FORM 10-K
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark one)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2023
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____ to ____
Commission File No: 0-11740
MESA LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
| Colorado |
84-0872291 |
| (State or other jurisdiction of |
(I.R.S. Employer |
| Incorporation or organization) |
Identification number) |
| 12100 West Sixth Avenue |
|
| Lakewood, Colorado |
80228 |
| (Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (303) 987-8000
Securities registered under Section 12(b) of the Act:
| Title of each class |
Trading Symbol |
Name of each exchange on which registered |
||
| Common stock, no par value |
MLAB |
The Nasdaq Stock Market LLC |
Securities registered under Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (check one):
| Large accelerated filer ☒ |
Accelerated filer ☐ |
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of voting stock held by non-affiliates of the registrant was $713 million based upon the closing market price and common shares outstanding as of September 30, 2022.
The number of outstanding shares of the Registrant’s common stock as of May 19, 2023 was 5,369,959.
This document (excluding exhibits) contains 74 pages.
DOCUMENTS INCORPORATED BY REFERENCE
Part III is incorporated by reference from the registrant’s definitive Proxy Statement for its 2023 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after the close of the registrant's fiscal year.
Forward-Looking Statements
This Report on Form 10-K contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The forward-looking statements in this Report on Form 10-K do not constitute guarantees of future performance. Investors are cautioned that statements in this Report on Form 10-K which are not strictly historical statements, including, without limitation, express or implied statements or guidance regarding current or future financial performance and position, potential impairment of future earnings, anticipated effects of, and future actions to be taken in response to, the COVID-19 pandemic, management’s strategy, plans and objectives for future operations or acquisitions, product development and sales, product research and development, regulatory approval, selling, general and administrative expenditures, intellectual property, development and manufacturing plans, availability of materials and product and adequacy of capital resources and financing plans constitute forward-looking statements. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which the Company operates, and management’s beliefs and assumptions. In addition, other written and oral statements that constitute forward-looking statements may be made by the Company or on the Company’s behalf. Words such as “expect,” “anticipate,” “intend,” “plan,” “seek,” “believe,” “could,” “estimate,” “may,” “target,” “project,” or variations of such words and similar expressions are intended to identify forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated, including those discussed in Item 1A. “Risk Factors,” and elsewhere in this report. We disclaim any obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise.
In this annual report on Form 10-K, Mesa Laboratories, Inc., a Colorado corporation, together with its subsidiaries is collectively referred to as “we,” “us,” “our,” the “Company,” or "Mesa." Mesa was organized in 1982 as a Colorado corporation.
General
We are a multinational manufacturer, developer, and seller of life sciences tools and critical quality control solutions, many of which are sold into niche markets driven by regulatory requirements. We have manufacturing operations in the United States and Europe, and our products are marketed by our sales personnel in North America, Europe and Asia Pacific, and by independent distributors in these areas as well as throughout the rest of the world. We prefer markets in which we can establish a strong presence and achieve high gross margins.
We are headquartered in Lakewood, Colorado and our common stock is listed for trading on the Nasdaq Global Market (“Nasdaq”) under the symbol MLAB.
Our fiscal year ends on March 31. References in this Annual Report on Form 10-K (“annual report”) to a particular “fiscal year,” “year” or “year-end” mean our fiscal year.
Strategy
We strive to create shareholder value and further our purpose of Protecting the Vulnerable® by growing our business both organically and through further strategic acquisitions, by improving our operating efficiency, and by continuing to hire, develop and retain top talent. As a business, we commit to our purpose of Protecting the Vulnerable® every day by taking a customer-focused approach to developing, building and delivering our products and services in order to help our customers ensure product integrity, increase patient and worker safety, and improve the quality of life throughout the world. We serve a broad set of industries, particularly the pharmaceutical, healthcare and medical device verticals.
Our revenues come from product sales, which include consumables and hardware; as well as services, which include discrete and ongoing maintenance, calibration, and testing services. We grow our revenues organically by expanding our customer base, increasing sales volumes, and implementing price increases, and inorganically through acquisitions.
Our acquisition strategy is focused on businesses that complement our existing portfolio and those that expand our presence further into life sciences tools and critical quality control solutions markets for regulated applications.
We focus on improving our operating efficiency through the Mesa Way, which is our customer-centric, lean based system for continuously improving and operating a set of high-margin, niche businesses. As part of our ongoing commitment to empowerment, improvement, and learning, we launched an enhanced version of the Mesa Way during fiscal year 2023. This iteration was developed by a cross-functional committee composed of employees from all job levels and divisions to help our employees engage deeply with Mesa’s vision and purpose. The Mesa Way is based on four pillars:
| – |
Measure what matters: We use “True North,” our customers’ perspectives, to measure what matters most and to set high standards for performance. We manage to leading indicators whenever possible, which drives us to proactively avoid problems before they are apparent to our customers. |
| – |
Empower Teams: We move decision making as close to the customer as possible and provide real-time communication forums to align the whole organization towards surpassing customer expectations. |
| – |
Sustainably Improve: We leverage a common and proven set of lean-based tools to identify sources of opportunities, prioritize our biggest opportunities, and enable change to be embraced and implemented quickly. |
| – |
Always Learn: We ensure that improvements are sustained, enabling us to raise performance expectations and repeat the cycle of improvement. Equally, this cycle strengthens the Mesa team by providing endless learning opportunities for our employees, and helps us become an employer of choice in our communities. |
We hire, develop, and retain top talent capable of taking on new challenges using a team approach to continuously improve our products, our services, and ourselves, resulting in long-term value creation for our stakeholders.
Our Segments
We report our financial performance in four segments, or divisions: (1) Clinical Genomics, (2) Sterilization and Disinfection Control, (3) Biopharmaceutical Development, and (4) Calibration Solutions. Unallocated corporate expenses and other business activities are reported within Corporate and Other.
Clinical Genomics
Our Clinical Genomics division develops, manufactures and sells highly sensitive, low cost, high-throughput genetic analysis tools and related consumables and services that enable genetic analysis for a broad range of diagnostic and research applications in several therapeutic areas.
Using Clinical Genomics’ MassARRAY® system and our proprietary consumables, including chips, panels, and chemical reagent solutions, our customers can analyze DNA samples for inherited genetic disease testing, pharmacogenetics, oncology testing, infectious disease testing, and other highly differentiated applications. The MassARRAY® system couples mass spectrometry with end-point polymerase chain reaction ("PCR") methods, enabling highly multiplexed reactions under universal cycling conditions to provide accurate, sensitive, rapid genetic analysis.
The MassARRAY® system is differentiated in the market by its ability to target up to 50 specific DNA variants in a single PCR reaction and run up to 384 samples on one SpectroCHIP® array, up to eight times in a full workday, with the flexibility to process additional samples overnight. The system allows for the testing of hundreds of mutations, including SNPs, insertions, deletions, translocations, copy number variation, and methylation makers, all in a single, efficient workflow. Using time-of-flight mass spectrometry, genetic variants are distinguished by analysis of their individual mass, eliminating the need for fluorescence. The system's integrated software provides a user-friendly interface to generate reports that identify targets and review spectra.
In addition to the MassARRAY® system and related consumable products, Clinical Genomics also sells services, including equipment maintenance contracts and custom laboratory services through which our scientists help customers develop specified assay designs.
About 70% of our Clinical Genomics revenues are from consumables used on a routine basis; sales of these products are less sensitive to general economic conditions. Approximately 20% of our Clinical Genomics revenues are from more discretionary hardware products that are more sensitive to general economic conditions. The remainder of Clinical Genomics revenues relate to services and support agreements.
Clinical Genomics sells its products and services predominantly to clinical labs, including large specialty, reference, and pathology labs, as well as to a variety of academic, hospital, and government facilities. The majority of revenues are derived from customers in the United States and China. Our Clinical Genomics products are manufactured in San Diego, California, primarily by assembling purchased subcomponents designed to our specifications into finished goods, and by processing and mixing reagents. Our Clinical Genomics products generate revenues through direct sales, and also through independent distributors in certain regions.
Sterilization and Disinfection Control
Our Sterilization and Disinfection Control division manufactures and sells biological, chemical and cleaning indicators used to assess the effectiveness of sterilization processes, including steam, gas, hydrogen peroxide, ethylene oxide, radiation, and other processes in the pharmaceutical, medical device, hospital, and dental industries. The Sterilization and Disinfection Control division also provides related testing services, mainly to the dental industry.
Biological indicators contain spores of certain microorganisms that provide defined resistance to specified sterilization processes. In use, biological indicators are exposed to a sterilization process and then tested to determine the presence of surviving organisms. We grow the microbiological spores used in our biological indicator products from raw materials and apply them to convenient carriers such as small pieces of filter paper or stainless steel discs for sale. To ensure our biological indicators accurately assess the effectiveness of sterilization, we undertake extensive quality control steps during manufacture to ensure the spores are well-characterized in terms of purity, the population of spores, and the spores’ resistance to sterilization following placement on or in the target carrier.
We offer a variety of product formats which allow our biological indicators to be used in many types of processes and environments. Our biological indicator products include inoculated carriers such as spore strips or discs which require post-processing transfer to a growth media; self-contained indicators, which have the growth media already pre-packaged in crushable ampoules; process challenge devices (“PCDs”), which increase the resistance of the biological indicators; and growth media. Our simple spore strips are used most often in small table-top steam sterilizers in dental offices, while our more complex self-contained biological indicators, which may be used with or without PCDs, are frequently used by medical device manufacturers to assure sterility in complex ethylene oxide sterilization processes. We also offer testing services in which customers return used dental sterilization spore strips to our microbiological laboratory for testing. Biological indicators and chemical indicators are often used together to monitor processes. Chemical indicators use a chemical change (generally determined by color) to assess the exposure to sterilization conditions.
Cleaning indicators are used to assess the effectiveness of cleaning processes, including washer-disinfectors and ultrasonic cleaners in healthcare settings. Cleaning is the critical first step performed prior to disinfection and sterilization. Debris left on an instrument may interfere with microbial inactivation and can compromise disinfection or sterilization processes. Our cleaning indicator products are manufactured by inoculating a test soil onto a stainless-steel coupon. The test soil is designed to mimic the challenge of removing blood and tissue from surgical instruments and evaluates the effectiveness of our customers' cleaning processes.
Our Bozeman, Montana and Munich, Germany locations manufacture our Sterilization and Disinfection Control division products, which include, among others, our EZTest®, Apex®, and Simicon biological indicators and PCDs. Our Bozeman, Montana facility provides sterility assurance testing services to dental offices in the United States and Canada. Sterilization and disinfection control products are disposable and are used on a routine basis, thus product sales are less sensitive to general economic conditions. We generate sales to end users through our direct sales personnel and independent distributors. Customers include industrial users involved in pharmaceutical and medical device manufacturing, hospitals, dental offices, and contract sterilization providers. Our sterilization and disinfection control products are used in highly regulated industries and compete on the basis of quality, flexibility, cost effectiveness, and suitability for intended use.
Biopharmaceutical Development
Our Biopharmaceutical Development division develops, manufactures, and sells automated systems for protein analysis (immunoassays) and peptide synthesis solutions. Protein analysis and peptide synthesis solutions accelerate the discovery, development, and manufacture of biological therapies, among other applications. Customers include biopharmaceutical research, development, and manufacturing teams at biopharmaceutical companies and their contract research organization partners, as well as academic research and development laboratories.
The Biopharmaceutical Development division sells two types of products: (1) protein analysis solutions, which are used to test for the existence or concentration of specific proteins in a sample, and (2) peptide synthesis solutions, which automate the synthesis of peptides from amino acids; both are primarily used in biopharmaceutical research, discovery and development, and bioprocessing applications.
Our Biopharmaceutical Development division develops and manufactures Gyrolab® xPand and Gyrolab xPlore™ hardware and software, as well as Gyrolab Bioaffy® consumable microfluidic disks (“CDs”), and Gyrolab kits and Rexxip® buffers for protein analysis in Uppsala, Sweden, while PurePep® Chorus, Sonata+ and Symphony® hardware and software for peptide synthesis are developed and manufactured in Tucson, Arizona. The recent addition of the PurePep® EasyClean products, a green chemistry solution to purify peptides, adds a peptide consumables stream to our peptide synthesis business.
Most of the products manufactured in Sweden are typically invoiced in U.S. dollars or euros, whereas the costs to produce the products are incurred in Swedish Krona. As a result, the Biopharmaceutical Development segment is susceptible to changes in foreign currency. For a discussion of risks related to our non-U.S. operations and foreign currency exchange, refer to Item 1A. Risk Factors, “Foreign currency exchange rates may adversely affect our financial statements.”
About one-third of our Biopharmaceutical Development revenues are from consumables used on a routine basis; sales of these products are less sensitive to general economic conditions. Approximately 45% of revenues are from more discretionary hardware purchases that are more sensitive to general economic conditions. The remainder of sales are related to service and support agreements. We generate sales to end users through direct sales as well as through independent foreign distributors. Marketing activities include industry conferences, user meetings, educational webinars, and all forms of digital marketing, in addition to market sensing and capturing user requirements for new product roadmaps.
The Biopharmaceutical Development division’s market success is primarily dependent upon creating innovative, high quality products that customers choose based on available features, cost-effectiveness, and performance. We believe we are one of the leading world-wide suppliers of protein analysis and peptide synthesis equipment to the biologics discovery and development markets. We further believe that enhancements of our product offerings and new product development driven by our research and development team, the recognized quality of our products and support, and the ability to continue to bring novel, cutting edge products and solutions to the market will allow us to remain competitive in the growing markets we serve.
Protein Analysis
We develop, manufacture, and market protein analysis equipment and consumable CDs, kits, and buffers that enable the detection and quantification of a target protein in a biological or bioprocess sample. Gyrolab technology is widely used across human and non-human applications, mainly for therapy development and bioprocess design. Customers, primarily pharmaceutical and biotech companies and their contract research organization partners developing protein-based therapies, use our consumable CDs to deposit their samples for mixing with application specific reagents. The CDs and reagents are loaded into one of our instruments for processing and analysis. Our proprietary software then facilitates the design of experiments, interprets results, provides useful data analysis for assay optimization and decision making, and supports end user regulatory compliance. Our protein analysis products accelerate the development and processing of assays to obtain accurate results for pre-clinical and clinical studies as well as for upstream and downstream bioprocessing of biological therapies, thus meeting critical data and time requirements. Our analytical protein technologies provide superior data consistency and accuracy while reducing labor and the attendant variability of more manual analysis methods.
Peptide Synthesis
Our peptide synthesis solutions enable customers to automate the chemical synthesis of peptides used in the creation of peptide therapies, biomaterials, cosmetics, and general research. Our peptide synthesizers and related consumables, including our new peptide purification consumables line, facilitate the ability to efficiently produce more complex and longer peptides with higher purity. Our synthesizers are designed to support regulatory compliance for end users. Customers of our peptide synthesizers include commercial and academic biopharmaceutical laboratories, as well as contract manufacturers of peptides.
Calibration Solutions
Our Calibration Solutions division develops, manufactures, sells, and services quality control products using principles of advanced metrology to calibrate or measure critical chemical or physical parameters such as temperature, pressure, pH, and humidity for health and safety purposes, primarily in hospital, medical device manufacturing, pharmaceutical manufacturing, and various laboratory environments. Generally, our Calibration Solutions products are used for quality control, safety validation, and regulatory compliance. As of March 31, 2023, our Lakewood, Colorado and Hanover, Germany facilities manufacture our Calibration Solutions products, which include dialysate meters and consumables, continuous monitoring systems, data loggers, gas flow calibration and air sampling equipment, and torque testing systems represented largely by the DialyGuard®, ViewPoint®, DryCal®, DataTrace®, BGI, IBP Medical, Torquo®, and SureTorque® brands.
The majority of our Calibration Solutions products have relatively long lifespans and their purchase by customers is discretionary, so sales are more sensitive to general economic conditions. Service demand is driven by customers’ quality control and regulatory environments, which require products to be recalibrated or recertified periodically. Our Calibration Solutions products are manufactured by assembling the products from purchased components and calibrating the final products. Our Calibration Solutions division's commercial efforts focus on offering metrology products to our customers that are required to meet regulatory requirements and quality control standards. We generate sales through our direct sales personnel and independent distributors.
Dialysate Meters and Consumables
Our dialysis medical meters are used to test various parameters of dialysis fluid (dialysate) and the proper calibration and operation of dialysis machines. Each meter measures some combination of temperature, pressure, pH, conductivity and flow to ensure that the dialysate has the proper composition to promote the transfer of waste products from the blood to the dialysate. The meters provide a digital readout verifying whether a dialysis machine is working within prescribed limits and delivering properly prepared dialysate. We manufacture two styles of medical meters; those designed for use by dialysis machine manufacturers and biomedical technicians, and those used primarily by dialysis clinicians. The meters for technicians are characterized by exceptional accuracy, stability and flexibility, and are used by the industry as the primary standard for the calibration of dialysis machines. The meters designed for use by dialysis clinicians are known primarily for their ease of use, and they incorporate a built-in syringe sampling system. These meters are used as the final quality control check on the dialysate just prior to starting treatment.
In addition to dialysate meters, we market a line of standard consumable solutions for use in dialysis clinics for calibration of our meters. These standard solutions are regularly consumed by dialysis clinics, and thus, along with the calibration services that we also provide, are less impacted by general economic conditions than sales of meters.
Customers that utilize our dialysate products include dialysis facilities, medical device manufacturers, and biomedical service companies. With technological advancements in dialysis machines that include built-in calibrators, we anticipate a reduction in sales of meters designed for clinicians. Refer to Item 1A. Risk Factors, “Changes to dialysis methods and equipment capabilities may decrease demand for our dialysis products and negatively impact our financial statements.”
Continuous Monitoring
Our continuous monitoring products are used to monitor various environmental parameters such as temperature, humidity, and differential pressure to ensure that critical storage and processing conditions are maintained. Continuous monitoring systems are used in controlled environments such as refrigerators, freezers, warehouses, laboratory incubators, clean rooms, and a number of other settings. Continuous monitoring systems consist of wireless sensors that are placed in controlled environments which communicate with cloud and local servers to transmit and store data continuously. A critical function of our systems is the ability to provide local alarms and notifications via e-mail, text, or telephone if established environmental conditions are exceeded. Among the important competitive differentiators of our continuous monitoring systems are (1) their high degree of reliability and up-time; (2) a large variety of sensor types to meet the needs of most applications; (3) a skilled, distributed installation and service team; and (4) a full-featured and 21 CFR Part 11 (Electronic records; Electronic signatures) validated software program, providing extensive reporting and alarm capability. We also offer support agreements and provide annual sensor recalibrations.
We have a strong, competitive position in North America but are not currently focused on international expansion. Key markets for our continuous monitoring systems are hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies, and laboratory environments, all located in North America.
Data Loggers
Our data loggers are self-contained, wireless, high precision instruments used in critical manufacturing and quality control processes in the pharmaceutical, medical device, food, and tool industries. They are used to measure temperature, humidity and pressure inside a process or a product during manufacture. In addition, data loggers can be used to validate the proper operation of laboratory or manufacturing equipment, either during installation or for annual re-certifications. The products consist of individual data loggers, a personal computer (“PC”) interface, software, and various accessories. Customers typically purchase a large number of data loggers along with a single PC interface and software package. In practice, the user programs the loggers to collect environmental data at pre-determined time intervals, places the data loggers into the product or process to be tested, and then collects stored process data from the data logger either through the PC interface or wirelessly via a radio link. The user can then prepare tabular and graphical reports using the software. Unique aspects of our data loggers are their ability to operate at elevated temperatures and in explosive environments, which are important differentiating factors in the marketplace. We face competition in data logger sales from several other companies, some of which have well-established commercial organizations, particularly in Europe.
Gas Flow Calibration and Air Sampling Equipment
We manufacture a variety of instruments and equipment for gas flow calibration and environmental air sampling. Our gas flow calibration instruments provide the precise standards required by laboratories and industry for the design, development, manufacture, installation and calibration of various gas flow meters and air sampling devices. Our flow calibrators are used by professionals in many industries, including (1) industrial hygienists and environmental technicians, (2) calibration and research laboratories, (3) manufacturers who design, develop and manufacture gas flow metering devices, and (4) industrial engineering and manufacturing companies that utilize gas flow metering devices. We see expanded opportunities in gas flow calibration as markets that heavily use and measure process gas are growing. There is competition in gas flow calibration; however, our products are distinguished by their unique dry piston technology and industry-leading accuracy and certifications.
In the air sampling area, our technology is used primarily for the determination of particulate concentrations in air as a measure of urban or industrial air pollution, and for industrial hygiene assessments. The primary products include air samplers, particle separators and pumps. While both the public and private sector continue to focus on air quality and its impact on the environment and the health of populations, technological advances in real-time monitoring have made the traditional air sampling market more limited. In the environmental area, our particle samplers were some of the first on the market and they were recognized early-on as “reference samplers” by the U.S. Environmental Protection Agency. This product has a competitive advantage in the market because our particle separation cyclones utilize the “federal reference method” for the measurement of PM2.5 in ambient air and are sold to most manufacturers of ambient particulate measurement instrumentation.
Torque Testing Systems
Our automated torque testing systems are durable and reliable motorized cap torque analyzers that measure the amount of force required to open a container. The primary advantages of our torque instruments are their high accuracy and long-term consistency of measurement. Industries utilizing these instruments include pharmaceutical and beverage and food processing companies. Given the niche nature of this product, there is a relatively low level of competition for this product line; however, the growth of this line is limited by the growth of new manufacturing facilities and packaging regulations in pharmaceutical manufacturing. Torque products are used by many of the same customers that purchase our data loggers, offering channel synergy opportunities.
Corporate and Other
Corporate and other consists of unallocated corporate expenses and other business activities.
Other Matters Relating to our Business as a Whole
Acquisitions
Year ended March 31, 2023 Acquisition
On November 17, 2022, we acquired substantially all of the assets and certain liabilities of Belyntic GmbH’s peptide purification business (“Belyntic” or the “Belyntic acquisition”). We paid $4.95 million on the date of acquisition, and we expect to pay an additional $1.5 million based on the probable approval of pending patent applications expected within 36 months of the acquisition date. The business complements our existing peptide synthesis business, part of the Biopharmaceutical Development segment, by adding a new peptide purification consumables line. We have prepared a preliminary analysis of the valuation of net assets acquired in the Belyntic acquisition, which is subject to revision as more detailed analyses are completed.
Year Ended March 31, 2022 Acquisition
On October 20, 2021, we completed the acquisition of 100% of the outstanding shares of Agena Bioscience, Inc. (“Agena” or “the Agena Acquisition”) for adjusted cash consideration of $300.8 million. Agena is a leading clinical genomics tools company that develops, manufactures, markets and supports proprietary instruments and related consumables that enable genetic analysis for a broad range of diagnostic and research applications. The acquisition of Agena moved our business toward the life sciences tools sector and expanded our market opportunities, particularly in Asia. Agena’s operations comprise our Clinical Genomics segment. We finalized our valuation of the net assets acquired in the Agena Acquisition during fiscal year 2023.
Manufacturing and Materials
Most of the components, raw materials, and other supplies used in our product lines are available from a number of different suppliers. We generally maintain multiple sources of supply, but we are dependent on sole or limited sources for certain items. We continue to emphasize reviewing our supply base and designs for limited source suppliers that might affect our ability to supply critical products to our customers. We also continue to work with our suppliers to understand existing and potential future supply chain conditions. Our actions to mitigate the impact of supply chain disruptions, including pre-ordering components in higher than usual quantities and sourcing new vendors have been somewhat successful; however, raw materials shortages impacted our Calibrations Solutions division through most of fiscal year 2023. See further discussion within Item 1A. Risk Factors, “We face numerous manufacturing and supply chain risks. In addition, our reliance upon sole or limited sources of supply for certain materials, components and services could cause production interruptions, delays and inefficiencies.”
Major Customers
Typically, no individual customer represents more than 10% of our consolidated accounts receivable or revenues. Due to large orders placed and shipped late in fiscal year 2023, one of our distributors represented approximately 18% of our trade receivables as of March 31, 2023, but we have since collected, and expect to fully collect, payments on the remaining outstanding balance. Otherwise, no customer has represented more than 10% of our accounts receivable or revenues in the past three fiscal years.
During fiscal year 2023, we were notified by Sema4 Holdings Corp. ("Sema4"), a customer of our Clinical Genomics division, that they are exiting the reproductive health screening business and as a result, they intend to significantly reduce the quantity of orders they place with us in the future. Revenues from sales to Sema4 were approximately $8.2 million during the first twelve months of our ownership of Agena. Following the notice, we evaluated our business operations and enacted several cost-cutting measures in the Clinical Genomics division, including a reduction-in-force, to preserve our financial model. These actions are expected to generate more than $4.0 million in future annualized savings.
Backlog
We define backlog as firm orders from customers for products and services where the order will be fulfilled within the next 12 months. Backlog as of March 31, 2023 and 2022 was approximately $38.1 million and $56.0 million, respectively. Approximately $9 million of the fiscal year end 2022 backlog related to Sema4. Incremental temporary and permanent manufacturing employees and reduced supply chain constraints in fiscal year 2023 enabled us to maintain a lower backlog as of March 31, 2023.
Research and Development
Research and development ("R&D") activities are primarily directed towards innovating new products and improving the quality and performance of our existing products or altering our current products to accommodate use of raw materials that are more readily available for purchase in our supply chain. Other R&D efforts seek to develop or improve software that will be sold, leased, or marketed in the future, and to improve manufacturing efficiencies.
Intellectual Property
We own numerous patents, trademarks, and other proprietary rights, each of which is important to the various facets of our business. Where appropriate, we seek patent protection for inventions and developments made by our personnel that are incorporated into our products or otherwise fall within our fields of interest. There can be no assurance, however, that any patent will provide adequate protection for the technology, system, product, service or process it covers. In addition, the process of obtaining and protecting patents can be long and expensive. We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our proprietary position. Our products and services are sold under various trade names, trademarks and brand names. We consider our trade names, trademarks and brand names to be valuable in the marketing of our products in each segment. We do not believe that the loss of any one patent or other proprietary right would have a material adverse effect on our overall business or on any of our reporting segments.
Regulatory Matters
Mesa's operations are global and are affected by complex state, federal and international laws relating to healthcare, environmental protection, antitrust, anti-corruption, marketing, fraud and abuse, import and export control, product safety and efficacy, employment, privacy, government contracts acquisition regulations, and other areas.
We are required to comply with certain International Standard Organization (“ISO”) standards, United States Pharmacopeia standards and Food and Drug Administration (“FDA”) requirements in order to sell some of our products. Our biological indicators are developed and manufactured according to ISO 11138 (Sterilization of health care products – Biological indicators) under a quality system that complies with ISO 13485:2016 (Medical devices – Quality management systems – Requirements for regulatory purposes and 21 CFR 820 (Quality system regulation). Specific Calibration Solutions products are compliant under ISO 13485:2016, ISO 17025:2017, and certain 21 CFR 820 regulations. Our Biopharmaceutical Division’s Uppsala, Sweden and Tucson, Arizona facilities are ISO 9001:2015 certified. Clinical Genomics operates a quality management system which complies with the requirements of ISO 13485:2016.
Several products in the Sterilization and Disinfection Control, Calibration Solutions, and Clinical Genomics divisions are classified by the FDA as medical devices subject to the provisions of the Federal Food, Drug and Cosmetic Act, which requires any company proposing to market a medical device to notify the FDA of its intention at least 90 days before doing so. We have received permission from the FDA to market all of our products requiring such permission. Some of our facilities are subject to FDA regulations and inspections, which may be time-consuming and costly. This includes ongoing compliance with the FDA’s current Good Manufacturing Practices regulations that require, among other things, the systematic control of design, manufacture, packaging, storage and transportation of products. Failure to comply with these practices renders the product adulterated and could subject us to an interruption of manufacturing and sales of these products, and possible regulatory action by the FDA.
The manufacture and sale of medical devices is also regulated by some states. Although there is substantial overlap between state regulations and the regulations of the FDA, compliance with some state laws may require additional cost or effort; however, we do not anticipate that complying with state regulations will create any significant issues or burdens.
Foreign countries also have laws regulating medical devices sold in those countries, which require additional resources for compliance. The time required to obtain approval from countries’ regulating bodies can be lengthy and resource consuming, particularly as each country’s requirements may differ.
We are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal or sensitive data in the course of our business, including the EU General Data Protection Regulation which imposes strict requirements on how we collect, transmit, process and retain personal data.
