UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2024
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________ to ________
Commission File Number 1-11460

Eterna Therapeutics Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 31-1103425 | |
|
(State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
|
| 1035 Cambridge Street, Suite 18A, Cambridge, MA | 02141 | |
| (Address of Principal Executive Offices) | (Zip Code) |
(212) 582-1199
(Registrant’s telephone number, including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on Which Registered |
||
| Common Stock, $0.005 par value | ERNA | Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the common stock held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter (June 30, 2024), computed by reference to the closing sale price of the common stock on the Nasdaq Capital Market on such date, was approximately $8.9 million. For purposes of this determination shares beneficially owned by executive officers, directors and ten percent stockholders have been excluded, which does not represent an admission by the registrant as to the affiliate status of such person.
As of March 10, 2025, the registrant had 52,244,929 shares of common stock outstanding.
TABLE OF CONTENTS
|
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements” as that term is defined under the Private Securities Litigation Reform Act of 1995 (“PSLRA”), Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements include statements related to future events, results, performance, prospects and opportunities, including statements related to our strategic plans, capital needs, and our financial position. Forward-looking statements are based on information currently available to us, on our current expectations, estimates, forecasts, and projections about the industries in which we operate and on the beliefs and assumptions of management. Forward looking statements often contain words such as “expects,” “anticipates,” “could,” “targets,” “projects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “may,” “will,” “would,” and similar expressions. In addition, any statements that refer to projections of our future financial performance, our anticipated growth and trends in our business, and other characterizations of future events or circumstances, are forward-looking statements. Forward-looking statements by their nature address matters that are, to different degrees, subject to risks and uncertainties that could cause actual results to differ materially and adversely from those expressed in any forward-looking statements. For us, particular factors that might cause or contribute to such differences include those identified in the “Summary of Principal Risk Factors” below and the other risks and uncertainties described in Part I, Item 1A “Risk Factors” of this Annual Report on Form 10-K and described in other documents we file from time to time with the Securities and Exchange Commission (the “SEC”), including our Quarterly Reports on Form 10-Q.
Readers are urged not to place undue reliance on the forward-looking statements in this Annual Report on Form 10-K, which speak only as of the date of this Annual Report on Form 10-K. We are including this cautionary note to make applicable, and take advantage of, the safe harbor provisions of the PSLRA. Except as required by law, we do not undertake, and expressly disclaim any obligation, to disseminate, after the date hereof, any updates or revisions to any such forward-looking statements to reflect any change in expectations or events, conditions or circumstances on which any such statements are based.
We believe that the expectations reflected in forward-looking statements in this Annual Report on Form 10-K are based upon reasonable assumptions at the time made. However, given the risks and uncertainties, you should not rely on any forward-looking statements as a prediction of actual results, developments or other outcomes. You should read these forward-looking statements with the understanding that we may be unable to achieve projected results, developments or other outcomes and that actual results, developments or other outcomes may be materially different from what we expect.
Unless stated otherwise or the context otherwise requires, all references in this Annual Report on Form 10-K to “Eterna” refer to Eterna Therapeutics Inc., references to “Eterna LLC” refer to Eterna Therapeutics LLC, and references to the “Company,” “we,” “us” or “our” refer to Eterna and its consolidated subsidiaries, including Eterna LLC, Novellus, Inc. and Novellus Therapeutics Limited.
SUMMARY OF PRINCIPAL RISK FACTORS
Below is a summary of the principal factors that make an investment in our securities speculative or risky. This summary does not address all of the risks that we face. We urge investors to carefully review and consider the additional discussion of the risks summarized in this risk factor summary, and other risks that we face, which can be found below under the heading “Risk Factors” in Item 1A of this Annual Report on Form 10-K, together with other information in this report, before making investment decisions regarding our securities.
Risks Related to our Business and Industry
| ● | We will require substantial additional capital to fund our operations, and if we fail to obtain the necessary financing, we may not be able to continue as a going concern. | |
| ● | We have incurred significant losses since our inception and expect to continue to incur losses for the foreseeable future, which, together with our limited financial resources and substantial capital requirements, make it difficult to assess our prospects. | |
| ● | We depend substantially, and expect in the future to continue to depend, on in-licensed intellectual property. Such licenses impose obligations on our business, and if we fail to comply with those obligations, we could lose license rights, which would substantially harm our business. | |
| ● | We rely heavily on in-licensed intellectual property from Factor Limited. Loss of this license or termination of the Factor L&C Agreement could significantly harm our product development and ability to enter co-development strategic partnerships, materially impacting our business.. | |
| ● | We have previously identified a material weakness in our internal control over financial reporting, which may adversely affect investor confidence in us, result in litigation and materially and adversely affect our business and operating results. |
|
|
Risks Related to New, Cutting Edge Technologies
| ● | Our product development relies on novel, inherently risky technologies. Stem cell therapy is a relatively new field, and our efforts may not result in effective treatments for human diseases. | |
| ● | We are in an industry with intense competition and rapid technological change and our competitors may develop therapies that are more advanced, safer or more effective than any therapy we may develop in the future, which may adversely affect our financial condition. | |
| ● | Negative public opinion and increased regulatory scrutiny due to ethical and other concerns surrounding the use of stem cell therapy or human tissue may damage public perception of our synthetic allogeneic iMSC product candidates or adversely affect our ability to conduct our business. | |
| ● | The manufacture of biotechnology products is complex, and manufacturers often encounter difficulties in production. |
Risks Related to Ownership of our Common Stock
| ● | Seven stockholders collectively own a significant percentage of our outstanding common stock, and as a result of such ownership, such stockholders may influence the election of directors and other matters submitted to stockholders. | |
| ● | Our failure to meet the continued listing requirements of Nasdaq could result in a delisting of our common stock. | |
| ● | Anti-takeover provisions of Delaware law and provisions in our charter and bylaws could make a third-party acquisition of us difficult. |
Risks Related to Regulatory Requirements and Our Intellectual Property
| ● | The regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable. If we are unable to obtain regulatory approval for our product candidates, our business will be substantially harmed. | |
| ● | If we are unable to obtain and maintain patent and other intellectual property protection, or if the scope of the patent and other intellectual property protection obtained is not sufficiently broad, our business, financial condition, results of operations, and/or prospects may be materially and adversely effected. | |
| ● | If we do not obtain patent term extension for future products that our strategic partners or collaborators may successfully develop, our business may be materially harmed. | |
| ● | Changes in patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect future products and product candidates that we or our strategic partners or collaborators may develop. | |
| ● | We may not be able to protect our intellectual property rights throughout the world. | |
| ● | We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time-consuming and unsuccessful. |
|
|
PART I
| ITEM 1. | Business |
Overview
We are a preclinical-stage synthetic allogeneic iMSC therapy company. Our vision is to improve the lives of patients with difficult-to-treat diseases through innovative, effective, and safe, but accessible cellular therapies, and our mission is to develop allogenic off-the-shelf cellular therapies, leveraging induced pluripotent stem cell (“iPSC”)-derived mesenchymal stem cells (“iMSCs”) to target solid tumors and autoimmune diseases.
Objectives and Business Strategy
Our lead product candidate ERNA-101 is allogenic IL-7 and IL-15-secreting iMSCs. ERNA-101 capitalizes on the intrinsic tumor-homing ability of MSCs to slip through the tumor’s defenses and to deliver potent pro-inflammatory factors directly to the tumor microenvironment (“TME”), limiting systemic exposure and potential toxicity while unleashing potent anti-cancer immune responses including enhancement of T-cell anti-tumor activity. Our initial focus is to develop ERNA-101 in platinum-resistant, ovarian cancer. We collaborated with the University of Texas MD Anderson Cancer Center to investigate the ability of ERNA-101 to induce and modulate antitumor immunity in an ovarian cancer model. We are expecting to complete the Investigational New Drug (“IND”) enabling studies and IND submission by 2026.
We are also investigating anti-inflammatory cytokine (e.g. IL-10)-secreting iMSCs in inflammatory/auto-immune disorders like Rheumatoid arthritis, which we refer to as ERNA-102. MSCs have an intrinsic ability to home to inflamed tissue and have been shown to dampen inflammation and drive/healing/regeneration through multiple secreted mediators and cell-cell interactions. We are investigating the ability of ERNA-102 to turbocharge these anti-inflammatory and regenerative effects.
Additionally, we are actively seeking strategic partnerships to co-develop or out-license therapeutic assets and engage with potential collaborators to expand developmental opportunities.
License Agreement
On September 24, 2024, we entered into the Exclusive License and Collaboration Agreement (“the Factor L&C Agreement”) with Factor Bioscience Limited (“Factor Limited”). The Factor L&C Agreement terminated the Amended and Restated Factor License Agreement (the “A&R Factor License Agreement”) entered into on November 14, 2023 as well as an exclusive license agreement we acquired from Dilos Bio (formerly known as Exacis Biotherapeutics Inc. (“Exacis”)) under an asset purchase agreement in April 2023.
Under the Factor L&C Agreement, we have obtained an exclusive license in the fields of cancer, autoimmune disorders, and rare diseases with respect to certain licensed technology and we have the right to develop the licensed technology directly or enter into co-development agreements with partners who can help bring such technology to market. The Factor L&C Agreement also provides for certain services and materials to be provided by Factor to facilitate our development of the licensed technology and to enable us to scale up production at third party facilities.
The initial term of the Factor L&C Agreement is one year after the effective date, and it automatically renews yearly thereafter. We may terminate the Factor L&C Agreement for any reason upon 90 days’ written notice to Factor, and the parties otherwise have customary termination rights, including in connection with certain uncured material breaches and specified bankruptcy events.
Pursuant to the Factor L&C Agreement, we will pay Factor $0.2 million per month for the first twelve months, $0.1 million per month for the first nine months toward patent costs, certain milestone payments, royalty payments on net sales of commercialized products and sublicensing fee payments.
|
|
Patent Portfolio
Our strategy is to develop and advance a pipeline of therapeutic products both internally and through strategic partnerships, leveraging our in-licensed synthetic allogeneic iMSC therapy, with the near-term focus on deploying our synthetic allogeneic iMSC therapy through strategic partnerships. As of March 10, 2025, we had in-licensed 13 patent families filed in the United States and other major markets worldwide, including 33 granted patents, 31 pending non-provisional patent applications or provisional patent applications, and 4 pre-nationalization PCT application. Patent protection for the iMSC technology platform includes:
| Family Number and Title | United
States or Foreign Jurisdiction |
Earliest
Effective Date of Patent Application |
|
| FAB-001: “Methods and Products for Transfecting Cells” |
Granted: US (Nos. 10829738, 10982229, 11692203, 10472611, 11466293, 10662410, 12227757);
EP (No. 2788033 (CH; DE; FR; GB; IE));
EP (No. 3260140 (BE; CH; DE; DK; FR; GB; IE; NL);
CA (No. 2,858,148);
JP (Nos. 6073916, 6294944);
KR (No. 10-2196339)
Pending: US (4X), EP, CA |
12/05/2011 | |
| FAB-003: “Methods and Products for Transfection” |
Granted: US (Nos. 8497124, 9127248, 11492600, 9399761, 9562218, 9695401, 9879228, 9969983, 10131882, 10301599, 10443045, 12227768)
Pending: US (3X) |
5/07/2012 | |
| FAB-005: “Methods and Products for Expressing Proteins in Cells” |
Granted: : JP (Nos. 6510416, 6890565, 6793146, 7436406); KR (No. 10-2121086); CA (No. 2890110)
Pending: JP, US |
11/01/2012 | |
| FAB-009: “Nucleic Acid Products and Methods of Administration Thereof” |
Granted: JP (Nos. 7199809, Not Assigned Yet)
Pending: CA, JP |
02/16/2016 |
|
|
| Family Number and Title | United
States or Foreign Jurisdiction |
Earliest
Effective Date of Patent Application |
|
| FAB-010: “Nucleic Acid Products and Methods of Administration Thereof” | Pending: US, CA, EP |
08/17/2017 | |
| FAB-011: “Nucleic Acid-Based Therapeutics” | Pending: US (2X), EP |
03/27/2019 | |
| FAB-013: “Engineered Gene-Editing Proteins” | Pending: US, EP | 05/12/2021 | |
| FAB-016: “Mesenchymal Stem Cell Therapies” |
Pending: US, EP, JP |
04/28/2021
|
|
| FAB-017: “Engineered Immune Cell Therapies” | Pending: US, CA, EP and JP | 03/04/2022 | |
| FAB-018: “Circular RNA” |
Pending: US, CA, EP and JP |
04/27/2022 | |
| FAB-019: “Methods for reprogramming and gene editing cells” | Pre-nationalization PCT: (1X) | 01/05/2022 | |
| FAB-021: ““Methods for reprogramming and gene editing cells” | Pre-nationalization PCT: (1X) | 05/01/2024 | |
| FAB-023: “Methods for reprogramming and gene editing cells” |
Pre-nationalization PCT: (1X) Pending: US |
09/20/2024 04/19/2024 |
|
|
US – United States of America EP – European Patent Convention PCT – Patent Cooperation Treaty BE – Belgium CA – Canada CH – Switzerland DE – Germany DK – Denmark FR – France GB – Great Britain IE – Ireland JP – Japan KR – Republic of Korea (South Korea) NL – Netherlands |
Patent Families
Descriptions of our patent families are as follows:
| ● | FAB-001: “Methods and Products for Transfecting Cells” - The present invention relates in part to nucleic acids encoding proteins, nucleic acids containing non-canonical nucleotides, therapeutics comprising nucleic acids, methods, kits, and devices for inducing cells to express proteins, methods, kits, and devices for transfecting, gene editing, and reprogramming cells, and cells, organisms, and therapeutics produced using these methods, kits, and devices. Methods for inducing cells to express proteins and for reprogramming and gene-editing cells using RNA are disclosed. Methods for producing cells from patient samples, cells produced using these methods, and therapeutics comprising cells produced using these methods are also disclosed. | |
| ● | FAB-003: “Methods and Products for Transfection” - The present invention relates in part to methods for producing tissue-specific cells from patient samples, and to tissue-specific cells produced using these methods. Methods for reprogramming cells using RNA are disclosed. Therapeutics comprising cells produced using these methods are also disclosed. |
|
|
| ● | FAB-005: “Methods and Products for Expressing Proteins in Cells” - The present invention relates in part to nucleic acids encoding proteins, therapeutics comprising nucleic acids encoding proteins, methods for inducing cells to express proteins using nucleic acids, methods, kits and devices for transfecting, gene editing, and reprogramming cells, and cells, organisms, and therapeutics produced using these methods, kits, and devices. Methods and products for altering the DNA sequence of a cell are described, as are methods and products for inducing cells to express proteins using synthetic RNA molecules. Therapeutics comprising nucleic acids encoding gene-editing proteins are also described. | |
| ● | FAB-009: “Nucleic Acid Products and Methods of Administration Thereof” - The present invention relates in part to nucleic acids, including nucleic acids encoding proteins, therapeutics and cosmetics comprising nucleic acids, methods for delivering nucleic acids to cells, tissues, organs, and patients, methods for inducing cells to express proteins using nucleic acids, methods, kits and devices for transfecting, gene editing, and reprogramming cells, and cells, organisms, therapeutics, and cosmetics produced using these methods, kits, and devices. | |
| ● | FAB-010: “Nucleic Acid Products and Methods of Administration Thereof” - The present invention relates in part to nucleic acids, including nucleic acids encoding proteins, therapeutics and cosmetics comprising nucleic acids, methods for delivering nucleic acids to cells, tissues, organs, and patients, methods for inducing cells to express proteins using nucleic acids, methods, kits and devices for transfecting, gene editing, and reprogramming cells, and cells, organisms, therapeutics, and cosmetics produced using these methods, kits, and devices. | |
| ● | FAB-011: “Nucleic Acid-Based Therapeutics” - The present invention relates in part to nucleic acids, including nucleic acids encoding proteins, therapeutics and cosmetics comprising nucleic acids, methods for delivering nucleic acids to cells, tissues, organs, and patients, methods for inducing cells to express proteins using nucleic acids, methods, kits and devices for transfecting, gene editing, and reprogramming cells, and cells, organisms, therapeutics, and cosmetics produced using these methods, kits, and devices. | |
| ● | FAB-013: “Engineered Gene-Editing Proteins” - The present invention relates in part to nucleic acids encoding gene editing proteins, including novel engineered variants. | |
| ● | FAB-016: “Mesenchymal Stem Cell Therapies” - Cell-based therapies based on MSCs are described. | |
| ● | FAB-017: “Engineered Immune Cell Therapies” - The present disclosure relates in part to engineered immune cells that are, inter alia, silenced from a host immune response. | |
| ● | FAB-018: “Circular RNA” - Nucleic acid structures that promote formation of circular RNAs (circRNAs), which may comprise hybridization of substantially complimentary regions within the nucleic acid and contact with an RNA ligase. The nucleic acid structures may be used in gene editing and/or therapeutic applications. In some embodiments, the nucleic acid comprises the structure: 5’-X-Y-A-IRES-B-CDS-C-Y’-Z-3’, wherein X, Y, Y’ and Z each independently comprise one or more nucleotides; Y and Y’ are substantially complementary; X and Z are not substantially complementary; IRES comprises an internal ribosome entry site; CDS comprises a coding sequence; and A, B, and C are each independently a spacer comprising one or more nucleotides or null. | |
| ● | FAB-019: “Methods for reprogramming and gene editing cells” The present disclosure provides improved methods for reprogramming and gene editing cells, including manufacturing a population of cells comprising cells of the lymphoid lineage and/or cells of the myeloid lineage. |
|
|
| ● | FAB-021: “Methods for reprogramming and gene editing cells” The present disclosure provides improved methods for reprogramming and gene editing cells. The present disclosure provides a method of inserting a sequence in a DNA site by introducing a single strand break followed by insertion of the sequence using a single-stranded repair template. In another aspect, the present disclosure provides a method of activating gene expression in a cell during its differentiation. In another aspect, the present disclosure methods and compositions related to iPSC-derived mesenchymal stroma/stem cells that overexpressed IDO1. In another aspect, the present disclosure provides a solid support comprising iPSC-derived mesenchymal stem cells which can be used to treat organ damage. | |
| ● | FAB-023: “Methods for reprogramming and gene editing cells” The present disclosure provides improved methods for reprogramming and gene editing cells. The present disclosure provides a method gene editing a cell using engineered chromatin opening sequence at the DNA target site. The cells can be induced pluripotent stem cells. The disclosure is also directed to the targeting the inhibition of tumor promoting genes. |
Patent Term and Term Extensions
Individual patents have terms for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States and the European Union are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office, or the USPTO, delay in issuing the patent as well as a portion of the term effectively lost as a result of the United States Food and Drug Administration (“FDA”) regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the restoration period cannot extend the patent term beyond 14 years from FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically are also 20 years from the earliest effective filing date. All taxes or annuities for a patent, as required by the USPTO and various foreign jurisdictions, must be timely paid in order for the patent to remain in force during this period of time.
The actual protection afforded by a patent may vary on a product-by-product basis, from country to country, and can depend upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Our patents and patent applications may be subject to procedural or legal challenges by others. We may be unable to obtain, maintain and protect the intellectual property rights necessary to conduct our business, and we may be subject to claims that we infringe or otherwise violate the intellectual property rights of others, which could materially harm our business. For more information, see Item 1A “Risk Factors-Risks Related to Our Intellectual Property” contained in this Annual Report on Form 10-K.
Supply and Manufacturing
We currently do not have any agreements for the supply or manufacturing of cell lines. However, together with our licensor, Factor Limited, we believe that we have considerable experience in developing engineered cell lines. Pursuant to the Factor L&C Agreement Factor Limited has agreed to provide us with certain synthetic iMSC cell engineering research support services, including (i) reasonable access to Factor Bioscience’s research laboratory facilities located in Cambridge, Massachusetts, (ii) training of our research staff in certain mRNA, iPSC, gene editing technologies and process of converting iPSC to iMSC, (iii) copies of protocol binders, formulations, Licensor Know-How (as defined in the Factor L&C Agreement) and sequences that may be useful for the development of synthetic iMSC products and (iv) in vitro transcription templates, mRNA constructs, and iPS and iMS cells that may be useful for the development of synthetic iMSC products.
We expect to rely on contract manufacturing relationships for any products that we may develop or acquire in the future. However, there can be no assurance that we will be able to successfully contract with such manufacturers on terms acceptable to us, or at all.
Contract manufacturers are subject to ongoing periodic and unannounced inspections by the FDA, the Drug Enforcement Administration (“DEA”) and corresponding state agencies to ensure strict compliance with current good manufacturing practices (“cGMPs”) and other state and federal regulations. Our contractors, if any, in Europe face similar challenges from the numerous European Union and member state regulatory agencies and authorized bodies. We do not have control over third-party manufacturers’ compliance with these regulations and standards, other than through contractual obligations. If our contractors are deemed out of compliance with cGMPs, product recalls could result, inventory could be destroyed, production could be stopped, and supplies could be delayed or otherwise disrupted, which could have a materially adverse effect on our business.
|
|
If we need to change manufacturers after commercialization, the FDA and corresponding foreign regulatory agencies must approve these new manufacturers in advance, which will involve testing and additional inspections and associated regulatory submissions to ensure compliance with FDA regulations and standards, which collectively may result in significant lead times, delay and cost. Furthermore, switching manufacturers may be difficult because the number of potential manufacturers is limited. It may be difficult or impossible for us to find a replacement manufacturer quickly or on terms acceptable to us, or at all.
Regulatory Matters
Government regulation and product approval
Drugs and biologics must be approved by the FDA through the New Drug Application (“NDA”) process or the Biologic License Application (“BLA”) process before they may be legally marketed in the United States. We use the terms “marketing application” or “MA” to apply to both.
There are two centers within the FDA that are responsible for the review and approval of drug and biologic marketing applications and general regulatory oversight: the Center for Drug Evaluation and Research (“CDER”) and the Center for Biologics Evaluation and Research (“CBER”). While all conventional drug products are regulated by CDER, biologic products can be regulated by either CDER or CBER, depending on the product’s classification.
The majority of BLA submissions are assigned to CBER; however, BLAs for certain biologic product categories are reviewed by CDER. These product categories include monoclonal antibodies for in vivo use, most proteins for therapeutic use, and categories such as cytokines, enzymes, and other novel proteins. Regardless of the category, NDAs for all drug products fall under the jurisdiction of CDER.
In the United States, drugs are subject to rigorous regulation by the FDA under the federal Food, Drug, and Cosmetic Act (“FDCA”) and implementing regulations, and biologics under the FDCA, the Public Health Services Act (“PHSA”), and their implementing regulations. Additionally, drugs and biologics are subject to other federal and state statutes. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable United States requirements at any time during the product development process, approval process, or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us. The process required by the FDA before a drug or biologic may be marketed in the United States generally involves the following:
| ● | completion of preclinical laboratory tests, animal studies and formulation studies according to the FDA’s good laboratory practice, or GLP, regulations; | |
| ● | submission of an investigational new drug application (“IND”), which must become effective before human clinical trials may begin and which must include approval by an institutional review board (“IRB”) at each clinical site before the trials are initiated; | |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use conducted in compliance with federal regulations and good clinical practice (“GCP”), an international standard meant to protect the rights and health of human clinical trial subjects and to define the roles of clinical trial sponsors, administrators, and monitors; | |
| ● | submission to, and acceptance by, the FDA of a MA; | |
| ● | satisfactory completion of an FDA inspection of our manufacturing facility or other facilities at which the drug or biologic is produced to assess compliance with current good manufacturing practice (“cGMP”), regulations to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; | |
| ● | potential FDA audit of the non-clinical and clinical trial sites that generated the data in support of the MA: and | |
| ● | FDA review and approval of the MA. |
The testing and approval process require substantial time, effort and financial resources, and the receipt and timing of any approval is uncertain.
|
|
United States drug development process
Once a pharmaceutical candidate is identified for development it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. Prior to beginning human clinical trials, a sponsor must submit an IND to the FDA, which includes the results of the preclinical tests, together with manufacturing information and analytical data. Some preclinical or non-clinical testing may continue even after the IND is submitted. In addition to including the results of the preclinical studies, the IND will also include a protocol detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated, if the trial lends itself to an efficacy evaluation. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the trial. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may, at any time, impose a clinical hold on ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization and then only under terms authorized by the FDA.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of one or more qualified investigators in accordance with federal regulations and GCP.
Clinical trials must be conducted under protocols detailing the objectives of the trial and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND. Further, an IRB affiliated with each institution participating in the clinical trial must review and approve each protocol before any clinical trial commences at that institution. All research subjects must provide informed consent, and informed consent information must be submitted to the IRB for approval prior to initiation of the trial and prior to providing it to potential subjects. Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if adverse events or other certain types of other changes occur.
Human clinical trials are typically conducted in three phases. A fourth, or post-approval, phase may include additional clinical studies. These phases generally include the following, and may be sequential, or may overlap or be combined:
| ● | Phase 1 clinical trials involve the initial introduction of the drug or biologic into human subjects. These studies are designed to determine the safety of usually single doses of the compound and determine any dose limiting intolerance, as well as evidence of the metabolism and pharmacokinetics of the drug in humans. For some products for severe or life-threatening diseases, especially if the product may be too toxic to administer to healthy humans, the initial clinical trials may be conducted in individuals having a specific disease for which use the tested product is indicated. | |
| ● | Phase 2 clinical trials usually involve studies in a limited patient population to evaluate the safety and efficacy of the drug or biologic for specific, targeted indications, to determine dosage tolerance and optimal dosage, and to identify possible adverse effects and safety risks. | |
| ● | In Phase 3, if a compound is found to be potentially effective and to have an acceptable safety profile in Phase 2 (or occasionally Phase 1) studies, the Phase 3 studies will be conducted to further confirm clinical efficacy, optimal dosage and safety within an expanded population which may involve geographically diverse clinical trial sites. Generally, but not always, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of a marketing application. | |
| ● | Phase 4 clinical trials are studies required of or agreed to by a sponsor that are conducted after the FDA has approved a product for marketing. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement. Failure to promptly conduct Phase 4 clinical trials where necessary could result in withdrawal of approval for products approved under accelerated approval regulations. |
While Phase 1, Phase 2, and Phase 3 studies are generally required for approval of a marketing application, certain drugs and biologics may not require one or more steps in the process depending on other testing and the situation involved. Additionally, the FDA, an IRB, or the sponsor may stop testing at any time if results show patients being exposed to unnecessary health risks or overly dangerous side effects. Prior to the initiation of a clinical trial or at any time during the conduct of studies with human subjects, the FDA may place a study on clinical hold where patients may not be enrolled and ongoing trial activities are suspended until questions around potential safety issues with investigational products are addressed.
|
|
In addition, the manufacturer of an investigational drug in a Phase 2 or Phase 3 clinical trial for a serious or life-threatening disease is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for expanded access to such investigational drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the mechanism of action and physical characteristics of the drug and finalize a process for manufacturing the product in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product and, among other requirements, the manufacturer must develop methods for testing the identity, strength, quality, potency, and purity of the final product. Additionally, appropriate packaging must be selected and validated, and stability studies must be conducted to demonstrate that the product does not undergo unacceptable deterioration over its shelf life.
Additional Regulation for Cell Therapy Clinical Trials
In addition to the regulations discussed elsewhere in this section, there are a number of additional standards that apply to clinical trials involving the use of cell therapy. The FDA has issued various guidance documents regarding cell therapies, which outline additional factors the FDA will consider at each of the above stages of development and relate to, among other things: the proper preclinical assessment of cell therapies; the CMC information that should be included in an IND application; the proper design of tests to measure product potency in support of an IND or BLA application; and measures to observe delayed adverse effects in subjects who have been exposed to investigational cell therapies when the risk of such effects is high. Further, the clinical study requirements set by the FDA vary significantly based on a product’s type, complexity, novelty, intended use, and target market. Obtaining regulatory approval for innovative therapies like ours can be more costly and time-consuming compared to more familiar or well-studied treatments. Additionally, negative outcomes in other cell therapy trials could prompt regulators to revise approval requirements for our product candidates.
United States drug review and approval process
Following completion of clinical studies, the results are evaluated and, depending on the outcome, submitted to the FDA in the form of an NDA or BLA in order to obtain FDA approval of the product and authorization to commence commercial marketing. In responding to an NDA or BLA, the FDA may require additional testing or information, may require that the product labeling be modified, may impose a post-approval study and other commitments or reporting requirements or other restrictions on product distribution, or may deny the application. The timing of final FDA review and action varies greatly but can take years in some cases and may involve the input of an FDA advisory committee of outside experts. Product sales in the United States may commence only upon FDA approval of an NDA or BLA.
FDA approval of a marketing application is required before marketing of the product may begin in the United States. The MA must include the results of product development, preclinical studies and clinical studies, together with other detailed information, including information on the chemistry, manufacture and controls utilized in manufacture of the product. In addition, an MA must also demonstrate purity, specifically in terms of showing that the final product does not contain extraneous material. The FDA has 60 days from its receipt of the MA to review the application to ensure that it is sufficiently complete for substantive review before accepting it for filing. The FDA may request additional information rather than accept an MA for filing. In this event, the MA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The submission of an MA is also subject to the payment of a substantial application fee (although a waiver of such fee may be obtained under certain limited circumstances, including when the drug that is subject of the application has received Orphan Drug Designation for the indication sought). Further, the sponsor of an approved MA is subject to an annual program fee. User fees typically increase annually. The approval process is lengthy and complex, and the FDA may refuse to approve an MA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee. The FDA reviews an application to determine, among other things, whether a product is safe and effective for its intended use. Before approving an MA, the FDA will inspect the facility or facilities where the product is manufactured to determine whether its manufacturing is cGMP–compliant to assure and preserve the product’s identity, potency, quality, purity and stability.
|
|
If the FDA’s evaluation of the marketing submission or manufacturing facilities is not favorable, the FDA will issue a complete response letter. The complete response letter outlines the deficiencies in the submission and often requires additional testing or information in order for the FDA to reconsider the application. Even after submitting this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. With limited exceptions, the FDA may withhold approval of an MA regardless of prior advice it may have provided or commitments it may have made to the sponsor.
Once an MA is approved, changes to the conditions of approval, including additional indications, are made by the submission of a supplement to the MA The supplemental NDA (“sNDA”) or the supplemental BLA (“sBLA”) must contain all of the information necessary to support the change. In the case of a new indication, that information usually consists of at least one clinical trial, and often more. Like an MA, FDA determines whether the supplemental application is sufficiently complete to permit review before it is filed. FDA then reviews the supplemental application. The FDA can either approve or issue a complete response letter outlining the deficiencies.
Manufacturing readiness
As part of the approval process, the FDA must inspect and approve each manufacturing facility. Among the conditions of approval is the requirement that a manufacturer’s quality control and manufacturing procedures conform to cGMP. Manufacturers must expend significant time, money and effort to ensure continued compliance, and the FDA conducts periodic inspections to verify compliance. If a manufacturer fails to comply or cannot remedy regulator identified deficiencies, then the FDA may prohibit the product from being marketed.
If the FDA grants approval, the approval will be limited to those conditions and patient populations for which the product is safe and effective, as demonstrated through clinical studies. Further, a product may be marketed only in those dosage forms and for those indications approved in the MA. Certain changes to an approved MA, including, with certain exceptions, any significant changes to labeling, require approval of a supplemental application before the drug may be marketed as changed. Any products manufactured or distributed pursuant to FDA approvals are subject to continuing monitoring and regulation by the FDA, including compliance with cGMP and the reporting of adverse experiences with the drugs. The nature of marketing claims that the FDA permits in the labeling and advertising of products will generally be limited to those specified in FDA approved labeling, and the advertising of products will be subject to comprehensive monitoring and regulation by the FDA. Products whose review was accelerated may carry additional restrictions on marketing activities, including the requirement that all promotional materials are pre-submitted to the FDA. Claims exceeding those contained in approved labeling will constitute a violation of the FDCA. Violations of the FDCA or regulatory requirements at any time during the product development process, approval process, or marketing and sale following approval may result in agency enforcement actions, including corrective advertising, cessation of violative promotion, withdrawal of approval, recall, seizure of products, warning letters, injunctions, fines and/or civil or criminal penalties.
In addition, federal, state and foreign laws and regulations regarding the manufacture and sale of new drugs are subject to future changes.
Post-approval requirements and consideration
Once an MA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA and Federal Trade Commission closely regulate the post-approval marketing and promotion of drugs and biologics, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. As a condition of MA approval, the FDA may also require a risk evaluation and mitigation strategy (“REMS”) to help ensure that the benefits of the drug or biologic outweigh the potential risks. REMS can include medication guides, communication plans for the healthcare professionals, and other Elements to Assure Safe Use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug or biologic.
|
|
Drugs and biologics may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new MA supplement before the change can be implemented. An MA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing MA supplements as it does in reviewing MAs.
Adverse event reporting and submission of periodic reports are required following FDA approval of an MA. The FDA also may require post-marketing testing, known as Phase 4 testing, and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control as well as drug manufacture, packaging, and labeling procedures must continue to conform to cGMPs after approval. Drug and biologic manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Foreign regulatory requirements
In addition to regulation by the FDA and certain state regulatory agencies, there are a variety of foreign regulations governing clinical trials and the marketing of products. Outside of the United States, the ability of a company to market a product depends upon receiving a marketing authorization from the appropriate regulatory agencies. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. In any country, however, a company will only be permitted to commercialize its products if the appropriate regulatory agency is satisfied that the company presented adequate evidence of safety, quality and efficacy. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must be obtained prior to the commencement of marketing of the product in those countries. The regulatory approval and oversight process in other countries includes all of the risks associated with regulation by the FDA and certain state regulatory agencies as described above.
Under the European Union regulatory system, applications for drug approval may be submitted either in a centralized or decentralized manner. Under the centralized procedure, a single application to the European Medicines Agency (“EMA”) may lead to an approval granted by the European Commission which permits marketing of the product throughout the European Union. The decentralized procedure provides for mutual recognition of nationally approved decisions and is used for products that do not comply with requirements for the centralized procedure. Under the decentralized procedure, the holders of national marketing authorization in one of the countries within the European Union may submit further applications to other countries within the European Union, who will be requested to recognize the original authorization based on an assessment report provided by the country in which marketing authorization is held.
Pharmaceutical pricing and reimbursement
In both United States and foreign markets, the ability of a company to commercialize its products successfully, and to attract commercialization partners for its products, depends in significant part on the availability of adequate financial coverage and reimbursement from third-party payors, including, in the United States, governmental payors such as Medicare and Medicaid, managed care organizations, private commercial health insurers and pharmacy benefit managers (“PBMs”). Third party payors are increasingly challenging the prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. Companies may need to conduct expensive pharmacoeconomic or other studies to further demonstrate the value of its products. Even with the availability of such studies, products may be considered less safe, less effective or less cost-effective than alternative products, and third-party payors may not provide coverage and reimbursement for any product, in whole or in part.
Political, economic and regulatory influences are subjecting the health care industry in the United States to fundamental changes. There have been, and we expect there will continue to be, legislative and regulatory proposals to change the healthcare system in ways that could significantly affect the development and commercialization of products, including the Patient Protection and Affordable Care Act of 2010 (the “Affordable Care Act”).
|
|
In the United States, Congress, state legislatures, and private sector entities are expected to continue to consider and may adopt healthcare policies intended to curb rising healthcare costs. These cost containment measures could include:
| ● | controls on government-funded reimbursement for drugs; | |
| ● | mandatory rebates or additional charges to manufacturers for their products to be covered on Medicare Part D formularies; | |
| ● | controls on healthcare providers; | |
| ● | controls on pricing of pharmaceutical products, including the possible reference of the pricing of United States drugs to non-United States drug pricing for the same product; | |
| ● | challenges to the pricing of drugs or limits or prohibitions on reimbursement for specific products through other means; | |
| ● | reform of drug importation laws; | |
| ● | entering into contractual agreements with payors; and | |
| ● | expansion of use of managed-care systems in which healthcare providers contract to provide comprehensive healthcare for a fixed cost per person |
The Inflation Reduction Act of 2022 (the “IRA”) contained several provisions designed to curb the prices of drugs and biologics to Medicare beneficiaries. For instance, the IRA will require the federal government to directly negotiate the prices of certain drugs and biologics beginning in 2026. Additionally, beginning in 2023, the IRA requires manufacturers of drugs and biologics to offer rebates if the price of the drug or biologic raises faster than inflation.
We are unable to predict what additional legislation, regulations or policies, if any, relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future or what effect such legislation, regulations or policies would have on our business.
Competition
Biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our novel iMSC technology, expertise, technological capabilities, and scientific resources give us a strong competitive edge, we face competition from many multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies, universities and other research organizations that are developing various approaches to the treatment of solid tumors and other inflammatory diseases.
Our synthetic iMSC technology competes with both existing MSC-based therapies and emerging technologies. Mesoblast an Australia-based regenerative medicine company was the first to launch an MSC-based therapy RYONCIL® in the U.S. for the treatment of steroid-refractory acute graft-versus-host disease (GVHD). Additionally, other U.S. based companies like BrainStorm Cell Therapeutics, RESTEM, Celltex, Baylx, Calidi Bio, Akan Biosciences, among others, are developing MSC therapies in solid tumor and inflammatory diseases, which we believe could be considered our primary competitors.
Many of our competitors have significantly greater financial, marketing, technical, research and human resources than we do, and may also have strategic partnerships and collaborative arrangements with leading companies and research institutions. Established pharmaceutical companies may also invest heavily to accelerate the discovery and development of technology that could make our technology obsolete. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. As a result of all of these factors, our competitors may succeed in obtaining patent protection and/or discovering, developing and commercializing technology that is competitive with or superior to our technology.
Human Capital Resources
Employees
We operate in a highly competitive industry and recognize that our success relies upon our ability to attract, develop and retain a diverse team of talented individuals. We place high value on the satisfaction and well-being of our employees and operate with fair labor standards and industry-competitive compensation and benefits. As of March 10, 2025, we had six full-time employees, which includes two research and development positions and four administrative positions. None of our employees are covered by collective bargaining agreements.
|
|
Compensation, Benefits and Development
Our approach to employee compensation and benefits is designed to deliver cash, equity and benefit programs that are competitive with those offered by leading companies in the biotechnology and pharmaceutical industries to attract, motivate and retain talent with a focus on encouraging performance, promoting accountability and adherence to our values and alignment with the interests of our stockholders.
Our base pay program aims to compensate our employees relative to the value of the contributions of their role, which takes into account the skills, knowledge and abilities required to perform each position, as well as the experience brought to the job. We may also provide our employees with opportunities to earn performance-based cash and equity compensation to reward the achievement of company-wide goals established annually and designed to drive aspects of our strategic priorities that support and advance our strategy across our company. Our employees are also eligible to receive equity awards under our long-term incentive program that are designed to align their interests with the interests of our stockholders. All employees also participate in a regular performance measurement process through which staff receive performance and development feedback, which is taken into account in determining annual compensation.
Our benefit programs are generally broad-based, promote health and overall well-being and emphasize saving for retirement. All employees are eligible to participate in the same health and retirement savings plans.
Code of Business Conduct and Ethics
We are committed to conducting business in accordance with the highest ethical standards. Our Code of Conduct and Ethics, which applies to all our employees, emphasizes the importance of integrity, honesty, forthrightness, respect and fairness.
Health, Safety and Well-Being
We actively promote the safety, health and well-being of our employees. For example, we focused on employee safety throughout the COVID-19 pandemic by implementing extensive safety measures, which included on-site COVID-19 testing protocols and flexible remote working options for most of our employees.
Corporate Information
Our principal executive offices are located at 1035 Cambridge Street, Suite 18A, Cambridge, Massachusetts 02141, and our phone number is (212) 582-1199. We maintain a website at www.eternatx.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to reports filed pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended, are available free of charge on our website at www.eternatx.com, as soon as reasonably practicable after such reports are available on the Securities and Exchange Commission (SEC) website at www.sec.gov. Additionally, copies of our Annual Report will be made available, free of charge, upon written request. Information contained on, or accessible through, our website is not a part of and is not incorporated by reference into this Annual Report on Form 10-K.
| ITEM 1A. | Risk Factors |
Our business, financial condition and operating results can be affected by many factors, whether currently known or unknown, many of which are not exclusively within our control, including but not limited to those described below, any one or more of which could, directly or indirectly, cause our financial condition and operating results to differ materially from historical or anticipated future financial condition and operating results. Any of these factors, in whole or in part, could materially and adversely affect our business, financial condition, operating results and stock price. We urge investors to carefully consider the risk factors described below in evaluating our stock and the information in this Annual Report on Form 10-K, including the consolidated financial statements and the notes thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
|
|
Risks Related to our Business and Industry
We will require substantial additional capital to fund our operations and execute our business strategy, and we may not be able to raise adequate capital on a timely basis, on favorable terms, or at all.
