000153412012/312025Q3FALSEhttp://fasb.org/us-gaap/2025#DerivativeLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2025#DerivativeLiabilitiesNoncurrenthttp://www.avalotherapeutics.com/20250930#PropertyPlantAndEquipmentAndOperatingLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortizationhttp://www.avalotherapeutics.com/20250930#PropertyPlantAndEquipmentAndOperatingLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortizationhttp://fasb.org/us-gaap/2025#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2025#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2025#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2025#OtherLiabilitiesNoncurrentP1YP6Mxbrli:sharesiso4217:USDiso4217:USDxbrli:sharesxbrli:pureavtx:class_of_stockavtx:employeeavtx:segment00015341202025-01-012025-09-3000015341202025-11-0300015341202025-09-3000015341202024-12-310001534120us-gaap:SeriesDPreferredStockMember2024-12-310001534120us-gaap:SeriesDPreferredStockMember2025-09-300001534120us-gaap:SeriesEPreferredStockMember2024-12-310001534120us-gaap:SeriesEPreferredStockMember2025-09-300001534120us-gaap:SeriesCPreferredStockMember2025-09-300001534120us-gaap:SeriesCPreferredStockMember2024-12-310001534120us-gaap:ProductMember2025-07-012025-09-300001534120us-gaap:ProductMember2024-07-012024-09-300001534120us-gaap:ProductMember2025-01-012025-09-300001534120us-gaap:ProductMember2024-01-012024-09-3000015341202025-07-012025-09-3000015341202024-07-012024-09-3000015341202024-01-012024-09-3000015341202025-06-300001534120us-gaap:CommonStockMember2025-06-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2025-06-300001534120us-gaap:AdditionalPaidInCapitalMember2025-06-300001534120us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-06-300001534120us-gaap:RetainedEarningsMember2025-06-300001534120us-gaap:CommonStockMember2025-07-012025-09-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2025-07-012025-09-300001534120us-gaap:AdditionalPaidInCapitalMember2025-07-012025-09-300001534120us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-07-012025-09-300001534120us-gaap:RetainedEarningsMember2025-07-012025-09-300001534120us-gaap:CommonStockMember2025-09-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2025-09-300001534120us-gaap:AdditionalPaidInCapitalMember2025-09-300001534120us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-09-300001534120us-gaap:RetainedEarningsMember2025-09-300001534120us-gaap:CommonStockMember2024-12-310001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2024-12-310001534120us-gaap:AdditionalPaidInCapitalMember2024-12-310001534120us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-12-310001534120us-gaap:RetainedEarningsMember2024-12-310001534120us-gaap:CommonStockMember2025-01-012025-09-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2025-01-012025-09-300001534120us-gaap:AdditionalPaidInCapitalMember2025-01-012025-09-300001534120us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-01-012025-09-300001534120us-gaap:RetainedEarningsMember2025-01-012025-09-3000015341202024-06-300001534120us-gaap:CommonStockMember2024-06-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2024-06-300001534120us-gaap:AdditionalPaidInCapitalMember2024-06-300001534120us-gaap:RetainedEarningsMember2024-06-300001534120avtx:ConversionOfConvertiblePreferredStockMemberus-gaap:CommonStockMember2024-07-012024-09-300001534120avtx:ConversionOfConvertiblePreferredStockMemberus-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001534120avtx:ConversionOfConvertiblePreferredStockMember2024-07-012024-09-300001534120us-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001534120us-gaap:RetainedEarningsMember2024-07-012024-09-3000015341202024-09-300001534120us-gaap:CommonStockMember2024-09-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2024-09-300001534120us-gaap:AdditionalPaidInCapitalMember2024-09-300001534120us-gaap:RetainedEarningsMember2024-09-3000015341202023-12-310001534120us-gaap:CommonStockMember2023-12-310001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PreferredStockMember2023-12-310001534120us-gaap:AdditionalPaidInCapitalMember2023-12-310001534120us-gaap:RetainedEarningsMember2023-12-310001534120us-gaap:CommonStockMember2024-01-012024-09-300001534120us-gaap:AdditionalPaidInCapitalMember2024-01-012024-09-300001534120avtx:AlmataBioTransactionMemberus-gaap:SeriesCPreferredStockMember2024-01-012024-09-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PrivatePlacementMember2024-01-012024-09-300001534120us-gaap:SeriesDPreferredStockMemberus-gaap:PrivatePlacementMember2024-01-012024-09-300001534120us-gaap:SeriesEPreferredStockMemberus-gaap:PrivatePlacementMember2024-01-012024-09-300001534120avtx:ConversionOfConvertiblePreferredStockMemberus-gaap:CommonStockMember2024-01-012024-09-300001534120avtx:ConversionOfConvertiblePreferredStockMemberus-gaap:AdditionalPaidInCapitalMember2024-01-012024-09-300001534120avtx:ConversionOfConvertiblePreferredStockMember2024-01-012024-09-300001534120us-gaap:RetainedEarningsMember2024-01-012024-09-300001534120srt:RevisionOfPriorPeriodReclassificationAdjustmentMember2024-12-310001534120srt:RevisionOfPriorPeriodReclassificationAdjustmentMember2024-09-300001534120avtx:AlmataBioTransactionMember2024-03-272024-03-270001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2024-03-272024-03-270001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2024-08-132024-08-130001534120avtx:AlmataBioTransactionMemberus-gaap:CommonStockMember2024-08-132024-08-130001534120avtx:AlmataBioTransactionMember2024-03-282024-03-280001534120avtx:AlmataBioTransactionMember2024-04-012024-04-300001534120avtx:MilestoneOneMemberavtx:AlmataBioTransactionMember2024-03-280001534120avtx:MilestoneTwoMemberavtx:AlmataBioTransactionMember2024-03-280001534120avtx:MilestoneOneMemberavtx:AlmataBioTransactionMember2024-10-012024-10-310001534120avtx:AlmataBioTransactionMember2024-01-012024-09-300001534120avtx:AlmataBioTransactionMember2024-09-300001534120avtx:AlmataBioTransactionMember2024-08-132024-08-130001534120avtx:AlmataBioTransactionMember2024-03-270001534120avtx:MillipredMember2021-07-012023-09-300001534120us-gaap:EmployeeStockOptionMember2025-07-012025-09-300001534120us-gaap:EmployeeStockOptionMember2025-01-012025-09-300001534120us-gaap:EmployeeStockOptionMember2024-07-012024-09-300001534120us-gaap:EmployeeStockOptionMember2024-01-012024-09-300001534120avtx:WarrantCommonStockMember2025-07-012025-09-300001534120avtx:WarrantCommonStockMember2025-01-012025-09-300001534120avtx:WarrantCommonStockMember2024-07-012024-09-300001534120avtx:WarrantCommonStockMember2024-01-012024-09-300001534120us-gaap:SeriesCPreferredStockMember2025-07-012025-09-300001534120us-gaap:SeriesCPreferredStockMember2025-01-012025-09-300001534120us-gaap:SeriesCPreferredStockMember2024-01-012024-09-300001534120us-gaap:SeriesCPreferredStockMember2024-07-012024-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2025-07-012025-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2024-07-012024-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-09-300001534120us-gaap:PerformanceSharesMember2025-01-012025-09-300001534120us-gaap:PerformanceSharesMember2025-07-012025-09-300001534120us-gaap:PerformanceSharesMember2024-07-012024-09-300001534120us-gaap:PerformanceSharesMember2024-01-012024-09-300001534120us-gaap:SeriesCPreferredStockMember2024-09-300001534120us-gaap:SeriesCPreferredStockMember2025-09-300001534120us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001534120us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001534120us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001534120us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001534120us-gaap:USTreasuryAndGovernmentMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001534120us-gaap:USTreasuryAndGovernmentMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001534120us-gaap:USTreasuryAndGovernmentMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001534120us-gaap:USTreasuryAndGovernmentMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001534120us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001534120us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001534120us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001534120us-gaap:FairValueMeasurementsRecurringMember2025-09-300001534120us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001534120us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001534120us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001534120us-gaap:FairValueMeasurementsRecurringMember2024-12-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2025-06-300001534120us-gaap:FairValueInputsLevel3Member2025-06-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2025-07-012025-09-300001534120us-gaap:FairValueInputsLevel3Member2025-07-012025-09-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2025-09-300001534120us-gaap:FairValueInputsLevel3Member2025-09-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2024-12-310001534120us-gaap:FairValueInputsLevel3Member2024-12-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2025-01-012025-09-300001534120us-gaap:FairValueInputsLevel3Member2025-01-012025-09-300001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2024-06-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2024-06-300001534120us-gaap:FairValueInputsLevel3Member2024-06-300001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2024-07-012024-09-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2024-07-012024-09-300001534120us-gaap:FairValueInputsLevel3Member2024-07-012024-09-300001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2024-09-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2024-09-300001534120us-gaap:FairValueInputsLevel3Member2024-09-300001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2023-12-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2023-12-310001534120us-gaap:FairValueInputsLevel3Member2023-12-310001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2024-01-012024-09-300001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueInputsLevel3Member2024-01-012024-09-300001534120us-gaap:FairValueInputsLevel3Member2024-01-012024-09-300001534120avtx:ESTherapeuticsMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX501Member2022-12-310001534120avtx:AVTX007Memberavtx:MilestoneOneMember2022-12-310001534120avtx:AVTX007Memberavtx:MilestoneTwoMember2022-12-310001534120avtx:AVTX611Member2022-12-310001534120avtx:AVTX501AndAVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX501Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-09-300001534120avtx:AVTX501AndAVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-07-012025-09-300001534120avtx:AVTX501AndAVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-01-012025-09-300001534120avtx:AVTX501Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberavtx:MeasurementInputProbabilityOfSuccessMember2022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberavtx:MeasurementInputSalesForecastPeakMember2022-10-012022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberavtx:MeasurementInputProbabilityOfSuccessMember2022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:MeasurementInputExpectedTermMember2022-10-012022-12-310001534120us-gaap:PrivatePlacementMemberus-gaap:WarrantMember2025-09-300001534120us-gaap:PrivatePlacementMemberus-gaap:WarrantMember2024-12-310001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2024-03-282024-03-280001534120us-gaap:PrivatePlacementMemberus-gaap:PreferredStockMember2022-10-012022-12-3100015341202022-10-012022-12-310001534120us-gaap:MoneyMarketFundsMember2025-09-300001534120us-gaap:USTreasuryAndGovernmentMember2025-09-300001534120us-gaap:CashAndCashEquivalentsMember2025-09-300001534120us-gaap:CashAndCashEquivalentsMember2024-12-310001534120us-gaap:ShortTermInvestmentsMember2025-09-300001534120us-gaap:ShortTermInvestmentsMember2024-12-310001534120stpr:PAus-gaap:BuildingMember2025-09-300001534120stpr:PAus-gaap:BuildingMember2025-01-012025-09-300001534120stpr:MDus-gaap:BuildingMember2024-10-012024-12-310001534120stpr:MDus-gaap:BuildingMember2025-01-012025-09-300001534120stpr:MDus-gaap:BuildingMember2025-09-300001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PrivatePlacementMember2024-03-282024-03-280001534120us-gaap:PrivatePlacementMemberus-gaap:WarrantMember2024-03-282024-03-280001534120us-gaap:PrivatePlacementMemberus-gaap:PreferredStockMember2024-03-282024-03-280001534120us-gaap:PrivatePlacementMember2024-03-282024-03-280001534120us-gaap:PrivatePlacementMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PrivatePlacementMember2024-08-132024-08-130001534120us-gaap:CommonStockMember2024-08-132024-08-130001534120us-gaap:CommonStockMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMember2024-10-012024-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2025-09-300001534120us-gaap:WarrantMemberus-gaap:FairValueInputsLevel3Member2024-10-012024-12-310001534120us-gaap:PrivatePlacementMemberus-gaap:AdditionalPaidInCapitalMember2024-10-012024-12-310001534120avtx:AlmataTransactionAndMarch2024FinancingMember2025-09-300001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2025-09-300001534120avtx:AlmataTransactionAndMarch2024FinancingMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2024-12-310001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataBioTransactionAndMarch2024FinancingMemberus-gaap:AdditionalPaidInCapitalMember2024-12-310001534120us-gaap:SeriesCPreferredStockMember2025-07-012025-09-300001534120us-gaap:SeriesCPreferredStockMember2025-01-012025-09-300001534120avtx:ATMAgreementMember2025-06-012025-06-300001534120avtx:ATMAgreementMember2025-01-012025-09-300001534120avtx:ATMAgreementMember2025-07-012025-09-300001534120avtx:CommonStockWarrantsExpirationJune2031Memberus-gaap:CommonClassAMember2025-09-300001534120avtx:A2016ThirdAmendedPlanMember2025-01-012025-09-300001534120avtx:A2016FourthAmendedPlanMember2025-01-012025-01-010001534120avtx:A2016ThirdAmendedPlanMember2025-09-300001534120avtx:InducementAwardPlanMember2025-09-300001534120avtx:InducementAwardPlanMember2025-01-012025-09-300001534120avtx:A2016ThirdAmendedPlanMemberus-gaap:EmployeeStockOptionMember2025-01-012025-09-300001534120us-gaap:EmployeeStockOptionMembersrt:MaximumMemberavtx:A2016ThirdAmendedPlanMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2025-01-012025-09-300001534120us-gaap:EmployeeStockOptionMembersrt:MinimumMemberavtx:A2016ThirdAmendedPlanMembersrt:DirectorMember2025-01-012025-09-300001534120us-gaap:EmployeeStockOptionMembersrt:MaximumMemberavtx:A2016ThirdAmendedPlanMembersrt:DirectorMember2025-01-012025-09-300001534120us-gaap:ResearchAndDevelopmentExpenseMember2025-07-012025-09-300001534120us-gaap:ResearchAndDevelopmentExpenseMember2024-07-012024-09-300001534120us-gaap:ResearchAndDevelopmentExpenseMember2025-01-012025-09-300001534120us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-09-300001534120us-gaap:GeneralAndAdministrativeExpenseMember2025-07-012025-09-300001534120us-gaap:GeneralAndAdministrativeExpenseMember2024-07-012024-09-300001534120us-gaap:GeneralAndAdministrativeExpenseMember2025-01-012025-09-300001534120us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-09-300001534120avtx:ServiceBasedOptionsMember2024-12-310001534120avtx:ServiceBasedOptionsMember2024-01-012024-12-310001534120avtx:ServiceBasedOptionsMember2025-01-012025-09-300001534120avtx:ServiceBasedOptionsMember2025-09-300001534120avtx:ServiceBasedOptionsMember2024-01-012024-09-300001534120avtx:ServiceBasedOptionsMember2025-07-012025-09-300001534120avtx:ServiceBasedOptionsMember2024-07-012024-09-300001534120srt:MinimumMemberavtx:ServiceBasedOptionsMember2025-09-300001534120srt:MaximumMemberavtx:ServiceBasedOptionsMember2025-09-300001534120srt:MinimumMemberavtx:ServiceBasedOptionsMember2025-01-012025-09-300001534120srt:MaximumMemberavtx:ServiceBasedOptionsMember2025-01-012025-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2024-12-310001534120us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2025-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2024-08-132024-08-130001534120us-gaap:RestrictedStockUnitsRSUMember2025-07-012025-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2024-07-012024-09-300001534120us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-09-300001534120avtx:A2016FourthAmendedPlanMemberus-gaap:PerformanceSharesMember2025-08-012025-08-310001534120avtx:A2016FourthAmendedPlanMemberus-gaap:PerformanceSharesMember2025-01-012025-09-300001534120us-gaap:PerformanceSharesMember2025-09-300001534120us-gaap:PerformanceSharesMembersrt:MaximumMemberavtx:A2016FourthAmendedPlanMember2025-01-012025-09-300001534120srt:MinimumMemberus-gaap:PerformanceSharesMember2025-09-300001534120srt:MaximumMemberus-gaap:PerformanceSharesMember2025-09-300001534120us-gaap:EmployeeStockMember2016-04-052016-04-050001534120us-gaap:EmployeeStockMember2016-04-050001534120us-gaap:EmployeeStockMember2025-01-012025-01-010001534120us-gaap:EmployeeStockMember2025-01-012025-09-300001534120us-gaap:EmployeeStockMember2025-07-012025-09-300001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2024-03-270001534120avtx:AVTX009LillyLicenseAgreementMemberavtx:LillyLicenseAgreementMembersrt:MinimumMember2024-03-270001534120avtx:AVTX009LillyLicenseAgreementMemberavtx:LillyLicenseAgreementMembersrt:MaximumMember2024-03-270001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2024-03-272024-03-270001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2025-01-012025-09-300001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2025-07-012025-09-300001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2025-09-300001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2021-03-250001534120avtx:MilestoneOneMemberavtx:AVTX002KKCLicenseAgreementMemberavtx:KyowaKirinCoLtdKKCMember2021-03-250001534120avtx:MilestoneTwoMemberavtx:AVTX002KKCLicenseAgreementMemberavtx:KyowaKirinCoLtdKKCMember2021-03-250001534120avtx:KyowaKirinCoLtdKKCMemberus-gaap:ContractTerminationMembersrt:MinimumMember2021-03-252021-03-250001534120avtx:KyowaKirinCoLtdKKCMemberus-gaap:ContractTerminationMembersrt:MaximumMember2021-03-252021-03-250001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2025-07-012025-09-300001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2025-01-012025-09-300001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2025-09-300001534120avtx:CHOPLicenseAgreementMemberavtx:AVTX002KKCLicenseAgreementMember2020-02-030001534120avtx:CHOPLicenseAgreementMemberavtx:AVTX002KKCLicenseAgreementMember2020-02-032020-02-030001534120avtx:CHOPLicenseAgreementMemberavtx:AVTX002KKCLicenseAgreementMember2025-09-300001534120avtx:CHOPLicenseAgreementMember2020-02-032020-02-030001534120avtx:CHOPLicenseAgreementMemberus-gaap:ContractTerminationMember2020-02-032020-02-030001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2019-07-150001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006Member2019-07-152019-07-150001534120avtx:AstellasPharmaIncAstellasMember2019-07-152019-07-150001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2025-01-012025-09-300001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2025-07-012025-09-300001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2025-09-300001534120avtx:AltoMemberavtx:AVTX301OutLicenseMember2021-05-280001534120avtx:AVTX301OutLicenseMember2021-05-282021-05-280001534120avtx:AltoMemberavtx:AVTX301OutLicenseMember2025-09-300001534120avtx:AVTX406LicenseAssignmentMemberavtx:MilestoneOneMemberavtx:ESMember2021-06-090001534120avtx:AVTX406LicenseAssignmentMemberavtx:MilestoneTwoMemberavtx:ESMember2021-06-090001534120avtx:ESMemberavtx:AVTX406LicenseAssignmentMember2025-09-300001534120avtx:AVTX800SeriesAssetSaleMemberavtx:PurchaseAgreementMemberavtx:AUGTherapeuticsLLCMember2023-10-272023-10-270001534120avtx:AVTX800SeriesAssetSaleMemberavtx:PurchaseAgreementMemberavtx:AUGTherapeuticsLLCMember2023-10-270001534120avtx:AVTX009Memberavtx:MilestoneTwoMember2024-04-300001534120avtx:AVTX009Memberavtx:MilestoneThreeMember2024-10-012024-10-3100015341202019-07-012019-07-310001534120avtx:ReportableSegmentMember2025-07-012025-09-300001534120avtx:ReportableSegmentMember2024-07-012024-09-300001534120avtx:ReportableSegmentMember2025-01-012025-09-300001534120avtx:ReportableSegmentMember2024-01-012024-09-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-Q
|
|
|
|
|
|
☑
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
for the quarterly period ended September 30, 2025 |
| OR |
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
COMMISSION FILE NUMBER: 001-37590
AVALO THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
Delaware
(State of incorporation)
|
45-0705648
(I.R.S. Employer Identification No.)
|
|
1500 Liberty Ridge Drive, Suite 321
Wayne, Pennsylvania 19087
(Address of principal executive offices)
|
(410) 522-8707
(Registrant’s telephone number,
including area code)
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading Symbol |
Name of each exchange on which registered |
| Common Stock, $0.001 par value |
AVTX |
Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b‑2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
Large accelerated filer ☐ |
|
Accelerated filer ☐ |
Non-accelerated filer ☑ |
|
Smaller reporting company ☑ |
Emerging growth company ☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Exchange Act). Yes ☐ No ☑
As of November 3, 2025, the registrant had 18,133,968 shares of common stock outstanding.
AVALO THERAPEUTICS, INC.
