UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): April 19, 2024
MANGOCEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Texas | 001-41615 | 87-3841292 | ||
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
15110 N. Dallas Parkway, Suite 600 Dallas, Texas |
75248 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (214) 242-9619
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.0001 Par Value Per Share | MGRX |
The Nasdaq Stock Market LLC (Nasdaq Capital Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 Entry into a Material Definitive Agreement
Patent Purchase Agreement
Effective on April 24, 2024, Mangoceuticals, Inc., a Texas corporation (the “Company”, “we” and “us”), entered into a Patent Purchase Agreement (the “IP Purchase Agreement”), with Intramont Technologies, Inc. (“Intramont”). Pursuant to the IP Purchase Agreement, we purchased certain patents and patent applications owned by Intramont, related to prevention of infections, including the common cold, respiratory diseases, and orally transmitted diseases such as human papillomavirus (HPV) (the “Patents”), in consideration for $20,000,000, which is payable to Intramont by (a) the issuance of 980,000 shares of the Company’s newly designated 6% Series C Convertible Preferred Stock (the “Series C Preferred Stock”), with a face value of $20.00 per share, for a total value of $19,600,000 (the “Series C Shares”); and (b) $400,000 in cash, (i) with $200,000 payable on or before June 30, 2024, (ii) $100,000 payable on or before August 31, 2024, and (iii) $100,000 payable on or before November 30, 2024 (collectively, the “Cash Payments”).
In the event any of the Cash Payments have not been made on or before the due date thereof as provided above, we have 30 days to cure such non-payment, and if not paid by the end of such 30 day period, we have the option of paying Intramont $15,000 for a thirty day extension period for each Cash Payment.
The IP Purchase Agreement, and the purchase of the Patents, closed on April 24, 2024, upon the parties entry into the IP Purchase Agreement, and the Series C Shares were also issued on April 24, 2024.
The rights and preferences of the Series C Preferred Stock are discussed in greater detail below under “Item 5.03 Amendments to Designation of Incorporation or Bylaws; Change in Fiscal Year”.
The IP Purchase Agreement included standard representations and warranties and confidentiality and indemnification obligations of the parties, for a transaction of that type and size.
The Company purchased the Patents through its newly formed wholly-owned subsidiary, MangoRx IP Holdings, LLC, a Texas limited liability company.
The IP Purchase Agreement also included a grant back license, whereby the Company provided Intramont, an irrevocable, co-exclusive, non-transferable and non-assignable (except in the event of a change of control), non-sublicensable, worldwide, license to use the Patents for the lives thereof (the “Grant Back-License”). The Grant Back-License is subject to Intramont paying the Company a royalty of ten percent (10%) of gross worldwide sales of products sold by Intramont which utilize the Patents, beginning on April 24, 2025, and continuing until the end of the life of the last Patent (the “Royalty Payments”). The Royalty Payments are to be paid to the Company on an annual basis, within 30 days after the end of the calendar year.
Finally, the IP Purchase Agreement granted Intramont a right of first refusal, which provides that, if at any time prior to April 24, 2027, if we receive an offer to purchase the Patents and determine to accept such offer, or we determine to sell the Patents to a third party, we are required to provide Intramont the right of first refusal to either match such offer, or negotiate different purchase terms for the Patents.
The Company intends to utilize the Patents by commencing research, development, clinical trial studies and efficacy testing on a variety of oral applications including, but not limited to, an oral dissolvable tablet (ODT), lozenge, toothpaste and/or mouthwash.
The foregoing description of the IP Purchase Agreement is only a summary of the material terms of such agreement and does not purport to be complete and is qualified in its entirety by reference to the full text of such agreement, which is filed as Exhibit 10.1, to this Current Report on Form 8-K and incorporated herein by reference.
Item 2.01 Completion of Acquisition or Disposition of Assets.
The information set forth in Item 1.01 above is incorporated into this Item 2.01 by reference.
Item 3.02 Unregistered Sales of Equity Securities.
The information set forth in Item 1.01 above and Item 5.03 below, is incorporated herein by reference.
The issuance of the Series C Shares was exempt from registration pursuant to an exemption from registration provided by Section 4(a)(2) and/or Rule 506 of Regulation D of the Securities Act of 1933, as amended (the “Securities Act”), since the foregoing issuance did not involve a public offering, the recipient took the securities for investment and not resale, we took appropriate measures to restrict transfer, and the recipient is an “accredited investor”. The securities are subject to transfer restrictions, and the certificates evidencing the securities contain an appropriate legend stating that such securities have not been registered under the Securities Act and may not be offered or sold absent registration or pursuant to an exemption therefrom. The securities were not registered under the Securities Act and such securities may not be offered or sold in the United States absent registration or an exemption from registration under the Securities Act and any applicable state securities laws.
If the Series C Shares were converted in full, without factoring in any dividends which can be paid in-kind and/or shares of common stock, a maximum of 1,960,000 shares of common stock would be due to the holder thereof.
Item 3.03 Material Modification to Rights of Security Holders.
The information contained in Item 5.03 relating to the Series C Designation (as discussed in Item 5.03), below, is incorporated in this Item 3.03 by reference.
Item 5.03 Amendments to Designation of Incorporation or Bylaws; Change in Fiscal Year.
On April 19, 2024, the Company submitted for filing to the Secretary of State of Texas, a Certificate of Designations of Mangoceuticals, Inc. Establishing the Designations, Preferences, Limitations and Relative Rights of Its 6% Series C Convertible Cumulative Preferred Stock (the “Series C Designation”), which was filed with the Secretary of State of Texas on April 23, 2024, effective as of April 19, 2024. The Series C Designation designated 6,250,000 shares of Series C Preferred Stock.
The Series C Designation provides for the Series C Preferred Stock to have the following terms:
6% Series C Convertible Cumulative Preferred Stock
Dividend Rights. From and after the issuance date of the Series C Preferred Stock, each share of Series C Preferred Stock is entitled to receive, when, as and if authorized and declared by the Board of Directors of the Company, out of any funds legally available therefor, cumulative dividends in an amount equal to (i) the 6% per annum on the stated value (initially $20 per share)(the “Stated Value”) as of the record date for such dividend (as described in the Series C Designation), and (ii) on an as-converted basis, any dividend or other distribution, whether paid in cash, in-kind or in other property, authorized and declared by the Board of Directors on the issued and outstanding shares of common stock in an amount determined by assuming that the number of shares of common stock into which such shares of Series C Preferred Stock could be converted on the applicable record date for such dividend or distribution.
Dividends payable pursuant to (i) above are payable quarterly in arrears, if, as and when authorized and declared by the Board of Directors, or any duly authorized committee thereof, to the extent not prohibited by law, on March 31, June 30, September 30 and December 31 of each year (unless any such day is not a business day, in which event such dividends are payable on the next succeeding business day, without accrual of interest thereon to the actual payment date), commencing on June 30, 2024.
Accrued dividends may be settled in cash, subject to applicable law, shares of common stock (valued at the closing price on the date the dividend is due) or in-kind, by increasing the Stated Value by the amount of the quarterly dividend.
Liquidation Preference. Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary (a “Liquidation”), the holders of the Series C Preferred Stock are entitled to receive out of the assets, whether capital or surplus, of the Company an amount equal to the Stated Value (the “Liquidation Preference”), for each share of Series C Preferred Stock, before any distribution or payment is made to the holders of any junior securities, but after the payment of any liquidation preference of any holder of senior securities, including the Series B Convertible Preferred Stock, which has a preferential right to payments in liquidation, and if the assets of the Company are insufficient to pay in full such amounts, then the entire assets to be distributed to the holders of the Series C Preferred Stock are to be ratably distributed among the holders of the Series C Preferred Stock in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full.
Conversion Rights. Each holder of Series C Preferred Stock may, at its option, convert its shares of Series C Preferred Stock into that number of shares of common stock equal to the Stated Value of such share of Series C Preferred Stock, divided by the conversion price of $10.00 per share (i.e., initially a 2-for-1 conversion ratio) (the “Conversion Price”), subject to adjustment for stock splits and stock dividends, with any fractional shares rounded up to the nearest whole share.
The Series C Designation includes a conversion limitation prohibiting any holder and their affiliates from converting the Series C Preferred Stock into common stock in the event that upon such conversion their beneficial ownership of the Company’s common stock would exceed 4.999% (which can be increased as to any holder, to up to 9.999%, with 61 days prior written notice by such holder). The Series C Designation also includes a general restriction prohibiting the issuance of more than 19.99% of the Company’s outstanding shares as of the date of entry into the IP Purchase Agreement, without the Company’s stockholders approving such issuance(s) under the rules of the Nasdaq Capital Market.
Voting Rights. The Series C Preferred Stock have no voting rights, except in connection with the protective provisions discussed below.
Protective Provisions. So long as any shares of Series C Preferred Stock are outstanding, the Company cannot without first obtaining the approval of the holders of a majority of the then outstanding shares of Series C Preferred Stock, voting together as a class:
(a) Amend any provision of the Series C Designation;
(b) Increase or decrease (other than by redemption or conversion) the total number of authorized shares of Series C Convertible Preferred Stock;
(c) Amend the Certificate of Formation of the Company (including by designating additional series of Preferred Stock) in a manner which adversely affects the rights, preferences and privileges of the Series C Preferred Stock;
(d) Effect an exchange, or create a right of exchange, cancel, or create a right to cancel, of all or any part of the shares of another class of shares into shares of Series C Preferred Stock; or
(e) Alter or change the rights, preferences or privileges of the shares of Series C Preferred Stock so as to affect adversely the shares of such series.
Redemption Rights. The Company may redeem the outstanding Series C Preferred Stock shares, from time to time, in whole or in part, at any time after April 24, 2025, and continuing indefinitely thereafter, at the option of the Company, for cash, at the aggregate Liquidation Preference of the shares redeemed.
* * * * * The foregoing description of the Series C Preferred Stock and Series C Designation is only a summary of the material terms of such Series C Preferred Stock and Series C Designation and does not purport to be complete and is qualified in its entirety by reference to the full text of the Series C Designation, which is filed as Exhibit 3.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 8.01. Other Events.
Press Release
On April 25, 2024, the Company issued a press release announcing the closing of the IP Purchase Agreement. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
Nasdaq Minimum Stockholders’ Equity Compliance
As previously disclosed in the Current Report on Form 8-K, filed by the Company with the Securities and Exchange Commission (the “Commission” or the “SEC”) on November 7, 2023, on November 3, 2023, the Company received a letter from The Nasdaq Stock Market LLC (“Nasdaq”) notifying the Company that it was not in compliance with the minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market. Nasdaq Listing Rule 5550(b)(1) (the “Rule”) requires companies listed on the Nasdaq Capital Market to maintain stockholders’ equity of at least $2,500,000. In the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, the Company reported stockholders’ equity of $1,354,821, which is below the minimum stockholders’ equity required for continued listing pursuant to the Rule. Additionally, the Company did not meet the alternative Nasdaq continued listing standards under Nasdaq Listing Rules.
Nasdaq provided the Company until December 18, 2023 to submit to Nasdaq a plan to regain compliance. We submitted the plan to regain compliance in a timely manner, and on January 24, 2024, as reported in the Current Report on Form 8-K filed by the Company with the Commission on January 25, 2024, Nasdaq advised the Company that it determined to grant the Company an extension to regain compliance with the Rule until April 29, 2024.
As a result of the purchase of the Patents as described in the IP Purchase Agreement in Item 1.01 above, and the funds raised pursuant to the initial closing of the April 4, 2024, Securities Purchase Agreement, entered into with an institutional accredited investor , as described in greater detail in the Company’s Current Report on Form 8-K filed with the Commission on April 11, 2024, as of the date of this Current Report on Form 8-K, the Company believes it has regained compliance with the Rule because it believes that its stockholders’ equity exceeds $2.5 million and that it also satisfies the minimum $5 million equity requirement for initial listing on The Nasdaq Capital Market. In that regard, the Company believes that as of the date of this Form 8-K filing, stockholders’ equity exceeds $5 million. The Company anticipates evidencing compliance with the Rule as of the date of its next periodic report.
Notwithstanding such expected compliance, Nasdaq will continue to monitor the Company’s ongoing compliance with the stockholders’ equity requirement and, if at the time of its next periodic report the Company does not evidence compliance, the Company may be subject to delisting.
Notwithstanding the above, the Company remains out of compliance with Nasdaq’s minimum bid price requirement as of the date of this Current Report on Form 8-K and intends to request a further 180 day extension from Nasdaq to obtain compliance with such requirement. If granted by Nasdaq, the Company plans to a reverse stock split during the extension period, if necessary, to cure such deficiency.
Item 9.01 Exhibits.
(d) Exhibits.
| Exhibit No. | Description | |
| 3.1* | Certificate of Designations, Preferences and Rights of 6% Series C Convertible Preferred Stock of Mangoceuticals, Inc., filed with the Secretary of State of Texas on April 19, 2024 | |
| 10.1*# | Patent Purchase Agreement dated April 24, 2024, by and between Mangoceuticals, Inc., as purchaser and Intramont Technologies, Inc., as seller | |
| 99.1* | Mangoceuticals, Inc. Press Release dated April 25, 2024 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document and included in Exhibit 101). |
* Filed herewith.
# Certain schedules and exhibits have been omitted pursuant to Item 601(b)(2)(ii) of Regulation S-K. A copy of any omitted schedule or Exhibit will be furnished supplementally to the Securities and Exchange Commission upon request; provided, however that Mangoceuticals, Inc. may request confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, for any schedule or Exhibit so furnished.
Forward-Looking Statements
This Current Report on Form 8-K, including the press release furnished as Exhibit 99.1, to this Current Report on Form 8-K, contains forward-looking statements within the meaning of the federal securities laws, including the Private Securities Litigation Reform Act of 1995, and, as such, may involve known and unknown risks, uncertainties and assumptions. You can identify these forward-looking statements by words such as “may,” “should,” “expect,” “anticipate,” “believe,” “estimate,” “intend,” “plan” and other similar expressions. These forward-looking statements relate to the Company’s current expectations and are subject to the limitations and qualifications set forth in the press release as well as in the Company’s other filings with the Securities and Exchange Commission, including, without limitation, that actual events and/or results may differ materially from those projected in such forward-looking statements. These statements also involve known and unknown risks, which may cause the results of the Company, its divisions and concepts to be materially different than those expressed or implied in such statements, including those referenced in the press release. Accordingly, readers should not place undue reliance on any forward-looking statements. Forward-looking statements may include comments as to the Company’s beliefs and expectations as to future financial performance, events and trends affecting its business and are necessarily subject to uncertainties, many of which are outside the Company’s control. More information on potential factors that could affect the Company’s financial results is included from time to time in the “Cautionary Statement Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of the Company’s most recently filed periodic reports on Form 10-K and Form 10-Q and subsequent filings with the SEC and available at www.sec.gov and in the “Investors–SEC Filings” section of the Company’s website at www.mangorx.com. Forward-looking statements speak only as of the date they are made. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise that occur after that date, except as otherwise provided by law.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| MANGOCEUTICALS, INC. | ||
| Date: April 25, 2024 | By: | /s/ Jacob D. Cohen |
| Jacob D. Cohen | ||
| Chief Executive Officer | ||
Exhibit 3.1


CERTIFICATE OF DESIGNATIONS
OF
MANGOCEUTICALS, INC.