Based on our current financial condition and forecasts of available cash, we will not have sufficient capital to fund our operations for the 12 months following the issuance date of the accompanying consolidated financial statements. We can provide no assurance that we will be able to obtain additional capital when needed, on favorable terms, or at all. If we cannot raise capital when needed, on favorable terms or at all, we will need to reevaluate our planned operations and may need to reduce expenses, file for bankruptcy, reorganize, merge with another entity, or cease operations. If we become unable to continue as a going concern, we may have to liquidate our assets, and might realize significantly less than the values at which they are carried on our financial statements, and stockholders may lose all or part of their investment in our common stock.
Our future funding requirements, both near- and long-term, will depend on many factors, including, but not limited to:
| ● | the timing, progress, costs and results of ERNA-101 and ERNA-102; | |
| ● | the costs of any other product development programs we may initiate, including the costs to conduct the studies; | |
| ● | the outcome, timing and cost of meeting regulatory requirements established by the FDA and other comparable foreign regulatory authorities; | |
| ● | the pace and success of our potential strategic partners in co-developing our product candidates and the proceeds to us, if any, as a result; | |
| ● | the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; | |
| ● | the cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us or any of our potential co-development strategic partners or collaborators; and | |
| ● | the effect of competing market developments. |
We may seek to raise additional capital through a variety of means, including through equity, equity-linked or debt securities offerings, collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties. Our past success in raising capital through equity and convertible note offerings should not be viewed as an indication we will be successful in raising capital through those or any other means in the future.
To the extent that we raise additional capital by issuing equity or equity-linked securities, existing stockholder ownership may experience substantial dilution, and the securities may include preferred shares with liquidation or other preferences that could harm the rights of a common stockholder. Servicing the interest and principal repayment obligations under any debt we incur will divert funds that might otherwise be available to support our operations. In addition, debt financing may involve covenants that restrict our ability to operate our business. To the extent we raise additional capital through arrangements with third parties, such arrangements would likely require us to relinquish valuable rights to our technologies or grant licenses on terms that may not be favorable to us.
Unstable and unfavorable market and economic conditions may harm our ability to raise additional capital.
An economic downturn, recession or recessionary concerns, increased inflation, rising interest rates, adverse developments affecting financial institutions or the financial services industry, or the occurrence or continued occurrence of events similar to those in recent years, such as the COVID-19 pandemic or other public health emergencies, geopolitical conflict, natural/environmental disasters, terrorist attacks, strained relations between the U.S. and a number of other countries, social and political discord and unrest in the U.S. and other countries, and government shutdowns, among others, increase market volatility and have long-term adverse effects on the U.S. and global economies and financial markets. Volatility and deterioration in the financial markets and liquidity constraints or other adverse developments affecting financial institutions may make equity or debt financings more difficult, more costly or more dilutive and may increase competition for, or limit the availability of, funding from other third-party sources, such as from strategic collaborations.
We cannot be certain that additional capital will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue our business activities, or potentially discontinue operations altogether. In addition, attempting to secure additional capital may divert the time and attention of our management from day-to-day activities and harm its ability to execute on our business strategy.
|
|
We have incurred significant losses since our inception and expect to continue to incur losses for the foreseeable future, which, together with our limited financial resources and substantial capital requirements, make it difficult to assess our prospects.
We have incurred significant net losses since inception. As of December 31, 2024, we had an accumulated deficit of approximately $231.5 million. Since inception, we have primarily financed our operations by raising capital through the sale of shares of our common stock, warrants to purchase shares of our common stock and convertible notes.
We have not been profitable since we commenced operations and may never achieve profitability. If we do successfully obtain regulatory approval to market any of our product candidates, our revenue will be dependent upon, in part and among other things, the size of the markets in the territories for which we gain regulatory approval, the number of competitors in such markets, the accepted price for any such product candidate. If the indication approved by regulatory authorities is narrower than we expect, or the treatment population is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of any of our product candidates, even if approved. Even if we do achieve profitability, we may not be able to sustain or increase profitability. Failure to become and remain profitable may adversely impact the market price of the common stock and our ability to raise capital and continue operations.
We depend substantially, and expect in the future to continue to depend, on in-licensed intellectual property. Such licenses impose obligations on our business, and if we fail to comply with those obligations, we could lose license rights, which would substantially harm our business.
We rely on patents, know-how and proprietary technology licensed from Factor Limited under the Factor L&C Agreement. We may in the future become party to additional license agreements pursuant to which we in-license key intellectual property. The Factor L&C Agreement imposes various sublicense fees and other obligations on us. For example, we are obligated to pay Factor Limited $0.2 million per month for the first twelve months, $0.1 million per month for the first nine months toward patent costs, certain milestone payments, royalty payments on net sales of commercialized products and sublicensing fee payments. The parties have customary termination rights under the Factor L&C Agreement, including in connection with certain uncured material breaches of the Factor L&C Agreement and specified bankruptcy events. Any termination of our existing or future licenses could result in the loss of significant rights and would harm our business significantly.
Disputes may also arise between us and our licensors regarding intellectual property subject to a license agreement, including:
| ● | the scope of rights granted under the license agreement and other interpretation-related issues; | |
| ● | whether and the extent to which our technology and processes infringe intellectual property of the licensor that is not subject to the licensing agreement; | |
| ● | our right to sublicense patents and other intellectual property to third parties under the license agreement; | |
| ● | our diligence obligations under the agreement and what activities satisfy those diligence obligations; | |
| ● | the priority of invention of patented technology; and | |
| ● | the ownership of inventions and know-how resulting from any joint creation or use of intellectual property by our licensors and us or our partners. |
If disputes over intellectual property that we have licensed, or license in the future, prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully enter into co-development strategic partnerships. In addition, the resolution of any such disputes could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Additionally, we may have limited control over the maintenance, prosecution or enforcement of rights we in-license, and we may also have limited control over activities previously or separately conducted by our licensors. For example, we cannot be certain that activities conducted by Factor Limited or any other present or future licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. We may also have limited control over other intellectual property that is not licensed to us but that may be related to our in-licensed intellectual property. We may have limited control over the manner in which our licensors initiate an infringement proceeding against a third-party infringer or the intellectual property or defend certain of the intellectual property that is licensed to us. It is possible that the licensors’ infringement proceedings or defense activities may be less vigorous than had we conducted them ourselves.
|
|
If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, we may have to abandon development of the relevant program or drug candidate and our business, financial condition, results of operations and prospects could suffer.
We are generally also subject to all of the same risks with respect to protection of intellectual property that we own, as we are for intellectual property that we license. If we or our licensors fail to adequately protect the intellectual property underlying our synthetic iMSC technology platform and any other in-licensed intellectual property, our ability to enter into co-development strategic partnerships could materially suffer.
Our intellectual property rights may not adequately protect our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business. For example:
| ● | we, or our license partners or current or future collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or may own in the future; | |
| ● | we, or our license partners or current or future collaborators, might not have been the first to file patent applications covering certain of our or their inventions; | |
| ● | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing, misappropriating or otherwise violating any of our owned or licensed intellectual property rights; | |
| ● | it is possible that our pending licensed patent applications or those that we may own in the future will not lead to issued patents; | |
| ● | issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors or other third parties; | |
| ● | our competitors or other third parties might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale; | |
| ● | we may not develop additional proprietary technologies that are patentable; | |
| ● | the patents of others may harm our business; | |
| ● | we may choose not to file a patent in order to maintain certain trade secrets or proprietary know- how, and a third party may subsequently file a patent covering such intellectual property; and | |
| ● | our trade secrets or proprietary know-how may be unlawfully disclosed, thereby losing their trade secret or proprietary status. |
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations and prospects.
We rely heavily on in-licensed intellectual property from Factor Limited. Loss of this license or termination of the Factor L&C Agreement could significantly harm our product development and ability to enter co-development strategic partnerships, materially impacting our business.
Our business is substantially dependent upon the synthetic iMSC technology licensed from Factor Limited. Pursuant to the Factor L&C Agreement, Factor Limited has customary termination rights, including in connection with certain uncured material breaches of the Factor L&C Agreement, failure to make payments and specified bankruptcy events. Our ability to develop therapeutics products or enter into co-development partnerships using the Factor Patents depends entirely on the effectiveness and continuation of the Factor L&C Agreement. If the Factor L&C Agreement is terminated, there is no guarantee that we will be able to enter into a new license agreement that aligns with our business strategy on the same or similar terms, if at all, and our competitors could in-license the technology, which would result in a significant market disadvantage to us.
|
|
We or our licensors may be subject to claims challenging the inventorship or ownership of the patents and other intellectual property that we own or license now or in the future.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an ownership interest in the patents and intellectual property that we in-license or that we may own or in-license in the future. While it is our policy to require our employees or contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own or such assignment may not be self-executing, for example, as part of employment or consulting agreements, or may be breached. Our licensors may face similar obstacles. Litigation may be necessary to defend against any claims challenging inventorship or ownership, including in derivation proceedings in the USPTO. If we or our licensors fail in defending any such claims, we may have to pay monetary damages and may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property, which could adversely impact our business, results of operations and financial condition.
We have previously identified a material weakness in our internal control over financial reporting. If we are unable to develop and maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results in a timely manner, which may adversely affect investor confidence in us, and materially and adversely affect our business and operating results.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected and corrected on a timely basis. Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud.
In prior periods, we identified a material weakness as discussed below. We were unable to timely file our Quarterly Report on Form 10-Q for the three months ended March 31, 2022 due to identifying errors in our financial statements reported in the Annual Report on Form 10-K for the years ended December 31, 2021 and 2020 during our preparation of the financial statements for the quarter ended March 31, 2022. On June 30, 2022, we filed an amendment to our Annual Report on Form 10-K for the years ended December 31, 2021 and 2020 to correct the errors in our financial statements for the years ended December 31, 2021 and 2020 and for the quarters ended June 30, 2020, September 30, 2020, March 31, 2021, June 30, 2021 and September 30, 2021. Management concluded that the errors were the result of accounting personnel’s lack of technical proficiency in the accounting for complex matters.
Management has implemented measures designed to ensure that the deficiencies contributing to the ineffectiveness of our internal control over financial reporting are remediated, such that the internal controls are designed, implemented and operating effectively. The remediation actions implemented to date include: enhancing the business process controls related to reviews over technical, complex, and non-recurring transactions; providing additional training to accounting personnel and using external accounting advisors to review management’s conclusions on certain technical, complex and non-recurring matters.
As a result of the above remediation measures, and as disclosed in Part II, Item 9A to this Annual Report on Form 10-K, our Chief Executive Officer and Senior Vice President of Finance concluded that the prior material weakness was remediated as of December 31, 2024, and our disclosure controls and procedures were effective and provided reasonable assurance of achieving the desired control objectives.
If we identify any additional material weaknesses in the future, any such newly identified material weakness could limit our ability to prevent or detect a misstatement of our accounts or disclosures and could result in a material misstatement of our annual or interim financial statements. In such case, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports, investors may lose confidence in our financial reporting and our stock price may decline as a result. We cannot assure you that the measures we have taken to date, or any measures we may take in the future, will be sufficient to avoid potential future material weaknesses.
Our business and operations would suffer in the event of system failures, cyber-attacks or a deficiency in our cyber-security.
Our computer systems, as well as those of various third parties on which we rely, may sustain damage from computer viruses, unauthorized access, data breaches, phishing attacks, cybercriminals, natural disasters (including hurricanes and earthquakes), terrorism, war and telecommunication and electrical failures. We rely on our third-party providers to implement effective security measures and identify and correct for any such failures, deficiencies or breaches. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development and other programs. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability and it could have a material adverse effect on our business, results of operations and financial condition. See Part I, Item 1C. Cybersecurity for more information on information regarding our cybersecurity risk management, strategy, and governance.
|
|
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive life science industry depends in large part upon the ability to attract highly qualified personnel. In order to induce valuable employees to remain with us, we intend to provide employees with stock options and/or restricted stock units that vest over time. The value to employees of stock options that vest over time will be significantly affected by movements in the price of the common stock that it will not be able to control and may at any time be insufficient to counteract more lucrative offers from other companies.
Competition for skilled personnel in our industry is intense and competition for experienced scientists may limit our ability to hire and retain highly qualified personnel on acceptable terms. Despite our efforts to retain valuable employees, our employees may terminate their employment with us on short notice.
Other companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than we do, and such companies also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we have to offer. If we are unable to continue to attract and retain high-quality personnel, our business, results of operations and financial condition may be materially adversely affected.
Risks Related to New, Cutting Edge Technologies
Our product development relies on novel, inherently risky technologies. Synthetic mesenchymal stem cell therapy is a relatively new field, and our efforts may not result in effective treatments for human diseases.
Cellular immunotherapies, synthetic mesenchymal stem cell therapies, and iPSC-derived product candidates represent relatively new therapeutic areas, and the FDA has cautioned consumers about potential safety risks associated with them. To date, there are relatively few approved cell therapies. As a result, the regulatory approval process for cellular therapy product candidates is uncertain and may be more expensive and take longer than the approval process for product candidates based on other, better known or more extensively studied technologies and therapeutic approaches.
Cell reprogramming technology and related cell therapy products using iPSC lines represent novel therapeutic approaches, and to our knowledge no iPSC-derived cell products are currently approved for commercial sale anywhere in the world. As such, it is difficult to accurately predict the type and scope of challenges that we may confront in developing and advancing a pipeline of iPSC-derived therapeutic products. We thus face uncertainties associated with the preclinical and clinical development, manufacture, and regulatory compliance for the initiation and conduct of clinical trials, regulatory approval, and reimbursement required for successful commercialization of future product candidates. Further, the processes and requirements imposed by the FDA or other applicable regulatory authorities may cause delays and additional costs in obtaining approvals for marketing authorization for any future product candidates. Because our platform is novel, and cell- based therapies are relatively new, regulatory agencies may lack experience in evaluating product candidates using our synthetic iMSC technology platform. This novelty may lengthen the regulatory review process, including the time it takes for the FDA to review IND applications if and when such applications are submitted, increase development costs, and delay or prevent commercialization of future products, if such products are approved for marketing.
Due to the rapid advancements in cellular technologies, regulatory processes and requirements in the United States and in other jurisdictions governing cellular therapy products are evolving and the FDA or other regulatory bodies may change the requirements, or identify different regulatory pathways, for the clinical testing and approval of these product candidates. For example, in recent years the FDA has issued several new guidance documents related to developing and manufacturing cellular therapy products. In addition, adverse developments in clinical trials of cellular therapy products conducted by others, or in treated patients after such products are commercialized, may cause the FDA or other oversight bodies to change the requirements for approval of any of our product candidates. For example, in November 2023, the FDA announced that it was investigating reports of T-cell malignancy in patients following their treatment with B cell maturation antigen-directed or CD19-directed autologous chimeric antigen receptor (CAR) T-cell immunotherapies, although more recent public statements by agency leadership indicate that the benefits of such treatments are expected to still outweigh those risks. Future adverse events or safety issues could lead to more significant regulatory action applicable to either a specific product or a broader product class, based on case-by-case science-based benefit-risk assessments. Similarly, the EMA oversees the development of cellular therapies in the EU and may issue new guidelines concerning the development and marketing authorization for cellular therapy products and require that we comply with these new guidelines. These regulatory agencies and committees and any new regulations, requirements or guidelines they promulgate may lengthen the regulatory review process, which may reduce the anticipated benefits of our co-development strategic partnerships or adversely affect the commercialization of any future therapeutic products we may develop.
|
|
Accordingly, we may be required to change regulatory strategies or to modify applications for clinical investigations or regulatory approval, which could delay and impair our ability to complete the preclinical and clinical development and manufacture of, and obtain regulatory approval for, our product candidates. Changes in regulatory authorities and advisory groups, or any new regulations, requirements or guidelines we promulgate, may lengthen the regulatory review process, require additional studies, increase development and manufacturing costs, lead to changes in regulatory pathways, positions and interpretations, delay or prevent approval and commercialization of product candidates we develop or lead to significant post-approval limitations or restrictions that may reduce the our anticipated benefits.
The clinical trial requirements of the FDA, the EMA and other regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of the product candidate. Due to the novelty and complexity of cellular products, the regulatory approval process for such product candidates is uncertain and may be more expensive and take longer than the approval process for product candidates based on other, better known or more extensively studied technologies. It is difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for product candidates using this technology in either the United States or the E.U. or how long it will take to commercialize any product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product candidate to market could decrease our ability to generate sufficient product revenue, and our business, financial condition, results of operations and prospects may be harmed.
We are in an industry with intense competition and rapid technological change and our competitors may develop therapies that are more advanced, safer, or more effective than any therapy we develop in the future, which may adversely affect our financial condition.
We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies, universities, and other research institutions. Many of our competitors have substantially greater financial, technical, research and human resources than we do, and may also have strategic partnerships and collaborative arrangements with leading companies and research institutions . Our competitors may succeed in developing, acquiring, or licensing on an exclusive basis, products that are more effective, safer, or less costly than any products that we may develop in the future, or achieve patent protection, marketing approval, product commercialization, and market penetration earlier than us. Additionally, technologies developed by our competitors may render any product candidates we are seeking to develop uneconomical or obsolete. For additional information regarding our competition, see “Part I, Item 1. Business—Competition”.
Negative public opinion and increased regulatory scrutiny due to ethical and other concerns surrounding the use of stem cell therapy or human tissue may damage public perception of our synthetic allogeneic iMSC product candidates or adversely affect our ability to conduct our business.
Concerns about the safety or ethics of cell therapy, even if unrelated to our product candidates, could lead to stricter regulations, public resistance, patient recruitment challenges, regulatory delays, labeling restrictions, and reduced demand for our therapies. Such developments could significantly affect our business, financial condition, and the commercialization of future cell therapy products.
The manufacture of biotechnology products is complex, and manufacturers often encounter difficulties in production.
The manufacture of biotechnology products, including cellular and gene therapy products, is complex and requires significant expertise and capital investment. Manufacturers for any product candidates developed using our synthetic iMSC technology platform will be required to comply with cGMP regulations and guidelines for clinical trial product manufacture and subsequently for commercial product manufacture. Manufacturers of biotechnology products often encounter difficulties in production, particularly in scaling up, addressing product quality, product comparability, validating production processes and mitigating potential sources of contamination. These problems include difficulties with raw material procurement, production costs and yields, quality control, product quality, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Any delay or interruption in the supply of preclinical study supplies (or clinical trial supplies in the future) could delay the completion of such studies, increase the costs associated with the affected development programs and, depending upon the period of delay, require new studies to be commenced at additional expense or terminated completely.
|
|
Risks Related to Ownership of our Common Stock
Seven stockholders collectively own a significant percentage of our outstanding common stock, and as a result of such ownership, such stockholders may influence the election of directors and other matters submitted to stockholders.
According to their most recent SEC filings and/or our corporate records, seven stockholders—Charles Cherington, Nicholas Singer, John D. Halpern, the George Denny Estate, Freebird Partners LP, IAF, LLC and Regolith Capital Investments LP—collectively own approximately 76% of our outstanding shares of common stock. Although, to our knowledge, such stockholders are not a “group” or “acting in concert,” they have and we expect them to continue to have, individually and/or collectively, the ability to influence the election of our board of directors and the outcome of other matters submitted to our stockholders. The interests of these stockholders may not always coincide with our interests or the interests of other stockholders, and such stockholders, individually or collectively, may act in a manner that advances their best interests and not necessarily those of other stockholders. One consequence to this substantial influence is that it may be difficult for investors to remove our management and it could also deter unsolicited takeovers, including transactions in which stockholders might otherwise receive a premium for their shares over then current market prices.
The sale of our common stock to Lincoln Park Capital Fund LLC (“Lincoln Park”) may cause dilution to our other stockholders and the subsequent sale of the shares of common stock acquired by Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
Lincoln Park committed to purchase up to $10.0 million of our common stock under a standby equity purchase agreement (“SEPA”). Through December 31, 2024, we have issued and sold approximately 214,000 shares of our common stock to Lincoln Park for approximately $0.3 million in gross proceeds under the SEPA, leaving an approximately $9.7 million balance of the $10.0 million total commitment. The purchase price for the shares that we may sell to Lincoln Park under the SEPA is subject to a pricing formula in the SEPA and will vary based on the price of our common stock at the time we initiate the sale. Depending on market liquidity at the time, sales of such shares may cause the trading price of our common stock to fall.
We generally have the right to control the timing and amount of any future sales of our shares to Lincoln Park under the SEPA. Sales of shares of our common stock to Lincoln Park under the SEPA, if any, will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park all, some or none of the shares of our common stock that may be available for us to sell pursuant to the SEPA. If and when we do sell shares to Lincoln Park, after Lincoln Park has acquired the shares, Lincoln Park may resell all, some or none of those shares at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us could result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales.
There may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
We are generally not restricted from issuing additional common stock, including any securities that are convertible into or exchangeable for, or that represent the right to receive, common stock. To raise additional capital, we may in the future sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that are lower than the prices paid by existing stockholders, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders, which could result in substantial dilution to the interests of existing stockholders. The market price of our common stock could decline as a result of sales of common stock or securities that are convertible into or exchangeable for, or that represent the right to receive, common stock or the perception that such sales could occur.
|
|
In addition, under the terms of the asset purchase agreement pursuant to which we acquired assets from Exacis, we agreed to issue to Exacis shares of our common stock as contingent consideration. If our market capitalization equals or exceeds $100 million during the three-year period commencing on April 26, 2023 and ending on the three-year anniversary thereof, the number of shares of common stock we would issue is determined by a formula specified in the asset purchase agreement. In addition, if our market capitalization equals or exceeds $200 million during the same three-year period, we agreed to issue to Exacis additional shares of our common stock determined by a formula specified in the asset purchase agreement. See Note 4 to the accompanying consolidated financial statements for additional information.
Our failure to meet the continued listing requirements of Nasdaq could result in a delisting of our common stock.
Our common stock is listed on The Nasdaq Capital Market. The Nasdaq Capital Market requires that listed companies satisfy certain continued listing requirements. Listing Rule 550(a)(2) requires that listed companies maintain a minimum bid price of their common stock of at least $1 per share (the “Bid Price Rule”). Listing Rule 5550(b) requires that listed companies have: (1) stockholders’ equity of at least $2.5 million (the “Stockholders’ Equity Rule”; (2) a market value of listed securities (the “MVLS Rule”) of at least $35 million; or (3) net income from continuing operations of $500,000 in the company’s most recently completed fiscal year or in two of the three most recently completed fiscal years.
On December 30, 2024, we received notice from Nasdaq that we no longer met the Bid Price Rule and were provided until June 30, 2025 to regain compliance with the Bid Price Rule. On January 6, 2025, we received notice from Nasdaq informing us that we no longer met the MVLS Rule and were provided until July 7, 2025 to regain compliance with the MLVS Rule. If at any time during the Bid Price Rule compliance period, our closing bid price is at least $1 per share for a minimum of 10 consecutive business days during the 180-day compliance period, Nasdaq will provide written confirmation that we regained compliance with that applicable rule. In the event we do not regain compliance with the Bid Price Rule by June 30, 2025, we may be eligible for consideration of a second 180-day compliance period if we meet the MLVS Rule and all other initial listing standards for Nasdaq’s Capital Market, with the exception of the Bid Price Rule. In addition, we would also be required to notify Nasdaq of our intent to cure the Bid Price Rule deficiency by effecting a reverse stock split, if necessary. If it appears to Nasdaq that we will not be able to cure the deficiency, or if we are otherwise not eligible, Nasdaq will provide use will its notice that our securities will be subject to delisting.
Likewise, if at any time during the MLVS Rule compliance period our MVLS closes at $35 million or more for a minimum of 10 consecutive business days, Nasdaq will provide written confirmation that we have regained compliance that applicable rule. In the event we do not regain compliance with the Market Value Standard by July 7, 2025, Nasdaq will provide us notice that our securities will be subject to delisting, at which time, we may appeal the delisting determination.
Additionally, our stockholders’ equity at December 31, 2024 was approximately $1.9 million and we do not currently meet the net income from continuing operations compliance standards described in Listing Rule 5500(b)(3). Accordingly, we also expect to receive a notice from Nasdaq informing us that we do not meet Listing Rule 5550(b)(1). If we receive such a notice, we expect to be afforded 45 days to submit a plan to regain compliance with the stockholders’ equity requirement for Nasdaq’s consideration, and if the plan is accepted, to be granted an extension period of up to 180 calendar days from the date of the deficiency notice to regain compliance. If the plan is not accepted or if we are unable to regain compliance within any extension period granted by Nasdaq, Nasdaq would be required to issue a delisting determination, which we expect we would be entitled to request a hearing before a Nasdaq Hearings Panel to present a plan to regain compliance and to request a further extension period to regain compliance.
If we fail to satisfy any of the Nasdaq continued listing requirements, Nasdaq may take steps to delist our common stock. In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with Nasdaq continued listing requirements would be successful.
If our common stock is ultimately delisted by Nasdaq, and we are not able to list our securities on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, then we could face significant material adverse consequences, including: a material reduction in the liquidity of our common stock and a corresponding material reduction in the trading price of our common stock; a more limited market quotations for our securities; a determination that our common stock is a “penny stock” that requires brokers to adhere to more stringent rules and possibly resulting in a reduced level of trading activity in the secondary trading market for our securities; more limited research coverage by stock analysts; loss of reputation; more difficult and more expensive equity financings in the future; the potential loss of confidence by investors; and fewer business development opportunities.
|
|
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” If our common stock remains listed on Nasdaq, our common stock will be covered securities. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. If our securities were no longer listed on Nasdaq and therefore not “covered securities,” we would be subject to regulation in each state in which we offer our securities.
Anti-takeover provisions of Delaware law and provisions in our charter and bylaws could make a third-party acquisition of us difficult.
Because we are a Delaware corporation, the anti-takeover provisions of Delaware law could make it more difficult for a third party to acquire control of us, even if the change in control would be beneficial to stockholders. We are subject to the provisions of Section 203 of the General Corporation Law of Delaware, which prohibits us from engaging in certain business combinations, unless the business combination is approved in a prescribed manner. In addition, our restated certificate of incorporation and restated bylaws also contain certain provisions that may make a third-party acquisition of us difficult, including the ability of our board of directors to issue preferred stock and the inability of our stockholders to call a special meeting or act by written consent.
Risks Related to our Financial Position and Capital Requirements
We may acquire businesses, assets or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We may acquire additional businesses, assets or products, form strategic alliances or create joint ventures with third parties that we believe will complement or augment our existing business. If we acquire businesses with promising intellectual property, markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new acquisition. Difficulties may prevent us from realizing its expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction.
Our ability to utilize our net operating loss carryforwards and tax credit carryforwards may be subject to limitations.
Our ability to use our federal and state net operating losses (“NOLs”) to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our NOLs.
Under Section 382 and Section 383 of the Code and corresponding provisions of state law, if a corporation undergoes an “ownership change,” its ability to use its pre-change NOL carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited. A Section 382 “ownership change” is generally defined as a greater than 50 percentage point change (by value) in its equity ownership by certain stockholders over a three-year period. Even if we achieve profitability, we may not be able to utilize a material portion of our NOL carryforwards and other tax attributes, which could have a material adverse effect on cash flow and results of operations. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. There is also a risk that due to regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, our existing NOLs could expire or otherwise be unavailable to offset future income tax liabilities.
|
|
Risks Related to Regulatory Requirements
We are subject to extensive and costly government regulation.
Product candidates employing medical technology are subject to extensive and rigorous domestic government regulation including regulation by the FDA, other divisions of the United States Department of Health and Human Services, the United States Department of Justice, state and local governments, and their respective foreign equivalents. If products employing our technologies are marketed abroad, they will also be subject to extensive regulation by foreign governments, whether or not they have obtained FDA approval for one or more uses. Such foreign regulation may be equally or more demanding than corresponding United States regulation.
Government regulation substantially increases the cost and risk of researching, developing, manufacturing, and selling medical products. Even if we or our strategic partners are able to obtain regulatory approval for a particular product candidate, the approval may limit the indicated medical uses for the product, may otherwise limit the ability to promote, sell, and distribute the product, may require costly post-marketing surveillance, and/or may require ongoing post-marketing studies. Material changes to an approved product, such as, for example, manufacturing changes or revised labeling, may require further regulatory review and approval. Once obtained, any approvals may be withdrawn, including, for example, if there is a later discovery of previously unknown problems with the product, such as a previously unknown safety issue.
In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of a product candidate. For example, regulatory agencies may approve a product candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. Regulators may approve a product candidate for a smaller patient population, a different drug formulation or a different manufacturing process, than we or our strategic partners are seeking.
The regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable. If we are ultimately unable to obtain regulatory approval for our product candidates, we may be unable to produce revenue and our business will be substantially harmed.
A product cannot be commercialized until the appropriate regulatory authorities have reviewed and approved the product candidate. The time required to obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable, typically takes many years following the commencement of clinical studies and depends upon numerous factors, including the type, complexity, and novelty of the product candidates involved. Regulatory authorities have substantial discretion in the approval process and may refuse to accept an application for review, or may decide that our data are insufficient for approval and require additional non-clinical, clinical or other studies.
We may never be able to obtain regulatory approval for any product candidates that we develop in the future. If our future product candidates are ultimately not approved for any reason, our business, prospects, results of operations and financial condition would be adversely affected.
In addition, even once clinical development of a future product candidate is initiated, such clinical studies may not start or be completed on schedule, if at all. The completion or commencement of clinical studies can be delayed or prevented for a number of reasons, including, among others:
| ● | the FDA or comparable foreign regulatory authorities may not authorize us or our future clinical investigators to commence planned clinical studies, or require that we suspend ongoing clinical studies through imposition of clinical holds; | |
| ● | negative results from our ongoing studies or other industry studies involving engineered or gene-edited cell therapy product candidates; | |
| ● | delays in reaching or failing to reach agreement on acceptable terms with prospective clinical research organizations (“CROs”) and clinical study sites, the terms of which can be subject to considerable negotiation and may vary significantly among different CROs and study sites; | |
| ● | inadequate quantity or quality of a product candidate or other materials necessary to conduct clinical studies, for example delays in the manufacturing of sufficient supply of finished drug product; | |
| ● | difficulties obtaining ethics committee or IRB, approval to conduct a clinical study at a prospective site or sites; | |
| ● | challenges in recruiting and enrolling subjects to participate in clinical studies, the proximity of subjects to study sites, eligibility criteria for the clinical study, the nature of the clinical study protocol, the availability of approved effective treatments for the relevant disease and competition from other clinical study programs for similar indications; |
|
|
| ● | severe or unexpected drug-related side effects experienced by subjects in a clinical study, such as severe neurotoxicity and cytokine release syndrome; | |
| ● | the FDA or comparable foreign regulatory authorities may disagree with a proposed clinical study design, implementation of clinical trials or our interpretation of data from clinical studies, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical studies; | |
| ● | reports from non-clinical or clinical testing of other competing candidates that raise safety or efficacy concerns; and | |
| ● | difficulties retaining subjects who have enrolled in a clinical study but may be prone to withdraw due to rigors of the clinical studies, lack of efficacy, side effects, personal issues, or loss of interest. |
Changes in regulatory requirements, agency guidance or unanticipated events during our non-clinical studies and future clinical studies of our future product candidates may occur, which may result in changes to non-clinical or clinical study protocols or additional non-clinical or clinical study requirements, which could result in increased costs to us and could delay our projected development timeline.
Changes in regulatory requirements or FDA or EMA guidance, or unanticipated events during our non-clinical studies and future clinical studies, may force us to amend non-clinical studies and future clinical study protocols. The FDA, EMA or comparable foreign regulatory authorities may also impose additional non-clinical studies and clinical study requirements. Amendments to protocols for or other aspects of our non-clinical studies may increase the cost or delay the timing or successful completion of those studies. If we experience delays completing, or if we terminate, any of our non-clinical or future clinical studies, or if we are required to conduct additional non-clinical or clinical studies, the commercial prospects for our future product candidates may be harmed and our ability to recognize product revenue will be delayed.
Disruptions at the FDA and other government agencies caused by funding shortages or other events or conditions outside of their control could negatively impact our business.
The ability of the FDA to review and approve INDs, proposed clinical trial protocols, or new product candidates can be affected by a variety of factors, including, but not limited to, government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, statutory, regulatory, and policy changes, and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other regulatory agencies may also slow the time necessary for new product candidates to be reviewed or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical employees and stop critical activities. In addition, during the COVID-19 pandemic, the FDA’s inspectional activities were interrupted and restarted on a risk-based basis, which had the effect of delaying review and potential approval of product candidate marketing applications.
If a prolonged government shutdown occurs, or if global health concerns prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA to timely review and process our future regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
|
|
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
We maintain quantities of various flammable and toxic chemicals in our facilities in Massachusetts that are used for our research and development activities. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. We believe our procedures for storing, handling and disposing these hazardous materials in our laboratory facilities comply with the relevant guidelines of the relevant local, state, and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of animals and biohazardous materials. Any insurance coverage we have may not be sufficient to cover these liabilities. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate, any of these laws or regulations which would adversely affect our business.
Healthcare legislative reform measures may have a material and adverse effect on our business, financial condition, results of operations, and prospects.
Third-party payors, whether domestic or foreign, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In both the United States and certain foreign jurisdictions, there have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal, and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations, and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
| ● | the demand for our therapeutic candidates, if we obtain marketing approval; | |
| ● | our ability to receive or set a price that we believe is fair for our future products; | |
| ● | our ability to generate revenue and achieve or maintain profitability; | |
| ● | the level of taxes that we are required to pay; and | |
| ● | the availability of capital. |
The Affordable Care Act of 2010 (“ACA”) includes measures that have significantly changed the way healthcare is financed by both governmental and private insurers in the United States. It also included the provisions that created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. The ACA continues to significantly impact the United States’s pharmaceutical industry.
Moreover, there has been heightened governmental scrutiny over the manner in which prescription drug and biological product manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. In August 2022, President Biden signed into the law the Inflation Reduction Act of 2022 (“IRA”), which includes (among other things) multiple provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States. A manufacturer of drug products covered by Medicare Parts B or D must pay a rebate to the federal government if their drug product’s price increases faster than the rate of inflation. The IRA is in the process of being implemented by CMS and its impact on the pharmaceutical industry in the United States remains uncertain at this time, in part because multiple large pharmaceutical companies and other stakeholders (e.g., the U.S. Chamber of Commerce) have initiated federal lawsuits against CMS arguing a separate price negotiation program is unconstitutional for a variety of reasons, among other complaints. Those lawsuits are currently ongoing.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, in recent years, several states have formed prescription drug affordability boards (“PDABs”). These PDABs have attempted to implement upper payment limits on drugs sold in their respective states in both public and commercial health plans. For example, in August 2023, Colorado’s PDAB announced a list of five prescription drugs that would undergo an affordability review. The effects of these efforts similarly remain uncertain pending the outcomes of several federal lawsuits challenging state authority to regulate prescription drug payment limits.
|
|
We expect that the ACA, the IRA, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, lower reimbursement, and new payment methodologies. This could lower the price that we receive for any future approved therapeutic product. Any denial in coverage or reduction in reimbursement from Medicare or other government-funded programs may result in a similar denial or reduction in payments from private payors, which may prevent us from being able to generate sufficient revenue, attain profitability, or commercialize our future therapeutic candidates, if approved.
In the European Union, similar political, economic and regulatory developments may affect our ability to profitably commercialize our current or any future products. In addition to continuing pressure on prices and cost containment measures, legislative developments at the European Union or member state level may result in significant additional requirements or obstacles that may increase our operating costs. In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. Our future products, if any, might not be considered medically reasonable and necessary for a specific indication or cost-effective by third-party payors, an adequate level of reimbursement might not be available for such products and third-party payors’ reimbursement policies might adversely affect our ability to sell any future products profitably.
Legislative and regulatory proposals have also been made to expand post-approval requirements and restrict sales and promotional activities for biologic therapeutics, and FDA’s statutory authorities are periodically amended by Congress. For example, as part of the Consolidated Appropriations Act for 2023, Congress provided FDA additional authorities related to the accelerated approval pathway for human drugs and biologics. Under these recent amendments to the FDCA, the agency may require a sponsor of a product granted accelerated approval to have a confirmatory trial underway prior to approval. The amendments also give FDA the option of using expedited procedures to withdraw product approval if the sponsor’s confirmatory trial fails to verify the claimed clinical benefits of the product. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our therapeutic candidates, if any, may be. Increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-approval testing and other requirements.
In addition, in April 2023 the European Commission issued a proposal that will revise and replace the existing general pharmaceutical legislation governing drug and biological products intended for the EU market. If adopted and implemented as currently proposed, these revisions will significantly change several aspects of drug development and approval in the EU.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, our therapeutic candidates may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
Risks Relating to Our Intellectual Property
If the licensors of our in-licensed technology are unable to obtain and maintain patent and other intellectual property protection, or if the scope of the patent and other intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to those derived from such intellectual property, and our ability to achieve profitability may be adversely affected.
Our ability to compete effectively will depend, in part, on maintaining the proprietary nature of our in-licensed technology and manufacturing processes. We rely on research, manufacturing and other know-how, patents, trade secrets, license agreements and contractual provisions to establish our intellectual property rights. These legal means, however, afford only limited protection and may not adequately protect our rights.
|
|
We cannot predict whether the patent applications related to our in-licensed technology will issue as patents, or whether the claims of any resulting patents will provide us with a competitive advantage or whether the licensor will be able to successfully pursue patent applications in the future relating to such products and product candidates. Moreover, the patent application and approval processes are expensive and time-consuming. The licensor may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. Furthermore, we, or any future partners, collaborators, or licensees, may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, we may miss potential opportunities for the licensor to seek additional patent protection. Defects of form in the preparation or filing of patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments. If the licensor fails to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If there are material defects in the form, preparation, prosecution or enforcement of our patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents.
Even if they are unchallenged, our in-licensed patents and patent applications, if issued, may not provide us with any meaningful protection or prevent competitors from designing around our patent claims by developing similar or alternative technologies or therapeutics in a non-infringing manner. For example, a third party may develop a competitive therapy that provides benefits similar to one or more of the future products and product candidates that we or our strategic partners or collaborators may develop but that falls outside the scope of our patent protection. If the patent protection provided by the patents and patent applications is not sufficiently broad to impede such competition, the successful commercialization of such product candidates could be negatively affected.
Other parties, many of whom have substantially greater resources and have made significant investments in competing technologies, have developed or may develop technologies that may be related or competitive with our approach, and may have filed or may file patent applications and may have been issued or may be issued patents with claims that overlap or conflict with our patent applications, either by claiming the same compositions, formulations or methods or by claiming subject matter that could dominate our patent position. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. As a result, any patents we may in-license in the future may not provide us with adequate and continuing patent protection sufficient to exclude others from commercializing products similar to future products and product candidates that we or our strategic partners or collaborators may develop.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain. No consistent policy regarding the breadth of claims allowed in biotechnology and pharmaceutical patents has emerged to date in the United States or in many foreign jurisdictions. The standards applied by the USPTO and foreign patent offices in granting patents are not always applied uniformly or predictably. In addition, the determination of patent rights with respect to pharmaceutical compounds commonly involves complex legal and factual questions, which has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our competitors may also seek approval to market their own products similar to or otherwise competitive with our products. Alternatively, our competitors may seek to market generic versions of any approved products by submitting ANDAs or ABLAs to the FDA in which they claim that the patents related to our in-licensed technology are invalid, unenforceable or not infringed. In these circumstances, we may need to defend or assert these patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find the in-licensed patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, we cannot offer any assurances about which, if any, patents will issue, the breadth of any such patents, whether any issued patents will be found invalid and unenforceable or will be threatened by third parties or whether any issued patents will effectively prevent others from commercializing competing technologies and drug candidates.
