UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
| (Mark One) | |
| ☒ |
Quarterly Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the Quarterly Period Ended September 30, 2023
| ☐ |
Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File Number: 001-39070
| MONOPAR THERAPEUTICS INC. |
| (Exact name of registrant as specified in its charter) |
| Delaware |
32-0463781 |
|
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. employer identification number) |
| 1000 Skokie Blvd., Suite 350, Wilmette, IL |
60091 |
|
| (Address of principal executive offices) |
(zip code) |
(847) 388-0349
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value |
MNPR |
The Nasdaq Stock Market LLC (Nasdaq Capital Market) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated Filer |
☒ |
Smaller reporting company |
☒ |
| Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The number of shares outstanding with respect to each of the classes of our common stock, as of November 7, 2023, is set forth below:
| Class |
Number of shares outstanding |
|
| Common Stock, par value $0.001 per share |
14,866,400 |
TABLE OF CONTENTS
| Page |
|||
| Condensed Consolidated Balance Sheets as of September 30, 2023 and December 31, 2022 |
|||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|||
Forward-Looking Statements
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Act”), and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical facts included in this Quarterly Report on Form 10-Q are forward-looking statements. The words “hopes,” “believes,” “anticipates,” “plans,” “seeks,” “estimates,” “projects,” “expects,” “intends,” “may,” “could,” “should,” “would,” “will,” “continue,” and similar expressions are intended to identify forward-looking statements. The following uncertainties and factors, among others, could affect future performance and cause actual results to differ materially from those matters expressed in or implied by forward-looking statements:
| ● |
our ability to raise sufficient funds within the next 12 months in order for us to (1) continue the clinical development of camsirubicin through and beyond our ongoing Phase 1b dose escalation clinical trial, (2) support further development of MNPR-101 for radiopharmaceutical use in advanced cancers, including through the initiation and completion of a first-in-human clinical trial, and (3) support further development of MNPR-202 and related compounds; as well as our ability to further raise additional funds in the future to support any future product candidate programs through completion of clinical trials, and our current and future product candidate programs through the approval processes and, if applicable, commercialization; |
|
|
|
| ● | our ability to regain compliance with Nasdaq's minimum bid price rule, including whether we can obtain a second 180-day period to regain compliance; |
| ● |
our ability to raise funds at acceptable terms; |
|
|
|
| ● |
our ability to find a suitable pharmaceutical partner or partners to further our development efforts, under acceptable financial terms; |
|
|
|
| ● |
risks and uncertainties associated with our research and development activities, including our clinical trials, regulatory submissions, and manufacturing and quality expenses; |
|
|
|
| ● |
estimated timeframes for our clinical trials and regulatory reviews for approval to market products are uncertain; |
|
|
|
| ● |
the rate of market acceptance and competitiveness in terms of pricing, efficacy and safety, of any products for which we receive marketing approval, and our ability to competitively market any such products as compared to larger pharmaceutical firms; |
|
|
|
| ● |
the difficulties of commercialization, marketing and product manufacturing and overall strategy; |
|
|
|
| ● |
uncertainties of intellectual property position and strategy including new discoveries and patent filings; |
|
|
|
| ● |
our ability to attract and retain experienced and qualified key personnel and/or to find and utilize external sources of experience, expertise and scientific, medical and commercialization knowledge to complete product development and commercialization of new products; |
|
|
|
| ● |
the risks inherent in our estimates regarding the level of needed expenses, capital requirements and the availability of required additional financing at acceptable terms; |
|
|
|
| ● |
the impact of government laws and regulations including increased governmental control of healthcare and pharmaceuticals, resulting in direct price controls driving lower prices, other governmental regulations affecting cost requirements and structures for selling therapeutic or imaging products, and recent governmental legislation affecting other industries which may indirectly increase our costs of obtaining goods and services; |
|
|
|
| ● |
the uncertain continuing impact of COVID-19 on our ability to advance our clinical programs and raise additional financing; |
|
|
|
| ● |
the cumulative impact of domestic and global inflation, volatility in financial markets and/or the potential for an economic recession increasing our costs of obtaining goods and services or making financing more difficult to obtain on acceptable terms or at all; |
|
|
|
| ● |
the uncertain impact of the Russia-Ukraine war or the Israel-Hamas war on our clinical material manufacturing expenses and timelines, as well as on general economic, trade and financial market conditions; and |
|
|
|
| ● |
uncertainty of our financial projections and operational timelines and the development of new competitive products and technologies. |
Although we believe that the risk assessments identified in such forward-looking statements are appropriate, we can give no assurance that such risks will materialize. Cautionary statements are disclosed in this Quarterly Report on Form 10-Q, including without limitation statements in the section entitled “Item 1A - Risk Factors,” addressing forward-looking statements. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements. We undertake no obligation to update any statements made in this Quarterly Report on Form 10-Q or elsewhere, including without limitation any forward-looking statements, except as required by law.
Any forward-looking statements in this Quarterly Report on Form 10-Q reflect our current views with respect to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances projected in this information.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in “Item 1A - Risk Factors” of our December 31, 2022 Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 23, 2023 and “Item 1A - Risk Factors” of this Quarterly Report on Form 10-Q. These risks include, among others, the following:
| ● |
We are a clinical stage biopharmaceutical company with a history of financial losses. We expect to continue to incur significant losses for the foreseeable future and may never achieve or maintain cash self-sufficiency or profitability, which could result in a decline in the market value of our common stock. |
|
|
|
||
| ● |
Funds raised to date are not sufficient to (1) continue the clinical development of camsirubicin through and beyond our ongoing Phase 1b dose escalation clinical trial; (2) support further development of MNPR-101 for radiopharmaceutical use in advanced cancers, including through the initiation and completion of a first-in-human clinical trial; or (3) support continued development of MNPR-202 and related compounds. If we are unable to raise enough funds within the next 12 months from the sale of our common stock or other financing efforts, or conclude a strategic agreement or collaboration such as out-licensing our product candidates, or entering into a clinical or commercial partnership, we will likely have to terminate one or more programs. There can be no assurance that we will be able to secure such financing or find a suitable development partner on satisfactory terms. |
|
|
|
||
| ● |
The termination of our Validive clinical trial at the end of March 2023 resulted in a decrease in our stock price. The closing bid price of our stock fell below $1.00 for more than 30 consecutive trading days and on August 28, 2023, we received a notice from Nasdaq stating that we are out of compliance with Nasdaq listing standards giving us 180 days to regain compliance. While we intend to apply for a second 180-day period to regain compliance, there can be no assurance that we would regain compliance within Nasdaq’s original or potential extended time limits and requirements. If we do not regain compliance, we would face delisting and it may have serious adverse consequences on our ability to raise funds, which may cause us to delay, restructure or otherwise reconsider our operations. |
|
| ● |
We do not have and may never have any approved products on the market. Our business is highly dependent upon receiving marketing approvals from various U.S. and international governmental agencies and would be severely harmed if we are not granted approvals to manufacture and sell our product candidates. |
|
|
|
||
| ● |
Our clinical trials may not yield sufficiently conclusive results for regulatory agencies to approve the use of our products, which would adversely affect our financial condition. |
|
|
|
||
| ● |
If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals will be delayed or prevented, which would materially delay our program schedules and adversely affect our financial condition. |
|
|
|
||
| ● |
If we or our licensees, development collaborators, or suppliers are unable to manufacture our products in sufficient quantities or at defined quality specifications, or are unable to obtain regulatory approvals for the manufacturing facility, we may be unable to develop and/or meet demand for our products and lose time to market and potential revenues. |
|
|
|
||
| ● |
We rely on qualified third parties to conduct our active pharmaceutical ingredient manufacturing, our drug product manufacturing, non-clinical studies, and our clinical trials. If these third parties do not or cannot successfully carry out their contractual duties and meet expected deadlines or performance goals, the initiation or conduct of our clinical trials would be delayed and we may be unable to obtain regulatory approval for, or commercialize, our current product candidates or any future products, and our financial condition would be adversely affected. |
|
|
|
||
| ● |
The Russia-Ukraine war, and resulting sanctions against Russia and Russian entities, and Russian reduction in gas shipments to the EU and other allies, have increased fuel costs, reduced access to critical supplies and may cause shipping delays. In addition, the Israel-Hamas war has created additional uncertainties. The broader economic, trade and financial market consequences are uncertain at this time, which may increase the cost of supplies for our clinical materials, may delay the manufacture of our clinical materials, may increase costs of other goods and services or make it more difficult or costly to raise additional financing, any of which could cause an adverse effect on our clinical programs and on our financial condition. |
| ● |
Market variables, such as inflation of product costs, labor rates and fuel, freight and energy costs, as well as geopolitical events could likely cause us to suffer significant increases in our operating and administrative expenses. |
|
|
|
||
| ● |
Unstable market and economic conditions, such as the recent volatility in the markets due to concerns about bank stability and economic challenges due to inflation, may have serious adverse consequences on our ability to raise funds, which may cause us to delay, restructure or otherwise reconsider our operations. |
|
|
|
||
| ● |
The effects of economic and political pressure to lower pharmaceutical prices are a major threat to the economic viability of new research-based pharmaceutical products, and any significant decrease in drug prices could materially and adversely affect the financial appeal of our products and investment prospects. |
|
|
|
||
| ● |
We face significant competition from other biotechnology and pharmaceutical companies, and from research-based academic medical institutions, in our targeted medical indications, and our operating results would be adversely affected if we fail to compete effectively. Many competitors have greater organizational capabilities in our industry, much higher available capital resources, and established marketing resources and sales in the targeted markets. Competition and technological change may make our product candidates obsolete or non-competitive. |
|
|
|
||
| ● |
The termination of third-party licenses would adversely affect our rights to important compounds or technologies which are essential to develop and market our products. |
|
|
|
||
| ● |
If we and our third-party licensors do not obtain and preserve protection for our respective intellectual property rights, our competitors may be able to develop and market competing drugs, which would adversely affect our financial condition. |
|
|
|
||
| ● |
If we lose key management leadership, and/or the expertise and experience of our scientific personnel, and if we cannot recruit qualified employees or other highly qualified and experienced personnel for future requirements, we would be at risk to experience significant program delays and increased compensation and operational costs, and our business would be materially disrupted. |
|
|
|
||
| ● |
The long-term effects of COVID-19 are highly uncertain, and their scope and impact could have a substantial negative bearing on our business, financial condition, operating results, stock price and ability to raise additional funds. |
FINANCIAL INFORMATION
Condensed Consolidated
Balance Sheets
(Unaudited)
| September 30, 2023 |
December 31, 2022* |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 5,515,353 | $ | 8,186,194 | ||||
| Investments |
2,979,498 | 4,933,550 | ||||||
| Other current assets |
73,193 | 45,982 | ||||||
| Total current assets |
8,568,044 | 13,165,726 | ||||||
| Operating lease right-of-use asset |
25,088 | 61,228 | ||||||
| Total assets |
$ | 8,593,132 | $ | 13,226,954 | ||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable, accrued expenses and other current liabilities |
$ | 1,981,802 | $ | 3,128,894 | ||||
| Total current liabilities |
1,981,802 | 3,128,894 | ||||||
| Non-current operating lease liability |
— | 8,408 | ||||||
| Total liabilities |
1,981,802 | 3,137,302 | ||||||
| Commitments and contingencies (Note 8) |
||||||||
| Stockholders’ equity: |
||||||||
| Common stock, par value of $0.001 per share, 40,000,000 shares authorized, 14,198,438 and 12,946,573 shares issued and outstanding at September 30, 2023, and December 31, 2022, respectively |
14,198 | 12,947 | ||||||
| Additional paid-in capital |
64,985,485 | 61,871,784 | ||||||
| Accumulated other comprehensive income |
3,949 | 8,942 | ||||||
| Accumulated deficit |
(58,392,302 | ) | (51,804,021 | ) | ||||
| Total stockholders’ equity |
6,611,330 | 10,089,652 | ||||||
| Total liabilities and stockholders’ equity |
$ | 8,593,132 | $ | 13,226,954 | ||||
* Derived from the Company’s audited consolidated financial statements.
