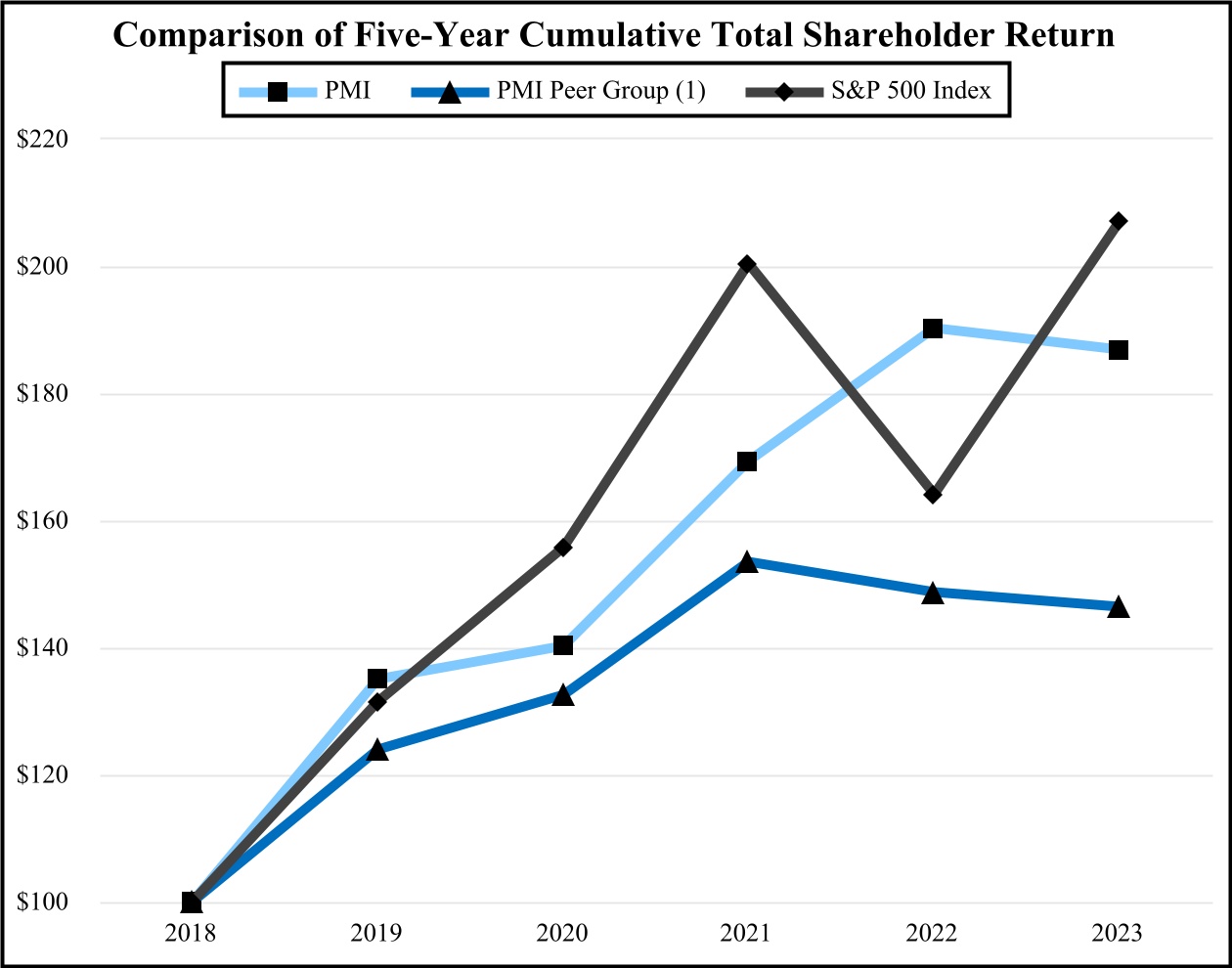
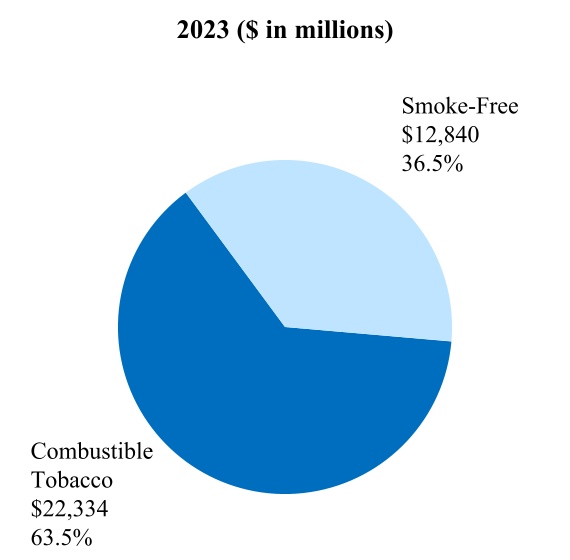
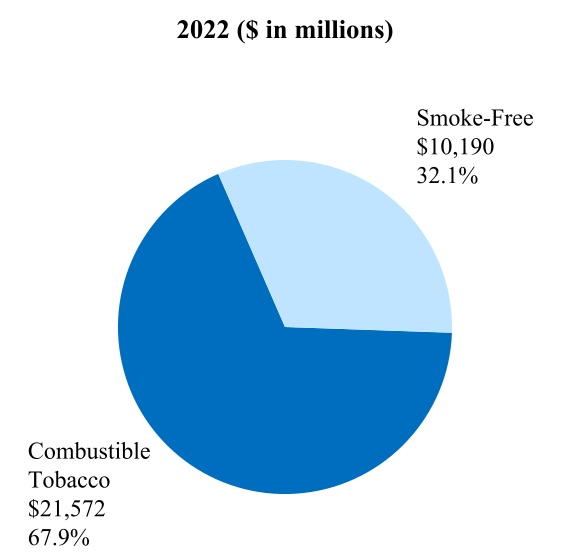
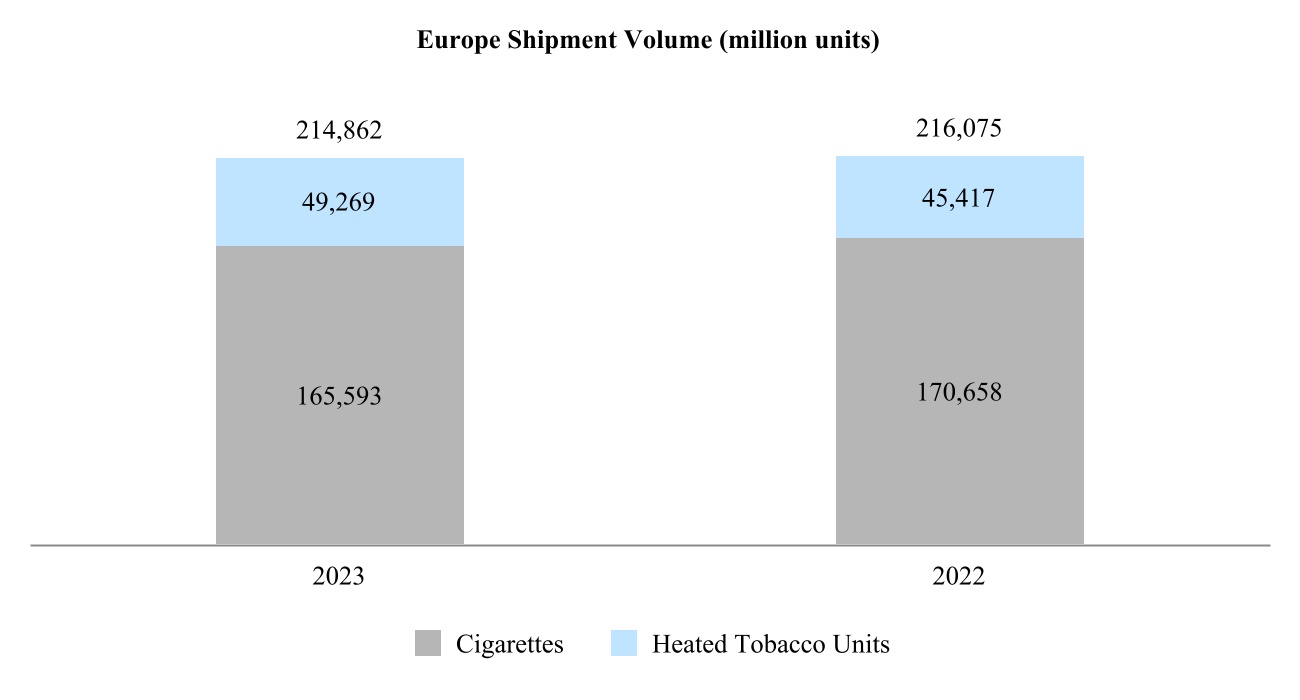
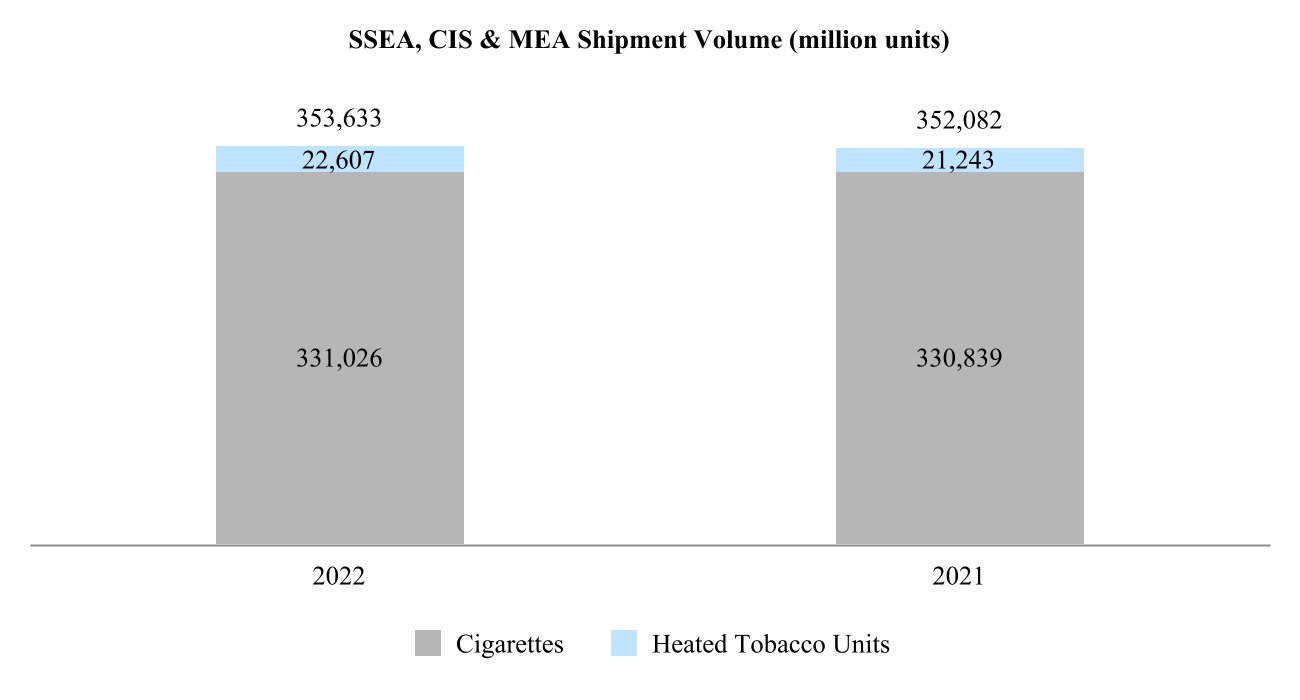
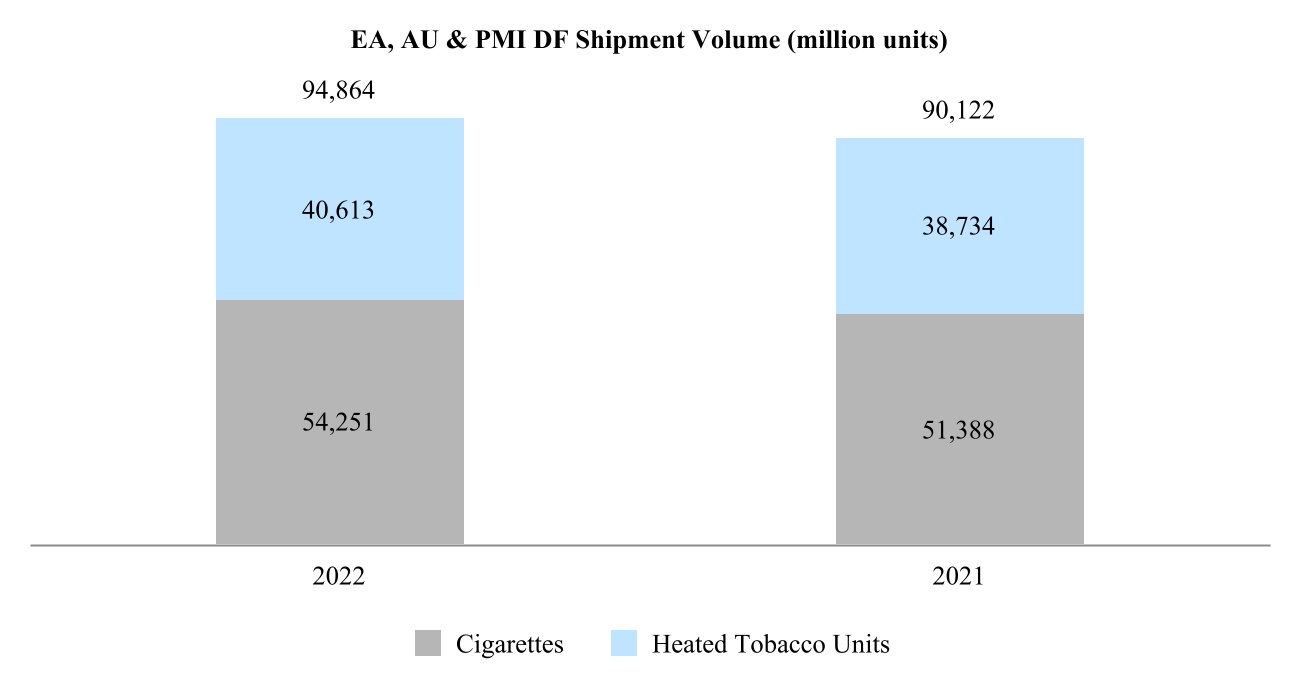
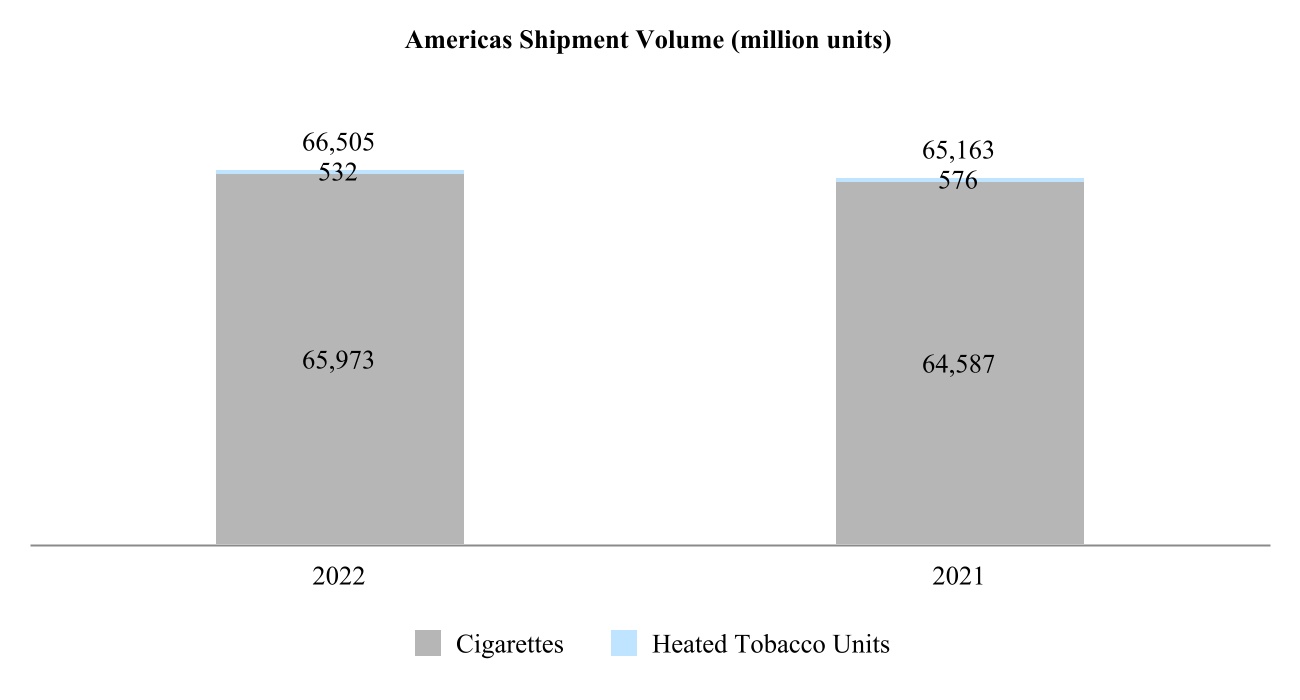
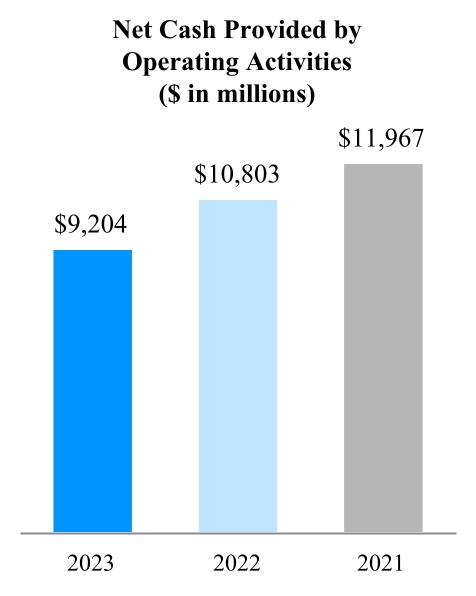
00014133292023FYfalsehttp://www.pmi.com/20231231#MarketingAdministrationAndResearchCostshttp://www.pmi.com/20231231#MarketingAdministrationAndResearchCostshttp://fasb.org/us-gaap/2023#InterestIncomeExpenseNethttp://fasb.org/us-gaap/2023#InterestIncomeExpenseNethttp://fasb.org/us-gaap/2023#InterestIncomeExpenseNethttp://fasb.org/us-gaap/2023#InterestIncomeExpenseNethttp://fasb.org/us-gaap/2023#InterestIncomeExpenseNethttp://fasb.org/us-gaap/2023#InterestIncomeExpenseNethttp://fasb.org/us-gaap/2023#MachineryAndEquipmentGrosshttp://fasb.org/us-gaap/2023#MachineryAndEquipmentGrosshttp://fasb.org/us-gaap/2023#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2023#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2023#LongTermDebtAndCapitalLeaseObligationsCurrenthttp://fasb.org/us-gaap/2023#LongTermDebtAndCapitalLeaseObligationsCurrenthttp://fasb.org/us-gaap/2023#OtherAccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2023#OtherAccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2023#LongTermDebtAndCapitalLeaseObligationshttp://fasb.org/us-gaap/2023#LongTermDebtAndCapitalLeaseObligationshttp://www.pmi.com/20231231#IncomeTaxesAndOtherLiabilitiesNoncurrenthttp://www.pmi.com/20231231#IncomeTaxesAndOtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#NetCashProvidedByUsedInOperatingActivitieshttp://fasb.org/us-gaap/2023#NetCashProvidedByUsedInOperatingActivities00014133292023-01-012023-12-310001413329us-gaap:CommonStockMember2023-01-012023-12-310001413329pm:A2.875Notesdue20241Member2023-01-012023-12-310001413329pm:A2.875Notesdue20242Member2023-01-012023-12-310001413329pm:A0.625Notesdue2024Member2023-01-012023-12-310001413329pm:A3.250Notesdue2024Member2023-01-012023-12-310001413329pm:A2.750Notesdue2025Member2023-01-012023-12-310001413329pm:A3.375Notesdue2025Member2023-01-012023-12-310001413329pm:A2.750Notesdue2026Member2023-01-012023-12-310001413329pm:A2.875Notesdue2026Member2023-01-012023-12-310001413329pm:A0.125Notesdue2026Member2023-01-012023-12-310001413329pm:A3.125Notesdue2027Member2023-01-012023-12-310001413329pm:A3.125Notesdue2028Member2023-01-012023-12-310001413329pm:A2.875Notesdue2029Member2023-01-012023-12-310001413329pm:A3.375Notesdue2029Member2023-01-012023-12-310001413329pm:A0.800Notesdue2031Member2023-01-012023-12-310001413329pm:A3.125Notesdue2033Member2023-01-012023-12-310001413329pm:A2.000Notesdue2036Member2023-01-012023-12-310001413329pm:A1.875Notesdue2037Member2023-01-012023-12-310001413329pm:A6.375Notesdue2038Member2023-01-012023-12-310001413329pm:A1.450Notesdue2039Member2023-01-012023-12-310001413329pm:A4.375Notesdue2041Member2023-01-012023-12-310001413329pm:A4.500Notesdue2042Member2023-01-012023-12-310001413329pm:A3.875Notesdue2042Member2023-01-012023-12-310001413329pm:A4.125Notesdue2043Member2023-01-012023-12-310001413329pm:A4.875Notesdue2043Member2023-01-012023-12-310001413329pm:A4.250Notesdue2044Member2023-01-012023-12-3100014133292023-06-30iso4217:USD00014133292024-01-31xbrli:shares00014133292022-01-012022-12-3100014133292021-01-012021-12-31iso4217:USDxbrli:shares0001413329us-gaap:RelatedPartyMember2023-01-012023-12-310001413329us-gaap:RelatedPartyMember2022-01-012022-12-310001413329us-gaap:RelatedPartyMember2021-01-012021-12-310001413329us-gaap:InProcessResearchAndDevelopmentMember2023-01-012023-12-310001413329pm:EAAUPMIDFSegmentMembercountry:KR2023-01-012023-12-3100014133292023-12-3100014133292022-12-310001413329us-gaap:RelatedPartyMember2023-12-310001413329us-gaap:RelatedPartyMember2022-12-310001413329pm:SwedishMatchABMember2023-01-012023-12-310001413329pm:SwedishMatchABMember2022-01-012022-12-310001413329pm:SwedishMatchABMember2021-01-012021-12-310001413329us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2023-01-012023-12-310001413329us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2022-01-012022-12-310001413329us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2021-01-012021-12-3100014133292021-12-3100014133292020-12-310001413329us-gaap:CommonStockMember2020-12-310001413329us-gaap:AdditionalPaidInCapitalMember2020-12-310001413329us-gaap:RetainedEarningsMember2020-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001413329us-gaap:TreasuryStockCommonMember2020-12-310001413329us-gaap:NoncontrollingInterestMember2020-12-310001413329us-gaap:RetainedEarningsMember2021-01-012021-12-310001413329us-gaap:NoncontrollingInterestMember2021-01-012021-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310001413329us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310001413329us-gaap:TreasuryStockCommonMember2021-01-012021-12-310001413329us-gaap:CommonStockMember2021-12-310001413329us-gaap:AdditionalPaidInCapitalMember2021-12-310001413329us-gaap:RetainedEarningsMember2021-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001413329us-gaap:TreasuryStockCommonMember2021-12-310001413329us-gaap:NoncontrollingInterestMember2021-12-310001413329us-gaap:RetainedEarningsMember2022-01-012022-12-310001413329us-gaap:NoncontrollingInterestMember2022-01-012022-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310001413329us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001413329us-gaap:TreasuryStockCommonMember2022-01-012022-12-310001413329us-gaap:CommonStockMember2022-12-310001413329us-gaap:AdditionalPaidInCapitalMember2022-12-310001413329us-gaap:RetainedEarningsMember2022-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001413329us-gaap:TreasuryStockCommonMember2022-12-310001413329us-gaap:NoncontrollingInterestMember2022-12-310001413329us-gaap:RetainedEarningsMember2023-01-012023-12-310001413329us-gaap:NoncontrollingInterestMember2023-01-012023-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001413329us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001413329us-gaap:TreasuryStockCommonMember2023-01-012023-12-310001413329us-gaap:CommonStockMember2023-12-310001413329us-gaap:AdditionalPaidInCapitalMember2023-12-310001413329us-gaap:RetainedEarningsMember2023-12-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001413329us-gaap:TreasuryStockCommonMember2023-12-310001413329us-gaap:NoncontrollingInterestMember2023-12-310001413329pm:GeographicalSegmentMember2023-01-012023-12-31pm:segment0001413329pm:GeographicalSegmentMember2022-01-012022-12-310001413329srt:MinimumMemberus-gaap:MachineryAndEquipmentMember2023-12-310001413329srt:MaximumMemberus-gaap:MachineryAndEquipmentMember2023-12-310001413329us-gaap:BuildingAndBuildingImprovementsMembersrt:MaximumMember2023-12-310001413329pm:NoncontrollingInterestPurchasePhilipMorrisTtnMamulleriSanayiVeTicaretAMember2022-03-31xbrli:pure0001413329pm:NoncontrollingInterestPurchasePhilipMorrisPazarlamaVeSatAMember2022-03-310001413329pm:NoncontrollingInterestPurchaseMember2022-01-012022-03-310001413329pm:NoncontrollingInterestPurchaseMember2022-03-3100014133292023-01-012023-01-310001413329us-gaap:AdditionalPaidInCapitalMember2023-01-012023-01-310001413329us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-01-310001413329pm:SwedishMatchABMember2022-11-110001413329pm:SwedishMatchABMember2022-12-310001413329pm:SwedishMatchABMember2022-11-112022-11-110001413329pm:SwedishMatchABMember2022-11-112022-12-310001413329pm:SwedishMatchABMember2023-02-170001413329pm:SwedishMatchABMember2023-09-122023-09-12iso4217:SEKxbrli:shares0001413329pm:SwedishMatchABMember2022-11-112023-11-110001413329pm:SwedishMatchABMember2023-11-110001413329pm:SwedishMatchABMember2022-11-112023-03-310001413329pm:SwedishMatchABMember2022-10-012022-12-310001413329us-gaap:TrademarksMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329srt:MinimumMemberus-gaap:TrademarksMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329us-gaap:TrademarksMembersrt:MaximumMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329us-gaap:TrademarksMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329us-gaap:TechnologyBasedIntangibleAssetsMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329srt:MinimumMemberus-gaap:CustomerRelationshipsMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329us-gaap:CustomerRelationshipsMembersrt:MaximumMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329us-gaap:CustomerRelationshipsMemberpm:SwedishMatchABMember2023-11-112023-11-110001413329pm:PMIAndSwedishMatchMember2022-01-012022-12-310001413329pm:PMIAndSwedishMatchMember2021-01-012021-12-310001413329pm:AGSnusAktieselskabMember2021-05-060001413329pm:AGSnusAktieselskabMember2021-05-062021-05-060001413329pm:AGSnusAktieselskabMember2022-10-012022-12-310001413329pm:FertinPharmaASMember2021-09-150001413329pm:FertinPharmaASMember2021-09-152021-09-15iso4217:DKK0001413329pm:CashConsiderationMemberpm:FertinPharmaASMember2021-09-152021-09-150001413329pm:ConsiderationForPaymentToSettleIndebtednessMemberpm:FertinPharmaASMember2021-09-152021-09-150001413329srt:MinimumMemberpm:FertinPharmaASMember2021-09-152021-09-150001413329pm:FertinPharmaASMembersrt:MaximumMember2021-09-152021-09-150001413329pm:VecturaGroupPlcMember2021-09-15iso4217:GBPxbrli:shares0001413329pm:VecturaGroupPlcMember2021-12-310001413329pm:VecturaGroupPlcMember2021-09-152021-09-15iso4217:GBP0001413329srt:MinimumMemberpm:VecturaGroupPlcMember2021-09-152021-09-150001413329pm:VecturaGroupPlcMembersrt:MaximumMember2021-09-152021-09-150001413329pm:VecturaGroupPlcMember2022-01-012022-12-310001413329pm:AltriaGroupMember2022-10-200001413329pm:AltriaGroupMember2023-07-140001413329pm:OtiTopicIncAssetAcquisitionMember2021-08-090001413329pm:OtiTopicIncAssetAcquisitionMember2021-08-092021-08-090001413329pm:OtiTopicIncAssetAcquisitionMember2021-01-012021-12-3100014133292022-03-312022-03-310001413329country:UA2023-06-202023-06-200001413329pm:WarInUkraineMembercountry:UA2023-12-310001413329country:RUpm:WarInUkraineMember2023-12-310001413329pm:WarInUkraineMemberus-gaap:CostOfSalesMembercountry:UA2023-01-012023-12-310001413329pm:WarInUkraineMemberpm:MarketingAdministrationAndResearchCostsMembercountry:UA2023-01-012023-12-310001413329pm:WarInUkraineMembercountry:UA2023-01-012023-12-310001413329pm:WarInUkraineMemberus-gaap:CostOfSalesMembercountry:UA2022-01-012022-12-310001413329pm:WarInUkraineMemberpm:MarketingAdministrationAndResearchCostsMembercountry:UA2022-01-012022-12-310001413329pm:WarInUkraineMembercountry:UA2022-01-012022-12-310001413329country:RUpm:WarInUkraineMemberus-gaap:CostOfSalesMember2023-01-012023-12-310001413329country:RUpm:WarInUkraineMemberpm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-310001413329country:RUpm:WarInUkraineMember2023-01-012023-12-310001413329country:RUpm:WarInUkraineMemberus-gaap:CostOfSalesMember2022-01-012022-12-310001413329country:RUpm:WarInUkraineMemberpm:MarketingAdministrationAndResearchCostsMember2022-01-012022-12-310001413329country:RUpm:WarInUkraineMember2022-01-012022-12-310001413329pm:WarInUkraineMemberus-gaap:CostOfSalesMember2023-01-012023-12-310001413329pm:WarInUkraineMemberpm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-310001413329pm:WarInUkraineMember2023-01-012023-12-310001413329pm:WarInUkraineMemberus-gaap:CostOfSalesMember2022-01-012022-12-310001413329pm:WarInUkraineMemberpm:MarketingAdministrationAndResearchCostsMember2022-01-012022-12-310001413329pm:WarInUkraineMember2022-01-012022-12-310001413329pm:WellnessAndHealthcareSegmentMember2023-01-012023-12-310001413329pm:EuropeSegmentMember2021-12-310001413329pm:SSEACISMEASegmentMember2021-12-310001413329pm:EAAUPMIDFSegmentMember2021-12-310001413329pm:AmericasSegmentMember2021-12-310001413329pm:SwedishMatchABSegmentMember2021-12-310001413329pm:WellnessAndHealthcareSegmentMember2021-12-310001413329pm:EuropeSegmentMember2022-01-012022-12-310001413329pm:SSEACISMEASegmentMember2022-01-012022-12-310001413329pm:EAAUPMIDFSegmentMember2022-01-012022-12-310001413329pm:AmericasSegmentMember2022-01-012022-12-310001413329pm:SwedishMatchABSegmentMember2022-01-012022-12-310001413329pm:WellnessAndHealthcareSegmentMember2022-01-012022-12-310001413329pm:EuropeSegmentMember2022-12-310001413329pm:SSEACISMEASegmentMember2022-12-310001413329pm:EAAUPMIDFSegmentMember2022-12-310001413329pm:AmericasSegmentMember2022-12-310001413329pm:SwedishMatchABSegmentMember2022-12-310001413329pm:WellnessAndHealthcareSegmentMember2022-12-310001413329pm:EuropeSegmentMember2023-01-012023-12-310001413329pm:SSEACISMEASegmentMember2023-01-012023-12-310001413329pm:EAAUPMIDFSegmentMember2023-01-012023-12-310001413329pm:AmericasSegmentMember2023-01-012023-12-310001413329pm:SwedishMatchABSegmentMember2023-01-012023-12-310001413329pm:EuropeSegmentMember2023-12-310001413329pm:SSEACISMEASegmentMember2023-12-310001413329pm:EAAUPMIDFSegmentMember2023-12-310001413329pm:AmericasSegmentMember2023-12-310001413329pm:SwedishMatchABSegmentMember2023-12-310001413329pm:WellnessAndHealthcareSegmentMember2023-12-310001413329us-gaap:TrademarksMember2023-12-310001413329us-gaap:TrademarksMember2022-12-310001413329us-gaap:DevelopedTechnologyRightsMember2023-12-310001413329us-gaap:DevelopedTechnologyRightsMember2022-12-310001413329us-gaap:OtherIntangibleAssetsMember2023-12-310001413329us-gaap:OtherIntangibleAssetsMember2022-12-310001413329us-gaap:CostOfSalesMember2023-01-012023-12-310001413329pm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-3100014133292022-07-012022-09-300001413329us-gaap:DevelopedTechnologyRightsMemberpm:WellnessAndHealthcareSegmentMember2022-12-310001413329pm:DefiniteLivedIntangiblesAndOtherAssetsMember2023-12-310001413329pm:DefiniteLivedIntangiblesAndOtherAssetsMember2022-12-310001413329pm:MegapolisMember2023-12-310001413329pm:EITAMember2023-12-310001413329pm:STAEMMember2023-12-310001413329pm:STAEMMemberpm:EITAMember2023-12-310001413329pm:ManagementEtDeveloppementDesActifsEtDesRessourcesHoldingMADARHoldingMemberpm:STAEMMember2023-12-310001413329pm:EIHMember2023-04-300001413329pm:UTCMember2023-04-300001413329pm:EIHMemberpm:UTCMember2023-04-300001413329pm:RothmansBensonAndHedgesInc.RBHMember2019-03-220001413329us-gaap:FairValueInputsLevel1Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-12-310001413329pm:TTIMemberpm:PMMMember2023-12-310001413329pm:IPMIndiaMember2023-12-310001413329us-gaap:RelatedPartyMemberpm:MegapolisMember2023-01-012023-12-310001413329us-gaap:RelatedPartyMemberpm:MegapolisMember2022-01-012022-12-310001413329us-gaap:RelatedPartyMemberpm:MegapolisMember2021-01-012021-12-310001413329pm:OtherRelatedPartyMemberus-gaap:RelatedPartyMember2023-01-012023-12-310001413329pm:OtherRelatedPartyMemberus-gaap:RelatedPartyMember2022-01-012022-12-310001413329pm:OtherRelatedPartyMemberus-gaap:RelatedPartyMember2021-01-012021-12-310001413329pm:OtherRelatedPartyMember2023-01-012023-12-310001413329pm:OtherRelatedPartyMember2022-01-012022-12-310001413329pm:OtherRelatedPartyMember2021-01-012021-12-310001413329us-gaap:RelatedPartyMemberpm:MegapolisMember2023-12-310001413329us-gaap:RelatedPartyMemberpm:MegapolisMember2022-12-310001413329pm:OtherRelatedPartyMemberus-gaap:RelatedPartyMember2023-12-310001413329pm:OtherRelatedPartyMemberus-gaap:RelatedPartyMember2022-12-310001413329us-gaap:CommercialPaperMember2023-12-310001413329us-gaap:CommercialPaperMember2022-12-310001413329us-gaap:BankLoanObligationsMember2023-12-310001413329us-gaap:BankLoanObligationsMember2022-12-310001413329pm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMember2022-12-310001413329pm:USDollarNotesMembersrt:MinimumMember2023-12-310001413329pm:USDollarNotesMembersrt:MaximumMember2023-12-310001413329pm:USDollarNotesMember2023-01-012023-12-310001413329pm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMember2022-12-310001413329srt:MinimumMemberpm:ForeignCurrencyObligationsMemberpm:EuroNotesPayableMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroNotesPayableMembersrt:MaximumMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroNotesPayableMember2023-01-012023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroNotesPayableMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroNotesPayableMember2022-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwissFrancNotesMember2023-01-012023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwissFrancNotesMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwissFrancNotesMember2022-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroBankLoanMember2023-01-012023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroBankLoanMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:EuroBankLoanMember2022-12-310001413329srt:MinimumMemberpm:ForeignCurrencyObligationsMemberpm:SwedishKronaNotesMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwedishKronaNotesMembersrt:MaximumMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwedishKronaNotesMember2023-01-012023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwedishKronaNotesMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:SwedishKronaNotesMember2022-12-310001413329pm:ForeignCurrencyObligationsMemberus-gaap:NotesPayableOtherPayablesMember2023-01-012023-12-310001413329pm:ForeignCurrencyObligationsMemberus-gaap:NotesPayableOtherPayablesMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberus-gaap:NotesPayableOtherPayablesMember2022-12-310001413329us-gaap:FairValueInputsLevel1Member2023-12-310001413329us-gaap:FairValueInputsLevel1Member2022-12-310001413329us-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel2Member2022-12-310001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-05-112022-05-110001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-05-110001413329pm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-06-23iso4217:EUR0001413329us-gaap:DebtInstrumentRedemptionPeriodOneMemberpm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-06-230001413329us-gaap:DebtInstrumentRedemptionPeriodOneMemberpm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-06-232022-06-230001413329us-gaap:DebtInstrumentRedemptionPeriodTwoMemberpm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-06-230001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-06-230001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-11-072022-11-100001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-11-072022-11-070001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-11-102022-11-100001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2022-11-212022-11-210001413329pm:SeniorUnsecuredBridgeFacilityMemberpm:SwedishMatchABMember2023-02-172023-02-170001413329pm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-11-072022-11-070001413329us-gaap:DebtInstrumentRedemptionPeriodOneMemberpm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-11-072022-11-070001413329us-gaap:DebtInstrumentRedemptionPeriodTwoMemberpm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-11-072022-11-070001413329pm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2023-12-310001413329pm:SeniorUnsecuredTermLoanMemberpm:SwedishMatchABMember2022-12-310001413329pm:TwoPointEightSevenFivePercentUSDollarNotesDueMay2024Memberpm:USDollarNotesMember2023-12-310001413329pm:ThreePointTwoFivePercentUSDollarNotesDueNovember2024Memberpm:USDollarNotesMember2023-12-310001413329pm:FivePointOneTwoFivePercentUSDollarNotesDueNovember2024Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:OnePointFiveZeroZeroPercentUSDollarNotesDueMay2025Member2023-12-310001413329pm:USDollarNotesMemberpm:ThreePointThreeSevenFivePercentUSDollarNotesDueAugust2025Member2023-12-310001413329pm:FivePointZeroPercentUSDollarNotesDueNovember2025Memberpm:USDollarNotesMember2023-12-310001413329pm:TwoPointSevenFivePercentUSDollarNotesDueFebruary2026Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:FourPointEightSevenFivePercentUSDollarNotesDueFebruary2026IssuedInFebruary2023Member2023-12-310001413329pm:FourPointEightSevenFivePercentUSDollarNotesDueFebruary2026IssuedInMay2023Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:ZeroPointEightSevenFivePercentUSDollarNotesDueMay2026Member2023-12-310001413329pm:ThreePointOneTwoFivePercentUSDollarNotesDueAugust2027Memberpm:USDollarNotesMember2023-12-310001413329pm:FivePointOneTwoFivePercentUSDollarNotesDueNovember2027Memberpm:USDollarNotesMember2023-12-310001413329pm:FourPointEightSevenFivePercentUSDollarNotesDueFebruary2028IssuedInFebruary2023Memberpm:USDollarNotesMember2023-12-310001413329pm:FourPointEightSevenFivePercentUSDollarNotesDueFebruary2028IssuedInMay2023Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:ThreePointOneTwoFivePercentUSDollarNotesDueMarch2028Member2023-12-310001413329pm:FourPointZeroPercentUSDollarNotesDueMay2028Memberpm:USDollarNotesMember2023-12-310001413329pm:FivePointTwoFivePercentUSDollarNotesDueSeptember2028Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:ThreePointThreeSevenFivePercentUSDollarNotesDueAugust2029Member2023-12-310001413329pm:FivePointSixTwoFivePercentUSDollarNotesDueNovember2029Memberpm:USDollarNotesMember2023-12-310001413329pm:FivePointOneTwoFivePercentUSDollarNotesDueFebruary2030IssuedFebruary2023Memberpm:USDollarNotesMember2023-12-310001413329pm:FivePointOneTwoFivePercentUSDollarNotesDueFebruary2030IssuedMay2023Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:TwoPointOneZeroZeroPercentUSDollarNotesDueMay2030Member2023-12-310001413329pm:USDollarNotesMemberpm:FivePointFivePercentUSDollarNotesDueSeptember2030Member2023-12-310001413329pm:OnePointSevenFiveZeroPercentUSDollarNotesDueNovember2030Memberpm:USDollarNotesMember2023-12-310001413329pm:FivePointSevenFiveZeroPercentUSDollarNotesDueNovember2032Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:FivePointThreeSevenFivePercentUSDollarNotesDueFebruary2033IssuedFebruary2023Member2023-12-310001413329pm:USDollarNotesMemberpm:FivePointThreeSevenFivePercentUSDollarNotesDueFebruary2033IssuedMay2023Member2023-12-310001413329pm:USDollarNotesMemberpm:FivePointSixTwoFivePercentUSDollarNotesDueSeptember2033Member2023-12-310001413329pm:USDollarNotesMemberpm:SixPointThreeSevenFivePercentUSDollarNotesDueMay2038Member2023-12-310001413329pm:FourPointThreeSevenFivePercentUSDollarNotesDueNovember2041Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:FourPointFivePercentUnitedStatesDollarNotesDueMarchTwoThousandFourtyTwoMember2023-12-310001413329pm:USDollarNotesMemberpm:ThreePointEightSevenFivePercentUnitedStatesDollarNotesDueAugustTwoThousandFourtyTwoMember2023-12-310001413329pm:USDollarNotesMemberpm:FourPointOneTwoFivePercentUsDollarNotesDueMarch2043Member2023-12-310001413329pm:FourPointEightSevenFivePercentUSDollarNotesDueNovember2043Memberpm:USDollarNotesMember2023-12-310001413329pm:USDollarNotesMemberpm:FourPointTwoFivePercentUSDollarNotesDueNovember2044Member2023-12-310001413329pm:FourPointTwoFivePercentUSDollarNotesIssuedMay2016DueNovember2044Memberpm:USDollarNotesMember2023-12-310001413329pm:TwoPointEightSevenFivePercentEuroNotesDueMay2024Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:PointEightSevenFivePercentEuroNotesDueSeptember2024Member2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:ZeroPointSixTwoFivePercentEuroNotesDueNovember2024Member2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:TwoPointSevenFivePercentEuroNotesDueMarch2025Member2023-12-310001413329pm:OnePointTwoPercentEuroNotesDueNovember2025Memberpm:ForeignCurrencyObligationsMemberpm:ComponentOneMember2023-12-310001413329pm:ComponentTwoMemberpm:OnePointTwoPercentEuroNotesDueNovember2025Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:OnePointTwoPercentEuroNotesDueNovember2025Memberpm:ForeignCurrencyObligationsMemberpm:ComponentThreeMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:TwoPointEightSevenFivePercentEuroNoteDueMarch2026Member2023-12-310001413329pm:ZeroPointOneTwoFivePercentEuroNotesDueAugust2026Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:PointEightSevenFivePercentEuroNotesDueFebruary2027Member2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:TwoPointEightSevenFivePercentEuroNoteDueMay2029Member2023-12-310001413329pm:ZeroPointEightZeroZeroPercentEuroNotesDueAugust2031Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:ThreePointOneTwoFivePercentEuroNotesDueJune2033Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:TwoPercentEuroNotesDueMay2036Member2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:OnePointEightSevenFivePercentEuroNotesDueNovember2037Member2023-12-310001413329pm:ForeignCurrencyObligationsMemberpm:OnePointFourFiveZeroPercentEuroNotesDueAugust2039Member2023-12-310001413329pm:OnePointSixTwoFivePercentSwissFrancNoteDueMay2024Memberpm:ForeignCurrencyObligationsMember2023-12-31iso4217:CHF0001413329pm:ForeignCurrencyObligationsMemberpm:TwoPointSevenOneZeroPercentSwedishKronaDueJanuary2026Member2023-12-31iso4217:SEK0001413329pm:OnePointThreeNineFivePercentSwedishKronaNoteDueFebruary2026Memberpm:ForeignCurrencyObligationsMemberpm:ComponentOneMember2023-12-310001413329pm:ComponentTwoMemberpm:OnePointThreeNineFivePercentSwedishKronaNoteDueFebruary2026Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:OnePointThreeNineFivePercentSwedishKronaNoteDueFebruary2026Memberpm:ForeignCurrencyObligationsMemberpm:ComponentThreeMember2023-12-310001413329pm:OnePointThreeNineFivePercentSwedishKronaNoteDueFebruary2026Memberpm:ForeignCurrencyObligationsMemberpm:ComponentFourMember2023-12-310001413329pm:TwoPointOneNineZeroPercentSwedishKronaDueApril2029Memberpm:ForeignCurrencyObligationsMember2023-12-310001413329pm:ThreeHundredSixtyFourDayRevolvingCreditExpiringJanuary302024Member2023-12-310001413329pm:MultiYearRevolvingCreditFacilityExpiringFebruary102026Member2023-12-310001413329pm:MultiYearRevolvingCreditFacilityExpiringSeptember292026Member2023-12-310001413329pm:ThreeHundredSixtyFourDayRevolvingCreditExpiringJanuary312023Member2023-01-012023-12-310001413329us-gaap:SubsequentEventMemberpm:ThreeHundredSixtyFourDayRevolvingCreditExpiringJanuary282025Member2024-01-240001413329pm:MultiYearRevolvingCreditFacilityExpiringFebruary102027Member2022-01-280001413329pm:MultiYearRevolvingCreditFacilityExpiringSeptember292027Member2022-09-200001413329pm:ShortTermCreditArrangementMember2023-12-310001413329pm:ShortTermCreditArrangementMember2022-12-310001413329pm:SharesIssuedMember2020-12-310001413329pm:SharesRepurchasedMember2020-12-310001413329pm:SharesOutstandingMember2020-12-310001413329pm:SharesRepurchasedMember2021-01-012021-12-310001413329pm:SharesOutstandingMember2021-01-012021-12-310001413329pm:SharesIssuedMember2021-12-310001413329pm:SharesRepurchasedMember2021-12-310001413329pm:SharesOutstandingMember2021-12-310001413329pm:SharesRepurchasedMember2022-01-012022-12-310001413329pm:SharesOutstandingMember2022-01-012022-12-310001413329pm:SharesIssuedMember2022-12-310001413329pm:SharesRepurchasedMember2022-12-310001413329pm:SharesOutstandingMember2022-12-310001413329pm:SharesRepurchasedMember2023-01-012023-12-310001413329pm:SharesOutstandingMember2023-01-012023-12-310001413329pm:SharesIssuedMember2023-12-310001413329pm:SharesRepurchasedMember2023-12-310001413329pm:SharesOutstandingMember2023-12-3100014133292021-06-110001413329srt:ScenarioForecastMembersrt:MinimumMember2021-06-112024-06-110001413329srt:ScenarioForecastMembersrt:MaximumMember2021-06-112024-06-1100014133292021-06-112021-06-1100014133292021-07-222022-03-3100014133292022-01-012022-03-3100014133292022-05-112022-05-110001413329pm:A2017PerformanceIncentivePlanMember2022-05-310001413329pm:A2017PerformanceIncentivePlanMember2023-12-310001413329pm:A2017NonEmployeeDirectorsPlanMember2017-05-310001413329pm:A2017NonEmployeeDirectorsPlanMember2023-12-310001413329us-gaap:RestrictedStockUnitsRSUMember2022-12-310001413329us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001413329us-gaap:RestrictedStockUnitsRSUMember2023-12-310001413329us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001413329us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-31pm:year0001413329pm:GrantDateFairValueMemberus-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001413329pm:FairValueMemberus-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001413329pm:GrantDateFairValueMemberus-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001413329pm:FairValueMemberus-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001413329pm:GrantDateFairValueMemberus-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-310001413329pm:FairValueMemberus-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-310001413329us-gaap:PerformanceSharesMember2023-01-012023-12-310001413329us-gaap:PerformanceSharesMember2023-12-310001413329us-gaap:PerformanceSharesMember2021-12-31pm:performanceMetric0001413329srt:MinimumMember2023-01-012023-12-310001413329srt:MaximumMember2023-01-012023-12-310001413329us-gaap:PerformanceSharesMember2022-12-310001413329pm:PerformanceShareUnitsOtherPerformanceFactorsMember2022-12-310001413329pm:PerformanceShareUnitsTSRRelativeToCustomerPeerGroupMember2022-12-310001413329pm:PerformanceShareUnitsOtherPerformanceFactorsMember2023-01-012023-12-310001413329pm:PerformanceShareUnitsTSRRelativeToCustomerPeerGroupMember2023-01-012023-12-310001413329pm:PerformanceShareUnitsOtherPerformanceFactorsMember2023-12-310001413329pm:PerformanceShareUnitsTSRRelativeToCustomerPeerGroupMember2023-12-310001413329pm:PerformanceShareUnitsOtherPerformanceFactorsMember2022-01-012022-12-310001413329pm:PerformanceShareUnitsTSRRelativeToCustomerPeerGroupMember2022-01-012022-12-310001413329us-gaap:PerformanceSharesMember2022-01-012022-12-310001413329pm:PerformanceShareUnitsOtherPerformanceFactorsMember2021-01-012021-12-310001413329pm:PerformanceShareUnitsTSRRelativeToCustomerPeerGroupMember2021-01-012021-12-310001413329us-gaap:PerformanceSharesMember2021-01-012021-12-310001413329pm:GrantDateFairValueMemberus-gaap:PerformanceSharesMember2023-01-012023-12-310001413329pm:FairValueMemberus-gaap:PerformanceSharesMember2023-01-012023-12-310001413329pm:GrantDateFairValueMemberus-gaap:PerformanceSharesMember2022-01-012022-12-310001413329pm:FairValueMemberus-gaap:PerformanceSharesMember2022-01-012022-12-310001413329pm:GrantDateFairValueMemberus-gaap:PerformanceSharesMember2021-01-012021-12-310001413329pm:FairValueMemberus-gaap:PerformanceSharesMember2021-01-012021-12-310001413329pm:SwedishMatchABMember2023-12-3100014133292021-10-012021-10-31iso4217:IDR0001413329country:RU2023-01-012023-12-310001413329country:RU2022-01-012022-12-310001413329country:RU2021-01-012021-12-310001413329us-gaap:PensionPlansDefinedBenefitMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2021-01-012021-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMember2023-01-012023-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMember2022-01-012022-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:WellnessAndHealthcareSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:WellnessAndHealthcareSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:WellnessAndHealthcareSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMember2022-01-012022-12-310001413329country:JPus-gaap:GeographicConcentrationRiskMember2023-01-012023-12-310001413329country:JPus-gaap:GeographicConcentrationRiskMember2022-01-012022-12-310001413329country:JPus-gaap:GeographicConcentrationRiskMember2021-01-012021-12-310001413329pm:EAAUPMIDFSegmentMemberpm:CustomerOneMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:RevenueFromContractWithCustomerMember2023-01-012023-12-310001413329pm:EAAUPMIDFSegmentMemberpm:CustomerOneMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:RevenueFromContractWithCustomerMember2022-01-012022-12-310001413329pm:EAAUPMIDFSegmentMemberpm:CustomerOneMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:RevenueFromContractWithCustomerMember2021-01-012021-12-310001413329pm:CustomerOneMemberpm:EuropeSegmentMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:RevenueFromContractWithCustomerMember2023-01-012023-12-310001413329pm:CustomerOneMemberpm:EuropeSegmentMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:RevenueFromContractWithCustomerMember2022-01-012022-12-310001413329pm:CustomerOneMemberpm:EuropeSegmentMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:RevenueFromContractWithCustomerMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMemberpm:CombustibleTobaccoProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMemberpm:CombustibleTobaccoProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMemberpm:CombustibleTobaccoProductsMember2021-01-012021-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMember2023-01-012023-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMember2022-01-012022-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:CombustibleTobaccoProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:CombustibleTobaccoProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:CombustibleTobaccoProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMemberpm:CombustibleTobaccoProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMemberpm:CombustibleTobaccoProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMemberpm:CombustibleTobaccoProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMemberpm:SwedishMatchABSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMemberpm:SwedishMatchABSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMemberpm:SwedishMatchABSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:CombustibleTobaccoProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMemberpm:SmokeFreeProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMemberpm:SmokeFreeProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMemberpm:SmokeFreeProductsMember2021-01-012021-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMember2023-01-012023-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMember2022-01-012022-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:SmokeFreeProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:SmokeFreeProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:SmokeFreeProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMemberpm:SmokeFreeProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMemberpm:SmokeFreeProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMemberpm:SmokeFreeProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMemberpm:SwedishMatchABSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMemberpm:SwedishMatchABSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMemberpm:SwedishMatchABSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsExcludingWellnessAndHealthcareMemberpm:SmokeFreeProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsExcludingWellnessAndHealthcareMemberpm:SmokeFreeProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsExcludingWellnessAndHealthcareMemberpm:SmokeFreeProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMemberpm:WellnessAndHealthcareSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMemberpm:WellnessAndHealthcareSegmentMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMemberpm:WellnessAndHealthcareSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMember2022-01-012022-12-310001413329us-gaap:OperatingSegmentsMemberpm:SmokeFreeProductsMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMember2021-01-012021-12-310001413329pm:SSEACISMEASegmentMemberpm:NetRevenuesMember2023-01-012023-03-310001413329pm:MarketingAdministrationAndResearchCostsMember2023-07-012023-09-300001413329pm:EuropeSegmentMemberpm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-310001413329pm:SSEACISMEASegmentMemberpm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-310001413329pm:EAAUPMIDFSegmentMemberpm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-310001413329pm:AmericasSegmentMemberpm:MarketingAdministrationAndResearchCostsMember2023-01-012023-12-310001413329pm:SSEACISMEASegmentMembercountry:SA2021-06-012021-06-300001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2021-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMember2023-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMember2022-12-310001413329pm:SSEACISMEASegmentMemberus-gaap:OperatingSegmentsMember2021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EastAsiaAndAustraliaMember2023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EastAsiaAndAustraliaMember2022-12-310001413329us-gaap:OperatingSegmentsMemberpm:EastAsiaAndAustraliaMember2021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2022-12-310001413329us-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2021-12-310001413329us-gaap:OperatingSegmentsMember2023-12-310001413329us-gaap:OperatingSegmentsMember2022-12-310001413329us-gaap:OperatingSegmentsMember2021-12-310001413329pm:AltriaGroupMember2023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AltriaGroupMember2022-12-310001413329us-gaap:OperatingSegmentsMemberpm:AltriaGroupMember2021-12-310001413329us-gaap:OperatingSegmentsMembercountry:CH2023-12-310001413329us-gaap:OperatingSegmentsMembercountry:CH2022-12-310001413329us-gaap:OperatingSegmentsMembercountry:CH2021-12-310001413329us-gaap:OperatingSegmentsMembercountry:ID2023-12-310001413329us-gaap:OperatingSegmentsMembercountry:ID2022-12-310001413329us-gaap:OperatingSegmentsMembercountry:ID2021-12-310001413329us-gaap:OperatingSegmentsMembercountry:IT2023-12-310001413329us-gaap:OperatingSegmentsMembercountry:IT2022-12-310001413329us-gaap:OperatingSegmentsMembercountry:IT2021-12-310001413329us-gaap:PensionPlansDefinedBenefitMember2023-01-012023-12-310001413329us-gaap:PensionPlansDefinedBenefitMember2021-01-012021-12-310001413329pm:PostemploymentBenefitPlansMember2023-01-012023-12-310001413329pm:PostemploymentBenefitPlansMember2022-01-012022-12-310001413329pm:PostemploymentBenefitPlansMember2021-01-012021-12-310001413329us-gaap:PostretirementBenefitCostsMember2023-01-012023-12-310001413329us-gaap:PostretirementBenefitCostsMember2022-01-012022-12-310001413329us-gaap:PostretirementBenefitCostsMember2021-01-012021-12-310001413329us-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMember2021-12-310001413329us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-12-310001413329us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-12-310001413329us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-01-012023-12-310001413329us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-01-012022-12-310001413329us-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-12-310001413329pm:BenefitObligationMembercountry:CHpm:PensionPlanPortfolioMember2023-01-012023-12-310001413329pm:BenefitObligationMembercountry:CHpm:PensionPlanPortfolioMember2022-01-012022-12-310001413329pm:FairValueOfPlanAssetsMembercountry:CHpm:PensionPlanPortfolioMember2023-01-012023-12-310001413329pm:FairValueOfPlanAssetsMembercountry:CHpm:PensionPlanPortfolioMember2022-01-012022-12-310001413329pm:BenefitObligationMembercountry:USpm:PensionPlanPortfolioMember2023-01-012023-12-310001413329pm:BenefitObligationMembercountry:USpm:PensionPlanPortfolioMember2022-01-012022-12-310001413329pm:FairValueOfPlanAssetsMembercountry:USpm:PensionPlanPortfolioMember2023-01-012023-12-310001413329pm:FairValueOfPlanAssetsMembercountry:USpm:PensionPlanPortfolioMember2022-01-012022-12-310001413329us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-01-012021-12-310001413329us-gaap:DefinedBenefitPlanEquitySecuritiesMember2023-12-310001413329us-gaap:DefinedBenefitPlanDebtSecurityMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2023-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2023-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMember2023-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329pm:InvestmentFundsMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberpm:InvestmentFundsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329pm:InvestmentFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel3Memberpm:InvestmentFundsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMember2023-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2023-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2023-12-310001413329us-gaap:OtherDebtSecuritiesMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:OtherDebtSecuritiesMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:OtherDebtSecuritiesMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:OtherDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2023-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2023-12-310001413329pm:USAndInternationalEquitiesMember2023-12-310001413329pm:USAndInternationalGovernmentBondsMember2023-12-310001413329pm:InvestmentFundsHoldingCorporateBondsMember2023-12-310001413329pm:RealEstateAndOtherMoneyMarketsMember2023-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanEquitySecuritiesUsMember2022-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanEquitySecuritiesNonUsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329pm:InvestmentFundsMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberpm:InvestmentFundsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329pm:InvestmentFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:FairValueInputsLevel3Memberpm:InvestmentFundsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberpm:DebtSecurityGovernmentUSAndForeignMember2022-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2022-12-310001413329us-gaap:OtherDebtSecuritiesMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:OtherDebtSecuritiesMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:OtherDebtSecuritiesMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:OtherDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:FairValueInputsLevel12And3Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2022-12-310001413329us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001413329us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2022-12-310001413329pm:USAndInternationalEquitiesMember2022-12-310001413329pm:USAndInternationalGovernmentBondsMember2022-12-310001413329pm:InvestmentFundsHoldingCorporateBondsMember2022-12-310001413329pm:RealEstateAndOtherMoneyMarketsMember2022-12-310001413329pm:PostemploymentBenefitPlansMember2023-12-310001413329pm:PostemploymentBenefitPlansMember2022-12-310001413329pm:PostemploymentBenefitPlansMember2021-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2023-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2022-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329us-gaap:OtherCurrentAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329us-gaap:OtherCurrentAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329pm:OtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329pm:OtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329us-gaap:OtherAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329us-gaap:OtherAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMemberus-gaap:OtherLiabilitiesMember2023-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMemberus-gaap:OtherLiabilitiesMember2022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:OtherCurrentAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:OtherCurrentAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-12-310001413329us-gaap:InterestRateContractMemberpm:OtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-12-310001413329us-gaap:InterestRateContractMemberpm:OtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:OtherAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:OtherAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherLiabilitiesMember2023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherLiabilitiesMember2022-12-310001413329us-gaap:OtherCurrentAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2023-12-310001413329us-gaap:OtherCurrentAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2022-12-310001413329pm:OtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2023-12-310001413329pm:OtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2022-12-310001413329us-gaap:OtherAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2023-12-310001413329us-gaap:OtherAssetsMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2022-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMemberus-gaap:OtherLiabilitiesMember2023-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMemberus-gaap:OtherLiabilitiesMember2022-12-310001413329us-gaap:OtherCurrentAssetsMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329us-gaap:OtherCurrentAssetsMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329pm:OtherAccruedLiabilitiesMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329pm:OtherAccruedLiabilitiesMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329us-gaap:OtherAssetsMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-12-310001413329us-gaap:OtherAssetsMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:OtherLiabilitiesMember2023-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:OtherLiabilitiesMember2022-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310001413329us-gaap:SalesMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310001413329us-gaap:SalesMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310001413329us-gaap:SalesMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310001413329us-gaap:ForeignExchangeContractMemberus-gaap:CostOfSalesMember2023-01-012023-12-310001413329us-gaap:ForeignExchangeContractMemberus-gaap:CostOfSalesMember2022-01-012022-12-310001413329us-gaap:ForeignExchangeContractMemberus-gaap:CostOfSalesMember2021-01-012021-12-310001413329pm:MarketingAdministrationAndResearchCostsMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310001413329pm:MarketingAdministrationAndResearchCostsMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310001413329pm:MarketingAdministrationAndResearchCostsMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310001413329us-gaap:ForeignExchangeContractMemberus-gaap:InterestExpenseMember2023-01-012023-12-310001413329us-gaap:ForeignExchangeContractMemberus-gaap:InterestExpenseMember2022-01-012022-12-310001413329us-gaap:ForeignExchangeContractMemberus-gaap:InterestExpenseMember2021-01-012021-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-01-012023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-01-012022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2021-01-012021-12-310001413329us-gaap:InterestRateContractMemberus-gaap:InterestExpenseMember2023-01-012023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:InterestExpenseMember2022-01-012022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:InterestExpenseMember2021-01-012021-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2023-01-012023-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2022-01-012022-12-310001413329us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CommodityContractMember2021-01-012021-12-310001413329us-gaap:CommodityContractMemberus-gaap:CostOfSalesMember2023-01-012023-12-310001413329us-gaap:CommodityContractMemberus-gaap:CostOfSalesMember2022-01-012022-12-310001413329us-gaap:CommodityContractMemberus-gaap:CostOfSalesMember2021-01-012021-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestExpenseMember2023-01-012023-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestExpenseMember2022-01-012022-12-310001413329us-gaap:InterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestExpenseMember2021-01-012021-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:InterestExpenseMember2023-01-012023-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:InterestExpenseMember2022-01-012022-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:InterestExpenseMember2021-01-012021-12-310001413329us-gaap:NondesignatedMemberpm:MarketingAdministrationAndResearchCostsMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310001413329us-gaap:NondesignatedMemberpm:MarketingAdministrationAndResearchCostsMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310001413329us-gaap:NondesignatedMemberpm:MarketingAdministrationAndResearchCostsMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310001413329us-gaap:ForeignExchangeContractMember2023-01-012023-12-310001413329us-gaap:ForeignExchangeContractMember2022-01-012022-12-310001413329us-gaap:ForeignExchangeContractMember2021-01-012021-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310001413329us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310001413329us-gaap:FairValueHedgingMember2023-12-310001413329us-gaap:NetInvestmentHedgingMemberpm:ForeignDebtMember2023-01-012023-12-310001413329us-gaap:NetInvestmentHedgingMemberpm:ForeignDebtMember2022-01-012022-12-310001413329us-gaap:NetInvestmentHedgingMemberpm:ForeignDebtMember2021-01-012021-12-310001413329us-gaap:OtherComprehensiveIncomeMember2022-12-310001413329us-gaap:OtherComprehensiveIncomeMember2021-12-310001413329us-gaap:OtherComprehensiveIncomeMember2020-12-310001413329us-gaap:OtherComprehensiveIncomeMember2023-01-012023-12-310001413329us-gaap:OtherComprehensiveIncomeMember2022-01-012022-12-310001413329us-gaap:OtherComprehensiveIncomeMember2021-01-012021-12-310001413329us-gaap:OtherComprehensiveIncomeMember2023-12-310001413329us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310001413329us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310001413329us-gaap:AccumulatedTranslationAdjustmentMember2021-12-310001413329us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-12-310001413329us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310001413329us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-12-310001413329us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-12-310001413329us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2022-12-310001413329us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2021-12-310001413329pm:SmokingAndHealthClassActionsMembercountry:CApm:ConseilQuebecoisSurLeTabacEtLaSanteandJeanYvesBlaisMember2015-05-272015-05-27pm:manufacturer0001413329pm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:ImperialTobaccoLtd.RothmansBensonAndHedgesInc.AndJTIMacdonaldCorp.Memberpm:ConseilQuebecoisSurLeTabacEtLaSanteandJeanYvesBlaisMember2015-05-272015-05-27iso4217:CAD0001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:ConseilQuebecoisSurLeTabacEtLaSanteandJeanYvesBlaisMember2015-05-272015-05-27pm:plaintiff0001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberpm:AppellateRulingMemberpm:CeciliaLetourneauConseilQuebecoisSurLaTabacEtLaSanteandJeanYvesBlaisCasesMembercountry:CA2015-10-012015-10-300001413329pm:SmokingAndHealthClassActionsMemberpm:AppellateRulingMemberpm:CeciliaLetourneauConseilQuebecoisSurLaTabacEtLaSanteandJeanYvesBlaisCasesMemberpm:ImperialTobaccoLtd.Membercountry:CA2015-10-012015-10-300001413329pm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:ImperialTobaccoLtd.RothmansBensonAndHedgesInc.AndJTIMacdonaldCorp.Memberpm:ConseilQuebecoisSurLeTabacEtLaSanteandJeanYvesBlaisMember2019-03-012019-03-010001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:ConseilQuebecoisSurLeTabacEtLaSanteandJeanYvesBlaisMember2019-03-012019-03-010001413329pm:SmokingAndHealthClassActionsMemberpm:CeciliaLetourneauConseilQuebecoisSurLaTabacEtLaSanteandJeanYvesBlaisCasesMemberus-gaap:JudicialRulingMembercountry:CApm:ImperialTobaccoLtd.RothmansBensonAndHedgesInc.AndJTIMacdonaldCorp.Member2019-03-012019-03-010001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberpm:AppellateRulingMemberpm:CeciliaLetourneauConseilQuebecoisSurLaTabacEtLaSanteandJeanYvesBlaisCasesMembercountry:CA2019-03-012019-03-010001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberpm:AppellateRulingMemberpm:CeciliaLetourneauConseilQuebecoisSurLaTabacEtLaSanteandJeanYvesBlaisCasesMembercountry:CA2019-01-012019-03-310001413329pm:SmokingAndHealthClassActionsMembercountry:CApm:CeciliaLetourneauMember2015-05-272015-05-270001413329pm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:ImperialTobaccoLtd.RothmansBensonAndHedgesInc.AndJTIMacdonaldCorp.Memberpm:CeciliaLetourneauMember2015-05-272015-05-270001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:CeciliaLetourneauMember2015-05-272015-05-270001413329pm:RothmansBensonAndHedgesInc.RBHMemberpm:SmokingAndHealthClassActionsMemberus-gaap:JudicialRulingMembercountry:CApm:CeciliaLetourneauMember2019-03-012019-03-010001413329pm:AdamsMemberus-gaap:PendingLitigationMembercountry:CA2009-07-102009-07-10pm:cigarette0001413329pm:SuzanneJacklinMemberus-gaap:PendingLitigationMembercountry:CA2012-06-202012-06-200001413329pm:IndividualSmokingAndHealthCasesMemberpm:CombustibleProductsMember2023-12-31pm:litigationCase0001413329pm:IndividualSmokingAndHealthCasesMemberpm:CombustibleProductsMember2022-12-310001413329pm:IndividualSmokingAndHealthCasesMemberpm:CombustibleProductsMember2021-12-310001413329pm:SmokingAndHealthClassActionsMemberpm:CombustibleProductsMember2023-12-310001413329pm:SmokingAndHealthClassActionsMemberpm:CombustibleProductsMember2022-12-310001413329pm:SmokingAndHealthClassActionsMemberpm:CombustibleProductsMember2021-12-310001413329pm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMember2023-12-310001413329pm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMember2022-12-310001413329pm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMember2021-12-310001413329pm:LabelRelatedClassActionMemberpm:CombustibleProductsMember2023-12-310001413329pm:LabelRelatedClassActionMemberpm:CombustibleProductsMember2022-12-310001413329pm:LabelRelatedClassActionMemberpm:CombustibleProductsMember2021-12-310001413329pm:IndividualLabelRelatedCasesMemberpm:CombustibleProductsMember2023-12-310001413329pm:IndividualLabelRelatedCasesMemberpm:CombustibleProductsMember2022-12-310001413329pm:IndividualLabelRelatedCasesMemberpm:CombustibleProductsMember2021-12-310001413329pm:PublicCivilActionsMemberpm:CombustibleProductsMember2023-12-310001413329pm:PublicCivilActionsMemberpm:CombustibleProductsMember2022-12-310001413329pm:PublicCivilActionsMemberpm:CombustibleProductsMember2021-12-310001413329pm:CaseDecidedInFavorOfPlaintiffMember2023-12-310001413329pm:CasesRemainingOnAppealMember2023-12-310001413329pm:SmokingAndHealthIndividualActionsMemberus-gaap:JudicialRulingMembercountry:ARpm:HugoLespadaMember2016-08-052016-08-05iso4217:ARS0001413329pm:ClaudiaMilanoMemberpm:SmokingAndHealthIndividualActionsMemberus-gaap:JudicialRulingMembercountry:AR2021-06-172021-06-170001413329pm:ClaudiaMilanoMemberpm:SmokingAndHealthIndividualActionsMemberus-gaap:JudicialRulingMembercountry:AR2021-07-022021-07-020001413329pm:ClaudiaMilanoMemberpm:SmokingAndHealthIndividualActionsMemberus-gaap:JudicialRulingMembercountry:AR2021-12-162021-12-160001413329pm:SmokingAndHealthIndividualActionsMemberpm:SenemYilmazelMembercountry:TRus-gaap:JudicialRulingMember2023-06-232023-06-23iso4217:TRY0001413329pm:IndividualSmokingAndHealthCasesMemberpm:CombustibleProductsMembercountry:AR2023-12-310001413329pm:IndividualSmokingAndHealthCasesMemberpm:CombustibleProductsMembercountry:CA2023-12-310001413329pm:IndividualSmokingAndHealthCasesMemberpm:CombustibleProductsMembercountry:CL2023-12-310001413329pm:IndividualSmokingAndHealthCasesMembercountry:TRpm:CombustibleProductsMember2023-12-310001413329country:BRpm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMember2023-12-310001413329pm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMembercountry:CA2023-12-310001413329country:KRpm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMember2023-12-310001413329country:NGpm:HealthCareCostRecoveryActionsMemberpm:CombustibleProductsMember2023-12-310001413329us-gaap:PendingLitigationMembercountry:NGpm:HealthCareCostRecoveryActionsMemberpm:TheAttorneyGeneralOfLagosStateMember2008-03-132008-03-130001413329us-gaap:PendingLitigationMemberpm:TheAttorneyGeneralOfKanoStateMembercountry:NGpm:HealthCareCostRecoveryActionsMember2007-05-092007-05-090001413329us-gaap:PendingLitigationMembercountry:NGpm:HealthCareCostRecoveryActionsMemberpm:TheAttorneyGeneralOfGombeStateMember2008-10-172008-10-170001413329us-gaap:PendingLitigationMembercountry:NGpm:HealthCareCostRecoveryActionsMemberpm:TheAttorneyGeneralOfOyoStateMember2007-05-252007-05-250001413329us-gaap:PendingLitigationMembercountry:NGpm:TheAttorneyGeneralOfOgunStateMemberpm:HealthCareCostRecoveryActionsMember2008-02-262008-02-260001413329country:KRpm:HealthCareCostRecoveryActionsMember2014-04-142014-04-14pm:patient0001413329country:ITpm:IndividualLabelRelatedCasesMemberpm:CombustibleProductsMember2023-12-310001413329pm:IndividualLabelRelatedCasesMemberpm:CombustibleProductsMembercountry:CL2023-12-310001413329pm:PublicCivilActionsMemberpm:CombustibleProductsMembercountry:VE2023-12-310001413329us-gaap:PendingLitigationMemberpm:TheDepartmentofSpecialInvestigationsoftheGovernmentofThailandMemberpm:OtherLitigationMembercountry:TH2016-01-182016-01-18pm:defendantiso4217:THB0001413329pm:TheDepartmentofSpecialInvestigationsoftheGovernmentofThailandMemberpm:OtherLitigationMembercountry:TH2019-11-012019-11-300001413329pm:TheDepartmentofSpecialInvestigationsoftheGovernmentofThailandMemberpm:OtherLitigationMembercountry:TH2022-06-012022-06-010001413329pm:TheDepartmentofSpecialInvestigationsoftheGovernmentofThailandMemberpm:OtherLitigationMembercountry:TH2017-01-262017-01-260001413329pm:TheDepartmentofSpecialInvestigationsoftheGovernmentofThailandMemberpm:OtherLitigationMembercountry:TH2020-03-012020-03-310001413329country:KRpm:TheSouthKoreanBoardOfAuditAndInspectionMemberpm:OtherLitigationMember2016-11-012017-03-31iso4217:KRW0001413329country:KRpm:TheSouthKoreanBoardOfAuditAndInspectionMemberpm:OtherLitigationMember2016-11-012016-12-310001413329country:KRpm:TheSouthKoreanBoardOfAuditAndInspectionMemberpm:OtherLitigationMember2018-01-012018-03-310001413329country:KRpm:TheSouthKoreanBoardOfAuditAndInspectionMemberpm:OtherLitigationMember2020-01-012020-01-310001413329country:KRpm:TheSouthKoreanBoardOfAuditAndInspectionMemberpm:OtherLitigationMember2023-07-202023-07-20pm:panelpm:hearing0001413329country:KRpm:TheSouthKoreanBoardOfAuditAndInspectionMemberpm:OtherLitigationMember2020-06-012020-06-3000014133292017-12-31pm:lawsuit00014133292020-03-03pm:study00014133292021-05-142021-05-14pm:patent00014133292021-09-212021-09-210001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2022-06-152022-06-150001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2021-12-312021-12-310001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2021-12-31pm:product0001413329pm:BritishAmericanTobaccoPlcMemberpm:OtherLitigationMember2023-03-300001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2023-05-100001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2023-05-102023-05-100001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2022-01-112022-01-110001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2022-03-302022-03-300001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2023-07-132023-07-130001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2023-09-142023-09-140001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2022-07-212022-07-210001413329pm:PMGmbHAndPMPSAMemberpm:OtherLitigationMember2021-06-012021-06-300001413329pm:BritishAmericanTobaccoPlcMemberpm:OtherLitigationMember2020-10-012020-10-310001413329pm:BritishAmericanTobaccoPlcMemberpm:OtherLitigationMember2022-12-232022-12-230001413329pm:PhilipMorrisProductsSAMemberpm:OtherLitigationMember2021-07-012021-07-310001413329pm:CanadianGovernmentMember2020-10-170001413329pm:CanadianGovernmentMember2022-03-310001413329us-gaap:FinancialGuaranteeMemberpm:MedicagoIncMember2022-08-31pm:arrangement0001413329pm:EVaporProductsManufacturingOptimizationMember2023-01-012023-03-310001413329us-gaap:ContractTerminationMemberpm:EVaporProductsManufacturingOptimizationMember2023-01-012023-03-310001413329pm:EVaporProductsManufacturingOptimizationMemberpm:FinanceLeaseTerminationMember2023-01-012023-03-310001413329pm:EVaporProductsManufacturingOptimizationMemberpm:AssetImpairmentMember2023-01-012023-03-310001413329us-gaap:ContractTerminationMemberpm:PhilipMorrisKoreaMember2022-01-012022-12-31pm:phase0001413329us-gaap:EmployeeSeveranceMember2021-01-012021-12-310001413329pm:OrganizationalDesignOptimizationMember2021-12-31pm:employee0001413329us-gaap:EmployeeSeveranceMemberpm:OrganizationalDesignOptimizationMember2021-12-310001413329pm:OrganizationalDesignOptimizationMemberpm:AssetImpairmentMember2021-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2023-01-012023-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:EuropeSegmentMember2021-01-012021-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:SSEACISMEASegmentMember2023-01-012023-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:SSEACISMEASegmentMember2021-01-012021-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMember2023-01-012023-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMember2021-01-012021-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2023-01-012023-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:AmericasSegmentMember2021-01-012021-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMember2023-01-012023-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMember2021-01-012021-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:WellnessAndHealthcareSegmentMember2023-01-012023-12-310001413329us-gaap:EmployeeSeveranceMemberus-gaap:OperatingSegmentsMemberpm:WellnessAndHealthcareSegmentMember2021-01-012021-12-310001413329us-gaap:EmployeeSeveranceMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:EuropeSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:EuropeSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:SSEACISMEASegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:SSEACISMEASegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberus-gaap:ContractTerminationMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberus-gaap:ContractTerminationMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:AmericasSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:AmericasSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:SwedishMatchABSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:SwedishMatchABSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:WellnessAndHealthcareSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberus-gaap:ContractTerminationMemberpm:WellnessAndHealthcareSegmentMember2021-01-012021-12-310001413329us-gaap:ContractTerminationMember2023-01-012023-12-310001413329us-gaap:ContractTerminationMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:EuropeSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:EuropeSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:SSEACISMEASegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:SSEACISMEASegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:AssetImpairmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:EAAUPMIDFSegmentMemberpm:AssetImpairmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:AmericasSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:AmericasSegmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMemberpm:AssetImpairmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:SwedishMatchABSegmentMemberpm:AssetImpairmentMember2021-01-012021-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:WellnessAndHealthcareSegmentMember2023-01-012023-12-310001413329us-gaap:OperatingSegmentsMemberpm:AssetImpairmentMemberpm:WellnessAndHealthcareSegmentMember2021-01-012021-12-310001413329pm:AssetImpairmentMember2023-01-012023-12-310001413329pm:AssetImpairmentMember2021-01-012021-12-310001413329srt:MinimumMember2023-12-310001413329srt:MaximumMember2023-12-310001413329us-gaap:MachineryAndEquipmentMember2023-12-310001413329us-gaap:MachineryAndEquipmentMember2022-12-310001413329us-gaap:OtherAssetsMember2023-12-310001413329us-gaap:OtherAssetsMember2022-12-310001413329pm:SuppliersUsingSupplyChainFinancingProgramMember2023-12-310001413329pm:SuppliersUsingSupplyChainFinancingProgramMember2022-12-3100014133292023-10-012023-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
|
|
|
|
|
| ☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2023
OR
|
|
|
|
|
|
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-33708
PHILIP MORRIS INTERNATIONAL INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
| Virginia |
|
13-3435103 |
(State or other jurisdiction of
incorporation or organization) |
|
(I.R.S. Employer
Identification No.) |
|
|
677 Washington Blvd, Suite 1100
|
|
|
| Stamford |
|
|
| Connecticut |
|
06901 |
| (Address of principal executive offices) |
|
(Zip Code) |
203-905-2410
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, no par value |
|
PM |
|
New York Stock Exchange |
| 2.875% Notes due 2024 |
|
PM24 |
|
New York Stock Exchange |
| 2.875% Notes due 2024 |
|
PM24C |
|
New York Stock Exchange |
| 0.625% Notes due 2024 |
|
PM24B |
|
New York Stock Exchange |
| 3.250% Notes due 2024 |
|
PM24A |
|
New York Stock Exchange |
| 2.750% Notes due 2025 |
|
PM25 |
|
New York Stock Exchange |
| 3.375% Notes due 2025 |
|
PM25A |
|
New York Stock Exchange |
| 2.750% Notes due 2026 |
|
PM26A |
|
New York Stock Exchange |
| 2.875% Notes due 2026 |
|
PM26 |
|
New York Stock Exchange |
| 0.125% Notes due 2026 |
|
PM26B |
|
New York Stock Exchange |
| 3.125% Notes due 2027 |
|
PM27 |
|
New York Stock Exchange |
| 3.125% Notes due 2028 |
|
PM28 |
|
New York Stock Exchange |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| 2.875% Notes due 2029 |
|
PM29 |
|
New York Stock Exchange |
| 3.375% Notes due 2029 |
|
PM29A |
|
New York Stock Exchange |
| 0.800% Notes due 2031 |
|
PM31 |
|
New York Stock Exchange |
| 3.125% Notes due 2033 |
|
PM33 |
|
New York Stock Exchange |
| 2.000% Notes due 2036 |
|
PM36 |
|
New York Stock Exchange |
| 1.875% Notes due 2037 |
|
PM37A |
|
New York Stock Exchange |
| 6.375% Notes due 2038 |
|
PM38 |
|
New York Stock Exchange |
| 1.450% Notes due 2039 |
|
PM39 |
|
New York Stock Exchange |
| 4.375% Notes due 2041 |
|
PM41 |
|
New York Stock Exchange |
| 4.500% Notes due 2042 |
|
PM42 |
|
New York Stock Exchange |
| 3.875% Notes due 2042 |
|
PM42A |
|
New York Stock Exchange |
| 4.125% Notes due 2043 |
|
PM43 |
|
New York Stock Exchange |
| 4.875% Notes due 2043 |
|
PM43A |
|
New York Stock Exchange |
| 4.250% Notes due 2044 |
|
PM44 |
|
New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☑ Accelerated filer ☐
Non-accelerated filer ☐ Smaller reporting company ☐
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☑
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
As of June 30, 2023, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $152 billion based on the closing sale price of the common stock as reported on the New York Stock Exchange.
|
|
|
|
|
|
|
|
|
|
|
|
| Class |
|
Outstanding at |
January 31, 2024 |
Common Stock,
no par value |
|
1,552,456,597 |
|
shares |
DOCUMENTS INCORPORATED BY REFERENCE
|
|
|
|
|
|
|
Parts Into Which Incorporated |
| Portions of the registrant’s definitive proxy statement for use in connection with its annual meeting of shareholders to be held on May 8, 2024, to be filed with the Securities and Exchange Commission on or about March 28, 2024. |
Part III |
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Page |
PART I |
|
| Item 1. |
|
Business |
|
| Item 1A. |
|
Risk Factors |
|
| Item 1B. |
|
Unresolved Staff Comments |
|
| Item 1C. |
|
Cybersecurity |
|
| Item 2. |
|
Properties |
|
| Item 3. |
|
Legal Proceedings |
|
| Item 4. |
|
Mine Safety Disclosures |
|
|
|
|
|
PART II |
|
| Item 5. |
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
|
| Item 6. |
|
|
|
| Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
| Item 7A. |
|
Quantitative and Qualitative Disclosures About Market Risk |
|
| Item 8. |
|
Financial Statements and Supplementary Data |
|
| Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
| Item 9A. |
|
Controls and Procedures |
|
| Item 9B. |
|
Other Information |
|
| Item 9C. |
|
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
|
|
|
|
|
PART III |
|
| Item 10. |
|
Directors, Executive Officers and Corporate Governance |
|
| Item 11. |
|
Executive Compensation |
|
| Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
| Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
| Item 14. |
|
Principal Accounting Fees and Services |
|
|
|
|
|
PART IV |
| Item 15. |
|
Exhibits and Financial Statement Schedules |
|
|
|
|
|
Signatures |
|
In this report, “PMI,” “we,” “us” and “our” refers to Philip Morris International Inc. and its subsidiaries.
Trademarks and service marks in this report are the registered property of, or licensed by, the subsidiaries of Philip Morris International Inc. and are italicized.
PART I
Item 1.Business.
General Development of Business
General
Philip Morris International Inc. is a Virginia holding company incorporated in 1987. We are a leading international tobacco company, actively delivering a smoke-free future and evolving our portfolio for the long term to include products outside of the tobacco and nicotine sector. Our current product portfolio primarily consists of cigarettes and smoke-free products, which include heat-not-burn, vapor, and oral nicotine products. Since 2008, we have invested $12.5 billion to develop, scientifically substantiate and commercialize innovative smoke-free products for adults who would otherwise continue to smoke, with the goal of completely ending the sale of cigarettes. This investment includes the building of world-class scientific assessment capabilities, notably in the areas of pre-clinical systems toxicology, clinical and behavioral research, as well as post-market studies. In November 2022, we acquired Swedish Match AB ("Swedish Match") – a leader in oral nicotine delivery – creating a global smoke-free combination led by the companies’ IQOS and ZYN brands. The U.S. Food and Drug Administration (the "FDA") has authorized versions of our IQOS Platform 1 devices and consumables, and Swedish Match's General snus, as Modified Risk Tobacco Products ("MRTPs"). We describe the MRTP orders in more detail in the "Business Environment" section of Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
In March 2008, we became a U.S. public company listed on the New York Stock Exchange and subject to the rules of the U.S. Securities and Exchange Commission (the "SEC").
In September 2021, we laid the foundation for our long-term growth ambitions beyond nicotine in wellness and healthcare, including the milestone acquisitions of Vectura Group plc and Fertin Pharma A/S, which provide essential capabilities for future product development. Now, through our Vectura Fertin Pharma business, with a strong foundation and significant expertise in life sciences, we aim to expand into wellness and healthcare areas.
Through our acquisition of Swedish Match, we acquired a market leader in oral nicotine delivery with a significant presence in the United States market. The Swedish Match acquisition was a key milestone in PMI’s transformation to becoming a smoke-free company. Our consolidated statements of earnings for the year ended December 31, 2022, include the results of operations of Swedish Match from November 11, 2022 (acquisition date) to December 31, 2022. The operating results of Swedish Match are included in a separate segment.
In the fourth quarter of 2022, we also completed an agreement with Altria Group, Inc. to end our commercial relationship in the U.S. covering IQOS as of April 30, 2024. Thereafter, PMI will have the full rights to commercialize IQOS in the U.S.
For further details of our 2021 and 2022 acquisitions, see Item 8, Note 3. Acquisitions and Note 13. Segment Reporting, and for additional details concerning the agreement with Altria, see Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation - Operating Results by Business Segment - Business Environment.
Smoke-free products ("SFPs") is the term we primarily use to refer to all of our products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral nicotine. In addition, SFPs include wellness and healthcare products, as well as consumer accessories such as lighters and matches.
Reduced-risk products ("RRPs") is the term we use to refer to products that present, are likely to present, or have the potential to present less risk of harm to smokers who switch to these products versus continuing to smoke. We have a range of RRPs in various stages of development, scientific assessment and commercialization. Our RRPs are smoke-free products that contain and/or generate far lower quantities of harmful and potentially harmful constituents than found in cigarette smoke.
Our RRPs and commercial activities for these products are designed for, and directed toward, current adult smokers and users of nicotine-containing products. We put significant effort to restrict access of our products from non-smokers and youth. We believe regulation must include measures designed to prevent youth initiation; and we also support and engage with relevant authorities to seek sensible regulation of flavors, mandated health warnings and minimum age laws.
Our IQOS smoke-free product brand portfolio includes heated tobacco and nicotine-containing vapor products. Our leading smoke-free platform ("Platform 1") uses a precisely controlled heating device into which a specially designed and proprietary tobacco unit is inserted and heated to generate an aerosol. Heated tobacco units ("HTU") is the term we use to refer to heated tobacco consumables, which include our BLENDS, DELIA, HEETS, HEETS Creations, HEETS Dimensions (defined collectively as "HEETS"), Marlboro HeatSticks, SENTIA, TEREA, TEREA CRAFTED, and TEREA Dimensions, as well as the KT&G-licensed brands, Fiit and Miix (outside of South Korea).
HTU's also include zero tobacco heat-not-burn consumables (LEVIA). Platform 1 was first introduced in Nagoya, Japan, in 2014. As of December 31, 2023, our smoke-free products were available for sale in 84 markets.
At the time of our acquisition of Swedish Match, it already had a leading nicotine pouch franchise in the U.S. under the ZYN brand name. The Swedish Match product portfolio is complementary to our existing smoke-free portfolio, permitting us to bring together a leading oral nicotine product with the leading heat-not-burn product. By joining forces with Swedish Match, we expect to accelerate the achievement of our joint smoke-free ambitions, switching more adults who would otherwise continue to smoke to better alternatives faster than either company could achieve separately.
Our cigarettes are sold in approximately 175 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands and is led by Marlboro, the world’s best-selling international cigarette, which accounted for approximately 39% of our total 2023 cigarette shipment volume. Marlboro is complemented in the premium-price category by Parliament. Our other leading international cigarette brands are Chesterfield, L&M, and Philip Morris. These five international cigarette brands contributed approximately 79% of our cigarette shipment volume in 2023. We also own a number of important local cigarette brands, such as Dji Sam Soe and Sampoerna A in Indonesia, and Fortune and Jackpot in the Philippines.
Source of Funds — Dividends
We are a legal entity separate and distinct from our direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions that are otherwise compliant with law.
Description of Business
To further support the growth of our smoke-free business, reinforce consumer centricity, and increase the speed of innovation and deployment, in January 2023, we rearranged our operations in four geographical segments, down from the previous six, as follows:
•Europe Region is headquartered in Lausanne, Switzerland, and covers all the European Union countries, Switzerland, the United Kingdom, and also Ukraine, Moldova and Southeast Europe;
•South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA") is headquartered in Dubai, United Arab Emirates. It covers South and Southeast Asia, the African continent, the Middle East, Turkey, as well as Israel, Central Asia, Caucasus and Russia;
•East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF") is headquartered in Hong Kong, and includes the consolidation of our international duty free business with East Asia & Australia; and
•Americas Region is headquartered in Stamford, Connecticut, and covers the United States, Canada and Latin America.
The operations of Swedish Match, which reflects our fourth quarter 2022 acquisition of the company, and our Wellness and Healthcare segment remained unchanged. The Wellness and Healthcare ("W&H") segment includes the operating results of our Wellness and Healthcare business, Vectura Fertin Pharma.
Following the combination and the progress in 2023 toward the integration of the Swedish Match business into the existing PMI regional segment structure, we will update our segment reporting by including Swedish Match results in the four existing geographical segments. As of the first quarter of 2024, we will report on this basis.
Our total shipment volume, including cigarettes and heated tobacco units, increased by 1.0% in 2023 to 738.2 billion units, with shipment volume of heated tobacco units reaching 125.3 billion units in 2023, up from 109.2 billion units in 2022. Shipment volume of our principal cigarette brand, Marlboro, decreased by 1.9% in 2023.
References in this Form 10-K to total international market, defined as worldwide cigarette and heated tobacco unit volume, excluding the United States, total industry (or total market) and market shares, are our estimates for tax-paid products based on the latest available data from a number of internal and external sources, and may, in defined instances, exclude China and/or our duty free business.
Unless otherwise stated, references to total industry (or total market), our shipment volume and our market share performance reflect cigarettes and heated tobacco units.
Key data regarding total market and market share were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
2022 |
2021 |
| Total Market, billion units (excluding China and the U.S.) |
2,580 |
2,622 |
2,620 |
|
|
|
|
Total International Market Share (1) |
28.3% |
27.7% |
27.2% |
| Cigarettes |
23.7% |
23.6% |
23.7% |
| HTU |
4.7% |
4.1% |
3.5% |
|
|
|
|
PMI Cigarette over Cigarette Market Share (2) |
25.2% |
25.0% |
24.8% |
Marlboro Cigarette over Cigarette Market Share (3) |
9.8% |
9.8% |
9.5% |
|
|
|
|
|
|
|
|
| (1) Defined as PMI's cigarette and heated tobacco unit in-market sales volume as a percentage of total industry cigarette and heated tobacco unit sales volume, excluding China and the U.S., including cigarillos in Japan |
| (2) Defined as PMI's cigarette in-market sales volume as a percentage of total industry cigarette sales volume, excluding China and the U.S., including cigarillos in Japan |
(3) Defined as Marlboro's cigarette in-market sales volume as a percentage of total industry cigarette sales volume, excluding China and the U.S., including cigarillos in Japan |
| Note: Sum of share of market by product categories might not foot to total due to roundings |
We have a market share of at least 15% in approximately 100 markets, including Algeria, Argentina, Australia, Austria, Belgium, Brazil, the Czech Republic, Egypt, France, Germany, Greece, Hong Kong, Hungary, Indonesia, Israel, Italy, Japan, Kazakhstan, Kuwait, Mexico, the Netherlands, the Philippines, Poland, Portugal, Romania, Russia, Saudi Arabia, the Slovak Republic, South Korea, Spain, Switzerland, Turkey and Ukraine.
Distribution & Sales
Our main types of distribution and sales are tailored to the characteristics of each market and are often used simultaneously:
•Direct sales and distribution, where we sell directly to the retailers;
•Distribution through independent distributors that often distribute other fast-moving consumer goods and are responsible for distribution in a particular market;
•Exclusive zonified distribution, where the dedicated multicategory product distributors are assigned to exclusive territories within a market;
•Distribution through national or regional wholesalers that then supply the retail trade;
•Our own e-commerce infrastructure for product sales to trade partners and to consumers; and
•Our own brand retail infrastructure for our RRPs and accessories for sales to consumers.
Competition
We are subject to highly competitive conditions in all aspects of our business. We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, R&D, innovation, packaging, customer service, marketing, advertising and retail price and, increasingly, adult smoker willingness to convert to our RRPs.
The competitive environment and our competitive position can be significantly influenced by weak economic conditions; erosion of consumer confidence; competitors' introduction of lower-price products or innovative products; novel products which given their taste characteristics may be more commercially successful; higher product taxes; higher absolute prices and larger gaps between retail price categories; and product regulation that diminishes the ability to differentiate tobacco products, restricts adult consumer access to truthful and non-misleading information about our RRPs, or disproportionately impacts the commercialization of our products in relation to our competitors.
Competitors in our industry include British American Tobacco plc, Japan Tobacco Inc., Imperial Brands plc, new market entrants, particularly with respect to innovative products, several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, Egypt, China, Taiwan, Thailand and Vietnam. Some competitors have different profit, volume and regulatory objectives, and some international competitors may be less susceptible than PMI to changes in currency exchange rates. Certain new market entrants in the non-combustible product category may alienate consumers from innovative products through inappropriate marketing campaigns, messaging and inferior product satisfaction, and without scientific substantiation based on appropriate R&D protocols and standards. The growing use of digital media could increase the speed and extent of the dissemination of inaccurate and misleading information about our RRPs, all of which could have a material adverse effect on our profitability and results of operations.
Procurement and Raw Materials
We purchase tobacco leaf of various types, grades and styles throughout the world, mostly through independent international tobacco suppliers. In 2023, we also contracted directly with farmers in several countries, including Argentina, Brazil, Italy, Pakistan and Poland. In 2023, direct sourcing from farmers represented approximately 18% of PMI’s global leaf requirements. The largest supplies of tobacco leaf are sourced from Argentina, Brazil, China, India, Italy, Indonesia (mostly for domestic use in kretek products), Malawi, Mozambique, the Philippines, Turkey and the United States. We believe that there is an adequate supply of tobacco leaf in the world markets to satisfy our current and anticipated production requirements.
Given the global reach of our value chain, properly managing land and water resources and utilizing a geographically diversified sourcing strategy for agricultural products are priorities as we seek to increase the resilience of our production systems and minimize operational risks. We conduct a global water risk assessment annually in tobacco-growing regions to identify potential hotspots for physical water risks that require adaptation measures. Our water stewardship strategy includes guidance for applying a landscape approach to water optimization projects, protecting natural resources and recharge areas, and improving the efficiency of irrigation systems to integrate better farm water management. These business practices are intended to mitigate the risk that climate change could influence weather patterns in ways that negatively impact the quality or cost of the agricultural products used to manufacture our products.
In addition to tobacco leaf, we purchase a wide variety of direct materials from a total of approximately 360 suppliers. In 2023, our top ten suppliers of direct materials combined represented approximately 60% of our total direct materials purchases. The four most significant direct materials that we purchase are printed paper board used in packaging, acetate tow used in filter making, fine paper used in the manufacturing of cigarettes and heated tobacco units, and susceptors used for the TEREA heated tobacco units. In addition, the adequate supply and procurement of cloves are of particular importance to our Indonesian business.
We discuss the details of our supply chain for our RRPs in Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations of this Annual Report on Form 10-K (“Item 7”) in Business Environment—Reduced-Risk Products.
Business Environment
Information called for by this Item is hereby incorporated by reference to the paragraphs in Item 7, Business Environment to this Annual Report on Form 10-K.
Other Matters
Customers
As described in more detail in “Distribution & Sales” above, in many of our markets we sell our products to distributors. In 2023, sales to a distributor in the Europe Region and a distributor in the EA, AU & PMI DF Region each amounted to 10 percent or more of our consolidated net revenues. See Item 8, Note 13. Segment Reporting for more information. We believe that none of our business segments is dependent upon a single customer or a few customers, the loss of which would have a material adverse effect on our consolidated results of operations. In some of our markets, particularly in the Europe, SSEA, CIS & MEA, and EA, AU & PMI DF Regions, a loss of a distributor may result in a temporary market disruption.
Human Capital
Our Workforce. At December 31, 2023, including Swedish Match's employees, we employed approximately 82,700 people worldwide of more than 130 different nationalities, including full-time, temporary and part-time staff. Our businesses are subject to a number of laws and regulations relating to our relationship with our employees. Generally, these laws and regulations are specific to the location of each business. We engage with legally recognized employee representative bodies and we have collective bargaining agreements in several of the countries in which we operate. In addition, in accordance with European Union requirements, we have established a European Works Council composed of management and elected members of our workforce. We believe we maintain good relations with our employees and their representative organizations.
Our Internal Transformation. To be successful in our transformation to a smoke-free future, we must continue transforming our culture and ways of working, align our talent with our business needs, successfully integrate acquired businesses and innovate to become a truly consumer-centric business. To achieve our strategic goals, we need to attract, retain and motivate the best global talent, talent that is diverse and has the right degree of experience, competencies and skills. Therefore, we strive to ensure the development of our existing talent while recruiting those with expertise in areas that are relatively new to us such as digital and technical solutions. Our compensation and benefit programs are set at the levels that we believe are necessary to attract the best talent and remain competitive with other consumer product companies.
Oversight and Management. Our Board of Directors (the "Board") provides oversight of various matters pertaining to our workforce. The Compensation and Leadership Development Committee of the Board is responsible for executive compensation matters and oversight of the risks and programs related to talent management. Our Code of Conduct highlights our commitment to ethical business conduct and honesty, respect, and fairness.
Diversity, Equity, and Inclusion. At PMI, we believe that a diverse workforce and an inclusive culture are strategic priorities that help fuel innovation and business success. We aspire to maintain a minimum of 40% female representation globally in management positions in most of our business functions and regions, and to have at least 35% of senior positions held by women globally by 2025. Given the size and continued growth of our business in Asia, it is also our aspiration to have at least 20% of senior roles held by Asian talent globally and at least 60% representation of local talent in our market management teams by 2025.
We were the first multinational company to receive a global EQUAL-SALARY certification from the EQUAL-SALARY Foundation in 2019. In 2022, we were re-certified as a global EQUAL-SALARY organization for the second time, verifying that PMI continues to pay female and male employees equally for equal work everywhere where we operate. This achievement is an important milestone toward the creation of a more diverse and inclusive workplace and the continuation of our reputation as a top employer. In 2023, we completed another year of market level reviews with success and maintained our global certification.
Creation of employee resource groups ("ERGs") was another important priority to drive further inclusion at PMI. Our ERGs are open to participation by all employees and we believe they help build an enhanced sense of belonging, visibility, and greater understanding of different experiences and dimensions of diversity in our company. Currently, we have established global ERGs for race, ethnicity and cultural diversity, LGBTQ+, gender, parents and caregivers, and disability dimensions concerning our employees. Each global ERG is sponsored by a member of the PMI senior leadership team to reinforce the fact that our strong commitment to diversity, equity and inclusion comes from the top. In 2023, we continued to focus on the growth of our global ERGs, and to expand them locally, to be able to meet the specific needs of different markets and regions.
Government Regulation
As a company with global operations in a heavily regulated industry, we are subject to multiple laws and regulations of the jurisdictions in which we operate. We discuss our regulatory environment in Item 7, Business Environment.
The regulatory landscape related to sustainability matters is rapidly evolving. We closely monitor these developments and implement initiatives addressing PMI’s priorities in line with our sustainability strategy. In particular, we are subject to international, national and local environmental laws and regulations in the countries in which we do business. We have specific programs across our business units designed to meet applicable environmental compliance requirements and reduce our carbon footprint, wastage, as well as water and energy consumption. We report externally about our climate change mitigation strategy, together with associated targets and results in reducing our carbon footprint, through CDP (formerly known as the Carbon Disclosure Project), the leading international non-governmental organization assessing the work of thousands of companies worldwide in the area of environmental impact, including climate change.
Our environmental and occupational health and safety management program includes policies, standard practices and procedures at all our manufacturing centers. Furthermore, we have engaged an external certification body to validate the effectiveness of this management program at our manufacturing centers around the world, in accordance with internationally recognized standards for safety and environmental management. Our subsidiaries expect to continue to make investments in order to drive improved performance and maintain compliance with environmental laws and regulations. We assess and report to our management the compliance status of all our legal entities on a regular basis. Based on current regulations, the management and controls we have in place and our review of climate change risks (both physical and regulatory), environmental expenditures have not had, and are not expected to have, a material adverse effect on our consolidated results of operations, capital expenditures, financial position, earnings or competitive position.
Based on current regulations, compliance with government regulations, including environmental regulations, has not had, and is not expected to have a material adverse effect on our results of operations, capital expenditures, financial position, earnings, or competitive position.
As discussed in more detail in Item 1A. Risk Factors, our financial results could be significantly affected by regulatory initiatives that could result in a significant decrease in demand for our brands or by climate-related regulations that increase our cost of operation. More specifically, any regulatory requirements that lead to a commoditization of tobacco products or impede adult consumers' ability to convert to our RRPs, as well as any significant increase in the cost of complying with new regulatory requirements could have a material adverse effect on our financial results. Further, tightened climate-related regulation may lead to additional carbon taxation or energy price increases impacting our cost of operation. These shifts in regulation and other market trends could, amongst others, impact current deforestation rates. Availability of deforestation-free materials could be impacted by increased demand for alternative energy sources and low-carbon fuels, such as biomass, which could result in increased sourcing costs.
We discuss additional information regarding regulatory matters relating to climate change in Item 7, Climate Change Laws and Regulations.
Information About Our Executive Officers
The disclosure regarding executive officers is hereby incorporated by reference to the discussion under the heading “Information about our Executive Officers as of February 8, 2024” in Part III, Item 10. Directors, Executive Officers and Corporate Governance of this Annual Report on Form 10-K (“Item 10”).
Intellectual Property
Our trademarks are valuable assets, and their protection and reputation are essential to us. We own the trademark rights to all of our principal brands, including Marlboro, HEETS, IQOS, IQOS ILUMA, TEREA, and ZYN, or have the right to use them in all countries in which these brands are advertised or sold.
In addition, we have a large number of granted patents and pending patent applications worldwide. Our patent portfolio, as a whole, is material to our business. However, no one patent, or group of related patents, is material to us. We also have registered industrial designs, as well as unregistered proprietary trade secrets, technology, know-how, processes and other unregistered intellectual property rights.
Effective January 1, 2008, PMI entered into an Intellectual Property Agreement with Philip Morris USA Inc., a wholly owned subsidiary of Altria Group, Inc. (“PM USA”). The Intellectual Property Agreement allocates ownership of jointly funded intellectual property as follows:
•PMI owns all rights to jointly funded intellectual property outside the United States, its territories and possessions; and
•PM USA owns all rights to jointly funded intellectual property in the United States, its territories and possessions.
The parties agreed to submit disputes under the Intellectual Property Agreement first to negotiation between senior executives and then to binding arbitration.
An agreement was reached with PM USA in 2022 relating to IQOS commercialization rights in the U.S. including, among other things, an agreement relating to intellectual property rights consistent with the commercialization rights for relevant IQOS products.
Seasonality
Our business segments are not significantly affected by seasonality, although in certain markets cigarette consumption may be lower during the winter months due to the cold weather and may rise during the summer months due to outdoor use, longer daylight, and tourism. However, we typically experience slower RRP adult user growth in the third quarter and an acceleration in the fourth quarter of each year due to seasonal influences.
Available Information
We are required to file with the SEC annual, quarterly and current reports, proxy statements and other information required by the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The SEC maintains an Internet website at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, from which investors can electronically access our SEC filings.
We make available free of charge on, or through, our website at www.pmi.com our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Investors can access our filings with the SEC by visiting www.pmi.com.
The information on our website is not, and shall not be deemed to be, a part of this report or incorporated into any other filings we make with the SEC.
Item 1A. Risk Factors.
The following risk factors should be read carefully in connection with evaluating our business and the forward-looking statements contained in this Annual Report on Form 10-K. Any of the following risks could materially adversely affect our business, our operating results, our financial condition and the actual outcome of matters as to which forward-looking statements are made in this Annual Report on Form 10-K.
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in this Annual Report on Form 10-K and other filings with the SEC, in reports to stockholders and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "aspires," "estimates," "intends," "projects," "aims," "goals," "targets," "forecasts" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Our RRPs constitute a relatively new product category that is less predictable than our mature cigarette business. Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities.
In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements. We elaborate on these and other risks we face throughout this document, particularly in Item 7, Business Environment. You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
Overall Business Risks
We may be unsuccessful in our attempts to introduce, commercialize, and grow reduced-risk products in existing and new markets, and regulators may prohibit or significantly restrict the commercialization of these products or the communication of scientifically substantiated information and claims.
Our key strategic priorities are to: (i) continue developing and commercializing products that present less risk of harm to adult smokers who switch to reduced-risk products versus continued cigarette smoking; and (ii) encourage and educate current adult smokers who would otherwise continue to smoke cigarettes to switch to those products. For our efforts to be successful, we must:
|
|
|
|
|
|
| • |
develop RRPs that adult smokers who would otherwise continue to smoke cigarettes find to be satisfying alternatives to smoking; |
| • |
for those adult smokers, our goal is to offer RRPs with a scientifically substantiated risk-reduction profile that approaches as closely as possible the risk-reduction profile associated with smoking cessation; |
| • |
substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on scientific evidence of the highest standard that is made available for scrutiny and review by external independent scientists and relevant regulatory bodies; and |
| • |
advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs, including the communication of scientifically substantiated information to enable adult smokers to make better choices. |
We might not succeed in our effort to introduce, commercialize, and grow our RRPs in existing and new markets. If we do not succeed, but others do, or if heat-not-burn products are inequitably regulated compared to other RRP categories without regard to the totality of the scientific evidence available for such products, we may be at a competitive disadvantage. In addition, actions of some market participants, such as the inappropriate marketing of e-vapor products to youth, as well as alleged health consequences associated with the use of certain e-vapor products, may unfavorably impact public opinion and/or mischaracterize the health consequences of all e-vapor products or other RRPs to consumers, regulators and policy makers without regard to the totality of scientific evidence available for specific products. This may impede our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs. We cannot predict the extent to which regulators will permit the sale and/or marketing of RRPs. Regulatory restrictions could limit the success of our RRPs.
The World Health Organization (the "WHO") study group on tobacco product regulation published their eighth report on the scientific basis of tobacco product regulation in May 2021. The report is based on a review of scientific evidence related to novel and emerging nicotine and tobacco products, such as electronic nicotine delivery systems ("ENDS"), electronic non-nicotine delivery systems and HTPs. The report concludes by making a number of policy recommendations on HTPs and ENDS that, if implemented, could restrict both the availability of these products and the access to accurate information about them. In August 2021, the FCTC Secretariat published two reports on novel and emerging tobacco products to the Ninth Session of the CoP of the FCTC, which are not materially different from the WHO study group report. Substantive decisions based on these reports were deferred to the Tenth Session of the CoP ("CoP 10"), which was scheduled for November 2023, but has been postponed to February 2024. In August 2023, the WHO Study Group on Tobacco Products Regulation ("TobReg") issued its ninth report, including recommendations on nicotine pouches, which were in line with previous policy recommendations on regulating flavors in tobacco and nicotine products. It is not possible to predict whether or to what extent these developments will be reflected in decisions adopted at CoP 10, following deliberations. In December 2023, the WHO issued a white paper on electronic cigarettes. While acknowledging that long-term health effects of using e-cigarettes are not fully understood, the WHO calls on countries to ban or strictly regulate these products in order to prevent youth uptake and counter nicotine addiction.
The WHO’s reports are not binding on the WHO Member States or on parties to the FCTC, and so it is not possible to predict the extent to which any proposals it adopts will be implemented. However, the WHO proposals could lead to restrictions on the availability of certain of our RRPs and access to accurate information about them in one or more of our markets, which could have a material adverse effect on our results of operations.
Additionally, any claims, regardless of merit, challenging our research and clinical data available to date, may impact the development of science-based regulatory frameworks for the commercialization of the RRP category and the commercialization of the RRP category in general.
Our RRPs and commercial activities for these products are designed for, and directed toward, current adult smokers and users of nicotine-containing products. We put significant effort to restrict access of our products from non-smokers and youth. Despite our efforts, technological, operational, regulatory and/or commercial developments might impact the implementation or effectiveness of youth access prevention mechanisms and surrounding infrastructure. If there is significant usage, whether actual or perceived, of our products or competitive products among youth or non-smokers, even in situations over which we have no control, our reputation and credibility may suffer, the regulatory approach to our products may become more restrictive, and our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs may be significantly impacted.
Moreover, the FDA’s premarket tobacco product and modified risk tobacco product authorizations of two versions of our Platform 1 product are subject to strict marketing, reporting and other requirements. Although we have received these authorizations from the FDA, there is no guarantee that the product will remain authorized for sale in the U.S., or that new versions of the product (Platform 1 or other smoke-free platforms) will receive necessary authorizations, particularly if there is a significant uptake in youth or non-smoker initiation. The commercialization of our products in the United States is dependent on successfully managing compliance with federal, state, and local laws, regulations, legal agreements, and related interpretations. Failure to successfully manage compliance and to resolve any disputes that may arise regarding the application of legal and administrative requirements to our products could negatively impact the timing, manner, or success of our commercialization plans in the United States.
Premarket tobacco applications for certain ZYN products, which are currently marketed in the U.S., were submitted in March 2020. The FDA has not completed its review of such applications but concluded that such ZYN products can continue to be marketed in the U.S., subject to the FDA’s enforcement discretion, because the applications were submitted prior to a September 9, 2020 deadline. We also submitted additional premarket tobacco applications for other ZYN products after the deadline, and we are unable to market these products until the FDA authorizes such applications. There is no guarantee that the ZYN products will receive the necessary authorizations from the FDA or that the FDA will allow us to continue to sell the ZYN products currently in the market, pending its review of the applications.
The financial and business performance of our reduced-risk products is less predictable than our cigarette business.
Our RRPs are novel products in a relatively new category, and the pace at which adult smokers adopt them may vary, depending on the competitive, regulatory, fiscal and cultural environment, and other factors in a specific market. There may be periods of accelerated growth and periods of slower growth for these products, the timing and drivers of which may be more difficult for us to predict versus our mature cigarette business. The impact of this lower predictability on our projected results for a specific period may be significant, due to geopolitical or macroeconomic events that negatively impact RRP availability or adoption, which in turn may have a material adverse effect on our results of operations.
We may be unsuccessful in our efforts to differentiate reduced-risk products and cigarettes with respect to taxation.
To date, we have been largely successful in demonstrating to regulators that our RRPs are not cigarettes due to the absence of combustion, and accordingly they are generally taxed either as a separate category or as other tobacco products, which typically yields more favorable tax rates than cigarettes. Nevertheless, we are unable to predict whether regulators will be issuing new regulations under which RRPs will be equally taxed in line with other tobacco products such as conventional cigarettes. If we cease to be successful in these efforts, RRP unit margins may be materially adversely affected, which in turn may have a material adverse effect on our results of operations, revenues, cash flows, and profitability.
Consumption of tax-paid cigarettes continues to decline in many of our markets.
This decline is due to multiple factors, including increased taxes and pricing, governmental actions, the diminishing social acceptance of smoking, health concerns, competition, continuing economic and geopolitical uncertainty, and the continuing prevalence of illicit products. These factors and their potential consequences are discussed more fully below and in Item 7, Business Environment. A continuous decline in the consumption of cigarettes could have a material adverse effect on our revenue, cash flow and profitability, which in turn may have a material adverse effect on our ability to fund our smoke-free transformation.
Cigarettes are subject to substantial taxes. Significant increases in cigarette-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions. These tax increases may disproportionately affect our profitability and make us less competitive versus certain of our competitors.
Tax regimes, including excise taxes, sales taxes and import duties, can disproportionately affect the retail price of cigarettes versus other combustible tobacco products, or disproportionately affect the relative retail price of our cigarette brands versus cigarette brands manufactured by certain of our competitors. Because our portfolio is weighted toward the premium-price cigarette category, tax regimes based on sales price can place us at a competitive disadvantage in certain markets.
Furthermore, our volume and profitability may be adversely affected in these markets.
In addition, increases in cigarette taxes are expected to continue to have an adverse impact on our sales of cigarettes, due to resulting lower consumption levels, a shift in sales from manufactured cigarettes to other combustible tobacco products and from the premium-price to the mid-price or low-price cigarette categories, where we may be under-represented, from local sales to cross-border purchases of lower price products, or to illicit products such as contraband, counterfeit and "illicit whites."
Each of these risks could have a material adverse effect on our business, operations, results of operations, revenues, cash flow and profitability.
Our business faces significant governmental action aimed at increasing regulatory requirements with the goal of reducing or preventing the use of tobacco or nicotine-containing products.
Governmental actions, combined with the diminishing social acceptance of smoking and private actions to restrict smoking, have resulted in reduced industry volumes for our products in many of our markets, and we expect that such factors will continue to reduce consumption levels and will increase down-trading and the risk of counterfeiting, contraband, "illicit whites" and cross-border purchases. Significant regulatory developments will continue to take place over the next few years in most of our markets, driven principally by the Framework Convention on Tobacco Control (the "FCTC"). Since it came into force in 2005, the FCTC has led to increased efforts by tobacco control advocates and public health organizations to promote increasingly restrictive regulatory measures on the marketing and sale of tobacco and nicotine-containing products to adult nicotine users. Regulatory initiatives that have been proposed, introduced or enacted by governmental authorities in various jurisdictions include:
|
|
|
|
|
|
| • |
restrictions on or licensing of outlets permitted to sell tobacco or nicotine-containing products; |
| • |
the levying of substantial and increasing tax and duty charges; |
| • |
restrictions or bans on advertising, marketing and sponsorship; |
| • |
the display of larger health warnings, graphic health warnings and other labeling requirements; |
| • |
restrictions on packaging design, including the use of colors, and mandating plain packaging; |
| • |
restrictions on packaging and cigarette formats and dimensions; |
| • |
restrictions or bans on the display of product packaging at the point of sale and restrictions or bans on vending machines; |
| • |
generation sales bans, under which the sale of certain tobacco or nicotine-containing products to people born after a certain year would be prohibited; |
| • |
requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and/or other smoke or product constituents; |
| • |
disclosure, restrictions, or bans of tobacco product ingredients, including bans on the flavors of certain tobacco and nicotine-containing products; |
| • |
increased restrictions on smoking and use of tobacco and nicotine-containing products in public and work places and, in some instances, in private places and outdoors; |
| • |
restrictions or prohibitions of novel tobacco or nicotine-containing products or related devices; |
| • |
elimination of duty free sales and duty free allowances for travelers; |
| • |
restrictions in terms of importing or exporting our products impacting our logistics activities and ability to ship our products; |
| • |
encouraging litigation against tobacco companies; and |
| • |
excluding tobacco companies from transparent public dialogue regarding public health and other policy matters. |
Our financial results could be materially affected by regulatory initiatives resulting in a significant decrease in demand for our brands. More specifically, requirements that lead to a commoditization of tobacco products or impede adult consumers' ability to convert to our RRPs, as well as any significant increase in the cost of complying with new regulatory requirements could have a material adverse effect on our financial results.
Changes in the earnings mix and changes in tax laws may result in significant variability in our effective tax rates. Our ability to receive payments from foreign subsidiaries or to repatriate royalties and dividends could be restricted by local country currency exchange controls and other regulations.
