |
RH71310 |

PIP-156 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-156 |
VWR |
ERGONOMIC HP |
B42761319 |
PIP-157 |
Single Channel Pipette |
0.5 to 10 uL SINGLE CHANNEL PIPETTE, PIP-157 |
VWR |
ERGONOMIC HP |
B42720414 |
PIP-158 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-158 |
VWR |
ERGONOMIC HP |
B42750380 |
PIP-159 |
Single Channel Pipette |
0.1 to 2 uL SINGLE CHANNEL PIPETTE, PIP-159 |
VWR |
ERGONOMIC HP |
A42710726 |
PIP-160 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-160 |
VWR |
ERGONOMIC HP |
B42730230 |
PIP-162 |
8-Channel Pipette |
10 to 100 uL 8 CHANNEL PIPETTE, PIP-162 |
Eppendorf |
Research Plus |
K69935K |
PIP-163 |
8-Channel Pipette |
0.5 to 10 uL 8 CHANNEL PIPETTE, PIP-163 |
Eppendorf |
Research Plus |
L50080K |
PIP-164 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-164 |
Eppendorf |
Research Plus |
M60331K |
PIP-165 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL |
Eppendorf |
Research Plus |
M28841K |

|
|
PIPETTE, PIP-165 |
|
|
|
PIP-166 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-166 |
Eppendorf |
Research Plus |
M28795K |
PIP-167 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-167 |
Eppendorf |
Research Plus |
M60330K |
PIP-168 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-168 |
Eppendorf |
Research Plus |
M56124K |
PIP-169 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-169 |
Eppendorf |
Research Plus |
M56442K |
PIP-170 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-170 |
Rainin |
Pipet-Lite SL-200 XLS |
C038113533 |
PIP-171 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-171 |
Rainin |
Pipet-Lite SL-20 XLS |
C038111643 |
PIP-172 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-172 |
Rainin |
Pipet-Lite SL-1000 XLS |
C037046686 |
PIP-173 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-173 |
VWR |
Catalog No. 76169-234 |
21k0085 |

PIP-174 |
Single Channel Pipette |
100 to 10000 uL SINGLE CHANNEL PIPETTE, PIP-174 |
VWR |
Catalog No. 76169-240 |
22B0203 |
PIP-175 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-175 |
VWR |
Catalog No. 76169-238 |
22B0735 |
PIP-176 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-176 |
VWR |
Catalog No. 76169-232 |
21K0226 |
PIP-177 |
12-Channel Pipette |
20 to 200 uL 12 CHANNEL PIPETTE, PIP-177 |
VWR |
Catalog No. 89079-956 |
C53870050 |
PIP-178 |
Single Channel Pipette |
0.2 to 2 uL SINGLE CHANNEL PIPETTE, PIP-178 |
VWR |
Catalog No. 76169-274 |
22A1179 |
PIP-179 |
Single Channel Pipette |
1 to 10 uL SINGLE CHANNEL PIPETTE, PIP-179 |
VWR |
Catalog No. 76169-232 |
22B0348 |
PIP-180 |
Single Channel Pipette |
2 to 20 uL SINGLE CHANNEL PIPETTE, PIP-180 |
VWR |
Catalog No. 76169-234 |
22B0787 |
PIP-181 |
Single Channel Pipette |
20 to 200 uL SINGLE CHANNEL PIPETTE, PIP-181 |
VWR |
Catalog No. 76169-238 |
22B0689 |
PIP-182 |
Single Channel Pipette |
100 to 1000 uL SINGLE CHANNEL PIPETTE, PIP-182 |
VWR |
Catalog No. 76169-240 |
22B0158 |

PIP-184 |
Repeater Pipette |
HandyStep S 500 uL REPETITIVE PIPETTE, PIP-184 |
BRAND |
DE-M22 |
22D58959 |
PIP-185 |
Single Channel Pipette |
0.5 to 5 mL SINGLE CHANNEL PIPETTE, PIP-185 |
EPPENDORF |
N/A |
G37495L |
PIP-186 |
12-Channel Pipette |
20 to 300 uL 12 CHANNEL PIPETTE, PIP-186 |
VWR |
76169-260 |
22C0106 |
PIP-187 |
Single Channel Pipette |
0.5 to 5 mL SINGLE CHANNEL PIPETTE, PIP-187 |
Eppendorf |
Research Plus |
G37503L |
PIP-N/A |
Repeater Pipette |
Repeater Pipet |
Eppendorf |
Repeater Plus |
L18883C |
PIP-N/A |
12-Channel Pipette |
Pipette (1200uL 12 Channel) |
Eppendorf |
Xplorer Plus |
I36933L |
PIP-N/A |
|
10uL Single Channel Pipette |
Eppendorf |
Research Plus |
N69392K |
PIP-N/A |
|
10uL Single Channel Pipette |
Eppendorf |
Research Plus |
N69471K |
PIP-N/A |
|
200uL Single Channel Pipette |
Eppendorf |
Research Plus |
P42051K |
PIP-N/A (New) |
Single Channel Pipette |
1200uL 12 Channel Pipette |
Eppendorf |
Xplorer Plus |
J27483L |
PIP-N/A (New) |
Single Channel Pipette |
200uL Single Channel Pipette |
VWR |
76169-238 |
22H0507 |
PIP-N/A (New) |
Single Channel Pipette |
10uL Single Channel Pipette |
VWR |
76189-232 |
22H0705 |

PIP-N/A (New) |
Single Channel Pipette |
20uL Single Channel Pipette |
VWR |
76189-234 |
22H0297 |
PIP-N/A (New) |
Single Channel Pipette |
1000uL Single Channel Pipette |
VWR |
76189-240 |
22G1014 |
PIP-N/A (New) |
Single Channel Pipette |
2uL Single Channel Pipette |
VWR |
76189-274 |
22A1186 |
PP-03 |
KrosFlo Research II Pump |
KrosFlo Research II Pump (Used for Depth Filtration) |
Spectrum Laboratories |
900-1612 |
E10003074 |
PP-06 |
Peristaltic Pump |
Masterflex L/S Pump |
Masterflex by Cole-Parmer Instrument Co. |
7524-40 |
M10001213 |
PP-07 |
Peristaltic Pump |
Peristaltic Pump |
Masterflex by Cole-Parmer Instrument Co. |
77410-10 |
F11000873 |
PP-08 |
Peristaltic Pump |
Peristaltic Pump |
Masterflex by Cole-Parmer Instrument Co. |
77410-10 |
F11000868 |
PP-09 |
Peristaltic Pump |
Peristaltic Pump |
Masterflex by Cole-Parmer Instrument Co. |
950-0000 |
K10002589 |
PP-10 |
Peristaltic Pump |
Peristaltic Pump |
Watson Marlow |
520Du |
L020909 |
PP-12 |
Peristaltic Pump |
Peristaltic Pump |
Watson Marlow |
323S/D |
K100409 |
PP-13 |
Peristaltic Pump |
Masterflex VP |
Masterflex by Cole-Parmer Instrument Co. |
77410-10 |
E11000743 |

PP-14 |
Peristaltic Pump |
Masterflex L/P |
Masterflex by Cole-Parmer Instrument Co. |
77601-10 |
N/A |
PP-15 |
Peristaltic Pump |
Masterflex I/P Pump |
Masterflex by Cole-Parmer Instrument Co. |
77601-10 |
G11004421 |
PP-16 |
Peristaltic Pump |
Peristaltic Pump |
Spectrum Laboratories |
708-13197-000 |
C16001928 |
PP-17 |
Peristaltic Pump |
MasterFlex 15/24/35/36 L/S Pump |
Masterflex by Cole-Parmer Instrument Co. |
07522-20 |
D19004907 |
PP-19 |
Peristaltic Pump |
Peristaltic Pump |
Masterflex by Cole-Parmer Instrument Co. |
77602-10 |
D20000965 |
PP-20 |
Peristaltic Pump |
Peristaltic Pump |
Masterflex by Cole-Parmer Instrument Co. |
77410-10 |
D20000964 |
PP-21 |
Peristaltic Pump |
Peristaltic Pump |
Watson Marlow |
323E/D |
200605-301958 |
PRC-03 |
|
Mastercycle |
Eppendorf |
nexus GX2 |
6336JL526723 |
PS-01 |
Electrophoresis Power Supply |
Mini-Gel Electrophoresis Power Supply |
Life Technologies |
PowerEase 90W PS0090 |
090091217 |
PS-02 |
Electrophoresis Power Supply |
Mini-Gel Electrophoresis Power Supply |
Life Technologies |
PowerEase 90W PS0090 |
090091217 |
PSS-01 |
|
PROTEIN STAINING SYSTEM, |
GENSCRIPT |
L00657 |
|

|
|
BUILDING 1 MB/PD LAB |
|
|
|
PU-18 |
Peristaltic Pump |
MasterFlex 73/82 L/S Pump; B1 |
Masterflex by Cole-Parmer Instrument Co. |
77420-10 |
F19003722 |
PU-502 |
|
Centrifugal Pump (Sanitary Centrifugal Pump) |
Fristam |
FZX2150 |
FZX21502002648 |
PW-02 |
|
ELGA Pure Water System |
ELGA |
Purelab Ultra |
UGG279578 |
RH-09 |
|
Temperature element; TraceableGO DATALOGGING HYGROMETER |
Control Company |
15-027-679 |
181361790 |
RH-15 |
Thermometer/Clock/Humidity Monitor |
Thermometer/Clock/Humidity Monitor |
VWR |
36934-164 |
192263914 |
RH-17 |
Thermometer/Clock/Humidity Monitor |
Thermometer/Clock/Humidity Monitor |
VWR |
36934-164 |
192263885 |
RH-36 |
Thermometer/Clock/Humidity Monitor |
Thermometer/Clock/Humidity Monitor |
VWR |
62344-734 |
170009673 |
RH-37 |
Thermometer/Clock/Humidity Monitor |
Thermometer/Clock/Humidity Monitor |
VWR |
62344-734 |
170009667 |
ROTO-01 |
|
Centrifuge Rotor |
Beckman Coulter |
SX4400 |
13D 1054 |
ROTO-02 |
|
Centrifuge Rotor |
Beckman Coulter |
S096 |
12D 1116 |
ROTO-03 |
|
Centrifuge Rotor |
Beckman Coulter |
|
21D 1128 |
RS-01 |
|
Hoopman Seed Sifter |
HOOPMAN EQUIPMENT AND |
RS-480 |
20E64-0297 |