ESTABLISHING THE DESIGNATIONS, PREFERENCES,
LIMITATIONS AND RELATIVE RIGHTS OF ITS
6% SERIES C CONVERTIBLE CUMULATIVE PREFERRED STOCK
Pursuant to Section 21.155 of the Texas Business Organizations Code (the “TBOC”), Mangoceuticals, Inc., a corporation organized and existing under the TBOC (the “Company”),
DOES HEREBY CERTIFY that pursuant to the authority conferred upon the Board of Directors by the Certificate of Formation of the Company, as amended (the “Certificate of Formation”), and pursuant to Section 21.155 of the TBOC, the Board of Directors, pursuant to Section 6.201 of the TBOC, by unanimous consent of all members of the Board of Directors on April 18, 2024, duly adopted a resolution providing for the designation of a series of Six Million Two Hundred and Fifty Thousand (6,250,000) shares of Series C Convertible Preferred Stock, which resolution is and reads as follows:
RESOLVED, that pursuant to the authority expressly granted to and invested in the Board of Directors by the provisions of the Certificate of Formation of the Company and Section 21.155 of the TBOC, the Company hereby establishes a new series of Preferred Stock, par value $0.0001 per share, of the Company and fixes the number of shares of such series and the powers, designations, preferences and relative rights of such series, and the qualifications, limitations or restrictions thereof as follows:
The new series of Preferred Stock, par value $0.0001 per share, of the Company shall be, and hereby is, designated “6% Series C Convertible Cumulative Preferred Stock” (the “Series C Preferred Stock” or the “Preferred Stock”), and the initial number of designated shares constituting such series shall be Six Million Two Hundred and Fifty Thousand (6,250,000) (the “Shares”). The powers and preferences, and the relative, participating, optional and other rights, and the qualifications, limitations, and restrictions thereon of the Series C Preferred Stock shall be set forth in this Certificate of Designations (the “Designation” or the “Certificate of Designation”) below:
1. Definitions. For purposes of the Shares and as used in this Designation, in addition to other terms defined throughout this Designation, the following terms have the following meanings when used herein:
“Board” shall mean the board of directors of the Company or any committee of members of the board of directors authorized by such board to perform any of its responsibilities with respect to the Shares.
“Business Day” shall mean any day other than a Saturday, Sunday or a day on which state or federally chartered banking institutions in New York, New York are not required to be open.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
“Call Date” shall mean the date fixed for redemption of the Shares and specified in the notice to Holders required under paragraph (b) of Section 4 hereof as the Call Date.
“Closing Sales Price” means the last sales price of the Company’s Common Stock on the Principal Market as reported by NASDAQ.com (or a comparable reporting service of national reputation)(collectively, “NASDAQ.com”), or if the foregoing does not apply, the last reported sales price of such security on a national exchange or in the over-the-counter market on the electronic bulletin board for such security as reported by NASDAQ.com, or, if no such price is reported for such security by NASDAQ.com, the average of the bid prices of all market makers for such security as reported in the “pink sheets” by OTC Markets, in each case for such date or, if such date was not a Trading Day for such security, on the next preceding date that was a Trading Day. If the Closing Sales Price cannot be calculated for such security as of either of such dates on any of the foregoing bases, the Closing Sales Price of such security on such date shall be the fair market value as reasonably determined by the Company.
“Common Shares” or “Common Stock” shall mean the shares of common stock, $0.0001 par value, of the Company.
“Company” shall mean Mangoceuticals, Inc., a Texas corporation.
“Conversion Price” means $10.00, subject to adjustment in connection with any Recapitalization.
“Conversion Rights” shall have the meaning set forth in Section 5 hereof.
“Designation” shall mean this Certificate of Designations of Mangoceuticals, Inc. Establishing the Designations, Preferences, Limitations and Relative Rights of Its 6% Series C Convertible Preferred Stock.
“Dividend Payment Date” shall have the meaning set forth in subparagraph (a) of Section 2 hereof.
“Dividend Periods” shall mean quarterly dividend periods commencing on the first day of each of January, April, July and October and ending on and including the day preceding the first day of the next succeeding Dividend Period.
“Dividend Rate” is 6% per annum.
“Dividend Record Date” shall have the meaning set forth in subparagraph (a) of Section 2 hereof.
“Exchange Act” shall mean the U.S. Securities Exchange Act of 1934, as amended and the rules and regulations promulgated thereunder.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
“Issue Date” shall mean the date that any Shares are first issued by the Company.
“Holder” shall mean the person or entity in which the Shares are registered on the books of the Company, which shall initially be the person or entity which such Shares are issued to, and shall thereafter be permitted and legal assigns which the Company is notified of by the Holder and which the Holder has provided a valid legal opinion in connection therewith to the Company and to whom such Shares are legally transferred.
“Junior Shares” shall have the meaning set forth in paragraph (a)(iii) of Section 7 hereof.
“Liquidation Preference” means the Stated Value.
“Parity Shares” shall have the meaning set forth in paragraph (a)(ii) of Section 7 hereof.
“Person” shall mean any individual, firm, partnership, limited liability company, corporation or other entity, and shall include any successor (by merger or otherwise) of such entity.
“Preferred Stock Certificates” means the stock certificate(s) issued by the Company representing the applicable Series C Preferred Stock shares.
“Principal Market” means initially the Nasdaq Capital Market and shall also include the New York Stock Exchange, NYSE American, the Nasdaq Global Market, the OTCQB, the OTCQX or the OTC Pink Market, whichever is at the time the principal trading exchange or market for the Common Shares, based upon share volume.
“Recapitalization” shall mean any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event described in Sections 10(b) through (c).
“Restricted Shares” means shares of the Company’s Common Stock which are restricted from being transferred by the Holder thereof unless the transfer is effected in compliance with the Securities Act and applicable state securities laws (including investment suitability standards, which shares shall bear the following restrictive legend (or one substantially similar)):
The securities represented by this certificate have not been registered under the Securities Act of 1933 or any state securities act. The securities have been acquired for investment and may not be sold, transferred, pledged or hypothecated unless (i) they shall have been registered under the Securities Act of 1933 and any applicable state securities act, or (ii) the corporation shall have been furnished with an opinion of counsel, satisfactory to counsel for the corporation, that registration is not required under any such acts.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
“Securities Act” means the Securities Act of 1933, as amended (and any successor thereto) and the rules and regulations promulgated thereunder.
“Senior Shares” shall have the meaning set forth in paragraph (a)(i) of Section 7 hereof.
“set apart for payment” shall be deemed to include, without any further action, the following: the recording by the Company in its accounting ledgers of any accounting or bookkeeping entry that indicates, pursuant to an authorization by the Board and a declaration of dividends or other distribution by the Company, the initial and continued allocation of funds to be so paid on any series or class of shares of stock of the Company; provided, however, that if any funds for any class or series of Junior Shares or any class or series of Parity Shares are placed in a separate account of the Company or delivered to a disbursing, paying or other similar agent, then “set apart for payment” with respect to the Shares shall mean irrevocably placing such funds in a separate account or irrevocably delivering such funds to a disbursing, paying or other similar agent.
“Shares” has the meaning set forth in the introductory paragraphs hereof.
“Simple Majority” means the holders of at least a majority of the then issued and outstanding Shares.
“Stated Value” means $20.00 per Share, as adjusted pursuant to Sections 2.5 and 2.6 hereof, as applicable.
“Trading Day” means any day on which the Common Shares are traded on the Principal Market, or, if the Principal Market is not the principal trading market for the Common Shares, then on the principal securities exchange or securities market on which the Common Shares is then traded; provided that “Trading Day” shall not include any day on which the Common Shares is scheduled to trade on such exchange or market for less than 4.5 hours or any day that the Common Shares is suspended from trading during the final hour of trading on such exchange or market (or if such exchange or market does not designate in advance the closing time of trading on such exchange or market, then during the hour ending at 4:00:00 p.m., New York Time).
“Transfer Agent” means ClearTrust, LLC, or such other agent or agents of the Company as may be designated by the Board or its duly authorized designee as the transfer agent, registrar and dividend disbursing agent for the Shares, including, at the option of the Board, the Company itself.
2. 2.1 Dividends in General. From and after the Issue Date of each Share, each Share shall be entitled to receive, when, as and if authorized and declared by the Board of Directors, out of any funds legally available therefor, cumulative dividends in an amount equal to (i) the Dividend Rate on the Stated Value of such Share as of the Record Date for such dividend (each such dividend on the Shares, a “Regular Dividend” and, collectively, the “Regular Dividends”), and (ii) on an as-converted basis, any dividend or other distribution, whether paid in cash, in-kind or in other property (including, for the avoidance of doubt, any securities other than Common Shares, but not in connection with any stock dividend paid solely in Common Shares), authorized and declared by the Board of Directors on the issued and outstanding Common Shares in an amount determined by assuming that the number of Common Shares into which such Share could be converted pursuant to Section 5 hereof, on the applicable Record Date for such dividend or distribution on the Common Shares were issued to, and held by, the Holder of such Share on such Record Date (each such dividend on the Shares pursuant to this clause (ii), a “Participating Dividend” and, collectively, the “Participating Dividends” and, together with the Regular Dividends, the “Dividends”).
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
2.2 “Record Date” shall mean, with respect to any dividend, distribution or other transaction or event in which the holders of Common Shares or shares of Shares, as applicable, have the right to receive any cash, securities or other property or in which the Common Shares or shares of Shares (or other applicable security), as applicable, are exchanged for or converted into any combination of cash, securities or other property, the date fixed for determination of shareholders entitled to receive such cash, securities or other property (whether such date is fixed by the Board of Directors or a committee thereof, or by statute, contract, this Certificate of Designations or otherwise). With respect to any Regular Dividend payable on any Regular Dividend Payment Date, the Record Date therefor will be the immediately preceding March 15, June 15, September 15 or December 15, as applicable.
2.3 Payment of Regular Dividends. Regular Dividends shall be payable quarterly in arrears, if, as and when authorized and declared by the Board of Directors, or any duly authorized committee thereof, to the extent not prohibited by law, on March 31, June 30, September 30 and December 31 of each year (unless any such day is not a Business Day, in which event such Regular Dividends shall be payable on the next succeeding Business Day, without accrual of interest thereon to the actual payment date), commencing on June 30, 2024 (each such payment date, a “Regular Dividend Payment Date,” and the period from, and including, the Issue Date to, and including, the first Regular Dividend Payment Date and each such quarterly period thereafter from, but excluding, the immediately preceding Regular Dividend Payment Date to, and including, the next occurring Regular Dividend Payment Date, a “Regular Dividend Period”). The amount of Regular Dividends payable in respect of each Share for any period shall be computed on the basis of a 360-day year consisting of twelve thirty-day months. Regular Dividends shall begin to accrue from the Issue Date whether or not declared and whether or not the Company has assets legally available to make payment thereof, at a rate equal to the Dividend Rate and, if not declared and paid, shall be cumulative, regardless of whether or not in any Regular Dividend Period there are funds of the Company legally available for the payment of such Regular Dividend. In the event that the Board of Directors has authorized the payment of any Regular Dividend, the Company may, in its sole discretion and notwithstanding anything to the contrary in this Certificate of Designations, settle such Regular Dividend in cash out of funds legally available therefor, in Common Shares pursuant to the terms and conditions of Section 2.4, in-kind pursuant to the terms and conditions of Section 2.4, or a combination of cash and in-kind settlement pursuant to the terms and conditions of Section 2.6, and the Company shall set aside sufficient funds for the portion of any Regular Dividend to be paid in whole or in part in cash before the Board of Directors or any other authorized Person may declare, set apart funds for or pay any dividend on the Junior Shares; provided, however, that, to the extent any such payment in cash is prohibited by applicable law, such payment will be made in Common Shares in accordance with the terms and conditions of Section 2.4 or in-kind in accordance with the terms and conditions of Section 2.4. Participating Dividends shall be payable as and when paid to the holders of Common Shares (each such date, a “Participating Dividend Payment Date” and, together with a Regular Dividend Payment Date, a “Dividend Payment Date”). Participating Dividends are payable on a cumulative basis once declared, regardless of whether or not there are then funds of the Company available for the payment of such Participating Dividend pursuant to law.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
2.4 Dividends Paid in Common Shares. With respect to each Share, any Regular Dividend or portion thereof in respect of such Share that has accrued during any applicable Regular Dividend Period but is not paid (in whole or in part) in cash or by a PIK Dividend (pursuant to Section 2.45 hereof) on the applicable Regular Dividend Payment Date may be paid at the option of the Company in Common Shares, by issuing each Holder a number of Common Shares equal to (x) the total amount of the Regular Dividend due to such Holder, divided by (y) the Closing Sales Price, rounded up to the nearest whole Common Share (“Dividend Shares”). Dividend Shares shall be issued to each Holder within five (5) Trading Days of the applicable Divided Payment Date.
2.5 Accrued or PIK Dividends. With respect to each Share, any Regular Dividend or portion thereof in respect of such Share that has accrued during any applicable Regular Dividend Period but is not paid (in whole or in part) in cash or Common Shares (pursuant to Section 2.4 hereof) on the applicable Regular Dividend Payment Date (the amount of any accrued and unpaid Regular Dividend with respect to any Share for any Regular Dividend Period, regardless of whether such Regular Dividend is paid in cash or kind, the “Accrued Dividend Amount” with respect to such Share for such Regular Dividend Period) shall, regardless of whether or not such Regular Dividend is authorized and declared by the Board of Directors, or whether the Company has assets legally available to make payment thereof, be added to the Stated Value of such Share immediately following the close of business on such Regular Dividend Payment Date. Any such addition of the Accrued Dividend Amount in respect of a Share to the Stated Value of such Share pursuant to this Section 2.4 is referred to herein as a “PIK Dividend.” The Accrued Dividend Amount in respect of any Regular Dividend Period that is not paid (in whole or in part) in cash or in Common Shares pursuant to Section 2.4, shall, without duplication of any prior PIK Dividends (if any) only be added to the Stated Value of such Share once. Regular Dividends with respect to each Share shall continue, from and after the date of each PIK Dividend, if any, to accrue in an amount per annum equal to the Dividend Rate of the Stated Value of such Share as of the relevant Record Date. Notwithstanding anything to the contrary in this Certificate of Designations, the Company will not be permitted to make any PIK Dividend election to the extent such election would violate the listing standards of the Principal Market; provided, however, that nothing herein will affect the compounding of any Regular Dividend that the Company does not pay in cash (which compounding will apply even if the Company is otherwise prohibited from electing to make any PIK Dividend pursuant to this sentence).
2.6 Cash and PIK Dividends. In the event that the Board of Directors has authorized and declared the payment of a Regular Dividend and the settlement of such Regular Dividend payment in part by payment of cash to each Holder of Shares and in part pursuant to a PIK Dividend (any such Regular Dividend, a “Cash and PIK Dividend”), the Company shall, on the applicable Regular Dividend Payment Date and in respect of each Share, (i) pay to the Holder thereof an amount of cash equal to the Cash and PIK Dividend Cash Settlement Amount in respect of such Share, and (ii) add to the Stated Value of such Share an amount equal to (A) the Accrued Dividend Amount with respect to such Share for the Regular Dividend Period ending on, and including, such Regular Dividend Payment Date, minus (B) the Cash and PIK Dividend Cash Settlement Amount in respect of such Share. If the Board of Directors declares a Cash and PIK Dividend, and any portion of the cash payment of such Cash and PIK Dividend per Share is not paid pursuant to the terms of this Section 3, then such portion shall be added to the Stated Value of such Share in accordance with the terms of this Section 2.6.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
2.7 In the event that the Board of Directors has authorized and declared the payment of a Participating Dividend, such Participating Dividend shall be paid in a manner consistent with the payments of dividends on the Common Shares. The Company will not declare any dividend or distribution on the Common Shares unless, concurrently therewith, the Company declares a corresponding Participating Dividend, except for dividends paid on Common Shares solely in Common Shares, which shall not be deemed Participating Dividends.
