Document
Exhibit 99.1
Castle Biosciences Reports Third Quarter 2023 Results
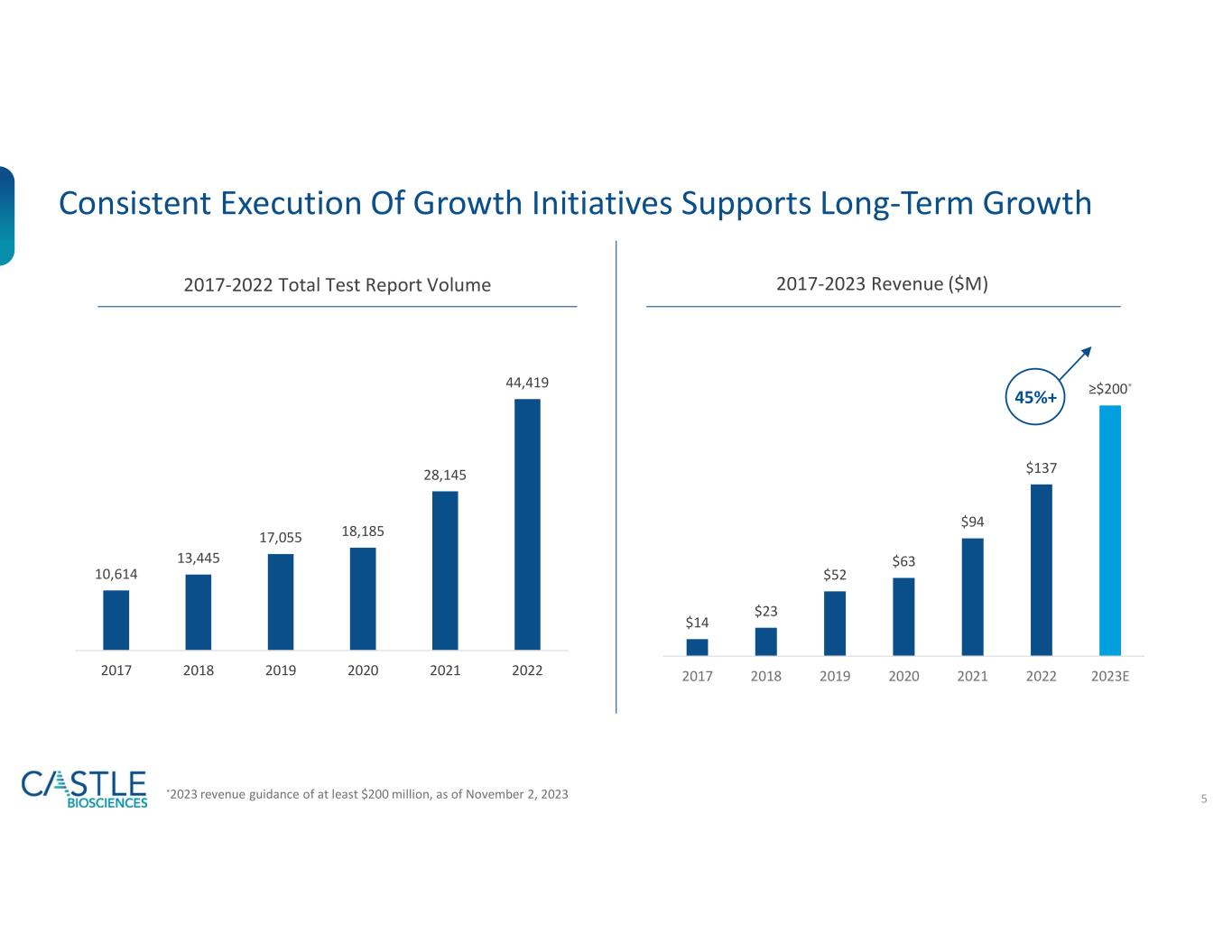
Q3 2023 revenue increased 66% over Q3 2022 to $61 million
Q3 2023 total test reports increased 52% over Q3 2022
Raising full year 2023 revenue guidance to at least $200 million from at least $180 million
Conference call and webcast today at 4:30 p.m. ET
FRIENDSWOOD, Texas - Nov. 2, 2023--Castle Biosciences, Inc. (Nasdaq: CSTL), a company improving health through innovative tests that guide patient care, today announced its financial results for the third quarter and nine months ended September 30, 2023.
“Our third quarter results were exceptional, demonstrating the strength of our business fundamentals and the innovative test portfolio we’ve built to provide patients and clinicians with actionable information to guide patient care,” said Derek Maetzold, president and chief executive officer of Castle Biosciences. “We delivered another quarter of significant test volume growth and revenue growth, which helped drive positive earnings and positive operating cash flow. Given our consistent performance year-to-date and confidence in our business, we are raising our 2023 revenue guidance to at least $200 million, up from at least $180 million.
“We continue to invest in our strategic growth initiatives across our entire test portfolio, while maintaining our firm focus on financial discipline. We believe our investments will continue to support our long-term value creation plans and the improvement of patient care. Our success is not possible without the continued dedication and efforts of our Castle team, and I would like to express my sincere appreciation for their contributions.”
Third Quarter Ended September 30, 2023, Financial and Operational Highlights
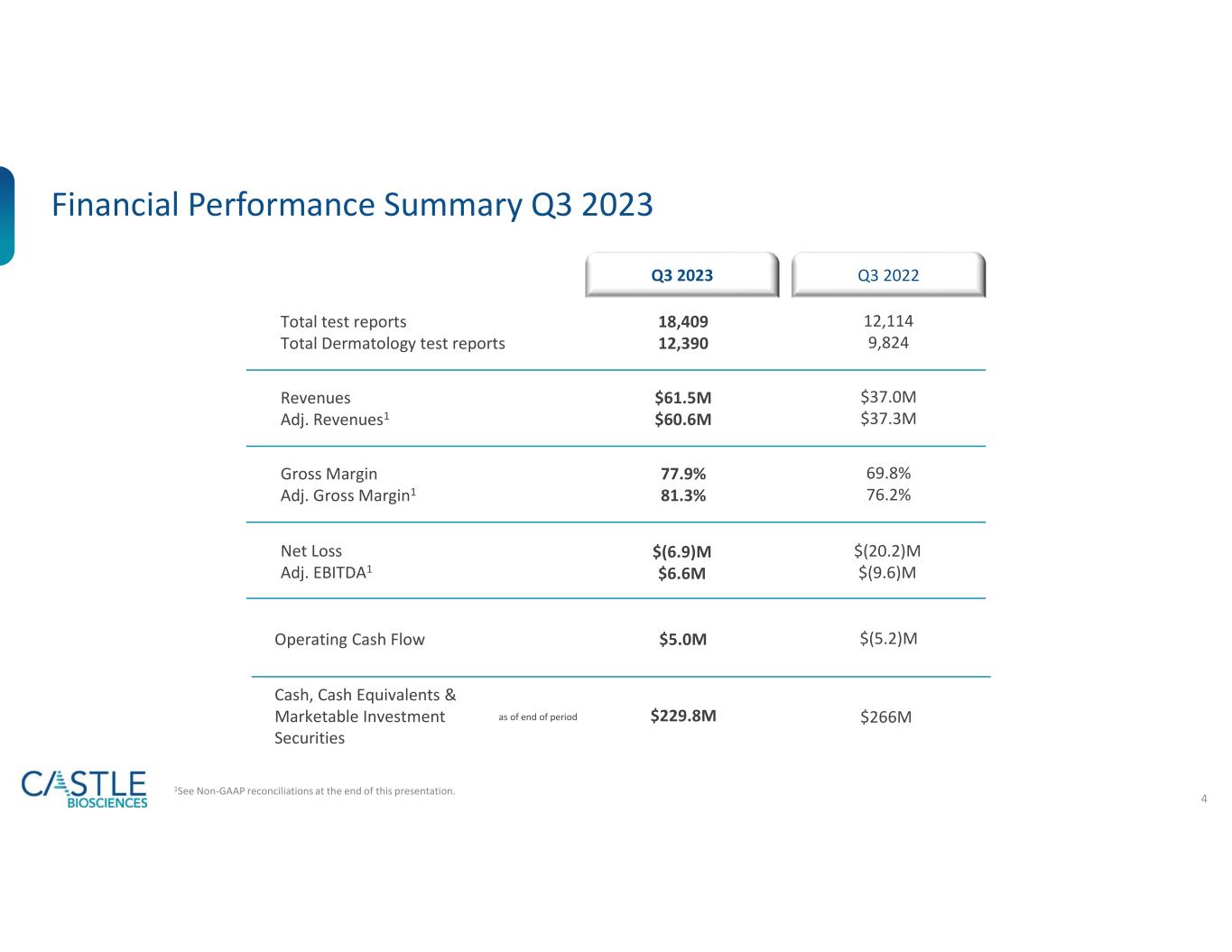
•Revenues were $61.5 million, a 66% increase compared to $37.0 million during the same period in 2022. Included in revenue for the period were revenue adjustments related to tests delivered in prior periods. These prior period revenue adjustments for the quarter ended September 30, 2023, were $0.9 million of net positive revenue adjustments, compared to $(0.3) million of net negative revenue adjustments for the same period in 2022.
•Adjusted revenues, which exclude the effects of revenue adjustments related to tests delivered in prior periods, were $60.6 million, a 63% increase compared to $37.3 million for the same period in 2022.
•Delivered 18,409 total test reports in the third quarter of 2023, an increase of 52% compared to 12,114 in the same period of 2022:
◦DecisionDx®-Melanoma test reports delivered in the quarter were 8,559, compared to 7,354 in the third quarter of 2022, an increase of 16%.
◦DecisionDx®-SCC test reports delivered in the quarter were 2,820, compared to 1,636 in the third quarter of 2022, an increase of 72%.
◦MyPath® Melanoma test reports delivered in the quarter were 1,011, compared to 834 MyPath Melanoma and DiffDx®-Melanoma aggregate test reports in the third quarter of 2022, an increase of 21%.
◦DecisionDx®-UM test reports delivered in the quarter were 399, compared to 392 in the third quarter of 2022, an increase of 2%.
◦TissueCypher® Barrett’s Esophagus test reports delivered in the quarter were 2,829, compared to 690 in the third quarter of 2022, an increase of 310%.
◦IDgenetix® test reports delivered in the quarter were 2,791, compared to 1,208 in the third quarter of 2022, an increase of 131%.
•Gross margin for the quarter ended September 30, 2023, was 78%, and adjusted gross margin was 81%.
•Net cash provided by operations was $5.0 million, compared to net cash used in operations of $5.2 million for the same period in 2022.
•Net loss for the third quarter, which includes non-cash stock-based compensation expense of $13.0 million, was $(6.9) million, compared to a net loss of $(20.2) million for the same period in 2022.
•Adjusted EBITDA for the third quarter was $6.6 million, compared to $(9.6) million for the same period in 2022.