Government Contracts
Although we transact business with various U.S. government agencies, no government contract or aggregate contracts are of such magnitude that a renegotiation of profits or termination of the contracts at the election of the government would have a material adverse effect on our financial results.
Environmental Matters
As a global corporate citizen, we recognize the importance of the environment to a healthy, sustainable future for our business, our customers, and our communities. We are committed to minimizing the environmental impacts of our business operations, and we actively evaluate ways to promote rigorous sustainability standards in our operations and products, including efforts to conserve water and energy and to reduce waste. More information about our environmental, social, and governance (“ESG”) efforts is included in our ESG brochure, which is available on our website at www.mesalabs.com/esg. The contents of our ESG brochure are not incorporated by reference into this annual report on Form 10-K.
Human Capital Management
As a company, our vision is to Protect the Vulnerable® and we believe that our vision is achieved in large part through the strength of our workforce. Every day, our talented employees strive to implement lean based tools to find ways to continuously improve our products and services so that we may better serve our customers. We recruit top talent from all backgrounds using a combination of industry expert recruiters and recruiting tools to reach a diverse pool of candidates across race, gender, disability, and veteran statuses. We support employees with compensation, benefits and development programs aimed at ensuring employees are productive and engaged.
Employees
As of March 31, 2023, we had 698 employees, of whom 272 are employed for manufacturing and quality assurance, 141 for research and development and engineering, 184 for sales and marketing, and 101 for administration. Our voluntary employee turnover decreased slightly during fiscal year 2023 compared to fiscal year 2022. As our overall headcount has grown, we have continued to attract and retain high-performing, diverse employees at all levels of the organization. We will continue efforts to increase employee satisfaction and retention in the future.
Diversity and Inclusion
We are committed to diversity and inclusion (“D&I”), and we are always working to improve in this area. We continue to evolve our talent acquisition process to focus on diversity for both external hires and succession planning. We make efforts to work with vendors and to consider candidates for employment from underrepresented categories. Our global cloud-based human capital management platform enables us to more accurately track employee representation and identify how we can better enhance our diversity around the world. We train managers annually on anti-discrimination and anti-harassment practices. Our executive officers have committed to help drive further D&I progress during our year ending March 31, 2024 and beyond. As of March 31, 2023, 57% of our directors are from under-represented categories.
Compensation and Benefits
We are intentional in providing fair and equitable compensation to all of our employees. Our compensation and benefits are competitive to market and create incentives to attract and retain employees. In determining merit increases, we evaluate individual performance—including measuring an individual's contribution to company goals and performing semi-annual performance reviews—to align financial incentives with individual contributions. Our compensation package includes market-competitive pay, cash bonuses, stock-based compensation to certain levels of employees, health care and retirement benefits, paid time off, paid caregiver leave, and 401(K) matching, among other benefits.
Communication and Engagement
We believe that our success depends in part on our employees understanding how their work contributes to our company purpose and strategy. To this end, we utilize a variety of channels to facilitate open and direct communication, including: (i) quarterly town hall meetings with our executive team; (ii) internally maintained websites; (iii) an anonymous whistleblower hotline that is advertised to our employees; and (iv) employee engagement surveys. We also measure employee net promoter scores, which is an employee ranking of how likely they are to recommend working at Mesa to a family member or friend. Our employee net promoter scores in fiscal year 2023 improved slightly compared to the prior year. We have undertaken several initiatives to improve employee engagement, including implementing salary increases and leadership development programs. Additionally, we recently launched an enhanced version of the Mesa Way, an initiative developed by a cross-functional committee of employees from all levels, divisions, and geographies in our company to connect our employees and the work we do with our purpose and values. We will continue tracking and making efforts to improve our net promoter scores and employee engagement in the future.
Available Information
We are subject to the reporting and other information requirements of the Securities Exchange Act of 1934, as amended (“Exchange Act”). We make available, free of charge, on or through our website at www.mesalabs.com under the link “Financials” in the Investor Relations section, our annual report on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, and other information. Information on our website is not incorporated into this annual report on Form 10-K and is not a part of this report. The Securities and Exchange Commission (“SEC”) also maintains a website at www.sec.gov containing reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Our code of ethics and Board of Directors committee charters and policies are also posted on the Investor Relations section of our website. The information on our website is not part of this or any other report Mesa files with, or furnishes to, the SEC.
In addition to the other information set forth in this Annual Report on Form 10-K and other documents we filed with the SEC, you should carefully consider the following factors, which could materially affect our business, financial condition or results of operations in future periods. The risks and uncertainties described below are those that we have identified as material, but these are not the only risks and uncertainties facing us. Our business is also subject to general risks and uncertainties that affect many other companies, such as market conditions, economic conditions, geopolitical events, changes in laws, regulations or accounting rules, fluctuations in interest rates, terrorism, wars or conflicts, major health concerns, natural disasters or other disruptions of expected business conditions. Additional risks and uncertainties not currently known to us or that we currently believe are immaterial also may impair our business, including our results of operations, liquidity and financial condition.
Business and Strategic Risks
Conditions in the global economy, the markets we serve, and financial markets may adversely affect our business, financial statements, and access to capital markets.
Our business is sensitive to general economic conditions. Slow or disrupted global economic growth, heightened inflation, volatility in the currency and credit markets, high levels of unemployment or underemployment, labor availability constraints, reduced levels of capital expenditures, changes or anticipation of potential changes in government fiscal, tax, trade and monetary policies, changes in capital requirements for financial institutions, government deficit reduction and budget negotiation dynamics, sequestration, austerity measures, sovereign debt defaults, and other challenges that adversely affect the global economy could adversely affect us and our distributors, customers and suppliers, including having the effect of:
| ● |
reducing demand for our products and services, limiting the financing available to our customers and suppliers, increasing order cancellations and resulting in longer sales cycles and slower adoption of new technologies; |
| ● |
increasing the difficulty in collecting accounts receivable and the risk of excess and obsolete inventories; |
| ● |
increasing price competition in our served markets; |
| ● |
supply interruptions, which could disrupt our ability to produce our products; |
| ● |
increasing the risk of impairment of goodwill and other long-lived assets, and the risk that we may not be able to fully recover the value of other assets such as tax assets; |
| ● |
increasing the risk that counterparties to our contractual arrangements will become insolvent or otherwise unable to fulfill their contractual obligations, which could increase the risks identified above; and |
| ● |
adversely impacting market sizes and growth rates. |
Inflationary conditions in recent periods have resulted in the U.S. Federal Reserve and other financial regulatory bodies implementing increases in interest rates, and these increases have slowed global growth and made a recession more likely. If growth in the global economy or in any of the markets we serve slows for a significant period, if there is significant deterioration in the global economy or such markets or if improvements in the global economy do not benefit the markets we serve, our business and financial results could be adversely affected. We cannot predict the likelihood, duration or severity of any disruption in financial markets or any adverse economic conditions in the U.S. and other countries.
Our growth could suffer if the markets into which we sell our products and services decline, do not grow as anticipated or experience volatility.
Our growth depends in part on the growth of the markets which we serve, and visibility into our markets is limited (particularly for markets into which we sell through distribution). Any decline or lower than expected growth in our served markets could diminish demand for our products and services, which would adversely affect our financial statements. Certain of our businesses’ demand depends on customers’ capital spending budgets as well as government funding policies, and matters of public policy and government budget dynamics as well as product and economic cycles can affect the spending decisions of these entities. Demand for our products and services is also sensitive to changes in customer order patterns, which may be affected by announced price changes, marketing or promotional programs, new product introductions, the timing of industry conferences, changes in distributor or customer inventory levels, or other factors. Any of these factors could adversely affect our growth and results of operations in any given period.
We face competition and if we are unable to compete effectively, we may experience decreased demand and market share resulting in decreased revenues. Even if we compete effectively, we may be required to reduce prices for our products and services resulting in decreased profit margins.
The markets for our current and potential products are competitive. Because of the range of products and services we sell and the variety of markets we serve, we encounter a wide variety of competitors, including several that possess both larger sales forces and greater capital resources.
In order to compete effectively, we must maintain relationships with major customers, continue to grow our business by establishing relationships with new customers, develop new products and services to maintain and expand our brand recognition, and penetrate new markets, including in developing countries and high growth markets. Our failure to compete effectively or pricing pressures resulting from competition may adversely impact our results of operations.
Changing industry trends may affect our results of operations.
Various changes within the industries we serve may limit future demand for our products and may include mergers within key industries we serve, making us more dependent on fewer, larger customers for our sales; decreased product demand driven by changes in customers' regulatory environments or standard industry practices; price competition for key products; and new competitor products that may result in customers discontinuing new orders.
Our growth depends in part on the timely development, commercialization, and customer acceptance of new and enhanced products and services based on technological innovation.
Our growth depends on the acceptance of our products and services in the marketplace, the penetration achieved by the companies to which we sell, and our ability to introduce new and innovative products that meet the needs of the various markets we serve. We can offer no assurance that we will be able to continue to introduce new and enhanced products, that the products we introduce, or have introduced, will be widely accepted by the marketplace, or that our direct sales team or independent distributors will successfully penetrate our various markets. Our failure to introduce new and enhanced products or gain widespread acceptance of our products and services could adversely affect our financial results. If we fail to accurately predict future customer needs and preferences, fail to produce viable technologies, or fail to protect the intellectual property of such technologies, we may invest heavily in research and development of products and services that do not lead to significant revenues, which could adversely affect our profitability. Even if we successfully innovate and develop new and enhanced products and services, we may incur substantial costs in doing so, and our profitability may suffer.
If we are unable to continue to hire and retain skilled personnel, we will have difficulty manufacturing and marketing our products.
Our success depends largely upon the continued service of our management and manufacturing employees and our ability to attract, retain and motivate manufacturing and management personnel, some of whom we are recruiting for in-person positions in competitive labor markets, particularly Bozeman, Montana. Loss of key personnel or our inability to hire and retain personnel could materially adversely affect our manufacturing efforts, harm our ability to meet compliance requirements, and increase backlog. Further, if we have to pay manufacturing employees higher wages to attract and retain them, our gross margins and overall profitability may decline.
Adverse changes in our relationships with, or the financial condition, performance, purchasing patterns or inventory levels of, distributors and other channel partners could adversely affect our financial statements.
We sell a significant number of products to distributors and other channel partners that have valuable relationships with customers and end-users. Some of these distributors and other partners also sell our competitors’ products or compete with us directly, and if they favor competing products for any reason, they may fail to market our products effectively. Adverse changes in our relationships with these distributors and other partners, or adverse developments in their financial condition, performance or purchasing patterns, could adversely affect our business and financial statements.
The levels of inventory maintained by our distributors and other channel partners, and changes in those levels, can also negatively impact our results of operations in any given period. In addition, the consolidation of distributors could adversely impact our business and financial statements. We cannot directly control the actions of our distributors. Our distributors may not comply with export laws, or follow the terms of the distribution agreements which require compliance with export laws, which could have legal or financial implications for us.
Our international operations subject us to a wide range of risks.
Our operations and sales outside of the United States have increased as a result of our strategic acquisitions and the continued expansion of our commercial organization. Risks related to these increased foreign operations include:
| ● | fluctuations in foreign currency exchange rates, which may affect reported results from operations as well as actual costs; | |
| ● |
interruption in the transportation of materials to us and finished goods to our customers; |
| ● |
differences in terms of sale, including longer payment terms than are typical in the United States; |
| ● |
local product preferences and product requirements; |
| ● |
trade protection measures, embargoes and import or export restrictions and requirements; |
| ● |
unexpected changes in laws or regulatory requirements, including changes in labor or tax laws; |
| ● |
capital controls and limitations on ownership and on repatriation of earnings and cash; |
| ● |
changes in general economic and political conditions in countries where we operate, particularly as a result of ongoing economic instability within foreign jurisdictions; |
| ● |
difficulty in staffing and managing widespread operations; |
| ● |
differing labor or employment regulations; |
| ● |
difficulties in implementing restructuring actions on a timely or comprehensive basis; |
| ● |
differing protection of intellectual property; and |
| ● |
greater uncertainty, risk, expense and delay in commercializing products in certain foreign jurisdictions, including with respect to product and other regulatory approvals. |
International business risks have in the past and may in the future negatively affect our business and financial statements. A deterioration in diplomatic relations between the United States and any country where we conduct business could adversely affect our future operations and lead to a decline in profitability. Changes in U.S. policy regarding international trade, including import and export regulation and international trade agreements, could also negatively impact our business. Tariffs imposed by the U.S. on a broad range of imports or trade measures imposed by other countries could result in an increase in supply chain costs that we may not be able to offset or that could otherwise adversely impact our results of operations.
Our international operations are governed by the U.S. Foreign Corrupt Practices Act and similar anti-corruption laws outside the United States. Global enforcement of anti-corruption laws has increased in recent years, with more enforcement proceedings by U.S. and foreign governmental agencies and the imposition of significant fines and penalties. Our international operations, which often involve customer relationships with foreign governments, create the risk that there may be unauthorized payments or offers of payments made by employees, consultants, or distributors. Any alleged or actual violations of these laws may subject us to government investigations and significant criminal or civil sanctions and other liabilities, and negatively affect our reputation.
The COVID-19 pandemic, or similar public health crises, could have an adverse impact and risk to our business.
Since December 2019, COVID-19 has spread to countries in which we or our customers and suppliers operate and has caused major disruption. The COVID-19 virus has led to the implementation of various responses, including government-imposed quarantines, extended business closures, travel restrictions and other public health safety measures, as well as reported adverse impacts on healthcare resources, facilities and providers across the United States and in other countries. Our business operations were impacted by such restrictions. Many of our customers and potential customers closed facilities or limited facility hours due to the spread of COVID-19. Such closures have resulted in, and may continue to result in, our inability to demonstrate and install some of our products, and lower demand for certain products. Any interruptions in the installation of ordered products could delay our ability to recognize revenues in a particular period.
We operate on a global basis with offices or operations in North America, Europe, and Asia, and global health crises, such as COVID-19, could result in a widespread economic downturn in the industries in which we and our customers operate. The extent to which outbreaks impact our business and the businesses of our customers will depend on future developments, which remain uncertain and cannot be predicted with confidence, such as the impact of new business closures and business disruptions. Government mandated shut-downs and restrictions in China adversely impacted revenues from our Clinical Genomics division during fiscal year 2023. Some effects of the COVID-19 pandemic that could delay or otherwise adversely affect our operations and performance include disruptions in our supply chains, limitations on travel that could interrupt our ability to market products and provide installations or maintenance services at customer sites, interruption in global shipping affecting the transport of our products and supplies, restrictions on business operations by governments, impacts to the valuation of our financial assets due to market volatility, and delays in partner clinical trials due to government-imposed restrictions or lockdowns in China, among others. The COVID-19 pandemic could also have the effect of heightening other risk factors described in this report.
Although the COVID-19 pandemic has largely subsided as a public health matter, we may experience material adverse impacts to our business as a result of the pandemic's adverse impact on the global economy, in-person collaboration and sales efforts, and our customers' changed purchasing behaviors and confidence.
Operational Risks
A significant disruption in, or breach in security of, our information technology systems or data could adversely affect our business, reputation and financial statements.
We rely on information technology systems, some of which are provided or managed by third-parties, to process, transmit and store electronic information (including sensitive data such as confidential business information and personally identifiable data relating to employees, customers, and other business partners), and to manage or support a variety of critical business processes and activities (such as receiving and fulfilling orders, billing, collecting and making payments, shipping products, providing services and support to customers and fulfilling contractual obligations). In addition, some products or software we sell to customers may connect to our systems for maintenance or other purposes, and we sell software as a service and cloud-based platforms. These systems, products and services (including those we acquire through business acquisitions) may be damaged, disrupted or shut down due to attacks by computer hackers, computer viruses, ransomware, human error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and in any such circumstances our system redundancy and other disaster recovery planning may be ineffective or inadequate. Attacks may also target hardware, software and information installed, stored or transmitted in our products after such products have been purchased and incorporated into third-party products, facilities or infrastructure. Security breaches of systems provided or enabled by us, regardless of whether the breach is attributable to a vulnerability in our products or services, could result in the misappropriation, destruction or unauthorized disclosure of confidential information or personal data belonging to us or to our employees, partners, customers, or suppliers. Our information technology systems have been subject to computer viruses, malicious codes, unauthorized access and other cyber-attacks and we expect the sophistication and frequency of such attacks to continue to increase. Unauthorized tampering, adulteration or interference with our products may also adversely affect product functionality and result in loss of data, risk to product safety and product recalls or field actions.
Any attacks, breaches or other disruptions or damage could interrupt our operations or the operations of our customers and partners, delay production and shipments, result in theft of our and our customers’ intellectual property and trade secrets, damage customer, business partner, and employee relationships, and our reputation, or result in defective products or services, legal claims and proceedings, liability and penalties under privacy laws and increased costs for security and remediation, each of which could adversely affect our business, reputation and financial statements.
Further, a significant number of our employees work remotely, which exposes us to greater cybersecurity risks. Any inability to maintain reliable information technology systems and appropriate controls with respect to global data privacy and security requirements and prevent data breaches can result in adverse regulatory consequences, business consequences and litigation.
We face numerous manufacturing and supply chain risks. In addition, our reliance upon sole or limited sources of supply for certain materials, components and services could cause production interruptions, delays and inefficiencies.
We purchase materials, components and equipment from third parties for use in our manufacturing operations. Our results of operations could be adversely impacted if we are unable to adjust our purchases to reflect changes in customer demand and market fluctuations. Suppliers may extend lead times, limit supplies or increase prices. If we cannot purchase sufficient products at competitive prices and of sufficient quality on a timely enough basis to meet increasing demand, we may not be able to satisfy market demand, product shipments may be delayed, our costs may increase, or we may breach our contractual commitments and incur liabilities.
In addition, some of our businesses purchase certain required products from sole or limited source suppliers for reasons of quality assurance, regulatory requirements, cost effectiveness, availability or uniqueness of design. If these or other suppliers encounter financial, operating or other difficulties or if our relationship with them changes, we might not be able to quickly establish or qualify replacement sources of supply. The supply chains for our businesses were impacted in fiscal year 2023 and could also be disrupted in the future by supplier capacity constraints, supplier bankruptcy or exiting of the business for other reasons, decreased availability of key raw materials or commodities and external events such as natural disasters, pandemics or other public health problems, war, terrorist actions, governmental actions and legislative or regulatory changes. Any of these factors could result in production interruptions, delays, extended lead times and inefficiencies.
Our revenues and other operating results depend in large part on our ability to manufacture and assemble our products in sufficient quantities and in a timely manner. Any interruptions we experience in the manufacture or shipment of our products or changes to the way we manufacture products could delay our ability to recognize revenues in a particular period. In addition, we must maintain sufficient production capacity in order to meet anticipated customer demand, which carries fixed costs that we may not be able to offset if orders slow, which would adversely affect our operating margins. If we are unable to manufacture our products consistently, in sufficient quantities, and on a timely basis, our revenues, gross margins and our other operating results will be materially and adversely affected.
Because we cannot always immediately adapt our production capacity and related cost structures to changing market conditions, our manufacturing capacity may at times exceed or fall short of our production requirements. Any or all of these problems could result in the loss of customers, provide an opportunity for competing products to gain market acceptance, and otherwise adversely affect our financial condition.
Our financial results are subject to fluctuations in the cost and availability of components and commodities that we use in our operations.
Our manufacturing operations employ a wide variety of components and raw materials and other commodities, including metallic-based components, electronic components, chemicals, and plastics and other petroleum-based products. Prices for and availability of these components, and raw materials and other commodities have fluctuated significantly in the past, and more recently have increased. Any sustained interruption in the supply of these items could disrupt production, delay customer order fulfillments, and adversely affect our business. In addition, due to the highly competitive nature of the industries that we serve, the cost-containment efforts of our customers and the terms of certain contracts we are party to, if components and commodity prices rise, we may be unable to pass along cost increases through higher prices. If we are unable to fully recover higher costs through price increases or offset these increases through cost reductions, or if there is a time delay between the increase in costs and our ability to recover or offset these costs, our margins and profitability could decline, and our financial statements could be adversely affected.
In addition, transportation costs have increased, which may reduce our gross profit margins unless and until we are able to pass the cost increases along to our customers. There are several reasons for the supply chain disruptions to components that we rely on to manufacture our products, including: increased demand for other products that use the same components as those we purchase, manufacturing shut-downs that reduced production of components, obsolescence of materials we have historically purchased, labor issues, and long lead times for raw materials used in the production of components. A continued shortage of components or other key materials that comprise the components could cause a significant disruption to our production schedule and have a substantial adverse effect on our financial condition or results of operations.
Significant developments or uncertainties stemming from the U.S. administration, including changes in U.S. trade policies, tariffs and the reaction of other countries thereto could have an adverse effect on our business.
Changes, potential changes or uncertainties in U.S. social, political, regulatory and economic conditions or laws and policies governing foreign trade, manufacturing, and development and investment in the territories and countries where we or our customers operate, or governing the health care system, can adversely affect our business and financial statements. For example, trade tensions between the United States and China remain high, and each country has continued to impose significant tariffs on a wide range of goods imported from the other country. China accounted for approximately 12% of our sales during the year ended March 31, 2023. These factors have adversely affected, and in the future could further adversely affect, our business and financial statements.
Macroeconomic pressures in the markets in which we operate may adversely affect our financial results.
Geopolitical issues around the world can impact macroeconomic conditions and could have a material adverse impact on our financial results. For example, the ultimate impact of the conflict in Ukraine on fuel prices, inflation, the global supply chain and other macroeconomic conditions is unknown and could materially adversely affect global economic growth, disrupting discretionary spending habits and generally decreasing demand for our products and services. While we do not purchase any significant raw materials directly from Russia, it is a significant global producer of fuel, nickel, and copper. Disruptions in the markets for those inputs could negatively impact the global economy. We cannot predict the extent or duration of sanctions in response to the conflict in Ukraine, nor can we predict the effects of legislative or other governmental actions or regulatory scrutiny of Russia and Belarus, Russia's other allies or other countries with which Russia has significant trade or financial ties, including China. While our sales to Russia have historically produced an immaterial amount of revenues and profitability compared to the overall company, we cannot predict the impact that the conflict may have on future financial results.
Violation of data privacy laws could adversely affect our business, reputation and financial statements.
If we are unable to maintain reliable information technology systems and appropriate controls with respect to global data privacy and security requirements and prevent data breaches, we may suffer adverse regulatory consequences, business consequences and litigation. As a multinational organization, we are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal and/or sensitive data in the course of our business. The EU General Data Protection Regulation imposes strict requirements on how we collect and process personal data, including, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. Data privacy laws in other jurisdictions, such as California and Colorado, also impose data privacy obligations. Government enforcement actions can be costly and interrupt the regular operation of our business, and data breaches or violations of data privacy laws can result in fines, reputational damage and civil lawsuits, any of which may adversely affect our business, reputation and financial statements. In addition, compliance with the varying data privacy regulations around the world may require significant expenditures and may require changes in our products or business models that reduce revenues.
Changes to dialysis methods and equipment capabilities may decrease demand for our dialysis products and negatively impact our financial statements.
Our Dialyguard product line accounts for approximately one-third of the revenues and gross margin associated with our Calibration Solutions division. The majority of revenues in our Dialyguard business are associated with products used in dialysis clinics, while a smaller portion of our sales relate to in-home care. Technological advancements, such as dialysis machines that feature built-in dialysis calibration functionalities, have and may continue to adversely affect demand for our dialysis products.
We may be unable to efficiently manage our growth as a larger and more geographically diverse organization.
Our strategic acquisitions and the continued organic expansion of our commercial sales operations have increased the scope and complexity of our business. As a result, we face challenges inherent in efficiently managing a more complex business with an increased number of employees over large geographic distances, including the need to implement appropriate systems, policies, benefits, and compliance programs. Our inability to manage successfully a substantially larger and geographically more diverse (including from a cultural perspective) organization could materially adversely affect our operating results and financial statements.
If we suffer loss to our facilities, supply chains, distribution systems or information technology systems due to a catastrophic event, our operations could be seriously harmed.
Our facilities, supply chains, distribution systems and information technology systems are subject to catastrophic loss due to fire, flood, earthquake, hurricane, pandemics and epidemics and other public health crises, war, terrorism or other natural or human-made disasters. If any of these facilities, supply chains or systems were to experience a catastrophic loss, it could disrupt our operations, delay production and shipments, result in defective products or services, damage customer relationships and our reputation and result in legal exposure and large repair or replacement expenses. Our insurance coverage with respect to natural disasters is limited and is subject to deductible and coverage limits and may be unavailable or insufficient to protect us against such losses.
The health care industry and related industries that we serve have undergone, and are in the process of undergoing, significant changes in an effort to reduce costs, which could adversely affect our financial statements.
Participants in the health care industry and related industries have implemented, and are implementing, significant changes in an effort to reduce costs. Many of the end-users to whom our customers supply products rely on government funding of and reimbursement for health care products and services and research activities. The U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the “PPACA”), health care austerity measures in other countries and other potential health care reform changes and government austerity measures have reduced and may further reduce the amount of government funding or reimbursement available to customers or end-users of our products and services and/or the volume of medical procedures using our products and services.
These changes as well as other impacts from market demand, government regulations, third-party coverage and reimbursement policies and societal pressures have started changing the way healthcare is delivered, reimbursed and funded and may cause participants in the health care industry and related industries that we serve to purchase fewer of our products and services, reduce the prices they are willing to pay for our products or services, reduce the amount of reimbursement and funding available for our products and services from governmental agencies or third-party payors, affect the acceptance rate of new technologies and products and increase our compliance and other costs. All of the factors described above could adversely affect our business and financial results.
The manufacture of many of our products is a highly exacting and complex process, and if we directly or indirectly encounter problems manufacturing products, our reputation, business and financial results could suffer.
The manufacture of many of our products is a highly exacting and complex process, due in part to strict regulatory requirements. Problems may arise during manufacturing for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials, natural disasters and environmental factors, and if not discovered before the product is released to market could result in recalls and product liability exposure. Because of the time required to approve and license certain regulated manufacturing facilities and other stringent regulations of the FDA and similar agencies regarding the manufacture of certain of our products, an alternative manufacturer may not be available on a timely basis to replace such production capacity. Any of these manufacturing problems could result in significant costs, liability, lost revenues, and loss of market share, as well as negative publicity and damage to our reputation that could reduce demand for our products.
Climate change, or legal or regulatory measures to address climate change and sustainability, may negatively affect us, and any actions we take or fail to take in response to such matters could damage our reputation.
Climate change resulting from increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere could present risks to our operations. Physical risk resulting from acute changes (such as hurricanes, tornados, wildfires or flooding) or chronic changes (such as droughts, heat waves or sea level changes) in climate patterns can adversely impact our facilities and operations and disrupt our supply chains and distribution systems. Concern over climate change can also result in new or additional legal or regulatory requirements designed to reduce greenhouse gas emissions and/or mitigate the effects of climate change on the environment (such as taxation of, or caps on the use of, carbon-based energy). Any such new or additional legal or regulatory requirements may increase the costs associated with, or disrupt, sourcing, manufacturing and distribution of our products, which may adversely affect our business and financial statements.
Acquisition Risks
Any inability to consummate acquisitions at our historical rate and at appropriate prices could negatively impact our growth rate and stock price.
Our ability to grow revenues, earnings and cash flows at or above our historic rates depends in part upon our ability to identify and successfully acquire and integrate businesses at appropriate prices and realize anticipated synergies. We may not be able to consummate acquisitions at rates similar to the past, which could adversely impact our growth rate and our stock price. Promising acquisitions are difficult to identify and execute for a number of reasons, including high valuations, competition among prospective buyers, the availability of affordable funding in the capital markets, and the need to satisfy applicable closing conditions. Changes in accounting or regulatory requirements, or instability in the credit markets, or global crises that prevent travelling or other activities necessary for acquisitions could also adversely impact our ability to consummate acquisitions.
Our acquisition of businesses could negatively impact our financial statements.
