UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of February 2024
Commission File Number: 001-38064
Aeterna Zentaris Inc.
(Translation of registrant’s name into English)
c/o
Norton Rose Fulbright Canada, LLP,
222 Bay Street, Suite 3000,
PO Box 53, Toronto ON M5K 1E7, Canada
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐

On February 15, 2024, Aeterna Zentaris Inc. (“Aeterna”) published a Notice of Special Meeting of Shareholders and Management Proxy Circular with respect to its previously announced intent to consummate a Plan of Arrangement (the “Plan of Arrangement”) with Ceapro Inc. (“Ceapro”) pursuant to an Arrangement Agreement, dated as of December 14, 2023 (the “Arrangement Agreement”), between Aeterna and Ceapro. A copy of the Notice of Special Meeting of Shareholders and Management Proxy Circular is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
On the same day, Aeterna published a news release related to the Special Meeting of Shareholders and the Management Proxy Circular. A copy of the news release is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
This Report on Form 6-K and Exhibit 99.1 included with this Report on Form 6-K are hereby incorporated by reference into Aeterna’s Registration Statements on Forms S-8 (No. 333-224737, No. 333-210561 and No. 333-200834) and Form F-3 (No. 333-254680) (collectively, the “Registration Statements”) and shall be deemed to be a part thereof from the date on which this Report on Form 6-K is furnished, to the extent not superseded by documents or reports subsequently filed or furnished. The information contained on the websites referenced in Exhibit 99.1 included with this Report on Form 6-K is not incorporated by reference or deemed to be a part of this Report on Form 6-K or any of the Registration Statements.
Aeterna has filed a Registration Statement on Form F-1 (including a prospectus) (File No. 333-277115) (the “Form F-1 Registration Statement”) with the U.S. Securities and Exchange Commission (the “SEC”) on February 15, 2024 for the issuance of common share purchase warrants and common shares issuable upon exercise thereof in connection with the Plan of Arrangement, but it has not yet become effective. The common share purchase warrants and common shares issuable upon the exercise thereof may not be sold nor may offers to buy them be accepted prior to the time the Form F-1 Registration Statement becomes effective. Before you invest, you should read the prospectus in the Form F-1 Registration Statement and the other documents incorporated by reference therein for more complete information about Aeterna, Ceapro, the Plan of Arrangement and the common share purchase warrant offering.
You may get copies of the Form F-1 Registration Statement for free by visiting EDGAR on the SEC website at www.sec.gov or at SEDAR+ at www.sedarplus.ca. Alternatively, you may obtain copies of them by contacting the following:
Aeterna’s Proxy Solicitor
Kingsdale Advisors
1-866-581-1513 (North American Toll Free) or
416-623-2513 (Outside North America — text and call enabled)
contactus kingsdaleadvisors.corn
www.AEZSmerger.com
Media Contact
Joel Shaffer
FGS Longview
joel.shaffer@fgslongview.comr
416-670-6468
| ( |
No Offer or Solicitation
This Report on Form 6-K and the exhibits attached hereto and incorporated by reference herein, and the information contained herein and therein are not, and do not, constitute an offer to sell any securities or a solicitation of an offer to buy any securities in the United States or any other state or jurisdiction, nor shall any securities of Aeterna be offered or sold in any jurisdiction in which such an offer, solicitation or sale would be unlawful. Neither the SEC nor any state securities commission has approved or disapproved of the transactions described herein or determined if this communication is truthful or complete. Any representation to the contrary is a criminal offense.
You should not construe the contents of this communication as legal, tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein.
Forward-Looking Statements
The information in this Report on Form 6-K and the exhibits attached hereto and incorporated herein by reference include forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, specifically Section 27A of the U.S. Securities Act of 1933, as amended, and Section 21E of the U.S. Securities Exchange Act of 1934, as amended. These forward-looking statements involve a number of known and unknown risks, uncertainties and other factors that could actual results and outcomes to be materially different from historical results or from any future results expressed or implied by such forward-looking statements.
Forward-looking statements include, but are not limited to, the ability of Aeterna and Ceapro to successfully consummate the Plan of Arrangement pursuant to the Arrangement Agreement within the time expected or at all and, if completed, the anticipated benefits and synergies as well as the assets, cost structure, financial position, cash flows and growth prospects of the combined company.
Risks and factors that could cause actual results or outcomes to differ materially from expectations include, among others, the following:
| ● | the failure of Aeterna or Ceapro to receive shareholder approval; | |
| ● | the failure of Aeterna or Ceapro to obtain regulatory approvals and securities exchange approvals, including from the Nasdaq and the TSX; | |
| ● | Aeterna’s ability to raise capital and obtain financing to continue its currently planned operations; | |
| ● | Aeterna’s ability to maintain compliance with the continued listing requirements of the NASDAQ and to maintain the listing of its Common Shares on the NASDAQ; | |
| ● | Aeterna’s ability to continue as a going concern, which is dependent, in part, on its ability to transfer cash from Aeterna Zentaris GmbH to Aeterna and its U.S. subsidiary and to secure additional financing; | |
| ● | Aeterna’s now heavy dependence on the success of Macrilen™ (macimorelin) and related out-licensing arrangements and the continued availability of funds and resources to successfully commercialize the product, including its heavy reliance on the success of the license and assignment agreement with Novo Nordisk A/S; | |
| ● | Aeterna’s ability to enter into out-licensing, development, manufacturing, marketing and distribution agreements with other pharmaceutical companies and keep such agreements in effect; | |
| ● | Aeterna’s reliance on third parties for the manufacturing and commercialization of Macrilen™ (macimorelin); | |
| ● | potential disputes with third parties, leading to delays in or termination of the manufacturing, development, out-licensing or commercialization of Aeterna’s product candidates, or resulting in significant litigation or arbitration; | |
| ● | uncertainties related to the regulatory process; | |
| ● | unforeseen global instability, including the instability due to the global pandemic of the novel coronavirus; | |
| ● | Aeterna’s ability to efficiently commercialize or out-license Macrilen™ (macimorelin); | |
| ● | Aeterna’s reliance on the success of the pediatric clinical trial in the European Union (“E.U.”) and U.S. for Macrilen™ (macimorelin); | |
| ● | the degree of market acceptance of Macrilen™ (macimorelin); | |
| ● | Aeterna’s ability to obtain necessary approvals from the relevant regulatory authorities to enable it to use the desired brand names for its product; |
| ( |
| ● | Aeterna’s ability to successfully negotiate pricing and reimbursement in key markets in the E.U. for Macrilen™ (macimorelin); | |
| ● | any evaluation of potential strategic alternatives to maximize potential future growth and shareholder value may not result in any such alternative being pursued, and even if pursued, may not result in the anticipated benefits; | |
| ● | Aeterna’s ability to protect its intellectual property; and | |
| ● | the potential of liability arising from shareholder lawsuits and general changes in economic conditions. |
Additional risk factors that could cause actual results to differ materially include those risks identified in Item 3. “Key Information – Risk Factors” contained in Aeterna’s most recent Annual Report on Form 20-F filed with the SEC and its other filings and submissions from time to time, including those containing its quarterly and annual results, with the SEC, which are available on Aeterna’s website located at www.aeterna.com.
Many of these risks and factors are beyond Aeterna’s control. Aeterna cautions you not to place undue reliance on these forward-looking statements. All written and oral forward-looking statements attributable to Aeterna and/or Ceapro, or persons acting on their behalf, are qualified in their entirety by these cautionary statements. Moreover, unless required by law to update these statements, Aeterna will not necessarily update any of these statements after the date hereof, either to conform them to actual results or to changes in their expectation.
DOCUMENTS INDEX
| Exhibit | Description | |
| 99.1 | Notice of Special Meeting of Shareholders and Management Proxy Circular | |
| 99.2 | News Release, dated February 15, 2024 |
| ( |

SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AETERNA ZENTARIS INC. | ||
| Date: February 16, 2024 | By: | /s/ Giuliano La Fratta |
| Giuliano La Fratta | ||
| Chief Financial Officer | ||
| ( |
Exhibit 99.1






































