UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark one)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended September 30, 2025
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934‐‐
For the transition period from ________________ to __________________
Commission File Number: 001-36291
DIAMEDICA THERAPEUTICS INC.
(Exact name of registrant as specified in its charter)
|
British Columbia, Canada (State or other jurisdiction of incorporation or organization) |
Not Applicable (I.R.S. Employer Identification No.) |
|
301 Carlson Parkway, Suite 210 Minneapolis, Minnesota 55305 (Address of principal executive offices) (Zip Code) |
|
(763) 496-5454
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol |
Name of each exchange on which registered |
|
Voting common shares, no par value per share |
DMAC |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐ |
Accelerated filer ☐ |
|
Non-accelerated filer ☒ |
Smaller reporting company ☒ Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 10, 2025, there were 52,077,439 voting common shares of the registrant outstanding.
DiaMedica Therapeutics Inc.
FORM 10-Q
September 30, 2025
TABLE OF CONTENTS
| Description | Page | |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
1 |
|
|
PART I. |
FINANCIAL INFORMATION | |
|
|
||
|
Item 1. |
Financial Statements |
2 |
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
14 |
|
Item 3. |
Quantitative and Qualitative Disclosures about Market Risk |
20 |
|
Item 4. |
Controls and Procedures |
20 |
|
PART II. |
OTHER INFORMATION | |
|
Item 1. |
Legal Proceedings |
21 |
|
Item 1A. |
Risk Factors |
21 |
|
Item 2. |
Unregistered Sales of Equity Securities and Use of Proceeds |
22 |
|
Item 3. |
Defaults Upon Senior Securities |
22 |
|
Item 4. |
Mine Safety Disclosures |
22 |
|
Item 5. |
Other Information |
22 |
|
Item 6. |
Exhibits |
23 |
|
SIGNATURE PAGE |
24 |
|
This quarterly report on Form 10-Q contains certain forward-looking statements that are within the meaning of Section 27A of the United States Securities Act of 1933, as amended, and Section 21E of the United States Securities Exchange Act of 1934, as amended, and are subject to the safe harbor created by those sections. For more information, see “Cautionary Note Regarding Forward-Looking Statements.”
As used in this report, references to “DiaMedica,” the “Company,” “we,” “our” or “us,” unless the context otherwise requires, refer to DiaMedica Therapeutics Inc. and its subsidiaries, all of which are consolidated in DiaMedica’s condensed consolidated financial statements. References in this report to “common shares” mean our voting common shares, no par value per share.
We own various unregistered trademarks and service marks, including our corporate logo. Solely for convenience, the trademarks and trade names in this report are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that the owner of such trademarks and trade names will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this report that are not descriptions of historical facts are forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition, prospects and share price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would,” the negative of these terms or other comparable terminology and the use of future dates.
The forward-looking statements in this report are subject to risks and uncertainties and include, among other things:
|
● |
our plans to develop, obtain an investigational new drug (IND) application for the clinical study of DM199 for PE and ultimately to obtain regulatory approval for and commercialize our DM199 product candidate for the treatment of preeclampsia (PE) and acute ischemic stroke (AIS); |
|
● |
our ability to conduct successful clinical testing of our DM199 product candidate for PE and AIS and meet certain anticipated or target milestones and dates thereof with respect to our clinical studies; |
|
● |
the ability of our physician collaborators to successfully complete the current Phase 2, proof-of-concept clinical trial of DM199 for the treatment of PE, our reliance on these physician collaborators to conduct the study, and our expectations related to the timing of Part 1a of this study and the ability of these physician collaborators to identify a suitable dose for use in Part 1b of this study; |
|
● |
our ability to meet anticipated site activations, enrollment and interim analysis timing with respect to our Phase 2/3 ReMEDy2 clinical trial of DM199 for the treatment of AIS, especially in the light of slower than expected site activations and enrollment which we believe are due, in part, to hospital and medical facility staffing shortages; inclusion/exclusion criteria in the study protocol; concerns managing logistics and protocol compliance for participants discharged from the hospital to an intermediate care facility; concerns regarding the prior clinically significant hypotension events and circumstances surrounding the clinical hold which was lifted in June 2023; use of artificial intelligence and telemedicine which have enabled smaller hospitals to retain AIS patients not eligible for mechanical thrombectomy instead of sending these patients to the larger stroke centers which are more likely to be sites in our trial; and competition for research staff and trial subjects due to other pending stroke and neurological clinical trials; |
|
● |
the success of the actions we are taking to mitigate the impact of the factors adversely affecting our ReMEDy2 trial site activations and enrollment rate, including significantly expanding our internal clinical team and bringing in-house certain trial activities, such as study site identification, qualification and activation, clinical site monitoring and overall program management; globally expanding the trial; and making additional changes to the study protocol; and risks associated with these mitigation actions; |
|
● |
uncertainties relating to regulatory applications and related filing and approval timelines, especially in light of recent changes in funding and staffing levels for the U.S. Food and Drug Administration (FDA) and other government agencies; |
|
● |
the possible occurrence of future adverse events associated with or unfavorable results from the Phase 2 investigator-sponsored PE trial or our ReMEDy2 trial and their potential to adversely effect current or future trials; |
|
● |
the adaptive design of our ReMEDy2 trial, which is intended to enroll approximately 300 patients at up to 100 sites globally, and the possibility that the final sample size, which will be determined based upon the results of an interim analysis of 200 participants, may be up to 728 patients, according to a pre-determined statistical plan, other possible changes in the trial, including as a result of input from the FDA, and the results of the interim analysis as determined by our independent data safety monitoring board; |
|
● |
our expectations regarding the perceived benefits of our DM199 product candidate over existing treatment options for PE and AIS; |
|
● |
our ability to partner with and generate revenue from biopharmaceutical or pharmaceutical partners to develop, obtain regulatory approval for, and commercialize our DM199 product candidate for PE and AIS; |
|
● |
the potential size of the markets for our DM199 product candidate for PE and AIS and our or any future partner’s ability to serve those markets, the rate and degree of market acceptance of and ability to obtain coverage and adequate reimbursement for, our DM199 product candidate for PE and AIS both in the United States and internationally; |
|
● |
the success, cost and timing of our clinical trials, as well as our reliance on our key executives, clinical personnel, advisors and third parties in connection with our trials; |
|
● |
our or any future partner’s ability to commercialize, market and manufacture DM199; |
|
● |
expectations regarding U.S. federal, state and foreign regulatory requirements and developments affecting our pending and future clinical trials and regulatory approvals of our DM199 product candidate for PE and AIS and future commercialization and manufacturing of such products if required regulatory approvals are obtained; |
|
● |
our expectations regarding our ability to obtain and maintain intellectual property protection for our DM199 product candidate; |
|
● |
expectations regarding competition and our ability to obtain data exclusivity for our DM199 product candidate for PE and AIS; and |
|
● |
our estimates regarding expenses, market opportunity for our product candidates, future revenue, and capital requirements; our anticipated use of the net proceeds from our prior private placements; how long our current cash resources will last; and our need for and ability to obtain additional financing to fund our operations, including funding necessary to complete our current clinical trials and obtain regulatory approvals for our DM199 product candidate for PE and/or AIS. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under “Part I. Item 1A. Risk Factors” in our annual report on Form 10-K for the fiscal year ended December 31, 2024 and those described above and elsewhere in this report, including under “Part II. Item 1A. Risk Factors.” Moreover, we operate in a very competitive and rapidly-changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Forward-looking statements should not be relied upon as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Except as required by law, including the securities laws of the United States, we do not intend to update any forward-looking statements to conform these statements to actual results or to changes in our expectations.
PART I - FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
DiaMedica Therapeutics Inc.
Condensed Consolidated Balance Sheets
(In thousands, except share amounts)
|
September 30, 2025 |
December 31, 2024 |
|||||||
|
(unaudited) |
||||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 3,326 | $ | 3,025 | ||||
|
Marketable securities |
51,992 | 41,122 | ||||||
|
Prepaid expenses and other assets |
445 | 227 | ||||||
|
Amounts receivable |
260 | 236 | ||||||
|
Deposits |
200 | — | ||||||
|
Total current assets |
56,223 | 44,610 | ||||||
|
Non-current assets: |
||||||||
|
Deferred offering costs |
456 | — | ||||||
|
Operating lease right-of-use asset, net |
218 | 279 | ||||||
|
Property and equipment, net |
150 | 148 | ||||||
|
Deposits |
— | 1,308 | ||||||
|
Total non-current assets |
824 | 1,735 | ||||||
|
Total assets |
$ | 57,047 | $ | 46,345 | ||||
|
LIABILITIES AND EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 1,920 | $ | 940 | ||||
|
Accrued liabilities |
3,239 | 4,347 | ||||||
|
Operating lease obligation |
99 | 90 | ||||||
|
Finance lease obligation |
10 | 13 | ||||||
|
Total current liabilities |
5,268 | 5,390 | ||||||
|
Non-current liabilities: |
||||||||
|
Operating lease obligation |
150 | 225 | ||||||
|
Finance lease obligation |
7 | 12 | ||||||
|
Total non-current liabilities |
157 | 237 | ||||||
|
Shareholders’ equity: |
||||||||
|
Common shares, no par value; unlimited authorized; 52,077,439 and 42,818,660 shares issued and outstanding, as of September 30, 2025 and December 31, 2024, respectively |
— | — | ||||||
|
Paid-in capital |
215,600 | 180,697 | ||||||
|
Accumulated other comprehensive income |
50 | 23 | ||||||
|
Accumulated deficit |
(164,028 | ) | (140,002 | ) | ||||
|
Total shareholders’ equity |
51,622 | 40,718 | ||||||
|
Total liabilities and shareholders’ equity |
$ | 57,047 | $ | 46,345 | ||||
See accompanying notes to the condensed consolidated financial statements.
DiaMedica Therapeutics Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
(Unaudited)
|
Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
Operating expenses: |
||||||||||||||||
|
Research and development |
$ | 6,437 | $ | 4,983 | $ | 17,915 | $ | 12,587 | ||||||||
|
General and administrative |
2,596 | 1,900 | 7,269 | 5,675 | ||||||||||||
|
OPERATING LOSS |
(9,033 | ) | (6,883 | ) | (25,184 | ) | (18,262 | ) | ||||||||
|
Other income, net |
419 | 616 | 1,176 | 1,739 | ||||||||||||
|
Loss before income tax expense |
(8,614 | ) | (6,267 | ) | (24,008 | ) | (16,523 | ) | ||||||||
|
Income tax expense |
(6 | ) | (7 | ) | (18 | ) | (21 | ) | ||||||||
|
Net loss |
(8,620 | ) | (6,274 | ) | (24,026 | ) | (16,544 | ) | ||||||||
|
Other comprehensive loss |
||||||||||||||||
|
Unrealized gain on marketable securities |
64 | 132 | 27 | 75 | ||||||||||||
|
Net loss and comprehensive loss |
$ | (8,556 | ) | $ | (6,142 | ) | $ | (23,999 | ) | $ | (16,469 | ) | ||||
|
Basic and diluted net loss per share |
$ | (0.17 | ) | $ | (0.15 | ) | $ | (0.53 | ) | $ | (0.42 | ) | ||||
|
Weighted average shares outstanding – basic and diluted |
49,630,119 | 42,751,577 | 45,168,749 | 39,604,179 | ||||||||||||
See accompanying notes to the condensed consolidated financial statements.
DiaMedica Therapeutics Inc.
Condensed Consolidated Statements of Shareholders’ Equity
For the Three Months Ended September 30, 2025 and 2024
(In thousands, except share amounts)
(Unaudited)
|
Common Shares |
Paid-In Capital |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
Total Shareholders’ Equity |
||||||||||||||||
|
Balances at December 31, 2024 |
42,818,660 | $ | 180,697 | $ | 23 | $ | (140,002 | ) | $ | 40,718 | ||||||||||
|
Issuance of common shares upon the vesting and settlement of restricted stock units |
3,805 | — | — | — | — | |||||||||||||||
|
Issuance of common shares upon the exercise of stock options |
37,000 | 94 | — | — | 94 | |||||||||||||||
|
Share-based compensation expense |
— | 867 | — | — | 867 | |||||||||||||||
|
Unrealized loss on marketable securities |
— | — | (18 | ) | — | (18 | ) | |||||||||||||
|
Net loss |
— | — | — | (7,707 | ) | (7,707 | ) | |||||||||||||
|
Balances at March 31, 2025 |
42,859,465 | $ | 181,658 | $ | 5 | $ | (147,709 | ) | $ | 33,954 | ||||||||||
|
Issuance of common shares in settlement of deferred stock units |
142,345 | — | — | — | — | |||||||||||||||
|
Issuance of common shares upon the vesting and settlement of restricted stock units |
3,803 | — | — | — | — | |||||||||||||||
|
Issuance of common shares upon the exercise of stock options |
66,875 | 163 | — | — | 163 | |||||||||||||||
|
Share-based compensation expense |
— | 771 | — | — | 771 | |||||||||||||||
|
Unrealized loss on marketable securities |
— | — | (19 | ) | — | (19 | ) | |||||||||||||
|
Net loss |
— | — | — | (7,699 | ) | (7,699 | ) | |||||||||||||
|
Balances at June 30, 2025 |
43,072,488 | $ | 182,592 | $ | (14 | ) | $ | (155,408 | ) | $ | 27,170 | |||||||||
|
Issuance of common shares under Private Placement net of offering costs of $0.1 million |
8,606,425 | 29,977 | — | — | 29,977 | |||||||||||||||
|
Sale of common shares, net of offering costs |
223,472 | 1,550 | — | — | 1,550 | |||||||||||||||
|
Issuance of common shares upon the exercise of stock options |
171,250 | 465 | — | — | 465 | |||||||||||||||
|
Issuance of common shares upon the vesting and settlement of restricted stock units |
3,804 | — | — | — | — | |||||||||||||||
|
Share-based compensation expense |
— | 1,016 | — | — | 1,016 | |||||||||||||||
|
Unrealized gain on marketable securities |
64 | 64 | ||||||||||||||||||
|
Net loss |
— | — | — | (8,620 | ) | (8,620 | ) | |||||||||||||
|
Balances at September 30, 2025 |
52,077,439 | $ | 215,600 | $ | 50 | $ | (164,028 | ) | $ | 51,622 | ||||||||||
|
Common Shares |
Paid-In Capital |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
Total Shareholders’ Equity |
||||||||||||||||
|
Balances at December 31, 2023 |
37,958,000 | $ | 166,609 | $ | 6 | $ | (115,558 | ) | $ | 51,057 | ||||||||||
|
Issuance of common shares upon the vesting and settlement of restricted stock units |
5,916 | — | — | — | — | |||||||||||||||
|
Share-based compensation expense |
— | 488 | — | — | 488 | |||||||||||||||
|
Unrealized loss on marketable securities |
— | — | (45 | ) | — | (45 | ) | |||||||||||||
|
Net loss |
— | — | — | (5,151 | ) | (5,151 | ) | |||||||||||||
|
Balances at March 31, 2024 |
37,963,916 | $ | 167,097 | $ | (39 | ) | $ | (120,709 | ) | $ | 46,349 | |||||||||
|
Issuance of common shares under Private Placement net of offering costs of $0.1 million |
4,720,000 | 11,747 | — | — | 11,747 | |||||||||||||||
|
Issuance of common shares upon the vesting and settlement of restricted stock units |
5,916 | — | — | — | — | |||||||||||||||
|
Issuance of common shares upon the exercise of common stock options |
2,750 | 7 | — | — | 7 | |||||||||||||||
|
Share-based compensation expense |
— | 443 | — | — | 443 | |||||||||||||||
|
Unrealized loss on marketable securities |
— | — | (12 | ) | — | (12 | ) | |||||||||||||
|
Net loss |
— | — | — | (5,119 | ) | (5,119 | ) | |||||||||||||
|
Balances at June 30, 2024 |
42,692,582 | $ | 179,294 | $ | (51 | ) | $ | (125,828 | ) | $ | 53,415 | |||||||||
|
Issuance of common shares upon the vesting of and settlement of restricted stock units |
5,915 | — | — | — | — | |||||||||||||||
|
Issuance of common shares upon the exercise of stock options |
68,000 | 126 | — | — | 126 | |||||||||||||||
|
Share-based compensation expense |
— | 565 | — | — | 565 | |||||||||||||||
|
Unrealized gain on marketable securities |
— | — | 132 | — | 132 | |||||||||||||||
|
Net loss |
— | — | — | (6,274 | ) | (6,274 | ) | |||||||||||||
|
Balances at September 30, 2024 |
42,766,497 | $ | 179,985 | $ | 81 | $ | (132,102 | ) | $ | 47,964 | ||||||||||
See accompanying notes to the condensed consolidated financial statements.
DiaMedica Therapeutics Inc.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
|
Nine Months Ended September 30, |
||||||||
|
2025 |
2024 |
|||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (24,026 |
) |
$ | (16,544 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Share-based compensation |
2,654 | 1,496 | ||||||
|
Amortization of discounts on marketable securities |
(690 | ) | (1,013 | ) | ||||
|
Non-cash lease expense |
61 | 56 | ||||||
|
Depreciation |
32 | 28 | ||||||
|
Changes in operating assets and liabilities: |
||||||||
|
Amounts receivable |
(24 | ) | 79 | |||||
|
Prepaid expenses and other assets |
(218 | ) | 131 | |||||
|
Deposits |
1,108 | (1,308 | ) | |||||
|
Accounts payable |
980 | 245 | ||||||
|
Accrued liabilities and operating lease liabilities |
(1,174 | ) | 1,188 | |||||
|
Net cash used in operating activities |
(21,297 | ) | (15,642 | ) | ||||
|
Cash flows from investing activities: |
||||||||
|
Purchase of marketable securities |
(51,224 |
) |
(39,623 |
) |
||||
|
Maturities and sales of marketable securities |
41,071 | 43,000 | ||||||
|
Purchases of property and equipment |
(34 |
) |
(18 |
) |
||||
|
Net cash provided by (used in) investing activities |
(10,187 | ) | 3,359 | |||||
|
Cash flows from financing activities: |
||||||||
|
Proceeds from the sale of common shares, net of offering costs |
31,527 | 11,747 | ||||||
|
Proceed from the exercise of common stock options |
722 | 133 | ||||||
|
Deferred offering costs |
(456 | ) | — | |||||
|
Principal payments on finance lease obligation |
(8 |
) |
(6 |
) |
||||
|
Net cash provided by financing activities |
31,785 | 11,874 | ||||||
|
Net increase (decrease) in cash and cash equivalents |
301 | (409 | ) | |||||
|
Cash and cash equivalents at beginning of period |
3,025 | 4,543 | ||||||
|
Cash and cash equivalents at end of period |
$ | 3,326 | $ | 4,134 | ||||
|
Supplemental disclosure of non-cash transactions: |
||||||||
|
Cash paid for income taxes |
$ | 18 | $ | 20 | ||||
|
Assets acquired under financing lease |
$ | — | $ | 30 | ||||
See accompanying notes to the condensed consolidated financial statements.
DiaMedica Therapeutics Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited)
1. Business
DiaMedica Therapeutics Inc. and its wholly owned subsidiaries, DiaMedica USA Inc. and DiaMedica Australia Pty Ltd. (collectively, we, us, our, DiaMedica and the Company), is a clinical stage biopharmaceutical company focused on developing novel treatments for preeclampsia (PE), fetal growth restriction (FGR) and acute ischemic stroke (AIS). DiaMedica’s lead product candidate, DM199, is the first pharmaceutically active recombinant (synthetic) form of the human tissue kallikrein-1 (KLK1) protein, an established therapeutic modality in Asia for the treatment of preeclampsia, acute ischemic stroke and other vascular diseases. Our common shares are publicly traded on The Nasdaq Capital Market under the symbol “DMAC.”
2. Risks and Uncertainties
DiaMedica operates in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the United States Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in the European Union, and comparable agencies in other countries. We are in the clinical stage of development of our lead product candidate, DM199, for the treatment of PE and AIS. We have not completed the development of any product candidate and do not generate any revenues from the commercial sale of any product candidate. Our lead product candidate, DM199, requires significant additional clinical testing and investment prior to seeking marketing approval and is not expected to be commercially available for at least three to four years, if at all.
With respect to our PE clinical program, a Phase 2 open-label, single center, single-arm, safety and pharmacodynamic, proof-of-concept, investigator-sponsored study of DM199 for the treatment of PE is currently being conducted at the Tygerberg Hospital in Cape Town, South Africa. This Phase 2 study consists of three studies in PE (Part 1a, dose-escalation; Part 1b, dose-expansion; and Part 2, expectant management) and a fourth study in fetal growth restriction (FGR, Part 3, expectant management). Part 1a topline study results are intended to identify a suitable dose for Parts 1b, 2, and 3. Up to 90 women with PE and potentially an additional 30 subjects with fetal growth restriction may be evaluated. The first subject in Part 1a was enrolled in the fourth quarter of 2024 and interim results from Part 1a of the study were released in July 2025. The interim results (N=28 subjects) demonstrated that DM199 appears safe and well-tolerated with clinically-relevant pharmacodynamic activity with no evidence of placental transfer. Additionally, subjects exhibited rapid, statistically significant reductions in blood pressure with duration of effect that was sustained up to 24 hours post-infusion compared to pre-treatment baseline. Preparations are underway to initiate Part 1b where up to 30 subjects with PE and expected delivery within 72 hours will be treated with a dose regimen identified from Part 1a.
With respect to our AIS clinical program, we are currently conducting a Phase 2/3, adaptive design, randomized, double-blind, placebo-controlled trial of DM199 for the treatment of AIS, (the ReMEDy2 trial). Our ReMEDy2 trial is intended to enroll approximately 300 participants at up to 100 sites globally. The adaptive design component includes an interim analysis by our independent data safety monitoring board to be conducted after the first 200 participants have completed the trial. Based on the results of the interim analysis, the study may then be stopped for futility or the final sample size will be determined, ranging between 300 and 728 patients, according to a pre-determined statistical plan. We have experienced and continue to experience slower than expected site activations and enrollment in our ReMEDy2 trial. We believe these conditions may be due to hospital and medical facility staffing shortages; inclusion/exclusion criteria in the study protocol; concerns managing logistics and protocol compliance for participants discharged from the hospital to an intermediate care facility; concerns regarding the prior clinically significant hypotension events and circumstances surrounding the previous clinical hold; use of artificial intelligence and telemedicine which have enabled smaller hospitals to retain AIS patients not eligible for mechanical thrombectomy instead of sending these patients to the larger stroke centers which are more likely to be sites in our trial; and competition for research staff and trial subjects due to other pending stroke and neurological trials. We continue to reach out to current and potential study sites to understand the specific issues at each study site. In an effort to mitigate the impact of these factors, we have significantly expanded our internal clinical team and have brought in-house certain trial activities, including site identification, qualification and activation, clinical site monitoring, and overall program management. We are currently conducting our ReMEDy2 trial in the United States and in the countries of Canada and Georgia. We are in the process of preparing regulatory filings and identifying and engaging study sites in an additional seven European countries and, on August 28, 2025, received approval for the conduct of this study in the United Kingdom. We continue to work closely with our contract research organizations and other advisors to develop procedures to support both U.S. and global study sites and potential participants as needed. We intend to continue to monitor the results of these efforts and, if necessary, implement additional actions to enhance site activations and enrollment in our ReMEDy2 trial; however, no assurances can be provided as to the success of these actions and if or when these issues will resolve. Failure to resolve these issues may result in further delays in our ReMEDy2 trial and increase the difficulty in forecasting enrollment. We currently estimate that the interim analysis will be completed in the second half of 2026.
Our future success is dependent upon the success of our development efforts, our ability to demonstrate clinical progress for our DM199 product candidate in the United States or other markets, our ability, or the ability of any future partner, to obtain required governmental approvals of our product candidate, our ability to license or market and sell our DM199 product candidate, and our ability to obtain additional financing to fund these efforts.