|
|
|
ENGINEERING |
|
|
RT-01 |
|
Rotap Sifter (RO-TAP SIEVE SHAKER) |
WSTyler |
RX-29 |
16924 |
SC-02 |
Scale/Balance |
1200g ANALYTICAL BALANCE, SC-02 |
Mettler Toledo |
XP1203S (VWR 11277-142) |
B104107101 |
SC-03 |
Scale/Balance |
400 g Benchtop Scale |
Ohaus |
SP402 |
7131450513 |
SC-05 |
Scale/Balance |
400 g Benchtop Scale |
Ohaus |
SP402 |
7131450567 |
SC-06 |
Scale/Balance |
4000 g Benchtop Scale |
Ohaus |
SP4001 |
B615338640 |
SC-07 |
Scale/Balance |
400 g Benchtop Scale |
Ohaus |
SP402 |
7131250842 |
SC-08 |
Scale/Balance |
4000 g Benchtop Scale |
Ohaus |
SP4001 |
7131282198 |
SC-09 |
Scale/Balance |
4000 g Benchtop Scale |
Ohaus |
SP4001 |
7130210631 |
SC-10 |
Scale/Balance |
300 kg Floor Scale |
Ohaus |
T32XW |
0048381-6JM |
SC-11 |
Scale/Balance |
200 kg Floor Scale |
Arlyn Scales |
SAW-KMPI-201-3 |
38382G |
SC-12 |
Scale/Balance |
60,000 g Benchtop Scale |
Arlyn Scales |
SAW-KML-12 |
39243A |
SC-15 |
Scale/Balance |
6200 g Benchtop Scale |
Ohaus |
SPX6201 |
B620493261 |
SC-16 |
Scale/Balance |
32100 g Benchtop Scale |
Mettler Toledo |
XS32001L (VWR 62410-854) |
B63806041 |
SC-17 |
Scale/Balance |
5000 lb Portable Floor |
Mettler Toledo |
Deckmate 2888 with |
1175478-1AN with |

|
|
Scale with Indicator |
|
IND570 Harsh |
04553356MM |
SC-18 |
Scale/Balance |
5000 lb Portable Floor Scale with Indicator |
Mettler Toledo |
Deckmate 2888 with IND570 Harsh |
1175483-1AN with 04553326MM |
SC-19 |
Scale/Balance |
300 kg Floor Scale |
Ohaus |
3000 Series T31P |
004221-6HM |
SC-20 |
Scale/Balance |
20 kg Benchtop Scale |
AND |
SK-20K |
M4131280 |
SC-21 |
Scale/Balance |
200 g Benchtop Scale |
Ohaus |
CS200 |
Unknown |
SC-22 |
Scale/Balance |
30000 g Benchtop Scale |
Ohaus |
V71P30T |
8337390095 |
SC-23 |
Scale/Balance |
100 kg Floor Scale |
Ohaus |
Defender 5000 T51XW |
B644224624 |
SC-24 |
Scale/Balance |
30000 g Benchtop Scale |
Ohaus |
V71P30T |
8337470178 |
SC-25 |
Scale/Balance |
5000 lb Floor Scale with Indicator |
Mettler Toledo |
PUA579 with IND570 Harsh |
B71493420 with B651462092 |
SC-26 |
Scale/Balance |
60 kg Floor Scale with Indicator |
Mettler Toledo |
PBA655-CC60 with IND246 |
B721166690 with B742830324 |
SC-27 |
Scale/Balance |
220 g Benchtop Scale |
Mettler Toledo |
ME204TE/00 |
B919631364 |
SC-28 |
Scale/Balance |
6200 g Benchtop Scale |
Mettler Toledo |
ML6001T/00 |
C030759821 |
SC-29 |
Scale/Balance |
600 kg Portable Floor Scale with Indicator |
Mettler Toledo |
Deckmate 2888 with IND236 |
C048577386 with C032837773 |
SC-30 |
Scale/Balance |
120 g Portable Toploading Balance |
VWR |
VWR-123P |
10282020121 |

SC-33 |
Scale/Balance |
1500 kg Portable Floor Scale with Indicator |
Mettler Toledo |
Deckmate 2888 with IND570 Harsh |
C219032181 with C129189754 |
SC-N/A (New) |
Scale/Balance |
60 kg KrosFlo Scale |
Repligen |
ACSS-60K |
633495 |
SC-N/A (New) |
Scale/Balance |
60 kg KrosFlo Scale |
Repligen |
ACSS-60K |
633501 |
SC-N/A (New) |
Scale/Balance |
1500 kg Portable Floor Scale with Indicator |
Mettler Toledo |
Deckmate 2888 with IND570 Harsh |
C210670680 with C208578114 |
SCN-01 |
Electrophoresis Gel Scanner |
Electropherisis Gel Scanner; Computer: DT-99 |
Microtek |
Bio-5000 plus |
SK6A2100119 |
SCN-01_DT-99 |
Electrophoresis Gel Scanner |
Desktop; Instrument: SCN-01 |
Dell |
|
|
SD-01 |
|
SMC Seeder (Seeder Portion) |
|
CXSM20-50-273L |
N/A |
SHK-01 |
Shaker |
Low speed orbital shaker |
CORNING |
S2030-LS-COR |
16103390 |
SHK-02 |
Shaker |
Digital Variable Speed Nutating Mixer |
Fisher Scientific |
88861043 |
G4CF61043015 |
SHK-03 |
Shaker |
Digital MicroPlate Shaker |
Thermo Scientific |
88882005 |
K3CT82005021 |
SHK-04 |
Shaker |
Digital Microplate Shaker |
Thermo Scientific |
88882005 |
KBCT82005172 |
SHK-05 |
Shaker |
Analog Low Speed Orbital Shaker (LES Orbital Shaker 120V) |
Corning |
6780-FP |
20040169 |
SHK-06 |
Shaker |
Analog Titer Plate Shaker |
Thermo Scientific |
4625 |
C1882110311321 |

SHK-07 |
Shaker |
Shaker (tilt) |
THERMO SCIENTIFIC |
4630 |
C1668101034877 |
SHK-08 |
Shaker |
LSE Low Speed Orbital Shaker |
CORNING |
S2030-LS-COR |
16033191 |
SHK-N/A (New) |
Shaker |
Digital MicroPlate Shaker |
Thermo Scientific |
88882005 |
KBCT82005184 |
SP-06 |
Stir Plate |
Analog Magnetic Stir Plate |
Thermo Scientific |
SP18425Q |
C1725160405796 |
SP-07 |
Stir Plate |
Analog Magnetic Stir Plate |
Thermo Scientific |
SP18425Q |
C1725160405797 |
SP-08 |
Stir Plate |
Digital Magnetic Stir Plate |
VWR |
97042-748 |
160419001 |
SP-09 |
Stir Plate |
Stir Plate; B1 |
COLE PARMER |
04661-29 |
010212C04714 |
SP-10 |
Stir Plate |
Analog Magnetic Stirrer |
VWR |
12620-994 |
110505009 |
SP-11 |
Stir Plate |
Digital Magnetic Stir Plate |
Corning |
6795-610D |
133710018020 |
SPC-01 |
|
Spectrophotometer |
Beckman Coulter |
DU 730 |
1321276 |
SPC-02 |
|
Spectrophotometer; Computer: LAB05 |
Beckman Coulter |
DU 800 |
1096311 |
SPC-02_DT-32 |
|
Desktop CPU; Instrument: SPC-02 |
Dell |
Optiplex 980 |
397BNN1 |
SPC-04 |
|
Chlorophyll Meter |
Konica Minolta |
SPAD-502 Plus |
20009831 |
SPC-05 |
|
Handheld Wavelength Light Meter |
Wave Illumination |
WAVEGO-VIS-50 |
W119040274 |
SPC-06 |
|
Spectrophotometer |
THERMOSCIENTIFIC |
GENESYS 30 |
9A1Y121114 |

