UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of May 2024
Commission File Number: 001-38064
Aeterna Zentaris Inc.
(Translation of registrant’s name into English)
c/o Norton Rose Fulbright Canada, LLP, 222 Bay Street, Suite 3000, PO Box 53, Toronto ON M5K 1E7
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1):
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7):
Exhibits 99.1 and 99.2, included with this report on Form 6-K are hereby incorporated by reference into the Registrant’s Registration Statements on Forms S-8 (No. 333-224737, No. 333-210561 and No. 333-200834) and Forms F-3 (No.333-232935 and No. 333-254680) and shall be deemed to be a part thereof from the date on which this report is furnished, to the extent not superseded by documents or reports subsequently filed or furnished.
DOCUMENTS INDEX
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AETERNA ZENTARIS INC. | |||
| Date: May 15, 2023 | By: | /s/ Klaus Paulini | |
| Klaus Paulini | |||
| President and Chief Executive Officer | |||
Exhibit 99.1

Aeterna Zentaris Inc.
Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars)
(Unaudited)
|
|
Aeterna Zentaris Inc.
Condensed Interim Consolidated Statements of Financial Position
(In thousands of US dollars)
(Unaudited)
|
As of March 31, 2024 |
As of December 31, 2023 |
|||||||
| $ | $ | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | 29,545 | 34,016 | ||||||
| Trade and other receivables | 190 | 222 | ||||||
| Inventory | 64 | 66 | ||||||
| Income taxes receivable | 118 | 121 | ||||||
| Prepaid expenses and other current assets | 1,278 | 1,942 | ||||||
| Total current assets | 31,195 | 36,367 | ||||||
| Non-current assets | ||||||||
| Restricted cash equivalents | 326 | 332 | ||||||
| Property and equipment | 271 | 317 | ||||||
| Total non-current assets | 597 | 649 | ||||||
| Total assets | 31,792 | 37,016 | ||||||
| LIABILITIES | ||||||||
| Current liabilities | ||||||||
| Payables and accrued liabilities (note 4) | 4,315 | 3,622 | ||||||
| Provisions | 422 | 429 | ||||||
| Income taxes payable | 109 | 111 | ||||||
| Deferred revenues (note 3) | 252 | 218 | ||||||
| Lease liabilities | 154 | 160 | ||||||
| Total current liabilities | 5,252 | 4,540 | ||||||
| Non-current liabilities | ||||||||
| Deferred revenues (note 3) | 1,471 | 1,544 | ||||||
| Lease liabilities | 80 | 119 | ||||||
| Employee future benefits (note 5) | 12,019 | 12,617 | ||||||
| Total non-current liabilities | 13,570 | 14,280 | ||||||
| Total liabilities | 18,822 | 18,820 | ||||||
| Shareholders’ equity | ||||||||
| Share capital (note 6) | 293,410 | 293,410 | ||||||
| Warrants | 5,085 | 5,085 | ||||||
| Contributed surplus | 90,718 | 90,710 | ||||||
| Deficit | (375,264 | ) | (369,831 | ) | ||||
| Accumulated other comprehensive loss | (979 | ) | (1,178 | ) | ||||
| Total Shareholders’ equity | 12,970 | 18,196 | ||||||
| Total liabilities and shareholders’ equity | 31,792 | 37,016 | ||||||
Commitments (note 11)
Subsequent event (note 12)
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Approved by the Board of Directors
| /s/ Carolyn Egbert | /s/ Dennis Turpin | |
| Carolyn Egbert, Chair of the Board | Dennis Turpin, Director |
|
|
Aeterna Zentaris Inc.
Condensed Interim Consolidated Statements of Changes in Shareholders’ Equity
For the three months ended March 31, 2024, and 2023
(In thousands of US dollars)
(Unaudited)
| Share capital | Warrants | Contributed surplus | Deficit | Accumulated other comprehensive loss | Total | |||||||||||||||||||
| $ | $ | $ | $ | $ | $ | |||||||||||||||||||
| Balance – December 31, 2023 | 293,410 | 5,085 | 90,710 | (369,831 | ) | (1,178 | ) | 18,196 | ||||||||||||||||
| Net loss | - | - | - | (5,752 | ) | - | (5,752 | ) | ||||||||||||||||
| Other comprehensive loss: | ||||||||||||||||||||||||
| Foreign currency translation adjustments | - | - | - | - | 199 | 199 | ||||||||||||||||||
| Actuarial gain on defined benefit plans (note 5) | - | - | - | 319 | - | 319 | ||||||||||||||||||
| Comprehensive loss | (5,433 | ) | 199 | (5,234 | ) | |||||||||||||||||||
| Share-based compensation costs | - | - | 8 | - | - | 8 | ||||||||||||||||||
| Balance – March 31, 2024 | 293,410 | 5,085 | 90,718 | (375,264 | ) | (979 | ) | 12,970 | ||||||||||||||||
| Share capital | Warrants | Contributed surplus | Deficit | Accumulated other comprehensive loss | Total | |||||||||||||||||||
| $ | $ | $ | $ | $ | $ | |||||||||||||||||||
| Balance – December 31, 2022 | 293,410 | 5,085 | 90,332 | (352,084 | ) | (967 | ) | 35,776 | ||||||||||||||||
| Net loss | - | - | - | (4,255 | ) | - | (4,255 | ) | ||||||||||||||||
| Other comprehensive loss: | ||||||||||||||||||||||||
| Foreign currency translation adjustments | - | - | - | - | (168 | ) | (168 | ) | ||||||||||||||||
| Actuarial loss on defined benefit plans | - | - | - | (162 | ) | - | (162 | ) | ||||||||||||||||
| Comprehensive income | (4,417 | ) | (168 | ) | (4,585 | ) | ||||||||||||||||||
| Share-based compensation costs | - | - | 17 | - | - | 17 | ||||||||||||||||||
| Balance – March 31, 2023 | 293,410 | 5,085 | 90,349 | (356,501 | ) | (1,135 | ) | 31,208 | ||||||||||||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
|
|
Aeterna Zentaris Inc.
Condensed Interim Consolidated Statements of Loss and Comprehensive Loss
For the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data)
(Unaudited)
| Three months ended | ||||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Revenues (note 3) | 4 | 2,128 | ||||||
| Expenses | ||||||||
| Cost of sales | 16 | 17 | ||||||
| Research and development | 2,584 | 4,012 | ||||||
| Selling, general and administrative (note 10) | 3,514 | 2,306 | ||||||
| Total expenses | 6,114 | 6,335 | ||||||
| Loss from operations | (6,110 | ) | (4,207 | ) | ||||
| Gain (loss) due to changes in foreign currency exchange rates | 77 | (59 | ) | |||||
| Interest income | 285 | 13 | ||||||
| Other finance costs | (4 | ) | (2 | ) | ||||
| Net finance income (costs) | 358 | (48 | ) | |||||
| Loss before income taxes | (5,752 | ) | (4,255 | ) | ||||
| Income tax recovery | - | - | ||||||
| Net loss | (5,752 | ) | (4,255 | ) | ||||
| Other comprehensive loss: | ||||||||
| Items that may be reclassified subsequently to profit or loss: | ||||||||
| Foreign currency translation adjustments | 199 | (168 | ) | |||||
| Items that will not be reclassified to profit or loss: | ||||||||
| Actuarial gain (loss) on defined benefit plans (note 5) | 319 | (162 | ) | |||||
| Comprehensive loss | (5,234 | ) | (4,585 | ) | ||||
| Basic and diluted loss per share (note 8) | (4.74 | ) | (3.51 | ) | ||||
| Weighted average number of shares outstanding (basic and diluted)1 | 1,213,969 | 1,213,969 | ||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
| 1 | On May [3], 2024, the Company’s shares were consolidated on a 1-for-4 reverse stock split, which has been reflected retrospectively in the financial statements and further discussed in note 6. |
|
|
Aeterna Zentaris Inc.
Condensed Interim Consolidated Statements of Cash Flows
For the three months ended March 31, 2024, and 2023
(In thousands of US dollars)
(Unaudited)
| Three months ended | ||||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Cash flows from operating activities | ||||||||
| Net loss for the period | (5,752 | ) | (4,255 | ) | ||||
| Items not affecting cash and cash equivalents: | ||||||||
| Depreciation and amortization | 44 | 40 | ||||||
| Share-based compensation costs | 8 | 17 | ||||||
| Employee future benefits | 130 | 135 | ||||||
| Net foreign exchange differences | (6 | ) | 8 | |||||
| Amortization of deferred revenues | - | (760 | ) | |||||
| Provisions | - | (8 | ) | |||||
| Other non-cash items | 5 | 1 | ||||||
| Changes in operating assets and liabilities (note 7) | 1,281 | 761 | ||||||
| Net cash used in operating activities | (4,290 | ) | (4,061 | ) | ||||
| Cash flows from financing activities | ||||||||
| Payments on lease liabilities | (42 | ) | (38 | ) | ||||
| Net cash used in financing activities | (42 | ) | (38 | ) | ||||
| Cash flows from investing activities | ||||||||
| Purchase of property and equipment | (5 | ) | (2 | ) | ||||
| Net cash used in investing activities | (5 | ) | (2 | ) | ||||
| Effect of exchange rate changes on cash and cash equivalents | (134 | ) | 50 | |||||
| Net change in cash and cash equivalents | (4,471 | ) | (4,051 | ) | ||||
| Cash and cash equivalents – Beginning of period | 34,016 | 50,611 | ||||||
| Cash and cash equivalents – End of period | 29,545 | 46,560 | ||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
1. Business overview
Summary of business
Aeterna Zentaris is a specialty biopharmaceutical company commercializing and developing therapeutics and diagnostic tests. The Company’s lead product, Macrilen® (macimorelin), is the first and only U.S. Food and Drug Administration (“FDA”) and European Medicines Agency (“EMA”) approved oral test indicated for the diagnosis of patients with adult growth hormone deficiency (“AGHD”). Macimorelin is currently marketed under the tradename Ghryvelin™ in the European Economic Area and the United Kingdom through an exclusive licensing agreement with Pharmanovia. The Company’s several other license and commercialization partners are also seeking approval for commercialization of macimorelin in Israel and the Palestinian Authority, the Republic of Korea, Turkey and several non-European Union Balkan countries. The Company is actively pursuing business development opportunities for the commercialization of macimorelin in North America, Asia and the rest of the world.