As of September 30, 2025, we have incurred losses of $164.0 million since our inception in 2000. For the nine months ended September 30, 2025, we incurred a net loss of $24.0 million and negative cash flows from operating activities of $21.3 million. We expect to continue to incur substantial operating losses until such time as any future product sales, licensing fees, milestone payments and/or royalty payments generate revenue sufficient to fund our continuing operations. For the foreseeable future, we expect to incur significant operating losses as we continue the development and clinical study of, and to seek regulatory approval for, our DM199 product candidate. As of September 30, 2025, we had combined cash, cash equivalents and marketable securities of $55.3 million, working capital of $51.0 million and shareholders’ equity of $51.6 million.
Our principal source of cash has been net proceeds from the issuance of equity securities. Although we have previously been successful in obtaining financing through equity securities offerings, there is no assurance that we will be able to do so in the future. This is particularly true if our clinical data are not positive or if economic and market conditions deteriorate.
We expect that we will need substantial additional capital to further our research and development activities and complete the required clinical studies, regulatory activities and manufacturing development for our product candidate, DM199, or any future product candidates, to a point where they may be licensed or commercially sold. We expect our current cash, cash equivalents and marketable securities to be sufficient to continue the Phase 2 PE trial, the ReMEDy2 trial and otherwise fund our planned operations for at least the next 12 months from the date of issuance of these condensed consolidated financial statements. The amount and timing of our future funding requirements will depend on many factors, including timing and results of our ongoing development efforts, including our current ReMEDy2 trial and the rate of site activation and participant enrollment in the study; the Phase 2 PE trial; the potential expansion of our current development programs; the effects of ongoing site staffing shortages; and other factors on our clinical trials and our operating expenses. We may require significant additional funds earlier than we currently expect and there is no assurance that we will not need or seek additional funding prior to such time, especially if market conditions for raising capital are favorable.
3. Summary of Significant Accounting Policies
Interim financial statements
We have prepared the accompanying condensed consolidated financial statements in accordance with accounting principles generally accepted in the United States (US GAAP) for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the Securities and Exchange Commission (SEC). Accordingly, the condensed consolidated financial statements do not include all of the information and footnotes required by US GAAP for complete financial statements. These condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals which, in the opinion of management, are necessary to present fairly our condensed consolidated financial position, condensed consolidated results of operations, condensed consolidated statement of shareholders’ equity and condensed consolidated cash flows for the periods and as of the dates presented. Our fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2024 was derived from our audited consolidated financial statements. These condensed consolidated financial statements should be read in conjunction with our annual consolidated financial statements and the notes thereto. The nature of our business is such that the results of any interim period may not be indicative of the results to be expected for the entire year. Certain prior year amounts have been reclassified to conform to the current year presentation.
Segments
We operate in a single segment focusing on researching and developing potentially transformative treatments for severe ischemic diseases. Consistent with our operational structure, our chief operating decision maker manages and allocates resources for the Company at a consolidated level. Therefore, the results of our operations are reported on a consolidated basis for purposes of segment reporting. Substantially all assets are held in the United States.
Cash and cash equivalents
The Company considers all bank deposits, including money market funds and other investments, purchased with an original maturity to the Company of three months or less, to be cash and cash equivalents. The carrying amount of our cash equivalents approximates fair value due to the short maturity of the investments.
Marketable securities
Our marketable securities may consist of obligations of the United States government and its agencies, bank certificates of deposit and investment grade corporate obligations, which are classified as available-for-sale. Marketable securities which mature within 12 months from their purchase date are included in current assets. Securities are generally valued based on market prices for similar assets using third party certified pricing sources and are carried at fair value. The amortized cost of marketable securities is adjusted for amortization of premiums or accretion of discounts to maturity. Such amortization or accretion is included in interest income. Realized gains and losses, if any, are calculated on the specific identification method. Interest income is included in other income in the condensed consolidated statements of operations.
We conduct periodic reviews to identify and evaluate each available-for-sale debt security that is in an unrealized loss position in order to determine whether an other-than-temporary impairment exists. An unrealized loss exists when the current fair value of an individual security is less than its amortized cost basis. Declines in fair value considered to be temporary and caused by noncredit-related factors of the issuer, are recorded in accumulated other comprehensive income or loss, which is a separate component of shareholders’ equity. Declines in fair value that are other than temporary or caused by credit-related factors of the issuer, are recorded within earnings as an impairment loss. There were no other-than-temporary unrealized losses as of September 30, 2025.
Fair value measurements
Under the authoritative guidance for fair value measurements, fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
The hierarchy is broken down into three levels defined as follows:
Level 1 Inputs — quoted prices in active markets for identical assets and liabilities;
Level 2 Inputs — observable inputs other than quoted prices in active markets for identical assets and liabilities; and
Level 3 Inputs — unobservable inputs.
As of September 30, 2025, the Company believes that the carrying amounts of its other financial instruments, including amounts receivable, accounts payable and accrued liabilities, approximate their fair value due to the short-term maturities of these instruments. See Note 4, titled “Marketable Securities” for additional information.
Deferred offering costs
Deferred offering costs represent legal, accounting and other direct costs related to the Company’s efforts to raise capital through a public sale of the Company’s common shares under the 2025 Sales Agreement, see Note 10. Costs related to the public sale of the Company’s common shares are deferred until the completion of the applicable offering, at which time such costs are reclassified to additional paid-in-capital as a reduction of the proceeds. See Note 10 titled “Shareholders’ Equity” for additional information.
4. Marketable Securities
The available-for-sale marketable securities are primarily comprised of investments in commercial paper, corporate bonds and government securities and consist of the following, measured at fair value on a recurring basis (in thousands):
|
Fair Value Measurements Using Inputs Considered as of: |
||||||||||||||||||||||||||||||||
|
September 30, 2025 |
December 31, 2024 |
|||||||||||||||||||||||||||||||
|
Total |
Level 1 |
Level 2 |
Level 3 |
Total |
Level 1 |
Level 2 |
Level 3 |
|||||||||||||||||||||||||
|
Government securities |
$ | 44,522 | $ | — | $ | 44,522 | $ | — | $ | 12,831 | $ | — | $ | 12,831 | $ | — | ||||||||||||||||
|
Commercial paper and corporate bonds |
7,470 | — | 7,470 | — | 28,291 | — | 28,291 | — | ||||||||||||||||||||||||
|
Total |
$ | 51,992 | $ | — | $ | 51,992 | $ | — | $ | 41,122 | $ | — | $ | 41,122 | $ | — | ||||||||||||||||
Maturities of individual securities are less than 12 months and the amortized cost of all securities approximated fair value as of September 30, 2025 and December 31, 2024. Accrued interest receivable on marketable securities is included in amounts receivable and was $228,000 and $235,000 as of September 30, 2025 and December 31, 2024, respectively.
There were no transfers of assets between Level 1 and Level 2 of the fair value measurement hierarchy during the nine months ended September 30, 2025.
5. Deposits
DiaMedica periodically advances funds to vendors engaged to support the performance of our clinical trials and related supporting activities. The funds advanced are held, interest free, for varying periods of time and may be recovered by the Company through partial reductions of ongoing invoices, application against final study/project invoices or refunded upon completion of services to be provided. Deposits are classified as current or non-current based upon their expected recovery time.
6. Amounts Receivable
Amounts receivable consisted primarily of accrued interest receivable on marketable securities of $228,000 and $235,000 as of September 30, 2025 and December 31, 2024, respectively.
7. Property and Equipment
Property and equipment, net, consisted of the following (in thousands):
|
September 30, 2025 |
December 31, 2024 |
|||||||
|
Computer equipment |
145 | 118 | ||||||
|
Furniture and equipment |
128 | 128 | ||||||
|
Leasehold improvements |
16 | 16 | ||||||
| 289 | 262 | |||||||
|
Less accumulated depreciation |
(139 | ) | (114 | ) | ||||
|
Property and equipment, net |
$ | 150 | $ | 148 | ||||
8. Accrued Liabilities
Accrued liabilities consisted of the following (in thousands):
|
September 30, 2025 |
December 31, 2024 |
|||||||
|
Clinical trial costs |
$ | 1,615 | $ | 2,277 | ||||
|
Compensation |
920 | 1,060 | ||||||
|
Research and development services |
600 | 888 | ||||||
|
Professional services fees |
97 | 112 | ||||||
|
Other |
7 | 10 | ||||||
|
Total accrued liabilities |
$ | 3,239 | $ | 4,347 | ||||
9. Operating Lease
Office lease
Our operating lease costs were $78,000 for each of the nine-month periods ended September 30, 2025 and 2024. Our variable lease costs were $67,000 and $64,000 for the nine months ended September 30, 2025 and 2024, respectively. Variable lease costs consist primarily of common area maintenance costs, insurance and taxes which are paid based upon actual costs incurred by the lessor.
Maturities of our operating lease obligation are as follows as of September 30, 2025 (in thousands):
|
2025 |
$ | 29 | ||
|
2026 |
116 | |||
|
2027 |
119 | |||
|
2028 |
10 | |||
|
Total lease payments |
274 | |||
|
Less interest portion |
(25 | ) | ||
|
Present value of operating lease obligation |
249 | |||
|
Less current portion of operating lease |
(99 | ) | ||
|
Operating lease obligation, non-current |
$ | 150 |
10. Shareholders’ Equity
Authorized capital shares
DiaMedica has authorized share capital of an unlimited number of voting common shares, and the shares do not have a stated par value. Common shareholders are entitled to receive dividends as declared by the Company, if any, and are entitled to one vote per share at the Company’s annual general meeting and any extraordinary or special general meeting.
On August 12, 2025, we entered into a Sales Agreement (the 2025 Sales Agreement) with TD Securities (USA) LLC (TD Cowen) under which the Company may, from time to time, sell common shares having an aggregate offering price of up to $100 million, through an “at-the-market” offering program (ATM Offering). TD Cowen will receive a commission from the Company of 2.5% of the aggregate gross proceeds of any common shares sold under the 2025 Sales Agreement. Any shares offered and sold in the ATM Offering are to be issued pursuant to the Company’s shelf registration statement on Form S-3 and the 424(b)(2) prospectus supplement dated August 22, 2025.
Equity issued during the nine months ended September 30, 2025
On July 21, 2025, we entered into securities purchase agreements with accredited investors, pursuant to which we agreed to issue and sell an aggregate 8,606,425 common shares at a purchase price of $3.50 per share in a private placement. As a result of the offering, which closed on July 23, 2025, we received gross proceeds of $30.1 million, which resulted in net proceeds to us of approximately $30.0 million, after deducting the offering expenses.
In connection with the July 2025 private placement, we entered into a registration rights agreement (2025 Registration Rights Agreement) with the investors pursuant to which we agreed to file with the SEC a registration statement registering the resale of the shares sold in the July 2025 private placement (2025 Resale Registration Statement). The 2025 Resale Registration Statement was filed with the SEC on August 1, 2025 and declared effective by the SEC on August 8, 2025. Under the terms of the 2025 Registration Rights Agreement, we agreed to keep the 2025 Resale Registration Statement effective at all times until the shares are no longer considered “Registrable Securities” under the 2025 Registration Rights Agreement and if we fail to keep the 2025 Resale Registration Statement effective, subject to certain permitted exceptions, we will be required to pay liquidated damages to the investors in an amount of up to 10% of the invested capital, excluding interest. We also agreed, among other things, to indemnify the selling holders under the 2025 Resale Registration Statement from certain liabilities and to pay all fees and expenses incident to our performance of or compliance with the 2025 Registration Rights Agreement.
During the nine months ended September 30, 2025, 11,412 common shares were issued upon the vesting and settlement of restricted stock units, 142,345 shares were issued upon the settlement of deferred share units, 275,125 common shares were issued upon the exercise of stock options for gross proceeds of $722,000 and 223,472 common shares were issued and sold under the ATM Offering for gross proceeds of $1.6 million.
Equity issued during the nine months ended September 30, 2024
On June 25, 2024, we entered into securities purchase agreements with accredited investors, pursuant to which we agreed to issue and sell an aggregate 4,720,000 common shares at a purchase price of $2.50 per share in a private placement. As a result of the offering, which closed on June 28, 2024, we received gross proceeds of $11.8 million, which resulted in net proceeds to us of approximately $11.7 million, after deducting the offering expenses.
In connection with the June 2024 private placement, we entered into a registration rights agreement (2024 Registration Rights Agreement) with the investors pursuant to which we agreed to file with the SEC a registration statement registering the resale of the shares sold in the June 2024 private placement (2024 Resale Registration Statement). The 2024 Resale Registration Statement was filed with the SEC on July 10, 2024 and declared effective by the SEC on July 18, 2024. Under the terms of the 2024 Registration Rights Agreement, we agreed to keep the 2024 Resale Registration Statement effective at all times until the shares are no longer considered “Registrable Securities” under the 2024 Registration Rights Agreement and if we fail to keep the 2024 Resale Registration Statement effective, subject to certain permitted exceptions, we will be required to pay liquidated damages to the investors in an amount of up to 10% of the invested capital, excluding interest. We also agreed, among other things, to indemnify the selling holders under the 2024 Resale Registration Statement from certain liabilities and to pay all fees and expenses incident to our performance of or compliance with the 2024 Registration Rights Agreement.
During the nine months ended September 30, 2024, 17,747 common shares were issued upon the vesting and settlement of restricted stock units and 70,750 common shares were issued upon the exercise of common stock options for gross proceeds of $133,000.
Common shares reserved for future issuance are as follows:
|
September 30, 2025 |
||||
|
Common shares issuable upon exercise of employee and non-employee stock options |
7,321,854 | |||
|
Common shares issuable upon settlement of deferred stock units |
174,515 | |||
|
Common shares issuable upon vesting and settlement of restricted stock units |
3,803 | |||
|
Common shares available for grant under the Amended and Restated 2019 Omnibus Incentive Plan |
976,252 | |||
|
Common shares available for grant under the 2021 Employment Inducement Incentive Plan |
600,625 | |||
|
Total |
9,077,049 | |||
11. Net Loss Per Share
We compute net loss per share by dividing our net loss (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Shares issued during the period and shares reacquired during the period, if any, are weighted for the portion of the period that they were outstanding. The computation of diluted earnings per share, or EPS, is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued. Our diluted EPS is the same as basic EPS due to common equivalent shares being excluded from the calculation, as their effect is anti-dilutive.
The following table summarizes our calculation of net loss per common share for the periods presented (in thousands, except share and per share data):
|
Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
Net loss |
$ | (8,620 |
) |
$ | (6,274 |
) |
$ | (24,026 | ) | $ | (16,544 | ) | ||||
|
Weighted average shares outstanding—basic and diluted |
49,630,119 | 42,751,577 | 45,168,749 | 39,604,179 | ||||||||||||
|
Basic and diluted net loss per share |
$ | (0.17 |
) |
$ | (0.15 |
) |
$ | (0.53 | ) | $ | (0.42 | ) | ||||
The following outstanding potential common shares were not included in the diluted net loss per share calculations as their effects were not dilutive:
|
Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
Common shares issuable upon exercise of employee and non-employee stock options |
7,321,854 | 4,632,438 | 7,321,854 | 4,632,438 | ||||||||||||
|
Common shares issuable upon settlement of deferred stock units |
174,515 | 284,886 | 174,515 | 284,886 | ||||||||||||
|
Common shares issuable upon vesting and settlement of restricted stock units |
3,803 | 5,913 | 3,803 | 5,913 | ||||||||||||
12. Share-Based Compensation
Amended and Restated 2019 Omnibus Incentive Plan (2019 Plan)
The 2019 Plan permits the Board, or a committee or subcommittee thereof, to grant to the Company’s eligible employees, non-employee directors and certain consultants non-statutory and incentive stock options, stock appreciation rights, restricted stock awards, restricted stock units (RSUs), deferred stock units (DSUs), performance awards, non-employee director awards and other share-based awards. We grant options to purchase common shares under the 2019 Plan at no less than the fair market value of the underlying common shares as of the date of grant. Options granted to employees and non-employee directors have a maximum term of ten years and generally vest over one to four years. Options granted to non-employees have a maximum term of five years and generally vest over one year. Subject to adjustment as provided in the 2019 Plan, the maximum number of the Company’s common shares authorized for issuance under the 2019 Plan is 7,000,000 shares. As of September 30, 2025, options to purchase an aggregate of 5,707,444 common shares were outstanding; 164,770 common shares were reserved for issuance upon settlement of DSUs; 3,803 shares were reserved for issuance upon the vesting and settlement of RSUs; and 976,252 shares remained available for issuance.
2021 Employment Inducement Incentive Plan (2021 Inducement Plan)
The 2021 Inducement Plan permits the Board, or a committee or subcommittee thereof, to grant of non-statutory options, stock appreciation rights, restricted stock awards, restricted stock units, performance awards and other share-based awards, to new employees who satisfy the standards for inducement grants under Nasdaq Listing Rule 5635(c)(4) or 5635(c)(3), as applicable. The Inducement Plan has a term of 10 years. The share reserve under the Inducement Plan may be increased at the discretion of and approval by the Board and on July 31, 2025, the Board increased the number of common shares reserved for issuance under the plan to 2,000,000. As of September 30, 2025, options to purchase an aggregate of 1,240,000 common shares were outstanding under the Inducement Plan and 600,625 shares remained available for issuance.
Prior Stock Option Plan
The Company ceased granting awards under its Amended and Restated Stock Option Plan in conjunction with shareholder approval of the 2019 Plan. Awards outstanding under the prior plan remain outstanding in accordance with and pursuant to the terms thereof. Options granted under the prior plan have terms similar to those used under the 2019 Plan. As of September 30, 2025, options to purchase an aggregate of 374,410 common shares were outstanding.
Prior Deferred Stock Unit Plan
The Company ceased granting awards under its Deferred Share Unit Plan in conjunction with shareholder approval of the 2019 Plan. Awards outstanding under that plan remain outstanding in accordance with and pursuant to the terms thereof. As of September 30, 2025, there were 9,745 common shares reserved for issuance upon settlement of outstanding deferred share units.
Share-based compensation expense for each of the periods presented is as follows (in thousands):
|
Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
General and administrative |
$ | 646 | $ | 418 | $ | 1,862 | $ | 1,047 | ||||||||
|
Research and development |
370 | 147 | 792 | 449 | ||||||||||||
|
Total share-based compensation |
$ | 1,016 | $ | 565 | $ | 2,654 | $ | 1,496 | ||||||||
We recognize share-based compensation for options awards based on the fair value of each award as estimated using the Black-Scholes option valuation model. Ultimately, the actual expense recognized over the vesting period will only be for those options that actually vest.
A summary of option activity is as follows (in thousands except share and per share amounts):
|
Shares Underlying Options |
Weighted Average Exercise Price Per Share |
Aggregate Intrinsic Value |
||||||||||
|
Balances at December 31, 2024 |
4,692,438 | $ | 3.33 | $ | 10,243 | |||||||
|
Granted |
3,358,416 | 4.79 | ||||||||||
|
Forfeitures |
(408,875 | ) | 3.67 | |||||||||
|
Exercised |
(275,125 | ) | 2.63 | |||||||||
|
Expired |
(45,000 | ) | 8.03 | |||||||||
|
Balances at September 30, 2025 |
7,321,854 | $ | 3.99 | $ | 21,221 | |||||||
Information about stock options outstanding, vested and expected to vest as of September 30, 2025, is as follows:
|
Outstanding, Vested and Expected to Vest |
Options Vested and Exercisable |
|||||||||||||||||||||
|
Per Share Exercise Price |
Shares |
Weighted Average Remaining Contractual Life (Years) |
Weighted Average Exercise Price |
Options Exercisable |
Weighted Average Remaining Contractual Life (Years) |
|||||||||||||||||
|
$1.00 |
- | $1.99 | 191,443 | 7.5 |
$ |
1.59 | 117,974 | 7.5 | ||||||||||||||
|
$2.00 |
- | $2.99 | 2,420,895 | 7.0 | 2.73 | 1,453,679 | 6.7 | |||||||||||||||
|
$3.00 |
- | $3.99 | 311,893 | 5.0 | 3.54 | 241,270 | 3.9 | |||||||||||||||
|
$4.00 |
- | $4.99 | 3,167,069 | 8.1 | 4.30 | 726,031 | 4.2 | |||||||||||||||
|
$5.00 |
- | $10.00 | 1,230,554 | 8.3 | 6.15 | 374,519 | 5.0 | |||||||||||||||
| 7,321,854 | 7.6 |
$ |
3.99 | 2,913,473 | 5.6 | |||||||||||||||||
13. Segment Information
An operating segment is identified as a component of an enterprise that engages in business activities about which separate discrete financial information and operating results is regularly reviewed by the chief operating decision-maker (CODM) in making decisions regarding resource allocation and assessing performance. DiaMedica's CODM is the Chief Executive Officer. The Company operates in a single operating segment focused on the development of its drug product candidate, DM199, for the treatment of severe ischemic disease. The CODM manages and allocates resources to the operations of the Company on a total company basis. Further, the CODM reviews and utilizes functional expenses (i.e., research, development and general and administrative) at the consolidated level to manage the Company's operations. Other segment items included in consolidated net loss are revenues, share-based compensation, interest income, other expense, net, and income tax expense, which are reflected in the condensed consolidated statements of operations and comprehensive loss. The measure of segment assets is reported on the condensed consolidated balance sheet as total consolidated assets.
The following table presents financial information, including significant segment expenses, which are regularly provided to the CODM and included within segment and consolidated net loss:
|
Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
Operating expenses, excluding share-based compensation |
||||||||||||||||
|
Research and development |
$ | 6,066 | $ | 4,835 | $ | 17,122 | $ | 12,137 | ||||||||
|
General and administrative |
1,951 | 1,483 | 5,408 | 4,629 | ||||||||||||
|
Total operating expenses, excluding share-based compensation |
8,017 | 6,318 | 22,530 | 16,766 | ||||||||||||
|
Share-based compensation |
||||||||||||||||
|
Research and development |
370 | 147 | 792 | 449 | ||||||||||||
|
General and administrative |
646 | 418 | 1,862 | 1,047 | ||||||||||||
|
Total share-based compensation |
1,016 | 565 | 2,654 | 1,496 | ||||||||||||
|
Operating loss |
(9,033 | ) | (6,883 | ) | (25,184 | ) | (18,262 | ) | ||||||||
|
Interest income |
440 | 618 | 1,197 | 1,758 | ||||||||||||
|
Other income (expense), net |
(21 | ) | (2 | ) | (21 | ) | (19 | ) | ||||||||
|
Income tax expense |
(6 | ) | (7 | ) | (18 | ) | (21 | ) | ||||||||
|
Segment and consolidated net loss |
$ | (8,620 | ) | $ | (6,274 | ) | $ | (24,026 | ) | $ | (16,544 | ) | ||||
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is based upon accounting principles generally accepted in the United States of America and discusses the financial condition and results of operations for DiaMedica Therapeutics Inc. and our subsidiaries for the three- and nine-month periods ended September 30, 2025 and 2024.
This discussion should be read in conjunction with our condensed consolidated financial statements and related notes included elsewhere in this report and our audited financial statements and related notes included in our annual report on Form 10-K for the year ended December 31, 2024. The following discussion contains forward-looking statements that involve numerous risks and uncertainties. Our actual results could differ materially from the forward-looking statements as a result of these risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements” for additional cautionary information.
Business Overview
We are a clinical stage biopharmaceutical company committed to improving the lives of people suffering from preeclampsia (PE) and acute ischemic stroke (AIS). Our lead candidate DM199 (rinvecalinase alfa) is the first pharmaceutically active recombinant (synthetic) form of the human tissue kallikrein-1 (KLK1) protein (serine protease enzyme) to be clinically studied in patients. KLK1 is an established therapeutic modality in Asia, with human urinary KLK1 for the treatment of AIS and porcine KLK1 for the treatment of cardio renal disease, including hypertension. Our current focus is on the treatment of PE and AIS. We plan to advance DM199 through required clinical trials to create shareholder value by establishing its clinical and commercial potential as a therapy for PE and AIS. We have also produced a potential novel treatment for severe acute pancreatitis, DM300, which is currently in the early preclinical stage of development.