The accompanying notes are an integral part of these condensed consolidated financial statements.
Condensed Consolidated
Statements of Operations and Comprehensive Loss
(Unaudited)
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2023 |
2022 |
2023 |
2022 |
|||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
$ | 1,316,520 | $ | 1,732,230 | $ | 4,564,602 | $ | 5,488,633 | ||||||||
| General and administrative |
749,474 | 675,115 | 2,354,645 | 2,139,246 | ||||||||||||
| Total operating expenses |
2,065,994 | 2,407,345 | 6,919,247 | 7,627,879 | ||||||||||||
| Loss from operations |
(2,065,994 | ) | (2,407,345 | ) | (6,919,247 | ) | (7,627,879 | ) | ||||||||
| Interest income |
112,260 | 7,698 | 330,966 | 8,579 | ||||||||||||
| Net loss |
(1,953,734 | ) | (2,399,647 | ) | (6,588,281 | ) | (7,619,300 | ) | ||||||||
| Other comprehensive income (loss): |
||||||||||||||||
| Foreign currency translation gain (loss) |
6,930 | 25,519 | (8,254 | ) | 46,145 | |||||||||||
| Unrealized gain (loss) on investments |
(10,203 | ) | — | 3,261 | ||||||||||||
| Comprehensive loss |
$ | (1,957,007 | ) | $ | (2,374,128 | ) | $ | (6,593,274 | ) | $ | (7,573,155 | ) | ||||
| Net loss per share: |
||||||||||||||||
| Basic and diluted |
$ | (0.14 | ) | $ | (0.19 | ) | $ | (0.49 | ) | $ | (0.60 | ) | ||||
| Weighted average shares outstanding: |
||||||||||||||||
| Basic and diluted |
14,118,397 | 12,754,685 | 13,551,776 | 12,664,387 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
Condensed Consolidated Statements of Stockholders’ Equity
Three and Nine Months Ended September 30, 2023
(Unaudited)
| Accumulated |
||||||||||||||||||||||||
| Additional |
Other |
Total |
||||||||||||||||||||||
| Common Stock |
Paid- |
Comprehensive |
Accumulated |
Stockholders’ |
||||||||||||||||||||
| Shares |
Amount |
in Capital |
Income (Loss) |
Deficit |
Equity |
|||||||||||||||||||
| Balance at January 1, 2023 |
12,946,573 | $ | 12,947 | $ | 61,871,784 | $ | 8,942 | $ | (51,804,021 | ) | $ | 10,089,652 | ||||||||||||
| Issuance of common stock under a Capital on DemandTM Sales Agreement with JonesTrading Institutional Services, LLC, net of commissions, fees and offering costs of $37,661 |
244,392 | 244 | 807,094 | — | — | 807,338 | ||||||||||||||||||
| Issuance of common stock to non-employee directors pursuant to vested restricted stock units |
10,132 | 10 | (10 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock to employees pursuant to vested restricted stock units, net of taxes |
20,959 | 21 | (16,848 | ) | — | — | (16,827 | ) | ||||||||||||||||
| Stock-based compensation (non-cash) |
— | — | 476,209 | — | — | 476,209 | ||||||||||||||||||
| Net loss |
— | — | — | — | (2,434,556 | ) | (2,434,556 | ) | ||||||||||||||||
| Other comprehensive income, net |
— | — | — | 22,845 | — | 22,845 | ||||||||||||||||||
| Balance at March 31, 2023 |
13,222,056 | 13,222 | 63,138,229 | 31,787 | (54,238,577 | ) | 8,944,661 | |||||||||||||||||
| Issuance of common stock under a Capital on DemandTM Sales Agreement with JonesTrading Institutional Services, LLC, net of commissions, fees and offering costs of $26,522 |
621,227 | 621 | 659,374 | — | — | 659,995 | ||||||||||||||||||
| Issuance of common stock to non-employee directors pursuant to vested restricted stock units |
10,136 | 10 | (10 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock to employees pursuant to vested restricted stock units, net of taxes |
44,662 | 45 | (16,658 | ) | — | — | (16,613 | ) | ||||||||||||||||
| Stock-based compensation (non-cash) |
— | — | 473,296 | — | — | 473,296 | ||||||||||||||||||
| Net loss |
— | — | — | — | (2,199,991 | ) | (2,199,991 | ) | ||||||||||||||||
| Other comprehensive loss, net |
— | — | — | (24,565 | ) | — | (24,565 | ) | ||||||||||||||||
| Balance at June 30, 2023 |
13,898,081 | 13,898 | 64,254,231 | 7,222 | (56,438,568 | ) | 7,836,783 | |||||||||||||||||
| Issuance of common stock under a Capital on DemandTM Sales Agreement with JonesTrading Institutional Services, LLC, net of commissions, fees and offering costs of $8,218 |
259,860 | 260 | 265,363 | — | — | 265,623 | ||||||||||||||||||
| Issuance of common stock to non-employee directors pursuant to vested restricted stock units |
10,132 | 10 | (10 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock to employees pursuant to vested restricted stock units, net of taxes |
30,365 | 30 | (8,237 | ) | — | — | (8,207 | ) | ||||||||||||||||
| Stock-based compensation (non-cash) |
— | — | 474,138 | — | — | 474,138 | ||||||||||||||||||
| Net loss |
— | — | — | — | (1,953,734 | ) | (1,953,734 | ) | ||||||||||||||||
| Other comprehensive loss, net |
— | — | — | (3,273 | ) | — | (3,273 | ) | ||||||||||||||||
| Balance at September 30, 2023 |
14,198,438 | $ | 14,198 | $ | 64,985,485 | $ | 3,949 | $ | (58,392,302 | ) | $ | 6,611,330 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
Monopar Therapeutics Inc.
Condensed Consolidated Statements of Stockholders’ Equity
Three and Nine Months Ended September 30, 2022
(Unaudited)
| Accumulated |
||||||||||||||||||||||||
| Additional |
Other |
Total |
||||||||||||||||||||||
| Common Stock |
Paid- |
Comprehensive |
Accumulated |
Stockholders’ |
||||||||||||||||||||
| Shares |
Amount |
in Capital |
Income (Loss) |
Deficit |
Equity |
|||||||||||||||||||
| Balance at January 1, 2022 |
12,598,125 | $ | 12,598 | $ | 60,220,016 | $ | (3,160 | ) | $ | (41,288,383 | ) | $ | 18,941,071 | |||||||||||
| Issuance of common stock to non-employee directors pursuant to vested restricted stock units |
11,436 | 12 | (12 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock to employees pursuant to vested restricted stock units, net of taxes |
11,031 | 11 | (16,677 | ) | — | — | (16,666 | ) | ||||||||||||||||
| Stock-based compensation (non-cash) |
— | — | 499,812 | — | — | 499,812 | ||||||||||||||||||
| Net loss |
— | — | — | — | (2,456,722 | ) | (2,456,722 | ) | ||||||||||||||||
| Other comprehensive loss |
— | — | — | (584 | ) | — | (584 | ) | ||||||||||||||||
| Balance at March 31, 2022 |
12,620,592 | 12,621 | 60,703,139 | (3,744 | ) | (43,745,105 | ) | 16,966,911 | ||||||||||||||||
| Issuance of common stock to non-employee directors pursuant to vested restricted stock units |
11,436 | 11 | (11 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock to employees pursuant to vested restricted stock units, net of taxes |
28,177 | 28 | (29,683 | ) | — | — | (29,655 | ) | ||||||||||||||||
| Issuance of common stock upon exercise of stock options |
40,532 | 41 | — | — | — | 41 | ||||||||||||||||||
| Stock-based compensation (non-cash) |
— | — | 357,293 | — | — | 357,293 | ||||||||||||||||||
| Offering costs |
— | — | (35,846 | ) | — | — | (35,846 | ) | ||||||||||||||||
| Net loss |
— | — | — | — | (2,762,931 | ) | (2,762,931 | ) | ||||||||||||||||
| Other comprehensive income |
— | — | — | 21,210 | — | 21,210 | ||||||||||||||||||
| Balance at June 30, 2022 |
12,700,737 | 12,701 | 60,994,892 | 17,466 | (46,508,036 | ) | 14,517,023 | |||||||||||||||||
| Issuance of common stock to non-employee directors pursuant to vested restricted stock units |
11,436 | 11 | (11 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock to employees pursuant to vested restricted stock units, net of taxes |
16,094 | 16 | (10,688 | ) | — | — | (10,672 | ) | ||||||||||||||||
| Issuance of common stock upon exercise of stock options |
127,468 | 128 | — | — | — | 128 | ||||||||||||||||||
| Stock-based compensation (non-cash) |
— | — | 390,741 | — | — | 390,741 | ||||||||||||||||||
| Offering costs |
— | — | (13,194 | ) | — | — | (13,194 | ) | ||||||||||||||||
| Net loss |
— | — | — | — | (2,399,647 | ) | (2,399,647 | ) | ||||||||||||||||
| Other comprehensive income |
— | — | — | 25,519 | — | 25,519 | ||||||||||||||||||
| Balance at September 30, 2022 |
12,855,735 | $ | 12,856 | $ | 61,361,740 | $ | 42,985 | $ | (48,907,683 | ) | $ | 12,509,898 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
Condensed Consolidated
Statements of Cash Flows (Unaudited)
| For the Nine Months Ended |
||||||||
| September 30, |
||||||||
| 2023 |
2022 |
|||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (6,588,281 | ) | $ | (7,619,300 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Stock-based compensation expense (non-cash) |
1,423,643 | 1,247,846 | ||||||
| Changes in operating assets and liabilities, net |
||||||||
| Other current assets |
(27,219 | ) | 162,017 | |||||
| Accounts payable, accrued expenses and other current liabilities |
(1,120,537 | ) | 282,435 | |||||
| Operating lease right-of-use assets and liabilities, net |
— | 249 | ||||||
| Net cash used in operating activities |
(6,312,394 | ) | (5,926,753 | ) | ||||
| Cash flows from investing activities: |
||||||||
| Purchase of short-term investments |
(7,891,330 | ) | — | |||||
| Maturities of short-term investments |
9,848,643 | — | ||||||
| Net cash provided by investing activities |
1,957,313 | — | ||||||
| Cash flows from financing activities: |
||||||||
| Net cash proceeds from the sales of common stock under a Capital on DemandTM Sales Agreement |
1,734,326 | — | ||||||
| Other offering costs |
— | (49,040 | ) | |||||
| Taxes paid related to net share settlement of vested restricted stock units |
(41,647 | ) | (56,993 | ) | ||||
| Cash proceeds from the issuance of stock upon exercise of stock options |
— | 169 | ||||||
| Net cash provided by (used in) financing activities |
1,692,679 | (105,864 | ) | |||||
| Effect of exchange rates |
(8,439 | ) | 45,209 | |||||
| Net decrease in cash and cash equivalents |
(2,670,841 | ) | (5,987,408 | ) | ||||
| Cash and cash equivalents at beginning of period |
8,186,194 | 20,303,869 | ||||||
| Cash and cash equivalents at end of period |
$ | 5,515,353 | $ | 14,316,461 | ||||
| Supplemental non-cash flow information: |
||||||||
| Lease liability arising out of obtaining right-of-use asset |
$ | — | $ | 92,359 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
Note 1 – Nature of Business and Liquidity
Nature of Business
Monopar Therapeutics Inc. (“Monopar” or the ”Company”) is a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients. Monopar currently has three compounds in development: 1) camsirubicin (generic name for MNPR-201, GPX-150; 5-imino-13-deoxydoxorubicin), a Phase 1b clinical stage novel analog of doxorubicin engineered specifically to retain anticancer activity while minimizing toxic effects on the heart; 2) MNPR-101 RIT and MNPR-101-Zr, a preclinical stage uPAR-targeted antibody being developed as a radioimmunotherapeutic and companion diagnostic for advanced cancers; and 3) an early stage camsirubicin analog, MNPR-202, for various cancers. On March 27, 2023, the Company discontinued its Validive Phase 2b/3 VOICE trial based upon its Data Safety Monitoring Board’s determination that the trial did not meet the pre-defined threshold for efficacy of a 15% absolute difference in severe oral mucositis prevention between Validive and placebo. The Company continued to record clinical site close-out related expenses for Validive in Q3 2023. The Company is not anticipating incurring any license or royalty obligations or incurring any significant expenses beyond Q3 2023 related to Validive.