Acquisitions involve a number of financial, accounting, managerial, operational, legal, compliance and other risks and challenges, including the following, any of which could adversely affect our business and our financial statements:
| ● |
any business, technology, service or product that we acquire could under-perform relative to our expectations and the price that we paid for it, or not perform in accordance with our anticipated timetable, or we could fail to make such business profitable; |
| ● |
we may incur or assume significant debt in connection with our acquisitions which could cause a deterioration of our credit rating, result in increased borrowing costs and interest expense and diminish our future access to the capital markets; |
| ● |
acquisitions could cause our results of operations to differ from our own or the investment community’s expectations in any given period, or over the long-term; |
| ● |
pre-closing and post-closing acquisition-related earnings charges could adversely impact our results of operations in any given period, and the impact may be substantially different from period to period; |
| ● |
acquisitions could create demands on our management, operational resources and financial and internal control systems that we are unable to effectively address, or for which we may incur additional costs; |
| ● |
we could experience difficulty in integrating personnel, operations, financial and other systems, and in retaining key employees and customers; |
| ● |
we may be unable to achieve cost savings or other synergies anticipated in connection with an acquisition; |
| ● |
we may assume by acquisition unknown liabilities, known contingent liabilities that become realized, known liabilities that prove greater than anticipated, internal control deficiencies or exposure to regulatory sanctions resulting from the acquired company’s activities. The realization of any of these liabilities or deficiencies may increase our expenses, adversely affect our financial position or cause us to fail to meet our public financial reporting obligations; |
| ● |
in connection with acquisitions, we may enter into post-closing financial arrangements such as purchase price adjustments, earn-out obligations and indemnification obligations, which may have unpredictable financial results; and |
| ● |
as a result of our acquisitions, we have recorded significant goodwill and intangible assets on our balance sheets. If we are not able to realize the value of these assets, we may be required to incur charges relating to the impairment of these assets, which could materially impact our financial results. |
The indemnification provisions of acquisition agreements by which we have acquired companies may not fully protect us and as a result we may face unexpected liabilities, or we may have acquisition agreements with no indemnification protection at all.
Certain of the acquisition agreements by which we have acquired companies require the former owners to indemnify us against certain liabilities related to the operation of the company before we acquired it. In most of these agreements, however, the liability of the former owners is limited, and certain former owners may be unable to meet their indemnification responsibilities. We cannot guarantee that these indemnification provisions will protect us fully or at all, and as a result we may face unexpected liabilities that could adversely impact our financial statements. In addition, we may enter into acquisition agreements that have no indemnification protection at all.
Future strategic transactions or acquisitions may require us to seek additional financing, which we may not be able to secure on favorable terms, or at all.
We actively evaluate various strategic transactions on an ongoing basis, and in order to complete such transactions, we may need to seek additional financing. We may not be able to secure such financing on favorable terms, or at all. In addition, future acquisitions may require the issuance or sale of additional equity or debt securities, which may result in dilution to our stockholders.
Legal, Regulatory, Compliance, and Reputational Risks
We are subject to lawsuits and regulatory proceedings.
We have been a defendant in a number of lawsuits, and in the future are subject to the possibility of a variety of litigation and regulatory proceedings, including claims for damages arising out of the use of products or services and claims relating to intellectual property matters, employment matters, tax matters, commercial disputes, product liability, marketing matters, insurance coverage, competition and sales and trading practices, environmental matters, product retirement, personal injury, and acquisition or divestiture-related matters, as well as regulatory investigations or enforcement. We may also become subject to lawsuits as a result of past or future acquisitions or as a result of liabilities retained from, or representations, warranties or indemnities provided in connection with, divested businesses. Any of these lawsuits may include claims for compensatory damages, punitive and consequential damages or injunctive relief. The defense of these lawsuits may divert our management’s attention, we may incur significant expenses in defending these lawsuits, and we may be required to pay damages or settlements or become subject to equitable remedies that could adversely affect our operations and financial results. Moreover, any insurance or indemnification rights that we may have may be insufficient or unavailable to protect us against such losses. In addition, developments in proceedings in any given period may require us to adjust loss contingency estimates that we have recorded in our financial statements, record estimates for liabilities or assets previously not susceptible of reasonable estimates or pay cash settlements or judgments. Any of these developments could adversely affect our financial results in any given period. We cannot make assurances that our liabilities in connection with litigation and other legal regulatory proceedings will not exceed our estimates or adversely affect our financial results and business. Please see Note 13. “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8. Financial Statements and Supplementary Data for additional discussion.
Our reputation, ability to do business and prepare financial statements may be impaired by improper conduct by any of our employees, agents or business partners.
We cannot provide assurance that our internal controls and compliance systems will always protect us from acts committed by employees, agents or business partners of ours (or of businesses we acquire or partner with) that would violate U.S. and/or non-U.S. laws, including the laws governing payments to government officials, bribery, fraud, kickbacks and false claims, pricing, sales and marketing practices, conflicts of interest, competition, export and import compliance, money laundering and data privacy.
If we do not or cannot adequately protect our intellectual property, if third parties infringe our intellectual property rights, or if we or our customers are alleged to infringe upon others’ intellectual property rights, we may suffer competitive injury or expend significant resources enforcing or defending our rights.
We own patents, trademarks, copyrights, trade secrets and other intellectual property and licenses to intellectual property owned by others, which in the aggregate are important to our business. The intellectual property rights that we obtain, however, may not be sufficiently broad or otherwise may not provide us a significant competitive advantage, and patents may not be issued for pending or future patent applications owned by or licensed to us. In addition, the steps that we and our licensors have taken to maintain and protect our intellectual property may not prevent it from being challenged, invalidated, circumvented or designed-around, particularly in countries where intellectual property rights are not highly developed or protected. In some circumstances, enforcement may not be available to us because an infringer has a dominant intellectual property position or for other business reasons, or countries may require compulsory licensing of our intellectual property. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements will adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets or other proprietary rights. In addition, we or our customers may be alleged to infringe upon the intellectual property of third parties. Our failure to obtain or maintain intellectual property rights that convey competitive advantages, adequately protect our intellectual property, detect or prevent circumvention or unauthorized use of such property, and limit the cost of enforcing our intellectual property rights or defending against any allegation of infringement, could adversely impact our competitive position and results of operations.
We are subject to extensive regulation.
The process of obtaining and maintaining required regulatory approvals is lengthy, expensive and uncertain. We can offer no assurance that delays will not occur in the future that could have a significant adverse effect on our ability to introduce new products on a timely basis. Regulatory agencies periodically inspect our manufacturing facilities to ascertain compliance with “good manufacturing practices” and can subject approved products to additional testing and surveillance programs.
Failure to comply with applicable regulatory requirements can, among other things, result in fines, suspension of regulatory approvals, product recalls, operating restrictions and criminal penalties. If we fail to comply with regulatory requirements, it could have an adverse effect on our results of operations and financial condition. We, our representatives and the industries in which we operate may at times be under review and/or investigation by regulatory authorities. Compliance with applicable regulations may affect our returns on investment, require us to incur significant expenses or modify our business model or impair our flexibility in modifying product, marketing, pricing or other strategies. Our products and operations are also often subject to the rules of industrial standards bodies such as the International Standards Organization, and failure to comply with these rules could result in withdrawal of certifications needed to sell our products and services and otherwise adversely impact our business and financial statements.
Certain of our products are medical devices and other products subject to regulation by the U.S. FDA, by other federal and state governmental agencies, or by comparable agencies of other countries and regions. We cannot guarantee that we will be able to obtain regulatory clearance (such as 510(k) clearance) or approvals for new products or modifications to (or additional indications or uses of) existing products within our anticipated timeframe or at all, and if we do obtain such clearance or approval, it may be time-consuming, costly and subject to restrictions. Our ability to obtain such regulatory clearances or approvals will depend on many factors and the process for obtaining such clearances or approvals could change over time and may require the withdrawal of products from the market until such clearances are obtained. The global regulatory environment has become increasingly stringent and unpredictable. Several countries that did not have regulatory requirements for medical devices have established such requirements in recent years, and other countries have expanded, or plan to expand, their existing regulations.
Ensuring that our internal operations and business arrangements with third parties comply with applicable laws and regulations involves substantial costs. It is also possible that government authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law. Noncompliance with applicable laws and regulations can result in, among other things, fines, expenses, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, failure to receive 510(k) clearance of devices, withdrawal of marketing approvals, reputational damage, business disruption, loss of customers, disbarment from selling to certain federal agencies, criminal prosecutions and other adverse effects. Further, defending against any such actions can be costly and time-consuming and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions brought against us, our business may be negatively impacted.
Off-label marketing of our products could result in substantial penalties.
The FDA strictly regulates the promotional claims that may be made about approved or cleared products. In particular, any clearances we may receive only permit us to market our products for the uses indicated on the labeling cleared by the FDA. We may request additional label indications for our current products, and the FDA may deny those requests outright, require additional data to support any additional indications or impose limitations on the intended use of any cleared products as a condition of clearance. If the FDA determines that we have marketed our products for off-label use, we can be subject to fines, injunctions or other penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, substantial monetary penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, and/or the curtailment of our operations. Any of these events could significantly harm our business and financial results.
Certain modifications to our products may require new 510(k) clearances or other marketing authorizations and may require us to recall or cease marketing our products.
Once a medical device is permitted to be legally marketed in the United States pursuant to a 510(k) clearance, a manufacturer may be required to notify the FDA of certain modifications to the device. Manufacturers determine in the first instance whether a change to a product requires a new 510(k) clearance or premarket submission, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances are necessary. We have made modifications to our products in the past and have determined based on our review of the applicable FDA regulations and guidance that in certain instances new 510(k) clearances or other premarket submissions were not required. We may make similar modifications or add additional features in the future that we believe do not require a new 510(k) clearance. If the FDA disagrees with our determinations and requires us to submit new 510(k) notifications, we may be required to cease marketing or to recall the modified product until we obtain clearance, and we may be subject to significant regulatory fines or penalties.
Changes in governmental regulations may reduce demand for our products or services or increase our expenses.
We compete in markets in which we and our customers must comply with federal, state, and other jurisdictional regulations, such as regulations governing health and safety, food and drugs, privacy and electronic communications. We develop, configure and market our products and services to meet customer needs created by these regulations. These regulations are complex, change frequently, have tended to become more stringent over time and may be inconsistent across jurisdictions. Any significant change in any of these regulations (or in the interpretation or application thereof) could reduce demand for, increase our costs of producing or delay the introduction of new or modified products and services, or could restrict our existing activities, products and services. In addition, in certain of our international markets our growth depends in part upon the introduction of new regulations. In these markets, the delay or failure of governmental and other entities to adopt or enforce new regulations, the adoption of new regulations which our products and services are not positioned to address or the repeal of existing regulations, could adversely affect demand. In addition, regulatory deadlines may result in substantially different levels of demand for our products and services from period-to-period.
Product liability suits against us, product defects or unanticipated use or inadequate disclosure with respect to our products or services could adversely affect our business, reputation and our financial statements.
Manufacturing or design defects in, unanticipated use of, safety or quality issues (or the perception of such issues) with respect to, or inadequate disclosure of risks relating to the use of products and services that we make or sell, including items that we source from third parties, can lead to personal injury, property damage or other liability. These events could lead to recalls or safety alerts, the removal of a product or service from the market and product liability or similar claims being brought against us. Recalls, removals and product liability and similar claims, regardless of their validity or ultimate outcome, can result in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products and services. Our product liability insurance may not adequately cover our costs arising from defects in our products or otherwise.
We are subject to export and import control laws and regulations that could impair our ability to compete in international markets or subject us to liability if we violate such laws and regulations.
We are subject to U.S. export controls and sanctions regulations that restrict the shipment or provision of certain products and services to certain countries, governments, and persons. While we take precautions to prevent our products and services from being exported in violation of these laws, we cannot guarantee that the precautions we take will prevent violations. If we are found to be in violation of U.S. sanctions or export control laws, it could result in substantial fines and penalties for us and for the individuals working for us. We may also be adversely affected through other penalties, reputational harm, loss of access to certain markets, or otherwise.
Complying with export control and sanctions regulations may be time-consuming and may result in the delay or loss of sales opportunities or impose other costs. Any change in export or import regulations, economic sanctions or related legislation, or change in the countries, governments, persons or technologies targeted by such regulations, could result in our decreased ability to export or sell certain products to existing or potential customers in affected jurisdictions.
We are subject to laws and regulations governing government contracts.
We are subject to laws and regulations governing government contracts, and failure to address these laws and regulations or comply with government contracts could harm our business by leading to a reduction in revenues associated with these customers. We have agreements relating to the sale of our products to government entities and, as a result, we are subject to various statutes and regulations that apply to companies doing business with the government. We are also subject to investigation for compliance with the regulations governing government contracts. A failure to comply with these regulations could result in suspension of these contracts, criminal, civil and administrative penalties or debarment.
Financial and Tax Risks
We have identified material weaknesses in our internal control over financial reporting. If we are unable to develop and maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results in a timely manner or prevent fraud, which may adversely affect investor confidence in our financial reporting and adversely affect our business and operating results and the market price for our common stock.
Under Section 404 of the Sarbanes-Oxley Act of 2002 and rules promulgated by the SEC, companies are required to conduct an annual comprehensive evaluation of their internal control over financial reporting. Further, each year our independent registered public accounting firm is required to attest to and report on the effectiveness of our internal control over financial reporting. Management concluded that as of March 31, 2023, our internal control over financial reporting was not effective. As described in "Part II, Item 9A — Controls and Procedures," we identified two material weaknesses in the design and operation of our internal control over financial reporting whereby (i) Management's review controls over fair value calculations including Management's preliminary valuation of the Belyntic Acquisition were insufficient, and (ii) Management's review controls over the qualitative assessment of goodwill impairment were insufficient to identify potential impairment triggers. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. As a result of these material weaknesses, management has concluded that our disclosure controls and procedures were not effective as of March 31, 2023.
The material weaknesses will not be considered remediated until management designs and implements effective controls, namely Management must use a valuation specialist to perform the final valuation over the net assets acquired in the Belyntic acquisition prior to the end of the measurement period and implement well documented evaluation of its process to monitor for triggers of potential impairment. We expect our remediation efforts to be effective, however, we can provide no assurance that they will be or that additional material weaknesses will not arise in the future. The existence of these material weaknesses and of any other ineffective controls over our financial reporting could have negative impacts including one or more of the following:
| ● |
Restatement of previously filed financial statements; |
| ● |
Failure to meet our reporting deadlines (which among other consequences would result in a default of our convertible Notes due 2025); |
| ● |
Loss of investor confidence; |
| ● |
Restrict our ability to access capital markets; |
| ● |
Require us to expend significant resources to correct the deficiencies; |
| ● |
Negative impact on the trading price of our common stock. |
Foreign currency exchange rates may adversely affect our financial statements.
As a global company with substantial operations outside the U.S., sales and purchases in currencies other than the U.S. dollar expose us to fluctuations in foreign currencies relative to the U.S. dollar and may adversely affect our financial statements. Increased strength of the U.S. dollar increases the effective price of our products sold in U.S. dollars into other countries, which may require us to lower our prices or adversely affect sales to the extent we do not increase local currency prices. Decreased strength of the U.S. dollar could adversely affect the cost of materials, products and services we purchase overseas. Sales and expenses of our non-U.S. businesses are also translated into U.S. dollars for reporting purposes and the strengthening or weakening of the U.S. dollar could result in unfavorable translation effects. In addition, certain of our businesses may invoice customers in a currency other than their functional currency, and movements in the invoiced currency relative to the functional currency could also result in unfavorable translation effects. We also face exchange rate risk from our investments in subsidiaries owned and operated in foreign countries. We do not enter into hedging arrangements to mitigate any foreign currency exposure.
We may be required to recognize impairment charges for our goodwill and other intangible assets.
As of March 31, 2023, the net carrying value of our goodwill and other intangible assets totaled $503.3 million. Significant negative industry or economic trends, disruptions to our business, loss of major customers, strategic shifts in our business, inability to effectively integrate acquired businesses, unexpected significant changes or planned changes in use of our assets, changes in the structure of our business, divestitures, market capitalization declines, or increases in associated discount rates may impair our goodwill and other intangible assets. Any charges relating to such impairments would adversely affect our financial statements in the periods recognized.
The loss of key customers, or reductions in their demand for our products and services, could have a significant negative impact on our revenues, results of operations, and financial position.
Certain of our reporting segments sell to customers who individually comprise greater than 10% of segment revenues. Our business, financial condition or results of operations could be adversely affected by the loss of any such customers, or by a reduction in their purchases of our products and services due to downturns in their business, changes in their business strategies, reduced capital spending, unfavorable macroeconomic conditions, or other factors.
Changes in accounting standards could affect our reported financial results.
New accounting standards or pronouncements that may become applicable from time to time, or changes in the interpretation of existing standards and pronouncements, could have a significant effect on our reported results of operations for the affected periods.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report financial results or prevent fraud. If we identify a material weakness in our internal control over financial reporting, our ability to meet our reporting obligations and the trading price of our stock could be negatively affected.
Effective internal controls are necessary to provide reliable financial reports and to assist in the effective prevention of fraud. Any inability to provide reliable financial reports or prevent fraud could harm our business. We regularly review and update our internal controls, disclosure controls and procedures, and corporate governance policies. In addition, we are required under the Sarbanes-Oxley Act of 2002 to report annually on our internal control over financial reporting. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. If we, or our independent registered public accounting firm, determine that our internal control over financial reporting is not effective, discover areas that need improvement in the future or discover a material weakness, as was the case as of March 31, 2023, these shortcomings could have an adverse effect on our business and financial results, and the price of our common stock could be negatively affected.
Failure to comply with reporting requirements could also subject us to sanctions and/or investigations by the SEC, the Nasdaq Stock Market or other regulatory authorities. We have previously implemented several significant ERP modules and have acquired businesses that were subsequently required to adopt our systems of internal controls. The implementation of these systems represents a change in our internal control over financial reporting. Although we continue to monitor and assess our internal control environment as changes are made, and we have taken steps to modify and enhance the design and effectiveness of our internal control over financial reporting, there is a risk that deficiencies may occur that could aggregate to a material weakness specifically related to our information technology general controls. If we fail to remedy any deficiencies or maintain the adequacy of our internal controls, we could be subject to regulatory scrutiny, civil or criminal penalties or shareholder litigation. In addition, failure to maintain adequate internal controls could result in financial statements that do not accurately reflect our operating results or financial condition.
Our failure to maintain appropriate environmental, social, and governance ("ESG") practices and disclosures could result in reputational harm, a loss of customer and investor confidence, and adverse business and financial results.
Governments, investors, customers, and employees are enhancing their focus on ESG practices and disclosures, and expectations in this area are rapidly evolving and increasing. While we monitor the various and evolving standards and associated reporting requirements, failure to adequately maintain appropriate ESG practices that meet stakeholder expectations may result in reputational harm, loss of business, reduced market valuation, an inability to attract customers, and an inability to attract and retain top talent.
Changes in our tax rates or exposure to additional income tax liabilities or assessments could affect our profitability. In addition, audits by tax authorities could result in additional tax payments for prior periods.
We are subject to income taxes in the U.S. and in various non-U.S. jurisdictions. The amount of income taxes we pay is subject to ongoing audits by U.S. federal, state and local tax authorities and by non-U.S. tax authorities, such as those audits described elsewhere in this report. If audits result in payments or assessments different from our reserves, our future results may include unfavorable adjustments to our tax liabilities and our financial statements could be adversely affected. Any further significant changes to the tax system in the United States or in other jurisdictions (including changes in the taxation of international income as further described below) could adversely affect our financial statements.
Our ability to use net operating losses and tax credit carryforwards and certain built-in losses to reduce future tax payments is limited by provisions of the Internal Revenue Code, and it is possible that certain transactions or a combination of certain transactions may result in material additional limitations on our ability to use our net operating loss and tax credit carryforwards.
Section 382 and 383 of the Internal Revenue Code of 1986, as amended, contain rules that limit the ability of a company that undergoes an ownership change, which is generally any change in ownership of more than 50% of its stock over a three-year period, to utilize its net operating loss and tax credit carryforwards and certain built-in losses recognized in years after the ownership change. These rules generally operate by focusing on ownership changes involving stockholders owning directly or indirectly 5% or more of the stock of a company and any change in ownership arising from a new issuance of stock by the company. Generally, if an ownership change occurs, the yearly taxable income limitation on the use of net operating loss and tax credit carryforwards and certain built-in losses is equal to the product of the applicable long-term, tax-exempt rate and the value of the company’s stock immediately before the ownership change. We may be unable to offset our taxable income with losses, or our tax liability with credits, before such losses and credits expire and therefore would incur larger federal income tax liability. Federal net operating losses generated after December 31, 2017 are not subject to expiration and generally may not be carried back to prior taxable years except that, under the Coronavirus Aid, Relief, and Economic Security Act, net operating losses generated in 2018, 2019 and 2020 may be carried back five taxable years. Additionally, for taxable years beginning after March 31, 2021, the deductibility of such deferral net operating losses is limited to 80% of our taxable income in any future taxable year.
Changes in tax law relating to multinational corporations could adversely affect our tax position.
The U.S. Congress, government agencies in non-U.S. jurisdictions where we and our affiliates do business, and the Organization for Economic Co-operation and Development (“OECD”) have recently focused on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” where profits are claimed to be earned for tax purposes in low-tax jurisdictions, or payments are made between affiliates from a jurisdiction with high tax rates to a jurisdiction with lower tax rates. The OECD has released several components of its comprehensive plan to create an agreed set of international rules for addressing base erosion and profit shifting. As a result, the tax laws in the United States and other countries in which we do business could change on a prospective or retroactive basis, and any such changes could adversely affect our business and financial statements.
Our business is subject to sales tax in numerous states.
The application of indirect taxes, such as sales tax, is a complex and evolving issue. A company is required to collect and remit state sales tax from certain of its customers if that company is determined to have “nexus” in a particular state. The determination of nexus varies by state and often requires knowledge of each jurisdiction’s tax case law. The application and implementation of existing, new or future laws could change the states in which we are required to collect and remit sales taxes. If any jurisdiction determines that we have “nexus” in additional locations that we have not contemplated, it could have an adverse effect on our financial results.
If global credit market conditions deteriorate, our financial performance could be adversely affected.
The cost and availability of credit are subject to changes in the global economic environment. If conditions in major credit markets deteriorate, our ability to obtain debt financing or the terms associated with that debt financing may be negatively affected, which could affect our results of operations.
Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business or the ability to raise capital to repay our 1.375% convertible senior notes due August 15, 2025 (the “2025 Notes”) at maturity or repurchase the notes in the event of a fundamental change, or if we borrow under our credit facility, swingline loan, and letters of credit (together referred to as the "Credit Facility") or if we incur more debt.
We incurred significant indebtedness in the amount of $172.5 million in the form of the 2025 Notes which mature on August 15, 2025, unless earlier converted. We also have a revolving Credit Facility and could borrow additional amounts under that at any time, incurring more debt.
At our option, we may settle the 2025 notes in shares of our common stock, cash, or a combination thereof. Holders of the 2025 Notes also have the right to require us to repurchase all or a portion of their 2025 Notes upon the occurrence of a fundamental change (as defined in the applicable indenture governing the 2025 Notes) at a repurchase price equal to 100% of the principal amount of the 2025 Notes to be repurchased, plus accrued and unpaid interest. In addition, if the 2025 Notes have not previously been converted or repurchased due to a decline in our share price, we may be required to repay the 2025 Notes in cash. Our ability to make required cash payments in connection with conversions of the 2025 Notes, repurchase the 2025 Notes in the event of a fundamental change, or to repay or refinance the 2025 Notes at maturity will depend on market conditions and our future performance, which is subject to economic, financial, competitive, and other factors beyond our control.
In addition, our ability to repurchase or to pay cash upon conversion or at maturity of the 2025 Notes may be limited by law or regulatory authority. Our failure to repurchase Notes following a fundamental change as required by the applicable indenture would constitute a default under such indenture. A default under the indenture or agreements governing our future indebtedness could have a material adverse effect on our business, results of operations, and financial condition. If the payment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the 2025 Notes or to pay cash upon conversion or at maturity.
Additional stock issuances could result in significant dilution to our stockholders.
We may issue additional equity securities to raise capital, make acquisitions, or for a variety of other purposes. Additional issuances of our stock may be made pursuant to the exercise or conversion of new or existing convertible debt securities, stock options, or other equity incentive awards. We rely on equity-based compensation as an important tool in recruiting and retaining employees. The amount of dilution due to equity-based compensation of our employees and other additional issuances could be substantial. In addition, in March 2022, we entered a sales agreement with Jefferies LLC ("Jefferies") to sell shares of our common stock, from time to time, with aggregate gross sales proceeds up to $150.0 million through an at-the-market equity offering program under which Jefferies will act as our sales agent. Further, we may settle all or a portion of the 2025 Notes in shares or in cash, at our option. We include shares of common stock issuable upon conversion of the 2025 Notes in our diluted earnings per share to the extent such shares are not anti-dilutive. We will reevaluate this policy from time to time if we conclude we intend to settle all or a portion of the 2025 notes in cash, or in the event conversion notices are received from noteholders and our stock price is above the strike price. If we issue common stock or securities convertible into common stock for the above reasons, or any other reason, our common stockholders would experience additional dilution and, as a result, our stock price may decline.
Our stock price may be volatile, which may subject us to a securities class action litigation.
The trading price of our common stock price may be volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
| ● |
general economic, industry and market conditions; |
| ● |
actions by institutional or other large stockholders; |
| ● |
the depth and liquidity of the market for our common stock; |
| ● |
volume and timing of orders for our products; |
| ● |
developments generally affecting medical device companies; |
| ● |
the announcement of new products or product enhancements by us or our competitors; |
| ● |
changes in earnings estimates or recommendations by securities analysts; |
| ● |
investor perceptions of us and our business, including changes in market valuations of medical device companies generally; and |
| ● |
our results of operations and financial performance. |
In addition, the stock market in general, and the Nasdaq Stock Market and the market for products and devices sold into the medical and healthcare industries in particular, have experienced substantial price and volume volatility that is often seemingly unrelated to the operating performance of particular companies, including recently in connection with the ongoing COVID-19 pandemic, the conflict in Ukraine and increased inflation and interest rates in the United States, which have resulted in decreased stock prices for many companies notwithstanding the lack of a fundamental change in their underlying business models or prospects. These broad market fluctuations may cause the trading price of our common stock to decline, regardless of our actual operating performance. In the past, securities class action litigation has at times been brought against a company after a period of volatility in the market price of its common stock. We may become involved in this type of litigation in the future. Any securities litigation claims brought against us could result in substantial expense and the diversion of management’s attention from our business.
Item 1B. Unresolved Staff Comments As of March 31, 2023, we owned two facilities and both are material to our business: one in Lakewood, Colorado and the other in Bozeman, Montana.
None.
Both facilities are used for manufacturing, engineering, research and development, marketing, and administration. Two of our four segments use the properties: Sterilization and Disinfectant Control and Calibration Solutions. We have eleven leased facilities which are individually immaterial.
For information regarding legal proceedings, refer to Note 13. “Commitments and Contingencies” in our Consolidated Financial Statements included in Item 8. Financial Statements and Supplementary Data.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded on the Nasdaq Global Market (“Nasdaq”) under the symbol “MLAB.”
While we have paid dividends to holders of our common stock on a quarterly basis since 2003, the declaration and payment of future dividends will depend on many factors, including, but not limited to, our earnings, financial condition, business development needs and regulatory considerations, and is at the sole discretion of our Board of Directors. At this time, we expect to continue paying dividends commensurate with our historical practice.
As of March 31, 2023, there were 60 holders of record of our common stock. This amount does not include “street name” holders or beneficial holders of our common stock, who holder their shares through banks, brokers or other financial institutions.
During the year ended March 31, 2023, we did not sell any equity securities that were not registered under the Securities Act of 1933, as amended.
On November 7, 2005, our Board of Directors adopted a share repurchase plan which allows for the repurchase of up to 300,000 of our common shares. This plan will continue until the maximum is reached or the plan is terminated by further action of the Board of Directors. We made no repurchases of our common stock during the years ended March 31, 2023, March 31, 2022, or March 31, 2021. As of March 31, 2023, 137,514 shares remained available to repurchase pursuant to the repurchase plan.
See Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters for information regarding securities authorized for issuance.
Set forth below is a line graph comparing, for the period March 31, 2018 through March 31, 2023, the cumulative total shareholder return on our common stock against the cumulative total return of (a) the S&P Composite Stock Index (b) the S&P Small Cap 600, and (c) a self-selected peer group, comprised of the following companies: Danaher Corp., Inc., Repligen Corp., Steris Corp., Utah Medical Products, Inc., Cantel Medical Corp., Fortive Corp., Merit Medical Systems, Inc., Mettler Toledo International, Inc., Transcat Inc., Elector-Sensors, Inc., Medtronic, P.L.C, and Illumina, Inc. The graph shows the value on March 31 of each year, assuming an original investment of $100 in each and reinvestment of cash dividends.