FORM 10-Q
For the Quarter Ended September 30, 2025
TABLE OF CONTENTS
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Balance Sheets
(In thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2025 |
|
December 31, 2024 |
|
|
(unaudited) |
|
|
| Assets |
|
|
|
|
| Current assets: |
|
|
|
|
| Cash and cash equivalents |
|
$ |
26,963 |
|
|
$ |
134,546 |
|
|
|
|
|
|
Short-term investments |
|
84,654 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
| Prepaid expenses and other current assets |
|
2,139 |
|
|
4,325 |
|
| Restricted cash, current portion |
|
90 |
|
|
19 |
|
| Total current assets |
|
113,846 |
|
|
138,890 |
|
| Property and equipment, net |
|
542 |
|
|
1,209 |
|
| Goodwill |
|
10,502 |
|
|
10,502 |
|
| Restricted cash, net of current portion |
|
210 |
|
|
131 |
|
| Total assets |
|
$ |
125,100 |
|
|
$ |
150,732 |
|
| Liabilities, mezzanine equity and stockholders’ equity |
|
|
|
|
| Current liabilities: |
|
|
|
|
| Accounts payable |
|
$ |
474 |
|
|
$ |
283 |
|
| Accrued expenses and other current liabilities |
|
7,498 |
|
|
6,317 |
|
| Derivative liability, current |
|
— |
|
|
360 |
|
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
7,972 |
|
|
6,960 |
|
| Royalty obligation |
|
2,000 |
|
|
2,000 |
|
| Deferred tax liability, net |
|
304 |
|
|
270 |
|
| Derivative liability, non-current |
|
23,160 |
|
|
8,120 |
|
| Other long-term liabilities |
|
117 |
|
|
350 |
|
| Total liabilities |
|
33,553 |
|
|
17,700 |
|
| Mezzanine equity: |
|
|
|
|
Series D Preferred Stock—$0.001 par value; 1 share of Series D Preferred Stock authorized at September 30, 2025 and December 31, 2024; 1 share of Series D Preferred Stock issued and outstanding at September 30, 2025 and December 31, 2024 |
|
— |
|
|
— |
|
Series E Preferred Stock—$0.001 par value; 1 share of Series E Preferred Stock authorized at September 30, 2025 and December 31, 2024; 1 share of Series E Preferred Stock issued and outstanding at September 30, 2025 and December 31, 2024 |
|
— |
|
|
— |
|
| Stockholders’ equity: |
|
|
|
|
Common stock—$0.001 par value; 200,000,000 shares authorized at September 30, 2025 and December 31, 2024; 17,827,635 and 10,471,934 shares issued and outstanding at September 30, 2025 and December 31, 2024, respectively |
|
18 |
|
|
10 |
|
Series C Preferred Stock—$0.001 par value; 34,326 shares of Series C Preferred Stock authorized at September 30, 2025 and December 31, 2024; 19,364 and 24,896 shares of Series C Preferred Stock issued and outstanding at September 30, 2025 and December 31, 2024, respectively |
|
— |
|
|
— |
|
| Additional paid-in capital |
|
526,290 |
|
|
503,285 |
|
| Accumulated other comprehensive income |
|
41 |
|
|
— |
|
| Accumulated deficit |
|
(434,802) |
|
|
(370,263) |
|
| Total stockholders’ equity |
|
91,547 |
|
|
133,032 |
|
| Total liabilities, mezzanine equity and stockholders’ equity |
|
$ |
125,100 |
|
|
$ |
150,732 |
|
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Statements of Operations and Comprehensive (Loss) Income (Unaudited)
(In thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
Nine Months Ended |
| |
|
September 30, |
|
September 30, |
| |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
| Revenues: |
|
|
|
|
|
|
|
|
| Product revenue, net |
|
$ |
— |
|
|
$ |
249 |
|
|
$ |
— |
|
|
$ |
249 |
|
|
|
|
|
|
|
|
|
|
| Total revenues, net |
|
— |
|
|
249 |
|
|
— |
|
|
249 |
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
| Cost of product sales |
|
$ |
— |
|
|
$ |
(714) |
|
|
$ |
— |
|
|
$ |
(453) |
|
| Research and development |
|
13,621 |
|
|
9,538 |
|
|
36,817 |
|
|
16,254 |
|
| General and administrative |
|
5,577 |
|
|
4,286 |
|
|
16,366 |
|
|
12,008 |
|
| Acquired in-process research and development |
|
— |
|
|
— |
|
|
— |
|
|
27,641 |
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
19,198 |
|
|
13,110 |
|
|
53,183 |
|
|
55,450 |
|
| Loss from operations |
|
(19,198) |
|
|
(12,861) |
|
|
(53,183) |
|
|
(55,201) |
|
| Other (expense) income: |
|
|
|
|
|
|
|
|
| Change in fair value of derivative liability |
|
(12,530) |
|
|
(1,100) |
|
|
(14,680) |
|
|
(6,260) |
|
| Interest income, net |
|
1,117 |
|
|
964 |
|
|
3,367 |
|
|
2,101 |
|
| Excess of initial warrant fair value over private placement proceeds |
|
— |
|
|
— |
|
|
— |
|
|
(79,276) |
|
| Change in fair value of warrant liability |
|
— |
|
|
36,025 |
|
|
— |
|
|
148,071 |
|
| Private placement transaction costs |
|
— |
|
|
— |
|
|
— |
|
|
(9,220) |
|
| Other expense, net |
|
(3) |
|
|
(5) |
|
|
(8) |
|
|
(5) |
|
| Total other (expense) income, net |
|
(11,416) |
|
|
35,884 |
|
|
(11,321) |
|
|
55,411 |
|
| (Loss) income before taxes |
|
(30,614) |
|
|
23,023 |
|
|
(64,504) |
|
|
210 |
|
| Income tax expense (benefit) |
|
11 |
|
|
(14) |
|
|
35 |
|
|
— |
|
Net (loss) income |
|
$ |
(30,625) |
|
|
$ |
23,037 |
|
|
$ |
(64,539) |
|
|
$ |
210 |
|
|
|
|
|
|
|
|
|
|
Net (loss) income per share of common stock - basic |
|
$ |
(2.19) |
|
|
$ |
0.98 |
|
|
$ |
(5.47) |
|
|
$ |
0.01 |
|
| Net loss per share of common stock - diluted |
|
$ |
(2.19) |
|
|
$ |
(2.83) |
|
|
$ |
(5.47) |
|
|
$ |
(22.63) |
|
Weighted average common shares outstanding - basic |
|
14,000,451 |
|
|
5,546,257 |
|
|
11,795,810 |
|
|
2,491,114 |
|
Weighted average common shares outstanding - diluted |
|
14,000,451 |
|
|
10,784,037 |
|
|
11,795,810 |
|
|
6,540,963 |
|
Comprehensive (loss) income: |
|
|
|
|
|
|
|
|
Net (loss) income |
|
$ |
(30,625) |
|
|
$ |
23,037 |
|
|
$ |
(64,539) |
|
|
$ |
210 |
|
Other comprehensive loss: |
|
|
|
|
|
|
|
|
| Unrealized income on investments, net |
|
75 |
|
|
— |
|
|
41 |
|
|
— |
|
Comprehensive (loss) income |
|
$ |
(30,550) |
|
|
$ |
23,037 |
|
|
$ |
(64,498) |
|
|
$ |
210 |
|
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Statements of Mezzanine and Stockholders’ Equity (Unaudited)
(In thousands, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Mezzanine preferred stock |
|
|
Common stock |
|
Series C preferred stock |
|
|
|
Additional paid-in |
|
Accumulated other |
|
Accumulated |
Total stockholders’ |
| |
Shares |
Amount |
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
|
|
|
|
capital |
|
comprehensive income |
|
deficit |
|
equity |
Three Months Ended September 30, 2025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, June 30, 2025 |
2 |
|
$ |
— |
|
|
|
10,837,356 |
|
|
$ |
11 |
|
|
24,696 |
|
|
$ |
— |
|
|
|
|
|
|
$ |
508,774 |
|
|
$ |
(34) |
|
|
$ |
(404,177) |
|
|
$ |
104,574 |
|
| Issuance of common stock in exchange for retirement of Series C Preferred Stock |
— |
|
— |
|
|
|
5,331,946 |
|
|
5 |
|
|
(5,332) |
|
|
— |
|
|
|
|
|
|
(5) |
|
|
— |
|
|
— |
|
|
— |
|
| Issuance of common stock pursuant to ATM Program, net |
— |
|
— |
|
|
|
1,658,333 |
|
|
2 |
|
|
— |
|
|
— |
|
|
|
|
|
|
14,368 |
|
|
— |
|
|
— |
|
|
14,370 |
|
| Unrealized gain on investments, net |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
75 |
|
|
— |
|
|
75 |
|
| Stock-based compensation |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
3,153 |
|
|
— |
|
|
— |
|
|
3,153 |
|
| Net loss |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
(30,625) |
|
|
(30,625) |
|
| Balance, September 30, 2025 |
2 |
|
$ |
— |
|
|
|
17,827,635 |
|
|
$ |
18 |
|
|
19,364 |
|
|
$ |
— |
|
|
|
|
|
|
$ |
526,290 |
|
|
$ |
41 |
|
|
$ |
(434,802) |
|
|
$ |
91,547 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Mezzanine preferred stock |
|
|
Common stock |
|
Series C preferred stock |
|
|
|
Additional paid-in |
|
Accumulated other |
|
Accumulated |
Total stockholders’ |
| |
Shares |
Amount |
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
|
|
|
|
capital |
|
comprehensive income |
|
deficit |
|
equity |
Nine Months Ended September 30, 2025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2024 |
2 |
|
$ |
— |
|
|
|
10,471,934 |
|
|
$ |
10 |
|
|
24,896 |
|
|
$ |
— |
|
|
|
|
|
|
$ |
503,285 |
|
|
$ |
— |
|
|
$ |
(370,263) |
|
|
$ |
133,032 |
|
Issuance of common stock in exchange for retirement of Series C Preferred Sock |
— |
|
— |
|
|
|
5,531,946 |
|
|
6 |
|
|
(5,532) |
|
|
— |
|
|
|
|
|
|
(6) |
|
|
— |
|
|
— |
|
|
— |
|
| Issuance of common stock pursuant to ATM Program, net |
— |
|
— |
|
|
|
1,658,333 |
|
|
2 |
|
|
— |
|
|
— |
|
|
|
|
|
|
14,368 |
|
|
— |
|
|
— |
|
|
14,370 |
|
Vesting of Restricted Stock Units net of shares withheld for taxes |
— |
|
— |
|
|
|
155,686 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
(510) |
|
|
— |
|
|
— |
|
|
(510) |
|
Shares purchased through employee stock purchase plan |
— |
|
— |
|
|
|
9,736 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
40 |
|
|
— |
|
|
— |
|
|
40 |
|
| Unrealized gain on investments, net |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
41 |
|
|
— |
|
|
41 |
|
| Stock-based compensation |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
9,113 |
|
|
— |
|
|
— |
|
|
9,113 |
|
Net loss |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
(64,539) |
|
|
(64,539) |
|
| Balance, September 30, 2025 |
2 |
|
$ |
— |
|
|
|
17,827,635 |
|
|
$ |
18 |
|
|
19,364 |
|
|
$ |
— |
|
|
|
|
|
|
$ |
526,290 |
|
|
$ |
41 |
|
|
$ |
(434,802) |
|
|
$ |
91,547 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Mezzanine preferred stock |
|
|
Common stock |
|
|
|
Series C preferred stock |
|
Additional paid-in |
|
Accumulated |
Total stockholders’ |
|
|
| |
Shares |
Amount |
|
|
Shares |
|
Amount |
|
|
|
|
|
Shares |
|
Amount |
|
capital |
|
deficit |
|
equity |
|
|
| Three Months Ended September 30, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, June 30, 2024 |
22,360 |
|
$ |
11,457 |
|
|
|
1,034,130 |
|
|
$ |
1 |
|
|
|
|
|
|
— |
|
|
$ |
— |
|
|
$ |
344,352 |
|
|
$ |
(357,961) |
|
|
$ |
(13,608) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Retirement of Series C Preferred Stock in exchange for issuance of common stock |
(8,648) |
|
(9,799) |
|
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
| Issuance of common stock in exchange for retirement of Series C Preferred Stock |
— |
|
— |
|
|
|
8,648,244 |
|
|
9 |
|
|
|
|
|
|
— |
|
|
— |
|
|
9,790 |
|
|
— |
|
|
9,799 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
1,848 |
|
|
— |
|
|
1,848 |
|
|
|
| Net income |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
23,037 |
|
|
23,037 |
|
|
|
| Balance, September 30, 2024 |
13,712 |
|
$ |
1,658 |
|
|
|
9,682,374 |
|
|
$ |
10 |
|
|
|
|
|
|
— |
|
|
$ |
— |
|
|
$ |
355,990 |
|
|
$ |
(334,924) |
|
|
$ |
21,076 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Mezzanine preferred stock |
|
|
Common stock |
|
Series C preferred stock |
|
Additional paid-in |
|
Accumulated |
Total stockholders’ |
| |
Shares |
Amount |
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
capital |
|
deficit |
|
equity |
Nine Months Ended September 30, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2023 |
— |
|
$ |
— |
|
|
|
801,746 |
|
|
$ |
1 |
|
|
— |
|
|
$ |
— |
|
|
$ |
342,437 |
|
|
$ |
(335,134) |
|
|
$ |
7,304 |
|
Impact of reverse split fractional share round-up |
— |
|
— |
|
|
|
60,779 |
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
Issuance of common stock pursuant to AlmataBio Transaction |
— |
|
— |
|
|
|
171,605 |
|
|
— |
|
|
— |
|
|
— |
|
|
815 |
|
|
— |
|
|
815 |
|
Issuance of Series C Preferred Stock pursuant to AlmataBio Transaction |
2,412 |
|
11,457 |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Issuance of Series C Preferred Stock in private placement |
19,946 |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Issuance of Series D Preferred Stock in private placement |
1 |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Issuance of Series E Preferred Stock in private placement |
1 |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Retirement of Series C Preferred Stock in exchange
for issuance of common stock |
(8,648) |
|
(9,799) |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Issuance of common stock in exchange for
retirement of Series C Preferred Stock |
— |
|
— |
|
|
|
8,648,244 |
|
|
9 |
|
|
— |
|
|
— |
|
|
9,790 |
|
|
— |
|
|
9,799 |
|
| Stock-based compensation |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
2,948 |
|
|
— |
|
|
2,948 |
|
| Net income |
— |
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
210 |
|
|
210 |
|
Balance, September 30, 2024 |
13,712 |
|
$ |
1,658 |
|
|
|
9,682,374 |
|
|
$ |
10 |
|
|
— |
|
|
$ |
— |
|
|
$ |
355,990 |
|
|
$ |
(334,924) |
|
|
$ |
21,076 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Statements of Cash Flows (Unaudited)
(Amounts in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
| |
|
2025 |
|
2024 |
| Operating activities |
|
|
|
|
| Net (loss) income |
|
$ |
(64,539) |
|
|
$ |
210 |
|
| Adjustments to reconcile net loss used in operating activities: |
|
|
|
|
| Depreciation and amortization |
|
328 |
|
|
101 |
|
| Stock-based compensation |
|
9,113 |
|
|
2,948 |
|
Accretion of available-for-sale investments, net |
|
(493) |
|
|
— |
|
| Acquired in-process research and development |
|
— |
|
|
27,641 |
|
| Excess of initial warrant fair value over private placement proceeds |
|
— |
|
|
79,276 |
|
| Change in fair value of warrant liability |
|
— |
|
|
(148,071) |
|
| Transaction costs paid pursuant to private placement |
|
— |
|
|
7,485 |
|
Contingent consideration paid pursuant to AlmataBio Transaction |
|
— |
|
|
(7,500) |
|
|
|
|
|
|
| Transaction costs payable upon exercise of warrants issued in private placement |
|
— |
|
|
1,734 |
|
|
|
|
|
|
| Change in fair value of derivative liability |
|
14,680 |
|
|
6,260 |
|
|
|
|
|
|
| Deferred taxes |
|
34 |
|
|
— |
|
| Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Prepaid expenses and other current assets |
|
2,186 |
|
|
(3,270) |
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable |
|
191 |
|
|
1,365 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued expenses and other current liabilities |
|
1,312 |
|
|
(2,110) |
|
| Lease liability, net |
|
(24) |
|
|
(81) |
|
| Net cash used in operating activities |
|
(37,212) |
|
|
(34,012) |
|
| Investing activities |
|
|
|
|
Purchases of investments |
|
(94,621) |
|
|
— |
|
| Maturities of investments |
|
10,500 |
|
|
— |
|
Cash assumed from AlmataBio Transaction |
|
— |
|
|
356 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities |
|
(84,121) |
|
|
356 |
|
| Financing activities |
|
|
|
|
| Proceeds from issuance of common stock pursuant to ATM Program, net |
|
14,370 |
|
|
— |
|
| Cash paid related to withholding shares to satisfy RSU tax withholding obligations |
|
(510) |
|
|
— |
|
| Proceeds from issuance of common stock under employee stock purchase plan |
|
40 |
|
|
— |
|
| Proceeds from private placement investment, gross |
|
— |
|
|
115,625 |
|
| Transaction costs paid pursuant to private placement |
|
— |
|
|
(7,485) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash provided by financing activities |
|
13,900 |
|
|
108,140 |
|
(Decrease) increase in cash, cash equivalents and restricted cash |
|
(107,433) |
|
|
74,484 |
|
| Cash, cash equivalents, and restricted cash at beginning of period |
|
134,696 |
|
|
7,546 |
|
| Cash, cash equivalents, and restricted cash at end of period |
|
$ |
27,263 |
|
|
$ |
82,030 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental disclosures of non-cash activities |
|
|
|
|
|
|
|
|
|
Issuance of common stock and Series C Preferred Stock pursuant to AlmataBio Transaction |
|
$ |
— |
|
|
$ |
12,272 |
|
|
|
|
|
|
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the condensed consolidated balance sheets that sum to the total of the same such amounts shown in the condensed consolidated statements of cash flows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, |
|
|
2025 |
|
2024 |
| Cash and cash equivalents |
|
$ |
26,963 |
|
|
$ |
81,858 |
|
| Restricted cash, current |
|
90 |
|
|
41 |
|
| Restricted cash, non-current |
|
210 |
|
|
131 |
|
| Total cash, cash equivalents and restricted cash |
|
$ |
27,263 |
|
|
$ |
82,030 |
|
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Notes to Unaudited Condensed Consolidated Financial Statements
1. Business
Avalo Therapeutics, Inc. (the “Company,” “Avalo” or “we”) is a clinical stage biotechnology company fully dedicated to developing IL-1β-based treatments for immune-mediated inflammatory diseases. Our lead asset, AVTX-009, is in a Phase 2 clinical trial for hidradenitis suppurativa (“HS”). The Company is also exploring additional opportunities to make an impact in prevalent indications that have significant remaining unmet needs.
Avalo was incorporated in Delaware and commenced operation in 2011, and completed its initial public offering in October 2015.
Liquidity
Since inception, we have incurred significant operating and cash losses from operations. We have primarily funded our operations to date through sales of equity securities, out-licensing transactions and sales of assets.
For the nine months ended September 30, 2025, Avalo generated a net loss of $64.5 million and negative cash flows from operations of $37.2 million. As of September 30, 2025, Avalo had $111.6 million in cash and cash equivalents and short-term investments.
In accordance with Accounting Standards Codification Topic 205-40, Presentation of Financial Statements - Going Concern, the Company evaluated its ability to continue as a going concern within one year after the date that the accompanying condensed consolidated financial statements are issued. Based on our current operating plans, we expect that our existing cash and cash equivalents and short-term investments are sufficient to fund operations for at least twelve months from the filing date of this Quarterly Report on Form 10-Q. The Company closely monitors its cash and cash equivalents and short-term investments and seeks to balance the level of cash and cash equivalents with our projected needs to allow us to withstand periods of uncertainty relative to the availability of funding on favorable terms. We may satisfy any future cash needs through sales of equity securities under the Company’s at-the-market program or other equity financings, out-licensing transactions, strategic alliances/collaborations, sale of programs, and/or mergers and acquisitions. There can be no assurance that any financing or business development initiatives can be realized by the Company, or if realized, what the terms may be. To the extent that we raise capital through the sale of equity, the ownership interest of our existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Further, if the Company raises additional funds through collaborations, strategic alliances or licensing arrangements with third parties, the Company might have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates.
2. Basis of Presentation and Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (the “FASB”). The unaudited condensed consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets, and the satisfaction of liabilities in the ordinary course of business.
In the opinion of management, the accompanying unaudited condensed consolidated financial statements include all adjustments, consisting of normal recurring adjustments, which are necessary to present fairly the Company’s financial position, results of operations, and cash flows. The condensed consolidated balance sheet at December 31, 2024 has been derived from audited financial statements at that date. The interim results of operations are not necessarily indicative of the results that may occur for the full fiscal year. Certain information and footnote disclosure normally included in the financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to instructions, rules, and regulations prescribed by the United States Securities and Exchange Commission (“SEC”).
The Company believes that the disclosures provided herein are adequate to make the information presented not misleading when these unaudited condensed consolidated financial statements are read in conjunction with the December 31, 2024 audited consolidated financial statements.
In the first quarter of 2025, the Company concluded that it would include other receivables within the prepaid and other current assets line in the Company’s unaudited condensed consolidated balance sheets and statement of cash flows. The Company reclassified $0.6 million from other receivables to prepaid and current assets as of December 31, 2024 within the unaudited condensed consolidated balance sheets and $0.9 million from other receivables to prepaid and other current assets for the nine months ended September 30, 2024 within the unaudited statement of cash flows, to conform with the current period presentation.
Unless otherwise indicated, all amounts in the following tables are in thousands except share and per share amounts.
Significant Accounting Policies
During the nine months ended September 30, 2025, there were no significant changes to the Company’s summary of significant accounting policies contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, as filed with the SEC on March 20, 2025, except for the policies as described below.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or fewer when purchased to be cash equivalents. These assets include investments in money market funds and U.S. Treasury securities. Cash equivalents are reflected at fair value, as further described in Note 6 - Fair Value Measurements.
Concentration of Credit Risk
The primary financial instruments that subject the Company to concentrated credit risk include cash, cash equivalents, and short-term investments. The Company maintains its cash, cash equivalents, and short-term investments with high-quality financial institutions and, consequently, the Company believes that such funds are subject to minimal credit risk. The Company’s cash equivalents consist of money market funds, which are invested in U.S. Treasury and government agency obligations, and the Company’s short-term investments consist of U.S. Treasury securities. Credit risk in these securities is reduced as a result of the Company’s investment policy to make high credit quality investments with its cash and cash equivalents. The Company’s investment policy limits investment instruments to investment-grade securities with the objective to preserve capital and to maintain liquidity until the funds can be used in business operations.
Investments
The Company generally invests its excess cash in money market funds and marketable debt securities. Such investments are included in either cash and cash equivalents (if the original maturity, from the date of purchase, is 90 days or fewer) or short-term investments (if the original maturity, from the date of purchase, is in excess of 90 days and less than 1 year) on the unaudited condensed consolidated balance sheet, as they represent the investment of funds readily convertible to cash to fund current operations. The Company classifies its investments as either trading, held-to-maturity or available-for-sale based on facts and circumstances present at the time it purchases the securities. As of September 30, 2025, all of our investments were classified as available-for-sale, which are reported at fair value at each balance sheet date, and for which fair value measurement data is obtained from independent pricing services. For securities with unrealized holding gains and losses (the adjustments to fair value), when the Company expects to receive cash flows sufficient to recover the amortized cost basis of a security, such gains and losses are included in “Accumulated other comprehensive income (loss)” as a component of stockholders’ equity. The Company identifies credit losses when it does not expect to receive cash flows sufficient to recover the amortized cost basis of a security. On a quarterly basis, the Company evaluates whether decreases in the fair values of its investments are below their amortized cost, and if so, it marks the investment to market through a charge to our unaudited condensed consolidated statements of operations and comprehensive (loss) income. Realized gains and losses, if any, are included in interest income, net on the unaudited condensed consolidated statements of operations and comprehensive (loss) income. The amortized costs of investments are adjusted for amortization of premiums and accretion of discounts to maturity, which is included in interest income, net on the unaudited condensed consolidated statements of operations and comprehensive (loss) income.
Recently Issued Accounting Pronouncements Not Yet Adopted
In September 2025, the FASB issued ASU 2025-07, Derivatives and Hedging (Topic 815) and Revenue from Contracts with Customers (Topics 606): Derivatives Scope Refinements and Scope Clarification for Share-based Noncash Consideration from a Customer in a Revenue Contract (“ASU 2025-07”), which, among other items, amends the application of derivative accounting to contracts with features based on the operations or activities of one of the parties to the contract. The amendments are expected to (a) reduce the cost and complexity of evaluating whether contracts with features based on the operations or activities of one of the parties to the contract are derivatives, (b) better portray the economics of those contracts in the financial statements, and (c) reduce diversity in practice resulting from the broad application of the current guidance and changing business environment. The standard is effective for fiscal years beginning after December 15, 2026, with early adoption permitted. The Company is currently evaluating the impact of adopting ASU 2025-07.
In November 2024, the FASB issued ASU 2024-03, Disaggregation of Income Statement Expenses, which requires disaggregation and disclosure of specified information about certain costs and expenses in the notes to the financial statements. The standard is effective for fiscal years beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027, with early adoption permitted. The Company is currently evaluating the impact of adopting ASU 2024-03.
In December 2023, the FASB issued ASU 2023-09, Income taxes (Topic 740): Improvements to Income Tax Disclosures, which amends guidance to enhance the transparency and decision usefulness of income tax disclosures. It is effective for fiscal years beginning after December 15, 2024. The Company is required to adopt this standard in its Form 10-K for the year ended December 31, 2025, and does not expect it to have a material impact on its consolidated financial statements and related disclosures.
3. Asset Acquisition
AlmataBio Transaction
On March 27, 2024, the Company acquired AVTX-009, an anti-IL-1β mAb, through a merger of AlmataBio, Inc. (“AlmataBio”) with and into its wholly owned subsidiary (the “AlmataBio Transaction”). The Company’s acquisition of AlmataBio was structured as a stock-for-stock transaction whereby all outstanding equity interests in AlmataBio were exchanged in a merger for a combination of the Company’s common stock and shares of the Company’s non-voting convertible preferred stock (the “Series C Preferred Stock”), resulting in the issuance of 171,605 shares of Company common stock and 2,412 shares of Series C Preferred Stock. Upon Company stockholder approval on August 13, 2024 and subject to beneficial ownership limitations, 2,063 shares of Series C Preferred Stock issued to former AlmataBio stockholders automatically converted into 2,062,930 shares of common stock.
In addition to the shares issued, a cash payment of $7.5 million was due to the former AlmataBio stockholders upon the closing of a private placement. The private placement closed on March 28, 2024 and the Company paid the $7.5 million in April 2024. The Company is also required to pay potential development milestone payments to the former AlmataBio stockholders, including $5.0 million due upon the first patient dosed in a Phase 2 trial in patients with HS for AVTX-009, and $15.0 million due upon the first patient dosed in a Phase 3 trial for AVTX-009, both of which are payable in cash or Avalo stock at the election of the former AlmataBio stockholders, subject to the terms and conditions of the definitive merger agreement. In October 2024, the first development milestone was met and the Company paid the $5.0 million cash payment.
The Company was the acquiring company for accounting purposes. In connection with the AlmataBio Transaction, substantially all of the consideration paid is allocable to the fair value of acquired in-process research and development (“IPR&D”), specifically AVTX-009, and as such the acquisition is treated as an asset acquisition. The Company initially recognized AlmataBio’s assets and liabilities by allocating the accumulated cost of the acquisition based on their relative fair values, as estimated by management. The net assets acquired as of the transaction date have been combined with the assets, liabilities, and results of operations of the Company on consummation of the AlmataBio Transaction. In accordance with ASC 730, Research and Development, the portion of the consideration allocated to the acquired IPR&D, specifically AVTX-009, based on its relative fair value, is included as an operating expense as there is no alternative future use.