DM199 is a recombinant form of KLK1 (rhKLK1), which is a synthetic version of the naturally occurring protease enzyme kallikrein-1, and the first and only rhKLK1 undergoing global clinical development studies in both PE and AIS. DM199 has been granted Fast Track designation from the FDA for the treatment of AIS. Naturally occurring KLK1 (extracted from human urine or porcine pancreas) has been an approved therapeutic agent in Asia for decades in the treatment of AIS and hypertension associated with cardiorenal disease. DM199 is produced using recombinant DNA technology without the need for extracted human or animal tissue sources and thereby eliminates risk of pathogen transmission.
KLK1 is a serine protease enzyme that plays an important role in the regulation of diverse physiological processes via a molecular mechanism that may enhance microcirculatory blood flow and tissue perfusion by increasing production of nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF). In preeclampsia, DM199 is intended to lower blood pressure, enhance endothelial health and improve perfusion to maternal organs and the placenta, potentially disease modifying outcomes improving both maternal and perinatal outcomes. In the case of AIS, DM199 is intended to enhance blood flow and boost neuronal survival in the ischemic penumbra by dilating arterioles surrounding the site of the vascular occlusion and inhibiting apoptosis (neuronal cell death) while also facilitating neuronal remodeling through the promotion of angiogenesis.
Our product development pipeline is as follows:
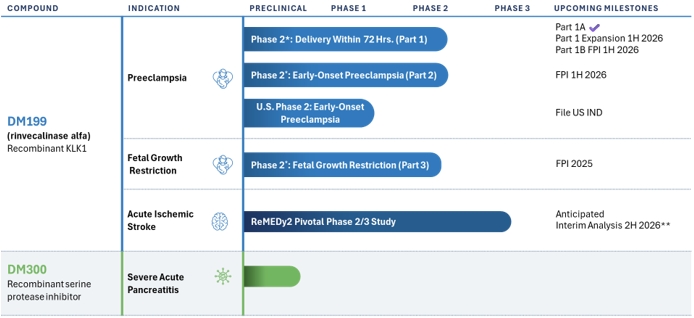
* Investigator sponsored trial
We are developing DM199 to address two major critical unmet needs. In PE, there are currently no approved agents in any global market to safely lower maternal blood pressure and/or reduce the risk of fetal growth restriction. Historically, the major issue with potential PE treatments has been that traditional vasodilators commonly used to reduce essential hypertension (e.g., beta-blockers, angiotensin converting enzyme inhibitors (ACEi)) can readily cross the placental barrier and enter into the fetal circulation and cause harm to the developing fetus. We believe that DM199 is uniquely suited to treat PE since its molecular size, approximately 26 kilodaltons (KD), is typically too large to cross the placental barrier, as was demonstrated in the interim result noted below, and therefore may reduce blood pressure and enhance microcirculatory perfusion to the maternal organs and placenta without entering fetal circulation, a potentially significant safety advantage. Additionally, we believe DM199 has the potential to not only address hypertension of PE, but also to confer disease modifying outcomes for both maternal and perinatal outcomes, including fetal growth restriction. In AIS, up to 80% of AIS patients are not eligible for treatment with currently approved clot-busting (thrombolytic) drugs or catheter-based clot removal procedures (mechanical thrombectomy). DM199 is intended to enhance collateral blood flow and boost neuronal survival in the ischemic penumbra by inhibiting neuronal cell death (apoptosis) and promoting neuronal remodeling and neoangiogenesis, and accordingly, offer a potential treatment option for AIS patients who otherwise have no therapeutic options.
Preeclampsia Program and Phase 2 Investigator-Sponsored Study
Our clinical development program in PE currently centers around an investigator-sponsored safety, tolerability and pharmacodynamic, proof-of-concept Phase 2 study in PE patients. This Phase 2 study consists of three studies in PE (Part 1a, dose-escalation; Part 1b, dose-expansion; and Part 2, expectant management) and a fourth study in fetal growth restriction (FGR, Part 3, expectant management). Part 1a topline study results are intended to identify a suitable dose for Parts 1b, 2, and 3. Up to 90 women with PE and potentially an additional 30 subjects with fetal growth restriction may be evaluated. The first subject in Part 1a was enrolled in the fourth quarter of 2024 and interim results from Part 1a of the study were released in July 2025. The interim results (N=28 subjects) demonstrate that DM199 appears safe and well-tolerated with clinically-relevant pharmacodynamic activity with no evidence of placental transfer. Additionally, subjects exhibited rapid, statistically significant reductions in blood pressure with duration of effect that was sustained up to 24 hours post-infusion compared to pre-treatment baseline, a durable effect extending up to 24 hours post-infusion. Preparations are underway to initiate Part 1b where up to 30 subjects with PE and expected delivery within 72 hours will be treated with a dose regimen identified from Part 1a.
Based in part upon these interim results, we believe DM199 has the potential to lower blood pressure, enhance endothelial health and improve perfusion to maternal organs and the placenta. We have completed studies on fertility, embryofetal development and pre- and post-natal development in animal models, which support the potential safety of DM199 in pregnant humans. Given the complexities inherent in conducting clinical studies involving pregnant women, a very vulnerable patient group, DiaMedica requested a pre-IND meeting with the FDA to obtain feedback prior to submitting an IND application for PE. The FDA granted DiaMedica an in-person meeting, which has been recently held and we plan to provide an update regarding the meeting once the final meeting minutes are received.
AIS Program and Phase 2/3 ReMEDy2 Trial
Our clinical program in AIS centers on our ReMEDy2 clinical trial of DM199 for the treatment of AIS. Our ReMEDy2 clinical trial is a Phase 2/3, adaptive design, randomized, double-blind, placebo-controlled trial intended to enroll approximately 300 participants at up to 100 sites globally. The adaptive design component includes an interim analysis by our independent data safety monitoring board to be conducted after the first 200 participants have completed the trial. Based on the results of the interim analysis, the study may be stopped for futility or the final sample size will be determined, ranging between 300 and 728 patients, according to a pre-determined statistical plan. As previously disclosed, we have experienced and continue to experience slower than expected site activations and enrollment in our ReMEDy2 trial. We believe these conditions may be due to hospital and medical facility staffing shortages; inclusion/exclusion criteria in the study protocol; concerns managing logistics and protocol compliance for participants discharged from the hospital to an intermediate care facility; concerns regarding the prior clinically significant hypotension events and circumstances surrounding the previous clinical hold; use of artificial intelligence and telemedicine which have enabled smaller hospitals to retain AIS patients not eligible for mechanical thrombectomy instead of sending these patients to the larger stroke centers which are more likely to be sites in our trial; and competition for research staff and trial subjects due to other pending stroke and neurological trials. We continue to reach out to current and potential study sites to understand the specific issues at each study site. In an effort to mitigate the impact of these factors, we have significantly expanded our internal clinical team and have brought in-house certain trial activities, including site identification, qualification and activation, clinical site monitoring and overall program management. We are currently conducting the trial in the United States and in the countries of Canada and Georgia. We are in the process of preparing regulatory filings and identifying and engaging study sites in an additional seven European countries and have submitted for approval of this study in the United Kingdom. We continue to work closely with our contract research organizations and other advisors to develop procedures to support both U.S. and global study sites and potential participants as needed. We intend to continue to monitor the results of these efforts and, if necessary, implement additional actions to enhance site activations and enrollment in our ReMEDy2 trial; however, no assurances can be provided as to the success of these actions and if or when these issues will resolve. Failure to resolve these issues may result in further delays in our ReMEDy2 trial and increase the difficulty in forecasting enrollment. We currently estimate that the interim analysis will be completed in the second half of 2026.
Financial Overview
We have not generated any revenues from product sales. Since our inception, we have financed our operations primarily from sales of equity securities, interest income on funds available for investment, and government grants. We have incurred a net loss in each year since our inception. Our net losses were $24.0 million and $16.5 million for the nine months ended September 30, 2025 and 2024, respectively. As of September 30, 2025, we had an accumulated deficit of $164.0 million. Substantially all of our operating losses resulted from expenses incurred in connection with our research and development (R&D) activities and general and administrative (G&A) support costs associated with our operations.
We expect to continue to incur significant expenses and operating losses for at least the next few years. We anticipate that our quarterly expenses will increase moderately relative to recent prior quarterly periods as we continue to advance our DM199 clinical development program into PE and we continue our ReMEDy2 trial, including additional site activations in the U.S. and globally and enrollment of participants in the trial. Our efforts to expand our team to provide support for our clinical and administrative operations will likely also contribute to such increases.
While we expect the level of future negative operating quarterly cash flows to generally increase moderately relative to recent prior quarterly periods as we expand our DM199 clinical development program into PE and we continue our ReMEDy2 trial, including our global expansion, we expect our current cash resources will be sufficient to allow us to continue our ReMEDy2 trial, support the Phase 2 PE trial, and otherwise fund our planned operations for at least the next 12 months from the date of issuance of the condensed consolidated financial statements included in this report. However, the amount and timing of our future funding requirements will depend on many factors, including timing and results of our ongoing development efforts, including the current Phase 2 PE trial, our current ReMEDy2 trial and in particular the rate of site activation and participant enrollment in the study, the potential further expansion of our current development programs and other factors. We may require or otherwise seek significant additional funds earlier than we currently need or expect. We may elect to raise additional funds even before we need them if market conditions for raising additional capital are favorable.
Components of Our Results of Operations
Research and Development Expenses
R&D expenses consist primarily of fees paid to external service providers such as contract research organizations; clinical support services; clinical development including clinical site costs; outside nursing services; and laboratory testing. R&D costs also include non-clinical testing; fees paid to our contract manufacturing and development organizations and outside laboratories for development of DM199 and related manufacturing processes; costs for production runs of DM199; consulting resources with specialized expertise related to the execution of our development plan for DM199; and personnel costs, including salaries, benefits, non-cash share-based compensation expense; and other personnel costs. Over the past approximately 10 years, our R&D efforts have been primarily focused on developing DM199. At this time, due to the risks inherent in the clinical development process and the clinical stage of our product development programs, we are unable to estimate with any certainty the costs we will incur in completing the development of DM199 through marketing approval. The process of conducting clinical studies necessary to obtain regulatory approval and manufacturing scale-up to support expanded development and potential future commercialization is costly and time consuming. Any failure by us or delay in completing clinical studies, manufacturing scale-up, or in obtaining regulatory approvals could lead to increased R&D expenses and, in turn, have a material adverse effect on our results of operations.
General and Administrative Expenses
G&A expenses consist primarily of salaries and benefits, including non-cash share-based compensation related to our executive, finance, business development and support functions. G&A expenses also include insurance, including directors’ and officers’ liability coverage, rent and utilities, travel expenses, patent costs, and professional fees, including for auditing, tax and legal services.
Other Income, Net
Other income, net consists primarily of interest income earned on marketable securities.
Results of Operations
Comparison of the Three and Nine Month Periods Ended September 30, 2025 and 2024
The following table summarizes our unaudited results of operations for the three and nine month periods ended September 30, 2025 and 2024 (in thousands):
|
Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
Research and development expenses |
$ | 6,437 | $ | 4,983 | $ | 17,915 | $ | 12,587 | ||||||||
|
General and administrative expenses |
2,596 | 1,900 | 7,269 | 5,675 | ||||||||||||
|
Other income, net |
$ | (419 | ) | $ | (616 | ) | $ | (1,176 | ) | $ | (1,739 | ) | ||||
Research and Development Expenses
R&D expenses increased to $6.4 million for the three months ended September 30, 2025, up from $5.0 million for the three months ended September 30, 2024. R&D expenses increased to $17.9 million for the nine months ended September 30, 2025, up from $12.6 million for the nine months ended September 30, 2024. The increases in both periods are primarily due to cost increases driven by the continuation of our ReMEDy2 clinical trial, including its global expansion, progress with the Phase 2 IST in PE and the expansion of our clinical team in the prior and current year periods. These increases were partially offset in both periods by cost reductions related to manufacturing process development work performed and completed in the prior year period. We expect that our R&D expenses will moderately increase in future periods relative to recent prior periods as we continue our ReMEDy2 trial, including our global expansion and continue the expansion of our DM199 clinical development program into PE.
General and Administrative Expenses
G&A expenses were $2.6 million and $1.9 million for the three months ended September 30, 2025 and 2024, respectively. G&A expenses were $7.3 million and $5.7 million for the nine months ended September 30, 2025 and 2024, respectively. These increases in both periods resulted primarily from increased non-cash share-based compensation expense and increased personnel costs incurred in conjunction with expanding our team. Increases in investor relations, patent and professional fees also contributed to the increase in both periods. We expect G&A expenses to remain steady in future periods as compared to recent prior periods.
Other Income, Net
Other income, net, was $419 thousand and $616 thousand for the three months ended September 30, 2025 and 2024, respectively, and $1.2 million and $1.7 million for the nine months ended September 30, 2025 and 2024, respectively. The decreases in both periods resulted from reduced interest income recognized during the current year period related to lower average marketable securities balances during the current year period as compared to the prior year period.
Liquidity and Capital Resources
On August 12, 2025, we entered into the 2025 Sales Agreement with TD Cowen under which the Company may, from time to time, sell common shares having an aggregate offering price of up to $100 million, through an ATM Offering program. TD Cowen will receive a commission from the Company of 2.5% of the aggregate gross proceeds of any common shares sold under the 2025 Sales Agreement. Any shares offered and sold in the ATM Offering are to be issued pursuant to the Company’s shelf registration statement on Form S-3 and the 424(b) prospectus supplement dated August 22, 2025. As of September 30, 2025, approximately $98.4 million remained available for issuance in the ATM Offering.
The following tables summarize our liquidity and capital resources as of September 30, 2025 and December 31, 2024, and our cash flows for each of the nine month periods ended September 30, 2025 and 2024, and are intended to supplement the more detailed discussion that follows (in thousands):
|
September 30, 2025 |
December 31, 2024 |
|||||||
|
Cash, cash equivalents and marketable securities |
$ | 55,318 | $ | 44,147 | ||||
|
Total assets |
57,047 | 46,345 | ||||||
|
Total current liabilities |
5,268 | 5,390 | ||||||
|
Total shareholders’ equity |
51,622 | 40,718 | ||||||
|
Working capital |
50,955 | 39,220 | ||||||
|
Nine Months Ended September 30, |
||||||||
|
2025 |
2024 |
|||||||
|
Cash Flow Data |
||||||||
|
Cash flow provided by (used in): |
||||||||
|
Operating activities |
$ | (21,297 | ) | $ | (15,642 | ) | ||
|
Investing activities |
(10,187 | ) | 3,359 | |||||
|
Financing activities |
31,785 | 11,874 | ||||||
|
Net increase (decrease) in cash |
$ | 301 | $ | (409 | ) | |||
Working Capital
We had aggregate cash, cash equivalents and marketable securities of $55.3 million, current liabilities of $5.3 million and working capital of $51.0 million as of September 30, 2025, compared to aggregate cash, cash equivalents and marketable securities of $44.1 million, $5.4 million in current liabilities and $39.2 million in working capital as of December 31, 2024. The increases in our combined cash, cash equivalents and marketable securities and in our working capital is due to the net proceeds received from our July 2025 private placement, partially offset by net cash used to fund our current operations.
Cash Flows
Operating Activities
Net cash used in operating activities for the nine months ended September 30, 2025 was $21.3 million compared to $15.6 million for the nine months ended September 30, 2024. The increase in cash used in operating activities resulted primarily from the increased net loss, partially offset by changes in operating assets and liabilities during the current year period.
Investing Activities
Investing activities consist primarily of purchases and maturities of marketable securities. Net cash used in investing activities was $10.2 million for the nine months ended September 30, 2025, compared to net cash provided by investing activities of $3.4 million for the nine months ended September 30, 2024. This change resulted primarily from the investment of proceeds from the July 2025 private placement, partially offset by maturities of our marketable securities during the current year quarter.
Financing Activities
Net cash provided by financing activities was $31.8 million and 11.9 million for the nine months ended September 30, 2025 and 2024, respectively. Net cash provided by financing activities during each period consisted primarily of net proceeds from the sale of common shares in our July 2025 and June 2024 private placements.
Capital Requirements
Since our inception, we have incurred losses while advancing the development of our DM199 product candidate. We have not generated any revenues from product sales and do not expect to do so for at least three to four years. We do not know when or if we will generate any revenues from product sales or out-licensing of our DM199 product candidate or any future product candidate. We will not generate any revenue from product sales unless and until we obtain required regulatory approvals. We expect to continue to incur substantial operating losses until such time as any future product sales, licensing fees, milestone payments and/or royalty payments are sufficient to generate revenues to fund our continuing operations. We expect our operating losses to moderately increase as compared to recent prior periods as we continue the research, development and clinical studies of, and seek regulatory approval for, our DM199 product candidate, including, in particular, the expansion of our clinical development program into PE and the continuation and global expansion of our ReMEDy2 trial. In the long-term, subject to obtaining regulatory approval of our DM199 product candidate, or any other product candidate, and if we are unable to secure the assistance of, or out-license to, a strategic partner, we expect to incur significant commercialization expenses for product marketing, sales, manufacturing and distribution.
Accordingly, we expect we will need substantial additional capital to complete our R&D activities, including current and anticipated future clinical studies, regulatory activities, and otherwise develop our product candidate, DM199, or any future product candidate, to a point where the product candidate may be out-licensed or commercially sold. Although we are striving to achieve these plans, there is no assurance that these and other strategies will be achieved or that additional funding will be obtained on favorable terms or at all. We expect our rate of future negative quarterly cash flows to vary depending on our clinical activities and the timing of expenses Accordingly, and notwithstanding the completion of our July 2025 private placement in which we received net proceeds of $30.0 million, we expect we will need substantial additional capital to complete our R&D activities, including current and anticipated future clinical studies, regulatory activities, and otherwise develop our product candidate, DM199, or any future product candidate, to a point where the product candidate may be out-licensed or commercially sold. Although we are striving to achieve these plans, there is no assurance that these and other strategies will be achieved or that additional funding will be obtained on favorable terms or at all. We expect our rate of future negative quarterly cash flows to vary depending on our clinical activities and the timing of expenses incurred and will increase moderately relative to recent prior quarterly periods as we expand our PE clinical development program and continue and globally expand our ReMEDy2 trial. We expect our current cash resources to be sufficient to support the expansion of our PE clinical development program, continue our ReMEDy2 trial and otherwise fund our planned operations for at least the next twelve months from the date of issuance of the condensed consolidated financial statements included in this report. The amount and timing of our future funding requirements will depend on many factors, including timing and results of our ongoing development efforts, including our current ReMEDy2 trial and the Phase 2 PE trial, the potential further expansion of our current development programs, and other factors on our operating expenses. We may require significant additional funds earlier than we currently expect and there is no assurance that we will not need or seek additional funding prior to such time, especially if market conditions for raising additional capital are favorable.
Historically, we have financed our operations primarily from sales of equity securities and interest income received on funds available for investment and we expect to continue this practice for the foreseeable future. Our most recent equity financing was our July 2025 private placement in which we issued and sold an aggregate of 8,606,425 common shares pursuant to a securities purchase agreement at a purchase price of $3.50 per share to accredited investors. As a result of the offering, we received gross proceeds of $30.1 million, which resulted in net proceeds to us of approximately $30.0 million, after deducting offering expenses. We do not have any existing credit facilities under which we could borrow funds. We may seek to raise additional funds through various sources, such as equity or debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This is particularly true if our clinical data is not positive or economic and market conditions deteriorate.
To the extent we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. Debt financing, if available, may involve agreements that include conversion discounts, pledging our intellectual property as collateral or covenants limiting or restricting our ability to take specific actions, such as incurring additional debt or making capital expenditures. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, or strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. The availability of financing will be affected by the status of our clinical trials; our clinical data and other results of scientific and clinical research; the ability to obtain regulatory approvals and other regulatory actions; market acceptance of our product candidates; the state of the capital markets generally with particular reference to pharmaceutical, biotechnology and medical companies; the status of strategic alliance agreements; and other relevant commercial considerations.
If adequate funding is not available to us when needed, we may be required to scale back our operations by taking actions that may include, among other things, implementing cost reduction strategies, such as reducing use of outside professional service providers, reducing the number of our employees or employee compensation, modifying or delaying the development of our DM199 product candidate; licensing to third parties the rights to commercialize our DM199 product candidate for PE, AIS or other indications that we would otherwise seek to pursue, or otherwise relinquishing significant rights to our technologies, future revenue streams, research programs or product candidates or granting licenses on terms that may not be favorable to us; and/or divesting assets or ceasing operations through a sale or liquidation of our Company.
Critical Accounting Estimates
There have been no material changes to our critical accounting estimates from the information provided in “Part II. Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates,” included in our annual report on Form 10-K for the fiscal year ended December 31, 2024.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company, we are not required to provide disclosure pursuant to this Item.
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the United States Securities Exchange Act of 1934, as amended (Exchange Act)) that are designed to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act, is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Our management evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered in this report. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of such period to provide reasonable assurance that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the three months ended September 30, 2025 that has materially affected or is reasonably likely to materially affect our internal control over financial reporting.
PART II - OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
Litigation with Pharmaceutical Research Associates Group B.V., acquired by ICON plc as of July 1, 2021, (ICON/PRA Netherlands)
On November 23, 2022, we filed a petition requesting leave for a prejudgment attachment of all relevant documents in possession of Pharmaceutical Research Associates Group B.V., acquired by ICON plc as of July 1, 2021, (ICON/PRA Netherlands), which was granted on November 28, 2022, by the District Court of Northern Netherlands. A representative of the District Court served ICON/PRA Netherlands with the prejudgment attachment on or about December 7 and 8, 2022. The case was formally introduced to the Netherlands Commercial Court (NCC) on December 28, 2022 and a hearing by the NCC to determine whether we are entitled to take possession of the records seized was scheduled and held on March 16, 2023.
On April 21, 2023, the NCC issued a judgement affirming our ownership of the physical documents, including 51 hardcopy folders and certain digital files, related to the clinical studies performed by ICON/PRA Netherlands and seized by the Dutch courts in December 2022. The NCC further ordered ICON/PRA Netherlands to allow and tolerate the surrender of the documents, including digital and source data. Additionally, the NCC found that we are not in breach of any obligation under the clinical study agreement and ICON/PRA Netherlands had no basis to suspend the fulfillment of its obligations under the clinical study agreement to provide us all clinical data and access to perform an audit of the study. On June 15, 2023, ICON/PRA Netherlands filed an appeal of this decision and requested a scheduling hearing with the NCC, which occurred on September 23, 2024.
The hearing addressing our claims for damages was conducted on December 7, 2023. On February 7, 2024, the NCC issued a judgment in which the NCC found that, although all data related to the study is the rightful property of DiaMedica, there was an insufficient causal link between PRA Netherlands withholding study data and the damages claimed by us. We notified the NCC and PRA Netherlands of our intent to appeal this decision and submitted our statement of grounds for appeal on October 15, 2024. The NCC issued a decision to consolidate both appeals and evaluate them concurrently at a single hearing, which occurred on March 20, 2025. In response to the NCC ruling issued on June 24, 2025, in which the court found that ICON/PRA Netherlands was not in default under the agreement and dismissed all counter claims against DiaMedica, we reached an agreement to enter into a mutual release agreement with ICON/PRA Netherlands. On October 31, 2025, we agreed to a Settlement Agreement with Pharmaceutical Research Associates Group B.V. and PRA Health Sciences, Inc., pursuant to which DiaMedica and Pharmaceutical Research Associates Group B.V. and PRA Health Sciences, Inc. agree to settle any and all disputes, claims and liabilities that have arisen, or will arise, out of their relationship, past and present, including – but not limited to – the dispute summarized above. Additionally, DiaMedica agreed to take necessary actions to release certain data being held by a judicial custodian pursuant to the judgment of the District Court of Northern Netherlands (Groningen) within five business days of the execution of the Settlement Agreement. Based on the disposition of the claims against Pharmaceutical Research Associates Group B.V. and PRA Health Sciences, Inc. summarized above, we do not expect to provide any further updates on this matter.
From time to time, we may be subject to other various ongoing or threatened legal actions and proceedings, including those that arise in the ordinary course of business, which may include employment matters and breach of contract disputes. Such matters are subject to many uncertainties and to outcomes that are not predictable with assurance and that may not be known for extended periods of time. There are no material pending legal proceedings to which DiaMedica or any of its subsidiaries is a party or of which any of our property is the subject.
