
Bexobrutideg Investor Update Investor Presentation October 2025

Important Notice and Disclaimers This presentation contains statements that relate to future events and expectations and as such constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. When or if used in this presentation, the words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “outlook,” “plan,” “predict,” “should,” “will,” and similar expressions and their variants, as they relate to Nurix Therapeutics, Inc. (“Nurix”, the “Company,” “we,” “us” or “our”), may identify forward-looking statements. All statements that reflect Nurix’s expectations, assumptions or projections about the future, other than statements of historical fact, are forward-looking statements, including, without limitation, statements regarding the therapeutic potential of bexobrutideg; Nurix's plans for the clinical development of bexobrutideg; the planned timing for the initiation and enrollment of patients in current and future clinical trials of bexobrutideg; the planned timing for the provision of updates and findings from Nurix's clinical trials; our future financial or business plans; our future performance, prospects and strategies; future conditions, trends, and other financial and business matters; our current and prospective drug candidates; the planned timing and conduct of the clinical trial programs for our drug candidates; the planned timing for the provision of updates and initial findings from our clinical studies; the potential benefits of our collaborations, including potential milestone and sales-related payments; the potential advantages of DEL-AI and our drug candidates; the extent to which our scientific approach, our drug discovery engine, targeted protein degradation, and degrader antibody conjugates may potentially address a broad range of diseases; the extent animal model data, in vitro potency data, and proteomics data predicts human efficacy; the timing and success of the development and commercialization of our current and anticipated drug candidates; the expected net proceeds and completion of our registered direct offering; and our ability to fund our operations into 2028. Forward-looking statements reflect Nurix’s current beliefs, expectations, and assumptions. Although Nurix believes the expectations and assumptions reflected in such forward-looking statements are reasonable, Nurix can give no assurance that they will prove to be correct. Forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and changes in circumstances that are difficult to predict, which could cause Nurix’s actual activities and results to differ materially from those expressed in any forward-looking statement. Such risks and uncertainties include, but are not limited to: (i) whether Nurix will be able to advance, obtain regulatory approval of and ultimately commercialize bexobrutideg; (ii) risks and uncertainties related to Nurix’s ability to advance its drug candidates, obtain regulatory approval of and ultimately commercialize its drug candidates; (iii) the timing and results of clinical trials; (iv) Nurix’s ability to fund development activities and achieve development goals; (v) risks and uncertainties relating to Nurix's collaboration partners, including the speed of development of partnered programs and the timing and receipt of payments from Nurix's collaboration partners, including milestone payments and royalties on future potential product sales; (vi) the impact of macroeconomic events and conditions, including increasing financial market volatility and uncertainty, inflation, interest rate fluctuations, instability in the global banking system, uncertainty with respect to the federal budget and debt ceiling, the impact of war, military or regional conflicts, and global health pandemics, on Nurix’s clinical trials and operations; (vii) Nurix’s ability to protect intellectual property and (viii) other risks and uncertainties described under the heading “Risk Factors” in Nurix’s Quarterly Report on Form 10-Q for the fiscal quarter ended August 31, 2025, and other SEC filings. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. The statements in this presentation speak only as of the date of this presentation, even if subsequently made available by Nurix on its website or otherwise. Nurix disclaims any intention or obligation to update publicly any forward-looking statements, whether in response to new information, future events, or otherwise, except as required by applicable law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Furthermore, while we believe our own internal estimates and research are reliable, such estimates and research have not been verified by any independent source. 2
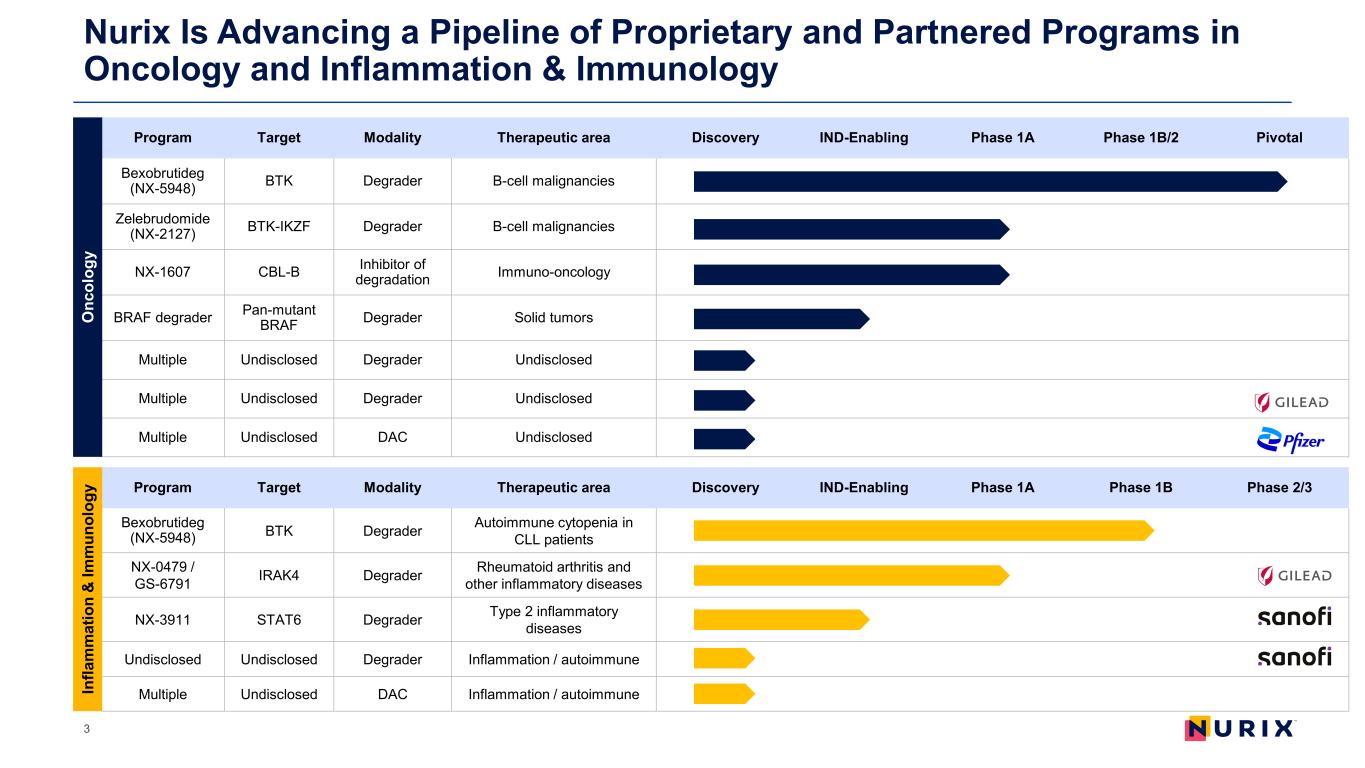
Nurix Is Advancing a Pipeline of Proprietary and Partnered Programs in Oncology and Inflammation & Immunology 3 Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B/2 Pivotal Bexobrutideg (NX-5948) BTK Degrader B-cell malignancies Zelebrudomide (NX-2127) BTK-IKZF Degrader B-cell malignancies NX-1607 CBL-B Inhibitor of degradation Immuno-oncology BRAF degrader Pan-mutant BRAF Degrader Solid tumors Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed DAC Undisclosed Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B Phase 2/3 Bexobrutideg (NX-5948) BTK Degrader Autoimmune cytopenia in CLL patients NX-0479 / GS-6791 IRAK4 Degrader Rheumatoid arthritis and other inflammatory diseases NX-3911 STAT6 Degrader Type 2 inflammatory diseases Undisclosed Undisclosed Degrader Inflammation / autoimmune Multiple Undisclosed DAC Inflammation / autoimmune O nc ol og y In fla m m at io n & Im m un ol og y
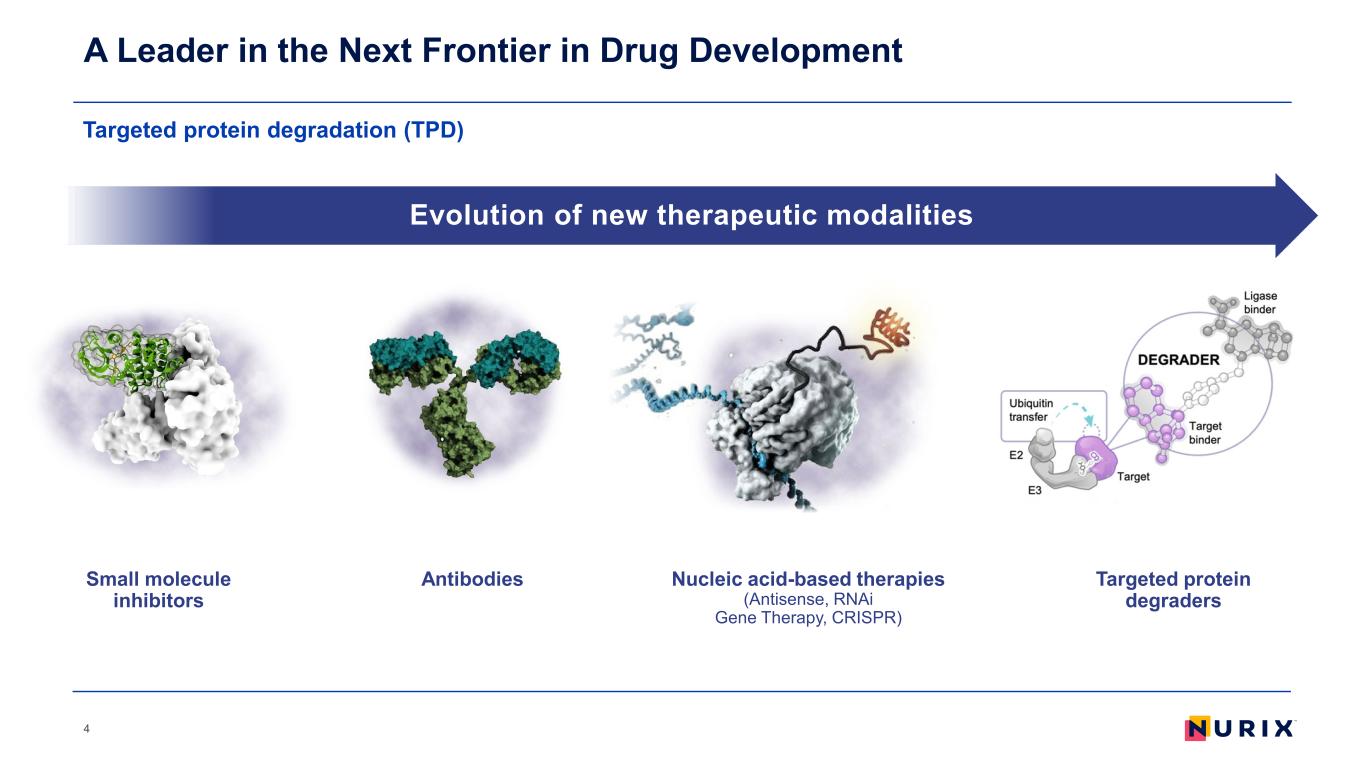
Targeted protein degradation (TPD) A Leader in the Next Frontier in Drug Development 4 Evolution of new therapeutic modalities AntibodiesSmall molecule inhibitors Nucleic acid-based therapies (Antisense, RNAi Gene Therapy, CRISPR) Targeted protein degraders

600 mg dose selected per Project Optimus Cleared to move ahead globally (FDA, MHRA, EMA) Pivotal Phase 2 trial initiated – DAYBreak CLL-201 Confirmatory Phase 3 trial initiation planned for H1 2026 New best-in-class in vitro potency data New proteomics highlighting best-in-class selectivity Next bexobrutideg clinical update at ASH 2025 Advancing bexobrutideg as a potential best-in-class BTK degrader Positioned for Success – Key Program Updates 5
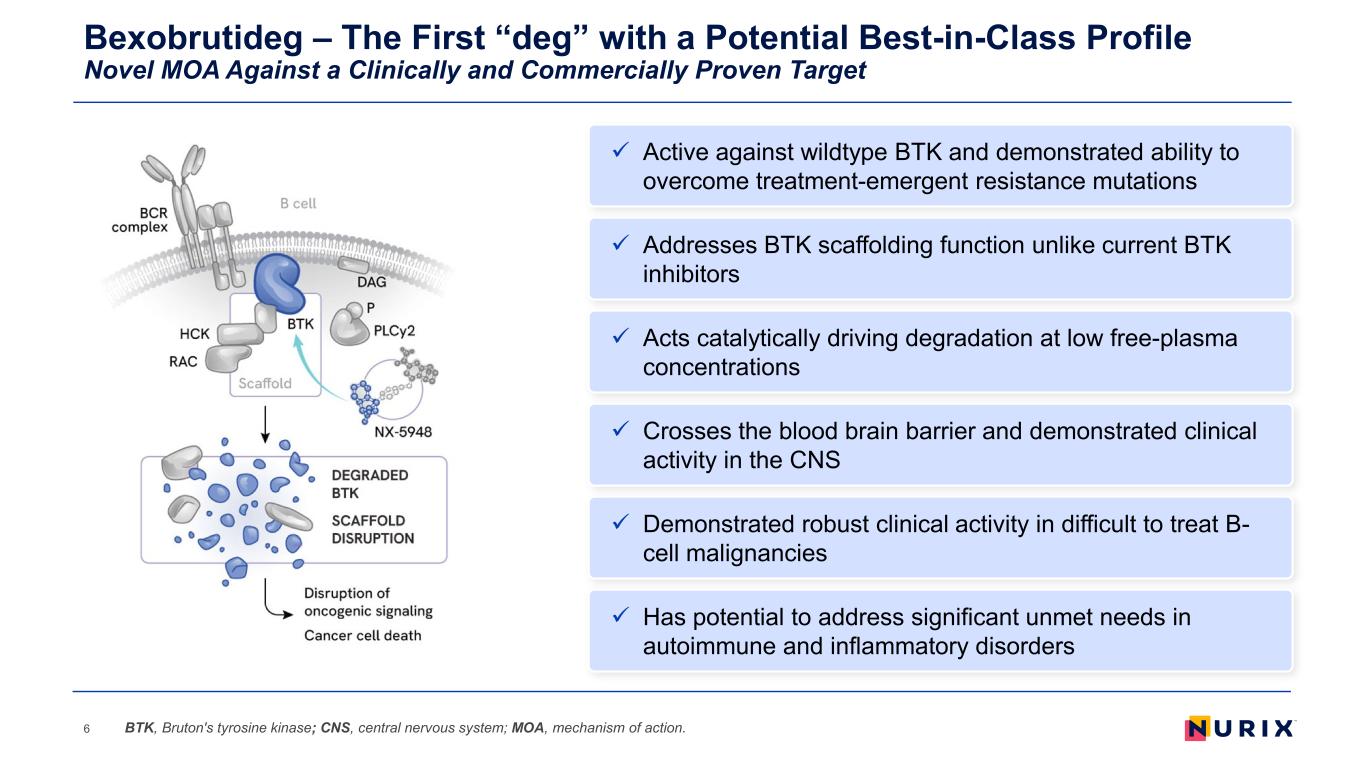
Bexobrutideg – The First “deg” with a Potential Best-in-Class Profile Novel MOA Against a Clinically and Commercially Proven Target 6 Addresses BTK scaffolding function unlike current BTK inhibitors Acts catalytically driving degradation at low free-plasma concentrations Demonstrated robust clinical activity in difficult to treat B- cell malignancies Active against wildtype BTK and demonstrated ability to overcome treatment-emergent resistance mutations Crosses the blood brain barrier and demonstrated clinical activity in the CNS Has potential to address significant unmet needs in autoimmune and inflammatory disorders BTK, Bruton's tyrosine kinase; CNS, central nervous system; MOA, mechanism of action.