The Company is also dedicated to the development of therapeutic assets and has taken steps to establish a pre-clinical pipeline to potentially address unmet medical needs across several indications with a focus on rare or orphan indications.
These unaudited condensed interim consolidated financial statements were approved by the Board of Directors (the “Board”) on May 14, 2024.
2. Basis of presentation
These unaudited condensed interim consolidated financial statements have been prepared in accordance with IAS 34, Interim Financial Reporting as issued by the International Accounting Standards Board.
The unaudited condensed interim consolidated financial statements do not include all the notes normally included in annual consolidated financial statements. Accordingly, these unaudited condensed interim consolidated financial statements should be read in conjunction with the Company’s annual consolidated financial statements as of and for the year ended December 31, 2023.
The accounting policies used in these condensed interim consolidated financial statements are consistent with those presented in the Company’s annual consolidated financial statements.
New standards and amendments
Several amendments apply for the first time in 2024, but do not have an impact on the interim condensed consolidated financial statements of the Company. The Company has not early adopted any standard, interpretation or amendment that has been issued but is not yet effective.
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
Critical accounting estimates and judgements
The preparation of condensed interim consolidated financial statements in accordance with IFRS requires management to make judgements, estimates and assumptions that affect the reported amounts of the Company’s assets, liabilities, revenues, expenses and related disclosures. Judgements, estimates and assumptions are based on historical experience, expectations, current trends and other factors that management believes to be relevant at the time at which the Company’s condensed interim consolidated financial statements are prepared.
Management reviews, on a regular basis, the Company’s accounting policies, assumptions, estimates and judgements in order to ensure that the condensed interim consolidated financial statements are presented fairly and in accordance with IFRS applicable to interim financial statements. Critical accounting estimates and assumptions, as well as critical judgements used in applying accounting policies in the preparation of the Company’s condensed interim consolidated financial statements, were the same as those applied to the Company’s annual consolidated financial statements as of and for the year ended December 31, 2023.
3. Revenue
The Company derives revenue from the transfer of goods and services over time and at a point in time in the following categories:
Summary of revenue from transfer of goods and services
| Three months ended | ||||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Royalties | 4 | 18 | ||||||
| License fees | - | 760 | ||||||
| Development services | - | 1,339 | ||||||
| Supply chain | - | 11 | ||||||
| Total | 4 | 2,128 | ||||||
The Company recorded revenue for the transfer of services overtime for the three months ended March 31, 2024, $nil (2023 – $2,110). Revenue recorded at a point in time for the three months ended March 31, 2024, was $4 (2023 – $18).
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
Liabilities related to contracts with customers
The following table provides a summary of deferred revenue balances:
Summary of deferred revenue
| March 31, 2024 | ||||||||||||
| Current | Non-current | Total | ||||||||||
| $ | $ | $ | ||||||||||
| Pharmanovia | 244 | 1,350 | 1,594 | |||||||||
| NK Meditech | 8 | 121 | 129 | |||||||||
| Contract liabilities | 252 | 1,471 | 1,723 | |||||||||
| December 31, 2023 | ||||||||||||
| Current | Non-current | Total | ||||||||||
| $ | $ | $ | ||||||||||
| Pharmanovia | 209 | 1,420 | 1,629 | |||||||||
| NK Meditech | 9 | 124 | 133 | |||||||||
| Contract liabilities | 218 | 1,544 | 1,762 | |||||||||
4. Payables and accrued liabilities
Summary of detailed information about payables and accrued liabilities
| Three months ended | ||||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Trade accounts payable | 2,402 | 1,866 | ||||||
| Accrued research and development costs | 438 | 804 | ||||||
| Accrued employee benefits | 411 | 343 | ||||||
| Payroll tax and other statutory liabilities | 60 | 78 | ||||||
| Other accrued liabilities | 1,004 | 531 | ||||||
| Payables and accrued liabilities | 4,315 | 3,622 | ||||||
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
5. Employee future benefits
The change in the Company’s employee future benefit obligations is summarized as follows:
Summary of net defined benefit liability asset
|
Three months ended March 31, 2024 |
Year ended December 31, 2023 | |||||||||||||||
| Pension | Other | |||||||||||||||
| benefit plans | benefit plans | Total | Total | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Change in plan liabilities | ||||||||||||||||
| Balances – Beginning of the year | 23,622 | 98 | 23,720 | 21,750 | ||||||||||||
| Current service cost (residual value) | 32 | 3 | 35 | 120 | ||||||||||||
| Interest cost | 185 | - | 185 | 807 | ||||||||||||
| Actuarial loss (gain) arising from changes in financial assumptions | (319 | ) | - | (319 | ) | 1,213 | ||||||||||
| Benefits paid | (183 | ) | - | (183 | ) | (815 | ) | |||||||||
| Impact of foreign exchange rate changes | (524 | ) | (3 | ) | (527 | ) | 645 | |||||||||
| Balances – End of the period | 22,813 | 98 | 22,911 | 23,720 | ||||||||||||
| Change in plan assets | ||||||||||||||||
| Balances – Beginning of the year | 11,103 | - | 11,103 | 10,591 | ||||||||||||
| Interest income from plan assets | 88 | - | 88 | 396 | ||||||||||||
| Employer contributions | 9 | - | 9 | 36 | ||||||||||||
| Employee contributions | 3 | - | 3 | 13 | ||||||||||||
| Benefits paid | (63 | ) | - | (63 | ) | (266 | ) | |||||||||
| Remeasurement of plan assets | - | - | - | 15 | ||||||||||||
| Impact of foreign exchange rate changes | (248 | ) | - | (248 | ) | 318 | ||||||||||
| Balances – End of the year | 10,892 | - | 10,892 | 11,103 | ||||||||||||
| Net liability of the unfunded plans | 11,184 | 98 | 11,282 | 11,682 | ||||||||||||
| Net liability of the funded plans | 737 | - | 737 | 935 | ||||||||||||
| Net amount recognized as Employee future benefits | 11,921 | 98 | 12,019 | 12,617 | ||||||||||||
| Amounts recognized: | ||||||||||||||||
| In net loss | 126 | 4 | 130 | 520 | ||||||||||||
| Actuarial gain (loss) on defined benefit plans in other comprehensive (gain) loss | 319 | - | 319 | (1,195 | ) | |||||||||||
The calculation of the employee future benefit obligation is sensitive to the discount rate assumption and other assumptions such as the rate of the pension benefit increase. Discount rates were 3.40% as of March 31, 2024 and 3.30% as of December 31, 2023, causing the variances in the actuarial gain on defined benefit plan during the three months ended March 31, 2024.
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
Share capital
The Company has authorized an unlimited number of common shares (being voting and participating shares) with no par value, as well as an unlimited number of preferred, first and second ranking shares, issuable in series, with rights and privileges specific to each class, with no par value.
Subsequent to the end of the reporting period of March 31, 2024, on May 1, 2024, the Company’s board of directors approved an
amendment to the Company’s articles of incorporation to effect a 1-for-4 reverse stock split (“reverse split”) of
the Company’s common shares, deferred share units (DSU), stock options and warrants outstanding as of the effective
date. Accordingly, all common shares, DSU, stock options, warrants and per share amounts in these interim condensed consolidated financial
statements have been retroactively adjusted for all periods presented to give effect to the reverse stock split. The reverse split was effected in the
markets on May 3, 2024.
| Common shares | Amount | |||||||
| # | $ | |||||||
| Balance – December 31, 2023 | 1,213,969 | 293,410 | ||||||
| - | - | |||||||
| Balance – March 31, 2024 | 1,213,969 | 293,410 | ||||||
Share-based compensation
During the three months ended March 31, 2024, the Company granted nil (2023 – 3,500) stock options and nil (2023 – nil) deferred share units under the Long-Term Incentive Plan. The compensation expense for the three months ended March 31, 2024, was $8 (2023 – $17) recognized over the vesting period. Option activity for the three months ended March 31, 2024, and 2023, was as follows:
| Stock options | Weighted average exercise price | |||||||
| # | $ | |||||||
| Balance – December 31, 2023 | 13,350 | 50.04 | ||||||
| Forfeited | (167 | ) | 42.04 | |||||
| Balance – March 31, 2024 | 13,183 | 50.14 | ||||||
| Stock options | Weighted average exercise price | |||||||
| # | $ | |||||||
| Balance – December 31, 2022 | 10,508 | 80.20 | ||||||
| Granted | 3,500 | 15.00 | ||||||
| Balance – March 31, 2023 | 14,008 | 63.91 | ||||||
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
7. Supplemental disclosure of cash flow information
Summary of changes in operating assets and liabilities
| Three months ended | ||||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Trade and other receivables | 74 | 203 | ||||||
| Inventory | - | 5 | ||||||
| Prepaid expenses and other current assets | 625 | 626 | ||||||
| Payables and accrued liabilities | 711 | 235 | ||||||
| Deferred revenues | - | (154 | ) | |||||
| Provision for restructuring and other costs | - | (9 | ) | |||||
| Employee future benefits | (129 | ) | (145 | ) | ||||
| Increase (decrease) in operating assets and liabilities | 1,281 | 761 | ||||||
Summary of pertinent data relating to computation of basic and diluted net loss per share
| Three months ended | ||||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Net loss | (5,752 | ) | (4,255 | ) | ||||
| Basic and diluted weighted-average shares outstanding | 1,213,969 | 1,213,969 | ||||||
| Basic and diluted loss per share | (4.74 | ) | (3.51 | ) | ||||
| Items excluded from the calculation of diluted net loss per share due to their anti-dilutive effect: | ||||||||
| Stock options and DSUs | 62,413 | 38,238 | ||||||
| Warrants | 114,405 | 114,405 | ||||||
9. Segment information
The Company operates in a single operating segment, being the biopharmaceutical segment.