Below is a summary of the total consideration, assets acquired and the liabilities assumed in connection with the AlmataBio Transaction (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, 2024 |
Stock consideration1 |
|
$ |
12,272 |
|
|
|
Milestone payment due upon close of private placement investment2 |
|
7,500 |
|
|
|
Milestone payment due upon first patient dosed in a Phase 2 trial2 |
|
5,000 |
|
|
|
| Transaction costs |
|
2,402 |
|
|
|
| Total GAAP Purchase Price at Close |
|
$ |
27,174 |
|
|
|
|
|
|
|
|
| Acquired IPR&D |
|
$ |
27,641 |
|
|
|
| Cash |
|
356 |
|
|
|
| Accrued expenses and other current liabilities |
|
(823) |
|
|
|
| Total net assets acquired and liabilities assumed |
|
$ |
27,174 |
|
|
|
1 Equal to the aggregate common stock issued of 171,605 and the aggregate shares of Series C Preferred Stock issued of 2,412 (as-convertible to 2,412,000 shares of common stock), multiplied by the Company’s closing stock price of $4.75 on March 27, 2024. On August 13, 2024 upon Company stockholder approval and subject to beneficial ownership limitations, 2,063 of the 2,412 shares of Series C Preferred Stock were converted into 2,062,930 shares of common stock.
2 Avalo deemed these milestones probable and estimable as of the transaction close date and therefore included them as part of the GAAP purchase price at close. The milestone payment due upon the close of the private placement was paid in April 2024. The milestone payment due upon the first patient dosed in a Phase 2 trial was paid in October 2024.
4. Revenue
The Company’s license and supply agreement for Millipred®, an oral prednisolone indicated across a wide variety of inflammatory conditions, expired, as planned, on September 30, 2023. Avalo considered Millipred® a non-core asset. There was no gross revenue recognized from sales of prescription drugs for the three and nine months ended September 30, 2025 or September 30, 2024. Historically, the Company sold Millipred® in the United States primarily through wholesale distributors, who accounted for substantially all of the Company’s net product revenues and trade receivables. The Company continues to monitor estimates for commercial liabilities for Millipred®, such as sales returns. As additional information becomes available, the Company could recognize expense (or a benefit) for differences between actuals or updated estimates to the reserves previously recognized.
Pursuant to the Millipred® license and supply agreement, Avalo was required to pay the supplier fifty percent of the net profit of the Millipred® product following each calendar quarter, with a $0.5 million quarterly minimum payment contingent on Avalo achieving certain net profit thresholds as stipulated in the agreement. The profit share commenced on July 1, 2021 and ended on September 30, 2023. The net profit share is subject to a reconciliation process, where estimated deductions to arrive at net profit will be reconciled to actuals, which might result in Avalo owing additional amounts to the supplier or vice versa, which would be recognized in cost of product sales.
5. Net (Loss) Income Per Share
The Company had two classes of stock outstanding during the three and nine months ended September 30, 2025 and September 30, 2024, common stock and preferred stock. The Company computes net (loss) income per share using the two-class method, as the Series C Preferred Stock participates in distributions with the Company’s common stock. The two-class method of computing net (loss) income per share is an earnings allocation formula that determines net loss for common stock and any participating securities according to dividends declared and participation rights in undistributed earnings. As the Company is in a net loss position for the three and nine months ended September 30, 2025, the two-class method of calculating net loss per share results in no allocation of undistributed losses to participating securities. For the three and nine months ended September 30, 2024, the two class method of calculating net income per share resulted in an allocation of a portion of the net income to participating securities because the Company had net income for the period.
Basic net (loss) income per share for common stock is computed by dividing the sum of distributed and undistributed earnings by the weighted average number of shares outstanding for the period.
Diluted net loss per share includes the potential dilutive effect of common stock equivalents as if such securities were converted or exercised during the period, when the effect is dilutive. Common stock equivalents include: (i) outstanding stock options and restricted stock units which are included under the “treasury stock method” when dilutive; (ii) common stock to be issued upon the exercise of outstanding warrants, which are included under the “treasury stock method” when dilutive, and (iii) preferred stock under the if-converted method. Although the impact of these items is generally anti-dilutive during periods of net loss, the Company will determine whether the common stock equivalents should be included in diluted loss per share pursuant to sequencing rules.
The following tables set forth the computation of basic and diluted net (loss) income per share of common stock for the three and nine months ended September 30, 2025 and September 30, 2024 (in thousands, except share and per share amounts):
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, 2025 |
|
|
| |
|
Common stock |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(30,625) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average shares |
|
14,000,451 |
|
|
|
Basic and diluted net loss per share |
|
$ |
(2.19) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, 2025 |
| |
|
Common stock |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(64,539) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average shares |
|
11,795,810 |
|
|
|
Basic and diluted net loss per share |
|
$ |
(5.47) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, 2024 |
| |
|
Common stock |
|
|
|
Basic income per share: |
|
|
Net income |
|
$ |
23,037 |
|
Net income attributed to Series C Preferred Stock |
|
(17,575) |
|
Net income - basic |
|
5,462 |
|
| Weighted average shares |
|
5,546,257 |
|
Basic net income per share |
|
$ |
0.98 |
|
|
|
|
Diluted loss per share: |
|
|
Numerator: |
|
|
Net income - basic |
|
$ |
5,462 |
|
Change in fair value of warrant liability |
|
(36,025) |
|
Net loss - diluted |
|
$ |
(30,563) |
|
Denominator: |
|
|
Effect of dilutive securities: |
|
|
Weighted average shares - basic |
|
5,546,257 |
Common shares issuable for warrants |
|
5,237,780 |
Weighted average shares - diluted |
|
10,784,037 |
Diluted net loss per share |
|
$ |
(2.83) |
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, 2024 |
| |
|
Common stock |
|
|
|
| Basic income loss per share: |
|
|
| Net income |
|
$ |
210 |
|
| Net income attributed to Series C preferred Stock |
|
(177) |
|
| Net income - basic |
|
33 |
|
| Weighted average shares |
|
2,491,114 |
|
Basic net loss per share |
|
$ |
0.01 |
|
|
|
|
Diluted loss per share: |
|
|
Numerator: |
|
|
| Net income - basic |
|
$ |
33 |
|
Change in fair value of warrant liability |
|
(148,071) |
|
Net loss - diluted |
|
$ |
(148,038) |
|
Denominator: |
|
|
Effect of dilutive securities: |
|
|
Weighted average shares - basic |
|
2,491,114 |
|
| Common shares issuable for warrants |
|
4,049,849 |
|
Weighted average shares - diluted |
|
6,540,963 |
|
Diluted net loss per share |
|
$ |
(22.63) |
|
The following outstanding securities have been excluded from the computation of diluted weighted shares outstanding for the three and nine months ended September 30, 2025 and 2024, as they could have been anti-dilutive:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three and Nine Months Ended |
|
|
|
|
September 30, |
|
|
| |
|
2025(2) |
|
2024(2) |
|
|
|
|
| Stock options |
|
4,138,621 |
|
2,000,056 |
|
|
|
|
Warrants on common stock |
|
148 |
|
148 |
|
|
|
|
Series C Preferred Stock (as-convertible to common stock)(1) |
|
19,363,974 |
|
13,709,653 |
|
|
|
|
| Restricted Stock Units |
|
421,397 |
|
632,100 |
|
|
|
|
Performance Stock Units(3) |
|
492,500 |
|
— |
|
|
|
|
1 Each share of the Company’s Series C Preferred Stock is convertible to 1,000 shares of common stock, subject to certain beneficial ownership limitations.
2 Pursuant to the AlmataBio Transaction, the Company is required to pay potential development milestone payments to the former AlmataBio stockholders in cash or Avalo stock at the election of the former AlmataBio stockholders; refer to Notes 3 and 10 for more information. In the event of a settlement in shares, the number of Avalo shares delivered will vary based on the Company’s stock price. These additional shares are not included in the computation of basic and diluted net (loss) income per share for the three and nine months ended September 30, 2025 and 2024 pursuant to the guidance on contingently issuable shares.
3 Calculated assuming 100% achievement of performance metric.
6. Fair Value Measurements
ASC 820, Fair Value Measurements and Disclosures (“ASC 820”) defines fair value as the price that would be received to sell an asset, or paid to transfer a liability, in the principal or most advantageous market in an orderly transaction between market participants on the measurement date. The fair value standard also establishes a three‑level hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability on the measurement date. The three levels are defined as follows:
•Level 1—inputs to the valuation methodology are quoted prices (unadjusted) for an identical asset or liability in an active market.
•Level 2—inputs to the valuation methodology include quoted prices for a similar asset or liability in an active market or model‑derived valuations in which all significant inputs are observable for substantially the full term of the asset or liability.
•Level 3—inputs to the valuation methodology are unobservable and significant to the fair value measurement of the asset or liability.
The Company measures the fair value of money market funds based on quoted prices in active markets for identical securities. Investments also include U.S. Treasury securities, which are valued either based on recent trades of securities in inactive markets or based on quoted market prices of similar instruments and other significant inputs derived from, or corroborated by, observable market data.
The following table presents, for each of the fair value hierarchy levels required under ASC 820, the Company’s assets and liabilities that are measured at fair value on a recurring basis (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
September 30, 2025 |
|
|
| |
|
Fair Value Measurements Using |
|
|
| |
|
Quoted prices in active markets for identical assets |
|
Significant other observable inputs |
|
Significant unobservable inputs |
|
|
| |
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
Total |
| Assets |
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
18,786 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
18,786 |
|
U.S. Treasury securities |
|
— |
|
|
1,246 |
|
|
— |
|
|
1,246 |
|
Marketable debt securities: |
|
|
|
|
|
|
|
|
U.S. Treasury securities |
|
— |
|
|
84,654 |
|
|
|
|
84,654 |
|
Total financial assets |
|
$ |
18,786 |
|
|
$ |
85,900 |
|
|
$ |
— |
|
|
$ |
104,686 |
|
| Liabilities |
|
|
|
|
|
|
|
|
| Derivative liability |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
23,160 |
|
|
$ |
23,160 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, 2024 |
|
|
| |
|
Fair Value Measurements Using |
|
|
| |
|
Quoted prices in active markets for identical assets |
|
Significant other observable inputs |
|
Significant unobservable inputs |
|
|
| |
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
Total |
| Assets |
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
133,148 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
133,148 |
|
Total financial assets |
|
133,148 |
|
|
— |
|
|
— |
|
|
133,148 |
|
| Liabilities |
|
|
|
|
|
|
|
|
| Derivative liability |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
8,480 |
|
|
$ |
8,480 |
|
The carrying amounts reported in the accompanying unaudited condensed consolidated financial statements for cash, restricted cash, prepaid and other current assets, accounts payable, and accrued expenses and other current liabilities approximate their respective fair values because of the short-term nature of these accounts.
Level 3 Valuation
The table below summarizes changes in the fair value of the Company’s Level 3 valuations for the three and nine months ended September 30, 2025 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Derivative liability |
|
Total |
Balance at June 30, 2025 |
|
|
|
$ |
10,630 |
|
|
$ |
10,630 |
|
| Change in fair value |
|
|
|
12,530 |
|
|
12,530 |
|
Balance at September 30, 2025 |
|
|
|
$ |
23,160 |
|
|
$ |
23,160 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Derivative liability |
|
Total |
Balance at December 31, 2024 |
|
|
|
$ |
8,480 |
|
|
$ |
8,480 |
|
|
|
|
|
|
|
|
| Change in fair value |
|
|
|
14,680 |
|
|
14,680 |
|
Balance at September 30, 2025 |
|
|
|
$ |
23,160 |
|
|
$ |
23,160 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Warrant liability |
|
Derivative liability |
|
Total |
Balance at June 30, 2024 |
|
$ |
82,855 |
|
|
$ |
10,710 |
|
|
$ |
93,565 |
|
|
|
|
|
|
|
|
| Change in fair value |
|
(36,025) |
|
|
1,100 |
|
|
(34,925) |
|
Balance at September 30, 2024 |
|
$ |
46,830 |
|
|
$ |
11,810 |
|
|
$ |
58,640 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Warrant liability |
|
Derivative liability |
|
Total |
Balance at December 31, 2023 |
|
$ |
— |
|
|
$ |
5,550 |
|
|
$ |
5,550 |
|
| Initial valuation of warrant liability |
|
194,901 |
|
|
— |
|
|
194,901 |
|
| Change in fair value |
|
(148,071) |
|
|
6,260 |
|
|
(141,811) |
|
Balance at September 30, 2024 |
|
$ |
46,830 |
|
|
$ |
11,810 |
|
|
$ |
58,640 |
|
Derivative liability
In the fourth quarter of 2022, Avalo sold its economic rights to future milestone and royalty payments for previously out-licensed assets AVTX-501, AVTX-007, and AVTX-611 to ES Therapeutics, LLC (“ES”), an affiliate of Armistice Capital LLC (“Armistice”), in exchange for $5.0 million (the “ES Transaction”). At the time of the transaction, Armistice was a significant stockholder of the Company whose chief investment officer, Steven Boyd, and managing director, Keith Maher, served on Avalo’s Board until August 8, 2022. The ES Transaction was approved in accordance with Avalo’s related party transaction policy.
The economic rights sold include (a) rights to a milestone payment of $20.0 million upon the filing and acceptance of an NDA for AVTX-501 pursuant to an agreement with Janssen Pharmaceutics, Inc., now Johnson & Johnson Innovative Medicine (“J&J”) (the “AVTX-501 Milestone”) and (b) rights to any future milestone payments and royalties relating to AVTX-007 under a license agreement with Apollo AP43 Limited (“Apollo”), including up to $6.25 million of development milestones, up to $67.5 million in sales-based milestones, and royalty payments over a ten year period of a low single digit percentage of annual net sales (which percentage increases to another low single digit percentage if annual net sales exceed a specified threshold) (the “AVTX-007 Milestones and Royalties”). In addition, Avalo waived all its rights to AVTX-611 sales-based payments of up to $20.0 million that were payable by ES.
The exchange of the economic rights of the AVTX-501 Milestone and AVTX-007 Milestones and Royalties for cash met the definition of a derivative instrument. The fair value of the derivative liability is determined using a combination of a scenario-based method and an option pricing method (implemented using a Monte Carlo simulation). The significant inputs including probabilities of success, expected timing, and forecasted sales as well as market-based inputs for volatility, risk-adjusted discount rates and allowance for counterparty credit risk are unobservable and based on the best information available to Avalo. Certain information used in the valuation is inherently limited in nature and could differ from J&J’s and Apollo’s internal estimates.
The fair value of the derivative liability as of the transaction date was approximately $4.8 million, of which $3.5 million was attributable to the AVTX-501 Milestone and $1.3 million was attributable to the AVTX-007 Milestones and Royalties. Subsequent to the transaction date, at each reporting period, the derivative liability is remeasured at fair value. As of September 30, 2025, the fair value of the derivative liability was $23.2 million, all of which was attributable to the AVTX-007 Milestone and Royalties and was classified as a non-current liability. For the three and nine months ended September 30, 2025, the $12.5 million and $14.7 million change in fair value was recognized in other expense, net in the accompanying unaudited condensed consolidated statements of operations and comprehensive (loss) income.
The fair value of the AVTX-501 Milestone was deemed to be de minimis, driven by less than 1% probability of success based on Avalo’s interpretation of an announcement from J&J in March 2025, noting the discontinuation of the aticaprant depression program (previously referred to as AVTX-501 by Avalo), which was the only indication publicly disclosed, paired with a lack of commitment to an alternative indication. The fair value of AVTX-007 Milestones and Royalties was primarily driven by sales forecasts with peak annual net sales reaching $2.0 billion in atopic dermatitis, an approximate 41% probability of success, and an estimated time to commercialization of approximately 7.3 years, based on Avalo’s interpretation of Apollo’s September 2025 announcement that the drug met the primary endpoint in its Phase 2a clinical trial in atopic dermatitis. We estimated these unobservable inputs based on limited publicly available information and therefore could differ from J&J’s and Apollo’s respective internal development plans, assessments of probability of success and other inputs of our fair value calculation. Any changes to these inputs may result in significant changes to the fair value measurement. Notably, the peak annual net sales forecast (for the AVTX-007 Milestones and Royalties) and the probability of success (for both the AVTX-501 Milestone and the AVTX-007 Milestone and Royalties) are the largest drivers of the fair value, so changes to either would likely result in significant changes to their respective fair values.
In the event that J&J and/or Apollo are required to make payment(s) to ES Therapeutics pursuant to the underlying agreements, Avalo will recognize revenue under its existing contracts with those customers for that amount when it is no longer probable there would be a significant revenue reversal with any differences between the fair value of the derivative liability related to that payment immediately prior to the revenue recognition and revenue recognized to be recorded as other expense. However, given Avalo is no longer entitled to collect these payments, the potential ultimate settlement of the payments in the future from J&J and/or Apollo to ES Therapeutics (and the future mark-to-market activity each reporting period) will not impact Avalo’s future cash flows.
Warrant liability
In March 2024, the Company closed a private placement investment with institutional investors in which the investors received shares of Series C Preferred Stock and warrants to purchase shares of Avalo’s common stock (or a number of shares of Series C Preferred Stock). Refer to Note 10 - Capital Structure and sub-header “March 2024 Financing” for more information.
The Company determined that the warrants did not satisfy the conditions to be accounted for as equity instruments. As the warrants did not meet the equity contract scope exception, the Company classified the warrants as a derivative liability upon issuance.
The Company’s warrant liability was measured at fair value on the issuance date and was measured at fair value each reporting period thereafter until the warrants were fully exercised in the fourth quarter of 2024. As of September 30, 2025 and December 31, 2024, there were no warrants associated with the private placement outstanding and thus no corresponding warrant liability.
For the initial warrant valuation in the first quarter of 2024 and subsequent fair value measurement at each reporting period prior to exercises, the Company utilized the Black-Scholes option pricing model to measure fair value of the warrants, which required assumptions that were subjective and required judgment. As such, the warrant liability was classified as a Level 3 instrument as its value was based on unobservable market inputs. The initial fair value measurement of the warrant liability was $194.9 million and exceeded the initial gross proceeds received from the private placement of $115.6 million, resulting in a $79.3 million loss at issuance of the excess of initial liability fair value. The warrants were fully exercised in the fourth quarter of 2024. Refer to Note 10 - Capital Structure for additional discussion regarding the issuance of the Series C Preferred Stock and common stock pursuant to the warrant exercises.
No changes in valuation techniques occurred during the three and nine months ended September 30, 2025 and 2024. No transfers of assets between Level 1 and Level 2 of the fair value measurement hierarchy occurred during the three and nine months ended September 30, 2025 and 2024.
7. Investments
The following table summarizes our investments classified as available-for-sale as of September 30, 2025 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Amortized Cost |
|
Unrealized Gains |
|
Unrealized Losses |
|
Fair Value |
Money market funds |
|
$ |
18,786 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
18,786 |
|
U.S. Treasury securities |
|
85,859 |
|
|
52 |
|
|
(11) |
|
|
85,900 |
|
Total |
|
$ |
104,645 |
|
|
$ |
52 |
|
|
$ |
(11) |
|
|
$ |
104,686 |
|
The fair values of our investments by classification in the unaudited condensed consolidated balance sheets were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of |
| |
|
September 30, 2025 |
|
December 31, 2024 |
Cash and cash equivalents |
|
$ |
20,032 |
|
|
$ |
— |
|
Short-term investments |
|
84,654 |
|
|
— |
|
Total |
|
$ |
104,686 |
|
|
$ |
— |
|
As of September 30, 2025, the aggregate fair value of securities that were in an unrealized loss position for fewer than twelve months was $18.6 million and no investments had been in a continuous unrealized loss position for longer than twelve months. The Company considers any losses to be temporary in nature and the Company has the intent and ability to hold its marketable debt securities until recovery. As a result, the Company determined it did not hold any investments with a credit loss at September 30, 2025.
As of September 30, 2025, accrued interest receivable on available-for-sale investments was $0.4 million, which was included within prepaid and other current assets on our unaudited condensed consolidated balance sheets.
8. Leases
Avalo leases its main administrative office space located in Wayne (Chesterbrook), Pennsylvania, which is classified as an operating lease. The initial annual base rent for this office is $0.2 million and the annual operating expenses are approximately $0.1 million. The annual base rent is subject to periodic increases of approximately 2.4% over the term of the lease. The lease has an initial term of 5.25 years from the lease commencement on December 1, 2021 and expires on February 28, 2027.
Additionally, Avalo has an operating lease for administrative office space in Rockville, Maryland, which the Company elected to early-terminate effective January 31, 2026 by providing notice and paying the $0.3 million contractual early termination fee in the fourth quarter of 2024, resulting in a remeasurement and reduction of the lease liability and right-of-use (“ROU”) asset by $0.3 million. Because the Company had vacated the property, the ROU asset related to the property had been fully amortized as of September 30, 2025. The lease liability and lease payments related to the property will continue through the early-termination date of January 31, 2026. The annual base rent for this office is $0.2 million and was subject to annual 2.5% increases over the term of the lease. The lease provided for a rent abatement for a period of 12 months following the Company’s date of occupancy. The lease had an initial term of 10 years from the date the Company made its first annual fixed rent payment, which occurred in January 2020.
The weighted average remaining term of the operating leases at September 30, 2025 was 1.3 years.
Supplemental balance sheet information related to the leased properties include (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of |
| |
|
September 30, 2025 |
|
December 31, 2024 |
Right-of-use assets |
|
$ |
402 |
|
|
$ |
741 |
|
|
|
|
|
|
Lease liability, current |
|
$ |
438 |
|
|
$ |
568 |
|
Lease liability, non-current |
|
117 |
|
|
350 |
|
| Total operating lease liabilities |
|
$ |
555 |
|
|
$ |
918 |
|
The operating lease ROU assets are included in property and equipment, net and the lease liabilities current and non-current are included in accrued expenses and other current liabilities and other long-term liabilities, respectively, in our unaudited condensed consolidated balance sheets. The Company utilized a weighted average discount rate of 10.0% to determine the present value of the lease payments.
The components of lease expense for the three and nine months ended September 30, 2025 and 2024 were as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
|
|
2025 |
|
2024 |
|
2025 |
|
2024 |
| Operating lease cost* |
|
$ |
103 |
|
|
$ |
104 |
|
|
$ |
397 |
|
|
$ |
326 |
|
*Includes short-term leases, which are immaterial.
The following table shows a maturity analysis of the operating lease liabilities as of September 30, 2025 (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
|
| |
|
Undiscounted Cash Flows |
| October 1, 2025 through December 31, 2025 |
|
$ |
140 |
|
| 2026 |
|
392 |
|
| 2027 |
|
63 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total lease payments |
|
$ |
595 |
|
| Less implied interest |
|
(40) |
|
| Total |
|
$ |
555 |
|
9. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities as of September 30, 2025 and December 31, 2024 consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of |
| |
|
September 30, 2025 |
|
December 31, 2024 |
| Research and development |
|
$ |
2,491 |
|
|
$ |
1,625 |
|
| Compensation and benefits |
|
3,381 |
|
|
2,883 |
|
| General and administrative |
|
570 |
|
|
380 |
|
|
|
|
|
|
|
|
|
|
|
| Commercial operations |
|
291 |
|
|
534 |
|
| Royalty payment |
|
327 |
|
|
327 |
|
| Lease liability, current |
|
438 |
|
|
568 |
|
|
|
|
|
|
| Total accrued expenses and other current liabilities |
|
$ |
7,498 |
|
|
$ |
6,317 |
|
10. Capital Structure
Pursuant to the Company’s amended and restated certificate of incorporation, the Company is authorized to issue two classes of stock, common stock and preferred stock. At September 30, 2025, the Company was authorized to issue 205,000,000 shares of capital stock, of which 200,000,000 was common stock and 5,000,000 was preferred stock. All shares of common and preferred stock have a par value of $0.001 per share.