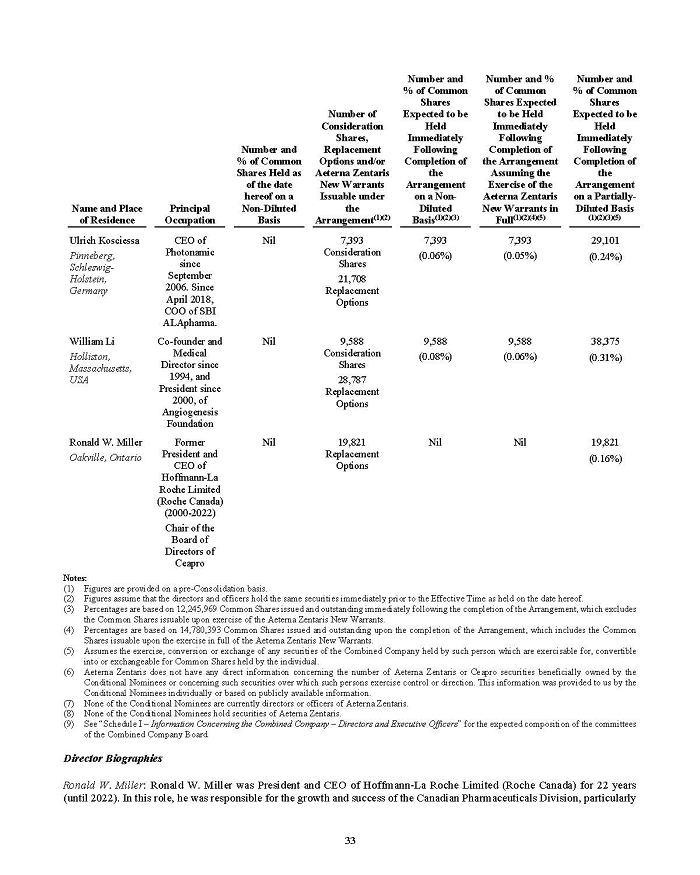



























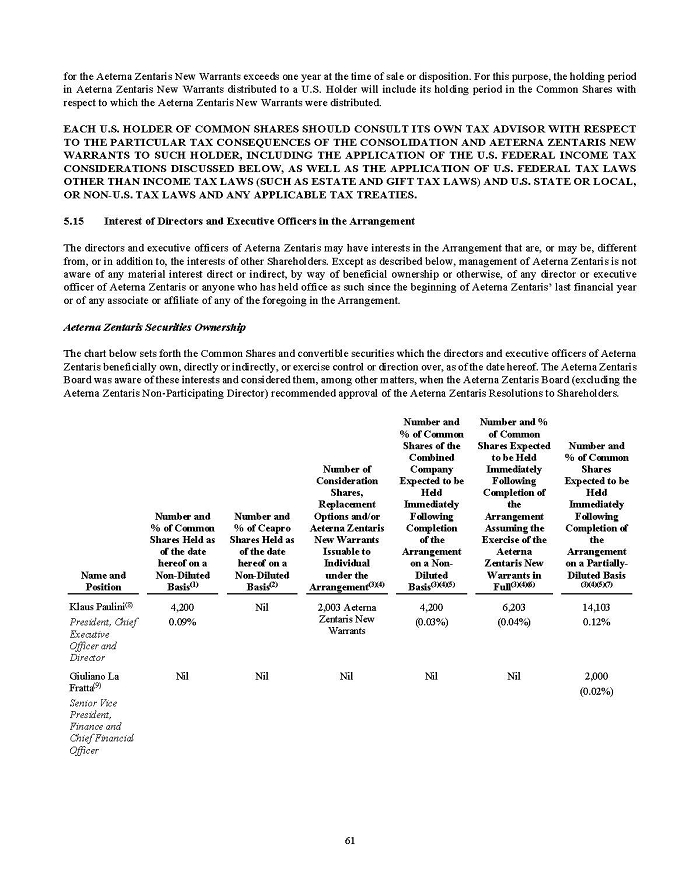
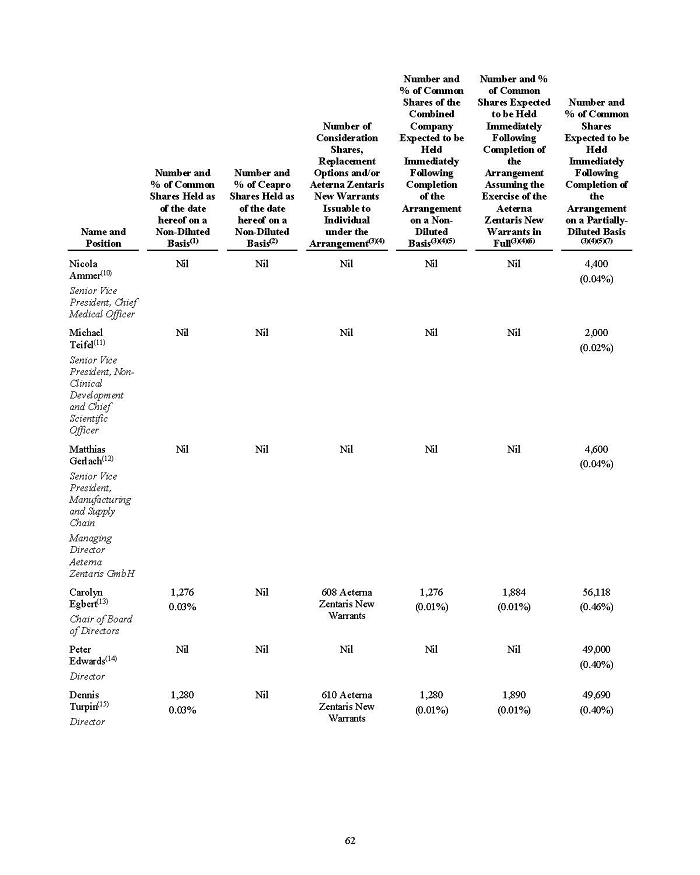



















































































































































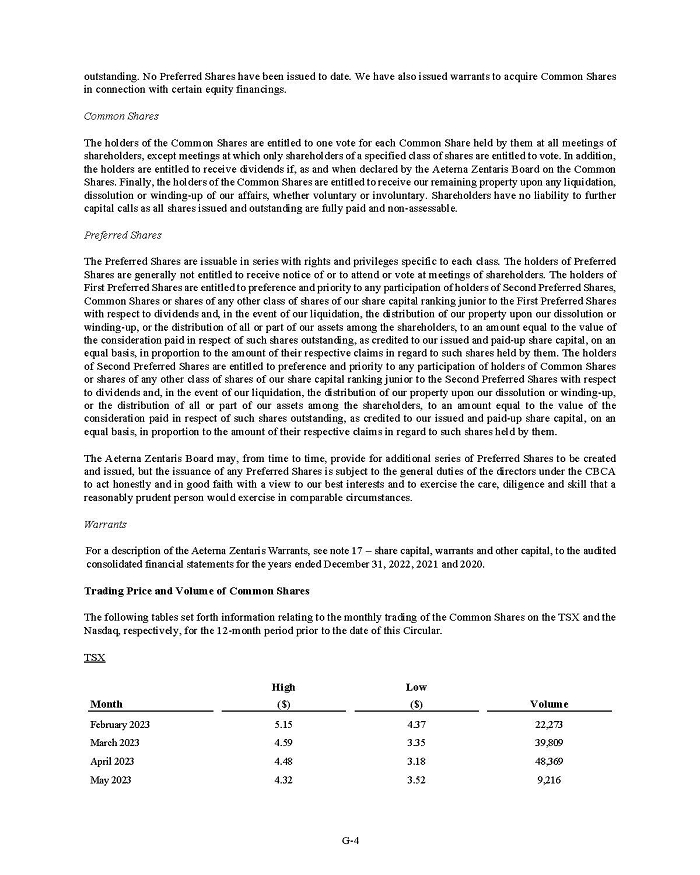









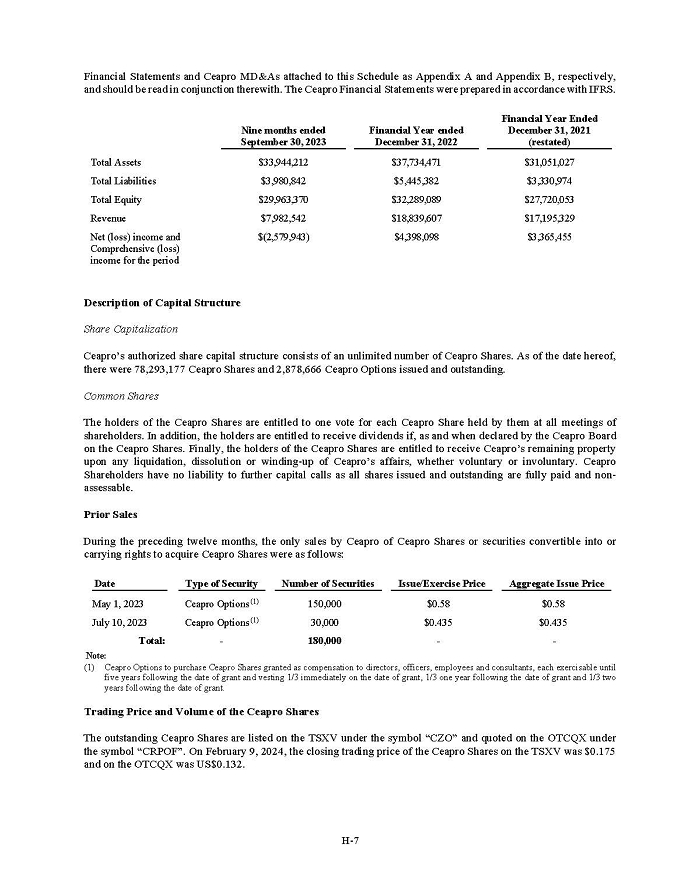
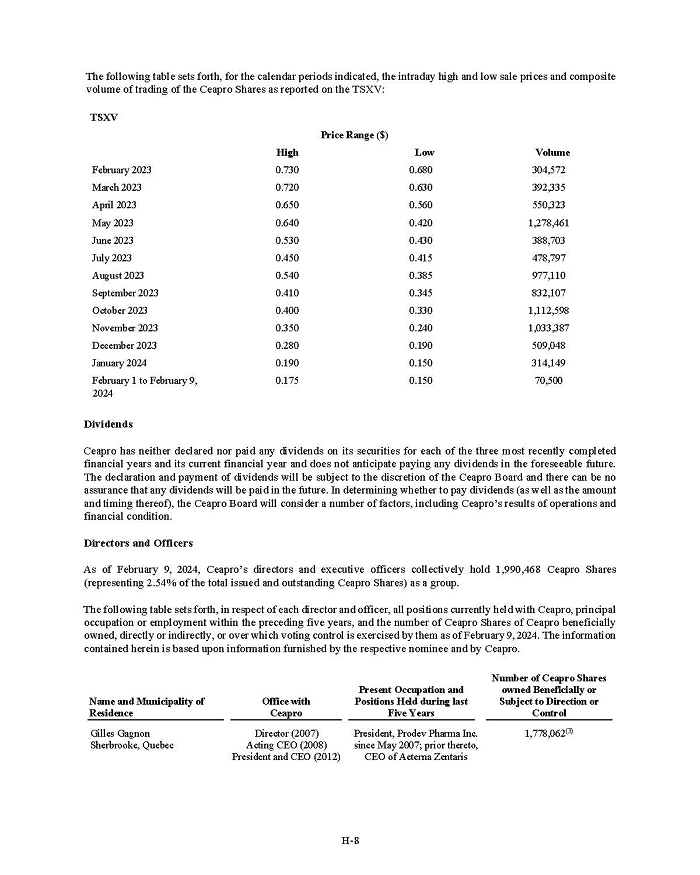





