UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2023
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM__________ TO__________
Commission File Number 001-37603
BIORESTORATIVE THERAPIES, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 30-1341024 | |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| 40 Marcus Drive, Suite 1, Melville, New York | 11747 | |
| (Address of principal executive offices) | (Zip Code) |
(631) 760-8100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Common Stock $0.0001 par value |
BRTX | Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter.
As of June 30, 2023, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $13,663,719 based on the closing sale price as reported on the Nasdaq Capital Market.
APPLICABLE ONLY TO REGISTRANTS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PRECEDING FIVE YEARS:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☒ No ☐
As of March 28, 2024, there were 6,769,919 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None
INDEX
|
|
PART I
Forward-Looking Statements
This Annual Report contains forward-looking statements as that term is defined in the federal securities laws. The events described in forward-looking statements contained in this Annual Report may not occur. Generally, these statements relate to business plans or strategies, projected or anticipated benefits or other consequences of our plans or strategies, projected or anticipated benefits from acquisitions to be made by us, or projections involving anticipated revenues, earnings or other aspects of our operating results. The words “may,” “will,” “expect,” “believe,” “anticipate,” “project,” “plan,” “intend,” “estimate,” and “continue,” and their opposites and similar expressions are intended to identify forward-looking statements. We caution you that these statements are not guarantees of future performance or events and are subject to a number of uncertainties, risks and other influences, many of which are beyond our control, that may influence the accuracy of the statements and the projections upon which the statements are based. Factors which may affect our results include, but are not limited to, the risks and uncertainties discussed in Item 1A of this Annual Report (“Risk Factors”).
Any one or more of these uncertainties, risks and other influences could materially affect our results of operations and whether forward-looking statements made by us ultimately prove to be accurate. Our actual results, performance and achievements could differ materially from those expressed or implied in these forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether from new information, future events or otherwise.
Intellectual Property
This Annual Report includes references to our federally registered trademarks, BioRestorative Therapies and Dragonfly design, BRTX-100, ThermoStem and BRTX. The Dragonfly logo is also registered with the U.S. Copyright Office. This Annual Report also includes references to trademarks, trade names and service marks that are the property of other organizations. Solely for convenience, trademarks and trade names referred to in this Annual Report appear without the ®, SM or ™ symbols, and copyrighted content appears without the use of the symbol ©, but the absence of use of these symbols does not reflect upon the validity or enforceability of the intellectual property owned by us or third parties.
| ITEM 1. | BUSINESS. |
(a) Business Development
As used in this Annual Report on Form 10-K, or the Annual Report, references to the “Company”, “we”, “us”, or “our” refer to BioRestorative Therapies, Inc.
We were incorporated in Nevada on June 13, 1997. On August 15, 2011, we changed our name from “Stem Cell Assurance, Inc.” to “BioRestorative Therapies, Inc.” Effective January 1, 2015, we reincorporated in Delaware. Effective December 31, 2022, we reincorporated in Nevada.
|
|
We develop therapeutic products and medical therapies using cell and tissue protocols, primarily involving adult stem cells.
We are currently pursuing our Disc/Spine Program with our initial investigational therapeutic product being called BRTX-100. In March 2022, a United States patent issued in our Disc/Spine Program. We submitted an IND application to the U.S. Food and Drug Administration, or the FDA, to obtain authorization to commence a Phase 2 clinical trial investigating the use of BRTX-100 in the treatment of chronic lower back pain arising from degenerative disc disease. We have received such authorization from the FDA and have commenced such clinical trial.
We have obtained an exclusive license to use technology for investigational adult stem cell treatment of disc and spine conditions, including protruding and bulging lumbar discs. The technology is an advanced stem cell injection procedure that may offer relief from lower back pain, buttock and leg pain, and numbness and tingling in the leg and foot.
We are also developing our ThermoStem Program. This pre-clinical program involves the use of brown adipose (fat) in connection with the cell-based treatment of type 2 diabetes and obesity as well as hypertension, other metabolic disorders and cardiac deficiencies. Patents related to the ThermoStem Program have been issued in the United States and other jurisdictions.
Further, we are seeking to develop a biologics-based cosmetic products business. Pursuant to such business, we would manufacture and sell cosmetics and hair growth products designed for cosmetic and aesthetic uses.
Material Events During 2023
In April 2023, we entered into a Capital On Demand™ Sales Agreement, or the Sales Agreement, with JonesTrading Institutional Services LLC, or the Agent, pursuant to which we may offer and sell, from time to time, through or to the Agent, shares of our common stock having an aggregate offering price of up to $6,109,000. To date, we have sold approximately $622,000 of our shares pursuant to the Sales Agreement.
In April 2023, we announced that we had completed enrollment for the safety run-in component of our Phase 2 clinical study of BRTX-100.
In May 2023, we announced that we had signed a clinical trial agreement with Northwell Health, New York State’s largest health care provider and private employer, pursuant to which Northwell Health is participating in our Phase 2 clinical study of BRTX-100.
In May 2023, we announced that we had received a license from the New York State Department of Health to act as a tissue bank for the processing of mesenchymal stem cells from autologous donors.
In June 2023, we announced that the final subject in our BRTX-100 Phase 2 clinical trial safety cohort had been dosed.
In June 2023, we announced that the independent Data Safety Monitoring Board, which is overseeing our Phase 2 clinical trial, unanimously recommended the continuation of our study in accordance with the version of the protocol with no changes.
|
|
In June 2023, a Japanese patent related to our ThermoStem Program was issued to us.
In June 2023, a United States patent related to our ThermoStem Program was issued to us.
In July 2023, a European patent related to our ThermoStem Program was issued to us.
In July 2023, we sold 685,033 shares of our common stock in a registered direct public offering. We received gross proceeds of approximately $2,100,000 from the offering.
In September 2023, we entered into a supply agreement with a supplier of biologic-based cosmetics. Pursuant to the supply agreement, using our cGMP ISO-7 certified clean room, we manufactured tissue-based biologics for cosmetic and aesthetic applications. In December 2023, we terminated the supply agreement.
In December 2023, a United States patent related to our ThermoStem Program was issued to us.
Material Events During 2024
In February 2024, we issued 2,000,000 shares of our common stock pursuant to the exercise of warrants. In connection with the issuance, we issued warrants for the purchase of 2,513,686 shares of our common stock and are obligated to issue an additional 1,351,580 shares of our common stock, which additional shares have been fully paid for but have not yet been issued due to a maximum beneficial ownership limitation for one of the warrantholders. We received gross proceeds of approximately $8,100,000 from the warrant exercise.
(b) Business
General
We develop therapeutic products, using cell and tissue protocols, primarily involving adult stem cells. Our two core programs, as described below, relate to the treatment of disc/spine disease and metabolic disorders:
| ● | Disc/Spine Program (brtxDisc). Our lead cell therapy candidate, BRTX-100, is a product formulated from autologous (or a person’s own) cultured mesenchymal stem cells, or MSCs, collected from the patient’s bone marrow. We intend that the product will be used for the non-surgical treatment of painful lumbosacral disc disorders or as a complimentary therapeutic to a surgical procedure. The BRTX-100 production process utilizes proprietary technology and involves collecting a patient’s bone marrow, isolating and culturing stem cells from the bone marrow and cryopreserving the cells. In an outpatient procedure, BRTX-100 is to be injected by a physician into the patient’s damaged disc. The treatment is intended for patients whose pain has not been alleviated by non-surgical procedures and who potentially face the prospect of surgery. We have received authorization from the FDA to commence a Phase 2 clinical trial using BRTX-100 to treat chronic lower back pain arising from degenerative disc disease. We have commenced such clinical trial through the execution of a CRO agreement with PRC Clinical, the execution of clinical trial site agreements, patient enrollment, the commencement of patient procedures, the purchase of manufacturing equipment and the expansion of our laboratory to include capabilities for clinical production. In March 2022, a United States patent related to our Disc/Spine Program was issued. We have been granted exclusive license rights with regard to the patent. See “Disc/Spine Program” below. |
|
|
| ● | Metabolic Program (ThermoStem). We are developing a cell-based therapy candidate to target obesity and metabolic disorders using brown adipose (fat) derived stem cells, or BADSC, to generate brown adipose tissue, or BAT. We refer to this as our ThermoStem Program. BAT is intended to mimic naturally occurring brown adipose depots that regulate metabolic homeostasis in humans. Initial preclinical research indicates that increased amounts of brown fat in animals may be responsible for additional caloric burning as well as reduced glucose and lipid levels. Researchers have found that people with higher levels of brown fat may have a reduced risk for obesity and diabetes. Patents related to the ThermoStem Program have been issued in the United States and other jurisdictions. See “Metabolic Brown Adipose (Fat) Program” below. |
We have also licensed an investigational curved needle device designed to deliver cells and/or other therapeutic products or material to the spine and discs (and other parts of the body). We anticipate that FDA approval or clearance will be necessary for this device prior to commercialization. We do not intend to utilize this device in connection with our Phase 2 clinical trial with regard to BRTX-100. See “Curved Needle Device” below.
The patents and patent applications for the Disc/Spine Program, the ThermoStem Program and the curved needle device are listed below under “Technology; Research and Development.”
We are also seeking to develop a biologics-based cosmetic products business. Pursuant to such business, we would manufacture and sell cosmetics and hair growth products designed for cosmetic and aesthetic uses.
Overview
Every human being has stem cells in his or her body. These cells exist from the early stages of human development until the end of a person’s life. Throughout our lives, our body continues to produce stem cells that regenerate to produce differentiated cells that make up various aspects of the body such as skin, blood, muscle and nerves. These are generally referred to as adult (non-embryonic) stem cells. These cells are important for the purpose of medical therapies aiming to replace lost or damaged cells or tissues or to otherwise treat disorders.
Regenerative cell therapy relies on replacing diseased, damaged or dysfunctional cells with healthy, functioning ones or repairing damaged or diseased tissue. A great range of cells can serve in cell therapy, including cells found in peripheral and umbilical cord blood, bone marrow and adipose (fat) tissue. Physicians have been using adult stem cells from bone marrow to treat various blood cancers for more than 65 years (the first successful bone marrow transplant was performed in 1956). Recently, physicians have begun to use stem cells to treat various other diseases. We intend to develop cell and tissue products and regenerative therapy protocols, primarily involving adult stem cells, to allow patients to undergo cellular-based treatments.
|
|
We are concentrating initially on therapeutic areas in which risk to the patient is low, recovery is relatively easy, results can be demonstrated through sufficient clinical data, and patients and physicians will be comfortable with the procedure. We believe that there will be readily identifiable groups of patients who will benefit from these procedures. We also believe that these procedures will be significantly less expensive than the most common surgical procedure alternatives and will compare favorably, over the long-term, to conservative treatment costs which may persist for years.
Accordingly, we have focused our initial developmental efforts on cellular-based therapeutic products and clinical development programs in selective areas of medicine for which the treatment protocol is minimally invasive. Such areas include the treatment of the disc and spine and metabolic-related disorders. Upon regulatory approval, we will seek to obtain third party reimbursement for our products and procedures; however; if we are not successful, patients may be required to pay for our products and procedures out of pocket in full and without the ability to be reimbursed by any governmental and other third party payers, which would adversely impact our prospects.
We have undertaken research and development efforts in connection with the development of investigational therapeutic products and medical therapies using cell and tissue protocols, primarily involving adult stem cells. See “Disc/Spine Program,” “Metabolic Brown Adipose (Fat) Program” and “Curved Needle Device” below. As a result of these programs, we have seven United States patents, thirteen foreign patents, one United States patent application, and four foreign patent applications related to research regarding our ThermoStem Program. We have also obtained licenses for four United States patent applications related to our Disc/Spine Program, one United States patent related to our Disc/Spine Program, and a license for one United States patent related to a curved needle device.
We have established a research laboratory facility with current Good Manufacturing Practice, or cGMP, capabilities to produce clinical grade products and we will seek to further develop cellular-based treatments, products and protocols, stem cell-related intellectual property, or IP, and translational research applications. See “Laboratory” below.
We have not generated any significant revenues to date. In November 2021, we completed a $23,000,000 public offering of our securities. In 2023, we raised approximately $2,680,000 in additional gross proceeds through public offerings of our securities. In February 2024, we also received approximately $8,100,000 in gross proceeds pursuant to the exercise of warrants. Such aggregate funds are sufficient for us to complete our Phase 2 clinical trial investigating the use of BRTX-100 in the treatment of chronic lower back pain arising from degenerative disc disease, as further described in this section, as well as to continue our pre-clinical research and development efforts with respect to our ThermoStem Program and to satisfy our current working capital needs; however, the implementation of our business plan, as discussed below, will require the receipt of additional financing to fund our research and development efforts, including our contemplated Phase 3 clinical trial with regard to BRTX-100 and our contemplated clinical trials relating to our ThermoStem Program, and otherwise fund our operations. We intend to seek to raise capital through investment bankers and from biotech funds, strategic partners and other financial institutions. We will require significant additional financing to complete our contemplated Phase 3 clinical trial investigating the use of BRTX-100. We will also require a substantial amount of additional funding to implement our other programs described in this section, and fund general operations. No assurance can be given that the amount of funding that we anticipate may be required for such purposes is correct or that we will be able to accomplish our goals within the timeframes projected. In addition, no assurance can be given that we will be able to obtain any required financing on commercially reasonable terms or otherwise. If we are unable to obtain adequate funding, we may be required to significantly curtail or discontinue our proposed operations.
|
|
Disc/Spine Program
General
Among the initiatives that we are currently pursuing is our Disc/Spine Program, with our initial product candidate being called BRTX-100. We have obtained an exclusive license (see “Exclusive License” below) that permits us to use technology for adult stem cell treatment of disc and spine conditions. The technology is an advanced stem cell culture and injection procedure into the intervertebral disc, or IVD, that may offer relief from lower back pain, buttock and leg pain, and numbness and tingling in the leg and foot.
Lower back pain is the most common, most disabling, and most costly musculoskeletal ailment faced worldwide. According to a 2016 market report from Trinity Partners, a global life sciences consulting firm, of the 250 million American adults, nearly 25 million have chronic lower back pain of which approximately 12 million have been diagnosed with and treated for disc degeneration and approximately 5.6 million have pain caused by a protruding or injured disc. We believe that between 500,000 and one million invasive surgical procedures are performed each year to try to alleviate the pain associated with these lower back conditions and that such procedures cost approximately $40 billion. Clinical studies have documented that the source of the pain is most frequently damage to the IVD. This can occur when forces, whether a single load or repetitive microtrauma, exceed the IVD’s inherent capacity to resist those loads. Aging, obesity, smoking, lifestyle, and certain genetic factors may predispose one to an IVD injury. Current surgical approaches to back pain are extremely invasive (often altering the spine’s biomechanics unfavorably and predisposing it to further disc degeneration) and are associated with unacceptably low success rates (with a second operation occurring 10% to 20% of the time). In addition, current surgical approaches are costly with spinal fusion surgery costing approximately $110,000, discectomy costing approximately $20,000 to $50,000 and disc replacement surgery costing approximately $80,000 to $150,000. Even conservative treatments can be costly, with oral medications costing between $1,000 and $2,000 per year, injection treatments costing approximately $8,000 per year and physical therapy costing approximately $20,000 annually. We anticipate that the cost of a single treatment using BRTX-100 will compare favorably to conservative treatments which may continue for years and will be less expensive than the most common surgical procedures.
While once thought to be benign, the natural history of lower back pain is often one of chronic recurrent episodes of pain leading to progressive disability. This is believed to be a direct result of the IVD’s poor healing capacity after injury. The IVD is the largest avascular (having few or no blood vessels) structure in the body and is low in cellularity. Therefore, its inherent capacity to heal after injury is poor. The clinical rationale of BRTX-100 is to deliver a high concentration of the patient’s own cultured MSCs into the site of pathology to promote healing and relieve pain.
|
|
We have developed a mesenchymal stem cell product candidate, BRTX-100, derived from autologous (or a person’s own) human bone marrow, cultured and formulated, in a proprietary method, specifically for introduction into a painful lumbar disc. The product candidate was developed utilizing in part the exclusive license described below under “Exclusive License.” As described below under “BRTX-100” and “Production and Delivery,” BRTX-100 is a hypoxic (low oxygen) stem cell product developed through a culturing process. In order to enhance the survivability of our bone marrow-derived MSCs in the avascular environment of the damaged disc, BRTX-100 is designed to expand under hypoxic conditions. This process is intended to result in a large cell count population with enhanced viability and therapeutic potential following injection into the injured disc.
In February 2017, pursuant to an IND application, we received authorization from the FDA to commence a Phase 2 clinical trial investigating the use of BRTX-100, our lead cell therapy candidate, in the treatment of chronic lower back pain arising from degenerative disc disease. We have commenced our Phase 2 clinical trial through the execution of a CRO agreement with PRC Clinical, the execution of clinical trial site agreements, patient enrollment, the commencement of patient procedures, the purchase of manufacturing equipment and the expansion of our laboratory to include capabilities for clinical production. We believe that, based upon our periodic reports to the FDA as to the commencement of the clinical trial, the existing IND remains effective.
In addition to developing BRTX-100, we may also seek to sublicense the technology to a strategic third party, who may assist in gaining FDA approval for a lumbar disc indication, or third parties for use in connection with cellular-based developmental programs with regard to disc and spine related conditions.
We have established a laboratory, which includes a clean room facility, to perform the production of cell products (including BRTX-100) for use in our clinical trials, for third party cell products or for general research purposes. We may also use this laboratory to develop our pipeline of future products and expand our stem cell-related IP. See “Laboratory” and “Technology; Research and Development” below.
In March 2022, a United States patent related to BRTX-100 was issued. We have been granted exclusive license rights with respect to the patent. See “Exclusive License” below.
BRTX-100
Our lead product candidate, BRTX-100, is an autologous hypoxic (low oxygen) cultured mesenchymal stem cell product derived from a patient’s own bone marrow and formulated with a proprietary biomaterial carrier (platelet lysate) to increase potency, viability and survivability. We have designed the cryopreserved sterile cellular product candidate to be provided in vials for injection into painful lumbar discs. We anticipate the product candidate will be delivered using a standard 20 gauge 3.5 inch introducer needle and a 25 gauge 6 inch needle that will extend into the disc center upon delivery. Upon regulatory approval, we plan to provide training to medical practitioners with regard to the approved injection procedure. It is anticipated that the delivery of the product candidate will be a 30 minute procedure.
|
|
Mesenchymal stem cells used in BRTX-100 are similar to other MSCs under development by others; however, in order to enhance the survivability of our bone marrow-derived MSCs in the avascular environment of the damaged disc, BRTX-100 is designed to expand under hypoxic conditions for a period of approximately three weeks. This process is intended to result in an approximate 40 million cell count population with enhanced viability and therapeutic potential following injection locally into injured spinal discs. Publications and scientific literature have indicated that MSCs preconditioned in hypoxic environment show enhanced skeletal muscle regeneration properties and improved impacts upon circulation and vascular formation compared to MSCs cultured under normoxic (normal oxygen) conditions.
In August 2018, the Journal of Translational Medicine published the results of our study evaluating the benefits of long-term hypoxic culturing of human bone marrow-derived MSCs.
In September 2021, we were awarded a National Institutes of Health Small Business Technology Transfer (STTR) Phase 1 grant for $256,000 to evaluate the therapeutic effects on our hypoxic cultured bone marrow derived mesenchymal stem cells (BRTX-100) after encapsulation with a PEG-peptide hydrogel. The work is being done in collaboration with Washington University of St. Louis.
Since June 2022, we have entered into clinical trial agreements with 16 sites to conduct our Phase 2 clinical trial targeting chronic lumbar disc disease.
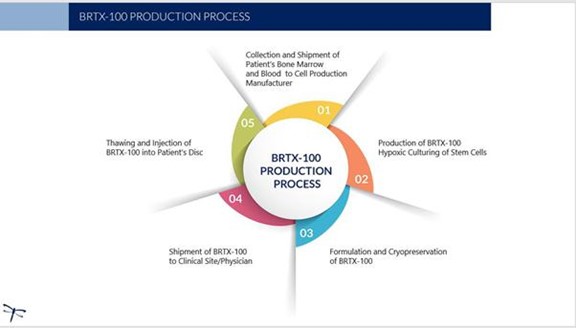
Production and Delivery
The production of our product candidate, BRTX-100, begins with the physician collecting bone marrow from the patient under local anesthesia. Peripheral blood is also collected from the patient. The physician will then send the patient’s bone marrow and blood samples to our laboratory (or a contract laboratory) for culturing and formulation. The hypoxic culturing process is intended to result in the selection of a cell population that is suitable for an improved possibility of survival in the internal disc environment. We anticipate that the cell culturing process and product formulation will take approximately three weeks, with an additional two weeks required for quality control testing required to meet product release criteria. We will then send the therapeutic cryopreserved stem cells (BRTX-100) in a sterile vial back to the physician’s offices where it will undergo a controlled thaw prior to the procedure. The price structure for the procedure and our services has not been determined and no assurances can be given as to the effect that such price structure will have on the marketability of such procedure and services. The following illustrates the process:
|
|
Exclusive License
Pursuant to our license agreement with Regenerative Sciences, LLC, or Regenerative, that became effective in April 2012, or the Regenerative License Agreement, we have obtained, among other things, a worldwide (excluding Asia and Argentina), exclusive, royalty-bearing license from Regenerative to utilize or sublicense a certain method for culturing cells for use in our developmental program involving disc and spine conditions, including protruding or painful discs and the treatment of avascular zones. The investigational technology that has been licensed is an advanced stem cell culture and injection procedure that may offer relief from lower back pain, buttock and leg pain, and numbness and tingling in the leg and foot. Pursuant to the Regenerative License Agreement, we have also obtained a worldwide, exclusive, royalty-bearing license from Regenerative to utilize or sublicense a certain investigational curved needle device for the administration of specific cells and/or cell products to the disc and/or spine (and other parts of the body). It will be necessary to advance the design of this investigational device to facilitate the delivery of substances, including living cells, to specific locations within the body and minimize the potential for damage to nearby structures.
The patents that are the subject of the Regenerative License Agreement have been assigned to Regenexx, LLC which we have been advised by Regenerative is an affiliate of Regenerative.
Animal Study
The efficacy and safety of our product candidate, BRTX-100, has been tested in a degenerative intervertebral rabbit disc model. In this study, 80 rabbits underwent surgery to create a puncture in the discs. Four weeks post-surgery, each rabbit had either contrast, a biomaterial carrier or BRTX-100 injected into the discs. In order to study the biodistribution and efficacy of BRTX-100, the rabbits were evaluated at day 56 and day 120.
The key safety findings of the animal study are as follows:
| ● | There was no evidence or observation of gross toxicity related to the administration of BRTX-100 at either time point. The clinical pathology across both groups and time points were within expected normal historical ranges and under the conditions of the test. No abnormalities (including fractures or overt signs of lumbar disc disease) were identified after review of the radiographic images taken at both endpoints for both groups. No toxicity or adverse finding was evident in the systemic tissues or the discs of animals receiving BRTX-100. |
|
|
| ● | There was no detectable presence of human cells (BRTX-100) observed at the day 56 interim time point. This is consistent with the proposed mechanism of action that BRTX-100 acts through a paracrine effect of secreted growth and immunomodulation factors. |
The key efficacy findings of the animal study are as follows:
| ● | BRTX-100 showed a statistically significant DHI (disc height increase) over the control group at day 120. | |
| ● | BRTX-100 showed a statistically significant improvement in disc histology over the control group at day 120 as graded by a validated histology scale. BRTX-100 showed a significant improvement in the cellularity and matrix of the disc when compared to the control at day 120. |
Clinical Trial
Pursuant to an IND application we submitted to the FDA, we have obtained authorization to commence a Phase 2 clinical trial investigating the use of BRTX-100, our lead cell therapy candidate, in the treatment of chronic lower back pain arising from degenerative disc disease. We have commenced our Phase 2 clinical trial through the execution of a CRO agreement with PRC Clinical, the execution of clinical trial agreements with 16 sites, patient enrollment, the commencement of patient procedures, the purchase of manufacturing equipment and the expansion of our laboratory to include capabilities for clinical production.
The following describes the Phase 2 clinical trial authorized by the FDA:
A Phase 2 Prospective, Double-Blinded, Placebo Controlled, Randomized Study
| ● | General |
| ● | 99 patients; randomized 2:1, BRTX-100 to control, 40 million cells/dose | |
| ● | 10-20 clinical trial sites (we intend to utilize 15 clinical trial sites) | |
| ● | Primary efficacy endpoint at 12 months | |
| ● | Patient safety and efficacy follow up at 24 months | |
| ● | Included subjects must have only one symptomatic diseased disc | |
| ● | Included subjects must have current diagnosis of chronic lumbar disc disease typical pain with degeneration of a single disc confirmed by history, exam, radiography, or other acceptable means | |
| ● | Included subjects must have exhausted previous conservative non-operative therapies |
|
|
| ● | Primary Efficacy Endpoint |
| ● | Responder endpoint - percentage of patients that meet the improvement in function and reduction in pain threshold | |
| ● | Improvement in function defined as at least a 30% increase in function based on the Oswestry questionnaires (ODI) | |
| ● | Reduction of pain defined as at least a 30% decrease in pain as measured using the Visual Analogue Scale (VAS) |
| ● | Additional or Secondary Endpoints |
| ● | Clinical response at 12 months | |
| ● | Changes from baseline in pain as assessed with the VAS score and ODI at weeks 2, 12, 26, 52 and 104 | |
| ● | Changes from baseline in function as assessed with the ODI at weeks 2, 12, 26, 52 and 104 | |
| ● | Changes from baseline in function as assessed by Roland Morris Disability Questionnaire (RMDQ) at weeks 26, 52 and 104 | |
| ● | Changes from baseline function as assessed by Functional Rating Index (FRI) at weeks 12, 52 and 104 | |
| ● | Changes from baseline Quality of Life assessment (SF-12 questionnaire) scores at weeks 2, 12, 26, 52 and 104 |
In December 2021, we entered into a Master Service Agreement with Professional Research Consulting Inc. d/b/a PRC Clinical, a contract research organization, or CRO, specializing in clinical trial management, to conduct our Phase 2 clinical trial.
In April 2023, we announced that we had completed enrollment for the safety run-in component of our Phase 2 clinical study of BRTX-100.
In May 2023, we announced that we had signed a clinical trial agreement with Northwell Health, New York State’s largest health care provider and private employer, pursuant to which Northwell Health is participating in our Phase 2 clinical study of BRTX-100.
In June 2023, we announced that the final subject in our BRTX-100 Phase 2 clinical trial safety cohort had been dosed.
In June 2023, we announced that the independent Data Safety Monitoring Board, which is overseeing our Phase 2 clinical trial, unanimously recommended the continuation of our study in accordance with the version of the protocol with no changes.
The FDA approval process can be lengthy, expensive and uncertain and there is no guarantee that the clinical trial(s) will be completed or that the product will ultimately receive approval or clearance.
As an alternative to undertaking any necessary clinical trials ourselves, we may explore the licensing of our rights with respect to our product candidate, BRTX-100, to a strategic partner. Such an arrangement could possibly eliminate or significantly reduce the need to raise the substantial capital needed to commence and complete the clinical trials and undertake the commercialization of BRTX-100 and would provide licensing-related revenue to us in lieu of product sales revenue. No assurance can be given that any licensing agreement will be entered into, whether upon commercially reasonable terms or otherwise.
|
|
Defined Health Report
In March 2018, we engaged Defined Health, a business development and strategy consulting firm, to conduct an independent review of BRTX-100. Defined Health has worked with many of the leading companies in the pharmaceutical, biotech and healthcare industries for over 25 years.
The review was intended to collect informed, independent opinions regarding BRTX-100 among key opinion leaders, or KOLs (i.e., orthopedic surgeons specializing in back and spine surgery with experience in stem cell therapy), who, upon studying applicable clinical material, could offer opinions regarding the future therapeutic potential of BRTX-100.
As noted in the Defined Health report, the KOLs indicated that stem cell therapies have great potential to treat chronic lumbar disc disease and other therapeutic areas. The KOLs reacted positively to the value proposition of our product candidate, BRTX-100, and were optimistic that the clinical data presented to date is likely to be mirrored in future clinical investigations. Given the opportunity, the KOLs indicated that they would likely participate in a clinical trial should it be offered at their center and that they would recommend the study to appropriately eligible patients. The report indicated that, if BRTX-100 were to be granted FDA approval, the KOLs anticipate that it would be integrated into the standard of care for eligible chronic lumbar disc disease patients.
Similar Therapies
Human data from studies of therapies comparative to BRTX-100 have shown reduced pain, increased function, and an absence of significant safety issues with a durable response, as shown below:

|
|
Impact on Public Health
The United States is the world’s leading consumer of hydrocodone (99%) and oxycodone (83%) and leads the world in per capital consumption of such drugs (twice as much as second ranked Canada). In 2020, 91,000 persons in the United States died from overdoses.
Total annual healthcare and lost productivity costs in the United States related to pain, including headache, back pain and neck pain, are estimated to be $600 billion, which is twice the annual costs related to heart disease and greater than the combined annual costs related to cancer and diabetes.
Metabolic Brown Adipose (Fat) Program
Since June 2011, we have been engaging in pre-clinical research efforts with respect to an investigational platform technology utilizing brown adipose (fat) derived stem cells, or BADSCs, for therapeutic purposes. We have labeled this initiative our ThermoStem Program.
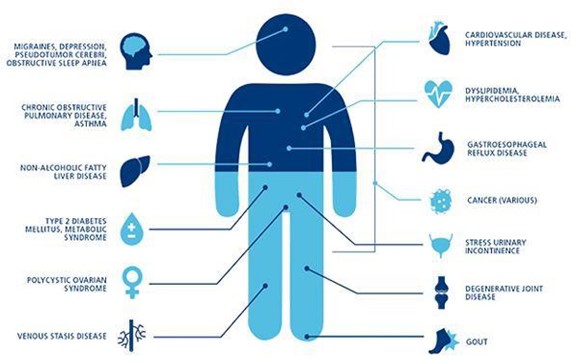
Brown fat is a specialized adipose (fat) tissue found in the human body that plays a key role in the evolutionarily conserved mechanisms underlying thermogenesis (generation of non-shivering body heat) and energy homeostasis in mammals - long known to be present at high levels in hibernating mammals and human newborns. Recent studies have demonstrated that brown fat is present in the adult human body and may be correlated with the maintenance and regulation of healthy metabolism, thus potentially being involved in caloric regulation. The pre-clinical ThermoStem Program involves the use of a cell-based (brown adipose tissue construct) treatment for metabolic disease, such as type 2 diabetes, obesity, hypertension and other metabolic disorders, as well as cardiac deficiencies. The diseases, disorders and syndromes that may be targeted by our ThermoStem Program are as follows:
|
|
We have had initial success in transplanting the brown adipose tissue construct in animals, and we are currently exploring ways to deliver into humans. Even though present, BAT mass is very low in healthy adults and even lower in obese populations. Therefore, it may not be sufficient to either naturally impact whole body metabolism, or to be targeted by drugs intended to increase its activity in the majority of the population. Increasing BAT mass is crucial in order to benefit from its metabolic activity and this is what our ThermoStem Program seeks to accomplish. We may also identify other naturally occurring biologics and chemically engineered molecules that may enhance brown adipose tissue performance and activity.
Obesity, the abnormal accumulation of white fat tissue, leads to a number of metabolic disorders and is the driving force behind the rise of type 2 diabetes and cardiovascular diseases worldwide. Pharmacological efforts to alter metabolic homeostasis through modulating central control of appetite and satiety have had limited market penetration due to significant psychological and physiological safety concerns directly attributed to modulating these brain centers. Adipose tissue is one of the largest organs in the human body and plays a key role in central energy balance and lipid homeostasis. White and brown adipose tissues are found in mammals. White adipose tissue’s function is to store energy, whereas BAT specializes in energy expenditure. As discussed in a 2020 article published in the International Journal of Molecular Sciences, recent advancements in unraveling the mechanisms that control the induction, differentiation, proliferation, and thermogenic activity of BAT, along with the application of imaging technologies for human BAT visualization, have generated optimism that these advances may provide novel strategies for targeting BAT activation/thermogenesis, leading to efficacious and safe obesity targeted therapies.
We are developing a cell-based product candidate to target obesity and metabolic disorders using BADSCs. Our goal is to develop a bioengineered implantable brown adipose tissue construct intended to mimic ones naturally occurring in the human body. We have isolated and characterized a human multipotent stem cell population that resides within BAT depots. We have expanded these stem cells to clinically relevant numbers and successfully differentiated them into functional brown adipocytes. We intend to use adult stem cells that may be differentiated into progenitor or fully differentiated brown adipocytes, or a related cell type, which can be used therapeutically in patients. We are focusing on the development of treatment protocols that utilize allogeneic cells (i.e., stem cells from a genetically similar but not identical donor).
|
|
In order to deliver these differentiated cells into target locations in vivo, we seeded BADSCs onto 3-dimensional biological scaffolds. Pre-clinical animal models of diet-induced obesity, that were transplanted with differentiated BADSCs supported by a biological scaffold, presented significant reductions in weight and blood glucose levels compared to scaffold only controls. We are identifying technology for in vivo delivery in small animal models. Having completed our proof of concept using our BAT in small animals, we are currently developing our next generation BAT. It is anticipated that this next version will contain a higher purity of BADSC and a greater percent of functional brown adipocytes, which is expected to increase the therapeutic effect compared to our first generation product. In addition, we are exploring the delivery of the therapeutic using encapsulation technology, which will only allow for reciprocal exchange of small molecules between the host circulation and the BAT implant. We expect that encapsulation may present several advantages over our current biological scaffolds, including prevention of any immune response or implant rejection that might occur in an immunocompetent host and an increase in safety by preventing the implanted cells from invading the host tissues. We have developed promising data on the loading of human stem cell-derived tissue engineered brown fat into an encapsulation device to be used as a cell delivery system for our metabolic platform program for the treatment of type 2 diabetes, obesity, hyperlipidemia and hypertension. This advancement may lead to successful transplantation of brown fat in humans. We are evaluating the next generation of BAT constructs that will first be tested in small animal models. No assurance can be given that this delivery system will be effective in vivo in animals or humans. Our allogeneic brown adipose derived stem cell platform potentially provides a therapeutic and commercial model for the cell-based treatment of obesity and related metabolic disorders.
In February 2014, our research with regard to the identification of a population of brown adipose derived stem cells was published in Stem Cells, a respected stem cell journal.
In March 2014, we entered into a Research Agreement with Pfizer Inc., a global pharmaceutical company. Pursuant to the Research Agreement with Pfizer, we were engaged to provide research and development services with regard to a joint study of the development and validation of a human brown adipose cell model. The Research Agreement with Pfizer provided for an initial payment to us of $250,000 and the payment of up to an additional $525,000 during the two-year term of the Agreement, all of which has been received. The Research Agreement expired upon completion of the services provided for therein.
In August 2015, we entered into a one year research collaboration agreement with the University of Pennsylvania with regard to the understanding of brown adipose biology and its role in metabolic disorders. In September 2018, we entered into a one year research collaboration agreement with the University of Pennsylvania pursuant to which the university was provided access to our proprietary brown adipose tissue cells for research purposes. No amounts were payable by or to us pursuant to either agreement.
|
|
In September 2015, a United States patent related to the ThermoStem Program was issued to us.
In April 2017, an Australian patent related to the ThermoStem Program was issued to us.
In December 2017, a Japanese patent related to the ThermoStem Program was issued to us.
In January 2019, a United States patent related to the ThermoStem Program was issued to us.
In October 2019, an Australian patent related to the ThermoStem Program was issued to us.
In October 2019, an Israeli patent related to the ThermoStem Program was issued to us.
In March 2020, a United States patent related to our ThermoStem Program was issued to us.
In March 2020, our collaboration with the University of Pennsylvania resulted in a publication in Cell Reports, a respected peer reviewed journal, with regard to our ThermoStem Program.
In April 2020, a European patent related to our ThermoStem Program was issued to us. This European patent was validated in Belgium, France, Germany, Italy, Poland, Spain, Sweden, Switzerland, and the United Kingdom.
In May 2020, an Israeli patent related to our ThermoStem Program was issued to us.
In January 2021, a European patent related to our ThermoStem Program was issued to us. This European patent was validated in France, Germany, Italy, Spain, and the United Kingdom.
In March 2021, a United States patent related to our ThermoStem Program was issued to us.
In June 2021, a Japanese patent related to our ThermoStem Program was issued to us.
In July 2021, a United States patent related to our ThermoStem Program was issued to us.
In August 2021, an Australian patent related to our ThermoStem Program was issued to us.
In February 2022, a Japanese patent related to our ThermoStem Program was issued to us.
In March 2022, an Israeli patent related to our ThermoStem Program was issued to us.
In December 2022, we announced that we were awarded a Small Business Innovation Research (SBIR) Phase 1 grant from Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health to enable the development and the evaluation of our ThermoStem Program for the treatment of polycystic ovary syndrome (PCOS). The work is to be done in collaboration with Dr. Sheng Wu, Associate Professor, Center for Metabolic Disease Research at Temple University.
In June 2023, a Japanese patent related to our ThermoStem Program was issued to us.
|
|
In June 2023, a United States patent related to our ThermoStem Program was issued to us.
In July 2023, a European patent related to our ThermoStem Program was issued to us. This European patent was validated in France, Germany, Italy, Spain, and the United Kingdom.
In December 2023, a United States patent related to our ThermoStem Program was issued to us.
We have completed proof of concept preclinical animal studies using our first generation brown adipose derived stem cells. We intend to undertake additional preclinical animal studies in order to optimize delivery and explore the feasibility of targeting additional indications. Such studies are planned to begin in 2024. Following the completion of such studies, if successful, we intend to file an IND with the FDA and initiate a clinical trial. The FDA approval process can be lengthy, expensive and uncertain and there is no guarantee of ultimate approval or clearance.
We anticipate that much of our development work in this area will take place at our laboratory facility, outside core facilities at academic, research or medical institutions, or contractors. See “Laboratory” below.
Curved Needle Device
Pursuant to the Regenerative License Agreement discussed under “Disc/Spine Program-Exclusive License” above, we have licensed and further developed an investigational curved needle device, or CND, that is a needle system with a curved inner cannula to allow access to difficult-to-locate regions for the delivery or removal of fluids and other substances. The investigational CND is intended to deliver stem cells and/or other therapeutic products or material to the interior of a human intervertebral disc, the spine region, or potentially other areas of the body. The device is designed to rely on the use of pre-curved nested cannulae that allow the cells or material to be deposited in the posterior and lateral aspects of the disc to which direct access is not possible due to outlying structures such as vertebra, spinal cord and spinal nerves. We anticipate that the use of the investigational CND will facilitate the delivery of substances, including living cells, to specific locations within the body and minimize the potential for damage to nearby structures. The investigational device may also have more general use applications. In August 2015, a United States patent for the CND was issued to the licensor, Regenerative. We anticipate that FDA approval or clearance will be necessary for the investigational CND prior to commercialization. We do not intend to utilize the CND in connection with our Phase 2 clinical trial with regard to BRTX-100. The FDA review and approval process can be lengthy, expensive and uncertain and there is no guarantee of ultimate approval or clearance.
Laboratory
We have established a laboratory in Melville, New York for research purposes and have built a cleanroom within the laboratory for the production of cell-based product candidates, such as BRTX-100, for use in a clinical trial, for third party cell products or for general research purposes.
We have expanded our laboratory to include capabilities for the clinical production of our pipeline of clinical and investigational cell therapy candidates. Our expanded cGMP facility includes process development space, ISO 7 cleanrooms and state-of-the-art equipment. We have expanded our research and development operations to include clinical manufacturing, a necessary step for our Phase 2 clinical trial for BRTX-100. The new facility has been designed to provide cGMP manufacturing according to FDA and European Medicines Agency regulations and guidelines to support clinical grade cell production. In May 2023, we announced that we had received a license from the New York State Department of Health to act as a tissue bank for the processing of mesenchymal stem cells from autologous donors.
|
|
As we develop our business and our stem cell product candidates, and we obtain regulatory approval, we will seek to establish ourselves as a key provider of adult stem cells for therapies and expand to provide cells in other market areas for stem cell therapy. We may also use outside laboratories specializing in cell therapy services and manufacturing of cell products.
Technology; Research and Development
We intend to utilize our laboratory or a third party laboratory in connection with cellular research activities. We also intend to obtain cellular-based therapeutic technology licenses and increase our IP portfolio. We intend to seek to develop potential stem cell delivery systems or devices. The goal of these specialized delivery systems or devices is to deliver cells into specific areas of the body, control the rate, amount and types of cells used in a treatment, and populate these areas of the body with sufficient stem cells so that there is a successful therapeutic result.
We also intend to perform research to develop certain stem cell optimization compounds, media designed to enhance cellular growth and regeneration for the purpose of improving pre-treatment and post-treatment outcomes.
In our Disc/Spine Program, thirteen patent applications have been filed with regard to technology that is the subject of the Regenerative License Agreement (see “Disc/Spine Program-Exclusive License” above). Regenerative has been issued a patent from one of these applications with regard to its curved needle therapeutic delivery device. This patent expires in March 2031. In addition, in March 2022, a United States patent related to BRTX-100 was issued. This patent expires in December 2029. Of the other eleven applications that were filed, four applications remain pending. The patents that are the subject of the Regenerative License Agreement have been assigned to Regenexx, LLC which we have been advised is an affiliate of Regenerative.
In our ThermoStem Program, we have one pending United States patent application and seven United States patents within three patent families. Four of the patents expire in June 2032 and three of the patents expire in April 2034. With regard to the first patent family in the ThermoStem Program, patent applications have been filed in five foreign jurisdictions (of which four applications have been granted as foreign patents and one application has lapsed). The patents expire in June 2032. With regard to the second patent family in the ThermoStem Program, patent applications have been filed in four foreign jurisdictions (of which four applications have been granted as foreign patents). The patents expire in April 2034. With regard to the third patent family in the ThermoStem Program, patent applications have been filed in four foreign jurisdictions.
Our patent applications and those of Regenexx, LLC are currently in prosecution (i.e., we and Regenexx, LLC are seeking issued patents).
|
|
In March 2014, we entered into a Research and Development Agreement with Rohto Pharmaceutical Co., Ltd., a Japanese pharmaceutical company, or Rohto. Pursuant to the Research and Development Agreement with Rohto, we were engaged to provide research and development services with regard to stem cells. The agreement with Rohto expired upon the completion of the services provided for therein.
We have secured registrations in the U.S. Patent and Trademark Office for the following trademarks:
| ● |  |
|
| ● | BRTX-100 | |
| ● | THERMOSTEM | |
| ● | BRTX |
The Dragonfly Logo is also registered with the U.S. Copyright Office.
We also have federal common law rights in the trademark BioRestorative Therapies and other trademarks and trade names used in the conduct of our business that are not registered.
Our success will depend in large part on our ability to develop and protect our proprietary technology. We intend to rely on a combination of patent, trade secret and know-how, copyright and trademark laws, as well as confidentiality agreements, licensing agreements, non-compete agreements and other agreements, to establish and protect our proprietary rights. Our success will also depend upon our ability to avoid infringing upon the proprietary rights of others, for if we are judicially determined to have infringed such rights, we may be required to pay damages, alter our services, products or processes, obtain licenses or cease certain activities.
During the years ended December 31, 2023 and 2022, we incurred $4,034,591 and $3,513,352, respectively, in research and development expenses.
Scientific Advisors
We have established a Scientific Advisory Board whose purpose is to provide advice and guidance in connection with scientific matters relating to our business. The Scientific Advisory Board has established a Disc Advisory Committee which focuses on matters relating to our Disc/Spine Program. Our Scientific Advisory Board members are Dr. Wayne Marasco (Chairman), Dr. Jason Lipetz, Dr. Wayne Olan, Dr. Joy Cavagnaro, Dr. Harvinder Sandhu and Dr. Christopher Plastaras. The Disc Advisory Committee members are Dr. Lipetz (Chairman), Dr. Olan, Dr. Sandhu and Dr. Plastaras. See Item 10 of this Annual Report (“Directors, Executive Officers and Corporate Governance – Scientific Advisory Board”) for a listing of the principal positions for Drs. Marasco, Lipetz, Olan, Cavagnaro, Sandhu and Plastaras.
|
|
Competition
We will compete with many pharmaceutical, biotechnology and medical device companies, as well as other private and public stem cell companies involved in the development and commercialization of cell-based medical technologies and therapies.
Regenerative medicine is rapidly progressing, in large part through the development of cell-based therapies or devices designed to isolate cells from human tissues. Most efforts involve cell sources, such as bone marrow, adipose tissue, embryonic and fetal tissue, umbilical cord and peripheral blood and skeletal muscle.
Companies working in the area of regenerative medicine with regard to the disc and spine include, among others, Mesoblast, SpinalCyte, DiscGenics and Isto Biologics. Companies that are developing products and therapies to combat obesity and diabetes include Novo Nordisk, Sanofi, Merck, Eli Lilly, Roche, Pfizer, Regeneron and Altimmune. The recent extensive use of both FDA-approved and compounded versions of glucagon-like peptide-1 (GLP-1) receptor agonist drug products, such as Wegovy and Ozempic (semaglutide) for the treatment of obesity has significantly increased the competition in the obesity market.
Many of our competitors and potential competitors have substantially greater financial, technological, research and development, marketing and personnel resources than we do. We cannot, with any accuracy, forecast when or if these companies are likely to bring their products and therapies to market in competition with those that we are pursuing.
The Biologics Price Competition and Innovation Act, or the BPCIA, sets forth an abbreviated pathway for the approval of biosimilar and interchangeable biological products that could be used by future competitors, if any, of our product candidates that are approved by the FDA as a biologic. For the FDA to approve a biosimilar product, it must find that there are no clinically meaningful differences between the reference product and the proposed biosimilar product. Interchangeability requires that a product is biosimilar to the reference product, and the product must be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. Under the BPCIA, an application for a biosimilar product cannot be submitted to the FDA until four years following approval of the reference product, and it may not be approved by the FDA until 12 years after the original branded product is approved under a biologics license application, or BLA.
We believe that, if any of our product candidates are approved as a biological product under a BLA, it should qualify for the 12-year period of exclusivity. However, there is a risk that the FDA could permit biosimilar applicants to reference approved biologics other than our therapeutic candidates, thus circumventing our exclusivity and potentially creating the opportunity for competition sooner than anticipated. Additionally, this period of regulatory exclusivity does not apply to companies pursuing regulatory approval via their own traditional BLA, rather than via the abbreviated pathway. Moreover, it is possible that a biosimilar product could be approved as “interchangeable” with our product and therefore substitutable for our product by a healthcare professional under applicable state laws.
|
|
We may also face increased competition from stem cell therapies performed by treatment centers that do not require FDA premarket approval. In August 2022, a federal District Court in the case of United States v. California Stem Cell Treatment Center, Inc. held that certain autologous adipose stem cell treatments were not “biological products” and therefore did not require FDA approval. The FDA has appealed the decision but, should it be upheld, we could face competition from stem cell clinics that would not be required to undergo the costly and time-consuming FDA approval process.
Set forth below is a comparison of BRTX-100 to Mesoblast’s adult stem cell biologic:
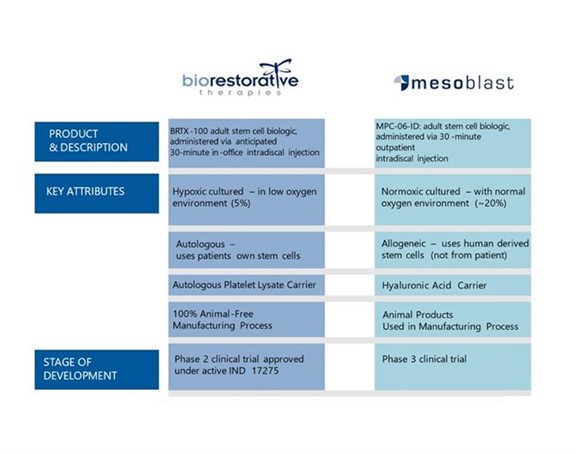
We believe that BRTX-100 has competitive advantages to Mesoblast’s product for the following reasons:
| ● | The use of autologous cells results in low to no risk of rejection, greater safety profile (introduction of viral/genetic) and a streamlined regulatory path | |
| ● | Hypoxic culturing creates increased cell proliferation, greater plasticity, increased paracrine effect and increased cell survival after application | |
| ● | Autologous platelet lysate provides growth factors that interact with the cells, allowing for better cell survival | |
| ● | Low to no risk of safety concerns related to immunological and zoonotic (animal to human) transmission |
|
|
Customers
Upon regulatory approval, our cell product candidates are intended to be marketed to physicians, other health care professionals, hospitals, research institutions, pharmaceutical companies and the military. It is anticipated that physicians who are trained and skilled in performing spinal injections will be the physicians most likely to treat discs with injections of BRTX-100 upon regulatory approval. These physicians would include interventional physiatrists (physical medicine physicians), pain management anesthesiologists, interventional radiologists and neurosurgeons.
Governmental Regulation
U.S. Government Regulation
The health care industry is highly regulated in the United States. The federal government, through various departments and agencies, state and local governments, and private third-party accreditation organizations, regulate and monitor the health care industry, associated products, and operations. The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, approval, manufacture, distribution and marketing of medical products, including drugs, biologics, and medical devices. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, post-approval monitoring, advertising, promotion, sampling and import and export of medical products. The following is a general overview of the laws and regulations pertaining to our business.
FDA Regulation of Stem Cell Treatment and Products
The FDA regulates the manufacture of human stem cell treatments and associated products under the authority of the Public Health Service Act, or PHSA, and the Federal Food, Drug, and Cosmetic Act, or FDCA. Stem cells can be regulated under the FDA’s Human Cells, Tissues, and Cellular and Tissue-Based Products Regulations, or HCT/Ps, or may also be subject to the FDA’s drug, biologic, or medical device regulations, each as discussed below.
Human Cells, Tissues, and Cellular and Tissue-Based Products Regulation
Under Section 361 of the PHSA, the FDA issued specific regulations governing the use of HCT/Ps in humans. Pursuant to Part 1271 of Title 21 of the Code of Federal Regulations, or CFR, or the HCT/P Regulations, the FDA established a unified registration and listing system for establishments that manufacture and process HCT/Ps. The regulations also include provisions pertaining to donor eligibility determinations; current good tissue practices covering all stages of production, including harvesting, processing, manufacture, storage, labeling, packaging, and distribution; and other procedures to prevent the introduction, transmission, and spread of communicable diseases.
The HCT/P Regulations define HCT/Ps as articles “containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion or transfer into a human recipient.” The HCT/P Regulations strictly constrain the types of products that may be regulated solely as HCT/P. Factors considered include the degree of manipulation, whether the product is intended for a homologous function, whether the product has been combined with noncellular or non-tissue components, and the product’s effect or dependence on the body’s metabolic function. In those instances where cells, tissues, and cellular and tissue-based products have been only minimally manipulated, are intended strictly for homologous use, have not been combined with noncellular or nontissue substances, and do not depend on or have any effect on the body’s metabolism, the manufacturer is only required to register with the FDA, submit a list of manufactured products, and adopt and implement procedures for the control of communicable diseases. If one or more of the above factors has been exceeded, the product would be regulated as a drug, biological product, or medical device rather than an HCT/P.
|
|
Because we are an enterprise in the early stages of operations and have not generated significant revenues from operations, it is difficult to anticipate the likely regulatory status of the array of products and services that we may offer. We believe that some of the adult autologous (self-derived) stem cells that will be used in our cellular therapy products and services, including the brown adipose (fat) tissue that we intend to use in our ThermoStem Program, may be regulated by the FDA as HCT/Ps under the HCT/P Regulations. However, the FDA may disagree with this position or conclude that some or all of our stem cell therapy products or services do not meet the applicable definitions and exemptions to the regulation. In July 2020, the FDA issued an updated guidance document entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use” that provides additional guidance on how FDA interprets the HCT/P Regulations, particularly the definition of the terms “minimally manipulated” and “homologous use.” In the guidance, the FDA stated it would exercise enforcement discretion until May 31, 2021 for products that do not comply with the HCT/P Regulations. As of that date, manufacturers of products marketed as HCT/Ps that do not comply with the HCT/P Regulations are subject to immediate FDA enforcement action. If we are not regulated solely under the HCT/P Regulations, we would need to expend significant resources to comply with the FDA’s broad regulatory authority under the FDCA. Historically, the U.S. federal courts have upheld the FDA’s authority to regulate stem cell products under the FDCA that do not comply with the FDA’s interpretations of the HCT/P Regulations. However, in August 2022, a federal District Court in the case of United States v. California Stem Cell Treatment Center, Inc. held that certain autologous adipose stem cell treatments that the FDA alleged were biological products were instead within the HCT/P definition and therefore did not require FDA approval. The FDA has appealed the decision but, should it be upheld, it could expand the types of stem cell products that are regulated solely under the HCT/P Regulations.
If regulated solely under the FDA’s HCT/P statutory and regulatory provisions, once our laboratory in the United States becomes operational, it will need to satisfy the following requirements, among others, to process and store stem cells:
| ● | registration and listing of HCT/Ps with the FDA; | |
| ● | donor eligibility determinations, including donor screening and donor testing requirements; | |
| ● | current good tissue practices, specifically including requirements for the facilities, environmental controls, equipment, supplies and reagents, recovery of HCT/Ps from the patient, processing, storage, labeling and document controls, and distribution and shipment of the HCT/Ps to the laboratory, storage, or other facility; | |
| ● | tracking and traceability of HCT/Ps and equipment, supplies, and reagents used in the manufacture of HCT/Ps; | |
| ● | adverse event reporting; | |
| ● | FDA inspection; and | |
| ● | abiding by any FDA order of retention, recall, destruction, and cessation of manufacturing of HCT/Ps. |
|
|
Non-reproductive HCT/Ps and non-peripheral blood stem/progenitor cells that are offered for import into the United States and regulated solely under Section 361 of the PHSA must also satisfy the requirements under 21 C.F.R. § 1271.420. Section 1271.420 requires that the importer of record of HCT/Ps notify the FDA prior to, or at the time of, importation and provide sufficient information for the FDA to make an admissibility decision. In addition, the importer must hold the HCT/P intact and under conditions necessary to prevent transmission of communicable disease until an admissibility decision is made by the FDA.
If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions including public warning letters, fines, consent decrees, orders of retention, recall or destruction of product, orders to cease manufacturing, and criminal prosecution. If any of these events were to occur, it could materially adversely affect us.
To the extent that our cellular therapy activities are limited to developing products and services outside the United States, as described in detail below, the products and services would not be subject to FDA regulation, but will be subject to the applicable requirements of the foreign jurisdiction. We intend to comply with all applicable foreign governmental requirements.
Drug and Biological Product Regulation
An HCT/P product that does not meet the criteria for being solely regulated under Section 361 of the PHSA will be regulated as a drug, device or biological product under the FDCA and/or Section 351 of the PHSA, and applicable FDA regulations. The FDA has broad regulatory authority over drugs and biologics marketed for sale in the United States. The FDA regulates the research, clinical testing, manufacturing, safety, effectiveness, labeling, storage, recordkeeping, promotion, distribution, and production of drugs and biological products. The FDA also regulates the export of drugs and biological products manufactured in the United States to international markets in certain situations.