AlmataBio Transaction
On March 27, 2024, the Company acquired AlmataBio in which the former AlmataBio stockholders received (i) 171,605 shares of the Company’s common stock and (ii) 2,412 shares of the Company’s Series C Preferred Stock. Upon Company stockholder approval, which was obtained on August 13, 2024 and subject to beneficial ownership limitations, 2,063 shares of the Series C Preferred Stock issued to the former AlmataBio stockholders automatically converted into 2,062,930 shares of common stock. Refer to Note 3 - Asset Acquisition for more information regarding the acquisition and refer to sub-header “Series C Preferred Stock” within the “March 2024 Financing” section below for more information regarding the Series C Preferred Stock.
March 2024 Financing
On March 28, 2024, the Company closed a private placement investment in which the investors received (i) 19,946 shares of non-voting convertible Series C Preferred Stock, and (ii) warrants to purchase up to an aggregate of 11,967,526 shares of Avalo’s common stock (or a number of shares of Series C Preferred Stock convertible into the number of shares of common stock the warrant was then exercisable into), resulting in upfront gross proceeds of $115.6 million. Net proceeds were $108.1 million after deducting transaction costs of $7.5 million. The private placement transaction costs were expensed within other (expense) income, net for the nine months ended September 30, 2024. The Company received an additional $69.4 million of gross proceeds upon the full exercise of the warrants in the fourth quarter of 2024. Net proceeds were $67.6 million after deducting $1.7 million of transaction costs. Upon Company stockholder approval, which was obtained on August 13, 2024 and subject to beneficial ownership limitations, 6,585 shares of Series C Preferred Stock issued pursuant to the financing automatically converted into 6,585,314 shares of common stock. Additionally, the Company issued 781,259 shares of common stock and 11,186.267 shares of Series C Preferred Stock as a result of the warrant exercises in the fourth quarter of 2024.
Warrants on common stock or Series C Preferred Stock issued in March 2024 Financing
The warrants were exercisable via gross physical settlement for $5.796933 per underlying share of common stock (or a number of shares of Series C Preferred Stock convertible into the number of shares of common stock the warrant was then exercisable into). The warrants were fully exercised in the fourth quarter of 2024. The warrants included anti-dilution protection provisions.
The Company determined that the warrants did not satisfy the conditions to be accounted for as equity instruments. As the warrants did not meet the equity contract scope exception, the Company classified the warrants as a derivative liability upon issuance. The initial measurement of the warrants at fair value exceeded the proceeds received such that the difference between the initial fair value of the warrants and net upfront cash proceeds was recognized in the income statement as a loss. Subsequently, the warrants were carried at fair value with changes in fair value recognized in the Company’s unaudited condensed consolidated statements of operations and comprehensive (loss) income until exercised. Upon exercise of the warrants in the fourth quarter of 2024, the warrant liability was valued at $73.3 million. The settlement of the $73.3 million warrant liability and related share issuance proceeds of $69.4 million resulted in a $142.7 million impact to additional-paid-in-capital in the fourth quarter of 2024. The classification of the Series C Preferred Stock in permanent equity is discussed below within the section “Series C Preferred Stock issued in the AlmataBio Transaction, March 2024 Financing and upon Warrant Exercises.”
The valuation of the warrants was considered under Level 3 of the fair value hierarchy due to the need to use assumptions in the valuation that are both significant to the fair value measurement and unobservable. Refer to Note 6 - Fair Value Measurements for additional information regarding the settlement and valuation of the warrant liability.
Series C Preferred Stock issued in the AlmataBio Transaction, March 2024 Financing and upon Warrant Exercises
As of September 30, 2025, the Company had 5,000,000 shares of Preferred Stock authorized, of which 34,326 have been designated as Series C Preferred Stock. As of September 30, 2025, there were 19,364 shares of Series C Preferred Stock outstanding. The Series C Preferred Stock has a par value of $0.001 per share. The Series C Preferred Stock has no voting rights, no liquidation preference, and is not redeemable. In the event of any liquidation, dissolution or winding up of the Company, holders of Series C Preferred Stock are entitled to be paid out of the assets with the Company legally available for distribution to its stockholders on an as-converted and pari-passu basis with common stock. The Series C Preferred Stock is subject to broad-based weighted average anti-dilution protection for certain issuances of common stock and securities convertible into common stock. The Series C Preferred Stock is entitled to receive dividends equal to and in the same form, and in the same manner, based on the then-current conversion ratio as dividends actually paid on shares of the common stock, when, as and if such dividends are paid on shares of the common stock.
As a result of a contract amendment in the fourth quarter of 2024, the Series C Preferred Stock met equity classification and was recognized as a component of permanent stockholders’ equity within additional paid-in-capital. Prior to the contract amendment, the Series C Preferred Stock was contingently redeemable outside the control of the Company such that the Series C Preferred Stock was recognized outside of permanent equity. During the fourth quarter of 2024, the remaining 349 shares of Series C Preferred Stock held by the former AlmataBio stockholders, with a carrying value of $1.7 million, were reclassified to permanent equity. Additionally, the 11,186.267 shares of Series C Preferred Stock issued as a result of the warrant exercise in the fourth quarter of 2024, with a carrying value of $133.0 million, was recognized as a component of permanent stockholders’ equity within additional-paid-in capital on the Company’s unaudited condensed consolidated balance sheet.
No amounts were allocated to the Series C Preferred Stock issued pursuant to the March 2024 Financing because the initial fair value of the warrants exceeded gross proceeds received for the issuance of the private placement bundle that included both Series C Preferred Stock and warrants.
During the three and nine months ended September 30, 2025, an aggregate of approximately 5,332 shares of Series C Preferred Stock were converted to 5,331,946 shares of common stock and 5,532 shares of Series C Preferred Stock were converted to 5,531,946 shares of common stock, respectively.
Series D and Series E Preferred Stock issued in the March 2024 Financing
As a condition to the March 2024 Financing, a single share of Series D Preferred Stock and a single Series E Preferred Stock were issued to two institutional investors that participated in the private placement. Both the Series D and the Series E Preferred Stock have a par value and liquidation preference of $0.001 per share. The Series D and Series E Preferred Stock do not have voting rights, are not entitled to dividends, and are not convertible into common stock. Each of the holders of the Series D and Series E Preferred Stock have the option to require the Company to redeem their shares at a price equal to the par value at any time. The Company retains the right to redeem the Series D and Series E Preferred Stock at a price equal to the par value if the holder owns less than a certain threshold of the Company’s outstanding common stock. The Series D and Series E Preferred Stock do not provide the holders with substantive economics, and were issued solely to allow for the institutional investors to appoint a director to the Company’s board of directors. Because the Series D and Series E Preferred Stock are redeemable at par value outside the control of the Company, they are recognized outside of permanent equity.
At-the-Market Offering Program
In June 2025, the Company entered into an “at-the-market” sales agreement with TD Securities (USA) LLC (“TD Cowen”), pursuant to which the Company may sell, from time to time, shares of its common stock having an aggregate offering price of up to $75.0 million through TD Cowen (the “ATM Program”). The Company sold 1.7 million shares of common stock for net proceeds of $14.4 million under the ATM Program during the three and nine months ended September 30, 2025.
Common Stock Warrants
At September 30, 2025, the following common stock warrants were outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of common shares |
|
|
Exercise price |
|
Expiration |
| underlying warrants |
|
|
per share |
|
date |
| 148 |
|
|
$ |
7,488.00 |
|
|
June 2031 |
11. Stock-Based Compensation
In April 2016, our board of directors adopted the 2016 Equity Incentive Plan, which was approved by our stockholders in May 2016 and which was subsequently amended and restated in May 2018 and August 2019 with the approval of our board of directors and our stockholders. In June 2024, our board of directors approved a fourth amended and restated equity incentive plan, which was subsequently approved by the Company’s stockholders in August 2024 (the “2016 Fourth Amended Plan”). During the term of the 2016 Fourth Amended Plan, the share reserve will automatically increase on the first trading day in January of each calendar year ending on (and including) January 1, 2034, by an amount equal to 5% of the total number of outstanding shares of common stock and Series C Preferred Stock (determined on an as-converted stock basis) plus all outstanding prefunded warrants to acquire shares of common stock (if any) as of December 31 of the preceding calendar year. On January 1, 2025, pursuant to the terms of the 2016 Fourth Amended Plan, an additional 1,768,393 shares were made available for issuance. As of September 30, 2025, there were 341,821 shares available for future issuance under the 2016 Fourth Amended Plan.
In September 2025, our board of directors adopted the 2025 Inducement Award Plan (the “Inducement Plan”). Grants under our Inducement Plan are awarded in accordance with Nasdaq Listing Rule 5635(c)(4). A total of 1,300,000 shares of our common stock were initially reserved for issuance under our Inducement Plan. As of September 30, 2025, there were 1,300,000 shares available for future issuance under the Inducement Plan. Subsequent to September 30, 2025, we granted stock options under our Inducement Plan to purchase an aggregate of 375,000 shares of our common stock with an exercise price of $12.96 per share. These options were granted as an inducement to two new employees entering employment with the Company.
Option grants expire after ten years. Employee options typically vest over four years. Employees typically receive a new hire option grant, as well as an annual grant in the first or second quarter of each year. Options granted to directors typically vest immediately or over periods of one or three years. In lieu of director fees, directors may elect to receive stock options, which vest immediately. For stock options granted to employees and non-employee directors, the estimated grant date fair market value of the Company’s stock-based awards is amortized ratably over the individuals’ service periods, which is the period in which the awards vest. Stock-based compensation expense includes expense related to stock options, restricted stock units and employee stock purchase plan shares. The amount of stock-based compensation expense recognized for the three and nine months ended September 30, 2025 and 2024 was as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
| Research and development |
|
$ |
1,189 |
|
|
$ |
762 |
|
|
$ |
3,606 |
|
|
$ |
1,250 |
|
| General and administrative |
|
1,964 |
|
|
1,086 |
|
|
5,507 |
|
|
1,698 |
|
| Total stock-based compensation |
|
$ |
3,153 |
|
|
$ |
1,848 |
|
|
$ |
9,113 |
|
|
$ |
2,948 |
|
Stock options with service-based vesting conditions
The Company has granted stock options that contain service-based vesting conditions. The compensation cost for these options is recognized on a straight-line basis over the vesting periods. A summary of option activity for the nine months ended September 30, 2025 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Options Outstanding |
| |
|
Number of shares |
|
Weighted average exercise price per share |
|
Weighted average grant date fair value per share |
|
Weighted average remaining contractual term (in years) |
| Balance at December 31, 2024 |
|
1,999,749 |
|
|
$ |
19.91 |
|
|
$ |
14.98 |
|
|
9.6 |
| Granted |
|
2,138,872 |
|
|
$ |
7.52 |
|
|
$ |
6.20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at September 30, 2025 |
|
4,138,621 |
|
|
$ |
13.51 |
|
|
$ |
10.44 |
|
|
9.0 |
|
|
|
|
|
|
|
|
|
| Exercisable at September 30, 2025 |
|
717,461 |
|
|
$ |
35.96 |
|
|
$ |
24.99 |
|
|
8.7 |
The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s common stock for those stock options that had exercise prices lower than the fair value of the Company’s common stock. As of September 30, 2025, the aggregate intrinsic value of options outstanding was $15.7 million. There were 711,962 options that vested during the nine months ended September 30, 2025 with a weighted average exercise price of $11.50 per share. The total grant date fair value of shares which vested during the nine months ended September 30, 2025 and 2024 was $7.0 million and $1.9 million, respectively.
The Company recognized stock-based compensation expense of $2.4 million and $7.0 million related to stock options with service-based vesting conditions for the three and nine months ended September 30, 2025, respectively. The Company recognized stock-based compensation expense of $1.4 million and $2.5 million related to stock options with service-based vesting conditions for the three and nine months ended September 30, 2024, respectively. At September 30, 2025, there was $22.6 million of total unrecognized compensation cost related to unvested service-based vesting condition awards. The unrecognized compensation cost is expected to be recognized over a weighted-average period of 2.8 years.
Stock-based compensation assumptions
The following table presents the assumptions used to compute stock-based compensation expense for stock options with service-based vesting conditions granted under the Black-Scholes valuation model for the nine months ended September 30, 2025.
|
|
|
|
|
|
|
|
|
| Service-based options |
|
|
| Expected term of option (in years) |
|
5.0 - 6.1 |
| Expected stock price volatility |
|
99.9% - 104.4% |
| Risk-free interest rate |
|
3.74% - 4.45% |
| Expected annual dividend yield |
|
0% |
|
|
Restricted Stock Units
The Company has granted RSUs that contain service-based vesting conditions. The Company measures the fair value of the RSUs using the stock price on the date of grant. The compensation cost for RSUs is recognized on a straight-line basis over the vesting period. A summary of RSU activity for the nine months ended September 30, 2025 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
RSUs Outstanding |
| |
|
Number of shares |
|
Weighted average grant date fair value |
Unvested RSUs at December 31, 2024 |
|
632,100 |
|
|
$ |
9.88 |
|
|
|
|
|
|
| Vested |
|
(210,703) |
|
|
$ |
9.88 |
|
Unvested RSUs at September 30, 2025 |
|
421,397 |
|
|
|
The RSUs, which were granted on August 13, 2024, vest annually over a three-year period beginning on March 28, 2025. Accordingly, the first tranche of RSUs vested on March 28, 2025. The Company recognized stock-based compensation expense of $0.6 million and $1.9 million related to RSUs for the three and nine months ended September 30, 2025, respectively. The Company recognized stock-based compensation expense of $0.4 million related to RSUs for the three and nine months ended September 30, 2024. At September 30, 2025, there was $3.1 million of total unrecognized compensation cost related to RSUs. The unrecognized compensation cost is expected to be recognized over a weighted-average period of 1.5 years.
Performance Stock Units
A Performance Stock Unit (“PSU”) represents one equivalent share of our common stock to be issued after achievement of the performance goals specified in the grant. The Company estimates the fair value of our PSUs as of the grant date based upon the expected likelihood of achievement of the performance goals specified in the grant and the closing market price of our common stock on the date of grant. The Company recognizes stock-based compensation expense over the requisite service period, if it is probable that the performance goal will be achieved.
In August 2025, the Company granted PSUs to executives under the 2016 Fourth Amended Plan with a grant date fair value of $8.90 per unit (the “PSU Awards”). The PSU Awards include 492,500 shares that will be available to vest assuming 100% achievement of the performance metric, with a maximum of 738,750 shares available to vest assuming maximum achievement. The performance metric for the PSU Awards is based on the timing of data release and efficacy of the Company’s Phase 2 LOTUS trial, and the achieved units may range from 0% to 150% of the target number depending on the level of achievement against the specified performance metric. Upon successful achievement of the performance metric, the PSU Awards will vest on the third anniversary of the grant date. As of September 30, 2025, the Company has not recognized any compensation expense related to the PSU Awards as the Company does not yet consider achievement of the performance metric to be probable.
Employee Stock Purchase Plan
On April 5, 2016, the Company’s board of directors approved the 2016 Employee Stock Purchase Plan, which was approved by the Company’s stockholders and became effective on May 18, 2016 (the “Initial ESPP”). In June 2024, our board of directors approved an amended and restated employee stock purchase plan, which was subsequently approved by the Company’s stockholders in August 2024 (the “ESPP”).
Under the ESPP, eligible employees can purchase common stock through accumulated payroll deductions at such times as are established by the administrator. The ESPP is administered by the compensation committee of the Company’s board of directors. Under the ESPP, eligible employees may purchase stock at 85% of the lower of the fair market value of a share of the Company’s common stock (i) on the first day of an offering period or (ii) on the purchase date. Eligible employees may contribute up to 15% of their earnings during the offering period. The Company’s board of directors may establish a maximum number of shares of the Company’s common stock that may be purchased by any participant, or all participants in the aggregate, during each offering period. Under the ESPP, a participant may not accrue rights to purchase more than $25,000 of the fair market value of the Company’s common stock for each calendar year in which such right is outstanding.
The Company initially reserved and authorized up to 174 shares of common stock for issuance under the Initial ESPP. Pursuant to the ESPP, on January 1 of each calendar year, the aggregate number of shares that may be issued under the ESPP automatically increases by a number equal to 1% of the Company’s outstanding shares of common stock and Series C Preferred Stock (determined on an as-converted basis) plus all outstanding prefunded warrants to acquire shares of common stock (if any), as of December 31 of the preceding calendar year. On January 1, 2025, the number of shares available for issuance under the ESPP increased by 353,679 shares. As of September 30, 2025, 570,520 shares remained available for issuance.
In accordance with the guidance in ASC 718-50, Employee Share Purchase Plans, the ability to purchase shares of the Company’s common stock at the lower of the offering date price or the purchase date price represents an option and, therefore, the ESPP is a compensatory plan under this guidance. Accordingly, stock-based compensation expense is determined based on the option’s grant-date fair value and is recognized over the requisite service period of the option. The Company used the Black-Scholes valuation model and recognized stock-based compensation expense of $0.1 million and $0.2 million for the three and nine months ended September 30, 2025 and minimal stock-based compensation expense in 2024.
12. Income Taxes
The Company recognized minimal income tax expense for the three and nine months ended September 30, 2025 and 2024 due to the significant valuation allowance against the Company’s deferred tax assets and the current and prior period losses.
On July 4, 2025, the One Big Beautiful Bill Act (“OBBB”) was enacted in the United States. The OBBB includes significant provisions, such as the permanent extension of certain expiring provisions of the Tax Cuts and Jobs Act, and restoration of favorable tax treatment for certain business provisions including the expensing of domestic research and development expenditures. The Company continues to assess the impact on the consolidated financial statements, however, no material changes have occurred as of September 30, 2025.
13. Commitments and Contingencies
Litigation
Litigation - General
The Company may become party to various contractual disputes, litigation, and potential claims arising in the ordinary course of business. Reserves are established in connection with such matters when a loss is probable and the amount of such loss can be reasonably estimated. The Company currently does not believe that the resolution of such matters will have a material adverse effect on its financial position or results of operations except as otherwise disclosed in this report.
Possible Future Milestone Payments for In-Licensed Compounds
General
Avalo is a party to license and development agreements with various third parties, which contain future payment obligations such as royalties and milestone payments. The Company recognizes a liability (and related expense) for each milestone if and when such milestone is probable and can be reasonably estimated. As typical in the biotechnology industry, each milestone has unique risks that the Company evaluates when determining the probability of achieving each milestone and the probability of success evolves over time as the programs progress and additional information is obtained. The Company considers numerous factors when evaluating whether a given milestone is probable including (but not limited to) the regulatory pathway, development plan, ability to dedicate sufficient funding to reach a given milestone and the probability of success.
AVTX-009
On March 27, 2024, Avalo obtained the rights to an anti-IL-1β mAb (AVTX-009), including the world-wide exclusive license from Eli Lilly and Company (“Lilly”) (the “Lilly License Agreement”), pursuant to its acquisition of AlmataBio. AlmataBio had previously purchased the rights, title and interest in the asset from Leap Therapeutics, Inc. (“Leap”) in 2023, which have since been assumed by Avalo pursuant to its acquisition of AlmataBio (the “Leap Agreement”). Avalo is responsible for the development and commercialization of the program.
Avalo is required to pay up to $70.0 million based on the achievement of specified development and regulatory milestones to Lilly. Upon commercialization, the Company is required to pay sales-based milestones aggregating up to $650.0 million payable to Lilly and $70.0 million payable to Leap. There are no annual or maintenance fees payable under the Lilly License Agreement and Leap Agreement. Additionally, Avalo is required to pay royalties to Lilly during a country-by-country royalty term in which the low end and the high end of the range fall between 5% and 15% of Avalo or its sublicensees’ annual net sales. The royalty term due to Lilly commences on the date of first commercial sale of the licensed product in a given territory and expires on a county-by-country basis; on the latest of (a) the tenth (10th) anniversary of the date of the first commercial sale, (b) the expiration of the last-to-expire licensed patent in the given territory, or (c) the expiration of any data exclusivity period for the licensed product in the given territory.
The Lilly License Agreement remains in effect until the expiration of the last-to-expire royalty term of any licensed products. Each party may terminate for cause or by mutual agreement though the Company may terminate at its sole discretion by giving one-hundred twenty (120) days’ prior written notice to Lilly, in which case all licenses and rights granted pursuant to the agreement will automatically terminate and revert to Lilly. There are no termination or expiration provisions under the Leap Agreement.
Avalo has not paid any milestones, royalties or any other amounts under the Lilly License Agreement or Leap Agreement.
No expense related to the agreements was recognized in the three and nine months ended September 30, 2025. There has been no cumulative expense recognized as of September 30, 2025 under the agreements. The Company will continue to monitor the milestones and royalties at each reporting period.
Refer to the sub-header below entitled “Acquisition Related and Other Contingent Liabilities” for information regarding future development milestones that are payable to the former AlmataBio stockholders.
Quisovalimab (AVTX-002)
KKC License Agreement
On March 25, 2021, the Company entered into a license agreement with Kyowa Kirin Co., Ltd. (“KKC”) for exclusive worldwide rights to develop, manufacture and commercialize quisovalimab, KKC’s first-in-class fully human anti-LIGHT (TNFSF14) monoclonal antibody for all indications (the “KKC License Agreement”). The KKC License Agreement replaced the Amended and Restated Clinical Development and Option Agreement between the Company and KKC dated May 28, 2020. Avalo is responsible for the development and commercialization of quisovalimab in all indications worldwide (other than the option in the KKC License Agreement that, upon exercise by KKC, allows KKC to develop, manufacture and commercialize quisovalimab in Japan). Avalo is not currently pursuing the clinical development of quisovalimab and is exploring strategic alternatives.
Under the KKC License Agreement, the Company paid KKC an upfront license fee of $10.0 million, which we recognized within research and development expenses in 2021. Avalo is also required to pay KKC up to an aggregate of $112.5 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is required to make milestone payments to KKC aggregating up to $75.0 million tied to the achievement of annual net sales targets. There are no annual or maintenance fees payable under the KKC License Agreement.
Additionally, the Company is required to pay KKC royalties during a country-by-country royalty term equal to a mid-teen percentage of annual net sales. The Company is required to pay KKC a mid-twenties percentage of the payments that the Company receives from sublicensing of its rights under the KKC License Agreement, subject to certain exclusions. The royalty term due to KKC commences on the date of first commercial sale of the licensed product in a given territory and expires on a county-by-country basis, on the latest of (a) the twelfth (12th) anniversary of the date of the first commercial sale, (b) the expiration of the last-to-expire licensed patent in the given territory, or (c) the expiration of any data exclusivity period for the licensed product in the given territory.
The KKC License Agreement remains in effect while the Company and its affiliates and sublicensees develop and commercialize quisovalimab subject to customary termination rights. Each party may terminate for cause though Avalo may terminate for convenience upon six (6) months’ prior written notice in the case where regulatory approval has not been obtained for the licensed product or upon twelve (12) months’ prior written notice where regulatory approval has been obtained for the licensed product.
As disclosed above, Avalo paid the $10.0 million upfront license fee in 2021. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to the KKC License Agreement was recognized in the three and nine months ended September 30, 2025. There has been no cumulative expense recognized as of September 30, 2025 related to the milestones, royalties or any other amounts other than the $10.0 million upfront license fee incurred in 2021 as disclosed above. The Company will continue to monitor the milestones and royalties at each reporting period.
CHOP License Agreement
Following its February 3, 2020 merger with Aevi Genomic Medicine, Inc. (“Aevi”), the Company became party to a license agreement with The Children’s Hospital of Philadelphia (“CHOP”) (as amended, the “CHOP License Agreement”). Quisovalimab became a covered product under this license agreement in 2021 and at that time became subject to the terms therein. Avalo is not currently pursuing the clinical development of quisovalimab and is exploring strategic alternatives.
An initial upfront fee of $0.5 million was paid to CHOP by Aevi, which Avalo acquired in 2020. Avalo is required to pay an additional $1.0 million to CHOP based on the achievement of specified regulatory and commercial milestones. Avalo is obligated to pay an annual license maintenance fee of $0.2 million to CHOP, of which Avalo has paid an aggregate of $1.1 million as of the filing date of this Quarterly Report on Form 10-Q.
The Company is also obligated to pay tiered royalties to CHOP on a country-to-country basis in which the low end and high end of the range are single-digit royalties based on the Company’s net sales of quisovalimab. The royalty term extends to the later of (a) fifteen years following the original date of the CHOP License Agreement, (b) the last-to-expire of the valid claims in the licensed patent rights covering the manufacture, sale, or use of quisovalimab and (c) the expiration of the regulatory exclusivity period for quisovalimab.