ITEM 1A. RISK FACTORS
Other than as set forth below, there have been no other material changes to our risk factors from those disclosed in our annual report on Form 10-K for the fiscal year ended December 31, 2024.
Subsequent trials may fail to replicate promising data seen in earlier preclinical studies and clinical trials.
Interim data from the ongoing Part 1a portion of the investigator-sponsored Phase 2 study of DM199 for the treatment of preeclampsia provided promising results. However, these results may not be replicated in ongoing and future studies or trials, and the final data analysis may differ from interim data analysis.
Even if ongoing and future trials of DM199 are conducted and completed as planned, the results may not replicate the results seen in preclinical studies and early clinical studies or meet the primary or secondary endpoints, or otherwise not prove sufficient to obtain regulatory approval or result in a restricted product label that could negatively impact commercialization. Success in preclinical testing does not ensure success in clinical trials, and success in early stage clinical trials does not ensure success in later clinical trials. This can be due to a variety of reasons, including variations in patient populations, or the inability of certain patients to complete all assessments required by the clinical trial protocol, adjustments to clinical trial protocols or designs as compared to earlier testing or trials, variations in the data that could produce inconclusive or uninterpretable results, or the use of additional trial sites or investigators.
Furthermore, if we fail to replicate the promising results from our preclinical studies or clinical trials in ongoing or future clinical trials, we may be unable to successfully develop, obtain regulatory approval for and commercialize our current or future product candidates.
Changes in funding and staffing for the FDA, SEC and other government agencies could prevent our new products from being developed, approved or commercialized in a timely manner or otherwise prevent those agencies from performing normal functions upon which the operation of our business substantially relies and which could negatively impact our business.
The ability and propensity of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels; payment of user fees and reauthorization of user fee programs; staffing and other resource limitations; its ability to hire and retain key personnel; statutory, regulatory and policy changes; and other business disruptions. Average review times at the FDA and foreign regulatory authorities have fluctuated in recent years as a result. In addition, the funding of government agencies that support research and development activities that pertain to FDA review, such as research to understand new technologies or establish new standards, is subject to the political process, which is inherently fluid and unpredictable. Such government agencies also have been subject to reductions in funding and downsizing of agency staffing levels, which could materially impact our business and operations. The current U.S. Presidential administration has implemented or proposed policies that may affect the FDA review process, including efforts to downsize the federal workforce, remove job elimination protections for federal workers, limit certain communications, and potentially impact user fee reauthorization. If political pressure, global health concerns, or other factors prevent the FDA or other regulatory authorities from conducting their regular reviews or other regulatory activities, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
Rule 10b5-1 Plan and Non-Rule 10b5-1 Trading Arrangement Adoptions, Terminations, and Modifications
During the quarterly period ended September 30, 2025, none of our directors or “officers” (as defined in Rule 16a-1(f) under the Exchange Act) adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408, respectively, of SEC Regulation S-K.
ITEM 6. EXHIBITS
The following exhibits are being filed or furnished with this quarterly report on Form 10-Q:
|
Exhibit No. |
Description |
Manner of Filing |
||
|
3.1 |
Notice of Articles of DiaMedica Therapeutics Inc. dated May 20, 2025 |
Incorporated by reference to Exhibit 3.1 to DiaMedica’s Quarterly Report on Form 10-Q for the period ended June 30, 2025 |
||
|
3.2 |
Amended and Restated Articles of DiaMedica Therapeutics Inc. Effective May 17, 2023 |
Incorporated by reference to Exhibit 3.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on May 18, 2023 |
||
|
10.1 |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on July 21, 2025 |
|||
|
10.2 |
Incorporated by reference to Exhibit 10.2 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on July 21, 2025 |
|||
| 10.3 | Amended and Restated 2021 Employment Inducement Incentive Plan date July 31, 2025 # | Filed herewith | ||
|
10.4 |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on August 6, 2025 |
|||
|
10.5 |
Filed herewith |
|||
| 10.6 | Sales Agreement between DiaMedica Therapeutics Inc. and TD Securities (USA) LLC, dated August 12, 2025 | Incorporated by reference to Exhibit 1.2 to DiaMedica’s Registration Statement on Form S-3 filed with the Securities and Exchange Commission on August 12, 2025 | ||
|
31.1 |
Filed herewith |
|||
|
31.2 |
Filed herewith |
|||
|
32.1 |
Furnished herewith |
|||
|
32.2 |
Furnished herewith |
|||
|
101 |
Financial statements from the quarterly report on Form 10-Q of DiaMedica Therapeutics Inc. for the three months ended September 30, 2025, formatted in Inline XBRL: (i) the Condensed Consolidated Balance Sheets, (ii) Condensed Consolidated Statements of Operations and Comprehensive Loss, (iii) Condensed Consolidated Statements of Shareholders’ Equity, (iv) Condensed Consolidated Statements of Cash Flows, (v) Notes to the Condensed Consolidated Financial Statements, and (vi) the information set forth in Part II, Item 5 |
Filed herewith |
||
|
104 |
Cover Page Interactive Data File |
Embedded within the Inline XBRL document |
* Certain exhibits and schedules have been omitted pursuant to Item 601 of Regulation S-K. A copy of any omitted exhibit can be furnished to the Commission upon its request*
+ Certain personal information, which would constitute an unwarranted invasion of personal privacy, has been redacted from this exhibit pursuant to Item 601 of Regulation S-K.
# Management contract or compensation plan or arrangement Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
SIGNATURES
|
DIAMEDICA THERAPEUTICS INC. |
|
|
Date: November 12, 2025 |
/s/ Rick Pauls |
|
Rick Pauls President and Chief Executive Officer |
|
|
(Principal Executive Officer) |
|
|
Date: November 12, 2025 |
/s/ Scott Kellen |
|
Scott Kellen Chief Financial Officer |
|
|
(Principal Financial Officer and Principal Accounting Officer) |
Exhibit 10.3

DIAMEDICA THERAPEUTICS INC.
2021 EMPLOYMENT INDUCEMENT INCENTIVE PLAN
Table of Contents
|
1. |
Purpose of Plan. |
1 |
|
2. |
Definitions. |
1 |
|
3. |
Plan Administration. |
6 |
|
4. |
Shares Available for Issuance. |
8 |
|
5. |
Participation. |
9 |
|
6. |
Options. |
9 |
|
7. |
Stock Appreciation Rights. |
10 |
|
8. |
Restricted Stock Awards and Restricted Stock Units. |
11 |
|
9. |
Performance Awards. |
13 |
|
10. |
Other Stock-Based Awards. |
14 |
|
11. |
Dividend Equivalents. |
15 |
|
12. |
Effect of Termination of Employment or Other Service. |
15 |
|
13. |
Payment of Withholding Taxes. |
18 |
|
14. |
Change in Control. |
19 |
|
15. |
Rights of Eligible Recipients and Participants; Transferability. |
21 |
|
16. |
Securities Law and Other Restrictions. |
22 |
|
17. |
Deferred Compensation; Compliance with Section 409A. |
22 |
|
18. |
Amendment, Modification and Termination. |
23 |
|
19. |
Effective Date and Duration of this Plan. |
23 |
|
20. |
Shareholder Approval. |
24 |
|
21. |
Miscellaneous. |
24 |
DIAMEDICA THERAPEUTICS INC.
2021 EMPLOYMENT INDUCEMENT INCENTIVE PLAN
|
1. |
Purpose of Plan. |
The purpose of the DiaMedica Therapeutics Inc. 2021 Employment Inducement Incentive Plan (this “Plan”) is to advance the interests of DiaMedica Therapeutics Inc., a corporation organized under the laws of British Columbia (the “Company”), and its shareholders by enabling the Company and its Subsidiaries to attract qualified new employees of the Company and its Subsidiaries, providing incentive compensation for such individuals that is linked to the growth and profitability of the Company and increases in shareholder value and aligning the interests of such employees with the interests of its shareholders through opportunities for equity participation in the Company. Only Eligible Recipients may receive Awards under this Plan.
|
2. |
Definitions. |
The following terms will have the meanings set forth below, unless the context clearly otherwise requires. Terms defined elsewhere in this Plan will have the same meaning throughout this Plan.
2.1 “Adverse Action” means any action or conduct by a Participant that the Committee, in its sole discretion, determines to be injurious, detrimental, prejudicial or adverse to the interests of the Company or any Subsidiary, including: (a) disclosing confidential information of the Company or any Subsidiary to any person not authorized by the Company or Subsidiary to receive it, (b) engaging, directly or indirectly, in any commercial activity that in the judgment of the Committee competes with the business of the Company or any Subsidiary or (c) interfering with the relationships of the Company or any Subsidiary and their respective employees, independent contractors, customers, prospective customers and vendors.
2.2 “Affiliate” means, with respect to any Person, any other Person directly or indirectly controlling, controlled by or under common control with, such Person where “control” will have the meaning given such term under Rule 405 of the Securities Act.
2.3 “Applicable Law” means any applicable law, including without limitation, (a) provisions of the Code, the Securities Act, the Exchange Act and any rules or regulations thereunder; (b) corporate, securities, tax or other laws, statutes, rules, requirements or regulations, whether federal, state, provincial, local or foreign; and (c) rules of any securities exchange, national market system or automated quotation system on which the Shares are listed, quoted or traded.
2.4 “Award” means, individually or collectively, an Option, Stock Appreciation Right, Restricted Stock Award, Restricted Stock Unit, Performance Award, or Other Stock-Based Award, in each case granted to an Eligible Recipient pursuant to this Plan.
2.5 “Award Agreement” means either: (a) a written or electronic (as provided in Section 21.7) agreement entered into by the Company and a Participant setting forth the terms and provisions applicable to an Award granted under this Plan, including any amendment or modification thereof, or (b) a written or electronic (as provided in Section 21.7) statement issued by the Company to a Participant describing the terms and provisions of such an Award, including any amendment or modification thereof.
2.6 “Board” means the Board of Directors of the Company.
2.7 “Broker Exercise Notice” means a written notice pursuant to which a Participant, upon exercise of an Option, irrevocably instructs a broker or dealer to sell a sufficient number of Shares to pay all or a portion of the exercise price of the Option and/or any related withholding tax obligations and remit such sums to the Company and directs the Company to deliver Shares to be issued upon such exercise directly to such broker or dealer or its nominee.
2.8 “Cause” means, unless otherwise provided in an Award Agreement, (a) “Cause” as defined in any employment, consulting, severance or similar agreement between the Participant and the Company or one of its Subsidiaries or Affiliates (an “Individual Agreement”), or (b) if there is no such Individual Agreement or if it does not define Cause: (i) dishonesty, fraud, misrepresentation, embezzlement or deliberate injury or attempted injury, in each case related to the Company or any Subsidiary; (ii) any unlawful or criminal activity of a serious nature; (iii) any intentional and deliberate breach of a duty or duties that, individually or in the aggregate, are material in relation to the Participant’s overall duties; (iv) any material breach by a Participant of any employment, service, confidentiality, non-compete or non-solicitation agreement entered into with the Company or any Subsidiary; or (v) before a Change in Control, such other events as will be determined by the Committee. Before a Change in Control, the Committee will, unless otherwise provided in an Individual Agreement, have the sole discretion to determine whether “Cause” exists with respect to subclauses (i), (ii), (iii), (iv) or (v) above, and its determination will be final.
2.9 “Change in Control” means, unless otherwise provided in an Award Agreement or any Individual Agreement, and except as provided in Section 17, an event described in Section 14.1 of this Plan.
2.10 “Code” means the United States Internal Revenue Code of 1986, as amended. Any reference to a section of the Code herein will be deemed to include a reference to any applicable regulations thereunder and any successor or amended section of the Code.
2.11 “Committee” means the Board or, if the Board so delegates, the Compensation Committee of the Board or a subcommittee thereof, or any other committee delegated authority by the Board to administer this Plan. If the Board determines appropriate, such committee may be comprised solely of directors designated by the Board to administer this Plan who are (a) “non-employee directors” within the meaning of Rule 16b-3 under the Exchange Act, and (b) “independent directors” within the meaning of the rules of the Nasdaq Stock Market (or other applicable exchange or market on which the Common Shares may be traded or quoted). The members of the Committee will be appointed from time to time by and will serve at the discretion of the Board. Any action duly taken by the Committee will be valid and effective, whether or not the members of the Committee at the time of such action are later determined not to have satisfied the requirements of membership provided herein.
2.12 “Common Shares” or “Shares” means the voting common shares, no par value, of the Company, or the number and kind of shares of stock or other securities into which such Common Shares may be changed in accordance with Section 4.4 of this Plan.
2.13 “Company” means DiaMedica Therapeutics Inc., a corporation organized under the laws of British Columbia, and any successor thereto as provided in Section 21.5 of this Plan.
2.14 “Director” means a member of the Board, as constituted from time to time.
2.15 “Disability” means, unless otherwise provided in an Award Agreement, with respect to a Participant who is a party to an Individual Agreement, which agreement contains a definition of “disability” or “permanent disability” (or words of like import) for purposes of termination of employment thereunder by the Company, “disability” or “permanent disability” as defined in the most recent of such agreements; or in all other cases, means the disability of the Participant such as would entitle the Participant to receive disability income benefits pursuant to the long-term disability plan of the Company or Subsidiary then covering the Participant or, if no such plan exists or is applicable to the Participant, the permanent and total disability of the Participant within the meaning of Section 22(e)(3) of the Code.
2.16 “Dividend Equivalents” has the meaning set forth in Section 3.2(m) of this Plan.
2.17 “Eligible Recipients” mean any prospective Employee who is commencing employment with the Company or a Subsidiary, or is being rehired following a “bona fide period of non-employment” by the Company or a Subsidiary, if he or she is granted an Award in connection with his or her commencement of employment with the Company or a Subsidiary and such grant is an inducement material to his or her entering into employment with the Company or a Subsidiary (within the meaning of Nasdaq Stock Market Rule 5635(d) or any successor rule, if the Company’s securities are traded on the Nasdaq Stock Market, and any interpretations by Nasdaq of such rule, and/or the applicable requirements of any other established stock exchange on which the Company’s securities are traded, as applicable, as such rules and requirements may be amended from time to time). Notwithstanding the foregoing, if the Company’s securities are traded on the Nasdaq Stock Market, an “Eligible Recipient” shall not include any prospective Employee who has previously been an Employee or a Director unless following a “bona fide period of non-employment” by the Company or a Subsidiary (within the meaning of Nasdaq Stock Market Rule 5635(d) or any successor rule, if the Company’s securities are traded on the Nasdaq Stock Market, and any interpretations by Nasdaq of such rule, and/or the applicable requirements of any other established stock exchange on which the Company’s securities are traded, as applicable, as such rules and requirements may be amended from time to time). The Committee may in its discretion adopt procedures from time to time to ensure that a prospective Employee is eligible to participate in the Plan prior to the granting of any Awards to such individual under the Plan (including without limitation a requirement that each such prospective Employee certify to the Company prior to the receipt of an Award under the Plan that he or she has had a bona fide period of non-employment, and that the grant of Awards under the Plan is an inducement material to his or her agreement to enter into employment with the Company or a Subsidiary).
2.18 “Employee” means any individual performing services for the Company or a Subsidiary and designated as an employee of the Company or a Subsidiary on the payroll records thereof. An Employee will not include any individual during any period he or she is classified or treated by the Company or Subsidiary as an independent contractor, a consultant, or any employee of an employment, consulting or temporary agency or any other entity other than the Company or Subsidiary, without regard to whether such individual is subsequently determined to have been, or is subsequently retroactively reclassified as a common-law employee of the Company or Subsidiary during such period. An individual will not cease to be an Employee in the case of: (a) any leave of absence approved by the Company, or (b) transfers between locations of the Company or between the Company or any Subsidiaries. Neither service as a Board member nor payment of a Board member’s fee by the Company will be sufficient to constitute “employment” by the Company.
2.19 “Exchange Act” means the United States Securities Exchange Act of 1934, as amended. Any reference to a section of the Exchange Act herein will be deemed to include a reference to any applicable rules and regulations thereunder and any successor or amended section of the Exchange Act.
2.20 “Fair Market Value” means, with respect to the Common Shares, as of any date a price that is equal to the closing sale price of a Common Share as of the end of the regular trading session on such date, as reported by the Nasdaq Stock Market or any national securities exchange on which the Common Shares are then listed (or, if no shares were traded on such date, as of the next preceding date on which there was such a trade) or if the Common Shares are not so listed, admitted to unlisted trading privileges or reported on any national exchange, the closing sale price as of the immediately preceding trading date at the end of the regular trading session, as reported by the OTC Bulletin Board, OTC Markets or other comparable quotation service (or, if no shares were traded or quoted on such date, as of the next preceding date on which there was such a trade or quote). In the event the Common Shares are not publicly traded at the time a determination of its value is required to be made hereunder, the determination of Fair Market Value shall be made by the Committee in such manner as it deems appropriate and in good faith in the exercise of its reasonable discretion, and consistent with the definition of “fair market value” under Section 409A of the Code. If determined by the Committee, such determination will be final, conclusive and binding for all purposes and on all persons, including the Company, the shareholders of the Company, the Participants and their respective successors-in-interest. No member of the Committee will be liable for any determination regarding the fair market value of the Common Shares that is made in good faith.
2.21 “Grant Date” means the date an Award is granted to a Participant pursuant to this Plan and as determined pursuant to Section 5 of this Plan.
2.22 “Independent Director” shall mean a Director of the Company who is not an Employee of the Company and who qualifies as “independent” within the meaning of Nasdaq Stock Market Rule 5605(a)(2), or any successor rule, if the Company’s securities are traded on the Nasdaq Stock Market, and/or the applicable requirements of any other established stock exchange on which the Company’s securities are traded, as applicable, as such rules and requirements may be amended from time to time.
2.23 “Individual Agreement” has the meaning set forth in Section 2.8 of this Plan.
2.24 “Option” means a right to purchase Common Shares granted to an Eligible Recipient pursuant to Section 6 of this Plan. Any Option granted under this Plan is not intended to meet the requirements of an Incentive Stock Option.
2.25 “Other Stock-Based Award” means an Award, denominated in Shares, not otherwise described by the terms of this Plan, granted pursuant to Section 10 of this Plan.
2.26 “Participant” means an Eligible Recipient who receives one or more Awards under this Plan.
2.27 “Performance Award” means a right granted to an Eligible Recipient pursuant to Section 9 of this Plan to receive an amount of cash, number of Shares, or a combination of both, contingent upon and the value of which at the time it is payable is determined as a function of the extent of the achievement of one or more Performance Goals during a specified Performance Period or the achievement of other objectives during a specified period.
2.28 “Performance Goals” mean with respect to any applicable Award, one or more targets, goals or levels of attainment required to be achieved during the specified Performance Period, as set forth in the related Award Agreement.
2.29 “Performance Period” means the period of time, as determined by the Committee, during which the Performance Goals must be met in order to determine the degree of payout or vesting with respect to an Award.
2.30 “Period of Restriction” means the period when a Restricted Stock Award or Restricted Stock Units are subject to a substantial risk of forfeiture (based on the passage of time, the achievement of Performance Goals, or upon the occurrence of other events as determined by the Committee, in its discretion), as provided in Section 8 of this Plan.
2.31 “Person” means an individual, partnership, corporation, limited liability company, business trust, joint stock company, trust, unincorporated association, joint venture, governmental authority or any other entity of whatever nature.
2.32 “Plan” means this DiaMedica Therapeutics Inc. 2021 Employment Inducement Incentive Plan, as may be amended from time to time.
2.33 “Plan Year” means the Company’s fiscal year.
2.34 “Previously Acquired Shares” means Shares that are already owned by the Participant or, with respect to any Award, that are to be issued to the Participant upon the grant, exercise, vesting or settlement of such Award.
2.35 “Restricted Stock Award” means an award of Common Shares granted to an Eligible Recipient pursuant to Section 8 of this Plan that is subject to the restrictions on transferability and the risk of forfeiture imposed by the provisions of such Section 8.
2.36 “Restricted Stock Unit” means an award denominated in Shares granted to an Eligible Recipient pursuant to Section 8 of this Plan.
2.37 “Retirement” means, unless otherwise defined in the Award Agreement or in an Individual Agreement between the Participant and the Company or one of its Subsidiaries or Affiliates, “Retirement” as defined from time to time for purposes of this Plan by the Committee or by the Company’s chief human resources officer or other person performing that function or, if not so defined, means voluntary termination of employment or service by the Participant on or after the date the Participant reaches age fifty-five (55) with the present intention to leave the Company’s industry or to leave the general workforce.
2.38 “Securities Act” means the United States Securities Act of 1933, as amended. Any reference to a section of the Securities Act herein will be deemed to include a reference to any applicable rules and regulations thereunder and any successor or amended section of the Securities Act.
2.39 “Stock Appreciation Right” means a right granted to an Eligible Recipient pursuant to Section 7 of this Plan to receive a payment from the Company upon exercise, in the form of Shares, cash or a combination of both, equal to the difference between the Fair Market Value of one or more Shares and the grant price of such shares under the terms of such Stock Appreciation Right.
2.40 “Stock-Based Award” means any Award, denominated in Shares, made pursuant to this Plan, including Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units, Performance Awards or Other Stock-Based Awards.
2.41 “Subsidiary” means any corporation or other entity, whether domestic or foreign, in which the Company has or obtains, directly or indirectly, an interest of more than fifty percent (50%) by reason of stock ownership or otherwise.
2.42 “Tax Date” means the date any withholding or employment related tax obligation arises under the Code or any Applicable Law for a Participant with respect to an Award.
2.43 “Tax Laws” has the meaning set forth in Section 21.8 of this Plan.
|
3. |
Plan Administration. |
3.1 The Committee. This Plan will be administered by the Committee. The Committee will act by majority approval of the members at a meeting or by unanimous written consent, and a majority of the members of the Committee will constitute a quorum. The Committee may exercise its duties, power and authority under this Plan in its sole discretion without the consent of any Participant or other party, unless this Plan specifically provides otherwise. The Committee will not be obligated to treat Participants or Eligible Recipients uniformly, and determinations made under this Plan may be made by the Committee selectively among Participants or Eligible Recipients, whether or not such Participants and Eligible Recipients are similarly situated. Each determination, interpretation or other action made or taken by the Committee pursuant to the provisions of this Plan will be final, conclusive and binding for all purposes and on all persons, and no member of the Committee will be liable for any action or determination made in good faith with respect to this Plan or any Award granted under this Plan.
3.2 Authority of the Committee. In accordance with and subject to the provisions of this Plan, the Committee will have full and exclusive discretionary power and authority to take such actions as it deems necessary and advisable with respect to the administration of this Plan, including the following:
(a) To adopt procedures from time to time intended to ensure that an individual is an Eligible Recipient prior to the granting of any Awards to such individual under this Plan (including without limitation a requirement, if any, that each such individual certify to the Company prior to the receipt of an Award under this Plan that such individual has not been previously employed, has had a bona fide period of non-employment, and that the grant of Awards under this Plan is an inducement material to such individual’s agreement to enter into employment with the Company or a Subsidiary);
(b) To designate the Eligible Recipients to be selected as Participants;
(c) To determine the nature, extent and terms of the Awards to be made to each Participant, including the amount of cash or number of Shares to be subject to each Award, any exercise price or grant price, the manner in which Awards will vest, become exercisable, settled or paid out and whether Awards will be granted in tandem with other Awards, and the form of Award Agreement, if any, evidencing such Award;
(d) To determine the time or times when Awards will be granted;
(e) To determine the duration of each Award;
(f) To determine the terms, restrictions and other conditions to which the grant of an Award or the payment or vesting of Awards may be subject;
(g) To construe and interpret this Plan and Awards granted under it, and to establish, amend and revoke rules and regulations for its administration and in so doing, to correct any defect, omission, or inconsistency in this Plan or in an Award Agreement, in a manner and to the extent it will deem necessary or expedient to make this Plan fully effective;
(h) To determine Fair Market Value in accordance with Section 2.20 of this Plan;
(i) To amend this Plan or any Award Agreement, as provided in this Plan;
(j) To adopt subplans or special provisions applicable to Awards regulated by the laws of a jurisdiction other than, and outside of, the United States, which except as otherwise provided in this Plan, such subplans or special provisions may take precedence over other provisions of this Plan;
(k) To authorize any person to execute on behalf of the Company any Award Agreement or any other instrument required to effect the grant of an Award previously granted by the Committee;
(l) To determine whether Awards will be settled in Shares, cash or in any combination thereof;
(m) To determine whether Awards will be adjusted for dividend equivalents, with “Dividend Equivalents” meaning a credit, made at the discretion of the Committee, to the account of a Participant in an amount equal to the cash dividends paid on one Common Share for each Common Share represented by an Award held by such Participant, subject to Section 11 of this Plan and any other provision of this Plan, and which Dividend Equivalents may be subject to the same conditions and restrictions as the Awards to which they attach and may be settled in the form of cash, Shares, or in any combination of both; and
(n) To impose such restrictions, conditions or limitations as it determines appropriate as to the timing and manner of any resales by a Participant or other subsequent transfers by the Participant of any Shares, including restrictions under an insider trading policy, stock ownership guidelines, restrictions as to the use of a specified brokerage firm for such resales or other transfers and other restrictions designed to increase equity ownership by Participants or otherwise align the interests of Participants with the Company’s shareholders.