Agenda 7 Q&A to follow Paula G. O’Connor, M.D. Chief Medical Officer Bexobrutideg Clinical Development Update Gwenn Hansen, Ph.D. Chief Scientific Officer Best-in-Class BTK Degrader: Potency, Coverage, and Selectivity Extending our leadership into I&I

Paula G. O’Connor, M.D. Chief Medical Officer DAYBreak: Registrational Pathway for Bexobrutideg in R/R CLL

Bexobrutideg 600 mg Once Daily Oral Dose Cleared by Global Regulators for Pivotal Monotherapy Trials in Relapsed/Refractory CLL • Highest dose tested in Phase 1 cleared by global regulators for pivotal monotherapy studies in r/r CLL – U.S. Food and Drug Administration (FDA), in accordance with Project Optimus – U.K Medicines and Healthcare products Regulatory Authority (MHRA) – European Medicines Agency (EMA) • Global designations in CLL support regulatory interactions – Fast Track Designation with FDA – PRIME designation with EMA • Pivotal Phase 2 trial underway – First site activated in October 2025 • Confirmatory Phase 3 trial initiation planned for H1 2026 – Key study start up activities underway 9
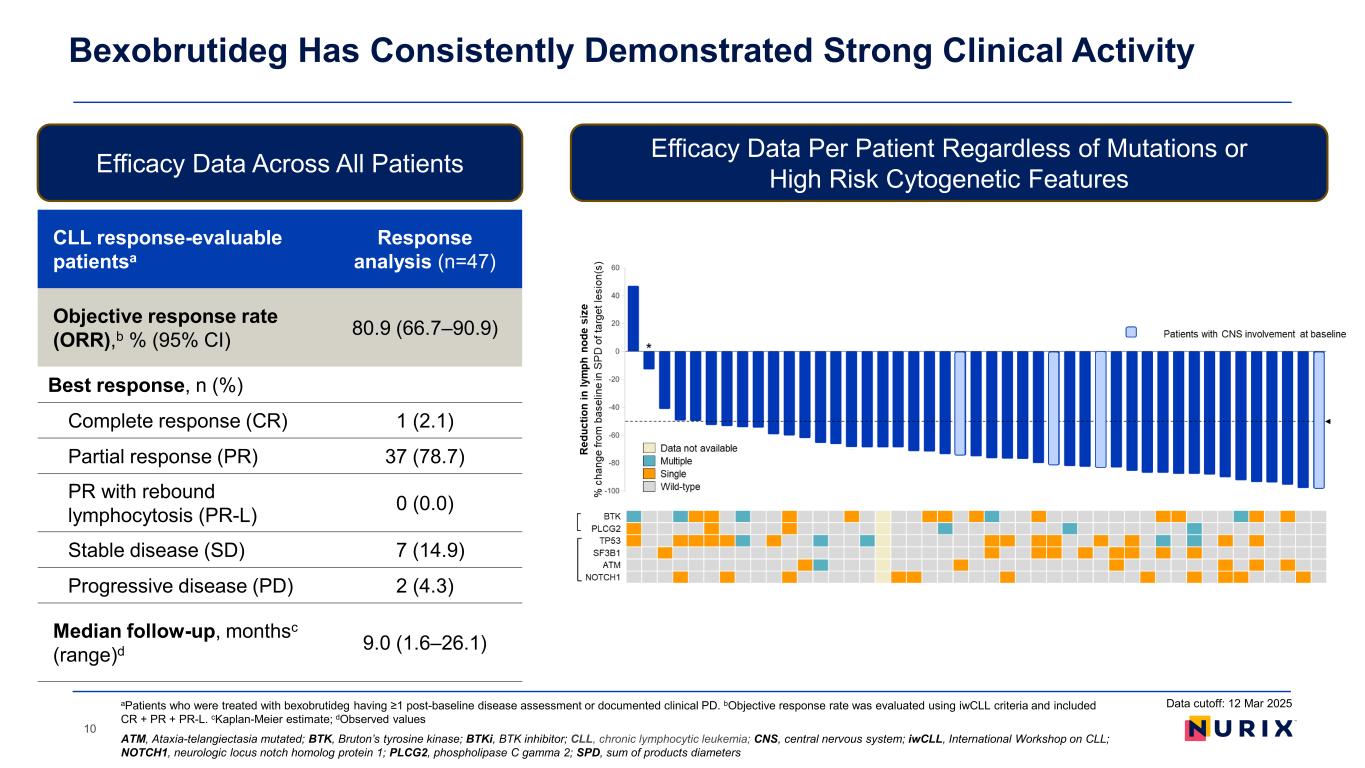
Bexobrutideg Has Consistently Demonstrated Strong Clinical Activity 10 Efficacy Data Across All Patients Efficacy Data Per Patient Regardless of Mutations or High Risk Cytogenetic Features CLL response-evaluable patientsa Response analysis (n=47) Objective response rate (ORR),b % (95% CI) 80.9 (66.7–90.9) Best response, n (%) Complete response (CR) 1 (2.1) Partial response (PR) 37 (78.7) PR with rebound lymphocytosis (PR-L) 0 (0.0) Stable disease (SD) 7 (14.9) Progressive disease (PD) 2 (4.3) Median follow-up, monthsc (range)d 9.0 (1.6–26.1) Data cutoff: 12 Mar 2025 ATM, Ataxia-telangiectasia mutated; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; CLL, chronic lymphocytic leukemia; CNS, central nervous system; iwCLL, International Workshop on CLL; NOTCH1, neurologic locus notch homolog protein 1; PLCG2, phospholipase C gamma 2; SPD, sum of products diameters aPatients who were treated with bexobrutideg having ≥1 post-baseline disease assessment or documented clinical PD. bObjective response rate was evaluated using iwCLL criteria and included CR + PR + PR-L. cKaplan-Meier estimate; dObserved values
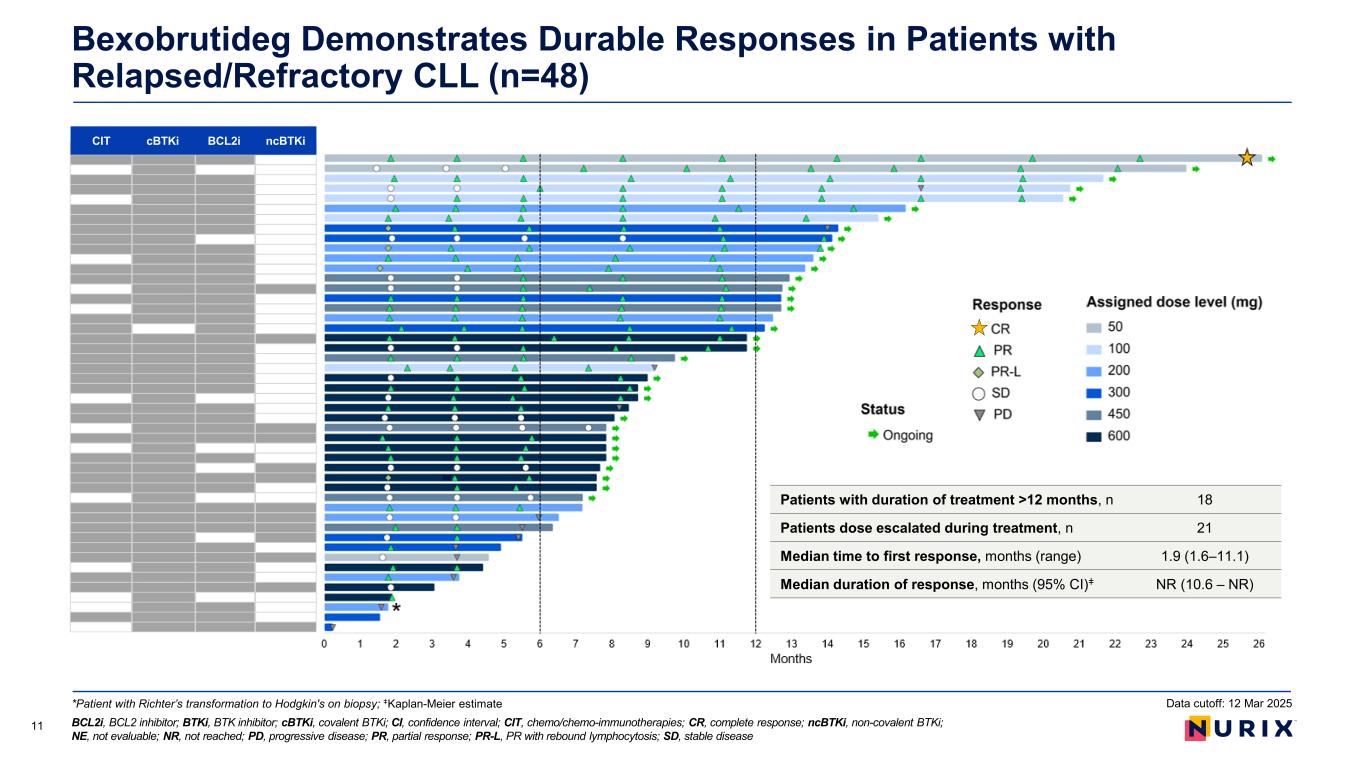
Bexobrutideg Demonstrates Durable Responses in Patients with Relapsed/Refractory CLL (n=48) BCL2i, BCL2 inhibitor; BTKi, BTK inhibitor; cBTKi, covalent BTKi; CI, confidence interval; CIT, chemo/chemo-immunotherapies; CR, complete response; ncBTKi, non-covalent BTKi; NE, not evaluable; NR, not reached; PD, progressive disease; PR, partial response; PR-L, PR with rebound lymphocytosis; SD, stable disease 11 CIT cBTKi BCL2i ncBTKi Data cutoff: 12 Mar 2025*Patient with Richter’s transformation to Hodgkin's on biopsy; ǂKaplan-Meier estimate Patients with duration of treatment >12 months, n 18 Patients dose escalated during treatment, n 21 Median time to first response, months (range) 1.9 (1.6–11.1) Median duration of response, months (95% CI)ǂ NR (10.6 – NR)
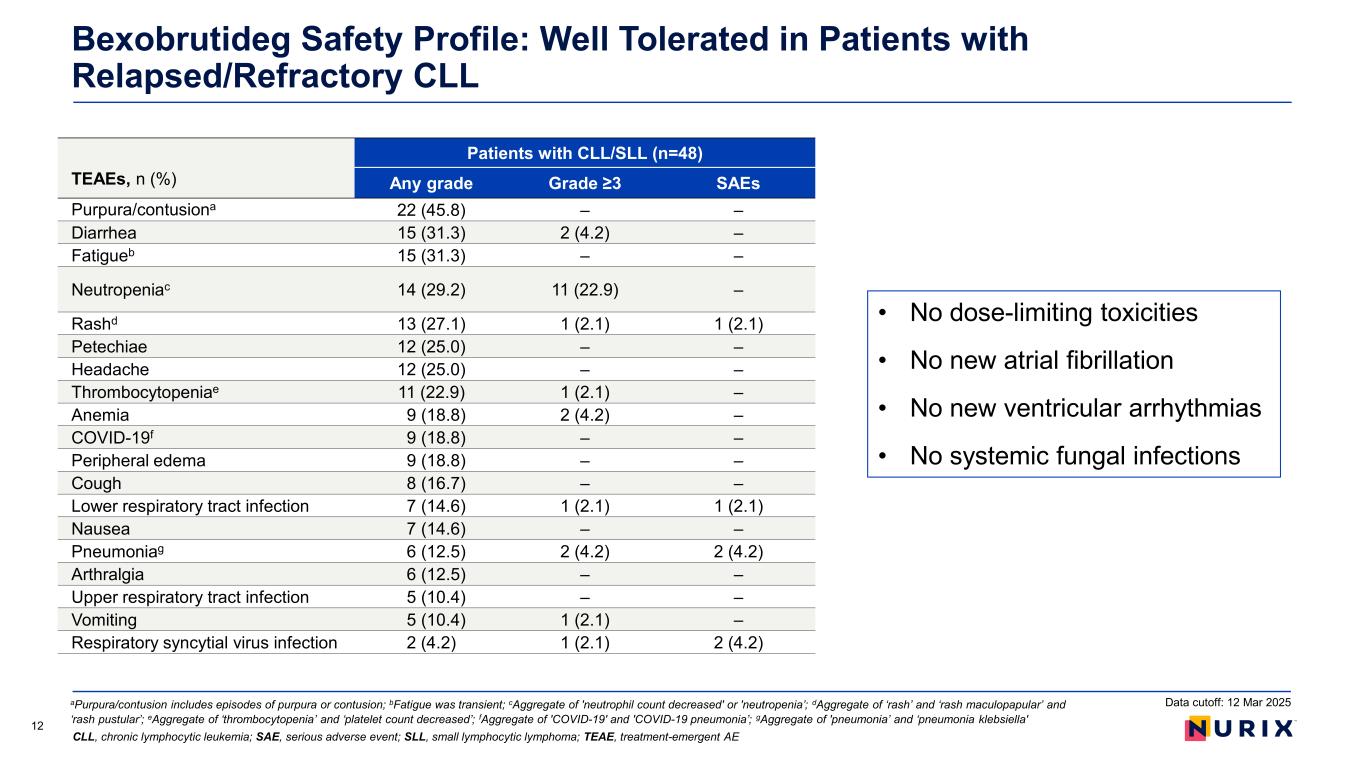
Bexobrutideg Safety Profile: Well Tolerated in Patients with Relapsed/Refractory CLL 12 TEAEs, n (%) Patients with CLL/SLL (n=48) Any grade Grade ≥3 SAEs Purpura/contusiona 22 (45.8) – – Diarrhea 15 (31.3) 2 (4.2) – Fatigueb 15 (31.3) – – Neutropeniac 14 (29.2) 11 (22.9) – Rashd 13 (27.1) 1 (2.1) 1 (2.1) Petechiae 12 (25.0) – – Headache 12 (25.0) – – Thrombocytopeniae 11 (22.9) 1 (2.1) – Anemia 9 (18.8) 2 (4.2) – COVID-19f 9 (18.8) – – Peripheral edema 9 (18.8) – – Cough 8 (16.7) – – Lower respiratory tract infection 7 (14.6) 1 (2.1) 1 (2.1) Nausea 7 (14.6) – – Pneumoniag 6 (12.5) 2 (4.2) 2 (4.2) Arthralgia 6 (12.5) – – Upper respiratory tract infection 5 (10.4) – – Vomiting 5 (10.4) 1 (2.1) – Respiratory syncytial virus infection 2 (4.2) 1 (2.1) 2 (4.2) aPurpura/contusion includes episodes of purpura or contusion; bFatigue was transient; cAggregate of 'neutrophil count decreased' or 'neutropenia’; dAggregate of ‘rash’ and ‘rash maculopapular’ and ‘rash pustular’; eAggregate of ‘thrombocytopenia’ and ‘platelet count decreased’; fAggregate of 'COVID-19' and 'COVID-19 pneumonia’; gAggregate of 'pneumonia’ and ‘pneumonia klebsiella' CLL, chronic lymphocytic leukemia; SAE, serious adverse event; SLL, small lymphocytic lymphoma; TEAE, treatment-emergent AE Data cutoff: 12 Mar 2025 • No dose-limiting toxicities • No new atrial fibrillation • No new ventricular arrhythmias • No systemic fungal infections