In addition to patent protection, we expect to rely heavily on trade secrets, know-how and other unpatented technology, which are difficult to protect. Although we seek such protection in part by entering into confidentiality agreements with our vendors, employees, consultants and others who may have access to proprietary information, we cannot be certain that these agreements will not be breached, adequate remedies for any breach would be available, or our trade secrets, know-how and other unpatented proprietary technology will not otherwise become known to or be independently developed by our competitors. If we are unsuccessful in protecting our intellectual property rights, sales of our products may suffer and our ability to generate revenue could be severely impacted.
|
|
If the licensor of our in-licensed technology does not obtain patent term extension for future products that we or our strategic partners or collaborators may successfully develop, our business may be materially harmed.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering future products and product candidates that we or our strategic partners or collaborators may develop are obtained, once the patent life has expired for a particular product, we or our strategic partners or collaborators may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are approved and commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
In the future, if we obtain an issued patent covering one of the product candidates that we or our strategic partners or collaborators may develop, depending upon the timing, duration and specifics of any FDA marketing approval of such product candidates, such patent may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, or Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process for drugs and biologics. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended. A patent may only be extended once and only based on a single approved product. However, the patent owner may not be granted an extension because of, for example, failure to obtain a granted patent before approval of a product candidate, failure to exercise due diligence during the testing phase or regulatory review process, failure to apply within applicable deadlines, failure to apply prior to expiration of relevant patents or otherwise our failure to satisfy applicable requirements. A patent licensed to us by a third party may not be available for patent term extension. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially.
Changes in patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect future products and product candidates that we or our strategic partners or collaborators may develop.
Changes in either the patent laws or the interpretation of the patent laws in the United States or other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. When implemented, the Leahy-Smith Act included several significant changes to U.S. patent law that impacted how patent rights could be prosecuted, enforced and defended. In particular, the Leahy-Smith Act also included provisions that switched the United States from a “first-to-invent” system to a “first-to-file” system, allowed third- party submission of prior art to the USPTO during patent prosecution and set forth additional procedures to attack the validity of a patent by the USPTO administered post grant proceedings. Under a first-to-file system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The USPTO developed new regulations and procedures governing the administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Some of the Company’s patents and patent applications have effective dates later than March 16, 2013 and thus will be subject to the provisions of the Leahy-Smith Act.
|
|
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent rulings from the U.S. Court of Appeals for the Federal Circuit and the U.S. Supreme Court have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining, defending and enforcing patents on products and product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those in the United States. The requirements for patentability may differ in certain countries, particularly in developing countries; thus, even in countries where we do pursue patent protection, there can be no assurance that any patents will issue with claims that cover our products. There can be no assurance that we will obtain or maintain patent rights in or outside the United States under any future license agreements. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from utilizing our inventions in all countries outside the United States, even in jurisdictions where we pursue patent protection, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not pursued and obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with future products and product candidates that we or our strategic partners or collaborators may develop and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing with us.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, laws of some countries outside of the United States and Europe do not afford intellectual property protection to the same extent as the laws of the United States and Europe. Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries including India and China, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology and pharmaceutical products, which could make it difficult for us to stop the infringement of our in-licensed patents or marketing of competing products in violation of our proprietary rights generally. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in certain countries outside the United States and Europe. In addition, many countries limit the enforceability of patents against government authorities or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
Proceedings to enforce our patent rights, even if obtained, in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. While we intend to protect our intellectual property rights in major markets for our products, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our products. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop.
|
|
We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of a third-party patent, which might adversely affect our ability to develop and market our products.
We cannot guarantee that any of our patent searches or analyses, including the identification of relevant patents, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending application in the United States and abroad that is relevant to or necessary for the commercialization of our drug candidates in any jurisdiction.
The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect. For example, we may incorrectly determine that our products are not covered by a third-party patent or may incorrectly predict whether a third-party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States or abroad that we consider relevant may be incorrect. Our failure to identify and correctly interpret relevant patents may negatively impact our ability to develop and market our products.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming ownership of what we regard as our own intellectual property.
Many of our current and former employees, including our senior management, were previously employed at universities or at other biotechnology or pharmaceutical companies, including some which may be competitors or potential competitors. Some of these employees may be subject to proprietary rights, non-disclosure and non- competition agreements, or similar agreements, in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our patents, trade secrets, or other intellectual property as an inventor or co- inventor. For example, we or our collaborators may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our drug candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership of our patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our drug candidates. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
|
|
We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel. In addition, our patents may become, involved in inventorship, priority, or validity disputes. To counter or defend against such claims can be expensive and time-consuming, and our adversaries may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, in addition to counterclaims asserting that our patents are invalid or unenforceable, or both.
In an infringement proceeding, a court may decide that a patent is invalid or unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating intellectual property rights we own or control. An adverse result in any litigation proceeding could put one or more of our owned or in-licensed patents at risk of being invalidated or interpreted narrowly. Further, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Even if resolved in our favor, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our personnel from their normal responsibilities. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing drug candidate. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. Furthermore, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities.
We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our marks of interest and our business may be adversely affected.
Our current or future trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We rely on both registration and common law protection for our trademarks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. During trademark registration proceedings, we may receive rejections. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, with the USPTO and with comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected. We may license our trademarks and trade names to third parties, such as distributors. Although these license agreements may provide guidelines for how our trademarks and trade names may be used, a breach of these agreements or misuse of our trademarks and tradenames by our licensees may jeopardize our rights in or diminish the goodwill associated with our trademarks and trade names.
|
|
Moreover, any proprietary name we have proposed to use with our drug candidates in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. Similar requirements exist in Europe. The FDA typically conducts a review of proposed proprietary product names, including an evaluation of potential for confusion with other product names. If the FDA (or an equivalent administrative body in a foreign jurisdiction) objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties, and be acceptable to the FDA. Furthermore, in many countries, owning and maintaining a trademark registration may not provide an adequate defense against a subsequent infringement claim asserted by the owner of a senior trademark. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. If we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
| ITEM 1B. | Unresolved Staff Comments |
We do not have any unresolved comments issued by the SEC Staff.
| ITEM 1C. | Cybersecurity |
Risk Management and Strategy
We have established policies and processes for assessing, identifying, and managing material risk from cybersecurity threats, and have integrated these processes into our overall risk management systems and processes. We monitor cybersecurity threats, including any potential unauthorized occurrence on or conducted through our information systems that we use through third party providers that may result in adverse effects on the confidentiality, integrity, or availability of our information systems or any information residing therein.
We engage consultants in connection with our risk assessment processes. These service providers assist us in designing and implementing our cybersecurity policies and procedures, as well as monitoring and testing our safeguards. We require each third-party service provider to certify that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company.
As of December 31, 2024 and through the date of the filing of this report, we are not aware of any cybersecurity incidents that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations or financial condition. For additional information regarding risks from cybersecurity threats, please refer to Item 1A, “Risk Factors,” in this report.
Governance
One of the key functions of our board of directors is informed oversight of our risk management process, including risks from cybersecurity threats. Our board of directors is responsible for monitoring and assessing strategic risk exposure, and our executive officers are responsible for the day-to-day management of the material risks we face. Our board of directors administers its cybersecurity risk oversight function through its audit committee, which provides oversight of our cybersecurity program as part of its periodic review of enterprise risk management.
Our President and Chief Executive Officer and Senior Vice President of Finance are primarily responsible for assessing and managing our material risks from cybersecurity threats. In this regard, our President and Chief Executive Officer and Senior Vice President of Finance have assistance from consultants.
Our President and Chief Executive Officer and Senior Vice President of Finance oversee our cybersecurity policies and processes, including those described in “Risk Management and Strategy” above. Under such policies and processes, our President and Chief Executive Officer and Senior Vice President are responsible for reporting to our audit committee regarding any cybersecurity incidents.
The audit committee, in turn, provides periodic reports to our board of directors regarding our cybersecurity processes, including the results of cybersecurity risk assessments.
| ITEM 2. | Properties |
We currently lease approximately 4,000 square feet of office and laboratory space in the aggregate in New York and Massachusetts. We sublease the New York office space to a sublessee. The terms of our leases expire from December 2026 through June 2028. We believe that our leased properties are generally well maintained, in good operating condition and meet our current business needs.
| ITEM 3. | Legal Proceedings |
For a description of our legal proceedings, refer to Note 13 to the consolidated financial statements, which is incorporated herein by reference.
| ITEM 4. | Mine Safety Disclosures |
Not Applicable.
|
|
PART II
| ITEM 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common stock is listed on The Nasdaq Capital Market under the symbol “ERNA.”
Holders of Common Stock
As of March 10, 2025, there were approximately 146 stockholders of record of our common stock. The number of stockholders of record is based upon the actual number of holders registered on our books at such date. A substantially greater number of holders of our common stock are “street name” or beneficial holders, whose shares are held by banks, brokers and other financial institutions.
Preferred Stock
We have 156,112 shares of Series A Preferred Stock issued and outstanding. The Series A Preferred Stock provides for a cumulative annual dividend of 10 cents per share, payable in semi-annual installments in June and December. Dividends may be paid in cash or in shares of our common stock. In 2024, we paid approximately $8,000 in cash and issued approximately 11,000 shares of common stock as payment of the dividends to the holders of our Series A Preferred Stock. We expect to pay the dividends on our Series A Preferred Stock in accordance with its terms.
Dividend Policy
We have not declared or paid any cash dividends on our common stock. We currently do not anticipate paying any cash dividends in the foreseeable future. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to applicable laws and contractual limitations, and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
Securities Authorized for Issuance under Equity Compensation Plans
Information about our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this report.
|
|
Recent Sales of Unregistered Securities
We did not sell any unregistered securities during the period covered by this report that were not previously reported in a Quarterly Report on Form 10-Q or Current Report on Form 8-K.
Issuer Purchases of Equity Securities
None.
| ITEM 6. | [Reserved] |
|
ITEM 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion should be read in conjunction with our consolidated financial statements and the notes thereto included in Part II, Item 8 of this report. The following discussion contains forward-looking statements. See “CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS” in Part I of this report. Forward-looking statements are not guarantees of future activities or results. Many factors could cause our actual activities or results to differ materially from those anticipated in forward-looking statements, including those discussed in “Item 1A. Risk Factors” of Part I of this report.
Overview
We are a preclinical-stage synthetic allogeneic iMSC therapy company. Our vision is to improve the lives of patients with difficult-to-treat diseases through innovative, effective, and safe, but accessible cellular therapies, and our mission is to develop allogenic off-the-shelf cellular therapies, leveraging induced pluripotent stem cell (“iPSC”)-derived mesenchymal stem cells (“iMSCs”) to target solid tumors and autoimmune diseases.
September 2024 Transactions
Exchange Transactions
Pursuant to exchange agreements we entered into on September 24, 2024 with the holders of certain of our warrants and convertible notes, on October 29, 2024, we issued an aggregate of 38.3 million shares of our common stock in exchange for: (i) warrants to purchase an aggregate of approximately 4.4 million shares of our common stock that we issued in December 2022 with an exercise price of $1.43 per share; (ii) $8.7 million in the aggregate principal amount of convertible notes that we issued in July 2023 and warrants to purchase an aggregate of approximately 6.1 million shares of our common stock that we issued in July 2023 with an exercise price of $1.43 per share; (iii) $9.2 million in the aggregate principal amount of convertible notes that we issued in December 2023 and warrants to purchase an aggregate of approximately 9.6 million shares of our common stock that we issued in December 2023 with an exercise price of $1.43 per share (the “exchange transactions”).
The holders of the warrants described in the paragraph above exchanged all their warrants for shares of our common stock at an exchange ratio of 0.5 of a share of common stock for every one share of common stock issuable upon exercise of the applicable warrant (rounded up to the nearest whole number), and the holders of the convertible notes described in the paragraph above exchanged all their convertible notes for shares of our common stock at an exchange ratio equal to (A) the sum expressed in U.S. dollars of (1) the principal amount of the applicable convertible note, plus (2) all accrued and unpaid interest thereon through the date the applicable convertible note is exchanged plus (3) all interest that would have accrued through, but not including, the maturity date of applicable convertible note if it was outstanding from the date such convertible note is exchanged through its maturity date, divided by (B) $1.00 (rounded up to the nearest whole number).
Conversion of Bridge Notes
On September 24, 2024, we closed a private placement in which we sold an aggregate principal amount of approximately $3.9 million of 12.0% senior convertible notes (the “bridge notes”).
On October 29, 2024, in accordance with the terms of the bridge notes, approximately $3.0 million of the principal amount of the bridge notes plus all accrued and unpaid interest thereon, plus such amount of interest that would have accrued on the principal amount through December 24, 2024, was automatically converted at a conversion price of $0.50 into 6.2 million shares of our common stock, and approximately $0.9 million of the principal amount of the bridge notes plus all accrued and unpaid interest thereon, plus such amount of interest that would have accrued on the principal amount through December 24, 2024, was automatically converted at a conversion price of $0.50 into pre-funded warrants to purchase 1.8 million shares of our common stock.
|
|
Private Placement
Pursuant to a securities purchase agreement we entered into with certain investors on September 24, 2024, on October 29, 2024, we closed a private placement (the “common stock private placement” and together with the bridge notes and the exchange transactions, the “September 2024 Transactions”) in which we sold an aggregate of 1.4 million shares of our common stock and pre-funded warrants to purchase 0.1 million shares of our common stock at a purchase price of $0.75 per share of common stock and $0.745 per pre-funded warrant. We received approximately $1.1 million in gross proceeds from the issuance of such securities. For additional information regarding this private placement, see Note 6 to the accompanying consolidated financial statements.
For additional information regarding the September 2024 Transactions, see Note 6 to the accompanying consolidated financial statements.
In total, the Company issued approximately 45.9 million shares of common stock and 1.9 million pre-funded warrants on October 29, 2024 pursuant to the private placement, the exchange transactions and the conversion of the bridge notes discussed above and had 51.4 million shares of common stock issued and outstanding after the closing of the September 2024 Transactions.
Termination of Sublease
In October 2022, we entered into a sublease for office and laboratory space in Somerville, Massachusetts. In connection with entering into the sublease, we delivered a security deposit in the form of a letter of credit in the amount of $4.1 million. The letter of credit was collateralized with $4.1 million of cash deposited in a restricted account.
On August 5, 2024, the sublessor drew down on the letter of credit for the full $4.1 million to cover the approximately $4.0 million of past due rent payments for February 2024 through August 2024, plus interest and penalties.
On August 9, 2024, we and the sublessor entered into a sublease termination agreement pursuant to which the parties agreed to terminate the sublease effective August 31, 2024. Pursuant to the sublease termination agreement, we agreed to surrender and vacate the premises, all of our right, title and interest in all furniture, fixtures and laboratory equipment at the premises will become the property of the sublessor, and both parties will be released of their obligations under the sublease. As a result of the sublease termination, we recognized a gain on lease termination of approximately $1.6 million for the year ended December 31, 2024, and we expect to save approximately $72 million in base rental payments, parking, operating expenses, taxes and utilities that we would have paid over the remaining lease term.
Basis of Presentation
Revenue
In February 2023, we entered into an exclusive option and license agreement (the “Lineage Agreement”) with Lineage Cell Therapeutics, Inc. (“Lineage”), under which we granted Lineage an option to obtain an exclusive sublicense to certain of our technology for preclinical, clinical and commercial purposes in exchange for a non-refundable up-front payment to us of $0.3 million. In August 2023, Lineage requested that we begin developing certain induced pluripotent stem cell lines in exchange for a cell line customization fee. Lineage paid us $0.4 million towards the customization fee, which we were recognizing ratably over the customization period.
On September 24, 2024, we entered into an agreement with Factor Bioscience whereby we assigned the Lineage Agreement to Factor Bioscience (the “Lineage Assignment Agreement”). The Lineage Assignment Agreement with Factor Bioscience. assigns all our rights and obligations under that the Lineage Agreement to Factor Bioscience. Payments to us related to the Lineage Agreement will now be subject to the Lineage Assignment Agreement, which provides for Factor Bioscience paying us thirty percent (30%) of all amounts it receives from Lineage in the event that Lineage obtains a sublicense from Factor Bioscience. Upon receipt of future payments for the customization activities set forth in the Lineage Agreement, Factor Bioscience will pay us twenty percent (20%) of all amounts Factor Bioscience receives from Lineage. Because we have no further obligations under the agreement with Lineage, we have fully recognized as revenue amounts previously recorded in deferred revenue of approximately $0.5 million for the year ended December 31, 2024. For additional information, see Note 5 to the accompanying consolidated financial statements. We have no other revenue generating contracts at this time.
|
|
Cost of Revenues
We recognize direct labor and supplies associated with generating our revenue as cost of revenues. As provided for in the A&R Factor License Agreement discussed in Note 11 to the accompanying consolidated financial statements, we were obligated to pay Factor Limited 20% of any amounts we receive from a customer that was related to the licensed technology under the A&R Factor License Agreement, which we also recognize as a cost of revenue.
Research and Development Expenses
We expense our research and development costs as incurred. Research and development expenses consist of costs incurred for company-sponsored research and development activities. Upfront payments and milestone payments made for the licensing of technology are expensed as research and development in the period in which they are incurred if the technology is not expected to have any alternative future uses other than the specific research and development project for which it was intended.
The major components of research and development costs include salaries and employee benefits, stock-based compensation expense, supplies and materials, preclinical study costs, expensed licensed technology, consulting, scientific advisors and other third-party costs, as well as allocations of various overhead costs related to our product development efforts.
We have contracted with third parties to perform various studies. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. We accrue for third party expenses based on estimates of the services received and efforts expended during the reporting period. If the actual timing of the performance of the services or the level of effort varies from the estimate, the accrual is adjusted accordingly. The expenses for some third-party services may be recognized on a straight-line basis if the expected costs are expected to be incurred ratably during the period. Payments under the contracts depend on factors such as the achievement of certain events or milestones, the allocation of responsibilities among the parties to the agreement, and the completion of portions of the preclinical study or similar conditions.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries, benefits and other costs, including equity-based compensation, for our executive and administrative personnel, legal and other professional fees, travel, insurance, and other corporate costs.
|
|
Comparison of the Years Ended December 31, 2024 and 2023
| Year ended December 31, | ||||||||||||
| (in thousands) | 2024 | 2023 | Change | |||||||||
| Revenue | $ | 582 | $ | 68 | $ | 514 | ||||||
| Cost of revenues | 96 | 236 | (140 | ) | ||||||||
| Gross income (loss) | 486 | (168 | ) | 654 | ||||||||
| Operating expenses: | ||||||||||||
| Research and development | 4,604 | 5,920 | (1,316 | ) | ||||||||
| General and administrative | 13,132 | 14,587 | (1,455 | ) | ||||||||
| Gain on lease termination | (1,576 | ) | - | (1,576 | ) | |||||||
| Acquisition of Exacis IPR&D | - | 460 | (460 | ) | ||||||||
| Total operating expenses | 16,160 | 20,967 | (4,807 | ) | ||||||||
| Loss from operations | (15,674 | ) | (21,135 | ) | 5,461 | |||||||
| Other (expense) income, net: | ||||||||||||
| Loss on extinguishment of debt | (22,440 | ) | - | (22,440 | ) | |||||||
| Change in fair value of convertible notes | 1,017 | - | 1,017 | |||||||||
| Change in fair value of bridge notes derivative liability | (1,459 | ) | - | (1,459 | ) | |||||||
| Change in fair value of warrant liabilities | 414 | 215 | 199 | |||||||||
| Change in fair value of contingent consideration | 66 | 118 | (52 | ) | ||||||||
| Loss on non-controlling investment | - | (59 | ) | 59 | ||||||||
| Interest income | 249 | 138 | 111 | |||||||||
| Interest expense | (6,752 | ) | (614 | ) | (6,138 | ) | ||||||
| Other income (expense), net | 70 | (334 | ) | 404 | ||||||||
| Total other expense, net | (28,835 | ) | (536 | ) | (28,299 | ) | ||||||
| Loss before income taxes | (44,509 | ) | (21,671 | ) | (22,838 | ) | ||||||
| (Provision) benefit for income taxes | (30 | ) | 3 | (33 | ) | |||||||
| Net loss | $ | (44,539 | ) | $ | (21,668 | ) | $ | (22,871 | ) | |||
Revenue
During the years ended December 31, 2024 and 2023, we recognized revenue related to the cell line customization activities we performed for Lineage. The increase in revenue is due to accelerating the recognition of approximately $0.5 million of deferred revenue related to nonrefundable payments we received from Lineage due to the Lineage Assignment Agreement we entered into on September 24, 2024 with Factor Bioscience discussed earlier. As of December 31, 2024, we did not have any deferred revenue balances on our consolidated balance sheet.
Cost of Revenue
During the years ended December 31, 2024 and 2023, our cost of revenues included direct labor and materials to perform the customization cell line activities for Lineage. The decrease in cost of revenue was primarily related to a 20% license fee paid to Factor Bioscience during the year ended December 31, 2023 related to the Lineage Agreement, which was not repeated in 2024.
Research and Development Expenses
| Years ended December 31, | ||||||||||||
| 2024 | 2023 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Professional fees | $ | 759 | $ | 1,181 | $ | (422 | ) | |||||
| MSA/license expense | 3,017 | 3,250 | (233 | ) | ||||||||
| Payroll-related | 502 | 701 | (199 | ) | ||||||||
| Stock-based compensation | 89 | 234 | (145 | ) | ||||||||
| Allocated occupancy expense | 192 | 186 | 6 | |||||||||
| Other expenses, net | 45 | 368 | (323 | ) | ||||||||
| Total research and development expenses | $ | 4,604 | $ | 5,920 | $ | (1,316 | ) | |||||
|
|
Total research and development expenses decreased by approximately $1.3 million for the year ended December 31, 2024 compared to the year ended December 31, 2023, primarily due to decreased professional fees due to a reduction in consultant services, MSA/license fees as a result of the new Factor L&C Agreement, payroll-related expenses and stock-based compensation from a reduction in headcount, and other expenses incurred during 2023 related to closing down a clinical trial we ended in 2022.
General and Administrative Expenses
| Years ended December 31, | ||||||||||||
| 2024 | 2023 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Professional fees | $ | 4,168 | $ | 6,464 | $ | (2,296 | ) | |||||
| Insurance | 497 | 1,140 | (643 | ) | ||||||||
| Payroll-related | 1,607 | 2,045 | (438 | ) | ||||||||
| Stock-based compensation | 1,431 | 1,008 | 423 | |||||||||
| Occupancy expense | 5,074 | 3,306 | 1,768 | |||||||||
| Other expenses, net | 355 | 624 | (269 | ) | ||||||||
| Total general and administrative expenses | $ | 13,132 | $ | 14,587 | $ | (1,455 | ) | |||||
Our general and administrative expenses decreased by approximately $1.5 million for the year ended December 31, 2024 compared to the year ended December 31, 2023 primarily due to decreases in professional fees related to legal services and consultants, insurance expense due to lower premiums and payroll-related expenses resulting from less severance expense during the year ended December 31, 2024 compared to the year ended December 31, 2023. These decreases were offset by increased occupancy expense related to our Somerville sublease that we began to incur expense for in July 2023 and was terminated effective August 31, 2024, as well as increased stock-based compensation due to stock option awards granted to the chief executive officer during the year ended December 31, 2024.
Gain on Lease Termination
On August 9, 2024, we and the sublessor of our Somerville sublease entered into a sublease termination agreement effective August 31, 2024. Pursuant to the sublease termination agreement, we agreed to surrender and vacate the premises, all of our right, title and interest in all furniture, fixtures and laboratory equipment at the premises will become the property of the sublessor, and both parties will be released of their obligations under the sublease. As a result of the sublease termination, we recognized a gain on lease termination of approximately $1.6 million for the year ended December 31, 2024. There was no similar transaction during the year ended December 31, 2023.
Acquisition of Exacis In-Process Research and Development
In April 2023, we acquired from Exacis substantially all of its intellectual property assets, including all of its right, title and interest in an exclusive license agreement between Exacis and Factor Limited (the “Purchased License”). The Purchased License was determined to be an in-process research and development (“IPR&D”) asset that has no alternative future use and no separate economic value from its original intended purpose, which is therefore expensed in the period the cost is incurred. As a result, we expensed the fair value of the Purchased License of approximately $0.5 million during the year ended December 31, 2023. For additional information, see Note 4 to the accompanying financial statements included in this report. There was no similar transaction during the year ended December 31, 2024.
Loss on Extinguishment of Debt
We recognized a $22.4 million loss on extinguishment of debt for the year ended December 31, 2024 related to the exchange transaction and common stock private placement entered into on September 24, 2024. There was no similar transaction during the year ended December 31, 2023. See Note 6 to the accompanying consolidated financial statements for more information on the exchange transaction and common stock private placement.
Change in Fair Value of Convertible Notes
Because the modification of our convertible notes was accounted for as an extinguishment of debt and marked to fair value as of September 24, 2024 upon modification, we recognized income of approximately $1.0 million during the year ended December 31, 2024 related to the change in fair value of the convertible notes. This was due to such convertible notes being marked to fair value as of October 29, 2024 when such convertible notes were converted to shares of common stock. There was no similar transaction during the year ended December 31, 2023.
|
|
Change in Fair Value of Bridge Notes Derivative Liability
We recognized expense of $1.6 million related to the initial measurement at September 24, 2024 of the incremental fair value of the bridge notes derivative liability over the carrying value due to bifurcation of the conversion feature (recognized as a derivative liability) from the bridge notes. This was offset by $0.1 million in income recognized for the change in fair value of the bridge notes derivative liability due to remeasuring the liability during the year ended December 31, 2024. There was no similar transaction during the year ended December 31, 2023. See Note 6 to the accompanying consolidated financial statements for more information on the bridge notes.
Change in Fair Value of Warrant Liabilities
We recognized income of $0.4 million and $0.2 million for the years ended December 31, 2024 and 2023, respectively, for the change in the fair value of our warrant liabilities. The change in fair value of warrant liabilities for the year ended December 31, 2024 includes certain warrants that were reclassified to a liability in September 2024 and then exchanged for shares of common stock in October 2024 as part of the September 2024 Transactions described above. See Note 6 to the accompanying consolidated financial statements for more information on the exchanged warrants.
Change in Fair Value of Contingent Consideration
On the closing date of the acquisition of assets from Exacis in April 2023, we recognized a contingent consideration liability of $0.2 million for future payments that may be payable to Exacis, which was included as part of the $0.5 million fair value of the Purchased License asset and expensed as IPR&D during the year ended December 31, 2023. This contingent consideration liability is remeasured at each period end, and any change in the fair value of the contingent liability is recognized in the statement of operations. As of December 31, 2024 and 2023, we remeasured the contingent liability and recognized income of $0.1 million for each of the years ended December 31, 2024 and 2023 due to the decrease in the fair value of the contingent consideration liability.
Loss on Non-Controlling Investment
We account for our 25% non-controlling investment in NoveCite, Inc. (“NoveCite”) under the equity method. We have not guaranteed any obligations of NoveCite, nor are we otherwise committed to providing further financial support for NoveCite. Therefore, we only record 25% of NoveCite’s losses up to our investment carrying amount. As a result, we did not recognize additional losses related to NoveCite for the year ended December 31, 2024. We recognized a loss of approximately $0.1 million for the year ended December 31, 2023.
Interest Income
We recognized an increase in interest income for the year ended December 31, 2024 compared to the year ended December 31, 2023 due to having our cash into interest bearing accounts for the full year of 2024 compared to 2023.
Interest Expense
We recognized an increase in interest expense for the year ended December 31, 2024 of approximately $6.1 million compared to the year ended December 31, 2023 primarily due to interest expense and amortization of debt issuance costs associated with the 2023 convertible note financings and the 2024 bridge notes.
Other Income (Expense), Net
| Years ended December 31, | ||||||||||||
| 2024 | 2023 | Change | ||||||||||
| (in thousands) | ||||||||||||
| SEPA fees | $ | - | $ | (280 | ) | $ | 280 | |||||
| Other income (expense), net | 70 | (54 | ) | 124 | ||||||||
| Total expense, net | $ | 70 | $ | (334 | ) | $ | 404 | |||||
For the year ended December 31, 2024, we recognized other income related to amounts earned from Factor Bioscience under the Lineage Assignment Agreement entered into in September 2024. For the year ended December 31, 2023, we recognized (a) commitment fees and other fees related to the SEPA we entered into with Lincoln Park in April 2023 and (b) other miscellaneous expense.
|
|
Provision for Income Taxes
During 2024, we expect to incur state income tax liabilities related to our operations. We have established a full valuation allowance for all deferred tax assets, including our net operating loss carryforwards, since we could not conclude that we were more likely than not able to generate future taxable income to realize these assets. The effective tax rate differs from the statutory tax rate due primarily to our full valuation allowance.
Liquidity and Capital Resources
As of December 31, 2024, we had cash of approximately $1.7 million, and we had an accumulated deficit of approximately $231.5 million. We have to date incurred operating losses, and we expect these losses to continue in the future. For the year ended December 31, 2024, we incurred a net loss of $44.5 million, and we used $15.8 million of cash in operating activities.
On March 11, 2025, we received $1.5 million in exchange for the issuance of a promissory note with an aggregate principal amount of $1.5 million to an investor. The promissory note matures on the earlier of (i) June 15, 2025 or (ii) upon us receiving greater than $5 million in aggregate proceeds from a subsequent capital raise. Interest accrues at a rate of 5.0% per annum, payable at maturity.
On October 29, 2024, we also received approximately $1.1 million upon the closing of the common stock private placement. Other than the proceeds raised under the bridge notes and the common stock private placement, our sole source of liquidity is through sales of our common stock under the SEPA, pursuant to which Lincoln Park committed to purchase up to $10.0 million of our common stock. Such sales of common stock by us, if any, are subject to certain conditions and limitations set forth in the SEPA, including a condition that we may not direct Lincoln Park to purchase any shares of common stock under the SEPA if such purchase would result in Lincoln Park beneficially owning more than 4.99% of our issued and outstanding shares of common stock. Sales under the SEPA may occur from time to time, at our sole discretion, through April 2025. To date, we have issued and sold approximately 214,000 shares of our common stock to Lincoln Park, including approximately 74,000 commitment shares, and have received approximately $0.3 million in gross proceeds from such sales. We sold no shares under the SEPA during the year ended December 31, 2024.
Based on our current financial condition and forecasts of available cash, we will not have sufficient capital to fund our operations for the 12 months following the issuance date of the accompanying consolidated financial statements. We can provide no assurance that we will be able to obtain additional capital when needed, on favorable terms, or at all. If we cannot raise capital when needed, on favorable terms or at all, we will need to reevaluate our planned operations and may need to reduce expenses, file for bankruptcy, reorganize, merge with another entity, or cease operations. If we become unable to continue as a going concern, we may have to liquidate our assets, and might realize significantly less than the values at which they are carried on our financial statements, and stockholders may lose all or part of their investment in our common stock. See the risk factor in Item 1A of Part II of this report titled, “We will require substantial additional capital to fund our operations, and if we fail to obtain the necessary financing, we may not be able to pursue our business strategy.”
Historically, the cash used to fund our operations has come from a variety of sources and predominantly from sales of shares of our common stock and convertible notes. We will continue to evaluate and plan to raise additional funds to support our working capital needs through public or private equity offerings, debt financings, strategic partnerships, out-licensing our intellectual property or other means. There can be no assurance that capital will be available when needed or that, if available, it will be obtained on terms favorable to us and our stockholders. Our ability to raise capital through sales of our common stock will depend on a variety of factors including, among others, market conditions, the trading price and volume of our common stock, and investor sentiment. In addition, macroeconomic factors and volatility in the financial market, which may be exacerbated in the short term by concerns over inflation, interest rates, impacts of the wars in Ukraine and the Middle East, strained relations between the U.S. and several other countries, and social and political discord and unrest in the U.S., among other things, may make equity or debt financings more difficult, more costly or more dilutive to our stockholders.
In addition, equity or debt financings may have a dilutive effect on the holdings of our existing stockholders, and debt financings may subject us to restrictive covenants, operational restrictions and security interests in our assets. If we raise capital through collaborative arrangements, we may be required to relinquish some rights to our technologies or grant sublicenses on terms that are not favorable to us.
We prepared the accompanying condensed consolidated financial statements on a going concern basis, which assumes that we will realize our assets and satisfy our liabilities in the normal course of business. As discussed above, there is substantial doubt about our ability to continue as a going concern because we do not have sufficient cash to satisfy our working capital needs and other liquidity requirements over at least the next 12 months from the date of issuance of the accompanying condensed consolidated financial statements. The accompanying condensed consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and reclassification of assets or the amounts and classifications of liabilities that may result from the outcome of the uncertainty of our ability to remain a going concern.
|
|
In addition, while we are not presently pursuing product development, we may do so in the future. Developing product candidates, conducting clinical trials and commercializing products requires substantial capital, and we would need to raise substantial additional funds if we were to pursue the development of one or more product candidates.
Cash Flows
Cash flows from operating, investing and financing activities, as reflected in the accompanying condensed consolidated statements of cash flows, are summarized as follows:
| For
the years ended December 31, |
||||||||||||
| (in thousands) | 2024 | 2023 | Change | |||||||||
| Cash (used in) provided by: | ||||||||||||
| Operating activities | $ | (15,836 | ) | $ | (20,408 | ) | $ | 4,572 | ||||
| Investing activities | (365 | ) | (19 | ) | (346 | ) | ||||||
| Financing activities | 6,260 | 16,556 | (10,296 | ) | ||||||||
| Net decrease in cash and cash equivalents | $ | (9,941 | ) | $ | (3,871 | ) | $ | (6,070 | ) | |||
Net Cash Used in Operating Activities
There was a decrease of approximately $4.6 million in cash used in operating activities for the year ended December 31, 2024 compared to the year ended December 31, 2023. This change was due a $4.5 million decrease in net loss, after giving effect to adjustments made for non-cash transactions, primarily due to an increase in recognition of revenue as well as a reduction in professional and consulting expenses, offset by a slight increase of $0.1 million in cash used in operating assets and liabilities for the year ended December 31, 2024 compared to the year ended December 31, 2023.
Net Cash Used in Investing Activities
We used approximately $0.4 million to pay for the purchases of property and equipment during the year ended December 31, 2024. There was an immaterial amount of investing activities during the year ended December 31, 2023.
Net Cash Provided by Financing Activities
Net cash provided by financing activities for the year ended December 31, 2024 includes approximately $6.4 million of gross proceeds received from the convertible note financing, the bridge note financing and the common stock private placement that occurred in January 2024, September 2024 and October 2024, respectively. Net cash provided by financing activities for the year ended December 31, 2023 includes approximately $16.5 million of gross proceeds from convertible note financings and $0.3 million of proceeds received from selling approximately 214,000 shares to Lincoln Park under the SEPA. The Company did not sell any shares under the SEPA during the year ended December 31, 2024.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under applicable SEC rules.
|
|
Critical Accounting Estimates
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires us to make judgments, estimates, and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported expenses during the reporting periods. We continually evaluate our judgments, estimates and assumptions. We base our estimates on the terms of underlying agreements, our expected course of development, historical experience and other factors we believe are reasonable based on the circumstances, the results of which form our management’s basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. We believe the following critical accounting estimates affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Goodwill Impairment
Goodwill represents the excess of the purchase price over the fair value of identifiable assets acquired and the liabilities assumed. Goodwill is not amortized but is tested for impairment annually or more frequently if events occur or circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, macroeconomic conditions, industry and market considerations, cost factors, overall financial performance and other relevant events. Management evaluates our company as a single reporting unit, therefore, our goodwill is tested for impairment at the entity level. Goodwill is tested for impairment as of December 31st of each year, or more frequently as warranted by events or changes in circumstances mentioned above. Accounting guidance also permits an optional qualitative assessment for goodwill to determine whether it is more likely than not that the carrying value of a reporting unit exceeds its fair value. If, after this qualitative assessment, we determine that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, then no further quantitative testing would be necessary. A quantitative assessment is performed if the qualitative assessment results in a more likely than not determination or if a qualitative assessment is not performed. The quantitative assessment considers whether the carrying amount of a reporting unit exceeds its fair value, in which case an impairment charge is recorded to the extent the reporting unit’s carrying value exceeds its fair value.
Recent Accounting Pronouncements
Recently Adopted Accounting Standards
In June 2022, the Financial Accounting Standard Board (the “FASB”) issued Accounting Standards Update (“ASU”) No. 2022-03, Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions (“ASU 2022-03”). The FASB issued ASU 2022-03 to (1) clarify the guidance in Topic 820, Fair Value Measurement, when measuring the fair value of an equity security subject to contractual restrictions that prohibit the sale of an equity security, (2) to amend a related illustrative example, and (3) to introduce new disclosure requirements for equity related securities subject to contractual sale restrictions that are measured at fair value in accordance with Topic 820. ASU 2022-03 clarifies that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The guidance was effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years with early adoption permitted. The adoption of this ASU did not have a material impact to our consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting – Improvements to Reportable Segment Disclosures, which provides updates to qualitative and quantitative reportable segment disclosure requirements, including enhanced disclosures about significant segment expenses and increased interim disclosure requirements, among others. ASU No. 2023-07 was effective for fiscal years beginning after December 15, 2023, and interim periods in fiscal years beginning after December 15, 2024. Early adoption was permitted, and the amendments should be applied retrospectively. The adoption of this ASU did not have an impact to our consolidated financial statements, but did result in additional disclosures made in the notes to the consolidated financial statements.
|
|
Recently Issued Accounting Standards to be Adopted
In October 2023, the FASB issued ASU No. 2023-06, Disclosure Improvements – Codification Amendment in Response to the SEC’s Disclosure Update and Simplification Initiative. This ASU modified the disclosure and presentation requirements of a variety of codification topics by aligning them with the SEC’s regulations. The amendments to the various topics should be applied prospectively, and the effective date will be determined for each individual disclosure based on the effective date of the SEC’s removal of the related disclosure. If the SEC has not removed the applicable requirements from Regulation S-X or Regulation S-K by June 30, 2027, then this ASU will not become effective. Early adoption is prohibited. The Company does not expect the amendments in this ASU to have a material impact on its consolidated financial statements.
In December 2023, the FASB issued ASU No. 2023-09, Improvements to Income Tax Disclosures, which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components of the effective tax rate reconciliation, and modifies other income tax-related disclosures. ASU No. 2023-09 is effective for fiscal years beginning after December 15, 2024 and allows for adoption on a prospective basis, with a retrospective option. Early adoption is permitted. We do not expect the adoption of this ASU to have a material impact on our consolidated financial statements.
In November 2024, the FASB issued ASU No. 2024-03, Income Statement – Reporting Comprehensive Income – Expense Disaggregation Disclosures (Subtopic 220-40). This ASU is intended to improve disclosures about a public business entity’s expenses by requiring disaggregated disclosure, in the notes to the financial statements, of prescribed categories of expenses within relevant income statement captions. ASU No. 2024-03 is effective for fiscal years beginning after December 15, 2026 and interim periods within fiscal years beginning after December 15, 2027 (as clarified in ASU No. 2025-01, Income Statement – Reporting Comprehensive Income – Expense Disaggregation Disclosures (Subtopic 220-40): Clarifying the Effective Date). Early adoption is permitted. The new standard may be applied either on a prospective or retrospective basis. We do not expect the adoption of this ASU to have a material impact on our consolidated financial statements.
In November 2024, the FASB issued ASU No. 2024-04, Debt – Debt with Conversion and Other Options (Subtopic 470-20): Induced Conversions of Convertible Debt Instruments. This ASU clarifies the requirements for determining whether certain settlements of convertible debt instruments should be accounted for as an induced conversion. ASU No. 2024-04 is effective for annual reporting periods beginning after December 15, 2025 and interim reporting periods within those annual reporting periods. Early adoption is permitted, and the amendments may be applied on either a prospective or retrospective basis. We do not expect the amendments in this ASU to have a material impact on our consolidated financial statements.
| ITEM 7A. | Quantitative and Qualitative Disclosures about Market Risk |
Under SEC rules and regulations, as a smaller reporting company we are not required to provide the information otherwise required by this item.
| ITEM 8. | Financial Statements and Supplementary Data |
See “Index to Consolidated Financial Statements” on page F-1 for the consolidated financial statements filed with this report.
| ITEM 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| ITEM 9A. | Controls and Procedures |
Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as such term is defined under Rule 13a-15(e) promulgated under the Exchange Act, designed to ensure that information required to be disclosed in our reports filed pursuant to the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and our principal financial officer, as appropriate, to allow timely decisions regarding required disclosures.