Nine Months Ended September 30, 2023, Financial and Operational Highlights
•Revenues were $153.7 million, a 56% increase compared to $98.7 million during the same period in 2022. Included in revenue for the period were revenue adjustments related to tests delivered in prior periods. These prior period revenue adjustments for the nine months ended September 30, 2023, were $(3.1) million of net negative revenue adjustments, compared to $(1.9) million of net negative revenue adjustments for the same period in 2022.
•Adjusted revenues, which exclude the effects of revenue adjustments related to tests delivered in prior periods, were $156.8 million, a 56% increase compared to $100.6 million for the same period in 2022.
•Delivered 50,145 total test reports in the nine months ended September 30, 2023, an increase of 58% compared to 31,775 in the same period of 2022:
◦DecisionDx-Melanoma test reports delivered in the nine months ended September 30, 2023, were 24,739, compared to 20,502 for the same period in 2022, an increase of 21%.
◦DecisionDx-SCC test reports delivered in the nine months ended September 30, 2023, were 7,912, compared to 4,122 for the same period in 2022, an increase of 92%.
◦MyPath Melanoma test reports delivered in the nine months ended September 30, 2023, were 2,944, compared to 2,739 MyPath Melanoma and DiffDx-Melanoma aggregate test reports for the same period in 2022, an increase of 7%.
◦DecisionDx-UM test reports delivered in the nine months ended September 30, 2023, were 1,269, compared to 1,279 for the same period in 2022, a decrease of 1%.
◦TissueCypher Barrett’s Esophagus test reports delivered in the nine months ended September 30, 2023, were 5,659, compared to 1,098 for the same period in 2022, following our initial offering of the test beginning in December 2021.
◦IDgenetix test reports delivered in the nine months ended September 30, 2023, were 7,622, compared to 2,035 for the same period in 2022, following our initial offering of the test beginning in April 2022.
•Gross margin for the nine months ended September 30, 2023, was 74%, and adjusted gross margin was 79%.
•Net cash used in operations was $24.2 million, compared to $35.7 million for the same period in 2022.
•Net loss for the nine months ended September 30, 2023, which includes non-cash stock-based compensation expense of $39.4 million, was $(54.9) million, compared to $(46.5) million for the same period in 2022.
•Adjusted EBITDA for the nine months ended September 30, 2023, was $(13.8) million, compared to $(32.2) million for the same period in 2022.
Cash, Cash Equivalents and Marketable Investment Securities
As of September 30, 2023, the Company’s cash, cash equivalents and marketable investment securities totaled $229.8 million.
2023 Outlook
Castle Biosciences is increasing its guidance for anticipated total revenue in 2023. The Company now anticipates generating at least $200 million in total revenue in 2023 compared to the previously provided guidance of at least $180 million.
Third Quarter and Recent Accomplishments and Highlights
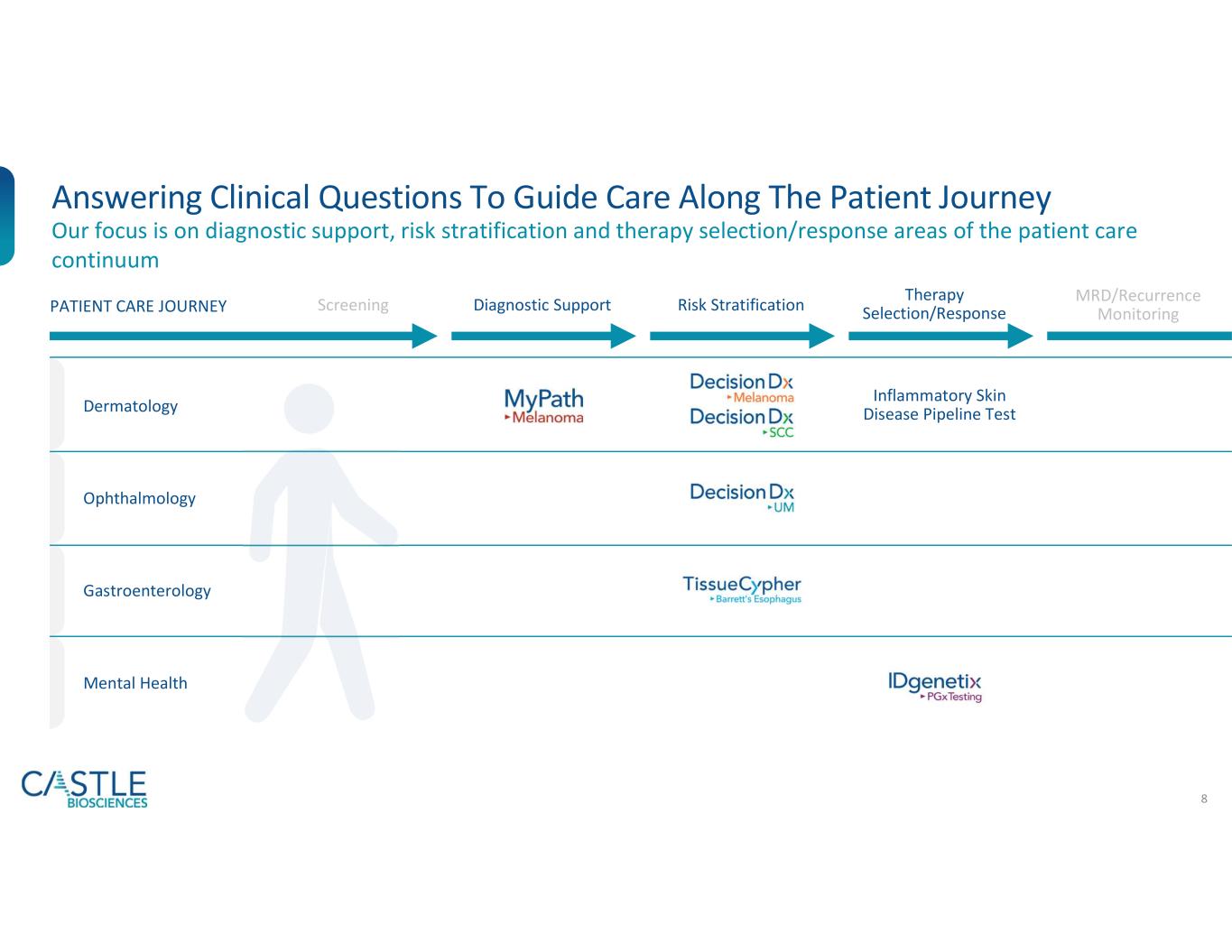
Dermatology
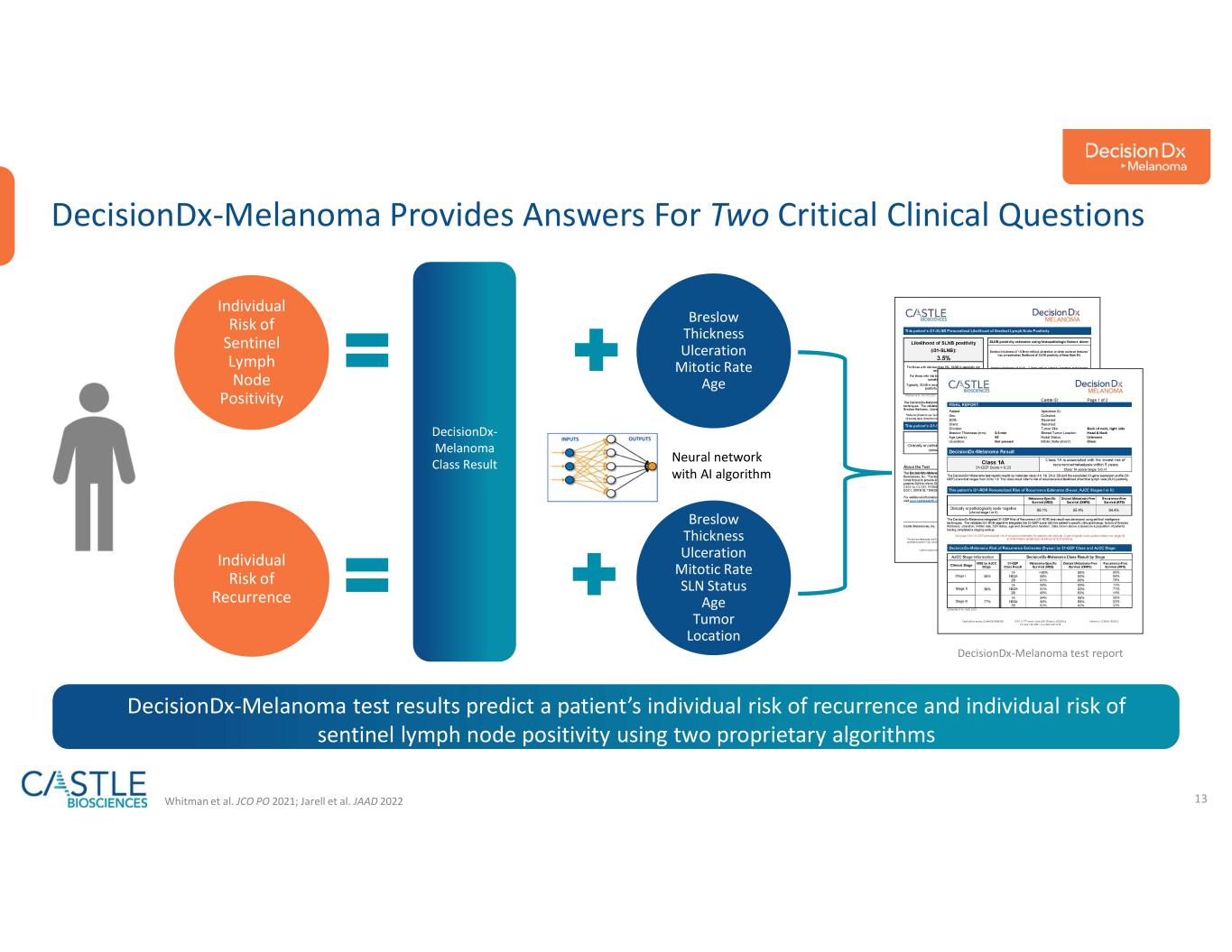
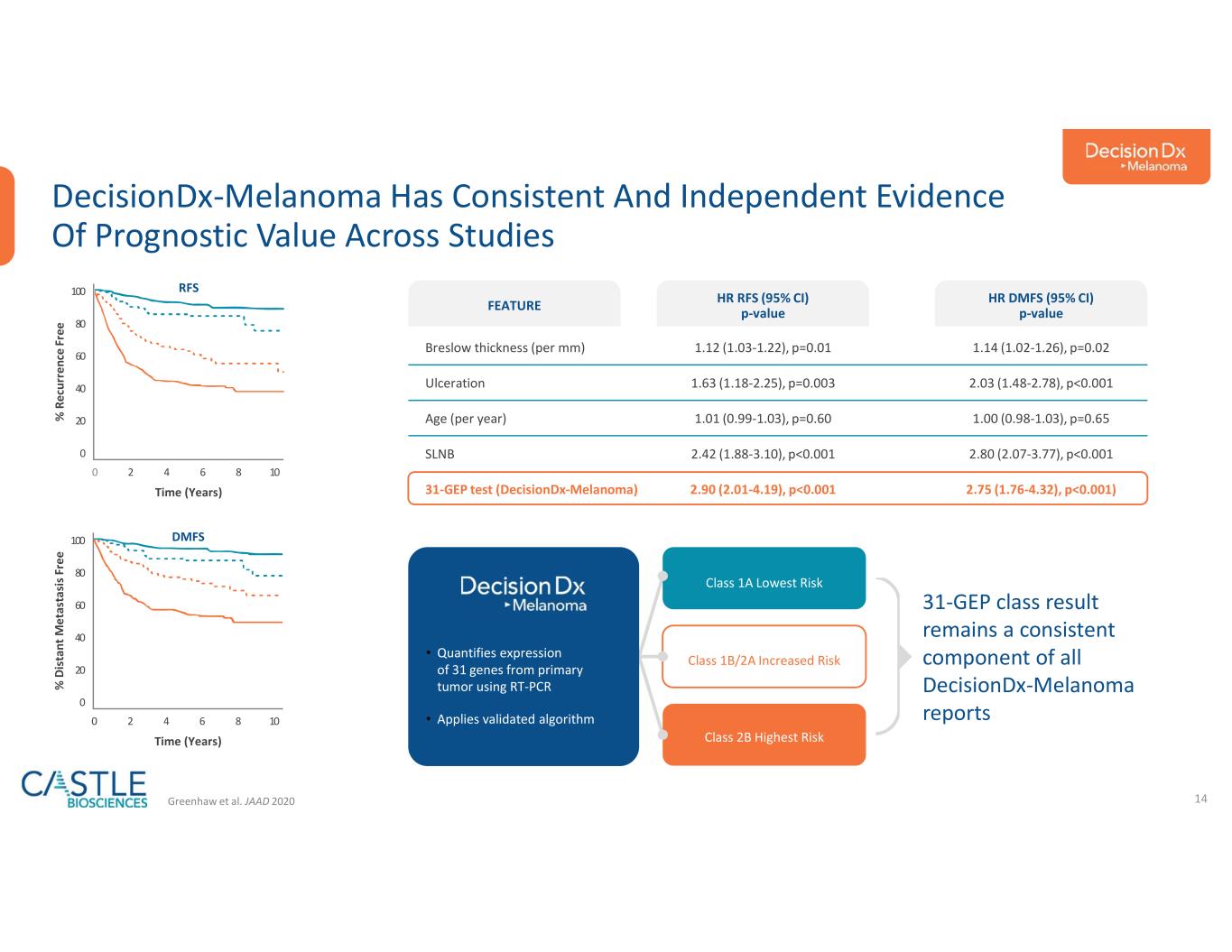
•DecisionDx-Melanoma: In October 2023, the Company announced a new study demonstrating DecisionDx-Melanoma outperforms a nomogram developed at the Memorial Sloan Kettering Cancer Center in predicting the risk of sentinel lymph node positivity in patients with cutaneous melanoma. The study can be found here.