We are subject to income tax laws in the United States and numerous foreign jurisdictions. Changes in the U.S. tax system, including significant increases in the U.S. corporate income tax rate and the minimum tax rate on certain earnings of foreign subsidiaries could be enacted. Such changes could have a material adverse impact on our effective tax rate thereby reducing our net earnings. Further changes in the tax laws of foreign jurisdictions could arise as a result of the base erosion and profit shifting project undertaken by the Organisation for Economic Co-operation and Development, which recommended changes to numerous long-standing tax principles. If implemented, such changes, as well as changes in taxing jurisdictions’ administrative interpretations, decisions, policies, or positions, could also have a material adverse impact on our effective tax rate thereby reducing our net earnings. In future periods, our ability to recover deferred tax assets could be subject to additional uncertainty as a result of such developments. Furthermore, changes in the earnings mix or applicable foreign tax laws may result in significant variability in our effective tax rates.
As a result of Russia’s invasion of Ukraine, certain taxing jurisdictions, including the U.S., have proposed punitive tax legislation applicable to companies doing business in Russia, which could also have a material adverse impact on our effective tax rate if enacted thereby reducing our net earnings.
Because we are a U.S. holding company, our most significant source of funds is distributions from our non-U.S. subsidiaries. Certain countries in which we operate have adopted or could institute currency exchange controls and other regulations or policies that limit or prohibit our local subsidiaries' ability to convert local currency into U.S. dollars or to make payments outside the country. This could subject us to the risks of local currency devaluation and business disruption.
Disruptions in the credit markets or changes to our credit ratings may adversely affect our business.
We currently generate significant cash flows from ongoing operations and have access to global credit markets through our various short- and long- term financing activities. Our financial performance, credit ratings, interest rates, the stability of financial institutions with which we partner, geopolitical or national developments, the stability and liquidity of the credit markets and the state of the global economy could affect the availability and cost of financing.
Disruption in the credit markets, limitations on our ability to borrow, slower than anticipated debt deleveraging, or a downgrade of our current credit rating could increase our future borrowing costs which could materially and adversely affect our financial condition and results of operations. In addition, tighter or more volatile credit markets may lead to business disruptions for certain of our suppliers, contract manufacturers or trade customers which could, in turn, adversely impact our business, results of operations, cash flow and financial condition.
We could decide, or be required to, recall products, which could have a material adverse effect on our business, reputation, results of operations, cash flows or financial position.
We could decide, or laws or regulations could require us, to recall products due to the failure, or alleged failure, to meet quality standards or specifications, suspected or confirmed and deliberate or unintentional product contamination, manufacturing defects, or other product adulteration, misbranding or tampering. A product recall or a product liability or other claim (even if unsuccessful or without merit) could generate negative publicity about us and our products, and our Company’s reputation or that of our brands may be adversely affected. In addition, if another company recalls or experiences negative publicity related to a product in a category in which we compete, adult nicotine consumers might reduce their overall consumption of products in that product category. Any of these events could have a material adverse effect on our business, reputation, results of operations, cash flows or financial position.
We may be required to write down assets due to impairment, which could have a material adverse effect on our results of operations or financial position.
We continuously monitor the values of our long-lived assets, reporting units, intangible assets, as well as investments in equity securities, including our continuing investment in Rothmans, Benson & Hedges ("RBH"), to determine whether events or changes in circumstances indicate that an impairment exists. Additionally, we test goodwill and non-amortizable intangible assets for impairment annually. The values of these assets may be affected by several factors, including general macroeconomic and geopolitical conditions; regulatory and legal developments; changes in product volume growth rates; changes in pricing strategies and costs bases; discount rates; success of planned new product expansions; competitive activity; and income and excise taxes. If an impairment is determined to exist, we will incur impairment losses, which could have a material adverse effect on our results of operations or financial position. See Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Estimates for additional information concerning impairment determination and calculation.
Risks Related to the Impact of the War in Ukraine on our Business
Our business, results of operations, cash flows and financial position may be adversely impacted by the continuation and consequences of the war in Ukraine.
In 2023, Russia accounted for around 9% of our total cigarette and heated tobacco unit shipment volume, and around 6% of our total net revenues. Ukraine accounted for around 2% of our total cigarette and heated tobacco unit shipment volume, and around 1% of our total net revenues. Historically, we also produced finished goods in Ukraine for export and manufactured products in Russia. In 2022, as a result of Russia’s invasion of Ukraine, we suspended planned investments and scaled down our manufacturing operations in Russia.
The full implications of the Russian invasion of Ukraine for our operations in those countries are impossible to predict at this time. The likelihood of retaliatory action by the Russian government against companies, including PMI, as a result of actions and statements made in response to the Russian invasion or otherwise, including the possibility of legal action against us or our employees; the deprivation of rights in, or access to, our Russian assets; or nationalization of foreign businesses or assets (including cash reserves held in Russia and intangible assets such as trademarks), is impossible to predict. We are continuously assessing the evolving situation in Russia, including regulatory constraints in the market entailing very complex terms and conditions that must be met for any divestment transaction to be granted approval by the authorities, and restrictions resulting from international regulations. In the event of a divestment, our ability to fully realize the value of the business would likely be subject to material impairment. In Ukraine, there is no way to know when and to what extent we will be able to fully normalize our operations or to what extent our workforce, facilities, inventory, and other assets will remain intact. These developments have and will continue to have a material adverse impact on our business, results of operations, cash flows and financial position, and may result in further impairment charges.
The conflict also continues to elevate the likelihood of supply chain disruptions, both in the region and globally, and may inhibit our ability to timely source materials and services needed to make and sell our products. For example, historically we sourced certain finished goods, production materials and components from both Russia and Ukraine, including printed materials and filters, and the invasion has, and may continue to, disrupt the availability of and impact our supply chain for these materials. These disruptions, to the extent we are unable to find alternative sources or otherwise address these supply constraints, may impact the availability and cost of our products in other markets, which would adversely impact our business, results of operations, cash flows and financial position, and may result in impairment charges. Furthermore, the imposition of various restrictions on transactions with parties from certain jurisdictions, the ban on exports of various products, and other economic and financial restrictions may adversely affect certain third parties with which we do business in Russia, such as customers, suppliers, intermediaries, service providers and banks.
The broader consequences of the invasion are also impossible to predict, but could include reputational consequences, further sanctions, financial or currency restrictions, punitive tax law changes, embargoes, regional instability, and geopolitical shifts as well as adverse effects on macroeconomic conditions, security conditions, currency exchange rates, and financial markets. Given the nature of our business and global operations, such geo-political instability and uncertainty could increase the costs of our materials and operations; reduce demand for our products; have a negative impact on our supply chains, manufacturing capabilities, or distribution capabilities; increase our exposure to currency fluctuations; constrain our liquidity or our ability to access capital markets; create staffing or operations difficulties; or subject us to increased cyber-attacks. While we will continue to monitor this fluid situation and develop contingency plans as necessary to address any disruptions to our business operations as they develop, the extent of the conflict’s effect on our business and results of operations as well as the global economy, cannot be predicted.
The conflict may also heighten many other risks disclosed in this Form 10-K, any of which could adversely affect our business, results of operations, cash flows or financial position. Such risks could affect, without limitation, the achievement of our strategic priorities, including achievement of our RRP growth targets; the availability of third-party manufacturing resources; the availability of attractive acquisition and strategic business opportunities and our ability to fully realize the benefits of these transactions; our ability to attract, motivate, and retain the best global talent; and our loss of revenue from counterfeiting and similar illicit activities.
Risks Related to Sourcing and Distribution of Products, Services and Materials
Use of third-parties may negatively impact the distribution, quality, and availability of our products and services, and we may be required to replace third-party contract distributors, manufacturers or service providers.
We increasingly rely on third-parties and their subcontractors/suppliers, sometimes concentrated in a specific geographic area, for product distribution and to manufacture some of our products and product parts (particularly, the electronic devices and accessories), as well as to provide services, including to support our finance, commercialization and information technology processes. While many of these arrangements improve efficiencies and decrease our operating costs, they also diminish our direct control. Such diminished control may lead to disruption in the distribution of our products and may have a material adverse effect on the quality and availability of products or services, our supply chain, and the speed and flexibility in our response to changing market conditions and adult consumer preferences, all of which may place us at a competitive disadvantage. In addition, we may be unable to renew these agreements on satisfactory terms for numerous reasons, including government regulations, and the distribution of our products may be disrupted in certain markets or our costs may increase significantly if we must replace such third parties with other partners or our own resources.
The effects of climate change, other environmental issues, and related legal or regulatory responses may have a negative impact on our business and results of operations.
While we seek to mitigate our business risks associated with environmental issues, such as climate change, by establishing environmental goals and standards and seeking business partners, including within our supply chain, that are committed to operating in ways that protect the environment or mitigate environmental impacts, we recognize that there are inherent environmental-related risks, including climate change-related risks, wherever business is conducted. Among other potential impacts, climate change could influence the quality and volume of the agricultural products we rely on, including tobacco, due to several factors beyond our control, including more frequent variations in weather patterns, extreme weather events causing unexpected downtime and inventory losses, other adverse weather conditions, and governmental restrictions on trade, all of which may lead to disruption of operations at factories, warehouses and other premises.
Furthermore, nature-related risks, including those related to natural ecosystems degradation, decreased agricultural productivity in certain regions of the world, biodiversity loss, water resource depletion and deforestation, which are partially driven or exacerbated by climate change, may negatively impact the resilience of, or otherwise disrupt, our business operations or those of our suppliers and business partners.
There is an increased focus by foreign, federal, state and local regulatory and legislative bodies on environmental policies, including those relating to climate change. New environmental-related legal or regulatory requirements may lead to additional carbon taxation, raw or other materials taxation, energy price increases, new compliance costs, increased distribution and supply chain costs, and other expenses impacting our cost of operations. Moreover, given that the regulatory framework in this regard is highly dynamic, additional uncertainties may be driven by further upcoming regulatory changes on which we might have limited visibility or limited time to implement, which could have an impact on several elements of our business, including elevating the cost or complexity of our operations. Even if we make changes to align ourselves with legal or regulatory requirements, we may still be subject to significant penalties if such laws or regulations are interpreted and applied in a manner inconsistent with our practices.
Government mandated prices, production control programs, and shifts in crops driven by economic conditions may increase the cost or reduce the quality of the tobacco and other agricultural products used to manufacture our products.
As with other agricultural commodities, the price of tobacco leaf and cloves can be influenced by imbalances in supply and demand and the impacts of natural disasters and pandemics such as COVID-19. Tobacco production in certain countries is subject to a variety of controls, including government mandated prices and production control programs. Changes in the patterns of demand for agricultural products could cause farmers to produce less tobacco or cloves. Any significant change in tobacco leaf and clove prices, quality and quantity could affect our profitability and our business.
A prolonged disruption of our production facilities could have a material adverse effect on our business, financial condition and results of operations.
A prolonged disruption at or shut-down of one or more of our production facilities, especially our ZYN production facility in Kentucky, which currently supplies substantially all of our capacity for ZYN sales in the U.S., due to natural- or man-made disasters or other events outside of our control, such as equipment malfunction or widespread outbreaks of acute illness, including COVID-19, or for any other reason, could limit our capacity to meet customer demands. Such an event could disrupt our operations; delay production, shipments and revenue; and result in significant expense to repair or replace our affected facilities. As a result, we could forgo revenue opportunities and potentially lose market share, which could materially and adversely affect our business, financial condition and results of operations.
Risks Related to our International Operations
Because we have operations in numerous countries, our results may be adversely impacted by economic, regulatory and political developments, natural disasters, pandemics or conflicts.
Some of the countries in which we operate face the threat of civil unrest and can be subject to regime changes. In others, nationalization, terrorism, conflict and the threats of war or acts of war may have a significant impact on the business environment. Factors beyond our control, such as, without limitation, natural disasters, extreme weather events, pandemics (including COVID-19), economic, political, regulatory, acts of war or threats of war, or other developments could disrupt or increase the expenses related to our supply chain, manufacturing capabilities, distribution capabilities, or the energy and other utility services required to operate our factories, warehouses, and other premises. Our business continuity plans and other safeguards might not always be effective to fully mitigate their impact. For example, the global pandemic outbreak of the COVID-19 virus in 2020 created significant societal and economic disruption and the closure of stores, factories and offices, restrictions on manufacturing, distribution and travel, and supply chain disruptions, among other impacts. Such developments – including the impact of geopolitical disruptions resulting from the conflict in the Middle East and the impact on energy prices and availability in the EU and elsewhere resulting from the invasion of Ukraine by Russia – could cause significant volume declines in our duty-free business and certain other key markets; disrupt or delay our distribution, manufacturing or supply chain; increase currency volatility; increase costs of our materials and operations and lead to loss of property or equipment that are critical to our business in certain markets and difficulty in staffing and managing our operations, all of which could have a material adverse effect on our business, operations, volumes, revenue, cash flows, financial position, net earnings and profitability. We discuss additional risks associated with Russia's invasion of Ukraine and climate change, above.
In certain markets, we are dependent on governmental approvals of various actions such as price changes, and failure to obtain such approvals could impair growth of our profitability.
In addition, despite our high ethical standards and rigorous controls and compliance policies aimed at preventing and detecting unlawful conduct, given the breadth and scope of our international operations, we may not be able to detect all potential improper or unlawful conduct by our employees and partners. Such improper or unlawful conduct (actual or alleged) could lead to litigation and regulatory action, cause damage to our reputation and that of our brands, and result in substantial costs.
Our reported results could be adversely affected by unfavorable currency exchange rates and currency fluctuations could impair our competitiveness. Our results could also be adversely affected by capital controls or by foreign currency exchange constraints or devaluations.
We conduct our business primarily in local currency and, for purposes of financial reporting, the local currency results are translated into U.S. dollars based on average exchange rates prevailing during a reporting period. Foreign currencies may fluctuate significantly against the U.S. dollar, reducing our net revenues, operating income and EPS. Our primary local currency cost bases may be different from our primary currency revenue markets, and U.S. dollar fluctuations against various currencies may have disproportionate negative impact on cash flows and on net revenues as compared to our gross profit and operating income margins.
Capital controls and/or foreign currency exchange constraints may affect the ability of our subsidiaries in impacted jurisdictions to settle foreign currency denominated imports of goods and services and/or to pay dividends and royalties. These factors may also increase foreign currency devaluation risks, which may have a negative impact on our net assets and results of operations in these jurisdictions. All of which could have a material adverse effect on our financial condition, including our leverage ratios, cash flows, net earnings, and profitability.
A sustained period of elevated inflation across the markets in which we operate could result in higher operating and financing costs and lead to reduced demand for our products.
Increasing inflationary pressures has and may continue to result in significant increases to our expenses, including direct materials, wages, energy, and transportation costs. While we take actions, wherever possible, to reduce the impact of the effects of inflation, in cases of sustained and elevated inflation across several of our major markets, it may be difficult to effectively control the increases to our costs. In recent periods, increased inflation has and may continue to lead to growing pressures on the cost of certain direct materials, wages, energy, transportation, and logistics as well as an increased cost of capital due to interest rate increases driven by the response to increased inflation. Inflationary pressures may also negatively impact consumer purchasing power, which could result in reduced demand for our products. We expect certain inflationary elements to ease, with a moderate increase in 2024. If we are unable to increase our prices sufficiently or take other actions to mitigate the effect of inflationary pressures, our profitability and financial position could be negatively impacted.
Risks Related to Legal Challenges and Investigations
Litigation related to tobacco use and exposure to environmental tobacco smoke could substantially reduce our profitability and could severely impair our liquidity.
There is litigation related to tobacco products pending in certain jurisdictions in which we operate. Damages claimed in some tobacco-related litigation are significant and, in certain cases in Brazil, Canada, and Nigeria, range into the billions of U.S. dollars. We anticipate that new cases will continue to be filed. The FCTC encourages litigation against tobacco product manufacturers. It is possible that our consolidated results of operations, cash flows or financial position could be materially adversely affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. We face various administrative and legal challenges related to certain RRP activities, including allegations concerning product classification, advertising restrictions, corporate communications, product coach activities, scientific substantiation, product liability, antitrust, and unfair competition. While we design our programs to comply with relevant regulations, we expect these or similar challenges to continue as we expand our efforts to commercialize RRPs and to communicate with the public. The outcomes of these matters may affect our RRP commercialization and public communication activities and performance in one or more markets. Also see Item 8, Note 18. Contingencies to our consolidated financial statements for a discussion of pending litigation.
From time to time, we are subject to governmental investigations on a range of matters.
Investigations include allegations of contraband shipments of cigarettes, allegations of unlawful pricing activities within certain markets, allegations of underpayment of income taxes, customs duties and/or excise taxes, allegations of false and misleading usage of descriptors, allegations of unlawful advertising, and allegations of unlawful labor practices. We cannot predict the outcome of those investigations or whether additional investigations may be commenced, and it is possible that our business could be materially adversely affected by an unfavorable outcome of pending or future investigations. See Item 8, Note 18. Contingencies—Other Litigation and Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations—Operating Results by Business Segment—Business Environment—Governmental Investigations for a description of certain governmental investigations to which we are subject.
We may be unable to adequately protect our intellectual property rights, and disputes relating to intellectual property rights could harm our business.
Our intellectual property rights are valuable assets, their protection is important to our business, and that protection may not be equally available in every country in which we operate or in which our products are sold. If the steps we take to protect our intellectual property rights globally, including through applying for, prosecuting, maintaining and enforcing, where relevant, a combination of trademark, design, copyright, patent, trade secrets and other intellectual property rights, are inadequate, or if others infringe or misappropriate our intellectual property rights, notwithstanding legal protection, our business, financial condition, and results of operations could be adversely impacted. Moreover, failing to manage our existing and/or future intellectual property may place us at a competitive disadvantage. Intellectual property rights of third parties may limit our ability to develop, manufacture and/or commercialize our products in one or more markets. Competitors or other third parties may claim that we infringe their intellectual property rights. Any such claims, regardless of merit, could divert management’s attention, be costly, disruptive, time-consuming and unpredictable and expose us to significant litigation costs and damages, and may impede our ability to develop, manufacture and/or commercialize new or existing RRPs and improve our products, and thus have a material adverse effect on our revenue and our profitability. In addition, if, as a result, we are unable to manufacture or sell our RRPs or improve their quality in one or more markets, our ability to convert adult smokers to our RRPs in such markets would be adversely affected. See Item 8, Note 18. Contingencies—Other Litigation to our consolidated financial statements for a description of certain intellectual property proceedings.
Risks Related to our Competitive Environment
We face intense competition, and our failure to compete effectively could have a material adverse effect on our profitability and results of operations.
We are subject to highly competitive conditions in all aspects of our business. We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, R&D, innovation, packaging, customer service, marketing, advertising and retail price and, increasingly, adult smoker willingness to convert to our RRPs. The competitive environment and our competitive position can be significantly influenced by weak economic conditions; erosion of consumer confidence; competitors' introduction of lower-price products or innovative products; novel products which given their taste characteristics may be more commercially successful; higher product taxes; higher absolute prices and larger gaps between retail price categories; and product regulation that diminishes the ability to differentiate tobacco products, restricts adult consumer access to truthful and non-misleading information about our RRPs, or disproportionately impacts the commercialization of our products in relation to our competitors.
Competitors in our industry include British American Tobacco plc, Japan Tobacco Inc., Imperial Brands plc, new market entrants, particularly with respect to innovative products, several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, Egypt, China, Taiwan, Thailand and Vietnam. Some competitors have different profit, volume and regulatory objectives, some international competitors may be less susceptible than PMI to changes in currency exchange rates, and some competitors may sell products in circumvention of applicable regulations that compete directly with our products. Certain new market entrants in the non-combustible product category may alienate consumers from innovative products through inappropriate marketing campaigns, messaging and inferior product satisfaction, and without scientific substantiation based on appropriate R&D protocols and standards. The growing use of digital media could increase the speed and extent of the dissemination of inaccurate and misleading information about our RRPs, all of which could have a material adverse effect on our profitability and results of operations. See Item 1, Business—Competition for a description of the competitive environment in which we operate.
We may be unable to anticipate changes in adult consumer preferences.
Our business is subject to changes in adult consumer preferences, which may be influenced by local economic conditions, accessibility to our products and availability of accurate information related to our products.
To be successful, we must:
|
|
|
|
|
|
| • |
promote brand equity successfully; |
| • |
anticipate and respond to new adult consumer trends; |
| • |
ensure that our products meet our quality standards; |
| • |
develop new products and markets and broaden brand portfolios; |
| • |
improve productivity; |
| • |
educate and encourage adult smokers to convert to our RRPs; |
| • |
ensure effective adult consumer engagement, including communication about product characteristics and usage of RRPs; |
| • |
mitigate the impact of developments that cause damage to our reputation and that of our brands; |
| • |
provide excellent customer care; |
| • |
ensure adequate production capacity to meet demand for our products; and |
| • |
be able to protect or enhance margins through price increases. |
In periods of economic uncertainty, adult consumers may tend to purchase low-price brands, and the volume of our premium-price and mid-price brands and our profitability could be materially adversely impacted as a result. Such down-trading trends may be reinforced by regulation that limits branding, communication and product differentiation. In addition to economic uncertainty (including recessions and inflation) unusual weather events and global or local epidemics, endemics or pandemics (such as COVID-19) has and may change the preferences of our adult consumers and lower demand for our products, particularly for our mid-price or premium-price brands.
Our ability to grow profitability may be limited by our inability to introduce new products, enter new markets, maintain sufficient production capacity, or improve our margins through higher pricing and improvements in our brand and geographic mix.
Our profit growth may be materially adversely impacted if we are unable to introduce new products or enter new markets successfully, to meet the demand for our products with increased production capacity, to raise prices, or to improve the proportion of our sales of higher margin products and in higher margin geographies.
We may be unable to expand our brand portfolio through acquisitions or the development of strategic business relationships, and the intended benefits from our investments may not materialize.
One element of our growth strategy is to expand our brand portfolio and market positions through selective acquisitions and the development of strategic business relationships. Acquisition and strategic business development opportunities are limited and present risks of failing to achieve efficient and effective integration, strategic objectives and/or anticipated revenue improvements and cost savings. There is no assurance that we will be able to acquire attractive businesses or enter into strategic business relationships on favorable terms ahead of our competitors, or that such acquisitions or strategic business development relationships will be accretive to earnings or improve our competitive position. In addition, we may not have a controlling position in certain strategic investments or relationships, which could impact the extent to which the intended financial growth and other benefits from these investments or relationships may ultimately materialize.
Our ability to achieve our strategic goals may be impaired if we fail to attract, motivate and retain the best global talent and effectively align our organizational design with the goals of our transformation.
To be successful, we must continue transforming our culture and ways of working, align our talent and organizational design with our increasingly complex business needs, and innovate and transform to a consumer-centric business. We compete for talent, including in areas that are relatively new to us such as digital, information technology, and life sciences, with companies in the consumer products, technology, pharmaceutical and other sectors that enjoy greater societal acceptance. As a result, we may be unable to attract, motivate and retain the best global talent with the right degree of diversity, experience and skills to achieve our strategic goals.
Risks Related to Illicit Trade
We lose revenues as a result of counterfeiting, contraband, cross-border purchases, "illicit whites," non-tax-paid volume produced by local manufacturers, and counterfeiting of our smoke-free products' devices and consumables.
Large quantities of counterfeit cigarettes are sold in the international market. We believe that Marlboro is the most heavily counterfeited international cigarette brand, although we cannot quantify the revenues we lose as a result of this activity. In addition, our revenues are reduced by contraband, cross-border purchases, "illicit whites" and non-tax-paid volume produced by local manufacturers. Our revenues and consumer satisfaction with our smoke-free products' devices and consumables may be materially adversely affected by counterfeit products that do not meet our product quality standards and scientific validation procedures.
Risks Related to Cybersecurity and Data Governance
We are significantly dependent on our and third-party information technology networks and systems, and a cybersecurity incident or attack against those networks or systems may adversely impact our business and operations.
We and our business partners heavily rely on information technology networks and systems, including those connected to the Internet, to help manage business processes and operations, including the collection, storage, interpretation, and processing of confidential, sensitive, personal and other data; internal and external communications; marketing and e-commerce activities; the manufacture, sale, and distribution of our products; management of third-party business relationships; engagement with governmental authorities; innovation through research and development; and other activities necessary for business operations. Some of these information systems and networks are developed, supplied, or managed by third-party service providers that may make us vulnerable to “supply chain” style cyberattacks. The failure or disruption of our information technology networks and systems, or those managed by third-party service providers or owned by our business partners and used in furtherance of PMI’s business, due to cybersecurity attacks; unauthorized attempts to corrupt or extract data; security vulnerabilities; misconfigurations; human error; or failure or inability by us, third-parties, or our business partners to adhere to cybersecurity industry best practices, could place us at a competitive disadvantage, cause reputational damage, impact our operations, result in data breaches, significant business disruption, litigation, regulatory action including significant fines or penalties, financial impact, loss of revenue or assets including our intellectual property, personal, confidential, or sensitive data.
Cyberattacks, security incidents and vulnerabilities impacting PMI, newly acquired companies, our business partners, or our third-party providers, continue to dynamically evolve in sophistication and volume, making it difficult for us to predict probability, frequency, and impact severity of security incidents. Further, it may be inherently difficult to detect vulnerabilities during due diligence, for long periods of time, or soon enough to mitigate exploitation. There can be no assurance that such security incidents or vulnerabilities will not have a material adverse effect on us in the future. While PMI works to mitigate these risks by implementing a cybersecurity risk program and a third-party cybersecurity risk management program, there can be no assurance that these programs are comprehensive or accurately identify and sufficiently mitigate all cybersecurity risks.
We continue to make investments in administrative, technical, and physical safeguards to maintain information security protections in line with industry standards and best practices. We evaluate the adequacy of preventative actions to reduce security incidents on an ongoing basis.
Our safeguards may not, however, be effective in mitigating the impact of service disruptions or other failures of these information technology networks and systems. Failure to timely respond and mitigate security incidents, could result in wide-ranging business interruptions. Such security incidents could place us at a competitive disadvantage; result in financial impacts, a loss of revenue, assets, including our intellectual property, personal or other sensitive data; result in litigation and regulatory action including significant fines or penalties; impact our operations; cause damage to our reputation and that of our brands; and result in significant remediation and other costs. See Item 1C. Cybersecurity for a description of our cybersecurity risk management and strategy and governance.
Our or our business partners’ failure or inability to adhere to privacy, data, artificial intelligence and information security laws could result in business disruption, loss of reputation and consumer trust, litigation, regulatory action including significant fines or penalties, financial impact, and loss of revenue, assets or personal, confidential, or sensitive data.
An actual or alleged failure to comply with complex and changing privacy, data, artificial intelligence and information security laws and regulations under the EU General Data Protection Regulation, various U.S. state and federal laws, and other similar privacy and information security laws across the jurisdictions in which PMI operates, such as the failure to protect personal data; implement appropriate technological and reasonable security measures; implement and maintain appropriate safeguards for personal data being transferred internationally; respect the privacy rights of data subjects; provide sufficient detailed notices of personal data processing; retrieve consent and provide opt-outs; meet stringent timeframe requirements for incident reporting to regulatory authorities; comply with artificial intelligence regulations; and others, could have a material adverse effect on us, subject us to substantial fines and/or legal challenges, and/or harm our business, reputation, financial condition, or operating results. Such laws and regulations across the jurisdictions in which PMI operates may vary, resulting in inconsistent or conflicting legal obligations.
Risks Related to Swedish Match and Vectura Fertin Pharma
We may be unable to fully realize the expected benefits from the acquisitions of Swedish Match or Vectura Fertin Pharma.
Since 2021, we have acquired Swedish Match, OtiTopic, Fertin Pharma and Vectura (collectively, the "Acquisitions"), and subsequently launched Vectura Fertin Pharma, our new Wellness and Healthcare business, consolidating OtiTopic, Fertin Pharma and Vectura. The anticipated benefits of the Acquisitions may not be realized fully, or at all, or may take longer to realize than expected. Furthermore, the success of the Acquisitions also depends on the continued successful commercialization and growth of Swedish Match's products in highly competitive markets and on the success of the research and development efforts of Vectura Fertin Pharma, including the ability to obtain regulatory approval for new products, and the ability to commercialize or license these new products developed by them. Moreover, our combustible product portfolio may stand in the way of introducing and growing new Wellness and Healthcare product categories and may prevent our business from developing a long-term sustainable ecosystem of products in the wellness, therapeutic, and healthcare categories.
Swedish Match and Vectura Fertin Pharma may have liabilities that are not known to us.
The businesses that we have acquired may have liabilities that we were unable to identify, or were unable to discover, in the course of performing our due diligence investigations during the Acquisitions thereof. There is no assurance that the indemnification available to us under the respective acquisition agreements, will be sufficient in amount, scope or duration to fully offset the possible liabilities associated with the respective business or property that we assumed upon consummation of each Acquisition. Furthermore, the acquisition of Swedish Match was structured as a direct purchase of shares from Swedish Match shareholders and therefore did not include an acquisition agreement or indemnification rights. Any such liabilities, individually or in the aggregate, could have a material adverse effect on our business, financial condition and results of operations.
Accounting adjustments related to the Acquisitions could adversely affect our financial results.
We accounted for the completion of the Acquisitions using the acquisition method of accounting. Given the nature of the assets acquired in the Acquisitions, we may not be able to avoid future impairments of those assets, which may also have a material impact on our future results of operation and financial position.
PMI, Swedish Match and Vectura Fertin Pharma may be subject to uncertainties that could adversely affect our respective businesses, and adversely affect the financial results of our combined businesses.
Our success following these Acquisitions depends in part upon our ability and the ability of each of Swedish Match and Vectura Fertin Pharma to maintain business relationships. The effect of the Acquisitions on customers, suppliers, employees and other constituencies of each of Swedish Match, Fertin Pharma and Vectura, may have a material adverse effect on us and/or the businesses that we have acquired through the Acquisitions. Customers, suppliers and others who do business with Swedish Match or Vectura Fertin Pharma may delay or defer business decisions, decide to terminate, modify or renegotiate their relationships, or take other actions, which could negatively affect the revenues, earnings and cash flows of our company or the businesses that we have acquired. Regulatory changes may have an impact on the development and/or commercialization of products which originate from the Swedish Match or Vectura Fertin Pharma value chains, as well as our revenues, earnings and cash flow. If we are unable to maintain the business and operational relationships of Swedish Match, or of Vectura Fertin Pharma, our financial position, results of operations or cash flows upon combining with these companies could be adversely affected.
Item 1B.Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
PMI relies heavily on the availability, reliability, and security of our information systems, networks, data, and intellectual property to, among other things, help manage our business processes and operations, collect and interpret data, and communicate internally and externally with employees, suppliers, consumers and customers, and business partners. We have a cross-functional cybersecurity risk program developed using standard industry practices, which monitors and manages cybersecurity threats to our business and information systems. We invest in administrative, technical, and physical safeguards, including continuity planning, to enhance resilience on our core processes, to maintain information security protections of our data and to safeguard the privacy of consumers, customers, employees and business partners.
Risk Management and Strategy
Our cybersecurity risk program, managed by our Chief Information Security Officer (“CISO”) and the information security team, is conducted under our enterprise risk management framework and operates on a risk-based approach in assessing risks from cybersecurity threats, as follows:
•Cybersecurity Threat Scenarios. Our cybersecurity risk assessment process consists of identifying and compiling a catalogue of top cybersecurity threat scenarios relevant to PMI, which facilitates risk assessments with our IT and business stakeholders.
•Cybersecurity Maturity Assessment. Our risk exposure from relevant cybersecurity threat scenarios is mitigated by evaluating existing cybersecurity capabilities and corresponding maturity to identify and address areas for improvement.
•Cybersecurity Threat Assessment. To establish PMI’s current and target cybersecurity risk exposure, residual risk exposure from the most relevant cybersecurity threat scenarios across IT platforms and regions is evaluated and measured based upon the cybersecurity maturity assessments.
•Cybersecurity Risk Program. PMI has a cybersecurity risk program to enhance its ability to identify, prevent, mitigate, respond and recover from disruptive cybersecurity threats and incidents and to reduce cybersecurity risk exposure. Improvements in our cybersecurity defense capabilities are prioritized based upon the results of cybersecurity threat assessments and cybersecurity maturity assessments. Identified issues from these assessments form the improvement initiatives under our cybersecurity risk program. As discussed in more detail below under “Governance,” the program’s key improvement initiatives, their implementation status, and the overall progression in our cybersecurity capability maturity are regularly presented to the applicable governing body within PMI. In addition, our cybersecurity risk program operates in coordination with the following:
Cyber Defense. Our dedicated cyber defense team provides services to identify, help prevent, detect and respond against cybersecurity threats and intrusions and collaborates with internal and external stakeholders to help protect PMI’s information, mitigate operational disruptions and maintain business continuity. The cyber defense team’s controls and procedures identify and enable escalation of cybersecurity incidents to the applicable governing body within PMI, as appropriate, to meet disclosure and reporting requirements for such incidents.
Third-Party Cyber Risk Management. Some of our information systems and networks are developed, supplied, or managed by third-party service providers. Our third-party cyber risk management process analyzes and seeks to control risks associated with outsourcing products or services, such as “supply chain” style cyberattacks, and identifies preventative and detective controls to mitigate third-party vendor and service provider cybersecurity risks that could adversely impact our business and operations.
Education and Awareness. PMI regularly provides its workforce with mandatory cybersecurity awareness education and training addressing information security related tasks in line with our evolving information security policies, standards, procedures, and practice as well as supplemental role-based training and awareness programs.
We engage external assessors and other third parties to independently evaluate our cybersecurity risk management process, including the relevance to PMI of identified cybersecurity scenarios and the results of cybersecurity maturity assessments. The outcome of such evaluations, audits or reviews are reported to the Corporate Risk Governance Committee and to the Audit & Risk Committee, and our cybersecurity policies, standards and processes are adjusted, as necessary.
PMI follows a risk evaluation process for issues identified through internal audits, security assessments, third-party cybersecurity risk assessments, or self-assessment disclosures, and resulting information technology risks are recorded for risk remediation, transfer, avoidance, or acceptance as appropriate. Some of our information systems are managed by specialist third-party service providers, and we work with internal specialists to protect systems and data from unauthorized access and other cybersecurity threats.
Governance
The Audit and Risk Committee of our Board of Directors oversees our policies and practices with respect to risk assessment and risk management, including a review, in coordination with our management, of PMI’s management of cybersecurity. Our CISO presents reports to the Audit and Risk Committee or to the full Board of Directors at least quarterly, which reports include cybersecurity risk status along with key performance indicators and key risk response strategies and plans.
The Corporate Risk Governance Committee receives quarterly reports on the Company’s overall cybersecurity risk exposure including the individual top cybersecurity threat scenario residual risk ratings and the plan and status of the cybersecurity risk program, to facilitate calibration with other enterprise risk domains and validation of the risk response plans. The Corporate Risk Governance Committee includes our Chief Executive Officer (“CEO”), Chief Financial Officer (“CFO”), General Counsel (“GC”), Senior Vice President Operations, and our Chief Digital & Information Officer (“CDIO”).
Cybersecurity incidents that have been determined to meet established SEC reporting consideration thresholds are promptly communicated to the Disclosure Committee, which is responsible for evaluating the potential materiality of such incidents and ensuring the accuracy, timeliness and completeness of related disclosures under applicable reporting obligations, and other relevant communications or presentations. The Disclosure Committee’s membership includes the following executives: the Corporate Secretary; the GC; the CFO; the Controller & Principal Accounting Officer; the Chief Risk Assurance Officer; and the Vice President, Investor Relations. In addition, the CISO serves as an advisor to the Disclosure Committee.
The CISO has served in various roles in information technology and information security for over 25 years, including in the telecommunications and management consultancy sectors and serving as the Chief Information Security Officer of two large public companies. The CDIO holds an engineering degree and has served in various senior positions in information technology for over 20 years, including serving as Senior Vice President, IT Sales, and Global Chief Information Officer at a public company. The CEO has served in various positions in finance and general management at PMI for over 30 years, including as Chief Financial Officer and Chief Operating Officer, and holds a master’s degree in economics. The CFO has over 15 years of experience in finance and management, having held several executive positions in charge of finance, legal affairs information systems and industry administration at various companies. The GC has served at PMI for 18 years in several positions within the Legal & Compliance department, including as Vice President and Associate General Counsel of various regions, and holds two master’s degrees having studied law, management and finance.
As of the date of this Annual Report on Form 10-K, PMI is not aware of any risks from cybersecurity threats, including as a result of any previous cybersecurity incidents, that have materially affected or are reasonably likely to materially affect PMI, its business strategy, results of operations or financial condition. For additional information concerning PMI’s risks related to cybersecurity, see Item 1.A. Risk Factors.
Item 2. Properties.
We own or lease various manufacturing, office and research and development facilities in locations around the world. We own properties in Switzerland where our operations center and state-of-the-art research and development facility are located.
At December 31, 2023, we operated and owned a total of 50 manufacturing facilities across our segments. Among them, 9 factories produced heated tobacco units and 7 factories produced oral nicotine products.
In 2023, certain of our facilities each manufactured over 30 billion units (cigarettes and heated tobacco units combined). Our largest manufacturing facilities, in terms of cigarette and heated tobacco unit volume, are located in Turkey (SSEA, CIS & MEA), Russia (SSEA, CIS & MEA), Indonesia (SSEA, CIS & MEA), Poland (Europe), Italy (Europe), Czech Republic (Europe), Lithuania (Europe) and the Philippines (SSEA, CIS & MEA). Our largest nicotine pouch manufacturing facility is located in the United States. As part of our global operating model, products manufactured in a particular manufacturing facility are not necessarily distributed in the operating segment where the facility is located.
We have integrated the production of our heated tobacco units into a number of our existing manufacturing facilities, and we are progressing with our plans to build manufacturing capacity for our other RRP and smoke-free platforms. We will continue to optimize our manufacturing infrastructure.
We believe the properties owned or leased by our subsidiaries are maintained in good condition and are believed to be suitable and adequate for our present needs.
Item 3.Legal Proceedings.
The information called for by this Item is incorporated herein by reference to Item 8, Note 18. Contingencies.
Item 4.Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
The principal stock exchange on which our common stock (no par value) is listed is the New York Stock Exchange (ticker symbol "PM"). At January 31, 2024, there were approximately 41,300 holders of record of our common stock.
Information regarding equity-based compensation plans required by Regulation S-K Item 201(d) is provided in Item 12 of this 10-K.
Performance Graph
The graph below compares the cumulative total shareholder return on PMI's common stock with the cumulative total return for the same period of PMI's Peer Group and the S&P 500 Index. The graph assumes the investment of $100 as of December 31, 2018, in PMI common stock (at prices quoted on the New York Stock Exchange), and each of the indices as of the market close and reinvestment of dividends on a quarterly basis.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Date |
|
PMI |
|
|
PMI Peer Group (1) |
|
S&P 500 Index |
| December 31, 2018 |
|
$100.00 |
|
|
$100.00 |
|
$100.00 |
| December 31, 2019 |
|
$135.00 |
|
|
$123.90 |
|
$131.50 |
| December 31, 2020 |
|
$140.20 |
|
|
$132.60 |
|
$155.70 |
| December 31, 2021 |
|
$169.30 |
|
|
$153.40 |
|
$200.40 |
| December 31, 2022 |
|
$190.30 |
|
|
$148.70 |
|
$164.10 |
| December 31, 2023 |
|
$186.90 |
|
|
$146.40 |
|
$207.20 |
(1) The PMI Peer Group presented in this graph is the same as that used in the prior year. The PMI Peer Group was established based on a review of four characteristics: global presence; a focus on consumer products; and net revenues and a market capitalization of a similar size to those of PMI. The review also considered the primary international tobacco companies. As a result of this review, the following companies constitute the PMI Peer Group: Altria Group, Inc., Anheuser-Busch InBev SA/NV, British American Tobacco p.l.c., The Coca-Cola Company, Colgate-Palmolive Co., Diageo plc, Heineken N.V., Imperial Brands PLC, Japan Tobacco Inc., Johnson & Johnson, Kimberly-Clark Corporation, The Kraft-Heinz Company, McDonald's Corp., Mondelēz International, Inc., Nestlé S.A., PepsiCo, Inc., The Procter & Gamble Company, Roche Holding AG, and Unilever NV and PLC.
Note: Figures are rounded to the nearest $0.10.
Issuer Purchases of Equity Securities During the Quarter Ended December 31, 2023
Our share repurchase activity for each of the three months in the quarter ended December 31, 2023, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Period |
|
Total
Number of
Shares
Repurchased |
|
Average
Price Paid
per Share |
|
Total Number
of Shares
Purchased as
Part of Publicly
Announced
Plans or
Programs |
|
Approximate
Dollar Value
of Shares that
May Yet be
Purchased
Under the Plans
or Programs |
October 1, 2023 –
October 31, 2023 (1) |
|
|
|
$ |
— |
|
|
10,481,359 |
|
|
$ |
6,016,847,275 |
|
November 1, 2023 –
November 30, 2023 (1) |
|
|
|
$ |
— |
|
|
10,481,359 |
|
|
$ |
6,016,847,275 |
|
December 1, 2023 –
December 31, 2023 (1) |
|
|
|
$ |
— |
|
|
10,481,359 |
|
|
$ |
6,016,847,275 |
|
|
Pursuant to Publicly Announced
Plans or Programs
|
|
— |
|
|
$ |
— |
|
|
|
|
|
October 1, 2023 –
October 31, 2023 (2) |
|
3,536 |
|
|
$ |
92.75 |
|
|
|
|
|
November 1, 2023 –
November 30, 2023 (2) |
|
6,873 |
|
|
$ |
89.12 |
|
|
|
|
|
December 1, 2023 –
December 31, 2023 (2) |
|
907 |
|
|
$ |
93.04 |
|
|
|
|
|
For the Quarter Ended
December 31, 2023 |
|
11,316 |
|
|
$ |
90.57 |
|
|
|
|
|
(1)On June 11, 2021, our Board of Directors authorized a new share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period that commenced in July 2021. These share repurchases have been made pursuant to the $7 billion program. On May 11, 2022, we announced the suspension of our three-year share repurchase program following the recommended public offer to acquire the outstanding shares of Swedish Match from its shareholders. For further details on the offer, see the Acquisitions and Other Business Arrangements section of Part II, Item 7 of this Form 10-K.
(2)Shares repurchased represent shares tendered to us by employees who vested in restricted and performance share unit awards and used shares to pay all, or a portion of, the related taxes.
Item 6. [Reserved].
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion should be read in conjunction with the other sections of this Annual Report on Form 10-K, including the consolidated financial statements and related notes contained in Item 8, and the discussion of risks and cautionary factors that may affect future results in Item 1A. Risk Factors.
Description of Our Company
We are a leading international tobacco company, actively delivering a smoke-free future. We are evolving our portfolio for the long term to include products outside of the tobacco and nicotine sector. Our current product portfolio primarily consists of cigarettes and smoke-free products. Since 2008, we have invested $12.5 billion to develop, scientifically substantiate and commercialize innovative smoke-free products for adults who would otherwise continue to smoke, with the goal of completely ending the sale of cigarettes. This investment includes the building of world-class scientific assessment capabilities, notably in the areas of pre-clinical systems toxicology, clinical and behavioral research, as well as post-market studies. In November 2022, we acquired Swedish Match AB ("Swedish Match"), a leader in oral nicotine delivery, creating a global smoke-free combination led by the companies’ IQOS and ZYN brands. The U.S. Food and Drug Administration (the "FDA") has authorized versions of our IQOS Platform 1 devices and consumables, and Swedish Match's General snus, as Modified Risk Tobacco Products ("MRTPs"). We describe the MRTP orders in more detail in the "Business Environment" section of this Item 7.
In January 2023, we began managing our business in four geographical segments, down from six previously, in addition to our continuing Swedish Match and Wellness and Healthcare segments: As of December 31, 2023, our operating segments were as follows:
•Europe Region;
•South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA");
•East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF");
•Americas Region;
•Swedish Match, which reflects our fourth quarter 2022 acquisition of the company; and
•Wellness and Healthcare ("W&H"), which includes the operating results of our Vectura Fertin Pharma business.
Following the combination and the progress in 2023 toward integrating the Swedish Match business into the existing PMI regional segment structure, we will update our segment reporting by including Swedish Match results in the four existing geographical segments. As of the first quarter of 2024, we will report on this basis.
Our cigarettes are sold in approximately 175 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands.
Smoke-free products (also referred to herein as "SFPs") is the term we primarily use to refer to all of our products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral nicotine. In addition, SFPs include wellness and healthcare products, as well as consumer accessories such as lighters and matches.
In addition to the manufacture and sale of cigarettes, we are engaged in the development and commercialization of reduced-risk products ("RRPs"). RRPs is the term we use to refer to products that present, are likely to present, or have the potential to present less risk of harm to smokers who switch to these products versus continuing smoking. We have a range of RRPs in various stages of development, scientific assessment and commercialization. Our RRPs are SFPs that contain and/or generate far lower quantities of harmful and potentially harmful constituents than found in cigarette smoke. IQOS is the leading brand in our SFPs portfolio. As of December 31, 2023, our smoke-free products were available for sale in 84 markets.
In 2021, we laid the foundation for our long-term growth ambitions beyond nicotine in wellness and healthcare, including the milestone acquisitions of Vectura Group plc ("Vectura") and Fertin Pharma A/S ("Fertin Pharma"), which provide essential capabilities for future product development. Now, through our Vectura Fertin Pharma business, with a strong foundation and significant expertise in life sciences, we aim to expand into wellness and healthcare areas.
In 2022, we acquired Swedish Match AB, a market leader in oral nicotine delivery with a significant presence in the United States market. The Swedish Match acquisition is a key milestone in PMI’s transformation to becoming a smoke-free company. Swedish Match has a leading nicotine pouch franchise in the U.S. under the ZYN brand name. The Swedish Match product portfolio is complementary to our existing portfolio, permitting us to bring together a leading oral nicotine product with the leading heat-not-burn product. By joining forces with Swedish Match, we expect to accelerate the achievement of our joint smoke-free ambitions, switching more adults who would otherwise continue to smoke cigarettes to better alternatives faster than either company could achieve separately.
In 2022, we also completed an agreement with Altria Group, Inc. to end our commercial relationship in the U.S. covering IQOS as of April 30, 2024. Thereafter, PMI will hold the full rights to commercialize IQOS in the U.S. On July, 14, 2023, we made the final payment to Altria under the terms of the agreement.
For further details of our 2021 and 2022 acquisitions, as well as the agreement with Altria Group, Inc., see Item 8, Note 3. Acquisitions and the "Business Environment" section of this Item 7
We use the term net revenues to refer to our operating revenues from the sale of our products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. Our net revenues and operating income are affected by various factors, including the volume of products we sell, the price of our products, changes in currency exchange rates and the mix of products we sell. Mix is a term used to refer to the proportionate value of premium-price brands to mid-price or low-price brands in any given market (product mix). Mix can also refer to the proportion of shipment volume in more profitable markets versus shipment volume in less profitable markets (geographic mix).
Our cost of sales consists principally of: tobacco leaf, non-tobacco raw materials, labor and manufacturing costs; shipping and handling costs; and the cost of devices produced by third-party electronics manufacturing service providers. Estimated costs associated with device warranty programs are generally provided for in cost of sales in the period the related revenues are recognized.
Our marketing, administration and research costs include the costs of marketing and selling our products, other costs generally not related to the manufacture of our products (including general corporate expenses), and costs incurred to develop new products. The most significant components of our marketing, administration and research costs are marketing and sales expenses and general and administrative expenses.
Philip Morris International Inc. is a legal entity separate and distinct from its direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions that are otherwise compliant with law.
Executive Summary
The following executive summary provides the business update and significant highlights from the Discussion and Analysis that follows.
Global Patent Settlement
On February 1, 2024, we entered into a global settlement agreement with British American Tobacco p.l.c. ("BAT") that resolves all ongoing patent infringement litigation between the parties related to heated tobacco and vapor products. The settlement includes non-monetary provisions between PMI and BAT that resolve all ongoing global patent infringement litigation, encompassing all related injunctions and exclusion orders, and prevents patent infringement and certain other future claims against current heated tobacco and vapor products. Under the settlement PMI and BAT also agreed to request rescission of the Limited Exclusion Order and Cease and Desist Order issued by the International Trade Commission prohibiting the importation of certain heat-not-burn products by PMI and its affiliates into the U.S. The settlement also allows each party to innovate and introduce product iterations. For further details, see Item 8, Note 18. Contingencies.
War in Ukraine
In Ukraine, our main priority remains the safety and security of our employees and their families in the country. We continue commercial activities in select locations where safety allows, in order to provide product availability and service to adult consumers, and supply the market from production centers outside Ukraine, as well as through a contract manufacturing arrangement. Production at our factory in Kharkiv remains suspended. On June 20, 2023, we announced the investment of $30 million in a new production facility in the Lviv region, in Western Ukraine. Preparatory work for the facility began in July 2023 and production is expected to commence in the first quarter of 2024. As of December 31, 2023, our Ukrainian operations had approximately $0.4 billion in total assets, excluding intercompany balances.
In Russia, we are continuously assessing the evolving situation in the country. This includes regulatory constraints in the market entailing very complex terms and conditions that must be met for any divestment transaction to be granted approval by the authorities, and restrictions resulting from international regulations. In the event of a divestment, our ability to fully realize the value of the business would likely be subject to material impairment. As of December 31, 2023, our Russian operations had approximately $2.7 billion in total assets, excluding intercompany balances, of which approximately $0.8 billion consisted of cash and equivalents held mostly in local currency (Russian rubles).
Additionally, we hold a 23% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis, PMI's distributor in Russia. For further details, see Item 8, Note 6. Related Parties – Equity Investments and Other.
These developments above have and will continue to have a material adverse impact on our business, results of operations, cash flows and financial position, and may result in impairment charges.
For further details, see Item 8, Note 4. War in Ukraine to our consolidated financial statements, as well as Item 1A. Risk Factors and the "Trade Policy" section of this Item 7.
Consolidated Operating Results
•Net Revenues – Net revenues of $35.2 billion for the year ended December 31, 2023, increased by $3.4 billion, or 10.7%, from the comparable 2022 amount. The change in our net revenues from the comparable 2022 amount was driven by the following (variances not to scale):
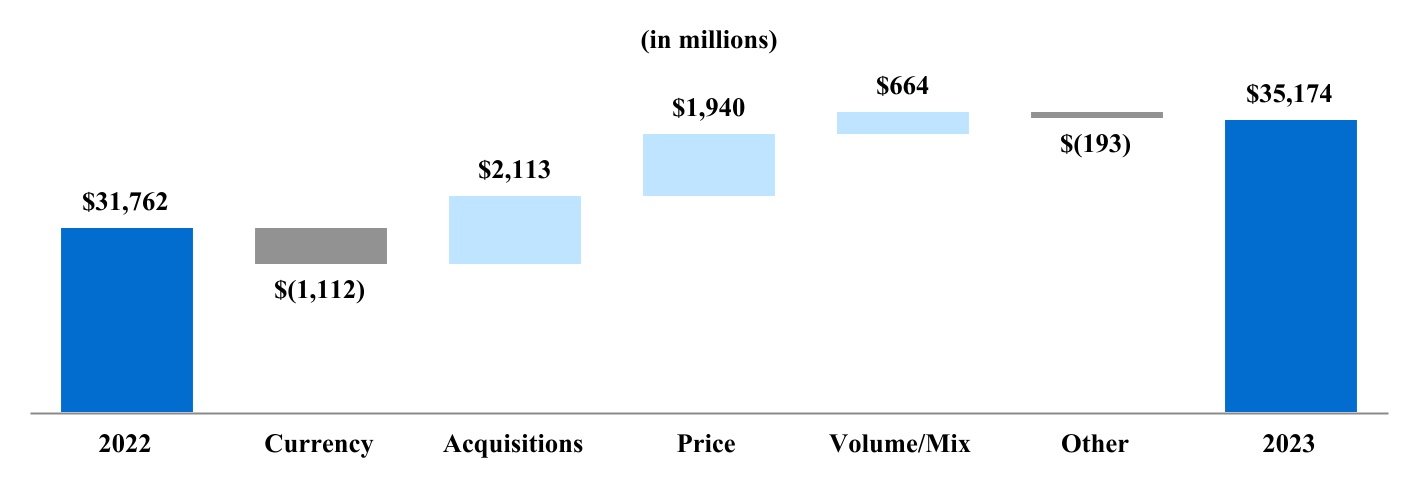
Net revenues increased by 10.7%, including the impact of the Swedish Match acquisition and currency. Net revenues, excluding currency and acquisitions, increased by 7.6%, mainly reflecting: a favorable pricing variance, primarily driven by higher combustible tobacco pricing, and favorable volume/mix, mainly driven by higher HTU volume, partially offset by lower cigarette volume. The increase was partly offset by lower fees for certain distribution rights billed to customers in certain markets and a charge to net revenues in 2023 of $80 million following the termination of a distribution arrangement in the Middle East, both shown in "Other." The termination of a distribution arrangement in the Middle East is further described in the following "Diluted Earnings Per Share" discussion.
Net revenues by product category for the years ended December 31, 2023 and 2022, are shown below:
•Diluted Earnings Per Share – The changes in our reported diluted earnings per share (“diluted EPS”) for the year ended December 31, 2023, from the comparable 2022 amounts, were as follows:
|
|
|
|
|
|
|
|
|
|
Diluted EPS |
% Change |
| For the year ended December 31, 2022 |
$ |
5.81 |
|
|
|
|
|
| 2022 Charges related to the war in Ukraine |
0.08 |
|
|
| 2022 Fair value adjustment for equity security investments |
(0.02) |
|
|
| 2022 Amortization of intangibles |
0.09 |
|
|
| 2022 Impairment of goodwill and other intangibles |
0.06 |
|
|
| 2022 Costs associated with Swedish Match AB offer |
0.06 |
|
|
| 2022 Swedish Match AB acquisition accounting related item |
0.06 |
|
|
| 2022 Income tax impact associated with Swedish Match AB financing |
(0.13) |
|
|
| 2022 Tax items |
(0.03) |
|
|
| Subtotal of 2022 items |
0.17 |
|
|
|
|
|
| 2023 Charges related to the war in Ukraine |
(0.03) |
|
|
| 2023 Asset impairment and exit costs |
(0.06) |
|
|
| 2023 South Korea indirect tax charge |
(0.11) |
|
|
| 2023 Termination of agreement with Foundation for a Smoke-Free World |
(0.07) |
|
|
| 2023 Fair value adjustment for equity security investments |
0.02 |
|
|
| 2023 Amortization of intangibles |
(0.25) |
|
|
| 2023 Impairment of goodwill and other intangibles |
(0.44) |
|
|
| 2023 Termination of distribution arrangement in the Middle East |
(0.04) |
|
|
| 2023 Swedish Match AB acquisition accounting related item |
(0.01) |
|
|
| 2023 Income tax impact associated with Swedish Match AB financing |
0.11 |
|
|
| 2023 Tax items |
(0.11) |
|
|
| Subtotal of 2023 items |
(0.99) |
|
|
|
|
|
| Currency |
(0.63) |
|
|
| Interest |
(0.21) |
|
|
| Change in tax rate |
0.03 |
|
|
| Operations |
0.84 |
|
|
| For the year ended December 31, 2023 |
$ |
5.02 |
|
(13.6) |
% |
Charges related to the war in Ukraine – During 2022, we recorded a pre-tax charge of $151 million (representing $128 million net of income tax and a diluted EPS charge of $0.08 per share), related to circumstances driven by the war, including machinery and inventory write-downs, additional allowances for receivables and the cost of PMI’s humanitarian efforts. During 2023, we recorded a pre-tax charge of $53 million (representing $43 million net of income tax and a diluted EPS charge of $0.03 per share), related to circumstances driven by the war, including the cost of PMI’s humanitarian efforts, severance payments, as well as an impairment of certain long-lived assets. For further details, see Item 8, Note 4. War in Ukraine.
Fair value adjustment for equity security investments – During 2022, we recorded a favorable fair value adjustment for our equity security investments in India and Sri Lanka ($0.02 per share increase in diluted EPS). During 2023, we recorded a favorable fair value adjustment for our equity security investments in India and Sri Lanka ($0.02 per share increase in diluted EPS). For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other.
Amortization of intangibles – During 2022, we recorded amortization of intangibles expense of $159 million (representing $129 million net of income tax or $0.09 per share decrease in diluted EPS). During 2023, we recorded amortization of intangibles expense of $497 million (representing $389 million net of income tax or $0.25 per share decrease in diluted EPS). The higher amortization expense in 2023 was primarily due to increased acquired intangible assets recorded as a result of our acquisitions in 2022. For further details, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
Impairment of goodwill and other intangibles – During 2022, we recorded an impairment charge related to definite-lived intangible assets of $112 million (representing $98 million net of income tax and a diluted EPS charge of $0.06 per share) reflecting the impact of general economic and market conditions resulting in a reduction in future estimated cash flows on certain products within the Wellness and Healthcare segment. This impairment charge was recorded within cost of sales in the consolidated statements of earnings for the year ended December 31, 2022.
During the second quarter of 2023, we completed our annual review of goodwill and non-amortizable intangible assets for potential impairment. Based on this review, it was determined that the estimated fair value of the Wellness and Healthcare reporting unit was lower than its carrying value. Consequently, we recorded a total non-cash impairment charge of $680 million (representing a $0.44 per share decrease in diluted EPS) consisting of a goodwill impairment charge of $665 million and a non-amortizable intangible asset pre-tax impairment charge of $15 million for an in-process research and development project related to one of our 2021 acquisitions. The impairment charge was recorded in impairment of goodwill ($665 million) and marketing, administration and research costs ($15 million) within the consolidated statements of earnings during the year ended December 31, 2023, and was included in the Wellness and Healthcare segment results.
For further details, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
Costs associated with Swedish Match AB offer – During 2022, we incurred pre-tax costs associated with the Swedish Match offer of $116 million (representing $99 million net of income tax and a diluted EPS charge of $0.06 per share) primarily related to financing costs, derivative financial instruments and certain transaction related costs. These pre-tax costs of $116 million were recorded in marketing, administration and research costs ($115 million expense) and interest expense, net ($1 million expense) on our consolidated statement of earnings for the year ended December 31, 2022.
Swedish Match AB acquisition accounting related item – Following the Swedish Match acquisition, we recorded in 2022 pre-tax purchase accounting adjustments of $125 million related to the sale of acquired inventories stepped up to fair value (representing $94 million net of income tax and a diluted EPS charge of $0.06 per share). During 2023, we recorded pre-tax purchase accounting adjustments of $18 million related to the sale of acquired inventories stepped up to fair value (representing $13 million net of income tax and a diluted EPS charge of $0.01 per share). These pre-tax adjustments were recorded in cost of sales in the consolidated statements of earnings for the years ended December 31, 2023 and 2022. For further details, see Item 8, Note 3. Acquisitions.
Asset impairment and exit costs – During 2023, we recorded pre-tax asset impairment and exit costs of $109 million, representing $96 million net of income tax and a diluted EPS charge of $0.06 per share, related to a project to fully outsource and restructure the manufacturing of e-vapor devices and consumables. For further details, see Item 8, Note 20. Asset Impairment and Exit Costs.
South Korea indirect tax charge – On July 13, 2023, our South Korean subsidiary, PM Korea, received an adverse ruling from the Supreme Court of South Korea related to cases alleging underpayment of excise taxes in connection with a 2015 excise tax increase and subsequent audit by the South Korean Board of Audit and Inspection. The Supreme Court ruling reversed previous decisions that were in PM Korea’s favor at the trial and appellate levels. As a result of the ruling, we concluded that an adverse outcome is probable. Consequently, we recorded a non-cash pre-tax charge of $204 million (representing $174 million net of income tax or $0.11 per share decrease in diluted EPS) in the second quarter results of 2023, reflecting the full amount previously paid by PM Korea. For further details, see Item 8, Note 18. Contingencies.
Termination of agreement with Foundation for a Smoke-Free World – On September 29, 2023, PMI and the Foundation for a Smoke-Free World (the "Foundation") entered into the Final Grant Agreement and Termination of the Second Amended and Restated Pledge Agreement ("Agreement"). Under the terms of the Agreement, PMI paid $140 million in the third quarter of 2023 in return for the termination of the pledge agreement between the parties. As a result, in the third quarter of 2023, PMI recorded a pre-tax charge of $140 million (representing $111 million net of income tax or $0.07 per share decrease in diluted EPS) commensurate with the early termination of the pledge agreement. The pre-tax charge was recorded in marketing, administration and research costs in the consolidated statements of earnings during the year ended December 31, 2023. For further details, see "Other Developments" within the Business Environment section of this Item 7.
Termination of distribution arrangement in the Middle East – Following the termination of a distribution arrangement in the Middle East, we recorded a pre-tax charge of $80 million in the first quarter of 2023 (representing $70 million net of income tax and a diluted EPS charge of $0.04 per share). The pre-tax charge was recorded as a reduction of net revenues in the consolidated statements of earnings and was included in the SSEA, CIS & MEA segment results.
Income taxes – The Income tax impact associated with Swedish Match AB financing that increased our 2022 diluted EPS by $0.13 per share and increased our 2023 diluted EPS by $0.11 per share in the table above was due to a deferred tax benefit for unrealized foreign currency losses on intercompany loans related to the Swedish Match acquisition financing reflected in the consolidated statements of earnings, while the underlying pre-tax foreign currency movements fully offset in the consolidated statements of earnings and were reflected as currency translation adjustments in the consolidated statements of stockholders' (deficit) equity.
The 2022 Tax items that increased our 2022 diluted EPS by $0.03 per share in the table above were due to a reduction in deferred tax liabilities related to pension plan assets of $40 million.
The 2023 tax items that decreased our 2023 diluted EPS by $0.11 per share in the table above were due to an increase in deferred tax liabilities related to the unremitted earnings of PMI's Russian subsidiaries due to the unilateral suspension of certain Russian double tax treaties by the Russian authorities on August 8, 2023, with respect to certain payments including dividends. The change in the tax rate that increased our diluted EPS by $0.03 per share in the table above was primarily due to changes in earnings mix by taxing jurisdiction.
Currency – The unfavorable impact of $0.63 per share during the reporting period primarily results from the fluctuations of the U.S. dollar, especially against the Argentine peso, Egyptian pound, Japanese yen, Russian ruble and Swiss franc, partly offset by the Euro. This unfavorable currency movement has impacted our profitability across our primary revenue markets and local currency cost bases.
Interest – The unfavorable impact of $0.21 per share from interest in the table above was due primarily to higher interest expense in connection with the Swedish Match acquisition, partially offset by higher net interest income driven by higher interest rates.
Operations – The increase in diluted EPS of $0.84 per share from our operations in the table above was due primarily to the following segments:
•Swedish Match: Reflecting the 2023 impact following the fourth quarter 2022 acquisition; and
•EA, AU & PMI DF: Favorable volume/mix, favorable pricing and lower supply chain costs;
partially offset by
•Americas: Unfavorable volume/mix and higher marketing, administration and research costs, partly offset by favorable pricing;
•SSEA, CIS & MEA: Higher marketing, administration and research costs, higher manufacturing costs, unfavorable volume/mix and the impact of lower fees for certain distribution rights billed to customers in certain markets, partly offset by favorable pricing;
•Wellness and Healthcare: Primarily reflecting commercial investments and higher administration costs; and
•Europe: Higher marketing, administration and research costs, higher manufacturing costs and unfavorable volume/mix, partly offset by favorable pricing.
For further details, see the Consolidated Operating Results and Operating Results by Business Segment sections of the following Discussion and Analysis.
Discussion and Analysis
Critical Accounting Estimates
Item 8, Note 2. Summary of Significant Accounting Policies to our consolidated financial statements includes a summary of the significant accounting policies and methods used in the preparation of our consolidated financial statements. In most instances, we must use a particular accounting policy or method because it is the only one that is permitted under U.S. GAAP.
The preparation of financial statements requires that we use estimates and assumptions that affect the reported amounts of our assets, liabilities, net revenues and expenses, as well as our disclosure of contingencies. If actual amounts differ from previous estimates, we include the revisions in our consolidated results of operations in the period during which we know the actual amounts. Historically, aggregate differences, if any, between our estimates and actual amounts in any year have not had a significant impact on our consolidated financial statements.
The selection and disclosure of our critical accounting estimates have been discussed with our Audit & Risk Committee. The following is a discussion of the more significant assumptions, estimates, accounting policies and methods used in the preparation of our consolidated financial statements:
Acquisitions - PMI accounts for business combinations using the acquisition method of accounting. PMI allocates the purchase price of an acquired business to the assets acquired and liabilities assumed based upon their estimated fair values at the acquisition date with the excess recorded as Goodwill. The fair value of the applicable assets acquired and liabilities assumed is determined through established valuation techniques, such as the income, cost or market approach. PMI may utilize third-party valuation experts to assist in the fair value determination of certain assets acquired and liabilities assumed. The determination of fair value requires management to make judgements and may involve the use of significant estimates, including assumptions with respect to estimated projected revenue growth, future cash flows, terminal growth rates, useful economic lives of intangible assets acquired, discount rates, royalty rates and other factors. Certain acquired intangibles are expected to have indefinite lives based on their history and PMI’s intent to continue to support and build the intangible.
Although PMI believes its estimates of fair value are reasonable, actual financial results could differ from those estimates. Changes in assumptions related to future financial results or other underlying assumptions could have a significant impact on the determination of the fair value of the intangible assets acquired.
See Item 8, Note 3. Acquisitions to our consolidated financial statements for details of the critical accounting estimates relevant to the business combinations in the periods presented in this Form 10-K.
Revenue Recognition - We recognize revenue as performance obligations are satisfied. Our primary performance obligation is the distribution and sales of cigarettes and smoke-free products, including heat-not-burn, e-vapor and oral nicotine products. Our performance obligations are typically satisfied upon shipment or delivery to our customers. PMI estimates the cost of sales returns based on historical experience, and these estimates are immaterial. Estimated costs associated with warranty programs for IQOS devices are generally provided for in cost of sales in the period the related revenues are recognized, based on a number of factors, including historical experience, product failure rates and warranty policies. The transaction price is typically based on the amount billed to the customer and includes estimated variable consideration where applicable. Such variable consideration is typically not constrained and is estimated based on the most likely amount that PMI expects to be entitled to under the terms of the contracts with customers, historical experience of discount or rebate redemption, where relevant, and the terms of any underlying discount or rebate programs, which may change from time to time as the business and product categories evolve.
Goodwill and Non-Amortizable Intangible Assets Valuation - We test goodwill and non-amortizable intangible assets for impairment annually or more frequently if events occur that would warrant such review, using either a qualitative or quantitative assessment for each of our reporting units. Where a qualitative assessment is followed, we determine whether it is more likely than not that an impairment exists based on qualitative factors such as macroeconomic conditions, industry and competitive conditions, legal and regulatory environment, historical financial performance and significant changes within the reporting unit. If the qualitative assessment indicates that it is more likely than not that an impairment exists, then a quantitative assessment is performed. The quantitative impairment analysis involves comparing the fair value of each reporting unit or non-amortizable intangible asset to the carrying value. If the carrying value exceeds the fair value, goodwill or a non-amortizable intangible asset is considered impaired. To determine the fair value of a reporting unit, we use the market approach using earnings multiples of comparable global companies within the tobacco industry and a discounted cash flow model. To determine the fair value of non-amortizable intangible assets, we primarily use a discounted cash flow model applying the relief-from-royalty method. These discounted cash flow models include management assumptions relevant for forecasting operating cash flows, which are subject to changes in business conditions, such as volumes and prices, costs to produce, discount rates and estimated capital needs. Management considers historical experience and all available information at the time the fair values are estimated, and we believe these assumptions are consistent with the assumptions a hypothetical marketplace participant would use.
During the second quarter of 2023, PMI completed its annual review of goodwill and non-amortizable intangible assets for potential impairment. We performed a quantitative impairment assessment for all of our reporting units and non-amortizable intangible assets with the exception of the three reporting units and non-amortizable intangible asset related to the Swedish Match segment for which we have performed a qualitative assessment. Based on this review, it was determined that the estimated fair value of the Wellness and Healthcare reporting unit was lower than its carrying value.
Consequently, PMI recorded a goodwill impairment charge of $665 million. Additionally, as a result of the impairment test of non-amortizable intangible assets, PMI recorded a pre-tax impairment charge of $15 million. There was no impairment charge of goodwill and non-amortizable intangible assets for our other reporting units. For additional information, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
At December 31, 2023, the carrying value of our goodwill was $16.8 billion, which is related to ten geographical reporting units, each of which consists of a group of markets with similar operating and economic characteristics, three reporting units related to the Swedish Match segment and the Wellness and Healthcare business. The estimated fair value of each of our fourteen reporting units and non-amortizable intangible assets exceeded the carrying value as of December 31, 2023.
Investment in non-marketable equity securities – We perform a sensitivity analysis to estimate the value of our continuing investment in RBH on an ongoing basis. The analysis includes estimating a range of fair values to determine whether any indicators of impairment exist. The estimated range of fair values of the underlying business and of the contingent liability is determined based on an income approach using a discounted cash flow analysis. The information used in the estimate includes observable inputs, such as discount rates, terminal growth rates, previous court rulings, total tobacco market size in Canada, RBH’s share of the market and tobacco-litigation related expense, as well as unobservable inputs such as operating budgets and strategic plans, various inflation scenarios, estimated shipment volumes, and expected product pricing and projected margins.
For further details, see Item 8, Note 6. Related Parties – Equity investments and Other to our consolidated financial statements
Marketing Costs - We incur certain costs to support our products through programs that include advertising, marketing, consumer engagement and trade promotions. The costs of our advertising and marketing programs are expensed in accordance with U.S. GAAP. Recognition of the cost related to our consumer engagement and trade promotion programs contain uncertainties due to the judgment required in estimating the potential performance and compliance for each program. For volume-based incentives provided to customers, management continually assesses and estimates, by customer, the likelihood of the customer's achieving the specified targets, and records the reduction of revenue as the sales are made. For other trade promotions, management relies on estimated utilization rates that have been developed from historical experience. Changes in the assumptions used in estimating the cost of any individual marketing program would not result in a material change in our financial position, results of operations or operating cash flows.
Employee Benefit Plans - As discussed in Item 8, Note 14. Benefit Plans to our consolidated financial statements, we provide a range of benefits to our employees and retired employees, including pensions, postretirement health care and postemployment benefits (primarily severance). We record annual amounts relating to these plans based on calculations specified by U.S. GAAP. These calculations include various actuarial assumptions, such as discount rates, assumed rates of return on plan assets, compensation increases, mortality, turnover rates and health care cost trend rates. We review actuarial assumptions on an annual basis and make modifications to the assumptions based on current rates and trends when it is deemed appropriate to do so. As permitted by U.S. GAAP, any effect of the modifications is generally amortized over future periods. We believe that the assumptions utilized in calculating our obligations under these plans are reasonable based upon our historical experience and advice from our actuaries.
Weighted-average discount rate assumptions for pension and postretirement plan obligations at December 31, 2023 and 2022 are as follows:
|
|
|
|
|
|
|
|
|
|
2023 |
2022 |
| Pension plans |
2.28% |
3.03% |
| Postretirement plans |
5.19% |
5.89% |
We anticipate that assumption changes will increase 2024 pre-tax pension and postretirement expense to approximately $154 million as compared with approximately $106 million in 2023, excluding amounts related to employee severance and early retirement programs. The anticipated increase is primarily due to higher amortization of unrecognized actuarial losses of $72 million, coupled with higher service cost of $39 million, partially offset by higher expected return on assets of $29 million, coupled with lower interest cost of $26 million and other movements of $8 million.
Weighted-average expected rate of return and discount rate assumptions have a significant effect on the amount of expense reported for the employee benefit plans. A fifty-basis-point decrease in our discount rate would increase our 2024 pension and postretirement expense by approximately $57 million, and a fifty-basis-point increase in our discount rate would decrease our 2024 pension and postretirement expense by approximately $48 million. Similarly, a fifty-basis-point decrease (increase) in the expected return on plan assets would increase (decrease) our 2024 pension expense by approximately $41 million.
Income Taxes - Income tax provisions for jurisdictions outside the United States, as well as state and local income tax provisions, are determined on a separate company basis, and the related assets and liabilities are recorded in our consolidated balance sheets.
The extent of our operations involves dealing with uncertainties and judgments in the application of complex tax regulations in a multitude of jurisdictions. The final taxes paid are dependent upon many factors, including negotiations with taxing authorities in various jurisdictions and resolution of disputes arising from federal, state, and international tax audits. In accordance with the authoritative guidance for income taxes, we evaluate potential tax exposures and record tax liabilities for anticipated tax audit issues based on our estimate of whether, and the extent to which, additional taxes will be due. We adjust these reserves in light of changing facts and circumstances; however, due to the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from our current estimate of the tax liabilities. If our estimate of tax liabilities proves to be less than the ultimate assessment, an additional charge to expense would generally result. If payment of these amounts ultimately proves to be less than the recorded amounts, the reversal of the liabilities would result in tax benefits being recognized in the period when we determine the liabilities are no longer necessary.
We are required to assess the likelihood of recovering deferred tax assets against future sources of taxable income. If we determine, using all available evidence, that we do not reach the more likely than not threshold for recovery, a valuation allowance is recorded. Significant judgment is required in determining the need for and amount of valuation allowances for deferred tax assets including estimates of future taxable income in the applicable jurisdictions and the feasibility of on-going tax planning strategies, as applicable.
The effective tax rates used for interim reporting are based on our full-year geographic earnings mix projections. Changes in currency exchange rates, earnings mix by taxing jurisdiction or future regulatory developments may have an impact on the effective tax rates. Significant judgment is required in determining income tax provisions and in evaluating tax positions.
For further details, see Item 8, Note 12. Income Taxes to our consolidated financial statements.
Hedging - As discussed below in “Market Risk,” we use derivative financial instruments principally to reduce exposures to market risks resulting from fluctuations in foreign currency exchange and interest rates by creating offsetting exposures. For derivative contracts that are designated and qualify as fair value hedges the gain or loss on the derivative, as well as the offsetting gain or loss on the hedged items attributable to the hedged risk, is recognized in the consolidated statement of earnings. For our other derivatives to which we have elected to apply hedge accounting, gains and losses on these derivatives are initially deferred in accumulated other comprehensive losses on the consolidated balance sheet and recognized in the consolidated statement of earnings into the same line item as the impact of the underlying transaction and in the periods when the related hedged transactions are also recognized in operating results. Gain (losses) related to derivatives contracts for which hedge accounting provisions have not been elected are recognized in the consolidated statement of earnings.
Contingencies - As discussed in Item 8, Note 18. Contingencies, to our consolidated financial statements, legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. At the present time, except as stated otherwise in Item 8, Note 18. Contingencies, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available to it: (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
Consolidated Operating Results
Our net revenues and operating income by segment were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
2023 |
2022 |
2021 |
Net Revenues |
|
|
|
| Europe |
$ |
13,598 |
|
$ |
12,869 |
|
$ |
13,155 |
|
SSEA, CIS & MEA |
10,629 |
|
10,467 |
|
9,858 |
|
EA, AU & PMI DF |
6,201 |
|
5,936 |
|
6,448 |
|
| Americas |
1,944 |
|
1,903 |
|
1,843 |
|
| Swedish Match |
2,496 |
|
316 |
|
— |
|
| Wellness and Healthcare |
306 |
|
271 |
|
101 |
|
| Net revenues |
$ |
35,174 |
|
$ |
31,762 |
|
$ |
31,405 |
|
Operating Income (Loss) |
|
|
|
| Europe |
$ |
6,012 |
|
$ |
5,802 |
|
$ |
6,409 |
|
SSEA, CIS & MEA |
3,047 |
|
3,864 |
|
3,295 |
|
EA, AU & PMI DF |
2,481 |
|
2,424 |
|
2,836 |
|
| Americas |
62 |
|
436 |
|
487 |
|
| Swedish Match |
824 |
|
(22) |
|
— |
|
| Wellness and Healthcare |
(870) |
|
(258) |
|
(52) |
|
| Operating income |
$ |
11,556 |
|
$ |
12,246 |
|
$ |
12,975 |
|
Items affecting the comparability of results from operations were as follows:
•Impairment of goodwill and other intangibles – For the year ended December 31, 2023, PMI recorded $680 million of goodwill and non-amortizable intangible assets impairment charges that was included in the Wellness and Healthcare segment. For the year ended December 31, 2022, PMI recorded an impairment charge related to definite-lived intangible assets of $112 million. This charge was included in the Wellness and Healthcare segment. For further details, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
•South Korea indirect tax charge – See Item 8, Note 18. Contingencies for details of the $204 million pre-tax charge included in the EA, AU & PMI DF segment results for the year ended December 31, 2023.
•Termination of distribution arrangement in the Middle East – In the first quarter of 2023, PMI recorded a pre-tax charge of $80 million following the termination of a distribution arrangement in the Middle East. This pre-tax charge was recorded as a reduction of net revenues in the consolidated statements of earnings, and was included in the SSEA, CIS & MEA segment results for the year ended December 31, 2023.
•Charges related to the war in Ukraine - See Item 8, Note 4. War in Ukraine for details of the $53 million and $151 million pre-tax charges in the Europe segment for the years ended December 31, 2023 and 2022, respectively.
•Swedish Match AB acquisition accounting related item - See Item 8, Note 3. Acquisitions for details of the $18 million and $125 million pre-tax purchase accounting adjustments related to the sale of acquired inventories stepped up to fair value included in the Swedish Match segment for the years ended December 31, 2023 and 2022, respectively.
•Asset impairment and exit costs - See Item 8, Note 20. Asset Impairment and Exit Costs for details of the $109 million and $216 million pre-tax charges for the year ended December 31, 2023 and 2021, respectively, as well as a breakdown of these costs by segment.
•Termination of agreement with Foundation for a Smoke-Free World – On September 29, 2023, PMI and the Foundation for a Smoke-Free World (the "Foundation") entered into the Final Grant Agreement and Termination of the Second Amended and Restated Pledge Agreement ("Agreement"). Under the terms of the agreement, PMI paid $140 million in the third quarter of 2023 in return for the termination of the pledge agreement between the parties. As a result, in the third quarter of 2023, PMI recorded a pre-tax charge of $140 million commensurate with the early termination of the pledge agreement. The pre-tax charge was recorded in marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2023 and was included in the operating results of the following segments: Europe ($62 million); SSEA, CIS & MEA ($44 million); EA, AU & PMI DF ($27 million); and Americas ($7 million).
•Saudi Arabia customs assessments - In June 2021, PMI recorded a pre-tax charge of $246 million in relation to additional customs duties in Saudi Arabia assessed for the periods of 2014 through 2020 in line with existing and contemplated arrangements with our distributors. In accordance with U.S. GAAP, the charge was recorded as a reduction in net revenues of combustible tobacco products included in the SSEA, CIS & MEA segment for the year ended December 31, 2021.
•Asset acquisition cost - See Item 8, Note 3. Acquisitions for the details of the $51 million pre-tax charge associated with the asset acquisition of OtiTopic, Inc. included in the Wellness and Healthcare segment within the operating income table above for the year ended December 31, 2021.
Our net revenues by product category were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| PMI Net Revenues by Product Category |
| (in millions) |
2023 |
2022 |
2021 |
| Combustible tobacco products |
|
|
|
| Europe |
$ |
8,037 |
|
$ |
7,694 |
|
$ |
8,767 |
|
SSEA, CIS & MEA |
9,321 |
|
9,173 |
|
8,734 |
|
EA, AU & PMI DF |
2,676 |
|
2,831 |
|
2,861 |
|
| Americas |
1,869 |
|
1,804 |
|
1,706 |
|
| Swedish Match |
431 |
|
70 |
|
— |
|
| Total combustible tobacco products |
22,334 |
|
21,572 |
|
22,067 |
|
| Smoke-free products |
|
|
|
| Smoke-free products excluding Wellness and Healthcare: |
|
|
|
| Europe |
5,561 |
|
5,175 |
|
4,388 |
|
| SSEA, CIS & MEA |
1,308 |
|
1,294 |
|
1,124 |
|
| EA, AU & PMI DF |
3,525 |
|
3,105 |
|
3,587 |
|
| Americas |
75 |
|
99 |
|
137 |
|
| Swedish Match |
2,065 |
|
246 |
|
— |
|
| Total smoke-free products excluding Wellness and Healthcare |
12,534 |
|
9,919 |
|
9,237 |
|
| Wellness and Healthcare |
306 |
|
271 |
|
101 |
|
| Total smoke-free products |
12,840 |
|
10,190 |
|
9,338 |
|
|
|
|
|
| Total PMI net revenues |
$ |
35,174 |
|
$ |
31,762 |
|
$ |
31,405 |
|
Note: Sum of product categories or Regions might not foot to total PMI due to rounding.
Net revenues related to combustible tobacco products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of PMI's cigarettes and other tobacco products that are combusted. Other tobacco products primarily include roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos and do not include smoke-free products.
Net revenues related to smoke-free products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes, if applicable. These net revenue amounts consist of the sale of all of PMI's products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral nicotine, also including wellness and healthcare products, as well as consumer accessories such as lighters and matches.
Net revenues related to wellness and healthcare products consist of operating revenues generated from the sale of products primarily associated with inhaled therapeutics, and oral and intra-oral delivery systems that are included in the operating results of PMI's Wellness and Healthcare business, Vectura Fertin Pharma.
PMI's heat-not-burn products include licensed KT&G heat-not-burn products.
References to "Cost/Other" in the Consolidated Financial Summary table of total PMI and the six segments throughout this "Discussion and Analysis" reflects the currency-neutral variances of: cost of sales (excluding the volume/mix cost component); marketing, administration and research costs (including asset impairment and exit costs); and amortization and impairment of intangibles. “Cost/Other” also includes the currency-neutral net revenue variance, unrelated to volume/mix and price components, attributable to: fees for certain distribution rights billed to customers in certain markets in the SSEA, CIS & MEA Region, the revenue adjustment for the termination of a distribution arrangement in the Middle East, and the Saudi Arabia customs assessment net revenue adjustment.
Our consolidated shipment volume is shown in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
| Consolidated Shipment Volume |
| Cigarettes and Heated Tobacco Units (million units) |
2023 |
2022 |
2021 |
| Cigarettes |
612,949 |
|
621,908 |
|
624,875 |
|
| Heated Tobacco Units |
125,263 |
|
109,169 |
|
94,976 |
|
| Total Cigarettes and Heated Tobacco Units |
738,212 |
|
731,077 |
|
719,851 |
|
|
|
|
|
Oral Product Shipment Volume (million cans) (1) |
|
|
|
| Nicotine Pouches |
421.1 |
|
42.5 |
|
1.1 |
|
| Snus |
240.4 |
|
54.8 |
|
6.2 |
|
| Moist Snuff |
133.7 |
|
16.0 |
|
— |
|
| Other |
4.2 |
|
— |
|
— |
|
| Total Oral Products |
799.3 |
|
113.2 |
|
7.3 |
|
(1) Excluding snuff, snuff leaf and U.S. chew |
|
|
|
Note: Sum may not foot to total due to roundings
Following the deconsolidation of our Canadian subsidiary, we continue to report the volume and corresponding royalty revenues of brands sold by RBH for which other PMI subsidiaries are the trademark owners. These include HEETS, Next, Philip Morris and Rooftop. The volume and corresponding royalty revenues of these brands sold by RBH were not material to PMI for all periods presented.
Heated tobacco units ("HTU") is the term we use to refer to heated tobacco consumables, which include our BLENDS, DELIA, HEETS, HEETS Creations, HEETS Dimensions (defined collectively as "HEETS"), Marlboro HeatSticks, SENTIA, TEREA, TEREA CRAFTED, and TEREA Dimensions, as well as the KT&G-licensed brands, Fiit and Miix (outside of South Korea). HTU's also include zero tobacco heat-not-burn consumables (LEVIA).
Unless otherwise stated, market share for HTUs is defined as the in-market sales volume for HTUs as a percentage of the total estimated industry sales volume for cigarettes and HTUs.
References to total industry (or total market), our shipment volume and our market share performance reflect cigarettes and heated tobacco units, unless otherwise stated.
Total industry volume, PMI in-market sales volume and PMI market share for the following geographies include the cigarillo category in Japan: the total international market, EA, AU & PMI DF Region, and Japanese domestic market.
In-market sales ("IMS") is defined as sales to the retail channel, depending on the market and distribution model.
References to total international market, defined as worldwide cigarette and heated tobacco unit volume excluding the United States, total industry (or total market) and market shares throughout this "Discussion and Analysis" are our estimates for tax-paid products based on the latest available data from a number of internal and external sources and may, in defined instances, exclude China and/or our duty free business.
From time to time, PMI’s shipment volumes are subject to the impact of distributor inventory movements (or wholesaler inventory movements in certain markets where PMI does not sell to distributors), and estimated total industry/market volumes are subject to the impact of inventory movements in various trade channels that include estimated trade inventory movements of PMI’s competitors arising from market-specific factors that significantly distort reported volume disclosures.
Such factors may include changes to the manufacturing supply chain, shipment methods, consumer demand, timing of excise tax increases or other influences that may affect the timing of sales to customers. In such instances, in addition to reviewing PMI shipment volumes and certain estimated total industry/market volumes on a reported basis, management reviews these measures on an adjusted basis that excludes the impact of distributor and/or estimated trade inventory movements. Management also believes that disclosing PMI shipment volumes and estimated total industry/market volumes in such circumstances on a basis that excludes the impact of distributor and/or estimated trade inventory movements improves the comparability of performance and trends for these measures over different reporting periods.
2023 compared with 2022
The following discussion compares our consolidated operating results for the year ended December 31, 2023, with the year ended December 31, 2022.
Total Market
Estimated international industry volume (excluding China and the U.S.) for cigarettes and HTUs of 2.6 trillion, decreased by 1.6%, reflecting declines in the SSEA, CIS & MEA Region, the Europe Region and the Americas Region, partly offset by an increase in the EA, AU & PMI DF Region, as described in the Regional sections of this Item 7.
For the full year 2024, we currently expect an estimated total international industry volume decline for cigarettes and HTUs, excluding China and the U.S., of -2% to flat.
Shipment Volume
Our total cigarette and HTU shipment volume increased by 1.0%, reflecting an 14.7% increase in HTU shipments across all regions, partly offset by a 1.4% decline in cigarette shipments due to declines in the Europe, EA, AU & PMI DF, and Americas Regions, partly offset by the SSEA, CIS & MEA Region. Cigarette shipment volume for Marlboro decreased by 1.9% to 240.0 billion units, due primarily to the Philippines.
Our total oral product shipment volume increased by +100%, driven by the Swedish Match acquisition. For comparison purposes, assuming the inclusion of Swedish Match's 2022 shipment volume prior to our acquisition, total oral product shipment volume increased by 16.8%, primarily reflecting growth in nicotine pouches (particularly in the U.S.), partly offset by a decline for snus (mainly in Scandinavia). Swedish Match's total oral product shipment volume increased by 17.1% versus its corresponding shipments in 2022. Volume comparisons versus Swedish Match's 2022 results reflect data sourced from its disclosures, available at www.swedishmatch.com/investors.
For the full year 2024, we currently expect the total cigarette, HTU and oral smoke-free product shipment volume growth for PMI of flat to +1% driven by smoke-free products.
For the full year 2024, we also expect nicotine pouch shipment volume in the U.S. of approximately 520 million cans.
Adjusted in-market sales for HTUs increased by 14.8% (in line with full-year HTUs shipment volume growth of 14.7%), including growth in Europe of 17.6% and Japan of 14.5%. Excluding Russia and Ukraine, adjusted in market sales for HTUs increased by 17.1%.
International Share of Market - Cigarette and HTUs (Excluding China and the United States)
|
|
|
|
|
|
|
|
|
|
|
|
|
Full-Year |
|
2023 |
2022 |
Change (pp) |
Total International Market Share (1) |
28.3 |
% |
27.7 |
% |
0.6 |
|
| Cigarettes |
23.7 |
% |
23.6 |
% |
0.1 |
|
| HTU |
4.7 |
% |
4.1 |
% |
0.6 |
|
|
|
|
|
Cigarette over Cigarette Market Share (2) |
25.2 |
% |
25.0 |
% |
0.2 |
|
|
|
|
|
| (1) Defined as PMI's cigarette and heated tobacco unit in-market sales volume as a percentage of total industry cigarette and heated tobacco unit sales volume, excluding China and the U.S., including cigarillos in Japan |
| (2) Defined as PMI's cigarette in-market sales volume as a percentage of total industry cigarette sales volume, excluding China and the U.S., including cigarillos in Japan |
| Note: Sum of product categories might not foot to total due to roundings |
Key Market Data
Key market data regarding total market size, our shipments and market share were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PMI Shipments (billion units) |
|
PMI Market Share (%)(2) |
| Market |
|
Total Market
(billion units) |
|
Total |
|
Cigarette |
|
Heated Tobacco Unit |
|
Total |
|
Heated Tobacco Unit |
|
|
2023 |
2022 |
|
2023 |
2022 |
|
2023 |
2022 |
|
2023 |
2022 |
|
2023 |
2022 |
|
2023 |
2022 |
Total (1) (2) |
|
2,579.9 |
2,621.5 |
|
738.2 |
731.1 |
|
612.9 |
621.9 |
|
125.3 |
109.2 |
|
28.3 |
27.7 |
|
4.7 |
4.1 |
| Europe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| France |
|
29.8 |
32.5 |
|
13.0 |
14.0 |
|
12.8 |
13.7 |
|
0.2 |
0.2 |
|
42.5 |
43.6 |
|
0.7 |
0.7 |
Germany (3) |
|
69.0 |
70.3 |
|
26.5 |
28.2 |
|
23.3 |
24.8 |
|
3.1 |
3.4 |
|
39.0 |
38.9 |
|
5.3 |
4.0 |
| Italy |
|
73.3 |
72.8 |
|
39.7 |
40.8 |
|
27.3 |
28.6 |
|
12.4 |
12.3 |
|
53.9 |
54.1 |
|
17.3 |
14.6 |
| Poland |
|
56.7 |
55.7 |
|
23.7 |
21.7 |
|
18.7 |
17.1 |
|
5.0 |
4.5 |
|
41.8 |
38.9 |
|
8.9 |
8.2 |
| Spain |
|
43.6 |
44.6 |
|
12.9 |
13.6 |
|
11.8 |
12.7 |
|
1.1 |
0.9 |
|
29.3 |
30.0 |
|
2.3 |
1.7 |
| SSEA, CIS & MEA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Egypt |
|
74.0 |
93.6 |
|
24.3 |
21.0 |
|
23.0 |
20.0 |
|
1.3 |
1.0 |
|
32.8 |
22.2 |
|
1.7 |
0.8 |
| Indonesia |
|
291.6 |
304.0 |
|
83.4 |
86.8 |
|
83.4 |
86.8 |
|
— |
— |
|
28.6 |
28.6 |
|
— |
— |
| Philippines |
|
42.9 |
53.4 |
|
23.8 |
32.2 |
|
23.5 |
32.0 |
|
0.2 |
0.2 |
|
55.4 |
60.3 |
|
0.5 |
0.4 |
| Russia |
|
203.4 |
208.8 |
|
64.8 |
64.7 |
|
47.9 |
49.3 |
|
16.9 |
15.4 |
|
31.8 |
31.2 |
|
8.0 |
7.6 |
| Turkey |
|
136.5 |
116.8 |
|
69.0 |
56.1 |
|
69.0 |
56.1 |
|
— |
— |
|
50.5 |
48.0 |
|
— |
— |
| EA, AU & PMI DF |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Australia |
|
7.2 |
8.9 |
|
2.5 |
3.0 |
|
2.5 |
3.0 |
|
— |
— |
|
34.8 |
33.4 |
|
— |
— |
Japan (2) |
|
149.0 |
148.3 |
|
60.9 |
55.5 |
|
17.9 |
21.1 |
|
43.0 |
34.4 |
|
39.6 |
37.6 |
|
26.7 |
23.6 |
| South Korea |
|
72.0 |
72.6 |
|
14.0 |
13.9 |
|
8.9 |
9.4 |
|
5.1 |
4.5 |
|
19.5 |
19.2 |
|
7.1 |
6.2 |
| Americas |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Argentina |
|
28.8 |
30.3 |
|
17.8 |
19.3 |
|
17.8 |
19.3 |
|
— |
— |
|
61.9 |
63.8 |
|
— |
— |
| Mexico |
|
30.0 |
32.2 |
|
18.9 |
21.0 |
|
18.8 |
20.8 |
|
0.1 |
0.1 |
|
63.1 |
65.2 |
|
0.5 |
0.4 |
|
| (1) Market share estimates are calculated using IMS data, unless otherwise stated |
| (2) Total market and market share estimates include cigarillos in Japan |
| (3) PMI market share reflects estimated adjusted in-market sales volume share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary |
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/Other |
| (in millions) |
|
|
|
Net Revenues (1) |
|
$ |
35,174 |
|
$ |
31,762 |
|
|
10.7 |
% |
7.6 |
% |
|
$ |
3,412 |
|
$ |
(1,112) |
|
$ |
2,113 |
|
$ |
1,940 |
|
$ |
664 |
|
$ |
(193) |
|
Cost of Sales (2) |
|
(12,893) |
|
(11,402) |
|
|
(13.1) |
% |
(8.4) |
% |
|
(1,491) |
|
167 |
|
(695) |
|
— |
|
(755) |
|
(208) |
|
Marketing, Administration and Research Costs (3) |
|
(10,060) |
|
(8,114) |
|
|
(24.0) |
% |
(13.5) |
% |
|
(1,946) |
|
(128) |
|
(724) |
|
— |
|
— |
|
(1,094) |
|
Impairment of Goodwill (4) |
|
(665) |
|
— |
|
|
— |
% |
— |
% |
|
(665) |
|
— |
|
— |
|
— |
|
— |
|
(665) |
|
| Operating Income |
|
$ |
11,556 |
|
$ |
12,246 |
|
|
(5.6) |
% |
(2.5) |
% |
|
$ |
(690) |
|
$ |
(1,073) |
|
$ |
694 |
|
$ |
1,940 |
|
$ |
(91) |
|
$ |
(2,160) |
|
(1) Cost/Other variance includes charges in 2023 of $80 million following the termination of a distribution arrangement in the Middle East.
(2) Cost/Other variance includes charges in 2023 of $15 million related to the war in Ukraine, more than offset by charges in 2022 of $112 million related to an impairment charge of other intangible assets, $62 million related to the war in Ukraine and $125 million of Swedish Match AB acquisition accounting related item. For more details, see Item 8, Note 3. Acquisitions, Note 4. War in Ukraine and Note 5. Goodwill and Other Intangible Assets, net.
(3) Cost/Other variance includes charges in 2023 of $109 million related to asset impairment and exit costs, the South Korea indirect tax charge of $204 million, an impairment charge for other intangibles of $15 million, the termination of a pledge agreement with the Foundation for a Smoke-Free World of $140 million, and $38 million related to the war in Ukraine, partially offset by charges in 2022 of $89 million related to the war in Ukraine and $115 million related to costs associated with the Swedish Match AB offer. For more details, see Item 8, Note 4. War in Ukraine, Note 5. Goodwill and Other Intangible Assets, net, Note 18. Contingencies and Note 20. Asset Impairment and Exit Costs.
(4) For details on the impairment of goodwill recorded in the second quarter of 2023, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
Net revenues increased by 10.7%, including the impact of the Swedish Match acquisition and currency. Net revenues, excluding currency and acquisitions, increased by 7.6%, mainly reflecting: a favorable pricing variance, primarily driven by higher combustible tobacco pricing, and favorable volume/mix, mainly driven by higher HTU volume, partially offset by lower cigarette volume. The increase was partly offset by lower fees for certain distribution rights billed to customers in certain markets and a charge to net revenues in 2023 of $80 million following the termination of a distribution arrangement in the Middle East, both shown in "Other".
The unfavorable currency in net revenues was due primarily to the Egyptian pound, Indonesian rupiah, Japanese yen and Russian ruble, partially offset by the Euro and Mexican peso.
Net revenues include $12.8 billion in 2023 and $10.2 billion in 2022 related to smoke-free products.
Cost of sales increased by 13.1%, including the impact of the Swedish Match acquisition and currency. Cost of sales, excluding currency and acquisitions, increased by 8.4%, primarily related to higher manufacturing costs (primarily due to inflationary impacts, notably related to direct materials, tobacco leaf and energy, partly offset by productivity) and unfavorable volume/mix, mainly reflecting unfavorable category mix (notably due to lower cigarette volume and higher HTU volume), as well as the technical impact of third-party manufacturing in Indonesia. This increase was partially offset by the Swedish Match AB acquisition accounting related item in 2022, the impairment charge of other intangible assets in 2022 and lower charges related to the War in Ukraine.
Operating income decreased by 5.6%, including the impact of the Swedish Match acquisition and currency. Operating income, excluding currency and acquisitions, decreased by 2.5%, primarily reflecting: the 2023 impairment charge for goodwill and other intangibles of $680 million, the impact of 2023 asset impairment and exit costs of $109 million, the termination of a distribution arrangement in the Middle East of $80 million in 2023, the South Korea indirect tax charge of $204 million in 2023 and the termination of a pledge agreement with the Foundation for a Smoke-Free World of $140 million in 2023, partly offset by the 2022 charges of $125 million related to the Swedish Match AB acquisition accounting related item, lower charges of $98 million related to the war in Ukraine compared to 2022, the 2022 costs associated with the Swedish Match AB offer of $115 million and $112 million related to an impairment charge of other intangible assets in 2022. In addition to these items, operating income was also negatively impacted by: higher marketing, administration and research costs (primarily due to inflationary impacts, notably related to wages, and lower commercial investments in the prior year period); higher manufacturing costs, as noted for cost of sales; and the impact of lower fees for certain distribution rights, as noted for net revenues, partially offset by a favorable pricing variance.
Amortization expense on a pre-tax basis for each of the next five years is estimated to be approximately $470 million or less, assuming no additional transactions occur that require the amortization of intangible assets. Additionally, the estimated future amortization expense could significantly increase following the reacquisition of IQOS commercialization rights in the U.S. from Altria Group, Inc. (see Item 8, Note 3, Acquisitions and the "Business Environment" section of this Item 7), the accounting for which will depend on the facts and circumstances effective May 1, 2024, when PMI will hold the full rights. We currently estimate that the incremental increase in amortization expense in 2024, as a result of the reacquisition of IQOS commercialization rights in the U.S., will be approximately $370 million on a pre-tax basis for the remaining 8 months of the year. For full year 2025 through 2028, we currently estimate that this incremental increase will be approximately $555 million on a pre-tax basis.
Like many other global companies, we have faced global inflationary pressures, primarily impacting cost of sales for our business (notably related to certain direct materials, energy, transportation and logistics) and overall inflationary impacts on marketing, administration and research costs (notably wages). For the year ended December 31, 2023, this impact on cost of sales was approximately $580 million. We expect certain inflationary elements to ease, with a moderate increase in 2024. For further details, see "Impact of Inflation on Our Business and Mitigation Efforts" within the Business Environment section of this Item 7.
Interest expense, net, of $1.1 billion increased by $473 million (80.4%), primarily due to higher interest expense in connection with the Swedish Match acquisition, partially offset by higher net interest income driven by higher interest rates.
Our effective tax rate increased by 3.1 percentage points to 22.4%. We estimate that our 2024 effective tax rate will be approximately 21% to 22%, excluding discrete tax events. For further details, see Item 8, Note 12. Income Taxes.
Net earnings attributable to PMI of $7.8 billion decreased by $1.2 billion or 13.6%. This decrease was due primarily to lower operating income, higher interest expense, net and a higher effective tax rate, as discussed above. Basic and diluted EPS of $5.02 decreased by 13.7% and 13.6%, respectively. Excluding an unfavorable currency impact of $0.63, diluted EPS decreased by 2.8%.
2022 compared with 2021
For a discussion comparing our consolidated operating results for the year ended December 31, 2022, with the year ended December 31, 2021, refer to Part II, Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation - Discussion and Analysis - Consolidated Operating Results in our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the U.S. Securities and Exchange Commission on February 10, 2023. This section is incorporated by reference into this Annual Report on Form 10-K for the year ended December 31, 2023.
Operating Results by Business Segment
Business Environment
Taxes, Legislation, Regulation and Other Matters Regarding the Manufacture, Marketing, Sale and Use of Tobacco Products
The tobacco industry and our company face a number of challenges that may adversely affect our business, volume, results of operations, cash flows and financial position. These challenges, which are discussed below and in “Cautionary Factors That May Affect Future Results,” include:
•regulatory restrictions on our products, including restrictions on the packaging, marketing, and sale of tobacco or other nicotine-containing products or related devices that could reduce our competitiveness, eliminate our ability to communicate with adult consumers, or even ban certain of our products;
•fiscal challenges, such as excessive excise tax increases and discriminatory tax structures;
•illicit trade in cigarettes and other tobacco and nicotine-containing products, including counterfeit, contraband and so-called "illicit whites";
•intense competition, including from non-tax paid volume by certain local manufacturers;
•pending and threatened litigation as discussed in Item 8, Note 18. Contingencies; and
•governmental investigations.
Regulatory Restrictions: The tobacco industry operates in a highly regulated environment. The well-known risks of smoking have led regulators to impose significant restrictions and high excise taxes on cigarettes.
Much of the regulation that shapes the business environment in which we operate is driven by the World Health Organization's (the "WHO") Framework Convention on Tobacco Control (the "FCTC"), which entered into force in 2005. The main objective of the FCTC is to establish a global agenda for tobacco regulation, with the purpose of reducing tobacco use. To date, 182 countries and the European Union ("EU") are Parties to the FCTC. The treaty requires Parties to have in place various tobacco control measures and recommends others. The FCTC governing body, the Conference of the Parties (“CoP”), has also adopted non-binding guidelines and policy recommendations related to certain articles of the FCTC that go beyond the text of the treaty. In October 2018, the CoP recognized the need for more scientific assessment and improved reporting to define policy on heated tobacco products. Similar to its previous policy recommendations on e-cigarettes, the CoP invited countries to regulate, restrict or prohibit heated tobacco products, as appropriate under their national laws.
Prior to the Ninth Session of the CoP ("CoP 9") to the FCTC, which took place in November 2021, the WHO and the WHO FCTC Secretariat published two reports on novel and emerging tobacco products. Discussions on these reports have been postponed to the Tenth Session of the CoP ("CoP 10"), which was scheduled for November 2023 but postponed to February 2024. In August 2023, the WHO Study Group on Tobacco Products Regulation issued its ninth report, including recommendations on nicotine pouches, in line with previous policy recommendations on regulating flavors in tobacco and nicotine products. It is not possible to predict whether or to what extent these developments will be reflected in decisions adopted at CoP 10 following deliberations. The WHO’s reports are not binding on the WHO Member States nor on Parties to the FCTC.
In December 2023, the WHO issued a white paper on e-cigarettes. While acknowledging that long-term health effects of using e-cigarettes are not fully understood, the Organization calls on countries to ban or strictly regulate these products, in order to prevent youth uptake and counter nicotine addiction.
We believe that when better alternatives to cigarettes exist, the discussion should not be whether these alternatives should be made available to the more than one billion people who smoke cigarettes today, but how fast, and within what regulatory framework to maximize their adoption while minimizing unintended use. Therefore, we advocate for regulatory frameworks that are based on a continuum of risk where non-combustible products fall below combustible cigarettes. Product regulation should include measures that encourage and accelerate switching to non-combustible products, for example, by allowing adult consumers who would not otherwise quit smoking cigarettes to receive truthful and non-misleading information about such alternatives to enable them to make informed decisions and by applying uniform product standards to enable manufacturers to demonstrate the reduction in harmful and potentially harmful constituents, as well as the absence of combustion. Regulation should also include specific rules for ingredients, labeling and consumer communication, and should ensure that the public is informed about the health risks of all combustible and non-combustible tobacco and nicotine-containing products. Importantly, regulation must include measures designed to prevent initiation by youth and non-smokers. We support mandated health warnings, minimum age laws, restrictions on advertising, and smoking restrictions in public spaces. We also support regulatory measures that help reduce illicit trade.
Certain measures are discussed in more detail below and in the Reduced-Risk Products (RRPs) section.
Fiscal Challenges: Excessive and disruptive excise, sales and other tax increases and discriminatory tax structures are expected to continue to have an adverse impact on our profitability, due to lower consumption and consumer down-trading to non-premium, discount, other low-price or low-taxed combustible tobacco products such as fine cut tobacco and illicit cigarettes. In addition, in certain jurisdictions, some of our combustible tobacco products are subject to tax structures that discriminate against premium-price products and manufactured cigarettes. We believe that such tax policies undermine public health by encouraging consumers to turn to illicit trade, and ultimately undercut government revenue objectives, disrupt the competitive environment, and encourage criminal activity. Other jurisdictions have imposed, or are seeking to impose, levies or other taxes specifically on tobacco companies, such as taxes on revenues and/or profits.
World Customs Organization Developments: In 2020, the World Customs Organization (the “WCO”) amended the Harmonized System ("HS") nomenclature to introduce dedicated custom codes for novel tobacco and nicotine products, including heated tobacco products, e-cigarettes and other nicotine-containing products. The amendments became effective as of January 1, 2022. The vast majority of countries where our RRPs are commercialized have adopted the amended HS, creating new dedicated customs codes for novel tobacco and nicotine products.
EU Tobacco Products Directive: In April 2014, the EU adopted a significantly revised TPD, which came into force in May 2016. All EU Member States have adopted laws transposing the TPD. The TPD sets forth a comprehensive set of regulatory requirements for tobacco products, including:
•health warnings covering 65% of the front and back panels of cigarette packs, with an option for Member States to further standardize tobacco packaging, including the introduction of plain packaging;
•a ban on characterizing flavors in some tobacco products, with a transition period for menthol that expired in May 2020;
•security features and tracking and tracing measures that became effective in May 2019; and
•a framework for the regulation of novel tobacco products and e-cigarettes, including requirements for health warnings and information leaflets, a prohibition on product packaging text related to reduced risk, and the introduction of notification requirements or authorization procedures in advance of commercialization.
In May 2021, the European Commission published its first report on the application of the TPD. The report identifies significant progress made due to the implementation of the TPD and where there is still room for improvement. Most notably, it finds that the EU legislation has enhanced tobacco control, which contributed to protecting the health of EU citizens by providing Member States with strong rules to address the use of tobacco products in the EU. The TPD reportedly achieved the 2% reduction target of the impact assessment with decreased smoking prevalence among youth. The report also concludes that there is scope for improvement in certain areas, such as enforcement at national level, assessment of ingredients, and a better consideration for novel and emerging products.
In November 2021, the European Commission published the implementation roadmap to Europe's Beating Cancer Plan (the "Plan"). According to the Plan, a revision of the TPD is planned for 2024.
EU Tobacco Excise Directive ("TED"): The EU Commission is preparing a legislative proposal for the revision of the 2011 EU Tobacco Excise Directive that may include definitions and tax treatment for novel tobacco and nicotine-containing products, including heated tobacco products, e-cigarettes and nicotine pouches. The proposal, after several delays, could still be adopted by the College of Commissioners during 2024. Depending on the adoption date by the College of Commissioners, the proposal will be discussed by the EU Council in the course of 2024-2025. Any final amendments to TED require unanimous agreement by all EU Member States, followed by transposition of TED into national legislation. A potential enforcement date for any changes to TED, after the transposition in Member States' national legislation, could be 2027.
Plain Packaging and Other Packaging Restrictions: Plain packaging legislation bans the use of branding, logos and colors on packaging other than the brand name and variant that may be printed only in specified locations and in a uniform font. To date, plain packaging laws have been adopted in certain markets in all of our operating segments, including the key markets of Australia, France, Saudi Arabia and Turkey. Some countries, such as Canada, Denmark and Israel, adopted plain packaging regulations that apply to all tobacco products, including RRPs. Other countries are also considering plain packaging legislation.
Some countries have adopted, or are considering adopting, packaging restrictions that could have an impact similar to plain packaging. Examples of such restrictions include standardizing the shape and size of packages, prohibiting certain colors or the use of certain descriptive phrases on packaging, and requiring very large graphic health warnings that leave little space for branding.
Restrictions and Bans on the Use of Ingredients: The WHO and others in the public health community have recommended restrictions or total bans on the use of some or all ingredients in tobacco products, including menthol. Broad restrictions and ingredient bans would require us to reformulate our American blend tobacco products and could reduce our ability to differentiate these products in the market in the long term. In many countries, menthol bans would eliminate the entire category of mentholated tobacco products. The EU banned cigarettes and roll-your-own tobacco products with characterizing flavors. Other tobacco products, including heated tobacco products, are currently exempted from this characterizing flavor ban. However, on November 23, 2022, the EU Commission published a delegated directive that will eliminate this exemption. All EU Member States are required to apply the delegated directive as of October 23, 2023, which bans the use of characterizing flavors in heated tobacco products, impacting a significant proportion of our RRP products currently sold in the EU. Based on high consumer switching to non-flavored products in reaction to past bans on flavors in other categories and markets, we anticipate that, while short-term volatility is possible, including in year-end trade inventories, the ban’s impact on our shipment volumes in the EU will be relatively limited in the near term. Our fundamental view remains that we do not expect a meaningful change in the structural growth of the category with consumers switching to non-flavored products partially mitigating the effect of the ban. A majority of EU Member States have transposed this directive, or are in the final stages of transposing it, withdrawing the heated tobacco product exemption from the characterizing flavor ban into national law. The remaining markets are expected to adopt this directive in 2024, and we will continue to actively monitor relevant developments in the EU market. Other countries may follow the EU’s approach toward tobacco product ingredients. Turkey banned menthol as of May 2020. Broader ingredient bans have been adopted by Brazil and Canada.
Bans on Display of Tobacco Products at Retail: In a number of our markets, including, but not limited to, Australia and Russia, governments have banned the display of tobacco products at the point of sale. Other countries are considering similar bans.
Bans and Restrictions on Advertising, Marketing, Promotions and Sponsorships: For many years, the FCTC has called for, and countries have imposed, partial or total bans on tobacco advertising, marketing, promotions and sponsorships, including bans and restrictions on advertising on radio and television, in print and on the Internet. The FCTC's non-binding guidelines recommend that governments prohibit all forms of communication with adult smokers.
Restrictions on Product Design: Some members of the public health community are calling for the further standardization of tobacco products by requiring, for example, that cigarettes have a certain minimum diameter, which would result in a ban on slim cigarettes, or requiring the use of standardized filter and cigarette paper designs. In addition, at its meeting in November 2016, the CoP adopted non-binding guidelines recommending that countries regulate product design features that increase the attractiveness of tobacco products, such as the diameter of cigarettes and the use of flavor capsules.
Restrictions on Public Smoking and Use of Nicotine-Containing Products in Public: The pace and scope of restrictions on the use of our products have increased significantly in most of our markets. Many countries around the world have adopted, or are likely to adopt, regulations that restrict or ban smoking and use of nicotine-containing products in public and/or work places, restaurants, bars and nightclubs. Some public health groups have called for, and some countries, regional governments and municipalities have adopted or proposed, bans on smoking in outdoor places, as well as bans on smoking in cars (typically, when minors are present) and private homes.
Other Regulatory Issues: Some regulators are considering, or in some cases have adopted, regulatory measures designed to reduce the supply of tobacco products. These include regulations intended to reduce the number of retailers selling tobacco products by, for example, reducing the overall number of tobacco retail licenses available or banning the sale of tobacco products within specified distances of certain public facilities. Other regulators are also considering generation sales bans, which prohibit the sale of certain tobacco or nicotine products to people born after a certain year.
On December 13, 2022 the New Zealand parliament passed a bill introducing regulatory measures restricting the sale and supply of smoked tobacco products, including reducing the number of retail outlets licensed to sell smoked tobacco products, imposing a maximum limit of nicotine content for smoked tobacco products and prohibiting the sale of smoked tobacco products to anyone born on or after January 1, 2009. These measures are limited to smoked tobacco products and do not apply to heated tobacco products and e-cigarettes. In Mexico, a new law came into force on December 12, 2022 prohibiting imports and exports of certain nicotine and non-nicotine delivery and consumption systems, as well as the consumables used in those systems, including much of our RRP portfolio.
On December 16, 2022, the Mexican Federal Government enacted an implementation regulation for the tobacco control law, which includes (i) a point of sale display ban of tobacco products; (ii) restrictions on where tobacco products can be consumed, and (iii) prohibition to communicate corporate social responsibility programs funded by the tobacco industry.
On January 1, 2023, a law regulating the marketing of nicotine pouches went into effect in Slovakia. The regulatory framework contains a minimum legal age (18 years) to purchase, a nicotine limit, and a labelling requirement. In Belgium, a Royal Decree banning nicotine pouches entered into force on July 1, 2023, with a sell-off period from retail to consumer as of October 1, 2023.
On March 22, 2023, a bill amending the Tobacco Hazards Prevention and Control Act in Taiwan went into effect. It regulates heated tobacco products and bans e-cigarettes. The amendment particularly specifies that designated tobacco products (including heated tobacco products) that are not cigarettes, cut tobacco, cigars, snuff nor chewing tobacco, must undergo a health risk assessment as part of an authorization system.
On March 28, 2023, the Argentinian Ministry of Health prohibited the import, distribution, commercialization and advertisement of heated tobacco products, including related devices. The country had previously banned the use of e-cigarettes in 2011.
In a limited number of markets, most notably Japan, we are dependent on governmental approvals that may limit our pricing flexibility.
The EU Single-Use Plastics Directive, which will require tobacco manufacturers and importers to cover the costs of public collection systems for tobacco product filters, under Extended Producer Responsibility ("EPR") schemes, came into force on July 2, 2019. To date, some member states transposed the Directive into national legislation. By the end of 2024, most EU Member States are expected to bring into force national legislation for mandatory EPR schemes and related EPR costs for tobacco manufacturers and importers.
While we cannot predict the impact of this initiative on our business at this time, we currently expect further adoption of similar laws in other jurisdictions, and we are monitoring developments in this area.
In some countries, including in the EU, cigarettes are subject to testing, disclosure and mandatory emissions limits for tar, nicotine, carbon monoxide and other smoke constituents. In the Netherlands, several public health organizations have requested that the Dutch enforcement body enforce the requirements for maximum tar, nicotine, and carbon monoxide ("TNCO") emissions levels for cigarettes using a test method other than the method currently set forth in the EU TPD and transposed into national legislation. This request followed publication of a report by the Dutch State Institute for Public Health & Environment, which found that all cigarette brands sold in the Netherlands exceeded the maximum TNCO levels when measured under an alternative method. The Dutch enforcement body declined the request, and the applicants have challenged such decision in pending legal proceedings. The case is currently pending before the Trade and Industry Appeals Tribunal in the Netherlands A decision was expected in the fourth quarter of 2023, but the Court recently announced that it intends to submit preliminary questions to the Court of Justice of the European Union. While we cannot predict the outcome, a decision to enforce the existing TNCO ceilings using the alternative test method could impact a significant portion of the manufactured cigarettes available on the market in the Netherlands and could lead to similar actions in other EU countries.
Illicit Trade: Illicit tobacco trade creates a cheap and unregulated supply of tobacco products, undermines efforts to reduce smoking prevalence, especially among youth, damages legitimate businesses and intellectual property rights, stimulates organized crime, increases corruption and reduces government tax revenue. We generally estimate that, excluding China and the U.S., illicit trade may account for as much as 14% of global cigarette consumption; this includes counterfeit, contraband and the persistent problem of “illicit whites,” which are cigarettes legally purchased in one jurisdiction for the sole purpose of being exported and illegally sold in another jurisdiction where they have no legitimate market. Currently, we estimate that illicit trade in the EU accounted for approximately 8% of total cigarette consumption in 2023.
A number of jurisdictions are considering actions to prevent illicit trade. In November 2012, the FCTC adopted the Protocol to Eliminate Illicit Trade in Tobacco Products (the “Protocol”), which includes supply chain control measures, such as licensing of manufacturers and distributors, enforcement of these control measures in free trade zones, controls on duty free and Internet channels and the implementation of tracking and tracing technologies. To date, 68 Parties, including the EU, have ratified it. The Protocol came into force in September 2018. Since then, implementation in national legislations has been ongoing. In November 2021, the second Meeting of the Parties to the Protocol decided, among other things, to focus on the implementation of a framework for global information sharing to combat illicit tobacco trade and enable the Parties to the Protocol to exchange tracking and tracing information of products in a secure manner. We welcome this decision and expect that other countries will ratify the Protocol.