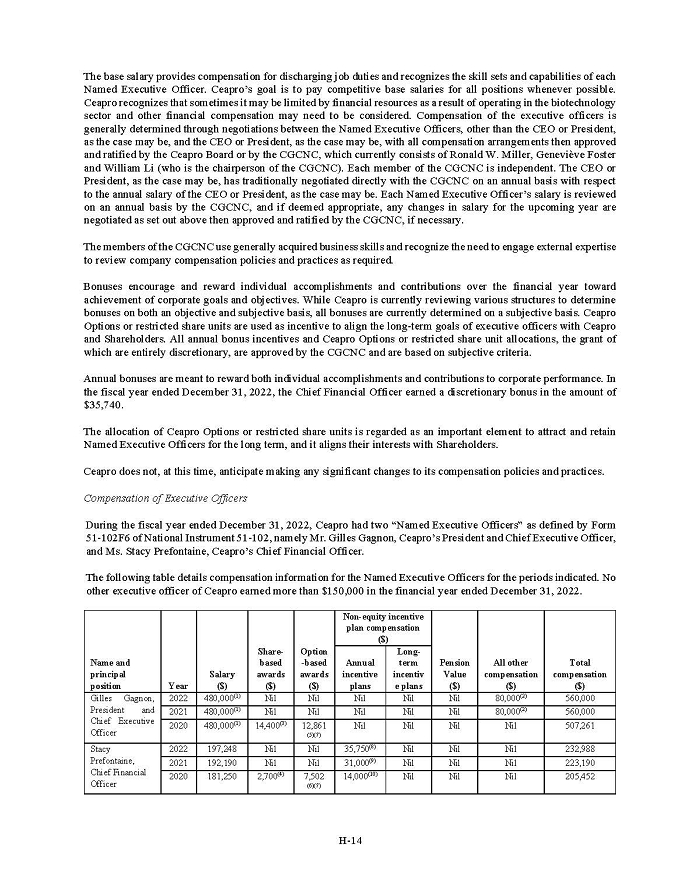



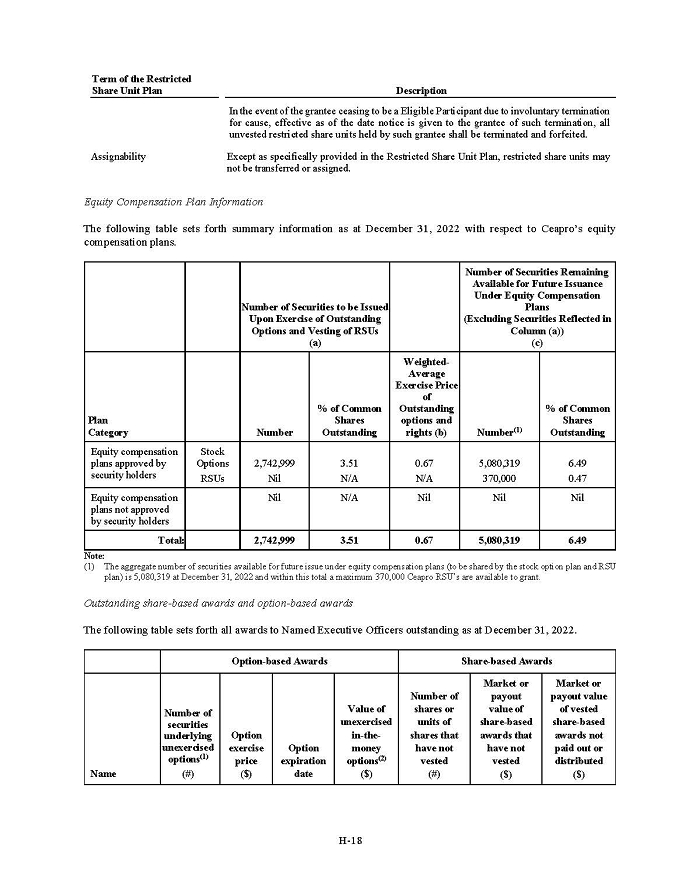























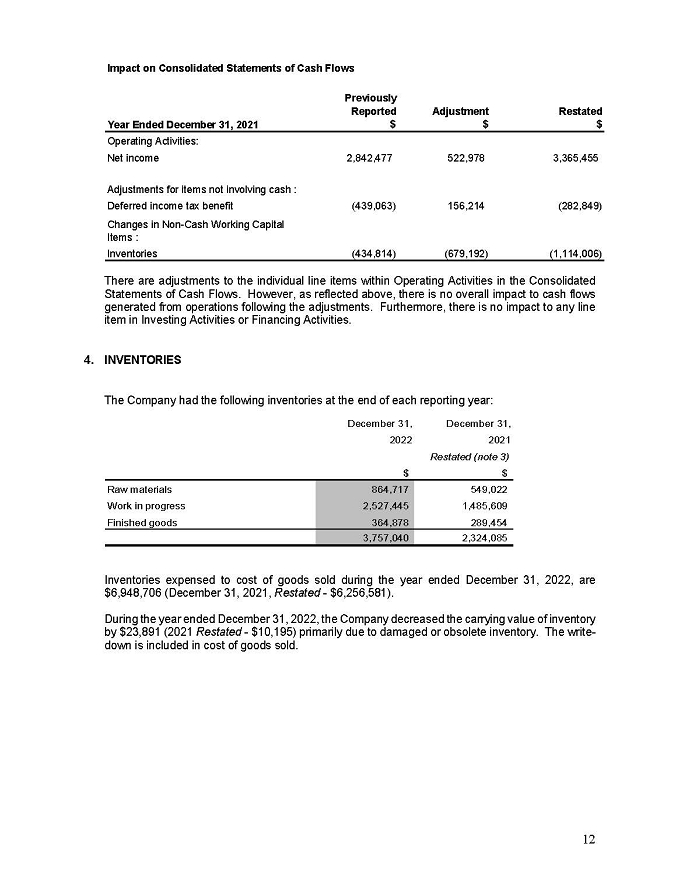
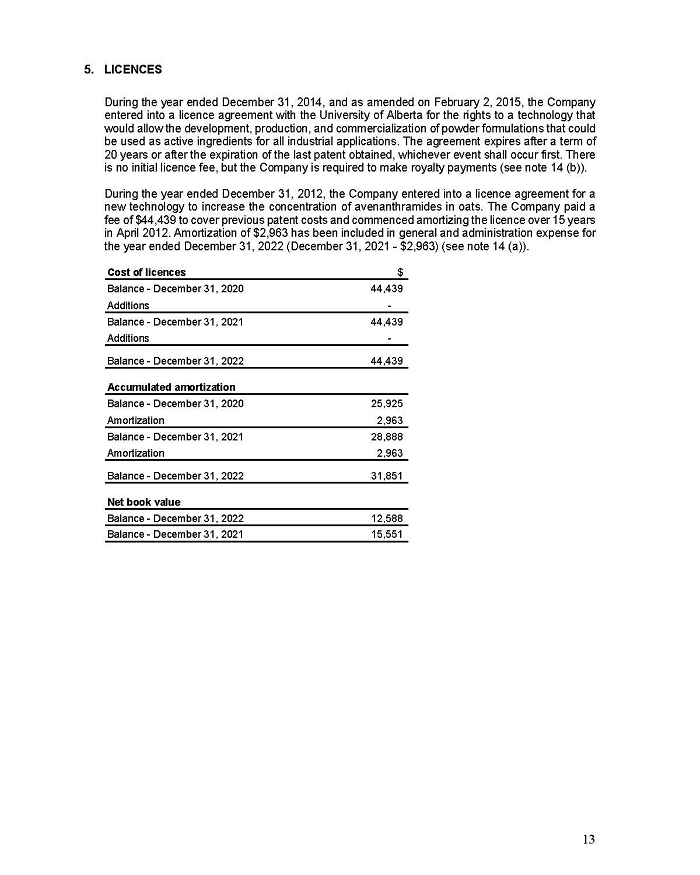