SPC-07 |
|
Spectrophotometer |
THERMOSCIENTIFIC |
GENESYS 30 |
9A1Y121108 |
SPC-08 |
|
Evolution 260 Bio UV-VISIBLE SPECTROPHOTOMETER, SPC-08; Computer: DT-70 |
THERMO FISHER SCIENTIFIC |
840-211000 |
5A6Y 073105 |
SPC-08_DT-70 |
|
Dell Desktop; Instrument: SPC-08 |
Dell |
|
|
SPC-N/A |
Fluorometer |
Qubit 4 Fluorometer |
Thermo Fisher Scientific |
Qubit 4 Fluorometer |
2322621070191 |
SPST-01 |
|
Certified Reference Materials |
Hellma Analytics |
Type 667-UV003 |
None |
SPST-02 |
|
Certified Reference Materials |
Hellma Analytics |
Type 666-F3 |
None |
SPST-03 |
|
Certified Reference Materials |
Hellma Analytics |
Type 666-F290 |
None |
SPST-04 |
|
Certified Reference Materials |
Starna Scientific |
87288-87293 |
33083 |
SPST-05 |
|
Certified Reference Materials |
Starna Scientific |
87311-87312 |
33084 |
SS-01 |
Autoclave |
PRIMUS Autoclave; B1 |
PRIMUS |
PSS8-B-SPSD |
17603 |
SS-02 |
Autoclave |
Autoclave |
Sanyo |
MLS-3781-L |
30641 |
SS-03 |
Autoclave |
Autoclave |
Sanyo |
MLS-3781-L |
30642 |
STAB-01 |
Incubator |
Stability Chamber (25 C / 60% RH STABILITY CHAMBER) |
BAHNSON ENVIRONMENTAL SPECIALTIES |
ES2000 CDM-C |
2107234827 |
STAB-02 |
Incubator |
Stability Chamber (25 |
BAHNSON ENVIRON |
ES2000 CDM-C |
2107234826 |

|
|
C / 60% RH STABILITY CHAMBER) |
MENTAL SPECIALTIES |
|
|
SW-01 |
Other |
Slide Warmer |
Premiere |
XH-2002 |
C&AU110161 |
TE-36 |
|
TempAlert TEMPERATURE SENSOR, TE-36 |
SMART SENSE by DIGI |
AC-TMP5PINDIN6 |
28FA96D4090000 |
TE-37 |
|
TempAlert TEMPERATURE SENSOR, TE-37 |
SMART SENSE by DIGI |
AC-TMP5PINDIN6 |
289022D4090000 |
TE-503 |
|
Tank 503 Regulator |
|
N/A |
N/A |
TFF-01 |
TFF |
Spectrum KTF-1000 System |
REPLIGEN |
SYTF-1000 |
2016-12-20-002 |
TFF-01_TK-01 |
TFF |
Spectrum TFF-01 Surge Tank |
REPLIGEN |
ZA1234289 |
80601138 |
TFF-02 |
TFF |
Spectrum KTF-1000 System |
REPLIGEN |
SYTF-1000 |
2016-12-20-001 |
TFF-02_TK-02 |
TFF |
Spectrum TFF-02 Surge Tank |
REPLIGEN |
ZA014860 |
80601115 |
TFF-03 |
TFF |
KrosFlo KMPi TFF System |
REPLIGEN |
|
S/N: C1600469 |

TFF-04 |
TFF |
KrosFlo KR2i TFF System |
REPLIGEN |
900-1893 |
Pump S/N: B16004036 |
TFF-05 |
TFF |
KrosFlo KR2i TFF System |
REPLIGEN |
900-1893 |
J19004878 |
TFF-06 |
TFF |
KrosFlo KMPi TFF System |
REPLIGEN |
900-1939 |
L19003012 |
TFF-07 |
TFF |
KrosFlo KMPi TFF System |
REPLIGEN |
900-1939 |
L19003011 |
TFF-08 |
TFF |
TFF Skid (MasterFlex); KrosFlo LDF37 TFF System |
REPLIGEN |
SYKL-121-01N or SYKL-122-01N |
A11000474 (Masterflex B/T Digital Modular Drive) |

|
|
|
|
01N (SpectrumLabs; Model: 900-1607) |
|
TFF-09 |
TFF |
Repligen KTF-250 System |
REPLIGEN |
SYTF-250 |
200626-001 |
TFF-10 |
TFF |
Repligen KTF-600 System |
REPLIGEN |
SYTF-600 |
200625-001 |
TFF-11 |
TFF |
KrosFlo KR2i TFF System |
REPLIGEN |
900-1893 |
L19005530 |
TFF-12 |
TFF |
Repligen KTF-2000 System |
REPLIGEN |
SYTF-2000 |
200323-001 |
TFF-13 |
TFF |
KrosFlo KR2i TFF System |
REPLIGEN |
900-1893 |
Unknown |
TFF-14 |
TFF |
Repligen KTF-200 System |
REPLIGEN |
SYTF-200 |
20059865-001 |
TM-01 |
Thermometer |
Digital Thermocouple Thermometer |
Fluke |
52 |
72840145 |
TM-02 |
Thermometer |
Digital Thermometer |
Fluke |
51 II |
13550436 |
TM-06 |
Thermometer |
LONG-STEM DIGITAL THERMOMETER, TM-06 |
VWR |
61220-416 |
160381824 |
TM-09 |
Thermometer |
Single Channel Datalogging Thermometer |
Control Company |
61161-336 |
111515290 |
TM-15 |
Thermometer |
2 Channel Datalogging Thermometer |
VWR |
10048-650 |
170320475 |
TM-17 |
Thermometer |
2 Channel Datalogging Thermometer |
VWR |
10048-650 |
170372115 |
TM-18 |
Thermometer |
2 Channel Datalogging Thermometer |
VWR |
10048-650 |
170372116 |
TM-19 |
Thermometer |
2 Channel Datalogging Thermometer |
VWR |
10048-660 |
170407774 |
TM-22 |
Thermometer |
Liquid in Glass Thermometer |
VWR |
61014-920 |
None |

TM-25 |
Thermometer |
Liquid in Glass Thermometer |
VWR |
61014-920 |
None |
TM-26 |
Thermometer |
Liquid in Glass Thermometer |
H-B |
61014-920 |
None |
TM-28 |
Thermometer |
Liquid in Glass Thermometer |
H-B |
60 to 120 deg F |
L58439 |
TM-30 |
Thermometer |
Long-Stem Digital Thermometer |
Digi-Sense |
61220-416 |
191952722 |
TM-32 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
Unknown |
TM-40 |
Thermometer |
Temperature Monitoring Datalogger |
Sensitech |
TempTale Ultra Dry Ice Probe |
GH22S003H0 |
TM-41 |
Thermometer |
Temperature Monitoring Datalogger |
Sensitech |
TempTale Ultra Dry Ice Probe |
GH22S003D0 |
TM-42 |
Thermometer |
Temperature Monitoring Datalogger |
Sensitech |
TempTale Ultra Dry Ice Probe |
GDS2S007V0 |
TM-43 |
Thermometer |
Temperature Monitoring Datalogger |
Sensitech |
TempTale Ultra Dry Ice Probe |
GDS2S007E0 |
TM-44 |
Thermometer |
Temperature Monitoring Datalogger |
Sensitech |
TempTale Ultra Dry Ice Probe |
GDS2S007B0 |
TM-46 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
200543970 |
TM-48 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
200110485 |
TM-49 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
200544006 |
TM-50 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369473 |

TM-51 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369451 |
TM-52 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
Unknown |
TM-53 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369458 |
TM-54 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369476 |
TM-55 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369460 |
TM-56 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369465 |
TM-57 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369454 |
TM-58 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369523 |
TM-59 |
Thermometer |
Long-Stem Digital Thermometer |
(Cole-Parmer) Digi-Sense |
90205-00 |
210369468 |
TM-60 |
Thermometer |
Total Immersion Liquid in Glass Thermometer |
VWR |
89095-612 |
725753 |
TM-61 |
Thermometer |
Total Immersion Liquid in Glass Thermometer |
VWR |
89095-612 |
725870 |
TM-62 |
Thermometer |
Total Immersion Liquid in Glass Thermometer |
VWR |
89095-612 |
725944 |

TOC-02 |
|
TOC Analyzer; Computer: DT-59 |
SUEZ |
M9 Laboratory |
21036186 |
TOC-02_AUTO-01 |
|
TOC Autosampler |
SUEZ |
AUTOSAMPLER |
A2EE-698B-43A9-94D1-0C2645DF294D |
TOC-02_DT-59 |
|
Dell Desktop/Sievers M9, TOC-02 (TOC) |
Dell |
|
|
TP-01 |
|
Schneider Control Panel |
|
N/A |
N/A |
TSW-20 |
Timer |
3 Channel Timer |
Fisherbrand |
06-662-46 |
181141321 |
TSW-22 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
191972038 |
TSW-31 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
181582595 |
TSW-33 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
181582673 |
TSW-34 |
Timer |
Nano Timer |
VWR |
21800-064 |
192577090 |
TSW-38 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
200398677 |
TSW-41 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
200398710 |
TSW-42 |
Timer |
Jumbo 2 Channel Digital Timer |
Fisherbrand |
06-662-47 |
200398665 |
TSW-43 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
200398670 |
TSW-45 |
Timer |
Jumbo 2 Channel Digital Timer |
VWR |
61161-346 |
200398735 |
TSW-48 |
Timer |
4 Channel Timer |
Control Company |
5004 |
200486051 |
TSW-51 |
Timer |
4 Channel Timer |
Control Company |
5004 |
200486056 |