We devote substantial resources to help prevent illicit trade in combustible tobacco products and RRPs. For example, we engage with governments, our business partners and other stakeholders to implement effective measures to combat illicit trade and, in some instances, pursue legal remedies to protect our intellectual property rights.
The tracking and tracing regulations for cigarettes and roll-your-own products manufactured or destined for the EU became effective on May 20, 2019. The effective date for other tobacco-containing products, including some of our RRPs such as heated tobacco units, is May 20, 2024. While we expect that this regulation will increase our operating expenses, we do not expect this increase to be significant.
In 2009, our Colombian subsidiaries entered into an Investment and Cooperation Agreement with the national and regional governments of Colombia to promote investment in, and cooperation on, anti-contraband and anti-counterfeit efforts. The agreement provides $200 million in funding over a 20-year period to address issues such as combating illegal cigarette trade and increasing the quality and quantity of locally grown tobacco.
In May 2016, PMI launched PMI IMPACT, a global initiative that supports third-party projects dedicated to fighting illicit trade and related crimes such as corruption, organized criminal networks and money laundering. The centerpiece of PMI IMPACT is a council of external independent experts in the fields of law, anti-corruption and law enforcement responsible for evaluating and approving funding proposals for PMI IMPACT grants. PMI has pledged $100 million to fund projects within PMI IMPACT over three funding rounds. The implementation of the grants assigned under the third funding round is ongoing.
Reduced-Risk Products (RRPs)
Our Approach to RRPs: We recognize that smoking cigarettes causes serious diseases and that the best way to avoid the harm of smoking is to never start or to quit. Nevertheless, it is predicted that by 2025, the number of smokers will remain largely unchanged from the current estimate of 1.1 billion, despite considerable efforts to discourage smoking.
Cigarettes burn tobacco, which produces smoke. As a result of the combustion process, the smoker inhales various toxic substances. In contrast, RRPs do not burn tobacco and therefore contain significantly lower levels of harmful and potentially harmful constituents ("HPHCs") than found in cigarette smoke.
Our RRPs and commercial activities for these products are designed for, and directed toward, current adult smokers and users of nicotine-containing products. We put significant effort to restrict access of our products from non-smokers and youth.
For adult smokers who would otherwise continue to smoke cigarettes, we believe that RRPs, while not risk-free, offer a much better choice. Accordingly, our key strategic priorities are to: (i) continue developing and commercializing products that present less risk of harm to adult smokers who switch to such products versus continued cigarette smoking; and (ii) educate and encourage current adult smokers who would otherwise continue to smoke cigarettes to switch to those products.
We recognize that this transformation from cigarettes to RRPs will take time and that the speed of transformation will depend in part upon factors beyond our control, such as the willingness of governments, regulators and other policy groups to embrace RRPs as a desired alternative to continued cigarette smoking. As a leading international cigarette manufacturer, we will continue to accelerate this transformation by using our extensive commercial and distribution infrastructure as an effective platform for the commercialization of our RRPs and communication with adult smokers and trade partners about the substantiated benefits of switching to our RRPs. As long as a significant number of adult smokers continue to smoke cigarettes, responsible leadership of the category is critical. We aim to maintain our competitive position in the cigarette market through selective investment.
While seeking to remain competitive in the cigarette market, we are judiciously reallocating resources from cigarettes to RRPs and are streamlining our cigarette portfolio.
We have a range of RRPs in various stages of development, scientific assessment, and commercialization. We are committed to conducting rigorous scientific assessments of our RRP platforms to substantiate that they reduce exposure to HPHCs and, ultimately, that these products present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to them versus continued cigarette smoking. We draw upon a team of expert scientists and engineers from a broad spectrum of scientific disciplines and our extensive learnings of adult consumer preferences to further develop and assess our RRPs. Our efforts are guided by the following key objectives:
•to develop RRPs that adult smokers who would otherwise continue to smoke cigarettes find to be satisfying alternatives to smoking;
•for those adult smokers, our goal is to offer RRPs with a scientifically substantiated risk-reduction profile that approaches as closely as possible the risk-reduction profile associated with smoking cessation;
•to substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on scientific evidence of the highest standard that is made available for scrutiny and review by external independent scientists and relevant regulatory bodies; and
•to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs, including the communication of scientifically substantiated information to enable adult smokers to make better choices.
Our RRP Platforms: Our product development is based on the elimination of combustion via tobacco heating and other innovative systems, which we believe are the most promising path to providing a better consumer choice for those who would otherwise continue to smoke cigarettes. We recognize that no single product will appeal to all adult smokers. Therefore, we are developing a portfolio of products intended to appeal to a variety of distinct adult consumer preferences and achieve population harm reduction.
Five PMI-developed or improved RRP platforms are in various stages of development and commercialization readiness:
Platform 1 uses a precisely controlled heating device incorporating our IQOS HeatControl technology, into which a specially designed and proprietary tobacco unit is inserted and heated to generate an aerosol. We have conducted a series of clinical studies for this platform, the results of which were included in our submissions to the U.S. Food and Drug Administration (“FDA”). In addition to the original version of Platform 1 which relies on a heating technology using a blade, a newer version of Platform 1 is now available using induction.
Most of the studies referenced above were conducted with the blade version of Platform 1 and additional research was conducted with the induction technology. We believe that there is full comparability between the subsequent Platform 1 versions, and that the data from the studies conducted with the blade version of Platform 1 remain valid and applicable to the newer version of Platform 1. In 2022, we also began the initial launch of a heated tobacco product, which utilizes external resistive heating technology and that is commercialized under the BONDS brand.
Platform 2 used a pressed carbon heat source which, when ignited, generated a nicotine-containing aerosol by heating tobacco. As a result of consumer testing feedback, the design of our current Platform 2 technology has been discontinued. We are assessing alternative designs for this consumer segment.
Platform 3 uses a nicotine salt and is composed of two parts: (1) a consumable that contains a highly soluble encapsulated nicotine salt powder and (2) a non-electric device that activates it. Once a consumable is inserted into the mechanical device, the nicotine salt powder is aerosolized upon inhalation. The results of our pharmacodynamic study related to this version indicate this product's potential as an acceptable alternative to continued cigarette smoking in terms of product satisfaction. We are working on product modifications to enable adult smokers, who are looking for better alternatives to cigarettes, to switch to a Platform 3 product.
Platform 4 covers e-vapor products, which are battery-powered devices that produce an aerosol by vaporizing a tobacco-free liquid solution. We developed new e-liquids for our e-vapor products to deliver real tobacco taste satisfaction. Using patented technology, flavors and nicotine are extracted directly from the tobacco leaves and captured in a tobacco-free liquid solution, without having to add flavoring ingredients.
In the first quarter of 2023, we initiated a project to fully outsource and restructure the manufacturing of e-vapor devices and consumables. As a result, PMI recorded pre-tax asset impairment and exit costs of $109 million. We intend to focus on commercializing these products in select markets, with an emphasis on profitability.
We also entered into a licensing agreement with Kaival Brands International, LLC, in June 2022 to distribute an e-vapor product, known in the U.S. as the BIDI® Stick. The agreement grants PMI certain intellectual property rights relating to the premium e-vapor devices and, potentially, other newly developed devices, to permit PMI to manufacture, promote, sell, and distribute the e-vapor device and, to the extent included, other newly developed devices in international markets outside of the U.S.
Platform 5 covers snus and modern oral nicotine pouches. Snus refers to dried loose tobacco, or snuff, which is consumed by sniffing the product through the nose, moist loose tobacco which is put in the mouth between the lower or upper lip and gum, and snus pouches which contain ground tobacco, water, salt and flavors. Modern oral nicotine pouches consist of white pre-conditioned pouches containing nicotine derived from tobacco. Users place a pouch between the upper lip and gum and leave it there while the nicotine and flavor are being released. At the end of the use, the user can dispose of the pouch. Nicotine pouches are inherently smoke-free as they are consumed orally, and no combustion process occurs during use. They contain primarily nicotine, flavors, and cellulose substrate. The nicotine used in the pouches is of pharmaceutical-grade like the nicotine used in medicinal products, such as gums and inhalers, while the flavors are approved for use in food in accordance with the product quality standards for nicotine pouches developed by the Swedish Institute for Standards. In 2021, PMI acquired AG Snus Aktieselskab ("AG Snus"), as well as Fertin Pharma A/S, two companies manufacturing and/or marketing nicotine pouches. In 2022, we significantly expanded our Platform 5 products portfolio with the acquisition of Swedish Match. The acquisition also represents an expansion of our RRP presence in the U.S. market, where Swedish Match's ZYN brand is the leading nicotine pouch franchise.
We aim to expand our brand portfolio and market positions with additional RRPs. In addition, we continue to use our expertise, technology and capabilities to explore new growth opportunities beyond our current business, including products that do not contain nicotine or tobacco.
The research and development expense for our smoke-free portfolio accounted for 99% of our total research and development expense for each of the three years ended December 31, 2023, 2022 and 2021. The research and development expense for the years ended December 31, 2023, 2022 and 2021, is set forth in Item 8, Note 15. Additional Information to the consolidated financial statements.
Commercialization of RRPs: We are developing a multiplatform approach and tailoring our commercialization strategy to the characteristics of each specific market. We focus our commercialization efforts on consumer retail experience, guided consumer trials and customer care, and increasingly, digital communication programs and e-commerce. In order to accelerate switching to our Platform 1 products, our initial market introductions typically entail one-on-one consumer engagement (in person or by digital means) and device discounts. These initial commercialization efforts require substantial investment, which we believe will moderate over time and further benefit from the increased use of digital engagement capabilities. PMI has, and continues to, accelerate its investments in digital consumer engagement.
In 2014, we introduced our Platform 1 product in pilot city launches in Nagoya, Japan, and in Milan, Italy. Since then, we have continuously expanded our commercialization activities.
As of December 31, 2023, PMI's smoke-free products were available for sale in 84 markets.
Data shows that only a very small percentage of adult smokers who convert to our Platform 1 product switch back to cigarettes.
We have integrated the production of our heated tobacco units into several of our existing manufacturing facilities, are progressing with our plans to build manufacturing capacity for our other RRP platforms, and continue to optimize our manufacturing infrastructure and expand our commercialization activities for new products and markets. We discuss certain risks related to the commercialization and supply of our RRP portfolio in Item 1.A. Risk Factors.
We discuss product warranties in more detail in Item 8, Note 7. Product Warranty. The significance of warranty claims depends on a number of factors, including device version mix, product failure rates, logistics and service delivery costs, and warranty policies, and may increase with the number of devices sold.
On October 20, 2022, PMI announced that it had reached an agreement with Altria Group, Inc. (“Altria”) to end the companies’ commercial relationship with respect to Platform 1 in the U.S. as of April 30, 2024 (the “2022 Agreement”). Thereafter, under the 2022 Agreement, PMI will hold the full rights to commercialize Platform 1 in the United States—the world’s largest smoke-free market. The 2022 Agreement provides a clear path to expanding Platform 1’s international success in a market where approximately 30 million adults continue to smoke cigarettes.
The U.S. government has contacted Altria and its affiliate, Philip Morris USA, Inc. (“PM USA”) in connection with the 2022 Agreement. Altria and its subsidiary PM USA are parties to a 2006 order (“2006 Order”) in the United States District Court for the District of Columbia holding that they violated the Racketeer Influenced and Corrupt Organizations Act (“RICO”). The 2006 Order imposes restrictions on defendants from selling or transferring their cigarette brands, brand names, cigarette product formulas or cigarette businesses without the transferee submitting to the jurisdiction of the court and subjecting itself to the 2006 Order as of the date of sale or transfer. The U.S. government has informed Altria that it believes the transaction contemplated by the 2022 Agreement (the “Transaction”) falls within the scope of this provision and that before the Transaction can be effectuated, PMI must submit to the 2006 Order. While we do not know the specific relief the U.S. government may seek from the court, we believe that there are strong arguments as to why the provision cited by the U.S. government is inapplicable to the Transaction and we also believe that there are paths available to minimize or eliminate potential impact on the timing or effectuation of the Transaction.
Our commercialization efforts for the other PMI-developed RRP platforms are as follows:
•In late 2022, we began commercializing our BONDS product in the Philippines and Colombia.
•Since August 2020, we have launched and expanded our portfolio of vaping products (branded VEEV) in 26 markets.
•Following our acquisition of Swedish Match, we have access to a strong portfolio of Swedish Match brands in both the snus and nicotine pouch categories. Swedish Match nicotine pouches are currently available in 23 markets.
•In addition to Swedish Match products, we have launched a reformulated version of the already commercialized nicotine pouches bearing the Shiro brand by our affiliate AG Snus in ten markets.
RRP Regulation and Taxation: RRPs contain nicotine and are not risk-free. As we describe in more detail above, we support science-based regulation and taxation of RRPs, and believe that regulation and taxation should differentiate between cigarettes and products that present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to these products versus continued smoking and should recognize a continuum of risk for tobacco and other nicotine-containing products. Regulation, as well as industry practices, should reflect the fact that youth should not consume nicotine in any form.
Some governments have banned or are seeking to ban or severely restrict emerging tobacco and nicotine-containing products such as our RRPs and communication of truthful and non-misleading information about such products.
These regulations might foreclose or unreasonably restrict adult consumer access even to products that might be shown to be a better consumer choice than continuing to smoke cigarettes. Since the COVID-19 pandemic, some governments have been and may continue to be temporarily unable to focus on the development of science-based regulatory frameworks for the development and commercialization of RRPs or on the enforcement or implementation of regulations that are significant to our business.
We oppose blanket bans and unreasonable restrictions of products that have the potential to present less risk of harm compared to continued cigarette smoking. By contrast, we support regulation that sets clear standards for all RRP categories and propels innovation to benefit adult smokers who would otherwise continue to smoke cigarettes.
In the United States, an established regulatory framework for assessing “Modified Risk Tobacco Products” ("MRTP") and “New Tobacco Products” exists under the jurisdiction of the FDA. We submitted to the FDA a Modified Risk Tobacco Product Application (“MRTPA”) for our Platform 1 product in December 2016, and a Premarket Tobacco Product Application (“PMTA”) for our Platform 1 product in March 2017.
On April 30, 2019, the FDA determined that a version of our Platform 1 product, namely, IQOS 2.4 and three related consumables, is appropriate for the protection of public health and authorized it for sale in the United States. The FDA’s decision followed its comprehensive assessment of our PMTA. On December 7, 2020, the FDA reached the same determination for the IQOS 3 device and authorized that version of our Platform 1 product for sale in the United States.
On July 7, 2020, the FDA determined that the available scientific evidence demonstrates that the issuance of an exposure modification order would be appropriate for the promotion of public health and authorized the marketing of a version of our Platform 1 product, namely IQOS 2.4 and three related consumables, as an MRTP. The FDA authorized the marketing of this product in the U.S. with the following information:
"AVAILABLE EVIDENCE TO DATE:
•the IQOS system heats tobacco but does not burn it.
•this significantly reduces the production of harmful and potentially harmful chemicals.
•scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals."
We must request and receive authorization from the FDA in order to continue marketing this product with the same modified exposure information before the order expires four years from the date of the orders.
The FDA may issue two types of MRTP orders: a “risk modification” order or an “exposure modification” order. We had requested both types of orders for IQOS 2.4 and an initial selection of 3 consumables' variants. After review, the FDA determined that the evidence did not support issuing a "risk modification" order at this time but that it did support issuing an "exposure modification" order for the product. This determination included a finding that issuance of the exposure modification order is expected to benefit the health of the population as a whole. We also received an exposure modification order for IQOS 3. We look forward to working with the FDA to provide any additional information they may require to market Platform 1 products with reduced risk claims.
The FDA’s PMTA and MRTP orders do not mean that the agency “approved” our Platform 1 product. These authorizations are subject to strict marketing, reporting and other requirements, and are not a guarantee that the product will remain authorized, particularly if there is a significant uptake in youth or non-smoker initiation. The FDA will monitor the marketing of the product.
On March 18, 2021, we submitted to the FDA a supplemental MRTPA ("sMRTPA") for IQOS 3 requesting authorization to market this version of the device as a MRTP with reduced exposure information like IQOS 2.4. In June 2021, the FDA formally accepted and filed our sMRTPA for substantive scientific review, following a period for the public to provide comments on our application. The FDA authorized our sMRTPA for IQOS 3 by issuing a Modified Risk Granted Order – Exposure Modification on March 11, 2022.
On January 26, 2023, the FDA authorized the marketing of two new tobacco-flavored consumables (Marlboro Sienna HeatSticks and Marlboro Bronze HeatSticks) and a modified version of the authorized Marlboro Amber HeatSticks. These products are line extensions and/or modified versions of the tobacco-flavored consumables for which the FDA had previously issued a marketing granted order. In its assessment, the FDA determined that the three variants of HeatSticks were comparable to the previously authorized tobacco-flavored consumables.
On April 28, 2023, we submitted the Annual Report for the IQOS Tobacco Heating System ("THS") to the FDA. The report included a systematic review of the literature covering publications related to the IQOS THS between March 1, 2022 and February 28, 2023. The report concluded that, although the scientific evidence continues to develop and evolve, the extensive data reviewed confirms that while Heated Tobacco Products (“HTPs”) are not risk-free, the use of HTPs are likely to present less risk of harm for both users and non-users against the well-proven risks of continued cigarette smoking, and therefore continues to support the "appropriate for the promotion of public health" status of IQOS THS.
On July 5, 2023, we submitted a renewal application to the FDA requesting re-authorization to market the IQOS products with a modified exposure order in the U.S. This renewal request was received by the FDA 360 days prior to the expiration date of the original marketing orders, and in early September 2023, the FDA formally accepted our renewal MRTPAs for review. As our application proceeds through the review process, the FDA may request additional information or conduct subsequent inspections to verify the information we submitted.
On October 20, 2023, we submitted bundled PMTAs for our IQOS ILUMA THS products together with MRTPAs requesting authorization of the exposure reduction marketing order previously granted for IQOS blade versions. We submitted these applications at the same time in order to allow the FDA to evaluate the PMTAs and MRTPAs concurrently.
Some states and municipalities in the U.S. have introduced severe restrictions for the sale of certain e-cigarettes and tobacco products, including those authorized by the FDA. We believe that such restrictions on FDA-authorized products will not advance public health and will unreasonably limit adult consumer access to products that are shown to be a better alternative to continued cigarette smoking.
In March 2020, the FDA issued a final rule to require new text and graphic health warnings on cigarette packs and advertisements. HTPs are technically covered by this rule, however the FDA stated that it would make product-specific decisions about health warnings when issuing or revising individual product or marketing orders. This approach would be consistent with the original marketing order granted for Heatsticks where the FDA required Philip Morris Products S.A. to remove the Surgeon General’s health warning for carbon monoxide from its packaging and advertising, and to use a nicotine addiction health warning instead. Philip Morris Products S.A. is committed to providing adult consumers with complete, accurate, and non-misleading information about possible health risks associated with its products. We shared our views with the FDA on the application of the new warnings to our HTPs. The final rule was the subject of litigation in the U.S. and was vacated nationwide by a federal court in November 2022. Philip Morris Products S.A. was not a party to this litigation.
On March 8, 2023, the FDA proposed new requirements for tobacco product manufacturers regarding the manufacture, design, packing and storage of their products. The FDA stated that these proposed requirements would help protect public health by, among other things, minimizing or preventing contamination and limiting additional risks by ensuring product consistency. The FDA held a public hearing on April 12, 2023, to gather additional comments from stakeholders, including the industry, the scientific community, advocacy groups, and the public. The proposed rule was also made available for public comment for 180 days. The FDA will review all comments as part of the rulemaking process for this rule. PMI welcomes the FDA’s rule under section 906(e) of the Federal Food, Drug, and Cosmetic Act and plans to share its views with the FDA on this important foundational rule.
FDA actions may influence the regulatory approach of other governments and regulatory agencies.
Currently, national standards in certain countries set minimum quality and safety requirements for heat-not-burn products with technical heat-not-burn specifications and/or methods for demonstrating the absence of combustion. These standards are mandatory in Armenia, Bahrain, Egypt, Jordan, Saudi Arabia, Tajikistan, Tunisia, the UAE and Uzbekistan, and voluntary in Algeria, Colombia, Costa Rica, Dominican Republic, Indonesia, Kazakhstan, Kyrgyzstan, Morocco, the Philippines, Russia, Vietnam, the U.K. and Ukraine. In Japan, a voluntary standard sets minimum safety requirements for tobacco heating devices.
For e-vapor products (e-cigarettes) national standards setting minimum quality and safety requirements have been adopted in several markets. These standards are mandatory in Armenia, Bahrain, China, Egypt, Jordan, New Zealand, United Arab Emirates, and Saudi Arabia and Tajikistan, and voluntary in Azerbaijan, Costa Rica, France, Indonesia, Kazakhstan, the Philippines, Russia, the U.K. and Ukraine.
Currently, industry standards setting minimum quality and safety requirements for tobacco-free oral nicotine products (nicotine pouches) have been adopted in Pakistan, Sweden, the U.K. and Ukraine. These standards are voluntary.
We expect other governments to consider similar product standards for all novel tobacco and nicotine-containing products and encourage making them mandatory.
All EU member states have transposed the EU TPD, including the provisions on novel tobacco products, such as heated tobacco units, and e-cigarettes. Most of the EU member states require a notification submitted six months before the intended placing on the market of such products, while some require pre-market authorizations for the introduction of such products. To date, we have filed a comprehensive dossier summarizing our scientific assessment of our Platform 1 product in over 20 member states.
On March 23, 2023, the Greek Ministry of Health authorized a claim for IQOS with HEETS AMBER to inform Greek IQOS users about reduction in emissions of toxicants when using such product compared to cigarette smoking. The decision authorized the following claim: “The concentration of chemical substances with recognized toxicity produced when using IQOS with HEETS AMBER tobacco sticks is lower compared to conventional smoking. A reduction in the concentration of chemical substances with recognized toxicity does not mean a corresponding reduction in risk for health. The aerosol of this tobacco product contains nicotine and other hazardous chemicals. This tobacco product harms your health and is addictive. The best choice is to quit tobacco and nicotine use altogether.” With this authorization, Greece is the second country officially recognizing the reduction in level of toxicants in the IQOS aerosol compared to cigarette smoke.
On September 12, 2022, Norway rejected a submission for authorization of HEETS as a novel tobacco product. Norway partially transposed the EU TPD under the European Free Trade Association agreement and introduced an authorization system for novel tobacco products following Article 19 of TPD. To date, Norway has not granted authorization of any novel tobacco product, and e-cigarettes and tobacco free nicotine pouches have not been granted access, either.
On October 31, 2019, our Australian subsidiary, Philip Morris Limited (“PML”), submitted an application to the Scheduling Committee of the Therapeutic Goods Administration of Australia (“TGA”) seeking to exempt HTPs from being prohibited in Australia. In August 2020, the TGA issued its decision denying the application and stating that the application did not present compelling evidence to establish a public health benefit from greater access to nicotine in HTPs.
To date, several governmental agencies have published their scientific findings that analyze the harm-reduction potential of certain RRPs versus continuing to smoke cigarettes, including:
In December 2017, at the request of the U.K. Department of Health and Public Health England, the U.K. Committee on Toxicity published its assessment of the risk of heat-not-burn products relative to cigarette smoking. This assessment included analysis of scientific data for two heat-not-burn products, one of which was our Platform 1 product. The assessment concluded that, while still harmful to health, compared with the known risks from cigarettes, heat-not-burn products are probably less harmful. Subsequently, in February 2018, Public Health England published a report stating that the available evidence suggests that heat-not-burn products may be considerably less harmful than cigarettes but more harmful than e-cigarettes.
In May 2018, the German Federal Institute for Risk Assessment (“BfR”) published a study on the Platform 1 aerosol relative to cigarette smoke using the Health Canada Intense Smoking Regimen. BfR found reductions in selected HPHCs in a range of 80-99%. This publication indicates that significant reductions in the levels of selected toxicants are likely to reduce toxicant exposure, which BfR stated might be regarded as a discrete benefit compared to combustible cigarettes.
In May 2018, the Dutch National Institute for Public Health and Environment (“RIVM”) published a factsheet on novel tobacco products that heat rather than burn tobacco, focusing on our Platform 1 product. RIVM analyzed the aerosol generated by our Platform 1 product and concluded that the use of this product, while still harmful to health, is probably less harmful than continuing to smoke cigarettes.
In June 2018, the Korean Food and Drug Administration (“KFDA”) issued a statement on products that heat rather than burn tobacco. The KFDA tested three heat-not-burn products, one of which was our Platform 1 product. The KFDA confirmed that the levels of the nine HPHCs tested in the aerosol of these products were on average approximately 90% lower compared to those measured in the cigarette smoke of the top five cigarette brands in South Korea. However, the KFDA stated that it could not establish that the tested heat-not-burn products are less harmful than cigarettes. In October 2018, our Korean subsidiary filed a request with a local court seeking information underlying KFDA’s analysis, conclusions and public statements. In May 2020, the court ordered KFDA to produce certain records. Subsequent to that decision, and after exchanges between the parties, the case was closed.
In August 2018, the Science & Technology Committee of the U.K. House of Commons published a report of its inquiry into e-cigarettes and heat-not-burn products. The report concluded that e-cigarettes are significantly less harmful to health than smoking tobacco. The report also observed that for those smokers who do not accept e-cigarettes, heat-not-burn products may offer a public health benefit despite their relative risk. The report called for a risk-proportionate regulatory environment for both e-cigarettes and heat-not-burn products and noted that e-cigarettes should remain the least taxed, cigarettes the most taxed, with heat-not-burn products falling between the two. The U.K. Committee on Advertising Practice announced the removal of a prohibition of health claims in the advertising of e-cigarettes in the U.K., effective November 2018.
In November 2018, the Eurasian Economic Commission (regulatory body of the Eurasian Union consisting of Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) published the results of its commissioned study on novel nicotine-containing products, including our Platform 1 product. The study confirms significantly lower levels of HPHCs in the aerosol generated by this product compared to cigarette smoke.
In January 2019, scientific media published the results of the study of the China National Tobacco Quality Supervision and Test Centre (“CNTQST”) comparing the aerosol generated by our Platform 1 product with cigarette smoke. The CNTQST found that the former contained fewer, and lower levels of, harmful constituents than the latter and concluded that the lower temperature of heating tobacco in our Platform 1 product contributed to the difference. The CNTQST stated that the reduction in emissions of harmful constituents cannot be interpreted as a harm/risk reduction for cigarette smokers in the same proportion.
In April 2020, the Superior Health Council of Belgium (“SHC”) published results of its inquiry into heat-not-burn products. The SHC concluded that heat-not-burn products, while not safe, have a more favorable toxicity profile than cigarettes. However, in light of the uncertainty of such products’ short and long-term impacts, the toxic effects of the dual use with cigarettes, and the existence of approved smoking cessation tools, the SHC recommended that current regulations for cigarettes should apply to heat-not-burn products.
In June 2022, the SHC published new advice on e-cigarettes in which they confirm that e-cigarettes are substantially less harmful than smoking cigarettes and, therefore, a better alternative for smokers. The SHC underlines that the vast majority of the risks of tobacco smoking are not caused by nicotine, but by the harmful substances that are released by the combustion of tobacco. Based on the cited science, the SHC calls for legislation that makes a clear distinction between cigarettes and e-cigarettes by focusing on better informing smokers about the benefits of the lower-risk (but not risk-free) alternative, as well as on protecting non-smokers and young people.
The foregoing scientific findings of government agencies may not be indicative of the measures that the relevant government authorities could take in regulating our products.
We make our scientific findings publicly available for scrutiny and peer review through several channels, including our websites. From time to time, adult consumers, competitors, members of the scientific community, and others inquire into our scientific methodologies, challenge our scientific conclusions or request further study of certain aspects of our RRPs and their health effects. We are committed to a robust and open scientific debate and believe that such debate should be based on accurate and reliable scientific information. We seek to provide accurate and reliable scientific information about our RRPs; nonetheless, we may not be able to prevent third-party dissemination of false, misleading or unsubstantiated information about these products. The dissemination of scientifically unsubstantiated information or studies with a strong confirmation bias by third parties may cause confusion among adult smokers and affect their decision to switch to better alternatives to continued smoking, such as our RRPs.
To date, we have been largely successful in demonstrating to regulators that our heated tobacco units are not cigarettes due to the absence of combustion, and as such, they are generally taxed either as a separate category or as other tobacco products, which typically yields more favorable tax rates than cigarettes. Although we believe that this is sensible from the public health perspective, we cannot guarantee that regulators will continue this approach.
There can be no assurance that we will succeed in our efforts to replace cigarettes with RRPs or that regulation will allow us to commercialize RRPs in all markets, to communicate about our RRPs, including making scientifically substantiated risk-reduction claims, or to treat RRPs differently from cigarettes.
Legal Challenges to RRPs: We face various administrative and legal challenges related to certain RRP activities, including allegations concerning product classification, advertising restrictions, corporate communications, product coach activities, scientific substantiation, product liability, and unfair competition. While we design our programs to comply with relevant regulations, we expect these or similar challenges to continue as we expand our efforts to commercialize RRPs and to communicate with the public. The outcomes of these matters may affect our RRP commercialization and public communication activities and performance in one or more markets.
In April 2020, affiliates of British American Tobacco p.l.c. ("BAT") filed a complaint against PMI, Philip Morris Products S.A., Altria Group, Inc., and its subsidiaries before the International Trade Commission ("ITC"). On September 29, 2021, the ITC issued its Final Determination ("FD"), Limited Exclusion Order ("LEO") and Cease and Desist Order ("CDO"). The ITC upheld the finding of infringement in the FD and found a subsequent violation. The ITC issued a LEO against Philip Morris Products S.A., prohibiting the importation of infringing tobacco heating articles and components thereof, and CDOs against Altria Client Services, LLC, and certain of its affiliates, which went into effect at the end of the 60-day Presidential review period on November 28, 2021. We appealed the patent issues. Furthermore, lawsuits based on the same patent families were repeatedly and universally rejected in European courts and the European Patent Office. The decision has no bearing outside the United States.
On February 1, 2024 we entered into a global settlement with BAT that resolves all ongoing patent infringement litigation between the parties related to heated tobacco and vapor products. Among other matters, under the settlement PMI and BAT agreed to request rescission of the LEO and CDO. For further details, see Item 8, Note 18. Contingencies to our consolidated financial statements.
Our RRP Business Development Initiatives: In December 2013, we established a strategic framework with Altria Group, Inc. (“Altria”) setting out terms on how the parties would collaborate to develop and commercialize e-vapor products and commercialize two of our RRPs in the U.S. In late 2018, Altria announced that it will participate in the e-vapor category only through another e-vapor company in which Altria acquired a minority interest. In September 2019, Altria's subsidiary, Philip Morris USA Inc. (“PM USA”), began commercialization of a version of our Platform 1 product in the U.S. Under the agreement, PM USA was required to achieve certain milestones in order to maintain its exclusive distribution right and additional milestones in order to extend the agreement after the initial 5-year term. On October 20, 2022, PMI announced that it had reached an agreement with Altria to terminate the companies' commercial relationship covering IQOS in the U.S., as of April 30, 2024. Thereafter, PMI will hold the full rights to commercialize IQOS in the U.S. For more details, see Item 8, Note 3. Acquisitions and Note 18. Contingencies to our consolidated financial statements.
In January 2020, we announced an agreement with KT&G, a leading tobacco and nicotine company in South Korea, for the commercialization of KT&G’s smoke-free products outside of South Korea on an exclusive basis. On January 30, 2023, we announced a renewal and extension of this arrangement. For more information, see Acquisitions and Other Business Arrangements below.
Other Developments: In September 2017, we announced our support of the Foundation for a Smoke-Free World (the "Foundation"). The Foundation is an independent, nonprofit organization dedicated to reducing the health impacts of smoking as set out in its Articles of Incorporation and its Bylaws. In September 2020, our pledge agreement with the Foundation was amended. We contributed $45 million in 2020, $40 million in 2021, $17.5 million in 2022, and had expected to contribute up to $35 million annually from 2023 through 2029, as specified in the amended pledge agreement. In 2023, the Foundation and PMI agreed to terminate the existing pledge agreement and PMI has made final grant payments totaling $140 million, commensurate with the early termination of the pledge agreement.
Governmental Investigations
From time to time, we are subject to governmental investigations on a range of matters, including tax, customs, antitrust, advertising, and labor practices. We describe certain matters pending in South Korea and Thailand in Item 8, Note 18. Contingencies.
In November 2010, a World Trade Organization ("WTO") panel issued its decision in a dispute between the Philippines and Thailand, concerning a series of Thai customs and tax measures affecting cigarettes imported by Philip Morris (Thailand) Limited ("PM Thailand") into Thailand. The decision concluded that Thailand had no basis to find that PM Thailand's declared customs values and taxes paid were too low, as alleged by the Thai government and created obligations for Thailand to revise its laws, regulations, or practices affecting the customs valuation and tax treatment of future cigarette imports. Thailand agreed to fully comply with the decision, but the Philippines asserts that to date Thailand has not fully complied with the WTO panel decision and commenced challenges at the WTO Appellate Body. The WTO Appellate Body is not operational, and the appeals by Thailand are suspended indefinitely. In December 2020, the Philippines and Thailand agreed to pursue facilitator-assisted discussions aimed at progressing and resolving outstanding issues and the countries have since agreed to seek the establishment of a bilateral consultative mechanism, with the goal of reaching a comprehensive settlement of their dispute, consistent with their rights and obligations under the WTO Agreements, as well as the recommendations and rulings of the WTO Dispute Settlement Body.
In July 2020, the Public Prosecutor’s office of Rome, Italy, notified our Italian subsidiary, Philip Morris Italia S.r.l. (“PM Italia”), as well as three former or current employees and a former external consultant of PM Italia in March 2020, that it concluded a preliminary investigation against them for alleged contravention of anti-corruption laws and related disruption of trade freedom. The Public Prosecutor alleges that the individuals involved promised certain personal favors to government officials from January to July of 2018 in exchange for favorable treatment for PM Italia, and that PM Italia lacked appropriate organizational controls to prevent the alleged actions by the individuals.
In September 2020, the Prosecutor issued his indictment and referred the matter to the court. At the preliminary hearing held on May 11, 2021, the judge decided to refer all charges/defendants (including our affiliate) to trial. The first trial hearing took place on September 22, 2021. BAT has filed a civil claim against PM Italia claiming vicarious liability for any wrongdoing of its former or current employees and seeking EUR 50 million (approximately $55.2 million) in damages. The court admitted the claim and issued summons for PM Italia to appear in the case. The court proceeded with the examination of witnesses beginning in September 2023. PM Italia believes the charges brought against it by the Public Prosecutor are without merit and will defend them vigorously.
Impact of Inflation on Our Business and Mitigation Efforts
Like many other global companies, we have experienced inflationary pressures in 2022 and 2023, including: growing pressures on the cost of certain direct materials, wages, energy, transportation, and logistics as well as an increased cost of capital due to interest rate increases driven by the response to increased inflation. For the year ended December 31, 2023, the impact on cost of sales was approximately $580 million and we expect certain inflationary elements to ease, with a moderate increase in 2024. This impact has been, and we expect it to continue to be, significantly offset by the positive elements of pricing, productivities and the mitigating factors as we progress through the year. The net result of the inflationary impacts and our efforts to mitigate these impacts were not material to PMI during these periods.
Inflationary impacts driven by higher wages have resulted from merit increases that reflect local inflation as we continuously evaluate our compensation and benefit offerings to be competitive with the current market. Increased transportation costs resulted from increased shipping rates for all modes of transportation (air, ocean and inland) due to ocean and air capacity constraints. Increases in cost of sales resulted from higher cost of direct materials due to the pass on of energy, transportation, labor and commodity price increases from suppliers as well as increases in utility costs, including gas and electricity prices, primarily in Europe resulting from the war in Ukraine. Raw materials such as tobacco leaf have longer inventory durations which resulted in insignificant inflationary impacts to our cost of sales in 2022; however tobacco leaf purchases in both 2022 and 2023 have been at higher prices due to inflationary impacts on fertilizer prices and labor costs, thus resulting in increases in the cost of inventory with corresponding impacts on our financial results in 2023. In addition, our cash flow from operations was impacted by the net working capital investment related to the procurement of tobacco leaf inventory and higher cost of direct materials. We expect certain of these inflationary elements to ease in 2024 as noted above.
We have taken several actions to mitigate these inflationary pressures. Mitigation efforts have included (i) indexation clauses related to commodity costs and energy pricing within contracts, (ii) tactical inventory purchases, (iii) identification of new suppliers in different geographical locations for incremental sourcing, (iv) increasing tobacco leaf inventory durations to secure additional volumes at favorable prices, (v) optimizing the mix of tobacco leaf origins and suppliers, (vi) continuous evaluation of shipping routes and methods of shipment, (vii) supplier negotiations, (viii) variable contract durations for energy costs, (ix) hedging strategies, and (x) other pricing, productivity and procurement initiatives.
Asset Impairment and Exit Costs
We discuss asset impairment and exit costs related to restructuring activities in Item 8, Note 20. Asset Impairment and Exit Costs to our consolidated financial statements.
U.S. GAAP Treatment of Highly Inflationary Economies
We apply highly inflationary accounting to the results of operations of our subsidiaries in Argentina, Turkey, Lebanon and Venezuela as the cumulative inflation rate in these economies for a three-year period meets or exceeds 100%, in accordance with U.S. GAAP. As a result, monetary assets and liabilities denominated in local currencies are remeasured to the U.S. Dollar at each balance sheet date, with remeasurement gains and losses recognized in consolidated statement of earnings.
This impact of currency fluctuations could negatively impact our financial condition and results of operations. For the years ended December 31, 2023, 2022 and 2021, we recognized exchange gains (losses) of $(194) million, $11 million and $9 million, respectively, resulting from remeasurement adjustments related to highly inflationary accounting.
Climate Change Laws and Regulations
While, to date, the effect of climate-related laws and regulations on PMI has not been material to our business, results of operations or financial condition, consideration of environmental and climate-related laws and regulations is an integral aspect of PMI’s climate-related risk assessment process. To this end, we actively monitor the existing and potential impact on PMI of significant pending or existing climate change-related legislation, regulations, international accords, reporting frameworks, standards, principles, and other forms of guidance. Examples include, but are not limited to, the EU Emissions Trading System, the 2015 Paris Climate Agreement, the work of the International Financial Reporting Standards Foundation, including the International Sustainability Standards Board proposed climate standard and the recommendations of the Task Force on Climate-related Financial Disclosures, the SEC’s proposed rules regarding climate-related disclosures, the Task Force on Nature-related Financial Disclosures, the EU Corporate Sustainability Reporting Directive, the EU Taxonomy Regulation, the EU Deforestation Regulation, the EU Proposal for a Corporate Sustainability Due Diligence Directive, CDP, the GHG Protocol, and carbon tax programs in Europe and Canada.
Acquisitions and Other Business Arrangements
We discuss our acquisitions in Item 8, Note 3. Acquisitions to our consolidated financial statements.
KT&G
On January 30, 2023, PMI announced a long-term collaboration with KT&G, South Korea’s leading tobacco and nicotine manufacturer, to continue to commercialize KT&G’s innovative smoke-free devices and consumables on an exclusive, worldwide basis (excluding South Korea).
The agreement covers fifteen years, to January 29, 2038, with performance-review cycles and associated commitments, based on volume, to be confirmed for each three-year period, to allow flexibility for evolving market conditions.
The agreement gives PMI continued exclusive access to KT&G’s smoke-free brands and product-innovation pipeline, including offerings for low- and middle-income markets, that will enhance PMI’s existing portfolio of smoke-free products.
Products sold under the agreement will be subject to assessment to ensure they meet the regulatory requirements in the markets where they are launched, as well as PMI’s high standards of quality and scientific substantiation. PMI and KT&G will seek any necessary regulatory approvals that may be required on a market-by-market basis.
Equity Investments
We discuss our equity investments in Item 8, Note 6. Related Parties - Equity Investments and Other to our consolidated financial statements.
Trade Policy
PMI complies with all applicable trade restrictions and requirements, including sanctions, in the markets in which it operates. We have taken appropriate actions in response to the latest sanctions to ensure full compliance with the relevant restrictions.
We are subject to various trade restrictions imposed by the U.S., the EU, Switzerland, the U.K., and other jurisdictions in which we do business (“Trade Sanctions”), including the trade and economic sanctions administered by the U.S. Department of the Treasury's Office of Foreign Assets Control (“OFAC”) and the U.S. Department of State. It is our policy to comply fully with these Trade Sanctions.
Pursuant to specific exemptions or licenses, or where sanctions do not apply to our business, PMI may make sales in countries subject to Trade Sanctions.
We do not do business or sell products in Iran, North Korea or Syria.
We sell cigarettes in Cuba under a distribution agreement. These sales are permitted by U.S. law under a License Exception for Agricultural Commodities, issued by the U.S. Department of Commerce (Bureau of Industry and Security), and specifically granted to our distributor.
Certain states within the U.S. have enacted legislation permitting or requiring state pension funds to divest or abstain from future investment in stocks of companies that do business with certain countries that are sanctioned by the U.S. Because we do business in certain of these countries, consistent with our policy to fully comply with Trade Sanctions and as described above, these state pension funds may have divested of our stock or may not invest in our stock. We do not believe such legislation has had a material effect on the price of our shares.
On June 24, 2021, the EU introduced sanctions in relation to Belarus aimed at specific sectors of the Belarus economy, including the tobacco sector. Subsequently, seven non-EU countries (Norway, Iceland, Liechtenstein, North Macedonia, Bosnia and Herzegovina, Montenegro, and Albania) announced that they “aligned themselves” with the majority of the EU sanctions. Switzerland and the U.K. have also imposed sanctions similar in scope to the EU sanctions.
On August 9, 2021, the U.S. imposed blocking sanctions on certain Belarusian individuals and entities pursuant to an Executive Order, which expanded the bases for the imposition of sanctions, including, among others, by authorizing the imposition by OFAC of blocking sanctions on persons operating in the tobacco sector of the Belarus economy. From 2021 to 2023, the U.S., the EU, the U.K., Switzerland and several other jurisdictions supplemented their respective sanctions lists by including additional Belarusian sanctions targets.
Following the start of the conflict in Ukraine on February 24, 2022, the U.S., the EU, the U.K., Switzerland, Canada, Australia, New Zealand, Singapore, South Korea, Japan and other countries introduced extensive economic sanctions and export controls in relation to Russia. While the introduced sanctions slightly vary from jurisdiction to jurisdiction, they are largely aligned. The restrictions are primarily targeted at the Russian financial, banking, oil, military, aviation and marine sectors. The U.S. has also introduced a prohibition on new investment in the Russian Federation by a U.S. person, wherever located, and authorized the imposition of blocking sanctions on anyone operating in the Russian manufacturing sector. The potential application of the latter for goods other than military goods remains unclear. Among sanctions targets are Russian political figures and military personnel, certain oligarchs and journalists, and companies operating in the above-mentioned sectors. Export to Russia of certain luxury goods, and goods and technology which might contribute to Russia’s technological enhancement was banned. Seven non-EU countries (Norway, Iceland, Liechtenstein, North Macedonia, Bosnia and Herzegovina, Montenegro, and Albania) announced that they “aligned themselves” with the majority of the EU sanctions. The U.S., the EU, Switzerland and Japan introduced additional trade restrictions banning, among many other goods, the export of certain non-tobacco materials used to produce cigarettes and heated tobacco consumables in Russia. The EU, Switzerland and the U.K. also prohibited technical assistance and other services related to restricted goods. The EU, Switzerland and the U.K. prohibited import into their territories of certain goods, including cigarettes, among others, which might generate significant revenues for Russia if they originate in Russia or are exported from Russia. The EU and Switzerland prohibited transfer and licensing of intellectual property rights in relation to restricted goods. Additionally, the EU, the U.S., the U.K., Switzerland, Canada, Australia, New Zealand and Ukraine imposed sanctions on Mr. Igor Kesaev, a non-majority shareholder of Megapolis Distribution B.V.
The U.S., the U.K., Switzerland and the EU banned the export of electric accumulators and static converters to Russia. In addition, the U.S. and the U.K. banned the export of electronic cigarettes and similar personal electric vaporizing devices to Russia. Certain countries also banned the delivery of services to Russia, such as information technology consultancy services, accounting and business and management consulting services.
Russia introduced certain countermeasures aimed at reducing the effect of Western sanctions. Countermeasures include restrictions on export of certain goods from Russia, including tobacco-related production equipment, restrictions on lending to foreign borrowers, repatriation of dividends and transactions with securities and real estate involving companies from “hostile” countries (i.e., those which introduced sanctions in relation to Russia).
PMI continues to monitor the development of new sanctions and ensure full compliance.
2023 compared with 2022
The following discussion compares operating results within each of our segments for 2023 with 2022.
Europe:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
13,598 |
|
$ |
12,869 |
|
|
5.7 |
% |
3.7 |
% |
|
$ |
729 |
|
$ |
249 |
|
$ |
— |
|
$ |
540 |
|
$ |
(60) |
|
$ |
— |
|
| Operating Income |
|
$ |
6,012 |
|
$ |
5,802 |
|
|
3.6 |
% |
0.4 |
% |
|
$ |
210 |
|
$ |
186 |
|
$ |
— |
|
$ |
540 |
|
$ |
(79) |
|
$ |
(437) |
|
Net revenues increased by 5.7%. Net revenues, excluding currency and acquisitions, increased by 3.7%, reflecting: a favorable pricing variance, mainly driven by higher combustible tobacco pricing; partially offset by unfavorable volume/mix, mainly due to lower cigarette volume, as well as unfavorable cigarette mix, partly offset by higher HTU volume.
The pricing variance for the years 2023 and 2022 was negatively impacted by the supplemental tax surcharge on heated tobacco units in Germany, which went into effect in 2022. The negative impact will continue until a ruling on the legality of the surcharge is issued. It is currently being assessed in court and the obligation to pay the surcharge is temporarily suspended. PMI currently accounts for the surcharge as a reduction in net revenues and in accrued liabilities in its results. The accrued liability balance will continue to increase with the continuation of the HTU selling activities and in the case of an unfavorable ruling would negatively impact PMI’s future cash provided by operating activities. A favorable ruling would positively impact future PMI’s operating results.
Operating income increased by 3.6%. Operating income, excluding currency and acquisitions, increased by 0.4%, primarily reflecting: lower charges in 2023 related to the war in Ukraine ($98 million), a favorable comparison to 2022 related to costs associated with the Swedish Match AB offer ($53 million) and a favorable pricing variance. The increase was partly offset by the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World ($62 million), the 2023 charges for asset impairment and exit costs ($49 million), higher marketing, administration and research costs (mainly due to inflationary impacts and lower commercial investments in the prior year period); higher manufacturing costs (primarily due to inflationary impacts); and unfavorable volume/mix, mainly due to the same factors as for net revenues.
Europe - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region decreased by 1.3% to 542.3 billion units, reflecting a 3.0% decline for cigarettes, partly offset by a 15.6% increase for HTUs. The decrease in the estimated total market was predominantly due to the United Kingdom (down by 15.4%), France (down by 8.2%), Germany (down by 1.8%) and Spain (down by 2.4%), partly offset by Poland (up by 1.8%).
|
|
|
|
|
|
|
|
|
|
|
|
Europe Key Data |
Full-Year |
|
|
|
Change |
|
2023 |
2022 |
% / pp |
| PMI Market Share |
|
|
|
| Cigarettes |
30.3 |
% |
31.1 |
% |
(0.8) |
|
| Heated Tobacco Units |
9.1 |
% |
7.8 |
% |
1.3 |
|
| Total Europe |
39.4 |
% |
39.0 |
% |
0.4 |
|
Note: Sum may not foot due to roundings.
Our total cigarette and HTU shipment volume in the Region decreased by 0.6% to 214.9 billion units, mainly due to Germany (down by 6.0%), Italy (down by 2.8%; or up by 0.4% excluding the net unfavorable impact of estimated distributor inventory movements) and France (down by 7.3%), partly offset by Poland (up by 9.4%).
Our estimated HTU adjusted in-market sales volume in the Region increased by 17.6%, including growth in Germany and Italy of 29.7% and 16.6%, respectively.
Our HTU share of the total cigarette and HTU market in the Region increased by 1.3 points, or by 1.5 points on an adjusted basis.
SSEA, CIS & MEA:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
10,629 |
|
$ |
10,467 |
|
|
1.5 |
% |
11.7 |
% |
|
$ |
162 |
|
$ |
(1,060) |
|
$ |
— |
|
$ |
1,008 |
|
$ |
400 |
|
$ |
(186) |
|
| Operating Income |
|
$ |
3,047 |
|
$ |
3,864 |
|
|
(21.1) |
% |
(4.2) |
% |
|
$ |
(817) |
|
$ |
(653) |
|
$ |
— |
|
$ |
1,008 |
|
$ |
(237) |
|
$ |
(935) |
|
Net revenues increased by 1.5%. Net revenues, excluding currency and acquisitions, increased by 11.7%, primarily reflecting: a favorable pricing variance, mainly driven by higher combustible tobacco pricing, with HTU pricing also higher; and favorable volume/mix, primarily driven by favorable cigarette mix, as well as higher volume for HTUs, partly offset by an unfavorable cigarette volume impact. This increase was partially offset by the 2023 termination of a distribution arrangement in the Middle East of $80 million and lower fees for certain distribution rights billed to customers in certain markets, both included in "Cost/Other" in the table above.
Operating income decreased by 21.1%. Operating income, excluding currency and acquisitions, decreased by 4.2%, primarily reflecting: higher marketing, administration and research costs; higher manufacturing costs (primarily due to inflationary impacts); unfavorable volume/mix, mainly due to an unfavorable cigarette volume impact and unfavorable cigarette mix, partly offset by higher HTU volume; and the termination of a distribution arrangement, coupled with the impact of lower fees for certain distribution rights, as noted for net revenues; as well as the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World of $44 million and the 2023 charges for asset impairment and exit costs of $34 million. The decrease was partially offset by the favorable pricing variance and a favorable comparison to 2022 related to costs associated with the Swedish Match AB offer of $33 million.
SSEA, CIS & MEA - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region decreased by approximately 2% to 1,528.6 billion units, due to a decline for cigarettes. The decrease in the estimated total market was predominantly due to Egypt (down by 20.9%) and Pakistan (down by 35.1%), partly offset by Turkey (up by 16.9%).
Our Regional market share increased by 0.8 points to 23.4%.
Our total cigarette and HTU shipment volume in the Region increased by 1.3% to 358.2 billion units, mainly driven by Turkey (up by 23.0%), partly offset by the Philippines (down by 26.2%). Our estimated HTU adjusted in-market sales volume increased by 8.2% including limited growth in Russia.
EA, AU & PMI DF:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
6,201 |
|
$ |
5,936 |
|
|
4.5 |
% |
11.2 |
% |
|
$ |
265 |
|
$ |
(400) |
|
$ |
— |
|
$ |
206 |
|
$ |
459 |
|
$ |
— |
|
| Operating Income |
|
$ |
2,481 |
|
$ |
2,424 |
|
|
2.4 |
% |
18.6 |
% |
|
$ |
57 |
|
$ |
(395) |
|
$ |
— |
|
$ |
206 |
|
$ |
326 |
|
$ |
(80) |
|
Net revenues increased by 4.5%. Net revenues, excluding currency and acquisitions, increased by 11.2%, reflecting: favorable volume/mix, mainly driven by higher HTU volume, partly offset by lower cigarette volume and unfavorable smoke-free product mix (for HTUs and devices); and a favorable pricing variance, driven by higher combustible tobacco and device pricing, partly offset by lower HTU (net) pricing (primarily related to Japan).
Operating income increased by 2.4%. Operating income, excluding currency and acquisitions, increased by 18.6%, mainly reflecting favorable volume/mix, primarily driven by higher HTU volume, partly offset by lower cigarette volume and unfavorable HTU mix; the favorable pricing variance; lower supply chain costs (primarily related to Japan); and a favorable comparison to 2022 related to costs associated with the Swedish Match AB offer ($24 million). The increase was partly offset by the 2023 charge related to the South Korea indirect tax ($204 million), the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World ($27 million) and the 2023 charges for asset impairment and exit costs ($21 million).
EA, AU & PMI DF - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding China, increased by 1% to 319.8 billion units, reflecting growth for HTUs, partly offset by a decline for cigarettes. The increase in the estimated total market was mainly driven by International Duty Free (up by 35.7%), partly offset by Taiwan (down by 7.4%) and Australia (down by 19.4%).
Our Regional market share increased by 1.3 points to 30.0%.
Our total cigarette and HTU shipment volume in the Region increased by 6.7% to 101.2 billion units, mainly driven by Japan (up by 9.7%) and International Duty Free (up by 14.5%).
PMI's estimated HTU adjusted in-market sales volume in the Region increased by 15.8%, including growth in Japan of 14.5%.
Americas:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
1,944 |
|
$ |
1,903 |
|
|
2.2 |
% |
(2.9) |
% |
|
$ |
41 |
|
$ |
96 |
|
$ |
— |
|
$ |
128 |
|
$ |
(177) |
|
$ |
(6) |
|
| Operating Income |
|
$ |
62 |
|
$ |
436 |
|
|
(85.8) |
% |
(40.6) |
% |
|
$ |
(374) |
|
$ |
(197) |
|
$ |
— |
|
$ |
128 |
|
$ |
(139) |
|
$ |
(166) |
|
Net revenues increased by 2.2%. Net revenues, excluding currency and acquisitions, decreased by 2.9%, primarily reflecting: unfavorable volume/mix, mainly due to lower cigarette volume and unfavorable cigarette mix; partly offset by a favorable pricing variance, driven by higher combustible tobacco pricing.
Operating income decreased by 85.8%. Operating income, excluding currency and acquisitions, decreased by 40.6%, mainly reflecting: higher marketing, administration and research costs (including incremental investments in the U.S. in preparation for smoke-free product commercialization); and unfavorable volume/mix, mainly due to the same factors as for net revenues; partly offset by the favorable pricing variance.
Americas - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding the U.S., decreased by around 1% to 189.2 billion units, driven by a decline for cigarettes. The decrease in the estimated total market was mainly due to Mexico (down by 6.8%), Canada (down by 12.6%) and Argentina (down by 5.0%), partly offset by Brazil (up by 10.1%).
Our Regional market share, excluding the U.S., decreased by 1.1 points to 33.7%.
Our total cigarette and HTU shipment volume in the Region decreased by 3.9% to 63.9 billion units, mainly due to Mexico (down by 9.8%) and Argentina (down by 7.9%), partly offset by Brazil (up by 12.8%).
Swedish Match:
As of November 11, 2022, PMI became the owner of a majority position in Swedish Match and started consolidating Swedish Match operating results. The business operations of our Swedish Match segment are evaluated separately from the geographical segments.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
2,496 |
|
$ |
316 |
|
|
+100% |
21.2 |
% |
|
$ |
2,180 |
|
$ |
— |
|
$ |
2,113 |
|
$ |
25 |
|
$ |
42 |
|
$ |
— |
|
| Operating Income / (Loss) |
|
$ |
824 |
|
$ |
(22) |
|
|
+100% |
+100% |
|
$ |
846 |
|
$ |
(8) |
|
$ |
694 |
|
$ |
25 |
|
$ |
38 |
|
$ |
97 |
|
We recorded net revenues of $2.5 billion in the Swedish Match segment, with smoke-free products accounting for over 80% of the segment's total net revenues.
We recorded operating income of $824 million for the year ended December 31, 2023. Operating income included $372 million related to the amortization of acquired intangibles and $18 million of charges related to an acquisition accounting-related item recorded in 2023.
Swedish Match - PMI Shipment Volume Commentary
|
|
|
|
|
|
|
|
|
|
|
|
|
Swedish Match Oral Product Shipment Volume (1)
(million cans)
|
Full-Year |
| Nicotine Pouches |
2023 |
2022 |
Change |
| U.S. |
384.8 |
|
34.5 |
|
+100% |
| Scandinavia |
28.7 |
|
3.7 |
|
+100% |
| Other |
4.6 |
|
1.2 |
|
+100% |
| Total Nicotine Pouches |
418.2 |
|
39.4 |
|
+100% |
| Snus |
|
|
|
| Scandinavia |
218.2 |
|
39.3 |
|
+100% |
| Other |
6.8 |
|
1.1 |
|
+100% |
| Total Snus |
224.9 |
|
40.4 |
|
+100% |
|
|
|
|
| Moist Snuff |
133.7 |
|
16.0 |
|
+100% |
|
|
|
|
| Other |
4.2 |
|
— |
|
— |
% |
|
|
|
|
| Total Oral Products |
781.0 |
|
95.8 |
|
+100% |
(1) Excluding U.S. chew
|
|
|
|
|
|
|
|
|
|
|
|
Swedish Match Combustible Tobacco Shipment Volume
(million units) |
Full-Year |
|
2023 |
2022 |
Change |
| Cigars |
1,578.6 |
|
259.6 |
|
+100% |
For comparison purposes, the following commentaries assumed the inclusion of Swedish Match's 2022 shipment volume for the full year, thereby providing the comparability of Swedish Match's volume performance between periods. Volume comparisons versus Swedish Match's 2022 results reflect data sourced from its disclosures, available at www.swedishmatch.com/investors.
Swedish Match's total shipment volume for oral products increased by 17.1% versus its corresponding shipments of 667.1 million cans in 2022.
Nicotine pouch shipment volume increased by 55.3% compared to Swedish Match's 2022 shipment volume of 269.2 million cans, reflecting 62.0% growth for ZYN in the U.S. In Scandinavia, shipment volume for nicotine pouches grew by 6.1%.
Shipment volume for snus declined by 13.8% compared to Swedish Match's 2022 shipment volume of 261.0 million cans.
Cigar shipment volume declined by 12.2% compared to Swedish Match's 2022 cigar shipment volume of 1,798.0 million units, primarily due to the impact of industry pricing effects.
Wellness and Healthcare:
The operating results of PMI’s Vectura Fertin Pharma business are reported in the Wellness and Healthcare segment. The business operations of our Wellness and Healthcare segment are evaluated separately from the geographical segments.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2023 |
2022 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
306 |
|
$ |
271 |
|
|
12.9 |
% |
11.8 |
% |
|
$ |
35 |
|
$ |
3 |
|
$ |
— |
|
$ |
33 |
|
$ |
— |
|
$ |
(1) |
|
| Operating Income / (Loss) |
|
$ |
(870) |
|
$ |
(258) |
|
|
-(100)% |
-(100)% |
|
$ |
(612) |
|
$ |
(6) |
|
$ |
— |
|
$ |
33 |
|
$ |
— |
|
$ |
(639) |
|
Net revenues increased by 12.9%. Net revenues, excluding currency and acquisitions, increased by 11.8%, notably reflecting the higher net revenues for smoking cessation products and select inhalation products.
The operating loss of $870 million in 2023 was primarily due to an impairment charge for goodwill and other intangibles of $680 million in the second quarter, as well as commercial investments and higher administration costs and the amortization of acquired intangibles. The operating loss of $258 million in 2022 included a charge for impairment of other intangible assets of $112 million and the amortization of acquired intangibles.
2022 compared with 2021
As previously disclosed in the Description of Our Company section of this Item 7, in January 2023, we began managing our business in four geographical segments, down from six previously, in addition to our continuing Swedish Match and Wellness and Healthcare segments. The following discussion compares operating results within each of our segments for 2022 with 2021 under this new operating segment structure.
Europe:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2022 |
2021 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
12,869 |
|
$ |
13,155 |
|
|
(2.2) |
% |
9.7 |
% |
|
$ |
(286) |
|
$ |
(1,576) |
|
$ |
10 |
|
$ |
(122) |
|
$ |
1,402 |
|
$ |
— |
|
| Operating Income |
|
$ |
5,802 |
|
$ |
6,409 |
|
|
(9.5) |
% |
6.6 |
% |
|
$ |
(607) |
|
$ |
(1,029) |
|
$ |
(2) |
|
$ |
(122) |
|
$ |
918 |
|
$ |
(372) |
|
Net revenues decreased by 2.2%. Net revenues, excluding currency and acquisitions, increased by 9.7%, reflecting: favorable volume/mix, mainly driven by higher HTU volume and device volume, partly offset by lower cigarette volume, unfavorable HTU mix, and unfavorable cigarette mix; partially offset by an unfavorable pricing variance, mainly due to lower HTU (net) pricing and lower device pricing, partly offset by higher combustible tobacco pricing.
The pricing variance for the full year 2022 was negatively impacted by the supplemental tax surcharge on heated tobacco units in Germany, which went into effect in 2022. The negative impact will continue until a ruling on the legality of the surcharge is issued. It is currently being assessed in court and the obligation to pay the surcharge is temporarily suspended. PMI currently accounts for the surcharge as a reduction in net revenues and in accrued liabilities in its results. The accrued liability balance will continue to increase with the continuation of the HTU selling activities and in the case of an unfavorable ruling would negatively impact PMI’s future cash provided by operating activities. A favorable ruling would positively impact future PMI’s operating results.
Operating income decreased by 9.5%.
Operating income, excluding currency and acquisitions, increased by 6.6%, primarily reflecting favorable volume/mix, mainly driven by higher HTU volume, partly offset by lower cigarette volume, unfavorable HTU mix, unfavorable cigarette mix and the unfavorable impact on profitability of higher device volume; partially offset by an unfavorable pricing variance; higher manufacturing costs; and higher marketing, administration and research costs (including the unfavorable impact of 2022 charges related to the war in Ukraine of $151 million, 2022 costs associated with the Swedish Match AB offer of $53 million and a favorable comparison versus the prior year period related to asset impairment and exit costs of $72 million).
Europe - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region decreased by 0.4% to 549.6 billion units, primarily driven by:
•Germany, down by 5.1%, primarily reflecting the impact of excise tax-driven price increases and higher cross-border (non-domestic) purchases due to the easing of pandemic-related measures;
•the U.K., down by 13.6%, notably reflecting the impact of increased out-bound tourism compared to the pandemic-affected prior year period; and
•Ukraine, down by 18.3%, due to cigarettes and HTUs;
partly offset by
•Italy, up by 3.4%, mainly reflecting the impact on adult smoker average daily consumption of the easing of pandemic-related measures (particularly in the first half of the year);
•Poland, up by 13.0%, primarily reflecting a lower estimated prevalence of illicit trade, as well as higher border sales (largely due to the easing of pandemic-related measures); and
•Romania, up by 4.2%, mainly reflecting a lower estimated prevalence of illicit trade, as well as higher border sales (largely due to the easing of pandemic-related measures).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Europe Key Data |
|
Full-Year |
|
|
|
|
Change |
|
|
2022 |
2021 |
% / pp |
|
|
|
|
|
|
|
|
|
|
| PMI Market Share |
|
|
|
|
| Cigarettes |
|
31.1 |
% |
32.2 |
% |
(1.1) |
|
| Heated Tobacco Units |
|
7.8 |
% |
6.1 |
% |
1.7 |
|
| Total Europe |
|
39.0 |
% |
38.3 |
% |
0.7 |
|
Note: Sum may not foot to total due to roundings
Our Regional market share increased by 0.7 points to 39.0%, with gains in Germany, Italy and Poland, partly offset by declines in France, Spain and Ukraine.
Our total cigarette and HTU shipment volume increased by 1.7% to 216.1 billion units, mainly driven by:
•Italy, up by 5.8%, primarily reflecting a higher market share driven by HTUs, as well as a higher total market;
•Poland, up by 17.6%, mainly reflecting the higher total market and a higher market share driven by HTUs; and
•Romania, up by 36.1%. Excluding the net favorable impact of estimated distributor inventory movements, total in-market sales volume increased by 27.3%, primarily reflecting a higher market share driven by HTUs, as well as the higher total market;
partly offset by
•France, down by 8.1%, primarily reflecting a lower total market and a lower market share; and
•Ukraine, down by 30.1%, due to cigarettes and HTUs.
SSEA, CIS & MEA:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2022 |
2021 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
10,467 |
|
$ |
9,858 |
|
|
6.2 |
% |
10.4 |
% |
|
$ |
609 |
|
$ |
(419) |
|
$ |
— |
|
$ |
582 |
|
$ |
193 |
|
$ |
253 |
|
| Operating Income |
|
$ |
3,864 |
|
$ |
3,295 |
|
|
17.3 |
% |
20.3 |
% |
|
$ |
569 |
|
$ |
(99) |
|
$ |
— |
|
$ |
582 |
|
$ |
(112) |
|
$ |
198 |
|
Net revenues increased by 6.2%. Net revenues, excluding currency and acquisitions, increased by 10.4%, notably reflecting; a favorable pricing variance, mainly driven by combustible tobacco pricing; a favorable comparison related to the Saudi Arabia customs assessments of $246 million in 2021, shown in "Cost/Other," and a favorable volume/mix, primarily driven by higher cigarette volume and higher HTU volume.
Operating income increased by 17.3%. Operating income, excluding currency and acquisitions, increased by 20.3%, notably reflecting: a favorable pricing variance; a favorable comparison related to the Saudi Arabia customs assessments in 2021 (as noted above for net revenues); and lower marketing, administration and research costs (including a favorable comparison versus the prior year period related to asset impairment and exit costs of $45 million, partially offset by the unfavorable impact of 2022 costs associated with the Swedish Match AB offer of $33 million); partly offset by higher manufacturing costs; and unfavorable volume/mix, mainly due to lower cigarette mix.
SSEA, CIS & MEA - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region decreased by 0.2% to 1,564.4 billion units, primarily due to:
•Algeria, down by 16.1%, or by 6.8% excluding the net unfavorable impact of estimated trade inventory movements, primarily reflecting industry supply chain disruptions, as well as the impact of excise tax-driven price increases in the first quarter of 2021;
•Bangladesh, down by 6.2%, primarily reflecting the impact of pandemic-related restrictions on mobility during February 2022, as well as the impact of second-quarter 2022 excise tax-driven price increases;
•the Philippines, down by 4.9%, mainly reflecting the impact of first-quarter 2022 excise tax-driven price increases;
•Russia, down by 3.7%, mainly due to the impact of price increases; and
•Turkey, down by 6.7%, mainly reflecting a higher estimated prevalence of illicit trade, partly offset by the impact on adult smoker average daily consumption of the easing of pandemic-related measures, coupled with increased in-bound tourism;
partly offset by
•India, up by 16.8%, primarily reflecting a favorable comparison versus the prior year, during which pandemic-related restrictions impacted the movement of certain products, including tobacco; and
•Indonesia, up by 3.6%, mainly reflecting the impact on adult smoker consumption of the easing of pandemic-related measures, which drove growth in the tax-advantaged 'below tier one' segment.
Our Regional market share increased by 0.2 points to 22.6%.
Our total cigarette and HTU shipment volume in the Region increased by 0.4% to 353.6 billion units, mainly driven by:
•Egypt, up by 8.2%, primarily reflecting a higher market share driven by cigarettes and HTUs;
•India, up by 73.9%, primarily reflecting a higher market share (driven by geographic expansion) and the higher total market; and
•Indonesia, up by 4.8%, mainly reflecting the higher total market;
partly offset by
•the Philippines, down by 6.3%, mainly reflecting the lower total market; and
•Russia, down by 6.0%, due to cigarettes and HTUs.
EA, AU & PMI DF:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2022 |
2021 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
5,936 |
|
$ |
6,448 |
|
|
(7.9) |
% |
1.9 |
% |
|
$ |
(512) |
|
$ |
(635) |
|
$ |
— |
|
$ |
(24) |
|
$ |
147 |
|
$ |
— |
|
| Operating Income |
|
$ |
2,424 |
|
$ |
2,836 |
|
|
(14.5) |
% |
(1.3) |
% |
|
$ |
(412) |
|
$ |
(376) |
|
$ |
— |
|
$ |
(24) |
|
$ |
(170) |
|
$ |
158 |
|
Net revenues decreased by 7.9%. Net revenues, excluding currency and acquisitions, increased by 1.9%, reflecting: favorable volume/mix, mainly driven by higher cigarette volume, higher HTU volume and higher device volume, partly offset by unfavorable device mix and unfavorable cigarette mix; partially offset by an unfavorable pricing comparison.
Operating income decreased by 14.5%. Operating income, excluding currency and acquisitions, decreased by 1.3%, mainly reflecting: unfavorable volume/mix, primarily due to unfavorable HTU mix, unfavorable cigarette mix and unfavorable device mix, partly offset by higher cigarette volume; higher manufacturing costs; and an unfavorable pricing comparison; partially offset by lower marketing, administration and research costs (including a favorable comparison versus the prior year period related to asset impairment and exit costs of $91 million, partly offset by the unfavorable impact of 2022 costs associated with the Swedish Match AB offer of $24 million).
EA, AU & PMI DF - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding China, increased by 1.3% to 316.4 billion units, mainly driven by:
•International Duty Free, up by 41.0%, primarily reflecting the impact of reduced government travel restrictions and increased passenger traffic in certain geographies;
partly offset by
•Japan, down by 1.5%, primarily reflecting the impact of the October 2021 excise tax-driven price increases.
Our Regional market share, excluding China, increased by 1.1 points to 28.7%.
Our total cigarette and HTU shipment volume increased by 5.3% to 94.9 billion units, mainly driven by:
•PMI Duty Free, up by 61.3%, or by 43.5% excluding the net favorable impact of estimated distributor inventory movements (primarily due to cigarettes), reflecting the higher total market and a higher market share; and
•Japan, up by 0.6%, or by 3.9% excluding the net unfavorable impact of estimated distributor inventory movements (primarily due to HTUs), reflecting a higher market share, partly offset by the lower total market;
partly offset by
•Australia, down by 5.1%, mainly reflecting a lower total market, partly offset by a higher market share; and
•South Korea, down by 1.6%, primarily reflecting a lower market share.
Americas:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2022 |
2021 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
1,903 |
|
$ |
1,843 |
|
|
3.3 |
% |
4.1 |
% |
|
$ |
60 |
|
$ |
(15) |
|
$ |
— |
|
$ |
102 |
|
$ |
(23) |
|
$ |
(4) |
|
| Operating Income |
|
$ |
436 |
|
$ |
487 |
|
|
(10.5) |
% |
(8.2) |
% |
|
$ |
(51) |
|
$ |
(11) |
|
$ |
— |
|
$ |
102 |
|
$ |
(6) |
|
$ |
(136) |
|
Net revenues increased by 3.3%. Net revenues, excluding currency and acquisitions, increased by 4.1%, primarily reflecting: a favorable pricing variance, driven by combustible tobacco pricing; partly offset by unfavorable volume/mix, mainly due to unfavorable cigarette mix.
Operating income decreased by 10.5%. Operating income, excluding currency and acquisitions, decreased by 8.2%, mainly reflecting: higher marketing, administration and research costs (including the unfavorable impact of 2022 costs associated with the Swedish Match AB offer of $5 million and a favorable comparison versus the prior year period related to asset impairment and exit costs of $8 million); and higher manufacturing costs; partly offset by a favorable pricing variance. Volume/mix was slightly unfavorable, mainly due to unfavorable cigarette mix, largely offset by higher cigarette volume.
Americas - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding the U.S., increased by 1.8% to 191.1 billion units, primarily driven by:
•Brazil, up by 7.6%, primarily reflecting a lower estimated prevalence of illicit trade;
partly offset by
•Canada, down by 12.8%, notably reflecting the impact of price increases and out-switching from cigarettes to e-vapor products.
Our Regional market share, excluding the U.S., increased by 0.3 points to 34.8%.
Our total cigarette and HTU shipment volume increased by 2.1% to 66.5 billion units, mainly driven by:
•Brazil, up by 13.3%, primarily reflecting the higher total market and a higher market share; and
•Mexico, up by 2.5%, mainly reflecting a higher total market and a higher market share for cigarettes;
partly offset by
•Argentina, down by 2.8%, primarily reflecting a lower market share due to adult smoker downtrading to ultra-low-price brands produced by local manufacturers, partly offset by a higher total market.
Swedish Match:
Our results for the Swedish Match operating segment for the full-year include Swedish Match's results beginning on November 11, 2022, when PMI became the owner of a majority position in Swedish Match, through December 31, 2022. The business operations of our Swedish Match segment are managed and evaluated separately from the geographical segments.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2022 |
2021 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
316 |
|
$ |
— |
|
|
— |
% |
— |
% |
|
$ |
316 |
|
$ |
— |
|
$ |
316 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
| Operating Income / (Loss) |
|
$ |
(22) |
|
$ |
— |
|
|
— |
% |
— |
% |
|
$ |
(22) |
|
$ |
— |
|
$ |
(22) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
We recorded net revenues of $316 million in the Swedish Match segment, with an operating loss of $22 million, primarily reflecting $125 million in an acquisition accounting-related item and $26 million related to the amortization of acquired intangibles.
Wellness and Healthcare:
In the third quarter of 2021, we acquired Fertin Pharma A/S, Vectura Group plc. and OtiTopic, Inc. On March 31, 2022, we launched a new Wellness and Healthcare business, Vectura Fertin Pharma, consolidating these entities. The operating results of this business are reported in the Wellness and Healthcare segment. The business operations of our Wellness and Healthcare segment are managed and evaluated separately from the geographical segments.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial Summary - Years Ended December 31, |
|
|
|
|
Change
Fav./(Unfav.) |
|
Variance
Fav./(Unfav.) |
|
2022 |
2021 |
|
Total |
Excl.
Curr. & Acquis. |
|
Total |
Cur-
rency |
Acqui-sitions |
Price |
Vol/
Mix |
Cost/
Other |
| (in millions) |
|
|
|
| Net Revenues |
|
$ |
271 |
|
$ |
101 |
|
|
+100% |
(7.9) |
% |
|
$ |
170 |
|
$ |
(11) |
|
$ |
189 |
|
$ |
(10) |
|
$ |
— |
|
$ |
2 |
|
| Operating Income / (Loss) |
|
$ |
(258) |
|
$ |
(52) |
|
|
-(100)% |
-(100)% |
|
$ |
(206) |
|
$ |
8 |
|
$ |
(72) |
|
$ |
(10) |
|
$ |
— |
|
$ |
(132) |
|
Net revenues increased over 100%. Net revenues, excluding currency and acquisitions, decreased by 7.9%, primarily reflecting lower product supply revenues and lower royalties.
The operating loss of $258 million in 2022 included $171 million of amortization and impairment of intangibles. The remaining operating loss in 2022 of $87 million mainly reflected investments in research and development, as well as expenses related to employee retention programs.
Financial Review
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in millions) |
2023 |
2022 |
2021 |
| Net cash provided by operating activities |
$ |
9,204 |
|
$ |
10,803 |
|
$ |
11,967 |
|
| Net cash used in investing activities |
(3,598) |
|
(15,679) |
|
(2,358) |
|
| Net cash provided by (used in) financing activities |
(5,582) |
|
3,806 |
|
(11,977) |
|
2023 compared with 2022
•Net Cash Provided by Operating Activities
Net cash provided by operating activities of $9.2 billion for the year ended December 31, 2023 decreased by $1.6 billion from the comparable 2022 period. Excluding unfavorable currency movements of $1.3 billion, the unfavorable variance of $0.3 billion was due primarily to higher working capital requirements of $1.2 billion, partly offset by higher currency-neutral net earnings, excluding non-cash depreciation and amortization expense and goodwill and other intangible impairment charges.
The unfavorable currency movements primarily related to the currency impact on net earnings and represented the fluctuations of the U.S. dollar, especially against the Argentine peso, Egyptian pound, Japanese yen, Russian ruble and Swiss franc, partly offset by the Euro.
The higher working capital requirements in 2023 as compared with 2022 were primarily due to less cash provided by accrued liabilities and other current assets, net of positive cash movements in inventories, mainly reflecting the timing of excise tax-paid inventory movements primarily related to excise tax increases and timing of the corresponding excise tax payments, and more cash used in accounts payable, which primarily reflects the 2023 payment for higher IQOS ILUMA device purchases in the fourth quarter of 2022 to meet the needs of ILUMA launches. Working capital requirements were also negatively impacted by the higher cost of tobacco leaf and other direct materials due to inflationary pressures (for further details, see “Impact of Inflation on Our Business and Mitigation Efforts” section of this Item 7), as well as tactical stock increases for certain direct materials. These increases in the working capital requirements were partly offset by more cash provided by accounts receivable mainly reflecting the timing of sales and cash collections, as well as higher usage of our factoring arrangements to sell trade receivables. For further detail on our factoring arrangements, see Item 8, Note 19. Sale of Accounts Receivable.
For the full year 2024, we currently expect net cash provided by operating activities of $10 billion to $11 billion at prevailing exchange rates, subject to year-end working capital requirements.
•Net Cash Used in Investing Activities
Net cash used in investing activities of $3.6 billion for the year ended December 31, 2023, decreased by $12.1 billion from the comparable 2022 period. This decrease in cash used was primarily due to the $14.0 billion of cash used in 2022 for the Swedish Match acquisition, net of acquired cash. This was partially offset by the higher second installment of $1,775 million (including interest) paid to Altria Group, Inc. ($773 million) for PMI to reacquire the IQOS commercialization rights in the U.S., and the unfavorable movements of $944 million in net investment hedges, which was principally related to changes in exchange rates between the Euro and the U.S. dollar, and higher capital expenditures. For further detail on our cash payments to Altria Group, Inc. and derivatives designated as net investment hedges, see Item 8, Note 3. Acquisitions and Note 16. Financial Instruments, and the "Business Environment" section of this Item 7.
Our capital expenditures were $1.3 billion in 2023 and $1.1 billion in 2022. The 2023 expenditures were primarily related to our ongoing investments in smoke-free product manufacturing capacity. We expect total capital expenditures in 2024 of approximately $1.2 billion, partly reflecting investments in ZYN capacity in the U.S.
•Net Cash Provided by (Used in) Financing Activities
Net cash used in financing activities of $5.6 billion for the year ended December 31, 2023, increased by $9.4 billion compared with net cash provided by financing activities of $3.8 billion for the year ended December 31, 2022. The change was primarily due to net borrowings of $9.9 billion under credit facilities related to the Swedish Match acquisition in 2022, coupled with the repayment on the related bridge facility in 2023. This was partly offset by higher long-term debt issuances in 2023, a favorable comparison resulting from transactions with noncontrolling interests related to our Turkish subsidiaries (sale of noncontrolling stakes in 2023 compared to purchase of such stakes in 2022) and lower payments in 2023 to acquire remaining issued and outstanding shares in Swedish Match. For further details on the transactions with noncontrolling interests in our Turkish subsidiaries and our purchase of remaining Swedish Match shares, see Item 8, Note 3. Acquisitions.
Dividends paid in 2023 and 2022 were $8.0 billion and $7.8 billion, respectively.
2022 compared with 2021
For a discussion comparing our net cash activities (operating, investing and financing) for the year ended December 31, 2022, with the year ended December 31, 2021, refer to Part II, Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation - Financial Review in our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the U.S. Securities and Exchange Commission on February 10, 2023. This section is incorporated by reference into this Annual Report on Form 10-K for the year ended December 31, 2023.
•Debt and Liquidity
We define cash and cash equivalents as short-term, highly liquid investments, readily convertible to known amounts of cash that mature within a maximum of three months and have an insignificant risk of change in value due to interest rate or credit risk changes. As a policy, we do not hold any investments in structured or equity-linked products. Our cash and cash equivalents are predominantly held with institutions that have investment-grade long-term credit rating. As part of our cash management strategy and in order to manage counterparty exposure, we also enter into reverse repurchase agreements. Such agreements are collateralized with government or corporate securities held by a custodial bank and, at maturity, cash is paid back to PMI, and the collateral is returned to the bank. For 2022, the activities for such reverse repurchase agreements were not material. For 2023, we did not enter into such reverse repurchase agreements.
In a number of jurisdictions, including Argentina, Egypt and Russia, we are impacted by various capital controls and/or foreign currency exchange constraints that affect the ability of our subsidiaries in these jurisdictions to settle foreign currency denominated imports of goods and services and/or to pay dividends. These factors increase foreign currency devaluation risks, which may have a negative impact on our financial condition, net assets and results of operations in these jurisdictions.
We utilize long-term and short-term debt financing, including a commercial paper program that is regularly used to finance ongoing liquidity requirements, as part of our overall cash management strategy. Our ability to access the capital and credit markets as well as overall dynamics of these markets may impact borrowing costs. We expect that the combination of our long-term and short-term debt financing, the commercial paper program and the committed credit facilities, coupled with our operating cash flows, will enable us to meet our liquidity requirements.
In August 2021, we published a business transformation-linked financing framework (“Framework”), which integrates PMI's smoke-free transformation into its financing strategy. The Framework outlines the guidelines that we will follow in issuing business transformation-linked financing instruments in the debt capital and loan markets, which may include public notes offerings, private placements, loans, and other relevant financing instruments.
Credit Ratings – The cost and terms of our financing arrangements as well as our access to commercial paper markets may be affected by applicable credit ratings. At February 8, 2024, our credit ratings and outlook by major credit rating agencies were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term |
Long-term |
Outlook |
| Moody’s |
P-1 |
A2 |
Stable |
| Standard & Poor’s |
A-2 |
A- |
Stable |
| Fitch |
F1 |
A |
Negative |
Revolving Credit Facilities – On January 24, 2024, we entered into an agreement to extend the term of our 364-day committed revolving credit facility in the amount of $1.7 billion from January 30, 2024, to January 28, 2025.
At February 8, 2024, our committed revolving credit facilities were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Type
(in billions)
|
|
Committed Revolving Credit Facilities |
|
|
| 364-day revolving credit, expiring January 28, 2025 |
|
$ |
1.7 |
|
|
|
Multi-year revolving credit, expiring February 10, 2026(1) |
|
2.0 |
|
|
|
Multi-year revolving credit, expiring September 29, 2026(2) (3) |
|
2.5 |
|
|
|
Total facilities |
|
$ |
6.2 |
|
|
|
|
|
|
|
|
(1) On January 28, 2022, we entered into an agreement, effective February 10, 2022, to amend and extend the term of our $2.0 billion multi-year revolving credit facility, for an additional year covering the period February 11, 2026 to February 10, 2027, in the amount of $1.9 billion.
(2) Includes business transformation-linked pricing adjustments that may result in the reduction or increase in both the interest rate and commitment fee under the credit agreement if PMI achieves, or fails to achieve, certain specified targets based on its business transformation goals.
(3) On September 20, 2022, we entered into an agreement, effective September 29, 2022, to amend and extend the term of our $2.5 billion multi-year revolving credit facility, for an additional year covering the period September 30, 2026 to September 29, 2027, in the amount of $2.3 billion. On September 20, 2023, PMI entered into an agreement, effective September 29, 2023, to amend and further extend the term to September 29, 2028.
At February 8, 2024, there were no borrowings under the committed revolving credit facilities, and the entire committed amounts were available for borrowing.
All banks participating in our committed revolving credit facilities have an investment-grade long-term credit rating from the credit rating agencies. We continuously monitor the credit quality of our banking group, and at this time we are not aware of any potential non-performing credit provider.
These committed revolving credit facilities do not include any credit rating triggers, material adverse change clauses or any provisions that could require us to post collateral. We expect to continue to meet our covenants.
In addition to the committed revolving credit facilities discussed above, PMI maintains certain short-term credit arrangements, including uncommitted credit lines, to primarily meet working capital needs. These credit arrangements amounted to approximately $2.7 billion at December 31, 2023 and approximately $1.9 billion at December 31, 2022. Borrowings under these arrangements and other bank loans amounted to $283 million at December 31, 2023, and $295 million at December 31, 2022.
Credit Facilities related to the Financing of the Swedish Match Acquisition – In connection with PMI’s all-cash recommended public offer to the shareholders of Swedish Match, on May 11, 2022, PMI entered into a credit agreement relating to a 364-day senior unsecured bridge facility. The facility provided for borrowings up to an aggregate principal amount of $17 billion, expiring 364 days after the occurrence of certain events unless extended. On June 23, 2022, PMI entered into a €5.5 billion (approximately $5.8 billion at the date of signing) senior unsecured term loan credit agreement consisting of a €3.0 billion (approximately $3.2 billion at the date of signing) tranche expiring three years after the occurrence of certain events and a €2.5 billion (approximately $2.6 billion at the date of signing) tranche expiring on June 23, 2027. In connection with the term loan facility, the aggregate principal amount of commitments under the 364-day senior unsecured bridge facility was reduced from $17 billion to $11 billion. On November 11, 2022, PMI acquired a controlling interest of 85.87% of the total issued shares in Swedish Match and acquired 94.81% of its outstanding shares as of December 31, 2022. In accordance with the Swedish Companies Act, PMI subsequently exercised its right to compulsorily redeem the remaining shares for which acceptances were not received and obtained legal title to 100% of the shares in Swedish Match on February 17, 2023.
PMI borrowed $8.4 billion under the bridge facility by delivering notices of borrowing for advances of $7.9 billion and $0.5 billion on November 7, 2022 and November 10, 2022, respectively. On November 21, 2022 and February 17, 2023, PMI repaid $4.0 billion and $4.4 billion, respectively, under the bridge facility. Effective February 20, 2023, the remaining outstanding commitments under the bridge facility were fully canceled and the bridge facility agreement was terminated in accordance with its terms.
On November 7, 2022, PMI also delivered notices of borrowing for advances totaling €5.5 billion under the term loan facility, of which €3.0 billion will become due on November 9, 2025 and €2.5 billion will become due on June 23, 2027 unless prepaid pursuant to the terms of the credit agreement. As of December 31, 2023 and 2022, the €5.5 billion (approximately $6 billion) term loan facility was fully drawn and remained outstanding.
The proceeds under the bridge facility and the term loan facility were used, directly or indirectly, to finance the acquisition, including, the payment of related fees and expenses. For further details, see Item 8, Note 3. Acquisitions to our consolidated financial statements.
Commercial Paper Program – We continue to have access to liquidity in the commercial paper market through programs in place in the U.S. and in Europe having an aggregate issuance capacity of $8.0 billion. At December 31, 2023, we had $1.7 billion of commercial paper outstanding. At December 31, 2022, we had $0.9 billion of commercial paper outstanding. The average commercial paper balance outstanding during 2023 and 2022 was $3.6 billion and $3.1 billion, respectively.
Sale of Accounts Receivable – To mitigate credit risk and enhance cash and liquidity management, we sell trade receivables to unaffiliated financial institutions. For further details, see Item 8, Note 19. Sale of Accounts Receivable to our consolidated financial statements.
Supply Chain Financing – We engage with unaffiliated global financial institutions that offer a voluntary supply chain financing program to some of our suppliers. For further details, see Item 8, Note 22. Supply Chain Financing to our consolidated financial statements.
Debt – Our total debt was $47.9 billion at December 31, 2023, and $43.1 billion at December 31, 2022. Our total debt is primarily fixed rate in nature. The weighted-average all-in financing cost of our total debt was 3.3% in 2023 and 2.5% in 2022. For further details, including the fair value of our debt, see Item 8, Note 8. Indebtedness. The amount of debt that we can issue is subject to approval by our Board of Directors.
On February 10, 2023, we filed a shelf registration statement with the U.S. Securities and Exchange Commission, under which we may from time to time sell debt securities and/or warrants to purchase debt securities over a three-year period.
Our debt issuances in 2023 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
|
|
|
|
|
|
|
|
| Type |
|
Face Value |
|
Interest Rate |
|
Issuance |
|
Maturity |
| U.S. dollar notes |
(a) |
$1,250 |
|
4.875% |
|
February 2023 |
|
February 2026 |
| U.S. dollar notes |
(a) |
$1,000 |
|
4.875% |
|
February 2023 |
|
February 2028 |
| U.S. dollar notes |
(a) |
$1,500 |
|
5.125% |
|
February 2023 |
|
February 2030 |
| U.S. dollar notes |
(a) |
$1,500 |
|
5.375% |
|
February 2023 |
|
February 2033 |
| U.S. dollar notes |
(a) (b) |
$450 |
|
4.875% |
|
May 2023 |
|
February 2026 |
| U.S. dollar notes |
(a) (c) |
$550 |
|
4.875% |
|
May 2023 |
|
February 2028 |
| U.S. dollar notes |
(a) (d) |
$700 |
|
5.125% |
|
May 2023 |
|
February 2030 |
| U.S. dollar notes |
(a) (e) |
$750 |
|
5.375% |
|
May 2023 |
|
February 2033 |
| U.S. dollar notes |
(f) |
$650 |
|
5.250% |
|
September 2023 |
|
September 2028 |
| U.S. dollar notes |
(f) |
$700 |
|
5.500% |
|
September 2023 |
|
September 2030 |
| U.S. dollar notes |
(f) |
$1,000 |
|
5.625% |
|
September 2023 |
|
September 2033 |
(a) Interest is payable semi-annually, commencing in August 2023
(b) These notes are a further issuance of the 4.875% notes issued in February 2023
(c) These notes are a further issuance of the 4.875% notes issued in February 2023
(d) These notes are a further issuance of the 5.125% notes issued in February 2023
(e) These notes are a further issuance of the 5.375% notes issued in February 2023
(f) Interest is payable semi-annually, commencing in March 2024
On February 17, 2023, PMI applied a portion of the net proceeds of the debt issuances to prepay $4.4 billion under its bridge facility, which represented all borrowings outstanding under the bridge facility. PMI used a portion of the May 2023 net proceeds to pay the remaining cash consideration due in accordance with the terms of its agreement with Altria. For further details on PMI's agreement with Altria, see Item 8, Note 3. Acquisitions and the "Business Environment" section of this Item 7. The remaining net proceeds of the February and May 2023 offerings, as well as the September 2023 offering have been used for general corporate purposes.
The weighted-average time to maturity of our long-term debt was approximately 7 years at the end of 2023 and 8 years at the end of 2022.
Cash Requirements – At December 31, 2023, our material short-term and long-term cash requirements for various contractual obligations and commitments primarily consisted of the following:
•principal payments related to long-term debt and the associated interest payments. For further details, see Item 8, Note 8. Indebtedness to our consolidated financial statements;
•accounts payable and accrued liabilities on our consolidated balance sheet (primarily short-term in nature);
•purchase obligations for inventory and production costs to be utilized in the normal course of business such as raw materials, electronic devices, indirect materials and supplies, packaging, co-manufacturing arrangements, storage and distribution, as well as capital expenditures. These purchase obligations are expected to be approximately $3.7 billion in 2024 and approximately $1.0 billion for years beyond;
•operating lease liabilities, on an undiscounted basis, which were included in our consolidated balance sheets. For further details, see Item 8, Note 21. Leases to our consolidated financial statements; and
•other long-term liabilities mainly related to transition tax. For further details, see Item 8, Note 12. Income Taxes to our consolidated financial statements.
•Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including special purpose entities, other than guarantees, and cash requirements discussed above.
Guarantees – At December 31, 2023, we have guarantees of our own performance, which are primarily related to excise taxes on the shipment of our products. There is no liability in the consolidated financial statements associated with these guarantees. These guarantees have not had, and are not expected to have, a significant impact on PMI’s liquidity. In October 2020, we guaranteed an obligation for a then equity method investee that was subsequently divested in 2022. For further details, see Item 8, Note 18. Contingencies to our consolidated financial statements.
Swedish Match Notes Consent Solicitation and PMI Guarantee – On June 15, 2023, our wholly owned subsidiary, Swedish Match AB ("Swedish Match"), initiated a public consent solicitation of eligible holders of certain outstanding series of its notes to amend certain terms and conditions of these respective notes. The eligible noteholders provided the requisite irrevocable consent instructions voting in favor of the amendments, which were subsequently passed by way of extraordinary resolution at the noteholders’ meeting held on July 28, 2023. As a result of the passage of the extraordinary resolution, Philip Morris International Inc. entered into a guarantee, which guarantees unconditionally and irrevocably to the noteholders the punctual payment when due, whether at stated maturity, by acceleration or otherwise, of the principal, premium, if any, and interest on the notes.
Equity and Dividends
We discuss our stock awards as of December 31, 2023, in Item 8, Note 10. Stock Plans to our consolidated financial statements.
On June 11, 2021, our Board of Directors authorized a new share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period. On July 22, 2021, we began repurchasing shares under this new share repurchase program. From July 22, 2021 through March 31, 2022, we repurchased 10.5 million shares of our common stock at a cost of approximately $1.0 billion. During the first three months of 2022, we repurchased 2.0 million shares of our common stock at a cost of $199 million.
On May 11, 2022, we announced the suspension of our three-year share repurchase program following the recommended public offer to acquire the outstanding shares of Swedish Match from its shareholders. Prior to the suspension of the program, we made no share repurchases during the second quarter of 2022. We did not make any share repurchases in 2023 and we do not currently anticipate restarting our share repurchase program during 2024.
Dividends paid in 2023 were $8.0 billion. During the third quarter of 2023, our Board of Directors approved a 2.4% increase in the quarterly dividend to $1.30 per common share. As a result, the present annualized dividend rate is $5.20 per common share.
Market Risk
Counterparty Risk - We predominantly work with financial institutions with strong short- and long-term credit ratings as assigned by Standard & Poor’s and Moody’s. These banks are also part of a defined group of relationship banks. Non-investment grade institutions are only used in certain emerging markets to the extent required by local business needs. We have a conservative approach when it comes to choosing financial counterparties and financial instruments. As such we do not invest or hold investments in any structured or equity-linked products. The majority of our cash and cash equivalents is currently invested with maturities of less than 30 days.
We continuously monitor and assess the credit worthiness of all our counterparties.
Derivative Financial Instruments - We operate in markets primarily outside of the United States of America, with manufacturing and sales facilities in various locations around the world. Consequently, we use certain financial instruments to manage our foreign currency and interest rate exposure. We use derivative financial instruments principally to reduce our exposure to market risks resulting from fluctuations in foreign exchange and interest rates by creating offsetting exposures. We are not a party to leveraged derivatives and, by policy, do not use derivative financial instruments for speculative purposes.
See Item 8, Note 16. Financial Instruments to our consolidated financial statements for further details on our derivative financial instruments and the related collateral arrangements.
Value at Risk - We use a value at risk computation to estimate the potential one-day loss in the fair value of our interest-rate-sensitive and foreign currency price-sensitive derivative financial instruments, representing the majority of our derivative financial instruments exposure. This computation includes our debt and foreign currency forwards, swaps and options. Anticipated transactions, foreign currency trade payables and receivables, and net investments in foreign subsidiaries, which the foregoing instruments are intended to hedge, were excluded from the computation.
The computation estimates were made assuming normal market conditions, using a 95% confidence interval and a one-day holding period using a "parametric delta-gamma" approximation technique to determine the observed interrelationships between movements in interest rates and various currencies and in calculating the risk of the underlying positions in the portfolio. These interrelationships were determined by observing interest rate and forward currency rate movements primarily over the preceding quarter for determining value at risk at December 31, 2023 and 2022, and primarily over each of the four preceding quarters for the calculation of average, high and low value at risk amounts during each year.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Impact |
(in millions) |
At December 31, 2023 |
|
Average |
|
High |
|
Low |
Instruments sensitive to: |
|
|
|
|
|
|
|
Foreign currency rates |
$77 |
|
$74 |
|
$82 |
|
$66 |
|
|
|
|
|
|
|
|
Interest rates |
$297 |
|
$332 |
|
$505 |
|
$219 |
|
|
|
|
|
|
|
|
|
Fair Value Impact |
(in millions) |
At December 31, 2022 |
|
Average |
|
High |
|
Low |
Instruments sensitive to: |
|
|
|
|
|
|
|
Foreign currency rates |
$33 |
|
$55 |
|
$73 |
|
$33 |
|
|
|
|
|
|
|
|
Interest rates |
$233 |
|
$253 |
|
$317 |
|
$195 |
The significant year-over-year increase in "average" and "high" impact on the value at risk computation above was primarily due to trends in foreign currency and interest rate exposures.
The value at risk computation is a risk analysis tool designed to statistically estimate the maximum probable daily loss from adverse movements in interest and foreign currency rates under normal market conditions. The computation does not purport to represent actual losses in fair value or earnings to be incurred by us, nor does it consider the effect of favorable changes in market rates. We cannot predict actual future movements in such market rates and do not present these results to be indicative of future movements in market rates or to be representative of any actual impact that future changes in market rates may have on our future results of operations or financial position.
Contingencies
See Item 3 and Item 8, Note 18. Contingencies to our consolidated financial statements for a discussion of contingencies.
Cautionary Factors That May Affect Future Results
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in filings with the SEC, in reports to stockholders and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "aspires," "estimates," "intends," "projects," "aims," "goals," "targets," "forecasts" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Our RRPs constitute a relatively new product category that is less predictable than our mature cigarette business. Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities. In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements.
We elaborate on these and other risks we face throughout this document, particularly in Item 1A. Risk Factors and Business Environment of this section. You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk.
The information called for by this Item is included in Item 7, Market Risk.
Item 8.Financial Statements and Supplementary Data.
Consolidated Statements of Earnings
(in millions of dollars, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
for the years ended December 31, |
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenues 1 & 2 (Notes 6 & 13) |
$ |
35,174 |
|
|
$ |
31,762 |
|
|
$ |
31,405 |
|
Cost of sales 3 (Notes 4 & 5) |
12,893 |
|
|
11,402 |
|
|
10,030 |
|
Gross profit |
22,281 |
|
|
20,360 |
|
|
21,375 |
|
Marketing, administration and research costs 4 (Notes 3, 4, 5, 13, 18 & 20) |
10,060 |
|
|
8,114 |
|
|
8,400 |
|
Impairment of goodwill (Note 5) |
665 |
|
|
— |
|
|
— |
|
Operating income |
11,556 |
|
|
12,246 |
|
|
12,975 |
|
Interest expense, net (Note 15) |
1,061 |
|
|
588 |
|
|
628 |
|
Pension and other employee benefit costs (Note 14) |
45 |
|
|
24 |
|
|
115 |
|
Earnings before income taxes |
10,450 |
|
|
11,634 |
|
|
12,232 |
|
Provision for income taxes (Note 12) |
2,339 |
|
|
2,244 |
|
|
2,671 |
|
Equity investments and securities (income)/loss, net |
(157) |
|
|
(137) |
|
|
(149) |
|
Net earnings |
8,268 |
|
|
9,527 |
|
|
9,710 |
|
Net earnings attributable to noncontrolling interests |
455 |
|
|
479 |
|
|
601 |
|
Net earnings attributable to PMI |
$ |
7,813 |
|
|
$ |
9,048 |
|
|
$ |
9,109 |
|
Per share data (Note 11): |
|
|
|
|
|
Basic earnings per share |
$ |
5.02 |
|
|
$ |
5.82 |
|
|
$ |
5.83 |
|
Diluted earnings per share |
$ |
5.02 |
|
|
$ |
5.81 |
|
|
$ |
5.83 |
|
(1) Includes net revenues from related parties of $3,553 million, $3,658 million and $3,330 million for the years ended December 31, 2023, 2022 and 2021, respectively
(2) Net of excise tax on products of $49,404 million, $48,958 million and $50,818 million for the years ended December 31, 2023, 2022 and 2021, respectively
(3) Includes an impairment charge for other intangibles of $112 million for the year ended December 31, 2022. For further details, see Note 5. Goodwill and Other Intangible Assets, net
(4) Includes an impairment charge for other intangibles of $15 million and a charge of $204 million for the South Korea indirect tax charge for the year ended December 31, 2023. For further details, see Note 5. Goodwill and Other Intangible Assets, net and Note 18. Contingencies
See notes to consolidated financial statements.
Consolidated Statements of Comprehensive Earnings
(in millions of dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| for the years ended December 31, |
2023 |
|
2022 |
|
2021 |
| Net earnings |
$ |
8,268 |
|
|
$ |
9,527 |
|
|
$ |
9,710 |
|
| Other comprehensive earnings (losses), net of income taxes: |
|
|
|
|
|
Change in currency translation adjustments: |
|
|
|
|
|
Unrealized gains (losses), net of income taxes of $156 in 2023, $(169) in 2022 and $(58) in 2021 |
(1,643) |
|
|
(1,268) |
|
|
58 |
|
(Gains)/losses transferred to earnings, net of income taxes of $0 in 2023, 2022 and 2021 |
12 |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
Change in net loss and prior service cost: |
|
|
|
|
|
Net gains (losses) and prior service costs, net of income taxes of $182 in 2023, $(132) in 2022 and $(210) in 2021 |
(861) |
|
|
843 |
|
|
1,055 |
|
|
Amortization of net losses, prior service costs and net transition costs, net of income taxes of $(28) in 2023, $(49) in 2022
and $(72) in 2021
|
87 |
|
|
217 |
|
|
323 |
|
|
|
|
|
|
|
Change in fair value of derivatives accounted for as hedges: |
|
|
|
|
|
|
Gains (losses) recognized, net of income taxes of $(30) in 2023,
$(99) in 2022 and $(20) in 2021
|
195 |
|
|
481 |
|
|
124 |
|
|
(Gains) losses transferred to earnings, net of income taxes of
$32 in 2023, $35 in 2022 and $7 in 2021
|
(220) |
|
|
(219) |
|
|
(35) |
|
|
|
|
|
|
|
| Total other comprehensive earnings (losses) |
(2,430) |
|
|
54 |
|
|
1,525 |
|
Total comprehensive earnings |
5,838 |
|
|
9,581 |
|
|
11,235 |
|
| Less comprehensive earnings attributable to: |
|
|
|
|
|
Noncontrolling interests |
281 |
|
|
515 |
|
|
522 |
|
|
|
|
|
|
|
| Comprehensive earnings attributable to PMI |
$ |
5,557 |
|
|
$ |
9,066 |
|
|
$ |
10,713 |
|
See notes to consolidated financial statements.
Consolidated Balance Sheets
(in millions of dollars, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
at December 31, |
2023 |
|
2022 |
Assets |
|
|
|
Cash and cash equivalents |
$ |
3,060 |
|
|
$ |
3,207 |
|
Trade receivables (less allowances of $79 in 2023 and $42 in 2022) (1) |
3,461 |
|
|
3,850 |
|
Other receivables (less allowances of $35 in 2023 and $32 in 2022) |
930 |
|
|
906 |
|
Inventories: |
|
|
|
Leaf tobacco |
1,942 |
|
|
1,674 |
|
Other raw materials |
2,293 |
|
|
2,028 |
|
Finished product |
6,539 |
|
|
6,184 |
|
|
10,774 |
|
|
9,886 |
|
|
|
|
|
Other current assets (Note 3) |
1,530 |
|
|
1,770 |
|
Total current assets |
19,755 |
|
|
19,619 |
|
Property, plant and equipment, at cost: |
|
|
|
Land and land improvements |
550 |
|
|
545 |
|
Buildings and building equipment |
4,617 |
|
|
4,291 |
|
Machinery and equipment |
10,713 |
|
|
9,549 |
|
Construction in progress |
1,200 |
|
|
1,058 |
|
|
17,080 |
|
|
15,443 |
|
Less: accumulated depreciation |
9,564 |
|
|
8,733 |
|
|
7,516 |
|
|
6,710 |
|
Goodwill (Note 5) |
16,779 |
|
|
19,655 |
|
Other intangible assets, net (Note 5) |
9,864 |
|
|
6,732 |
|
Equity investments (Note 6) |
4,929 |
|
|
4,431 |
|
Deferred income taxes |
814 |
|
|
603 |
|
Other assets (less allowances of $25 in 2023 and $20 in 2022) (Note 3) |
5,647 |
|
|
3,931 |
|
Total Assets |
$ |
65,304 |
|
|
$ |
61,681 |
|
(1) Includes trade receivables from related parties of $710 million and $688 million as of December 31, 2023, and 2022, respectively. For further details, see Note 6. Related Parties - Equity Investments and Other.
See notes to consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
at December 31, |
2023 |
|
2022 |
Liabilities |
|
|
|
Short-term borrowings (Note 8) |
$ |
1,968 |
|
|
$ |
5,637 |
|
Current portion of long-term debt (Note 8) |
4,698 |
|
|
2,611 |
|
Accounts payable |
4,143 |
|
|
4,076 |
|
Accrued liabilities: |
|
|
|
Marketing and selling |
862 |
|
|
695 |
|
Taxes, except income taxes |
7,514 |
|
|
7,440 |
|
Employment costs |
1,262 |
|
|
1,168 |
|
Dividends payable |
2,041 |
|
|
1,990 |
|
Other |
2,737 |
|
|
2,679 |
|
Income taxes |
1,158 |
|
|
1,040 |
|
|
|
|
|
Total current liabilities |
26,383 |
|
|
27,336 |
|
Long-term debt (Note 8) |
41,243 |
|
|
34,875 |
|
Deferred income taxes |
2,335 |
|
|
1,956 |
|
Employment costs |
3,046 |
|
|
1,984 |
|
Income taxes and other liabilities (Note 12) |
1,743 |
|
|
1,841 |
|
Total liabilities |
74,750 |
|
|
67,992 |
|
Contingencies (Note 18) |
|
|
|
|
Stockholders’ (Deficit) Equity
|
|
|
|
Common stock, no par value (2,109,316,331 shares issued in 2023 and 2022) (Note 9) |
— |
|
|
— |
|
Additional paid-in capital |
2,285 |
|
|
2,230 |
|
Earnings reinvested in the business |
34,090 |
|
|
34,289 |
|
Accumulated other comprehensive losses (Note 17) |
(11,815) |
|
|
(9,559) |
|
|
24,560 |
|
|
26,960 |
|
Less: cost of repurchased stock (556,891,800 and 559,098,620 shares in 2023 and 2022, respectively) |
35,785 |
|
|
35,917 |
|
Total PMI stockholders’ deficit |
(11,225) |
|
|
(8,957) |
|
Noncontrolling interests |
1,779 |
|
|
2,646 |
|
Total stockholders’ deficit |
(9,446) |
|
|
(6,311) |
|
Total Liabilities and Stockholders’ (Deficit) Equity |
$ |
65,304 |
|
|
$ |
61,681 |
|
See notes to consolidated financial statements.
Consolidated Statements of Cash Flows
(in millions of dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
for the years ended December 31, |
2023 |
|
2022 |
|
2021 |
|
CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES |
|
|
|
|
|
|
Net earnings |
$ |
8,268 |
|
|
$ |
9,527 |
|
|
$ |
9,710 |
|
|
Adjustments to reconcile net earnings to operating cash flows: |
|
|
|
|
|
|
| Depreciation and amortization expense |
1,398 |
|
|
1,077 |
|
|
998 |
|
|
| Impairment of goodwill and other intangibles (Note 5) |
680 |
|
|
112 |
|
|
— |
|
|
Deferred income tax (benefit) provision |
(330) |
|
|
(234) |
|
|
(17) |
|
|
Asset impairment and exit costs, net of cash paid (Note 20) |
30 |
|
|
(93) |
|
|
(22) |
|
|
Cash effects of changes, net of the effects from acquired companies: |
|
|
|
|
|
|
Receivables, net (1) |
314 |
|
|
(871) |
|
|
(198) |
|
|
Inventories |
(862) |
|
|
(1,287) |
|
|
549 |
|
|
Accounts payable |
(288) |
|
|
719 |
|
|
653 |
|
|
Accrued liabilities and other current assets |
(232) |
|
|
1,862 |
|
|
623 |
|
|
Income taxes |
(232) |
|
|
(261) |
|
|
(260) |
|
|
Pension plan contributions, net of refunds (Note 14) |
(21) |
|
|
3 |
|
|
(269) |
|
|
Other |
479 |
|
|
249 |
|
|
200 |
|
|
Net cash provided by operating activities |
9,204 |
|
|
10,803 |
|
|
11,967 |
|
|
CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES |
|
|
|
|
|
|
Capital expenditures |
(1,321) |
|
|
(1,077) |
|
|
(748) |
|
|
| Acquisition of Swedish Match AB, net of acquired cash (Note 3) |
— |
|
|
(13,976) |
|
|
— |
|
|
| Other acquisitions, net of acquired cash (Note 3) |
— |
|
|
— |
|
|
(2,111) |
|
|
| Altria Group, Inc. agreement (Note 3) |
(1,775) |
|
|
(1,002) |
|
|
— |
|
|
Equity investments |
(111) |
|
|
(20) |
|
|
(34) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net investment hedges and other derivatives (Note 16) |
(660) |
|
|
284 |
|
|
466 |
|
|
Other |
269 |
|
|
112 |
|
|
69 |
|
|
Net cash used in investing activities |
(3,598) |
|
|
(15,679) |
|
|
(2,358) |
|
|
(1) Includes amounts from related parties of $(154) million, $(166) million and $(149) million in 2023, 2022 and 2021, respectively
See notes to consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| for the years ended December 31, |
2023 |
|
2022 |
|
2021 |
| CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES |
|
|
|
|
|
| Short-term borrowing activity by original maturity: |
|
|
|
|
|
| Net issuances (repayments) - maturities of 90 days or less |
$ |
530 |
|
|
$ |
876 |
|
|
$ |
— |
|
| Issuances - maturities longer than 90 days |
1,366 |
|
|
934 |
|
|
— |
|
| Repayments - maturities longer than 90 days |
(1,172) |
|
|
(795) |
|
|
— |
|
| Borrowings under credit facilities related to Swedish Match AB acquisition |
— |
|
|
13,920 |
|
|
— |
|
| Repayments under credit facilities related to Swedish Match AB acquisition |
(4,430) |
|
|
(4,000) |
|
|
— |
|
| Long-term debt proceeds |
9,959 |
|
|
5,965 |
|
|
— |
|
| Long-term debt repaid |
(2,551) |
|
|
(2,724) |
|
|
(3,042) |
|
| Repurchases of common stock |
— |
|
|
(209) |
|
|
(775) |
|
|
|
|
|
|
|
| Dividends paid |
(7,964) |
|
|
(7,812) |
|
|
(7,580) |
|
|
|
|
|
|
|
| Payments to acquire Swedish Match AB noncontrolling interests (Note 3) |
(883) |
|
|
(1,495) |
|
|
— |
|
Payments to noncontrolling interests and Other (Note 3) |
(437) |
|
|
(854) |
|
|
(580) |
|
| Net cash provided by (used in) financing activities |
(5,582) |
|
|
3,806 |
|
|
(11,977) |
|
| Effect of exchange rate changes on cash, cash equivalents and restricted cash |
(95) |
|
|
(213) |
|
|
(417) |
|
Cash, cash equivalents and restricted cash(1): |
|
|
|
|
|
| Increase (Decrease) |
(71) |
|
|
(1,283) |
|
|
(2,785) |
|
Balance at beginning of year |
3,217 |
|
|
4,500 |
|
|
7,285 |
|
Balance at end of year |
$ |
3,146 |
|
|
$ |
3,217 |
|
|
$ |
4,500 |
|
|
|
|
|
|
|
| Cash Paid: |
|
|
|
|
|
| Interest |
$ |
1,342 |
|
|
$ |
717 |
|
|
$ |
716 |
|
| Income taxes |
$ |
2,952 |
|
|
$ |
2,751 |
|
|
$ |
2,936 |
|
(1) The amounts for cash, cash equivalents and restricted cash shown above include restricted cash of $86 million, $10 million and $4 million as of December 31, 2023, 2022 and 2021, respectively, which were included in other current assets in the consolidated balance sheets.
See notes to consolidated financial statements.
Consolidated Statements of Stockholders' (Deficit) Equity
(in millions of dollars, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PMI Stockholders’ (Deficit) Equity |
|
|
|
|
|
|
|
Common
Stock |
|
Additional
Paid-in
Capital |
|
Earnings Reinvested
in the Business |
|
Accumulated Other
Comprehensive
Losses |
|
Cost of
Repurchased
Stock |
|
Noncontrolling
Interests |
|
|
Total |
|
| Balances, January 1, 2021 |
$ |
— |
|
|
$ |
2,105 |
|
|
$ |
31,638 |
|
|
$ |
(11,181) |
|
|
$ |
(35,129) |
|
|
$ |
1,936 |
|
|
|
$ |
(10,631) |
|
|
| Net earnings |
|
|
|
|
9,109 |
|
|
|
|
|
|
601 |
|
|
|
9,710 |
|
|
| Other comprehensive earnings (losses), net of income taxes |
|
|
|
|
|
|
1,604 |
|
|
|
|
(79) |
|
|
|
1,525 |
|
|
| Issuance of stock awards (Note 10) |
|
|
119 |
|
|
|
|
|
|
78 |
|
|
|
|
|
197 |
|
|
Dividends declared ($4.90 per share) |
|
|
|
|
(7,665) |
|
|
|
|
|
|
|
|
|
(7,665) |
|
|
| Dividends paid to noncontrolling interests |
|
|
|
|
|
|
|
|
|
|
(560) |
|
|
|
(560) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock repurchased |
|
|
|
|
|
|
|
|
(785) |
|
|
|
|
|
(785) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other |
|
|
1 |
|
|
|
|
|
|
|
|
— |
|
|
|
1 |
|
|
| Balances, December 31, 2021 |
— |
|
|
2,225 |
|
|
33,082 |
|
|
(9,577) |
|
|
(35,836) |
|
|
1,898 |
|
|
|
(8,208) |
|
|
| Net earnings |
|
|
|
|
9,048 |
|
|
|
|
|
|
479 |
|
|
|
9,527 |
|
|
| Other comprehensive earnings (losses), net of income taxes |
|
|
|
|
|
|
189 |
|
|
|
|
(135) |
|
|
|
54 |
|
|
| Issuance of stock awards (Note 10) |
|
|
37 |
|
|
|
|
|
|
118 |
|
|
|
|
|
155 |
|
|
Dividends declared ($5.04 per share) |
|
|
|
|
(7,841) |
|
|
|
|
|
|
|
|
|
(7,841) |
|
|
| Dividends paid to noncontrolling interests |
|
|
|
|
|
|
|
|
|
|
(472) |
|
|
|
(472) |
|
|
| Common stock repurchased |
|
|
|
|
|
|
|
|
(199) |
|
|
|
|
|
(199) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Acquisitions (Note 3) |
|
|
|
|
|
|
|
|
|
|
2,379 |
|
|
|
2,379 |
|
|
| Purchases of shares from noncontrolling interests (Note 3) |
|
|
(32) |
|
|
|
|
(171) |
|
|
|
|
(1,503) |
|
|
|
(1,706) |
|
|
| Balances, December 31, 2022 |
— |
|
|
2,230 |
|
|
34,289 |
|
|
(9,559) |
|
|
(35,917) |
|
|
2,646 |
|
|
|
(6,311) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net earnings |
|
|
|
|
7,813 |
|
|
|
|
|
|
455 |
|
|
|
8,268 |
|
|
| Other comprehensive earnings (losses), net of income taxes |
|
|
|
|
|
|
(2,436) |
|
|
|
|
6 |
|
|
|
(2,430) |
|
|
| Issuance of stock awards (Note 10) |
|
|
61 |
|
|
|
|
|
|
132 |
|
|
|
|
|
193 |
|
|
Dividends declared ($5.14 per share) |
|
|
|
|
(8,012) |
|
|
|
|
|
|
|
|
|
(8,012) |
|
|
| Dividends paid to noncontrolling interests |
|
|
|
|
|
|
|
|
|
|
(497) |
|
|
|
(497) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sale (purchases) of subsidiary shares to/(from) noncontrolling interests (Note 3) |
|
|
(6) |
|
|
|
|
180 |
|
|
|
|
(831) |
|
|
|
(657) |
|
|
| Balances, December 31, 2023 |
$ |
— |
|
|
$ |
2,285 |
|
|
$ |
34,090 |
|
|
$ |
(11,815) |
|
|
$ |
(35,785) |
|
|
$ |
1,779 |
|
|
|
$ |
(9,446) |
|
|
See notes to consolidated financial statements.
Notes to Consolidated Financial Statements
Background and Basis of Presentation:
Background
Philip Morris International Inc. is a holding company incorporated in Virginia, U.S.A. (also referred to herein as the U.S., the United States or the United States of America), whose subsidiaries and affiliates and their licensees are primarily engaged in the manufacture and sale of cigarettes and smoke-free products. Throughout these financial statements, the term "PMI" refers to Philip Morris International Inc. and its subsidiaries.