The process required by the FDA before a drug or biologic may be marketed in the United States generally involves the following:
● completion of non-clinical laboratory tests, animal studies and formulation studies conducted according to Good Laboratory Practice, or GLP, or other applicable regulations;
● submission of an IND, which allows clinical trials to begin unless the FDA objects within 30 days;
● performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug or biologic for its intended use or uses conducted in accordance with FDA regulations and Good Clinical Practices, or GCP, which are international ethical and scientific quality standards meant to ensure that the rights, safety and well-being of trial participants are protected and that the integrity of the data is maintained;
|
|
● registration of clinical trials of FDA-regulated products and certain clinical trial information;
● preparation and submission to the FDA of a new drug application, or NDA, in the case of a drug or BLA in the case of a biologic;
● review of the product by an FDA advisory committee, where appropriate or if applicable;
● satisfactory completion of pre-approval inspection of manufacturing facilities and clinical trial sites at which the product, or components thereof, are produced to assess compliance with cGMP requirements and of selected clinical trial sites to assess compliance with GCP requirements; and
● FDA approval of an NDA or BLA which must occur before a drug or biologic can be marketed or sold.
Approval of an NDA requires a showing that the drug is safe and effective for its intended use and that the methods, facilities, and controls used for the manufacturing, processing, and packaging of the drug are adequate to preserve its identity, strength, quality, and purity. To obtain a BLA, a manufacturer must show that the proposed product is safe, pure, and potent and that the facility in which the product is manufactured, processed, packed, or held meets established quality control standards.
For purposes of an NDA or BLA approval by the FDA, human clinical trials are typically conducted in the following phases (which may overlap):
● Phase 1: The investigational product is initially given to healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. These trials may also provide early evidence on effectiveness. During Phase 1 clinical trials, sufficient information about the investigational product’s pharmacokinetics and pharmacologic effects may be obtained to permit the design of well-controlled and scientifically valid Phase 2 clinical trials.
● Phase 2: These clinical trials are conducted in a limited number of human subjects in the target population to identify possible adverse effects and safety risks, to determine the efficacy of the investigational product for specific targeted diseases and to determine dosage tolerance and dosage levels. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more costly Phase 3 clinical trials.
● Phase 3: Phase 3 clinical trials are undertaken after Phase 2 clinical trials demonstrate that a dosage range of the investigational product appears effective and has a tolerable safety profile. The Phase 2 clinical trials must also provide sufficient information for the design of Phase 3 clinical trials. Phase 3 clinical trials are conducted to provide statistically significant evidence of clinical efficacy and to further test for safety risks in an expanded human subject population at multiple clinical trial sites. These clinical trials are intended to further evaluate dosage, effectiveness and safety, to establish the overall benefit-risk profile of the investigational product and to provide an adequate basis for product labeling and approval by the FDA. In most cases, the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of an investigational drug or biologic.
|
|
All clinical trials must be conducted in accordance with FDA regulations, GCP requirements and their protocols in order for the data to be considered reliable for regulatory purposes. Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. These government regulations may delay or prevent approval of product candidates for a considerable period of time and impose costly procedures upon our business operations.
The FDA may require, or companies may pursue, additional clinical trials, referred to as Phase 4 clinical trials, after a product is approved. Such trials may be made a condition to be satisfied for continuing drug approval. The results of Phase 4 clinical trials can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA has authority to require sponsors to conduct post-marketing trials to specifically address safety issues identified by the agency.
Under the Pediatric Research Equity Act, or PREA, certain NDAs and BLAs and certain supplements to an NDA or BLA must contain data to assess the safety and efficacy of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of pediatric data or full or partial waivers. A sponsor who is planning to submit a marketing application for a drug that includes a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration submit an initial Pediatric Study Plan, or PSP, to the FDA within 60 days of an end-of-Phase 2 meeting or, if there is no such meeting, as early as practicable before the initiation of the Phase 3 or Phase 2/3 study. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach an agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from preclinical studies, early phase clinical trials, and/or other clinical development programs.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, manufacturing processes or facilities, require submission and FDA approval of a new NDA or BLA, or an NDA or BLA supplement, before the change can be implemented. An NDA or BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA and BLA supplements as it does in reviewing NDAs and BLAs.
Drug and biological products must also comply with applicable requirements, including monitoring and recordkeeping activities, manufacturing requirements, reporting to the applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling, or off-label use, limitations on industry-sponsored scientific and educational activities and requirements for promotional activities involving the internet. Although physicians may, in their independent professional medical judgment, prescribe legally available drugs for off-label uses, manufacturers typically may not market or promote such off-label uses.
|
|
We have determined that, under the FDA’s current interpretation of the applicable law, our BRTX-100 product candidate will be regulated as a biological product under the PHSA. Therefore, we will need to expend significant resources to ensure regulatory compliance. There is no assurance as to whether or when we will receive FDA approval of the BRTX-100 product candidate. The process of designing, conducting, compiling and submitting the non-clinical and clinical studies required for BLA approval is time-consuming, expensive and unpredictable. The process can take many years, depending on the product and the FDA’s requirements.
In addition, even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of onerous risk management plans, restrictions on distribution or use, or post-marketing trial requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product, including safety labeling or imposition of a Risk Evaluation and Mitigation Strategy, or REMS, the requirement to conduct post-market studies or clinical trials or even complete withdrawal of the product from the market. Delay in obtaining, or failure to obtain, regulatory approval for our products, or obtaining approval but for significantly limited use, would harm our business. Further, we cannot predict what adverse governmental regulations may arise from future United States or foreign governmental action.
If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure of our products, total or partial shutdown of our production, withdrawal of approvals, and criminal prosecutions. If any of these events were to occur, it could materially adversely affect us.
FDA Expedited Review Programs
The FDA is authorized to expedite the review of NDAs and BLAs in several ways. Under the Fast Track program, the sponsor of a drug or biologic product candidate may request the FDA to designate the product for a specific indication as a Fast Track product concurrent with or after the filing of the IND. Drug and biologic products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product candidate and the specific indication for which it is being studied.
In addition to other benefits, such as the ability to have greater interactions with the FDA, the FDA may initiate review of sections of a Fast Track NDA or BLA before the application is complete, a process known as rolling review.
|
|
Any product submitted to the FDA for marketing, including under a Fast Track program, may also be eligible for the following other types of FDA programs intended to expedite development and review:
● Breakthrough therapy designation. To qualify for the breakthrough therapy program, product candidates must be intended to treat a serious or life-threatening disease or condition, and preliminary clinical evidence must indicate that such product candidates may demonstrate substantial improvement on one or more clinically significant endpoints over existing therapies. The FDA will seek to ensure the sponsor of a breakthrough therapy product candidate receives intensive guidance on an efficient drug development program, intensive involvement of senior managers and experienced staff on a proactive, collaborative and cross-disciplinary review, and rolling review.
● Priority review. A product candidate is eligible for priority review if it treats a serious condition and, if approved, it would be a significant improvement in the safety or effectiveness of the treatment, diagnosis or prevention of a serious condition compared to marketed products. The FDA aims to complete its review of priority review applications within six months as opposed to ten months for standard review.
● Accelerated approval. Drug or biologic products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval. Accelerated approval means that a product candidate may be approved on the basis of adequate and well-controlled clinical trials establishing that the product candidate has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity and prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug or biologic product candidate receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials. As a result of the FDA’s controversial use of the accelerated approval pathway for an Alzheimer’s drug (aducanumab), Congress revised the accelerated approval process as a part of the Food and Drug Omnibus Reform Act of 2022 to provide the FDA with additional authorities to enforce the post-approval study requirements and to withdraw approvals when those requirements are not met.
Fast Track designation, breakthrough therapy designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Further, the FDA is authorized to accelerate review and approval of products designated as regenerative advanced therapies. A product is eligible for this designation if it is a regenerative medicine advanced therapy, or RMAT (which may include a cell therapy), that is intended to treat, modify, reverse or cure a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such disease or condition. The benefits of a RMAT designation include early interactions with the FDA to expedite development and review, benefits available to breakthrough therapies, potential eligibility for priority review and accelerated approval based on surrogate or intermediate endpoints.
|
|
Medical Device Regulation
The FDA also has broad authority over the regulation of medical devices marketed for sale in the United States. The FDA regulates the research, clinical testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, promotion, distribution, and production of medical devices. The FDA also regulates the export of medical devices manufactured in the United States to international markets.
Under the FDCA, medical devices are classified into one of three classes, Class I, Class II, or Class III, depending upon the degree of risk associated with the medical device and the extent of control needed to ensure safety and effectiveness. Class I devices are subject to the lowest degree of regulatory scrutiny because they are considered low risk devices and need only comply with the FDA’s General Controls. The General Controls include compliance with the registration, listing, adverse event reporting requirements, and applicable portions of the Quality System Regulation as well as the general misbranding and adulteration prohibitions.
Class II devices are subject to the General Controls as well as certain Special Controls such as 510(k) premarket notification. Class III devices are subject to the highest degree of regulatory scrutiny and typically include life supporting and life sustaining devices and implants. They are subject to the General Controls and Special Controls that include a premarket approval application, or PMA. “New” devices are automatically regulated as Class III devices unless they are shown to be low risk, in which case they may be subject to de novo review to be moved to Class I or Class II. Clinical research of an investigational device is subject to the FDA’s Investigational Device Exemption, or IDE, regulations. Nonsignificant risk devices are subject to abbreviated requirements that do not require a submission to the FDA but must have Institutional Review Board (IRB) approval and comply with other requirements pertaining to informed consent, labeling, recordkeeping, reporting, and monitoring. Significant risk devices require the submission of an IDE application to the FDA and the FDA’s approval of the IDE application.
The FDA premarket clearance and approval process can be lengthy, expensive and uncertain. It generally takes three to twelve months from submission to obtain 510(k) premarket clearance, although it may take longer. Approval of a PMA could take one to four years, or more, from the time the application is submitted and there is no guarantee of ultimate clearance or approval. Securing FDA clearances and approvals may require the submission of extensive clinical data and supporting information to the FDA. Additionally, the FDA actively enforces regulations prohibiting marketing and promotion of devices for indications or uses that have not been cleared or approved by the FDA. In addition, modifications or enhancements of products that could affect the safety or effectiveness or effect a major change in the intended use of a device that was either cleared through the 510(k) process or approved through the PMA process may require further FDA review through new 510(k) or PMA submissions.
In the event we develop processes, products or services which qualify as medical devices subject to FDA regulation, we intend to comply with such regulations. If the FDA determines that our products are regulated as medical devices and we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions from public warning letters, application integrity proceedings, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure of our products, total or partial shutdown of our production, withdrawal of approvals, and criminal prosecutions. If any of these events were to occur, it could materially adversely affect us.
|
|
Current Good Manufacturing Practices and other FDA Regulations of Cellular Therapy Products
Products that fall outside of the HCT/P regulations and are regulated as drugs, biological products, or devices must comply with applicable cGMP regulations. These cGMPs and related quality standards are designed to ensure the products that are processed at a facility meet the FDA’s applicable requirements for identity, strength, quality, sterility, purity, and safety. In the event that our domestic United States operations are subject to the FDA’s drug, biological product, or device regulations, we intend to comply with the applicable cGMPs and quality regulations.
If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure of our products, total or partial shutdown of our production, withdrawal of approvals, and criminal prosecutions. If any of these events were to occur, it could materially adversely affect us.
Promotion of Foreign-Based Cellular Therapy Treatment— “Medical Tourism”
We may establish, or license technology to third parties in connection with their establishment of, adult stem cell therapy facilities outside the United States. We also intend to work with hospitals and physicians to make the stem cell-based therapies available for patients who travel outside the United States for treatment. “Medical tourism” is defined as the practice of traveling across international borders to obtain health care.
The Federal Trade Commission, or the FTC, has the authority to regulate and police advertising of medical treatments, procedures, and regimens in the United States under the Federal Trade Commission Act, or the FTCA. The FTC has regulatory authority to prevent unfair and deceptive practices and false advertising. Specifically, the FTC requires advertisers and promoters to have a reasonable basis to substantiate and support claims. The FTC has many enforcement powers, one of which is the power to order disgorgement by promoters deemed in violation of the FTCA of any profits made from the promoted business and can order injunctions from further violative promotion. Advertising that we may utilize in connection with our medical tourism operations will be subject to FTC regulatory authority, and we intend to comply with such regulatory régime. Similar laws and requirements are likely to exist in other countries and we intend to comply with such requirements.
Federal Regulation of Clinical Laboratories
The federal Clinical Laboratory Improvement Amendments, or CLIA, provides the Centers for Medicare and Medicaid Services, or CMS, authority over all laboratory testing, except research, that is performed on humans in the United States. The Division of Laboratory Services, within the Survey and Certification Group, under the Center for Medicaid and State Operations, or CMSO, has the responsibility for implementing the CLIA program.
|
|
The CLIA program is designed to establish quality laboratory testing by ensuring the accuracy, reliability, and timeliness of patient test results. Under CLIA, a laboratory is a facility that does laboratory testing on specimens derived from humans and used to provide information for the diagnosis, prevention, treatment of disease, or impairment of, or assessment of health. Laboratories that handle stem cells and other biologic matter are, therefore, included under the CLIA program. Under the CLIA program, laboratories must be certified by the government, satisfy governmental quality and personnel standards, undergo proficiency testing, be subject to inspections, and pay fees. To the extent that our business activities require CLIA certification, we intend to obtain and maintain such certification. If we are subject to CLIA, the failure to comply with CLIA standards could result in suspension, revocation, or limitation of a laboratory’s CLIA certificate. In addition, fines or criminal penalties could also be levied. If any of these events were to occur, it could impact our business operations.
Health Insurance Portability and Accountability Act—Protection of Patient Health Information
We may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The Health Insurance Portability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information on certain types of individuals and organizations. In addition, certain state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other and from HIPAA in significant ways and may not have the same effect, thus complicating compliance efforts. Further, we may need to also comply with additional federal or state privacy laws and regulations that may apply to certain diagnoses, such as HIV/AIDS, to the extent that they apply to us.
The Department of Health and Human Services, or HHS, through its Office for Civil Rights, investigates breach reports and determines whether administrative or technical modifications are required and whether civil or criminal sanctions should be imposed. Companies failing to comply with HIPAA and the implementing regulations may also be subject to civil money penalties or in the case of knowing violations, potential criminal penalties, including monetary fines, imprisonment, or both. In some cases, the State Attorneys General may seek enforcement and appropriate sanctions in federal court.
Other Applicable U.S. Laws
In addition to the above-described regulation by United States federal and state government, the following are other federal and state laws and regulations that could directly or indirectly affect our ability to operate the business:
| ● | state and local licensure, registration, and regulation of the development of pharmaceuticals and biologics; | |
| ● | state and local licensure of medical professionals; | |
| ● | state statutes and regulations related to the corporate practice of medicine; |
|
|
| ● | laws and regulations administered by U.S. Customs and Border Protection related to the importation of biological material into the United States; | |
| ● | other laws and regulations administered by the FDA; | |
| ● | other laws and regulations administered by HHS; | |
| ● | state and local laws and regulations governing human subject research and clinical trials; | |
| ● | the federal physician self-referral prohibition, also known as Stark Law, and any state equivalents to Stark Law; | |
| ● | the federal False Claims Act, or FCA; | |
| ● | the federal Anti-Kickback Statute, or AKS, and any state equivalent statutes and regulations; | |
| ● | federal and state coverage and reimbursement laws and regulations; | |
| ● | state and local laws and regulations for the disposal and handling of medical waste and biohazardous material; | |
| ● | Occupational Safety and Health Administration, or OSHA, regulations and requirements; | |
| ● | the Intermediate Sanctions rules of the IRS providing for potential financial sanctions with respect to “excess benefit transactions” with tax-exempt organizations; | |
| ● | the Physician Payments Sunshine Act (in the event that our products are classified as drugs, biologics, devices or medical supplies and are reimbursed by Medicare, Medicaid or the Children’s Health Insurance Program); | |
| ● | state and other federal laws addressing the privacy of health information; and | |
| ● | state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare professionals and other potential referral sources, state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare professionals or marketing expenditures, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Violation of any of the laws described above or any other governmental laws and regulations may result in penalties, including civil and criminal penalties, damages, fines, the curtailment or restructuring of operations, the exclusion from participation in federal and state healthcare programs and imprisonment. Furthermore, efforts to ensure that business activities and business arrangements comply with applicable healthcare laws and regulations can be costly for manufacturers of branded prescription products.
Foreign Government Regulation
In general, we will need to comply with the government regulations of each individual country in which our therapy centers are located and products are to be distributed and sold. These regulations vary in complexity and can be as stringent, and on occasion even more stringent, than FDA regulations in the United States. Due to the fact that there are new and emerging cell therapy regulations that have recently been drafted and/or implemented in various countries around the world, the application and subsequent implementation of these new and emerging regulations have little to no precedence. Therefore, the level of complexity and stringency is not always precisely understood for each country, creating greater uncertainty for the international regulatory process. Furthermore, government regulations can change with little to no notice and may result in up-regulation of our product(s), thereby creating a greater regulatory burden for our cell processing technology products. We have not yet thoroughly explored the applicable laws and regulations that we will need to comply with in foreign jurisdictions. It is possible that we may not be permitted to expand our business into one or more foreign jurisdictions.
|
|
We do not have any definitive plans or arrangements with respect to the establishment by us of stem cell therapy clinics in any country. We intend to explore any such opportunities as they arise.
Offices
Our principal executive offices are located at 40 Marcus Drive, Suite One, Melville, New York, and our telephone number is (631) 760-8100. Our website is www.biorestorative.com. Our internet website and the information contained therein or connected thereto are not intended to be incorporated by reference into this Annual Report.
Employees
We currently have 11 employees, all of whom are full-time employees. We believe that our employee relations are good.
| ITEM 1A. | RISK FACTORS. |
The risk factors listed in this section provide examples of risks, uncertainties and events that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. Readers should be aware that the occurrence of any of the events described in these risk factors could have a material adverse effect on our business, results of operations and financial condition. We undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events, or otherwise.
Preceding the full risk factors is a list of certain of the risk factors that follow. Reference is made to the complete risk factors for a full description of the risks involved.
Risks Related to Our Business Generally
| ● | We have a limited operating history; we have incurred substantial losses since inception; we expect to continue to incur losses for the near term. | |
| ● | We will need to obtain a significant amount of financing to complete our clinical trials and implement our business plan. | |
| ● | We will need to enter into agreements in order to implement our business strategy. | |
| ● | We depend on our executive officers and on our ability to attract and retain additional qualified personnel. |
|
|
Risks Related to Our Cell Therapy Product Development Efforts
| ● | Our future success is significantly dependent on the timely and successful development and commercialization of BRTX-100, our lead product candidate for the treatment of chronic lumbar disc disease; if we encounter delays or difficulties in the development of this product candidate, as well as any other product candidates, our business prospects would be significantly harmed. | |
| ● | We may experience delays and other difficulties in enrolling a sufficient number of patients in our clinical trials which could delay or prevent the receipt of necessary regulatory approvals. | |
| ● | The development of our cell therapy product candidates is subject to uncertainty because autologous cell therapy is inherently variable. | |
| ● | Any disruption to our access to the media (including cell culture media) and reagents we are using in the clinical development of our cell therapy product candidates could adversely affect our ability to perform clinical trials and seek future regulatory submissions. | |
| ● | Our clinical trials may fail to demonstrate adequately the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization. | |
| ● | Even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize a product candidate, and the approval may be for a narrower indication than we seek. | |
| ● | We may never obtain FDA approval for any of our product candidates in the United States and, even if we do, we may never obtain approval for or commercialize any of our product candidates in any foreign jurisdiction, which would limit our ability to realize our full market potential. | |
| ● | We presently lack manufacturing capabilities to produce our product candidates at commercial scale quantities and do not have an alternate manufacturing supply at this time, which could negatively impact our ability to meet any future demand for the products. | |
| ● | The commercial potential and profitability of our products are unknown and subject to significant risk and uncertainty. | |
| ● | We may have difficulties in sourcing brown adipose (fat) tissue. | |
| ● | If safety problems are encountered by us or others developing new stem cell-based therapies, our stem cell initiatives could be materially and adversely affected. | |
| ● | We are vulnerable to competition and technological change, and also to physicians’ inertia. | |
| ● | We have limited experience in the development and marketing of cell therapies and may be unsuccessful in our efforts to establish a profitable business. | |
| ● | Our cell therapy business is based on novel technologies that are inherently expensive, risky and may not be understood by or accepted in the marketplace, which could adversely affect our future value. | |
| ● | Our cell therapy product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated. | |
| ● | We may be subject to significant product liability claims and litigation, including potential exposure from the use of our product candidates in human subjects, and our insurance may be inadequate to cover claims that may arise. | |
| ● | Our internal computer systems, or those that are expected to be used by our clinical investigators, clinical research organizations or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of development programs for our product candidates. | |
| ● | Our inability to obtain reimbursement for our products and services from private and governmental insurers could negatively impact demand for our products and services. |
|
|
Risks Related to Our Intellectual Property
| ● | We may not be able to protect our proprietary rights. | |
| ● | Changes to United States patent law may have a material adverse effect on our intellectual property rights. | |
| ● | In certain countries, patent holders may be required to grant compulsory licenses, which would likely have a significant and detrimental effect on any future revenues in such country. |
Risks Related to Government Regulation
| ● | Even if we obtain regulatory approval for a product candidate, our products will remain subject to regulatory oversight. | |
| ● | We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties. | |
| ● | The failure to receive regulatory approvals for our cell therapy product candidates would likely have a material and adverse effect on our business and prospects. | |
| ● | If we are unable to conduct clinical studies in accordance with regulations and accepted standards, we may be delayed in receiving, or may never receive, regulatory approvals of our product candidates from the FDA and other regulatory authorities. | |
| ● | Health care companies have been the subjects of federal and state investigations, and we could become subject to investigations in the future. | |
| ● | It is uncertain to what extent the government, private health insurers and third-party payors will approve coverage or provide reimbursement for the therapies and products to which our services relate. Availability for such reimbursement may be further limited by reductions in Medicare, Medicaid and other federal healthcare program funding in the United States. | |
| ● | Competitor companies or hospitals in the EU may be able to take advantage of EU rules permitting sales of unlicensed medicines for individual patients to sell competing products without a marketing authorization. |
Risks Related to Our Common Stock
| ● | We pay no dividends. | |
| ● | Stockholders who hold unregistered shares of our common stock are subject to resale restrictions pursuant to Rule 144 due to our former status as a “shell company.” | |
| ● | Material weaknesses in our internal control over financial reporting may cause us to fail to timely and accurately report our financial results or result in a material misstatement of our consolidated financial statements. | |
| ● | There may be significant future issuances or resales of our common stock which may materially and adversely dilute stockholders’ ownership interest and affect the market price of our securities. | |
| ● | Anti-takeover provisions and the regulations to which we may be subject may make it more difficult for a third party to acquire control of us, even if the change in control would be beneficial to our securityholders. |
|
|
Risks Associated with Our Nasdaq Listing
| ● | We cannot assure you that we will be able to continue to comply with the minimum bid price requirement of Nasdaq. | |
| ● | The market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve. |
Risks Related to Our Business Generally
We have a limited operating history; we have incurred substantial losses since inception; we expect to continue to incur losses for the near term.
We have a limited operating history. Since our inception, we have incurred net losses. As of December 31, 2023, our accumulated deficit was $167,056,381.
We will need to obtain a significant amount of financing to complete our clinical trials and implement our business plan.
Since our inception, we have not generated revenues from our operations and have funded our operations through the sale of our equity securities and debt securities. The implementation of our business plan, as discussed in this Annual Report under Item 1 (“Business”), will require the receipt of sufficient equity and/or debt financing to purchase necessary equipment, technology and materials, fund our clinical trials and other research and development efforts and otherwise fund our operations. We will require significant additional funding to complete our clinical trials using BRTX-100. We will also require a substantial amount of additional funding to implement our other programs described in this Annual Report under Item 1 (“Business”), including our metabolic ThermoStem Program, and fund general operations. No assurance can be given that the amount of funding that we anticipate may be required for such purposes is correct or that we will be able to accomplish our goals within the timeframes projected. In addition, no assurance can be given that we will be able to obtain any required financing on commercially reasonable terms or otherwise. In the event we do not obtain the financing required for the above purposes, we may have to curtail our development, marketing and promotional activities, which would have a material adverse effect on our business, financial condition and results of operations, and ultimately we could be forced to discontinue our operations and liquidate.
Our business strategy is high risk.
We are focusing our resources and efforts primarily on the development of cellular-based products and services which will require extensive cash for research, development and commercialization activities. This is a high-risk strategy because there is no assurance that our products and services, including our Disc/Spine Program and our ThermoStem metabolic brown fat research initiative, will ever become commercially viable (commercial risk), that we will prevent other companies from depriving us of market share and profit margins by offering services and products based on our inventions and developments (legal risk), that we will successfully manage a company in a new area of business, regenerative medicine, and on a different scale than we have operated in the past (operational risk), that we will be able to achieve the desired therapeutic results using stem and regenerative cells (scientific risk), or that our cash resources will be adequate to develop our products and services until we become profitable, if ever (financial risk). We are using our cash in one of the riskiest industries in the economy (strategic risk). This may make our securities an unsuitable investment for many investors.
|
|
We will need to enter into agreements in order to implement our business strategy.
Except for a certain license agreement with Regenerative Sciences, LLC and agreements relating to the conduct of our Phase 2 clinical trial, we do not have any material agreements or understandings in place with respect to the implementation of our business strategy. No assurances can be given that we will be able to enter into any necessary agreements with respect to the development of our business. Our inability to enter into any such agreements would have a material adverse effect on our results of operations and financial condition.
We depend on our executive officers and on our ability to attract and retain additional qualified personnel.
Our performance is substantially dependent on the performance of Lance Alstodt, our Chief Executive Officer. We rely upon him for strategic business decisions and guidance. We are also dependent on the performance of Francisco Silva, our Vice President of Research and Development. Each of Messrs. Alstodt and Silva is subject to an employment agreement with us. We do not have any key-man insurance policies on the lives of either of our executive officers. We believe that our future success in developing marketable products and services and achieving a competitive position will depend in large part upon whether we can attract and retain additional qualified management and scientific personnel. Competition for such personnel is intense, and there can be no assurance that we will be able to attract and retain such personnel. The loss of the services of Mr. Alstodt and/or Mr. Silva or the inability to attract and retain additional personnel and develop expertise as needed would have a substantial negative effect on our results of operations and financial condition.
The impact of COVID-19 and related risks could materially affect our results of operations and prospects.
Beginning in March 2020, the global pandemic related to the novel coronavirus COVID-19 began to impact the global economy. Because of the size and breadth of this pandemic, all of the direct and indirect consequences of COVID-19 are not yet known and may not emerge for some time. Risks presented by the ongoing effects of COVID-19 include, among others, the following:
Clinical Trials. We anticipate that the COVID-19 pandemic may negatively impact our clinical trials. Due to the worldwide efforts being taken to combat COVID-19 and the increased clinical work being done in this respect, we believe that it may be difficult for certain needed laboratory supplies, equipment and other materials to be obtained in order to conduct our clinical trials. We also anticipate that, due to a fear of COVID-19 transmission, there may be a hesitancy on the part of certain individuals to become clinical trial participants. We believe that these possible negative effects have lessened as more of the population has become vaccinated; however, the impact that the vaccinations will have is uncertain at this time.
|
|
Adverse Legislative and/or Regulatory Action. Federal, state and local government actions to address and contain the impact of COVID-19 may adversely affect us. For example, we may be subject to legislative and/or regulatory action that negatively impacts the manner in which the clinical trials may be conducted.
Operational Disruptions and Heightened Cybersecurity Risks. Our operations could be disrupted if key members of our senior management or a significant percentage of our workforce are unable to continue to work because of illness, government directives or otherwise. In addition, in connection with increased remote working arrangements, we face a heightened risk of cybersecurity attacks or data security incidents and are more dependent on internet and telecommunications access and capabilities.
Risks Related to Our Cell Therapy Product Development Efforts
Our future success is significantly dependent on the timely and successful development and commercialization of BRTX-100, our lead product candidate for the treatment of chronic lumbar disc disease; if we encounter delays or difficulties in the development of this product candidate, as well as any other product candidates, our business prospects would be significantly harmed.
We are dependent upon the successful development, approval and commercialization of our product candidates. Before we are able to seek regulatory approval of our product candidates, we must conduct and complete extensive clinical trials to demonstrate their safety and efficacy in humans. Our lead product candidate, BRTX-100, is in early stages of development and we have just recently commenced a Phase 2 clinical trial using BRTX-100 to treat chronic lower back pain due to degenerative disc disease related to protruding/bulging discs.
Clinical testing is expensive, difficult to design and implement, and can take many years to complete. Importantly, a failure of one or more of these or any other clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to complete our clinical studies, receive regulatory approval or commercialize our cell therapy product candidates, including the following:
| ● | suspensions, delays or changes in the design, initiation, enrollment, implementation or completion of required clinical trials; adverse changes in our financial position or significant and unexpected increases in the cost of our clinical development program; changes or uncertainties in, or additions to, the regulatory approval process that require us to alter our current development strategy; clinical trial results that are negative, inconclusive or less than desired as to safety and/or efficacy, which could result in the need for additional clinical studies or the termination of the product’s development; delays in our ability to manufacture the product in quantities or in a form that is suitable for any required clinical trials; | |
| ● | intellectual property constraints that prevent us from making, using, or commercializing any of our cell therapy product candidates; |
|
|
| ● | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of these product candidates may be insufficient or inadequate; the inability to generate sufficient pre-clinical, toxicology, or other in vivo or in vitro data, to support the initiation of clinical studies; | |
| ● | delays in reaching agreement on acceptable terms with prospective clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different clinical study sites; | |
| ● | delays in obtaining required Institutional Review Board, or IRB, approval at each clinical study site; | |
| ● | imposition of a temporary or permanent clinical hold by regulatory agencies for a number of reasons, including after review of an IND application or amendment, or equivalent application or amendment; as a result of a new safety finding that presents unreasonable risk to clinical trial participants; a negative finding from an inspection of our clinical study operations or study sites; developments on trials conducted by competitors or approved products post-market for related technology that raise FDA concerns about risk to patients of the technology broadly; or if the FDA finds that the investigational protocol or plan is clearly deficient to meet its stated objectives; | |
| ● | difficulty collaborating with patient groups and investigators; | |
| ● | failure by our CRO, other third parties, or us to adhere to clinical study requirements; | |
| ● | failure to perform in accordance with the FDA’s current Good Clinical Practices, or GCP, requirements, or applicable regulatory guidelines in other countries; | |
| ● | delays in having patients qualify for or complete participation in a study or return for post-treatment follow-up; | |
| ● | patients dropping out of a study; | |
| ● | occurrence of adverse events associated with the product candidate that are viewed to outweigh its potential benefits; | |
| ● | changes in the standard of care on which a clinical development plan was based, which may require new or additional trials; | |
| ● | transfer of manufacturing processes from any academic collaborators to larger-scale facilities operated by either a contract manufacturing organization, or CMO, or by us, and delays or failure by our CMOs or us to make any necessary changes to such manufacturing process; | |
| ● | delays in our clinical trials caused by the COVID-19 pandemic or other health emergencies; | |
| ● | delays in manufacturing, testing, releasing, validating, or importing/exporting sufficient stable quantities of our product candidates for use in clinical studies or the inability to do any of the foregoing; | |
| ● | the FDA not accepting clinical data from trials that are conducted at clinical sites in countries where the standard of care is potentially different from the United States; and | |
| ● | failure to raise sufficient funds to complete our clinical trials. |
Any inability to successfully complete pre-clinical and clinical development could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may be required, or we may elect, to conduct additional studies to bridge our modified product candidates to earlier versions. Clinical study delays could also shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
|
|
Even if we are able to successfully complete our clinical development program for our product candidates, and ultimately receive regulatory approval to market one or more of the products, we may, among other things:
| ● | obtain approval for indications that are not as broad as the indications we sought; | |
| ● | have the product removed from the market after obtaining marketing approval; | |
| ● | encounter issues with respect to the manufacturing of commercial supplies; | |
| ● | be subject to additional post-marketing testing requirements; and/or | |
| ● | be subject to restrictions on how the product is distributed or used. |
We anticipate that we will not be able to commercialize our BRTX-100 product candidate for at least five years.
We may experience delays and other difficulties in enrolling a sufficient number of patients in our clinical trials which could delay or prevent the receipt of necessary regulatory approvals.
We may not be able to initiate or complete as planned any clinical trials if we are unable to identify and enroll a sufficient number of eligible patients to participate in the clinical trials required by the FDA or other regulatory authorities. We also may be unable to engage a sufficient number of clinical trial sites to conduct our trials.
We may face challenges in enrolling patients to participate in our clinical trials due to the novelty of our cell-based therapies, the size of the patient populations and the eligibility criteria for enrollment in the trial. In addition, some patients may have concerns regarding cell therapy that may negatively affect their perception of therapies under development and their decision to enroll in the trials. Furthermore, patients suffering from diseases within target indications may enroll in competing clinical trials, which could negatively affect our ability to complete enrollment of our trials. Enrollment challenges in clinical trials often result in increased development costs for a product candidate, significant delays and potentially the abandonment of the clinical trial.
We may have other delays in completing our clinical trials and we may not complete them at all.
We have just recently commenced the clinical trials necessary to obtain FDA approval to market our product candidate, BRTX-100. Since we lack significant experience in completing clinical trials and bringing a drug through commercialization, we have hired outside consultants with such experience. Clinical trials for BRTX-100 and other product candidates in development may be delayed or terminated as a result of many factors, including the following:
| ● | patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects, or other reasons; | |
| ● | failure by regulators to authorize us to commence a clinical trial; | |
| ● | suspension or termination by regulators of clinical research for many reasons, including concerns about patient safety, the failure of study sites and/or investigators in our clinical research program to comply with GCP requirements, or our failure, or the failure of our contract manufacturers, to comply with current cGMP requirements; | |
| ● | delays or failure to obtain clinical supply for our products necessary to conduct clinical trials from contract manufacturers; |
|
|
| ● | treatment candidates demonstrating a lack of efficacy during clinical trials; | |
| ● | treatment candidates demonstrating significant safety signals; and/or | |
| ● | inability to continue to fund clinical trials or to find a partner to fund the clinical trials. |
Any delay or failure to complete clinical trials and obtain FDA approval for our product candidates could have a material adverse effect on our cost to develop and commercialize, and our ability to generate revenue from, a particular product candidate.
The development of our cell therapy product candidates is subject to uncertainty because autologous cell therapy is inherently variable.
When manufacturing an autologous cell therapy, the number and composition of the cell population varies from patient to patient. Such variability in the number and composition of these cells could adversely affect our ability to manufacture autologous cell therapies in a cost-effective or profitable manner and meet acceptable product release specifications for use in a clinical trial or, if approved, for commercial sale. As a consequence, the development and regulatory approval process for autologous cell therapy products could be delayed or may never be completed.
Any disruption to our access to the media (including cell culture media) and reagents we are using in the clinical development of our cell therapy product candidates could adversely affect our ability to perform clinical trials and seek future regulatory submissions.
Certain media (including cell culture media) and reagents, as well as devices, materials and systems, that we intend to use in our clinical trials, and that we may need or use in commercial production, are provided by unaffiliated third parties. Any lack of continued availability of these media, reagents, devices, materials and systems for any reason would have a material adverse effect on our ability to complete these studies and could adversely impact our ability to achieve commercial manufacture of our planned therapeutic products. Although other available sources for these media, reagents, devices, materials and systems may exist in the marketplace, we have not evaluated their cost, effectiveness, or intellectual property foundation and therefore cannot guarantee the suitability or availability of such other potential sources.
Products that appear promising in research and development may be delayed or may fail to reach later stages of clinical development.
The successful development of cellular based products is highly uncertain. Product candidates that appear promising in preclinical and early research and development may be delayed or fail to reach later stages of development. Decisions regarding the further development of product candidates must be made with limited and incomplete data, which makes it difficult to ensure or even accurately predict whether the allocation of limited resources and the expenditure of additional capital on specific product candidates will result in desired outcomes. Pre-clinical and clinical data can be interpreted in different ways, and negative or inconclusive results or adverse events during a clinical trial could delay, limit or prevent the development of a product candidate. Positive preclinical data may not continue or occur for future subjects in our clinical studies and may not be repeated or observed in ongoing or future studies involving our product candidates. Furthermore, our product candidates may also fail to show the desired safety and efficacy in later stages of clinical development despite having successfully advanced through initial clinical studies. In addition, regulatory delays or rejections may be encountered as a result of many factors, including changes in regulatory policy during the period of product development.
|
|
Our clinical trials may fail to demonstrate adequately the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization.
The clinical trials of our product candidates are, and the manufacturing and marketing of our products will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and market our product candidates. Before obtaining regulatory approvals for the commercial sale of any of our product candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that our product candidates are both safe and effective for use in each target indication. In particular, because some of our product candidates are subject to regulation as biological drug products, we will need to demonstrate that those products are safe, pure, and potent for use in their target indications. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors and may include decrease or elimination of pain, adequate duration of response, a delay in the progression of the disease, an improvement in function and/or decrease in disability.
In addition, even if such trials are successfully completed, we cannot guarantee that the FDA will interpret the results as we do, and more trials could be required before we submit our product candidates for approval. To the extent that the results of the trials are not satisfactory to the FDA for support of a marketing application, we may be required to expend significant resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates.
Even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize a product candidate, and the approval may be for a narrower indication than we seek.
We cannot commercialize a product candidate until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates meet their safety and efficacy endpoints in clinical trials, the regulatory authorities may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions or conditions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory authority policy during the period of product development, clinical trials and the review process. Regulatory authorities also may approve a product candidate for more limited indications than requested or they may impose significant limitations in the form of narrow indications, contraindications or a Risk Evaluation and Mitigation Strategy, or REMS. These regulatory authorities may require warnings or precautions with respect to conditions of use or they may grant approval subject to the performance of costly post-marketing clinical trials. In addition, regulatory authorities may not approve the labeling claims or allow the promotional claims that are necessary or desirable for the successful commercialization of our product candidates. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates and materially and adversely affect our business, financial condition, results of operations and prospects.
|
|
We may never obtain FDA approval for any of our product candidates in the United States and, even if we do, we may never obtain approval for or commercialize any of our product candidates in any foreign jurisdiction, which would limit our ability to realize our full market potential.
In order to eventually market any of our product candidates in any particular foreign jurisdiction, we must establish and comply with numerous and varying regulatory requirements regarding safety and efficacy on a jurisdiction-by-jurisdiction basis. Approval by the FDA in the United States, if obtained, does not ensure approval by regulatory authorities in other countries or jurisdictions. In addition, preclinical studies and clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country.
Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and costs for us and require additional preclinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our product candidates in those countries. The foreign regulatory approval process involves similar risks to those associated with FDA approval. We do not have any product candidates approved for sale in any jurisdiction, including international markets, nor have we attempted to obtain such approval. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our products may be unrealized.
We presently lack manufacturing capabilities to produce our product candidates at commercial scale quantities and do not have an alternate manufacturing supply at this time, which could negatively impact our ability to meet any future demand for the products.
We have utilized our laboratory to provide the cell processing services necessary for clinical production of BRTX-100 for our Phase 2 disc clinical trial. We believe that we have sufficient laboratory capacity to provide such services with regard to the balance of the Phase 2 trial; however, we would need to significantly expand our manufacturing capabilities to provide such cell processing services to meet potential commercial demand for BRTX-100 and any other of our product candidates, if approved, as well as any of our other product candidates that might attain regulatory approval. Such expansion would require additional regulatory approvals. Even if we increase our manufacturing capabilities, it is possible that we may still lack sufficient capacity to meet demand. Ultimately, if we are unable to supply our products to meet commercial demand, whether because of processing constraints or other disruptions, delays or difficulties that we experience, sales of the products and their long-term commercial prospects could be significantly damaged.
We may seek to utilize a third-party manufacturer for BRTX-100 or any of our other product candidates; however, we do not have any arrangements in place with a third-party manufacturer. If our facilities at which these product candidates would be manufactured or our equipment were significantly damaged or destroyed, or if there were other disruptions, delays or difficulties affecting manufacturing capacity, our planned and future clinical studies and commercial production for these product candidates would likely be significantly disrupted and delayed. It would be both time consuming and expensive to replace this capacity with third parties, particularly since any new facility would need to comply with the regulatory requirements.
|
|
Ultimately, if we are unable to supply our cell therapy product candidates to meet commercial demand (assuming commercial approval is obtained), whether because of processing constraints or other disruptions, delays or difficulties that we experience, our production costs could dramatically increase and sales of the product and its long-term commercial prospects could be significantly damaged.
The commercial potential and profitability of our products are unknown and subject to significant risk and uncertainty.
Even if we successfully develop and obtain regulatory approval for our cell therapy product candidates, the market may not understand or accept the products, which could adversely affect both the timing and level of future sales. Ultimately, the degree of market acceptance of our product candidates (or any of our future product candidates) will depend on a number of factors, including:
| ● | the clinical effectiveness, safety and convenience of the product particularly in relation to alternative treatments; | |
| ● | our ability to distinguish our products (which involve adult cells) from any ethical and political controversies associated with stem cell products derived from human embryonic or fetal tissue; and | |
| ● | the cost of the product, the reimbursement policies of government and third-party payors and our ability to obtain sufficient third-party coverage or reimbursement. |
Even if we are successful in achieving sales of our product candidates, it is not clear to what extent, if any, the products will be profitable. The costs of goods associated with production of cell therapy products are significant. In addition, some changes in manufacturing processes or procedures generally require FDA or foreign regulatory authority review and approval prior to implementation. We may need to conduct additional pre-clinical studies and clinical trials to support approval of any such changes. Furthermore, this review process could be costly and time-consuming and could delay or prevent the commercialization of product candidates.
We may have difficulties in sourcing brown adipose (fat) tissue.
We use brown adipose (fat) tissue to identify and characterize brown adipose derived stem cells for use in our pre-clinical ThermoStem Program. There is no certainty that we will be able to continue to collect brown adipose samples through any relationships that we have, have had or may establish with potential sources of brown adipose tissue. The inability to procure brown fat tissue would have a material adverse effect upon our ability to advance our ThermoStem Program.
|
|
If safety problems are encountered by us or others developing new stem cell-based therapies, our stem cell initiatives could be materially and adversely affected.
The use of stem cells for therapeutic indications is still in the very early stages of development. If an adverse event occurs during clinical trials related to one of our proposed products and/or services or those of others, the FDA and other regulatory authorities may halt clinical trials or require additional studies. The occurrence of any of these events would delay, and increase the cost of, our development efforts and may render the commercialization of our proposed products and/or services impractical or impossible.
We are vulnerable to competition and technological change, and also to physicians’ inertia.
We will compete with many domestic and foreign companies in developing our technology and products, including biotechnology, medical device and pharmaceutical companies. Many current and potential competitors have substantially greater financial, technological, research and development, marketing, and personnel resources. There is no assurance that our competitors will not succeed in developing alternative products and/or services that are more effective, easier to use, or more economical than those which we may develop, or that would render our products and/or services obsolete and non-competitive. In general, we may not be able to prevent others from developing and marketing competitive products and/or services similar to ours or which perform similar functions or which are marketed before ours.
Competitors may have greater experience in developing products, therapies or devices, conducting clinical trials, obtaining regulatory clearances or approvals, manufacturing and commercialization. It is possible that competitors may obtain patent protection, approval or clearance from the FDA or achieve commercialization earlier than we can, any of which could have a substantial negative effect on our business.
We will compete against cell-based therapies derived from alternate sources, such as bone marrow, adipose tissue, umbilical cord blood and potentially embryos. Doctors historically are slow to adopt new technologies like ours, whatever the merits, when older technologies continue to be supported by established providers. Overcoming such inertia often requires very significant marketing expenditures or definitive product performance and/or pricing superiority.
We expect that physicians’ inertia and skepticism will also be a significant barrier as we attempt to gain market penetration with our future products and services. We may need to finance lengthy time-consuming clinical studies (so as to provide convincing evidence of the medical benefit) in order to overcome this inertia and skepticism.
We may form or seek collaborations or strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We may form or seek strategic alliances, create joint ventures or collaborations, or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute the shares of our existing stockholders, or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy. To date, such efforts have not been successful.
|
|
Further, collaborations involving our product candidates, such as our collaborations with third-party research institutions, are subject to numerous risks, which may include the following:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration; | |
| ● | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding, or other external factors, such as a business combination that diverts resources or creates competing priorities; | |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials, or require a new formulation of a product candidate for clinical testing; | |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; | |
| ● | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to their marketing and distribution; | |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; | |
| ● | disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our product candidates, or that result in costly litigation or arbitration that diverts management attention and resources; | |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates; and | |
| ● | collaborators may own or co-own intellectual property covering our products that results from our collaborating with them, and in such cases, we would not have the exclusive right to commercialize such intellectual property. |
As a result, if we enter into collaboration agreements and strategic partnerships or license our products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction. Any delays in entering into new collaborations or strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates in certain geographies for certain indications, which would harm our business prospects, financial condition, and results of operations.
|
|
We have limited experience in the development and marketing of cell therapies and may be unsuccessful in our efforts to establish a profitable business.
Our business plan has been focused historically on capturing a piece of the burgeoning field of cell therapy. We have limited experience in the areas of cell therapy product development and marketing, and in the related regulatory issues and processes. Although we have recruited a team that has experience with designing and conducting clinical trials and have hired FDA consultants, as a company, we have limited experience in conducting clinical trials and no experience in conducting clinical trials through to regulatory approval of any product candidate. In part because of this lack of experience, we cannot be certain that planned clinical trials will begin or be completed on time, if at all. We cannot assure that we will successfully achieve our clinical development goals or fulfill our plans to capture a piece of the cell therapy market.
Our cell therapy business is based on novel technologies that are inherently expensive, risky and may not be understood by or accepted in the marketplace, which could adversely affect our future value.
The clinical development, commercialization and marketing of cell and tissue-based therapies are at an early-stage, substantially research-oriented, and financially speculative. To date, very few companies have been successful in their efforts to develop and commercialize a cell therapy product. In general, cell-based or tissue-based products may be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy, or other characteristics that may prevent or limit their approval or commercial use. In addition, BRTX-100 is a cell-based candidate that is produced by using a patient’s own stem cells derived from bone marrow. Regulatory approval of novel product candidates such as BRTX-100, which is manufactured using novel manufacturing processes, can be more complex and expensive and take longer than other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to the FDA’s lack of experience with them. To our knowledge, the FDA has not yet approved a disc related stem cell therapy product. This lack of experience may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, which would increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions. Furthermore, the number of people who may use cell or tissue-based therapies is difficult to forecast with accuracy. Our future success is dependent on the establishment of a large global market for cell- and tissue-based therapies and our ability to capture a share of this market with our product candidates.
Our cell therapy product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.
The enactment of the Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated regulatory pathway for the approval of products demonstrated to be biosimilar, or “highly similar,” to or “interchangeable” with an FDA-approved innovator (original) biologic product. The abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing reference product. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the original branded product is approved under a biologics license application, or BLA. The FDA has developed considerable experience with the biosimilar and interchangeable biosimilar processes since the enactment of the BPCIA in 2009. Should any of our product candidates be approved via the BLA pathway, we expect that biosimilar applicants will seek approval of biosimilar, and/or interchangeable, versions of our product that could result in lower prices for our products.
|
|
We believe that, if any of our product candidates are approved as a biological product under a BLA, it should qualify for the 12-year period of exclusivity. However, there is a risk that the FDA could approve biosimilar applicants for other reference products that no longer have such exclusivity, thus potentially creating the opportunity for greater competition sooner than anticipated.
We may also face increased competition from stem cell therapies performed by treatment centers that do not require FDA premarket approval. In August 2022, a federal District Court in the case of United States v. California Stem Cell Treatment Center, Inc. held that certain autologous adipose stem cell treatments were not “biological products” and therefore did not require FDA approval. The FDA has appealed the decision but, should it be upheld, we could face competition from stem cell clinics that would not be required to undergo the costly and time-consuming FDA approval process.
The FDA’s regulation of regenerative medicine products remains unpredictable and we are not certain what impact this will have on the potential approval of our products
The FDA’s regulation of therapies derived from stem cell products and technologies is evolving and may continue to evolve. In December 2016, the 21st Century Cures Act, or the Cures Act, was signed into law in the United States to advance access to medical innovations. Among other things, the Cures Act established a new FDA regenerative medicine advanced therapy, or RMAT, designation. This designation offers a variety of benefits to product candidates, including enhanced FDA support during clinical development, priority review on application filing, accelerated approval based on potential surrogate endpoints, and the potential use of patient registry data and other forms of real world evidence for post-approval confirmatory studies. There is no certainty that any of our product candidates will receive RMAT designation or any other type of expedited review program designation from the FDA. In any event, the receipt of an FDA RMAT designation or other expedited review program designation may not result in a faster development process, review or approval compared to products considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA.
We may be subject to significant product liability claims and litigation, including potential exposure from the use of our product candidates in human subjects, and our insurance may be inadequate to cover claims that may arise.
Our business exposes us to potential product liability risks inherent in the testing, processing and marketing of cell therapy products. Such liability claims may be expensive to defend and result in large judgments against us. We face an inherent risk of product liability exposure related to the testing of our current and any future product candidates in human clinical trials and will face an even greater risk with respect to any commercial sales of our products should they be approved. No product candidate has been widely used over an extended period of time, and therefore safety data is limited. Cell therapy companies derive the raw materials for manufacturing of product candidates from human cell sources, and therefore the manufacturing process and handling requirements are extensive, which increases the risk of quality failures and subsequent product liability claims.
|
|
We will need to maintain insurance coverage adequate to cover our clinical trials and increase that coverage before commercializing product candidates, if ever. At any time during our clinical trials or after commercialization, if that occurs, we may not be able to obtain or maintain product liability insurance on acceptable terms with adequate coverage or at all, or if claims against us substantially exceed our coverage, then our financial position could be significantly impaired.
Whether or not we are ultimately successful in any product liability litigation that may arise, such litigation could consume substantial amounts of our financial and managerial resources, result in decreased demand for our products and injure our reputation.
We seek to maintain errors and omissions, directors and officers, workers’ compensation and other insurance at levels we believe to be appropriate to our business activities. If, however, we were subject to a claim in excess of this coverage or to a claim not covered by our insurance and the claim succeeded, we would be required to pay the claim from our own limited resources, which could have a material adverse effect on our financial condition, results of operations and business. Additionally, liability or alleged liability could harm our business by diverting the attention and resources of our management and damaging our reputation.
Our internal computer systems, or those that are expected to be used by our clinical investigators, clinical research organizations or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of development programs for our product candidates.
We rely on information technology systems to keep financial records, maintain laboratory and corporate records, communicate with staff and external parties and operate other critical functions. Any significant degradation or failure of these computer systems could cause us to inaccurately calculate or lose data. Despite the implementation of security measures, these internal computer systems and those used by our clinical investigators, clinical research organizations, and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunication and electrical failures. The techniques that could be used by criminal elements or foreign governments to attack these computer systems are sophisticated, change frequently and may originate from less regulated and remote areas of the world. While we have not experienced any such system failure, theft of information, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our clinical development activities. For example, the loss of clinical trial data from historical or future clinical trials could result in delays in regulatory approval efforts and significantly increase costs to recover or reproduce the data. To the extent that any disruption, theft of information, or security breach were to result in a loss of or damage to data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the clinical development and the future development of our product candidates could be delayed.
|
|
To operate and sell in international markets carries great risk.
We intend to market our products and services both domestically and in foreign markets. A number of risks are inherent in international transactions. In order for us to market our products and services in non-U.S. jurisdictions, we need to obtain and maintain required regulatory approvals or clearances in these countries and must comply with the country specific regulations regarding safety, manufacturing processes and quality. These regulations, including the requirements for approvals or clearances to market, may differ from the FDA regulatory scheme. International operations and sales also may be limited or disrupted by political instability, price controls, trade restrictions and changes in tariffs. Additionally, fluctuations in currency exchange rates may adversely affect demand for our services and products by increasing the price of our products and services in the currency of the countries in which the products and services are offered.
There can be no assurance that we will obtain regulatory approvals or clearances in all of the countries where we intend to market our products and services, that we will not incur significant costs in obtaining or maintaining foreign regulatory approvals or clearances, or that we will be able to successfully commercialize our products and services in various foreign markets. Delays in receipt of approvals or clearances to market our products and services in foreign countries, failure to receive such approvals or clearances or the future loss of previously received approvals or clearances could have a substantial negative effect on our results of operations and financial condition.
Our inability to obtain reimbursement for our products and services from private and governmental insurers could negatively impact demand for our products and services.
Market acceptance and sales of our product candidates may depend on coverage and reimbursement policies and health care reform measures. Decisions about formulary coverage as well as levels at which government authorities and third-party payors, such as private health insurers and health maintenance organizations, reimburse patients for the price they pay for our product candidates, as well as levels at which these payors pay directly for our product candidates, where applicable, could affect whether we are able to successfully commercialize these products. We cannot guarantee that reimbursement will be available for any of our product candidates. We also cannot guarantee that coverage or reimbursement amounts will not reduce the demand for, or the price of, our product candidates.
If coverage and reimbursement are not available or are available only at limited levels, we may not be able to successfully commercialize our products. The Patient Protection and Affordable Care Act, or PPACA, as well as the Inflation Reduction Act, passed in August 2022, and other health reforms include measures that would limit or prohibit payments for certain medical treatments or subject the pricing of drugs and biologics to government control. In addition, in many foreign countries, particularly the countries of the European Union, or the EU, the pricing of drugs and biologics is subject to government control. If our products are or become subject to government regulation that limits or prohibits payment for our products, or that subjects the price of our products to government control, we may not be able to generate revenue, attain profitability or commercialize our products.
|
|
In addition, third-party payors are increasingly limiting both coverage and the level of reimbursement of new drugs and biologics. They may also impose strict prior authorization requirements and/or refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly-approved drugs and biologics. If we are unable to obtain adequate levels of reimbursement for our product candidates, our ability to successfully market and sell our product candidates will be harmed.
Risks Related to Our Intellectual Property
We may not be able to protect our proprietary rights.
Our commercial success will depend in large part upon our ability to protect our proprietary rights. There is no assurance, for example, that any additional patents will be issued based on our or our licensor’s pending applications or, if issued, that such patents will not become the subject of a re-examination, will provide us with competitive advantages, will not be challenged by any third parties, or that the patents of others will not prevent the commercialization of products and services incorporating our technology. Furthermore, there can be no guarantee that others will not independently develop similar products and services, duplicate any of our products and services, or design around any patents we obtain.