2.8 Partial Dividend Payments. Except as otherwise provided herein, if at any time the Company pays, in cash, less than the total amount of Dividends then accrued, but unpaid, with respect to the Shares, such cash payment shall be distributed pro rata among the Holders thereof based upon the Stated Value of all Shares held by each such Holder as of the Record Date for such payment. When Dividends are not paid in full upon the Shares, all dividends declared on Shares and any other class or series of stock which has parity rights with the Shares (“Parity Stock”) shall be paid pro rata so that the amount of dividends so declared on the Shares and each such other class or series of Parity Stock shall in all cases bear to each other the same ratio as accrued, but unpaid, Dividends (for the full amount of dividends that would be payable for the most recently completed Regular Dividend Period if dividends were declared in full on non-cumulative Parity Stock) on the Shares and such other class or series of Parity Stock bear to each other.
2.9 No dividend on the Shares will be declared by the Company or paid or set apart for payment by the Company in cash at such time as the terms and provisions of Senior Shares or any agreement of the Company, including any agreement relating to its indebtedness, prohibit such declaration, payment or setting apart for payment or provide that such declaration, payment or setting apart for payment would constitute a breach thereof or a default thereunder, or if such declaration, payment or setting aside of funds is restricted or prohibited under Texas law or other applicable law; provided, however, notwithstanding anything to the contrary contained herein, dividends on the Shares shall continue to accrue without interest and accumulate regardless of whether: (i) any or all of the foregoing restrictions exist; (ii) the Company has earnings or profits; (iii) there are funds legally available for the payment of such dividends; or (iv) such dividends are authorized by the Board. Accrued and unpaid dividends on the Shares will accumulate as of the Dividend Payment Date on which they first become payable or on the date of redemption of the Shares, as the case may be. Notwithstanding anything in this Section 2, no Dividends shall be paid on the Shares prior to the payment in full of all dividends due on Senior Shares.
2.10 Within one Business Day of the Record Date for any Regular Dividend, the Company will send written notice to each Holder of shares of Shares stating (i) whether such Regular Dividend will be paid in cash, in Common Shares pursuant to Section 2.4 hereof, by increasing the Stated Value of each Share pursuant to Section 2.4, or pursuant to a Cash and PIK Dividend pursuant to Section 2.6, and (ii) if such Regular Dividend will be paid, at least in part, by increasing the Stated Value of a Share pursuant to Section 2.4 or pursuant to a Cash and PIK Dividend pursuant to Section 2.6, the Stated Value of each Share immediately before and immediately after the applicable increase. If the Company fails to send such written notice at or before the Close of Business on the Business Day immediately following the Record Date for any Regular Dividend, then the Company will be deemed to have irrevocably elected to pay such Regular Dividend by increasing the Stated Value of each Share pursuant to Section 2.4.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
2.11 Non-Cash Distributions. Whenever a Participating Dividend shall be payable in property other than cash, the value of such Participating Dividend shall be deemed to be the fair market value of such property as determined in good faith by the Board of Directors.
2.12 Restricted Securities. Unless the Dividend Shares are covered by a valid and effective registration under the Securities Act or the Holder provides the Company a valid opinion from an attorney stating that such Dividend Shares can be issued free of restrictive legend, which shall be determined by the Company (or the Transfer Agent) in its sole discretion, such shares shall be issued as Restricted Shares.
2. Liquidation Preference.
(a) Subject to the priority rights of the holders of Senior Shares and the rights of the Parity Shares, in the event of any liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, after the payment in full of all amounts due to the holders of Senior Shares, but before any payment or distribution of the assets of the Company (whether capital or surplus) shall be made to or set apart for the holders of Junior Shares as to the distribution of assets on any liquidation, dissolution or winding up of the Company, each Holder of the Shares shall be entitled to receive an amount of cash equal to the Liquidation Preference. If, upon any liquidation, dissolution or winding up of the Company, after the payment in full to all holders of the Senior Shares, the assets of the Company, or proceeds thereof, distributable among the Holders of the Shares shall be insufficient to pay in full the preferential amount aforesaid and liquidating payments on any other shares of any class or series of Parity Shares as to the distribution of assets on any liquidation, dissolution or winding up of the Company, then such assets, or the proceeds thereof, shall be distributed among the Holders of Shares and any such other Parity Shares ratably in accordance with the respective amounts that would be payable on such Shares and any such other Parity Shares if all amounts payable thereon were paid in full.
(b) Written notice of any such liquidation, dissolution or winding up of the Company, stating the payment date or dates when, and the place or places where, the amounts distributable in such circumstances shall be payable, shall be given by first class mail, postage pre-paid, not less than twenty (20) nor more than sixty (60) days prior to the payment date stated therein, to each record Holder of Shares at the respective address of such Holders as the same shall appear on the stock transfer records of the Company.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
(c) Subject to the rights of the holders of Senior Shares and Parity Shares upon liquidation, dissolution or winding up, upon any liquidation, dissolution or winding up of the Company, after payment shall have been made in full to the Holders of the Shares, as provided in this Section 3, any other series or class or classes of Junior Shares shall, subject to the respective terms and provisions (if any) applying thereto, be entitled to receive any and all assets remaining to be paid or distributed, and the Holders of the Shares shall not be entitled to share therein.
(d) If any assets of the Company distributed to stockholders in connection with any liquidation, dissolution, or winding up of the Company are other than cash, then the value of such assets shall be their fair market value as determined in good faith by the Board of Directors.
3. Company Call Option.
(a) Optional Redemption at Election of Company. The Company may redeem the Shares, from time to time, in whole or in part, at any time after the first anniversary of the Issue Date and continuing indefinitely thereafter, at the option of the Company, for cash, at the aggregate Liquidation Preference of the Shares redeemed (the “Redemption Amount”). If fewer than all of the outstanding Shares are to be redeemed pursuant to the Company’s exercise of its redemption right under this Section 4(a), the Shares to be redeemed shall be selected pro rata (as nearly as practicable without creating fractional shares) or by lot or in such other equitable method prescribed by the Company.
(b) Redemption Procedures. Notice of the redemption of any Shares under paragraph (a) of this Section 4 shall be mailed by first class mail or via electronic mail to each Holder of record of Shares to be redeemed at the address of each such Holder as shown on the Company’s records, not less than twenty (20) nor more than sixty (60) days prior to the Call Date. Neither the failure to deliver any notice required by this paragraph (b), nor any defect therein or in the delivery thereof, to any particular Holder, shall affect the sufficiency of the notice or the validity of the proceedings for redemption with respect to the other Holders. Any notice delivered in the manner herein provided shall be conclusively presumed to have been duly given on the date mailed whether or not the Holder receives the notice. Each such mailed notice shall state, as appropriate: (1) the Call Date; (2) the number of Shares to be redeemed and, if fewer than all the Shares held by such Holder are to be redeemed, the number of such Shares to be redeemed from such Holder; (3) the redemption price per Share (determined as set forth in paragraph (a) of this Section 4); (4) if any Shares are represented by certificates, the place or places at which certificates for such Shares are to be surrendered; and (5) any other information required by law or by the applicable rules of any exchange or national securities market upon which the Shares may be listed or admitted for trading. Notice having been delivered as aforesaid, from and after the Call Date (unless the Company shall fail to make available an amount of cash necessary to effect such redemption), (i) said Shares shall no longer be deemed to be outstanding, and (ii) all rights of the Holders thereof as Holders of Shares shall cease (except the right to receive cash payable upon such redemption, without interest thereon, upon surrender and endorsement of their certificates if so required).
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
(c) Set Asides. The Company’s obligation to provide cash in accordance with the preceding subsection shall be deemed fulfilled if, on or before the Call Date, the Company shall irrevocably deposit funds necessary for such redemption, in trust, with a bank or trust company that has, or is an affiliate of a bank or trust company that has, capital and surplus of at least $50 million, with irrevocable instructions that such cash be applied to the redemption of the Shares so called for redemption, in which case the notice to Holders of the Shares will (i) state the date of such deposit, (ii) specify the office of such bank or trust company as the place of payment of the redemption price and (iii) require such Holders to surrender the certificates, if any, representing such Shares at such place on or about the date fixed in such redemption notice (which may not be later than the Call Date) against payment of the redemption price. No interest shall accrue for the benefit of the Holders of Shares to be redeemed on any cash so set aside by the Company. Subject to applicable escheat laws, any such cash unclaimed at the end of six months from the Call Date shall revert to the general funds of the Company after which reversion the Holders of such Shares so called for redemption shall look only to the general funds of the Company for the payment of such cash.
(d) Holder Obligations. Without limiting the obligation of each Holder set forth herein (including in the subsequent clause (e)), the Company and/or the Company’s Transfer Agent shall be authorized to take whatever action necessary, if any, following the issuance and delivery of the Redemption Amount to reflect the cancellation of the Series C Preferred Stock subject to the redemption, which shall not require the approval and/or consent of any Holder (a “Cancellation”).
(e) Further Assurances. Notwithstanding the above, each Holder, by accepting such Preferred Stock certificates (if any) hereby covenants that it will, whenever and as reasonably requested by the Company and the Transfer Agent, at the Company’s sole cost and expense, do, execute, acknowledge and deliver any and all such other and further acts, deeds, assignments, transfers, conveyances, confirmations, powers of attorney and any instruments of further assurance, approvals and consents as the Company or the Transfer Agent may reasonably require in order to complete, insure and perfect the Cancellation, if such may be reasonably required by the Company and/or the Company’s Transfer Agent.
4. Conversion. The Holders of the Shares shall have conversion rights as follows (the “Conversion Rights”):
(a) Right to Convert. Each Share shall be convertible (each a “Conversion”) into that number of fully-paid, nonassessable Common Shares determined by dividing (i) the Stated Value, by (ii) the Conversion Price (such total number of Common Shares due, the “Conversion Shares”), at the option of the Holder thereof, in whole or in part at any time after the second anniversary of the Issue Date and continuing indefinitely thereafter, at the option of the Holder, by providing a notice in the form attached hereto, to the Company, at the office of the Company or any transfer agent for such stock. If any conversion of Series C Preferred Stock would result in the issuance of a fractional number of Common Shares (aggregating all shares of Series C Preferred Stock being converted), then, the number of shares of Common Shares issuable upon conversion of the Series C Preferred Stock shall be the next higher whole number of shares.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
(b) Mechanics of Conversion. Before any Holder of Shares shall be entitled to convert the same into Common Shares, they shall surrender the certificate or certificates therefor, duly endorsed, at the office of this Company or of any transfer agent for the Shares, and shall give written notice to this Company at its principal corporate office, of the election to convert the same and shall state therein the name or names in which the certificate or certificates for Common Shares are to be issued. This Company shall, as soon as practicable thereafter, issue and deliver at such office to such Holder of Shares, or to the nominee or nominees of such Holder, a certificate or certificates for the number of Common Shares to which such Holder shall be entitled as aforesaid. Such conversion shall be deemed to have been made immediately prior to the close of business on the date of such surrender of the Shares to be converted, and the person or persons entitled to receive the Common Shares issuable upon such conversion shall be treated for all purposes as the record Holder or Holders of such Common Shares as of such date.
(c) No Impairment. The Company will not, by amendment of its Certificate of Formation or through any reorganization, recapitalization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms to be observed or performed hereunder by this Company, but will at all times in good faith assist in the carrying out of all the provisions of this Section and in the taking of all such action as may be necessary or appropriate in order to protect the Conversion Rights of the Holders of the Shares against impairment.
(d) Notice. Any notice required by the provisions of this Section to be given to the Holders of Shares shall be deemed given if deposited in the United States mail, postage prepaid, and addressed to each Holder of record at his address appearing on the books of the Company.
(e) Restricted Shares. Unless the Conversion Shares are covered by a valid and effective registration under the Securities Act or the Holder provides the Company a valid opinion from an attorney stating that such Conversion Shares can be issued free of restrictive legend, which shall be determined by the Company (or the Transfer Agent) in its sole discretion, such shares shall be issued as Restricted Shares.
(f) Beneficial Ownership Limitation for Conversion; Exchange Cap.
(i) No Holder Conversion shall result in the conversion of more than that number of shares of Series C Preferred Stock, if any, such that, upon such Conversion, the aggregate beneficial ownership of the Company’s Common Shares (calculated pursuant to Rule 13d-3 of the Exchange Act) of such Holder and all persons affiliated with such Holder as described in Rule 13d-3 is more than 4.999% of the Company’s Common Shares then outstanding (the “Maximum Percentage”). By written notice to the Company, a Holder may increase or decrease the Maximum Percentage to any percentage not in excess of 9.999% as specified in such written notice; provided that (A) any such increase will not be effective until the 61st day after such notice is received by the Company; and (B) any such increase or decrease will apply only to the requesting Holder and not to any other Holder. In the event any Conversion would result in the issuance of shares of Common Shares to any Holder in excess of the Maximum Percentage, only that number of shares of Series C Preferred Stock which when Converted would not result in such Holder exceeding the Maximum Percentage shall be subject to such applicable Conversion, if any, and Holder shall continue to hold any remaining shares of Series C Convertible Preferred Stock, the conversion of which would result in Holder exceeding the Maximum Percentage.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
(ii) The Company’s Transfer Agent shall be authorized to promptly disclose the total outstanding Common Shares to the Holder from time to time at the request of the Holder in order for the Holder to determine its compliance with the Maximum Percentage.
(iii) The Company shall not be required to verify or investigate or confirm whether any Conversion would exceed the Maximum Percentage, and instead the Company shall be able to rely on any notice of holder conversion as prima facie evidence of, and as a representation by, the applicable Holder, that such applicable conversion described in the notice of holder conversion would not result in a violation of the Maximum Percentage.
(iv) The Maximum Percentage shall not apply to any Common Shares issued by the Company in connection with a redemption as described in Section 4 hereof.
(v) Until the Company obtains approval to waive this Section 5(f)(v) by the requisite number of its stockholders at a duly called special or annual meeting of stockholders, the Company shall not deliver Common Shares, Dividend Shares, and/or upon Conversion Shares under Section 5 hereof, to the extent that (A) the aggregate Common Shares issued by the Company to all Holders pursuant to Section 2.4 as Dividend Shares, (B) the aggregate Common Shares issued by the Company to all Holders pursuant to Section 5, as Conversion Shares, plus (C) the aggregate Common Shares issued by the Company hereunder would exceed 19.99% of the Common Shares issued and outstanding on the date of this Designation, subject to adjustment in connection with any Recapitalization. No Holder shall vote any Common Shares in favor of any proposal to waive this Section 5(f)(v) at any meeting of Common Stockholders.
5. Status of Acquired Shares. All Shares issued, redeemed by the Company or converted by the Holder in accordance with Sections 4 or 5 hereof, or otherwise acquired by the Company, shall be cancelled by the Company.
6. Ranking.
(a) Any class or series of shares of stock of the Company that shall be deemed to rank:
(i) prior to the Shares, as to the payment of dividends and as to distribution of assets upon liquidation, dissolution or winding up, if the Holders of such class or series shall be entitled to the receipt of dividends or of amounts distributable upon liquidation, dissolution or winding up, as the case may be, in preference or priority to the Holders of Shares, and such shares shall be defined as “Senior Shares”. For the sake of clarity and in an abundance of caution, the Series B Convertible Preferred Stock of the Company shall be deemed Senior Shares;
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
(ii) on a parity with the Shares, as to the payment of dividends and as to distribution of assets upon liquidation, dissolution or winding up, whether or not the dividend rates, dividend payment dates or redemption or liquidation prices per share thereof be different from those of the Shares, if the Holders of such class or series and the Shares shall be entitled to the receipt of dividends and of amounts distributable upon liquidation, dissolution or winding up in proportion to their respective amounts of accrued and unpaid dividends per share or liquidation preferences, without preference or priority one over the other (“Parity Shares”); and
(iii) junior to the Shares, as to the payment of dividends and as to the distribution of assets upon liquidation, dissolution or winding up, if such class or series shall be the Common Shares or any other class or series of shares of stock of the Company now or hereafter issued and outstanding over which the Shares have preference or priority in the payment of dividends and in the distribution of assets upon any liquidation, dissolution or winding up of the Company (“Junior Shares”).