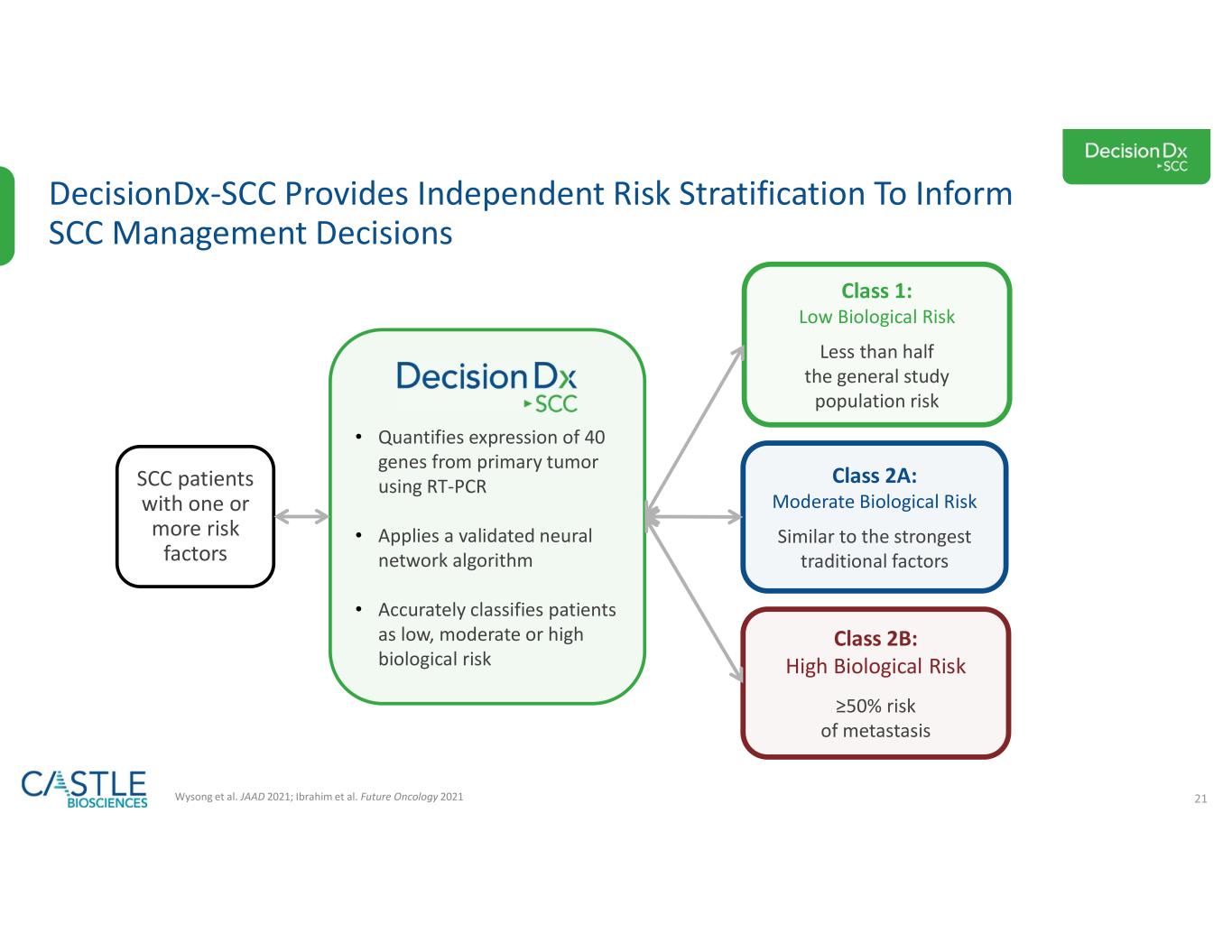
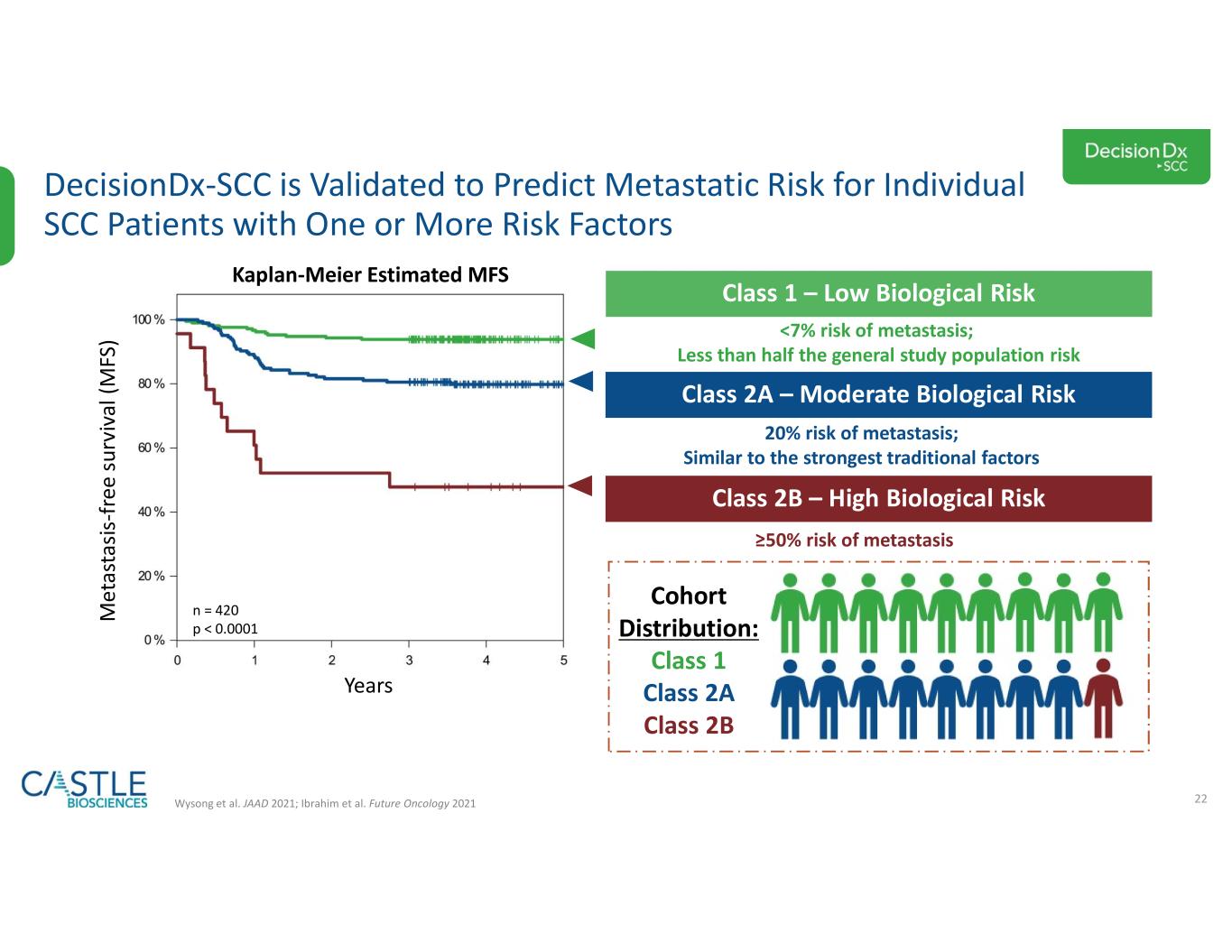
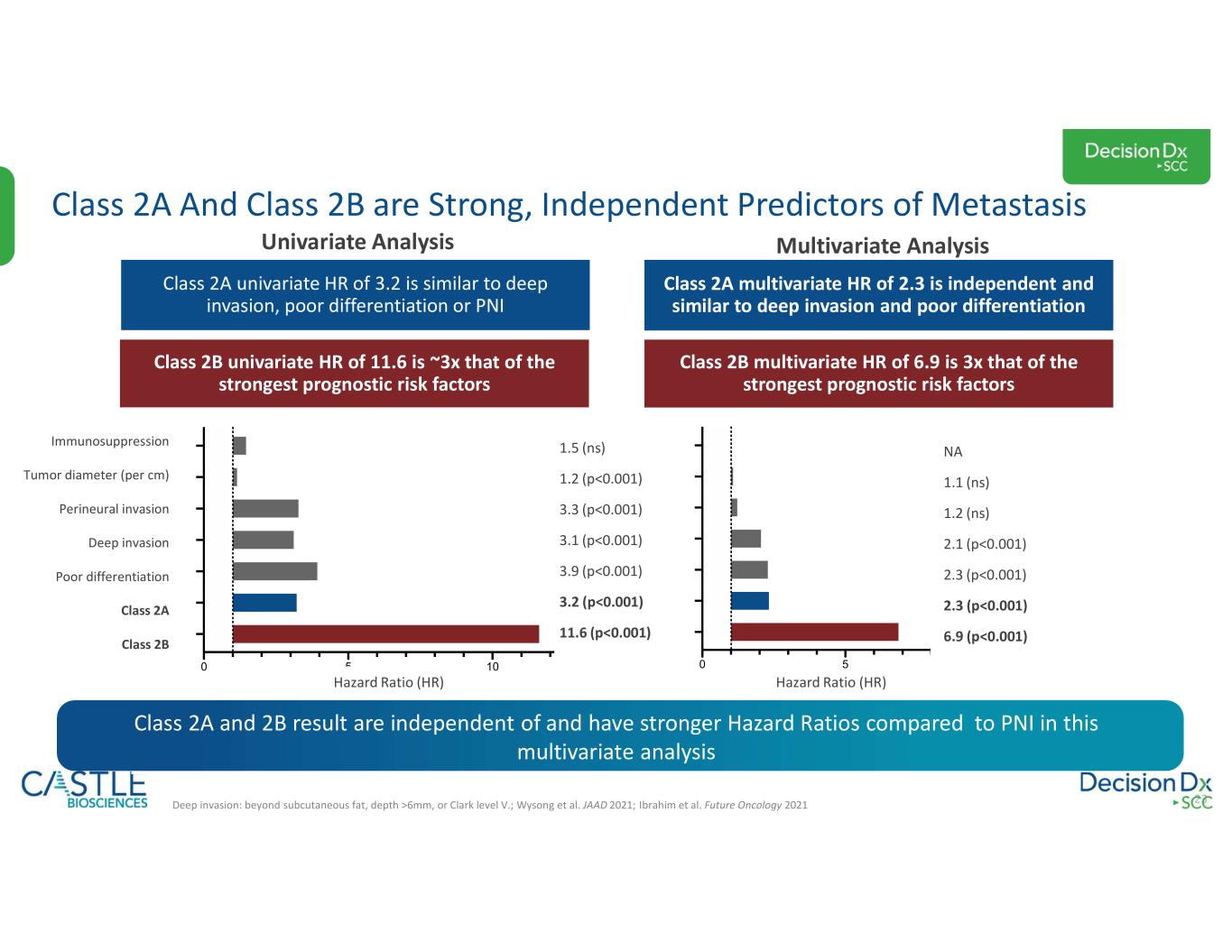
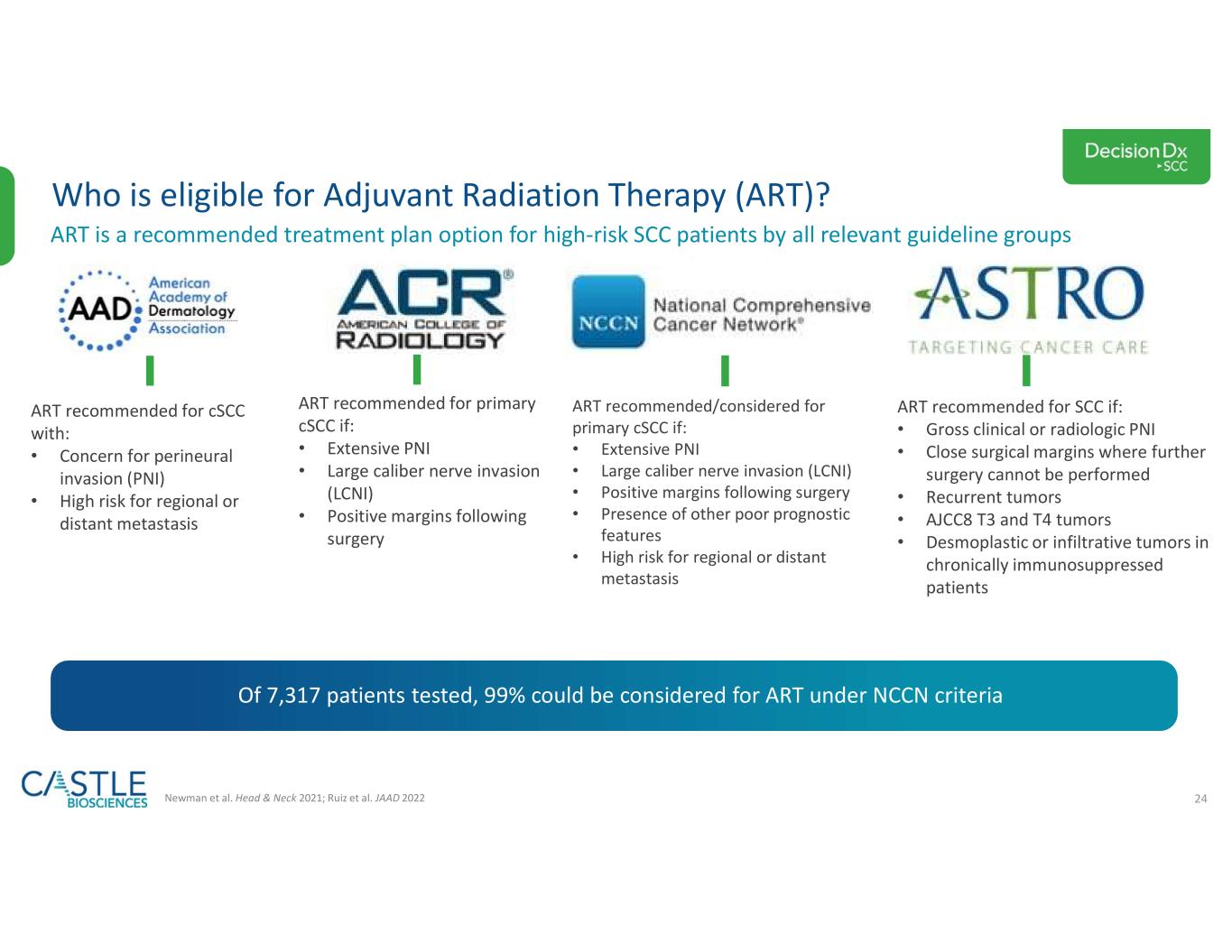
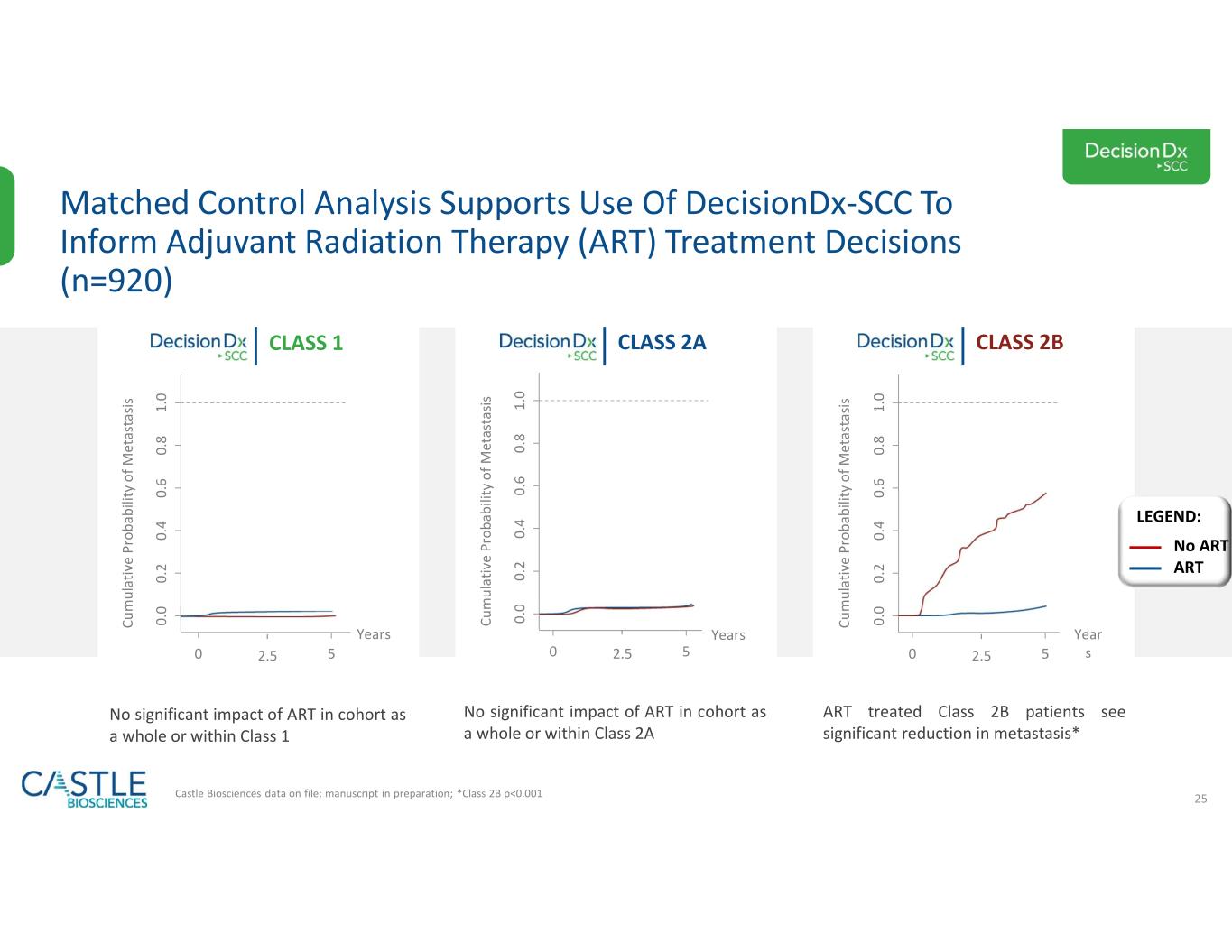
•DecisionDx-SCC: In October 2023, the Company shared new data demonstrating the ability of its DecisionDx-SCC test to identify localized high-risk cutaneous squamous cell carcinoma patients at a higher risk of metastasis who may benefit from adjuvant radiation therapy. See the Company’s news release from October 3, 2023, for more information.