TSW-52 |
Timer |
4 Channel Timer |
Control Company |
5004 |
200486054 |
TSW-54 |
Timer |
4 Channel Timer |
Control Company |
5004 |
200486052 |
TSW-58 |
Timer |
Digital Stopwatch |
VWR |
82023-864 |
WA07906 |
TSW-60 |
Timer |
Digital Stopwatch |
VWR |
82023-864 |
WA07914 |
TSW-64 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931180 |
TSW-65 |
Timer |
Nano Timer |
VWR |
21800-064 |
210727529 |
TSW-66 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931190 |
TSW-67 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210931191 |
TSW-68 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931189 |
TSW-69 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931195 |
TSW-70 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210931185 |
TSW-71 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931177 |
TSW-72 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931198 |
TSW-73 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931245 |
TSW-74 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931182 |
TSW-75 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210931200 |
TSW-77 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210727543 |
TSW-78 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210931201 |
TSW-80 |
Timer |
Nano Timer |
VWR |
21800-064 |
210931179 |
TSW-81 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210931205 |
TSW-82 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210931176 |
TSW-83 |
Timer |
Timer (Nano 2 Channel) |
VWR |
21800-064 |
210727550 |
TT-25 |
|
Temperature Alert |
DIGI |
TM-ZP300-SREVA |
11666000000168690138 |
TT-27 |
|
Temperature Alert |
DIGI |
TM-ZP300-SREVA |
11666000000168690138 |
TT-38 |
|
TempAlert WIRELESS TEMPERATURE |
SMART SENSE by DIGI |
TM-ZP300-S Rev A |
11666000000-178600001 |

|
|
TRANSMITTER, TT-38 |
|
|
|
TT-43 |
|
TempAlert WIRELESS TEMPERATURE TRANSMITTER, TT-43 |
SMART SENSE by DIGI |
TM-ZP300-DS Rev B |
11666000000-189718014 |
TT-45 |
|
TempAlert WIRELESS TEMPERATURE TRANSMITTER, TT-45 |
SMART SENSE by DIGI |
TM-ZP300-DS Rev B |
11666000000-189753969 |
TT-46 |
|
TempAlert WIRELESS TEMPERATURE TRANSMITTER, TT-46 |
SMART SENSE by DIGI |
TM-ZP300-DS Rev B |
1.1666E+19 |
TT-48 |
|
TempAlert WIRELESS TEMPERATURE TRANSMITTER, TT-48 |
SMART SENSE by DIGI |
TM-ZP300-DS Rev B |
11666000000-189680788 |
TT-51 |
|
TempAlert WIRELESS TEMPERATURE TRANSMITTER, TT-51 |
SMART SENSE by DIGI |
TM-ZP300-DS Rev B |
1.1666E+19 |
TT-52 |
|
Smart Sense Moisture Sensor |
SMART SENSE by DIGI |
TM-ZP300-DS Rev B |
11666000000-189702013 |
TURB-01 |
Handheld Turbidity Meter |
Handheld Turbidity Meter |
Hach |
LPG439.01.0002 |
12070C018761 |
TY-01 |
Tally Counter |
Tally Counter |
Cole Parmer |
20610-00 |
3128-201 |
TY-02 |
Tally Counter |
Tally Counter |
Cole Parmer |
20610-00 |
N/A |
TY-04 |
Tally Counter |
Handheld Tally Counter |
Uline |
H-7350 |
None |

UB-01 |
Ultrasonic waterbath |
Water Bath (Ultrasonic) |
VWR |
97043-972 |
1015A0217 |
UB-02 |
Ultrasonic waterbath |
Digital Ultrasonic Cleaner |
VWR |
97043-936 |
1013E041X |
VAC-FH-324 |
|
SFI Vacuum Tank |
|
855955-1-1 |
5475-1 |
VC-01 (ANZL-01) |
Cell Counter |
Vi-Cell BLU Cell Viability Analyzer |
Beckman Coulter |
ViCell Blu |
C1919622C077 |
VC-100 |
|
Perry Industries Recirculation Table |
|
VA-1200 |
P1053 |
VP-02 |
Dry Vacuum Pump/Compressor |
DRY VACUUM PUMP/COMPRESSOR, VP-02 |
Welch |
2511B-75 B |
21600001068 |
VP-03 |
Dry Vacuum Pump/Compressor |
Vacuum Pump |
VACUUBRAND |
MZ 2C NT |
41194003 |
VP-04 |
Dry Vacuum Pump/Compressor |
Dry Vacuum Pump/Compressor |
Welch |
25118-75 B |
041200000866 |
VP-05 |
Dry Vacuum Pump/Compressor |
Vacuum Pump |
Welch |
2511B-75 C |
042000001173 |
VPC-01 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
MILLIPORE SIGMA |
MAS-100VF |
201288 |
VPC-02 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
MILLIPORE SIGMA |
MAS-100VF |
201289 |
VPC-03 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
EMD Millipore |
MAS-100 VF |
201290 |
VPC-04 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
EMD Millipore |
MAS-100 VF |
202390 |
VPC-05 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
MILLIPORE SIGMA |
MAS-100VF |
202722 |

VPC-06 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
EMD Millipore |
MAS-100 VF |
202723 |
VPC-07 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
MILLIPORE SIGMA |
MAS-100VF |
202724 |
VPC-08 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
MILLIPORE SIGMA |
MAS-100VF |
202725 |
VPC-09 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
MILLIPORE SIGMA |
MAS-100VF |
202726 |
VPC-10 |
Air Sampler |
Viable Air Sampler, QC EM Devices |
EMD Millipore |
MAS-100 VF |
202727 |
VPE-01 |
Spectrometer |
SoloVPE SPECTROMETER, VPE-01; Computer: BCS-SOLOVPE-01 |
C TECHNOLOGIES, Inc |
IN-VPE-SOLO5 |
CTS2201049 |
VX-01 |
Vortex mixer |
Analog Vortex Mixer |
VWR |
58816-121 |
101216023 |
VX-02 |
Vortex mixer |
VWR Vortex Mixer |
VWR |
10153-688 |
018133022489 |
VX-03 |
Vortex mixer |
VWR Vortex Mixer |
VWR |
14005-824 (945303) |
70111001 |
VX-05 |
Vortex mixer |
Vortexer |
THERMO SCIENTIFIC |
88882009 |
JBCT82009043 |
VX-06 |
Vortex mixer |
Vortexer (9454FIDGUS) |
Fisher Scientific |
02215418 |
200811005 |
VX-07 |
Vortex mixer |
VWR Advanced Vortex Mixer |
VWR |
97043-564 |
200708001 |
VX-08 |
Vortex mixer |
Analog Vortex Mixer |
VWR |
58816-121 |
100729032 |
VX-N/A |
|
IKA MS 3 Vortexer |
IKA |
MS 3 B S36 |
03.527864 |
VX-N/A (New) |
Vortex mixer |
VWR Digital Vortex Mixer |
VWR |
10153-842 |
C224266120 |
VX-N/A (New) |
Vortex mixer |
VWR Digital Vortex Mixer |
VWR |
10153-842 |
C224266141 |

VX-N/A (New) |
Vortex mixer |
Advanced Vortex Mixer |
VWR |
10153-842 |
C224266119 |
VX-N/A (New) |
Vortex mixer |
Advanced Vortex Mixer |
VWR |
9453VWHDUSA |
C234665787 |
WB-01 |
Water Bath |
Heating Bath 10LA |
VWR |
97025-118 |
1H1120169 |
WB-02 |
Water Bath |
Water Bath (General Purpose) |
VWR |
WB-02 (or WB20) |
W41681341 |
WB-04 (New) |
Water Bath |
Digital Water Bath |
Benchmark Scientific |
B2000-08 |
MBG6119U-707 |
WB-N/A |
Water Bath |
Digital Water Bath |
PolyScience |
WBE05 |
E12170211 |
WPW-01 |
Microplate Reader |
Well Plate Washer BioTek ELX 508 |
BioTek |
05404-0998 |
254624 |
WS-01 |
|
Glassware Washer, QC EM Devices |
Miele Professional |
N/A |
74350297 |
FZ-04 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 |
Panasonic |
U76VA-PA |
16117N0304 |
FZ-06 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-70 |
Panasonic |
MDF-U76VA-PA |
16067N0208 |
FRZ-18 |
Dual Chamber Freezer (18-1 and 18-2) |
-20 |
PHCBI |
MDF-MU549DHL-PA |
210460070 |
FZ-03 |
SANYO Biomedical Freezer (-20C) |
-20 |
Sanyo |
MDF-U730 |
10099311 |
FZ-05 |
Freezer (-20C) |
-20 |
Panasonic |
MDF-U731M-PA |
16089305 |

FZ-07 |
|
-20 |
VWR |
SCBMF-1420 |
SYM-WB20961796 |
STAB-05 |
Freezer (-20C) |
-20 |
PHCBI |
MDF-MU549DHL-PA |
210360005 |
STAB-03 |
Refrigerator |
2 to 8 DEG C |
PHCBI |
MIR-554-PA |
21040077 |
FRZ-22-TE-01 |
Temperature probe |
|
BURNS ENGINEERING, Inc. |
23435-10A-035-120 |
4268334 |
STAB-04 |
Refrigerator |
2 to 8 DEG C |
PHCBI |
MIR-554-PA |
21040079 |
FRZ-20 |
|
-20 deg C Setpoint +/-5 deg C |
VECTOR LAB PRODUCTS |
VFZR25-LB-RSD |
21POP 003477 |
FRZ-21 |
|
-20 deg C Setpoint +/-5 deg C |
VECTOR LAB PRODUCTS |
VFZR25-LB-RSD |
21POP 003469 |
FZ-01-TE-01 |
Temperature probe |
|
|
|
|
FZ-15 |
Combination Refrigerator/Freezer |
-20 deg C Setpoint +/-5 deg C |
PHCBI |
MPR-715F-PA |
191090101 |
FZ-01 |
Freezer (-20C) |
-23 deg C Setpoint +/-5 deg C |
Sanyo |
MDF-U730M |
10119429 |
|
|
|
|
|
|
REF-10 |
Refrigerator |
5 deg C Setpoint +/-3 deg C |
VECTOR LAB PRODUCTS |
VREF25-LB-RSD |
21POP 028904 |
REF-11 |
Refrigerator |
5 deg C Setpoint +/-3 deg C |
VECTOR LAB PRODUCTS |
VREF25-LB-RSD |
21POP 019802 |
RF-07 |
Refrigerator |
5 deg C Setpoint +/-3 deg C |
PANASONIC |
MPR-721-PA |
16120850 |
RF-01 |
Sanyo Refrigerator |
5 deg C Setpoint +/-3 deg C, double |
SANYO |
MPR-1410 |
8120328 |

|
|
door refrigerator |
|
|
|
RF-02 |
Sanyo Refrigerator |
5 deg C Setpoint +/-3 deg C, double door refrigerator |
SANYO |
MPR-1411 |
10110326 |
FRZ-22 |
Freezer (-80C) |
-80 deg C Setpoint +/-10 deg C |
HAIER |
DW-86L729BPT |
BE0GY 6EBA0 0QGMA E0010 |
FRZ-23 |
Freezer (GMP) |
-80 deg C Setpoint +/-10 deg C |
HAIER |
DW-86L729BPT |
BE0GY 6EBA0 0QGMA E0020 |
FZ-02 |
Ultra-Low Temperature Freezer |
-80 to -60 deg C |
Sanyo |
MDF-U74VC |
10090862 |
FZ-08 |
Ultra-Low Temperature Freezer |
-80 to -60 deg C |
Sanyo |
MDF-U53VC |
8110241 |
FZ-09 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU502VXC-PA |
18070036 |
FZ-10 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU502VXC-PA |
18100058 |
FZ-11 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU702VXC-PA |
19020098 |