3.3 Delegation. All Awards granted hereunder shall be approved by (i) the Committee, comprised of a majority of the Company’s Independent Directors or (ii) a majority of the Company’s Independent Directors and (y) the authority to grant Awards shall not be delegated under any circumstances.
3.4 No Re-pricing. Notwithstanding any other provision of this Plan other than Section 4.4 of this Plan, the Committee may not, without prior approval of the Company’s shareholders, seek to effect any re-pricing of any previously granted, “underwater” Option or Stock Appreciation Right by: (a) amending or modifying the terms of the Option or Stock Appreciation Right to lower the exercise price or grant price; (b) canceling the underwater Option or Stock Appreciation Right in exchange for (i) cash; (ii) replacement Options or Stock Appreciation Rights having a lower exercise price or grant price; or (iii) other Awards; or (c) repurchasing the underwater Options or Stock Appreciation Rights and granting new Awards under this Plan. For purposes of this Section 3.4, an Option or Stock Appreciation Right will be deemed to be “underwater” at any time when the Fair Market Value of the Common Shares is less than the exercise price of the Option or grant price of the Stock Appreciation Right.
3.5 Participants Based Outside of the United States. In addition to the authority of the Committee under Section 3.2(j) and notwithstanding any other provision of this Plan, the Committee may, in its sole discretion, amend the terms of this Plan or Awards with respect to Participants resident outside of the United States or employed by a non-U.S. Subsidiary in order to comply with local legal requirements, to otherwise protect the Company’s or Subsidiary’s interests or to meet objectives of this Plan, and may, where appropriate, establish one or more sub-plans (including the adoption of any required rules and regulations) for the purposes of qualifying for preferred tax treatment under foreign tax laws. The Committee will have no authority, however, to take action pursuant to this Section 3.5: (a) to reserve Shares or grant Awards in excess of the limitations provided in Section 4.1 of this Plan; (b) to effect any re-pricing in violation of Section 3.4 of this Plan; (c) to grant Options or Stock Appreciation Rights having an exercise price or grant price less than one hundred percent (100%) of the Fair Market Value of one Share on the Grant Date in violation of Section 6.3 or Section 7.3 of this Plan; or (d) for which shareholder approval would then be required pursuant to Section 18.2 of this Plan.
3.6 Actions Required Upon Grant of Award. Following the issuance of any Award under this Plan, the Company shall comply with any applicable announcement and notification requirements set forth in the listing requirements of the applicable securities exchange.
|
4. |
Shares Available for Issuance. |
4.1 Maximum Number of Shares Available. Subject to adjustment as provided in Section 4.4 of this Plan, the maximum number of Shares that will be available for issuance under this Plan shall not exceed 2,000,000.
4.2 Accounting for Awards. Shares that are issued under this Plan or that are subject to outstanding Awards will be applied to reduce the maximum number of Shares remaining available for issuance under this Plan only to the extent they are used; provided, however, that the full number of Shares subject to a stock-settled Stock Appreciation Right or other Stock-Based Award will be counted against the Shares authorized for issuance under this Plan, regardless of the number of Shares actually issued upon settlement of such Stock Appreciation Right or other Stock-Based Award. Furthermore, any Shares withheld to satisfy tax withholding obligations on Awards issued under this Plan, any Shares withheld to pay the exercise price or grant price of Awards under this Plan and any Shares not issued or delivered as a result of the “net exercise” of an outstanding Option pursuant to Section 6.5 or settlement of a Stock Appreciation Right in Shares pursuant to Section 7.7 will be counted against the Shares authorized for issuance under this Plan and will not be available again for grant under this Plan. Shares subject to Awards settled in cash will again be available for issuance pursuant to Awards granted under the Plan. Any Shares repurchased by the Company on the open market using the proceeds from the exercise of an Award will not increase the number of Shares available for future grant of Awards. Any Common Shares related to Awards granted under this Plan that terminate by expiration, forfeiture, cancellation or otherwise without the issuance of the Shares, will be available again for grant under this Plan. The Shares available for issuance under this Plan may be authorized and unissued shares or treasury shares.
4.3 Stock Distributed. Any Shares distributed pursuant to an Award may consist, in whole or in part, of authorized and unissued Common Shares, treasury Common Shares or Common Shares purchased on the open market.
4.4 Adjustments to Shares and Awards.
(a) In the event of any reorganization, merger, consolidation, recapitalization, liquidation, reclassification, stock dividend, stock split, combination of shares, rights offering, divestiture or extraordinary dividend (including a spin off) or any other similar change in the corporate structure or Shares the Company, the Committee (or, if the Company is not the surviving corporation in any such transaction, the board of directors of the surviving corporation) will make appropriate adjustment or substitutions (which determination will be conclusive) as to: (i) the number and kind of securities or other property (including cash) available for issuance or payment under this Plan, and (ii) in order to prevent dilution or enlargement of the rights of Participants, the number and kind of securities or other property (including cash) subject to outstanding Awards and the exercise price of outstanding Awards; provided, however, that this Section 4.4 will not limit the authority of the Committee to take action pursuant to Section 14 of this Plan in the event of a Change in Control. The determination of the Committee as to the foregoing adjustments and/or substitutions, if any, will be final, conclusive and binding on Participants under this Plan.
(b) Notwithstanding anything else herein to the contrary, without affecting the number of Shares reserved or available hereunder, the limit in Section 4.1 of this Plan, the Committee may authorize the issuance or assumption of benefits under this Plan in connection with any merger, consolidation, acquisition of property or stock or reorganization upon such terms and conditions as it may deem appropriate, subject to compliance with the rules under Sections 422, 424 and 409A of the Code, or any successor regulations, as and where applicable.
|
5. |
Participation. |
Participants in this Plan will be those Eligible Recipients who, in the judgment of the Committee, are expected to contribute to the achievement of the objectives of the Company or its Subsidiaries. Eligible Recipients may be granted from time to time one or more Awards, singly or in combination or in tandem with other Awards, as may be determined by the Committee in its sole discretion. Awards will be deemed to be granted as of the date specified in the grant resolution of the Committee, which date will be the Grant Date of any related Award Agreement with the Participant.
|
6. |
Options. |
6.1 Grant. An Eligible Recipient may be granted one or more Options under this Plan, and such Options will be subject to such terms and conditions, consistent with the other provisions of this Plan, as may be determined by the Committee in its sole discretion; provided, however, that any Option granted under this Plan shall comply with Applicable Law and applicable stock exchange rules.
6.2 Award Agreement. Each Option grant will be evidenced by an Award Agreement that will specify the exercise price of the Option, the maximum duration of the Option, the number of Shares to which the Option pertains, the conditions upon which an Option will become vested and exercisable, and such other provisions as the Committee will determine which are not inconsistent with the terms of this Plan or applicable stock exchange rules.
6.3 Exercise Price. The per share price to be paid by a Participant upon exercise of an Option granted pursuant to this Section 6 will be determined by the Committee in its sole discretion at the time of the Option grant; provided, however, that such price will not be less than one hundred percent (100%) of the Fair Market Value of one Share on the Grant Date.
6.4 Exercisability and Duration. An Option will become exercisable at such times and in such installments and upon such terms and conditions as may be determined by the Committee in its sole discretion at the time of grant, including (a) the achievement of one or more of the Performance Goals; or that (b) the Participant remain in the continuous employment or service with the Company or a Subsidiary for a certain period; provided, however, that no Option may be exercisable after ten (10) years from the Grant Date. Notwithstanding the foregoing, if the exercise of an Option that is exercisable in accordance with its terms is prevented by the provisions of Section 16 of this Plan, the Option will remain exercisable until thirty (30) days after the date such exercise first would no longer be prevented by such provisions, but in any event no later than the expiration date of such Option.
6.5 Payment of Exercise Price.
(a) The total purchase price of the Shares to be purchased upon exercise of an Option will be paid entirely in cash (including check, bank draft or money order); provided, however, that the Committee, in its sole discretion and upon terms and conditions established by the Committee, may allow such payments to be made, in whole or in part, by (i) tender of a Broker Exercise Notice; (ii) by tender, either by actual delivery or attestation as to ownership, of Previously Acquired Shares; (iii) a “net exercise” of the Option (as further described in paragraph (b), below); (iv) by a combination of such methods; or (v) any other method approved or accepted by the Committee in its sole discretion and permitted under applicable law. Notwithstanding any other provision of this Plan to the contrary, no Participant who is an “executive officer” of the Company within the meaning of Section 13(k) of the Exchange Act will be permitted to make payment with respect to any Awards granted under this Plan, or continue any extension of credit with respect to such payment with a loan from the Company or a loan arranged by the Company in violation of Section 13(k) of the Exchange Act.
(b) In the case of a “net exercise” of an Option, the Company will not require a payment of the exercise price of the Option from the Participant but will reduce the number of Shares issued upon the exercise by the largest number of whole shares that has a Fair Market Value on the exercise date that does not exceed the aggregate exercise price for the shares exercised under this method. Shares will no longer be outstanding under an Option (and will therefore not thereafter be exercisable) following the exercise of such Option to the extent of (i) shares used to pay the exercise price of an Option under the “net exercise,” (ii) shares actually delivered to the Participant as a result of such exercise and (iii) any shares withheld for purposes of tax withholding pursuant to Section 13 of this Plan.
(c) For purposes of such payment, Previously Acquired Shares tendered or covered by an attestation will be valued at their Fair Market Value on the exercise date of the Option.
6.6 Manner of Exercise. An Option may be exercised by a Participant in whole or in part from time to time, subject to the conditions contained in this Plan and in the Award Agreement evidencing such Option, by delivery in person, by facsimile or electronic transmission or through the mail of written notice of exercise to the Company at its principal executive office (or to the Company’s designee as may be established from time to time by the Company and communicated to Participants) and by paying in full the total exercise price for the Shares to be purchased in accordance with Section 6.5 of this Plan.
|
7. |
Stock Appreciation Rights. |
7.1 Grant. An Eligible Recipient may be granted one or more Stock Appreciation Rights under this Plan, and such Stock Appreciation Rights will be subject to such terms and conditions, consistent with the other provisions of this Plan, as may be determined by the Committee in its sole discretion. Stock Appreciation Rights may be granted to an Eligible Recipient for services provided to a Subsidiary only if, with respect to such Eligible Recipient, the underlying Shares constitute “service recipient stock” within the meaning of Treas. Reg. Sec. 1.409A-1(b)(5)(iii) promulgated under the Code.
7.2 Award Agreement. Each Stock Appreciation Right will be evidenced by an Award Agreement that will specify the grant price of the Stock Appreciation Right, the term of the Stock Appreciation Right, and such other provisions as the Committee will determine which are not inconsistent with the terms of this Plan.
7.3 Grant Price. The grant price of a Stock Appreciation Right will be determined by the Committee, in its discretion, at the Grant Date; provided, however, that such price may not be less than one hundred percent (100%) of the Fair Market Value of one Share on the Grant Date.
7.4 Exercisability and Duration. A Stock Appreciation Right will become exercisable at such times and in such installments as may be determined by the Committee in its sole discretion at the time of grant; provided, however, that no Stock Appreciation Right may be exercisable after ten (10) years from its Grant Date. Notwithstanding the foregoing, if the exercise of a Stock Appreciation Right that is exercisable in accordance with its terms is prevented by the provisions of Section 16 of this Plan, the Stock Appreciation Right will remain exercisable until thirty (30) days after the date such exercise first would no longer be prevented by such provisions, but in any event no later than the expiration date of such Stock Appreciation Right.
7.5 Manner of Exercise. A Stock Appreciation Right will be exercised by giving notice in the same manner as for Options, as set forth in Section 6.6 of this Plan, subject to any other terms and conditions consistent with the other provisions of this Plan as may be determined by the Committee in its sole discretion.
7.6 Settlement. Upon the exercise of a Stock Appreciation Right, a Participant will be entitled to receive payment from the Company in an amount determined by multiplying:
(a) The excess of the Fair Market Value of a Share on the date of exercise over the per share grant price; by
(b) The number of Shares with respect to which the Stock Appreciation Right is exercised.
7.7 Form of Payment. Payment, if any, with respect to a Stock Appreciation Right settled in accordance with Section 7.6 of this Plan will be made in accordance with the terms of the applicable Award Agreement, in cash, Shares or a combination thereof, as the Committee determines.
|
8. |
Restricted Stock Awards and Restricted Stock Units. |
8.1 Grant. An Eligible Recipient may be granted one or more Restricted Stock Awards or Restricted Stock Units under this Plan, and such Awards will be subject to such terms and conditions, consistent with the other provisions of this Plan, as may be determined by the Committee in its sole discretion. Restricted Stock Units will be similar to Restricted Stock Awards except that no Shares are actually awarded to the Participant on the Grant Date of the Restricted Stock Units. Restricted Stock Units will be denominated in Shares but paid in cash, Shares or a combination of cash and Shares as the Committee, in its sole discretion, will determine, and as provided in the Award Agreement.
8.2 Award Agreement. Each Restricted Stock Award or Restricted Stock Unit grant will be evidenced by an Award Agreement that will specify the type of Award, the period(s) of restriction, the number of Shares subject to a Restricted Stock Award, or the number of Restricted Stock Units granted, and such other provisions as the Committee will determine that are not inconsistent with the terms of this Plan.
8.3 Conditions and Restrictions. Subject to the terms and conditions of this Plan, the Committee will impose such conditions or restrictions on a Restricted Stock Award or Restricted Stock Units granted pursuant to this Plan as it may deem advisable including a requirement that Participants pay a stipulated purchase price for each Share underlying a Restricted Stock Award, Restricted Stock Unit, restrictions based upon the achievement of specific Performance Goals, time-based restrictions on vesting following the attainment of the Performance Goals, time-based restrictions, restrictions under Applicable Laws or holding requirements or sale restrictions placed on the Shares by the Company upon vesting of such Restricted Stock Award, Restricted Stock Units.
8.4 Voting Rights. Unless otherwise determined by the Committee and set forth in a Participant’s Award Agreement, to the extent permitted or required by Applicable Law, as determined by the Committee, Participants holding a Restricted Stock Award granted hereunder will be granted the right to exercise full voting rights with respect to the Shares underlying such Restricted Stock Award during the Period of Restriction. A Participant will have no voting rights with respect to any Restricted Stock Units granted hereunder.
8.5 Dividend Rights.
(a) Unless otherwise determined by the Committee and set forth in a Participant’s Award Agreement, to the extent permitted or required by Applicable Law, as determined by the Committee, Participants holding a Restricted Stock Award granted hereunder will have the same dividend rights as the Company’s other shareholders. Notwithstanding the foregoing any such dividends as to a Restricted Stock Award that is subject to vesting requirements will be subject to forfeiture and termination to the same extent as the Restricted Stock Award to which such dividends relate and the Award Agreement may require that any cash dividends be reinvested in additional Shares subject to the Restricted Stock Award and subject to the same conditions and restrictions as the Restricted Stock Award with respect to which the dividends were paid. In no event will dividends with respect to Restricted Stock Awards that are subject to vesting be paid or distributed until the vesting provisions of such Restricted Stock Award lapse.
(b) Unless otherwise determined by the Committee and set forth in a Participant’s Award Agreement, to the extent permitted or required by Applicable Law, as determined by the Committee, prior to settlement or forfeiture, any Restricted Stock Units awarded under this Plan may, at the Committee’s discretion, carry with it a right to Dividend Equivalents. Such right entitles the Participant to be credited with an amount equal to all cash dividends paid on one Share while the Restricted Stock Unit is outstanding. Dividend Equivalents may be converted into additional Restricted Stock and may (and will, to the extent required below) be made subject to the same conditions and restrictions as the Restricted Stock Units to which they attach. Settlement of Dividend Equivalents may be made in the form of cash, in the form of Shares, or in a combination of both. Dividend Equivalents as to Restricted Stock Units will be subject to forfeiture and termination to the same extent as the corresponding Restricted Stock Units as to which the Dividend Equivalents relate. In no event will Participants holding Restricted Stock Units be entitled to receive the payment of any Dividend Equivalents on such Restricted Stock Units until the vesting provisions of such Restricted Stock Units lapse.
8.6 Enforcement of Restrictions. To enforce the restrictions referred to in this Section 8, the Committee may place a legend on the stock certificates representing Restricted Stock Awards referring to such restrictions and may require the Participant, until the restrictions have lapsed, to keep the stock certificates, together with duly endorsed stock powers, in the custody of the Company or its transfer agent, or to maintain evidence of stock ownership, together with duly endorsed stock powers, in a certificateless book entry stock account with the Company’s transfer agent. Alternatively, Restricted Stock Awards may be held in non-certificated form pursuant to such terms and conditions as the Company may establish with its registrar and transfer agent or any third-party administrator designated by the Company to hold Restricted Stock Awards on behalf of Participants.
8.7 Lapse of Restrictions; Settlement. Except as otherwise provided in this Plan, including without limitation this Section 8 and 15.4 of this Plan, Shares underlying a Restricted Stock Award will become freely transferable by the Participant after all conditions and restrictions applicable to such shares have been satisfied or lapse (including satisfaction of any applicable tax withholding obligations). Upon the vesting of a Restricted Stock Unit, the Restricted Stock Unit will be settled, subject to the terms and conditions of the applicable Award Agreement, (a) in cash, based upon the Fair Market Value of the vested underlying Shares, (b) in Shares or (c) a combination thereof, as provided in the Award Agreement, except to the extent that a Participant has properly elected to defer income that may be attributable to a Restricted Stock Unit under a Company deferred compensation plan or arrangement.
8.8 Section 83(b) Election for Restricted Stock Award. If a Participant makes an election pursuant to Section 83(b) of the Code with respect to a Restricted Stock Award, the Participant must file, within thirty (30) days following the Grant Date of the Restricted Stock Award, a copy of such election with the Company and with the Internal Revenue Service, in accordance with the regulations under Section 83 of the Code. The Committee may provide in the Award Agreement that the Restricted Stock Award is conditioned upon the Participant’s making or refraining from making an election with respect to the award under Section 83(b) of the Code.
|
9. |
Performance Awards. |
9.1 Grant. An Eligible Recipient may be granted one or more Performance Awards under this Plan, and such Awards will be subject to such terms and conditions, consistent with the other provisions of this Plan, as may be determined by the Committee in its sole discretion, including the achievement of one or more Performance Goals.
9.2 Award Agreement. Each Performance Award will be evidenced by an Award Agreement that will specify the amount of cash, Shares, other Awards, or combination of both to be received by the Participant upon payout of the Performance Award, any Performance Goals upon which the Performance Award is subject, any Performance Period during which any Performance Goals must be achieved and such other provisions as the Committee will determine which are not inconsistent with the terms of this Plan.
9.3 Vesting. Subject to the terms of this Plan, the Committee may impose such restrictions or conditions, not inconsistent with the provisions of this Plan, to the vesting of such Performance Awards as it deems appropriate, including the achievement of one or more of the Performance Goals.
9.4 Earning of Performance Award Payment. Subject to the terms of this Plan and the Award Agreement, after the applicable Performance Period has ended, the holder of Performance Awards will be entitled to receive payout on the value and number of Performance Awards earned by the Participant over the Performance Period, to be determined as a function of the extent to which the corresponding Performance Goals have been achieved and such other restrictions or conditions imposed on the vesting and payout of the Performance Awards has been satisfied.
9.5 Form and Timing of Performance Award Payment. Subject to the terms of this Plan, after the applicable Performance Period has ended, the holder of Performance Awards will be entitled to receive payment on the value and number of Performance Awards earned by the Participant over the Performance Period, to be determined as a function of the extent to which the corresponding Performance Goals have been achieved. Payment of earned Performance Awards will be as determined by the Committee and as evidenced in the Award Agreement. Subject to the terms of this Plan, the Committee, in its sole discretion, may pay earned Performance Awards in the form of cash, in Shares or other Awards (or in a combination thereof) eq ual to the value of the earned Performance Awards at the close of the applicable Performance Period. Payment of any Performance Award will be made as soon as practicable after the Committee has determined the extent to which the applicable Performance Goals have been achieved and not later than the fifteenth (15th) day of the third (3rd) month immediately following the later of the end of the Company’s fiscal year in which the Performance Period ends and any additional vesting restrictions are satisfied or the end of the calendar year in which the Performance Period ends and any additional vesting restrictions are satisfied, except to the extent that a Participant has properly elected to defer payment that may be attributable to a Performance Award under a Company deferred compensation plan or arrangement. The determination of the Committee with respect to the form and time of payment of Performance Awards will be set forth in the Award Agreement pertaining to the grant of the Performance Award. Any Shares or other Awards issued in payment of earned Performance Awards may be granted subject to any restrictions deemed appropriate by the Committee, including that the Participant remain in the continuous employment or service with the Company or a Subsidiary for a certain period.
9.6 Evaluation of Performance. The Committee may provide in any such Award Agreement including Performance Goals that any evaluation of performance may include or exclude any of the following events that occurs during a Performance Period: (a) items related to a change in accounting principles; (b) items relating to financing activities; (c) expenses for restructuring or productivity initiatives; (d) other non-operating items; (e) items related to acquisitions; (f) items attributable to the business operations of any entity acquired by the Company during the Performance Period; (g) items related to the disposal of a business or segment of a business; (h) items related to discontinued operations that do not qualify as a segment of a business under applicable accounting standards; (i) items attributable to any stock dividend, stock split, combination or exchange of stock occurring during the Performance Period; (j) any other items of significant income or expense which are determined to be appropriate adjustments; (k) items relating to unusual or extraordinary corporate transactions, events or developments; (l) items related to amortization of acquired intangible assets; (m) items that are outside the scope of the Company’s core, on-going business activities; (n) items related to acquired in-process research and development; (o) items relating to changes in tax laws; (p) items relating to major licensing or partnership arrangements; (q) items relating to asset impairment charges; (r) items relating to gains or losses for litigation, arbitration and contractual settlements; (s) foreign exchange gains and losses; or (t) items relating to any other unusual or nonrecurring events or changes in applicable laws, accounting principles or business conditions.
9.7 Adjustment of Performance Goals, Performance Periods or other Vesting Criteria. The Committee may amend or modify the vesting criteria (including any Performance Goals or Performance Periods) of any outstanding Awards based in whole or in part on the financial performance of the Company (or any Subsidiary or division, business unit or other sub-unit thereof) in recognition of unusual or nonrecurring events (including the events described in Sections 9.6 or 4.4(a) of this Plan) affecting the Company or the financial statements of the Company or of changes in applicable laws, regulations or accounting principles, whenever the Committee determines that such adjustments are appropriate in order to prevent unintended dilution or enlargement of the benefits or potential benefits intended to be made available under this Plan. The determination of the Committee as to the foregoing adjustments, if any, will be final, conclusive and binding on Participants under this Plan.