Gastroenterology
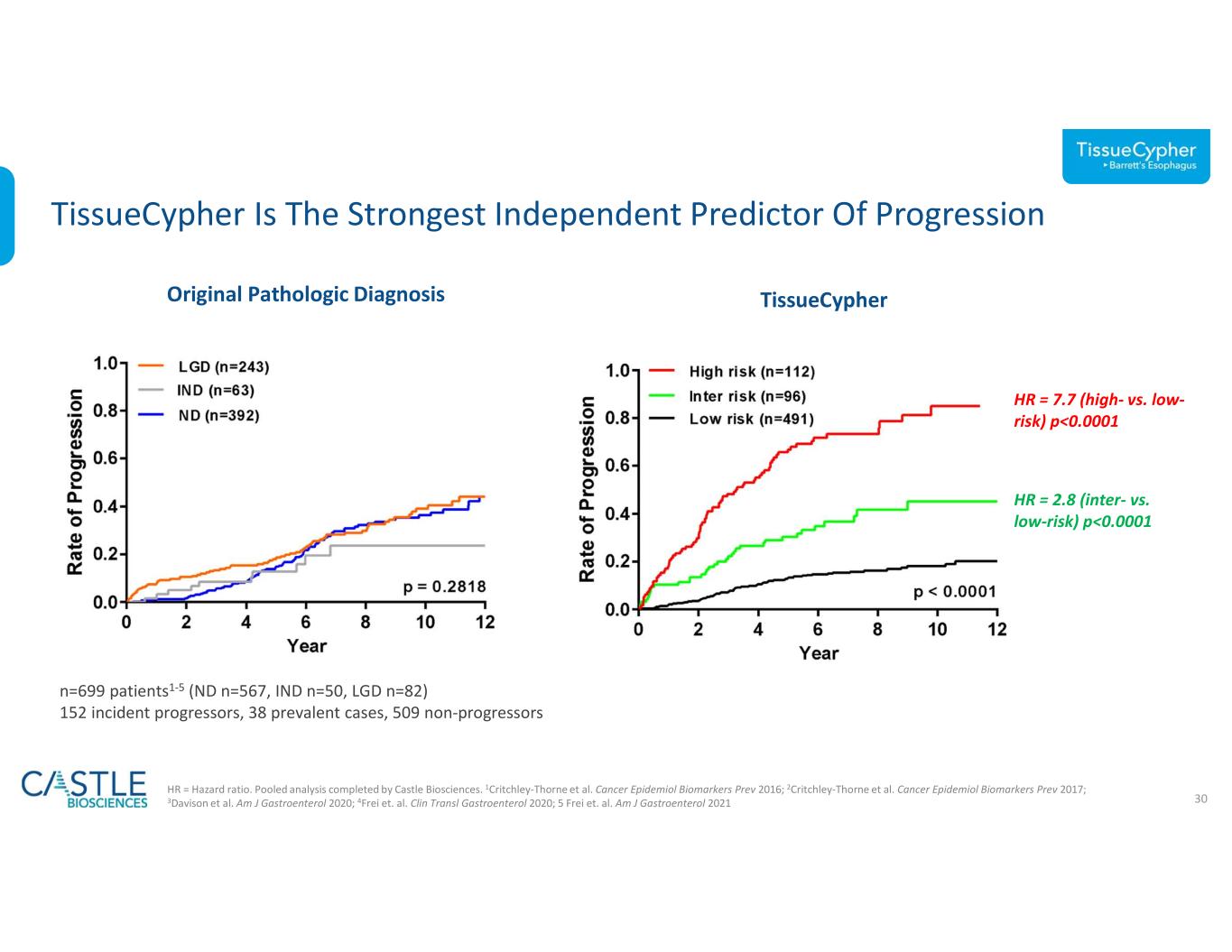
•In October, the Company announced new data demonstrating the significant clinical utility of its TissueCypher Barrett’s Esophagus test in guiding risk-aligned upstaging of care for patients with non-dysplastic Barrett’s esophagus (BE) at a higher risk of progression to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) than indicated by their clinicopathologic risk factors. See the Company’s news release from October 2, 2023, for more information.
•In September, the Company announced the publication of data demonstrating that the TissueCypher Barrett’s Esophagus test outperformed standard of care pathology review in predicting malignant progression to HGD and EAC in BE patients with an initial diagnosis of low-grade dysplasia (LGD).The study can be found here.
•In September, the Company announced new data demonstrating its TissueCypher Barrett’s Esophagus test can identify patients at a higher or lower risk of developing esophageal cancer than indicated by pathologic diagnoses and clinical risk factors to guide escalated or de-escalated patient management. See the Company’s news release from September 8, 2023, for more information.
•In August, the Company announced a new study published in The American Journal of Gastroenterology showing how use of TissueCypher Barrett’s Esophagus test results can significantly improve management decisions for BE patients with LGD to improve health outcomes. The study can be found here.
Mental Health
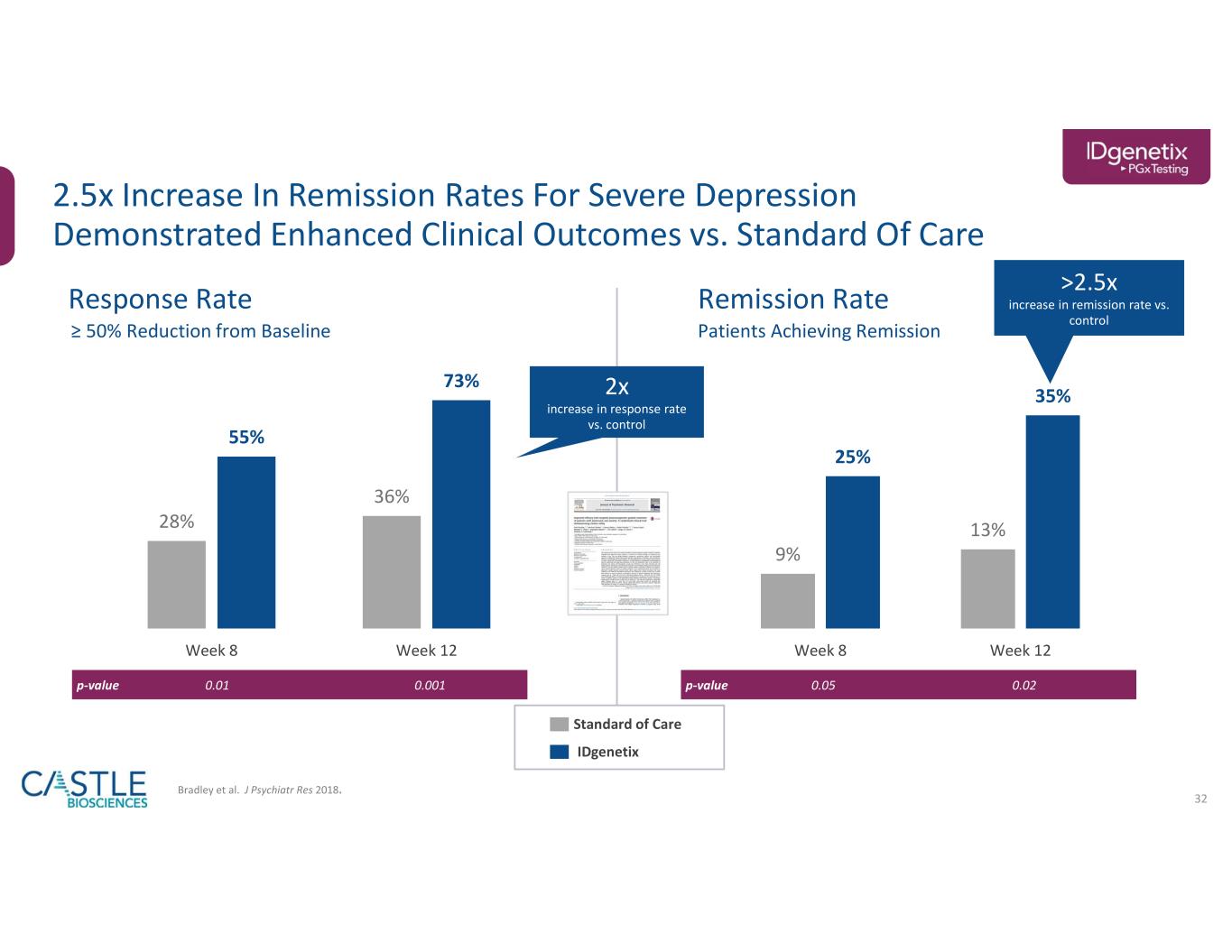
•In September, the Company announced data from a study showing the addition of drug-drug interactions and lifestyle factors to drug-gene interactions provided by its IDgenetix test significantly impacted the number of drug recommendations and contributed to improved remission rates for patients with moderate to severe depression. See the Company’s new release from September 9, 2023, for more information.
Corporate
•In September, the Company announced that it had earned a Top Workplaces National Industry Award, ranking third among 84 Top Workplaces in the healthcare industry. Castle has earned several additional Top Workplace awards this year, including Top Workplaces USA and Arizona Top Workplace awards, both for the second consecutive year, as well as the Culture Excellence Awards for Innovation, Work-Life Flexibility, Compensation & Benefits, Leadership and Purpose & Values. See the Company’s news release from September 19, 2023, for more information.
•In September, the Company announced that it had received its Clinical Laboratory Permit from the New York State Department of Health for its laboratory in Pittsburgh. Castle operates clinical laboratories in Pittsburgh and Phoenix. With the recent successful inspection in Pittsburgh, both laboratories are now permitted to provide test services to patients and physicians in the state of New York. Castle’s Phoenix laboratory received its permit in early 2018. See the Company’s news release from September 12, 2023, for more information.
Conference Call and Webcast Details
Castle Biosciences will hold a conference call on Thursday, Nov. 2, 2023, at 4:30 p.m. Eastern time to discuss its third quarter 2023 results and provide a corporate update.
A live webcast of the conference call can be accessed here: https://events.q4inc.com/attendee/479909909 or via the webcast link on the Investor Relations page of the Company’s website, https://ir.castlebiosciences.com/overview/default.aspx. Please access the webcast at least 10 minutes before the conference call start time. An archive of the webcast will be available on the Company’s website until Nov. 23, 2023.
To access the live conference call via phone, please dial 833 470 1428 from the United States, or +1 404 975 4839 internationally, at least 10 minutes prior to the start of the call, using the conference ID 925738.
There will be a brief Question & Answer session following management commentary.
Use of Non-GAAP Financial Measures (UNAUDITED)
In this release, we use the metrics of Adjusted Revenues, Adjusted Gross Margin and Adjusted EBITDA, which are non-GAAP financial measures and are not calculated in accordance with generally accepted accounting principles in the United States (GAAP). Adjusted Revenues and Adjusted Gross Margin reflect adjustments to GAAP net revenues to exclude net positive and/or net negative revenue adjustments recorded in the current period associated with changes in estimated variable consideration related to test reports delivered in previous periods. Adjusted Gross Margin further excludes acquisition-related intangible asset amortization. Adjusted EBITDA excludes from net loss interest income, interest expense, income tax expense (benefit), depreciation and amortization expense, stock-based compensation expense, change in fair value of contingent consideration and acquisition related transaction costs.