In designing and evaluating the disclosure controls and procedures, we recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and we were required to apply our judgment in evaluating the cost-benefit relationship of possible controls and procedures. We have carried out an evaluation as of the end of the period covered by this Annual Report on Form 10-K under the supervision, and with the participation, of our management, including our President and Chief Executive Officer (who serves as our principal executive officer) and our Senior Vice President of Finance (who serves as our principal financial officer) of the effectiveness of the design and operation of our disclosure controls and procedures.
|
|
Based on that evaluation, our Chief Executive Officer and Senior Vice President of Finance concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Annual Report on Form 10-K in providing reasonable assurance of achieving the desired control objectives.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
We previously identified a material weakness in our internal control over financial reporting. We were unable to timely file our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2022 due to identifying errors in our financial statements reported in our Annual Report on Form 10-K for the years ended December 31, 2021 and 2020 during our preparation of the financial statements for the quarter ended March 31, 2022. Management concluded that the errors were the result of accounting personnel’s lack of technical proficiency in complex matters. On June 30, 2022, we filed an amendment to our Annual Report on Form 10-K for the years ended December 31, 2021 and 2020 to correct the errors in our financial statements for the years ended December 31, 2021 and 2020 and for the quarters ended June 30, 2020, September 30, 2020, March 31, 2021, June 30, 2021 and September 30, 2021.
Management implemented measures designed to ensure that the deficiencies contributing to the ineffectiveness of our internal control over financial reporting were remediated, such that the internal controls are designed, implemented and operating effectively. The remediation actions taken include the following:
| ● | enhance the business process controls related to reviews over technical, complex, and non-recurring transactions; | |
| ● | provide additional training to accounting personnel; and | |
| ● | use external accounting advisors to review management’s conclusions on technical, complex and non-recurring matters. |
We have completed the documentation and review of the corrective actions described above, and our management has concluded that the design and operation of our financial reporting process as it relates to technical accounting proficiency in complex matters is effective and therefore that the related previously identified material weakness has been fully remediated as of December 31, 2024.
We are committed to developing a strong internal control environment, and we believe the remediation efforts that we have implemented resulted in significant improvements in our control environment. Our management continues to monitor and evaluate the relevance of our risk-based approach and the effectiveness of our internal controls and procedures over financial reporting on an ongoing basis and is committed to taking actions and implementing enhancements or improvements, as necessary.
Accordingly, our management, with the participation of our Chief Executive Officer and Senior Vice President of Finance, evaluated the effectiveness of our internal control over financial reporting as of December 31, 2024, and concluded that our internal control over financial reporting was effective as of December 31, 2024. In making this assessment, we utilized the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control — Integrated Framework (2013).
Changes in Internal Control over Financial Reporting
Except for the actions taken to remediate the material weakness as described above, there was no change in our internal control over financial reporting during the most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | Other Information |
During the quarter ended December 31, 2024, no director or officer of the Company adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
| ITEM 9C. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
Not Applicable.
|
|
PART III
| ITEM 10. | Directors, Executive Officers and Corporate Governance |
Directors and Executive Officers
The names of our directors and executive officers and their respective ages, positions, biographies and, in the case of directors, their qualifications to serve as directors, are set forth below as of March 10, 2025.
| Name | Age | Position | ||
| Sanjeev Luther | 63 | President and Chief Executive Officer and Director | ||
| Sandra Gurrola | 58 | Senior Vice President, Finance | ||
| James Bristol | 78 | Chairman of the Board | ||
| Peter Cicala | 63 | Director | ||
| Elena Ratner | 48 | Director | ||
| William Wexler | 65 | Director |
Sanjeev Luther has served as President, Chief Executive Officer and as a member of our board of directors since January 2024. Prior to that, Mr. Luther served as President, Chief Executive Officer and a board member of Cornerstone Pharmaceuticals from November 2017 to December 2023 and as its Chief Operations Officer and Chief Business Officer from December 2014 to November 2017. Prior to that, Mr. Luther served in various leadership roles at Bristol-Myers Squibb, Novartis, Bausch and Lomb and GE Healthcare. Mr. Luther holds an MBA in Marketing and a B.S. in Marketing and Business Administration from the State University of New York at Buffalo.
Mr. Luther’s qualifications to serve on our board of directors include his expertise in the healthcare industry, his business training and education, and his extensive experience managing life science companies.
Sandra Gurrola has served as our Senior Vice President of Finance since May 2023 and served as our Vice President of Finance from June 2021 until May 2023. Prior to that, she served as the Senior Vice President of eGames.com Holdings, LLC from March 2021 to June 2021 and as a consultant to us. Ms. Gurrola served as Senior Vice President of Finance to NTN Buzztime, Inc. from September 2019 to March 2021 and its Vice President of Finance from 2014 until 2019. From 2009 to 2014, Ms. Gurrola served NTN Buzztime, Inc. in various leadership accounting roles, including Controller, Director of Accounting, and Director of Financial Reporting and Compliance. Previously, she was a senior manager of financial reporting for Metabasis Therapeutics, Inc., a biotechnology company. Ms. Gurrola received a B.A. in English from San Diego State University.
James Bristol has served as a member of our board of directors since October 2023. Dr. Bristol worked for 32 years in drug discovery, research and preclinical development at Schering-Plough Corporation, Parke-Davis, and Pfizer Inc. (“Pfizer”), serving in various senior research and development roles. From 2003 until his retirement in 2007, Dr. Bristol served as Senior Vice President of Worldwide Drug Discovery Research at Pfizer Global Research & Development, where he oversaw 3,000 scientists at seven Pfizer sites as they produced an industry leading number of drug development candidates in 11 therapeutic areas. In 2009, Dr. Bristol joined Frazier Healthcare Partners as a Senior Advisor. From August 2007 until Dec 2024, Dr. Bristol served as a member of the board of directors of Deciphera Pharmaceuticals, and since 2018 he has served as a member of the board of directors of Erasca, Inc., both of which are publicly traded life science companies. Dr. Bristol also served on the board of directors of Ignyta from 2014 until its acquisition by Roche in 2018 and served on the board of directors of SUDO Biosciences, Inc. from June 2021 until December 2023, and of Cadent Therapeutics, Inc. from 2011 until 2020. Dr. Bristol is the author of over 100 publications, abstracts and patents, and he conducted postdoctoral research at the University of Michigan (NIH Postdoctoral Fellow) and at The Squibb Institute for Medical Research. Dr. Bristol holds a Ph.D. in organic chemistry from the University of New Hampshire and a B.S. in Chemistry from Bates College.
Dr. Bristol’s qualifications to serve on our board of directors include his vast experience in the biopharmaceutical industry, including in management and as a director, as well as his expertise in drug discovery and development.
Peter Cicala has served as a member of our board of directors since February 2024. Mr. Cicala currently serves as General Counsel for a private biotechnology company, where he has been since March of 2021. In November of 2019, he co-founded Pretzel Therapeutics, Inc., a biotechnology company, and still serves as an executive advisor. From March 2020 until March 2021, Mr. Cicala served as Chief Intellectual Property Counsel for Intercept Pharmaceuticals, Inc. and from March 2014 until November 2019, he served as Chief Patent Counsel for Celgene Corporation, both publicly traded biopharmaceutical companies. Mr. Cicala has practiced law for over 25 years, and also has over 10 years of experience as a medicinal chemist. He received his B.S. in chemistry from Fairleigh Dickinson University and a J.D. from Seton Hall University School of Law.
Mr. Cicala’s qualifications to serve on our board of directors include his expertise in pharmaceutical and biotechnology intellectual property law and in strategic management of proprietary technology and products.
Elena Ratner has served as a member of our board of directors since January 2025. Since July 2019, Dr. Ratner has been serving as a professor in the Department of Obstetrics, Gynecology and Reproductive Sciences at Yale University School of Medicine and also serves as the director of the Discovery to Cure Early Ovarian Detection program. Dr. Ratner’s clinical research has focused on new targeted drugs for ovarian cancer and on reversing chemotherapy resistance in ovarian and uterine cancers. She received her B.S. in premedical studies from Columbia University and her M.D. from State University of New York Medical College.
Dr. Ratner’s qualifications to serve on our board of directors include her vast expertise in obstetrics, gynecology and reproductive sciences, and specifically in ovarian cancer research and treatment.
|
|
William Wexler has served as a member of our board of directors since June 2022. Prior to joining our board of directors, Mr. Wexler worked on over 150 individual projects, serving in various capacities including as Chairman, Chief Executive Officer, Chief Restructuring Officer and other designated roles of senior responsibility. Mr. Wexler has served as the Managing Member of WEXLER Consulting LLC, a management consulting firm, since 2012. From 2012 to 2019, he served in various roles, including as Chairman of the Board, interim Chief Executive Officer, Chief Executive Officer and sole director and stockholder representative of Upstate New York Power Products, Inc., a holding company that owned and operated power plants throughout upstate New York. From 2012 to 2013, Mr. Wexler served as Chief Restructuring Officer of VMR Electronics, LLC, a manufacturer of cable assembly products for the electronics interconnect industry. Prior to that, he served as a Managing Director and national finance practice lead at BBK, Ltd., a turn-around advisory firm, from 2006 to 2011. Mr. Wexler served as group Managing Director of corporate restructuring at Huron Consulting Group, LLC from 2002 to 2005. Previously, he was a Managing Director at Berenson Minella & Co., a boutique investment-banking firm, from 2000 to 2002. Between 1986 and 2000 he served as a Senior Director at BNP Paribas, where he established and led Paribas Properties, Inc., a real estate investment arm of the bank, and also where he was a lead officer of the then newly created U.S. asset workout group. Mr. Wexler started his professional career in 1981 in commercial lease brokerage, asset management and investment sales at Jones Lang Wootton (now Jones Lang LaSalle) where he worked until 1986. He earned a B.A. in Political Science from Johns Hopkins University.
Mr. Wexler’s qualifications to serve on our board of directors include his experience in investment and senior management roles, as well as his business training and education.
Family Relationships
There are no family relationships between any of our officers or directors.
Involvement in Certain Legal Proceedings
None of our directors or executive officers is involved in any legal proceeding that requires disclosure under Item 401(f) of Regulation S-K.
Code of Ethics.
Our board of directors has adopted a Code of Business Conduct and Ethics that applies to all of our employees, officers and directors, including our Chief Executive Officer, Chief Financial Officer and other executive and senior financial officers. A copy of our Code of Business Conduct and Ethics is available under the “Governance” tab of the “Investor Relations” section of our website located at www.eternatx.com. We intend to disclose any changes in our Code of Business Conduct and Ethics or waivers from it that apply to our principal executive officer, principal financial officer, or principal accounting officer by posting such information on the same website or by filing with the SEC a Current Report on Form 8-K, in each case if such disclosure is required by SEC or Nasdaq rules. The information on our website is not intended to form a part of or be incorporated by reference into this Proxy Statement.
Audit Committee
We have a standing audit committee established in accordance with Section 3(a)(58)(A) of the Exchange Act. Our audit committee consists of William Wexler (Chair), James Bristol and Peter Cicala, all of whom meet the requirements for independence of audit committee members under applicable Nasdaq and SEC rules, including Rule 10A-3 promulgated under the Exchange Act. All of the members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq. In addition, Mr. Wexler qualifies as our “audit committee financial expert,” as such term is defined in Item 407 of Regulation S-K.
Changes in Stockholder Nomination Procedures
There have been no material changes to the procedures by which stockholders may recommend nominees to our board of directors since such procedures were last described in our proxy statement filed with the SEC on October 7, 2024.
Insider Trading Policy
We have adopted an insider trading policy governing the purchase, sale, and other dispositions of our securities by directors, senior management, and employees. A copy of the Insider Trading Policy has been filed as exhibit 19 to this report.
|
|
| ITEM 11. | Executive Compensation |
Overview
When determining executive officer compensation, and the various components that comprise it, our compensation committee evaluates and considers publicly available executive officer compensation survey data to present a competitive compensation package to attract and retain top talent, including an appropriate level of salary, performance-based bonus and equity incentives. Typically, our compensation committee evaluates competitive market benchmark data for a given executive role. Additionally, our compensation committee is authorized to engage outside advisors and experts to assist and advise our compensation committee on matters relating to executive compensation. In 2023, our compensation committee retained the services of Pearl Meyer, an independent compensation consultant, to review the cash and equity compensation package that was offered to Mr. Luther prior to his appointment as our President and Chief Executive Officer.
Our President and Chief Executive Officer presents compensation recommendations to our compensation committee with respect to the executive officers other than himself. Our compensation committee considers such recommendations, in conjunction with possible input from our compensation committee’s independent compensation consultant, in making compensation decisions or recommendations to the full board of directors. The full board participates in evaluating the performance of our executive officers, except that our Chief Executive Officer does not participate when our board of directors evaluates his performance and is not present during voting or deliberations regarding his performance or compensation matters.
Compensation-Related Risk Assessment
Our compensation committee assesses and monitors whether any of our compensation policies and programs are reasonably likely to have a material adverse effect on our Company. Our compensation committee and management do not believe that the Company presently maintains compensation policies or practices that are reasonably likely to have a material adverse effect on the Company’s risk management or create incentives that could lead to excessive or inappropriate risk taking by employees. In reaching this conclusion, our compensation committee considered all components of our compensation program and assessed any associated risks. Our compensation committee also considered the various strategies and measures employed by the company that mitigate such risk, including: (i) the overall balance achieved through our use of a mix of cash and equity, annual and long-term incentives and time-and performance-based compensation; (ii) our use of multi-year vesting periods for equity grants; and (ii) the oversight exercised by our compensation committee over performance metrics, if any, established for performance-based bonuses and its administration of our equity incentive plans.
Compensation Recoupment (Clawback) Policy
In 2023, we adopted a clawback policy providing for the recovery of erroneously-awarded incentive-based compensation related to the three fiscal years preceding the date on which the company is required to prepare an accounting restatement. The clawback policy complies with the requirements of Nasdaq’s listing rules.
Named Executive Officers
Under applicable SEC rules and regulations, our “named executive officers” are all individuals who served as our principal executive officer during 2024, our two most highly compensated executive officers (other than our principal executive officer) who were serving as executive officers at December 31, 2024, and up to two additional individuals who would have been one of our top two most highly compensated executive officer had they been serving as an executive officer at the end of 2024. Our 2024 named executive officers are identified in the table below:
| Name | Title | |
| Sanjeev Luther | President and Chief Executive Officer | |
| Sandra Gurrola | Senior Vice President of Finance |
|
|
Summary Compensation Table
The following table sets out the compensation for our Named Executive Officers for the years ended December 31, 2024 and December 31, 2023:
| 2024 Summary Compensation Table | |||||||||||||||||||||||||||||||||||
| Name and Principal Position | Fiscal Year | Salary (US$) | Bonus (US$) | Stock-Based Awards (US$)(1) | Option-Based Awards (US$)(1) | Non-Equity Incentive Plan Compensation (US$) | Nonqualified deferred compensation earnings (US$) | All Other Compensation (US$) | Total Compensation (US$) | ||||||||||||||||||||||||||
| Sanjeev Luther, President and Chief Executive Officer | 2024 | $ | 550,000 | $ | 75,000 | (2) | $ | — | $ | 2,422,818 | $ | — | $ | — | $ | — | ) | $ | 3,047,818 | ||||||||||||||||
| Sandra Gurrola, Sr. Vice President of Finance | 2024 | $ | 275,000 | $ | — | $ | — | $ | 110,198 | $ | — | $ | — | $ | — | $ | 385,198 | ||||||||||||||||||
| 2023 | $ | 255,833 | $ | 50,050 | (3) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 305,883 | ||||||||||||||||||
| 1. |
The amounts reported in this column represent the aggregate grant date fair value of stock options granted during the applicable year. These amounts were calculated in accordance with FASB ASC Topic 718, Compensation – Stock Compensation, except that any estimate of forfeitures was disregarded. For a description of the assumptions used in computing the dollar amount recognized for financial statement reporting purposes, see Note 15, Stock-Based Compensation, in the Notes to the Consolidated Financial Statements contained in this Annual Report on Form 10-K. |
| 2. | Mr. Luther was appointed as our President and Chief Executive Officer effective January 1, 2024 and amount represents a cash signing bonus pursuant to his employment agreement. |
| 3. | Represents a discretionary spot bonus paid to Ms. Gurrola and approved by our board of directors. |
Narrative to Summary Compensation Table
The following is a discussion of each component of our executive compensation program for 2024.
Base Salary
Each of our named executive officers receives a base salary. The base salary is the fixed cash compensation component of our executive compensation program and it recognizes individual performance, time in role, scope of responsibility, leadership skills and experience. The base salary compensates an executive for performing his or her job responsibilities on a day-to-day basis. Generally, base salaries are reviewed annually company-wide and adjusted (upward or downward) when appropriate based upon individual performance, expanded duties, changes in the competitive marketplace and, with respect to upward adjustments, if we are, financially and otherwise, able to pay it. We try to offer competitive base salaries to help attract and retain executive talent.
Bonus and Incentive Compensation
In addition to base salaries, our compensation committee has the authority to award discretionary annual bonuses to our named executive officers based on corporate and individual performance. Each year, our compensation committee or our board of directors may establish performance goals, which may be based on measures such as revenue, achievement of certain research and development milestones, completion of a strategic transaction, and other metrics the directors and management believe to provide proper incentives for achieving long-term shareholder value. Our board of directors retains full discretion over performance evaluation and the amount of any bonuses to be paid to a named executive officer. Annual bonuses, if any, are intended to reward the individual performance of each named executive officer. In addition to an assessment of corporate and individual performance, the determination of the amount of a named executive officer’s bonus may vary from year to year depending on our financial condition and conditions in the industry in which we operate. The amount of such bonuses increase with executive rank so that, as rank increases, a greater portion of total annual cash compensation is based on annual corporate and individual performance. For the year ended December 31, 2024, no performance goals were established for any named executive officer.
As further described below in Named Executive Officer Employment Agreements and Change in Control Arrangements, in January 2024, we paid a signing bonus to Mr. Luther in the amount of $75,000 pursuant to his employment agreements.
|
|
Equity-Based Compensation Programs
Historically we have issued stock options to our employees, including our named executive officers, to provide a means whereby our employees may develop a sense of proprietorship and personal involvement in our development and financial success, and to encourage them to devote their best efforts to us, thereby advancing our interests and the interests of stockholders. Our board of directors believes that the granting of equity awards promotes continuity of management and increases incentive and personal interest in our welfare by those who are primarily responsible for shaping and carrying out our long-range plans and pursuing our growth and financial success.
We do not maintain any written policies on the timing of issuing equity-based incentive awards. Our compensation committee has responsibility for granting equity-based incentive awards to our named executive officers and considers whether there is any material nonpublic information (“MNPI”) about the Company when determining the timing and terms of stock option awards. The Compensation Committee generally does not time the grant of stock options in relation to our public disclosure of MNPI. We have not timed the release of MNPI for the purpose of affecting the value of executive compensation. Vesting of equity awards is generally tied to continuous service with us and serves as an additional retention measure.
In January 2024, we granted a stock option award to Mr. Luther pursuant to his employment agreement. For more information regarding this award, see Named Executive Officer Employment Agreements and Change in Control Arrangements below.
In April 2024, we granted to Ms. Gurrola a time-based non-qualified stock option covering 80,000 shares of common stock, of which one-third will vest on the one-year anniversary of the grant date and the remaining shares will vest in 24 substantially equal monthly installments thereafter, subject to her continuous service.
During fiscal year 2024, no named executive officer received a grant of stock options during the period beginning four business days before, and ending one business day after, the filing of a periodic report on Form 10-Q or Form 10-K, or the filing or furnishing of a current report on Form 8-K that discloses material nonpublic information.
Benefits and Perquisites
Employee Benefit Plans
Named executive officers are eligible to participate in our employee benefit plans, including our medical, disability and life insurance plans, in each case, on the same basis as all of our other employees. Our employee benefit plans are designed to assist in attracting and retaining skilled employees. We also maintain a 401(k) plan for the benefit of our eligible employees, including the named executive officers, as discussed below.
401(k) Plan
We maintain a retirement savings plan, or 401(k) plan, that provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Under the 401(k) Plan, eligible employees may defer up to 90% of their compensation subject to applicable annual contribution limits imposed by the Internal Revenue Code of 1986, as amended (the “Code”), and limits imposed by non-discrimination testing. Our employees’ pre-tax contributions are allocated to each participant’s individual account and participants are immediately and fully vested in their contributions. The 401(k) plan is intended to be qualified under Section 401(a) of the Code with the 401(k) plan’s related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan. We match employees’ contributions at a rate of 100% of the first 3% of the employee’s contribution and 50% of the next 2% of the employee’s contribution, for a maximum match of 4%.
Pension Benefits
We do not maintain any pension benefit or retirement plans other than the 401(k) Plan.
Nonqualified Deferred Compensation
We do not maintain any nonqualified deferred compensation plans.
|
|
Named Executive Officer Employment Agreements and Change in Control Arrangements
The following descriptions summarize the principal terms of our employment agreements with our named executive officers.
Sanjeev Luther
Sanjeev Luther was appointed as our President and Chief Executive Officer effective January 1, 2024. We entered into an employment agreement, dated as of December 19, 2023, with Mr. Luther, which provides for at-will employment until terminated by us or Mr. Luther. Mr. Luther’s employment agreement provides for an annual base salary of $550,000, which amount is subject to periodic review by our board of directors or our compensation committee. Mr. Luther also received a one-time signing bonus of $75,000.
Mr. Luther is eligible to receive an annual cash bonus award in an amount up to 50% of his base salary upon achievement of agreed upon performance targets. The bonus will be determined by our board of directors or our compensation committee and paid annually by March 15 in the year following the performance year on which such bonus is based.
In accordance with the terms of his employment agreement, Mr. Luther was granted an equity award on January 1, 2024, consisting of 1,685,218 non-qualified stock options, which will vest over a four-year period, with 25% of the options vesting on the first anniversary of the grant date, and the remaining options vesting monthly over the remaining three years. On April 26, 2024, the compensation committee approved a modification to Mr. Luther’s stock option award to reduce the vesting term to three years rather than four years, with 25% of the shares subject to the stock option award still vesting on the first anniversary of the grant date, and the balance of the shares vesting monthly over the remaining two years. Vesting generally requires Mr. Luther’s continued employment through the relevant vesting date.
If Mr. Luther’s employment is terminated by us without Cause (as defined in his employment agreement) or by Mr. Luther for Good Reason (as defined in his employment agreement), we will pay Mr. Luther all amounts accrued but unpaid as of the effective date of such termination, as well as a lump sum payment equal to nine months of his salary, as well as up to nine months of continued benefits. Mr. Luther will also be paid a pro-rata performance bonus equal to (x) the performance bonus Mr. Luther would have received based on actual performance for such fiscal year if Mr. Luther had remained employed for the entire fiscal year multiplied by (y) a fraction, the numerator of which is the number of days Mr. Luther was employed during such fiscal year. Notwithstanding the foregoing, if a termination without Cause or for Good Reason occurs beginning upon the occurrence of a Change in Control (as defined in the employment agreement) and ending on the first anniversary of the occurrence of the Change in Control (“Change in Control Protection Period”), Mr. Luther will receive the benefits described in the preceding sentence, but the lump sum severance payment and the payment of benefits will be for a 12-month period and he will receive 100% of his target bonus. In addition, all outstanding and unvested equity awards granted to Mr. Luther during his employment will become immediately vested and exercisable upon such date of termination during the Change in Control Protection Period and will be exercisable for a period of 12 months following the date of termination during the Change in Control Protection Period. Any such severance benefits under the employment agreement are contingent on Mr. Luther entering into and not revoking a general release of claims in favor of our company.
Sandra Gurrola
We entered into an employment agreement, dated as of June 16, 2021, with Sandra Gurrola, which provides for our at-will employment of Ms. Gurrola commencing on June 21, 2021 and continuing until terminated by us or Ms. Gurrola. Ms. Gurrola’s employment agreement provides for an annual base salary of $220,000, which amount is subject to periodic review by our board of directors or our compensation committee. In December 2023, upon the recommendation of our compensation committee, our board of directors approved an increase to Ms. Gurrola’s annual base salary from $220,000 to $275,000. In addition, our board of directors approved a lump sum payment of $33,542 to Ms. Gurrola, representing the additional amount of salary Ms. Gurrola would have received had the increase to her annual base salary taken effect as of May 5, 2023.
Ms. Gurrola is also eligible to receive an annual cash bonus award in an amount up to 35% of her base salary upon achievement of agreed upon performance targets. The bonus will be determined by our board of directors or our compensation committee and paid annually by March 15 in the year following the performance year on which such bonus is based.
In accordance with her employment agreement, in June 2021, Ms. Gurrola was granted 1,750 restricted stock units, 25% of which vests on each anniversary of the grant date over four years. Vesting generally requires Ms. Gurrola’s continued employment through the relevant vesting date.
If Ms. Gurrola’s employment is terminated by us without Cause (as defined in the employment agreement) or by Ms. Gurrola for Good Reason (as defined in the employment agreement), we will pay Ms. Gurrola all amounts accrued but unpaid as of the effective date of such termination, as well as continuation of her salary and benefits for the following six-month period. Notwithstanding the foregoing, if a termination of employment without Cause or for Good Reason occurs within 90 days before or 12 months after a Change in Control (as defined in the employment agreement), Ms. Gurrola will receive the benefits described in the preceding sentence, but the continuation of her salary and benefits will be for 12-month period, and, in addition, Ms. Gurrola will receive a lump-sum payment of her target bonus and the restricted stock units granted to her in June 2021 will fully vest. Any such severance benefits under the employment agreement are contingent on Ms. Gurrola entering into and not revoking a general release of claims in favor of our company.
|
|
Outstanding Equity Awards at 2024 Fiscal Year-End
The following table summarizes the number of shares of our common stock underlying outstanding equity incentive plan awards for each named executive officer as of December 31, 2024.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||||
| Name | Grant Date | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | Option exercise price ($) |
Option expiration date |
Number of shares or units of stock that have not vested (#) |
Market value of shares of units of stock that have not vested ($) | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested shares ($) |
||||||||||||||||||||||||||||
| Sanjeev Luther, President and Chief Executive Officer |
1/1/2024 | (1) | — | 1,685,218 | — | 1.80 | 1/01/2034 | — | — | — | — | |||||||||||||||||||||||||||
| Sandra Gurrola, Sr. Vice President of Finance |
6/21/2021 | (2) | — | — | — | — | — | 437 | 127 | — | — | |||||||||||||||||||||||||||
| 3/11/2022 | (3) | 5,725 | 478 | — | 38.60 | 3/11/2032 | — | — | — | — | ||||||||||||||||||||||||||||
| 4/26/2024 | (4) | — | 80,000 | — | 1.74 | 4/26/2034 | — | — | — | — | ||||||||||||||||||||||||||||
| 1. | The stock option vests over three years, with 25% vesting on the one-year anniversary of the grant date, and the remaining stock options vesting in 24 substantially equal monthly installments thereafter. |
| 2. | The restricted stock units vest at a rate of 25% of the shares subject to the award in four substantially equal annual installments on the anniversary date of the grant date. |
| 3. | The stock option vests in 36 substantially equal monthly installments. |
| 4. | The stock option vests over three years, with one-third vesting on the one-year anniversary of the grant date, and the remaining stock options vesting in 24 substantially equal monthly installments thereafter. |
|
|
Director Compensation
We have a non-employee director compensation program to compensate our non-employee directors for their service in such capacity with annual retainers and equity compensation as described below. However, since August 2022, we have not compensated our non-employee directors in accordance with our non-employee director compensation program.
During 2024, we did not compensate any of our directors, in either cash or equity, for their service in such capacity. On January 1, 2024, we granted to Dorothy Clarke a stock option to purchase 84,261 shares of our common stock as compensation for her services as a member of our board of directors from August 28, 2023 until December 31, 2023, for which she had previously not been compensated.
In April 2024, we awarded each of Jim Bristol and Peter Cicala a stock option grant to purchase 124,525 and 88,943 shares of our common stock, respectively, which vest in full on the one-year anniversary of the grant date.
In connection with her appointment as a member of our board of directors on January 7, 2025, we awarded Elena Ratner a stock option grant to purchase 140,078 shares of our common stock, which vests over three years, with one-third vesting on the one-year anniversary of the grant date and the remaining options vesting in 24 substantially equal monthly installments thereafter.
Our compensation committee and Board are assessing our non-employee director compensation program, and if and when we restart compensating our non-employee directors for their service in such capacity, the elements of our non-employee director compensation program may be different from what is described below.
| Compensation Element | Amount | |
| Annual Board Member Compensation | Paid in cash or stock options at our board’s discretion. Cash paid in quarterly installments or upon the effective date of an earlier resignation of the non-employee director. Stock Options to vest quarterly over one year from grant date: | |
| Board Member: $40,000 | ||
| Board Chair: $70,000 | ||
| Committee Member Retainers | Paid in cash or stock options at our board’s discretion. Cash paid in quarterly installments or upon the effective date of an earlier resignation of the non-employee director. Stock Options to vest quarterly over one year from grant date: | |
| Audit Committee: $7,500 | ||
| Compensation Committee: $5,000 | ||
| Nominating/Governance Committee: $4,000 | ||
| Leadership Supplemental Retainer | Paid in cash or stock options, ‘s discretion. Cash paid in quarterly installments or upon the effective date of an earlier resignation of the non- employee director. Stock Options to vest quarterly over one year from grant date: | |
| Audit Committee Chair: $15,000 | ||
| Compensation Committee Chair: $10,000 | ||
| Nominating/Governance Committee Chair: $8,000 | ||
| New Director Equity Award (outside directors) |
Option for 8,290 shares of Common Stock, which option shall have an exercise price equal to the fair market value per share of common stock, as determined under the 2020 Plan, and, subject to continued service on our board of directors, vest in an initial installment of one-third of the shares on the first anniversary of the grant date, with the remaining shares to vest in 24 substantially equal installments thereafter. |
Our board of directors and our compensation committee designed our non-employee director compensation program to reward directors for their contributions to our success, align the director compensation program with stockholder interests, and provide competitive compensation necessary to attract and retain high quality non-employee directors. We do not pay fees to any of our directors for meeting attendance.
|
|
2024 Director Compensation
The following table sets forth the compensation of each director, who is not a named executive officer, for service during 2024. This table excludes Mr. Luther, who is a named executive officers and does not receive any compensation from us for his service as a director. See the section above entitled “Executive Officer Compensation” for information about Mr. Luther’s compensation.
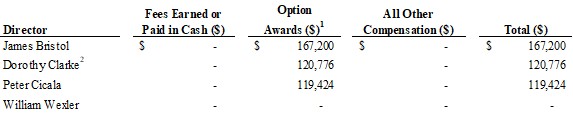
| (1) | The amounts reported in this column represent the aggregate grant date fair value of stock options granted during 2024. These amounts were calculated in accordance with FASB ASC Topic 718, Compensation – Stock Compensation, except that any estimate of forfeitures was disregarded. For a description of the assumptions used in computing the dollar amount recognized for financial statement reporting purposes, see Note 15, Stockholders’ Equity, in the Notes to the Consolidated Financial Statements contained in this Annual Report on Form 10-K. |
| (2) | Amount excludes compensation Ms. Clarke received as an employee. |
| ITEM 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The following table sets forth information known to us regarding beneficial ownership of common stock as of March 10, 2025 (the “Measurement Date”) by:
| ● | each person known by us to be the beneficial owner of more than 5% of outstanding common stock; |
| ● | each of our named executive officers and directors; and |
| ● | all of our executive officers and directors as a group. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days after the Measurement Date. In computing the number of shares beneficially owned by a person or entity and the percentage ownership of that person or entity in the table below, all shares subject to options, warrants and restricted stock units held by such person or entity were deemed outstanding if such securities are currently exercisable, or exercisable or would vest based on service-based vesting conditions within 60 days of the Measurement Date, assuming that the liquidity event vesting conditions had been satisfied as of such date. These shares were not deemed outstanding, however, for the purpose of computing the percentage ownership of any other person or entity.
The beneficial ownership of our common stock is based on 52,244,929 shares of our common stock outstanding as of the Measurement Date.
Unless otherwise indicated, we believe that each person named in the table below has sole voting and investment power with respect to all shares of common stock beneficially owned by him.
|
|
Unless otherwise noted, the business address of each of these stockholders is c/o Eterna Therapeutics, Inc., 1035 Cambridge Street, Suite 18A, Cambridge, MA 02141.
| Name and Address of Beneficial Owner | Common
Shares Beneficially |
Percentage of Common Shares Beneficially Owned |
||||||
| Greater than 5% Stockholders: | ||||||||
| Charles Cherington(1) | 16,647,302 | 31.86 | % | |||||
| John Halpern(2)^ | 5,228,571 | 9.99 | % | |||||
| Freebird Partners LP(3)^ | 5,228,571 | 9.99 | % | |||||
| George Denny Estate (4) | 4,954,285 | 9.48 | % | |||||
| IAF, LLC(5) | 2,679,214 | 5.13 | % | |||||
| Regolith Capital Investments LP (6) | 2,641,814 | 5.06 | % | |||||
| Named Executive Officers and Directors: | ||||||||
| Sanjeev Luther(7) | 631,956 | 1.20 | % | |||||
| Sandra Gurrola(8) | 33,577 | * | ||||||
| James Bristol(7) | 124,525 | * | ||||||
| Peter Cicala(7) | 88,943 | * | ||||||
| Elena Ratner | — | — | ||||||
| William Wexler(7) | 15,895 | * | ||||||
All current directors and executive officers as a group (6 persons)(9) |
894,896 | 1.68 | % | |||||
Less than 1%
The securities beneficially owned by this stockholder include prefunded warrants that include a 9.99% blocker. The number of common shares beneficially owned, the percentage of common shares beneficially owned and the percentage of total voting power shown in the table gives effect to such blocker. Pursuant to the terms of the prefunded warrants, the number of shares of common stock that may be acquired by the holder thereof upon exercise of the prefunded warrants is limited, to the extent necessary, to ensure that following such exercise, the number of shares of common stock then beneficially owned by the holder and any other persons or entities whose beneficial ownership of common stock would be attributed to the holder for purposes of Section 13(d) of the Exchange Act does not exceed 9.99% of the total number of shares of our common stock then outstanding. Upon delivery of a written notice to us, the holder may from time-to-time increase (with such increase not effective until the 61st day after delivery of such notice) or decrease the blocker to any other percentage not in excess of 9.99%.
| (1) | The number of common shares beneficially owned consists of (i) 16,633,205 shares of common stock and (ii) 14,097 shares of common stock issuable upon the conversion of shares of Series A convertible preferred stock (assuming a conversion rate of 5.0583 per share). Mr. Cherington’s address is c/o Ara Partners, LLC, 200 Berkeley Street, 26th Floor, Boston, MA, 02116. |
| (2) | The number of common shares beneficially owned consists of (i) 5,136,571 shares of common stock held by the John D. Halpern Revocable Trust, of which, Mr. Halpern and Katherine H. Halpern are trustees and (ii) 92,000 shares of common stock issuable upon exercise of prefunded warrants. Mr. Halpern and Ms. Halpern share voting and dispositive powers. Mr. Halpern’s address is PO Box 540 Portsmouth, New Hampshire 03802. |
| (3) | The number of common shares beneficially owned consists of (i) 5,136,686 shares of common stock and (ii) 91,885 shares of common stock issuable upon exercise of prefunded warrants. Freebird Investments LLC serves as the general partner of Freebird Partners LP. Curtis Huff is the sole member and 100% owner of Freebird Investments LLC, the President of Freebird Partners LP and the Managing Member of Freebird Investments LLC. By virtue of these relationships, each of Freebird Investments LLC and Mr. Huff may be deemed to share beneficial ownership of the securities held of record by Freebird Partners LP. The principal business address of Freebird Partners LP is 2800 Post Oak Blvd, Suite 2000, Houston, Texas 77056. |
| (4) |
Denny Family Partners II, LLC owns 270,583 shares of common stock and the George Denny III 2021 Trust (the “Denny Trust”) owns 4,720,058 shares of common stock. Amos Denny is the managing partner of Denny Family Partners II, LLC and in such capacity has the sole voting and dispositive power over the shares owned by such entity. Amos Denny disclaims beneficial ownership of the shares held by Denny Family Partners II, LLC except to the extent of his pecuniary interest therein. The Denny Trust has four trustees who share voting and dispositive power over the shares owned by the Denny Trust. Each of the trustees disclaims beneficial ownership of the shares held by the Denny Trust except to the extent of their respective pecuniary interest therein, if any. The address for each of Denny Family Partners II, LLC and Denny Trust is PO Box 423, Poland, ME 04274. The number of common shares beneficially owned consists of (i) 4,940,188 shares of common stock and (ii) 14,097 shares of common stock issuable upon the conversion of shares of Series A convertible preferred stock (assuming a conversion rate of 5.0583 per share). |
| (5) | Represents outstanding shares of common stock. David Laughlin is the manager of IAF, LLC and has sole voting and dispositive power over the shares held by such entity. Mr. Laughlin disclaims beneficial ownership of the shares held by IAF, LLC except to the extent of his pecuniary interest therein. IAF LLC’s address is 115 Church Street, Charleston, SC 29401. |
|
|
| (6) | Includes (i) 2,478,881 shares of common stock held by Regolith Capital Investments LP (“Regolith”) and (ii) 162,933 shares of common stock held by Shameek Konar. Mr. Konar and his spouse are the General Partner of Regolith. By virtue of these relationships, each of Mr. Konar and his spouse may be deemed to share beneficial ownership of the shares held by Regolith. Regolith’s address is 10608 Stoppard View Way, Knoxville, TN, 37922. |
| (7) | Represents shares of common stock issuable upon exercise of options. |
| (8) | Includes 32,391 shares of common stock issuable upon exercise of options. |
| (9) | Includes 893,711 shares of common stock issuable upon exercise of options. |
Securities Authorized for Issuance under Equity Compensation Plans
The following table contains information as of December 31, 2024 with respect to compensation plans under which our equity securities are authorized for issuance.
| Equity Compensation Plan Information | ||||||||||||
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by securityholders(1) | 854,682 | $ | 3.06 | 380,108 | ||||||||
| Equity compensation plans not approved by securityholders(2) | 1,774,826 | $ | 9.69 | 71,363 | ||||||||
| Total | 2,629,508 | $ | 7.53 | 451,471 | ||||||||
| (1) | At our 2021 annual meeting of stockholders, our stockholders approved a restatement of the Eterna Therapeutics Inc. Restated 2020 Stock Incentive Plan (the “Restated 2020 Plan”). The Restated 2020 Plan is a broad-based incentive plan, which allows for the grant of stock options, restricted stock, restricted stock units, performance awards, unrestricted stock awards and similar kinds of equity-based compensation to employees, directors, consultants and prospective employees. |
| (2) | In May 2021, our board of directors adopted our 2021 Inducement Stock Incentive Plan (the “2021 Inducement Plan”). The 2021 Inducement Plan was adopted without stockholder approval pursuant to Section 711 of the Company Guide of the NYSE American LLC, the stock exchange on which our common stock was listed at the time the 2021 Inducement Plan was adopted by our board of directors. The 2021 Inducement Plan provides for the grant of equity-based awards, including non-qualified stock options, performance shares, performance units, restricted stock, restricted stock units, and stock appreciation rights. The awards available for grant under the 2021 Inducement Plan are available only to new employees and incentive stock options may not be issued under the 2021 Inducement Plan. |
| ITEM 13. | Certain Relationships and Related Transactions, and Director Independence |
Except as described in Note 11 (Related Party Transactions) to the consolidated financial statements of this Annual Report on Form 10-K, which is incorporated by reference into this Item 13, since January 1, 2023, there has not been nor are there currently proposed any transactions or series of similar transactions to which we were or are to be a party in which the amount involved exceeds the lesser of $120,000 or one percent (1%) of the average of our total assets at year-end for the last two completed fiscal years and in which any director, executive officer, holder of more than 5% of the common stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
Related Party Transaction Policy
Our audit committee is responsible for the review, approval, or ratification of any potential conflict of interest transaction involving any of our directors or executive officers, director nominees, any person known by us to be the beneficial owner of more than 5% of our outstanding capital stock, or any family member of or related party to such persons, including any transaction required to be reported under Item 404(a) of Regulation S-K promulgated by the SEC.
|
|
In reviewing any such proposed transaction, our audit committee is tasked with considering all relevant facts and circumstances, including the commercial reasonableness of the terms, the benefit or perceived benefit, or lack thereof, to us, opportunity costs of alternate transactions, the materiality and character of the related person’s direct or indirect interest and the actual or apparent conflict of interest of the related person.