(b) The Company’s Common Shares shall be considered Junior Shares relative to the Shares.
7. Voting Rights. Except for the Protective Provisions described below, and as expressly required by the NRS, the Series C Preferred Stock shall not have any voting rights. For so long as any Shares are outstanding, the Company shall not, without first obtaining the approval (at a meeting duly called or by written consent, as provided by law) of a Simple Majority (collectively, the “Protective Provisions”):
(a) Amend any provision of this Designation;
(b) Increase or decrease (other than by redemption or conversion) the total number of authorized shares of Series C Preferred Stock of the Company;
(c) Amend the Certificate of Formation of the Company (including by designating additional series of Preferred Stock) in a manner which adversely affects the rights, preferences and privileges of the Series C Convertible Preferred Stock;
(d) Effect an exchange, or create a right of exchange, cancel, or create a right to cancel, of all or any part of the shares of another class of shares into shares of Series C Preferred Stock; or
(e) Alter or change the rights, preferences or privileges of the shares of Series C Preferred Stock so as to affect adversely the shares of such series.
Except as required by applicable provisions of Texas law, the Shares shall not have any relative, participating, optional or other special voting rights and powers other than as set forth herein, and the consent of the Holders thereof shall not be required for the taking of any corporate action. No amendment to these terms of the Shares shall require the vote of the holders of Common Shares (except as required by law).
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
8. Record Holders. The Company and the Transfer Agent shall deem and treat the record Holder of any Shares as the true and lawful owner thereof for all purposes, and neither the Company nor the Transfer Agent shall be affected by any notice to the contrary.
9. Adjustments for Recapitalizations.
(a) Equitable Adjustments for Recapitalizations. (i) The Liquidation Preference, the Stated Value, and the Conversion Price, and any provisions hereof which are expressly tied to the price or value of Shares (each, as and if applicable) (the “Preferred Stock Adjustable Provisions”), and; (ii) any and all other terms, conditions, amounts and provisions of this Designation which (x) pursuant to the terms of this Designation provide for equitable adjustment in the event of a Recapitalization (the “Other Equitable Adjustable Provisions”); or (y) the Board of Directors of the Company determines in their reasonable good faith judgment is required to be equitably adjusted in connection with any Recapitalizations, shall each be subject to equitable adjustment as provided in Sections 10(b) through (c), below, as determined by the Board of Directors in their sole and reasonable discretion.
(b) Adjustments for Subdivisions or Combinations of Common Shares. In the event the outstanding Common Shares shall be subdivided (by stock split, by payment of a stock dividend or otherwise), into a greater number of Common Shares, without a corresponding subdivision of the Series C Preferred Stock and the Other Equitable Adjustable Provisions (if any) in effect immediately prior to such subdivision shall, concurrently with the effectiveness of such subdivision, be proportionately and equitably adjusted. In the event the outstanding Common Shares shall be combined (by reclassification or otherwise) into a lesser number of shares of Common Stock, without a corresponding combination of the Series C Preferred Stock and the Other Equitable Adjustable Provisions (if any) in effect immediately prior to such combination shall, concurrently with the effectiveness of such combination, be proportionately and equitably adjusted.
(c) Adjustments for Subdivisions or Combinations of Series C Preferred Stock. In the event the outstanding shares of Series C Preferred Stock shall be subdivided (by stock split, by payment of a stock dividend or otherwise), into a greater number of shares of Series C Preferred Stock, the applicable Preferred Stock Adjustable Provisions and the Other Equitable Adjustable Provisions (if any) in effect immediately prior to such subdivision shall, concurrently with the effectiveness of such subdivision, be proportionately and equitably adjusted. In the event the outstanding shares of Series C Preferred Stock shall be combined (by reclassification or otherwise) into a lesser number of shares of Series C Preferred Stock, the applicable Preferred Stock Adjustable Provisions and the Other Equitable Adjustable Provisions (if any) in effect immediately prior to such combination shall, concurrently with the effectiveness of such combination, be proportionately and equitably adjusted. Provided however that the result of any concurrent adjustment in the Common Shares (as provided under Section (b)) and Series C Preferred Stock (as provided under Section (c)) shall only be to affect the equitable adjustable provisions hereof once.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
(d) Other Adjustments. The Board of Directors of the Company shall also adjust equitably, and shall have the right to adjust equitably, any or all of the Preferred Stock Adjustable Provisions or Other Equitable Adjustable Provisions from time to time, if the Board of Directors of the Company determines in their reasonable good faith judgment that such values and/or provisions are required to be equitably adjusted in connection with any Company action.
(f) Certificate as to Adjustments. Upon the occurrence of each adjustment or readjustment pursuant to this Section 10, the Company at its expense shall promptly compute such adjustment or readjustment in accordance with the terms hereof and furnish to each Holder a certificate setting forth such adjustment or readjustment and showing in detail the facts upon which such adjustment or readjustment is based. The Company shall, upon the reasonable written request at any time of any Holder, furnish or cause to be furnished to such Holder a like certificate setting forth (i) such adjustments and readjustments, (ii) the Conversion Price at the time in effect, and (iii) the number of Common Shares and the amount, if any, of other property which at the time would be received upon the conversion of the Series C Preferred Stock.
10. Sinking Fund. The Shares shall not be entitled to the benefits of any retirement or sinking fund.
11. Uncertificated Book-Entry Securities. At the option of the Company, the Shares may be issued as book-entry securities directly registered in the stockholder’s name on the Company’s or Transfer Agent’s books and records. If entered as book-entry, the Shares need not be represented by certificates, but instead would be uncertificated securities of the Company.
12. Other Rights. Except as otherwise stated herein, there are no other rights, privileges, or preferences attendant or relating to in any way to the Shares, including by way of illustration but not limitation, those concerning participation, or anti-dilution rights or preferences.
13. Reports. The Company shall mail to all Holders of Series C Preferred Stock those reports, proxy statements and other materials that it mails to all of its Holders of Common Shares.
14. Replacement Preferred Stock Certificates. In the event that any Holder notifies the Company that a Preferred Stock Certificate evidencing shares of Series C Preferred Stock has been lost, stolen, destroyed or mutilated, the Company shall issue a replacement stock certificate evidencing the Series C Preferred Stock identical in tenor and date (or if such certificate is being issued for shares not covered in a redemption or conversion, in the applicable tenor and date) to the original Preferred Stock Certificate evidencing the Series C Preferred Stock, provided that the Holder executes and delivers to the Company and/or its Transfer Agent, as applicable, an affidavit of lost stock certificate and an agreement reasonably satisfactory to the Company and its Transfer Agent to indemnify the Company from any loss incurred by it in connection with such Series C Preferred Stock certificate, and provides the Company and/or its Transfer Agent such other information, documents and if applicable, bonds and indemnities as the Company or its Transfer Agent customarily requires for reissuances of stock certificates (collectively the “Lost Certificate Materials”); provided, however, the Company shall not be obligated to re-issue replacement stock certificates if the Holder contemporaneously requests the Company to convert or redeem the full number of shares evidenced by such lost, stolen, destroyed or mutilated certificate.
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
15. Construction. When used in this Designation, unless a contrary intention appears: (i) a term has the meaning assigned to it; (ii) “or” is not exclusive; (iii) “including” means including without limitation; (iv) words in the singular include the plural and words in the plural include the singular, and words importing the masculine gender include the feminine and neuter genders; (v) any agreement, instrument or statute defined or referred to herein or in any instrument or certificate delivered in connection herewith means such agreement, instrument or statute as from time to time amended, modified or supplemented and includes (in the case of agreements or instruments) references to all attachments thereto and instruments incorporated therein; (vi) the words “hereof”, “herein” and “hereunder” and words of similar import when used in this Designation shall refer to this Designation as a whole and not to any particular provision hereof; (vii) references contained herein to Article, Section, Schedule and Exhibit, as applicable, are references to Articles, Sections, Schedules and Exhibits in this Designation unless otherwise specified; (viii) references to “dollars”, “Dollars” or “$” in this Designation shall mean United States dollars; (ix) reference to a particular statute, regulation or law means such statute, regulation or law as amended or otherwise modified from time to time; (x) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein); (xi) unless otherwise stated in this Designation, in the computation of a period of time from a specified date to a later specified date, the word “from” means “from and including” and the words “to” and “until” each mean “to but excluding”; (xii) references to “days” shall mean calendar days; and (xiii) the paragraph and Section headings contained in this Designation are for convenience only, and shall in no manner affect the interpretation of any of the provisions of this Designation.
——————————————————————————
NOW THEREFORE BE IT RESOLVED, that the Designation is hereby approved, affirmed, confirmed, and ratified; and it is further
RESOLVED, that each officer of the Company be and hereby is authorized, empowered and directed to execute and deliver, in the name of and on behalf of the Company, any and all documents, and to perform any and all acts necessary to reflect the Board of Directors approval and ratification of the resolutions set forth above; and it is further
RESOLVED, that in addition to and without limiting the foregoing, each officer of the Company and the Company’s attorney be and hereby is authorized to take, or cause to be taken, such further action, and to execute and deliver, or cause to be delivered, for and in the name and on behalf of the Company, all such instruments and documents as he may deem appropriate in order to effect the purpose or intent of the foregoing resolutions (as conclusively evidenced by the taking of such action or the execution and delivery of such instruments, as the case may be) and all action heretofore taken by such officer in connection with the subject of the foregoing recitals and resolutions be, and it hereby is approved, ratified and confirmed in all respects as the act and deed of the Company; and it is further
RESOLVED, that this Designation may be executed in several counterparts, each of which is an original; that it shall not be necessary in making proof of this Designation or any counterpart hereof to produce or account for any of the other.
[Remainder of page left intentionally blank. Signature page follows.]
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
IN WITNESS WHEREOF, Mangoceuticals, Inc. has approved and caused this “Certificate of Designation of Mangoceuticals, Inc. Establishing the Designations, Preferences, Limitations and Relative Rights of Its 6% Series C Convertible Preferred Stock” to be duly executed and approved this 18th day of April 2024.
| /s/ Jacob D. Cohen | |
| Jacob D. Cohen | |
| Chief Executive Officer |
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
NOTICE OF CONVERSION
(TO
BE EXECUTED BY THE REGISTERED HOLDER IN ORDER TO CONVERT
PREFERRED SHARES)
The undersigned hereby elects to convert the number of 6% Series C Convertible Preferred Shares indicated below into common shares, $0.0001 par value (the “Common Shares”), of Mangoceuticals Inc. (the “Company”), a corporation organized under the laws of the State of Texas, according to the conditions hereof, as of the date written below.
Conversion
calculations:
Date to Effect Conversion:
| Number of Preferred Shares owned prior to Conversion: | |
| Number of Preferred Shares to be Converted: | |
| Stated Value of the Preferred Shares to be Converted: | |
| Number of Common Shares to be Issued: | |
| Applicable Conversion Price: |
| Number of Preferred Shares subsequent to Conversion: |
_________________
| Address for Delivery: |
or
DWAC Instructions:
Broker no:__________
Account no:__________
| HOLDER | ||
| By: | ||
| Name: | ||
| Title: | ||
| Mangoceuticals, Inc.: Certificate of Designations of 6% Series C Convertible Preferred Stock | Page |
Exhibit 10.1
PATENT PURCHASE AGREEMENT
This PATENT PURCHASE AGREEMENT (“Agreement”) is entered into and made effective as of this 24th day of April 2024 (“Effective Date”) by and between Mangoceuticals, Inc., a Texas corporation with a place of business at 15110 Dallas Parkway, Suite 600, Dallas, TX 75248 (“Purchaser”), and Intramont Technologies, Inc., a New Jersey corporation, with a place of business at 185 Prospect Avenue, Unit 7i, Hackensack, NY 07601 (“Seller”) (each of Seller and Purchaser is defined herein as a “Party”, and collectively referred to as the “Parties”).
WITNESSETH:
WHEREAS, Seller owns certain patents and patent applications set forth in Exhibit A hereto; and
WHEREAS, Purchaser desires to purchase from Seller, and Seller desires to sell and assign to Purchaser, such patents and patent applications set forth in Exhibit A hereto, on the terms and conditions set forth herein.
NOW, THEREFORE, in consideration of the premises and of the mutual covenants set forth herein, the sufficiency and receipt of which the Parties hereby acknowledge, the Parties, intending to be legally bound, hereby agree as follows:
ARTICLE I
DEFINITIONS
1.1 Capitalized Terms. In addition to those terms defined in the body of this Agreement, the following capitalized terms shall have the meanings set forth below:
(a) “Affiliate” means a legal entity that Controls, is Controlled by, or is under common Control with a Party. Such legal entity shall constitute an Affiliate of the Party only when and for so long as the Control exists.
(b) “Assigned Patents” means each of the following, whether or not pending, issued, expired, abandoned or closed: (a) the Patents listed on Exhibit A (“Listed Patents”), attached hereto and incorporated herein, (b) any and all Patents that are part of the same Patent Families as the Listed Patents, (c) any and all of the inventions, invention disclosures, and discoveries existing as of the Effective Date to the extent disclosed or claimed in subitems (a) and (b) above, and (d) any rights of priority created by such Patents under any treaty relating thereto.
(c) “Assignment Agreements” means any executed agreements assigning, changing, confirming or correcting ownership (including without limitation original patent assignment agreements) of any part, portion or all rights in the Assigned Patents from the Inventor(s) and/or any prior owner to any prior owner or Seller.
(d) “Bona Fide Owned and Controlled” means for the purpose of a design that ownership and control was not transferred or provided for purposes of providing a license to cover an offering of a third party under the licenses granted herein.
(e) “Change of Control” means (a) any transaction or series of transactions whereby any person or entity directly or indirectly acquires Control of another person or entity; or (b) the consummation (whether directly or indirectly through one or more intermediaries) of a sale or other disposition of all or substantially all of another person’s or entity’s assets in any single transaction or series of related transactions.
(f) “Control” (including the correlative meanings of the terms “Controls”, “Controlled by” and “under common Control with”) means the direct or indirect ownership of more than fifty percent (50%) of an entity, or the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of a person or an entity, whether through the ability to exercise voting power, by contract or otherwise.
| Page |
(g) “Disclaimer Issue” means a terminal disclaimer (including under 35 U.S.C. Sec. 253 or 37 CFR 1.321 or the equivalent laws or regulation of any other patent authority) that exists or is or should reasonably be required to be made in a patent or patent application to address a double patenting issue, including such an issue raised in a judicial or administrative proceeding (including any proceeding with the U.S. Patent and Trademark Office or any corresponding foreign patent authority).
(h) “Encumbrance” means with respect to any of the Assigned Patents, any mortgage, lien, pledge, charge, Commitment, security interest, express or implied license, Grant, judgment, stipulation, court order or decree, or other restriction regarding transfer or licensing, or any other commitment to a third party which would result in any such Encumbrance whether currently existing or arising in the future.
(i) “Governmental Entity” means any court, administrative agency or commission or other federal, state, county, local or foreign governmental authority, instrumentality, agency commission or subdivision thereof, including but not limited to the U.S. Patent and Trademark Office (“PTO”) and the European Patent Office (“EPO”).