• Accelerated approval strategy depends on: − FDA’s determination of unmet need at time of regulatory review − Adequate enrollment in confirmatory Phase 3 trial • Trial designed to support potential accelerated approval in a high unmet need treatment setting − Post-cBTKi, post-ncBTKi, and post-BCL-2i (triple exposed) • First site activated October 2025 – 600 mg cleared for initiation of pivotal studies Phase 2 Single-Arm Study for Potential Accelerated Approval 13 Primary efficacy endpoint ORR per iwCLL as assessed by IRC Relapsed/refractory CLL/SLL Triple-exposed (post-cBTKi, ncBTKi & BCL-2i) N= ~100 Bexobrutideg orally 600mg QD Response assessment every 8 weeks Safety follow-up Response assessment & survival follow-up CLL, chronic lymphocytic leukemia; ORR, objective response rate; iwCLL, International Workshop on CLL; IRC, Independent Review Committee; QD, once daily; SLL, small lymphocytic lymphoma; cBTKi, covalent BTK inhibitor; ncBTKi, non-covalent BTK inhibitor; BCL-2i, BCL-2 inhibitor Triple-exposed CLL patients who progressed on or did not respond to prior therapy
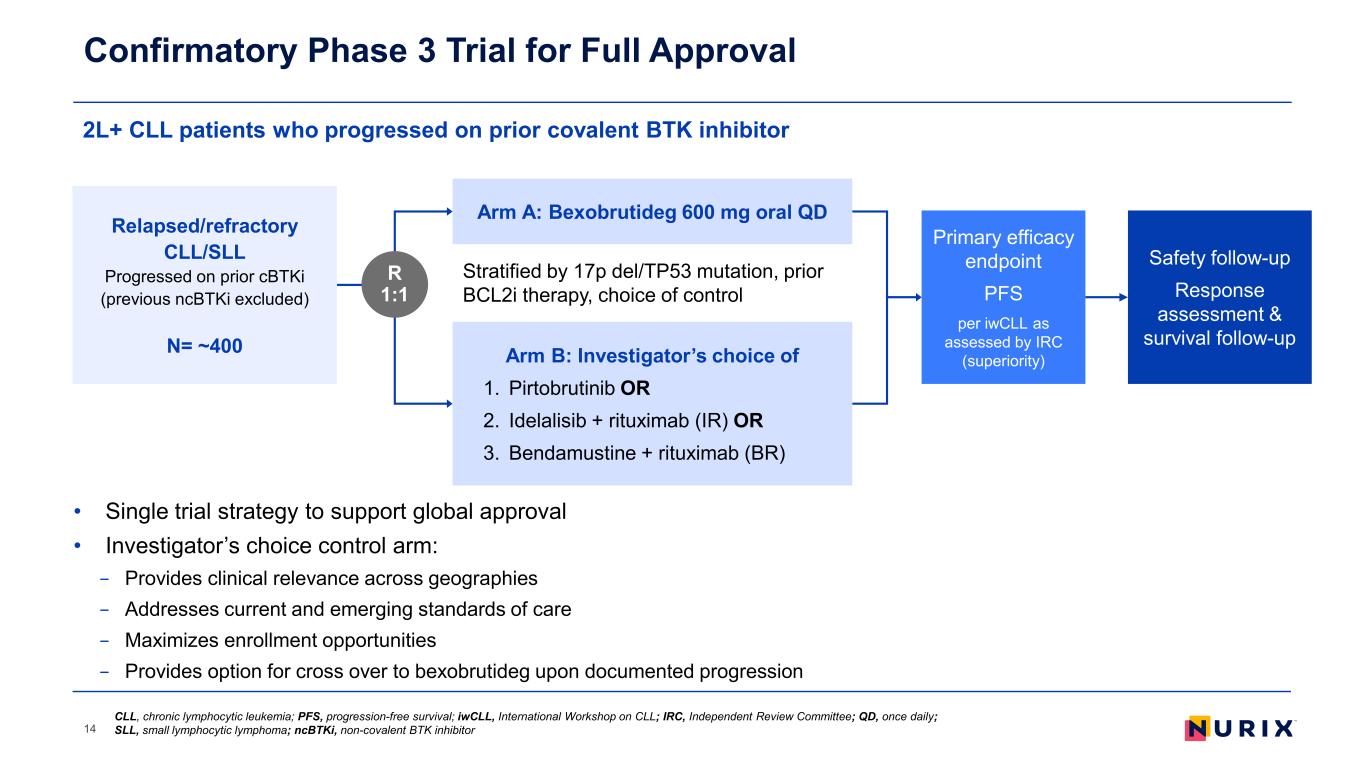
• Single trial strategy to support global approval • Investigator’s choice control arm: − Provides clinical relevance across geographies − Addresses current and emerging standards of care − Maximizes enrollment opportunities − Provides option for cross over to bexobrutideg upon documented progression 2L+ CLL patients who progressed on prior covalent BTK inhibitor Confirmatory Phase 3 Trial for Full Approval 14 Stratified by 17p del/TP53 mutation, prior BCL2i therapy, choice of control Primary efficacy endpoint PFS per iwCLL as assessed by IRC (superiority) Relapsed/refractory CLL/SLL Progressed on prior cBTKi (previous ncBTKi excluded) N= ~400 R 1:1 Arm A: Bexobrutideg 600 mg oral QD Arm B: Investigator’s choice of 1. Pirtobrutinib OR 2. Idelalisib + rituximab (IR) OR 3. Bendamustine + rituximab (BR) Safety follow-up Response assessment & survival follow-up CLL, chronic lymphocytic leukemia; PFS, progression-free survival; iwCLL, International Workshop on CLL; IRC, Independent Review Committee; QD, once daily; SLL, small lymphocytic lymphoma; ncBTKi, non-covalent BTK inhibitor
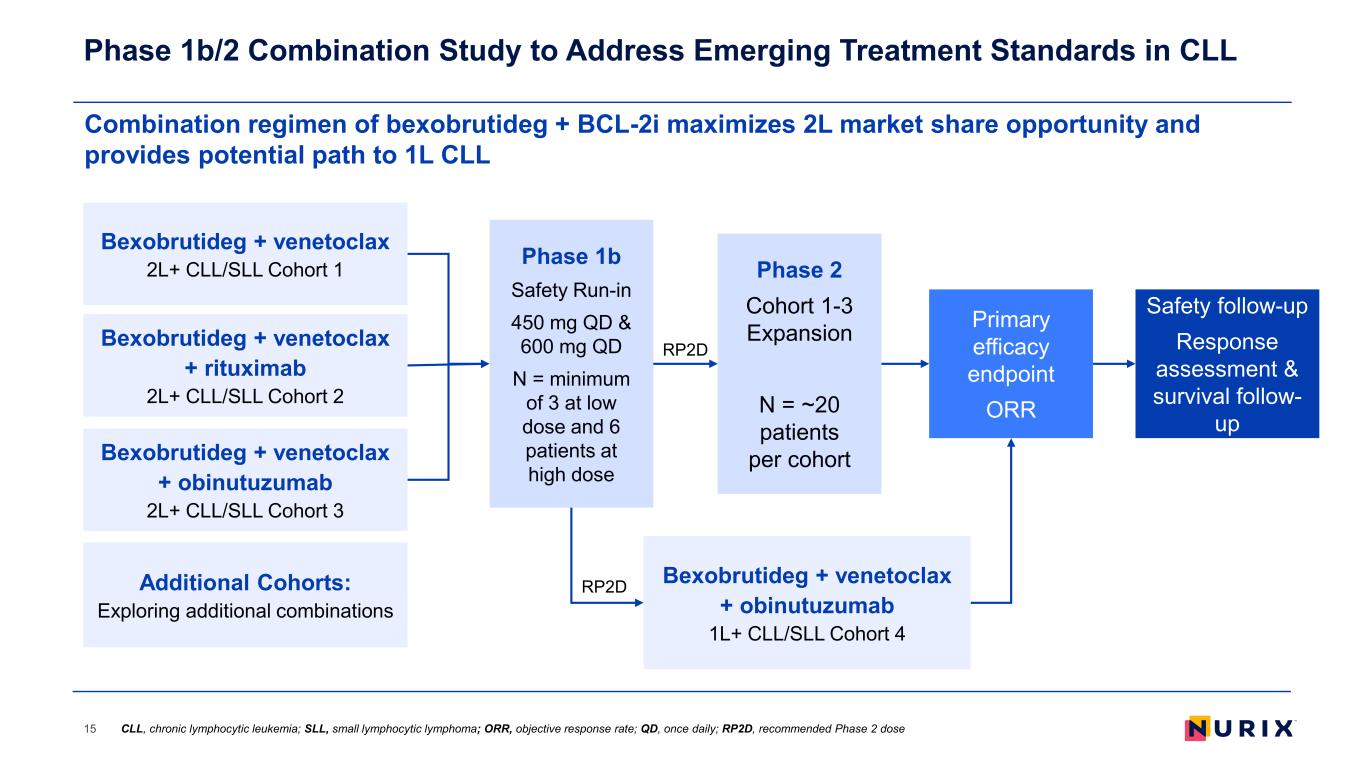
Combination regimen of bexobrutideg + BCL-2i maximizes 2L market share opportunity and provides potential path to 1L CLL Phase 1b/2 Combination Study to Address Emerging Treatment Standards in CLL 15 Bexobrutideg + venetoclax 2L+ CLL/SLL Cohort 1 Bexobrutideg + venetoclax + rituximab 2L+ CLL/SLL Cohort 2 Bexobrutideg + venetoclax + obinutuzumab 2L+ CLL/SLL Cohort 3 Phase 1b Safety Run-in 450 mg QD & 600 mg QD N = minimum of 3 at low dose and 6 patients at high dose Primary efficacy endpoint ORR Safety follow-up Response assessment & survival follow- up Phase 2 Cohort 1-3 Expansion N = ~20 patients per cohort Additional Cohorts: Exploring additional combinations Bexobrutideg + venetoclax + obinutuzumab 1L+ CLL/SLL Cohort 4 RP2D RP2D CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; ORR, objective response rate; QD, once daily; RP2D, recommended Phase 2 dose

• Phase 2 DAYBreak trial underway for monotherapy in r/r CLL • Confirmatory Phase 3 for monotherapy in r/r CLL planned to start in H1 2026 • Combination trial planned to start H1 2026 to expand clinical opportunity across lines of therapy in CLL – Initial focus on combinations with standards of care in CLL (BCL-2i & anti-CD20) • Maturing data from the ongoing Phase 1a/1b study to inform further development and potential paths for additional accelerated approvals – Multiple bexobrutideg abstracts accepted for presentation at the upcoming American Society of Hematology (ASH) Annual Meeting in December 2025 We aim to establish bexobrutideg as a cornerstone therapy across the CLL landscape Building a World Class Hematology/Oncology Franchise 16

Gwenn Hansen, Ph.D. Chief Scientific Officer Potential Best-in-Class BTK Degrader: Potency, Coverage, and Selectivity