Under our policy, employees are required to report any material transaction or relationship that could result in a conflict of interest to our compliance officer.
All transactions disclosed in Note 11 (Related Party Transactions) to the consolidated financial statements of this Annual Report on Form 10-K were approved by our audit committee in accordance with our related party transaction policy.
Director Independence
Our board of directors undertook a review of the independence of each director. Based on information provided by each director concerning his or her background, employment, and affiliations, our board of directors determined that our board of directors meets independence standards under the applicable rules and regulations of the SEC and the listing standards of Nasdaq. Our board of directors has affirmatively determined that all of our current directors are “independent” as defined in the listing standards of Nasdaq, other than Mr. Luther, who is also an employee. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our Company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
| ITEM 14. | Principal Accounting Fees and Services |
Fees and Services of Independent Registered Public Accounting Firm
The table below summarizes the fees billed to us by Grant Thornton for each of the last two fiscal years.
| Year | Audit Fees | Audit- Related Fees | Tax Fees | All Other Fees | Total | |||||||||||||||
| 2023 | $ | 516,224 | $ | — | $ | — | $ | — | $ | 516,750 | ||||||||||
| 2024 | $ | 399,130 | $ | — | $ | — | $ | — | $ | 399,130 | ||||||||||
Audit Fees. Audit fees consist of fees for professional services rendered for the audit of our consolidated financial statements (including tax services performed to fulfill the auditor’s responsibility under generally accepted auditing standards), reviews of the interim financial statements included in Forms 10-Q and for services that are normally provided by the auditor in connection with statutory and regulatory filings or engagements.
Audit-Related Fees. Audit-related fees consist of fees for assurance and related services (e.g., due diligence) that are reasonably related to the performance of the audit or review of our financial statements and are not reported under audit fees. The nature of those services is comprised of services for employee benefit plan audits, due diligence related to mergers and acquisitions, accounting consultations and audits in connection with proposed or consummated acquisitions, internal control reviews, attest services related to financial reporting that are not required by statute or regulation, and consultation concerning financial accounting and reporting standards.
Tax Fees. Tax fees consist of fees for professional services rendered for tax compliance, tax consulting and tax planning.
All Other Fees. All other fees are fees for products and services other than services in respect of which the fees are reported as audit, audit-related or tax fees.
Policy for Approval of Audit and Permitted Non-Audit Services
All audit and permissible non-audit services provided by the independent auditors are pre-approved by the Audit Committee (or the Chair of the Audit Committee, pursuant to a delegation of authority). These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to one year and any pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget. The independent auditors and management are required to periodically report to the Audit Committee regarding the extent of services provided by the independent auditors in accordance with this pre-approval, and the fees for the services performed to date. The Audit Committee may also pre-approve particular services on a case-by-case basis.
|
|
PART IV
| ITEM 15. | Exhibits, Financial Statement Schedules |
(a) The following documents are filed as a part of this Annual Report on Form 10-K:
(1) Consolidated Financial Statements. The consolidated financial statements of the Company and its consolidated subsidiaries are set forth in the “Index to Consolidated Financial Statements” on page F-1.
(2) Financial Statement Schedules. None
(3) Exhibits. The following exhibits are submitted with this Annual Report on Form 10-K or, where indicated, incorporated by reference to other filings.
|
|
|
|
| * | Indicates management contract or compensatory plan. |
| ** | Pursuant to Item 601(a)(5) of Regulation S-K, schedules and similar attachments to this exhibit have been omitted because they do not contain information material to an investment or voting decision and such information is not otherwise disclosed in such exhibit. The Company will supplementally provide a copy of any omitted schedule or similar attachment to the U.S. Securities and Exchange Commission or its staff upon request. |
| # | Pursuant to Regulation S-K Item 601(b)(2), certain exhibits and schedules to this exhibit have been omitted. The Company agrees to furnish supplementally a copy of any omitted exhibit or schedule to the SEC upon its request. |
| ^ | Pursuant to Item 601(b)(10) of Regulation S-K, certain confidential portions of this exhibit were omitted by means of marking such portions with an asterisk because such information is both not material and is the type that the Company treats as private or confidential. |
| ITEM 16. | Form 10-K Summary |
None.
|
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ETERNA THERAPEUTICS INC. | ||
| Date: March 12, 2025 | By: | /s/ Sanjeev Luther |
| Sanjeev Luther | ||
|
President, Chief Executive Officer, and Director (Principal Executive Officer) |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/ Sanjeev Luther | President, Chief Executive Officer, and Director (Principal Executive Officer) | March 12, 2025 | ||
| Sanjeev Luther | ||||
| /s/ Sandra Gurrola | Senior Vice President of Finance (Principal Financial Officer and Principal Accounting Officer) | March 12, 2025 | ||
| Sandra Gurrola | ||||
| /s/ James Bristol | Chairman of the Board | March 12, 2025 | ||
| James Bristol | ||||
| /s/ Peter Cicala | Director | March 12, 2025 | ||
| Peter Cicala | ||||
| /s/ Elena Ratner | Director | March 12, 2025 | ||
| Elena Ratner | ||||
| /s/ William Wexler | Director | March 12, 2025 | ||
| William Wexler |
|
|
ETERNA THERAPEUTICS INC. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F- |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Eterna Therapeutics Inc.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Eterna Therapeutics Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of December 31, 2024 and 2023, the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Going concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company incurred a net loss of approximately $44.5 million during the year ended December 31, 2024, and had an accumulated deficit of approximately $231.5 million as of December 31, 2024. These conditions, along with other matters as set forth in Note 2, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical audit matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2022.
Iselin, New Jersey
March
12, 2025
| F- |
ETERNA THERAPEUTICS INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value amounts)
| December
31, 2024 |
December
31, 2023 |
|||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 1,729 | $ | 7,575 | ||||
| Other receivables | 437 | 425 | ||||||
| Prepaid expenses and other current assets | 186 | 1,599 | ||||||
| Total current assets | 2,352 | 9,599 | ||||||
| Restricted cash | - | 4,095 | ||||||
| Property and equipment, net | 85 | 493 | ||||||
| Right-of-use assets - operating leases | 670 | 32,781 | ||||||
| Goodwill | 2,044 | 2,044 | ||||||
| Other assets | 118 | 120 | ||||||
| Total assets | $ | 5,269 | $ | 49,132 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,721 | $ | 1,067 | ||||
| Accrued expenses | 1,007 | 1,893 | ||||||
| Income taxes payable | 3 | 2 | ||||||
| Operating lease liabilities, current | 207 | 2,216 | ||||||
| Due to related party, current | - | 1,205 | ||||||
| Deferred revenue, current | - | 190 | ||||||
| Total current liabilities | 2,938 | 6,573 | ||||||
| Convertible notes, net | - | 6,773 | ||||||
| Warrant liabilities | 1 | 116 | ||||||
| Operating lease liabilities, non-current | 477 | 32,854 | ||||||
| Deferred revenue, non-current | - | 392 | ||||||
| Contingent consideration liability | 41 | 107 | ||||||
| Other liabilities | 111 | 84 | ||||||
| Total liabilities | 3,568 | 46,899 | ||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $0.005 par value, 1,000 shares authorized, 156 designated and outstanding of Series A convertible preferred stock at December 31, 2024 and 2023, $156 liquidation preference | 1 | 1 | ||||||
| Common stock, $0.005 par value, 100,000 shares authorized at December 31, 2024 and 2023; 51,386 and 5,410 issued and outstanding at December 31, 2024 and 2023, respectively | 257 | 27 | ||||||
| Additional paid-in capital | 232,979 | 189,186 | ||||||
| Accumulated deficit | (231,536 | ) | (186,981 | ) | ||||
| Total stockholders’ equity | 1,701 | 2,233 | ||||||
| Total liabilities and stockholders’ equity | $ | 5,269 | $ | 49,132 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
ETERNA THERAPEUTICS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| Year ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Revenue | $ | 582 | $ | 68 | ||||
| Cost of revenues | 96 | 236 | ||||||
| Gross profit (loss) | 486 | (168 | ) | |||||
| Operating expenses: | ||||||||
| Research and development | 4,604 | 5,920 | ||||||
| General and administrative | 13,132 | 14,587 | ||||||
| Gain on lease termination | (1,576 | ) | - | |||||
| Acquisition of Exacis in-process research and development | - | 460 | ||||||
| Total operating expenses | 16,160 | 20,967 | ||||||
| Loss from operations | (15,674 | ) | (21,135 | ) | ||||
| Other (expense) income, net: | ||||||||
| Loss on extinguishment of debt | (22,440 | ) | - | |||||
| Change in fair value of convertible notes | 1,017 | - | ||||||
| Change in fair value of bridge notes derivative liability | (1,459 | ) | - | |||||
| Change in fair value of warrant liabilities | 414 | 215 | ||||||
| Change in fair value of contingent consideration | 66 | 118 | ||||||
| Loss on non-controlling investment | - | (59 | ) | |||||
| Interest income | 249 | 138 | ||||||
| Interest expense | (6,752 | ) | (614 | ) | ||||
| Other income (expense), net | 70 | (334 | ) | |||||
| Total other expense, net | (28,835 | ) | (536 | ) | ||||
| Loss before income taxes | (44,509 | ) | (21,671 | ) | ||||
| (Provision) benefit for income taxes | (30 | ) | 3 | |||||
| Net loss | (44,539 | ) | (21,668 | ) | ||||
| Series A preferred stock dividend | (16 | ) | (16 | ) | ||||
| Net loss attributable to common stockholders | $ | (44,555 | ) | $ | (21,684 | ) | ||
| Net loss per common share - basic and diluted | $ | (3.26 | ) | $ | (4.08 | ) | ||
| Weighted average shares outstanding - basic and diluted | 13,647 | 5,314 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
ETERNA THERAPEUTICS INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
For the years December 31, 2024 and 2023
(In thousands)
| Series A Preferred Stock | Common Stock | Additional Paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||
| Balances at January 1, 2023 | 156 | $ | 1 | 5,127 | $ | 26 | $ | 177,377 | $ | (165,297 | ) | $ | 12,107 | |||||||||||||||
| Issuance of common stock in connection with Exacis asset acquisition | - | - | 69 | - | 208 | - | 208 | |||||||||||||||||||||
| Issuance of common stock related to stock purchase agreement with Lincoln Park Capital Fund, LLC, net | - | - | 214 | 1 | 579 | - | 580 | |||||||||||||||||||||
| Issuance of warrants in connection with convertible notes financing | - | - | - | - | 9,014 | - | 9,014 | |||||||||||||||||||||
| Repricing of warrants in connection with convertible notes financing | - | - | - | - | 766 | - | 766 | |||||||||||||||||||||
| Cash dividends to Series A preferred stockholders | - | - | - | - | - | (16 | ) | (16 | ) | |||||||||||||||||||
| Stock-based compensation | - | - | - | - | 1,242 | - | 1,242 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (21,668 | ) | (21,668 | ) | |||||||||||||||||||
| Balances at December 31, 2023 | 156 | $ | 1 | 5,410 | $ | 27 | $ | 189,186 | $ | (186,981 | ) | $ | 2,233 | |||||||||||||||
| Issuance of note warrants | - | - | - | - | 720 | - | 720 | |||||||||||||||||||||
| Fair value of forward sale contract pursuant to common stock offering | - | - | - | - | 576 | - | 576 | |||||||||||||||||||||
| Reclassification of warrants to liability | - | - | - | - | (11,244 | ) | - | (11,244 | ) | |||||||||||||||||||
| Issuance of common stock in exchange of convertible notes | - | - | 28,351 | 142 | 31,045 | - | 31,187 | |||||||||||||||||||||
| Issuance of common stock in exchange of warrants | - | - | 9,951 | 50 | 10,895 | - | 10,945 | |||||||||||||||||||||
| Issuance of common stock and prefunded warrants upon | ||||||||||||||||||||||||||||
| the conversion of bridge notes | - | - | 6,244 | 31 | 9,247 | - | 9,278 | |||||||||||||||||||||
| Issuance of common stock and prefunded warrants in | ||||||||||||||||||||||||||||
| connection with private placement, net | - | - | 1,402 | 7 | 995 | - | 1,002 | |||||||||||||||||||||
| Issuance of common stock to consultant for services | - | - | 17 | - | 23 | - | 23 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 1,520 | - | 1,520 | |||||||||||||||||||||
| Issuance of common stock to Series A preferred stockholders | ||||||||||||||||||||||||||||
| in lieu of cash dividends | - | - | 11 | - | 16 | (16 | ) | - | ||||||||||||||||||||
| Net loss | - | - | - | - | - | (44,539 | ) | (44,539 | ) | |||||||||||||||||||
| Balances at December 31, 2024 | 156 | $ | 1 | 51,386 | $ | 257 | $ | 232,979 | $ | (231,536 | ) | $ | 1,701 | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
ETERNA THERAPEUTICS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| For
years ended December 31, |
||||||||
| 2024 | 2023 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (44,539 | ) | $ | (21,668 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 139 | 84 | ||||||
| Stock-based compensation | 1,520 | 1,242 | ||||||
| Amortization of right-of-use asset | 1,499 | 1,039 | ||||||
| Gain on lease termination | (1,576 | ) | - | |||||
| Accrued interest expense | 174 | 176 | ||||||
| Paid-in-kind interest expense | 1,261 | 113 | ||||||
| Amortization of debt discount and debt issuance costs | 5,259 | 303 | ||||||
| Loss on extinguishment of debt | 22,440 | - | ||||||
| Change in fair value of convertible notes | (1,017 | ) | - | |||||
| Change in fair value of bridge notes derivative liability | 1,459 | - | ||||||
| Change in fair value of warrant liabilities | (414 | ) | (215 | ) | ||||
| Change in fair value of contingent consideration liability | (66 | ) | (118 | ) | ||||
| Commitment shares issued to Lincoln Park Capital, LLC | - | 249 | ||||||
| Loss on shares sold to Lincoln Park Capital, LLC | - | 11 | ||||||
| Non-cash component of acquisition of Exacis in-process research and development | - | 433 | ||||||
| Loss on disposal of fixed assets | - | 1 | ||||||
| Loss on non-controlling investment | - | 59 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Other receivables | (12 | ) | 527 | |||||
| Prepaid expenses and other current assets | 1,319 | (556 | ) | |||||
| Other non-current assets | 2 | 1,014 | ||||||
| Accounts payable and accrued expenses | 183 | (2,898 | ) | |||||
| Operating lease liability | (1,707 | ) | 1,338 | |||||
| Due to related party | (1,205 | ) | (1,750 | ) | ||||
| Deferred revenue | (582 | ) | 582 | |||||
| Other liabilities | 27 | (374 | ) | |||||
| Net cash used in operating activities | (15,836 | ) | (20,408 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of property and equipment | (369 | ) | (19 | ) | ||||
| Proceeds received from the sale of fixed assets | 4 | - | ||||||
| Net cash used in investing activities | (365 | ) | (19 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds received from bridge notes financing | 3,887 | - | ||||||
| Proceeds received from common stock and prefunded warrants offering | 1,137 | - | ||||||
| Fees paid related to the common stock and prefunded warrant offering | (135 | ) | - | |||||
| Proceeds received from convertible notes financings | 1,405 | 16,503 | ||||||
| Fees paid related to convertible notes financings | (34 | ) | (251 | ) | ||||
| Proceeds received under promissory note | - | 1,500 | ||||||
| Payment made on promissory note | - | (1,500 | ) | |||||
| Proceeds from sale of common stock pursuant to stock | ||||||||
| purchase agreement with Lincoln Park Capital Fund, LLC | - | 320 | ||||||
| Dividends paid to Series A preferred stockholders | - | (16 | ) | |||||
| Net cash provided by financing activities | 6,260 | 16,556 | ||||||
| Net decrease in cash and cash equivalents | (9,941 | ) | (3,871 | ) | ||||
| Cash, cash equivalents and restricted cash at beginning of period | 11,670 | 15,541 | ||||||
| Cash, cash equivalents and restricted cash at end of period | $ | 1,729 | $ | 11,670 | ||||
| Supplemental disclosures of cash flow information: | ||||||||
| Cash paid during the period for: | ||||||||
| Interest | $ | 48 | $ | 20 | ||||
| Income taxes | $ | 2 | $ | 4 | ||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Exchange of warrants for common stock | $ | 10,945 | $ | - | ||||
| Exchange of convertible notes for common stock | $ | 31,187 | $ | - | ||||
| Conversion of bridge notes for common stock | $ | 9,278 | $ | - | ||||
| Reclassification of warrants to liabilities | $ | 11,244 | $ | - | ||||
| Note warrants issued | $ | 755 | $ | 9,219 | ||||
| Unpaid fees incurred in connection with the convertible note financings | $ | 32 | $ | 116 | ||||
| Paid in-kind interest added to convertible notes principal | $ | 1,447 | $ | 113 | ||||
| Repricing of warrants in connection with the December 2023 financing | $ | - | $ | 766 | ||||
| Adjustment to lease liability and ROU asset due to remeasurement | $ | 4,245 | $ | (1,620 | ) | |||
| Stock issued to Series A preferred stockholders in lieu of cash dividend | $ | 16 | $ | - | ||||
| Initial measurement of ROU assets | $ | - | $ | 34,410 | ||||
| Initial measurement of lease liability | $ | - | $ | 34,170 | ||||
| Accrual for purchase of property and equipment | $ | - | $ | 323 | ||||
| Contingent consideration for Exacis asset acquisition | $ | - | $ | 225 | ||||
| Issuance of common stock for Exacis asset acquisition | $ | - | $ | 208 | ||||
| Reconciliation of cash, cash equivalents and restricted cash at end of period: | ||||||||
| Cash and cash equivalents | $ | 1,729 | $ | 7,575 | ||||
| Restricted cash | - | 4,095 | ||||||
| Total cash, cash equivalents and restricted cash at end of period | $ | 1,729 | $ | 11,670 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
ETERNA THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the Years Ended December 31, 2024 and 2023
1) Organization and Description of Business Operations
Eterna Therapeutics Inc. (the “Company”) is a preclinical-stage synthetic allogeneic iMSC therapy company. Its vision is to improve the lives of patients with difficult-to-treat diseases through innovative, effective, and safe, but accessible cellular therapies, and its mission is to develop allogenic off-the-shelf cellular therapies, leveraging induced pluripotent stem cell (“iPSC”)-derived mesenchymal stem cells (“iMSCs”) to target solid tumors and autoimmune diseases.
As used herein, the “Company” or “Eterna” refers collectively to Eterna and its consolidated subsidiaries (Eterna Therapeutics LLC, Novellus, Inc. and Novellus Therapeutics Limited) unless otherwise stated or the context otherwise requires.
2) Liquidity and Capital Resources
The Company has incurred significant operating losses and has an accumulated deficit as a result of its efforts to develop product candidates and provide general and administrative support for operations. As of December 31, 2024, the Company had a cash balance of approximately $1.7 million and an accumulated deficit of approximately $231.5 million. For the year ended December 31, 2024, the Company incurred a net loss of $44.5 million, which includes a $22.4 million non-cash charge for loss on extinguishment of debt, and the Company used cash of $15.8 million in operating activities.
In October 2022, the Company entered into a sublease for approximately 45,500 square feet of office and laboratory space in Somerville, Massachusetts. Pursuant to the sublease, the Company delivered to the sublessor a security deposit in the form of a letter of credit in the amount of $4.1 million. The letter of credit was issued by the Company’s commercial bank, which required that the Company cash collateralize the letter of credit by depositing $4.1 million in a restricted cash account with such bank.
On August 5, 2024, the sublessor drew down on the letter of credit for the full $4.1 million to cover past due rent, plus penalties and interest. On August 9, 2024, the Company and the sublessor entered into a sublease termination agreement, effective August 31, 2024. See Note 8 for additional information regarding the sublease and sublease termination agreement.
In April 2023, the Company entered into a standby equity purchase agreement (the “SEPA”) and a registration rights agreement with Lincoln Park Capital Fund, LLC (“Lincoln Park”), pursuant to which Lincoln Park committed to purchase up to $10.0 million of the Company’s common stock in an “equity line” financing arrangement. During the year ended December 31, 2023, the Company issued and sold approximately 214,000 shares of common stock under the SEPA for gross proceeds of $0.3 million. No shares were sold under the SEPA during the year ended December 31, 2024.
In July and December 2023, the Company received $16.5 million in aggregate gross proceeds from the issuance of convertible notes, and on January 11, 2024 it received $1.4 million in gross proceeds from the issuance of additional convertible notes. On September 24, 2024, the Company received $3.9 million in aggregate gross proceeds from the issuance of bridge notes, and on October 29, 2024, the Company received $1.1 million in gross proceeds from the sale of shares of the Company’s common stock and prefunded warrants. See Note 6 for additional information regarding these financings.
On March 11, 2025, the Company received $1.5 million in exchange for the issuance of a promissory note with an aggregate principal amount of $1.5 million to an investor. See Note 19 for more information on this subsequent event.
In connection with preparing the accompanying consolidated financial statements as of and for the year ended December 31, 2024, the Company’s management concluded that there is substantial doubt regarding the Company’s ability to continue as a going concern because it does not expect to have sufficient cash or working capital resources to fund operations for the twelve-month period subsequent to the issuance date of these consolidated financial statements. The Company will need to raise additional capital, which could be through the sales of shares of its common stock under the SEPA, public or private equity offerings, debt financings, out-licensing the Company’s intellectual property, strategic partnerships or other means. Other than the SEPA, the Company currently has no arrangements for capital, and no assurances can be given that it will be able to raise capital when needed, on acceptable terms, or at all.
The accompanying consolidated financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The accompanying consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
| F- |
3) Basis of Accounting Presentation and Summary of Significant Accounting Policies
Basis of Accounting Presentation
The consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”). All significant intercompany balances and transactions have been eliminated in consolidation.
Summary of Significant Accounting Policies
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect: (a) the reported amounts of assets and liabilities; (b) disclosure of contingent assets and liabilities at the date of the consolidated financial statements; (c) the reported amounts of expenses during the reporting period; and (d) the reported amount of the fair value of assets acquired in connection with business combinations. On an ongoing basis, the Company evaluates its estimates, including those related to the recoverability and useful lives of long-lived assets; stock-based compensation assumptions; valuation assumptions of warrants and liabilities associated with the September 2024 Transactions; contingencies; contingent consideration and the provision for income taxes, including the valuation allowance. The Company bases its estimates on a combination of historical experience and various other assumptions that it believes are reasonable under the circumstances. Actual results may differ materially from these estimates.
Cash, Cash Equivalents and Restricted Cash
The Company classifies highly liquid investments with a remaining contractual maturity at date of purchase of three months or less as cash equivalents. The Company had no cash equivalents as of December 31, 2024 or 2023.
Restricted cash as of December 31, 2023 consisted of a cash collateralization of $4.1 million for a security deposit in the form of a letter of credit issued by the Company’s commercial bank and delivered to the sublessor of office and laboratory space the Company subleases in Somerville, Massachusetts.
Property and Equipment
Property and equipment are recorded at cost and are depreciated over their estimated useful lives using the straight-line method. Laboratory and manufacturing equipment are depreciated over an estimated useful life of seven years. Leasehold improvements are depreciated over the shorter of their estimated useful life, or the lease term. Furniture and fixtures are depreciated over an estimated useful life of five years. Computer equipment are depreciated over an estimated useful life of three years. Upon retirement or other disposition of these assets, the cost and related accumulated depreciation of these assets are removed from the accounts and the resulting gain or losses are reflected in the results of operations. Expenditures for maintenance and repairs are charged to operations. Renewals and betterments are capitalized.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of identifiable assets acquired and liabilities assumed. Goodwill is not amortized but is tested for impairment annually or more frequently if events occur or circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, macroeconomic conditions, industry and market considerations, cost factors, overall financial performance and other relevant events. Management evaluates the Company as a single reporting unit, therefore, goodwill is tested for impairment at the entity level. Goodwill is tested for impairment as of December 31st of each year, or more frequently as warranted by events or changes in circumstances mentioned above. Accounting guidance also permits an optional qualitative assessment for goodwill to determine whether it is more likely than not that the carrying value of a reporting unit exceeds its fair value. If, after this qualitative assessment, the Company determines that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, then no further quantitative testing will be necessary. A quantitative assessment is performed if the qualitative assessment results in a more likely than not determination or if a qualitative assessment is not performed. The quantitative assessment considers whether the carrying amount of a reporting unit exceeds its fair value, in which case an impairment charge is recorded to the extent the reporting unit’s carrying value exceeds its fair value.
| F- |
Revenue Recognition
The Company recognizes revenue under ASC 606, Revenue from Contracts with Customers (“ASC 606”) when a customer obtains control of promised services or goods in an amount that reflects the consideration to which the Company expects to receive in exchange for those goods or services.
In general, the Company applies the following steps when recognizing revenue from contracts with customers: (i) identify the contract, (ii) identify the performance obligations, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations and (v) recognize revenue when a performance obligation is satisfied. Recognition of revenue is driven by satisfaction of the performance obligations using one of two methods: revenue is either recognized over time or at a point in time. Contracts containing multiple performance obligations classify those performance obligations into separate units of account either as standalone or combined units of account. Allocation of revenue to individual elements that qualify for separate accounting is based on the separate selling prices determined for each component, and total contract consideration is then allocated across the components of the arrangement. If separate selling prices are not available, the Company will use its best estimate of such selling prices, consistent with the overall pricing strategy and after consideration of relevant market factors.
The Company estimates the amount of consideration it expects to recognize as revenue that is not probable of having a significant reversal of such recognized revenue, and it places a constraint on the remaining contractual consideration. As it becomes evident that the constrained amounts are no longer at risk of a significant reversal of revenue, the Company will remove the constraint from the related revenue and recognize a cumulative catch-up adjustment to revenue in the period in which the constraint was removed.
The Company had one revenue generating contract relating to an option and license agreement as well as certain development activities. See Note 5.
Contract Assets:
A contract asset is an entity’s right to payment for goods and services already transferred to a customer if that right to payment is conditional on something other than the passage of time. Generally, an entity will recognize a contract asset when it has fulfilled a contract obligation but must perform other obligations before being entitled to payment. Contract assets consist primarily of the cost of project contract work performed by third parties whereby the Company expects to recognize any related revenue at a later date, upon satisfaction of the contract obligations. The Company had no contract assets as of December 31, 2024 or 2023.
Contract Liabilities:
Contract liabilities consist primarily of consideration received, usually in the form of payment, on project work to be performed whereby the Company expects to recognize the related revenue at a later date, upon satisfaction of the contract obligations. As of December 31, 2023, contract liabilities were $0.6 million and were recognized as deferred revenue in the accompanying consolidated balance sheet. The Company recognized $0.6 million and $0.1 million of revenue during the years ended December 31, 2024 and 2023, respectively, from contract liabilities that arose in 2023. There were no contract liabilities that arose during the year ended December 31, 2024, and there was no contract liabilities balance as of December 31, 2024.
Research and Development
The Company expenses its research and development costs as incurred. Research and development expenses consist of costs incurred for company-sponsored research and development activities. Upfront payments and milestone payments made for the licensing of technology are expensed as research and development in the period in which they are incurred if the technology is not expected to have any alternative future uses other than the specific research and development project for which it was intended.
The major components of research and development costs include salaries and employee benefits, stock-based compensation expense, supplies and materials, preclinical study costs, expensed licensed technology, consulting, scientific advisors and other third-party costs, as well as allocations of various overhead costs related to our product development efforts.
The Company has contracted with third parties to perform various studies. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. The Company accrues for third party expenses based on estimates of the services received and efforts expended during the reporting period. If the actual timing of the performance of the services or the level of effort varies from the estimate, the accrual is adjusted accordingly. The expenses for some third-party services may be recognized on a straight-line basis if the expected costs are expected to be incurred ratably during the period. Payments under the contracts depend on factors such as the achievement of certain events or milestones, the allocation of responsibilities among the parties to the agreement, and the completion of portions of the preclinical study or similar conditions.
| F- |
Income Taxes
The Company records deferred tax liabilities and assets based on the differences between the consolidated financial statements carrying amounts and the tax basis of assets and liabilities, using enacted tax rates in effect in the years the differences are expected to reverse and establishing a valuation allowance when it was more likely than not that some portion or all of the deferred tax assets would not be realized. Income tax expense consists of the tax payable for the period and the change during the period in deferred tax assets and liabilities.
Tax benefits from uncertain tax positions are recognized only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the consolidated financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate resolution. The Company has no material uncertain tax positions for any of the reporting periods presented.
The Company calculates basic and diluted net loss per share attributable to common stockholders in conformity with the two-class method required for participating securities. The Company’s convertible notes contractually entitled the holders of such notes to participate in dividends but did not contractually require the holders to participate in the Company’s losses. As such, the two-class method is not applicable during periods with a net loss.
Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period, including the weighted average effect of prefunded warrants the Company issued in connection with the September 2024 Transactions (see Note 6 and Note 16), and without consideration for potentially dilutive securities. The Company determined that the exercise of the prefunded warrants requires nominal consideration for the delivery of shares of common stock, and as a result, has considered the 1,879,000 shares underlying the prefunded warrants to be outstanding effective October 29, 2024 for purposes of calculated basic net loss per share.
Diluted net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding, including the weighted average effect of the prefunded warrants, plus dilutive securities. Shares of common stock issuable upon exercise, conversion or vesting of stock options, restricted stock units, warrants and the outstanding Series A convertible preferred stock are considered potential shares of common stock and are included in the calculation of diluted net loss per share using the treasury method when their effect is dilutive. The Company’s convertible notes were also considered potential shares of common stock for the year ended December 31, 2023 and were included in the calculation of diluted net loss per share using the “if-converted” method as of such period, and the more dilutive of either the two-class method or the if-converted method was reported. There were no convertible notes outstanding as of December 31, 2024. Diluted net loss per share is the same as basic net loss per share for periods in which the effect of potentially dilutive shares of common stock is antidilutive.
Segment Reporting
The Company operates within a single reportable operating segment being the research and development of cellular therapies. The Company has identified its president and chief executive officer as its chief operating decision maker (“CODM”), who regularly reviews the Company’s performance and allocates resources based on information reported at the consolidated entity level.
Concentration of Credit Risk
The Company maintains its cash balances in financial institutions located in the United States. Accounts at each institution are insured by the Federal Deposit Insurance Corporation (“FDIC”) up to $250,000. The Company’s cash balances are uninsured for deposit accounts that exceed the FDIC insurance limit.
In the Company’s business, vendor concentrations could be indicative of vulnerabilities in the Company’s supply chain, which could ultimately impact the Company’s ability to continue its research and development activities. For the years ended December 31, 2024 and 2023, there was no vendor concentration related to the Company’s research and development activities.
| F- |
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset, or paid to transfer a liability, in an orderly transaction between willing market participants. A fair value hierarchy has been established for valuation inputs that gives the highest priority to quoted prices in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The fair value hierarchy is as follows:
● Level 1 Inputs – Valued based on quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date.
● Level 2 Inputs – Valued based on inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. These might include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (such as interest rates, volatilities, prepayment speeds, credit risks, etc.) or inputs that are derived principally from or corroborated by market data by correlation or other means.
● Level 3 Inputs – Valued based on inputs for which there is little or no market value, which require the reporting entity to develop its own assumptions.
The carrying amounts reported on the consolidated balance sheet for cash and restricted cash, other receivables, prepaid assets and other current assets, accounts payable and accrued expenses, other current liabilities and other liabilities approximate fair value due to their short maturities.
Leases
The Company accounts for its leases under ASC Topic 842, Leases. Operating lease liabilities represent the present value of lease payments not yet paid. Right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset and are based upon the operating lease liabilities adjusted for prepaid or accrued lease payments, initial direct costs, lease incentives and impairment of operating lease assets. If the interest rate implicit in the lease is not readily determinable, the Company uses the incremental borrowing rates for collateralized borrowings in an amount equal to the lease payments under similar terms.
The Company has elected the practical expedient to not separate non-lease components from the lease components to which they relate and instead account for each as a single lease component for all underlying asset classes. Some leasing arrangements require variable payments that are dependent on usage or may vary for other reasons, such as payments for insurance, tax payments and other miscellaneous costs. The variable portion of payments contemplated in the lease that do not depend on an index or rate are not included in the ROU assets or lease liabilities. Rather, variable payments that do not depend on an index or rate are expensed when the obligation for those payments is incurred and are included in lease expenses. Accordingly, all expenses associated with a lease contract are accounted for as lease expenses.
The Company has also elected not to recognize ROU and lease liabilities for short-term leases that have a term of 12 months or less.
The Company accounts for lease modifications as a separate contract when the modification (i) grants the lessee an additional right of use not included in the original lease contract, and (ii) increases the lease payments commensurate with the stand-alone price for the additional right of use. In this case, the Company would be treated as a new lease and measured in accordance with ASC 842 at the commencement date of the new lease without any impact on the existing lease. Otherwise, the Company accounts for lease modifications as a continuance of the existing lease, in which case, the Company reassesses the lease classification, remeasures the lease liability using an updated discount rate, and unless there is a full or partial termination of the lease, adjusts the ROU asset by the amount of change to the lease liability. For a full or partial lease termination, the lessee reduces the carrying amount of the ROU asset on a basis proportionate to the full or partial termination of the lease, and any difference between the adjustment to the ROU asset and the lease liability is recognized as a gain or loss in the current period.
Commitment and Contingencies
The Company follows ASC 450-20, Loss Contingencies, to report accounting for contingencies. Liabilities for loss contingencies arising from claims, assessments, litigation, fines and penalties, and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
| F- |
The Company recognizes stock-based compensation expense for equity awards granted to employees, directors and certain consultants. The Company estimates the fair value of stock options using the Black-Scholes option pricing model. The fair value of stock options granted is recognized as expense over the requisite service period on a straight-line basis.
Warrants
The Company accounts for common stock warrants as either equity-classified or liability-classified instruments based on an assessment of the specific terms of the warrants and applicable authoritative guidance in ASC 480, Distinguishing Liabilities from Equity, and ASC 815, Derivatives and Hedging. The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, or meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own stock and whether the holders of the warrants could potentially require net cash settlement in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
Convertible Notes
The Company accounts for its convertible notes as a long-term liability equal to the proceeds received from issuance, including the embedded conversion feature, plus any interest paid-in-kind, net of the unamortized debt issuance costs and debt discount on the consolidated balance sheets. The Company evaluates all embedded features contained in the convertible notes, such as the conversion feature, the paid-in-kind feature and the redemption feature in the event of a default, to determine if such features require bifurcation as a derivative. The conversion feature included in the convertible notes is not required to be accounted for separately as an embedded derivative because the conversion feature is considered both indexed to the Company’s own stock and qualifies to be classified in stockholders’ equity. The paid-in-kind feature is considered to be a commitment to originate a loan, and the terms of the additional loans have the same terms as the original debt instrument. Therefore, the paid-in-kind feature qualifies for the scope exception under the applicable accounting guidance and is not required to be bifurcated as a derivative. The redemption feature in the event of a default was determined to be clearly and closely related to the convertible notes and not required to be bifurcated as a derivative.
Proceeds from the sale of convertible notes with stock purchase warrants are allocated to the two elements based on their relative fair values. The portion of the proceeds allocated to warrants are recorded as a debt discount to the convertible note proceeds and presented on a net basis in the consolidated balance sheet. Debt issuance costs directly attributable to the transaction are capitalized and allocated to the convertible notes and warrants in the same manner as the proceeds. The amount of debt issuance costs allocated to the convertible notes represent a reduction of the face value of the convertible note proceeds. The Company amortizes debt issuance costs and debt discounts over the contractual term of the convertible notes, using the effective interest method, as interest expense on the consolidated statements of operations.
Recent Accounting Standards
Recently Adopted Accounting Standards
In June 2022, the Financial Accounting Standard Board (the “FASB”) issued Accounting Standards Update (“ASU”) No. 2022-03, Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions (“ASU 2022-03”). The FASB issued ASU 2022-03 to (1) clarify the guidance in Topic 820, Fair Value Measurement, when measuring the fair value of an equity security subject to contractual restrictions that prohibit the sale of an equity security, (2) to amend a related illustrative example, and (3) to introduce new disclosure requirements for equity related securities subject to contractual sale restrictions that are measured at fair value in accordance with Topic 820. ASU 2022-03 clarifies that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The guidance was effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years with early adoption permitted. The adoption of this ASU did not have a material impact to the Company’s consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting – Improvements to Reportable Segment Disclosures, which provides updates to qualitative and quantitative reportable segment disclosure requirements, including enhanced disclosures about significant segment expenses and increased interim disclosure requirements, among others. ASU No. 2023-07 was effective for fiscal years beginning after December 15, 2023, and interim periods in fiscal years beginning after December 15, 2024. Early adoption was permitted, and the amendments should be applied retrospectively. The adoption of this ASU did not have an impact to the Company’s consolidated financial statements, but it did result in additional disclosures made in the notes to the consolidated financial statements.
| F- |
Recently Issued Accounting Standards to be Adopted
In October 2023, the FASB issued ASU No. 2023-06, Disclosure Improvements – Codification Amendment in Response to the SEC’s Disclosure Update and Simplification Initiative. This ASU modified the disclosure and presentation requirements of a variety of codification topics by aligning them with the SEC’s regulations. The amendments to the various topics should be applied prospectively, and the effective date will be determined for each individual disclosure based on the effective date of the SEC’s removal of the related disclosure. If the SEC has not removed the applicable requirements from Regulation S-X or Regulation S-K by June 30, 2027, then this ASU will not become effective. Early adoption is prohibited. The Company does not expect the amendments in this ASU to have a material impact on its consolidated financial statements.
