|
Delaware
|
001-37942
|
30-0645032
|
||
|
(State or other jurisdiction
of incorporation)
|
(Commission
File Number)
|
(I.R.S. Employer
Identification No.)
|
|
20 Park Plaza, Suite 424
Boston, Massachusetts
|
02116
|
|||
|
(Address of principal executive offices)
|
(Zip Code)
|
|||
|
☐
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
☐
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
☐
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
|
Title of each class
|
Trading
Symbol(s)
|
Name of each exchange
on which registered
|
||
|
Common Stock, $0.001 par value
|
CRVO
|
NASDAQ Capital Market
|
|
Item 7.01
|
Regulation FD Disclosure
|
|
Item 8.01
|
Other Events
|
|
Item 9.01
|
Financial Statements and Exhibits
|
|
Exhibit No.
|
Description
|
|
99.1
|
|
|
99.2
|
|
|
104
|
Cover Page Interactive Data File (embedded within the Inline XBRL document).
|
|
Date: January 31, 2025
|
CervoMed Inc.
|
||
|
By:
|
/s/ William Elder
|
||
|
Name:
|
William Elder
|
||
| Title: |
Chief Financial Officer & General Counsel
|
||
Exhibit 99.1

CervoMed Provides Update on Neflamapimod DLB Program as Part of Presentation at the 8th International Lewy Body Dementia Conference
— Data from the first 16 weeks of the open-label extension phase of the RewinD-LB trial are expected in 1Q 2025 and will include data from participants who have received capsules from a more recently manufactured batch of neflamapimod —
— Pharmacokinetic data obtained in a food-effect study in healthy volunteers in 4Q 2024 indicates that the new capsules achieved targeted mean plasma concentrations —
— Within-subject comparison of certain participants who received older capsules during the double-blind phase of the RewinD-LB trial and new capsules in the open-label extension phase indicated that participants receiving newer batch capsules, on average, achieved targeted plasma concentrations —
Boston, January 31, 2025 – CervoMed Inc. (NASDAQ: CRVO) (“CervoMed” or the “Company”), a clinical-stage company focused on developing treatments for age-related neurologic disorders, today provided an update on its neflamapimod development program in dementia with Lewy bodies (DLB) as part of an oral presentation at the 8th International Lewy Body Dementia Conference (ILBDC) of the topline results for the blinded portion of the Company’s Phase 2b RewinD-LB trial.
Key Findings from RewinD-LB Trial (Blinded 16-Week Portion)
|
• |
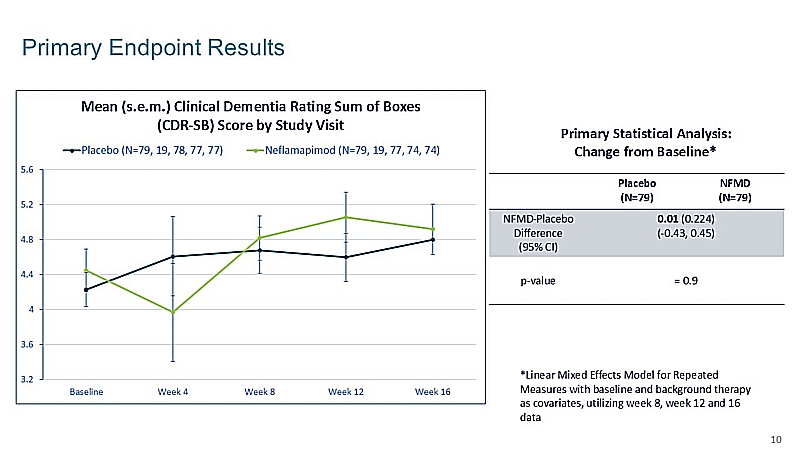
No discernible differences between neflamapimod 40mg dosed three times daily (TID) and placebo treatment groups during the 16-week double-blind phase of the clinical study. |
|
• |
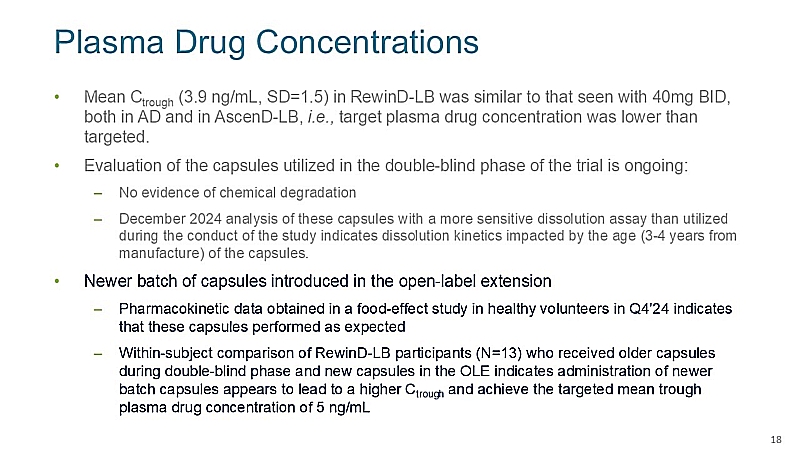
Measured trough plasma drug concentrations during the double-blind phase were, on average, similar to those seen with 40mg twice-daily (BID) dosing in a previous study in Alzheimer’s Disease, a potential explanation for the current results. |
|
• |