9.8 Dividend Rights. Participants holding Performance Awards granted under this Plan will not receive any cash dividends or Dividend Equivalents based on the dividends declared on Shares that are subject to such Performance Awards during the period between the date that such Performance Awards are granted and the date such Performance Awards are settled.
|
10. |
Other Stock-Based Awards. |
10.1 Other Stock-Based Awards. Subject to such terms and conditions, consistent with the other provisions of this Plan, as may be determined by the Committee in its sole discretion, the Committee may grant Other Stock-Based Awards to Eligible Recipients not otherwise described by the terms of this Plan (including the grant or offer for sale of unrestricted Shares) in such amounts and subject to such terms and conditions as the Committee will determine. Such Awards may involve the transfer of actual Shares to Participants as a bonus or in lieu of obligations to pay cash or deliver other property under this Plan or under other plans or compensatory arrangements, or payment in cash or otherwise of amounts based on the value of Shares, and may include Awards designed to comply with or take advantage of the applicable local laws of jurisdictions other than the United States.
10.2 Value of Other Stock-Based Awards. Each Other Stock-Based Award will be expressed in terms of Shares or units based on Shares, as determined by the Committee. The Committee may establish Performance Goals in its discretion for any Other Stock-Based Award. If the Committee exercises its discretion to establish Performance Goals for any such Awards, the number or value of Other Stock-Based Awards that will be paid out to the Participant will depend on the extent to which the Performance Goals are met.
10.3 Payment of Other Stock-Based Awards. Payment, if any, with respect to an Other Stock-Based Award will be made in accordance with the terms of the Award, in cash or Shares for any Other Stock-Based Award, as the Committee determines, except to the extent that a Participant has properly elected to defer payment that may be attributable to an Other Stock-Based Award under a Company deferred compensation plan or arrangement.
|
11. |
Dividend Equivalents. |
Subject to the provisions of this Plan and any Award Agreement, any Participant selected by the Committee may be granted Dividend Equivalents based on the dividends declared on Shares that are subject to any Award (including any Award that has been deferred), to be credited as of dividend payment dates, during the period between the date the Award is granted and the date the Award is exercised, vests, settles, is paid or expires, as determined by the Committee. Such Dividend Equivalents will be converted to cash or additional Shares by such formula and at such time and subject to such limitations as may be determined by the Committee and the Committee may provide that such amounts (if any) will be deemed to have been reinvested in additional Shares or otherwise reinvested. Notwithstanding the foregoing, the Committee may not grant Dividend Equivalents based on the dividends declared on Shares that are subject to an Option or Stock Appreciation Right or unvested Performance Awards; and further, no dividend or Dividend Equivalents will be paid out with respect to any unvested Awards.
|
12. |
Effect of Termination of Employment or Other Service. |
12.1 Termination Due to Cause. Unless otherwise expressly provided by the Committee in its sole discretion in an Award Agreement or the terms of an Individual Agreement between the Participant and the Company or one of its Subsidiaries or Affiliates or a plan or policy of the Company applicable to the Participant specifically provides otherwise, and subject to Sections 12.4 and 12.5 of this Plan, in the event a Participant’s employment or other service with the Company or any Subsidiary is terminated for Cause:
(a) All outstanding Options and Stock Appreciation Rights held by the Participant as of the effective date of such termination will be immediately terminated and forfeited;
(b) All outstanding but unvested Restricted Stock Awards, Restricted Stock Units, Performance Awards and Other Stock-Based Awards held by the Participant as of the effective date of such termination will be terminated and forfeited; and
(c) All other outstanding Awards to the extent not vested will be immediately terminated and forfeited.
12.2 Termination Due to Death, Disability or Retirement. Unless otherwise expressly provided by the Committee in its sole discretion in an Award Agreement between the Participant and the Company or one of its Subsidiaries or Affiliates or the terms of an Individual Agreement or a plan or policy of the Company applicable to the Participant specifically provides otherwise, and subject to Sections 12.4, 12.5 and 14 of this Plan, in the event a Participant’s employment or other service with the Company and all Subsidiaries is terminated by reason of death or Disability of a Participant, or in the case of a Participant that is an Employee, Retirement:
(a) All outstanding Options and Stock Appreciation Rights held by the Participant as of the effective date of such termination or Retirement will, to the extent exercisable as of the date of such termination or Retirement, remain exercisable for a period of one (1) year after the date of such termination or Retirement (but in no event after the expiration date of any such Option or Stock Appreciation Right) and Options and Stock Appreciation Rights not exercisable as of the date of such termination or Retirement will be terminated and forfeited;
(b) All outstanding unvested Restricted Stock Awards held by the Participant as of the effective date of such termination or Retirement will be terminated and forfeited; and
(c) All outstanding unvested Restricted Stock Units, Performance Awards, and Other Stock-Based Awards held by the Participant as of the effective date of such termination or Retirement will be terminated and forfeited; provided, however, that with respect to any such Awards the vesting of which is based on the achievement of Performance Goals, if a Participant’s employment or other service with the Company or any Subsidiary, as the case may be, is terminated prior to the end of the Performance Period of such Award, but after the conclusion of a portion of the Performance Period (but in no event less than one year), the Committee may, in its sole discretion, cause Shares to be delivered or payment made (except to the extent that a Participant has properly elected to defer income that may be attributable to such Award under a Company deferred compensation plan or arrangement) with respect to the Participant’s Award, but only if otherwise earned for the entire Performance Period and only with respect to the portion of the applicable Performance Period completed at the date of such event, with proration based on the number of months or years that the Participant was employed or performed services during the Performance Period. The Committee will consider the provisions of Section 12.5 of this Plan and will have the discretion to consider any other fact or circumstance in making its decision as to whether to deliver such Shares or other payment, including whether the Participant again becomes employed.
12.3 Termination for Reasons Other than Death, Disability or Retirement. Unless otherwise expressly provided by the Committee in its sole discretion in an Award Agreement or the terms of an Individual Agreement between the Participant and the Company or one of its Subsidiaries or Affiliates or a plan or policy of the Company applicable to the Participant specifically provides otherwise, and subject to Sections 12.4, 12.5 and 14 of this Plan, in the event a Participant’s employment or other service with the Company and all Subsidiaries is terminated for any reason other than for Cause or death or Disability of a Participant, or in the case of a Participant that is an Employee, Retirement:
(a) All outstanding Options and Stock Appreciation Rights held by the Participant as of the effective date of such termination will, to the extent exercisable as of such termination, remain exercisable for a period of three (3) months after such termination (but in no event after the expiration date of any such Option or Stock Appreciation Right) and Options and Stock Appreciation Rights not exercisable as of such termination will be terminated and forfeited. If the Participant dies within the three (3) month period referred to in the preceding sentence, the Option or Stock Appreciation Right may be exercised by those entitled to do so under the Participant’s will or by the laws of descent and distribution within a period of one (1) year following the Participant’s death (but in no event after the expiration date of any such Option or Stock Appreciation Right).
(b) All outstanding unvested Restricted Stock Awards held by the Participant as of the effective date of such termination will be terminated and forfeited;
(c) All outstanding unvested Restricted Stock Units, Performance Awards, and Other Stock-Based Awards held by the Participant as of the effective date of such termination will be terminated and forfeited; provided, however, that with respect to any such Awards the vesting of which is based on the achievement of Performance Goals, if a Participant’s employment or other service with the Company or any Subsidiary, as the case may be, is terminated by the Company without Cause prior to the end of the Performance Period of such Award, but after the conclusion of a portion of the Performance Period (but in no event less than one year), the Committee may, in its sole discretion, cause Shares to be delivered or payment made (except to the extent that a Participant has properly elected to defer income that may be attributable to such Award under a Company deferred compensation plan or arrangement) with respect to the Participant’s Award, but only if otherwise earned for the entire Performance Period and only with respect to the portion of the applicable Performance Period completed at the date of such event, with proration based on the number of months or years that the Participant was employed or performed services during the Performance Period.
12.4 Modification of Rights upon Termination. Notwithstanding the other provisions of this Section 12, upon a Participant’s termination of employment or other service with the Company or any Subsidiary, as the case may be, the Committee may, in its sole discretion (which may be exercised at any time on or after the Grant Date, including following such termination) cause Options or Stock Appreciation Rights (or any part thereof) held by such Participant as of the effective date of such termination to terminate, become or continue to become exercisable or remain exercisable following such termination of employment or service, and Restricted Stock, Restricted Stock Units, Performance Awards and Other Stock-Based Awards held by such Participant as of the effective date of such termination to terminate, vest or become free of restrictions and conditions to payment, as the case may be, following such termination of employment or service, in each case in the manner determined by the Committee; provided, however, that (a) no Option or Stock Appreciation Right may remain exercisable beyond its expiration date; and (b) any such action by the Committee adversely affecting any outstanding Award will not be effective without the consent of the affected Participant (subject to the right of the Committee to take whatever action it deems appropriate under Section 4.4, 12.5, 14 or 18 of this Plan).
12.5 Additional Forfeiture Events.
(a) Effect of Actions Constituting Cause or Adverse Action. Notwithstanding anything in this Plan to the contrary and in addition to the other rights of the Committee under this Plan, including this Section 12.5, if a Participant is determined by the Committee, acting in its sole discretion, to have taken any action that would constitute Cause or an Adverse Action during or within one (1) year after the termination of employment or other service with the Company or a Subsidiary, irrespective of whether such action or the Committee’s determination occurs before or after termination of such Participant’s employment or other service with the Company or any Subsidiary and irrespective of whether or not the Participant was terminated as a result of such Cause or Adverse Action, (i) all rights of the Participant under this Plan and any Award Agreements evidencing an Award then held by the Participant will terminate and be forfeited without notice of any kind, and (ii) the Committee in its sole discretion will have the authority to rescind the exercise, vesting or issuance of, or payment in respect of, any Awards of the Participant that were exercised, vested or issued, or as to which such payment was made, and to require the Participant to pay to the Company, within ten (10) days of receipt from the Company of notice of such rescission, any amount received or the amount of any gain realized as a result of such rescinded exercise, vesting, issuance or payment (including any dividends paid or other distributions made with respect to any Shares subject to any Award). The Company may defer the exercise of any Option or Stock Appreciation Right for a period of up to six (6) months after receipt of the Participant’s written notice of exercise or the issuance of share certificates upon the vesting of any Award for a period of up to six (6) months after the date of such vesting in order for the Committee to make any determination as to the existence of Cause or an Adverse Action. The Company will be entitled to withhold and deduct from future wages of the Participant (or from other amounts that may be due and owing to the Participant from the Company or a Subsidiary) or make other arrangements for the collection of all amounts necessary to satisfy such payment obligations. Unless otherwise provided by the Committee in an applicable Award Agreement, this Section 12.5(a) will not apply to any Participant following a Change in Control.
(b) Forfeiture or Clawback of Awards Under Applicable Law and Company Policy. If the Company is required to prepare an accounting restatement due to the material noncompliance of the Company, as a result of misconduct, with any financial reporting requirement under the securities laws, then any Participant who is one of the individuals subject to automatic forfeiture under Section 304 of the Sarbanes-Oxley Act of 2002 will reimburse the Company for the amount of any Award received by such individual under this Plan during the 12-month period following the first public issuance or filing with the Securities and Exchange Commission, as the case may be, of the financial document embodying such financial reporting requirement. The Company also may seek to recover any Award made as required by the provisions of the Dodd-Frank Wall Street Reform and Consumer Protection Act or any other clawback, forfeiture or recoupment provision required by Applicable Law or under the requirements of any stock exchange or market upon which the Shares are then listed or traded. In addition, all Awards under this Plan will be subject to forfeiture or other penalties pursuant to any clawback or forfeiture policy of the Company, as in effect from time to time, and such forfeiture and/or penalty conditions or provisions as determined by the Committee and set forth in the applicable Award Agreement.
|
13. |
Payment of Withholding Taxes. |
13.1 General Rules. The Company is entitled to (a) withhold and deduct from future wages of the Participant (or from other amounts that may be due and owing to the Participant from the Company or a Subsidiary), or make other arrangements for the collection of, all amounts the Company reasonably determines are necessary to satisfy any and all federal, foreign, state, provincial and local withholding and employment related tax requirements attributable to an Award, including the grant, exercise, vesting or settlement of, or payment of dividends with respect to, an Award, or (b) require the Participant promptly to remit the amount of such withholding to the Company before taking any action, including issuing any Shares, with respect to an Award. When withholding Shares for taxes is effected under this Plan, it will be withheld only up to an amount based on the maximum statutory tax rates in the Participant’s applicable tax jurisdiction or such other rate that will not trigger a negative accounting impact on the Company.
13.2 Special Rules. The Committee may, in its sole discretion and upon terms and conditions established by the Committee, permit or require a Participant to satisfy, in whole or in part, any withholding or employment related tax obligation described in Section 13.1 of this Plan by withholding Shares underlying an Award, by electing to tender, or by attestation as to ownership of, Previously Acquired Shares, by delivery of a Broker Exercise Notice or a combination of such methods. For purposes of satisfying a Participant’s withholding or employment-related tax obligation, Shares withheld by the Company or Previously Acquired Shares tendered or covered by an attestation will be valued at their Fair Market Value on the Tax Date.
|
14. |
Change in Control. |
14.1 Definition of Change in Control. Unless otherwise provided in an Award Agreement or Individual Agreement between the Participant and the Company or one of its Subsidiaries or Affiliates, a “Change in Control” will mean the occurrence of any of the following:
(a) The acquisition, other than from the Company, by any individual, entity or group (within the meaning of Section 13(d)(3) or 14(d)(2) of the Exchange Act) of beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act) of fifty percent (50%) or more of either the then outstanding Shares of the Company or the combined voting power of the then outstanding voting securities of the Company entitled to vote generally in the election of directors; or
(b) The consummation of a reorganization, merger or consolidation of the Company, in each case, with respect to which all or substantially all of the individuals and entities who were the respective beneficial owners of the Common Shares and voting securities of the Company immediately prior to such reorganization, merger or consolidation do not, following such reorganization, merger or consolidation, beneficially own, directly or indirectly, more than fifty percent (50%) of, respectively, the then outstanding Shares and the combined voting power of the then outstanding voting securities entitled to vote generally in the election of directors, as the case may be, of the corporation resulting from such reorganization, merger or consolidation; or
(c) a complete liquidation or dissolution of the Company or the sale or other disposition of all or substantially all of the assets of the Company.
14.2 Effect of Change in Control. Subject to the terms of the applicable Award Agreement or an Individual Agreement, in the event of a Change in Control, the Committee (as constituted prior to such Change in Control) may, in its discretion:
(a) require that shares of stock of the corporation resulting from such Change in Control, or a parent corporation thereof, be substituted for some or all of the Shares subject to an outstanding Award, with an appropriate and equitable adjustment to such Award as shall be determined by the Board in accordance with Section 4.4;
(b) provide that (i) some or all outstanding Options shall become exercisable in full or in part, either immediately or upon a subsequent termination of employment, (ii) the restrictions or vesting applicable to some or all outstanding Restricted Stock Awards and Restricted Stock Units shall lapse in full or in part, either immediately or upon a subsequent termination of employment, (iii) the Performance Period applicable to some or all outstanding Awards shall lapse in full or in part, and/or (iv) the Performance Goals applicable to some or all outstanding Awards shall be deemed to be satisfied at the target or any other level; and/or
(c) require outstanding Awards, in whole or in part, to be surrendered to the Company by the holder, and to be immediately cancelled by the Company, and to provide for the holder to receive (A) a cash payment in an amount determined pursuant to Section 14.3 below; (B) shares of capital stock of the corporation resulting from or succeeding to the business of the Company pursuant to such Change in Control, or a parent corporation thereof, having a fair market value not less than the amount determined under clause (A) above; or (C) a combination of the payment of cash pursuant to clause (A) above and the issuance of shares pursuant to clause (B) above.
14.3 Alternative Treatment of Incentive Awards. In connection with a Change in Control and subject to Section 17, the Committee, in its sole discretion, either in an Award Agreement at the time of grant of an Award or at any time after the grant of such an Award, in lieu of providing a substitute award to a Participant pursuant to Section 14.2(a), may determine that any or all outstanding Awards granted under this Plan, whether or not exercisable or vested, as the case may be, will be canceled and terminated and that in connection with such cancellation and termination the holder of such Award will receive for each Share subject to such Award a cash payment (or the delivery of shares of stock, other securities or a combination of cash, stock and securities with a fair market value (as determined by the Committee in good faith) equivalent to such cash payment) equal to the difference, if any, between the consideration received by shareholders of the Company in respect of a Share in connection with such Change in Control and the purchase price per share, if any, under the Award, multiplied by the number of Shares subject to such Award (or in which such Award is denominated); provided, however, that if such product is zero ($0) or less or to the extent that the Award is not then exercisable, the Award may be canceled and terminated without payment therefor. If any portion of the consideration pursuant to a Change in Control may be received by holders of Shares on a contingent or delayed basis, the Committee may, in its sole discretion, determine the fair market value per share of such consideration as of the time of the Change in Control on the basis of the Committee’s good faith estimate of the present value of the probable future payment of such consideration. Notwithstanding the foregoing, any Shares issued pursuant to an Award that immediately prior to the effectiveness of the Change in Control are subject to no further restrictions pursuant to this Plan or an Award Agreement (other than pursuant to the securities laws) will be deemed to be outstanding Shares and receive the same consideration as other outstanding Shares in connection with the Change in Control.
14.4 Limitation on Change in Control Payments. Notwithstanding anything in this Section 14 to the contrary, if, with respect to a Participant, the acceleration of the vesting of an Award or the payment of cash in exchange for all or part of a Stock-Based Award (which acceleration or payment could be deemed a “payment” within the meaning of Section 280G(b)(2) of the Code), together with any other “payments” that such Participant has the right to receive from the Company or any corporation that is a member of an “affiliated group” (as defined in Section 1504(a) of the Code without regard to Section 1504(b) of the Code) of which the Company is a member, would constitute a “parachute payment” (as defined in Section 280G(b)(2) of the Code), then the “payments” to such Participant pursuant to Section 14.2 or Section 14.3 of this Plan will be reduced (or acceleration of vesting eliminated) to the largest amount as will result in no portion of such “payments” being subject to the excise tax imposed by Section 4999 of the Code; provided, however, that such reduction will be made only if the aggregate amount of the payments after such reduction exceeds the difference between (a) the amount of such payments absent such reduction minus (b) the aggregate amount of the excise tax imposed under Section 4999 of the Code attributable to any such excess parachute payments; and provided, further that such payments will be reduced (or acceleration of vesting eliminated) by first eliminating vesting of Options with an exercise price above the then Fair Market Value of a Share that have a positive value for purposes of Section 280G of the Code, followed by reducing or eliminating payments or benefits pro rata among Awards that are deferred compensation subject to Section 409A of the Code, and, if a further reduction is necessary, by reducing or eliminating payments or benefits pro rata among Awards that are not subject to Section 409A of the Code. Notwithstanding the foregoing sentence, if a Participant is subject to a separate agreement with the Company or a Subsidiary that expressly addresses the potential application of Section 280G or 4999 of the Code, then this Section 14.4 will not apply and any “payments” to a Participant pursuant to Section 14 of this Plan will be treated as “payments” arising under such separate agreement; provided, however, such separate agreement may not modify the time or form of payment under any Award that constitutes deferred compensation subject to Section 409A of the Code if the modification would cause such Award to become subject to the adverse tax consequences specified in Section 409A of the Code.
14.5 Exceptions. Notwithstanding anything in this Section 14 to the contrary, individual Award Agreements or Individual Agreements between a Participant and the Company or one of its Subsidiaries or Affiliates may contain provisions with respect to vesting, payment or treatment of Awards upon the occurrence of a Change in Control, and the terms of any such Award Agreement or Individual Agreement will govern to the extent of any inconsistency with the terms of this Section 14. The Committee will not be obligated to treat all Awards subject to this Section 14 in the same manner. The timing of any payment under this Section 14 may be governed by any election to defer receipt of a payment made under a Company deferred compensation plan or arrangement.
|
15. |
Rights of Eligible Recipients and Participants; Transferability. |
15.1 Employment. Nothing in this Plan or an Award Agreement will interfere with or limit in any way the right of the Company or any Subsidiary to terminate the employment or service of any Eligible Recipient or Participant at any time, nor confer upon any Eligible Recipient or Participant any right to continue employment or other service with the Company or any Subsidiary.
15.2 No Rights to Awards. No Participant or Eligible Recipient will have any claim to be granted any Award under this Plan.
15.3 Rights as a Shareholder. Except as otherwise provided in the Award Agreement, a Participant will have no rights as a shareholder with respect to Shares covered by any Stock-Based Award unless and until the Participant becomes the holder of record of such Shares and then subject to any restrictions or limitations as provided herein or in the Award Agreement.
15.4 Restrictions on Transfer.
(a) Except pursuant to testamentary will or the laws of descent and distribution or as otherwise expressly permitted by subsections (b) and (c) below, no right or interest of any Participant in an Award prior to the exercise (in the case of Options or Stock Appreciation Rights) or vesting, issuance or settlement of such Award will be assignable or transferable, or subjected to any lien, during the lifetime of the Participant, either voluntarily or involuntarily, directly or indirectly, by operation of law or otherwise.
(b) A Participant will be entitled to designate a beneficiary to receive an Award upon such Participant’s death, and in the event of such Participant’s death, payment of any amounts due under this Plan will be made to, and exercise of any Options or Stock Appreciation Rights (to the extent permitted pursuant to Section 12 of this Plan) may be made by, such beneficiary. If a deceased Participant has failed to designate a beneficiary, or if a beneficiary designated by the Participant fails to survive the Participant, payment of any amounts due under this Plan will be made to, and exercise of any Options or Stock Appreciation Rights (to the extent permitted pursuant to Section 12 of this Plan) may be made by, the Participant’s legal representatives, heirs and legatees. If a deceased Participant has designated a beneficiary and such beneficiary survives the Participant but dies before complete payment of all amounts due under this Plan or exercise of all exercisable Options or Stock Appreciation Rights, then such payments will be made to, and the exercise of such Options or Stock Appreciation Rights may be made by, the legal representatives, heirs and legatees of the beneficiary.
(c) Upon a Participant’s request, the Committee may, in its sole discretion, permit a transfer of all or a portion of a Non-Statutory Stock Option, other than for value, to such Participant’s child, stepchild, grandchild, parent, stepparent, grandparent, spouse, former spouse, sibling, niece, nephew, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, any person sharing such Participant’s household (other than a tenant or employee), a trust in which any of the foregoing have more than fifty percent (50%) of the beneficial interests, a foundation in which any of the foregoing (or the Participant) control the management of assets, and any other entity in which these persons (or the Participant) own more than fifty percent (50%) of the voting interests. Any permitted transferee will remain subject to all the terms and conditions applicable to the Participant prior to the transfer. A permitted transfer may be conditioned upon such requirements as the Committee may, in its sole discretion, determine, including execution or delivery of appropriate acknowledgements, opinion of counsel, or other documents by the transferee.
(d) The Committee may impose such restrictions on any Shares acquired by a Participant under this Plan as it may deem advisable, including minimum holding period requirements, restrictions under applicable federal securities laws, under the requirements of any stock exchange or market upon which the Common Shares are then listed or traded, or under any blue sky or state securities laws applicable to such Shares or the Company’s insider trading policy.