Our commercial success will also depend upon our ability to avoid infringing patents issued to others. If we were judicially determined to be infringing on any third-party patent, we could be required to pay damages, alter our products, services or processes, obtain licenses, or cease certain activities. If we are required in the future to obtain any licenses from third parties for some of our products and/or services, there can be no guarantee that we would be able to do so on commercially favorable terms, if at all. United States and foreign patent applications are not immediately made public, so we might be surprised by the grant to someone else of a patent on a technology we are actively using. Although we conducted a freedom to operate, or FTO, search years ago on the licensed technology associated with our Disc/Spine Program, modifications made, and/or further developments that may be made, to that technology may not be covered by the initial FTO. No FTO has been undertaken with respect to our ThermoStem brown fat initiative.
Litigation, which would result in substantial costs to us and the diversion of effort on our part, may be necessary to enforce or confirm the ownership of any patents issued or licensed to us, or to determine the scope and validity of third-party proprietary rights. If our competitors claim technology also claimed by us and prepare and file patent applications in the United States, we may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office, or the Patent Office, or a foreign patent office to determine priority of invention, which could result in substantial costs and diversion of effort, even if the eventual outcome is favorable to us. Any such litigation or interference proceeding, regardless of outcome, could be expensive and time-consuming.
Successful challenges to our patents through oppositions, re-examination proceedings or interference proceedings could result in a loss of patent rights in the relevant jurisdiction. If we are unsuccessful in actions we bring against the patents of other parties, and it is determined that we infringe upon the patents of third parties, we may be subject to litigation, or otherwise prevented from commercializing potential products and/or services in the relevant jurisdiction, or may be required to obtain licenses to those patents or develop or obtain alternative technologies, any of which could harm our business. Furthermore, if such challenges to our patent rights are not resolved in our favor, we could be delayed or prevented from entering into new collaborations or from commercializing certain products and/or services, which could adversely affect our business and results of operations.
|
|
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential or sensitive information could be compromised by disclosure in the event of litigation. In addition, during the course of litigation there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
In addition to patents, we rely on unpatented trade secrets and proprietary technological expertise. Some of our intended future cell-related therapeutic products and/or services may fit into this category. We also rely, in part, on confidentiality agreements with our partners, employees, advisors, vendors, and consultants to protect our trade secrets and proprietary technological expertise. There can be no guarantee that these agreements will not be breached, or that we will have adequate remedies for any breach, or that our unpatented trade secrets and proprietary technological expertise will not otherwise become known or be independently discovered by competitors.
Failure to obtain or maintain patent protection, failure to protect trade secrets, third-party claims against our patents, trade secrets, or proprietary rights or our involvement in disputes over our patents, trade secrets, or proprietary rights, including involvement in litigation, could divert our efforts and attention from other aspects of our business and have a substantial negative effect on our results of operations and financial condition.
We may not be able to protect our intellectual property in countries outside of the United States.
Intellectual property law outside the United States is uncertain and, in many countries, is currently undergoing review and revisions. The laws of some countries do not protect our patent and other intellectual property rights to the same extent as United States laws. Third parties may attempt to oppose the issuance of patents to us in foreign countries by initiating opposition proceedings. Opposition proceedings against any of our patent filings in a foreign country could have an adverse effect on our corresponding patents that are issued or pending in the United States. It may be necessary or useful for us to participate in proceedings to determine the validity of our patents or our competitors’ patents that have been issued in countries other than the United States. This could result in substantial costs, divert our efforts and attention from other aspects of our business, and could have a material adverse effect on our results of operations and financial condition.
Changes to United States patent law may have a material adverse effect on our intellectual property rights.
The Leahy-Smith America Invents Act, or AIA, which was signed into law in 2011, significantly changes United States patent law. It may take some time to establish what the law means, since it is just being interpreted by the lower courts, Federal Circuit Courts of Appeal, and the Supreme Court. The effects of these decisions are still not known. The first major change is that AIA switches the United States patent system from a “first to invent” system to a “first to file” system. Now that the first to file system is in effect, there is a risk that another company may independently develop identical or similar patents at approximately the same time, and be awarded the patents instead of us. Further, for the second major change, AIA abolished interference proceedings, and establishes derivation proceedings to replace interference proceedings in all cases in which the time period for instituting an interference proceeding has not lapsed where an inventor named in an earlier application derived the claimed invention from a named inventor. Now that the derivation proceedings are in effect, there is a risk that the inventorship of any pending patent application can be challenged for reasons of derivation. The third major change is that AIA established post-grant opposition proceedings that will apply only to patent applications filed after “first to file” became effective. Post-grant opposition will enable a person who is not the patent owner to initiate proceedings in the Patent Office within nine months after the grant of a patent that can result in cancellation of a patent as invalid. In addition to AIA, recent court decisions have created uncertainty with regard to our ability to obtain and maintain patents. Therefore there is a risk that any of our patents once granted may be subject to post-grant opposition, which will increase uncertainty on the validity of any newly granted patent or may ultimately result in cancellation of the patent.
|
|
In addition, the Supreme Court has recently taken more limiting positions as to what constitutes patentable subject matter. As a result, many patents covering what were previously patentable inventions are now determined to cover inventions which are deemed non-statutory subject matter and are now invalid. As a result of this and subsequent opinions by the Court of Appeals for the Federal Circuit, the Patent Office is now applying more stringent limitations to claims in patent applications and is refusing to grant patents in areas of technology where patents were previously deemed available. Therefore there is a risk that we will be unable to acquire patents to cover our products and if such patents are granted they may subsequently be found to be invalid.
In certain countries, patent holders may be required to grant compulsory licenses, which would likely have a significant and detrimental effect on any future revenues in such country.
Many countries, including some countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, most countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may be limited to monetary relief and may be unable to enjoin infringement, which could materially diminish the value of the patent. Compulsory licensing of life-saving products is also becoming increasingly common in developing countries, either through direct legislation or international initiatives. Such compulsory licenses could be extended to our product candidates, which may limit our potential revenue opportunities, including with respect to any future revenues that may result from our product candidates.
Risks Related to Government Regulation
Even if we obtain regulatory approval for a product candidate, our products will remain subject to regulatory oversight.
Our product candidates for which we obtain regulatory approval will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information. Any regulatory approvals that we receive for our product candidates also may be subject to a REMS or the specific obligations imposed as a condition for marketing authorization by equivalent authorities in a foreign jurisdiction, limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the quality, safety and efficacy of the product. For example, in the United States, the holder of an approved new drug application, or NDA, or BLA is obligated to monitor and report adverse events and any failure of a product to meet the specifications in the NDA or BLA. The holder of an approved NDA or BLA also must submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with the Federal Food, Drug and Cosmetic Act, or FDCA, and implementing regulations and are subject to FDA oversight and post-marketing reporting obligations, in addition to other potentially applicable federal and state laws.
|
|
In addition, product manufacturers and their facilities may be subject to payment of application and program fees and are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP requirements and adherence to commitments made in the NDA, BLA or foreign marketing application. If we or a regulatory authority discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or if a regulatory authority disagrees with the promotion, marketing or labeling of our product, a regulatory authority may impose restrictions relative to that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements for any product candidate following approval, a regulatory authority may:
| ● | issue a warning or untitled letter asserting that we are in violation of the law; | |
| ● | seek an injunction or impose administrative, civil or criminal penalties or monetary fines; | |
| ● | suspend or withdraw regulatory approval; | |
| ● | suspend any ongoing clinical trials; | |
| ● | refuse to approve a pending BLA or comparable foreign marketing application (or any supplements thereto) submitted by us or our strategic partners; | |
| ● | restrict the marketing or manufacturing of the product; | |
| ● | seize or detain the product or otherwise demand or require the withdrawal or recall of the product from the market; | |
| ● | refuse to permit the import or export of products; | |
| ● | request and publicize a voluntary recall of the product; or | |
| ● | refuse to allow us to enter into supply contracts, including government contracts. |
|
|
Any government enforcement action or investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and adversely affect our business, financial condition, results of operations and prospects.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
In the United States, the research, manufacturing, distribution, sale, and promotion of drugs and biologic products are subject to regulation by various federal, state, and local authorities, including the FDA, the Centers for Medicare and Medicaid Services, or CMS, other divisions the Department of Health and Human Services, or HHS (e.g., the Office of Inspector General), the United States Department of Justice offices of the United States Attorney, the Federal Trade Commission and state and local governments. Our operations are directly, or indirectly through our prescribers, customers and purchasers, subject to various federal and state fraud and abuse laws and regulations, including the federal Anti-Kickback Statute, or AKS, the federal civil and criminal False Claims Act, or FCA, the Physician Payments Sunshine Act and regulations and equivalent provisions in other countries. In addition, we may be subject to patient privacy laws by both the federal government and the states in which we conduct our business.
State and federal regulatory and enforcement agencies continue actively to investigate violations of health care laws and regulations, and the United States Congress continues to strengthen the arsenal of enforcement tools. For example, the Bipartisan Budget Act of 2018 increased the criminal and civil penalties that can be imposed for violating certain federal health care laws, including the AKS. Enforcement agencies also continue to pursue novel theories of liability under these laws. Government agencies have recently increased regulatory scrutiny and enforcement activity with respect to programs supported or sponsored by pharmaceutical companies, including reimbursement and co-pay support, funding of independent charitable foundations and other programs that offer benefits for patients. Several investigations into these programs have resulted in significant civil and criminal settlements.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the laws described above or any other government regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our financial condition and divert the attention of our management from operating our business.
|
|
Further, in the event we determine to operate in foreign jurisdictions, including conducting clinical trials, we will need to comply with the United States Foreign Corrupt Practices Act of 1977, or the FCPA. The FCPA prohibits a corporation, including its subsidiaries, third-party contractors, distributors, consultants and employees, from corruptly making or offering to make payments to foreign officials for the purpose of obtaining or enhancing business. Under the law, “foreign officials” include employees of health systems operated by government entities. The FCPA also establishes specific record-keeping and internal accounting controls. Violations of the FCPA can result in the imposition of civil penalties or criminal prosecution. Failure to comply with the FCPA will adversely affect our business.
In addition to the FCPA, we will also need to comply with the foreign government laws and regulations of each individual country in which any therapy centers that we may establish are located and products are to be distributed and sold. These regulations vary in complexity and can be as stringent, and on occasion even more stringent, than FDA regulations in the United States. Due to the fact that there are new and emerging stem cell and cell therapy regulations that have recently been drafted and/or implemented in various countries around the world, the application and subsequent implementation of these new and emerging regulations have little to no precedence. Therefore, the level of complexity and stringency is not always precisely understood today for each country, creating greater uncertainty for the international regulatory process. Furthermore, there can be no guarantee that laws and regulations will not be implemented, amended and/or reinterpreted in a way that will negatively affect our business. Likewise, there can be no assurance that we will be able, or will have the resources, to maintain compliance with all such healthcare laws and regulations. Failure to comply with such healthcare laws and regulations, as well as the costs associated with such compliance or with enforcement of such healthcare laws and regulations, may have a material adverse effect on our operations or may require restructuring of our operations or impair our ability to operate profitably.
Our current and future employees, consultants and advisors and our future principal investigators, medical institutions and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk of fraud or other misconduct by our current and future employees, consultants, advisors, principal investigators, medical institutions and commercial partners, including contract laboratories, and CROs. Misconduct by these parties could include intentional failures to comply with FDA regulations or the regulations applicable in other jurisdictions, provide accurate information to the FDA and other regulatory authorities, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us.
We currently do not and in the future may not independently conduct all aspects of our product candidate research and preclinical and clinical testing and product candidate manufacturing. If we rely on third parties, including CROs, medical institutions, and contract laboratories to monitor and manage data for our ongoing preclinical and clinical programs, we will still maintain responsibility for ensuring their activities are conducted in accordance with the applicable study protocol, legal, regulatory and scientific standards. We and our third-party vendors will be required to comply with current cGMP, GCP, and Good Laboratory Practice, or GLP, requirements, which are a collection of laws and regulations enforced by the FDA, the EU and comparable foreign authorities for all of our product candidates in clinical development.
|
|
In addition, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct also could involve the improper use of information obtained in the course of clinical trials or interactions with the FDA or other regulatory authorities, which could result in regulatory sanctions and cause serious harm to our reputation.
The precautions we take to detect and prevent employee and third-party misconduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, financial condition, results of operations and prospects, including the imposition of significant fines or other sanctions.
The failure to receive regulatory approvals for our cell therapy product candidates would likely have a material and adverse effect on our business and prospects.
To date, we have not received regulatory approval to market any of our product candidates in any jurisdiction. If we seek approval of any of our cell therapy product candidates, we will be required to submit to the FDA and potentially other regulatory authorities extensive pre-clinical and clinical data supporting its safety and efficacy, as well as information about the manufacturing process and to undergo inspection of our manufacturing facility or other contract manufacturing facilities, if utilized, among other things. The process of obtaining FDA and other regulatory approvals is expensive, generally takes many years and is subject to numerous risks and uncertainties, particularly with complex and/or novel product candidates such as our cell-based product candidates. Changes in regulatory approval requirements, policies, or court decisions may cause delays in the approval or rejection of an application, make it easier for our competitors to gain regulatory approval to enter the marketplace, or allow competitors to enter the market without having to obtain FDA approval. Ultimately, the FDA and other regulatory agencies have substantial discretion in the approval process and may refuse to accept any application or may decide that our product candidate data are insufficient for approval without the submission of additional preclinical, clinical or other studies. In addition, varying agency interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any difficulties or failures that we encounter in securing regulatory approval for our product candidates would likely have a substantial adverse impact on our ability to generate product sales, and could make any search for a collaborative partner more difficult. Similarly, any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
|
|
If we are unable to conduct clinical studies in accordance with regulations and accepted standards, we may be delayed in receiving, or may never receive, regulatory approvals of our product candidates from the FDA and other regulatory authorities.
To obtain marketing approvals for our product candidates in the United States and abroad, we must, among other requirements, complete adequate and well-controlled clinical trials sufficient to demonstrate to the FDA and other regulatory bodies that the product candidate is safe and effective for each indication for which approval is sought. If the FDA finds that patients enrolled in the trial are or would be exposed to an unreasonable and significant risk of illness or injury, due to, among other things, occurrence of a serious adverse event in an ongoing clinical trial, the FDA can place one or more of our clinical trials on hold. If safety concerns develop, we may, or the FDA or an institutional review board may require us to, stop the affected trials before completion.
The completion of our clinical trials also may be delayed or terminated for a number of other reasons, including if:
| ● | third-party clinical investigators do not perform the clinical trials on the anticipated schedule or consistent with the clinical trial protocol, good clinical practices required by the FDA and other regulatory requirements, or other third parties do not perform data collection and analysis in a timely or accurate manner; | |
| ● | inspections of clinical trial sites by the FDA or other regulatory authorities reveal violations that require us to undertake corrective action, suspend or terminate one or more sites, or prohibit use of some or all of the data in support of marketing applications; or |
| ● | the FDA or one or more institutional review boards suspends or terminates the trial at an investigational site, or precludes enrollment of additional subjects. |
Our development costs will increase if there are material delays in our clinical trials, or if we are required to modify, suspend, terminate or repeat a clinical trial. If we are unable to conduct our clinical trials properly, we may never receive regulatory approval to market our product candidates.
Health care companies have been the subjects of federal and state investigations, and we could become subject to investigations in the future.
Both federal and state government agencies have heightened civil and criminal enforcement efforts. There are numerous ongoing investigations of health care companies, as well as their executives and managers. In addition, amendments to the federal FCA, including under healthcare reform legislation, have made it easier for private parties to bring “qui tam” (or whistleblower) lawsuits against companies under which the whistleblower may be entitled to receive a percentage of any money paid to the government. The FCA provides, in part, that an action can be brought against any person or entity that has knowingly presented, or caused to be presented, a false or fraudulent request for payment from the federal government, or who has made a false statement or used a false record to get a claim approved. The government has taken the position that claims presented in violation of the federal AKS, Stark Law or other healthcare-related laws, including laws enforced by the FDA, may be considered a violation of the FCA. Penalties include substantial fines for each false claim, plus three times the amount of damages that the federal government sustained because of the act of that person or entity and/or exclusion from the Medicare and Medicaid programs. In addition, a majority of states have adopted similar state whistleblower and false claims provisions.
We are not aware of any government investigations involving any of our facilities or management. While we believe that we are in compliance with applicable governmental healthcare laws and regulations, any future investigations of our business or executives could cause us to incur substantial costs, and result in significant liabilities or penalties, as well as damage to our reputation.
|
|
It is uncertain to what extent the government, private health insurers and third-party payors will approve coverage or provide reimbursement for the therapies and products to which our services relate. Availability for such reimbursement may be further limited by reductions in Medicare, Medicaid and other federal healthcare program funding in the United States.
To the extent that health care providers cannot obtain coverage or reimbursement for our products and therapies, they may elect not to provide such products and therapies to their patients and, thus, may not need our services. Further, as cost containment pressures are increasing in the health care industry, government and private payors may adopt strategies designed to limit the amount of reimbursement paid to health care providers.
Similarly, the trend toward managed health care and bundled pricing for health care services in the United States, could significantly influence the purchase of healthcare products and services, resulting in lower prices and reduced demand for our therapeutic products under development.
We may directly or indirectly receive revenues from federal health care programs, such as Medicare. Federal health care programs are subject to changes in coverage and reimbursement rules and procedures, including retroactive rate adjustments. These contingencies could materially decrease the range of services covered by such programs or the reimbursement rates paid directly or indirectly for our products and services. To the extent that any health care reform favors the reimbursement of other therapies over our therapeutic products under development, such reform could affect our ability to sell our services, which may have a material adverse effect on our revenues.
The limitation on reimbursement available from private and government payors may reduce the demand for, or the price of, our products and services, which could have a material adverse effect on our revenues. Additional legislation or regulation relating to the health care industry or third-party coverage and reimbursement may be enacted in the future which could adversely affect the revenues generated from the sale of our products and services.
Furthermore, there has been a trend in recent years towards reductions in overall funding for Medicare, Medicaid and other federal health care programs. The reduced funding of governmental programs could have a negative impact on the demand for our services to the extent it relates to products and services which are reimbursed by government and private payors.
Unintended consequences of healthcare reform in the United States may adversely affect our business.
The healthcare industry is undergoing fundamental changes resulting from political, economic and regulatory influences. In the United States, the PPACA was signed into law in 2010 under the Obama administration. By implementing comprehensive reforms, the law seeks to, among other things, increase access to healthcare for the uninsured and control the escalation of healthcare expenditures within the economy.
|
|
In addition, other legislative changes have been adopted since the PPACA was enacted. These changes include aggregate reductions in Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2018, will remain in effect through 2030 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Congress has since considered additional reductions in Medicare reimbursement for drugs and devices as part of legislation to reduce the budget deficit. Similar legislation could be enacted in the future. The Medicare regulations and interpretive determinations that determine how drugs, devices and services are covered and reimbursed also are subject to change. These laws, regulations, and interpretive determinations may result in additional reductions in Medicare and other health care funding, which could impact our business.
In August 2022, President Biden signed the Inflation Reduction Act, or the IRA, which provides for (i) the government to set or negotiate prices for select high-cost Medicare Part D (beginning in 2026) and Medicare Part B drugs (beginning in 2028) that are more than nine years (for small-molecule drugs) or 13 years (for biological products) from their FDA approval, (ii) manufacturers to pay a rebate for Medicare Part B and Part D drugs when prices increase faster than inflation beginning in 2022 for Medicare Part D and 2023 for Medicare Part B drugs, and (iii) Medicare Part D redesign which replaces the current coverage gap provisions and establishes a $2,000 cap for out-of-pocket limits costs for Medicare beneficiaries beginning in 2025, with manufacturers being responsible for 10% of costs up to the $2,000 cap and 20% after that cap is reached. Implementation of the IRA is expected to be carried out through upcoming actions by regulatory authorities, the outcome of which is uncertain.
Healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and decreased reimbursement. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our product candidates. It is difficult to predict how enforcement initiatives under the PPACA, the IRA, and/or additional legislation or regulation enacted in the future may impact our business. If the PPACA, the IRA, and/or additional legislation or regulation enacted in the future cause such unintended consequences or indirect impact, they could have a material adverse effect on our business, financial condition and results of operations.
Competitor companies or hospitals in the EU may be able to take advantage of EU rules permitting sales of unlicensed medicines for individual patients to sell competing products without a marketing authorization.
The EU medicines rules allow individual member states to permit the supply of a medicinal product without a marketing authorization to fulfill special needs, where the product is supplied in response to a bona fide unsolicited order, formulated in accordance with the specifications of a healthcare professional and for use by an individual patient under his direct personal responsibility. This may, in certain countries, also apply to products manufactured in a country outside the EU and imported to treat specific patients or small groups of patients. In addition, advanced therapy medicinal products do not need a marketing authorization if they are prepared on a non-routine basis and are used within the same EU member state in a hospital in accordance with a medical prescription for an individual patient.
|
|
These exemptions could allow our competitors to make sales in the EU without having obtained a marketing authorization and without undergoing the expense of clinical trials, especially if those competitors have cell processing facilities in the relevant EU member state. Similarly, certain hospitals may be able to compete with us on the basis of these rules.
Risks Related to Our Common Stock
We pay no dividends.
We have never paid cash dividends in the past, and currently do not intend to pay any cash dividends in the foreseeable future. We intend to retain earnings, if any, to finance the development and expansion of our business. Our future dividend policy will be subject to the discretion of our Board of Directors and will be contingent upon future earnings, if any, our financial condition, capital requirements, general business conditions, and other factors. Therefore, we can give no assurance that any dividends of any kind will ever be paid to holders of our common stock.
There is no assurance that an active trading market for our common stock will be sustained.
Our common stock is listed on Nasdaq. However, no assurance can be given that an active market for our common stock will be sustained. In addition, although there have been market makers in our common stock, we cannot assure that these market makers will continue to make a market in our securities or that other factors outside of our control will not cause them to stop market making in our securities. Making a market in securities involves maintaining bid and ask quotations and being able to effect transactions in reasonable quantities at those quoted prices, subject to various securities laws and other regulatory requirements. Furthermore, the development and maintenance of a public trading market depends upon the existence of willing buyers and sellers, the presence of which is not within our control or that of any market maker. Market makers are not required to maintain a continuous two-sided market, are required to honor firm quotations for only a limited number of securities, and are free to withdraw firm quotations at any time. Even with a market maker, factors such as our past losses from operations and the small size of our company mean that there can be no assurance of an active and liquid market for our securities developing in the foreseeable future. Even if there is a market for our securities, we cannot assure that securityholders will be able to resell their securities at any price.
Stockholders who hold unregistered shares of our common stock are subject to resale restrictions pursuant to Rule 144 due to our former status as a “shell company.”
We previously were a “shell company” pursuant to Rule 144, promulgated under the Securities Act, or Rule 144, and, as such, sales of our securities pursuant to Rule 144 cannot be made unless, among other things, we continue to remain subject to Section 13 or 15(d) of the Exchange Act, and we file all of our required periodic reports with the SEC under the Exchange Act. Because our unregistered securities cannot be sold pursuant to Rule 144 unless we continue to meet such requirements, any unregistered securities we sell in the future or issue to consultants or employees, in consideration for services rendered or for any other purpose, will have no liquidity unless we continue to comply with such requirements. As a result, it may be more difficult for us to obtain financing to fund our operations and pay our consultants and employees with our securities instead of cash.
|
|
We have incurred, and will continue to incur, increased costs as a result of being an SEC reporting company.
The Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as a variety of related rules implemented by the SEC, have required changes in corporate governance practices and generally increased the disclosure requirements of public companies. As a reporting company, we incur significant legal, accounting and other expenses in connection with our public disclosure and other obligations. Based upon SEC regulations currently in effect, we are required to establish, evaluate and report on our internal control over financial reporting. We believe that compliance with the myriad of rules and regulations applicable to reporting companies and related compliance issues will continue to require a significant amount of time and attention from our management.
Material weaknesses in our internal control over financial reporting may cause us to fail to timely and accurately report our financial results or result in a material misstatement of our consolidated financial statements.
We identified control deficiencies in the design and operation of our internal control over financial reporting that constituted a material weakness, as further described in Item 9A of this Annual Report (“Controls and Procedures”). A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our consolidated financial statements will not be prevented or detected on a timely basis. Our material weakness related to the following control deficiencies:
| ● | Lack of adherence to formal policies and procedures; |
| ● | Lack of risk assessment procedures on internal controls to detect financial reporting risks on a timely manner; and |
| ● | Lack of sufficient formal management testing over documented formal procedures and controls, and time to evaluate continuous effectiveness of controls to achieve complete and accurate financial reporting and disclosures, including documented controls over the preparation and review of journal entries and account reconciliations. |
The deficiencies described above, if not remedied, could result in a misstatement of one or more account balances or disclosures in our annual or interim consolidated financial statements that would not be prevented or detected, and, accordingly, we determined that these control deficiencies constitute a material weakness.
To address our material weakness, we have added accounting and finance personnel and implemented new financial accounting processes. We intend to continue to take steps to remediate the material weakness described above through implementing enhancements and controls within our accounting systems, hiring additional qualified accounting and finance resources and further evolving our accounting and quarterly and annual close processes. We will not be able to remediate these control deficiencies until these steps have been completed and have been operating effectively for a sufficient period of time and Management has concluded, through testing, that the controls are operating effectively. The redesign and implementation of improvements to our accounting and proprietary systems and controls may be costly and time consuming and the cost to remediate may impair our results of operations in the future.
|
|
If we fail to remediate our material weakness, identify future material weaknesses in our internal control over financial reporting or fail to meet the demands that have been placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, we may be unable to accurately report our financial results or report them within the timeframes required by law or stock exchange regulations. Failure to comply with Section 404 of the Sarbanes-Oxley Act could also potentially subject us to sanctions or investigations by the SEC or other regulatory authorities. If additional material weaknesses exist or are discovered in the future, and we are unable to remediate any such material weakness, our reputation, results of operations and financial condition could suffer.
Our stock price may fluctuate significantly and be highly volatile and this may make it difficult for a securityholder to resell our securities at the volume, prices and times the securityholder finds attractive.
The market price of our common stock may be subject to significant fluctuations and be highly volatile, which may make it difficult for a securityholder to resell our securities at the volume, prices and times the securityholder finds attractive. There are many factors that will impact our stock price and trading volume, including, but not limited to, the factors listed above under “Risks Related to Our Business Generally,” “Risks Related to Our Cell Therapy Product Development Efforts,” “Risks Related to Our Intellectual Property,” “Risks Related to Government Regulation,” “Risks Related to Our Common Stock” and “Risks Associated with Our Nasdaq Listing.”
Stock markets, in general, experience significant price and volume volatility, and the market price of our securities may continue to be subject to such market fluctuations that may be unrelated to our operating performance and prospects. Increased market volatility and fluctuations could result in a substantial decline in the market price of our securities.
There may be significant future issuances or resales of our common stock which may materially and adversely dilute stockholders’ ownership interest and affect the market price of our securities .
We currently have authorization to issue up to 75,000,000 shares of common stock of which, as of March 28, 2024, 6,769,919 shares were issued and outstanding. In addition, as discussed in Item 1 (“Business – Business Development – Material Events During 2024”), we are obligated to issue an additional 1,351,580 shares of common stock. We are not restricted from issuing additional shares of our common stock in the future, including securities convertible into, or exchangeable or exercisable for, shares of our common stock. In addition, there are 1,398,158 shares of Series B preferred stock issued and outstanding. Such shares are convertible under certain circumstances into an equal number of shares of common stock.
Pursuant to our November 2021 public offering of securities, we issued warrants for the purchase of an aggregate of 2,645,000 shares of common stock (of which warrants for the purchase of an aggregate of 1,675,000 shares of common stock have been exercised) as well as underwriter warrants for the purchase of 235,970 shares of common stock. We have an effective registration statement on Form S-3 under the Securities Act registering the issuance of such shares. The shares issuable pursuant to the registration statement on Form S-3 will be freely tradable in the public market, except for shares held by affiliates. In addition, in connection with the public offering and pursuant to exchange agreements entered into with holders of convertible notes and warrants, we issued an aggregate of 313,789 shares of common stock and warrants for the purchase of an aggregate of 1,856,938 shares of common stock (of which warrants for the purchase of an aggregate of 1,676,580 shares of common stock have been exercised). The shares of common stock issued to such holders are eligible for resale in the open market (subject to Rule 144 volume limitations applicable to affiliates), potentially causing sales in the market to increase and our stock price to decline. We have registered the resale of the shares of common stock issuable upon exercise of such warrants. The issuance of shares of common stock upon exercise of the above warrants would dilute the ownership of our stockholders.
|
|
In addition, in February 2024, in connection with the warrant exercises discussed in Item 1 (“Business – Business Development – Material Events During 2024”), we issued warrants for the purchase of an aggregate of 2,513,686 shares of common stock. We have agreed to file a registration statement with the SEC to register the resale of the shares of common stock underlying such warrants.
We also have effective registration statements on Form S-8 under the Securities Act registering an aggregate of 3,850,000 shares of our common stock issuable under our 2021 Stock Incentive Plan, or the 2021 Plan (of which 319,797 shares have been issued). As of March 28, 2024, options to purchase 3,401,608 shares of our common stock were outstanding under the 2021 Plan. The shares issued and issuable pursuant to the registration statements on Form S-8 will be freely tradable in the public market, except for shares held by affiliates. We may include a resale prospectus in a registration statement on Form S-8 with regard to the 2021 Plan covering the resale of the shares issuable to Messrs. Alstodt and Silva (and other affiliates) upon their exercise of options held by them and the vesting of the above described RSUs. The resale of such shares will be currently subject to the volume limitations imposed by Rule 144.
Further, we have an effective shelf registration statement on Form S-3 under the Securities Act registering $75,000,000 of our equity and debt securities. Pursuant to the requirements of Form S-3, we currently may sell pursuant to such Form S-3, during any 12 month period, securities having an aggregate market value of no more than one-third of the aggregate market value of the shares of our common stock held by non-affiliates.
The sale of a substantial number of shares of our common stock or securities convertible into, or exchangeable or exercisable for, shares of our common stock, whether directly by us in future offerings or by our existing stockholders in the secondary market, the perception that such issuances or resales could occur or the availability for future issuances or resale of shares of our common stock or securities convertible into, or exchangeable or exercisable for, shares of our common stock could materially and adversely affect the market price of our securities and our ability to raise capital through future offerings of equity or equity-related securities on attractive terms or at all.
In addition, our Board of Directors is authorized to designate and issue 18,456,842 shares of preferred stock without further stockholder approval, containing such rights and preferences as our Board of Directors shall determine. We may also issue other equity and equity-related securities that are senior to our common stock in the future for a number of reasons, including, without limitation, to support operations and growth, and to comply with any future changes in regulatory standards.
|
|
Anti-takeover provisions and the regulations to which we may be subject may make it more difficult for a third party to acquire control of us, even if the change in control would be beneficial to our securityholders.
We are incorporated in Nevada. Anti-takeover provisions in Nevada law and our articles of incorporation and bylaws could make it more difficult for a third party to acquire control of us and may prevent stockholders from receiving a premium for their securities. Our certificate of incorporation provides that our Board of Directors may issue up to 20,000,000 shares of preferred stock, in one or more series, without stockholder approval and with such terms, preferences, rights and privileges as the Board of Directors may deem appropriate. Of such 20,000,000 authorized shares, 1,398,158 shares of Series B preferred stock are issued and outstanding. These provisions and other factors may hinder or prevent a change in control, even if the change in control would be perceived as beneficial to, or sought by, our other stockholders.
Risks Associated with Our Nasdaq Listing
We cannot assure you that we will be able to continue to comply with the minimum bid price requirement of Nasdaq.
Although the market price of our common stock satisfied the initial listing minimum bid price requirement for Nasdaq, there can be no assurance that the market price of our common stock will remain at the $1.00 per share level required for continuing compliance with that requirement. There are many factors, such as negative financial or operational results, that could adversely affect the market price of our common stock and jeopardize our ability to maintain Nasdaq’s minimum bid price requirement.
The market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that our Nasdaq listing has helped generate greater and broader investor interest, including institutional investors, there can be no assurances in that regard. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. |
Not applicable.
| ITEM 1C. | CYBERSECURITY. |
We recognize the critical importance of cybersecurity in safeguarding sensitive information, maintaining operational resilience, and protecting stakeholders’ interests.
|
|
We are in the process of establishing a cybersecurity policy designed to establish a comprehensive framework for identifying, assessing, mitigating, and responding to cybersecurity risks across the organization and implement protocols to evaluate, recognize, and address significant risks, including those posed by cybersecurity threats. This strategy will encompass the utilization of standard traffic monitoring tools, educating personnel to identify and report abnormal activities, and partnering with reputable service providers capable of upholding security standards equivalent to or exceeding our own.
These measures are to be integrated into our broader operational risk management framework aimed at minimizing exposure to unnecessary risks across our operations. For cybersecurity, we collaborate with consultants and third-party service providers to implement industry-standard strategies aimed at identifying and mitigating potential threats or vulnerabilities within our systems. Additionally, the policy strategy will have a comprehensive cybersecurity crisis response plan to manage high severity security incidents, ensuring efficient coordination across the organization.
Cybersecurity threats have not significantly impacted our operations, and we do not anticipate such risks materially affecting our business, strategy, financial condition, or results of operations. However, given the escalating sophistication of cybersecurity threats, our preventive measures may not always suffice. Despite well-designed controls, we acknowledge the inability to foresee all security breaches, including those stemming from third-party misuse of AI technologies, and the potential challenges in implementing timely preventive measures. See Item 1A (“Risk Factors - Our internal computer systems, or those that are expected to be used by our clinical investigators, clinical research organizations or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of development programs for our product candidates.”) for further insights into cybersecurity attack-related risks that we may face.
Our Chief Financial Officer will oversee our information security programs, including cybersecurity initiatives, and our cybersecurity incident response process. The Audit Committee of the Board oversees cybersecurity risk management activities, supported by our management, the Board of Directors, and external consultants. We assess and prioritize risks based on potential impact, implement technical controls, and monitor third-party vendors’ security practices.
| ITEM 2. | PROPERTIES. |
Our principal executive offices and laboratory are located at 40 Marcus Drive, Suite One, Melville, New York. We occupy 6,800 square feet of space at the premises pursuant to a lease that expires in December 2024. The lease provides for a current annual base rental of $173,060. Our premises are suitable and adequate for our current operations.
| ITEM 3. | LEGAL PROCEEDINGS. |
None.
| ITEM 4. | MINE SAFETY DISCLOSURES. |
Not applicable.
|
|
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information
Transactions in our common stock are currently reported under the symbol “BRTX” on the Nasdaq Capital Market.
Holders
As of March 28, 2024, there were 352 record holders of our shares of common stock.
Dividends
Not applicable.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
| ITEM 6. | [RESERVED] |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
The following discussion and analysis of the consolidated results of operations and financial condition of BioRestorative Therapies, Inc. as of December 31, 2023 and 2022 and for the years ended December 31, 2023 and 2022 should be read in conjunction with our financial statements and the notes to those financial statements that are included elsewhere in this Annual Report following Item 16 (“Form 10-K Summary”). References in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” to “us,” “we,” “our,” and similar terms refer to BioRestorative Therapies, Inc.. This Annual Report contains forward-looking statements as that term is defined in the federal securities laws. The events described in forward-looking statements contained in this Annual Report may not occur. Generally, these statements relate to business plans or strategies, projected or anticipated benefits or other consequences of our plans or strategies, projected or anticipated benefits from acquisitions that may be made by us, or projections involving anticipated revenues, earnings or other aspects of our operating results. The words “may,” “will,” “expect,” “believe,” “anticipate,” “project,” “plan,” “intend,” “estimate,” and “continue,” and their opposites and similar expressions, are intended to identify forward-looking statements. We caution you that these statements are not guarantees of future performance or events and are subject to a number of uncertainties, risks and other influences, many of which are beyond our control, which may influence the accuracy of the statements and the projections upon which the statements are based. Reference is made to Item 1A of this Annual Report (“Risk Factors”) for a discussion of some of the uncertainties, risks and assumptions associated with these statements.
|
|
Overview
We develop therapeutic products and medical therapies using cell and tissue protocols, primarily involving adult stem cells.
We are currently pursuing our Disc/Spine Program with our initial investigational therapeutic product being called BRTX-100. In March 2022, a United States patent issued in our Disc/Spine Program. We have received authorization from the FDA to commence a Phase 2 clinical trial investigating the use of BRTX-100 in the treatment of chronic lower back pain arising from degenerative disc disease. We have commenced such clinical trial through the execution of a CRO agreement with PRC Clinical, the execution of clinical trial site agreements, patient enrollment, the commencement of patient procedures, the purchase of manufacturing equipment and the expansion of our laboratory to include capabilities for clinical production. We have obtained an exclusive license to use technology for investigational adult stem cell treatment of disc and spine conditions, including protruding and bulging lumbar discs. The technology is an advanced stem cell injection procedure that may offer relief from lower back pain, buttock and leg pain, and numbness and tingling in the leg and foot.
We are also developing our ThermoStem Program. This pre-clinical program involves the use of brown adipose (fat) in connection with the cell-based treatment of type 2 diabetes and obesity as well as hypertension, other metabolic disorders and cardiac deficiencies. United States patents related to the ThermoStem Program were issued in September 2015, January 2019, March 2020, March 2021, July 2021, June 2023 and December 2023; Australian patents related to the ThermoStem Program were issued in April 2017, October 2019 and August 2021; Japanese patents related to the ThermoStem Program were issued in December 2017, June 2021, February 2022 and June 2023; Israeli patents related to our ThermoStem Program were issued in October 2019, May 2020 and March 2022; and European patents related to the ThermoStem Program were issued in April 2020, January 2021 and July 2023.
We have licensed a patented curved needle device that is a needle system designed to deliver cells and/or other therapeutic products or materials to the spine and discs or other potential sites. We anticipate that FDA approval or clearance will be necessary for this device prior to commercialization. We do not intend to utilize this device in connection with our Phase 2 clinical trial with regard to BRTX-100.
We are seeking to develop a biologics-based cosmetic products business. Pursuant to such business, we would manufacture and sell cosmetics and hair growth products designed for cosmetic and aesthetic uses.
Our offices are located in Melville, New York where we have established a laboratory facility in order to increase our capabilities for the further development of possible cellular-based treatments, products and protocols, stem cell-related intellectual property and translational research applications.
|
|
As of December 31, 2023, our accumulated deficit was $167,056,381. We have historically only generated a modest amount of revenue, and our losses have principally been operating expenses incurred in research and development, marketing and promotional activities in order to commercialize our products and services, plus costs associated with meeting the requirements of being a public company. We expect to continue to incur substantial costs for these activities over at least the next year.
In November 2021, we completed a $23,000,000 underwritten public offering of units of securities pursuant to which an aggregate of 2,300,000 shares of our common stock and warrants for the purchase of an aggregate of 2,645,000 shares of our common stock were issued. We are using the net proceeds from the offering as follows: (i) undertaking of clinical trials with respect to BRTX-100 and its related collection and delivery procedure; (ii) pre-clinical research and development with respect to our ThermoStem Program; and (iii) general corporate and working capital purposes. In connection with the public offering, our common stock was listed on the Nasdaq Capital Market.
In November 2021, concurrently with the consummation of the public offering, we issued an aggregate of 313,789 shares of our common stock, 1,543,158 shares of our Series A preferred stock and warrants for the purchase of an aggregate of 1,856,938 shares of our common stock in exchange for convertible promissory notes in the aggregate principal amount of $10,046,897, together with accrued interest thereon, and warrants for the purchase of an aggregate of 3,677,997 shares of our common stock. Such indebtedness and warrants were exchanged at a price of $10.00 per unit of securities, consistent with the public offering price of our units of common stock and warrants. The newly issued warrants are exercisable for a period of five years at an exercise price of $10.00 per share. In September 2022, the 1,543,158 shares of Series A preferred stock were exchanged for an equal number of shares of Series B preferred stock. As of March 28, 2024, there were 1,398,158 shares of Series B preferred stock issued and outstanding.
In April 2023, we entered into a Capital on Demand Sales Agreement with JonesTrading Institutional Services LLC, or the Sales Agent, under which we currently have the ability to issue and sell shares of our common stock, from time to time, through the Sales Agent, up to an aggregate offering price of approximately $6,109,000 in what is commonly referred to as an “at-the-market”, or ATM, program. During the year ended December 31, 2023, we sold 132,827 shares of our common stock under the ATM program with the Sales Agent at a weighted-average gross price of approximately $4.68 per share and raised approximately $622,000 of gross proceeds. The total commissions and related legal fees were approximately $127,000, and we received net proceeds of approximately $495,000. As of December 31, 2023, we had remaining capacity to sell up to an additional $5,487,000 of common stock under the ATM program.
In July 2023, we sold an aggregate of 685,033 shares of our common stock in a registered direct public offering. We received net proceeds of approximately $1,854,000 from the offering.
The net proceeds received from our November 2021 public offering, the ATM program and the registered direct offering are sufficient for us to complete our Phase 2 clinical trial with regard to BRTX-100; however, we will require significant additional funding to complete our contemplated Phase 3 BRTX-100 clinical trial (assuming the receipt of no revenues). We will also require a substantial amount of additional funding to implement our other programs as discussed in this Annual Report under the caption Item 1 (“Business”), including our metabolic ThermoStem Program, and fund general operations. No assurance can be given that the amount of funding that we anticipate may be required for such purposes is correct or that we will be able to accomplish our goals within the timeframes projected. In addition, no assurance can be given that we will be able to obtain any required financing on commercially reasonable terms or otherwise.
|
|
Consolidated Results of Operations
Year Ended December 31, 2023 Compared with Year Ended December 31, 2022
The following table presents selected items in our consolidated statements of operations for the years ended December 31, 2023 and 2022, respectively:
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenues, net | $ | 145,800 | $ | 119,800 | ||||
| Operating Expenses: | ||||||||
| Research and development | 4,034,591 | 3,513,352 | ||||||
| General and administrative | 11,331,983 | 15,580,473 | ||||||
| Total Operating Expenses | 15,366,574 | 19,093,825 | ||||||
| Loss From Operations | (15,220,774 | ) | (18,974,025 | ) | ||||
| Other Income: | ||||||||
| Interest income | (552,293 | ) | (11,650 | ) | ||||
| Gain on PPP loan forgiveness | - | (250,000 | ) | |||||
| Grant income | (83,333 | ) | (110,518 | ) | ||||
| Other income | (169,664 | ) | (107,088 | ) | ||||
| Total Other Income | (805,290 | ) | (479,256 | ) | ||||
| Net Loss | $ | (14,415,484 | ) | $ | (18,494,769 | ) | ||
Revenues
For the years ended December 31, 2023 and 2022, we generated $145,800 and $119,800, respectively, of royalty revenue in connection with our sublicense agreement.
Research and development
Research and development expenses include cash and non-cash compensation of (a) our Vice President of Research and Development; (b) our Scientific Advisory Board members; and (c) laboratory staff and costs related to our brown fat and disc/spine initiatives. Research and development expenses are expensed as they are incurred. For the year ended December 31, 2023, research and development expenses increased by $521,239, or 15%, to $4,034,591 compared to $3,513,352 for the year ended December 31, 2022. The increase was primarily driven by increased compensation of $857,294 due to salary increases and bonuses, increased lab supplies expenses of $193,298, increased repair and maintenance expenses of $30,521, increased license agreements and fees expenses of $25,155 and increased consulting fees of $112,581, offset by a decrease in lab site fees of $53,029, and a decrease in PRC service expenses of $644,245 as the result of non-recurring start-up and execution expenses incurred during the year ended December 31, 2022.
|
|
We expect that our research and development expenses will continue to increase with the continuation of the aforementioned initiatives.
General and administrative
General and administrative expenses consist primarily of salaries, bonuses, payroll taxes and stock-based compensation to employees (excluding any cash or non-cash compensation of our Vice President of Research and Development and our laboratory staff), as well as corporate expenses such as legal and professional fees, investor relations and occupancy related expenses. For the year ended December 31, 2023, general and administrative expenses decreased by $4,248,490, or 27% to $11,331,983 from $15,580,473 for the year ended December 31, 2022. The decrease was primarily driven by a $4,753,377 decrease in stock-based compensation, decreased franchise tax expense of $130,850, decreased amortization expense of $104,630, decreased travel expense of $84,122, and decreased insurance expense of $53,858, all offset by an increase in compensation of $775,678 due to salary increases and bonuses, increased investor relations expense of $80,957, increased professional fees of $44,056 and an increase in office supplies expense of $10,616.
We expect that our general and administrative expenses related to operations will increase as we expand our staff, develop our infrastructure and incur additional costs to support the growth of our business.
Interest income
For the year ended December 31, 2023, interest income increased $540,643, or 4641%, to $552,293 from $11,650 for the year ended December 31, 2022. The increase was due to the interest and dividend income of the investments held in marketable securities.
Gain on PPP loan forgiveness
Under the terms of the U.S. Small Business Administration’s Paycheck Protection Program, or PPP, our $250,000 PPP loan was forgiven during the year ended December 31, 2022.
Grant income
Grant income of $83,333 during the year ended December 31, 2023 consisted of funding received under a National Institutes of Health Small Business Technology Transfer (STTR) Phase 1 grant, offset by related expenses. Grant income of $110,518 during the year ended December 31, 2022 consisted of funding received under a $256,000 National Institutes of Health Small Business Technology Transfer (STTR) Phase 1 grant, which we were awarded in September 2021.
Other income, net
For the year ended December 31, 2023, other income, net of $169,664 primarily related to an Employee Retention Tax Credit, gains from settlements of certain accrued expenses and realized and unrealized gain on investments. For the year ended December 31, 2022, Other income, net of $107,088 primarily related to gains from settlements of certain accrued expenses and unrealized gain on investments.
|
|
Liquidity and Capital Resources
Liquidity
We measure our liquidity in a number of ways, including the following:
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash, Cash Equivalents and Investments | $ | 11,065,995 | $ | 14,749,408 | ||||
| Working Capital | $ | 10,327,134 | $ | 14,688,188 | ||||
Availability of Additional Funds
Based upon our accumulated deficit of $167,056,381 as of December 31, 2023, along with our forecast for continued operating losses and our need for financing to fund our contemplated clinical trials, as of such date, we require additional equity and/or debt financing to continue our operations. However, based on cash on hand and investments as of December 31, 2023 and the receipt of approximately $8,100,000 in gross proceeds in February 2024 pursuant to the warrant exercises discussed below, management believes that we have sufficient cash to fund operations for the twelve months from the issuance of the financial statements included in this Annual Report.
Our operating needs include the planned costs to operate our business, including amounts required to fund our clinical trials, working capital and capital expenditures. Our future capital requirements and the adequacy of our available funds will depend on many factors, including our ability to successfully commercialize our products and services, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement our product and service offerings.
We may be unable to raise sufficient additional capital when we need it or raise capital on favorable terms. Future financing may require us to pledge certain assets and enter into covenants that could restrict certain business activities or our ability to incur further indebtedness and may contain other terms that are not favorable to our stockholders or us. If we are unable to obtain adequate funds on reasonable terms, we may be required to significantly curtail or discontinue operations or obtain funds by entering into financing agreements on unattractive terms.
“At-the-Market” Offering
In April 2023, we entered into a Capital on Demand Sales Agreement with JonesTrading Institutional Services LLC, or the Sales Agent, under which we currently have the ability to issue and sell shares of our common stock, from time to time, through the Sales Agent, up to an aggregate offering price of approximately $6,109,000 in what is commonly referred to as an “at-the-market”, or ATM, program. During the year ended December 31, 2023, we sold 132,827 shares of our common stock under the ATM program with the Sales Agent at a weighted-average gross price of approximately $4.68 per share and raised approximately $622,000 of gross proceeds. The total commissions and related legal fees were approximately $127,000, and we received net proceeds of approximately $495,000. As of December 31, 2023, we had remaining capacity to sell up to an additional approximately $5,487,000 of common stock under the ATM program.
|
|
Registered Direct Offering
In July 2023, we sold an aggregate of 685,033 shares of our common stock in a registered direct public offering. We received net proceeds of approximately $1,831,000 from the offering.
Warrant Exercises
In February 2024, we received gross proceeds of approximately $8,100,000 pursuant to the exercise of outstanding warrants. See Item 1 (“Business – Business Development – Material Events During 2024”) for additional information.
Cash Flows
During the years ended December 31, 2023 and 2022, our sources and uses of cash were as follows:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Net cash used in operating activities | $ | (6,430,211 | ) | $ | (5,913,100 | ) | ||
| Net cash provided by (used in) investing activities | 3,252,043 | (13,399,855 | ) | |||||
| Net cash provided by financing activities | 2,351,343 | - | ||||||
| Net decrease in cash | $ | (826,825 | ) | $ | (19,312,955 | ) | ||
Net Cash Used in Operating Activities
Net cash used in operating activities was $6,430,211 for the year ended December 31, 2023, primarily due to cash used to fund the net loss of $14,415,484, which was partially offset by non-cash expenses of $7,469,947 related primarily to stock-based compensation and $515,326 of cash provided by changes in our operating assets and liabilities. Net cash used in operating activities was $5,913,100 for the year ended December 31, 2022, primarily due to cash used to fund the net loss of $18,494,769 and a non-cash gain of $250,000 on forgiveness of our PPP loan, which was partially offset by non-cash expenses of $12,567,525 related primarily to stock-based compensation and $14,144 of cash provided by changes in operating assets and liabilities.
Net Cash Provided by (Used in) Investing Activities
Net cash provided by investing activities increased by $16,731,432 for the year ended December 31, 2023 compared to the year ended December 31, 2022, primarily due to the initial use of cash in the amount of $16,000,000 for the purchase of investments held in marketable securities made in 2022 for which there was no corresponding activity in 2023.
|
|
Net Cash Provided by Financing Activities
Net cash provided by financing activities increased by $2,351,343 for the year ended December 31, 2023 compared to the year ended December 31, 2022 due to the net proceeds from the ATM and registered direct public offerings of our common stock.
Effects of Inflation
We do not believe that inflation had a material impact on our business, revenues or operating results during the periods presented.
Critical Accounting Estimates
We prepare our consolidated financial statements in accordance with U.S. generally accepted accounting principles, or GAAP, which require our management to make estimates that affect the reported amounts of assets, liabilities and disclosures of contingent assets and liabilities at the balance sheet dates, as well as the reported amounts of revenues and expenses during the reporting periods. To the extent that there are material differences between these estimates and actual results, our financial condition or results of operations would be affected. We base our estimates on our own historical experience and other assumptions that we believe are reasonable after taking account of our circumstances and expectations for the future based on available information. We evaluate these estimates on an ongoing basis.
We consider an accounting estimate to be critical if: (i) the accounting estimate requires us to make assumptions about matters that were highly uncertain at the time the accounting estimate was made, and (ii) changes in the estimate that are reasonably likely to occur from period to period or the use of different estimates that we reasonably could have used in the current period would have a material impact on our financial condition or results of operations. There are items within our financial statements that require estimation but are not deemed critical, as defined above.
For a detailed discussion of our significant accounting policies and related judgments, see Note 2 of the Notes to Consolidated Financial Statements in “Item 8. Financial Statements and Supplementary Data” of this Annual Report.
Recently Issued Accounting Pronouncements
See Note 2 to our consolidated financial statements for the years ended December 31, 2023 and 2022 included elsewhere in this Annual Report following Item 16 (“Form 10-K Summary”).
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
|
|
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
Not applicable.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
The financial statements required by this Item 8 of this Annual Report are included in this Annual Report following Item 16 (“Form 10-K Summary”). As a smaller reporting company, we are not required to provide supplementary financial information.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as that term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act), that are designed to ensure that information required to be disclosed in our reports under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosures. In designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Any controls and procedures, no matter how well designed and operated, can provide only reasonable, not absolute, assurance of achieving the desired control objectives.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we are required to perform an evaluation of our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) under the Exchange Act, as of December 31, 2023.
Management has completed such evaluation and has concluded that our disclosure controls and procedures were not effective to provide reasonable assurance that information required to be disclosed by us in reports we file or submit under the Exchange Act is appropriate to allow timely decisions regarding required disclosures. As a result of the material weakness in internal controls over financial reporting described below, we concluded that our disclosure controls and procedures as of December 31, 2023 were not effective.
|
|
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is a process designed under the supervision of our principal executive and principal financial officer and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our consolidated financial statements for external reporting purposes in accordance with GAAP.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Material Weaknesses in Internal Control over Financial Reporting
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 based on the framework established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, management has determined that our internal control over financial reporting as of December 31, 2023 was not effective.
A material weakness, as defined in the standards established by the Sarbanes-Oxley is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
The ineffectiveness of our internal control over financial reporting was due to the following material weaknesses:
| ● | Lack of adherence to formal policies and procedures; |
| ● | Lack of risk assessment procedures on internal controls to detect financial reporting risks in a timely manner; |
| ● | Lack of sufficient formal management testing over documented formal procedures and controls, and time to evaluate continuous effectiveness of controls to achieve complete and accurate financial reporting and disclosures, including documented controls over the preparation and review of journal entries and account reconciliations. |
Management’s Plan to Remediate the Material Weakness
Management has been implementing and continues to implement measures designed to ensure that control deficiencies contributing to the material weakness are remediated, such that these controls are designed, implemented, and operating effectively. The remediation actions include:
| ● | Management personnel, including our Chief Financial Officer, are overseeing the financial reporting process and implementation of enhanced controls and governance; |
| ● | Engagement of external financial consulting firm to continue to enhance financial reporting, financial operations and internal controls; and |
| ● | Documentation of key procedures and controls using a risk-based approach. |
|
|
Management is committed to maintaining a strong internal controls environment and implementing measures designed to help ensure that control deficiencies contributing to the material weakness are remediated as soon as possible. We have documented key procedures and controls using a risk-based approach and have, therefore, made progress toward remediation. We continue to implement our remediation plan, which includes continued engagement of an external financial consulting firm to enhance financial reporting and operations as well as design and implementation of controls. We will consider the material weakness remediated after the applicable controls operate for a sufficient period of time, and Management has concluded, through testing, that the controls are operating effectively.
We will continue to monitor and evaluate the effectiveness of our internal controls and procedures over financial reporting on an ongoing basis and is committed to taking further action and implementing additional enhancements or improvements, as necessary and as funds allow.
This Annual Report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our independent registered public accounting firm pursuant to rules of the Securities and Exchange Commission that exempt smaller reporting companies from this requirement.