Liquidity
The Company has incurred an accumulated deficit of approximately $58.4 million as of September 30, 2023. To date, the Company has primarily funded its operations with the net proceeds from the Company’s initial public offering of its common stock on Nasdaq, sales of its common stock in the public market through at-the-market sales agreements, private placements of convertible preferred stock and of common stock and cash provided in the camsirubicin asset purchase transaction. Management estimates that currently available cash will provide sufficient funds to enable the Company to meet its obligations at least through November 2024. The Company’s ability to fund its future operations, including the continued clinical development of camsirubicin and continued development of its radiopharmaceutical program, is dependent upon its ability to execute its business strategy, to obtain additional funding and/or to execute collaborative research agreements. There can be no certainty that future financing or collaborative research agreements will occur in the amounts required or at a time needed to maintain operations, if at all.
The termination of the Company’s Validive clinical trial at the end of March 2023 resulted in a decrease in the Company’s stock price. The closing bid price of the Company’s stock fell below $1.00 for more than 30 consecutive trading days, and on August 28, 2023, the Company received a notice from Nasdaq stating that it is out of compliance with Nasdaq listing standards giving the Company 180 days to regain compliance. While the Company intends to apply for a second 180-day period to regain compliance, there can be no assurance that the Company will regain compliance within Nasdaq’s original or potential extended time limits and requirements. If the Company does not regain compliance, we would face delisting and it may have serious adverse consequences on the Company’s ability to raise funds, which may cause Monopar to delay, restructure or otherwise reconsider its operations.
Market variables over which the Company has no control, such as inflation of product costs, higher capital costs, labor rates and fuel, freight and energy costs, as well as geopolitical events could cause the Company to suffer significant increases in its operating and administrative expenses.
The Russia-Ukraine war, and resulting sanctions against Russia and Russian entities or allies, have increased fuel costs and may cause shipping delays. In addition, the Israel-Hamas war has created additional uncertainties. The broader economic, trade and financial market consequences are uncertain at this time, which may increase the cost of supplies for the Company’s clinical materials, may delay the manufacture of its clinical materials, may increase costs of other goods and services, or make it more difficult or costly to raise additional financing, any of which could cause an adverse effect on the Company’s clinical and development program and on the Company’s financial condition.
The coronavirus disease (“COVID-19”) continues to affect economies and business around the world. Due to many uncertainties, the Company is unable to estimate COVID-19’s financial impact or duration in light of treatment options and potential surges of new cases from current or future COVID-19 variants or its potential impact on the Company’s current clinical trial and development programs, including COVID-19’s effect on drug candidate manufacturing, shipping, patient recruitment at clinical sites and regulatory agencies around the globe.
Note 2 – Significant Accounting Policies
Basis of Presentation
These condensed consolidated financial statements include the financial results of Monopar Therapeutics Inc., its wholly-owned French subsidiary, Monopar Therapeutics, SARL, and its wholly-owned Australian subsidiary, Monopar Therapeutics Australia Pty Ltd, and have been prepared in accordance with accounting principles generally accepted in the U.S. (“GAAP”) and include all disclosures required by GAAP for financial reporting. All intercompany accounts have been eliminated. The principal accounting policies applied in the preparation of these condensed consolidated financial statements are set out below and have been consistently applied in all periods presented. The Company has been primarily involved in performing research activities, developing product candidates, and raising capital to support and expand these activities.
The accompanying interim unaudited condensed consolidated financial statements contain all normal, recurring adjustments necessary to present fairly the Company’s condensed consolidated financial position as of September 30, 2023, and the Company’s condensed consolidated results of operations and comprehensive loss for the three and nine months ended September 30, 2023 and 2022, and the Company’s condensed consolidated cash flows for the nine months ended September 30, 2023 and 2022.
The interim condensed consolidated results of operations and comprehensive loss and condensed consolidated cash flows for the periods presented are not necessarily indicative of the condensed consolidated results of operations or cash flows which may be reported for the remainder of 2023 or for any future period. Certain information and footnote disclosures normally included in financial statements prepared in accordance with GAAP have been condensed or omitted. The accompanying unaudited interim condensed consolidated financial statements should be read in conjunction with the audited financial statements and notes thereto for the year ended December 31, 2022, included in the Company’s Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 23, 2023.
Functional Currency
The Company’s consolidated functional currency is the U.S. Dollar. The Company’s Australian subsidiary and French subsidiary use the Australian Dollar and European Euro, respectively, as their functional currency. At each quarter-end, each foreign subsidiary’s balance sheets are translated into U.S. Dollars based upon the quarter-end exchange rate, while their statements of operations and comprehensive loss and statements of cash flows are translated into U.S. Dollars based upon an average exchange rate during the period.
Comprehensive Loss
Comprehensive loss represents net loss plus any income or losses not reported in the condensed consolidated statements of operations and comprehensive loss, such as foreign currency translations gains and losses and unrealized gains and losses on debt security investments that are reflected on the Company’s condensed consolidated statements of stockholders’ equity.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities, and reported amounts of expenses in the condensed consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Going Concern Assessment
The Company applies Accounting Standards Codification 205-40 (“ASC 205-40”), Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, which the Financial Accounting Standards Board (“FASB”) issued to provide guidance on determining when and how reporting companies must disclose going concern uncertainties in their financial statements. ASC 205-40 requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern within one year of the date of issuance of the entity’s financial statements (or within one year after the date on which the financial statements are available to be issued, when applicable). Further, a company must provide certain disclosures if there is “substantial doubt about the entity’s ability to continue as a going concern.” In September 2023, the Company analyzed its cash requirements at least through November 2024 and has determined that, based upon the Company’s current available cash, the Company has no substantial doubt about its ability to continue as a going concern.
Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity of three months or less on the date of purchase to be cash equivalents. Cash equivalents as of September 30, 2023 and December 31, 2022, consisted of two money market accounts and U.S. Treasury Bills.
Investments
The Company considers all of its investments in debt securities (U.S. Government or Agencies), with maturities at the date of purchase from over three months to one year to be available-for-sale securities. These investments are recorded at fair value with the unrealized gains and losses reflected in accumulated other comprehensive income (loss) on the Company’s condensed consolidated balance sheets. Realized gains and losses from the sale of investments, if any are determined, are recorded net in the condensed consolidated statements of operations and comprehensive loss. The investments selected by the Company have a low level of inherent credit risk given they are issued by the U.S. government and any changes in their fair value are primarily attributable to changes in interest rates and market liquidity. Investments as of September 30, 2023 and December 31, 2022, consisted of U.S. Treasury Bills with maturities of over three months to one year.
Prepaid Expenses
Prepayments are expenditures for goods or services before the goods are used or the services are received and are charged to operations as the benefits are realized. Prepaid expenses may include payments to development collaborators in excess of actual expenses incurred by the collaborator measured at the end of each reporting period. Prepayments also include insurance premiums, dues and subscriptions and software costs of $10,000 or more per year that are expensed monthly over the life of the contract, which is typically one year. Prepaid expenses are reflected on the Company’s condensed consolidated balance sheets as other current assets.
Leases
Lease agreements are evaluated to determine whether an arrangement is or contains a lease in accordance with ASC 842, Leases. Right-of-use lease assets and lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at the commencement date. The right-of-use lease asset on the Company’s condensed consolidated balance sheets includes any lease payments made and excludes lease incentives. The incremental borrowing taking into consideration the Company’s credit quality and borrowing rate for similar assets is used in determining the present value of future payments. Lease expense is recorded as general and administrative expenses on the Company’s condensed consolidated statements of operations and comprehensive loss.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist of cash and cash equivalents. The Company maintains cash and cash equivalents at two reputable financial institutions. As of September 30, 2023, the balance at one financial institution was in excess of the $250,000 Federal Deposit Insurance Corporation (“FDIC”) insurable limit. The Company has not experienced any losses on its deposits since inception and management believes the Company is not exposed to significant risks with respect to these financial institutions.
Fair Value of Financial Instruments
For financial instruments consisting of cash and cash equivalents, investments, accounts payable, accrued expenses, and other current liabilities, the carrying amounts are reasonable estimates of fair value due to their relatively short maturities.
The Company adopted ASC 820, Fair Value Measurements and Disclosures, as amended, which addresses the measurement of the fair value of financial assets and financial liabilities. Under this standard, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e., the “exit price”) in an orderly transaction between market participants at the measurement date.
The standard establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs reflect assumptions market participants would use in pricing an asset or liability based on market data obtained from independent sources. Unobservable inputs reflect a reporting entity’s pricing an asset or liability developed based on the best information available under the circumstances. The fair value hierarchy consists of the following three levels:
Level 1 - instrument valuations are obtained from real-time quotes for transactions in active exchange markets involving identical assets.
Level 2 - instrument valuations are obtained from readily available pricing sources for comparable instruments.
Level 3 - instrument valuations are obtained without observable market values and require a high-level of judgment to determine the fair value.
Determining which category an asset or liability falls within the hierarchy requires significant judgment. The Company evaluates its hierarchy disclosures each reporting period. There were no transfers between Level 1, 2 or 3 of the fair value hierarchy during the three and nine months ended September 30, 2023 and 2022. The following table presents the assets and liabilities that are reported at fair value on our condensed consolidated balance sheets on a recurring basis. No values were recorded in Level 2 or Level 3 as of September 30, 2023 and December 31, 2022.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
| September 30, 2023 |
Level 1 |
Total |
||||||
| Assets: |
||||||||
| Cash equivalents(1) |
$ | 4,670,605 | $ | 4,670,605 | ||||
| Investments(2) |
2,979,498 | 2,979,498 | ||||||
| Total |
$ | 7,650,103 | $ | 7,650,103 | ||||
| December 31 2022 |
Level 1 |
Total |
||||||
| Assets: |
||||||||
| Cash equivalents(1) |
$ | 7,248,946 | $ | 7,248,946 | ||||
| Investments(2) |
4,933,550 | 4,933,550 | ||||||
| Total |
$ | 12,182,496 | $ | 12,182,496 | ||||
| (1) |
Cash equivalents as of September 30, 2023 and December 31, 2022, represent the fair value of the Company’s investment in two money market accounts and U.S. Treasury Bills with maturities at the date of purchase of three months or less. |
|
|
|
| (2) |
Investments represents the fair value of the Company’s investment in U.S. Treasury Bills with maturities at the date of purchase from over three months to one year. |
Net Loss per Share
Net loss per share for the three and nine months ended September 30, 2023 and 2022, is calculated by dividing net loss by the weighted-average shares of common stock outstanding during the periods. Diluted net loss per share for the three and nine months ended September 30, 2023 and 2022, is calculated by dividing net loss by the weighted-average shares of the sum of a) weighted average common stock outstanding (14,118,397 and 12,754,685 shares for the three months ended September 30, 2023 and 2022 respectively, and 13,551,776 and 12,664,387 shares for the nine months ended September 30, 2023 and 2022, respectively) and b) potentially dilutive shares of common stock (such as stock options and restricted stock units) outstanding during the period. As of September 30, 2023 and 2022, potentially dilutive securities included stock-based awards to purchase up to 2,580,829 and 1,928,152 shares of the Company’s common stock, respectively. For the three and nine months ended September 30, 2023 and 2022, potentially dilutive securities are excluded from the computation of fully diluted net loss per share as their effect is anti-dilutive.