FZ-13 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU702VXC-PA |
18120545 |
FZ-14 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU702VXC-PA |
19010021 |
RF-01-TE-01 |
temperature probe |
|
|
|
|
FZ-19 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU702VXC |
2180472 |
RF-02-TE-01 |
Temperature probe |
|
|
|
|
STAB-06 |
Ultra Low-Temp Upright Freezer Panasonic MDF-DU502VXC-PA |
-80 to -60 deg C |
PHCBI |
MDF-DU702VXC-PA |
21050263 |
RF-03-TE-01 |
Temperature probe |
|
|
|
|
RF-03 |
VWR Refrigerator |
Double Door Refrigerator |
VWR |
GDM-49-SCI-HC-LD |
9049901 |
RF-06 |
Refrigerator |
Double Door Refrigerator |
VWR |
SCCP-49 |
SYM-7468326-1207 |
RF-08 |
Refrigerator |
Double Door Refrigerator |
VWR |
GDM-49-SCI-HS-TSL01 |
10005976 |

RF-09 |
Refrigerator |
Double Door Refrigerator |
VWR |
GDM-49-SCI-HS-TSL01 |
10009572 |
PGCH-01 |
Plant Grow Chamber |
gBrite LED PLANT GROWTH CHAMBER |
CARON |
7312-50-2 |
7312-50-2-013 |
PGCH-02 |
Plant Grow Chamber |
gBrite LED PLANT GROWTH CHAMBER |
CARON |
7312-50-2 |
7312-50-2-014 |
RF-04 |
Refrigerator |
Refrigerator |
SANYO |
MPR-311D(H) |
10110824 |
CR-114.1 |
Walk-in cold room (Room 114) |
|
|
|
|
FRZ-24 |
Combination Refrigerator/Freezer |
|
PHCBI |
MPR-715F-PA |
211290288 |
N/A |
Chart Liquid Nitrogen Tank |
|
Chart Inc |
MVE512 |
CAB2116450639 |
LA-10 |
Labeler |
Newman Stainless steel labeler |
Newman Labelling |
NV2 |
10450 |
FKL-01 |
Forklift |
Raymond electric forklift |
Raymond |
960-CSR30T |
960-10-01406 |
FKL-02 |
Forklift |
Raymond electric forklift |
Raymond |
960-CSR30T |
960-10-01407 |
FKL-03 |
Forklift |
Raymond electric forklift |
Raymond |
960-CSR30T |
960-10-01408 |
BR-03 |
Controller/chiller |
Bioreactor controller/Chiller |
Sartorius/Polyscience |
8843415/DCA304D1VX-U01 |
07172/1A10B1373 |
BR-03_TCU-01 |
Chiller |
VWR Chilled Recirculator |
VWR |
1177PD |
3F1081196 |
BR-03_TCU-03 |
Chiller |
VWR Chilled Recirculator |
VWR |
1177MD |
108800807 |
BR-01_TCU-01 |
Chiller |
Polyscience Chilled Recirculator |
VWR |
5870T87XC751 |
1C10A1640 |

BR-03_TCU-02 |
Chiller |
VWR Chilled Recirculator |
VWR |
1177PD |
108C00855 |
|
Electric Forklift |
Raymond electric forklift walk behind, 2020 |
Raymond |
6210 |
###-##-#### |
|
Crane |
Vestil aluminum rolling gantry crane |
Vestil |
|
|
|
Pump |
Busch rolling vacuum pump cart |
|
RS-Ra-0100F |
|
|
Cabinet |
All flammable cabinets |
|
|
|
|
Cabinet |
All corrosive cabinets |
|
|
|
|
Bin |
All PVC rolling trash bins |
|
|
|
|
Ladder |
All rolling ladders |
|
|
|
|
Lockers |
Two cylinder lockers |
|
|
|
|
Racks |
All Rolling multi tier metro racks |
|
|
|
|
Shelving |
three stainless steel multi shelf wall units, bookshelves |
|
|
|
|
Tables |
All stainless steel tables |
|
|
|
|
Ladders |
All fiberglass/aluminum step ladders |
|
|
|
|
Carts |
All stainless rolling carts |
|
|
|
|
Jacks |
All hydraulic pallet jacks |
|
|
|
|
Lift |
hydraulic die lifting cart |
|
|
|

|
Benches |
All Rolling lab benches |
|
|
|
|
Lifts/Dollies |
All Rolling barrel lifts/dollies |
|
|
|
|
Columns |
All rolling stainless chromotography columns |
|
|
|
|
Scrubber |
Tennant ECH2 walk behind floor scrubber |
Tennant |
ECH2 |
|
|
Totes |
All collapsible totes/bag holders |
|
|
|
|
Columns |
Pallet of glass Columns in the warehouse |
|
|
|
|
Pump |
Goulds pump |
Goulds |
5SVFA30 |
|
|
Racking |
five stainless tray racks |
|
|
|
|
Racking |
All wire racks |
|
|
|
|
Compressor |
Air compressor mounted on stainless table, warehouse |
|
|
|
|
Conveyors |
Two rolling conveyors |
|
|
|
|
Glassware |
Lab Glassware |
|
|
|
|
Vacuums |
Two shop vaccs in warehouse |
|
|
|
|
Fans |
Two Fans in warehouse |
|
|
|
|
Lights |
All Grow lights in warehouse |
|
|
|
|
Tank |
Carbon steel mixing tank in warehouse |
|
|
|

|
Sealer |
Hot lips sealer |
Wave |
28411704 |
1446852 |
|
Pump |
Mini peristaltic pump |
Krosflo |
Minikro pilot |
G10000004 |
|
Transformers |
All transformers in warehouse |
|
|
|
|
Switchboxes |
Pallet of Switch boxes |
|
|
|
|
Pumps |
Two Grundfos pumps in warehouse |
|
|
|
|
Lift |
rolling boom lift |
|
|
|
|
Filter |
Donaldson Stainless filter |
|
|
|
|
Filter |
New in crate Donaldson stainless filter |
|
|
|
|
Filter |
Stainless steel filter |
Pentair |
ES22NA-15 |
|
HPLC-01 |
VWR Refrigerator |
Hewlett Packard HPLC |
Hewlett packard |
1100 |
|
|
UV-Vis |
UV Vision unit |
Cary |
60 UV-Vis |
G6860A |
|
Shaker |
Incubated shaker |
Jeio Tech |
SI-600 |
M039118 |
|
Lift |
Autoquip 1,000 lb capacity lift in warehouse |
|
|
|
|
TA System |
New in box TA System |
Waters |
P/N 533002.901 |
5332-3071 |
|
Lift |
Autoquip 2,500 lb lift |
Autoquip |
STE30-25-4 |
211108791 |
|
Controller |
Axichrom Master controller |
Axichrom |
300-1000 |
44 |
|
Cabinet |
Stainless steel cabinet |
|
|
|
|
Collector |
Fraction collector |
GE Healthcare |
F9-R |
1779850 |
|
Cryo rack |
Cryo rack system |
VWR |
BR-3 |
NPB2012200846 |

|
Tank |
Cryo tank |
MVE |
SC 4/2V |
|
CV-100 |
Table |
Accumulation table |
M&O perry |
VA-1200 |
P-1053 |
FTP-01 |
FTP Unit |
Lab FTP unit |
|
|
|
|
Sinks |
All stainless steel sinks |
|
|
|
ICE-02 |
Ice machine |
Ice machine |
|
|
|
TK-3441-A |
Tank |
Plastic mixing tank |
|
|
|
TK-3441-B |
Tank |
Plastic mixing tank |
|
|
|
TK-3441-C |
Tank |
Plastic mixing tank |
|
|
|
|
Charger |
Forklift Battery Charger |
Enersys |
EQ3-15-1 |
IJ70608 |
|
Charger |
Forklift Battery Charger |
Enersys |
EQ3-15-1 |
IJ70606 |
|
Charger |
Forklift Battery Charger |
Enersys |
EQ3-15-1 |
IJ70607 |
|
Charger |
Forklift Battery Charger |
Enersys |
EQ3-15-1 |
IJ70605 |
TK-401 |
SS Tank |
T&C 200 gallon pressurized tank with pump |
T&C Stainless |
|
TC7354 |
TK-501 |
|
Harvest T&C Tank Vessel (TK-501) |
T&C STAINLESS |
|
TC7355 |
TK-501_TT-501 |
|
Tank 501 Sensor TT-501 |
ROSEMOUNT |
644HANAJ6M5Q4F6 |
287705 |
TK-503 |
|
Harvest T&C Tank Vessel (TK-503) |
T&C STAINLESS |
|
SN: TC7363, NB: 1092 |
TK-503 |
|
T&C Tank (503) |
|
N/A |
TC7354 |
TK-503_ TT-503 |
|
Tank 503 Temperature Sensor |
ROSEMOUNT |
644HANAJ6M5Q4F6 |
285370 |
CF-502 |
Centrifuge |
HARVEST CENTRIFUGE, CF-502 |
FLOTTWEG |
AC1200-420 HYG Disc Stack |
200017880 |