Changes in Internal Control Over Financial Reporting
Other than described above, there have been no changes in our internal control over financial reporting that occurred during our fourth quarter of 2023 that have materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION. |
None.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS. |
Not applicable.
|
|
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
Directors and Executive Officers
Information regarding our directors and executive officers is set forth below. Each of our officers devotes his full business time in providing services on our behalf.
| Name | Age | Positions Held | ||
| Lance Alstodt | 53 | Chief Executive Officer, President and Chairman of the Board | ||
| Francisco Silva | 49 | Vice President of Research and Development, Secretary and Director | ||
| Robert E. Kristal | 57 | Chief Financial Officer | ||
| Robert Paccasassi | 55 | Vice President of Quality Assurance/Regulatory Compliance | ||
| Nickolay Kukekov, Ph.D. | 50 | Director, Compensation Committee Chair | ||
| Patrick F. Williams | 51 | Director, Audit Committee Chair | ||
| David Rosa | 60 | Director, Nominating Committee Chair |
Lance Alstodt
Lance Alstodt has served as our Chief Executive Officer, President and Chairman of the Board since November 2020. He served as our Executive Vice President and Chief Strategy Officer from October 2018 to February 2020. Since 2013, Mr. Alstodt has served as Chief Executive Officer of MedVest Consulting Corporation, an advisory and capital firm that focuses exclusively on the healthcare industry. Prior to MedVest, he was an investment banker with over 23 years of experience with respect to healthcare investment banking, including mergers and acquisitions. From 2011 to 2013, Mr. Alstodt was a Managing Director at Leerink Partners where he helped lead its medical technology sector. From 2009 to 2011, he was a Managing Director and Head of Medical Technology at Oppenheimer & Co. From 2000 to 2009, Mr. Alstodt was a Managing Director in the Healthcare Group and Global Mergers and Acquisitions Group at Bank of America Merrill Lynch. He previously spent seven years as a Vice President in the Global Mergers and Acquisitions Group at J.P. Morgan Chase, where he worked extensively on acquisitions, leveraged buyouts, private and public financings, exclusive sales and general advisory assignments. Mr. Alstodt received a degree in Economics from the State University of New York at Albany, with a secondary concentration in Finance and Marketing. We believe that Mr. Alstodt’s executive-level management experience with us and other healthcare businesses and his extensive experience in the investment banking field relating to the healthcare sector give him the qualifications to serve as one of our directors.
Francisco Silva
Francisco Silva has served as our Vice President of Research and Development since March 2013, having also previously served in such position from April 2011 until March 2012. Mr. Silva was elected our Secretary and a director in November 2020. He served as our Research Scientist from March 2012 to June 2012 and as our Chief Scientist from June 2012 to March 2013. From 2007 to 2011, Mr. Silva served as Chief Executive Officer of DV Biologics LLC, and as President of DaVinci Biosciences, LLC, companies engaged in the commercialization of human based biologics for both research and therapeutic applications. From 2003 to 2007, Mr. Silva served as Vice President of Research and Development for PrimeGen Biotech LLC, a company engaged in the development of cell based platforms. From 2002 to 2003, he was a Research Scientist with PrimeGen Biotech and was responsible for the development of experimental designs that focused on germ line reprogramming stem cell platforms. Mr. Silva has taught courses in biology, anatomy and advanced tissue culture at California State Polytechnic University. He has obtained a number of patents relating to stem cells and has had numerous articles published with regard to stem cell research. Mr. Silva graduated from California State Polytechnic University with a degree in Biology. He also obtained a Graduate Presidential Fellowship and MBRS Fellowship from California State Polytechnic University. We believe that Mr. Silva’s executive-level management experience with us since April 2011 and his extensive knowledge of the science related to our business give him the qualifications to serve as one of our directors.
|
|
Robert E. Kristal
Robert E. Kristal has served as our Chief Financial Officer since November 2021. Mr. Kristal is an experienced Wall Street and Bay Street professional who has served in various management roles within multiple business lines of investment banks. From 2016 to 2020, he was Head of Equity Research at H.C. Wainwright. Mr. Kristal provided investment banking and merchant banking services from 2013 to 2016 at H.C. Wainwright and T.R. Winston. He is a Charted Financial Analyst. Mr. Kristal received a Bachelor of Arts degree in Economics from Wilfrid Laurier University and a Bachelor of Commerce (Honors) degree in Finance from the University of Windsor.
Robert Paccasassi
Robert Paccasassi has served as our Vice President of Quality Assurance/Regulatory Compliance since December 2021, having previously served in such position from August 2016 to September 2020, and having previously served as our Director of Quality and Compliance from September 2015 to August 2016. Mr. Paccasassi has over 20 years of experience in highly regulated product operations, with specific expertise in GMP (large and small molecule) clinical and commercial quality assurance and regulatory compliance leadership roles. He was the Director of Quality Systems (GMP) at Merck KGaA (Dermstadt, Germany) from 2011 to 2014. In this role, Mr. Paccasassi was responsible for leading the ongoing development and implementation of the Corporate Quality Unit’s global GMP policies, processes and directives. He held key quality and compliance management roles at EMD Serono, Biogen Idec, Millennium Pharmaceuticals and Regeneron Pharmaceuticals. Mr. Paccasassi was a Chief Technologist/Site Head overseeing all day to day technical and quality operations of two cGMP biologic production laboratories for Curative Health Services. He was also a Medical Technologist working in the field of immunohematology at Brigham & Women’s Hospital, Boston, Massachusetts. Mr. Paccasassi received a Masters in Business Administration (MBA) degree from Johnson & Wales University and a Bachelor of Science degree in Medical Technology/Biology from the University of Rhode Island.
Nickolay Kukekov, Ph.D.
Nickolay Kukekov, Ph.D. has served as one of our directors since March 2021 and Chair of our Board’s Compensation Committee since November 2021. For more than the past fifteen years, Dr. Kukekov has held a number of healthcare investment banking positions. He has served as Senior Managing Director of Paulson Investment Company, LLC since 2020. From 2012 to 2020, Dr. Kukekov was a founding partner of Highline Research Advisors LLC. He served as a Managing Director of Summer Street Research Partners from 2010 to 2012. From 2007 to 2009, Dr. Kukekov was a Managing Director of Paramount Capital. He served as a Vice President of Rodmen & Renshaw from 2006 to 2007. He serves as a director of Brain Scientific, Inc. and Omnia Wellness Inc. whose shares are publicly traded. Dr. Kukekov received a Bachelor of Arts degree in molecular, cellular and developmental biology from the University of Colorado at Boulder and a Ph.D. in neuroscience from Columbia University College of Physicians and Surgeons. We believe that Dr. Kukekov’s extensive experience in the investment banking field relating to the healthcare sector and his strong background in regenerative medicine give him the qualifications to serve as one of our directors.
|
|
Patrick F. Williams
Patrick F. Williams has served as one of our directors and Chair of our Board’s Audit Committee since November 2021. Mr. Williams has more than 20 years of experience across medical device, consumer product goods and technology sectors. Appointed as Chief Financial Officer of STAAR Surgical Company, or STAAR, in July 2020, Mr. Williams is responsible for optimizing the financial performance of STAAR and ensuring the scalability of various functions to support high growth expansion. From 2016 to 2019, he served as the Chief Financial Officer of Sientra, Inc. before transitioning to General Manager for its miraDry® business unit. From 2012 to 2016, Mr. Williams served as Chief Financial Officer of ZELTIQ Aesthetics, Inc., a publicly-traded medical device company that was acquired by Allergan. Previously, he served as Vice President in finance, strategy and investor relations roles from 2007 to 2012 at NuVasive, Inc., a San-Diego based medical device company servicing the spine sector. He has also held finance roles with Callaway Golf and Kyocera Wireless. Mr. Williams received an MBA in Finance and Management from San Diego State University and a Bachelor of Arts in Economics from the University of California, San Diego. We believe that Mr. Williams’ executive-level management experience with healthcare-related businesses, including his financial management expertise, give him the qualifications to serve as one of our directors.
David Rosa
David Rosa has served as one of our directors and Chair of our Board’s Nominating Committee since November 2021. Mr. Rosa has served as the Chief Executive Officer, President and a director of NeuroOne Medical Technologies Corporation, or NeuroOne (Nasdaq: NMTC), since July 2017 and served as Chief Executive Officer and a director of NeuroOne, Inc., formerly its wholly-owned subsidiary, from October 2016 until December 2019, when NeuroOne, Inc. merged with and into NeuroOne. NeuroOne is committed to providing minimally invasive and hi-definition solutions for EEG recording, brain stimulation and ablation solutions for patients suffering from epilepsy, Parkinson’s disease, dystonia, essential tremors, chronic pain due to failed back surgeries and other related neurological disorders that may improve patient outcomes and reduce procedural costs. From November 2009 to November 2015, Mr. Rosa served as the Chief Executive Officer and President of Sunshine Heart, Inc., n/k/a CHF Solutions, Inc. (Nasdaq: CHFS), a publicly-held early-stage medical device company. From 2008 to November 2009, he served as Chief Executive Officer of Milksmart, Inc., a company that specializes in medical devices for animals. From 2004 to 2008, Mr. Rosa served as the Vice President of Global Marketing for Cardiac Surgery and Cardiology at St. Jude Medical, Inc. He serves as a director on the board of directors of Biotricity Inc. (Nasdaq:BTCY) and Healthcare Triangle, Inc. (Nasdaq:HCTI). Mr. Rosa is Chairman of Neuro Event Labs, a privately-held company in Finland, and an Advisory Board member of SYNAPS Dx, a privately-held company in Bethesda, Maryland. We believe that Mr. Rosa’s senior leadership experience in the medical device industry and his strong technical, strategic, and operational expertise give him the qualifications to serve as one of our directors.
|
|
Scientific Advisory Board
The following persons are the members of our Scientific Advisory Board:
| Name | Principal Positions | |
|
Wayne Marasco, M.D., Ph.D. Chairman |
Professor, Department of Cancer Immunology & AIDS, Dana-Farber Cancer Institute; Professor of Medicine, Harvard Medical School; Principal Faculty Member, Harvard Stem Cell Institute |
|
|
Jason Lipetz, M.D. Chairman, Disc Advisory Committee |
Founder, Long Island Spine Rehabilitation Medicine; Chief of Spine Medicine, Northwell Health Spine Center; Clinical Assistant Professor, Department of Physical Medicine and Rehabilitation, Zucker School of Medicine at Hofstra/Northwell |
|
| Wayne J. Olan, M.D. |
Director, Interventional and Endovascular Neurosurgery; Associate Professor, Neurosurgery and Radiology, George Washington University Medical Center; Consulting Physician, Department of Radiology, National Institutes of Health |
|
|
Joy Cavagnaro, Ph.D., DABT, RAC |
President and Founder, Access BIO, L.C.; Fellow, Academy of Toxicological Sciences and the Regulatory Professional Society; Formerly Senior Pharmacologist and Director of Quality Assurance, Food and Drug Administration’s Center for Biologics Evaluation and Research |
|
| Harvinder Sandhu, M.D. |
Orthopedic Spine Surgeon, Hospital for Special Surgery; Formerly Chief of Spinal Surgery Service, UCLA Medical Center |
|
| Christopher Plastaras, M.D. |
Clinical Director of Musculoskeletal Spine and Sports Rehabilitation Medicine and Physiatrist, MossRehab; Formerly Director of The Penn Spine and Rehabilitation Center; Formerly Director of Spine, Sports and Musculoskeletal Medicine Fellowship, University of Pennsylvania |
Family Relationships
There are no family relationships among any of our executive officers, directors and Scientific Advisory Board members.
|
|
Term of Office
We have a classified Board of Directors. The directors will hold office until the respective annual meetings of stockholders indicated below and until their respective successors are elected and qualified or until their earlier resignation or removal.
| Name | Class | Term Expires | ||
| Lance Alstodt | III | 2026 | ||
| Francisco Silva | II | 2025 | ||
| Nickolay Kukekov | I | 2024 | ||
| Patrick F. Williams | III | 2026 | ||
| David Rosa | II | 2025 |
Each executive officer will hold office until the initial meeting of the Board of Directors following the next annual meeting of stockholders and until his successor is elected and qualified or until his or her earlier resignation or removal.
Audit Committee
The Audit Committee of the Board of Directors is responsible for overseeing our accounting and financial reporting processes and the audits of our financial statements. The members of the Audit Committee are Mr. Williams (Chair), Dr. Kukekov and Mr. Rosa.
Audit Committee Financial Expert
Our Board has determined that Mr. Williams qualifies as an “audit committee financial expert,” as that term is defined in Item 407(d)(5) of Regulation S-K.
Delinquent Section 16(a) Beneficial Ownership Reports
Section 16 of the Exchange Act requires that reports of beneficial ownership of common stock and changes in such ownership be filed with the Securities and Exchange Commission by Section 16 “reporting persons,” including directors, certain officers, holders of more than 10% of the outstanding common stock and certain trusts of which reporting persons are trustees. We are required to disclose in this Annual Report each reporting person whom we know to have failed to file any required reports under Section 16 on a timely basis during the fiscal year ended December 31, 2023. To our knowledge, based solely on a review of copies of Forms 3, 4 and 5 filed with the Securities and Exchange Commission, during the fiscal year ended December 31, 2023, our officers, directors and 10% stockholders complied with all Section 16(a) filing requirements applicable to them, except that each of Mr. Alstodt and Mr. Silva filed one Form 4 late (each reporting one transaction) and Dale Broadrick, a 10% stockholder, filed three Forms 4 late (reporting an aggregate of eight transactions).
Code of Ethics for Senior Financial Officers
Our Board of Directors has adopted a Code of Ethics for our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the Code of Ethics is posted on our website, www.biorestorative.com. We intend to satisfy the disclosure requirement under Item 5.05(c) of Form 8-K regarding an amendment to, or a waiver from, our Code of Ethics by posting such information on our website, www.biorestorative.com.
|
|
| ITEM 11. | EXECUTIVE COMPENSATION. |
Summary Compensation Table
The following Summary Compensation Table sets forth all compensation earned in all capacities during the fiscal years ended December 31, 2023 and 2022 by (i) our principal executive officer, and (ii) our two most highly compensated executive officers, other than our principal executive officer, who were serving as an executive officer as of December 31, 2023 and whose total compensation for the 2023 fiscal year, as determined by Regulation S-K, Item 402, exceeded $100,000 (the individuals falling within categories (i) and (ii) are collectively referred to as the Named Executive Officers):
| Name and Principal Position | Year | Salary | Bonus | Stock Awards(1) | Option Awards(1) | All Other Compensation | Total | |||||||||||||||||||
| Lance Alstodt | 2023 | $ | 479,167 | $ | 475,000 | (2) | $ | - | $ | 300,000 | $ | - | $ | 1,254,167 | ||||||||||||
| Chief Executive Officer | 2022 | $ | 400,000 | $ | - | (2) | $ | 52,364 | $ | - | $ | - | $ | 452,364 | ||||||||||||
| Francisco Silva | 2023 | $ | 454,167 | $ | 437,500 | (3) | $ | - | $ | 300,000 | $ | - | $ | 1,191,667 | ||||||||||||
| VP, Research and Development | 2022 | $ | 375,000 | $ | - | (3) | $ | 52,364 | $ | - | $ | - | $ | 427,364 | ||||||||||||
| Robert Kristal | 2023 | $ | 240,624 | $ | 127,500 | (4) | $ | - | $ | 250,000 | $ | - | $ | 618,124 | ||||||||||||
| Chief Financial Officer | 2022 | $ | 175,000 | $ | - | (4) | $ | - | $ | - | $ | - | $ | 175,000 | ||||||||||||
| (1) | Amounts reflect the aggregate grant date fair value of grants made in the fiscal year computed in accordance with stock-based accounting rules (FASB ASC Topic 718-Stock Compensation). Assumptions used in the calculations of these amounts are included in Note 8 to our consolidated financial statements included in this Annual Report. |
| (2) | The 2023 Bonus amount represents (a) a discretionary bonus is the amount of $250,000 in consideration of 2023 services which was paid in 2024 and (b) a discretionary bonus in the amount of $225,000 in consideration of 2022 services which was paid in 2023. |
| (3) | The 2023 Bonus amount represents (a) a discretionary bonus in the amount of $225,000 in consideration of 2023 services which was paid in 2024 and (b) a discretionary bonus in the amount of $212,500 in consideration of 2022 services which was paid in 2023. |
| (4) | The 2023 Bonus amount represents (a) a discretionary bonus in the amount of $75,000 in consideration of 2023 services which was paid in 2024 and (b) a discretionary bonus in the amount of $52,500 in consideration of 2022 services which was paid in 2023. |
|
|
Outstanding Equity Awards at Fiscal Year-End
The following table provides information on outstanding equity awards as of December 31, 2023 to the Named Executive Officers:
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name | Number of securities underlying unexercised options exercisable | Number of securities underlying unexercised options unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options | Option exercise price | Option expiration date | Number of shares or units of stock that have not vested | Market value of shares of units that have not vested | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested | |||||||||||||||||||||||||||
| Lance Alstodt | 293,479 | - | - | $ | 5.08 | 3/18/2031 | - | $ | - | - | $ | - | ||||||||||||||||||||||||
| Lance Alstodt | 34,174 | 7,885 | (1) | - | $ | 5.08 | 11/4/2031 | - | $ | - | - | $ | - | |||||||||||||||||||||||
| Lance Alstodt | 53,381 | 53,381 | (2) | - | $ | 2.91 | 2/17/2033 | - | $ | - | - | $ | - | |||||||||||||||||||||||
| Lance Alstodt | - | - | - | $ | - | - | 48,913 | (3) | $ | 85,104 | - | $ | - | |||||||||||||||||||||||
| Francisco Silva | 3 | $ | 18,800 | 2/18/2024 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 1 | $ | 18,800 | 3/12/2024 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 9 | $ | 18,800 | 10/23/2024 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 6 | $ | 18,800 | 9/4/2025 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 15 | $ | 14,920 | 6/10/2026 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 20 | $ | 11,200 | 7/12/2027 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 25 | $ | 4,920 | 10/29/2028 | - | $ | - | - | $ | - | ||||||||||||||||||||||||||
| Francisco Silva | 293,479 | - | - | $ | 5.08 | 3/18/2031 | - | $ | - | - | $ | - | ||||||||||||||||||||||||
| Francisco Silva | 34,174 | 7,885 | (1) | - | $ | 5.08 | 11/4/2031 | - | $ | - | - | $ | - | |||||||||||||||||||||||
| Francisco Silva | 53,381 | 53,381 | (2) | - | $ | 2.91 | 2/17/2033 | - | $ | - | - | $ | - | |||||||||||||||||||||||
| Francisco Silva | - | - | - | $ | - | - | 48,913 | (3) | $ | 85,104 | - | $ | - | |||||||||||||||||||||||
| Robert Kristal | 10,490 | - | - | $ | 5.08 | 11/4/2031 | - | $ | - | - | $ | - | ||||||||||||||||||||||||
| Robert Kristal | 44,484 | 44,484 | (2) | - | $ | 2.91 | 2/17/2033 | - | $ | - | - | $ | - | |||||||||||||||||||||||
| (1) | Option becomes exercisable in three nearly equal quarterly installments beginning on February 4, 2024. |
| (2) | Option becomes exercisable in eight nearly equal quarterly installments beginning on February 17, 2024. |
| (3) | Restricted stock vests on March 18, 2024. |
Employment Agreements
Lance Alstodt
Effective November 16, 2020, Mr. Alstodt was elected our Chief Executive Officer, President and Chairman of the Board. On March 18, 2021, we entered into an employment agreement with Mr. Alstodt which provides for a term ending on March 18, 2026. Pursuant to the employment agreement, Mr. Alstodt currently is entitled to receive an annual salary of $550,000 (giving effect to a $150,000 performance salary increase received in November 2021 and $50,000 annual increases in salary pursuant to his employment agreement). Concurrently with the execution of the employment agreement, we granted to Mr. Alstodt pursuant to the 2021 Plan (i) a ten year option for the purchase of 293,479 shares of our common stock at an exercise price of $47.60 per share (which exercise price was subsequently reduced to $13.50 per share and further reduced to $5.08 per share) and (ii) 146,740 restricted stock units, or RSUs. The option vested to the extent of 50% thereof on the date of grant, 12.5% on November 4, 2021 and the balance in six equal quarterly installments commencing on December 18, 2021. The RSUs vest in three equal annual installments on the first, second and third anniversaries of the date of grant. In the event that Mr. Alstodt’s employment is terminated by us without “cause”, or Mr. Alstodt terminates his employment for “good reason” (each as defined in the employment agreement), Mr. Alstodt will be entitled to receive severance in an amount up to one time his then annual base salary. If Mr. Alstodt’s employment with us is terminated without cause, the option granted to Mr. Alstodt will remain exercisable until its expiration date notwithstanding such termination of employment with us. In addition, the RSUs granted to Mr. Alstodt will vest in the event of the termination of his employment without cause or in the event of a change in control (as defined in the 2021 Plan). In March 2022, we and Mr. Alstodt agreed that, in lieu of a $50,000 increase in his annual salary (as provided for in his employment agreement), we issued to Mr. Alstodt 12,438 RSUs (having a value of $50,000), which RSUs vested in twelve equal monthly installments. Such grant was in consideration of Mr. Alstodt deferring his right to receive the $50,000 increase in his salary for one year.
|
|
Francisco Silva
On March 18, 2021, we and Mr. Silva entered into an employment agreement which provides for a term ending on March 18, 2026. Pursuant to the employment agreement, Mr. Silva is currently entitled to receive an annual salary of $525,000 (giving effect to a $150,000 performance salary increase received in November 2021 and $50,000 annual increases in salary pursuant to his employment agreement). Concurrently with the execution of the employment agreement, we granted to Mr. Silva pursuant to the 2021 Plan (i) a ten year option for the purchase of 293,479 shares of our common stock at an exercise price of $47.60 per share (which exercise price was subsequently reduced to $13.50 per share and further reduced to $5.08 per share) and (ii) 146,740 RSUs. The option vested to the extent of 50% thereof on the date of grant, 12.5% on November 4, 2021 and the balance in six equal quarterly installments commencing on December 18, 2021. The RSUs vest in three equal annual installments on the first, second and third anniversaries of the date of grant. In the event that Mr. Silva’s employment is terminated by us without “cause”, or Mr. Silva terminates his employment for “good reason” (each as defined in the employment agreement), Mr. Silva will be entitled to receive severance in an amount up to one time his then annual base salary. If Mr. Silva’s employment with us is terminated without cause, the option granted to Mr. Silva will remain exercisable until its expiration date notwithstanding such termination of employment with us. In addition, the RSUs granted to Mr. Silva will vest in the event of the termination of his employment without cause or in the event of a change in control (as defined in the 2021 Plan). In March 2022, we and Mr. Silva agreed that, in lieu of a $50,000 increase in his annual salary (as provided for in his employment agreement), we issued to Mr. Silva 12,438 RSUs (having a value of $50,000), which RSUs vested in twelve equal monthly installments. Such grant was in consideration of Mr. Silva deferring his right to receive the $50,000 increase in his salary for one year.
Director Compensation
The following table sets forth certain information concerning the compensation of our non-employee directors for the fiscal year ended December 31, 2023:
| Name | Fees Earned or Paid in Cash | Stock Awards | Option Awards | Non-Equity Incentive Plan Compensation | Nonqualified Deferred Compensation Earnings | All Other Compensation | Total | |||||||||||||||||||||
| Nickolay Kukekov | $ | 30,000 | $ | - | $ | 90,000 | (1) | $ | - | $ | - | $ | - | $ | 120,000 | |||||||||||||
| Patrick F. Williams | $ | 30,000 | $ | - | $ | 90,000 | (2) | $ | - | $ | - | $ | - | $ | 120,000 | |||||||||||||
| David Rosa | $ | 30,000 | $ | - | $ | 90,000 | (3) | $ | - | $ | - | $ | - | $ | 120,000 | |||||||||||||
| (1) | As of December 31, 2023, Dr. Kukekov held options for the purchase of 57,264 shares of common stock. |
| (2) | As of December 31, 2023, Mr. Williams held options for the purchase of 42,518 shares of common stock. |
| (3) | As of December 31, 2023, Mr. Rosa held options for the purchase of 42,518 shares of common stock. |
Dr. Kukekov and Messrs. Williams and Rosa, our non-employee directors, as compensation for their services as a director, are entitled to receive per annum $30,000 in cash and $90,000 in option grants.
|
|
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
Principal Stockholders
The following table sets forth certain information regarding the beneficial ownership of our common stock, as of March 20, 2024, known by us, through transfer agent records and reports filed with the SEC, to be held by: (i) each person who beneficially owns 5% or more of the shares of common stock then outstanding; (ii) each of our directors; (iii) each of our Named Executive Officers (as defined above); and (iv) all of our directors and executive officers as a group. The following table also sets forth certain information regarding the beneficial ownership of our Series B preferred stock as of March 20, 2024.
The information in this table reflects “beneficial ownership” as defined in Rule 13d-3 of the Exchange Act. To our knowledge, and unless otherwise indicated, each stockholder has sole voting power and investment power over the shares listed as beneficially owned by such stockholder, subject to community property laws where applicable. Percentage ownership is based on 6,769,919 shares of common stock (exclusive of the shares of common stock issuable to Auctus Fund, LLC, or Auctus, as discussed in footnote (10) to the following table) and 1,398,158 shares of Series B preferred stock outstanding as of March 20, 2024.
|
Name and Address of Beneficial Owner |
Number of Owned |
Approximate Percent of Class |
Number of Shares of Series B Preferred Stock Beneficially Owned |
Approximate Percent of Class |
||||||||||||
| Directors and Executive Officers | ||||||||||||||||
| Lance Alstodt(1) | 790,805 | (2) | 10.7 | % | - | - | ||||||||||
| Francisco Silva(1) | 756,666 | (3) | 10.3 | % | - | - | ||||||||||
| Robert Kristal(1) | 205,668 | (4) | 3.0 | % | - | - | ||||||||||
| Nickolay Kukekov | 95,510 | (5) | 1.4 | % | - | - | ||||||||||
| Patrick F. Williams | 82,341 | (5) | 1.2 | % | - | - | ||||||||||
| David Rosa | 82,341 | (5) | 1.2 | % | - | - | ||||||||||
| All directors and executive officers as a group (7 persons) | 2,186,862 | (6) | 25.4 | % | - | - | ||||||||||
| Certain Beneficial Owners | ||||||||||||||||
| Dale Broadrick(7) | 926,126 | (8) | 13.7 | % | - | - | ||||||||||
|
Auctus Fund, LLC(9) Auctus Fund Management LLC(9) Alfred Sollami(9) Louis Posner(9) |
679,753 |
(10) | 9.99 | % | 1,398,158 | (11) | 100 | % | ||||||||
| * | Less than 1% |
| (1) | Address is c/o BioRestorative Therapies, Inc., 40 Marcus Drive, Suite One, Melville, New York 11747. |
| (2) | Includes 618,933 shares of common stock issuable upon the exercise of options that are exercisable currently or within 60 days. |
| (3) | Includes 597,079 shares of common stock issuable upon the exercise of options that are exercisable currently or within 60 days and 12,136 shares of common stock held by Mr. Silva in a retirement account. |
| (4) | Includes 197,674 shares of common stock issuable upon the exercise of options that are exercisable currently or within 60 days. |
| (5) | Represents shares of common stock issuable upon the exercise of options that are exercisable currently or within 60 days. |
| (6) | Includes 1,847,409 shares of common stock issuable upon the exercise of options that are exercisable currently or within 60 days. |
| (7) | Address is 3003 Brick Church Pike, Nashville, Tennessee |
| (8) | Based upon Amendment No. 9 to Schedule 13D and Forms 4 filed with the SEC. Includes 1,109 shares common stock issuable upon the exercise of currently exercisable warrants and 477,972 shares of common stock held by Fleetco Inc. of which Mr. Broadrick is a 93% stockholder. |
| (9) | Address is 545 Boylston Street, 2nd Floor, Boston, Massachusetts 02116. |
|
|
| (10) | Based upon Amendment No. 2 to Schedule 13G filed with the SEC and other filings made by us with the SEC. Auctus holds a warrant for the purchase of up to 1,257,435 shares of our common stock. In addition, Auctus’ shares of Series B preferred stock are convertible into an aggregate of 1,398,158 shares of our common stock. In connection with the transaction in which the warrants were issued to Auctus, we issued to Auctus certain shares of our common stock and have agreed to issue to Auctus, upon receipt of notice from Auctus, subject to the limitation discussed below, 1,351,580 shares of our common stock, or the Additional Shares. However, such warrant is not exercisable for the purchase of our common stock, such Series B preferred stock is not convertible into shares of our common stock and the Additional Shares are not issuable to the extent Auctus would beneficially own, after such exercise, conversion or issuance, more than 9.99% of our outstanding shares of common stock. Auctus has advised that, as of March 20, 2024, it owned 645,320 shares of common stock, which represented 9.5% of the then 6,769,919 outstanding shares of common stock, and that the Additional Shares are issuable to it, upon notice from it, to the extent that such issuances would not result in Auctus beneficially owning after such issuances more than 9.99% of our outstanding shares of common stock. Based upon the foregoing, as of March 20, 2024, 34,433 Additional Shares are issuable to Auctus (to comply with the 9.99% beneficial ownership limitation), the remaining Additional Shares are not issuable, Auctus’ warrant is not currently exercisable for the purchase of shares of common stock and its Series B preferred stock is not currently convertible into shares of common stock. The number of shares of common stock beneficially owned by Auctus includes the 34,433 Additional Shares currently issuable to it. Without the 9.99% limitation discussed above, Auctus would have beneficial ownership of 4,652,493 shares of common stock. |
| (11) | Pursuant to the Certificate of Designations of Preferred Stock with regard to the Series B preferred stock, Auctus, as the sole holder of the 1,398,158 outstanding shares of Series B preferred stock, is entitled to vote such shares based on the number of shares of common stock into which such shares are convertible (currently 1,398,158); however, pursuant to such Certificate of Designations of Preferred Stock, as indicated in footnote (10), such Series B preferred stock is not convertible into shares of common stock to the extent Auctus would beneficially own, after such conversion, more than 9.99% of our then outstanding shares of common stock. Since Auctus has advised that, as of March 20, 2024, it owned 645,320 shares of common stock, which represented 9.5% of the outstanding shares of common stock, and, as indicated in footnote (10) above, 34,433 Additional Shares are issuable to it, Auctus’ Series B preferred stock is not currently convertible into shares of common stock. Accordingly, as of March 20, 2024, Auctus, as the sole holder of the Series B preferred stock, is not entitled to any votes for such shares. |
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth information as of December 31, 2023 with respect to compensation plans (including individual compensation arrangements) under which our common stock are authorized for issuance, aggregated as follows:
| ● | All compensation plans previously approved by security holders; and | |
| ● | All compensation plans not previously approved by security holders. |
|
|
EQUITY COMPENSATION PLAN INFORMATION
| Number of securities to be issued upon exercise of outstanding options (a) |
Weighted-average exercise price of outstanding options (b) |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
||||||||||
| Equity compensation plans approved by security holders | 1,466,892 | $ | 4.11 | 2,063,311 | (1) | |||||||
| Total | 1,466,892 | $ | 4.11 | 2,063,311 | ||||||||
| (1) | Includes 97,824 unvested restricted stock units outstanding at December 31, 2023. |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
Director Independence
Board of Directors
Our Board of Directors is comprised of Lance Alstodt (Chair), Francisco Silva, Nickolay Kukekov, Patrick F. Williams and David Rosa. Each of Dr. Kukekov, Mr. Williams and Mr. Rosa is an “independent director” based on the definition of independence in Listing Rule 5605(a)(2) of The Nasdaq Stock Market.
Audit Committee
Mr. Williams (Chair), Dr. Kukekov and Mr. Rosa are the members of our Board’s Audit Committee. Each of Mr. Williams, Dr. Kukekov and Mr. Rosa is an “independent director” based on the definition of independence in Listing Rule 5605(a)(2) of The Nasdaq Stock Market and Rule 10A-3(b)(1) under the Exchange Act. Our Board of Directors has determined that Mr. Williams qualifies as an “audit committee financial expert,” as that term is defined in Item 407(d)(5) of Regulation S-K.
Nominating Committee
Mr. Rosa (Chair), Dr. Kukekov and Mr. Williams are the members of our Board’s Nominating Committee. Each of Mr. Rosa, Dr. Kukekov and Mr. Williams is an “independent director” based on the definition of independence in Listing Rule 5605(a)(2) of The Nasdaq Stock Market.
Compensation Committee
Dr. Kukekov (Chair), Mr. Williams and Mr. Rosa are the members of our Board’s Compensation Committee. Each of Dr. Kukekov, Mr. Williams and Mr. Rosa is an “independent director” based on the definition of independence in Listing Rule 5605(a)(2) of The Nasdaq Stock Market.
|
|
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES. |
Marcum LLP served as our independent registered public accountants for the years ended December 31, 2023 and 2022.
The following is a summary of the fees billed or expected to be billed to us by Marcum LLP, our independent registered public accountants, for professional services rendered with respect to the fiscal years ended December 31, 2023 and 2022 and by Friedman LLP, our former independent registered public accountants, for professional services rendered with respect to the fiscal year ended December 31, 2022:
| 2023 | 2022 | |||||||||||
| Marcum LLP | Marcum LLP | Friedman LLP | ||||||||||
| Audit Fees (1) | $ | 101,500 | $ | 65,000 | $ | 30,000 | ||||||
| Audit-Related Fees (2) | 37,000 | 2,500 | 4,000 | |||||||||
| Tax Fees (3) | - | - | - | |||||||||
| All Other Fees (4) | - | - | - | |||||||||
| $ | 138,500 | $ | 67,500 | $ | 34,000 | |||||||
| (1) | Audit Fees consist of fees billed and expected to be billed for services rendered for the audit of our consolidated financial statements for the fiscal years ended December 31, 2023 and 2022, and the review of our condensed consolidated financial statements included in our Quarterly Reports on Form 10-Q. |
| (2) | Audit-Related Fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit of our financial statements and in connection with the filing of Forms S-1, S-3 and S-8 registration statements and are not reported under “Audit Fees.” |
| (3) | Tax Fees consist of fees billed for professional services related to preparation of our U.S. federal and state income tax returns and tax advice. |
| (4) | All Other Fees consist of fees billed for products and services provided by our independent registered public accountants, other than those disclosed above. |
The Audit Committee is responsible for the appointment, compensation and oversight of the work of the independent registered public accountants, and approves in advance any services to be performed by the independent registered public accountants, whether audit-related or not. The Audit Committee reviews each proposed engagement to determine whether the provision of services is compatible with maintaining the independence of the independent registered public accountants. The fees shown above were pre-approved either by our Board or our Audit Committee.
|
|
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
|
|
|
|
|
|
* Filed herewith
** Furnished herewith
| ITEM 16. | FORM 10-K SUMMARY. |
Not applicable
|
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| BIORESTORATIVE THERAPIES, INC. | ||
| Dated: March 29, 2024 | By: | /s/ Lance Alstodt |
| Lance Alstodt | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Capacity | Date | ||
| /s/ Lance Alstodt | Chief Executive Officer, President, Chairman of the Board and Director | March 29, 2024 | ||
| Lance Alstodt | (Principal Executive Officer) | |||
| /s/ Francisco Silva | Vice President, Research and Development and Director | March 29, 2024 | ||
| Francisco Silva | ||||
| /s/ Robert E. Kristal | Chief Financial Officer | March 29, 2024 | ||
| Robert E. Kristal | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Nickolay Kukekov | Director | March 29, 2024 | ||
| Nickolay Kukekov | ||||
| /s/ Patrick F. Williams | Director | March 29, 2024 | ||
| Patrick F. Williams | ||||
| /s/ David Rosa | Director | March 29, 2024 | ||
| David Rosa |
|
|
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
BIORESTORATIVE THERAPIES, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F- |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
BioRestorative Therapies, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioRestorative Therapies, Inc. (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of operations, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
Marcum LLP
We have served as the Company’s auditor since 2020.
Marlton, New Jersey
March 29, 2024
| F- |
BIORESTORATIVE THERAPIES, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 884,377 | $ | 1,713,772 | ||||
| Investments held in marketable securities | 10,181,618 | 13,035,636 | ||||||
| Accounts receivable | 19,300 | 16,000 | ||||||
| Prepaid expenses and other current assets | 305,231 | 363,082 | ||||||
| Total Current Assets | 11,390,526 | 15,128,490 | ||||||
| Property and equipment, net | 356,055 | 261,003 | ||||||
| Right-of-use assets | 151,447 | 241,760 | ||||||
| Intangible assets, net | 713,692 | 803,438 | ||||||
| Total Assets | $ | 12,611,720 | $ | 16,434,691 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable | $ | 189,389 | $ | 170,902 | ||||
| Accrued expenses and other current liabilities | 711,686 | 130,072 | ||||||
| Lease liability, current portion | 162,317 | 139,328 | ||||||
| Total Current Liabilities | 1,063,392 | 440,302 | ||||||
| Lease liability, net of current portion | - | 162,317 | ||||||
| Total Liabilities | 1,063,392 | 602,619 | ||||||
| Commitments and contingencies | - | - | ||||||
| Stockholders’ Equity: | ||||||||
| Preferred stock, $0.01 par value; 20,000,000 shares authorized; | ||||||||
| Series B Convertible Preferred Stock; 1,543,158 shares designated, 1,398,158 and 1,518,158 shares issued and outstanding at December 31, 2023 and 2022, respectively | 13,982 | 15,182 | ||||||
| Common stock, $0.0001 par value; 75,000,000 shares authorized; 4,706,917 and 3,677,775 shares issued and outstanding at December 31, 2023 and 2022, respectively | 471 | 369 | ||||||
| Additional paid-in capital | 178,590,256 | 168,457,418 | ||||||
| Accumulated deficit | (167,056,381 | ) | (152,640,897 | ) | ||||
| Total Stockholders’ Equity | 11,548,328 | 15,832,072 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 12,611,720 | $ | 16,434,691 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
BIORESTORATIVE THERAPIES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenues, net | $ | 145,800 | $ | 119,800 | ||||
| Operating Expenses: | ||||||||
| Research and development | 4,034,591 | 3,513,352 | ||||||
| General and administrative | 11,331,983 | 15,580,473 | ||||||
| Total Operating Expenses | 15,366,574 | 19,093,825 | ||||||
| Loss From Operations | (15,220,774 | ) | (18,974,025 | ) | ||||
| Other Income: | ||||||||
| Interest income | (552,293 | ) | (11,650 | ) | ||||
| Gain on PPP loan forgiveness | - | (250,000 | ) | |||||
| Grant income | (83,333 | ) | (110,518 | ) | ||||
| Other income | (169,664 | ) | (107,088 | ) | ||||
| Total Other Income | (805,290 | ) | (479,256 | ) | ||||
| Net Loss | $ | (14,415,484 | ) | $ | (18,494,769 | ) | ||
| Net Loss Per Share - Basic and Diluted | $ | (3.42 | ) | $ | (5.11 | ) | ||
| Weighted Average Common Shares Outstanding - | ||||||||
| Basic and Diluted | 4,218,347 | 3,617,858 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
BIORESTORATIVE THERAPIES, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| Series A Convertible | Series B Convertible | Additional | ||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Common Stock | Paid-In | Accumulated | ||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||||||||
| Balance - January 1, 2022 | 1,543,158 | $ | 15,432 | - | $ | - | 3,520,391 | $ | 353 | $ | 155,727,292 | $ | (134,146,128 | ) | $ | 21,596,949 | ||||||||||||||||||||
| Issuance of Series B Convertible Preferred Stock in exchange for Series A Convertible Preferred Stock | (1,543,158 | ) | (15,432 | ) | 1,543,158 | 15,432 | - | - | - | - | - | |||||||||||||||||||||||||
| Warrants issued in connection with license exclusivity agreement | - | - | - | - | - | - | 117,030 | - | 117,030 | |||||||||||||||||||||||||||
| Conversion of Series B Convertible Preferred Stock into common stock | - | - | (25,000 | ) | (250 | ) | 25,000 | 3 | 247 | - | - | |||||||||||||||||||||||||
| Stock-based compensation: | ||||||||||||||||||||||||||||||||||||
| Restricted share units | - | - | - | - | 116,486 | 11 | 4,735,097 | - | 4,735,108 | |||||||||||||||||||||||||||
| Options | - | - | - | - | - | - | 7,741,864 | - | 7,741,864 | |||||||||||||||||||||||||||
| Common stock | - | - | - | - | 15,898 | 2 | 135,888 | - | 135,890 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (18,494,769 | ) | (18,494,769 | ) | |||||||||||||||||||||||||
| Balance - December 31, 2022 | - | - | 1,518,158 | 15,182 | 3,677,775 | 369 | 168,457,418 | (152,640,897 | ) | 15,832,072 | ||||||||||||||||||||||||||
| Stock-based compensation: | ||||||||||||||||||||||||||||||||||||
| Restricted share units | - | - | - | - | 89,840 | 9 | 4,633,655 | - | 4,633,664 | |||||||||||||||||||||||||||
| Options | - | - | - | - | - | - | 3,141,803 | - | 3,141,803 | |||||||||||||||||||||||||||
| Common stock | - | - | - | - | 1,442 | - | 7,500 | - | 7,500 | |||||||||||||||||||||||||||
| Conversion of Series B Convertible Preferred Stock into common stock | - | - | (120,000 | ) | (1,200 | ) | 120,000 | 12 | 1,188 | - | - | |||||||||||||||||||||||||
| Issuance and sale of common stock, net of issuance costs | - | - | - | - | 817,860 | 81 | 2,348,692 | - | 2,348,773 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (14,415,484 | ) | (14,415,484 | ) | |||||||||||||||||||||||||
| Balance - December 31, 2023 | - | $ | - | 1,398,158 | $ | 13,982 | 4,706,917 | $ | 471 | $ | 178,590,256 | $ | (167,056,381 | ) | $ | 11,548,328 | ||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
BIORESTORATIVE THERAPIES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash Flows From Operating Activities: | ||||||||
| Net loss | $ | (14,415,484 | ) | $ | (18,494,769 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 165,735 | 120,545 | ||||||
| Dividend and interest income | (569,068 | ) | (76,004 | ) | ||||
| Stock-based compensation | 7,782,967 | 12,612,862 | ||||||
| Gain on PPP loan forgivesness | - | (250,000 | ) | |||||
| Non-cash lease expense | 90,313 | 160,122 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (3,300 | ) | (11,000 | ) | ||||
| Prepaid expenses and other current assets | 57,851 | 73,099 | ||||||
| Accounts payable | 18,487 | 120,075 | ||||||
| Accrued expenses and other current liabilities | 581,616 | (4,898 | ) | |||||
| Lease liability | (139,328 | ) | (163,132 | ) | ||||
| Net Cash Used In Operating Activities | (6,430,211 | ) | (5,913,100 | ) | ||||
| Cash Flows From Investing Activities: | ||||||||
| Sale of marketable securities | 20,964,373 | 9,492,304 | ||||||
| Purchase of marketable securities | (17,541,287 | ) | (22,451,936 | ) | ||||
| Purchase of intangible assets | - | (175,000 | ) | |||||
| Purchases of equipment | (171,043 | ) | (265,223 | ) | ||||
| Net Cash Provided By (Used In) Investing Activities | 3,252,043 | (13,399,855 | ) | |||||
| Cash Flows From Financing Activities: | ||||||||
| Net proceeds from issuance of common stock in ATM transactions | 497,353 | - | ||||||
| Net proceeds from issuance of common stock in registered direct offering | 1,853,990 | - | ||||||
| Net Cash Provided By Financing Activities | 2,351,343 | - | ||||||
| Net Decrease In Cash and Cash Equivalents | (826,825 | ) | (19,312,955 | ) | ||||
| Cash and Cash Equivalents - Beginning of the Year | 1,713,772 | 21,026,727 | ||||||
| Cash and Cash Equivalents - End of the Year | $ | 886,947 | $ | 1,713,772 | ||||
| Supplemental Disclosures of Cash Flow Information: | ||||||||
| Cash paid during the year for: | ||||||||
| Interest | $ | - | $ | - | ||||
| Income taxes | $ | - | $ | - | ||||
| Non-cash investing and financing activities: | ||||||||
| Warrants issued as consideration for intangible assets | $ | - | $ | 117,030 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F- |
BIORESTORATIVE THERAPIES, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 - ORGANIZATION, LIQUIDITY AND BUSINESS OPERATIONS
Corporate History
BioRestorative Therapies, Inc. has one wholly-owned subsidiary, Stem Pearls, LLC (“Stem Pearls”). BioRestorative Therapies, Inc. and its subsidiary are referred to collectively as “BRT” or the “Company”.
On December 23, 2022, the Company reincorporated from Delaware to Nevada by filing Articles of Incorporation with the state of Nevada. The reincorporation was structured as a statutory merger.
Liquidity
The accompanying consolidated financial statements have been prepared on the basis that the Company will continue as a going concern, which contemplates realization of assets and satisfying liabilities in the normal course of business. For the year ended December 31, 2023, the Company had a net loss of $14.4 million (of which, $7.8 million was attributable to non-cash stock-based compensation) and negative cash flows from operations of $6.4 million. The Company anticipates that it will continue to incur net losses and negative cash flows from operations as it executes its development plans for 2024 and beyond, as well as other potential strategic and business development initiatives. The Company has previously funded, and plans to continue funding, these losses primarily through current cash on hand, investments in marketable securities and additional infusions of cash from equity and debt financing. Subsequent to December 31, 2023, the Company raised gross proceeds of approximately $8.1 million in connection with a warrant exercise program, which is further discussed in Note 10 – Subsequent Events.
Based on cash on hand as of the date these financial statements were issued, which includes $8.1 million of gross proceeds from the warrant exercise program, the Company believes it has sufficient cash on hand in order to fund operations for at least the twelve months after the issuance date of these financial statements.
Current funds noted above will not be sufficient to enable the Company to fully complete its development activities or attain profitable operations. If the Company is unable to obtain such needed additional financing on a timely basis, the Company may have to curtail its development, marketing and promotional activities, which would have a material adverse effect on the Company’s business, financial condition and results of operations, and ultimately the Company could be forced to discontinue its operations and liquidate.
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”), which contemplate continuation of the Company as a going concern and the realization of assets and satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the consolidated financial statements do not necessarily purport to represent realizable or settlement values. The accompanying consolidated financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern.
Business Operations
BRT develops therapeutic products and medical therapies using cell and tissue protocols, primarily involving adult stem cells. BRT’s website is at www.biorestorative.com. The information contained in the website or connected thereto is not intended to be incorporated by reference into the accompanying financial statements or these footnotes. BRT is currently developing a Disc/Spine Program referred to as “brtxDISC”. Its lead cell therapy candidate, BRTX-100, is a product formulated from autologous (or a person’s own) cultured mesenchymal stem cells collected from the patient’s bone marrow. The product is intended to be used for the non-surgical treatment of painful lumbosacral disc disorders or as a complimentary therapeutic to a surgical procedure. BRT is also engaging in research efforts with respect to a platform technology utilizing brown adipose (fat) for therapeutic purposes to treat type 2 diabetes, obesity and other metabolic disorders and has labeled this initiative its ThermoStem Program. Further, BRT has licensed a patented curved needle device that is a needle system designed to deliver cells and/or other therapeutic products or material to the spine and discs or other potential sites.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with GAAP. The summary of significant accounting policies presented below is designed to assist in understanding the Company’s consolidated financial statements. Such consolidated financial statements and accompanying notes are the representations of Company’s management, who is responsible for their integrity and objectivity.
| F- |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Stem Pearls. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of the consolidated financial statements in conformity with GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, together with amounts disclosed in the related notes to the financial statements. The Company bases its estimates and assumptions on historical experience, known or expected trends and various other assumptions that it believes to be reasonable. As future events and their effects cannot be determined with precision, actual results could differ from these estimates which may cause the Company’s future results to be affected.
Reclassifications
Certain prior period balance sheet, statement of operations and statement of cash flows amounts have been reclassified to conform to the Company’s fiscal 2023 presentation. These reclassifications have no impact on the Company’s previously reported net loss.
Concentrations
Financial instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution. The Company maintains deposits in its cash account in excess of the Federal Depository Insurance Coverage limit of $250,000. As of December 31, 2023, the Company had not experienced losses on this account.
The royalties related to the Company’s sublicense comprised 100% of the Company’s revenue during the years ended December 31, 2023 and 2022. See “Revenue Recognition” below.
Revenue Recognition
The Company accounts for revenue in accordance with Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers.
The Company derives all of its revenue pursuant to a license agreement between the Company and a stem cell treatment company (“SCTC”) entered into in January 2012. In November 2022, the Company’s license rights under the agreement became exclusive. Pursuant to the license agreement, the SCTC granted to the Company a license to use certain intellectual property related to, among other things, stem cell disc procedures and the Company has granted to the SCTC a sublicense to use, and the right to sublicense to third parties the right to use, in certain locations in the United States and the Cayman Islands, certain of the licensed intellectual property. In consideration of the sublicenses, the SCTC has agreed to pay the Company royalties on a per disc procedure basis.
The Company’s contracted transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. The Company’s contracts have a single performance obligation which is not separately identifiable from other promises in the contracts and is, therefore, not distinct. The Company’s performance obligation is satisfied upon the transfer of risk of loss to the customer. The timing of the Company’s revenue recognition may differ from the timing of receiving royalty payments. A receivable is recorded when revenue is recognized prior to receipt of a royalty payment and the Company has an unconditional right to the royalty payment. Alternatively, when a royalty payment precedes the provision of the related services, the Company records deferred revenue until the performance obligations are satisfied. During the years ended December 31, 2023 and 2022, the Company recognized $145,800 and $119,800, respectively, of revenue related to the Company’s sublicenses.
Practical Expedients
As part of ASC 606, the Company has adopted several practical expedients including:
| ● | Significant Financing Component - the Company does not adjust the promised amount of consideration for the effects of a significant financing component since the Company expects, at contract inception, that the period between when the Company transfers a promised good or service to the customer and when the customer pays for that good or service will be one year or less. |
| ● | Unsatisfied Performance Obligations - all performance obligations related to contracts with a duration for less than one year; the Company has elected to apply the optional exemption provided in ASC Topic 606 and, therefore, is not required to disclose the aggregate amount of transaction price allocated to performance obligations that are unsatisfied or partially satisfied at the end of the reporting period. |
| ● | Right to Invoice - the Company has a right to consideration from a customer in an amount that corresponds directly with the value to the customer of the Company’s performance completed to date; the Company may recognize revenue in the amount to which the entity has a right to invoice. |
| F- |
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents. There were no cash equivalents as of December 31, 2023 or 2022.
Accounts Receivable
Accounts receivable are carried at their contractual amounts, less a provision for current expected credit losses. The reserve represents the Company’s best estimate of expected credit losses it may experience in the Company’s receivable portfolio. Management estimates the allowance for credit losses based on an ongoing review of existing economic conditions, the financial conditions of the customers, historical trends in credit losses, and the amount and age of past due accounts. The Company writes off accounts receivable against the provision for current expected credit losses when a balance is determined to be uncollectible. The Company did not record a provision for current expected credit losses as of December 31, 2023 or 2022.
Property and Equipment
Property and equipment are recorded at cost. Depreciation is computed using straight-line method over the estimated useful lives of the related assets, generally three to fifteen years. Expenditures that enhance the useful lives of the assets are capitalized and depreciated. Computer equipment costs are capitalized, as incurred, and depreciated on a straight-line basis over a range of 3 - 5 years.
Leasehold improvements are amortized over the lesser of (i) the useful life of the asset, or (ii) the remaining lease term. Maintenance and repairs are charged to expense as incurred. The Company capitalizes cost attributable to the betterment of property and equipment when such betterment extends the useful life of the assets. At the time of retirement or other disposition of property and equipment, the cost and accumulated depreciation will be removed from the accounts and the resulting gain or loss, if any, will be reflected in operations.
Intangible Assets
The Company records its intangible assets at cost in accordance with ASC 350, Intangibles - Goodwill and Other. Definite lived intangible assets are amortized over their estimated useful life using the straight-line method, which is determined by identifying the period over which the cash flows from the asset are expected to be generated.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including definite-lived intangible assets and right-of-use assets from operating leases, for impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Recoverability of these assets is determined by comparing the forecasted undiscounted net cash flows of the operation to which the assets relate to the carrying amount. If the operation is determined to be unable to recover the carrying amount of its assets, then these assets are written down first, followed by other long-lived assets of the operation to fair value. Fair value is determined based on discounted cash flows or appraised values, depending on the nature of the assets. For the years ended December 31, 2023 and 2022, we determined that there was no impairment charge for our long-lived assets.
Fair Value of Financial Instruments
Fair value is defined as the amount that would be received for selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date and is measured using inputs in one of the following three categories:
Level 1 measurements are based on unadjusted quoted prices in active markets for identical assets or liabilities that we have the ability to access. Valuation of these items does not entail a significant amount of judgment.
Level 2 measurements are based on quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active or market data other than quoted prices that are observable for the assets or liabilities.
Level 3 measurements are based on unobservable data that are supported by little or no market activity and are significant to the fair value of the assets or liabilities.
| F- |
The Company considers cash and cash equivalents, investments held in marketable securities, accounts receivable, accounts payable and accrued liabilities to meet the definition of financial instruments. As of December 31, 2023 and 2022, the carrying amount of cash and cash equivalents, investments held in marketable securities, accounts receivable, accounts payable and accrued liabilities approximate their fair value due to the relatively short period of time between their origination and their expected realization or payment.
SCHEDULE OF FAIR VALUE RECURRING BASIS
| Fair value measurements at reporting date using: | ||||||||||||||||
| Fair value | Quoted
prices in active markets for identical liabilities (Level 1) |
Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||||
| Assets: | ||||||||||||||||
| Marketable securities as of December 31, 2023 | $ | 10,181,618 | $ | 10,181,618 | - | - | ||||||||||
| Marketable securities as of December 31, 2022 | $ | 13,035,636 | $ | 13,035,636 | - | - | ||||||||||
During the years ended December 31, 2023 and 2022, the Company recognized aggregate dividend and interest income of $569,068 and $76,004 respectively, on its marketable securities, which was included within other income on its consolidated statements of operations.
Net loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the period. The dilutive effect, if any, of stock options and warrants are calculated using the treasury stock method. All outstanding convertible preferred stock is considered common stock at the beginning of the period or at the time of issuance, if later, pursuant to the if-converted method. Since the effect of common stock equivalents is anti-dilutive with respect to losses, options, warrants, and convertible preferred stock have been excluded from the Company’s computation of diluted net loss per common share for the periods presented.
SCHEDULE OF WEIGHTED AVERAGE DILUTIVE COMMON SHARES
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Stock options | 1,466,892 | 864,639 | ||||||
| Warrants | 4,791,019 | 4,791,082 | ||||||
| Unvested RSUs | 97,827 | 201,870 | ||||||
| Convertible Preferred Stock | 1,398,158 | 1,518,158 | ||||||
| Total | 7,753,896 | 7,375,749 | ||||||
The Company measures the cost of services received in exchange for an award of equity instruments based on the fair value of the award. The fair value of the award is measured on the grant date and then is recognized over the period during which services are required to be provided in exchange for the award, usually the vesting period, on a straight-line basis. The Company computes the fair value of equity-classified warrants and options granted using the Black-Scholes option pricing model. Option forfeitures are recorded as incurred as a reduction of amounts previously expensed.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets, including tax loss and credit carry forwards, and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
| F- |
The Company utilizes ASC 740, “Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. The Company accounts for income taxes using the asset and liability method to compute the differences between the tax basis of assets and liabilities and the related financial amounts, using currently enacted tax rates. A valuation allowance is recorded when it is “more likely-than-not” that a deferred tax asset will not be realized.
For uncertain tax positions that meet a “more likely than not” threshold, the Company recognizes the benefit of uncertain tax positions in the consolidated financial statements. The Company’s practice is to recognize interest and penalties, if any, related to uncertain tax positions in income tax expense in the consolidated statements of operations.
Leases
The Company determines whether an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets and operating lease liabilities in our consolidated balance sheets.
ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent its obligation to make lease payments arising from the lease. ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that it will exercise the option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
Recently Adopted Accounting Pronouncements
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses, which requires entities to estimate all expected credit losses for financial assets measured at amortized cost basis, including trade receivables, held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. The Company adopted this guidance on January 1, 2023. The adoption of this accounting standard did not have a material impact on the Company’s consolidated financial statements.
NOTE 3 - PROPERTY AND EQUIPMENT
Property and equipment consists of the following:
SCHEDULE OF PROPERTY PLANT AND EQUIPMENT
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Medical equipment | $ | 352,133 | $ | 352,133 | ||||
| Furniture and fixtures | 123,486 | 123,486 | ||||||
| Computer software and equipment | 136,205 | 117,544 | ||||||
| Office equipment | 18,779 | 18,779 | ||||||
| Manufacturing equipment | 395,232 | 242,852 | ||||||
| Leasehold improvements | 342,048 | 342,048 | ||||||
| 1,367,883 | 1,196,842 | |||||||
| Less: accumulated depreciation | (1,011,828 | ) | (935,839 | ) | ||||
| Property and equipment, net | $ | 356,055 | $ | 261,003 | ||||
Total depreciation expense for the years ended December 31, 2023 and 2022 was $75,989 and $42,212, respectively. Depreciation expense is reflected in general and administrative expenses and research and development expenses in the consolidated statements of operations.
NOTE 4 - INTANGIBLE ASSETS
The Company is a party to a license agreement with the SCTC (as amended) (the “SCTC Agreement”). Pursuant to the SCTC Agreement, the Company obtained, among other things, a worldwide (excluding Asia and Argentina), exclusive, royalty-bearing license from the SCTC to utilize or sublicense a certain method for culturing cells and a worldwide, exclusive, royalty-bearing license from the SCTC to utilize or sublicense a certain medical device patent for the administration of specific cells and/or cell products to the disc and/or spine (and other parts of the body). Pursuant to the license agreement with the SCTC, certain performance milestones (or payouts in lieu of performance milestones) had to be satisfied in order for the Company to maintain its exclusive rights with regard to the disc/spine technology. The Company did not timely satisfy the third of these performance milestones (which needed to be satisfied by February 2022). Accordingly, such rights became non-exclusive. However, the Company and the SCTC entered into an amended agreement under which the Company paid $175,000 and issued 51,370 warrants, with a fair value of $117,030, in exchange for renewed exclusivity. The consideration transferred to the SCTC in exchange for exclusivity was capitalized to intangible assets on the Company’s consolidated balance sheet as of December 31, 2022.
| F- |
In February 2017, the Company received authorization from the Food and Drug Administration (the “FDA”) to proceed with a Phase 2 clinical trial. In February 2022, the Company announced that the United States Patent and Trademark Office issued a notice of allowance for a patent application relating to the Company’s BRTX-100 clinical program. This patent was issued in March 2022.
Intangible assets consist of the following:
SCHEDULE OF INTANGIBLE ASSETS
| Patents and Trademarks | Licenses | Accumulated Amortization | Total | |||||||||||||
| Balance as of January 1, 2022 | $ | 3,676 | $ | 1,301,500 | $ | (715,436 | ) | $ | 589,740 | |||||||
| Considertion transferred for license exclusivity | - | 292,030 | - | 292,030 | ||||||||||||
| Amortization expense | - | - | (78,332 | ) | (78,332 | ) | ||||||||||
| Balance as of December 31, 2022 | 3,676 | 1,593,530 | (793,768 | ) | 803,438 | |||||||||||
| Amortization expense | - | - | (89,746 | ) | (89,746 | ) | ||||||||||
| Balance as of December 31, 2023 | $ | 3,676 | $ | 1,593,530 | $ | (883,514 | ) | $ | 713,692 | |||||||
| Weighted average remaining amortization period at December 31, 2023 (in years) | - | 10.9 | ||||||||||||||
Amortization of intangible assets consists of the following:
SCHEDULE OF FINITE LIVED INTANGIBLE ASSETS AMORTIZATION EXPENSES
| Patents and Trademarks | Licenses | Accumulated Amortization | ||||||||||
| Balance as of January 1, 2022 | $ | 3,676 | $ | 711,760 | $ | 715,436 | ||||||
| Amortization expense | - | 78,332 | 78,332 | |||||||||
| Balance as of December 31, 2022 | 3,676 | 790,092 | 793,768 | |||||||||
| Amortization expense | - | 89,746 | 89,746 | |||||||||
| Balance as of December 31, 2023 | $ | 3,676 | $ | 879,838 | $ | 883,514 | ||||||
Amortization expense for the next five years is as follows:
SCHEDULE OF FINITE LIVED INTANGIBLE ASSETS FUTURE AMORTIZATION EXPENSES
| For the Years Ending December 31, | Total | |||
| 2024 | $ | 89,746 | ||
| 2025 | 89,746 | |||
| 2026 | 89,746 | |||
| 2027 | 89,746 | |||
| 2028 | 89,746 | |||
| Total | $ | 448,730 | ||
| F- |
NOTE 5 - ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other current liabilities consist of:
SCHEDULE OF ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Accrued bonuses | $ | 638,000 | $ | 26,250 | ||||
| Accrued general and adminstrative expenses | 73,686 | 103,822 | ||||||
| Total accrued expenses and other current liabilities | $ | 711,686 | $ | 130,072 | ||||
NOTE 6 - NOTES PAYABLE
A summary of the notes payable activity during the years ended December 31, 2023 and 2022 is presented below:
SCHEDULE OF NOTES PAYABLE ACTIVITY
| Outstanding, Janaury 1, 2022 | $ | 250,000 | ||
| Issuance | - | |||
| Forgiveness | (250,000 | ) | ||
| Outstanding, December 31, 2022 | - | |||
| Issuance | - | |||
| Forgiveness | - | |||
| Outstanding, December 31, 2023 | $ | - |
NOTE 7 - STOCKHOLDERS’ EQUITY
Series A Preferred Stock
On November 8, 2021, in connection with the Company’s public offering, the Company’s Board of Directors adopted a resolution allowing for the designation and issuance of 1,543,158 shares of the Company’s Preferred Stock, $.01 par value per share, designated as Series A Preferred Stock (“Series A”). The Series A had a liquidation preference of $0.001 per share. On September 8, 2022, the Company issued 1,543,158 shares of Series B Preferred Stock (“Series B”) to Auctus Fund, LLC (“Auctus”) in exchange for an equal number of shares of the Company’s outstanding Series A. Simultaneously, the stock certificate representing the Series A shares was being returned to the Company for cancellation. On such date and upon such exchange, the Company’s Board of Directors cancelled the Series A.
Series B Preferred Stock
Effective September 8, 2022, the Company issued 1,543,158 shares of Series B to Auctus in exchange for an equal number of shares of the Company’s outstanding Series A. The terms of the Series B are substantially identical to those of the Series A, except that, among other things, the limitation on beneficial ownership of common stock of the Company upon a conversion of the Series B into Common Stock, and the limitation on the number of votes attributable to the Series B, is 9.99% of the then outstanding Common Stock of the Company instead of 4.99% as provided for the Series A. The Company is required, at all times, to reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the full conversion of the Series B. The Series B is not subject to redemption by the Company or any Series B holder. The exchange of Series A for Series B had no impact on the Company’s financial statements as of December 31, 2022.
Dividends
Series B holders shall be entitled to receive, when and as declared by the Board of Directors, dividends on a pari passu basis with the holders of the shares of Common Stock based upon the number of shares of Common Stock into which the Series B is then convertible.
| F- |
Voting Rights
Series B holders shall be entitled to vote on all matters presented to the stockholders of the Company for a vote at a meeting of stockholders of the Company or a written consent in lieu of a meeting of stockholders of the Company, and shall be entitled to such number of votes for each share of Series B entitled to vote at such meetings or pursuant to such consent, voting together with the holders of shares of Common Stock and other shares of preferred stock who are entitled to vote, and not as a separate class, except as required by law. The number of votes to which the Series B holders shall be entitled to vote for each share of Series B shall equal the number of shares of Common Stock into which such Series B is then convertible; provided, however, that in no event shall a Series B holder be entitled to vote more than 9.99% of the then outstanding shares of Common Stock.
Conversion
Optional Conversion - Each share of Series B shall be convertible, at any time and from time to time, at the option of the Series B holder, into one share of Common Stock; provided, however, that in no event shall a Series B holder be entitled to convert any shares of Series B to the extent that such conversion would result in beneficial ownership by such Series B holder of more than 9.99% of the outstanding shares of common stock.
Automatic Conversion - From time to time, in the event that an event occurs which has the effect of reducing a Series B holder’s beneficial ownership of shares of Common Stock to less than 9.5% of the then publicly disclosed outstanding shares of Common Stock, then, within five business days, the Series B holder is required to provide notice to the Company to such effect, which notice shall state the number of shares of Common Stock beneficially owned by the Series B holder and shall provide reasonable detail with regard thereto, including the number of derivative securities compromising a portion of such beneficial share amount. Such notice shall have the effect of a notice of conversion with respect to the conversion of such number of shares of Series B as would increase the Series B holder’s beneficial ownership of Common Stock to 9.99% of the then publicly disclosed outstanding shares of Common Stock.
On October 25, 2022, Auctus converted 25,000 shares of Series B into 25,000 shares of Common Stock. The number of shares of Series B remaining outstanding after this conversion was 1,518,158.
On April 4, 2023, Auctus converted 120,000 shares of Series B into 120,000 shares of Common Stock. As of December 31, 2023, the number of shares of Series B remaining outstanding after giving effect to such conversion was 1,398,158.
2021 Stock Incentive Plan
On March 18, 2021, the Company’s Board of Directors adopted the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “2021 Plan”). The 2021 Plan was approved by the Company’s stockholders on August 17, 2021. Pursuant to the 2021 Plan, a total of 1,175,000 shares of common stock were initially authorized to be issued pursuant to the grant of stock options, restricted stock units, restricted stock, stock appreciation rights and other incentive awards. On December 10, 2021, the Company’s Board of Directors approved an amendment to increase the number of shares of Common Stock authorized to be issued from 1,175,000 to 2,500,000. Such amendment was approved by the Company’s stockholders on November 3, 2022. On July 13, 2023, the Company’s Board of Directors approved an amendment to the 2021 Plan to increase the number of shares of Common Stock authorized to be issued from 2,500,000 to 3,850,000. Such amendment was approved by the Company’s stockholders on September 13, 2023.
Compensatory Common Stock Issuance
During the year ended December 31, 2022, the Company issued 15,898 shares of immediately vested common stock with an aggregate value of $135,888 to third parties for services rendered. During the year ended December 31, 2023, the Company issued 1,442 shares of immediately vested common stock with a value of $7,500 to a consultant for services rendered.
Sales of Common Stock
In April 2023, the Company entered into a Capital on Demand Sales Agreement with JonesTrading Institutional Services LLC (the “Sales Agent”) under which the Company currently has the ability to issue and sell shares of its Common Stock, from time to time, through the Sales Agent, up to an aggregate offering price of approximately $6,109,000 in what is commonly referred to as an “at-the-market” (“ATM”) program. During the year ended December 31, 2023, the Company sold 132,827 shares of its Common Stock at a weighted average price of $4.68 per share and raised $494,782 in net proceeds under the ATM program. As of December 31, 2023, the Company had remaining capacity to sell up to an additional $5,487,000 of common stock under the ATM program.
On July 13, 2023, the Company sold an aggregate of 685,033 shares of Common Stock to several institutional buyers and accredited investors in a registered direct offering at an offering price of $3.03 per share. The offering closed on July 13, 2023 with net proceeds of $1,853,990. The Company intends to use the net proceeds from the offering in connection with its clinical trials with respect to its lead cell therapy candidate, BRTX-100, pre-clinical research and development with respect to its metabolic ThermoStem Program and for general corporate purposes and working capital.
| F- |
Warrant and Option Valuation
The Company has computed the fair value of warrants and options granted using the Black-Scholes option pricing model. The expected term used for warrants and options issued to non-employees is the contractual life and the expected term used for options issued to employees and directors is the estimated period of time that options granted are expected to be outstanding. The Company utilizes the “simplified” method to develop an estimate of the expected term of “plain vanilla” employee option grants. The Company is utilizing an expected volatility figure based on a review of the historical volatilities, over a period of time, equivalent to the expected life of the instrument being valued, of similarly positioned public companies within its industry. The risk-free interest rate was determined from the implied yields from U.S. Treasury zero-coupon bonds with a remaining term consistent with the expected term of the instrument being valued.
Warrant Activity Summary
A summary of the warrant activity during the year ended December 31, 2023 is presented below:
SCHEDULE OF WARRANT ACTIVITY
| Weighted | ||||||||||||
| Weighted | Average | |||||||||||
| Average | Remaining | |||||||||||
| Number of | Exercise | Life | ||||||||||
| Warrants | Price | In Years | ||||||||||
| Outstanding, January 31, 2023 | 4,791,082 | $ | 10.71 | |||||||||
| Granted | - | |||||||||||
| Exercised | - | |||||||||||
| Expired | (63 | ) | 10,397 | |||||||||
| Outstanding, December 31, 2023 | 4,791,019 | $ | 10.57 | 2.9 | ||||||||
| Exercisable, December 31, 2023 | 4,791,019 | $ | 10.57 | 2.9 | ||||||||
SCHEDULE OF WARRANTS GRANTED ASSUMPTIONS
| For the Year Ended | ||||
| December 31, 2022 | ||||
| Risk free interest rate | 4.40 | % | ||
| Expected term (years) | 5.00 | |||
| Expected volatility | 313.55 | % | ||
| Expected dividends | 0.00 | % | ||
The weighted average estimated fair value of the warrants granted during the year ended December 31, 2022 was $2.28 per warrant. The Company did not issue any warrants during the year ended December 31, 2023.
| F- |
SCHEDULE OF STOCK WARRANTS
| Warrants Outstanding | Warrants Exercisable | |||||||||||||
| Weighted | ||||||||||||||
| Outstanding | Average | Exercisable | ||||||||||||
| Exercise | Number of | Remaining Life | Number of | |||||||||||
| Price | Warrants | In Years | Warrants | |||||||||||
| $ | 2.92 | 51,370 | 3.9 | 51,370 | ||||||||||
| $ | 10.00 | 4,501,938 | 2.9 | 4,501,938 | ||||||||||
| $ | 12.00 | 235,970 | 2.9 | 235,970 | ||||||||||
| $ | 60.00 | 250 | 1.0 | 250 | ||||||||||
| $ | 800.00 | 869 | 0.8 | 869 | ||||||||||
| $ | 2,240.00 | 39 | 0.5 | 39 | ||||||||||
| $ | 2,800.00 | 264 | 0.3 | 264 | ||||||||||
| $ | 3,400.00 | 264 | 0.3 | 264 | ||||||||||
| $ | 4,000.00 | 55 | 0.1 | 55 | ||||||||||
| 4,791,019 | 4,791,019 | |||||||||||||
Stock Options
SCHEDULE OF STOCK OPTION ACTIVITY
| Weighted | ||||||||||||||||
| Weighted | Average | |||||||||||||||
| Average | Remaining | |||||||||||||||
| Number of | Exercise | Life | Intrinsic | |||||||||||||
| Options | Price | In Years | Value | |||||||||||||
| Outstanding, January 31, 2023 | 864,639 | $ | 5.08 | |||||||||||||
| Granted | 629,017 | 2.91 | ||||||||||||||
| Exercised | - | |||||||||||||||
| Forfeited | (26,764 | ) | 4.87 | |||||||||||||
| Outstanding, December 31, 2023 | 1,466,892 | $ | 4.11 | 7.4 | $ | - | ||||||||||
| Exercisable, December 31, 2023 | 1,201,526 | $ | 4.33 | 7.1 | $ | - | ||||||||||
The weighted average grant date fair value of the stock options granted during the years ended December 31, 2023 and 2022 was approximately $3.00 and $4.88, respectively.
SCHEDULE OF INFORMATION RELATED TO STOCK OPTIONS
| Options Outstanding | Options Exercisable | |||||||||||||
| Weighted | ||||||||||||||
| Outstanding | Average | Exercisable | ||||||||||||
| Exercise | Number of | Remaining Life | Number of | |||||||||||
| Price | Options | In Years | Options | |||||||||||
| $ | 2.91 | 654,017 | 7.5 | 412,604 | ||||||||||
| $ | 5.08 | 812,875 | 6.9 | 788,922 | ||||||||||
| 1,466,892 | 1,201,526 | |||||||||||||
| F- |
SCHEDULE OF STOCK OPTION GRANTED ASSUMPTIONS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Risk free interest rate | 4.22 | % | 2.42 | % | ||||
| Expected term (years) | 3.50 | 3.50 | ||||||
| Expected volatility | 175.00 | % | 285.91 | % | ||||
| Expected dividends | 0.00 | % | 0.00 | % | ||||
Restricted Share Units
Pursuant to the 2021 Plan, the Company may grant restricted stock units (“RSUs”) to employees, consultants or non-employee directors (“Eligible Recipients”). The number, terms and conditions of the RSUs that are granted to Eligible Recipients are determined on an individual basis by the 2021 Plan administrator. On the distribution date, the Company shall issue to the Eligible Recipient one unrestricted, fully transferable share of the Company’s common stock (or the fair market value of one such share in cash) for each vested and nonforfeitable RSU.
A summary of the unvested RSUs as of December 31, 2023 is as follows:
SCHEDULE OF UNVESTED RESTRICTED STOCK UNITS
| Number of Shares | ||||
| Non-vested at January 1, 2023 | 201,870 | |||
| Granted | - | |||
| Vested | (104,043 | ) | ||
| Forfeited | - | |||
| Non-vested at December 31, 2023 | 97,827 | |||
Stock-Based Compensation Expense
SCHEDULE OF STOCK OPTION EXPENSE
| For the Years Ended | Unrecognized at |
Weighted Average Remaining Amortization |
||||||||||||||
| December 31, | December 31, | Period | ||||||||||||||
| 2023 | 2022 | 2023 | (Years) | |||||||||||||
| General and administrative | $ | 7,782,967 | $ | 12,612,862 | $ | 1,557,071 | 0.83 | |||||||||
| Total | $ | 7,782,967 | $ | 12,612,862 | $ | 1,557,071 | 0.83 | |||||||||
NOTE 8 - INCOME TAXES
The Company identified its federal and New York tax returns as its “major” tax jurisdictions. The Company is no longer subject to income tax income examinations by these tax authorities for taxable years ended December 31, 2018, and prior. The Company reviewed the prior New York state income tax filings and concluded that the prior year return will be amended to change the apportionment from zero to 100%. The Company believes its income tax filing positions and deductions will be sustained on audit and does not anticipate any adjustments that would result in a material change to its financial position. Therefore, no liabilities for uncertain tax positions have been recorded.
At December 31, 2023, the Company had approximately $63,500,000 and $13,600,000, respectively, of federal and state net operating losses that may be available to offset future taxable income. As a result of the Tax Cuts and Jobs Act of 2017 (the “Tax Act”), certain future carryforwards do not expire. At December 31, 2023, approximately $7,800,000 of federal net operating losses will expire from 2030 to 2038 and approximately $55,700,000 have no expiration but are subject to a utilization limit equal to 80% of current taxable income.
In accordance with Section 382 of the Internal Revenue Code, the usage of the Company’s net operating loss carryforwards are subject to annual limitations due to several greater than 50% ownership changes.
The Section 382 limitations resulted in approximately $28,200,000 of federal NOLs not being realizable as of December 31, 2023, and 2022 and the cumulative reversal of approximately $9,600,000 of net operating loss deferred tax assets.
| F- |
The Company has not performed a formal analysis for the year ended December 31, 2023, but it believes its ability to use such net operating losses and tax credit carryforwards in the future is subject to annual limitations due to change of control provisions under Sections 382 and 383 of the Internal Revenue Code, which will significantly impact its ability to realize these deferred tax assets.
Management assesses the available positive and negative evidence to estimate whether sufficient future taxable income will be generated to permit use of the existing deferred tax assets. A significant piece of objective negative evidence evaluated was the cumulative loss incurred over the three-year period ended December 31, 2023. For the period ended December 31, 2023, the Company has recorded a full valuation allowance recorded against the gross deferred tax asset balance as management believes that it is more likely than not that the results of operations will not generate sufficient taxable income to realize any of the deferred tax assets.
As of the date of this filing, the Company has not filed its 2023 federal and state corporate income tax returns. The Company expects to file these documents as soon as practicable.
The Company’s net deferred tax assets, liabilities and valuation allowance as of December 31, 2023 and 2022 are summarized as follows:
SCHEDULE OF NET DEFERRED TAX ASSETS AND LIABILITIES
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Deferred Tax Assets: | ||||||||
| Net Operating Loss Carryforwards | $ | 14,311,000 | $ | 13,200,000 | ||||
| Stock based Compensation | 15,788,000 | 13,810,000 | ||||||
| Research & Development Costs | 1,676,000 | 655,000 | ||||||
| Research & Development Credits | 330,000 | 330,000 | ||||||
| ROU Asset | 25,000 | - | ||||||
| Other DTA | 4,000 | - | ||||||
| Total Deferred Tax Assets | 32,134,000 | 27,995,000 | ||||||
| Deferred Tax Liabilities: | ||||||||
| Depreciation | (122,000 | ) | (106,000 | ) | ||||
| Intangible assets | (47,000 | ) | (113,000 | ) | ||||
| Lease Liability | (39,000 | ) | - | |||||
| Other DTL | (86,000 | ) | - | |||||
| Total Deferred Liabilities | (294,000 | ) | (219,000 | ) | ||||
| Net Deferred Tax Asset | 31,840,000 | 27,776,000 | ||||||
| Valuation Allowance | $ | (31,840,000 | ) | $ | (27,776,000 | ) | ||
| Deferred Tax Asset, Net of Valuation Allowance | $ | - | $ | - | ||||
| Change in VA | $ | (4,064,000 | ) | $ | (5,822,000 | ) | ||
The income tax provision (benefit) as of December 31, 2023 and 2022 consists of the following:
SCHEDULE OF INCOME TAX PROVISION (BENEFIT)
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Federal: | ||||||||
| Current | $ | - | $ | - | ||||
| Deferred | - | - | ||||||
| State & Local: | ||||||||
| Current | - | - | ||||||
| Deferred | - | - | ||||||
| Total Income Tax Provision (Benefit) | $ | - | $ | - | ||||
| F- |
A reconciliation of the statutory federal income tax benefit to actual tax benefit for the years ended December 31, 2023 and 2022 is as follows:
SCHEDULE OF STATUTORY FEDERAL INCOME TAX RATE
| 2023 | 2022 | |||||||
| Federal Statutory Rate | 21.0 | % | 21.0 | % | ||||
| State rate Net, Net of Fed Benefit | 1.3 | % | 5.6 | % | ||||
| Permanent Difference | -0.7 | % | -1.8 | % | ||||
| Tax Return to Provision Adjustment | -0.2 | % | 6.7 | % | ||||
| Change in Valuation Allowance | -21.4 | % | -31.5 | % | ||||
| Effective tax rate | 0.0 | % | 0.0 | % | ||||
NOTE 9 – LEASES
The Company is a party to a lease for 6,800 square feet of space located in Melville, New York (the “Melville Lease”) with respect to its corporate and laboratory operations. The Melville Lease was scheduled to expire in March 2020 (subject to extension at the option of the Company for a period of five years) and provided for an annual base rental during the initial term ranging between $132,600 and $149,260. In June 2019, the Company exercised its option to extend the Melville Lease and entered into a lease amendment with the lessor whereby the five-year extension term commenced on January 1, 2020 with annual base rent ranging between $153,748 and $173,060.
When measuring lease liabilities for leases that were classified as operating leases, the Company discounted lease payments using its estimated incremental borrowing rate at August 1, 2019. The weighted average incremental borrowing rate applied was 12%.
The following table presents net lease cost and other supplemental lease information:
SCHEDULE OF NET LEASE COST AND OTHER SUPPLEMENTAL LEASE INFORMATION
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Lease Costs | ||||||||
| Operating lease cost (cost resulting from lease payments) | $ | 168,028 | $ | 163,132 | ||||
| Net lease costs | $ | 168,028 | $ | 163,132 | ||||
| Operating lease - operating cash flows (fixed payments) | $ | 168,028 | $ | 163,132 | ||||
| Operating lease - operating cash flows (liability reduction) | $ | 139,328 | $ | 119,055 | ||||
| Non-current leases - right of use assets | $ | 151,447 | $ | 241,760 | ||||
| Current liabilities - operating lease liabilities | $ | 162,317 | $ | 139,328 | ||||
| Non-current liabilities - operating lease liabilities | $ | - | $ | 162,317 | ||||
Future minimum payments under non-cancelable leases for operating leases for the remaining terms of the leases following the year ended December 31, 2023:
SCHEDULE OF FUTURE MINIMUM PAYMENTS UNDER NON-CANCELABLE LEASES FOR OPERATING LEASES
| Fiscal Year | Operating Leases | |||
| 2024 | $ | 173,060 | ||
| Total future minimum lease payments | 173,060 | |||
| Amount representing interest | (10,743 | ) | ||
| Present value of net future minimum lease payments | $ | 162,317 | ||
| F- |
NOTE 10 - SUBSEQUENT EVENTS
Warrant Exercise and Issuance
On February 6, 2024, the Company entered into agreements with certain holders of its existing warrants exercisable for an aggregate of 3,351,580 shares of its Common Stock (collectively, the “Existing Warrants”), to exercise their warrants at a reduced exercise price of $2.33 per share, in exchange for new warrants (the “New Warrants”) as described below. The aggregate gross proceeds from the exercise of the Existing Warrants and the payment of the New Warrants, as described below, was approximately $8.1 million, before deducting financial advisory fees. The reduction of the exercise price of the Existing Warrants and the issuance of the New Warrants was structured as an at-market transaction under Nasdaq rules. Of the 3,351,580 shares of Common Stock issuable upon the exercise of the Existing Warrants, to date, the Company has issued an aggregate of 2,000,000 shares of Common Stock. The remaining 1,351,580 shares of Common Stock, which are issuable to Auctus, are being held in abeyance due to Auctus’ maximum beneficial ownership limitation. Such shares have been fully paid for and are issuable upon notice from Auctus to the Company.
In consideration for the immediate exercise of the Existing Warrants for cash and the payment of $0.125 per share underlying the New Warrants, the exercising holders received the New Warrants to purchase shares of Common Stock in a private placement pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”). The New Warrants will be exercisable for a period of five years into an aggregate of up to 2,513,686 shares of Common Stock at an exercise price of $2.43 per share. The securities offered in the private placement have not been registered under the Securities Act or applicable state securities laws. Accordingly, the securities may not be offered or sold in the United States except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act and such applicable state securities laws. As part of the transaction, the Company agreed to file a resale registration statement with the SEC to register the resale of the shares of Common Stock underlying the New Warrants issued in the private placement.
In connection with the transaction described above, the Company entered into a financial advisory services agreement, dated February 5, 2024, with Roth Capital Partners, LLC (“Roth”), pursuant to which the Company has paid Roth a cash fee of approximately $528,000 for its services, in addition to reimbursement for certain expenses.
Option Grants
Subsequent to December 31, 2023, the Company granted options to purchase an aggregate 1,934,716 shares of the Company’s common stock at an exercise price of $1.45 per share to employees, the Company’s board of directors and a member of the Company’s scientific advisory board. The options had an aggregate grant date fair value of $2,140,000 and vest as follows: (i) options to purchase an aggregate 513,663 shares of common stock vest monthly over one year, and (ii) options to purchase an aggregate of 1,421,053 shares of common stock vest 50% immediately with the remainder vesting quarterly over two years commencing one year from the date of grant. The Company will recognize the grant date fair value of the options proportionate to the vesting period.
| F- |
Exhibit 10.7
As Amended
September 13, 2023
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
ARTICLE 1 PURPOSE
The purpose of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”) is to promote the success and enhance the value of BioRestorative Therapies, Inc., a Delaware corporation (the “Company”), and its Subsidiaries (as defined below) by linking the individual interests of Employees, Consultants and members of the Board to those of the Company’s stockholders and by providing such individuals with an incentive for outstanding performance to generate superior returns to the Company’s stockholders. The Plan is further intended to provide flexibility to the Company and its Subsidiaries in their ability to motivate, attract, and retain the services of those individuals upon whose judgment, interest, and special effort the successful conduct of the Company’s operation is largely dependent.
ARTICLE 2 DEFINITIONS AND CONSTRUCTION
Wherever the following terms are used in the Plan they shall have the meanings specified below, unless the context clearly indicates otherwise. The singular pronoun shall include the plural where the context so indicates.
2.1 “Administrator” shall mean the entity that conducts the general administration of the Plan as provided in Article 10 hereof. With reference to the duties of the Administrator under the Plan which have been delegated to one or more persons pursuant to Section 10.6 hereof, or which the Board has assumed, the term “Administrator” shall refer to such person(s) unless the Committee or the Board has revoked such delegation or the Board has terminated the assumption of such duties.
2.2 “Applicable Accounting Standards” shall mean Generally Accepted Accounting Principles in the United States, International Financial Reporting Standards or such other accounting principles or standards as may apply to the Company’s financial statements under United States federal securities and other applicable laws from time to time.
2.3 “Applicable Law” shall mean any applicable law, including without limitation, (a) provisions of the Code, the Securities Act, the Exchange Act and any rules or regulations thereunder; (b) corporate, securities, tax or other laws, statutes, rules, requirements or regulations, whether federal, state, local or foreign; and (c) rules of any securities exchange or automated quotation system on which the Shares are listed, quoted or traded.
2.4 “Award” shall mean an Option, a Restricted Stock award, a Dividend Equivalent award, a Stock Payment award, a Restricted Stock Unit award, a Performance Share award, an Other Incentive Award, or a Stock Appreciation Right, which may be awarded or granted under the Plan.
2.5 “Award Agreement” shall mean any written notice, agreement, contract or other instrument or document evidencing an Award, including through electronic medium, which shall contain such terms and conditions with respect to an Award as the Administrator shall determine, consistent with the Plan.
2.6 “Board” shall mean the Board of Directors of the Company.
2.7 “Cause” shall mean (a) the Administrator’s determination that the Participant failed to substantially perform the Participant’s duties (other than any such failure resulting from the Participant’s Disability); (b) the Administrator’s determination that the Participant failed to carry out, or comply with, any lawful and reasonable directive of the Board or the Participant’s immediate supervisor; (c) the Participant’s commission of any act which, if the Participant were convicted would constitute, or the Participant’s conviction, plea of no contest, plea of nolo contendere, or imposition of unadjudicated probation for, any (i) felony, (ii) indictable offense or (iii) crime involving moral turpitude; (d) the Participant’s unlawful use (including being under the influence) or possession of illegal drugs on the premises of the Company or any of its Subsidiaries or while performing the Participant’s duties and responsibilities; or (e) the Participant’s commission of an act of fraud, embezzlement, misappropriation, willful or gross misconduct, or breach of fiduciary duty against the Company or any of its Subsidiaries. Notwithstanding the foregoing, if the Participant is a party to a written employment, consulting, or other agreement with the Company or any of its Subsidiaries in which the term “cause” is defined, then “Cause” shall be as such term is defined in the applicable written employment or consulting agreement.
2.8 “Change in Control” shall mean the occurrence of any of the following events:
(a) A “change in ownership,” as described in Section 1.409A-3(i)(5)(v) of the Treasury Regulations.
(b) A “change in effective control,” as described in Section 1.409A-3(i)(5)(vi) of the Treasury Regulations (but substituting “50 percent” for “30 percent” in the first sentence of Section 1.409A-3(i)(5)(vi)(A)(1)).
(c) A “change in ownership of a substantial portion of the assets,” as described in Section 1.409A-3(i)(5)(vii) of the Treasury Regulations (but substituting “50 percent” for “40 percent” in the first sentence thereof).
2.9 “Code” shall mean the Internal Revenue Code of 1986, as amended from time to time, together with the regulations and official guidance promulgated thereunder, whether issued prior or subsequent to the grant of any Award.
2.10 “Committee” shall mean the committee appointed by the Board to administer the Plan or the Board if no committee is appointed.
2.11 “Common Stock” shall mean the common stock of the Company.
2.12 “Company” shall mean BioRestorative Therapies, Inc., a Delaware corporation.
2.13 “Consultant” shall mean any consultant or advisor to the Company or any Subsidiary who provides consulting or advisory services, other than as an Employee or Director, and such consultant or advisor (a) is a natural person (or an entity wholly-owned, directly or indirectly, by a natural person), (b) has provided and/or will provide bona fide services to the Company, and (c) such services are not in connection with the offer or sale of securities in a capital-raising transaction and do not directly or indirectly promote or maintain a market for the Company’s securities.
2.14 “Director” shall mean a member of the Board, as constituted from time to time.
|
|
2.15 “Disability,” unless otherwise specified in an Award Agreement or under the terms of a Program, shall mean total and permanent disability as defined in Section 22(e)(3) of the Code. For purposes of the Plan, a Participant shall be deemed to have incurred a Disability if the Participant is determined to be totally disabled by the Social Security Administration or in accordance with the applicable disability insurance program of the Company. Notwithstanding the foregoing, if the Participant is a party to a written employment, consulting, or other agreement with the Company or any of its Subsidiaries in which the term “disability” is defined, then “Disability” shall be as such term is defined in the applicable written employment or consulting agreement.
2.16 “Dividend Equivalent” shall mean a right to receive the equivalent value (in cash or Shares) of dividends paid on Shares, awarded under Section 8.1 hereof.
2.17 “Effective Date” shall mean the date the Plan is approved by the Board.
2.18 “Eligible Individual” shall mean any person who is an Employee, a Consultant or a Non-Employee Director, as determined by the Administrator.
2.19 “Employee” shall mean any officer or other employee (within the meaning of Section 3401(c) of the Code) of the Company or any Subsidiary.
2.20 “Equity Restructuring” shall mean a nonreciprocal transaction between the Company and its stockholders, such as a stock dividend, stock split, reverse stock split, spin-off, rights offering or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of Shares (or other securities of the Company) or the share price of Common Stock (or other securities) and causes a change in the per share value of the Common Stock underlying outstanding stock-based Awards.
2.21 “Exchange Act” shall mean the Securities Exchange Act of 1934, as amended from time to time.
2.22 “Expiration Date” shall have the meaning given to such term in Section 11.1(b).
2.23 “Fair Market Value” shall mean, as of any given date, the value of a Share, determined as follows:
(a) if the Common Stock is listed on any established stock exchange or a national market system, including, without limitation, Nasdaq, its Fair Market Value shall be the closing sales price for such stock (or the closing bid, if no sales were reported) as quoted on such exchange or system on the day immediately preceding the day of determination (or, if the determination is made after the close of business for trading, then on the day of determination) (or, if no closing sales price or closing bid was reported on that date, as applicable, on the last trading date such closing sales price or closing bid was reported), as reported in The Wall Street Journal or such other source as the Administrator deems reliable;
(b) if the Common Stock is regularly quoted on an automated quotation system (including the OTCQB market) or by a recognized securities dealer, its Fair Market Value shall be the closing sales price for such stock (or the mean between the high bid and low asked prices for the Common Stock, if selling prices are not reported), as quoted on such system or by such securities dealer on the day immediately preceding the day of determination (or, if the determination is made after the close of business for trading, then on the day of determination) (or, if no such prices were reported on that date, as applicable, on the last date such prices were reported), as reported in The Wall Street Journal or such other source as the Administrator deems reliable; or
|
|
(c) in the absence of an established market for the Common Stock, the Fair Market Value shall be determined by the Administrator in good faith, using such criteria as it shall determine, in its sole discretion, to be appropriate for valuation, provided that the determination shall be consistent with the requirements of Sections 422 and 409A of the Code, if applicable.
2.24 “Greater Than 10% Stockholder” shall mean an individual then-owning (within the meaning of Section 424(d) of the Code) more than ten percent (10%) of the total combined voting power of all classes of stock of the Company or any “parent corporation” or “subsidiary corporation” (as defined in Sections 424(e) and 424(f) of the Code, respectively).
2.25 “Incentive Stock Option” shall mean an Option that is intended to qualify as an incentive stock option and conforms to the applicable provisions of Section 422 of the Code.
2.26 “Non-Employee Director” shall mean a Director of the Company who is not an Employee.
2.27 “Non-Qualified Stock Option” shall mean an Option that is not an Incentive Stock Option or which is designated as an Incentive Stock Option but does not meet the applicable requirements of Section 422 of the Code.
2.28 “Option” shall mean a right to purchase Shares at a specified exercise price, granted under Article 5 hereof. An Option shall be either a Non-Qualified Stock Option or an Incentive Stock Option; provided, however, that Options granted to Non-Employee Directors and Consultants shall only be Non-Qualified Stock Options.
2.29 “Organizational Documents” shall mean, collectively, (a) the Company’s articles of incorporation, certificate of incorporation, bylaws or other similar organizational documents relating to the creation and governance of the Company, and (b) the Committee’s charter or other similar organizational documentation relating to the creation and governance of the Committee.
2.30 “Other Incentive Award” shall mean an Award denominated in, linked to or derived from Shares or value metrics related to Shares, granted pursuant to Section 8.5 hereof.
2.31 “Participant” shall mean a person who has been granted an Award pursuant to the Plan.
2.32 “Performance Share” shall mean a contractual right awarded under Section 8.4 hereof to receive a number of Shares or the Fair Market Value of such number of Shares in cash based on the attainment of specified performance goals or other criteria determined by the Administrator.
2.33 “Plan” shall mean this BioRestorative Therapies, Inc. 2021 Stock Incentive Plan, as it may be amended, supplemented, and restated from time to time.
2.34 “Program” shall mean any program adopted by the Administrator pursuant to the Plan containing the terms and conditions intended to govern a specified type of Award granted under the Plan and pursuant to which such type of Award may be granted under the Plan.
2.35 “Restricted Stock” shall mean an award of Shares made under Article 7 hereof that is subject to certain restrictions and may be subject to risk of forfeiture.
2.36 “Restricted Stock Unit” shall mean a contractual right awarded under Section 8.3 hereof to receive in the future a Share or the Fair Market Value of a Share in cash.
|
|
2.37 “Securities Act” shall mean the Securities Act of 1933, as amended.
2.38 “Share Limit” shall have the meaning provided in Section 3.1(a) hereof.
2.39 “Shares” shall mean shares of Common Stock.
2.40 “Stock Appreciation Right” shall mean an Award entitling the Participant (or other person entitled to exercise pursuant to the Plan) to exercise all or a specified portion thereof (to the extent then exercisable pursuant to its terms) and to receive from the Company an amount determined by multiplying the difference obtained by subtracting the exercise price per share of such Award from the Fair Market Value on the date of exercise of such Award by the number of Shares with respect to which such Award shall have been exercised, subject to any limitations the Administrator may impose.
2.41 “Stock Payment” shall mean a payment in the form of Shares awarded under Section 8.2 hereof.
2.42 “Subsidiary” shall mean (a) a corporation, association or other business entity of which fifty percent (50%) or more of the total combined voting power of all classes of capital stock is owned, directly or indirectly, by the Company and/or by one or more Subsidiaries, (b) any partnership or limited liability company of which fifty percent (50%) or more of the equity interests are owned, directly or indirectly, by the Company and/or by one or more Subsidiaries, and (c) any other entity not described in clauses (a) or (b) above of which fifty percent (50%) or more of the ownership or the power (whether voting interests or otherwise), pursuant to a written contract or agreement, to direct the policies and management or the financial and the other affairs thereof, are owned or controlled by the Company and/or by one or more Subsidiaries.
2.43 “Substitute Award” shall mean an Award granted under the Plan in connection with a corporate transaction, such as a merger, combination, consolidation or acquisition of property or stock, in any case, upon the assumption of, or in substitution for, an outstanding equity award previously granted by a company or other entity that is a party to such transaction; provided, however, that in no event shall the term “Substitute Award” be construed to refer to an award made in connection with the cancellation and repricing of an Option or Stock Appreciation Right.
2.44 “Termination of Service” shall mean, unless otherwise determined by the Administrator:
(a) As to a Consultant, the time when the engagement of a Participant as a Consultant to the Company and its Subsidiaries is terminated for any reason, with or without cause, including, without limitation, by resignation, discharge, death, Disability or retirement, but excluding terminations where the Consultant simultaneously commences or remains in employment and/or service as an Employee and/or Director of the Company or any Subsidiary.
(b) As to a Non-Employee Director, the time when a Participant who is a Non-Employee Director ceases to be a Director for any reason, including, without limitation, a termination by resignation, failure to be elected, removal, death, Disability or retirement, but excluding terminations where the Participant simultaneously commences or remains in employment and/or service as an Employee of and/or Consultant to the Company or any Subsidiary.
|
|
(c) As to an Employee, the time when the employee-employer relationship between a Participant and the Company and its Subsidiaries is terminated for any reason, including, without limitation, a termination by resignation, discharge, death, Disability or retirement, but excluding terminations where the Participant simultaneously commences or remains in service as a Consultant to and/or Director of the Company or any Subsidiary.
The Administrator, in its sole discretion, shall determine the effect of all matters and questions relating to any Termination of Service, including, without limitation, whether a Termination of Service has occurred, whether any Termination of Service resulted from a discharge for Cause and whether any particular leave of absence constitutes a Termination of Service; provided, however, that, with respect to Incentive Stock Options, unless the Administrator otherwise provides in the terms of any Program, Award Agreement or otherwise, or as otherwise required by Applicable Law, a leave of absence, change in status from an employee to an independent contractor or other change in the employee-employer relationship shall constitute a Termination of Service only if, and to the extent that, such leave of absence, change in status or other change interrupts employment for the purposes of Section 422(a)(2) of the Code. For purposes of the Plan, a Participant’s employee-employer relationship or consultancy relationship shall be deemed to be terminated in the event that the Subsidiary employing or contracting with such Participant ceases to remain a Subsidiary following any merger, sale of stock or other corporate transaction or event (including, without limitation, a spin-off).
ARTICLE 3 SHARES SUBJECT TO THE PLAN
3.1 Number of Shares.
(a) Subject to Sections 3.1(b) and 11.2 hereof, the aggregate number of Shares which may be issued or transferred pursuant to Awards under the Plan is Three Million Eight Hundred Fifty Thousand (3,850,000) Shares (the “Share Limit”). No more than Three Million Eight Hundred Fifty Thousand (3,850,000) Shares may be issued upon the exercise of Incentive Stock Options.
(b) If any Shares subject to an Award are forfeited or expire or such Award is settled for cash (in whole or in part), the Shares subject to such Award shall, to the extent of such forfeiture, expiration or cash settlement, again be available for future grants of Awards under the Plan and shall be added back to the Share Limit in the same number of Shares as were debited from the Share Limit in respect of the grant of such Award (as may be adjusted in accordance with Section 11.2 hereof). In addition, the following Shares shall be added back to the Share Limit and will be available for future grants of Awards: (i) Shares tendered by a Participant or withheld by the Company in payment of the exercise price of an Option or Stock Appreciation Right; (ii) Shares tendered by the Participant or withheld by the Company to satisfy any tax withholding obligation with respect to an Award; and (iii) Shares subject to a Stock Appreciation Right that are not issued in connection with the stock settlement of the stock appreciation right on exercise thereof. Any Shares forfeited by the Participant or repurchased by the Company under Section 7.4 hereof at the same price paid by the Participant so that such Shares are returned to the Company will again be available for Awards. The payment of Dividend Equivalents in cash in conjunction with any outstanding Awards shall not be counted against the Shares available for issuance under the Plan. Notwithstanding the provisions of this Section 3.1(b), no Shares may again be optioned, granted or awarded if such action would cause an Incentive Stock Option to fail to qualify as an incentive stock option under Section 422 of the Code.
|
|
(c) Substitute Awards shall not reduce the Shares authorized for grant under the Plan, except to the extent required by reason of Section 422 of the Code. Additionally, in the event that a company acquired by the Company or any Subsidiary, or with which the Company or any Subsidiary combines, has shares available under a pre-existing plan approved by its stockholders and not adopted in contemplation of such acquisition or combination, the shares available for grant pursuant to the terms of such pre-existing plan (as adjusted, to the extent appropriate, using the exchange ratio or other adjustment or valuation ratio or formula used in such acquisition or combination to determine the consideration payable to the holders of common stock of the entities party to such acquisition or combination) may be used for Awards under the Plan and shall not reduce the Shares authorized for grant under the Plan to the extent that grants of Awards using such available shares are (i) permitted without stockholder approval under the rules of the principal securities exchange on which the Common Stock is then listed, if applicable, and (ii) made only to individuals who were not employed by or providing services to the Company or its Subsidiaries immediately prior to such acquisition or combination.
ARTICLE 4 GRANTING OF AWARDS
4.1 Participation. The Administrator may, from time to time, select from among all Eligible Individuals, those to whom one or more Awards shall be granted and shall determine the nature and amount of each Award, which shall not be inconsistent with the requirements of the Plan. No Eligible Individual or other Person shall have any right to be granted an Award pursuant to the Plan.
4.2 Award Agreement. Each Award shall be evidenced by an Award Agreement stating the terms and conditions applicable to such Award, consistent with the requirements of the Plan and any applicable Program. Award Agreements evidencing Incentive Stock Options shall contain such terms and conditions as may be necessary to meet the applicable provisions of Section 422 of the Code.
4.3 At-Will Service. Nothing in the Plan or in any Program or Award Agreement hereunder shall confer upon any Participant any right to continue as an Employee, Director or Consultant of the Company or any Subsidiary, or shall interfere with or restrict in any way the rights of the Company or any Subsidiary, which rights are hereby expressly reserved, to discharge any Participant at any time for any reason whatsoever, with or without Cause, and with or without notice, or to terminate or change all other terms and conditions of any Participant’s employment or engagement, except to the extent expressly provided otherwise in a written agreement between the Participant and the Company or any Subsidiary.
4.4 Stand-Alone and Tandem Awards. Awards granted pursuant to the Plan may, in the sole discretion of the Administrator, be granted either alone, in addition to, or in tandem with, any other Award granted pursuant to the Plan. Awards granted in addition to or in tandem with other Awards may be granted either at the same time as or at a different time from the grant of such other Awards.
ARTICLE 5 GRANTING OF OPTIONS AND STOCK APPRECIATION RIGHTS
5.1 Granting of Options and Stock Appreciation Rights to Eligible Individuals. The Administrator is authorized to grant Options and Stock Appreciation Rights to Eligible Individuals from time to time, in its sole discretion, on such terms and conditions as it may determine which shall not be inconsistent with the Plan.
|
|
5.2 Qualification of Incentive Stock Options. No Incentive Stock Option shall be granted to any person who is not an Employee of the Company or any “parent corporation” or “subsidiary corporation” of the Company (as defined in Sections 424(e) and 424(f) of the Code, respectively). No person who qualifies as a Greater Than 10% Stockholder may be granted an Incentive Stock Option unless such Incentive Stock Option conforms to the applicable provisions of Section 422 of the Code. Any Incentive Stock Option granted under the Plan may be modified by the Administrator, with the consent of the Participant, to disqualify such Option from treatment as an “incentive stock option” under Section 422 of the Code. To the extent that the aggregate fair market value of stock with respect to which “incentive stock options” (within the meaning of Section 422 of the Code, but without regard to Section 422(d) of the Code) are exercisable for the first time by a Participant during any calendar year under the Plan and all other plans of the Company or any “parent corporation” or “subsidiary corporation” of the Company (as defined in Section 424(e) and 424(f) of the Code, respectively) exceeds one hundred thousand dollars ($100,000), the Options shall be treated as Non-Qualified Stock Options to the extent required by Section 422 of the Code. The rule set forth in the preceding sentence shall be applied by taking Options and other “incentive stock options” into account in the order in which they were granted and the fair market value of stock shall be determined as of the time the respective options were granted. In addition, to the extent that any Options otherwise fail to qualify as Incentive Stock Options, such Options shall be treated as Nonqualified Stock Options. Any interpretations and rules under the Plan with respect to Incentive Stock Options shall be consistent with the provisions of Section 422 of the Code.
5.3 Option and Stock Appreciation Right Exercise Price. The exercise price per Share subject to each Option and Stock Appreciation Right shall be set by the Administrator, but shall not be less than one hundred percent (100%) of the Fair Market Value of a Share on the date the Option or Stock Appreciation Right, as applicable, is granted (or, as to Incentive Stock Options, on the date the Option is modified, extended or renewed for purposes of Section 424(h) of the Code). In addition, in the case of Incentive Stock Options granted to a Greater Than 10% Stockholder, such price shall not be less than one hundred ten percent (110%) of the Fair Market Value of a Share on the date the Option is granted (or the date the Option is modified, extended or renewed for purposes of Section 424(h) of the Code). Notwithstanding the foregoing, in the case of an Option or Stock Appreciation Right that is a Substitute Award, the exercise price per share of the Shares subject to such Option or Stock Appreciation Right, as applicable, may be less than the Fair Market Value per share on the date of grant; provided that the exercise price of any Substitute Award shall be determined in accordance with the applicable requirements of Sections 424 and 409A of the Code.
5.4 Option and SAR Term. The term of each Option and the term of each Stock Appreciation Right shall be set by the Administrator in its sole discretion; provided, however, that the term shall not be more than ten (10) years from the date the Option or Stock Appreciation Right, as applicable, is granted, or five (5) years from the date an Incentive Stock Option is granted to a Greater Than 10% Stockholder. Except as limited by the requirements of Section 409A or Section 422 of the Code, and subject to the limitations set forth in the previous sentence, the Administrator may extend the term of any outstanding Option or Stock Appreciation Right, and may extend the time period during which vested Options or Stock Appreciation Rights may be exercised, in connection with any Termination of Service of the Participant or otherwise.
5.5 Termination of Services.
(a) Unless otherwise provided in an Award Agreement, if a Participant has a Termination of Service for any reason other than for Cause or due to death or Disability, the Participant may exercise any vested outstanding Option or Stock Appreciation Right, to the extent exercisable on the date of Termination of Service, at any time within three months after the date of such Termination of Service.
|
|
(b) Unless otherwise provided in an Award Agreement, if a Participant has a Termination of Service due to death or Disability, the Participant (or in the case of death, the Participant’s estate or the beneficiary who acquired the right to exercise the Option or Stock Appreciation Right by bequest or inheritance or otherwise by reason of the death of the Participant) may exercise any vested outstanding Option or Stock Appreciation Right, to the extent exercisable on the date of Termination of Service, at any time within twelve months after the date of such Termination of Service.
(c) Unless otherwise provided in an Award Agreement, if a Participant has a Termination of Service for Cause, any Option or Stock Appreciation Right held by the Participant under the Plan, to the extent not exercised prior to the Termination of Service, and whether or not vested, will terminate immediately.
(d) Notwithstanding the foregoing, nothing in this Section 5.5 will extend the exercise period for any Option or Stock Appreciation Right beyond the stated term of the Option or Stock Appreciation Right.
5.6 Option and SAR Vesting.
(a) The terms and conditions pursuant to which an Option or Stock Appreciation Right vests in the Participant and becomes exercisable shall be determined by the Administrator and set forth in the applicable Award Agreement. Such vesting may be based on service with the Company or any Subsidiary, specified performance goals, or any other criteria selected by the Administrator. At any time after the grant of an Option or Stock Appreciation Right, the Administrator may, in its sole discretion and subject to whatever terms and conditions it selects, accelerate the vesting of the Option or Stock Appreciation Right.
(b) Unless otherwise permitted by the Plan, no portion of an Option or Stock Appreciation Right which is unexercisable at a Participant’s Termination of Service shall thereafter become exercisable.
5.7 Substitution of Stock Appreciation Rights. The Administrator may, in its sole discretion, substitute an Award of Stock Appreciation Rights for an outstanding Option at any time prior to or upon exercise of such Option; provided, however, that such Stock Appreciation Rights shall be exercisable with respect to the same number of Shares for which such substituted Option would have been exercisable, and shall also have the same exercise price and remaining term as the substituted Option.
ARTICLE 6 EXERCISE OF OPTIONS AND STOCK APPRECIATION RIGHTS
6.1 Exercise and Payment. An exercisable Option or Stock Appreciation Right may be exercised in whole or in part. However, an Option or Stock Appreciation Right shall not be exercisable with respect to fractional shares and the Administrator may require that, by the terms of the Option or Stock Appreciation Right, a partial exercise must be with respect to a minimum number of Shares. Payment of the amounts payable with respect to Stock Appreciation Rights pursuant to this Article 6 shall be in cash, Shares (based on its Fair Market Value as of the date the Stock Appreciation Right is exercised), or a combination of both, as determined by the Administrator.
|
|
6.2 Manner of Exercise. All or a portion of an exercisable Option or Stock Appreciation Right shall be deemed exercised upon delivery of all of the following to the Secretary of the Company, the Administrator or such other person or entity designated by the Administrator, or his, her or its office, as applicable:
(a) A written or electronic notice complying with the applicable rules established by the Administrator stating that the Option or Stock Appreciation Right, or a portion thereof, is exercised. The notice shall be signed by the Participant or other person then entitled to exercise the Option or Stock Appreciation Right or such portion thereof;
(b) Such representations and documents as the Administrator, in its sole discretion, deems necessary or advisable to effect compliance with Applicable Law. The Administrator may, in its sole discretion, also take such additional actions as it deems appropriate to effect such compliance including, without limitation, placing legends on share certificates and issuing stop-transfer notices to agents and registrars;
(c) In the event that the Option or Stock Appreciation Right shall be exercised pursuant to Section 9.3 hereof by any person or persons other than the Participant, appropriate proof of the right of such person or persons to exercise the Option or Stock Appreciation Right, as determined in the sole discretion of the Administrator; and
(d) Full payment of the exercise price and applicable withholding taxes for the Shares with respect to which the Option or Stock Appreciation Right, or portion thereof, is exercised, in a manner permitted by the Administrator in accordance with Sections 9.1 and 9.2 hereof.
6.3 Notification Regarding Disposition. The Participant shall give the Company prompt written or electronic notice of any disposition of Shares acquired by exercise of an Incentive Stock Option which occurs within (a) two (2) years after the date of granting (including the date the Option is modified, extended or renewed for purposes of Section 424(h) of the Code) of such Option to such Participant, or (b) one (1) year after the date of transfer of such Shares to such Participant.
ARTICLE 7 RESTRICTED STOCK
7.1 Award of Restricted Stock.
(a) The Administrator is authorized to grant Restricted Stock to Eligible Individuals, and shall determine the terms and conditions, including the restrictions applicable to each award of Restricted Stock, which terms and conditions shall not be inconsistent with the Plan or any applicable Program, and may impose such conditions on the issuance of such Restricted Stock as it deems appropriate.
(b) The Administrator shall establish the purchase price, if any, and form of payment for Restricted Stock; provided, however, that if a purchase price is charged, such purchase price shall be no less than the par value of the Shares to be purchased, unless otherwise permitted by Applicable Law. In all cases, legal consideration shall be required for each issuance of Restricted Stock to the extent required by Applicable Law.
|
|
7.2 Rights as Stockholders. Subject to Section 7.4 hereof, upon issuance of Restricted Stock, the Participant shall have, unless otherwise provided by the Administrator, all the rights of a stockholder with respect to the Shares, subject to the restrictions in the Plan, an applicable Program or in the applicable Award Agreement, including the right to receive all dividends and other distributions paid or made with respect to the Shares; provided, however, that, in the sole discretion of the Administrator, any extraordinary distributions with respect to the Shares may be subject to the restrictions set forth in Section 7.3 hereof. In addition, with respect to Restricted Stock that is subject to performance-based vesting (including the continuation of services for a specified period of time), dividends which are paid prior to vesting shall only be paid out to the Participant to the extent that the performance-based vesting conditions are subsequently satisfied and the share of Restricted Stock vests.