Research and Development Expenses
Research and development (“R&D”) costs are expensed as incurred. Major components of R&D expenses include salaries and benefits paid to the Company’s R&D staff, compensation expenses of G&A personnel performing R&D, fees paid to consultants and to the entities that conduct certain R&D activities on the Company’s behalf and costs of materials and supplies which were used in R&D activities during the reporting period.
Clinical Trials Accruals
The Company accrues and expenses the costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations, service providers, and clinical trial sites. The Company estimates the amounts to accrue based upon discussions with internal clinical personnel and external service providers as to progress or stage of completion of trials or services and the agreed upon fees to be paid for such services. Costs of setting up clinical trial sites for participation in the trials are expensed immediately as R&D expenses. Clinical trial site costs related to patient screening and enrollment are accrued as patients are screened/entered into the trial.
Collaborative Agreements
The Company and its collaborative partners are active participants in collaborative agreements and all parties would be exposed to significant risks and rewards depending on the technical and commercial success of the activities. Contractual payments to the other parties in collaboration agreements and costs incurred by the Company when the Company is deemed to be the principal participant for a given transaction are recognized on a gross basis in R&D expenses. Royalties and license payments are recorded as earned.
During the three and nine months ended September 30, 2023 and 2022, no milestones were met, and no royalties were earned, therefore, the Company did not pay or accrue/expense any license or royalty payments.
Licensing Agreements
The Company has various agreements licensing technology utilized in the development of its product or technology programs. The licenses contain success milestone obligations and royalties on future sales. During the three and nine months ended September 30, 2023 and 2022, no milestones were met, and no royalties were earned, therefore, the Company did not pay or accrue/expense any license or royalty payments under any of its license agreements.
Patent Costs
The Company expenses costs relating to issued patents and patent applications, including costs relating to legal, renewal and application fees, as a component of general and administrative expenses in its condensed consolidated statements of operations and comprehensive loss.
Income Taxes
The Company uses an asset and liability approach for accounting for deferred income taxes, which requires recognition of deferred income tax assets and liabilities for the expected future tax consequences of events that have been recognized in its financial statements but have not been reflected in its taxable income. Estimates and judgments are required in the calculation of certain tax liabilities and in the determination of the recoverability of certain deferred income tax assets, which arise from temporary differences and carryforwards. Deferred income tax assets and liabilities are measured using the currently enacted tax rates that apply to taxable income in effect for the years in which those tax assets and liabilities are expected to be realized or settled.
The Company regularly assesses the likelihood that its deferred income tax assets will be realized from recoverable income taxes or recovered from future taxable income. To the extent that the Company believes any amounts are not “more likely than not” to be realized, the Company records a valuation allowance to reduce the deferred income tax assets. In the event the Company determines that all or part of the net deferred tax assets are not realizable in the future, an adjustment to the valuation allowance would be charged to earnings in the period such determination is made. Similarly, if the Company subsequently determines deferred income tax assets that were previously determined to be unrealizable are now realizable, the respective valuation allowance would be reversed, resulting in an adjustment to earnings in the period such determination is made.
Internal Revenue Code Sections 382 and 383 (“Sections 382 and 383”) limit the use of net operating loss (“NOL”) carryforwards and R&D credits, after an ownership change. To date, the Company has not conducted a Section 382 or 383 study, however, because the Company will continue to raise significant amounts of equity in the coming years, the Company expects that Sections 382 and 383 will limit the Company’s usage of NOLs and R&D credits in the future.
ASC 740, Income Taxes, requires that the tax benefit of net operating losses, temporary differences, and credit carryforwards be recorded as an asset to the extent that management assesses that realization is “more likely than not.” Realization of the future tax benefits is dependent on the Company’s ability to generate sufficient taxable income within the carryforward period. The Company has reviewed the positive and negative evidence relating to the realizability of the deferred tax assets and has concluded that the deferred tax assets are not “more likely than not” to be realized. As a result, the Company recorded a full valuation allowance as of September 30, 2023 and December 31, 2022. U.S. Federal R&D tax credits from 2016 to 2019 were utilized to reduce payroll taxes in future periods and were recorded as other current assets (anticipated to be received within 12 months), on the Company’s condensed consolidated balance sheets. The Company intends to maintain the valuation allowance until sufficient evidence exists to support its reversal. The Company regularly reviews its tax positions. For a tax benefit to be recognized, the related tax position must be “more likely than not” to be sustained upon examination. Any amount recognized is generally the largest benefit that is “more likely than not” to be realized upon settlement. The Company’s policy is to recognize interest and penalties related to income tax matters as an income tax expense. For the three and nine months ended September 30, 2023 and 2022, the Company did not have any interest or penalties associated with unrecognized tax benefits.
The Company is subject to U.S. Federal, Illinois and California state income taxes. In addition, the Company is subject to local tax laws of France and Australia. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. Monopar was originally formed as an LLC in December 2014, then incorporated on December 16, 2015. The Company is subject to U.S. Federal, state and local tax examinations by tax authorities for the tax years 2015 through 2022. The Company does not anticipate significant changes to its current uncertain tax positions through September 30, 2023.
Stock-Based Compensation
The Company accounts for stock-based compensation arrangements with employees, non-employee directors and consultants using a fair value method, which requires the recognition of compensation expense for costs related to all stock-based awards, including stock option and restricted stock unit (“RSU”) grants. The fair value method requires the Company to estimate the fair value of stock-based payment awards on the date of grant using an option pricing model or the closing stock price on the date of grant in the case of RSUs.
Stock-based compensation expense for awards granted to employees, non-employee directors and consultants are based on the fair value of the underlying instrument calculated using the Black-Scholes option-pricing model on the date of grant for stock options and using the closing stock price on the date of grant for RSUs and recognized as expense on a straight-line basis over the requisite service period, which is the vesting period. Determining the appropriate fair value model and related assumptions requires judgment, including estimating the future stock price volatility and expected terms. The expected volatility rates are estimated based on the Company’s historical actual volatility over the two-year period from its initial public offering on December 18, 2019 through December 31, 2021 for stock-based awards granted in 2022. For awards granted during the nine months ended September 30, 2023, the expected volatility rates were estimated based on the Company’s historical actual volatility over the three-year period from its initial public offering on December 18, 2019, through December 31, 2022. The expected term for options granted to date is estimated using the simplified method. Forfeitures only include known forfeitures to-date as the Company accounts for forfeitures as they occur due to a limited history of forfeitures. The Company has not paid dividends and does not anticipate paying a cash dividend in the future vesting period and, accordingly, uses an expected dividend yield of zero. The risk-free interest rate is based on the rate of U.S. Treasury securities with maturities consistent with the estimated expected term of the awards.
Note 3 - Investments
As of September 30, 2023, the Company had two money market accounts recorded in cash and cash equivalents and available-for-sale investments with contractual maturities of one year or less as follows:
| As of September 30, 2023 |
Cost Basis |
Unrealized Gains |
Aggregate Fair Value |
|||||||||
| U.S. Treasury Bills |
$ | 4,944,340 | $ | 18,300 | $ | 4,962,640 | ||||||
| Money Market Accounts - Cash Equivalents |
2,687,463 | — | 2,687,463 | |||||||||
| Total |
$ | 7,631,803 | $ | 18,300 | $ | 7,650,103 | ||||||
As of September 30, 2023, there were no available-for-sale securities in an unrealized-loss position. U.S. Treasury Bills classified as Investments on the condensed consolidated balance sheet as of September 30, 2023 were $3.0 million.
As of December 31, 2022 the Company had two money market accounts recorded in cash and cash equivalents and available-for-sale investments with contractual maturities of one year or less as follows:
| As of December 31, 2022 |
Cost Basis |
Unrealized Gains |
Aggregate Fair Value |
|||||||||
| U.S. Treasury Bills |
$ | 6,905,171 | $ | 15,039 | $ | 6,920,210 | ||||||
| Money Market Accounts - Cash Equivalents |
5,262,286 | — | 5,262,286 | |||||||||
| Total |
$ | 12,167,457 | $ | 15,039 | $ | 12,182,496 | ||||||
As of December 31, 2022, there were no available-for-sale securities in an unrealized-loss position and there were no sales of available-for-sale securities made during 2022. U.S. Treasury Bills classified as Investments on the condensed consolidated balance sheet as of December 31, 2022 were $4.9 million.
See Note 2 for additional discussion regarding the Company’s fair value measurements.
Note 4 - Capital Stock
Holders of the common stock are entitled to receive such dividends as may be declared by the Board of Directors out of funds legally available therefor. To date no dividends have been declared. Upon dissolution and liquidation of the Company, holders of the common stock are entitled to a ratable share of the net assets of the Company remaining after payments to creditors of the Company. The holders of shares of common stock are entitled to one vote per share for the election of each director nominated to the Board and one vote per share on all other matters submitted to a vote of stockholders.
The Company’s amended and restated certificate of incorporation authorizes the Company to issue 40,000,000 shares of common stock with a par value of $0.001 per share.
Sales of Common Stock
On April 20, 2022, the Company entered into a Capital on Demand™ Sales Agreement with JonesTrading Institutional Services LLC (“JonesTrading”), pursuant to which Monopar may offer and sell, from time to time, through or to JonesTrading, as sales agent or principal, shares of Monopar’s common stock. On April 20, 2022, the Company filed a prospectus supplement with the U.S. Securities and Exchange Commission relating to the offer and sale of its common stock from time to time pursuant to the agreement up to an aggregate amount of $4,870,000. In addition, the Company filed a new Form S-3, which included therein a prospectus to increase the aggregate amount under this agreement to $6,505,642. The Form S-3 was declared effective by the Securities and Exchange Commission on January 4, 2023, at which time the prospectus included therein replaced the prior prospectus supplement. Expenses related to these financing activities were recorded as offering costs (a reduction of additional paid in capital) on the Company’s condensed consolidated statement of stockholders’ equity for the period. During the nine months ended September 30, 2023, the Company sold 1,125,479 shares of its common stock at an average gross price per share of $1.60 for net proceeds of $1,760,197, after fees and commissions of $45,161. In addition, the Company incurred and accrued legal, accounting and other fees totaling $27,241 for net proceeds after fees, commissions and expenses of $1,732,956. During the nine months ended September 30, 2022, the Company did not sell any shares of common stock.
As of September 30, 2023, the Company had 14,198,438 shares of common stock issued and outstanding.
Note 5 - Stock Incentive Plan
In April 2016, the Company’s Board of Directors and stockholders representing a majority of the Company’s outstanding stock at that time, approved the Monopar Therapeutics Inc. 2016 Stock Incentive Plan, as amended (the “Plan”), allowing the Company to grant up to an aggregate 700,000 shares of stock-based awards in the form of stock options, restricted stock units, stock appreciation rights and other stock-based awards to employees, non-employee directors and consultants. In October 2017, the Company’s Board of Directors voted to increase the stock award pool to 1,600,000 shares of common stock, which subsequently was approved by the Company’s stockholders. In April 2020, the Company’s Board of Directors voted to increase the stock award pool to 3,100,000 (an increase of 1,500,000 shares of common stock), which was approved by the Company’s stockholders in June 2020. In April 2021, the Company’s Board of Directors voted to approve an amendment to the 2016 Stock Incentive Plan to remove certain individual award limits and other provisions related to I.R.C. Section 162(m) and to update the limit on Incentive Stock Options to no more that 100% of the maximum aggregate number of shares which may be granted under the plan, which was approved by the Company’s stockholders in June 2021. In March 2022, the Company’s Board of Directors voted to increase the stock award pool to 5,100,000 (an increase of 2,000,000 shares of common stock), which was approved by the Company’s stockholders in June 2022.
There were no stock awards granted during the three months ended September 30, 2023.
Under the Plan, the per share exercise price for the shares to be issued upon exercise of an option shall be determined by the Plan Administrator, except that the per share exercise price shall be no less than 100% of the fair market value per share on the grant date. Fair market value is the Company’s closing price on the grant date on Nasdaq. Stock options generally expire after 10 years.