10. Related party transactions
During the three months ended March 31, 2024, the Company recorded a termination benefit payment of $324 (€300) within selling, general and administrative expenses.
|
|
Aeterna Zentaris Inc.
Notes to the Condensed Interim Consolidated Financial Statements
As of March 31, 2024, and for the three months ended March 31, 2024, and 2023
(In thousands of US dollars, except share and per share data and as otherwise noted)
(Unaudited)
11. Commitments
Significant expenditure contracted for at the end of the reporting period but not recognized as liabilities is as follows:
Schedule of expected future minimum lease payments
| TOTAL | ||||
| $ | ||||
| Less than 1 year | 4,335 | |||
| 1 - 5 years | 75 | |||
| Total | 4,410 | |||
In 2021, the Company executed various agreements including in-licensing and similar arrangements with development partners. Such agreements may require the Company to make payments on achievement of stages of development, launch or revenue milestones, although the Company generally has the right to terminate these agreements at no penalty. The Company may have to pay up to $38,756 upon achieving certain sales volumes, regulatory or other milestones related to specific products.
12. Subsequent event
On December 14, 2023, the Company and Ceapro Inc. (“Ceapro”) jointly announced the signing of a definitive agreement to combine their operations in an all-stock merger of equals. Pursuant to the agreement, the transaction will be effected by way of a plan of arrangement under the Canada Business Corporations Act pursuant to which, following the 1-for-4 reverse split on May 3, 2024, each outstanding Ceapro common share will be exchanged for 0.02360 of an Aeterna common share with the result that Ceapro will become a wholly-owned subsidiary of Aeterna (the “Transaction”). When completed, the Transaction will be considered a reverse acquisition and the historical financial statements following the business combination will be those of Ceapro. Additionally, as part of the Transaction, Aeterna will issue to its shareholders immediately prior to the closing of the transaction, 0.47698 of a share purchase warrant (“Transaction Warrants”) for each Aeterna common share held as of such date. Holders of Aeterna’s currently outstanding warrants will also be issued transaction warrants in accordance with the anti-dilution provisions of such warrants. Each whole transaction warrant will be exercisable to purchase one common share of Aeterna at a nominal exercise price of $0.01. The transaction also provides the outstanding options to acquire Ceapro common shares to be replaced by options allowing current holders to acquire common shares of Aeterna on similar terms, as adjusted by the exchange ratio in the transaction. Following the closing of the Transaction, the former shareholders of Ceapro will own 50% of Aeterna and the pre-transaction securityholders of Aeterna will own the remaining 50%, assuming the exercise of all Transaction Warrants. The transaction required the approval of shareholders of both Companies and the Alberta court, which were obtained on March 12, 2024, and March 28, 2024 respectively, and is subject to closing conditions customary for transactions of this nature, including applicable stock exchange approvals. It is anticipated that the transaction will close in the second quarter of 2024, subject to the satisfaction of the conditions of the agreement.
|
|
Exhibit 99.2

Management’s Discussion and Analysis of Financial Condition and Results of Operations
Introduction
This Management’s Discussion and Analysis (“MD&A”) provides a review of the results of operations, financial condition and cash flows of Aeterna Zentaris Inc. for the three-month period ended March 31, 2024. In this MD&A, “Aeterna Zentaris”, “Aeterna”, the “Company”, “we”, “us” and “our” mean Aeterna Zentaris Inc. and its subsidiaries. This discussion should be read in conjunction with the information contained in the Company’s unaudited consolidated financial statements and the notes thereto as of March 31, 2024, and for the three-month periods ended March 31, 2024, and 2023. Our unaudited consolidated financial statements have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”).
The Company’s common shares are listed on both The Nasdaq Capital Market (“Nasdaq”) and on the Toronto Stock Exchange (“TSX”) under the symbol “AEZS”.
All amounts in this MD&A are presented in thousands of United States (“U.S.”) dollars, except for share and per share data, or as otherwise noted. This MD&A was approved by the Company’s Board of Directors (the “Board”) on May 14, 2024. This MD&A is dated May 14, 2024.
About Forward-Looking Statements
This document contains statements that may constitute forward-looking statements within the meaning of U.S. and Canadian securities legislation and regulations, and such statements are made pursuant to the safe-harbor provision of the U.S. Private Securities Litigation Reform Act of 1995. In some cases, these forward-looking statements can be identified by words or phrases such as “forecast”, “may”, “will”, “expect”, anticipate”, “estimate”, “intend”, “plan”, “indicate”, “believe”, “direct”, or “likely”, or the negative of these terms, or other similar expressions intended to identify forward-looking statements. In addition, any statements that refer to expectations, intentions, projections and other characterizations of future events or circumstances contain forward-looking information.
Forward-looking statements are based on the opinions and estimates of the Company as of the date of this MD&A, and they are subject to known and unknown risks, uncertainties, assumptions and other factors that may cause the actual results, level of activity, performance or achievements to be materially different from those expressed or implied by such forward-looking statements, including but not limited to the factors described under Item 3, D. – “Risk factors” in our most recent Annual Report on Form 20-F and those relating to: Aeterna’s expectations with respect to the DETECT-trial (as defined below) (including the enrollment of subjects in the DETECT-trial, the application of the macimorelin growth hormone stimulation tests and the completion of the DETECT-trial); Aeterna’s expectations regarding conducting pre-clinical research to identify and characterize an AIM Biologicals-based development candidate for the treatment of neuromyelitis optica spectrum disorder (“NMOSD”), as well as Parkinson’s disease (“PD”), and developing a manufacturing process for selected candidates; Aeterna’s expectations regarding conducting assessments in relevant PD models; the University of Queensland’s undertaking a subsequent investigator initiated clinical trial evaluating macimorelin as a potential therapeutic for the treatment of amyotrophic lateral sclerosis (“ALS”), also known as Lou Gehrig’s disease, and Aeterna’s formulating a pre-clinical development plan for same; the commencement of Aeterna’s formal pre-clinical development of AEZS-150 (as a potential therapeutic in chronic hypoparathyroidism as defined below) in preparation for a potential investigational new drug (“IND”) filing for conducting the first in-human clinical study of AEZS-150; the impacts associated with the termination of the license agreement with Novo Nordisk Healthcare AG (“Novo Nordisk” or “Novo”), as discussed below ; the expected outcomes and benefits of the Arrangement (as defined below); the ability of Aeterna and Ceapro Inc. (“Ceapro”) to complete the Arrangement on the terms described herein, or at all; the anticipated timeline for the completion of the Arrangement; and receipt of regulatory and stock exchange approvals (including approval of the continued listing of Aeterna’s common shares on the Nasdaq and the TSX).
|
|
Forward-looking statements involve known and unknown risks and uncertainties and other factors which may cause the actual results, performance or achievements stated herein to be materially different from any future results, performance or achievements expressed or implied by the forward-looking information. Such risks and uncertainties include, among others: Aeterna’s and Ceapro’s present and future business strategies; operations performance within expected ranges; anticipated future cash flows; local and global economic conditions and the environment in which the combined operations will operate in the future; anticipated capital and operating costs; and the availability and timing of required stock exchange, regulatory and other approvals for the completion of the Arrangement; our reliance on the success of the pediatric clinical trial in the European Union and U.S. for Macrilen® (macimorelin); potential delays associated with the completion of the DETECT-trial; we may be unable to enroll the expected number of subjects in the DETECT-trial, and the result of the DETECT-trial may not support receipt of regulatory approval in childhood-onset growth hormone deficiency (“CGHD”); results from ongoing or planned pre-clinical studies of macimorelin by the University of Queensland or for our other products under development may not be successful or may not support advancing the product to human clinical trials; our ability to raise capital and obtain financing to continue our currently planned operations; our dependence on the success of Macrilen® (macimorelin) and related out-licensing arrangements, including the continued availability of funds and resources to successfully commercialize the product; our ability to enter into additional out-licensing, development, manufacturing, marketing and distribution agreements with other pharmaceutical companies and to keep such agreements in effect; and our ability to continue to list our common shares on the Nasdaq or the TSX. These risk factors are not intended to represent a complete list of the risk factors that could affect the Company. These factors and assumptions, however, should be considered carefully. More detailed information about these and other factors is included under Item 3, D. – “Risk factors” in our most recent Annual Report on Form 20-F.
Although the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements, there may be other factors that cause results not to be as anticipated, estimated or intended. Many of these factors are beyond our control. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements. The Company does not undertake to update any forward-looking statements contained herein, except as required by applicable securities laws. New factors emerge from time to time, and it is not possible for the Company to predict all of these factors, or to assess in advance the impact of each such factor on the Company’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement.
Certain forward-looking statements contained herein about prospective results of operations, financial position or cash flows may constitute a financial outlook. Such statements are based on assumptions about future events, are given as of the date hereof and are based on economic conditions, proposed courses of action and management’s assessment of currently available relevant information. The Company’s management has approved the financial outlook as of the date hereof. Readers are cautioned that such financial outlook information contained herein should not be used for purposes other than for which it is disclosed herein.