|
|
|
|
Sep 2 Phase |
|
CF-502_FI-01 |
Centrifuge |
Endress-Hauser Sensor, Flow Indicator |
Endress+Hauser |
Promag 10 Transmitter Model No.: 10H40-UFOA1AA |
N3031119000 |
CF-502_PI-01 |
Centrifuge |
Pressure Sensor |
Endress+Hauser |
PTP33B-IEW0/0 |
N303AA0116E |
CF-502_PI-02 |
Centrifuge |
Endress-Hauser Sensor, Pressure Indicator |
Endress+Hauser |
PTP33B-IEW0/0 |
N303180116E |
CF-502_PI-03 |
Centrifuge |
Filter Regulator |
Festo |
356 759 JN |
N/A |
CF-502_PI-04 |
Centrifuge |
Murr Sensor, Pressure Indicator |
|
|
2.56953E+17 |
CF-502_TRB-02 |
Centrifuge |
SELI Sensor, Turbidity Meter |
|
N/A |
2172756910 |
CF-502-TRB-01 |
Centrifuge |
Turbidity Sensor |
|
N/A |
2172465703 |
UV-302 |
Water Purifier |
UV Purification System |
Wyckomar Inc |
|
|
UV-301 |
Water Purifier |
UV Purification System |
Wyckomar Inc |
|
|
SK-3441, P 3441-F |
Pump skid |
Irrigation skid with pump |
|
|
|
TK-310 |
Tank |
Plastic tank |
|
|
|
TK-311 |
Tank |
Plastic tank |
|
|
|
TK-321 |
Tank |
Plastic tank |
|
|
|
TK-320 |
Tank |
Plastic tank |
|
|
|
TK-312 |
Tank |
Plastic tank |
|
|
|
TK-309 |
Tank |
Plastic tank |
|
|
|
P-310 |
Pump |
1/2HP convertible well pump. |
Wayne |
|
|

TK-304 |
|
Germination Tank |
CHEMTAINER |
TC3581IC-BLA |
1.9002E+11 |
TK-305 |
|
Germination Tank |
CHEMTAINER |
TC3581IC-BLA |
1.9002E+11 |
TK-306 |
|
Germination Tank |
CHEMTAINER |
TC3581IC-BLA |
1.9002E+11 |
TK-307 |
|
Germination Tank |
CHEMTAINER |
TC3581IC-BLA |
1.9002E+11 |
TK-308 |
|
Germination Return Tank |
CHEMTAINER |
TC3581IC-BLA |
1.9002E+11 |
TK-313 |
|
Post Infiltration Tank |
|
N/A |
N/A |
TK-314 |
|
Post Infiltration Tank |
|
N/A |
N/A |
TK-318 |
|
Seeder Tank |
|
N/A |
N/A |
TK-319 |
|
Transplant Tank |
CHEMTAINER |
TC3581IC-BLA |
NONE |
|
|
Softwalls (2) one in germ, one in warehouse |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equipment to be sold at seller's discretion | |||||
|
POD |
Growing pod, approximately 7' x 12' |
|
|
|
|
POD |
Growing pod, approximately 7' x 12' |
|||
|
POD |
Growing pod, approximately 7' x 12' |
|||
|
POD |
Growing pod, approximately 7' x 12' |
|||
|
POD |
Growing pod, approximately 7' x 12' |
|||

|
POD |
Green house. 12' x 42' approximately |
|||
|
Infiltrator |
Plant infiltrator system from Autoquip lift to autoquip lift |
|||
|
Pallet elevator |
pallet elevator |
|
||
|
Pallet elevator |
pallet elevator |
|
EXHIBIT B
[Purchaser Terms of Sale]

An 18% Buyer's Premium will apply.
PAYMENT TIMES: WITHIN 48 HOURS OF COMPLETION OF THE SALE.
ALL BIDS ARE MADE AND RECEIVED UNDER THE TERMS HEREINAFTER SET FORTH TO WHICH TERMS BIDDERS AGREE BY MAKING THEIR BID AT THE SALE. THESE TERMS CANNOT BE ALTERED EXCEPT BY THE AUCTIONEER. NO EMPLOYEE HAS AUTHORITY TO MODIFY SAME. THE OWNER AND/OR AUCTIONEER RESERVE THE RIGHT TO ADD TO OR MODIFY THE TERMS OF THE SALES.
This auction may require a credit or debit card 100% refundable deposit. Your credit/debit card added through the Bidspotter.com fully secure and PCI compliant registration process will be assessed a 100% refundable $1,000.00 (US Bidder) or $2,500.00 (International Bidder) deposit in order to be approved to bid online within this auction. If you are unsuccessful in the auction, your card will be refunded of your deposit within 24 hours post auction.
Holland Industrial Group LLC. DOES NOT TAKE DEBIT CARDS OR CREDIT CARDS FOR AUCTION INVOICE PAYMENTS.
All payments must be made by Wire Transfer or Company Check with Bank Letter of Guarantee to Holland Industrial Group LLC. Here is an example of what your letter should be: [Mr. “Customer Name” is a customer of this bank. This bank will guarantee unqualified payment of Holland Industrial Group LLC. on the account listed herein up to the amount of $. This letter is good until (insert expiration date 30 days from sale date)]. By registering, the Bidder hereby expressly authorizes HIG to charge the Credit Card if the Bidder fails to pay any invoice within forty-eight (48) hours after each such invoice is issued to Bidder.
All bidders are required to give full name, address, phone, fax, and bank information when registering. All bidders must register before bidding.
All bids must be paid in full within two business days after the bids have closed. The Auctioneer shall determine the bidding increments. Some items may be auctioned with reserve.
All purchases must be removed within two weeks after the bids have closed. No lot can, on any account, be removed prior to HIG having been paid in full. Purchases will be delivered only on presentation of paid bill. Auctioneer shall not be responsible for goods not removed within the aforesaid time.
Purchasers must remove equipment at their expense, risk, and liability. Purchasers must engage the riggers. Riggers, movers, electricians, or buyer performing work at the Auction Sites must provide Auctioneer with evidence of insurance. The insurance coverage must be at a level acceptable to Auctioneer and to the Seller.

The insurance must name both Holland Industrial Group LLC and IBIO INC as additional insured. Purchaser is required to remove all the equipment within a period not later than April 28. Purchaser is required to get their riggers, before starting removal, to furnish a certificate of insurance with both IBIO and Auctioneers listed as additional insureds. Purchasers are agreeing that they’ll lose their equipment if it’s not removed by April 28.
Purchasers are solely responsible for disconnecting any utilities and for rigging and shipping the equipment. All the equipment has to be removed by the Purchasers from facility by April 28. Purchasers must get the equipment removed in a workmanlike manner. Purchasers must remove oils or other fluids in the equipment before removal.
All checks for deposits and balances due shall be payable to the order of Holland Industrial Group LLC. unless instructed otherwise. All bills must be paid to the Opa-locka headquarters offices of the Auctioneer at 13105 NW 42nd Ave Opa-locka FL 33054, or by wire transfer directly to the bank specified in the wire transfer instructions provided for this on-line auction. The full purchase price on all lots sold to the same buyer must be paid within the time fixed and before removal of any of the goods.
THE AUCTIONEER SHALL NOT BE RESPONSIBLE FOR THE CORRECT DESCRIPTION, GENUINENESS, AUTHENTICITY OF, OR DEFECT IN ANY LOT, AND MAKES NO WARRANTY IN CONNECTION THEREWITH. NO SALE SHALL BE SET ASIDE NOR ALLOWANCE MADE ON ACCOUNT OF ANY INCORRECTNESS, ERROR IN CATALOGING, OR ANY IMPERFECTION NOT NOTED. NO DEDUCTION ALLOWED ON DAMAGED ARTICLES, ALL ARTICLES BEING EXPOSED FOR PUBLIC EXHIBITION, AND SOLD “AS IS” AND WITHOUT RECOURSE. ARTICLES ARE NOT WARRANTED AS MERCHANTABLE OR FIT FOR ANY PARTICULAR PURPOSE, AND NO CLAIM MAY BE MADE BY PURCHASER RELATING TO THE CONDITION OR USE OF ARTICLES PURCHASED, OR FOR PROXIMATE OR CONSEQUENTIAL DAMAGES ARISING THEREFROM.
Articles purchased may not incorporate approved activating mechanisms, operating safety devices or safety guards, as required by OSHA or otherwise. It is Purchaser’s responsibility that articles purchased be so equipped and safeguarded to meet OSHA and any other requirements before placing such articles into operation.
Purchaser agrees to indemnify and hold Auctioneer harmless from and against all claims and liabilities relating to the condition or use of the articles purchased or failure of user to follow instructions, warnings or recommendations of the manufacturer, or to comply with federal, state and local laws applicable to such articles, including OSHA requirements, or for proximate or consequential damages, costs or legal expenses arising therefrom.