In December 2023, the FASB issued ASU No. 2023-09, Improvements to Income Tax Disclosures, which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components of the effective tax rate reconciliation, and modifies other income tax-related disclosures. ASU No. 2023-09 is effective for fiscal years beginning after December 15, 2024 and allows for adoption on a prospective basis, with a retrospective option. Early adoption is permitted. The Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In November 2024, the FASB issued ASU No. 2024-03, Income Statement – Reporting Comprehensive Income – Expense Disaggregation Disclosures (Subtopic 220-40). This ASU is intended to improve disclosures about a public business entity’s expenses by requiring disaggregated disclosure, in the notes to the financial statements, of prescribed categories of expenses within relevant income statement captions. ASU No. 2024-03 is effective for fiscal years beginning after December 15, 2026 and interim periods within fiscal years beginning after December 15, 2027 (as clarified in ASU No. 2025-01, Income Statement – Reporting Comprehensive Income – Expense Disaggregation Disclosures (Subtopic 220-40): Clarifying the Effective Date). Early adoption is permitted. The new standard may be applied either on a prospective or retrospective basis. The Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In November 2024, the FASB issued ASU No. 2024-04, Debt – Debt with Conversion and Other Options (Subtopic 470-20): Induced Conversions of Convertible Debt Instruments. This ASU clarifies the requirements for determining whether certain settlements of convertible debt instruments should be accounted for as an induced conversion. ASU No. 2024-04 is effective for annual reporting periods beginning after December 15, 2025 and interim reporting periods within those annual reporting periods. Early adoption is permitted, and the amendments may be applied on either a prospective or retrospective basis. The Company does not expect the amendments in this ASU to have a material impact on its consolidated financial statements.
| 4) | Asset Acquisition |
On April 26, 2023, the Company entered into an asset purchase agreement (the “Exacis Purchase Agreement”), with Dilos Bio (formerly known as Exacis Biotherapeutics Inc. (“Exacis”)), the stockholders party thereto and, with respect to specified provisions therein, Factor Limited. Pursuant to the Exacis Purchase Agreement, the Company acquired from Exacis substantially all of Exacis’ intellectual property assets (the “Exacis Assets”), including all of Exacis’ right, title and interest in and to an exclusive license agreement between Exacis and Factor Limited (the “Purchased License”). The Company assumed none of Exacis’ liabilities, other than liabilities under the Purchased License that accrue subsequent to the closing date. The transactions contemplated by the Exacis Purchase Agreement (the “Exacis Acquisition”) closed on April 26, 2023.
| F- |
In consideration for the Exacis Assets, on the closing date of the transaction, the Company issued to Exacis approximately 69,000 shares of common stock, which shares were subject to a 12-month lockup that expired in April 2024. The shares were issued to Exacis at a price based on the Company having an assumed equity valuation of $75.0 million, divided by the number of issued and outstanding shares of common stock as of the close of business two trading days prior to the closing date. For accounting purposes, the shares issued were valued at $3.00 per share, which was the closing price of the Company’s common stock on the date of issuance. Additionally, the Company agreed to make the following contingent payments:
| (i) | if, at any time during the three-year period commencing on the closing date and ending on the three-year anniversary of the closing date, the Company’s market capitalization equals or exceeds $100.0 million for at least ten consecutive trading days, then the Company will issue to Exacis a number of shares of common stock equal to (x) $2.0 million divided by (y) the quotient of $100.0 million divided by the number of the Company’s then issued and outstanding shares of common stock; | |
| (ii) | if, at any time during the three-year period commencing on the closing date and ending on the three-year anniversary of the closing date, the Company’s market capitalization equals or exceeds $200.0 million for at least ten consecutive trading days, then the Company will issue to Exacis a number of additional shares of common stock equal to (x) $2.0 million divided by (y) the quotient of $200.00 million divided by the number of the Company’s then issued and outstanding shares of common stock (collectively with (i) above, the “Market Cap Contingent Consideration”); and | |
| (iii) | during the five-year period commencing on the closing date and ending on the five-year anniversary of the closing date, the Company will pay or deliver to Exacis 20% of all cash or other consideration (collectively, “License Contingent Consideration”) actually received by the Company during such five-year period from (i) third-party licensees or sublicensees of the intellectual property rights acquired by the Company from Exacis pursuant to the Exacis Purchase Agreement, or (ii) subject to certain exceptions, the sale of such intellectual property rights; provided, that the License Contingent Consideration shall not in any event exceed $45.0 million. |
The Company accounted for the Exacis Acquisition as an asset acquisition because it determined that substantially all of the fair value of the assets acquired was concentrated in the Purchased License. Assets acquired in an asset acquisition are recognized based on their cost to the acquirer and generally allocated to the assets on a relative fair value basis. The Company’s cost for acquiring the Exacis Assets includes the issuance of the Company’s common stock, direct acquisition-related costs and contingent consideration.
The Market Cap Contingent Consideration is indexed to or settled in the Company’s own shares. As a result, the Company classified the Market Cap Contingent Consideration as a liability measured at fair value because the financial instrument embodied a conditional obligation (the Company would only issue the shares on the condition that the market capitalization thresholds are met), and at inception, the monetary value of the obligation is based solely on a fixed monetary amount ($2.0 million of shares for each target), which will be settleable with a variable number of the Company’s shares. The Company used a Monte Carlo simulation model to estimate the fair value of the Market Cap Contingent Consideration as of the acquisition date using the following assumptions:
Schedule of Fair Valuation of Assumptions
| Stock price | $ | 3.00 | ||
| Risk-free rate | 3.58 | % | ||
| Volatility | 100 | % | ||
| Dividend yield | 0 | % | ||
| Expected term | 3.0 years |
See Note 9 for more information on the fair value measurement of the Market Cap Contingent Consideration as of December 31, 2024 and 2023..
The License Contingent Consideration is to be settled in cash and is generally recognized when the liability is probable and estimable. As of the acquisition date, the Company concluded that paying the License Contingent Consideration was not probable or estimable. Therefore, there was no initial liability recognized for the License Contingent Consideration. The Company also did not record a liability at December 31, 2024 or 2023, as the Company continued to conclude that such payment was not probable.
The table below shows the total fair value of the consideration paid for the Exacis Assets (in thousands).
Schedule of Fair Value Measurement of Assets Acquired
| Fair
Value of Consideration |
||||
| Shares issued | $ | 208 | ||
| Contingent consideration | 225 | |||
| Direct costs | 27 | |||
| Total fair value | $ | 460 | ||
The Company allocated 100% of the fair value of the consideration to the Purchased License, which the Company determined is an in-process research and development (“IPR&D”) asset. IPR&D assets acquired through an asset purchase that have no alternative future uses and no separate economic values from their original intended purpose are expensed in the period the cost is incurred. As a result, the Company expensed the fair value of the Purchased License during the year ended December 31, 2023.
On September 24, 2024, in connection with entering into the Exclusive License and Collaboration Agreement (“the Factor L&C Agreement”) with Factor Bioscience Limited (“Factor Limited”), the Purchased License was assigned back to Factor Limited. See Note 11 for more information on the Factor L&C Agreement.
| F- |
| 5) | Contract with Customer |
On February 21, 2023, the Company and Lineage Cell Therapeutics, Inc. (“Lineage”) entered into an exclusive option and license agreement (the “Lineage Agreement”), which provided Lineage with the option (the “Option Right”) to obtain an exclusive sublicense of intellectual property from the Company and to request the Company to develop a customized cell line (the intellectual property that would be sublicensed by Lineage is currently licensed by the Company from Factor Limited). The Lineage Agreement was amended in August 2023 to provide for changes specifically related to the cell line customization activities such as (i) payment terms, (ii) certain definitions, (iii) certain courses of action if the customized cell line selected by Lineage is not successful and (iv) documentation requirements. Lineage paid the Company a $0.3 million non-refundable up-front payment (the “Option Fee”) for the Option Right and paid an initial payment of $0.4 million to commence the cell line customization activities, per the amended payment terms. If Lineage obtained the sublicense, the Company would be entitled to receive additional license fees, including milestone payments and royalties.
On September 24, 2024, the Company and Factor Bioscience (as defined in Note 11) entered into an agreement (the “Lineage Assignment Agreement”) under which the Company assigned the Lineage Agreement to Factor Bioscience. The Company’s rights and obligations under the agreement are now the responsibility of Factor Bioscience.
Payments to the Company related to the Lineage Agreement will be subject to the Lineage Assignment Agreement, which provides for Factor Bioscience paying the Company thirty percent (30%) of all amounts it actually receives from Lineage in the event that Lineage exercises its Option Right. Upon receipt of payment for the customization activities set forth in the Lineage Agreement, Factor Bioscience will pay the Company twenty percent (20%) of all amounts Factor Bioscience receives from Lineage.
Prior to the Lineage Assignment Agreement entered into on September 24, 2024, the Company accounted for the Lineage Agreement under ASC 606 and determined that the Option Right was an unexercised right held by Lineage under the Lineage Agreement at contract inception, as the cell line customization activities and the sublicense were optional purchases at contract inception. These optional purchases of goods and services would be treated as separate contracts if and when Lineage determines that it would make such purchases. Therefore, 100% of the Option Fee was allocated to the Option Right. The Option Fee would remain in deferred revenue until such time that Lineage entered into the sublicense or when the Option Right expired. However, as a result of the Lineage Assignment Agreement, and there being no further obligations regarding the nonrefundable payment related to the Option Right, the Company recognized the $0.3 million Option Right payment in full as revenue during the year ended December 31, 2024.
The Option Right and the cell line customization activities were accounted for as separate contracts, and the Company determined that the amended terms discussed above represented a modification to the cell line customization contract. Because there were no goods or services transferred to Lineage before entering into the amendment, and therefore, no previously recognized revenue, there was no catch-up adjustment to revenue required at the time of the amendment.
Lineage was to make payments to the Company for the cell line customization activities over the development period. The Company would only earn the remaining full amount of the cell line customization fee if it made certain progress towards delivery of the customized cell line. The Company determined that $0.4 million of consideration received could be recognized without the probability of being reversed, and it placed a constraint on the remaining contractual customization fee. The $0.4 million was being recognized equally over the development period. However, as a result of the Lineage Assignment Agreement, and there being no further obligations the Company must fulfill for the customization activities, the Company accelerated the recognition of the remaining deferred revenue and recognized approximately $0.3 million during the year ended December 31, 2024. The Company recognized approximately $0.1 million in revenue during the year ended December 31, 2023 related to the customization activities.
The Company recognized direct labor and supplies used in the customization activities as incurred, which are recorded as a cost of revenue. As provided for in the A&R Factor License Agreement discussed in Note 11, the Company was obligated to pay Factor Limited 20% of any amounts the Company received from a customer that was related to the licensed technology under the A&R Factor License Agreement, which is also recorded as a cost of revenue. For the year ended December 31, 2023, the Company recognized $0.1 million in license fees, which is recorded in cost of revenues, due to Factor Limited. There was no such license fee incurred during the year ended December 31, 2024.
As provided for in the Lineage Assignment Agreement, the Company recorded a receivable of approximately $0.1 million during the year ended December 31, 2024 related to amounts Factor Bioscience owes to the Company related to the customization activities, which is recognized in other income (expense), net in the accompanying consolidated statement of operations. There were no amounts due from Factor Bioscience during the year ended December 31, 2023.
| F- |
| 6) | Debt and Equity Financings |
Promissory Notes
On December 8, 2023, the Company received $1.5 million in exchange for the issuance of 6% promissory note with an aggregate principal amount of $1.5 million to an investor. The promissory note was to mature on January 8, 2024, and interest accrued at a rate of 6.0% per annum, payable at maturity. On December 14, 2023, the Company repaid the $1.5 million of principal and $1,500 of accrued interest due under the promissory note. There are no further obligations under the promissory note.
On March 11, 2025, the Company received $1.5 million in exchange for the issuance of a promissory note with an aggregate principal amount of $1.5 million to an investor. See Note 19 for more information on this subsequent event.
Convertible Notes Financings
On July 14, 2023, the Company received $8.7 million from a private placement in which the Company issued $8.7 million in aggregate principal amount of convertible notes (the “July 2023 Convertible Notes”) and warrants to purchase an aggregate of approximately 6.1 million shares of its common stock (the “July 2023 Warrants”). The Company recognized approximately $0.2 million in fees associated with the transaction.
On December 14, 2023, the Company entered into a purchase agreement with certain purchasers for the private placement of $9.2 million of convertible notes (the “December 2023 Convertible Notes” and together with the July 2023 Convertible Notes, the “Convertible Notes”) and warrants to purchase an aggregate of approximately 9.6 million shares of the Company’s common stock (the “December 2023 Warrants” and together with the July 2023 Warrants, the “Note Warrants”).
There were two closings under the December 14, 2023 purchase agreement – one on December 15, 2023 and the second on January 11, 2024. At the first closing, the Company received $7.8 million and issued $7.8 million of December 2023 Convertible Notes and December 2023 Warrants to purchase approximately 8.1 million shares of its common stock. At the second closing, the Company received $1.4 million and issued $1.4 million of December 2023 Convertible Notes and December 2023 Warrants to purchase approximately 1.5 million shares of its common stock.
See Note 16 for more information on the Note Warrants.
The interest rates for the July 2023 Convertible Notes and the December 2023 Convertible Notes were 6% per year and 12% per year, respectively, both of which were payable quarterly in arrears. At the Company’s election, it may pay interest either in cash or in-kind by increasing the outstanding principal amount of the Convertible Notes. The Convertible Notes were to mature on the five-year anniversary of the date of their issuance, unless earlier converted or repurchased. The Company did not have the option to redeem any of the Convertible Notes prior to maturity.
The Company recognized approximately $2.8 million and $0.6 million in interest expense for the years ended December 31, 2024 and 2023 for the Convertible Notes, respectively, which includes both the amortization of debt issuance costs and interest recognized on the Convertible Notes as follows (in thousands):
Schedule of Interest Expense
| Year ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Interest | $ | 1,399 | $ | 291 | ||||
| Debt issuance costs | 1,372 | 303 | ||||||
| Total interest expense | $ | 2,771 | $ | 594 | ||||
The $1.4 million and $0.3 million in interest for the years ended December 31, 2024 and 2023, respectively, were paid in-kind and added to the principal of the Convertible Notes, which became part of the Exchange Transactions discussed below.
| F- |
At the option of the holders, the Convertible Notes may be converted into shares of the Company’s common stock at an initial conversion price of, with respect to the July 2023 Convertible Notes, $2.86 per share and, with respect to the December 2023 Convertible Notes, $1.9194 per share, subject to customary adjustments for stock splits, stock dividends, recapitalization and the like.
In connection with the issuance of the December 2023 Convertible Notes, the Company agreed to reduce the exercise price of the warrants the Company issued in a private placement in December 2022 (the “December 2022 Warrants”) (see Note 16) to purchase an aggregate of approximately 4.4 million shares of the Company’s common stock from $3.28 to $1.43 per share and of the July 2023 Warrants from $2.61 to $1.43 per share. The effect of the reduction of the exercise price of these warrants was approximately $1.6 million and measured as the excess of the fair value of the modified instruments over the fair value of the instruments immediately before they were modified. The change in the fair value of the repriced warrants was considered an issuance cost to the December 2023 Convertible Notes and December 2023 Warrants. As such, the $1.6 million was allocated to each of those respective instruments based on their relative fair values, or approximately $0.8 million to each of the December 2023 Convertible Notes and December 2023 Warrants.
The Company determined that there were no embedded derivatives within the Convertible Notes that required bifurcation from the host agreement. The Company allocated the gross proceeds received, the fees incurred, and as applicable, the impact of repricing the warrants discussed above, over the July 2023 Convertible Notes and July 2023 Warrants and over the December 2023 Convertible Notes and December 2023 Warrants, as applicable, based on their relative fair values as follows (in thousands):
Schedule of Based on Relative Fair Value Allocation of Proceeds and Costs
| Allocation of Proceeds and Costs: | Allocation of | |||||||||||||||||||
| Relative Fair Value |
Allocation Percentage |
Proceeds | Costs | Proceeds, Net |
||||||||||||||||
| July 2023 Convertible Notes | $ | 8,715 | 39.94 | % | $ | 3,481 | $ | (80 | ) | (766) | $ | 3,401 | ||||||||
| July 2023 Warrants | 13,103 | 60.06 | % | 5,234 | (121 | ) | 5,113 | |||||||||||||
| $ | 21,818 | 100.00 | % | $ | 8,715 | $ | (201 | ) | $ | 8,514 | ||||||||||
| Allocation of Proceeds and Costs: | Allocation of | |||||||||||||||||||||||
| December 2023 Closing | Relative Fair Value |
Allocation Percentage |
Proceeds | Costs | Warrant Repricing |
Proceeds, Net |
||||||||||||||||||
| December 2023 Convertible Notes | $ | 9,059 | 48.83 | % | $ | 3,803 | $ | (81 | ) | $ | (766 | ) | $ | 2,956 | ||||||||||
| December 2023 Warrants | 9,495 | 51.17 | % | 3,985 | (85 | ) | (802 | ) | 3,098 | |||||||||||||||
| $ | 18,554 | 100.00 | % | $ | 7,788 | $ | (166 | ) | $ | (1,568 | ) | $ | 6,054 | |||||||||||
| Allocation of Proceeds and Costs: | Allocation of | |||||||||||||||||||
| January 2024 Closing | Relative Fair Value |
Allocation Percentage |
Proceeds | Costs | Proceeds, Net |
|||||||||||||||
| December 2023 Convertible Notes | $ | 1,750 | 46.24 | % | $ | 650 | $ | (31 | ) | $ | 619 | |||||||||
| December 2023 Warrants | 2,035 | 53.76 | % | 755 | (35 | ) | 720 | |||||||||||||
| $ | 3,785 | 100.00 | % | $ | 1,405 | $ | (66 | ) | $ | 1,339 | ||||||||||
The Company estimated the fair values of the Convertible Notes as of July 14, 2023, December 15, 2023 and January 11, 2024 based off a valuation performed by a third-party specialist using a binomial tree model and the following assumptions:
Schedule of Fair Value Assumptions
| Date | Stock Price |
Credit Spread |
Volatility | Risk-Free Rate |
||||||||||||||
| July 2023 Convertible Notes | 7/14/2023 | $ | 2.81 | 2,500 | 108 | % | 4.60 | % | ||||||||||
| December 2023 Convertible Notes | 12/15/2023 | $ | 1.51 | 2,000 | 109 | % | 3.90 | % | ||||||||||
| December 2023 Convertible Notes | 1/11/2024 | $ | 1.75 | 2,000 | 109 | % | 3.90 | % | ||||||||||
| F- |
The fair value of the Note Warrants, all of which qualified for equity classification, was determined using the Black-Scholes pricing model as of each of July 14, 2023, December 15, 2023 and January 11, 2024 using the following assumptions:
| Date | Stock Price |
Exercise Price |
Expected Life |
Volatility | Dividend | Risk-Free Rate |
||||||||||||||||||
| July 2023 warrants | 7/14/2023 | $ | 2.81 | $ | 2.61 | 5 years | 98 | % | 0.00 | % | 4.04 | % | ||||||||||||
| December 2023 warrants | 12/15/2023 | $ | 1.51 | $ | 1.43 | 5 years | 101 | % | 0.00 | % | 3.91 | % | ||||||||||||
| December 2023 warrants | 1/11/2024 | $ | 1.75 | $ | 1.43 | 5 years | 102 | % | 0.00 | % | 3.90 | % | ||||||||||||
The amount of proceeds allocated to the Note Warrants resulted in a corresponding reduction in the carrying value of the respective convertible notes as a debt discount, which is amortized with the debt issuance costs as a component of interest expense based on the effective interest rate method over the contractual terms of the convertible notes.
On October 29, 2024, all of the Convertible Notes were exchanged for common stock pursuant to the Exchange Transactions (as discussed further below) and as part of the September 2024 Transactions (as defined below) that the Company’s stockholders approved at the Company’s annual meeting of stockholders on October 29, 2024 (the “Annual Meeting). As of December 31, 2024, there were no Convertible Notes outstanding.
Bridge Notes Financing
On September 24, 2024, the Company entered into a purchase agreement with certain purchasers for the private placement of $3.9 million of convertible notes (the “Bridge Notes”). The interest rate on the Bridge Notes was 12% per year, payable quarterly in arrears. At the Company’s election, it may pay interest either in cash or in-kind by increasing the outstanding principal amount of the Bridge Notes. The Bridge Notes were to mature on the one-year anniversary of the date of their issuance, unless earlier converted or repurchased. The Company did not have the option to redeem any of the Bridge Notes prior to maturity. The Bridge Notes financing closed on September 24, 2024.
The only conversion event for the Bridge Notes was upon stockholder approval at the Annual Meeting, in which case, 100% of the principal amount of the Bridge Notes plus all accrued and unpaid interest thereon, and interest that would have accrued on the principal amount through December 24, 2024, would automatically convert into shares of the Company’s common stock at a conversion price of $0.50. Otherwise, the Bridge Notes could only be paid in cash upon maturity.
The Company was required to bifurcate the conversion feature from the Bridge Notes and record it as a derivative liability at its fair value. The Company determined the fair value of the derivative liability by taking the difference between the fair value of the Bridge Notes with the conversion feature and without the conversion feature, which resulted in the Company recording a $5.5 million derivative liability, with a corresponding $3.9 million reduction in the carrying value of the Bridge Notes recorded as a debt discount and a $1.6 million charge to expense for the incremental fair value of the derivative liability as of September 24, 2024. The debt discount was amortized as a component of interest expense.
The Company remeasured the fair value of the Bridge Notes derivative liability at each reporting period and recorded a reduction in the liability of $0.2 million for the year ended December 31, 2024.
On October 29, 2024, all of the Bridge Notes were converted to common stock as part of the September 2024 Transactions (as defined below) that the Company’s stockholders approved at the Annual Meeting.
The Company recognized $3.9 million in interest expense for the year ended December 31, 2024 for the Bridge Notes, which includes both the acceleration of the amortized debt issuance costs as a result of the conversion of the Bridge Notes to common stock and interest recognized on the Bridge notes as follows (in thousands):
Schedule of Interest Expense
| Year ended
December 31, |
||||
| 2024 | ||||
| Interest | $ | 45 | ||
| Debt issuance costs | 3,887 | |||
| Total interest expense | $ | 3,932 | ||
There was no interest expense recognized on the Bridge Notes for the year ended December 31, 2023. The interest recognized on the Bridge Notes of less than $0.1 million was paid in-kind and added to the principal of the Bridge Notes as part of the conversion to common stock that occurred in October 2024. As of December 31, 2024, there were no Bridge Notes outstanding.
| F- |
Exchange Transaction
On September 24, 2024, the Company entered into exchange agreements (the “Exchange Agreements”) with the holders of (i) warrants to purchase an aggregate of approximately 4.4 million shares of our common stock the Company issued in December 2022 with an exercise price of $1.43 per share (the “December 2022 Warrants”); (ii) the Note Warrants (and when combined with the December 2022 Warrants, the “Exchanged Warrants”); and (iii) the Convertible Notes. The parties to the Exchange Agreements represented the holders of all the outstanding Convertible Notes and all the outstanding Exchanged Warrants described above except for a December 2022 Warrant to purchase approximately 0.1 million shares of our common stock.
Subject to approval by the Company’s stockholders at the Annual Meeting, under the Exchange Agreements (i) the holders of the Exchanged Warrants agreed to exchange all their warrants for shares of the Company’s common stock at an exchange ratio of 0.5 of a share of common stock for every one share of common stock issuable upon exercise of the applicable warrant (rounded up to the nearest whole number), and (ii) the holders of the Convertible Notes agreed to exchange all their Convertible Notes for shares of the Company’s common stock at an exchange ratio equal to (A) the sum expressed in U.S. dollars of (1) the principal amount of the applicable Convertible Note, plus (2) all accrued and unpaid interest thereon through the date the applicable Convertible Note is exchanged plus (3) all interest that would have accrued through, but not including, the maturity date of applicable Convertible Note if it was outstanding from the date such Convertible Note is exchanged through its maturity date (the sum of (A) totaling approximately $28.4 million), divided by (B) $1.00 (rounded up to the nearest whole number) (the “Exchange Transactions”).
The Company determined that the modifications to the Convertible Notes at September 24, 2024 should be accounted for as an extinguishment of debt because there was at least a 10% change in the cash flows of the modified debt instrument compared to the carrying amount of the original debt instrument, and as such, the difference between the reacquisition price (which includes any premium) and the net carrying amount of the debt being extinguished (which includes any deferred debt issuance costs) should be recognized as a gain or loss when the debt is extinguished.
As of September 24, 2024, prior to entering into the Exchange Agreements, there was approximately $10.1 million of net carrying amount of the Convertible Notes, which was comprised of $19.4 million of principal and accrued interest through such date, offset by approximately $9.3 million of unamortized debt issuance costs. The fair value of the Convertible Notes was $32.0 million and was determined by multiplying approximately 28,351,000 shares the Company would be issuing on October 29, 2024 by the closing stock price of $1.13 per share on September 24, 2024. The difference between the reacquisition price and the net carrying amount of the Convertible Notes being extinguished was approximately $21.9 million. Accordingly, the Company increased the carrying value of the reacquired Convertible Notes to $32.0 million and recognized a loss on extinguishment of debt of approximately $21.9 million. As discussed further below, upon conversion of the Convertible Notes to shares of common stock on October 29, 2024, the Company recorded $1.0 million in income for the change in fair value of the shares of common stock being issued.
Because shareholder approval was required for the Exchange Transactions to occur, the Company determined that the modifications to the Exchanged Warrants resulted in a change in classification from equity to liability. A provision that requires shareholder approval precludes equity classification because such approval is not an input into a fixed-for-fixed valuation model. As a result, the Company recorded the Exchanged Warrants at fair value as of September 24, 2024 by taking the number of shares of common stock issuable from the exchanged warrants multiplied by the closing stock price of $1.13 and reclassifying approximately $11.2 million from equity to warrant liabilities. The Company then marked-to-market the Exchanged Warrants at each reporting period by taking the same quantity of shares multiplied by the closing stock price on such date and for the year ended December 31, 2024, recognized a reduction to the warrant liabilities of $0.3 million.
Equity Financing
On September 24, 2024, the Company entered into a securities purchase agreement (the “SPA”) with certain accredited investors to sell in a private placement an aggregate of approximately 1,517,000 shares of the Company’s common stock (or, in lieu thereof, pre-funded warrants to purchase one share of our common stock) for a purchase price of $0.75 per share of common stock and $0.745 per pre-funded warrant (the “Common Stock Private Placement” and together with the Bridge Notes and the Exchange Transactions, the “September 2024 Transactions”). The closing of the Common Stock Private Placement was conditioned upon receiving stockholder approval at the Annual Meeting.
The SPA represented a forward sale contract obligating the Company to sell a fixed number of shares of its common stock at a fixed price per share upon obtaining shareholder approval at the Annual Meeting. The Company measured the fair value of the forward sale contract as the difference between (A) the fair value of the expected shares to be purchased by the investors as of the date the Company entered into the SPA and (B) the purchase price of the shares and recorded approximately $0.6 million to additional paid-in capital as of September 24, 2024. Because of the concurrent execution of the SPA and the Exchange Agreements, and because the investors in the SPA are also parties to the Exchange Transactions, the $0.6 million was added to the $21.9 million loss on extinguishment of debt discussed above for a total loss of $22.4 million during the year ended December 31, 2024.
| F- |
On October 29, 2024, the Company held its Annual Meeting, the Company’s stockholders approved the September 2024 Transactions, and as a result, the following occurred on October 29, 2024:
| ● | Under the Common Stock Private Placement, the Company issued approximately 1,402,000 shares of common stock and pre-funded warrants to purchase 115,000 shares of common stock and received approximately $1.1 million in gross proceeds from the issuance of such securities. The pre-funded warrants have an exercise price of $0.005 per share, are exercisable at any time and will not expire until exercised in full. | |
| ● | Under the Bridge Notes, approximately $3.0 million of the principal amount of the Bridge Notes plus all accrued and unpaid interest thereon, plus such amount of interest that would have accrued on the principal amount through December 24, 2024, was automatically converted at a conversion price of $0.50 into approximately 6,244,000 shares of the Company’s common stock and approximately $0.9 million of the principal amount of the Bridge Notes plus all accrued and unpaid interest thereon, plus such amount of interest that would have accrued on the principal amount through December 24, 2024, was automatically converted at a conversion price of $0.50 into pre-funded warrants to purchase 1,764,000 shares of common stock. The pre-funded warrants have an exercise price of $0.005 per share, are exercisable at any time and will not expire until exercised in full. As of October 29, 2024, there were no Bridge Notes outstanding. | |
| ● | Under the Exchange Transactions, (i) the holders of the Exchanged Warrants exchanged approximately 19,902,000 warrants for approximately 9,951,000 shares of the Company’s common stock, and (ii) the holders of the Convertible Notes exchanged all their Convertible Notes for approximately 28,351,000 shares of our common stock for a total of 38,302,000 shares of our common stock under the Exchange Transactions. As of October 29, 2024, there were no Convertible Notes outstanding. |
| 7) | Property and Equipment |
Property and equipment consist of the following (in thousands):
Schedule of Property and Equipment
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Laboratory and manufacturing equipment | $ | 28 | $ | 40 | ||||
| Furniture and fixtures | 19 | 19 | ||||||
| Leasehold improvements | - | 274 | ||||||
| Computer equipment and programs | 210 | 274 | ||||||
| Property and equipment, gross | 257 | 607 | ||||||
| Less accumulated depreciation and amortization | (172 | ) | (114 | ) | ||||
| Property and equipment, net | $ | 85 | $ | 493 | ||||
During the year ended December 31, 2024, the Company recognized a loss on disposal of assets of approximately $0.5 million in connection with the sublease termination agreement related to the Somerville, Massachusetts lease, which is recorded as part of the gain on lease termination on the accompanying consolidated statement of operations for the year ended December 31, 2024 (See Note 8 for more details on the sublease termination agreement). During the year ended December 31, 2023, the Company recognized a de minimis loss on disposal of fixed assets.
Depreciation expense was approximately $0.1 million for each of the years ended December 31, 2024 and 2023. No depreciation expense is recorded on fixed assets in process until such time as the assets are completed and are placed into service.
| F- |
| 8) | Leases |
Operating Leases
The Company currently has operating leases for office in the borough of Manhattan in New York, New York, and Cambridge, Massachusetts, which expire in 2026 and 2028, respectively.
In addition, in October 2022, the Company entered into a sublease with a subsidiary of Bristol-Myers Squibb Company, as sublessor (“Sublessor”), for office, laboratory and research and development space of approximately 45,500 square feet in Somerville, Massachusetts. The sublease provided for base rental payments of approximately $0.5 million per month as well as monthly payments for parking and the Company’s share of traditional lease expenses, including certain taxes, operating expenses and utilities. The Company paid the Sublessor a security deposit in the form of a letter of credit in the amount of approximately $4.1 million.
The Sublessor provided the Company with a tenant improvement allowance (“TIA”) of $190 per rentable square foot, or $8.6 million, for assets that were determined to be owned by the sublessor/lessor and considered a reimbursement rather than a lease incentive. As of December 31, 2023, the Company received the entire $8.6 million TIA. The Company incurred out-of-pocket tenant improvements costs of approximately $1.6 million, which was in excess of the $8.6 million TIA. These out-of-pocket expenses were considered non-cash lease payments and were added to the consideration in the contract.
The Company recorded an initial lease liability of $34.1 million and a corresponding ROU asset of $34.4 million during the year ended December 31, 2023. During the years ended December 31, 2023 and 2024, the Company remeasured the lease liability due to changes in out-of-pocket expenses for sublessor/lessor owned assets and timing of rent payments and recorded adjustments to the lease liability and ROU asset of approximately a $1.6 million reduction as of December 31, 2023 and an increase of $4.2 million for the year ended December 31, 2024.
On May 3, 2024, the Company received a notice from the Sublessor regarding past due rent payments of approximately $2.3 million, including amounts related to property taxes and common area maintenance costs, that the Company did not pay for the months of February, March, April and May 2024. Failure to pay the past due rent payments in full, plus approximately $70,000 in late fees and interest, within five business days from the date of the notice constituted an event of default under the sublease.
The Company also did not pay the rent for June, July or August 2024 and, as of August 1, 2024, owed approximately $4.0 million in the aggregate in past due rent. On August 5, 2024, the Sublessor drew down on the letter of credit for the full $4.1 million to cover the approximately $4.0 million of past due rent payments, plus interest and penalties.
On August 9, 2024, the Company and Sublessor entered into a sublease termination agreement, effective August 31, 2024. The sublease was originally scheduled to expire in 2033. Pursuant to the sublease termination agreement, the Company agreed to the following: to surrender and vacate the premises; that the Company’s right, title and interest in all furniture, fixtures and laboratory equipment at the premises will become the property of the sublessor; and that both parties will be released of their obligations under the sublease. As a result of the sublease termination, the Company recognized a gain on lease termination of approximately $1.6 million for the year ended December 31, 2024, which includes a loss on disposal of fixed assets of approximately $0.5 million.
For the years ended December 31, 2024 and 2023, the net operating lease expenses were as follows (in thousands):
Schedule of Net Operating Lease Expense
| Years ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Operating lease expense | $ | 4,447 | $ | 3,399 | ||||
| Sublease income | (84 | ) | (84 | ) | ||||
| Variable lease expense | 893 | 136 | ||||||
| Total lease expense | $ | 5,256 | $ | 3,451 | ||||
| F- |
The tables below show the beginning balances of the operating ROU assets and lease liabilities as of January 1, 2024 and the ending balances as of December 31 2024, including the changes during the period (in thousands).
Schedule of Operating Lease Right-of-use Assets and Liabilities
| Operating
Lease ROU Assets |
||||
| Operating lease ROU assets at January 1, 2024 | $ | 32,781 | ||
| Adjustment to ROU asset for remeasurement of | ||||
| Somerville Sublease liability | 4,245 | |||
| Write-off of Somerville Sublease ROU asset | (34,857 | ) | ||
| Amortization of operating lease ROU assets | (1,499 | ) | ||
| Operating lease ROU assets at December 31, 2024 | $ | 670 | ||
| Operating
Lease Liabilities |
||||
| Operating lease liabilities at January 1, 2024 | $ | 35,070 | ||
| Adjustment to lease liability due to remeasurement | ||||
| of Somerville Sublease | 4,245 | |||
| Accretion of interest for Somerville Sublease | 2,465 | |||
| Write-off of Somerville Sublease liability | (36,924 | ) | ||
| Principal payments on operating lease liabilities | (4,172 | ) | ||
| Operating lease liabilities at December 31, 2024 | 684 | |||
| Less non-current portion | 477 | |||
| Current portion at December 31, 2024 | $ | 207 | ||
As of December 31, 2024, the Company’s operating leases had a weighted-average remaining life of 3.1 years with a weighted-average discount rate of 10.23%. The maturities of the operating lease liabilities are as follows (in thousands):
Schedule of Maturities of Operating Lease Liabilities
| As
of December 31, 2024 |
||||
| 2025 | $ | 274 | ||
| 2026 | 267 | |||
| 2027 | 163 | |||
| 2028 | 82 | |||
| Total payments | 786 | |||
| Less imputed interest | (102 | ) | ||
| Total operating lease liabilities | $ | 684 | ||
Manhattan Sublease
In April 2019, the Company entered into a sublease with an unaffiliated third party (the “Subtenant”), whereby the Subtenant agreed to sublease approximately 999 square feet of space rented by the Company in the borough of Manhattan in New York, New York commencing on May 15, 2019. The term of this sublease expires on October 31, 2026 with no option to extend. Rent payments by the Subtenant under the sublease began on September 1, 2019. The sublease stipulates an annual rent increase of 2.25%. The Subtenant is also responsible for paying to the Company all tenant energy costs, annual operating costs, and annual tax costs attributable to the subleased space during the term of the sublease.
The Company received sublease payments of approximately $0.1 million for each of the years ended December 31, 2024 and 2023, respectively. The Company treats the sublease as a separate lease, as the Company was not relieved of the primary obligation under the related lease. The Company continues to account for the related lease as a lessee and in the same manner as prior to the commencement date of the sublease. The Company accounts for the sublease as a lessor of the lease. The sublease is classified as an operating lease, as it does not meet the criteria of a sale-type or direct financing lease.
| F- |
The following tables shows the future payments the Company expects to receive from the Subtenant over the remaining term of the sublease (in thousands):
Schedule of Future Lease Payments from Sublease Agreement
| As
of December 31, 2024 |
||||
| 2025 | $ | 88 | ||
| 2026 | 75 | |||
| Total payments | $ | 163 | ||
| 9) | Fair Value of Financial Instruments |
The Company issued approximately 343,000 warrants in connection with a private placement during the first quarter of 2022 (the “Q1-22 warrants”), which were determined to be classified as a liability. The Company also recorded the Market Cap Contingent Consideration liability related to the Exacis Acquisition. See Note 4 for more information related to the Exacis Acquisition.
In connection with the Bridge Notes, the Company recorded a derivative liability as of September 24, 2024. In connection with the Exchange Transactions, on September 24, 2024, the Company reclassified the Exchanged Warrants from equity to a liability. See Note 6 for more information related to the Bridge Notes and Exchange Transactions.
The Company uses a Black-Scholes option pricing model to estimate the fair value of the Q1-22 warrant liabilities and a Monte Carlo simulation model to estimate the fair value of the contingent consideration related to the Market Cap Contingent Consideration, both of which are considered a Level 3 fair value measurement.
The Company determined the fair value of the derivative liability by taking the difference between the fair value of the Bridge Notes with the conversion feature and without the conversion feature. Pursuant to the approval of the September 2024 Transactions by the Company’s stockholders at the Annual Meeting, the Bridge Notes were converted to shares of the Company’s common stock, and the outstanding principal and interest of the Bridge Notes as well as the derivative liability were reclassified to equity. As of December 31, 2024, there was no derivative liability balance.
The Company determined the fair value of the Exchanged Warrants as of September 24, 2024 by taking the number of shares of common stock issuable from the Exchanged Warrants multiplied by the closing stock price of $1.13 and reclassified approximately $11.2 million from equity to warrant liabilities.
The Company remeasures the fair value of the warrant liabilities, the Bridge Notes derivative liability and the Market Cap Contingent Consideration at each reporting period and changes in the fair values are recognized in the consolidated statement of operations.
The following tables summarize the liabilities that are measured at fair value as of December 31, 2024 and December 31, 2023 (in thousands):
Schedule of Liabilities Measured at Fair Value
| Description | Level | December
31, 2024 |
December
31, 2023 |
|||||||
| Liabilities: | ||||||||||
| Warrant liabilities - Q1-22 warrants | 3 | $ | 1 | $ | 116 | |||||
| Market Cap Contingent Consideration | 3 | $ | 41 | $ | 107 | |||||
Certain inputs used in Black-Scholes and Monte Carlo models may fluctuate in future periods based upon factors that are outside of the Company’s control. A significant change in one or more of these inputs used in the calculation of the fair value may cause a significant change to the fair value of the Company’s warrant liabilities or contingent consideration liabilities, which could also result in material non-cash gains or losses being reported in the Company’s condensed consolidated statement of operations.
| F- |
The following table presents the changes in the liabilities measured at fair value from January 1, 2024 through December 31, 2024 (in thousands):
Schedule of Changes in Warrant Liabilities
| Warrant Liabilities |
Derivative Liability |
Contingent Consideration |
||||||||||
| Fair value at January 1, 2024 | $ | 116 | $ | - | $ | 107 | ||||||
| Reclassification of Exchanged Warrants from equity to liability | 11,244 | - | - | |||||||||
| Initial measurement of Bridge Notes derivative liability | - | 5,566 | - | |||||||||
| Change in fair value | (414 | ) | (220 | ) | (66 | ) | ||||||
| Reclassification of Exchanged Warrants and Bridge Notes | ||||||||||||
| derivative liability to equity | (10,945 | ) | (5,346 | ) | - | |||||||
| Fair value at December 31, 2024 | $ | 1 | $ | - | $ | 41 | ||||||
Based off valuations performed during 2024 and as of December 31, 2024, the Company recognized a change in fair value of the Market Cap Contingent Consideration of approximately $0.1 million for the year ended December 31, 2024.
The Company remeasured the Bridge Notes derivative liability by taking the difference between the fair value of the Bridge Notes with the conversion feature and without the conversion feature at each reporting period and recorded a $0.2 million credit for the change in fair value during the year ended December 31, 2024.
In connection with the approval of the September 2024 Transactions by the Company’s stockholders at the Annual Meeting on October 29, 2024, the Exchanged Warrants were exchanged for and the Bridge Notes were converted to shares of the Company’s common stock. The liability related to the Exchanged Warrants and the outstanding principal and interest of the Bridge Notes as well as the derivative liability were reclassified to equity. As of December 31, 2024, there were no liability balances related to the derivative liability or the Exchange Warrants.
The table below is provided for comparative purposes only and presents information about the fair value of the Company’s Convertible Notes relative to the carrying values recognized in the condensed consolidated balance sheet as of December 31, 2023 (in thousands).
Schedule of Fair Value and Carrying Values of Convertible Notes
| December 31, 2023 | ||||||||||
| Level | Carrying Value |
Fair Value |
||||||||
| Convertible Notes | 3 | $ | 16,616 | $ | 17,594 | |||||
In connection with the approval of the September 2024 Transactions by the Company’s stockholders at the Annual Meeting on October 29, 2024, the Convertible Notes were exchanged for shares of the Company’s common stock. As of December 31, 2024, there were no Convertible Notes outstanding.
The Company assessed the fair value of the Convertible Notes as of December 31, 2023 using a binomial model, which is considered a Level 3 measurement. The inputs used for the assessment were risk-free rate of 4.07%, expected term of 2.3 years, stock price of $1.80, volatility of 108% and dividend yield of 0% done.
| 10) | Goodwill |
The Company recorded goodwill in the amount of $2.0 million related to a 2018 acquisition that was accounted for as a business combination. The Company performed its annual qualitative assessment as of December 31, 2024, and based on that assessment, the Company was unable to conclude that it was more likely than not that the fair value of the entity exceeded its carrying value as of such date. As a result, the Company performed a step-one quantitative assessment and concluded that the fair value of the reporting unit was greater than the carrying value as of December 31, 2024, and the goodwill was considered not impaired. Therefore, the Company did not recognize an impairment charge during the year ended December 31, 2024. .
