UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 7, 2025
CALIDI BIOTHERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-40789 | 86-2967193 | ||
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
|
4475 Executive Drive, Suite 200, San Diego, California |
92121 | |
| (Address of principal executive offices) | (Zip Code) |
(858) 794-9600
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered | ||
| Common stock, par value $0.0001 per share | CLDI | NYSE American LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On November 7, 2025, Calidi Biotherapeutics, Inc. (the “Company”) presented new data on its first therapeutic candidate from its RedTail platform, CLD-401, at the Society of Immunotherapy for Cancer (SITC) Annual Meeting. The Company presented the new data in a webinar (the “Presentation”) and a poster (the “Poster”). Furnished as Exhibit 99.1 and Exhibit 99.2 hereto and incorporated by reference herein are the Presentation and Poster, respectively.
By filing this Current Report on Form 8-K and furnishing the information contained herein, the Company makes no admission as to the materiality of any information in this report that is required to be disclosed solely by reason of Regulation FD. The information in this Item 7.01 disclosure, including Exhibit 99.1 and Exhibit 99.2, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities under that Section. In addition, the information in this Item 7.01 disclosure, including Exhibits 99.1 and Exhibit 99.2, shall not be incorporated by reference into the filings of the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events |
On November 10, 2025, the Company issued a press release announcing the presentation of new data on its first therapeutic candidate from its RedTail platform, CLD-401, at the Society of Immunotherapy for Cancer (SITC) Annual Meeting. A copy of the press release is included as Exhibit 99.3 hereto and is incorporated by reference herein.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit | Exhibit Description | |
| 99.1 | Presentation | |
| 99.2 | Poster | |
| 99.3 | Press Release dated November 10, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| CALIDI BIOTHERAPEUTICS, INC. | ||
| Dated: November 10, 2025 | ||
| By: | /s/ Andrew Jackson | |
| Name: | Andrew Jackson | |
| Title: | Chief Financial Officer | |