Smoke-free products (also referred to herein as "SFPs") is the term PMI primarily uses to refer to all of its products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral nicotine. In addition, SFPs include wellness and healthcare products, as well as consumer accessories such as lighters and matches.
Reduced-risk products ("RRPs") is the term PMI uses to refer to products that present, are likely to present, or have the potential to present less risk of harm to smokers who switch to these products versus continuing smoking. PMI has a range of RRPs in various stages of development, scientific assessment and commercialization. PMI's RRPs are smoke-free products that contain and/or generate far lower quantities of harmful and potentially harmful constituents than found in cigarette smoke.
"Platform 1" is the term PMI uses to refer to PMI’s reduced-risk product that uses a precisely controlled heating device into which a specially designed and proprietary tobacco unit is inserted and heated to generate an aerosol.
Basis of presentation
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the dates of the financial statements and the reported amounts of net revenues and expenses during the reporting periods. Significant estimates and assumptions include, among other things: pension and benefit plan assumptions; useful lives and valuation assumptions of goodwill and other intangible assets; valuation assumptions for non-marketable equity securities; marketing programs, and income taxes. Actual results could differ from those estimates.
The consolidated financial statements include PMI, as well as its wholly owned and majority-owned subsidiaries. Investments in which PMI exercises significant influence (generally 20%-50% ownership interest) are accounted for under the equity method of accounting. Investments not accounted for under the equity method of accounting are measured at fair value, if it is readily determinable, with changes in fair value recognized in net income. Investments without readily determinable fair values, non-marketable equity securities, are measured and recorded using a measurement alternative that values the security at cost minus any impairment. All intercompany transactions and balances have been eliminated.
In the fourth quarter of 2022, PMI acquired a controlling interest of the total issued shares in Swedish Match AB (“Swedish Match”). The operating results of Swedish Match are included in a separate segment. In the third quarter of 2021, PMI acquired Fertin Pharma A/S, Vectura Group plc. and OtiTopic, Inc. On March 31, 2022, PMI launched a Wellness and Healthcare business consolidating these entities, Vectura Fertin Pharma. The operating results of this business are reported in the Wellness and Healthcare segment. For further details on these acquisitions, see Note 3. Acquisitions and Note 13. Segment Reporting.
To further support the growth of PMI's smoke-free business, reinforce consumer centricity, and increase the speed of innovation and deployment, in January 2023, PMI began managing its business in four geographical segments, down from six previously, in addition to its continuing Swedish Match and Wellness and Healthcare segments. The four geographical segments are as follows: Europe Region; South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA"); East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF"); and Americas Region.
Certain prior years' amounts have been reclassified to conform with the current year's presentation. As a result of the new regional structure discussed above, certain goodwill amounts under the former six geographical segments were reallocated to the four geographical segments under the new structure. For further details, see Note 5. Goodwill and Other Intangible Assets, net. These reclassifications did not impact PMI's consolidated financial position, results of operations or cash flows in any of the periods presented.
Summary of Significant Accounting Policies:
Acquisitions
PMI uses the acquisition method of accounting for acquired businesses. Under the acquisition method, PMI’s consolidated financial statements reflect the operations of an acquired business starting from the closing date of the acquisition. PMI allocates the purchase price to the tangible and identifiable intangible assets acquired and liabilities assumed based on the estimated fair values as of the acquisition date. Any residual purchase price is recorded as goodwill. The fair value of assets acquired and liabilities assumed in certain cases may be subject to revision based on the final determination of fair value during a period of time not to exceed 12 months from the acquisition date. Contingent consideration liabilities are recognized at the estimated fair value on the acquisition date. Subsequent changes to the fair value of contingent consideration are recognized in marketing, administration and research costs in the consolidated statement of earnings. Transaction costs are expensed as incurred.
If PMI determines that assets acquired do not meet the definition of a business, the transaction will be accounted for as an acquisition of assets rather than a business combination and, therefore, no goodwill will be recorded. In an asset acquisition, acquired in-process research and development ("IPR&D") with no alternative future use is charged to expense.
Cash and cash equivalents
Cash equivalents include demand deposits with banks and all highly liquid investments with original maturities of three months or less.
Depreciation and Amortization
Property, plant and equipment are stated at historical cost and depreciated primarily using the straight-line method over the estimated useful lives of the assets. Machinery and equipment are depreciated primarily over periods ranging from 3 to 15 years, and buildings and building improvements primarily over periods up to 40 years.
Definite-lived intangible assets are amortized over their useful lives. For further details, see Note 5. Goodwill and Other Intangible Assets, net.
Employee benefit plans
PMI provides a range of benefits to its employees and retired employees, including pensions, postretirement health care and postemployment benefits (primarily severance). PMI records annual amounts relating to these plans based on calculations specified under U.S. GAAP. PMI recognizes the funded status of its defined pension and postretirement plans on the consolidated balance sheets. The funded status is measured as the difference between the fair value of the plans assets and the benefit obligation. PMI measures the plan assets and liabilities at the end of the fiscal year. For defined benefit pension plans, the benefit obligation is the projected benefit obligation. For the postretirement health care plans, the benefit obligation is the accumulated postretirement benefit obligation. Any plan with an overfunded status is recognized as an asset, and any plan with an underfunded status is recognized as a liability. Any gains or losses and prior service costs or credits that have not been recognized as a component of net periodic benefit costs are recorded as a component of other comprehensive earnings (losses), net of deferred taxes. PMI elects to recognize actuarial gains/(losses) using the corridor approach.
Fair value measurements
PMI follows ASC 820, Fair Value Measurements and Disclosures with respect to assets and liabilities that are measured at fair value. The guidance defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The guidance also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The guidance describes three levels of input that may be used to measure fair value. Level 1 inputs are quoted prices in active markets for identical assets or liabilities. Level 2 inputs include quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Level 3 are unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
Foreign currency translation
PMI translates the results of operations of its subsidiaries and affiliates, except those operating in highly inflationary economies, using average exchange rates during each period, whereas balance sheet accounts are translated using exchange rates at the end of each period. Currency translation adjustments are recorded as a component of stockholders’ (deficit) equity. In addition, some of PMI’s subsidiaries have assets and liabilities denominated in currencies other than their functional currencies, and to the extent those are not designated as net investment hedges, these assets and liabilities generate transaction gains and losses when translated into their respective functional currencies.
PMI applies highly inflationary accounting if the cumulative inflation rate in an economy for a three-year period meets or exceeds 100%. Subsidiaries operating in highly inflationary economies use the U.S. dollar as the functional currency. Monetary assets and liabilities are translated at exchange rates in effect at the balance sheet date while non-monetary assets and liabilities are translated at historical exchange rates. Exchange gains and losses resulting from remeasurement adjustments are recorded within marketing, administration and research costs in the consolidated statements of earnings.
Goodwill and non-amortizable intangible assets valuation
PMI tests goodwill and non-amortizable intangible assets for impairment annually or more frequently if events occur that would warrant such review. PMI performs its annual impairment analysis in the second quarter of each year. The impairment analysis involves comparing the fair value of each reporting unit or non-amortizable intangible asset to the carrying value. If the carrying value exceeds the fair value, goodwill or a non-amortizable intangible asset is considered impaired.
Hedging instruments
Derivative financial instruments are recorded at fair value on the consolidated balance sheets as either assets or liabilities. Changes in the fair value of derivatives are recorded each period either in accumulated other comprehensive losses on the consolidated balance sheet or in earnings, depending on whether a derivative is designated and effective as part of a hedge transaction and, if it is, the type of hedge transaction. Gains and losses on derivative instruments reported in accumulated other comprehensive losses are reclassified to the consolidated statements of earnings, into the same line item as the impact of the underlying transaction, in the periods in which operating results are affected by the hedged item. Cash flows from hedging instruments are classified in the same manner as the affected hedged item in the consolidated statements of cash flows.
Impairment of long-lived assets
PMI reviews long-lived assets, including amortizable intangible assets, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. PMI performs undiscounted operating cash flow analyses to determine if an impairment exists. For purposes of recognition and measurement of an impairment for assets held for use, PMI groups assets and liabilities at the lowest level for which cash flows are separately identifiable. If an impairment is determined to exist, any related impairment loss is calculated based on fair value. Impairment losses on assets to be disposed of, if any, are based on the lower of carrying value or estimated proceeds to be received less costs of disposal.
Investment in non-marketable equity securities
Non-marketable equity securities are subject to periodic impairment reviews during which PMI considers both qualitative and quantitative factors that may have a significant impact on the investees' fair value. Upon determining that an impairment may exist, the security’s fair value is calculated and compared to its carrying value, and an impairment is recognized immediately if the carrying value exceeds the fair value.
Impairment of equity method investments
Equity method investments are evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of the investments may not be recoverable. An impairment loss would be recorded whenever a decline in value of an equity investment below its carrying amount is determined to be other than temporary. PMI determines whether a loss is other than temporary by considering the length of time and extent to which the fair value of the equity investment has been less than the carrying amount, the financial condition of the equity investment, and the intent to retain the investment for a period of time is sufficient to allow for any anticipated recovery in market value.
Income taxes
Income taxes are provided on all earnings for jurisdictions outside the United States. These provisions, as well as state and local income tax provisions, are determined on a separate company basis, and the related assets and liabilities are recorded in PMI’s consolidated balance sheets. Significant judgment is required in determining income tax provisions and in evaluating tax positions. PMI recognizes accrued interest and penalties associated with uncertain tax positions as part of the provision for income taxes on the consolidated statements of earnings. PMI recognizes income taxes associated with Global Intangible Low-Taxed Income ("GILTI") taxes as current period expense rather than including these amounts in the measurement of deferred taxes.
Inventories
Inventories are stated at the lower of cost or net realizable value. The first-in, first-out and average cost methods are used to cost substantially all inventories. It is a generally recognized industry practice to classify leaf tobacco inventory as a current asset, although part of such inventory, because of the duration of the aging process, ordinarily would not be utilized within one year.
Leases
PMI determines that a contract contains a lease if the contract conveys a right to control the use of the identified asset for a period of time in exchange for consideration. Operating lease expense is recognized on a straight-line basis over the lease term. Finance lease expense is amortized based on production activity or the lease term. Lease expense is recorded in cost of sales or marketing, administration and research costs depending on the nature of the leased item. At lease commencement, PMI recognizes lease liabilities and the corresponding right-of-use assets (at the present value of future payments) for predominately all of its leases. The recognition of the right-of-use asset and lease liability includes renewal options when it is reasonably certain that they will be exercised. Certain of PMI’s leases include payments that are based on changes to an index or on actual usage. These lease payments are adjusted periodically and are included within variable lease costs. PMI accounts for lease and nonlease components as a single-lease component with the exception of its vehicle leases, of which PMI accounts for the lease components separately from the nonlease components. Additionally, leases with an initial term of 12 months or less are not included in the right-of-use asset or lease liability on the consolidated statement of financial position.
Marketing costs
PMI supports its products with advertising, adult consumer engagement and trade promotions. Such programs include, but are not limited to, discounts, rebates, in-store display incentives, e-commerce, mobile and other digital platforms, adult consumer activation and promotion activities, as well as costs associated with adult consumer experience outlets and other adult consumer touchpoints and volume-based incentives. Advertising, as well as certain consumer engagement and trade activities costs, are expensed as incurred. Trade promotions are recorded as a reduction of revenues based on amounts estimated as being due to customers at the end of a period, based principally on historical utilization. For interim reporting purposes, advertising and certain consumer engagement expenses are charged to earnings based on estimated sales and related expenses for the full year.
Revenue recognition
PMI recognizes revenue primarily through the manufacture and sale of cigarettes and smoke-free products, including heat-not-burn, vapor and oral nicotine products. The majority of PMI revenues are generated by sales through direct and indirect distribution networks with short-term payment conditions and where control is typically transferred to the customer either upon shipment or delivery of goods. PMI evaluates the transfer of control through evidence of the customer’s receipt and acceptance, transfer of title, PMI’s right to payment for those products and the customer’s ability to direct the use of those products upon receipt. Typically, PMI’s performance obligations are satisfied and revenue is recognized either upon shipment or delivery of goods.
In certain instances, PMI facilitates shipping and handling activities after control has transferred to the customer. PMI has elected to record all shipping and handling activities as costs to fulfill a contract. The shipping and handling costs that have not been incurred at the time revenue is recognized are accrued. The transaction price is typically based on the amount billed to the customer and includes estimated variable consideration, where applicable. Such variable consideration is typically not constrained and is estimated based on the most likely amount that PMI expects to be entitled to under the terms of the contracts with customers, historical experience of discount or rebate redemption, where relevant, and the terms of any underlying discount or rebate programs, which may change from time to time as the business and product categories evolve. PMI has elected to exclude excise taxes collected from customers from the measurement of the transaction price, thereby presenting revenues net of excise taxes. Estimated costs associated with warranty programs are generally provided for in cost of sales in the period the related revenues are recognized.
Research and Development and Acquired In-Process Research and Development ("IPR&D")
Research and development costs are expensed as incurred.
In a business combination, the fair value of IPR&D acquired is initially capitalized and accounted for as indefinite-lived intangible assets until completion or abandonment of the projects. Upon completion, a determination as to the useful life is performed and the intangible asset is accounted for as a definite-lived intangible asset. Both the indefinite and definite-lived intangible assets are subject to impairment testing annually or more frequently if indicators exist. In an asset acquisition, the initial cost to acquire the IPR&D is expensed in the consolidated statements of earnings when the project has no alternative future use. PMI records these costs within marketing, administration and research costs in its consolidated statements of earnings.
Stock-based compensation
PMI measures compensation cost for all stock-based awards at fair value on date of grant and recognizes the compensation costs over the service periods for awards expected to vest. PMI’s accounting policy is to estimate the number of awards expected to be forfeited and adjust the expense when it is no longer probable that the employee will fulfill the service condition. For further details, see Note 10. Stock Plans.
Acquisitions:
Transactions With Noncontrolling Interests
Turkey – In the first quarter of 2022, PMI acquired the remaining 25% stake of its holding in Philip Morris Tütün Mamulleri Sanayi ve Ticaret A.Ş. ("PMTM") (formerly Philsa Philip Morris Sabanci Sigara ve Tütüncülük Sanayi ve Ticaret A.Ş.) and 24.75% stake in Philip Morris Pazarlama ve Satiş A.Ş. ("PMPS") (formerly Philip Morris SA, Philip Morris Sabanci Pazarlama ve Satiş A.Ş.) from its Turkish partners, Sabanci Holding for a total acquisition price including transaction costs and remaining dividend entitlements of approximately $223 million. As a result of this acquisition, PMI owned 100% of these Turkish subsidiaries as of December 31, 2022. The purchase of the remaining stakes in these holdings resulted in a decrease to PMI's additional paid-in capital of $30 million and an increase to accumulated other comprehensive losses of $171 million primarily following the reclassification of accumulated currency translation losses from noncontrolling interests to PMI’s accumulated other comprehensive losses during the first quarter of 2022.
In January 2023, PMI sold the acquired stakes of its holdings in PMTM and PMPS to Pioneers Tutun Yatirim Anonim Sirketi (“Pioneers”) for a consideration of approximately $258 million, including transaction costs and dividend entitlements. The sale resulted in an increase to PMI's additional paid-in capital of $36 million and a decrease to accumulated other comprehensive losses of $179 million, following the reclassification of accumulated other comprehensive losses from PMI’s accumulated other comprehensive losses to noncontrolling interests.
Business Combinations
Swedish Match AB – On November 11, 2022 (the acquisition date), Philip Morris Holland Holdings B.V. (“PMHH”), a wholly owned subsidiary of PMI, acquired a controlling interest of 85.87% of the total issued shares in Swedish Match AB (“Swedish Match”) and acquired 94.81% of its outstanding shares as of December 31, 2022. The shares were acquired through acceptances of the tender offer and a series of open market and over-the-counter purchases. PMI funded the acquisition through cash on-hand and debt proceeds, as described in Note 8. Indebtedness. The aggregate cash paid as of the acquisition date was $14,460 million (or $13,976 million net of cash acquired), which was included in investing activities in the consolidated statements of cash flows for the year ended December 31, 2022. The cash paid in connection with the additional purchases of the noncontrolling interests after the acquisition date and through December 31, 2022 amounted to $1,495 million and was included in financing activities in the consolidated statements of cash flows for the year ended December 31, 2022.
In accordance with the Swedish Companies Act, PMI subsequently exercised its right to initiate arbitral proceedings to compulsorily redeem the remaining shares for which acceptances were not received and obtained legal title to 100% of the shares in Swedish Match on February 17, 2023. Cash paid in connection with such legal title, together with an immaterial amount attributable to open market purchases that were executed in December 2022 but settled in January 2023, amounted to $883 million and was included in financing activities in the consolidated statements of cash flows for the year ended December 31, 2023. While PMI paid the referenced amounts and acquired legal title to the shares, under the Swedish Companies Act the redemption process was not complete until the final redemption price was determined by an arbitral tribunal. On September 12, 2023, the arbitral tribunal determined the final redemption price to be Swedish krona (SEK) 115.07, unchanged from the SEK 115.07 that PMI paid per share in connection with obtaining legal title to the shares. This process was completed in the fourth quarter of 2023 when the opportunity to appeal the arbitral tribunal determination ended.
Swedish Match is a market leader in oral nicotine delivery with a significant presence in the United States market. The acquisition will accelerate PMI’s transformation to become a smoke-free company with a comprehensive global smoke-free portfolio with leadership positions in heat-not-burn, and the fastest growing category of oral nicotine, with the potential for accelerated international expansion.
In November 2023, PMI finalized all measurement period adjustments related to the Swedish Match acquisition. The table below summarizes the final purchase price allocation for the fair value of assets acquired and liabilities assumed as of the acquisition date:
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
Preliminary Purchase Price Allocation Recognized as of the acquisition date |
Measurement Period Adjustments during 2023 |
Final Purchase Price Allocation Recognized as of
the acquisition date |
| Cash and cash equivalents |
$ |
484 |
|
$ |
— |
|
$ |
484 |
|
| Trade receivables |
135 |
|
— |
|
135 |
|
| Other receivables |
53 |
|
— |
|
53 |
|
| Inventories |
444 |
|
(7) |
|
437 |
|
| Other current assets |
524 |
|
(109) |
|
415 |
|
| Property, plant and equipment |
627 |
|
50 |
|
677 |
|
| Other intangible assets |
4,512 |
|
3,356 |
|
7,868 |
|
| Other non-current assets |
214 |
|
2 |
|
216 |
|
| Current portion of long-term debt |
224 |
|
— |
|
224 |
|
| Accounts payable |
120 |
|
— |
|
120 |
|
| Other current liabilities |
531 |
|
1 |
|
532 |
|
| Income taxes |
14 |
|
— |
|
14 |
|
| Long-term debt |
1,126 |
|
(5) |
|
1,121 |
|
| Deferred income taxes |
1,253 |
|
717 |
|
1,970 |
|
| Other non-current liabilities |
187 |
|
9 |
|
196 |
|
| Identifiable net assets acquired |
3,538 |
|
2,570 |
|
6,108 |
|
| Noncontrolling interest |
2,379 |
|
— |
|
2,379 |
|
| Goodwill |
13,301 |
|
(2,570) |
|
10,731 |
|
| Total consideration transferred |
$ |
14,460 |
|
$ |
— |
|
$ |
14,460 |
|
The total fair value step-up adjustment for inventories was $146 million, of which $125 million was recognized in cost of sales in the fourth quarter of 2022 and the remaining balance in the first quarter of 2023.
The fair value of long-term debt was primarily determined using readily available market prices as of the acquisition date and the total purchase price adjustment of $(107) million is being amortized as an increase to interest expense, net over the lives of the related debt.
Goodwill is primarily attributable to future growth opportunities, anticipated synergies in the U.S. and intangible assets that did not qualify for separate recognition. The goodwill is not deductible for income tax purposes.
Identifiable intangible assets of Swedish Match consist of:
|
|
|
|
|
|
|
|
|
|
|
|
|
Type |
Useful Life |
Estimated Fair Value (in millions) |
| Trademarks |
Non-amortizable |
|
$ |
3,133 |
|
| Trademarks |
Amortizable |
20 - 30 years |
1,067 |
|
| Developed technology, including patents |
|
10 years |
113 |
|
| Customer relationships |
|
6 - 15 years |
3,555 |
|
|
|
|
|
| Total identifiable intangible assets |
|
|
$ |
7,868 |
|
The significant assumptions used in determining the fair values of the identifiable intangible assets included royalty rates, revenue growth rates, profit margins, customer attrition rates and discount rates.
Trademarks primarily relate to $3,133 million for the ZYN trademark, which has been determined to have an indefinite life due to the fast growth and the leading position of the brand in the U.S. market. All other trademarks have been determined to have a useful life ranging between 20 - 30 years. The trademarks have been valued using the relief from royalty method supported by revenue growth rate assumptions and royalty rates disaggregated at the individual trademark level.
Developed technology, including patents, relates to the nicotine pouch technology of $113 million. These patents have been assigned a useful life of 10 years, which is in line with their protection period and have been valued using the comparable transactions and income methods.
Customer relationships have been valued by categories of customers and geographic locations, namely the U.S. market, Scandinavia, and other markets using the multiple periods excess earnings method. The significant assumptions included customer attrition rates disaggregated at the customer category level, the revenue growth rates, as well as profit margins.
PMI consolidated statements of earnings for the year ended December 31, 2022, include $316 million of net revenues and $(26) million of net losses associated with the results of operations of Swedish Match from the acquisition date to December 31, 2022. The operating results of Swedish Match are included in a separate segment.
Acquisition related transaction costs, which were comprised primarily of regulatory, financial advisory and legal fees, totaled $59 million for the year ended December 31, 2022, and were included in marketing, administration and research costs in the consolidated statements of earnings. Bridge and term loan credit agreement related fees associated with the issuance of debt amounted to $54 million, of which $37 million were capitalized at the acquisition date. The fair value of the noncontrolling interest was based on the tender offer as of the acquisition date.
Under the EU Merger Regulation, approval by the European Commission of PMI's acquisition of Swedish Match was conditional on PMHH's divestiture of Swedish Match's subsidiary, SMD Logistics AB ("SMDL"), following the completion of the offer to tender all shares in Swedish Match to PMHH. As a result, these assets were accounted for as assets held for sale and included within other current assets and other accrued liabilities in PMI’s consolidated balance sheets at March 31, 2023 and December 31, 2022. PMI subsequently sold SMDL on June 30, 2023 and the transaction did not have a material impact on the consolidated statements of earnings for the year ended December 31, 2023.
The unaudited pro forma combined financial information was prepared using the acquisition method of accounting and was based on the historical financial information of PMI and Swedish Match. In order to reflect the occurrence of the acquisition on January 1, 2021, as required, the unaudited pro forma financial information includes adjustments to reflect the following:
•incremental amortization expense to be incurred based on the current fair values of the identifiable intangible assets acquired;
•incremental cost of products sold related to the fair value adjustments associated with acquisition date inventory;
•additional interest expense associated with the issuance of debt to finance the acquisition, including the effects of the related derivative financial instruments designated to hedge interest rate risks as well as economic hedges;
•reclassification of non-recurring acquisition-related costs incurred during the year ended December 31, 2022, to the year ended December 31, 2021;
•impact of a deferred tax cost of $430 million in 2022 and $321 million in 2021 related to the theoretical unrealized foreign currency gains on intercompany loans related to the acquisition financing. These theoretical unrealized pre-tax foreign currency movements were fully offset in the consolidated statements of earnings and were reflected as currency translation adjustments in PMI's consolidated statements of stockholders' (deficit) equity, while the corresponding deferred tax impacts were reflected in PMI's consolidated statements of earnings; and
•other immaterial items (i.e., the alignment of accounting policies from IFRS to US GAAP.)
The unaudited pro forma financial information is not necessarily indicative of what the consolidated results of operations would have been had the acquisition been completed on January 1, 2021. In addition, the unaudited pro forma financial information is not a projection of future results of operations of the combined company, nor does it reflect the expected realization of any synergies or cost savings associated with the acquisition.
The unaudited pro forma financial information is as follows:
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in millions) |
2022 |
2021 |
| Net revenues |
$ |
33,579 |
|
$ |
33,488 |
|
| Net earnings attributable to PMI |
$ |
8,779 |
|
$ |
8,484 |
|
AG Snus - On May 6, 2021, PMI acquired 100% of AG Snus Aktieselskab ("AG Snus"), a company based in Denmark, and its Swedish subsidiary Tobacco House of Sweden AB fully owned by AG Snus, which operates in the oral tobacco (i.e. snus) and modern oral (i.e. nicotine pouches) product categories. The purchase price was $28 million in cash, net of cash acquired, with additional contingent payments of up to $10 million, primarily relating to product development and performance targets over a less than two-year period. In the fourth quarter of 2022, the additional contingent payment was settled for $9 million. The operating results of AG Snus are included in the Europe segment, and were not material.
Fertin Pharma – On September 15, 2021, PMI acquired 100% of Fertin Pharma A/S (“Fertin Pharma”), a company based in Denmark. Fertin Pharma is a developer and manufacturer of pharmaceutical and well-being products based on oral and intra-oral delivery systems. The acquisition was funded with existing cash. The total consideration of $821 million (DKK 5.2 billion) included cash of $580 million and the payment of $241 million related to the settlement of Fertin Pharma’s indebtedness. The purchase price of $821 million was allocated to cash ($24 million), current assets including receivables and inventories ($69 million), non-current assets including property, plant and equipment ($228 million), goodwill ($378 million), and other intangible assets ($245 million, which primarily consisted of customer relationships, developed technology, and in-process research and development ("IPR&D")), partially offset by current liabilities ($44 million, which primarily consisted of accrued liabilities and accounts payable) and non-current liabilities ($79 million, primarily deferred income tax). Goodwill is primarily attributable to future growth opportunities provided by acquired R&D capabilities and any intangibles that did not qualify for separate recognition. The goodwill is not deductible for income tax purposes. The amortizable intangible assets are being amortized over their estimated useful lives of 8 to 19 years. During 2022, PMI did not record any measurement period adjustments to the purchase price allocation. The final purchase price allocation was reflected in the consolidated balance sheets as of December 31, 2022.
Vectura – During the third quarter and up to September 15, 2021, PMI acquired a controlling interest of 74.77% of the total issued shares in Vectura Group plc (“Vectura”), an inhaled therapeutics company based in the United Kingdom. The shares were acquired through a series of open market purchases and acceptances of the tender offer at a price of 165 pence per share. As a result of additional acceptances of the offer and the exercise of the right to acquire compulsorily the Vectura shares, in accordance with the applicable English law, PMI completed the acquisition of 100% of Vectura in the fourth quarter of 2021. The acquisition was funded with existing cash from a designated account operated solely for the purpose of funding this acquisition.
The total purchase price of $1,384 million (GBP 1.0 billion) for 100% of the Vectura shares was allocated to cash ($136 million), current assets including receivables and inventories ($89 million), non-current assets including property, plant and equipment ($67 million), goodwill ($780 million), and other intangible assets ($486 million, which primarily consisted of developed technology, and IPR&D), partially offset by current liabilities ($100 million, primarily accrued liabilities), and non-current liabilities ($74 million, primarily deferred income tax). Goodwill is primarily attributable to future growth opportunities provided by acquired R&D capabilities and any intangibles that did not qualify for separate recognition. The goodwill is not deductible for income tax purposes. The amortizable intangible assets are being amortized over their estimated useful lives of 3 to 13 years. During 2022, PMI made certain measurement period adjustments to the purchase price allocation to reflect facts and circumstances in existence as of the acquisition date, which resulted in an increase to goodwill of $190 million. The increase was primarily due to a decrease in other intangible assets ($233 million), and a decrease in deferred income tax liabilities ($43 million). The final purchase price allocation was reflected in the consolidated balance sheets as of December 31, 2022.
Pro forma results of operations for AG Snus, Fertin Pharma and Vectura have not been presented as the aggregate impact is not material to PMI's consolidated statements of earnings.
Altria Group, Inc. Agreement
On October 20, 2022, PMI announced that it had reached an agreement with Altria Group, Inc. ("Altria") to end the companies' relationship regarding the IQOS commercialization rights in the U.S. as of April 30, 2024. As a result of PMI reacquiring these rights, effective May 1, 2024, PMI will hold the full rights to commercialize IQOS in the U.S. As part of the agreement, PMI agreed to pay a total cash consideration of $2.7 billion, with $1.0 billion paid at the inception of the agreement and the remaining $1.7 billion (plus interest, at a per annum rate equal to six percent (6%)), to be paid by July 2023 at the latest. The cash consideration paid at the inception of the agreement of $1.0 billion has been accounted for within other assets in PMI’s consolidated balance sheets as of December 31, 2023 and 2022. The remaining consideration of $1.7 billion plus interest was paid to Altria on July 14, 2023 and has been accounted for within other assets in PMI's consolidated balance sheets as of December 31, 2023. PMI will finalize the accounting for this transaction by assigning the consideration to the respective assets in May 2024, when PMI can exercise its ability to commercialize IQOS in the U.S. For further details on PMI's agreement with Altria, see Note 18. Contingencies.
Asset Acquisition
On August 9, 2021, PMI acquired 100% of OtiTopic, Inc., a U.S. respiratory drug development company with a late-stage dry powder inhalation aspirin treatment for acute myocardial infarction. The transaction price was $38 million in cash, plus transaction costs, with additional contingent payment of $13 million, primarily related to certain key milestones that PMI deemed probable. Additionally, PMI may owe up to $25 million in future additional contingent payments dependent upon the achievement of certain milestones. PMI accounted for this transaction as an asset acquisition since the IPR&D of the dry powder inhalation aspirin treatment represented substantially all of the fair value of the gross assets acquired. At the date of acquisition, PMI determined that the acquired IPR&D had no alternative future use. As a result, PMI recorded a charge of $51 million to research and development costs within marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2021.
As previously discussed in Note 1. Background and Basis of Presentation on March 31, 2022, PMI launched a Wellness and Healthcare business, Vectura Fertin Pharma, which consolidated Fertin Pharma, Vectura and OtiTopic, Inc. into one operating segment.
War in Ukraine:
Since the onset of the war in Ukraine in February 2022, PMI's main priority has been the safety and security of its employees and their families in the country.
Ukraine
PMI temporarily suspended its commercial and manufacturing operations in Ukraine, including the closing of its factory in Kharkiv at the end of February 2022, in order to preserve the safety of its employees. PMI subsequently resumed some retail activities where safety allowed, in order to provide product availability and service to adult consumers, and began to supply the market from production centers outside Ukraine, as well as through a contract manufacturing arrangement. Production at the factory in Kharkiv remains suspended. PMI is not aware of any major damage to its production facilities, inventories or other assets in Ukraine. On June 20, 2023, PMI announced the investment of $30 million in a new production facility in the Lviv region, in Western Ukraine. In the fourth quarter of 2023, as a result of the completion of certain preparatory work for this new production facility, PMI recorded impairment of certain long-lived assets. As of December 31, 2023, PMI’s Ukrainian operations had approximately $446 million in total assets, excluding intercompany balances. These total assets included $82 million and $304 million in receivables and inventories, respectively.
Russia
PMI has suspended its planned investments in the Russian Federation including all new product launches and commercial, innovation, and manufacturing investments. PMI has also taken steps to scale down its manufacturing operations in Russia amid ongoing supply chain disruptions and the evolving regulatory environment. PMI is continuously assessing the evolving situation in Russia, including recent regulatory constraints in the market that entail very complex terms and conditions that must be met for any divestment transaction to be granted approval by the authorities, and restrictions resulting from international regulations. As a result of PMI continuing operations within Russia as of December 31, 2023, it has not recorded an impairment of long-lived and other assets. However, PMI recorded specific asset write downs in 2022 as referred to in the table below. PMI’s Russian operations as of December 31, 2023 had approximately $2.7 billion in total assets, excluding intercompany balances. These total assets included $773 million, $494 million, $948 million, $261 million and $167 million in cash (primarily held in local currency), receivables, inventories, property, plant and equipment and goodwill, respectively.
In addition, there was approximately $1,182 million of cumulative foreign currency translation losses reflected in accumulated other comprehensive losses in the consolidated statement of stockholders’ equity as of December 31, 2023.
For the years ended December 31, 2023 and 2022, PMI recorded in its consolidated statements of earnings pre-tax charges related to circumstances driven by the war as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
2023 |
|
2022 |
|
|
|
Cost of sales |
Marketing, administration and research costs |
Total |
|
Cost of sales |
Marketing, administration and research costs |
Total |
|
|
|
|
Ukraine 1 |
$ |
15 |
|
$ |
38 |
|
$ |
53 |
|
|
$ |
42 |
|
$ |
36 |
|
$ |
78 |
|
|
|
|
|
Russia 2 |
— |
|
— |
|
— |
|
|
20 |
|
53 |
|
73 |
|
|
|
|
|
| Total |
$ |
15 |
|
$ |
38 |
|
$ |
53 |
|
|
$ |
62 |
|
$ |
89 |
|
$ |
151 |
|
|
|
|
|
1 The 2023 pre-tax charges were primarily due to the cost of PMI’s humanitarian efforts, which includes salary continuation for its employees, severance payments, as well as an impairment of certain long-lived assets in the fourth quarter of 2023. The 2022 pre-tax charges were primarily due to an inventory write-down, additional allowance for receivables and the cost of PMI’s humanitarian efforts, which includes salary continuation for its employees.
2 The 2022 pre-tax charges were primarily due to machinery and inventory write downs related to the commercial decisions noted above.
PMI will continue to monitor the situation as it evolves and will determine if further charges are needed.
Goodwill and Other Intangible Assets, net:
2023 Annual impairment review of goodwill and non-amortizable intangible assets
During the second quarter of 2023, PMI completed its annual review of goodwill and non-amortizable intangible assets for potential impairment. Based on this review, it was determined that the estimated fair value of the Wellness and Healthcare reporting unit was lower than its carrying value. Consequently, PMI recorded a goodwill impairment charge of $665 million in the consolidated statements of earnings for the year ended December 31, 2023, reflecting the impact of reduced estimated future cash flows, which were primarily attributable to clinical trial results that became available in June 2023 for an inhalable aspirin product being developed by the Wellness and Healthcare business. While it was observed that the experimental product had a rapid onset of effect, which is the key medical advantage sought, there was significant variability in inhaled dose among subjects. The study was therefore deemed unsuccessful and, as a result, product design improvements are required. PMI had planned to file a new drug application for this product with the U.S. Food and Drug Administration later this year. However, additional time is now required to evaluate design improvements and the corresponding less certain outcome. The cash flow estimates were also adversely impacted by slower-than-anticipated development of the contract development and manufacturing organization ("CDMO") business, including challenges associated with increased cost related to certain key products. The goodwill impairment charge is not deductible for income tax purposes. Additionally, as a result of the impairment test of non-amortizable intangible assets, PMI recorded a pre-tax impairment charge of $15 million for an in-process research and development project related to one of PMI's 2021 acquisitions. This pre-tax impairment charge of $15 million was recorded within marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2023.
The Wellness and Healthcare reporting unit's fair value was determined using the discounted cash flow model. PMI will continue to monitor this reporting unit as any changes in assumptions and estimates, unfavorable clinical trial results, failure to obtain regulatory approvals or other market factors could result in additional future goodwill and other intangible asset impairments. Certain Wellness and Healthcare products include components or gases which may be subject to enhanced regulations that could impact the related product development and market strategies. This may also lead to supply disruptions that could result in additional future impairments.
While PMI’s remaining reporting units have fair values substantially in excess of their carrying values, there are still risks related to PMI’s Russian reporting unit’s assets as the fair value of these assets is difficult to predict due to the volatility in foreign currency and commodity markets, supply chain, and current economic, political and social conditions. For more information see Note 4. War in Ukraine. PMI performed a quantitative impairment assessment for all of its reporting units and non-amortizable intangible assets with the exception of the Swedish Match segment. As the purchase price allocation for the acquisition of Swedish Match was preliminary at that time of the annual review, PMI performed a qualitative impairment assessment and concluded that it was not more likely than not that the fair value of the Swedish Match reporting units and its non-amortizable intangible assets were less than the respective carrying amounts.
Goodwill
The movements in goodwill were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
Europe |
SSEA, CIS & MEA |
EA, AU & PMI DF |
Americas |
Swedish Match |
Wellness & Healthcare |
Total |
| Balances at January 1, 2022 |
$ |
1,455 |
|
$ |
3,143 |
|
$ |
540 |
|
$ |
611 |
|
$ |
— |
|
$ |
931 |
|
$ |
6,680 |
|
| Changes due to: |
|
|
|
|
|
|
|
| Acquisitions |
— |
|
— |
|
— |
|
— |
|
13,301 |
|
— |
|
13,301 |
|
| Currency |
(85) |
|
(274) |
|
(47) |
|
4 |
|
(5) |
|
(109) |
|
(516) |
|
| Other |
— |
|
— |
|
— |
|
— |
|
— |
|
190 |
|
190 |
|
| Balances, December 31, 2022 |
1,370 |
|
2,869 |
|
493 |
|
615 |
|
13,296 |
|
1,012 |
|
19,655 |
|
| Changes due to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Impairment |
— |
|
— |
|
— |
|
— |
|
— |
|
(665) |
|
(665) |
|
| Currency |
69 |
|
8 |
|
(1) |
|
89 |
|
151 |
|
43 |
|
359 |
|
| Measurement period adjustments |
— |
|
— |
|
— |
|
— |
|
(2,570) |
|
— |
|
(2,570) |
|
|
|
|
|
|
|
|
|
| Balances, December 31, 2023 |
$ |
1,439 |
|
$ |
2,877 |
|
$ |
492 |
|
$ |
704 |
|
$ |
10,877 |
|
$ |
390 |
|
$ |
16,779 |
|
As discussed in Note 1. Background and Basis of Presentation, in January 2023, PMI began managing its business in four geographical segments, Swedish Match segment and Wellness and Healthcare segment. As a result, the January 1, 2022 and December 31, 2022 goodwill balances in the table above included the reclassifications from the former six geographical segments to the four geographical segments under the new structure.
The increase in goodwill in 2022 was due primarily to the final purchase price allocation associated with Vectura Group plc acquisition in 2021 (reflected in "changes due to other" in Wellness and Healthcare segment) and the preliminary purchase price allocation associated with the Swedish Match AB acquisition in the fourth quarter of 2022, partially offset by currency movements. For further details on these business combinations, see Note 3. Acquisitions.
The decrease in goodwill in 2023 was primarily due to the measurement period adjustments to the Swedish Match final purchase price allocation (see Note 3, Acquisitions), coupled with the impairment discussed above and partially offset by currency movements.
At December 31, 2023, goodwill primarily reflects PMI’s acquisitions of Swedish Match AB, Fertin Pharma A/S and Vectura Group plc., as well as acquisitions in Greece, Indonesia, Mexico, the Philippines and Serbia.
Other Intangible Assets
Details of other intangible assets were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
December 31, 2022 |
(in millions) |
Weighted-Average Remaining Useful Life |
Gross Carrying Amount |
Accumulated Amortization |
Net |
|
Gross Carrying Amount |
Accumulated Amortization |
Net |
| Non-amortizable intangible assets |
|
$ |
4,543 |
|
|
$ |
4,543 |
|
|
$ |
3,346 |
|
|
$ |
3,346 |
|
| Amortizable intangible assets: |
|
|
|
|
|
|
|
|
| Trademarks |
16 years |
2,267 |
|
$ |
784 |
|
1,483 |
|
|
2,050 |
|
$ |
674 |
|
1,376 |
|
|
|
|
|
|
|
|
|
|
| Developed technology, including patents |
7 years |
774 |
|
329 |
|
445 |
|
|
975 |
|
243 |
|
732 |
|
| Customer relationships and other |
12 years |
3,843 |
|
450 |
|
3,393 |
|
|
1,390 |
|
112 |
|
1,278 |
|
| Total other intangible assets |
|
$ |
11,427 |
|
$ |
1,563 |
|
$ |
9,864 |
|
|
$ |
7,761 |
|
$ |
1,029 |
|
$ |
6,732 |
|
Non-amortizable intangible assets substantially consist of the ZYN trademark and other trademarks related to acquisitions in Indonesia and Mexico. The increase since December 31, 2022 was due to the measurement period adjustments to the Swedish Match final purchase price allocation in the amount of $1,056 million (see Note 3, Acquisitions), coupled with currency movements of $156 million, partially offset by an impairment for an in-process research and development project related to one of PMI's 2021 acquisitions discussed above.
The increase in the gross carrying amount of amortizable intangible assets from December 31, 2022, was mainly due to the measurement period adjustments to the Swedish Match final purchase price allocation in the amount of $2,300 million (see Note 3, Acquisitions), coupled with currency movements of $161 million.
The change in the accumulated amortization from December 31, 2022, was mainly due to the 2023 amortization of $497 million, coupled with currency movements of $37 million. The amortization of intangibles for the years ended December 31, 2023 was recorded in cost of sales of $58 million and in marketing, administration and research costs of $439 million on PMI's consolidated statements of earnings.
Amortization expense on a pre-tax basis for each of the next five years is estimated to be approximately $470 million or less, assuming no additional transactions occur that require the amortization of intangible assets. Additionally, the estimated future amortization expense could significantly increase following the reacquisition of IQOS commercialization rights in the U.S. from Altria Group, Inc. (see Note 3, Acquisitions), the accounting for which will depend on the facts and circumstances effective May 1, 2024, when PMI will hold the full rights.
2022 Impairment of Other Intangibles
In the third quarter of 2022, PMI recorded a pre-tax impairment charge of $112 million, reflecting the impact of general economic and market conditions resulting in a reduction in future estimated cash flows on certain products within the Wellness and Healthcare segment. The impairment reduced the carrying values of developed technology definite-lived intangible assets in the Wellness and Healthcare segment to $325 million. The fair value of these intangible assets was primarily determined using the multi-period excess earnings method. This impairment charge was recorded within cost of sales in the consolidated statements of earnings for the year ended December 31, 2022.
Related Parties - Equity Investments and Other:
Equity Method Investments:
At December 31, 2023 and 2022, PMI had total equity method investments of $1,309 million and $1,000 million, respectively. Equity method investments are initially recorded at cost. Under the equity method of accounting, the investment is adjusted for PMI's proportionate share of earnings or losses, dividends, capital contributions, changes in ownership interests and movements in currency translation adjustments. The carrying value of our equity method investments at December 31, 2023 and 2022, exceeded our share of the investees' book value by $907 million and $750 million, respectively. The difference between the investment carrying value and the amount of underlying equity in net assets is mainly attributable to equity method goodwill, convertible debt instruments, and definite-lived intangible assets and other assets. The difference related to the definite-lived intangibles and other assets at December 31, 2023 and 2022 of $31 million and $35 million, respectively, is amortized on a straight-line basis and is included in Equity investments and securities (income)/loss, net on the consolidated statements of earnings. At December 31, 2023 and 2022, PMI received year-to-date dividends from equity method investees of $57 million and $9 million, respectively.
PMI holds a 23% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis, PMI's distributor in Russia (SSEA, CIS & MEA segment), which as of December 31, 2023 had a carrying value of $385 million. While as of December 31, 2023, there have been no impairment indicators based on the business’ performance, there are still risks related to this investment as the fair value of these assets is difficult to predict due to the volatility in foreign currency and commodity markets, supply chain, and current economic, political and social conditions. For more information, see Note 4. War in Ukraine. Additionally, there was approximately $561 million of cumulative foreign currency translation losses associated with Megapolis Distribution BV reflected in accumulated other comprehensive losses in the consolidated statement of stockholders’ equity as of December 31, 2023.
PMI holds a 49% equity interest in United Arab Emirates-based Emirati Investors-TA (FZC) (“EITA”). PMI holds an approximate 25% economic interest in Société des Tabacs Algéro-Emiratie (“STAEM”), an Algerian joint venture that is 51% owned by EITA and 49% by the Algerian state-owned enterprise Management et Développement des Actifs et des Ressources Holding ("MADAR Holding"), which manufactures and distributes under license some of PMI’s brands (SSEA, CIS & MEA segment).
In April 2023, PMI increased its equity ownership and acquired 66.73% of Egyptian Investment Holding (“EIH”), a United Arab Emirates based company and as a result, acquired an approximate economic interest of 25% in United Tobacco Company ("UTC"). UTC is an entity incorporated in Egypt, which is 38% owned by EIH and manufactures products under license for Philip Morris Misr LCC (“PMM”), an entity incorporated in Egypt which is consolidated in PMI’s financial statements in the SSEA, CIS & MEA segment.
The initial investments in Megapolis Distribution BV, EITA and UTC have been recorded at cost and are included in equity investments on the consolidated balance sheets. Transactions between these equity method investees and PMI subsidiaries are considered to be related-party transactions and are included in the tables below.
Equity securities:
On March 22, 2019, PMI’s wholly owned subsidiary in Canada, Rothmans, Benson & Hedges Inc. (“RBH”) obtained an initial order from the Ontario Superior Court of Justice granting it protection under the Companies’ Creditors Arrangement Act ("CCAA"), which is a Canadian federal law that permits a Canadian business to restructure its affairs while carrying on its business in the ordinary course with minimal disruption to its customers, suppliers and employees. The administration of the CCAA process, principally relating to the powers provided to the court under the CCAA and the oversight provided by the court appointed monitor, removes certain elements of control of the business from both PMI and RBH. As a result, PMI determined that it no longer had a controlling financial interest over RBH as defined in ASC 810 (Consolidation), and deconsolidated RBH as of the date of the CCAA filing. For further details, see Note 18, Contingencies.
Since the deconsolidation of RBH on March 22, 2019, PMI has accounted for its continuing investment in RBH in accordance with ASC 321 (Investments-Equity Securities) as an equity security, without readily determinable fair value, and recorded its continuing investment in RBH at fair value of $3,280 million at the date of deconsolidation, within equity investments. Developments in the CCAA process, including resolution through a plan of arrangement or compromise of some or all tobacco-related litigation pending in Canada may have a material adverse impact on the fair value of PMI’s continuing investment in RBH and may result in impairment charges. Transactions between PMI and RBH are considered to be related-party transactions from the date of deconsolidation and are included in the tables below.
The fair value of PMI’s other equity securities, which have been classified within Level 1, was $375 million and $326 million for the years ended December 31, 2023 and 2022, respectively. Unrealized pre-tax gains (losses) of $49 million and $43 million ($38 million and $33 million net of tax) on these equity securities were recorded in equity investments and securities (income)/loss, net on the consolidated statements of earnings for the years ended December 31, 2023 and 2022, respectively. For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Note 2. Summary of Significant Accounting Policies.
Other related parties:
United Arab Emirates-based Trans-Emirates Trading and Investments (FZC) ("TTI") holds a 33% non-controlling interest in Philip Morris Misr LLC ("PMM"), an entity incorporated in Egypt which is consolidated in PMI’s financial statements in the SSEA, CIS & MEA segment. PMM sells, under license, PMI brands in Egypt through an exclusive distribution agreement with a local entity that is also controlled by TTI.
Godfrey Phillips India Ltd ("GPI") is one of the non-controlling interest holders in IPM India, which is a 56.3% owned PMI consolidated subsidiary in the SSEA, CIS & MEA segment. GPI also acts as contract manufacturer and distributor for IPM India.
Financial activity with the above related parties:
PMI’s net revenues and expenses with the above related parties were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in millions) |
|
2023 |
2022 |
2021 |
| Net revenues: |
|
|
|
|
| Megapolis Group |
|
$ |
2,267 |
|
$ |
2,485 |
|
$ |
2,207 |
|
| Other |
|
1,286 |
|
1,173 |
|
1,123 |
|
Net revenues (a) |
|
$ |
3,553 |
|
$ |
3,658 |
|
$ |
3,330 |
|
|
|
|
|
|
| Expenses: |
|
|
|
|
| Other |
|
$ |
186 |
|
$ |
119 |
|
$ |
69 |
|
| Expenses |
|
$ |
186 |
|
$ |
119 |
|
$ |
69 |
|
(a) Net revenues exclude excise taxes and VAT billed to customers.
PMI’s balance sheet activity with the above related parties was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, |
| (in millions) |
|
2023 |
2022 |
| Receivables: |
|
|
|
| Megapolis Group |
|
$ |
474 |
|
$ |
478 |
|
| Other |
|
236 |
|
210 |
|
| Receivables |
|
$ |
710 |
|
$ |
688 |
|
|
|
|
|
| Payables: |
|
|
|
| Other |
|
$ |
18 |
|
$ |
31 |
|
| Payables |
|
$ |
18 |
|
$ |
31 |
|
|
|
|
|
|
|
|
|
The activities with the above related parties are in the ordinary course of business, and are primarily for distribution, service fees, contract manufacturing and license agreements. PMI eliminated its respective share of all significant intercompany transactions with the equity method investees.
Product Warranty:
PMI's heat-not-burn devices and e-vapor products are subject to standard product warranties generally for a period of 12 months from the date of purchase or such other periods as required by law. PMI generally provides in cost of sales for the estimated cost of warranty in the period the related revenue is recognized. PMI assesses the adequacy of its accrued product warranties and adjusts the amounts as necessary based on actual experience and changes in future estimates. Factors that affect product warranties may vary across markets but typically include device version mix, product failure rates, logistics and service delivery costs, and warranty policies. PMI accounts for its product warranties within other accrued liabilities. At December 31, 2023 and December 31, 2022, these amounts were as follows:
|
|
|
|
|
|
|
|
|
|
At December 31, |
| (in millions) |
2023 |
2022 |
| Balance at beginning of period |
$ |
104 |
|
$ |
113 |
|
| Changes due to: |
|
|
| Warranties issued |
60 |
|
107 |
|
| Settlements |
(83) |
|
(114) |
|
| Currency/Other |
(1) |
|
(2) |
|
| Balance at end of period |
$ |
80 |
|
$ |
104 |
|
Indebtedness:
Short-Term Borrowings
At December 31, 2023 and 2022, PMI’s short-term borrowings and related average interest rates consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
December 31, 2022 |
| (in millions) |
Amount Outstanding |
|
Average Year-End Rate |
|
Amount Outstanding |
|
Average Year-End Rate |
Commercial paper |
$ |
1,685 |
|
|
5.6 |
% |
|
$ |
912 |
|
|
4.4 |
% |
Bank loans |
283 |
|
|
8.9 |
|
|
295 |
|
|
7.5 |
|
| U.S. dollar credit facility borrowings related to Swedish Match AB acquisition |
— |
|
|
— |
|
|
4,430 |
|
|
4.9 |
|
|
$ |
1,968 |
|
|
|
|
$ |
5,637 |
|
|
|
Given the mix of PMI's legal entities and their respective local economic environments, the average interest rate for bank loans above can vary significantly from day to day and country to country.
The fair values of PMI’s short-term borrowings at December 31, 2023 and 2022, based on current market interest rates, approximate carrying value.
Long-Term Debt
At December 31, 2023 and 2022, PMI’s long-term debt consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
(in millions) |
2023 |
|
2022 |
U.S. dollar notes, 0.875% to 6.375% (average interest rate 4.446%), due through 2044 |
$ |
30,272 |
|
|
$ |
22,596 |
|
| Foreign currency obligations: |
|
|
|
Euro notes, 0.125% to 3.125% (average interest rate 1.877%), due through 2039 |
8,526 |
|
|
8,116 |
|
Swiss franc note, 1.625%, due 2024 |
299 |
|
|
378 |
|
Euro credit facility borrowings related to Swedish Match AB acquisition, (average interest rate 4.453%), due through 2027 |
6,121 |
|
|
5,850 |
|
Swedish krona notes, 1.395% to 2.710% (average interest rate 2.016%), due through 2029 |
236 |
|
|
343 |
|
Other (average interest rate 6.027%), due through 2031 (a) |
487 |
|
|
203 |
|
| Carrying value of long-term debt |
45,941 |
|
|
37,486 |
|
Less current portion of long-term debt |
4,698 |
|
|
2,611 |
|
|
$ |
41,243 |
|
|
$ |
34,875 |
|
(a) Includes long-term bank loans at subsidiaries, as well as $53 million and $54 million in finance leases at December 31, 2023 and 2022, respectively.
The fair value of PMI’s outstanding long-term debt, which is utilized solely for disclosure purposes, is determined using quotes and market interest rates currently available to PMI for issuances of debt with similar terms and remaining maturities. At December 31, 2023 and 2022 the fair value of PMI's outstanding long-term debt, excluding the aforementioned finance leases, was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
(in millions) |
2023 |
|
2022 |
| Level 1 |
$ |
38,259 |
|
|
$ |
28,919 |
|
| Level 2 |
6,687 |
|
|
6,142 |
|
For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Note 2. Summary of Significant Accounting Policies.
Credit Facilities related to the Financing of the Swedish Match Acquisition
In connection with PMI's all-cash recommended public offer to the shareholders of Swedish Match, on May 11, 2022, PMI entered into a credit agreement relating to a 364-day senior unsecured bridge facility. The facility provided for borrowings up to an aggregate principal amount of $17 billion, expiring 364 days after the occurrence of certain events unless extended. On June 23, 2022, PMI entered into a €5.5 billion (approximately $5.8 billion at the date of signing) senior unsecured term loan credit agreement consisting of a €3.0 billion (approximately $3.2 billion at the date of signing) tranche expiring three years after the occurrence of certain events and a €2.5 billion (approximately $2.6 billion at the date of signing) tranche expiring on June 23, 2027. In connection with the term loan facility, the aggregate principal amount of commitments under the 364-day senior unsecured bridge facility was reduced from $17 billion to $11 billion. On November 11, 2022, PMI acquired a controlling interest of 85.87% of the total issued shares in Swedish Match and acquired 94.81% of its outstanding shares as of December 31, 2022. In accordance with the Swedish Companies Act, PMI subsequently exercised its right to compulsorily redeem the remaining shares for which acceptances were not received and obtained legal title to 100% of the shares in Swedish Match on February 17, 2023.
PMI borrowed $8.4 billion under the bridge facility by delivering notices of borrowing for advances of $7.9 billion and $0.5 billion on November 7, 2022 and November 10, 2022, respectively. On November 21, 2022 and February 17, 2023, PMI repaid $4.0 billion and $4.4 billion, respectively, under the bridge facility. Effective February 20, 2023, the remaining outstanding commitments under the bridge facility were fully canceled and the bridge facility agreement was terminated in accordance with its terms.
On November 7, 2022, PMI also delivered notices of borrowing for advances totaling €5.5 billion under the term loan facility, of which €3.0 billion will become due on November 9, 2025 and €2.5 billion will become due on June 23, 2027 unless prepaid pursuant to the terms of the credit agreement. As of December 31, 2023 and 2022, the €5.5 billion (approximately $6 billion) term loan facility was fully drawn and remained outstanding.
The proceeds under the bridge facility and the term loan facility were used, directly or indirectly, to finance the acquisition, including, the payment of related fees and expenses. For further details on this acquisition, see Note 3. Acquisitions.
Notes Outstanding:
PMI’s notes outstanding at December 31, 2023, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
|
|
|
|
|
|
|
|
Type |
|
Face Value |
|
Interest
Rate |
|
Issuance |
|
Maturity |
U.S. dollar notes |
|
$900 |
|
2.875% |
|
May 2019 |
|
May 2024 |
U.S. dollar notes |
|
$750 |
|
3.250% |
|
November 2014 |
|
November 2024 |
U.S. dollar notes |
|
$1,000 |
|
5.125% |
|
November 2022 |
|
November 2024 |
U.S. dollar notes |
|
$750 |
|
1.500% |
|
May 2020 |
|
May 2025 |
U.S. dollar notes |
|
$750 |
|
3.375% |
|
August 2015 |
|
August 2025 |
U.S. dollar notes |
|
$750 |
|
5.000% |
|
November 2022 |
|
November 2025 |
U.S. dollar notes |
|
$750 |
|
2.750% |
|
February 2016 |
|
February 2026 |
U.S. dollar notes |
|
$1,250 |
|
4.875% |
|
February 2023 |
|
February 2026 |
U.S. dollar notes |
(a) |
$450 |
|
4.875% |
|
May 2023 |
|
February 2026 |
U.S. dollar notes |
|
$750 |
|
0.875% |
|
November 2020 |
|
May 2026 |
U.S. dollar notes |
|
$500 |
|
3.125% |
|
August 2017 |
|
August 2027 |
U.S. dollar notes |
|
$1,500 |
|
5.125% |
|
November 2022 |
|
November 2027 |
U.S. dollar notes |
|
$1,000 |
|
4.875% |
|
February 2023 |
|
February 2028 |
U.S. dollar notes |
(b) |
$550 |
|
4.875% |
|
May 2023 |
|
February 2028 |
U.S. dollar notes |
|
$500 |
|
3.125% |
|
November 2017 |
|
March 2028 |
U.S. dollar notes |
(c) |
$50 |
|
4.000% |
|
May 2013 |
|
May 2028 |
U.S. dollar notes |
|
$650 |
|
5.250% |
|
September 2023 |
|
September 2028 |
U.S. dollar notes |
|
$750 |
|
3.375% |
|
May 2019 |
|
August 2029 |
U.S. dollar notes |
|
$1,250 |
|
5.625% |
|
November 2022 |
|
November 2029 |
U.S. dollar notes |
|
$1,500 |
|
5.125% |
|
February 2023 |
|
February 2030 |
U.S. dollar notes |
(d) |
$700 |
|
5.125% |
|
May 2023 |
|
February 2030 |
U.S. dollar notes |
|
$750 |
|
2.100% |
|
May 2020 |
|
May 2030 |
U.S. dollar notes |
|
$700 |
|
5.500% |
|
September 2023 |
|
September 2030 |
U.S. dollar notes |
|
$750 |
|
1.750% |
|
November 2020 |
|
November 2030 |
U.S. dollar notes |
|
$1,500 |
|
5.750% |
|
November 2022 |
|
November 2032 |
U.S. dollar notes |
|
$1,500 |
|
5.375% |
|
February 2023 |
|
February 2033 |
U.S. dollar notes |
(e) |
$750 |
|
5.375% |
|
May 2023 |
|
February 2033 |
U.S. dollar notes |
|
$1,000 |
|
5.625% |
|
September 2023 |
|
September 2033 |
U.S. dollar notes |
|
$1,500 |
|
6.375% |
|
May 2008 |
|
May 2038 |
U.S. dollar notes |
|
$750 |
|
4.375% |
|
November 2011 |
|
November 2041 |
U.S. dollar notes |
|
$700 |
|
4.500% |
|
March 2012 |
|
March 2042 |
U.S. dollar notes |
|
$750 |
|
3.875% |
|
August 2012 |
|
August 2042 |
U.S. dollar notes |
|
$850 |
|
4.125% |
|
March 2013 |
|
March 2043 |
U.S. dollar notes |
|
$750 |
|
4.875% |
|
November 2013 |
|
November 2043 |
U.S. dollar notes |
|
$750 |
|
4.250% |
|
November 2014 |
|
November 2044 |
U.S. dollar notes |
(f) |
$500 |
|
4.250% |
|
May 2016 |
|
November 2044 |
EURO notes |
(g) |
€600 (approximately $761) |
|
2.875% |
|
May 2012 |
|
May 2024 |
EURO notes |
(c) |
€300 (approximately $308) |
|
0.875% |
|
September 2016 |
|
September 2024 |
EURO notes |
(g) |
€500 (approximately $582) |
|
0.625% |
|
November 2017 |
|
November 2024 |
EURO notes |
(g) |
€750 (approximately $972) |
|
2.750% |
|
March 2013 |
|
March 2025 |
EURO notes |
(c) |
€200 (approximately $205) |
|
1.200% |
|
November 2017 |
|
November 2025 |
EURO notes |
(c) |
€50 (approximately $51) |
|
1.200% |
|
December 2020 |
|
November 2025 |
EURO notes |
(c) |
€50 (approximately $51) |
|
1.200% |
|
June 2021 |
|
November 2025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
|
|
|
|
|
|
|
|
Type |
|
Face Value |
|
Interest
Rate |
|
Issuance |
|
Maturity |
EURO notes |
(g) |
€1,000 (approximately $1,372) |
|
2.875% |
|
March 2014 |
|
March 2026 |
EURO notes |
(g) |
€500 (approximately $557) |
|
0.125% |
|
August 2019 |
|
August 2026 |
EURO notes |
(c) |
€300 (approximately $308) |
|
0.875% |
|
February 2020 |
|
February 2027 |
EURO notes |
(g) |
€500 (approximately $697) |
|
2.875% |
|
May 2014 |
|
May 2029 |
EURO notes |
(g) |
€750 (approximately $835) |
|
0.800% |
|
August 2019 |
|
August 2031 |
EURO notes |
(g) |
€500 (approximately $648) |
|
3.125% |
|
June 2013 |
|
June 2033 |
EURO notes |
(g) |
€500 (approximately $578) |
|
2.000% |
|
May 2016 |
|
May 2036 |
EURO notes |
(g) |
€500 (approximately $582) |
|
1.875% |
|
November 2017 |
|
November 2037 |
EURO notes |
(g) |
€750 (approximately $835) |
|
1.450% |
|
August 2019 |
|
August 2039 |
Swiss franc notes |
(g) |
CHF250 (approximately $283) |
|
1.625% |
|
May 2014 |
|
May 2024 |
| Swedish krona notes |
(c) |
SEK1,000 (approximately $95) |
|
2.710% |
|
January 2019 |
|
January 2026 |
| Swedish krona notes |
(c) |
SEK700 (approximately $67) |
|
1.395% |
|
February 2021 |
|
February 2026 |
| Swedish krona notes |
(c) |
SEK100 (approximately $10) |
|
1.395% |
|
March 2021 |
|
February 2026 |
| Swedish krona notes |
(c) |
SEK200 (approximately $19) |
|
1.395% |
|
September 2021 |
|
February 2026 |
| Swedish krona notes |
(c) |
SEK200 (approximately $19) |
|
1.395% |
|
January 2022 |
|
February 2026 |
| Swedish krona notes |
(c) |
SEK300 (approximately $29) |
|
2.190% |
|
April 2021 |
|
April 2029 |
(a) These notes are a further issuance of the 4.875% notes issued in February 2023.
(b) These notes are a further issuance of the 4.875% notes issued in February 2023.
(c) Notes issued by Swedish Match AB. USD equivalents for foreign currency notes were calculated based on exchange rates on the date of acquisition.
(d) These notes are a further issuance of the 5.125% notes issued in February 2023.
(e) These notes are a further issuance of the 5.375% notes issued in February 2023.
(f) These notes are a further issuance of the 4.250% notes issued by PMI in November 2014.
(g) USD equivalents for foreign currency notes were calculated based on exchange rates on the date of issuance.
The net proceeds from the sale of the securities listed in the table above were primarily used for general corporate purposes, including working capital requirements and repurchase of PMI's common stock. On February 17, 2023, PMI applied a portion of the net proceeds of the February 2023 debt issuances to prepay $4.4 billion under its bridge facility, which represented all borrowings outstanding under the bridge facility. PMI used a portion of the May 2023 net proceeds to pay the remaining cash consideration due in accordance with the terms of its agreement with Altria. For further details on PMI's agreement with Altria, see Note 3. Acquisitions. The remaining net proceeds of the February and May 2023 offerings, as well as the September 2023 offering have been used for general corporate purposes.
Aggregate maturities:
Aggregate maturities of long-term debt are as follows:
|
|
|
|
|
|
(in millions) |
|
| 2024 |
$ |
4,709 |
|
| 2025 |
6,785 |
|
| 2026 |
5,117 |
|
| 2027 |
5,141 |
|
| 2028 |
2,771 |
|
| 2029-2033 |
13,312 |
|
| 2034-2038 |
2,613 |
|
| Thereafter |
5,885 |
|
|
46,333 |
|
| Debt discounts and fair value adjustments |
(392) |
|
| Total long-term debt |
$ |
45,941 |
|
Revolving Credit Facilities
At December 31, 2023, PMI’s total committed revolving credit facilities were as follows:
|
|
|
|
|
|
|
|
|
|
Type
(in billions) |
Committed Revolving Credit Facilities |
|
|
364-day revolving credit, expiring January 30, 2024 (1) |
$ |
1.8 |
|
|
|
Multi-year revolving credit, expiring February 10, 2026 (2) |
2.0 |
|
|
|
Multi-year revolving credit, expiring September 29, 2026 (3) (4) |
2.5 |
|
|
|
Total facilities |
$ |
6.3 |
|
|
|
|
|
|
|
(1) On January 24, 2024, PMI entered into an agreement to extend the term of its 364-day committed revolving credit facility in the amount of $1.7 billion from January 30, 2024, to January 28, 2025.
(2) On January 28, 2022, PMI entered into an agreement, effective February 10, 2022, to amend and extend the term of its $2.0 billion multi-year revolving credit facility, for an additional year covering the period February 11, 2026 to February 10, 2027, in the amount of $1.9 billion.
(3) Includes pricing adjustments that may result in the reduction or increase in both the interest rate and commitment fee under the credit agreement if PMI achieves, or fails to achieve, certain specified targets.
(4) On September 20, 2022, PMI entered into an agreement, effective September 29, 2022, to amend and extend the term of its $2.5 billion multi-year revolving credit facility, for an additional year covering the period September 30, 2026 to September 29, 2027, in the amount of $2.3 billion. On September 20, 2023, PMI entered into an agreement, effective September 29, 2023, to amend and further extend the term to September 29, 2028.
At December 31, 2023, there were no borrowings under these committed revolving credit facilities, and the entire committed amounts were available for borrowing.
In addition to the committed revolving credit facilities discussed above, PMI maintains certain short-term credit arrangements, including uncommitted credit lines, to primarily meet working capital needs. These credit arrangements amounted to approximately $2.7 billion at December 31, 2023, and approximately $1.9 billion at December 31, 2022. Borrowings under these arrangements and other bank loans amounted to $283 million at December 31, 2023, and $295 million at December 31, 2022.
Capital Stock:
Shares of authorized common stock are 6.0 billion; issued, repurchased and outstanding shares were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares Issued |
|
Shares
Repurchased |
|
Shares
Outstanding |
| Balances, January 1, 2021 |
2,109,316,331 |
|
|
(551,942,600) |
|
|
1,557,373,731 |
|
| Repurchase of shares |
|
|
(8,514,629) |
|
|
(8,514,629) |
|
| Issuance of stock awards |
|
|
1,310,891 |
|
|
1,310,891 |
|
| Balances, December 31, 2021 |
2,109,316,331 |
|
|
(559,146,338) |
|
|
1,550,169,993 |
|
| Repurchase of shares |
|
|
(1,966,730) |
|
|
(1,966,730) |
|
| Issuance of stock awards |
|
|
2,014,448 |
|
|
2,014,448 |
|
| Balances, December 31, 2022 |
2,109,316,331 |
|
|
(559,098,620) |
|
|
1,550,217,711 |
|
| Repurchase of shares |
|
|
— |
|
|
— |
|
| Issuance of stock awards |
|
|
2,206,820 |
|
|
2,206,820 |
|
| Balances, December 31, 2023 |
2,109,316,331 |
|
|
(556,891,800) |
|
|
1,552,424,531 |
|
On June 11, 2021, PMI's Board of Directors authorized a new share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period. On July 22, 2021, PMI began repurchasing shares under this new share repurchase program. From July 22, 2021 through March 31, 2022, PMI repurchased 10.5 million shares of its common stock at a cost of approximately $1.0 billion. During the first three months of 2022, PMI repurchased 2.0 million shares of its common stock at a cost of $199 million. On May 11, 2022, PMI announced the suspension of its three-year share repurchase program following the recommended public offer to acquire the outstanding shares of Swedish Match from its shareholders. For further details, see Note 3. Acquisitions. Prior to the suspension of the program, PMI made no share repurchases during the second quarter of 2022.
At December 31, 2023, 30,505,637 shares of common stock were reserved for stock awards under PMI’s stock plans, and 250 million shares of preferred stock, without par value, were authorized but unissued. PMI currently has no plans to issue any shares of preferred stock.
Stock Plans:
In May 2022, PMI’s shareholders approved the Philip Morris International Inc. 2022 Performance Incentive Plan (the “2022 Plan”). Under the 2022 Plan, PMI may grant to eligible employees restricted shares and restricted share units, performance-based cash incentive awards and performance-based equity awards. Up to 25 million shares of PMI’s common stock may be issued under the 2022 Plan. At December 31, 2023, shares available for grant under the 2022 Plan were 22,171,530.
In May 2017, PMI’s shareholders approved the Philip Morris International Inc. 2017 Stock Compensation Plan for Non-Employee Directors (the “2017 Non-Employee Directors Plan”). A non-employee director is defined as a member of the PMI Board of Directors who is not a full-time employee of PMI or of any corporation in which PMI owns, directly or indirectly, stock possessing at least 50% of the total combined voting power of all classes of stock entitled to vote in the election of directors in such corporation. Up to 1 million shares of PMI common stock may be awarded under the 2017 Non-Employee Directors Plan. At December 31, 2023, shares available for grant under the plan were 876,226.
Restricted share unit (RSU) awards
PMI may grant RSU awards to eligible employees; recipients may not sell, assign, pledge or otherwise encumber such awards. Such awards are subject to forfeiture if certain employment conditions are not met. RSU awards generally vest on the third anniversary of the grant date. RSU awards do not carry voting rights, although they do earn dividend equivalents.
During 2023, the activity for RSU awards was as follows:
|
|
|
|
|
|
|
|
|
|
Number of
Shares |
Weighted-
Average Grant
Date Fair Value
Per Share |
| Balance at January 1, 2023 |
4,519,470 |
|
$ |
91.26 |
|
| Granted |
1,756,750 |
|
101.96 |
|
| Vested |
(1,483,356) |
|
87.30 |
|
| Forfeited |
(189,543) |
|
96.96 |
|
| Balance at December 31, 2023 |
4,603,321 |
|
$ |
96.38 |
|
During the years ended December 31, 2023, 2022 and 2021, the grant date fair value of the RSU awards granted to PMI employees and the recorded compensation expense related to RSU awards were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions, except per RSU award granted) |
Total Grant Date Fair Value of RSU Awards Granted |
|
Weighted-Average Grant Date Fair Value Per RSU Award Granted |
Compensation Expense related to RSU Awards |
| 2023 |
$ |
179 |
|
|
$ |
101.96 |
|
$ |
153 |
|
| 2022 |
$ |
174 |
|
|
$ |
104.75 |
|
$ |
135 |
|
| 2021 |
$ |
166 |
|
|
$ |
82.17 |
|
$ |
139 |
|
The fair value of the RSU awards at the date of grant is amortized to expense over the restriction period, typically three years after the date of the award, or upon death, disability or reaching the age of 58. As of December 31, 2023, PMI had $160 million of total unrecognized compensation costs related to non-vested RSU awards. These costs are expected to be recognized over a weighted-average period of approximately seventeen months, or upon death, disability or reaching the age of 58.
During the years ended December 31, 2023, 2022 and 2021, share and fair value information for PMI RSU awards that vested were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (dollars in millions) |
Shares of RSU Awards that Vested |
|
Grant Date Fair Value of Vested Shares of RSU Awards |
Total Fair Value of RSU Awards that Vested |
| 2023 |
1,483,356 |
|
|
$ |
129 |
|
$ |
148 |
|
| 2022 |
1,603,571 |
|
|
$ |
126 |
|
$ |
174 |
|
| 2021 |
1,256,441 |
|
|
$ |
121 |
|
$ |
111 |
|
Performance share unit (PSU) awards
PMI may grant PSU awards to certain executives; recipients may not sell, assign, pledge or otherwise encumber such awards. The PSU awards require the achievement of certain performance metrics, which are predetermined at the time of grant, typically over a three-year performance cycle. The performance metrics for such PSU's granted during 2023 and 2022 consisted of PMI's Total Shareholder Return ("TSR") relative to a predetermined peer group and on an absolute basis (40% weight), PMI’s currency-neutral compound annual adjusted diluted earnings per share growth rate (30% weight), and a Sustainability Index, which consists of two drivers:
•Product Sustainability (20% weight) measuring progress primarily on PMI's efforts to maximize the benefits of smoke-free products, purposefully phase out cigarettes, and reduce post-consumer waste; and
•Operational Sustainability (10% weight) measuring progress on PMI's efforts to tackle climate change, preserve nature, improve the quality of life of people in its supply chain, and foster an empowered, and inclusive workplace.
The performance metrics for such PSU's granted in 2021 consisted of PMI's TSR relative to a predetermined peer group and on an absolute basis (40% weight), PMI’s currency-neutral compound annual adjusted diluted earnings per share growth rate (30% weight), and PMI’s performance against specific measures of PMI’s transformation, defined as net revenues from PMI's RRPs and any other non-combustible products as a percentage of PMI's total net revenues in the last year of the performance cycle (30% weight).
The aggregate of the weighted performance factors for the three metrics in each such PSU award determines the percentage of PSUs that will vest at the end of the three-year performance cycle. The minimum percentage of such PSUs that can vest is zero, with a target percentage of 100 and a maximum percentage of 200. Each such vested PSU entitles the participant to one share of common stock. An aggregate weighted PSU performance factor of 100 will result in the targeted number of PSUs being vested. At the end of the performance cycle, participants are entitled to an amount equivalent to the accumulated dividends paid on common stock during the performance cycle for the number of shares earned. PSU awards do not carry voting rights.
During 2023, the activity for PSU awards was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
Shares |
|
Weighted-
Average PSU Grant Date
Fair Value Subject to Other Performance Factors |
Weighted-
Average PSU Grant Date
Fair Value Subject to TSR Performance Factors |
|
|
(Per Share) |
(Per Share) |
| Balance at January 1, 2023 |
1,507,190 |
|
|
$ |
90.31 |
|
$ |
115.45 |
|
| Granted |
482,360 |
|
|
102.02 |
|
133.54 |
|
| Vested |
(902,232) |
|
|
85.99 |
|
98.45 |
|
| Adjustments for performance achievement |
400,992 |
|
|
85.99 |
|
98.45 |
|
| Forfeited |
(61,030) |
|
|
98.24 |
|
131.39 |
|
| Balance at December 31, 2023 |
1,427,280 |
|
|
$ |
95.45 |
|
$ |
126.86 |
|
During the years ended December 31, 2023, 2022 and 2021, the grant date fair value of the PSU awards granted to PMI employees and the recorded compensation expense related to PSU awards were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions, except per PSU award granted) |
Weighted-
Average PSU Grant Date
Fair Value Subject to Other Performance Factors |
|
Weighted-
Average PSU Grant Date
Fair Value Subject to TSR Performance Factors |
|
Compensation Expense related to PSU Awards |
|
Total |
Per PSU Award |
|
Total |
Per PSU Award |
|
Total |
| 2023 |
$ |
29 |
|
$ |
102.02 |
|
|
$ |
26 |
|
$ |
133.54 |
|
|
$ |
59 |
|
| 2022 |
$ |
30 |
|
$ |
104.92 |
|
|
$ |
27 |
|
$ |
143.89 |
|
|
$ |
48 |
|
| 2021 |
$ |
28 |
|
$ |
81.86 |
|
|
$ |
25 |
|
$ |
106.93 |
|
|
$ |
71 |
|
The grant date fair value of the PSU awards subject to the other performance factors was determined by using the market price of PMI’s stock on the date of the grant. The grant date fair value of the PSU market-based awards subject to the TSR performance factor was determined by using the Monte Carlo simulation model. The following assumptions were used to determine the grant date fair value of the PSU awards subject to the TSR performance factor for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
2023 |
|
2022 |
|
2021 |
Average risk-free interest rate (a) |
4.1 |
% |
|
1.7 |
% |
|
0.2 |
% |
Average expected volatility (b) |
24.3 |
% |
|
28.3 |
% |
|
31.7 |
% |
(a) Based on the U.S. Treasury yield curve.
(b) Determined using the observed historical volatility.
The fair value of the PSU award at the date of grant is amortized to expense over the performance period, which is typically three years after the date of the award, or upon death, disability or reaching the age of 58. As of December 31, 2023, PMI had $39 million of total unrecognized compensation cost related to non-vested PSU awards. This cost is recognized over a weighted-average performance cycle period of approximately seventeen months, or upon death, disability or reaching the age of 58.
During the years ended December 31, 2023, 2022 and 2021, share and fair value information for PMI PSU awards that vested were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (dollars in millions) |
Shares of PSU Awards that Vested |
|
Grant Date Fair Value of Vested Shares of PSU Awards |
Total Fair Value of PSU Awards that Vested |
| 2023 |
902,232 |
|
|
$ |
83 |
|
$ |
91 |
|
| 2022 |
669,960 |
|
|
$ |
54 |
|
$ |
74 |
|
| 2021 |
189,839 |
|
|
$ |
21 |
|
$ |
16 |
|
Earnings per Share:
Unvested share-based payment awards that contain non-forfeitable rights to dividends or dividend equivalents are participating securities and therefore are included in PMI’s earnings per share calculation pursuant to the two-class method.
Basic and diluted earnings per share (“EPS”) were calculated using the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
| Net earnings attributable to PMI |
$ |
7,813 |
|
|
$ |
9,048 |
|
|
$ |
9,109 |
|
| Less distributed and undistributed earnings attributable to share-based payment awards |
22 |
|
|
24 |
|
|
26 |
|
| Net earnings for basic and diluted EPS |
$ |
7,791 |
|
|
$ |
9,024 |
|
|
$ |
9,083 |
|
| Weighted-average shares for basic EPS |
1,552 |
|
|
1,550 |
|
|
1,558 |
|
Plus contingently issuable performance stock units (PSUs) (1) |
1 |
|
|
2 |
|
|
1 |
|
| Weighted-average shares for diluted EPS |
1,553 |
|
|
1,552 |
|
|
1,559 |
|
(1) Including rounding adjustment
For the 2023, 2022 and 2021 computations, there were no antidilutive stock awards.
Income Taxes:
Earnings before income taxes and provision for income taxes consisted of the following for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
2023 |
|
2022 |
|
2021 |
Earnings before income taxes |
$ |
10,450 |
|
|
$ |
11,634 |
|
|
$ |
12,232 |
|
Provision for income taxes: |
|
|
|
|
|
United States federal and state: |
|
|
|
|
|
Current |
$ |
201 |
|
|
$ |
(75) |
|
|
$ |
73 |
|
Deferred |
(368) |
|
|
(139) |
|
|
27 |
|
Total United States |
(167) |
|
|
(214) |
|
|
100 |
|
Outside United States: |
|
|
|
|
|
Current |
2,468 |
|
|
2,553 |
|
|
2,616 |
|
Deferred |
38 |
|
|
(95) |
|
|
(45) |
|
Total outside United States |
2,506 |
|
|
2,458 |
|
|
2,571 |
|
Total provision for income taxes |
$ |
2,339 |
|
|
$ |
2,244 |
|
|
$ |
2,671 |
|
On August 16, 2022, the Inflation Reduction Act ("the Act") was signed into law in the U.S. The Act includes a new corporate alternative minimum tax and an excise tax on stock buybacks effective after December 31, 2022. As of December 31, 2023, PMI has determined that the Act had no tax impacts on its consolidated financial statements.
On March 11, 2021, the American Rescue Plan Act of 2021 ("the ARP Act") was signed into law in the U.S. to provide certain relief as a result of the COVID-19 pandemic. PMI has determined that the ARP Act had no significant impact on PMI's effective tax rate.
At December 31, 2017, PMI recorded a one-time transition tax liability on its accumulated foreign earnings, which is payable over an eight-year period beginning in 2018. At December 31, 2023 and December 31, 2022, $0.3 billion and $0.7 billion of PMI's remaining long-term portion of transition tax liability, respectively, was recorded in "income taxes and other liabilities" on PMI's consolidated balance sheets.
At December 31, 2023, applicable U.S. federal income taxes have not been provided on approximately $0.6 billion of accumulated earnings of Swedish Match subsidiaries that are expected to be permanently reinvested. PMI does not foresee a need to repatriate these earnings since its U.S. cash requirements are supported by distributions of earnings from PMI foreign entities that have not been designated as permanently reinvested and existing credit facilities. At December 31, 2023, PMI has determined the amount of deferred tax liabilities related to these unremitted Swedish Match earnings is approximately $71 million.
At December 31, 2023 and 2022, U.S. federal and foreign deferred income taxes have been provided on all accumulated earnings of PMI's foreign subsidiaries.
PMI is regularly examined by tax authorities around the world and is currently under examination in a number of jurisdictions. The U.S. federal statute of limitations on assessment remains open for the years 2019 and onward. Foreign and U.S. state jurisdictions have statutes of limitations generally ranging from 3 to 5 years after the filing of a return. Years still open to examination by foreign tax authorities in major jurisdictions include Germany (2018 onward), Indonesia (2019 onward), Italy (2017 onward), Russia (2020 onward) and Switzerland (2019 onward).
At December 31, 2023, subsidiaries of PMI in Indonesia, principally PT Hanjaya Mandala Sampoerna Tbk ("HMS"), have recorded income tax receivables in the amount of 4.0 trillion Indonesian rupiah (approximately $255 million) relating to corporate income tax assessments paid to avoid potential penalties, primarily for domestic and other intercompany transactions for the years 2014 to 2020. Objection letters have been filed with the Tax Office and these assessments are being challenged at various levels in court. These income tax receivables are included in other assets in PMI’s consolidated balance sheets at December 31, 2023.
It is reasonably possible that within the next 12 months certain tax examinations will close, which could result in a change in unrecognized tax benefits along with related interest and penalties. An estimate of any possible change cannot be made at this time.
A reconciliation of the beginning and ending amount of unrecognized tax benefits was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
2023 |
|
2022 |
|
2021 |
| Balance at January 1, |
$ |
72 |
|
|
$ |
89 |
|
|
$ |
72 |
|
Additions based on tax positions related to the current year |
7 |
|
|
12 |
|
|
12 |
|
Additions for tax positions of previous years |
1 |
|
|
2 |
|
|
15 |
|
Reductions for tax positions of prior years |
(23) |
|
|
(18) |
|
|
(1) |
|
Reductions due to lapse of statute of limitations |
(3) |
|
|
(6) |
|
|
(3) |
|
Settlements |
— |
|
|
(4) |
|
|
— |
|
Other |
1 |
|
|
(3) |
|
|
(6) |
|
| Balance at December 31, |
$ |
55 |
|
|
$ |
72 |
|
|
$ |
89 |
|
Unrecognized tax benefits and PMI’s liability for contingent income taxes, interest and penalties were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
December 31, 2023 |
|
December 31, 2022 |
|
December 31, 2021 |
Unrecognized tax benefits |
$ |
55 |
|
|
$ |
72 |
|
|
$ |
89 |
|
Accrued interest and penalties |
9 |
|
|
13 |
|
|
18 |
|
Tax credits and other indirect benefits |
(1) |
|
|
(3) |
|
|
(7) |
|
Liability for tax contingencies |
$ |
63 |
|
|
$ |
82 |
|
|
$ |
100 |
|
The amount of unrecognized tax benefits that, if recognized, would impact the effective tax rate was $55 million at December 31, 2023. The remainder, if recognized, would principally affect deferred taxes.
For the years ended December 31, 2023, 2022 and 2021, PMI recognized income (expense) in its consolidated statements of earnings of $5 million, $2 million and $(3) million, respectively, related to interest and penalties associated with uncertain tax positions.
The effective income tax rate on pre-tax earnings differed from the U.S. federal statutory rate for the following reasons for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| U.S. federal statutory rate |
21.0 |
% |
|
21.0 |
% |
|
21.0 |
% |
| Increase (decrease) resulting from: |
|
|
|
|
|
| Foreign rate differences |
(1.0) |
|
|
(0.5) |
|
|
(0.3) |
|
| Dividend repatriation cost |
0.8 |
|
|
0.7 |
|
|
0.6 |
|
|
|
|
|
|
|
| Global intangible low-taxed income |
2.0 |
|
|
1.0 |
|
|
0.8 |
|
| U.S. state taxes |
(0.1) |
|
|
0.1 |
|
|
0.2 |
|
| Foreign derived intangible income |
(0.9) |
|
|
(0.8) |
|
|
(0.7) |
|
| Foreign exchange |
(1.6) |
|
|
(1.7) |
|
|
— |
|
| Non-deductible goodwill impairment |
1.3 |
|
|
— |
|
|
— |
|
| Unremitted earnings of Russian subsidiaries |
1.7 |
|
|
— |
|
|
— |
|
| Other |
(0.8) |
|
|
(0.5) |
|
|
0.2 |
|
| Effective tax rate |
22.4 |
% |
|
19.3 |
% |
|
21.8 |
% |
The 2023 effective tax rate increased 3.1 percentage points to 22.4%. The change in the effective tax rate for 2023, as compared to 2022, was unfavorably impacted by: (i) an increase in deferred tax liabilities related to the unremitted earnings of PMI's Russian subsidiaries due to the unilateral suspension of certain Russian double tax treaties by the Russian authorities on August 8, 2023, with respect to certain payments including dividends; (ii) the non-deductible Wellness and Healthcare goodwill impairment charge and (iii) an increase in foreign tax credit limitation related to GILTI, partially offset by changes in earnings mix by taxing jurisdiction.
The 2022 effective tax rate decreased 2.5 percentage points to 19.3%. The change in the effective tax rate for 2022, as compared to 2021, was favorably impacted by changes in income tax reserves, a deferred tax benefit for unrealized foreign currency losses on intercompany loans related to the Swedish Match acquisition financing reflected in the consolidated statements of earnings ($203 million), while the underlying pre-tax foreign currency movements fully offset in the consolidated statements of earnings and were reflected as currency translation adjustments in its consolidated statements of stockholders' (deficit) equity, and by a reduction in deferred tax liabilities related to pension plan assets ($40 million), partially offset by an increase in deferred tax liabilities related to the fair value adjustment of equity securities held by PMI ($10 million). For further details, see Note 6. Related Parties - Equity Investments and Other.
The tax effects of temporary differences that gave rise to deferred income tax assets and liabilities consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, |
(in millions) |
2023 |
|
2022 |
| Deferred income tax assets: |
|
|
|
| Accrued postretirement and postemployment benefits |
$ |
223 |
|
|
$ |
217 |
|
| Accrued pension costs |
450 |
|
|
277 |
|
| Inventory |
27 |
|
|
22 |
|
| Accrued liabilities |
191 |
|
|
158 |
|
| Net operating loss, tax credit, and other carryforwards |
501 |
|
|
384 |
|
| Foreign exchange |
149 |
|
|
— |
|
| Other |
19 |
|
|
— |
|
| Total deferred income tax assets |
1,560 |
|
|
1,058 |
|
| Less: valuation allowance |
(369) |
|
|
(378) |
|
| Deferred income tax assets, net of valuation allowance |
1,191 |
|
|
680 |
|
| Deferred income tax liabilities: |
|
|
|
| Intangible assets |
(2,136) |
|
|
(1,485) |
|
| Property, plant and equipment |
(218) |
|
|
(200) |
|
| Unremitted earnings |
(358) |
|
|
(141) |
|
| Foreign exchange |
— |
|
|
(175) |
|
| Other |
— |
|
|
(32) |
|
Total deferred income tax liabilities |
(2,712) |
|
|
(2,033) |
|
| Net deferred income tax assets (liabilities) |
$ |
(1,521) |
|
|
$ |
(1,353) |
|
At December 31, 2023, PMI recorded deferred tax assets for net operating loss, tax credit, and other carryforwards of $501 million, with varying dates of expiration, primarily after 2028, including $274 million with an unlimited carryforward period. At December 31, 2023, PMI has recorded a valuation allowance of $369 million against deferred tax assets that do not meet the more-likely-than not recognition threshold.
At December 31, 2022, PMI recorded deferred tax assets for net operating loss, tax credit, and other carryforwards of $384 million, with varying dates of expiration, primarily after 2027, including $173 million with an unlimited carryforward period. At December 31, 2022, PMI has recorded a valuation allowance of $378 million against deferred tax assets that do not meet the more-likely-than-not recognition threshold.
Segment Reporting:
PMI’s subsidiaries and affiliates are primarily engaged in the manufacture and sale of cigarettes and smoke-free products, including heat-not-burn, e-vapor and oral nicotine products. Excluding the Wellness and Healthcare segment and the 2022 acquisition of Swedish Match, PMI's segments are generally organized by geographic region and managed by segment managers who are responsible for the operating and financial results of the regions inclusive of combustible tobacco and smoke-free product categories sold in the region. Effective in January 2023, PMI began managing its business in four geographical segments, down from six previously, in addition to its continuing Swedish Match and Wellness and Healthcare segments. The four geographical segments are as follows: Europe Region; South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA"); East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF"); and Americas Region.
The Swedish Match segment represents the fourth quarter 2022 acquisition of the company. The Wellness and Healthcare segment reflects the operating results of Vectura Fertin Pharma. For further details on these acquisitions, see Note 3. Acquisitions. PMI records net revenues and operating income to its geographical segments based upon the geographic area in which the customer resides.
PMI’s chief operating decision maker evaluates geographical segment performance and allocates resources based on regional operating income, which includes results from all product categories sold in each region, excluding Swedish Match and Wellness and Healthcare products. Business operations in the Swedish Match segment and the Wellness and Healthcare segment are evaluated separately. Interest expense, net, and provision for income taxes are centrally managed and, accordingly, such items are not presented by segment since they are excluded from the measure of segment profitability reviewed by management. Information about total assets by segment is not disclosed because such information is not reported to or used by PMI’s chief operating decision maker. Segment goodwill and other intangible assets, net, are disclosed in Note 5. Goodwill and Other Intangible Assets, net. The accounting policies of the segments are the same as those described in Note 2. Summary of Significant Accounting Policies.
PMI disaggregates its net revenues from contracts with customers by product category for each of PMI's four geographical segments and for the Swedish Match segment. For the Wellness and Healthcare business, Vectura Fertin Pharma discussed above, net revenues from contracts with customers are included in the Wellness and Healthcare segment. PMI believes this best depicts how the nature, amount, timing and uncertainty of its revenue and cash flows are affected by economic factors.
Net revenues by segment were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in millions) |
2023 |
|
2022 |
|
2021 |
| Net revenues: |
|
|
|
|
|
| Europe |
$ |
13,598 |
|
|
$ |
12,869 |
|
|
$ |
13,155 |
|
SSEA, CIS & MEA |
10,629 |
|
|
10,467 |
|
|
9,858 |
|
EA, AU & PMI DF |
6,201 |
|
|
5,936 |
|
|
6,448 |
|
| Americas |
1,944 |
|
|
1,903 |
|
|
1,843 |
|
| Swedish Match |
2,496 |
|
|
316 |
|
|
— |
|
| Wellness and Healthcare |
306 |
|
|
271 |
|
|
101 |
|
| Net revenues |
$ |
35,174 |
|
|
$ |
31,762 |
|
|
$ |
31,405 |
|
Total net revenues attributable to customers located in Japan, PMI's largest market in terms of net revenues, were $3.9 billion, $3.9 billion and $4.6 billion in 2023, 2022 and 2021, respectively. PMI had one customer in the EA, AU & PMI DF segment that accounted for 11%, 12% and 15% of PMI’s consolidated net revenues, and one customer in the Europe segment that accounted for 12%, 13% and 13% of PMI’s consolidated net revenues in 2023, 2022 and 2021, respectively.
PMI's net revenues by product category were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in millions) |
2023 |
|
2022 |
|
2021 |
| Combustible tobacco products: |
|
|
|
|
|
| Europe |
$ |
8,037 |
|
|
$ |
7,694 |
|
|
$ |
8,767 |
|
SSEA, CIS & MEA |
9,321 |
|
|
9,173 |
|
|
8,734 |
|
EA, AU & PMI DF |
2,676 |
|
|
2,831 |
|
|
2,861 |
|
| Americas |
1,869 |
|
|
1,804 |
|
|
1,706 |
|
| Swedish Match |
431 |
|
|
70 |
|
|
— |
|
| Total combustible tobacco products |
22,334 |
|
|
21,572 |
|
|
22,067 |
|
| Smoke-free products: |
|
|
|
|
|
| Smoke-free products excluding Wellness and Healthcare: |
|
|
|
|
|
| Europe |
5,561 |
|
|
5,175 |
|
|
4,388 |
|
SSEA, CIS & MEA |
1,308 |
|
|
1,294 |
|
|
1,124 |
|
EA, AU & PMI DF |
3,525 |
|
|
3,105 |
|
|
3,587 |
|
| Americas |
75 |
|
|
99 |
|
|
137 |
|
| Swedish Match |
2,065 |
|
|
246 |
|
|
— |
|
| Total smoke-free products excluding Wellness and Healthcare |
12,534 |
|
|
9,919 |
|
|
9,237 |
|
| Wellness and Healthcare |
306 |
|
|
271 |
|
|
101 |
|
| Total smoke-free products |
12,840 |
|
|
10,190 |
|
|
9,338 |
|
|
|
|
|
|
|
| Total PMI net revenues |
$ |
35,174 |
|
|
$ |
31,762 |
|
|
$ |
31,405 |
|
Note: Sum of product categories or Regions might not foot to total PMI due to roundings.
Net revenues related to combustible tobacco products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of PMI's cigarettes and other tobacco products that are combusted. Other tobacco products primarily include roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos and do not include smoke-free products.
Net revenues related to smoke-free products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes, if applicable. These net revenue amounts consist of the sale of all of PMI's products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral nicotine, also including wellness and healthcare products, as well as consumer accessories such as lighters and matches.
Net revenues related to wellness and healthcare products consist of operating revenues generated from the sale of products primarily associated with inhaled therapeutics, and oral and intra-oral delivery systems that are included in the operating results of PMI's Wellness and Healthcare business, Vectura Fertin Pharma.
Operating income (loss) by segment were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
Operating income (loss): |
|
|
|
|
|
| Europe |
$ |
6,012 |
|
|
$ |
5,802 |
|
|
$ |
6,409 |
|
SSEA, CIS & MEA |
3,047 |
|
|
3,864 |
|
|
3,295 |
|
EA, AU & PMI DF |
2,481 |
|
|
2,424 |
|
|
2,836 |
|
| Americas |
62 |
|
|
436 |
|
|
487 |
|
| Swedish Match |
824 |
|
|
(22) |
|
|
— |
|
| Wellness and Healthcare |
(870) |
|
|
(258) |
|
|
(52) |
|
Operating income |
$ |
11,556 |
|
|
$ |
12,246 |
|
|
$ |
12,975 |
|
Items affecting the comparability of results from operations were as follows:
•Impairment of goodwill and other intangibles – For the year ended December 31, 2023, PMI recorded $680 million of goodwill and non-amortizable intangible assets impairment charges that was included in the Wellness and Healthcare segment. For the year ended December 31, 2022, PMI recorded an impairment charge related to definite-lived intangible assets of $112 million. This charge was included in the Wellness and Healthcare segment. For further details, see Note 5. Goodwill and Other Intangible Assets, net.
•South Korea indirect tax charge – See Note 18. Contingencies for details of the $204 million pre-tax charge included in the EA, AU & PMI DF segment results for the year ended December 31, 2023.
•Termination of distribution arrangement in the Middle East – In the first quarter of 2023, PMI recorded a pre-tax charge of $80 million following the termination of a distribution arrangement in the Middle East. This pre-tax charge was recorded as a reduction of net revenues in the consolidated statements of earnings, and was included in the SSEA, CIS & MEA segment results for the year ended December 31, 2023.
•Charges related to the war in Ukraine - See Note 4. War in Ukraine for details of the $53 million and $151 million pre-tax charges in the Europe segment for the years ended December 31, 2023 and 2022, respectively.
•Swedish Match AB acquisition accounting related item - See Note 3. Acquisitions for details of the $18 million and $125 million pre-tax purchase accounting adjustments related to the sale of acquired inventories stepped up to fair value included in the Swedish Match segment for the years ended December 31, 2023 and 2022, respectively.
•Asset impairment and exit costs - See Note 20. Asset Impairment and Exit Costs for details of the $109 million and $216 million pre-tax charges for the year ended December 31, 2023 and 2021, respectively, as well as a breakdown of these costs by segment.
•Termination of agreement with Foundation for a Smoke-Free World – On September 29, 2023, PMI and the Foundation for a Smoke-Free World (the "Foundation") entered into the Final Grant Agreement and Termination of the Second Amended and Restated Pledge Agreement ("Agreement"). Under the terms of the agreement, PMI paid $140 million in the third quarter of 2023 in return for the termination of the pledge agreement between the parties. As a result, in the third quarter of 2023, PMI recorded a pre-tax charge of $140 million commensurate with the early termination of the pledge agreement. The pre-tax charge was recorded in marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2023 and was included in the operating results of the following segments: Europe ($62 million); SSEA, CIS & MEA ($44 million); EA, AU & PMI DF ($27 million); and Americas ($7 million).
•Saudi Arabia customs assessments - In June 2021, PMI recorded a pre-tax charge of $246 million in relation to additional customs duties in Saudi Arabia assessed for the periods of 2014 through 2020 in line with existing and contemplated arrangements with our distributors. In accordance with U.S. GAAP, the charge was recorded as a reduction in net revenues of combustible tobacco products included in the SSEA, CIS & MEA segment for the year ended December 31, 2021.
•Asset acquisition cost - See Note 3. Acquisitions for the details of the $51 million pre-tax charge associated with the asset acquisition of OtiTopic, Inc. included in the Wellness and Healthcare segment within the operating income table above for the year ended December 31, 2021.
Other segment data were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
Depreciation and amortization expense: |
|
|
|
|
|
| Europe |
$ |
333 |
|
|
$ |
377 |
|
|
$ |
371 |
|
| SSEA, CIS & MEA |
309 |
|
|
340 |
|
|
354 |
|
| EA, AU & PMI DF |
148 |
|
|
167 |
|
|
168 |
|
| Americas |
77 |
|
|
74 |
|
|
71 |
|
| Swedish Match |
447 |
|
|
34 |
|
|
— |
|
| Wellness and Healthcare |
84 |
|
|
85 |
|
|
34 |
|
Total depreciation and amortization expense |
$ |
1,398 |
|
|
$ |
1,077 |
|
|
$ |
998 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
Capital expenditures: |
|
|
|
|
|
| Europe |
$ |
778 |
|
|
$ |
642 |
|
|
$ |
481 |
|
| SSEA, CIS & MEA |
287 |
|
|
258 |
|
|
149 |
|
| EA, AU & PMI DF |
38 |
|
|
25 |
|
|
36 |
|
| Americas |
57 |
|
|
92 |
|
|
54 |
|
| Swedish Match |
127 |
|
|
15 |
|
|
— |
|
| Wellness and Healthcare |
34 |
|
|
45 |
|
|
28 |
|
Total capital expenditures |
$ |
1,321 |
|
|
$ |
1,077 |
|
|
$ |
748 |
|
PMI’s total property, plant and equipment, net and other assets by geographic area were:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
Long-lived assets: |
|
|
|
|
|
| Europe |
$ |
5,697 |
|
|
$ |
5,179 |
|
|
$ |
4,918 |
|
| SSEA, CIS & MEA |
2,197 |
|
|
2,047 |
|
|
2,181 |
|
| East Asia and Australia |
481 |
|
|
675 |
|
|
742 |
|
| Americas |
1,310 |
|
|
1,282 |
|
|
666 |
|
| Total long-lived assets |
9,685 |
|
|
9,183 |
|
|
8,507 |
|
| Altria Group, Inc. agreement |
2,777 |
|
|
1,002 |
|
|
— |
|
| Financial instruments |
701 |
|
|
456 |
|
|
210 |
|
Total property, plant and equipment, net and Other assets |
$ |
13,163 |
|
|
$ |
10,641 |
|
|
$ |
8,717 |
|
Long-lived assets consist of non-current assets other than goodwill; other intangible assets, net; deferred tax assets, equity investments, financial instruments and payment under the agreement with Altria Group, Inc., see Note 3, Acquisitions and Note 18, Contingencies. PMI's largest markets in terms of long-lived assets are Switzerland, Indonesia and Italy. Total long-lived assets located in Switzerland, which is reflected in the Europe segment above, were $1.6 billion, $1.4 billion and $1.3 billion at December 31, 2023, 2022 and 2021, respectively. Total long-lived assets located in Indonesia, which is reflected in the SSEA, CIS & MEA segment above, were $1.1 billion, $0.9 billion and $0.9 billion at December 31, 2023, 2022 and 2021, respectively. Total long-lived assets located in Italy, which is reflected in the Europe segment above, were $1.0 billion, $0.9 billion and $0.9 billion at December 31, 2023, 2022 and 2021, respectively.
Benefit Plans:
Pension coverage for employees of PMI’s subsidiaries is provided, to the extent deemed appropriate, through separate plans, many of which are governed by local statutory requirements. In addition, PMI provides health care and other benefits to certain U.S. retired employees and certain non-U.S. retired employees. In general, health care benefits for non-U.S. retired employees are covered through local government plans.
Pension and other employee benefit costs per the consolidated statements of earnings consisted of the following for December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
2023 |
|
2022 |
|
2021 |
| Net pension costs (income) |
$ |
(84) |
|
|
$ |
(93) |
|
|
$ |
(1) |
|
| Net postemployment costs |
117 |
|
|
107 |
|
|
108 |
|
| Net postretirement costs |
12 |
|
|
10 |
|
|
8 |
|
| Total pension and other employee benefit costs |
$ |
45 |
|
|
$ |
24 |
|
|
$ |
115 |
|
Pension and Postretirement Benefit Plans
Obligations and Funded Status
The projected benefit obligations, plan assets and funded status of PMI’s pension plans, and the accumulated benefit obligation, plan assets and net amount accrued for PMI's postretirement health care plans, at December 31, 2023 and 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension(1) |
|
Postretirement |
(in millions) |
2023 |
|
2022 |
|
2023 |
|
2022 |
| Benefit obligation at January 1 |
$ |
8,606 |
|
|
$ |
10,998 |
|
|
$ |
229 |
|
|
$ |
198 |
|
Service cost |
174 |
|
|
233 |
|
|
4 |
|
|
2 |
|
Interest cost |
258 |
|
|
78 |
|
|
12 |
|
|
6 |
|
Benefits paid |
(520) |
|
|
(429) |
|
|
(13) |
|
|
(9) |
|
| Employee contributions |
145 |
|
|
141 |
|
|
— |
|
|
— |
|
Settlement, curtailment and plan amendment |
(17) |
|
|
(17) |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
Actuarial losses (gains) |
1,209 |
|
|
(2,294) |
|
|
24 |
|
|
(46) |
|
Currency |
763 |
|
|
(434) |
|
|
(4) |
|
|
(5) |
|
| Acquisition of Swedish Match |
— |
|
|
316 |
|
|
— |
|
|
85 |
|
Other |
(51) |
|
|
14 |
|
|
(6) |
|
|
(2) |
|
Benefit obligation at December 31, |
10,567 |
|
|
8,606 |
|
|
246 |
|
|
229 |
|
Fair value of plan assets at January 1, |
7,939 |
|
|
9,337 |
|
|
3 |
|
|
— |
|
Actual return on plan assets |
643 |
|
|
(1,061) |
|
|
— |
|
|
— |
|
Employer contributions, net of refunds |
21 |
|
|
(3) |
|
|
13 |
|
|
9 |
|
Employee contributions |
145 |
|
|
141 |
|
|
— |
|
|
— |
|
Benefits paid |
(520) |
|
|
(429) |
|
|
(13) |
|
|
(9) |
|
Settlement |
(17) |
|
|
(14) |
|
|
— |
|
|
— |
|
Currency |
639 |
|
|
(333) |
|
|
— |
|
|
— |
|
| Acquisition of Swedish Match |
— |
|
|
303 |
|
|
— |
|
|
3 |
|
| Other |
1 |
|
|
(2) |
|
|
— |
|
|
— |
|
Fair value of plan assets at December 31, |
8,851 |
|
|
7,939 |
|
|
3 |
|
|
3 |
|
Net pension and postretirement liability recognized at December 31, |
$ |
(1,716) |
|
|
$ |
(667) |
|
|
$ |
(243) |
|
|
$ |
(226) |
|
(1) Primarily non-U.S. based defined benefit retirement plans.
At December 31, 2023 , actuarial losses (gains) consisted primarily of losses for assumption changes related to lower discount rates year-over-year for Swiss, German and Dutch plans. At December 31, 2022 actuarial losses (gains) consisted primarily of gains for assumption changes related to higher discount rates year-over-year for Swiss, German and Dutch plans.
At December 31, 2023 and 2022, the Swiss pension plan represented 67% and 64% of the benefit obligation, respectively, and approximately 62% and 60% of the fair value of plan assets at December 31, 2023 and 2022, respectively. At December 31, 2023 and 2022, the U.S. pension plans represented 6% and 7% of the benefit obligation, respectively, and approximately 6% and 6% of the fair value of plan assets at December 31, 2023 and 2022, respectively.
At December 31, 2023 and 2022, the amounts recognized on PMI's consolidated balance sheets for the pension and postretirement plans were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension |
|
Postretirement |
(in millions) |
2023 |
|
2022 |
|
2023 |
|
2022 |
Other assets |
$ |
294 |
|
|
$ |
410 |
|
|
|
|
|
Accrued liabilities — employment costs |
(31) |
|
|
(32) |
|
|
$ |
(12) |
|
|
$ |
(11) |
|
Long-term employment costs |
(1,979) |
|
|
(1,045) |
|
|
(231) |
|
|
(215) |
|
|
$ |
(1,716) |
|
|
$ |
(667) |
|
|
$ |
(243) |
|
|
$ |
(226) |
|
The accumulated benefit obligation, which represents benefits earned to date, for the pension plans was $10.0 billion and $8.2 billion at December 31, 2023 and 2022, respectively.
For pension plans with accumulated benefit obligations in excess of plan assets, the accumulated benefit obligation and fair value of plan assets were $8.8 billion and $7.2 billion, respectively, as of December 31, 2023. The accumulated benefit obligation and fair value of plan assets were $5.8 billion and $5.0 billion, respectively, as of December 31, 2022.
For pension plans with projected benefit obligations in excess of plan assets, the projected benefit obligation and fair value of plan assets were $9.2 billion and $7.2 billion, respectively, as of December 31, 2023. The projected benefit obligation and fair value of plan assets were $6.4 billion and $5.4 billion, respectively, as of December 31, 2022.
The following weighted-average assumptions were used to determine PMI’s pension and postretirement benefit obligations at December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension |
|
Postretirement |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
Discount rate |
2.28 |
% |
|
3.03 |
% |
|
5.19 |
% |
|
5.89 |
% |
Rate of compensation increase |
2.05 |
|
|
1.98 |
|
|
|
|
|
Interest crediting rate |
2.99 |
|
|
2.97 |
|
|
|
|
|
Health care cost trend rate assumed for next year |
|
|
|
|
6.54 |
|
|
6.14 |
|
Ultimate trend rate |
|
|
|
|
4.49 |
|
|
4.78 |
|
Year that rate reaches the ultimate trend rate |
|
|
|
|
2047 |
|
2046 |
The discount rate for the largest pension plans is based on a yield curve constructed from a portfolio of high quality corporate bonds that produces a cash flow pattern equivalent to each plan’s expected benefit payments. The discount rate for the remaining plans is developed from local bond indices that match local benefit obligations as closely as possible.
Components of Net Periodic Benefit Cost
Net periodic pension and postretirement health care costs consisted of the following for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension |
|
Postretirement |
(in millions) |
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
Service cost |
$ |
174 |
|
|
$ |
233 |
|
|
$ |
291 |
|
|
$ |
4 |
|
|
$ |
2 |
|
|
$ |
2 |
|
Interest cost |
258 |
|
|
78 |
|
|
50 |
|
|
12 |
|
|
6 |
|
|
5 |
|
Expected return on plan assets |
(365) |
|
|
(352) |
|
|
(371) |
|
|
— |
|
|
— |
|
|
— |
|
Amortization: |
|
|
|
|
|
|
|
|
|
|
|
Net losses |
18 |
|
|
181 |
|
|
314 |
|
|
(1) |
|
|
2 |
|
|
3 |
|
Prior service cost (credit) |
(2) |
|
|
(2) |
|
|
1 |
|
|
— |
|
|
— |
|
|
— |
|
Net transition obligation |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Settlement and curtailment |
7 |
|
|
2 |
|
|
5 |
|
|
1 |
|
|
2 |
|
|
— |
|
| Net periodic pension and postretirement costs |
$ |
90 |
|
|
$ |
140 |
|
|
$ |
290 |
|
|
$ |
16 |
|
|
$ |
12 |
|
|
$ |
10 |
|
Settlement and curtailment charges were due primarily to employee severance and early retirement programs.
The following weighted-average assumptions were used to determine PMI’s net pension and postretirement health care costs:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension |
|
Postretirement |
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
Discount rate - service cost |
3.27 |
% |
|
1.03 |
% |
|
0.72 |
% |
|
5.89 |
% |
|
3.08 |
% |
|
2.84 |
% |
Discount rate - interest cost |
3.03 |
|
|
0.71 |
|
|
0.44 |
|
|
5.89 |
|
|
3.08 |
|
|
2.84 |
|
Expected rate of return on plan assets |
4.42 |
|
|
4.17 |
|
|
4.43 |
|
|
|
|
|
|
|
Rate of compensation increase |
1.98 |
|
|
1.77 |
|
|
1.79 |
|
|
|
|
|
|
|
Interest crediting rate |
2.97 |
|
|
3.15 |
|
|
3.20 |
|
|
|
|
|
|
|
Health care cost trend rate |
|
|
|
|
|
|
6.14 |
|
|
6.27 |
|
|
6.21 |
|
PMI’s expected rate of return on pension plan assets is determined by the plan assets’ historical long-term investment performance, current asset allocation and estimates of future long-term returns by asset class.
PMI and certain of its subsidiaries sponsor defined contribution plans. Amounts charged to expense for defined contribution plans totaled $111 million, $82 million and $71 million for the years ended December 31, 2023, 2022 and 2021, respectively.
Plan Assets
PMI’s investment strategy for pension plans is based on an expectation that equity securities will outperform debt securities over the long term. Accordingly, the target allocation of PMI’s plan assets is broadly characterized as approximately 55% in equity securities and approximately 45% in debt securities and other assets. The strategy primarily utilizes indexed U.S. equity securities, international equity securities and investment-grade debt securities. PMI attempts to mitigate investment risk by rebalancing between equity and debt asset classes once a year or as PMI’s contributions and benefit payments are made.
The fair value of PMI’s pension plan assets at December 31, 2023 and 2022, by asset category was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Asset Category
(in millions) |
At December 31, 2023 |
|
Quoted Prices
In Active
Markets for
Identical
Assets/Liabilities
(Level 1) |
|
Significant
Other
Observable
Inputs
(Level 2) |
|
Significant
Unobservable
Inputs
(Level 3) |
|
Cash and cash equivalents |
$ |
117 |
|
|
$ |
117 |
|
|
|
|
|
|
Equity securities: |
|
|
|
|
|
|
|
|
U.S. securities |
158 |
|
|
158 |
|
|
|
|
|
|
International securities |
569 |
|
|
569 |
|
|
|
|
|
|
Investment funds(a) |
7,123 |
|
|
5,366 |
|
|
$ |
1,757 |
|
|
|
|
Government bonds |
255 |
|
|
183 |
|
|
72 |
|
|
|
|
Corporate bonds |
320 |
|
|
320 |
|
|
|
|
|
|
Other |
37 |
|
|
— |
|
|
5 |
|
|
32 |
|
(c) |
Total assets in the fair value hierarchy |
$ |
8,579 |
|
|
$ |
6,713 |
|
|
$ |
1,834 |
|
|
$ |
32 |
|
|
Investment funds measured at net asset value(b) |
272 |
|
|
|
|
|
|
|
|
Total assets |
$ |
8,851 |
|
|
|
|
|
|
|
|
(a) Investment funds whose objective seeks to replicate the returns and characteristics of specified market indices (primarily MSCI — Europe, Switzerland, North America, Asia Pacific, Japan, Emerging Markets for equities, and FTSE EMU, FTSE Non-EGBI EuroBIG, SBI AAA-BBB and JP Morgan EMBI for bonds), primarily consist of mutual funds, common trust funds and commingled funds. Of these funds, 57% are invested in U.S. and international equities; 15% are invested in U.S. and international government bonds; 15% are invested in corporate bonds and 13% are invested in real estate.
(b) In accordance with FASB ASC Subtopic 820-10, certain investments measured at fair value using the net asset value per share practical expedient have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented in the statement of financial position.
(c) Amount relates to annuity policies of which the fair value is calculated using an actuarial model.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Asset Category
(in millions) |
At December 31, 2022 |
|
Quoted Prices
In Active
Markets for
Identical
Assets/Liabilities
(Level 1) |
|
Significant
Other
Observable
Inputs
(Level 2) |
|
Significant
Unobservable
Inputs
(Level 3) |
|
Cash and cash equivalents |
$ |
79 |
|
|
$ |
79 |
|
|
|
|
|
|
Equity securities: |
|
|
|
|
|
|
|
|
U.S. securities |
140 |
|
|
140 |
|
|
|
|
|
|
International securities |
521 |
|
|
521 |
|
|
|
|
|
|
Investment funds(a) |
6,419 |
|
|
4,870 |
|
|
$ |
1,549 |
|
|
|
|
Government bonds |
178 |
|
|
117 |
|
|
61 |
|
|
|
|
Corporate bonds |
302 |
|
|
302 |
|
|
|
|
|
|
Other |
35 |
|
|
— |
|
|
3 |
|
|
32 |
|
(c) |
Total assets in the fair value hierarchy |
$ |
7,674 |
|
|
$ |
6,029 |
|
|
$ |
1,613 |
|
|
$ |
32 |
|
|
Investment funds measured at net asset value(b) |
265 |
|
|
|
|
|
|
|
|
Total assets |
$ |
7,939 |
|
|
|
|
|
|
|
|
(a) Investment funds whose objective seeks to replicate the returns and characteristics of specified market indices (primarily MSCI — Europe, Switzerland, North America, Asia Pacific, Japan; Russell 3000, S&P 500 for equities and Citigroup EMU, Citigroup Non-EGBI EuroBIG, SBI AAA-BBB and JP Morgan EMBI for bonds), primarily consist of mutual funds, common trust funds and commingled funds. Of these funds, 57% were invested in U.S. and international equities; 15% were invested in U.S. and international government bonds; 16% were invested in corporate bonds, and 12% were invested in real estate.
(b) In accordance with FASB ASC Subtopic 820-10, certain investments measured at fair value using the net asset value per share practical expedient have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented in the statement of financial position.
(c) Amount relates to annuity policies of which the fair value is calculated using an actuarial model.
For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Note 2. Summary of Significant Accounting Policies.
PMI makes, and plans to make, contributions, to the extent that they are tax deductible and meet specific funding requirements of its funded pension plans. Currently, PMI anticipates making contributions of approximately $119 million in 2024 to its pension plans, based on current tax and benefit laws. However, this estimate is subject to change as a result of changes in tax and other benefit laws, as well as asset performance significantly above or below the assumed long-term rate of return on pension assets, or changes in interest and currency rates.
The estimated future benefit payments from PMI pension plans at December 31, 2023, are as follows:
|
|
|
|
|
|
(in millions) |
|
| 2024 |
$ |
417 |
|
| 2025 |
430 |
|
| 2026 |
428 |
|
| 2027 |
438 |
|
| 2028 |
457 |
|
| 2029 - 2033 |
2,490 |
|
PMI's expected future annual benefit payments for its postretirement health care plans are estimated to be not material through 2032.
Postemployment Benefit Plans
PMI and certain of its subsidiaries sponsor postemployment benefit plans covering certain designated salaried and hourly employees. The cost of these plans is charged to expense over the working life of the covered employees. Net postemployment costs were $213 million, $184 million and $228 million for the years ended December 31, 2023, 2022 and 2021, respectively.
The amounts recognized in accrued postemployment costs net of plan assets on PMI's consolidated balance sheets at December 31, 2023 and 2022, were $915 million and $807 million, respectively.