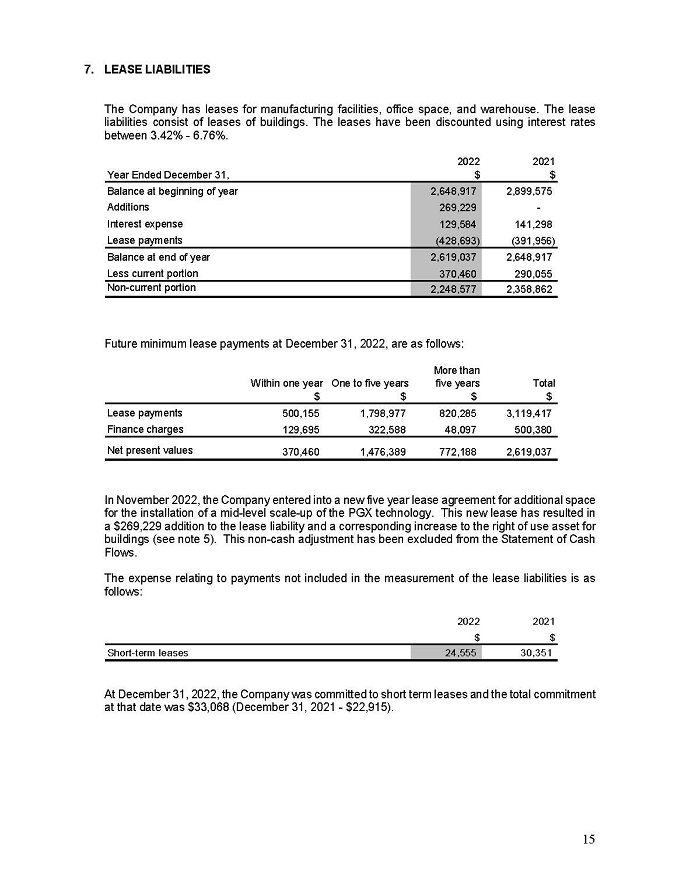
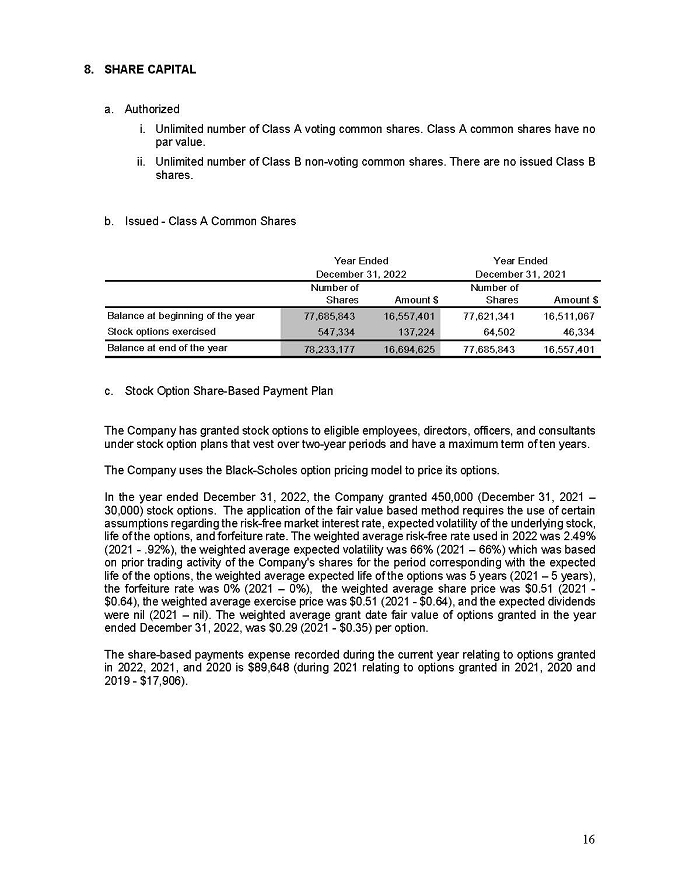