(j) “Grant” means a license, waiver of any rights of enforcement (including but not limited to any covenant not to sue, covenant not to assert, or standstill agreement), release of any claim, or other grant of any right.
(k) “Grant Back License” has the meaning set forth in Section 2.3.
(l) “Importation Information” means an electronic file provided by Seller, in the form provided by Purchaser, containing certain information requested by Purchaser regarding the Assigned Patents.
(m) “including” means including without limitation.
(n) “Inventor” means each of the named inventors of each of the Assigned Patents as well as any inventor who should be or should have been named on each of the Assigned Patents.
(o) “or” means “and/or”.
(p) “Patent Documents” means (i) all prosecution files (physical and electronic) and docket reports (capturing a time period no shorter than ninety (90) days following the Effective Date) for all of the Assigned Patents in the possession or control of Seller, its counsel or its agents; (ii) all Assignment Agreements; (iii) all documents, records and files in the possession or control of Seller, its counsel or its agents (and including any and all of each Inventor) with respect to (A) the conception and reduction to practice (and diligence in reduction to practice) of the inventions of any of the Assigned Patents, (B) the disclosure of, acquisition, prosecution, registration, reissuance, correction, enforcement, defense, and maintenance of the Assigned Patents (including without limitation ribbon copies of any letters patent), (C) Seller’s marking activities and program(s) with respect to the Assigned Patents, and (D) Seller’s licensing or sales activities with respect to the Assigned Patents; and (iv) all other material documentation or information in the possession or control of Seller, its counsel or its agents related to the Assigned Patents. Notwithstanding the foregoing, Patent Documents shall exclude any documents or information in clauses (i) - (iv) of this Section which are subject to the doctrines of attorney-client privilege, attorney work-product, joint defense, common interest and/or any other applicable privilege or immunity (collectively, “Common Interest Privilege”) if providing such documents or information to Purchaser cannot be accomplished in a manner that protects such Common Interest Privilege.
| Page |
(q) “Patent Family” means a set comprised of all Patents (a) that are linked or entitled to be linked through one or more claims of benefit or priority pursuant to 35 U.S.C. §§ 120 or 119 (or the equivalent laws or regulation of any other patent authority) or by a terminal disclaimer pursuant to 35 U.S.C. § 253 or 37 CFR § 1.321 (or the equivalent laws or regulation of any other patent authority) or (b) that are, or are entitled to be, foreign counterparts, reissues, divisionals, extensions, continuations or continuations-in-part with respect to any other Patent in such set.
(r) “Patents” means any United States, foreign or international patents and patent applications, patents and patent applications resulting or issuing therefrom, certificates of invention, utility models or any other grants by any Governmental Entity for the protection of inventions, including all non-provisionals, provisional, reissues, divisionals, continuations, continuations-in-part, re-examinations and extensions of any of the foregoing; provided, however, that when the term “Patent” is used in the context of, or to refer to, a particular patent or patent application, or a patent or patent application on a schedule, the term shall mean only that particular patent or patent application, as the case may be.
(s) “Seller Product” means any product associated with the Assigned Patents, which as of the Effective Date, meets all of the following: (i) the design of the product is Bona Fide Owned and Controlled by Seller, is sold by the Seller, or in development with the intention by Seller to commercialize, and (ii) in the absence of a license or other authorization, the product would infringe one or more of the Assigned Patents.
(t) “Subsidiary” means a legal entity that is Controlled by a Party. Such legal entity shall constitute a Subsidiary of the Party only when and for so long as the Control exists.
(u) “Transfer Documents” means the fully executed patent transfer documents, in a form approved by Purchaser suitable for filing with the relevant Governmental Authority, in each jurisdiction where the Assigned Patents issued from or have been filed, as the case may be, in each case to record the change of ownership of the Assigned Patents from Seller to Purchaser. Unless otherwise directed by Purchaser, Transfer Documents for U.S. Assigned Patents shall be as provided in the form of Exhibit B (“Patent Assignment”).
| Page |
ARTICLE II
TRANSFER OF ASSIGNED PATENTS AND COOPERATION
2.1 Initial Transfer. Effective as of the Effective Date, Seller hereby irrevocably sells, transfers, conveys and assigns, and shall cause its Affiliates to irrevocably sell, transfer, convey and assign, to Purchaser (or its designee, as to any or all of the Assigned Patents), and Purchaser hereby acquires from Seller or its Affiliates, all right, title and interest in and to (i) all Assigned Patents, including without limitation the right to sue, license and collect and receive all income, royalties, damages, payments due, injunctive relief and any other settlements or remedies (including, without limitation, causes of action and rights to damages and payments for past, present or future infringements or misappropriations) with respect thereto, in each case, in all countries relating to the Assigned Patents and (ii) the Patent Documents and rights (including copyrights) with respect thereto. The Parties understand and agree that no license agreements or other contracts, obligations or other liabilities of Seller, Seller’s Affiliates or prior owners, whether listed in Exhibit C of this Agreement or not, are assigned, delegated or otherwise transferred to or assumed by Purchaser hereunder, whether expressly, by implication, by reason of estoppel or otherwise.
(a) Additional Transfers.
1. To the extent all right, title and interest in and to any Assigned Patent is not transferred pursuant to Section 2.1 (Initial Transfer) for whatever reason, Seller hereby irrevocably sells, transfers, conveys and assigns to Purchaser, and shall cause its Affiliates to irrevocably sell, transfer, convey and assign, and Purchaser hereby acquires from Seller or its Affiliates, all right, title and interest in and to such Assigned Patent in accordance with the terms of this Agreement, including the right to sue, license and collect and receive all income, royalties, damages, payments due, injunctive relief and any other settlements or remedies (including, without limitation, causes of action and rights to damages and payments for past, present or future infringements or misappropriations) with respect thereto, effective as of the Effective Date.
2. If, after the Effective Date, any Assigned Patent is subject to a Disclaimer Issue linking it to a Patent of Seller, Seller shall, without additional consideration, nunc pro tunc transfer and does hereby convey as of the Effective Date (to the extent not previously effectively assigned by Seller to Purchaser pursuant to Section 2.1), ownership of such Seller Patent to Purchaser and such Patent shall be considered an Assigned Patent hereunder.
(b) Undisclosed Patents. If at any time it is determined that a Patent (including patent applications, regardless of status) exists or existed that is part of the same Patent Family corresponding to one or more of the Assigned Patents and such Patent was not identified as an Assigned Patent, then, in addition to any other remedies Purchaser may have under this Agreement, Seller shall upon notice from Purchaser promptly assign such Patent (and any Patents that may have issued therefrom) to Purchaser.
2.2 Delivery. On the Effective Date, Seller shall have delivered to Purchaser the following:
(a) All Transfer Documents, fully executed and notarized where appropriate;
(b) Electronic copies of the prosecution files, docket reports and Assignment Agreements referred to in clauses (i) and (ii) of the definition of Patent Documents; and
(c) The Importation Information.
| Page |
2.3 Grant Back License.
(a) Subject to the terms of this Section 2.3, and effective as of the Effective Date, Purchaser hereby grants back to Seller and each of its Subsidiaries (but only as long as such Subsidiary is and remains a Subsidiary of Seller), an irrevocable, Co-Exclusive (defined in Section 2.3(b)((3) below), non-transferable and non-assignable (except in the event of a Change of Control as set forth below), non-sublicensable, worldwide, license under the Assigned Patents and for the lives thereof, to make, have made (to the extent substantially designed by Seller or its Subsidiaries), import, use, offer to sell, sell and otherwise dispose of Seller Products to any third party. The license and rights set forth in this Section 2.3 shall apply only to the Assigned Patents assigned by Seller to Purchaser under this Agreement and shall not apply to any other Patents of Purchaser or any of its Affiliates, whether by implication, estoppel or otherwise (the “Grant Back License”). The Grant Back License, as to any Affiliate of Seller, will terminate as to such Affiliate if and when such Affiliate ceases to meet the requirements of being an Affiliate of Seller.
(b) Further Limitations on Grant Back License.
1. The Grant Back License from Purchaser to Seller and its Subsidiaries is expressly set forth in Section 2.3 above and no other licenses, authorizations or rights are granted or conveyed, whether expressly or by implication or otherwise, all of which are expressly disclaimed.
2. Notwithstanding anything to the contrary, the Grant Back License of Section 2.3: (i) excludes the right to grant sublicenses; (ii) is non-transferable and non-assignable (except in the event of a Change of Control as set forth below)(by operation of law or otherwise); (iii) excludes the right to further place any Encumbrance on the Assigned Patents; (iv) excludes any covenant, license (except for the grants expressly set forth in Section 2.3(a) above), authorization, or other right, express or implied or by estoppel or otherwise, to make, have made, import, use, offer to sell, sell and otherwise dispose of any products other than Seller Products (even if such products are used in combination with Seller Products as described and limited by Section 2.3); (v) does not include the right under any Assigned Patent to manufacture or have manufactured products or Seller Products as a foundry or contract manufacturer for a third party, or to otherwise manufacture, sell, or otherwise distribute products or Seller Products for or on behalf of any third parties, or to otherwise sell, lease or transfer any product without material modification back to the same supplier or customer or an affiliate thereof; and (vi) shall not be deemed or construed to grant, make or constitute any license, covenant, immunity, authorization or right, whether by implication, estoppel, acquiescence, reliance or otherwise, with respect to any activities that Seller undertakes for or on behalf of any third party where the purpose of such third party choosing Seller is obtaining rights under one or more Assigned Patents (i.e., patent laundering). In the event of a Change of Control, Seller’s successor-in-interest shall continue to hold the license granted under Section 2.3, but only as it relates to Seller Products in existence as of the time of such Change of Control, as may carry the trademark, service mark or brand name of such successor-in-interest, and to successor versions of such Seller Products designed by or for such successor-in-interest.
3. Co-Exclusive License. Purchaser and Seller acknowledge and agree that the licenses granted herein permit both Purchaser and Seller and their respective Subsidiaries to sell or distribute through dealers or sell direct to end-users, without requirement for any additional license to the Assigned Patents, any product which is made by, made for (by a contract manufacturer), used by, designed by, or sold by such Party or its Subsidiaries (“Co-Exclusive License”), subject to the Royalty Payments of Section 2.3(b)(4) below to be paid by Seller to Purchaser.
| Page |
4. Royalty Payments. Seller agrees to pay Purchaser a royalty of ten percent (10%) of gross worldwide sales of Seller Products, which shall go into effect on the first anniversary of the Effective Date and extend throughout the life of the last to expire of the Assigned Patents (the “Royalty Payments”). The Royalty Payments shall be paid to the Purchaser on an annual basis, within 30 days after the end of the calendar year. Seller further agrees to maintain complete and accurate books and records at its principal office, which may be inspected by Purchaser during normal operating hours (unless otherwise agreed by the Parties) upon reasonable notice during the term of the Grant Back License and two (2) years thereafter. For the avoidance of doubt, the Royalty Payments shall not be applicable to, and the Seller shall not owe any Royalty Payments, on any sales of products by Seller, other than the making, using, selling and offering for sale of Seller Products by Seller and/or any permitted sub-licensees, if any.
5. First Right of Refusal. At any time after a period of three (3) years from the Effective Date, the Seller shall have a first right of refusal with respect to the acquisition of the Assigned Patents. In the event that the Purchaser receives an offer to purchase the Assigned Patents and the Purchaser in its sole discretion determines to accept such offer, Purchaser will notify Seller in writing of the existence and terms of such offer in writing within five (5) days of Purchaser’s determination to accept such offer. Seller shall then have thirty (30) days to exercise its right of first refusal to purchase the Assigned Patents either at the same price and on the same terms as contained in such offer or at a price mutually agreed upon by both Purchaser and Seller (the “Offer Terms”). In the event that Seller elects not to exercise its rights under this Section 2.3(b)(5) to purchase the Assigned Patents or fails to timely respond to the Offer Terms, Purchaser may sell the Patents to the offering party on the Offer Terms, subject to the License set forth herein. In the event the offering party does not purchase the Assigned Patents on the Offer Terms then Seller’s rights of first refusal pursuant to this Section 2.3(b)(5) shall continue to apply to the Assigned Patents. The rights of Seller under this Section 2.3(b)(5) shall apply with respect to each proposed sale of the Assigned Patents to any third party by Purchaser.
6. Assumption of Rights. Purchaser agrees and covenants that it will not sell, transfer or assign to a third party (“Buyer”) any of its intellectual property or property rights in the Assigned Patents (i) without honoring the First Right of Refusal of Section 2.4(b)(5) above, or (ii) without obligating such Buyer to honor all rights of Purchaser under this Agreement with respect to such intellectual property rights and securing such Buyer’s assumption of all obligations of Purchaser to Seller under this Agreement with respect to such intellectual property rights.
| Page |
2.4 Further Assurances.
(a) Further Cooperation.
i. Seller shall, and Seller shall direct its Affiliates and its and their employees (including all employed Inventors) to, fully cooperate with and assist Purchaser and any successor, its counsel and similar agents in securing the Purchaser’s rights in the Assigned Patents in any and all countries and in the enforcement, prosecution and maintenance of the Assigned Patents without additional consideration, including facilitating the cooperation of the Inventors (whether employed by Seller or not). Without limiting the generality of the foregoing, following the Effective Date, Seller agrees that its cooperation and assistance hereunder will include (if requested by Purchaser), without limitation, (a) the full disclosure to Purchaser of all pertinent factual or other information and data reasonably available to Seller, including providing contact information for Inventors, (b) the execution of all applications, specifications, papers, documents, oaths, assignments, declarations, affidavits and all other instruments which Purchaser shall request as may be necessary and proper to obtain and vest such rights and in order to assign and convey to Purchaser, its successors, assigns, and nominees the sole and exclusive right, title and interest in and to the Assigned Patents and otherwise completely effect consummation of the transactions contemplated by this Agreement, (c) making factual witnesses available upon the reasonable request of Purchaser and participation in any litigation defenses including the giving of testimony in any suit, legal action, hearing, investigation, or other proceeding relating to the Assigned Patents, (d) if requested by Purchaser, joinder as a necessary party plaintiff or in another capacity reasonably requested by Purchaser, and (e) reasonably cooperating with and assisting Purchaser, in any legal or equitable action, litigation, arbitration or other legal, regulatory or administrative proceeding regarding any of the Assigned Patents or the scope, infringement or validity thereof (“Patent Proceeding”), including, without limitation, enforcement of any of the Assigned Patents against potential infringers in any court proceeding or before the International Trade Commission (ITC) and proceedings regarding any of the Assigned Patents before the PTO, EPO or any other similar agency; provided, however, that Purchaser shall have sole control and discretion over any and all such Patent Proceedings and any settlement thereof, and the exclusive right to receive, retain and enforce all damages, awards, and other remedies of any kind in connection with any such Patent Proceeding, or (f) the performance of any other acts as may be necessary and proper to vest full title and transfer all rights and interest in and to the Assigned Patents in Purchaser (or its designee) and otherwise completely effect consummation of the transactions contemplated by this Agreement. With respect to items (c), (d) and (e) of this paragraph, Purchaser will reimburse Seller for all preapproved, reasonable, out-of-pocket costs incurred in connection with providing the assistance and cooperation requested by Purchaser hereunder, and Purchaser shall reimburse Seller, its employees, attorneys, agents and inventors at a reasonable hourly rate mutually agreed upon by the Parties for time spent in connection with providing assistance or cooperation requested by Purchaser hereunder, with respect to all other subsections of this Agreement, Seller shall pay all out-of-pocket costs and expenses and Purchaser shall not be required to reimburse Sellers for such amounts.
ii. Within thirty (30) days following the Effective Date, Seller shall provide to Purchaser or its designee all Patent Documents. For the avoidance of doubt, the obligations set forth in this Section are in addition to, and not intended to replace or contradict, the obligations set forth in Section 2.2(b).
iii. Seller agrees to provide Purchaser on or before the Effective Date any and all information required to create an implementation plan that defines the various activities and/or due dates for responses and/or payments in any of the Assigned Patents that are due and/or payable within ninety (90) days of the Effective Date, including (but not limited to) a global docket report for all Assigned Patents, and a schedule of international annuity payments and/or US maintenance fees due for the Assigned Patents (“Implementation Plan”). Within five (5) business days following the Effective Date, Seller shall, at Seller’s expense, notify all foreign and domestic counsel identified in the Implementation Plan that the Assigned Patents have been transferred to Purchaser (or Purchaser’s designee) as of the Effective Date and direct such counsel to (i) digitize and electronically transfer all Patent Documents to Purchaser (or Purchaser’s agent) within thirty (30) days of such notification, (ii) ship all physical Patent Documents to a location specified by Purchaser within thirty (30) days of such notification, (iii) provide Purchaser with a manifest and tracking number for all materials shipped to Purchaser, (iv) send Purchaser a copy of all notices regarding the Assigned Patents which it receives, and (v) satisfy any reasonable information requests by Purchaser (at Seller’s expense) and otherwise take direction from Purchaser with respect to the Assigned Patents. For the avoidance of doubt, the obligations set forth in this Section are in addition to, and not intended to replace or contradict, the obligations set forth in Section 2.2(b).
| Page |
iv. Seller shall, on or before the Effective Date, pay all maintenance, annuity, renewal and issuance fees and the like with respect to the Assigned Patents that are due and payable prior to the end of the ninety (90) day period following the Effective Date.