About Material Information
This MD&A includes information that we believe to be material to investors after considering all circumstances. We consider information and disclosures to be material if they result in, or would reasonably be expected to result in, a significant change in the market price or value of our securities, or where it is likely that a reasonable investor would consider the information and disclosures to be important in making an investment decision.
We are a reporting issuer under the securities legislation of all of the provinces of Canada, and our securities are registered with the U.S. Securities and Exchange Commission (“SEC”). We are therefore required to file or furnish continuous disclosure information, such as interim and annual financial statements, management’s discussion and analysis, proxy or information circulars, annual reports on Form 20-F, material change reports and press releases with the appropriate securities regulatory authorities. Additional information about the Company and copies of these documents may be obtained free of charge upon request from our Corporate Secretary or on the Internet at the following addresses: www.zentaris.com, www.sedarplus.ca and www.sec.gov.
|
|
Company Overview
Aeterna Zentaris is a specialty biopharmaceutical company commercializing and developing therapeutics and diagnostic tests. The Company’s lead product, Macrilen® (macimorelin), is the first and only U.S. Food and Drug Administration (“FDA”) and European Medicines Agency (“EMA”) approved oral test indicated for the diagnosis of patients with adult growth hormone deficiency (“AGHD”). Macimorelin is currently marketed under the tradename Ghryvelin™ in the European Economic Area and under the tradename “Macimorelin 60 mg granules for oral suspension in sachet” in the United Kingdom through an exclusive licensing agreement with Pharmanovia. The Company’s several other license and commercialization partners have already received or are seeking approval for commercialization of macimorelin in Israel and the Palestinian Authority, the Republic of Korea, Turkey and several non-European Union Balkan countries. The Company is actively pursuing business development opportunities for the commercialization of macimorelin in North America, Asia and the rest of the world. We are also leveraging the clinical success and compelling safety profile of macimorelin to develop the compound for the diagnosis of CGHD, an area of significant unmet need.
The Company is also dedicated to the development of therapeutic assets and has established a pre-clinical development pipeline to potentially address unmet medical needs across a number of indications, with a focus on rare and/or orphan indications, including, Neuromyelitis Optica Spectrum Disorder (NMOSD), Parkinson’s disease (PD), chronic hypoparathyroidism (DC-PTH) and ALS (Lou Gehrig’s disease).
Subsequent to the end of the reporting period of March 31, 2024 and in relation to the Plan of Arrangement with Ceapro, on May 1, 2024, the Company’s board of directors approved an amendment to the Company’s articles of incorporation to effect a 1-for-4 reverse stock split (“reverse split”) of the Company’s common shares, deferred share units, stock options and warrants outstanding as of the effective date. The reverse split was effected in the markets on May 3, 2024.
Plan of Arrangement with Ceapro
On December 14, 2023, Aeterna Zentaris entered into an arrangement agreement (the “Arrangement Agreement”) with Ceapro Inc. (“Ceapro”) which was subsequently amended on January 16, 2024, pursuant to which Aeterna Zentaris and Ceapro agreed that, subject to the terms and conditions set forth in the Arrangement Agreement, including the receipt of the approval of the securityholders of Aeterna Zentaris and Ceapro which was obtained on March 12 2024, the final order of the Court of King’s Bench of Alberta which was issued on March 27, 2024, and certain regulatory and exchange approvals described below, Aeterna Zentaris will acquire 100 percent of the common shares of Ceapro (the “Ceapro Shares”) pursuant to a court-approved plan of arrangement under the Canada Business Corporations Act such that Ceapro will become a wholly-owned subsidiary of Aeterna Zentaris and Aeterna Zentaris will continue the operations of Aeterna Zentaris and Ceapro on a combined basis (the “Arrangement”). Additionally, as part of the Arrangement, Aeterna Zentaris will issue to its shareholders immediately prior to the closing of the Arrangement, 0.47698 of a share purchase warrant (“Aeterna Zentaris New Warrant”) for each Aeterna Zentaris common share held as of such date. The terms of the Arrangement Agreement were the result of arm’s length negotiation between Aeterna Zentaris and Ceapro and their respective advisors.
On the closing of the Arrangement, existing shareholders of Aeterna Zentaris and former shareholders of Ceapro would own approximately 50% of the outstanding Aeterna Zentaris common shares assuming the exercise of all of the Aeterna Zentaris New Warrants and based on the number of Aeterna Zentaris common shares and Ceapro Shares issued and outstanding as of market close on December 13, 2023. When completed, the Arrangement will be considered a reverse acquisition and the historical financial statements following the business combination will be those of Ceapro.
Aeterna Zentaris has applied to list all of its common shares issuable upon the exercise of the Aeterna Zentaris New Warrants on the TSX and has filed an initial listing application with the Nasdaq as the exchange has determined that the Arrangement constitutes a “change of control” under its rules and regulations. The parties intend to rely upon the exemption from the registration requirements of the U.S. Securities Act pursuant to Section 3(a)(10) thereof and applicable state securities laws with respect to the issuance of the Aeterna Zentaris common shares and the replacement options pursuant to the Arrangement. Aeterna Zentaris and Ceapro anticipate completing the Arrangement in the second quarter of 2024, subject to obtaining all required approvals and satisfying all required conditions.
Following closing, Aeterna Zentaris and Ceapro have agreed to use their commercially reasonable efforts to delist the Ceapro Shares from the TSXV. Aeterna Zentaris and Ceapro also intend to apply for a decision for Ceapro to cease to be a reporting issuer under the securities laws of each jurisdiction of Canada in which Ceapro is a reporting issuer, if permitted by applicable laws.
|
|
The Arrangement Agreement contains customary representations and warranties and is subject to customary conditions to closing and other restrictive covenants, including, but not limited to, the following:
| ● | Directors and Officers: Upon the closing of the Arrangement, certain directors of Aeterna Zentaris will resign, the number of director seats on the Aeterna Zentaris Board will be increased by four and Ronald W. Miller, Ulrich Kosciessa, Geneviève Foster and William Li, each a nominee of Ceapro, will be appointed to fill such vacancies on the Aeterna Zentaris board, to the extent permitted by law. The Chief Executive Officer of the combined company will be Gilles Gagnon, the current Chief Executive Officer of Ceapro and a director of Aeterna Zentaris and the Chief Financial Officer of the combined company will be Giuliano La Fratta, the Chief Financial Officer of Aeterna Zentaris. | |
| ● | Non-Solicitation: Subject to certain exceptions, neither party will solicit or assist in the initiation of proposals that could result in an Acquisition Proposal (as defined in the Arrangement Agreement) by a third-party. | |
| ● | Notification of Proposals: A party that receives an acquisition solicitation has to notify the other party within 24 hours of its receipt of such solicitation and must provide certain information and details relating to such acquisition solicitation. | |
| ● | Superior Proposal: Notwithstanding other restrictions contained in the Arrangement Agreement, in the event a party receives a superior proposal from a third-party, such party may, subject to compliance with the terms of the Arrangement Agreement, enter into a definitive agreement with a party providing for an Acquisition Proposal so long as such Acquisition Proposal constitutes a Superior Proposal (as defined in the Arrangement Agreement). | |
| ● | Termination of Arrangement Agreement: The parties may terminate the Arrangement Agreement upon the occurrence of certain conditions, and in any event, if the effective date has not occurred on or before June 14, 2024. | |
| ● | Termination Fees: Upon the occurrence of certain termination events pursuant to the terms of the Arrangement Agreement, Aeterna Zentaris shall be entitled to a fee of US$0.5 million to be paid by Ceapro within the time(s) specified in the Arrangement Agreement in respect to each termination event. |
For additional information with respect to the Arrangement and the representations and warranties, conditions to closing and other terms in the Arrangement Agreement please refer to the section entitled “The Plan of Arrangement – Principal Terms of the Plan of Arrangement” in the Company’s Registration Statement on Form F-1 filed with the SEC on February 15, 2024 (including any supplements or amendments to such registration statement subsequently filed by the Company with the SEC) and to the Arrangement Agreement incorporated by reference as Exhibit 2.1 to the Registration Statement on Form F-1. Further information is also available in the management information circular dated February 9, 2024 which is available on the Company’s SEDAR+ profile at www.sedarplus.ca.
|
|
Key Operational Developments
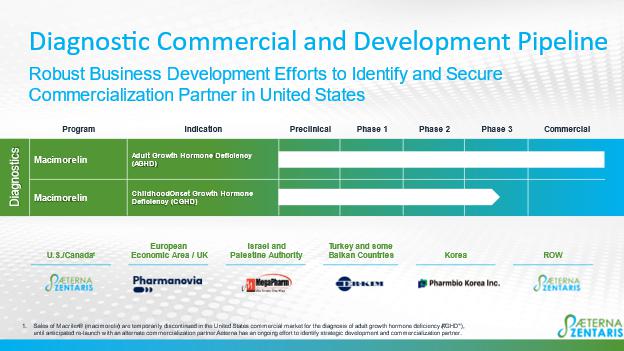
Macimorelin Commercialization Program
On March 15, 2023, with the Company’s consent, Consilient Health Limited (“Consilient” or “CH”) entered into an assignment agreement with Atnahs Pharma UK Ltd. (Pharmanovia) to transfer the current licensing agreement for the commercialization of macimorelin in the European Economic Area and the United Kingdom to Pharmanovia, as well as the current supply agreement pursuant to which the Company agreed to provide the licensed product. Also on March 15, 2023, the Company and Pharmanovia entered into an amendment agreement, pursuant to which the Company provided its acknowledgement and consent to the Assignment Agreement and agreed to certain amended terms which do not materially differ from the previous license and supply agreement with CH. To date, we have received total pricing milestone payments from CH of $0.5 million (€0.5 million) relating to Ghryvelin™/Macimorelin 60 mg approved list prices in the United Kingdom, Germany and Spain. We shipped initial batches of macimorelin (Ghryvelin™/Macimorelin 60 mg) to Consilient in the first quarter of 2022. Consilient launched the product meanwhile in the United Kingdom, Sweden, Denmark, Finland, Germany and Austria. More EU countries will follow pending re-imbursement negotiations. On April 19, 2022, we announced that the European Patent Office had issued a patent providing intellectual property protection of macimorelin in 27 countries within the European Union as well as additional European non-EU countries, such as the UK and Turkey, for macimorelin for use to diagnose GHD in adults. In the meantime, the related PCT patent application has been granted in Canada, Japan, South Korea, Eurasia and New Zealand.