The accrued postemployment costs were determined using a weighted-average discount rate of 4.3% and 5.6% in 2023 and 2022, respectively; an assumed ultimate annual weighted-average turnover rate of 2.8% and 2.9% in 2023 and 2022, respectively; assumed compensation cost increases of 2.4% in 2023 and 2.8% in 2022, and assumed benefits as defined in the respective plans. In accordance with local regulations, certain postemployment plans are funded. As a result, the accrued postemployment costs disclosed above are presented net of the related assets of $33 million and $30 million at December 31, 2023 and 2022, respectively. Postemployment costs arising from actions that offer employees benefits in excess of those specified in the respective plans are charged to expense when incurred.
Comprehensive Earnings (Losses)
The amounts recorded in accumulated other comprehensive losses at December 31, 2023, consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
Pension |
|
Post-
retirement |
|
Post-
employment |
|
Total |
Net (losses) gains |
$ |
(2,325) |
|
|
$ |
(36) |
|
|
$ |
(770) |
|
|
$ |
(3,131) |
|
Prior service (cost) credit |
77 |
|
|
1 |
|
|
(21) |
|
|
57 |
|
Net transition (obligation) asset |
(3) |
|
|
— |
|
|
— |
|
|
(3) |
|
Deferred income taxes |
283 |
|
|
19 |
|
|
186 |
|
|
488 |
|
Losses to be amortized |
$ |
(1,968) |
|
|
$ |
(16) |
|
|
$ |
(605) |
|
|
$ |
(2,589) |
|
The amounts recorded in accumulated other comprehensive losses at December 31, 2022, consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
Pension |
|
Post-
retirement |
|
Post-
employment |
|
Total |
Net (losses) gains |
$ |
(1,437) |
|
|
$ |
(14) |
|
|
$ |
(753) |
|
|
$ |
(2,204) |
|
Prior service (cost) credit |
70 |
|
|
1 |
|
|
(21) |
|
|
50 |
|
Net transition (obligation) asset |
(3) |
|
|
— |
|
|
— |
|
|
(3) |
|
Deferred income taxes |
138 |
|
|
14 |
|
|
183 |
|
|
335 |
|
Losses to be amortized |
$ |
(1,232) |
|
|
$ |
1 |
|
|
$ |
(591) |
|
|
$ |
(1,822) |
|
The amounts recorded in accumulated other comprehensive losses at December 31, 2021, consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
Pension |
|
Post-
retirement |
|
Post-
employment |
|
Total |
Net (losses) gains |
$ |
(2,495) |
|
|
$ |
(64) |
|
|
$ |
(884) |
|
|
$ |
(3,443) |
|
Prior service (cost) credit |
71 |
|
|
1 |
|
|
(22) |
|
|
50 |
|
Net transition (obligation) asset |
(3) |
|
|
— |
|
|
— |
|
|
(3) |
|
Deferred income taxes |
278 |
|
|
24 |
|
|
214 |
|
|
516 |
|
Losses to be amortized |
$ |
(2,149) |
|
|
$ |
(39) |
|
|
$ |
(692) |
|
|
$ |
(2,880) |
|
The movements in other comprehensive earnings (losses) during the year ended December 31, 2023, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
Pension |
|
Post-
retirement |
|
Post-
employment |
|
Total |
Amounts transferred to earnings: |
|
|
|
|
|
|
|
Amortization: |
|
|
|
|
|
|
|
Net losses (gains) |
$ |
19 |
|
|
$ |
1 |
|
|
$ |
76 |
|
|
$ |
96 |
|
Prior service cost (credit) |
7 |
|
|
— |
|
|
— |
|
|
7 |
|
Net transition obligation (asset) |
— |
|
|
— |
|
|
— |
|
|
— |
|
Other income/expense: |
|
|
|
|
|
|
|
Net losses (gains) |
11 |
|
|
1 |
|
|
— |
|
|
12 |
|
Prior service cost (credit) |
— |
|
|
— |
|
|
— |
|
|
— |
|
Deferred income taxes |
(9) |
|
|
(1) |
|
|
(18) |
|
|
(28) |
|
|
28 |
|
|
1 |
|
|
58 |
|
|
87 |
|
Other movements during the year: |
|
|
|
|
|
|
|
Net (losses) gains |
(918) |
|
|
(24) |
|
|
(93) |
|
|
(1,035) |
|
Prior service (cost) credit |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred income taxes |
154 |
|
|
6 |
|
|
21 |
|
|
181 |
|
|
(764) |
|
|
(18) |
|
|
(72) |
|
|
(854) |
|
Total movements in other comprehensive earnings (losses) |
$ |
(736) |
|
|
$ |
(17) |
|
|
$ |
(14) |
|
|
$ |
(767) |
|
The movements in other comprehensive earnings (losses) during the year ended December 31, 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
Pension |
|
Post-
retirement |
|
Post-
employment |
|
Total |
Amounts transferred to earnings: |
|
|
|
|
|
|
|
Amortization: |
|
|
|
|
|
|
|
Net losses (gains) |
$ |
178 |
|
|
$ |
3 |
|
|
$ |
85 |
|
|
$ |
266 |
|
Prior service cost (credit) |
(4) |
|
|
— |
|
|
— |
|
|
(4) |
|
|
|
|
|
|
|
|
|
Other income/expense: |
|
|
|
|
|
|
|
Net losses (gains) |
2 |
|
|
1 |
|
|
— |
|
|
3 |
|
Prior service cost (credit) |
— |
|
|
— |
|
|
1 |
|
|
1 |
|
Deferred income taxes |
(28) |
|
|
(1) |
|
|
(20) |
|
|
(49) |
|
|
148 |
|
|
3 |
|
|
66 |
|
|
217 |
|
Other movements during the year: |
|
|
|
|
|
|
|
Net (losses) gains |
878 |
|
|
46 |
|
|
46 |
|
|
970 |
|
Prior service (cost) credit |
3 |
|
|
— |
|
|
— |
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Deferred income taxes |
(112) |
|
|
(9) |
|
|
(11) |
|
|
(132) |
|
|
769 |
|
|
37 |
|
|
35 |
|
|
841 |
|
Total movements in other comprehensive earnings (losses) |
$ |
917 |
|
|
$ |
40 |
|
|
$ |
101 |
|
|
$ |
1,058 |
|
The movements in other comprehensive earnings (losses) during the year ended December 31, 2021, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
Pension |
|
Post-
retirement |
|
Post-
employment |
|
Total |
Amounts transferred to earnings: |
|
|
|
|
|
|
|
Amortization: |
|
|
|
|
|
|
|
Net losses (gains) |
$ |
294 |
|
|
$ |
4 |
|
|
$ |
85 |
|
|
$ |
383 |
|
Prior service cost (credit) |
7 |
|
|
(1) |
|
|
— |
|
|
6 |
|
|
|
|
|
|
|
|
|
Other income/expense: |
|
|
|
|
|
|
|
Net losses (gains) |
5 |
|
|
1 |
|
|
— |
|
|
6 |
|
Prior service cost (credit) |
— |
|
|
— |
|
|
— |
|
|
— |
|
| Deferred income taxes |
(51) |
|
|
(1) |
|
|
(20) |
|
|
(72) |
|
|
255 |
|
|
3 |
|
|
65 |
|
|
323 |
|
Other movements during the year: |
|
|
|
|
|
|
|
Net (losses) gains |
1,353 |
|
|
(5) |
|
|
(130) |
|
|
1,218 |
|
Prior service (cost) credit |
42 |
|
|
— |
|
|
— |
|
|
42 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Deferred income taxes |
(241) |
|
|
1 |
|
|
30 |
|
|
(210) |
|
|
1,154 |
|
|
(4) |
|
|
(100) |
|
|
1,050 |
|
Total movements in other comprehensive earnings (losses) |
$ |
1,409 |
|
|
$ |
(1) |
|
|
$ |
(35) |
|
|
$ |
1,373 |
|
Additional Information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
| Depreciation expense |
$ |
901 |
|
|
$ |
918 |
|
|
$ |
902 |
|
Research and development expense |
$ |
709 |
|
|
$ |
642 |
|
|
$ |
617 |
|
Advertising expense |
$ |
965 |
|
|
$ |
777 |
|
|
$ |
807 |
|
Foreign currency net transaction (gains)/losses |
$ |
305 |
|
|
$ |
199 |
|
|
$ |
45 |
|
Interest expense |
$ |
1,526 |
|
|
$ |
768 |
|
|
$ |
737 |
|
Interest income |
(465) |
|
|
(180) |
|
|
(109) |
|
Interest expense, net |
$ |
1,061 |
|
|
$ |
588 |
|
|
$ |
628 |
|
Financial Instruments:
Overview
PMI operates in markets primarily outside of the United States of America, with manufacturing and sales facilities in various locations around the world and is exposed to risks such as changes in foreign currency exchange rates and interest rates. As a result, PMI uses deliverable and non-deliverable forward foreign exchange contracts, foreign currency swaps and foreign currency options, (collectively referred to as "foreign exchange contracts"), and interest rate contracts to mitigate its exposure to changes in foreign currency exchange and interest rates related to net investments in foreign operations, third-party and intercompany actual and forecasted transactions. The primary currencies to which PMI is exposed include the Euro, Egyptian pound, Indonesian rupiah, Japanese yen, Mexican peso, Philippine peso, Russian ruble and Swiss franc.
Additionally, certain materials that PMI uses in the manufacturing of its products are exposed to market price risks. PMI uses commodity derivative contracts (“commodity contracts") to manage its exposure to the market price volatility of certain commodity components of these materials.
These foreign exchange contracts, interest rate contracts and commodity contracts are collectively referred to as "derivative contracts". PMI is not a party to leveraged derivatives and, by policy, does not use derivative financial instruments for speculative purposes. Substantially all of PMI's derivative financial instruments are subject to master netting arrangements, whereby the right to offset occurs in the event of default by a participating party. While these contracts contain the enforceable right to offset through close-out netting rights, PMI elects to present them on a gross basis in the consolidated balance sheets. Collateral associated with these arrangements is in the form of cash and is unrestricted. Financial instruments qualifying for hedge accounting must maintain a specified level of effectiveness between the hedging instrument and the item being hedged, both at inception and throughout the hedged period. PMI formally documents the nature and relationships between the hedging instruments and hedged items, as well as its risk-management objectives, strategies for undertaking the various hedge transactions and method of assessing hedge effectiveness. Additionally, for hedges of forecasted transactions, the significant characteristics and expected terms of the forecasted transaction must be specifically identified, and it must be probable that each forecasted transaction will occur. If it were deemed probable that the forecasted transaction would not occur, the gain or loss would be recognized in earnings.
The gross notional amounts for outstanding derivatives as of December 31, 2023 and 2022, were as follows:
|
|
|
|
|
|
|
|
|
| (in millions) |
2023 |
2022 |
| Derivative contracts designated as hedging instruments: |
|
|
| Foreign exchange contracts |
$ |
21,987 |
|
$ |
17,627 |
|
| Interest rate contracts |
3,600 |
|
1,019 |
|
| Commodity contracts |
20 |
|
— |
|
|
|
|
| Derivative contracts not designated as hedging instruments: |
|
|
| Foreign exchange contracts |
17,658 |
|
21,755 |
|
| Total |
$ |
43,265 |
|
$ |
40,401 |
|
The fair value of PMI’s derivative contracts included in the consolidated balance sheets as of December 31, 2023 and 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative Assets |
|
Derivative Liabilities |
|
|
|
Fair Value |
|
|
|
Fair Value |
(in millions) |
Balance Sheet
Classification
|
|
2023 |
|
2022 |
|
Balance Sheet
Classification
|
|
2023 |
|
2022 |
| Derivative contracts designated as hedging instruments: |
|
|
|
|
|
|
|
|
|
|
|
| Foreign exchange contracts |
Other current assets |
|
$ |
345 |
|
|
$ |
376 |
|
|
Other accrued liabilities |
|
$ |
249 |
|
|
$ |
126 |
|
|
Other assets |
|
153 |
|
|
341 |
|
|
Income taxes and other liabilities |
|
449 |
|
|
147 |
|
| Interest rate contracts |
Other current assets |
|
1 |
|
|
— |
|
|
Other accrued liabilities |
|
78 |
|
|
27 |
|
|
Other assets |
|
— |
|
|
— |
|
|
Income taxes and other liabilities |
|
18 |
|
|
56 |
|
| Commodity contracts |
Other current assets |
|
— |
|
|
— |
|
|
Other accrued liabilities |
|
5 |
|
|
— |
|
|
Other assets |
|
— |
|
|
— |
|
|
Income taxes and other liabilities |
|
1 |
|
|
— |
|
| Derivative contracts not designated as hedging instruments: |
|
|
|
|
|
|
|
|
|
|
|
| Foreign exchange contracts |
Other current assets |
|
85 |
|
|
156 |
|
|
Other accrued liabilities |
|
425 |
|
|
165 |
|
|
Other assets |
|
— |
|
|
— |
|
|
Income taxes and other liabilities |
|
143 |
|
|
16 |
|
Total gross amount derivatives contracts presented in the consolidated balance sheets |
|
|
$ |
584 |
|
|
$ |
873 |
|
|
|
|
$ |
1,368 |
|
|
$ |
537 |
|
| Gross amounts not offset in the consolidated balance sheets |
|
|
|
|
|
|
|
|
|
|
|
| Financial instruments |
|
|
(374) |
|
|
(346) |
|
|
|
|
(374) |
|
|
(346) |
|
| Cash collateral received/pledged |
|
|
(109) |
|
|
(341) |
|
|
|
|
(551) |
|
|
(48) |
|
| Net amount |
|
|
$ |
101 |
|
|
$ |
186 |
|
|
|
|
$ |
443 |
|
|
$ |
143 |
|
PMI assesses the fair value of its derivative contracts using standard valuation models that use, as their basis, readily observable market inputs. The fair value of PMI’s foreign exchange forward contracts, foreign currency swaps and interest rate contracts is determined by using the prevailing foreign exchange spot rates and interest rate differentials, and the respective maturity dates of the instruments.
The fair value of PMI’s currency options is determined by using a Black-Scholes methodology based on foreign exchange spot rates and interest rate differentials, currency volatilities and maturity dates. The fair value of PMI’s commodity contracts is determined by using the prevailing market spot and futures prices and the respective maturity dates of the instruments. PMI’s derivative contracts have been classified within Level 2 at December 31, 2023 and 2022.
For the years ended December 31, 2023, 2022 and 2021, PMI's derivative contracts impacted the consolidated statements of earnings and comprehensive earnings as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (pre-tax, in millions) |
For the Years Ended December 31, |
|
Amount of Gain/(Loss) Recognized in Other Comprehensive Earnings/(Losses) on Derivatives |
|
Statement of Earnings
Classification of Gain/(Loss)
on Derivatives |
|
Amount of Gain/(Loss) Reclassified from Other Comprehensive Earnings/(Losses) into Earnings |
|
Amount of Gain/(Loss) Recognized in Earnings |
|
|
2023 |
2022 |
2021 |
|
|
|
2023 |
2022 |
2021 |
|
2023 |
2022 |
2021 |
|
| Derivative contracts designated as hedging instruments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow hedges: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign exchange contracts |
$ |
195 |
|
$ |
288 |
|
$ |
138 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenues |
|
$ |
194 |
|
$ |
233 |
|
$ |
59 |
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
Marketing, administration and research costs |
|
27 |
|
30 |
|
(10) |
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
(15) |
|
(7) |
|
(6) |
|
|
|
|
|
|
| Interest rate contracts |
37 |
|
292 |
|
6 |
|
|
Interest expense, net |
|
46 |
|
(2) |
|
(1) |
|
|
|
|
|
|
| Commodity contracts |
(7) |
|
— |
|
— |
|
|
Cost of sales |
|
— |
|
— |
|
— |
|
|
|
|
|
|
| Fair value hedges: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate contracts |
|
|
|
|
Interest expense, net (a) |
|
|
|
|
|
$ |
(14) |
|
$ |
(83) |
|
$ |
1 |
|
|
Net investment hedges (b): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign exchange contracts |
(788) |
|
300 |
|
484 |
|
|
Interest expense, net (c) |
|
|
|
|
|
268 |
|
181 |
|
150 |
|
|
| Derivative contracts not designated as hedging instruments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign exchange contracts |
|
|
|
|
Interest expense, net |
|
|
|
|
|
301 |
|
112 |
|
55 |
|
|
|
|
|
|
|
Marketing, administration and research costs (d) |
|
|
|
|
|
(575) |
|
(169) |
|
215 |
|
|
| Total |
$ |
(563) |
|
$ |
880 |
|
$ |
628 |
|
|
|
|
$ |
252 |
|
$ |
254 |
|
$ |
42 |
|
|
$ |
(20) |
|
$ |
41 |
|
$ |
421 |
|
|
(a) The gains (losses) from these contracts are offset by the changes in the fair value of the hedged item
(b) Amount of gains (losses) on hedges of net investments principally related to changes in exchange and interest rates between the Euro and U.S. dollar
(c) Represent the gains for amounts excluded from the effectiveness testing
(d) The gains (losses) from these contracts attributable to changes in foreign currency exchange rates are partially offset by the (losses) and gains generated by the underlying intercompany and third-party loans being hedged
Cash Flow Hedges
PMI has entered into derivative contracts to hedge the foreign currency exchange, interest rate and commodity price risks related to certain forecasted transactions. Gains and losses associated with qualifying cash flow hedge contracts are deferred as components of accumulated other comprehensive losses until the underlying hedged transactions are reported in PMI’s consolidated statements of earnings. As of December 31, 2023, PMI has hedged forecasted transactions with derivative contracts expiring at various dates through May 2028. The impact of these hedges is primarily included in operating cash flows on PMI’s consolidated statements of cash flows.
Fair Value Hedges
PMI has entered into fixed-to-floating interest rate contracts, designated as fair value hedges to minimize exposure to changes in the fair value of fixed rate U.S. dollar-denominated debt that results from fluctuations in benchmark interest rates. For derivative contracts that are designated and qualify as fair value hedges the gain or loss on the derivative, as well as the offsetting gain or loss on the hedged items attributable to the hedged risk, is recognized in current earnings. The carrying amount of the debt hedged, which includes the cumulative adjustment for fair value gains/losses, as of December 31, 2023 was $937 million, and is recorded in long-term debt in the consolidated balance sheets. The cumulative amount of fair value gains/(losses) included in the carrying amount of the debt hedged was $60 million as of December 31, 2023.
Hedges of Net Investments in Foreign Operations
PMI designates derivative contracts and certain foreign currency denominated debt and other financial instruments as net investment hedges, primarily of its Euro net assets. The amount of pre-tax gain/(loss) related to the non-derivative financial instruments, that was reported as a component of accumulated other comprehensive losses within currency translation adjustments, was $48 million, $521 million and $278 million, for the years ended December 31, 2023, 2022 and 2021, respectively. The premiums paid for, and settlements of, net investment hedges are included in investing cash flows on PMI’s consolidated statements of cash flows.
Other Derivatives
PMI has entered into derivative contracts to hedge the foreign currency exchange and interest rate risks related to intercompany loans between certain subsidiaries, third-party loans and acquisition related transactions. While effective as economic hedges, no hedge accounting is applied for these contracts; therefore, the gains (losses) relating to these contracts are reported in PMI’s consolidated statements of earnings. Acquisition related transactions are included in investing cash flows on PMI’s consolidated statements of cash flows.
Qualifying Hedging Activities Reported in Accumulated Other Comprehensive Losses
Derivative gains or losses reported in accumulated other comprehensive losses are a result of qualifying hedging activity. Transfers of these gains or losses to earnings are offset by the corresponding gains or losses on the underlying hedged item. Hedging activity affected accumulated other comprehensive losses, net of income taxes, as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
Gain/(loss) as of January 1, |
$ |
266 |
|
|
$ |
4 |
|
|
$ |
(85) |
|
Derivative (gains)/losses transferred to earnings |
(220) |
|
|
(219) |
|
|
(35) |
|
Change in fair value |
195 |
|
|
481 |
|
|
124 |
|
Gain/(loss) as of December 31, |
$ |
241 |
|
|
$ |
266 |
|
|
$ |
4 |
|
At December 31, 2023, PMI expects $78 million of derivative gains that are included in accumulated other comprehensive losses to be reclassified to the consolidated statement of earnings within the next 12 months. These gains are expected to be substantially offset by the statement of earnings impact of the respective hedged transactions.
Contingent Features
PMI’s derivative instruments do not contain contingent features.
Credit Exposure and Credit Risk
PMI is exposed to credit loss in the event of non-performance by counterparties. While PMI does not anticipate non-performance, its risk is limited to the fair value of the financial instruments less any cash collateral received or pledged. PMI actively monitors its exposure to credit risk through the use of credit approvals and credit limits and by selecting and continuously monitoring a diverse group of major international banks and financial institutions as counterparties.
Accumulated Other Comprehensive Losses:
PMI's accumulated other comprehensive losses, net of taxes, consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (Losses) Earnings |
At December 31, |
(in millions) |
2023 |
|
2022 |
|
2021 |
Currency translation adjustments |
$ |
(9,467) |
|
|
$ |
(8,003) |
|
|
$ |
(6,701) |
|
Pension and other benefits |
(2,589) |
|
|
(1,822) |
|
|
(2,880) |
|
Derivatives accounted for as hedges |
241 |
|
|
266 |
|
|
4 |
|
|
|
|
|
|
|
Total accumulated other comprehensive losses |
$ |
(11,815) |
|
|
$ |
(9,559) |
|
|
$ |
(9,577) |
|
Reclassifications from Other Comprehensive Earnings
The movements in accumulated other comprehensive losses and the related tax impact, for each of the components above, that are due to current period activity and reclassifications to the income statement are shown on the consolidated statements of comprehensive earnings for the years ended December 31, 2023, 2022, and 2021. For additional information, see Note 3. Acquisitions (Transactions With Noncontrolling Interests) for disclosures related to currency translation adjustments, Note 14. Benefit Plans for disclosures related to PMI's pension and other benefits and Note 16. Financial Instruments for disclosures related to derivative financial instruments.
Contingencies:
Tobacco-Related Litigation
Legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. Our indemnitees include distributors, licensees, and others that have been named as parties in certain cases and that we have agreed to defend, as well as to pay costs and some or all of judgments, if any, that may be entered against them. Pursuant to the terms of the Distribution Agreement between Altria Group, Inc. ("Altria") and PMI, PMI will indemnify Altria and Philip Morris USA Inc. ("PM USA"), a U.S. tobacco subsidiary of Altria, for tobacco product claims based in substantial part on products manufactured by PMI or contract manufactured for PMI by PM USA, and PM USA will indemnify PMI for tobacco product claims based in substantial part on products manufactured by PM USA, excluding tobacco products contract manufactured for PMI.
It is possible that there could be adverse developments in pending cases against us and our subsidiaries. An unfavorable outcome or settlement of pending tobacco-related litigation could encourage the commencement of additional litigation.
Damages claimed in some of the tobacco-related litigation are significant and, in certain cases in Brazil, Canada and Nigeria, range into the billions of U.S. dollars. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. However, as discussed below, we have to date been largely successful in defending tobacco-related litigation.
We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. At the present time, except as stated otherwise in this Note 18. Contingencies, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available to it (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
It is possible that our consolidated results of operations, cash flows or financial position could be materially affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. Nevertheless, although litigation is subject to uncertainty, we and each of our subsidiaries named as a defendant believe, and each has been so advised by counsel handling the respective cases, that we have valid defenses to the litigation pending against us, as well as valid bases for appeal of adverse verdicts. All such cases are, and will continue to be, vigorously defended. However, we and our subsidiaries may enter into settlement discussions in particular cases if we believe it is in our best interests to do so.
CCAA Proceedings and Stay of Tobacco-Related Cases Pending in Canada
As a result of the Court of Appeal of Quebec’s decision in both the Létourneau and Blais cases described below, our subsidiary, Rothmans, Benson & Hedges Inc. (“RBH”), and the other defendants, JTI Macdonald Corp., and Imperial Tobacco Canada Limited, sought protection in the Ontario Superior Court of Justice under the Companies’ Creditors Arrangement Act (“CCAA”) on March 22, March 8, and March 12, 2019, respectively. CCAA is a Canadian federal law that permits a Canadian business to restructure its affairs while carrying on its business in the ordinary course. The initial CCAA order made by the Ontario Superior Court on March 22, 2019 authorizes RBH to pay all expenses incurred in carrying on its business in the ordinary course after the CCAA filing, including obligations to employees, vendors, and suppliers. RBH's financial results have been deconsolidated from our consolidated financial statements since March 22, 2019. As part of the CCAA proceedings, there is currently a comprehensive stay up to and including March 29, 2024 of all tobacco-related litigation pending in Canada against RBH and the other defendants, including PMI and our indemnitees (PM USA and Altria), namely, the smoking and health class actions filed in various Canadian provinces and health care cost recovery actions. These proceedings are presented below under the caption “Stayed Litigation — Canada.” Ernst & Young Inc. has been appointed as monitor of RBH in the CCAA proceedings. In accordance with the CCAA process, as the parties work towards a plan of arrangement or compromise in a confidential mediation, it is anticipated that the court will set additional hearings and further extend the stay of proceedings. On April 17, 2019, the Ontario Superior Court ruled that RBH and the other defendants will not be allowed to file an application to the Supreme Court of Canada for leave to appeal the Court of Appeal’s decision in the Létourneau and the Blais cases so long as the comprehensive stay of all tobacco-related litigation in Canada remains in effect and that the time period to file the application would be extended by the stay period. While RBH believes that the findings of liability and damages in both Létourneau and the Blais cases were incorrect, the CCAA proceedings will provide a forum for RBH to seek resolution through a plan of arrangement or compromise of all tobacco-related litigation pending in Canada. It is not possible to predict the resolution of the underlying legal proceedings or the length of the CCAA process.
Stayed Litigation — Canada
Smoking and Health Litigation — Canada
In the first class action pending in Canada, Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais v. Imperial Tobacco Canada Ltd., Rothmans, Benson & Hedges Inc. and JTI-Macdonald Corp., Quebec Superior Court, Canada, filed in November 1998, RBH and other Canadian cigarette manufacturers (Imperial Tobacco Canada Ltd. and JTI-Macdonald Corp.) are defendants. The plaintiffs, an anti-smoking organization and an individual smoker, sought compensatory and punitive damages for each member of the class who suffers allegedly from certain smoking-related diseases. The class was certified in 2005. The trial court issued its judgment on May 27, 2015. The trial court found RBH and two other Canadian manufacturers liable and found that the class members’ compensatory damages totaled approximately CAD 15.5 billion (approximately $11.5 billion), including pre-judgment interest. The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion (approximately $2.3 billion) including pre-judgment interest). In addition, the trial court awarded CAD 90,000 (approximately $67,000) in punitive damages, allocating CAD 30,000 (approximately $22,000) to RBH. The trial court estimated the disease class at 99,957 members. RBH appealed to the Court of Appeal of Quebec. In October 2015, the Court of Appeal ordered RBH to furnish security totaling CAD 226 million (approximately $167 million) to cover both the Létourneau and Blais cases, which RBH has paid in installments through March 2017. The Court of Appeal ordered Imperial Tobacco Canada Ltd. to furnish security totaling CAD 758 million (approximately $561 million) in installments through June 2017. JTI Macdonald Corp. was not required to furnish security in accordance with plaintiffs’ motion. The Court of Appeal ordered that the security is payable upon a final judgment of the Court of Appeal affirming the trial court’s judgment or upon further order of the Court of Appeal.
On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court’s findings of liability and the compensatory and punitive damages award while reducing the total amount of compensatory damages to approximately CAD 13.5 billion (approximately $10 billion), including interest due to the trial court’s error in the calculation of interest. The compensatory damages award is on a joint and several basis with an allocation of 20% to RBH (approximately CAD 2.7 billion (approximately $2 billion), including pre-judgment interest). The Court of Appeal upheld the trial court’s findings that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking and by conspiring to prevent consumers from learning of the dangers of smoking. The Court of Appeal further held that the plaintiffs either need not prove, or had adequately proven, that these faults were a cause of the class members’ injuries. In accordance with the judgment, defendants were required to deposit their respective portions of the damages awarded in both the Létourneau case described below and the Blais case, approximately CAD 1.1 billion (approximately $813 million), into trust accounts within 60 days.
RBH’s share of the deposit was approximately CAD 257 million (approximately $194 million). PMI recorded a pre-tax charge of $194 million in its consolidated results, representing $142 million net of tax, as tobacco litigation-related expense, in the first quarter of 2019. The charge reflects PMI’s assessment of the portion of the judgment that represents probable and estimable loss prior to the deconsolidation of RBH and corresponds to the trust account deposit required by the judgment.
In the second class action pending in Canada, Cecilia Létourneau v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI-Macdonald Corp., Quebec Superior Court, Canada, filed in September 1998, RBH and other Canadian cigarette manufacturers (Imperial Tobacco Canada Ltd. and JTI-Macdonald Corp.) are defendants. The plaintiff, an individual smoker, sought compensatory and punitive damages for each member of the class who is deemed addicted to smoking. The class was certified in 2005. The trial court issued its judgment on May 27, 2015. The trial court found RBH and two other Canadian manufacturers liable and awarded a total of CAD 131 million (approximately $97 million) in punitive damages, allocating CAD 46 million (approximately $34 million) to RBH. The trial court estimated the size of the addiction class at 918,000 members but declined to award compensatory damages to the addiction class because the evidence did not establish the claims with sufficient accuracy. The trial court found that a claims process to allocate the awarded punitive damages to individual class members would be too expensive and difficult to administer. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court’s findings of liability and the total amount of punitive damages awarded allocating CAD 57 million (approximately $42 million), including interest to RBH. See the Blais description above for further detail concerning the security order pertaining to both Létourneau and Blais cases and the impact of the decision on PMI’s financial statements.
RBH and PMI believe the findings of liability and damages in both Létourneau and the Blais cases were incorrect and in contravention of applicable law on several grounds including, the following: (i) defendants had no obligation to warn class members who knew, or should have known, of the risks of smoking; (ii) defendants cannot be liable to class members who would have smoked regardless of what warnings were given; and (iii) defendants cannot be liable to all class members given the individual differences among class members.
In the third class action pending in Canada, Kunta v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Winnipeg, Canada, filed June 12, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic obstructive pulmonary disease (“COPD”), severe asthma, and mild reversible lung disease resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products.
In the fourth class action pending in Canada, Adams v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Saskatchewan, Canada, filed July 10, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, emphysema, heart disease, or cancer, as well as restitution of profits.
In the fifth class action pending in Canada, Semple v. Canadian Tobacco Manufacturers' Council, et al., The Supreme Court (trial court), Nova Scotia, Canada, filed June 18, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and COPD resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products.
In the sixth class action pending in Canada, Dorion v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Alberta, Canada, filed June 15, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic bronchitis and severe sinus infections resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. To date, we, our subsidiaries, and our indemnitees have not been properly served with the complaint.
In the seventh class action pending in Canada, McDermid v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada, filed June 25, 2010, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and heart disease resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from heart disease allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed.
In the eighth class action pending in Canada, Bourassa v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada, filed June 25, 2010, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, the heir to a deceased smoker, alleges that the decedent was addicted to tobacco products and suffered from emphysema resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from chronic respiratory diseases allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed. In December 2014, plaintiff filed an amended statement of claim.
In the ninth class action pending in Canada, Suzanne Jacklin v. Canadian Tobacco Manufacturers' Council, et al., Ontario Superior Court of Justice, filed June 20, 2012, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, heart disease, or cancer, as well as restitution of profits.
Health Care Cost Recovery Litigation — Canada
In the first health care cost recovery case pending in Canada, Her Majesty the Queen in Right of British Columbia v. Imperial Tobacco Limited, et al., Supreme Court, British Columbia, Vancouver Registry, Canada, filed January 24, 2001, we, RBH, our indemnitee (PM USA), and other members of the industry are defendants. The plaintiff, the government of the province of British Columbia, brought a claim based upon legislation enacted by the province authorizing the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, resulting from a “tobacco related wrong.”
In the second health care cost recovery case filed in Canada, Her Majesty the Queen in Right of New Brunswick v. Rothmans Inc., et al., Court of Queen's Bench of New Brunswick, Trial Court, New Brunswick, Fredericton, Canada, filed March 13, 2008, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of New Brunswick based on legislation enacted in the province. This legislation is similar to the law introduced in British Columbia that authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the third health care cost recovery case filed in Canada, Her Majesty the Queen in Right of Ontario v. Rothmans Inc., et al., Ontario Superior Court of Justice, Toronto, Canada, filed September 29, 2009, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Ontario based on legislation enacted in the province. This legislation is similar to the laws introduced in British Columbia and New Brunswick that authorize the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the fourth health care cost recovery case filed in Canada, Attorney General of Newfoundland and Labrador v. Rothmans Inc., et al., Supreme Court of Newfoundland and Labrador, St. Johns, Canada, filed February 8, 2011, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Newfoundland and Labrador based on legislation enacted in the province that is similar to the laws introduced in British Columbia, New Brunswick and Ontario. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the fifth health care cost recovery case filed in Canada, Attorney General of Quebec v. Imperial Tobacco Limited, et al., Superior Court of Quebec, Canada, filed June 8, 2012, we, RBH, our indemnitee (PM USA), and other members of the industry are defendants. The claim was filed by the government of the province of Quebec based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the sixth health care cost recovery case filed in Canada, Her Majesty in Right of Alberta v. Altria Group, Inc., et al., Supreme Court of Queen's Bench Alberta, Canada, filed June 8, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Alberta based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the seventh health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Manitoba v. Rothmans, Benson & Hedges, Inc., et al., The Queen's Bench, Winnipeg Judicial Centre, Canada, filed May 31, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Manitoba based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces.
The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the eighth health care cost recovery case filed in Canada, The Government of Saskatchewan v. Rothmans, Benson & Hedges Inc., et al., Queen's Bench, Judicial Centre of Saskatchewan, Canada, filed June 8, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Saskatchewan based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the ninth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Prince Edward Island v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Prince Edward Island (General Section), Canada, filed September 10, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Prince Edward Island based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the tenth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Nova Scotia v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Nova Scotia, Canada, filed January 2, 2015, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Nova Scotia based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
__________
The table below lists the number of tobacco-related cases pertaining to combustible products pending against us and/or our subsidiaries or indemnitees as of December 31, 2023, December 31, 2022 and December 31, 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Type of Case1 |
|
Number of Cases Pending as of December 31, 2023 |
|
Number of Cases Pending as of December 31, 2022 |
|
Number of Cases Pending as of December 31, 2021 |
| Individual Smoking and Health Cases |
|
45 |
|
40 |
|
40 |
| Smoking and Health Class Actions |
|
9 |
|
9 |
|
9 |
| Health Care Cost Recovery Actions |
|
17 |
|
17 |
|
17 |
| Label-Related Class Actions |
|
— |
|
— |
|
— |
| Individual Label-Related Cases |
|
4 |
|
6 |
|
3 |
| Public Civil Actions |
|
1 |
|
1 |
|
1 |
______
¹ Includes cases pending in Canada.
Since 1995, when the first tobacco-related litigation was filed against a PMI entity, 544 Smoking and Health, Label-Related, Health Care Cost Recovery, and Public Civil Actions in which we and/or one of our subsidiaries and/or indemnitees were a defendant have been terminated in our favor. Fifteen cases have had decisions in favor of plaintiffs. Ten of these cases have subsequently reached final resolution in our favor and five remain on appeal, or are subject to an appeal, or our subsidiary may file an appeal.
The table below lists the verdict and significant post-trial developments in the five pending cases where a verdict was returned in favor of the plaintiff:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Date |
|
Location of
Court/Name of
Plaintiff |
|
Type of
Case |
|
Verdict |
|
Post-Trial
Developments |
| May 27, 2015 |
|
Canada/Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais
|
|
Class Action |
|
On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Blais class on liability and found the class members’ compensatory damages totaled approximately CAD 15.5 billion (approximately $11.5 billion), including pre-judgment interest. The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion including pre-judgment interest (approximately $2.3 billion)). The trial court awarded CAD 90,000 (approximately $67,000) in punitive damages, allocating CAD 30,000 (approximately $22,000) to our subsidiary. The trial court ordered defendants to pay CAD 1 billion (approximately $740 million) of the compensatory damage award, CAD 200 million (approximately $148 million) of which is our subsidiary’s portion, into a trust within 60 days. |
|
In June 2015, RBH commenced the appellate process with the Court of Appeal of Quebec. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court's decision. (See “Stayed Litigation — Canada” for further detail.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Date |
|
Location of
Court/Name of
Plaintiff |
|
Type of
Case |
|
Verdict |
|
Post-Trial
Developments |
| May 27, 2015 |
|
Canada/Cecilia Létourneau
|
|
Class Action |
|
On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Létourneau class on liability and awarded a total of CAD 131 million (approximately $97 million) in punitive damages, allocating CAD 46 million (approximately $34 million) to RBH. The trial court ordered defendants to pay the full punitive damage award into a trust within 60 days. The court did not order the payment of compensatory damages.
|
|
In June 2015, RBH commenced the appellate process with the Court of Appeal of Quebec. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court's decision. (See “Stayed Litigation — Canada” for further detail.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Date |
|
Location of
Court/Name of
Plaintiff |
|
Type of
Case |
|
Verdict |
|
Post-Trial
Developments |
| August 5, 2016 |
|
Argentina/Hugo Lespada |
|
Individual Action |
|
On August 5, 2016, the Civil Court No. 14 - Mar del Plata, issued a verdict in favor of plaintiff, an individual smoker, and awarded him ARS 110,000 (approximately $133), plus interest, in compensatory and moral damages. The trial court found that our subsidiary failed to warn plaintiff of the risk of becoming addicted to cigarettes. |
|
On August 23, 2016, our subsidiary filed its notice of appeal. On October 31, 2017, the Civil and Commercial Court of Appeals of Mar del Plata ruled that plaintiff's claim was barred by the statute of limitations and it reversed the trial court's decision. On May 17, 2021, plaintiff filed a federal extraordinary appeal. On November 1, 2021, the Supreme Court of the Province of Buenos Aires dismissed plaintiff's federal extraordinary appeal. On November 10, 2021, plaintiff filed a direct appeal before the Federal Supreme Court. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Date |
|
Location of
Court/Name of
Plaintiff |
|
Type of
Case |
|
Verdict |
|
Post-Trial
Developments |
| June 17, 2021 |
|
Argentina/Claudia Milano |
|
Individual Action |
|
On June 17, 2021, the Civil Court No. 9 - Mar del Plata, issued a verdict in favor of plaintiff, an individual smoker, and awarded her smoking cessation treatments, ARS 150,000 (approximately $181), in compensatory and moral damages, and ARS 4,000,000 (approximately $4,825) in punitive damages, plus interest and costs. The trial court found that our subsidiary failed to warn plaintiff of the risk of becoming addicted to cigarettes. |
|
On July 2, 2021, our subsidiary filed its notice of appeal. In addition, plaintiff filed an appeal challenging the dismissal of the claim for psychological damages. As required by local law, our subsidiary deposited the damages awarded, plus interest and costs, in total ARS 6,114,428 (approximately $7,375), into a court escrow account. Our subsidiary challenged the amount determined by the court. The Civil and Commercial Court of Appeals of Mar del Plata granted our subsidiary's challenge to the escrow amount determined by the trial court. As a result, on December 16, 2021, ARS 893,428 (approximately $1,078) was returned to our subsidiary. If our subsidiary ultimately prevails, the remaining deposited amounts will be returned to our subsidiary. On May 31, 2022, the Civil and Commercial Court of Appeals of Mar del Plata ruled that the statute of limitations barred plaintiff's claim and reversed the trial court's decision. On June 15, 2022, plaintiff filed an extraordinary appeal.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Date |
|
Location of
Court/Name of
Plaintiff |
|
Type of
Case |
|
Verdict |
|
Post-Trial
Developments |
| June 23, 2023 |
|
Turkey/ Senem Yilmazel |
|
Individual Action |
|
On June 23, 2023, the Ankara Consumer Court published its decision in favor of plaintiff, the daughter of an individual smoker, against our subsidiary and a BAT subsidiary, awarding her TRY 10,000 (approximately $327) in damages. The trial court found that the plaintiff’s father died as a result of lung cancer and COPD caused by his cigarette consumption. |
|
On September 8, 2023, our subsidiary filed its appeal. On September 25, 2023, the plaintiff filed an appeal challenging the damages amount determined by the court. |
Pending claims related to tobacco products generally fall within the following categories:
Smoking and Health Litigation: These cases primarily allege personal injury and are brought by individual plaintiffs or on behalf of a class or purported class of individual plaintiffs. Plaintiffs' allegations of liability in these cases are based on various theories of recovery, including negligence, gross negligence, strict liability, fraud, misrepresentation, design defect, failure to warn, breach of express and implied warranties, violations of deceptive trade practice laws and consumer protection statutes. Plaintiffs in these cases seek various forms of relief, including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include licit activity, failure to state a claim, lack of defect, lack of proximate cause, assumption of the risk, contributory negligence, and statute of limitations.
As of December 31, 2023, there were a number of smoking and health cases pending against us, our subsidiaries or indemnitees, as follows:
•45 cases brought by individual plaintiffs in Argentina (31), Canada (2), Chile (11), and Turkey (1), compared with 40 such cases on December 31, 2022, and 40 cases on December 31, 2021; and
•9 cases brought on behalf of classes of individual plaintiffs, compared with 9 such cases on December 31, 2022 and 9 such cases on December 31, 2021.
The class actions pending in Canada are described above under the caption “Smoking and Health Litigation — Canada.”
Health Care Cost Recovery Litigation: These cases, brought by governmental and non-governmental plaintiffs, seek reimbursement of health care cost expenditures allegedly caused by tobacco products. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including unjust enrichment, negligence, negligent design, strict liability, breach of express and implied warranties, violation of a voluntary undertaking or special duty, fraud, negligent misrepresentation, conspiracy, public nuisance, defective product, failure to warn, sale of cigarettes to minors, and claims under statutes governing competition and deceptive trade practices. Plaintiffs in these cases seek various forms of relief including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include lack of proximate cause, remoteness of injury, failure to state a claim, adequate remedy at law, “unclean hands” (namely, that plaintiffs cannot obtain equitable relief because they participated in, and benefited from, the sale of cigarettes), and statute of limitations.
As of December 31, 2023, there were 17 health care cost recovery cases pending against us, our subsidiaries or indemnitees in Brazil (1), Canada (10), Korea (1) and Nigeria (5), compared with 17 such cases on December 31, 2022 and 17 such cases on December 31, 2021.
The health care cost recovery actions pending in Canada are described above under the caption “Health Care Cost Recovery Litigation — Canada.”
In the health care cost recovery case in Brazil, The Attorney General of Brazil v. Souza Cruz Ltda., et al., Federal Trial Court, Porto Alegre, Rio Grande do Sul, Brazil, filed May 21, 2019, we, our subsidiaries, and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases in certain prior years, payment of anticipated costs of treating future alleged smoking-related diseases, and moral damages. Defendants filed answers to the complaint in May 2020.
In the first health care cost recovery case in Nigeria, The Attorney General of Lagos State v. British American Tobacco (Nigeria) Limited, et al., High Court of Lagos State, Lagos, Nigeria, filed March 13, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the second health care cost recovery case in Nigeria, The Attorney General of Kano State v. British American Tobacco (Nigeria) Limited, et al., High Court of Kano State, Kano, Nigeria, filed May 9, 2007, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of challenging the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the third health care cost recovery case in Nigeria, The Attorney General of Gombe State v. British American Tobacco (Nigeria) Limited, et al., High Court of Gombe State, Gombe, Nigeria, filed October 17, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In February 2011, the court ruled that the plaintiff had not complied with the procedural steps necessary to serve us. As a result of this ruling, plaintiff must re-serve its claim. We have not yet been re-served.
In the fourth health care cost recovery case in Nigeria, The Attorney General of Oyo State, et al., v. British American Tobacco (Nigeria) Limited, et al., High Court of Oyo State, Ibadan, Nigeria, filed May 25, 2007, we and other members of the industry are defendants. Plaintiffs seek reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We challenged service as improper. In June 2010, the court ruled that plaintiffs did not have leave to serve the writ of summons on the defendants and that they must re-serve the writ. We have not yet been re-served.
In the fifth health care cost recovery case in Nigeria, The Attorney General of Ogun State v. British American Tobacco (Nigeria) Limited, et al., High Court of Ogun State, Abeokuta, Nigeria, filed February 26, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In May 2010, the trial court rejected our objections to the court's jurisdiction. We have appealed. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the health care cost recovery case in Korea, the National Health Insurance Service v. KT&G, et. al., filed April 14, 2014, our subsidiary and other Korean manufacturers are defendants. Plaintiff alleges that defendants concealed the health hazards of smoking, marketed to youth, added ingredients to make their products more harmful and addictive, and misled consumers into believing that Lights cigarettes are safer than regular cigarettes. The National Health Insurance Service seeks to recover damages allegedly incurred in treating 3,484 patients with small cell lung cancer, squamous cell lung cancer, and squamous cell laryngeal cancer from 2003 to 2012. The trial court dismissed the case in its entirety on November 20, 2020. The Appellate court granted the Plaintiff a de novo appeal in 2021 and determined that the appellate proceedings will take place in stages: wrongful conduct/product defect allegations first, then causation and finally issues such as standing/direct action.
Label-Related Cases: These cases, now brought only by individual plaintiffs, allege that the use of the descriptor “Lights” or other alleged misrepresentations or omissions of labeling information constitute fraudulent and misleading conduct. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including misrepresentation, deception, and breach of consumer protection laws. Plaintiffs seek various forms of relief including restitution, injunctive relief, and compensatory and other damages. Defenses raised include lack of causation, lack of reliance, assumption of the risk, and statute of limitations.
As of December 31, 2023, there were 4 label-related cases brought by individual plaintiffs in Italy (1) and Chile (3) pending against our subsidiaries, compared with 6 such cases on December 31, 2022, and 3 such cases on December 31, 2021.
Public Civil Actions: Claims have been filed either by an individual, or a public or private entity, seeking to protect collective or individual rights, such as the right to health, the right to information or the right to safety. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including product defect, concealment, and misrepresentation. Plaintiffs in these cases seek various forms of relief including injunctive relief such as banning cigarettes, descriptors, smoking in certain places and advertising, as well as implementing communication campaigns and reimbursement of medical expenses incurred by public or private institutions.
As of December 31, 2023, there was 1 public civil action pending against our subsidiary in Venezuela (1), compared with 1 such case on December 31, 2022, and 1 such case on December 31, 2021.
In a public civil action in Venezuela, Federation of Consumers and Users Associations (“FEVACU”), et al. v. National Assembly of Venezuela and the Venezuelan Ministry of Health, Constitutional Chamber of the Venezuelan Supreme Court, filed April 29, 2008, we were not named as a defendant, but the plaintiffs published a notice pursuant to court order, notifying all interested parties to appear in the case. In January 2009, our subsidiary appeared in the case in response to this notice. The plaintiffs purport to represent the right to health of the citizens of Venezuela and claim that the government failed to protect adequately its citizens' right to health. The claim asks the court to order the government to enact stricter regulations on the manufacture and sale of tobacco products. In addition, the plaintiffs ask the court to order companies involved in the tobacco industry to allocate a percentage of their “sales or benefits” to establish a fund to pay for the health care costs of treating smoking-related diseases. In October 2008, the court ruled that plaintiffs have standing to file the claim and that the claim meets the threshold admissibility requirements. In December 2012, the court admitted our subsidiary and a subsidiary of British American Tobacco plc as interested third parties. In February 2013, our subsidiary answered the complaint.
U.S. Government Matter: The U.S. government has contacted Altria and PM USA in connection with an agreement between PMI and Altria to end their commercial relationship with respect to Platform 1 in the U.S. as of April 30, 2024 (“Altria Agreement"). Altria and PM USA are parties to a 2006 order in the United States District Court for the District of Columbia holding that they violated the Racketeer Influenced and Corrupt Organizations Act (“2006 Order”). PMI was not a defendant in that proceeding. The 2006 Order imposed injunctive relief on defendants including, but not limited to, enjoining false, misleading, or deceptive statements concerning cigarettes; prohibiting express or implied health statements for any cigarette brand; and requiring defendants to make certain corrective statements at point-of sale and on websites. The 2006 Order also imposed restrictions on defendants from selling or transferring their cigarette brands, brand names, cigarette product formulas or cigarette businesses without the transferee submitting to the jurisdiction of the court and subjecting itself to the 2006 Order as of the date of sale or transfer. The U.S. government has informed Altria that it believes the transaction contemplated by the Altria Agreement falls within the scope of this provision and that, before it can be effectuated, PMI must submit to the 2006 Order. While we do not know the specific relief the U.S. government may seek from the court, we believe that there are strong arguments as to why the provision cited by the U.S. government is inapplicable to the Altria Agreement.
Other Litigation
The Department of Special Investigations of the government of Thailand ("DSI") conducted an investigation into alleged underpayment by our subsidiary, Philip Morris (Thailand) Limited ("PM Thailand"), of customs duties and excise taxes relating to imports from the Philippines covering the period 2003-2007. On January 18, 2016, the Public Prosecutor filed charges against our subsidiary and seven former and current employees in the Bangkok Criminal Court alleging that PM Thailand and the individual defendants jointly and with the intention to defraud the Thai government, under-declared import prices of cigarettes to avoid full payment of taxes and duties in connection with import entries of cigarettes from the Philippines during the period of July 2003 to June 2006. The government sought a fine of approximately THB 80.8 billion (approximately $2.3 billion). In May 2017, Thailand enacted a new customs act. The new act, which took effect in November 2017, substantially limits the amount of fines that Thailand could seek in these proceedings. PM Thailand believes that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and other Thai governmental agencies. Trial in the case began in November 2017 and concluded in September 2019. In November 2019, the trial court found our subsidiary guilty of under-declaration of the prices and imposed a fine of approximately THB 1.2 billion (approximately $33.5 million). The trial court dismissed all charges against the individual defendants. In December 2019, as required by the Thai law, our subsidiary paid the fine. This payment is included in other assets on the consolidated balance sheets and negatively impacted net cash provided by operating activities in the consolidated statements of cash flows in the period of payment. Both our subsidiary and the Public Prosecutor filed an appeal of the trial court's decision. The appellate court issued its decision on the appeals on June 1, 2022. The appellate court affirmed the findings of under-declaration of import prices of cigarettes but reduced the fine to approximately THB 122 million (approximately $3.4 million) finding the trial court erred in its calculation of the under-declaration and fine. The appellate court affirmed the acquittals of the individual defendants. Our subsidiary has appealed the decision to the Supreme Court of Thailand. The Public Prosecutor has also filed an appeal challenging the dismissal of charges against the individual defendants and the amount of the fine imposed. Thailand is required to refund any payment made by our subsidiary in excess of any fine asserted by the courts.
The DSI also conducted an investigation into alleged underpayment by PM Thailand of customs duties and excise taxes relating to imports from Indonesia covering the period 2000-2003. On January 26, 2017, the Public Prosecutor filed charges against PM Thailand and its former Thai employee in the Bangkok Criminal Court alleging that PM Thailand and its former employee jointly and with the intention to defraud the Thai government under-declared import prices of cigarettes to avoid full payment of taxes and duties in connection with import entries during the period from January 2002 to July 2003. The government is seeking a fine of approximately THB 19.8 billion (approximately $553 million). In May 2017, Thailand enacted a new customs act. The new act, which took effect in November 2017, substantially limits the amount of fines that Thailand could seek in these proceedings. PM Thailand believes that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law, and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and a Thai court. Trial in the case began in November 2018 and concluded in December 2019. In March 2020, the trial court found our subsidiary guilty of under-declaration of the prices and imposed a fine of approximately THB 130 million (approximately $3.6 million). The trial court dismissed all charges against the individual defendant. In April 2020, as required by Thai law, our subsidiary paid the fine. This payment is included in other assets on the consolidated balance sheets and negatively impacted net cash provided by operating activities in the consolidated statements of cash flows in the period of payment. Our subsidiary filed an appeal of the trial court's decision. In addition, the Public Prosecutor filed an appeal of the trial court's decision challenging the dismissal of charges against the individual defendant and the amount of the fine imposed. The appellate court issued its decision on the appeals on January 31, 2023. The appellate court affirmed the findings of under-declaration of import prices of cigarettes but reduced the fine imposed by the trial court. The appellate court directed the Public Prosecutor to coordinate with customs officials to calculate such reduced fine in accordance with the appellate court’s decision. The appellate court affirmed the acquittal of the individual defendant. Our subsidiary has appealed the decision to the Supreme Court of Thailand. The Public Prosecutor has filed an appeal to the Supreme Court of Thailand challenging the dismissal of charges against the individual defendant and the amount of the fine.
Thailand is required to refund any payment made by our subsidiary in excess of any fine assessed by the courts.
The South Korean Board of Audit and Inspection (“BAI”) conducted an audit of certain Korean government agencies and the tobacco industry into whether inventory movements ahead of the January 1, 2015 increase of cigarette-related taxes and funds by tobacco companies, including Philip Morris Korea Inc. ("PM Korea"), our South Korean subsidiary, were in compliance with South Korean laws. In November 2016, the tax authorities completed their audit and assessed allegedly underpaid taxes and penalties. In order to avoid nonpayment financial costs, PM Korea paid approximately KRW 272 billion (approximately $204 million), of which KRW 100 billion (approximately $75 million) was paid in 2016 and KRW 172 billion (approximately $129 million) was paid in the first quarter of 2017. These paid amounts negatively impacted net cash provided by operating activities in the consolidated statements of cash flows in the period of payment. PM Korea appealed the assessments. In January 2020, a trial court ruled that PM Korea did not underpay taxes in the amount of approximately KRW 218 billion (approximately $164 million). The tax authorities appealed this decision to the appellate court. In September 2020, the appellate court upheld the trial court's decision. The tax authorities appealed to the Supreme Court of South Korea. On July 13, 2023, the Supreme Court reversed the appellate court's decision and remanded the tax cases to the appellate court. Two separate panels at the appellate court were assigned to implement the Supreme Court decision, one related to the local level tobacco consumption tax (“TCT”) and the other related to the national level individual consumption tax (“ICT”). The first two hearings for the TCT case took place on October 18, 2023, and December 6, 2023, and the next hearing is scheduled on March 20, 2024. The first hearing for the ICT case took place on November 17, 2023, and the next hearing is put on hold until all issues are cleared in the TCT case hearings. PM Korea made factual and legal arguments which were not previously reviewed and factored in the Supreme Court decision. Based on the decision issued by the Supreme Court of South Korea on July 13, 2023, management has concluded that an adverse outcome is probable. In June 2020, another trial court ruled that PM Korea did not underpay approximately KRW 54 billion (approximately $40 million) of alleged funds underpayments. The government agencies appealed this decision. In January 2021, the appellate court upheld the trial court's decision. The government agencies appealed to the Supreme Court of South Korea. This funds case is still pending review at the Supreme Court without a specific decision date. Based on the decision issued by the Supreme Court of South Korea on July 13, 2023, management has concluded that an adverse outcome is probable. Consequently, in the second quarter of 2023, PMI recorded a non-cash pre-tax charge of $204 million in marketing, administration and research costs, reflecting the full amount previously paid by PM Korea.
A putative shareholder class action lawsuit, In re Philip Morris International Inc. Securities Litigation, is pending in the United States District Court for the Southern District of New York, purportedly on behalf of purchasers of Philip Morris International Inc. stock between July 26, 2016 and April 18, 2018. The lawsuit names Philip Morris International Inc. and certain officers and employees as defendants and includes allegations that the defendants made false and/or misleading statements and/or failed to disclose information about PMI’s business, operations, financial condition, and prospects, related to product sales of, and alleged irregularities in clinical studies of, PMI’s Platform 1 product. The lawsuit seeks various forms of relief, including damages. In November 2018, the court consolidated three putative shareholder class action lawsuits with similar allegations previously filed in the Southern District of New York (namely, City of Westland Police and Fire Retirement System v. Philip Morris International Inc., et al., Greater Pennsylvania Carpenters’ Pension Fund v. Philip Morris International Inc., et al., and Gilchrist v. Philip Morris International Inc., et al.) into these proceedings. A putative shareholder class action lawsuit, Rubenstahl v. Philip Morris International Inc., et al., that had been previously filed in December 2017 in the United States District Court for the District of New Jersey, was voluntarily dismissed by the plaintiff due to similar allegations in these proceedings. On February 4, 2020, the court granted defendants’ motion in its entirety, dismissing all but one of the plaintiffs’ claims with prejudice. The court noted that one of plaintiffs’ claims (allegations relating to four non-clinical studies of PMI’s Platform 1 product) did not state a viable claim but allowed plaintiffs to replead that claim by March 3, 2020. On February 18, 2020, the plaintiffs filed a motion for reconsideration of the court's February 4th decision; this motion was denied on September 21, 2020. On September 28, 2020, plaintiffs filed an amended complaint seeking to replead allegations relating to four non-clinical studies of PMI's Platform 1 product. On September 10, 2021, the court granted defendant's motion to dismiss plaintiffs' amended complaint in its entirety. On December 26, 2023, the U.S. Court of Appeals for the Second Circuit upheld the dismissal of plaintiffs' claims.
On February 1, 2024, Philip Morris Products S.A. ("PMPSA") entered into a settlement agreement (the “Settlement Agreement”) with Nicoventures Trading Limited (“NTV”), an affiliate of British American Tobacco p.l.c. (“BAT”). Under the Settlement Agreement, PMPSA, NTV and their respective affiliates (the “Parties”) have agreed, among other things, to: (i) dismiss with prejudice, subject to certain limited exceptions, and without admission of liability certain pending legal proceedings (the “Proceedings”) between them and concerning certain of their respective products; (ii) request rescission of the limited exclusion order and the cease-and-desist order issued by the International Trade Commission (“ITC”) on September 29, 2021, and (iii) fully and finally discharge without admission of liability any injunctions granted to the Parties in the Proceedings.
In April 2020, affiliates of BAT commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Altria Client Services LLC, et al., in the federal court in the Eastern District of Virginia, where PMI's subsidiary, PMPSA, as well as Altria Group, Inc.'s subsidiaries, are defendants. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade products in the United States.
In April 2020, BAT affiliates filed a complaint against PMI, PMPSA, Altria Group, Inc., and its subsidiaries before the ITC. Plaintiffs sought an order to prevent the importation of Platform 1 products into the United States. The ITC evidentiary hearing closed on February 1, 2021. On May 14, 2021, the administrative law judge issued an Initial and Recommended Determination ("ID/RD") finding that the Platform 1 blade products infringe two of the three patents asserted by Plaintiffs, recommending that the ITC issue a Limited Exclusion order against infringing products, and recommending against a cease-and-desist, as well as recommending against a bond pending Presidential review of the ITC's Final Determination ("FD"). Defendants and Plaintiffs filed separate Petitions for Review with the ITC of the ID/RD on May 28, 2021; on July 27, 2021, the ITC granted each of the petitions in part, deciding to review certain issues in the ID/RD. Plaintiffs and Defendants also submitted brief statements of the public interest factors in issue to the ITC on June 15, 2021. On September 29, 2021, the ITC issued its FD finding a violation of section 337 of the U.S. Tariff Act and issued (a) a limited exclusion order against PMPSA, prohibiting, inter alia, the importation of Platform 1 product and infringing components; and (b) a cease-and-desist order against Altria Client Services, LLC and its affiliate prohibiting, inter alia, sales of imported Platform 1 products. The ITC predicated the orders on its finding that Platform 1 blade products infringe two patents owned by a BAT affiliate. The ITC also found that Platform 1 blade products do not infringe a third patent owned by a BAT affiliate. The ITC further held that there were insufficient concerns over public interest to prevent the issuance of remedial orders. Following the Presidential Review period, the orders became effective and Defendants filed a petition for review of the FD with the U.S. Court of Appeals for the Federal Circuit. Defendants also filed motions in the ITC and Federal Circuit for a stay of the orders pending disposition of the appeal; the ITC denied the motion on January 20, 2022 and the Federal Circuit denied the motion on January 25, 2022. The Federal Circuit heard oral argument on defendants' appeal of the FD on October 3, 2022 and, on March 31, 2023, the Federal Circuit affirmed the FD. The Eastern District of Virginia and ITC cases filed by BAT are among the Proceedings to be dismissed pursuant to the Settlement Agreement including through the Parties’ request for rescission of the limited exclusion order and the cease-and-desist order issued by the ITC on September 29, 2021.
In the Eastern District of Virginia case, the defendants also counterclaimed that BAT infringed their patents relating to certain e-vapor products, seeking damages for, and injunctive relief against, the commercialization of these products by BAT. The trial of Defendant PMPSA’s counterclaims took place from June 8-14, 2022 and, on June 15, 2022, the jury returned a verdict for PMPSA awarding approximately $10.8 million in damages for infringement up to December 31, 2021 of two PMPSA patents by BAT’s affiliate and two of BAT’s e-vapor products; the jury also found BAT’s affiliate did not infringe one of the two PMPSA patents and that the BAT affiliates had failed to prove one of the two PMPSA patents was invalid. PMPSA filed a motion for an injunction or, in the alternative, an ongoing royalty on August 12, 2022. On March 30, 2023, the court denied PMPSA's motion for an injunction and granted PMPSA an ongoing royalty against two of BAT's U.S. e-vapor products. On May 1, 2023, the court entered partial final judgment under Rule 54(b) on PMPSA's claim against BAT's affiliate. That same day, BAT's affiliate filed a notice of appeal to the U.S. Court of Appeals for the Federal Circuit. On May 10, 2023, PMPSA filed a notice of cross-appeal. This case is among the Proceedings to be dismissed pursuant to the Settlement Agreement. Upon petition of PMPSA, the Patent Trial and Appeal Board ("PTAB") of the United States Patent and Trademark Office has instituted review of certain claims pertaining to four of the six patents asserted by BAT affiliates in both proceedings. On January 11, 2022, PTAB issued its final decision on one of the two patents underlying the ITC's FD, invalidating all challenged claims of BAT's patent. On March 30, 2022, PTAB issued its final decision on the second of the two patents underlying the ITC's FD, finding the challenged claims patentable. The parties have filed appeals of these PTAB results to the U.S. Court of Appeals for the Federal Circuit. Oral argument was held on July 13, 2023. On July 17, 2023, the Federal Circuit issued a decision summarily affirming PTAB’s decision to invalidate all challenged claims in one of the two patents underlying the ITC’s FD. The Federal Circuit issued the mandate notifying the USPTO to record the invalidity of the challenged claims on August 23, 2023. On September 14, 2023, the Federal Circuit issued a decision affirming the PTAB's decision finding certain claims in the second of the two patents underlying the ITC’s FD patentable. PMPSA’s counterclaim in the Eastern District of Virginia challenging the validity of the remaining claims in the second of the two patents underlying the ITC’s FD is currently stayed. On July 21, 2022, PMPSA filed a Request for Rehearing of PTAB's November 2020 decision not to institute review of certain claims in the second of the two patents underlying the ITC's FD; PTAB denied the Request on October 13, 2022.
In April 2020, BAT’s affiliate commenced patent infringement proceedings, Nicoventures Trading Limited v. PM GmbH, et al., against PMI’s German subsidiary, Philip Morris GmbH, and PMPSA, in the Regional Court in Munich, Germany. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade products in Germany. In June 2021, the court stayed the proceeding in respect of one of the two patents asserted by BAT’s Affiliate. Following the December 2022 confirmation of the revocation of the other BAT patent by the European Patent Office Board of Appeal, BAT withdrew its initial claim based on that patent; the stayed action based on the second patent remains pending and is stayed pending final resolution of the revocation action. This case is among the Proceedings to be dismissed pursuant to the Settlement Agreement.
In September 2020, BAT’s affiliates commenced patent infringement and unfair competition proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Products S.A., et al., against PMPSA and PMI’s Italian subsidiaries, Philip Morris Manufacturing & Technology Bologna S.p.A. and Philip Morris Italia S.r.l., in the Court of Milan, Italy. Plaintiffs seek damages, as well as injunctive relief against the manufacture in Italy of the Platform 1 blade heated tobacco units allegedly infringing the asserted patents and the commercialization of the Platform 1 blade products in Italy. As part of this proceeding, in October 2020, BAT’s affiliates filed a request based on one of the two asserted patents seeking preliminary injunctive relief against the manufacture and commercialization of the Platform 1 blade products in Italy. In July 2022, the court dismissed plaintiffs’ request for preliminary injunction in its entirety and plaintiffs did not appeal this ruling.
The merits proceeding remains pending; the next hearing is currently scheduled to occur in the first quarter of 2024. This case is among the Proceedings to be dismissed pursuant to the Settlement Agreement.
In October 2020, BAT’s affiliates commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Japan, Limited, et al., against PMI’s Japanese subsidiary, Philip Morris Japan Limited, and a third-party distributor in the Tokyo District Court. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade products in Japan. On December 23, 2022, the Court dismissed BAT’s claims with respect to one of the two patents that it asserted, finding no infringement; BAT filed an appeal of this dismissal. On September 21, 2023, the IP High Court issued its judgment dismissing BAT's appeal regarding the first patent. BAT appealed this decision to the Supreme Court on November 2, 2023. On November 29, 2023, the Tokyo District Court issued a first instance decision favorable to PMI, finding no infringement of the second patent BAT asserted and dismissing BAT's claim. BAT appealed this decision to the IP High Court on January 12, 2024. These cases are among the Proceedings to be dismissed pursuant to the Settlement Agreement.
In November 2020, BAT’s affiliates commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Romania SRL, et al., against PMI’s Romanian subsidiaries, Philip Morris Romania S.R.L. and Philip Morris Trading S.R.L., and a third-party distributor in the Court of Law of Bucharest, Civil Registry. Plaintiffs seek damages and preliminary and permanent injunctive relief against the manufacture and commercialization of the Platform 1 blade products in Romania. In February 2021, the court dismissed plaintiffs’ request for a preliminary injunction. In April 2021, the appellate court denied plaintiffs' appeal, confirming the dismissal of plaintiffs' request for preliminary injunction. Plaintiffs' proceeding requesting damages and a permanent injunction remains pending before the Court of Law of Bucharest, Civil Registry. In an October 14, 2021 hearing, the court stayed the proceeding. This case is among the Proceedings to be dismissed pursuant to the Settlement Agreement.
In March 2021, BAT’s affiliates commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Korea, Co., Ltd., against PM Korea in the Seoul Central District Court. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade heated tobacco units in South Korea. This case is among the Proceedings to be dismissed pursuant to the Settlement Agreement. On May 30, 2022, the Korean Patent Office issued a decision that all of the challenged claims in the patent asserted by Plaintiffs are invalid; Plaintiffs filed an appeal of this decision. Following BAT’s unsuccessful correction action at the Korean Patent Office, the court held the first hearing in the appeal of the infringement proceeding on September 12, 2023. An additional hearing in the appeal is currently scheduled to occur in the fourth quarter of 2024.
In July, 2021, PMPSA filed a claim at the High Court of Justice of England and Wales against BAT affiliates Nicoventures Trading Limited and British American Tobacco (Investments) Limited seeking revocation of the UK parts of two BAT European patents. In March, the BAT affiliates stated that they would consent to revocation of one of the patents and filed a counterclaim against PMPSA and Philip Morris Limited seeking from the court a declaration that the remaining BAT affiliate patent is infringed by Platform 1 induction products, as well as damages and injunctive relief against the commercialization of the Platform 1 induction products in the U.K. The trial took place from September 21-28, 2022. On October 25, 2023, the Court issued its decision finding that the remaining BAT patent was invalid and not infringed. BAT has elected not to appeal this decision.
Other patent challenges by both parties are pending in various jurisdictions.
On December 21, 2023, we were informed that Future Technology K.K. (“FTKK”) filed an application with Tokyo Customs against Sojitz Corporation (“Sojitz”), Philip Morris Japan Limited’s (“PMJL”) importer and distributor, due to alleged infringement of JP7299432. FTKK is seeking an order stopping the importation of TEREA consumables. At this time, FTKK is not seeking any monetary damages or costs. PMJL has entered an appearance in the proceeding as an interested party and filed its response to FTKK's application on January 31, 2024. We believe that this lawsuit is without merit and will defend it vigorously. On January 26, 2024, PMJL filed a declaratory judgment action in Tokyo District Court seeking a declaration that JP7299432 is invalid and/or infringed.
We are also involved in additional litigation arising in the ordinary course of our business. While the outcomes of these proceedings are uncertain, management does not expect that the ultimate outcomes of other litigation, including any reasonably possible losses in excess of current accruals, will have a material adverse effect on our consolidated results of operations, cash flows or financial position.
Third-Party Guarantees
Until November 1, 2022, Medicago Inc. ("Medicago") was an equity method investee of Philip Morris Investments B.V. (“PMIBV”), a PMI subsidiary. On October 17, 2020, Medicago had entered into a contribution agreement with the Canadian government (the “Contribution Agreement”) whereby the Canadian government agreed to contribute up to CAD 173 million (approximately $131 million on the date of signing) to Medicago, to support its on-going COVID-19 vaccine development and clinical trials ("First Stage"), and for the construction of its Quebec City manufacturing facility ("Second Stage", and together with the First Stage, the “Project”). On March 31, 2022, the Contribution Agreement was amended (the “Contribution Agreement Amendment”) to reflect an additional contribution from the Canadian government up to CAD 27 million (approximately $22 million on the date of signing) to Medicago for the Second Stage.
In August 2022, Medicago received the final tranche of the contribution from the Canadian government in relation to the First Stage, confirming thereby the completion of such first stage and consequently reducing by approximately CAD 123 million (approximately $93 million on the date of signing) the Repayment Obligations (as defined below).
PMIBV and Mitsubishi Tanabe Pharma Corporation (“MTPC”) are also parties to the Contribution Agreement and the Contribution Agreement Amendment as guarantors of Medicago’s obligations thereunder on a joint and several basis (“Co-Guarantors”). The Co-Guarantors agreed to repay amounts contributed by the Canadian government plus interest, if Medicago fails to do so (the "Repayment Obligations"), and could be responsible for the costs of Medicago’s other obligations (such as the achievement of specific milestones of the Project). The guarantees are in effect through March 31, 2026. Prior to the release of PMIBV from its obligations as guarantor described below, it was reasonably possible that PMI could have been responsible for a portion of those costs and obligations.
On November 1, 2022, PMIBV transferred all of the shares it owned in Medicago to MTPC Holdings Canada Inc., the majority shareholder of Medicago. MTPC assumed and agreed to perform all of PMIBV's obligations under the guarantees and to indemnify and save PMIBV harmless in respect of any and all claims related to the guaranteed obligations. On February 3, 2023, PMI learned through a public announcement that a decision had been taken to cease all operations at Medicago and to proceed with an orderly wind up of Medicago’s business and operations.
On September 27, 2023, the Canadian government released PMIBV from all its obligations as guarantor under the Contribution Agreement and the Contribution Agreement Amendment.
Sale of Accounts Receivable:
To mitigate risk and enhance cash and liquidity management PMI sells trade receivables to unaffiliated financial institutions. These arrangements allow PMI to sell, on an ongoing basis, certain trade receivables without recourse. The trade receivables sold are generally short-term in nature and are removed from the consolidated balance sheets. PMI sells trade receivables under two types of arrangements, servicing and non-servicing. For servicing arrangements, PMI continues to service the sold trade receivables on an administrative basis and does not act on behalf of the unaffiliated financial institutions. When applicable, a servicing liability is recorded for the estimated fair value of the servicing. The amounts associated with the servicing liability were not material for the years ended December 31, 2023 and 2022. Under the non-servicing arrangements, PMI does not provide any administrative support or servicing after the trade receivables have been sold to the unaffiliated financial institutions.
Cumulative trade receivables sold, including excise taxes, for the years ended December 31, 2023 and 2022, were $13.3 billion and $11.9 billion, respectively. PMI’s operating cash flows were positively impacted by the amount of the trade receivables sold and derecognized from the consolidated balance sheets, which remained outstanding with the unaffiliated financial institutions. The trade receivables sold that remained outstanding under these arrangements as of December 31, 2023, 2022 and 2021, were $1.6 billion, $1.0 billion and $0.9 billion, respectively. The net proceeds received are included in cash provided by operating activities in the consolidated statements of cash flows. The difference between the carrying amount of the trade receivables sold and the sum of the cash received is recorded as a loss on sale of trade receivables within marketing, administration and research costs in the consolidated statements of earnings. For the years ended December 31, 2023, 2022 and 2021 the loss on sale of trade receivables was $49 million, $26 million and $9 million, respectively.
Asset Impairment and Exit Costs:
For the years ended December 31, 2023 and 2021, PMI recorded total pre-tax asset impairment and exit costs of $109 million and $216 million, respectively, related to restructuring activities. These pre-tax asset impairment and exit costs were included in marketing, administration and research costs on the consolidated statements of earnings. For the year ended December 31, 2022, PMI did not record any charges for asset impairment and exit costs related to restructuring activities.
For the year ended December 31, 2023, PMI recorded a pre-tax impairment charge on goodwill and other intangibles of $680 million within the Wellness and Healthcare segment. For the year ended December 31, 2022, PMI recorded a pre-tax impairment charge on other intangibles of $112 million within the Wellness and Healthcare segment.
For further details, see Note 5. Goodwill and Other Intangible Assets, net.
For the year ended December 31, 2023, PMI recorded an impairment of certain long-lived assets in Ukraine. See Note 4. War in Ukraine for the impact of the war on PMI.
e-Vapor Products Manufacturing Optimization
In the first quarter of 2023, PMI initiated a project to fully outsource and restructure the manufacturing of e-vapor devices and consumables. As a result, PMI recorded pre-tax asset impairment and exit costs of $109 million. This amount included contract termination costs for suppliers of $78 million, including $21 million of embedded finance lease terminations, payable in cash. This amount also included asset impairment costs of $31 million, primarily related to machinery and equipment, which were non-cash charges.
South Korea
In 2021, PM Korea implemented a new business operating model, which required the restructuring of its current distribution agreements. As a result, PMI recorded exit costs of $57 million in the year ended December 31, 2021, related to contract terminations and restructuring with certain distributors.
Organizational Design Optimization
As part of PMI’s transformation to a smoke-free future, PMI sought to optimize its organizational design, which included the elimination, relocation and outsourcing of certain operations center and centralized activities. In January 2020, PMI commenced a multi-phase restructuring project in Switzerland. PMI initiated the employee consultation procedures, as required under Swiss law, for the impacted employees. The consultation procedures for the first two phases were completed in 2020 with the final phases initiated and completed in 2021. Additionally, since the commencement of this multi-phase restructuring project in 2020, PMI launched a voluntary separation program in Switzerland for certain eligible employees and announced the outsourcing of certain activities in Argentina, Indonesia, Poland and the United States. This multi-phase restructuring project was completed in the fourth quarter of 2021.
For the year ended December 31, 2021, PMI recorded pre-tax charges of $159 million related to the organizational design optimization. Since inception of this multi-phase restructuring project in January 2020 through December 31, 2021, approximately 1,020 positions in total were impacted, resulting in cumulative pre-tax charges of $308 million related to the organizational design optimization program. Of this cumulative pre-tax amount, $300 million related to separation program charges and $8 million related to asset impairment charges.
Asset Impairment and Exit Costs by Segment
During 2023 and 2021, PMI recorded the following pre-tax asset impairment and exit costs by segment related to restructuring activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in millions) |
2023 |
|
|
|
2021 |
Separation programs: (1) |
|
|
|
|
|
| Europe |
$ |
— |
|
|
|
$ |
72 |
| SSEA, CIS & MEA |
— |
|
|
|
45 |
| EA, AU & PMI DF |
— |
|
|
|
34 |
| Americas |
— |
|
|
|
8 |
| Swedish Match |
— |
|
|
|
— |
| Wellness and Healthcare |
— |
|
|
|
— |
| Total separation programs |
— |
|
|
|
|
159 |
|
Contract termination charges: (1) |
|
|
|
|
|
| Europe |
35 |
|
|
|
— |
| SSEA, CIS & MEA |
25 |
|
|
|
— |
| EA, AU & PMI DF |
15 |
|
|
|
57 |
| Americas |
3 |
|
|
|
— |
| Swedish Match |
— |
|
|
|
— |
| Wellness and Healthcare |
— |
|
|
|
— |
| Total contract termination charges |
78 |
|
|
|
|
57 |
Asset impairment charges (1) |
|
|
|
|
|
| Europe |
14 |
|
|
|
— |
| SSEA, CIS & MEA |
9 |
|
|
|
— |
| EA, AU & PMI DF |
6 |
|
|
|
— |
| Americas |
2 |
|
|
|
— |
| Swedish Match |
— |
|
|
|
— |
| Wellness and Healthcare |
— |
|
|
|
— |
| Total asset impairment charges |
31 |
|
|
|
|
— |
| Asset impairment and exit costs |
$ |
109 |
|
|
|
|
216 |
(1) Organizational design optimization pre-tax charges in 2021 and e-vapor products manufacturing optimization charges in 2023 were allocated across all geographical segments.
Movement in Exit Cost Liabilities
The movement in exit cost liabilities for the year ended December 31, 2023 was as follows:
|
|
|
|
|
|
| (in millions) |
|
| Liability balance, January 1, 2023 |
$ |
40 |
|
| Charges, net |
78 |
|
| Cash spent |
(79) |
|
| Currency/other |
(10) |
|
| Liability balance, December 31, 2023 |
$ |
29 |
|
Future cash payments for exit costs incurred to date are anticipated to be substantially paid by the end of 2024.
Leases:
PMI has operating and finance leases that are principally for real estate (office space, warehouses and retail store space), machinery and equipment, and vehicles. Lease terms range from 1 year to 70 years, some of which include options to renew, which are reasonably certain to be renewed. Lease terms may also include options to terminate the lease. The exercise of a lease renewal or termination option is at PMI’s discretion.
PMI’s operating and finance leases at December 31, 2023 and 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, |
| (in millions) |
2023 |
2022 |
|
Operating Leases |
Finance Leases |
Operating Leases |
Finance Leases |
| Assets: |
|
|
|
|
| Machinery and equipment |
$ |
— |
|
$ |
111 |
|
$ |
— |
|
$ |
123 |
|
| Other assets |
631 |
|
— |
|
594 |
|
— |
|
| Total lease assets |
$ |
631 |
|
$ |
111 |
|
$ |
594 |
|
$ |
123 |
|
|
|
|
|
|
| Liabilities: |
|
|
|
|
| Current |
|
|
|
|
| Current portion of long-term debt |
$ |
— |
|
$ |
30 |
|
$ |
— |
|
$ |
34 |
|
| Accrued liabilities - Other |
197 |
|
— |
|
178 |
|
— |
|
| Noncurrent |
|
|
|
|
| Long-term debt |
— |
|
23 |
|
— |
|
20 |
|
| Income taxes and other liabilities |
456 |
|
— |
|
436 |
|
— |
|
| Total lease liabilities |
$ |
653 |
|
$ |
53 |
|
$ |
614 |
|
$ |
54 |
|
The components of PMI’s lease cost were as follows for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
| (in millions) |
2023 |
2022 |
2021 |
|
| Operating lease cost |
$ |
266 |
|
$ |
248 |
|
$ |
259 |
|
|
| Finance lease cost: |
|
|
|
|
| Amortization of right-of-use assets |
49 |
|
83 |
|
54 |
|
|
| Interest on lease liabilities |
1 |
|
1 |
|
1 |
|
|
| Short-term lease cost |
59 |
|
59 |
|
55 |
|
|
| Variable lease cost |
28 |
|
23 |
|
25 |
|
|
| Total lease cost |
$ |
403 |
|
$ |
414 |
|
$ |
394 |
|
|
Maturity of PMI’s lease liabilities, on an undiscounted basis, as of December 31, 2023, were as follows:
|
|
|
|
|
|
|
|
|
| (in millions) |
Operating Leases |
Finance Leases |
| 2024 |
$ |
228 |
|
$ |
31 |
|
| 2025 |
159 |
|
12 |
|
| 2026 |
109 |
|
6 |
|
| 2027 |
72 |
|
5 |
|
| 2028 |
45 |
|
2 |
|
| Thereafter |
163 |
|
— |
|
| Total lease payments |
776 |
|
56 |
|
| Less: Interest |
123 |
|
3 |
|
| Present value of lease liabilities |
$ |
653 |
|
$ |
53 |
|
Other information related to PMI’s leases was as follows for the years ended December 31, 2023, 2022 and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
| (in millions) |
2023 |
2022 |
2021 |
|
Operating Leases |
Finance Leases |
Operating Leases |
Finance Leases |
Operating Leases |
Finance Leases |
Cash paid for amounts included in the measurement of lease liabilities in operating cash flows (1) |
$ |
265 |
|
$ |
— |
|
$ |
243 |
|
$ |
— |
|
$ |
259 |
|
$ |
— |
|
| Cash paid for amounts included in the measurement of lease liabilities in financing cash flows |
$ |
— |
|
$ |
27 |
|
$ |
— |
|
$ |
76 |
|
$ |
— |
|
$ |
26 |
|
| Leased assets obtained in exchange for new lease liabilities |
$ |
205 |
|
$ |
55 |
|
$ |
255 |
|
$ |
100 |
|
$ |
64 |
|
$ |
89 |
|
| Weighted-average remaining lease term (years) |
10.2 |
2.6 |
10.3 |
2.1 |
8.3 |
1.7 |
Weighted-average discount rate(2) (3) |
5.1 |
% |
4.9 |
% |
3.4 |
% |
4.4 |
% |
3.6 |
% |
5.3 |
% |
(1) Cash paid included in the operating cash flows for finance leases is not material.
(2) PMI’s weighted-average discount rate for operating leases is based on its estimated pre-tax cost of debt adjusted for country-specific risk.
(3) PMI’s weighted-average discount rate for finance leases, excluding embedded leases, is based on its estimated pre-tax cost of debt adjusted for country-specific risk and where applicable the interest rate explicit in lease contracts.
Supply Chain Financing:
PMI has engaged with unaffiliated global financial institutions that offer a voluntary supply chain financing ("SCF") program to some of our suppliers. Under the SCF program, the suppliers may elect, at their sole discretion, to sell PMI's payment obligations to these financial institutions. The suppliers independently negotiate the sale arrangements directly with these financial institutions. PMI does not participate in these negotiations, nor does it have any economic interest in these agreements, or in the designated suppliers’ voluntary decision to sell PMI's payment obligations to these financial institutions. No guarantees or securities are provided by PMI or any of its subsidiaries under the SCF programs. PMI's obligations to its suppliers, including amounts due and scheduled payment terms are not impacted by the suppliers’ decision to sell amounts under the SCF program. The payment terms of PMI’s suppliers generally do not exceed 120 days. All outstanding payable amounts related to suppliers that are participating in the SCF program are recorded in accounts payable in PMI's consolidated balance sheets. The associated payments are included in cash flows from operating activities within PMI's consolidated statement of cash flows. As of December 31, 2023 and 2022, the total amount due to suppliers participating in the SCF program was approximately $0.9 billion and $1.1 billion, respectively.
New Accounting Standards:
Improvements to Reportable Segment Disclosures
On November 27, 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update ASU 2023-07, “Improvements to Reportable Segment Disclosures” (“ASU 2023-07”). ASU 2023-07 improves reportable segment disclosures, primarily through enhanced disclosures about significant segment expenses regularly provided to the chief operating decision maker that impact segment profit or loss.
The amendments are effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024, on a retrospective basis, with early adoption permitted. PMI is currently evaluating the impact of ASU 2023-07 on its disclosures.
Improvements to Income Tax Disclosures
On December 14, 2023, the FASB issued Accounting Standards Update ASU 2023-09, “Improvements to Income Tax Disclosures” (“ASU 2023-09”). ASU 2023-09 enhances the transparency of income tax disclosures, primarily by requiring public business entities to disclose specific categories in the rate reconciliation tabular presentation, as well as by providing additional information for reconciling items that meet a quantitative threshold. The ASU also requires disaggregated disclosures of federal, state and foreign income tax taxes paid.
ASU 2023-09 is effective for annual periods beginning after December 15, 2024, and early adoption is permitted. The amendments are applicable on a prospective basis, although retrospective basis is also permitted. PMI is currently evaluating the impact of ASU 2023-09 on its disclosures.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Philip Morris International Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Philip Morris International Inc. and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of earnings, comprehensive earnings, stockholders’ (deficit) equity and cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Report of Management on Internal Control Over Financial Reporting. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Tobacco-Related Litigation for Smoking and Health Class Actions and Health Care Cost Recovery Actions
As described in Note 18 to the consolidated financial statements, the Company has 9 smoking and health class actions and 17 health care cost recovery actions pending. The Company records provisions in the consolidated financial statements for pending litigation when management determines that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. Except as stated otherwise in Note 18, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available, (i) management has not concluded that it is probable that a loss has been incurred in any of the pending smoking and health class actions and health care cost recovery cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending smoking and health class actions and health care cost recovery cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any.
The principal considerations for our determination that performing procedures relating to tobacco-related litigation for smoking and health class actions and health care cost recovery actions is a critical audit matter are that there was significant judgment by management when determining the probability of a loss being incurred and an estimate of the amount or range of the potential loss for each case, which in turn led to a high degree of auditor subjectivity, judgment and effort in evaluating management’s assessment related to the loss contingencies associated with smoking and health class actions and health care cost recovery actions related claims.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s evaluation of smoking and health class actions and health care cost recovery actions, including controls over determining the probability and range of loss as well as controls over financial statement disclosures. These procedures also included, among others, obtaining and evaluating the letters of audit inquiry with external and internal legal counsel, evaluating the reasonableness of management’s assessment regarding whether an unfavorable outcome is reasonably possible or probable and reasonably estimable, and evaluating the sufficiency of the Company’s smoking and health class actions and health care cost recovery actions contingencies disclosures.
Acquisition of Swedish Match AB - Valuation of Trademarks and Customer Relationships
As described in Note 3 to the consolidated financial statements, the Company acquired a controlling interest in Swedish Match AB for consideration of $14.5 billion in 2022, which resulted in $7.9 billion of intangible assets being recorded, of which $7.8 billion relate to trademarks and customer relationships. Management applied significant judgment in estimating the fair value of intangible assets acquired, which involved the use of significant estimates and assumptions with respect to the revenue growth rates, royalty rates, and discount rates for trademarks, and revenue growth rates, profit margins, customer attrition rates, and discount rates for customer relationships.
The principal considerations for our determination that performing procedures relating to the valuation of trademarks and customer relationships acquired in the acquisition of Swedish Match AB is a critical audit matter are the significant judgment by management when developing the fair value estimate of the trademarks and customer relationships acquired, which in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions of revenue growth rates, royalty rates, and discount rates for trademarks, and revenue growth rates, profit margins, customer attrition rates, and discount rates for customer relationships. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the acquisition accounting, including controls over management’s valuation of the trademarks and customer relationships acquired and controls over the development of significant assumptions related to revenue growth rates, profit margins, customer attrition rates, royalty rates, and discount rates.
These procedures also included, among others, testing management’s process for estimating the fair value of trademarks and customer relationships. Testing management’s process included evaluating the appropriateness of the valuation methods, testing the completeness and accuracy of data provided by management, and evaluating the reasonableness of significant assumptions related to revenue growth rates, profit margins, customer attrition rates, royalty rates, and discount rates. Evaluating the reasonableness of the revenue growth rates and profit margins involved considering the past performance of the acquired business, as well as economic and industry forecasts. Professionals with specialized skill and knowledge were used to assist in the evaluation of management’s valuation methods, and the reasonableness of the customer attrition rate, royalty rate, and discount rate assumptions.
|
|
|
|
|
|
|
|
|
/S/ PRICEWATERHOUSECOOPERS SA |
|
|
PricewaterhouseCoopers SA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lausanne, Switzerland |
|
|
| February 8, 2024 |
|
|
We have served as the Company’s auditor since 2008.
Report of Management on Internal Control Over Financial Reporting
Management of Philip Morris International Inc. (“PMI” or "we") is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. PMI’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes those written policies and procedures that:
•pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of PMI;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America;
•provide reasonable assurance that receipts and expenditures of PMI are being made only in accordance with the authorization of management and directors of PMI; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements.
Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and actions taken to correct deficiencies as identified.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of PMI’s internal control over financial reporting as of December 31, 2023. Management based this assessment on criteria for effective internal control over financial reporting described in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included an evaluation of the design of PMI’s internal control over financial reporting and testing of the operational effectiveness of its internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of our Board of Directors.
Based on this assessment, management determined that, as of December 31, 2023, PMI maintained effective internal control over financial reporting.
PricewaterhouseCoopers SA, an independent registered public accounting firm, who audited and reported on the consolidated financial statements of PMI included in this report, has audited the effectiveness of PMI’s internal control over financial reporting as of December 31, 2023, as stated in their report herein.
February 8, 2024
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A.Controls and Procedures.
PMI carried out an evaluation, with the participation of PMI’s management, including PMI’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of PMI’s disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report. Based upon that evaluation, PMI’s Chief Executive Officer and Chief Financial Officer concluded that PMI’s disclosure controls and procedures are effective. There have been no changes in PMI’s internal control over financial reporting during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, PMI’s internal control over financial reporting.
The Report of Management on Internal Control over Financial Reporting and the Report of Independent Registered Public Accounting Firm are included in Item 8.
Item 9B.Other Information.
On February 6, 2024, Jun Makihara informed PMI's board of directors (the “Board”) that he will not stand for re-election to the Board at our 2024 annual meeting of shareholders. Mr. Makihara’s decision not to stand for re-election to the Board was not a result of any disagreement with the Company.
During the three months ended December 31, 2023, no director or officer of PMI adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as such terms are defined in Item 408(a) of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
Except for the information relating to the executive officers set forth in Item 10 and the information relating to equity compensation plans set forth in Item 12, the information called for by Items 10-14 is hereby incorporated by reference to PMI’s definitive proxy statement for use in connection with its annual meeting of stockholders to be held on May 8, 2024, that will be filed with the SEC on or about March 28, 2024 (the “proxy statement”), and, except as indicated therein, made a part hereof.
Item 10.Directors, Executive Officers and Corporate Governance.
Information About Our Executive Officers as of February 8, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
|
Office |
|
Age |
|
| Jacek Olczak |
|
Chief Executive Officer |
|
59 |
|
|
| Massimo Andolina |
|
President, Europe Region |
|
55 |
|
|
| Emmanuel Babeau |
|
Chief Financial Officer |
|
56 |
|
|
| Werner Barth |
|
President, Combustibles Category & Global Combustibles Marketing |
|
59 |
|
|
| Lars Dahlgren |
|
President, Smoke-Free Oral Products & Chief Executive Officer Swedish Match |
|
53 |
|
|
| Frederic de Wilde |
|
President, South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region |
|
56 |
|
|
| Reginaldo Dobrowolski |
|
Vice President and Controller |
|
49 |
|
|
| Yann Guérin |
|
Senior Vice President and General Counsel |
|
47 |
|
|
| Stacey Kennedy |
|
President, Americas Region & CEO of PMI's U.S. Business |
|
51 |
|
|
| Paul Riley |
|
President, East Asia, Australia, and PMI Duty Free Region |
|
58 |
|
|
| Stefano Volpetti |
|
President, Smoke-Free Inhalable Products & Chief Consumer Officer |
|
52 |
|
|
Jacek Olczak – Age 59
Mr. Olczak was appointed as our Chief Executive Officer in May 2021. From January 2018 until May 2021, Mr. Olczak has served as our Chief Operating Officer, and from August 2012 until December 31, 2017, he served as our Chief Financial Officer. He joined PMI’s Polish affiliate in 1993 and progressed through various roles in finance and general management positions across Europe, including as Managing Director of PMI’s markets in Poland and Germany and as President of the European Union Region, before being appointed Chief Financial Officer. Prior to joining PMI, Mr. Olczak worked for BDO, an international network of public accounting, tax, consulting and business advisory firms.
Massimo Andolina – Age 55
Mr. Andolina was appointed as our President, Europe Region in January 2023, prior to which he served as our Senior Vice President, Operations since January 2018. He joined PMI in 2008 as Director, Operations Planning, and has held several various roles at PMI, including Vice President, Operations of Latin America & Canada Region from December 2010 to July 2013; Vice President, EU Operations, from August 2013 to June 2016; and Vice President, PMI Transformation from July 2016 to December 2017. Prior to joining PMI, Mr. Andolina held a variety of international positions in strategic marketing and general management for Tetra Pak International and in operations for R.J. Reynolds International.
Emmanuel Babeau – Age 56
Mr. Babeau was appointed as our Chief Financial Officer in May 2020. Prior to joining PMI in May 2020, Mr. Babeau served as the Deputy Chief Executive Officer of Schneider Electric, an energy and automation digital solutions company. In this position, he was in charge of Finance and Legal Affairs. Mr. Babeau joined Schneider Electric in 2009 as Executive Vice President Finance and a member of the Management Board. Mr. Babeau also served on the board of Sanofi S.A., a French multinational healthcare company, from 2018 to 2020. Mr. Babeau started his career in 1990 at Arthur Andersen, and from 1993 to 2009, he progressed through various positions at Pernod Ricard, a beverage company, the latest being Chief Financial Officer and Group Deputy Managing Director. Mr. Babeau also served as a non-executive director at Sodexo, a French food services and facilities management company, from January 2016 until December 2021. He currently sits on the board of Davide Campari-Milano N.V.
Werner Barth – Age 59
Mr. Barth was appointed as our President, Combustibles Category & Global Combustibles Marketing in November 2021. Mr. Barth joined PMI in 1990 as Marketing Trainee at Philip Morris Germany and throughout his career he progressed through various roles at PMI in marketing, product management, brand supervision and general management. Prior to his current position, from 2015, Mr. Barth held the role of Senior Vice President, Marketing & Sales, and from 2018, he held the role of Senior Vice President, Commercial.
Lars Dahlgren – Age 53
Mr. Dahlgren was appointed as our President Smoke-Free Oral Products and CEO Swedish Match in January 2023. Prior to PMI’s acquisition of Swedish Match, he served as President and Chief Executive Officer of Swedish Match since June 2008, and as its Chief Financial Officer and Senior Vice President from July 2004 until June 2008. Prior to that, from April 2004 to July 2004, he was Acting Chief Financial Officer and Vice President of Finance at Swedish Match. Mr. Dahlgren joined Swedish Match in 1996 and has been a member of its Group Management Team since 2004.
Frederic de Wilde – Age 56
Mr. de Wilde was appointed as our President, South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Regions in January 2023, prior to which he served as President, European Union Region from July 2015. From July 2011 until July 2015, Mr. de Wilde held the role of Senior Vice President, Marketing & Sales. Mr. de Wilde joined PMI in 1992 as Brand Manager L&M at Philip Morris Belgium, and throughout his career, he progressed through various roles at PMI in marketing, sales and general management.
Reginaldo Dobrowolski – Age 49
Mr. Dobrowolski was appointed as our Vice President and Controller in August 2021. From May 2019 until August 2021, Mr. Dobrowolski was our Vice President, Corporate Financial Planning, Data & Reporting. Prior to that, Mr. Dobrowolski held various roles in our Finance department, including Director Corporate Financial Planning & Reporting from October 2014 until May 2019.
Yann Guérin - Age 47
Mr. Guérin was appointed as Senior Vice President and General Counsel in July 2023, having served as Senior Vice President and Global Head of Law and Compliance for June 2023. Previously, he served as Vice President and Associate General Counsel, Corporate from July 2021 to May 2023; as Vice President and Associate General Counsel, South & Southern Asia from November 2019 to June 2021; and as Vice President and Associate General Counsel, Middle-East, Africa & Global Duty Free from January 2018 to October 2019. Prior to that, since joining PMI in 2006, Mr. Guérin held a variety of legal roles across the company’s businesses, regions and functions. Before joining PMI, he was an attorney at Skadden Arps.
Stacey Kennedy – Age 51
Ms. Kennedy was appointed as our President, Americas Region & CEO of PMI's U.S. Business in January 2023. Previously, she served as our President, South and Southeast Asia Region from January 2018. From 2015 until 2018, Ms. Kennedy served as Managing Director for Germany, Austria, Croatia, and Slovenia. Ms. Kennedy began her career with Philip Morris USA in 1995 as a Territory Sales Manager. Throughout her career, she held a number of positions of increasing responsibility in commercial and general management.
Paul Riley – Age 58
Mr. Riley was appointed as our President, East Asia, Australia, and PMI Duty Free Region in January 2023. Previously, he served as our President, East Asia and Australia Region from January 2018. From 2015 until 2018, Mr. Riley served as President of Philip Morris Japan. Mr. Riley joined Philip Morris Australia in 1988. Over the following two decades, he held a number of positions in Australia, Hong Kong, and Japan, before being named Managing Director, Serbia & Montenegro in 2010. Mr. Riley returned to the Asia Region in 2013, when he became President of Philip Morris Fortune Tobacco Corporation in the Philippines.
Stefano Volpetti – Age 52
Mr. Volpetti was appointed as our President Smoke-Free Inhalable Products & Chief Consumer Officer in January 2023, having served as President Smoke-Free Products Category & Chief Consumer Officer from November 2021. Mr. Volpetti joined PMI in June 2019 as Chief Consumer Officer. From February 2016 until May 2019, Mr. Volpetti served as the Vice President & Brand Franchise Leader of a multi-functional, global business unit at Procter & Gamble, a multinational consumer goods company. Mr. Volpetti spent 22 years at Procter & Gamble, progressing through various roles with increasing responsibility locally in Italy and Mexico, and on a regional level for the European market. Mr. Volpetti also served as Chief Marketing Officer at Luxottica Group S.p.A, an Italian eyewear conglomerate, in 2015.
Codes of Ethics and Corporate Governance
We have adopted a code of ethics, which we call the Code of Conduct. The Code of Conduct complies with requirements set forth in Item 406 of Regulation S-K, applies to all of our employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, and persons performing similar functions. We have also adopted a code of business conduct and ethics that applies to the members of our Board of Directors. These documents are available free of charge on our website at www.pmi.com.
In addition, we have adopted corporate governance guidelines and charters for our Audit and Risk, Compensation and Leadership Development, Science and Technology and Nominating and Corporate Governance committees of the Board of Directors. All of these documents are available free of charge on our website at www.pmi.com. Any waiver granted by Philip Morris International Inc. to its principal executive officer, principal financial officer or controller, or any person performing similar functions under our code of ethics, or certain amendments to the code of ethics, will be disclosed on our website at www.pmi.com.
The information on our website is not, and shall not be deemed to be, a part of this Report or incorporated into any other filings made with the SEC.
Also refer to Board Operations and Governance—Committees of the Board, Election of Directors—Process for Nominating Directors, Election of Directors—Director Nominees and Stock Ownership Information and Availability of Reports, Other Matters and 2024 Annual Meetings—2024 Annual Meeting sections of the proxy statement.
Item 11.Executive Compensation.
Refer to Compensation Discussion and Analysis, Compensation Tables, Compensation of Directors, and Pay Ratio sections of the proxy statement.
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The number of shares to be issued upon exercise or vesting and the number of shares remaining available for future issuance under PMI’s equity compensation plans at December 31, 2023, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Securities
to be Issued upon
Exercise of Outstanding
Options and Vesting of RSUs and PSUs
(a)
|
|
Weighted Average
Exercise Price of
Outstanding Options
(b)
|
|
Number of Securities
Remaining Available for
Future Issuance Under
Equity Compensation Plans
(excluding Securities
reflected in column (a))
(c)
|
|
Equity compensation plans
approved by stockholders |
7,457,881 |
|
1 |
$ |
— |
|
|
23,047,756 |
|
|
1 Represents 4,603,321 shares of common stock that may be issued upon vesting of the restricted share units and 2,854,560 shares that may be issued upon vesting of the performance share units if maximum performance targets are achieved for each performance cycle. PMI has not granted options since the spin-off from Altria on March 28, 2008.
Also refer to Stock Ownership Information—Ownership of Equity Securities section of the proxy statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Refer to Related Person Transactions and Code of Conduct and Election of Directors—Independence of Nominees sections of the proxy statement.
Item 14.Principal Accounting Fees and Services.
Refer to Audit and Risk Committee Matters section of the proxy statement.
PART IV
Item 15.Exhibits and Financial Statement Schedules.
(a) Index to Consolidated Financial Statements and Schedules
|
|
|
|
|
|
|
Page |
Consolidated Statements of Earnings for the years ended December 31, 2023, 2022 and 2021 |
|
|
Consolidated Statements of Comprehensive Earnings for the years ended December 31, 2023,
2022 and 2021
|
|
Consolidated Balance Sheets at December 31, 2023 and 2022 |
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2023, 2022
and 2021
|
|
|
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended
December 31, 2023, 2022 and 2021
|
|
| Notes to Consolidated Financial Statements |
|
Report of Independent Registered Public Accounting Firm (PCAOB ID 1358) |
|
| Report of Management on Internal Control Over Financial Reporting |
|
Schedules have been omitted either because such schedules are not required or are not applicable.
(b) The following exhibits are filed as part of this Report:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2.1 |
|
— |
|
|
| 2.2 |
|
— |
|
|
| 3.1 |
|
— |
|
|
| 3.2 |
|
— |
|
|
| 4.1 |
|
— |
|
|
| 4.2 |
|
— |
|
|
| 4.3 |
|
— |
|
|
| 4.4 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.4 |
|
__ |
|
Extension Agreement, effective February 7, 2017, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders party thereto, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 30, 2017). |
| 10.5 |
|
__ |
|
Extension Agreement, effective January 31, 2014, to Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders party thereto and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.3 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2014). |
| 10.6 |
|
__ |
|
Extension Agreement, effective as of February 10, 2015, to Credit Agreement dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2015). |
| 10.7 |
|
__ |
|
Amendment No. 1, dated as of July 20, 2015, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, The Royal Bank of Scotland plc, as resigning administrative agent, and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as successor administrative agent (incorporated by reference to Exhibit 10.52 to the Annual Report on Form 10-K for the year ended December 31, 2015). |
| 10.8 |
|
— |
|
Credit Agreement, dated as of October 1, 2015, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed October 5, 2015). |
| 10.9 |
|
—
|
|
|
| 10.10 |
|
—
|
|
Extension Agreement, effective as of October 1, 2016, to the Credit Agreement dated as of October 1, 2015, among Philip Morris International Inc., lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed August 31, 2016). |
| 10.11 |
|
— |
|
Extension Agreement, effective as of October 1, 2017, to the Credit Agreement, dated as of October 1, 2015, among Philip Morris International Inc., the lenders party thereto and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed August 29, 2017). |
| 10.12 |
|
—
|
|
Extension Agreement, effective as of February 6, 2018, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2018). |
| 10.13 |
|
—
|
|
Extension Agreement, effective as of February 5, 2019, to the Credit Agreement dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2019). |
| 10.14 |
|
— |
|
Amendment and Extension Agreement, effective February 4, 2020, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., each lender named therein and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 3, 2020). |
| 10.15 |
|
— |
|
Credit Agreement, dated as of February 10, 2020, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch, as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 11, 2020). |
| 10.16 |
|
— |
|
Amendment and Extension Agreement, effective February 2, 2021, to the Credit Agreement, dated as of February 12, 2013, among PMI, the lenders named therein and Citibank Europe PLC, UK Branch (legal successor to Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 2, 2021). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.17 |
|
— |
|
Amendment and Extension Agreement, effective February 10, 2021, to the Credit Agreement, dated as of February 10, 2020, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed February 2, 2021). |
| 10.18 |
|
— |
|
Credit Agreement, dated as of September 29, 2021, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.1to the Current Report on Form 8-K filed September 30, 2021). |
| 10.19 |
|
— |
|
Amendment and Extension Agreement, effective February 1, 2022, to the Credit Agreement, dated as of February 12, 2013, among PMI, the lenders named therein and Citibank Europe PLC, UK Branch (legal successor to Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 1, 2022). |
| 10.20 |
|
— |
|
Amendment and Extension Agreement, effective February 10, 2022, to the Credit Agreement, dated as of February 10, 2020, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed February 1, 2022). |
| 10.21 |
|
— |
|
|
| 10.22 |
|
— |
|
|
| 10.23 |
|
— |
|
|
| 10.24 |
|
— |
|
|
| 10.25 |
|
— |
|
Amendment and Extension Agreement, dated as of September 20, 2022, to the Credit Agreement, dated as of September 29, 2021, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed September 23, 2022). |
| 10.26 |
|
— |
|
|
| 10.27 |
|
— |
|
Amendment and Extension Agreement, dated as of September 20, 2023 among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed September 20, 2023). |
| 10.28 |
|
— |
|
|
| 10.29 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.30 |
|
— |
|
|
| 10.31 |
|
— |
|
|
| 10.32 |
|
— |
|
|
| 10.33 |
|
— |
|
|
| 10.34 |
|
— |
|
|
| 10.35 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.36 |
|
— |
|
|
| 10.37 |
|
— |
|
|
| 10.38 |
|
— |
|
|
| 10.39 |
|
— |
|
|
| 10.40 |
|
— |
|
|
| 10.41 |
|
— |
|
|
| 10.42 |
|
— |
|
|
| 10.43 |
|
— |
|
|
| 10.44 |
|
— |
|
|
| 10.45 |
|
— |
|
|
| 10.46 |
|
— |
|
|
| 10.47 |
|
— |
|
|
| 10.48 |
|
— |
|
|
| 10.49 |
|
— |
|
|
| 10.50 |
|
— |
|
|
| 10.51 |
|
— |
|
|
| 10.52 |
|
— |
|
|
| 10.53 |
|
— |
|
|
| 10.54 |
|
— |
|
|
| 10.55 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.56 |
|
— |
|
|
| 10.57 |
|
— |
|
|
| 10.58 |
|
— |
|
|
| 10.59 |
|
— |
|
|
| 10.60 |
|
— |
|
|
| 10.61 |
|
— |
|
|
| 10.62 |
|
— |
|
|
| 10.63 |
|
— |
|
|
| 10.64 |
|
— |
|
|
| 10.65 |
|
— |
|
|
| 10.66 |
|
— |
|
|
| 10.67 |
|
— |
|
|
| 10.68 |
|
— |
|
|
| 10.69 |
|
— |
|
|
| 10.70 |
|
— |
|
|
| 10.71 |
|
— |
|
|
| 10.72 |
|
— |
|
|
| 10.73 |
|
— |
|
|
| 10.74 |
|
— |
|
|
| 10.75 |
|
— |
|
|
| 10.76 |
|
— |
|
|
| 10.77 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.78 |
|
— |
|
|
| 10.79 |
|
— |
|
|
| 10.80 |
|
— |
|
|
| 10.81 |
|
— |
|
|
| 10.82 |
|
— |
|
|
| 10.83 |
|
— |
|
|
| 10.84 |
|
— |
|
|
| 10.85 |
|
— |
|
|
| 10.86 |
|
— |
|
|
| 10.87 |
|
— |
|
|
| 10.88 |
|
— |
|
|
| 10.89 |
|
— |
|
|
| 10.90 |
|
— |
|
|
| 10.91 |
|
— |
|
|
| 10.92 |
|
— |
|
|
| 10.93 |
|
— |
|
|
| 10.94 |
|
— |
|
|
| 10.95 |
|
— |
|
|
| 10.96 |
|
— |
|
|
| 10.97 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 21 |
|
— |
|
|
| 23 |
|
— |
|
|
| 31.1 |
|
— |
|
|
| 31.2 |
|
— |
|
|
| 32.1 |
|
— |
|
|
| 32.2 |
|
— |
|
|
| 97 |
|
— |
|
|
| 101.INS |
|
— |
|
XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document.
|
| 101.SCH |
|
— |
|
XBRL Taxonomy Extension Schema. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 101.CAL |
|
— |
|
XBRL Taxonomy Extension Calculation Linkbase. |
| 101.DEF |
|
— |
|
XBRL Taxonomy Extension Definition Linkbase. |
| 101.LAB |
|
— |
|
XBRL Taxonomy Extension Label Linkbase. |
| 101.PRE |
|
— |
|
XBRL Taxonomy Extension Presentation Linkbase. |
| 104 |
|
— |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
________
* Denotes management contract or compensatory plan or arrangement in which directors or executive officers are eligible to participate.
** Schedules and certain portions of this exhibit have been omitted pursuant to Item 601(a)(5) and Item 601(b)(10)(iv) of Regulation S-K.
x Denotes exhibits filed herewith.
The exhibits filed herewith do not include certain instruments with respect to long-term debt of PMI, inasmuch as the total amount of debt authorized under any such instrument does not exceed 10 percent of the total assets of PMI on a consolidated basis. PMI agrees, pursuant to Item 601(b)(4)(iii) of Regulation S-K, that it will furnish a copy of any such instrument to the SEC upon request.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
| PHILIP MORRIS INTERNATIONAL INC. |
|
|
| By: |
/s/ JACEK OLCZAK |
|
(Jacek Olczak
Chief Executive Officer) |
Date: February 8, 2024
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jacek Olczak, Emmanuel Babeau, and Darlene Quashie Henry and each of them, acting individually, as his or her true and lawful attorney-in-fact, each with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K for the year ended December 31, 2023, and other documents in connection herewith and therewith, and to file the same, with all exhibits thereto, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection herewith and therewith and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated:
|
|
|
|
|
|
|
|
|
| Signature |
Title |
Date |
|
|
|
| /s/ JACEK OLCZAK |
Chief Executive Officer and Director |
February 8, 2024 |
| (Jacek Olczak) |
| /s/ EMMANUEL BABEAU |
Chief Financial Officer |
February 8, 2024 |
| (Emmanuel Babeau) |
| /s/ REGINALDO DOBROWOLSKI |
Vice President and Controller |
February 8, 2024 |
| (Reginaldo Dobrowolski) |
| /s/ ANDRÉ CALANTZOPOULOS |
Executive Chairman |
February 8, 2024 |
| (André Calantzopoulos) |
| /s/ BONIN BOUGH |
Director |
February 8, 2024 |
| (Bonin Bough) |
| /s/ MICHEL COMBES |
Director |
February 8, 2024 |
| (Michel Combes) |
| /s/ DR. JUAN JOSÉ DABOUB |
Director |
February 8, 2024 |
(Juan José Daboub) |
|
|
|
|
|
|
|
|
|
/s/ WERNER GEISSLER |
Director |
February 8, 2024 |
(Werner Geissler) |
/s/ VICTORIA HARKER |
Director |
February 8, 2024 |
| (Victoria Harker) |
/s/ LISA A. HOOK |
Director |
February 8, 2024 |
| (Lisa A. Hook) |
/s/ JUN MAKIHARA |
Director |
February 8, 2024 |
(Jun Makihara) |
/s/ KALPANA MORPARIA |
Director |
February 8, 2024 |
(Kalpana Morparia) |
/s/ ROBERT B. POLET |
Director |
February 8, 2024 |
(Robert B. Polet) |
/s/ DESSISLAVA TEMPERLEY |
Director |
February 8, 2024 |
(Dessislava Temperley) |
/s/ SHLOMO YANAI |
Director |
February 8, 2024 |
(Shlomo Yanai) |
EX-4.3
2
pm-ex43_123123xq4.htm
EX-4.3
Document
Exhibit 4.3
DESCRIPTION OF THE REGISTRANT’S SECURITIES
REGISTERED PURSUANT TO SECTION 12 OF THE
SECURITIES EXCHANGE ACT OF 1934
DESCRIPTION OF CAPITAL STOCK
The following description is a summary of the material terms that are included in our amended and restated articles of incorporation and our amended and restated bylaws. This summary is qualified in its entirety by the specific terms and provisions contained in our amended and restated articles of incorporation and our amended and restated bylaws, copies of which are incorporated by reference to exhibits to the Annual Report on Form 10-K for the year ended December 31, 2023, and by the provisions of applicable law. We encourage you to read our amended and restated articles of incorporation and our amended and restated bylaws.
Common Stock
Authorized Common Stock. Our authorized capital stock consists of 6,000,000,000 shares of common stock, no par value, and 250,000,000 shares of preferred stock, no par value.
Authorized But Unissued Capital Stock. Virginia law does not require shareholder approval for any issuance of authorized shares other than in connection with certain mergers to which we may be a party. However, the NYSE rules require shareholder approval of certain issuances of common stock or securities convertible into or exchangeable for common stock equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of our common stock. These additional shares may be used for a variety of corporate purposes, including future public offerings to raise additional capital or to facilitate corporate acquisitions.
Voting. The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the shareholders. Approval of an amendment of our articles of incorporation, a merger, a share exchange, a sale of all our property or a dissolution must be approved by a majority of all votes entitled to be cast.
Dividends. The holders of our common stock are entitled to receive dividends and other distributions as may be declared by our Board, subject to the preferential rights of any outstanding preferred stock.
Other Rights. Upon our liquidation, dissolution or winding-up, after payment in full of the amounts required to be paid to holders of any outstanding shares of preferred stock, if any, all holders of our common stock will be entitled to receive a pro rata distribution of all of our assets and funds legally available for distribution.
No shares of our common stock are subject to redemption or have preemptive rights to purchase additional shares of our common stock or any of our other securities.
Listing of Common Stock
A primary listing for our common stock is on the NYSE under the trading symbol “PM.” The secondary listing for our common stock is on the SIX Swiss Exchange under the trading symbol “PMI.”
Transfer Agent and Registrar
The transfer agent and registrar of our common stock is Computershare Trust Company, N.A.
Preferred Stock
Our Board of Directors has the authority, without action by the shareholders, to designate and issue preferred stock in one or more series or classes and to designate the rights, preferences and privileges of each series or class, which may be greater than the rights of our common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock upon the rights of holders of our common stock until our Board of Directors determines the specific rights of the holders of the preferred stock.
However, the effects might include:
|
|
|
|
|
|
|
|
|
|
|
|
| |
• |
|
restricting dividends on our common stock; |
|
|
|
|
|
|
|
|
|
|
|
|
| |
• |
|
diluting the voting power of our common stock; |
|
|
|
|
|
|
|
|
|
|
|
|
| |
• |
|
impairing liquidation rights of our common stock; or |
|
|
|
|
|
|
|
|
|
|
|
|
| |
• |
|
delaying or preventing a change in control of us without further action by our shareholders. |
We have no present plans to issue any shares of preferred stock.
Board of Directors; Removal; Vacancies
Virginia law provides that the Board of Directors of a Virginia corporation shall consist of a number of individuals specified in or fixed in accordance with the bylaws of the corporation or, if not specified in or fixed in accordance with the bylaws, then a number specified in or fixed in accordance with the articles of incorporation of the corporation. We do not have a classified board of directors. All directors are elected annually.
Under Virginia law, our Board of Directors may amend the bylaws from time to time to increase or decrease the number of directors; provided, that any decrease in the number of directors may not shorten any incumbent director’s term or reduce any quorum or voting requirements until the person ceases to be a director.
Under Virginia law, a member of our Board of Directors may be removed with or without cause by a majority of the votes entitled to be cast at a meeting of shareholders called expressly for that purpose at which a quorum is present. If a director is elected by a voting group of shareholders, only the shareholders of that voting group may participate in the vote to remove the director.
Our bylaws provide that any vacancy occurring on our Board of Directors may be filled by the affirmative vote of the majority of the remaining directors, though less than a quorum.
No Cumulative Voting
Our articles of incorporation and bylaws do not provide for cumulative voting in the election of directors.
Majority Voting for Directors
The Company’s bylaws provide that, where the number of nominees for director does not exceed the number of directors to be elected, directors shall be elected by a majority rather than by a plurality vote. Under applicable law, a director’s term extends until his or her successor is duly elected and qualified. Thus, an incumbent director who fails to receive a majority vote would continue to serve as a holdover director. To address that possibility, our Corporate Governance Guidelines require a director who receives less than a majority of the votes cast to offer to resign. The Nominating and Corporate Governance Committee would then consider, and recommend to the Board, whether to accept or reject the offer. In a contested election in which one or more nominees are properly proposed by shareholders, a director-nominee will be elected by a plurality of the votes cast in such election.
Shareholder Nominations and Proposals
Our bylaws set forth the procedures a shareholder must follow to nominate directors or to bring other business before shareholder meetings. Our bylaws provide that, subject to the rights of holders of any outstanding shares of preferred stock, a shareholder may nominate one or more persons for election as directors, or bring other matters, at a meeting only if advance written notice of the shareholder’s nomination has been given, either by personal delivery or certified mail, to and received by, our Corporate Secretary whose address is Avenue de Rhodanie 50, 1007 Lausanne, Switzerland, within such time periods and following such procedures as set forth in the bylaws.