7.3 Restrictions. All shares of Restricted Stock (including any shares received by Participants thereof with respect to shares of Restricted Stock as a result of stock dividends, stock splits or any other form of recapitalization) shall be subject to such restrictions and vesting requirements as the Administrator shall provide in the applicable Program or Award Agreement. By action taken after the Restricted Stock is issued, the Administrator may, on such terms and conditions as it may determine to be appropriate, accelerate the vesting of such Restricted Stock by removing any or all of the restrictions imposed by the terms of any Program or by the applicable Award Agreement.
7.4 Repurchase or Forfeiture of Restricted Stock. Except as otherwise determined by the Administrator, if no purchase price was paid by the Participant for the Restricted Stock, upon a Termination of Service, the Participant’s rights in unvested Restricted Stock then subject to restrictions shall lapse and be forfeited, and such Restricted Stock shall be surrendered to the Company and cancelled without consideration on the date of such Termination of Service. If a purchase price was paid by the Participant for the Restricted Stock, upon a Termination of Service the Company shall have the right to repurchase from the Participant the unvested Restricted Stock then-subject to restrictions at a cash price per share equal to the price paid by the Participant for such Restricted Stock or such other amount as may be specified in an applicable Program or the applicable Award Agreement. The Administrator in its sole discretion may provide that, upon certain events, including without limitation a Change in Control, the Participant’s death, retirement or Disability, any other specified Termination of Service or any other event, the Participant’s rights in unvested Restricted Stock shall not terminate, such Restricted Stock shall vest and cease to be forfeitable and, if applicable, the Company shall cease to have a right of repurchase.
7.5 Certificates/Book Entries for Restricted Stock. Restricted Stock granted pursuant to the Plan may be evidenced in such manner as the Administrator shall determine. Certificates or book entries evidencing shares of Restricted Stock must include an appropriate legend referring to the terms, conditions, and restrictions applicable to such Restricted Stock, and the Company may, in its sole discretion, retain physical possession of any stock certificate until such time as all applicable restrictions lapse.
7.6 Section 83(b) Election. If a Participant makes an election under Section 83(b) of the Code to be taxed with respect to the Restricted Stock as of the date of transfer of the Restricted Stock rather than as of the date or dates upon which the Participant would otherwise be taxable under Section 83(a) of the Code, the Participant shall be required to deliver a copy of such election to the Company promptly after filing such election with the Internal Revenue Service.
|
|
ARTICLE 8 DIVIDEND EQUIVALENTS; STOCK PAYMENTS; RESTRICTED STOCK UNITS; PERFORMANCE SHARES; OTHER INCENTIVE AWARDS
8.1 Dividend Equivalents.
(a) Subject to Section 8.1(b) hereof, Dividend Equivalents may be granted by the Administrator, either alone or in tandem with another Award, based on dividends declared on Common Stock, to be credited as of dividend payment dates during the period between the date the Dividend Equivalents are granted to a Participant and the date such Dividend Equivalents terminate or expire, as determined by the Administrator. Such Dividend Equivalents shall be converted to cash or additional Shares by such formula and at such time and subject to such limitations as may be determined by the Administrator. In addition, Dividend Equivalents with respect to an Award that is subject to performance-based vesting that are based on dividends paid prior to the vesting of such Award shall only be paid out to the Participant to the extent that the performance-based vesting conditions are subsequently satisfied and the Award vests.
(b) Notwithstanding the foregoing, no Dividend Equivalents shall be payable with respect to Options or Stock Appreciation Rights.
8.2 Stock Payments. The Administrator is authorized to make one or more Stock Payments to any Eligible Individual. The number or value of Shares of any Stock Payment shall be determined by the Administrator and may be based upon one or more specific performance criteria or any other specific criteria, including service to the Company or any Subsidiary, determined by the Administrator. Stock Payments may, but are not required to, be made in lieu of base salary, bonus, fees or other cash compensation otherwise payable to such Eligible Individual.
8.3 Restricted Stock Units. The Administrator is authorized to grant Restricted Stock Units to any Eligible Individual. The number and terms and conditions of Restricted Stock Units shall be determined by the Administrator. The Administrator shall specify the date or dates on which the Restricted Stock Units shall become fully vested and nonforfeitable, and may specify such conditions to vesting as it deems appropriate, including conditions based on one or more specific performance criteria or other specific criteria, including service to the Company or any Subsidiary, in each case, on a specified date or dates or over any period or periods, as determined by the Administrator. The Administrator shall specify, or may permit the Participant to elect, the conditions and dates upon which the Shares underlying the Restricted Stock Units shall be issued, which dates shall not be earlier than the date as of which the Restricted Stock Units vest and become nonforfeitable and which conditions and dates shall be consistent with the applicable provisions of Section 409A of the Code or an exemption therefrom. On the distribution dates, the Company shall issue to the Participant one unrestricted, fully transferable Share (or the Fair Market Value of one such Share in cash) for each vested and nonforfeitable Restricted Stock Unit.
8.4 Performance Share Awards. Any Eligible Individual selected by the Administrator may be granted one or more Performance Share awards which shall be denominated in a number or range of Shares and the vesting of which may be linked to any specific performance criteria (in each case on a specified date or dates or over any period or periods determined by the Administrator) and/or time-vesting or other criteria, as determined by the Administrator.
8.5 Other Incentive Awards. The Administrator is authorized to grant Other Incentive Awards to any Eligible Individual, which Awards may cover Shares or the right to purchase Shares or have a value derived from the value of, or an exercise or conversion privilege at a price related to, or that are otherwise payable in or based on, Shares, stockholder value or stockholder return, in each case, on a specified date or dates or over any period or periods determined by the Administrator. Other Incentive Awards may be linked to such specific performance criteria as determined appropriate by the Administrator. Other Incentive Awards may be paid in cash, Shares, or a combination of cash and Shares, as determined by the Administrator.
|
|
8.6 Other Terms and Conditions. All applicable terms and conditions of each Award described in this Article 8, including without limitation, as applicable, the term, vesting conditions and exercise/purchase price applicable to the Award, shall be set by the Administrator in its sole discretion, provided, however, that the value of the consideration paid by a Participant for an Award, if any, shall not be less than the par value of a Share, unless otherwise permitted by Applicable Law.
8.7 Exercise upon Termination of Service. Awards described in this Article 8 are exercisable or distributable, as applicable, only while the Participant is an Employee, Director or Consultant, as applicable. Except as otherwise provided in the Plan, the Administrator, however, in its sole discretion may provide that such Award may be exercised or distributed subsequent to a Termination of Service as provided under an applicable Program, Award Agreement, payment deferral election and/or in certain events, including without limitation, a Change in Control, the Participant’s death, retirement or Disability or any other specified Termination of Service.
ARTICLE 9 ADDITIONAL TERMS OF AWARDS
9.1 Payment. The Administrator shall determine the method or methods by which payments by any Participant with respect to any Awards granted under the Plan shall be made, including, without limitation: (a) cash or check, (b) Shares (including, in the case of payment of the exercise price of an Award, Shares issuable pursuant to the exercise of the Award) held for such minimum period of time as may be established by the Administrator, in each case, having a Fair Market Value on the date of delivery equal to the aggregate payments required, (c) other form of legal consideration acceptable to the Administrator, or (d) any combination of the foregoing. The Administrator shall also determine the methods by which Shares shall be delivered or deemed to be delivered to Participants.
9.2 Tax Withholding and Tax Bonuses.
(a) The Company and its Subsidiaries shall have the authority and the right to deduct or withhold, or require a Participant to remit to the Company or a Subsidiary, an amount sufficient to satisfy federal, state, local and foreign taxes (including the Participant’s social security, Medicare and any other employment tax obligation) required by law to be withheld with respect to any taxable event concerning a Participant arising in connection with any Award. The Administrator may in its sole discretion and in satisfaction of the foregoing requirement allow a Participant to satisfy such obligations by any payment means described in Section 9.1 hereof, including without limitation, by allowing such Participant to elect to have the Company or a Subsidiary withhold Shares otherwise issuable under an Award (or allow the surrender of Shares). Notwithstanding anything in this Section 9.2 to the contrary, the Company shall not allow a Participant to make any such election if the election would cause a violation of Section 409A of the Code.
(b) The Committee, in its discretion, shall have the authority, at the time of grant of any Award under the Plan or at any time thereafter, to approve cash bonuses to designated Participants to be paid upon their exercise or receipt of (or the lapse of restrictions relating to) Awards in order to provide funds to pay all or a portion of federal and state taxes due as a result of such exercise or receipt (or the lapse of such restrictions). The Committee shall have full authority in its discretion to determine the amount of any such tax bonus. Notwithstanding the foregoing, tax bonuses shall not be granted with respect to Awards that are Stock Appreciation Rights or Options.
|
|
9.3 Transferability of Awards.
(a) No Award under the Plan may be sold, pledged, assigned or transferred in any manner other than by will or the laws of descent and distribution, unless and until such Award has been exercised, or the Shares underlying such Award have been issued, and all restrictions applicable to such Shares have lapsed. No Award or interest or right therein shall be liable for or otherwise subject to the debts, contracts or engagements of the Participant or the Participant’s successors in interest or shall be subject to disposition by transfer, alienation, anticipation, pledge, hypothecation, encumbrance, assignment or any other means whether such disposition be voluntary or involuntary or by operation of law by judgment, levy, attachment, garnishment or any other legal or equitable proceedings (including bankruptcy) unless and until such Award has been exercised, or the Shares underlying such Award have been issued, and all restrictions applicable to such Shares have lapsed, and any attempted disposition of an Award prior to the satisfaction of these conditions shall be null and void and of no effect.
(b) During the lifetime of the Participant, only the Participant may exercise any exercisable portion of an Award granted to him under the Plan. Notwithstanding the foregoing, a Non-Qualified Stock Option granted under the Plan may be transferred, in whole or in part, during a Participant’s lifetime, upon the approval of the Administrator, to a Participant’s “family members” (as such term is defined in Rule 701(c)(3) of the Securities Act and General Instructions A(1)(a)(5) to Form S-8) through a gift or domestic relations order. The transferred portion of a Non-Qualified Stock Option may only be exercised by the person or entity who acquires a proprietary interest in such Option pursuant to the transfer. The terms applicable to the transferred portion shall be the same as those in effect for the Option immediately prior to such transfer and shall be set forth in such documents issued to the transferee as the Administrator may deem appropriate. After the death of the Participant, any exercisable portion of an Award may, prior to the time when such portion becomes unexercisable under the Plan or the applicable Program or Award Agreement, be exercised by the Participant’s personal representative or by any person empowered to do so under the deceased Participant’s will or under the then-applicable laws of descent and distribution.
(c) Notwithstanding Section 9.3(a) hereof, a Participant may, in the manner determined by the Administrator, designate a beneficiary to exercise the rights of the Participant and to receive any distribution with respect to any Award upon the Participant’s death. A beneficiary, legal guardian, legal representative, or other person claiming any rights pursuant to the Plan is subject to all terms and conditions of the Plan and any Program or Award Agreement applicable to the Participant, and to any additional restrictions deemed necessary or appropriate by the Administrator. If the Participant is married or a domestic partner in a domestic partnership qualified under Applicable Law and resides in a “community property” state, a designation of a person other than the Participant’s spouse or domestic partner, as applicable, as his or her beneficiary with respect to more than fifty percent (50%) of the Participant’s interest in the Award shall not be effective without the prior written or electronic consent of the Participant’s spouse or domestic partner. If no beneficiary has been designated or survives the Participant, payment shall be made to the person entitled thereto pursuant to the Participant’s will or the laws of descent and distribution. Subject to the foregoing, a beneficiary designation may be changed or revoked by a Participant at any time provided the change or revocation is delivered to the Administrator in writing prior to the Participant’s death.
9.4 Conditions to Issuance of Shares.
(a) The Administrator shall determine the methods by which Shares shall be delivered or deemed to be delivered to Participants. Notwithstanding anything herein to the contrary, neither the Company nor its Subsidiaries shall be required to issue or deliver any certificates or make any book entries evidencing Shares pursuant to the exercise of any Award, unless and until the Administrator has determined, with advice of counsel, that the issuance of such Shares is in compliance with Applicable Law, and the Shares are covered by an effective registration statement or applicable exemption from registration. In addition to the terms and conditions provided herein, the Administrator may require that a Participant make such reasonable covenants, agreements, and representations as the Administrator, in its discretion, deems advisable in order to comply with any such Applicable Law.
|
|
(b) All Share certificates delivered pursuant to the Plan and all Shares issued pursuant to book entry procedures are subject to any stop-transfer orders and other restrictions as the Administrator deems necessary or advisable to comply with Applicable Law. The Administrator may place legends on any Share certificate or book entry to reference restrictions applicable to the Shares.
(c) The Administrator shall have the right to require any Participant to comply with any timing or other restrictions with respect to the settlement, distribution or exercise of any Award, including a window-period limitation, as may be imposed in the sole discretion of the Administrator.
(d) No fractional Shares shall be issued and the Administrator shall determine, in its sole discretion, whether cash shall be given in lieu of fractional Shares or whether such fractional Shares shall be eliminated by rounding down.
(e) The Company, in its sole discretion, may (i) retain physical possession of any stock certificate evidencing Shares until any restrictions thereon shall have lapsed and/or (ii) require that the stock certificates evidencing such Shares be held in custody by a designated escrow agent (which may but need not be the Company) until the restrictions thereon shall have lapsed, and that the Participant deliver a stock power, endorsed in blank, relating to such Shares.
(f) Notwithstanding any other provision of the Plan, unless otherwise determined by the Administrator or required by Applicable Law, the Company and/or its Subsidiaries may, in lieu of delivering to any Participant certificates evidencing Shares issued in connection with any Award, record the issuance of Shares in the books of the Company (or, as applicable, its transfer agent or stock plan administrator).
9.5 Market Stand-Off. In connection with any underwritten public offering by the Company of its equity securities pursuant to an effective registration statement filed under the Securities Act, including the Company’s initial public offering, the Participant will not directly or indirectly sell, make any short sale of, loan, hypothecate, pledge, offer, grant or sell any Option or other contract for the purchase of, any Option or other contract for the sale of, or otherwise dispose of or transfer, or agree to engage in any of the foregoing transactions with respect to, any Shares acquired under this Plan or any Award issued under this Plan without the prior written consent of the Company or its underwriters. Such restriction (the “Market Stand-Off”) will be in effect for such period of time following the date of the final prospectus for the offering as may be requested by the Company or such underwriters. In no event, however, will such period exceed a) 180 days or b) such other period as may be requested by the Company or the underwriters to accommodate regulatory restrictions on (i) the publication or other distribution of research reports and (ii) analyst recommendations and opinions, including, but not limited to, the restrictions contained in NASD Rule 2711(f)(4) or NYSE Rule 472(f)(4), or any successor provisions or amendments thereto). In the event of the declaration of a stock dividend, a spin-off, a stock split, an adjustment in conversion ratio, a recapitalization or a similar transaction affecting the Company’s outstanding securities without receipt of consideration, any new, substituted or additional securities which are by reason of such transaction distributed with respect to any Stock shall be subject to the Market Stand-Off. The Company may impose stop-transfer instructions with respect to any and all Stock or Options previously referred to in this Section until the end of the applicable stand-off period. The Company’s underwriters will be beneficiaries of the agreement set forth in this Section. A Participant will be subject to this Section only if the directors and officers of the Company are subject to similar arrangements.
|
|
9.6 Forfeiture and Claw-Back Provisions.
(a) Unless otherwise provided in an Award Agreement: (i) any proceeds, gains or other economic benefit actually or constructively received by the Participant upon any receipt or exercise of the Award, or upon the receipt or resale of any Shares underlying the Award, must be paid to the Company, and (ii) the Award shall terminate and any unexercised portion of the Award (whether or not vested) shall be forfeited, if (x) a Termination of Service occurs within six months following receipt or exercise of the Award, (y) the Participant at any time engages in any activity in competition with the Company, or which is inimical, contrary or harmful to the interests of the Company, as further defined by the Administrator or (z) the Participant incurs a Termination of Service for Cause; and
(b) All Awards (including any proceeds, gains or other economic benefit actually or constructively received by a Participant upon any receipt or exercise of any Award or upon the receipt or resale of any Shares underlying the Award) shall be subject to the applicable provisions of any claw-back policy implemented by the Company, whether implemented prior to or after the grant of such Award, including without limitation, any claw-back policy adopted to comply with the requirements of Applicable Law, to the extent set forth in such claw-back policy and/or in the applicable Award Agreement.
9.7 Leave of Absence. Unless the Administrator provides otherwise, vesting of Awards granted hereunder shall not be suspended during any unpaid leave of absence.
ARTICLE 10 ADMINISTRATION
10.1 Administrator. The Committee (or another committee or a subcommittee of the Board assuming the functions of the Committee under the Plan) shall administer the Plan (except as otherwise permitted herein) unless otherwise determined by the Board. Except as may otherwise be provided in the Organizational Documents, appointment of Committee members shall be effective upon acceptance of appointment, Committee members may resign at any time by delivering written or electronic notice to the Board, and vacancies in the Committee may only be filled by the Board. Notwithstanding the foregoing, (a) the full Board, acting by a majority of its members in office, shall conduct the general administration of the Plan with respect to Awards granted to Non-Employee Directors of the Company and (b) the Board or Committee may delegate its authority hereunder to the extent permitted by Section 10.6 hereof.
10.2 Duties and Powers of Administrator. It shall be the duty of the Administrator to conduct the general administration of the Plan in accordance with its provisions. The Administrator shall have the power to interpret the Plan and all Programs and Award Agreements, and to adopt such rules for the administration, interpretation and application of the Plan and any Program as are not inconsistent with the Plan, to interpret, amend or revoke any such rules and to amend any Program or Award Agreement provided that the rights or obligations of the holder of the Award that is the subject of any such Program or Award Agreement are not materially adversely affected by such amendment, unless the consent of the Participant is obtained or such amendment is otherwise permitted under Section 9.5, Section 11.2, Section 11.7, or Section 11.10 hereof. Without limiting the generality of the foregoing, the Administrator shall have the power to reduce the exercise price of an Option granted pursuant to the Plan (either directly or pursuant to the cancellation of the Option and the regrant of an Option) to an exercise price equal to the Fair Market Value of a Share at the time of the exercise price reduction or Option regrant. Any such interpretations and rules with respect to Incentive Stock Options shall be consistent with the provisions of Section 422 of the Code. In its sole discretion, the Board may at any time and from time to time exercise any and all rights and duties of the Committee in its capacity as the Administrator under the Plan.
|
|
10.3 Action by the Committee. Unless otherwise established by the Board or in the Organizational Documents or as required by Applicable Law, a majority of the Administrator shall constitute a quorum and the acts of a majority of the members present at any meeting at which a quorum is present, and acts approved in writing by a majority of the members of the Administrator in lieu of a meeting, shall be deemed the acts of the Administrator. Each member of the Administrator is entitled to, in good faith, rely or act upon any report or other information furnished to that member by any officer or other employee of the Company or any Subsidiary, the Company’s independent certified public accountants, or any executive compensation consultant or other professional retained by the Company to assist in the administration of the Plan.
10.4 Authority of Administrator. Subject to any specific designation in the Plan and Applicable Law, the Administrator has the exclusive power, authority and sole discretion to:
(a) Designate Eligible Individuals to receive Awards;
(b) Determine the type or types of Awards to be granted to each Eligible Individual;
(c) Determine the number of Awards to be granted and the number of Shares to which an Award will relate;
(d) Determine the terms and conditions of any Award granted pursuant to the Plan, including, but not limited to, the exercise price, grant price, or purchase price, any specific performance criteria, any restrictions or limitations on the Award, any schedule for vesting, lapse of forfeiture restrictions or restrictions on the exercisability of an Award, and accelerations or waivers thereof, and any provisions related to non-competition and recapture of gain on an Award, based in each case on such considerations as the Administrator in its sole discretion determines;
(e) Determine whether, to what extent, and under what circumstances an Award may be settled in, or the exercise price of an Award may be paid in cash, Shares, other Awards, or other property, or an Award may be canceled, forfeited, or surrendered;
(f) Prescribe the form of each Award Agreement, which need not be identical for each Participant;
(g) Decide all other matters that must be determined in connection with an Award;
(h) Establish, adopt, or revise any Programs, rules and regulations as it may deem necessary or advisable to administer the Plan;
(i) Interpret the terms of, and any matter arising pursuant to, the Plan, any Program or any Award Agreement; and
(j) Make all other decisions and determinations that may be required pursuant to the Plan or as the Administrator deems necessary or advisable to administer the Plan.
|
|
10.5 Decisions Binding. The Administrator’s interpretation of the Plan, any Awards granted pursuant to the Plan, any Program, any Award Agreement and all decisions and determinations by the Administrator with respect to the Plan are final, binding, and conclusive on all parties.
10.6 Delegation of Authority. To the extent permitted by Applicable Law, the Board or Committee may from time to time delegate to a committee of one or more members of the Board or one or more officers of the Company the authority to grant or amend Awards or to take other administrative actions pursuant to this Article 10; provided, however, that in no event shall an officer of the Company be delegated the authority to grant Awards to, or amend Awards held by, officers of the Company (or Directors) to whom authority to grant or amend Awards has been delegated hereunder; provided, further, that any delegation of administrative authority shall only be permitted to the extent it is permissible under the Organizational Documents, and other Applicable Law. Any delegation hereunder shall be subject to the restrictions and limits that the Board or Committee specifies at the time of such delegation or that are otherwise included in the applicable Organizational Documents, and the Board or Committee, as applicable, may at any time rescind the authority so delegated or appoint a new delegatee. At all times, the delegatee appointed under this Section 10.6 shall serve in such capacity at the pleasure of the Board or the Committee, as applicable, and the Board or the Committee may abolish any committee at any time and re-vest in itself any previously delegated authority.
ARTICLE 11 MISCELLANEOUS PROVISIONS
11.1 Amendment, Suspension or Termination of the Plan.
(a) Except as otherwise provided in this Section 11.1, the Plan may be wholly or partially amended or otherwise modified, suspended or terminated at any time or from time to time by the Board; provided that, except as provided in Section 9.5, Section 11.2, Section 11.7, or Section 11.10 hereof, no amendment, suspension or termination of the Plan shall, without the consent of the Participant, impair any rights or obligations under any Award theretofore granted or awarded, unless the Award itself otherwise expressly so provides.
(b) No Awards may be granted or awarded during any period of suspension or after termination of the Plan, and notwithstanding anything herein to the contrary, in no event may any Award be granted under the Plan after the tenth (10th) anniversary of the date on which the Plan was adopted by the Board (the “Expiration Date”). Any Awards that are outstanding on the Expiration Date shall remain in force according to the terms of the Plan, the applicable Program and the applicable Award Agreement.
(c) A Participant shall not have the right to a change in the exercise price of an Option, an increase in the exercise period for exercise of an Option, or a change to the number of securities received on exercise of an Option.
11.2 Changes in Common Stock or Assets of the Company, Acquisition or Liquidation of the Company and Other Corporate Events.
(a) In the event of any stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of Company assets to stockholders, or any other change affecting the shares of the Company’s stock or the share price of the Company’s stock other than an Equity Restructuring, the Administrator may make equitable adjustments, if any, to reflect such change with respect to (i) the aggregate number and kind of shares that may be issued under the Plan; (ii) the number and kind of Shares (or other securities or property) subject to outstanding Awards; (iii) the terms and conditions of any outstanding Awards (including, without limitation, any applicable performance targets or criteria with respect thereto); and/or (iv) the grant or exercise price per share for any outstanding Awards under the Plan.
|
|
(b) In the event of any transaction or event described in Section 11.2(a) hereof or any unusual or nonrecurring transactions or events affecting the Company, any Subsidiary, or the financial statements of the Company or any Subsidiary, or of changes in Applicable Law or Applicable Accounting Standards, the Administrator, in its sole discretion, and on such terms and conditions as it deems appropriate, either by the terms of the Award or by action taken prior to the occurrence of such transaction or event, is hereby authorized to take any one or more of the following actions whenever the Administrator determines that such action is appropriate in order to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the Plan or with respect to any Award under the Plan, to facilitate such transactions or events or to give effect to such changes in Applicable Law or Applicable Accounting Standards:
(i) To provide for the termination of any such Award in exchange for an amount of cash and/or other property, if any, equal to the amount that would have been attained upon the exercise of such Award or realization of the Participant’s rights (and, for the avoidance of doubt, if as of the date of the occurrence of the transaction or event described in this Section 11.2, the Administrator determines in good faith that no amount would have been attained upon the exercise of such Award or realization of the Participant’s rights, then such Award may be terminated by the Company without payment);
(ii) To provide that such Award be assumed by the successor or survivor corporation, or a parent or subsidiary thereof, or shall be substituted for by similar options, rights or awards covering the stock of the successor or survivor corporation, or a parent or subsidiary thereof, with appropriate adjustments as to the number and kind of shares and applicable exercise or purchase price;
(iii) To make adjustments in the number and type of securities subject to outstanding Awards and Awards which may be granted in the future and/or in the terms, conditions and criteria included in such Awards (including the grant or exercise price, as applicable);
(iv) To provide that such Award shall be exercisable or payable or fully vested with respect to all securities covered thereby, notwithstanding anything to the contrary in the Plan or an applicable Program or Award Agreement;
(v) To replace such Award with other rights or property selected by the Administrator in its sole discretion; and/or
(vi) To provide that the Award cannot vest, be exercised or become payable after such event.
(c) In connection with the occurrence of any Equity Restructuring, and notwithstanding anything to the contrary in Sections 11.2(a) and 11.2(b) hereof:
(i) The number and type of securities subject to each outstanding Award and the exercise price or grant price thereof, if applicable, shall be equitably adjusted; and/or
|
|
(ii) The Administrator shall make such equitable adjustments, if any, as the Administrator in its discretion may deem appropriate to reflect such Equity Restructuring with respect to the aggregate number and kind of shares that may be issued under the Plan.
The adjustments provided under this Section 11.2(c) shall be nondiscretionary and shall be final and binding on the affected Participant and the Company.
(d) Except as may otherwise be provided in any applicable Award Agreement or other written agreement entered into between the Company (or a Subsidiary) and a Participant, if a Change in Control occurs and a Participant’s outstanding Awards are not continued, converted, assumed, or replaced by the surviving or successor entity in such Change in Control, then, immediately prior to the Change in Control, such outstanding Awards, to the extent not continued, converted, assumed, or replaced, shall become fully vested and, as applicable, exercisable, and all forfeiture, repurchase and other restrictions on such Awards shall lapse. Upon, or in anticipation of, a Change in Control, the Administrator may cause any and all Awards outstanding hereunder to terminate at a specific time in the future, including but not limited to the date of such Change in Control, and shall give each Participant the right to exercise such Awards during a period of time as the Administrator, in its sole and absolute discretion, shall determine. For the avoidance of doubt, if the value of an Award that is terminated in connection with this Section 11.2(d) is zero or negative at the time of such Change in Control, such Award shall be terminated upon the Change in Control without payment of consideration therefor.
(e) The Administrator may, in its sole discretion, include such further provisions and limitations in any Award, agreement or certificate, as it may deem equitable and in the best interests of the Company that are not inconsistent with the provisions of the Plan.
(f) Unless otherwise determined by the Administrator, no adjustment or action described in this Section 11.2 or in any other provision of the Plan shall be authorized to the extent it would (i) cause the Plan to violate Section 422(b)(1) of the Code or (ii) cause an Award to fail to be exempt from or fail to comply with Section 409A of the Code.
(g) The existence of the Plan, any Program, any Award Agreement and/or any Award granted hereunder shall not affect or restrict in any way the right or power of the Company or the stockholders of the Company or any Subsidiary to make or authorize any adjustment, recapitalization, reorganization or other change in the Company’s or such Subsidiary’s capital structure or its business, any merger or consolidation of the Company or any Subsidiary, any issue of stock or of options, warrants or rights to purchase stock or of bonds, debentures, preferred or prior preference stocks whose rights are superior to or affect the Common Stock, the securities of any Subsidiary, or the rights thereof or which are convertible into or exchangeable for Common Stock or securities of any Subsidiary, or any sale or transfer of all or any part of its assets or business, or any other corporate act or proceeding, whether of a similar character or otherwise.
(h) In the event of any pending stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of Company assets to stockholders, or any other change affecting the Shares or the share price of the Common Stock including any Equity Restructuring, for reasons of administrative convenience, the Company in its sole discretion may refuse to permit the exercise of any Award during a period of thirty (30) days prior to the consummation of any such transaction.
11.3 Approval of Plan by Stockholders. The Plan shall be submitted for the approval of the Company’s stockholders within twelve (12) months after the date of the Board’s initial adoption of the Plan. Awards may be granted or awarded prior to such stockholder approval; and, provided that if such approval has not been obtained at the end of said 12-month period, no Options previously granted or awarded under the Plan shall qualify as Incentive Stock Options.
|
|
11.4 No Stockholders Rights. Except as otherwise provided herein or in an applicable Program or Award Agreement, a Participant shall have none of the rights of a stockholder with respect to Shares covered by any Award until the Participant becomes the record owner of such Shares, including the right to participate in any new issues of stock of the Company.
11.5 Paperless Administration. In the event that the Company establishes, for itself or using the services of a third party, an automated system for the documentation, granting or exercise of Awards, such as a system using an internet website or interactive voice response, then the paperless documentation, granting or exercise of Awards by a Participant may be permitted through the use of such an automated system.
11.6 Effect of Plan upon Other Compensation Plans. The adoption of the Plan shall not affect any other compensation or incentive plans in effect for the Company or any Subsidiary. Nothing in the Plan shall be construed to limit the right of the Company or any Subsidiary: (a) to establish any other forms of incentives or compensation for Employees, Directors or Consultants of the Company or any Subsidiary or (b) to grant or assume options or other rights or awards otherwise than under the Plan in connection with any proper corporate purpose including without limitation, the grant or assumption of options in connection with the acquisition by purchase, lease, merger, consolidation or otherwise, of the business, stock or assets of any corporation, partnership, limited liability company, firm or association.
11.7 Compliance with Laws. The Plan, the granting and vesting of Awards under the Plan, the issuance and delivery of Shares and the payment of money under the Plan or under Awards granted or awarded hereunder are subject to compliance with all Applicable Law and to such approvals by any listing, regulatory or governmental authority as may, in the opinion of counsel for the Company, be necessary or advisable in connection therewith. Any securities delivered under the Plan shall be subject to such restrictions, and the person acquiring such securities shall, if requested by the Company, provide such assurances and representations to the Company as the Company may deem necessary or desirable to assure compliance with all Applicable Law. The Administrator, in its sole discretion, may take whatever actions it deems necessary or appropriate to effect compliance with Applicable Law, including, without limitation, placing legends on share certificates and issuing stop-transfer notices to agents and registrars. Notwithstanding anything to the contrary herein, the Administrator may not take any actions hereunder, and no Awards shall be granted, that would violate Applicable Law. To the extent permitted by Applicable Law, the Plan and Awards granted or awarded hereunder shall be deemed amended to the extent necessary to conform to such Applicable Law.
11.8 Titles and Headings, References to Sections of the Code or Exchange Act. The titles and headings of the sections in the Plan are for convenience of reference only and, in the event of any conflict, the text of the Plan, rather than such titles or headings, shall control. References to sections of the Code or the Exchange Act shall include any amendment or successor thereto.
11.9 Governing Law. The Plan and any Programs or Award Agreements hereunder shall be administered, interpreted and enforced under the internal laws of the State of Delaware without regard to conflicts of laws thereof.
|
|
11.10 Section 409A. To the extent that the Administrator determines that any Award granted under the Plan is subject to Section 409A of the Code, the Plan, any applicable Program and the Award Agreement covering such Award shall be interpreted in accordance with Section 409A of the Code. Notwithstanding any provision of the Plan to the contrary, in the event that, following the Effective Date, the Administrator determines that any Award may be subject to Section 409A of the Code, the Administrator may adopt such amendments to the Plan, any applicable Program and the Award Agreement or adopt other policies and procedures (including amendments, policies and procedures with retroactive effect), or take any other actions, that the Administrator determines are necessary or appropriate to avoid the imposition of taxes on the Award under Section 409A of the Code, either through compliance with the requirements of Section 409A of the Code or with an available exemption therefrom. The Company makes no representations or warranties as to the tax treatment of any Award under Section 409A of the Code or otherwise. The Company shall have no obligation under this Section 11.10 or otherwise to take any action (whether or not described herein) to avoid the imposition of taxes, penalties or interest under Section 409A of the Code with respect to any Award and shall have no liability to any Participant or any other person if any Award, compensation or other benefits under the Plan are determined to constitute non-compliant, “nonqualified deferred compensation” subject to the imposition of taxes, penalties and/or interest under Section 409A of the Code.
11.11 No Rights to Awards. No Eligible Individual or other person shall have any claim to be granted any Award pursuant to the Plan, and neither the Company nor the Administrator is obligated to treat Eligible Individuals, Participants or any other persons uniformly.
11.12 Unfunded Status of Awards. The Plan is intended to be an “unfunded” plan for incentive compensation. With respect to any payments not yet made to a Participant pursuant to an Award, nothing contained in the Plan or any Program or Award Agreement shall give the Participant any rights that are greater than those of a general creditor of the Company or any Subsidiary.
11.13 Indemnification. To the extent allowable pursuant to Applicable Law and the Organizational Documents, each member of the Board and any officer or other employee to whom authority to administer any component of the Plan is delegated shall be indemnified and held harmless by the Company from any loss, cost, liability, or expense that may be imposed upon or reasonably incurred by such member in connection with or resulting from any claim, action, suit, or proceeding to which he or she may be a party or in which he or she may be involved by reason of any action or failure to act pursuant to the Plan and against and from any and all amounts paid by him or her in satisfaction of judgment in such action, suit, or proceeding against him or her; provided, however, that he or she gives the Company an opportunity, at its own expense, to handle and defend the same before he or she undertakes to handle and defend it on his or her own behalf. The foregoing right of indemnification shall not be exclusive of any other rights of indemnification to which such persons may be entitled pursuant to the Organizational Documents, as a matter of law, or otherwise, or any power that the Company may have to indemnify them or hold them harmless.
11.14 Relationship to other Benefits. No payment pursuant to the Plan shall be taken into account in determining any benefits under any pension, retirement, savings, profit sharing, group insurance, welfare or other benefit plan of the Company or any Subsidiary except to the extent otherwise expressly provided in writing in such other plan or an agreement thereunder.
11.15 Expenses. The expenses of administering the Plan shall be borne by the Company and its Subsidiaries.
|
|
Exhibit 10.12
EXECUTIVE EMPLOYMENT AGREEMENT
This Executive Employment Agreement (this “Agreement”) is made as of November 4, 2021 (the “Effective Date”), by and between BioRestorative Therapies, Inc., a Delaware corporation (the “Company”), and Robert E. Kristal (the “Executive”) (Company and Executive are collectively the “Parties”). Certain capitalized terms used in this Agreement are defined in Section 13.
RECITALS
WHEREAS, the Company and the Executive desire to enter into an employment agreement which will set forth the terms and conditions upon which the Executive shall be employed by the Company and upon which the Company shall compensate the Executive.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto agree as follows:
1. Employment. The Company will employ the Executive, and the Executive accepts employment with the Company, upon the terms and conditions set forth in this Agreement, for the period beginning on the Effective Date and ending as provided in Sections 2 and/or 5 below (the “Employment Period”).
2. Initial Employment Term. This Agreement shall commence on the Effective Date and will continue for a period of one (1) year or until either Party terminates the Agreement in accordance with Section 5 hereof (the “Initial Employment Term”). Notwithstanding the foregoing, nothing herein shall limit or restrict the Parties extending the Initial Employment Term, provided such extension is memorialized in writing by the Parties.
3. Position and Duties. During the Employment Period, the Executive will serve in the position set forth on Schedule A to this Agreement and will render such managerial, analytical, administrative, financial and other executive services to, and shall have such responsibilities on behalf of, the Company and its Subsidiaries, as are from time to time necessary in connection with the management and affairs of the Company and its Subsidiaries and are consistent with his position, in each case subject to the authority of the CEO and the Board of Directors of the Company (the “Board”) to define and limit such executive services. The Executive’s primary responsibilities shall include, without limitation, those set forth on Schedule A attached hereto. The Executive will devote substantially all of his business time and attention to the business and affairs of the Company and its Subsidiaries, provided that the Executive will be permitted to (i) serve as a member of the board of directors or advisory board of charitable organizations and/or, subject to Sections 6-8, perform services for other business organizations with which the Executive has or may become associated, (ii) engage in charitable activities and community affairs, and (iii) manage his personal investments and affairs, except that the Executive will limit the time devoted to the activities described in clauses (i), (ii), and (iii) so as not to materially interfere, individually or in the aggregate, with the performance of his duties and responsibilities hereunder. The Executive will perform his duties and responsibilities to the best of his abilities in a diligent, trustworthy, businesslike and efficient manner, and Executive acknowledges and agrees that he owes a fiduciary duty of loyalty to the Company. While subject to change in the sole discretion of the Company, the Executive will report to the person(s) set forth on Schedule A. During the Employment Period, the Executive’s primary work location shall be 4719 Cole Avenue, Dallas, Texas 75205.
4. Salary and Benefits.
(a) Salary. During the Employment Period, the Company will pay the Executive a salary at not less than the rate set forth on Schedule A to this Agreement (as in effect from time to time, the “Salary”) as compensation for services. The Salary will be payable in regular installments in accordance with the general payroll practices of the Company and its Subsidiaries and subject to applicable withholding requirements. .
(b) Discretionary Bonus. In the sole discretion of the Board of Directors of the Company (the “Board”) (including the approval of a majority of the independent members of the Board), and subject to many factors, including but not limited to Executive’s performance and the financial performance of the Company, Executive is eligible for an annual bonus of up to 30% of Executive’s Salary.
(c) Benefits. During the Employment Period, the Company will provide the Executive with benefits under such plans as the Board may establish or maintain from time to time for similarly-situated employees. The Executive will be entitled to the number of weeks of paid vacation each year set forth on Schedule A attached hereto, prorated for any partial calendar year. To the extent that the Executive does not use all the vacation time in any year, calculated on a calendar year basis, up to two (2) weeks (or such lesser prorated amount) of the unused vacation may be carried over to the next year and any remaining unused amounts will be lost and forfeited by Executive. In no circumstance shall Executive earn or possess more than six (6) weeks of vacation at any time. Vacation will cease to be earned once Executive has 6 weeks of earned but unused vacation and will not earn additional vacation until such time as vacation is taken by Executive.
(d) Reimbursement of Expenses. During the Employment Period, the Company will reimburse the Executive for all reasonable out-of-pocket expenses incurred by Executive in the course of performing Executive’s duties that are consistent with the Company’s policies in effect from time to time with respect to travel, entertainment and other business expenses, subject to the Company’s requirements with respect to reporting and documentation of such expenses.
(e) Equity. Upon the Effective Date, the Company entered into a Stock Option Award Agreement with Executive pursuant to which Executive was granted a ten year option to purchase 10,490 shares of common stock of the Company at an exercise price of $13.50 per share.
|
|
5. Termination.
(a) The Employment Period will continue until the earliest of: (i) the Executive’s resignation for any or no reason; (ii) the death or Disability of the Executive; (iii) the giving of notice of termination by the Company: (A) for Cause or (B) for any other reason or for no reason (a termination described in this clause (iii)(B) being a termination by the Company “Without Cause”); (iv) the Executive’s resignation for Good Reason; or (v) the end of the Initial Employment Term in the event it is not extended in writing by the Parties.
(b) If the Company terminates the Employment Period Without Cause or the Executive resigns for Good Reason, the Executive will be entitled to receive each of the following: all accrued and unpaid Salary and earned, but unused vacation time for the then-current annual period (with the right to vacation time being pro-rated for such period through the Termination Date) and all unreimbursed business expenses incurred through the Termination Date and payable pursuant to Section 4(d), which accrued and unpaid salary, unused vacation and unreimbursed expenses shall be payable in a lump sum (less applicable taxes and withholdings) on the later of the next regularly-scheduled payroll date or five (5) days after the Termination Date (the amounts described in the immediately preceding sentence are referred to herein as the “Accrued Obligations”). The Executive shall also be entitled to any COBRA benefits to which the Executive is entitled by law. COBRA benefits shall be at the Executive’s sole expense. In addition, if the Company terminates the Executive’s employment without Cause or the Executive resigns for Good Reason, subject to paragraph (d) hereof, (i) the Executive shall be entitled to an aggregate payment equal to the lesser of the Salary Executive would receive during the remainder of the Initial Employment Term or twenty-five percent (25%) of his then current Salary (the “Cash Severance Amount”).
(c) If the Employment Period terminates by reason of (i) the Company’s termination with Cause, (ii) the Executive’s resignation without Good Reason, or (iii) the Initial Employment Term expires without written extension by the Parties, then the Executive (or his estate in the case of his death) will be entitled to receive the Accrued Obligations, as well as any COBRA benefits to which the Executive is entitled by law (at the Executive’s sole expense).
(d) The Cash Severance Amount, if applicable, will be paid in equal biweekly installments over a period of no more than three (3) months following the Termination Date in accordance with the general payroll practices of the Company and subject to all applicable withholding requirements; provided, however, that the payment of the Cash Severance Amount shall be conditioned upon the Executive (i) executing and delivering to the Company a general release of all past and present claims against the Company and its Subsidiaries substantially in the form attached hereto as Exhibit A (the “Form of Release”), within twenty-one (21) days of the date the Company delivers such general release (the “Release”) to the Executive, and (ii) not exercising the Executive’s revocation right during the period for revocation described in the Form of Release and/or Release; provided, further, that, in the event of the Executive’s breach of this Agreement, then the Company’s obligation to pay the Cash Severance Amount shall terminate and be of no further force or effect and the Executive shall be obligated to reimburse the Company for all Cash Severance Amount previously made. To the extent that Cash Severance Amount payments are payable, they shall be made or commence on the later of the first payroll date following the effective date in the Release or the fortieth (40th) day following the Termination Date.
|
|
(e) Upon the Termination Date, the Executive will be obligated to and be deemed to have resigned from each position (if any) that he then holds as an officer or director of the Company or any Subsidiary, and the Executive will take any action that the Company or any Subsidiary may reasonably request in order to confirm or evidence such resignation.
(f) Neither the termination or expiration of this Agreement nor the termination of the Executive’s employment with the Company, whether by the Company or the Executive, whether for Cause or Without Cause, whether for Good Reason or without Good Reason, and whether voluntary or involuntary, shall affect the continuing operation and effect of Sections 6-8 hereof, which shall continue in full force and effect according to their terms. In addition, neither the termination or expiration of this Agreement nor the termination of the Executive’s employment with the Company, whether by the Company or the Executive, whether for Cause or Without Cause, whether for Good Reason or without Good Reason, and whether voluntary or involuntary, will result in a termination or waiver of any rights and remedies that the Company may have under this Agreement and applicable law.
(g) In the event of the termination of this Agreement or the Executive’s employment, whether by the Company or the Executive, whether for Cause or Without Cause, whether for Good Reason or without Good Reason, and whether voluntary or involuntary, except as expressly provided for herein, the Executive shall not be entitled to any further compensation or benefits.
6. Confidentiality/Non-Disclosure. Executive agrees that during the Employment Period and thereafter, other than in connection with his duties on behalf of Company, Executive will not disclose Confidential Information, proprietary information, or trade secrets, related to any business of Company including, without limitation, and whether or not such information is specifically designated as confidential or proprietary: (a) business plans; (b) business processes; (c) practices; (d) marketing strategies; (e) documents; (f) information concerning existing and prospective markets, suppliers and customers; (g) financial information; (h) information concerning the development of new products and services; (i) operations; (j) techniques; (k) agreements; (l) contracts; (m) terms of agreements; (n) transactions; (o) potential transactions; (p) know-how; (q) technical and non-technical data related to software programs, design, specifications, compilations, inventions, improvements, patent applications, studies, research, methods, devices, prototypes, processes, procedures and techniques (“Confidential Information”). Notwithstanding anything to the contrary contained herein, Confidential Information, proprietary information, or trade secrets shall not be deemed to include any of the foregoing: (i) information that is within the public domain or known generally in the trade as of the Effective Date of this Agreement, except to the extent that such information entered the public domain through an act or omission of Executive (other than lawfully on behalf of Company); (ii) information that enters the public domain after the Effective Date of this Agreement by no act or omission of Executive (other than by Executive lawfully on behalf of Company); (iii) disclosures required to enforce any rights hereunder or any other agreement with Company; or (iv) disclosures required by any subpoena or other legal process; provided, Executive will, to the extent permitted by law, promptly notify Company of such fact so that Company may seek an appropriate remedy to prevent such disclosure.
|
|
Under the federal Defend Trade Secrets Act of 2016, Executive shall not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that: (a) is made (i) in confidence to a federal, state, or local government official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose of reporting or investigating a suspected violation of law; or (b) is made to Executive’s attorney in relation to a lawsuit for retaliation against Executive for reporting a suspected violation of law; or (c) is made in a complaint or other document filed in a lawsuit or other proceeding, if such filing is made under seal.
7. Restrictive Covenants.
(a) The services of the Executive are unique and extraordinary and essential to the business of the Company, especially since the Executive shall have access to the Company’s customer lists, trade secrets and other privileged and confidential information essential to the Company’s business. Therefore, the Executive agrees that, as a material inducement to, and a condition precedent to the Company’s payment obligations hereunder and its other covenants herein, if the term of the Executive’s employment hereunder shall expire or the Executive’s employment shall at any time terminate for any reason whatsoever, for Cause or Without Cause, for Good Reason or without Good Reason, the Executive will not at any time within one (1) year after such expiration or termination (the “Restrictive Covenant Period”), without the prior written approval of the Company, directly or indirectly, whether individually or as a principal, officer, stockholder, equity participant, employee, partner, joint venturer, member, manager, director or agent of, or lender, consultant or independent contractor to, any Person, or in any other capacity, other than on behalf of or for the benefit of the Company:
(i) anywhere in the United States of America, directly or indirectly, engage or participate in a business which, as of such expiration or termination date, is similar to or competitive with the business of the Company as of the Termination Date, as described in the Company’s Securities and Exchange Commission (the “SEC”) filings (or, in the event that the Company is not subject to the SEC disclosure requirements, then as described on the Company’s website (the “Business”)), wherein Executive shall be performing the same or similar duties as he performed for the Company, or if Executive is likely to use or disclose confidential or proprietary information of the Company. For the sake of clarity, the Company’s Business is focused on stem-cell based therapies, including autologous hypoxic disc treatments and the use of brown adipose stem cells for treating metabolic disorders. Notwithstanding the foregoing, Executive may acquire, as a passive investor, up to five percent (5%) of the outstanding voting stock of any entity whose securities are listed on a stock exchange or NASDAQ;
(ii) cause, seek to persuade or solicit any director, officer, employee, customer, account, agent or supplier of, or consultant or independent contractor to, the Company or others with whom the Company has had a business relationship (collectively, “Business Associates”) to discontinue or materially modify the status, employment or other relationship of such Business Associate with the Company, or to become employed in any activity similar to or competitive with the Business of the Company;
(iii) cause, seek to persuade or solicit, any prospective customer, account, supplier or other Business Associate of the Company (which at the date of cessation of the Executive’s employment with the Company was then, to the knowledge of the Executive, actively being solicited by the Company) to determine not to enter into a business relationship with the Company or to materially modify its contemplated business relationship;
|
|
(iv) hire, retain, engage or associate in a business relationship with, directly or indirectly, any employee of the Company; or
(v) solicit from or cause or authorize to be solicited from, for or on behalf of the Executive or any third party, any business which is similar to or competitive with the Business of the Company, or enter into a similar or competitive business relationship with, (a) others who are, or were within one (l) year prior to the cessation of the Executive’s employment with the Company, customers, accounts or other Business Associates of the Company, or (b) any prospective customer, account or other Business Associate of the Company which at the date of such cessation was then, to the Executive’s knowledge, actively being solicited by the Company.
The foregoing restrictions set forth in this Section 7 shall apply likewise during the Employment Period and all references to “Business” shall be deemed to refer to the then Business of the Company during the Employment Period.
(b) For purposes of this Section 7, the term “Company” shall mean and include the Company and any and all Subsidiaries and Affiliates of the Company in existence from time to time.
(c) Executive acknowledges the benefits provided or made available to him pursuant to the provisions of this Agreement, including, without limitation, the agreement on the part of the Company to employ the Executive during the Employment Period (subject to the terms and conditions hereof) constitute sufficient consideration for the restrictions contained in this Section 7 . The Executive also acknowledges and agrees that the covenants set forth in this Section 7 are reasonable and necessary in order to protect and maintain the proprietary and other legitimate business interests of the Company and that the enforcement thereof would not prevent the Executive from earning a livelihood.
(d) Company and Executive agree (a) that the provisions of this Section 7 do not impose an undue hardship on Executive and are not injurious to the public, (b) that these provisions are necessary to protect the Business of Company, (c) that the nature of Executive’s responsibilities with Company under this Agreement provide and/or will provide Executive with access to Confidential Information, proprietary information, or trade secrets that are valuable and confidential to Company, (d) that Company would not have entered into this Agreement with Executive if Executive did not agree to the provisions of this Section 7, (e) that Company would not have provided the Cash Severance Amount or any other consideration outlined in this Agreement if Executive did not agree to the provisions of this Section 7, (f) that the provisions of this Section 7 are reasonable in terms of length of time and scope, and (g) that adequate consideration supports the provisions of this Section 7. In the event that a court determines that any provision of this Section 7 are unreasonably broad or extensive, Executive agrees that such court should narrow such provision to the extent necessary to make it reasonable and enforce the provisions as narrowed. If such partial enforcement is not possible, the provision shall be deemed severed from this Agreement. Company reserves all rights to seek any and all remedies and damages permitted under law including, but not limited to, injunctive relief, equitable relief, and compensatory damages for any breach of Executive’s obligations under this Section 7, without the obligation to post or provide any bond. In the event Executive breaches or threatens to breach any of the provisions of this Section 7, Company shall immediately cease payment of any Cash Severance Amount, or other amounts due under this Agreement, as the case may be.
|
|
8. Developments and Inventions. All modifications, alterations, enhancements, betterments, ideas or discoveries which are the result, directly or indirectly, of Executive’s employment and/or affiliation with the Company and/or Executive’s access to Confidential Information (collectively “Developments”) shall be the sole and exclusive property of Company and are considered a “work made for hire” for the purposes of Company’s rights under copyright and other laws. To the extent that any Developments may not be considered “work made for hire,” Executive shall assign to Company such Developments and all rights therein, to the extent permitted by law. Executive shall take all necessary steps, without compensation, whether during or after his Employment Period: (a) to assign all right, title, and interest in any Developments to Company, and (b) to assist Company in registering, prosecuting, perfecting, protecting, maintaining and enforcing any and all patent, copyright, trade secret or other right or interest in any Developments for any and all countries. If any Developments assigned hereunder is based upon, or is incorporated into or is an improvement or derivative of, or cannot reasonably be made, used, reproduced and/or distributed without using or violating technology or rights owned or licensed by Company and not assigned hereunder, Executive shall grant Company a perpetual, worldwide, royalty-free, non-exclusive and sub-licensable right and license to exploit and exercise all such technology and rights in support of Company’s exercise or exploitation of any such assigned Development(s) (including any modifications, improvements and derivatives thereof). Specifically excluded from this Section is anything developed entirely on Executive’s own time and without the use of any equipment, supplies, facilities, other resources and/or Confidential Information of Company, provided that the Development does not relate to the Business of Company.
9. Omitted.
10. Deductions and Withholding. The Executive agrees that the Company shall withhold from any and all payments required to be made to the Executive pursuant to this Agreement all federal, state, local and/or other taxes that are required to be withheld in accordance with applicable statutes and/or regulations from time to time in effect.
11. Code Section 409A.
(a) The intent of the Parties is that payments and benefits under this Agreement comply with Section 409A of the Internal Revenue Code of 1986, as amended (together with the regulations and guidance promulgated thereunder, “Code Section 409A”), and, accordingly, to the maximum extent permitted, this Agreement shall be interpreted to be in compliance therewith. To the extent that any provision hereof is modified in order to comply with Code Section 409A, such modification shall be made in good faith and shall, to the maximum extent reasonably possible, maintain the original intent and economic benefit to the parties hereto of the applicable provision without violating the provisions of Code Section 409A. In no event whatsoever shall the Company be liable for any additional tax, interest or penalty that may be imposed on the Executive by Code Section 409A.
|
|
(b) A termination of employment shall not be deemed to have occurred for purposes of any provision of this Agreement providing for the payment of any amounts or benefits constituting deferred compensation under Code Section 409A upon or following a termination of employment unless such termination of employment is also a “separation from service” within the meaning of Code Section 409A and, for purposes of any such provision of this Agreement, references to a termination of employment or like terms shall mean “separation from service.” If the Executive is deemed on the date of termination to be a “specified employee” within the meaning of that term under Code Section 409A(a)(2)(B), then with regard to any payment or the provision of any benefit that is considered deferred compensation under Code Section 409A payable on account of a “separation from service,” such payment or benefit shall be made or provided at the date which is the earlier of (i) the expiration of the six (6) month period measured from the date of such “separation from service” of the Executive, and (ii) the date of the Executive’s death (the “Delay Period”). Upon the expiration of the Delay Period, all payments and benefits delayed pursuant to this Section 11(b) (whether they would have otherwise been payable in a single sum or in installments in the absence of such delay) shall be paid or reimbursed to the Executive in a lump sum, and any remaining payments and benefits due under this Agreement shall be paid or provided in accordance with the normal payment dates specified herein.
(c) All expenses or other reimbursements under this Agreement shall be made as soon as practicable and in any event on or prior to the last day of the taxable year following the taxable year in which such expenses were incurred by the Executive (provided that if any such reimbursements constitute taxable income to the Executive, such reimbursements shall be paid no later than March 15th of the calendar year following the calendar year in which the expenses to be reimbursed were incurred), and no such reimbursement or expenses eligible for reimbursement in any taxable year shall in any way affect the expenses eligible for reimbursement in any other taxable year.
(d) For purposes of Code Section 409A, the Executive’s right to receive any installment payments pursuant to this Agreement shall be treated as a right to receive a series of separate and distinct payments. Whenever a payment under this Agreement specifies a payment period with reference to a number of days (e.g., “payment shall be made within sixty (60) days”), the actual date of payment within the specified period shall be within the sole discretion of the Company.
(e) In no event shall any payment under this Agreement that constitutes “deferred compensation” for purposes of Code Section 409A be offset by any other payment pursuant to this Agreement or otherwise.