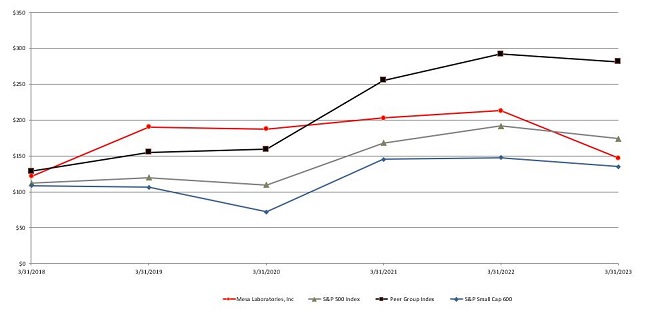
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
(dollars in thousands, unless specified)
Overview
We are a multinational manufacturer, developer, and seller of life sciences tools and critical quality control products and services, many of which are sold into niche markets driven by regulatory requirements. We have manufacturing operations in the United States and Europe, and our products are marketed by our sales personnel in North America, Europe, and Asia Pacific, as well as by independent distributors in these areas and throughout the rest of the world. We prefer markets in which we can establish a strong presence and achieve high gross profit margins. As of March 31, 2023, we managed our operations in four reportable segments, or divisions: Clinical Genomics, Sterilization and Disinfection Control, Biopharmaceutical Development, and Calibration Solutions. Each of our divisions are described further in "Results of Operations" below. Unallocated corporate expenses and other business activities are reported within Corporate and Other.
Corporate Strategy
We strive to create shareholder value and further our purpose of Protecting the Vulnerable® by growing our business both organically and through acquisitions, by improving our operating efficiency, and by continuing to hire, develop and retain top talent. As a business, we commit to our purpose of Protecting the Vulnerable® every day by taking a customer-focused approach to developing, building, and delivering our products. We serve a broad set of industries, in particular the pharmaceutical, healthcare services, and medical device verticals, in which the safety, quality, and efficacy of products is critical. By delivering the highest quality products possible, we are committed to protecting the communities we serve.
Organic Revenues Growth
Organic revenues growth is driven by the expansion of our customer base, increases in sales volumes, new product offerings, and price increases, and may be affected positively or negatively by changes in foreign currency rates. Our ability to increase organic revenues is affected by general economic conditions, both domestic and international, customer capital spending trends, competition, and the introduction of new products. Our policy is to price our products competitively and, where possible, we pass along cost increases to our customers in order to maintain our margins. We typically evaluate costs and pricing annually with price increases effective January 1; however, as a result of high inflation in recent quarters, we implemented an additional mid-year price increase late in the second quarter of fiscal year 2023.
Inorganic Revenues Growth - Acquisitions
During the third quarter of fiscal year 2023, we completed the Belyntic acquisition. We paid $4,950 on the date of acquisition, and we expect to pay an additional $1,500 of contingent consideration based on the probable approval of pending patent applications expected within 36 months of the acquisition date. The acquisition provided a natural complement to our peptide synthesis business by adding a consumables product line.
During the third quarter of fiscal year 2022, we completed the acquisition of Agena for an aggregate net purchase price of $300,793. Agena is a leading clinical genomics tools company that develops, manufactures, and sells highly sensitive, low-cost, high-throughput genetic analysis tools used by clinical labs to perform genomic clinical testing in several therapeutic areas, such as screenings for hereditary diseases, pharmacogenetics and oncology related applications. The acquisition of Agena accelerated our strategic trajectory towards higher growth applications within the regulated segments of the life sciences tools market.
Over the past decade, we have consummated a number of acquisitions as part of our growth strategy. These acquisitions have allowed us to expand our product offerings, globalize our company, and increase the scale at which we operate, which in turn affords us the ability to improve our operating efficiency, extend our customer base, and further the pursuit of our purpose: Protecting the Vulnerable®.
Improving Our Operating Efficiency
We maximize value in both our existing businesses and those we acquire by implementing efficiencies in our manufacturing, commercial, engineering, and administrative operations. We achieve efficiencies using the four pillars that make up the Mesa Way, which is our customer-centric, lean-based system for continuously improving and operating a set of high-margin, niche businesses. The Mesa Way is focused on: Measuring What Matters using our customers' perspective and setting high standards for performance; Empowering Teams to improve operationally and exceed customer expectations; Sustainably Improving using lean-based tools designed to help us identify the root cause of opportunities and prioritize the biggest opportunities; and Always Learning so that performance continuously improves.
Gross profit is affected by many factors including our product mix, manufacturing efficiencies, costs of products and labor, foreign currency rates, and price competition. Historically, as we have integrated our acquisitions and taken advantage of manufacturing efficiencies, our gross profit percentages for some products have improved. There are, however, differences in gross profit percentages between product lines, and ultimately the mix of sales will continue to impact our overall gross profit.
Hire, Develop, and Retain Top Talent
At the center of our organization are talented people who are capable of taking on new challenges using a team approach. It is our exceptionally talented workforce that works together and uses our lean-based tool set to find ways to continuously improve our products, our services, and ourselves, resulting in long-term value creation for our shareholders.
General Trends
We are a global company, with multinational operations. During our fiscal year 2023, approximately 46% of our revenues were derived from revenues earned outside of the United States. Since Mesa serves a number of industries across a variety of global markets, we may be affected by world-wide, regional, or industry-specific economic or political factors. However, our diversity in industry, geography, and product and service offerings may limit the impact of changes in specific industry trends or local economic changes to a single geographic area on our consolidated operating results. We actively monitor trends affecting industries that we operate in, including monitoring key competitors and customers, as well as staying abreast of changes to local economies and how they may affect our divisions.
Exchange rates were volatile throughout fiscal year 2023. A weakening or strengthening of foreign currencies against the United States dollar ("USD") increases or decreases our reported revenues, gross profit margins, and operating expenses, and impacts the comparability of our results between periods. Overall, currency exchange rates negatively impacted our reported revenues for fiscal year 2023 compared to fiscal year 2022. Generally, the USD strengthening against major currencies adversely impacts our reported revenues, but to a lesser extent, positively impacts our reported expenses; conversely, the weakening of the U.S. dollar against major currencies positively impacts our reported revenues but negatively impacts our reported expenses. The ultimate impact to gross profit as a percentage of revenue depends on the magnitude of changes in foreign currencies.
Inflation was significant in the regions that we operate in during fiscal year 2023. Current and future inflationary effects may continue to be impacted by a variety of macroeconomic forces, including, but not limited to: supply chain disruptions, government fiscal policies, changes in interest rates, and changing demand for goods and services. Inflationary pressures have affected our business in a number of ways, including increasing the cost of raw materials, labor, and freight, and the rate of interest we pay on borrowings under our Credit Facility. Our actions to mitigate the impact of supply chain disruptions and inflation, including pre-ordering components in higher than usual quantities, sourcing new vendors and increasing prices have been somewhat successful; however, raw materials shortages impacted our Calibrations Solutions division throughout much of fiscal year 2023.
COVID-19 negatively impacted commercial execution in different ways throughout fiscal year 2023, and in some cases, limited sales of Clinical Genomics consumables to existing customers and instruments to new customers. Specifically, we experienced disruptions to our business in China resulting from government mandated shut-downs and restrictions during fiscal year 2023. Although the COVID-19 pandemic has largely subsided as a public health matter, we may experience material adverse impacts to our business as a result of the pandemic's adverse impact on the global economy, in-person collaboration and sales efforts, and our customers’ changed purchasing behaviors and confidence.
During fiscal year 2023, we were notified by Sema4 Holdings Corp. ("Sema4"), a customer of our Clinical Genomics division, that they are exiting the reproductive health screening business, and as a result, they intend to significantly reduce the quantity of orders they place with us in the future. Revenues from sales to Sema4 were approximately $8,200 during the first twelve months of our ownership of Agena and were approximately $4,600 during fiscal year 2023. Following the notice, we evaluated our business operations and enacted several cost-cutting measures in the Clinical Genomics division, including a reduction-in-force, to preserve our financial model. These actions are expected to generate more than $4,000 in future annualized savings.
Results of Operations
Our results of operations and year-over-year changes are discussed in the following section. The tables and discussion below should be read in conjunction with the accompanying Consolidated Financial Statements and the notes thereto appearing in Item 8. Financial Statements and Supplementary Data (in thousands, except percent data). Refer to Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended March 31, 2022, filed on May 31, 2022, for a comparison of results of operations for the years ended March 31, 2022 and March 31, 2021.
Revenues from our reportable segments increased 19% for fiscal year 2023 as compared to fiscal year 2022. Revenues growth for fiscal year 2023 was primarily attributable to the acquisition of Agena, and to a lesser extent, organic revenues growth of 0.6%.
Gross profit as a percentage of revenues increased two percentage points for fiscal year 2023 as a result of the recognition of a $7,462 non-cash inventory step-up charge, part of purchase accounting for the Agena Acquisition, during fiscal year 2022, partially offset by unfavorable product mix, increased cost of labor, and adverse changes in foreign currency on our reported revenues.
Results by reportable segment are as follows:
| Revenues |
Organic Revenues Growth |
Gross Profit as a % of Revenues |
||||||||||||||||||||||
| Year Ended March 31, 2023 |
Year Ended March 31, 2022 |
Year Ended March 31, 2023 |
Year Ended March 31, 2022 |
Year Ended March 31, 2023 |
Year Ended March 31, 2022 |
|||||||||||||||||||
| Clinical Genomics |
$ | 62,299 | $ | 32,840 | (12.9 | %) | N/A | 52 | % | 36 | % | |||||||||||||
| Sterilization and Disinfection Control |
64,609 | 59,044 | 9.4 | % | 11 | % | 72 | % | 74 | % | ||||||||||||||
| Biopharmaceutical Development |
47,365 | 45,579 | 3.8 | % | 34 | % | 64 | % | 63 | % | ||||||||||||||
| Calibration Solutions |
44,807 | 46,872 | (4.4 | %) | 0 | % | 54 | % | 53 | % | ||||||||||||||
| Reportable segments |
$ | 219,080 | $ | 184,335 | 0.6 | % | 13 | % | 61 | % | 59 | % | ||||||||||||
Our condensed consolidated results of operations are as follows:
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Revenues |
$ | 219,080 | $ | 184,335 | $ | 133,937 | 19 | % | 38 | % | ||||||||||
| Gross profit |
133,693 | 109,090 | 87,014 | 23 | % | 25 | % | |||||||||||||
| Operating expenses |
130,373 | 104,388 | 74,656 | 25 | % | 40 | % | |||||||||||||
| Operating income |
3,320 | 4,702 | 12,358 | (29 | %) | (62 | %) | |||||||||||||
| Net income |
$ | 930 | $ | 1,871 | $ | 3,274 | (50 | %) | (43 | %) | ||||||||||
Reportable Segments
Clinical Genomics
The Clinical Genomics division develops, manufactures and sells highly sensitive, low-cost, high-throughput genetic analysis tools and related consumables and services that enable clinical labs to perform genomic testing for a broad range of diagnostic and research applications in several therapeutic areas, such as screenings for hereditary diseases, pharmacogenetics, and oncology related applications.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Revenues |
$ | 62,299 | $ | 32,840 | $ | - | 90 | % | N/A | |||||||||||
| Gross profit |
32,485 | 11,941 | - | 172 | % | N/A | ||||||||||||||
| Gross profit as a % of revenues |
52 | % | 36 | % | N/A | 16 | % | N/A | ||||||||||||
Revenues in the Clinical Genomics division represent revenues from October 20, 2021 until March 31, 2023. Clinical Genomics revenues increased 90% for fiscal year 2023 compared to fiscal year 2022 due to a significantly shorter period of ownership of Agena in fiscal year 2022 compared to fiscal year 2023, partially offset by adverse foreign currency exchange rates. We recognized approximately $4,600 in revenues from sales to Sema4 during fiscal year 2023, and we expect a significant reduction of revenues resulting from the loss of Sema4's business in future quarters. However, two of our distribution partners, Guangzhou Darui Biotechnology Co., Ltd. and Jiangsu Simcere Medical Device Co., Ltd., recently received approval from China's National Medical Products Administration for Class III in vitro diagnostics ("IVD") panels. One of these panels addresses hereditary deafness and the other covers pharmacogenetics with the intended use of guiding personal drug therapy. These are the first approved Class III IVD panels in China from our distribution partner program, and we expect some increase in revenues resulting from these programs beginning in fiscal year 2024.
Gross profit percentage for the Clinical Genomics division increased 16 percentage points for fiscal year 2023 compared to fiscal year 2022 primarily due to the amortization of a $7,462 inventory step-up required under the purchasing accounting standards in the third quarter of fiscal year 2022. Excluding the inventory step-up, gross profit as a percentage of revenue decreased seven percentage points as a result of lower revenues due to unfavorable foreign currency impacts and the loss of Sema4, on a partially fixed cost base.
Sterilization and Disinfection Control
Our Sterilization and Disinfection Control division manufactures and sells biological, cleaning, and chemical indicators which are used to assess the effectiveness of sterilization and disinfection processes, including steam, gas, hydrogen peroxide, ethylene oxide, radiation, and other processes in the medical device, pharmaceutical, and hospital industries. The division also provides testing and laboratory services, mainly to the dental industry.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Revenues |
$ | 64,609 | $ | 59,044 | $ | 53,119 | 9 | % | 11 | % | ||||||||||
| Gross profit |
46,520 | 43,720 | 39,870 | 6 | % | 10 | % | |||||||||||||
| Gross profit as a % of revenues |
72 | % | 74 | % | 75 | % | (2 | %) | (1 | %) | ||||||||||
Sterilization and Disinfection Control revenues increased 9% for fiscal year 2023 compared to fiscal year 2022, despite the USD strengthening against the euro which resulted in lower reported revenues derived from sales in Europe. During fiscal year 2023, we added temporary and permanent manufacturing headcount at our Bozeman Montana facility, which enabled us to fulfill approximately $1,800 of customer orders that were backlogged as of March 31, 2022. Fiscal year 2023 also benefited from favorable product mix and, to a lesser extent, price increases.
Sterilization and Disinfection Control's gross profit percentage decreased two percentage points during the year ended March 31, 2023 primarily due to increased labor and benefit costs, including the cost of temporary headcount, inflation in freight expense, and the result of foreign currency negatively impacting our reported revenues.
Biopharmaceutical Development
Our Biopharmaceutical Development division develops, manufactures and sells automated systems for protein analysis (immunoassays) and peptide synthesis solutions. Immunoassays and peptide synthesis solutions accelerate the discovery, development, and manufacture of biotherapeutic therapies, among other applications.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Revenues |
$ | 47,365 | $ | 45,579 | $ | 33,892 | 4 | % | 34 | % | ||||||||||
| Gross profit |
30,340 | 28,605 | 21,035 | 6 | % | 36 | % | |||||||||||||
| Gross profit as a % of revenues |
64 | % | 63 | % | 62 | % | 1 | % | 1 | % | ||||||||||
Biopharmaceutical Development's revenues increased 4% for fiscal year 2023 compared to fiscal year 2022. The division's increase in reported revenue was a result of increased product adoption and price increases, partially offset by unfavorable changes in foreign currency exchange rates on our reported revenues.
Biopharmaceutical Development's gross profit percentage increased one percentage point during the year ended March 31, 2023 as a result of higher revenues on a partially-fixed cost base, partially offset by unfavorable product mix and foreign currency fluctuations negatively impacting our reported revenues.
Calibration Solutions
The Calibration Solutions division develops, manufactures and sells quality control products using principles of advanced metrology to measure or calibrate critical chemical or physical parameters in various dialysis, process monitoring, instrument monitoring, environmental monitoring, gas flow, environmental air quality, and torque applications, primarily in hospital, medical device manufacturing, pharmaceutical manufacturing, and laboratory environments.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Revenues |
$ | 44,807 | $ | 46,872 | $ | 46,926 | (4 | %) | - | % | ||||||||||
| Gross profit |
24,388 | 24,989 | 26,112 | (2 | %) | (4 | %) | |||||||||||||
| Gross profit as a % of revenues |
54 | % | 53 | % | 56 | % | 1 | % | (3 | %) | ||||||||||
Calibration Solutions' revenues decreased 4% for fiscal year 2023 compared to fiscal year 2022, primarily as a result of supply constraints limiting our ability to manufacture ordered quantities of certain products, partially offset by slightly higher service revenues as our service technicians had access to client facilities for substantially all of fiscal year 2023, and the benefit of modest price increases. Production difficulties resulted in longer lead times for customer orders, which negatively impacted the timing of new orders; however, beginning in the fourth quarter of fiscal year 2023, production difficulties abated somewhat and revenues increased modestly compared to each of the first three quarters of fiscal year 2023.
Calibration Solutions' gross profit percentage increased one percentage point during the year ended March 31, 2023 primarily as a result of favorable product mix.
Corporate and Other
Corporate and Other consists of unallocated corporate expenses and other business activities.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Revenues |
$ | - | $ | - | $ | - | N/A | N/A | ||||||||||||
| Gross (loss) profit |
(40 | ) | (165 | ) | (3 | ) | (76 | %) | 5400 | % | ||||||||||
| Gross profit as a % of revenues |
N/A | N/A | N/A | N/A | N/A | |||||||||||||||
Operating Expenses
Operating expenses for the year ended March 31, 2023 increased 25% in total compared to the year ended March 31, 2022 primarily as a result of the increased costs of operations resulting from the Agena Acquisition which was consummated about halfway through fiscal year 2022.
Selling
Selling expense is driven primarily by labor costs, including salaries and commissions; accordingly, it may vary with sales levels.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Selling expense |
$ | 37,439 | $ | 28,310 | 18,480 | 32 | % | 53 | % | |||||||||||
| As a percentage of revenues |
17 | % | 15 | % | 14 | % | 2 | % | 1 | % | ||||||||||
Selling expense increased 32% for the year ended March 31, 2023. Excluding the impact of Agena, selling expense increased 13% for the year ended March 31, 2023, as we continued to execute on our previously-announced plan to invest in sales and marketing resources in order to increase organic revenues growth. We hired several sales employees, resulting in higher labor-related costs. Further, travel-related costs increased as we continued to resume in-person meetings, tradeshows, and sales events. Increases were partially offset by lower commissions and bonus expense.
General and Administrative
Labor costs, non-cash stock-based compensation, and amortization of intangible assets drive the substantial majority of general and administrative expense.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| General and administrative expense |
$ | 72,444 | $ | 60,311 | $ | 45,788 | 20 | % | 32 | % | ||||||||||
| As a percentage of revenues |
33 | % | 33 | % | 34 | % | - | % | (1 | %) | ||||||||||
General and administrative expenses increased 20% for the year ended March 31, 2023. Excluding the impact of Agena, general and administrative expenses increased 1% for the year ended March 31, 2023. The increase was a result of higher personnel costs, including increased stock-based compensation expense as we expanded the number of employee participants in the program. Increases to general and administrative costs were partially offset by lower intangible amortization expense as a result of the strengthening of the USD, lower annual bonus accruals based on our financial results for the year ended March 31, 2023, and decreased acquisition and integration-related costs.
Research and Development
Research and development expense is predominantly comprised of labor costs and third-party consultants.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Research and development expense |
$ | 20,490 | $ | 15,767 | $ | 10,388 | 30 | % | 52 | % | ||||||||||
| As a percentage of revenues |
9 | % | 9 | % | 8 | % | - | % | 1 | % | ||||||||||
Research and development expenses for the year ended March 31, 2023 increased 30%. Excluding the impact of Agena, research and development costs for the year ended March 31, 2023 increased 3% primarily as a result of our purchase of in process research and development technology that we are further developing in order to enhance a product offering in our Sterilization and Disinfection Control division, as well as higher personnel costs as we continue enhancing existing products and developing new products and features.
Nonoperating Expense
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Nonoperating expense |
$ | 3,709 | 1,128 | 10,055 | 229 | % | (89 | %) | ||||||||||||
Nonoperating expense for fiscal year 2023 is composed primarily of interest expense and amortization of the debt discount associated with the 2025 Notes and the Credit Facility as well as gains and losses on foreign currency transactions. Nonoperating expense was higher in fiscal year 2023 compared to fiscal year 2022 due to interest expense on the Credit Facility, which had an average interest rate of 4.5% during fiscal year 2023 compared with an average interest rate of 1.7% for the periods during which a balance was outstanding during fiscal year 2022, partially offset by net foreign currency gains.
Interest expense and amortization of debt discount was lower for the year ended March 31, 2022 compared to the year ended March 31, 2021 due to our adoption of Accounting Standards Update No. 2020-06, Debt with Conversion and Other Options and Derivatives and Hedging Accounting for Convertible Instruments and Contracts in an Entity's Own Equity ("ASU 2020-06"), which resulted in a $4,090 reduction in non-cash interest expense related to the 2025 Notes.
Income Taxes
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2023 |
2022 |
2021 |
2023 vs. 2022 |
2022 vs. 2021 |
||||||||||||||||
| Income tax (benefit) expense |
$ | (1,319 | ) | $ | 1,703 | $ | (971 | ) | (177 | %) | (275 | %) | ||||||||
| Effective tax rate |
339 | % | 48 | % | (42 | %) | 291 | % | 90 | % | ||||||||||
Our income tax rate varies based upon many factors, but in general we anticipate that on a go-forward basis, our effective tax rate will be approximately 26%, plus or minus the impact of excess tax benefits and deficiencies associated with share-based payment awards to employees (please see Note 12. “Income Taxes” within Item 8. Financial Statements and Supplementary Data) and purchase price accounting for any future acquisitions. The change in our effective tax rate during the year ended March 31, 2023 is primarily due to being in a net loss position before taxes, a decrease in the provision for limitations under Section 162(m) of the Internal Revenue Code, partially offset by lower tax benefits associated with stock option exercises in fiscal year 2023. Tax benefits and deficiencies associated with share-based payment awards to our employees have caused and, in the future, may cause large fluctuations in our realized effective tax rate based on timing, volume, and nature of stock options exercised under our share-based payment program.
Net Income
Net income for the year ended March 31, 2023 varied with the changes in revenues, gross profit, and operating expenses (including, respectively, $28,821 and $12,538 of non-cash amortization of intangible assets acquired in a business combination, and stock-based compensation expense).
Non-GAAP reconciliation
Adjusted operating income (which excludes the non-cash impact of amortization of intangible assets acquired in a business combination, stock-based compensation and impairment of goodwill and long-lived assets) is used by management as a supplemental performance measure in order to compare current financial performance to historical performance, assess the ability of our assets to generate cash, and evaluate potential acquisitions.
Adjusted operating income should not be considered an alternative to, or more meaningful than, net income, operating income, cash flow from operating activities or any other measure of financial performance presented in accordance with GAAP as measures of operating performance or liquidity.
The following table sets forth our reconciliation of adjusted operating income, a non-GAAP measure, to operating income:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Operating income |
$ | 3,320 | $ | 4,702 | $ | 12,358 | ||||||
| Amortization of intangible assets acquired in a business combination |
28,821 | 21,806 | 14,513 | |||||||||
| Stock-based compensation |
12,538 | 11,391 | 9,268 | |||||||||
| Adjusted Operating Income |
$ | 44,679 | $ | 37,899 | $ | 36,139 | ||||||
Liquidity and Capital Resources
Our sources of liquidity include cash generated from operations, cash and cash equivalents on hand, cash available from our Credit Facility and the Open Market Sale AgreementSM described below, working capital, and potential additional equity and debt offerings. We believe that cash flows from operating activities and potential cash provided by borrowings from our Credit Facility or funds from our Open Market Sale AgreementSM, when necessary, will be sufficient to meet our ongoing short-term and long-term operating requirements, scheduled interest payments on debt, dividend payments, and anticipated capital expenditures. At our option, we may settle the 2025 Notes in shares of our common stock or in cash, or we may re-finance the debt, depending on conditions in the market and the share price of our common stock.
Our more significant uses of resources have historically included acquisitions, payments on debt and interest obligations, long-term capital expenditures, and quarterly dividends to shareholders. Working capital is the amount by which current assets exceed current liabilities. We had working capital of $75,616 and $76,263 on March 31, 2023 and 2022, respectively. We also had $32,910 and $49,346 of cash and cash equivalents as of March 31, 2023 and 2022, respectively.
As of March 31, 2023, $172,500 was outstanding under the 2025 Notes and $13,000 was outstanding under the Credit Facility. In April 2023, we paid an additional $3,000 on our Credit Facility.
We have evaluated our risk from concentration of cash deposits and taken appropriate steps to mitigate such risk. We maintain relationships and cash deposits at multiple banking institutions across the world in an effort to diversify and reduce risk of loss.
In April 2022, we entered into an Open Market Sale AgreementSM pursuant to which we may issue and sell, from time to time, shares of our common stock with an aggregate value of up to $150,000. We did not sell any shares under this agreement during fiscal year 2023.
We routinely evaluate opportunities for strategic acquisitions. Future material acquisitions may require that we obtain additional capital, assume additional third-party debt or incur other long-term obligations. We believe that we have the ability to issue more equity or debt in the future in order to finance our acquisition and investment activities; however, additional equity or debt financing, or other transactions, may not be available on acceptable terms, if at all.
We may from time to time repurchase or take other steps to reduce our debt. These actions may include retirements or refinancing of outstanding debt, privately negotiated transactions or otherwise. The amount of debt that may be retired, if any, could be material and would be decided at the sole discretion of our Board of Directors and would depend on market conditions, our cash position, and other considerations.
Dividends
We have paid regular quarterly dividends since 2003. We declared and paid dividends of $0.16 per share each quarter of the years ended March 31, 2023, 2022, and 2021.
In April 2023, our Board of Directors declared a quarterly cash dividend of $0.16 per share of common stock, payable on June 15, 2023, to shareholders of record at the close of business on May 31, 2023.
Cash Flows
Our cash flows from operating, investing, and financing activities were as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Net cash provided by operating activities |
$ | 27,983 | $ | 39,223 | $ | 37,073 | ||||||
| Net cash (used in) investing activities |
(9,494 | ) | (305,225 | ) | (1,992 | ) | ||||||
| Net cash (used in) provided by financing activities |
(33,328 | ) | 52,576 | 146,228 | ||||||||
Cash flows from operating activities for the year ended March 31, 2023 provided $27,983. The $11,240 decrease in cash flows from operating activities primarily resulted from changes in our working capital accounts including higher purchases of inventories to mitigate potential supply chain issues as well as lower accrued liabilities, particularly bonus, partially offset by non-cash adjustments to net income including higher stock compensation expense and higher depreciation and amortization expense. Net income and non-cash adjustments totaled $45,095 for the year ended March 31, 2023 compared to $46,415 for the year ended March 31, 2022, while cash provided by operating assets and liabilities decreased by $9,920. Cash used in investing activities was lower during the year ended March 31, 2023 compared to the year ended March 31, 2022 due to cash expended on the Agena Acquisition fiscal year 2022, partially offset by the Belyntic Acquisition in fiscal year 2023. Cash used in financing activities primarily resulted from our repayment of $36,000 on our Credit Facility. The fiscal year 2022 draw on our Credit Facility was used to fund a portion of the purchase price of the Agena Acquisition. Our equity raise completed during the year ended March 31, 2021 provided $145,935.
Critical Accounting Policies and Estimates
Our Consolidated Financial Statements are prepared in accordance with accounting principles generally accepted in the United States, which require management to make estimates, judgments, and assumptions that affect the amounts reported in our Consolidated Financial Statements and accompanying notes. We believe that the following are the more critical judgment areas in the application of accounting policies that currently affect our financial condition and results of operations. Management has discussed the development, selection, and disclosure of critical accounting policies and estimates with the Audit Committee of our Board of Directors. While our estimates and assumptions are based on our knowledge of current events and circumstances and actions we may take in the future, actual results may ultimately differ from these estimates and assumptions. For a discussion of our significant accounting policies, see Note 1. “Description of Business and Summary of Significant Accounting Policies” in Item 8. Financial Statements and Supplementary Data.
Purchase Accounting for Acquisitions
We account for all business combinations in which we obtain control over another entity using the acquisition method of accounting, which requires most assets (both tangible and intangible) and liabilities (including any applicable contingent consideration) to be recognized at fair value at the date of acquisition. The excess of the purchase price over the fair value of assets less liabilities is recognized as goodwill. We determine fair value using widely accepted valuation techniques, primarily discounted cash flow and market multiple analyses. These types of analyses require us to make and monitor assumptions and estimates regarding industry and economic factors, the profitability of future business strategies, discount rates and cash flow. Certain adjustments to the assessed fair values of acquired assets or liabilities made subsequent to the acquisition date but within a one-year measurement period are recorded as adjustments to goodwill. Any adjustments subsequent to the measurement period are recorded within earnings. We expense all costs as incurred related to an acquisition in selling, general, and administrative expenses.