Retrospective analysis of the capsules suggests the lower–than-expected bioavailability may be related primarily to the age of the drug batch utilized during the double-blind phase of the study. |
|
• |
The Company believes there is potential to obtain efficacy endpoint data at the targeted plasma drug concentrations during the open-label extension (OLE) phase of the study, during which a portion of participants received capsules from a more recently manufactured drug batch. |
|
• |
Pharmacokinetic (PK) findings from a food effect study in healthy volunteers conducted in 4Q 2024 with the newer capsules and within-subject comparison of 13 participants during the OLE phase each indicate that, on average, participants achieved target plasma concentrations with the newer capsules. |
|
• |
Topline data from the first 16 weeks of the OLE phase of the RewinD-LB trial are expected in 1Q 2025. |

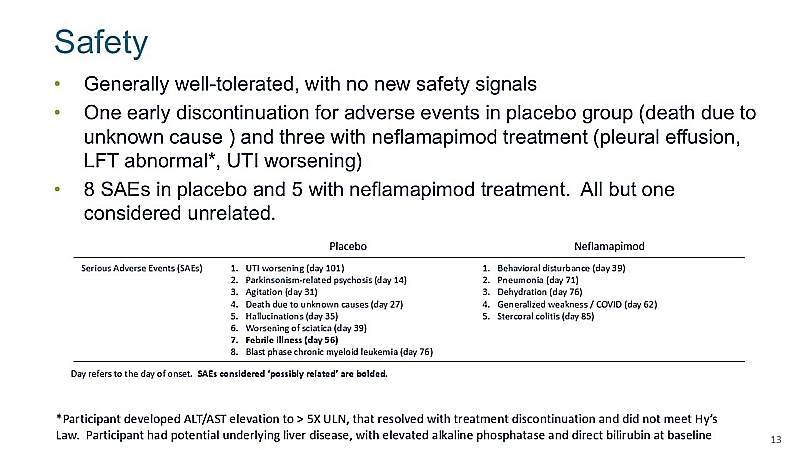
“After extensive analysis of the clinical and safety data sets and the drug product administered to participants during the double-blind phase of the study, we continue to believe neflamapimod may have potential as a treatment for DLB when administered at a therapeutically effective level,” said John Alam, MD, Chief Executive Officer of CervoMed. “Our next data readout, from the first 16 weeks of the OLE phase of the trial, will be available in the first quarter of 2025 and will include data on a range of global, cognitive and motor efficacy endpoints, as well as pharmacokinetic data, obtained from a sizeable subgroup of participants who are receiving capsules of neflamapimod manufactured more recently than the capsules utilized during the double-blind phase of the study. These data will play an important role in our evaluation of the next steps in the development of neflamapimod.”
The data set from the double-blind phase of the RewinD-LB trial presented today at ILBDC is now accessible in the Investor section of the CervoMed website https://www.cervomed.com/.
About Dementia with Lewy Bodies (DLB)
DLB is the third most common degenerative disease of the brain (after Alzheimer’s disease and Parkinson’s disease), with approximately 700,000 individuals affected in each of the United States (U.S.) and European Union. Patients with this disease accumulate protein deposits, called Lewy bodies, in the brain’s nerve cells. This negatively affects cognitive ability, including attention, judgement, and reasoning, along with motor function. Patients with DLB incur higher healthcare costs, have longer hospitalizations, report lower quality of life, and have caregivers with higher levels of distress when compared to patients with Alzheimer’s disease. No treatments for DLB have been approved by the U.S. Food and Drug Administration or European Medicines Agency, and there are few drugs in development. The current standard of care is cholinesterase inhibitor therapy, which is approved for use in Alzheimer’s disease, but in DLB patients typically improves cognition transiently, and does not impact the motor component of the disease.