Bexobrutideg – Defining a Best-in-Class Profile 18 Best-in-class mutational coverage Best-in-class degrader selectivity Best-in-class degrader potency FDA clearance to proceed with highest tested dose BTK, Bruton's tyrosine kinase; CNS, central nervous system Clear evidence of clinical activity in the CNS
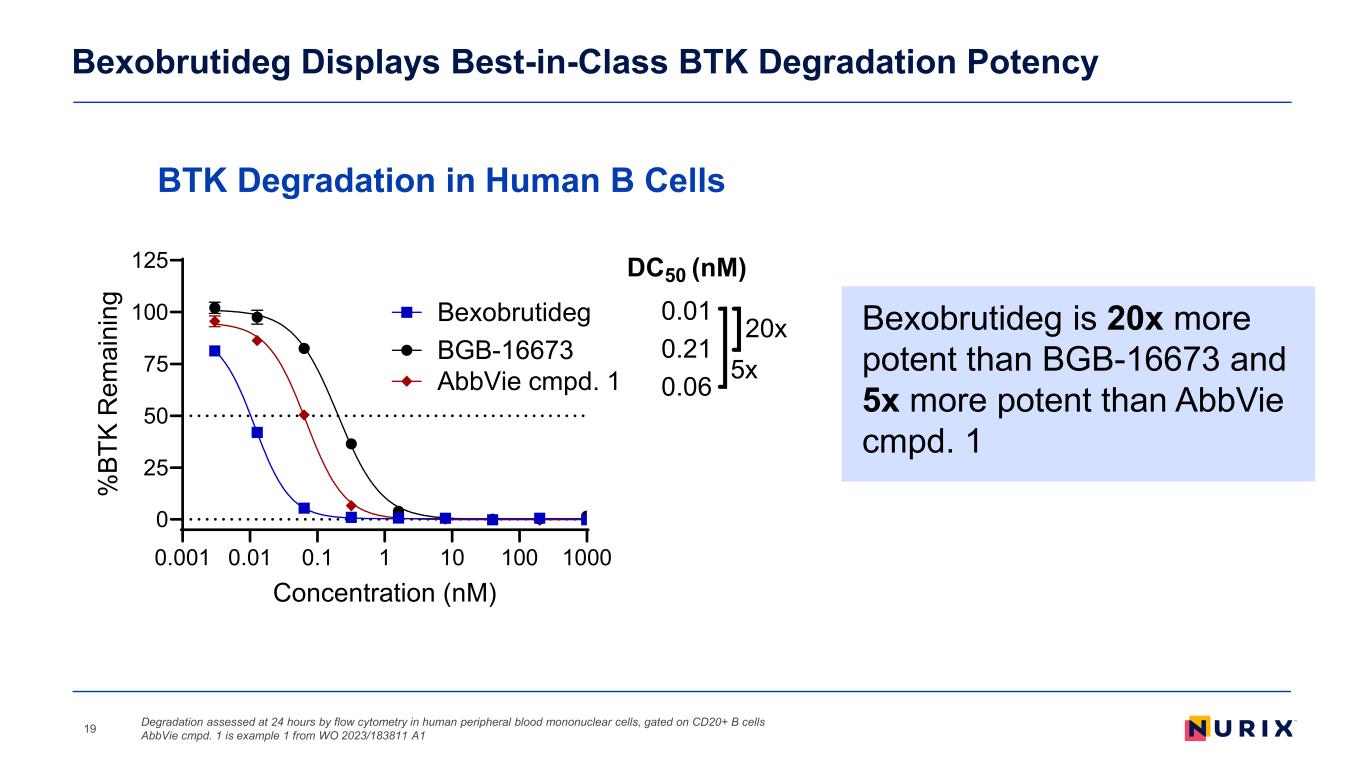
Bexobrutideg Displays Best-in-Class BTK Degradation Potency Degradation assessed at 24 hours by flow cytometry in human peripheral blood mononuclear cells, gated on CD20+ B cells AbbVie cmpd. 1 is example 1 from WO 2023/183811 A119 Bexobrutideg is 20x more potent than BGB-16673 and 5x more potent than AbbVie cmpd. 1 0.001 0.01 0.1 1 10 100 1000 0 25 50 75 100 125 Concentration (nM) % BT K R em ai ni ng Bexobrutideg AbbVie cmpd. 1 BGB-16673 DC50 (nM) 0.01 0.21 0.06 20x 5x BTK Degradation in Human B Cells
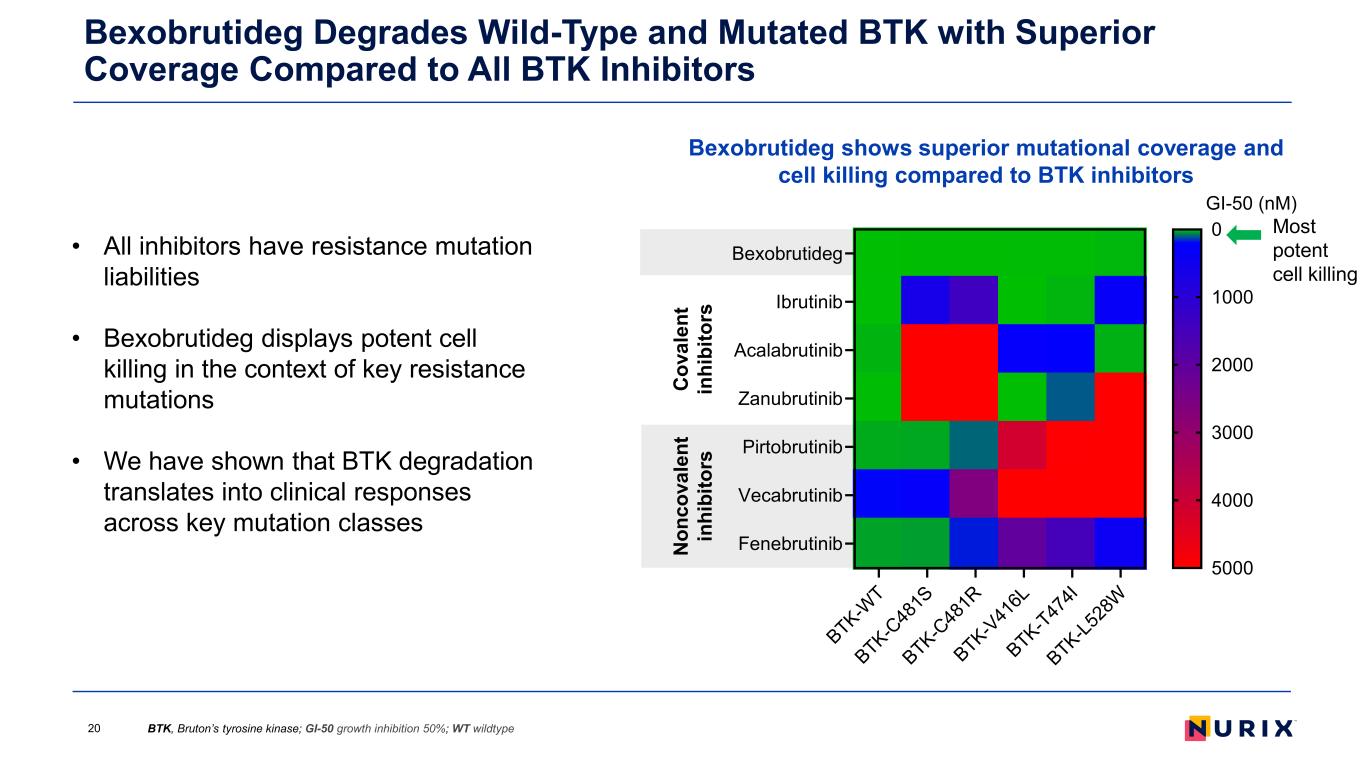
Bexobrutideg Degrades Wild-Type and Mutated BTK with Superior Coverage Compared to All BTK Inhibitors BTK, Bruton’s tyrosine kinase; GI-50 growth inhibition 50%; WT wildtype20 Bexobrutideg shows superior mutational coverage and cell killing compared to BTK inhibitors Most potent cell killing N on co va le nt in hi bi to rs C ov al en t in hi bi to rs • All inhibitors have resistance mutation liabilities • Bexobrutideg displays potent cell killing in the context of key resistance mutations • We have shown that BTK degradation translates into clinical responses across key mutation classes BTK-W T BTK-C 48 1S BTK-C 48 1R BTK-V 41 6L BTK-T47 4I BTK-L5 28 W Bexobrutideg Ibrutinib Acalabrutinib Zanubrutinib Pirtobrutinib Vecabrutinib Fenebrutinib GI-50 (nM) 0 1000 2000 3000 4000 5000
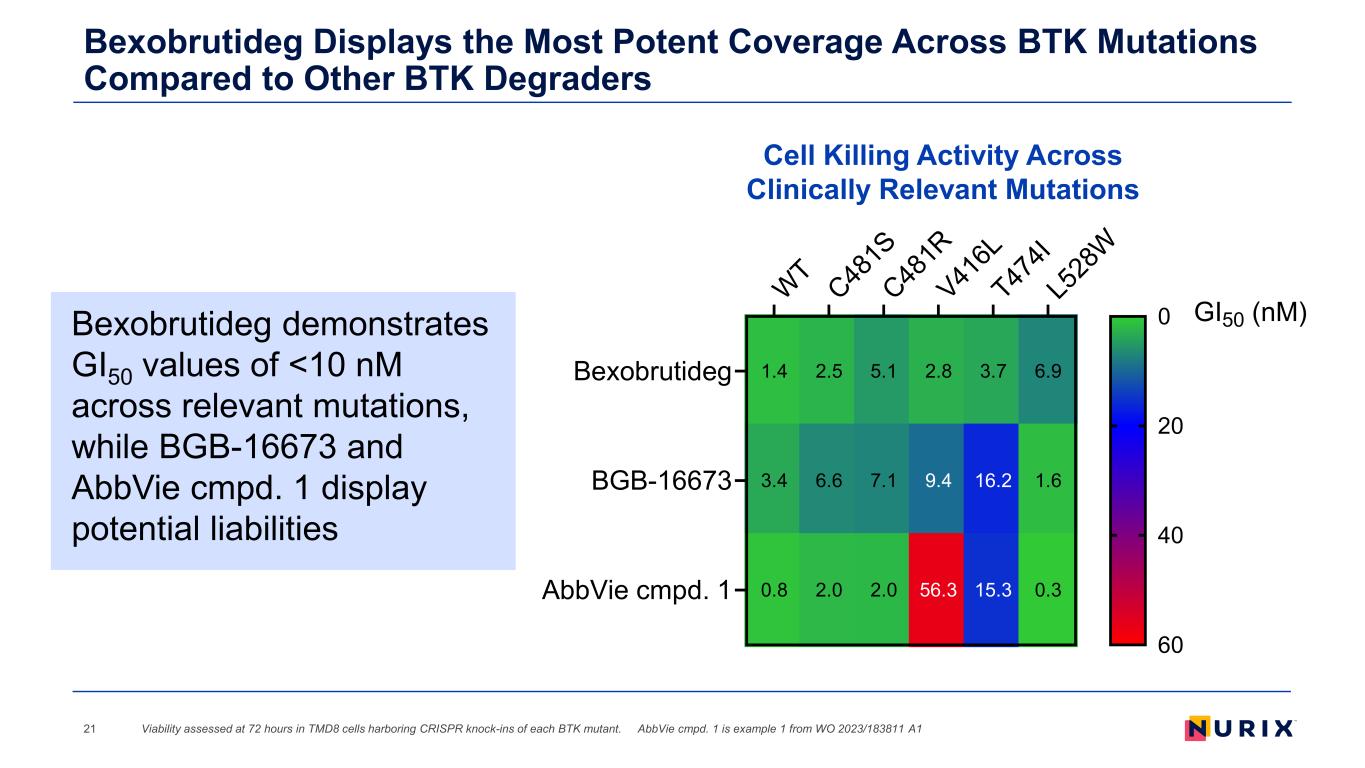
1.4 3.4 0.8 2.5 6.6 2.0 5.1 7.1 2.0 2.8 9.4 56.3 3.7 16.2 15.3 6.9 1.6 0.3 WT C48 1S C48 1R V41 6L T47 4I L5 28 W Bexobrutideg BGB-16673 AbbVie cmpd. 1 GI50 (nM)0 20 40 60 Bexobrutideg Displays the Most Potent Coverage Across BTK Mutations Compared to Other BTK Degraders 21 Cell Killing Activity Across Clinically Relevant Mutations Viability assessed at 72 hours in TMD8 cells harboring CRISPR knock-ins of each BTK mutant. AbbVie cmpd. 1 is example 1 from WO 2023/183811 A1 Bexobrutideg demonstrates GI50 values of <10 nM across relevant mutations, while BGB-16673 and AbbVie cmpd. 1 display potential liabilities
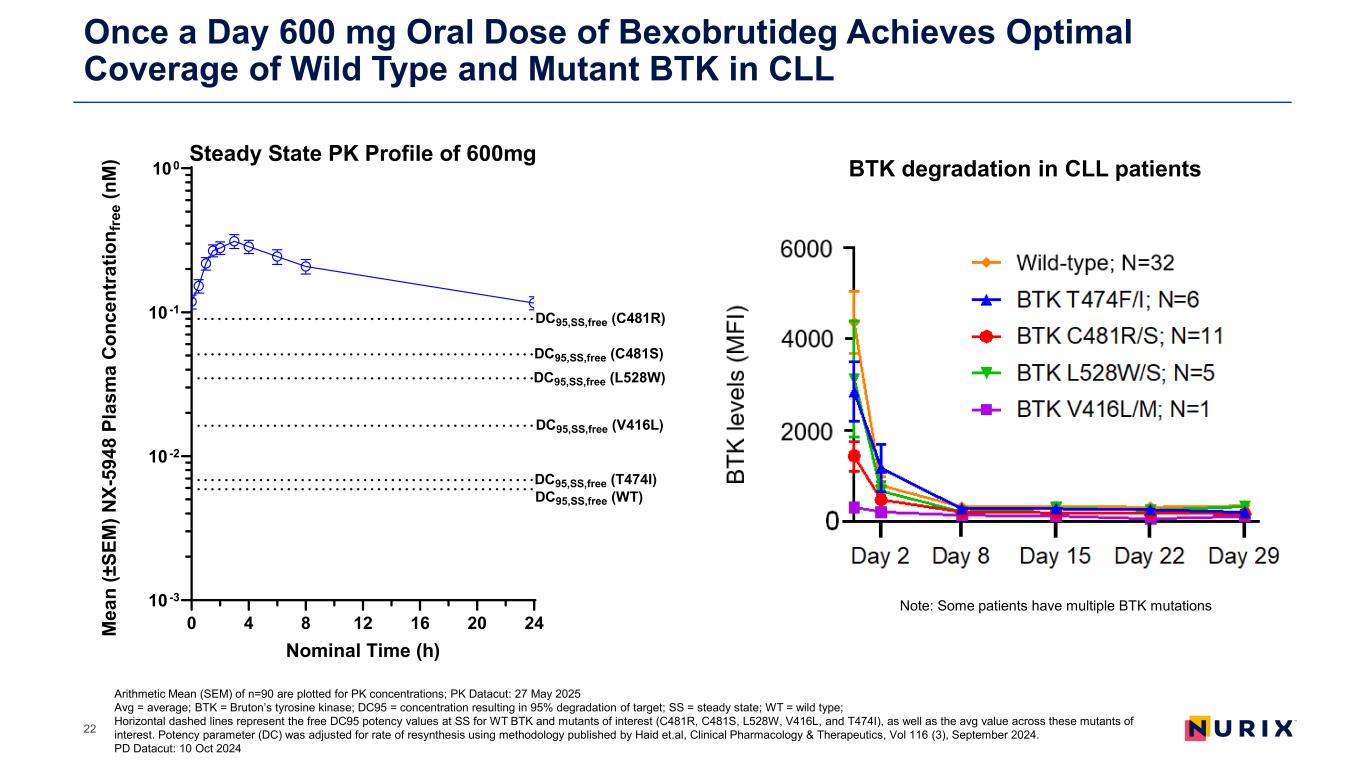
Once a Day 600 mg Oral Dose of Bexobrutideg Achieves Optimal Coverage of Wild Type and Mutant BTK in CLL 22 Note: Some patients have multiple BTK mutations BTK degradation in CLL patients Arithmetic Mean (SEM) of n=90 are plotted for PK concentrations; PK Datacut: 27 May 2025 Avg = average; BTK = Bruton’s tyrosine kinase; DC95 = concentration resulting in 95% degradation of target; SS = steady state; WT = wild type; Horizontal dashed lines represent the free DC95 potency values at SS for WT BTK and mutants of interest (C481R, C481S, L528W, V416L, and T474I), as well as the avg value across these mutants of interest. Potency parameter (DC) was adjusted for rate of resynthesis using methodology published by Haid et.al, Clinical Pharmacology & Therapeutics, Vol 116 (3), September 2024. PD Datacut: 10 Oct 2024 0 4 8 12 16 20 24 10-3 10-2 10-1 100 Steady State PK Profile of 600mg Nominal Time (h) M ea n (± SE M ) N X- 59 48 P la sm a C on ce nt ra tio n f re e ( nM ) DC95,SS,free (WT) DC95,SS,free (C481R) DC95,SS,free (C481S) DC95,SS,free (V416L) DC95,SS,free (T474I) DC95,SS,free (L528W)
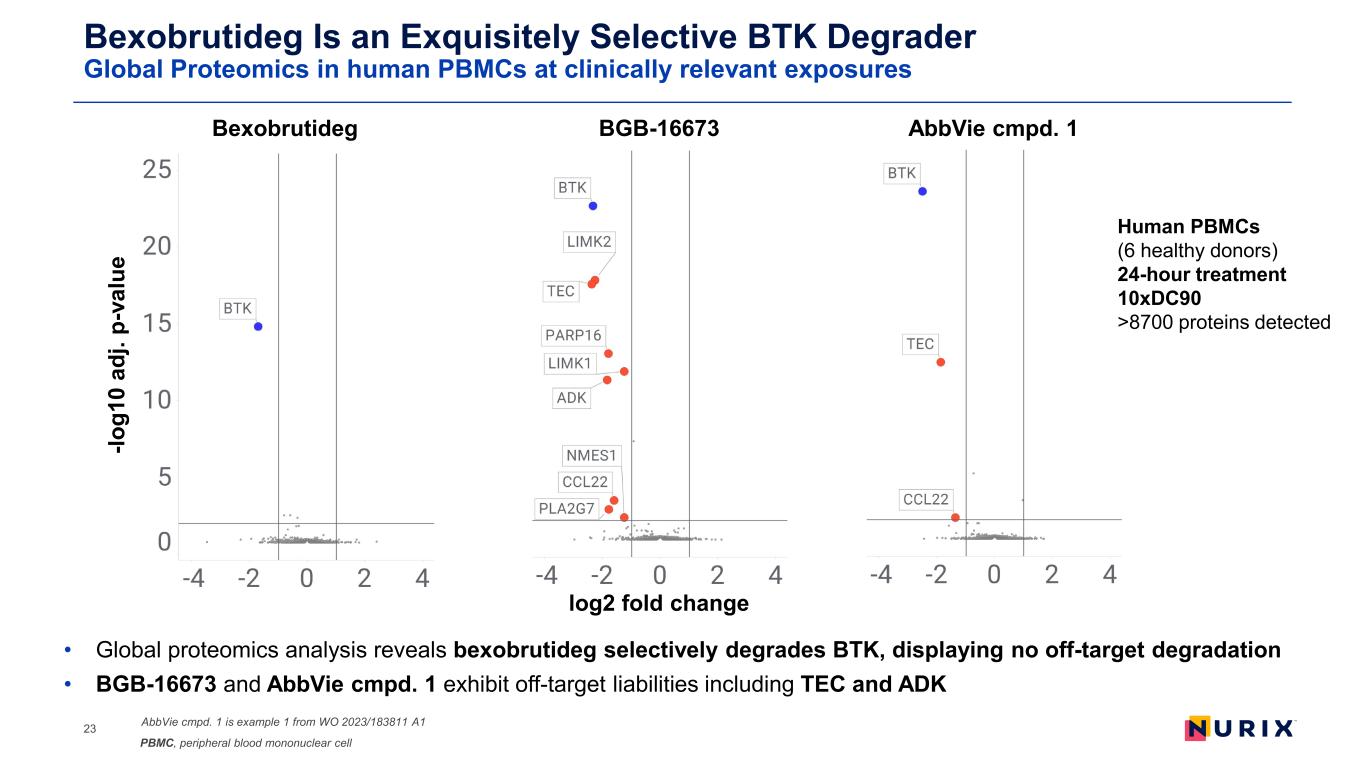
Bexobrutideg Is an Exquisitely Selective BTK Degrader Global Proteomics in human PBMCs at clinically relevant exposures • Global proteomics analysis reveals bexobrutideg selectively degrades BTK, displaying no off-target degradation • BGB-16673 and AbbVie cmpd. 1 exhibit off-target liabilities including TEC and ADK 23 -lo g1 0 ad j. p- va lu e log2 fold change Bexobrutideg AbbVie cmpd. 1BGB-16673 Human PBMCs (6 healthy donors) 24-hour treatment 10xDC90 >8700 proteins detected AbbVie cmpd. 1 is example 1 from WO 2023/183811 A1 PBMC, peripheral blood mononuclear cell
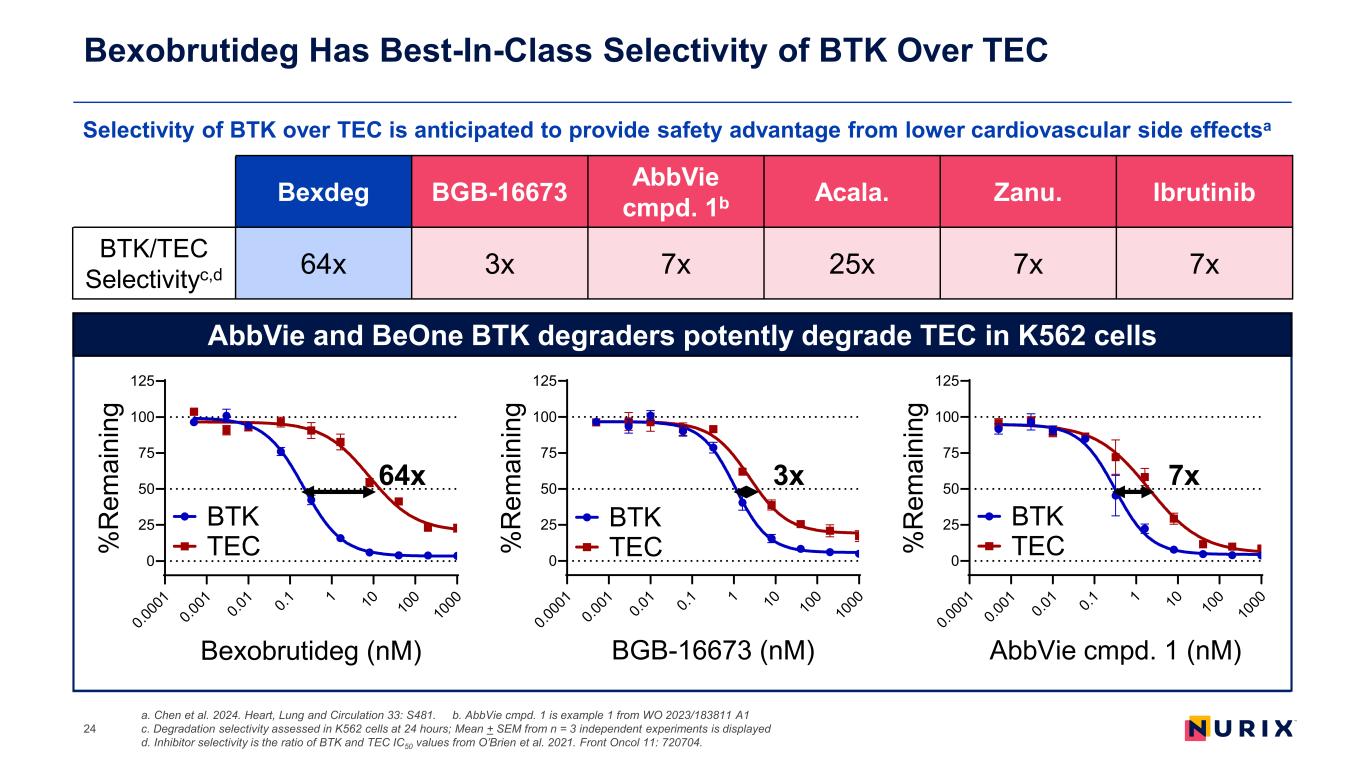