Results of operations of the acquired company are included in our Consolidated Financial Statements from the date of the acquisition forward. If actual results are not consistent with our assumptions and estimates, or if our assumptions and estimates change due to new information, we may be exposed to an impairment charge in the future.
Acquired Intangible Assets
Our business acquisitions typically result in the recognition of goodwill and other intangible assets, which affect the amount of future period amortization expense and possible impairment charges we may incur.
Intangible assets with finite lives are amortized over their useful lives using the straight-line method and amortization expense is recorded within cost of products or selling, general and administrative expense in the Consolidated Statements of Income. Impairment assessments are conducted if events or conditions indicate that asset carrying amounts may not be recoverable, including changes in the competitive landscape, any internal decisions to pursue new or different technology strategies, losses of significant customers, or significant changes in the marketplace, including adverse changes in the prices paid for our products or changes in the size of the market for our products. If impairment indicators are present, we determine whether the carrying value of the underlying intangible asset is recoverable through undiscounted estimated future cash flows. If the asset is not found to be recoverable, we estimate the asset's fair value using Level 3 inputs and record an impairment to write down the asset's carrying value to the estimated fair value. If the estimate of an intangible asset’s remaining useful life is changed, the remaining carrying amount of the intangible asset is amortized prospectively over the revised remaining useful life. We continue to believe that our finite lived intangible assets are recoverable as of March 31, 2023.
We test goodwill and indefinite lived intangible assets for impairment on an annual basis during the fourth quarter of each year, or more frequently if events and circumstances indicate it is more likely than not that the fair value of the respective asset is less than its carrying value. Events that would indicate impairment and trigger interim impairment assessments include but are not limited to: current economic and market conditions, including a decline in market capitalization; a significant adverse change in legal factors; business climate or operational performance of the business; and an adverse action or assessment by a regulator. Our impairment tests for indefinite lived intangible assets other than goodwill are generally conducted at the individual asset level. We accounted for the economic uncertainty caused by the macro-economic environment, including rising interest rates and high inflation, when conducting our impairment analyses of goodwill and other indefinite lived intangible assets during the fourth quarter of our year ended March 31, 2023.
Our impairment tests typically begin with optional qualitative assessments to determine whether it is more likely than not that the carrying value of a goodwill reporting unit or other intangible asset exceeds its fair value, as permitted by the accounting guidance. If, after this qualitative assessment, we determine it is more likely than not that the fair value is greater than the carrying amount, no further quantitative testing is necessary. A quantitative assessment is performed if the qualitative assessment results in a more-likely-than-not determination or if a qualitative assessment is not performed. The quantitative assessment measures whether the carrying amount of a reporting unit or indefinite lived intangible asset exceeds its fair value, in which case an impairment charge is recorded to the extent carrying value exceeds fair value. Fair value is determined using an income approach, which relies heavily on Level 3 inputs. In fiscal year 2023, we performed initial qualitative assessments over reporting units associated with our Clinical Genomics, Biopharmaceutical Development, and Calibration Solutions reportable segments. We supplemented our qualitative analysis over Clinical Genomics with a quantitative assessment as described further below. We also performed a quantitative assessment over the Sterilization and Disinfection Control reportable segment due to the length of time elapsed since our last quantitative assessment. Through our testing, we concluded that no impairment exists as of March 31, 2023.
Due to the loss of a significant customer, in the third quarter of fiscal year 2023 we used Level 3 inputs to quantitatively test the recoverability of the Clinical Genomics division’s intangible asset group and to evaluate the division’s goodwill for impairment. After considering all information available to us as of that testing date, we concluded that no impairment was indicated. As of March 31, 2023, our annual qualitative impairment analyses continued to support the conclusion that Clinical Genomics’ goodwill and intangible assets are not impaired; however, we chose to perform a quantitative impairment test in light of the division’s fourth quarter financial performance compared to original forecasts. We will continue to carefully monitor Clinical Genomics’ goodwill and other intangible assets for impairment in future periods. It is reasonably possible that the division’s goodwill, intangibles, or both, may be impaired in the near term. Impairment would result in non-cash charges, which could have a material adverse effect on our financial condition and results of operations.
The total net carrying values of Clinical Genomics’ intangible assets and goodwill potentially subject to future impairment are $140,700 and $135,811, respectively, as of March 31, 2023. The value of finite-lived intangible assets decreases about $15.5 million each year as we record amortization expense, and the value of goodwill and intangible assets may be affected by future events, including changes in our customer or product mix, market conditions, or our operating performance falling short of current forecasts.
Stock-based Compensation
We recognize compensation expense for equity awards over the vesting period based on the fair value of the awards. We use the Black-Scholes valuation model to estimate the fair value of our stock options. The Black-Scholes model requires assumptions to be made regarding our stock price volatility, the expected life of awards, and expected dividend rates. The volatility assumption and the expected life assumptions are based on our historical data. The compensation expense related to performance share awards is based in part on the estimated probability of achieving performance goals associated with particular levels of payout for performance shares. We determine the probability of achievement of future levels of performance by comparing the relevant performance level with our internal estimates of future performance. Those estimates are based on a number of assumptions, and different assumptions may result in different conclusions regarding the probability of achieving future levels of performance relevant to the payout levels for the awards. Had we arrived at different assumptions of stock price volatility or expected lives of our options, or different assumptions regarding the probability of our achieving future levels of performance with respect to performance share awards, our stock-based compensation expense and results of operations could have been different.
Income Taxes
Our provision for income taxes requires the use of estimates in determining the timing and amounts of deductible and taxable items, including impacts on effective tax rates, deferred tax items and valuation allowances based on management’s interpretation and application of complex tax laws and accounting guidance. We establish reserves for uncertain tax positions for material, known tax exposures relating to deductions, transactions and other matters involving uncertainty as to the measurement and recognition of the item. While we believe that our reserves are adequate, issues raised by a tax authority may be finally resolved at an amount different than the related reserve and could materially increase or decrease our income tax provision in the current and/or future periods.
Recent Accounting Standards and Pronouncements
For a discussion of the new accounting standards impacting the Company, refer to Note 1. “Description of Business and Summary of Significant Accounting Policies” in Item 8. Financial Statements and Supplementary Data.
Contractual Obligations
We are party to many contractual obligations that involve commitments to make payments to third parties in the ordinary course of business. For a description of our contractual obligations and other commercial commitments as of March 31, 2022, see our Annual Report on Form 10-K for the fiscal year ended March 31, 2022, filed with the Securities and Exchange Commission on May 31, 2022.
On a consolidated basis, at March 31, 2023, we had contractual obligations for open purchase orders of approximately $17,270 for routine purchases of supplies and inventory, of which the substantial majority are payable in less than one year.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We have no derivative instruments and minimal exposure to commodity market risks.
Foreign Currency Exchange Rates
We face exchange rate risk from transactions with customers in countries outside the United States and from intercompany transactions between affiliates. Transactional exchange rate risk arises from the purchase and sale of goods and services in currencies other than our functional currency or the functional currency of the applicable subsidiary. We also face translational exchange rate risk related to the translation of financial statements of our foreign operations into U.S. dollars, our functional currency. Costs incurred and sales recorded by subsidiaries operating outside of the United States are translated into U.S. dollars using exchange rates effective during the respective period. As a result, we are exposed to movements in the exchange rates of various currencies against the U.S. dollar. Our Biopharmaceutical Development division is particularly susceptible to currency exposures since it incurs a substantial portion of its expenses in Swedish Krona, while most revenue contracts are in U.S. dollars and euros. Therefore, when the Swedish Krona strengthens or weakens against the U.S. dollar, operating profits are increased or decreased, respectively. The effect of a change in currency exchange rates on our international subsidiaries' assets and liabilities is reflected in the accumulated other comprehensive income component of stockholders’ equity.
To the extent material, we have discussed the impact of the change in foreign currency within Item 7. "Results of Operations." A hypothetical 10 percent increase in currency exchange rates compared to the U.S. dollar (U.S. dollar strengthening) would result in an estimated $875 after tax reduction in net earnings over a one-year period. Actual changes in market prices or rates may differ from hypothetical changes.
Interest Rates
During our year ended March 31, 2021, we entered into the Credit Facility which bears interest at either a base rate or a SOFR rate, plus an applicable spread. Based on our interest rate and balance outstanding as of March 31, 2023, we estimate that if interest rates increased 1 percentage point, we would incur approximately $130 of additional interest expense per year.
Inflation Risk
Inflation generally impacts us by increasing our costs of labor, materials, and freight. The rates of inflation experienced in recent years have not had a significant impact on our financial statements as inflationary cost increases have been offset by annual price increases. However, any price increases imposed may lead to declines in sales volume if competitors do not similarly adjust prices. We cannot reasonably estimate our ability to successfully recover any impact of inflation cost increases into the future.
Item 8. Financial Statements and Supplementary Data
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Stockholders and Board of Directors
Mesa Laboratories, Inc.
Lakewood, Colorado
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Mesa Laboratories, Inc. (the “Company”) as of March 31, 2023 and 2022, the related consolidated statements of income, comprehensive (loss) income, stockholders' equity, and cash flows for each of the years in the three-year period ended March 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the three-year period ended March 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of March 31, 2023 based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and our report dated May 30, 2023 expressed an adverse opinion thereon.
Basis for Opinion
The Company's management is responsible for these financial statements. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical audit matters or on the accounts or disclosures to which they relate.
Valuation of Goodwill – Clinical Genomics Reporting Unit - Refer to Notes 1 and 6
Critical Audit Matter Description
As discussed in Note 1 of the consolidated financial statements, goodwill is tested for impairment at least annually at the reporting unit level. This requires management to estimate the fair value of the reporting units with goodwill allocated to them. The Company estimates the fair value based on a discounted cash flow method. As of the annual impairment testing date, the Clinical Genomics reporting unit goodwill balance totaled $135.8 million.
Auditing management's goodwill impairment test of the Clinical Genomics reporting unit involved especially subjective judgments due to the significant estimation required in determining the fair value of the reporting unit. In particular, the estimate of the fair value for the reporting unit is sensitive to changes in assumptions such as the discount rate, the long-term growth rate and expected future net cash flows, including projected revenues and operating expenses, which are affected by expectations about future market and economic conditions.
How the Critical Audit Matter was Addressed in the Audit
Our audit procedures performed to address this critical audit matter included the following, among others:
| ● |
We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company's goodwill impairment review process. For example, we tested controls over the estimation of the fair value of the reporting unit, including the Company's controls over the valuation model, the mathematical accuracy of the valuation model and development of underlying assumptions used to estimate the fair value of the reporting unit. |
| ● |
To test the estimated fair value of the Company's Clinical Genomics reporting unit, our audit procedures included, among others, |
| ● |
Assessing the valuation methodology and the underlying data used by the Company in its analysis, including testing the significant assumptions discussed above. |
| ● |
We compared the significant assumptions discussed above used by management to current industry and economic trends, changes to the Company's business model and other relevant factors, including considering contradictory evidence. |
| ● |
We performed sensitivity analyses of these significant assumptions to evaluate the changes in the fair value of the reporting unit that would result from changes in these assumptions. |
| ● |
We involved valuation specialists to assist in our evaluation of the valuation methodology and the significant assumptions used in determining the fair value of the reporting unit. |
| ● |
Evaluating the Company’s disclosures related to the goodwill impairment testing. |
Income Taxes – Refer to Notes 1 and 12
Critical Audit Matter Description
The Company’s income tax expense includes U.S., state, local and international income taxes. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial reporting basis and the tax basis of existing assets and liabilities. The tax rate used to determine the deferred tax assets and liabilities is based on the enacted tax rate for the year and the manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
We identified management’s calculation of the provision for income taxes as a critical audit matter because of the significant judgments and estimates management makes to determine these amounts. Performing audit procedures to evaluate the reasonableness of management’s interpretation of tax law in various domestic and foreign jurisdictions, and its estimate of the associated provisions and tax charges required a high degree of auditor judgment and increased effort.
How the Critical Audit Matter was Addressed in the Audit
Our audit procedures performed to address this critical audit matter included the following, among others:
| ● |
We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over income tax balances and disclosures, including the provision for income taxes. |
| ● |
We assessed the Company’s income tax provision by: |
| ● |
Testing the provision for income taxes, including the effective tax rate reconciliation, permanent and temporary differences and uncertain tax positions, by evaluating communications with tax advisors, and testing the underlying data for completeness and accuracy. |
| ● |
Utilizing personnel with specialized knowledge and skill in domestic and international tax to assist in (i) evaluating management’s application of domestic and foreign tax laws and (ii) evaluating the calculation of the deferred tax attributes. |
| ● |
Evaluating the significant assumptions used by management in establishing and measuring tax-related assets and liabilities, including the application of recent tax laws and regulations. |
| ● |
Evaluating the Company’s disclosures related to the provision for income taxes. |
/s/ Plante & Moran, PLLC
We have served as the Company’s auditor since 1986.
Denver, Colorado
May 30, 2023
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors of Mesa Laboratories, Inc.
Adverse Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting as of March 31, 2023 of Mesa Laboratories, Inc. (the “Company”), based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO framework”). In our opinion, because of the effect of the material weaknesses described in the following paragraphs on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of March 31, 2023, based on criteria established in the COSO framework.
A material weakness is a control deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management’s assessment:
| 1. |
Management failed to utilize resources with an appropriate level of knowledge and expertise in performing and reviewing the preliminary valuation of the Belyntic acquisition. As a result, we identified errors in the preliminary valuation as part of our audit procedures after the preliminary valuation had been reviewed internally by management. Accordingly, we concluded that management’s review controls in this area were not properly designed or operating effectively to achieve the control objective. |
| 2. |
Management’s review controls over the qualitative assessment of goodwill impairment were insufficient to identify potential impairment triggers. As a result, we identified potential impairment triggers that required management to further evaluate whether an impairment had occurred. Accordingly, we concluded that management’s review controls in this area were not properly designed or operating effectively to achieve the control objective. |
These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the March 31, 2023 financial statements, and this report does not affect our report dated May 30, 2023, on those financial statements.
We also have audited the accompanying consolidated balance sheets of the Company as of March 31, 2023 and 2022, the related consolidated statements of income, comprehensive (loss) income, stockholders' equity, and cash flows for each of the years in the three-year period ended March 31, 2023, and the related notes (collectively referred to as the “financial statements”), in accordance with the standards of the Public Company Accounting Oversight Board (United States). Our report dated May 30, 2023, expresses an unqualified opinion.
Basis for Opinion
The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Item 9A, Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Plante & Moran, PLLC
We have served as the Company’s auditor since 1986.
Denver, Colorado
May 30, 2023
Mesa Laboratories, Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
| March 31, |
March 31, |
|||||||
| 2023 |
2022 |
|||||||
| ASSETS |
||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 32,910 | $ | 49,346 | ||||
| Accounts receivable, less allowances of $849 and $630, respectively |
42,551 | 41,224 | ||||||
| Inventories, net |
34,642 | 24,606 | ||||||
| Prepaid expenses and other |
8,872 | 9,142 | ||||||
| Total current assets |
118,975 | 124,318 | ||||||
| Noncurrent assets |
||||||||
| Property, plant and equipment, net |
28,149 | 28,620 | ||||||
| Deferred tax asset |
1,076 | 1,318 | ||||||
| Other assets |
10,373 | 11,830 | ||||||
| Customer relationships, net |
152,189 | 176,688 | ||||||
| Intellectual property, net |
46,400 | 53,273 | ||||||
| Other intangibles, net |
18,226 | 20,156 | ||||||
| Goodwill |
286,444 | 291,166 | ||||||
| Total assets |
$ | 661,832 | $ | 707,369 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 6,134 | $ | 7,897 | ||||
| Accrued payroll and benefits |
9,433 | 14,717 | ||||||
| Unearned revenues |
14,407 | 13,830 | ||||||
| Other accrued expenses |
13,385 | 11,611 | ||||||
| Total current liabilities |
43,359 | 48,055 | ||||||
| Noncurrent liabilities |
||||||||
| Deferred tax liability |
34,028 | 39,224 | ||||||
| Other long-term liabilities |
7,693 | 7,924 | ||||||
| Credit facility |
13,000 | 49,000 | ||||||
| Convertible senior notes, net of discounts and debt issuance costs |
170,272 | 169,365 | ||||||
| Total liabilities |
268,352 | 313,568 | ||||||
| Stockholders’ equity |
||||||||
| Common stock, no par value; authorized 25,000,000 shares; issued and outstanding, 5,369,466 and 5,265,627 shares, respectively |
332,076 | 313,460 | ||||||
| Retained earnings |
74,199 | 76,675 | ||||||
| Accumulated other comprehensive (loss) income |
(12,795 | ) | 3,666 | |||||
| Total stockholders’ equity |
393,480 | 393,801 | ||||||
| Total liabilities and stockholders’ equity |
$ | 661,832 | $ | 707,369 | ||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Income
(In thousands, except per share data)
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Revenues |
||||||||||||
| Product |
$ | 180,520 | $ | 149,422 | $ | 107,028 | ||||||
| Service |
38,560 | 34,913 | 26,909 | |||||||||
| Total revenues |
219,080 | 184,335 | 133,937 | |||||||||
| Cost of revenues |
||||||||||||
| Cost of products |
60,937 | 54,747 | 33,120 | |||||||||
| Cost of services |
24,450 | 20,498 | 13,803 | |||||||||
| Total cost of revenues |
85,387 | 75,245 | 46,923 | |||||||||
| Gross profit |
133,693 | 109,090 | 87,014 | |||||||||
| Operating expenses |
||||||||||||
| Selling |
37,439 | 28,310 | 18,480 | |||||||||
| General and administrative |
72,444 | 60,311 | 45,788 | |||||||||
| Research and development |
20,490 | 15,767 | 10,388 | |||||||||
| Total operating expenses |
130,373 | 104,388 | 74,656 | |||||||||
| Operating income |
3,320 | 4,702 | 12,358 | |||||||||
| Nonoperating expenses |
||||||||||||
| Interest expense and amortization of debt discount |
4,770 | 3,885 | 8,024 | |||||||||
| Other (income) expense, net |
(1,061 | ) | (2,757 | ) | 2,031 | |||||||
| Total nonoperating expense |
3,709 | 1,128 | 10,055 | |||||||||
| (Loss) earnings before income taxes |
(389 | ) | 3,574 | 2,303 | ||||||||
| Income tax (benefit) expense |
(1,319 | ) | 1,703 | (971 | ) | |||||||
| Net income |
$ | 930 | $ | 1,871 | $ | 3,274 | ||||||
| Earnings per share |
||||||||||||
| Basic |
$ | 0.17 | $ | 0.36 | $ | 0.66 | ||||||
| Diluted |
$ | 0.17 | $ | 0.35 | $ | 0.64 | ||||||
| Weighted-average common shares outstanding |
||||||||||||
| Basic |
5,321 | 5,212 | 4,975 | |||||||||
| Diluted |
5,361 | 5,335 | 5,124 | |||||||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Comprehensive (Loss) Income
(In thousands except per share data)
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Net income |
$ | 930 | $ | 1,871 | $ | 3,274 | ||||||
| Other comprehensive (loss) income |
||||||||||||
| Foreign currency translation adjustments |
(16,461 | ) | (12,450 | ) | 26,485 | |||||||
| Comprehensive (loss) income |
$ | (15,531 | ) | $ | (10,579 | ) | $ | 29,759 | ||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
| Common Stock |
||||||||||||||||||||
| Number of Shares |
Amount |
Retained Earnings |
AOCI* |
Total |
||||||||||||||||
| March 31, 2020 |
4,387,140 | $ | 158,023 | $ | 72,359 | $ | (10,369 | ) | $ | 220,013 | ||||||||||
| Proceeds from issuance of common stock, net of issuance costs of $9,315 |
690,000 | 145,935 | - | - | 145,935 | |||||||||||||||
| Exercise of stock options and vesting of restricted stock units |
65,532 | 4,943 | - | - | 4,943 | |||||||||||||||
| Tax withholding on restricted stock units |
(2,104 | ) | (517 | ) | - | - | (517 | ) | ||||||||||||
| Dividends paid, $0.64 per share |
- | - | (3,165 | ) | - | (3,165 | ) | |||||||||||||
| Stock-based compensation expense |
- | 9,268 | - | - | 9,268 | |||||||||||||||
| Foreign currency translation |
- | - | - | 26,485 | 26,485 | |||||||||||||||
| Adoption of accounting standards, net |
- | - | (9 | ) | - | (9 | ) | |||||||||||||
| Net income |
- | - | 3,274 | - | 3,274 | |||||||||||||||
| March 31, 2021 |
5,140,568 | 317,652 | 72,459 | 16,116 | 406,227 | |||||||||||||||
| Exercise of stock options and vesting of restricted stock units |
128,337 | 8,027 | - | - | 8,027 | |||||||||||||||
| Tax withholding on restricted stock units |
(3,278 | ) | (875 | ) | - | - | (875 | ) | ||||||||||||
| Dividends paid, $0.64 per share |
- | - | (3,339 | ) | - | (3,339 | ) | |||||||||||||
| Stock-based compensation expense |
- | 11,391 | - | - | 11,391 | |||||||||||||||
| Foreign currency translation |
- | - | - | (12,450 | ) | (12,450 | ) | |||||||||||||
| Cumulative adjustment due to adoption of ASU 2020-06 |
- | (22,735 | ) | 5,684 | - | (17,051 | ) | |||||||||||||
| Net income |
- | - | 1,871 | - | 1,871 | |||||||||||||||
| March 31, 2022 |
5,265,627 | 313,460 | 76,675 | 3,666 | 393,801 | |||||||||||||||
| Exercise of stock options and vesting of restricted stock units |
108,737 | 6,997 | - | - | 6,997 | |||||||||||||||
| Tax withholding on restricted stock units |
(4,898 | ) | (919 | ) | - | - | (919 | ) | ||||||||||||
| Dividends paid, $0.64 per share |
- | - | (3,406 | ) | - | (3,406 | ) | |||||||||||||
| Stock-based compensation expense |
- | 12,538 | - | - | 12,538 | |||||||||||||||
| Foreign currency translation |
- | - | - | (16,461 | ) | (16,461 | ) | |||||||||||||
| Net income |
- | - | 930 | - | 930 | |||||||||||||||
| March 31, 2023 |
5,369,466 | $ | 332,076 | $ | 74,199 | $ | (12,795 | ) | $ | 393,480 | ||||||||||
*Accumulated Other Comprehensive (Loss) Income.
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net income |
$ | 930 | $ | 1,871 | $ | 3,274 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
| Depreciation and amortization |
33,824 | 25,068 | 17,660 | |||||||||
| Stock-based compensation |
12,538 | 11,391 | 9,268 | |||||||||
| Non-cash interest and debt amortization |
907 | 1,029 | 5,397 | |||||||||
| Deferred taxes |
(3,494 | ) | 128 | (3,503 | ) | |||||||
| Amortization of step-up in inventory basis |
- | 7,462 | (436 | ) | ||||||||
| Other |
390 | (534 | ) | 161 | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable, net |
(2,121 | ) | (6,752 | ) | (647 | ) | ||||||
| Inventories |
(10,182 | ) | (1,045 | ) | 929 | |||||||
| Prepaid expenses and other assets |
(510 | ) | (3,606 | ) | 2,878 | |||||||
| Accounts payable |
(1,545 | ) | 1,370 | 967 | ||||||||
| Other accrued expenses |
(3,360 | ) | 255 | (317 | ) | |||||||
| Unearned revenues |
606 | 2,586 | 1,442 | |||||||||
| Net cash provided by operating activities |
27,983 | 39,223 | 37,073 | |||||||||
| Cash flows from investing activities: |
||||||||||||
| Acquisitions, net of cash acquired |
(4,950 | ) | (300,793 | ) | - | |||||||
| Purchases of property, plant and equipment |
(4,544 | ) | (4,432 | ) | (1,992 | ) | ||||||
| Net cash (used in) investing activities |
(9,494 | ) | (305,225 | ) | (1,992 | ) | ||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from the issuance of common stock, net |
- | - | 145,935 | |||||||||
| Proceeds from the issuance of debt |
- | 70,000 | - | |||||||||
| Payments of debt |
(36,000 | ) | (21,000 | ) | - | |||||||
| Dividends |
(3,406 | ) | (3,339 | ) | (3,165 | ) | ||||||
| Proceeds from the exercise of stock options |
6,997 | 8,027 | 4,943 | |||||||||
| Payment of tax withholding obligation on vesting of restricted stock |
(919 | ) | (875 | ) | (517 | ) | ||||||
| Payments of contingent consideration |
- | (237 | ) | (304 | ) | |||||||
| Payment of debt issuance costs |
- | - | (664 | ) | ||||||||
| Net cash (used in) provided by financing activities |
(33,328 | ) | 52,576 | 146,228 | ||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(1,597 | ) | (1,093 | ) | 1,176 | |||||||
| Net (decrease) increase in cash and cash equivalents |
(16,436 | ) | (214,519 | ) | 182,485 | |||||||
| Cash and cash equivalents at beginning of period |
49,346 | 263,865 | 81,380 | |||||||||
| Cash and cash equivalents at end of period |
$ | 32,910 | $ | 49,346 | $ | 263,865 | ||||||
| Cash paid for: |
||||||||||||
| Income taxes |
$ | 1,356 | $ | 3,048 | $ | 1,367 | ||||||
| Interest |
$ | 3,485 | $ | 2,762 | $ | 2,372 |
| Supplemental non-cash activity: |
||||||||||||
| Contingent consideration from acquisitions |
$ | 1,190 | $ | - | $ | 490 |
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Notes to Consolidated Financial Statements
(dollar and share amounts in thousands, unless otherwise specified)
Note 1. Description of Business and Summary of Significant Accounting Policies
Description of Business
In this Annual Report on Form 10-K, Mesa Laboratories, Inc., a Colorado corporation, together with its subsidiaries is collectively referred to as “we,” “us,” “our,” the “Company,” or "Mesa."
We are a multinational manufacturer, developer, and seller of life sciences tools and critical quality control products and services, many of which are sold into niche markets driven by regulatory requirements. We have manufacturing operations in the United States and Europe, and our products are marketed by our sales personnel in North America, Europe, and Asia Pacific, as well as by independent distributors in these areas and throughout the rest of the world. We prefer markets in which we can establish a strong presence and achieve high gross profit margins.
As of March 31, 2023, we managed our operations in four reportable segments, or divisions:
| ● |
Clinical Genomics - develops, manufactures and sells highly sensitive, low-cost, high-throughput genetic analysis tools and related consumables and services that enable clinical labs to perform genomic testing for a broad range of diagnostic and research applications in several therapeutic areas, such as screenings for hereditary diseases, pharmacogenetics, and oncology related applications. |
| ● |
Sterilization and Disinfection Control - manufactures and sells biological, cleaning, and chemical indicators which are used to assess the effectiveness of sterilization and disinfection processes, including steam, gas, hydrogen peroxide, ethylene oxide, radiation, and other processes in the hospital, dental, medical device and pharmaceutical industries. The division also provides testing and laboratory services, mainly to the dental industry. |
| ● | Biopharmaceutical Development - develops, manufactures and sells automated systems for protein analysis (immunoassays) and peptide synthesis solutions. Immunoassays and peptide synthesis solutions accelerate the discovery, development, and manufacture of biotherapeutic therapies, among other applications. |
| ● |
Calibration Solutions - develops, manufactures and sells quality control products using principles of advanced metrology to measure or calibrate critical chemical or physical parameters in various dialysis, process monitoring, instrument monitoring, environmental monitoring, gas flow, environmental air quality, and torque applications, primarily in hospital, medical device manufacturing, pharmaceutical manufacturing, and laboratory environments. |
Unallocated corporate expenses and other business activities are reported within Corporate and Other.
Principles of Consolidation and Basis of Presentation
Our Consolidated Financial Statements are prepared in accordance with the rules and regulations of the Securities and Exchange Commission and in accordance with accounting principles generally accepted in the United States (“GAAP”), and include our accounts and those of our wholly owned subsidiaries after elimination of all intercompany accounts and transactions.