Stock option activity under the Plan was as follows:
| Options Outstanding |
||||||||
| Number of Shares Subject to Options |
Weighted-Average Exercise Price |
|||||||
| Balances at December 31, 2022 |
1,642,950 | 4.28 | ||||||
| Granted(1) |
508,902 | 3.14 | ||||||
| Forfeited(2) |
(42,851 | ) | 3.93 | |||||
| Balances at September 30, 2023 |
2,109,001 | 4.01 | ||||||
| Unvested options outstanding expected to vest(3) |
685,120 | 3.41 | ||||||
| (1) |
508,902 options vest as follows: options to purchase 443,182 shares of the Company’s common stock vest 6/48ths on the six-month anniversary of grant date and 1/48th per month thereafter; options to purchase 55,720 shares of the Company’s common stock vest quarterly over one year; and options to purchase 10,000 shares of the Company’s common stock vest monthly over one year. |
|
|
|
| (2) |
Forfeited options represent unvested shares and vested, unexercised and expired shares related to employee terminations. |
|
|
|
| (3) |
Forfeitures only include known forfeitures to-date as the Company accounts for forfeitures as they occur due to a limited history of forfeitures. |
A summary of options outstanding as of September 30, 2023, is shown below:
| Exercise Prices |
Number of Shares Subject to Options Outstanding |
Weighted-Average Remaining Contractual Term in Years |
Number of Shares Subject to Options Fully Vested and Exercisable |
Weighted-Average Remaining Contractual Term in Years |
||||||||||||
| $0.001 - $5.00 |
1,371,895 | 7.21 | 762,978 | 5.79 | ||||||||||||
| $5.01 - $10.00 |
617,942 | 5.75 | 543,017 | 5.51 | ||||||||||||
| $10.01 - $15.00 |
113,039 | 6.34 | 111,761 | 6.34 | ||||||||||||
| $15.01 - $20.00 |
6,125 | 6.34 | 6,125 | 6.34 | ||||||||||||
| 2,109,001 | 6.73 | 1,423,881 | 5.73 | |||||||||||||
Restricted stock unit activity under the Plan was as follows:
| Weighted- Average |
||||||||
| Restricted |
Grant Date |
|||||||
| Stock Units |
Fair Value |
|||||||
| (#) |
per Unit ($) |
|||||||
| Unvested balance at December 31, 2022 |
272,650 | 4.00 | ||||||
| Granted |
368,345 | 3.16 | ||||||
| Vested |
(169,167 | ) | 3.84 | |||||
| Unvested Balance at September 30, 2023 |
471,828 | 3.83 | ||||||
Stock option grants and fair values under the Plan were as follows:
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2023 |
2022 |
2023 |
2022 |
|||||||||||||
| Stock options granted |
— | 4,000 | 508,902 | 582,064 | ||||||||||||
| Fair value of shares vested |
$ | 226,054 | $ | 205,397 | $ | 754,414 | $ | 757,976 | ||||||||
As of September 30, 2023, the aggregate intrinsic value of outstanding vested stock options was approximately $0.2 million (there were no unvested stock options that had intrinsic value) and the weighted-average exercise price in aggregate was $4.01 which includes $4.30 for fully vested stock options and $3.41 for stock options expected to vest. As of September 30, 2023, unamortized unvested balance of stock-based compensation was $3.1 million, to be amortized over the following 2.6 years.
During the three months ended September 30, 2023 and 2022, the Company recognized $256,670 and $ 204,360 of employee, non-employee director and consultant stock-based compensation expense as general and administrative expenses, respectively, and $217,468 and $186,382 as research and development expenses, respectively. During the nine months ended September 30, 2023 and 2022, the Company recognized $757,303 and $613,525 of employee, non-employee director and consultant stock-based compensation expense as general and administrative expenses, respectively, and $666,340 and $634,321 as research and development expenses, respectively. The stock-based compensation expense is allocated on a departmental basis, based on the classification of the stock-based award holder. No income tax benefits have been recognized in the condensed consolidated statements of operations and comprehensive loss for stock-based compensation arrangements.
Note 6 - Related Party Transactions
As of September 30, 2023, Tactic Pharma, LLC (“Tactic Pharma”), the Company’s initial investor, beneficially owned 30.1% of Monopar’s common stock and during the three and nine months ended September 30, 2023, there were no transactions between Tactic Pharma and Monopar.
None of the related parties discussed in this paragraph received compensation other than market-based salary, market-based stock-based compensation and benefits and performance-based incentive bonus or in the case of non-employee directors, market-rate Board fees and market-rate stock-based compensation. The Company considers the following individuals as related parties: Two of the Company’s board members were also Managing Members of Tactic Pharma as of September 30, 2023. Chandler D. Robinson is a Company Co-Founder, Chief Executive Officer, common stockholder, Managing Member of Tactic Pharma, former Manager of the predecessor LLC, Manager of CDR Pharma, LLC and Board member of Monopar as a C Corporation. Michael Brown is a Managing Member of Tactic Pharma (as of February 1, 2019, with no voting power as it relates to Monopar), a previous managing member of Monopar as an LLC, common stockholder and Board member of Monopar as a C Corporation.
Note 7 – Commitments and Contingencies
License, Development and Collaboration Agreements
Onxeo S.A.
In June 2016, the Company executed an option and license agreement with Onxeo S.A. (“Onxeo”), a public French company, which gave Monopar the exclusive option to license (on a world-wide exclusive basis) Validive to pursue treating severe oral mucositis in patients undergoing chemoradiation treatment for head and neck cancers. The pre-negotiated Onxeo license agreement for Validive as part of the option agreement includes clinical, regulatory, developmental and sales milestones and escalating royalties on net sales. On September 8, 2017, the Company exercised the license option, and therefore paid Onxeo the $1 million fee under the option and license agreement.
On March 27, 2023, Monopar announced the discontinuation of its Validive Phase 2b/3 VOICE trial based upon the Data Safety Monitoring Board (“DSMB”) determination that the trial did not meet the pre-defined threshold for efficacy of a 15% absolute difference in severe oral mucositis prevention between Validive and placebo. The Company is planning to terminate the license and does not anticipate any future license or royalty obligations under the Onxeo license agreement.
Grupo Español de Investigación en Sarcomas (“GEIS”)
In June 2019, the Company executed a clinical collaboration agreement with GEIS for the development of camsirubicin in patients with advanced soft tissue sarcoma (“ASTS”). Following completion of the Company’s Phase 1b clinical trial in the U.S. that Monopar initiated in the third quarter of 2021 with the first patient dosed in October 2021, the Company continues to expect that GEIS will sponsor and lead a multi-country, randomized, open-label Phase 2 clinical trial to evaluate camsirubicin head-to-head against doxorubicin, the current first-line treatment for ASTS. The Company will provide study drug and supplemental financial support for the clinical trial. During the three and nine months ended September 30, 2023 and September 30, 2022, no expenses were incurred under the GEIS agreement. The Company can terminate the agreement by providing GEIS with advance notice, and without affecting the Company’s rights and ownership to any related intellectual property or clinical data. In the second quarter of 2021, due to regulatory delays in Spain, Monopar decided to conduct an open-label Phase 1b clinical trial of camsirubicin in the U.S., therefore no expenses were incurred related to the GEIS collaboration beyond March 31, 2021.
XOMA Ltd.
The intellectual property rights contributed by Tactic Pharma to the Company included the non-exclusive license agreement with XOMA Ltd. for the humanization technology used in the development of MNPR-101. Pursuant to such license agreement, the Company is obligated to pay XOMA Ltd. clinical, regulatory and sales milestones for MNPR-101 that could reach up to $14.925 million if the Company achieves all milestones. The agreement does not require the payment of sales royalties. There can be no assurance that the Company will reach any milestones under the XOMA agreement. As of September 30, 2023, the Company had not reached any milestones and has not been required to pay XOMA Ltd. any funds under this license agreement.
Legal Contingencies
The Company may be subject to claims and assessments from time to time in the ordinary course of business. No claims have been asserted to date.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnification. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but that have not yet been made. To date, the Company has not paid any claims nor been required to defend any action related to its indemnification obligations. However, the Company may record charges in the future as a result of future claims against these indemnification obligations.
In accordance with its second amended and restated certificate of incorporation, amended and restated bylaws and the indemnification agreements entered into with each officer and non-employee director, the Company has indemnification obligations to its officers and non-employee directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacities. There have been no indemnification claims to date.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our condensed consolidated financial statements and related notes contained in this Quarterly Report on Form 10-Q. Some of the information contained in this discussion and analysis are set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business and related financing activities, includes forward-looking statements that involve risks and uncertainties.
Overview
We are a clinical stage biopharmaceutical company focused on developing innovative treatments for cancer patients. We are building a drug development pipeline through the licensing and acquisition of therapeutics in late preclinical or in clinical development stages. We leverage our scientific and clinical experience to help reduce the risk of and accelerate the clinical development of our drug product candidates.
Financial Status
Our cash, cash equivalents and investments as of September 30, 2023, were $8.5 million. As discussed further below and elsewhere in this Quarterly Report, we expect that our current funds will be sufficient at least through November 2024 for us to obtain topline results from our ongoing open-label Phase 1b camsirubicin clinical trial by mid-2024 (but as discussed below, this may not be the case if camsirubicin reaches even higher dose levels than we are anticipating and topline results are deferred as dosing continues beyond mid-2024), advance our MNPR-101 radiopharmaceutical program into its first-in-human clinical trial, and close out our terminated Validive Phase 2b/3 (VOICE) clinical program. We will require additional funding to further advance our clinical and preclinical programs and we anticipate that we will seek to raise additional capital within the next 12 months to fund our future operations.
Our primary funding source over the past three years was sales of shares of our common stock under at-the-market sales programs through Capital on Demand™ Sales Agreements with JonesTrading Institutional Services LLC (“Jones Trading”). For the nine months ended September 30, 2023, we sold 1,125,479 shares of our common stock at an average gross price per share of $1.60 for net proceeds of $1,760,197, after fees and commissions of $45,161. In addition, we incurred and accrued legal, accounting and other fees totaling $27,241 for net proceeds after fees, commissions and expenses of $1,732,956.
Discontinuation of Validive Development
On March 27, 2023, we announced the completion of a pre-specified interim analysis for our Validive Phase 2b/3 VOICE trial for the prevention of severe oral mucositis (“SOM”) in patients undergoing chemoradiotherapy (“CRT”) for oropharyngeal cancer (“OPC”). The interim analysis included the first approximately 50% of the total planned patients to be enrolled. It was conducted by an independent Data Safety Monitoring Board (“DSMB”), which informed us that the trial did not meet the pre-defined threshold for efficacy of a 15% absolute difference in SOM prevention between Validive and placebo. The DSMB also reported that there were no safety concerns attributed to Validive. Based on not meeting the pre-specified efficacy threshold, in March 2023 we terminated the study and discontinued the development of Validive.
Camsirubicin Clinical Update
The Phase 1b open-label, dose-escalating clinical trial of camsirubicin in patients with advanced soft tissue sarcoma (“ASTS”) is currently ongoing and is in the fifth dose-level cohort (650 mg/m2), which is nearly 2.5x the highest dose evaluated in any prior camsirubicin clinical trial (265 mg/m2). We have dosed two patients in the fifth dose cohort so far, and both experienced reductions in their tumor dimensions, one of approximately 18% and the other of approximately 20%.
To date, 9 out of the 14 patients that have been enrolled into the Phase 1b trial have achieved stable disease (“SD”, as defined by RECIST 1.1 criteria) after camsirubicin treatment, including all patients in the fourth (520 mg/m2) and fifth dose cohorts. One patient in the fourth dose cohort went from having what was initially determined to be an unresectable cancer to, after several cycles of camsirubicin treatment and a corresponding 21% reduction in tumor dimensions, being determined to be resectable. This changed the course of treatment for this patient, who subsequently underwent successful surgical resection of the cancer with clear margins. The Phase 1b data to date show an improvement in median progression free survival from what was observed in the prior camsirubicin Phase 2 trial (265 mg/m2). No dose-limiting toxicity, as defined in the protocol, has been observed in the Phase 1b trial to-date. A medically complex patient in the fifth dose cohort has an ongoing left ventricular ejection fraction (“LVEF”) decrease and is being assessed for potential anthracycline (camsirubicin) induced cardiotoxicity. This patient has a BMI of 42.5, one kidney, hypertension, a long-standing heart murmur, and a maternal history of heart failure.