We use Adjusted Revenues, Adjusted Gross Margin and Adjusted EBITDA internally because we believe these metrics provide useful supplemental information in assessing our revenue and operating performance reported in accordance with GAAP, respectively. We believe that Adjusted Revenues, when used in conjunction with our test report volume information, facilitates investors’ analysis of our current-period revenue performance and average selling price performance by excluding the effects of revenue adjustments related to test reports delivered in prior periods, since these adjustments may not be indicative of the current or future performance of our business. We believe that providing Adjusted Revenues may also help facilitate comparisons to our historical periods. Adjusted Gross Margin is calculated using Adjusted Revenues and therefore excludes the impact of revenue adjustments related to test reports delivered in prior periods, which we believe is useful to investors as described above. We further exclude acquisition-related intangible asset amortization in the calculation of Adjusted Gross Margin. We believe that excluding acquisition-related intangible asset amortization may facilitate gross margin comparisons to historical periods and may be useful in assessing current-period performance without regard to the historical accounting valuations of intangible assets, which are applicable only to tests we acquired rather than internally developed. We believe Adjusted EBITDA may enhance an evaluation of our operating performance because it excludes the impact of prior decisions made about capital investment, financing, investing and certain expenses we believe are not indicative of our ongoing performance. However, these non-GAAP financial measures may be different from non-GAAP financial measures used by other companies, even when the same or similarly titled terms are used to identify such measures, limiting their usefulness for comparative purposes.
These non-GAAP financial measures are not meant to be considered in isolation or used as substitutes for net revenues, gross margin, or net loss reported in accordance with GAAP; should be considered in conjunction with our financial information presented in accordance with GAAP; have no standardized meaning prescribed by GAAP; are unaudited; and are not prepared under any comprehensive set of accounting rules or principles. In addition, from time to time in the future, there may be other items that we may exclude for purposes of these non-GAAP financial measures, and we may in the future cease to exclude items that we have historically excluded for purposes of these non-GAAP financial measures. Likewise, we may determine to modify the nature of adjustments to arrive at these non-GAAP financial measures. Because of the non-standardized definitions of non-GAAP financial measures, the non-GAAP financial measure as used by us in this press release and the accompanying reconciliation tables have limits in their usefulness to investors and may be calculated differently from, and therefore may not be directly comparable to, similarly titled measures used by other companies. Accordingly, investors should not place undue reliance on non-GAAP financial measures. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures are presented in the tables at the end of this release.
About Castle Biosciences
Castle Biosciences (Nasdaq: CSTL) is a leading diagnostics company improving health through innovative tests that guide patient care. The Company aims to transform disease management by keeping people first: patients, clinicians, employees and investors.
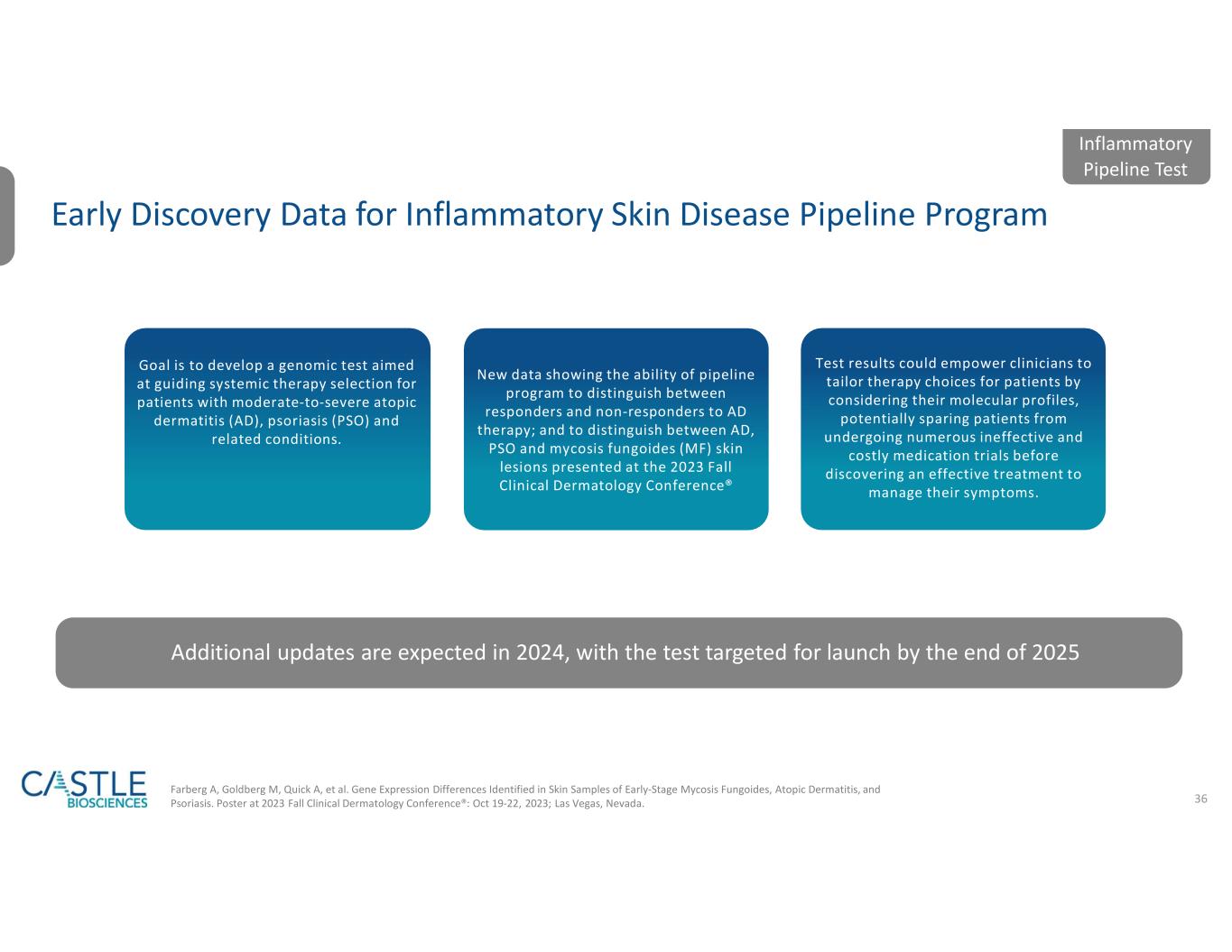
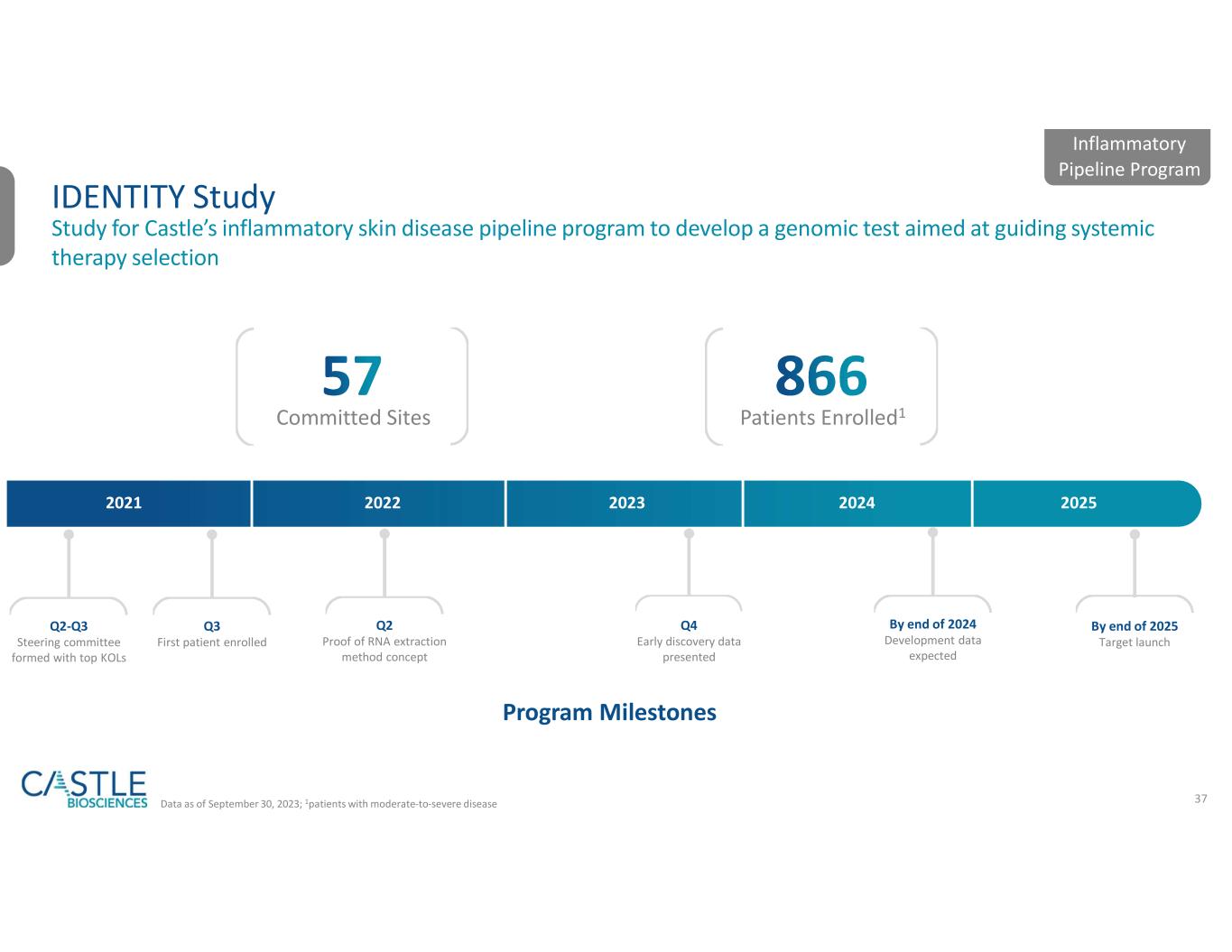
Castle’s current portfolio consists of tests for skin cancers, uveal melanoma, Barrett’s esophagus and mental health conditions. Additionally, the Company has active research and development programs for tests in other diseases with high clinical need, including its test in development to help guide systemic therapy selection for patients with moderate-to-severe, atopic dermatitis, psoriasis and related conditions.
To learn more, please visit www.CastleBiosciences.com and connect with us on LinkedIn, Facebook, X and Instagram.