Prior Period Reclassification
During fiscal year 2022 we combined our historical Instruments and Continuous Monitoring reportable segments to create the Calibration Solutions reportable segment. Prior year amounts from fiscal year 2021 have been recast to conform to current year presentation, consistent with our Annual Report on Form 10-K for the year ended March 31, 2022. Our change in financial reporting segments has not resulted in any change to consolidated amounts reported in the Consolidated Financial Statements for any periods presented in this Annual Report on Form 10-K.
Certain amounts presented in Note 2. "Revenue" in prior periods of fiscal year 2022 and 2023 have been reclassified. Specifically, we reclassified a portion of the Biopharmaceutical Development division's revenues from consumables into revenues from hardware and services. Certain revenues related to Clinical Genomics division have been reclassified out of revenues from hardware and into revenues from consumables. These reclassifications allow for consistency of presentation across divisions and have not resulted in any change to consolidated or segment amounts reported in the Consolidated Financial Statements for any periods presented in this Annual Report on Form 10-K.
Management Estimates
The preparation of our Consolidated Financial Statements in conformity with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our Consolidated Financial Statements and accompanying notes. Actual results could differ from our estimates under different assumptions or conditions.
Summary of Significant Accounting Policies
Foreign Currency
Exchange rate adjustments resulting from foreign currency transactions are recognized in net earnings, whereas effects resulting from the translation of financial statements are reflected as a component of accumulated other comprehensive income within stockholders’ equity. Assets and liabilities of subsidiaries operating outside the United States with a functional currency other than the U.S. dollar are translated into U.S. dollars at period end exchange rates, and revenue and expense accounts are translated at weighted average period rates.
Fair Value Measurements
Fair value is the price we would receive to sell an asset or pay to transfer a liability (exit price) in an orderly transaction between market participants. We determine fair value based on the following input hierarchy:
Level 1: Quoted prices for identical assets or liabilities in active markets.
Level 2: Observable inputs other than prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or that can be corroborated with observable market data.
Level 3: Unobservable inputs supported by little or no market activity. Pricing models, discounted cash flow methodologies, and other similar techniques involving significant management judgment or estimation typically require unobservable inputs.
Assets recognized or disclosed at fair value in the Consolidated Financial Statements on a nonrecurring basis are measured at fair value if determined to be impaired or if purchased pursuant to our acquisition of a business, including items such as inventory, property and equipment, operating lease assets, goodwill, and other intangible assets. Fair values assigned to assets acquired and liabilities assumed in acquisitions, except deferred revenues, are measured using Level 3 inputs.
Revenue Recognition
Our revenues come from product sales, which include consumables and hardware; as well as services, which include discrete and ongoing maintenance, calibration, and testing services. Revenues are recognized when or as we satisfy our performance obligations under the terms of a contract, which occurs when control of the promised products or services transfers to our customers. We recognize the amount of consideration we expect to receive in exchange for transferring products or services to our customers (the transaction price) as revenue. For all revenue contracts, prices are fixed at the time of purchase and no price protections or variables are offered. The significant majority of our revenues and related receivables are generated from contracts with customers that are 12 months or less in duration.
We generally recognize revenues as follows:
Product sales: Our performance obligations related to product sales generally consist of the promise to sell tangible goods and integrated software to distributors or end users. Control of these goods is typically transferred upon shipment, at which time our performance obligation is satisfied and revenue is recognized. Purchase orders typically provide evidence of an arrangement for product sales. Products sold include an assurance-type warranty which is accounted for as part of accrued warranty expense.
Services: We generate service revenues from discrete and ongoing maintenance, calibration, and testing services performed on our physical products. For discrete services, our performance obligation to complete specified work is satisfied and revenue is recognized upon performance of the service. Performance obligations arising from ongoing service contracts in which we promise to stand ready to provide maintenance or other services on an as-needed basis are satisfied by completing any services that are contractually required during the contract period, if requested by the customer, or simply by the passage of time if no services are requested. For ongoing service contracts, revenue is recognized on a straight-line basis over the life of the contract in a faithful depiction of our obligation to provide services over the contract period. Evidence of a service arrangement may be in the form of a formal contract or a purchase order.
Collectability is reasonably assured through our customer review process, and payment is typically due within 60 days or less.
Upon adoption of Accounting Standards Codification 606, we elected the practical expedients to expense commission costs (typically our only significant incremental cost to obtain a contract) as incurred and to account for shipping and handling costs as fulfillment costs. The substantial majority of our contracts have original durations of one year or less, and we have elected not to disclose the expected timing or allocated transaction prices of future performance obligations such as obligations to perform maintenance and repair services. Additionally, we have elected to not assess whether a significant financing component exists when the period between when we perform our performance obligation and when the customer remits payment is one year or less. None of our contracts contained significant financing components as of or for the fiscal years ended March 31, 2023 or 2022.
Contracts with customers may contain multiple performance obligations. For such arrangements, the transaction price is allocated to each performance obligation based on the estimated relative standalone selling prices of the promised products or services underlying each performance obligation. Standalone selling prices are based on the price at which the performance obligation is sold separately. If the standalone selling price is not observable through past transactions, we estimate the standalone selling price considering available information such as market conditions and internally approved pricing guidelines. In limited circumstances, for obligations with highly variable or unobservable standalone selling prices, we may assign standalone prices to obligations based on the residual transaction price after all observable standalone selling prices have been determined. Discounts may be approved at the time of purchase and are included within a contract’s fixed transaction price. Discounts are typically allocated to the performance obligations included in the contract based on the standalone values of such obligations. All expected and actual consideration from customers is included in the transaction price.
Shipping and Handling
Payments made by customers to us for shipping and handling costs are included in revenues on the Consolidated Statements of Income, and our expenses are included in cost of revenues. We account for shipping and handling costs arising from contracts with customers as fulfillment costs. Shipping and handling for inventory and materials we purchase is included as a component of inventory on the Consolidated Balance Sheets, and expensed to cost of revenues when products are sold.
Unearned Revenues
Certain of our products may be sold with associated time-based service contracts whereby we provide repairs, technical support, parts, and various analytical or maintenance services. In the event these contracts are paid in advance by the customer, the associated amounts are recorded as an unearned revenue liability and recognized as revenue ratably over the term of the service period, generally one year. Prepayments from customers with respect to other products and services are likewise recorded as unearned revenue liabilities and are recognized to revenue when earned.
Accrued Warranty Expense
We typically provide assurance-type limited product warranties on our products and, accordingly, accrue for estimates of related warranty expenses.
Accounts Receivable and Allowance for Doubtful Accounts
All trade accounts receivable are reported at net realizable value on the accompanying Consolidated Balance Sheets, adjusted for any write-offs and net of allowances for doubtful accounts. Allowances for doubtful accounts represent our best estimate and current expectation of future credit losses from trade accounts. We estimate credit losses based on historical information, current and expected future economic and market conditions, and reviews of the current status of customers’ trade accounts receivable. In circumstances in which we become aware of a specific customer’s inability to meet its financial obligations, a specific reserve is recorded against amounts due to reduce the recognized receivable to the amount reasonably expected to be collected.
We do not believe our trade accounts receivable represent significant concentrations of credit risk due to our diversified portfolio of individual customers and geographical areas. See Note 3. “Fair Value Measurements” for further discussion and for information on how we manage credit risk.
Differences may arise between estimated and actual losses, which could materially affect the provision for credit losses and, therefore, net earnings. We recorded $736, $304, and $100 of expense associated with doubtful accounts for the years ended March 31, 2023, 2022, and 2021, respectively. The increase in bad debt expense reflects the uncertainty in market and macro-economic conditions.
Inventories
Inventories are stated at the lower of cost or net realizable value and are relieved to cost of products upon sale using a weighted average costing methodology. Inventories acquired in an acquisition are recorded at fair market value. Our work in process and finished goods inventories include the costs of raw materials, labor and overhead, which are estimated based on trailing twelve months of expense and standard labor hours for each product. We evaluate labor and overhead costs annually unless specific circumstances necessitate a mid-year evaluation for specific items.
We monitor inventory costs relative to selling prices and perform physical cycle count procedures on inventories throughout the year to determine if a lower of cost or net realizable value reserve is necessary. We estimate and maintain an inventory reserve as needed for such matters as excess or obsolete inventory, shrinkage, and scrap. This reserve may fluctuate as our assumptions change due to new information, discrete events, or changes in our business, such as entering new markets or discontinuing a specific product; however, once inventory is written down, a new cost basis is established that is not subsequently written back up in future fiscal years.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost, less allowances for depreciation, except for assets acquired in acquisitions, which are recorded at fair value. Expenditures for major renewals and improvements that extend the life of the asset are capitalized, while expenditures for minor replacements, maintenance, and repairs are expensed as incurred.
Depreciation is calculated using the straight-line method over the assets’ estimated useful lives. Upon asset retirement or disposal, accounts are relieved of cost and accumulated depreciation, and any related gain or loss is reflected in our results of operations. In some cases, particularly with respect to business consolidation or closure activities, accelerated depreciation may be required for the revised remaining useful lives of assets designated to be abandoned in the future.
At least annually, we evaluate and adjust as necessary the estimated lives of property, plant and equipment. Any changes in estimated useful lives are recorded prospectively. Estimated useful lives of significant classes of depreciable assets are as follows:
| Category |
Useful Lives in Years |
| Buildings and building improvements | 40 (or less) |
| Manufacturing equipment | 7 (or less) |
| Office, lab and other equipment |
7 (or less) |
| Computer equipment |
3 (or less) |
| Leasehold improvements | Lesser of the economic life or the remaining term in the respective lease |
Land is not depreciated and construction in progress is not depreciated until placed in service, at which time it is assigned a useful life consistent with the nature of the asset.
Leases
Under ASC 842, we determine whether contractual arrangements contain a lease at the inception of the arrangement. If a lease is identified in an arrangement, we recognize a right-of-use asset ("ROU") and liability on our Consolidated Balance Sheets and determine whether the lease should be classified as a finance or operating lease. We do not have any finance leases. We do not recognize assets or liabilities for leases with terms of less than 12 months, and our short-term leases are not material.
A contract is a lease or contains one when (1) the contract contains an explicitly or implicitly identified asset and (2) the customer obtains substantially all of the economic benefits from the use of that underlying asset and directs how and for what purpose the asset is used during the term of the contract in exchange for consideration. Operating lease assets and liabilities are recognized at the lease commencement date. Operating lease liabilities represent the present value of lease payments not yet paid. Operating lease assets represent our right to use an underlying asset and are based upon the operating lease liabilities adjusted for prepayments. Adjustments would also be made for accrued lease payments, initial direct costs, lease incentives, and impairment of operating lease assets, none of which are present in any of our current lease contracts. When readily determinable, the discount rate used to calculate the lease liability is the rate implicit in the lease, otherwise we use our incremental borrowing rate based on the information available at lease commencement. When we acquire a business, we retain the acquiree's classification of its leases. We evaluate the ROU assets and liabilities in accordance with ASC 842.
Our leases typically contain rent escalations over the lease term. We recognize expense for these leases on a straight-line basis over the lease term. Lease expense is recorded in cost of products, selling, general and administrative, or research and development on our Consolidated Statements of Income, depending on the nature of use of the underlying asset. Many of our leases include one or more renewal or termination options exercisable at our discretion, which are included in the determination of the lease term if we are reasonably certain to exercise the option. We have also entered into lease agreements that have variable payments related to certain indexes. Variable lease payments are recognized in the period in which those payments are incurred. All non-lease components are readily identifiable in our lease contract. We account for non-lease components separately from the lease component to which it is related.
Acquired Intangible Assets
Our goodwill and other intangible assets result from acquisitions of existing businesses. Upon acquisition, we record the fair values of separately identifiable indefinite and definite lived intangible assets using, among other sources of relevant information, independent appraisals, or actuarial or other valuations. Intangible assets affect the amount of future amortization expense and possible impairment charges we may incur.
Goodwill and indefinite lived intangible assets (certain tradenames we intend to renew and continue using indefinitely) are not subject to amortization and are tested for impairment qualitatively, and if necessary, quantitatively, at least annually during the fourth quarter of our fiscal year, or when events or changes in circumstances indicate it may be more likely than not that carrying value exceeds fair value. We perform impairment tests of goodwill at the reporting unit level and tests for other indefinite lived intangible assets at the asset level.
Intangible assets deemed to have finite lives are amortized on a straight-line basis over their useful lives, generally ranging from five to fifteen years (See Note 6. “Goodwill and Intangible Assets”). We determine the useful lives of finite intangible assets based on the specific facts and circumstances related to each asset, and we evaluate the appropriateness of assigned useful lives at least annually. Factors we consider when determining useful lives include the contractual term of any agreement related to the asset, the historical performance of the asset, our long-term strategy for using the asset, any laws or other local regulations which could impact the useful life of the asset, and economic factors such as competition or specific market conditions. Finite-lived intangible assets are tested for impairment if events or changes in circumstances indicate that the carrying amount of a long-lived asset or asset group might not be recoverable.
The fair value measurements used in testing intangible asset impairments are typically based on discounted cash flow projection models, using Level 3 inputs. See “Fair Value of Financial Instruments” for a description of input levels. Significant assumptions include, among others, the weighted average cost of capital, net sales growth, and terminal growth rates. In certain cases, management uses other market information when available to estimate fair value. Impairment charges represent the excess carrying amount over estimated fair value. We do not believe our goodwill and other intangible assets are impaired as of March 31, 2023.
Research & Development Costs
We conduct research and development activities for the purpose of developing new products and enhancing the functionality, effectiveness, reliability, and accuracy of existing products. Research and development costs are expensed as incurred. Research and development expense is predominantly comprised of labor costs and third-party consultants, but we may from time to time purchase in-process research and development with the intention of developing a saleable product.
Convertible Debt
We issue shares in the form of stock options and full-value awards as part of employee and non-employee director compensation pursuant to the Mesa Laboratories, Inc. 2014 Equity Plan (the "2014 Equity Plan") and the Mesa Laboratories, Inc. 2021 Equity Incentive Plan (the "2021 Equity Plan" or together, "the Equity Plans").
The Equity Plans are administered by the Compensation Committee of the Board of Directors, which has the authority to grant equity awards, or to delegate its authority under the plan to make grants (subject to certain legal and regulatory restrictions), including the authority to determine the individuals to whom awards will be granted, the type of awards and when the awards are to be granted, the number of shares to be covered by each award, the vesting schedule, and all other terms and conditions of the awards.
For purposes of counting the shares remaining under the 2021 Equity Plan, each share underlying a stock option or a full value award counts as one share used. For purposes of counting the shares remaining available under the 2014 Equity Plan, each share issuable pursuant to outstanding full value awards counts as five shares issued, whereas each share underlying a stock option counts as one share issued. We issue new shares of common stock upon the exercise of stock options and the vesting of time-based restricted stock units ("RSUs") and performance-based RSUs ("PSUs").
Earnings Per Share
Basic earnings per share (“EPS”) is computed by dividing net income by the weighted-average number of common shares outstanding during the reporting period. Diluted earnings per share (“diluted EPS”) is computed similarly to basic earnings per share, except it includes the effects of potential dilution that could occur if dilutive securities were exercised. Potentially dilutive securities include stock options, RSUs and PSUs (collectively “stock awards”), as well as common shares underlying the 2025 Notes. Potentially dilutive securities are excluded from the calculation of diluted EPS in the event they are subject to performance conditions that have not yet been achieved or if they would otherwise be antidilutive. Diluted EPS considers the impact of potentially dilutive securities except in periods in which there is a loss; in such cases the inclusion of the potential common shares would have an antidilutive effect. See Note 10. “Earnings per Share” for EPS calculations for the years ended March 31, 2023, 2022 and 2021.
Income Taxes
Income tax expense includes U.S., state, local and international income taxes. Deferred tax assets and liabilities are recognized and reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the tax basis of existing assets and liabilities used for income tax purposes. The tax rate used to determine the deferred tax assets and liabilities is based on the enacted tax rate for the year and the manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
From time to time, we engage in transactions in which the tax consequences may be subject to uncertainty, such as acquisitions. Significant judgment is required in assessing and estimating the tax consequences of these transactions. We prepare and file tax returns based on interpretation of tax laws and regulations. In the normal course of business, our tax returns are subject to examination by various taxing authorities. Such examinations may result in future tax, interest and penalty assessments by these taxing authorities. In determining our income tax provision for financial reporting purposes, we establish a reserve for uncertain tax income positions unless we determine it is not more likely than not that such positions would be sustained upon examination, based on their technical merits.. That is, for financial reporting purposes, we only recognize tax benefits taken on the tax return that we believe are more likely than not of being sustained. There is considerable judgment involved in determining whether positions taken on the tax return are more likely than not of being sustained. We adjust our tax reserve estimates periodically because of ongoing examinations by, and settlements with, the various taxing authorities, as well as changes in tax laws, regulations and interpretations. The consolidated income tax provision of any given year includes adjustments to prior year income tax accruals that are considered appropriate and any related estimated interest. Our policy is to recognize, when applicable, interest and penalties on uncertain income tax positions as part of general administrative expense. (See Note 12. “Income Taxes”).
Acquisition Related Contingent Consideration Liabilities
Acquisition related contingent consideration liabilities consist of estimated amounts due under various acquisition agreements and may be based on revenues growth, specified profitability growth metrics, or the attainment of milestones such as patent approvals. At each reporting period, we evaluate the expected future payments and any associated discount rate to determine the fair value of the contingent consideration. We re-evaluate the fair value of contingent liabilities at each reporting period and record any necessary adjustments in other expense, net on the Consolidated Statements of Income. See Note 13. “Commitments and Contingencies” for information regarding existing contingent consideration liabilities as of March 31, 2023.
Legal Contingencies
We are party to various claims and legal proceedings that arise in the normal course of business. We record an accrual for legal contingencies when we determine it is probable we have incurred a liability and can reasonably estimate the amount of the loss (See Note 13. “Commitments and Contingencies”).
Purchase Accounting for Acquisitions
We account for all business combinations in which we obtain control over another entity using the acquisition method of accounting, which requires most assets (both tangible and intangible) and liabilities (including any applicable contingent consideration, but excluding deferred revenue, which is measured at book value) to be recorded at fair value at the date of acquisition. The excess of the purchase price over the fair value of acquired assets less liabilities is recognized as goodwill. We determine fair value using widely accepted valuation techniques, primarily discounted cash flow and market multiple analyses, which rely heavily on Level 3 inputs. These types of analyses require us to make and monitor assumptions and estimates regarding industry and economic factors, the profitability of future business strategies, discount rates and cash flow. Certain adjustments to the assessed fair values of acquired assets or liabilities made subsequent to the acquisition date but within the measurement period are recorded as adjustments to goodwill. Any adjustments subsequent to the measurement period are recorded within earnings. We expense all acquisition costs as incurred related to an acquisition in selling, general, and administrative expenses.
Results of operations of acquired companies are included in our Consolidated Financial Statements from the date of the acquisition forward. If actual results are not consistent with our assumptions and estimates, or if our assumptions and estimates change due to new information, we may be exposed to an impairment charge in the future. For the years ended March 31, 2023, 2022 and 2021, our acquisitions of businesses (net of cash acquired and including contingent consideration) totaled $6,140, $300,793, and $0, respectively.
Business Consolidation Costs
We estimate liabilities for business closure activities by gathering detailed estimates of costs and, if applicable, asset sale proceeds, for each business consolidation initiative. For a typical business consolidation initiative, we estimate costs of employee severance, impairment of property and equipment and other assets including estimating net realizable value, if necessary, accelerated depreciation, termination payments for contracts and leases, and any other qualifying costs related to the exit plan. Such charges represent our best estimates; however, they require assumptions about plans that may change over time. The estimated costs are grouped by specific projects within the overall exit plan and are monitored at each reporting period. Any subsequent changes to the original estimates are recorded in current earnings.
Risks and Uncertainties
The preparation of financial statements requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities at the reporting date and revenues and expenses during the reporting periods. These estimates represent management's judgement about the outcome of future events. The current global business environment continues to be impacted directly and indirectly by the effects of the novel coronavirus ("COVID-19"), the conflict in Ukraine, and other factors. It is not possible to accurately predict the future impact of such events and circumstances. However, we have reviewed the estimates used in preparing the financial statements and have identified the following factors that have a reasonable possibility of being materially affected in the near term:
| ● |
Estimates regarding the future financial performance of the business used in the impairment tests for goodwill and long-lived assets acquired in a business combination; however, our impairment tests conducted during the quarter ended March 31, 2023 concluded that goodwill is not impaired; |
| ● |
Estimates regarding the recoverability of deferred tax assets and estimates regarding cash needs and associated indefinite reinvestment assertions; |
| ● |
Estimates regarding recoverability for customer receivables; |
| ● |
Estimates of the net realizable value of inventory. |
Recently Issued Accounting Pronouncements
We have reviewed all recently issued accounting pronouncements and have concluded that they are either not applicable to us or are not expected to have a significant impact on our consolidated financial statements.
Recently Adopted Accounting Pronouncements
There have been no accounting pronouncements applicable to us that we were required to adopt or that we have elected to adopt during fiscal year 2023.
Note 2. Revenue
We develop, manufacture, market, sell and maintain life sciences tools and quality control instruments and related software, consumables, and services.
Hardware sales include physical products such as instruments used for molecular and genetic analysis, protein synthesizers, medical meters, wireless sensor systems, and data loggers. Hardware sales may be offered with accompanying perpetual or annual software licenses, which in some cases are required for the hardware to function.
Consumables are typically used on a one-time basis and require frequent replacement in our customers' operating cycles. Consumables such as reagents used for molecular and genetic analysis or solutions used for protein synthesis are critical to the ongoing use of our instruments. Consumables such as biological indicator test strips are used on a standalone basis.
We also offer maintenance, calibration, and testing service contracts. These contracts result in revenues recognized over time, for example, when we are obligated to perform labor and replace parts on an as-needed basis over a contractually specified period of time, or at a point in time, upon completion of a specific, discrete service. In many cases, our contracts contain both revenues recognized over time and revenues recognized at a point in time.
We evaluate our revenues internally based on operating segment, the nature of goods and services provided, and the timing of revenue generation.
The following tables present disaggregated revenues from contracts with customers for the years ended March 31, 2023, 2022 and 2021:
| Year Ended March 31, 2023 |
||||||||||||||||||||
| Clinical Genomics (1) |
Sterilization and Disinfection Control |
Biopharmaceutical Development |
Calibration Solutions |
Total |
||||||||||||||||
| Consumables |
$ | 43,374 | $ | 55,605 | $ | 15,800 | $ | 3,062 | $ | 117,841 | ||||||||||
| Hardware and Software |
13,347 | 692 | 22,079 | 26,561 | 62,679 | |||||||||||||||
| Services |
5,578 | 8,312 | 9,486 | 15,184 | 38,560 | |||||||||||||||
| Total Revenues |
$ | 62,299 | $ | 64,609 | $ | 47,365 | $ | 44,807 | $ | 219,080 | ||||||||||
| Year Ended March 31, 2022 |
||||||||||||||||||||
| Clinical Genomics (1) |
Sterilization and Disinfection Control |
Biopharmaceutical Development |
Calibration Solutions |
Total |
||||||||||||||||
| Consumables |
$ | 22,271 | $ | 50,311 | $ | 15,551 | $ | 3,675 | $ | 91,808 | ||||||||||
| Hardware and Software |
6,726 | 700 | 21,651 | 28,537 | 57,614 | |||||||||||||||
| Services |
3,843 | 8,033 | 8,377 | 14,660 | 34,913 | |||||||||||||||
| Total Revenues |
$ | 32,840 | $ | 59,044 | $ | 45,579 | $ | 46,872 | $ | 184,335 | ||||||||||
| Year Ended March 31, 2021 |
||||||||||||||||||||
| Clinical Genomics (1) |
Sterilization and Disinfection Control |
Biopharmaceutical Development |
Calibration Solutions |
Total |
||||||||||||||||
| Consumables |
$ | - | $ | 45,869 | $ | 13,942 | $ | 3,198 | $ | 63,009 | ||||||||||
| Hardware and Software |
- | 505 | 13,545 | 29,969 | 44,019 | |||||||||||||||
| Services |
- | 6,745 | 6,405 | 13,759 | 26,909 | |||||||||||||||
| Total Revenues |
$ | - | $ | 53,119 | $ | 33,892 | $ | 46,926 | $ | 133,937 | ||||||||||
(1) Revenues in the Clinical Genomics division represent transactions subsequent to the acquisition of Agena Bioscience, Inc. on October 20, 2021.
Contract Balances
Our contracts have varying payment terms and conditions. Some customers prepay for products and services, resulting in either unearned revenues or customer deposits, called contract liabilities. Short-term contract liabilities are included within other accrued expenses and unearned revenues in the accompanying Consolidated Balance Sheets, and long-term contract liabilities are included within other long-term liabilities in the accompanying Consolidated Balance Sheets. The significant majority of our revenues and related receivables and contract liabilities are generated from contracts with customers with original expected durations of 12 months or less. Contract liabilities will be recognized to revenue as we satisfy our obligations under the terms of the contracts.
A summary of contract liabilities is as follows:
| Contract liabilities as of March 31, 2022 |
$ | 15,069 | ||
| Prior year liabilities recognized in revenues during the year ended March 31, 2023 |
(8,643 | ) | ||
| Contract liabilities added during the year ended March 31, 2023, net of revenues recognized |
9,672 | |||
| Contract liabilities balance as of March 31, 2023 |
$ | 16,098 |
Note 3. Fair Value Measurements
Our financial instruments generally consist of cash and cash equivalents, trade accounts receivable, obligations under trade accounts payable, and debt. Due to their short-term nature, the carrying values of cash and cash equivalents, trade accounts receivable, and trade accounts payable approximate fair value.
The financial instruments that subject us to the highest concentration of credit risk are cash and accounts receivable. We maintain relationships and cash deposits at multiple banking institutions across the world in an effort to diversify and reduce risk of loss. Concentration of credit risk with respect to accounts receivable is limited to customers to whom we make significant sales. Unusually, one of our distributors accounted for approximately 18% of total trade receivables as of March 31, 2023. Some of this balance was attributable to orders placed in the last months of fiscal year 2023, but a substantial portion was aged from earlier months; we have since collected payments for all aged balances and have continued to collect currently due amounts.
To manage credit risk, we consider the creditworthiness of new and existing customers, establish credit limits, and regularly review outstanding balances and payment histories. We may require pre-payments from customers under certain circumstances and may limit future purchases until payments are made on past due amounts.
We have outstanding $172,500 aggregate principal of 1.375% convertible senior notes due August 15, 2025, which we refer to as our 2025 Notes. We estimate the fair value of the 2025 Notes based on the last actively traded price or observable market input preceding the end of the reporting period. The estimated fair value and carrying value of the 2025 Notes were as follows:
| March 31, 2023 |
March 31, 2022 |
|||||||||||||||
| Carrying Value |
Fair Value (Level 2) |
Carrying Value |
Fair Value (Level 2) |
|||||||||||||
| 2025 Notes |
$ | 170,272 | $ | 161,072 | $ | 169,365 | $ | 185,438 | ||||||||
There were no transfers between the levels of the fair value hierarchy during the fiscal years ended March 31, 2023 and 2022.
Our financial liabilities based upon Level 3 inputs include a contingent consideration arrangement relating to our acquisition of substantially all the assets and certain liabilities of Belyntic GmbH’s peptide purification business (the "Belyntic acquisition," see Note 4. "Significant Transactions"). We are obligated to pay contingent consideration of $1,500 cash upon approval of pending patent applications, expected within 36 months of the acquisition date. The fair value of the contingent consideration was $1,190 as of March 31, 2023, and was recorded in other long-term liabilities on the accompanying Consolidated Balance Sheets. We estimated the fair value of the contingent consideration at inception using a probability-weighted outcome analysis based on our expectations of patent approval, leveraging our historical experience and expert input. The amount ultimately paid for the contingency could range from $0 to $1,500.
Note 4. Significant Transactions
Acquisitions
Belyntic, GmbH
On November 17, 2022, we acquired substantially all of the assets and certain liabilities of Belyntic GmbH’s peptide purification business. We paid $4,950 on the date of acquisition, and we expect to pay an additional $1,500 based on the probable approval of pending patent applications expected within 36 months of the acquisition date. The business complements our existing peptide synthesis business, part of the Biopharmaceutical Development segment, by adding a new consumables line. We have prepared a preliminary analysis of the valuation of net assets acquired in the Belyntic acquisition, which is subject to revision as more detailed analyses are completed.
Agena Bioscience, Inc.