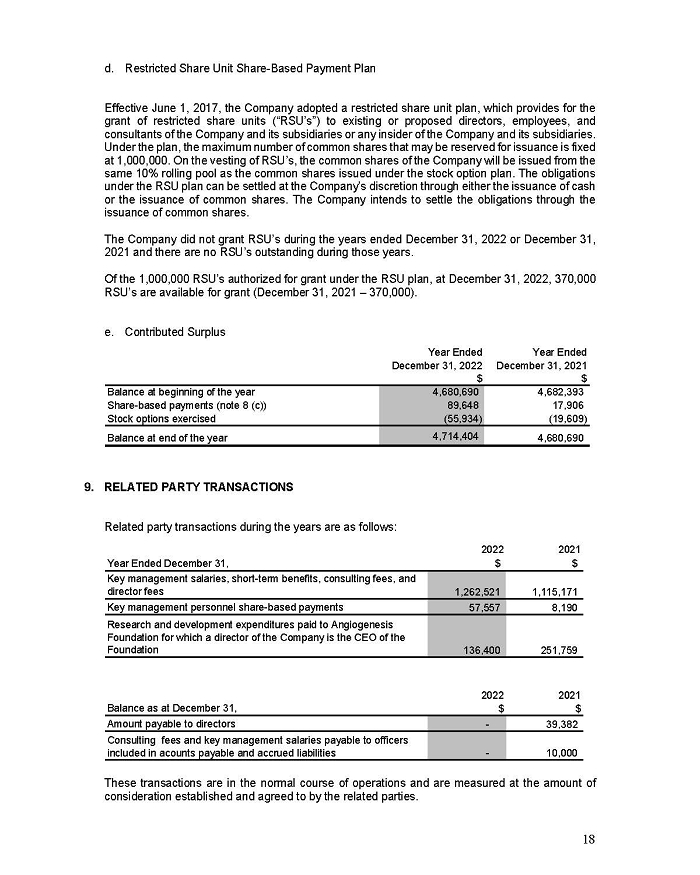


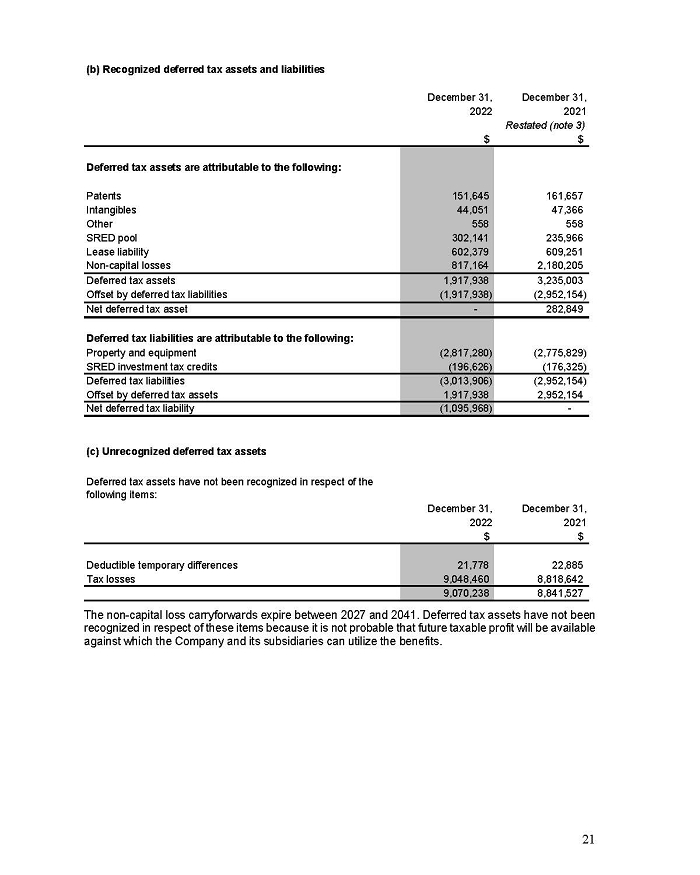


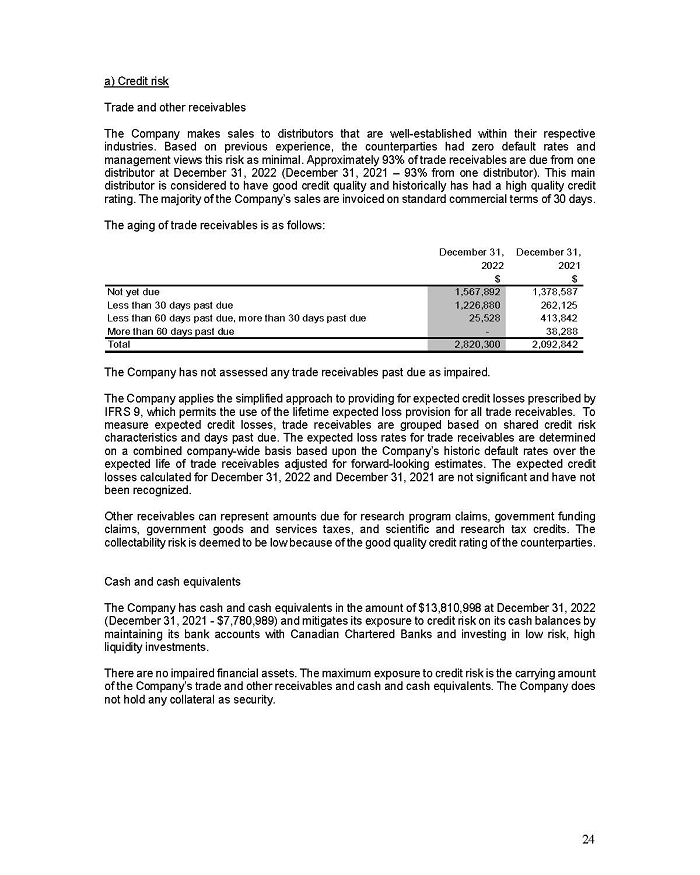


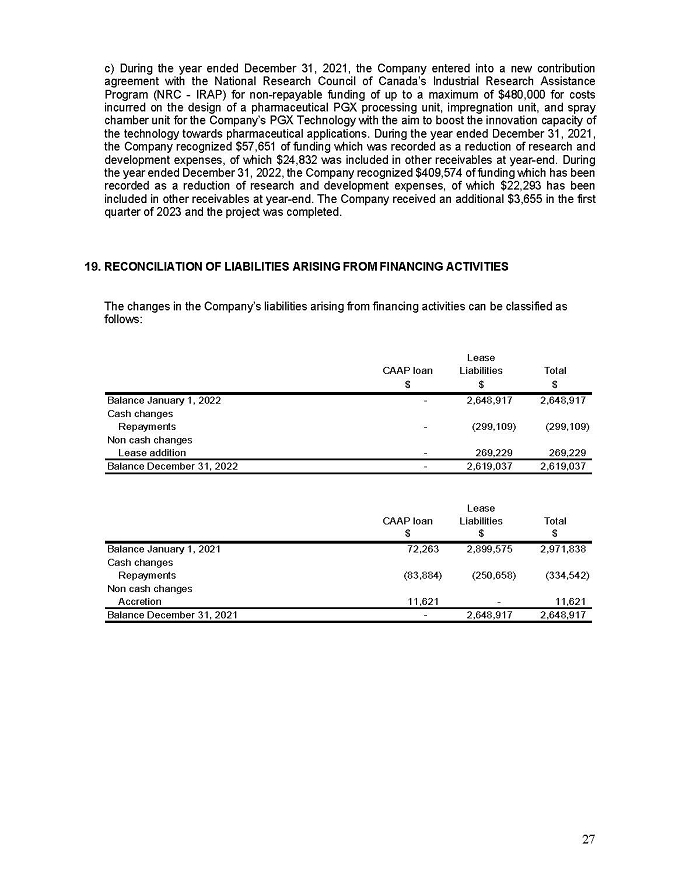




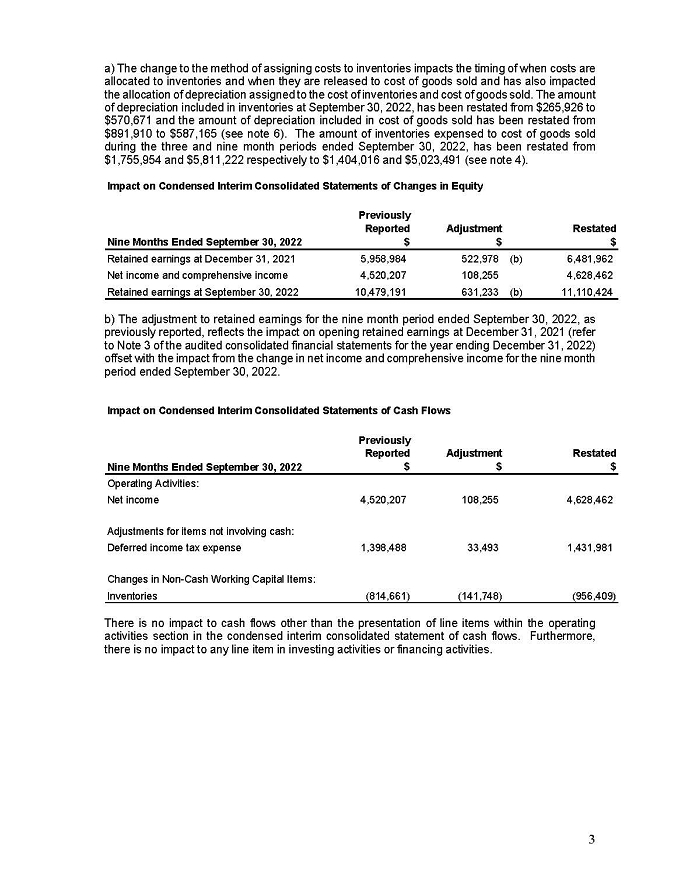
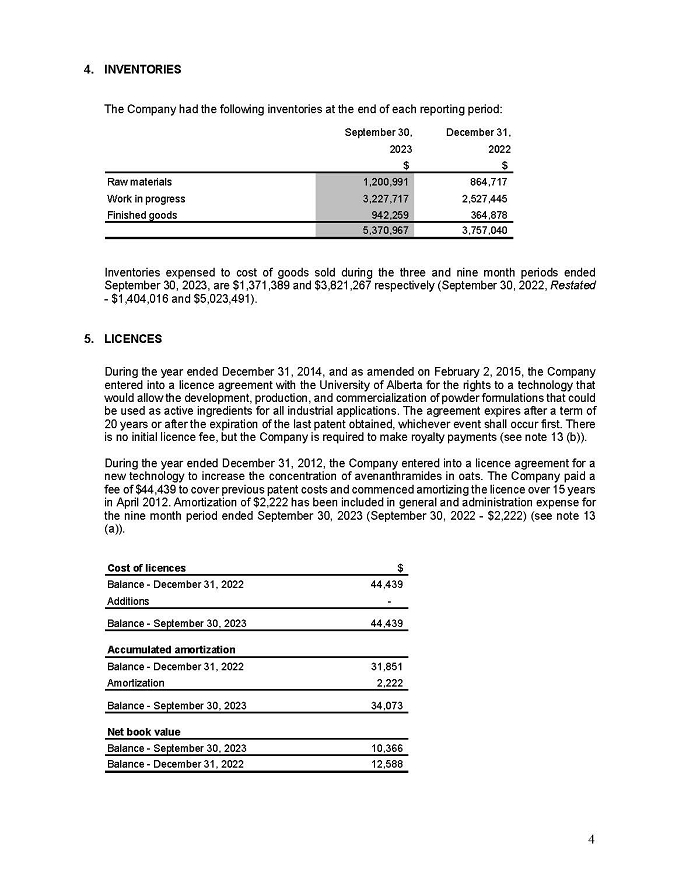
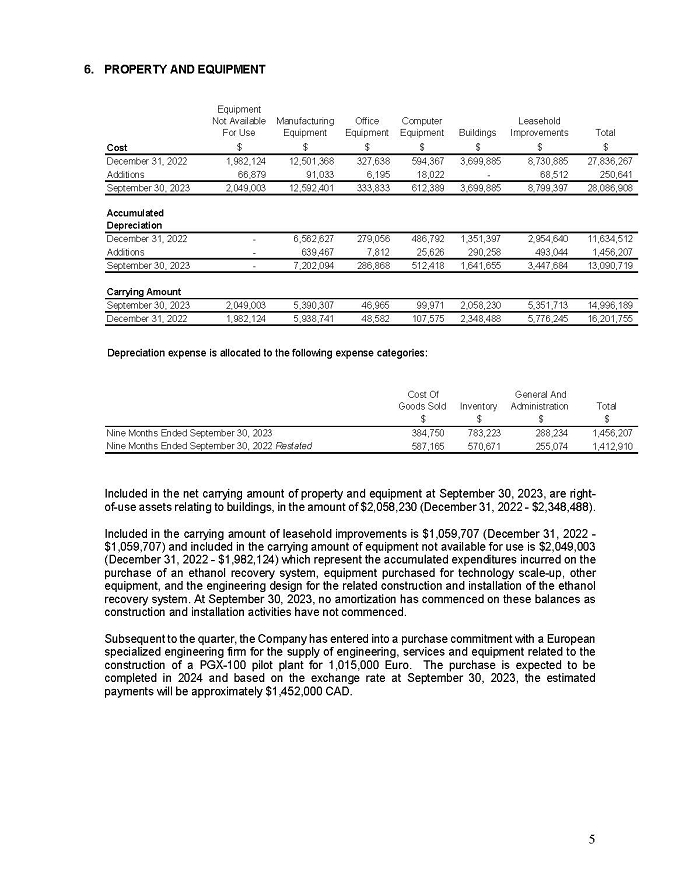