In addition, our bylaws permit an eligible shareholder or group of shareholders who have owned 3% or more of our common stock for at least three years to nominate and include in our proxy statement director candidates to occupy up to 20% of the authorized Board seats.
Business brought before a meeting that does not comply with our by-law provisions will not be transacted.
Special Shareholder Meetings
Under our bylaws, only our Board of Directors or our chairman may call special meetings of shareholders.
Anti-Takeover Statutes
Affiliated Transactions Statute. Virginia law contains provisions governing affiliated transactions. In general, these provisions prohibit a Virginia corporation from engaging in affiliated transactions with an interested shareholder, which is any holder of more than 10% of any class of its outstanding voting shares, for a period of three years following the date that such person became an interested shareholder, unless:
|
|
|
|
|
|
|
|
|
|
|
|
| |
• |
|
a majority of disinterested directors; and |
|
|
|
|
|
|
|
|
|
|
|
|
| |
• |
|
the holders of two-thirds of the voting shares, other than the shares beneficially owned by the interested shareholder, approve the affiliated transaction. |
Affiliated transactions subject to this approval requirement include mergers, share exchanges, material dispositions of corporate assets not in the ordinary course of business, any dissolution of the corporation proposed by or on behalf of an interested shareholder or any reclassification, including reverse stock splits, recapitalizations or mergers of the corporation with its subsidiaries, which increases the percentage of voting shares owned beneficially by an interested shareholder by more than 5%.
Control Share Acquisitions Statute. We have opted out of the Virginia anti-takeover law regulating control share acquisitions.
EX-4.4
3
pmi-exhibit44x10xk2023.htm
EX-4.4
Document
Exhibit 4.4
DESCRIPTION OF DEBT SECURITIES
(as of December 31, 2023)
The following description of the Company’s 2.875% notes due 2024 (the “2.875% USD 2024 Notes”), 2.875% notes due 2024 (the “2.875% EURO 2024 Notes”), 0.625% notes due 2024 (the “0.625% 2024 Notes”), 3.250% notes due 2024 (the “3.250% 2024 Notes”), 5.125% notes due 2024 (the “5.125% 2024 Notes”), 2.750% notes due 2025 (the “2.750% 2025 Notes”), 1.500% notes due 2025 (the “1.500% 2025 Notes), 3.375% notes due 2025 (the “3.375% 2025 Notes”), 5.000% notes due 2025 (the “5.000% 2025 Notes”), 4.875% notes due 2026 (the “4.875% 2026 Notes”), 2.750% notes due 2026 (the “2.750% 2026 Notes”), 2.875% notes due 2026 (the “2.875% 2026 Notes”), 0.875% notes due 2026 (the “0.875% 2026 Notes”), 0.125% notes due 2026 (the “0.125% 2026 Notes”), 3.125% notes due 2027 (the “3.125% 2027 Notes”), 5.125% notes due 2027 (the “5.125% 2027 Notes”), 4.875% notes due 2028 (the “4.875% 2028 Notes”), 3.125% notes due 2028 (the “3.125% 2028 Notes”), 5.250% notes due 2028 (the “5.250% 2028 Notes”), 2.875% notes due 2029 (the “2.875% 2029 Notes”), 3.375% notes due 2029 (the “3.375% 2029 Notes”), 5.625% notes due 2029 (the “5.625% 2029 Notes”), 5.125% notes due 2030 (the “5.125% 2030 Notes”), 2.100% notes due 2030 (the “2.100% 2030 Notes”), 5.500% notes due 2030 (the “5.500% 2030 Notes”), 1.750% notes due 2030 (the “1.750% 2030 Notes”), 0.800% notes due 2031 (the “2031 Notes”), 5.750% notes due 2032 (the “2032 Notes”), 5.375% notes due 2033 (the “5.375% 2033 Notes”), 3.125% notes due 2033 (the “3.125% 2033 Notes”), 5.625% notes due 2033 (the “5.625% 2033 Notes”), 2.000% notes due 2036 (the “2036 Notes”), 1.875% notes due 2037 (the “2037 Notes”), 6.375% notes due 2038 (the “2038 Notes”), 1.450% notes due 2039 (the “2039 Notes”), 4.375% notes due 2041 (the “2041 Notes”), 4.500% notes due 2042 (the “4.500% 2042 Notes”), 3.875% notes due 2042 (the “3.875% 2042 Notes”), 4.125% notes due 2043 (the “4.125% 2043 Notes”), 4.875% notes due 2043 (the “4.875% 2043 Notes”) and 4.250% notes due 2044 (the “2044 Notes”), which we refer to collectively as the “notes,” is a summary and is not complete.
This summary is qualified in its entirety by the specific terms and provisions contained in the indenture, dated as of April 25, 2008, between Philip Morris International Inc. (“PMI,” “we” or “us”) and HSBC Bank USA, National Association, as trustee, under which the notes were issued (the “indenture”).
General
The notes are PMI’s direct unsecured obligations and rank equally with all of PMI’s other unsecured and unsubordinated debt. The indenture does not limit the amount of debt we may issue under the indenture and provides that additional notes may be issued up to the aggregate principal amount authorized by a board resolution. We may issue notes from time to time in one or more series with the same or various maturities, at par, at a discount or at a premium.
There is no requirement that any other notes that we issue be issued under the indenture. Thus, any other notes that we issue may be issued under other indentures or documentation containing provisions different from those included in the indenture or the applicable prospectus to one or more issues of the notes.
We may, without the consent of the holders of the notes, issue additional notes of a certain series having the same ranking and the same interest rate, maturity and other terms as the notes of such series, except for the public offering price and issue date. Any additional notes of a series having such similar terms, together with the applicable series of notes, will constitute a single series of notes under the indenture. No additional notes of a series may be issued if an event of default has occurred with respect to such series of notes.
2.875% USD 2024 Notes
We issued $900,000,000 aggregate principal amount of 2.875% USD 2024 Notes, maturing May 1, 2024 and bearing interest at a rate of 2.875% per annum, payable semiannually on May 1 and November 1 of each year. As of December 31, 2023, $900,000,000 aggregate principal amount of the 2.875% USD 2024 Notes was outstanding.
2.875% EURO 2024 Notes
We issued €600,000,000 aggregate principal amount of 2.875% EURO 2024 Notes, maturing May 30, 2024 and bearing interest at a rate of 2.875% per annum, payable annually on May 30 of each year. As of December 31, 2023, €600,000,000 aggregate principal amount of the 2.875% EURO 2024 Notes was outstanding.
0.625% 2024 Notes
We issued €500,000,000 aggregate principal amount of 0.625% 2024 Notes, maturing November 8, 2024 and bearing interest at a rate of 0.625% per annum, payable annually on November 8 of each year. As of December 31, 2023, €500,000,000 aggregate principal amount of the 0.625% 2024 Notes was outstanding.
3.250% 2024 Notes
We issued $750,000,000 aggregate principal amount of 3.250% 2024 Notes, maturing November 10, 2024 and bearing interest at a rate of 3.250% per annum, payable semiannually on May 10 and November 10 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 3.250% 2024 Notes was outstanding.
5.125% 2024 Notes
We issued $1,000,000,000 aggregate principal amount of 5.125% 2024 Notes, maturing November 15, 2024 and bearing interest at a rate of 5.125% per annum, payable semiannually on May 15 and November 15 of each year. As of December 31, 2023, $1,000,000,000 aggregate principal amount of the 5.125% 2024 Notes was outstanding.
2.750% 2025 Notes
We issued €750,000,000 aggregate principal amount of 2.750% 2025 Notes, maturing March 19, 2025 and bearing interest at a rate of 2.750% per annum, payable annually on March 19 of each year. As of December 31, 2023, €750,000,000 aggregate principal amount of the 2.750% 2025 Notes was outstanding.
1.500% 2025 Notes
We issued $750,000,000 aggregate principal amount of 1.500% 2025 Notes maturing May 1, 2025 and bearing interest at a rate of 1.500% per annum, payable semiannually on May 1 and November 1 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 1.500% 2025 Notes was outstanding.
3.375% 2025 Notes
We issued $750,000,000 aggregate principal amount of 3.375% 2025 Notes, maturing August 11, 2025 and bearing interest at a rate of 3.375% per annum, payable semiannually on February 11 and August 11 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 3.375% 2025 Notes was outstanding.
5.000% 2025 Notes
We issued $750,000,000 aggregate principal amount of 5.000% 2025 Notes, maturing November 17, 2025 and bearing interest at a rate of 5.000% per annum, payable semiannually on May 17 and November 17 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 5.000% 2025 Notes was outstanding.
4.875% 2026 Notes
We issued $1,700,000,000 aggregate principal amount of 4.875% 2026 Notes, maturing February 13, 2026 and bearing interest at a rate of 4.875% per annum, payable semiannually on February 13 and August 13 of each year. As of December 31, 2023, $1,700,000,000 aggregate principal amount of the 4.875% 2026 Notes was outstanding.
2.750% 2026 Notes
We issued $750,000,000 aggregate principal amount of 2.750% 2026 Notes, maturing February 25, 2026 and bearing interest at a rate of 2.750% per annum, payable semiannually on February 25 and August 25 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 2.750% 2026 Notes was outstanding.
2.875% 2026 Notes
We issued €1,000,000,000 aggregate principal amount of 2.875% 2026 Notes, maturing March 3, 2026 and bearing interest at a rate of 2.875% per annum, payable annually on March 3 of each year. As of December 31, 2023, €1,000,000,000 aggregate principal amount of the 2.875% 2026 Notes was outstanding.
0.875% 2026 Notes
We issued $750,000,000 aggregate principal amount of 0.875% 2026 Notes, maturing May 1, 2026 and bearing interest at a rate of 0.875% per annum, payable semiannually on May 1 and November 1 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 0.875% 2026 Notes was outstanding.
0.125% 2026 Notes
We issued €500,000,000 aggregate principal amount of 0.125% 2026 Notes, maturing August 3, 2026 and bearing interest at a rate of 0.125% per annum, payable annually on August 3 of each year. As of December 31, 2023, €500,000,000 aggregate principal amount of the 0.125% 2026 Notes was outstanding.
3.125% 2027 Notes
We issued $500,000,000 aggregate principal amount of 3.125% 2027 Notes, maturing August 17, 2027 and bearing interest at a rate of 3.125% per annum, payable semiannually on February 17 and August 17 of each year. As of December 31, 2023, $500,000,000 aggregate principal amount of the 3.125% 2027 Notes was outstanding.
5.125% 2027 Notes
We issued $1,500,000,000 aggregate principal amount of 5.125% 2027 Notes, maturing November 17, 2027 and bearing interest at a rate of 5.125% per annum, payable semiannually on May 17 and November 17 of each year. As of December 31, 2023, $1,500,000,000 aggregate principal amount of the 5.125% 2027 Notes was outstanding.
4.875% 2028 Notes
We issued $1,550,000,000 aggregate principal amount of 4.875% 2028 Notes, maturing February 15, 2028 and bearing interest at a rate of 4.875% per annum, payable semiannually on February 15 and August 15 of each year. As of December 31, 2023, $1,550,000,000 aggregate principal amount of the 4.875% 2028 Notes was outstanding.
3.125% 2028 Notes
We issued $500,000,000 aggregate principal amount of 3.125% 2028 Notes, maturing March 2, 2028 and bearing interest at a rate of 3.125% per annum, payable semiannually on March 2 and September 2 of each year. As of December 31, 2023, $500,000,000 aggregate principal amount of the 3.125% 2028 Notes was outstanding.
5.250% 2028 Notes
We issued $650,000,000 aggregate principal amount of 5.250% 2028 Notes, maturing September 7, 2028 and bearing interest at a rate of 5.250% per annum, payable semiannually on March 7 and September 7 of each year. As of December 31, 2023, $650,000,000 aggregate principal amount of the 5.250% 2028 Notes was outstanding.
2.875% 2029 Notes
We issued €500,000,000 aggregate principal amount of 2.875% 2029 Notes, maturing May 14, 2029 and bearing interest at a rate of 2.875% per annum, payable annually on May 14 of each year. As of December 31, 2023, €500,000,000 aggregate principal amount of the 2.875% 2029 Notes was outstanding.
3.375% 2029 Notes
We issued $750,000,000 aggregate principal amount of 3.375% 2029 Notes, maturing August 15, 2029 and bearing interest at a rate of 3.375% per annum, payable semiannually on February 15 and August 15 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 3.375% 2029 Notes was outstanding.
5.625% 2029 Notes
We issued $1,250,000,000 aggregate principal amount of 5.625% 2029 Notes, maturing November 17, 2029 and bearing interest at a rate of 5.625% per annum, payable semiannually on May 17 and November 17 of each year. As of December 31, 2023, $1,250,000,000 aggregate principal amount of the 5.625% 2029 Notes was outstanding.
5.125% 2030 Notes
We issued $2,200,000,000 aggregate principal amount of 5.125% 2030 Notes, maturing February 15, 2030 and bearing interest at a rate of 5.125% per annum, payable semiannually on February 15 and August 15 of each year. As of December 31, 2023, $2,200,000,000 aggregate principal amount of the 5.125% 2030 Notes was outstanding.
2.100% 2030 Notes
We issued $750,000,000 aggregate principal amount of 2.100% 2030 Notes, maturing May 1, 2030 and bearing interest at a rate of 2.100% per annum, payable semiannually on May 1 and November 1 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 2.100% 2030 Notes was outstanding.
5.500% 2030 Notes
We issued $700,000,000 aggregate principal amount of 5.500% 2030 Notes, maturing September 7, 2030 and bearing interest at a rate of 5.500% per annum, payable semiannually on March 7 and September 7 of each year. As of December 31, 2023, $700,000,000 aggregate principal amount of the 5.500% 2030 Notes was outstanding.
1.750% 2030 Notes
We issued $750,000,000 aggregate principal amount of 1.750% 2030 Notes, maturing November 1, 2030 and bearing interest at a rate of 1.750% per annum, payable semiannually on May 1 and November 1 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 1.750% 2030 Notes was outstanding.
2031 Notes
We issued €750,000,000 aggregate principal amount of 2031 Notes, maturing August 1, 2031 and bearing interest at a rate of 0.800% per annum, payable annually on August 1 of each year. As of December 31, 2023, €750,000,000 aggregate principal amount of the 2031 Notes was outstanding.
2032 Notes
We issued $1,500,000,000 aggregate principal amount of 2032 Notes, maturing November 17, 2032 and bearing interest at a rate of 5.750% per annum, payable semiannually on May 17 and November 17 of each year.
As of December 31, 2023, $1,500,000,000 aggregate principal amount of the 2032 Notes was outstanding.
5.375% 2033 Notes
We issued $2,250,000,000 aggregate principal amount of 5.375% 2033 Notes, maturing February 15, 2033 and bearing interest at a rate of 5.375% per annum, payable semiannually on February 15 and August 15 of each year. As of December 31, 2023, $2,250,000,000 aggregate principal amount of the 5.375% 2033 Notes was outstanding.
3.125% 2033 Notes
We issued €500,000,000 aggregate principal amount of 3.125% 2033 Notes, maturing June 3, 2033 and bearing interest at a rate of 3.125% per annum, payable annually on June 3 of each year. As of December 31, 2023, €500,000,000 aggregate principal amount of the 3.125% 2033 Notes was outstanding.
5.625% 2033 Notes
We issued $1,000,000,000 aggregate principal amount of 5.625% 2033 Notes, maturing September 7, 2033 and bearing interest at a rate of 5.625% per annum, payable semiannually on March 7 and September 7 of each year. As of December 31, 2023, $1,000,000,000 aggregate principal amount of the 5.625% 2033 Notes was outstanding.
2036 Notes
We issued €500,000,000 aggregate principal amount of 2036 Notes, maturing May 9, 2036 and bearing interest at a rate of 2.000% per annum, payable annually on May 9 of each year. As of December 31, 2023, €500,000,000 aggregate principal amount of the 2036 Notes was outstanding.
2037 Notes
We issued €500,000,000 aggregate principal amount of 2037 Notes, maturing November 6, 2037 and bearing interest at a rate of 1.875% per annum, payable annually on November 6 of each year. As of December 31, 2023, €500,000,000 aggregate principal amount of the 2037 Notes was outstanding.
2038 Notes
We issued $1,500,000,000 aggregate principal amount of 2038 Notes, maturing May 16, 2038 and bearing interest at a rate of 6.375% per annum, payable semiannually on May 16 and November 16 of each year. As of December 31, 2023, $1,500,000,000 aggregate principal amount of the 2038 Notes was outstanding.
2039 Notes
We issued €750,000,000 aggregate principal amount of 2039 Notes, maturing August 1, 2039 and bearing interest at a rate of 1.450% per annum, payable annually on August 1 of each year. As of December 31, 2023, €750,000,000 aggregate principal amount of the 2039 Notes was outstanding.
2041 Notes
We issued $750,000,000 aggregate principal amount of 2041 Notes, maturing November 15, 2041 and bearing interest at a rate of 4.375% per annum, payable semiannually on May 15 and November 15 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 2041 Notes was outstanding.
4.500% 2042 Notes
We issued $700,000,000 aggregate principal amount of 4.500% 2042 Notes, maturing March 20, 2042 and bearing interest at a rate of 4.500% per annum, payable semiannually on March 20 and September 20 of each year. As of December 31, 2023, $700,000,000 aggregate principal amount of the 4.500% 2042 Notes was outstanding.
3.875% 2042 Notes
We issued $750,000,000 aggregate principal amount of 3.875% 2042 Notes, maturing August 21, 2042 and bearing interest at a rate of 3.875% per annum, payable semiannually on February 21 and August 21 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 3.875% 2042 Notes was outstanding.
4.125% 2043 Notes
We issued $850,000,000 aggregate principal amount of 4.125% 2043 Notes, maturing March 4, 2043 and bearing interest at a rate of 4.125% per annum, payable semiannually on March 4 and September 4 of each year. As of December 31, 2023, $850,000,000 aggregate principal amount of the 4.125% 2043 Notes was outstanding.
4.875% 2043 Notes
We issued $750,000,000 aggregate principal amount of 4.875% 2043 Notes, maturing November 15, 2043 and bearing interest at a rate of 4.875% per annum, payable semiannually on May 15 and November 15 of each year. As of December 31, 2023, $750,000,000 aggregate principal amount of the 4.875% 2043 Notes was outstanding.
2044 Notes
We issued $1,250,000,000 aggregate principal amount of 2044 Notes, maturing November 10, 2044 and bearing interest at a rate of 4.250% per annum, payable semiannually on May 10 and November 10 of each year. As of December 31, 2023, $1,250,000,000 aggregate principal amount of the 2044 Notes was outstanding.
Optional Redemption with Payment of “Make-Whole”
5.125% 2024 Notes; 5.000% 2025 Notes; 4.875% 2026 Notes
At any time prior to the scheduled maturity date for the 5.125% 2024 Notes, 5.000% 2025 Notes and 4.875% 2026 Notes, we may, at our option, redeem the 5.125% 2024 Notes, 5.000% 2025 Notes and 4.875% 2026 Notes, in whole at any time or in part from time to time (equal to $2,000 or an integral multiple of $1,000 in excess thereof). The redemption price will be equal to the greater of (i) 100% of the principal amount of each of the 5.125% 2024 Notes, 5.000% 2025 Notes or 4.875% 2026 Notes to be redeemed and (ii) the sum of the present values of each remaining scheduled payment of principal and interest of the notes to be redeemed that would be due if such notes matured on the maturity date of the applicable notes (exclusive of interest accrued to the date of redemption) discounted to the redemption date, on a semiannual basis (assuming a 360-day year consisting of twelve 30-day months), at a rate equal to the applicable Treasury Rate (as defined below) plus 15 basis points in the case of the 5.125% 2024 Notes, 5.000% 2025 Notes and 4.875% 2026 Notes plus, in each case, accrued and unpaid interest, if any, thereon to, but excluding, the redemption date.
2.875% USD 2024 Notes; 1.500% 2025 Notes; 0.875% 2026 Notes; 5.125% 2027 Notes; 4.875% 2028 Notes; 5.250% 2028 Notes
Prior to the date that is one month prior to the scheduled maturity date for the 2.875% USD 2024 Notes, 1.500% 2025 Notes, 0.875% 2026 Notes, 5.125% 2027 Notes, 4.875% 2028 Notes and 5.250% 2028 Notes, we may, at our option, redeem the 2.875% USD 2024 Notes, 1.500% 2025 Notes, 0.875% 2026 Notes, 5.125% 2027 Notes, 4.875% 2028 Notes and 5.250% 2028 Notes, in whole at any time or in part from time to time (equal to $2,000 or an integral multiple of $1,000 in excess thereof). The redemption price will be equal to the greater of (i) 100% of the principal amount of each of the 2.875% USD 2024 Notes, 1.500% 2025 Notes, 0.875% 2026 Notes, 5.125% 2027 Notes, 4.875% 2028 Notes or 5.250% 2028 Notes to be redeemed and (ii) the sum of the present values of each remaining scheduled payment of principal and interest of the notes to be redeemed that would be due if such notes matured on the date that is one month prior to the scheduled maturity date (exclusive of interest accrued to the date of redemption) discounted to the redemption date, on a semiannual basis (assuming a 360-day year consisting of twelve 30-day months), at a rate equal to the applicable Treasury Rate (as defined below) plus 10 basis points in the case of the 0.875% 2026 Notes, 12.5 basis points in the case of the 2.875% USD 2024 Notes, 20 basis points in the case of the 1.500% 2025 Notes, 4.875% 2028 Notes and 5.250% 2028 Notes, and 25 basis points in the case of the 5.125% 2027 Notes, plus, in each case, accrued and unpaid interest, if any, thereon to, but excluding, the redemption date.
5.625% 2029 Notes; 5.125% 2030 Notes; 5.500% 2030 Notes
Prior to the date that is two months prior to the scheduled maturity date for the 5.625% 2029 Notes, 5.125% 2030 Notes and 5.500% 2030 Notes, we may, at our option, redeem the 5.625% 2029 Notes, 5.125% 2030 Notes and 5.500% 2030 Notes, in whole at any time or in part from time to time (equal to $2,000 or an integral multiple of $1,000 in excess thereof). The redemption price will be equal to the greater of (i) 100% of the principal amount of each of the 5.625% 2029 Notes, 5.125% 2030 Notes or 5.500% 2030 Notes to be redeemed and (ii) the sum of the present values of each remaining scheduled payment of principal and interest of the notes to be redeemed that would be due if such notes matured on the date that is two months prior to the scheduled maturity date (exclusive of interest accrued to the date of redemption) discounted to the redemption date, on a semiannual basis (assuming a 360-day year consisting of twelve 30-day months), at a rate equal to the applicable Treasury Rate (as defined below) plus 25 basis points in the case of the 5.125% 2030 Notes and 5.500% 2030 Notes, and 30 basis points in the case of the 5.625% 2029 Notes, plus, in each case, accrued and unpaid interest, if any, thereon to, but excluding, the redemption date.
3.375% 2025 Notes; 2.750% 2026 Notes; 3.125% 2027 Notes; 3.125% 2028 Notes; 3.375% 2029 Notes; 2.100% 2030 Notes; 1.750% 2030 Notes; 2032 Notes; 5.375% 2033 Notes; 5.625% 2033 Notes
Prior to the date that is three months prior to the scheduled maturity date for the 3.375% 2025 Notes, 2.750% 2026 Notes, 3.125% 2027 Notes, 3.125% 2028 Notes, 3.375% 2029 Notes, 2.100% 2030 Notes, 1.750% 2030 Notes, 2032 Notes, 5.375% 2033 Notes and 5.625% 2033 Notes, we may, at our option, redeem the 3.375% 2025 Notes, 2.750% 2026 Notes, 3.125% 2027 Notes, 3.125% 2028 Notes, 3.375% 2029 Notes, 2.100% 2030 Notes, 1.750% 2030 Notes, 2032 Notes, 5.375% 2033 Notes and 5.625% 2033 Notes, in whole at any time or in part from time to time (equal to $2,000 or an integral multiple of $1,000 in excess thereof). The redemption price will be equal to the greater of (i) 100% of the principal amount of each of the 3.375% 2025 Notes, 2.750% 2026 Notes, 3.125% 2027 Notes, 3.125% 2028 Notes, 3.375% 2029 Notes, 2.100% 2030 Notes, 1.750% 2030 Notes, 2032 Notes, 5.375% 2033 Notes or 5.625% 2033 Notes to be redeemed and (ii) the sum of the present values of each remaining scheduled payment of principal and interest of the notes to be redeemed that would be due if such notes matured on the date that is three months prior to the scheduled maturity date (exclusive of interest accrued to the date of redemption) discounted to the redemption date, on a semiannual basis (assuming a 360-day year consisting of twelve 30-day months), at a rate equal to the applicable Treasury Rate (as defined below) plus 15 basis points in the case of the 3.125% 2027 Notes, 3.125% 2028 Notes and 3.375% 2029 Notes, 20 basis points in the case of the 3.375% 2025 Notes, 2.750% 2026 Notes and 1.750% 2030 Notes, 25 basis points in the case of the 2.100% 2030 Notes and 5.625% 2033 Notes, and 30 basis points in the case of the 2032 Notes and 5.375% 2033 Notes, plus, in each case, accrued and unpaid interest, if any, thereon to, but excluding, the redemption date.
0.625% 2024 Notes; 0.125% 2026 Notes; 2031 Notes; 2036 Notes; 2037 Notes; 2039 Notes
Prior to the date that is three months prior to the scheduled maturity date, we may, at our option, redeem the 0.625% 2024 Notes, 0.125% 2026 Notes, 2031 Notes, 2036 Notes, 2037 Notes and 2039 Notes, in whole at any time or in part from time to time (in €1,000 increments, provided that any remaining principal amount thereof shall be at least the minimum authorized denomination thereof), at a redemption price equal to the greater of (i) 100% of the principal amount of each of the 0.625% 2024 Notes, 0.125% 2026 Notes, 2031 Notes, 2036 Notes, 2037 Notes or 2039 Notes to be redeemed and (ii) the sum of the present values of each remaining scheduled payment of principal and interest of the notes to be redeemed that would be due if such notes were due on the date that is three months prior to the scheduled maturity date for the notes (exclusive of interest accrued to the date of redemption) discounted to the redemption date on an annual basis (Actual/Actual (ICMA)), at a rate equal to the applicable Comparable Government Bond Rate (as defined below) plus 15 basis points in the case of the 0.625% 2024 Notes and the 0.125% 2026 Notes, 20 basis points in the case of the 2036 Notes, 2037 Notes and 25 basis points in the case of the 2031 Notes and the 2039 Notes, plus, in each case, accrued and unpaid interest, if any, thereon to, but excluding, the redemption date.
Optional Redemption at Par
We may, at our option, redeem the notes of an applicable series to which a Par Call Date relates, in whole at any time or in part from time to time (equal to $2,000 or an integral multiple of $1,000 in excess thereof), on or after the applicable Par Call Date, at a redemption price equal to 100% of the principal amount of the notes being redeemed, plus, in each case, accrued and unpaid interest, if any, thereon to, but excluding, the redemption date.
“Par Call Date” means (i) with respect to the 2.875% USD 2024 Notes, 1.500% 2025 Notes, 0.875% 2026 Notes, 5.125% 2027 Notes, 4.875% 2028 Notes and 5.250% 2028 Notes, the date that is one month prior to the maturity date of the applicable notes, (ii) with respect to the 5.625% 2029 Notes, 5.125% 2030 Notes and 5.500% 2030 Notes, the date that is two months prior to the maturity date of the applicable notes and (iii) with respect to the 0.625% 2024 Notes, 3.375% 2025 Notes, 2.750% 2026 Notes, 0.125% 2026 Notes, 3.125% 2027 Notes, 3.125% 2028 Notes, 3.375% 2029 Notes, 2.100% 2030 Notes, 1.750% 2030 Notes, 2031 Notes, 2032 Notes, 5.375% 2033 Notes, 5.625% 2033 Notes, 2036 Notes, 2037 Notes and 2039 Notes, the date that is three months prior to the maturity date of the applicable notes.
Payment of Additional Amounts
Subject to the exceptions and limitations set forth below, we will pay to the beneficial owner of any note who is a non-United States person (as defined below) such additional amounts as may be necessary to ensure that every net payment on such note, after deduction or withholding by us or any of our paying agents for or on account of any present or future tax, assessment or other governmental charge imposed upon or as a result of such payment by the United States or any political subdivision or taxing authority of the United States, will not be less than the amount provided in such note to be then due and payable. However, we will not pay additional amounts if the beneficial owner is subject to taxation solely for reasons other than its ownership of the note, nor will we pay additional amounts for or on account of:
(a) any tax, assessment or other governmental charge that is imposed or withheld solely by reason of the existence of any present or former connection (other than the mere fact of being a beneficial owner of a note) between the beneficial owner (or between a fiduciary, settlor, beneficiary or person holding a power over such beneficial owner, if the beneficial owner is an estate or trust, or a member or shareholder of the beneficial owner, if the beneficial owner is a partnership or corporation) of a note and the United States, including, without limitation, such beneficial owner (or such fiduciary, settlor, beneficiary, person holding a power, member or shareholder) being or having been a citizen or resident of the United States or treated as being or having been a resident thereof;
(b) any tax, assessment or other governmental charge that is imposed or withheld solely by reason of the beneficial owner (or a fiduciary, settlor, beneficiary or person holding a power over such beneficial owner, if the beneficial owner is an estate or trust, or a member or shareholder of the beneficial owner, if the beneficial owner is a partnership or corporation) (1) being or having been present in, or engaged in a trade or business in, the United States, (2) being treated as having been present in, or engaged in a trade or business in, the United States, or (3) having or having had a permanent establishment in the United States;
(c) any tax, assessment or other governmental charge that is imposed or withheld solely by reason of the beneficial owner (or a fiduciary, settlor, beneficiary or person holding a power over such beneficial owner, if the beneficial owner is an estate or trust, or a member or shareholder of the beneficial owner, if the beneficial owner is a partnership or corporation) being or having been with respect to the United States a personal holding company, a controlled foreign corporation, a passive foreign investment company or a foreign private foundation or other foreign tax exempt organization, or being a corporation that accumulates earnings to avoid United States federal income tax;
(d) any tax, assessment or other governmental charge imposed on a beneficial owner that actually or constructively owns 10% or more of the total combined voting power of all of our classes of stock that are entitled to vote within the meaning of Section 871(h)(3) of the Internal Revenue Code of 1986, as amended, or the Code;
(e) any tax, assessment or other governmental charge that is payable by any method other than withholding or deduction by us or any paying agent from payments in respect of such note;
(f) any gift, estate, inheritance, sales, transfer, personal property or excise tax or any similar tax, assessment or other governmental charge;
(g) any tax, assessment or other governmental charge required to be withheld by any paying agent from any payment in respect of any note if such payment can be made without such withholding by at least one other paying agent;
(h) any tax, assessment or other governmental charge that is imposed or withheld by reason of a change in law, regulation, or administrative or judicial interpretation that becomes effective more than 15 days after the payment becomes due or is duly provided for, whichever occurs later;
(i) any tax, assessment or other governmental charge imposed as a result of the failure of the beneficial owner to comply with applicable certification, information, documentation or other reporting requirements concerning the nationality, residence, identity or connection with the United States of the holder or beneficial owner of a note, if such compliance is required by statute or regulation of the United States as a precondition to relief or exemption from such tax, assessment or other governmental charge;
(j) any tax, assessment or other governmental charge imposed by reason of the failure of the beneficial owner to fulfill the statement requirements of Section 871(h) or Section 881(c) of the Code;
(k) any tax, assessment or other governmental charge imposed pursuant to the provisions of Sections 1471 through 1474 of the Code; or
(l) any combination of items (a), (b), (c), (d), (e), (f), (g), (h), (i), (j) and (k).
In addition, we will not pay additional amounts to a beneficial owner of a note that is a fiduciary, partnership, limited liability company or other fiscally transparent entity, or to a beneficial owner of a note that is not the sole beneficial owner of such note, as the case may be. This exception, however, will apply only to the extent that a beneficiary or settlor with respect to the fiduciary, or a beneficial owner or member of the partnership, limited liability company or other fiscally transparent entity, would not have been entitled to the payment of an additional amount had the beneficiary, settlor, beneficial owner or member received directly its beneficial or distributive share of the payment. The term “beneficial owner” includes any person holding a note on behalf of or for the account of a beneficial owner.
As used herein, the term “non-United States person” means a person that is not a United States person. The term United States person means a citizen or resident of the United States or, a corporation or partnership created or organized in or under the laws of the United States or any political subdivision thereof, an estate the income of which is subject to United States federal income taxation regardless of its source, a trust subject to the primary supervision of a court within the United States and the control of one or more United States persons as described in Section 7701(a)(30) of the Code, or a trust that existed on August 20, 1996, and elected to continue its treatment as a domestic trust. “United States” means the United States of America (including the States and the District of Columbia), its territories, its possessions and other areas subject to its jurisdiction (including the Commonwealth of Puerto Rico).
Redemption for Tax Reasons
We may redeem a series of notes prior to maturity in whole as specified in the particular prospectus supplement at a redemption price equal to the principal amount of such notes plus any accrued interest and additional amounts to the date fixed for redemption upon the occurrence of events specified in the indenture. If we exercise our option to redeem a series of notes, we will deliver to the trustee a certificate signed by an authorized officer stating that we are entitled to redeem the notes and the written opinion of independent legal counsel if required.
Issuance in Euro
With respect to the euro denominated notes, initial holders were required to pay for the notes in euro, and all payments of interest and principal, including payments made upon any redemption of the notes, are payable in euro. If we are unable to obtain euro due to the imposition of exchange controls or other circumstances beyond our control (including the dissolution of the European Monetary Union) or if the euro is no longer being used by the then member states of the European Monetary Union that have adopted the euro as their currency or for the settlement of transactions by public institutions of or within the international banking community, then all payments in respect of the notes will be made in U.S. dollars until the euro is again available to us or so used. In such circumstances, the amount payable on any date in euro will be converted into U.S. dollars at the rate mandated by the U.S. Federal Reserve Board as of the close of business on the second business day prior to the relevant payment date or, in the event the U.S. Federal Reserve Board has not mandated a rate of conversion, on the basis of the then most recent euro/U.S. dollar exchange rate available on or prior to the second business day prior to the relevant payment date as determined by us in our sole discretion. Any payment in respect of the notes so made in U.S. dollars will not constitute an event of default under the notes or the indenture governing the notes. Neither the trustee nor the paying agent shall have any responsibility for any calculation or conversion in connection with the foregoing.
Consolidation, Merger or Sale
Under the indenture, we may not consolidate with or merge into any other corporation or convey or transfer our properties and assets substantially as an entirety to any person unless:
•the corporation formed by such consolidation or into which we are merged or the person which acquires by conveyance or transfer our properties and assets substantially as an entirety is a corporation organized and existing under the laws of the United States, any state thereof or the District of Columbia and expressly assumes, by a supplemental indenture, payment of the principal of and any premium and interest (including any additional amounts payable) on all the notes and the performance of every covenant of the indenture;
•after giving effect to the transaction, no Event of Default (as defined below) with respect to any series of notes, and no event which, after notice or lapse of time or both, would become an Event of Default, will have happened and be continuing;
•the successor corporation assuming the notes agrees, by supplemental indenture, to indemnify the individuals liable therefor for the amount of United States federal estate tax paid solely as a result of such assumption in respect of notes held by individuals who are not citizens or residents of the United States at the time of their death; and
•we deliver to the trustee an officers’ certificate and an opinion of counsel, each stating that the consolidation, merger, conveyance or transfer and the supplemental indenture comply with these provisions. (Section 801)
The successor corporation will assume all of our obligations under the indenture as if it were an original party to the indenture. After assuming such obligations, the successor corporation will have all of our rights and powers under the indenture. (Section 802)
Waivers Under the Indenture
Under the indenture, the holders of not less than a majority in aggregate principal amount of all affected series of the outstanding notes (voting as a single class), may on behalf of all holders of such affected series:
•waive our compliance with certain covenants of the indenture; and (Section 1009)
•waive any past default under the indenture, except:
•a default in the payment of the principal of, or any premium or interest on, any notes; and
•a default with respect to a covenant or provision of the indenture which itself cannot be modified or amended without the consent of the holder of each affected note.
(Section 513)
Events of Default
When we use the term “Event of Default” in the indenture with respect to a particular series of notes, we mean any of the following:
•we fail to pay any installment of interest on any note of that series for 30 days after payment was due;
•we fail to make payment of the principal of, or any premium on, any notes of that series when due;
•we fail to make any sinking fund payment when due with respect to notes of that series;
•we fail to perform any other covenant or warranty in respect of any notes of that series contained in the indenture or in such notes or in the applicable board resolution under which such series is issued and this failure continues for 90 days after we receive written notice of it from the trustee or holders of 25% in aggregate principal amount of all outstanding series of the notes or, with respect to any such covenant or agreement which is not applicable to all series of notes, by the holders of at least 25% in aggregate principal amount of the affected series (in each case voting as a single class);
•we or a court take certain actions relating to bankruptcy, insolvency or reorganization of our company; or
•any other event of default that may be specified for the notes of the series or in the board resolution with respect to the notes of that series. (Section 501)
A default with respect to a single series of notes under the indenture will not necessarily constitute a default with respect to any other series of notes issued under the indenture. A default under our other indebtedness will not be a default under the indenture. The trustee may withhold notice to the holders of notes of any default, except for defaults that involve our failure to pay principal or any premium or interest, if it determines in good faith that the withholding of notice is in the interest of the holders. (Section 602)
If an Event of Default for any series of notes occurs and continues (other than an Event of Default involving our bankruptcy, insolvency or reorganization), either the trustee or the holders of at least 25% in aggregate principal amount of all outstanding series of the notes to which it is applicable or, if such default is not applicable to all series of notes, the holders of at least 25% in principal amount of all series to which it is applicable (in each case voting as a single class) may require us upon notice in writing to us, to immediately repay the entire principal of all notes of such series together with accrued interest on the notes.
If an Event of Default occurs which involves our bankruptcy, insolvency or reorganization, then all unpaid principal amounts of all notes of such series together with accrued interest on the notes and accrued interest on all notes of each series then outstanding will immediately become due and payable, without any action by the trustee or any holder of notes. (Section 502)
Subject to certain conditions, the holders of a majority in principal amount of the outstanding notes of a series may rescind a declaration of acceleration if all Events of Default, other than the failure to pay principal or interest due solely because of the declaration of acceleration, have been cured or waived. (Section 502)
Other than its duties in case of an Event of Default, the trustee is not obligated to exercise any of its rights or powers under the indenture at the request, order or direction of any holders, unless the holders offer the trustee reasonable indemnity. (Section 507) The holders of a majority in principal amount outstanding of any series of notes may, subject to certain limitations, direct the time, method and place of conducting any proceeding for any remedy available to the trustee, or exercising any power conferred upon the trustee, for any series of notes. (Section 512)
The indenture requires us to file each year with the trustee, an officer’s certificate that states that:
•the signing officer has supervised a review of our activities during such year and performance under the indenture; and
•to the best of his or her knowledge, based on the review, we comply with all conditions and covenants of the indenture. (Section 1005)
A judgment for money damages by courts in the United States, including a money judgment based on an obligation expressed in a foreign currency, will ordinarily be rendered only in U.S. dollars. New York statutory law provides that a court shall render a judgment or decree in the foreign currency of the underlying obligation and that the judgment or decree shall be converted into U.S. dollars at the exchange rate prevailing on the date of entry of the judgment or decree. If a court requires a conversion to be made on a date other than a judgment date, the indenture requires us to pay additional amounts necessary to ensure that the amount paid in U.S. dollars to a holder is equal to the amount due in such foreign currency or currency unit. (Section 515)
Restrictive Covenants
The indenture includes the following restrictive covenants:
Limitations on Liens
The indenture limits the amount of liens that we or our subsidiaries may incur or otherwise create, in order to secure indebtedness for borrowed money, upon any Principal Facility (as defined below) or any shares of capital stock that any of our subsidiaries owning any Principal Facility has issued to us or any of our subsidiaries. If we or any of our subsidiaries incur such liens, then we will secure the notes to the same extent and in the same proportion as the debt that is secured by such liens. This covenant does not apply, however, to any of the following:
•in the case of a Principal Facility, liens incurred in connection with the issuance by a state or political subdivision thereof of any securities the interest on which is exempt from federal income taxes by virtue of Section 103 of the Internal Revenue Code of 1986, as amended, or any other laws or regulations in effect at the time of such issuance;
•liens existing on the date of the indenture;
•liens on property or shares of capital stock existing at the time we or any of our subsidiaries acquire such property or shares of stock (including acquisition through merger, share exchange or consolidation) or securing the payment of all or part of the purchase price, construction or improvement thereof incurred prior to, at the time of, or within 180 days after the later of the acquisition, completion of construction or improvement or commencement of full operation of such property for the purpose of financing all or a portion of such purchase or construction or improvement; or
•liens for the sole purpose of extending, renewing or replacing in whole or in part the indebtedness secured by any lien referred to in the foregoing three bullet points or in this bullet point; provided, however, that the principal amount of indebtedness secured thereby shall not exceed the principal amount of indebtedness so secured at the time of such extension, renewal or replacement, and that such extension, renewal or replacement shall be
limited to all or a part of the property which secured the lien so extended, renewed or replaced (plus improvements on such property).
Notwithstanding the foregoing, we and/or any of our subsidiaries may create, assume or incur liens that would otherwise be subject to the restriction described above, without securing notes issued under the indenture equally and ratably, if the aggregate value of all outstanding indebtedness secured by the liens plus the value of Sale and Leaseback Transactions does not at the time exceed 15% of Consolidated Net Tangible Assets. (Section 1007)
Sale and Leaseback Transactions
A Sale and Leaseback Transaction by us or any of our subsidiaries of any Principal Facility is prohibited, unless within 90 days of the effective date of the arrangement, an amount equal to the greater of the net proceeds of the sale of the property leased pursuant to the Sale and Leaseback Transaction or the fair value of the property at the time of entering into the Sale and Leaseback Transaction as determined by our board of directors (“value”) is applied by us to the retirement of non-subordinated indebtedness for money borrowed with more than one year stated maturity, including our notes, except that such sales and leasebacks are permitted to the extent that the “value” thereof plus the other secured debt referred to in the last paragraph of the subsection entitled “Restrictive Covenants—Limitations on Liens” does not at the time exceed 15% of our Consolidated Net Tangible Assets. (Section 1008)
There are no other restrictive covenants in the indenture.
Defined Terms
“Consolidated Net Tangible Assets” means the excess over current liabilities of all assets appearing on our most recent quarterly or annual consolidated balance sheet, less goodwill and other intangible assets and the minority interests of others in subsidiaries. (Section 101)
“Comparable Government Bond Rate” means, with respect to any redemption date, the price, expressed as a percentage, at which the gross redemption yield on the applicable series of notes to be redeemed, if they were to be purchased at such price on the third business day prior to the date fixed for redemption, would be equal to the gross redemption yield on such business day of the Reference Bond on the basis of the middle market price of the Reference Bond prevailing at 11:00 a.m. (London time) on such dealing day as determined by the Independent Investment Banker.
“Comparable Treasury Issue” means the U.S. Treasury security or securities selected by an Independent Investment Banker as having an actual or interpolated maturity comparable to the remaining term of the applicable notes that would be utilized, at the time of selection and in accordance with customary financial practice, in pricing new issues of corporate notes of a comparable maturity to the remaining term of such notes.
“Comparable Treasury Price” means, with respect to any redemption date (1) the average of the Reference Treasury Dealer Quotations for such redemption date, after excluding the highest and lowest such Reference Treasury Dealer Quotation or (2) if the Independent Investment Banker obtains fewer than four such Reference Treasury Dealer Quotations, the average of all such quotations.
“Independent Investment Banker” means one of the Reference Bond Dealers that we appoint as the Independent Investment Banker from time to time.
“Principal Facility” means as all real property constituting part of any manufacturing plant or distribution facility owned and operated by us or any subsidiary, together with such manufacturing plant or distribution facility, including all attached plumbing, electrical, ventilating, heating, cooling, lighting and other utility systems, ducts and pipes, but excluding trade fixtures, business machinery, equipment, motorized vehicles, tools, supplies and materials, security systems, cameras, inventory and other personal property and materials. The term Principal Facility shall not include any particular manufacturing plant or distribution facility as of any particular date unless its net book value exceeds 0.75% of Consolidated Net Tangible Assets. (Section 1007)
“Reference Bond” means, in relation to any Comparable Government Bond Rate calculation, a German government bond whose maturity is closest to the maturity of the applicable series of notes, or if we or the Independent Investment Banker considers that such similar bond is not in issue, such other German government bond as we or the Independent Investment Banker, with the advice of three brokers of, and/or market makers in, German government bonds selected by us or the Independent Investment Banker, determine to be appropriate for determining the Comparable Government Bond Rate.
“Reference Bond Dealer” means each of the applicable bond dealers for the applicable series of notes as defined in the respective prospectus supplements or their respective affiliates or any other broker of, and/or market maker in, German government bonds, selected by us.
“Reference Treasury Dealer” means each of the institutions identified in the applicable prospectus supplement or their affiliates, which are primary United States government securities dealers and one other leading primary U.S. government securities dealer in New York City reasonably designated by us; provided, however, that if any of the foregoing shall cease to be a primary U.S. government securities dealer in New York City (a “Primary Treasury Dealer”), we will substitute therefor another Primary Treasury Dealer.
“Reference Treasury Dealer Quotation” means, with respect to each Reference Treasury Dealer and any redemption date, the average, as determined by the Independent Investment Banker, of the bid and asked prices for the Comparable Treasury Issue (expressed in each case as a percentage of its principal amount) quoted in writing to the Independent Investment Banker by such Reference Treasury Dealer at 2:00 pm New York time on the third business day preceding such redemption date.
“Sale and Leaseback Transaction” means the sale or transfer of a Principal Facility with the intention of taking back a lease of the property, except a lease for a temporary period of less than three years, including renewals, with the intent that the use by us or any subsidiary will be discontinued on or before the expiration of such period. (Section 1008)
“Treasury Rate” means, with respect to any redemption date, the rate per annum equal to the semiannual equivalent yield to maturity or interpolated maturity (on a day count basis) of the Comparable Treasury Issue, assuming a price for the Comparable Treasury Issue (such price expressed as a percentage of its principal amount) equal to the Comparable Treasury Price for such redemption date.
Defeasance
The indenture provides that we may elect either (1) to defease and be discharged from any and all obligations with respect to the notes of a series (except for, among other things, our obligations to register notes mutilated, destroyed, lost or stolen and the maintenance of an office or agency for payment and money for payments held in trust and to issue temporary notes of that series with respect to such notes) (“legal defeasance”) or (2) to be released from our obligations to comply with the restrictive covenants under the indenture, and any omission to comply with such obligations will not constitute a default or an event of default with respect to any defeased series of notes (“covenant defeasance”). Legal defeasance or covenant defeasance, as the case may be, will be conditioned upon, among other things, the deposit by us with the trustee, in trust, of an amount of cash in the currency or currency unit in which the applicable series of notes is payable, that will generate sufficient cash, in the opinion of an internationally recognized firm of independent public accountants, to make interest, principal, premium and any other payments on that series of notes on their due date or redemption date.
We will be required to deliver to the trustee an opinion of counsel that the deposit and related defeasance will not cause the holders and beneficial owners of the notes of that series to recognize income, gain or loss for U.S. federal income tax purposes as a result of the legal or covenant defeasance, as the case may be. If we elect legal defeasance, that opinion of counsel must be based upon a ruling from the U.S. Internal Revenue Service or a change in applicable U.S. federal income tax law to that effect. (Sections 402-404)
Book-Entry; Delivery and Form; Global Notes
The notes denominated in euro were offered and sold in denominations of €100,000 and integral multiples of €1,000 in excess thereof and the notes denominated in U.S. dollars were offered and sold in principal amounts of $2,000 and integral multiples of $1,000 in excess thereof. The notes of each series were initially represented by one or more fully registered global notes. Each such global note denominated in euro was deposited with, or on behalf of, a common depositary, and registered in the name of the nominee of the common depositary for the accounts of Clearstream and Euroclear. Each such global note denominated in U.S. dollars was deposited with, or on behalf of, DTC or any successor thereto, as depositary, or Depositary, and registered in the name of Cede & Co. (as nominee of DTC).
Except as set forth in the applicable prospectus supplement, the global notes may be transferred, in whole and not in part, only to Euroclear or Clearstream or their respective nominees in the case of the notes denominated in euro and only to DTC in the case of the notes denominated in U.S. dollars. Unless and until it is exchanged in whole or in part for notes in definitive form, no global note denominated in U.S. dollars may be transferred except as a whole by the Depositary to a nominee of such Depositary. Investors may elect to hold interests in the global notes denominated in U.S. dollars through either the Depositary (in the United States) or through Clearstream or Euroclear, if they are participants in such systems, or indirectly through organizations that are participants in such systems. Holders of the notes denominated in euro may hold their interests in the global notes in Europe through Clearstream or Euroclear, either as a participant in such systems or indirectly through organizations that are participants in such systems. Clearstream and Euroclear will hold the applicable interests in the global notes on behalf of their respective participating organizations or customers through customers’ securities accounts in Clearstream’s or Euroclear’s names on the books of their respective depositaries. Book-entry interests in the notes and all transfers relating to the notes will be reflected in the book-entry records of Clearstream and Euroclear or DTC, as the case may be.
Notices
Notices to holders of the notes will be sent by mail or email to the registered holders and will be published, whether the notes are in global or definitive form, and, so long as the notes of each series are listed on the New York Stock Exchange, in a daily newspaper of general circulation in the City of New York. It is expected that publication will be made in the City of New York in The Wall Street Journal. Any such notice shall be deemed to have been given on the date of such publication or, if published more than once, on the date of the first such publication.
Concerning the Trustee
HSBC Bank USA, National Association is the trustee under the indenture. HSBC Bank USA, National Association or its affiliates make loans to and perform certain other services for us and certain of our subsidiaries and affiliates. Among other services, HSBC Bank USA, National Association or its affiliates provide us and our affiliates with investment banking and cash management services, foreign exchange and investment custody account services, and participate in our credit facilities and those of our affiliates.
Governing Law
The laws of the State of New York govern the indenture and the notes. (Section 112)
EX-10.28
4
exhibit1028.htm
EX-10.28
Document
AMENDMENT NO. 2
This Amendment No. 2 (this “Agreement”) to the Credit Agreement (as defined below) is dated as of November 10, 2023, among PHILIP MORRIS INTERNATIONAL INC., a Virginia corporation (“PMI”), the Lenders party hereto and CITIBANK EUROPE PLC, UK BRANCH, as Facility Agent.
WHEREAS, PMI, the Lenders and the Facility Agent are parties to that certain Term Loan Credit Agreement, dated as of June 23, 2022 (as amended by that certain Amendment No. 1 to the Credit Agreement, dated as of September 2, 2022, and as further amended or modified from time to time prior to the date hereof, the “Credit Agreement”); and
WHEREAS, PMI, the Lenders party hereto and Facility Agent desire to amend certain provisions under the Credit Agreement.
NOW, THEREFORE, in consideration of the premises set forth above and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1.Definitions. Capitalized terms used and not defined in this Agreement shall have the respective meanings given them in the Credit Agreement.
2.Amendment to Credit Agreement. Section 9.7(a)(i) of the Credit Agreement is amended and restated in its entirety as follows:
“each such assignment shall be of a constant, and not a varying, percentage of all rights and obligations under one or more Tranches under this Agreement;”
3.Limited Effect. Except as expressly provided hereby, all of the terms and provisions of the Credit Agreement and other related documents are and shall remain in full force and effect and are hereby ratified and confirmed. The amendments contained herein shall not be construed as a waiver or amendment of any other provision of the Credit Agreement or other related documents or for any purpose except as expressly set forth herein.
4.Condition Precedent. This Agreement shall become effective on and as of the first date this Agreement shall have been duly executed and delivered by PMI, the Required Lenders and the Facility Agent.
5.Headings. Section headings included herein are for convenience of reference only and shall not constitute a part of this Agreement for any other purpose or be given any substantive effect.
6.Binding Effect. This Agreement shall be binding upon and inure to the benefit of PMI, the Facility Agent and each Lender party hereto, and each of their respective successors and assigns.
7.Governing Law. This Agreement shall be governed by, and construed in accordance with, the laws of the State of New York.
8.Execution in Counterparts. This Agreement may be executed in any number of counterparts and by different parties hereto in separate counterparts, each of which when so executed shall be deemed to be an original and all of which taken together shall constitute one and the same agreement. Delivery of an executed counterpart of a signature page to this Agreement in .PDF format or by facsimile shall be effective as delivery of a manually executed counterpart of this Agreement.
[SIGNATURE PAGES FOLLOW]
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be executed by their respective officers thereunto duly authorized, as of the date first above written.
|
|
|
|
|
|
| PHILIP MORRIS INTERNATIONAL INC. |
| By: |
/s/ FRANK DE ROOIJ |
|
Name: Frank de Rooij |
|
Title: Vice President
Treasury and Corporate Finance |
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
CITIBANK EUROPE PLC, UK BRANCH, as Facility Agent |
| By: |
/s/ HENRIK S. SLOTSAA |
|
Name: Henrik S. Slotsaa |
|
Title: Vice President |
|
|
|
|
|
|
CITIBANK N.A., as Facility Agent |
| By: |
|
|
Name: |
|
Title: |
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
CITIBANK EUROPE PLC, UK BRANCH, as Facility Agent |
| By: |
|
|
Name: |
|
Title: |
|
|
|
|
|
|
CITIBANK, N.A., as Lender |
| By: |
/s/ CARYN BELL |
|
Name: Caryn Bell
Title: Managing Director
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
BANK OF AMERICA, N.A., LONDON BRANCH, as Lender |
| By: |
/s/ DEFNE GABAY |
|
Name: Defne Gabay
Title: Vice President
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
MIZUHO BANK, LTD., as Lender |
| By: |
/s/ TRACY RAHN |
|
Name: Tracy Rahn
Title: Executive Director
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
BANCO BILBAO VIZCAYA ARGENTARIA, S.A. NEW YORK BRANCH, as Lender |
| By: |
/s/ CARA YOUNGER |
|
Name: Cara Younger
Title: Managing Director
|
| By: |
/s/ ARMEN SEMIZIAN |
|
Name: Armen Semizian
Title: Managing Director
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
SUMITOMO MITSUI BANKING CORPORATION, as Lender |
| By: |
/s/ HARUHISA OKAMOTO |
|
Name: Haruhisa Okamoto
Title: Managing Director
|
| By: |
/s/ PHILIP MENDRZYK |
|
Name: Philip Mendrzyk
Title: Managing Director
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
WELLS FARGO BANK, N.A., LONDON BRANCH, as Lender |
| By: |
/s/ JONATHAN CHILDS |
|
Name: Jonathan Childs
Title: Director
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
CREDIT SUISSE AG, NEW YORK BRANCH, as Lender |
| By: |
/s/ VIPUL DHADDA |
|
Name: Vipul Dhadda
Title: Authorized Signatory
|
| By: |
/s/ HEESU SIN |
|
Name: Heesu Sin
Title: Authorized Signatory
|
|
|
|
|
|
|
CREDIT SUISSE (SWITZERLAND) LTD., as Lender |
| By: |
/s/ URSULA SCHWARZENBERGER |
|
Name: Ursula Schwarzenberger
Title: Authorized Signatory
|
| By: |
/s/ CHRISTOPH BISCHOFBERGER |
|
Name: Christoph Bischofberger
Title: Authorized Signatory
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
DEUTSCHE BANK AG NEW YORK BRANCH, as Lender |
| By: |
/s/ MING K. CHU |
|
Name: Ming K. Chu
Title: Director
|
| By: |
/s/ ALISON LUGO |
|
Name: Alison Lugo
Title: Vice President
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
BARCLAYS BANK PLC, as Lender |
| By: |
/s/ CHRIS BICHENO |
|
Name: Chris Bicheno
Title: Vice President
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
HSBC CONTINENTAL EUROPE, as Lender |
| By: |
/s/ ERIC BEAUTHEAC |
|
Name: Eric Beautheac
Title: Director Head of Multinationals France HSBC Continental Europe
|
| By: |
/s/ GOEFFROY DE TREDERN |
|
Name: Goeffroy de Tredern
Title: Vice President Multinationals Coverage HSBC Continental Europe
|
|
|
|
|
|
|
HSBC BANK PLC, as Lender |
| By: |
|
|
Name:
Title:
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
HSBC CONTINENTAL EUROPE, as Lender |
| By: |
|
|
Name:
Title:
|
| By: |
|
|
Name:
Title:
|
|
|
|
|
|
|
HSBC BANK PLC, as Lender |
| By: |
/s/ ROD STOYLE |
|
Name: Rod Stoyle
Title: Vice President
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
BANCO SANTANDER, S.A., as Lender |
| By: |
/s/ ALVARO DEL VILLAR RUBIN |
|
Name: Alvaro del Villar Rubin
Title: Authorized Signature
|
| By: |
/s/ FERNANDO MUNOZ GARCIA |
|
Name: Fernando Munoz Garcia
Title:
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
COMMERZBANK AG, NEW YORK BRANCH, as Lender |
| By: |
/s/ PEDRO BELL |
|
Name: Pedro Bell
Title: Managing Director
|
| By: |
/s/ ROBERT SULLIVAN |
|
Name: Robert Sullivan
Title: Vice President
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
STANDARD CHARTERED BANK, as Lender |
| By: |
/s/ A.W. MCALISTER |
|
Name: A.W. McAlister
Title: Head UK Corporates
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
MUFG BANK, LTD., as Lender |
| By: |
/s/ SIMON LELLO |
|
Name: Simon Lello
Title: Managing Director, Co-head of UKI Coverage
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
BANK OF CHINA (EUROPE) S.A., as Lender |
| By: |
/s/ ZHAO YI |
|
Name: Zhao Yi
Title: Assistant General Manager
|
[Signature Page to Term Loan Amendment No. 2]
|
|
|
|
|
|
BANKINTER S.A., as Lender |
| By: |
/s/ AMAYA RUANO |
|
Name: Amaya Ruano
Title:
|
| By: |
/s/ JOSE M. RODRIGUEZ |
|
Name: Jose M. Rodriguez |
[Signature Page to Term Loan Amendment No. 2]
EX-10.33
5
exhibit1033.htm
EX-10.33
Document
Exhibit 10.33
PHILIP MORRIS INTERNATIONAL INC. AMENDED AND RESTATED
AUTOMOBILE POLICY
(as of September 1, 2023)
The Registrant has a policy under which company owned or leased automobiles are provided to key executives for business use when required and for personal use at other times, or at any executive’s election, a cash allowance or travel pass is provided instead, as applicable. Such executives are required to include any taxable benefit under this policy in their annual tax returns.
EX-10.91
6
exhibit1091.htm
EX-10.91
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
RESTRICTED STOCK UNIT AGREEMENT
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Restricted Stock Unit Award (the “Award”) dated Month Day, Year (the “Award Date”) with respect to the number of shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “RSUs”), all in accordance with and subject to the following terms and conditions:
1.Definitions.
For purposes of the Award and this Restricted Stock Unit Agreement (this “Agreement”) the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meanings ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2. Normal Vesting.
The Award is subject to a Restricted Period, and during the Restricted Period the Award is subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, the RSUs shall become fully vested on the Vesting Date set forth in the Award Statement (the “Vesting Date”), provided that the Employee remains an employee of the PMI Group during the entire period commencing on the Award Date and ending on the Vesting Date.
3.Termination of Employment Before Vesting Date.
(a)In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to (i) death, Disability or (ii) Normal Retirement, or (iii) early retirement or termination of employment (other than for Cause), in either case by mutual agreement between the PMI Group and the Employee and after the Employee has attained age 58, then the RSUs shall become fully vested on the date of death, Disability, Normal Retirement, or such early retirement or termination of employment or the date specified in such mutual agreement.
(b)Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment with the PMI Group is terminated prior to the Vesting Date in circumstances not specified in items (i), (ii) or (iii) of the preceding paragraph, the Employee shall forfeit all rights to the unvested RSUs immediately upon the date of termination. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, accelerate the vesting of some or all of such unvested RSUs.
(c)If within the period of 12 months prior to the date of termination of employment, the Employee was an Executive Officer (as designated by the Board of Directors of the Company within the meaning of Section 16 of the Securities Exchange Act of 1934, as amended) and the termination of employment of such Employee is due to a reason other than death or Disability, any shares of Common Stock that are received by such Employee as a result of accelerated vesting provisions of Section 3(a) or (b), shall be automatically subject to a holding period that expires 12 consecutive months from the date of termination of employment.
(d) Subject to the provisions of Section 9 of the Agreement, the Company shall have the exclusive discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Tax on Dividend Equivalents.
The Employee does not have the right to vote the RSUs or receive dividends prior to the date, if any, such RSUs are paid to the Employee in the form of Common Stock pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall receive cash amounts (less applicable withholding taxes) equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock issuable with respect to the Award, as such dividends are paid.
5. Transfer Restrictions.
This Award and the RSUs issuable thereunder are non-transferable and may not be transferred, assigned, hypothecated, pledged or otherwise disposed of and shall not be subject to execution, attachment or similar process, or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the RSUs shall be forfeited.
These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below. In addition, shares of Common Stock subject to the holding period described in Section 3(c) of this Agreement may not be transferred, assigned, hypothecated, pledged or otherwise encumbered for the duration of the applicable holding period.
6. Withholding Taxes.
(a)With respect to Common Stock issuable upon vesting, the Employee understands and
acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations, which may exceed the amount, if any, actually withheld by the Company.
(b)To the extent permitted by law or applicable regulation, the Company shall have the right at
its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from the Award by either (i) deducting the number of shares of Common Stock payable under the RSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the RSUs becoming subject to current taxation (net settlement), or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested RSUs by the Employee (sell-to-cover). Shares of Common Stock payable under the RSUs deducted from the Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to this Section 6(b).
(c)If the Company or its tax advisor determines that the Employee is subject to withholding tax
in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying tax withholding obligations due in all applicable jurisdictions. If at any point the Employee is or was previously on an international assignment during the Restricted Period, the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s RSUs in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d) In the event that the Company’s obligation to withhold tax arises prior to the delivery of shares of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e) The Company makes no representations or undertakings regarding the tax treatment in
connection with any aspect of the Award, including the grant or vesting of the RSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the RSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested RSUs shall be registered in the name of the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
8. Settlement of RSUs.
Each RSU granted pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions and other terms of this Agreement, to issue to the Employee one share of Common Stock. Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) as soon as reasonably practicable following the Vesting Date pursuant to Section 2 or 3 of this Agreement and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement). However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably practicable thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s RSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement. In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to U.S. Federal income tax on any part of the payment of the RSUs and the Award is subject to Section 409A, then the RSUs shall be subject to the following provisions of this Section 9. If the Employee is a “specified employee” within the meaning of Section 409A, any payment of RSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to receive cash amounts equal to dividends on Common Stock pursuant to Section 4 of this Agreement, and such amounts shall be paid to the Employee as such dividends are paid.
In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the RSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control as provided by section 6(a) of the Plan, and shall instead be paid at the time the RSUs would otherwise be paid pursuant to this Agreement. References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its Affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying RSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee, or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence. Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to RSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting. The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time. This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, the Employee acknowledges and agrees that:
(a)The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b)As a condition to, and in consideration of, the grant, vesting, and settlement of RSUs, and in receiving the Award of RSUs, shares of Common Stock, or any other benefit relating to the RSUs, the Employee acknowledges, understands and agrees that the future value of the RSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty. Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such RSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the RSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c)The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d)The granting of the Award does not constitute, nor is evidence of, any agreement or understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries either to create an employment relationship that does not already exist or to continue to employ the Employee for any specific period of time;
(e)The Award, and any Common Stock acquired under it, are separate from any remuneration or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f)The Award does not create any contractual or other right to receive future Awards under the Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g)If the Employee is a resident in a country where English is not an official language, the
Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h)The Company may, in its sole discretion, deliver any documents related to this Agreement, the Award or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i)Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same;
(j)If any provision of this Agreement is held invalid or unenforceable under applicable laws, then such provision will be excluded from this Agreement, and the invalidity or unenforceability of such provision shall not affect the remaining parts of this Agreement, and the balance of this Agreement shall be interpreted, enforced and construed as if such provision were so excluded.
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Restricted Stock Unit Agreement has been duly executed as of Month Day, Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.92
7
exhibit1092.htm
EX-10.92
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
PERFORMANCE SHARE UNIT AGREEMENT
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
Performance Period: Month Day, Year to Month Day, Year
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Performance Share Unit Award (the “Award”) dated Month Day, Year (the “Award Date”) representing a right to receive shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “PSUs”), all in accordance with and subject to the following terms and conditions:
1. Definitions.
For purpose of the Award and this Performance Share Unit Agreement (this “Agreement”), the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meaning ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2. Normal Vesting.
(a) The Award is subject to a Restricted Period, and during the Restricted Period the Award is subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, a number of PSUs shall become vested on the Vesting Date set forth in the Award Statement (the “Vesting Date”) provided that the Employee remains an employee of the PMI Group during the entire period commencing on the Award Date and ending on the Vesting Date.
(b) The actual number of PSUs that become vested on the Vesting Date is equal to a percentage of the target number of PSUs (the “Performance Percentage”), which percentage is determined based on the performance achieved during the applicable performance period, as shown on the Award Statement and as determined by the Committee. The minimum percentage of PSUs that can vest is zero, while the maximum is twice the targeted number, subject to the limitations of the Plan. For the avoidance of doubt, if the date on which the Committee certifies the Performance Percentage is after the Vesting Date, then the actual number of PSUs that become vested shall not be determined until such later date of certification, and such later date of certification shall be treated as the Vesting Date for purposes of cash payments with respect to dividends and the timing of payment of the PSUs pursuant to Sections 4 and 8 of this Agreement. The Committee shall certify the Performance Percentage no later than June 30 immediately following the year in which the performance period ends.
3. Termination of Employment Before Vesting Date.
(a) In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to (i) Normal Retirement, or (ii) early retirement or termination of employment (other than for Cause), in either case by mutual agreement between the PMI Group and the Employee and after the Employee has attained age 58, then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2(b) of this Agreement. In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to death or Disability, then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested at the date of such termination shall be equal to the target number of PSUs set forth on the Award Statement.
(b) Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment with the PMI Group is terminated prior to the Vesting Date for any reason not specified in the preceding paragraph, the Employee shall forfeit all rights to the unvested PSUs immediately upon date of termination. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, treat the requirement that the Employee remain an employee of the PMI Group through the Vesting Date as deemed satisfied with respect to some or all of the PSUs, and in such case the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2(b) of this Agreement multiplied by the target number of PSUs for which the Committee treats the continued employment requirement as deemed satisfied.
(c) If the requirement that the Employee remain an employee of the PMI Group through the Vesting Date is deemed satisfied under this Section 3 for any reason other than the Employee’s death or Disability, but the Employee dies before the Committee’s certification of the Performance Percentage, then the number of PSUs that become vested shall be equal to the target number of PSUs for which the continued employment requirement is deemed satisfied under this Section 3.
(d) Subject to the provisions of Section 9 of the Agreement, the Company shall have the exclusive discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Taxes on Dividend Equivalents.
The Employee does not have the right to vote the PSUs or receive dividends prior to the date, if any, PSUs become vested and Common Stock becomes issuable to the Employee pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall be credited with cash amounts equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock that become issuable as of the Vesting Date, with such cash credits calculated without interest and paid, less applicable tax withholdings, in accordance with this Agreement.
5. Transfer Restrictions.
The Award and the PSUs issuable thereunder are non-transferable and may not be assigned, hypothecated, pledged, or otherwise disposed of and shall not be subject to execution, attachment or similar process or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the PSUs shall be forfeited. These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below.
6. Withholding Taxes.
(a) With respect to Common Stock issuable upon vesting, the Employee understands and acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations, which may exceed the amount, if any, actually withheld by the Company.
(b) To the extent permitted by law or applicable regulation, the Company shall have the right at its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from the Award by either (i) deducting the number of shares of Common Stock payable under the PSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the PSUs becoming subject to current taxation (net settlement), or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested PSUs by the Employee (sell-to-cover). Shares of Common Stock payable under the PSUs deducted from the Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to this Section 6(b).
(c) If the Company or its tax advisor determines that the Employee is subject to withholding tax in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying tax withholding obligations due in all applicable jurisdictions. If at any point the Employee is, or was previously on, an international assignment during the Restricted Period, the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s PSUs, in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d) In the event that the Company’s obligation to withhold tax arises prior to the delivery of shares
of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e) The Company makes no representations or undertakings regarding the tax treatment in connection with any aspect of the Award, including the grant or vesting of the PSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the PSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested PSUs shall be registered in the name of the estate of the Employee, and any cash amounts credited with respect to dividends shall be paid to the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the PSUs will be settled in the form of cash.
8. Settlement of PSUs.
The grant pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions, achievement of performance targets and other conditions set forth in of this Agreement, to issue to the Employee for each vested PSU one share of Common Stock and to pay to the Employee in a single lump sum any cash amounts credited on such vested PSU with respect to dividends. Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance and lump sum payment shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) (a) in all cases other than those set forth in clause (b), as soon as reasonably practicable following the Vesting Date and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement), and (c) in the case of termination of employment by reason of death or Disability or the Employee’s death after a termination of employment in the circumstances specified in Section 3 of this Agreement, as soon as reasonably practicable following such termination of employment or death. However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably practicable thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s PSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the calendar year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement. In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the PSUs will be settled in the form of cash.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to US Federal income tax on any part of the payment of the PSUs and the Award is subject to Section 409A, then the PSUs shall be subject to the following provisions of this Section 9. If the Employee is a “specified employee” within the meaning of Section 409A, any issuance or payment in respect of the PSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to be credited with cash amounts equal to dividends on Common Stock for the applicable Award pursuant to Section 4 of this Agreement, and such amounts shall accrue without interest and shall be paid in a lump sum at the time specified in the preceding sentence. In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the PSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control or termination of employment as provided by section 6(a) of the Plan, and shall instead be paid at the time the PSUs would otherwise be settled at the end of the applicable performance period in accordance with Section 8 of this Agreement. References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its Affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying PSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee, or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence.
Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to PSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting. The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time. This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, you acknowledge and agree that:
(a) The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b) As a condition to, and in consideration of, the grant, vesting, and settlement of PSUs, and in receiving the Award of PSUs, shares of Common Stock, or any other benefit relating to the PSUs, the Employee acknowledges, understands and agrees that the future value of the PSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty. Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such PSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the PSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c) The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d) The granting of the Award does not constitute, or be evidence of, any agreement or
understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries to continue to employ the Employee for any specific period of time;
(e) The Award, and any Common Stock acquired under it, are separate from any remuneration
or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f) The Award does not create any contractual or other right to receive future Awards under the
Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g) If the Employee is a resident in a country where English is not an official language, the
Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h) The Company may, in its sole discretion, deliver any documents related to this Agreement,
the Award or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i) Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same;
(j) The Employee voluntarily acknowledges and consents to the collection, use, processing and transfer of personal information for purposes of administration and management of the Employee’s participation in the Plan. The Employee may, at any time, review the data, require necessary amendments to it or withdraw consents in writing by contacting the Company; however withdrawing consent may affect the Employee’s ability to participate in the Plan.
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Performance Share Unit Agreement has been duly executed as of Month Day, Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.93
8
exhibit1093.htm
EX-10.93
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
RESTRICTED STOCK UNIT AGREEMENT
(Vesting in Installments)
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Restricted Stock Unit Award (the “Award”) dated Month Day, Year (the “Award Date”) with respect to the number of shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “RSUs”), all in accordance with and subject to the following terms and conditions:
1.Definitions.
For purposes of the Award and this Restricted Stock Unit Agreement (this “Agreement”) the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meaning ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2.Normal Vesting.
The Award is subject to a Restricted Period, and during the Restricted Period the Award is subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, the RSUs shall vest ratably in three (3) installments over a three (3) year period as set forth in the Award Statement (each such annual period, a “Vesting Period” with the vesting date for such period, the “Period Vesting Date”), provided that the Employee remains an employee of the PMI Group during each such Vesting Period as set out in the Award Statement.
3. Termination of Employment Before Vesting Date.
(a) In the event of the termination of the Employee’s employment with the PMI Group during any Vesting Period prior to a Period Vesting Date due to (i) death, Disability or (ii) Normal Retirement, or (iii) early retirement or termination of employment (other than for cause), in either case by mutual agreement between the PMI Group and the Employee and after the Employee has attained age 58, then the RSUs shall become fully vested on the date of death, Disability, Normal Retirement, or such early retirement or termination of employment or the date specified in such mutual agreement.
(b) Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment
with the PMI Group is terminated prior to a Period Vesting Date in circumstances not specified in items (i), (ii) or (iii) of the preceding paragraph, the Employee shall forfeit all rights to the unvested RSUs immediately upon date of termination for the Vesting Period during which the termination occurred and any subsequent Vesting Periods. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, accelerate the vesting of some or all such unvested RSUs.
(c) If within the period of 12 months prior to the date of termination of employment, the
Employee was an Executive Officer (as designated by the Board of Directors of the Company within the meaning of Section 16 of the Securities Exchange Act of 1934, as amended) and the termination of employment of such Employee is due to a reason other than death or Disability, any shares of Common Stock that are received by such Employee as a result of accelerated vesting provisions of Section 3(a) or (b), shall be automatically subject to a holding period that expires 12 consecutive months from the date of termination of employment.
(d) Subject to the provisions of Section 9 of the Agreement, the Company shall have the exclusive discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Tax on Dividend Equivalents.
The Employee does not have the right to vote the RSUs or receive dividends prior to the date, if any, such RSUs are paid to the Employee in the form of Common Stock pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall receive cash amounts (less applicable withholding taxes) equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock issuable with respect to the Award, as such dividends are paid.
5. Transfer Restrictions.
This Award and the RSUs issuable thereunder are non-transferable and may not be transferred, assigned, hypothecated, pledged or otherwise disposed of and shall not be subject to execution, attachment or similar process or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the RSUs shall be forfeited. These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below. In addition, shares of Common Stock subject to the holding period described in Section 3(c) of this Agreement may not be transferred, assigned, hypothecated, pledged or otherwise encumbered for the duration of the applicable holding period.
6. Withholding Taxes on Common Stock upon Vesting.
(a) With respect to Common Stock issuable upon vesting, the Employee understands and
acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations due, which may exceed the amount, if any, actually withheld by the Company.
(b) To the extent permitted by law or applicable regulation, the Company shall have the right at
its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from the Award by either (i) deducting the number of shares of Common Stock payable under the RSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the RSUs becoming subject to current taxation (net settlement), or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested RSUs by the Employee (sell-to-cover). Shares of Common Stock payable under the RSUs deducted from this Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to this Section 6(b).
(c) If the Company or its tax advisor determines that the Employee is subject to withholding tax
in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying withholding taxes due in all applicable jurisdictions. If at any point the Employee is on, or was previously on, an international assignment during the Restricted Period, the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s RSUs, in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d) In the event that the Company’s obligation to withhold tax arises prior to the delivery of shares
of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e)The Company makes no representations or undertakings regarding the tax treatment in
connection with any aspect of the Award, including the grant or vesting of the RSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the RSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested RSUs shall be registered in the name of the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
8. Settlement of RSUs.
Each RSU granted pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions and other terms set forth in this Agreement, to issue to the Employee one share of Common Stock on the applicable Period Vesting Date. Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) as soon as reasonably practicable following the Period Vesting Date pursuant to Section 2 or 3 of this Agreement and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement). However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably possible thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s RSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement. In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the RSUs will be settled in the form of cash and provided further that any applicable waiting period under HSR has expired or been terminated.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to US Federal income tax on any part of the payment of the RSUs, and the Award is subject to Section 409A, then the RSUs shall be subject to the following provisions of this Section 9. If the Employee is a “specified employee” within the meaning of Section 409A, any payment of RSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month, or thirteenth month in situations described in Section 3(c) of this Agreement if applicable, beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to receive cash amounts equal to dividends on Common Stock pursuant to Section 4 of this Agreement, and such amounts shall be paid to the Employee as such dividends are paid. In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the RSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control as provided by section 6(a) of the Plan, and shall instead be paid at the time the RSUs would otherwise be paid pursuant to this Agreement. References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying RSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee, or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence. Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to RSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting.
The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time. This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, you acknowledge and agree that:
(a) The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b) As a condition to, and in consideration of, the grant, vesting, and settlement of RSUs, and in receiving the Award of RSUs, shares of Common Stock, or any other benefit relating to the RSUs, the Employee acknowledges, understands and agrees that the future value of the RSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty. Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such RSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the RSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c) The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d) The granting of the Award does not constitute, or be evidence of, any agreement or understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries to continue to employ the Employee for any specific period of time;
(e) The Award, and any Common Stock acquired under it, are separate from any remuneration
or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f)The Award does not create any contractual or other right to receive future Awards under the
Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g) If the Employee is a resident in a country where English is not an official language, the Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h) The Company may, in its sole discretion, deliver any documents related to this Agreement, the Award or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i) The Company makes no representations or undertakings with regard to the foreign exchange rates used to determine the value of the Award granted, reporting of income and/or taxes paid at grant or vesting, the subsequent sale of shares acquired upon vesting and the receipt of any dividend equivalents and does not commit to structure the terms of the Plan, this Agreement or any aspect of Award administration to produce a beneficial outcome for the Employee in this respect;
(j) Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same.
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Restricted Stock Unit Agreement has been duly executed as of Month Day, Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.94
9
exhibit1094.htm
EX-10.94
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
RESTRICTED STOCK UNIT AGREEMENT
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Restricted Stock Unit Award (the “Award”) dated Month Day, Year (the “Award Date”) with respect to the number of shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “RSUs”), all in accordance with and subject to the following terms and conditions:
1. Definitions
For purposes of the Award and this Restricted Stock Unit Agreement the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meanings ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2. Normal Vesting.
The Award is subject to a Restricted Period, and during the Restricted Period the Award is subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, the RSUs shall become fully vested on the Vesting Date set forth in the Award Statement (the “Vesting Date”), provided that the Employee remains an employee of the PMI Group during the entire period commencing on the Award Date and ending on the Vesting Date.
3. Termination of Employment Before Vesting Date.
(a)In the event of the termination of the Employee’s employment with the PMI Group prior to
the Vesting Date due to (i) death, Disability, (ii) Normal Retirement, (iii) termination of employment unilaterally by the Company (other than for Cause), as set forth in the agreement with the Employee effective as of May 1, 2020 (the “Employment Agreement”), or (iv) early retirement or termination of employment (other than for Cause) in either case by mutual agreement [between the PMI Group and the Employee] and after the Employee has attained age 58, then the RSUs shall become fully vested on the date of death, Disability, Normal Retirement, or such early retirement or termination of employment or the date specified in such mutual agreement; provided, however, that the event of the termination set forth in item (iii) of this Section 3(a), the vesting would be further subject to the terms of the Employment Agreement.
(b)Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment with the
PMI Group is terminated prior to the Vesting Date in circumstances not specified in items (i), (ii), (iii) or (iv) of the preceding paragraph, the Employee shall forfeit all rights to the unvested RSUs immediately upon the date of termination. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, accelerate the vesting of some or all such unvested RSUs.
(c)If within the period of 12 months prior to the date of termination of employment, the
Employee was an Executive Officer (as designated by the Board of Directors of the Company within the meaning of Section 16 of the Securities Exchange Act of 1934, as amended) and the termination of employment of such Employee is due to a reason other than death or Disability, any shares of Common Stock that are received by such Employee as a result of accelerated vesting provisions of Section 3(a) or (b), shall be automatically subject to a holding period that expires 12 consecutive months from the date of termination of employment.
(d)Subject to the provisions of Section 9 of the Agreement, the Company shall have the
exclusive discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Tax on Dividend Equivalents.
The Employee does not have the right to vote the RSUs or receive dividends prior to the date, if any, such RSUs are paid to the Employee in the form of Common Stock pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall receive cash amounts (less applicable withholding taxes) equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock issuable with respect to the Award, as such dividends are paid.
5. Transfer Restrictions.
This Award and the RSUs issuable thereunder are non-transferable and may not be transferred, assigned, hypothecated, pledged or otherwise disposed of and shall not be subject to execution, attachment or similar process, or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the RSUs shall be forfeited. These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below. In addition, shares of Common Stock subject to the holding period described in Section 3(c) of this Agreement may not be transferred, assigned, hypothecated, pledged or otherwise encumbered for the duration of the applicable holding period.
6. Withholding Taxes.
(a)With respect to Common Stock issuable upon vesting, the Employee understands and
acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations, which may exceed the amount, if any, actually withheld by the Company.
(b)To the extent permitted by law or applicable regulation, the Company shall have the right at
its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from the Award by either (i) deducting the number of shares of Common Stock payable under the RSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the RSUs becoming subject to current taxation (net settlement) or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested RSUs by the Employee (sell-to-cover). Shares of Common Stock payable under the RSUs deducted from the Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to this Section 6(b).
(c)If the Company or its tax advisor determines that the Employee is subject to withholding tax
in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying tax withholding obligations due in all applicable jurisdictions. If at any point the Employee is or was previously on an international assignment during the Restricted Period, the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s RSUs, in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d)In the event that the Company’s obligation to withhold tax arises prior to the delivery of
shares of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e) The Company makes no representations or undertakings regarding the tax treatment in connection with any aspect of the Award, including the grant or vesting of the RSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the RSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested RSUs shall be registered in the name of the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
8. Settlement of RSUs.
Each RSU granted pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions and other terms of this Agreement, to issue to the Employee one share of Common Stock. Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) as soon as reasonably practicable following the Vesting Date pursuant to Section 2 or 3 of this Agreement and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement). However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably practicable thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s RSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement. In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to US Federal income tax on any part of the payment of the RSUs and the Award is subject to Section 409A, then the RSUs shall be subject to the following provisions of this Section 9.
If the Employee is a “specified employee” within the meaning of Section 409A, any payment of RSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to receive cash amounts equal to dividends on Common Stock pursuant to Section 4 of this Agreement, and such amounts shall be paid to the Employee as such dividends are paid. In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the RSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control as provided by section 6(a) of the Plan, and shall instead be paid at the time the RSUs would otherwise be paid pursuant to this Agreement. References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its Affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying RSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee, or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence. Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to RSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting. The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time. This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, you acknowledge and agree that:
(a) The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b) As a condition to, and in consideration of, the grant, vesting, and settlement of RSUs, and in receiving the Award of RSUs, shares of Common Stock, or any other benefit relating to the RSUs, the Employee acknowledges, understands and agrees that the future value of the RSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty. Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such RSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the RSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c) The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d) The granting of the Award does not constitute, or be evidence of, any agreement or understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries to continue to employ the Employee for any specific period of time;
(e)The Award, and any Common Stock acquired under it, are separate from any remuneration
or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f)The Award does not create any contractual or other right to receive future Awards under the
Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g)If the Employee is a resident in a country where English is not an official language, the
Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h)The Company may, in its sole discretion, deliver any documents related to this Agreement,
the Award, or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i)Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same;
(j) The Employee voluntarily acknowledges and consents to the collection, use, processing and transfer of personal information for purposes of administration and management of the Employee’s participation in the Plan. The Employee may, at any time, review the data, require necessary amendments to it or withdraw consents in writing by contacting the Company; however withdrawing consent may affect the Employee’s ability to participate in the Plan;
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Restricted Stock Unit Agreement has been duly executed as of Month Day, Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.95
10
exhibit1095.htm
EX-10.95
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
PERFORMANCE SHARE UNIT AGREEMENT
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
Performance Period: Month Day, Year to Month Day, Year
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Performance Share Unit Award (the “Award”) dated Month Day, Year (the “Award Date”) representing a right to receive shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “PSUs”), all in accordance with and subject to the following terms and conditions:
1. Definitions.
For purposes of the Award and this Performance Share Unit Agreement (this “Agreement”), the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meanings ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2. Normal Vesting.
(a)The Award is subject to a Restricted Period, and during the Restricted Period the Award is
subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, a number of PSUs shall become vested on the Vesting Date set forth in the Award Statement (the “Vesting Date”), provided that the Employee remains an employee of the PMI Group during the entire period commencing on the Award Date and ending on the Vesting Date.
(b)The actual number of PSUs that become vested on the Vesting Date is equal to a percentage
of the target number of PSUs (the “Performance Percentage”), which percentage is determined based on the performance achieved during the applicable performance period, as shown on the Award Statement and as determined by the Committee. The minimum percentage of PSUs that can vest is zero, while the maximum is twice the targeted number, subject to the limitations of the Plan. For the avoidance of doubt, if the date on which the Committee certifies the Performance Percentage is after the Vesting Date, then the actual number of PSUs that become vested shall not be determined until such later date of certification, and such later date of certification shall be treated as the Vesting Date for purposes of cash payments with respect to dividends and the timing of payment of the PSUs pursuant to Sections 4 and 8 of this Agreement. The Committee shall certify the Performance Percentage no later than June 30 immediately following the year in which the performance period ends.
3. Termination of Employment Before Vesting Date.
(a)In the event of the termination of the Employee’s employment with the PMI Group prior to
the Vesting Date due to (i) Normal Retirement, or (ii) early retirement or termination of employment (other than for Cause), in either case by mutual agreement between the PMI Group and the Employee and after the Employee has attained age 58, then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2(b) of this Agreement. In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to death or Disability, then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested at the date of such termination shall be equal to the target number of PSUs set forth on the Award Statement.
(b)In the event that the termination of the Employee’s employment with the PMI Group prior
to the Vesting Date by the Company (other than for Cause), as set forth in the agreement with the Employee effective as of May 1, 2020 (the “Employment Agreement”), then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2(b) of this Agreement and shall be prorated based on the number of months of employment between the award date and the vesting date of the award; provided, however, that the event of the termination set forth in this Section 3(b), the vesting would be further subject to the terms of the Employment Agreement.
(c)Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment with the PMI Group is terminated prior to the Vesting Date for any reason not specified in the preceding paragraphs, the Employee shall forfeit all rights to the unvested PSUs immediately upon date of termination. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, treat the requirement that the Employee remain an employee of the PMI Group through the Vesting Date as deemed satisfied with respect to some or all of the PSUs, and in such case the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2 of this Agreement multiplied by the target number of PSUs for which the Committee treats the continued employment requirement as deemed satisfied.
(d)If the requirement that the Employee remain an employee of the PMI Group through the
Vesting Date is deemed satisfied under this Section 3 for any reason other than the Employee’s death or Disability, but the Employee dies before the Committee’s certification of the Performance Percentage, then the number of PSUs that become vested shall be equal to the target number of PSUs for which the continued employment requirement is deemed satisfied under this Section 3.
(e) Subject to the provisions of Section 9 of the Agreement, the Company shall have the exclusive discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Taxes on Dividend Equivalents.
The Employee does not have the right to vote the PSUs or receive dividends prior to the date, if any, PSUs become vested and Common Stock becomes issuable to the Employee pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall be credited with cash amounts equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock that become issuable as of the Vesting Date, with such cash credits calculated without interest and paid, less applicable tax withholdings, in accordance with this Agreement.
5. Transfer Restrictions.
The Award and the PSUs issuable thereunder are non-transferable and may not be assigned, hypothecated, pledged or otherwise disposed of and shall not be subject to execution, attachment or similar process or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the PSUs shall be forfeited. These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below.
6. Withholding Taxes on Common Stock upon Vesting.
(a)With respect to Common Stock issuable upon vesting, the Employee understands and
acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations due, which may exceed the amount, if any, actually withheld by the Company.
(b)To the extent permitted by law or applicable regulation, the Company shall have the right at
its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from this Award by either (i) deducting the number of shares of Common Stock payable under the PSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the PSUs becoming subject to current taxation (net settlement) or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested PSUs by the Employee (sell-to-cover).
Shares of Common Stock payable under the PSUs deducted from this Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to this Section 6(b).
(c)If the Company or its tax advisor determines that the Employee is subject to withholding tax
in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying tax withholding obligations due in all applicable jurisdiction. If at any point the Employee is, or was previously on, an international assignment, during the Restricted Period the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s PSUs, in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d)In the event that the Company’s obligation to withhold tax arises prior to the delivery of
shares of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e)The Company makes no representations or undertakings regarding the tax treatment in
connection with any aspect of the Award, including the grant or vesting of the PSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the PSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested PSUs shall be registered in the name of the estate of the Employee, and any cash amounts credited with respect to dividends shall be paid to the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the PSUs will be settled in the form of cash.
8. Settlement of PSUs.
The grant pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions, achievement of performance targets and other conditions set forth in of this Agreement, to issue to the Employee for each vested PSU one share of Common Stock and to pay to the Employee in a single lump sum any cash amounts credited on such vested PSU with respect to dividends. Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance and lump sum payment shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) (a) in all cases other than those set forth in clause (b), as soon as reasonably practicable following the Vesting Date and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement), and (c) in the case of termination of employment by reason of death or Disability or the Employee’s death after a termination of employment in the circumstances specified in Section 3 of this Agreement, as soon as reasonably practicable following such termination of employment or death.
However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably practicable thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s PSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the calendar year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement. In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the PSUs will be settled in the form of cash, and further notwithstanding the foregoing.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to US Federal income tax on any part of the payment of the PSUs and the Award is subject to Section 409A, then the PSUs shall be subject to the following provisions of this Section 9. If the Employee is a “specified employee” within the meaning of Section 409A, any issuance or payment in respect of the PSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to be credited with cash amounts equal to dividends on Common Stock for the applicable Award pursuant to Section 4 of this Agreement, and such amounts shall accrue without interest and shall be paid in a lump sum at the time specified in the preceding sentence. In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the PSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control or termination of employment as provided by section 6(a) of the Plan, and shall instead be paid at the time the PSUs would otherwise be settled at the end of the applicable performance period in accordance with Section 8 of this Agreement. References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying PSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee , or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence. Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to PSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting. The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time. This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, you acknowledge and agree that:
(a) The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b) As a condition to, and in consideration of, the grant, vesting, and settlement of PSUs, and in receiving the Award of PSUs, shares of Common Stock, or any other benefit relating to the PSUs, the Employee acknowledges, understands and agrees that the future value of the PSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty. Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such PSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the PSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c)The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d)The granting of the Award does not constitute, or be evidence of, any agreement or
understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries to continue to employ the Employee for any specific period of time;
(e)The Award, and any Common Stock acquired under it, are separate from any remuneration
or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f)The Award does not create any contractual or other right to receive future Awards under the
Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g)If the Employee is a resident in a country where English is not an official language, the
Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h)The Company may, in its sole discretion, deliver any documents related to this Agreement,
the Award, or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i) Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same;
(j) The Employee voluntarily acknowledges and consents to the collection, use, processing and transfer of personal information for purposes of administration and management of the Employee’s participation in the Plan. The Employee may, at any time, review the data, require necessary amendments to it or withdraw consents in writing by contacting the Company; however, withdrawing consent may affect the Employee’s ability to participate in the Plan;
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Performance Share Unit Agreement has been duly executed as of Month Day, Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.96
11
exhibit1096.htm
EX-10.96
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
RESTRICTED STOCK UNIT AGREEMENT
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Restricted Stock Unit Award (the “Award”) dated Month Day. Year (the “Award Date”) with respect to the number of shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “RSUs”), all in accordance with and subject to the following terms and conditions:
1.Definitions.
For purposes of the Award and this Restricted Stock Unit Agreement (this “Agreement”) the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meanings ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2. Normal Vesting.
The Award is subject to a Restricted Period, and during the Restricted Period the Award is subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, the RSUs shall become fully vested on the Vesting Date set forth in the Award Statement (the “Vesting Date”), provided that the Employee remains an employee of the PMI Group during the entire period commencing on the Award Date and ending on the Vesting Date.
3.Termination of Employment Before Vesting Date.
(a) In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to (i) death, Disability or (ii) Normal Retirement, or (iii) early retirement or termination of employment (other than for Cause), in either case by mutual agreement [between the PMI Group and the Employee] and after the Employee has attained age 58, then the RSUs shall become fully vested on the date of death, Disability, Normal Retirement, or such early retirement or termination of employment or the date specified in such mutual agreement.
(b) In the event of the termination (other than for Cause) of the Employee’s employment with the PMI Group prior to the Vesting Date by mutual agreement between the PMI Group and the Employee, and the Employee is below age 58 at time of such termination, and (i) if the termination date occurs before the one year anniversary of the Award Date, then the Employee will forfeit their rights to all unvested RSUs immediately upon the termination date, or (ii) if the termination date occurs after the one year anniversary of the Award Date but before the two year anniversary of the Award Date, then one-third of the unvested RSUs will vest immediately upon the termination date while the Employee forfeits their rights to two-thirds of the unvested RSUs immediately upon the termination date, or (iii) if the termination date occurs after the two year anniversary of the Award Date, then two-thirds of the unvested RSUs will vest immediately upon the termination date, and the Employee forfeits their rights to one-third of the unvested RSUs immediately upon the termination date.
(c) Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment with the PMI Group is terminated prior to the Vesting Date in circumstances not specified in Sections 3(a) or 3(b) of this Agreement, the Employee shall forfeit all rights to the unvested RSUs immediately upon the date of termination. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, accelerate the vesting of some or all of such unvested RSUs.
(d) If within the period of 12 months prior to the date of termination of employment, the Employee was an Executive Officer (as designated by the Board of Directors of the Company within the meaning of Section 16 of the Securities Exchange Act of 1934, as amended) and the termination of employment of such Employee is due to a reason other than death or Disability, any shares of Common Stock that are received by such Employee as a result of accelerated vesting provisions of Section 3(a) or (b), shall be automatically subject to a holding period that expires 12 consecutive months from the date of termination of employment.
(e) Subject to the provisions of Section 9 of the Agreement, the Company shall have the exclusive
discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Tax on Dividend Equivalents.
The Employee does not have the right to vote the RSUs or receive dividends prior to the date, if any, such RSUs are paid to the Employee in the form of Common Stock pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall receive cash amounts (less applicable withholding taxes) equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock issuable with respect to the Award, as such dividends are paid.
5. Transfer Restrictions.
This Award and the RSUs issuable thereunder are non-transferable and may not be transferred, assigned, hypothecated, pledged or otherwise disposed of and shall not be subject to execution, attachment or similar process, or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the RSUs shall be forfeited. These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below. In addition, shares of Common Stock subject to the holding period described in Section 3(c) of this Agreement may not be transferred, assigned, hypothecated, pledged or otherwise encumbered for the duration of the applicable holding period.
6. Withholding Taxes.
(a)With respect to Common Stock issuable upon vesting, the Employee understands and
acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations, which may exceed the amount, if any, actually withheld by the Company.
(b)To the extent permitted by law or applicable regulation, the Company shall have the right at
its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from the Award by either (i) deducting the number of shares of Common Stock payable under the RSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the RSUs becoming subject to current taxation (net settlement), or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested RSUs by the Employee (sell-to-cover). Shares of Common Stock payable under the RSUs deducted from the Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to Section 6(b).
(c)If the Company or its tax advisor determines that the Employee is subject to withholding tax in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying tax withholding obligations due in all applicable jurisdictions. If at any point the Employee is or was previously on an international assignment during the Restricted Period, the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s RSUs in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d)In the event that the Company’s obligation to withhold tax arises prior to the delivery of
shares of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e) The Company makes no representations or undertakings regarding the tax treatment in connection with any aspect of the Award, including the grant or vesting of the RSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the RSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested RSUs shall be registered in the name of the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
8. Settlement of RSUs.
Each RSU granted pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions and other terms of this Agreement, to issue to the Employee one share of Common Stock. Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) as soon as reasonably practicable following the Vesting Date pursuant to Section 2 or 3 of this Agreement and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement). However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably practicable thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s RSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement.
In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the RSUs will be settled in the form of cash.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to U.S. Federal income tax on any part of the payment of the RSUs and the Award is subject to Section 409A, then the RSUs shall be subject to the following provisions of this Section 9. If the Employee is a “specified employee” within the meaning of Section 409A, any payment of RSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to receive cash amounts equal to dividends on Common Stock pursuant to Section 4of this Agreement, and such amounts shall be paid to the Employee as such dividends are paid. In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the RSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control as provided by section 6(a) of the Plan, and shall instead be paid at the time the RSUs would otherwise be paid pursuant to this Agreement. References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its Affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying RSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee, or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence. Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to RSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting. The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time.
This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, you acknowledge and agree that:
(a) The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b) As a condition to, and in consideration of, the grant, vesting, and settlement of RSUs, and in receiving the Award of RSUs, shares of Common Stock, or any other benefit relating to the RSUs, the Employee acknowledges, understands and agrees that the future value of the RSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty. Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such RSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the RSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c) The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d) The granting of the Award does not constitute, or be evidence of, any agreement or
understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries to continue to employ the Employee for any specific period of time;
(e)The Award, and any Common Stock acquired under it, are separate from any remuneration
or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f)The Award does not create any contractual or other right to receive future Awards under the
Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g)If the Employee is a resident in a country where English is not an official language, the
Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h)The Company may, in its sole discretion, deliver any documents related to this Agreement,
the Award or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i) Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same.
(j) The Employee voluntarily acknowledges and consents to the collection, use, processing and transfer of personal information for purposes of administration and management of the Employee’s participation in the Plan. The Employee may, at any time, review the data, require necessary amendments to it or withdraw consents in writing by contacting the Company; however withdrawing consent may affect the Employee’s ability to participate in the Plan;
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Restricted Stock Unit Agreement has been duly executed as of Month Day. Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.97
12
exhibit1097.htm
EX-10.97
Document
PHILIP MORRIS INTERNATIONAL INC.
2022 PERFORMANCE INCENTIVE PLAN
PERFORMANCE SHARE UNIT AGREEMENT
FOR PHILIP MORRIS INTERNATIONAL INC. COMMON STOCK
(Month Day, Year)
Performance Period: Month Day, Year to Month Day, Year
PHILIP MORRIS INTERNATIONAL INC. (the “Company”), a Virginia corporation, hereby
grants to the employee identified in the Award Statement (the “Employee”) under the Philip Morris International Inc. 2022 Performance Incentive Plan (the “Plan”), a Performance Share Unit Award (the “Award”) dated Month Day, Year (the “Award Date”) representing a right to receive shares of the Common Stock of the Company (the “Common Stock”) set forth in the Award Statement (the “PSUs”), all in accordance with and subject to the following terms and conditions:
1. Definitions.
For purpose of the Award and this Performance Share Unit Agreement (this “Agreement”), the following terms shall have the following meanings. Capitalized terms not otherwise defined herein shall have the meaning ascribed to them in the Plan.
(a)“Affiliate” shall have the meaning set forth in the Plan.
(b)“Cause” shall have the meaning set forth in the Plan.
(c)“Committee” shall have the meaning set forth in the Plan.
(d)“Disability” shall mean the permanent and total disability as determined under procedures established by the Company for purposes of the Plan.
(e)“Normal Retirement” shall mean the retirement from active employment under a pension plan of any member of the PMI Group or under an employment contract with any member of the PMI Group on or after the date specified as the normal retirement age in the pension plan or employment contract, if any, under which the Employee is at that time accruing pension benefits for their current service (or, in the absence of a specified normal retirement age, the age at which pension benefits under such plan or contract become payable without reduction for early commencement and without any requirement of a particular period of prior service). In any case in which (i) the meaning of “Normal Retirement” is uncertain under the definition contained in the prior sentence or (ii) a termination of employment at or after age 65 would not otherwise constitute “Normal Retirement,” an Employee’s termination of employment shall be treated as a “Normal Retirement” under such circumstances as the Committee, in its sole discretion, deems equivalent to retirement.
(f)“PMI Group” shall mean the Company and each of its Subsidiaries and Affiliates.
(g)“Restricted Period” shall have the meaning set forth in the Plan.
(h)“Section 409A” shall mean section 409A of the Code and the regulations thereunder.
(i)“Subsidiary” shall have the meaning set forth in the Plan.
2. Normal Vesting.
(a) The Award is subject to a Restricted Period, and during the Restricted Period the Award is subject to forfeiture until the time at which it becomes fully vested. Subject to Section 3 of this Agreement below, a number of PSUs shall become vested on the Vesting Date set forth in the Award Statement (the “Vesting Date”) provided that the Employee remains an employee of the PMI Group during the entire period commencing on the Award Date and ending on the Vesting Date.
(b) The actual number of PSUs that become vested on the Vesting Date is equal to a percentage of the target number of PSUs (the “Performance Percentage”), which percentage is determined based on the performance achieved during the applicable performance period, as shown on the Award Statement and as determined by the Committee. The minimum percentage of PSUs that can vest is zero, while the maximum is twice the targeted number, subject to the limitations of the Plan. For the avoidance of doubt, if the date on which the Committee certifies the Performance Percentage is after the Vesting Date, then the actual number of PSUs that become vested shall not be determined until such later date of certification, and such later date of certification shall be treated as the Vesting Date for purposes of cash payments with respect to dividends and the timing of payment of the PSUs pursuant to Sections 4 and 8 of this Agreement. The Committee shall certify the Performance Percentage no later than June 30 immediately following the year in which the performance period ends.
3. Termination of Employment Before Vesting Date.
(a) In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to (i) Normal Retirement, or (ii) early retirement or termination of employment (other than for Cause), in either case by mutual agreement between the PMI Group and the Employee and after the Employee has attained age 58, then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2(b) of this Agreement. In the event of the termination of the Employee’s employment with the PMI Group prior to the Vesting Date due to death or Disability, then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested at the date of such termination shall be equal to the target number of PSUs set forth on the Award Statement.
(b) In the event of the termination of the Employee’s employment (other than for Cause) with the PMI Group prior to the Vesting Date by mutual agreement between the PMI Group and the Employee, and the Employee is below age 54 at the time of such termination, then the Employee will forfeit their rights to all unvested PSUs immediately upon the termination date. In the event that such termination occurs when the Employee has attained age 54 but has not yet attained age 56 at time of such termination, and the termination date is more than two years after the Award Date then the requirement that the Employee remain an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the actual number of PSUs that actually become vested shall be determined based on the Performance Percentage as certified by the Compensation Committee in accordance with Section 2(b) of this Agreement, or if the termination date is less than two years after the Award Date, the Employee will forfeit their rights to all unvested PSUs immediately upon the termination date. In the event that such termination occurs when the Employee has attained age 56 but has not yet attained age 58, and the termination date is more than one year after the Award Date then the requirement that the Employee remains an employee of the PMI Group through the Vesting Date shall be deemed satisfied, and the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Compensation Committee in accordance with Section 2(b) of this Agreement; if the termination date is less than one year after the Award Date, the Employee will forfeit their rights to all unvested PSUs immediately upon the termination date.
(c) Subject to the provisions of section 6(a) of the Plan, if the Employee’s employment with the PMI Group is terminated prior to the Vesting Date for any reason not specified in Sections 3(a) or 3(b) of this Agreement, the Employee shall forfeit all rights to the unvested PSUs immediately upon date of termination. Notwithstanding the foregoing and except as provided in section 6(a) of the Plan, upon the termination of an Employee’s employment with the PMI Group, the Committee or its designee may, in its sole discretion, treat the requirement that the Employee remain an employee of the PMI Group through the Vesting Date as deemed satisfied with respect to some or all of the PSUs, and in such case the number of PSUs that become vested shall be determined based on the Performance Percentage as certified by the Committee in accordance with Section 2(b) of this Agreement multiplied by the target number of PSUs for which the Committee treats the continued employment requirement as deemed satisfied.
(d) If the requirement that the Employee remain an employee of the PMI Group through the Vesting Date is deemed satisfied under this Section 3 for any reason other than the Employee’s death or Disability, but the Employee dies before the Committee’s certification of the Performance Percentage, then the number of PSUs that become vested shall be equal to the target number of PSUs for which the continued employment requirement is deemed satisfied under this Section 3.
(e) Subject to the provisions of Section 9 of the Agreement, the Company shall have the exclusive discretion to determine when the Employee is no longer an Employee of the PMI Group for purposes of vesting or forfeiture of the Award under this Agreement for end of employment related cases or activities.
4. Voting and Dividend Rights; Withholding Taxes on Dividend Equivalents.
The Employee does not have the right to vote the PSUs or receive dividends prior to the date, if any, PSUs become vested and Common Stock becomes issuable to the Employee pursuant to the terms hereof. However, unless otherwise determined by the Committee, the Employee shall be credited with cash amounts equal to the dividends paid from the date the Award is granted through the date of payment under Section 8 of this Agreement with respect to shares of Common Stock that become issuable as of the Vesting Date, with such cash credits calculated without interest and paid, less applicable tax withholdings, in accordance with this Agreement.
5. Transfer Restrictions.
The Award and the PSUs issuable thereunder are non-transferable and may not be assigned, hypothecated, pledged, or otherwise disposed of and shall not be subject to execution, attachment or similar process or otherwise encumbered. Upon any attempt to effect any such disposition, or upon the levy of any such process, the Award shall immediately become null and void and the PSUs shall be forfeited. These restrictions shall not apply, however, to any payments received pursuant to Section 8 of this Agreement below.
6. Withholding Taxes.
(a) With respect to Common Stock issuable upon vesting, the Employee understands and acknowledges that, regardless of any action taken by the Company, they are responsible for the tax consequences of receiving the Award granted by the Company and at time of grant will review the personal tax implications with a tax advisor. The Employee acknowledges that the Company does not commit to structure the terms of the grant or any aspect of the Award to reduce or eliminate the Employee’s liability for withholding taxes or future personal tax obligations, which may exceed the amount, if any, actually withheld by the Company.
(b) To the extent permitted by law or applicable regulation, the Company shall have the right at its sole and absolute discretion to collect, directly from the Employee or from other compensation amounts due to the Employee from the Company, any and all amounts required to satisfy the actual statutory withholding taxes, and/or hypothetical withholding tax amounts if applicable, arising from the Award by either (i) deducting the number of shares of Common Stock payable under the PSUs having an aggregate value equal to the amount of withholding taxes due from the total number of shares of Common Stock payable under the PSUs becoming subject to current taxation (net settlement), or (ii) the remittance of the required amounts from any proceeds realized upon the open-market sale of the Common Stock received in payment of vested PSUs by the Employee (sell-to-cover). Shares of Common Stock payable under the PSUs deducted from the Award in satisfaction of tax withholding shall be valued at the fair market value of the Common Stock on the date as of which the amount giving rise to the withholding requirement first became includible in the gross income of the Employee under applicable tax laws. The Employee will have no further rights with respect to any shares of Common Stock that are retained or sold by the Company pursuant to this Section 6(b).
(c) If the Company or its tax advisor determines that the Employee is subject to withholding tax in more than one jurisdiction, the Employee acknowledges that the Company may be required to withhold or otherwise make arrangements for satisfying tax withholding obligations due in all applicable jurisdictions. If at any point the Employee is, or was previously on, an international assignment during the Restricted Period, the Company will calculate the amount of hypothetical tax which will be imposed on the Employee’s PSUs, in accordance with the Company’s guidelines in force at the time the withholding obligation arises.
(d) In the event that the Company’s obligation to withhold tax arises prior to the delivery of shares
of Common Stock (or cash proceeds) to the Employee (e.g. at time of grant) or it is determined after the delivery of shares of Common Stock (or cash proceeds) that the amount withheld by the Company’s withholding obligation was greater than the amount withheld by the Company, the Employee agrees to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
(e) The Company makes no representations or undertakings regarding the tax treatment in connection with any aspect of the Award, including the grant or vesting of the PSUs, the subsequent sale of shares of Common Stock acquired upon vesting and the receipt of any dividends or dividend equivalents, and does not commit to structure the terms of the Plan, this Agreement or any aspect of the Award to reduce or eliminate the Employee’s personal tax liability or other tax obligations.
7. Death of Employee.
If any of the PSUs shall vest upon the death of the Employee, any Common Stock received in payment of the vested PSUs shall be registered in the name of the estate of the Employee, and any cash amounts credited with respect to dividends shall be paid to the estate of the Employee. If the Company determines that settlement in the form of Common Stock is impractical or impermissible under the Estate laws of the Employee’s country of residence, the PSUs will be settled in the form of cash.
8. Settlement of PSUs.
The grant pursuant to the Award represents an unfunded and unsecured promise of the Company, subject to the vesting conditions, achievement of performance targets and other conditions set forth in of this Agreement, to issue to the Employee for each vested PSU one share of Common Stock and to pay to the Employee in a single lump sum any cash amounts credited on such vested PSU with respect to dividends.
Except as otherwise expressly provided in the Award Statement and subject to the terms of this Agreement, such issuance and lump sum payment shall be made to the Employee (or, in the event of their death to the Employee’s estate as provided above) (a) in all cases other than those set forth in clause (b), as soon as reasonably practicable following the Vesting Date and no later than December 31 of the year in which the Vesting Date occurs (except as otherwise provided in Section 9 of this Agreement), and (c) in the case of termination of employment by reason of death or Disability or the Employee’s death after a termination of employment in the circumstances specified in Section 3 of this Agreement, as soon as reasonably practicable following such termination of employment or death. However, if a scheduled Vesting Date falls on a Saturday, Sunday or federal holiday, such issuance date shall instead fall on the next following day that the principal office of the Company responsible for processing such transactions and the principle executive offices of the Company are open for business, or as soon as reasonably practicable thereafter.
Notwithstanding the foregoing, in the event that Employee is subject to the Company’s policy permitting officers and directors to sell shares only during certain “window” periods, in effect from time to time or Employee is otherwise prohibited from selling shares of the Company’s Common Stock in the public market and any shares covered by Employee’s PSUs are scheduled to be issued on a day (the “Original Distribution Date”) that does not occur during an open “window period” applicable to Employee, as determined by the Company in accordance with such policy (“Insider Trading Policy”), or does not occur on a date when Employee is otherwise permitted to sell shares of the Company’s Common Stock in the open market, and the Company elects not to satisfy its tax withholding obligations by withholding shares from Employee’s distribution (net settlement), then either (i) such shares shall not be issued and delivered on such Original Distribution Date and shall instead be issued and delivered during the next occurring open “window period” applicable to Employee pursuant to such policy (regardless of whether Employee is still providing continuous services at such time) or during the next period when Employee are not prohibited from selling shares of the Company’s Common Stock in the open market, but in no event later than December 31 of the calendar year in which the Original Distribution Date occurs, or (ii) the Company shall rely on any such similar process it may adopt from time to time consistent with the Insider Trading Policy, the Plan and this Agreement. In the event the Company determines that settlement in the form of Common Stock is impractical or impermissible under the laws of the Employee’s country of residence, the PSUs will be settled in the form of cash.
9. Compliance with Code Section 409A.
Notwithstanding anything in this Agreement to the contrary, if the Employee is subject to US Federal income tax on any part of the payment of the PSUs and the Award is subject to Section 409A, then the PSUs shall be subject to the following provisions of this Section 9. If the Employee is a “specified employee” within the meaning of Section 409A, any issuance or payment in respect of the PSUs under Section 8 of this Agreement above that is on account of the Employee’s separation from service and is scheduled to be paid within six months after such separation from service shall accrue without interest and shall be paid as soon as reasonably practicable after the first day of the seventh month beginning after the date of the Employee’s separation from service or, if earlier, as soon as reasonably practicable following the Employee’s death. During such delayed distribution period, the Employee shall continue to be credited with cash amounts equal to dividends on Common Stock for the applicable Award pursuant to Section 4 of this Agreement, and such amounts shall accrue without interest and shall be paid in a lump sum at the time specified in the preceding sentence. In the event of a “Change in Control” under section 6(b) of the Plan that is not also a “change in control event” within the meaning of Treas. Reg. §1.409A-3(i)(5)(i), the PSUs shall vest as set forth in section 6(a) of the Plan, but shall not be paid upon such Change in Control or termination of employment as provided by section 6(a) of the Plan, and shall instead be paid at the time the PSUs would otherwise be settled at the end of the applicable performance period in accordance with Section 8 of this Agreement.
References to termination of employment and separation from service shall be interpreted to mean a separation from service, within the meaning of Section 409A, with the Company and all of its Affiliates treated as a single employer under Section 409A. This Agreement shall be construed in a manner consistent with Section 409A. For purposes of Section 409A, the payment of dividend equivalents under Section 4 of this Agreement shall be construed as earnings and the time and form of payment of such dividend equivalents shall be treated separately from the time and form of payment of the underlying PSUs.
10. Clawback.
Notwithstanding anything in this Agreement to the contrary, if the Board of Directors of the Company or an appropriate Committee of the Board determines that, as a result of fraud, misconduct, a restatement of the Company’s financial statements, or a significant write-off not in the ordinary course of business affecting the Company’s financial statements, an Employee, or former Employee, has received more compensation in connection with this Award than would have been paid absent the fraud, misconduct, write-off or incorrect financial statement, the Board or Committee, in its discretion, shall take such action with respect to this Award as it deems necessary or appropriate to address the events that gave rise to the fraud, misconduct, write-off or restatement and to prevent its recurrence. Such action may include, to the extent permitted by applicable law, causing the partial or full cancellation of this Award and, with respect to PSUs that have vested, requiring the Employee to repay to the Company the partial or full fair market value of the Award determined at the time of vesting. The Employee agrees by accepting this Award that the Board or Committee may make such a cancellation, impose such a repayment obligation, or take other necessary or appropriate action in such circumstances.
In consideration for the Award, the Employee acknowledges and agrees that Employee is subject to any clawback or recoupment policy or other written agreement or arrangement the Company may have now or in the future with the Employee to the extent required by applicable law or rule of any securities exchange or market on which shares of Common Stock are listed or admitted for trading, as determined by the Committee in its sole discretion (the “Clawback Policy”) and that the Employee’s rights with respect to the Award and any other Awards granted to the Employee shall be subject to the Clawback Policy, as amended from time to time. This Agreement shall in all events be subject to all rights and obligations that the Company may have regarding the clawback of “incentive-based compensation” under Section 10D of the Exchange Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and any applicable rules and regulations promulgated thereunder from time to time by the U.S. Securities and Exchange Commission.
11. Other Terms and Acknowledgements.
By entering into this Agreement and accepting the Award, you acknowledge and agree that:
(a) The terms and provisions of the Plan (a copy of which will be furnished to the Employee upon written request to the Office of the Secretary, Avenue de Rhodanie 50, 1007 Lausanne, Switzerland) are incorporated herein by reference. To the extent any provision of this Agreement is inconsistent or in conflict with any term or provision of the Plan, the Plan shall govern;
(b) As a condition to, and in consideration of, the grant, vesting, and settlement of PSUs, and in receiving the Award of PSUs, shares of Common Stock, or any other benefit relating to the PSUs, the Employee acknowledges, understands and agrees that the future value of the PSUs, or the underlying shares of Common Stock upon settlement thereof pursuant to Section 8 of this Agreement, is unknown, indeterminable and cannot be predicted with certainty.