Exhibit 99.1


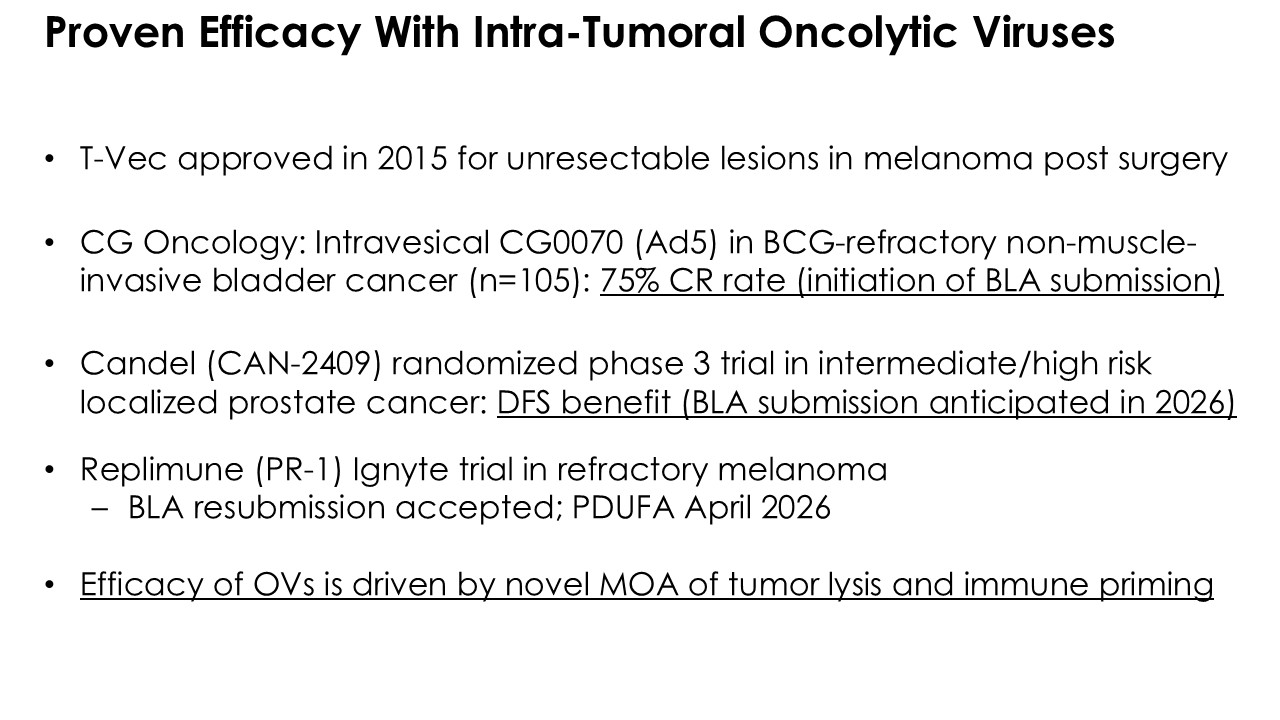
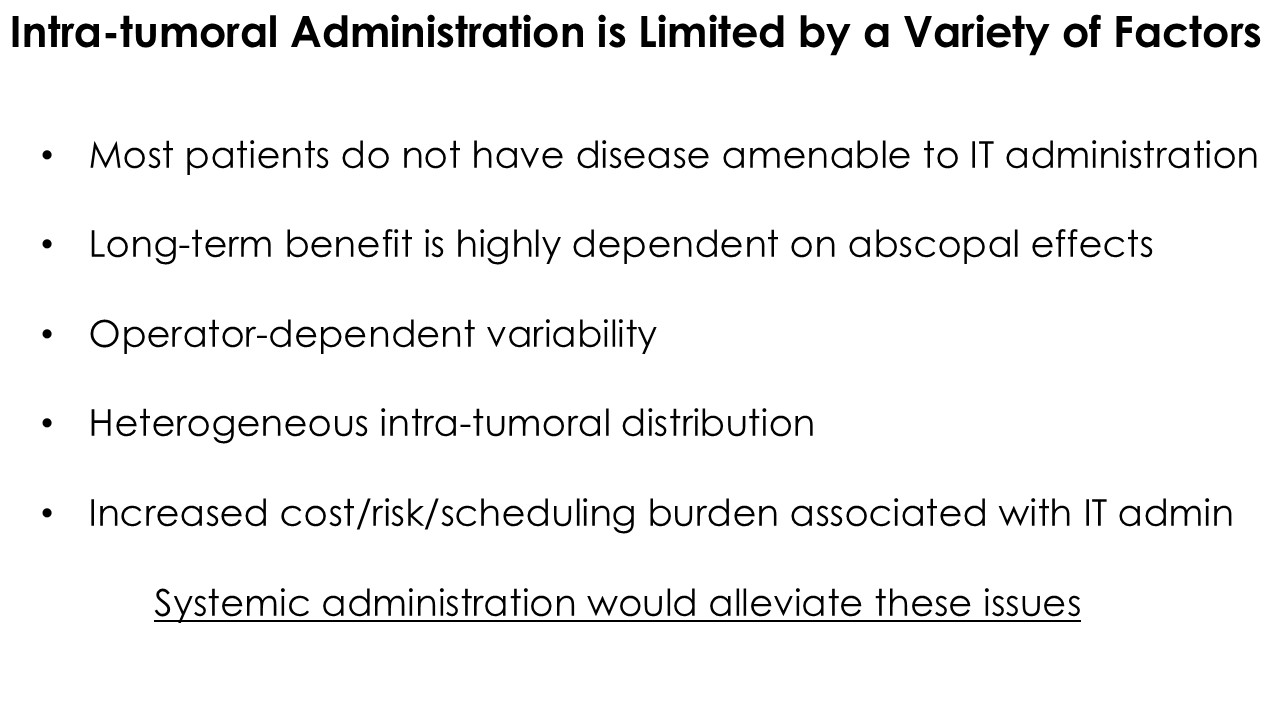
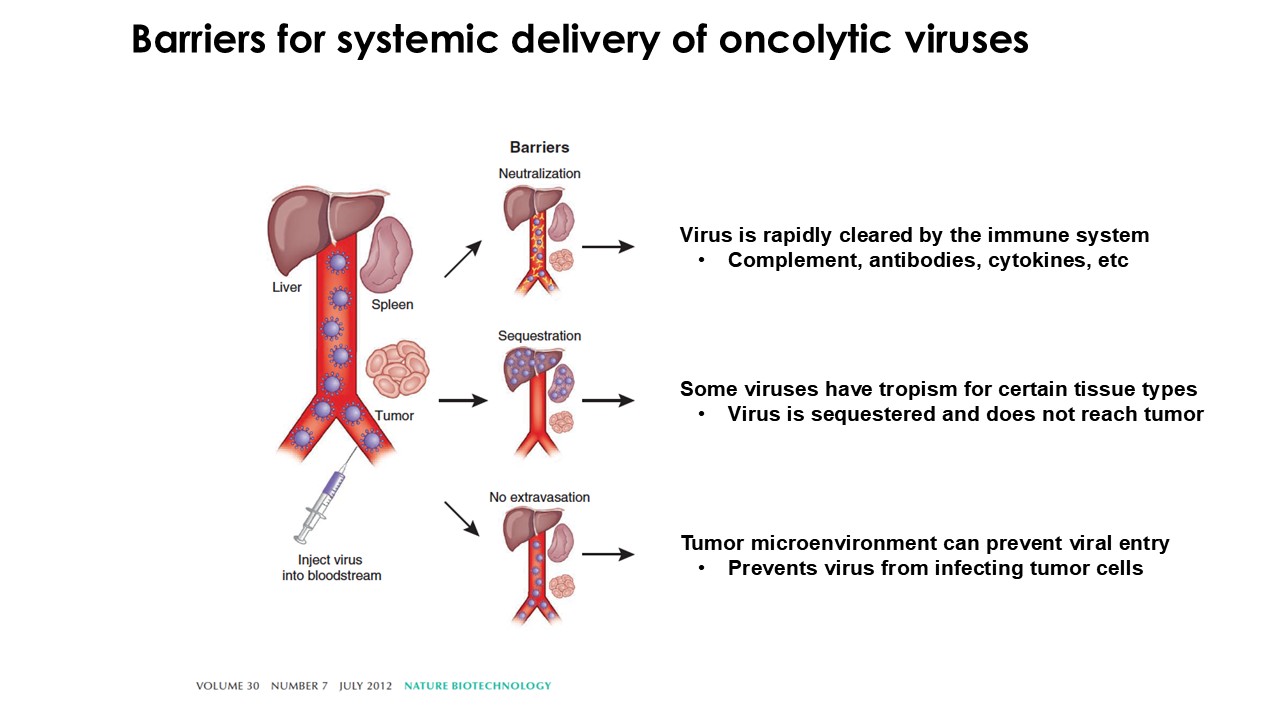
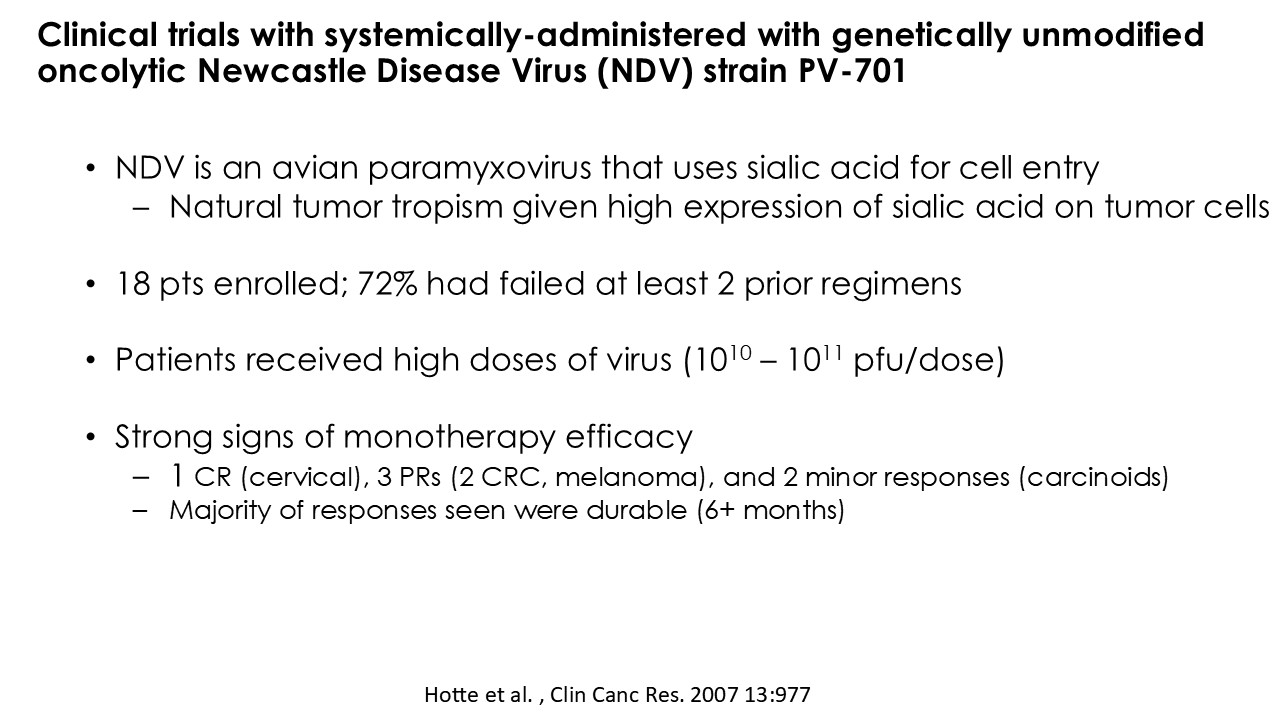
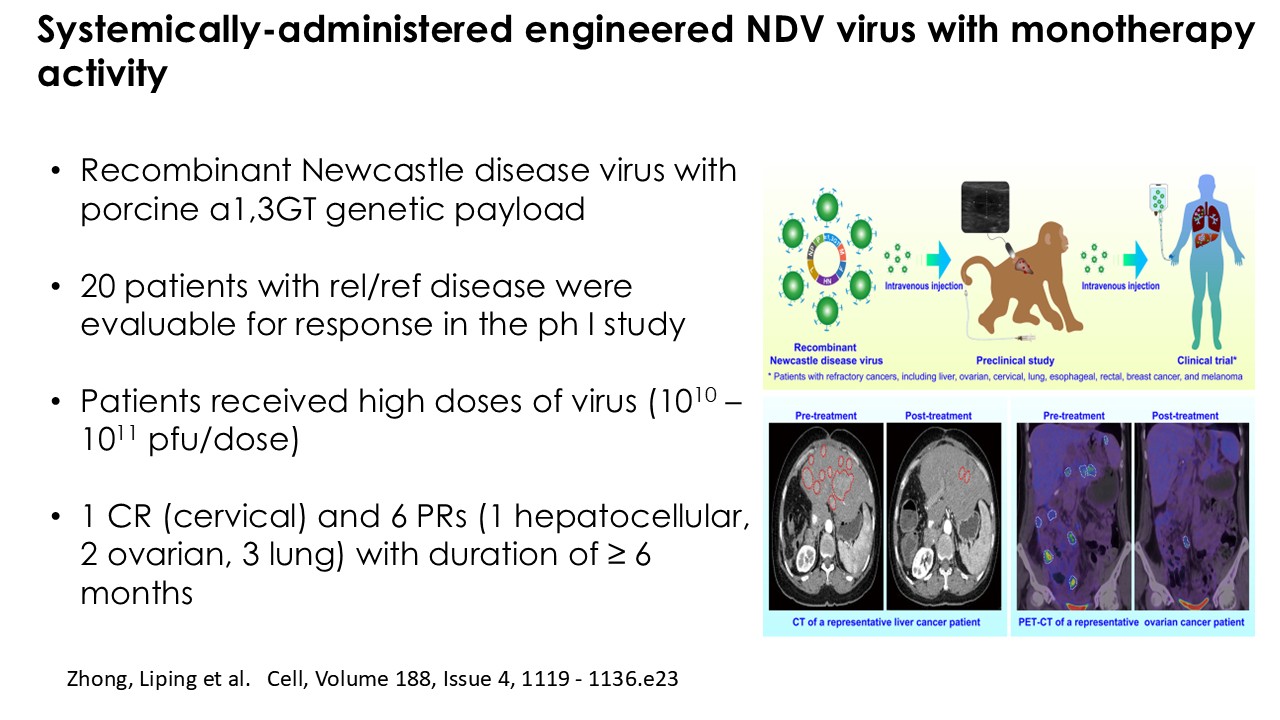
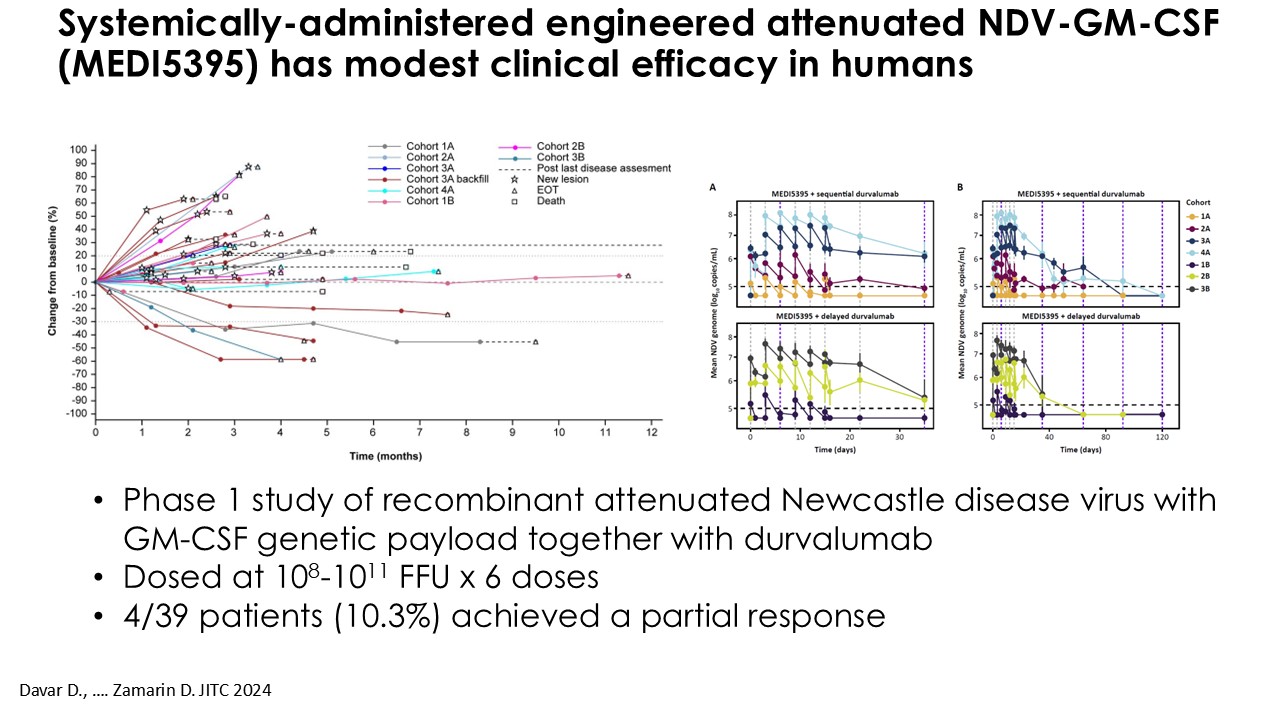

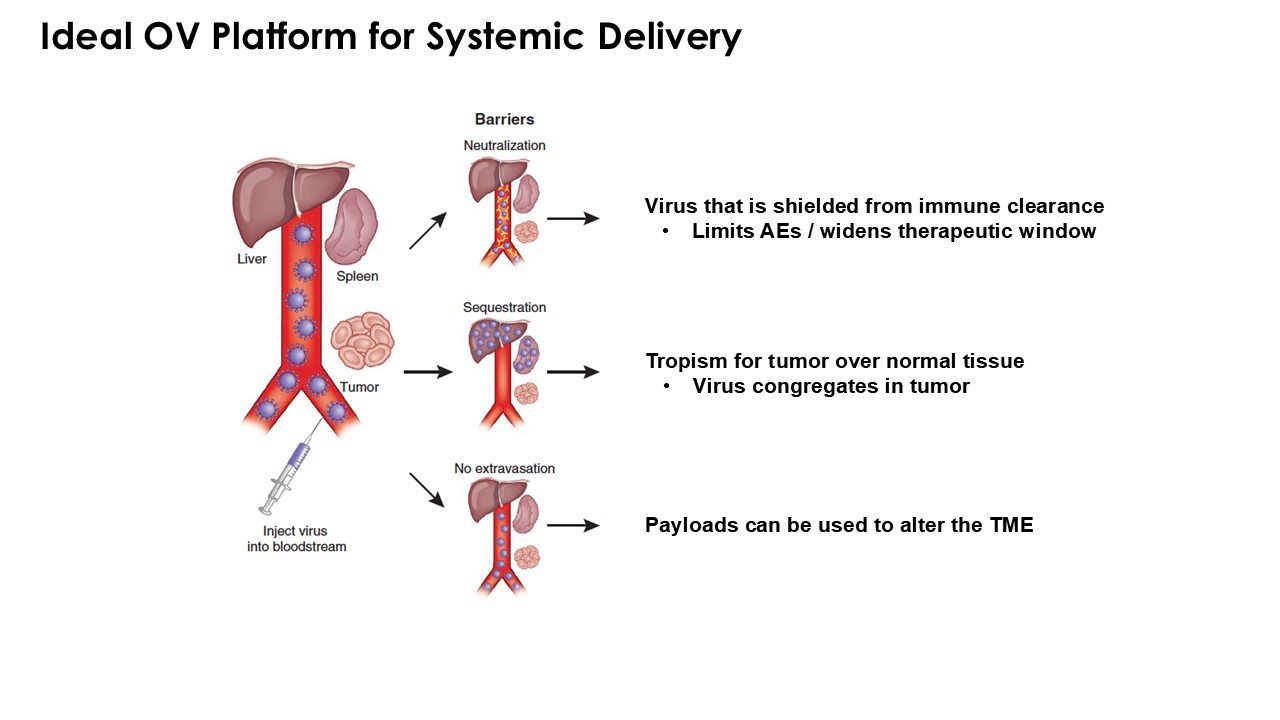
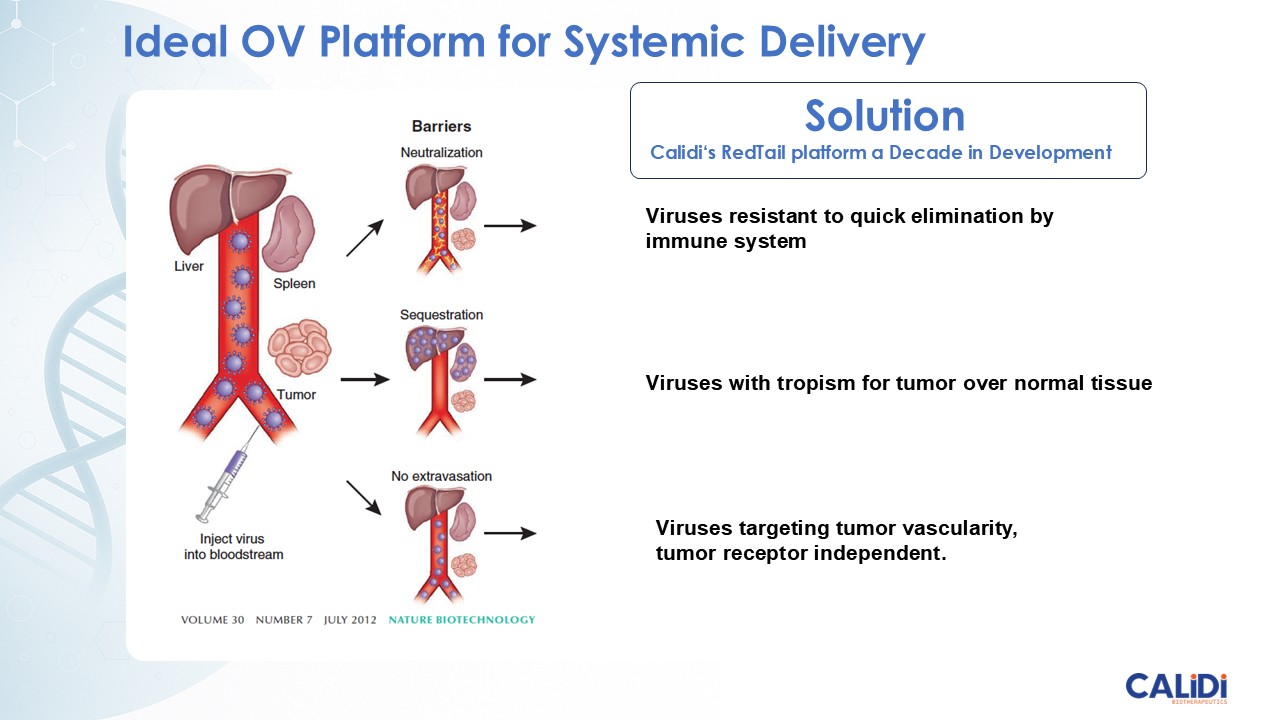
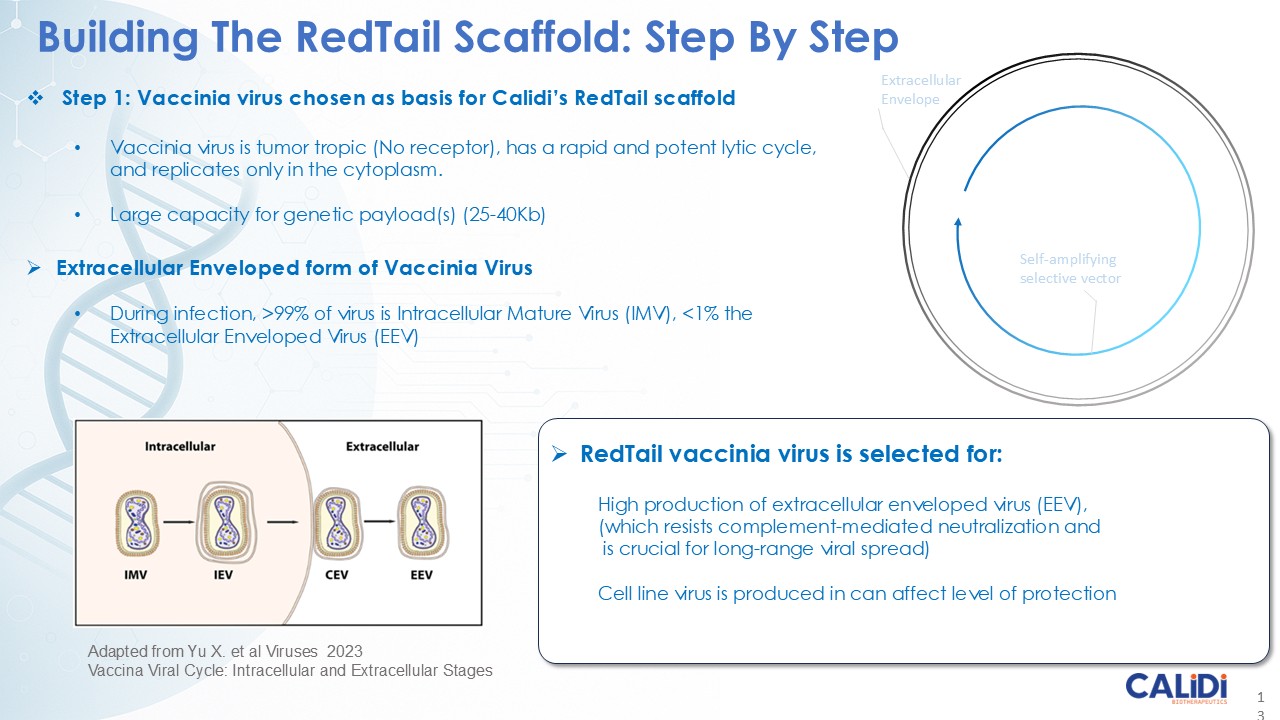
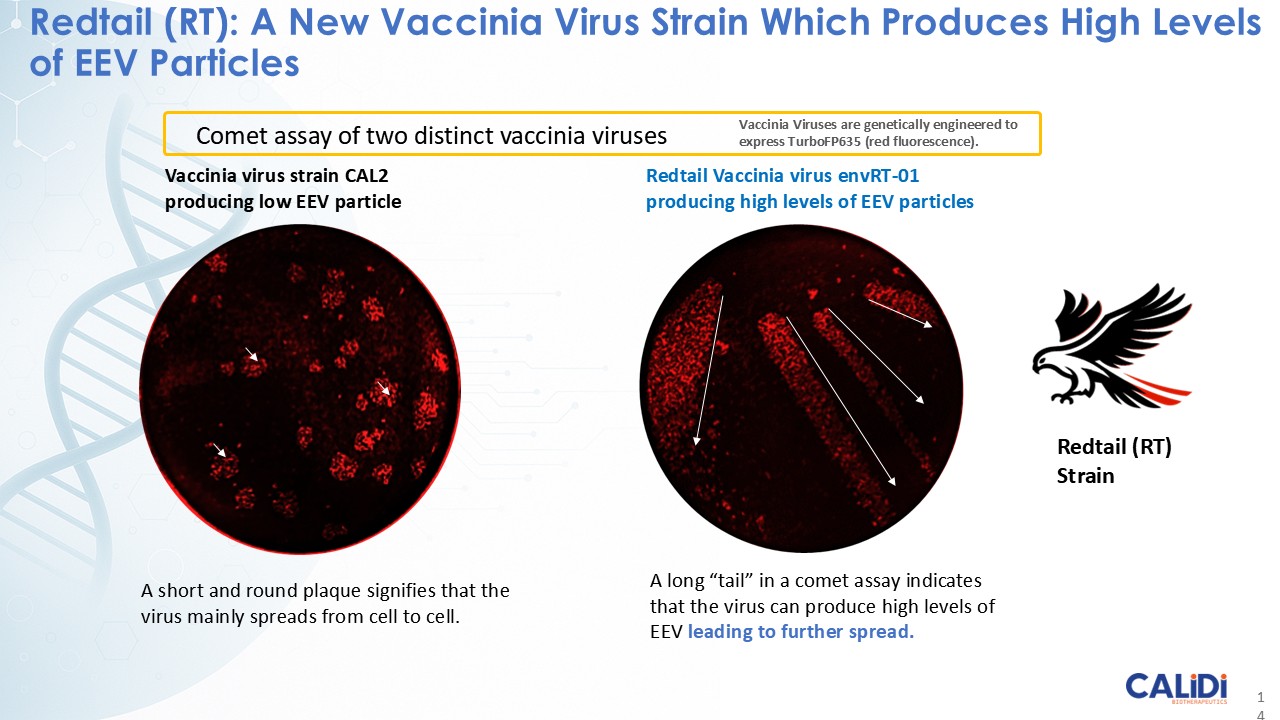
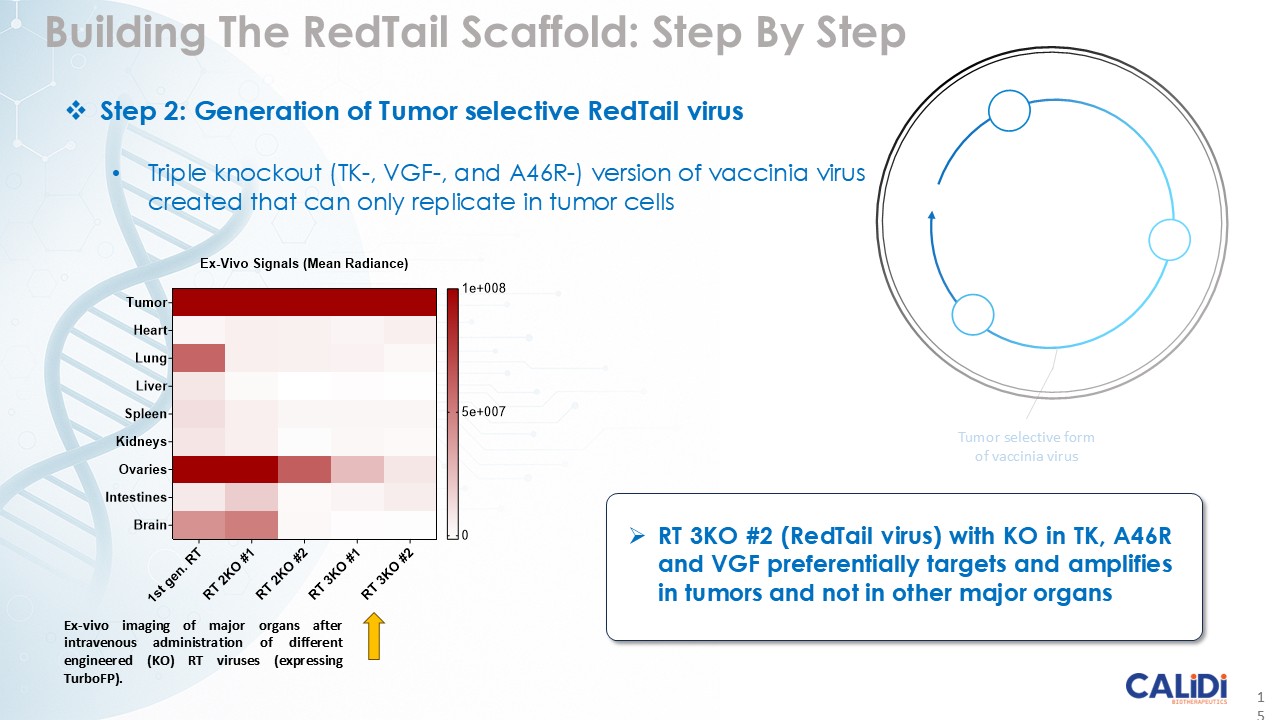



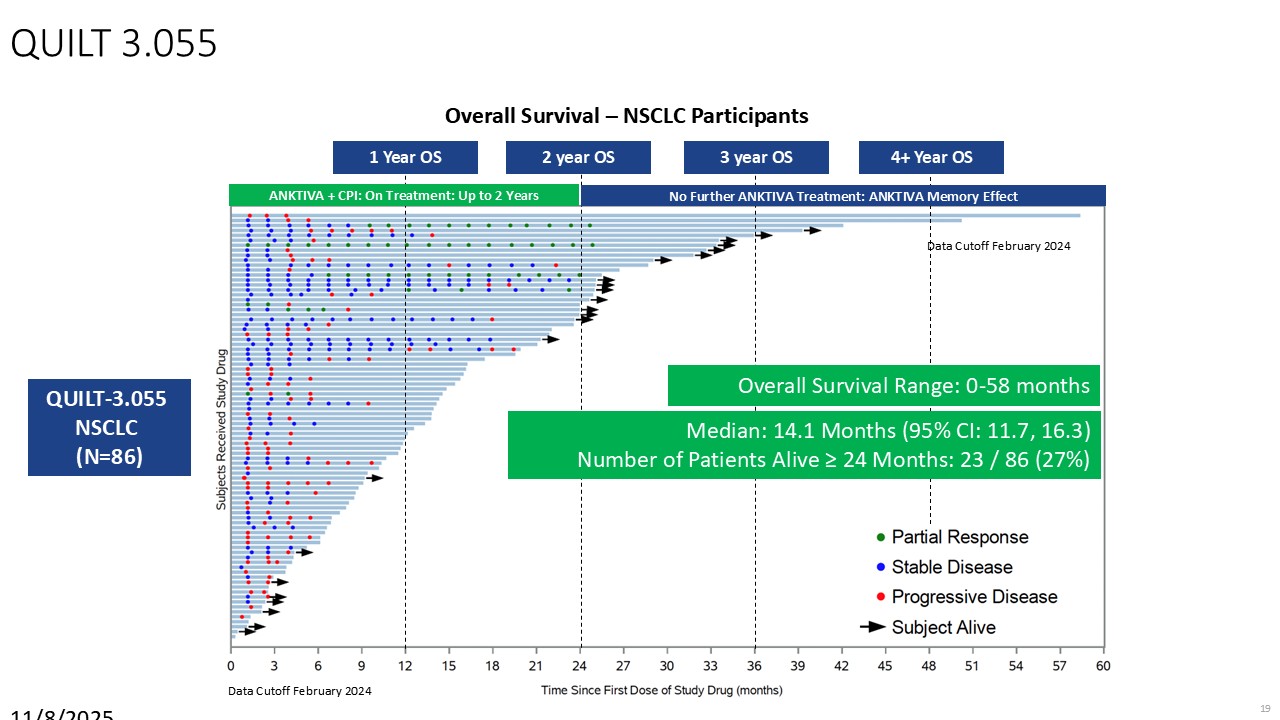
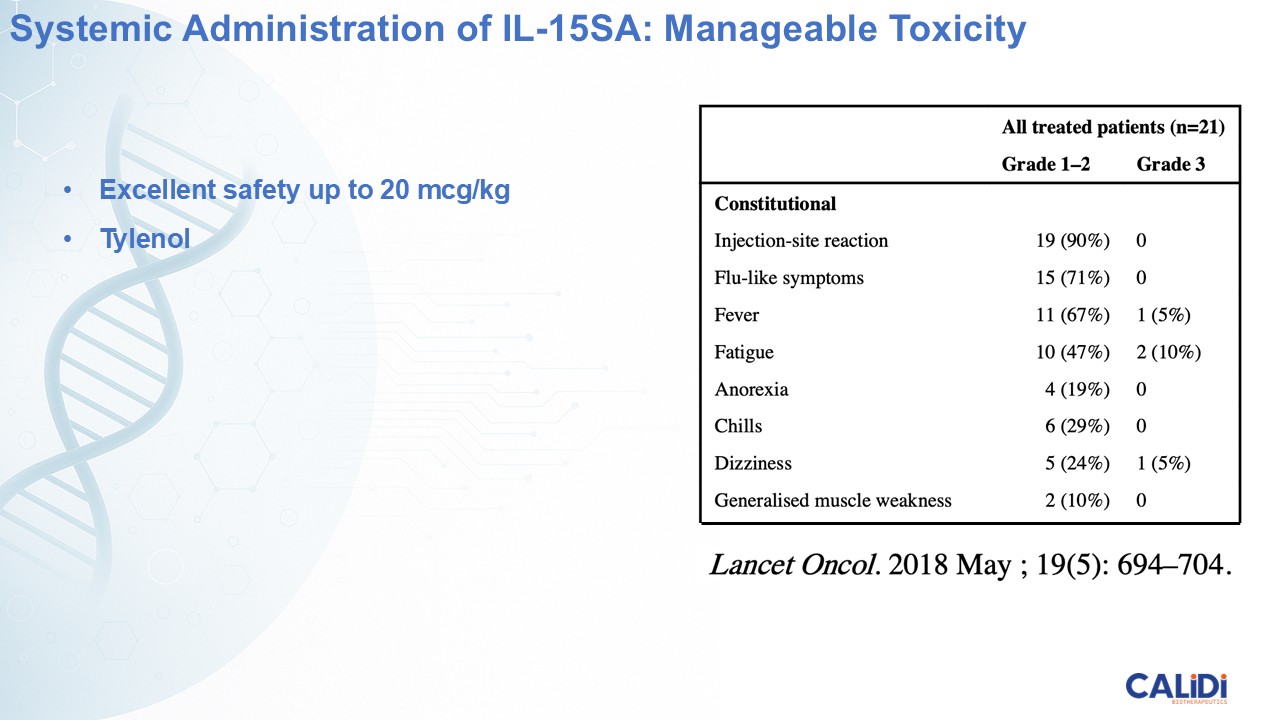


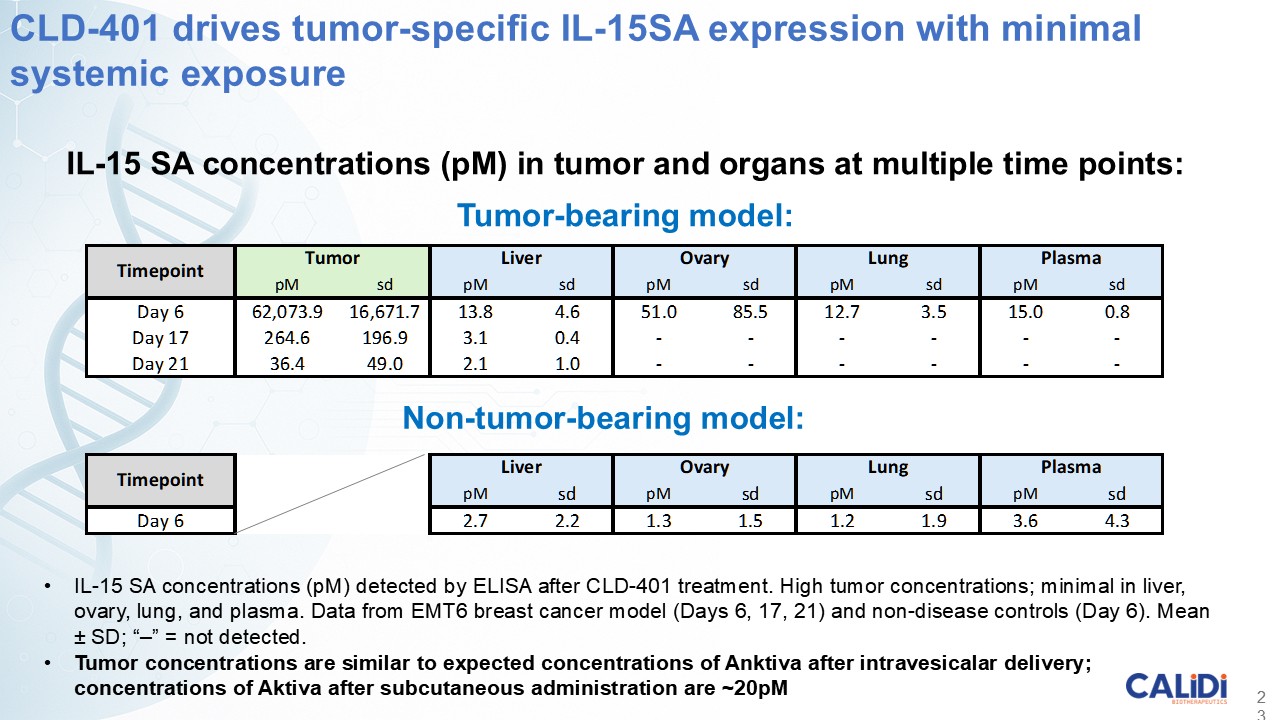
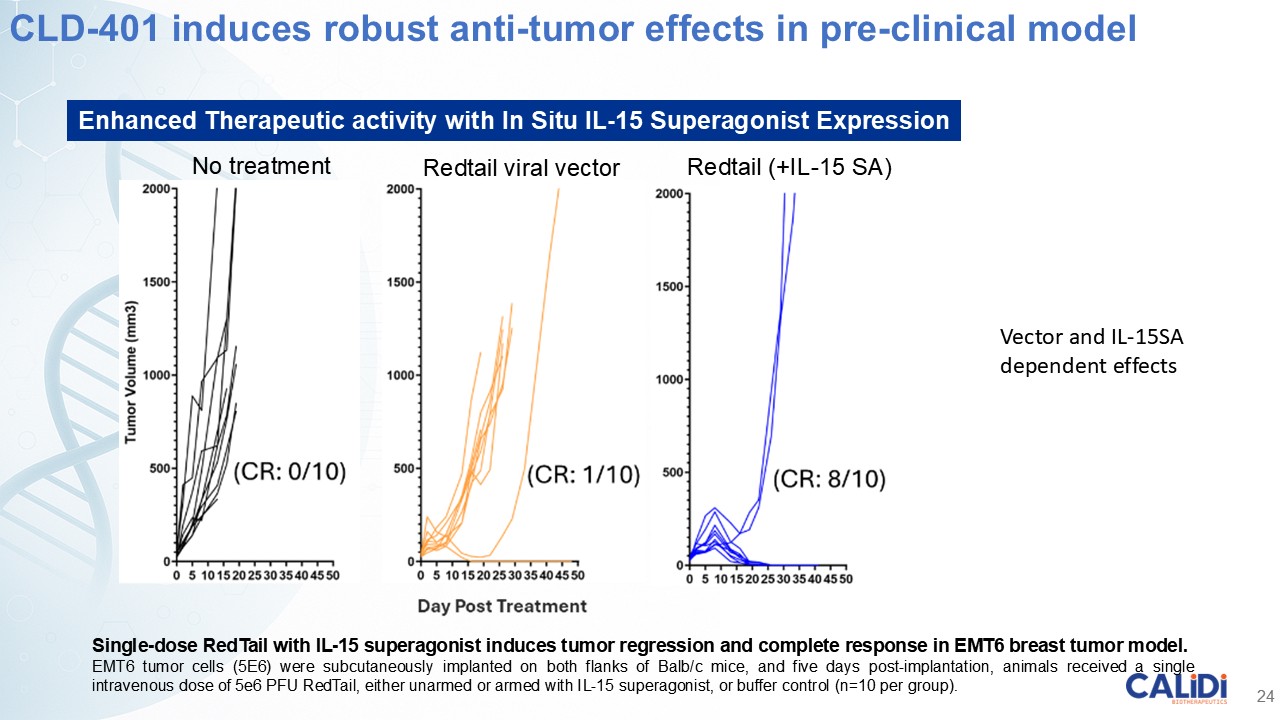
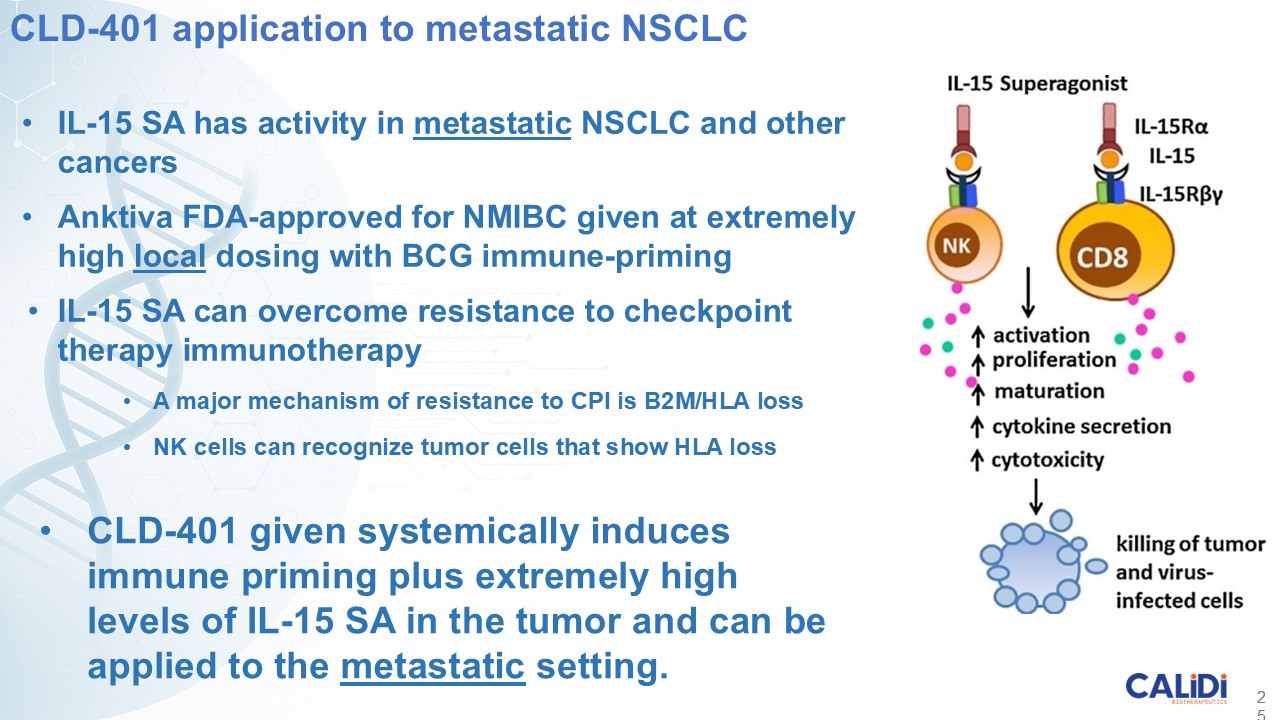


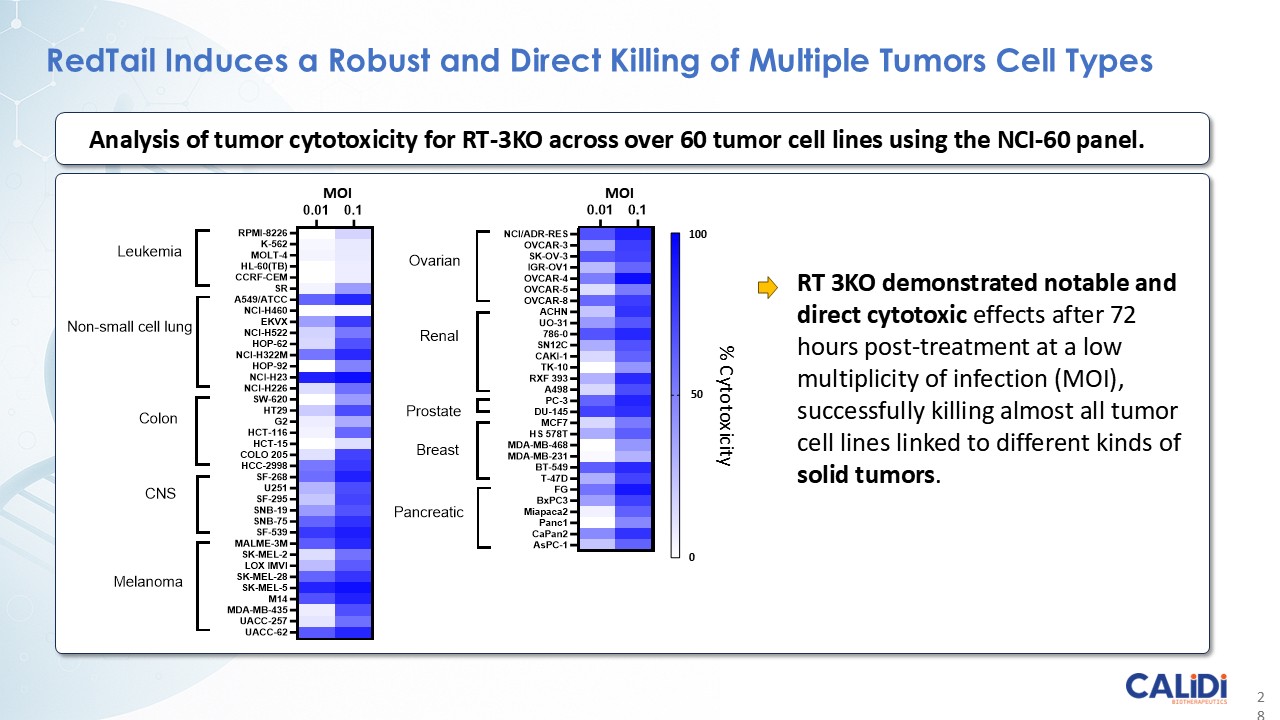
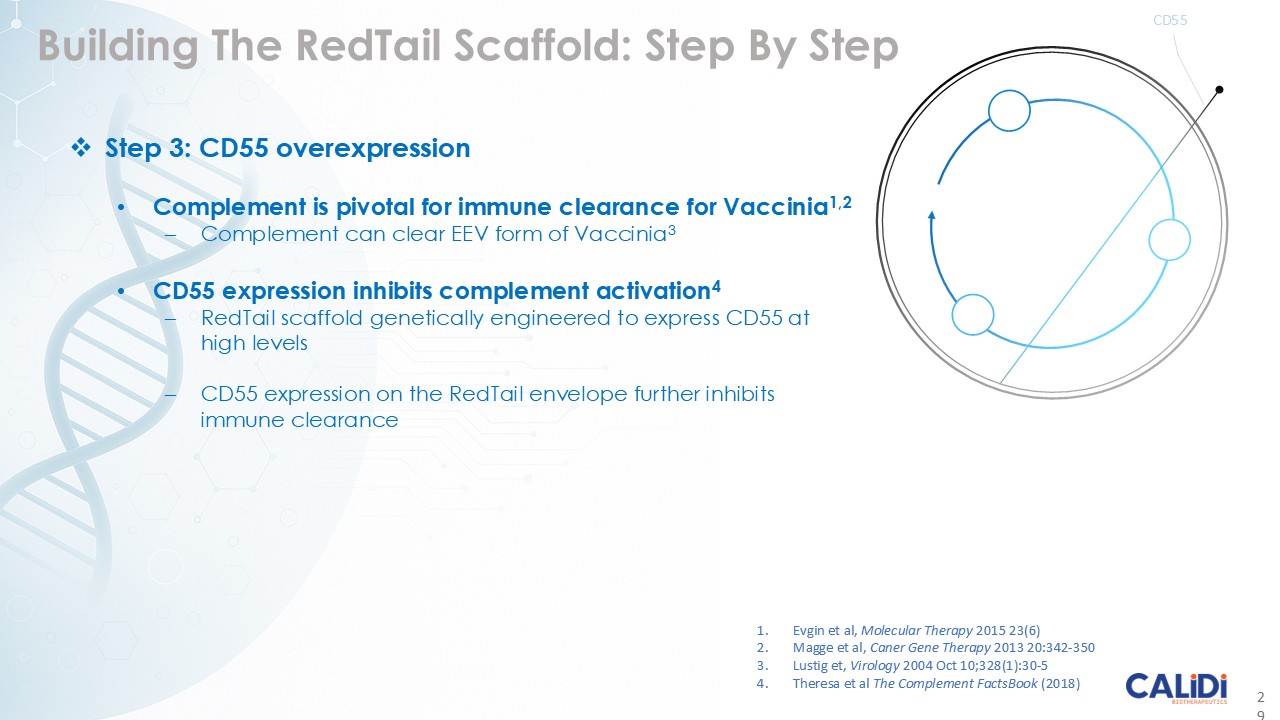
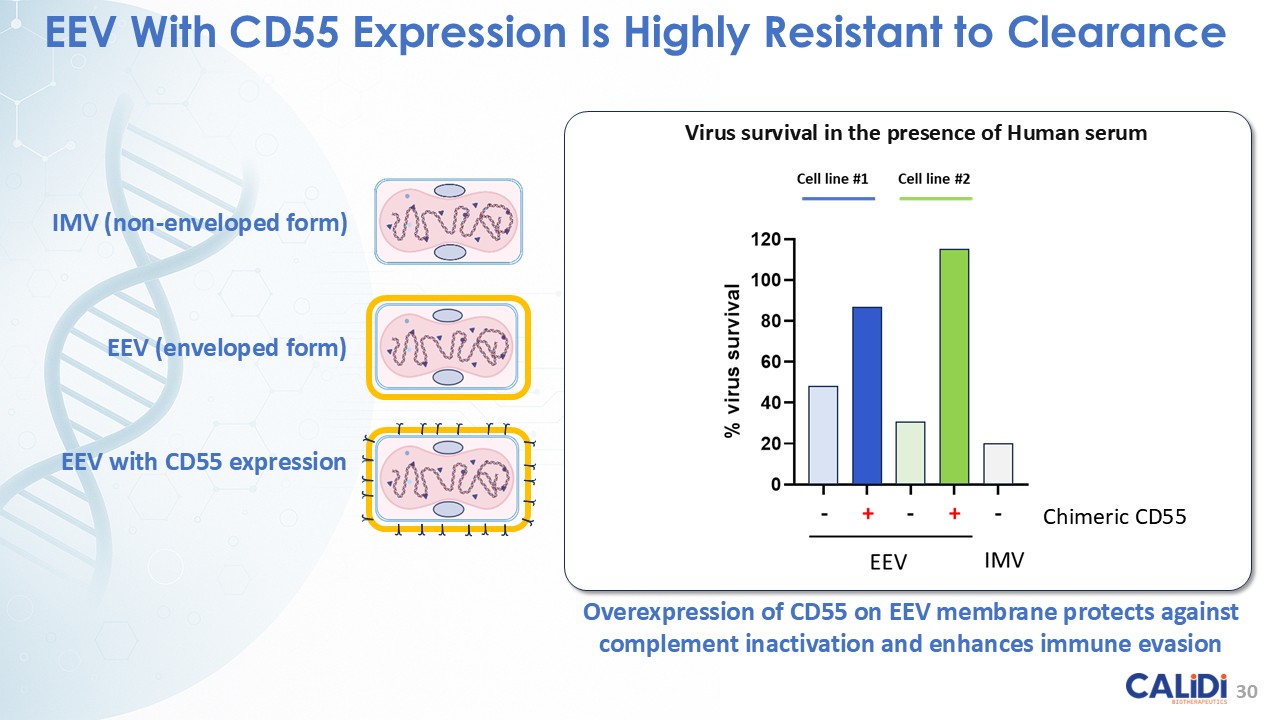
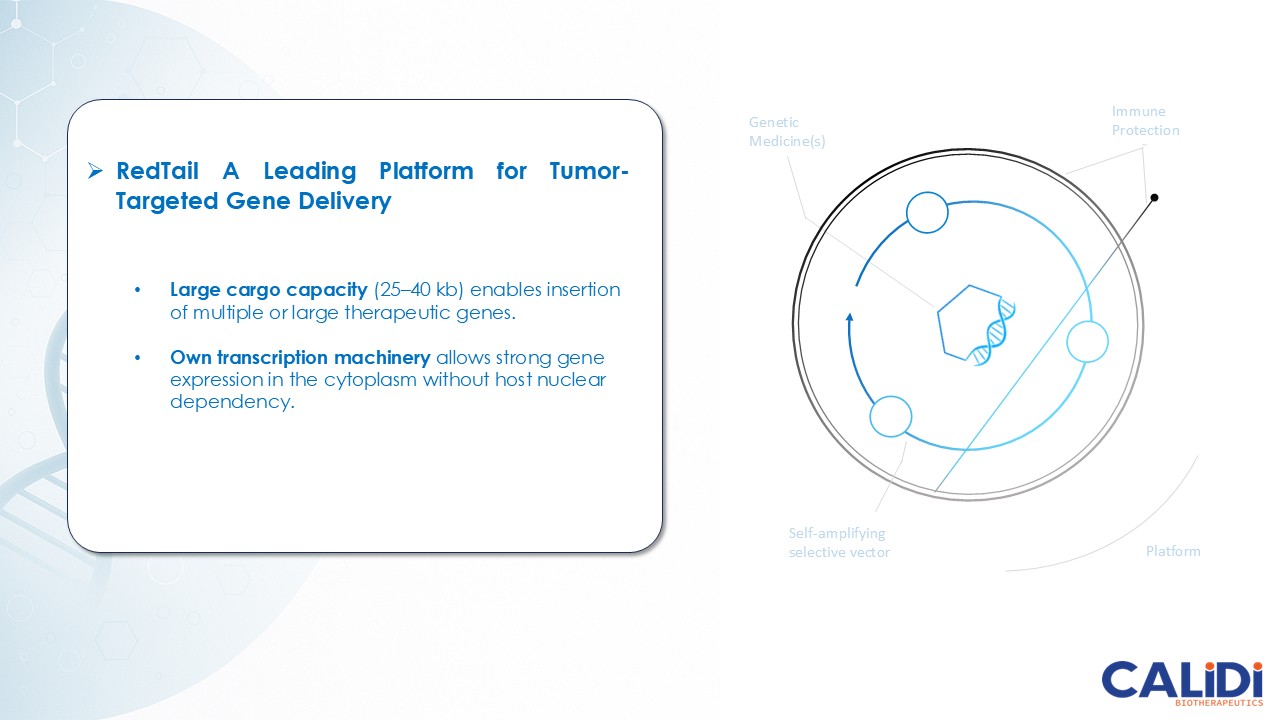
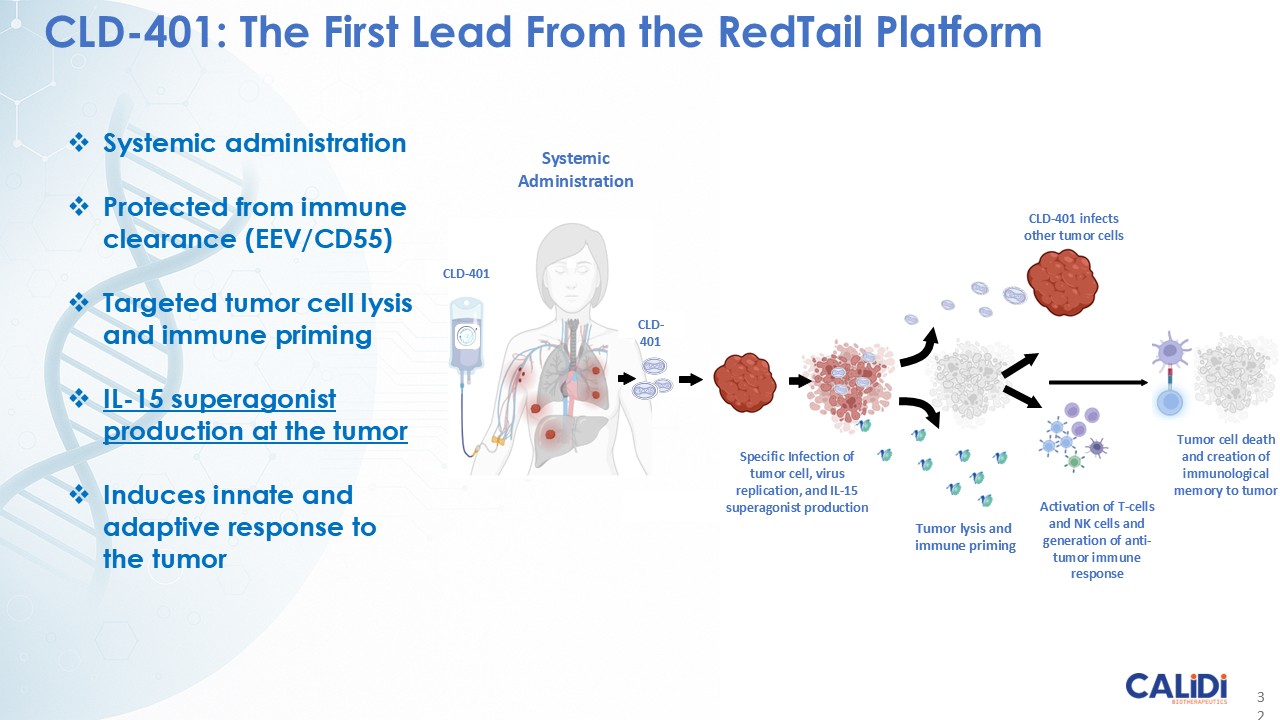
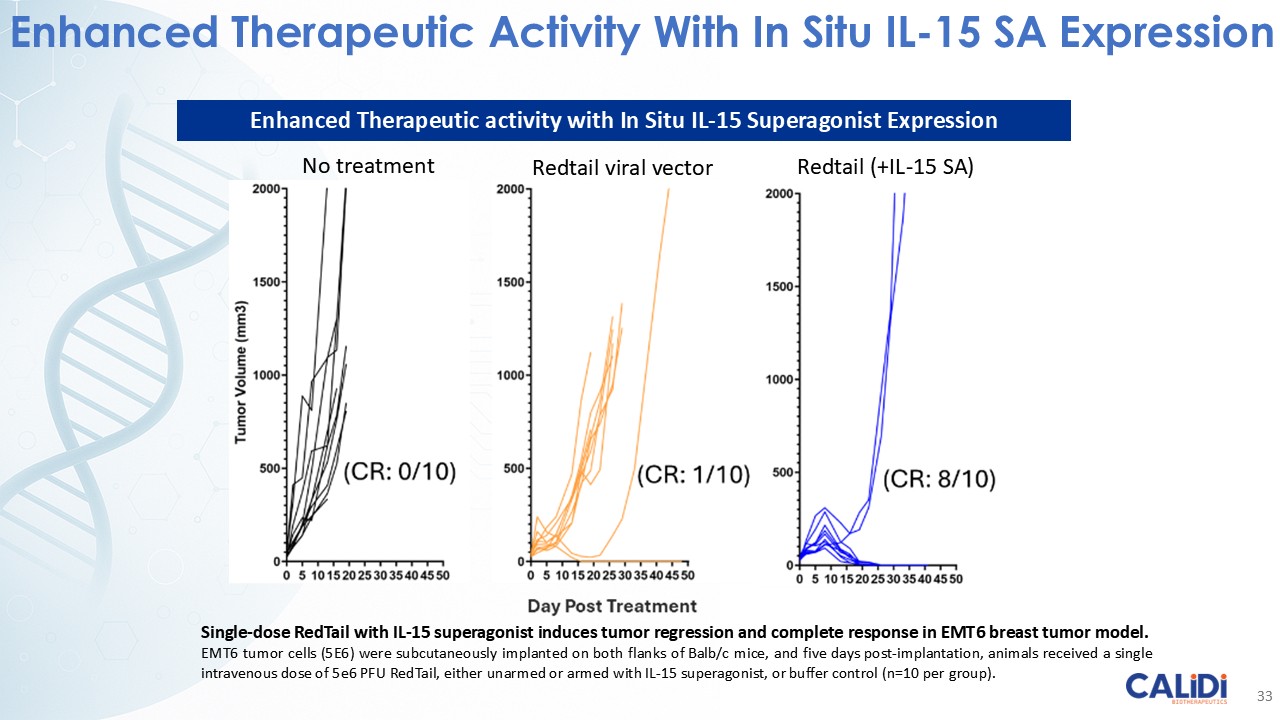
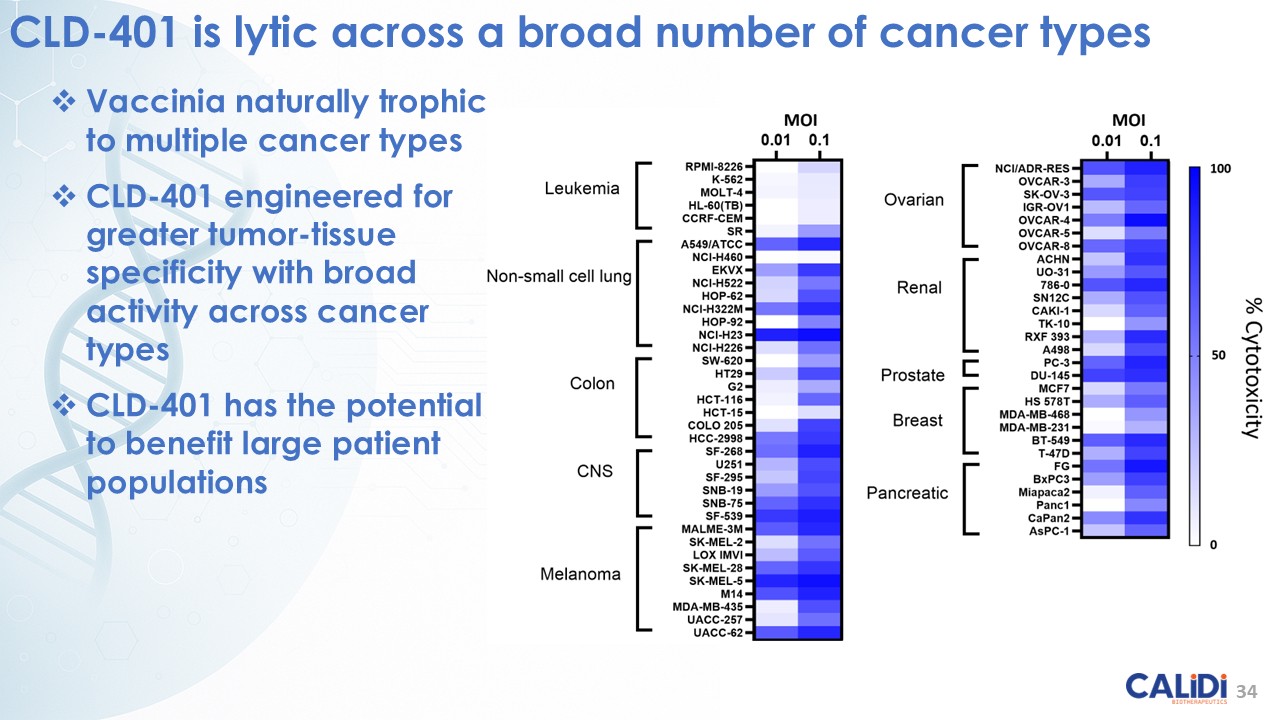
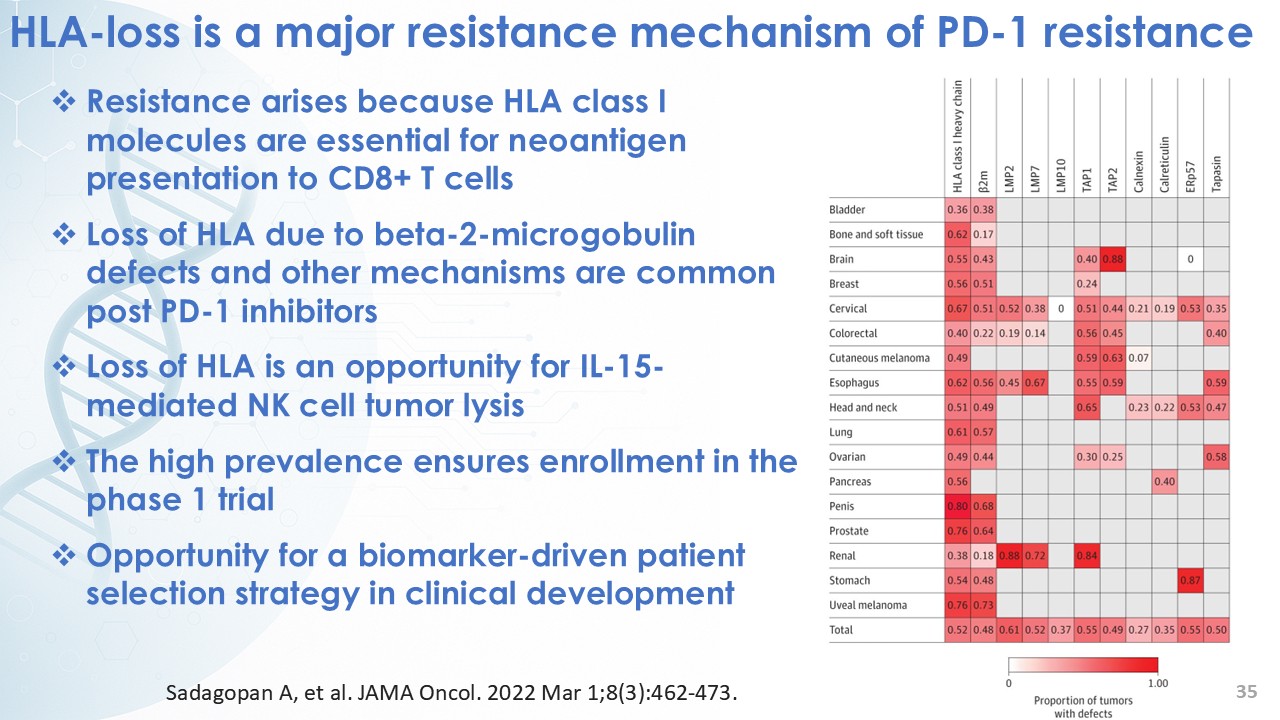

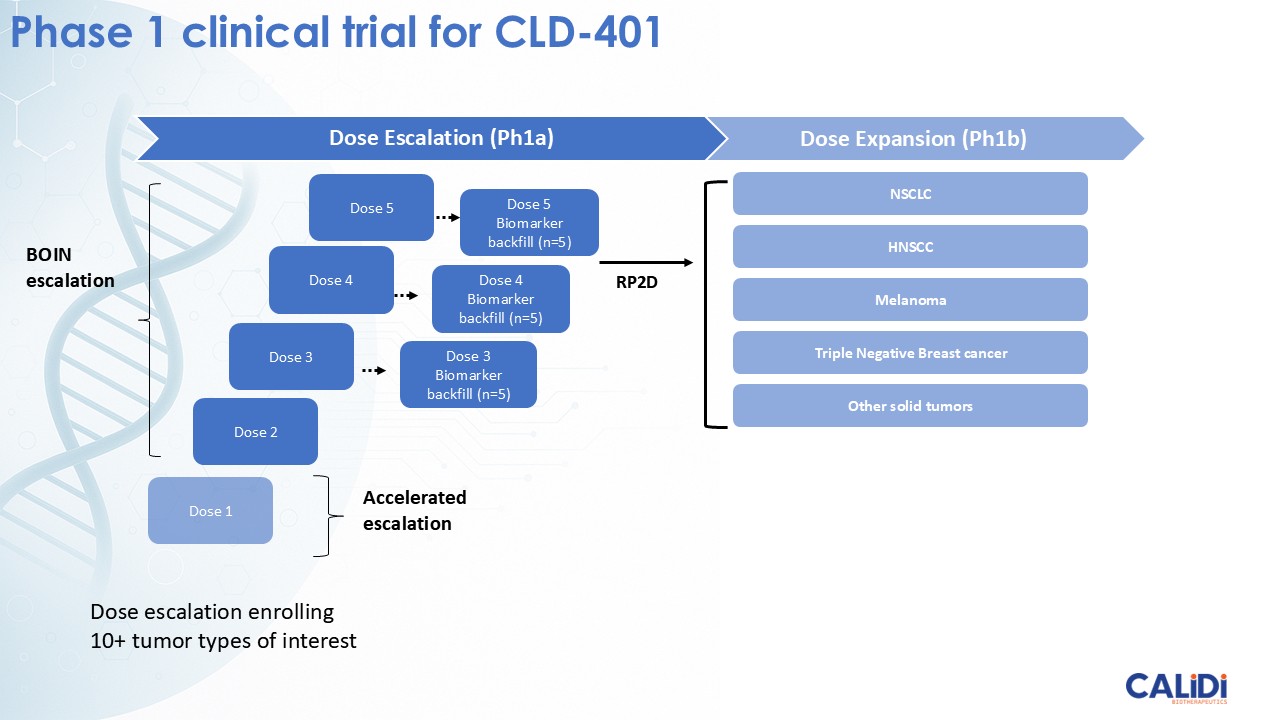


Exhibit 99.2

Exhibit 99.3
Calidi Biotherapeutics Presents New Data on its Therapeutic Lead, CLD-401, at the 2025 SITC Annual Meeting and Holds Its First Investor Day at SITC