15.5 Non-Exclusivity of this Plan. Nothing contained in this Plan is intended to modify or rescind any previously approved compensation plans or programs of the Company or create any limitations on the power or authority of the Board to adopt such additional or other compensation arrangements as the Board may deem necessary or desirable.
|
16. |
Securities Law and Other Restrictions. |
Notwithstanding any other provision of this Plan or any Award Agreements entered into pursuant to this Plan, the Company will not be required to issue any Shares under this Plan, and a Participant may not sell, assign, transfer or otherwise dispose of Shares issued pursuant to Awards granted under this Plan, unless (a) there is in effect with respect to such Shares a registration statement under the Securities Act and any applicable securities laws of a state or foreign jurisdiction or an exemption from such registration under the Securities Act and applicable state or foreign securities laws, and (b) there has been obtained any other consent, approval or permit from any other U.S. or foreign regulatory body which the Committee, in its sole discretion, deems necessary or advisable. The Company may condition such issuance, sale or transfer upon the receipt of any representations or agreements from the parties involved, and the placement of any legends on certificates representing Shares, as may be deemed necessary or advisable by the Company in order to comply with such securities law or other restrictions.
|
17. |
Deferred Compensation; Compliance with Section 409A. |
It is intended that all Awards issued under this Plan be in a form and administered in a manner that will comply with the requirements of Section 409A of the Code, or the requirements of an exception to Section 409A of the Code, and the Award Agreements and this Plan will be construed and administered in a manner that is consistent with and gives effect to such intent. The Committee is authorized to adopt rules or regulations deemed necessary or appropriate to qualify for an exception from or to comply with the requirements of Section 409A of the Code. With respect to an Award that constitutes a deferral of compensation subject to Code Section 409A: (a) if any amount is payable under such Award upon a termination of service, a termination of service will be treated as having occurred only at such time the Participant has experienced a Separation from Service; (b) if any amount is payable under such Award upon a Disability, a Disability will be treated as having occurred only at such time the Participant has experienced a “disability” as such term is defined for purposes of Code Section 409A; (c) if any amount is payable under such Award on account of the occurrence of a Change in Control, a Change in Control will be treated as having occurred only at such time a “change in the ownership or effective control of the corporation or in the ownership of a substantial portion of the assets of the corporation” as such terms are defined for purposes of Code Section 409A; (d) if any amount becomes payable under such Award on account of a Participant’s Separation from Service at such time as the Participant is a “specified employee” within the meaning of Code Section 409A, then no payment will be made, except as permitted under Code Section 409A, prior to the first business day after the earlier of (i) the date that is six months after the date of the Participant’s Separation from Service or (ii) the Participant’s death; and (e) no amendment to or payment under such Award will be made except and only to the extent permitted under Code Section 409A.
|
18. |
Amendment, Modification and Termination. |
18.1 Generally. Subject to other subsections of this Section 18 and Sections 3.4 and 18.3 of this Plan, the Board at any time may suspend or terminate this Plan (or any portion thereof) or terminate any outstanding Award Agreement and the Committee, at any time and from time to time, may amend this Plan or amend or modify the terms of an outstanding Award. The Committee’s power and authority to amend or modify the terms of an outstanding Award includes the authority to modify the number of Shares or other terms and conditions of an Award, extend the term of an Award, accept the surrender of any outstanding Award or, to the extent not previously exercised or vested, authorize the grant of new Awards in substitution for surrendered Awards; provided, however that the amended or modified terms are permitted by this Plan as then in effect and that any Participant adversely affected by such amended or modified terms has consented to such amendment or modification.
18.2 Shareholder Approval. No amendments to this Plan will be effective without approval of the Company’s shareholders if: (a) shareholder approval of the amendment is then required pursuant to the rules of the primary stock exchange or stock market on which the Common Shares are then traded, applicable corporate laws or regulations, or other Applicable Law, and the applicable laws of any foreign country or jurisdiction where Awards are, or will be, granted under this Plan; or (b) such amendment would: modify Section 3.4 of this Plan or reduce the minimum exercise price or grant price as set forth in Sections 6.3 and 7.3 of this Plan.
18.3 Awards Previously Granted. Notwithstanding any other provision of this Plan to the contrary, no termination, suspension or amendment of this Plan may adversely affect any outstanding Award without the consent of the affected Participant; provided, however, that this sentence will not impair the right of the Committee to take whatever action it deems appropriate under Sections 4.4, 9.7, 12, 14, 16 or 18.4 of this Plan.
18.4 Amendments to Conform to Law. Notwithstanding any other provision of this Plan to the contrary, the Committee may amend this Plan or an Award Agreement, to take effect retroactively or otherwise, as deemed necessary or advisable for the purpose of conforming this Plan or an Award Agreement to any present or future law relating to plans of this or similar nature, and to the administrative regulations and rulings promulgated thereunder. By accepting an Award under this Plan, a Participant agrees to any amendment made pursuant to this Section 18.4 to any Award granted under this Plan without further consideration or action.
|
19. |
Effective Date and Duration of this Plan. |
This Plan was approved by the Board on December 3, 2021 and became effective immediately. This Plan will terminate at midnight on December 2, 2031 and may be terminated prior to such time by Board action. No Award will be granted after termination of this Plan, but Awards outstanding upon termination of this Plan will remain outstanding in accordance with their applicable terms and conditions and the terms and conditions of this Plan.
|
20. |
Shareholder Approval. |
It is expressly intended that approval of the Company’s shareholders not be required as a condition of the effectiveness of this Plan, and this Plan’s provisions shall be interpreted in a manner consistent with such intent for all purposes. Specifically, Nasdaq Stock Market Rule 5635(c) generally requires shareholder approval for stock option plans or other equity compensation arrangements adopted by companies whose securities are listed on the Nasdaq Stock Market pursuant to which stock awards or stock may be acquired by officers, directors, employees or consultants of such companies. Nasdaq Stock Market Rule 5635(c)(4) provides an exemption in certain circumstances for “employment inducement” awards (within the meaning of Nasdaq Stock Market Rule 5635(c)(4)). Notwithstanding anything to the contrary herein, if the Company’s securities are traded on the Nasdaq Stock Market, then Awards under the Plan may only be made to Employees who have not previously been an Employee or Board member of the Company or a Subsidiary, in each case as an inducement material to the Employee’s entering into employment with the Company or a Subsidiary. Awards under the Plan will be approved by (a) the Committee, comprised of a majority of the Company’s Independent Directors, or (b) a majority of the Company’s Independent Directors. Accordingly, pursuant to Nasdaq Stock Market Rule 5635(c)(4), the issuance of Awards and the Common Shares issuable upon exercise or vesting of such Awards pursuant to the Plan are not subject to the approval of the Company’s shareholders.
|
21. |
Miscellaneous. |
21.1 Usage. In this Plan, except where otherwise indicated by clear contrary intention, (a) any masculine term used herein also will include the feminine, (b) the plural will include the singular, and the singular will include the plural, (c) “including” (and with correlative meaning “include”) means including without limiting the generality of any description preceding such term, and (d) “or” is used in the inclusive sense of “and/or”.
21.2 Relationship to Other Benefits. Neither Awards made under this Plan nor Shares or cash paid pursuant to such Awards under this Plan will be included as “compensation” for purposes of computing the benefits payable to any Participant under any pension, retirement (qualified or non-qualified), savings, profit sharing, group insurance, welfare, or benefit plan of the Company or any Subsidiary unless provided otherwise in such plan.
21.3 Fractional Shares. No fractional Shares will be issued or delivered under this Plan or any Award. The Committee will determine whether cash, other Awards or other property will be issued or paid in lieu of fractional Shares or whether such fractional Shares or any rights thereto will be forfeited or otherwise eliminated by rounding up or down.
21.4 Governing Law. Except to the extent expressly provided herein or in connection with other matters of corporate governance and authority (all of which will be governed by the laws of the Company’s jurisdiction of incorporation), the validity, construction, interpretation, administration and effect of this Plan and any rules, regulations and actions relating to this Plan will be governed by and construed exclusively in accordance with the laws of the State of Delaware, notwithstanding the conflicts of laws principles of any jurisdictions.
21.5 Successors. All obligations of the Company under this Plan with respect to Awards granted hereunder will be binding on any successor to the Company, whether the existence of such successor is the result of a direct or indirect purchase, merger, consolidation or otherwise, of all or substantially all of the business or assets of the Company.
21.6 Construction. Wherever possible, each provision of this Plan and any Award Agreement will be interpreted so that it is valid under the Applicable Law. If any provision of this Plan or any Award Agreement is to any extent invalid under the Applicable Law, that provision will still be effective to the extent it remains valid. The remainder of this Plan and the Award Agreement also will continue to be valid, and the entire Plan and Award Agreement will continue to be valid in other jurisdictions.
21.7 Delivery and Execution of Electronic Documents. To the extent permitted by Applicable Law, the Company may: (a) deliver by email or other electronic means (including posting on a Web site maintained by the Company or by a third party under contract with the Company) all documents relating to this Plan or any Award hereunder (including prospectuses required by the Securities and Exchange Commission) and all other documents that the Company is required to deliver to its security holders (including annual reports and proxy statements), and (b) permit Participants to use electronic, internet or other non-paper means to execute applicable Plan documents (including Award Agreements) and take other actions under this Plan in a manner prescribed by the Committee.
21.8 No Representations or Warranties Regarding Tax Effect. Notwithstanding any provision of this Plan to the contrary, the Company and its Subsidiaries, the Board, and the Committee neither represent nor warrant the tax treatment under any federal, state, local, or foreign laws and regulations thereunder (individually and collectively referred to as the “Tax Laws”) of any Award granted or any amounts paid to any Participant under this Plan including, but not limited to, when and to what extent such Awards or amounts may be subject to tax, penalties, and interest under the Tax Laws.
21.9 Unfunded Plan. Participants will have no right, title or interest whatsoever in or to any investments that the Company or its Subsidiaries may make to aid it in meeting its obligations under this Plan. Nothing contained in this Plan, and no action taken pursuant to its provisions, will create or be construed to create a trust of any kind, or a fiduciary relationship between the Company and any Participant, beneficiary, legal representative, or any other individual. To the extent that any individual acquires a right to receive payments from the Company or any Subsidiary under this Plan, such right will be no greater than the right of an unsecured general creditor of the Company or the Subsidiary, as the case may be. All payments to be made hereunder will be paid from the general funds of the Company or the Subsidiary, as the case may be, and no special or separate fund will be established and no segregation of assets will be made to assure payment of such amounts except as expressly set forth in this Plan.
21.10 Indemnification. Subject to any limitations and requirements of the British Columbia Business Corporation Act or other Applicable Law, each individual who is or will have been a member of the Board, or a Committee appointed by the Board, or an officer or Employee of the Company to whom authority was delegated in accordance with Section 3.3 of this Plan, will be indemnified and held harmless by the Company against and from any loss, cost, liability or expense that may be imposed upon or reasonably incurred by him or her in connection with or resulting from any claim, action, suit or proceeding to which he or she may be a party or in which he or she may be involved by reason of any action taken or failure to act under this Plan and against and from any and all amounts paid by him or her in settlement thereof, with the Company’s approval, or paid by him or her in satisfaction of any judgment in any such action, suit or proceeding against him or her, provided he or she will give the Company an opportunity, at its own expense, to handle and defend the same before he or she undertakes to handle and defend it on his/her own behalf. The foregoing right of indemnification will not be exclusive of any other rights of indemnification to which such individuals may be entitled under the Company’s Articles, as a matter of law, or otherwise, or pursuant to any agreement with the Company, or any power that the Company may have to indemnify them or hold them harmless.
Approved by Board of Directors July 31, 2025
Exhibit 10.5
EMPLOYMENT AGREEMENT
This Employment Agreement (“Agreement”) is effective as of August 11, 2025 (“Effective Date”), by and between DiaMedica USA, Inc. a Delaware corporation (the “Company”), and Julie Krop, an individual (“Executive”). The Company and Executive are sometimes referred to as the “Parties” or “Party” in this Agreement, and the Company may designate the parent company of the Company or a subsidiary to be the employer of the Executive.
In consideration of the mutual promises, covenants and agreements contained in this Agreement, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
|
1. |
EMPLOYMENT AND DUTIES. |
A. Job Title and Responsibilities. The Company hereby employs Executive, and Executive hereby agrees to be employed, as Chief Medical Officer (together with such other position or positions consistent with Executive’s title as the Company’s Chief Executive Officer (the “CEO”) may specify from time to time), initially reporting to the Chief Executive Officer, and will have such duties and responsibilities commensurate with such title. In addition, Executive will also serve as the Chief Medical Officer of Parent and all operating subsidiaries of Parent.
B. Full-Time Best Efforts. Executive agrees to devote Executive’s full professional time and attention to the business of the Company (and its subsidiaries, affiliates, or related entities) and the performance of Executive’s obligations under this Agreement, and will at all times faithfully, industriously and to the best of Executive’s ability, experience and talent, perform all of Executive’s obligations hereunder. Executive shall not, at any time during Executive’s employment by the Company, directly or indirectly, act as a partner, officer, director, consultant or Executive, or provide services in any other capacity to any other business enterprise that conflicts with the Company’s business or Executive’s duty of loyalty to the Company. Executive shall seek the written consent of the Company prior to accepting any outside board positions.
C. Duty of Loyalty. Executive acknowledges that during Executive’s employment with the Company, Executive has participated in and will participate in relationships with existing and prospective clients, customers, partners, suppliers, service providers and vendors of the Company that are essential elements of the Company’s goodwill. The parties acknowledge that Executive owes the Company a fiduciary duty to conduct all affairs of the Company in accordance with all applicable laws and the highest standards of good faith, trust, confidence and candor, and to endeavor, to the best of Executive’s ability, to promote the best interests of the Company.
D. Conflict of Interest. Executive agrees that while employed by the Company, and except with the advance written consent of the CEO, Executive will not enter into, on behalf of the Company, or cause the Company or any of its affiliates to enter into, directly or indirectly, any transactions with any business organization in which Executive or any member of Executive’s immediate family may be interested as a shareholder, partner, member, trustee, director, officer, employee, consultant, lender or guarantor or otherwise; provided, however, that nothing in this Agreement shall restrict transactions between the Company and any company whose stock is listed on a national securities exchange or actively traded in the over-the-counter market and over which Executive does not have the ability to control or significantly influence policy decisions.
|
2. |
COMPENSATION. |
A. Base Pay. The Company agrees to pay Executive gross annual compensation of $525,000 (“Base Salary”), less usual and customary withholdings, which shall be payable in arrears in accordance with the Company’s customary payroll practices. The Base Salary will be subject to normal periodic review, and such review will consider Executive’s contributions to the Company and the Company’s overall performance.
B. Bonus and Incentive Compensation. Executive shall be eligible for discretionary bonus and incentive based compensation approved by the Board (or a committee thereof) from time to time at its sole discretion as to eligibility and timing of payments. The initial target bonus rate is 40%.
C. Equity Award. Subject to approval by the Board (or a committee thereof), Executive shall be eligible to receive equity-based compensation awards from time to time as determined by the Board pursuant to the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, or any successor plan thereto (such plan, the “Plan”). The type of equity award(s), grant timing and vesting terms will be in the sole discretion of the Board (or a committee thereof).
D. Benefits. During Executive’s employment, Executive will be eligible to participate in the Company’s benefit programs, as governed by the terms of the official plan documents. Executive acknowledges that the Company may amend or terminate any of its benefit plans or programs at any time and for any reason.
E. Clawback. Executive agrees that any incentive or other compensation or benefits provided by the Company under this Agreement or otherwise will be subject to recoupment or clawback by the Company under any applicable clawback or recoupment policy of the Company as may be in effect from time to time or as required by applicable law, regulation or stock exchange listing requirement.
|
3. |
CONFIDENTIAL INFORMATION. |
A. Non-Disclosure, Non-Use and Definition of Confidential Information. Executive understands that during Executive’s employment relationship with the Company, the Company intends to provide Executive with information, including Confidential Information (as defined herein), without which Executive would not be able to perform Executive’s duties to the Company. Executive agrees, at all times during the term of Executive’s employment relationship and thereafter, to hold in strictest confidence, and not to use or disclose, except for the benefit of the Company to the extent necessary to perform Executive’s obligations to the Company, any Confidential Information that Executive obtains, accesses or creates during the term of the relationship, whether or not during working hours, until such Confidential Information becomes publicly and widely known and made generally available through no wrongful act of Executive or of others under confidentiality obligations as to the information involved. Executive understands that “Confidential Information” means information and physical material not generally known or available outside the Company and information and physical material entrusted to the Company by third parties under an obligation of non-disclosure or non-use or both. “Confidential Information” includes, without limitation, inventions, technical data, trade secrets, know-how, clinical data, regulatory information and strategies, marketing ideas or plans, research, product or service ideas or plans, business strategies, investments, investment opportunities, potential investments, market studies, industry studies, historical financial data, financial information and results, budgets, identity of customers, forecasts (financial or otherwise), possible or pending transactions, customer lists and domain names, price lists, and pricing methodologies. Any information that Executive knows or should reasonably know is Confidential Information, or that Employer treats as Confidential Information, will be presumed to be Confidential Information.
B. Exceptions. At all times, both during Executive’s employment and after its termination, Executive will keep and hold all such Confidential Information in strict confidence and trust. Executive will not use or disclose any Confidential Information without the prior written consent of the Company, except as may be necessary to perform Executive’s duties as an Executive of the Company for the benefit of the Company. Executive may disclose information that Executive is required to disclose by valid order of a government agency or court of competent jurisdiction, provided that Executive will:
1. Notify the Company in writing immediately upon learning that such an order may be sought or issued,
2. Cooperate with the Company as reasonably requested if the Company seeks to contest such order or to place protective restrictions on the disclosure pursuant to such order, and
3. Comply with any protective restrictions in such order and disclose only the information specified in the order.
C. Return of Confidential Information. Upon termination of employment with the Company, Executive will promptly deliver to the Company all documents and materials of any nature pertaining to Executive’s work with the Company.
D. Copyright Information. Executive agrees not to infringe the copyrights of the Company, its customers or third parties (including, without limitation, Executive’s previous employers, customers, etc.) by unauthorized or unlawful copying, modifying or distributing of copyrighted material, including plans, drawings, reports, financial analyses, market studies, computer software and the like.
|
4. |
COVENANT NOT TO COMPETE. |
A. Non-Competition Covenant. Executive agrees that during the Restricted Period (as defined below), without the prior written consent of the Company, Executive shall not, directly or indirectly within the Territory (as defined below): (i) personally, by agency, as an Executive, independent contractor, consultant, officer, director, manager, agent, associate, investor (other than as a passive investor holding less than five percent (5%) of the outstanding equity of an entity), or by any other artifice or device, engage in any Competitive Business (as defined below), (ii) assist others, including but not limited to Executives of the Company, to engage in any Competitive Business, or (iii) own, purchase, finance or organize a Competitive Business.
B. Definitions.
1. “Competitive Business” means (i) any person, entity or organization which is engaged in, consulting regarding or engaged in the development, production, marketing or selling of any pharmaceutical-based product, process, technology, invention or service which resembles, competes with or is intended to resemble or compete with a product, process, technology, device, invention or service under or being considered for research or development or being promoted, marketed, sold or serviced by the Company or any subsidiary; or (ii) any other line of business that the Company or any subsidiary, is actively preparing to pursue at any time during the term of Executive’s employment with the Company and in which Executive is involved.
2. “Territory” means the United States of America or locations where the Company is directly or indirectly developing or selling products or services.
3. “Restricted Period” means the period of Executive’s employment with the Company and for a period of twelve (12) months following the termination of Executive’s employment.
|
5. |
NON-SOLICITATION AND NON-INTERFERENCE COVENANTS. |
A. Non-Solicitation of Employees and Others. During the Restricted Period, (i) Executive shall not, directly or indirectly, solicit, recruit, or induce, or attempt to solicit, recruit or induce any employee, consultant, independent contractor, vendor, supplier, or agent to terminate or otherwise adversely affect his or her employment or other business relationship (or prospective employment or business relationship) with the Company, and (ii) Executive shall not, directly or indirectly, solicit, recruit, or induce, or attempt to solicit, recruit or induce any employee to work for Executive or any other person or entity, other than the Company or its affiliates or related entities.
B. Non-Solicitation of Customers. During the Restricted Period, Executive shall not, directly or indirectly, solicit, recruit, or induce any Customer (as defined below) for the purpose of (i) providing any goods or services related to a Competitive Business, or (ii) interfering with or otherwise adversely affecting the contracts or relationships, or prospective contracts or relationships, between the Company (including any related or affiliated entities) and such Customers. “Customer” means a person or entity with which Executive had contact or about whom Executive gained information while an employee of the Company, and to which the Company was selling or providing products or services, was in active negotiations for the sale of its products or services, or was otherwise doing business as of the date of the cessation of Executive’s employment with the Company or for whom the Company had otherwise done business within the twelve (12) month period immediately preceding the cessation of Executive’s employment with the Company.
|
6. |
ACKNOWLEDGEMENTS. Executive acknowledges and agrees that: |
A. The geographic and duration restrictions contained in Sections 4 and 5 of this Agreement are fair, reasonable, and necessary to protect the Company’s legitimate business interests and trade secrets, given the geographic scope of the Company’s business operations, the competitive nature of the Company’s business, and the nature of Executive’s position with the Company;
B. Executive’s employment creates a relationship of confidence and trust between Executive and the Company with respect to the Confidential Information, and Executive will have access to Confidential Information (including but not limited to trade secrets) that would be valuable or useful to the Company’s competitors;
C. The Company’s Confidential Information is a valuable asset of the Company, and any violation of the restrictions set forth in this Agreement would cause substantial injury to the Company;
D. The restrictions contained in this Agreement will not unreasonably impair or infringe upon Executive’s right to work or earn a living after Executive’s employment with the Company ends; and
E. This Agreement is a contract for the protection of trade secrets under applicable law and is intended to protect the Confidential Information (including trade secrets) identified above.
|
7. |
“BLUE PENCIL” AND SEVERABILITY PROVISION. |
If a court of competent jurisdiction declares any provision of this Agreement invalid, void, voidable, or unenforceable, the court shall reform such provision(s) to render the provision(s) enforceable, but only to the extent absolutely necessary to render the provision(s) enforceable and only in view of the parties’ express desire that the Company be protected to the greatest possible extent under applicable law from improper competition and the misuse or disclosure of trade secrets and Confidential Information. To the extent such a provision (or portion thereof) may not be reformed so as to make it enforceable, it may be severed and the remaining provisions shall remain fully enforceable.
|
8. |
INVENTIONS. |
A. Inventions Retained and Licensed. Executive acknowledges and agrees that Executive has no rights in any Inventions (as that term is defined below) other than inventions and information created, discovered or developed by Executive, whether or not patentable or registrable under patent, copyright or similar statutes, made or conceived or reduced to practice or learned by Executive, either alone or with others before Executive’s employment with the Company, which list of inventions Executive has provided the Company in writing on or prior to the Effective Date (“Prior Inventions”). Executive shall not incorporate, or permit to be incorporated, any Prior Invention owned by Executive or in which Executive has an interest in a Company product, process or machine without the Company’s prior written consent. Notwithstanding the foregoing, if, in the course of Executive’s employment with the Company, Executive directly or indirectly incorporates into a Company product, process or machine a Prior Invention owned by Executive or in which Executive has an interest, the Company is hereby granted and shall have a non-exclusive, royalty-free, irrevocable, perpetual, world-wide license to make, have made, modify, use, create derivative works from and sell such Prior Invention as part of or in connection with such product, process or machine.
B. Assignment of Inventions. Executive shall promptly make full, written disclosure to the Company, will hold in trust for the sole right and benefit of the Company, and hereby irrevocably transfers and assigns, and agrees to transfer and assign, to the Company, or its designee, all Executive’s right, title and interest in and to any and all inventions, original works of authorship, developments, concepts, improvements, designs, discoveries, ideas, trademarks (and all associated goodwill), mask works, or trade secrets, whether or not they may be patented or registered under copyright or similar laws, which Executive may solely or jointly conceive or develop or reduce to practice, or cause to be conceived or developed or reduced to practice, during Executive’s employment by the Company (the “Inventions”). Executive further acknowledges that all original works of authorship which are made by Executive (solely or jointly with others) within the scope of and during the period of Executive’s employment with the Company and which may be protected by copyright are “Works Made For Hire” as that term is defined by the United States Copyright Act. Executive understands and agrees that the decision whether to commercialize or market any Invention developed by Executive solely or jointly with others is within the Company’s sole discretion and the Company’s sole benefit and that no royalty will be due to Executive as a result of the Company’s efforts to commercialize or market any such invention.
Executive recognizes that Inventions relating to Executive’s activities while working for the Company and conceived or made by Executive, whether alone or with others, within one (1) year after cessation of Executive’s employment, may have been conceived in significant part while employed by the Company. Accordingly, Executive acknowledges and agrees that such Inventions shall be presumed to have been conceived during Executive’s employment with the Company and are to be, and hereby are, assigned to the Company unless and until Executive has established the contrary.
The requirements of this Section 8B do not apply to any intellectual property for which no equipment, supplies, facility or trade secret information of the Company was used, and which was developed entirely on the Executive’s own time, and (i) which does not relate (x) directly to the Company’s business or (y) to the Company’s actual or demonstrably anticipated research and development or (ii) which does not result from any work the Executive performed for the Company.