(b) Limited Power of Attorney. Seller hereby irrevocably constitutes and appoints Purchaser, with full power of substitution, to be its true and lawful attorney, and in its name, place or stead, to execute, acknowledge, swear to and file, all applications, specifications, papers, documents, oaths, assignments, declarations, affidavits and all other instruments, and to take any action which shall be necessary, appropriate or desirable to effectuate the transfer, or prosecution of the Assigned Patents in accordance with the terms of this Agreement; provided, however, that such power shall be exercised by the Purchaser only in the event that Seller fails to take the necessary actions required hereunder to effect or record such transfer, or prosecution of such Assigned Patents within thirty (30) days of Purchaser’s reasonable request, or ten (10) days prior to the deadline for taking the required action if earlier. This power of attorney shall be deemed to be coupled with an interest and shall be irrevocable until the Assigned Patents are purchased by or otherwise returned to Seller or a third-party.
(c) Additional Releases. To the extent any Assigned Patents have any liens or security interests upon them after the Effective Date in violation of Section 4.12, without limiting Seller’s other obligations hereunder, Seller shall promptly seek, obtain and record from its lenders or other third party the release of any liens or security interest that they may have on any of the Assigned Patents, including any liens on any Patent that is determined to be an Assigned Patent but that was not an Assigned Patent as of the Effective Date and Seller hereby waives any remedies with respect to the release of any such liens or security interests.
(d) Common Interest Privileged Information. Seller shall: (a) use best efforts to maintain common interest privilege with respect to all materials or information protected under a common interest privilege (“Privileged Information”) existing as of the Effective Date that relates to the Assigned Patents; and (b) provide Purchaser with at least thirty (30) days prior written notice before disclosing to any third party, or waiving any such common interest privilege with respect to, any such Privileged Information. In the event that Seller subsequently waives a common interest privilege with respect to Privileged Information related to the Assigned Patents, Seller shall also provide such Privileged Information to Purchaser. Upon Purchaser’s request following the Effective Date, Seller and Purchaser shall negotiate and enter into a common interest agreement under which Seller may have access to, while preserving the common interest privilege thereof, Privileged Information that Purchaser believes necessary for Purchaser’s licensing or enforcement of the Assigned Patents, at no additional cost to Purchaser other than Seller’s reasonable out-of-pocket expenses incurred in the course of fulfilling its obligations under such agreement.
(e) Conduct. Seller shall not engage in any act or conduct, or omit to perform any necessary act, the result of which would invalidate any portion of any of the Assigned Patents or render any portion of them unenforceable.
(f) Patent Maintenance, Enforcement, and Licensing. After the Effective Date, Purchaser shall have the sole right, but not the obligation, to prosecute, maintain, enforce, license, and take any other actions with respect to the Assigned Patents in its sole discretion.
| Page |
ARTICLE III PAYMENT AND TAXES
3.1 Payment. Upon the terms and subject to the conditions of this Agreement (including, without limitation, Seller’s compliance with Section 2.2), in full payment for the sale, conveyance, assignment, transfer and delivery of the Assigned Patents and all rights thereto, Purchaser agrees to remit to Seller a sum equal to Twenty Million US Dollars ($20,000,000) (the “Purchase Price”) which shall be paid to the Seller as follows:
a) The issuance of 980,000 shares of the Company’s 6% Series C Convertible Cumulative Preferred Shares, face value of $20.00 per share, for a total value of $19,600,000 (the “Series C Preferred Shares”). The Series C Preferred Shares shall carry a cumulative, annual coupon rate of six percent (6%) per annum and each share of the Series C Preferred Shares shall be convertible into two (2) shares of the Purchaser’s common stock. The Series C Preferred Shares shall be issued to the Seller within one (1) business day from the Effective Date; and
b) Three (3) cash payments by Purchaser to Seller for a total of Four Hundred Thousand US Dollars ($400,000) with (i) the first tranche payment on or before June 30, 2024, in the amount of Two Hundred Thousand US Dollars ($200,000), (ii) the second tranche payment on or before August 31, 2024 in the amount of One Hundred Thousand US Dollars ($100,000), and (iii) the third tranche payment on or before November 30, 2024 in the amount of One Hundred Thousand US Dollars ($100,000) (collectively, (i) through (iii), the “Cash Payments”).
In the event any of the above Cash Payments have not been made on or before the dates listed above, the Purchaser shall have thirty (30) days to cure such instance. In the event the Cash Payments have not been made by the thirty day cure period, Purchaser has the option of paying $15,000 for each additional thirty day extension period.
3.2 Transfer Taxes. Seller shall be solely responsible for the payment of, and shall pay when due, any federal, state, local, foreign or other tax, duty, levy, impost, fee, assessment or other governmental charge, including without limitation income, gross receipts, business, occupation, sales, stamp, value-added, excise (or similar transfer taxes), use, or other tax of any kind whatsoever and any premium, together with any interest, penalties, surcharges, fines and additions attributable to or imposed with respect to the foregoing (collectively “Taxes”) that may be payable in connection with the sale or purchase of the Assigned Patents and Seller shall indemnify Purchaser against any such Taxes as provided in Section 6.2 (Indemnification).
| Page |
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF SELLER
Seller hereby represents and warrants to Purchaser, as of the Effective Date, the following:
4.1 Corporate Organization. Seller is a corporation duly organized, validly existing and in good standing under the respective laws of its jurisdiction of incorporation, is duly qualified and is in good standing under the laws of each jurisdiction in which the character of the properties and assets now owned or held by it or the nature of the business now conducted by it requires it to be so licensed or qualified. Seller has full corporate power and authority to carry on its business as now being conducted.
4.2 Authority. Seller has full corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder. The execution and delivery of this Agreement by Seller and the performance by Seller of its obligations hereunder have been duly authorized by all necessary corporate action. This Agreement has been duly executed and delivered by Seller and constitutes the legal, valid and binding obligation of Seller, enforceable against it in accordance with its terms and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
4.3 No Conflict; No Consents. The execution and delivery of this Agreement and the performance of the obligations of Seller hereunder will not (i) violate or be in conflict with any provision of law, any order, rule or regulation of any court or other agency of government, or any provision of Seller’s articles of incorporation or bylaws, (ii) violate, be in conflict with, result in a breach of, constitute (with or without notice or lapse of time or both) a default under, or result in the acceleration of any obligations under, any indenture, agreement, lease or other instrument to which Seller is a party or by which it or any of its properties are bound, or (iii) result in the creation or imposition of any Encumbrance upon any of the Assigned Patents. No consent, approval or authorization of or declaration or filing with any Governmental Entity or other person or entity on the part of Seller is required in connection with the execution or delivery of this Agreement or the consummation of the transactions contemplated hereby.
4.4 List of Assigned Patents. Exhibit A sets forth a true, accurate and complete list of Assigned Patents and for each such Patent, the title, the Inventors’ names, the Patent number or serial number (as applicable), the filing date or issue date, the country in which the relevant Patent has been issued or applied for.
4.5 Prosecution. Each Assigned Patent has been prosecuted in compliance with the rules and processes of the United States Patent and Trademark Office (or the equivalent rules or processes of any other applicable patent authority anywhere in the world) and all applications for the Assigned Patents are true and correct in all material respects, including with respect to inventorship. To the extent “small entity” fees were paid to the United States Patent and Trademark Office for any Patent, such reduced fees were then appropriate because the payor qualified to pay “small entity” fees at the time of such payment in accordance with applicable law.
4.6 Assignment Agreements. For each Assigned Patent, Seller has obtained one or more Assignment Agreements which collectively assign all rights in such Patents to Seller. Seller has properly recorded all such previously executed Assignment Agreements with respect to the Assigned Patents as necessary to fully perfect its rights and title therein in accordance with governing laws and regulations in each respective jurisdiction.
4.7 Public Use, Disclosure or Sale. For each Assigned Patent, no acts or omissions of Seller, or any party acting on behalf of or at the direction of Seller, have or shall invalidate or hinder enforcement of such Patent under the laws of any jurisdiction (including under 35 U.S.C. §102(b)) including as a result of (i) disclosure of the invention or a printed publication that describes the claimed invention, (ii) public use of the claimed invention, or (iii) sale or offer for sale of the claimed invention prior to the application for such Patent.
| Page |
4.8 Knowledge of Invalidity. None of the Assigned Patents has ever been found invalid or unenforceable for any reason in any administrative, arbitration, judicial or other proceeding, and Seller does not know of and has not received any notice or information of any kind from any source suggesting that the Assigned Patents may be invalid or unenforceable. To Seller’s Knowledge (as defined below), none of the Assigned Patents is invalid or unenforceable, nor is Seller aware of any facts or circumstances that would render any Assigned Patent invalid or unenforceable.
4.9 Ownership and Encumbrances.
(a) Seller is the sole legal and beneficial owner of all right, title and interest, and has valid title, to all the Assigned Patents (including all rights to sue and collect damages for past, present and future infringement), free and clear of any Encumbrances, except as set forth in Exhibit C (“Encumbrances”), attached hereto and incorporated herein. Except as set forth in Exhibit C, upon transfer of the Assigned Patents from Seller to Purchaser hereunder, none of the Assigned Patents will be subject to any restrictions with respect to the transfer or licensing of such Patents or is subject, or will be subject, to any Encumbrance as a result of any facts, circumstances or agreements existing before the Effective Date. To the extent any exceptions to the foregoing are listed on Exhibit C, such exhibit includes a complete and accurate list and description of all Encumbrances, including, but not limited to, any relevant dates and parties.
(b) The Assigned Patents are not subject to any exclusive Grant to a third party.
(c) Except as set forth on Exhibit C, Purchaser will not be subject to any covenant not to sue, license or other similar restriction on its enforcement or enjoyment of the Assigned Patents as a result of any prior transaction related to the Assigned Patents.
(d) Seller has provided Purchaser with complete copies of all documentation reflecting the Encumbrances identified in Exhibit C and all such copies are complete in all material respects and no information has been deleted, omitted or redacted from such copies.
(e) Except for Purchaser, there are no existing contracts, agreements, options, commitments, proposals, bids, offers, or rights with, to, or in any person to acquire any of the Assigned Patents.
(f) As of the Effective Date, none of Seller, except as expressly set forth in Section 2.3 above, any prior owner of the Assigned Patents, or any Inventor will have any right or interest in and to any of the Assigned Patents.
(g) Any Grant currently in effect with respect to the Assigned Patents does not provide sublicensing rights.
4.10 Standards Bodies.
(a) Seller has (i) listed on Exhibit D (“Standards”), attached hereto and incorporated herein, (A) any standards body, patent pool, or similar formal or informal organization (“Standards Body”) that Seller or its Affiliates have participated with, been affiliated with, or have been a member of; (B) any commitments to, offers made to, or agreements with (“Commitments”) such Standards Bodies applicable to any Assigned Patent, and the terms of such Commitments; and (C) the Assigned Patents applicable to such Commitments; and (ii) provided Purchaser with complete copies of all documentation with respect to Seller’s Commitments, and all such copies and documentation are complete in all material respects, and no information has been deleted, omitted or redacted from such copies and documentation.
| Page |
(b) Seller is in compliance with the requirements of all Standards Bodies, and has not made any (i) Commitments to any Standards Bodies, to any entities, or to the public, to license or grant any rights with respect to any of the Assigned Patents (including any Commitment to license any of the Assigned Patents on a royalty-free basis or at a specified rate, or on any specific terms), other than a general commitment to license on reasonable and nondiscriminatory (RAND) or fair, reasonable and non-discriminatory (FRAND) terms; or (ii) misrepresentations to any Standards Bodies.
4.11 Documents and Importation Information. The Patent Documents delivered by Seller to Purchaser hereunder represent all material records and files of Seller, its counsel and agents of which Seller is or should be aware of, that are related to the Assigned Patents, with respect to the acquisition, prosecution, registration, reissuance, enforcement, defense, and maintenance of the Assigned Patents. The Importation Information delivered by Seller to Purchaser hereunder is true, accurate and complete.
4.12 No Impairment. Neither the execution, delivery or performance of this Agreement nor the consummation of the transactions contemplated herein will impair the right of Purchaser to use, possess, sell, license or dispose of any of the Assigned Patents. There are no royalties, honoraria, fees or other payments payable by Seller to any third party by reason of the ownership, use, possession, license, sale, or disposition of any Assigned Patents. There are no actions, suits, investigations, claims or proceedings threatened, pending or in progress relating in any way to the Assigned Patents.
4.13 No Notice. Seller has not put any third party on notice of actual or potential infringement of any Assigned Patent. Seller has not invited any third party to enter into a license under any of the Assigned Patents. Seller has not initiated any enforcement action with respect to any of the Assigned Patents.
4.14 Disclosure. Seller has disclosed to Purchaser in writing all facts and circumstances known to, or reasonably ascertainable by, Seller on or prior to the Effective Date as possibly having an adverse effect on the validity or enforceability of the Assigned Patents.
4.15 Marking. Seller and its Affiliates affix on its products or product packaging, manuals, or instructions associated with such products, a label indicating the Assigned Patents applicable to the respective products.
4.16 Fees and other Actions. Seller has paid all maintenance, annuity, renewal and issuance fees and the like with respect to the Assigned Patents that are due and payable prior to the end of the ninety (90) day period following the Effective Date. Except as set forth in Exhibit E (“Fees and Other Actions List”), attached hereto, there are no actions that must be taken or fees that must be paid within ninety (90) days following the Effective Date, including the payment of any filing, registration, maintenance, annuity, renewal or issuance fees or the filing of any responses with any Government Entity, including office action responses, documents, applications or certificates for the purposes of prosecuting, maintaining, perfecting, preserving or renewing any of the Assigned Patents.
4.17 Outstanding Judgment. No Assigned Patents are subject to any proceeding or outstanding decree, order, judgment, settlement agreement or stipulation. None of the Assigned Patents has been or are currently involved in any reexamination, reissue, opposition, interference proceeding, or any similar proceeding, and no such proceedings are pending or threatened.
| Page |
4.18 Lawsuits and Other Proceedings. No Assigned Patent has been involved in any past or pending action, suit, investigation, claim or proceeding (including any reexamination), nor has any Assigned Patent been threatened with any such action, suit, investigation, claim or proceeding, other than patent prosecution proceedings in the ordinary course.