DecisionDx-Melanoma, DecisionDx-CMSeq, DecisionDx-SCC, MyPath Melanoma, DiffDx-Melanoma, DecisionDx-UM, DecisionDx-PRAME, DecisionDx-UMSeq, TissueCypher and IDgenetix are trademarks of Castle Biosciences, Inc.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning our expectations regarding: (i) our full year 2023 revenue guidance of at least $200 million; (ii) our continued investment in our strategic growth across our entire test portfolio and the ability of this investment to continue to support our long-term value creation plans and the improvement of patient care; (iii) the potential clinical value and utility of our tests, including with respect to findings in the studies highlighted in this press release; (iv) our belief that the DecisionDx-SCC test can identify localized high-risk cutaneous squamous cell carcinoma patients at a higher risk of metastasis who may benefit from adjuvant radiation therapy; (v) our belief that the TissueCypher Barrett’s Esophagus test can identify patients at a higher or lower risk of developing esophageal cancer and that such test results can significantly improve management decisions for BE patients with LGD to improve health outcomes; and (vi) our belief that the IDgenetix test significantly impacted the number of drug recommendations and contributed to improved remission rates for patients with moderate to severe depression. The words “anticipate,” “can,” “could,” “expect,” “goal,” “may,” “plan” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions, or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from those in the forward-looking statements, including, without limitation: the accuracy of our assumptions and expectations underlying our fiscal 2023 revenue guidance (including, without limitation, our assumptions or expectations regarding continued reimbursement for our DecisionDx-SCC test at the current rate and reimbursement for our other products and subsequent coverage decisions, our estimated total addressable markets for our products and product candidates and the related expenses, capital requirements and potential needs for additional financing, the anticipated cost, timing and success of our product candidates, and our plans to research, develop and commercialize new tests and our ability to successfully integrate new businesses, assets, products or technologies acquired through acquisitions), the effects of macroeconomic events and conditions, including inflation and monetary supply shifts, labor shortages, liquidity concerns at, and failures of, banks and other financial institutions or other disruptions in the banking system or financing markets and recession risks, supply chain disruptions, outbreaks of contagious diseases (such as the COVID-19 pandemic) and geopolitical events (such as the ongoing Israel-Hamas War and Ukraine-Russia conflict), among others, on our business and our efforts to address its impact on our business; subsequent study or trial results and findings may contradict earlier study or trial results and findings or may not support the results discussed in this press release, including with respect to the tests discussed in this press release; our planned installation of additional equipment and supporting technology infrastructures and implementation of certain process efficiencies may not enable us to increase the future scalability of our TissueCypher Test; actual application of our tests may not provide the aforementioned benefits to patients; our newer gastroenterology and mental health franchises may not contribute to the achievement of our long-term financial targets as anticipated; and the risks set forth under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2022, our Quarterly Report on Form 10-Q for the three months ended September 30, 2023, each filed or to be filed with the SEC, and in our other filings with the SEC. The forward-looking statements are applicable only as of the date on which they are made, and we do not assume any obligation to update any forward-looking statements, except as may be required by law.
Investor Relations Contact:
Camilla Zuckero
czuckero@castlebiosciences.com
281-906-3868
Media Contact:
Allison Marshall
amarshall@castlebiosciences.com
###
CASTLE BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| NET REVENUES |
$ |
61,493 |
|
|
$ |
37,011 |
|
|
$ |
153,668 |
|
|
$ |
98,701 |
|
| OPERATING EXPENSES AND OTHER OPERATING INCOME |
|
|
|
|
|
|
|
| Cost of sales (exclusive of amortization of acquired intangible assets) |
11,319 |
|
|
8,859 |
|
|
32,559 |
|
|
22,489 |
|
| Research and development |
12,923 |
|
|
10,907 |
|
|
40,624 |
|
|
33,594 |
|
| Selling, general and administrative |
44,619 |
|
|
36,626 |
|
|
136,062 |
|
|
104,577 |
|
| Amortization of acquired intangible assets |
2,272 |
|
|
2,306 |
|
|
6,742 |
|
|
6,051 |
|
| Change in fair value of contingent consideration |
— |
|
|
(151) |
|
|
— |
|
|
(17,987) |
|
|
|
|
|
|
|
|
|
| Total operating expenses, net |
71,133 |
|
|
58,547 |
|
|
215,987 |
|
|
148,724 |
|
| Operating loss |
(9,640) |
|
|
(21,536) |
|
|
(62,319) |
|
|
(50,023) |
|
| Interest income |
2,769 |
|
|
1,293 |
|
|
7,504 |
|
|
1,693 |
|
| Interest expense |
(2) |
|
|
(6) |
|
|
(9) |
|
|
(13) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss before income taxes |
(6,873) |
|
|
(20,249) |
|
|
(54,824) |
|
|
(48,343) |
|
Income tax expense (benefit) |
32 |
|
|
— |
|
|
62 |
|
|
(1,823) |
|
| Net loss |
$ |
(6,905) |
|
|
$ |
(20,249) |
|
|
$ |
(54,886) |
|
|
$ |
(46,520) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss per share, basic and diluted |
$ |
(0.26) |
|
|
$ |
(0.77) |
|
|
$ |
(2.05) |
|
|
$ |
(1.79) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted-average shares outstanding, basic and diluted |
26,834 |
|
|
26,316 |
|
|
26,725 |
|
|
25,938 |
|
|
|
|
|
|
|
|
|
Stock-Based Compensation Expense
Stock-based compensation expense is included in the condensed consolidated statements of operations as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Cost of sales (exclusive of amortization of acquired intangible assets) |
$ |
1,245 |
|
|
$ |
975 |
|
|
$ |
3,719 |
|
|
$ |
2,725 |
|
| Research and development |
2,682 |
|
|
1,948 |
|
|
7,755 |
|
|
5,607 |
|
| Selling, general and administrative |
9,116 |
|
|
6,273 |
|
|
27,943 |
|
|
18,066 |
|
| Total stock-based compensation expense |
$ |
13,043 |
|
|
$ |
9,196 |
|
|
$ |
39,417 |
|
|
$ |
26,398 |
|
CASTLE BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(UNAUDITED)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Net loss |
$ |
(6,905) |
|
|
$ |
(20,249) |
|
|
$ |
(54,886) |
|
|
$ |
(46,520) |
|
| Other comprehensive income (loss): |
|
|
|
|
|
|
|
| Net unrealized gain (loss) on marketable investment securities |
73 |
|
|
(189) |
|
|
310 |
|
|
(189) |
|
| Comprehensive loss |
$ |
(6,832) |
|
|
$ |
(20,438) |
|
|
$ |
(54,576) |
|
|
$ |
(46,709) |
|
CASTLE BIOSCIENCES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
| ASSETS |
(unaudited) |
|
|
| Current Assets |
|
|
|
| Cash and cash equivalents |
$ |
91,223 |
|
|
$ |
122,948 |
|
| Marketable investment securities |
138,530 |
|
|
135,677 |
|
| Accounts receivable, net |
37,003 |
|
|
23,476 |
|
| Inventory |
5,769 |
|
|
3,980 |
|
| Prepaid expenses and other current assets |
7,097 |
|
|
6,207 |
|
| Total current assets |
279,622 |
|
|
292,288 |
|
| Long-term accounts receivable, net |
1,338 |
|
|
1,087 |
|
| Property and equipment, net |
22,273 |
|
|
14,315 |
|
| Operating lease assets |
11,613 |
|
|
12,181 |
|
| Goodwill and other intangible assets, net |
119,607 |
|
|
126,348 |
|
| Other assets – long-term |
1,566 |
|
|
1,110 |
|
| Total assets |
$ |
436,019 |
|
|
$ |
447,329 |
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
| Current Liabilities |
|
|
|
| Accounts payable |
$ |
6,929 |
|
|
$ |
4,731 |
|
| Accrued compensation |
22,405 |
|
|
24,358 |
|
|
|
|
|
| Operating lease liabilities |
1,091 |
|
|
1,777 |
|
| Other accrued and current liabilities |
5,899 |
|
|
5,262 |
|
| Total current liabilities |
36,324 |
|
|
36,128 |
|
|
|
|
|
| Noncurrent operating lease liabilities |
13,435 |
|
|
11,533 |
|
| Deferred tax liability |
441 |
|
|
428 |
|
| Other liabilities |
36 |
|
|
90 |
|
| Total liabilities |
50,236 |
|
|
48,179 |
|
|
|
|
|
| Stockholders’ Equity |
|
|
|
Common stock |
27 |
|
|
27 |
|
| Additional paid-in capital |
601,618 |
|
|
560,409 |
|
| Accumulated deficit |
(215,791) |
|
|
(160,905) |
|
| Accumulated other comprehensive loss |
(71) |
|
|
(381) |
|
| Total stockholders’ equity |
385,783 |
|
|
399,150 |
|
| Total liabilities and stockholders’ equity |
$ |
436,019 |
|
|
$ |
447,329 |
|
CASTLE BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
|
|
|
| OPERATING ACTIVITIES |
|
|
|
| Net loss |
$ |
(54,886) |
|
|
$ |
(46,520) |
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
| Depreciation and amortization |
9,106 |
|
|
7,702 |
|
| Stock-based compensation expense |
39,417 |
|
|
26,398 |
|
| Change in fair value of contingent consideration |
— |
|
|
(17,987) |
|
| Deferred income taxes |
13 |
|
|
(1,839) |
|
| Accretion of discounts on marketable investment securities |
(3,851) |
|
|
(184) |
|
| Other |
284 |
|
|
186 |
|
| Change in operating assets and liabilities: |
|
|
|
| Accounts receivable |
(13,779) |
|
|
(5,678) |
|
| Prepaid expenses and other current assets |
(892) |
|
|
(1,870) |
|
| Inventory |
(1,789) |
|
|
(1,502) |
|
| Operating lease assets |
(590) |
|
|
694 |
|
| Other assets |
(455) |
|
|
533 |
|
| Accounts payable |
2,693 |
|
|
2,155 |
|
| Operating lease liabilities |
1,093 |
|
|
(559) |
|
| Accrued compensation |
(1,953) |
|
|
3,669 |
|
|
|
|
|
| Other accrued and current liabilities |
1,376 |
|
|
(853) |
|
|
|
|
|
| Net cash used in operating activities |
(24,213) |
|
|
(35,655) |
|
|
|
|
|
| INVESTING ACTIVITIES |
|
|
|
| Purchases of property and equipment |
(9,828) |
|
|
(3,845) |
|
| Asset acquisition, adjustment to purchase price |
— |
|
|
547 |
|
| Acquisition of business, net of cash and cash equivalents acquired |
— |
|
|
(26,966) |
|
| Proceeds from sale of property and equipment |
10 |
|
|
9 |
|
| Purchases of marketable investment securities |
(136,693) |
|
|
(131,808) |
|
| Proceeds from maturities of marketable investment securities |
138,000 |
|
|
— |
|
|
|
|
|
|
|
|
|
| Net cash used in investing activities |
(8,511) |
|
|
(162,063) |
|
|
|
|
|
| FINANCING ACTIVITIES |
|
|
|
|
|
|
|
| Proceeds from exercise of common stock options |
197 |
|
|
675 |
|
| Payment of employees’ taxes on vested restricted stock units |
(1,119) |
|
|
(134) |
|
| Proceeds from contributions to the employee stock purchase plan |
2,027 |
|
|
1,812 |
|
| Repayment of principal portion of finance lease liabilities |
(106) |
|
|
(88) |
|
| Net cash provided by financing activities |
999 |
|
|
2,265 |
|
|
|
|
|
| NET CHANGE IN CASH AND CASH EQUIVALENTS |
(31,725) |
|
|
(195,453) |
|
| Beginning of period |
122,948 |
|
|
329,633 |
|
| End of period |
$ |
91,223 |
|
|
$ |
134,180 |
|
CASTLE BIOSCIENCES, INC.