CHOP may terminate the CHOP License Agreement for the material default or insolvency of the Company, and the Company may terminate the CHOP License Agreement at will with six (6) months’ written notice.
As disclosed above, Aevi paid the $0.5 million upfront license fee and Avalo has paid $1.1 million of annual license fees. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to the milestones and royalties due under the CHOP Agreement was recognized for the three and nine months ended September 30, 2025. Avalo has not recognized any cumulative expense under the agreement related to the milestone or royalties as of September 30, 2025. The Company will continue to monitor the milestones and royalties at each reporting period.
AVTX-008 Sanford Burnham Prebys License Agreement
On June 21, 2021, the Company entered into an Exclusive Patent License Agreement with Sanford Burnham Prebys Medical Discovery Institute (the “Sanford Burnham Prebys License Agreement”) under which the Company obtained an exclusive license to a portfolio of issued patents and patent applications covering an immune checkpoint program (AVTX-008). Avalo was responsible for the development and commercialization of the program. During the second quarter of 2025, the Company provided notice to terminate the Sanford Burnham Prebys License Agreement in accordance with its terms, effective September 2025. Due to changes in the Company’s operations and strategy, the Sanford Burnham Prebys License Agreement was no longer considered material by the Company at the time of termination.
AVTX-006 Astellas License Agreement
On July 15, 2019, the Company entered into an exclusive license agreement with OSI Pharmaceuticals, LLC, an indirect wholly owned subsidiary of Astellas Pharma, Inc. (“Astellas”), for the worldwide development and commercialization of the novel, second generation mTORC1/2 inhibitor (AVTX-006). Avalo is fully responsible for the development and commercialization of the program. Avalo is not currently pursuing the clinical development of AVTX-006 and is exploring strategic alternatives.
Under the terms of the license agreement, there was an upfront license fee of $0.5 million. The Company is required to pay Astellas up to an aggregate of $5.5 million based on the achievement of specified development and regulatory milestones. There are no annual maintenance fees payable under the Astellas license agreement. Additionally, the Company is required to pay Astellas a tiered mid-to-high single digit percentage of the payments that Avalo receives from any sublicensing of its rights under the Astellas license agreement, subject to certain exclusions. Upon commercialization, the Company is required to pay Astellas royalties during a country-by-country royalty term equal to a tiered mid-to-high single digit percentage of annual net sales during the period beginning upon the date of the first commercial sale of such licensed product in such country and ending on the later to occur of (a) the expiry of the last valid claim of an OSI product patent covering such licensed product in such country, (b) expiration of regulatory exclusivity in such country, and (c) ten (10) years from the first commercial sale of such licensed product in such country.
The Astellas License Agreement remains in effect on a country-by-country and licensed product-by-licensed product basis (in the territory), unless the license agreement is terminated earlier in accordance with the license agreement. Avalo may terminate the agreement at any time upon providing sixty (60) days’ written notice to Astellas and may terminate the agreement in its entirety without cause.
As disclosed above, Avalo paid the $0.5 million upfront license fee. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to this license agreement was recognized in the three and nine months ended September 30, 2025. There has been $0.5 million of cumulative expense recognized as of September 30, 2025 related to the milestones under this license agreement. The Company will continue to monitor the remaining milestones and royalties at each reporting period.
Possible Future Milestone Proceeds for Out-Licensed Compounds
AVTX-301 Out-License
On May 28, 2021, the Company out-licensed its rights in respect of its non-core asset, AVTX-301, to Alto Neuroscience, Inc. (“Alto”). The Company initially in-licensed the compound from an affiliate of Merck & Co., Inc. in 2013. Alto is fully responsible for the development and commercialization of the program.
Under the out-license agreement, the Company received a mid-six digit upfront payment from Alto, which we recognized as license revenue in 2021. The Company is also eligible to receive up to an aggregate of $18.6 million based on the achievement of specified development, regulatory and commercial sales milestones. Additionally, the Company is entitled to a less than single digit percentage royalty based on annual net sales.
The out-license agreement remains in effect on a licensed product-by-licensed product and country-by-country basis until the later of (i) the expiration of the last to expire valid patent claim covering such licensed product in such country, or (ii) 10 (ten) years after the first commercial sale of such licensed product in such country. Upon expiration of the agreement, the licenses shall become a fully paid-up, royalty-free, irrevocable, perpetual non-exclusive license and sublicense.
The Company had not recognized any milestones as of September 30, 2025 or received any payments other than the upfront payment as disclosed above.
AVTX-406 License Assignment
On June 9, 2021, the Company assigned its rights, title, interest, and obligations under an in-license covering its non-core asset, AVTX-406, to ES, a wholly owned subsidiary of Armistice, who was a significant stockholder of the Company at the time of the transaction and whose chief investment officer, Steven Boyd, and managing director, Keith Maher, served on Avalo’s Board until August 8, 2022. The transaction with ES was approved in accordance with Avalo’s related party transaction policy. ES is fully responsible for the development and commercialization of the program.
Under the assignment agreement, the Company received a low-six digit upfront payment from ES, which we recognized as license revenue in 2021. The Company is also eligible to receive up to an aggregate of $6.0 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is eligible to receive sales-based milestone payments aggregating up to $20.0 million tied to annual net sales targets.
The Company had not recognized any milestones as of September 30, 2025 or received any payments other than the upfront payment as disclosed above.
AVTX-800 Series Asset Sale
On October 27, 2023, the Company sold its rights, title and interests in AVTX-801, AVTX-802 and AVTX-803 (collectively, the “800 Series”) to AUG Therapeutics, LLC (“AUG”). AUG is fully responsible for the development and commercialization of the program.
Pursuant to the Purchase Agreement with AUG, the Company received an upfront payment of $0.2 million. Additionally, AUG assumed aggregate liabilities of $0.4 million, which included certain liabilities incurred prior to the date of the Purchase Agreement, costs due and payable between the date of the Purchase Agreement and the closing date, and obligations under 800 Series contracts assumed by AUG. Avalo is also entitled to a contingent milestone payment of 20% of certain amounts, if any, granted to AUG upon sale of any priority review voucher related to the 800 Series compounds granted to AUG by the FDA, net of any selling costs, or $15.0 million for each compound (for a potential aggregate of $45.0 million) if the first FDA approval is for any indication other than a Rare Pediatric Disease (as defined in the Purchase Agreement).
The Company had not recognized any revenue related to the milestones as of September 30, 2025 or received any payments other than the upfront payment and reimbursement for certain liabilities as disclosed above.
Acquisition Related and Other Contingent Liabilities
AlmataBio Transaction Possible Future Milestone Payments
On March 27, 2024, the Company acquired AVTX-009 through its acquisition of AlmataBio. Pursuant to the AlmataBio Transaction, the Company made a cash payment of $7.5 million in April 2024 to the former AlmataBio stockholders, which was due upon the initial closing of the private placement on March 28, 2024 (the “Initial Milestone”). Further, a portion of the consideration for the AlmataBio transaction includes development milestones to the former AlmataBio stockholders including $5.0 million due upon the first patient dosed in a Phase 2 trial in patients with HS for AVTX-009 (the “Second Milestone”), which was met and paid in October 2024 as discussed below, and $15.0 million due upon the first patient dosed in a Phase 3 trial for AVTX-009 (the “Third Milestone”), both of which are payable in cash or stock of Avalo at the election of the former AlmataBio stockholders. In the absence of timely notice of such election, Avalo may elect to pay the milestones in cash or common stock of Avalo.
The Company paid the Initial Milestone payment in April 2024 and recognized the payment within acquired in-process research and development expense. In addition, the Company concluded the Second Milestone was probable as of the acquisition date and therefore recognized the $5.0 million milestone within acquired in-process research and development expense in the condensed consolidated statements of operations and comprehensive income (loss) for the nine months ended September 30, 2024 and the corresponding liability as contingent consideration as of September 30, 2024. The Company made a cash payment of $5.0 million in October 2024 upon meeting the Second Milestone. The Company will continue to monitor the Third Milestone each reporting period.
AVTX-006 Royalty Agreement with Certain Related Parties
In July 2019, Aevi entered into a royalty agreement, and liabilities thereunder were assumed by Avalo upon close of the Aevi Merger in February 2020. The royalty agreement provided certain investors, including LeoGroup Private Investment Access, LLC on behalf of Garry Neil, the Company’s current Chief Executive Officer and former Chairman of the Board, and Mike Cola, the Company’s former Chief Executive Officer (collectively, the “Investors”), a royalty stream, in exchange for a one-time aggregate payment of $2.0 million (the “Royalty Agreement”). Pursuant to the Royalty Agreement, the Investors will be entitled collectively to an aggregate amount equal to a low-single digit percentage of the aggregate net sales of the Company’s second generation mTORC1/2 inhibitor, AVTX-006 for a royalty term consistent with the royalty term disclosed in the AVTX-006 Astellas License Agreement section above. Avalo considers AVTX-006 a non-core asset and is exploring strategic alternatives. At any time beginning three years after the date of the first public launch of AVTX-006, Avalo may exercise, at its sole discretion, a buyout option that terminates any further obligations under the Royalty Agreement in exchange for a payment to the Investors of an aggregate of 75% of the net present value of the royalty payments. A majority of the independent members of the board of directors and the audit committee of Aevi approved the Royalty Agreement.
Avalo assumed this Royalty Agreement upon closing of the Aevi Merger and it is recorded as a royalty obligation within the Company's accompanying unaudited condensed consolidated balance sheet as of September 30, 2025 and December 31, 2024. Because there is a significant related party relationship between the Company and the Investors, the Company has treated its obligation to make royalty payments under the Royalty Agreement as an implicit obligation to repay the funds advanced by the Investors. As the Company makes royalty payments in accordance with the Royalty Agreement, it will reduce the liability balance. At the time that such royalty payments become probable and estimable, and if such amounts exceed the liability balance, the Company will impute interest accordingly on a prospective basis based on such estimates, which will result in a corresponding increase in the liability balance.
14. Segments
The Company’s chief operating decision maker (“CODM”), who is our Chief Executive Officer, views the Company’s operations and manages the business as one operating segment. The presentation of financial results as one reportable segment is consistent with the way we operate our business and the manner in which our CODM evaluates performance and makes resource and operating decisions for the business. The accounting policies of the business segment are the same as those described in the summary of significant accounting policies as contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024.
The CODM evaluates performance and makes resource and operating decisions for the business based on net loss as reported on the unaudited condensed consolidated statement of operations and total assets as reported on the unaudited condensed consolidated balance sheet. The CODM’s primary evaluation of the Company’s success is its ability to progress its research and development pipeline programs toward commercialization or opportunistically out-license rights to indications or geographies. The CODM uses net loss compared to budget and/or forecast amounts to evaluate this progress to make resource and operating decisions, such as whether to issue equity and/or make new investments in additional indications or pipeline assets. Additionally, the Company’s CODM periodically reviews research and development expense, as stated on the unaudited condensed consolidated statement of operations, and treats it as a significant segment expense. The CODM considers research and development expense in the context of achieving the next expected milestone in the pipeline, and will make resource and operating decisions accordingly, such as decisions on raising additional capital and/or pursuing additional indications or programs. The following table summarizes our research and development expenses for the three and nine months ended September 30, 2025 and 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, 2025 |
|
|
2025 |
|
2024 |
|
2025 |
|
2024 |
| Nonclinical expenses |
|
$ |
184 |
|
|
$ |
154 |
|
|
$ |
445 |
|
|
$ |
501 |
|
| Clinical expenses |
|
7,250 |
|
|
5,078 |
|
|
18,315 |
|
|
6,719 |
|
| CMC expenses |
|
2,556 |
|
|
1,978 |
|
|
7,802 |
|
|
2,971 |
|
|
|
|
|
|
|
|
|
|
| Internal expenses: |
|
|
|
|
|
|
|
|
| Salaries, benefits and related costs |
|
2,383 |
|
|
1,511 |
|
|
6,414 |
|
|
4,644 |
|
| Stock-based compensation expense |
|
1,189 |
|
|
761 |
|
|
3,606 |
|
|
1,250 |
|
| Other |
|
59 |
|
|
56 |
|
|
235 |
|
|
169 |
|
|
|
$ |
13,621 |
|
|
$ |
9,538 |
|
|
$ |
36,817 |
|
|
$ |
16,254 |
|
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Quarterly Report on Form 10-Q and the information incorporated herein by reference contain forward-looking statements that involve a number of risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially from those expressed or implied by such forward-looking statements. Forward-looking statements can be identified by the use of forward-looking words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” “pro forma” or other similar words (including their use in the negative), or by discussions of future matters such as: the future financial and operational outlook; the development of product candidates; and other statements that are not historical. Although our forward-looking statements reflect the good faith judgment of our management, these statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those set out in our Annual Report on Form 10-K filed with the SEC on March 20, 2025, and in our other filings with the SEC. Statements made herein are as of the date of the filing of this Quarterly Report on Form 10-Q with the SEC and should not be relied upon as of any subsequent date. Unless otherwise required by applicable law, we do not undertake, and we specifically disclaim any obligation to update any forward-looking statements to reflect occurrences, developments, unanticipated events or circumstances after the date of such statement.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited financial statements and related notes that appear in Item 1 of this Quarterly Report on Form 10-Q and with our audited financial statements and related notes for the year ended December 31, 2024 appearing in our Annual Report on Form 10-K filed with the SEC on March 20, 2025.
Overview
Avalo Therapeutics, Inc. (the “Company,” “Avalo” or “we”) is a clinical stage biotechnology company fully dedicated to developing IL-1β-based treatments for immune-mediated inflammatory diseases. Our lead asset, AVTX-009, is in a Phase 2 clinical trial for hidradenitis suppurativa (“HS”). The Company is also exploring additional opportunities to make an impact in prevalent indications that have significant remaining unmet needs.
Our focus in 2025 is continuing to execute operationally on the development of AVTX-009, most notably the progression of the Phase 2 (“LOTUS”) trial of AVTX-009, an anti-IL-1β (mAb), in HS. We expect to release topline results from this trial in mid-2026.
Management’s primary evaluation of the success of the Company is the ability to progress its pipeline forward toward commercialization or opportunistically out-licensing rights to indications or geographies. We believe the ability to achieve the anticipated milestone as presented in the following chart represents our most immediate evaluation point as to the progress of our goal to move the pipeline forward.
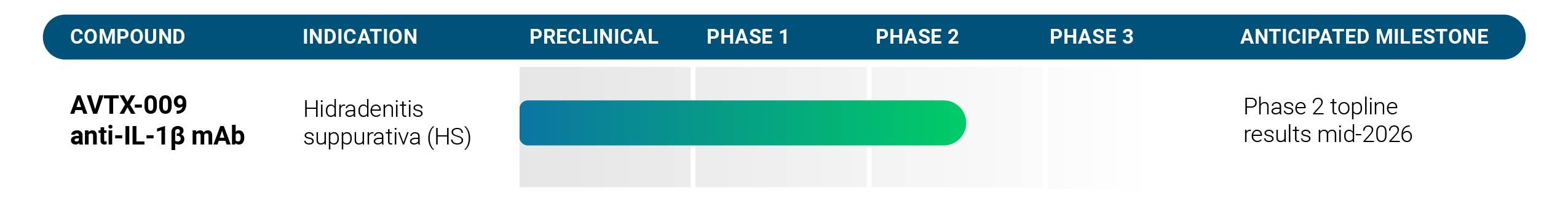
The Company’s Phase 2 LOTUS trial in HS, is a randomized, double-blind, placebo-controlled, parallel-group Phase 2 trial with two AVTX-009 dose regimens to evaluate the efficacy, safety and tolerability of AVTX-009 in approximately 250 adults with moderate to severe HS. Subjects were randomized (1:1:1) to receive either one of two doses of AVTX-009 or placebo. The primary efficacy endpoint is the proportion of subjects achieving Hidradenitis Suppurativa Clinical Response (HiSCR75) at Week 16. Avalo is the study sponsor and the current trial locations include the United States, Canada, France, Germany, Italy, Spain, Bulgaria, Czech Republic, Greece, Poland, Australia, Turkey, and Slovakia.
Recent Developments
In October 2025, the Company announced that it has completed enrollment in its Phase 2 LOTUS trial of AVTX-009 for the treatment of HS.
In September 2025, the Company announced the appointment of Kevin Lind to its Board of Directors. Further, in October 2025, we announced the expansion of our leadership team with key appointments in business development and human resources.
Liquidity
Since inception, we have incurred significant operating and cash losses from operations. We have primarily funded our operations to date through sales of equity securities, out-licensing transactions and sales of assets.
For the nine months ended September 30, 2025, Avalo generated a net loss of $64.5 million and negative cash flows from operations of $37.2 million. As of September 30, 2025, Avalo had $111.6 million in cash and cash equivalents and short-term investments.
Based on our current operating plans, we expect that our existing cash and cash equivalents and short-term investments are sufficient to fund operations for at least twelve months from the filing date of this Quarterly Report on Form 10-Q and we expect to fund operations into 2028. The Company closely monitors its cash and cash equivalents and short-term investments and seeks to balance the level of cash and cash equivalents with our projected needs to allow us to withstand periods of uncertainty relative to the availability of funding on favorable terms. We may satisfy any future cash needs through sales of equity securities under the Company’s at-the-market program or other equity financings, out-licensing transactions, strategic alliances/collaborations, sale of programs, and/or mergers and acquisitions. There can be no assurance that any financing or business development initiatives can be realized by the Company, or if realized, what the terms may be. To the extent that we raise capital through the sale of equity, the ownership interest of our existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Further, if the Company raises additional funds through collaborations, strategic alliances or licensing arrangements with third parties, the Company might have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates.
Our Strategy
Our strategy for increasing stockholder value includes:
•Advancing our pipeline through development to regulatory approval. Most notably and in the near term, completing our Phase 2 LOTUS trial in HS, preparing for the next stage of development for that indication and considering further indication expansion for AVTX-009;
•Acquiring or in-licensing rights to and/or developing targeted, complementary differentiated preclinical and clinical stage compounds that treat immune mediated disease; and
•Opportunistically out-licensing rights to compounds, indications or geographies.
There is no guarantee that our products will obtain regulatory approval by the United States Food and Drug Administration (the “FDA”) or comparable foreign regulatory authorities. The FDA approval process is complex, time-consuming, and expensive. It typically involves the following prior to submitting a new drug application (“NDA”) or biologics license application (“BLA”): preclinical laboratory and animal testing, submission of an Investigational New Drug (“IND”) application, and human clinical trials to establish safety and efficacy. Human clinical trials typically include: Phase 1 studies to evaluate the safety and tolerability of the drug, generally in normal, healthy volunteers; Phase 2 studies to evaluate safety and efficacy, as well as appropriate doses; these studies are typically conducted in patient volunteers who suffer from the particular disease condition that the drug is designed to treat; and Phase 3 studies to evaluate safety and efficacy of the product at specific doses in one or more larger pivotal trials. Upon submission of an NDA or BLA, the FDA reviews the application including potentially an FDA advisory committee review and typically inspects manufacturing facilities and clinical study sites prior to FDA approval or rejection of the application. Even if a product receives FDA approval, the agency may impose post-approval requirements or withdraw approval if safety or efficacy issues arise. The processes for obtaining marketing approvals in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
Results of Operations
Comparison of the Three Months Ended September 30, 2025 and 2024
Product Revenue, Net
The Company recognized no product revenue for the three months ended September 30, 2025 compared to minimal revenue for the three months ended September 30, 2024. The Company’s license and supply agreement for Millipred®, an oral prednisolone indicated across a wide variety of inflammatory conditions, expired on September 30, 2023, as planned. The Company continues to monitor estimates for commercial liabilities, such as sales returns. As additional information becomes available, the Company could recognize expense (or benefit) for differences between actuals or updated estimates to the reserves previously recognized.
Cost of Product Sales
We recognized no cost of product sales for the three months ended September 30, 2025 and a benefit of $0.7 million for the three months ended September 30, 2024, which related to the change in an estimate of commercial liabilities related to the Millipred® product. The Company ceased selling Millipred® in September 2023.
The Company will continue to monitor estimates for commercial liabilities related to the Millipred® product, such as sales returns and profit share with the supplier pursuant to the reconciliation process. As additional information becomes available, the Company could recognize expense (or a benefit) for differences between actuals or updated estimates to the reserves previously recognized, which could be recognized in cost of product sales.
Research and Development Expenses
The following table summarizes our research and development expenses for the three months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended September 30, |
| |
|
2025 |
|
2024 |
| Nonclinical expenses |
|
$ |
184 |
|
|
$ |
154 |
|
| Clinical expenses |
|
7,250 |
|
|
5,078 |
|
| CMC expenses |
|
2,556 |
|
|
1,978 |
|
|
|
|
|
|
| Internal expenses: |
|
|
|
|
| Salaries, benefits and related costs |
|
2,383 |
|
|
1,511 |
|
| Stock-based compensation expense |
|
1,189 |
|
|
761 |
|
| Other |
|
59 |
|
|
56 |
|
| |
|
$ |
13,621 |
|
|
$ |
9,538 |
|
Research and development expenses increased $4.1 million for the three months ended September 30, 2025 compared to the three months ended September 30, 2024. This increase was mainly driven by a $2.2 million increase in clinical expenses, $0.9 million increase in salaries, benefits and related costs, and $0.6 million in chemistry, manufacturing, and controls (“CMC”) expenses.
Clinical expenses increased due to the ongoing activities related to the Phase 2 LOTUS trial in HS incurred in the third quarter of 2025, including patient trial costs and clinical trial work performed by our contract research organization (“CRO”), compared to limited expenses for trial initiation activities in the third quarter of 2024. CMC expenses increased due to continued drug manufacturing activities to support the trial, compared to raw materials purchases in the prior year period.
Salaries, benefits and related costs increased $0.9 million compared to the three months ended September 30, 2024 due primarily to headcount additions during the current year. Stock-based compensation expense increased $0.4 million related to option and restricted stock unit grants made in the second half of 2024 and in 2025, including the annual employee grants in August 2024 and January 2025, as well as headcount additions throughout 2025.
We expect future research and development expenses in 2025 to increase as compared to the comparable period in 2024 due to the ongoing execution of the Phase 2 LOTUS trial in HS and supporting activities. Research and development expenses beyond mid-2026 are difficult to predict given they will be highly dependent on the outcome of the Phase 2 LOTUS trial.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the three months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended September 30, |
| |
|
2025 |
|
2024 |
|
|
|
|
|
| Salaries, benefits and related costs |
|
$ |
1,449 |
|
|
$ |
1,160 |
|
| Legal, consulting and other professional expenses |
|
1,794 |
|
|
1,461 |
|
| Stock-based compensation expense |
|
1,964 |
|
|
1,086 |
|
Commercial planning and marketing expenses |
|
22 |
|
|
142 |
|
| Other |
|
348 |
|
|
437 |
|
| |
|
$ |
5,577 |
|
|
$ |
4,286 |
|
General and administrative expenses increased $1.3 million for the three months ended September 30, 2025 compared to the prior year period. The increase was driven by a $0.9 million increase in stock-based compensation expense due to option and restricted stock unit grants made in the second half of 2024 and in 2025, including the annual employee grants in August 2024 and January 2025, as well as headcount additions throughout 2025. Salaries, benefits and related costs increased $0.3 million compared to the prior year period due to headcount additions.
Although we expect most of the increase in operating expenses in 2025 to be attributable to increased research and development activities to progress AVTX-009, we also expect moderate increases to general and administrative expenses for the remainder of 2025 as compared to the comparable period in 2024, related to supporting the AVTX-009 program. General and administrative expenses beyond mid-2026 are difficult to predict given they will be highly dependent on the outcome of the Phase 2 LOTUS trial in HS.
Other (Expense) Income, Net
The following table summarizes our other (expense) income, net for the three months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended September 30, |
| |
|
2025 |
|
2024 |
| Change in fair value of derivative liability |
|
(12,530) |
|
|
(1,100) |
|
Interest income, net |
|
1,117 |
|
|
964 |
|
|
|
|
|
|
|
|
|
|
|
| Change in fair value of warrant liability |
|
— |
|
|
36,025 |
|
| Other expense, net |
|
(3) |
|
|
(5) |
|
|
|
$ |
(11,416) |
|
|
$ |
35,884 |
|
Other expense, net was $11.4 million for the three months ended September 30, 2025 compared to other income, net of $35.9 million for the prior year period. The $47.3 million change was primarily driven by (i) the accounting impact of the warrant liability associated with the warrants issued in the March 2024 financing that were subsequently exercised in the fourth quarter of 2024, and (ii) the change in the fair value of the derivative liability in the current period driven by changes in assumptions related to the AVTX-007 Milestones and Royalties (as defined in Note 6 to the unaudited condensed consolidated financial statements). Refer to Note 6 - Fair Value Measurements of the unaudited condensed consolidated financial statements for more information.