4.19 Co-Development. None of the Assigned Patents were developed by, on behalf of, jointly with, or with the funding of, a third party.
4.20 Government Funding. None of the Assigned Patents were developed by, on behalf of, jointly with, or using grants or funding of any Governmental Entity, college, university, or educational institution.
4.21 No Patent License. Except for the rights expressly granted under Section 2.3, Seller is not acquiring any licenses or rights, whether by implication, estoppel or otherwise under or to any Patents of Purchaser or any of its Affiliates because of the Parties entering into this Agreement. The Parties entering into this Agreement will not result in any previous owner of the Assigned Patents or any third party obtaining any license, right or Grant whether by implication, estoppel or otherwise, under or to any Patent of Purchaser or any of its Affiliates.
4.22 The term “Knowledge” is defined to mean (x) an individual will be deemed to have “Knowledge” of a particular fact or other matter if that individual is actually aware of that fact or matter or a prudent individual could be expected to discover or otherwise become aware of that fact or matter in the course of conducting a reasonably comprehensive investigation to ascertain and establish the accuracy of each representation, warranty and statement in this Agreement, and (y) “Seller’s Knowledge” or “Knowledge of Seller” means the Knowledge of Seller’s and Seller’s Affiliates’ officers, directors, shareholders, partners, members, or employees, in-house or outside counsel, and/or any current employee that is or was directly involved in (i) any prosecution activities associated with one or more of the Assigned Patents, or (ii) the acquisition and divestiture of the Assigned Patents, or (iii) the maintenance, analysis, administration, evaluation of commercial value and strategic assessment of the Assigned Patents during Seller’s ownership of or control over the Assigned Patents.
4.23 No Defense. It shall not be a defense to a suit for damages by Purchaser against Seller, that any misrepresentation or breach of covenant or warranty that Seller is known by Purchaser, or that Purchaser had reason to know contained untrue statements.
4.24 Securities Representations.
(a) Purchase for Own Account. The Series C Preferred Shares and shares of common stock of the Purchaser issuable upon conversion thereof (the “Conversion Shares”) to be issued to Seller hereunder will be acquired for investment for Seller’s own account, not as a nominee or agent, and not with a view to the public resale or distribution thereof within the meaning of the Securities Act of 1933, as amended (the “Securities Act”), and Seller has no present intention of selling, granting any participation in, or otherwise distributing the same.
(b) Disclosure of Information.
(i) Seller has received or has had full access to all the information Seller considers necessary or appropriate to make an informed investment decision with respect to the Series C Preferred Shares to be issued to Seller hereunder. Seller has had an opportunity to ask questions and receive answers from the Purchaser regarding the Purchaser and the Series C Preferred Shares, and all such questions, if any, have been satisfactorily answered as of the date of this Agreement.
| Page |
(ii) Without limiting or reducing in any way Section 4.24(a)(i), above, the Seller acknowledges that it (A) is aware of, has received and had an opportunity to review (x) the Purchaser’s Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (“SEC”) on April 1, 2024 (the “Annual Report”); and (y) Purchaser’s current reports on Form 8-K from January 1, 2024, to the date of this Agreement (which filings can be accessed by going to https://www.sec.gov/edgar/searchedgar/companysearch.html, typing “Mangoceuticals” in the “Name, ticker symbol, or CIK” field, and clicking the “Search” button), in each case (x) through (z), including, but not limited to, the audited and unaudited financial statements, description of business, risk factors, results of operations, certain transactions and related business disclosures described therein (collectively the “Disclosure Documents”) and an independent investigation made by it of Purchaser; and (B) is not relying on any oral representation of Purchaser or any other person, nor any written representation or assurance from Purchaser; in connection with Seller’s acceptance of the Series C Preferred Shares and investment decision in connection therewith.
(c) Illiquid Securities. Seller realizes that the Series C Preferred Shares and Conversion Shares cannot readily be sold as they will be restricted securities and therefore the Series C Preferred Shares and Conversion Shares must not be accepted unless such Seller has liquid assets sufficient to assure that holding such Series C Preferred Shares and Conversion Shares indefinitely will cause no undue financial difficulties and such Seller can provide for current needs and possible personal contingencies.
(d) Discussions with Advisors. Seller has carefully considered and has, to the extent it believes such discussion necessary, discussed with its professional, legal, tax and financial advisors, the suitability of an investment in the Series C Preferred Shares for its particular tax and financial situation and its advisers, if such advisors were deemed necessary, have determined that the Series C Preferred Shares are a suitable investment for it.
(e) No General Solicitation. Seller has not become aware of and has not been offered the Series C Preferred Shares by any form of general solicitation or advertising, including, but not limited to, advertisements, articles, notices or other communications published in any newspaper, magazine, or other similar media or television or radio broadcast or any seminar or meeting where, to such Seller’s knowledge, those individuals that have attended have been invited by any such or similar means of general solicitation or advertising.
(f) No Registration Rights. Seller confirms and acknowledges that Purchaser is not under any obligation to register or seek an exemption under any federal and/or state securities acts for any sale or transfer of the Series C Preferred Shares or Conversion Shares, and such Seller is solely responsible for determining the status, in its hands, of the Series C Preferred Shares and Conversion Shares acquired hereunder and the availability, if required, of exemptions from registration for purposes of sale or transfer of the Series C Preferred Shares and Conversion Shares.
(g) Investment Experience. Seller understands that the acquisition of Series C Preferred Shares and Conversion Shares involves substantial risk. Seller acknowledges that Seller can bear the economic risk of Seller’s investment in the Series C Preferred Shares, and has sufficient knowledge and experience in financial or business matters such that Seller is capable of evaluating the merits and risks of this investment in the Series C Preferred Shares and protecting its own interests in connection with this investment. Seller hereby represents that it is an “accredited investor,” as such term is defined under Rule 501(a) of Regulation D promulgated under the Securities Act.
| Page |
(h) Restricted Shares. Seller understands that the Series C Preferred Shares and Conversion Shares are characterized as “restricted securities” under the Securities Act inasmuch as they are being acquired from Purchaser in a transaction not involving a public offering and that, under the Securities Act and applicable regulations thereunder, such securities may be resold without registration under the Securities Act only in certain limited circumstances. In this connection, Seller represents that Seller is familiar with Rule 144 as promulgated under the Securities Act and as presently in effect, and understands the resale limitations imposed thereby and by other applicable provisions of the Securities Act.
(7) Legend. Seller acknowledges and understands that the certificates or book-entry statements evidencing the Series C Preferred Shares and Conversion Shares will bear the legend set forth below:
“THE SECURITIES REPRESENTED HEREBY [AND ISSUABLE UPON CONVERSION HEREOF] HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR UNDER THE SECURITIES LAWS OF CERTAIN STATES. THESE SECURITIES ARE SUBJECT TO RESTRICTIONS ON TRANSFERABILITY AND RESALE AND MAY NOT BE TRANSFERRED OR RESOLD EXCEPT AS PERMITTED UNDER THE ACT AND THE APPLICABLE STATE SECURITIES LAWS, PURSUANT TO REGISTRATION OR EXEMPTION THEREFROM. INVESTORS SHOULD BE AWARE THAT THEY MAY BE REQUIRED TO BEAR THE FINANCIAL RISKS OF THIS INVESTMENT FOR AN INDEFINITE PERIOD OF TIME. THE ISSUER OF THESE SECURITIES MAY REQUIRE AN OPINION OF COUNSEL IN FORM AND SUBSTANCE SATISFACTORY TO THE ISSUER TO THE EFFECT THAT ANY PROPOSED TRANSFER OR RESALE IS IN COMPLIANCE WITH THE ACT AND ANY APPLICABLE STATE SECURITIES LAWS.”
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF PURCHASER
Purchaser hereby represents and warrants to Seller as of the Effective Date as follows:
5.1 Corporate Organization. Purchaser is a corporation duly organized, validly existing and, to the extent applicable, in good standing under the respective laws of the jurisdiction of its incorporation, is duly qualified and, to the extent applicable, is in good standing under the laws of each jurisdiction in which the character of the properties and assets now owned or held by it or the nature of the business now conducted by it requires it to be so licensed or qualified, except to the extent such failure would not have a material adverse effect on the Purchaser. Purchaser has full corporate power and authority to carry on its business as now being conducted.
5.2 Authority. Purchaser has full corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder. The execution and delivery of this Agreement by Purchaser and the performance by Purchaser of its obligations hereunder have been duly authorized by all necessary corporate action. This Agreement has been duly executed and delivered by Purchaser and constitutes the legal, valid and binding obligation of Purchaser, enforceable against it in accordance with its terms, subject to applicable laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
5.3 No Conflict; No Consents. The execution and delivery of this Agreement and the performance of the obligations of Purchaser hereunder will not violate or be in conflict with any provision of law, any order, rule or regulation of any Governmental Entity, or any provision of Purchaser’s certificate of incorporation or bylaws. No consent, approval or authorization of or declaration or filing with any Governmental Entity or other person or entity on the part of Purchaser is required in connection with the execution or delivery of this Agreement or the consummation of the transactions contemplated hereby.
| Page |
ARTICLE VI
SURVIVAL OF REPRESENTATIONS AND WARRANTIES; INDEMNIFICATION
6.1 Survival of Representations and Warranties. Except with respect to Seller’s representations and warranties under Sections 4.9 (Ownership and Encumbrances), 4.10 (Standards Bodies), 4.21 (No Patent License) and 4.24 (Securities Representations), which shall survive indefinitely, all of Seller’s and Purchaser’s representations and warranties contained in this Agreement shall terminate as of the last day of the twelfth (12th) month following the month in which the Effective Date occurs.
6.2 Indemnification.
(a) Seller shall defend, indemnify and hold harmless Purchaser, its Affiliates, and each of their Subsidiaries, shareholders, directors, officers, employees, agents, successors, and assigns from and against all damages, claims, liabilities, expenses and costs (including reasonable attorneys’ fees) arising, directly or indirectly, from any material breach of this Agreement by Seller, including, without limitation, any material breach of any representation or warranty made by Seller.
(b) Purchaser shall defend, indemnify and hold harmless Seller, its Subsidiaries, and each of their shareholders, directors, officers, employees, agents, successors, and assigns from and against all damages, claims, liabilities, expenses and costs (including reasonable attorneys’ fees) arising, directly or indirectly, from any material breach of this Agreement by Purchaser, including, without limitation, any material breach of any representation or warranty made by Purchaser.
(c) A person or entity that intends to claim indemnification under this Article VI (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) of any claim, damage, liability, cause of action or cost with respect to which the Indemnitee intends to claim such indemnification, provided that the failure of the Indemnitee to give notice as provided herein shall not relieve the Indemnitor of its obligations under this Article II unless the failure to give such notice is materially prejudicial to an Indemnitor’s ability to defend such action. Indemnitor, after it determines that indemnification is required of it, shall assume the defense thereof with counsel of its own choosing; provided, however, that an Indemnitee shall have the right to retain its own counsel, with the expenses to be paid by the Indemnitor if Indemnitor does not assume the defense. The Indemnitee shall, and shall cause its Subsidiaries and its own and its Subsidiaries’ employees and agents to, cooperate fully with the Indemnitor and its legal representatives in the investigation and defense of any claim, damage, liability, cause of action or cost covered by this indemnification. The maximum liability of each Indemnitor under this Agreement for a breach of warranty under Article IV or Article V, as applicable, and/or for a claim of indemnification as set forth in this Article VI, in the aggregate shall be equal to the Purchase Price.
| Page |
ARTICLE VIII MISCELLANEOUS
7.1 Confidentiality. The Parties shall maintain as strictly confidential this Agreement and any proprietary information disclosed under, or as a result of or during the negotiation of, this Agreement, which obligation shall survive the consummation of the transactions contemplated herein, and shall only use such information for the purpose of performing under and/or enforcing this Agreement or the Assigned Patents, except that each Party, or its Affiliates, may disclose or use this Agreement or any such proprietary information as follows:
(a) As reasonably necessary to prosecute or enforce the Assigned Patents;
(b) as reasonably necessary for Purchaser to record or otherwise perfect Purchaser’s interest in the Assigned Patents;
(c) to the extent required by law;
(d) to the extent such information is public information, except as a result of the breach of this Section 7.1;
(e) as is required by a court or an arbitral order which has been precipitated by a third party request; provided, that the entity making such disclosure or use shall seek appropriate confidentiality protections (e.g., having such disclosures covered by a protective order or other comparable protections) prior to making such disclosure or use;
(f) to satisfy SEC, NASDAQ or other statutory, regulatory, taxation, or administrative requirements;
(g) in a legal proceeding between the Parties or their Affiliates;
(h) to a potential acquirer, in connection with a potential acquisition of all or any material part of any business of such Party; or
(i) in confidence, to its accountants, bankers, attorneys, or their Affiliates.
Notwithstanding the foregoing, the Parties acknowledge that Purchaser or its Affiliates shall have the right, at its sole discretion, to publish and distribute a press release or a Current Report on Form 8-K or Periodic Report filed pursuant to the Securities Exchange Act of 1934, as amended, announcing the execution of this Agreement, describing the material terms hereof and filing such Agreement as an exhibit thereto.
7.2 Expenses. Except as otherwise provided in this Agreement, each Party will pay all fees and expenses incurred by it in connection with this Agreement and the transactions contemplated hereby.
7.3 Governing Law/Venue. This Agreement is governed by the laws of the State of Texas, excluding its conflict-of-laws principles. The state and federal courts in the State of Texas shall have exclusive jurisdiction over any claim, suit or proceeding (each, a “Proceeding”) related to this Agreement (including without limitation the breach or threatened breach thereof), and each Party irrevocably (a) consents to the jurisdiction of such courts for any Proceeding, (b) consents to service of process in any Proceeding in such courts by globally recognized overnight courier service at the address set forth above, as well as other means of service permitted by law; and (c) waives any objections on the grounds of venue, residence, domicile or inconvenient forum to any Proceeding brought in such courts.
7.4 Waivers. The failure of any Party to insist upon the performance of any of the terms or conditions of this Agreement or to exercise any right hereunder, shall not be construed as a waiver or relinquishment of any such right, term or condition. No waiver by any Party of any provision of this Agreement or any default, misrepresentation, or breach of warranty or covenant hereunder shall be valid unless the same shall be in writing and signed by the Party making such waiver.
| Page |
7.5 Severability. The provisions of this Agreement shall be severable, and if any of them are held invalid or unenforceable, then that provision shall be construed to the maximum extent permitted by law. The invalidity or unenforceability of one provision shall not necessarily affect any other.
7.6 Notices. All notices or other communications required or permitted under this Agreement shall be in writing and shall be delivered by personal delivery, registered mail, return receipt requested, or a qualified overnight delivery service addressed as indicated on page 1 of this Agreement. Facsimiles shall be sent to Seller at 185 Prospect Avenue, Unit 7i, Hackensack, NY 07601 and to Purchaser at 15110 Dallas Parkway, Suite 600, Dallas, Texas 75248. All such notices shall be deemed delivered at the time of delivery, except a facsimile shall be deemed delivered at the time of electronic confirmation of delivery.
7.7 Asset Purchase. The transaction contemplated under this Agreement is strictly an asset purchase, and Purchaser is not taking any assignment of any debt, obligation, or other Encumbrance on any of the Assigned Patents.