SAN DIEGO, November 10, 2025 (GLOBE NEWSWIRE) – Calidi Biotherapeutics, Inc. (NYSE American: CLDI) (“Calidi” or the “Company”), a clinical-stage biotechnology company pioneering the development of systemically delivered, targeted genetic medicines, presented new data on its first therapeutic candidate from its RedTail platform, CLD-401, at the Society of Immunotherapy for Cancer (SITC) Annual Meeting.
CLD-401 is a tumor-tropic oncolytic virus designed to home to metastatic sites after systemic administration, replicate only in tumors cells, induce an immune priming event at the tumor site, and express high levels of IL-15 superagonist, a potent cytokine that induces NK and T-cell responses to the tumor, in the tumor microenvironment (TME).
“Our latest data demonstrate that in our syngeneic murine models, our RedTail platform is protected from immune clearance after systemic administration and can find and specifically replicate in tumor cells at metastatic sites,” said Antonio F. Santidrian, PhD, Chief Scientific Officer and Head of Technical Operations at Calidi. “The data also demonstrate that the platform can effectively express genetic medicines at the tumor site in concentrations that are similar to what is achievable with localized dosing while avoiding systemic exposure.”
“In our syngeneic models, we are demonstrating levels of IL-15 superagonist in the tumor microenvironment after systemic administration that are comparable to levels seen with intrathecal administration of Anktiva in the bladder,” added Eric Poma, PhD, Chief Executive Officer. “The level of IL-15 superagonist in serum or organs in these models is several orders of magnitude lower than what is expressed in the tumor. We believe this represents an unprecedented level of targeted genetic medicine delivery and expression.”
Link to SITC poster https://www.calidibio.com/wp-content/uploads/2025/11/2025-SITC-Calidi-CLD-401.pdf
Calidi is currently conducting IND-enabling studies for CLD-401 and anticipates submitting an Investigational New Drug (IND) application by the end of 2026. The Company is also actively pursuing strategic partnerships to accelerate clinical development and broaden the impact of its RedTail platform.