Bexdeg BGB-16673 AbbVie cmpd. 1b Acala. Zanu. Ibrutinib BTK/TEC Selectivityc,d 64x 3x 7x 25x 7x 7x 0.0 00 1 0.0 01 0.0 1 0.1 1 10 10 0 10 00 0 25 50 75 100 125 Bexobrutideg (nM) % R em ai ni ng BTK TEC 0.0 00 1 0.0 01 0.0 1 0.1 1 10 10 0 10 00 0 25 50 75 100 125 BGB-16673 (nM) % R em ai ni ng BTK TEC 0.0 00 1 0.0 01 0.0 1 0.1 1 10 10 0 10 00 0 25 50 75 100 125 AbbVie cmpd. 1 (nM) % R em ai ni ng BTK TEC Selectivity of BTK over TEC is anticipated to provide safety advantage from lower cardiovascular side effectsa Bexobrutideg Has Best-In-Class Selectivity of BTK Over TEC a. Chen et al. 2024. Heart, Lung and Circulation 33: S481. b. AbbVie cmpd. 1 is example 1 from WO 2023/183811 A1 c. Degradation selectivity assessed in K562 cells at 24 hours; Mean + SEM from n = 3 independent experiments is displayed d. Inhibitor selectivity is the ratio of BTK and TEC IC50 values from O’Brien et al. 2021. Front Oncol 11: 720704. 24 64x 3x 7x AbbVie and BeOne BTK degraders potently degrade TEC in K562 cells
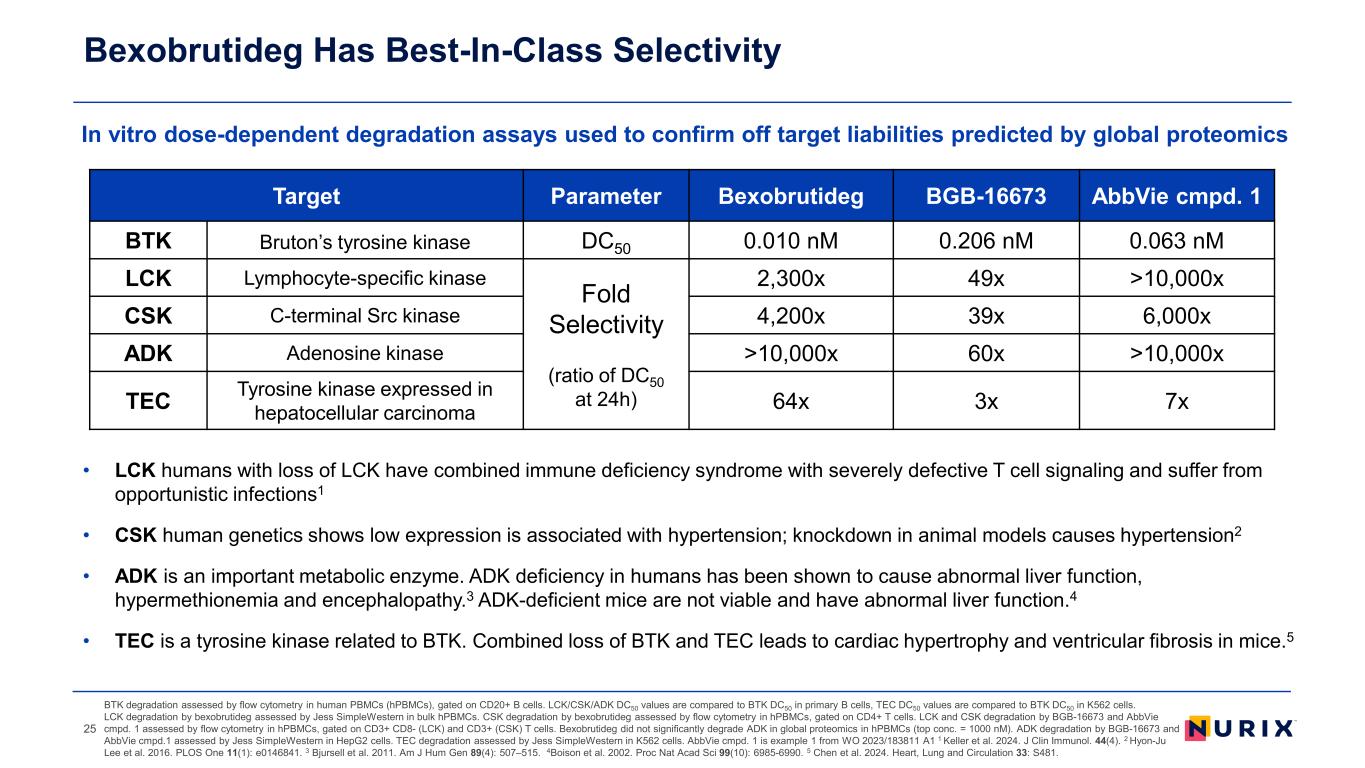
• LCK humans with loss of LCK have combined immune deficiency syndrome with severely defective T cell signaling and suffer from opportunistic infections1 • CSK human genetics shows low expression is associated with hypertension; knockdown in animal models causes hypertension2 • ADK is an important metabolic enzyme. ADK deficiency in humans has been shown to cause abnormal liver function, hypermethionemia and encephalopathy.3 ADK-deficient mice are not viable and have abnormal liver function.4 • TEC is a tyrosine kinase related to BTK. Combined loss of BTK and TEC leads to cardiac hypertrophy and ventricular fibrosis in mice.5 In vitro dose-dependent degradation assays used to confirm off target liabilities predicted by global proteomics Bexobrutideg Has Best-In-Class Selectivity BTK degradation assessed by flow cytometry in human PBMCs (hPBMCs), gated on CD20+ B cells. LCK/CSK/ADK DC50 values are compared to BTK DC50 in primary B cells, TEC DC50 values are compared to BTK DC50 in K562 cells. LCK degradation by bexobrutideg assessed by Jess SimpleWestern in bulk hPBMCs. CSK degradation by bexobrutideg assessed by flow cytometry in hPBMCs, gated on CD4+ T cells. LCK and CSK degradation by BGB-16673 and AbbVie cmpd. 1 assessed by flow cytometry in hPBMCs, gated on CD3+ CD8- (LCK) and CD3+ (CSK) T cells. Bexobrutideg did not significantly degrade ADK in global proteomics in hPBMCs (top conc. = 1000 nM). ADK degradation by BGB-16673 and AbbVie cmpd.1 assessed by Jess SimpleWestern in HepG2 cells. TEC degradation assessed by Jess SimpleWestern in K562 cells. AbbVie cmpd. 1 is example 1 from WO 2023/183811 A1 1 Keller et al. 2024. J Clin Immunol. 44(4). 2 Hyon-Ju Lee et al. 2016. PLOS One 11(1): e0146841. 3 Bjursell et al. 2011. Am J Hum Gen 89(4): 507–515. 4Boison et al. 2002. Proc Nat Acad Sci 99(10): 6985-6990. 5 Chen et al. 2024. Heart, Lung and Circulation 33: S481. 25 Target Parameter Bexobrutideg BGB-16673 AbbVie cmpd. 1 BTK Bruton’s tyrosine kinase DC50 0.010 nM 0.206 nM 0.063 nM LCK Lymphocyte-specific kinase Fold Selectivity (ratio of DC50 at 24h) 2,300x 49x >10,000x CSK C-terminal Src kinase 4,200x 39x 6,000x ADK Adenosine kinase >10,000x 60x >10,000x TEC Tyrosine kinase expressed in hepatocellular carcinoma 64x 3x 7x

Extending our leadership into I&I with potential best- in-class agents 26
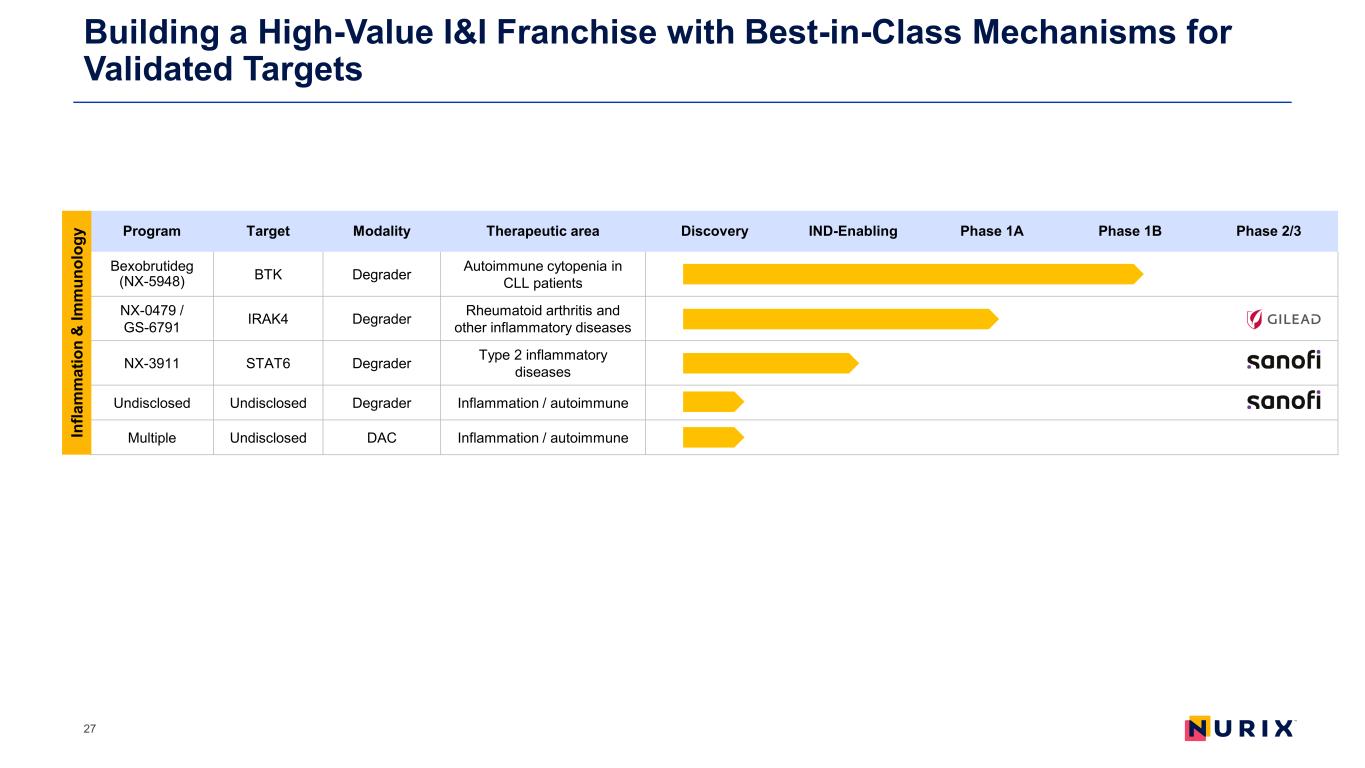
Building a High-Value I&I Franchise with Best-in-Class Mechanisms for Validated Targets 27 Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B Phase 2/3 Bexobrutideg (NX-5948) BTK Degrader Autoimmune cytopenia in CLL patients NX-0479 / GS-6791 IRAK4 Degrader Rheumatoid arthritis and other inflammatory diseases NX-3911 STAT6 Degrader Type 2 inflammatory diseases Undisclosed Undisclosed Degrader Inflammation / autoimmune Multiple Undisclosed DAC Inflammation / autoimmuneIn fla m m at io n & Im m un ol og y
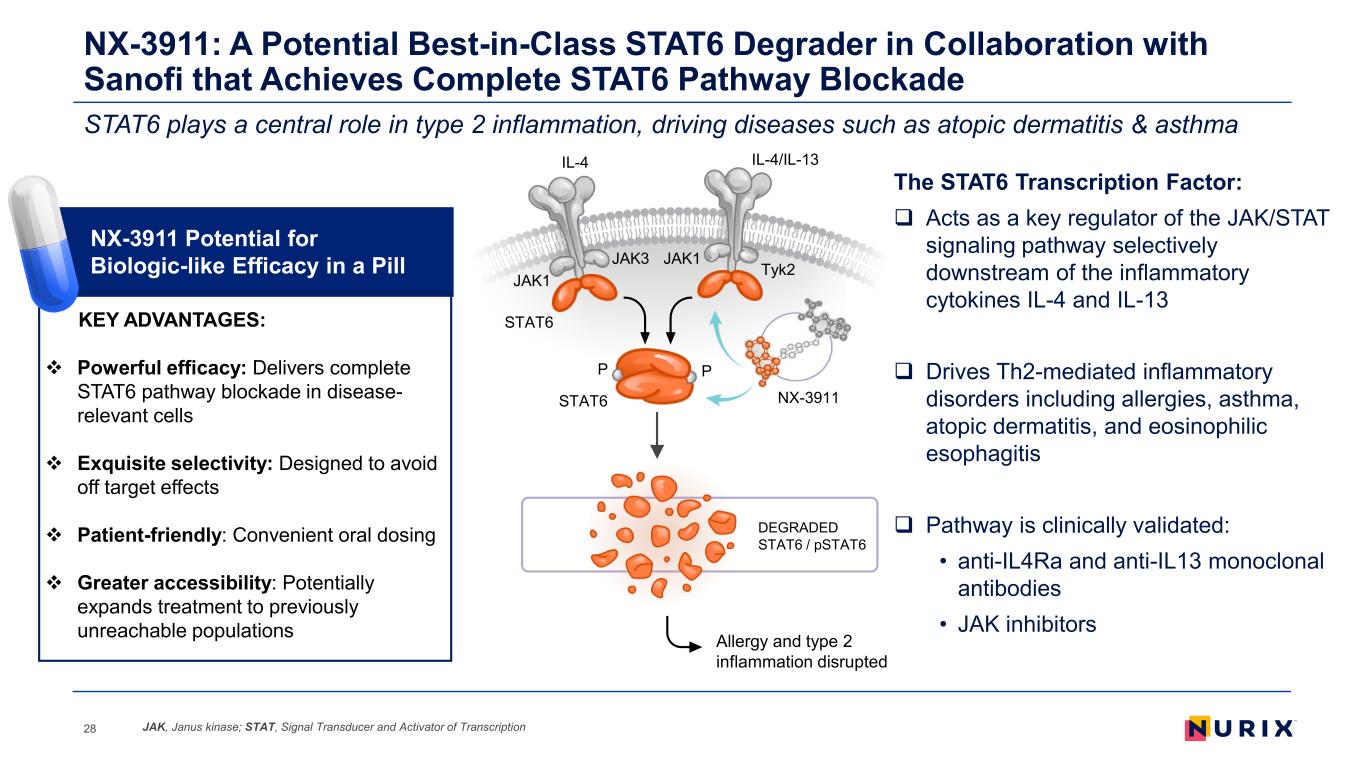