Neither the Company nor any Subsidiary or Affiliate will be liable for any decrease in the value of such PSUs, or underlying shares of Common Stock, or for any foreign exchange rate fluctuations between the Employee’s local currency and the United States Dollar that may affect the value of any benefit the Employee may receive in relation to the PSUs, or the underlying shares of Common Stock to be issued pursuant to the settlement thereof;
(c) The Company has granted the Employee this Award. The Employee’s employing Affiliate
or Subsidiary does not have power or authority over the terms and conditions of the Award, which are controlled solely by the Company, this Agreement, and the Plan;
(d) The granting of the Award does not constitute, or be evidence of, any agreement or
understanding, express or implied, on the part of the Company or its Affiliates or Subsidiaries to continue to employ the Employee for any specific period of time;
(e) The Award, and any Common Stock acquired under it, are separate from any remuneration
or benefits provided to the Employee by the Employee’s employing Affiliate or Subsidiary. Accordingly, the Award’s value and/or any Common Stock acquired under it will not be included in the basis for calculations of compensation, earnings, salaries, or other similar compensation terms used when calculating the Employee’s benefits under any employee benefit plan sponsored by the Company or any Affiliate or Subsidiary, including but not limited to any severance payments or similar termination compensation or indemnities that the Employee may be entitled to under local laws or collective agreements;
(f) The Award does not create any contractual or other right to receive future Awards under the
Plan, or benefits in lieu of the Awards, even if Awards have been awarded repeatedly in the past;
(g) If the Employee is a resident in a country where English is not an official language, the
Employee acknowledges and agrees that it is their express intent that this Agreement and the Plan and all other documents and notices given or instituted pursuant to the Award be drawn up in English. Further, the Employee acknowledges that they are sufficiently proficient in English to understand the terms and conditions of this Agreement and any documents related to the Plan or have had the ability to consult with an advisor who is sufficiently proficient in the English language. If the Employee received this Agreement or any other document related to the Plan translated into a language other than English and if the meaning of the translated version is different than the English version, the English version will control;
(h) The Company may, in its sole discretion, deliver any documents related to this Agreement, the Award or future Awards that may be granted under the Plan by electronic means. The Employee consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online electronic system established and maintained by the Company or another third party designated by the Company;
(i) Depending upon the country to which laws the Employee is subject, the Employee may have certain foreign asset/account and/or tax reporting requirements that may affect the Employee's ability to acquire or hold shares of Common Stock under the Plan or cash received from participating in the Plan (including from any dividends or sale proceeds arising from the sale of shares of Common Stock) in a brokerage or bank account outside the Employee 's country of residence. The Employee's country may require that the Employee report such accounts, assets or transactions to the applicable authorities in the Employee's country. The Employee also may be required to repatriate cash received from participating in the Plan to the Employee's country within a certain period of time after receipt. The Employee is responsible for knowledge of and compliance with any such regulations and should speak with the Employee's personal tax, legal and financial advisors regarding same;
(j) The Employee voluntarily acknowledges and consents to the collection, use, processing and transfer of personal information for purposes of administration and management of the Employee’s participation in the Plan. The Employee may, at any time, review the data, require necessary amendments to it or withdraw consents in writing by contacting the Company; however withdrawing consent may affect the Employee’s ability to participate in the Plan;
12.Country-Specific Terms, Conditions, and Notices.
If the Employee is a citizen of, resides in, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries set forth in the Country-Specific Equity Terms, Conditions, and Notifications Annex (the “Annex”), this Agreement is subject to, and its delivery is qualified by, the special terms, conditions, and notifications applicable to such country set forth in the Annex, subject to the qualifications set forth therein. Moreover, if Employee relocates to, or otherwise is deemed a citizen or resident of, or is otherwise determined by the Company in its discretion to be subject to the laws of one of the countries included in the Annex during the term of this Agreement, the special terms, conditions, and/or notifications for such country set forth in the Annex will apply to him or her unless determined otherwise by the Company.
IN WITNESS WHEREOF, this Performance Share Unit Agreement has been duly executed as of Month Day, Year.
PHILIP MORRIS INTERNATIONAL INC.
By: /s/ DARLENE QUASHIE HENRY
Name: Darlene Quashie Henry
Title: Vice President, Associate General Counsel & Corporate Secretary
EX-10.98
13
redactedsettlementagreement.htm
EX-10.98
Document
1 FEBRUARY 2024
(1) PHILIP MORRIS PRODUCTS S.A.
AND
(2) NICOVENTURES TRADING LIMITED
THE USE OF THE FOLLOWING NOTATION IN THIS EXHIBIT INDICATES THAT THE CONFIDENTIAL PORTION HAS BEEN OMITTED PURSUANT TO ITEM 601(b)(10)(iv) WHEREBY CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED BECAUSE IT IS BOTH NOT MATERIAL AND THE REGISTRANT CUSTOMARILY AND ACTUALLY TREATS SUCH INFORMATION AS PRIVATE OR CONFIDENTIAL: [***]
CONTENTS
|
|
|
|
|
|
| SCHEDULE 2.1(A) - PROCEEDINGS |
|
|
|
|
|
|
|
| SCHEDULE 2.1(B) - AGREED FORM ORDERS |
|
| SCHEDULE 2.5 - OTHER AGREED FORM DOCUMENTS IN RELATION TO THE PROCEEDINGS |
|
| SCHEDULE 3.1 - FORM ITC PETITION, LETTER AND LICENSE |
|
| SCHEDULE 3.3 - OTHER INJUNCTIONS |
|
| SCHEDULE 18.3 - ROYALTY MECHANISM EXAMPLES |
|
| SCHEDULE 19.7 - INDEPENDENT ACCOUNTANT |
|
| SCHEDULE 28.2 - CONFIDENTIALITY UNDERTAKINGS |
|
| SCHEDULE 29.2 - PMP DISCLOSURES AGAINST WARRANTIES |
|
| SCHEDULE 29.3 - BAT DISCLOSURES AGAINST WARRANTIES |
|
| SCHEDULE 35.2 - PRESS RELEASE |
|
| SCHEDULE 1.1.92 EXISTING DAC |
|
| SCHEDULE 1.1.94 - EXISTING PRODUCTS |
|
| SCHEDULE 1.1.220 - RELEASED PATENT FAMILIES |
|
| SCHEDULE 1.1.222 - RELEASED PRINCIPAL BRANDS |
|
| SCHEDULE 1.1.249 - ROYALTY [***] |
|
| EXHIBIT A - ADR EXHIBIT |
|
| EXHIBIT B - STANDSTILL EXHIBIT |
|
| CONFIDENTIAL EXHIBIT 3A - PMP COMFORT LETTER |
|
| CONFIDENTIAL EXHIBIT 3B - BAT COMFORT LETTER |
|
This Settlement Agreement (“Agreement”) by and between: (1) Philip Morris Products S.A., a corporation organised under the laws of Switzerland, with offices at Quai Jeanrenaud 3, 2000 Neuchâtel, Switzerland (“PMP”); and (2) Nicoventures Trading Limited, a company registered in England and Wales with registered office at Globe House, 1 Water Street, London, WC2R 3LA (“BAT”) is entered into as of the Effective Date.
Capitalised terms used but not defined in context have the meanings given them in Schedule 1 of this Agreement.
BACKGROUND
A. Each Party develops and/or commercialises a range of potentially reduced-risk tobacco and nicotine products, including the Products, and each Party has a wide portfolio of Patents including those which relate to the Products.
B. PMP owns Patents which it considers to be valid and infringed by Products of BAT. BAT owns Patents which it considers to be valid and infringed by Products of PMP.
C. The Parties are party to the Proceedings relating to certain Patents and certain Products.
D. To avoid the time and expense of the Proceedings and without any admission of liability or fault, the Parties desire to enter into a full, final, complete and global settlement of the Proceedings. The settlement of the Proceedings is intended to “wipe the slate clean” (i.e., bring an end to the Proceedings and release certain past and future claims) and protect Existing Products, in the form and presentation as sold on or before [***], from further proceedings in the Territory other than Excepted Claims.
E. In order to limit future disputes between the Parties and their respective Supply Chain Entities, whilst promoting continued innovation, the Parties have agreed a number of inter-related mechanisms to prospectively avoid certain Patent and other Intellectual Property Rights disputes relating to certain Products. The mechanisms include potentially royalty-bearing Covenants not to Sue and licences in the Territory and the ADR Procedure. In view of the inter-relationship between these mechanisms, the Parties recognise that no royalty rates can be viewed in isolation, being part of a heavily negotiated settlement of existing and prospective disputes between two uniquely situated Parties.
F. The Products include a product [***] referred to in this Agreement as [***]. In order to provide the certainty of a Covenant not to Sue for [***], the Parties have agreed that the potential royalty liability of BAT to PMP for [***] will be based upon the claims of certain Patents of PMP, including certain patent [***].
G. Any further agreement between the Parties in relation to products outside of the aegis of this Agreement, may be the subject of an Agreement Part 2.
H. In view of the complexity of agreed arrangements encompassed by this Agreement, the following roadmap is provided for orientation purposes only:
(a) the Parties have agreed to dismiss proceedings between them in accordance with Section 2 (including the rescission of the ITC Limited Exclusion Order and/or Cease and Desist Order and rescission of the injunctions in accordance with Section 3). The Parties will bear their own costs of the Proceedings, save to the extent any costs orders have been made and paid prior to the Effective Date. The Parties provide the mutual releases and Covenants not to Sue in relation to the Released Claims in accordance with Sections 4 and 5;
(b) perpetual Covenants not to Sue are given in the Territory in relation to:
(i) Existing Products as these were marketed on or before [***] basis (Section 5);
(ii) [***] on either a royalty-free or royalty-bearing basis (Sections 9 and 10);
(iii) Changed Products on a potentially royalty-bearing basis (Sections 11, 12, 13 and 14);
(iv) the Parties’ respective Changed Products or [***] in respect of Patent Families used in the Parties’ respective Existing Products (Section 6);
(v) (1) the Parties’ respective Changed Products or [***] in the Parties’ respective Existing Products (excluding [***]); (2) [***] Changed Products [***]; and (3) Other HNB and Vapour Changed Products [***] in the applicable Party’s Existing Products in the same Product [***] (Section 7); and
(vi) Accessories on a [***] basis; and Replacement Parts and Upgrade Parts on a [***] basis (Section 24),
(collectively, the Existing Products, [***] and the Changed Products are referred to hereinafter as the “Part 1 Products”);
(c) to benefit from the Covenants not to Sue, [***] and each of the Changed Products must be Compliant in accordance with the applicable criteria (as set out in Sections 9.4, 11.3, 12.2 and 13.2 and as assessed using the protocol in Section 16);
(d) the protocol in Section 17 and the assessment procedure in Section 15 is used to determine the Relevant Patent Families for royalty purposes.
(e) for [***], the royalty rate is determined based on the royalty [***] set out in Section 10 and[***], and, if [***] is determined to be royalty-bearing, then this royalty rate is determined and applied [***] for HNB Products [***] in accordance with Section 10.
(f) for Changed Products, the royalty rate (if any) for each Device or Consumable is calculated using [***] set out in Section 14 and applying the Royalty Mechanism of Section 18 to that Device or Consumable in accordance with Section 18, then this royalty rate is applied [***] of that Device or Consumable [***]
(g) Provision is included for the [***] to change during the Term. Payment is made as set out in Section 19.
(h) in order to streamline and simplify the assessment of whether royalties are due, the Parties have agreed to use a [***] Patent [***] for HNB Products [***]. However, in some cases for HNB Products where for the [***] Patent there is no [***] designation, [***] may be substituted although English law would be applied to the assessment and if that [***] designation is invalidated through a Validity Challenge that [***] Patent would not be available for assessment. For Vapour Products the [***] are used as [***] Patents;
(i) the Parties are mindful of the considerable disruption which may be caused by patent proceedings being brought against manufacturing facilities and therefore a Covenant not to Sue is given in the Territory in relation to manufacture, which also covers the manufacture of Products and their Components that are not Part 1 Products and the manufacture of ARUs and their Components, in accordance with Section 23;
(j) the Parties are mindful of the considerable disruption which may be caused by Measures and therefore the Parties will not seek, assist, or permit entry or enforcement of Measures based on Patents in accordance with Section 32;
(k) [***] grants to [***] a licence to the [***] Patents in [***] as set out in Section 20;
(l) each Party provides assurances to the other Party regarding their performance and compliance of its [***] Companies and Representatives in accordance with Section 1 and further assurances in relation to Third Parties in Section 25;
(m) the Parties have established an Advisory Committee as a forum to discuss and mitigate potential disputes under this Agreement and facilitate communication between the Parties relating to the good operation of this Agreement as set out in Section 36;
(n) the Parties have established a comprehensive and exclusive dispute resolution framework as set out in the ADR Exhibit for any Dispute between them as to the scope, rights and obligations granted under this Agreement including but not limited to resolving whether particular products are Part 1 or Part 2 Products or other products; and
(o) the Parties have agreed to a litigation standstill in respect of certain products as set out in the Standstill Exhibit.
Now therefore, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
1.OBLIGATIONS OF THE PARTIES
1.1Each of Philip Morris Products S.A. and Nicoventures Trading Limited hereby unconditionally and irrevocably agrees with the other, subject to Section 37, to procure the compliance, including the performance of all obligations, by its Associated Companies and its and their Representatives with the terms and conditions of this Agreement as if they too were parties to this Agreement.
1.2Each of Philip Morris Products S.A. and Nicoventures Trading Limited shall be liable for the non-compliance of its Affiliates and its and their Representatives and shall indemnify and hold harmless the other, its Affiliates and their Representatives and Supply Chain Entities in respect of (subject to Sections 29.7, 29.8, and 37) all losses, damages, costs, expenses, charges, penalties and other liabilities (including reasonable legal and other professional fees) incurred or suffered by the other, its Affiliates and their Representatives or Supply Chain Entities arising out of or in connection with: (1) any breach of a Covenant not to Sue by the indemnifying party or its Representatives; or (2) any breach of a Covenant not to Sue or any non-performance or non-compliance of any kind by the indemnifying party’s Associated Companies or their respective Representatives under this Agreement.
1.3PMP and BAT are each individually referred to herein as a “Party” and collectively as the “Parties”. Unless expressly specified otherwise, references in this Agreement to a Party (including references to “PMP” and to “BAT”) shall be deemed to include that Party’s Affiliates, and an obligation on a Party shall include an obligation to procure that its Affiliates comply with the same obligation, and a right of a Party shall be for the benefit of that Party and its Affiliates. References in this Agreement to Philip Morris Products S.A. and Nicoventures Trading Limited shall be references to those companies excluding their Affiliates, and Philip Morris Products S.A. and Nicoventures Trading Limited are each individually referred to herein as the “Signatory” as the case may be.
1.4A reference to a Supply Chain Entity shall be deemed to include its Representatives for the purposes of Sections 25.2, 28, 31, and 41.8.
1.5The Mutual Release in Section 4.1 and, subject to Sections 1.6 and 1.7, each Covenant not to Sue or licence (including those granted under Sections 3 and 20.1) under this Agreement or undertaking to procure that a Third Party Covenants not to Sue, given by BAT or PMP, is for the benefit of the other Party and its Representatives and Connected Persons (solely to the extent that the acts of such Connected Persons relate to the applicable Products of such Party), subject to such Connected Person undertaking all applicable acts described in Section 25.4.
1.6For the purposes of Sections 6.1, 7.1, 7.3, and 9.2, the Covenant not to Sue is a Covenant not to Sue: (1) BAT and its Representatives; and (2) solely to the extent that their acts relate to [***] and Changed Products of BAT, Connected Persons of BAT, subject to such Connected Persons undertaking all applicable acts described in Section 25.4.
1.7For the purposes of Sections 6.2, 7.2, and 7.4, the Covenant not to Sue is a Covenant not to Sue: (1) PMP and its Representatives; and (2) solely to the extent that their acts relate to Changed Products of PMP, Connected Persons of PMP, subject to such Connected Persons undertaking all applicable acts described in Section 25.4.
1.8Without prejudice to Section 1.1, the Covenants not to Sue in this Agreement shall not apply to, and the Released Claims shall not include, any claims in respect of any breach of this Agreement.
1.9The Covenants not to Sue granted hereunder shall be deemed to incorporate an obligation on the granting Party to procure that its [***] Companies shall comply with such Covenants not to Sue as if those [***] Companies had granted such Covenants not to Sue, for so long as such [***] Companies are an Associated Company of the granting Party.
1.10The Covenants not to Sue with respect to Patents granted hereunder are intended to, and shall be construed to have, the same effect for the purposes of patent exhaustion, as they would if they were non-exclusive licences of the same scope.
1.11The Covenants not to Sue granted in this Agreement that apply to Products that are Launched will, upon such Launch, [***] apply and be deemed effective [***], but only to the extent such Product is within a Covenant not to Sue.
1.12All of the licences, Covenants not to Sue and releases provided for in this Agreement are granted in relation to Intellectual Property Rights owned or, subject to Sections 22 and 25, [***] licensed by the Party granting the licence, Covenant not to Sue or release. For the avoidance of doubt, insofar as any of the licences, Covenants not to Sue and releases provided for in this Agreement are granted by reference to particular Patent Families, such licences, Covenants not to Sue and releases only extend to Patents within the Patent Family that are owned or, subject to Sections 22 and 25, [***] licensed by the Party granting the licence, Covenant not to Sue or release.
2.DISMISSAL OF PROCEEDINGS
2.1The Parties shall co-operate and take all actions necessary to cause the Proceedings to be fully and finally dismissed, with prejudice (to the extent possible in each such jurisdiction or forum save to the extent Schedule 2.1(A) provides otherwise), as between the Parties, without admission of liability, on the basis of the agreed form orders (listed at Schedule 2.1(B) with no monetary damages assessed or awarded on or before the Effective Date to be paid.
2.2Each Party has listed in Schedule 2.1(A) the parties to the respective Proceedings.
2.3Prior to the execution of this Agreement each Party has executed and completed all other required formalities on all orders and other documents necessary from that Party to fully and finally dismiss the Proceedings. Simultaneously with the execution of this Agreement each Party shall provide to the other copies of all such documents. Each Party shall take all actions specified in Schedule 2.1(A) in accordance with the respective timings specified therein and by reference to the agreed form orders listed in Schedule 2.1(B). If any of the agreed form orders listed in Schedule 2.1(B) are not accepted by the relevant Governmental Authority, each Party shall promptly co-operate and take all further actions necessary to cause the full and final dismissal of the Proceedings in accordance with Section 2.1 above.
2.4Each Party shall pay all of its own costs incurred in the Proceedings except to the extent that any costs orders have been made and paid prior to the Effective Date.
2.5Where any bond, security or guarantee has been paid or provided in respect of any of the Proceedings, each Party shall use their Best Efforts to procure the release or return of the monies underlying such instrument to the Party which provided such bond, security or guarantee, including without limitation the submission of the agreed documents listed in Schedule 2.5. If any of the agreed form documents listed in Schedule 2.5 are not accepted by the relevant Governmental Authority, each Party shall promptly co-operate and take all further actions necessary to cause such release or return of the monies in accordance with this Section 2.5. Each Party warrants that it has not, and represents that it shall not, seek to call upon any such bond, security or guarantee.
2.6The dismissal of the Proceedings shall not limit, prevent or otherwise restrict any Party from asserting, continuing or defending against any proceedings with Third Parties, including in relation to any Patent Family Member of the Released Patent Families, save as set out in Sections 4 and 5.
3.RECISSION OF THE ITC EXCLUSION ORDER AND TERMINATION OF INJUNCTIONS
3.1In furtherance of Section 2.1, simultaneously with the execution of this Agreement the Parties shall execute, and within [***] Business Days of the Effective Date, the Parties shall jointly file with the International Trade Commission (“ITC”), a Joint Unopposed Petition to Rescind Remedial Orders in the form attached in Schedule 3.1. Simultaneously with the execution of this Agreement BAT also will execute: (1) a joint letter to the Chief, Exclusion Order Branch, US Customs and Border Protection in the form set forth in Schedule 3.1; and (2) an express licence and authorisation to the Patents at issue in the ITC proceeding, in the form set forth in Schedule 3.1. BAT will use its Best Efforts to assist PMP to procure that the ITC rescinds the currently-existing prohibition on the importation into the USA of all products and components thereof that may be or are subject to, or excluded from entry into the USA by, the ITC Limited Exclusion Order and/or Cease and Desist Order. BAT shall also use its Best Efforts to assist PMP to procure that none of PMP or its Affiliates are subject to the ITC Limited Exclusion Order and/or Cease and Desist Order if it becomes necessary to take measures other than jointly filing the Joint Unopposed Petition to Rescind Remedial Orders [***].
3.2The Parties shall co-operate and take all actions necessary to cause any injunctions granted in the Proceedings other than that referenced in Section 3.1, and any applications therefor, to be fully and finally discharged, as between the Parties, without admission of liability (“Other Injunctions”).
3.3Each Party has listed in Schedule 3.3 all Other Injunctions obtained by or on behalf of it, its [***] Companies or their respective Representatives, or in which it, its [***] Companies or their respective Representatives (when acting as a Representative to such Person) have assisted, aided or abetted. Each Party shall take all actions necessary to discharge the Other Injunctions, including all such actions specified in Schedule 3.3 in accordance with the respective timings specified therein. A Party’s failure to list in Schedule 3.3 any Other Injunctions shall not relieve that Party from its obligations set forth in the previous sentence and in Section 3.2 above.
4.MUTUAL RELEASE
4.1Subject to Section 4.2, this Agreement is in full and final settlement of, and each Party (the “Releasing Party”) hereby provides a full release and forever discharges, all and/or any actions, claims, rights, demands and set-offs, whether or not presently known to the Parties, or their respective Representatives or to the law, and whether in law or equity, they had, have or may in the future have against the other Party (the “Released Party”) including, for the avoidance of doubt, all such actions, claims, rights, demands and set-offs arising out of or relating to activities or conduct occurring before, during or after the Term:
(a)in the Territory arising out of or relating to the Proceedings including orders connected thereto (including any potential liabilities in connection with any injunctions granted) save for any confidentiality or protective orders; or
(b)in the Territory relating to infringement of each Released Patent Family by or in relation to any and all of the Released Party’s Products in the Product Category or Product Categories in which the Released Patent Family has been released as specified in Schedule 1.1.233, Accessories and Replacement Parts Sold for Use with such Products and Components of all the foregoing;
(c)in relation to Patents in respect of the use, manufacture, marketing, distribution and sale in the Territory of the Released Party’s:
(i)Existing Products, Existing Accessories, Existing Upgrade Parts and their respective Components [***] as sold on or before [***] insofar as such Patents are used in or cover such Existing Products, Existing Accessories, Existing Upgrade Parts or their respective Components as sold on or before [***]; or (d)in relation to Intellectual Property Rights in the Territory: (1) other than Patents; and (2) other than Intellectual Property Rights in brand names (whether registered or unregistered), in respect of the use, manufacture, marketing, distribution and sale in the Territory of the Released Party’s:
(ii)Replacement Parts for any of the foregoing in Section (i) and their Components; or
(i)Existing Products, Existing Accessories, Existing Upgrade Parts and their respective Components [***] as sold on or before [***], insofar as such Intellectual Property Rights are used in or cover such Existing Products, Existing Accessories, Existing Upgrade Parts or their respective Components as sold on or before [***]; or
(ii)Replacement Parts for any of the foregoing in Section (i) and their Components; or
(e)in relation to Intellectual Property Rights in the Territory (1) other than Patents and (2) other than Intellectual Property Rights in brand names (whether registered or unregistered), in respect of the use, manufacture, marketing, distribution and sale in the Territory of [***], any of the Released Party’s Changed Products and their respective ARUs or Components of such Products or ARUs, where such [***] were used in respect of such Product’s or such ARU’s related Existing Products, Existing Accessories, Existing Replacement Parts, Existing Upgrade Parts and their respective Components and/or Packaging [***]; for the purpose of this Section the related Existing Product of [***] shall be deemed to be [***] of Schedule 1.1.94, for the avoidance of doubt, a Non-Notifiable [***] may, or may not, constitute [***] for the purposes of this subsection (e); or
(f)in relation to Intellectual Property Rights of the Releasing Party in the Released Territorial Brands of the Released Party insofar as they are used by the Released Party in respect of the use, manufacture, marketing, distribution, or sale of the Released Party’s Existing Products, Existing Accessories, Existing Replacement Parts, Existing Upgrade Parts and/or their respective Components and/or Packaging thereof in a particular jurisdiction within the Territory, provided that such use:
(i)[***];
(ii)[***]; and
(iii)involves the same corresponding act(s) (i.e., use, manufacture, marketing, distribution, or sale, as applicable).
as the use of such Released Territorial Brands undertaken by the Released Party [***] in respect of the applicable use, manufacture, marketing, distribution, or sale of the Released Party’s Existing Products, Existing Accessories, Existing Replacement Parts, Existing Upgrade Parts, and/or their respective Components and/or Packaging thereof (the “Released Territorial Brands Claims”); “Released Territorial Brands” means any brand names used in a jurisdiction within the Territory, including without limitation as a word or logo, in each case whether registered or unregistered, other than the Released Principal Brands. “Released Principal Brands” means the brand names of the Released Party listed in Schedule 1.1.222. For the avoidance of doubt, the release under this subsection (f) shall not extend to any brand name or other sign used with a Released Territorial Brand that is itself not a Released Territorial Brand of the Released Party; or
(g)in relation to use by the Released Party of any of the Released Party’s Released Principal Brands, including without limitation as a word or logo (or part thereof) or on its own or with another brand name or other sign, in respect of the Released Party’s Part 1 Products in the Territory. For the avoidance of doubt, the release under this subsection (g) shall not extend to any brand name or other sign used with a Released Principal Brand that is itself not a Released Principal Brand of the Released Party.
((a) to (g) collectively the “Released Claims”).
4.2Section 4.1:
(a)does not, save for in relation to Section 4.1(a), release or discharge any claim in respect of: (1) misappropriated trade secrets, use or disclosure of misappropriated information or technology or information or technology obtained in any other unauthorised manner or in breach of a confidentiality obligation; (2) violation of Competition Law ((1) to (2) collectively the “Excepted Claims”). For the avoidance of doubt, Excepted Claims do not include unfair competition law claims (which for the avoidance of doubt does not include Competition Law claims);
(b)is subject to Section 31; and
(c)does not affect the obligation to pay royalties pursuant to this Agreement.
4.3The release in Section 4.1 shall not limit, prevent or otherwise restrict any Party, [***] Company or their Representatives from asserting, continuing or defending against any proceedings with Third Parties, including in relation to any Patent Family Member of the Released Patent Families, save to the extent such release applies to acts of a Connected Person of a Party with respect to such Products as are released as provided by Sections 4 and 5.
5.RELEASED CLAIMS COVENANT NOT TO SUE
5.1Subject to Section 25.6, each Party, on a perpetual, basis, Covenants not to Sue the other Party under any of their Released Claims including all such claims arising out of or relating to activities or conduct occurring before, during or after the Term:
(a)in the Territory, for all Released Claims other than [***]; and
(b)in the specific jurisdictions of the Territory to which the claims under the [***] relate, for all [***],
((a) and (b) collectively, the “Released Claims Covenant not to Sue”). The Released Claims Covenant not to Sue is [***].
6.EXISTING PRODUCTS’ PATENT FAMILIES COVENANTS NOT TO SUE
6.1PMP, on a perpetual, [***] basis and in the Territory, Covenants not to Sue BAT for Patent infringement by:
(a)[***] or any Changed Product of BAT which is a HNB Product (including [***]), or any of their Components, in relation to Section 6 PMP Patent Families – HNB; “Section 6 PMP Patent Families – HNB” means each of those PMP Patent Families having, at the Effective Date, [***]; and
(b)any Changed Product of BAT which is a Vapour Product, or any of its Components, in relation to Section 6 PMP Patent Families – Vapour; “Section 6 PMP Patent Families - Vapour” means each of those PMP Patent Families having, at the Effective Date, [***].
6.2BAT, on a perpetual, [***] basis and in the Territory, Covenants not to Sue PMP for Patent infringement by any:
(a)Other HNB Changed Product of PMP, or any of its Components, in relation to Section 6 BAT Patent Families – Other HNB; “Section 6 BAT Patent Families – Other HNB” means each of those BAT Patent Families having, at the Effective Date, [***]; and
(b)[***] Changed Product or any of its Components, in relation to Section 6 BAT Patent Families – Other HNB and Section 6 BAT Patent Families - [***]; “Section 6 BAT Patent Families – [***]” means each of those BAT Patent Families having, at the Effective Date, [***]; and
(c)Vapour Changed Product of PMP, or any of their Components, in relation to Section 6 BAT Patent Family – Vapour; “Section 6 BAT Patent Families – Vapour” means each of those BAT Patent Families having, at the Effective Date, [***].
6.3If there is a dispute, in the context of determining the application of Section 12.2(c), in which the Patent Owning Party disputes the application of Section 6, then any such dispute will be resolved under the ADR Exhibit as an Expedited Dispute.
7.[***] COVENANTS NOT TO SUE
7.1PMP, on a perpetual, [***] basis and in the Territory, Covenants not to Sue BAT for Patent infringement by:
(a)[***] Changed Products, or any of their Components, in relation to [***] in such Products and Components [***] in Existing Products of BAT [***] (as determined pursuant to Section 15.13); and/or
(b)any Changed Products of BAT (other than [***] Changed Products), or any of their Components in relation to [***] in such Products and Components [***] in Existing Products of BAT [***] (as determined pursuant to Section 15.13).
7.2BAT, on a perpetual, [***] basis and in the Territory, Covenants not to Sue PMP for Patent infringement by any:
(a)[***] Changed Products, or any of their Components, in relation to [***] in such Products and Components [***] in Existing Products of PMP [***] (as determined pursuant to Section 15.13); and/or
(b)Changed Products of PMP (other than [***] Changed Products), or any of their Components, in relation to [***] in such Products and Components [***] in Existing Products of PMP [***] (as determined pursuant to Section 15.13).
7.3PMP, on a perpetual, [***] basis and in the Territory, Covenants not to Sue BAT for Patent infringement by [***] any Changed Products of BAT, or any of their Components, in relation to [***] such Changed Products and Components [***] (as determined pursuant to Section 15.13) in Part 1 Products of BAT where [***].
7.4BAT, on a perpetual, [***] basis and in the Territory, Covenants not to Sue PMP for Patent infringement by any Changed Products, of PMP, or any of their Components, in relation to [***] such Changed Products and Components [***] (as determined pursuant to Section 15.13) in Part 1 Products of PMP where [***].
7.5If there is a dispute, in the context of determining the application of Section 12.2(c), in which the Patent Owning Party disputes the application of Section 7, then any such dispute will be resolved under the ADR Exhibit as an Expedited Dispute.
8.SCHEDULE OF EXISTING PRODUCTS
8.1If a Party does not Identify a HNB Device or a Vapour Device, in each case: (1) that was [***] Launched; and (2) that is Compatible with a Consumable in the HNB Category or the Vapour Category respectively that is Identified (an “Omitted Device”):
(a)then the Party shall have for that Omitted Device the benefit of the release in Section 4, the Covenants not to Sue in Sections 5, 23 and 24 and the licences granted pursuant to Sections 3, 20 and 41.7, subject to the royalty obligations in Section 18, provided that:
(i)the Omitted Device differs from a Device that is Identified and that is in the [***]; or
(ii)the Omitted Device would have been [***] Product that is Identified and that is in the [***] if the Omitted Device was Launched after [***].
(b)The Party demonstrates the foregoing by following the protocol for the assessment of Compliance in Section 16 by providing the email notification in accordance with and containing the information set out in Section 16.3 and enclosing with the samples of the Omitted Device contemporaneous evidence that the Omitted Device was [***] Launched.
8.2If a Party does not Identify (1) a HNB Consumable; or (2) or a Vapour Consumable, in each case that was [***] Launched (an “Omitted Consumable”):
(a)if the Omitted Consumable is notified by the omitting Party to the other Party before the [***] anniversary of the Effective Date, that Omitted Consumable shall be deemed to be an Existing Product, unless such Omitted Consumable contains [***] in one or more Existing Products [***] as the Omitted Consumable;
(b)if the Omitted Consumable is notified by the omitting Party to the other Party on or after the [***] anniversary of the Effective Date, that Omitted Consumable shall be deemed to be an Existing Product, unless such Omitted Consumable contains [***] that: (1) are [***] Existing Products [***] as the Omitted Consumable; or (2) for HNB [***] Products are [***] Existing Products;
(c)(in addition to the requirements of Sections 8.2(a) and 8.2(b) for HNB Consumables only, save for HNB [***] Products, if an HNB Consumable is not Compatible with [***] then it is not deemed to be an Existing Product;
(d)if the Omitted Consumable is not deemed an Existing Product pursuant to Section 8.2(a), 8.2(b) or 8.2(c), then the Party shall have for that Omitted Consumable the benefit of:
(i)the release in Section 4, the Covenants not to Sue in Sections 5, 23 and 24 and the licences granted pursuant to Sections 3, 20 and 41.7, subject to the royalty obligations in Section 18; and
(ii) Sections 6 and 7, but only in relation to [***] that are also present in one or more Existing Products and, save for HNB [***] Products, that are in the [***] where such Existing Products are Identified, but only if:
(iii)the Omitted Consumable differs from a Consumable that is Identified and, save for HNB [***] Products, that is in the [***] or the Omitted Consumable would have [***] Product that is Identified and, save for HNB [***] Products, [***].
(iv)The Party demonstrates the foregoing by following the protocol for the assessment of Compliance in Section 16 by providing the email notification in accordance with, and containing the information set out in, Section 16.3 and enclosing with the samples of the Omitted Consumable contemporaneous evidence that the Omitted Consumable was [***] Launched.
9.[***] COVENANT NOT TO SUE
9.1It is a condition precedent of the [***] Covenant not to Sue (defined below) that [***] Launch occurs on or before [***]. If [***] Launch does not occur on or before [***], then the Parties agree that there is no [***] under this Agreement and provisions of this Agreement relating thereto shall be of no effect; any [***] shall not be a Part 1 Product.
9.2Subject to fulfilling the condition precedent in Section 9.1 and subject to Section 9.4 below, PMP on a perpetual (subject to Section 9.3) basis and in the Territory, Covenants not to Sue BAT for Patent infringement by [***] or any of its Components (“[***] Covenant not to Sue”). The [***] Covenant not to Sue may be royalty-bearing or royalty-free, with this being determined in accordance with Section 10.
9.3PMP grants the [***] Covenant not to Sue set forth in Section 9.2 above only in respect of: (1) an [***] Device that is Usable only with Consumables that each result in a [***]; and (2) [***] Device or [***] Consumable that is Usable only with any other BAT product(s) that each result in a [***] (the “[***] Field of Use”). If BAT sells product(s) (whether directly or through its Supply Chain Entities) that when Used with [***] Device or [***] Consumable [***] that is outside the [***] Field of Use, then the [***] Covenant not to Sue shall terminate with respect to any [***] Device or [***] Consumable with which the [***]. The determination of whether a Product or product is Usable with [***] that is within the [***] Field of Use shall be based upon the Product or product, in each case as [***]. For the avoidance of doubt, nothing in this Agreement is intended to restrict or limit the ability of BAT to launch a product that is outside the scope of [***] Covenant not to Sue, [***] Field of Use, or any other characteristic set forth in this Agreement for a Device or Consumable to be within the ambit of the [***] Covenant not to Sue, but such product shall not have the benefit of the [***] Covenant not to Sue.
9.4BAT shall within [***] Business Days of the date that the first [***] is sold by BAT [***] (the “[***] Sale”) notify PMP of such sale including the date of such sale and dispatch a sample thereof.
9.5In order for a Device or Consumable to be within the [***] Covenant not to Sue, that Device or Consumable must be Compliant as assessed using the protocol in Section 16, where “Compliant” (for [***] means that the Device or Consumable must fulfil all the following criteria:
(a)the Devices and Consumables must be contained in the [***] Launch Notice, in relation to which BAT shall comply with the requirements of Section 16.3 and which [***] Launch Notice shall not include any items Launched [***] after the date of the [***] Sale; (b)the Devices shall be HNB Devices and Consumables shall be HNB Consumables that are Usable [***];
(c)each [***] Consumable shall not, when tested using the [***] Test Method, and Used with any Devices within [***] (in normal operation) in the form such [***] Devices existed on [***], exhibit [***] of such [***] Devices (in normal operation) in the form such [***] Devices existed on [***]. Such testing shall be carried out as follows, provided that PMP may, in its sole discretion, waive the requirement for such test to be carried out. BAT shall select a [***] Consumable from each [***] Consumable Set. If the [***] Consumable fails the test, then the [***] Consumable Set to which it belongs will not be a Part 1 Product. If such [***] Consumable satisfies the test, then the [***] Consumable Set from which it is selected is deemed to satisfy the test. However, PMP may within [***] Business Days of receipt of the test results, and at its sole discretion and cost (subject to Section 16.9(a)), elect to test further [***] Consumables from within such [***] Consumable Set. If such further [***] Consumables fail such test, then those further[***] Consumables are excluded from the applicable [***] Consumable Set and will be deemed never to have been Part 1 Products, and PMP may within [***] Business Days of receipt of the test results, and at its sole discretion and cost (subject to Section 16.9(a)), elect to test further [***] Consumables from within such [***] Consumable Set. PMP may invoke the provisions of the previous sentence on two or more occasions in relation to an [***] Consumable Set that fails such test on two or more occasions. Either Party may elect, at its cost, (subject to Section 16.9(a)), to have any such tests repeated [***] and such repeat results shall supersede the initial results. Further disputes will be resolved under the ADR Exhibit as an Expedited Dispute;
(d)each [***] Consumable will be optimised for the [***] Devices. When determining whether an [***] Consumable has been optimised for the [***] Devices, a comparison is made, using the [***] Test Method, between: (1) the [***] Consumable Used with the [***] Devices and; (2) the same [***] Consumable when Used with the [***] Devices (in normal operation) in the form that existed on [***]. Such testing shall be carried out as follows. BAT shall select a [***] Consumable from each [***] Consumable Set. An [***] Consumable Set will be deemed optimised [***] Devices if the performance of the [***] Consumable in each [***] Device [***] delivery result of that [***] Consumable in all [***] Devices. If such [***] Consumable fails the test, then the [***] Consumable Set from which it is selected will not be a Part 1 Product. If such [***] Consumable satisfies the test, then the [***] Consumable Set from which it is selected is deemed to satisfy the test. However, PMP may within [***] Business Days of receipt of the test results, and at its sole discretion and cost (subject to Section 16.9(a)), elect to test [***] Consumables from within such [***] Consumable Set. If such further [***] Consumables fail such test, then those further [***] Consumables are excluded from the applicable [***]
Consumable Set and will be deemed never to have been Part 1 Products, and PMP may within [***] Business Days of receipt of the test results, and at its sole discretion and cost (subject to Section 16.9(a)), elect to test [***] Consumables from within such [***] Consumable Set. PMP may invoke the provisions of the previous sentence on two or more occasions in relation to an [***] Consumable Set that fails such test on two or more occasions. Either Party may elect, at its cost, to have any such tests repeated [***] and such repeat results shall supersede the initial results. Further disputes will be resolved under the ADR Exhibit as an Expedited Dispute. The provisions of this sub-Section relating to costs of [***] Tests are subject to the provisions of Section 16.9(a);
(e)the Devices and Consumables shall not form [***]:
(i)[***]; or
(ii)[***];
(f)each [***] Consumable shall be Compatible with each [***] Device;
(g)[***] shall consist of [***] Devices which shall all have [***]:
(i)[***];
(ii)[***]; and
(iii)[***];
[***] (“[***] Devices”);
(h)[***] shall consist of [***] Consumable Sets. “[***] Consumable Set” means a set of Consumables which all have:
(i)[***];
(ii)[***]; and
(iii)[***];
“[***] Consumable” means any member of any [***] Consumable Set;
(i)all of the [***] Consumable Sets and all members of the [***] Consumable Sets shall:
(i)[***];
(ii)either all consist of [***] Consumables or all consist of [***] Consumables [***] with all [***] Devices; and
(iii)in combination with [***] Devices, [***] which either all have a Device [***], or all have a Consumable [***]; and
(j)[***].
10.[***] ROYALTY
10.1[***] will be assessed for royalties on Relevant Patent Families in accordance with Section 15. Excluding the Excluded Patent Families, [***] can use (as determined in Section 10.5), [***] basis: [***]. For the avoidance of doubt, references in this Agreement to [***] are to all [***].
10.2When calculating the number of such Additional Patent Families [***]. Additional Patent Families that are so deemed to be PMP [***] Patent Family or PMP [***] Patent Family will thereafter be treated as such for all purposes under this Agreement [***].
10.3An “Additional Patent Family” is a Patent Family (or part of a Patent Family) that (subject to the deeming provisions of Section 10.2) is owned by PMP (for clarity excluding Patent Families included in [***]) and is neither a PMP [***] Patent Family nor a PMP [***] Patent Family, but that is a Patent Family comprising a [***] that had a member of that Patent Family [***] and where such member could be a [***] Patent or a [***] in accordance with Sections 15.3 and 15.4.
10.4If [***] is within the claims of any Patent of PMP that is not within a PMP [***] Patent Family, a PMP [***] Patent Family or an Additional Patent Family, the [***].
10.5The royalty applicable to [***] (if any) shall be calculated in accordance with the protocol set out in Section 17 determined in accordance with Section 15 by:
(a)determining the total number of [***] Patent Families identified in the Final Patent Report for [***] then identifying: (1) the number of such Patent Families that are classified as [***]; (2) the number of such Patent Families that are classified as [***]; and (3) the number of such Patent Families that are classified as [***];
(b)if in the Final Patent Report there is [***], BAT shall select and identify to PMP a number of such [***] Patent Families up to [***]; and
(c)BAT will pay to PMP [***] a [***] royalty multiplied by [***] (“[***] Royalty”) [***] for HNB Products [***].
11.CHANGED PRODUCTS COVENANT NOT TO SUE – [***] CHANGED PRODUCTS
11.1Each Party, on a perpetual (subject to Section 11.2) basis and in the Territory, Covenants not to Sue the other Party for Patent infringement by any [***] Changed Products or any of its Components Launched by the other Party, after [***], provided that the [***] Changed Products are Compliant by satisfying the terms in this Section. The Covenant not to Sue in this Section 11.1 may be royalty-bearing or royalty-free, with this being determined in accordance with Sections 14, 15, 17 and 18.
11.2PMP grants the Covenant not to Sue set forth in Section 11.1 above only in respect of: (1) a [***] Changed Product that is a Device that is Usable only with Consumables that each result in a [***]; and (2) any [***] Changed Product that is Usable only with other BAT product(s) that each result in a [***] (the “[***] Changed Product Field of Use”). If BAT sells product(s) (whether directly or through its Supply Chain Entities) that when Used with a [***] Changed Product [***] that is outside the [***] Changed Product Field of Use, then the Covenant not to Sue set forth in Section 11.1 above shall terminate with respect to [***] Changed Product [***]. The determination of whether a Product or a product is Usable with a [***] Changed Product [***] that is within the [***] Changed Product Field of Use shall be based upon [***]. For the avoidance of doubt, nothing in this Agreement is intended to restrict or limit the ability of BAT to launch a product that is outside the scope of the Covenant not to Sue set forth in Section 11.1 above, [***] Changed Product Field of Use, or any other characteristic set forth in this Agreement for a Device or Consumable to be within the ambit of the Covenant not to Sue set forth in Section 11.1 above, but such product shall not have the benefit of the Covenant not to Sue set forth in Section 11.1.
11.3In order for a Device or a Consumable, [***], to qualify as an “[***] Changed Product”, it must be Compliant as assessed using the protocol in Section 16, where “Compliant” (for [***] Changed Product) means that the Device or Consumable must fulfil all the following criteria:
(a)in the case of all Devices and Consumables:
(i)its [***] Product is: (1) in the case of Devices and Consumables of PMP, [***]; or (2) in the case of Devices and Consumables of BAT,[***]; (iii)it is an HNB Product changed such that [***]; and
(ii)the Device or Consumable must be [***];
(iv) the Device and Consumable must be contained in the Launch Notice in relation to which Launching Party shall comply with the requirements of Section 16.3.
(b)and in addition, if it is a Device or Consumable of BAT, then it shall not:
(i)[***]; or
(ii)be Usable with [***];
(c)and in addition, in the case of a Consumable, such Consumable is not Usable [***].
12.CHANGED PRODUCTS COVENANT NOT TO SUE – OTHER HNB CHANGED PRODUCTS
12.1Each Party, on a perpetual basis and in the Territory, Covenants not to Sue the other Party for Patent infringement by any Other HNB Changed Products Launched by the other Party after [***] or any of their Components, provided that those Changed Products are Compliant by satisfying the terms in this Section. The Covenant not to Sue in this Section 12.1 may be royalty-bearing or royalty-free, with this being determined in accordance with Sections 14, 15, 17 and 18.
12.2In order for a Device or a Consumable, [***], to qualify as an “Other HNB Changed Product” it must be Compliant as assessed using the protocol in Section 16, where “Compliant” (for Other HNB Changed Product) means that the Device or Consumable must fulfil all the following criteria, subject to Section 12.3:
(a)in the case of all Devices and Consumables:
(i)its [***] Product is a HNB Product [***] (in the case of Devices or Consumables of PMP) or [***] (in the case of Devices or Consumables of BAT); (iii)the Device or Consumable is changed such that [***]; and
(ii)the Device or Consumable must be [***];
(iv)the Device and Consumable must be contained in the Launch Notice in relation to which Launching Party shall comply with the requirements of Section 16.3.
(b)for a Device or Consumable of BAT, then it shall not:
(i)[***]; or
(ii)be Usable with [***];
(c)for Devices, each Device cannot [***] (as determined by Section 15) [***] one or more of the following: [***];
(d)and in addition, in the case of a Consumable, such Consumable is not Usable [***].
12.3 Only in the case of HNB [***] Products:
(a)the criteria in Section 12.2(a)(ii) shall not apply; and
(b)in applying the criteria in Section 12.2(a)(iii), limb (2) of the definition of Excluded Changes shall be disregarded.
13.CHANGED PRODUCTS COVENANT NOT TO SUE – VAPOUR PRODUCTS
13.1Each Party, on a perpetual basis and in the Territory, Covenants not to Sue the other Party for Patent infringement by any Vapour Changed Products Launched by the other Party after [***] or any of their Components, provided that those Changed Products are Compliant by satisfying the terms in this Section. The Covenant not to Sue in this Section 13.1 may be royalty-bearing or royalty-free, with this being determined in accordance with Sections 14, 15, 17 and 18.
13.2In order for a Device or a Consumable [***] to qualify as a “Vapour Changed Product” it must be Compliant as assessed using the protocol in Section 16, where “Compliant” (for a Vapour Changed Product) means that the Device or Consumable must fulfil all the following criteria:
(a)the Device or Consumable is a Product that [***];
(b)the Device or Consumable is not Usable[***];
(c)the Device or Consumable is not Usable [***]; and
(d)the Device and Consumable must be contained in the Launch Notice in relation to which the Launching Party shall comply with the requirements of Section 16.3.
14.ROYALTY
14.1Royalties in respect of a Changed Product, and royalties in respect of Existing Products if they become royalty-bearing pursuant to Section 18, shall be calculated as set out in this Section and Sections 14, 15 and 18.
14.2The Launching Party shall pay to the Patent Owning Party a royalty, calculated at a rate established in accordance with Section 18 using [***].
14.3Save as indicated in Section 14.4 below, the royalty rates for each Existing Product that is a HNB Product, [***] Changed Product and Other HNB Changed Product, [***], are calculated [***] in accordance with Section 18 per such Product [***] being:
(a)[***]; and
(b)[***];
subject to a [***] which is an HNB Product. For the avoidance of doubt, the royalty rates in this Section 14.3 apply to Hybrid Products save as set out in Section 14.4.
14.4Where the Existing Product that is a HNB Product or the Other HNB Changed Product is a Consumable of a [***] Device wherein the Vapour Consumable is Usable [***] then the royalty rates applied to that Consumable are those applicable to a Vapour Product as set out in Section 14.5 below.
14.5The royalty rates for each Existing Product that is a Vapour Product and each Vapour Changed Product, [***] are calculated [***] in accordance with Section 18 per such Product [***] being:
(a)[***]; and
(b)[***];
subject to a [***].
14.6In addition and subject to Sections 15.25 and 15.27, BAT will pay to PMP the [***] Royalty [***] for HNB Products [***] for all [***] Changed Products (as if, in addition to being a [***] Changed Product [***]), as set out in Section 10 above. For the avoidance of doubt, to the extent a Device or Consumable that is a [***] Changed Product requires [***] (as determined under Section 15), then the applicable royalty rate is calculated using [***] set out in Section 14 and applying the Royalty Mechanism of Section 18 [***] in accordance with Section 18. This royalty rate is then applied, [***].
15.ASSESSMENT OF A PRODUCT AGAINST A PATENT FAMILY
15.1The assessment of: (1) the relevance of a Patent Family of the Patent Owning Party to a Device or Consumable of the Launching Party; and (2) whether that Patent is a [***] Patent or a [***] Patent, is conducted in accordance with this Section. If the assessment relates to Compliance, it is conducted in accordance with the protocol set out in Section 16. If the assessment relates to the royalty obligations arising in relation to a Device or Consumable, the assessment is conducted in accordance with the protocol set out in Section 17.
15.2A Patent or Patent Family of the Patent Owning Party is “Relevant” to:
(a)[***] if:
(i)in relation to a PMP [***] Patent Family or a PMP [***] Patent Family, [***] is Within the Scope of any claims of a [***] Patent or [***] of the PMP [***] Patent Family or the PMP [***] Patent Family as established under this Section 15 in each case, for the avoidance of doubt, where such [***] Patent or [***]; or
(ii)in relation to an Additional Patent Family, a [***] Patent or [***] (as determined in accordance with Section 15.4) is Enforceable [***] pursuant to the ADR Procedure, as established under this Section 15; the ADR Procedure shall be final and binding as to Enforceability for the purpose of this Section 15.2(a)(ii) and any decisions relating to such [***] Patent or [***] in other fora including patent offices and courts, including in relation to amendments, grant and validity, shall be disregarded for the purposes of determining such Enforceability with respect to [***];
(b)a Device or Consumable of any Changed Product of the Launching Party, if a [***] Patent [***] (as determined in accordance with Section 15.4) is Enforceable [***].
15.3The Parties shall apply [***] for HNB Products and [***] for Vapour Products (each a “[***]”) and the Patent Owning Party shall specify, in the applicable Draft Patent Report, a single “[***] Patent” or a single “[***]” that it owns [***] for each Patent Family it asserts to be Relevant for the Device and/or Consumable [***]. If a Device or Consumable has not been Launched [***] for the assessment in relation to such Device or Consumable. Once a Patent has been specified in the Draft Patent Report as a [***] Patent or [***] for a particular Product under assessment, then the Patent Owning Party [***].
15.4The [***] Patents and [***] are as follows:
(a)for Vapour Products:
(i)[***]; and
(ii)[***];
(b)for HNB Products other than [***]:
(i)[***];
(ii)[***]; and
(iii)[***];
(c)for [***]:
(i)[***];
(ii)[***]; and
(iii)[***];
Notwithstanding the foregoing provisions of this Section 15.4(c), a [***] the applicable Patent Family is in the International Phase [***]. For the avoidance of doubt, if for a particular Patent Family in the International Phase, [***] “International Phase” means, with respect to a Patent Family and a country, the period beginning on the earliest priority date for such Patent Family and ending upon the earlier of: (1) [***] months thereafter; or (2) the date on which early national processing is requested under [***]. For any Patent Family that [***] is no longer in the International Phase [***]. For the avoidance of doubt, such [***] are considered to be the [***] referred to in Sections 15.4(c)(i), 15.4(c)(ii) and 15.4(c)(iii).
15.5In relation to Section 15.4(b)(ii) and Section 15.4(c)(ii), [***] was not at the Effective Date in force but a [***] was in force and is in force at the time of assessment under this Section:
(a)save for the purposes of Validity Challenges referenced in Section 15.5(b), the assessment is carried out under the law of [***], or if none, a certified translation thereof;
(b)for the purposes of Validity Challenges (as permitted under Section 31), [***] is the [***] Patent [***]. For the avoidance of doubt if [***], Section 15.6 will apply; and for the avoidance of doubt, if there was [***] in force at the Effective Date [***] is not, and does not become, the [***] Patent.
15.6If a patent that has been specified as a [***] Patent for a particular Product under assessment is amended or is found to be invalid or the Patent Owning Party is found to lack Entitlement to such Patent by a patent office or other Governmental Authorities but such amendment or finding is not final or unappealable at the date of that assessment, then [***] including the generation of the relevant Draft Patent Report unless the Parties agree otherwise. [***] in relation to such [***] Patent will be determined under Section 15.26.
15.7The governing patent laws applied in the assessment of the Relevance of [***] Patents and [***] shall be: [***]. The Parties may also agree in any assessment that [***] governing laws shall be applied.
15.8When considering whether: (1) a [***] Patent is Relevant to a Part 1 Product; and/or (2) a [***] of a PMP [***] Patent Family, a PMP [***] Patent Family or an Additional Patent Family is [***]. The determination of the Relevance of such [***] Patent or [***] is made in the following way:
(a)for [***]:
(i)[***] is not assessed against [***] when determining Compliance; and
(ii)when considering the royalty rates for [***] PMP can only include patents [***] that are [***] (as determined in accordance with Sections 15.3 and 15.4); provided [***]: (1) such patent or [***]; (2) any [***] of such patent or [***] in any of the [***] Patent Offices [***]; or (3) any Patent Family Member that is [***]; and;
(A)in the case of PMP [***] Patent Families and PMP [***] Patent Families, the assessment is made against the [***]
publicly available on [***];
(B)in the case of Additional Patent Families, the assessment is made against the [***]:
(a)[***] in the [***] publicly available on [***] in the respective [***] Patent Offices for HNB Products, [***], (“Category 1 Representatives”); however
(b)[***] in the form [***] on [***] (“Category 2 Representatives”);
[***] for a Patent [***] to be a [***], in each case on or before [***].
(b)for Changed Products:
(i)the only Changed Products assessed against the [***]. When considering whether a Device that is an Other HNB Changed Product is Compliant, then the assessment is made against a [***]. In order to be Compliant, a Device that is an Other HNB Changed Product must be Compliant [***]. For the avoidance of doubt, if no [***] Patents in a Patent Family [***] for such Device that is an Other HNB Changed Product;
(ii)when considering the royalty rates for Changed Products, the assessment of the Changed Product is made against the [***] and subject to the requirements of Section 15.8(b)(iii) to (v);
(iii)when considering a Device of an Other HNB Changed Product, a Relevant Patent Family is available on a potentially royalty-bearing basis even if [***]; (iv)when considering the royalty rates for Changed Products other than [***] Changed Products, for each Changed Product the Patent Owning Party can only include patents that are [***] (as determined in accordance with Section 15.3 and 15.4) [***]:
(A)such [***] Patent [***] of such Changed Products;
(B)at least one of the following is [***] of such Changed Product: [***]; and
for a Patent [***] to be a [***], in each case on or before [***];
(v)when considering the royalty rates for [***] Changed Products, for each [***] Changed Product, BAT can only include patents that are [***] Patents (as determined in accordance with Section 15.3 and 15.4) [***] where:
(A)such [***] Patent has [***] of such [***] Changed Products;
(B)at least one of the following is [***]; and
for a Patent [***] to be a [***], in each case on or before [***].
15.9In all cases, [***] all Patent Family Members of “Excluded Patent Families” may not be [***] Patents or [***], such that they cannot be Relevant Patents or Relevant Patent Families as determined under Section 15.2. The Excluded Patent Families are the Patent Families which are or were:
(a)the Released Patent Families, [***] released; (b)within the Covenants not to Sue in Section 6, in relation to the Device and/or Consumable under assessment;
(c)the subject of the [***] Licence where the Device and/or Consumable under assessment is a [***];
(d)only in the case of the assessment of [***], any Patent Family which is [***] in accordance with Section 10.4; or
(e)any Patent Family made available [***] to a Party pursuant to Section 5.12 of the ADR Exhibit. Such Patent Family shall be [***] with effect from the start of the first full [***] after the Party benefiting from the [***] Patent Family has nominated that Patent Family in accordance with Section 5.12 of the ADR Exhibit.
15.10In all cases, Excluded Patent Family Members may not be [***] Patents or [***] in respect of a Product under assessment. “Excluded Patent Family Members” means any Patent that:
(a)in relation to any Product other than [***], has been [***] of the relevant Device and/or Consumable;
(b)[***] for the same Product or which has the benefit under a Covenant not to Sue for the same Product. Examples of such Patents include [***]. In such case, and for the purpose of this Section 15.10(b), where such [***] Patents or [***] do not form part of the same Patent Family, then the invention(s) of such [***] Patents and [***] are also assessed in the following manner. [***] For the avoidance of doubt: (1) where the [***] Patents or [***] share the same priority date, then the Launching Party can elect which is the earlier [***] Patent or [***]; and (2) [***] shall apply (and shall not be disregarded) for the purposes of this Section 15.10(b); (c)at the date of Launch of the Product was [***], provided that such earlier Launched Changed Product [***] is Within the Scope of that [***] Patent [***] Launch of the later Changed Product under assessment.
For the avoidance of doubt, no Excluded Patent Family Member can be a Relevant Patent as determined under Section 15.2 for the Product under assessment.
15.11In all cases, Excluded Claims in [***] Patents or [***] are excluded from consideration in Product assessment. “Excluded Claims” mean the claims of a Patent:
(a)that claim an invention that is a [***];
(b)that claim an invention that is a [***];
(c)that claim an invention that is a Product in respect of which an arbitrator or judge, with the advice of a person of skill in the art, would not be able to determine whether the Product was Within the Scope of the relevant claims of the Patent [***];
(d)that claim an invention that is alleged in the Draft Patent Report for such Product [***];
(e)that claim an invention that is alleged in the Draft Patent Report for such Product [***];
(f)that claim an invention that is alleged in the Draft Patent Report for such Product [***];
(g)in relation to any Product other than [***], that is subject to: (1) a [***]; or (2) a [***] that, in each case (1) or (2), [***], excluding obvious corrections of any error of translation or transcription, clerical error or mistake in the specification (meaning that it is immediately evident that nothing else could have been intended in the uncorrected specification in accordance with the requirements of [***], irrespective of the governing law of the [***] Patent) provided that [***];
for the avoidance of doubt, no Excluded Claim can be considered when determining whether a [***] Patent or [***] is Relevant under Section 15.2 for the Product under assessment.
15.12A [***] Patent that is Relevant to a Changed Product is determined to be either a [***] Patent or a [***] Patent by determining [***].
15.13A [***] is assessed [***] in: (1) a Changed Product of the Launching Party or [***] in the case of BAT (the “Later Product”); and (2) a Product of the Launching Party that is either (A) subject to a Launch Notice and Launched by the Launching Party before the Launch of the Later Changed Product or (B) an Existing Product of the Launching Party (the “Earlier Product”) if:
(a)[***] Patent or [***] (in the case of [***] for PMP [***] Patent Families or a PMP [***] Patent Families) asserted by the Patent Owning Party to be Relevant [***] to the Later Product is identified by [***]. For the avoidance of any doubt: (1) this means that [***] and (2) the validity (or otherwise) of the independent claim(s) is not to be taken into account in the [***];
(b)the Launching Party will then provide a [***] identified in 15.13(a) above, and thereby all aspects of [***], are implemented by the Earlier Product that the Launching Party asserts implements [***] the Patent Owning Party prepared [***] in relation to such Later Product that it asserts is Relevant to that [***] Patent or [***] (as the case may be). If when comparing those [***] there are:
(i)[***]; or
(ii)[***] the changes made from such Earlier Product to such Later Product are considered collectively:
(A)if those changes, when considered collectively, [***] then such Earlier Product and such Later Product [***] in respect of the [***] Patent or [***];
(B)if those changes, when considered collectively, [***] and:
(a)if the [***] in such Later Product is not either: (1) [***]; or (2) disclosed in a manner that it is plausible that the Patent Owning Party had [***], then such Earlier and Later Products use the [***] in respect of [***] Patent or [***]; but
(b)if the [***] in such Later Product is either: (1) [***]; or (2) disclosed in a manner that it is plausible that the Patent Owning Party had [***], then such Earlier Product and Later Product do not use [***] in respect of the [***] Patent or [***].
15.14If the assessment under Sections 15.13(a) and 15.13(b) determines that such Earlier and Later Products use the [***] then:
(a)if the Later Product is [***] Changed Product, and the Earlier Product is an Existing Product of BAT which is an HNB Product, then the [***] benefits from the Covenant not to Sue in Section 7.1(a) in the Territory. Consequently: (1) the [***] Patent or [***] is not [***]; and (2) the Patent [***] is excluded from the[***]. That Patent [***] is then identified in the Final Patent Report of the Later Product as being subject to Section 7.1(a) in respect of [***]; or
(b)if the Later Product is a Changed Product of BAT (other than [***] Changed Product), and the Earlier Product is an Existing Product of BAT in the same Product [***], then the [***] benefits from the Covenant not to Sue in Section 7.1(b). Consequently: (1) that [***] Patent is not a [***] Patent [***]; and (2) the Patent [***] is excluded from the [***] of the Later Product in the Territory. That Patent [***] is then identified in the Final Patent Report of the Later Product as being subject to Section 7.1(b) in respect of [***];
(c)if the Later Product is an [***] Changed Product, and the Earlier Product is an Existing Product of PMP which is an HNB Product, then the [***] benefits from the Covenant not to Sue in Section 7.2(a). Consequently: (1) that [***] Patent is not a [***] Patent for the Later Product; and (2) the Patent [***] is excluded from the [***] of the Later Product in the Territory. That Patent [***] is then identified in the Final Patent Report of the Later Product as being subject to Section 7.2(a) in respect of [***];
(d)if the Later Product is a Changed Product of PMP (other than [***] Changed Product), and the Earlier Product is an Existing Product of PMP (excluding [***]) in the same Product [***], then the [***] benefits from the Covenant not to Sue in Section 7.2(b). Consequently: (1) the [***] Patent is not a [***] Patent for the Later Product; and (2) the Patent [***] is excluded from the [***] of the Later Product in the Territory. That Patent [***] is then identified in the Final Patent Report of the Later Product as being subject to Section 7.2(b) in respect of that [***];
(e)if the Later Product is a Changed Product of the Launching Party, and the Earlier Product is a Product of the Launching Party [***] as the Later Product (with [***] being [***]), then the [***] Patent is excluded from the [***] in the Later Product; or
(f)for the avoidance of doubt: (1) in the case of this Section 15.14, if the Earlier Product is a Changed Product and a [***] Patent in the same Patent [***] Patent of the Later Product was a [***] Patent for that Earlier Product, then the [***] Patent of the Earlier Product continues to be a [***] Patent for the Later Product unless the [***] Patent of the Earlier Product is not Enforceable against the Later Product; and (2) [***] is [***].
15.15If, for an Earlier Product (as defined in Section 15.13) that is a Changed Product that was subject to a Launch Notice, a Patent Family is not Relevant [***], for example because:
(a)no patent [***] capable of being a [***] Patent or [***] of the Patent Family [***] for that Earlier Product was [***]:
(i)[***] in the case of an [***] Changed Product, as required by Section 15.8(b)(v)(B); or
(ii)[***] in the case of [***] Changed Product, as required by Section 15.8(b)(iv)(B);
(b)no [***] Patent of the Patent Family had [***] of that Earlier Product, as required by Section 15.8(b)(iv)(A) or Section 15.8(b)(v)(A) as applicable;
(c)a [***] Patent of the Patent Family had [***] of that Earlier Product but that Earlier Product was not Within the Scope of that Patent, or the [***] Patent was not Enforceable against that Earlier Product;
(d)the Patent Family has been excluded from the [***] of the Earlier Product pursuant to Section 15.14;
(e)the Patent Owning Party issued a Draft Patent Report in accordance with Section 17.3 for that Earlier Product and the Patent Owning Party did not include a [***] Patent [***] in its Draft Patent Report even though that Earlier Product would have been Within the Scope of the [***] Patent at the relevant time; or
(f)no Patent Report was issued even though that Earlier Product would have been Within the Scope of a [***] Patent of the Patent Family at the relevant time,
then for a subsequently Launched Changed Product in the same [***] as the Earlier Product that was subject to the Launch Notice (a Later Product (as defined in Section 15.13)), that Patent Family is not Relevant [***] in such Earlier Product, and will therefore, in respect of [***] in such Later Product, be a “Changed Product [***] Family”.
15.16If, for an Earlier Product (as defined in Section 15.13) that is [***], a Patent Family [***] is not Relevant, for example because:
(a)Section 15.9(e) applies;
(b)No patent [***] capable of being a [***] Patent or [***] for [***] was [***] as required by Section 15.8(a)(ii);
(c)The Patent Family has been excluded from the [***] of [***] pursuant to Section 15.14;
(d)PMP issued a Draft Patent Report under Section 17.9 for [***] and PMP did not include the [***] Patent or [***] (which are limited to PMP[***] Patent Families, PMP [***] Patent Families or Additional Patent Families) in that Draft Patent Report even though [***] was Within the Scope of the [***] Patent or [***] as it was on [***]; or
(e)no Patent Report was issued by PMP under Section 17.9 for [***] even though [***] was Within the Scope of the [***] Patent or [***] (which are limited to PMP [***] Patent Families, PMP [***] Patent Families or Additional Patent Families) as it was on [***],
then, for a [***] Changed Product (a Later Product (as defined in Section 15.13)), that Patent Family is not Relevant [***] in relation to [***] used in such [***] Changed Product as [***], and will therefore, in respect of such [***] in such [***] Changed Product, be an “[***] Family”.
15.17A Patent Family will continue to be subject to Section 15.15 or 15.16 [***] (as determined in accordance with Section 15.13) in any Later Product in the same Changed Product [***] as an Earlier Product (with [***] being within the Changed Product [***]) where that Patent Family was an [***] Family or a Changed Product [***] Family in respect of the Earlier Product, unless, and in the case of Section 15.15 only, the [***]. For the avoidance of doubt, the requirement to Establish does not apply to Section 15.16 and to [***] and therefore the [***] Family is unaffected.
If the Launching Party fails to Establish the Earlier Product, such Patent Family ceases to be subject to Section 15.15, and [***] the Launching Party shall pay applicable royalties [***] in respect of such Patent Family on [***] all Later Product in respect of which such Patent Family was treated as subject to Section 15.15 [***] where that Patent Family is Relevant and[***] Product pursuant to Section 18.
For the avoidance of doubt, to the extent that a Patent Family is Relevant to the Earlier Product [***] and is not otherwise subject to Section 15.15 or 15.16, including as either a Changed Product [***] Family or an [***] Family, then if such Patent Family continues to be Relevant for such Later Product or any subsequent Changed Product in the same Changed Product [***], then royalties will be payable in relation to that Patent Family as determined under Section 10 if that Patent Family is the subject of the [***] Royalty or if not in accordance with Section 18.
15.18For the avoidance of doubt, Sections 15.9, 15.10 and 15.11 do not otherwise preclude the filing or assertion of Patents within Excluded Patent Families, Excluded Patent Family Members or Excluded Claims against Third Parties other than against the respective Connected Persons of Parties to the extent that such Connected Persons have the benefit of this Agreement.
15.19Under this Section 15:
(a)if [***] Patents and [***] of a Patent Family are Excluded Patent Family Members for [***] or the Changed Product under assessment, then that Patent Family is an Excluded Patent Family for [***] or the Changed Product under assessment [***];
(b)if [***] Patent or [***] consists entirely of Excluded Claims for [***] or the Changed Product under assessment, then that Patent is an Excluded Patent Family Member for [***] or the Changed Product under assessment[***]; and
(c)for the avoidance of doubt, subject to Section 15.8, in calculating royalties [***]: (1) no consideration is given to the [***]; and (2) there is no requirement for the [***]. However, the Covenants not to Sue in this Agreement shall [***] be for all the Territory.
15.20Each Party shall be entitled, on a one-time only basis, to add [***] in the “HNB Products” category, and to add [***] in the “Vapour Products” category, by giving a single written notice to the other Party of all such additions at least [***] months prior to the [***] anniversary of the Effective Date, provided that such notice may not specify the [***], the addition of which shall be subject to the separate requirements of Sections 15.21 to 15.24). Such additions shall take effect from the start of the first [***] to start on or after the [***] anniversary of the Effective Date. The Parties may by mutual agreement in accordance with Section 41.5, [***].
15.21Should at any time during the Term it become possible for both BAT and PMP to sell any and all of their respective HNB Products in the [***], and for the Parties to pay to and receive from each other related royalties, without either Party: (i) violating Sanctions; or (ii) reasonably considering (acting in good faith) that it or any of its Affiliates would otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, due in all cases to the lifting of all or a majority of the Sanctions which would realistically impact the trade of the HNB Products (including Sanctions targeting any HNB Products, Intellectual Property Rights or any third parties to which HNB Products may be sold directly or indirectly by either Party or the tobacco sector in general) (“Relevant HNB Sanctions”) that have been imposed on the [***] since [***], such that the Relevant HNB Sanctions imposed on the [***] revert to a position materially similar to, or more permissive than, the Relevant HNB Sanctions position immediately prior to [***] (for the purpose of this Section 15.21, the “Condition”), then either Party may give notice referencing this Section and asserting that the Condition has been met. The Condition shall be deemed to have been met if [***] (“[***] HNB Market Re-entry”), and notice within the meaning of this Section 15.21 shall be deemed to have been given and received as of the date upon which such [***] HNB Market Re-entry occurs. Then with effect from the start of the first [***] to start on or after the date which is [***] Business Days after the date the notice in this Section 15.21 is received or deemed to be received (the “Section 15.21 Addition Date”), the [***] will be deemed: [***] If the receiving Party of any notice, or deemed notice as a result of a [***] HNB Market Re-entry, disputes that the Condition has been fulfilled, or reasonably considers (acting in good faith) that the particular deemed amendments would cause it or any of its Affiliates to violate Sanctions or otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, it shall provide objections in writing within [***] Business Days of receipt of such notice specifying its objections, otherwise such notice shall be deemed to be valid. Any dispute in relation to this Section 15.21 shall be resolved in accordance with the ADR Exhibit as an Expedited Dispute (for the purpose of this Section 15.21, a “Section 15.21 Dispute”). However, if: (a) as of the Effective Date and prior to the Section 15.21 Addition Date, a Party, its Associated Companies or their respective Representatives has asserted against the other Party any Intellectual Property Rights in the [***] that would otherwise be within the scope of any of the licences, Covenants not to Sue or releases in respect of HNB Products provided for under the deemed amendments in this Section 15.21; (b) a Party has breached Section 29.2(j) (in the case of PMP) or Section 29.3(j) (in the case of BAT), in respect of Relevant [***] IPR relating to HNB Products, and the recipient of such Relevant [***] IPR relating to HNB Products or rights thereunder takes enforcement action against the other Party; or (c) a Party has, at the Section 15.21 Addition Date, breached Section 21.5 in respect of Relevant [***] IPR relating to HNB Products, and the recipient of such Relevant [***] IPR relating to HNB Products or rights thereunder takes enforcement action against the other Party, then that Party [***].
Following any deemed amendment under this Section 15.21, either Party may provide notice (for the purpose of this Section 15.21, a “Section 15.21 Sanctions Reimposition Notice”) to the other in such circumstances that it reasonably considers (acting in good faith) the Condition to have ceased to have been fulfilled or that it is exposed to a risk of breach, penalty or punitive consequences under Sanctions owing to the inclusion of the [***] in the Territory [***], in which case the [***] will be deemed: [***] in each case from the date upon which the Condition ceased to have been fulfilled or risk owing to the inclusion of the [***] in the Territory [***] arose. The other Party may raise a Section 15.21 Dispute within [***] Business Days of receipt of a Section 15.21 Sanctions Reimposition Notice, following which these changes may be reversed if the Party raising such Section 15.21 Dispute is successful in its challenge. For the purposes of this Section 15.21, in assessing whether the Condition has been fulfilled, no account shall be taken of any contractual restrictions between a Party and any Third Party.
15.22Should at any time during the Term it become possible for both BAT and PMP to sell any and all of their respective Vapour Products in the [***], and for the Parties to pay to and receive from each other related royalties, without either Party: (i) violating Sanctions; or (ii) reasonably considering (acting in good faith) that it or any of its Affiliates would otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, due in all cases to the lifting of all or a majority of the Sanctions which would realistically impact the trade of the Vapour Products (including Sanctions targeting any Vapour Products, Intellectual Property Rights or any third parties to which Vapour Products may be sold directly or indirectly by either Party or the tobacco sector in general) (“Relevant Vapour Sanctions”) that have been imposed on the [***] since [***], such that the Relevant Vapour Sanctions imposed on the [***] revert to a position materially similar to, or more permissive than, the Relevant Vapour Sanctions position immediately prior to [***] (for the purpose of this Section 15.22, the “Condition”), then either Party may give notice referencing this Section and asserting that the Condition has been met. The Condition shall be deemed to have been met if [***] (“[***] Vapour Market Re-entry”), and notice within the meaning of this Section 15.22 shall be deemed to have been given and received as of the date upon which such [***] Vapour Market Re-entry occurs. Then with effect from the start of the first [***] to start on or after the date which is [***] Business Days after the date the notice in this Section 15.22 is received or deemed to be received (the “Section 15.22 Addition Date”), the [***] will be deemed: [***] If the receiving Party of any notice, or deemed notice as a result of a [***] Vapour Market Re-entry, disputes that the Condition has been fulfilled, or reasonably considers (acting in good faith) that the particular deemed amendments would cause it or any of its Affiliates to violate Sanctions or otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, it shall provide objections in writing within [***] Business Days of receipt of such notice specifying its objections, otherwise such notice shall be deemed to be valid. Any dispute in relation to this Section 15.22 shall be resolved in accordance with the ADR Exhibit as an Expedited Dispute (for the purpose of this Section 15.22, a “Section 15.22 Dispute”).
However, if: (a) as of the Effective Date and prior to the Section 15.22 Addition Date, a Party, its Associated Companies or their respective Representatives has asserted against the other Party any Intellectual Property Rights in the [***] that would otherwise be within the scope of any of the licences, Covenants not to Sue or releases in respect of Vapour Products provided for under the deemed amendments in this Section 15.22; (b) a Party has breached Section 29.2(j) (in the case of PMP) or Section 29.3(j) (in the case of BAT), in respect of Relevant [***] IPR relating to Vapour Products, and the recipient of such Relevant [***] IPR relating to Vapour Products or rights thereunder takes enforcement action against the other Party; or (c) a Party has, at the Section 15.22 Addition Date, breached Section 21.5 in respect of Relevant [***] IPR relating to Vapour Products, and the recipient of such Relevant [***] IPR relating to Vapour Products or rights thereunder takes enforcement action against the other Party, [***]. Following any deemed amendment under this Section 15.22, either Party may provide notice (for the purpose of this Section 15.22, a “Section 15.22 Sanctions Reimposition Notice”) to the other in such circumstances that it reasonably considers (acting in good faith) the Condition to have ceased to have been fulfilled or that it is exposed to a risk of breach, penalty or punitive consequences under Sanctions owing to the inclusion of the [***] in the Territory [***], in which case the [***] will be deemed [***] in each case from the date upon which the Condition ceased to have been fulfilled or risk owing to the inclusion of the [***] in the Territory [***] arose. The other Party may raise a Section 15.22 Dispute within [***] Business Days of receipt of a Section 15.22 Sanctions Reimposition Notice, following which these changes may be reversed if the Party raising such Section 15.22 Dispute is successful in its challenge. For the purposes of this Section 15.22, in assessing whether the Condition has been fulfilled, no account shall be taken of any contractual restrictions between a Party and any Third Party.
15.23Should at any time during the Term it become possible for both BAT and PMP to sell any and all of their respective HNB Products in [***], and for the Parties to pay to and receive from each other related royalties, without either Party: (i) violating Sanctions; or (ii) reasonably considering (acting in good faith) that it or any of its Affiliates would otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, due in all cases to the lifting of all or a majority of the Sanctions which would realistically impact the trade of the HNB Products (including Sanctions targeting any HNB Products, Intellectual Property Rights or any third parties to which HNB Products may be sold directly or indirectly by either Party or the tobacco sector in general) (“Relevant HNB Sanctions”) that have been imposed on [***] since [***], such that the Relevant HNB Sanctions imposed on [***] revert to a position materially similar to, or more permissive than, the Relevant HNB Sanctions position immediately prior to [***] (for the purpose of this Section 15.23, the “Condition”), then either Party may give notice referencing this Section and asserting that the Condition has been met. The Condition shall be deemed to have been met if [***] (“[***] HNB Market Re-entry”), and notice within the meaning of this Section 15.23 shall be deemed to have been given and received as of the date upon which such [***] HNB Market Re-entry occurs.
Then with effect from the start of the first [***] to start on or after the date which is [***] Business Days after the date the notice in this Section 15.23 is received or deemed to be received (the “Section 15.23 Addition Date”), [***] will be deemed: [***] If the receiving Party of any notice, or deemed notice as a result of a [***] HNB Market Re-entry, disputes that the Condition has been fulfilled, or reasonably considers (acting in good faith) that the particular deemed amendments would cause it or any of its Affiliates to violate Sanctions or otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, it shall provide objections in writing within [***] Business Days of receipt of such notice specifying its objections, otherwise such notice shall be deemed to be valid. Any dispute in relation to this Section 15.23 shall be resolved in accordance with the ADR Exhibit as an Expedited Dispute (for the purpose of this Section 15.23, a “Section 15.23 Dispute”). However, if: (a) as of the Effective Date and prior to the Section 15.23 Addition Date, a Party, its Associated Companies or their respective Representatives has asserted against the other Party any Intellectual Property Rights in [***] that would otherwise be within the scope of any of the licences, Covenants not to Sue or releases in respect of HNB Products provided for under the deemed amendments in this Section 15.23; (b) a Party has breached Section 29.2(j) (in the case of PMP) or Section 29.3(j) (in the case of BAT), in respect of Relevant [***] IPR relating to HNB Products, and the recipient of such Relevant [***] IPR relating to HNB Products or rights thereunder takes enforcement action against the other Party; or (c) a Party has, at the Section 15.23 Addition Date, breached Section 21.5 in respect of Relevant [***] IPR relating to HNB Products, and the recipient of such Relevant [***] IPR relating to HNB Products or rights thereunder takes enforcement action against the other Party, [***] Following any deemed amendment under this Section 15.23, either Party may provide notice (for the purpose of this Section 15.23, a “Section 15.23 Sanctions Reimposition Notice”) to the other in such circumstances that it reasonably considers (acting in good faith) the Condition to have ceased to have been fulfilled or that it is exposed to a risk of breach, penalty or punitive consequences under Sanctions owing to the inclusion of [***] in the Territory [***], in which case [***] will be deemed [***] in each case from the date upon which the Condition ceased to have been fulfilled or risk owing to the inclusion of [***] in the Territory [***] arose. The other Party may raise a Section 15.23 Dispute within [***] Business Days of receipt of a Section 15.23 Sanctions Reimposition Notice, following which these changes may be reversed if the Party raising such Section 15.23 Dispute is successful in its challenge. For the purposes of this Section 15.23, in assessing whether the Condition has been fulfilled, no account shall be taken of any contractual restrictions between a Party and any Third Party.
15.24Should at any time during the Term it become possible for both BAT and PMP to sell any and all of their respective Vapour Products in [***], and for the Parties to pay to and receive from each other related royalties, without either Party: (i) violating Sanctions; or (ii) reasonably considering (acting in good faith) that it or any of its Affiliates would otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, due in all cases to the lifting of all or a majority of the Sanctions which would realistically impact the trade of the Vapour Products (including Sanctions targeting any Vapour Products, Intellectual Property Rights or any third parties to which Vapour Products may be sold directly or indirectly by either Party or the tobacco sector in general) (“Relevant Vapour Sanctions”) that have been imposed on [***] since [***], such that the Relevant Vapour Sanctions imposed on [***] revert to a position materially similar to, or more permissive than, the Relevant Vapour Sanctions position immediately prior to [***] (for the purpose of this Section 15.24, the “Condition”), then either Party may give notice referencing this Section and asserting that the Condition has been met. The Condition shall be deemed to have been met [***] (“[***] Vapour Market Re-entry”), and notice within the meaning of this Section 15.24 shall be deemed to have been given and received as of the date upon which such [***] Vapour Market Re-entry occurs. Then with effect from the start of the first [***] to start on or after the date which is [***] Business Days after the date the notice in this Section 15.24 is received or deemed to be received (the “Section 15.24 Addition Date”), [***] will be deemed: [***] If the receiving Party of any notice, or deemed notice as a result of a [***] Vapour Market Re-entry, disputes that the Condition has been fulfilled, or reasonably considers (acting in good faith) that the particular deemed amendments would cause it or any of its Affiliates to violate Sanctions or otherwise be exposed to a risk of breach, penalty or punitive consequences under Sanctions, it shall provide objections in writing within [***] Business Days of receipt of such notice specifying its objections, otherwise such notice shall be deemed to be valid. Any dispute in relation to this Section 15.24 shall be resolved in accordance with the ADR Exhibit as an Expedited Dispute (for the purpose of this Section 15.24, a “Section 15.24 Dispute”). However, if: (a) as of the Effective Date and prior to the Section 15.24 Addition Date, a Party, its Associated Companies or their respective Representatives has asserted against the other Party any Intellectual Property Rights in [***] that would otherwise be within the scope of any of the licences, Covenants not to Sue or releases in respect of Vapour Products provided for under the deemed amendments in this Section 15.24; (b) a Party has breached Section 29.2(j) (in the case of PMP) or Section 29.3(j) (in the case of BAT), in respect of Relevant [***] IPR relating to Vapour Products, and the recipient of such Relevant [***] IPR relating to Vapour Products or rights thereunder takes enforcement action against the other Party; or (c) a Party has, at the Section 15.24 Addition Date, breached Section 21.5 in respect of Relevant [***] IPR relating to Vapour Products, and the recipient of such Relevant [***] IPR relating to Vapour Products or rights thereunder takes enforcement action against the other Party [***]. Following any deemed amendment under this Section 15.24, either Party may provide notice (for the purpose of this Section 15.24, a “Section 15.24 Sanctions Reimposition Notice”) to the other in such circumstances that it reasonably considers (acting in good faith) the Condition to have ceased to have been fulfilled or that it is exposed to a risk of breach, penalty or punitive consequences under Sanctions owing to the inclusion of [***] in the Territory [***], in which case [***] will be deemed: [***] in each case from the date upon which the Condition ceased to have been fulfilled or risk owing to the inclusion of [***] in the Territory [***] arose.
The other Party may raise a Section 15.24 Dispute within [***] Business Days of receipt of a Section 15.24 Sanctions Reimposition Notice, following which these changes may be reversed if the Party raising such Section 15.24 Dispute is successful in its challenge. For the purposes of this Section 15.24, in assessing whether the Condition has been fulfilled, no account shall be taken of any contractual restrictions between a Party and any Third Party.
15.25Royalties are payable in respect of Relevant Patents until:
(a)subject to Section 15.27, in the case of [***] and the [***] Royalty as applied to [***] Changed Products for all the applicable [***] for HNB Products until the later of: (1) the expiry [***] of the [***] Patents, provided that (A) any extension of such [***] Patent’s term by a Governmental Authority is excluded and (B) each [***] Patent shall be deemed to expire as of 21 years after the filing date of the earliest patent application for which a claim of priority has been made (including any provisional application or other document from which priority can be claimed) in such [***] Patent’s Patent Family or otherwise 20 years after the filing date, regardless of whether such [***] Patent has actually expired as of such date; or (2) in the case of a [***] Patent [***], then until the date 20 years after its earliest filing date, and in no event later than 21 years after the earliest filing date of any of its Patent Family Members for which a claim of priority has been made (including any provisional application or other document from which priority can be claimed) or otherwise 20 years after the filing date . When a Patent Family ceases to be royalty-bearing pursuant to this Section (a), the [***] Patent Family Number and the [***] Patent Family Number and the royalty rate shall be recalculated pursuant to Section 10.5. For the avoidance of doubt, during any such recalculation, the allocation of Patent Families between PMP [***] Patents, PMP [***] Patents and Additional Patent Families shall remain as it was for the calculation of the [***] Royalty; and
(b)subject to Section 15.26, in the case of royalty on [***] Patents, for the [***] from the grant of the applicable [***] Patent until the expiry or final, non-appealable or non-appealed determination of invalidity or lack of Entitlement of the [***] Patent [***] provided that: (1) [***] is excluded; and (2) each [***] Patent shall be deemed to expire as of 21 years after the filing date of the earliest patent application in such [***] Patent’s Patent Family for which a claim of priority has been made (including any provisional application or other document from which priority can be claimed) or otherwise 20 years after the filing date, if that is earlier than actual expiry of such [***] Patent.
15.26In relation to Changed Products, royalty entitlement shall [***] for: (1) Relevant Patents granted at Launch of that Changed Products from Launch [***] and (2) for all Relevant Patents [***].
However, if there is a Validity Challenge to any [***] Patent of the Patent Owning Party (as permitted under Section 31), which is Relevant in relation to any Changed Product:
(a)the decision of the First Instance Level shall [***]. If the decision of the First Instance Level is non-appealable or not appealed, royalties shall either continue to accrue ([***]) or cease accruing [***] on the date of such decision of the First Instance Level;
(b)any intervening decision between the decisions of the First Instance Level and the First Appellate Level shall [***] If such intervening decision is non-appealable or not appealed, royalties shall either continue to accrue [***] or cease accruing[***] on the date of such intervening decision. However, there shall be [***] for the period between the decision of the First Instance Level and the intervening decision;
(c)if the decision of the First Instance Level is appealed to the First Appellate Level, royalties shall either continue to accrue [***] or cease accruing [***] on the date of the decision of the First Appellate Level, [***] However, there shall be [***] for the period between the decisions of the First Instance Level and the First Appellate Level;
(d)if the decision of the First Appellate Level is appealed, there shall be [***]Royalties shall either restart accruing [***] or cease accruing [***] on the date of such final non-appealable or non-appealed decision. In addition, the accrual of royalties shall be [***], for the period between the decision of the First Appellate Level and the final, non-appealable or non-appealed decision;
(e)if: (1) the claims are upheld by the First Appellate Level in an amended form which the Launching Party alleges the relevant Device or Consumable is no longer Within the Scope of, or there is a final, non-appealable or non-appealed determination to that effect; or (2) the claims are amended at the request of the Patent Owning Party (regardless of whether there is a Validity Challenge), to a form which the Launching Party alleges the relevant Device or Consumable is no longer Within the Scope of, the Launching Party may seek a reassessment of such Changed Product in accordance with Section 15.26(g) below. If the result of such reassessment confirms that the relevant Device or Consumable is no longer Within the Scope of the [***] Patent, then royalties shall [***] for the period between the decision by the First Appellate Level in the case of (1) or the date on which the claims are amended at the request of the Patent Owning Party in the case of (2), and such confirmation;
(f)if there is a final, non-appealable or non-appealed determination that: (1) reverses the decision of the First Appellate Level finding that the claims are upheld in an amended form which the relevant Device or Consumable is no longer Within the Scope of; and (2) permits claims in a different form which the Patent Owning Party alleges the relevant Device or Consumable are Within the Scope of (whether as a result of a Validity Challenge or not), then the Patent Owning Party may seek a reassessment of such Changed Product in accordance with Section 15.26(g) below. If the result of such reassessment confirms that the relevant Device or Consumable is Within the Scope of the [***] Patent, then the [***] for the period between the decision of the First Appellate Level and the final, non-appealable and non-appealed decision;
(g)a reassessment under this Section 15.26 is requested by the Assessment Representative of the Party seeking reassessment by providing email notification to the Assessment Representative of the other Party:
(i)attaching a copy of the relevant order, decision or judgment of the First Appellate Level or the final, unappealable determination;
(ii)attaching a copy of the relevant [***] Patent and the claims as amended;
(iii)identifying the relevant Products for which they are seeking reassessment;
(iv)stating that Party’s position on whether the relevant Products fall Within the Scope of the amended claims of the relevant [***] Patent including a detailed explanation [***]; and
(v)if that Party’s position is that as a result of the amendment the relevant [***] Patent that was a [***] Patent is now a [***] Patent, or vice-versa, an explanation as to why this is its position.
The Assessment Representative of the other Party must then respond to that email notification within [***] Business Days to confirm whether it accepts the position of the Party seeking reassessment and any failure to respond within this period is deemed to be acceptance, provided that after such [***] Business Days the Party seeking reassessment notifies the other Party of such deemed acceptance and the Patent Owning Party does not send a response to the email within [***] Business Days of such notice. If the other Party disputes the position of the Party seeking reassessment, then any such dispute will be resolved under the ADR Exhibit as an Expedited Dispute. For the avoidance of doubt, if there is a reassessment dispute that is to be resolved as an Expedited Dispute under the ADR Exhibit, the Enforceability of the [***] Patents or [***] are not considered when resolving this dispute.
15.27If the Final Patent Report for [***] indicates that BAT is entitled to initiate [***] Royalty in accordance with this Section 15.27:
(a)The [***] Royalty will be [***], provided that:
(i)for a [***] Changed Product BAT has [***] one of the Patent Families indicated as Relevant in the Final Patent Report for [***], when the applicable [***] Changed Product is assessed:
(A)against all the [***] Patents and [***] identified in the Final Patent Report for [***] for that Patent Family [***], and
(B)if the [***] Changed Product is a Device, when assessed in the context of all the [***] Consumables, and
(C)if the [***] Changed Product is a Consumable, when assessed in the context of all the [***] Devices, and
(ii)the Patent Family would no longer be Relevant to [***] if an equivalent [***] were made to [***] for which that Patent Family is Relevant (as indicated in the Final Patent Report for [***]).
(b)The assessment of such [***] is conducted [***] using the same [***] Patents and [***] as used in the assessment of the original [***] Royalty. The [***] Patent Family must be a Patent Family which, if removed from the Final Patent Report for [***] would result in the [***] Royalty being lower than the original [***] Royalty [***] (“[***]”). Such [***] Royalty shall be undertaken as set out in the remainder of this Section 15.27. If, with respect to a [***] Changed Product BAT has [***] more than one such Patent Family, the process set out in this Section 15.27 shall be undertaken concurrently in relation to each such Patent Family.
(c)BAT shall, in the Launch Notice for a proposed [***] Changed Product that BAT believes [***] a Patent Family indicated as Relevant in the Final Patent Report for [***] (“Proposed [***] Changed Product”) identify:
(i)the Patent Family BAT believes it has [***] (“Proposed [***] Patent Family”), together with an explanation as to why BAT believes it has [***] the Proposed [***] Patent Family; (ii)the respective [***] Device(s) and [***] Consumable(s) to which the respective Proposed [***] Patent Family is Relevant (“Proposed Removed [***] Products”);
(iii)[***] of the [***] Device or [***] Consumable that are to be changed or removed to achieve [***] (“Proposed [***]”);
(iv)the [***] Changed Product(s) that have been Launched that are Within the Scope of the Proposed [***] Patent Family, as assessed [***] using the same [***] Patents and [***] as used in the assessment of the original [***] Royalty (“Proposed Removed [***] Changed Products”); and
(v)whether the [***] Patents and [***] of the Proposed [***] Patent Family in respect of [***] in the Final Patent Report for[***], which shall for the purposes of this assessment and its application to Section 18 be deemed to be [***] Patents, are [***] Patents or [***] Patents.
(d)The Compliance of, and royalty obligations relating to, a Proposed [***] Changed Product, shall be assessed in the same way as any other Changed Product and additionally assessment pursuant to this Section 15.27 shall proceed as follows. Within [***] months of determination that the Proposed [***] Changed Product is Compliant, PMP shall notify BAT as to whether the matters identified by BAT pursuant to Sections 15.27(c)(i) to 15.27(c)(v) are agreed by PMP (“[***] Reply”):
(i)if PMP does not agree one or more of the matters identified by BAT pursuant to Sections 15.27(c)(i) to 15.27(c)(v), PMP shall identify the disputed matters and provide reasons therefor in the [***] Reply and the Parties shall, in the [***] Business Day period from receipt of the [***] Reply [***] Business Days), seek to resolve the disagreement by consulting and exchanging further information, with the provision of any Confidential Information being at the sole discretion of the Party providing such information and such exchange being in accordance with the requirements of Section 28. If after this period a dispute remains, then BAT must initiate an ADR Procedure as an Expedited Dispute within [***] Business Days, or the process pursuant to this Section 15.27 shall cease and the [***] Royalty shall remain unchanged. Further, and for the avoidance of doubt, any communications made between the Parties prior to the dispute being subject to an ADR Procedure are made on a without prejudice basis to any argument they may later make under the ADR Procedure or in any proceedings;
(ii)if by agreement of the Parties, or upon resolution of any disputed matters pursuant to the ADR Procedure, it is determined that the Proposed [***] Changed Product does not [***] the Proposed [***] Patent Family, the process pursuant to this Section 15.27 shall cease and the [***] Royalty shall remain unchanged;
(iii)if PMP agrees all the matters identified by BAT pursuant to Sections 15.27(c)(i) to 15.27(c)(v), or if upon resolution of any disputed matters pursuant to the ADR Procedure, it is determined that the Proposed [***] Changed Product does [***] the Proposed [***] Patent Family, then subject to any corrections to the matters identified by BAT pursuant to Sections 15.27(c)(i) to 15.27(c)(v) as agreed by the Parties or determined by the ADR Procedure, the Proposed [***] Changed Product shall be a “[***] Changed Product”, such Proposed [***] Patent Family shall be a “[***] Patent Family”, the Proposed [***] Features shall be a “[***] Features”, the Proposed Removed [***] Products shall be “Removed [***] Products”, the Proposed Removed [***] Changed Products shall be “Removed [***] Changed Products”;
for the avoidance of doubt, if it is determined that a Proposed [***] Changed Product does not [***] a Proposed [***] Patent Family for any reason, that shall not prevent BAT from citing that Patent Family in relation to a subsequent exercise of BAT’s rights pursuant to this Section 15.27.
(e)If it is determined that the Proposed [***] Patent Family is a [***] Patent Family:
(i)that [***] Patent Family is removed from the Final Patent Report for [***] and the [***] Patent Family number is recalculated pursuant to Section 10.5 to give the recalculated rate for[***] Royalty for [***];
(ii)for the royalty calculations for Removed [***] Products and Removed [***] Changed Products (together referred to as “[***] Section 18 Products”), each [***] Patent Family generates 1 [***] Patent [***] for each [***] Section 18 Product for the purposes of Section 18.2(b); and
(iii)for the avoidance of doubt, for the purposes of Section 18 the Removed [***] Products that are Consumables shall be grouped as Consumable Families in accordance with the requirements of the definition of Consumable Families to enable the application of Section 18 to such Consumables.
(f)If the [***] Royalty has been reduced pursuant to this Section 15.27, then for [***] Changed Products that are Launched after the Launch Notice for the [***] Changed Product, the [***] Features cannot be referred to as [***] of the Earlier Product pursuant to Sections 15.14, 15.15 and 15.16.
(g)BAT shall reimburse to PMP the reasonable external costs of PMP incurred in the assessment of a Proposed [***] Changed Product under this Section 15.27 including reasonable external legal costs and expert adviser costs, which shall not include any costs related to any ADR Procedure or Dispute related to any such Product or assessment under this Section 15.27.
(h)BAT may initiate the process set out in this Section 15.27 in the period after BAT has received the Draft Patent Report for [***] but prior to the determination whether royalty is payable on [***]. In such case:
(i)references in this Section 15.27 to the Final Patent Report for [***] shall be deemed to be references to the Draft Patent Report for [***];
(ii)if the Proposed [***] Patent Family is removed from the Draft Patent Report, prior to the conclusion of the process set out in this Section 15.27, this process shall cease in relation to the Proposed [***] Patent Family; and
(iii)if the Proposed [***] Patent Family is determined to be a [***] Patent Family pursuant to this Section 15.27, the consequences set out in Section 15.27(e) shall apply.
(i)If at any time following the issue of the Final Patent Report for [***], the [***] Royalty is [***], then no further [***] Royalty can be initiated, and any [***] will be terminated.
16.PROTOCOL FOR THE ASSESSMENT OF COMPLIANCE
16.1Unless agreed in writing between the Assessment Representatives of the Parties for a specific Product (or for all Products by variation of this Agreement under Section 41.5), assessment of whether a Device or Consumable of either [***] or a Changed Product is Compliant is made in accordance with this Section. If it is necessary to agree whether a Device has a Relevant Patent Family as part of determining whether it is Compliant (as is required by Section 12.2(c)), then that is assessed in accordance with Section 15.
16.2When considering the Compliance of a Product:
(a)a Party shall consider the Product in the form provided as samples pursuant to Section 16.4, and as otherwise detailed in the Draft Launch Notice;
(b)Compliance is assessed as of [***]; and
(c)in limited circumstances a limited exception may be made to certain Compliance requirements of this Agreement as set out in Sections 16.2(c) and 16.2(d). If:
(i)due to: (1) a change after the Effective Date in the Applicable Regulatory Requirements; or (2) a change after the Effective Date in interpretation by Applicable Regulators of Applicable Regulatory Requirements, a Party is, or will be, [***] of (A) an Existing Product and all Devices and/or all Consumables in the Changed Product [***] of that Existing Product, or (B) any other Part 1 Product that has previously been determined to be Compliant and all Devices and/or all Consumables in the Changed Product [***] of that Part 1 Product (and for the purposes of this reference only, the definition of the Changed Product [***] shall include any Existing Products in the group), (such change, a “Regulatory Change” and such Product, an “Affected Product”);
(ii)as a result of such Regulatory Change the affected Party is, or will be, required to [***]; and
(iii)it is not commercially and technically viable (“Viable”) to [***] Affected Product would be Compliant,
such [***] Product fulfilling the conditions of (i), (ii) and (iii) above, (the “Proposed Regulatory Changed Product”). Any Proposed Regulatory Changed Product shall, if the affected Party wishes to rely upon Sections 16.2(c) and 16.2(d), be reviewed through the process set out below.
(d)The process for review of a Proposed Regulatory Changed Product is as follows:
(i)the affected Party shall, no later than [***] Business Days before the Regulatory Change comes into force, notify the other Party of:
(A)details of the Regulatory Change;
(B)the identity of the Affected Product;
(C)an explanation as to how the Affected Product is affected by the Regulatory Change;
(D)reasonable details of the proposed modifications to the Affected Product;
(E)an explanation of the respects in which the Proposed Regulatory Changed Product would cease to be Compliant. However, a Proposed Regulatory Changed Product will only be a “Regulatory Changed Product” provided that:
(a)the lack of Compliance is [***] to conform with the Regulatory Change to achieve a Proposed Regulatory Changed Product that is Viable;
(b)if that Party is BAT, the proposed Regulatory Changed Product does not include [***], (c)if the Proposed Regulatory Changed Product is an Other HNB Changed Product, it complies with Section 12.2, and
(d)the Proposed Regulatory Changed Product is a Consumable, it is [***];
(F)an explanation as to why the proposed modification is necessary to comply with the Regulatory Change; and
(G)an explanation as to why there is no Viable way of conforming with the Regulatory Change such that the modified Affected Product would remain Compliant;
(ii)the other Party shall then have [***] Business Days upon receiving notification pursuant to Section 16.2(d)(i) to notify the affected Party whether it agrees that the Proposed Regulatory Changed Product is a Regulatory Changed Product including without limitation those set out in Sections 16.2(d)(i)(E), 16.2(d)(i)(F) and 16.2(d)(i)(G), providing in reasonable detail an explanation of its position in case of disagreement including, if applicable, an explanation as to why [***] the modified Affected Product would be remain Compliant;
(iii)if the other Party notifies the affected Party under Section 16.2(d)(ii) that it disagrees that the Proposed Regulatory Changed Product is a Regulatory Changed Product, and the Parties are unable to resolve the disagreement within [***] Business Days of the affected Party receiving such notification, [***] pursuant to the ADR Exhibit, which determination may be sought by the Party seeking to establish that as an Expedited Dispute;
(iv)if the other Party notifies the affected Party under Section 16.2(d)(ii) that it agrees that the Proposed Regulatory Changed Product is a Regulatory Changed Product, the Regulatory Changed Product shall be a Changed Product that is deemed to be part of the same Changed Product [***] as the Affected Product, [***], provided that:
(A)if the Regulatory Changed Product uses any Relevant Patents in addition to the Relevant Patents of the Affected Product (subject to the requirements of Section 12.2(c)), then those Patents will be assessed for royalties in accordance with Sections 15 to 18; (B)the Affected Product shall be the [***] Product for the Regulatory Changed Product but may not be relied upon as a [***] Product [***] after that Party has served a notice pursuant to Section 16.2(d)(i), [***]; and
(C)the Affected Product may not be relied upon as [***] Product pursuant to Section 7 or Section 15.13 in relation [***] after that Party has served a notice pursuant to Section 16.2(d)(i), [***].
(e)If a Party is unable to rely upon the provisions of Sections 16.2(c) and 16.2(d): (1) due to the provisos in Section 16.2(d)(i)(E); or (2) because the relevant product safety and/or regulatory requirements are applicable to Products [***], in either case, the Parties shall discuss whether any variation to this Agreement shall be made.
16.3BAT when Launching [***] and the Party which has Launched the Changed Product (in both cases the “Launching Party”) shall, subject to Section 16.5 and Section 16.7, provide email notification to the Assessment Representative of the other Party (the “Patent Owning Party”) within [***] Business Days of Launch of the Changed Product (or, with respect to any Changed Product that is Launched within [***] months after the Effective Date, [***] Business Days) or with respect to [***] Business Days after [***] Launch, including:
(a) the date of [***] Launch or the Launch of the Changed Product;
(b)the jurisdiction(s) it was Launched in;
(c)the name of the Product Launched (including, in the case of [***], the name of [***]);
(d)a unique identification number for the Product Launched (in the case of [***], a unique identification for [***]) in each case as ascribed by the Launching Party for the purposes of this Agreement; and
(e)for any Changed Product, if a Draft Launch Notice is required it shall also state:
(i)save for [***] Products, identification of the [***] Product to which that Changed Product relates, a copy of any Final Patent Report in relation to the [***] Product and identification of any [***] Patents of such [***] Product (“[***] Product Patents”) that the Launching Party believes are not Relevant to the Changed Product and the reason therefor; (ii)an outline of any changes the Launching Party wishes to draw to the attention of the Patent Owning Party to assist with the assessment of Compliance;
(iii)evidence of the Launch of the Changed Product, and if the Launching Party relies upon Section 15.17 [***] of the relevant HNB Changed Product;
(iv)identification of the Devices or Consumables with which the Changed Product is Compatible; and
(v)identification of any [***] Devices and their respective [***] Families;
(16.3(a) to (e) together the “Draft Launch Notice”, with the Draft Launch Notice for [***] being the “[***] Launch Notice”). Subject to Section 15.27, only one Draft Launch Notice shall be permitted for [***].
16.4The Launching Party shall, within [***] Business Days of the Draft Launch Notice (or, with respect to any Changed Product that is Launched between [***] and the Effective Date or within [***] months after the Effective Date, [***] Business Days), dispatch to the Patent Owning Party’s Assessment Representative, and provide documentary evidence to the Patent Owning Party, either through the Advisory Committee or as otherwise directed by the Advisory Committee, of such dispatch, of:
(a)(1) in relation to HNB Products: [***] samples of the Device and [***] samples of each Consumable (including HNB [***] Products) as Launched [***]; and (2) in relation to Vapour Products, [***] of the Device, and [***] of the Consumables (including Vapour [***] Products) provided, that in each case, the Launching Party shall within [***] Business Days of receipt of a request from the Patent Owning Party dispatch to the Patent Owning Party up to: (i) in relation to HNB Products, [***] of the specific Consumables; and (ii) in relation to Vapour Products, [***] of the specific Consumables; in each case if requested by the Patent Owning Party; and
(b)where a [***] Product is required for the assessment whether the Changed Product is Compliant, [***] samples of any Devices, in relation to HNB Products, [***] samples of any Consumables of [***] Product or in relation to Vapour Products, [***] samples of any Consumables of [***] Product; however, where such samples are unavailable, then copies of such [***] Test results and [***].
The Launching Party will make available to the Patent Owning Party a reasonable number of additional samples of such Devices or Consumables upon the Patent Owning Party’s reasonable request, including for the purpose of any additional testing PMP elects to do pursuant to Section 9.5(c) or Section 9.5(d). Such additional samples are limited in the case of [***] Products to the extent such samples exist. Numbers of samples of Consumables in this Section refer to stock keeping units. All samples of [***] Changed Product provided pursuant to this Agreement shall be accompanied by their respective unique identification numbers referenced in Section 16.3.
The applicable Covenants not to Sue in this Agreement in respect of [***] and Changed Product shall in each case be limited to the form of the samples provided in the respective Draft Launch Notices, subject to Section 16.7.
16.5The Parties shall consult and exchange that information with each other that is necessary to reasonably assist: (1) after Launch, the Launching Party, at the Launching Party’s request, with the preparation of the Draft Launch Notice; (2) after receipt of a Launch Notice, and subject to Section 16.16, the Patent Owning Party, at the Patent Owning Party’s request, to assess whether [***] or the changes embodied in a Changed Product are Compliant with the scope of Sections 9.3, 11.3, 12.2 or 13.2 as the case may be. Where reasonably practicable, each Party will respond to the other Party within [***] Business Days of a request for information from the other Party which may be extended by the responding Party by a further [***] Business Days if necessary to provide such information. If a Party requests samples of products from the other Party for the purposes of this Agreement other than pursuant to Section 16.4, the other Party shall give reasonable consideration to such request.
16.6For the purposes of this Section, if a Changed Product was Launched between [***] and the Effective Date, then the Launching Party may provide a Draft Launch Notice to the Patent Owning Party’s Assessment Representative within [***] Business Days of the Effective Date.
16.7The Launching Party:
(a)is not required to provide a Draft Launch Notice for Changed Products that are Non-Notifiable [***]. “Non-Notifiable [***]” are:
(i)any Device where such Device differs solely in one or more of the following manners, compared to an Existing Product, [***] or another Changed Product that has already been Launched by that Party pursuant to a Draft Launch Notice:
(A)[***]
(B)[***]
(C)[***];
(ii)any Consumable where such Consumable differs solely in one or more of the following manners, compared to an Existing Product, [***] Consumable or another Changed Product that has already been Launched by that Party pursuant to a Draft Launch Notice:
(A)[***]
(B)[***]
(C)[***]
(D)[***]
(E)[***];
(F)[***]; and/or
(G)[***]
excluding in all cases any Changed Product that has a [***] Patent other than [***] Product Patents;
(b)shall, no later than [***] Business Day after the end of the [***], provide the Patent Owning Party with a report of Non-Notifiable [***] in the form to be agreed by the Advisory Committee.
16.8If a Party does not provide a Draft Launch Notice in respect of a Changed Product to the Patent Owning Party in accordance with Section 16.3 above, then:
(a)if the Patent Owning Party reasonably believes that a Draft Launch Notice is required, for example because the Changed Product has a [***] Patent other than [***] Product Patents, the Patent Owning Party’s Assessment Representative may at any time, by written notice to the Launching Party’s Assessment Representative, require the Launching Party to provide a Draft Launch Notice within [***] Business Days of receipt of such notice. Thereafter assessment of such Changed Product shall proceed in accordance with Section 15 save that references in that Section [***]. Such notice from a Patent Owning Party shall not be taken to be an admission by the Patent Owning Party [***]; and (b)save for the circumstances provided in Section 16.8(a) above, then if as a result of the assessment pursuant to Section 16.8(a):
(i)such Changed Product is determined to be Compliant and to have no [***] Patents other than the [***] Product Patents, then the Launching Party will be deemed to have provided a timely Draft Launch Notice under Section 16.3 and such Changed Product will be entitled to the benefit of each applicable Covenant not to Sue granted under this Agreement, and royalties, if any, required to be paid by the Launching Party under this Agreement in respect of the [***] Product Patents will be due with respect to such Changed Product, [***];
(ii)such Changed Product is determined to be Compliant and to have [***] Patents that are [***] but no [***] Patents that are [***] Patents other than [***] Product Patents, then the Draft Launch Notice will be deemed to have been timely provided by the Launching Party on the date of such determination and such Changed Product will be entitled to the benefit of each applicable Covenant not to Sue granted under this Agreement and royalties, if any, required to be paid by the Launching Party under this Agreement will be due [***];
(iii)such Changed Product is determined to be Compliant and to have [***] Patents that are [***] Patents other than [***] Product Patents (regardless of whether it also has [***] Patents that are [***] Patents), then the Draft Launch Notice will be deemed provided by the Launching Party on the date of such determination and such Changed Product will be entitled to the benefit of each applicable Covenant not to Sue granted under this Agreement and royalties, if any, required to be paid by the Launching Party under this Agreement will be due [***].
However, in calculating such royalty, if the Patent Owning Party’s notice was within [***], the royalty rate applicable[***] shall be [***] For example, in relation to a HNB Product, if the royalty rate applicable to the [***] Product is [***]; (iv)such Changed Product is determined to be Compliant and to have [***] Patents that are [***] Patents other than [***] Patents (regardless of whether it also has [***] Patents that are [***]), then the Draft Launch Notice will be deemed provided by the Launching Party on the date of such determination and such Changed Product will be entitled to the benefit of each applicable Covenant not to Sue granted under this Agreement and royalties, if any, required to be paid by the Launching Party under this Agreement will be due [***]. However, in calculating such royalty, if the Patent Owning Party’s notice was [***], then [***], the royalty rate applicable [***] shall be [***]; and
(v)such Changed Product is determined not to be Compliant and/or to have [***] Patents that are [***] Patents, then the Launching Party will be deemed to have never issued a Draft Launch Notice and the Launching Party will not be entitled to the benefit of the Covenants not to Sue granted under this Agreement with respect to such Changed Product; and
(c)insofar as the [***] Changed Product that gives rise to [***] royalty rate pursuant to Section 16.8(b)(iii) or 16.8(b)(iv), is used in other later Changed Product [***] of the Launching Party as determined pursuant to Section 15.10(c), such [***] royalty rate shall also be applied in respect of such other Changed Product [***].
16.9Where [***] Tests are required to determine Compliance, the Patent Owning Party shall commission the [***] Tests within [***] Business Days of receipt of the applicable Product samples required to be provided in accordance with Section 16.4, and:
(a)for [***], the reasonable costs of such [***] Tests (except for [***] Tests PMP elects to do pursuant to Sections 9.5(c) and/or 9.5(d) in each case that are satisfied by the relevant Consumable) shall be reimbursed by BAT to PMP upon receipt of an invoice attaching a copy of the [***] Tests invoice; and
(b)for a Changed Product, the Patent Owning Party can elect to waive the requirement for such testing. If such [***] Tests are conducted, then the cost of such testing is only reimbursed by the Launching Party if such testing establishes that the Changed Product is not Compliant.
16.10Where either Party provides to the other a Product with [***] that will be subject to [***] Test pursuant to this Agreement, the providing Party shall provide [***] samples specified in this Agreement [***] to enable the [***] Tests to be carried out in half of such samples, and shall ensure that the samples [***] otherwise function the same [***].
Notwithstanding the foregoing, if it is not possible to provide samples with the [***], if samples provided with [***], or if [***] prevents such testing, then the Patent Owning Party can waive the testing; or alternatively, the Parties can work together to identify a technically feasible protocol to allow such testing [***]. If this is not possible, such [***] Tests shall be deemed to have been failed.
16.11Within the period of time specified in this Section from receipt of the Draft Launch Notice, the Patent Owning Party shall send an email notification to the Launching Party’s Assessment Representative stating whether it agrees that each of the Devices and Consumables contained in the Draft Launch Notice are Compliant and, if it deems any non-Compliant, provide the reasoning why such Device or Consumable is not Compliant (the “Compliance Reply”). The relevant period of time being:
(a)[***] months where the assessment of Compliance requires an assessment of whether there are any [***] Patents of the Patent Owning Party Relevant to the Products in the Draft Launch Notice; and the Patent Owning Party shall notify the Launching Party within [***] months after receipt of the Launch Notice if it asserts that such assessment of[***] Patents is required; or
(b)in all other cases, a [***] month period, which may be extended by agreement of the Parties and shall be extended if [***] Tests are required and the results thereof have not been made available within [***] Business Days of commission of the [***] Tests.
16.12If the Patent Owning Party does not send a Compliance Reply within the relevant period of time set out in Section 16.11 above, then all Devices and Consumables contained in the Draft Launch Notice are deemed [***], provided that following such failure the Launching Party notifies the Patent Owning Party of such deemed finalisation and the Patent Owning Party does not send a Compliance Reply within [***] Business Days of such notice.
16.13If the Patent Owning Party contests that any Device or Consumable is Compliant in its Compliance Reply, then the Parties shall consult and exchange further information within a [***] Business Day period from receipt of the Compliance Reply. If after that period a dispute remains, then the Launching Party must initiate an ADR Procedure within [***] month, or the contested Products will be deemed not to be Compliant, provided that after the expiry of such [***] month period the Patent Owning Party notifies the Launching Party of such deemed non-Compliance and the Launching Party does not initiate the ADR Procedure within [***] Business Days of such notice.
[***].
16.14If either pursuant to Section 16.13, by agreement between the Parties, or as finally determined in accordance with the ADR Exhibit, a Device or Consumable is found not to be Compliant for any reason [***], then, that Device or Consumable will be removed from the Draft Launch Notice and, subject to Sections 9.2, 9.3 and 11.2 and subject to the consequences of testing pursuant to Sections 9.5(c) and 9.5(d), will not be a Part 1 Product and, save for that in Section 23, shall not benefit from the Covenants not to Sue in this Agreement.
16.15For the avoidance of doubt, nothing in this Agreement shall prevent either Party from:
(a)commercialising any Product while its Compliance is being assessed;
(b)developing or Launching Products that are not Compliant, but such new Products would not be Part 1 Products and, save for the Covenant not to Sue in Section 23, shall not benefit from the Covenants not to Sue in this Agreement; or
(c)changing their HNB Products or Vapour Products to resultant Products other than [***] Changed Products, Other HNB Changed Products or Vapour Changed Products, but such resultant Products would not be Part 1 Products and, save for the Covenant not to Sue in Section 23, shall not benefit from the Covenants not to Sue in this Agreement.
16.16The Parties expect that the information to be exchanged under this Section 16 related to the assessment of Compliance will be either publicly available or derivable from information that is publicly available. Prior to any Dispute under this Section 16 being subject to an ADR Procedure, it is at the sole discretion of a Party as to whether it wishes to provide to the other Party any information that is not publicly available. If a Party chooses to exchange information that is not publicly available, the Parties will exchange such information in accordance with Section 28 and other protocols related to such information as are necessary to ensure compliance with Competition Law.
17.PROTOCOL FOR AGREEING THE RELEVANT ROYALTY-BEARING PATENT FAMILIES
17.1Unless agreed in writing between the Assessment Representatives of the Parties for a specific Product (or for all Products by variation of this Agreement under Section 41.5), assessment of whether a Patent Family is Relevant to the assessment of royalties for a Device or Consumable of either [***] or a Changed Product is made in accordance with this Section.
17.2Within a [***]-month period from each of:
(a)for [***], the later of: (1) the date that is [***] of the Draft Launch Notice; or (2) the date at which it is determined that [***] is Compliant; (b)for a Device of an Other HNB Changed Product, the later of: (1) [***] of the Draft Launch Notice; or (2) the date at which it is determined that it is Compliant when assessed pursuant to Section 12.2(c); or
(c)for any Changed Product other than those referenced in Section 17.2(b), the date that is [***] of the Draft Launch Notice for that Changed Product;
the Patent Owning Party shall assess each of the Devices and Consumables in the Draft Launch Notice (including, for the avoidance of doubt, the [***] Launch Notice) to identify the [***] Patents and [***] it asserts to be Relevant to determining whether any of the Devices or Consumables are royalty-bearing and if so, the applicable royalty rates. The Launching Party shall cooperate and support this assessment by providing, within [***] Business Days [***] responses and particulars to the inquiries made by the Patent Owning Party about the features or function or functioning of the Devices and Consumables subject to the Draft Launch Notice.
17.3For all Changed Products, at the conclusion of the [***] month period in Section 17.2, the Patent Owning Party may, in its sole discretion, send a report by email to the Launching Party’s Assessment Representative (the “Draft Patent Report”). Where that Draft Patent Report relates to a Changed Product for which a previously Launched Changed Product [***] of that Changed Product has been the subject of an earlier Final Patent Report, then the Patent Owning Party can rely on that earlier Final Patent Report if it identifies it in the Draft Patent Report for the Changed Product under consideration, such that such later Draft Patent Report only needs to identify the further Relevant [***] Patents in the Changed Product under consideration.