MNPR-101 for Radiopharmaceutical Use, Development Update
Pursuant to our 50/50 cost-sharing collaboration agreement with NorthStar Medical Radioisotopes, LLC (“NorthStar”) to develop potential radioimmunotherapeutics based on MNPR-101 (“MNPR-101 RITs”), we have coupled MNPR-101, a highly selective antibody against the urokinase plasminogen activator receptor ("uPAR"), to imaging and therapeutic radioisotopes. The resulting conjugates, MNPR-101-Zr and MNPR-101-PCTA-Ac225, are designed to be highly selective agents that have the potential to image and kill certain cancer cells. By eradicating these cancer cells with a uPAR-targeted RIT (“uPRIT”), the therapeutic goal is to spare healthy cells while quickly killing the cancer cells.
Based on promising preclinical imaging results with MNPR-101-Zr showing high and tumor-specific uptake across multiple tumor types, and with preclinical therapeutic efficacy and biodistribution studies utilizing the radioisotopes actinium-225 (“Ac-225”) and lutetium-177 (“Lu-177”), we and NorthStar committed to additional funding with the aim of initiating a first-in-human imaging study with MNPR-101-Zr as early as end of this year. MNPR-101-Zr is a zirconium-89 labeled version of MNPR-101. Positron emission tomography (“PET”) imaging of preclinical mouse models for triple-negative breast, colorectal, and pancreatic tumors displayed high, selective, and durable uptake of MNPR-101-Zr in these uPAR-expressing tumors. Additionally, preclinical triple-negative breast and pancreatic cancer mouse model studies with Ac-225 radiolabeled MNPR-101 have shown a promising dose-dependent-anti-cancer-effect. Favorable biodistribution profiles have been shown for Ac-225 and Lu-177 labeled MNPR-101. These proof-of-concept studies provide support for a first-in-human PET imaging study with MNPR-101-Zr and a future therapeutic study using Ac-225 or Lu-177 labeled MNPR-101 RIT. Overall, the imaging and therapeutic results demonstrate the potential utility of MNPR-101 as a precision targeting agent for both imaging and treatment in multiple cancer indications.
In July 2023, we announced a collaboration with the Cancer Science Institute of Singapore (“CSI Singapore”), one of Asia's premier cancer research centers, at the National University of Singapore (“NUS”) (consistently ranked as one of the world's top universities) to evaluate radiopharmaceutical versions of MNPR-101 in several aggressive cancers. Dr. Anand Jeyasekharan, MBBS MRCP (UK) PhD, of CSI Singapore, NUS, will be the Principal Investigator on the collaboration. Dr. Jeyasekharan is a physician-scientist who runs a research laboratory investigating the molecular and biological responses of cancer cells to oncology drugs, as well as treats cancer patients and leads early phase oncology clinical trials at NUS. In this collaboration, Dr. Jeyasekharan will initially investigate uPAR expression levels in tissue samples from patients with various subtypes of ASTS. Studies have shown uPAR to be a promising target for ASTS, which is a cancer Dr. Jeyasekharan specializes in treating. He plans to assess retrospective patient samples to identify which subtypes of ASTS have the highest expression of uPAR, thus making them the most promising to pursue in a human clinical trial.
MNPR-202 and Related Analogs Updates
In June 2021, we entered into a collaboration agreement with CSI Singapore, at NUS to evaluate the activity of MNPR-202 and related analogs in multiple types of cancer. MNPR-202 was designed to retain the same potentially non-cardiotoxic backbone as camsirubicin but is modified at other positions which may enable it to work in certain cancers that are resistant to camsirubicin and doxorubicin. In collaboration with Dr. Anand Jeyasekharan of CSI Singapore, we presented an abstract and poster of the preclinical data of MNPR-202 at the American Society of Hematology 64th Annual Meeting in New Orleans, LA. The poster presented the following promising data about MNPR-202:
| - |
has a similar cytotoxic potency to doxorubicin |
|
| - |
generates increased DNA damage in the cancer cells compared to doxorubicin |
|
| - |
has a unique immune activation profile versus doxorubicin |
|
| - |
demonstrates increased apoptosis (programmed cell death) compared to doxorubicin |
|
| - |
causes a distinct set of genes to be upregulated and downregulated versus doxorubicin and |
|
| - |
may also be superior to doxorubicin in certain combination treatment regimens. |
A combination drug screen with 183 compounds was performed, revealing distinct differences in the synergy profile between doxorubicin versus MNPR-202 when used along with other compounds. For example, MNPR-202 demonstrated a more favorable synergy profile with the experimental anti-cancer agent volasertib compared to doxorubicin. In a preclinical study using a human iPSC cardiomyocyte model to evaluate heart toxicity, cells treated with MNPR-202 maintained a greater contractile amplitude and a more consistent and regular beat rate relative to doxorubicin-treated cells, indicating that MNPR-202 has a broader therapeutic window than doxorubicin with respect to cardiotoxicity.
Our Product Pipeline
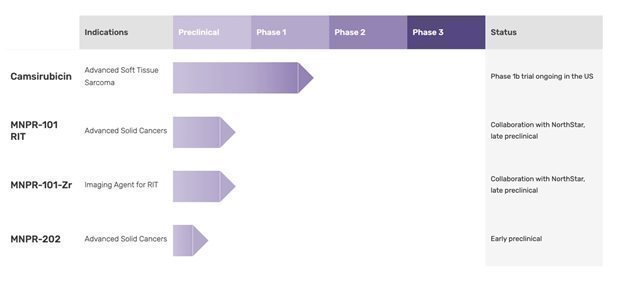
Our Strategy
Our management team has extensive experience in developing therapeutics and medical technologies through global regulatory approval and commercialization. In aggregate, companies they co-founded have achieved four drug approvals and three diagnostic medical imaging device approvals in the U.S. and the EU, successfully sold an asset developed by management which went on to have a positive Phase 3 clinical trial, sold two oncology-focused diagnostic imaging businesses to Fortune Global 1000 firms, and completed the clinical and commercial development and ultimately the sale of a commercial biopharmaceutical company for over $800 million in cash. In addition, the team has supported multiple regulatory submissions with the FDA and the European Medicines Agency (“EMA”) and launched multiple drugs in the U.S and the EU. Understanding the preclinical, clinical, regulatory and commercial development processes and hurdles are key factors in successful drug development and the expertise demonstrated by our management team across all of these areas increases the probability of success in advancing the product candidates in our product pipeline. Our strategic goal is to acquire, develop and commercialize promising oncology product candidates that address important unmet medical needs of cancer patients. Five key elements of our strategy to achieve this goal are to:
| ● |
Advance the clinical development of camsirubicin, by pursuing indications where doxorubicin has demonstrated efficacy. ASTS will be the first indication, which is anticipated to allow camsirubicin to go head-to-head against doxorubicin, the current first-line treatment. In this indication, camsirubicin previously demonstrated clinical benefit (stable disease or partial response) in 52.6% of patients evaluable for tumor progression in a single-arm Phase 2 study. Clinical benefit was proportional to dose and was consistently observed at higher cumulative doses of camsirubicin (>1000 mg/m2 cumulative dose). Our current ongoing Phase 1b clinical trial continues towards establishing a new, higher recommended dose for the next Phase 2 ASTS clinical trial. |
|
|
|
| ● |
Continue the development of MNPR-101 for radiopharmaceutical use as a therapeutic as well as a diagnostic imaging agent. We have prioritized our development of radiopharmaceuticals based on promising data from our imaging and efficacy animal model studies in multiple cancers including triple-negative breast and pancreatic cancers. Based on promising preclinical imaging and therapeutic results utilizing radiolabeled MNPR-101, we aim to initiate a first-in-human imaging study with MNPR-101-Zr as early as the end of this year. |
|
|
|
| ● |
Continue the development of MNPR-202 and related analogs in multiple types of cancers. The 2-pyrrilino camsirubicin analog (MNPR-202) and related analogs represent proprietary compositions of matter designed to retain the non-cardiotoxic backbone of camsirubicin yet exhibit novel features in terms of antitumor activity and mechanism that distinguish these analogs from camsirubicin as well as from doxorubicin, potentially addressing camsirubicin- and doxorubicin-resistant cancers. |
|
|
|
| ● |
Expand our drug development pipeline through advancing current assets, in-licensing, and acquisition of oncology product candidates. We plan to continue the expansion of our drug development pipeline through acquiring or in-licensing additional oncology product candidates, particularly those that leverage existing scientific and clinical data that helps reduce the risks of the next steps in clinical development. To be executed, such expansion will require additional funds. |
|
|
|
| ● |
Utilize the expertise and prior experience of our team in the areas of asset acquisition, drug development and commercialization to establish ourselves as a leading biopharmaceutical company. Our senior executive team has relevant experience in biopharmaceutical in-licensing and acquisitions as well as developing product candidates through approval and commercialization. In aggregate, our team has co-founded BioMarin Pharmaceutical (Nasdaq: BMRN), Sensant Corp (acquired by Siemens), American BioOptics (assets acquired by Olympus), Raptor Pharmaceuticals ($800 million sale to Horizon Therapeutics), Wilson Therapeutics (acquired by Alexion in June 2018 for $764 million; Alexion was subsequently acquired by AstraZeneca) and Tactic Pharma, LLC (“Tactic Pharma”). |
Revenues
We are an emerging growth company. We have no approved drugs and have not generated any revenues. To date, we have engaged in acquiring or in-licensing drug product candidates, entering into collaboration agreements for testing and clinical development of our drug product candidates and providing the infrastructure to support the clinical development of our drug product candidates. We do not anticipate commercial revenues from operations until we complete testing and development of one of our drug product candidates and obtain marketing approval or we sell, enter into a collaborative marketing arrangement, or out-license one of our drug product candidates to another party. See “Liquidity and Capital Resources”.
Recently Issued and Adopted Accounting Pronouncements
During the three months ended September 30, 2023, there were no relevant recently issued accounting pronouncements that would impact our financial position and our condensed consolidated statements of operations and comprehensive loss or cashflows.
Critical Accounting Policies and Use of Estimates
While our significant accounting policies are described in more detail in Note 2 of our condensed consolidated financial statements included elsewhere in this Quarterly Report on Form 10-Q, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our condensed consolidated financial statements.
Going Concern Assessment
We apply Accounting Standards Codification 205-40 ("ASC 205-40"), Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which the Financial Accounting Standards Board ("FASB") issued to provide guidance on determining when and how reporting companies must disclose going concern uncertainties in their financial statements. ASC-205-40 requires management to perform interim and annual assessments of an entity's ability to continue as a going concern within one year of the date of issuance of the entity's financial statements (or within one year after the date on which the financial statements are available to be issued when applicable). Further, a company must provide certain disclosures if there is "substantial doubt about the entity's ability to continue as a going concern." In September 2023, we analyzed our cash requirements at least through November 2024 and we have determined that, based upon our current available cash, we have no substantial doubt about our ability to continue as a going concern.
Clinical Trials Accruals
We accrue and expense the costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations, service providers, and clinical trial sites. We estimate the amounts to accrue based upon discussions with internal clinical personnel and external service providers as to progress or stage of completion of trials or services and the agreed upon fees to be paid for such services. Costs of setting up clinical trial sites for participation in the trials are expensed immediately as research and development expenses. Clinical trial site costs related to patient screening and enrollment are accrued as patients are screened/entered into the trial.
Stock-Based Compensation
We account for stock-based compensation arrangements with employees, non-employee directors and consultants using a fair value method, which requires the recognition of compensation expense for costs related to all stock-based compensation grants, including stock option and restricted stock unit (“RSU”) grants. The fair value method requires us to estimate the fair value of stock-based payment awards on the date of grant using an option pricing model or the closing stock price on the date of grant in the case of RSUs.