NX-3911: A Potential Best-in-Class STAT6 Degrader in Collaboration with Sanofi that Achieves Complete STAT6 Pathway Blockade 28 The STAT6 Transcription Factor: Acts as a key regulator of the JAK/STAT signaling pathway selectively downstream of the inflammatory cytokines IL-4 and IL-13 Drives Th2-mediated inflammatory disorders including allergies, asthma, atopic dermatitis, and eosinophilic esophagitis Pathway is clinically validated: • anti-IL4Ra and anti-IL13 monoclonal antibodies • JAK inhibitors NX-3911 Potential for Biologic-like Efficacy in a Pill NX-3911 IL-4 IL-4/IL-13 DEGRADED STAT6 / pSTAT6 P P STAT6 Tyk2 JAK3 JAK1 JAK1 STAT6 STAT6 plays a central role in type 2 inflammation, driving diseases such as atopic dermatitis & asthma Allergy and type 2 inflammation disrupted KEY ADVANTAGES: Powerful efficacy: Delivers complete STAT6 pathway blockade in disease- relevant cells Exquisite selectivity: Designed to avoid off target effects Patient-friendly: Convenient oral dosing Greater accessibility: Potentially expands treatment to previously unreachable populations JAK, Janus kinase; STAT, Signal Transducer and Activator of Transcription
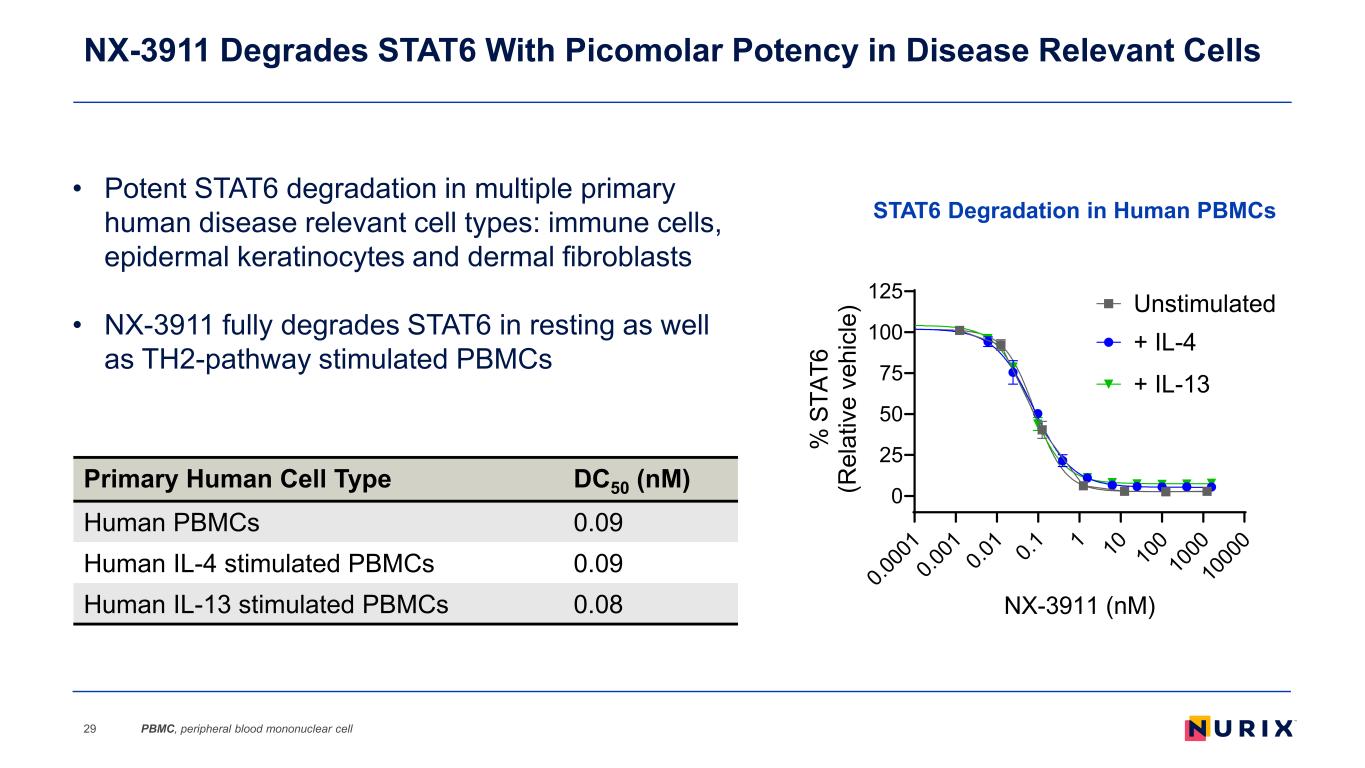
NX-3911 Degrades STAT6 With Picomolar Potency in Disease Relevant Cells 29 Primary Human Cell Type DC50 (nM) Human PBMCs 0.09 Human IL-4 stimulated PBMCs 0.09 Human IL-13 stimulated PBMCs 0.08 • Potent STAT6 degradation in multiple primary human disease relevant cell types: immune cells, epidermal keratinocytes and dermal fibroblasts • NX-3911 fully degrades STAT6 in resting as well as TH2-pathway stimulated PBMCs 0.0 00 1 0.0 01 0.0 1 0.1 1 10 10 0 10 00 10 00 0 0 25 50 75 100 125 NX-3911 (nM) % S TA T6 (R el at iv e ve hi cl e) + IL-4 + IL-13 Unstimulated STAT6 Degradation in Human PBMCs PBMC, peripheral blood mononuclear cell
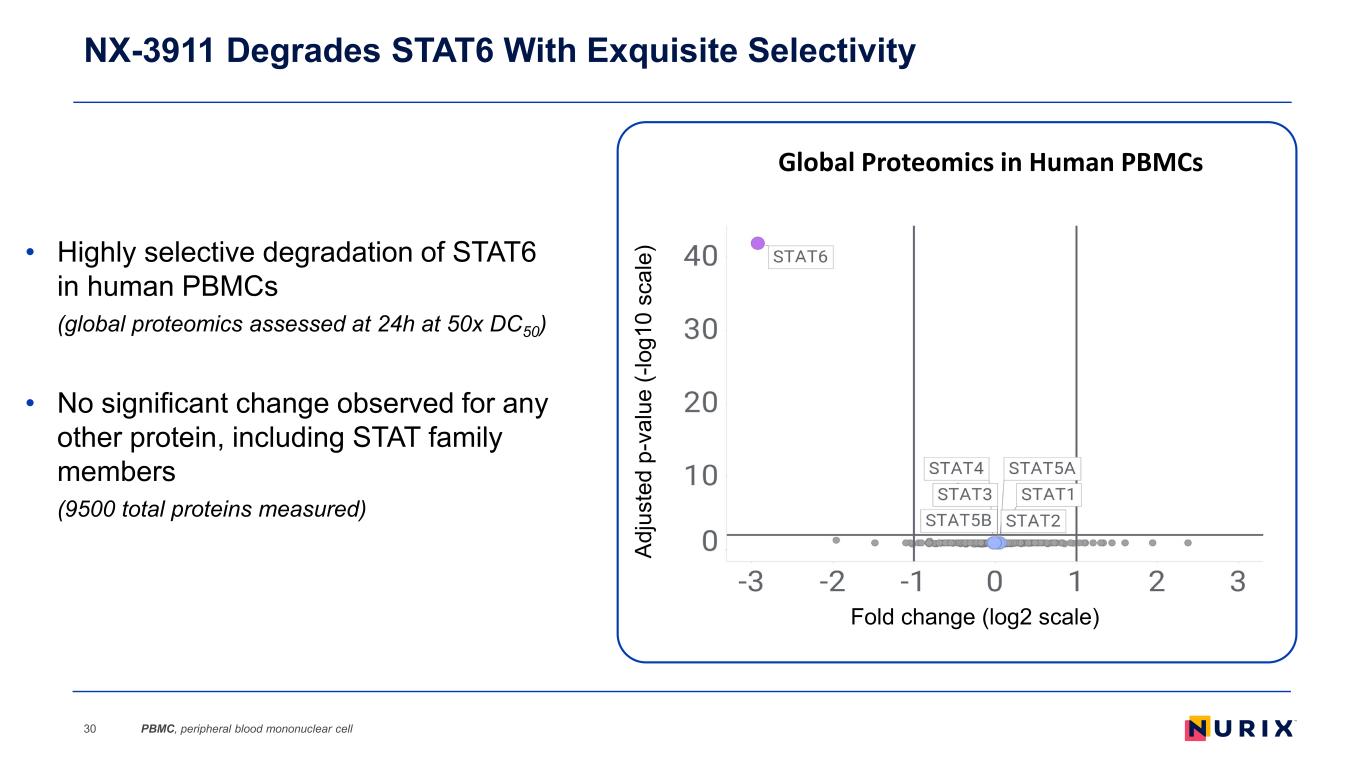
NX-3911 Degrades STAT6 With Exquisite Selectivity 30 Global Proteomics in Human PBMCs Ad ju st ed p -v al ue (- lo g1 0 sc al e) Fold change (log2 scale) • Highly selective degradation of STAT6 in human PBMCs (global proteomics assessed at 24h at 50x DC50) • No significant change observed for any other protein, including STAT family members (9500 total proteins measured) PBMC, peripheral blood mononuclear cell
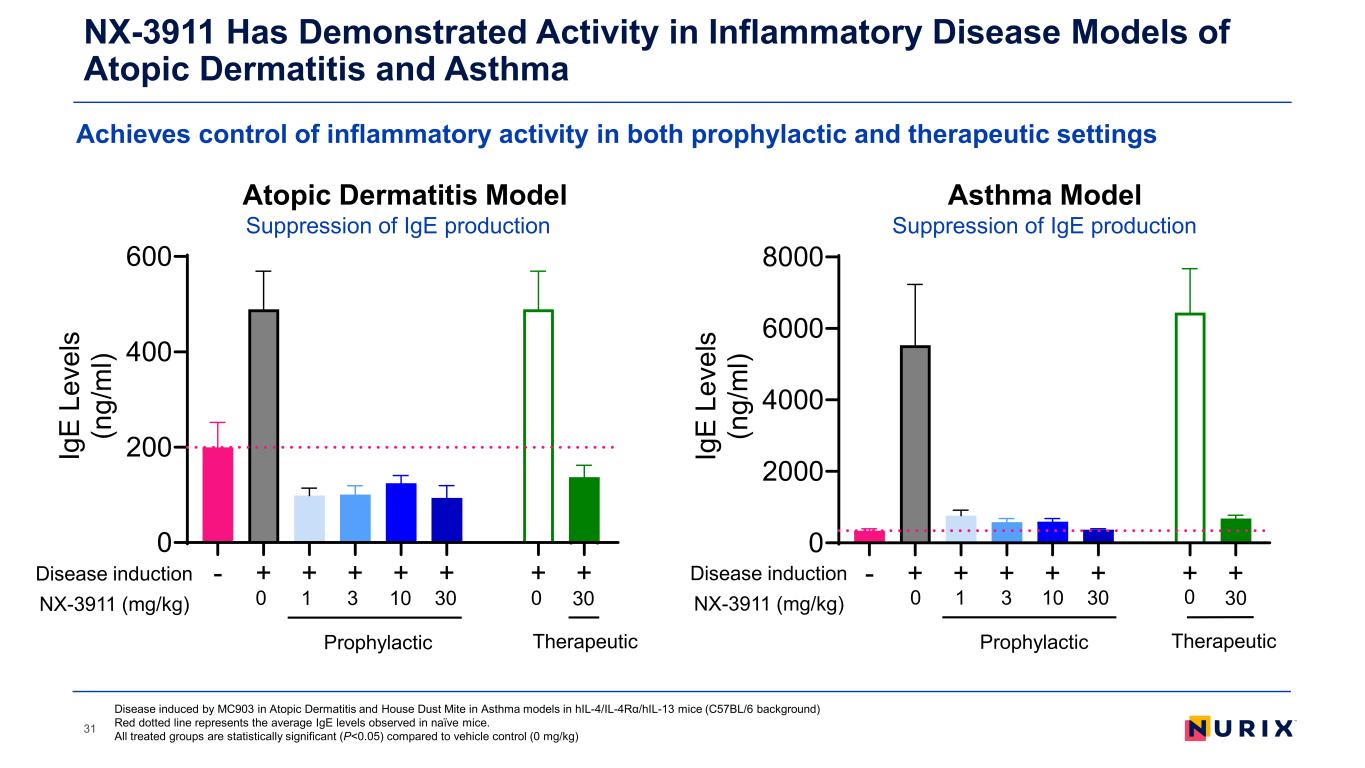
NX-3911 Has Demonstrated Activity in Inflammatory Disease Models of Atopic Dermatitis and Asthma 31 - + + + + + + + 0 200 400 600 Ig E Le ve ls (n g/ m l) - + + + + + + + 0 2000 4000 6000 8000 Ig E Le ve ls (n g/ m l) Asthma Model Suppression of IgE production Atopic Dermatitis Model Suppression of IgE production Disease induced by MC903 in Atopic Dermatitis and House Dust Mite in Asthma models in hIL-4/IL-4Rα/hIL-13 mice (C57BL/6 background) Red dotted line represents the average IgE levels observed in naïve mice. All treated groups are statistically significant (P<0.05) compared to vehicle control (0 mg/kg) Prophylactic Disease induction NX-3911 (mg/kg) Therapeutic 1 3 10 30 300 0 Prophylactic Disease induction NX-3911 (mg/kg) Therapeutic 1 3 10 30 300 0 Achieves control of inflammatory activity in both prophylactic and therapeutic settings
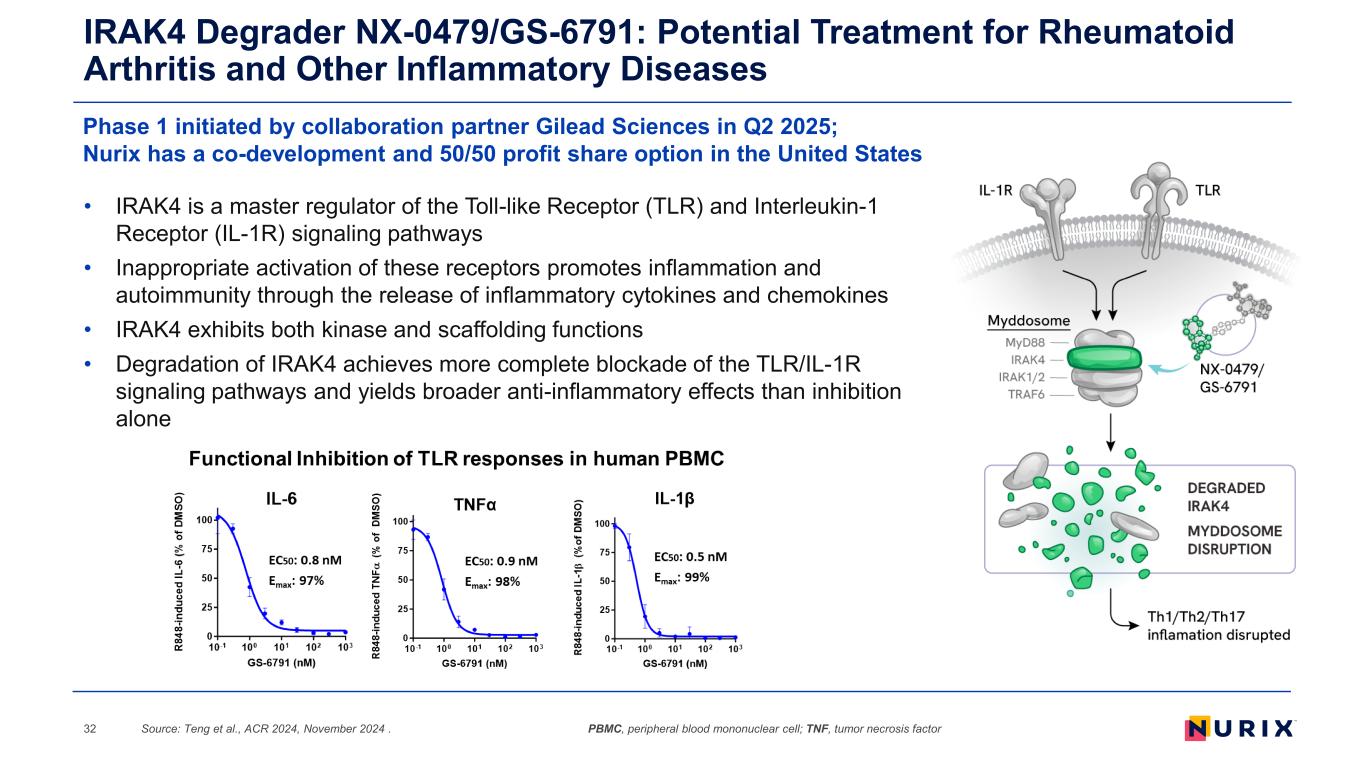
IRAK4 Degrader NX-0479/GS-6791: Potential Treatment for Rheumatoid Arthritis and Other Inflammatory Diseases Source: Teng et al., ACR 2024, November 2024 .32 Phase 1 initiated by collaboration partner Gilead Sciences in Q2 2025; Nurix has a co-development and 50/50 profit share option in the United States • IRAK4 is a master regulator of the Toll-like Receptor (TLR) and Interleukin-1 Receptor (IL-1R) signaling pathways • Inappropriate activation of these receptors promotes inflammation and autoimmunity through the release of inflammatory cytokines and chemokines • IRAK4 exhibits both kinase and scaffolding functions • Degradation of IRAK4 achieves more complete blockade of the TLR/IL-1R signaling pathways and yields broader anti-inflammatory effects than inhibition alone PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor
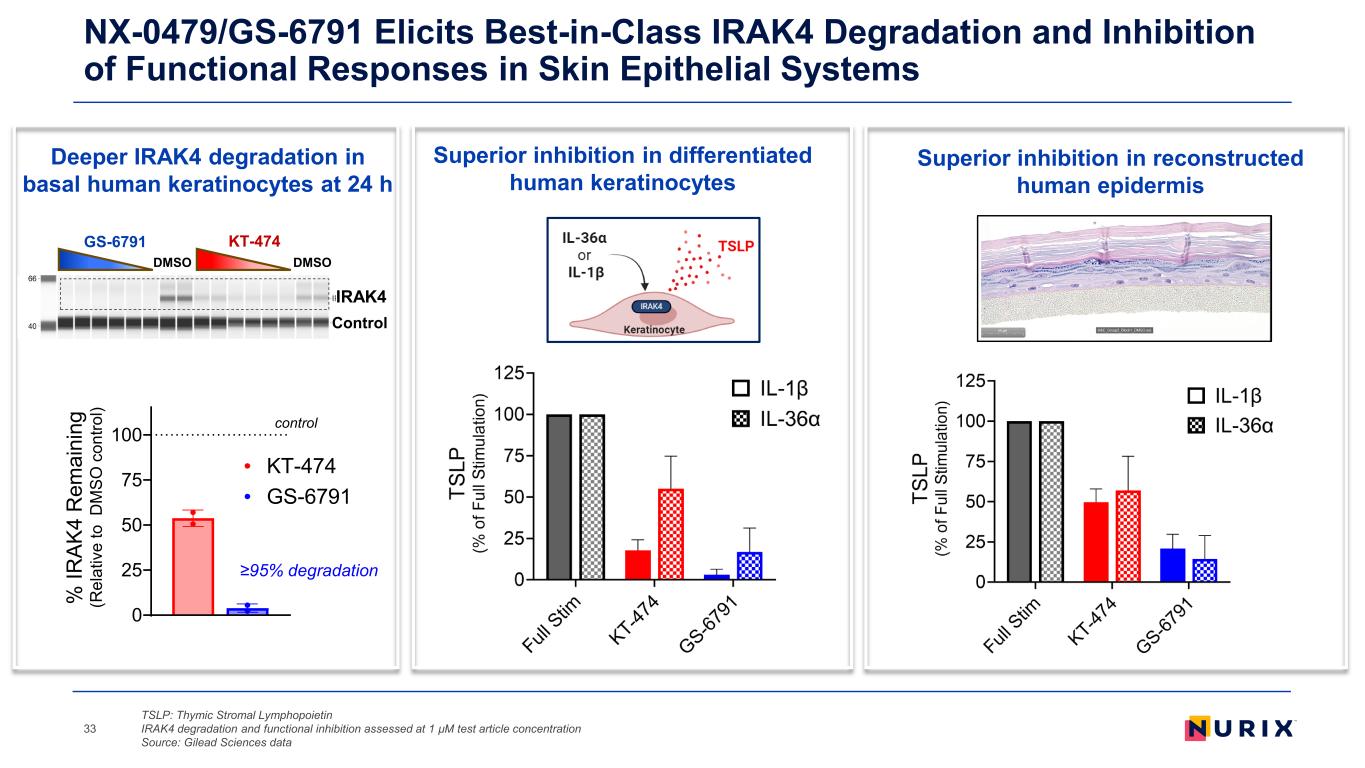
NX-0479/GS-6791 Elicits Best-in-Class IRAK4 Degradation and Inhibition of Functional Responses in Skin Epithelial Systems TSLP: Thymic Stromal Lymphopoietin IRAK4 degradation and functional inhibition assessed at 1 µM test article concentration Source: Gilead Sciences data 33 Deeper IRAK4 degradation in basal human keratinocytes at 24 h Superior inhibition in differentiated human keratinocytes Superior inhibition in reconstructed human epidermis IRAK4 Control DMSODMSO GS-6791 KT-474 0 25 50 75 100 % IR AK 4 R em ai ni ng (R el at iv e to D M SO c on tro l) GS-6791 KT-474 control ≥95% degradation

Arthur T. Sands, M.D., Ph.D. President and Chief Executive Officer Driving Value with Wholly Owned and Partnered Programs