The Company performed its annual qualitative assessment as of December 31, 2023, and based on that assessment, the Company determined that it was more likely than not that the fair value of the entity exceeded its carrying value for such year and that the performance of the quantitative impairment test was not required. Therefore, no impairment was required for the year ended December 31, 2023.
| F- |
| 11) | Related Party Transactions |
Agreements with Factor Bioscience Inc. and Affiliates
As of December 31, 2024, the Company had entered into the agreements described below with Factor Bioscience Inc. and/or Dr. Matthew Angel. These agreements have been deemed related party transactions because the Company’s former chief executive officer, Dr. Angel, is the chairman and chief executive officer of Factor Bioscience Inc. and a director of its subsidiary, Factor Bioscience Limited (“Factor Limited” and together with Factor Bioscience Inc. and its other affiliates, “Factor Bioscience”). Dr. Angel resigned as the Company’s chief executive officer effective December 31, 2023.
In May 2024, the Company entered into the First Amendment to Work Order 1 (the “Amended Work Order”) under a Master Services Agreement (the “MSA”) that the Company entered into with Factor Bioscience in September 2022, including the first work order under the MSA (“Work Order 1”). The Amended Work Order allowed the Company to terminate Work Order 1 on or after the second anniversary of the date of the MSA, subject to providing Factor Bioscience with 75 days’ prior notice if such notice is provided no later than June 30, 2024, rather than 120 days’ notice originally required. On June 26, 2024, the Company provided Factor Bioscience with its notice to terminate Work Order 1, which became effective on September 9, 2024.
Under Work Order 1, Factor Bioscience was providing the Company with mRNA cell engineering research support services, including access to certain facilities, equipment, materials and training, and the Company paid Factor Bioscience an initial fee of $5.0 million, payable in 12 equal monthly installments of approximately $0.4 million. Of the $5.0 million, the Company allocated $3.5 million to the License Fee Obligation (as defined below). Following the initial 12-month period, the Company continued paying Factor Bioscience the monthly fee of $0.4 million until such time as Work Order 1 was terminated.
In September 2022, Novellus Inc. (“Novellus”) and the Company entered into a Second Amendment to the Limited Waiver and Assignment Agreement (the “Waiver and Assignment Agreement”) with Drs. Matthew Angel and Christopher Rohde (the “Founders”) whereby the Company agreed to be responsible for all future, reasonable and substantiated legal fees, costs, settlements and judgments incurred by the Founders, the Company or Novellus for certain claims and actions and any pending or future litigation brought against the Founders, Novellus and/or the Company by or on behalf of the Westman and Sowyrda legal matters described in Note 11 (the “Covered Claims”). The Founders will continue to be solely responsible for any payments made to satisfy a judgement or settlement of any pending or future wage act claims. Under the Waiver and Assignment Agreement, the Founders agreed that they are not entitled to, and waived any right to, indemnification or advancement of past, present or future legal fees, costs, judgments, settlements or other liabilities they may have been entitled to receive from the Company or Novellus in respect of the Covered Claims. The Company and the Founders will share in any recoveries up to the point at which the parties have been fully compensated for legal fees, costs and expenses incurred, with the Company retaining any excess recoveries. The Company has the sole authority to direct and control the prosecution, defense and settlement of the Covered Claims.
On February 20, 2023, the Company, entered into an exclusive license agreement (the “Feb 2023 Factor Exclusive License Agreement”) with Factor Limited, pursuant to which Factor Limited granted to the Company an exclusive, sublicensable, worldwide license under certain patents owned by Factor Limited for the purpose of, among other things, identifying and pursuing certain opportunities to develop products in respect of such patents and to otherwise grant to third parties sublicenses to such patents. The Feb 2023 Factor Exclusive License Agreement, which terminated and superseded the Amended Factor License Agreement, was subsequently terminated and superseded by the A&R Factor License Agreement (as defined below).
On November 14, 2023, the Company entered into an amended and restated exclusive license agreement (the “A&R Factor License Agreement”) with Factor Limited to replace in its entirety the exclusive license agreement between the parties dated February 20, 2023 and the amendment thereto. Under the A&R Factor License Agreement, Factor Limited granted to the Company an exclusive, sublicensable license under certain patents owned by Factor Limited (the “Factor Patents”). The A&R Factor License Agreement also provides for, among other things, the expansion of the Company’s license rights to include (i) the field of use of the Factor Patents to include veterinary uses (ii) know-how that is necessary or reasonably useful to practice to the licensed patents, (iii) the ability to sublicense through multiple tiers (as opposed to only permitting a direct sublicense) and (iv) the transfer of technology to the Company, subject to the use restrictions in the A&R Factor License Agreement. The A&R Factor License Agreement was subsequently terminated and superseded by the Factor L&C Agreement discussed below.
On September 24, 2024, the Company entered into the Factor L&C Agreement, effective as of September 9, 2024, with Factor Limited. The Factor L&C Agreement terminated the A&R Factor License Agreement as well as the Purchased License that Exacis entered into with Factor Bioscience on November 4, 2020, which the Company acquired pursuant to the Exacis Purchase Agreement with Exacis and certain stockholders of Exacis on April 26, 2023.
| F- |
Under the Factor L&C Agreement, the Company has obtained exclusive licenses in the fields of cancer, autoimmune disorders, and rare diseases with respect to certain licensed technology and has the right to develop the licensed technology directly or enter into co-development agreements with partners who can help bring such technology to market. The Factor L&C Agreement also provides for certain services and materials to be provided by Factor Bioscience to facilitate the development of the licensed technology and to enable the Company to scale up production at third party facilities.
The initial term of the Factor L&C Agreement is one year after the effective date, and it automatically renews yearly thereafter. The Company may terminate the Factor L&C Agreement for any reason upon 90 days’ written notice to Factor Bioscience, and the parties otherwise have customary termination rights, including in connection with certain uncured material breaches and specified bankruptcy events.
Pursuant to the Factor L&C Agreement, the Company will pay Factor Bioscience approximately $0.2 million per month for the first twelve months, approximately $0.1 million per month for the first nine months toward patent costs, certain milestone payments, royalty payments on net sales of commercialized products and sublicensing fee payments.
Exacis Asset Acquisition
On April 26, 2023, the Company closed the Exacis Acquisition. See Note 4 for additional information.
The Exacis Acquisition was deemed a related party transaction because, at the time of the acquisition, (i) Dr. Gregory Fiore was both the chief executive officer of Exacis and a member of the Company’s board of directors, (ii) Dr. Angel was both the Company’s chief executive officer and chairman of Exacis’ scientific advisory board, and (iii) an affiliate of Factor Bioscience was the majority stockholder of Exacis.
Consulting Agreement with Former Director
In May 2023, the Company entered into a consulting agreement with Dr. Fiore, whereby Dr. Fiore agreed to provide business development consulting services to the Company for a monthly retainer of $20,000. The consulting agreement was terminable for any reason by either party upon 15 days’ written notice. The Company terminated the consulting agreement, effective July 31, 2023. Dr. Fiore served on the Company’s board of directors from June 2022 to October 4, 2023.
July 2023, December 2023 and September 2024 Financings
Investors in the July 2023 Convertible Note financing included Brant Binder, Richard Wagner, Charles Cherington and Nicholas Singer, and investors in the December 2023 Convertible Note financing and the September 2024 financing included Messrs. Cherington and Singer. Each of them participated in the applicable financing under the same terms and subject to the same conditions as all the other investors. See Note 6 for additional information regarding the financings. Mr. Binder served on the Company’s board of directors from July 6, 2023 to August 8, 2023, Mr. Wagner served on the Company’s board of directors from July 6, 2023 to August 8, 2023, Mr. Cherington served on the Company’s board of directors from March 2021 to July 6, 2023, and Mr. Singer served on the Company’s board of directors from June 2022 to July 6, 2023.
| 12) | Accrued Expenses |
Accrued expenses at December 31, 2024 and 2023 consisted of the following (in thousands):
Schedule of Accrued Expenses
| December 31, 2024 | December 31, 2023 | |||||||
| Professional fees | $ | 238 | $ | 239 | ||||
| Legal fees | 323 | 643 | ||||||
| Accrued compensation | 12 | 109 | ||||||
| Convertible notes interest | - | 176 | ||||||
| Somerville facility | - | 218 | ||||||
| Other | 434 | 508 | ||||||
| Total accrued expenses | $ | 1,007 | $ | 1,893 | ||||
| F- |
| 13) | Commitments and Contingencies |
Litigation Matters
The Company is involved in litigation and arbitrations from time to time in the ordinary course of business. Legal fees and other costs associated with such actions are expensed as incurred. In addition, the Company assesses the need to record a liability for litigation and contingencies. The Company reserves for costs relating to these matters when a loss is probable, and the amount can be reasonably estimated.
Novellus, Inc. v. Sowyrda et al., C.A. No. 2184CV02436-BLS2
On October 25, 2021 Novellus, Inc. filed a complaint in the Superior Court of Massachusetts, Suffolk County, against former Novellus, Inc. employees Paul Sowyrda and John Westman and certain other former investors in Novellus LLC (Novellus, Inc.’s former parent company prior to our acquisition of Novellus, Inc.), alleging breach of fiduciary duty, breach of contract and civil conspiracy. Eterna acquired Novellus, Inc. on July 16, 2021. On May 27, 2022 Novellus, Inc. amended the complaint to withdraw all claims against all defendants except Paul Sowyrda and John Westman. On July 1, 2022, Westman filed a motion to compel arbitration or in the alternative, to stay the litigation pending the disposition of certain litigation in the Court of Chancery for the State of Delaware filed by Mr. Sowyrda against Novellus LLC, Dr. Christopher Rohde, Dr. Matthew Angel, Leonard Mazur and Factor Bioscience, Inc. captioned Zelickson et al., v. Angel et al., C.A. 2021-1014-JRS and by Westman against Novellus LLC captioned Westman v. Novellus LLC, C.A. No. 2021-0882-NAC (together, the “Delaware Actions”). On July 1, 2022, Sowyrda answered the complaint and asserted counterclaims against Novellus, Inc, and third-party defendants Dr. Matthew Angel and Dr. Christopher Rohde alleging violations of the Massachusetts Wage Act, Massachusetts Minimum Fair Wage Law, the Fair Labor Standards Act, breach of contract, unjust enrichment and quantum meruit. Sowyrda also joined in Westman’s motion to stay the case pending the Delaware Actions. Novellus, Inc.’s claims and Mr. Sowyrda’s counterclaims relate to alleged conduct that took place before Eterna acquired Novellus, Inc.
On November 15, 2022, prior to a decision on Westman’s and Sowyrda’s motion to compel or stay, the parties agreed to voluntarily dismiss and consolidate the Delaware Actions with this action. On December 15, 2022, Sowyrda filed an Amended Answer to the Amended Complaint, asserted affirmative defenses and filed Amended Counterclaims against Dr. Angel, Dr. Rohde, Novellus LLC, Novellus Inc., Factor Bioscience Inc., and Eterna Therapeutics Inc. (collectively, the “Counterclaim Defendants”) alleging against various Counterclaim Defendants breach of contract, breaches of the implied duty of good faith and fair dealing, breaches of fiduciary duty, breaches of the operating agreement, aiding and abetting breaches of fiduciary duty, tortious interference with contract, equitable accounting, violations of the Massachusetts Wage Act, Massachusetts Minimum Fair Wage Law, the Fair Labor Standards Act, unjust enrichment, and quantum meruit. Also on December 15, 2022, Westman filed an answer to the Amended Complaint and asserted similar counterclaims against the same Counterclaim Defendants. Westman and Sowyrda each asserted claims for indemnification and/or advancement against Novellus, Inc. On January 11, 2023, Westman and Sowyrda served a joint motion to enforce their advancement and/or indemnification rights against Novellus Inc. Novellus Inc. vigorously opposes this motion and served its opposition on January 27, 2023. On February 8, 2023, Westman and Sowyrda served a reply in support of their motion to enforce indemnification/advancement rights, and submitted the motion to the Court. Novellus Inc. answered Westman and Sowyrda’s counterclaims on January 27, 2023, denying liability. The remaining Counterclaim Defendants served a motion to dismiss most of the remaining counterclaims on January 27, 2023. The Court entered an order granting the Counterclaim Defendants’ motion to dismiss and denying Sowyrda and Westman’s motion to enforce on June 15, 2023. The Court’s order dismissed all of Westman’s claims against Counterclaim Defendants except his claim for indemnification, and all of Sowyrda’s claims except his claim for indemnification and his employment-related claims, which Counterclaim Defendants did not move to dismiss. On July 6, 2023, Westman and Sowyrda filed a petition for interlocutory review with a single justice of the Massachusetts Appeals Court, seeking to overturn the judge’s decision granting the Counterclaim Defendants’ motion to dismiss most of the remaining counterclaims, but not the decision denying Westman and Sowyrda’s motion to enforce advancement rights. On July 25, 2023, the parties to the appeal filed a joint motion to the single justice in the appellate court to stay the appeal to allow for amended counterclaims to be filed by Counterclaim Plaintiffs and a motion to dismiss to be filed by Counterclaim Defendants. Counterclaim Plaintiffs filed an initial set of amended counterclaims on August 15, 2023. Counterclaim Plaintiffs amended and refiled their amended counterclaims on September 29, 2023. Counterclaim Defendants served their motion to dismiss all of the amended counterclaims, except for Sowyrda’s employment-related claims, on October 13, 2023. On June 13, 2024, the motion to dismiss was denied and the court set a schedule for discovery limited to a threshold factual issue. Discovery as to all other issues pertaining to the counterclaims was stayed. On July 15, 2024, Westman and Sowyrda requested that the single justice in the appellate court continue to stay the appeal pending the outcome of the limited discovery ordered by the Court. On July 31, 2024, Counterclaim Defendants and Sowyrda informed the Court that they had reached a settlement and requested that all claims pending between them be dismissed with prejudice, and on August 9, 2024, the Court approved the motion for approval of dismissal of all such claims with prejudice. Pursuant to the Court’s June 13, 2024 order, Counterclaim Defendants engaged in limited discovery with Westman.. The Counterclaim Defendants and Westman are currently in settlement discussions, and the Counterclaim Defendants and Westman requested a stay of all remaining deadlines pending memorialization of such discussions. The Court granted that motion on February 11, 2025. The Company has accrued approximately $0.2 million for this matter in the year ended December 31, 2024.
| F- |
eTheRNA Immunotherapies NV and eTheRNA Inc. v. Eterna Therapeutics Inc. C.A. No. 123CV11732
On July 31, 2023, eTheRNA Immunotherapies NV and eTheRNA Inc. filed a complaint against the Company alleging the following claims: (1) federal trademark infringement; (2) federal unfair competition; (3) Massachusetts state common law trademark infringement; (4) Massachusetts state unfair competition. On April 2, 2024, the parties settled the claims and stipulated to dismiss the complaint with prejudice. Per the settlement agreement entered into between the parties on March 19, 2024, the Company planned to phase-out its current use of the ETERNA trademark by October 31, 2024.
On October 6, 2024, the parties entered into an addendum to the settlement agreement extending the deadline for phasing out the Company’s use of the ETERNA trademark until March 31, 2025. If the Company continues to use the Eterna Therapeutics name as of April 1, 2025, it will be obligated to pay €667 per day that it continues to do so.
Licensing Agreements
On September 24, 2024, the Company entered into the Factor L&C Agreement. See Note 11 for details of this agreement.
Retirement Savings Plan
The Company established a defined contribution plan, organized under Section 401(k) of the Internal Revenue Code, which allows employees to defer up to 90% of their pay on a pre-tax basis. Beginning on January 1, 2023, the Company began matching employees’ contributions at a rate of 100% of the first 3% of the employee’s contribution and 50% of the next 2% of the employee’s contribution, for a maximum Company match of 4%. The Company matched less than $0.1 million towards employees’ 401k contributions for each of the years ended December 31, 2024 and 2023.
| 14) | Basic and Diluted Net Loss per Common Share |
Schedule of Computation of Net Loss Per Share Basic and Diluted
| Years ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Numerator: | ||||||||
| Net loss attributable to common stockholders | $ | (44,555 | ) | $ | (21,684 | ) | ||
| Denominator: | ||||||||
| Weighted average shares outstanding - basic and diluted | 13,647 | 5,314 | ||||||
| Net loss per common share - basic and diluted | $ | (3.26 | ) | $ | (4.08 | ) | ||
Since the Company was in a net loss position for all periods presented, the net loss per share attributable to common stockholders was the same on a basic and diluted basis, as the inclusion of all potential common equivalent shares outstanding would have been anti-dilutive.
Schedule of Securities Excluded from the Computation of Diluted Net Loss per Common Stock
| Years ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Stock options | 2,629 | 389 | ||||||
| Warrants | 484 | 18,922 | ||||||
| Preferred stock converted into common stock | 31 | 18 | ||||||
| Convertible Notes converted into common stock | - | 7,877 | ||||||
| RSUs | - | 1 | ||||||
| Total potential common shares excluded from computation | 3,144 | 27,207 | ||||||
| F- |
| 15) | Stock-Based Compensation |
Equity Incentive Plans
The Company’s stock-based compensation plans consist of the Restated 2020 Equity Incentive Plan (the “Restated 2020 Plan”) and the 2021 Inducement Equity Incentive Plan (the “2021 Inducement Plan”). The Company’s board of directors has designated its compensation committee as the administrator of the foregoing plans (the “Plan Administrator”). Among other things, the Plan Administrator selects persons to receive awards under the foregoing plans and determines the number of shares subject to each award and the terms, conditions, performance measures, if any, and other provisions of the award.
The Restated 2020 Plan provides for (a) approximately 724,000 shares of common stock that can be issued under the Restated 2020 Plan, which includes an increase to the Restated 2020 Plan of 300,000 that was approved by the Company’s stockholders at the 2023 annual meeting of stockholders in June 2023, and (b) an annual increase in the number of shares reserved for issuance on January 1 of each year from 2022 through 2031 equal to the lesser of (i) 5% of the number of shares of common stock outstanding on the immediately preceding December 31 and (ii) such smaller number of shares of common stock as may be determine by the board of directors (the provision providing for the increase described in clause (b) is referred to as the “evergreen provision”). Pursuant to the evergreen provision, shares issuable under the Restated 2020 Plan was increased by approximately 527,000 in the aggregate.
Awards under the Restated 2020 Plan may be granted to officers, directors, employees and consultants of the Company. Stock options granted under the Restated 2020 Plan may either be incentive stock options or nonqualified stock options, may have a term of up to ten years, and are exercisable at a price per share not less than the fair market value, as defined in the Restated 2020 Plan, on the date of grant.
As of December 31, 2024, there were approximately 855,000 stock options outstanding and no RSUs outstanding under the Restated 2020 Plan. As of December 31, 2024, there were approximately 380,000 shares of common stock remaining to be issued under the Restated 2020 Plan.
The 2021 Inducement Plan provides for the grant of up to 75,000 share-based awards as material inducement awards to new employees in accordance with the employment inducement grant rules set forth in Section 711(a) of the NYSE American LLC Company Guide (the Company’s common stock was listed on the NYSE American at the time the 2021 Inducement Plan was adopted). The 2021 Inducement Plan expires in May 2031. As of December 31, 2024, there were approximately 71,000 shares of common stock remaining to be issued under the 2021 Inducement Plan. As of December 31, 2023, there were no stock options outstanding and less than 1,000 RSUs outstanding under the 2021 Inducement Plan.
Equity Awards
Stock Options
Schedule of Weighted-Average Assumptions Used for Stock Options Granted
| Year ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Weighted average risk-free rate | 4.44 | % | 3.82 | % | ||||
| Weighted average volatility | 98.09 | % | 95.15 | % | ||||
| Dividend yield | 0 | % | 0 | % | ||||
| Expected term | 6.04 years | 5.44 years | ||||||
The risk-free rate is based on the observed interest rates appropriate for the expected life. The expected life (estimated period of time outstanding) of the stock options granted is estimated using the “simplified” method as permitted by the SEC’s Staff Accounting Bulletin No. 110, Share-Based Payment. Expected volatility is based on the volatility of the Company’s peer group over the expected life of the stock option granted, and the Company assumes no dividends. Forfeitures are recognized as incurred.
| F- |
Schedule of Stock Option Activity
| Outstanding Options |
Weighted Average Exercise Price per Share |
Weighted Average Remaining Contractual Life (in years) |
Aggregate
Intrinsic Value |
|||||||||||||
| Outstanding January 1, 2023 | 359 | $ | 57 | 7.57 | $ | - | ||||||||||
| Granted | 237 | 4 | ||||||||||||||
| Cancelled | (207 | ) | 19 | |||||||||||||
| Outstanding December 31, 2023 | 389 | 45 | 7.04 | - | ||||||||||||
| Granted | 2,488 | 2 | ||||||||||||||
| Cancelled | (248 | ) | 8 | |||||||||||||
| Outstanding December 31, 2024 | 2,629 | $ | 8 | 8.77 | $ | - | ||||||||||
| Options vested and exercisable at December 31, 2024 | 257 | $ | 61 | 5.58 | $ | - | ||||||||||
The per-share weighted average grant-date fair value of stock options granted during the year ended December 31, 2024 and 2023 was $1.39 and $2.99, respectively.
As of December 31, 2024, the unamortized stock-based compensation expense related to outstanding unvested options was approximately $2.3 million with a weighted average remaining requisite service period of 1.91 years. The Company expects to amortize this expense over the remaining requisite service period of these stock options.
Vesting of all stock options is subject to continuous service with the Company through their applicable vesting dates.
On January 1, 2024, Sanjeev Luther was appointed as President, Chief Executive Officer and a director of the Company. Upon his appointment, he was granted a non-qualified stock option to purchase approximately 1,685,000 shares of the Company’s common stock. The stock option has an exercise price of $1.80 per share, which was equal to the fair market value (as defined in the 2020 Restated Equity Incentive Plan) of the Company’s common stock on the date of grant, will vest over four years, with 25% of the shares vesting on the first anniversary of the grant date and the remaining 75% of the shares vesting in equal monthly installments over the three years thereafter, in each case, subject to continued service. The stock option was granted pursuant to the terms of Mr. Luther’s employment agreement and as a material inducement to his joining the Company in accordance with Nasdaq Listing Rule 5635(c)(4).
On April 26, 2024, the vesting terms of Mr. Luther’s stock option award were amended so that the option vests over three years, with 25% of the shares vesting on the first anniversary of the grant date and the remaining 75% of the shares will vest in equal monthly installments over the remaining two years, in each case, subject to continued service.
Since the only modification to Mr. Luther’s stock option award was to the vesting terms, there was no change to the fair value of the stock option and the total compensation cost was unchanged. However, the total compensation cost will be recognized over three years rather than four years, and as a result, the Company recognized approximately $0.1 million in additional stock-based compensation expense during the year ended December 31, 2024 as a result of the modification.
| F- |
Restricted Stock Units
Schedule of RSU Activity
| Outstanding Restricted Stock Units |
Weighted Average Fair Value per Share |
|||||||
| January 1, 2023 | 4 | $ | 236 | |||||
| Cancelled | (3 | ) | 199 | |||||
| December 31, 2023 | 1 | 322 | ||||||
| December 31, 2024 | 1 | $ | 322 | |||||
| Balance expected to vest at December 31, 2024 | 1 | $ | 322 | |||||
The Company recognizes the fair value of RSUs granted as expense on a straight-line basis over the requisite service period. For performance based RSUs, the Company begins recognizing the expense once the achievement of the related performance goal is determined to be probable.
Outstanding RSUs are settled in an equal number of shares of common stock on the vesting date of the award. An RSU award is settled only to the extent vested. Vesting generally requires the continued employment or service by the award recipient through the respective vesting date. Because RSUs are settled in an equal number of shares of common stock without any offsetting payment by the recipient, the measurement of cost is based on the quoted market price of the stock at the measurement date, which is the grant date.
In lieu of paying cash to satisfy withholding taxes due upon the settlement of vested RSUs, at the Company’s discretion, an employee may elect to have shares of common stock withheld that would otherwise be issued at settlement, the value of which is equal to the amount of withholding taxes payable. During the years ended December 31, 2024 and 2023, less than 1,000 RSUs vested in each year.
Stock-Based Compensation Expense
Schedule of Stock-Based Compensation Expense
| Years ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Research and development | $ | 89 | $ | 234 | ||||
| General and administrative | 1,431 | 1,008 | ||||||
| Total | $ | 1,520 | $ | 1,242 | ||||
| 16) | Stockholders’ Equity and Warrants |
Warrants
During the year ended December 31, 2024, the Company had the following warrant activity (in thousands):
Schedule of Warrants Outstanding
| Outstanding January 1, 2024 |
Granted | Exchanged Warrants |
Outstanding December 31, 2024 |
|||||||||||||
| Q1-22 Warrants | 343 | - | - | 343 | ||||||||||||
| December 2022 Warrants | 4,370 | - | (4,228 | ) | 142 | |||||||||||
| July 2023 Warrants | 6,094 | - | (6,094 | ) | - | |||||||||||
| December 2023 Warrants | 8,115 | 1,464 | (9,579 | ) | - | |||||||||||
| Prefunded Warrants | - | 1,879 | - | 1,879 | ||||||||||||
| Total | 18,922 | 3,343 | (19,901 | ) | 2,364 | |||||||||||
As discussed in Note 6 and further below, as a result of stockholder approval of the September 2024 Transactions at the Annual Meeting on October 29, 2024, the Exchanged Warrants were exchanged for approximately 9,951,000 shares of common stock, and 1,879,000 prefunded warrants were issued in connection with the conversion of the Bridge Notes and the closing of the Common Stock Private Placement. The prefunded warrants have an exercise price of $0.005 per share, are exercisable at any time and will not expire until exercised in full.
| F- |
The Q1-22 Warrants are classified as a liability, have an exercise price of $38.20 per share and expire on September 9, 2027. The remaining December 2022 Warrants qualify for equity classification, have an exercise price of $1.43 per warrant share and expire on June 2, 2028.
As of December 31, 2024, the weighted average remaining contractual life of the warrants outstanding was 2.90 years and the weighted average exercise price was $27.45, which does not include the prefunded warrants.
Cumulative Convertible Preferred Stock
The Company has authorized 156,000 shares of preferred stock, all of which is designated as Series A Cumulative Convertible Preferred Stock (the “Series A Preferred Stock”), and all of which were issued and outstanding as of December 31, 2024 and 2023.
The Series A Preferred Stock provides for a cumulative annual dividend of $0.10 per share, payable in semi-annual installments in June and December. Dividends may be paid in cash or with shares of common stock. The Company paid approximately $8,000 in cash and issued approximately 11,000 shares of common stock for payment of dividends during the year ended December 31, 2024. The Company paid approximately $16,000 in cash for payment of dividends during the year ended December 31, 2023.
The Series A Preferred Stock has no voting rights and has a $1.00 per share liquidation preference over the Company’s common stock. The holder of shares of Series A Preferred Stock has the right at any time to convert such shares into that number of shares of common stock that equals the number of shares of Series A Preferred Stock divided by the conversion rate. At December 31, 2024, the conversion rate was 5.0670 and, based on that conversion rate, one share of Series A Preferred Stock would have converted into approximately 0.20 shares of common stock, and all the outstanding shares of the Series A Preferred Stock would have converted into approximately 31,000 shares of common stock in the aggregate. There were no conversions during the years ended December 31, 2024 and 2023. There is no mandatory conversion term, date or any redemption features associated with the Series A Preferred Stock. The conversion rate will adjust under the following circumstances:
1. If the Company (a) pays a dividend or makes a distribution in shares of its common stock, (b) subdivides its outstanding shares of common stock into a greater number of shares, (c) combines its outstanding shares of common stock into a smaller number of shares, or (d) issues by reclassification of its shares of common stock any shares of its common stock (other than a change in par value, or from par value to no par value, or from no par value to par value), then the conversion rate in effect immediately prior to the applicable event will be adjusted so that the holders of the Series A Preferred Stock will be entitled to receive the number of shares of common stock which they would have owned or have been entitled to receive immediately following the happening of the event, had the Series A Preferred Stock been converted immediately prior to the record or effective date of the applicable event.
2. If the outstanding shares of the Company’s common stock are reclassified (other than a change in par value, or from par value to no par value, or from no par value to par value, or as a result of a subdivision, combination or stock dividend), or if the Company consolidates with or merge into another corporation and the Company is not the surviving entity, or if the Company sells all or substantially all of its property, assets, business and goodwill, then the holders of the Series A Preferred Stock will thereafter be entitled upon conversion to the kind and amount of shares of stock or other equity securities, or other property or assets which would have been receivable by such holders upon such reclassification, consolidation, merger or sale, if the Series A Preferred Stock had been converted immediately prior thereto.
3. If the Company issues common stock without consideration or for a consideration per share less than the then applicable Equivalent Preference Amount (as defined below), then the Equivalent Preference Amount will immediately be reduced to the amount determined by dividing (A) an amount equal to the sum of (1) the number of shares of common stock outstanding immediately prior to such issuance multiplied by the Equivalent Preference Amount in effect immediately prior to such issuance and (2) the consideration, if any, received by the Company upon such issuance, by (B) the total number of shares of common stock outstanding immediately after such issuance. The “Equivalent Preference Amount” is the value that results when the liquidation preference of one share of Series A Preferred Stock (which is $1.00) is multiplied by the conversion rate in effect at that time; thus the conversion rate applicable after the adjustment in the Equivalent Preference Amount as described herein will be the figure that results when the adjusted Equivalent Preference Amount is divided by the liquidation preference of one share of Series A Preferred Stock.
| F- |
SEPA
On April 5, 2023, the Company entered into the SEPA with Lincoln Park, pursuant to which Lincoln Park committed to purchase up to $10.0 million of the Company’s common stock. Such sales of common stock by the Company, if any, are subject to certain conditions and limitations set forth in the SEPA, including a condition that the Company may not direct Lincoln Park to purchase any shares of common stock under the SEPA if such purchase would result in Lincoln Park beneficially owning more than 4.99% of the Company’s issued and outstanding shares of common stock. Sales under the SEPA may occur from time to time, at the Company’s sole discretion, through April 2025.
In consideration of Lincoln Park’s entry into the SEPA, the Company issued to Lincoln Park approximately 74,000 shares of common stock (the “Commitment Shares”). The value of the Commitment Shares was recorded as a period expense and included in other expense, net, in the accompanying consolidated statements of operations for year ended December 31, 2023.
The Company evaluated the contract that includes the right to require Lincoln Park to purchase shares of common stock in the future (“put right”) considering the guidance in ASC 815-40, Derivatives and Hedging — Contracts on an Entity’s Own Equity and concluded that it is an equity-linked contract that does not qualify for equity classification, and therefore requires fair value accounting. The Company analyzed the terms of the freestanding put right and concluded that it has an immaterial value as of December 31, 2024 and 2023.
During the year ended December 31, 2023, the Company issued and sold approximately 214,000 shares of common stock under the SEPA, including the 74,000 Commitment Shares, for gross proceeds of approximately $0.3 million. The Company did not sell any shares of common stock under the SEPA during the year ended December 31, 2024. As of December 31, 2024, there were approximately 2,860,000 shares remaining to be sold under the SEPA.
September 2024 Transactions
As discussed in Note 6, on September 24, 2024, the Company entered into the September 2024 Transactions. On October 29, 2024, the Company held its Annual Meeting, whereby the Company’s stockholders approved the September 2024 Transactions, and as a result, the Company issued approximately 45,948,000 shares of common stock and 1,879,000 prefunded warrants and had approximately 51,386,000 shares of common stock issued and outstanding following such transactions. See Note 6 for details on the September 2024 Transactions.
Stock Repurchase Program
In November 2024, the Company’s Board authorized a stock repurchase program (the “Repurchase Program”) of up to $1.0 million of the Company’s outstanding common stock. Under the Repurchase Program, the repurchases may be made by the Company from time to time through open market purchases, privately negotiated transactions or other means in accordance with applicable securities laws. The timing and amount of repurchases will be determined by the Company, taking into consideration market conditions, stock price, and other factors. The Repurchase Program does not have a set expiration date and may be suspended, modified or discontinued at any time without prior notice. The Company did not repurchase any of its shares under the Repurchase Program during the year ended December 31, 2024. There was no such repurchase program during the year ended December 31, 2023.
| F- |
| 17) | Income Taxes |
Loss before income taxes consist of the following (in thousands):
Schedule of Loss Before Income Taxes
| Years ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| (in thousands) | ||||||||
| Domestic | $ | (44,529 | ) | $ | (21,654 | ) | ||
| Foreign | 20 | (17 | ) | |||||
| Total loss before income taxes | $ | (44,509 | ) | $ | (21,671 | ) | ||
For each of the years ended December 31, 2024 and 2023, current tax provisions and current deferred tax provisions were recorded as follows (in thousands):
Schedule of Income Tax Provision
| Years ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Current Tax Provision | ||||||||
| Federal | $ | - | $ | - | ||||
| State | 3 | 1 | ||||||
| Foreign | - | - | ||||||
| Current tax provision | 3 | 1 | ||||||
| Deferred Tax Provision | ||||||||
| Federal | - | - | ||||||
| State | 27 | (4 | ) | |||||
| Foreign | - | - | ||||||
| Deferred tax provision | 27 | (4 | ) | |||||
| Total tax provision (benefit) for income taxes | $ | 30 | $ | (3 | ) | |||
Deferred income taxes reflect the net tax effects of temporary differences between carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Realization of net deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. The table below consists of the Company’s net deferred tax assets and liabilities as of December 31, 2024 and December 31, 2023 (in thousands). Deferred tax assets have been substantially reserved for by a valuation allowance since it is more likely than not that such tax benefits will not be realized.
Schedule of Deferred Tax Assets and Liabilities
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Deferred Tax Assets: | ||||||||
| Net operating losses | $ | 16,496 | $ | 12,740 | ||||
| Foreign net operating losses | 782 | 784 | ||||||
| Stock compensation | 2,646 | 2,141 | ||||||
| In-process research and development | 1,030 | 1,009 | ||||||
| Capitalized research and development expenses | 4,548 | 3,105 | ||||||
| Accrued expenses | 192 | - | ||||||
| R&D credit carryforwards | 437 | 437 | ||||||
| ROU Liabilities | 187 | 8,932 | ||||||
| Other | 29 | 135 | ||||||
| Total gross deferred tax assets | 26,347 | 29,283 | ||||||
| Valuation allowance | (26,023 | ) | (18,302 | ) | ||||
| Net deferred tax assets | 324 | 10,981 | ||||||
| Deferred Tax Liabilities: | ||||||||
| Fixed assets | - | (6 | ) | |||||
| ROU Assets | (183 | ) | (8,349 | ) | ||||
| Convertible debt | - | (2,507 | ) | |||||
| Intangibles - goodwill | (229 | ) | (179 | ) | ||||
| Total deferred tax liabilities | (412 | ) | (11,041 | ) | ||||
| Net deferred taxes | $ | (88 | ) | $ | (60 | ) | ||
| F- |
The reconciliation between the Company’s effective tax rate on income from continuing operations and the federal statutory tax rate of 21% for the years ended December 31, 2024 and 2023 is as follows:
Schedule of Reconciliation of Computed Expected Income Taxes to Effective Income Taxes
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Tax at federal income tax rate | 21.00 | % | 21.00 | % | ||||
| State income tax, net of federal tax | 2.95 | % | 4.92 | % | ||||
| Foreign tax differential | 0.00 | % | (0.01 | %) | ||||
| Non-deductible expenses/excludable items | (0.05 | %) | (0.74 | %) | ||||
| Convertible debt | (9.16 | %) | (11.92 | %) | ||||
| Credits | (0.18 | %) | 0.00 | % | ||||
| Other | 2.72 | % | (3.34 | %) | ||||
| Change in valuation allowance | (17.35 | %) | (9.90 | %) | ||||
| (Provision) benefit for income taxes | (0.07 | %) | 0.01 | % | ||||
The net increase in the total valuation allowance for the year ended December 31, 2024 was an increase of approximately $7.7 million. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during periods in which those temporary differences become deductible. Management considered the scheduled reversal of deferred tax liabilities, projected future taxable income and planning strategies in making this assessment. Based on the level of historical operating results and projections for the taxable income for the future, management has determined that it is more likely than not that the deferred taxes assets will not be utilized. Accordingly, the Company has recorded a full valuation allowance. The net deferred tax liability represents an indefinite life intangible liability related to tax deductible goodwill, partially offset by an indefinite life deferred tax asset.
At December 31, 2024 and 2023, the Company has available net operating loss (“NOL”) carryforwards of approximately $62.1 million and $48.4 million for federal income tax purposes, respectively, of which approximately $61.4 million can be carried forward indefinitely. The Company has available $52.6 million and $39.6 million state NOLs for the years ended December 31, 2024 and 2023, respectively, which begin to expire in 2041. The Company also has foreign NOL carryforwards of approximately $6.3 million for each of the years ended December 31, 2024 and 2023, which carry forward indefinitely. Section 382 of the Internal Revenue Code (“IRC”) imposes limits on the ability to use NOL carryforwards that existed prior to a change in control to offset future taxable income. Such limitations would reduce, potentially significantly, the gross deferred tax assets disclosed in the table above related to the NOL carryforwards. The Company continues to disclose the NOL carryforwards at their original amount in the table above as no potential limitation has been quantified. The Company has also established a full valuation allowance for all deferred tax assets, including the NOL carryforwards, since the Company could not conclude that it was more likely than not able to generate future taxable income to realize these assets.
The Company has federal and state income tax credit carryforwards of approximately $0.4 million at both December 31, 2024 and 2023.. The credits begin to expire in 2041.
In accordance with authoritative guidance, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. The following table summarizes amounts the Company recorded for uncertain tax positions as of December 31, 2024 and 2023 (in thousands):
Schedule of Uncertain Tax Positions
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Beginning balance of uncertain tax positions | $ | 393 | $ | 121 | ||||
| Additions based on current year’s tax positions | - | - | ||||||
| Net changes based on prior year’s tax positions | - | 272 | ||||||
| Ending balance of uncertain tax positions | $ | 393 | $ | 393 | ||||
It is reasonably possible that unrecognized tax benefits may increase or decrease within the next twelve months due to tax examination changes, expiration of statute of limitations, or changes in tax law. The Company does not anticipate any significant changes to unrecognized tax benefits over the next 12 months.
The Company recognizes interest and penalties related to unrecognized tax positions within the income tax expense line in the accompanying consolidated statements of operations. There were no accrued interest and penalties associated with uncertain tax positions as of December 31, 2024 or December 31, 2023.
The Company is subject to U.S. federal, state, and foreign income tax. The Company’s income tax returns are subject to examination by the relevant taxing authorities. As of December 31, 2024, the 2021 – 2024 tax years remain subject to examination in the U.S. federal tax, various state, and foreign tax jurisdictions. The Company is not currently under examination by federal state, or foreign jurisdictions.
On August 16, 2022, the Inflation Reduction Act of 2022 (the “IRA”) was enacted into law. Among other changes to the tax code, the IRA imposes a 1% excise tax on certain repurchases of corporate stock by certain publicly traded corporations. The 1% stock buyback tax applies to redemptions by domestic corporations occurring in taxable years beginning after December 31, 2022. A number of exceptions to the stock buyback tax are available including exceptions to certain reorganizations. However, while these exceptions may be helpful in limiting the application of the stock buyback tax in situations in which it was not intended to apply, more guidance will be necessary for taxpayers to analyze the potential application of these exceptions and whether they will be able to rely upon them.
| F- |
| 18) | Segment Reporting |
The CODM uses consolidated net loss as a measure of profit and loss and assesses Company performance through the achievement of its business strategy goals. The CODM is regularly provided with forecasted expense information that is used to determine the Company’s liquidity needs and cash allocation to execute its business strategy, and he uses cash as a measure of segment assets in managing the Company. The Company operates in the United States, and all of its assets are located in the United States.
The table below provides a breakdown of the Company’s significant operating expenses for the years ended December 31, 2024 and 2023 with a reconciliation to net loss for each of those years.
The Company’s revenue and its cost of revenues for the years ended Decembe4 2024 and 2023 relate to the Lineage Agreement. Depreciation and amortization expense was $0.1 million for each of the years ended December 31, 2024 and 2023. During the year ended December 31, 2024, the Company recognized $22.6 million in other expense, net, related to the September Transactions. There were no such transactions for the year ended December 31, 2023. The Company recognized $6.5 million in interest expense, net, during the year ended December 31, 2024 compared to $0.5 million during the year ended December 31, 2023.