Stock-based compensation costs for stock awards granted to our employees, non-employee directors and consultants are based on the fair value of the underlying instruments calculated using the Black-Scholes option-pricing model on the date of grant for stock options and using the closing stock price on the date of grant for RSUs and recognized as expense on a straight-line basis over the requisite service period, which is the vesting period. Determining the appropriate fair value model and related assumptions requires judgment, including selecting methods for estimating our future stock price volatility and expected holding term. The expected volatility rates are estimated based on our actual historical volatility over the two-year period from our initial public offering on December 18, 2019, through December 31, 2021 for stock-based awards granted in 2022. The Company did not grant any stock awards during the three months ended September 30, 2023. For awards granted during the nine months ended September 30, 2023, the expected volatility rates are estimated based on our actual historical volatility over the three-year period from our initial public offering on December 18, 2019, through December 31, 2022. The expected term for stock options granted during the three and nine months ended September 30, 2023, and 2022, was estimated using the simplified method. Forfeitures only include actual forfeitures to-date as the Company accounts for forfeitures as they occur due to a limited history of forfeitures. We have not paid dividends and do not anticipate paying a cash dividend in future vesting periods and, accordingly, use an expected dividend yield of zero. The risk-free interest rate is based on the rate of U.S. Treasury securities with maturities consistent with the estimated expected term of the awards.
Results of Operations
Comparison of the Three and Nine Months Ended September 30, 2023 and 2022
The following table summarizes the results of our operations for the three and nine months ended September 30, 2023 and 2022:
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||||||||||
| (Unaudited) |
(Unaudited) |
|||||||||||||||||||||||
| (in thousands) |
2023 |
2022 |
Variance |
2023 |
2022 |
Variance |
||||||||||||||||||
| Research and development expenses |
$ | 1,317 | $ | 1,732 | $ | (415 | ) | $ | 4,564 | $ | 5,489 | $ | (925 | ) | ||||||||||
| General and administrative expenses |
749 | 675 | 74 | 2,355 | 2,139 | 216 | ||||||||||||||||||
| Total operating expenses |
2,066 | 2,407 | (341 | ) | 6,919 | 7,628 | (709 | ) | ||||||||||||||||
| Operating loss |
(2,066 | ) | (2,407 | ) | 341 | (6,919 | ) | (7,628 | ) | 709 | ||||||||||||||
| Interest income |
112 | 8 | 104 | 331 | 9 | 322 | ||||||||||||||||||
| Net loss |
$ | (1,954 | ) | $ | (2,399 | ) | $ | 445 | $ | (6,588 | ) | $ | (7,619 | ) | $ | 1,031 | ||||||||
Research and Development (“R&D”) Expenses
R&D expenses for the three months ended September 30, 2023 were $1,317,000, compared to $1,732,000 for the three months ended September 30, 2022. This represents a decrease of $415,000 attributed to a decrease of $673,000 in Validive clinical trial-related and clinical material manufacturing-related expenses offset by (1) an increase of $161,000 in non-clinical studies related to MNPR-101 uPRIT activity, (2) an increase of $78,000 in camsirubicin clinical trial expenses including patient dosing and manufacturing-related expenses, and (3) a net increase of $19,000 due to other R&D expenses.
R&D expenses for the nine months ended September 30, 2023 were $4,564,000, compared to $5,489,000 for the nine months ended September 30, 2022. This represents a decrease of $925,000 attributed to (1) a decrease of $688,000 in camsirubicin clinical trial expenses including patient dosing and manufacturing-related expenses, (2) a decrease of $503,000 in Validive clinical trial-related and clinical material manufacturing-related expenses, and (3) a net decrease of $13,000 in other R&D expenses offset by an increase of $279,000 in non-clinical studies related to MNPR-101 uPRIT activity.
General and Administrative (“G&A”) Expenses
G&A expenses for the three months ended September 30, 2023 were $749,000, compared to $675,000 for the three months ended September 30, 2022. This represents an increase of $74,000 primarily attributed to an increase in G&A salaries and benefits.
G&A expenses for the nine months ended September 30, 2023 were $2,355,000, compared to $2,139,000 for the nine months ended September 30, 2022. This represents an increase of $216,000 primarily attributed to an increase in G&A salaries and benefits.
Interest Income
Interest income for the three months ended September 30, 2023, increased by $104,000 versus the three months ended September 30, 2022, due to interest earned on treasury bills and money market accounts that yielded higher interest rates.
Interest income for the nine months ended September 30, 2023, increased by $322,000 versus the nine months ended September 30, 2022, due to interest earned on treasury bills and money market accounts that yielded higher interest rates.
Liquidity and Capital Resources
Sources of Liquidity
We have incurred losses and cumulative negative cash flows from operations since we commenced operations resulting in an accumulated deficit of approximately $58.4 million as of September 30, 2023. We anticipate that we will continue to incur losses for the foreseeable future. We expect that our R&D and G&A expenses will increase to enable the execution of our strategic plan. As a result, we anticipate that we will seek to raise additional capital within the next 12 months to fund our future operations. We will seek to obtain needed capital through a combination of equity offerings, including the usage of our Capital on DemandTM Sales Agreement with JonesTrading, debt financings, strategic collaborations and grant funding. To date, we have funded our operations through net proceeds from the initial public offering of our common stock, net proceeds from sales of our common stock through at-the-market sales programs, private placements of our preferred and common stock, and the net receipt of funds related to our acquisition of camsirubicin. We anticipate that the currently available funds as of October 27, 2023, will fund our planned operations at least through November 2024.
We invest our cash equivalents in two money market accounts and U.S. Treasury Bills.
Cash Flows
The following table provides information regarding our cash flows for the nine months ended September 30, 2023 and 2022.
| Nine Months Ended September 30, |
||||||||||||
| (Unaudited) |
||||||||||||
| (in thousands) |
2023 |
2022 |
Variance |
|||||||||
| Net cash used in operating activities |
$ | (6,312 | ) | $ | (5,927 | ) | $ | (385 | ) | |||
| Net cash provided by investing activities |
1,957 | — | 1,957 | |||||||||
| Net cash provided by (used in) financing activities |
1,692 | (106 | ) | 1,798 | ||||||||
| Effect of exchange rates |
(8 | ) | 45 | (53 | ) | |||||||
| Net decrease in cash and cash equivalents |
$ | (2,671 | ) | $ | (5,988 | ) | $ | 3,317 | ||||
In December 2022, the Company began investing its idle cash due to the rising interest rates. During the nine months ended September 30, 2023 and 2022 we had net cash outflows of $2,671,000 and $5,988,000, respectively, an outflow decrease of $3,317,000. During the nine months ended September 30, 2023, versus the nine months ended September 30, 2022, we had net cash provided by investing activities as certain investments matured (offset by the purchase of additional investments) and we had net cash provided by financing activities as funds were raised from sales of our common stock under an at-the-market sales program.
Cash Flow Used in Operating Activities
The increase of $385,000 in cash flow used in operating activities during the nine months ended September 30, 2023, compared to the nine months ended September 30, 2022, was primarily a result of changes in accounts payable, accrued expenses and other current liabilities.
Cash Flow Provided by Investing Activities
The cash flow provided by investing activities during the nine months ended September 30, 2023 of $1,957,000 was a result of our investment in U.S. Treasury Bills maturing during the nine months ended September 30, 2023 offset by the purchase of additional U.S. Treasury Bills during the period. During the nine months ended September 30, 2022, idle cash was invested in money market accounts and recorded as cash equivalents.
Cash Flow Provided by (Used in) Financing Activities
The increase in cash flow provided by (used in) financing activities during the nine months ended September 30, 2023, compared to the nine months ended September 30, 2022, of $1,798,000 was primarily due to the proceeds from sales of our common stock under an at-the-market sales program during the nine months ended September 30, 2023. We did not have any sales of our common stock during the nine months ended September 30, 2022.
Future Funding Requirements
To date, we have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate any revenue from product sales or royalties unless and until we obtain regulatory approval of and commercialize any of our current or future drug product candidates or we out-license or sell a drug product candidate to another party. At the same time, we expect our expenses to increase in connection with our ongoing development activities, particularly as we continue the research, development, future preclinical studies and clinical trials of, and seek regulatory approval for, our current and future drug product candidates. If we obtain regulatory approval of any of our current or future drug product candidates, we will need substantial additional funding for commercialization requirements and our continuing drug product development operations.
As a company, we have not completed development through marketing approvals of any therapeutic or imaging products. We expect to continue to incur significant increases in expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially as we:
| ● |
advance the clinical development and execute the regulatory strategy for camsirubicin; |
|
|
|
||
| ● |
continue the preclinical activities and potentially enter clinical development of MNPR-101-derived radioimmunotherapeutics and companion diagnostics, to image and treat cancer; |
|
|
|
||
| ● |
continue the preclinical activities, and potentially later-on enter clinical development, of MNPR-202 (and related analogs) for various cancer indications; |
|
|
|
||
| ● |
acquire and/or license additional pipeline drug product candidates and pursue the future preclinical and clinical development and regulatory requirements of such drug product candidates; |
|
|
|
||
| ● |
seek regulatory approvals for any of our current and future drug product candidates that successfully complete registration clinical trials; |
|
|
|
||
| ● |
establish or purchase the services of a sales, marketing and distribution infrastructure to commercialize any products for which we obtain marketing approval; |
|
|
|
||
| ● |
develop, or contract for, manufacturing/quality capabilities- to establish a reliable, high quality supply chain sufficient to support our clinical and specialized radiopharmaceutical requirements and to provide sufficient capacity to launch and supply the market for any product for which we obtain marketing approval; and |
|
|
|
||
| ● |
add or contract for required operational, financial, human resources and management information systems and capabilities and other specialized expert personnel to support our drug product candidate development and planned commercialization efforts. |
We anticipate that the funds available as of October 27, 2023, will fund our obligations at least through November 2024. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our drug product candidates, and the extent to which we enter into collaborations with third parties to participate in the development and commercialization of our drug product candidates, we are unable to accurately estimate with high reliability the amounts and timing required for increased capital outlays and operating expenditures associated with our current and anticipated drug product candidate development programs.