• Bexobrutideg has the potential to produce a pipeline of value over the course of its lifecycle • CLL, WM, NHL, and potentially I&I indications • Advancing bexobrutideg one step closer to registration and commercialization 600 mg dose selected per Project Optimus Regulatory alignment on dose with FDA, MHRA, EMA Pivotal Phase 2 trial initiated – DAYBreak CLL-201 Confirmatory Phase 3 trial initiation planned for H1 2026 • Potential best-in-class profile emerging for bexobrutideg • Partnered programs advancing toward significant regulatory and clinical milestones and have the potential to create substantial value for Nurix shareholders with our 50-50 U.S. profit share opt in rights • STAT6 and IRAK4 • Upon completion of our registered direct offering, pro forma cash is anticipated to be $678.8 million, providing expected runway into 2028* Building an Oncology and Immunology Powerhouse 35 EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; MHRA, U.K. Medicines and Healthcare products Regulatory Agency * Pro forma cash reflects expected net proceeds from the October 2025 offering added to Nurix’s cash, cash equivalents, and marketable securities balance as of August 31, 2025.
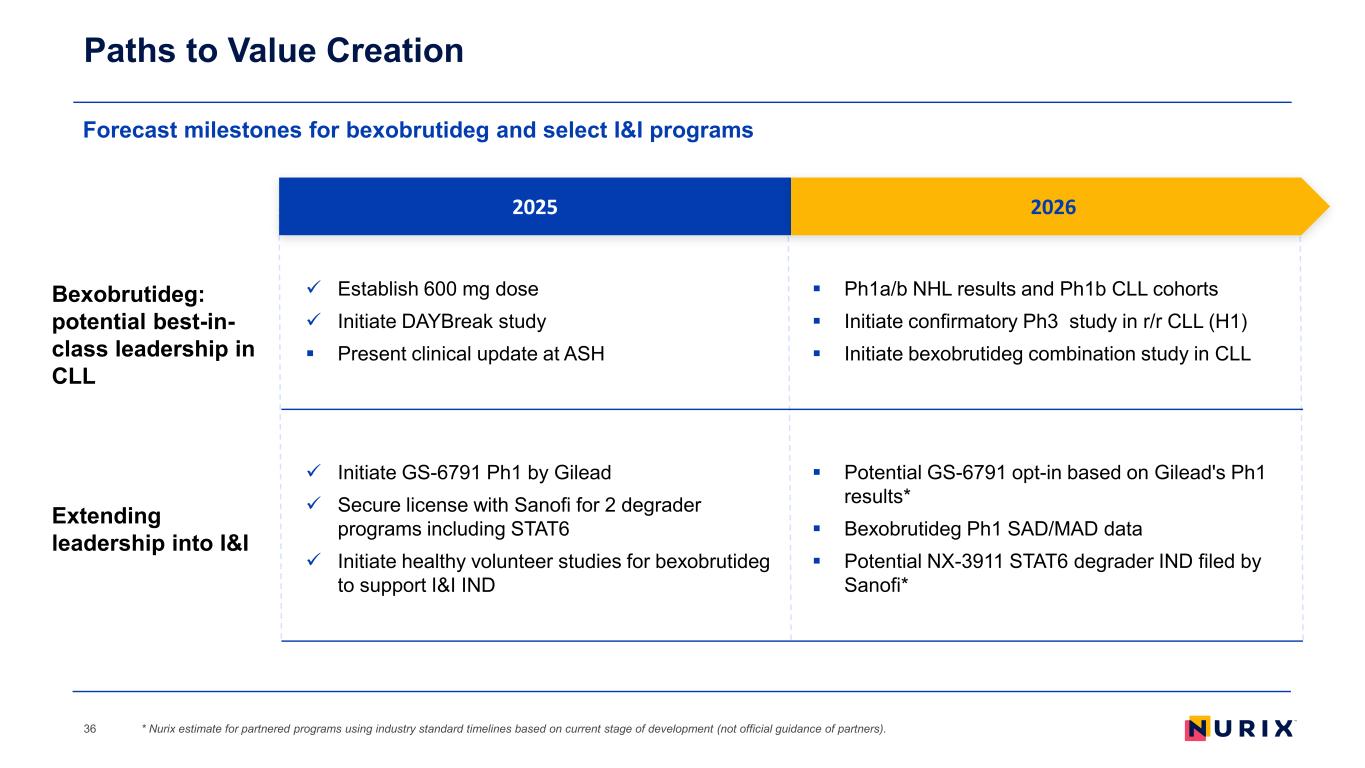
Forecast milestones for bexobrutideg and select I&I programs Paths to Value Creation * Nurix estimate for partnered programs using industry standard timelines based on current stage of development (not official guidance of partners).36 2025 Bexobrutideg: potential best-in- class leadership in CLL Extending leadership into I&I Establish 600 mg dose Initiate DAYBreak study Present clinical update at ASH Initiate GS-6791 Ph1 by Gilead Secure license with Sanofi for 2 degrader programs including STAT6 Initiate healthy volunteer studies for bexobrutideg to support I&I IND Ph1a/b NHL results and Ph1b CLL cohorts Initiate confirmatory Ph3 study in r/r CLL (H1) Initiate bexobrutideg combination study in CLL Potential GS-6791 opt-in based on Gilead's Ph1 results* Bexobrutideg Ph1 SAD/MAD data Potential NX-3911 STAT6 degrader IND filed by Sanofi* 2026

Q&A CONFIDENTIAL

Thank you CONFIDENTIAL

ESMO 2025 poster presentation Presented October 18, 2025 Appendix: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors
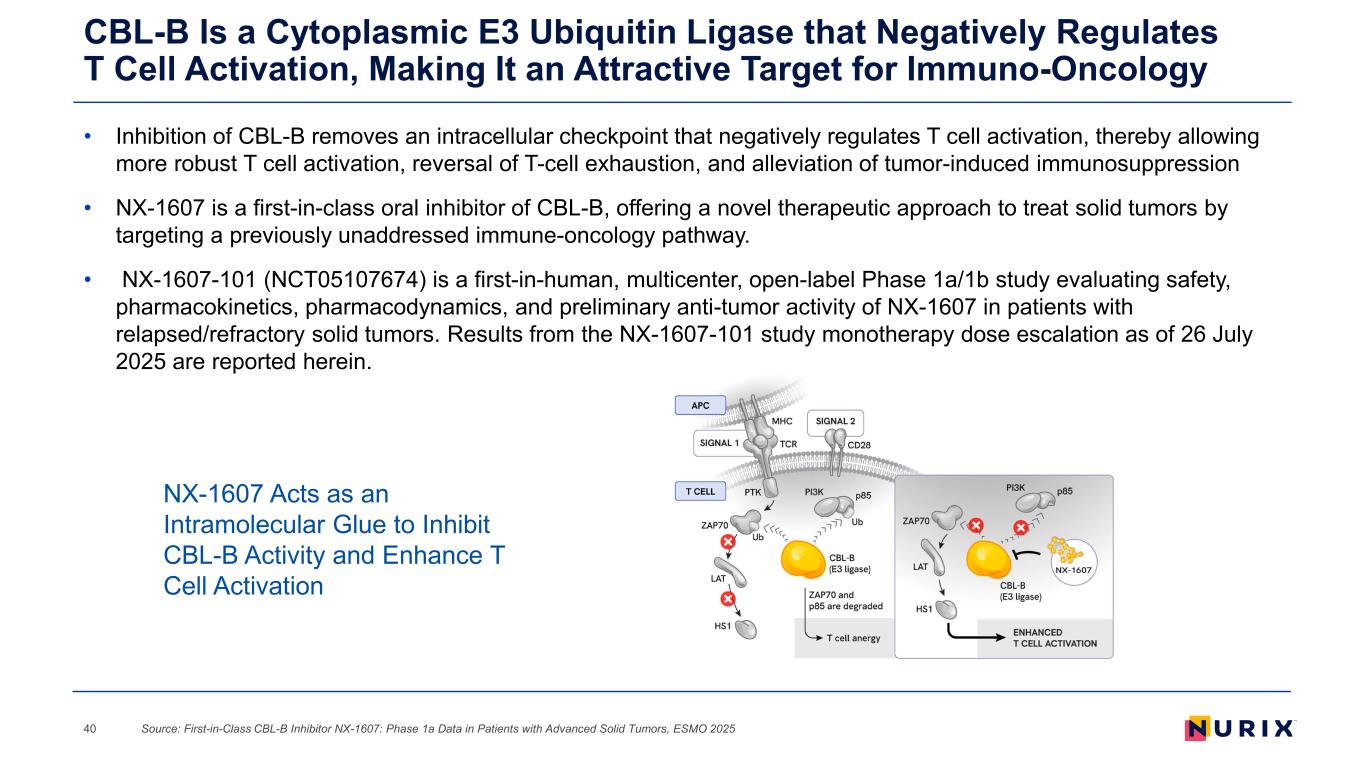
• Inhibition of CBL-B removes an intracellular checkpoint that negatively regulates T cell activation, thereby allowing more robust T cell activation, reversal of T-cell exhaustion, and alleviation of tumor-induced immunosuppression • NX-1607 is a first-in-class oral inhibitor of CBL-B, offering a novel therapeutic approach to treat solid tumors by targeting a previously unaddressed immune-oncology pathway. • NX-1607-101 (NCT05107674) is a first-in-human, multicenter, open-label Phase 1a/1b study evaluating safety, pharmacokinetics, pharmacodynamics, and preliminary anti-tumor activity of NX-1607 in patients with relapsed/refractory solid tumors. Results from the NX-1607-101 study monotherapy dose escalation as of 26 July 2025 are reported herein. CBL-B Is a Cytoplasmic E3 Ubiquitin Ligase that Negatively Regulates T Cell Activation, Making It an Attractive Target for Immuno-Oncology Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202540 NX-1607 Acts as an Intramolecular Glue to Inhibit CBL-B Activity and Enhance T Cell Activation
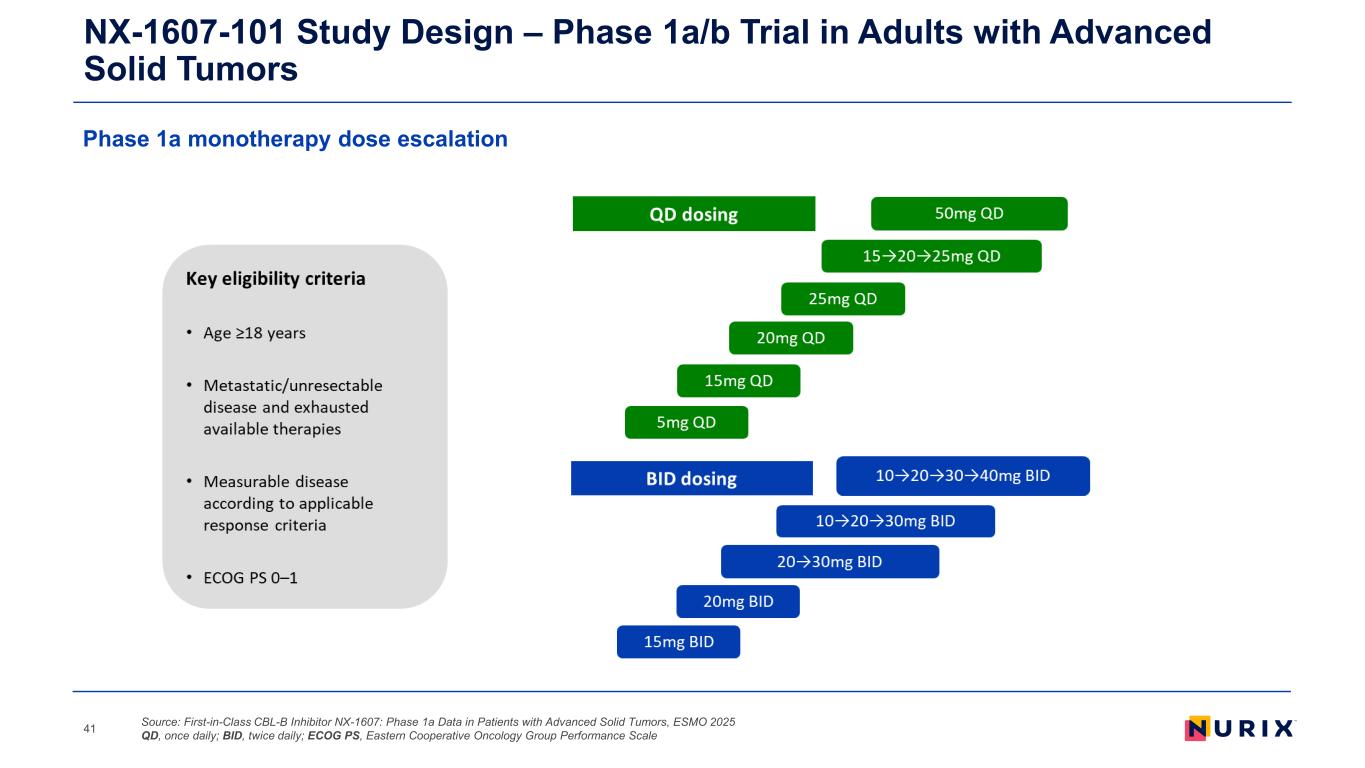
Phase 1a monotherapy dose escalation NX-1607-101 Study Design – Phase 1a/b Trial in Adults with Advanced Solid Tumors Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 2025 QD, once daily; BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group Performance Scale41
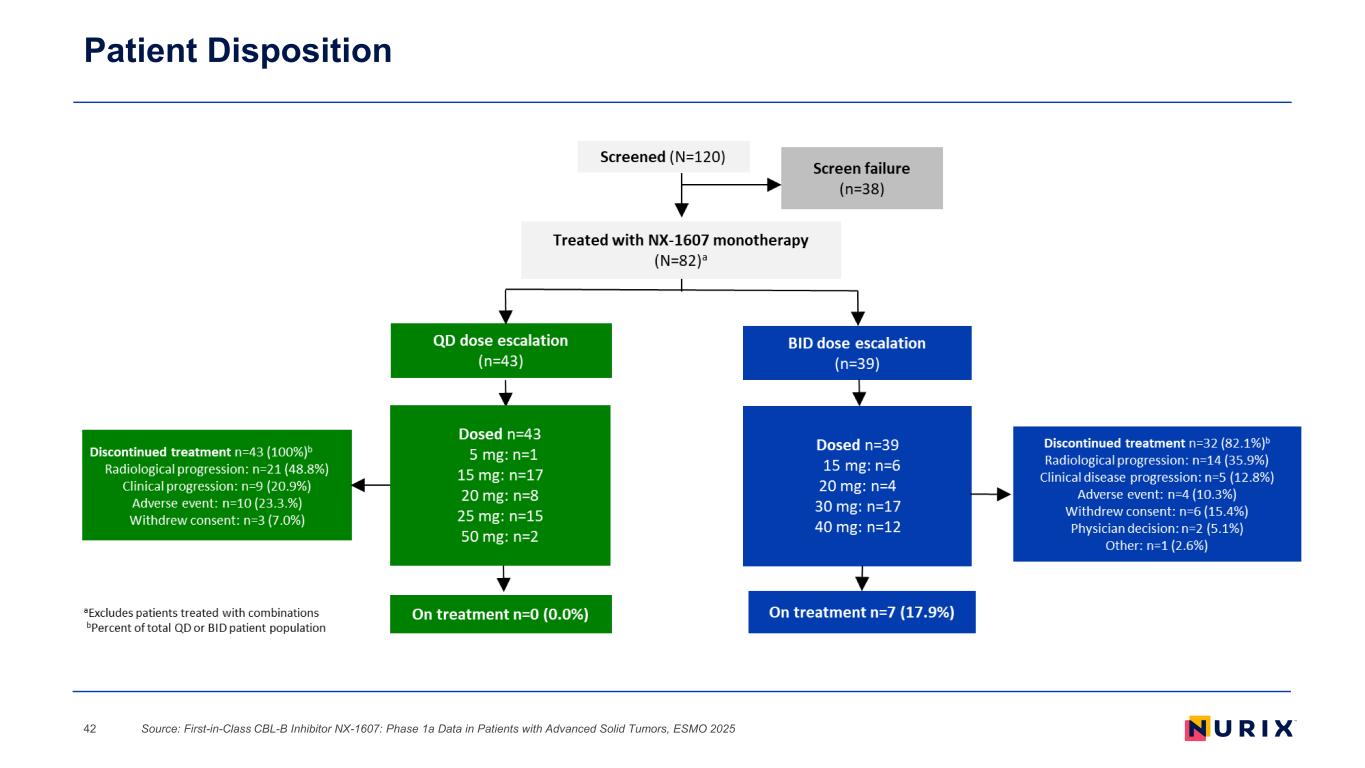
Patient Disposition Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202542
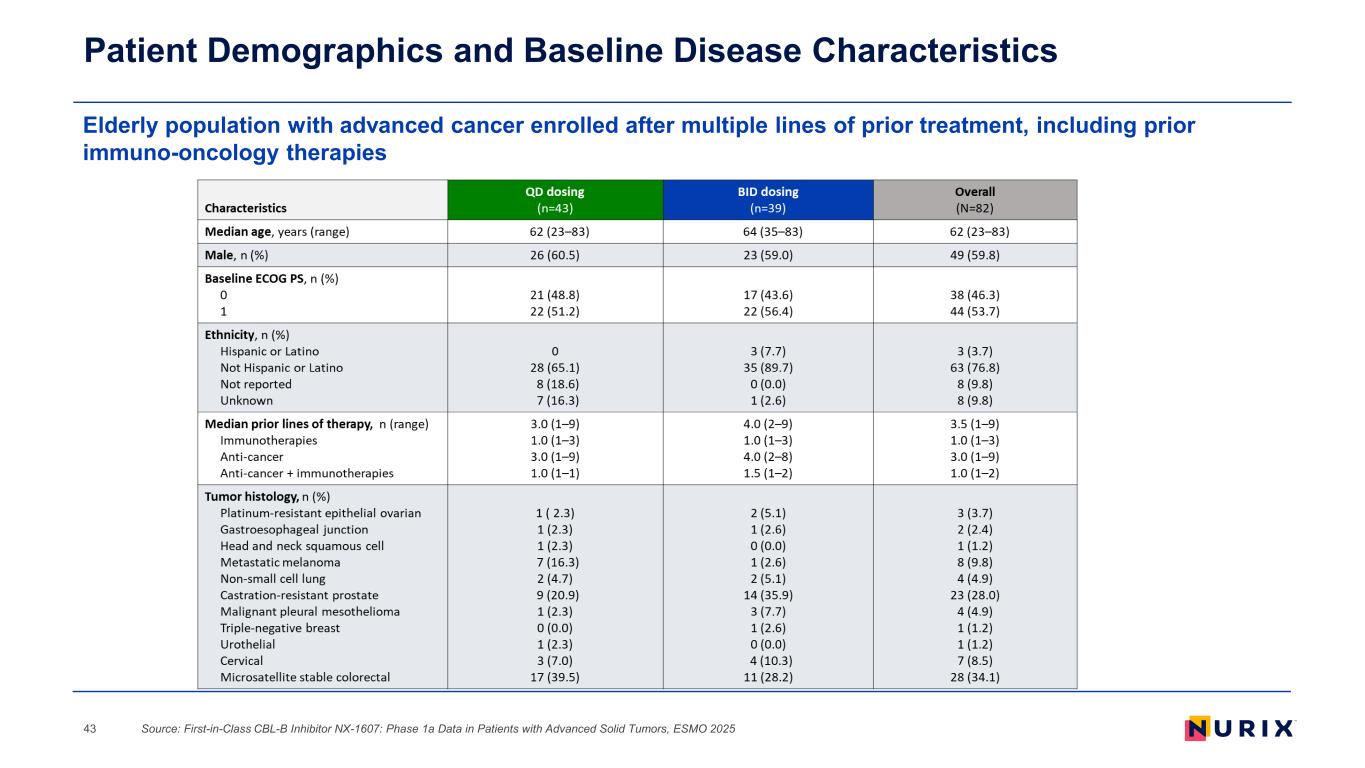
Elderly population with advanced cancer enrolled after multiple lines of prior treatment, including prior immuno-oncology therapies Patient Demographics and Baseline Disease Characteristics Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202543
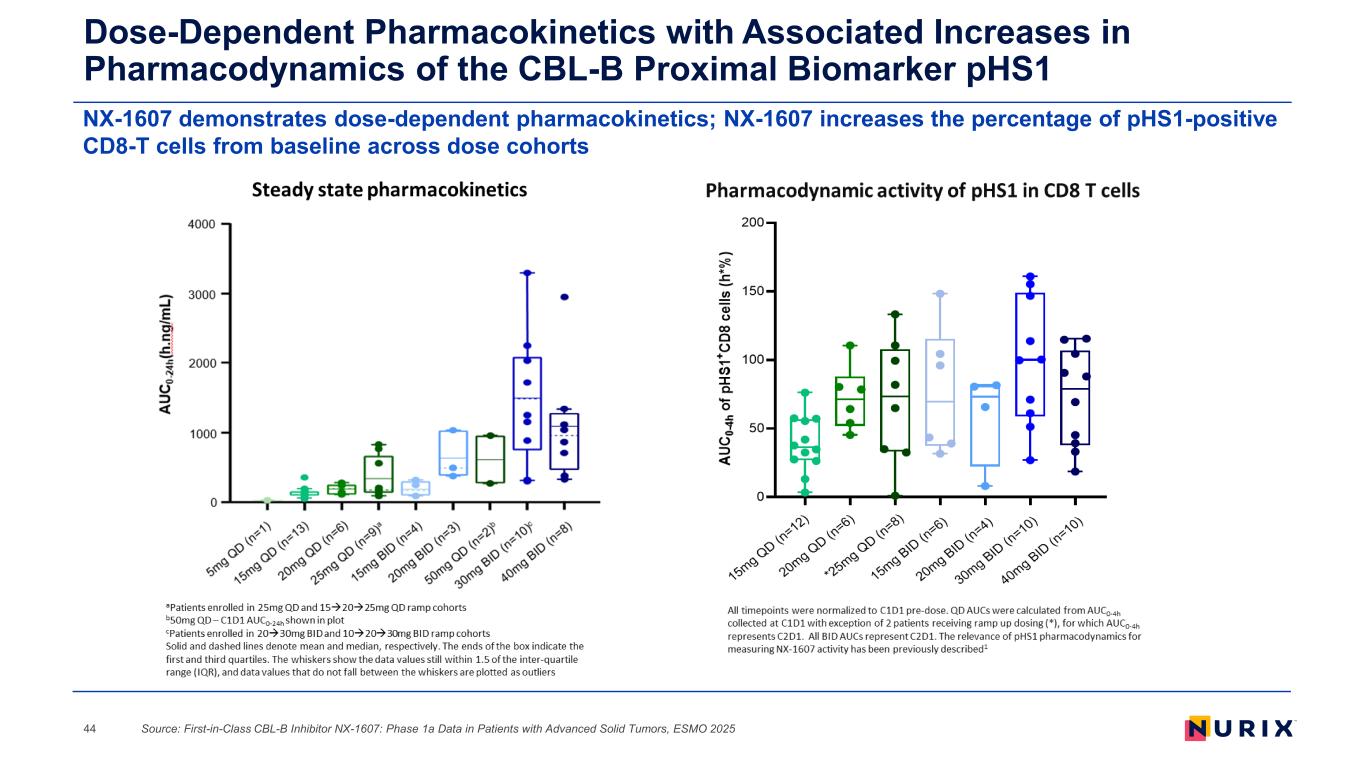
NX-1607 demonstrates dose-dependent pharmacokinetics; NX-1607 increases the percentage of pHS1-positive CD8-T cells from baseline across dose cohorts Dose-Dependent Pharmacokinetics with Associated Increases in Pharmacodynamics of the CBL-B Proximal Biomarker pHS1 Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202544