7.8 Entire Agreement/Amendment. This Agreement contains the complete and final agreement between the Parties, and supersedes all previous understandings, relating to the subject matter hereof whether oral or written. This Agreement may only be modified by a written agreement signed by duly authorized representatives of the Parties.
7.9 No Assignment. Except for Purchaser’s right to assign or delegate this Agreement and its rights and duties as set forth herein to a present or future Affiliate, neither this Agreement nor any rights or duties under this Agreement may be assigned or delegated, in whole or in part, by either Party without the prior written consent of the non-assigning Party, which consent may be withheld by the non-assigning Party for any or no reason. Any attempted assignment and/or delegation in breach of this Section 7.9 will be null and void. The Parties understand and agree that a Change of Control event is considered an assignment for the purposes of this Section 7.9.
7.10 Survival. Notwithstanding anything in this Agreement to the contrary except as set forth in Section 6.1, all representations, warranties, obligations, responsibilities, terms or conditions which by a fair reading of their nature are intended to survive shall be deemed to survive.
7.11 Limitation on Consequential Damages. EXCEPT IN THE CASE OF FRAUD BY SELLER, NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR LOSS OF PROFITS, OR ANY OTHER INDIRECT OR SPECIAL, CONSEQUENTIAL, PUNITIVE OR INCIDENTAL DAMAGES, HOWEVER CAUSED, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGE. THE PARTIES ACKNOWLEDGE THAT THESE LIMITATIONS ON POTENTIAL LIABILITIES WERE AN ESSENTIAL ELEMENT IN SETTING CONSIDERATION UNDER THIS AGREEMENT.
7.12 Arm’s Length Negotiations. Each Party herein expressly represents and warrants to all other Parties hereto that (a) before executing this Agreement, said Party has fully informed itself of the terms, contents, conditions and effects of this Agreement; (b) said Party has relied solely and completely upon its own judgment in executing this Agreement; (c) said Party has had the opportunity to seek and has obtained the advice of its own legal, tax and business advisors before executing this Agreement; (d) said Party has acted voluntarily and of its own free will in executing this Agreement; and (e) this Agreement is the result of arm’s length negotiations conducted by and among the Parties and their respective counsel.
| Page |
7.13 Amendments. Every right and remedy provided herein shall be cumulative with every other right and remedy, whether conferred herein, at law, or in equity, and may be enforced concurrently herewith, and no waiver by any Party of the performance of any obligation by the other shall be construed as a waiver of the same or any other default then, theretofore, or thereafter occurring or existing. This Agreement may be amended by a writing signed by all Parties hereto.
7.14 Remedies. The remedies provided in this Agreement shall be cumulative and in addition to all other remedies available under this Agreement, at law or in equity (including a decree of specific performance and/or other injunctive relief).
7.15 Counterparts, Effect of Facsimile, Emailed and Photocopied Signatures. This Agreement and any signed agreement or instrument entered into in connection with this Agreement, and any amendments hereto or thereto, may be executed in one or more counterparts, all of which shall constitute one and the same instrument. Any such counterpart, to the extent delivered by means of a facsimile machine or by .pdf, .tif, .gif, .jpeg or similar attachment to electronic mail (any such delivery, an “Electronic Delivery”) shall be treated in all manner and respects as an original executed counterpart and shall be considered to have the same binding legal effect as if it were the original signed version thereof delivered in person. At the request of any Party, each other Party shall re execute the original form of this Agreement and deliver such form to all other Parties. No Party shall raise the use of Electronic Delivery to deliver a signature or the fact that any signature or agreement or instrument was transmitted or communicated through the use of Electronic Delivery as a defense to the formation of a contract, and each such Party forever waives any such defense, except to the extent such defense relates to lack of authenticity.
7.16 Assignment to Wholly-Owned Subsidiary of Purchaser. The Parties agree and confirm, and the Seller acknowledges and agrees, that the Purchaser intends for its newly formed wholly-owned subsidiary, MangoRx IP Holdings, LLC, a Texas limited liability company (“MangoRx Holdings”), to hold the Assigned Patents, and Seller agrees to execute whatever documents and materials as the Purchaser may reasonably request, to affect such transfer and assignment. MangoRx Holdings shall be bound by the terms of this Agreement relating to the Assigned Patents as if a party hereto upon such assignment, and Purchaser shall take whatever action as may be necessary for MangoRx Holdings to be bound thereby, and comply with such applicable terms.
[Remainder of page left intentionally blank. Signature page follows.]
| Page |
IN WITNESS WHEREOF, the Parties have executed this Agreement by their duly authorized representatives as of the dates below to be effective as of the Effective Date.
| Mangoceuticals, Inc. | Intramont Technologies, Inc. | ||||
| By: | /s/ Jacob Cohen | By: | /s/ Jim Intrater | ||
| Name: | Jacob Cohen | Name: | Jim Intrater | ||
| Title: | CEO | Title: | CEO | ||
| Date: | April 24, 2024 | Date: | April 24, 2024 | ||
| Page |
EXHIBIT A
List of Patents
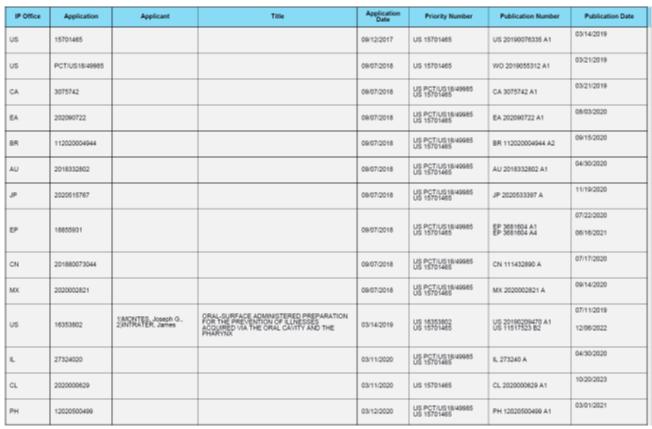
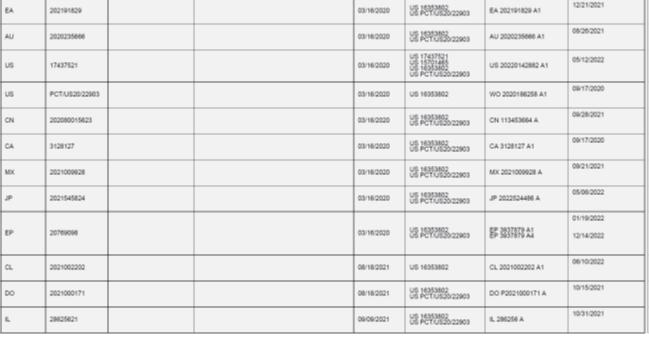
Exhibit 99.1
Mangoceuticals Acquires Global Patent Portfolio
to Revolutionize Preventive Care
Provides MangoRx with entrance into the non-Rx based, nutraceutical product space for sales on both direct-to-consumer online platforms and through retail locations
Dallas, Texas / April 25, 2024 / Mangoceuticals, Inc. (NASDAQ: MGRX) (“MangoRx” or the “Company”), a company focused on developing, marketing, and selling a variety of men’s health and wellness products in the area of erectile dysfunction (ED), hair growth and hormone replacement therapies is excited to announce that it has acquired a global patent portfolio for a pioneering oral solution and application aimed to combat and prevent a spectrum of infections, including the common cold, respiratory diseases, and orally transmitted diseases such as human papillomavirus (HPV) (the “Patent Portfolio”) from Intramont Technologies, Inc. (“Intramont”), pursuant to a Patent Purchase Agreement (“PPA”).
We believe that the acquired technology represents a breakthrough in preventive care. The acquired technology is protected by a global patent portfolio including US and international patent rights. The solution is specifically designed to prevent illnesses acquired through the oral cavity and the pharynx through the application of an orally available solution such as a toothpaste, oral dissolvable tablet (ODT), lozenge or mouthwash.
The keys to what we believe are the effectiveness of this oral health innovation are its unique ingredients and formulation. The patented formulation incorporates a proprietary blend, featuring GALALCOOL®️, zinc protoporphyrin IX, and select tannins. These components are believed to synergistically inhibit the acquisition of various pathogens, providing users with a comprehensive defense against oral and respiratory infections.
Jacob Cohen, Co-Founder and CEO of MangoRx commented, “We are thrilled to integrate this groundbreaking solution into our current portfolio of products, as this further signifies our entrance into non-prescription, or non-Rx, based products, which can be sold both via direct-to-consumer online or in retail locations. This acquisition aligns perfectly with our mission to enhance well-being through innovative health solutions. Through working to commercialize this technology, we aim to unlock significant value for our shareholders while advancing global health and addressing significant market needs.”
According to a recent market study performed by Fortune Business Insights, the global toothpaste market is a significant growth opportunity for MangoRx’s newly acquired technology. With the market size standing at $18.11 billion in 2022 and projected to reach $25.71 billion by 2030, a compound annual growth rate (CAGR) of 4.7%, the demand for high-quality oral health products is on the rise. As per the World Health Organization’s global oral health status report in 2022, nearly 3.5 billion people (3 out of 4) suffer from oral diseases globally, underscoring the urgent need for innovative preventive measures.
“Intramont is pleased to enter into a Patent Purchase Agreement with MangoRx”, commented Jim Intrater, CEO and Founder of Intramont Technologies, Inc., who continued, “MangoRx’s expertise in sales and marketing are of great potential help to our desires to bring forth new consumer health products to the general public. MangoRx has demonstrated its unique ability to develop, commercialize and market its products and we see this patented product fitting excellently among MangoRx’s product portfolio as well as through its other sales and distribution channels, both domestically and abroad.”
Pursuant to the PPA, the Company agreed to pay Intramont a total of $20,000,000 for the Patent Portfolio, through the issuance of 980,000 shares of its newly created 6% Series C Convertible Preferred shares (with a stated value and liquidation preference of $20.00 per share and convertible into shares of the Company’s common stock at $10.00 per share), which were issued at closing, and through the payment to Intramont of $400,000 in cash over the course of 7 months.
For more information on the transaction and the PPA, please see the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission earlier today.
About MangoRx
MangoRx is focused on developing a variety of men’s health and wellness products and services via a secure telemedicine platform. To date, the Company has identified men’s wellness telemedicine services and products as a growing sector and especially related to the area of erectile dysfunction (ED), hair growth and hormone replacement therapies. Interested consumers can use MangoRx’s telemedicine platform for a smooth experience. Prescription requests will be reviewed by a physician and, if approved, fulfilled and discreetly shipped through MangoRx’s partner compounding pharmacy and right to the patient’s doorstep. To learn more about MangoRx’s mission and other products, please visit www.MangoRx.com or on social media @Mango.Rx.
Cautionary Note Regarding Forward-Looking Statements
Certain statements made in this press release contain forward-looking information within the meaning of applicable securities laws, including within the meaning of the Private Securities Litigation Reform Act of 1995 (“forward-looking statements”). These forward-looking statements represent the Company’s current expectations or beliefs concerning future events and can generally be identified using statements that include words such as “estimate,” “expects,” “project,” “believe,” “anticipate,” “intend,” “plan,” “foresee,” “forecast,” “likely,” “will,” “target” or similar words or phrases. These forward-looking statements are subject to risks, uncertainties and other factors, many of which are outside of the Company’s control which could cause actual results to differ materially from the results expressed or implied in the forward-looking statements, including, but not limited to: our ability to commercialize the Patent Portfolio; our ability to obtain Comisión Federal para la Protección contra Riesgos Sanitarios for our ED product in Mexico, the costs thereof and timing associated therewith; our ability to obtain additional funding and generate revenues to support our operations; risks associated with our ED product which have not been, and will not be, approved by the U.S. Food and Drug Administration (“FDA”) and have not had the benefit of the FDA’s clinical trial protocol which seeks to prevent the possibility of serious patient injury and death; risks that the FDA may determine that the compounding of our planned products does not fall within the exemption from the Federal Food, Drug, and Cosmetic Act (“FFDCA Act”) provided by Section 503A; risks associated with related party relationships and agreements; the effect of data security breaches, malicious code and/or hackers; competition and our ability to create a well-known brand name; changes in consumer tastes and preferences; material changes and/or terminations of our relationships with key parties; significant product returns from customers, product liability, recalls and litigation associated with tainted products or products found to cause health issues; our ability to innovate, expand our offerings and compete against competitors which may have greater resources; our significant reliance on related party transactions; the projected size of the potential market for our technologies and products; risks related to the fact that our Chairman and Chief Executive Officer, Jacob D. Cohen has significant voting control over the Company; risks related to the significant number of shares in the public float, our share volume, the effect of sales of a significant number of shares in the marketplace, and the fact that the majority of our shareholders paid less for their shares than the public offering price of our common stock in our recent initial public offering; dilution caused by recent offerings; the fact that we have a significant number of outstanding warrants to purchase shares of common stock at $1.00 per share, the resale of which underlying shares have been registered under the Securities Act of 1933, as amended; our ability to build and maintain our brand; cybersecurity, information systems and fraud risks and problems with our websites; changes in, and our compliance with, rules and regulations affecting our operations, sales, marketing and/or our products; shipping, production or manufacturing delays; regulations we are required to comply with in connection with our operations, manufacturing, labeling and shipping; our dependency on third-parties to prescribe and compound our ED product; our ability to establish or maintain relations and/or relationships with third-parties; potential safety risks associated with our Mango ED product, including the use of ingredients, combination of such ingredients and the dosages thereof; the effects of changing rates of inflation and interest rates, and economic downturns, including potential recessions, as well as macroeconomic, geopolitical, health and industry trends, pandemics, acts of war (including the ongoing Ukraine/Russian conflict and war in Israel) and other large-scale crises; our ability to protect intellectual property rights; our ability to attract and retain key personnel to manage our business effectively; our ability to maintain the listing of our common stock on the Nasdaq Capital Market, including our current non-compliance with Nasdaq’s continued listing standards; overhang which may reduce the value of our common stock; volatility in the trading price of our common stock; and general consumer sentiment and economic conditions that may affect levels of discretionary customer purchases of the Company’s products, including potential recessions and global economic slowdowns. Although we believe that our plans, intentions and expectations reflected in or suggested by the forward-looking statements we make in this release are reasonable, we provide no assurance that these plans, intentions or expectations will be achieved. Consequently, you should not consider any such list to be a complete set of all potential risks and uncertainties.
More information on potential factors that could affect the Company’s financial results is included from time to time in the “Cautionary Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of the Company’s filings with the SEC, including the Company’s Annual Report on Form 10-K for the year ended December 31, 2023. These filings are available at www.sec.gov and at our website at https://www.mangoceuticals.com/sec-filings. All subsequent written and oral forward-looking statements attributable to the Company or any person acting on behalf of the Company are expressly qualified in their entirety by the cautionary statements referenced above. Other unknown or unpredictable factors also could have material adverse effects on the Company’s future results. The forward-looking statements included in this press release are made only as of the date hereof. The Company cannot guarantee future results, levels of activity, performance or achievements. Accordingly, you should not place undue reliance on these forward-looking statements. Finally, the Company undertakes no obligation to update these statements after the date of this release, except as required by law, and takes no obligation to update or correct information prepared by third parties that are not paid for by the Company. If we update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
Follow Mangoceuticals and MangoRx on social media:
https://www.instagram.com/mango.rx
https://twitter.com/Mangoceuticals
https://www.facebook.com/MangoRxOfficial
FOR PUBLIC RELATIONS
Lucky Break Public Relations
Sahra Simpson
Sahra@luckybreakpr.com
(323) 602-0091 ext. 704
FOR INVESTOR RELATIONS
Mangoceuticals Investor Relations
Email: investors@mangorx.com
SOURCE: Mangoceuticals Inc.