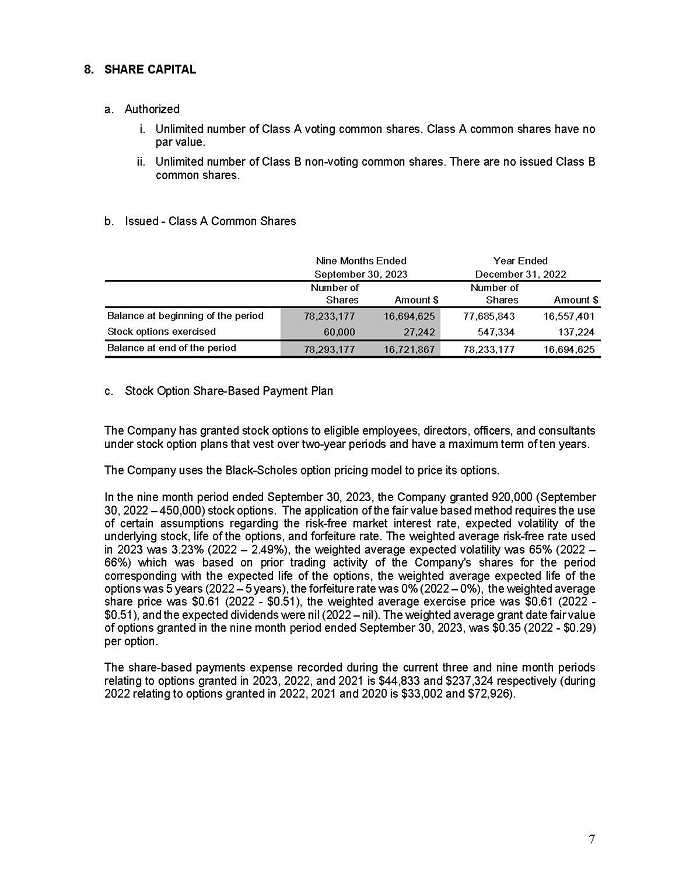



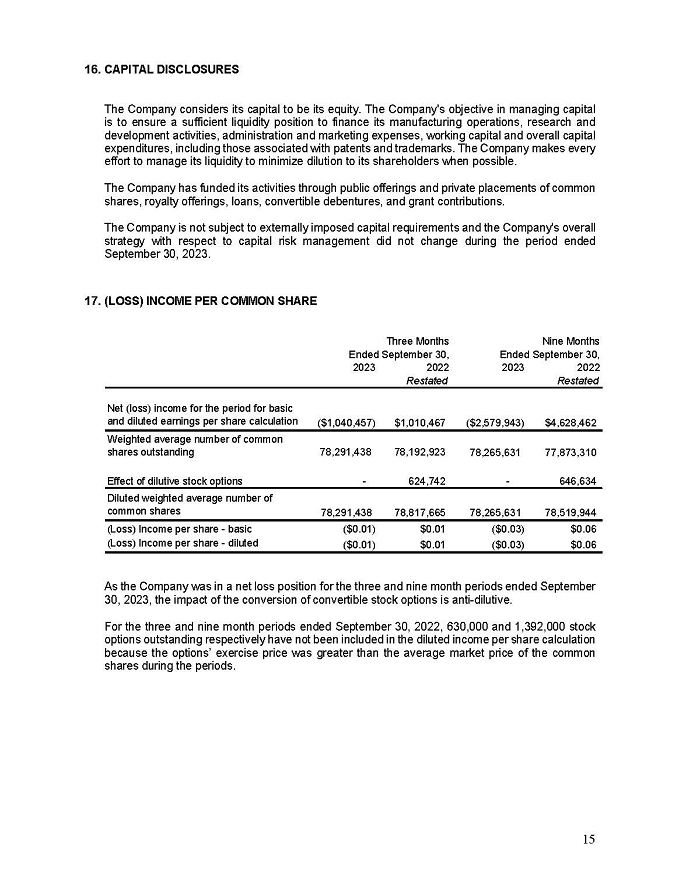



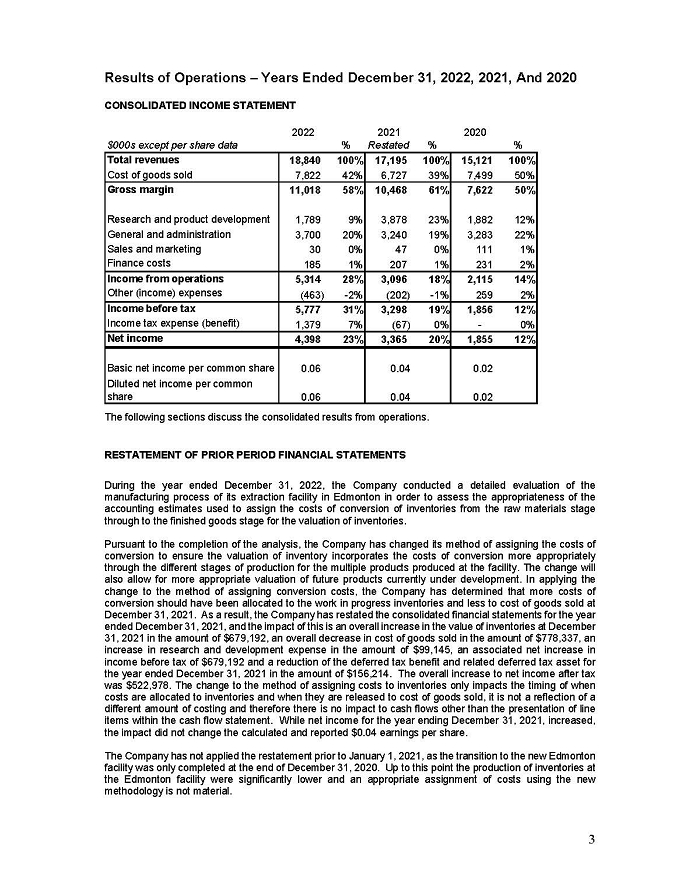

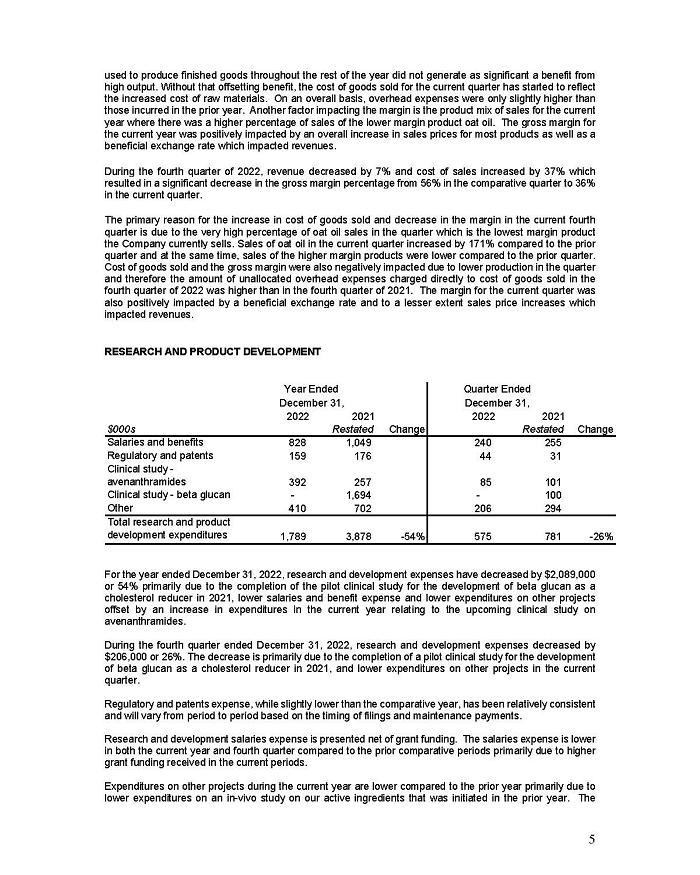
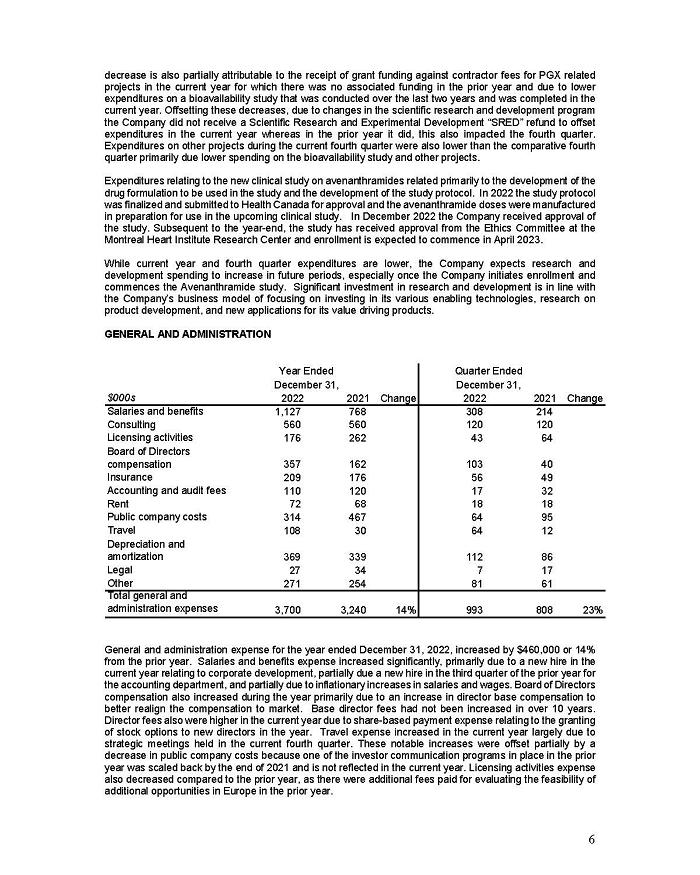