On October 20, 2021, we completed the acquisition of Agena Bioscience, Inc. for $300,793, net of cash acquired but inclusive of working capital adjustments. The Agena Acquisition aligned with our overall acquisition strategy, moved our business towards the life sciences tools sector, and expanded our market opportunities, particularly in Asia.
We funded the acquisition and transactions relating thereto with cash on hand and borrowings under the Credit Facility (as defined below). Of the cash consideration we paid, approximately $267,000 represented cash consideration to holders of Agena’s preferred and common stock, approximately $2,000 represented cash consideration paid for the settlement of Agena’s warrants, and approximately $31,800 represented cash consideration for the settlement of Agena's vested stock options as of the closing date.
Allocation of Purchase Price
The allocation of purchase price is based on the fair value of assets acquired and liabilities assumed, except deferred revenue recorded at book value, as of the acquisition date, based on the final valuation of Agena. The relief from royalty method was used to value our trade names and developed technology, while the multi-period excess earnings method, a form of the income approach, was used to value our customer relationships. These methods involve the use of significant estimates and assumptions depending on the underlying asset being valued, but may include internal rate of return, revenue growth rates, customer attrition rate, and royalty rates, all of which are considered Level 3 inputs. We obtained the information used to prepare the valuation during due diligence and from other sources. These estimates were based on assumptions that we believe to be reasonable; however, actual results may differ from these estimates. We have made appropriate adjustments to deferred taxes and tax-related balances within the measurement period during the year ended March 31, 2023.
The following table summarizes the allocation of the purchase price as of October 20, 2021:
| Life (in years) |
Amount |
|||||||
| Cash and cash equivalents |
$ | 7,544 | ||||||
| Accounts receivable |
11,100 | |||||||
| Other current assets |
25,480 | |||||||
| Total current assets |
44,124 | |||||||
| Property, plant and equipment/noncurrent assets |
15,832 | |||||||
| Deferred tax asset |
811 | |||||||
| Intangible assets: |
||||||||
| Goodwill |
N/A | 135,728 | ||||||
| Customer relationships |
12 | 103,800 | ||||||
| Intellectual property |
8 | 45,400 | ||||||
| Tradenames |
12 | 15,700 | ||||||
| Total assets acquired |
$ | 361,395 | ||||||
| Accounts payable |
2,174 | |||||||
| Unearned revenues |
2,713 | |||||||
| Other current liabilities |
11,052 | |||||||
| Total current liabilities |
15,939 | |||||||
| Deferred tax liability |
28,856 | |||||||
| Other noncurrent liabilities |
8,263 | |||||||
| Total liabilities assumed |
$ | 53,058 | ||||||
| Total purchase price, net of cash acquired |
$ | 300,793 | ||||||
Acquired Goodwill
Acquired goodwill of $135,728 as of the acquisition date, all of which is allocated to the Clinical Genomics reportable segment, represents the value expected to arise from expanded market opportunities, expected synergies, and assembled workforce, none of which qualify as amortizable intangible assets. The goodwill acquired is not deductible for income tax purposes.
Note 5. Leases
We have operating leases for buildings and office equipment. The following table presents the lease balances within the Consolidated Balance Sheets related to our operating leases:
| Lease Assets and Liabilities |
Balance Sheet Location |
March 31, 2023 |
March 31, 2022 |
||||||
| Operating lease ROU asset |
Other assets |
$ | 8,693 | $ | 10,201 | ||||
| Current operating lease liabilities |
Other accrued expenses |
2,868 | 2,768 | ||||||
| Noncurrent operating lease liabilities |
Other long-term liabilities |
5,752 | 7,436 | ||||||
The components of lease costs, the weighted average remaining lease term and the weighted average discount rate were as follows:
| Year Ended March 31, |
||||||||
| 2023 |
2022 |
|||||||
| Operating lease expense |
$ | 3,064 | $ | 1,973 | ||||
| Variable lease expense |
704 | 419 | ||||||
| Total lease expense |
$ | 3,768 | $ | 2,392 | ||||
| Weighted average remaining lease term in years |
3.3 | 4.3 | ||||||
| Weighted average discount rate |
2.0 | % | 1.7 | % | ||||
Supplemental cash flow information related to leases was as follows:
| Year Ended March 31, |
||||||||
| 2023 |
2022 |
|||||||
| Cash paid for amounts included in the measurements of lease liabilities |
$ | 3,017 | $ | 1,896 | ||||
| Operating lease assets obtained in exchange for operating lease obligations |
1,426 | 10,577 | ||||||
Maturities of lease liabilities are as follows as for the years ending March 31:
| 2024 |
$ | 3,018 | ||
| 2025 |
2,384 | |||
| 2026 |
1,984 | |||
| 2027 |
1,490 | |||
| Future value of lease liabilities |
8,876 | |||
| Less: imputed interest |
256 | |||
| Present value of lease liabilities |
$ | 8,620 |
Note 6. Goodwill and Intangible Assets
Goodwill arises from the excess purchase price of acquired businesses over the fair value of acquired tangible and intangible assets, less assumed liabilities.
Changes in the carrying amount of goodwill were as follows:
| Clinical Genomics |
Sterilization and Disinfection Control |
Biopharmaceutical Development |
Calibration Solutions |
Total |
||||||||||||||||
| March 31, 2021 |
$ | - | $ | 30,153 | 93,399 | $ | 37,289 | $ | 160,841 | |||||||||||
| Effect of foreign currency translation |
34 | (403 | ) | (5,134 | ) | (52 | ) | (5,555 | ) | |||||||||||
| Goodwill related to Agena Acquisition |
135,880 | - | - | - | 135,880 | |||||||||||||||
| March 31, 2022 |
$ | 135,914 | $ | 29,750 | $ | 88,265 | $ | 37,237 | 291,166 | |||||||||||
| Effect of foreign currency translation |
49 | (191 | ) | (7,381 | ) | (20 | ) | (7,543 | ) | |||||||||||
| Goodwill related to Belyntic Acquisition |
- | - | 2,973 | - | 2,973 | |||||||||||||||
| Measurement period adjustment - Agena Acquisition |
(152 | ) | - | - | - | (152 | ) | |||||||||||||
| March 31, 2023 |
$ | 135,811 | $ | 29,559 | $ | 83,857 | $ | 37,217 | $ | 286,444 | ||||||||||
Other intangible assets were as follows:
| March 31, 2023 |
March 31, 2022 |
|||||||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
|||||||||||||||||||
| Customer relationships |
$ | 238,247 | $ | (86,058 | ) | $ | 152,189 | $ | 244,157 | $ | (67,469 | ) | $ | 176,688 | ||||||||||
| Intellectual property |
65,950 | (19,550 | ) | 46,400 | 65,893 | (12,620 | ) | 53,273 | ||||||||||||||||
| Other intangibles |
24,793 | (6,567 | ) | 18,226 | 25,350 | (5,194 | ) | 20,156 | ||||||||||||||||
| Total |
$ | 328,990 | $ | (112,175 | ) | $ | 216,815 | $ | 335,400 | $ | (85,283 | ) | $ | 250,117 | ||||||||||
The range of useful lives and weighted-average remaining useful lives of amortizable intangible assets as of March 31, 2023 were as follows:
| Est. Useful |
Weighted Avg. |
|||||||
| Life |
Remaining Life |
|||||||
| Description |
(Years) |
(Years) |
||||||
| Customer relationships |
10 - 14 |
8.8 |
||||||
| Intellectual property |
8 - 14 |
6.5 |
||||||
| Other intangibles |
3 - 12 |
10.4 |
The following is estimated amortization expense for the years ending March 31:
| 2024 |
$ | 28,580 | ||
| 2025 |
26,999 | |||
| 2026 |
26,233 | |||
| 2027 |
25,733 | |||
| 2028 |
25,275 |
Amortization expense for finite-lived intangible assets acquired in a business combination was as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Amortization in Cost of revenues |
$ | 6,796 | $ | 3,806 | $ | 1,430 | ||||||
| Amortization in General and administrative |
22,025 | 18,000 | 13,083 | |||||||||
| Total |
$ | 28,821 | $ | 21,806 | $ | 14,513 | ||||||
Note 7. Supplemental Balance Sheets Information
Property, plant and equipment consisted of the following:
| March 31, 2023 |
March 31, 2022 |
|||||||
| Land |
$ | 889 | $ | 889 | ||||
| Buildings and building improvements |
22,005 | 21,537 | ||||||
| Manufacturing equipment |
14,481 | 17,336 | ||||||
| Computer equipment |
4,413 | 4,519 | ||||||
| Other |
4,394 | 1,578 | ||||||
| Construction in progress |
1,735 | 487 | ||||||
| Gross total |
47,917 | 46,346 | ||||||
| Accumulated depreciation |
(19,768 | ) | (17,726 | ) | ||||
| Property, plant and equipment, net |
$ | 28,149 | $ | 28,620 | ||||
Depreciation expense was as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Depreciation expense in Cost of revenues |
$ | 3,163 | $ | 2,243 | $ | 1,859 | ||||||
| Depreciation expense in Operating expense |
1,150 | 1,019 | 1,100 | |||||||||
| Total depreciation expense |
$ | 4,313 | $ | 3,262 | $ | 2,959 | ||||||
Inventories consisted of the following:
| March 31, 2023 |
March 31, 2022 |
|||||||
| Raw materials |
$ | 20,064 | $ | 14,172 | ||||
| Work in process |
617 | 4,419 | ||||||
| Finished goods |
13,961 | 6,015 | ||||||
| Inventories, net |
$ | 34,642 | $ | 24,606 | ||||
Accrued payroll and benefits consisted of the following:
| March 31, 2023 |
March 31, 2022 |
|||||||
| Bonus payable |
$ | 4,461 | $ | 7,468 | ||||
| Wages and paid-time-off payable |
2,329 | 3,677 | ||||||
| Payroll related taxes |
1,982 | 2,069 | ||||||
| Other benefits payable |
661 | 1,503 | ||||||
| Total accrued payroll and benefits |
$ | 9,433 | $ | 14,717 | ||||
Other accrued expenses consisted of the following:
| March 31, 2023 |
March 31, 2022 |
|||||||
| Accrued business taxes |
$ | 5,941 | $ | 4,967 | ||||
| Current operating lease liabilities |
2,868 | 2,768 | ||||||
| Customer deposits |
1,287 | 751 | ||||||
| Income taxes payable |
992 | 928 | ||||||
| Other |
2,297 | 2,197 | ||||||
| Total other accrued expenses |
$ | 13,385 | $ | 11,611 | ||||
Note 8. Indebtedness
Credit Facility
On March 5, 2021, we entered into a four-year senior secured credit agreement that includes 1) a revolving credit facility in an aggregate principal amount of up to $75,000, 2) a swingline loan in an aggregate principal amount not exceeding $5,000, and 3) letters of credit in an aggregate stated amount not exceeding $2,500 at any time. The agreement also provides for an incremental term loan or an increase in revolving commitments in an aggregate principal amount of at a minimum $25,000 and at a maximum $75,000, subject to the satisfaction of certain conditions and lender considerations. We refer to the facility and related agreement as the “Credit Facility”.
We borrowed $70,000 under the Credit Facility during fiscal year 2022 to provide a portion of the cash needed to complete the Agena Acquisition. We repaid $36,000 against our outstanding balance during the year ended March 31, 2023. As of March 31, 2023, the outstanding balance under our Credit Facility was $13,000. In April 2023 we repaid $3,000 on our line of credit.
On December 22, 2022, Mesa and the lenders amended the Credit Facility to replace references to the Eurodollar Rate with references to the Secured Overnight Financing Rate ("SOFR").
Amounts borrowed under the Credit Facility bear interest at either a base rate or a SOFR rate, plus an applicable spread. The interest rate on borrowings under our line of credit as of March 31, 2023 was 6.7%. We are obligated to pay quarterly unused commitment fees of between 0.15% and 0.35% of the Credit Facility’s aggregate principal amount, based on our leverage ratio. We incurred unused commitment fees of $107 and $78 for the years ended March 31, 2023, and March 31, 2022, respectively. The balance of unamortized customary lender fees was $312 and $484 as of March 31, 2023 and 2022, respectively.
The financial covenants in the Credit Facility include a maximum leverage ratio of 5.50 to 1.00 for the first four testing dates on which the line of credit is outstanding; 5.0 to 1.0 on each of the fifth, sixth, seventh, and eighth testing dates; and 4.5 to 1.0 on each testing date following the eighth testing date, except that we may have a leverage ratio of 5.75 to 1.0 for a period of four consecutive quarters following a permitted acquisition. The Credit Facility also stipulates a minimum fixed charge coverage ratio of 1.25 to 1.0. Other covenants include restrictions on our ability to incur debt, grant liens, make fundamental changes, engage in certain transactions with affiliates, or conduct asset sales. As of March 31, 2023, we were in compliance with all required covenants.
Convertible Notes
On August 12, 2019, we issued an aggregate principal amount of $172,500 of 2025 Notes. The 2025 Notes mature on August 15, 2025, unless earlier repurchased or converted, and bear interest at a rate of 1.375% payable semi-annually in arrears on February 15 and August 15 each year beginning on February 15, 2020. The 2025 Notes are initially convertible at a conversion rate of 3.5273 shares of common stock per $1,000 principal amount of Notes, which is equivalent to an initial conversion price of approximately $283.50 per share of common stock. Noteholders may convert their 2025 Notes at their option only in the following circumstances:
| (i) | during any calendar quarter commencing after the calendar quarter ended on December 31, 2019 (and only during such calendar quarter), if the last reported sale price per share of our common stock exceeds 130% of the conversion price for each of at least 20 trading days during the 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter; |
| (ii) | during the five consecutive business days immediately after any 10 consecutive trading day period (such 10 consecutive trading day period, the “measurement period”) in which the trading price per $1,000 principal amount of Notes for each trading day of the measurement period was less than 98% of the product of the last reported sale price per share of our common stock on such trading day and the conversion rate on such trading day; |
| (iii) | upon the occurrence of certain corporate events or distributions on our common stock, including certain distributions, the occurrence of a fundamental change (as defined in the indenture governing the 2025 Notes) or a transaction resulting in the Company’s common stock converting into other securities or property or assets; and |
| (iv) | at any time from, and including, April 15, 2025 until the close of business on the second scheduled trading day immediately before the maturity date. |
Upon conversion, we will pay or deliver, as the case may be, cash, shares of our common stock, or a combination of cash and shares of our common stock, at our election. We will reevaluate this policy from time to time as we receive conversion notices from note holders. The circumstances necessary for conversion were not met during the year ended March 31, 2023. As of March 31, 2023, the 2025 Notes are classified as a long-term liability on our Consolidated Balance Sheets as the circumstances necessary for conversion were not satisfied as of the end of the period. The if-converted value of the 2025 Notes did not exceed the principal balance as of March 31, 2023.
Debt issuance costs related to the 2025 Notes are comprised of discounts and commissions payable to the initial purchasers of $5,175 and third party offering costs of $255. The debt issuance costs are being amortized to interest expense using the effective interest method over the six-year contractual term of the 2025 Notes.
The net carrying amount of the 2025 Notes was as follows:
| March 31, 2023 |
March 31, 2022 |
|||||||
| Principal outstanding |
$ | 172,500 | $ | 172,500 | ||||
| Unamortized debt issuance costs |
(2,228 | ) | (3,135 | ) | ||||
| Net carrying value |
$ | 170,272 | $ | 169,365 | ||||
We recognized interest expense on the 2025 Notes as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Coupon interest expense at 1.375% |
$ | 2,372 | $ | 2,372 | $ | 2,372 | ||||||
| Amortization of debt discounts and issuance costs |
907 | 890 | 5,397 | |||||||||
| Total |
$ | 3,279 | $ | 3,262 | $ | 7,769 | ||||||
The effective interest rate of the liability component of the 2025 Notes is approximately 1.9%. Interest expense and amortization of debt discount was lower for the year ended March 31, 2022 compared to the year ended March 31, 2021 due to our adoption of ASU 2020-06.
Note 9. Stock Transactions and Stock-Based Compensation
(dollars and shares in thousands, except per share values)
In November 2005, our Board of Directors approved a program to repurchase up to 300 shares of our outstanding common stock. Under the program, shares of common stock may be purchased from time to time in the open market at prevailing prices or in negotiated transactions off the market. Shares of common stock repurchased will be cancelled and repurchases of shares of common stock will be funded through existing cash reserves. There were no repurchases of our shares of common stock under this plan during the years ended March 31, 2023, 2022 and 2021. As of March 31, 2023, we have repurchased 162 shares under this plan.
Under applicable law, Colorado corporations are not permitted to retain treasury stock. The price paid for repurchased shares is allocated between common stock and retained earnings based on management’s estimate of the original sales price of the underlying shares.
Public Offerings of Common Stock
On June 12, 2020, we completed the sale and issuance of a total of 600 shares of our common stock, and on June 19, 2020, our underwriters exercised in full their option to purchase an additional 90 shares of our common stock. The offering price to the public was $225.00 per share. The total proceeds we received from the offering, net of underwriting discounts and commissions and other offering expenses we paid, was $145,935.
Stock-Based Compensation
We issue shares in the form of stock options, RSUs and PSUs to employees and non-employee directors pursuant to the 2014 and 2021 Equity Plans. Our shareholders approved the 2021 Equity Plan during fiscal year 2022. The plan authorizes the issuance of 330 shares of common stock to eligible participants. 145 shares were available for future grants as of March 31, 2023. Under the 2014 Equity Plan, 1,100 shares of common stock have been authorized and reserved for eligible participants, all of which have been issued and 95 of which remain outstanding as of March 31, 2023.
Stock-based compensation expense recognized in the Consolidated Financial Statements was as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Stock-based compensation expense |
$ | 12,538 | $ | 11,391 | $ | 9,268 | ||||||
| Amount of income tax (benefit) recognized in earnings |
(1,169 | ) | (4,055 | ) | (1,816 | ) | ||||||
| Stock-based compensation expense, net of tax |
$ | 11,369 | $ | 7,336 | $ | 7,452 | ||||||
Stock Options
We use the Black-Scholes option-pricing model to estimate the fair value of stock option awards granted. The weighted average assumptions utilized in the model were as follows:
| 2023 |
2022 |
2021 |
||||||||||
| Risk-free interest rate |
3.55 | % |
0.46 | % |
0.27 | % |
||||||
| Expected life (years) |
3.52 | 3.52 | 3.86 | |||||||||
| Expected dividend yield |
0.07 | % |
0.06 | % |
0.10 | % |
||||||
| Volatility |
37.29 | % |
38.82 | % |
38.83 | % |
||||||
| Weighted-average Black-Scholes fair value per share at date of grant |
$ | 58.94 | $ | 76.02 | $ | 67.66 | ||||||
The amounts shown above for the estimated fair value per option granted are before the estimated effect of forfeitures, which reduces the amount of expense recorded in our Consolidated Statements of Income.
Stock option activity under the 2021 Equity Plan and legacy plans as of March 31, 2023, and changes for the year then ended are presented below (shares and dollars in thousands, except per-share data):
| Stock Options |
||||||||||||||||
| Shares Subject to Options |
Weighted- Average Exercise Price per Share |
Weighted-Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding as of March 31, 2022 |
202 | $ | 167.14 | 2.9 | $ | 18,261 | ||||||||||
| Awards granted |
43 | 185.60 | ||||||||||||||
| Awards forfeited or expired |
(9 | ) | 218.13 | |||||||||||||
| Awards exercised or distributed |
(73 | ) | 97.34 | |||||||||||||
| Outstanding as of March 31, 2023 |
163 | $ | 200.62 | 3.3 | $ | 1,643 | ||||||||||
| Exercisable as of March, 31, 2023 |
83 | $ | 193.05 | 2.3 | $ | 1,405 | ||||||||||
| Exercisable and expected to vest, March 31, 2023 |
160 | $ | 200.62 | 3.2 | $ | 1,641 | ||||||||||
The total intrinsic value of stock options exercised during the years ended March 31, 2023, 2022 and 2021 was $6,902, $15,209, and $9,559, respectively. Unrecognized stock-based compensation expense for stock options expected to vest as of March 31, 2023 was $2,835 and is expected to be recognized over a weighted average period of 1.7 years. The total fair value of options vested was $2,763, $2,856, and $2,005 during the years ended March 31, 2023, 2022 and 2021, respectively. The weighted-average grant price of awards granted during the years ended March 31, 2022 and 2021 was $268.81 and $226.72, respectively.
Time-Based Restricted Stock Units (RSUs)
RSU activity under the 2014 and 2021 Equity Plans was as follows (shares and dollars in thousands, except per-share data):
| Time-Based Restricted Stock Units |
||||||||||||||||
| Number of Shares |
Weighted- Average Grant Date Fair Value per Share |
Weighted- Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Nonvested at March 31, 2022 |
51 | $ | 252.86 | 1.0 | $ | 13,019 | ||||||||||
| Awards granted |
43 | 187.21 | ||||||||||||||
| Awards forfeited or expired |
(10 | ) | 229.52 | |||||||||||||
| Awards distributed |
(27 | ) | 250.85 | |||||||||||||
| Nonvested as of March 31, 2023 |
57 | $ | 209.27 | 1.0 | $ | 9,993 | ||||||||||
| Expected to vest |
53 | $ | 209.43 | 1.8 | $ | 9,254 | ||||||||||
For the years ended March 31, 2022 and 2021, the weighted average fair value per RSU granted was $274.55 and $231.61, respectively. Unrecognized stock-based compensation expense for RSUs that we have determined are probable of vesting was $6,893 as of March 31, 2023 and is expected to be recognized over a weighted average period of 1.8 years. The total fair value of RSUs vested was $6,751, $5,320, $1,819 during the years ended March 31, 2023, 2022 and 2021, respectively. The total intrinsic value of time-based RSUs distributed during the years ended March 31, 2023, 2022 and 2021 was $5,004, $5,320, and $2,429, respectively.
Performance-Based Restricted Stock Units (PSUs)
Performance-based RSUs vest upon completion of the service period described in the award agreement and based on achievement of the financial targets described in the award agreements. We recognize the expense relating to the performance-based RSUs based on the probable outcome of achievement of the financial targets on a straight-line basis over the service period.
PSU activity under the 2014 and 2021 Equity Plans was as follows (shares and dollars in thousands, except per-share data):
| Performance-Based Restricted Stock Units |
||||||||||||||||
| Number of Shares |
Weighted- Average Grant Date Fair Value per Share |
Weighted- average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Nonvested at March 31, 2022 at target |
55 | $ | 288.45 | 4.3 | $ | 14,093 | ||||||||||
| Awards granted |
19 | 182.14 | ||||||||||||||
| Performance adjustment |
(20 | ) | ||||||||||||||
| Awards distributed |
(10 | ) | 202.00 | |||||||||||||
| Nonvested as of March 31, 2023 at target |
44 | $ | 286.02 | 3.5 | $ | 7,958 | ||||||||||
| Expected to vest |
42 | $ | 287.48 | 3.4 | 7,306 | |||||||||||
For the year ended March 31, 2022, the average fair value per PSU granted was $302.15. Unrecognized stock-based compensation expense for PSUs that we have determined probable of vesting was $7,642 as of March 31, 2023 and is expected to be recognized over a weighted average period of 3.4 years. Total fair value of PSUs vested was $1,926 and $5,671 during the years ended March 31, 2023 and 2022, respectively. The total intrinsic value of PSUs distributed during the years ended March 31, 2023, 2022 and 2021 was $1,776, $7,549, and $0, respectively. There were no PSUs granted or distributed during the year ended March 31, 2021.
During the year ended March 31, 2023, the Compensation Committee of the Board of Directors created a plan to award 19 PSUs at target (the "FY23 PSUs") that are subject to both service and performance conditions to eligible employees. The performance period for the FY23 PSUs is from April 1, 2022 until March 31, 2023 and the service period is from April 1, 2022 until March 31, 2025. Of the total FY23 PSUs granted, 13 vest based on our achievement of specific performance criteria during fiscal year 2023 and they have a grant date fair value of $185.57. Based on actual performance during the performance period, we reduced the number of awards expected to vest to 0. The remaining awards will be settled in shares of our common stock, but they are subject to performance criteria that are subjective and as such their grant date was assigned as of March 31, 2023 when the criteria were defined and the number of awards was decided. Five shares are expected to be issued upon vesting based on determinations made by the Board of Directors.
During fiscal year 2022, we awarded 7 PSUs to key employees of Agena subject to both service and performance conditions. Based on actual performance through the period ended March 31, 2023, the awards did not vest.
On October 28, 2021, the Compensation Committee of the Board of Directors granted a special long-term equity award consisting of performance stock units covering a target of 40 shares (“PSUs”) that is subject to both performance and service conditions to our Chief Executive Officer. The performance period of the award is the three-year period from April 1, 2021 through March 31, 2024 and the service periods commence on October 28, 2021 and ends on October 27, 2024, October 27, 2025, and October 27, 2026, on which dates eligible PSUs will vest and be distributed. The performance metrics are cumulative GAAP revenues over the performance period and cumulative adjusted operating income over the performance period. The quantity of shares that will be issued upon vesting will range from 0 to 40; if financial performance targets are not met, then no shares will vest. Based on actual performance through the period ended March 31, 2023, the award is estimated to vest at 93%.
During the year ended March 31, 2023, we adjusted our estimate of PSUs expected to vest under all outstanding plans based on actual results achieved through the performance period. We recorded a cumulative effect release of ($1,787) during the period ($1,322, net of tax as well as $0.25 per basic and diluted share), which is recorded in general and administrative and selling expense on our Condensed Consolidated Statements of Income.
In the future, we expect non-cash stock-based compensation expense to decrease approximately $402 per quarter as a result of our new estimate of performance share units expected to vest.
Note 10. Earnings Per Share
(dollars and shares in thousands, except per share values)
The following table presents a reconciliation of the denominators used in the computation of basic and diluted earnings per share:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Net earnings available for shareholders |
$ | 930 | $ | 1,871 | $ | 3,274 | ||||||
| Weighted average outstanding shares of common stock |
5,321 | 5,212 | 4,975 | |||||||||
| Dilutive effect of stock options |
26 | 100 | 125 | |||||||||
| Dilutive effect of unvested stock awards |
14 | 23 | 24 | |||||||||
| Fully diluted shares |
5,361 | 5,335 | 5,124 | |||||||||
| Basic earnings per share |
$ | 0.17 | $ | 0.36 | $ | 0.66 | ||||||
| Diluted earnings per share |
$ | 0.17 | $ | 0.35 | $ | 0.64 | ||||||
The impact of the assumed conversion of the 2025 Notes calculated under the if-converted method was anti-dilutive, and as such shares underlying the 2025 Notes were excluded from the diluted EPS calculation for the fiscal years ended March 31, 2023, 2022, and 2021.
The following stock awards were excluded from the calculation of diluted EPS:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Assumed conversion of convertible debt |
608 | 608 | 608 | |||||||||
| Stock awards that were anti-dilutive |
154 | 40 | 44 | |||||||||
| Stock awards subject to performance conditions |
48 | 26 | 14 | |||||||||
| Total stock awards excluded from diluted EPS |
810 | 674 | 666 | |||||||||
Note 11. Employee Benefit Plans
We adopted the Mesa Laboratories, Inc. 401(K) Retirement Plan effective January 1, 2000. Under this plan, we match 100% of the first 4% of pay contributed by each eligible employee, and contributions vest immediately. Participation is voluntary, and employees are eligible on the first day of the month following their start date.
During the years ended March 31, 2023, 2022 and 2021, respectively, we contributed $1,768, $1,185, and $935 to Mesa Laboratories, Inc. 401(K) retirement plans on behalf of employees. Our employer match has increased over the years as employees from acquired companies have joined our 401(K) Retirement Plan.