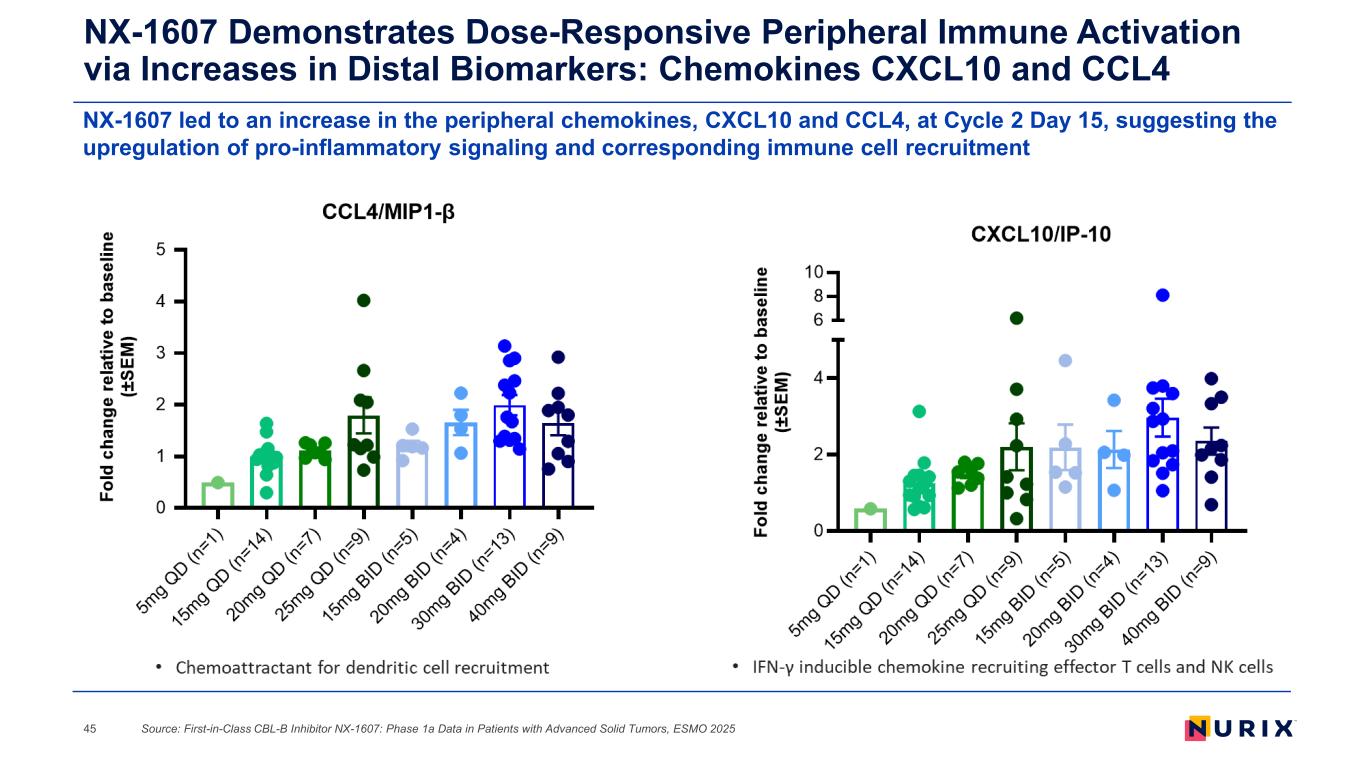
NX-1607 led to an increase in the peripheral chemokines, CXCL10 and CCL4, at Cycle 2 Day 15, suggesting the upregulation of pro-inflammatory signaling and corresponding immune cell recruitment NX-1607 Demonstrates Dose-Responsive Peripheral Immune Activation via Increases in Distal Biomarkers: Chemokines CXCL10 and CCL4 Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202545
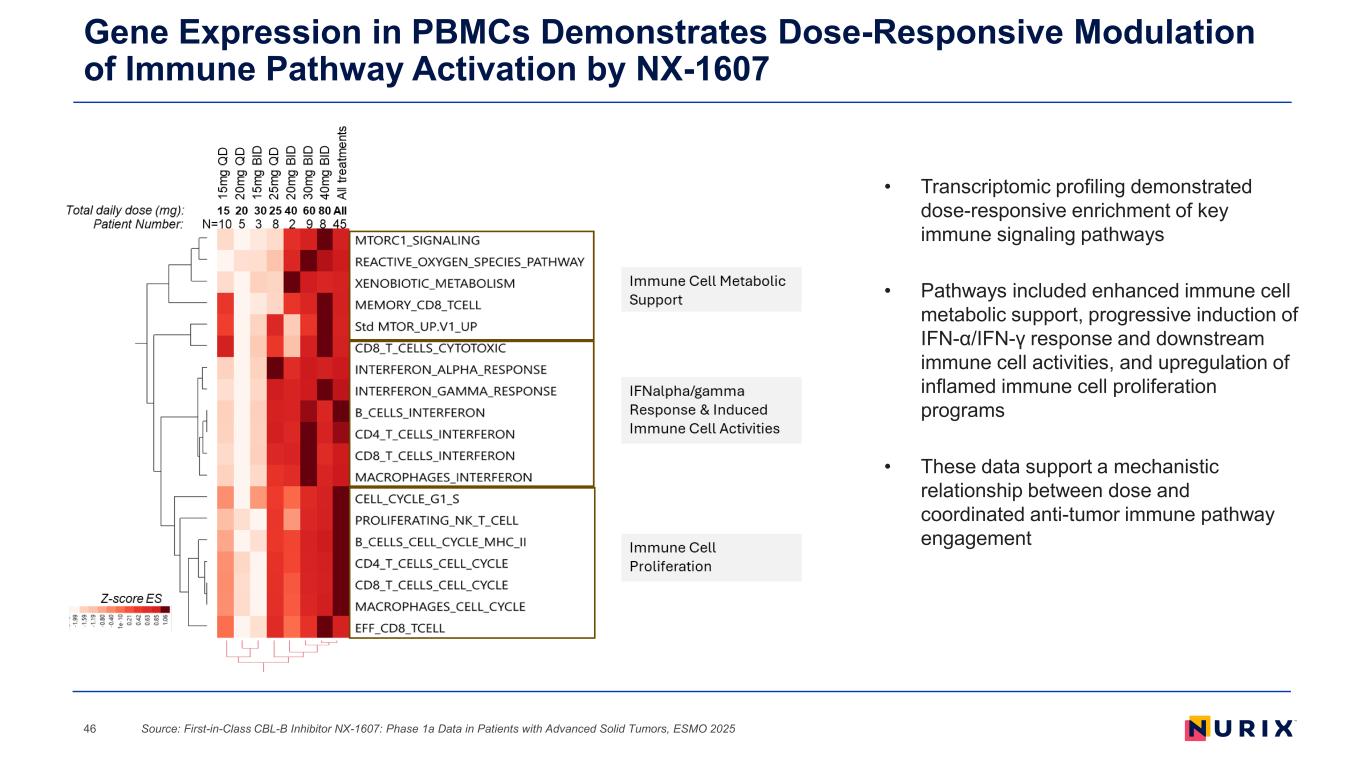
Gene Expression in PBMCs Demonstrates Dose-Responsive Modulation of Immune Pathway Activation by NX-1607 Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202546 • Transcriptomic profiling demonstrated dose-responsive enrichment of key immune signaling pathways • Pathways included enhanced immune cell metabolic support, progressive induction of IFN-α/IFN-γ response and downstream immune cell activities, and upregulation of inflamed immune cell proliferation programs • These data support a mechanistic relationship between dose and coordinated anti-tumor immune pathway engagement
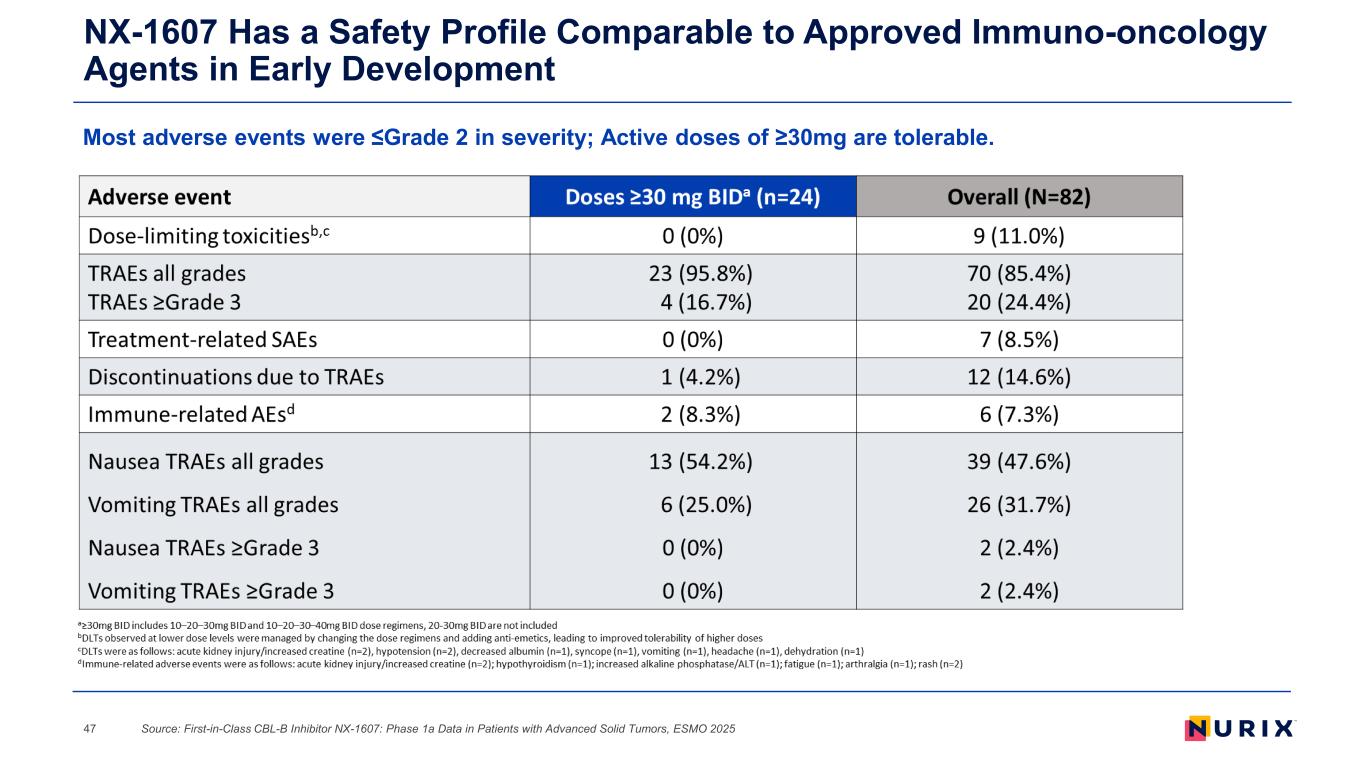
Most adverse events were ≤Grade 2 in severity; Active doses of ≥30mg are tolerable. NX-1607 Has a Safety Profile Comparable to Approved Immuno-oncology Agents in Early Development Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202547
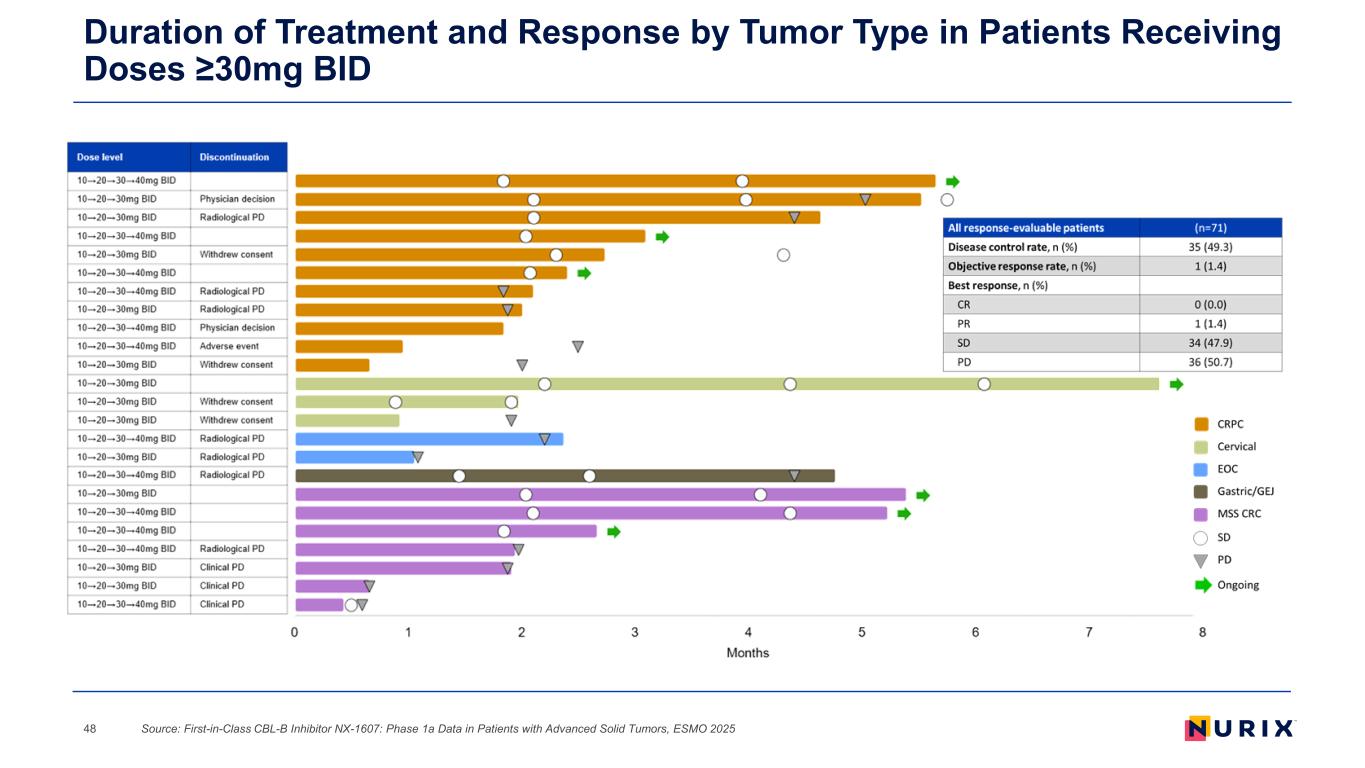
Duration of Treatment and Response by Tumor Type in Patients Receiving Doses ≥30mg BID Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202548
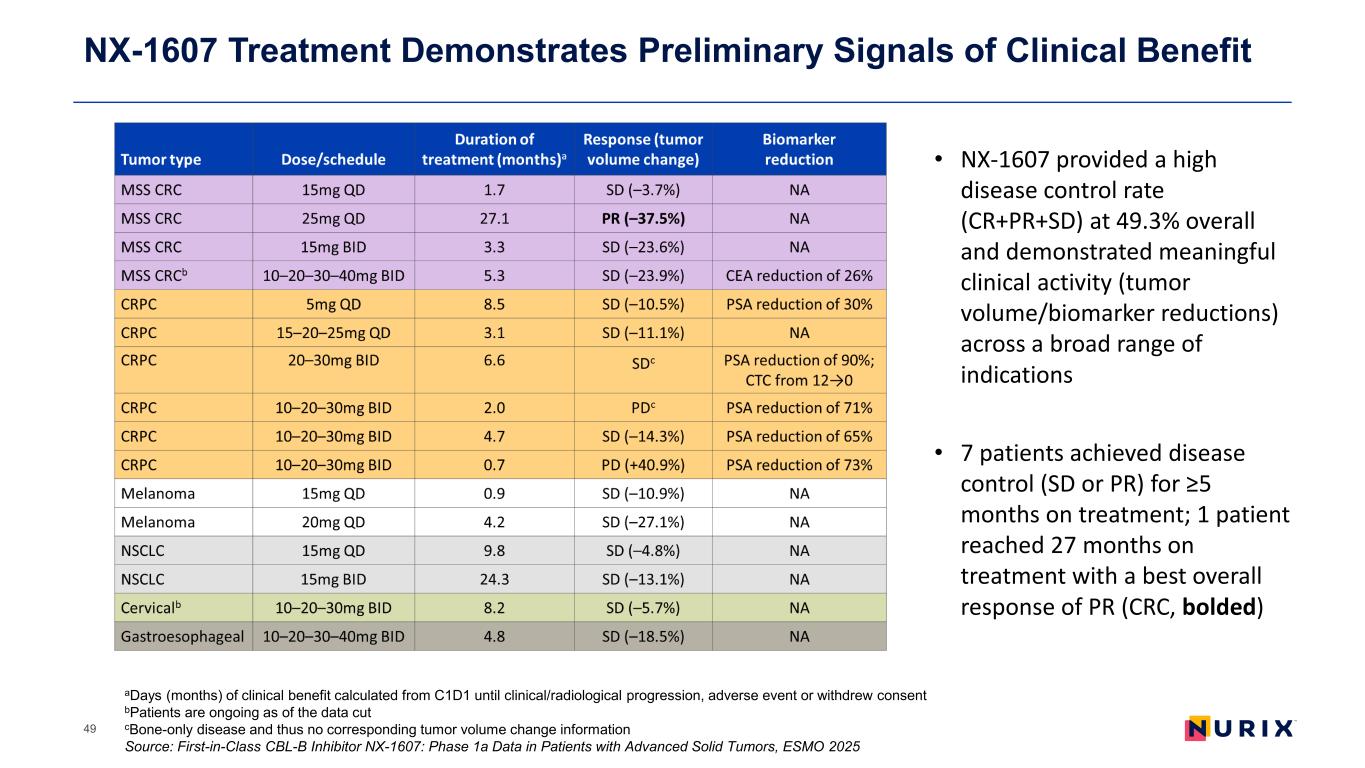
NX-1607 Treatment Demonstrates Preliminary Signals of Clinical Benefit 49 aDays (months) of clinical benefit calculated from C1D1 until clinical/radiological progression, adverse event or withdrew consent bPatients are ongoing as of the data cut cBone-only disease and thus no corresponding tumor volume change information Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 2025 • NX-1607 provided a high disease control rate (CR+PR+SD) at 49.3% overall and demonstrated meaningful clinical activity (tumor volume/biomarker reductions) across a broad range of indications • 7 patients achieved disease control (SD or PR) for ≥5 months on treatment; 1 patient reached 27 months on treatment with a best overall response of PR (CRC, bolded)
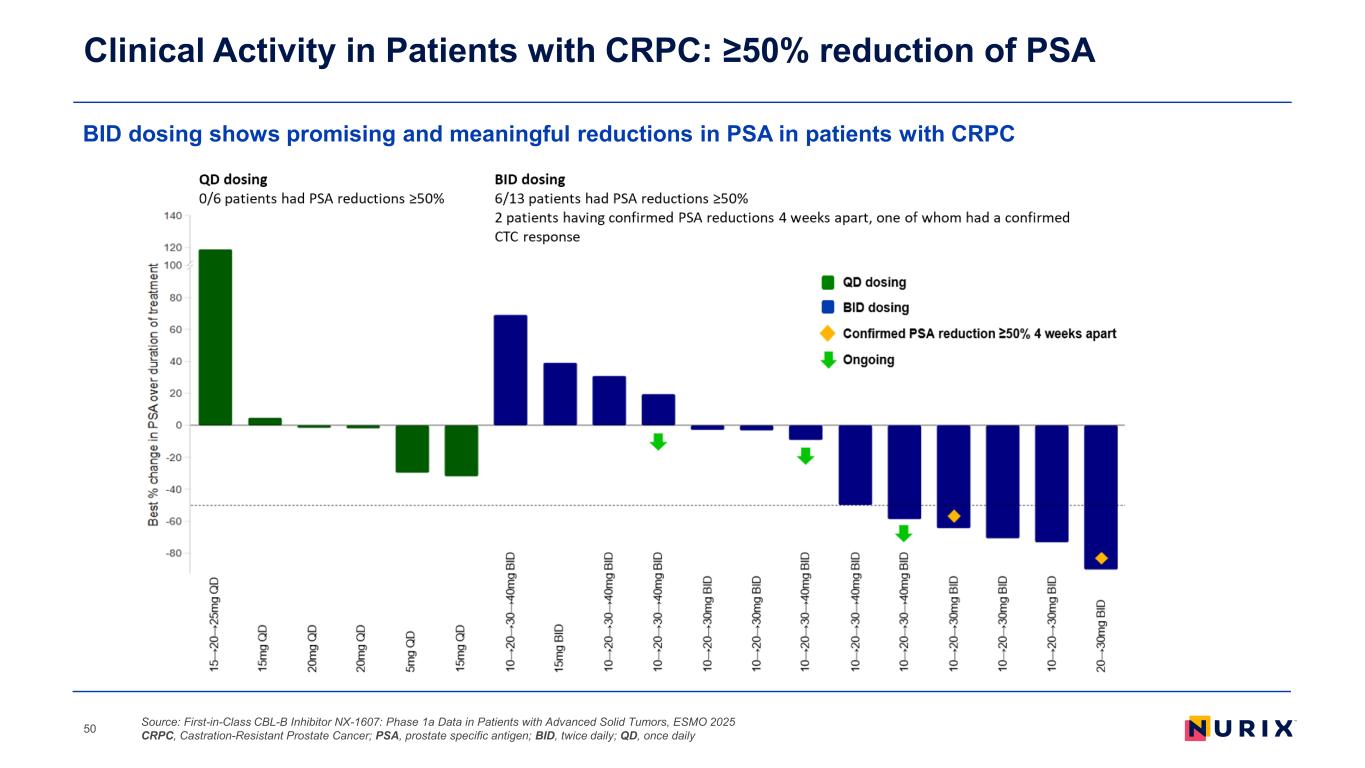
BID dosing shows promising and meaningful reductions in PSA in patients with CRPC Clinical Activity in Patients with CRPC: ≥50% reduction of PSA Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 2025 CRPC, Castration-Resistant Prostate Cancer; PSA, prostate specific antigen; BID, twice daily; QD, once daily50
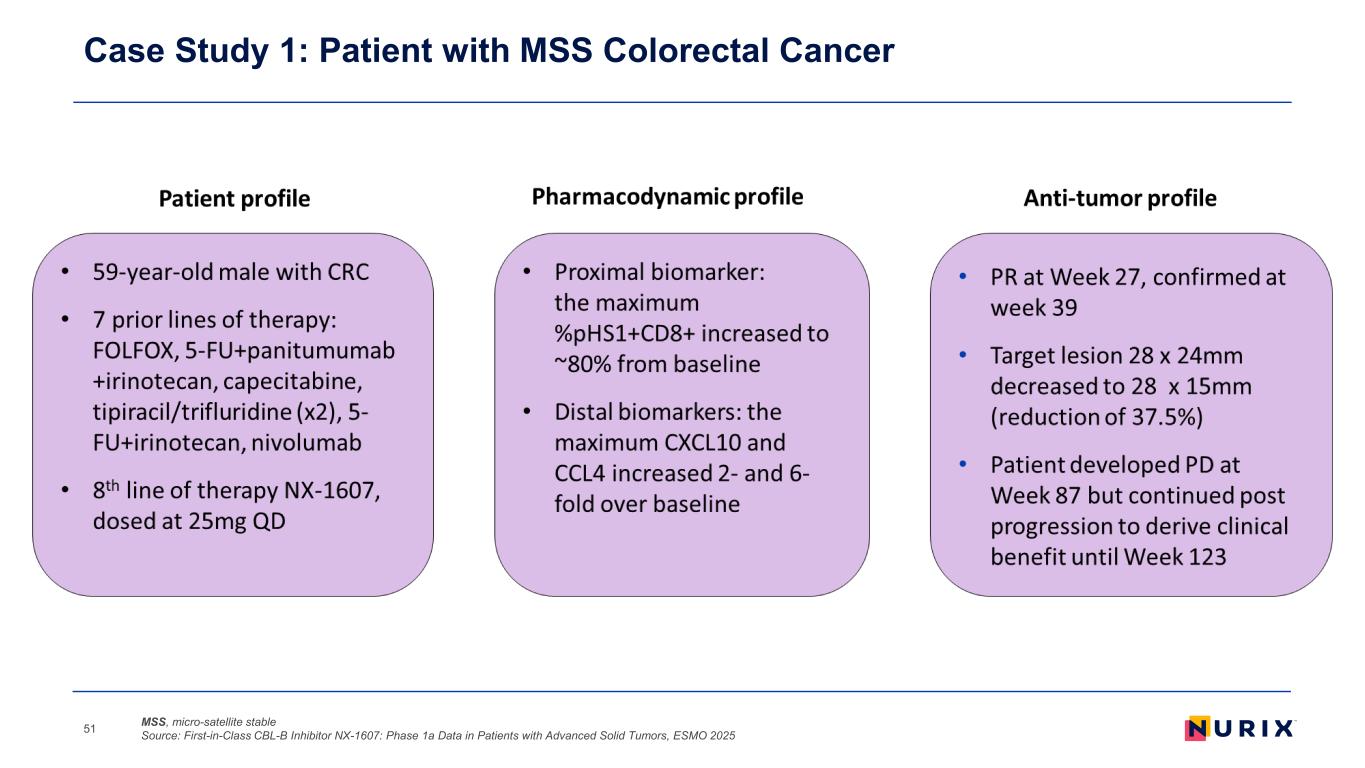
Case Study 1: Patient with MSS Colorectal Cancer MSS, micro-satellite stable Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202551
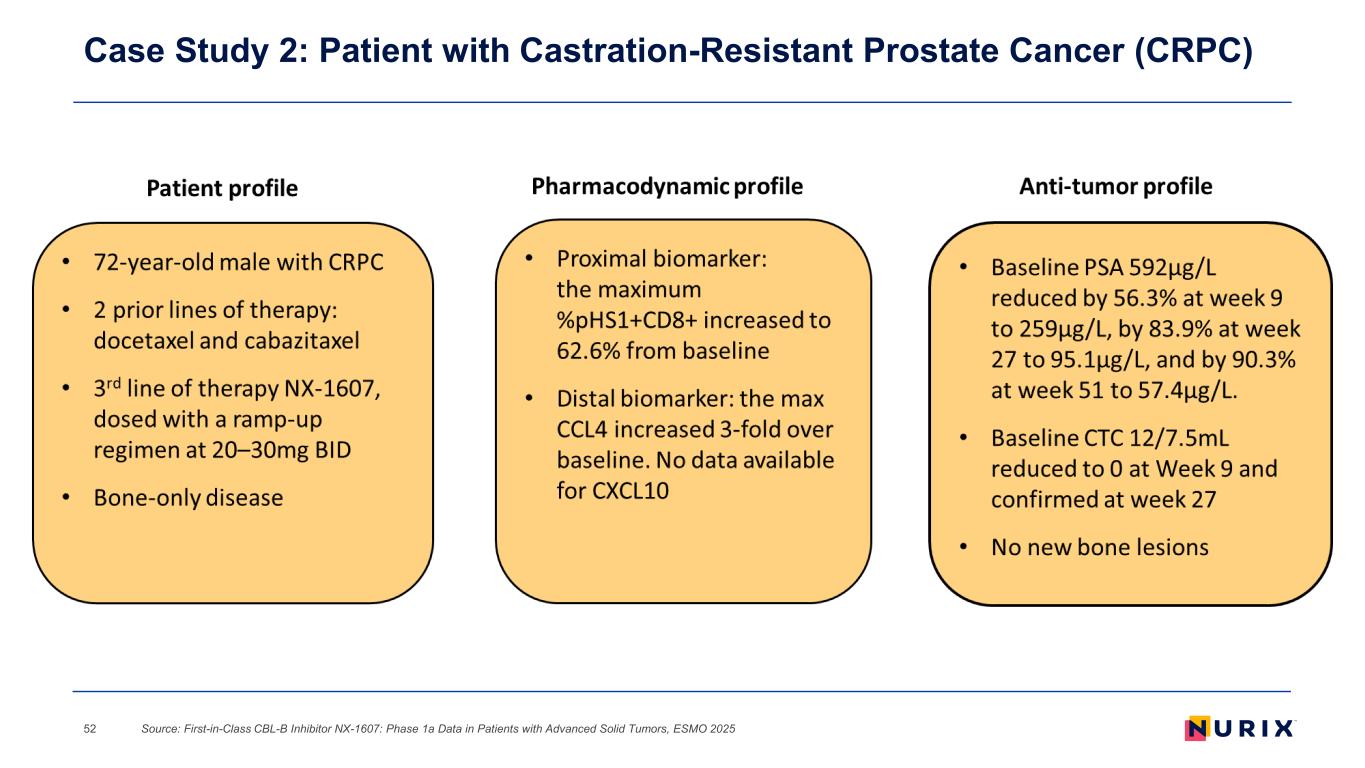
Case Study 2: Patient with Castration-Resistant Prostate Cancer (CRPC) Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202552
• NX-1607 is a first-in-class oral CBL-B inhibitor demonstrating a novel immune checkpoint mechanism distinct from PD-1/PD-L1 • NX-1607 is tolerable at pharmacologically active doses • Oral dosing of NX-1607 demonstrated dose-dependent exposure, increases in proximal and distal biomarkers, evidence of peripheral immune activation and reductions in tumor volume and cancer biomarkers, which together provide clinical proof that CBL-B inhibition can confer anti-tumor activity • NX-1607 monotherapy showed a high disease control rate of 49.3% with encouraging signals of clinical activity observed across multiple tumor types in heavily pretreated patients as with other successful I/O agents during early development, such as anti-PD11 and anti-CTLA42 • Data support the continued development of NX-1607 as monotherapy or in combination with other agents for the treatment of advanced solid tumors NX-1607 monotherapy demonstrates the characteristics of an active immuno-oncology agent Conclusions Source: First-in-Class CBL-B Inhibitor NX-1607: Phase 1a Data in Patients with Advanced Solid Tumors, ESMO 202553




















