On May 9, 2023, the USPTO issued patent US11,644,474 to the Company protecting the use of macimorelin for the diagnosis of GHD in pediatrics.
Since November 2020, Novo marketed macimorelin under the tradename Macrilen® through a license agreement and an amended license agreement (collectively the “Novo Amendment”) for the diagnosis of AGHD. On August 26, 2022, the Company announced that Novo had exercised its right to terminate the Novo Amendment. Following a 270-day notice period, Aeterna regained full rights to Macrilen® in the U.S. and Canada on May 23, 2023, and the sales of Macrilen® are temporarily discontinued in the U.S. commercial market for the diagnosis of AGHD, until an anticipated re-launch with an alternate commercialization partner. The Company continues to actively strategize and seek alternate development and commercialization partners for Macrilen® in the U.S. and other territories. The decision to temporarily discontinue sales of Macrilen® in the United States does not have any impact on the sales and commercialization efforts in the UK and European Economic Area.
|
|
On June 25, 2020, we announced that we entered into an exclusive distribution and related quality agreement with MegaPharm Ltd., a leading Israel-based biopharmaceutical company, for the commercialization in Israel and in the Palestinian Authority of macimorelin, to be used in the diagnosis of patients with AGHD and in clinical development for the diagnosis of CGHD. Under the terms of the agreement, MegaPharm Ltd. will be responsible for obtaining registration to market macimorelin in Israel and the Palestinian Authority, while the Company will be responsible for manufacturing, product supply, quality assurance and control, regulatory support, and maintenance of the relevant intellectual property. In June 2021, MegaPharm Ltd. filed an application to the Ministry of Health of Israel for regulatory approval of macimorelin in Israel, which received the Israeli license in November 2022 and final approval in February 2024.
We entered into license and supply agreements with NK Meditech Ltd. (“NK”), a subsidiary of PharmBio Korea, effective November 30, 2021, and a distribution and commercialization agreement with ER-Kim Pharmaceuticals Bulgaria Eood (“ER-Kim”), effective February 1, 2022. The agreements with NK are related to the development and commercialization of macimorelin for the diagnosis of AGHD and CGHD in the Republic of Korea, while the agreement with ER-Kim is related to the commercialization of macimorelin for the diagnosis of growth hormone deficiency in children and adults in Turkey and some non-European Union Balkan countries. The South Korean marketing authorization was granted in September 2023.
Macimorelin Clinical Program
On January 28, 2020, we announced the successful completion of patient recruitment for the first pediatric study of macimorelin as a growth hormone stimulation test for the evaluation of GHD in children. This study, AEZS-130-P01 (“Study P01”), was the first of two studies as agreed with the EMA in our Pediatric Investigation Plan (the “PIP”) for macimorelin as a GHD diagnostic. Macimorelin, a ghrelin agonist, is an orally active small molecule that stimulates the secretion of growth hormone from the pituitary gland into the circulatory system. The goal of Study P01 was to establish a dose that can both be safely administered to pediatric patients and cause a clear rise in growth hormone concentration in subjects ultimately diagnosed as not having GHD. The recommended dose derived from Study P01 is being evaluated in the pivotal second study, Study P02, on diagnostic efficacy and safety (the “DETECT-trial”). Study P01 was an international, multicenter study, which was conducted in Hungary, Poland, Ukraine, Serbia, Belarus and Russia. Study P01 was an open label, group comparison, dose escalation trial designed to investigate the safety, tolerability, and pharmacokinetic/pharmacodynamic (“PK/PD”) of macimorelin acetate after ascending single oral doses of macimorelin at 0.25, 0.5, and 1.0 milligram per kilogram body weight in pediatric patients from 2 to less than 18 years of age with suspected CGHD. We enrolled a total of 24 pediatric patients across the three cohorts of the study. Per study protocol, all enrolled patients completed four study visits after successful completion of the screening period. At Visit 1 and Visit 3, a provocative growth hormone stimulation test was conducted according to the study sites’ local practices. At Visit 2, the macimorelin test was performed, and following the oral administration of the macimorelin solution, blood samples were taken at predefined times for PK/PD assessment. Visit 4 was a safety follow-up visit at study end.
The final study results from Study P01 were published in the second quarter of 2020 indicating positive safety and tolerability data for use of macimorelin in CGHD, as well as PK/PD data observed in a range as expected from the adult studies.
On April 7, 2020, we announced the decision of the EMA to accept our modification request of our PIP as originally approved in March 2017, which covered the conduct of two pediatric studies and defined relevant key elements in the outline of these studies. We believe this EMA decision supports the development of one globally harmonized study protocol for test validation, specifically Study P02, which we expect to be accepted both in Europe and the U.S.
In late 2020, we entered into the start-up phase for the clinical safety and efficacy study, AEZS-130-P02 (“DETECT-trial”), evaluating macimorelin for the diagnosis of CGHD. The DETECT-trial is an open-label, single dose, multicenter and multinational study expected to enroll approximately 100 subjects worldwide (incl. sites in U.S: and EU), with at least 40 pre-pubertal and 40 pubertal subjects. The study design is expected to be suitable to support a claim for potential stand-alone testing, if successful. On April 22, 2021, the U.S. FDA Investigational New Drug Application associated with this clinical trial became active, (see: https://clinicaltrials.gov/ct2/show/NCT04786873), and on May 13, 2021, we announced the opening of the first clinical site in the U.S. Under the Novo Amendment, and following Novo’s notice to terminate the Novo Amendment, Novo has funded DETECT-trial costs up to $10.1 million (€9.4 million). Any additional trial costs incurred over $10.1 million (€9.4 million) will be paid by Aeterna.
|
|
On January 26, 2022, we announced that the DETECT-trial had experienced unavoidable delays in site initiation and patient enrollment due to the rise of the Omicron variant in the COVID-19 pandemic. Furthermore, in February 2022, due to the Russian invasion of Ukraine, the clinical trial activities planned in both Russia and Ukraine were halted and consequently, no patients have been enrolled in either of these countries’ clinical sites to date. On January 17, 2023, we provided a business update, highlighting that bolstered enrollment was expected by the engagement of an additional Clinical Research Organization (CRO) and the replacement of inactive countries and sites with three new countries (Armenia, Slovakia, and Turkey) as well as additional sites in the U.S. In March 2023, we received approval for and activated our first site in Slovakia. Sites in Armenia and Turkey were approved and activated in early 2024. With enrollment in the DETECT-trial now completed, we expect the conclusion of the active part of the trial in Q2 2024 and top-line data in Q3 2024.
Pipeline Expansion Opportunities
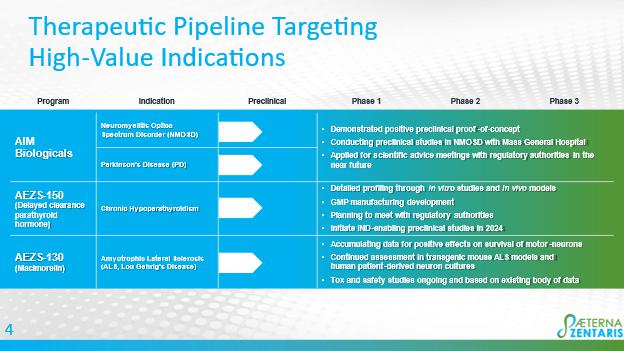
AIM Biologicals: Targeted, highly specific autoimmunity modifying therapeutics for the potential treatment of neuromyelitis optica spectrum disorder (NMOSD) and Parkinson’s disease
AIM Biologicals are based on a natural process during pregnancy, which induces immunogenic tolerance of the maternal immune system to the partially foreign fetal antigens. Fetal proteins are processed and presented on certain immunosuppressive major histocompatibility complex class I molecules to induce this tolerance. In an autoimmune disease, the immune system is misdirected and targets the body’s own protein. With AIM Biologicals, we aim to restore the tolerance against such proteins to treat autoimmune diseases. Our AIM Biologics program is focused on the rare and orphan indication NMOSD and on the second most common neurodegenerative disorder, Parkinson’s disease.
|
|
In January 2021, we entered into an exclusive patent license and research agreement with the University of Wuerzburg, Germany, for worldwide rights to develop, manufacture, and commercialize AIM Biologicals for the potential treatment of NMOSD. Additionally, we have engaged Prof. Dr. Joerg Wischhusen from the University Hospital in Wuerzburg as well as neuro-immunologist Dr. Michael Levy from the Massachusetts General Hospital (“MGH”) in Boston as consultants for scientific support and advice in the field of inflammatory central nervous system “CNS” disorders, autoimmune diseases of the nervous system, and NMOSD. In September 2021, we entered into an additional exclusive license with the University of Wuerzburg for early pre-clinical development towards the potential treatment of Parkinson’s disease. On May 12, 2022 we announced positive pre-clinical results in an innovative mouse model of Parkinson’s disease, where treatment with α-Synuclein specific AIM Biologicals showed a trend towards improved motoric function, as well as significant induction of regulatory T cells and rescue of substantia nigra neurons. The data were presented at IMMUNOLOGY2022™, the annual event of the American Association of Immunologists, held on May 6-10, 2022 in Portland, Oregon. On June 13, 2022, we announced that we had achieved proof-of-concept for the treatment of NMOSD in both in-vitro and in mouse models. These findings were presented at the 13th International Congress on Autoimmunity on June 10-13, 2022, in Athens, Greece. In October 2022, we entered into a research and development agreement with MGH in Boston and Dr. Michael Levy, to conduct pre-clinical ex-vivo and in-vivo studies in NMOSD.