No claims will be allowed after removal of goods from premises.
Auctioneer shall not, in any event, be liable for non-delivery or any other matter or thing, to any Purchaser of any lot, other than for the return to the Purchaser of the deposit or sum paid on said lot, should the Purchaser be entitled thereto.
In default of payment of bills in full within the time therein specified, the Auctioneer in addition to all other remedies allowed by law, may retain all monies received as deposit or otherwise, as liquidated damages. Lots not paid for and removed within the time allowed herein may be resold at public or private sale without further notice, and any deficiency, together with all expenses and charges of re-sale, will be charged to the defaulting Purchaser.
Persons attending during exhibition, sale or removal of goods assume all risks of damage of or loss to person and property and specifically release the Auctioneer from liability therefor. Neither the Auctioneer nor his principal shall be liable by reason of defect in or condition of the premises on which the sale is held.
The Auctioneer reserves the right to withdraw from sale any of the property listed or to sell at this sale property not listed, and also reserves the right to group one or more lots into one or more selling lots or to subdivide into two or more selling lots. Whenever the best interest of a Seller will be served, the Auctioneer reserves the right to sell all the property listed, in bulk.
Where items are sold by estimated weight, count or measure, the Purchaser will be billed for and required to pay for the estimated weight, count or measure. If, upon delivery, any shortage exists, the Purchaser will receive a refund at the rate of purchase. If there be an excess, the Purchaser will be required to pay for such excess, at the rate of purchase.
The Auctioneer reserves the right to reject any and all bids, alter the order of sale, and accept bids made on behalf of the Seller. Auctioneer is not responsible for internet lags, delays, blackouts, or disconnects.
The record of sale kept by the Auctioneer and bookkeeper will be taken as final in the event of any dispute.
The Auctioneer is acting as agent only and is not responsible for the acts of its principals. Purchaser acknowledges, as an inducement to Seller to commit Purchaser to bid, that Purchaser has independently inspected the goods bid upon by the Purchaser, and acknowledges to the Seller that Purchaser is acquiring all goods “AS IS, WITH ALL FAULTS” and “WITH REMOVAL AT PURCHASERS’ RISK AND EXPENSE” and agrees to protect, indemnify and hold harmless the Seller from any and all claims, demands, causes of actions, liabilities and judgments relating to the conditions or use of the goods.