Reconciliation of Non-GAAP Financial Measures (UNAUDITED)
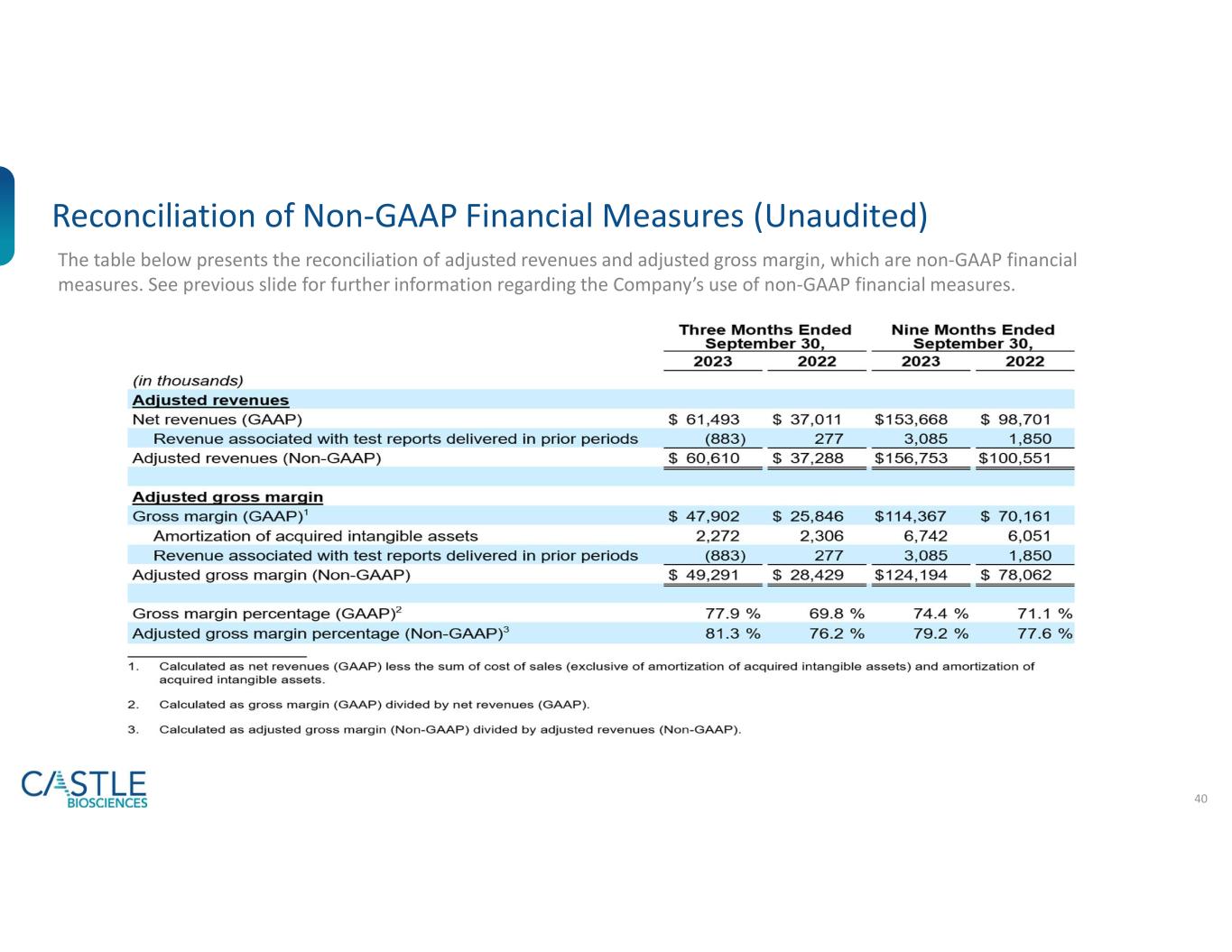
The table below presents the reconciliation of adjusted revenues and adjusted gross margin, which are non-GAAP financial measures. See "Use of Non-GAAP Financial Measures (UNAUDITED)" above for further information regarding the Company's use of non-GAAP financial measures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| (in thousands) |
|
|
|
|
|
|
|
| Adjusted revenues |
|
|
|
|
|
|
|
| Net revenues (GAAP) |
$ |
61,493 |
|
|
$ |
37,011 |
|
|
$ |
153,668 |
|
|
$ |
98,701 |
|
|
|
|
|
|
|
|
|
| Revenue associated with test reports delivered in prior periods |
(883) |
|
|
277 |
|
|
3,085 |
|
|
1,850 |
|
| Adjusted revenues (Non-GAAP) |
$ |
60,610 |
|
|
$ |
37,288 |
|
|
$ |
156,753 |
|
|
$ |
100,551 |
|
|
|
|
|
|
|
|
|
| Adjusted gross margin |
|
|
|
|
|
|
|
Gross margin (GAAP)1 |
$ |
47,902 |
|
|
$ |
25,846 |
|
|
$ |
114,367 |
|
|
$ |
70,161 |
|
| Amortization of acquired intangible assets |
2,272 |
|
|
2,306 |
|
|
6,742 |
|
|
6,051 |
|
| Revenue associated with test reports delivered in prior periods |
(883) |
|
|
277 |
|
|
3,085 |
|
|
1,850 |
|
| Adjusted gross margin (Non-GAAP) |
$ |
49,291 |
|
|
$ |
28,429 |
|
|
$ |
124,194 |
|
|
$ |
78,062 |
|
|
|
|
|
|
|
|
|
Gross margin percentage (GAAP)2 |
77.9 |
% |
|
69.8 |
% |
|
74.4 |
% |
|
71.1 |
% |
Adjusted gross margin percentage (Non-GAAP)3 |
81.3 |
% |
|
76.2 |
% |
|
79.2 |
% |
|
77.6 |
% |
1.Calculated as net revenues (GAAP) less the sum of cost of sales (exclusive of amortization of acquired intangible assets) and amortization of acquired intangible assets.
2.Calculated as gross margin (GAAP) divided by net revenues (GAAP).
3.Calculated as adjusted gross margin (Non-GAAP) divided by adjusted revenues (Non-GAAP).
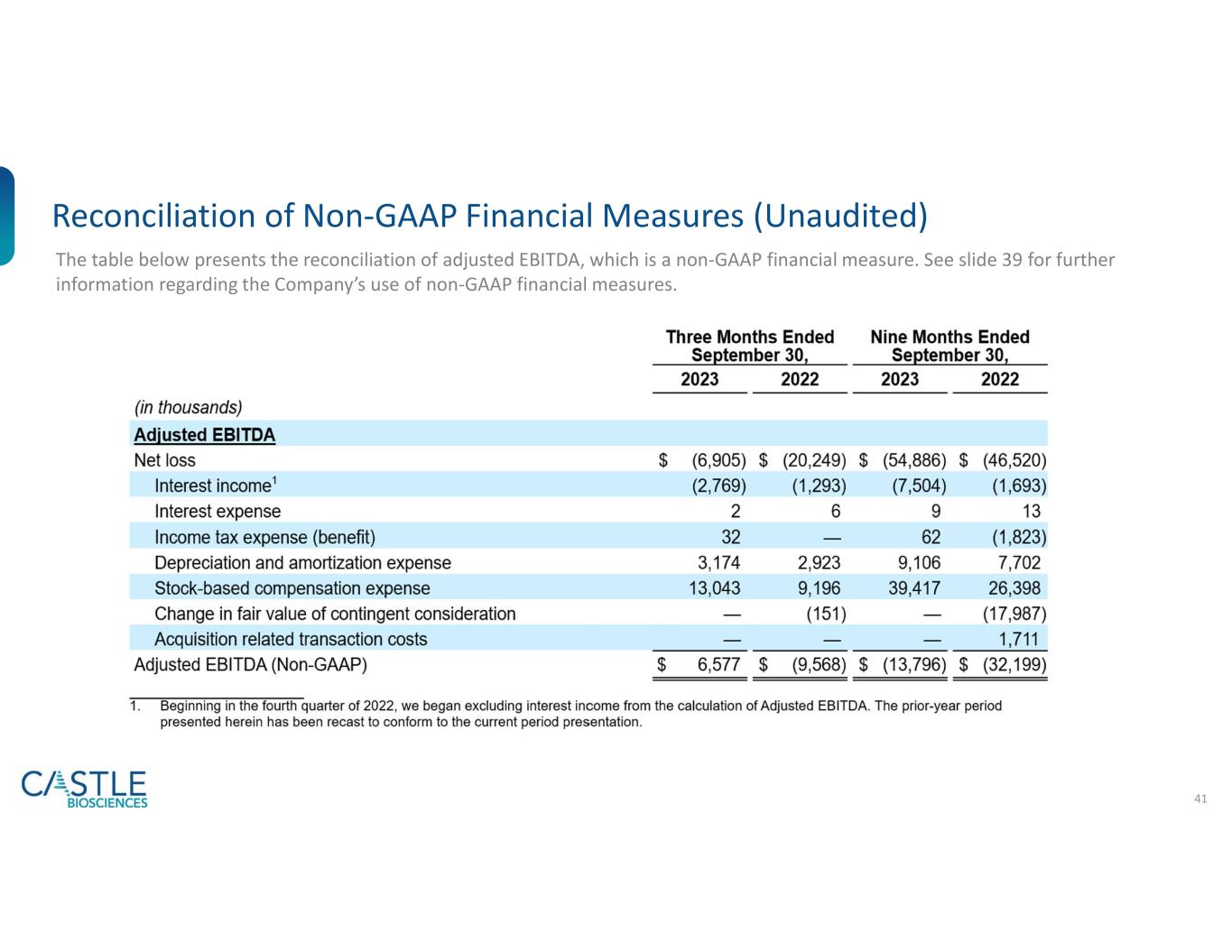
The table below presents the reconciliation of adjusted EBITDA, which is a non-GAAP financial measure. See "Use of Non-GAAP Financial Measures (UNAUDITED)" above for further information regarding the Company's use of non-GAAP financial measures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| (in thousands) |
|
|
|
|
|
|
|
| Adjusted EBITDA |
|
|
|
|
|
|
|
| Net loss |
$ |
(6,905) |
|
|
$ |
(20,249) |
|
|
$ |
(54,886) |
|
|
$ |
(46,520) |
|
Interest income1 |
(2,769) |
|
|
(1,293) |
|
|
(7,504) |
|
|
(1,693) |
|
| Interest expense |
2 |
|
|
6 |
|
|
9 |
|
|
13 |
|
Income tax expense (benefit) |
32 |
|
|
— |
|
|
62 |
|
|
(1,823) |
|
| Depreciation and amortization expense |
3,174 |
|
|
2,923 |
|
|
9,106 |
|
|
7,702 |
|
| Stock-based compensation expense |
13,043 |
|
|
9,196 |
|
|
39,417 |
|
|
26,398 |
|
| Change in fair value of contingent consideration |
— |
|
|
(151) |
|
|
— |
|
|
(17,987) |
|
| Acquisition related transaction costs |
— |
|
|
— |
|
|
— |
|
|
1,711 |
|
| Adjusted EBITDA (Non-GAAP) |
$ |
6,577 |
|
|
$ |
(9,568) |
|
|
$ |
(13,796) |
|
|
$ |
(32,199) |
|
1. Beginning in the fourth quarter of 2022, we began excluding interest income from the calculation of Adjusted EBITDA. The prior-year period presented herein has been recast to conform to the current period presentation.