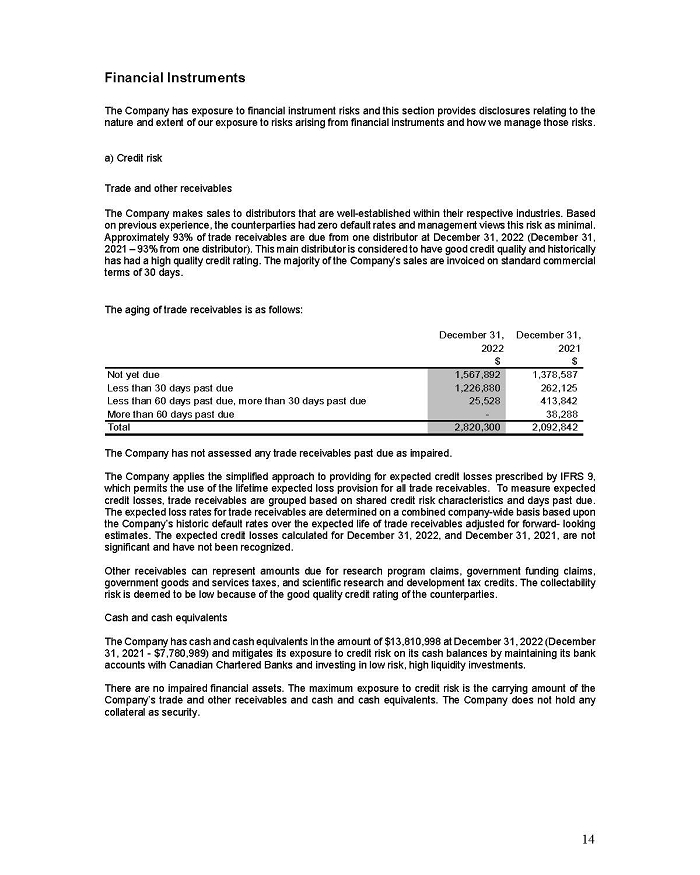
































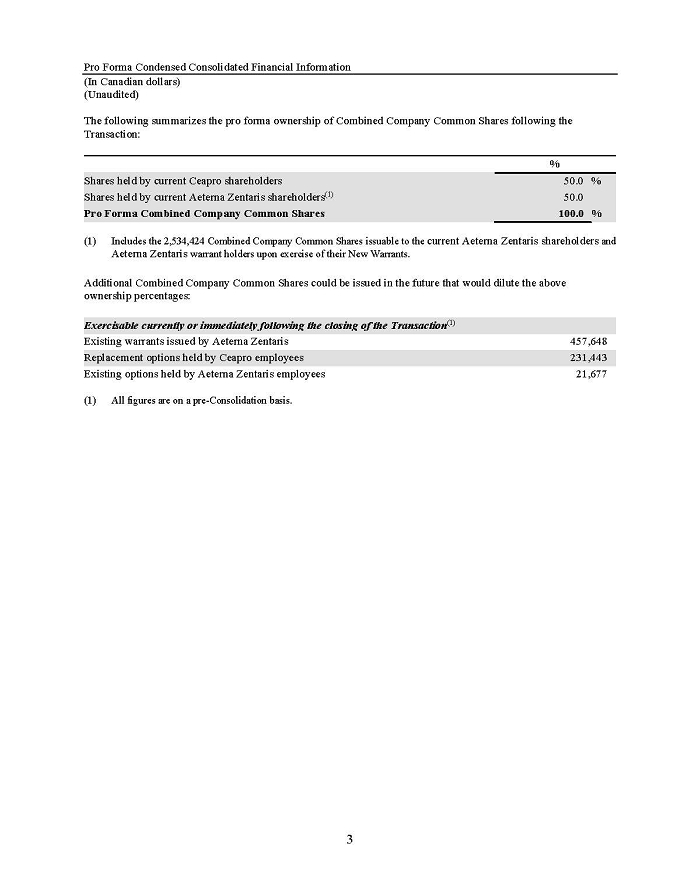

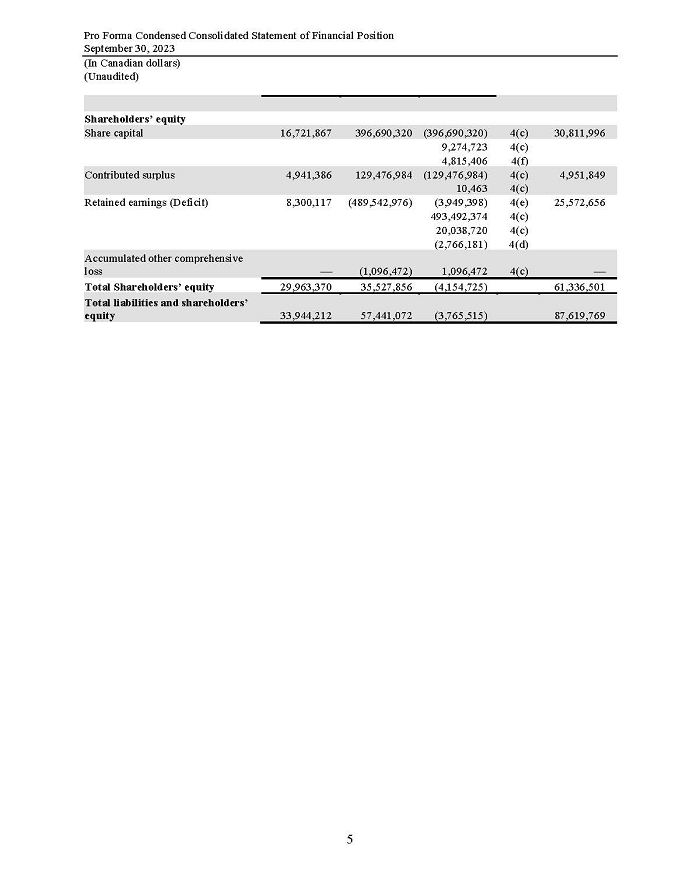





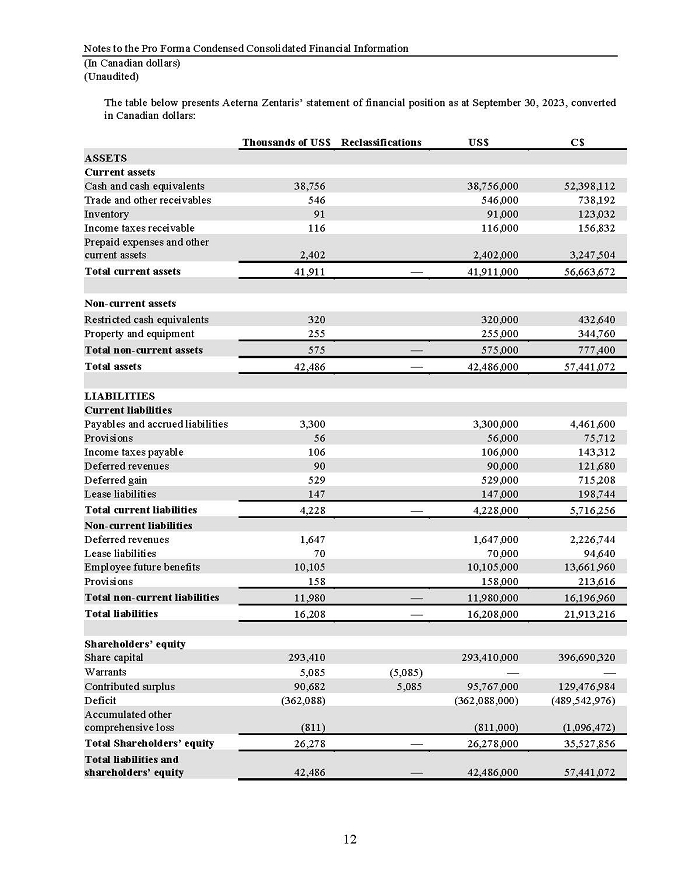




















Exhibit 99.2

Aeterna Zentaris Issues Letter to Shareholders and Management Proxy Circular Ahead of Special Meeting of Shareholders to Approve Merger of Equals with Ceapro
| ● | Aeterna Zentaris Board recommends shareholders vote FOR all resolutions, to create a diversified biopharmaceutical company with a compelling value proposition | |
| ● | More information and materials available at www.AEZSmerger.com | |
| ● | Shareholders with questions or who require assistance voting their shares should contact Kingsdale Advisors at 1-866-581-1513 (North American Toll Free) or 416-623-2513 (outside North America – text and call enabled) or contactus@kingsdaleadvisors.com |
TORONTO, February 15, 2024 — Aeterna Zentaris Inc. (NASDAQ: AEZS) (TSX: AEZS) (“Aeterna”) today announced that it has issued a Letter to Shareholders and Management Proxy Circular ahead of its special meeting of shareholders to approve its merger of equals transaction with Ceapro Inc. (TSXV: CZO) (OTCQX: CRPOF) (“Ceapro”).
The two innovative biopharmaceutical development companies previously announced that they had entered into a definitive agreement to combine operations in an all-stock merger of equals transaction (the “Transaction”). The combined company is expected to be listed on the Nasdaq Capital Market (“Nasdaq”) and the Toronto Stock Exchange (the “TSX”), subject to the receipt of all necessary approvals. A new name for the combined company is expected to be announced following the closing of the Transaction.
Aeterna’s board of directors has been actively working for over two years to find a transformational opportunity for Aeterna, and strongly believe that the Transaction with Ceapro is attractive for both companies and their respective shareholders. Currently, Ceapro’s revenue-generating cosmeceutical based business includes the production of extracts and “active ingredients” from renewable plant resources that are found in leading skincare products, including brands such as Aveeno, Jergens, Neutrogena, Lubriderm, and other leading brand names. With Aeterna’s pipeline and development expertise in higher margin pharmaceutical products, Aeterna believes the combined company has the potential to generate significant value in the near and long-term as a result of, but not limited to, the following:
| ● | Greater potential for stable cashflow to support R&D of potentially higher return pharmaceutical products. Ceapro currently generates revenues from two main active ingredients, oat beta glucan and avenanthramides, extracted and purified using its proprietary technology. Cash from these products is planned to be used along with Aeterna’s revenue from the commercialization or licensing of Aeterna’s macimorelin product to support the development of the combined company’s roster of exciting, high potential-return products, ideally creating growing and sustainable revenue for the combined company and investors. | |
| ● | Greater diversification of commercial and development product pipeline lowers risk. The combined company is expected to benefit from an extensive and diversified pipeline of innovative products in development, including Ceapro’s quicker-to-market biotechnology products and Aeterna’s exciting potentially higher return, but longer-horizon, products. With this pipeline rejuvenation, the combined company is anticipated to boast: |
| ○ | more products in the pipeline that are closer to potential commercialization; | |
| ○ | an enhanced ability to strategically focus financial and company resources in a manner that provides the most value to the company and shareholders; and | |
| ○ | a more compelling value proposition and lower risk profile. |
| ● | Expanded pharmaceutical research and development capabilities. Both Aeterna and Ceapro bring deep expertise and knowledge that are expected to play a key role in advancing the combined company and development pipeline. The combined company will have the infrastructure to support development activities and potentially offer improved efficiencies, in addition to cost savings. The combined company will also have an expanded development pipeline of products which we are committed to prioritizing as we evaluate what will provide the best overall potential for the combined company, shareholders, and consumers. | |
| ● | Compelling North American + European combination. Ceapro has an operational presence in North America, which addresses another strategic consideration for Aeterna, a Canadian company in North American markets but whose current operational footprint is largely European. While Aeterna expects to continue to maintain some presence in Europe, Aeterna believes it needs to re-focus operations within the North American biotechnology market. Aeterna believes that combining with Ceapro, a company with an established presence in North America, provides better exposure to potential new investors, business development opportunities and talent. | |
| ● | Expertise and efficiencies. Both companies have expertise that can build upon each other, which is expected to result in a stronger combined company. For example, Aeterna is adept at navigating the conduct of human clinical trials and the crucial regulatory approval process required to bring pharmaceutical products to market. The combined company plans to leverage this expertise with the higher value pharmaceutical opportunities being advanced by Ceapro for its active ingredients and technologies. |
In short, Aeterna’s board believes this business combination will provide both companies with the tools needed to begin to effect real change and to provide significant growth and value. To learn more, shareholders are urged to visit www.AEZSmerger.com.
Time is Short. Vote FOR all Resolutions Today
Aeterna’s board of directors recommends a vote in favour of all resolutions at the upcoming special meeting to be held at 11:00 a.m. (Eastern time) on March 12, 2024. In order to be counted at the meeting, your proxy must be received prior to the proxy voting deadline of 11:00 a.m. (Eastern time) on March 8, 2024 or, if the meeting is adjourned or postponed, by no later than 48 hours prior to the time fixed for the adjourned or postponed meeting.
If Aeterna shareholders have any questions or require assistance with voting their proxy, please contact Aeterna Zentaris’ strategic shareholder advisor and proxy solicitation agent, Kingsdale Advisors, at 1-866-581-1513 (North American Toll Free) or 416-623-2513 (Outside North America – text and call enabled), or by email at: contactus@kingsdaleadvisors.com.
Advisors and Counsel
Aeterna has engaged Raymond James as its financial advisor, Norton Rose Fulbright as its Canadian and U.S. legal advisor, FGS Longview as its communications advisor and Kingsdale Advisors as its proxy solicitor.
About Aeterna Zentaris Inc.
Aeterna is a specialty biopharmaceutical company developing and commercializing a diversified portfolio of pharmaceutical and diagnostic products focused on areas of significant unmet medical need. Aeterna’s lead product, macimorelin (Macrilen; Ghryvelin), is the first and only U.S. FDA and European Commission approved oral test indicated for the diagnosis of adult growth hormone deficiency (AGHD). Aeterna is leveraging the clinical success and compelling safety profile of macimorelin to develop it for the diagnosis of childhood-onset growth hormone deficiency (CGHD), an area of significant unmet need.
Aeterna is also dedicated to the development of its therapeutic assets and has established a pre-clinical development pipeline to potentially address unmet medical needs across a number of indications, including neuromyelitis optica spectrum disorder (NMOSD), Parkinson’s disease (PD), hypoparathyroidism and amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease). For more information, please visit www.zentaris.com and connect with Aeterna on LinkedIn and Facebook.
About Ceapro Inc.
Ceapro is a Canadian biotechnology company involved in the development of proprietary extraction technology and the application of this technology to the production of extracts and “active ingredients” from oats and other renewable plant resources.
Ceapro adds further value to its extracts by supporting their use in cosmeceutical, nutraceutical and therapeutics products for humans and animals. Ceapro has a broad range of expertise in natural product chemistry, microbiology, biochemistry, immunology and process engineering. These skills merge in the fields of active ingredients, biopharmaceuticals and drug-delivery solutions. For more information on Ceapro, please visit Ceapro’s website at www.ceapro.com.
Forward-Looking Statements