Income Tax Expense
The Company recognized minimal income tax expense for both the three months ended September 30, 2025 and 2024.
Comparison of the Nine Months Ended September 30, 2025 and 2024
Product Revenue, Net
The Company recognized no product revenue for the nine months ended September 30, 2025 compared to minimal revenue for the nine months ended September 30, 2024. The Company’s license and supply agreement for Millipred® expired on September 30, 2023 as planned. The Company continues to monitor estimates for commercial liabilities, such as sales returns. As additional information becomes available, the Company could recognize expense (or benefit) for differences between actuals or updated estimates to the reserves previously recognized.
Cost of Product Sales
We recognized no cost of product sales for the nine months ended September 30, 2025 compared to a benefit of $0.5 million for the same period in 2024, which related to the change in an estimate of commercial liabilities related to the Millipred® product. The Company ceased selling Millipred® in September 2023.
The Company will continue to monitor estimates for commercial liabilities related to the Millipred® product, such as sales returns and profit share with the supplier pursuant to the reconciliation process. As additional information becomes available, the Company could recognize expense (or a benefit) for differences between actuals or updated estimates to the reserves previously recognized, which could be recognized in cost of product sales.
Research and Development Expenses
The following table summarizes our research and development expenses for the nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
| |
|
2025 |
|
2024 |
| Nonclinical expenses |
|
$ |
445 |
|
|
$ |
501 |
|
| Clinical expenses |
|
18,315 |
|
|
6,719 |
|
| CMC expenses |
|
7,802 |
|
|
2,971 |
|
|
|
|
|
|
| Internal expenses: |
|
|
|
|
| Salaries, benefits and related costs |
|
6,414 |
|
|
4,644 |
|
| Stock-based compensation expense |
|
3,606 |
|
|
1,250 |
|
| Other |
|
235 |
|
|
169 |
|
| |
|
$ |
36,817 |
|
|
$ |
16,254 |
|
Research and development expenses increased $20.6 million for the nine months ended September 30, 2025. The increase was driven by increases in clinical and CMC expenses of $11.6 million and $4.8 million, respectively. Clinical expenses increased due to progress in the current year for the Phase 2 LOTUS trial in HS, including site activations, patient trial costs and clinical trial work performed by our CRO, as compared to trial enabling and activation activities incurred in the prior year period. CMC expenses increased due to raw material purchases and drug manufacturing activities to support the trial during the current year.
Stock-based compensation increased $2.4 million compared to the nine months ended September 30, 2024 due to option and restricted stock unit grants made during the second half of 2024 and in 2025, including the annual employee grants in August 2024 and January 2025, as well as headcount additions. Salaries, benefits and related costs increased $1.8 million compared to the nine months ended September 30, 2024 primarily due to headcount additions.
We expect future research and development expenses in 2025 to increase as compared to the comparable period in 2024 due to the ongoing execution of the Phase 2 LOTUS trial in HS and supporting activities. Research and development expenses beyond mid-2026 are difficult to predict given they will be highly dependent on the outcome of the Phase 2 LOTUS trial.
Acquired In-Process Research and Development
In the first quarter of 2024, we acquired AVTX-009 through the AlmataBio Transaction (as defined in Note 3 to the unaudited condensed consolidated financial statements), resulting in us acquiring $27.6 million of in-process research and development (“IPR&D”) in the nine months ended September 30, 2024. There was no acquired IPR&D for the nine months ended September 30, 2025.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
| |
|
2025 |
|
2024 |
|
|
|
|
|
| Salaries, benefits and related costs |
|
$ |
4,557 |
|
|
$ |
3,453 |
|
| Legal, consulting and other professional expenses |
|
4,986 |
|
|
5,318 |
|
| Stock-based compensation expense |
|
5,507 |
|
|
1,698 |
|
Commercial planning and marketing expenses |
|
89 |
|
|
300 |
|
| Other |
|
1,227 |
|
|
1,239 |
|
| |
|
$ |
16,366 |
|
|
$ |
12,008 |
|
General and administrative expenses increased $4.4 million for the nine months ended September 30, 2025 compared to the prior year period. The increase was driven primarily by a $3.8 million increase in stock-based compensation expense due to option and restricted stock unit grants made during the second half of 2024 and in 2025, including the annual grants in August 2024 and January 2025 as well as new hire grants. Salaries, benefits and related costs increased $1.1 million compared to the nine months ended September 30, 2024 due to headcount additions.
This increase was partially offset by a $0.3 million decrease in legal, consulting and other professional expenses compared to the prior year period related to increased expenses incurred in the prior period for accounting, reporting and consulting services incurred following the AlmataBio Transaction and concurrent private placement financing in March 2024.
Although we expect most of the increase in operating expenses in 2025 to be attributable to increased research and development activities to progress AVTX-009, we also expect moderate increases to general and administrative expenses for the remainder of 2025, as compared to the comparable period in 2024, related to supporting the AVTX-009 program. General and administrative expenses beyond mid-2026 are difficult to predict given they will be highly dependent on the outcome of the Phase 2 LOTUS trial in HS.
Other (Expense) Income, Net
The following table summarizes our other (expense) income, net for the nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
| |
|
2025 |
|
2024 |
| Change in fair value of derivative liability |
|
(14,680) |
|
|
(6,260) |
|
Interest income, net |
|
3,367 |
|
|
2,101 |
|
| Excess of initial warrant fair value over private placement proceeds |
|
— |
|
|
(79,276) |
|
| Change in fair value of warrant liability |
|
— |
|
|
148,071 |
|
| Private placement transaction costs |
|
— |
|
|
(9,220) |
|
| Other expense, net |
|
(8) |
|
|
(5) |
|
|
|
$ |
(11,321) |
|
|
$ |
55,411 |
|
Other expense, net was $11.3 million for the nine months ended September 30, 2025 compared to other income, net of $55.4 million for the prior year period. The $66.7 million change was primarily driven by (i) the accounting impact in the prior period of the warrant liability associated with the warrants issued in the March 2024 financing that were subsequently exercised in the fourth quarter of 2024, and (ii) the change in the fair value of the derivative liability in the current period driven by changes in assumptions related to the AVTX-007 Milestones and Royalties (as defined in Note 6 to the unaudited condensed consolidated financial statements). Refer to Note 6 - Fair Value Measurements of the unaudited condensed and consolidated financial statements for more information. Further, the Company incurred $9.2 million of private placement transaction costs in the prior year period that did not repeat in the current period, largely consisting of the placement agent fee of $7.0 million, and $1.7 million fee payable upon exercise of the warrants issued in the private placement investment.
Income Tax Expense
The Company recognized minimal income tax expense for both the nine months ended September 30, 2025 and 2024.
Liquidity and Capital Resources
Uses of Liquidity
The Company primarily uses cash to fund the ongoing development of AVTX-009 and costs associated with its organizational infrastructure. As of September 30, 2025, Avalo had $111.6 million in cash and cash equivalents and short-term investments.
Cash Flows
The following table summarizes our cash flows for the nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
| |
|
2025 |
|
2024 |
|
|
|
|
|
| Net cash (used in) provided by: |
|
|
|
|
| Operating activities |
|
$ |
(37,212) |
|
|
$ |
(34,012) |
|
| Investing activities |
|
(84,121) |
|
|
356 |
|
| Financing activities |
|
13,900 |
|
|
108,140 |
|
Net (decrease) increase in cash and cash equivalents |
|
$ |
(107,433) |
|
|
$ |
74,484 |
|
Net cash used in operating activities
Net cash used in operating activities was $37.2 million for the nine months ended September 30, 2025 and consisted primarily of net loss of $64.5 million, partially offset by net non-cash charges of $23.7 million and changes in our operating assets and liabilities of $3.7 million. The non-cash charges consisted primarily of a $14.7 million change in the fair value of the derivative liability and stock-based compensation of $9.1 million. Changes in our operating assets and liabilities consisted primarily of a $2.2 million decrease in prepaid expenses and other current assets due to our ongoing clinical work and a $1.3 million increase in accrued expenses and other liabilities primarily due to continued activity related to the LOTUS trial and the timing of vendor invoices.
Net cash used in operating activities was $34.0 million for the nine months ended September 30, 2024 and consisted primarily of net income of $0.2 million and adjustments to reconcile net income to net cash used in operating activities including the change in fair value of the warrant liability of $148.1 million, excess of initial warrant fair value over private placement investment proceeds of $79.3 million, acquired IPR&D of $27.6 million, $7.5 million milestone payment made to the former AlmataBio stockholders upon the closing of a private placement investment, change in fair value of the derivative liability of $6.3 million and stock-based compensation of $2.9 million. Prepaid expense increased $2.4 million primarily due to advances paid for AVTX-009 contracts and the timing of insurance prepayments. Accrued expenses and other liabilities increased $2.1 million primarily related to non-equity incentive compensation and increased research and development and general and administrative activities to support the development of AVTX-009.
Net cash (used in) provided by investing activities
Net cash used in investing activities for the nine months ended September 30, 2025 consisted of $94.6 million of purchases of available-for-sale investments, partially offset by $10.5 million of proceeds from maturities of available-for-sale investments.
Net cash provided by investing activities for the nine months ended September 30, 2024 consisted of the cash acquired as part of the AlmataBio Transaction.
Net cash provided by financing activities
Net cash provided by financing activities for the nine months ended September 30, 2025 consisted of net proceeds of $14.4 million from the sales of shares under our “at-the-market” sales agreement, partially offset by $0.5 million in cash paid to tax authorities related to withholding shares to satisfy RSU vesting withholding obligations on behalf of employees.
Net cash provided by financing activities for the nine months ended September 30, 2024 consisted of gross proceeds of $115.6 million from the private placement investment that closed on March 28, 2024, partially offset by transaction costs related to the private placement investment of $7.5 million.
Critical Accounting Policies, Estimates, and Assumptions
This Management’s Discussion and Analysis of Financial Condition and Results of Operations is based on our unaudited condensed consolidated financial statements included in this Quarterly Report on Form 10-Q, which have been prepared in accordance with GAAP. In preparing the financial statements in conformity with GAAP, the Company makes estimates and assumptions that have an impact on assets, liabilities, revenue and expenses reported. These estimates can also affect supplemental information disclosed by us, including information about contingencies, risk, and financial condition. In our unaudited condensed consolidated financial statements, estimates are used for, but not limited to, clinical trial accruals and research and development costs, stock-based compensation, fair value measurements, the valuation of derivative liabilities, revenue recognition, cost of product sales and cash flows used in management’s going concern assessment. The Company believes, given current facts and circumstances, that our estimates and assumptions are reasonable, adhere to GAAP and are consistently applied. Inherent in the nature of an estimate or assumption is the fact that actual results may differ from estimates, and estimates may vary as new facts and circumstances arise. Our most critical accounting estimates and assumptions are included in our Annual Report on Form 10-K for the year ended December 31, 2024 filed with the SEC on March 20, 2025. Except as described in Note 2 - Basis of Presentation and Significant Accounting Policies, there have been no significant changes to our critical accounting policies during the nine months ended September 30, 2025.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined by applicable SEC rules and regulations.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As a smaller reporting company, we are not required to provide the information required by this Item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
As required by Rule 13a-15(b) and Rule 15d-15(b) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, our management, including our principal executive officer and our principal financial officer, conducted an evaluation as of the end of the period covered by this Quarterly Report on Form 10-Q of the effectiveness of the design and operation of our disclosure controls and procedures. In designing and evaluating our disclosure controls and procedures, our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of the end of the period covered by this Quarterly Report on Form 10-Q.
Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the period covered by this Quarterly Report on Form 10-Q that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
Item 1. Legal Proceedings.
The information set forth in Note 13 - Commitments and Contingencies, under the heading “Litigation” to our unaudited condensed consolidated financial statements, included in Part I, Item 1 of this Quarterly Report on Form 10-Q, is incorporated herein by reference.
Item 1A. Risk Factors.
In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the factors discussed in Part I, “Item 1A. Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 20, 2025 (the “2024 10-K”), which could materially affect our business, financial condition, or future results. Our risk factors as of the date of this Quarterly Report on Form 10-Q have not changed materially from those described in the 2024 10-K referenced above. The risks described in the 2024 10-K referenced above, however, are not the only risks facing our Company. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially adversely affect our business, financial condition, or future results of operations and the trading price of our common stock.
Item 2. Unregistered Sales of Equity Securities, Use of Proceeds, and Issuer Purchases of Equity Securities.
(a)None.
(b)None.
(c)None.
Item 3. Defaults Upon Senior Securities.
Not applicable.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
Insider Trading Arrangements
During the quarter ended September 30, 2025, none of our directors or officers (as defined in rule 16a-1 (f) under the Exchange Act) adopted, modified or terminated a “Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading arrangement” (as such terms are defined in Item 408 of Regulation S-K).
Item 6. Exhibits.
|
|
|
|
|
|
|
|
|
Exhibit
Number |
|
Description of Exhibit |
|
|
|
| 10.1* |
|
|
|
|
|
| 10.2+ |
|
|
|
|
|
| 31.1+ |
|
|
|
|
|
| 31.2+ |
|
|
|
|
|
| 32.1† |
|
|
|
|
|
| 101 |
|
Interactive data files pursuant to Rule 405 of Regulation S-T: (i) Condensed Consolidated Balance Sheets as of September 30, 2025 (Unaudited) and December 31, 2024; (ii) Condensed Consolidated Statements of Operations and Comprehensive (Loss) Income (Unaudited) for the Three and Nine Months Ended September 30, 2025 and 2024; (iii) Condensed Consolidated Statements of Cash Flows (Unaudited) for the Nine Months Ended September 30, 2025 and 2024; (iv) Condensed Consolidated Statements of Mezzanine and Stockholders’ Equity (Unaudited) for the Three and Nine Months Ended September 30, 2025 and 2024; and (v) Notes to Unaudited Financial Statements. |
|
|
|
| 104 |
|
Cover Page Interactive Data File, formatted in XBRL (included in Exhibit 101). |
|
|
|
* Management contract or compensation plan or arrangement
+ Filed herewith.
† This certification is being furnished solely to accompany this Quarterly Report on Form 10-Q pursuant to 18 U.S.C. Section 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SIGNATURES |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized. |
|
|
|
|
|
|
|
|
|
Avalo Therapeutics, Inc. |
|
|
|
|
|
|
Date: November 6, 2025 |
|
/s/ Christopher Sullivan |
|
|
|
|
Christopher Sullivan |
|
|
|
|
Chief Financial Officer |
|
|
|
|
(on behalf of the registrant and as the registrant’s principal financial officer) |
|
|
|
|
|
EX-10.2
2
ex-102avtxx2025inducementa.htm
EX-10.2
Document
AVALO THERAPEUTICS, INC.
2025 INDUCEMENT AWARD PLAN
ADOPTED BY THE BOARD OF DIRECTORS: September 16, 2025
1. GENERAL.
(a) Purpose. The Company, by means of the Plan, intends to provide (i) inducements material to certain individuals to enter into employment with the Company within the meaning of Rule 5635(c)(4) of the Nasdaq Marketplace Rules, (ii) incentives for such persons to exert maximum efforts for the success of the Company and any Affiliate, and (iii) means by which Eligible Employees may be given an opportunity to benefit from increases in value of the Common Stock through the granting of Awards.
(b) Eligible Award Recipients. The only persons eligible to receive grants of Awards under this Plan are individuals who satisfy the standards for inducement grants under Nasdaq Marketplace Rule 5635(c)(4) and the related guidance under Nasdaq IM 5635-1. A person who previously served as an Employee or Director will not be eligible to receive Awards under the Plan, other than following a bona fide period of non-employment. Persons eligible to receive grants of Awards under this Plan are referred to in this Plan as “Eligible Employees.” Persons engaged as Directors and Consultants are not eligible to receive Awards under this Plan.
(c) Approval. Awards under this Plan must be approved by the Company’s compensation committee, provided such committee is comprised solely of “Independent Directors” (as such term is defined in Nasdaq Marketplace Rule 5605(a)(2)) of the Company in order to comply with the exemption from the stockholder approval requirement for “inducement grants” provided under the Inducement Award Rules (such committee, the “Independent Compensation Committee”).
(d) Available Awards. The Plan provides for the grant of the following types of Awards: (i) Nonstatutory Stock Options; (ii) Stock Appreciation Rights; (iii) Restricted Stock Awards; (iv) Restricted Stock Unit Awards; and (v) Other Stock Awards.
2. ADMINISTRATION.
(a) Administration by Board. The Board will administer the Plan; provided, however, that Awards may only be granted by either (i) a majority of the Company’s Independent Directors or (ii) the Independent Compensation Committee. Subject to those constraints and the other constraints of the Inducement Award Rules, the Board may delegate some of its powers of administration of the Plan to a Committee or Committees, as provided in Section 2(c).
(b) Powers of Board. The Board will have the power, subject to, and within the limitations of, the express provisions of the Plan and the Inducement Award Rules:
(i) To determine: (A) who will be granted Awards; (B) when and how each Award will be granted; (C) what type of Award will be granted; (D) the provisions of each Award (which need not be identical), including when a Participant will be permitted to exercise or otherwise receive cash or Common Stock under the Award; (E) the number of shares of Common Stock subject to, or the cash value of, an Award; and (F) the Fair Market Value applicable to a Stock Award; provided, however, that Awards may only be granted by either (i) a majority of the Company’s Independent Directors or (ii) the Independent Compensation Committee.
(ii) To construe and interpret the Plan and Awards granted under it, and to establish, amend and revoke rules and regulations for administration of the Plan and Awards. The Board, in the exercise of these powers, may correct any defect, omission or inconsistency in the Plan or in any Award Agreement, in a manner and to the extent it will deem necessary or expedient to make the Plan or Award fully effective.
(iii) To settle all controversies regarding the Plan and Awards granted under it.
(iv) To accelerate, in whole or in part, the time at which an Award may be exercised or vest (or at which cash or shares of Common Stock may be issued).
(v) To suspend or terminate the Plan at any time. Except as otherwise provided in the Plan (including Section 2(b)(viii)) or an Award Agreement, suspension or termination of the Plan will not materially impair a Participant’s rights under an outstanding Award without his or her written consent.
(vi) To amend the Plan in any respect the Board deems necessary or advisable, provided, however, that the Company will seek stockholder approval of any amendment of the Plan that changes the terms of the Plan in such a way as to require stockholder approval under applicable law or the listing standards of any national securities exchange or association on which the Company’s securities are listed. Except as otherwise provided in the Plan (including Section 2(b)(viii)) or an Award Agreement, no amendment of the Plan will materially impair a Participant’s rights under an outstanding Award without his or her written consent.
(vii) To submit any amendment to the Plan for stockholder approval.
(viii) To approve forms of Award Agreements for use under the Plan and to amend the terms of any one or more outstanding Awards, including, but not limited to, amendments to provide terms more favorable to the Participant than previously provided in the Award Agreement, subject to any specified limits in the Plan that are not subject to Board discretion; provided, however, that except as otherwise provided in the Plan (including this Section 2(b)(viii)) or an Award Agreement, the Board may not amend the terms of an outstanding Award if the Board, in its sole discretion, determines that the amendment, taken as a whole, will materially impair the Participant’s rights under such Award without his or her written consent.
Notwithstanding the foregoing or anything in the Plan to the contrary, unless prohibited by applicable law, the Board may amend the terms of any outstanding Award or the Plan, or may suspend or terminate the Plan, without the affected Participant’s consent, (A) to clarify the manner of exemption from, or to bring the Award or the Plan into compliance with, Section 409A of the Code, or (B) to comply with other applicable laws or listing requirements.
(ix) Generally, to exercise such powers and to perform such acts as the Board deems necessary or expedient to promote the best interests of the Company and that are not in conflict with the provisions of the Plan or Awards.
(x) To adopt such procedures and sub-plans as are necessary or appropriate to permit participation in the Plan by Eligible Employees who are foreign nationals or employed outside the United States (provided that Board approval will not be necessary for immaterial modifications to the Plan or any Award Agreement that are required for compliance with the laws of the relevant foreign jurisdiction).
(c) Delegation to Committee. Subject to the terms of Section 2(b), the Board may delegate some or all of the administration of the Plan to a Committee or Committees. Such Committee(s) shall consist solely of two or more Non-Employee Directors in accordance with Rule 16b-3. If administration of the Plan is delegated to a Committee, the Committee will have, in connection with the administration of the Plan, the powers theretofore possessed by the Board that have been delegated to the Committee, including the power to delegate to a subcommittee of the Committee any of the administrative powers the Committee is authorized to exercise (and references in this Plan to the Board will thereafter be to the Committee or subcommittee, as applicable). Any delegation of administrative powers will be reflected in resolutions, not inconsistent with the provisions of the Plan, adopted from time to time by the Board or Committee (as applicable). The Committee may, at any time, abolish the subcommittee and/or revest in the Committee any powers delegated to the subcommittee. The Board may retain the authority to concurrently administer the Plan with the Committee and may, at any time, revest in the Board some or all of the powers previously delegated.
(d) Effect of Board’s Decision. All determinations, interpretations and constructions made by the Board in good faith will not be subject to review by any person and will be final, binding and conclusive on all persons.
(e) Cancellation and Re-Grant of Stock Awards. Neither the Board nor any Committee will have the authority to (i) reduce the exercise or strike price of any outstanding Option or SAR under the Plan or (ii) cancel any outstanding Option or SAR that has an exercise or strike price greater than the then-current Fair Market Value of the Common Stock in exchange for cash or other Stock Awards under the Plan, unless the stockholders of the Company have approved such an action within 12 months prior to such an event.
3. SHARES SUBJECT TO THE PLAN.
(a) Share Reserve.
(i) Subject to Section 9(a) relating to Capitalization Adjustments, the aggregate number of shares of Common Stock that may be issued pursuant to Stock Awards from and after the Effective Date will not exceed 1,300,000 shares (the “Share Reserve”).
(ii) For clarity, the Share Reserve in this Section 3(a) is a limitation on the number of shares of Common Stock that may be issued pursuant to the Plan. Shares may be issued in connection with a merger or acquisition as permitted by Nasdaq Listing Rule 5635(c), and such issuance will not reduce the number of shares available for issuance under the Plan.
(b) Reversion of Shares to the Share Reserve. If a Stock Award or any portion thereof (i) expires or otherwise terminates without all of the shares covered by such Stock Award having been issued or (ii) is settled in cash (i.e., the Participant receives cash rather than stock), such expiration, termination or settlement will not reduce (or otherwise offset) the number of shares of Common Stock that may be available for issuance under the Plan. If any shares of Common Stock issued pursuant to a Stock Award are forfeited back to or repurchased by the Company because of the failure to meet a contingency or condition required to vest such shares in the Participant, then the shares that are forfeited or repurchased will revert to and again become available for issuance under the Plan. Any shares reacquired by the Company in satisfaction of tax withholding obligations on a Stock Award or as consideration for the exercise or purchase price of a Stock Award will again become available for issuance under the Plan.
(c) Source of Shares. The stock issuable under the Plan will be shares of authorized but unissued or reacquired Common Stock, including shares repurchased by the Company on the open market or otherwise.
4. ELIGIBILITY.
(a) Eligible Award Recipients. Awards may only be granted to persons who are Eligible Employees described in Section 1(b) of the Plan, where the Award is an inducement material to the individual’s entering into employment with the Company or an Affiliate within the meaning of Rule 5635(c)(4) of the Nasdaq Marketplace Rules or is otherwise permitted pursuant to Rule 5635(c) of the Nasdaq Marketplace Rules.