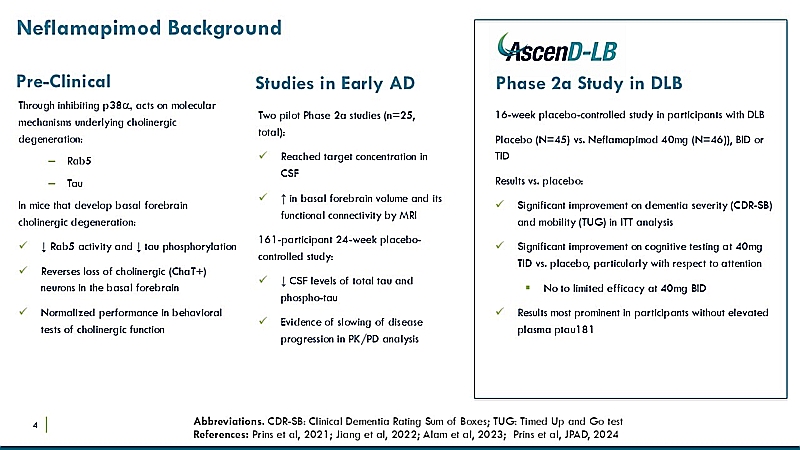
About Neflamapimod
Neflamapimod is an investigational, orally administered small molecule brain penetrant drug designed to inhibit alpha isoform of the p38MAP kinase. Following preclinical studies in which neflamapimod reversed synaptic dysfunction, results from CervoMed’s AscenD-LB Phase 2a clinical trial demonstrated that, compared to placebo, treatment with neflamapimod 40 mg TID significantly improved dementia severity, functional mobility and performance on a cognitive test battery, with the treatment response most substantial among participants with early-stage DLB. With a design guided by learnings from AscenD-LB, CervoMed’s RewinD-LB trial was the first trial to successfully enroll an exclusively early-stage DLB patient population.

About the RewinD-LB Phase 2b Trial in Dementia with Lewy Bodies
CervoMed’s Phase 2b trial, RewinD-LB, is a randomized, 16-week, double-blind, placebo-controlled clinical trial evaluating oral neflamapimod (40mg TID) in 159 participants with early-stage DLB. In early-stage DLB patients – who are estimated to comprise more than 50% of the total diagnosed DLB patient population at any given time – the disease has not progressed to a point where the patient has significant neuronal loss in the hippocampus. Patients with advanced DLB – in whom there is a significant, irreversible neuronal loss in the hippocampus and associated Alzheimer’s Disease co-pathology – were excluded from the trial. The primary endpoint in the trial is a change in CDR-SB, and secondary endpoints include the TUG test, a cognitive test battery, and the CGIC. The RewinD-LB trial is funded by a $21.3 million grant from the National Institutes of Health’s National Institute on Aging, which is being disbursed over the course of the trial as costs are incurred. The trial includes 43 sites across the United States, the United Kingdom, and the Netherlands).
About CervoMed
CervoMed Inc. is a clinical-stage company focused on developing treatments for age-related neurologic disorders. The Company is currently developing neflamapimod, an investigational, orally administered small molecule brain penetrant designed to inhibit p38 mitogen-activated protein kinase alpha. Neflamapimod has the potential to treat synaptic dysfunction, the reversible aspect of the underlying neurodegenerative processes that causes disease in certain major neurological disorders.
Forward-Looking Statements
This press release includes express and implied forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, regarding the intentions, plans, beliefs, expectations or forecasts for the future of the Company, including, but not limited to, the therapeutic potential of neflamapimod in DLB or any other indication; the anticipated timing and achievement of clinical and development milestones, including the announcement of additional data from the OLE phase of the RewinD-LB trial; any other expected or implied benefits or results, including that any initial clinical results observed with respect to neflamapimod in the AscenD-LB Trial or RewinD-LB Trial will be replicated in later trials; and the results of the Company’s ongoing investigation of the lower than expected blood concentration levels observed in the double-blind phase of the RewinD-LB trial, including the effect of more recently manufactured capsules. Terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “aims,” “seeks,” “intends,” “may,” “might,” “could,” “might,” “will,” “should,” “approximately,” “potential,” “target,” “project,” “contemplate,” “predict,” “forecast,” “continue,” or other words that convey uncertainty of future events or outcomes (including the negative of these terms) may identify these forward-looking statements. Although there is believed to be reasonable basis for each forward-looking statement contained herein, forward-looking statements by their nature involve risks and uncertainties, known and unknown, many of which are beyond the Company’s control and, as a result, actual results could differ materially from those expressed or implied in any forward-looking statement. Particular risks and uncertainties include, among other things, those related to: the Company’s available cash resources and the availability of additional funds on acceptable terms; the results of the Company’s clinical trials, including RewinD-LB; the likelihood and timing of any regulatory approval of neflamapimod or the nature of any feedback the Company may receive from the U.S. Food and Drug Administration; the ability to implement business plans, forecasts, and other expectations in the future; general economic, political, business, industry, and market conditions, inflationary pressures, and geopolitical conflicts; and the other factors discussed under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 filed with the U.S. Securities and Exchange Commission (SEC) on March 29, 2024, and other filings that the Company may file from time to time with the SEC. Any forward-looking statements in this press release speak only as of the date hereof (or such earlier date as may be identified). The Company does not undertake any obligation to update such forward-looking statements to reflect events or circumstances after the date of this press release, except to the extent required by law.
Investor Contact:
PJ Kelleher
LifeSci Advisors
Investors@cervomed.com
617-430-7579
Exhibit 99.2