In addition to the data presented, Calidi also held an investor day featuring members of its Scientific Advisory Board. Dr. Dimitri Zamarin, a world-renowned expert in virotherapy, from the Icahn School of Medicine at Mount Sinai and Dr John Wrangle, a thoracic medical oncologist with pioneering clinical work on IL-15 superagonist in patients, at the Medical University of South Carolina (MUSC). Both investigators spoke on the promise and differentiation of CLD-401 and the RedTail platform.
Link to Investor Day at SITC https://www.youtube.com/watch?v=ZGqtE6-kgmM Calidi Biotherapeutics (NYSE American: CLDI) is a clinical-stage company pioneering the development of targeted therapies with the potential to deliver genetic medicines to distal sites of disease.
About Calidi
The company’s proprietary Redtail platform features an engineered enveloped oncolytic virus designed for systemic delivery and targeting of metastatic sites. This advanced enveloped technology is intended to shield the virus from immune clearance, allowing virotherapy to effectively reach tumor sites, induce tumor lysis, and deliver potent genetic medicine(s) to metastatic locations.
CLD-401, the lead candidate from the Redtail platform, currently in IND-enabling studies, targets non-small cell lung cancer, head and neck cancer, and other tumor types with high unmet medical need.
Calidi Biotherapeutics is headquartered in San Diego, California. For more information, please visit www.calidibio.com or view Calidi’s Corporate Presentation here.
Forward-Looking Statements
This press release may contain forward-looking statements for purposes of the “safe harbor” provisions under the United States Private Securities Litigation Reform Act of 1995. Terms such as “anticipates,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “towards,” “would” as well as similar terms, are forward-looking in nature, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements include, but are not limited to, statements concerning key milestones, including certain pre-clinical data, planned clinical trials, and statements relating to the safety and efficacy of Calidi’s therapeutic candidates in development. Any forward-looking statements contained in this discussion are based on Calidi’s current expectations and beliefs concerning future developments and their potential effects and are subject to multiple risks and uncertainties that could cause actual results to differ materially and adversely from those set forth or implied in such forward-looking statements. These risks and uncertainties include, but are not limited to, the risk that Calidi is not able to raise sufficient capital to support its current and anticipated clinical trials, the risk that early results of clinical trials do not necessarily predict final results and that one or more of the clinical outcomes may materially change following more comprehensive review of the data, and as more patient data becomes available, the risk that Calidi may not receive FDA approval for some or all of its therapeutic candidates. Other risks and uncertainties are set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in the Company’s annual report filed with the SEC on Form 10-K on March 31, 2025, as may be amended or supplemented by other reports we file with the SEC from time to time. We disclaim any obligation to update any forward-looking statement to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events.
For Investors:
Dave
Gentry, CEO
RedChip Companies, Inc.
1-407-644-4256
CLDI@redchip.com