NMOSD is an autoimmune disease targeting the protein aquaporin 4, primarily found in optic nerves and the spinal cord. The disease leading to blindness and paralysis has a prevalence of 0.7-10 in 100,000, more common in persons with Asian or African compared to European ancestors, and nine times more prevalent among women compared to men. NMOSD progresses in often life-threatening relapses, which are aggressively treated with high-dose steroids and plasmapheresis. Current treatment options include treatment with immunosuppressive monoclonal antibodies, which carries risk of serious infections. Our pre-clinical plans include expanding the already available proof-of-concept data for the treatment of NMOSD in both in-vitro and in-vivo assessments to select an AIM Biologicals-based development candidate; and manufacturing process development for the selected candidate.
Parkinson’s disease is a neurological disease commonly associated with motoric problems with a slow and fast progression form. It is the second most common neurodegenerative disease affecting 10 million people worldwide. The hallmark of PD is the neuronal inclusion of mainly α-synuclein protein (αSyn) associated with the death of dopamine-producing cells. Dopaminergic medication is the mainstay treatment of PD symptoms. Up to now there is no pharmacological therapy available to prevent or delay disease progression. Alternate treatments, such as deep brain stimulation with short electric bursts, are being investigated for the treatment of symptoms. For the development of AIM Biologicals as potential PD therapeutics, Aeterna utilizes, among others, an innovative animal model on neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson’s disease mice, which has recently been published by University of Wuerzburg researchers. We are continuing in-vitro and in-vivo testing of antigen-specific AIM Biologics candidate molecules for the potential treatment of Parkinson’s disease.
AEZS-150 - Delayed Clearance (“DC”) Parathyroid Hormone (“PTH”) (“DC-PTH”) Fusion Polypeptides: Potential treatment for chronic hypoparathyroidism
On March 11, 2021, we entered into an exclusive license agreement with The University of Sheffield, United Kingdom, for the intellectual property relating to PTH fusion polypeptides covering the field of human use, which will initially be studied by Aeterna for the potential therapeutic treatment of chronic hypoparathyroidism (“HypoPT”). Under the terms of the exclusive patent and know-how license agreement entered into with the University of Sheffield, we obtained worldwide rights to develop, manufacture and commercialize PTH fusion polypeptides covered by the licensed patent applications for all human uses for an up-front cash payment, and milestone payments to be paid upon the achievement of certain development, regulatory and sales milestones, as well as low single digit royalty payments on net sales of those products and certain fees payable in connection with sublicensing. We will be responsible for the further development, manufacturing, approval, and commercialization of the licensed products. We also engaged the University of Sheffield under a research contract to conduct certain research activities to be funded by Aeterna, the results of which will be included within the scope of the license granted to Aeterna.
|
|
The researchers at the University of Sheffield have developed a method to increase the serum clearance time of peptides, which the Company is applying to the development of a treatment for HypoPT. HypoPT is an orphan disease where the PTH level is abnormally low or absent, with a prevalence per 100,000 of 37 in the U.S., 22 in Denmark, 9.4 in Norway, and 5.3 to 27 in Italy. Standard treatment is calcium and vitamin D supplementation. In consultation with the University of Sheffield, Aeterna has selected AEZS-150 (14A7) as the lead candidate in its DC-PTH program. AEZS-150 is being developed to provide a weekly treatment option of chronic hypoparathyroidism in adults. Recent progress includes the successful verification and reproduction of previous in-vivo data from the University of Sheffield, in a rat model of hypoparathyroidism, as well as ongoing development of the manufacturing process for AEZS-150 with the Company’s contract development and manufacturing organization, establishment of a master cell bank for a cell line expressing AEZS-150 and the development of a production process suitable for larger scale good manufacturing practices. Our next steps include working with The University of Sheffield to continue with in depth characterization of development candidate (in-vitro and in-vivo); meeting with regulatory authorities to formalize the pre-clinical development of AEZS-150 in preparation for a potential IND filing for conducting the first in-human clinical study.
AEZS-130 - Macimorelin Pre-clinical Program
On January 13, 2021, we entered into a material transfer agreement with Queensland University to provide macimorelin for the conduct of preclinical and clinical studies evaluating macimorelin as a therapeutic for the treatment of ALS. ALS is a rare progressive neurological disease primarily affecting the neurons controlling voluntary movement, leading to the disability to control movements such as walking, talking, and chewing. Most people with ALS die from respiratory failure, usually between 3-5 years after diagnosis. Currently there is no cure for ALS and no effective treatment to halt or reverse the progression of the disease. Ghrelin is a hormone with wide-ranging biological actions, most known for stimulating growth hormone release, which is demonstrating emerging evidence as therapeutic for ALS. As a ghrelin agonist, macimorelin has the potential as a treatment for ALS, which is evaluated in this research collaboration.
In July 2022, we entered a research and option to license agreement with UniQuest Pty Ltd., the commercialization company of The University of Queensland (UQ), Brisbane, Australia, to advance the development of macimorelin as a potential therapeutic for the treatment of ALS. We have developed an alternative formulation suitable for use in ALS patients and are accumulating data for positive effects of AEZS-130 treatment on survival of motor-neurons. We are continuing to evaluate AEZS-130 in transgenic mouse ALS models as well as in human patient-derived neuron cultures to demonstrate the therapeutic potential of macimorelin in this indication. Our next steps include completion of the ongoing toxicology and safety studies to support treatment over prolonged periods and following potential achievement of proof-of-concept, scientific advice with regulatory authorities to discuss program development to support first in human studies.
|
|
Condensed Interim Consolidated Statements of Loss and Comprehensive Loss Data
| (in thousands of US dollars, except loss per share) | Three months ended | |||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Revenues | 4 | 2,128 | ||||||
| Expenses | ||||||||
| Cost of sales | 16 | 17 | ||||||
| Research and development | 2,584 | 4,012 | ||||||
| Selling, general and administrative | 3,514 | 2,306 | ||||||
| Total expenses | 6,114 | 6,355 | ||||||
| Loss from operations | (6,110 | ) | (4,207 | ) | ||||
| Gain (loss) due to changes in foreign currency exchange rates | 77 | (59 | ) | |||||
| Interest income | 285 | 13 | ||||||
| Other finance costs | (4 | ) | (2 | ) | ||||
| Net finance income (costs) | 358 | (48 | ) | |||||
| Loss before income taxes | (5,752 | ) | (4,255 | ) | ||||
| Income tax recovery | - | - | ||||||
| Net loss | (5,752 | ) | (4,255 | ) | ||||
| Other comprehensive loss: | ||||||||
| Foreign currency translation adjustments | 199 | (168 | ) | |||||
| Actuarial gain (loss) on defined benefit plans | 319 | (162 | ) | |||||
| Comprehensive loss | (5,234 | ) | (4,585 | ) | ||||
| Basic and diluted loss per share 1 | (4.74 | ) | (3.51 | ) | ||||
1 On May 3, 2024, the Company’s shares were consolidated on a 1-for-4 reverse stock split, which has been reflected retrospectively in the MD&A.
|
|
Summarized Interim Consolidated Statements of Financial Position Data
| (in thousands of US dollars) |
March 31, 2024 |
December 31, 2023 |
||||||
| $ | $ | |||||||
| Cash and cash equivalents | 29,545 | 34,016 | ||||||
| Trade and other receivables and other current assets | 1,586 | 2,285 | ||||||
| Inventory | 64 | 66 | ||||||
| Restricted cash equivalents | 326 | 332 | ||||||
| Property and equipment | 271 | 317 | ||||||
| Total assets | 31,792 | 37,016 | ||||||
| Payables and accrued liabilities and income taxes payable | 4,424 | 3,733 | ||||||
| Current portion of provisions | 422 | 429 | ||||||
| Current portion of deferred revenues | 252 | 218 | ||||||
| Lease liabilities | 234 | 279 | ||||||
| Non-financial non-current liabilities (1) | 13,490 | 14,161 | ||||||
| Total liabilities | 18,822 | 18,820 | ||||||
| Shareholders’ equity | 12,970 | 18,196 | ||||||
| Total liabilities and shareholders’ equity | 31,792 | 37,016 | ||||||
| (1) | Comprised mainly of employee future benefits, provisions and non-current portion of deferred revenues. |
Critical Accounting Policies, Estimates and Judgments
The preparation of condensed interim consolidated financial statements in accordance with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of the Company’s assets, liabilities, revenues, expenses and related disclosures. Judgments, estimates and assumptions are based on historical experience, expectations, current trends and other factors that management believes to be relevant at the time at which the Company’s condensed interim consolidated financial statements are prepared.
Management reviews, on a regular basis, the Company’s accounting policies, assumptions, estimates and judgments in order to ensure that the consolidated financial statements are presented fairly and in accordance with IFRS applicable to interim financial statements. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Critical accounting estimates and assumptions, as well as critical judgments used in applying accounting policies in the preparation of the Company’s condensed interim consolidated financial statements, were the same as those applied to the Company’s annual consolidated financial statements for the year ended December 31, 2023.