Note 12. Income Taxes
Provision for Income Taxes
Earnings before income taxes were as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Domestic |
$ | 1,887 | $ | 4,579 | $ | 6,297 | ||||||
| Foreign |
(2,276 | ) | (1,005 | ) | (3,994 | ) | ||||||
| Total (loss) earnings before income taxes |
$ | (389 | ) | $ | 3,574 | $ | 2,303 | |||||
The components of our provision for income taxes were as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Current tax provision: |
||||||||||||
| U.S. Federal |
$ | 593 | $ | (83 | ) | $ | 1,500 | |||||
| U.S. State |
538 | 286 | 628 | |||||||||
| Foreign |
1,070 | 1,372 | 404 | |||||||||
| Total current tax expense |
2,201 | 1,575 | 2,532 | |||||||||
| Deferred tax provision: |
||||||||||||
| U.S. Federal |
(1,432 | ) | 1,707 | (2,410 | ) | |||||||
| U.S. State |
(210 | ) | 337 | (619 | ) | |||||||
| Foreign |
(1,878 | ) | (1,916 | ) | (474 | ) | ||||||
| Total deferred tax (benefit) expense |
(3,520 | ) | 128 | (3,503 | ) | |||||||
| Total income tax (benefit) expense |
$ | (1,319 | ) | $ | 1,703 | $ | (971 | ) | ||||
A reconciliation of our income tax provision and the amounts computed by applying statutory rates to earnings before income taxes was as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Federal income taxes at statutory rates |
$ | (82 | ) | $ | 751 | $ | 483 | |||||
| State income taxes, net of federal benefit |
(1,075 | ) | 628 | (221 | ) | |||||||
| Tax benefit of stock option exercises |
(1,169 | ) | (4,055 | ) | (1,816 | ) | ||||||
| Research and development credit |
(1,010 | ) | (495 | ) | (165 | ) | ||||||
| Limitation for 162(m) |
2,675 | 4,039 | 1,113 | |||||||||
| Return to provision adjustment |
(125 | ) | (68 | ) | (172 | ) | ||||||
| Subpart F, GILTI, & FDII |
(127 | ) | 6 | (999 | ) | |||||||
| Foreign rate differential |
(439 | ) | 152 | 810 | ||||||||
| Permanent Difference |
33 | 64 | 15 | |||||||||
| Interest reserve adjustment |
- | 668 | - | |||||||||
| Other |
- | 13 | (19 | ) | ||||||||
| Total income tax (benefit) expense |
$ | (1,319 | ) | $ | 1,703 | $ | (971 | ) | ||||
The Company has elected to recognize U.S. taxes on global intangible low-taxed income ("GILTI") as a period expense in the year the tax is incurred.
Deferred Tax Assets and Liabilities
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets (liabilities) were as follows:
| March 31, 2023 |
March 31, 2022 |
|||||||
| Deferred tax assets: |
||||||||
| Net operating loss |
$ | 6,945 | $ | 11,274 | ||||
| Credits |
4,769 | 5,321 | ||||||
| Allowances and reserves |
2,376 | 1,977 | ||||||
| Capitalized research expenditures(1) |
3,124 | - | ||||||
| Stock compensation deductible differences |
1,384 | 2,137 | ||||||
| Inventories |
1,348 | 1,316 | ||||||
| Other |
188 | 394 | ||||||
| Total deferred tax assets |
20,134 | 22,419 | ||||||
| Deferred tax liabilities: |
||||||||
| Goodwill and intangible assets |
(49,781 | ) | (56,145 | ) | ||||
| Property, plant and equipment |
(2,502 | ) | (3,284 | ) | ||||
| Other |
(221 | ) | (188 | ) | ||||
| Total deferred tax liabilities |
(52,504 | ) | (59,617 | ) | ||||
| Valuation allowance |
(582 | ) | (708 | ) | ||||
| Net deferred tax (liability) |
$ | (32,952 | ) | $ | (37,906 | ) | ||
| (1) Under the Tax Cut and Jobs Act of 2017, research and development costs are no longer fully deductible and are required to be capitalized and amortized for U.S tax purposes effective January 1, 2022. The mandatory capitalization requirement increases our deferred tax assets and cash tax liabilities. |
Valuation Allowance
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. In evaluating the need for a valuation allowance, management takes into account various factors, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies, and results of recent operations. Based on this evaluation, the Company has concluded that its U.S. operations and the majority of foreign operations have a sufficient source of income to realize our existing deferred tax assets as of March 31, 2023. The Company’s valuation allowance movement during fiscal year 2023 is mainly related to a change of judgement regarding the realizability of deferred tax assets in Canada and Germany.
The following table summarizes the changes in our valuation allowance for deferred tax assets:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Beginning balance |
$ | 708 | $ | 404 | $ | 391 | ||||||
| Additions charged to income tax expense and other accounts |
567 | 304 | 13 | |||||||||
| Deductions from reserves |
(693 | ) | - | - | ||||||||
| Ending balance |
$ | 582 | $ | 708 | $ | 404 | ||||||
Net Operating Loss Credit and Carryforwards
As of March 31, 2023, the Company had U.S. and Foreign net operating loss (“NOL”) carryforwards consisting of the following:
| March 31, 2023 |
Expiration Date |
|||||||
| Pre-2018 federal NOL carryforwards |
$ | - | N/A | |||||
| Post-2018 federal NOL carryforwards |
2,819 | Indefinite |
||||||
| State NOL carryforwards |
7,210 | March 31, 2037 |
||||||
| Foreign NOL carryforwards |
22,262 | Indefinite |
||||||
As of March 31, 2023, the Company had U.S. tax credit carryforwards consisting of the following:
| March 31, 2023 |
Expiration Date |
||||||
| Federal research tax credit carryforwards |
$ | 2,428 | March 31, 2038 |
||||
| State research tax credits carryforwards |
2,944 | March 31, 2034 |
|||||
| Federal foreign tax credit carryforwards |
15 | March 31, 2036 | |||||
As a result of the Agena acquisition in fiscal year 2022, an ownership change as defined in Section 382 of the Internal Revenue Code occurred resulting in limitations on the Company’s use of acquired federal and state net operating losses, as well as certain tax credits. As of March 31, 2023, $1,513 of the Company’s federal tax loss carryforwards, and $1,360 of the Company’s federal research and development credit carryforwards are subject to Section 382 and other restrictions.
Undistributed earnings in foreign subsidiaries
For the year ended March 31, 2023, provisions have not been made for income taxes on $65,028 of undistributed earnings that were deemed permanently reinvested in foreign subsidiaries at March 31, 2023. Determination of the amount of unrecognized deferred income tax liabilities on these earnings is not practicable because such liability, if any, depends on certain circumstances existing if and when remittance occurs. A deferred tax liability will be recognized if and when the Company no longer plans to permanently reinvest these undistributed earnings.
Uncertain Tax Positions
Uncertain tax positions, if ever recognized in the financial statements, would be recorded in the consolidated statements of operations as part of the income tax provision. A reconciliation of the beginning and ending amount of unrecognized tax benefits, exclusive of interest and penalties, included in the deferred tax liability on the accompanying Consolidated Balance Sheets of the Company is as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Beginning balance |
$ | 1,329 | $ | 64 | $ | 653 | ||||||
| (Decrease) increase related to prior period tax positions |
(1,272 | ) | 1,179 | (629 | ) | |||||||
| Increases related to current period tax positions |
35 | 86 | 40 | |||||||||
| Ending balance |
$ | 92 | $ | 1,329 | $ | 64 | ||||||
As of March 31, 2023, the Company recorded gross unrecognized tax benefits of $92, all of which, if recognized, would affect the Company’s effective tax rate. The Company recognizes interest and penalties accrued on uncertain income tax positions in other expense and general and administrative expense, respectively. Interest and penalties included in other long-term liabilities on the accompanying Consolidated Balance Sheets of the Company were $0 for each of the years ended March 31, 2023, 2022 and 2021. The Company does not expect a material change in unrecognized tax benefits or interest reversal in the next 12 months.
The Company files income tax returns in the U.S. various states and foreign jurisdictions. In the normal course of business, the Company is subject to examination by taxing authorities throughout the world. The following tax years remain subject to examination:
| Significant Jurisdictions | Open Years | |||
| U.S. Federal | 2019 - 2021 | |||
| U.S. States | 2018 - 2021 | |||
| Foreign | 2016 - 2021 | |||
In various jurisdictions, years prior to those listed above remain open solely for the purposes of examination of the Company’s NOL and credit carryforwards.
Note 13. Commitments and Contingencies
We are party to various legal proceedings arising in the ordinary course of business. As of March 31, 2023, we are not party to any legal proceeding that management believes could have a material adverse effect on our consolidated financial position, results of operations, or cash flows.
As part of the Belyntic acquisition, we have agreed to pay up to an additional $1,500 to the sellers upon approval of contractually specified pending patents. We believe it is probable the patents will be issued and that we will pay the sellers in full within 36 months from the date of acquisition. The liability is recorded at an estimated fair value of $1,190 in other long-term liabilities on the accompanying Consolidated Balance Sheets.
Note 14. Segment Data
Segment information is prepared on the same basis that our CEO and chief operating decision maker uses to manage our segments, evaluate financial results, and make key operating decisions. Our four reportable segments are organized primarily by the nature of the goods and services they sell. When determining our reportable segments, we aggregated operating segments based on their similar economic and operating characteristics. We evaluate the performance of our operating segments based on revenues, organic revenues growth, and gross profit. The accounting policies of the operating segments are the same as those described in Note 1. "Description of Business and Summary of Significant Accounting Policies."
The following tables set forth our segment information:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| Revenues (a): |
||||||||||||
| Clinical Genomics |
$ | 62,299 | $ | 32,840 | $ | - | ||||||
| Sterilization and Disinfection Control |
64,609 | 59,044 | 53,119 | |||||||||
| Biopharmaceutical Development |
47,365 | 45,579 | 33,892 | |||||||||
| Calibration Solutions |
44,807 | 46,872 | 46,926 | |||||||||
| Reportable segment revenues |
219,080 | 184,335 | 133,937 | |||||||||
| Corporate and Other (b) |
- | - | - | |||||||||
| Total revenues |
$ | 219,080 | $ | 184,335 | $ | 133,937 | ||||||
| Gross profit: |
||||||||||||
| Clinical Genomics |
$ | 32,485 | $ | 11,941 | $ | - | ||||||
| Sterilization and Disinfection Control |
46,520 | 43,720 | 39,870 | |||||||||
| Biopharmaceutical Development |
30,340 | 28,605 | 21,035 | |||||||||
| Calibration Solutions |
24,388 | 24,989 | 26,112 | |||||||||
| Reportable segment gross profit |
133,733 | 109,255 | 87,017 | |||||||||
| Corporate and Other (b) |
(40 | ) | (165 | ) | (3 | ) | ||||||
| Gross profit |
$ | 133,693 | $ | 109,090 | $ | 87,014 | ||||||
| Reconciling items: |
||||||||||||
| Operating expenses |
130,373 | 104,388 | 74,656 | |||||||||
| Operating income |
3,320 | 4,702 | 12,358 | |||||||||
| Nonoperating expense |
3,709 | 1,128 | 10,055 | |||||||||
| Earnings before income taxes |
$ | (389 | ) | $ | 3,574 | $ | 2,303 | |||||
| (a) |
Intersegment revenues are not significant and are eliminated to arrive at consolidated totals. |
| (b) |
Unallocated corporate expenses and other business activities are reported within Corporate and Other. |
The following table sets forth net inventories by reportable segment. Our chief operating decision maker is not provided with any other segment asset information.
| March 31, |
March 31, |
|||||||
| 2023 |
2022 |
|||||||
| Clinical Genomics |
$ | 13,985 | $ | 11,802 | ||||
| Sterilization and Disinfection Control |
3,492 | 2,176 | ||||||
| Biopharmaceutical Development |
8,384 | 4,495 | ||||||
| Calibration Solutions |
8,781 | 6,133 | ||||||
| Reportable segment inventory |
34,642 | 24,606 | ||||||
| Corporate and Other |
- | - | ||||||
| Total inventories, net |
$ | 34,642 | $ | 24,606 | ||||
The following table sets forth a summary of long-lived assets by geographic area. Long-lived assets exclude goodwill and intangible assets acquired in a business combination and deferred tax assets.
| As of March 31, |
||||||||
| 2023 |
2022 |
|||||||
| United States |
$ | 34,729 | $ | 36,475 | ||||
| Foreign |
3,793 | 3,975 | ||||||
| Total |
$ | 38,522 | $ | 40,450 | ||||
Revenues from external customers are attributed to individual countries based upon locations to which the product is shipped or exported, as follows:
| Year Ended March 31, |
||||||||||||
| 2023 |
2022 |
2021 |
||||||||||
| United States |
$ | 117,281 | $ | 99,068 | $ | 71,387 | ||||||
| China |
25,797 | 16,518 | 6,612 | |||||||||
| Other |
76,002 | 68,749 | 55,938 | |||||||||
| Total revenues |
$ | 219,080 | $ | 184,335 | $ | 133,937 | ||||||
No customer accounts for 10% or more of our consolidated revenues. No foreign country other than China exceeds 10% of total revenues.
Note 15. Subsequent Events
None.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure Item 9A.
None.
Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) that are designed to reasonably ensure that information required to be disclosed by us in the reports we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Our management evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of March 31, 2023. Based on that evaluation, and as a result of the material weakness described below, our management concluded that our disclosure controls and procedures were not effective as of March 31, 2023.
Nevertheless, based on the performance of additional procedures by management designed to ensure reliability of financial reporting, our management has concluded that, notwithstanding the material weaknesses described below, the consolidated financial statements, included in this Annual Report on Form 10-K, fairly present, in all material respects, our financial position, results of operations, and cash flows as of the dates, and for each of the periods presented, in conformity with U.S. GAAP.
Management's Annual Report on Internal Control Over Financial Reporting
Our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles in the United States. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness for future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management evaluated the effectiveness of our internal control over financial reporting as of March 31, 2023, using the framework in “Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013. Based on that evaluation, our management concluded that our internal control over financial reporting was not effective as of March 31, 2023 due to the material weaknesses described below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
There were two material weaknesses identified as of March 31, 2023:
1) Management's review controls over fair value calculations including Management's preliminary valuation of the Belyntic Acquisition were insufficient. Specifically, Management failed to utilize resources with an appropriate level of knowledge and expertise in performing and reviewing the fair value calculations including the preliminary Belyntic valuation. In fiscal year 2023, Mesa's acquisitions of businesses (net of cash acquired and including contingent consideration) totaled $6.1 million. Our auditors, Plante & Moran, PLLC identified errors in the preliminary valuation of Belyntic as part of their audit procedures after the preliminary valuation had been reviewed internally by Management.
2) Management's review controls over the qualitative assessment of goodwill impairment were insufficient to identify potential impairment triggers.
Remediation Plan for Material Weaknesses in Internal Control Over Financial Reporting
In response to the material weaknesses identified in "Management's Reporting on Internal Controls Over Financial Reporting," we, with the oversight from the Audit Committee of the Board of Directors, developed a plan to remediate the material weaknesses. Our remediation plan will require that, going forward, including for the final valuation of the Belyntic acquisition, Management will utilize a valuation specialist with the requisite knowledge to perform such valuations for all acquisitions of businesses. Management has committed to formally evaluate and document impairment triggers on a quarterly basis and ensure that such documentation is reviewed by a person competent to perform such a review.
We believe the use of a specialist with appropriate knowledge and experience valuing business combinations and putting in place a more robust process for identifying and evaluating potential impairment triggering events will effectively remediate the material weaknesses described in "Management's Report on Internal Control Over Financial Reporting."
Our independent auditor, Plante & Moran, PLLC, a registered public accounting firm, is appointed by the Audit Committee of our Board of Directors. Plante & Moran, PLLC has issued an adverse opinion on the effectiveness of our internal controls over financial reporting as of March 31, 2023, which appears in Item 8. Financial Statements and Supplementary Data of this Annual Report on Form 10-K.
Changes in internal control over financial reporting
The Agena Acquisition was completed on October 21, 2021, and the financial results of Agena are included in our Consolidated Financial Statements as of March 31, 2023 and for the period then ended and as of March 31, 2022 and for the year then ended. During the time since acquisition, we have assessed the control environment of Agena; made certain changes to Agena's internal controls over financial reporting, including design changes that were required as we brought Agena onto our enterprise resource planning system; and performed testing over the operating effectiveness of Agena's internal controls. We now consider Agena to be included in the scope of our assessment of internal controls over financial reporting.
Other than the remediation measures discussed above and the incorporation of Agena into our internal controls over financial reporting, there were no other changes during the quarter ended March 31, 2023 in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections Item 10.
Not applicable.
PART III
Directors, Executive Officers and Corporate Governance
Incorporated by reference from the definitive Proxy Statement for our 2023 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2023.
Item 11. Executive Compensation
Incorporated by reference from the definitive Proxy Statement for our 2023 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2023.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Securities Authorized for Issuance Under Equity Compensation Plans
The following table presents information regarding options and rights outstanding under our equity compensation plans as of March 31, 2023. All options reflected are options to purchase common stock.
| (a) Number of Securities to be Issued upon Exercising of Outstanding Options and Rights (1) |
(b) Weighted-Average Exercise Price of Outstanding Options and Rights (1) |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) (2) |
||||||||||
| Equity Compensation Plan Approved by Security Holders |
261,883 | $ | 216.41 | 144,588 | ||||||||
| Equity Compensation Plans Not Approved by Security Holders |
None |
N/A | None |
|||||||||
| Total |
261,883 | $ | 216.41 | 144,588 | ||||||||
| 1. |
Includes shares issuable in connection with awards with performance conditions, which will be issued based on achievement of performance criteria associated with the awards, with the number of shares issuable dependent on our level of performance. We have accounted for the shares based on the current achievement as of March 31, 2023. The weighted average exercise price in column (b) includes the weighted average exercise price of options only. |
| 2. |
Includes 144,588 shares remaining available under the 2021 Equity Plan. Each share underlying a full value award such as restricted stock or performance shares count as one share used against the total number of securities authorized under the plan. |
Additional information for this item is incorporated by reference from the definitive Proxy Statement for our 2023 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2023.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Incorporated by reference from the definitive Proxy Statement for our 2023 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2023.
Item 14. Principal Accountant Fees and Services
Plante & Moran, PPLC, Denver, Colorado, PCAOB ID 166 is the Company's independent registered public accounting firm.
Incorporated by reference from the definitive Proxy Statement for our 2023 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2023.
PART IV
Item 15. Exhibits and Financial Statement Schedules
| a) |
Consolidated Financial Statements |
The following documents included in Part II, Item 8. Financial Statements and Supplementary Data are filed as part of this Annual Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets - March 31, 2023 and 2022
Consolidated Statements of Income - Years ended March 31, 2023, 2022 and 2021
Consolidated Statements of Comprehensive Income - Years ended March 31, 2023, 2022 and 2021
Consolidated Statements of Stockholders' Equity - Years ended March 31, 2023, 2022 and 2021
Consolidated Statements of Cash Flows - Years ended March 31, 2023, 2022 and 2021
Notes to Consolidated Financial Statements
All financial statement schedules have been omitted either because they are not applicable or required, or the information that would be required to be included is disclosed in the notes to the Consolidated Financial Statements.
| b) |
Exhibits |
| 31.1 |
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a). |
| 31.2 |
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a). |
| 32.1 |
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) and 18 U.S.C. Section 1350. |
| 32.2 |
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) and 18 U.S.C. Section 1350. |
| 101.INS+ | Inline XBRL Instance Document-the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH+ | Inline XBRL Taxonomy Extension Schema Document. |
| 101.CAL+ | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF+ | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB+ | Inline XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE+ | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104+ | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101.*). |
* Indicates a management contract or compensatory plan, contract or arrangement.
α Mesa Laboratories, Inc. has entered into an Executive Employment Agreement with each of Gary M. Owens, John V. Sakys, and Brian Archbold.
+ Filed electronically herewith.
Item 16. Form 10-K Summary Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
None.
| MESA LABORATORIES, INC. | |||
| Registrant | |||
|
|
|
|
|
|
|
|
|
|
| Date: May 30, 2023 |
By: |
/s/ Gary M. Owens |
|
|
|
|
Gary M. Owens |
|
|
|
|
Chief Executive Officer |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name |
Title |
Date |
|
| /s/John J. Sullivan, Ph.D. |
Chairman of the Board of Directors |
May 30, 2023 | |
| John J. Sullivan |
|||
| /s/Gary M. Owens |
Chief Executive Officer, President, and Director |
May 30, 2023 | |
| Gary M. Owens |
|
||
| /s/John V. Sakys |
Chief Financial Officer and |
May 30, 2023 | |
| John V. Sakys |
Chief Accounting Officer, and Treasurer |
||
| /s/Jennifer S. Alltoft |
Director |
May 30, 2023 | |
| Jennifer S. Alltoft |
|||
| /s/Shannon Hall | Director |
May 30, 2023 | |
| Shannon Hall | |||
| /s/Shiraz Ladiwala | Director |
May 30, 2023 | |
| Shiraz Ladiwala | |||
| /s/John B. Schmieder | Director |
May 30, 2023 | |
| John B. Schmieder | |||
| /s/Tony Tripeny | Director | May 30, 2023 | |
| Tony Tripeny |
Exhibit 4.3
Description of the Registrant’s Securities
Registered Pursuant to Section 12 of the
Securities and Exchange Act of 1934
As of March 31, 2023, Mesa Laboratories, Inc. (the “Company,” “we,” or “our”) had one class of securities, our common stock, registered under Section 12 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
The following summary of certain terms of our capital stock does not purport to be complete and is subject to, and qualified in its entirety by, our Restated Certificate of Incorporation (the “Certificate of Incorporation”), our Amended and Restated Bylaws (the “Bylaws”), the forms of which are included as exhibits to the Annual Report on Form 10-K of which this Exhibit 4(d) is also included, as well as the relevant portions of the Colorado Business Corporation Act.
Our authorized capital stock consists of 25,000,000 shares of common stock, no par value, and 1,000,000 shares of preferred stock, no par value.
Common Stock
As of May 19, 2023, there were 5,369,959 shares of common stock issued and outstanding.
Voting
The holders of our common stock are entitled to one vote per share on all matters to be voted upon by the stockholders.
Dividends
The holders of common stock are entitled to receive ratably dividends, if any, as may be declared from time to time by the board of directors out of funds legally available for that purpose.
Liquidation Rights
In the event of our liquidation, dissolution or winding up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities.
Other Matters
The common stock has no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. Issued shares of common stock for which appropriate consideration have been received are non-assessable and shareholders are not liable for the debts or other obligations of the company.
Preferred Stock
As of May 19, 2023, there were no shares of preferred stock issued and outstanding.
Our Articles of Incorporation authorize our board of directors, subject to any limitations prescribed by law, without further stockholder approval, to establish and to issue from time to time one or more classes or series of preferred stock, no par value per share, covering up to an aggregate of 1,000,000 shares of preferred stock. Each class or series of preferred stock will cover the number of shares and will have the powers, preferences, rights, qualifications, limitations and restrictions determined by the board of directors.
Certain Provisions of Our Articles of Incorporation and Bylaws and Colorado Law
Certain provisions of our Articles of Incorporation and our Bylaws could make our acquisition by a third party, a change in our incumbent directors, or a similar change of control more difficult. These provisions, which are summarized below, may discourage certain types of takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. These provisions of our Articles of Incorporation and Bylaws may also be significant because they define certain of the aspects of our corporate governance.
Election of Directors. Our Bylaws provide that the board of directors may increase the size of our board and designate the directors to fill the vacancies.
No Cumulative Voting. Our Articles of Incorporation provide that no shareholder is permitted to cumulate its votes in the election of directors.
Advance Notice Bylaws. Our Bylaws require a shareholder seeking to nominate a candidate for election as director or to propose other business at a meeting of shareholders to provide us notice of the proposed candidate or business within a specified period in advance of the meeting.
Exclusive Forum. Our Bylaws provide that unless the Company consents in writing to the selection of an alternative forum, the sole and exclusive forum for any of the following shall be a state court within the State of Colorado, or, if no state court located within the State of Colorado has jurisdiction, the federal district court for the District of Colorado: (i) any claim that is based upon a violation of a duty under the laws of Colorado by a current or former director, officer, or shareholder in such capacity, (ii) any derivative action or proceeding brought on behalf of the Company, (iii) any action asserting a claim arising pursuant to any provision of the Colorado Business Corporation Act, the Articles of Incorporation or the Bylaws, (iv) any action asserting a claim governed by the internal affairs doctrine that is not included in (i) through (iii).
Limitations on Liability. Our Articles of Incorporation provide that no person who is or was a director will be personally liable to us or to our shareholders for monetary damages for breach of fiduciary duty as a director, so long as such director acted in good faith, subject to certain exceptions under the Colorado Business Corporation Act.
We have also obtained policies of directors' and officers' liability insurance. These policies insure our directors and officers against the cost of defense, settlement or payment of a judgment under certain circumstances. The existence of such limitation on liability, indemnification and insurance may impede a change of control of us to the extent that a hostile acquirer seeks to litigate its contest for control with our directors and officers.
Transfer Agent
The transfer agent for our common stock is Computershare Trust Company, N.A.
Listing
Our common stock is quoted on the Nasdaq Global Market, or Nasdaq under the trading symbol "MLAB."
Exhibit 21.1
Subsidiaries of Mesa Laboratories, Inc.
March 31, 2023
The following is a listing of subsidiaries of Mesa Laboratories, Inc., a Colorado corporation
|
Name |
Country of Incorporation or Organization |
|
| Agena Bioscience China | China | |
| Agena Bioscience GmbH | Germany | |
| Agena Bioscience HK | China | |
| Agena Bioscience, Inc. | United States | |
|
Gyros DE GmbH |
Germany |
|
|
Gyros Finans AB |
Sweden |
|
|
Gyros France Sarl |
France |
|
| Gyros Japana KK | Japan | |
|
Gyros Patent AB |
Sweden |
|
|
Gyros Protein Technology AB |
Sweden |
|
|
Gyros Protein Technology Holding AB |
Sweden |
|
|
Gyros U.K. Ltd. |
United Kingdom |
|
|
Gyros U.S. Inc. |
United States |
|
|
IBP Medical GmbH |
Germany |
|
|
Mesa Canada, Inc. |
Canada |
|
|
Mesa France SAS |
France |
|
|
Mesa Germany GmbH |
Germany |
|
|
MLI Sweden Holdco AB |
Sweden |
|
|
Protein Technologies, Inc. |
United States |
|
|
Simicon GmbH |
Germany |
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in Mesa Laboratories, Inc.’s Registration Statements on Form S-3 (File No. 333-264137) and Form S-8 (File Nos. 333-259154, 333-206551) of our reports dated May 30, 2023 with respect to the consolidated balance sheets of Mesa Laboratories, Inc. as of March 31, 2023 and 2022, the related consolidated statements of income, comprehensive (loss) income, stockholders’ equity, and cash flows for each of the years in the three-year period ended March 31, 2023, and the related notes, and the effectiveness of internal control over financial reporting as of March 31, 2023, which appear in this Annual Report on Form 10-K.
/s/ Plante & Moran, PLLC
Denver, Colorado
May 30, 2023
Exhibit 31.1 Certifications Pursuant to Rule 13a-14(a)
I, Gary M. Owens, certify that:
|
1. |
I have reviewed this Annual Report on Form 10-K of Mesa Laboratories, Inc.; |
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
|
4. |
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
(a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
(b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
(c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
(d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent quarter (the registrant’s fourth quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
|
5. |
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
|
(a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
|
(b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Date: May 30, 2023 |
/s/Gary M. Owens Gary M. Owens Chief Executive Officer |
Exhibit 31.2 Certifications Pursuant to Rule 13a-14(a)
I, John V. Sakys, certify that:
|
1. |
I have reviewed this Annual Report on Form 10-K of Mesa Laboratories, Inc.; |
|
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
|
4. |
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
(a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
(b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
(c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
(d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent quarter (the registrant’s fourth quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
|
5. |
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
|
(a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
|
(b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Date: May 30, 2023 |
/s/John V. Sakys John V. Sakys Chief Financial Officer
|
Exhibit 32.1 Certification Pursuant to Rule 13a-14(b) and 18 U.S.C. Section 1350
In connection with the Annual Report of Mesa Laboratories, Inc. (the “Company”) on Form 10-K for the year ended March 31, 2023, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Gary M. Owens, Chief Executive Officer of the Company, certify, pursuant to Rule 13a-14(b) and 18 U.S.C. § 1350, that:
|
(1) |
The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
(2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| Date: May 30, 2023 |
/s/Gary M. Owens Gary M. Owens Chief Executive Officer |
Exhibit 32.2 Certification Pursuant to Rule 13a-14(b) and 18 U.S.C. Section 1350
In connection with the Annual Report of Mesa Laboratories, Inc. (the “Company”) on Form 10-K for the year ended March 31, 2023, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, John V. Sakys, Chief Financial Officer of the Company, certify, pursuant to Rule 13a-14(b) and 18 U.S.C. § 1350, that:
|
(1) |
The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
(2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| Date: May 30, 2023 |
/s/John V. Sakys John V. Sakys Chief Financial Officer |