12. Representations and Warranties. The Executive represents and warrants to the Company and its Subsidiaries that: (a) the Executive is not a party to or bound by any employment, noncompete, nonsolicitation, or similar agreement with any other Person; (b) the Executive is not a party to or bound by any nondisclosure, confidentiality or similar agreement with any other Person that would affect the Executive’s ability to perform his responsibilities on behalf of the Company; and (c) this Agreement constitutes a valid and legally binding obligation of the Executive, enforceable against him in accordance with its terms. The Company represents that this Agreement constitutes a valid and legally binding obligation of the Company, enforceable against it in accordance with its terms. All representations and warranties contained herein will survive the execution and delivery of this Agreement.
|
|
13. Certain Definitions. When used in this Agreement, the following terms will have the following meanings:
“Affiliate” means, with respect to any Person, any other Person that, directly or indirectly, through one or more of its intermediaries, controls, is controlled by or is under common control with such Person.
“Cause” means any one or more of the following: (i) in the reasonable judgment of the Board, (A) the Executive acts (including a failure to act) in a manner that constitutes gross misconduct or gross negligence or that is otherwise materially injurious to the Company or its Subsidiaries; (ii) the Executive breaches any material term of this Agreement, which breach remains uncured to the reasonable satisfaction of the Board following ten (10) days’ written notice from the Company of such breach; (iii) the Executive has committed an act of fraud or misappropriation, or other act of dishonesty or illegal business practices relating to the Company or any of its Subsidiaries, customers or suppliers; (iv) the Executive’s commission of any act which, if the Executive were convicted, would constitute a felony, a crime of moral turpitude or a crime involving the illegal use of drugs, or the Executive’s entry of a plea of guilty or no contest thereto; (v) the Executive’s willful, material and continued failure or willful refusal to perform specific, lawful directives of the Board; (vi) any alcohol or other substance abuse on the part of the Executive that adversely impacts his ability to perform the material functions of his position and is materially injurious to the Company; (vii) any excessive absence of the Executive from his employment during normal working hours for reasons other than vacation or Disability that is materially injurious to the Company; (viii) the Executive’s breach of any other material obligation under this Agreement; or (ix) any material misrepresentation on the Executive’s part herein set forth. Notwithstanding the foregoing, if any act or omission described in the above definition of “Cause” is susceptible to cure (as determined in the reasonable discretion of the Board), the Executive shall have ten (10) days after notice from the Board to cure such violation to the reasonable satisfaction of the Board. Any notice to the Executive of termination for “Cause” shall be in writing and shall specify in reasonable detail the Executive’s acts or omissions that the Company considers to be “Cause”, provided however, that the Company will provide Executive a three (3) day period to review the notice and, in the sole discretion of the Company, may allow the Executive to suggest any reasonable modifications to the notice. No act or failure to act by the Executive shall be considered “willful” unless it is done or omitted to be done by the Executive in bad faith and without reasonable belief that he was acting in the best interests of the Company.
“CEO” means the Chief Executive Officer of the Company.
|
|
“Change in Control” means any one or more of the following events, which shall be deemed to occur on the date of such event: (A) any person or group (other than the Company) (I) acquires more than fifty percent (50%) of the corporate authority to direct the operations of the Company (including, without limitation, a change in the sole corporate member of the Company to a previously unrelated entity, regardless of whether such change in membership alters the composition of the Board), or (II) acquires (or has acquired during the twelve (12) month period that ends on the date of the most recent acquisition by such person or group) ninety percent (90%) or more of the total gross fair market value of all assets of the Company, determined without consideration of any liabilities associated with such assets acquired; (B) the Board executes an amendment to (or enters into a contractual agreement to amend) the Certificate of Incorporation or Bylaws (or their equivalent) of the Company such that a person or group of persons not previously affiliated with or controlled by the Board has the power, directly or indirectly, to designate or elect more than fifty percent (50%) of the Board; or (C) any merger, reorganization, or other corporate event which results in all or substantially all of the operations and assets of the Company (i.e., seventy-five percent (75%) or more of the Company’s value immediately prior to the merger, reorganization, or other corporate event) being controlled by an entity or person that is not tax-exempt under Section 501(c) of the Code.
“COBRA” means the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended from time to time.
“Disability” has the meaning given to such term under the Company’s long-term disability insurance plan or, if no such plan exists, then “Disability” means that the Executive is unable, due to illness, accident or other physical or mental incapacity, to perform substantially all of his duties and responsibilities (provided that, in any such case, the Executive shall have satisfied such criteria for a period of at least [six (6)] consecutive months or for at least [one hundred twenty-five (125)] business days during any [nine (9)] month period).
“Good Reason” means, without the Executive’s written consent, (i) the assignment to the Executive of duties materially inconsistent with the duties of his title and position and as described on Schedule A hereto or materially diminishes the Executive’s authority, duties or responsibilities in any way or requires him to report to any person or entity other than the Board; (ii) a material reduction in the Executive’s Salary or other benefits, except as part of a Company-wide reduction in compensation and/or benefits for similar employees (provided that the Executive’s reduction is consistent, on a proportional basis, with the reductions imposed on all of the Company’s executive level officers); (iii) the relocation of the Executive to an office more than fifty (50) miles from the Executive’s primary work location as set forth in Section 3; (iv) any other material breach by the Company of the provisions hereof; or (v) the Company experiences a “Change in Control” (as defined in this Section 13) event; provided, however, that none of the foregoing events shall constitute Good Reason unless (1) the Executive gives written notice of termination to the Company specifying the condition or event constituting Good Reason within thirty (30) days of the existence thereof; (2) the Company fails to cure the condition or event constituting Good Reason within thirty (30) days following receipt of the Executive’s notice; and (3) the Executive actually terminates his employment within thirty (30) days of the end of the cure period.
“Person” means an individual, a partnership, a corporation, an association, a limited liability company, a joint stock company, a trust, a joint venture, an unincorporated organization or any other entity (including any governmental entity or any department, agency or political subdivision thereof).
|
|
“Subsidiaries” means, with respect to any Person, any corporation, limited liability company, partnership, association or other business entity of which (i) if a corporation, a majority of the total voting power of shares of stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors thereof is at the time owned or controlled, directly or indirectly, by such Person or one (1) or more of the other Subsidiaries of such Person or a combination thereof, or (ii) if a limited liability company, partnership, association or other business entity, a majority of the partnership or other similar ownership interests thereof is at the time owned or controlled, directly or indirectly, by any Person or one (1) or more Subsidiaries of such Person or entity or a combination thereof. For purposes hereof, a Person or Persons will be deemed to have a majority ownership interest in a limited liability company, partnership, association or other business entity if such Person or Persons will be allocated a majority of limited liability company, partnership, association or other business entity gains or losses or will be or control any managing director, managing member, or general partner of such limited liability company, partnership, association or other business entity. Unless stated to the contrary, as used in this Agreement the term Subsidiary means a Subsidiary of the Company.
“Termination Date” means the date on which the Employment Period ends pursuant to Section 5(a).
14. Cooperation in Legal Matters. The Executive will cooperate with the Company and its Subsidiaries during the term of the Executive’s employment and, subject to Executive’s other personal and business obligations, thereafter, with respect to any pending or threatened claim, action, suit, or proceeding, whether civil, criminal, administrative, or investigative (the “Claims”), by being reasonably available to testify on behalf of the Company or any Subsidiaries, and to assist the Company and its Subsidiaries by providing information, meeting and consulting with the Company and its Subsidiaries or their representatives or counsel, as reasonably requested and at the Company’s sole expense. The Executive agrees not to disclose to or discuss with anyone who is not assisting the Company or any Subsidiary with the Claims, other than the Executive’s personal attorney, the fact of or the subject matter of the Claims, except as required by law. The Executive further agrees to maintain the confidences and privileges of the Company and its Subsidiaries, and acknowledges that any such confidences and privileges belong solely to the Company and its Subsidiaries and can only be waived by the Company or any Subsidiary, not the Executive. In the event that the Executive is subpoenaed to testify, or otherwise requested to provide information in any matter relating to the Company or any Subsidiary, the Executive agrees to promptly notify the Company after receipt of such subpoena, summons or request for information, to reasonably cooperate with the Company or any Subsidiary with respect to such subpoena, summons or request for information, and to not voluntarily provide any testimony or information unless required by law or permitted by the Company.
15. Background Check. The Executive agrees that the Company may undertake a background check with respect to the Executive in connection with the subject matter of this Agreement in such manner as the Company determines to be appropriate. The Company reserves the right to terminate this Agreement in the event the results of the background check are not satisfactory to the Company in its sole discretion. In the event of any such termination, this Agreement shall be deemed null and void.
|
|
16. Miscellaneous.
(a) Notices. All notices, demands or other communications to be given or delivered by reason of the provisions of this Agreement will be in writing and will be deemed to have been given (i) on the date of personal delivery to the recipient or an officer of the recipient, (ii) when sent by telecopy or facsimile machine to the number shown below on the date of such confirmed facsimile or telecopy transmission (provided that a confirming copy is sent via overnight mail), or (iii) when properly deposited for delivery by a nationally recognized commercial overnight delivery service, prepaid, or by deposit in the United States mail, certified or registered mail, postage prepaid, return receipt requested. Such notices, demands and other communications will be sent to each party at the address indicated for such party below:
if to the Executive, to:
Robert E. Kristal
4719 Cole Avenue
Dallas, Texas 75205
if to the Company, to:
40 Marcus Drive, Suite One
Melville, New York 11747
Facsimile: (631) 760-8414
Attention: CEO
with copies, which will not constitute notice to the Company, to:
Saul Ewing Arnstein & Lehr LLP
33 South Sixth Street, Suite 4750
Minneapolis, MN 55402
Facsimile: (612) 677-3844
Attention: Doug Ramler
and
Certilman Balin Adler & Hyman, LLP
90 Merrick Avenue, 9th Floor
East Meadow, New York 11554
Facsimile: (516) 296-7111
Attention: Fred Skolnik, Esq.
or to such other address or to the attention of such other person as the recipient party has specified by prior written notice to the sending party.
(b) Consent to Amendments. No modification, amendment or waiver of any provision of this Agreement will be effective against any Party hereto unless such modification, amendment or waiver is approved in writing by such Party. No other course of dealing among the Company, the Subsidiaries, and the Executive or any delay in exercising any rights hereunder will operate as a waiver by any of the Parties hereto of any rights hereunder.
|
|
(c) Assignability and Binding Effect. This Agreement will be binding upon and inure to the benefit of the Executive and his heirs, legal representatives, executors, administrators or successors, and will be binding upon and inure to the benefit of the Company and its successors and assigns. The Parties acknowledge and agreement that this Agreement is freely assignable by the Company and that the Executive may not assign, transfer, pledge, encumber, hypothecate or otherwise dispose of this Agreement, or any of his rights or obligations hereunder, and any such attempted assignment or disposition shall be null and void and without effect.
(d) Severability. Whenever possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be prohibited by or invalid under applicable law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Agreement.
(e) Headings and Sections. The headings in this Agreement are inserted for convenience only and are in no way intended to describe, interpret, define, or limit the scope, extent or intent of this Agreement or any provision of this Agreement. Unless the context requires otherwise, all references in this Agreement to Sections, Exhibits or Schedules will be deemed to mean and refer to Sections, Exhibits or Schedules of or to this Agreement.
(f) Governing Law. All issues and questions concerning the application, construction, validity, interpretation and enforcement of this Agreement and any exhibits and schedules to this Agreement shall be governed by, and construed in accordance with, the laws of the State of New York, without giving effect to any choice of law or conflict of law rules or provisions that would cause the application of the laws of any jurisdiction other than the State of New York.
(g) Waiver of Jury Trial. EACH PARTY TO THIS AGREEMENT HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR (II) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES HERETO IN RESPECT OF THIS AGREEMENT, IN EACH CASE WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. EACH PARTY TO THIS AGREEMENT HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION WILL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES TO THIS AGREEMENT MAY FILE AN ORIGINAL COUNTERPART OR A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY.
|
|
(h) Submission to Jurisdiction. ANY AND ALL SUITS, LEGAL ACTIONS OR PROCEEDINGS ARISING OUT OF THIS AGREEMENT WILL BE BROUGHT IN THE COURTS OF THE STATE OF NEW YORK OR THE UNITED STATES DISTRICT COURT IN THE EASTERN DISTRICT OF NEW YORK, AND EACH PARTY HEREBY SUBMITS TO AND ACCEPTS THE EXCLUSIVE JURISDICTION OF SUCH COURTS FOR THE PURPOSE OF SUCH SUITS, LEGAL ACTIONS OR PROCEEDINGS. TO THE FULLEST EXTENT PERMITTED BY LAW, EACH PARTY HERETO HEREBY IRREVOCABLY WAIVES ANY OBJECTION WHICH IT MAY NOW OR HEREAFTER HAVE TO THE LAYING OF VENUE OR ANY SUCH SUIT, LEGAL ACTION OR PROCEEDING IN ANY SUCH COURT AND HEREBY FURTHER WAIVES ANY CLAIM THAT ANY SUIT, LEGAL ACTION OR PROCEEDING BROUGHT IN ANY SUCH COURT HAS BEEN BROUGHT IN AN INCONVENIENT FORUM.
(i) Service of Process. WITH RESPECT TO ANY AND ALL SUITS, LEGAL ACTIONS OR PROCEEDINGS ARISING OUT OF THIS AGREEMENT, EACH PARTY WAIVES PERSONAL SERVICE OF ANY SUMMONS, COMPLAINT OR OTHER PROCESS AND AGREES THAT SERVICE THEREOF MAY BE MADE BY ANY MEANS SPECIFIED FOR NOTICE PURSUANT TO SECTION 14(a).
(j) Confidentiality. The Parties agree that this Agreement and the Release (if and when executed) are confidential and each Party agrees not to disclose any information regarding the terms of this Agreement or the Release to any Person, except that the Company may disclose information regarding the terms of this Agreement or the Release to its Affiliates and any lenders or as required by law or regulation or the rules of any stock exchange or market on which the Company’s securities are listed or traded, and the Executive may disclose information regarding the terms of this Agreement or the Release to his immediate family. Each Party may also disclose this information to its tax, legal or other counsel. Each Party shall instruct each of the foregoing not to disclose the same to anyone.
(k) No Strict Construction. The Parties hereto have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement will be construed as if drafted jointly by the Parties hereto, and no presumption or burden of proof will arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement.
(l) Entire Agreement. Except as otherwise expressly set forth in this Agreement, this Agreement and the other agreements referred to in this Agreement embody the complete agreement and understanding among the Parties to this Agreement with respect to the subject matter of this Agreement, and supersede and preempt any prior understandings, agreements, or representations by or among the Parties or their predecessors, written or oral, that may have related to the subject matter of this Agreement in any way. This Agreement will be deemed effective on the date hereof upon the execution hereof.
(m) Time. Whenever the last day for the exercise of any privilege or the discharge or any duty hereunder falls upon a day that is not a business day, the Party having such privilege or duty may exercise such privilege or discharge such duty on the next succeeding day that is a business day.
(n) Certain Terms. The use of the word “including” herein means “including without limitation.” Any definitions used herein defined in the plural will be deemed to include the singular as the context may require and any definitions used herein defined in the singular will be deemed to include the plural as the context may require. References to “Dollars” or “$” are references to the lawful currency of the United States of America.
[Remainder of page intentionally left blank. Signature page follows.]
|
|
IN WITNESS WHEREOF, the Parties hereto have executed this Executive Employment Agreement as of the date first written above.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| Chief Executive Officer | ||
| ROBERT E. KRISTAL | ||
|
|
SCHEDULE A
| Position: | Chief Financial Officer |
| Per Annum Salary: | $175,000, less applicable taxes and withholdings. |
| Responsibilities: | Executive will be primarily responsible for the areas set forth below, subject to the direction of the Company’s CEO and Board of Directors. |
| ● | Providing leadership, direction and management of the finance and accounting team |
| ● | Providing strategic recommendations to the CEO, President and members of the executive management team |
| ● | Managing the processes for financial forecasting and budgets, and overseeing the preparation of all financial reporting |
| ● | Advising on long-term business and financial planning (including cash management) |
| ● | Interacting with auditors, accountants and tax advisors |
| ● | Responsible for participating in all SEC filings |
| ● | Establishing and developing relations with existing and prospective stakeholders |
| ● | Partnering with the CEO on investor relations/press releases |
| ● | Attending in person and virtual banking/investor conferences |
| ● | Reviewing Company presentations used in connection with investors |
| ● | Reviewing all formal finance, HR and IT related procedures | |
| ● | Supervising HR/IT/Finance (controller) |
| Report to: | CEO and Board of Directors |
| Vacation: | Three (3) weeks. |
EXHIBIT A
GENERAL RELEASE
I, Robert E. Kristal, in consideration of and subject to the performance by BioRestorative Therapies, Inc., a Delaware corporation (the “Company”), of its obligations under the Executive Employment Agreement by and between the Company and myself, dated as of November 4, 2021 (as amended from time to time, the “Agreement”), do hereby release and forever discharge as of the date hereof, (i) the Company and (ii) each of its subsidiaries, affiliates and predecessors (including, without limitation, and to the extent that they could be liable in respect of their position with any of the foregoing, each of the present and former managers, directors, officers, direct or indirect equity holders, agents, representatives, employees, subsidiaries, affiliates, predecessors, successors, assigns, beneficiaries, heirs, executors, insurers, personal representatives, and attorneys of the parties referenced in clauses (i) and (ii) above) (collectively, the “Released Parties”) to the extent provided below.
1. I understand that any payments or benefits paid or granted to me under Section 5(b) of the Agreement represent, in part, consideration for signing this General Release and are not salary, wages or benefits to which I was already entitled. I understand and agree that I will not receive the payments and benefits specified in Section 5(b) of the Agreement if I execute this General Release and revoke this General Release within the time period permitted hereafter, if I do not execute the General Release in the requested time frame, or if I breach this General Release or anything in the Agreement. I also acknowledge and represent that I have received all payments and benefits the payment and provision of which were due to me, as of the date hereof, by virtue of my employment by the Company.
2. Except as provided in Paragraph 4 below, I knowingly and voluntarily (for myself, my heirs, executors, administrators and assigns) release and forever discharge the Released Parties from any and all claims, suits, controversies, actions, causes of action, cross-claims, counter-claims, demands, debts, compensatory damages, liquidated damages, punitive or exemplary damages, other damages, claims for costs and attorneys’ fees, or liabilities of any nature whatsoever in law and in equity, both past and present (through the date this General Release becomes effective and enforceable) and whether known or unknown, suspected, or claimed against any of the Released Parties which I, my spouse, or any of my heirs, executors, administrators or assigns, may have, which arise out of or are connected with my employment with, or my separation or termination from, the Company (including, but not limited to, any allegation, claim or violation, arising under: Title VII of the Civil Rights Act of 1964, as amended; the Civil Rights Act of 1991; the Age Discrimination in Employment Act of 1967, as amended (including the Older Workers Benefit Protection Act); the Equal Pay Act of 1963, as amended; the Americans with Disabilities Act of 1990; the Family and Medical Leave Act of 1993; the Worker Adjustment Retraining and Notification Act; the Employee Retirement Income Security Act of 1974; any applicable Executive Order Programs; the Fair Labor Standards Act; or their state or local counterparts; or under any other federal, state or local civil or human rights law, or under any other federal, state or local law, regulation or ordinance; or under any public policy, contract or tort, or under common law; or arising under any policies, practices or procedures of the Company or any of its affiliates; or any claim for wrongful discharge, breach of contract, infliction of emotional distress or defamation; or any claim for costs, fees, or other expenses, including attorneys’ fees incurred in these matters) (all of the foregoing collectively referred to herein as the “Claims”). Notwithstanding any other provision of this General Release to the contrary, this General Release does not encompass, and I do not release, waive or discharge the obligations of any of the Released Parties (a) to make the payments and provide the other benefits contemplated by the Agreement, (b) under any restricted stock agreement, option agreement or other agreement pertaining to my equity ownership, (c) under any indemnification or similar agreement with me.
3. I represent that I have made no assignment or transfer of any right, claim, demand, cause of action, or other matter covered by Paragraph 2 above.
4. I agree that this General Release does not waive or release any rights or claims that I may have under the Age Discrimination in Employment Act of 1967 which arise after the date I execute this General Release. I acknowledge and agree that my separation from employment with the Company in compliance with the terms of the Agreement shall not serve as the basis for any claim or action (including, without limitation, any claim under the Age Discrimination in Employment Act of 1967).
5. In signing this General Release, I acknowledge and intend that it shall be effective as a bar to each and every one of the Claim(s) hereinabove mentioned or implied. I expressly consent that this General Release shall be given full force and effect according to each and all of its express terms and provisions, including those relating to unknown and unsuspected Claims (notwithstanding any state statute that expressly limits the effectiveness of a general release of unknown, unsuspected and unanticipated Claims), if any, as well as those relating to any other Claims hereinabove mentioned or implied. I acknowledge and agree that this waiver is an essential and material term of this General Release and that without such waiver the Company would not have agreed to the terms of the Agreement. I further agree that in the event I should bring a Claim seeking damages against any Released Party, or in the event I should seek to recover damages against any Released Party in any Claim brought by a governmental agency on my behalf, this General Release shall serve as a complete defense to such Claim and I shall waive and not accept any monetary award or value in conjunction with such Claim. I further agree that I have not filed and am not aware of any pending Claim as of the execution of this General Release.
6. I agree that neither this General Release, nor the furnishing of the consideration for this General Release, shall be deemed or construed at any time to be an admission by the Company, any Released Party or myself of any improper or unlawful conduct.
7. Nothing in this Agreement shall be construed to preclude me from participating or cooperating in any investigation or proceeding conducted by the Equal Employment Opportunity Commission or any other state or federal administrative agency. However, in the event that a charge or complaint is filed against the Released Parties, or any of them, with any administrative agency or in the event of an authorized investigation, charge or lawsuit filed against the Released Parties, or any of them, by any administrative agency, I expressly waive and shall not accept any award or damages therefrom.
8. Notwithstanding anything in this General Release to the contrary, this General Release shall not relinquish, diminish, or in any way affect any of my rights or claims arising out of any breach by the Company after the date hereof of the Agreement if and to the extent those rights, in each case by their specific terms, survive termination of my employment with the Company.
9. Whenever possible, each provision of this General Release shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this General Release is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other provision or any other jurisdiction, but this General Release shall be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision had never been contained herein. However, should Section 2 of this General Release be declared or determined by any tribunal, administrative agency or court of competent jurisdiction to be illegal or invalid, and should I thereupon seek to institute any Claims that would have been within the scope of Section 2, the Company shall be entitled to immediate repayment, and I shall immediately return, all of the severance payments that I have received, and the Company shall not be obligated to make any further severance payments.
10. BY SIGNING THIS GENERAL RELEASE, I REPRESENT AND AGREE THAT:
A. I HAVE READ IT CAREFULLY;
B. I UNDERSTAND ALL OF ITS TERMS AND KNOW THAT I AM GIVING UP IMPORTANT RIGHTS, INCLUDING BUT NOT LIMITED TO, RIGHTS UNDER THE AGE DISCRIMINATION IN EMPLOYMENT ACT OF 1967, AS AMENDED, TITLE VII OF THE CIVIL RIGHTS ACT OF 1964, AS AMENDED, THE EQUAL PAY ACT OF 1963, THE AMERICANS WITH DISABILITIES ACT OF 1990, AND THE EMPLOYEE RETIREMENT INCOME SECURITY ACT OF 1974, AS AMENDED;
C. I VOLUNTARILY CONSENT TO EVERYTHING IN IT;
D. I HAVE BEEN ADVISED TO CONSULT WITH AN ATTORNEY BEFORE EXECUTING IT AND I HAVE DONE SO OR, AFTER CAREFUL READING AND CONSIDERATION I HAVE CHOSEN NOT TO DO SO OF MY OWN VOLITION;
E. I HAVE BEEN ADVISED I HAVE TWENTY-ONE (21) CALENDAR DAYS TO REVIEW THIS GENERAL RELEASE;
F. I AGREE THAT ANY MODIFICATIONS, MATERIAL OR OTHERWISE, MADE TO THIS GENERAL RELEASE DO NOT RESTART OR AFFECT IN ANY MANNER THE ORIGINAL TWENTY-ONE (21) CALENDAR DAY CONSIDERATION PERIOD;
G. I UNDERSTAND THAT I HAVE SEVEN (7) DAYS AFTER THE EXECUTION OF THIS GENERAL RELEASE TO REVOKE IT AND THAT THIS GENERAL RELEASE SHALL NOT BECOME EFFECTIVE OR ENFORCEABLE UNTIL THE REVOCATION PERIOD HAS EXPIRED. IN ORDER TO REVOKE, I AGREE THAT I MUST DELIVER A WRITTEN LETTER OF REVOCATION TO THE COMPANY (ATTN: SECRETARY, 40 MARCUS DRIVE, SUITE ONE, MELVILLE, NEW YORK 11747) BEFORE THE EXPIRATION OF THE REVOCATION PERIOD;
H. I HAVE SIGNED THIS GENERAL RELEASE KNOWINGLY AND VOLUNTARILY AND WITH THE ADVICE OF ANY COUNSEL RETAINED TO ADVISE ME WITH RESPECT TO IT; AND
I. I AGREE THAT THE PROVISIONS OF THIS GENERAL RELEASE MAY NOT BE AMENDED, WAIVED, CHANGED OR MODIFIED EXCEPT BY AN INSTRUMENT IN WRITING SIGNED BY AN AUTHORIZED REPRESENTATIVE OF THE COMPANY AND BY ME.
DATE:____________________________
_________________________________
ROBERT E. KRISTAL
Exhibit 10.27
EXECUTIVE EMPLOYMENT AGREEMENT
This Executive Employment Agreement (this “Agreement”) is made as of December ___, 2021 (the “Effective Date”), by and between BioRestorative Therapies, Inc., a Delaware corporation (the “Company”), and Robert Paccasassi (the “Executive”) (Company and Executive are collectively the “Parties”). Certain capitalized terms used in this Agreement are defined in Section 13.
RECITALS
WHEREAS, the Company and the Executive desire to enter into an employment agreement which will set forth the terms and conditions upon which the Executive shall be employed by the Company and upon which the Company shall compensate the Executive.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto agree as follows:
1. Employment. The Company will employ the Executive, and the Executive accepts employment with the Company, upon the terms and conditions set forth in this Agreement, for the period beginning on the Effective Date and ending as provided in Sections 2 and/or 5 below (the “Employment Period”).
2. Initial Employment Term. This Agreement shall commence on the Effective Date and will continue for a period of one (1) year or until either Party terminates the Agreement in accordance with Section 5 hereof (the “Initial Employment Term”). Notwithstanding the foregoing, nothing herein shall limit or restrict the Parties extending the Initial Employment Term, provided such extension is memorialized in writing by the Parties.
3. Position and Duties. During the Employment Period, the Executive will serve in the position set forth on Schedule A to this Agreement and will render such managerial, analytical, administrative, financial and other executive services to, and shall have such responsibilities on behalf of, the Company and its Subsidiaries, as are from time to time necessary in connection with the management and affairs of the Company and its Subsidiaries and are consistent with his position, in each case subject to the authority of the CEO of the Company (the “CEO”) and the Board of Directors of the Company (the “Board”) to define and limit such executive services. The Executive’s primary responsibilities shall include, without limitation, those set forth on Schedule A attached hereto. The Executive will devote substantially all of his business time and attention to the business and affairs of the Company and its Subsidiaries, provided that the Executive will be permitted to (i) serve as a member of the board of directors or advisory board of charitable organizations and/or, subject to Sections 6-8, perform services for other business organizations with which the Executive has or may become associated, (ii) engage in charitable activities and community affairs and (iii) manage his personal investments and affairs, except that the Executive will limit the time devoted to the activities described in clauses (i), (ii), and (iii) so as not to materially interfere, individually or in the aggregate, with the performance of his duties and responsibilities hereunder. The Executive shall be permitted to continue to provide consulting services through RPharma Consulting LLC (“RPharma”) as long as such consulting services (x) are provided only to entities that are clients of RPharma as of the date hereof, (y) are not provided to an entity whose business is competitive, directly or indirectly, with that of the Company and (z) do not materially interfere with the performance of his duties and responsibilities hereunder. The Executive will perform his duties and responsibilities to the best of his abilities in a diligent, trustworthy, businesslike and efficient manner, and Executive acknowledges and agrees that he owes a fiduciary duty of loyalty to the Company. While subject to change in the sole discretion of the Company, the Executive will report to the person set forth on Schedule A. During the Employment Period, the Executive’s primary work location shall be 40 Marcus Drive, Suite 1 Melville, New York 11747; provided, however, that the Executive shall be permitted to telecommute from time to time during the Employment Period provided that the Executive’s telecommuting does not materially impact his ability to perform his duties and responsibilities under this Agreement.
4. Salary and Benefits.
(a) Salary. During the Employment Period, the Company will pay the Executive a salary at not less than the rate set forth on Schedule A to this Agreement (as in effect from time to time, the “Salary”) as compensation for services. The Salary will be payable in regular installments in accordance with the general payroll practices of the Company and its Subsidiaries and subject to applicable withholding requirements.
(b) Discretionary Bonus. In the sole discretion of the Board (including the approval of a majority of the independent members of the Board), and subject to many factors, including but not limited to Executive’s performance (including, but not limited to, his satisfaction of the Bonus performance criteria set forth on Schedule A to this Agreement (the “Bonus Performance Criteria”)) and the financial performance of the Company, Executive shall be eligible for an annual bonus of up to 30% of Executive’s Salary (the “Bonus”). The Executive acknowledges and agrees that satisfaction of the Bonus Performance Criteria will not automatically entitle Executive to receive the Bonus as Executive acknowledges and agrees that many additional factors will be included in the Bonus determination.
(c) Benefits. During the Employment Period, the Executive will be entitled to participate in such Company benefit plans as the Board may establish or maintain from time to time in its sole discretion, at Executive’s sole cost and expense. The Executive will be entitled to the number of weeks of paid vacation each year set forth on Schedule A attached hereto, prorated for any partial calendar year. To the extent that the Executive does not use all the vacation time in any year, calculated on a calendar year basis, up to two (2) weeks (or such lesser prorated amount) of the unused vacation may be carried over to the next year and any remaining unused amounts will be lost and forfeited by Executive. In no circumstance shall Executive earn or possess more than six (6) weeks of vacation at any time. Vacation will cease to be earned once Executive has six (6) weeks of earned but unused vacation and will not earn additional vacation until such time as vacation is taken by Executive.
|
|
(d) Reimbursement of Expenses. During the Employment Period, the Company will reimburse the Executive for all reasonable out-of-pocket expenses incurred by Executive in the course of performing Executive’s duties that are consistent with the Company’s policies in effect from time to time with respect to travel, entertainment and other business expenses, subject to the Company’s requirements with respect to reporting and documentation of such expenses.
(e) Equity. The Executive acknowledges that, on November 4, 2021, the Company entered into a Stock Option Award Agreement with Executive (the “Option Agreement”) pursuant to which Executive was granted a ten year option (the “Option”) to purchase 8,277 shares of common stock of the Company at an exercise price of $13.50 per share, which exercise price has been reduced to $5.08 per share (subject to shareholder approval thereof). Subject to the terms and conditions of the Option Agreement, the Option is exercisable to the extent of one-eighth (1/8) thereof on a quarterly basis commencing as of February 4, 2022.
5. Termination.
(a) The Employment Period will continue until the earliest of: (i) the Executive’s resignation for any or no reason; (ii) the death or Disability of the Executive; (iii) the giving of notice of termination by the Company: (A) for Cause or (B) for any other reason or for no reason (a termination described in this clause (iii)(B) being a termination by the Company “Without Cause”); (iv) the Executive’s resignation for Good Reason; or (v) the end of the Initial Employment Term in the event it is not extended in writing by the Parties.
(b) If the Company terminates the Employment Period Without Cause, the Executive resigns for Good Reason, or the Initial Employment Term expires without written extension by the Parties, the Executive will be entitled to receive each of the following: all accrued and unpaid Salary and earned, but unused vacation time for the then-current annual period (with the right to vacation time being pro-rated for such period through the Termination Date), all vested stock options and all unreimbursed business expenses incurred through the Termination Date and payable pursuant to Section 4(d), which accrued and unpaid salary, unused vacation and unreimbursed expenses shall be payable in a lump sum (less applicable taxes and withholdings) on the later of the next regularly-scheduled payroll date or five (5) days after the Termination Date (the amounts described in the immediately preceding sentence are referred to herein as the “Accrued Obligations”). The Executive shall also be entitled to any COBRA benefits to which the Executive is entitled by law. COBRA benefits shall be at the Executive’s sole expense. In addition, if the Company terminates the Executive’s employment without Cause or the Executive resigns for Good Reason, subject to paragraph (d) hereof, the Executive shall be entitled to an aggregate payment equal to the lesser of the Salary Executive would receive during the remainder of the Initial Employment Term or twenty-five percent (25%) of his then current Salary.
(c) If the Employment Period terminates by reason of (i) the Company’s termination with Cause or (ii) the Executive’s resignation without Good Reason, then the Executive (or his estate in the case of his death) will be entitled to receive the Accrued Obligations, as well as any COBRA benefits to which the Executive is entitled by law (at the Executive’s sole expense).
|
|
(d) The Cash Severance Amount, if applicable, will be paid in equal biweekly installments over a period of no more than three (3) months following the Termination Date in accordance with the general payroll practices of the Company and subject to all applicable withholding requirements; provided, however, that the payment of the Cash Severance Amount shall be conditioned upon the Executive (i) executing and delivering to the Company a general release of all past and present claims against the Company and its Subsidiaries substantially in the form attached hereto as Exhibit A (the “Form of Release”), within twenty-one (21) days of the date the Company delivers such general release (the “Release”) to the Executive, and (ii) not exercising the Executive’s revocation right during the period for revocation described in the Form of Release and/or Release; provided, further, that, in the event of the Executive’s breach of this Agreement, then the Company’s obligation to pay the Cash Severance Amount shall terminate and be of no further force or effect and the Executive shall be obligated to reimburse the Company for all Cash Severance Amount previously made. To the extent that Cash Severance Amount payments are payable, they shall be made or commence on the later of the first payroll date following the effective date in the Release or the fortieth (40th) day following the Termination Date.
(e) Upon the Termination Date, the Executive will be obligated to and be deemed to have resigned from each position (if any) that he then holds as an officer or director of the Company or any Subsidiary, and the Executive will take any action that the Company or any Subsidiary may reasonably request in order to confirm or evidence such resignation.
(f) Neither the termination or expiration of this Agreement nor the termination of the Executive’s employment with the Company, whether by the Company or the Executive, whether for Cause or Without Cause, whether for Good Reason or without Good Reason, and whether voluntary or involuntary, shall affect the continuing operation and effect of Sections 6-8 hereof, which shall continue in full force and effect according to their terms. In addition, neither the termination or expiration of this Agreement nor the termination of the Executive’s employment with the Company, whether by the Company or the Executive, whether for Cause or Without Cause, whether for Good Reason or without Good Reason, and whether voluntary or involuntary, will result in a termination or waiver of any rights and remedies that the Company may have under this Agreement and applicable law.
(g) In the event of the termination of this Agreement or the Executive’s employment, whether by the Company or the Executive, whether for Cause or Without Cause, whether for Good Reason or without Good Reason, and whether voluntary or involuntary, except as expressly provided for herein, the Executive shall not be entitled to any further compensation or benefits.
6. Confidentiality/Non-Disclosure. Executive agrees that during the Employment Period and thereafter, other than in connection with his duties on behalf of Company, Executive will not disclose Confidential Information, proprietary information, or trade secrets, related to any business of Company including, without limitation, and whether or not such information is specifically designated as confidential or proprietary: (a) business plans; (b) business processes; (c) practices; (d) marketing strategies; (e) documents; (f) information concerning existing and prospective markets, suppliers and customers; (g) financial information; (h) information concerning the development of new products and services; (i) operations; (j) techniques; (k) agreements; (l) contracts; (m) terms of agreements; (n) transactions; (o) potential transactions; (p) know-how; (q) technical and non-technical data related to software programs, design, specifications, compilations, inventions, improvements, patent applications, studies, research, methods, devices, prototypes, processes, procedures and techniques (“Confidential Information”). Notwithstanding anything to the contrary contained herein, Confidential Information, proprietary information, or trade secrets shall not be deemed to include any of the foregoing: (i) information that is within the public domain or known generally in the trade as of the Effective Date of this Agreement, except to the extent that such information entered the public domain through an act or omission of Executive (other than lawfully on behalf of Company); (ii) information that enters the public domain after the Effective Date of this Agreement by no act or omission of Executive (other than by Executive lawfully on behalf of Company); (iii) disclosures required to enforce any rights hereunder or any other agreement with Company; or (iv) disclosures required by any subpoena or other legal process; provided, Executive will, to the extent permitted by law, promptly notify Company of such fact so that Company may seek an appropriate remedy to prevent such disclosure.
|
|
Under the federal Defend Trade Secrets Act of 2016, Executive shall not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that: (a) is made (i) in confidence to a federal, state, or local government official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose of reporting or investigating a suspected violation of law; or (b) is made to Executive’s attorney in relation to a lawsuit for retaliation against Executive for reporting a suspected violation of law; or (c) is made in a complaint or other document filed in a lawsuit or other proceeding, if such filing is made under seal.
7. Restrictive Covenants.
(a) The services of the Executive are unique and extraordinary and essential to the business of the Company, especially since the Executive shall have access to the Company’s customer lists, trade secrets and other privileged and confidential information essential to the Company’s business. Therefore, the Executive agrees that, as a material inducement to, and a condition precedent to the Company’s payment obligations hereunder and its other covenants herein, if the term of the Executive’s employment hereunder shall expire or the Executive’s employment shall at any time terminate for any reason whatsoever, for Cause or Without Cause, for Good Reason or without Good Reason, the Executive will not at any time within one (1) year after such expiration or termination (the “Restrictive Covenant Period”), without the prior written approval of the Company, directly or indirectly, whether individually or as a principal, officer, stockholder, equity participant, employee, partner, joint venturer, member, manager, director or agent of, or lender, consultant or independent contractor to, any Person, or in any other capacity, other than on behalf of or for the benefit of the Company:
(i) anywhere in the United States of America, directly or indirectly, engage or participate in a business which, as of such expiration or termination date, is similar to or competitive with the business of the Company as of the Termination Date, as described in the Company’s Securities and Exchange Commission (the “SEC”) filings (or, in the event that the Company is not subject to the SEC disclosure requirements, then as described on the Company’s website (the “Business”)), wherein Executive shall be performing the same or similar duties as he performed for the Company, or if Executive is likely to use or disclose confidential or proprietary information of the Company. For the sake of clarity, the Company’s Business is focused on stem-cell based therapies, including autologous hypoxic disc treatments and the use of brown adipose stem cells for treating metabolic disorders. Notwithstanding the foregoing, Executive may acquire, as a passive investor, up to five percent (5%) of the outstanding voting stock of any entity whose securities are listed on a stock exchange or NASDAQ;
|
|
(ii) cause, seek to persuade or solicit any director, officer, employee, customer, account, agent or supplier of, or consultant or independent contractor to, the Company or others with whom the Company has had a business relationship (collectively, “Business Associates”) to discontinue or materially modify the status, employment or other relationship of such Business Associate with the Company, or to become employed in any activity similar to or competitive with the Business of the Company;
(iii) cause, seek to persuade or solicit, any prospective customer, account, supplier or other Business Associate of the Company (which at the date of cessation of the Executive’s employment with the Company was then, to the knowledge of the Executive, actively being solicited by the Company) to determine not to enter into a business relationship with the Company or to materially modify its contemplated business relationship;
(iv) hire, retain, engage or associate in a business relationship with, directly or indirectly, any employee of the Company; or
(v) solicit from or cause or authorize to be solicited from, for or on behalf of the Executive or any third party, any business which is similar to or competitive with the Business of the Company, or enter into a similar or competitive business relationship with, (a) others who are, or were within one (l) year prior to the cessation of the Executive’s employment with the Company, customers, accounts or other Business Associates of the Company, or (b) any prospective customer, account or other Business Associate of the Company which at the date of such cessation was then, to the Executive’s knowledge, actively being solicited by the Company.
The foregoing restrictions set forth in this Section 7 shall apply likewise during the Employment Period and all references to “Business” shall be deemed to refer to the then Business of the Company during the Employment Period.
(b) For purposes of this Section 7, the term “Company” shall mean and include the Company and any and all Subsidiaries and Affiliates of the Company in existence from time to time.
(c) Executive acknowledges the benefits provided or made available to him pursuant to the provisions of this Agreement, including, without limitation, the agreement on the part of the Company to employ the Executive during the Employment Period (subject to the terms and conditions hereof) constitute sufficient consideration for the restrictions contained in this Section 7 . The Executive also acknowledges and agrees that the covenants set forth in this Section 7 are reasonable and necessary in order to protect and maintain the proprietary and other legitimate business interests of the Company and that the enforcement thereof would not prevent the Executive from earning a livelihood.
|
|
(d) Company and Executive agree (a) that the provisions of this Section 7 do not impose an undue hardship on Executive and are not injurious to the public, (b) that these provisions are necessary to protect the Business of Company, (c) that the nature of Executive’s responsibilities with Company under this Agreement provide and/or will provide Executive with access to Confidential Information, proprietary information, or trade secrets that are valuable and confidential to Company, (d) that Company would not have entered into this Agreement with Executive if Executive did not agree to the provisions of this Section 7, (e) that Company would not have provided the Cash Severance Amount or any other consideration outlined in this Agreement if Executive did not agree to the provisions of this Section 7, (f) that the provisions of this Section 7 are reasonable in terms of length of time and scope, and (g) that adequate consideration supports the provisions of this Section 7. In the event that a court determines that any provision of this Section 7 are unreasonably broad or extensive, Executive agrees that such court should narrow such provision to the extent necessary to make it reasonable and enforce the provisions as narrowed. If such partial enforcement is not possible, the provision shall be deemed severed from this Agreement. Company reserves all rights to seek any and all remedies and damages permitted under law including, but not limited to, injunctive relief, equitable relief, and compensatory damages for any breach of Executive’s obligations under this Section 7, without the obligation to post or provide any bond. In the event Executive breaches or threatens to breach any of the provisions of this Section 7, Company shall immediately cease payment of any Cash Severance Amount, or other amounts due under this Agreement, as the case may be.
8. Developments and Inventions. All modifications, alterations, enhancements, betterments, ideas or discoveries which are the result, directly or indirectly, of Executive’s employment and/or affiliation with the Company and/or Executive’s access to Confidential Information (collectively “Developments”) shall be the sole and exclusive property of Company and are considered a “work made for hire” for the purposes of Company’s rights under copyright and other laws. To the extent that any Developments may not be considered “work made for hire,” Executive shall assign to Company such Developments and all rights therein, to the extent permitted by law. Executive shall take all necessary steps, without compensation, whether during or after his Employment Period: (a) to assign all right, title, and interest in any Developments to Company, and (b) to assist Company in registering, prosecuting, perfecting, protecting, maintaining and enforcing any and all patent, copyright, trade secret or other right or interest in any Developments for any and all countries. If any Developments assigned hereunder is based upon, or is incorporated into or is an improvement or derivative of, or cannot reasonably be made, used, reproduced and/or distributed without using or violating technology or rights owned or licensed by Company and not assigned hereunder, Executive shall grant Company a perpetual, worldwide, royalty-free, non-exclusive and sub-licensable right and license to exploit and exercise all such technology and rights in support of Company’s exercise or exploitation of any such assigned Development(s) (including any modifications, improvements and derivatives thereof). Specifically excluded from this Section is anything developed entirely on Executive’s own time and without the use of any equipment, supplies, facilities, other resources and/or Confidential Information of Company, provided that the Development does not relate to the Business of Company.
|
|
9. Omitted.
10. Deductions and Withholding. The Executive agrees that the Company shall withhold from any and all payments required to be made to the Executive pursuant to this Agreement all federal, state, local and/or other taxes that are required to be withheld in accordance with applicable statutes and/or regulations from time to time in effect.
11. Code Section 409A.
(a) The intent of the Parties is that payments and benefits under this Agreement comply with Section 409A of the Internal Revenue Code of 1986, as amended (together with the regulations and guidance promulgated thereunder, “Code Section 409A”), and, accordingly, to the maximum extent permitted, this Agreement shall be interpreted to be in compliance therewith. To the extent that any provision hereof is modified in order to comply with Code Section 409A, such modification shall be made in good faith and shall, to the maximum extent reasonably possible, maintain the original intent and economic benefit to the parties hereto of the applicable provision without violating the provisions of Code Section 409A. In no event whatsoever shall the Company be liable for any additional tax, interest or penalty that may be imposed on the Executive by Code Section 409A.
(b) A termination of employment shall not be deemed to have occurred for purposes of any provision of this Agreement providing for the payment of any amounts or benefits constituting deferred compensation under Code Section 409A upon or following a termination of employment unless such termination of employment is also a “separation from service” within the meaning of Code Section 409A and, for purposes of any such provision of this Agreement, references to a termination of employment or like terms shall mean “separation from service.” If the Executive is deemed on the date of termination to be a “specified employee” within the meaning of that term under Code Section 409A(a)(2)(B), then with regard to any payment or the provision of any benefit that is considered deferred compensation under Code Section 409A payable on account of a “separation from service,” such payment or benefit shall be made or provided at the date which is the earlier of (i) the expiration of the six (6) month period measured from the date of such “separation from service” of the Executive, and (ii) the date of the Executive’s death (the “Delay Period”). Upon the expiration of the Delay Period, all payments and benefits delayed pursuant to this Section 11(b) (whether they would have otherwise been payable in a single sum or in installments in the absence of such delay) shall be paid or reimbursed to the Executive in a lump sum, and any remaining payments and benefits due under this Agreement shall be paid or provided in accordance with the normal payment dates specified herein.
(c) All expenses or other reimbursements under this Agreement shall be made as soon as practicable and in any event on or prior to the last day of the taxable year following the taxable year in which such expenses were incurred by the Executive (provided that if any such reimbursements constitute taxable income to the Executive, such reimbursements shall be paid no later than March 15th of the calendar year following the calendar year in which the expenses to be reimbursed were incurred), and no such reimbursement or expenses eligible for reimbursement in any taxable year shall in any way affect the expenses eligible for reimbursement in any other taxable year.
|
|
(d) For purposes of Code Section 409A, the Executive’s right to receive any installment payments pursuant to this Agreement shall be treated as a right to receive a series of separate and distinct payments. Whenever a payment under this Agreement specifies a payment period with reference to a number of days (e.g., “payment shall be made within sixty (60) days”), the actual date of payment within the specified period shall be within the sole discretion of the Company.
(e) In no event shall any payment under this Agreement that constitutes “deferred compensation” for purposes of Code Section 409A be offset by any other payment pursuant to this Agreement or otherwise.
12. Representations and Warranties. The Executive represents and warrants to the Company and its Subsidiaries that: (a) the Executive is not a party to or bound by any employment, noncompete, nonsolicitation, or similar agreement with any other Person; (b) the Executive is not a party to or bound by any nondisclosure, confidentiality or similar agreement with any other Person that would affect the Executive’s ability to perform his responsibilities on behalf of the Company; and (c) this Agreement constitutes a valid and legally binding obligation of the Executive, enforceable against him in accordance with its terms. The Company represents that this Agreement constitutes a valid and legally binding obligation of the Company, enforceable against it in accordance with its terms. All representations and warranties contained herein will survive the execution and delivery of this Agreement.
13. Certain Definitions. When used in this Agreement, the following terms will have the following meanings:
“Affiliate” means, with respect to any Person, any other Person that, directly or indirectly, through one or more of its intermediaries, controls, is controlled by or is under common control with such Person.
“Cause” means any one or more of the following: (i) in the reasonable judgment of the Board, (A) the Executive acts (including a failure to act) in a manner that constitutes gross misconduct or gross negligence or that is otherwise materially injurious to the Company or its Subsidiaries; (ii) the Executive breaches any material term of this Agreement, which breach remains uncured to the reasonable satisfaction of the Board following ten (10) days’ written notice from the Company of such breach; (iii) the Executive has committed an act of fraud or misappropriation, or other act of dishonesty or illegal business practices relating to the Company or any of its Subsidiaries, customers or suppliers; (iv) the Executive’s commission of any act which, if the Executive were convicted, would constitute a felony, a crime of moral turpitude or a crime involving the illegal use of drugs, or the Executive’s entry of a plea of guilty or no contest thereto; (v) the Executive’s willful, material and continued failure or willful refusal to perform specific, lawful directives of the Board; (vi) any alcohol or other substance abuse on the part of the Executive that adversely impacts his ability to perform the material functions of his position and is materially injurious to the Company; (vii) any excessive absence of the Executive from his employment during normal working hours for reasons other than vacation or Disability that is materially injurious to the Company; (viii) the Executive’s breach of any other material obligation under this Agreement; or (ix) any material misrepresentation on the Executive’s part herein set forth. Notwithstanding the foregoing, if any act or omission described in the above definition of “Cause” is susceptible to cure (as determined in the reasonable discretion of the Board), the Executive shall have ten (10) days after notice from the Board to cure such violation to the reasonable satisfaction of the Board. Any notice to the Executive of termination for “Cause” shall be in writing and shall specify in reasonable detail the Executive’s acts or omissions that the Company considers to be “Cause”, provided however, that the Company will provide Executive a three (3) day period to review the notice and, in the sole discretion of the Company, may allow the Executive to suggest any reasonable modifications to the notice. No act or failure to act by the Executive shall be considered “willful” unless it is done or omitted to be done by the Executive in bad faith and without reasonable belief that he was acting in the best interests of the Company.
|
|
“CEO” means the Chief Executive Officer of the Company.
“Change in Control” means any one or more of the following events, which shall be deemed to occur on the date of such event: (A) any person or group (other than the Company) (I) acquires more than fifty percent (50%) of the corporate authority to direct the operations of the Company (including, without limitation, a change in the sole corporate member of the Company to a previously unrelated entity, regardless of whether such change in membership alters the composition of the Board), or (II) acquires (or has acquired during the twelve (12) month period that ends on the date of the most recent acquisition by such person or group) ninety percent (90%) or more of the total gross fair market value of all assets of the Company, determined without consideration of any liabilities associated with such assets acquired; (B) the Board executes an amendment to (or enters into a contractual agreement to amend) the Certificate of Incorporation or Bylaws (or their equivalent) of the Company such that a person or group of persons not previously affiliated with or controlled by the Board has the power, directly or indirectly, to designate or elect more than fifty percent (50%) of the Board; or (C) any merger, reorganization, or other corporate event which results in all or substantially all of the operations and assets of the Company (i.e., seventy-five percent (75%) or more of the Company’s value immediately prior to the merger, reorganization, or other corporate event) being controlled by an entity or person that is not tax-exempt under Section 501(c) of the Code.
“COBRA” means the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended from time to time.
“Disability” has the meaning given to such term under the Company’s long-term disability insurance plan or, if no such plan exists, then “Disability” means that the Executive is unable, due to illness, accident or other physical or mental incapacity, to perform substantially all of his duties and responsibilities (provided that, in any such case, the Executive shall have satisfied such criteria for a period of at least three (3) consecutive months or for at least sixty (60) business days during any six (6) month period).
|
|
“Good Reason” means, without the Executive’s written consent, (i) the assignment to the Executive of duties materially inconsistent with the duties of his title and position and as described on Schedule A hereto or materially diminishes the Executive’s authority, duties or responsibilities in any way or requires him to report to any person or entity other than the Board; (ii) a material reduction in the Executive’s Salary or other benefits, except as part of a Company-wide reduction in compensation and/or benefits for similar employees (provided that the Executive’s reduction is consistent, on a proportional basis, with the reductions imposed on all of the Company’s executive level officers); (iii) the relocation of the Executive to an office more than fifty (50) miles from the Executive’s primary work location as set forth in Section 3; (iv) any other material breach by the Company of the provisions hereof; or (v) the Company experiences a “Change in Control” (as defined in this Section 13) event; provided, however, that none of the foregoing events shall constitute Good Reason unless (1) the Executive gives written notice of termination to the Company specifying the condition or event constituting Good Reason within thirty (30) days of the existence thereof; (2) the Company fails to cure the condition or event constituting Good Reason within thirty (30) days following receipt of the Executive’s notice; and (3) the Executive actually terminates his employment within thirty (30) days of the end of the cure period.
“Person” means an individual, a partnership, a corporation, an association, a limited liability company, a joint stock company, a trust, a joint venture, an unincorporated organization or any other entity (including any governmental entity or any department, agency or political subdivision thereof).
“Subsidiaries” means, with respect to any Person, any corporation, limited liability company, partnership, association or other business entity of which (i) if a corporation, a majority of the total voting power of shares of stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors thereof is at the time owned or controlled, directly or indirectly, by such Person or one (1) or more of the other Subsidiaries of such Person or a combination thereof, or (ii) if a limited liability company, partnership, association or other business entity, a majority of the partnership or other similar ownership interests thereof is at the time owned or controlled, directly or indirectly, by any Person or one (1) or more Subsidiaries of such Person or entity or a combination thereof. For purposes hereof, a Person or Persons will be deemed to have a majority ownership interest in a limited liability company, partnership, association or other business entity if such Person or Persons will be allocated a majority of limited liability company, partnership, association or other business entity gains or losses or will be or control any managing director, managing member, or general partner of such limited liability company, partnership, association or other business entity. Unless stated to the contrary, as used in this Agreement the term Subsidiary means a Subsidiary of the Company.
“Termination Date” means the date on which the Employment Period ends pursuant to Section 5(a).
14. Cooperation in Legal Matters. The Executive will cooperate with the Company and its Subsidiaries during the term of the Executive’s employment and, subject to Executive’s other personal and business obligations, thereafter, with respect to any pending or threatened claim, action, suit, or proceeding, whether civil, criminal, administrative, or investigative (the “Claims”), by being reasonably available to testify on behalf of the Company or any Subsidiaries, and to assist the Company and its Subsidiaries by providing information, meeting and consulting with the Company and its Subsidiaries or their representatives or counsel, as reasonably requested and at the Company’s sole expense. The Executive agrees not to disclose to or discuss with anyone who is not assisting the Company or any Subsidiary with the Claims, other than the Executive’s personal attorney, the fact of or the subject matter of the Claims, except as required by law. The Executive further agrees to maintain the confidences and privileges of the Company and its Subsidiaries, and acknowledges that any such confidences and privileges belong solely to the Company and its Subsidiaries and can only be waived by the Company or any Subsidiary, not the Executive. In the event that the Executive is subpoenaed to testify, or otherwise requested to provide information in any matter relating to the Company or any Subsidiary, the Executive agrees to promptly notify the Company after receipt of such subpoena, summons or request for information, to reasonably cooperate with the Company or any Subsidiary with respect to such subpoena, summons or request for information, and to not voluntarily provide any testimony or information unless required by law or permitted by the Company.
|
|
15. Background Check. The Executive agrees that the Company may undertake a background check with respect to the Executive in connection with the subject matter of this Agreement in such manner as the Company determines to be appropriate. The Company reserves the right to terminate this Agreement in the event the results of the background check are not satisfactory to the Company in its sole discretion. In the event of any such termination, this Agreement shall be deemed null and void.