Our future capital requirements will depend on many factors, including:
| ● |
the progress of clinical development and regulatory interactions and potential approvals of camsirubicin; |
|
|
|
||
| ● |
the costs, timing and outcomes of seeking, obtaining, and maintaining FDA and international regulatory approvals; |
| ● |
the progress of preclinical and potential clinical development of MNPR-101-derived radioimmunotherapeutics and companion diagnostics, to image and treat cancer, including activities through our collaboration with NorthStar; |
|
|
|
||
| ● |
the progress of preclinical and potential clinical development of MNPR-202 (and related analogs); |
|
|
|
||
| ● |
the number and characteristics of other drug product candidates that we may license, acquire, invent or otherwise pursue; |
|
|
|
||
| ● |
the scope, progress, timing, cost and results of research, preclinical development and clinical trials and regulatory requirements for future drug product candidates; |
| ● |
the costs associated with establishing or contracting for manufacturing/quality requirements and establishing or contracting for sales, marketing and distribution capabilities; |
|
|
|
||
| ● |
our ability and related costs to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make in connection with the licensing, filing, defense and enforcement of any patents or other intellectual property rights; |
|
|
|
||
| ● |
our need and ability to hire or contract for additional management, administrative, scientific, medical, sales and marketing, and manufacturing/quality and other specialized personnel or external expertise; |
|
|
|
||
| ● |
the effect and timing of entry of competing products or new therapies that may limit market penetration or prevent the introduction of our drug product candidates or reduce the commercial potential of our product portfolio; |
|
|
|
||
| ● |
our need to implement additional required internal management, operational, record keeping, and other systems and infrastructure; and |
|
|
|
||
| ● |
the economic and other terms, timing and success of our existing collaboration and licensing arrangements and any collaboration, licensing or other arrangements into which we may enter into in the future, including the timing of receipt of or payment to or from others of any license, milestone or royalty payments under these arrangements. |
Expenditures may increase in the future for:
| ● |
process development, manufacturing costs, clinical trial expenses and clinical database management of camsirubicin in connection with the Phase 1b dose escalation clinical trial and other future clinical development; |
|
|
|
||
| ● |
support of the development of MNPR-101-derived radioimmunotherapeutics and companion diagnostics to image and treat cancer, including activities through our collaboration with NorthStar; |
|
| ● |
preclinical studies (and if successful, clinical studies) of MNPR-202 (and related analogs); |
|
|
|
||
| ● |
increased employee compensation and consultant fees to support the increased scope of activities required for the progress of our product candidate programs including camsirubicin, MNPR-101 RIT (uPRIT and related compounds) and companion diagnostics and MNPR-202 (and related analogs); and |
|
|
|
||
| ● |
increased sales, marketing, distribution, quality, medical, pharmacovigilance, regulatory and compliance employees and/or consultants to support any of our programs if approved for marketing in any major market. |
Due to the termination of our Validive development, we do not anticipate significant additional expenditures for this program beyond the third quarter of 2023. We have initiated and commenced dosing in our Phase 1b camsirubicin clinical trial, and we are moving towards a first-in-human clinical trial of our MNPR-101-Zr imaging agent as early as end of this year. We intend to continue evaluating drug product candidates for the purpose of growing our pipeline. Identifying and securing high-quality compounds usually takes time and related expenses; however, our spending could be significantly accelerated in the future if additional drug product candidates are acquired and enter clinical development. In this event, we may be required to expand our management team, and pay higher contract manufacturing costs, contract research organization fees, other clinical development costs and insurance costs that are not currently projected. Beyond our need to raise additional funding within the next 12 months, substantial additional long-term funding is needed to further develop camsirubicin, our MNPR-101 RIT and companion diagnostic program and our MNPR-202 program, if successful, and otherwise generally to support our future product candidates through clinical trials, approval processes and, if applicable, commercialization.
Until we can generate a sufficient amount of product revenue to finance our cash requirements, we expect to finance our future cash needs primarily through a combination of equity offerings, including the usage of our Capital on DemandTM Sales Agreement with JonesTrading, debt financings, strategic collaborations and grant funding. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our current stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our current stockholders’ rights. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with other parties, we likely will have to share or relinquish valuable rights to our technologies, future revenue streams, research programs or drug product candidates or grant licenses on terms that may not be favorable to us, which will reduce our future returns and affect our future operating flexibility. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our pipeline product development or commercialization efforts or grant rights to others to develop and market drug product candidates that we would otherwise prefer to develop and market ourselves.
The termination of our Validive clinical trial due to the no-go decision at the end of March 2023 resulted in a decrease in our stock price. The closing bid price of our stock fell below $1.00 for more than 30 consecutive trading days, and on August 28, 2023, we received a notice from Nasdaq stating that we are out of compliance with Nasdaq listing standards giving us 180 days to regain compliance. While we intend to apply for a second 180-day extension to regain compliance, there can be no assurance that we would regain compliance within Nasdaq’s original or proposed extended time limits and requirements. If we don't regain compliance, it may have serious adverse consequences on our ability to raise funds, which may cause us to delay, restructure or otherwise reconsider our operations.
Contractual Obligations and Commitments
License, Development and Collaboration Agreements
Onxeo S.A.
In June 2016, we executed an agreement with Onxeo S.A., a French public company, which gave us the exclusive option to license (on a world-wide exclusive basis) Validive (clonidine hydrochloride mucobuccal tablet; clonidine HCI MBT) a mucoadhesive tablet of clonidine based on the Lauriad mucoadhesive technology. The agreement includes clinical, regulatory, developmental and sales milestones and escalating royalties on net sales. In September 2017, we exercised the option to license Validive from Onxeo for $1 million.
On March 27, 2023, we announced the discontinuation of our Validive Phase 2b/3 VOICE trial based upon the DSMB determination that the trial did not meet the pre-defined threshold for efficacy of a 15% absolute difference in SOM prevention between Validive and placebo. We are planning to terminate the license and do not anticipate any future license or royalty obligations under the Onxeo license agreement.
Grupo Español de Investigación en Sarcomas (“GEIS”)
In June 2019, we executed a clinical collaboration with GEIS for the development of camsirubicin in patients with advanced soft tissue sarcoma (“ASTS”). Following completion of our Phase 1b clinical trial in the U.S. that we initiated in the third quarter of 2021 with the first patient dosed in October 2021, we continue to expect that GEIS will sponsor and lead a multi-country, randomized, open-label Phase 2 clinical trial to evaluate camsirubicin head-to-head against doxorubicin, the current first-line treatment for ASTS. We will provide study drug and supplemental financial support for the clinical trial. During the three months ended September 30, 2023 and September 30, 2022, no expenses were incurred under the GEIS agreement. We can terminate the agreement by providing GEIS with advance notice, and without affecting our rights and ownership to any related intellectual property or clinical data. In the second quarter of 2021, due to regulatory delays in Spain, we decided to conduct an open-label Phase 1b clinical trial of camsirubicin in the U.S., therefore no expenses were incurred related to the GEIS collaboration beyond March 31, 2021.
XOMA Ltd.
Pursuant to a non-exclusive license agreement with XOMA Ltd. for the humanization technology used in the development of MNPR-101, we are obligated to pay XOMA Ltd. clinical, regulatory and sales milestones which could reach up to $14.925 million if we achieve all milestones for MNPR-101. The agreement does not require the payment of sales royalties. There can be no assurance that we will achieve any milestones. As of October 27, 2023, we had not reached any milestones and had not been required to pay XOMA Ltd. any funds under this license agreement.
Service Providers
In the normal course of business, we contract with service providers to assist in the performance of R&D, including drug product manufacturing, process development, clinical and preclinical development, and G&A including financial strategy, audit, tax and legal support. We can elect to discontinue the work under these agreements at any time. We could also enter into collaborative research and development, contract research, manufacturing and supplier agreements in the future, which may require upfront payments and/or long-term commitments of cash.
Office Lease
We are currently leasing office space for our executive headquarters at 1000 Skokie Blvd., in the Village of Wilmette, Illinois for $4,238 per month through February 2024, and we anticipate that we will lease additional space in the future as we hire additional personnel.
Legal Contingencies
We are currently not, and to date have never been, a party to any adverse material legal proceedings.
Indemnification
In the normal course of business, we enter into contracts and agreements that contain a variety of representations and warranties and provide for general indemnification. Our exposure under these agreements is unknown because it involves claims that may be made against us in the future, but that have not yet been made. To date, we have not paid any claims or been required to defend any action related to our indemnification obligations. However, we may record charges in the future as a result of these indemnification obligations.
In accordance with our Second Amended and Restated Certificate of Incorporation, Amended and Restated Bylaws and the indemnification agreements entered into with each officer and non-employee director, we have indemnification obligations to our officers and non-employee directors for certain events or occurrences, subject to certain limits, while they are serving at our request in such capacity. There have been no claims to date.
Item 4. Controls and Procedures
Our Chief Executive Officer and Chief Financial Officer have provided certifications filed as Exhibits 31.1 and 31.2, respectively, and Exhibit 32.1. Such certifications should be read in conjunction with the information contained in this Item 4 for a more complete understanding of the matters covered by those certifications.
(a) Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures as of September 30, 2023, pursuant to Rules 13a15(e) and 15d15(e) under the Exchange Act. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures, as of such date, were effective.
(b) Changes in Internal Control over Financial Reporting
We have concluded that the condensed consolidated financial statements and other financial information included in this Quarterly Report on Form 10-Q fairly present in all material respects our financial condition, results of operations and comprehensive loss and cash flows as of, and for, the periods presented.
There have been no changes in our internal control over financial reporting during the three months ended September 30, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Other than the additional risk factors below, there have been no material changes in information regarding our risk factors as described in Item 1A of our Annual Report on Form 10-K as filed with the SEC on March 23, 2023.
The interim analysis for our ongoing Validive Phase 2b/3 clinical program yielded a no-go decision resulting in a reduction of our stock price. If our stock price does not increase before the Nasdaq original or potential extended deadline, our business could be adversely impacted.
The termination of our Validive clinical trial due to the no-go decision at the end of March 2023 resulted in a decrease in our stock price. The closing bid price of our stock fell below $1.00 for more than 30 consecutive trading days and on August 28, 2023 we received a notice from Nasdaq stating that we are out of compliance with Nasdaq listing standards giving us 180 days to regain compliance. While we intend to apply for a second 180-day period to regain compliance, there can be no assurance that we would regain compliance. If we do not regain compliance, we would face delisting and it may have serious adverse consequences on our ability to raise funds, which may cause us to delay, restructure or otherwise reconsider our operations.
The following exhibits are filed as part of this Quarterly Report on Form 10-Q.
| Exhibit |
Document |
Incorporated by Reference From: |
||
| 31.1 |
Certification of Chandler D. Robinson, Chief Executive Officer |
Filed herewith |
||
| 31.2 |
Filed herewith |
|||
| 32.1 |
Filed herewith |
|||
| 101.INS |
Inline XBRL Instance Document |
|||
| 101.SCH |
Inline XBRL Taxonomy Extension Schema |
|||
| 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase |
|||
| 101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase |
|||
| 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase |
|||
| 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase |
|||
| 104 |
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| MONOPAR THERAPEUTICS INC. |
|||
| Dated: November 9, 2023 |
By: |
/s/ Chandler D. Robinson |
|
| Name: |
Chandler D. Robinson |
||
| Title: |
Chief Executive Officer and Director (Principal Executive Officer) |
||
| MONOPAR THERAPEUTICS INC. |
|||
| Dated: November 9, 2023 |
By: |
/s/ Kim R. Tsuchimoto |
|
| Name: |
Kim R. Tsuchimoto |
||
| Title: |
Chief Financial Officer and Director (Principal Financial Officer) |
||
EXHIBIT 31.1
CERTIFICATION
I, Chandler D. Robinson, certify that:
|
1. |
I have reviewed this Quarterly Report on Form 10-Q of Monopar Therapeutics Inc.; |
|
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
|
|
4. |
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
|
(a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
|
(b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
|
(c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
|
(d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
|
|
5. |
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
|
|
(a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
|
|
(b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
|
|
Date: November 9, 2023 |
|||
|
/s/ Chandler D. Robinson |
|||
|
Chandler D. Robinson |
|||
|
Chief Executive Officer |
EXHIBIT 31.2
CERTIFICATION
I, Kim R. Tsuchimoto, certify that:
|
1. |
I have reviewed this Quarterly Report on Form 10-Q of Monopar Therapeutics Inc.; |
|
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
|
|
4. |
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
|
(a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
|
(b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
|
(c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, the end of the period covered by this report based on such evaluation; and |
|
|
(d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
|
|
5. |
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
|
|
(a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
|
|
(b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
|
|
Date: November 9, 2023 |
|||
|
/s/ Kim R. Tsuchimoto |
|||
|
Kim R. Tsuchimoto |
|||
|
Chief Financial Officer |
EXHIBIT 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of Monopar Therapeutics Inc. (the Company) for the three and nine months ended September 30, 2023, as filed with the Securities and Exchange Commission on the date hereof (the Report), we, Chandler D. Robinson, and Kim R. Tsuchimoto, hereby certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, that:
|
(1) |
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
|
|
||
|
(2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
|
/s/ Chandler D. Robinson |
|||
|
Chandler D. Robinson |
|||
|
Chief Executive Officer |
|||
|
November 9, 2023 |
|||
|
/s/ Kim R. Tsuchimoto |
|||
|
Kim R. Tsuchimoto |
|||
|
Chief Financial Officer |
|||
| November 9, 2023 |
This certification accompanies the Form 10-Q to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Monopar Therapeutics Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-Q), irrespective of any general incorporation language contained in such filing.