Schedule of Breakdown of Significant Operating Expenses
| Year ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Revenue | $ | 582 | $ | 68 | ||||
| Cost of revenues | 96 | 236 | ||||||
| Gross profit (loss) | 486 | (168 | ) | |||||
| Operating expenses: | ||||||||
| Research and development by significant expense: | ||||||||
| MSA/license fees | 3,017 | 3,250 | ||||||
| Professional fees | 759 | 1,181 | ||||||
| Payroll and related | 502 | 701 | ||||||
| Other1 | 326 | 788 | ||||||
| Research and development | 4,604 | 5,920 | ||||||
| General and administrative by significant expense: | ||||||||
| Occupancy expense | 5,074 | 3,306 | ||||||
| Professional fees | 4,168 | 6,464 | ||||||
| Payroll and related | 1,607 | 2,045 | ||||||
| Stock-based compensation | 1,431 | 1,008 | ||||||
| Other2 | 852 | 1,764 | ||||||
| General and administrative | 13,132 | 14,587 | ||||||
| Gain on lease termination | (1,576 | ) | - | |||||
| Acquisition of Exacis in-process research and development | - | 460 | ||||||
| Total operating expenses | 16,160 | 20,967 | ||||||
| Loss from operations | (15,674 | ) | (21,135 | ) | ||||
| Other expense, net | (28,835 | ) | (536 | ) | ||||
| Loss before income taxes | (44,509 | ) | (21,671 | ) | ||||
| (Provision) benefit for income taxes | (30 | ) | 3 | |||||
| Net loss | $ | (44,539 | ) | $ | (21,668 | ) | ||
| Cash | $ | 1,729 | $ | 7,575 | ||||
| 1 | Other includes certain lab supply expenses, amounts related to the close out of a former clinical trial, allocated occupancy costs, stock-based compensation, and depreciation. |
| 2 | Other includes expenses related to insurance, information technology, travel, banking, depreciation and other miscellaneous expenses. |
| 19) | Subsequent Event |
On March 11, 2025, the Company received $1.5 million in exchange for the issuance of a promissory note with an aggregate principal amount of $1.5 million to an investor. The promissory note matures on the earlier of (i) June 15, 2025 or (ii) upon the Company receiving $5 million in gross proceeds from a subsequent capital raise. Interest accrues at a rate of 5.0% per annum, payable at maturity.
| F- |
Exhibit 19
ETERNA THERAPEUTICS INC.
SECURITIES TRADING POLICY
(Compliance with U.S. Securities Laws and Security Trading)
This Securities Trading Policy (this “Policy”) contains the following sections:
| 1.0 | General |
| 2.0 | Definitions |
| 3.0 | Statement of Policy |
| 4.0 | Certain Exceptions |
| 5.0 | Special Blackout Periods, Pre-clearance of Trades and Other Procedures |
| 6.0 | 10b5-1 Plans/Margin Accounts and Pledges/Short Sales |
| 7.0 | Potential Criminal and Civil Liability and/or Disciplinary Action |
| 8.0 | Broker Requirements for Section 16 Persons |
| 9.0 | Confidentiality |
| 10.0 | Legal Effect of this Policy |
| 11.0 | Reporting Violations/Seeking Advice |
| 1.0 | General |
| 1.1 | Eterna Therapeutics Inc., a Delaware corporation, and its subsidiaries (collectively, the “Company”, “we”, “us” or “our”), their respective directors, officers and employees (collectively, “Personnel”), Family Members (as defined below) of Personnel and trusts, corporations and other entities controlled by any of such persons (collectively, “Insiders”) must, at all times, comply with the securities laws of the United States and all applicable jurisdictions. The Company may also from time to time determine that additional persons and entities, including certain of the Company’s contractors or consultants, shall be deemed Personnel under this Policy (collectively, “Non-Employee Personnel”). |
| 1.2 | Federal securities laws prohibit trading in the securities of the Company or any other entity on the basis of “inside” information about the Company or that other entity obtained in the course of your position with the Company. These transactions are commonly known as “insider trading.” It is also illegal to recommend to others (commonly called “tipping”) that they buy, sell or retain the securities to which such inside information relates. Anyone violating these laws is subject to personal liability and could face criminal penalties, including a jail term. Federal securities laws also create a strong incentive for the Company to deter insider trading by its employees. In the normal course of business, Personnel may come into possession of inside information concerning the Company, transactions in which the Company proposes to engage or other entities with which the Company does business that is not available to the investing public. Therefore, the Company has established this Policy with respect to trading in its securities or securities of another company. Any violation of this Policy could subject you to disciplinary action, up to and including termination. See Section 7.0. |
| 1.3 | This Policy addresses compliance as it pertains to the disclosure of inside information regarding the Company or another company and to trading in securities (including transactions in common stock, preferred stock, bonds and other debt securities, options to purchase common stock, convertible debentures and warrants, as well as derivative securities whether or not issued by the Company, such as exchange-traded put or call options or swaps relating to the Company’s securities) while in possession of such inside information. In addition to requiring that Insiders comply with the letter of the law, it is the Company’s policy that Insiders exercise judgment so as to also comply with the spirit of the law and avoid even the appearance of impropriety. |
| 1.4 | This Policy is intended to protect Insiders and the Company from insider trading violations. However, the matters set forth in this Policy are merely guidelines and are not intended to replace your responsibility to understand and comply with the legal prohibition on insider trading. Appropriate judgment should be exercised in connection with all securities trading. Each person subject to this Policy is individually responsible for complying with this Policy and ensuring the compliance of any Family Member, household member or entity whose transactions are subject to this Policy. |
| 1.5 | In all cases, responsibility for determining whether an individual is in possession of Material Nonpublic Information (as defined below) rests with that individual, and any action on the part of the Company or any other employee pursuant to this Policy (or otherwise) does not in any way constitute legal advice or insulate an individual from liability under applicable securities laws. If you have specific questions regarding this Policy or applicable law, please contact the Compliance Officer for this Policy, who will initially be Sandra Gurrola, at sandra.gurrola@eternatx.com (the “Compliance Officer”). |
| 2.0 | Definitions |
| 2.1 |
“Family Members.” For purposes of this Policy, the term “Family Members” includes spouses, minor children, adult family members who reside with you (including adult children away at school), anyone else who shares the same household and any immediate family members and family members who do not share the same household but whose transactions in the Company’s securities are directed by you or are subject to your influence or control. Personnel are responsible for the transactions of their Family Members and therefore should make them aware of the need to confer with you before they trade in the Company’s securities or securities of companies we do business with. This Policy does not, however, apply to personal securities transactions of Family Members where the purchase or sale decision is made by a third party not controlled by, influenced by or related to an Insider or such person’s Family Members.
Certain family trusts and other entities of this type having an independent, professional trustee who makes investment decisions on behalf of the entity, and with whom the person subject to this Policy does not share Material Non-public Information, may be eligible for an exemption from this Policy. Please contact the Compliance Officer if you have any questions regarding this exception. An Insider should assume that this exception is not available unless such person has first obtained the approval of the Compliance Officer. |
| 2.2 | “Material.” Under Company policy and United States laws, information is “material” if a reasonable investor would consider the information important in determining whether to trade in a security. Information may be material even if it relates to future, speculative or contingent events and even if it is significant only when considered in combination with publicly available information. The information may concern the Company or another company and may be positive or negative. In addition, it should be emphasized that material information does not have to relate to a company’s business; information about the contents of a forthcoming publication in the financial press that is expected to affect the market price of a security could also be material. Nonpublic information can be material even with respect to companies that do not have publicly-traded stock, such as those with outstanding bonds. Employees should assume that information that would affect their trading decisions, or which might tend to influence the price of the security, is material. |
| Page |
| Depending on the facts and circumstances, examples of information that could be considered material include, but are not limited to: |
| ● | quarterly or annual results; | |
| ● | revenues, operating results (including EBITDA, Adjusted EBITDA and other key financial and operating metrics), estimates or guidance on operating results and changes in previously released results, estimates or guidance; | |
| ● | other unpublished financial results; | |
| ● | expansion or curtailment of operations and business disruptions; | |
| ● | a cybersecurity incident that may adversely impact the Company’s business, reputation or share value; | |
| ● | new inventions or discoveries; | |
| ● | a merger, acquisition, tender offer or divestiture proposal or agreement; | |
| ● | investments, joint ventures or changes in assets; | |
| ● | new service offerings or significant news relating to service offerings; | |
| ● | change in control of the Company or extraordinary management developments; | |
| ● | liquidity problems or impending bankruptcy, restructuring or layoffs; | |
| ● | changes in auditors or notification that the Company may no longer rely on an auditor’s reports; | |
| ● | significant changes in compensation policy; | |
| ● | changes in analyst recommendations or debt ratings; | |
| ● | financings and other events regarding the Company’s securities (e.g., defaults on loans or securities, calls of securities for redemption, share repurchase plans, stock splits, public or private sales of securities and changes to the rights of security holders); | |
| ● | dividend information; | |
| ● | the gain or loss of a significant customer or supplier; or | |
| ● | pending or threatened litigation or governmental investigations. |
| Information that something may happen, regardless of the likelihood, can be material. Courts often resolve close cases in favor of finding information material. Therefore, Insiders should err on the side of caution. Insiders should keep in mind that the Securities and Exchange Commission’s (“SEC”) rules and regulations provide that the mere fact that a person is aware of Material Non- Public Information is a bar to trading. It is no excuse that such person’s reasons for trading were not based on the information. | |
| 2.3 | “Non-Public Information.” Information is considered to be “Non-Public” unless it has been adequately disclosed to the public. This means that the information must be publicly disseminated and sufficient time must have passed for the securities markets to digest the information. |
| Page |
| It is important to note that information is not necessarily public merely because it has been discussed in the press or on social media, which will sometimes report rumors. In other words, the fact that rumors, speculation, or statements attributed to unidentified sources are public is insufficient to be considered widely disseminated even when the information is accurate. You should presume that information is non-public, unless you can point to its official release by the Company in at least one of the following ways: |
| ● | it has appeared in a publicly available filing with the SEC; or | |
| ● | issuance of a press release via major newswire such as the Dow Jones Broad Tape or the Associated Press. |
| You may not attempt to “beat the market” by trading simultaneously with, or shortly after, the official release of material information. After the information has been disseminated, a sufficient period of time must pass for the information to be absorbed by the general public. As a general rule, information should not be considered fully absorbed until the second trading day on the Nasdaq Stock Market LLC (“Nasdaq”) after the day on which the information is disseminated in a national news medium or disclosed in a filing with the SEC. Although there is no fixed period for how long it takes the market to absorb information, out of prudence a person in possession of Material Non-Public Information should from refrain from any trading activity until such time. | |
| Twenty-Twenty Hindsight. If securities transactions ever become the subject of scrutiny, they are likely to be viewed after-the-fact with the benefit of hindsight. As a result, before engaging in any transaction you should carefully consider how the transaction may be construed in the bright light of hindsight. If you have any questions or uncertainties about this Policy or a proposed transaction, please ask the Compliance Officer at sandra.gurrola@eternatx.com. | |
| 2.4 | “Security” or “Securities.” The term “security” or “securities” is defined very broadly by the securities laws and includes, among other things, stock (common and preferred), stock options, warrants, bonds, notes, debentures, convertible instruments, put or call options (e.g., exchange- traded options), and other similar instruments. |
| 2.5 | “Trade” or “Trading.” For purposes of this Policy, the term “trade” or “trading” means broadly any purchase, sale or other transaction to acquire, transfer or dispose of securities, including market option exercises, gifts or other contributions, exercises of stock options granted under the Company’s stock plans, sales of stock acquired upon the exercise of options and trades made under an employee benefit plan such as a 401(k) plan. |
| 3.0 | Statement of Policy |
| 3.1 | No Insider may trade in the Company’s securities at any time when the Insider has Material Non-Public Information concerning the Company. NOTE: it is your responsibility to determine whether you possess Material Non-Public Information, and any action on the part of the Company or any other employee pursuant to this Policy (or otherwise) does not in any way constitute legal advice or insulate an individual from liability under applicable securities laws. If you have any question whether information in your possession is Material Non-Public Information, then you should contact the Compliance Officer at ajackson@brooklynitx.com. |
| 3.2 | No Insider may trade in securities of another entity at any time when the Insider has Material Non-Public Information about that entity, including, without limitation, any of our customers, vendors, suppliers or partners, when that information was obtained as a result of the Insider’s employment or relationship to the Company. |
| Page |
| 3.3 | In addition to trading while in possession of Material Non-Public Information, no Insider may disclose (“tip”) Material Non-Public Information to any other person (including Family Members or entities, such as a trust or corporation), and no Insider may make buy or sell recommendations on the basis of Material Non-Public Information. In addition, Insiders should take care before trading on the recommendation of others to ensure that the recommendation is not the result of an illegal “tip.” |
| 3.4 | No Insider who receives or has access to the Company’s Material Non-Public Information may comment on stock price movements or rumors of other corporate developments (including discussions in Internet “chat rooms”) that are of possible significance to the investing public unless it is part of the Insider’s job (such as Investor Relations) or the Insider has been specifically authorized by the Compliance Officer. If you comment on stock price movements or rumors or disclose Material Non-Public Information to a third party you must contact the Compliance Officer immediately at sandra.gurrola@eternatx.com. |
| 3.5 | In addition, it is generally the practice of the Company not to respond to inquiries and/or rumors concerning the Company’s affairs. If you receive inquiries concerning the Company from the media or inquiries from securities analysts or other members of the financial community, you should refer such inquiries, without comment, to the Company’s Compliance Officer. Under no circumstances should you attempt to handle these inquiries without prior authorization from the Investor Relations Department. Only Company individuals specifically authorized to do so may answer questions about or disclose information concerning the Company. |
| 3.6 | Due to the heightened risk associated with the following transactions, no Insider, whether or not he or she possesses Material Non-Public Information, may engage in the following: |
| ● | Publicly Traded Options. Insiders may not trade in options, warrants, puts and calls or similar instruments on the Company’s securities. Given the relatively short term of publicly-traded options, transactions in options may create the appearance that an Insider is trading based on Material Nonpublic Information and focus an Insider’s attention on short-term performance at the expense of the Company’s long-term objectives. | |
| ● | Short Sales. Insiders may not sell securities of the Company “short.” A short sale has occurred if the seller: (i) does not own the securities sold; or (ii) does own the securities sold, but does not deliver them within twenty (20) days or place them in the mail within five (5) days of the sale. Short sales may reduce a seller’s incentive to seek to improve the Company’s performance, and often have the signal to the market that the seller lacks confidence in the Company’s prospects. | |
| ● | Margin Accounts and Pledges. Because a margin sale or foreclosure sale may occur at a time when a pledger is aware of Material Non-Public Information or otherwise is not permitted to trade in Company securities, directors, officers and Family Members of any of such persons and trusts, corporations and other entities over whom such person exercises influence or control over his, her or its securities trading decisions, may not hold Company securities in a margin account or otherwise pledge Company securities as collateral for a loan. See Section 6.2. | |
| ● | Hedging Transactions. No Insiders may engage in any hedging transactions (including variable forward contracts, equity swaps, collars and exchange funds) that are designed to hedge or offset any decrease in the market value of the Company’s equity securities. Hedging transactions may allow an Insider to continue to own Company securities, but without the full risks and rewards of ownership. This may lead to the Insider no longer having the same objectives as the Company’s other stockholders. |
| Page |
| ● | Standing and Limit Orders. Insiders may not place standing or limit orders on Company securities other than in connection with a Rule 10b5-1 trading plan (See Section 6.1). Standing and limit orders create heightened risks for insider trading violations because there is no control over the timing of purchases or sales that result from standing instructions to a broker, and as a result the broker could execute a transaction when an Insider is in possession of Material Non-Public Information. |
| 3.7 | This Policy continues to apply to transactions in Company securities even after termination of service with the Company. An Insider who is aware of Material Non-Public Information when he or she ceases to be an Insider may not trade in the Company’s securities until that information has become public or is no longer material. Questions or concerns on whether any continuing Non-Public information remains Material should be directed to the Compliance Officer. |
| 4.0 | Certain Exceptions |
| The prohibition on trading in the Company’s securities set forth in Section 3.0 above does not apply to: |
| ● | No Change in Beneficial Ownership. The trading restrictions in this Policy do not apply to transferring shares to an entity that does not involve a change in the beneficial ownership of the shares (for example, to an inter vivos trust of which you are the sole beneficiary during your lifetime). | |
| ● | Gifts of Securities. Bona fide gifts of securities are not transactions subject to this Policy unless the person making the gift has reason to believe that the recipient intends to sell the Company securities while the Insider is aware of Material Non-Public Information, or the person making the gift is subject to the trading restrictions specified in Section 5.0 (in which case pre-clearance is required). Gifts of securities may include gifts to trusts for estate planning purposes, as well as donations to a charitable organization. Whether a gift is “bona fide” may depend on various circumstances surrounding the gift. Accordingly, Insiders are encouraged to consult the Compliance Officer when contemplating a gift. | |
| ● | Stock Option Plan. The trading restrictions in this policy do not apply to the exercise of stock options pursuant to our stock plans where no Company common stock is sold in the market to fund the option exercise price or related taxes (i.e., a cashless exercise or where cash is paid to exercise the option) or to the exercise pursuant to which a person has elected to have the Company withhold shares subject to an option to satisfy tax withholding requirements. The trading restrictions do apply, however, to subsequent sales of any such stock received upon the exercise of options in which the proceeds are used to fund the option exercise price (i.e., a cashless exercise of options) or related taxes. In addition, the Company reserves the right to limit or restrict stock option exercises or tax withholdings not made pursuant to standing elections in appropriate circumstances. | |
| ● | Trading Plans. The execution of transactions pursuant to a trading plan that complies with SEC Rule 10b5-1 and which has been approved by the Company. See Section 6.1. |
| Page |
| ● | 401(k) Plan. To the extent the Company offers its securities as an investment option in the Company’s 401(k) plan, the trading restrictions in this Policy do not apply to the purchase of stock through the Company’s 401(k) plan resulting from periodic contributions of money to the plan through regular payroll deduction elections; however, the trading restrictions do apply to elections made under the 401(k) plan to (a) increase or decrease the percentage of periodic contributions that will be allocated to the Company stock fund, (b) make an intra-plan transfer of an existing account balance into or out of the Company stock fund, (c) borrow money against a 401(k) plan account if the loan will result in a liquidation of some or all of a Company stock fund balance and (d) pre-pay a plan loan if the pre-payment will result in allocation of loan proceeds to the Company stock fund. | |
| ● | Employee Stock Purchase Plan. To the extent the Company offers its securities as an investment option in an employee stock purchase plan, the trading restrictions in this Policy do not apply to the purchase of stock through the Company’s employee stock purchase plan resulting from periodic payroll contributions to the plan under an election made at the time of enrollment in the plan. The trading restrictions also do not apply to purchases of Company securities resulting from lump sum contributions to the plan provided that you elected to participate by lump sum payment at the beginning of the applicable enrollment period. However, the trading restrictions do apply to subsequent sales of any such stock purchased under the plan, an election to participate in the plan outside of an open enrollment period and changing instructions regarding the level of withholding contributions which are used to purchase stock made outside of an open enrollment period is subject to this Policy. | |
| ● | Restricted Stock Awards. The trading restrictions in this Policy do not apply to the vesting of shares of restricted stock, or the exercise of a tax withholding right pursuant to which you elect to have the Company withhold shares of stock to satisfy tax withholding requirements upon the vesting of any restricted stock. The trading restrictions do apply, however, to any market sale of restricted stock. | |
| ● | Registered Public Offering. The trading restrictions in this Policy do not apply to sales of the Company’s securities by selling stockholders in a registered public offering in accordance with applicable securities laws. | |
| ● | Other Similar Transactions. Any other purchase of Company securities directly from the Company or sales of Company securities directly to the Company may be exempted from the trading restrictions of this Policy with approval by the Compliance Officer or the Board of Directors. |
| NOTE: if you are a Section 16 Person (as defined in Section 5.1), you must notify the Compliance Officer prior to executing any transaction in reliance on any of the above exceptions. | |
| 5.0 | Special Blackout Periods, Pre-clearance of Trades and Other Procedures |
| 5.1 | Special Blackout Period Applicability. This Section 5.0 explains the requirements and procedures, which apply to all members of the Board of Directors and the executive officers (collectively, “Section 16 Persons”), as well as to others who may from time to time have access to Material Nonpublic Information about the Company. The Company may from time to time designate other individuals and/or positions that are subject to this Section 5.0. |
| Page |
| From time to time, the Company will notify persons that they are subject to the trading restrictions set forth in this Section 5.0 if the Company believes that, in the normal course of their duties, they have access to Material Non-Public Information. Such persons may include Non-Employee Personnel. | |
| Occasionally, Section 16 Persons and certain other individuals may have access to Material Non-Public Information for a limited period of time. During such a period, such persons, which shall always include all Section 16 Persons, and may include other Personnel and Non-Employee Personnel, may be notified that they are “Special Blackout Persons” who will be subject to the special blackout provisions set forth in Section 5.2. | |
| 5.2 | Special Blackout Period Suspension of Trading. From time to time, the Company may require that Special Blackout Persons suspend trading in the Company’s securities because of developments that have not yet been disclosed to the public. All Special Blackout Persons shall not trade in the Company’s securities while the suspension is in effect, and shall not disclose to any other person that the Company has suspended trading for certain individuals. Though these blackouts generally will arise because an event may occur that is material to the Company and is known by only a few directors, officers and/or employees, they may be declared for any reason. If the Company declares a special blackout period, the Company will deliver an e-mail (or other communication) notifying all Special Blackout Persons subject to the special blackout period when the special blackout period begins and when it ends. If, however, a person that is not a Special Blackout Person but whose trades are subject to pre-clearance requests permission to trade in the Company’s securities during the special blackout period, the Compliance Officer (or another designated individual) will inform the requesting person of a blackout period, without disclosing the reason for the blackout. Any person made aware of the existence of a special blackout period shall not disclose the existence of the blackout to any other person. NOTE: the Company’s delivery or non-delivery of these e-mails (or other communication) does not relieve you of your obligation to only trade in the Company’s securities in full compliance with this Policy. |
| 5.3 | Hardship Exemptions. Those subject to a special blackout period pursuant to Section 5.2 may request a hardship exemption during a special blackout period if they are not in possession of Material Non-Public Information and are not otherwise prohibited from trading pursuant to this Policy. Hardship exemptions are granted infrequently and only in exceptional circumstances. Any request for a hardship exemption should be made to the Compliance Officer at sandra.gurrola@eternatx.com. |
| 5.4 | Pre-Clearance of Trades Applicability. Section 16 Persons and Family Members of such persons and any other person or entity (including trusts, corporations and other entities) over whom the Section 16 Person exercises influence or control over his, her or its trading decisions (collectively, “Permanent Restricted Persons”), as well as Other Restricted Persons (as defined below), must obtain the advance approval of the Compliance Officer (or another designated individual) in accordance with Section 5.5 before effecting transactions in the Company’s securities, including any exercise of an option (whether cashless or otherwise), gifts, loans, pledges, rights or warrant to purchase or sell such securities, contribution to a trust or other transfers, whether the transaction is for the individual’s own account, one over which he or she exercises control or one in which he or she has a beneficial interest. Each proposed transaction will be evaluated to determine if it raises insider trading concerns or other concerns under federal laws and regulations. Any advice will relate solely to the restraints imposed by law and will not constitute advice regarding the investment aspects of any transaction. The requirements set forth in this Section 5.4 apply at all times for Permanent Restricted Persons. |
| From time to time, the Company will notify Personnel and Non-Employee Personnel (other than Permanent Restricted Persons) that they are subject to the pre-clearance requirements set forth in Section 5.5 if the Company believes that, in the normal course of their duties, they are likely to have regular access to Material Non-Public Information (together with Family Members of any of such persons and trusts, corporations and other entities controlled by any of such persons, “Other Restricted Persons”). |
| Page |
| Occasionally, individuals other than Permanent Restricted Persons and Other Restricted Persons may have access to Material Non-Public Information for a limited period of time (together with Family Members of any of such persons and trusts, corporations and other entities controlled by any of such persons, “Special Restricted Persons”). During such a period, such persons may be notified that they are Special Restricted Persons who will be subject to the pre-clearance requirements set forth in Section 5.5. | |
| 5.5 | Pre-Clearance Procedures. Subject to Section 6.1, Permanent Restricted Persons, Other Restricted Persons and Special Restricted Persons shall submit a request for pre-clearance to the Compliance Officer (or another designated individual) at least two business days in advance of the proposed transaction and by completing the attached “Request for Approval” form. Clearance of a transaction must be re-requested if the transaction order is not placed within twenty-four hours after obtaining pre-clearance. If pre-clearance is denied, the fact of such denial must be kept confidential by the person requesting such clearance. Approval must be in writing, dated and signed, specifying the securities involved. Pre-clearance correspondence with the Compliance Officer may be directed to the following email address: sandra.gurrola@eternatx.com. |
| When requesting pre-clearance, the requestor should carefully consider whether he or she may be aware of any Material Non-Public Information about the Company, and should describe fully those circumstances to the Compliance Officer. The requestor should also indicate whether he or she has effected any non-exempt “opposite-way” transactions within the past six months, and should be prepared to report the proposed transaction on an appropriate Form 4 or 5, if applicable. | |
| The requestor should also be prepared to comply with SEC Rule 144 and file Form 144, if advisable, at the time of any sale. | |
| 5.6 | General Restrictions Apply. For the avoidance of doubt, the provisions of Section 3 of this Policy continue to apply regardless of whether such Insider has obtained pre-clearance approval. You must consult the Compliance Officer whenever you are in doubt. |
| 5.7 | Questions. Because of the technical nature of some aspects of the federal securities laws, all directors and officers should review this material carefully and contact the Compliance Officer if at any time (i) you have questions about this Policy or its application to a particular situation; or (ii) you plan to trade in the Company’s securities, but are unsure as to whether the transaction might be in conflict with the securities laws and/or this Company Policy (including this Section 5.0). |
| 5.8 | Acknowledgement. All directors, officers and other employees subject to the procedures set forth in this Section 5.0 must acknowledge their understanding of, and intent to comply with, the Company’s Securities Trading Policy and this Section 5.0 on the form attached to this Policy. |
| 6.0 | 10b5-1 Plans/Margin Accounts and Pledges/Short Sales |
| 6.1 | 10b5-1 Trading Plans. Notwithstanding the prohibition against insider trading, Rule 10b5-1 (“Rule 10b5-1”) provides an affirmative defense against insider trading under Rule 10b-5. A person subject to this Policy can rely on this defense and trade in the Company’s securities, regardless of their awareness of inside information, if the transaction occurs pursuant to a pre-arranged written trading plan that was entered into when the person was not in possession of Material Non-Public Information and that complies with the requirements of Rule 10b5-1. A Rule 10b5-1 trading plan is a binding, written contract between you and your broker that specifies the price, amount, and date of trades to be executed in your account in the future, or provides a formula or mechanism that your broker will follow. A 10b5-1 trading plan can be established only when you do not possess Material Non-Public Information. Therefore, Insiders cannot enter into these plans at any time when in possession of Material Non-Public Information. In addition, a 10b5-1 trading plan must not permit you to exercise any subsequent influence over how, when, or whether the purchases or sales are made. |
| Page |
| As noted above, you have an affirmative defense against any claim by the SEC against you for insider trading if your trade was made under a 10b5-1 trading plan that you entered into when you were not aware of Material Non-Public Information. The rules regarding 10b5-1 trading plans are complex and you must fully comply with them. You should consult with your legal advisor before proceeding. | |
| Each Insider must pre-clear with the Compliance Officer his or her proposed 10b5-1 trading plan at least five business days prior to the entry into such plan. The Company reserves the right to withhold preclearance of any 10b5-1 trading plan that the Company determines is not consistent with the rules regarding such plans. Notwithstanding any pre-clearance of a 10b5-1 trading plan, the Company assumes no liability for the consequences of any transaction made pursuant to such plan. | |
| For Insiders, any modification or termination of a pre-approved 10b5-1 trading plan requires pre-clearance by the Compliance Officer. In addition, any modification or termination of a pre-approved 10b5-1 trading plan must occur before you become aware of any Material Non-Public Information and must comply with the requirements of the rules regarding 10b5-1 trading plans. | |
| Further, after entering into or amending a pre-cleared 10b5-1 trading plan, no Insider shall effect any transaction pursuant to such plan for thirty business days following such entry or amendment. Transactions effected pursuant to a pre-cleared 10b5-1 trading plan will not require further pre-clearance at the time of the transaction if the plan specifies the dates, prices and amounts of the contemplated trades, or establishes a formula for determining the dates, prices and amounts. | |
| Finally, if you are a Section 16 Person, 10b5-1 trading plans require special care. Because you can specify conditions that trigger a purchase or sale in a 10b5-1 trading plan, you may not even be aware that a transaction has taken place and you may not be able to comply with the SEC’s requirement that you report your transaction to the SEC within two business days after its execution. Therefore, for Section 16 Persons, a transaction executed according to a 10b5-1 trading plan is not permitted unless the 10b5-1 trading plan requires that your broker notify the Company before the close of business on the day of the execution of the transaction. See Section 8.0. | |
| You may amend or replace a Trading Plan only during periods when trading is permitted in accordance with this Policy, and you must submit any proposed amendment or replacement of a Trading Plan to the Compliance Officer for approval prior to adoption. You must provide notice to the Compliance Officer prior to terminating a Trading Plan. You should understand that frequent modifications or terminations of a Trading Plan may call into question your good faith in entering into the plan (and therefore may jeopardize the availability of the affirmative defense against insider trading allegations). | |
| 6.2 | Margin Accounts and Pledges. Securities purchased on margin may be sold by the broker without the customer’s consent if the customer fails to meet a margin call. Similarly, securities held in an account which may be borrowed against or are otherwise pledged (or hypothecated) as collateral for a loan may be sold in foreclosure if the borrower defaults on the loan. Accordingly, if you purchase securities on margin or pledge them as collateral for a loan, a margin sale or foreclosure sale may occur at a time when you are aware of Material Non-Public Information or otherwise are not permitted to trade in our securities. The sale, even though not initiated at your request, is still a sale for your benefit and may subject you to liability under the insider trading rules if made at a time when you are aware of Material Non-Public Information. Similar cautions apply to a bank or other loans for which you have pledged stock as collateral. |
| Page |
| Therefore, no Insiders and Family Members of any of such persons and trusts, corporations and other entities over whom such person exercises influence or control over his, her or its securities trading decisions, whether or not in possession of Material Non-Public Information, may purchase the Company’s securities on margin, or borrow against any account in which the Company’s securities are held, or pledge the Company’s securities as collateral for a loan. Such persons or entities who have pledged shares or hold securities in margin accounts must unwind or remove such securities. | |
| 7.0 | Potential Criminal and Civil Liability and/or Disciplinary Action |
| 7.1 | Individual Responsibility. Each Insider is individually responsible for complying with the securities laws and this Policy, regardless of whether the Company has prohibited trading by that Insider or any other Insiders. Trading in securities outside of any suspension periods should not be considered a “safe harbor.” |
| You should also bear in mind that any proceeding alleging improper trading will necessarily occur after the trade has been completed and is particularly susceptible to second-guessing with the benefit of hindsight. Therefore, as a practical matter, before engaging in any transaction you should carefully consider how enforcement authorities and others might view the transaction in hindsight. | |
| 7.2 | Controlling Persons. The securities laws provide that, in addition to sanctions against an individual who trades illegally, penalties may be assessed against what are known as “controlling persons” with respect to the violator. The term “controlling person” is not defined, but includes employers (i.e., the Company), its directors, officers and managerial and supervisory personnel. The concept is broader than what would normally be encompassed by a reporting chain. Individuals may be considered “controlling persons” with respect to any other individual whose behavior they have the power to influence. Liability can be imposed only if two conditions are met. First, it must be shown that the “controlling person” knew or recklessly disregarded the fact that a violation was likely. Second, it must be shown that the “controlling person” failed to take appropriate steps to prevent the violation from occurring. For this reason, the Company’s supervisory personnel are directed to take appropriate steps to ensure that those they supervise, understand and comply with the requirements set forth in this Policy. |
| 7.3 | Potential Sanctions. |
| (i) | Liability for Insider Trading and Tipping. Insiders, controlling persons and the Company may be subject to civil penalties, criminal penalties and/or jail for trading in securities when they have Material Non-Public Information or for improper transactions by any person (commonly referred to as a “tippee”) to whom they have disclosed Material Non-Public Information, or to whom they have made recommendations or expressed opinions on the basis of such information about trading securities. In addition to injunctive relief, disgorgement, and other ancillary remedies, U.S. law empowers the government to seek significant civil penalties against persons found liable of insider trading, including as tippers or tippees. The SEC has imposed large penalties even when the disclosing person did not profit from the trading. The SEC, the stock exchanges and the Financial Industry Regulatory Authority use sophisticated electronic surveillance techniques to uncover insider trading. |
| Page |
| (ii) | Possible Disciplinary Actions. Subject to applicable law, Personnel who violate this Policy will be subject to disciplinary action, up to and including termination of employment for cause, whether or not the Personnel’s failure to comply results in a violation of law. Needless to say, a violation of law, or even an SEC investigation that does not result in prosecution, can tarnish one’s reputation and irreparably damage a career. |
| 7.4 | Questions and Violations. Anyone with questions concerning this Policy or its application should contact the Compliance Officer. Any violation or perceived violation should be reported immediately to the Compliance Officer. |
| 8.0 | Broker Requirements for Section 16 Persons |
| The timely reporting of transactions requires tight interface with brokers handling transactions for our Section 16 Persons. A knowledgeable, alert broker can also serve as a gatekeeper, helping to ensure compliance with our pre-clearance procedures and helping prevent inadvertent violations. Therefore, in order to facilitate timely compliance by the directors and executive officers of the Company with the requirements of Section 16 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), brokers for Section 16 Persons need to comply with the following requirements: |
| ● | not to enter any order (except for orders under pre-approved Rule 10b5-1 plans) without first verifying with the Company that your transaction was pre-cleared and complying with the brokerage firm’s compliance procedures (e.g., Rule 144); and | |
| ● | to report before the close of business on the day of the execution of the transaction to the Company by telephone and in writing via e-mail to the Compliance Officer, the complete (i.e., date, type of transaction, number of shares and price) details of every transaction involving the Company’s stock, including gifts, transfers, pledges and all 10b5-1 transactions. |
| Because it is the legal obligation of the trading person to cause any filings on Form 3, Form 4, Form 5 or Form 144 (or as may otherwise be required) to be made, you are strongly encouraged to confirm with your broker, following any transaction, that he or she has telephoned and e-mailed the required information to the Company. | |
| 9.0 | Confidentiality |
| If material information relating to the Company or its business is considered Non-Public Information, such information must be kept in strict confidence and should be discussed only in the ordinary course of business with persons who have a “need to know” the information for legitimate business purposes and then only when the Personnel disclosing the information has no reason to believe that the recipient will misuse or improperly disclose the information. When such information is disclosed, the recipient must be told that such information may be used only for the business purpose related to its disclosure and that the information must be held in strict confidence. The utmost care and circumspection must be exercised at all times in order to protect the Company’s confidential information. The following practices should be followed to help prevent the misuse of confidential information: |
| ● | Always put confidential documents away when not in use and, based upon the sensitivity of the material, keep such documents in a locked desk or office. Written information should be appropriately safeguarded and should not be left where it may be seen by persons who do not have a need to know the contents of the documents. |
| Page |
| ● | Non-Public Information should not be discussed with any person within the Company under circumstances where it could be overheard by people who do not have a valid need to know such information, including public areas such as elevators, restaurants and airplanes. See also Section 7.2 above. | |
| ● | Take great care when discussing confidential information on speaker phones or on cellular phones in locations where you may be overheard. Do not discuss such information with relatives or social acquaintances. | |
| ● | Do not share your computer or other account IDs and passwords with any other person. Password protect computers and log off when they are not in use. |
| In addition to other circumstances where it may be applicable, this confidentiality policy must be strictly adhered to in responding to inquiries about the Company that may be made by the press, securities analysts or other members of the financial community. It is important that responses to any such inquiries be made on behalf of the Company by a duly designated officer. Accordingly, Personnel should not respond to any such inquiries and should refer all such inquiries to the Company’s Investor Relations Officer or the Compliance Officer. See also Sections 3.4 and 3.5 above. | |
| 10.0 | Legal Effect of this Policy |
| The terms of this Policy, including the procedures that implement this Policy, are not intended to serve as precise recitations of the legal prohibitions against insider trading and tipping which are highly complex, fact specific and evolving. Certain of the procedures are designed to prevent even the appearance of impropriety and in some respects may be more restrictive than the securities laws. Therefore, these procedures are not intended to serve as a basis for establishing civil or criminal liability that would not otherwise exist. | |
| 11.0 | Reporting Violations/Seeking Advice |
| Suspected violations of this Policy should be referred to the Compliance Officer. In addition, if you: (a) receive Material Non-Public Information that you are not authorized to receive or that you do not legitimately need to know to perform your employment responsibilities, or (b) receive confidential information and are unsure if it is within the definition of Material Non-Public Information or whether its release might be contrary to a fiduciary or other duty or obligation, you should not share it with anyone. To seek advice about what to do under those circumstances, you should contact the Compliance Officer. Consulting your colleagues can have the effect of exacerbating the problem. Containment of the information, until the legal implications of possessing it are determined, is critical. |
| Page |
EXHIBIT 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We have issued our report dated March 12, 2025, with respect to the consolidated financial statements included in the Annual Report of Eterna Therapeutics Inc. on Form 10-K for the year ended December 31, 2024. We consent to the incorporation by reference of said report in the Registration Statements of Eterna Therapeutics Inc. on Forms S-1 (File No. 333-261185, File No. 333-256570, File No. 333-271279 and File No. 333-283003), on Form S-3 (File No. 333-264585) and on Forms S-8 (File No. 333-260200, File No. 333-256760 and File No. 333-276521).
/s/ GRANT THORNTON LLP
Iselin, New Jersey
March 12, 2025
EXHIBIT 31.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER PURSUANT TO
SECURITIES EXCHANGE ACT RULES 13a-14(a) AND 15(d)-14(a), AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Sanjeev Luther, certify that:
1. I have reviewed this annual report on Form 10-K of Eterna Therapeutics Inc. (the “Company”);
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; | |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; | |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and | |
| (d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and | |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Dated: March 12, 2025 | /s/ Sanjeev Luther | |
|
Sanjeev Luther President and Chief Executive Officer Eterna Therapeutics Inc. |
EXHIBIT 31.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER PURSUANT TO
SECURITIES EXCHANGE ACT RULES 13a-14(a) AND 15(d)-14(a), AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Sandra Gurrola, certify that:
1. I have reviewed this annual report on Form 10-K of Eterna Therapeutics Inc. (the “Company”);
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; | |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; | |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and | |
| (d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and | |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Dated: March 12, 2025 | /s/ Sandra Gurrola | |
|
Sandra Gurrola Senior Vice President of Finance Eterna Therapeutics Inc. |
EXHIBIT 32.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED
PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Eterna Therapeutics Inc. (the “Registrant”) on Form 10-K for the year ended December 31, 2024 (the “Report”), I, Sanjeev Luther, President and Chief Executive Officer of the Registrant, do hereby certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, that, to my knowledge:
(1) the Report, as filed with the Securities and Exchange Commission, fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Registrant.
| Dated: March 12, 2025 | /s/ Sanjeev Luther | |
|
Sanjeev Luther President and Chief Executive Officer Eterna Therapeutics Inc. |
EXHIBIT 32.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED
PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Eterna Therapeutics Inc. (the “Registrant”) on Form 10-K for the year ended December 31, 2024 (the “Report”), I, Sandra Gurrola, Senior Vice President of Finance of the Registrant, do hereby certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, that, to my knowledge:
(1) the Report, as filed with the Securities and Exchange Commission, fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Registrant.
| Dated: March 12, 2025 | /s/ Sandra Gurrola | |
|
Sandra Gurrola Senior Vice President of Finance Eterna Therapeutics Inc. |