Financial Risk Factors and Other Financial Instruments
The nature and extent of our exposure to risks arising from financial instruments, including credit risk, liquidity risk and market risk and how we manage those risks are described in note 23 to the Company’s audited consolidated financial statements for the year ended December 31, 2023. There were no significant changes in the three-month period ended March 31, 2024, as compared to the December 31, 2023, disclosures.
|
|
Revenues
We generate revenue from license and collaboration agreements with customers (license fees, milestone revenue, royalties), the provision of development services, the sale of certain active pharmaceutical ingredients (“API”), semi-finished goods and finished goods, and from certain supply chain activities, which are comprised largely of oversight or supervisory support services related to stability studies or development activities carried out with respect to API batch production as specified in underlying contracts with customers.
| (in thousands of US dollars, except percentages) | Three months ended March 31, | |||||||||||||||
| 2024 | 2023 | Change | Change | |||||||||||||
| $ | $ | $ | % | |||||||||||||
| Revenue | ||||||||||||||||
| Royalties | 4 | 18 | (14 | ) | -78 | % | ||||||||||
| License fees | - | 760 | (760 | ) | -100 | % | ||||||||||
| Development services | - | 1,339 | (1,339 | ) | -100 | % | ||||||||||
| Supply chain | - | 11 | (11 | ) | -100 | % | ||||||||||
| Total revenue | 4 | 2,128 | (2,124 | ) | -100 | % | ||||||||||
Our total revenue for the three-month period ended March 31, 2024, decreased by $2.1 million. The decrease was due to the termination of the Company’s amended agreement with Novo Nordisk Healthcare in May 2023 and as a result, no license fee or development services revenue was recognized in Q1, 2024.
Research and development expenses
The following table summarizes our research and development expenses incurred during the periods indicated:
| (in thousands of US dollars, except percentages) | Three months ended March 31, | |||||||||||||||
| 2024 | 2023 | Change | Change | |||||||||||||
| $ | $ | $ | % | |||||||||||||
| Direct research and development expenses: | ||||||||||||||||
| Macimorelin pediatric DETECT-trial | 1,342 | 1,111 | 231 | 21 | % | |||||||||||
| AEZS-130 – Macimorelin ALS | 161 | 1,137 | (976 | ) | -86 | % | ||||||||||
| AEZS-150 – DC-PTH | 58 | 568 | (510 | ) | -90 | % | ||||||||||
| Aim Biologics - Parkinson’s Disease | 184 | 190 | (6 | ) | -3 | % | ||||||||||
| Aim Biologics - NMOSD | 340 | 118 | 222 | 188 | % | |||||||||||
| Bacterial Vaccine Platform - Covid-19 | - | 214 | (214 | ) | -100 | % | ||||||||||
| Bacterial Vaccine Platform - Chlamydia | - | 218 | (218 | ) | -100 | % | ||||||||||
| Additional programs | 72 | 34 | 38 | 112 | % | |||||||||||
| Total direct research and development expenses | 2,157 | 3,590 | (1,433 | ) | -40 | % | ||||||||||
| Employee-related expenses | 347 | 327 | 20 | 6 | % | |||||||||||
| Facilities, depreciation, and other expenses | 80 | 95 | (15 | ) | -16 | % | ||||||||||
| Total research and development expenses | 2,584 | 4,012 | (1,428 | ) | -36 | % | ||||||||||
Our total research and development expenses for the three-month period ended March 31, 2024, were $2.6 million as compared to $4.0 million for the same period in 2023. This decrease of $1.4 million was primarily driven by a $1.4 million decrease in direct research and development expenses. Direct research and development expenses include expenses incurred under arrangements with third parties, such as contract research organizations, contract manufacturers, and consultants.
|
|
The decrease in research and development expenses was primarily attributable to:
| ● | a $1.0 million and $0.5 million decrease in spending on the Company’s ALS and DC-PTH trials respectively driven by the timing of various pre-clinical activities associated with these projects; | |
| ● | a $0.4 million decrease in the Company’s Bacterial Vaccine platform projects as the Company ceased development of both the COVID-19 and Chlamydia vaccine trials in Q1, 2023; offset by | |
| ● | a combined $0.5 million increase in costs related to the DETECT and AIM Biologics projects due to the timing of various pre-clinical and clinical activities associated with these projects. |
Selling, general and administrative expenses
The following table summarizes our Selling, general and administrative expenses incurred during the period indicated:
| (in thousands of US dollars, except percentages) | Three months ended March 31, | |||||||||||||||
| 2024 | 2023 | Change | Change | |||||||||||||
| $ | $ | $ | % | |||||||||||||
| Selling, general and administrative expenses: | ||||||||||||||||
| Salaries & benefits | 1,016 | 759 | 257 | 34 | % | |||||||||||
| Insurance | 259 | 428 | (169 | ) | -39 | % | ||||||||||
| Professional fees | 1,497 | 830 | 667 | 80 | % | |||||||||||
| Other office & general expenses | 742 | 289 | 453 | 157 | % | |||||||||||
| Total selling, general and administrative expenses | 3,514 | 2,306 | 1,208 | 52 | % | |||||||||||
Our total selling, general and administrative expenses for the three-month period ended March 31, 2024, were $3.5 million as compared to $2.3 million for the same period in 2023. This increase of $1.2 million was primarily attributable to the following:
| ● | a $0.3 million increase in salaries & benefits expenses primarily due to the accrual of termination benefits; | |
| ● | a $0.7 million and $0.5 million increase in professional fees and other office & general expenses respectively due primarily to an increase in payments made to the Company’s legal and financial advisors to assist in strategic review and the Plan of Arrangement, which is described above under “Plan of Arrangement with Ceapro”; offset by | |
| ● | a $0.2 million decrease in insurance expenses as the Company negotiated a lower premium for the current year. |
Net finance income (costs)
For the three-month period ended March 31, 2024, our net finance income was $0.4 million as compared to net finance costs of $0.0 million for the same period in the prior year, an increase of $0.4 million. This increase was primarily due to a $0.3 million increase in interest income and a $0.1 million increase in gains due to changes in foreign currency exchange rates.
|
|
Net loss
For the three-month period ended March 31, 2024, we reported a consolidated net loss of $5.8 million, or $4.74 loss per common share (basic), as compared with a consolidated net loss of $4.3 million, or $3.51 loss per common share (basic) for the same period in 2023. The $1.5 million increase in net loss is attributable to:
| ● | a $2.1 million decrease in revenue as a result of the termination of the Novo Amended agreement in May 2023; |
| ● | a $1.2 million increase in selling, general and administrative expenses primarily due to professional services provided in connection with the announced plan of arrangement between the Company and Ceapro Inc. as well as the accrual of termination benefits; offset by |
| ● | a $1.4 million decrease in research & development expenses due to timing of various pre-clinical and clinical activities undertaken during the period; and |
| ● | a $0.4 million increase in net finance income (costs) primarily due to an increase in interest income earned. |
Selected quarterly financial data
| Three months ended | ||||||||||||||||
| (in thousands of US dollars, except for per share data) |
March 31, 2024 |
December 31, 2023 |
September 30, 2023 |
June 30, 2023 |
||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Revenues | 4 | 121 | 3 | 2,246 | ||||||||||||
| Net loss | (5,752 | ) | (5,635 | ) | (4,145 | ) | (2,518 | ) | ||||||||
| Net loss per share (basic and diluted) (1) | (4.74 | ) | (4.64 | ) | (3.41 | ) | (2.07 | ) | ||||||||
| Three months ended | ||||||||||||||||
| (in thousands of US dollars, except for per share data) | March 31, 2023 |
December 31, 2022 |
September 30, 2022 |
June 30, 2022 |
||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Revenues | 2,128 | 2,485 | 1,860 | (222 | ) | |||||||||||
| Net loss | (4,255 | ) | (12,451 | ) | (3,420 | ) | (4,216 | ) | ||||||||
| Net loss per share (basic and diluted) (1) | (3.51 | ) | (10.26 | ) | (2.82 | ) | (3.47 | ) | ||||||||
| (1) | Net loss per share is based on the weighted average number of shares outstanding during each reporting period, which may differ on a quarter-to-quarter basis. As such, the sum of the quarterly net loss per share amounts may not equal full-year net loss per share. |
Historical quarterly results of operations and net loss cannot be taken as reflective of recurring revenue or expenditure patterns of predictable trends, largely given the non-recurring nature of certain components of our revenues, unpredictable quarterly variations in net finance income and of foreign exchange gains and losses.
The decrease in revenue for the Q3, 2023 onwards, in contrast to earlier periods presented, is due to the termination of the Company’s amended agreement with Novo Nordisk Healthcare in May 2023.
The increase in net loss for the three-month period ended December 31, 2022, in contrast to the other quarterly periods presented, was due to the recording of an impairment charge on the Company’s goodwill and intangible assets for an amount of $7,642 and $372 respectively.
The decrease in revenue and increase in net loss for the three months ended June 30, 2022, was driven by the reversal of revenue of $0.4 million in license fees and $0.8 million in development services which took place in Q2, 2022 as a result of a change in management’s best estimate of additional costs associated to the DETECT-trial.
|
|
Liquidity and capital resources
The Company’s objective in managing capital, consisting of shareholders’ equity, with cash and cash equivalents being its primary components, is to ensure sufficient liquidity to fund research and development costs, selling expenses, general and administrative expenses and working capital requirements. Over the past several years, we have raised capital via public and private equity offerings and issuances and have entered licensing and collaborative arrangements, consideration from which, together with proceeds from equity issuances, has been our primary source of liquidity. The capital management objective of the Company remains the same as that in previous periods. The policy on dividends is to retain cash to keep funds available, to finance the activities required to advance the Company’s product development portfolio and to pursue appropriate commercial opportunities as they may arise. The Company is not subject to any capital requirements imposed by any regulators or by any other external source.