Seller, if not the same as the Auctioneer, shall have the rights, protections and benefits afforded to the Auctioneer as set forth herein.
Although all information has been obtained from sources deemed reliable, the auctioneer and seller make no warranty or guarantee, expressed or implied, as to the accuracy of the information herein contained, or contained in our catalog. It is for this reason that buyers should avail themselves of the opportunity to make inspection prior to the auction. Although it is not likely, auction sales are subject to cancellation or postponement. Kindly contact auctioneer prior to attendance.
ANY ADDITIONAL PROVISIONS OR AMENDMENTS TO THE ABOVE LISTED TERMS WILL BE POSTED BY THE AUCTIONEER PRIOR TO THE START OF BIDDING.
Exhibit 10.8
FIFTH AMENDMENT TO CREDIT AGREEMENT
THIS FIFTH AMENDMENT TO CREDIT AGREEMENT (this “Fifth Amendment”) is entered into as of the Fifth Amendment Closing Date (as defined below) by and between IBIO CDMO LLC, a Delaware limited liability company (“Borrower”), and WOODFOREST NATIONAL BANK, a national banking association, as lender (in such capacity, “Lender”).
RECITALS
A.Borrower and Lender entered into that certain Credit Agreement dated November 1, 2021 (as amended by the First Amendment thereto dated as of October 11, 2022, the Second Amendment thereto dated as of February 9, 2023, the Third Amendment thereto dated as of February 20, 2023, the Fourth Amendment thereto dated as of March 24, 2023 and as otherwise amended, restated, supplemented or modified from time to time, the “Credit Agreement”).
B.On April 14, 2023, Borrower did not provide Lender with an executed Purchase Agreement with respect to the sale of the iBio Property, which resulted in a Default as of such date under and in accordance with the terms of Section 1(d) of the Fourth Amendment, without the benefit of any grace period otherwise provided under Section 11.2(b) of the Credit Agreement (herein, the “PSA Default”).
C.Borrower has requested Lender to waive the PSA Default and Lender is willing to waive the PSA Default.
D.Borrower and Lender are willing to enter into the requested waiver and certain agreements and amendments set forth herein, subject to and conditioned upon the terms and conditions set forth in this Fifth Amendment.
AGREEMENT
NOW, THEREFORE, in consideration of the promises herein contained, the mutual benefits to be derived herefrom and other good and valuable consideration received by each party, and each intending to be legally bound hereby, the parties agree as follows:
I.Agreements and Amendments to Credit Agreement. Borrower and Lender agree as follows:
(a)Section 1.1, Definitions, of the Credit Agreement is hereby amended by adding the following definitions in their proper alphabetical order:
“Fifth Amendment” means the Fifth Amendment to Credit Agreement dated as of the Fifth Amendment Closing Date by and between Borrower and Lender.
“Fifth Amendment Closing Date” means May 10, 2023.
“Payment in Full” means all Obligations (other than contingent Obligations with respect to indemnity and reimbursement of expenses as to which no claim has been made as of the time of determination) have been paid in full in cash.
(b)Section 3.3, Interest, of the Credit Agreement is hereby amended and restated by replacing such section in its entirety with the following language:
Interest. From and after the Fifth Amendment Closing Date, the Term Loan shall accrue interest at a fixed rate per annum equal to 5.25%. In addition to the foregoing, from and after the Fifth Amendment Closing Date, the Term Loan shall further accrue additional interest, payable in kind and added to the balance of the Term Principal Amount, at a fixed rate per annum equal to (a) 1.00%, if Payment in Full occurs on or before June 30, 2023, (b) 2.00%, if Payment in Full occurs after June 30, 2023, but on or before September 30, 2023, or (c) 3.00%, if Payment in Full occurs after September 30, 2023, such interest accruing (at either 1.00%, 2.00% or 3.00%, as applicable, for the entirety of the accrual period), in each case, commencing on the Fifth Amendment Closing Date and continuing through and including the date that Payment in Full occurs.
(c)Section I(d) of the Fourth Amendment of the Credit Agreement is hereby amended and restated in its entirety by replacing such section with the following language:
(d)Sale of iBio Property. On or before the Maturity Date, if Borrower enters into a purchase and sale agreement between Borrower, as seller, and a third party, as buyer, regarding the purchase and sale of the iBio Property (a “Purchase Agreement”), Borrower shall deliver to Lender an executed copy of such Purchase Agreement and undertake all reasonable efforts to consummate the sale of the iBio Property. Upon the reasonable written request of Lender, Borrower shall provide Lender with copies of any other documents directly related to the sale of the iBio Property.
(e)Section I(e) of the Fourth Amendment of the Credit Agreement is hereby amended and restated in its entirety by replacing such section with the following language:
Fee. On the earlier of (i) the date that Payment in Full occurs or (ii) the Maturity Date, Borrower shall pay to Lender, in immediately available funds, a fee, which fee, in any case, is fully earned as of the Fifth Amendment Closing Date, in the amount of (x) $75,000, if Payment in Full occurs on or before June 30, 2023, (y) $100,000, if Payment in Full occurs after June 30, 2023, but on or before September 30, 2023, or (z) $125,000, if Payment in Full does not occur in accordance with sub clauses (x) or (y) above.
(f)Release of Reserved Funds.
2
Notwithstanding anything to the contrary in Section I(f) of the Fourth Amendment, so long as no Potential Default or Default exists or would be caused thereby and Borrower and Parent Guarantor are otherwise in compliance with the Loan Documents, (i) when the outstanding Term Principal Amount is reduced to $10,000,000, Lender shall release $500,000 of the Reserved Funds from the Reserved Funds Deposit Account to Borrower, and (ii) for each additional $2,500,000 reduction of the outstanding Term Principal Amount (i.e. the first reduction from $10,000,000 to $7,500,000), Lender shall release an additional $750,000 of the Reserved Funds from the Reserved Funds Deposit Account to Borrower, in each case, in immediately available funds.
(g)Payment in Full. So long as Borrower provides reasonable notice to Lender of a payoff, Lender shall, at least one Business Day prior to the date Payment in Full occurs, provide Borrower with a written payoff letter, which shall, among other things, contain an itemized break down of all outstanding Obligations to be paid on the date that Payment in Full occurs.
II.Waiver.
(a)Lender hereby waives the PSA Default, including any failure to give notice thereof.
(b)The waiver granted by Lender hereunder does not indicate an intent to establish any course of dealing between Lender and Borrower with regard to future waivers, consents, agreements to forbear or any other modifications that may be requested. Lender’s agreement to the waiver set forth herein should not be construed as an indication that Lender would be willing to agree to any further or future consents, waivers, agreements to forbear or any modifications to any of the terms of the Credit Agreement, as amended hereby, or other Loan Documents, or in respect of any Potential Default or Default that may exist or that may occur.
III.Conditions Precedent to the Effectiveness of Fifth Amendment. This Fifth Amendment shall be effective upon the satisfaction of the following conditions precedent:
(a)Lender shall have received this Fifth Amendment duly executed by Borrower and Parent Guarantor;
(b)Lender shall have received an Officer’s Certificate and authorizing consent for each of Borrower and Parent Guarantor, in Proper Form;
(c)To the extent outstanding and unpaid, the Borrower shall have paid to Lender (i) any fees and expenses due and owing under the Credit Agreement and (ii) all costs and expenses, including reasonable legal fees, payable in connection with this Fifth Amendment to the extent invoiced on or prior to the Fifth Amendment Closing Date; and
(d)After giving effect to this Fifth Amendment, no Potential Default or Default shall have occurred and be continuing.
IV.Reaffirmation of Representations and Warranties.
3
To induce Lender to enter into this Fifth Amendment, Borrower hereby reaffirms, as of the Fifth Amendment Closing Date (except as otherwise provided herein or to the extent such representations and warranties speak as to an earlier date or a date certain), its representations and warranties contained in Section 7 of the Credit Agreement (other than the representation set forth in the last sentence of Section 7.10 of the Credit Agreement), and in all other documents executed pursuant thereto, and additionally represents and warrants as follows:
(a)The execution and delivery of this Fifth Amendment and the performance by Borrower of its obligations under this Fifth Amendment are within Borrower’s power, have been duly authorized by all necessary company action, have received all necessary governmental approval (if any shall be required), and do not and will not contravene or conflict with any provision of law or of the Organizational Documents of Borrower or of any agreement binding upon Borrower.
(b)This Fifth Amendment represents the legal, valid and binding obligations of Borrower enforceable against Borrower in accordance with its terms subject as to enforcement only to bankruptcy, insolvency, reorganization, moratorium or other similar laws affecting the enforcement of creditors’ rights generally.
(c)After giving effect to this Fifth Amendment, no change, event or state of affairs has occurred and is continuing which would constitute a Potential Default or a Default.
(d) No exhibit or schedule to the Credit Agreement is required to be supplemented, amended or modified in connection with the transactions contemplated by this Fifth Amendment.
V.Defined Terms. Terms used herein that are defined in the Credit Agreement, as amended hereby, shall have the same meanings herein, unless the context otherwise requires.
VI.Reaffirmation of Credit Agreement. This Fifth Amendment shall be deemed to be an amendment to the Credit Agreement, and the Credit Agreement, as amended hereby, is hereby ratified, adopted and confirmed in each and every respect.
VII.Ratification of Liens; Release. The Borrower acknowledges and ratifies, as of the Fifth Amendment Closing Date, the existence and priority of the Liens granted by the Borrower in favor of Lender pursuant to the Security Documents in and to the Collateral and represents, warrants and covenants that such Liens are valid, existing and in full force and effect. THE BORROWER HEREBY RELEASES, DISCHARGES AND ACQUITS LENDER AND EACH AFFILIATE THEREOF AND THEIR RESPECTIVE DIRECTORS, OFFICERS, OWNERS, EMPLOYEES, AGENTS, REPRESENTATIVES AND ADVISORS AND THEIR RESPECTIVE SUCCESSORS AND ASSIGNS, FROM ANY AND ALL CLAIMS, DEMANDS, ACTIONS, CAUSES OF ACTION, REMEDIES, AND LIABILITIES OF EVERY KIND OR NATURE (INCLUDING WITHOUT LIMITATION, LENDER LIABILITY) ARISING OUT OF ANY ACT, OCCURRENCE, TRANSACTION OR OMISSION OCCURRING IN CONNECTION WITH THE CREDIT AGREEMENT AND THE OTHER LOAN DOCUMENTS PRIOR TO THE FIFTH AMENDMENT CLOSING DATE.
VIII.Governing Law. THIS FIFTH AMENDMENT SHALL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF TEXAS.
4
IX.Invalid Provisions. If any provision of this Fifth Amendment is held to be illegal, invalid or unenforceable, (a) the legality, validity and enforceability of the remaining provisions of this Fifth Amendment shall not be affected or impaired thereby and (b) the parties shall engage in good faith negotiations to replace the illegal, invalid or unenforceable provisions, with valid provisions the economic effect of which comes as close as possible to that of the illegal, invalid or unenforceable provisions. The invalidity of a provision in a particular jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction.
X.Multiple Counterparts and Electronic Signatures. This Fifth Amendment may be executed in any number of counterparts with the same effect as if all signatories had signed the same document. All counterparts must be construed together to constitute one and the same instrument. This Fifth Amendment may be transmitted and signed by facsimile, portable document format (PDF), or other electronic means, and shall have the same effect as manually-signed originals and shall be binding on the Loan Parties and Lender, with originals signatures to be delivered to Lender at Lender’s request.
XI.Section Headings. Section headings in this Fifth Amendment are included for convenience of reference only and shall not affect the interpretation of this Fifth Amendment.
XII.Successors and Assigns. This Fifth Amendment is binding upon, and inures to the benefit of, the parties hereto and their respective successors and permitted assigns.
XIII.Reservation of Rights; No Waiver of Defaults. Lender hereby reserves all of its rights and remedies under the Credit Agreement and the other Loan Documents in all respects and for all purposes in addition to all other rights and remedies available to it under applicable Law or in equity. Other than as set forth herein, this Fifth Amendment is not intended to operate as a waiver of Lender’s rights and remedies, and, other than as set forth herein, does not constitute or operate as (a) a waiver of (or a consent to) any existing Potential Default or Default or any other violation of or noncompliance with any provision of the Credit Agreement, as amended hereby, or any other Loan Document, (b) an agreement to waive any existing or future Potential Default or Default, or (c) a waiver of Lender’s right to insist upon strict compliance with each term, covenant, condition and provision of the Credit Agreement, as amended hereby, and the other Loan Documents.
XIV.ENTIRETY. THIS FIFTH AMENDMENT REPRESENTS THE FINAL AGREEMENT AMONG BORROWER, GUARANTORS AND LENDER AND MAY NOT BE CONTRADICTED BY EVIDENCE OF PRIOR, CONTEMPORANEOUS, OR SUBSEQUENT ORAL AGREEMENTS BY BORROWER, GUARANTORS AND LENDER. THERE ARE NO UNWRITTEN ORAL AGREEMENTS AMONG BORROWER, GUARANTORS AND LENDER.
[Signature pages follow.]
5
IN WITNESS WHEREOF, the parties hereto have caused this Fifth Amendment to be duly executed on the Fifth Amendment Closing Date.
|
BORROWER: |
|
|
|
|
|
IBIO CDMO LLC, |
|
|
a Delaware limited liability company |
|
|
|
|
|
By: |
/s/ Felipe Duran |
|
|
Felipe Duran |
|
|
Authorized Person |
Signature Page to Fifth Amendment to Credit Agreement
|
LENDER: |
|
|
|
|
|
WOODFOREST NATIONAL BANK |
|
|
|
|
|
By: |
/s/ Cameron D. Jones |
|
|
Cameron D. Jones |
|
|
Senior Vice President |
Signature Page to Fifth Amendment to Credit Agreement
GUARANTOR’S CONSENT AND AGREEMENT
As an inducement to Lender to execute, and in consideration of Lender’s execution of, this Fifth Amendment, IBIO, INC., a Delaware corporation (“Guarantor”), hereby consents to this Fifth Amendment, and agrees that this Fifth Amendment shall in no way release, diminish, impair, reduce or otherwise adversely affect the obligations and liabilities of the undersigned under the Guaranty executed November 1, 2021 (as amended by the Guaranty First Amendment, the Guaranty Second Amendment, the Guaranty Third Amendment, the Guaranty Fourth Amendment and as otherwise amended, restated, supplemented or modified from time to time, the “Guaranty”) executed by Guarantor in connection with the Credit Agreement. Guarantor further represents and warrants to Lender that (a) the representations and warranties in the Guaranty are true and correct in all material respects on and as of the Fifth Amendment Closing Date as though made on such date (except to the extent that such representations and warranties specifically relate to an earlier date), (b) it is in full compliance with all covenants and agreements contained in the Guaranty, (c) after giving effect to the Fifth Amendment, no Potential Default or Default has occurred and is continuing under the Guaranty and (d) the execution and delivery of this Guarantor’s Consent and Agreement are within Guarantor’s power and have been duly authorized by all necessary company action. This Guarantor’s Consent and Agreement shall be binding upon Guarantor, and its successors and permitted assigns, and shall inure to the benefit of Lender, and its successors and permitted assigns.
[Signature page follows.]
|
GUARANTOR: |
|
|
|
|
|
IBIO, INC., |
|
|
a Delaware corporation |
|
|
|
|
|
By: |
/s/ Felipe Duran |
|
|
Felipe Duran |
|
|
Interim Chief Financial Officer |
Signature Page to Guarantor’s Consent and Agreement to
Fifth Amendment to Credit Agreement I, Martin Brenner, certify that:
Exhibit 31.1
CERTIFICATION PURSUANTTO RULE 13a-14(a) OR RULE 15d-14(a)
OF THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF
THE SARBANES-OXLEY ACT OF 2002
1. |
I have reviewed this Quarterly Report on Form 10-Q for the quarter ended March 31, 2023 (the “report”) of iBio, Inc. (the “registrant”); |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. |
The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
c. |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
d. |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. |
The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
|
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
|
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: May 15, 2023 |
|
|
|
By: |
/s/ Martin Brenner |
|
|
Name: Martin Brenner |
|
|
Title: Interim Chief Executive Officer and Chief Scientific Officer (Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION PURSUANTTO RULE 13a-14(a) OR RULE 15d-14(a)
OF THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF
THE SARBANES-OXLEY ACT OF 2002
I, Felipe Duran, certify that:
1. |
I have reviewed this Quarterly Report on Form 10-Q for the quarter ended March 31, 2023 (the “report”) of iBio, Inc. (the “registrant”); |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. |
The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
c. |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
d. |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. |
The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
|
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
|
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: May 15, 2023 |
|
|
|
By: |
/s/ Felipe Duran |
|
|
Name: Felipe Duran |
|
|
Title: Interim Chief Financial Officer (Interim Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of iBio, Inc. (the “Company”) for the quarterly period ended March 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Martin Brenner, Interim Chief Executive Officer and Chief Scientific Officer (Principal Executive Officer) of the Company, certify pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
1. |
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
2. |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: |
May 15, 2023 |
/s/ Martin Brenner |
Martin Brenner |
||
Interim Chief Executive Officer and Chief Scientific Officer |
||
(Principal Executive Officer) |
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of iBio, Inc. (the “Company”) for the quarterly period ended March 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Felipe Duran, Interim Chief Financial Officer (Principal Financial Officer) of the Company, certify pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
1. |
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
2. |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: |
May 15, 2023 |
/s/ Felipe Duran |
Felipe Duran |
||
Interim Chief Financial Officer |
||
(Interim Principal Financial Officer) |