(b) Eligibility for Specific Stock Awards. Stock Awards may not be granted to Eligible Employees who are providing Continuous Service only to any “parent” of the Company, as such term is defined in Rule 405, unless (i) the stock underlying such Stock Awards is treated as “service recipient stock” under Section 409A of the Code (for example, because the Stock Awards are granted pursuant to a corporate transaction such as a spin off transaction), (ii) the Company, in consultation with its legal counsel, has determined that such Stock Awards are otherwise exempt from Section 409A of the Code, or (iii) the Company, in consultation with its legal counsel, has determined that such Stock Awards comply with the distribution requirements of Section 409A of the Code.
5. PROVISIONS RELATING TO OPTIONS AND STOCK APPRECIATION RIGHTS.
Each Option or SAR Agreement will be in such form and will contain such terms and conditions as the Board deems appropriate. All Options will be Nonstatutory Stock Options. The terms and conditions of separate Option or SAR Agreements need not be identical; provided, however, that each Award Agreement will conform to (through incorporation of the provisions hereof by reference in the applicable Award Agreement or otherwise) the substance of each of the following provisions:
(a) Term. No Option or SAR will be exercisable after the expiration of 10 years from the date of its grant or such shorter period specified in the Award Agreement.
(b) Exercise Price. The exercise or strike price of each Option or SAR will be not less than 100% of the Fair Market Value of the Common Stock subject to the Option or SAR on the date the Award is granted. Notwithstanding the foregoing, an Option or SAR may be granted with an exercise or strike price lower than 100% of the Fair Market Value of the Common Stock subject to the Award if such Award is granted pursuant to an assumption of or substitution for another option or stock appreciation right pursuant to a Corporate Transaction and in a manner consistent with the provisions of Section 409A of the Code. Each SAR will be denominated in shares of Common Stock equivalents.
(c) Purchase Price for Options. The purchase price of Common Stock acquired pursuant to the exercise of an Option may be paid, to the extent permitted by applicable law and as determined by the Board in its sole discretion, by any combination of the methods of payment set forth below. The Board will have the authority to grant Options that do not permit all of the following methods of payment (or that otherwise restrict the ability to use certain methods) and to grant Options that require the consent of the Company to use a particular method of payment. The permitted methods of payment are as follows:
(i) by cash (including electronic funds transfers), check, bank draft or money order payable to the Company;
(ii) pursuant to a program developed under Regulation T as promulgated by the Federal Reserve Board that, prior to the issuance of the stock subject to the Option, results in either the receipt of cash (or check) by the Company or the receipt of irrevocable instructions to pay the aggregate exercise price to the Company from the sales proceeds;
(iii) by delivery to the Company (either by actual delivery or attestation) of shares of Common Stock;
(iv) by a “net exercise” arrangement pursuant to which the Company will reduce the number of shares of Common Stock issuable upon exercise by the largest whole number of shares with a Fair Market Value that does not exceed the aggregate exercise price; provided, however, that the Company will accept a cash or other payment from the Participant to the extent of any remaining balance of the aggregate exercise price not satisfied by such reduction in the number of whole shares to be issued. Shares of Common Stock will no longer be subject to an Option and will not be exercisable thereafter to the extent that (A) shares issuable upon exercise are used to pay the exercise price pursuant to the “net exercise,” (B) shares are delivered to the Participant as a result of such exercise, and (C) shares are withheld to satisfy tax withholding obligations; or
(v) in any other form of legal consideration that may be acceptable to the Board and specified in the applicable Award Agreement.
(d) Exercise and Payment of a SAR. To exercise any outstanding SAR, the Participant must provide written notice of exercise to the Company in compliance with the provisions of the Award Agreement evidencing such SAR. The appreciation distribution payable on the exercise of a SAR will be not greater than an amount equal to the excess of (A) the aggregate Fair Market Value (on the date of the exercise of the SAR) of a number of shares of Common Stock equal to the number of Common Stock equivalents in which the Participant is vested under such SAR, and with respect to which the Participant is exercising the SAR on such date, over (B) the aggregate strike price of the number of Common Stock equivalents with respect to which the Participant is exercising the SAR on such date. The appreciation distribution may be paid in Common Stock, in cash, in any combination of the two or in any other form of consideration, as determined by the Board and contained in the Award Agreement evidencing such SAR.
(e) Transferability of Options and SARs. The Board may, in its sole discretion, impose such limitations on the transferability of Options and SARs as the Board will determine. In the absence of such a determination by the Board to the contrary, the following restrictions on the transferability of Options and SARs will apply:
(i) Restrictions on Transfer. An Option or SAR will not be transferable, except by will or by the laws of descent and distribution (or pursuant to Sections 5(e)(ii) and 5(e)(iii)), and will be exercisable during the lifetime of the Participant only by the Participant. The Board may permit transfer of the Option or SAR in a manner that is not prohibited by applicable tax and securities laws. Except as explicitly provided in the Plan, neither an Option nor a SAR may be transferred for consideration.
(ii) Domestic Relations Orders. Subject to the approval of the Board or a duly authorized Officer, an Option or SAR may be transferred pursuant to the terms of a domestic relations order, official marital settlement agreement or other divorce or separation instrument.
(iii) Beneficiary Designation. Subject to the approval of the Board or a duly authorized Officer, a Participant may, by delivering written notice to the Company, in a form approved by the Company (or the designated broker), designate a third party who, upon the death of the Participant, will thereafter be entitled to exercise the Option or SAR and receive the Common Stock or other consideration resulting from such exercise. In the absence of such a designation, upon the death of the Participant, the executor or administrator of the Participant’s estate will be entitled to exercise the Option or SAR and receive the Common Stock or other consideration resulting from such exercise. However, the Company may prohibit designation of a beneficiary at any time, including due to any conclusion by the Company that such designation would be inconsistent with the provisions of applicable laws.
(f) Vesting Generally. The total number of shares of Common Stock subject to an Option or SAR may vest and become exercisable in periodic installments that may or may not be equal. The Option or SAR may be subject to such other terms and conditions on the time or times when it may or may not be exercised (which may be based on the satisfaction of performance goals or other criteria) as the Board may deem appropriate. The vesting provisions of individual Options or SARs may vary. The provisions of this Section 5(f) are subject to any Option or SAR provisions governing the minimum number of shares of Common Stock as to which an Option or SAR may be exercised.
(g) Termination of Continuous Service. Except as otherwise provided in the applicable Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service terminates (other than for Cause and other than upon the Participant’s death or Disability), the Participant may exercise his or her Option or SAR (to the extent that the Participant was entitled to exercise such Option or SAR as of the date of termination of Continuous Service), but only within such period of time ending on the earlier of (i) the date that is three months following such termination of Continuous Service (or such longer or shorter period specified in the Award Agreement), and (ii) the expiration of the term of the Option or SAR as set forth in the Award Agreement. If, after such termination of Continuous Service, the Participant does not exercise his or her Option or SAR (as applicable) within the applicable time frame, the Option or SAR (as applicable) will terminate.
(h) Extension of Termination Date. Except as otherwise provided in the applicable Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if the exercise of an Option or SAR following the termination of a Participant’s Continuous Service (other than for Cause and other than upon the Participant’s death or Disability) would be prohibited at any time solely because the issuance of shares of Common Stock would violate the registration requirements under the Securities Act, then the Option or SAR will terminate on the earlier of (i) the expiration of a total period of time (that need not be consecutive) equal to the applicable post-termination exercise period after the termination of the Participant’s Continuous Service during which the exercise of the Option or SAR would not be in violation of such registration requirements, or (ii) the expiration of the term of the Option or SAR as set forth in the applicable Award Agreement. In addition, except as otherwise provided in the applicable Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if the sale of any Common Stock received upon exercise of an Option or SAR following the termination of a Participant’s Continuous Service (other than for Cause) would violate the Company’s insider trading policy, then the Option or SAR will terminate on the earlier of (i) the expiration of a total period of time (that need not be consecutive) equal to the applicable post-termination exercise period after the termination of the Participant’s Continuous Service during which the sale of the Common Stock received upon exercise of the Option or SAR would not be in violation of the Company’s insider trading policy, or (ii) the expiration of the term of the Option or SAR as set forth in the applicable Award Agreement.
(i) Disability of Participant. Except as otherwise provided in the applicable Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service terminates as a result of the Participant’s Disability, the Participant may exercise his or her Option or SAR (to the extent that the Participant was entitled to exercise such Option or SAR as of the date of termination of Continuous Service), but only within such period of time ending on the earlier of (i) the date that is 12 months following such termination of Continuous Service (or such longer or shorter period specified in the Award Agreement), and (ii) the expiration of the term of the Option or SAR as set forth in the Award Agreement. If, after such termination of Continuous Service, the Participant does not exercise his or her Option or SAR (as applicable) within the applicable time frame, the Option or SAR (as applicable) will terminate.
(j) Death of Participant. Except as otherwise provided in the applicable Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if (i) a Participant’s Continuous Service terminates as a result of the Participant’s death, or (ii) a Participant dies within the period (if any) specified in the Award Agreement for exercisability after the termination of the Participant’s Continuous Service (for a reason other than death), then the Participant’s Option or SAR may be exercised (to the extent that the Participant was entitled to exercise such Option or SAR as of the date of death) by the Participant’s estate, by a person who acquired the right to exercise the Option or SAR by bequest or inheritance, or by a person designated to exercise the Option or SAR upon the Participant’s death, but only within such period of time ending on the earlier of (i) the date that is 18 months following the date of death (or such longer or shorter period specified in the Award Agreement), and (ii) the expiration of the term of the Option or SAR as set forth in the Award Agreement. If, after the Participant’s death, the Option or SAR (as applicable) is not exercised within the applicable time frame, the Option or SAR (as applicable) will terminate.
(k) Termination for Cause. Except as explicitly provided otherwise in the applicable Award Agreement or other individual written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service is terminated for Cause, the Participant’s Option or SAR will terminate immediately upon such termination of Continuous Service, and the Participant will be prohibited from exercising his or her Option or SAR from and after the time of such termination of Continuous Service.
(l) Non-Exempt Employees. If an Option or SAR is granted to an Employee who is a non-exempt employee for purposes of the Fair Labor Standards Act of 1938, as amended, the Option or SAR will not be first exercisable for any shares of Common Stock until at least six months following the date of grant of the Option or SAR (although the Award may vest prior to such date). Consistent with the provisions of the Worker Economic Opportunity Act, (i) if such non-exempt employee dies or suffers a Disability, (ii) upon a Corporate Transaction in which such Option or SAR is not assumed, continued or substituted, (iii) upon a Change in Control, or (iv) upon the Participant’s retirement (as such term may be defined in the Participant’s Award Agreement, in another written agreement between the Participant and the Company or an Affiliate, or, if no such definition, in accordance with the Company’s then current employment policies and guidelines), the vested portion of any Options and SARs may be exercised earlier than six months following the date of grant. The foregoing provision is intended to operate so that any income derived by a non-exempt employee in connection with the exercise or vesting of an Option or SAR will be exempt from his or her regular rate of pay. To the extent permitted and/or required for compliance with the Worker Economic Opportunity Act to ensure that any income derived by a non-exempt employee in connection with the exercise, vesting or issuance of any shares under any other Stock Award will be exempt from the employee’s regular rate of pay, the provisions of this Section 5(l) will apply to all Stock Awards and are hereby incorporated by reference into such Stock Award Agreements.
6. PROVISIONS OF STOCK AWARDS OTHER THAN OPTIONS AND SARS.
(a) Restricted Stock Awards. Each Restricted Stock Award Agreement will be in such form and will contain such terms and conditions as the Board deems appropriate. To the extent consistent with the Company’s bylaws, at the Board’s election, shares of Common Stock underlying a Restricted Stock Award may be (i) held in book entry form subject to the Company’s instructions until any restrictions relating to the Restricted Stock Award lapse, or (ii) evidenced by a certificate, which certificate will be held in such form and manner as determined by the Board. The terms and conditions of separate Restricted Stock Award Agreements need not be identical; provided, however, that each Restricted Stock Award Agreement will conform to (through incorporation of the provisions hereof by reference in the applicable Award Agreement or otherwise) the substance of each of the following provisions:
(i) Consideration. A Restricted Stock Award may be awarded in consideration for (A) cash (including electronic funds transfers), check, bank draft or money order payable to the Company, (B) past services to the Company or an Affiliate, or (C) any other form of legal consideration (including future services) that may be acceptable to the Board, in its sole discretion, and permissible under applicable law.
(ii) Vesting. Shares of Common Stock awarded under a Restricted Stock Award Agreement may be subject to forfeiture to or repurchase by the Company in accordance with a vesting schedule to be determined by the Board (which may be based on the satisfaction of performance goals or other criteria).
(iii) Termination of Continuous Service. If a Participant’s Continuous Service terminates, the Company may receive through a forfeiture condition or a repurchase right any or all of the shares of Common Stock held by the Participant that have not vested as of the date of such termination under the terms of the Participant’s Restricted Stock Award Agreement.
(iv) Transferability. Rights to acquire shares of Common Stock under a Restricted Stock Award Agreement will be transferable by the Participant only upon such terms and conditions as are set forth in the Restricted Stock Award Agreement, as the Board will determine in its sole discretion, so long as Common Stock awarded under the Restricted Stock Award Agreement remains subject to the terms of the Restricted Stock Award Agreement.
(v) Dividends. A Restricted Stock Award Agreement may provide that any dividends paid on Restricted Stock will be subject to the same vesting and forfeiture restrictions as apply to the shares subject to the Restricted Stock Award to which they relate.
(b) Restricted Stock Unit Awards. Each Restricted Stock Unit Award Agreement will be in such form and will contain such terms and conditions as the Board deems appropriate. The terms and conditions of separate Restricted Stock Unit Award Agreements need not be identical; provided, however, that each Restricted Stock Unit Award Agreement will conform to (through incorporation of the provisions hereof by reference in the applicable Award Agreement or otherwise) the substance of each of the following provisions:
(i) Consideration. At the time of grant of a Restricted Stock Unit Award, the Board will determine the consideration, if any, to be paid by the Participant upon delivery of each share of Common Stock subject to the Restricted Stock Unit Award. The consideration to be paid (if any) by the Participant for each share of Common Stock subject to a Restricted Stock Unit Award may be paid in any form of legal consideration that may be acceptable to the Board, in its sole discretion, and permissible under applicable law.
(ii) Vesting. At the time of the grant of a Restricted Stock Unit Award, the Board may impose such restrictions on or conditions to the vesting of the Restricted Stock Unit Award as it, in its sole discretion, deems appropriate (which may be based on the satisfaction of performance goals or other criteria).
(iii) Payment. A Restricted Stock Unit Award may be settled by the delivery of shares of Common Stock, their cash equivalent, any combination thereof or in any other form of consideration, as determined by the Board and contained in the Restricted Stock Unit Award Agreement.
(iv) Additional Restrictions. At the time of the grant of a Restricted Stock Unit Award, the Board, as it deems appropriate, may impose such restrictions or conditions that delay the delivery of the shares of Common Stock (or their cash equivalent) subject to the Restricted Stock Unit Award to a time after the vesting of the Restricted Stock Unit Award.
(v) Dividend Equivalents. Dividend equivalents may be credited in respect of shares of Common Stock covered by a Restricted Stock Unit Award, as determined by the Board and contained in the Restricted Stock Unit Award Agreement. At the sole discretion of the Board, such dividend equivalents may be converted into additional shares of Common Stock covered by the Restricted Stock Unit Award in such manner as determined by the Board. Any additional shares covered by the Restricted Stock Unit Award credited by reason of such dividend equivalents will be subject to all of the same terms and conditions of the underlying Restricted Stock Unit Award Agreement to which they relate.
(vi) Termination of Continuous Service. Except as otherwise provided in the applicable Restricted Stock Unit Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service terminates, any portion of the Participant’s Restricted Stock Unit Award that has not vested as of the date of such termination will be forfeited upon such termination.
(c) Other Stock Awards. Other forms of Stock Awards valued in whole or in part by reference to, or otherwise based on, Common Stock, including the appreciation in value thereof (e.g., options or stock appreciation rights with an exercise price or strike price less than 100% of the Fair Market Value of the Common Stock at the time of grant) may be granted either alone or in addition to Stock Awards granted under Section 5 and this Section 6. Subject to the provisions of the Plan, the Independent Compensation Committee will have sole and complete authority to determine the persons to whom and the time or times at which such Other Stock Awards will be granted, the number of shares of Common Stock (or the cash equivalent thereof) to be granted pursuant to such Other Stock Awards and all other terms and conditions of such Other Stock Awards.
7. COVENANTS OF THE COMPANY.
(a) Availability of Shares. The Company will keep available at all times the number of shares of Common Stock reasonably required to satisfy then-outstanding Stock Awards.
(b) Securities Law Compliance. The Company will seek to obtain from each regulatory commission or agency having jurisdiction over the Plan the authority required to grant Stock Awards and to issue and sell shares of Common Stock upon exercise of the Stock Awards; provided, however, that this undertaking will not require the Company to register under the Securities Act the Plan, any Stock Award or any Common Stock issued or issuable pursuant to any such Stock Award. If, after reasonable efforts and at a reasonable cost, the Company is unable to obtain from any such regulatory commission or agency the authority that counsel for the Company deems necessary for the lawful issuance and sale of Common Stock under the Plan, the Company will be relieved from any liability for failure to issue and sell Common Stock upon exercise of such Stock Awards unless and until such authority is obtained. A Participant will not be eligible for the grant of an Award or the subsequent issuance of cash or Common Stock pursuant to the Award if such grant or issuance would be in violation of any applicable securities law.
(c) No Obligation to Notify or Minimize Taxes. The Company will have no duty or obligation to any Participant to advise such holder as to the time or manner of exercising a Stock Award. Furthermore, the Company will have no duty or obligation to warn or otherwise advise such holder of a pending termination or expiration of an Award or a possible period in which the Award may not be exercised. The Company has no duty or obligation to minimize the tax consequences of an Award to the holder of such Award.
8. MISCELLANEOUS.
(a) Use of Proceeds from Sales of Common Stock. Proceeds from the sale of shares of Common Stock issued pursuant to Stock Awards will constitute general funds of the Company.
(b) Corporate Action Constituting Grant of Awards. Corporate action constituting a grant by the Company of an Award to any Participant will be deemed completed as of the date of such corporate action, unless otherwise determined by the Board, regardless of when the instrument, certificate or letter evidencing the Award is communicated to, or actually received or accepted by, the Participant. In the event that the corporate records (e.g., Board consents, resolutions or minutes) documenting the corporate action constituting the grant contain terms (e.g., exercise price, vesting schedule or number of shares) that are inconsistent with those in the Award Agreement or related grant documents as a result of a clerical error in the papering of the Award Agreement or related grant documents, the corporate records will control and the Participant will have no legally binding right to the incorrect term in the Award Agreement or related grant documents.
(c) Stockholder Rights. No Participant will be deemed to be the holder of, or to have any of the rights of a holder with respect to, any shares of Common Stock subject to an Award unless and until (i) such Participant has satisfied all requirements for exercise of, or the issuance of shares of Common Stock under, the Award pursuant to its terms, and (ii) the issuance of the Common Stock subject to such Award has been entered into the books and records of the Company.
(d) No Employment or Other Service Rights. Nothing in the Plan, any Award Agreement or any other instrument executed thereunder or in connection with any Award granted pursuant thereto will confer upon any Participant any right to continue to serve the Company or an Affiliate in the capacity in effect at the time the Award was granted or will affect the right of the Company or an Affiliate to terminate (i) the employment of an Employee with or without notice and with or without cause, (ii) the service of a Consultant pursuant to the terms of such Consultant’s agreement with the Company or an Affiliate, or (iii) the service of a Director pursuant to the bylaws of the Company or an Affiliate, and any applicable provisions of the corporate law of the state in which the Company or the Affiliate is incorporated, as the case may be.
(e) Change in Time Commitment. In the event a Participant’s regular level of time commitment in the performance of his or her services for the Company or any Affiliate is reduced (for example, and without limitation, if the Participant is an Employee of the Company and the Employee has a change in status from a full-time Employee to a part-time Employee or takes an extended leave of absence) after the date of grant of any Award to the Participant, the Board has the right in its sole discretion to (i) make a corresponding reduction in the number of shares or cash amount subject to any portion of such Award that is scheduled to vest or become payable after the date of such change in time commitment, and (ii) in lieu of or in combination with such a reduction, extend the vesting or payment schedule applicable to such Award. In the event of any such reduction, the Participant will have no right with respect to any portion of the Award that is so reduced or extended.
(f) Investment Assurances. The Company may require a Participant, as a condition of exercising or acquiring Common Stock under any Award, (i) to give written assurances satisfactory to the Company as to the Participant’s knowledge and experience in financial and business matters and/or to employ a purchaser representative reasonably satisfactory to the Company who is knowledgeable and experienced in financial and business matters and that he or she is capable of evaluating, alone or together with the purchaser representative, the merits and risks of exercising the Award, and (ii) to give written assurances satisfactory to the Company stating that the Participant is acquiring Common Stock subject to the Award for the Participant’s own account and not with any present intention of selling or otherwise distributing the Common Stock. The foregoing requirements, and any assurances given pursuant to such requirements, will be inoperative if (A) the issuance of the shares upon the exercise or acquisition of Common Stock under the Stock Award has been registered under a then currently effective registration statement under the Securities Act, or (B) as to any particular requirement, a determination is made by counsel for the Company that such requirement need not be met in the circumstances under the then applicable securities laws. The Company may, upon advice of counsel to the Company, place legends on stock certificates issued under the Plan as such counsel deems necessary or appropriate in order to comply with applicable securities laws, including, but not limited to, legends restricting the transfer of the Common Stock.
(g) Withholding Obligations. Unless prohibited by the terms of an Award Agreement, the Company may, in its sole discretion, satisfy any federal, state or local tax withholding obligation relating to an Award by any of the following means or by a combination of such means: (i) causing the Participant to tender a cash payment; (ii) withholding shares of Common Stock from the shares of Common Stock issued or otherwise issuable to the Participant in connection with the Stock Award; provided, however, that no shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; (iii) withholding cash from an Award settled in cash; (iv) withholding payment from any amounts otherwise payable to the Participant; or (v) by such other method as may be set forth in the Award Agreement.
(h) Electronic Delivery. Any reference herein to a “written” agreement or document will include any agreement or document delivered electronically, filed publicly at www.sec.gov (or any successor website thereto) or posted on the Company’s intranet (or other shared electronic medium controlled by the Company to which the Participant has access).
(i) Deferrals. To the extent permitted by applicable law, the Board, in its sole discretion, may determine that the delivery of Common Stock or the payment of cash, upon the exercise, vesting or settlement of all or a portion of any Award may be deferred and may establish programs and procedures for deferral elections to be made by Participants. Deferrals by Participants will be made in accordance with Section 409A of the Code. Consistent with Section 409A of the Code, the Board may provide for distributions while a Participant is still an employee or otherwise providing services to the Company. The Board is authorized to make deferrals of Awards and determine when, and in what annual percentages, Participants may receive payments, including lump sum payments, following the Participant’s termination of Continuous Service, and implement such other terms and conditions consistent with the provisions of the Plan and in accordance with applicable law.
(j) Section 409A Compliance. Unless otherwise expressly provided for in an Award Agreement, the Plan and Award Agreements will be interpreted to the greatest extent possible in a manner that makes the Plan and the Awards granted hereunder exempt from Section 409A of the Code, and, to the extent not so exempt, in compliance with Section 409A of the Code. If the Board determines that any Award granted hereunder is not exempt from and is therefore subject to Section 409A of the Code, the Award Agreement evidencing such Award will incorporate the terms and conditions necessary to avoid the consequences specified in Section 409A(a)(1) of the Code and to the extent an Award Agreement is silent on terms necessary for compliance, such terms are hereby incorporated by reference into the Award Agreement. Notwithstanding anything to the contrary in this Plan (and unless the Award Agreement specifically provides otherwise), if the shares of Common Stock are publicly traded, and if a Participant holding an Award that constitutes “deferred compensation” under Section 409A of the Code is a “specified employee” for purposes of Section 409A of the Code, no distribution or payment of any amount that is due because of a “separation from service” (as defined in Section 409A of the Code without regard to alternative definitions thereunder) will be issued or paid before the date that is six months following the date of the Participant’s “separation from service” or, if earlier, the date of the Participant’s death, unless such distribution or payment may be made in a manner that complies with Section 409A of the Code, and any amounts so deferred will be paid in a lump sum on the day after such six month period elapses, with the balance paid thereafter on the original schedule.