Cash flows
The following table shows a summary of our consolidated cash flows for the periods indicated:
| (in thousands of US dollars) | Three months ended | |||||||
| March 31, | ||||||||
| 2024 | 2023 | |||||||
| $ | $ | |||||||
| Cash and cash equivalents – Beginning of period | 34,016 | 50,611 | ||||||
| Cash used in operating activities | (4,290 | ) | (4,061 | ) | ||||
| Cash flows (used in) provided by financing activities | (42 | ) | (38 | ) | ||||
| Cash flows provided (used in) by investing activities | (5 | ) | (2 | ) | ||||
| Effect of exchange rate changes on cash and cash equivalents | (134 | ) | 50 | |||||
| Cash and cash equivalents – End of period | 29,545 | 46,560 | ||||||
Operating Activities
Cash used by operating activities totaled $4.3 million for the three-month period ended March 31, 2024, as compared to $4.1 million in the same period in 2022. This $0.2 million increase in operating cash outflows is attributed primarily to:
| ● | a decrease in development services, product sales, royalty and supply chain revenues of $1.3 million; and |
| ● | an increase in selling, general and administrative expenses of $1.2 million, as described above; offset by |
| ● | a decrease in working capital outflows of $0.5 million, primarily related to the recognition of deferred revenue upon termination of the Novo Amendment agreement; |
| ● | a decrease in research and development expenses of $1.4 million, as described above; and |
| ● | an increase in net finance income (costs) of $0.4 million, as described above. |
Adequacy of financial resources
Since inception, the Company has incurred significant expenses in its efforts to develop and co-promote products. Our current business focus is to: investigate further therapeutic uses of Macrilen™, expand pipeline development activities, further expand the commercialization of macimorelin in available territories and fund ongoing clinical trial costs. Consequently, the Company has incurred operating losses and has generated negative cash flow from operations and in each of the last several years except for the year ended December 31, 2018, when the Company earned revenue from the sale of a license for the adult indication of Macrilen™ in the U.S. and Canada. The Company expects to incur significant expenses and operating losses for the foreseeable future as it advances its product candidates through preclinical and clinical development, seeks regulatory approval, and pursues commercialization of any approved product candidates. We expect that our research and development costs will increase in connection with our planned research and development activities.
As of March 31, 2024, the Company had an accumulated deficit of $375.3 million. The Company also had a net loss of $5.8 million and negative cash flows from operations of $4.3 million for the three-month period ended March 31, 2024. We believe that our existing cash on hand will be sufficient to fund our anticipated operating and capital expenditure requirements for at least the next 12 months. We plan to finance our future operations and capital expenditures primarily through cash on hand. We also believe that our existing cash on hand will be sufficient to fund our anticipated operating and capital expenditure requirements beyond the next 12 months and through 2025. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect. We may also require additional capital to pursue in-licenses or acquisitions of other product candidates.
|
|
Our forecast of the period through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially as a result of a number of factors. Our future capital requirements are difficult to forecast and will depend on many factors, including:
| ● | the terms and timing of any other collaboration, licensing, and other arrangements that we may establish; | |
| ● | the initiation, progress, timing, and completion of preclinical studies and clinical trials for our current and future potential product candidates; | |
| ● | our alignment with the FDA on regulatory approval requirements; | |
| ● | the number and characteristics of product candidates that we pursue; | |
| ● | the outcome, timing, and cost of regulatory approvals; | |
| ● | delays that may be caused by changing regulatory requirements; | |
| ● | the cost and timing of hiring new employees to support our continued growth; | |
| ● | the costs involved in filing and prosecuting patent applications and enforcing and defending patent claims; | |
| ● | the costs of filing and prosecuting intellectual property rights and enforcing and defending any intellectual property-related claims; | |
| ● | the costs of responding to and defending ourselves against complaints and potential litigation; | |
| ● | the costs and timing of procuring clinical and commercial supplies for our product candidates; and | |
| ● | the extent to which we acquire or in-license other product candidates and technologies. |
Contractual obligations and commitments as of March 31, 2024
Significant expenditure contracted for at the end of the reporting period but not recognized as liabilities is as follows:
| (in thousands of US dollars) | TOTAL | |||
| $ | ||||
| Less than 1 year | 4,335 | |||
| 1 - 5 years | 75 | |||
| 4,410 | ||||
In 2021, the Company executed various agreements including in-licensing and similar arrangements with development partners. Such agreements may require the Company to make payments on achievement of stages of development, launch or revenue milestones, although the Company generally has the right to terminate these agreements at no penalty. The Company may have to pay up to $38,756 upon achieving certain sales volumes, regulatory or other milestones related to specific products.
Contingencies
In the normal course of operations, the Company may become involved in various claims and legal proceedings related to, for example, contract terminations and employee-related and other matters.
Related Party Transactions
Other than employment agreements and indemnification agreements with our management, there are no related party transactions.
Off-Balance Sheet Arrangements
As of March 31, 2024, we did not have any interests in special purpose entities or any other off-balance sheet arrangements.
|
|
Risk Factors and Uncertainties
An investment in our securities involves a high degree of risk. In addition to the other information included in this MD&A and in the related consolidated financial statements, investors are urged to carefully consider the risks described under the heading “Risk Factors” in our most recent Annual Report on Form 20-F for the year ended December 31, 2023, for a discussion of the various risks that may materially affect our business. The risks and uncertainties not presently known to us or that we currently deem immaterial may also materially harm our business, operating results and financial condition and could result in a complete loss of your investment.
Our most recent Annual Report on Form 20-F was filed with the relevant Canadian and U.S. securities’ regulatory authorities at www.sedarplus.ca and with the SEC at www.sec.gov. Investors are urged to consult the risk factors in these documents.
Disclosure Controls and Procedures
The Chief Executive Officer and the Chief Financial Officer of the Corporation are responsible for establishing and maintaining our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act and Canadian securities legislation). Our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports we file or submit under U.S. and Canadian securities legislation is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including the Chief Executive Officer and the Chief Financial Officer, to allow timely decisions regarding required disclosures.
Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objective and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. There have been no significant changes to our disclosure controls and procedures for the three-month period ended March 31, 2024, that have materially affected, or are reasonably likely to materially affect, the disclosure controls and procedures.
Internal Controls over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act and Canadian securities legislation). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS as issued by the IASB.
Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of Aeterna Zentaris; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS as issued by the IASB, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the issuer; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of Company assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Controls over Financial Reporting
There have been no significant changes to our internal controls over financial reporting for the three-month period ended March 31, 2024, that have materially affected, or are reasonably likely to materially affect, internal controls over financial reporting.
|
|
Exhibit 99.3
FORM 52-109F2
CERTIFICATION OF INTERIM FILINGS
FULL CERTIFICATE
I, Klaus Paulini, Chief Executive Officer, Aeterna Zentaris Inc., certify the following:
| 1. | Review: I have reviewed the interim financial report and interim MD&A (together, the “interim filings”) of Aeterna Zentaris Inc. (the “issuer”) for the interim period ended March 31, 2024. | ||
| 2. | No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings. | ||
| 3. | Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings. | ||
| 4. | Responsibility: The issuer’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as those terms are defined in National Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings, for the issuer. | ||
| 5. | Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer’s other certifying officer(s) and I have, as at the end of the period covered by the interim filings | ||
| (a) | designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that | ||
| (i) | material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings are being prepared; and | ||
| (ii) | information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation; and | ||
| (b) | designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the issuer’s GAAP. | ||
| 5.1 | Control framework: The control framework the issuer’s other certifying officer(s) and I used to design the issuer’s ICFR is Internal Control – Integrated Framework: 2013, issued by the Committee of Sponsoring Organizations of the Treadway Commission. | ||
| 5.2 | N/A | ||
| 5.3 | N/A | ||
| 6. | Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer’s ICFR that occurred during the period beginning on January 1, 2024 and ended on March 31, 2024 that has materially affected, or is reasonably likely to materially affect, the issuer’s ICFR. | ||
Date: May 15, 2024
| /s/ Klaus Paulini | |
| Klaus Paulini | |
| Chief Executive Officer |
Exhibit 99.4
FORM 52-109F2
CERTIFICATION OF INTERIM FILINGS
FULL CERTIFICATE
I, Giuliano La Fratta, Chief Financial Officer, Aeterna Zentaris Inc., certify the following:
| 1. | Review: I have reviewed the interim financial report and interim MD&A (together, the “interim filings”) of Aeterna Zentaris Inc. (the “issuer”) for the interim period ended September 30, 2023. | ||
| 2. | No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings. | ||
| 3. | Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings. | ||
| 4. | Responsibility: The issuer’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as those terms are defined in National Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings, for the issuer. | ||
| 5. | Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer’s other certifying officer(s) and I have, as at the end of the period covered by the interim filings | ||
| (a) | designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that | ||
| (i) | material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings are being prepared; and | ||
| (ii) | information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation; and | ||
| (b) | designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the issuer’s GAAP. | ||
| 5.1 | Control framework: The control framework the issuer’s other certifying officer(s) and I used to design the issuer’s ICFR is Internal Control – Integrated Framework: 2013, issued by the Committee of Sponsoring Organizations of the Treadway Commission. | ||
| 5.2 | N/A | ||
| 5.3 | N/A | ||
| 6. | Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer’s ICFR that occurred during the period beginning on July 1, 2023 and ended on September 30, 2023 that has materially affected, or is reasonably likely to materially affect, the issuer’s ICFR. | ||
Date: November 8, 2023
| /s/ Giuliano La Fratta | |
| Giuliano La Fratta | |
| Chief Financial Officer |