16. Miscellaneous.
(a) Notices. All notices, demands or other communications to be given or delivered by reason of the provisions of this Agreement will be in writing and will be deemed to have been given (i) on the date of personal delivery to the recipient or an officer of the recipient, (ii) when sent by telecopy or facsimile machine to the number shown below on the date of such confirmed facsimile or telecopy transmission (provided that a confirming copy is sent via overnight mail), or (iii) when properly deposited for delivery by a nationally recognized commercial overnight delivery service, prepaid, or by deposit in the United States mail, certified or registered mail, postage prepaid, return receipt requested. Such notices, demands and other communications will be sent to each party at the address indicated for such party below:
if to the Executive, to:
Robert Paccasassi
___________________
___________________
if to the Company, to:
40 Marcus Drive, Suite One
Melville, New York 11747
Facsimile: (631) 760-8414
Attention: CEO
|
|
with copies, which will not constitute notice to the Company, to:
Certilman Balin Adler & Hyman, LLP
90 Merrick Avenue, 9th Floor
East Meadow, New York 11554
Facsimile: (516) 296-7111
Attention: Fred Skolnik, Esq.
or to such other address or to the attention of such other person as the recipient party has specified by prior written notice to the sending party.
(b) Consent to Amendments. No modification, amendment or waiver of any provision of this Agreement will be effective against any Party hereto unless such modification, amendment or waiver is approved in writing by such Party. No other course of dealing among the Company, the Subsidiaries, and the Executive or any delay in exercising any rights hereunder will operate as a waiver by any of the Parties hereto of any rights hereunder.
(c) Assignability and Binding Effect. This Agreement will be binding upon and inure to the benefit of the Executive and his heirs, legal representatives, executors, administrators or successors, and will be binding upon and inure to the benefit of the Company and its successors and assigns. The Parties acknowledge and agreement that this Agreement is freely assignable by the Company and that the Executive may not assign, transfer, pledge, encumber, hypothecate or otherwise dispose of this Agreement, or any of his rights or obligations hereunder, and any such attempted assignment or disposition shall be null and void and without effect.
(d) Severability. Whenever possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be prohibited by or invalid under applicable law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Agreement.
(e) Headings and Sections. The headings in this Agreement are inserted for convenience only and are in no way intended to describe, interpret, define, or limit the scope, extent or intent of this Agreement or any provision of this Agreement. Unless the context requires otherwise, all references in this Agreement to Sections, Exhibits or Schedules will be deemed to mean and refer to Sections, Exhibits or Schedules of or to this Agreement.
(f) Governing Law. All issues and questions concerning the application, construction, validity, interpretation and enforcement of this Agreement and any exhibits and schedules to this Agreement shall be governed by, and construed in accordance with, the laws of the State of New York, without giving effect to any choice of law or conflict of law rules or provisions that would cause the application of the laws of any jurisdiction other than the State of New York.
|
|
(g) Waiver of Jury Trial. EACH PARTY TO THIS AGREEMENT HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR (II) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES HERETO IN RESPECT OF THIS AGREEMENT, IN EACH CASE WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. EACH PARTY TO THIS AGREEMENT HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION WILL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES TO THIS AGREEMENT MAY FILE AN ORIGINAL COUNTERPART OR A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY.
(h) Submission to Jurisdiction. ANY AND ALL SUITS, LEGAL ACTIONS OR PROCEEDINGS ARISING OUT OF THIS AGREEMENT WILL BE BROUGHT IN THE COURTS OF THE STATE OF NEW YORK OR THE UNITED STATES DISTRICT COURT IN THE EASTERN DISTRICT OF NEW YORK, AND EACH PARTY HEREBY SUBMITS TO AND ACCEPTS THE EXCLUSIVE JURISDICTION OF SUCH COURTS FOR THE PURPOSE OF SUCH SUITS, LEGAL ACTIONS OR PROCEEDINGS. TO THE FULLEST EXTENT PERMITTED BY LAW, EACH PARTY HERETO HEREBY IRREVOCABLY WAIVES ANY OBJECTION WHICH IT MAY NOW OR HEREAFTER HAVE TO THE LAYING OF VENUE OR ANY SUCH SUIT, LEGAL ACTION OR PROCEEDING IN ANY SUCH COURT AND HEREBY FURTHER WAIVES ANY CLAIM THAT ANY SUIT, LEGAL ACTION OR PROCEEDING BROUGHT IN ANY SUCH COURT HAS BEEN BROUGHT IN AN INCONVENIENT FORUM.
(i) Service of Process. WITH RESPECT TO ANY AND ALL SUITS, LEGAL ACTIONS OR PROCEEDINGS ARISING OUT OF THIS AGREEMENT, EACH PARTY WAIVES PERSONAL SERVICE OF ANY SUMMONS, COMPLAINT OR OTHER PROCESS AND AGREES THAT SERVICE THEREOF MAY BE MADE BY ANY MEANS SPECIFIED FOR NOTICE PURSUANT TO SECTION 14(a).
(j) Confidentiality. The Parties agree that this Agreement and the Release (if and when executed) are confidential and each Party agrees not to disclose any information regarding the terms of this Agreement or the Release to any Person, except that the Company may disclose information regarding the terms of this Agreement or the Release to its Affiliates and any lenders or as required by law or regulation or the rules of any stock exchange or market on which the Company’s securities are listed or traded, and the Executive may disclose information regarding the terms of this Agreement or the Release to his immediate family. Each Party may also disclose this information to its tax, legal or other counsel. Each Party shall instruct each of the foregoing not to disclose the same to anyone.
|
|
(k) No Strict Construction. The Parties hereto have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement will be construed as if drafted jointly by the Parties hereto, and no presumption or burden of proof will arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement.
(l) Entire Agreement. Except as otherwise expressly set forth in this Agreement, this Agreement and the other agreements referred to in this Agreement embody the complete agreement and understanding among the Parties to this Agreement with respect to the subject matter of this Agreement, and supersede and preempt any prior understandings, agreements, or representations by or among the Parties or their predecessors, written or oral, that may have related to the subject matter of this Agreement in any way. This Agreement will be deemed effective on the date hereof upon the execution hereof.
(m) Time. Whenever the last day for the exercise of any privilege or the discharge or any duty hereunder falls upon a day that is not a business day, the Party having such privilege or duty may exercise such privilege or discharge such duty on the next succeeding day that is a business day.
(n)
Certain Terms. The use of the word “including” herein means “including
without limitation.” Any definitions used herein defined in the plural will be deemed to include the singular as the context may
require and any definitions used herein defined in the singular will be deemed to include the plural as the context may require. References
to “Dollars”
or “$” are references to the lawful currency of the United States of America.
[Remainder of page intentionally left blank. Signature page follows.]
|
|
IN WITNESS WHEREOF, the Parties hereto have executed this Executive Employment Agreement as of the date first written above.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| Chief Executive Officer | ||
| ROBERT PACCASASSI | ||
SCHEDULE A
| Position: | Vice President of Quality Assurance / Regulatory Compliance |
| Per Annum Salary: | $175,000, less applicable taxes and withholdings. |
| Responsibilities: | Executive will be primarily responsible for the areas set forth below, subject to the direction of the CEO: |
| ● | Identify, develop and oversee governing Quality Policies, Standards and Procedures covering GMP requirements within the Company |
| ● | Provide input regarding cGMP compliance risks and risk mitigation strategies |
| ● | Executive oversight over Quality Assurance/ Regulatory Compliance activities supporting clinical/ commercial products and product operations |
| ● | Attending in-person and virtual regulatory meetings |
| ● | Propose and develop strategies to facilitate regulatory approval for clinical use of the Company’s product pipeline |
| Report to: | CEO |
| Vacation: | Three (3) weeks |
| Bonus Performance Criteria: | By March 31, 2022 |
| ● | Manufacturing: Clean room qualification/certification completed. Vendor contracts completed/selected for product release. Media fill completed. Engineering runs completed. |
| ● | Clinical: BRTX-100 clinical program site selection complete, IRB/DSMB structure defined, In-house GCP SOPs in place. Ready for late Q2 FPI. |
| ● | QA: QMS completed/batch review process in place. |
| ● | Pre-clinical: Path forward defined for handoff of lead pre-clinical indication toward Phase I IND initiation. IND team meetings initiated in-house. |
| By June 30, 2022 |
| ● | Manufacturing: Production of initial 10 subjects initiated |
| ● | Clinical: BRTX-100 clinical program initiated - no critical /major clinical deviations identified with regard to FPI. |
| ● | QA: Initiate GLP QA quality procedures (for external studies). Critical Quality and Process parameters defined/management review process defined and initiated (quarterly reporting) |
| ● | Pre-clinical: IND CMC draft defined for first pre-clinical candidate/IND team meetings ongoing. Second IND timeline defined. IND section key person(s) responsibilities defined and internal/ external development of key sections initiated. |
| ● | Organizational Structure: Identify minimum requirements for second clinical program support/obtain go/no go decision. |
| By December 31, 2022 |
| ● | Manufacturing: BRTX-100 production ongoing. Pathway for changeover to next clinical program (production train) defined. Clinical manufacturing production scheme defined for next early stage candidate. |
| ● | QA: Define path forward for in-house QA/QC structure for two or more clinical programs in 2022 and beyond. |
| ● | Pre-clinical: Regulatory path defined for Phase 1 IND. Initiate dossier design team in-house/external regulatory support for Phase 1. CMC first pass draft defined. |
| ● | Organizational Structure: Provide Quality/Tech Ops expanded structure for support of two or more clinical programs targeted for 2022 and beyond. |
| ● | Regulatory: Revisit expedited review process timeline in support of clinical data - provide proposal for submission to Food and Drug Administration for expedited review for Phase III data, as feasible. |
EXHIBIT A
GENERAL RELEASE
I, Robert Paccasassi, in consideration of and subject to the performance by BioRestorative Therapies, Inc., a Delaware corporation (the “Company”), of its obligations under the Executive Employment Agreement by and between the Company and myself, dated as of December XX, 2021 (as amended from time to time, the “Agreement”), do hereby release and forever discharge as of the date hereof, (i) the Company and (ii) each of its subsidiaries, affiliates and predecessors (including, without limitation, and to the extent that they could be liable in respect of their position with any of the foregoing, each of the present and former managers, directors, officers, direct or indirect equity holders, agents, representatives, employees, subsidiaries, affiliates, predecessors, successors, assigns, beneficiaries, heirs, executors, insurers, personal representatives, and attorneys of the parties referenced in clauses (i) and (ii) above) (collectively, the “Released Parties”) to the extent provided below.
1. I understand that any payments or benefits paid or granted to me under Section 5(b) of the Agreement represent, in part, consideration for signing this General Release and are not salary, wages or benefits to which I was already entitled. I understand and agree that I will not receive the payments and benefits specified in Section 5(b) of the Agreement if I execute this General Release and revoke this General Release within the time period permitted hereafter, if I do not execute the General Release in the requested time frame, or if I breach this General Release or anything in the Agreement. I also acknowledge and represent that I have received all payments and benefits the payment and provision of which were due to me, as of the date hereof, by virtue of my employment by the Company.
2. Except as provided in Paragraph 4 below, I knowingly and voluntarily (for myself, my heirs, executors, administrators and assigns) release and forever discharge the Released Parties from any and all claims, suits, controversies, actions, causes of action, cross-claims, counter-claims, demands, debts, compensatory damages, liquidated damages, punitive or exemplary damages, other damages, claims for costs and attorneys’ fees, or liabilities of any nature whatsoever in law and in equity, both past and present (through the date this General Release becomes effective and enforceable) and whether known or unknown, suspected, or claimed against any of the Released Parties which I, my spouse, or any of my heirs, executors, administrators or assigns, may have, which arise out of or are connected with my employment with, or my separation or termination from, the Company (including, but not limited to, any allegation, claim or violation, arising under: Title VII of the Civil Rights Act of 1964, as amended; the Civil Rights Act of 1991; the Age Discrimination in Employment Act of 1967, as amended (including the Older Workers Benefit Protection Act); the Equal Pay Act of 1963, as amended; the Americans with Disabilities Act of 1990; the Family and Medical Leave Act of 1993; the Worker Adjustment Retraining and Notification Act; the Employee Retirement Income Security Act of 1974; any applicable Executive Order Programs; the Fair Labor Standards Act; or their state or local counterparts; or under any other federal, state or local civil or human rights law, or under any other federal, state or local law, regulation or ordinance; or under any public policy, contract or tort, or under common law; or arising under any policies, practices or procedures of the Company or any of its affiliates; or any claim for wrongful discharge, breach of contract, infliction of emotional distress or defamation; or any claim for costs, fees, or other expenses, including attorneys’ fees incurred in these matters) (all of the foregoing collectively referred to herein as the “Claims”). Notwithstanding any other provision of this General Release to the contrary, this General Release does not encompass, and I do not release, waive or discharge the obligations of any of the Released Parties (a) to make the payments and provide the other benefits contemplated by the Agreement, (b) under any restricted stock agreement, option agreement or other agreement pertaining to my equity ownership, (c) under any indemnification or similar agreement with me.
3. I represent that I have made no assignment or transfer of any right, claim, demand, cause of action, or other matter covered by Paragraph 2 above.
4. I agree that this General Release does not waive or release any rights or claims that I may have under the Age Discrimination in Employment Act of 1967 which arise after the date I execute this General Release. I acknowledge and agree that my separation from employment with the Company in compliance with the terms of the Agreement shall not serve as the basis for any claim or action (including, without limitation, any claim under the Age Discrimination in Employment Act of 1967).
5. In signing this General Release, I acknowledge and intend that it shall be effective as a bar to each and every one of the Claim(s) hereinabove mentioned or implied. I expressly consent that this General Release shall be given full force and effect according to each and all of its express terms and provisions, including those relating to unknown and unsuspected Claims (notwithstanding any state statute that expressly limits the effectiveness of a general release of unknown, unsuspected and unanticipated Claims), if any, as well as those relating to any other Claims hereinabove mentioned or implied. I acknowledge and agree that this waiver is an essential and material term of this General Release and that without such waiver the Company would not have agreed to the terms of the Agreement. I further agree that in the event I should bring a Claim seeking damages against any Released Party, or in the event I should seek to recover damages against any Released Party in any Claim brought by a governmental agency on my behalf, this General Release shall serve as a complete defense to such Claim and I shall waive and not accept any monetary award or value in conjunction with such Claim. I further agree that I have not filed and am not aware of any pending Claim as of the execution of this General Release.
6. I agree that neither this General Release, nor the furnishing of the consideration for this General Release, shall be deemed or construed at any time to be an admission by the Company, any Released Party or myself of any improper or unlawful conduct.
7. Nothing in this Agreement shall be construed to preclude me from participating or cooperating in any investigation or proceeding conducted by the Equal Employment Opportunity Commission or any other state or federal administrative agency. However, in the event that a charge or complaint is filed against the Released Parties, or any of them, with any administrative agency or in the event of an authorized investigation, charge or lawsuit filed against the Released Parties, or any of them, by any administrative agency, I expressly waive and shall not accept any award or damages therefrom.
8. Notwithstanding anything in this General Release to the contrary, this General Release shall not relinquish, diminish, or in any way affect any of my rights or claims arising out of any breach by the Company after the date hereof of the Agreement if and to the extent those rights, in each case by their specific terms, survive termination of my employment with the Company.
9. Whenever possible, each provision of this General Release shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this General Release is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other provision or any other jurisdiction, but this General Release shall be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision had never been contained herein. However, should Section 2 of this General Release be declared or determined by any tribunal, administrative agency or court of competent jurisdiction to be illegal or invalid, and should I thereupon seek to institute any Claims that would have been within the scope of Section 2, the Company shall be entitled to immediate repayment, and I shall immediately return, all of the severance payments that I have received, and the Company shall not be obligated to make any further severance payments.
10. BY SIGNING THIS GENERAL RELEASE, I REPRESENT AND AGREE THAT:
A. I HAVE READ IT CAREFULLY;
B. I UNDERSTAND ALL OF ITS TERMS AND KNOW THAT I AM GIVING UP IMPORTANT RIGHTS, INCLUDING BUT NOT LIMITED TO, RIGHTS UNDER THE AGE DISCRIMINATION IN EMPLOYMENT ACT OF 1967, AS AMENDED, TITLE VII OF THE CIVIL RIGHTS ACT OF 1964, AS AMENDED, THE EQUAL PAY ACT OF 1963, THE AMERICANS WITH DISABILITIES ACT OF 1990, AND THE EMPLOYEE RETIREMENT INCOME SECURITY ACT OF 1974, AS AMENDED;
C. I VOLUNTARILY CONSENT TO EVERYTHING IN IT;
D. I HAVE BEEN ADVISED TO CONSULT WITH AN ATTORNEY BEFORE EXECUTING IT AND I HAVE DONE SO OR, AFTER CAREFUL READING AND CONSIDERATION I HAVE CHOSEN NOT TO DO SO OF MY OWN VOLITION;
E. I HAVE BEEN ADVISED I HAVE TWENTY-ONE (21) CALENDAR DAYS TO REVIEW THIS GENERAL RELEASE;
F. I AGREE THAT ANY MODIFICATIONS, MATERIAL OR OTHERWISE, MADE TO THIS GENERAL RELEASE DO NOT RESTART OR AFFECT IN ANY MANNER THE ORIGINAL TWENTY-ONE (21) CALENDAR DAY CONSIDERATION PERIOD;
G. I UNDERSTAND THAT I HAVE SEVEN (7) DAYS AFTER THE EXECUTION OF THIS GENERAL RELEASE TO REVOKE IT AND THAT THIS GENERAL RELEASE SHALL NOT BECOME EFFECTIVE OR ENFORCEABLE UNTIL THE REVOCATION PERIOD HAS EXPIRED. IN ORDER TO REVOKE, I AGREE THAT I MUST DELIVER A WRITTEN LETTER OF REVOCATION TO THE COMPANY (ATTN: SECRETARY, 40 MARCUS DRIVE, SUITE ONE, MELVILLE, NEW YORK 11747) BEFORE THE EXPIRATION OF THE REVOCATION PERIOD;
H. I HAVE SIGNED THIS GENERAL RELEASE KNOWINGLY AND VOLUNTARILY AND WITH THE ADVICE OF ANY COUNSEL RETAINED TO ADVISE ME WITH RESPECT TO IT; AND
I. I AGREE THAT THE PROVISIONS OF THIS GENERAL RELEASE MAY NOT BE AMENDED, WAIVED, CHANGED OR MODIFIED EXCEPT BY AN INSTRUMENT IN WRITING SIGNED BY AN AUTHORIZED REPRESENTATIVE OF THE COMPANY AND BY ME.
DATE:____________________________
_________________________________
ROBERT PACCASASSI
Exhibit 10.36
INCENTIVE
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Incentive Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and Lance Alstodt (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 438,596 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be an Incentive Stock Option within the meaning of Section 422 of the Code to the maximum extent permitted by Applicable Law, although the Company makes no representation or guarantee that the Option will qualify as an Incentive Stock Option.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable with respect to 50% of the Option Shares on the Grant Date, with the remainder vesting quarterly in eight nearly equal installments (at a rate of 1/16 per quarter) with the first quarterly installment vesting on the one-year anniversary of the Grant Date and continuing every three months thereafter until fully vested.
In the event of the Participant’s involuntary Termination of Service by the Company without Cause, 100% of the Shares subject to the Option shall become immediately vested and exercisable. Upon the Participant’s Termination of Service for any other reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan. The Expiration Date shall not be more than ten years from the Grant Date (or, if the Option is being granted to a Greater Than 10% Stockholder, not more than five years from the Grant Date). To the extent the Expiration Date listed above is inconsistent with this paragraph, this paragraph shall control.
2.3 The Option shall not be subject to Section 9.6(a) of the Plan.
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option at any time until the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 24 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 24 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
|
|
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
|
|
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash-Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
|
|
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
10. Qualification as an Incentive Stock Option. It is understood that this Option is intended to qualify as an incentive stock option as defined in Section 422 of the Code to the extent permitted under Applicable Law. Accordingly, the Participant understands that in order to obtain the benefits of an incentive stock option, no sale or other disposition may be made of Shares for which incentive stock option treatment is desired within one (1) year following the date of exercise of the Option or within two (2) years from the Grant Date. The Participant understands and agrees that the Company shall not be liable or responsible for any additional tax liability the Participant incurs in the event that the Internal Revenue Service for any reason determines that this Option does not qualify as an incentive stock option within the meaning of the Code.
11. Disqualifying Disposition. If the Participant disposes of the Shares of Common Stock prior to the expiration of either two (2) years from the Grant Date or one (1) year from the date the Shares are transferred to the Participant pursuant to the exercise of the Option (a “Disqualifying Disposition”), the Participant shall notify the Company in writing within thirty (30) days after such disposition of the date and terms of such disposition. The Participant also agrees to provide the Company with any information concerning any such dispositions as the Company requires for tax purposes.
12. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
13. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
14. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
|
|
15. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
16. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
17. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
18. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
19. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
20. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
21. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
22. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
23. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
***Signature Page to Follow***
|
|
***Signature Page to Incentive Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Robert Kristal | ||
| CFO | ||
| PARTICIPANT: | |
| Lance Alstodt |
|
|
Exhibit 10.37
INCENTIVE
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Incentive Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and Francisco Silva (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 394,737 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be an Incentive Stock Option within the meaning of Section 422 of the Code to the maximum extent permitted by Applicable Law, although the Company makes no representation or guarantee that the Option will qualify as an Incentive Stock Option.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable with respect to 50% of the Option Shares on the Grant Date, with the remainder vesting quarterly in eight nearly equal installments (at a rate of 1/16 per quarter) with the first quarterly installment vesting on the one-year anniversary of the Grant Date and continuing every three months thereafter until fully vested.
In the event of the Participant’s involuntary Termination of Service by the Company without Cause, 100% of the Shares subject to the Option shall become immediately vested and exercisable. Upon the Participant’s Termination of Service for any other reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan. The Expiration Date shall not be more than ten years from the Grant Date (or, if the Option is being granted to a Greater Than 10% Stockholder, not more than five years from the Grant Date). To the extent the Expiration Date listed above is inconsistent with this paragraph, this paragraph shall control.
2.3 The Option shall not be subject to Section 9.6(a) of the Plan.
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option at any time until the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 24 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 24 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
|
|
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
|
|
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash-Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
|
|
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
10. Qualification as an Incentive Stock Option. It is understood that this Option is intended to qualify as an incentive stock option as defined in Section 422 of the Code to the extent permitted under Applicable Law. Accordingly, the Participant understands that in order to obtain the benefits of an incentive stock option, no sale or other disposition may be made of Shares for which incentive stock option treatment is desired within one (1) year following the date of exercise of the Option or within two (2) years from the Grant Date. The Participant understands and agrees that the Company shall not be liable or responsible for any additional tax liability the Participant incurs in the event that the Internal Revenue Service for any reason determines that this Option does not qualify as an incentive stock option within the meaning of the Code.
11. Disqualifying Disposition. If the Participant disposes of the Shares of Common Stock prior to the expiration of either two (2) years from the Grant Date or one (1) year from the date the Shares are transferred to the Participant pursuant to the exercise of the Option (a “Disqualifying Disposition”), the Participant shall notify the Company in writing within thirty (30) days after such disposition of the date and terms of such disposition. The Participant also agrees to provide the Company with any information concerning any such dispositions as the Company requires for tax purposes.
12. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
13. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
14. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
|
|
15. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
16. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
17. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
18. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
19. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
20. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
21. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
22. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
23. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
***Signature Page to Follow***
|
|
***Signature Page to Incentive Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Robert Kristal | ||
| CFO | ||
| PARTICIPANT: | |
| Francisco Silva |
|
|
Exhibit 10.38
INCENTIVE
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Incentive Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and Robert Kristal (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 263,158 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be an Incentive Stock Option within the meaning of Section 422 of the Code to the maximum extent permitted by Applicable Law, although the Company makes no representation or guarantee that the Option will qualify as an Incentive Stock Option.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable with respect to 50% of the Option Shares on the Grant Date, with the remainder vesting quarterly in eight nearly equal installments (at a rate of 1/16 per quarter) with the first quarterly installment vesting on the one-year anniversary of the Grant Date and continuing every three months thereafter until fully vested.
Upon the Participant’s Termination of Service for any reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan. The Expiration Date shall not be more than ten years from the Grant Date (or, if the Option is being granted to a Greater Than 10% Stockholder, not more than five years from the Grant Date). To the extent the Expiration Date listed above is inconsistent with this paragraph, this paragraph shall control.
|
|
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date three months following the Participant’s Termination of Service, or (b) the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Option (whether vested or unvested) shall immediately terminate and cease to be exercisable.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
|
|
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
|
|
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
|
|
10. Qualification as an Incentive Stock Option. It is understood that this Option is intended to qualify as an incentive stock option as defined in Section 422 of the Code to the extent permitted under Applicable Law. Accordingly, the Participant understands that in order to obtain the benefits of an incentive stock option, no sale or other disposition may be made of Shares for which incentive stock option treatment is desired within one (1) year following the date of exercise of the Option or within two (2) years from the Grant Date. The Participant understands and agrees that the Company shall not be liable or responsible for any additional tax liability the Participant incurs in the event that the Internal Revenue Service for any reason determines that this Option does not qualify as an incentive stock option within the meaning of the Code.
11. Disqualifying Disposition. If the Participant disposes of the Shares of Common Stock prior to the expiration of either two (2) years from the Grant Date or one (1) year from the date the Shares are transferred to the Participant pursuant to the exercise of the Option (a “Disqualifying Disposition”), the Participant shall notify the Company in writing within thirty (30) days after such disposition of the date and terms of such disposition. The Participant also agrees to provide the Company with any information concerning any such dispositions as the Company requires for tax purposes.
12. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
13. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
14. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
15. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
|
|
16. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
17. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
18. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
19. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
20. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
21. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
22. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
23. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
|
|
***Signature Page to Incentive Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| President and CEO | ||
| PARTICIPANT: | |
| Robert Kristal |
|
|
Exhibit 10.39
INCENTIVE
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Incentive Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and Robert Paccasassi (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 219,298 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be an Incentive Stock Option within the meaning of Section 422 of the Code to the maximum extent permitted by Applicable Law, although the Company makes no representation or guarantee that the Option will qualify as an Incentive Stock Option.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable with respect to 50% of the Option Shares on the Grant Date, with the remainder vesting quarterly in eight nearly equal installments (at a rate of 1/16 per quarter) with the first quarterly installment vesting on the one-year anniversary of the Grant Date and continuing every three months thereafter until fully vested.
Upon the Participant’s Termination of Service for any reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan. The Expiration Date shall not be more than ten years from the Grant Date (or, if the Option is being granted to a Greater Than 10% Stockholder, not more than five years from the Grant Date). To the extent the Expiration Date listed above is inconsistent with this paragraph, this paragraph shall control.
|
|
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date three months following the Participant’s Termination of Service, or (b) the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Option (whether vested or unvested) shall immediately terminate and cease to be exercisable.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
|
|
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
|
|
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
|
|
10. Qualification as an Incentive Stock Option. It is understood that this Option is intended to qualify as an incentive stock option as defined in Section 422 of the Code to the extent permitted under Applicable Law. Accordingly, the Participant understands that in order to obtain the benefits of an incentive stock option, no sale or other disposition may be made of Shares for which incentive stock option treatment is desired within one (1) year following the date of exercise of the Option or within two (2) years from the Grant Date. The Participant understands and agrees that the Company shall not be liable or responsible for any additional tax liability the Participant incurs in the event that the Internal Revenue Service for any reason determines that this Option does not qualify as an incentive stock option within the meaning of the Code.
11. Disqualifying Disposition. If the Participant disposes of the Shares of Common Stock prior to the expiration of either two (2) years from the Grant Date or one (1) year from the date the Shares are transferred to the Participant pursuant to the exercise of the Option (a “Disqualifying Disposition”), the Participant shall notify the Company in writing within thirty (30) days after such disposition of the date and terms of such disposition. The Participant also agrees to provide the Company with any information concerning any such dispositions as the Company requires for tax purposes.
12. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
13. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
14. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
15. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
|
|
16. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
17. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
18. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
19. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
20. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
21. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
22. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
23. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
|
|
***Signature Page to Incentive Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| President and CEO | ||
| PARTICIPANT: | |
| Robert Paccasassi |
|
|
Exhibit 10.40
NON-QUALIFIED
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Non-Qualified Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and Nickolay Kukekov (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 79,646 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be a Non-Qualified Stock Option and not an Incentive Stock Option within the meaning of Section 422 of the Code.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable in twelve equal monthly installments (at a rate of 1/12 per month) with the first installment vesting on the one-month anniversary of the Grant Date and continuing every month thereafter until fully vested.
Upon the Participant’s Termination of Service for any reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan.
|
|
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date three months following the Participant’s Termination of Service, or (b) the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Option (whether vested or unvested) shall immediately terminate and cease to be exercisable.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
|
|
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
|
|
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
|
|
10. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
11. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
12. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
13. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
14. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
15. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
16. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
|
|
17. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
18. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
19. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
20. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
21. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
***Signature Page to Follow***
|
|
***Signature Page to Non-Qualified Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| President and CEO | ||
| PARTICIPANT: | |
| Nickolay Kukekov |
|
|
Exhibit 10.41
NON-QUALIFIED
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Non-Qualified Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and Patrick F. Williams (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 79,646 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be a Non-Qualified Stock Option and not an Incentive Stock Option within the meaning of Section 422 of the Code.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable in twelve equal monthly installments (at a rate of 1/12 per month) with the first installment vesting on the one-month anniversary of the Grant Date and continuing every month thereafter until fully vested.
Upon the Participant’s Termination of Service for any reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan.
|
|
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date three months following the Participant’s Termination of Service, or (b) the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Option (whether vested or unvested) shall immediately terminate and cease to be exercisable.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
|
|
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
|
|
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
|
|
10. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
11. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
12. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
13. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
14. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
15. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
16. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
|
|
17. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
18. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
19. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
20. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
21. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
***Signature Page to Follow***
|
|
***Signature Page to Non-Qualified Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| President and CEO | ||
| PARTICIPANT: | |
| Patrick F. Williams |
|
|
Exhibit 10.42
NON-QUALIFIED
STOCK OPTION AWARD AGREEMENT
UNDER THE
BIORESTORATIVE THERAPIES, INC.
2021 STOCK INCENTIVE PLAN
This Non-Qualified Stock Option Award Agreement (this “Agreement”) is made and entered into as of February 13, 2024 by and between BioRestorative Therapies, Inc., a Nevada corporation (the “Company”), and David Rosa (the “Participant”).
| Grant Date: | February 13, 2024 |
| Exercise Price per Share: | $1.45 |
| Number of Option Shares: | 79,646 |
| Expiration Date: | February 13, 2034 |
1. Grant of Option.
1.1. Grant; Type of Option. The Company hereby grants to the Participant an option (the “Option”) to purchase the number of Shares of Common Stock of the Company equal to the number of Option Shares set forth above. The Option is being granted pursuant to the terms of the BioRestorative Therapies, Inc. 2021 Stock Incentive Plan (the “Plan”). The Option is intended to be a Non-Qualified Stock Option and not an Incentive Stock Option within the meaning of Section 422 of the Code.
1.2. Consideration; Subject to Plan. The grant of the Option is made in consideration of the services to be rendered by the Participant to the Company and is subject to the terms and conditions of the Plan. Capitalized terms used but not otherwise defined herein have the meanings given to them in the Plan.
2. Exercise Period; Vesting.
2.1 Vesting Schedule. The Option will become vested and exercisable in twelve equal monthly installments (at a rate of 1/12 per month) with the first installment vesting on the one-month anniversary of the Grant Date and continuing every month thereafter until fully vested.
Upon the Participant’s Termination of Service for any reason, the unvested portion of the Option will be forfeited and will not be exercisable.
2.2 Expiration. The Option will expire on the Expiration Date set forth above, or earlier as provided in this Agreement or the Plan.
|
|
3. Termination of Service.
3.1 Termination for Reasons Other Than Cause, Death, Disability. If the Participant has a Termination of Service for any reason other than Cause, death or Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date three months following the Participant’s Termination of Service, or (b) the Expiration Date.
3.2 Termination for Cause. If the Participant has a Termination of Service for Cause, the Option (whether vested or unvested) shall immediately terminate and cease to be exercisable.
3.3 Termination due to Disability. If the Participant has a Termination of Service as a result of the Participant’s Disability, the Participant may exercise the vested portion of the Option, but only within such period of time ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service or (b) the Expiration Date.
3.4 Termination due to Death. If the Participant has a Termination of Service as a result of the Participant’s death, the vested portion of the Option may be exercised by the Participant’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by the person designated to exercise the Option upon the Participant’s death, but only within the time period ending on the earlier of: (a) the date 12 months following the Participant’s Termination of Service, or (b) the Expiration Date.
4. Manner of Exercise.
4.1 Election to Exercise. To exercise the Option, the Participant (or in the case of exercise after the Participant’s death or incapacity, the Participant’s executor, administrator, heir or legatee, as the case may be) must deliver to the Company an executed stock option exercise agreement in such form as is approved by the Committee from time to time (the “Exercise Agreement”), which shall set forth, inter alia:
(a) the Participant’s election to exercise the Option;
(b) the number of Shares of Common Stock being purchased;
(c) any restrictions imposed on the Shares; and
(d) any representations, warranties and agreements regarding the Participant’s investment intent and access to information as may be required by the Company to comply with applicable securities laws.
If someone other than the Participant exercises the Option, then such person must submit documentation reasonably acceptable to the Company verifying that such person has the legal right to exercise the Option.
|
|
4.2 Payment of Exercise Price. The entire Exercise Price of the Option shall be payable in full at the time of exercise to the extent permitted by applicable statutes and regulations, either:
(a) in cash or by certified or bank check at the time the Option is exercised;
(b) by delivery to the Company of other Shares of Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Exercise Price (or portion thereof) due for the number of Shares being acquired, or by means of attestation whereby the Participant identifies for delivery specific Shares that have a Fair Market Value on the date of attestation equal to the Exercise Price (or portion thereof) and receives a number of Shares equal to the difference between the number of Shares thereby purchased and the number of identified attestation Shares (a “Stock for Stock Exchange”);
(c) through a “cashless exercise program” established with a broker;
(d) by reduction in the number of Shares otherwise deliverable upon exercise of such Option with a Fair Market Value equal to the aggregate Exercise Price at the time of exercise;
(e) by any combination of the foregoing methods; or
(f) in any other form of legal consideration that may be acceptable to the Committee.
4.3 Withholding. Prior to the issuance of Shares upon the exercise of the Option, the Participant must make arrangements satisfactory to the Company to pay or provide for any applicable federal, state and local withholding obligations of the Company. The Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the Option by any of the following means:
(a) tendering a cash payment;
(b) authorizing the Company to withhold Shares of Common Stock from the Shares of Common Stock otherwise issuable to the Participant as a result of the exercise of the Option; provided, however, that no Shares of Common Stock are withheld with a value exceeding the maximum amount of tax required to be withheld by law; or
(c) delivering to the Company previously owned and unencumbered Shares of Common Stock.
The Company has the right to withhold from any compensation paid to the Participant.
4.4 Issuance of Shares. Provided that the Exercise Agreement and payment are in form and substance satisfactory to the Company, the Company shall issue the Shares of Common Stock registered in the name of the Participant, the Participant’s authorized assignee, or the Participant’s legal representative which shall be evidenced by stock certificates representing the Shares with the appropriate legends affixed thereto, appropriate entry on the books of the Company or of a duly authorized transfer agent, or other appropriate means as determined by the Company.
|
|
5. No Right to Continued Employment; No Rights as Shareholder. Neither the Plan nor this Agreement shall confer upon the Participant any right to be retained in any position, as an Employee, Consultant or Director of the Company. Further, nothing in the Plan or this Agreement shall be construed to limit the discretion of the Company to terminate the Participant’s employment or service with the Company at any time, with or without Cause. The Participant shall not have any rights as a shareholder with respect to any Shares of Common Stock subject to the Option unless and until certificates representing the Shares have been issued by the Company to the holder of such Shares, or the Shares have otherwise been recorded on the books of the Company or of a duly authorized transfer agent as owned by such holder.
6. Transferability. The Option is only transferable by will or the laws of descent and distribution or in accordance with the limited conditions set forth in Section 9.3(b) of the Plan. No assignment or transfer of the Option, or the rights represented thereby, whether voluntary or involuntary, by operation of law or otherwise (except to a designated beneficiary, upon death, by will or the laws of descent or distribution or pursuant to Section 9.3(b) of the Plan) will vest in the assignee or transferee any interest or right herein whatsoever, but immediately upon such assignment or transfer the Option will terminate and become of no further effect.
7. Change in Control.
7.1 Acceleration of Vesting. Notwithstanding any provision of the Plan or this Agreement to the contrary, in the event of a Change in Control prior to the date that the Option is fully vested and exercisable, the Option shall become immediately vested and exercisable with respect to 100% of the Shares in each remaining vesting tranche. To the extent practicable, such acceleration of vesting and exercisability shall occur in a manner and at a time which allows the Participant the ability to participate in the Change in Control with respect to the Shares of Common Stock received.
7.2 Cash Out. In the event of a Change in Control, the Committee may, in its discretion and upon at least ten (10) days’ advance notice to the Participant, cancel the Option and pay to the Participant the value of the Option based upon the price per Share of Common Stock received or to be received by other shareholders of the Company in the event. Notwithstanding the foregoing, if at the time of a Change in Control the Exercise Price of the Option equals or exceeds the price paid for a Share of Common Stock in connection with the Change in Control, the Committee may cancel the Option without the payment of consideration therefor.
8. Adjustments. The Shares of Common Stock subject to the Option may be adjusted or terminated in any manner as contemplated by the Plan.
9. Tax Liability and Withholding. Notwithstanding any action the Company takes with respect to any or all income tax, social insurance, payroll tax, or other tax-related withholding (“Tax-Related Items”), the ultimate liability for all Tax-Related Items is and remains the Participant’s responsibility and the Company (a) makes no representation or undertakings regarding the treatment of any Tax-Related Items in connection with the grant, vesting, or exercise of the Option or the subsequent sale of any Shares acquired on exercise; and (b) does not commit to structure the Option to reduce or eliminate the Participant’s liability for Tax-Related Items.
|
|
10. Compliance with Law. The exercise of the Option and the issuance and transfer of Shares of Common Stock shall be subject to compliance by the Company and the Participant with all applicable requirements of federal and state securities laws and with all applicable requirements of any stock exchange on which the Company’s Shares of Common Stock may be listed. No Shares of Common Stock shall be issued pursuant to this Option unless and until any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel. The Participant understands that the Company is under no obligation to register the Shares with the Securities and Exchange Commission, any state securities commission or any stock exchange to effect such compliance.
11. Notices. Any notice required to be delivered to the Company under this Agreement shall be in writing and addressed to the Secretary of the Company at the Company’s principal corporate offices. Any notice required to be delivered to the Participant under this Agreement shall be in writing and addressed to the Participant at the Participant’s address as shown in the records of the Company. Either party may designate another address in writing (or by such other method approved by the Company) from time to time.
12. Governing Law. This Agreement will be construed and interpreted in accordance with the laws of the State of Nevada without regard to conflict of law principles.
13. Interpretation. Any dispute regarding the interpretation of this Agreement shall be submitted by the Participant or the Company to the Committee for review. The resolution of such dispute by the Committee shall be final and binding on the Participant and the Company.
14. Options Subject to Plan. This Agreement is subject to the Plan as approved by the Company’s shareholders. The terms and provisions of the Plan as it may be amended from time to time are hereby incorporated herein by reference. In the event of a conflict between any term or provision contained herein and a term or provision of the Plan, the applicable terms and provisions of the Plan will govern and prevail.
15. Successors and Assigns. The Company may assign any of its rights under this Agreement. This Agreement will be binding upon and inure to the benefit of the successors and assigns of the Company. Subject to the restrictions on transfer set forth herein, this Agreement will be binding upon the Participant and the Participant’s beneficiaries, executors, administrators and the person(s) to whom this Agreement may be transferred by will or the laws of descent or distribution.
16. Severability. The invalidity or unenforceability of any provision of the Plan or this Agreement shall not affect the validity or enforceability of any other provision of the Plan or this Agreement, and each provision of the Plan and this Agreement shall be severable and enforceable to the extent permitted by law.
|
|
17. Discretionary Nature of Plan. The Plan is discretionary and may be amended, cancelled or terminated by the Company at any time, in its discretion. The grant of the Option in this Agreement does not create any contractual right or other right to receive any Options or other Awards in the future. Future Awards, if any, will be at the sole discretion of the Company. Any amendment, modification, or termination of the Plan shall not constitute a change or impairment of the terms and conditions of the Participant’s employment with the Company.
18. Amendment. The Committee has the right to amend, alter, suspend, discontinue or cancel the Option, prospectively or retroactively; provided, that, no such amendment shall adversely affect the Participant’s material rights under this Agreement without the Participant’s consent.
19. No Impact on Other Benefits. The value of the Participant’s Option is not part of his or her normal or expected compensation for purposes of calculating any severance, retirement, welfare, insurance or similar employee benefit.
20. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Counterpart signature pages to this Agreement transmitted by facsimile transmission, by electronic mail in portable document format (.pdf), or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing an original signature.
21. Acceptance. The Participant hereby acknowledges receipt of a copy of the Plan and this Agreement. The Participant has read and understands the terms and provisions thereof, and accepts the Option subject to all of the terms and conditions of the Plan and this Agreement. The Participant acknowledges that there may be adverse tax consequences upon exercise of the Option or disposition of the underlying Shares and that the Participant should consult a tax advisor prior to such exercise or disposition.
***Signature Page to Follow***
|
|
***Signature Page to Non-Qualified Stock Option Agreement***
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| BIORESTORATIVE THERAPIES, INC. | ||
| By: | ||
| Lance Alstodt | ||
| President and CEO | ||
| PARTICIPANT: | |
| David Rosa |
|
|
Exhibit 23
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the incorporation by reference in the Registration Statement of BioRestorative Therapies, Inc. on Forms S-3 (Nos. 333-269631, 333-265052 and 333-258611) and S-8 (Nos. 333-255681, 333-196299, 333-203310, 333-210555, 333-214621, 333-228434, 333-233309, 333-270909 and 333-275571), of our report dated March 29, 2024, with respect to our audits of the consolidated financial statements of BioRestorative Therapies, Inc. as of December 31, 2023 and 2022 and for each of the two years ended December 31, 2023, which report is included in this Annual Report on Form 10-K of BioRestorative Therapies, Inc. for the year ended December 31, 2023.
/s/ Marcum llp
Marcum llp
Marlton, New Jersey
March 29, 2024
Exhibit 31.1
SECTION 302 CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
I, Lance Alstodt, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of BioRestorative Therapies, Inc. |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report. |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report. |
| 4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting. |
| 5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| /s/ Lance Alstodt | ||
| Date: March 29, 2024 | Lance Alstodt | |
| Principal Executive Officer |
Exhibit 31.2
SECTION 302 CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER
I, Robert Kristal, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of BioRestorative Therapies, Inc. |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report. |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report. |
| 4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting. |
| 5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| /s/ Robert Kristal | ||
| Date: March 29, 2024 | Robert Kristal | |
| Principal Financial Officer |
Exhibit 32
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
AND PRINCIPAL FINANCIAL OFFICER
PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to 18 U.S.C. § 1350, the undersigned officers of BioRestorative Therapies, Inc. (the “Company”) hereby certify that the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 (the “Report”) fully complies with the requirements of Section 13(a) or 15(d), as applicable, of the Securities Exchange Act of 1934 and that the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| /s/ Lance Alstodt | ||
| Date: March 29, 2024 | Lance Alstodt | |
| Principal Executive Officer | ||
| /s/ Robert Kristal | ||
| Robert Kristal | ||
| Principal Financial Officer |
The foregoing certification is being furnished solely pursuant to 18 U.S.C. § 1350 and is not being filed as part of the Report or as a separate disclosure document.
Exhibit 97
BIORESTORATIVE THERAPIES, INC.
CLAWBACK POLICY
1. Purpose. The Board of Directors (the “Board”) of BioRestorative Therapies, Inc. (the “Company”) has adopted this clawback policy, as amended (the “Clawback Policy”), which describes the circumstances in which Covered Individuals will be required to repay or return Erroneously Awarded Compensation to the Company in the event of an Accounting Restatement in accordance with Clawback Rules issued by the United States Securities and Exchange Commission (the “SEC”) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Nasdaq Stock Market LLC (the “Exchange”). Capitalized terms used and not otherwise defined herein shall have the meanings given in Section 2 or 4 below.
2. Covered Persons. A “Covered Individual” under this Clawback Policy shall mean (i) each individual who served as an Executive Officer at any time during the performance period applicable to the Incentive Compensation, whether or not still employed by the Company (and regardless of whether such Incentive Compensation was received during or after such person’s service as an Executive Officer) and (ii) such other current or former employees of the Company who may from time to time be deemed subject to this Clawback Policy by the Administrator. Each Covered Individual shall be required to sign and return to the Company a Clawback Policy Acknowledgement and Acceptance Form pursuant to which, among other things, such Covered Individual will acknowledge that he or she is bound by the terms of this Clawback Policy; provided, however, that this Clawback Policy shall apply to, and be enforceable against, any Covered Individual and his or her successors (as specified in Section 9 of this Clawback Policy) regardless of whether or not such Covered Individual properly signs and returns to the Company such Clawback Policy Acknowledgement and Acceptance Form and regardless of whether or not such Covered Individual is aware of his or her status as such.
3. Administration. Except as specifically set forth herein, this Clawback Policy shall be administered by the Administrator. Any determinations made by the Administrator shall be final and binding on all affected Covered Individuals and need not be uniform with respect to each Covered Individual. Subject to any limitation under applicable law, the Administrator may authorize and empower any officer or employee of the Company to take any and all actions necessary or appropriate to carry out the purpose and intent of this Clawback Policy (other than with respect to any recovery under this Clawback Policy involving such officer or employee).
4. Definitions. For purposes of this Clawback Policy, the following capitalized terms shall have the meanings set forth below:
| a. | “Accounting Restatement” shall mean an accounting restatement: (i) due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements (a “Big R” restatement); or (ii) that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (a “little r” restatement). |
|
|
| b. | “Administrator” shall mean the Committee. |
| c. | “Clawback Period” shall mean, with respect to any Accounting Restatement, the three completed fiscal years of the Company immediately preceding the Restatement Date and any transition period (that results from a change in the Company’s fiscal year) of less than nine months within or immediately following those three completed fiscal years. |
| d. | “Clawback Rules” shall mean Section 10D of the Exchange Act and any applicable rules or standards adopted by the SEC thereunder (including Rule 10D-1 under the Exchange Act) or the Exchange pursuant to Rule 10D-1 under the Exchange Act (including Nasdaq Stock Market Listing Rule 5608), in each case as may be in effect from time to time. |
| e. | “Committee” shall mean the Compensation Committee of the Board or any of the other committee designated by the Board to administer this Clawback Policy. |
| f. | “Erroneously Awarded Compensation” means, with respect to any Covered Individual, the excess of (i) the Covered Individual’s actual Incentive Compensation over (ii) the Incentive Compensation the Covered Individual otherwise would have earned, become vested in or been granted during the Clawback Period if the Company’s financial statements had reflected the restated amounts in an Accounting Restatement. Erroneously Awarded Compensation will be computed without regard to taxes paid. Erroneously Awarded Compensation shall not include any Incentive Compensation received by a Covered Individual prior to a Covered Individual’s service as an Executive Officer. Calculation of Erroneously Awarded Compensation with respect to Incentive Compensation based on stock price or total shareholder return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement, shall be based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive Compensation was received, and the Company shall maintain documentation of the determination of such reasonable estimate and provide such documentation to the Exchange in accordance with the Clawback Rules. Incentive Compensation is deemed received, earned or vested when the Financial Reporting Measure is attained, not when the actual payment, grant or vesting occurs. Incentive Compensation received by a Covered Individual prior to the Effective Date shall be subject to the terms of this Clawback Policy as if in effect prior to the Effective Date. |
| g. | “Effective Date” shall mean March __, 2024. |
|
|
| h. | “Executive Officer” means the Company’s president, principal financial officer, principal accounting officer (or, if there is no such accounting officer, the controller), any vice-president of the Company in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, or any other person who performs similar policymaking functions for the Company. Executive officers of the Company’s parent(s) or subsidiaries are deemed executive officers of the Company if they perform such policy making functions for the Company. |
| i. | “Financial Reporting Measures” shall mean measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures that are derived wholly or in part from such measures. Stock price and total shareholder return shall for purposes of this Clawback Policy be considered Financial Reporting Measures. For the avoidance of doubt, a Financial Reporting Measure need not be presented within the Company’s financial statements or included in a filing with the SEC. |
| j. | “Incentive Compensation” means any compensation that is granted, earned or vested based wholly or in part upon the attainment, during the Clawback Period, of any Financial Reporting Measure. |
| k. | “Impracticable” shall mean, in accordance with the good faith determination of the Administrator, or if the Administrator does not consist of independent directors, a majority of the independent directors serving on the Board, that either: (i) the direct expenses paid to a third party to assist in enforcing the Clawback Policy against a Covered Individual would exceed the amount to be recovered, after the Company has made a reasonable attempt to recover the applicable Erroneously Awarded Compensation, documented such reasonable attempt(s) and provided such documentation to the Exchange; or (ii) recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder. |
| l. | “Restatement Date” shall mean the earlier to occur of: (i) the date the Board, a committee of the Board or the officer or officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement; or (ii) the date a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement. |
5. Recoupment; Method of Recoupment.
| a. | In the event the Company is required to prepare an Accounting Restatement, the Administrator shall reasonably promptly, in accordance with the applicable Clawback Rules, determine the amount of any Erroneously Awarded Compensation for each Covered Individual in connection with such Accounting Restatement, and shall reasonably promptly thereafter provide each Covered Individual with written notice containing the amount of Erroneously Awarded Compensation and a demand for repayment or return, as applicable. |
|
|
| b. | The method of recovery of Erroneously Awarded Compensation shall be determined by the Administrator and shall include, without limitation: (i) recoupment of cash or shares of Company stock; (ii) forfeiture of unvested awards; (iii) cancellation of outstanding vested awards; (iv) offset of other amounts owed to the Covered Individual; (v) reduction of future compensation; and (vi) any other remedial or recovery action permitted by law. If the Erroneously Awarded Compensation includes shares of Company stock (or derivatives thereof), the Administrator may seek to require that the Covered Individual repay to the Company any dividends or dividend equivalents paid with respect to those securities, and any profits realized, directly or indirectly, from the sale or other disposition of those securities. For the avoidance of doubt, except to the extent permitted pursuant to the Clawback Rules, in no event may the Company accept an amount that is less than the amount of Erroneously Awarded Compensation in satisfaction of a Covered Individual’s obligations hereunder. Notwithstanding anything herein to the contrary, the Company shall not be required to take the actions contemplated in this Section 5(b) if recovery would be Impracticable. In implementing the actions contemplated in this Section 5(b), the Administrator will act in accordance with the listing standards and requirements of the Exchange and with the applicable Clawback Rules. |
| c. | Subject to the discretion of the Administrator, an applicable Covered Individual may be required to reimburse the Company for any and all expenses reasonably incurred (including legal fees) by the Company in recovering Erroneously Awarded Compensation in accordance with this Section 5. |
6. Indemnification Prohibition. No member of the Company shall be permitted to indemnify any Covered Individual against the loss of any Erroneously Awarded Compensation that is repaid, returned or recovered pursuant to the terms of this Clawback Policy and/or pursuant to the Clawback Rules, including any payment or reimbursement for the cost of third-party insurance purchased by any Covered Individual to cover any such loss under this Clawback Policy and/or pursuant to the Clawback Rules. Further, the Company shall not enter into any agreement that exempts any Incentive Compensation from the application of this Clawback Policy or that waives the Company’s right to recovery of any Erroneously Awarded Compensation, and this Clawback Policy shall supersede any such agreement, whether entered into before, on or after the Effective Date). Any such purported indemnification, whether oral or in writing, shall be null and void.
7. Interpretation. The Administrator is authorized to interpret and construe this Clawback Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Clawback Policy. It is intended that this Clawback Policy be interpreted in a manner that is consistent with the requirements of the Clawback Rules. The terms of this Clawback Policy shall also be construed and enforced in such a manner as to comply with applicable law, including the Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, and any other law or regulation that the Administrator determines is applicable. In the event any provision of this Clawback Policy is determined to be unenforceable or invalid under applicable law, such provision shall be applied to the maximum extent permitted by applicable law and shall automatically be deemed amended in a manner consistent with its objectives to the extent necessary to conform to any limitations required by applicable law.
8. Amendment; Termination. The Administrator may modify or amend this Clawback Policy, in whole or in part, from time to time, in its discretion and shall amend any or all of the provisions of this Clawback Policy as it deems appropriate and necessary, including as and when it determines that it is legally required by the Clawback Rules, any federal securities laws, state blue-sky laws, SEC rule or Exchange rule. The Administrator may terminate this Clawback Policy at any time, and this Clawback Policy shall remain in effect only so long as the Clawback Rules apply to the Company. Unless otherwise determined by the Administrator or as otherwise amended, this Clawback Policy shall automatically be deemed amended in a manner necessary to comply with any change in the Clawback Rules.
9. Successors. This Clawback Policy shall be binding and enforceable against all Covered Individuals and their beneficiaries, estates, heirs, executors, administrators or other legal representatives to the extent required by the Clawback Rules or as otherwise determined by the Administrator.
10. Tax Considerations. To the extent that, pursuant to this Clawback Policy, the Company is entitled to recover any Erroneously Awarded Compensation that is received by a Covered Individual, the gross amount received (i.e., the amount the Covered Individual received, or was entitled to receive, before any deductions for tax withholding or other payments) shall be returned by the Covered Individual.
|
|
CLAWBACK POLICY ACKNOWLEDGEMENT AND ACCEPTANCE FORM
The Board of Directors of BioRestorative Therapies, Inc. has adopted a Clawback Policy which is applicable to the Company’s Covered Individuals.
I, the undersigned, acknowledge that I have received a copy of the Clawback Policy, as it may be amended, restated, supplemented or modified from time to time, and that I have read it, understand it, and acknowledge that I am fully bound by, and subject to, all of the terms and conditions thereof.
In the event of any inconsistency between the terms of the Clawback Policy and the terms of any employment agreement to which I am a party, or the terms of any compensation plan, program, or arrangement under which Incentive Compensation has been granted, awarded, earned, or paid to me, whether or not deferred, the terms of the Clawback Policy shall govern.
If the Board of Directors or a committee of the Board of Directors determines that any Incentive Compensation I have received must be forfeited, repaid, or otherwise recovered by the Company, I shall promptly take whatever action is necessary to effectuate such forfeiture, repayment, or recovery.
I acknowledge that I am not entitled to indemnification in connection with the Company’s enforcement of the Clawback Policy.
I understand that any delay or failure by the Company to enforce any requirement contained in the Clawback Policy will not constitute a waiver of the Company’s right to do so in the future. Any capitalized terms used in this Clawback Policy Acknowledgment and Acceptance Form that are not otherwise defined shall have the meanings ascribed to them in the Clawback Policy.
| [Name] | |
| [Date] |
|
|