The information in this news release has been prepared as at February 15, 2024. Certain statements in this news release, referred to herein as “forward-looking statements”, constitute “forward-looking statements” within the meaning of the United States Private Securities Litigation Reform Act of 1995, specifically, Section 27A of the U.S. Securities Act of 1933, as amended, and Section 21E of the U.S. Securities Exchange Act, as amended, and “forward-looking information” under the provisions of Canadian securities laws. All statements, other than statements of historical fact, that address circumstances, events, activities, or developments that could or may or will occur are forward-looking statements. When used in this press release, words such as “anticipate”, “assume”, “believe”, “continue”, “could”, “expect”, “forecast”, “future”, “goal”, “guidance”, “indicate”, “intend”, “likely”, “maintain”, “may”, “objective”, “outlook”, “plan”, “potential”, “project”, “seek”, “strategy”, “synergies”, “view”, “will”, “would” or the negative or comparable terminology as well as terms usually used in the future and the conditional are generally intended to identify forward-looking statements, although not all forward-looking statements include such words.
Forward-looking statements in this news release include, but are not limited to statements and comments relating to: the rationale of Aeterna’s and Ceapro’s boards for entering into the Arrangement Agreement; the expected outcomes of the Transaction, including the combined company’s assets, cost structure, financial position, cash flows and growth prospects; the anticipated benefits and synergies of the combined operations; the ability of Aeterna and Ceapro to complete the Transaction on the terms described herein, or at all; the anticipated timeline for the completion of the Transaction and the special meeting; and receipt of regulatory, stock exchange and shareholder approvals (including approval of the continued listing of Aeterna’s common shares on Nasdaq and the TSX).
Forward-looking statements are necessarily based upon a number of factors and assumptions that, while considered reasonable by Aeterna as of the date of such statements, are inherently subject to significant business, economic, operational and other risks, uncertainties, contingencies and other factors, including those described below, which could cause actual results, performance or achievements of Aeterna and Ceapro to be materially different from results, performance or achievements expressed or implied by such forward-looking statements and, as such, undue reliance must not be placed on them. Forward-looking statements are also based on numerous material factors and assumptions, including as described in this news release, with respect to, among other matters: Aeterna’s and Ceapro’s present and future business strategies; operations performance within expected ranges; anticipated future cash flows; local and global economic conditions and the environment in which the combined operations will operate in the future; anticipated capital and operating costs; and the availability and timing of required stock exchange, regulatory, shareholder and other approvals for the completion of the Transaction.
Many factors, known and unknown, could cause actual results to be materially different from those expressed or implied by such forward-looking statements. Such risks include, but are not limited to: the ability to consummate the Transaction; the ability to obtain requisite shareholder approvals and the satisfaction of other conditions to the consummation of the Transaction on the proposed terms in the time assumed; the ability to obtain necessary stock exchange, regulatory or other approvals in the time assumed; the ability to realize the anticipated benefits of the Transaction or implementing the business plan for the combined company, including as a result of a delay in completing the Transaction or difficulty in integrating the businesses of the companies involved; significant Transaction costs or unknown liabilities; the potential payment of a termination fee by either Ceapro or Aeterna to the other in certain circumstances if the Transaction is not completed or if the Arrangement Agreement is terminated by either Aeterna or Ceapro to accept a superior proposal; directors and officers of Aeterna and Ceapro may have interests in the Transaction that may be different from those of Aeterna and Ceapro shareholders generally; the focus of both management’s time and attention on the Transaction may detract from other aspects of their respective businesses; the tax treatment of the Transaction may be subject to uncertainties; risks relating to the retention of key personnel during the interim period; the ability to realize synergies and cost savings at the times, and to the extent anticipated; the potential impact on research and development activities; the potential impact of the announcement or consummation of the Transaction on relationships, including with regulatory bodies, employees, suppliers, customers, competitors and other key stakeholders; Aeterna’s and Ceapro’s economic model and liquidity risks; technology risks; changes in or enforcement of national and local government legislation, taxation, controls or regulations and/or changes in the administration of laws, policies and practices; legal or regulatory developments and changes; the impact of foreign exchange rates; pricing pressures; and local and global political and economic conditions.
Information contained in forward-looking statements is based upon certain material assumptions that were applied in drawing a conclusion or making a forecast or projection, including Aeterna’s management perceptions of historical trends, current conditions and expected future developments, as well as other considerations that are believed to be appropriate in the circumstances. Aeterna considers these assumptions to be reasonable based on all currently available information but caution the reader that these assumptions regarding future events, many of which are beyond their control, may ultimately prove to be incorrect since they are subject to risks and uncertainties that affect Aeterna and Ceapro and their businesses.
Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date made. For a more detailed discussion of such risks and other factors that may affect Aeterna’s and Ceapro’s ability to achieve the expectations set forth in the forward-looking statements contained in this news release, see Aeterna’s Annual Report on Form 20-F and MD&A filed under Aeterna’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov and Ceapro’s MD&A filed under Ceapro’s profile on SEDAR+ at www.sedarplus.ca, as well as Aeterna’s and Ceapro’s other filings with the Canadian securities regulators and the U.S. Securities and Exchange Commission (the “SEC”). Other than as required by law, Aeterna does not intend, and does not assume any obligation to, update these forward-looking statements.
Information Concerning the Registration Statement
Aeterna has furnished the Management Proxy Circular for the upcoming special meeting of shareholders (the “Special Meeting”) to which this communication relates with the SEC as Exhibit 99.1 to a Current Report on Form 6-K on February 15, 2024, and on SEDAR+, and has mailed the Management Proxy Circular and other proxy solicitation materials to record holders as of January 12, 2024. Before you vote, you should read the Management Proxy Circular and other documents that Aeterna has furnished and filed with the SEC and on SEDAR+ for more complete information about Aeterna, Ceapro, the Special Meeting and the resolutions to be voted on.
Aeterna has filed a Registration Statement on Form F-1 (including a prospectus) (File No. 333-277115) (the “Registration Statement”) with the SEC for the issuance of common share purchase warrants and common shares issuable upon exercise thereof in connection with the Transaction discussed in this communication, but it has not yet become effective. The common share purchase warrants and common shares issuable upon exercise thereof may not be sold nor may offers to buy them be accepted prior to the time the Registration Statement becomes effective. Before you invest, you should read the prospectus in that Registration Statement and the other documents incorporated by reference therein for more complete information about Aeterna, Ceapro, the Transaction and the common share purchase warrant offering.
You may get copies of the Management Proxy Circular and the prospectus and Registration Statement for free by visiting EDGAR on the SEC website at www.sec.gov or at SEDAR+ at www.sedarplus.ca. Alternatively, you may obtain copies of them by contacting Aeterna’s proxy solicitor at the details provided below.
For Further Information
Aeterna’s Proxy Solicitor
Kingsdale Advisors
1-866-581-1513 (North American Toll Free) or
416-623-2513 (Outside North America – text and call enabled)
contactus@kingsdaleadvisors.com
www.AEZSmerger.com
Media Contact
Joel
Shaffer
FGS Longview
joel.shaffer@fgslongview.com
416-670-6468