(k) Clawback/Recovery. All Awards granted under the Plan will be subject to reduction, cancellation, forfeiture or recoupment in accordance with any clawback policy that the Company is required to adopt pursuant to the listing standards of any national securities exchange or association on which the Company’s securities are listed or as is otherwise required by the Dodd-Frank Wall Street Reform and Consumer Protection Act or other applicable law. By accepting an Award, the Participant is agreeing to be bound by each such clawback policy, as in effect or as may be adopted and/or modified from time to time by the Company in its discretion (including, without limitation, to comply with applicable law or stock exchange listing requirements). In addition, the Board may impose such other clawback, recovery or recoupment provisions in an Award Agreement as the Board determines necessary or appropriate, including, but not limited to, a reacquisition right in respect of previously acquired shares of Common Stock or other cash or property upon the occurrence of Cause. No recovery of compensation under such a clawback policy will be an event giving rise to a right to resign for “good reason” or “constructive termination” (or similar term) under any agreement with the Company.
9. ADJUSTMENTS UPON CHANGES IN COMMON STOCK; OTHER CORPORATE EVENTS.
(a) Capitalization Adjustments. In the event of a Capitalization Adjustment, the Board will appropriately and proportionately adjust: (i) the class(es) and maximum number of securities subject to the Plan pursuant to Section 3(a); and (ii) the class(es) and number of securities and price per share of stock subject to outstanding Stock Awards. The Board will make such adjustments, and its determination will be final, binding and conclusive.
(b) Dissolution or Liquidation. Except as otherwise provided in the applicable Stock Award Agreement or other written agreement between a Participant and the Company or an Affiliate, in the event of a dissolution or liquidation of the Company, all outstanding Stock Awards (other than Stock Awards consisting of vested and outstanding shares of Common Stock not subject to a forfeiture condition or the Company’s right of repurchase) will terminate immediately prior to the completion of such dissolution or liquidation, and the shares of Common Stock subject to a forfeiture condition or the Company’s right of repurchase may be reacquired or repurchased by the Company notwithstanding the fact that the holder of such Stock Award is providing Continuous Service; provided, however, that the Board may, in its sole discretion, cause some or all Stock Awards to become fully vested, exercisable and/or no longer subject to forfeiture or repurchase (to the extent such Stock Awards have not previously expired or terminated) before the dissolution or liquidation is completed but contingent on its completion.
(c) Corporate Transactions. In the event of a Corporate Transaction, notwithstanding any other provision of the Plan, the Board may take one or more of the following actions with respect to Stock Awards, contingent upon the closing or consummation of the Corporate Transaction, unless otherwise provided in the instrument evidencing the Stock Award, in any other written agreement between the Company or any Affiliate and the Participant or in any director compensation policy of the Company, or unless otherwise expressly provided by the Board at the time of grant of the Stock Award:
(i) arrange for the surviving corporation or acquiring corporation (or the surviving or acquiring corporation’s parent company) to assume or continue the Stock Award or to substitute a similar stock award for the Stock Award (including, but not limited to, an award to acquire the same consideration paid to the stockholders of the Company pursuant to the Corporate Transaction);
(ii) arrange for the assignment of any reacquisition or repurchase rights held by the Company in respect of Common Stock issued pursuant to the Stock Award to the surviving corporation or acquiring corporation (or the surviving or acquiring corporation’s parent company);
(iii) accelerate the vesting, in whole or in part, of the Stock Award (and, if applicable, the time at which the Stock Award may be exercised) to a date prior to the effective time of such Corporate Transaction as the Board determines (or, if the Board does not determine such a date, to the date that is five days prior to the effective date of the Corporate Transaction), with such Stock Award terminating if not exercised (if applicable) at or prior to the effective time of the Corporate Transaction; provided, however, that the Board may require Participants to complete and deliver to the Company a notice of exercise before the effective date of a Corporate Transaction, which exercise is contingent upon the effectiveness of such Corporate Transaction;
(iv) arrange for the lapse, in whole or in part, of any reacquisition or repurchase rights held by the Company with respect to the Stock Award;
(v) cancel or arrange for the cancellation of the Stock Award, to the extent not vested or not exercised prior to the effective time of the Corporate Transaction, and pay such cash consideration (including no consideration) as the Board, in its sole discretion, may consider appropriate; and
(vi) cancel or arrange for the cancellation of the Stock Award, to the extent not vested or not exercised prior to the effective time of the Corporate Transaction, in exchange for a payment, in such form as may be determined by the Board equal to the excess, if any, of (A) the per share amount payable to holders of Common Stock in connection with the Corporate Transaction, over (B) the per share exercise price under the applicable Award. For clarity, this payment may be zero ($0) if the value of the property is equal to or less than the exercise price. In addition, any escrow, holdback, earnout or similar provisions in the definitive agreement for the Corporate Transaction may apply to such payment to the same extent and in the same manner as such provisions apply to the holders of Common Stock.
The Board need not take the same action or actions with respect to all Stock Awards or portions thereof or with respect to all Participants. The Board may take different actions with respect to the vested and unvested portions of a Stock Award.
(d) Change in Control. A Stock Award may be subject to additional acceleration of vesting and exercisability upon or after a Change in Control as may be provided in the Stock Award Agreement for such Stock Award, in any other written agreement between the Company or any Affiliate and the Participant or in any director compensation policy of the Company, but in the absence of such provision, no such acceleration will occur.
10. TERMINATION OR SUSPENSION OF THE PLAN.
(a) The Board may suspend or terminate the Plan at any time. No Awards may be granted under the Plan while the Plan is suspended or after it is terminated.
(b) No Impairment of Rights. Suspension or termination of the Plan will not materially impair rights and obligations under any Award granted while the Plan is in effect except with the written consent of the affected Participant or as otherwise permitted in the Plan (including Section 2(b)(viii)) or an Award Agreement.
11. EFFECTIVE DATE OF PLAN.
This Plan will become effective on the Effective Date.
12. CHOICE OF LAW.
The laws of the State of Delaware will govern all questions concerning the construction, validity and interpretation of this Plan, without regard to that state’s conflict of laws rules.
13. DEFINITIONS. As used in the Plan, the following definitions will apply to the capitalized terms indicated below:
(a) “Affiliate” means, at the time of determination, any “parent” or “subsidiary” of the Company as such terms are defined in Rule 405. The Board will have the authority to determine the time or times at which “parent” or “subsidiary” status is determined within the foregoing definition.
(b) “Award” or “Stock Award” means any right to receive Common Stock granted under the Plan, including a Nonstatutory Stock Option, a Stock Appreciation Right, a Restricted Stock Award, a Restricted Stock Unit Award, or any Other Stock Award.
(c) “Award Agreement” means a written agreement between the Company and a Participant evidencing the terms and conditions of an Award.
(d) “Board” means the Board of Directors of the Company.
(e) “Capital Stock” means each and every class of common stock of the Company, regardless of the number of votes per share.
(f) “Capitalization Adjustment” means any change that is made in, or other events that occur with respect to, the Common Stock subject to the Plan or subject to any Stock Award after the Effective Date without the receipt of consideration by the Company through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, stock split, reverse stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or any similar equity restructuring transaction, as that term is used in Statement of Financial Accounting Standards Board Accounting Standards Codification Topic 718 (or any successor thereto). Notwithstanding the foregoing, the conversion of any convertible securities of the Company will not be treated as a Capitalization Adjustment.
(g) “Cause” will have the meaning ascribed to such term in any written agreement between a Participant and the Company or an Affiliate defining such term and, in the absence of such agreement, such term means, with respect to a Participant, the occurrence of any of the following events: (i) such Participant has breached his or her employment or service contract with the Company or an Affiliate, (ii) such Participant has engaged in disloyalty to the Company or an Affiliate, including, without limitation, fraud, embezzlement, theft, commission of a felony or proven dishonesty, (iii) such Participant has disclosed trade secrets or confidential information of the Company or an Affiliate to persons not entitled to receive such information, (iv) such Participant has breached any written non-competition, non-solicitation, invention assignment or confidentiality agreement between the Participant and the Company or an Affiliate or (v) such Participant has engaged in such other behavior detrimental to the interests of the Company or an Affiliate as the Company determines. The determination that a termination of the Participant’s Continuous Service is either for Cause or without Cause will be made by the Company, in its sole discretion. Any determination by the Company that the Continuous Service of a Participant was terminated with or without Cause for the purposes of outstanding Awards held by such Participant will have no effect upon any determination of the rights or obligations of the Company or such Participant for any other purpose.
(h) “Change in Control” means the occurrence, in a single transaction or in a series of related transactions, of any one or more of the following events:
(i) any Exchange Act Person becomes the Owner, directly or indirectly, of securities of the Company representing more than 50% of the combined voting power of the Company’s then outstanding securities other than by virtue of a merger, consolidation or similar transaction. Notwithstanding the foregoing, a Change in Control will not be deemed to occur (A) on account of the acquisition of securities of the Company directly from the Company, (B) on account of the acquisition of securities of the Company by an investor, any affiliate thereof or any other Exchange Act Person that acquires the Company’s securities in a transaction or series of related transactions the primary purpose of which is to obtain financing for the Company through the issuance of equity securities, or (C) solely because the level of Ownership held by any Exchange Act Person (the “Subject Person”) exceeds the designated percentage threshold of the outstanding voting securities as a result of a repurchase or other acquisition of voting securities by the Company reducing the number of shares outstanding, provided that if a Change in Control would occur (but for the operation of this sentence) as a result of the acquisition of voting securities by the Company, and after such share acquisition, the Subject Person becomes the Owner of any additional voting securities that, assuming the repurchase or other acquisition had not occurred, increases the percentage of the then outstanding voting securities Owned by the Subject Person over the designated percentage threshold, then a Change in Control will be deemed to occur;
(ii) there is consummated a merger, consolidation or similar transaction involving (directly or indirectly) the Company and, immediately after the consummation of such merger, consolidation or similar transaction, the stockholders of the Company immediately prior thereto do not Own, directly or indirectly, either (A) outstanding voting securities representing more than 50% of the combined outstanding voting power of the surviving Entity in such merger, consolidation or similar transaction or (B) more than 50% of the combined outstanding voting power of the parent of the surviving Entity in such merger, consolidation or similar transaction, in each case in substantially the same proportions as their Ownership of the outstanding voting securities of the Company immediately prior to such transaction;
(iii) there is consummated a sale, lease, exclusive license or other disposition of all or substantially all of the consolidated assets of the Company and its Subsidiaries, other than a sale, lease, license or other disposition of all or substantially all of the consolidated assets of the Company and its Subsidiaries to an Entity, more than 50% of the combined voting power of the voting securities of which are Owned by stockholders of the Company in substantially the same proportions as their Ownership of the outstanding voting securities of the Company immediately prior to such sale, lease, license or other disposition; or
(iv) individuals who, on the Effective Date, are members of the Board (the “Incumbent Board”) cease for any reason to constitute at least a majority of the members of the Board; provided, however, that if the appointment or election (or nomination for election) of any new Board member was approved or recommended by a majority vote of the members of the Incumbent Board then still in office, such new member will, for purposes of this Plan, be considered as a member of the Incumbent Board.
Notwithstanding the foregoing definition or any other provision of this Plan, (A) the term Change in Control will not include a sale of assets, merger or other transaction effected exclusively for the purpose of changing the domicile of the Company, and (B) the definition of Change in Control (or any analogous term) in an individual written agreement between a Participant and the Company or an Affiliate will supersede the foregoing definition with respect to Awards subject to such agreement; provided, however, that (1) if no definition of Change in Control (or any analogous term) is set forth in such an individual written agreement, the foregoing definition will apply; and (2) no Change in Control (or any analogous term) will be deemed to occur with respect to Awards subject to such an individual written agreement without a requirement that the Change in Control (or any analogous term) actually occur.
If required for compliance with Section 409A of the Code, in no event will an event be deemed a Change in Control if such event is not also a “change in the ownership of” the Company, a “change in the effective control of” the Company, or a “change in the ownership of a substantial portion of the assets of” the Company, each as determined under Treasury Regulations Section 1.409A-3(i)(5) (without regard to any alternative definition thereunder). The Board may, in its sole discretion and without a Participant’s consent, amend the definition of “Change in Control” to conform to the definition of a “change in control event” under Section 409A of the Code and the regulations thereunder.
(i) “Code” means the Internal Revenue Code of 1986, as amended, including any applicable regulations and guidance thereunder.
(j) “Committee” means a committee of one or more Directors to whom authority has been delegated by the Board in accordance with Section 2(c).
(k) “Common Stock” means the common stock of the Company.
(l) “Company” means Avalo Therapeutics, Inc., a Delaware corporation.
(m) “Consultant” means any person, including an advisor, who is (i) engaged by the Company or an Affiliate to render consulting or advisory services and is compensated for such services, or (ii) serving as a member of the board of directors of an Affiliate and is compensated for such services. However, service solely as a Director, or payment of a fee for such service, will not cause a Director to be considered a “Consultant” for purposes of the Plan. Notwithstanding the foregoing, a person is treated as a Consultant under this Plan only if a Form S-8 Registration Statement under the Securities Act is available to register either the offer or the sale of the Company’s securities to such person.
(n) “Continuous Service” means that the Participant’s service with the Company or an Affiliate, whether as an Employee, Director or Consultant, is not interrupted or terminated. A change in the capacity in which the Participant renders service to the Company or an Affiliate as an Employee, Director or Consultant or a change in the Entity for which the Participant renders such service, provided that there is no interruption or termination of the Participant’s service with the Company or an Affiliate, will not terminate a Participant’s Continuous Service; provided, however, that if the Entity for which a Participant is rendering services ceases to qualify as an Affiliate, as determined by the Board, in its sole discretion, such Participant’s Continuous Service will be considered to have terminated on the date such Entity ceases to qualify as an Affiliate. For example, a change in status from an Employee of the Company to a Consultant of an Affiliate or to a Director will not constitute an interruption of Continuous Service. To the extent permitted by law, the Board or the chief executive officer of the Company, in that party’s sole discretion, may determine whether Continuous Service will be considered interrupted in the case of (i) any leave of absence approved by the Board or chief executive officer, including sick leave, military leave or any other personal leave, or (ii) transfers between the Company, an Affiliate, or their successors. Notwithstanding the foregoing, a leave of absence will be treated as Continuous Service for purposes of vesting in an Award only to such extent as may be provided in the Company’s leave of absence policy, in the written terms of any leave of absence agreement or policy applicable to the Participant, or as otherwise required by law.
(o) “Corporate Transaction” means the consummation, in a single transaction or in a series of related transactions, of any one or more of the following events:
(i) a sale or other disposition of all or substantially all, as determined by the Board, in its sole discretion, of the consolidated assets of the Company and its Subsidiaries;
(ii) a sale or other disposition of more than 50% of the outstanding securities of the Company;
(iii) a merger, consolidation or similar transaction following which the Company is not the surviving corporation; or
(iv) a merger, consolidation or similar transaction following which the Company is the surviving corporation but the shares of Common Stock outstanding immediately preceding the merger, consolidation or similar transaction are converted or exchanged by virtue of the merger, consolidation or similar transaction into other property, whether in the form of securities, cash or otherwise.
If required for compliance with Section 409A of the Code, in no event will an event be deemed a Corporate Transaction if such event is not also a “change in the ownership of” the Company, a “change in the effective control of” the Company, or a “change in the ownership of a substantial portion of the assets of” the Company, each as determined under Treasury Regulations Section 1.409A-3(i)(5) (without regard to any alternative definition thereunder). The Board may, in its sole discretion and without a Participant’s consent, amend the definition of “Corporate Transaction” to conform to the definition of a “change in control event” under Section 409A of the Code and the regulations thereunder.
(p) “Director” means a member of the Board.
(q) “Disability” means, with respect to a Participant, the inability of such Participant to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment that can be expected to result in death or that has lasted or can be expected to last for a continuous period of not less than 12 months, as provided in Sections 22(e)(3) and 409A(a)(2)(c)(i) of the Code, and will be determined by the Board on the basis of such medical evidence as the Board deems warranted under the circumstances.
(r) “Effective Date” means the date on which the Plan was adopted by the Board.
(s) “Employee” means any person employed by the Company or an Affiliate. However, service solely as a Director, or payment of a fee for such services, will not cause a Director to be considered an “Employee” for purposes of the Plan.
(t) “Entity” means a corporation, partnership, limited liability company or other entity.
(u) “Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
(v) “Exchange Act Person” means any natural person, Entity or “group” (within the meaning of Section 13(d) or 14(d) of the Exchange Act), except that “Exchange Act Person” will not include (i) the Company or any Subsidiary of the Company, (ii) any employee benefit plan of the Company or any Subsidiary of the Company or any trustee or other fiduciary holding securities under an employee benefit plan of the Company or any Subsidiary of the Company, (iii) an underwriter temporarily holding securities pursuant to an offering of such securities, (iv) an Entity Owned, directly or indirectly, by the stockholders of the Company in substantially the same proportions as their Ownership of stock of the Company, or (v) any natural person, Entity or “group” (within the meaning of Section 13(d) or 14(d) of the Exchange Act) that, as of the Effective Date, is the Owner, directly or indirectly, of securities of the Company representing more than 50% of the combined voting power of the Company’s then outstanding securities.
(w) “Fair Market Value” means, as of any date, the value of the Common Stock determined as follows:
(i) If the Common Stock is listed on any established stock exchange or traded on any established market, the Fair Market Value of a share of Common Stock will be, unless otherwise determined by the Board, the closing sales price for such stock as quoted on such exchange or market (or the exchange or market with the greatest volume of trading in the Common Stock) on the date of determination, as reported in a source the Board deems reliable.
(ii) Unless otherwise provided by the Board, if there is no closing sales price for the Common Stock on the date of determination, then the Fair Market Value will be the closing sales price on the last preceding date for which such quotation exists.
(iii) In the absence of such markets for the Common Stock, the Fair Market Value will be determined by the Board in good faith and in a manner that complies with Section 409A of the Code.
(x) “Inducement Award Rules” means Nasdaq Marketplace Rule 5635(c)(4) and the related guidance under Nasdaq IM 5635-1, together with any analogous rules or guidance effective after the date hereof.
(y) “Non-Employee Director” means a Director who either (i) is not a current employee or officer of the Company or an Affiliate, does not receive compensation, either directly or indirectly, from the Company or an Affiliate for services rendered as a consultant or in any capacity other than as a Director (except for an amount as to which disclosure would not be required under Item 404(a) of Regulation S-K promulgated pursuant to the Securities Act (“Regulation S-K”)), does not possess an interest in any other transaction for which disclosure would be required under Item 404(a) of Regulation S-K, and is not engaged in a business relationship for which disclosure would be required pursuant to Item 404(b) of Regulation S-K, or (ii) is otherwise considered a “non-employee director” for purposes of Rule 16b-3.
(z) “Nonstatutory Stock Option” means an option granted pursuant to Section 5 that does not qualify as an “incentive stock option” within the meaning of Section 422 of the Code.
(aa) “Officer” means a person who is an officer of the Company within the meaning of Section 16 of the Exchange Act.
(bb) “Option” means a Nonstatutory Stock Option to purchase shares of Common Stock granted pursuant to the Plan.
(cc) “Option Agreement” means a written agreement between the Company and a holder of an Option evidencing the terms and conditions of an Option grant. Each Option Agreement will be subject to the terms and conditions of the Plan.
(dd) “Other Stock Award” means an award based in whole or in part by reference to the Common Stock which is granted pursuant to the terms and conditions of Section 6(c).
(ee) “Other Stock Award Agreement” means a written agreement between the Company and a holder of an Other Stock Award evidencing the terms and conditions of an Other Stock Award grant. Each Other Stock Award Agreement will be subject to the terms and conditions of the Plan.
(ff) “Own,” “Owned,” “Owner,” “Ownership” means a person or Entity will be deemed to “Own,” to have “Owned,” to be the “Owner” of, or to have acquired “Ownership” of securities if such person or Entity, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise, has or shares voting power, which includes the power to vote or to direct the voting, with respect to such securities.
(gg) “Participant” means a person to whom an Award is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Award.
(hh) “Plan” means this Avalo Therapeutics, Inc. 2025 Inducement Award Plan.
(ii) “Restricted Stock Award” means an award of shares of Common Stock which is granted pursuant to the terms and conditions of Section 6(a).
(jj) “Restricted Stock Award Agreement” means a written agreement between the Company and a holder of a Restricted Stock Award evidencing the terms and conditions of a Restricted Stock Award grant. Each Restricted Stock Award Agreement will be subject to the terms and conditions of the Plan.
(kk) “Restricted Stock Unit Award” means a right to receive shares of Common Stock which is granted pursuant to the terms and conditions of Section 6(b).
(ll) “Restricted Stock Unit Award Agreement” means a written agreement between the Company and a holder of a Restricted Stock Unit Award evidencing the terms and conditions of a Restricted Stock Unit Award grant. Each Restricted Stock Unit Award Agreement will be subject to the terms and conditions of the Plan.
(mm) “Rule 16b-3” means Rule 16b-3 promulgated under the Exchange Act or any successor to Rule 16b-3, as in effect from time to time.
(nn) “Rule 405” means Rule 405 promulgated under the Securities Act.
(oo) “Securities Act” means the Securities Act of 1933, as amended.
(pp) “Stock Appreciation Right” or “SAR” means a right to receive the appreciation on Common Stock that is granted pursuant to the terms and conditions of Section 5.
(qq) “Stock Appreciation Right Agreement” or “SAR Agreement” means a written agreement between the Company and a holder of a Stock Appreciation Right evidencing the terms and conditions of a Stock Appreciation Right grant. Each Stock Appreciation Right Agreement will be subject to the terms and conditions of the Plan.
(rr) “Stock Award Agreement” means a written agreement between the Company and a Participant evidencing the terms and conditions of a Stock Award grant. Each Stock Award Agreement will be subject to the terms and conditions of the Plan.
(ss) “Subsidiary” means, with respect to the Company, (i) any corporation of which more than 50% of the outstanding capital stock having ordinary voting power to elect a majority of the board of directors of such corporation (irrespective of whether, at the time, stock of any other class or classes of such corporation will have or might have voting power by reason of the happening of any contingency) is at the time, directly or indirectly, Owned by the Company, and (ii) any partnership, limited liability company or other entity in which the Company has a direct or indirect interest (whether in the form of voting or participation in profits or capital contribution) of more than 50%.
EX-31.1
3
ex-3113q2025.htm
EX-31.1
Document
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Garry Neil, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Avalo Therapeutics, Inc. (the “registrant”);
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
Date: November 6, 2025 |
/s/ Garry Neil, M.D. |
| |
Garry Neil, M.D.
Chief Executive Officer
(Registrant’s Principal Executive Officer) |
|
|
EX-31.2
4
ex-3123q2025.htm
EX-31.2
Document
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Christopher Sullivan, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Avalo Therapeutics, Inc. (the “registrant”);
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
Date: November 6, 2025 |
/s/ Christopher Sullivan |
| |
Christopher Sullivan
Chief Financial Officer
(Registrant’s Principal Financial Officer) |
|
|
EX-32.1
5
ex-3213q2025.htm
EX-32.1
Document
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
AND PRINCIPAL FINANCIAL OFFICER
PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of Avalo Therapeutics, Inc. (the “Registrant”) on Form 10-Q for the period ended September 30, 2025 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Garry Neil, Chief Executive Officer (principal executive officer) of the Registrant, and I, Christopher Sullivan, Chief Financial Officer (principal financial officer) of the Registrant, each hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that, to my knowledge:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition at the end of the period covered by the Report and the results of operations of the Registrant for the periods covered by the Report.
|
|
|
|
|
|
|
|
|
| Date: November 6, 2025 |
By: |
/s/ Garry Neil, M.D. |
| |
Name: |
Garry Neil, M.D. |
| |
Title: |
Chief Executive Officer
(Registrant’s Principal Executive Officer) |
| |
|
|
| Date: November 6, 2025 |
By: |
/s/ Christopher Sullivan |
| |
Name: |
Christopher Sullivan |
| |
Title: |
Chief Financial Officer
(Registrant’s Principal Financial Officer) |
The foregoing certifications are not deemed filed with the Securities and Exchange Commission for purposes of section 18 of the Securities Exchange Act of 1934, as amended (Exchange Act), and are not to be incorporated by reference into any filing of Avalo Therapeutics, Inc. under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