C. Maintenance of Records. Executive agrees to keep and maintain adequate and current written records of all Inventions made by Executive (solely or jointly with others) during Executive’s employment with the Company. The records will be in the form of notes, sketches, drawings and any other format that may be specified by the Company. The records will be available to and remain the sole property of the Company at all times.
D. Patent, Trademark and Copyright Registrations. Executive agrees to assist the Company, or its designee, at the Company’s expense, in every proper way to secure the Company’s rights in the Inventions and any copyrights, patents, trademarks, service marks, mask works, or any other intellectual property rights in any and all countries relating thereto, including, but not limited to, the disclosure to the Company of all pertinent information and data with respect thereto, the execution of all applications, specifications, oaths, assignments and all other instruments the Company reasonably deems necessary in order to apply for and obtain such rights and in order to assign and convey to the Company, its successors, assigns, and nominees the sole and exclusive rights, title, and interest in and to such inventions, and any copyrights, patents, trademarks, service marks, mask works, or any other intellectual property rights relating thereto. Executive further agrees that Executive’s obligation to execute or cause to be executed, when it is in Executive’s power to do so, any such instrument or paper shall continue after termination or expiration of this Agreement or the cessation of Executive’s employment with the Company. If the Company is unable because of Executive’s mental or physical incapacity or for any other reason, after reasonably diligent efforts, to secure Executive’s signature to apply for or to pursue any application for any United States or foreign patents, trademarks or copyright registrations covering inventions or original works of authorship assigned to the Company as above, then Executive hereby irrevocably designates and appoints the Company and its duly authorized officers and agents as Executive’s agent and attorney-in-fact to act for and in Executive’s behalf and stead to execute and file any such applications and to do all other lawfully permitted acts to further the prosecution and issuance of letters patent, trademarks or copyright registrations thereon with the same legal force and effect as if executed by Executive; this power of attorney shall be a durable power of attorney which shall come into existence upon Executive’s mental or physical incapacity.
|
9. |
SURVIVAL AND REMEDIES. |
Executive’s obligations of nondisclosure, non-solicitation, non-interference, and non-competition under this Agreement shall survive the cessation of Executive’s employment with the Company and shall remain enforceable. In addition, Executive acknowledges that upon a breach or threatened breach of any obligation of nondisclosure, non-solicitation, non-interference, or non-competition of this Agreement, the Company may suffer irreparable harm and damage for which money alone cannot fully compensate the Company. Executive therefore agrees that upon such breach or threat of imminent breach of any such obligation, the Company shall be entitled to seek a temporary restraining order, preliminary injunction, permanent injunction or other injunctive relief, without posting any bond or other security, barring Executive from violating any such provision. This Section 9 shall not be construed as an election of any remedy, or as a waiver of any right available to the Company under this Agreement or the law, including the right to seek damages from Executive for a breach of any provision of this Agreement and the right to require Executive to account for and pay over to the Company all profits or other benefits derived or received by Executive as the result of such a breach, nor shall this Section 9 be construed to limit the rights or remedies available under state law for any violation of any provision of this Agreement.
|
10. |
TERMINATION. |
A. Termination By Either Party. Either Party may terminate the Executive’s at-will employment at any time with or without notice, and with or without cause. Except as provided in this Section 10, upon termination of employment, Executive shall only be entitled to Executive’s accrued but unpaid Base Salary, any earned but unpaid bonus for the year prior to the date of termination, and other benefits earned under any Company-provided plans, policies and arrangements for the period preceding the effective date of the termination of employment. With respect to any earned but unpaid bonus for the year prior to the date of termination, the terms of which bonus plan require Executive to be an employee of the Company as of the date of payment, no payment will be made to Executive (or if applicable, the Executive’s beneficiary) if Executive’s employment with the Company terminates voluntarily by Executive, other than for Good Reason pursuant to Section 10C, or if Executive’s employment with the Company is terminated by the Company for Cause, but will be paid if Executive’s employment with the Company terminates due to Executive’s death or disability.
B. Termination Without Cause. If the Company terminates Executive’s employment without Cause (defined below), Executive shall be entitled to receive, in addition to the amounts due under Section 10A, as continuing severance pay at a rate equal to Executive’s Base Salary, as then in effect, for nine (9) months from the date of termination of employment, plus a lump-sum payment equal to a pro rata portion of Executive’s target annual bonus for the year in which the date of termination occurs (based on the date of termination), in each case, less all required tax withholdings and other applicable deductions, payable in accordance with the Company’s standard payroll procedures, commencing on the effective date of a Separation Agreement and Release of claims against the Company and after the end of any applicable rescission or revocation period, and provided that Executive has not revoked or rescinded (or attempted to revoke or rescind) any claims under such Release, in substantially the form of Exhibit A attached hereto, the timely execution and performance by Executive of which is specifically a condition to Executive’s receipt of any of the payments and benefits provided under this Section 10B; provided that (1) such Separation Agreement and Release shall be executed and be fully effective within sixty (60) days of the Executive’s termination of employment; (2) the first payment shall include any amounts that would have been paid to Executive if payment had commenced on the date of termination of employment; and (3) Executive shall not be required to execute a release of any claims arising from the Company’s failure to comply with its obligations under Section 10A. Subject to Executive’s execution and non-revocation of the Separation Agreement and Release, if Executive timely and effectively elects continuation coverage under the Company’s group health plan pursuant to COBRA or similar state law, the Company will pay or reimburse the premiums for such coverage of Executive (and Executive’s dependents, as applicable) at the same rate it pays for active employees for a period for nine (9) months from the date of termination of employment; provided that the Company’s obligation to make such payments shall immediately expire if Executive ceases to be eligible for continuation coverage under COBRA or similar state law or otherwise terminates such coverage. Notwithstanding the foregoing, any of the foregoing payments due under this Section 10B shall commence within seventy (70) days of Executive’s termination of employment, provided that if such seventy (70)-day period spans two (2) calendar years, payments shall commence in the latter calendar year. In addition to the foregoing and subject to Executive’s timely execution of a Separation Agreement and Release that has been executed and not revoked within any applicable rescission period that has expired within sixty (60) days of the Executive’s termination of employment. Executive shall be entitled to the immediate vesting of all outstanding equity awards.
C. Termination Upon a Change in Control. If the Company or any successor in interest to the Company terminates Executive’s employment without Cause in connection with or within twelve (12) months after a Change in Control (defined below) or if Executive terminates Executive’s employment for Good Reason (defined below) within twelve (12) months after a Change in Control, Executive shall be entitled to receive, in addition to the amounts due under Section 10A, a lump-sum payment equal to twelve (12) months of Executive’s Base Salary, as then in effect or as in effect immediately prior to a material reduction of Executive’s Base Salary which was the reason Executive resigned for Good Reason, plus a lump-sum payment equal to a pro rata portion of Executive’s target annual bonus for the year in which the date of termination occurs (based on the date of termination), in each case, less all tax withholdings and other applicable deductions the Company reasonably determines are required to be made, payable on the first regular payroll date after the effective date of a Separation Agreement and Release that has been executed and not revoked within any applicable rescission period that has expired within sixty (60) days of the Executive’s termination of employment, in substantially the form of Exhibit A attached hereto, the execution and performance by Executive of which is specifically a condition to Executive’s receipt of any of the payments and benefits provided under this Section 10C; provided that Executive shall not be required to execute a release of any claims arising from the Company’s failure to comply with its obligations under Section 10A. Subject to Executive’s execution and non-revocation of the Separation Agreement and Release, if Executive timely and effectively elects continuation coverage under the Company’s group health plan pursuant to COBRA or similar state law, the Company will pay or reimburse the premiums for such coverage of Executive (and Executive’s dependents, as applicable) at the same rate it pays for active employees for a period for twelve (12) months from the date of termination of employment; provided that the Company’s obligation to make such payments shall immediately expire if Executive ceases to be eligible for continuation coverage under COBRA or similar state law or otherwise terminates such coverage. Notwithstanding the previous provisions of this Section 10C, any payments due under this Section 10C shall commence within seventy (70) days of Executive’s termination of employment, provided that if such seventy (70)-day period spans two calendar years, payments shall commence in the latter calendar year. In addition to the foregoing and subject to Executive’s timely execution of a Separation Agreement and Release that has been executed and not revoked within any applicable rescission period that has expired within sixty (60) days of the Executive’s termination of employment. Executive shall be entitled to the immediate vesting of all outstanding equity awards. The payments and benefits described in this Section 10C are in lieu of, and not in addition to, the payments and benefits described in Section 10B, it being understood by Executive that Executive shall be paid and receive only one set of severance payments and benefits.
Notwithstanding any other provisions of this Agreement, if any “payments” (including, without limitation, any benefits or transfers of property or the acceleration of the vesting of any benefits) in the nature of compensation under any arrangement that is considered contingent on a “change in control” for purposes of Section 280G of the Internal Revenue Code of 1986, as amended (the “Code”), together with any other payments that Executive has the right to receive from the Company or any corporation that is a member of an “affiliated group” (as defined in Section 1504(a) of the Code without regard to Section 1504(b) of the Code) of which the Company is a member, would constitute a “parachute payment” (as defined in Section 280G(b)(2) of the Code), such “payments” may, at Executive’s sole election, be reduced to the largest amount as will result in no portion of such “payments” being subject to the excise tax imposed by Section 4999 of the Code. Any reduction of the payments shall be made in the following order: (1) options with an exercise price above the fair market value of the stock, provided the options give rise to a payment; (2) pro rata among amounts that constitute deferred compensation under Code Section 409A; and (3) reduction of any remaining payments in the manner determined at the discretion of Executive.
The accounting firm engaged by the Company for general audit purposes as of the day prior to the effective date of the change in control shall perform the foregoing calculations. The Company shall bear all expenses with respect to the determinations by such accounting firm required to be made hereunder. The accounting firm shall provide its calculations to the Company and Executive within sixty (60) calendar days after the date on which Executive’s right to a payment is triggered and the payment will be paid to Executive within seventy-four (74) calendar days of the date on which Executive’s right to a payment is triggered. Any good faith determinations of the accounting firm made hereunder shall be final, binding and conclusive upon the Company and Executive.
D. Termination for Cause, Death or Disability, or Resignation. If Executive’s employment with the Company terminates voluntarily by Executive, other than for Good Reason pursuant to Section 10C above, or if Executive’s employment with the Company is terminated by the Company for Cause or due to Executive’s death or disability, then payments of compensation by the Company to Executive hereunder will terminate immediately, except that Executive (or the Executive’s beneficiary if Executive’s termination is on account of death) will be entitled to the amounts due under Section 10A.
E. Definitions.
1. “Cause.” For all purposes under this Agreement, “Cause” is defined as (a) gross negligence or willful failure to perform Executive’s duties and responsibilities to the Company; (b) commission of any act of fraud, theft, embezzlement, financial dishonesty or any other willful misconduct that has caused or is reasonably expected to result in injury to the Company; (c) conviction of, or pleading guilty or nolo contendere to, any felony or a lesser crime involving dishonesty or moral turpitude; (d) material breach by Executive of any of Executive’s obligations under this Agreement or any written agreement or covenant with the Company, including the policies adopted from time to time by the Company applicable to all Executives, that has not been cured within thirty (30) days of notice of such breach or (e) the Company terminates the employment of Executive in connection with a liquidation, dissolution or winding down of the Company.
2. “Good Reason.” For all purposes under this Agreement, “Good Reason” is defined as Executive’s resignation within thirty (30) days following the expiration of any Company cure period (discussed below) following the occurrence of one or more of the following, without Executive’s express written consent: (a) a material reduction of Executive’s duties, authority, reporting level, or responsibilities, relative to Executive’s duties, authority, reporting level, or responsibilities in effect immediately prior to such Change in Control; (b) a material reduction in Executive’s base compensation; (c) the Company’s requiring of Executive to change the principal location at which Executive is to perform Executive’s services by more than fifty (50) miles, or (d) material breach of the Agreement by the Company. Executive will not resign for Good Reason without first providing the Company with written notice within thirty (30) days of the initial occurrence of the event that Executive believes constitutes “Good Reason” specifically identifying the acts or omissions constituting the grounds for Good Reason and providing Company a reasonable cure period of not less than thirty (30) days following the date of such notice and during which such condition has not been cured.
3. “Change in Control.” For all purposes under this Agreement, a “Change in Control” will mean the occurrence of any of the following:
a. the acquisition, other than from the Company or Parent (as defined below), by any individual, entity or group (within the meaning of Section 13(d)(3) or 14(d)(2) of the Securities Exchange Act of 1934, as amended (“Exchange Act”)) of beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act) of fifty percent (50%) or more of either the then outstanding common shares, no par value (“Common Shares”), of DiaMedica Therapeutics Inc., a company organized under the laws of Canada (“Parent”), or the combined voting power of the then outstanding voting securities of Parent entitled to vote generally in the election of directors, but excluding, for this purpose, any such acquisition by Parent or any of its subsidiaries, or any employee benefit plan (or related trust) of Parent or its subsidiaries, or any entity with respect to which, following such acquisition, more than fifty percent (50%) of, respectively, the then outstanding equity of such entity and the combined voting power of the then outstanding voting equity of such entity entitled to vote generally in the election of all or substantially all of the members of such entity’s governing body is then beneficially owned, directly or indirectly, by the individuals and entities who were the beneficial owners, respectively, of the Common Shares and voting securities of Parent immediately prior to such acquisition in substantially the same proportion as their ownership, immediately prior to such acquisition, of the then outstanding Common Shares or the combined voting power of the then outstanding voting securities of Parent entitled to vote generally in the election of directors, as the case may be; or
b. the consummation of a reorganization, merger or consolidation of Parent, in each case, with respect to which all or substantially all of the individuals and entities who were the respective beneficial owners of the Common Shares and voting securities of Parent immediately prior to such reorganization, merger or consolidation do not, following such reorganization, merger or consolidation, beneficially own, directly or indirectly, more than fifty percent (50%) of, respectively, the then outstanding Common Shares and the combined voting power of the then outstanding voting securities entitled to vote generally in the election of directors, as the case may be, of the corporation resulting from such reorganization, merger or consolidation; or
c. the sale or other disposition of all or substantially all of the assets of Parent; provided the occurrence under (a), (b) or (c), constitutes a “change in the ownership or effective control of a corporation, or a change in the ownership of a substantial portions of the assets of a corporation” under Section 409A of the Code.
F. No Other Benefits. In the event of a termination of Executive’s employment with the Company, the provisions of this Section 10 are Executive’s exclusive right to severance benefits and are in lieu of participation in any other severance policy or plan to which Executive might otherwise be entitled.
G. Termination from any Offices Held. Upon Executive’s termination of employment with the Company, Executive agrees that any and all offices held with Parent or any subsidiary, including the Company, if applicable, shall be automatically terminated. Executive agrees to cooperate with the Company and execute any documents reasonably required by the Company or competent authorities to effect this provision.
H. Return of Company Property. All devices, records, reports, data, notes, compilations, lists, proposals, correspondence, specifications, equipment, drawings, blueprints, manuals, planners, calendars, schedules, discs, financial plans and information, or other recorded matter, whether in hard copy, electronic media or otherwise (including all copies or reproductions made or maintained, whether on the Company’s premises or otherwise), pertaining to Executive’s work for the Company, or relating to the Company or the Company’s Confidential Information, whether created or developed by Executive alone or jointly during Executive’s employment with the Company, are the exclusive property of the Company. Executive shall surrender the same (as well as any other property of the Company) to the Company upon its request or promptly upon the cessation of employment.
|
11. |
NO CONFLICTING AGREEMENTS OR IMPROPER USE OF THIRD-PARTY INFORMATION. |
During Executive’s employment with the Company, Executive shall not improperly use or disclose any Confidential information or trade secrets of any former employer or other person or entity, and Executive shall not bring on to the premises of the Company any unpublished document or Confidential information belonging to any such former employer, person or entity, unless consented to in writing by the former employer, person or entity. Executive represents that Executive has not improperly used or disclosed any Confidential information or trade secrets of any other person or entity during the application process or while employed or affiliated with the Company. Executive also acknowledges and agrees that Executive is not subject to any contract, agreement, or understanding that would prevent Executive from performing Executive’s duties for the Company or otherwise complying with this Agreement. To the extent Executive violates this provision, or Executive’s employment with the Company constitutes a breach or threatened breach of any contract, agreement, or obligation to any third party, Executive shall indemnify and hold the Company harmless from all damages, expenses, costs (including reasonable attorneys’ fees) and liabilities incurred in connection with, or resulting from, any such violation or threatened violation.
|
12. |
GENERAL PROVISIONS. |
A. Governing Law; Consent To Personal Jurisdiction. The laws of the State of Minnesota shall govern the Executive’s employment and this Agreement without regard to conflict of laws principles. Executive and the Company each hereby consents to the personal jurisdiction of the state courts located in Hennepin County, State of Minnesota, and the federal district court sitting in Hennepin County, State of Minnesota, if that court otherwise possesses jurisdiction over the matter, for any legal proceeding concerning Executive’s employment or termination of employment, or arising from or related to this Agreement or any other agreement executed between Executive and the Company.
B. Entire Agreement. This Agreement, together with the Exhibits hereto, sets forth this entire Agreement between the Company (and any of its related or affiliated entities, officers, agents, owners or representatives) and Executive relating to the subject matter herein, and supersedes any and all prior discussions and agreements, whether written or oral, on the subject matter hereof, including without limitation that certain offer letter agreement dated as of July 28, 2025. To the extent that this Agreement may conflict with the terms of another written agreement between Executive and the Company, the terms of this Agreement will control.
C. Modification. No modification of or amendment to this Agreement will be effective unless in writing and signed by Executive and an authorized representative of the Company.
D. Waiver. The Company’s failure to enforce any provision of this Agreement shall not act as a waiver of its ability to enforce that provision or any other provision. The Company’s failure to enforce any breach of this Agreement shall not act as a waiver of that breach or any future breach. No waiver of any of the Company’s rights under this Agreement will be effective unless in writing. Any such written waiver shall not be deemed a continuing waiver unless specifically stated, and shall operate only as to the specific term or condition waived and shall not constitute a waiver of such term or condition for the future or as to any act other than that specifically waived.
E. Successors and Assigns. This Agreement shall be assignable to, and shall inure to the benefit of and bind, the Company’s, affiliates, subsidiaries, successors and assigns. Executive shall not have the right to assign Executive’s rights or obligations under this Agreement.
F. Construction. The language used in this Agreement will be deemed to be language chosen by Executive and the Company to express their mutual intent, and no rules of strict construction will be applied against either Party.
G. Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be enforceable, and all of which together shall constitute one agreement. Signatures of the parties that are transmitted in person or by facsimile or e-mail shall be accepted as originals.
H. Further Assurances. Executive agrees to execute any proper oath or verify any document required to carry out the terms of this Agreement.
I. Title and Headings. The titles, captions and headings of this Agreement are included for ease of reference only and will be disregarded in interpreting or construing this Agreement.
J. Notices. All notices and communications that are required or permitted to be given under this Agreement shall be in writing and shall be sufficient in all respects if given and delivered in person, by electronic mail, by facsimile, by overnight courier, or by certified mail, postage prepaid, return receipt requested, to the receiving Party at such Party’s address shown in the signature blocks below or to such other address as such Party may have given to the other by notice pursuant to this Section. Notice shall be deemed given (i) on the date of delivery in the case of personal delivery, electronic mail or facsimile, or (ii) on the delivery or refusal date as specified on the return receipt in the case of certified mail or on the tracking report in the case of overnight courier.
K. Code Section 409A. The amounts payable under this Agreement are intended to be exempt from the requirements of Section 409A of the Code (“Section 409A”). For purposes of Section 409A, any right to a series of installment payments is to be treated as a right to a series of separate payments. Any payments due under this Agreement on account of a termination of employment shall only be payable if the termination constitutes a “separation from service” within the meaning of Section 409A. To the extent that any such payments are determined to be deferred compensation subject to Section 409A, (i) the terms of this Agreement shall be interpreted to avoid incurring any penalties under Section 409A, and (ii) any payments due to a “specified Executive” of a publicly-traded company upon a separation from service shall be delayed until the first day of the seventh month following such separation from service. Notwithstanding the foregoing, in no event shall the Company be responsible for any taxes or penalties due under Section 409A.
|
13. |
EXECUTIVE’S ACKNOWLEDGMENTS. |
Executive consents to becoming an officer of the Company and acknowledges that Executive is executing this Agreement voluntarily and without duress or undue influence by the Company or anyone else and that Executive has carefully read this Agreement and fully understands the terms, consequences, and binding effect of this Agreement.
[Remainder of page intentionally left blank]
IN WITNESS WHEREOF, and intending to be legally bound, the Parties have executed this Employment Agreement as of the date first written above.
|
EXECUTIVE |
DIAMEDICA USA, INC. |
|||
|
Signature: |
/s/ Julie Krop |
Signature: |
/s/ Rick Pauls |
|
|
Print Name: |
Julie Krop, MD |
Print Name: |
Rick Pauls |
|
|
Date: |
August 11, 2025 |
Title: |
Chief Executive Officer |
|
|
Address: |
|
Date: |
August 11, 2025 |
|
|
Email: |
|
|||
Exhibit 31.1
CERTIFICATION PURSUANT TO RULE 13a-14(a) OR 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED, AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Rick Pauls, certify that:
|
1. |
I have reviewed this Quarterly Report on Form 10-Q of DiaMedica Therapeutics Inc.(Registrant); |
|
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the Registrant as of, and for, the periods presented in this report; |
|
|
4. |
The Registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Registrant and have: |
|
|
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
|
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
|
c. |
Evaluated the effectiveness of the Registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
|
d. |
Disclosed in this report any change in the Registrant’s internal control over financial reporting that occurred during the Registrant’s most recent fiscal quarter (the Registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the Registrant’s internal control over financial reporting; and |
|
|
5. |
The Registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Registrant’s auditors and the audit committee of the Registrant’s board of directors (or persons performing the equivalent functions): |
|
|
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Registrant’s ability to record, process, summarize and report financial information; and |
|
|
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the Registrant’s internal control over financial reporting. |
|
|
Dated: November 12, 2025 |
/s/ Rick Pauls |
||
|
Rick Pauls |
|||
|
President and Chief Executive Officer |
|||
|
(Principal Executive Officer) |
|||
Exhibit 31.2
CERTIFICATION PURSUANT TO RULE 13a-14(a) OR 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED, AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Scott Kellen, certify that:
|
1. |
I have reviewed this Quarterly Report on Form 10-Q of DiaMedica Therapeutics Inc.(Registrant); |
|
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the Registrant as of, and for, the periods presented in this report; |
|
|
4. |
The Registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Registrant and have: |
|
|
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
|
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
|
c. |
Evaluated the effectiveness of the Registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
|
d. |
Disclosed in this report any change in the Registrant’s internal control over financial reporting that occurred during the Registrant’s most recent fiscal quarter (the Registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the Registrant’s internal control over financial reporting; and |
|
|
5. |
The Registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Registrant’s auditors and the audit committee of the Registrant’s board of directors (or persons performing the equivalent functions): |
|
|
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Registrant’s ability to record, process, summarize and report financial information; and |
|
|
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the Registrant’s internal control over financial reporting. |
|
|
Dated: November 12, 2025 |
/s/ Scott Kellen |
|
|
Scott Kellen |
||
|
Chief Financial Officer |
||
|
(Principal Financial Officer) |
||
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
I, Rick Pauls, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
|
(1) |
the Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2025 of DiaMedica Therapeutics Inc. (the Report) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
(2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of DiaMedica Therapeutics Inc. |
Dated: November 12, 2025
|
/s/ Rick Pauls |
|
|
Rick Pauls |
|
|
President and Chief Executive Officer |
|
|
(Principal Executive Officer) |
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
I, Scott Kellen, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
|
(1) |
the Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2025 of DiaMedica Therapeutics Inc. (the Report) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
(2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of DiaMedica Therapeutics Inc. |
Dated: November 12, 2025
|
/s/ Scott Kellen |
|
|
Scott Kellen |
|
|
Chief Financial Officer |
|
|
(Principal Financial Officer) |
|