(a)If the Patent Owning Party fails to send a Draft Patent Report [***] month period or sends a Draft Patent Report which does not include any Relevant [***] Patents, then all Devices and Consumables contained in the Draft Launch Notice are deemed to be:
(i)if there is a Final Patent Report for the [***] Product, subject to the same royalty obligations as the [***] Product, [***] Patents within such Final Patent Report that the Launching Party indicated in the Draft Launch Notice were not Relevant to the Changed Product; or
(ii)if there is no Final Patent Report for the [***] Product, [***] unless: (1) [***] pursuant to Section 17.2; or (2) the Changed Product is [***] in which latter case (2) the Changed Product shall be subject to [***] as described in Section 16.7; provided in each case that after such failure, the Launching Party notifies the Patent Owning Party of such deemed outcome and the Patent Owning Party does not send a Draft Patent Report, or sends a Draft Patent Report which does not include any Relevant [***] Patents, within [***] Business Days of such notice.
(b)If the Patent Owning Party sends a Draft Patent Report, then that Draft Patent Report must identify:
(i)each [***] Patent(s) and [***] the Patent Owning Party asserts to be Relevant [***] to each Device and Consumable in that Draft Launch Notice;
(ii)any [***] Patents;
(iii)the claims that the Patent Owning Party is asserting and a detailed claim chart in respect of each asserted claim of the Relevant [***] Patent(s) and [***]; and
(iv)in the case of a Changed Product, an indication as to whether the Patent Owning Party considers the nominated [***] Patent(s) and [***] to be, in relation to that Changed Product: [***].
17.4If the Patent Owning Party does not include a [***] Patent or [***] for a Patent Family in the Draft Patent Report (or any other Patent Report referenced therein) in respect of a given Device or Consumable within the relevant Draft Patent Report, such [***] Patent or [***] and the applicable Patent Family shall not be Relevant to such Device or Consumable.
17.5If the Patent Owning Party sends a Draft Patent Report, then Launching Party must within [***] Business Days of receipt of the Draft Patent Report [***] raise any objection to that Draft Patent Report including identification of any [***] Patents or [***] in the Draft Patent Report that the Launching Party asserts not to be Relevant to the Changed Product under assessment and the respective relevant sub-sections of Sections 15.8, 15.9, 15.11 and 15.13 relied upon and:
(a)if the Draft Patent Report for a Changed Product refers to an earlier Final Patent Report of the relevant [***] Product, identification of any [***] Patent in that Final Patent Report for such [***] Product that the Launching Party asserts is not Relevant to the Changed Product under consideration together with the reasoning therefor;
(b)if the Launching Party relies upon Section 15.9(b), identification of what the Launching Party asserts to be:
(i)the relevant Existing Product of the Launching Party (as referenced in Section 6);
(ii)the granted Patent Family Member of the Patent Owning Party (as referenced in Section 6) that the Launching Party’s relevant Existing Product was Within the Scope of at the relevant date; and
(iii)a reasonably detailed explanation [***] in support of the assertion that such Existing Product is Within the Scope of such granted Patent Family Member;
(c)if the Launching Party relies upon Section 15.10(b), identification of the other [***] Patent or [***] for which royalty is claimed and [***] of such [***] Patent or [***]; and
(d)if the Launching Party relies upon Section 15.15, identification of the Earlier Product(s) relied upon as the source of the Changed Product [***] Family;
(i)if the Earlier Product relied upon as the source of the Changed Product [***] Family is a HNB Product that is [***];
(ii)a description of [***]; and
(iii)if it relies upon Section 15.15(d), a copy of the Final Patent Report referenced therein;
(e)if the Launching Party relies upon Section 15.15, identification of the Earlier Product relied upon as the source of the [***] Family;
(f)if the Launching Party relies upon the [***] Covenant Not to Sue (Section 7), identification of:
(i)the [***] Patent or [***] to which Section 7 applies;
(ii)what it asserts to be the [***] of the foregoing [***] Patent or [***] relied upon and [***] of the Product under assessment to which Section 7 applies;
(iii)[***] of the Existing Product (in the case of Sections 7.1 and 7.2) or the applicable Part 1 Products (in the case of Sections 7.3 and 7.4 respectively) relied upon for the purpose of Section 7; and (iv)an explanation of how Section 15.13 applies [***] in Section 17.5(f)(iii) and the [***] of [***] or the Changed Product (as the case may be).
17.6In a [***] Business Day period from receipt of the Launching Party’s objection, the Parties shall consult and exchange further information, with the provision of any Confidential Information being at the sole discretion of the Party providing such information and such exchange being in accordance with the requirements of Section 28. If after this period a dispute remains as to the inclusion of any [***] Patent or [***], then the Patent Owning Party must initiate an ADR Procedure under the ADR Exhibit within [***] Business Days [***] the Draft Patent Report. If the Launching Party does not within [***] Business Days of receipt of the Draft Patent Report (or within the extended deadline) raise any objection to that Draft Patent Report including with respect to the inclusion of particular [***] Patents or [***] within that Draft Patent Report, then that Draft Patent Report is deemed finalised, [***].
17.7If either pursuant to Section 17.6, by agreement between the Parties, or as finally determined in accordance with the ADR Exhibit, a [***] Patent or [***] is found not to be Relevant to a Device or Consumable, then the Draft Patent Report shall be deemed to be amended by the removal of that [***] Patent or [***] from the Draft Patent Report in relation to that Device or Consumable [***] and the Patent Family of such [***] Patent or [***] shall not be Relevant to such Device or Consumable. The so finalised Patent Report (“Final Patent Report”) shall be appended to the finalised Launch Notice (after that has been finalised in accordance with Section 16 (the “Final Launch Notice”)).
17.8The Final Launch Notice and Final Patent Report are then used to determine the royalty rate for the Products that were the subject of the Final Launch Notice in accordance with Section 10 (in relation to [***]), Section 14 (in relation to Changed Product) and using the Royalty Rate Mechanism in Section 18. The Final Patent Report for a Changed Product [***] or if the Patent Owning Party is found to lack Entitlement to a [***] Patent [***] pursuant to Section 15.26.
17.9PMP at its sole discretion may determine whether to issue a Draft Patent Report for [***]. If PMP issues a Draft Patent Report for [***] it shall contain the information specified in Section 16.3(a) to 16.3(d) inclusive and shall specify whether each Patent Family in the Draft Patent Report is a PMP [***] Patent Family, PMP [***] Patent Family or Additional Patent Family. For each Patent Family in the Draft Patent Report, PMP shall nominate at least one [***] Patent or [***] and may nominate a [***] Patent or [***] for HNB Products.
However, if PMP does not issue a Draft Patent Report for [***] within the period specified in Section 17.2, then it is deemed that the [***] Royalty is [***], provided that after such period BAT notifies the PMP of such deemed outcome and PMP does not send a draft Patent Report within [***] Business Days of such notice. If PMP believes that an [***] Royalty is due and issues a Draft Patent Report, [***] shall follow the same procedure as for Changed Product as set out in Sections 17.3 to 17.8 except that the royalty rate for [***] is determined in accordance with Section 10.5 and the Final Patent Report shall specify which Additional Patent Families (if any) are deemed to be PMP [***] Patent Families or PMP [***] Patent Families pursuant to Section 10.2.
17.10If BAT disputes the number of Additional Patent Families that PMP alleges in the Draft Patent Report are Relevant for [***] then:
(a)any dispute as to the number of PMP [***] Patent Families and/or PMP [***] Patent Families that are Relevant for [***] shall be determined, as a preliminary matter, as an Expedited Dispute. In such Expedited Dispute BAT shall, in the Pre-Arbitration Procedure, nominate the PMP [***] Patent Families and/or PMP [***] Patent Families it asserts are not Relevant to [***] (each, a “Challenged PMP [***] Patent Family” or “Challenged PMP [***] Patent Family”, as applicable). The Expedited Dispute procedure will determine which Challenged PMP [***] Patent Families and PMP [***] Patent Families are Relevant for [***] (the “Upheld [***] Patent Families”) and those not Relevant for[***] (the “Refused [***] Patent Families”). The total number of PMP [***] Patent Families and PMP [***] Patent Families Relevant for [***] then being the sum of: (1) the Upheld [***] Patent Families; and (2) the [***] Patent Families and/or PMP [***] Patent Families that BAT agreed as Relevant for [***] without challenging their Relevance;
17.11Upon conclusion of any Expedited Dispute referenced in Section 17.10(a), BAT shall be entitled (whether there is such a dispute or not), in its response to the Draft Patent Report for [***] prepared by PMP, to nominate, [***], the Additional Patent Families (if any) that shall be deemed as PMP [***] Patent Families and/or PMP [***] Patent Families pursuant to Section 10.2, and therefore subject to the [***] and the [***] respectively. Subject to Section 15.27, BAT shall not raise any further challenge to the Relevance of the Patent Families so nominated. The Additional Patent Families remaining after the nomination pursuant to this Section are the “Remaining Additional Patent Families”; and
(a)from the Remaining Additional Patent Families, BAT shall identify the Additional Patent Families it alleges are not Relevant to [***], for resolution via the ADR, subject to a maximum number of Additional Patent Families so identified. [***] 17.10(a)
[***] BAT shall not dispute the Relevance of any Additional Patent Families other than pursuant to this Sub-Section.
17.12The Parties expect that the information to be exchanged under this Section 17 related to the assessment of whether a Patent Family is Relevant to the assessment of royalties for a Device or Consumable will be either publicly available or derivable from information that is publicly available. Prior to any Dispute under this Section 17 being subject to an ADR Procedure, it is at the sole discretion of a Party as to whether it wishes to provide to the other Party any information that is not publicly available. If a Party chooses to exchange information that is not publicly available, the Parties will exchange such information in accordance with Section 28 and other protocols related to such information as are necessary to ensure compliance with Competition Law.
18.ROYALTY MECHANISM TO DETERMINE ROYALTY RATES RESULTING FROM THE LAUNCH OF A CHANGED PRODUCT AND FOR [***] SECTION 18 PRODUCTS
18.1The royalty consequences provided in this Section 18 (as further described in Schedule 18.1) shall arise from:
(a)[***] Section 18 Products; and
(b)a Changed Product (Device or Consumable) (including [***] Changed Products) that is Launched by a Launching Party, which has been determined to be royalty-bearing as one or more of the Patents of the Patent Owning Party are Relevant to the Changed Product, as set out in a Final Patent Report pursuant to Section 17.7.
18.2For the purposes of this Section 18:
(a)the “Paying Party” is:
(i)for [***] Section 18 Products: BAT;
(ii)for Changed Products to which this Section 18 applies: the Launching Party;
(b)the “[***] Patents” are:
(i)for Removed [***] Products: the respective [***] Patent(s) that are Relevant to such Products;
(ii)for Removed [***] Changed Products: the respective [***] Patent(s) that are Relevant to such Products, together with any Patents of the Patent Owning Party that are Relevant to the Removed [***] Changed Product as set out in a Final Patent Report pursuant to Section 17.7 (if applicable); (iii)for any other Changed Products to which this Section 18 applies: the Patents of the Patent Owning Party that are Relevant to the Changed Product as set out in a Final Patent Report pursuant to Section 17.7.
18.3Schedule 18.3 sets out worked examples of those royalty consequences. In the event of any conflict between Schedule 18.3 and this Section 18, this Section shall take precedence.
18.4If a Device to which this Section 18 applies has been determined, [***], to be royalty bearing under [***] Patent(s) of the Patent Owning Party, the Paying Party shall pay to the Patent Owning Party a royalty [***] on the Device at a royalty rate that is the sum of the following (but subject [***] Section 14):
(a)[***]; plus
(b)[***]:
(i)[***]; plus
(ii)[***]; plus
(c)[***]:
(i)[***]; or
(ii)[***].
18.5If a Consumable to which this Section 18 applies has been determined, [***] to be royalty bearing under [***] Patent(s) of the Patent Owning Party, the Paying Party shall pay to the Patent Owning Party a royalty [***] on the Consumable at a royalty rate that is the sum of the following [***]
(a)[***]; plus
(b)[***]:
(i)[***]; plus
(ii)[***]; plus
(c)[***]
18.6For the purposes of Section 18.5(c), [***] if:
(a)Where the Product is an HNB Product:
(i)[***]
(ii)[***]; and
(b)where the Product is a Vapour Product, [***]
18.7For the purposes of this Section 18, any Non-Notifiable [***], will be treated as [***], provided that both the Non-Notifiable [***] and the [***]: (1) [***]; and (2) [***].
18.8The mechanism set out in this Section 18:
(a)shall apply to Vapour Products which are not [***] Products provided that:
(i)[***]; and
(ii)[***];
(b)shall not apply to [***] Products. Royalty on [***] Products shall be calculated based on the number of [***] Patents used in the [***] Product in accordance with the royalty rates in Sections 14.3 (for HNB [***] Products) and 14.5 (for Vapour [***] Products) and subject to Section 15.
18.9When a Device is de-listed in every market in the Territory by the Paying Party such that it is no longer available from a Party to its Supply Chain Entities (“De-listed”), the [***] Patents of such Device shall, [***] in the calculation of royalties payable [***]. For the avoidance of doubt, this Section 18.9 will continue in effect following termination or expiry of this Agreement.
18.10When a Consumable Family is De-listed in every market in the Territory by the Paying Party, the [***] Patents of such Consumable shall [***] in the calculation of royalties payable [***].
18.11Each Party shall maintain an up-to-date record [***], in like form to the tables in Schedule 16.7, determining the applicable royalty rate per Product for its own Products and for the other Party’s Products from time to time pursuant to this Section 18 (“Royalty Rate Records”). Any difference between the Parties respective Royalty Rate Records as maintained pursuant to Section 36.1(b)(iv) shall be resolved in accordance with the ADR Exhibit.
18.12[***]
19.ROYALTY PAYMENT
19.1Royalties and other sums payable under this Agreement are exclusive of VAT (or similar tax) which shall be paid in addition to royalties, and such amounts shall be paid free and clear of all deductions and withholdings whatsoever, unless the deduction or withholding is required by law. If any deduction or withholding is required by law (“Tax Deduction”) the paying Party shall pay to the non-paying Party such sum as will, after the deduction or withholding has been made, leave the non-paying Party with the same amount as it would have been entitled to receive in the absence of any such requirement to make a deduction or withholding. If the paying Party is required to make a Tax Deduction, the paying Party shall, within [***] Business Days of making the deduction or withholding, provide a statement in writing showing the gross amount of the payment, the amount of the sum deducted and the actual amount paid.
19.2To the extent that any Tax Deduction applies to any payments made under this Agreement and the obligation on the paying Party to make the Tax Deduction can be relieved (or the amount of the Tax Deduction can be reduced to a lesser amount) on an application for relief by the non-paying Party, the non-paying Party shall (at the paying Party’s request and expense) as soon as reasonably practicable, make such application (including but not limited to seeking relief under any applicable double taxation treaty or convention) to enable the non-paying Party to receive amounts payable under this Agreement without any Tax Deduction (or to receive amounts at a reduced rate of tax deduction. The obligation (under Section 19.1) on the paying Party to pay increased amounts to the non-paying Party shall not apply to the extent that the non-paying Party would be entitled to receive amounts payable under this Agreement without any Tax Deduction applying (or would be entitled to receive amounts at a lower rate of Tax Deduction), but is not so entitled due to the non-paying Party’s failure to complete any necessary procedural formalities to receive amounts without such Tax Deduction (or to receive the amounts at the lower rate of tax deduction).
The non-paying Party will reimburse the paying Party for all, or a portion, of any increased amount paid by the paying Party to the non-paying Party on account of a Tax Deduction pursuant to Section 19.1 to the extent necessary to prevent the non-paying Party from receiving a net tax benefit from the payment of the Tax Deduction by the paying Party. Whether the non-paying Party would receive a net tax benefit shall be determined by comparing (1) the overall tax liability of the non-paying Party, taking into account any inclusion in taxable income resulting from the payment of the Tax Deduction and any amount that the non-paying Party is able to recover against its domestic corporate tax liability or any other liability on account of such payment, with (2) the overall tax liability of the non-paying Party if there had been no Tax Deduction.
19.3Royalties and any other sums payable under this Agreement shall be paid in United States dollars (USD) if the paying Party is PMP and British pounds sterling (GBP) if the paying Party is BAT. In each case payment shall be made to the credit of a bank account to be designated in writing by the non-paying Party. Each Party will make all payments hereunder in accordance with Applicable Law.
19.4Within [***] Business Days of [***] the paying Party shall notify the non-paying Party of the total amount of royalties payable in respect of [***]; within [***] Business Days of [***] the non-paying Party shall provide to the paying Party an invoice for such amount; and within [***] Business Days of [***] the paying Party shall pay such royalties to the non-paying Party. The issuance of such invoice shall be without prejudice to the non-paying Party’s right to dispute, pursuant to Section 19.9 the amount of royalty due from the paying Party or to charge interest on late payments of royalties in accordance with Section 19.6 where the amount of royalties was not notified in accordance with the timelines set out above.
19.5For the purpose of [***] the currency of payment, the rate of exchange to be applied shall be the simple average of daily FX rates as of [***] close of business, as available from Bloomberg LP, for the relevant [***].
19.6In the event of any delay in paying any sum due under this Agreement by the due date, the paying Party shall pay to the non-paying Party interest [***] on the overdue payment from the date when such payment was due to the date of actual payment at a rate of [***] as available from Bloomberg LP, for the relevant day. The Parties acknowledge that following [***] Launch or the Launch of a Changed Product, it will take some time to determine the applicable royalty rate pursuant to the terms of this Agreement and that whilst royalties may thereafter be due retrospectively in respect of such period, no interest will be due in respect of such period save to the extent that the paying Party has failed to comply with the deadlines in this Agreement applicable to such determination.
19.7The Parties shall comply with the provisions of Schedule 19.7 in relation to the Independent Accountant.
19.8Annually, by the last Business Day in [***] each year, including the year after royalties cease to be payable pursuant to this Agreement, the paying Party shall submit or cause to be submitted to the Independent Accountant a statement in writing recording the calculation of all royalties payable [***] and in particular:
(a)the [***] for which the royalties were calculated;
(b)the number of each royalty-bearing Product (identified by its respective unique identification number referenced in Section 16.3) supplied [***] during the [***];
(c)the royalty rate [***] applied to each royalty-bearing Product during the [***], including the timing and amount of any changes to such royalty rate during the [***];
(d)the [***] in respect of each royalty-bearing Product supplied during the [***];
(e)the [***] in the calculation of [***];
(f)the amount and mechanism of calculation of royalties due and payable;
(g)the amount of any withholding or other income taxes deductible or due to be deducted in the calculation of royalties due and payable;
(h)identification of any Devices that have been De-listed and the date of such De-listing;
(i)the calculation of any royalties that have retrospectively become due or repayable to the paying Party (for example pursuant to Section 15.26); and
(j)any other particulars the Independent Accountant may reasonably require to verify the accuracy of the royalty calculation.
19.9The Independent Accountant shall review the data provided pursuant to Section 19.8 and shall within [***] Business Days [***] make a preliminary report to each Party as to the accuracy of that Party’s own royalty calculation. Each Party shall have [***] Business Days to respond to the preliminary report it has received and the Independent Accountant’s final report as to the accuracy of each Party’s royalty calculation shall be provided to both Parties within [***] Business Days. Any dispute as to the outcome of the Independent Accountant’s review shall be resolved in accordance with the ADR Exhibit.
19.10If such final report of the royalties payable by the Parties to each other should reveal a net underpayment or overpayment from one Party to the other, the under-paying or over-receiving Party shall make a corrective payment to the other Party when making the next royalty payment pursuant to Section 19.4 together with interest pursuant to Section 19.6 in the case of an underpayment.
19.11The paying Party shall keep proper records and books of account showing [***] of royalty-bearing Products supplied or put into use. Such records and books shall be kept separate from any records and books not relating solely to royalty-bearing Products.
19.12If the paying Party is prohibited by Applicable Law or a Governmental Authority in any country from making any payment due under this Agreement, then the paying Party shall, within the prescribed period for making such payment, use its Best Efforts to secure from the relevant authority permission to make such payment and shall make it within [***] Business Days of receiving such permission. If such permission is not received within [***] Business Days of the paying Party making a request for such permission then, at the option of the non-paying Party, to the extent permitted by Applicable Law or the relevant Governmental Authority, the paying Party shall either deposit the payment in the currency of the relevant country in a bank account designated by the non-paying Party within such country, or make the payment to an Affiliate of the non-paying Party designated by the non-paying Party and having an office in the relevant country or in another country designated by the non-paying Party.
19.13If [***] for a Product in respect of [***] is a negative amount, the applicable royalty shall be zero; the paying Party shall not be entitled to [***]
19.14The provisions of this Section 19 shall remain in effect notwithstanding termination or expiry of this Agreement until the settlement of all subsisting claims by the non-paying Party.
20.LICENCE TO [***] OF SPECIFIC [***] PATENTS
20.1In respect of the good and valuable consideration set out in this Agreement, to the extent that these are owned by [***], [***] grants to [***] a non-exclusive, perpetual, royalty-free licence in the Territory for the benefit of [***] and (solely to the extent that the acts of such Connected Persons relate to the Products of [***]) its Connected Persons (sub-licensable only for the purpose of granting such benefit), solely in respect of the Part 1 Products of [***] which are [***] Products Launched prior to the expiry of the Term, of the Patent Families containing the following publications:
(a)[***]
(b)[***]
(c)[***]
(d)[***]
20.2However, the benefit for [***] Connected Persons pursuant to this Section 20 is subject to the Connected Person undertaking all applicable acts described in Section 25.4.
21.ACQUIRED OR DISPOSED PATENTS AND OTHER INTELLECTUAL PROPERTY RIGHTS
21.1Subject to the terms of the respective Covenants not to Sue, all of the Covenants not to Sue in this Agreement shall extend to the Patents or Intellectual Property Rights (as respectively specified in the relevant Covenant not to Sue) in respect of which legal title is acquired by a Party from:
(a)a Party’s Contractors, so that Intellectual Property Rights acquired from a Party’s Contractors are treated for the purposes of this Agreement, as if they were generated by that Party’s employees. “Contractors” means any Persons to the extent they are creating Intellectual Property Rights for a Party pursuant to a contract for services (for the avoidance of doubt, as defined by the law of England and Wales); and
(b)a Third Party other than a Party’s Contractors (“Acquired IPR”) subject to Section 21.2.
21.2In calculating royalties due pursuant to this Agreement, Patents within Acquired IPR shall be taken into account only in respect of Changed Products Launched on or after the date the Acquired IPR is acquired, subject to the royalty provisions of this Agreement.
21.3In connection with: (1) any assignment or transfer of (A) any Patents relating to technologies relevant to Products, and/or (B) any Intellectual Property Rights other than Patents within the scope of Section 4; (such Patents and other Intellectual Property Rights together, “Relevant IPR”), by a Party to any Third Party; (2) any licence granted by a Party to any Third Party of any Relevant IPR or other grant of rights that includes the right to enforce such Relevant IPR; or (3) any disposal or other change of Control of any Associated Company of a Party that, in either case, owns or, subject to Sections 22 and 25, [***] is able to licence any Relevant IPR, in each case (1), (2) and (3), to the extent on or after the Effective Date, and in the case of (3) limited to Relevant IPR consisting of, (x) Patent Families (including Patent Families comprising a single Patent) filed before such disposal or other change of Control, (y) other Intellectual Property Rights within the scope of Section 4 owned or [***] by the Associated Company at the date of such disposal or other change of Control, and (z) Relevant IPR that the Associated Company is otherwise able to licence at the date of such disposal or other change of Control, the granting Party agrees, that all of the licences, releases, Covenants not to Sue and other rights granted by them and all their obligations set forth in this Agreement including without limitation the release in Section 4, the Covenants not to Sue in Sections 5, 6, 7, 9, 11, 12, 13, 23 and 24, the licences granted pursuant to Sections 3, 20 and 41.7, the royalty obligations in Sections 17, 18 and 1918, the governing law provisions in Section 39 and the dispute resolution provisions in Section 40 (the “IPR Obligations”) shall run with such Relevant IPR, and that such granting Party shall ensure (in all cases subject to Section 38 and compliance with all Applicable Laws), that any assignee, transferee, successor or licensee to such Relevant IPR (including the acquiring or surviving entity in connection with any disposal or other change of Control of such granting Party’s Associated Company), or any other Person that obtains any enforcement rights with respect to such Relevant IPR agrees in writing, prior to such assignment, transfer, grant or licence, to be bound by the relevant IPR Obligations (including the obligation to obtain, and require that all subsequent assignees, transferees, successors, grantees or licensees though multiple tiers obtain, such written agreement from any subsequent assignee, transferee, successor, grantee or licensee).
Such Relevant IPR includes such Intellectual Property Rights as they evolve after the relevant grant of rights, including without limitation, reissues, extensions, re-examinations, continuations and divisionals. Upon the disposal or other change of Control which results in an Affiliate of a Signatory ceasing to be an Affiliate thereof, that entity shall cease to have the benefit of this Agreement. For the avoidance of doubt, references in this Section 21.3 to “Party” in (1) and (2) above extend to [***] Companies pursuant to Section 1.2.
21.4If a Party or any of its assignees, transferees, successors, licensees, grantees (through multiple tiers) or former Associated Companies takes or enables actions to be taken that are a breach of the IPR Obligations that are required of it pursuant to Section 21.3 without limitation of any other remedies the other Party may have, such Party (or if such Party is not a Signatory, the Signatory with respect which such Party is an Affiliate) shall indemnify and hold harmless the other Party in respect of (subject to Section 29.8) all losses, damages, costs, expenses, charges, penalties and other liabilities (including reasonable legal and other professional fees) incurred or suffered by the other arising out of or in connection with any such breach, including from any assertion of any Intellectual Property Right that would have been prevented by compliance with Section 21.3.
21.5Save for as a result of any forced state acquisition, appropriation, permanent or temporary transfer (for the duration of such transfer if temporary) by the [***], as relevant, including through any acts that would constructively amount to such acquisition, appropriation or transfer (“Involuntary Transfer”), no Party will assign to any Third Party or Designated Associated Company any of its: (1) Patents (which term means, solely for the purposes of this Section 21.5, “Patents” as otherwise defined in this Agreement but with deletion of the phrase “in the Territory”) relating to technologies relevant to Products; or (2) registered designs relating to Products ((1) and (2) together in the [***] “Relevant [***] IPR”, and in [***] “Relevant [***] IPR”) or grant to any Third Party or Designated Associated Company any licence or other right to any such Relevant [***] IPR or Relevant [***] IPR:
(a)in the [***], such that it entitles that Third Party or Designated Associated Company to enforce any such Relevant [***] IPR, where such enforcement would constitute a breach of Covenants not to Sue or licenses granted pursuant to this Agreement if such enforcement was undertaken by such Party and the [***] was part of the Territory, unless and until the [***] becomes part of the Territory pursuant to Section 15.21 or 15.22 (and Section 21.3 will thereafter apply to any such assignment or grant of rights).
(b)in [***] such that it entitles a Third Party or Designated Associated Company to enforce any such Relevant [***] IPR, where such enforcement would constitute a breach of Covenants not to Sue or licenses granted pursuant to this Agreement if such enforcement was undertaken by such Party and [***] was part of the Territory, unless and until [***] becomes part of the Territory pursuant to Section 15.23 or 15.24 (and Section 21.3 will thereafter apply to any such assignment or grant of rights).
For the avoidance of doubt, nothing in this Section 21.5 constitutes a Covenant not to Sue granted by either Party in the [***] unless and until, in each case, those countries become part of the Territory in accordance with Sections 15.21 to 15.24, as applicable.
21.6Any Product or group of related Products acquired from a Third Party by a Party (for the avoidance of doubt, including its Affiliates) after [***], which group of Products is acquired with respect to one or more countries (“Subject Countries”) by company acquisition, merger or asset purchase (but explicitly excluding licenses, co-promotion, co-marketing and distribution arrangements), (“Product Acquisition”), shall not benefit from the other Party’s IPR Obligations save that such Products shall be deemed to be Changed Products and will benefit from such other Party’s IPR Obligations solely with respect to the Subject Countries if:
(a)prior to the completion of such Product Acquisition, such Products have been Launched (mutatis mutandis) by such Third Party;
(b)in connection with such Product Acquisition the acquiring Party has acquired all rights of such Third Party in the Subject Countries to sell such Products;
(c)subsequent to the completion of such Product Acquisition, such Products shall be branded, labelled and packaged under the acquiring Party’s own brand;
(d)such Product Acquisition also includes the acquisition of legal title to one or more Patents;
(e)such Products would constitute Compliant Changed Product if developed by the acquiring Party, subject to the Draft Launch Notice required for Compliance of such Products being provided within [***] Business Days of completion of such acquisition. The Existing Product used as the [***] Product against which the acquired Product is assessed for Compliance is identified by the acquiring Party and stated in the Draft Launch Notice; and
(f)such Product Acquisition is completed prior to the expiry of the Term.
For the avoidance of doubt, if the other Party has or acquires entitlement to royalty payments with respect to such product line by virtue of some agreement other than this Agreement, such royalty payments pursuant to such other agreement shall [***] where such Products are deemed to be Changed Products under this Section 21.6.
21.7If a Party or its Affiliates disposes of legal title to all rights to sell a product line (other than to another Affiliate), whether by company disposal, merger, asset sale (but explicitly excluding licenses, co-promotion, co-marketing and distribution arrangements), that product line shall upon such completion of such disposal, including fulfilment of any conditions precedent, cease to benefit from the IPR Obligations, subject to Section 21.8.
21.8Subject to compliance with Section 21.3, each Signatory shall have the right to novate this Agreement in its entirety (including for the avoidance of doubt, the licence granted [***] pursuant to Section 3, to the extent any Patents subject to such licence are subject to the novation) provided that:
(a)it is in connection with a merger, acquisition or other sale or transfer of all or substantially all of the business, stock and assets related to both HNB Products and Vapour Products, and which includes all or substantially all of the related Intellectual Property Rights, and provided that, as a result of such transaction, such Party is no longer active in the business of HNB Products and Vapour Products;
(b)there shall be no novation prior to the [***] of the Effective Date;
(c)each of the party to which the Agreement is novated (“Incoming Party”) and the Signatory from which the Agreement is novated (“Outgoing Party”) shall continue to comply with the Standstill Exhibit with the remaining Signatory (“Remaining Party”), if the Standstill Exhibit is still in force at the date of novation;
(d)for the avoidance of doubt: (1) the novated Agreement shall apply to all applicable Intellectual Property Rights of the Incoming Party, not limited to the Intellectual Property Rights acquired by the Incoming Party from the Outgoing Party; and (2) to the extent that the Remaining Party and the Incoming Party are, at the date of novation, party to any proceedings which are within any Covenant not to Sue under this Agreement, they shall withdraw such proceedings in respect of any activities after the date of novation;
(e)such novation shall not relieve the Outgoing Party from any liabilities arising prior to such novation; and
(f)in the event that the Outgoing Party is Nicoventures Trading Limited and any Patents are retained by BAT, with effect from the date of the novation, BAT: (1) [***]; and (2) Covenants not to Sue for infringement of any such retained Patents on like terms to those granted in Sections 5, 6, 7, 11, 12, 13 and 24 save that:
(i)the products subject to such grant of rights shall be [***] the respective Sections 5, 11, 12, 13 and 24;
(ii)such grant of rights shall be [***]; and
(iii)[***] in respect of such grant of rights; and
(g)in the event that the Outgoing Party is Philip Morris Products S.A. and any Patents are retained by PMP, with effect from the date of the novation, PMP: (1) [***]; and; (2) Covenants not to Sue for infringement of any such retained Patents on like terms to those granted in Sections 5, 6, 7, 9, 11, 12, 13 and 24 save that:
(i)the products subject to such grant of rights shall be [***] the respective Sections 5, 9, 11, 12, 13 and 24;
(ii)such grant of rights shall be [***]; and
(iii)[***] in respect of such grant of rights;
(h)the Outgoing Party shall grant to the Remaining Party a Covenant not to Sue on like terms to that granted in Section 23, save that the term of such Covenant not to Sue shall be until the [***] anniversary of the Effective Date. For the avoidance of doubt, the expiry of such Covenant not to Sue is without prejudice to any licence or Covenant not to Sue in relation to the manufacture of Novation Items that is granted pursuant to Sections 21.8(f) and 21.8(g) above; and
(i)with effect from the date of the novation, the Outgoing Party, on a perpetual, [***] basis and in the Territory, Covenants not to Sue the Remaining Party for Patent infringement of any Patent that has an earliest priority date prior to the [***] anniversary of the date of novation (whether such Patent was filed by the Outgoing Party or acquired by the Outgoing Party) by any Part 1 Products, ARUs or their respective Components (in each case regardless of whether on the market prior to novation), of the Remaining Party.
22.SUBLICENSABLE INTELLECTUAL PROPERTY RIGHTS
22.1Except in relation to Intellectual Property Rights licenced or owned by Connected Persons (which are subject to Section 25.1 below) and subject to Section 22.2, insofar as a Party grants a Covenant not to Sue or a licence pursuant to this Agreement in respect of its Intellectual Property Rights, such Covenant not to Sue or licence shall, include, to the full extent permitted under the respective agreement or terms of [***], as amended from time to time by the parties thereto, or by changes in Applicable Law, and for the term of such agreement [***]:
(a)the [***] Sublicensable IPR;
(b)at the option of the grantee Party, and subject to the payment by the grantee Party of any payment or liability thereby incurred by the grantor Party, [***] Sublicensable IPR;
(c) [***] where that Party has the right to grant a licence or Covenant not to Sue under such Intellectual Property Rights [***] and without any obligation to pay, or liability for, any royalty, fee, or other payment or value by the grantor Party under such Intellectual Property Rights for, as a result of, or on the occasion of such licence or Covenant not to Sue, or exercise of any rights granted under such licence or Covenant not to Sue; and
(d)at the option of the grantee Party, and subject to the payment by the grantee Party of any payment or liability thereby incurred by the grantor Party, [***] where that Party has the right to grant a licence or Covenant not to Sue under such Intellectual Property Rights [***] and that are subject to the grantor Party having an obligation to pay, or liability for, a royalty, fee or other payment or value (other than nominal) by the grantor Party under such Intellectual Property Rights for, as a result of, or on the occasion of such licence or Covenant not to Sue, or exercise of any rights granted under such licence or Covenant not to Sue.
22.2The term of the Covenant not to Sue or licence granted by the grantor Party pursuant to Section 22.1 shall be for so long as the grantor Party has such rights in respect of [***].
22.3Patents included in any [***] shall not be taken into account in the calculation of any royalties under Section 18 of this Agreement or in any assessment of Compliance unless and until [***].
23.MANUFACTURING AND R&D COVENANT NOT TO SUE
23.1Each Party on a perpetual, royalty-free basis and in the Territory, Covenants not to Sue: (1) the other Party; and (2) solely to the extent that their acts relate to the Products, ARUs or their respective Components, of the other Party, the other Party’s Contractors, for Patent infringement by any Product (whether a Part 1 Product or a Part 2 Product), ARUs or their respective Components, in respect of:
(a)the manufacture [***] of any Products ARUs or their respective Components and, insofar as they are ancillary to such manufacture, [***];
(b)[***] or
(c)the research and development (“R&D”) of any Product (whether a Part 1 or a Part 2 Product), ARU or their respective Components, to the extent that it relates to R&D activities in relation to that Product [***].
For the avoidance of doubt, such R&D activities include but are not limited to: [***]
Nothing in this Section 23 shall limit the applicability of any research exemptions under any Applicable Law.
24.ACCESSORIES, REPLACEMENT PARTS, AND UPGRADE PARTS COVENANT NOT TO SUE
24.1Each Party on a perpetual, [***] basis and in the Territory, Covenants not to Sue the other Party for Patent infringement by any Accessory Sold for Use with a Part 1 Product by the other Party, or its Components.
24.2Each Party on a perpetual basis and in the Territory, Covenants not to Sue the other Party for Patent infringement by any Replacement Part Sold for Use with a Part 1 Product by the other Party, or its Components.
24.3For Existing Products, the Covenant not to Sue granted in Section 24.2 is on a [***] basis. For [***], the Launching Party shall pay to the Patent Owning Party a royalty on the [***] of Replacement Parts Sold for Use with Part 1 Products[***] at the royalty rate (if any) applicable to the Part 1 Product that the Replacement Part is Sold for Use with [***]. If the Replacement Part is Sold for Use [***], then the [***] of such Replacement Part[***] shall be allocated [***] in that [***], and the royalty rate (if any) for [***] of the Replacement Part. For example:
(a)[***]
(b)[***]
(i)[***]
(ii)[***]
(c)[***]
(d)[***]
24.4Each Party on a perpetual basis and in the Territory, Covenants not to Sue the other Party for Patent infringement by any Upgrade Part Sold for Use with a Part 1 Product by the other Party, or its Components, provided that [***].
The Upgrade Part shall be assessed for such qualification in accordance with the protocol for the assessment of Compliance in Section 16 including by provision of the email notification in accordance with, and containing the information set out in, Section 16.3, and the following information:
(a)[***]
(b)[***] and
(c)[***]
24.5The Launching Party shall pay to the Patent Owning Party a royalty on the [***] of Upgrade Parts Sold for Use with Part 1 Products, calculated in the same manner as royalties for Replacement Parts pursuant to Section 24.3. However, prior to the calculation, the Upgrade Part, [***] shall be assessed pursuant to Sections 16 and 17. If the Final Patent Report generated pursuant to Section 16 [***] contains Patents Families that are not in the Final Patent Report [***], then these further Patent Families relating to the Upgrade Parts are added to the respective Final Patent Reports [***], and then the royalty rate for [***] shall be re-determined pursuant to Section 18.
24.6For the purpose of application of the foregoing Sections 24.4 and 24.5, the following changes shall be deemed made to Sections 15, 16, 17 and 18:
(a)references to Launch replaced with references to [***] in the Territory [***]
(b)references to Devices and Consumables under assessment replaced with references to [***] and
(c)references to Product under assessment replaced with references [***]
25.THIRD PARTIES
25.1If a Party is a licensee or sublicensee or beneficiary of a Covenant not to Sue under any Patent that is owned [***] by that Party’s Connected Persons and that claims any aspect or feature of the other Party’s Part 1 Products or ARUs, and if such Party can, under the terms of its agreement with such Connected Person, grant a sublicense or extend a Covenant not to Sue thereunder to the other Party in connection with the other Party’s Part 1 Products or ARUs, then such Party shall grant, or use its Best Efforts to procure the grant of, such sublicense or Covenant not to Sue, as the case may be to the full extent permitted under such agreement, and such sublicensed Patents or Patents to which a Covenant not to Sue relates shall be treated under this Agreement as a Patent of such Party (subject to the terms and conditions of this Agreement) to the full extent permitted under such agreement.
If there are any royalties or other charges of any sort that are or become payable to a Connected Person due to the procuring of or exercise of such a sublicense or Covenant not to Sue, then, in order to receive the benefit of such a sublicense or Covenant not to Sue, the other Party shall either: (1) timely pay all such charges when they come due; or (2) promptly decline such sublicense or Covenant not to Sue. [***]
25.2If a Party has the right under any agreement with its Supply Chain Entities to cause any of its Supply Chain Entities not to sue the other Party or their respective Supply Chain Entities under any Patent over which a Supply Chain Entity has enforcement rights that would come within a Covenant not to Sue under this Agreement if owned by such Party, such Party will use Best Efforts to exercise that right, to the full extent permitted under such agreement.
25.3Each Party will use: (1) Best Efforts to ensure that its Supply Chain Entities are aware of the principles of this Agreement (including, without limitation, the Covenants not to Sue under this Agreement (as set forth in publicly available version of this Agreement filed with the relevant securities exchange (collectively, the “Principles”))); and (2) reasonable endeavours to encourage its Supply Chain Entities to respect such Principles to the fullest extent possible.
25.4If a Party to whom a Connected Person is connected becomes aware that such Connected Person has initiated, aided or abetted in any Connected Party Actions, such Party shall use Best Efforts to procure that such Connected Person withdraws and causes to be dismissed (on the basis that it makes no admissions) all such Connected Party Actions it has initiated, until the expiry of the Term or until it ceases to be a Connected Person, [***] “Connected Party Actions” means any action that would constitute a breach of any of the Covenants not to Sue contained in this Agreement if the Connected Person had given such Covenants not to Sue under the terms of this Agreement against the other Party in relation to Products. For the purposes of this Section 25 the reference to “Representatives” in the definition of “Connected Person” is limited to Representatives of a Party insofar as they act outside the course of their duties to that Party.
25.5Patents that are [***] shall not be taken into account in the calculation of any royalties under Section 18 of this Agreement or in any assessment of Compliance [***] For the avoidance of doubt, the Patents [***] (including Patents included in [***].
[***].
25.6Moreover, with respect to [***] Existing Products, the Released Claims Covenant not to Sue [***] shall extend to [***] Existing Products only for as long as 25.7In respect of BAT Changed Products and [***] the Parties shall meet after [***] and endeavour to agree (subject to prior agreement between PMP and [***]) on provisions to be appended to this Agreement regarding [***] and Changed Products of BAT vis-à-vis Intellectual Property Rights of [***] that may be applicable to [***] and/or any Changed Products of BAT.
26.INTELLECTUAL PROPERTY RIGHTS MANAGEMENT
Each Party shall, in its sole discretion, determine how it shall or shall not file, prosecute and maintain the Intellectual Property Rights in respect of which it provides releases, Covenants not to Sue or licences under this Agreement and shall owe no obligations pursuant to this Agreement to the other Party or any other Person in respect of the filing, prosecution and maintenance, renewal or enforcement against Third Parties of such Intellectual Property Rights.
27.TERM AND TERMINATION
27.1The Agreement will take effect from the Effective Date and remain in effect until the eighth (8th) anniversary hereof, subject to Sections 27.2 and 27.3 (the “Term”).
27.2Without affecting any other right or remedy available to it, either Signatory may terminate this Agreement with immediate effect by giving written notice to the other Signatory only if:
(a)the other Party is in breach of its obligations to cause the Proceedings to be fully and finally dismissed in accordance with Section 2.1 and/or Section 2.3;
(b)the other Party is in breach of the obligation to cause all injunctions to be discharged in accordance with Section 3.2;
(c)the other Signatory was in breach on the Effective Date of Section 29.2(a), 29.2(c), 29.2(d), 29.2(e) or 29.2(h) (if the breaching Signatory is Philip Morris Products S.A) or Section 29.3(a), 29.3(c), 29.3(d), 29.3(e) or 29.3(h) (if the breaching Signatory is Nicoventures Trading Limited), provided that:
(i) in the case of Sections 29.2(a) and 29.2(d) (insofar as such breach of Section 29.2(d) relates to the Released Claims in Sections 4.1(d), 4.1(e), 4.1(f) or 4.1(g)) if the breaching Signatory is Philip Morris Products S.A, and Sections 29.3(a) and 29.3(d) (insofar as such breach of Section 29.3(d) relates to the Released Claims in Sections 4.1(d), 4.1(e), 4.1(f) or 4.1(g)) if the breaching Signatory is Nicoventures Trading Limited, the breach is a material breach; and
(ii)in all cases pursuant to this Section 27.2(c), such notice is given within [***] months of becoming aware of such breach; or (d)the other Party is in repudiatory breach of this Agreement,
provided that, in each case, the non-breaching Party gives the breaching Party notice of such breach and the breaching Party fails to cure such breach within [***] Business Days of such notice. A breach of any of the warranties referred to in Section 27.2(c) will be considered cured for the purposes of this Section 27.2 if the other Party ensures that the non-breaching Party is put in materially the same position in all respects that it would have been had the warranty been true and does not suffer any material loss or damage as a result of such breach.
27.3Without affecting any other right or remedy available to it, Philip Morris Products S.A. may terminate this Agreement with immediate effect by giving written notice to Nicoventures Trading Limited if BAT breaches its obligations under the first sentence of Section 3.1, provided that, if the breach is remediable, Philip Morris Products S.A. gives Nicoventures Trading Limited notice of such breach and BAT fails to cure such breach within [***] Business Days of such notice.
27.4At the end of the Term (whether by termination or expiry):
(a)Sections 1, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15.1-15.19, 15.25-15.27, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 (except for 25.7) 28, 29.7-29.8, 32, and 38:
(i)Existing Products, [***] and Changed Products Launched prior to the end of the Term, for the avoidance of doubt, the reference in this Section 27.4(a)(i) to “Existing Product” includes both limbs (1) and (2) of that defined term;
(ii)Accessories and Upgrade Parts, in each case Sold for Use with Part 1 Products, which Accessories and Upgrade Parts are on the market prior to the end of the Term; and
(iii)Replacement Parts Sold for Use with Part 1 Product, which Part 1 Products are Launched prior to the end of the Term, regardless of whether the Replacement Parts are on the market prior to or after the end of the Term;
in each case, subject to any applicable on-going royalty obligations including all the provisions of this Agreement relating to the calculation of royalties, such continuing rights and obligations shall not continue beyond the [***] anniversary of the termination;
(b)both the release in Section 4 and the Released Claims Covenant not to Sue in Section 5 shall survive and continue in perpetuity in accordance with their terms, notwithstanding the termination or expiry of the Term and accordingly no such provision may be terminated by any rights including pursuant to any common law rights of repudiation or otherwise;
(c)the Covenant not to Sue in Section 23 shall survive and continue until the [***] anniversary of the Effective Date, notwithstanding the termination or expiry of the Term before that date and accordingly such provision may not be terminated earlier by any rights including pursuant to any common law rights of repudiation or otherwise;
(d)each Signatory shall be entitled to a copy of the content of the central repository maintained by the Advisory Committee pursuant to Section 36.1(b) and thereafter such central repository shall be deleted and closed;
(e)each Party shall promptly irretrievably destroy all Confidential Information of the other Party that was provided or made accessible pursuant to this Agreement, and any copy or manifestation thereof, and make reasonable endeavours to irretrievably delete any electronic copy of any of the foregoing that may remain in the possession or control of such Party (other than copies of such Confidential Information retained for purposes of abiding by such Party’s legal obligations and in complying with such Party’s established document retention policies (including the copy of the central repository referenced above), provided that such Party shall treat such retained Confidential Information in accordance with obligations imposed upon it by this Agreement), all except to the extent that and for so long as such Confidential Information is necessary for the exercise of any rights under this Agreement and/or the continuing licences and Covenants not to Sue under this Section 27.4 and/or to perform any obligations under this Agreement or any Applicable Law or regulatory requirement surviving the end of the Term and/or to resolve any ongoing dispute pursuant to this Agreement; and
(f)each Party shall certify, upon request by the other Party, to such other Party such Party’s compliance with its obligations under Section 27.4(e); and
(g)for the avoidance of doubt, Products Launched by either Party after the end of the Term shall not benefit from any licence or Covenant not to Sue under this Agreement other than pursuant to Sections 27.4(b) and 27.4(c).
27.5The following Sections of this Agreement will remain in effect notwithstanding expiry or termination of this Agreement: Sections 2, 3, 26, 27.4, 27.5, 27.6, 29.1-29.6 (solely until the [***] anniversary of such expiry or termination), 31, 34, 39, 40, and 41.
27.6Termination or expiry of this Agreement shall not affect any rights, remedies, obligations or liabilities of the Parties that have accrued up to the date of termination or expiry, including the right to claim damages in respect of any breach of the Agreement.
27.7For the avoidance of doubt neither Party may rescind the Agreement for misrepresentation other than fraud or fraudulent misrepresentation.
28.CONFIDENTIALITY
28.1All Confidential Information that is provided or made accessible by the Disclosing Party to the Receiving Party pursuant to this Agreement will be provided, made, accessible and/or disclosed only in accordance with Sections 28.2 through 28.5 (except that Section 28.2 shall not apply to Confidential Information that comprises the terms of this Agreement, other than the Patent Lists referenced below) and will be used solely to the extent necessary for such Receiving Party to perform any obligations or other activities of the Receiving Party under this Agreement and to exercise any licence and right that the Receiving Party gives or is granted or has, in or under this Agreement or for the purpose of resolving any dispute under this Agreement and will be preserved and maintained in confidence by the Receiving Party using at least the same measures it uses to protect its own Confidential Information, but no less than reasonable measures.
Notwithstanding anything to the contrary in the [***]: (1) the BAT [***] Patent Families and BAT [***] Patent Families shall be Confidential Information of BAT and PMP shall be deemed a Receiving Party of such Confidential Information; (2) the PMP [***] Patent Families and PMP [***] Patent Families shall be Confidential Information of PMP and BAT shall be deemed a Receiving Party of such Confidential Information; and (3) each such Receiving Party shall be permitted to use the foregoing pursuant to the terms and conditions of this Section 28. Each Signatory agrees that, for the Term, [***] in accordance with [***].
28.2Each Party shall provide the other Party, within [***] Business Days of the Effective Date, with a list of up to five (unless the Parties agree upon a different number) directors, officers or employees of that Party (whether employed directly or via a service company) nominated to receive Confidential Information of the other Party (a “Confidentiality Ring”). Each Representative within a Confidentiality Ring shall provide written confidentiality undertakings substantially in the form attached in Schedule 28.2, after which Confidential Information may be disclosed to such Representative. Each Party may change the members of its Confidentiality Ring on a one-in-one-out basis provided that the total number of members at any time shall not exceed five unless otherwise agreed. Each Party may disclose Confidential Information to its external legal advisers on confidentiality terms no less strict than those of this Agreement. [***] The Parties shall ensure that all disclosures of Confidential Information between permitted persons shall be made on a “need-to-know” basis only. However, each Party will be free to disclose the existence of this Agreement and the scope of the releases, licences and Covenants not to Sue granted in this Agreement to its Supply Chain Entities, officers, directors and agents.
28.3From and after the Effective Date, except as expressly permitted hereunder, no Party shall disclose the other Party’s Confidential Information without such Party’s written consent except: (1) as required by any Governmental Authority having jurisdiction and specifically requiring such disclosure; (2) in response to a witness summons, subpoena, court order or as otherwise may be required by law; (3) in confidence, to a Party’s (A) directors, (B) senior managers with responsibility for making decisions regarding the Agreement to the extent necessary for that purpose, (C) accountants, (D) legal counsel, (E) tax advisors and other financial, legal advisors and, (F) subject to the provisions of Section 28.2, its Confidentiality Ring [***]; (4) as required during the course of litigation or pursuant to the ADR Exhibit and subject to a confidentiality agreement, protective order or other appropriate confidentiality regime under the auspices of the relevant tribunal; (5) as required to be disclosed in connection with any filings, reports or disclosures that may be required to comply with the requirements of regulatory authorities or stock exchanges under Applicable Laws; (6) with obligations of confidentiality at least as stringent as those contained herein, to a counterparty in connection with a proposed merger, acquisition, disposal, financing or similar transaction or a proposed exclusive licence, sale or transfer of rights to any Patents under which a licence or Covenant not to Sue is granted under this Agreement; and (7) as required to enforce the terms of this Agreement; provided, however, that prior to any disclosure pursuant to paragraphs (1), (2), (4) or (5) herein, the Party making any such disclosure shall: (A) take reasonable actions in an effort to minimise the nature and extent of such disclosure; and (B) if permissible under Applicable Laws provide at least [***] Business Days advance written notice to the other Party of such disclosure, other than such known disclosure that the Parties are required to make on a quarterly, annual or ordinary course pursuant to the requirements of regulatory authorities or stock exchanges under Applicable Laws.
28.4The Parties shall develop Competition Law guidelines for required exchanges of information under this Agreement, including but not limited to the Confidentiality Ring, the Advisory Committee and any related meetings.
28.5Nothing in this Agreement shall: (1) prevent a Party from making a disclosure to, and co-operating with, (A) a regulator regarding any misconduct, wrongdoing or reportable breach of any regulatory requirement or (B) any law enforcement agency regarding any crime, or (2) impose obligations on the Parties that would be contrary to or impede compliance with their obligations under any Applicable Laws or regulatory requirements, provided however that this Section shall not have the effect of extending the scope of any Covenant not to Sue granted under this Agreement.
28.6The provisions of this Section 28 shall be in effect continuously during the Term and for a period of [***] years after the end of the Term.
29.WARRANTIES, DISCLAIMERS, AND DUTY TO MITIGATE
29.1Each of Philip Morris Products S.A. and Nicoventures Trading Limited warrants to the other that as of the Effective Date:
(a)it is a company duly incorporated and validly existing under the laws of its place of incorporation;
(b)it has the capacity and authority to enter into this Agreement;
(c)the person(s) entering into this Agreement on its behalf have been duly authorised to do so; and
(d)this Agreement and the obligations created hereunder are binding upon it and enforceable against it in accordance with their terms (subject to applicable principles of equity) and do not and will not breach the terms of any other agreement, or any judgment or court order, to which it is bound.
29.2Philip Morris Products S.A. warrants that:
(a)as of the Effective Date: (1) PMP is the exclusive owner of all right, title, and interest in and to all of the Patents in the PMP [***] Patent Families, the PMP[***] Patent Families and the PMP [***] Patent Families (other than as set out in Schedule 29.2), including all rights to claim and recover for alleged infringement thereof; and (2) PMP otherwise has all legal rights necessary to grant the licences, Covenants not to Sue, and releases provided for in this Agreement;
(b)as of the Effective Date it is unaware of any asserted or threatened dispute, claim or allegation, in each case made in writing, that any Third Party has any right, title, or interest in any Patents owned by PMP that are Patent Family Members of: (1) the PMP [***] Patent Families; (2) the PMP [***] Patent Families; (3) the PMP [***] Patent Families; and (4) any other Patent Families that are subject to the licences and Covenants not to Sue given under this Agreement, or any right to claim and recover for alleged infringement thereof;
(c)Philip Morris Products S.A. has the full right and authority to enter into this Agreement;
(d)as of the Effective Date, PMP has not transferred or assigned or purported to transfer or assign to any Person any action or right to bring an action with respect to any of the Released Claims;
(e)as of the Effective Date, PMP has not instituted any lawsuits or proceeding against BAT in any jurisdiction or forum in any court or tribunal in which an allegation of Patent infringement against Existing Products of BAT and/or other Products of BAT Launched after [***] but prior to the Effective Date is made, or any claims (for example unfair competition claims (which for the avoidance of doubt does not include Competition Law claims)) that arise from such patent lawsuit or proceedings (excluding Validity Challenges), or enforcement actions thereof, other than the Proceedings listed in Schedule 2.1(A) and Schedule 3.3;
(f)PMP has not between [***] and the Effective Date participated in any way (directly or indirectly) in any Intellectual Property Rights transaction in the Territory (as the Territory exists as of the Effective Date) the purpose of which is, or which BAT can reasonably demonstrate has or will have the effect of, avoiding or preventing extending to BAT or its Connected Persons, any material part of the benefit of any of the rights, licences, Covenants not to Sue or releases in the Territory hereunder, for the avoidance of doubt, a transaction will not have such purpose or effect merely because it promotes the commercial success of PMP for example distribution agreements of PMP and other agreements with its Connected Persons;
(g)as of the Effective Date PMP does not to the best of its knowledge Co-own any Patents in any Patent Family the earliest Patent Family Member of which was filed on or after [***] and does not to the best of its knowledge Co-own any registered trade marks, in each case which would, if wholly owned by PMP, be within any of the licences, Covenants not to Sue and releases provided for in this Agreement; (h)PMP has not between [***] and the Effective Date sought, assisted, aided or abetted in seeking, permitted entry of or enforcement of, Measures or permanent injunctions against BAT or its respective Connected Persons (with respect to any Products of BAT), or the respective Products of BAT, in relation to Patent infringement, save as listed in Schedule 2.1(A) and Schedule 3.3;
(i)as of [***] PMP has not sold any Existing Product, Existing Accessory, Existing Replacement Part, or Existing Upgrade Part, or any respective Component therefor under or by reference to the [***] of BAT excluding any reference to such [***] of BAT that is permitted under the Applicable Law (e.g., descriptive uses and permitted comparative advertising);
(j)the facts set out in Confidential Exhibit 3A are true and accurate in all material respects; and
(k)it is a wholly-owned subsidiary of Philip Morris International Inc..
29.3Nicoventures Trading Limited warrants that:
(a)as of the Effective Date: (1) BAT is the exclusive owner of all right, title, and interest in and to all of the Patents in the BAT [***] Patent Families, the BAT [***] Patent Families and the BAT [***] Patent Families (other than as set out in Schedule 29.3), including all rights to claim and recover for alleged infringement thereof; and (2) BAT otherwise has all legal rights necessary to grant the licences, Covenants not to Sue, and releases provided for in this Agreement;
(b)as of the Effective Date it is unaware of any asserted or threatened dispute, claim or allegation, in each case made in writing, that any Third Party has any right, title, or interest in any Patents owned by BAT that are Patent Family Members of: (1) the BAT [***] Patent Families; (2) the BAT [***] Patent Families; (3) the BAT [***] Patent Families; and (4) any other Patent Families that are subject to the licences and Covenants not to Sue given under this Agreement, or any right to claim and recover for alleged infringement thereof;
(c)Nicoventures Trading Limited has the full right and authority to enter into this Agreement;
(d)as of the Effective Date, BAT has not transferred or assigned or purported to transfer or assign to any Person any action or right to bring an action with respect to any of the of the Released Claims;
(e)as of the Effective Date, BAT has not instituted any lawsuits or proceeding against PMP in any jurisdiction or forum in any court or tribunal in which an allegation of Patent infringement against Existing Products of PMP and/or other Products of PMP Launched after [***] but prior to the Effective Date is made, or any claims (for example unfair competition claims (which for the avoidance of doubt does not include Competition Law claims)) that arise from such patent lawsuit or proceedings (excluding Validity Challenges), or enforcement actions thereof, other than the Proceedings listed Schedule 2.1(A) and Schedule 3.3;
(f)BAT has not between [***] and the Effective Date participated in any way (directly or indirectly) in any Intellectual Property Rights transaction in the Territory (as the Territory exists as of the Effective Date) the purpose of which is, or which PMP can reasonably demonstrate has or will have the effect of, avoiding or preventing extending to PMP or its Connected Persons, any material part of the benefit of any of the rights, licences, Covenants not to Sue or releases in the Territory hereunder, for the avoidance of doubt, a transaction will not have such purpose or effect merely because it promotes the commercial success of BAT for example distribution agreements of BAT and other agreements with its Connected Persons;
(g)as of the Effective Date BAT does not to the best of its knowledge Co-own any Patents in any Patent Family the earliest Patent Family Member of which was filed or after [***] and does not to the best of its knowledge Co-own any registered trade marks, in each case which would, if wholly owned by BAT, be within any of the licences, Covenants not to Sue and releases provided for in this Agreement;
(h)BAT has not between [***] and the Effective Date sought, assisted, aided or abetted in seeking, permitted entry of or enforcement of, Measures or permanent injunctions against PMP or its respective Connected Persons (with respect to any Products of PMP), or the respective Products of PMP, in relation to Patent infringement, save as listed in Schedule 2.1(A) and Schedule 3.3;
(i)as of [***] BAT has not sold any Existing Product, Existing Accessory, Existing Replacement Part, or Existing Upgrade Part, or any respective Component therefor under or by reference to the [***] of PMP excluding any reference to such [***] of PMP that is permitted under the Applicable Law (e.g., descriptive uses and permitted comparative advertising);
(j)the facts set out in Confidential Exhibit 3B are true and accurate in all material respects; and
(k)its ultimate parent undertaking and ultimate controlling party is British American Tobacco p.l.c..
29.4Any warranty expressed to be given “so far as [x] is aware” or “[x] is unaware” or “to the best of [x]’s knowledge”, or otherwise qualified by reference to the knowledge of Philip Morris Products S.A. or Nicoventures Trading Limited, shall mean the actual knowledge of:
(a)in the case of the warranties in Sections 29.2(g) and 29.3(g), [***] of the Parties (or equivalent positions) as of the Effective Date;
(b)in the case of the warranties in Sections 37.3(a)(ii)(2) and 37.4(a)(ii)(2), [***] of the Parties (or equivalent positions) as of the Effective Date; and (c)in the case of the warranties in Sections 29.2(b) and 29.3(b), [***] of the Parties (or equivalent positions) as of the Effective Date;
in each case after due, diligent and careful enquiry.
29.5If:
(a)Philip Morris Products S.A. was in breach of the warranties in Section 29.2(e) on the Effective Date or Nicoventures Trading Limited was in breach of the warranties in Section 29.3(e) on the Effective Date, without limiting the other Party’s remedies in respect of the same, such omitted proceeding shall be deemed to be listed in Schedule 2.1(A);
(b)Philip Morris Products S.A. was in breach of any of the warranties on the Effective Date in Section 29.2(a), 29.2(c), 29.2(d), 29.2(e) or 29.2(h) or Nicoventures Trading Limited was in breach of any of the warranties in Section 29.3(a), 29.3(c), 29.3(d), 29.3(e) or 29.3(h) on the Effective Date then, without limiting the other Party’s remedies in respect of the same (including rights pursuant to Section 30.2(a)) or the right to claim for any loss or damage, in contract or otherwise, the breaching Signatory shall indemnify and hold harmless the other Party in respect of (subject to Section 29.8) all losses, damages, costs, expenses, charges, penalties and other liabilities (including reasonable legal and other professional fees) incurred or suffered by the other Party arising out of or in connection with any such breach.
29.6With the sole exception of a Signatory’s representations and warranties expressly set forth in this Agreement, neither Signatory makes any representation or warranty or condition, and each Signatory hereby disclaims all representations and warranties and conditions of any kind, express, implied and statutory. Except solely for the representations and warranties in this Agreement, each Party grants any licence or Covenant not Sue by it “as is” and expressly disclaims any and all representations and warranties and conditions related to any and all Patents and other Intellectual Property Rights.
29.7Each Party must take reasonable steps and provide reasonable assistance to the other Party to avoid or mitigate any loss arising from a claim or any loss which, in the absence of mitigation, might give rise to a claim under, or in respect of breach of any provision of, this Agreement, and nothing in this Agreement shall restrict or limit such obligation.
29.8Subject to Section 29.7, in no event shall a Party be liable to the other Party or any Person claiming through the other Party for:
(a)any incidental, consequential, indirect, punitive, special or liquidated damages or losses, in each case under or in connection with this Agreement, unless such damages or losses:
(i)arise from a breach entitling the other Party to terminate this Agreement pursuant to Section 27.2 or 27.3, regardless of whether such right to terminate is exercised, and regardless of whether such damages or losses are recovered pursuant to an indemnity under this Agreement; (ii)arise from a Specified Breach, regardless of whether the Limitation Right is exercised, and regardless of whether such damages or losses are recovered pursuant to an indemnity under this Agreement; or
(iii)are recovered pursuant to the indemnity in Section 29.5(b);
(b)any liability that arises from or in connection with a beneficiary Party’s exercise of any rights under the Patents or other Intellectual Property Rights of the granting Party pursuant to a licence or Covenant not to Sue under this Agreement or any other rights pursuant to such licence or Covenant not to Sue; or
(c)any Product Claims,
provided that nothing in this Section 29.8 shall affect any liability for royalties payable pursuant to this Agreement.
29.9The Signatories hereby accept and acknowledge that in respect of each of the warranties given pursuant to this Agreement each of the disclosures set out in Schedules 29.2 and 29.3 shall be deemed fairly disclosed against and qualify each of those warranties, such that a breach of those warranties may not be asserted or claimed in respect of those matters and facts that are the subject of those disclosures.
30.LIMITATION RIGHT
30.1The following are the “Specified Breaches”:
(a)the bringing of any Disputes before any Governmental Authority in breach of Section 40.1;
(b)the seeking of Measures or permanent injunctions in breach of Sections 32.1 to 32.3; and
(c)any breach of Section 21.3, following which the assignee or licensee of a Party’s Patents seeks Measures or permanent injunctions in a manner that would, had such been sought by a Party, been in breach of Sections 32.1 to 32.3.
30.2If either Party commits any of the Specified Breaches (the “Breaching Party”) after the earlier of: (1) [***] Launch; or (2) the date that is [***] months after the Effective Date, then:
(a)if the other Party (the “Innocent Party”) gives the Breaching Party notice of such breach and the Breaching Party fails to cure such breach within [***] Business Days of such notice, then the Innocent Party may serve a further notice on the Breaching Party that the Innocent Party is exercising its right to limit the Breaching Party’s rights under the Agreement (the “Limitation Right”). If such further notice is not served within [***] Business Days after the expiry of the [***] Business Days period following the initial notice, the Innocent Party will be taken to have waived its right to exercise the Limitation Right.
(b)if the Innocent Party exercises the Limitation Right then from the date of such exercise:
(i)the rights of the Breaching Party under this Agreement shall be limited to those set out in Section 27.4 and the Breaching Party shall comply with the obligations set out in Section 27.4, mutatis mutandis;
(ii)[***]; and
(iii)Section 40.1 shall not prevent the bringing of proceedings in respect of the Post-Exercise Changed Products before any Governmental Authority;
but the Agreement shall otherwise continue to have full force and effect.
(c) a Party shall be entitled to challenge any purported exercise of the Limitation Right as an Expedited Dispute under the ADR Exhibit. Before such dispute is resolved pursuant to the ADR Exhibit the Limitation Right will not take effect. If it is finally determined pursuant to the ADR Exhibit that the Limitation Right was effectively invoked by the Innocent Party, then the Limitation Right will thereafter take effect [***] the date on which the Limitation Right was exercised by the Innocent Party.
(d)for the avoidance of doubt, a purported but wrongful exercise of the Limitation Right shall not, of itself, give rise to a right to terminate or constitute repudiation of this Agreement.
31.VALIDITY CHALLENGES, CHALLENGES TO THE ENTITLEMENT OF A PATENT AND CHALLENGES TO REGISTERABLE INTELLECTUAL PROPERTY RIGHTS
31.1This Agreement shall not prevent a Party, [***] Companies, or any of their Connected Persons, on or after the Effective Date from:
(a)continuing Validity Challenges whether or not the Patent is the subject of a licence or Covenant not to Sue (except to the extent that any such Validity Challenge is by way of counterclaim in any of the Proceedings and is to be terminated or discontinued as a direct consequence of the dismissal pursuant to Section 2.1) or recommencing, commencing or filing any Validity Challenge; or
(b)continuing any challenge to the registration of any registerable Intellectual Property Rights other than Patents whether or not such Intellectual Property Rights is the subject of a release, licence or Covenant not to Sue or recommencing, commencing or filing any challenge to the registration of any registerable Intellectual Property Rights other than Patents.
32.INJUNCTIONS AND PRE-ACTION COMMUNICATION
32.1With respect to Products (whether Part 1 Products or Part 2 Products and including Products under assessment) and ARUs, in each case Sold for Use with Products, (provided in the case of Accessories and Upgrade Parts that they are Sold for Use with Products during the Term), and Components of all the foregoing, the Parties shall not seek, assist, aid or abet in seeking, permit entry of, or enforcement of, Measures against each other based on Patent infringement by such Products, ARUs or Components. For the avoidance of doubt, with respect to Part 2 Products, subject to Section 32.2, this shall not affect the availability of permanent injunctions (or any enforcement measures resulting from such permanent injunctions) in connection with any such Part 2 Products and ARUs Sold for Use with Part 2 Products and Components of all the foregoing, unless the Parties agree otherwise in any Agreement Part 2.
32.2If either Party intends to seek a permanent injunction or any other remedy (except Measures with respect to the Products, ARUs and Components referenced in Section 32.1, which shall be governed by Section 32.1, not by this Section 32.2), based on Patent infringement in relation to a Device or Consumable Launched during the Term it believes to be a Part 2 Product, or an ARU Sold for Use with such a Part 2 Product or a Component of any of the foregoing, or an Out-of-Scope Item or component thereof (only to the extent used therein) it shall notify the other Party in writing. If the other Party within [***] Business Days of such notice, fails to: (1) dispute in writing that it is such an item; or (2) request a [***] Business Day moratorium to enable settlement discussions, the Party may seek such permanent injunction or other remedy. If the other Party does within[***] Business Days of such notice, dispute in writing that it is such an item, the Party shall not seek such permanent injunction or other remedy without first referring such dispute, as whether it is such an item, to be determined pursuant to the ADR Exhibit as an Expedited Dispute. If the other Party does within [***] Business Days of such notice request a [***] Business Day moratorium to enable settlement discussion, the Party shall not seek such permanent injunction or other remedy during such moratorium and the Parties shall during such period seek to settle the matter.
32.3For the avoidance of doubt nothing in this Agreement shall prevent either Party from seeking, assisting aiding or abetting in seeking, permitting entry of, or enforcement of, Measures or permanent injunctions, in respect of its Intellectual Property Rights other than Patents, except that the Parties shall not seek, assist, aid or abet in seeking, permit entry of, or enforcement of, Measures or permanent injunctions against each other in respect of its Intellectual Property Rights other than Patents to the extent such Intellectual Property Rights relate to Products, their respective ARUs, and the Components of such Products or ARUs, which in each case are the subject of the Released Claims or the Released Claims Covenant not to Sue.
32.4In any proceedings or Measures permitted under this Agreement, neither Party will rely upon the delay of the other Party, where such delay results from: (1) the actions of the Party against which the proceedings or Measures are instituted; or (2) compliance with any procedures under this Agreement.
32.5In relation to Part 2 Products, the provisions of this Section 32 are subject to the provisions of the Standstill Exhibit.
33.[***] STANDSTILL AGREEMENT
33.1The Parties hereby agree that:
(a)they shall comply with the provisions of the Standstill Exhibit;
(b)the [***]; and
(c)if there is any conflict between this Agreement (other than Section 32 and Section 37.6) and the Standstill Exhibit, this Agreement shall take precedence.
34.NO PRECEDENT
34.1Each Party acknowledges and agrees that this Agreement has been reached in order to settle existing and potential future disputes and has been heavily negotiated representing a comprehensive, unique and particularised resolution based on the distinctive characteristics of wide-ranging and long running disputes between the Parties in a broad range of rapidly evolving markets. The unique factors and context have resulted in this Agreement. As such, each Party agrees that none of the terms or conditions of this Agreement, including any royalty rates or any other consideration payable hereunder, can be deemed to be a precedent for any other agreement or transaction, nor can any such terms or conditions have any relevance whatsoever in any determination of the terms or conditions that might be adequate or appropriate for any other agreement or transaction, whether or not such agreement or transaction is between or among the Parties or involves any Third Party. Without in any way limiting the foregoing, each Party agrees that the royalty rates and other consideration payable hereunder reflects the comprehensive and Territory-wide nature of the Agreement, the number and diversity of Intellectual Property Rights and Products involved, each of the Parties’ unique situations in rapidly evolving markets, their current and perceived future Intellectual Property Rights portfolios including their geographic coverage, the Parties’ current and future ranges of Products which may come into consideration under this Agreement and no such royalty rate or any other consideration represents, shall be an indication of, or shall be used in the determination of, what constitutes or may constitute adequate, appropriate or reasonable consideration or an adequate, appropriate or reasonable royalty with respect to any particular Intellectual Property Right or product or any set of Intellectual Property Rights or products in any particular jurisdiction or set of jurisdictions worldwide. The Parties further agree that neither the specific terms nor the existence of the Agreement shall be taken as an indication that damages would be an adequate remedy for the infringement of the Parties’ Intellectual Property Rights.
34.2Use of the designations, “PMP [***] Patent Family”, “PMP [***] Patent Family”, “BAT [***] Patent Family”, “BAT [***] Patent Family”, “Additional Patent Family”, “[***]” and “[***]” in this Agreement is a definitional scheme used solely in this Agreement for convenience and in no way is meant to reflect value or any other attribute or quality of a Patent of the Party, and each Party agrees that such designations are solely for the purposes hereof, and cannot be used in connection with any proceeding or any other matter.
35.ANNOUNCEMENTS
35.1Subject to Sections 28.3, 35.2 and 35.3 neither Party shall make, or permit any person to make, any public announcement, communication or circular concerning the existence, subject matter or terms of this Agreement, the wider transactions contemplated by it, or the relationship between the Parties arising out of this Agreement, without the prior written consent of the other Parties (such consent not to be unreasonably withheld, conditioned or delayed).
35.2The Parties consent to the use of the Q&A and the issuance of Press Releases, in each case in the form in Schedule 35.2, from a date mutually agreed to by the Parties following the Effective Date.
35.3Each Party may make any public statements in response to questions by the press, analysts, investors or those attending industry conferences or analyst or investor conference calls, without prior notification to the other Party, so long as such statements are consistent with, and do not go beyond, any of the Press Releases or the Q&A (in each case in the form attached in Schedule 35.2), either Party’s relevant Form 8-K, 6-K or 20-F as applicable (including attachments) filed in connection with the Agreement, or any other statements agreed to jointly by the Parties pursuant to Section 35.1 above.
36.ADVISORY COMMITTEE
36.1Immediately following the Effective Date, the Parties shall establish an advisory committee to facilitate communication between the Parties relating to the Agreement (“Advisory Committee”). The Advisory Committee role shall include the following, within the parameters of this Agreement and the requirements of Competition Law:
(a)promotion of the performance of the obligations under this Agreement;
(b)the establishment and maintenance of an independently-hosted, virtual central repository, the cost of which shall be borne equally by the Parties, accessible to all Members of the Advisory Committee that includes at least the following information:
(i)current status of actions under Sections 15, 16, 17 and 18, with each Party being responsible for updating the same in relation to its actions;
(ii)Final Launch Notices;
(iii)Final Patent Reports; and
(iv)the respective Party’s Royalty Rate Records;
(c)consideration and recommendation to the respective Parties of amendments to the Agreement including:
(i) to correct what the Advisory Committee believes to be omissions or errors in, or unintended consequences of, the Agreement; (ii)to co-ordinate the instruction and remuneration of the Independent Accountant;
(iii)as to the frequency with which the Independent Accountant is required to verify the Parties’ royalty reports;
(iv)pursuant to Section 16.2;
(v)as to the definition of Non-Notifiable [***]; and
(vi)as to the timings specified in Sections 15, 16 and 17;
(d)consideration and recommendation to the respective Parties as to whether it would be beneficial for the Parties to develop, for the assistance of the Arbitral Tribunal, guidance as to the operation of this Agreement, and if so the matter that could be addressed in such guidance. This Agreement would take precedence over any such guidance; and
(e)determination of the form of the Non-Notifiable [***] report referenced in Section 16.7(b);
however, for the avoidance of doubt, the Advisory Committee’s role is an advisory one; the Advisory Committee shall have no power to amend the Agreement; amendment of the Agreement remains subject to Section 41.5.
36.2The Advisory Committee shall be established and run by the Parties as follows:
(a)The Advisory Committee shall comprise 6 members (“Members”) comprising an equal number of appointees from each of the Parties. All Members must also be members of a Confidentiality Ring throughout their tenure as Member. The initial members of the Advisory Committee shall be as follows:
|
|
|
|
|
|
| PMP Members |
BAT Members |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
(b)Each of the Parties shall be entitled to remove any Member appointed by it and to appoint any person to fill a vacancy arising from the removal or retirement of such Member. The Parties shall give each other prior written notice of any proposed changes in the identity of their Members.
(c)The Parties shall use reasonable endeavours to ensure that their appointed Members are of a level of expertise and seniority to deal with the issues that may arise for the Advisory Committee.
(d)The Advisory Committee shall meet following the Effective Date at intervals of not more than [***] and at any time upon the request of a Party in each case at dates and times to be mutually agreed.
(e)The meetings shall take place by video call unless agreed otherwise by the Parties in which case each Party shall bear its own travel and subsistence costs.
(f)The Parties shall alternately host the Advisory Committee meetings. At least [***] Business Days’ written notice of each proposed meeting of the Advisory Committee shall be given to each Member by the Party hosting the meeting.
(g)The quorum for meetings of Advisory Committee shall be 2 Members from each Party. Members may be represented at any meeting by another Member designated in writing by the absent Member.
(h)The Advisory Committee shall appoint from the Members a chairperson to chair meetings and a secretary to assist the chairperson. Such appointments shall alternate between the PMP Members and the BAT Members on a [***] basis. In the first instance, the chairperson shall be a PMP Member and the secretary shall be a PMP Member.
(i)The minutes of each meeting of the Advisory Committee shall be prepared by the secretary and retained in the central repository accessible to all Members. A copy of the minutes of each meeting shall be sent to each of the Members within [***] Business Days of each meeting.
37.DESIGNATED ASSOCIATED COMPANIES
37.1The rights and obligations under this Agreement, solely in relation to Designated Associated Companies, are amended pursuant to this Section 37.
37.2Each Signatory shall (and shall procure that each of its Associated Companies, other than a Designated Associated Company, shall):
(a)not take any action or omission that would (either directly or indirectly) result in:
(i)a Designated Associated Company of that Party (that is not an Identified PAC DAC) ceasing to be a [***] Company; or
(ii)an Identified PAC DAC of that Party obtaining rights to any Patents relating to [***], in each case as of the Effective Date; (including, in each case, (i)-(ii) by making its relevant Intellectual Property Rights or Products available to that Designed Associated Company);
(b)use Best Efforts to ensure that:
(i)each Designated Associated Company of that Party (that is not an Identified PAC DAC) remains a [***] Company
(ii)no Identified PAC DAC of that Party obtains any Patents relating to [***], in each case as of the Effective Date
(including, in each case, (i)-(ii) by exercising all voting rights, powers and other rights (direct and indirect) available to it);
(c)not disclose any Confidential Information of the other Party to a Designated Associated Company (or its Representatives) of that Party, except that a Party may disclose the terms of the Agreement (pursuant to the confidentiality terms of this Agreement) to any of those Designated Associated Companies of that Party in so far as it is necessary to do so to secure compliance with this Agreement by those Designated Associated Companies;
(d)ensure that its Representatives that have any Confidential Information of the other Party (including, for the avoidance of doubt, information about the terms of this Agreement other than the information about the terms of this Agreement disclosed pursuant to Section 37.2(c)), including members of its Confidentiality Ring, are not Representatives of any of its Designated Associated Companies and shall have no communications with Representatives of its Designated Associated Companies related to or concerning this Agreement, its subject matter, or matters pertaining to it; and,
(e)take any such action as is required to ensure that such Signatory and its Affiliates (excluding for the purposes of this sub-Section (e) its Designated Associated Companies) can prevent the making of, or procure the withdrawal of, any Prohibited Assertion Claim made or asserted by a New DAC of such Signatory, including through securing rights (enforceable against such New DAC) to procure the same;
provided that the foregoing obligations under subsections (a), (b) and (e) shall not oblige a Signatory to do anything that is unlawful.
37.3Nicoventures Trading Limited:
(a)warrants to PMP that: (1) the Associated Companies of BAT set out in Schedule 1.1.92 comprise the only Designated Associated Companies of BAT as at the Effective Date; and (2) as at the Effective Date, save for the Identified PAC DACs, each of those Designated Associated Companies:
(i)is not involved directly or indirectly in [***]; and
(ii)does not own (solely or jointly), and has no right to enforce (1) [***] or (2) as far as Nicoventures Trading Limited is aware, [***] subject to the Released Claims set out in Section 4.1(g); and
(b)undertakes to notify PMP of: (1) a Person becoming a New DAC and whether it is a [***] Company; or a Person ceasing to be a Designated Associated Company of BAT; and/or (2) a Designated Associated Company of BAT that is a [***] Company ceasing to be a [***] Company; and such notification being made in the case of (1) within [***] months of such change, and in the case of (2) by no later than [***] in the subsequent calendar year following such a change.
37.4Philip Morris Products S.A:
(a)warrants to BAT that: (1) the Associated Companies of PMP set out in Schedule 1.1.92 comprise the only Designated Associated Companies of PMP as at the Effective Date; and (2) as at the Effective Date, save for the Identified PAC DACs, to each of those Designated Associated Companies:
(i)is not involved directly or indirectly in [***]; and
(ii)does not own (solely or jointly), and has no right to enforce (1) [***]; or (2) as far as Philip Morris Products S.A is aware, [***] subject to the Released Claims set out in Section 4.1(g); and
(b)undertakes to notify BAT of: (1) a Person becoming a New DAC and whether it is a [***] Company, or a Person ceasing to be a Designated Associated Company of PMP; and/or (2) a Designated Associated Company of PMP that is a [***] Company ceasing to be a [***] Company; and such notification being made in the case of (1) within [***] months of such change, and in the case of (2) by no later than [***] in the subsequent calendar year following such a change.
37.5Each of PMP’s and BAT’s respective aggregate liability under or pursuant to this Agreement (in respect of every kind of liability including, but not limited to, liability in contract, tort (including negligence), misrepresentation, restitution or otherwise) for the actions or omissions of all of its Existing DACs and New DACs in so far as the relevant actions or omissions occur at a time at which such Person is a Designated Associated Company shall, subject to Section 37.7 below, be capped at [***] (the “DAC Cap”) For the avoidance of doubt, neither Signatory’s liability for a breach of Sections 37.2, 37.3, or 37.4 shall be subject to the DAC CAP.
37.6Subject to Section 37.9(c), each Signatory acknowledges and agrees that any action of any Designated Associated Company of the other Signatory, provided that the same is not solicited, procured or actively encouraged by a Signatory or its Associated Companies (excluding its Designated Associated Companies), cannot result in the termination of the Agreement or the termination or loss of any other rights or benefits of that other Signatory under this Agreement, including pursuant to Sections 9.3, 11.2, 27.2, 27.3 and 30.2.
37.7The DAC Cap shall not apply to, or (in whole or part) limit or exclude, a Party’s liability (including the liabilities of or for any of its Designated Associated Companies’ actions or omissions) in respect of:
(a)any of the following amounts, sums, liabilities or obligations arising under or in respect of the Agreement (and, for the avoidance of doubt, such amounts, sums, liabilities or obligations shall not count towards the DAC Cap):
(i)any royalties paid or to be paid (whether by the sale or manufacture by a Party, its Associated Companies, Designated Associated Company or otherwise) pursuant to this Agreement;
(ii)any other payments or expenses specified in this Agreement to be paid by a Party including payments in respect of Intellectual Property Rights owned [***] by third parties pursuant to Sections 22.1(b), 22.1(d) and 25.1, expenses payable pursuant to Section 16.9 ([***] Tests), Section 15.27(g) (PMP costs of assessment of [***]), Section 36 (operation of the Advisory Committee) and Schedule 19.7 (fees of Independent Accountant);
(iii)any taxes on the foregoing payments under Sections 37.7(a)(i) or 37.7(a)(ii) paid or to be paid by the paying Party; and
(iv)any interest on, additional damages, and enforcement costs incurred or awarded in respect of any of the foregoing amounts or payments due pursuant to the Agreement; and
(b)any liabilities or losses (1) in respect of fraud or fraudulent misrepresentation; (2) in respect of death or personal injury caused by or arising from negligence; or (3) to the extent or that cannot be excluded by Applicable Law.
37.8If:
(a)a Designated Associated Company (an “Enforcing DAC”) of a Party (such Party (excluding the Enforcing DAC) being a “Benefiting Party”) recovers damages or other financial compensation, payments or awards (including royalty awards, account of profits, recovery of legal costs or other penalties and payments) from the other Party (and/or its Affiliates, for the avoidance of doubt) (collectively the “Compensating Party”), by pursuing any claim or action against the Compensating Party which, if the same claim or action is pursued by the Signatory of the Benefiting Party, is or would be a breach of this Agreement, such as the Enforcing DAC asserting Patent rights that are the subject of a Covenant not to Sue under the Agreement (collectively “DAC Breaches”); or (b)the Benefiting Party (or any of its other Associated Companies, other than the Enforcing DAC) otherwise benefits economically or financially, directly or indirectly, from a DAC Breach by an Enforcing DAC of the Benefiting Party, including through the consequences of an injunction or seizure being granted against the Compensating Party;
then in either case of (a) or (b) any amount that is attributable to: (1) those damages or other financial compensation, payment or awards under Section 37.8(a) that a Benefiting Party or any of its other Associated Companies receives, is paid, or otherwise benefits from, through its investment in the Enforcing DAC; or (2) the economic or financial benefit of the Benefiting Party or any of its other Associated Companies derived under Section 37.8(b) then the Benefiting Party shall pay such amount (or an amount equivalent to such benefit) to the Compensating Party without delay, and in any event within [***] Business Days of the Benefiting Party receiving the same amounts, and payment shall be without prejudice to the Compensating Party’s other rights and remedies under this Agreement including those pursuant to Section 37.9 (but, for the avoidance of doubt, the Compensating Party shall not be entitled to recover damages more than once in respect of the same loss resulting from a DAC Breach).
37.9Without prejudice to the DAC Cap or Section 37.8, if an Enforcing DAC of a Benefiting Party asserts or brings any claim, proceeding or litigation in respect of or against a Compensating Party (or any Connected Persons of the Compensating Party, solely to the extent the actions of the Connected Person relates to the Products of the Compensating Party) for infringement of any Patents that are the subject of a Covenant not to Sue or a licence under this Agreement in respect of the Compensating Party’s (or, where applicable, its Connected Person’s) activities in so far as they are activities that are within the scope and/or grant of the Covenant not to Sue or licence so granted hereunder (such claims “Prohibited Assertion Claims”):
(a)the Benefiting Party shall use Best Efforts to procure that such Enforcing DAC promptly, and within [***] Business Days, withdraws and causes to be dismissed all such Prohibited Assertion Claims it has made or initiated [***] until the expiry of the Term or until it ceases to be an Associated Company, provided that the foregoing shall not oblige a Benefiting Party to do anything that is unlawful;
(b)the Benefiting Party shall, acting in good faith cooperate with the Compensating Party in respect of, and shall disclose to the Compensating Party, all material actions, steps and communications that the Benefiting Party has and is taking to fulfil its obligations under Section 37.9(a);
(c)notwithstanding the DAC Cap, if: (1) (A) a final injunction; (B) an interim injunction that was either granted or upheld following an inter partes hearing, or (C) an interim injunction that was obtained on an ex parte basis and which remains in place for [***] months [***], despite the Best Efforts of the Compensating Party to have it lifted, is secured against the Compensating Party (including any of its Affiliates) pursuant to or in respect of such Prohibited Assertion Claims (irrespective of compliance with Section 37.9(a)); or (2) such Prohibited Assertion Claims are not withdrawn and dismissed in accordance with Section 37.9(a), in any such case of (1) or (2):
(i)the Enforcing DAC of the Benefiting Party shall immediately cease to benefit from all Covenants not to Sue and licences granted by the Compensating Party for the remainder of the Term of the Agreement; and
(ii)notwithstanding anything else in this Agreement, provided that such Prohibited Assertion Claims have not been withdrawn and dismissed in accordance with Section 37.9(a), the Compensating Party shall be thereafter entitled to assert against the Benefiting Party [***] Patents [***] (irrespective of being within the same Patent Families) as have been asserted by the Enforcing DAC in the Prohibited Assertion Claims in that Product Category. The [***] Patents permitted to be asserted under this Section 37.9(c)(ii) may be asserted in:
(A)the same jurisdiction in which the Prohibited Assertion Claims have been asserted in respect of or against the Compensating Party (“Country X”); or
(B)in any other single jurisdiction (“Country Y”), provided that [***]. For the purposes of this Section 37.9(c), in the event that audited financial statements are not available for a relevant jurisdiction for the relevant Product Category, [***] in accordance with that Party’s internal policies and procedures.
For clarity, the right to assert Patents pursuant to this Section 37.9(c)(ii) includes bringing proceedings before any Governmental Authority, and for the further avoidance of doubt, including by seeking Measures and permanent injunctions; and upon the occurrence of any Prohibited Assertion Claim, notwithstanding the provisions of Sections 28 and 35 of this Agreement, the Compensating Party and the Benefiting Party shall each be entitled to issue public communications in response to such Prohibited Assertion Claim regarding its rights under this Agreement, including in relation to any steps taken to assert any Patents against the Enforcing DAC and/or Benefiting Party, pursuant to this Section 37.9.
38.SANCTIONS
38.1The Parties agree to comply with Sanctions in the performance of this Agreement. No licence, Covenant not to Sue, indemnity or other rights granted by either Party under this Agreement shall be effective to the extent this is or would be in breach of Sanctions for the grantor.
38.2No Party shall be required to perform any obligation under this Agreement, and no Party shall be liable for failure to perform or delay in performing any obligation under this Agreement, to the extent that such performance would be in breach of Sanctions or such Party reasonably considers (acting in good faith) that such performance would expose the Party or any of its Affiliates to a risk of breach, penalty or other punitive consequences under Sanctions.
38.3To the extent that Sanctions are imposed which prevent performance by a Party in accordance with Section 38.2, the Parties agree to, within a reasonable period, negotiate in good faith a practical solution in a manner that is compliant with Sanctions.
39.GOVERNING LAW
This Agreement and any dispute or claim (including non-contractual disputes or claims) arising out of or in connection with it or its subject matter or formation shall be governed by and construed in accordance with the law of England and Wales, subject to the provisions of section 5 of the licence entered into in the form set forth in Schedule 3.1.
40.JURISDICTION
40.1All Disputes, shall be resolved in accordance with the ADR Exhibit, which shall be the sole, final, and exclusive procedure for the resolution of any such Dispute. The Parties irrevocably consent to and accept the exclusive jurisdiction of the courts of England and Wales in respect of any other dispute, controversy or claim arising out of or in connection with this Agreement. For the avoidance of doubt this shall not prevent the parties from seeking Measures from other Governmental Authorities where the seeking of such Measures is permitted under this Agreement.
40.2For the avoidance of doubt:
(a)in resolving such matters under the ADR Exhibit, the Arbitral Tribunal (as defined in the ADR Exhibit) shall apply the mechanisms set forth in this Agreement including those set forth in Sections 15 to 18;
(b)any determination of the Arbitral Tribunal as to whether a Patent is Enforceable or a product is Within the Scope of a Patent, shall not affect questions of Patent validity, enforceability or infringement beyond this Agreement.
41.GENERAL
41.1No Other Rights. No rights are granted under this Agreement, by implication, estoppel, statute or otherwise, except as expressly set forth herein.
41.2Further Assurances. Each Party shall, at its own cost, promptly execute and deliver all such documents, and do all such things, as the other Party may from time to time reasonably require for the purpose of giving full effect to the provisions of this Agreement and to secure for the other Party, its Connected Persons, the full benefit of the rights, powers, and remedies conferred upon it under this Agreement.
41.3Entire Agreement and Remedies.
(a)This Agreement sets out the entire agreement between the Parties relating to the subject matter hereof and, save to the extent expressly set out in this Agreement, supersedes and extinguishes any prior drafts, agreements, undertakings, representations, warranties, promises, assurances and arrangements of any nature whatsoever, whether or not in writing, relating thereto. Except as otherwise expressly provided herein, this Agreement and all rights and obligations hereunder are granted and become effective as of, and only as of, the Effective Date.
(b)Notwithstanding Section 41.3(a) above and Section 41.3(e) below, this Agreement does not affect or override: (1) the [***] (2) any executed agreement between the Parties relating to the Parties’ ownership or use of Intellectual Property Rights other than Patents.
(c)Notwithstanding Section 41.3(a) above and Section 41.3(e) below, the Parties acknowledge and agree that this Agreement shall govern any Confidential Information disclosed on or after the Effective Date; to the extent it relates to any Confidential Information not governed by this Agreement, the [***] shall not be affected by this Agreement.
(d)The rights, powers, privileges, and remedies provided in this Agreement are cumulative and not exclusive of any rights, powers, privileges, or remedies provided by law.
(e)If there is any conflict between the terms of this Agreement and any other agreement entered into between the Parties in relation to the Products, this Agreement will prevail unless:
(i)such other agreement expressly states that it overrides this Agreement in the relevant respect; and
(ii)the Parties hereto are either also parties to that other agreement or otherwise expressly agree in writing that such other agreement will override this Agreement in that respect.
(f)This Agreement does not exclude any liability for, or remedy in respect of, death or personal injury caused by Party’s negligence, fraud or fraudulent misrepresentation or for any liability or remedy in so far as the same may not be excluded or limited by law.
(g)Each Party acknowledges that:
(i)it has not entered into this Agreement in reliance upon, and it will have no remedy in respect of, any misrepresentation, representation or statement (whether made by a Party or any other Person) which is not expressly set out in this Agreement; and
(ii)the only remedies available for any misrepresentation or breach of any representation or statement which was made prior to entry into this Agreement and which is expressly set out in this Agreement will be for breach of contract to the exclusion of all other rights and remedies (including those in tort or arising under statute).
41.4Waiver. Save as expressly set out in this Agreement, a failure or delay by a Party to exercise any right or remedy provided under this Agreement or by law, whether by conduct or otherwise, will not constitute a waiver of that or any other right or remedy, nor will it preclude or restrict any further exercise of that or any other right or remedy; and no single or partial exercise of any right or remedy provided under this Agreement or by law, whether by conduct or otherwise, will preclude or restrict the further exercise of that or any other right or remedy, except where otherwise specifically provided in this Agreement. Save as expressly set out in this Agreement, a waiver of any right or remedy under this Agreement will only be effective if given in writing and will not be deemed a waiver of any subsequent breach or default.
41.5Variation. No variation or amendment of this Agreement will be valid unless it is in writing and duly executed by or on behalf of each of the Signatories to this Agreement. Unless expressly agreed, no variation or amendment will constitute a general waiver of any provision of this Agreement, nor will it affect any rights or obligations under or pursuant to this Agreement which have already accrued up to the date of variation or amendment, and the rights and obligations under or pursuant to this Agreement will remain in full force and effect except and only to the extent that they are varied or amended.
41.6Invalidity. Where any provision of this Agreement is or becomes illegal, invalid, or unenforceable in any respect under the law of any jurisdiction then such provision will be deemed to be severed from this Agreement and, if possible, replaced with a lawful provision which, as closely as possible, gives effect to the intention of the Parties under this Agreement and, where permissible, that will not affect or impair the legality, validity, or enforceability in that, or any other, jurisdiction of any other provision of this Agreement.
41.7Invalidity or Ineffectiveness of Covenants not to Sue. Without prejudice to the generality of Section 41.2 and Section 41.6, if any Covenant not to Sue granted pursuant to this Agreement is illegal, invalid, ineffective or unenforceable in any respect under the law of any jurisdiction then the granting Party shall grant to the other Party a non-exclusive licence in such jurisdiction which as closely as possible gives effect to the intentions of the Parties in respect of such Covenant not to Sue. A Covenant not to Sue granted pursuant to this Agreement will be deemed to be ineffective to the extent it relates to [***] of the underlying Intellectual Property Rights is entitled to sue a Party to whom such Covenant not to Sue is granted, [***].
41.8No Grant of Rights Related to [***]. Notwithstanding any other provision of this Agreement, PMP does not grant pursuant to this Agreement to BAT, its Representatives or Supply Chain Entities, any release, licence, Covenant not to Sue, waiver or other right in respect of any product with, or that is Usable with another product or products and results [***], including without limitation pursuant to Section 23 or Section 32.
41.9No Partnership or Agency. Nothing in this Agreement is intended to, or will be deemed to, establish any partnership or joint venture between any of the Parties, make any Party the agent of another Party, or authorise any Party to make or enter into any commitments for or on behalf of any other Party.
41.10Notices. Any notice or other communication given under this Agreement or in connection with the matters contemplated herein will, except where otherwise specifically provided, be between Philip Morris Products S.A. and Nicoventures Trading Limited, including, for the avoidance of doubt, notices and communications pursuant to the ADR Exhibit; and shall be in writing in the English language, addressed as provided in Section 41.11 and served:
(a)by sending it by internationally recognised courier in which case it will be deemed to have been given upon delivery to that address;
(b)by email, in which case it will be deemed to have been given when dispatched subject to confirmation of delivery by a delivery receipt,
provided that in the case of sub-Section (b) above any notice despatched other than during the Working Hours at the destination will be deemed given at the start of the next period of Working Hours.
41.11Notices. Notices under this Agreement will be sent to the attention of the Persons and to the addresses or email addresses, subject to Section 41.12, as set out below:
For Philip Morris Products S.A. and its Affiliates where this Agreement specifies that notice is to be sent to the Assessment Representative:
Name: Philip Morris International
For the attention of: [***]
Address: Quai Jeanrenaud 5,Neuchatel 2000, Switzerland
E-mail address: [***]
with a copy to:
Name: Philip Morris International
For the attention of: [***]
Address: Quai Jeanrenaud 5,Neuchatel 2000, Switzerland
E-mail address: [***]
For Nicoventures Trading Limited and its Affiliates where this Agreement specifies that notice is to be sent to the Assessment Representative:
Name: British American Tobacco
For the attention of: [***]
Address: Globe House, 1 Water Street, London, WC2R 2PG
E-mail address: [***]
with a copy to:
Name: British American Tobacco
For the attention of: [***]
Address: Globe House, 1 Water Street, London, WC2R 2PG
E-mail address: [***]
For Philip Morris Products S.A. and its Affiliates for all other notices:
Name: Philip Morris International
For the attention of: [***]
Address: Avenue du Rhodanie 50, Lausanne Vaud 1001, Switzerland
E-mail address: [***]
with a copy to:
Name: Philip Morris International
For the attention of: [***]
Address: Quai Jeanrenaud 5,Neuchatel 2000, Switzerland
E-mail address: [***]
For Nicoventures Trading Limited and its Affiliates for all other notices:
Name: Nicoventures Trading Limited
For the attention of: [***], Head of Patents
Address: Globe House, 1 Water Street, London, WC2R 3LA
E-mail address: [***]
with a copy to:
Name: Nicoventures Trading Limited
For the attention of: [***]
Address: Globe House, 1 Water Street, London, WC2R 3LA
E-mail address: [***]
41.12Changes to Notice Address. Philip Morris Products S.A. and Nicoventures Trading Limited may notify each other of any change to its address or other details specified in the foregoing Section provided that such notification will only be effective on the date specified in such notice or 5 Business Days after the notice is given, whichever is later.
41.13Rights of Third Parties. A Person who is not a party to this Agreement will have no right under the Contracts (Rights of Third Parties) Act 1999 to enforce any of its terms, except, however, that the respective Affiliates and Connected Persons of Philip Morris Products S.A. and Nicoventures Trading Limited, will have such rights in relation to the respective rights granted to them pursuant to this Agreement, provided that such Affiliates and Connected Persons enforce their respective rights in accordance with the ADR Exhibit. The rights of Philip Morris Products S.A. and Nicoventures Trading Limited to rescind or vary this Agreement are not subject to the consent of their respective Affiliates and Connected Persons or any other Person.
41.14Indemnities. The indemnified Party shall inform the indemnifying Party promptly after becoming aware of any Third Party claim subject to indemnification pursuant to Section 1.2, 21.4 or 29.5(b) and shall give the indemnifying Party [***] Business Days to secure the complete withdrawal of such claim upon terms that impose no liabilities or restrictions upon the indemnified Party. Thereafter the indemnified Party shall retain full conduct of the claim save that the indemnified Party shall take reasonable steps to mitigate its liabilities in respect of any such claim and shall not settle any such claim on terms which oblige the indemnifying Party to make any payment to the indemnified Party under this indemnity without the prior written consent of the indemnifying Party, such consent not to be unreasonably withheld, conditioned or delayed.
41.15Construction; Language. As used in this Agreement, the words “include” and “including” and variations thereof will not be deemed to be terms of limitation, but rather will be deemed to be followed by the words “without limitation”. The headings in this Agreement and paragraph (I) of the “Background” at the beginning of this Agreement or any of its Exhibits (which has been included only to assist in orientating the reader of the Agreement) will not be referred to in connection with the construction or interpretation of this Agreement. This Agreement is in the English language only, which language shall be controlling in all respects, and all notices under this Agreement shall be in the English language.
For the avoidance of doubt, where brands names are used in this Agreement this is for convenience and any changes in brand names shall not affect the rights or obligations of the Parties. All references to the singular shall include the plural where the context so admits and vice versa, unless indicated to the contrary. The Schedules and Exhibits to this Agreement form part of this Agreement and shall have effect as if set out in full in the body of this Agreement. Any reference to this Agreement includes the Schedules and Exhibits to this Agreement. As used in this Agreement, the words “Patent infringement” include without limitation direct, indirect, induced and contributory infringement.
41.16Calculation of Time Periods. In relation to calculating any time period under this Agreement: (1) any period of time is calculated exclusive of the day from which the time period is expressed to run or the day upon which the event occurs which causes the period to start running; (2) where the day on or by which anything is to be done is not a Business Day, that thing must be done on or by the next Business Day; (3) where a time period is expressed in months or years, this means calendar months or years, such that, subject to (2) above and (4) below, the deadline on or by which anything is to be done is the same day of the subsequent month or year; and (4) where a deadline would expire on a date that does not exist in a subsequent month, then the deadline on or by which anything is to be done is the first Business Day in the following month.
41.17Signature
(a)This Agreement may be executed in any number of counterparts. Each counterpart will constitute an original of this Agreement but all the counterparts together will constitute but one and the same instrument.
(b)This Agreement shall be valid, binding and enforceable against a Party only when executed and delivered by an authorised individual on behalf of the Party by means of:
(i)a DocuSign® or other electronic signature;
(ii)an original, manual signature; or
(iii)a faxed, scanned or photocopied manual signature,
and each DocuSign® or other electronic, faxed, scanned or photocopied manual signature shall for all purposes have the same validity, legal effect and admissibility in evidence as an original manual signature and the Parties hereby waive any objection to the contrary.
(c)Within 5 Business Days of execution of this Agreement: (1) PMP shall provide to BAT a letter in the form set out in Confidential Exhibit 3A signed by Philip Morris Products S.A., and (2) BAT shall provide to PMP a letter in the form set out in Confidential Exhibit 3B signed by Nicoventures Trading Limited.
41.18Force Majeure. Neither Party shall be in breach of this Agreement nor liable for delay in performing, or failure to perform, any of its obligations under this Agreement if such delay or failure result from events, circumstances or causes beyond its reasonable control.
In such circumstances the time for performance of the Party affected by such events, circumstances or causes shall be extended by a period equivalent to the period during which performance of the obligation has been delayed or failed to be performed and the unaffected Party may also extend the time for its performance in so far as it is affected by the other Party’s delay.
41.19English law terms and legislation. An England and Wales legal term for any action, remedy, method of judicial proceeding, legal document, legal status, court, official or any legal concept or thing includes, in respect of any jurisdiction other than England, a reference to what most nearly approximates in that jurisdiction to the England and Wales legal term and a reference to any legislation includes, in respect of any jurisdiction other than England and Wales, a reference to any legislation of that jurisdiction that most nearly corresponds to the legislation referred to.
[Rest of Page Intentionally Left Blank; Signature Page Follows]
In Witness Whereof, BAT and PMP have executed this Agreement by their duly authorised representatives below on the date specified at the beginning of this Agreement:
|
|
|
|
|
|
|
Signed by Zafar Aslam Khan
for and on behalf of
Nicoventures Trading Limited
|
/s/ ZAFAR ASLAM KHAN
(Signature of authorized person)
|
|
Signed by Ralf Wittenberg
for and on behalf of
Nicoventures Trading Limited
|
/s/ RALF WITTENBERG
(Signature of authorized person)
|
Authorised signatory of Philip Morris Products S.A.:
Signed: /s/ EMMANUEL BABEAU
Emmanuel Babeau, Authorised Representative
Date: 1 February 2024
Authorised signatory of Philip Morris Products S.A.:
Signed: /s/ MICHELE CATTONI
Michele Cattoni, Authorized Representative
Date: 1 February 2024
SCHEDULE 1 - DEFINITIONS
1.1“Accessories” means, an ancillary item Sold for Use with a Product including [***]. For the avoidance of doubt, Accessories shall exclude: (1) Devices; (2) Consumables; (3) Replacement Parts; (4) Upgrade Parts; (5)[***]; and (6) Packaging.
1.2“Acquired IPR” has the meaning given in Section 21.1(b).
1.3“[***]” has the meaning given in Section 10.1.
1.4“Additional Patent Family” has the meaning given in Section 10.3.
1.5“ADR Exhibit” means the alternative dispute resolution mechanism between the Parties at Exhibit A.
1.6“ADR Procedure” has the meaning given in the ADR Exhibit.
1.7“Advisory Committee” has the meaning given in Section 36.1.
1.8“[***] Test Method” means: (1)[***]. References in this Agreement to the requirements for the outcome of testing with the [***] Test Method take into account the variance within the [***] Test Method and no further allowance shall be made for such variance.
1.9“[***] Tests” means the tests referenced in Sections 9.5(c), 9.5(d), 16.4, 16.9, 16.10 and 16.11.
1.10“Affected Product” has the meaning given in 16.2(c)(i).
1.11“Affiliates” means Associated Companies, however [***] Companies of the Parties, are deemed not to be Affiliates of the respective Parties.
1.12“Agreement Part 2” means any future agreement between the Parties relating to Part 2 Products.
1.13“Applicable Law” means all laws, statutes, rules, orders, regulations, by-laws, ordinances, subordinate legislation, judgments, decisions, decrees, and other pronouncements having the effect of law of any national, multinational, regional, state, provincial, county, city or other political subdivision, agency or other body, domestic or foreign, including recognised stock exchanges, to the extent any of the foregoing is applicable to a Party, its Connected Persons or any other Person in any jurisdiction in connection with its activities under this Agreement, including Sanctions.
1.14“ARUs” means Accessories, Replacement Parts, and Upgrade Parts.
1.15“Assessment Representative” has the meaning given in Section 41.11.
1.16“Associated Companies” means any Person, from time to time during the Term, which, directly or indirectly through one or more intermediaries, Controls, is Controlled by or is under common Control with a Party provided that such Person shall be deemed an Associated Company of such Party only during the period that such Control exists.
1.17[***]
1.18“Base Product” has the meaning given in Section 1.288 of this Schedule.
1.19“BAT [***] Patent Families” means those Patent Families [***].
1.20“BAT [***] Patent Families” means those patent families [***].
1.21“BAT [***] Patent Families” means all Patent Families which include one or more [***] Patents owned by BAT.
1.22“[***] HNB Market Re-entry” has the meaning given in Section 15.23.
1.23“[***] Vapour Market Re-entry” has the meaning given in Section 15.24.
1.24“Benefiting Party” has the meaning given in Section 37.8(a).
1.25“Best Efforts” means, with respect to a Person, diligent, reasonable, and good-faith efforts to accomplish the applicable objective not less than the level of effort a similarly situated Person would expend to achieve the same objective for its own account. Best Efforts do not require such Person to enter into any litigation, arbitration or other legal or quasi-legal proceeding.
1.26“Breaching Party” has the meaning given in Section 30.2.
1.27“Business Day” means a day other than Saturday, Sunday or any day on which commercial banks located in London, United Kingdom or the Canton of Vaud, Switzerland are authorised or obligated by Applicable Law to close.
1.28“Category 1 Representative” has the meaning given in Section 15.8(a)(ii)(B)(a).
1.29“Category 2 Representative” has the meaning given in Section 15.8(a)(ii)(B)(b).
1.30“Challenged PMP [***] Patent Family” has the meaning given in Section 17.10(a).
1.31“Challenged PMP [***] Patent Family” has the meaning given in Section 17.10(a).
1.32“Changed Product [***]” means a group comprising all Devices and Consumables that are Part 1 Products that are, or have been, [***], excluding from the group any Existing Products. For example: (1) for a Device that is a Changed Product the group is: [***]. However, solely for the purpose of the Changed Project [***] Family:
(a)in relation to a Vapour Product other than a [***] Product, the Changed Product [***] may (in addition to the above Devices and Consumables) also include [***] already existing Changed Products that are Vapour Products, one or more of which may be Vapour [***] Products;
(b)in relation to a Vapour [***] Product, it means a group comprising that Vapour [***] Product and [***] already existing Changed Products that are Vapour Products, one or more of which may be Vapour [***] Products; and
(c)in relation to a HNB [***] Product, solely for the purposes of the Changed Product [***] Family, it means a group comprising all HNB [***] Products that are Changed Products;
and “Changed Product [***] Members” shall be interpreted accordingly; all Changed Product [***] Members of a Changed Product [***] are within the same Product [***].
1.33“Changed Product Inter-operable” means in relation to: (1) any Changed Product which is a Device, that such Device when tested with a Consumable of each Consumable Family with which its Previous Device was [***], generates [***] generated by the Previous Device with such Consumables, as measured according to the [***] Method; and (2) any Changed Product which is a Consumable, that such Consumable [***].
1.34“Changed Product [***] Family” has the meaning given in Section 15.15.
1.35“Changed Products” means [***] Changed Products, Other HNB Changed Products and Vapour Changed Products.
1.36“Co-owned” means co-owned, disregarding any co-ownership arising from PCT applications or international trade mark applications with co-applicants. For the avoidance of doubt, a right is not “Co-owned” where there is split ownership in a right, such that one Person owns such right in one jurisdiction and another Person owns the equivalent right in a different jurisdiction. “Co-owned” shall be interpreted accordingly.
1.37“Compatible” means, in relation to a Consumable and a Device, that they are for use together by consumers: (1) as demonstrated by [***] (2) [***] as demonstrated by [***]; or (3) as otherwise demonstrated [***].
1.38“Compensating Party” has the meaning given in Section 37.8(a).
1.39“Competition Law” means competition law and antitrust law, including laws regulating anti-competitive agreements, anti-competitive practices, cartels, monopoly or abuse of a dominant position, and merger control, including but not limited to the United States antitrust laws, including the Federal Trade Commission Act, the Sherman Act, and the antitrust laws of each state, Articles 101 and 102 of the Treaty on the Functioning of the European Union, Chapters 1 and 2 of the United Kingdom Competition Act 1998, and other similar national, regional, or local law.
1.40“Compliance Reply” has the meaning given in Section 16.11.
1.41“Compliant” in relation to: (1) [***], has the meaning in Section 9.5; (2) [***] Changed Products, has the meaning set out in Section 11.3; (3) Other HNB Changed Products, has the meaning set out in Section 12.2; and (4) Vapour Changed Products, has the meaning set out in Section 13.2. Compliance shall be interpreted accordingly.
1.42“Component” means, in relation to a Product or ARU, a component of that Product or ARU, only to the extent used in that Product or ARU.
1.43“Condition” has the meaning given in Sections 15.21, 15.22, 15.23 or 15.24, as applicable.
1.44“Confidential Information” means the terms of this Agreement, and information of any form, whether oral or written, including documents, electronically stored information, and tangible things, that is disclosed by the Disclosing Party to the Receiving Party, including via the Advisory Committee, and that: (1) relates to the subject matter of this Agreement and (2) concerns the Disclosing Party and its products, prototypes, proofs of concept, operations, research and development efforts, Intellectual Property Rights, computer software, plans, intentions, market opportunities, processes, recipes, formulae, vendor and customer relationships, finances and other business operations and affairs, and any other information that, by its nature, the Receiving Party should reasonably know is confidential, and all electronic and tangible embodiments of such information, in each case of (1) to (2), above, whether or not marked or otherwise indicated as being confidential or proprietary and whether delivered by the Disclosing Party in writing, orally, visually and/or in other materials, or manners, including through the Receiving Party’s access to premises, equipment or facilities of the Disclosing Party, or by oral communication with Representatives of the Disclosing Party and all copies, excerpts, summaries, compilations, information, findings, data or analysis derived from the foregoing. For the purposes of this Agreement, the Confidential Information of a Party’s Connected Persons that is disclosed by the Disclosing Party to the Receiving Party is the Confidential Information of such Party. Confidential Information does not include information that the Receiving Party can show by reasonable contemporaneous evidence: (A) was in the public domain at the time it was communicated to the Receiving Party or thereafter becomes generally available to the public other than as a result of the public use, disclosure, or fault of the Receiving Party, (B) was available to the Receiving Party on a non-confidential basis prior to its disclosure to the Receiving Party by the Disclosing Party, (C) becomes available to the Receiving Party on a non-confidential basis from a source other than the Disclosing Party or any of its Connected Persons, prior to the public use or disclosure by the Receiving Party provided that such source is not bound by a confidentiality agreement or otherwise prohibited from transmitting the Confidential Information by a contractual legal or fiduciary obligation, or (D) is developed or acquired by the Receiving Party independently of any Confidential Information communicated to the Receiving Party by the Disclosing Party. The fact that the Parties or their Connected Persons are involved in any dispute resolution procedure in accordance with the terms of this Agreement will be considered Confidential Information of both Parties.
1.45“Confidentiality Ring” has the meaning given in Section 28.2.
1.46“Connected Party Actions” has the meaning given in Section 25.4.
1.47“Connected Person” means a Party’s Supply Chain Entities and their Representatives.
1.48“Consumable” means any substrate, together with any wrapper or housing therefor, including any electronics, filtration or cooling elements or any other parts that are, or are to be, integrated when sold to and used by a consumer with the wrapper or housing, wherein the substrate is intended to be heated to generate an aerosol for inhalation. Where a Consumable is marketed or sold as multiple Components that are to be combined together by the consumer, such Components together are the Consumable. For the avoidance of doubt, [***], when sold to a consumer and when in operation, will be part of that Consumable [***].
1.49“Consumable Family” means a Consumable, and any variants of that Consumable which: [***]. Within a Consumable Family, the Consumable and such variant Consumables: [***].
1.50“[***]” means a [***] of [***] that is used in [***].
1.51“Contractors” has the meaning given in Section 21.1(a).
1.52“Control” (including correlative meanings, the terms “Controlled by” and “under common Control with”) as used with respect to a Person means: (1) direct or indirect ownership of fifty percent (50%) or more of the voting securities or other voting interest of any Person; or (2) the possession, directly or indirectly, of the power to direct or cause the direction of the management, affairs and policies of such Person, whether through ownership of voting securities, by contract, as a general partner, as a manager, or otherwise.
1.53“[***] Patent” means a [***] Patent – HNB or a [***] Patent – Vapour that is not a [***] Patent.
1.54“[***] Patent” means a [***] Patent – HNB or a [***] Patent – Vapour in each case wherein the [***] is solely implemented by means of [***] of a Device.
1.55“[***] Patent – HNB” means any Patent of a Party claiming a [***] comprising one or more of the following technologies: [***].
1.56“[***] Patent – Vapour” means any Patent of a Party claiming a [***] comprising one or more of the following: [***].
1.57“[***] Patents” means [***] Patents and [***] Patents.
1.58“Country X” has the meaning given in Section 37.9(c)(ii)(A)
1.59“Country Y” has the meaning give in Section 37.9(c)(ii)(B).
1.60“Covenant not to Sue” means a covenant not to sue or bring or maintain or join in any other action, claim, suit, or proceeding in any forum, or otherwise assist, aid, abet or otherwise cooperate with any other Person to do any of the foregoing and “Covenants not to Sue” shall be interpreted accordingly.
1.61“DAC Breaches” has the meaning given in Section 37.8(a).
1.62“DAC Cap” has the meaning given in Section 37.5.
1.63“[***] Launched” means, in relation to a Product, that at any time prior to [***] the Product: (1) was readily available in at least one country in the Territory for consumers, who are independent of a Party and its Supply Chain Entities, to purchase from a Party or its Supply Chain Entities; and (2) (A) [***], and (B) had been provided by the Party, to its Supply Chain Entities which are not its Affiliates, in each case excluding any supply which is solely for the purpose of gifting or consumer testing.
1.64“De-listed” has the meaning given in Section 18.9 and “De-listing” shall be interpreted accordingly.
1.65“Designated Associated Company” means, in respect of a Party at any given time, an Associated Company of that Party in respect of which, at that time, that Party [***] of such Associated Company, whether through [***];
1.66“[***]” has the meaning given in Section 15.27(b) and “[***]” shall be interpreted accordingly.
1.67“[***] Features” has the meaning given in Section 15.27(d)(iii).
1.68“[***] Changed Product” has the meaning given in Section 15.27(d)(iii).
1.69“[***] Patent Family” has the meaning given in Section 15.27(d)(iii).
1.70“[***] Reply” has the meaning given in Section 15.27(d).
1.71“Device” means a means an electrically powered device that is involved in or affects aerosol production, other than a Consumable, which is Usable with a Consumable. Where such Device is marketed or sold as multiple Components that are to be combined together by the consumer, such Components together are the Device. For example, where a Device is a “two-part Device”, wherein one Component is a built-in charger unit, then both Components together are the Device. For the avoidance of doubt an [***] does not form part of the Device but is part of the Consumable.
1.72“Disclosing Party” means the Party or its Representatives disclosing information to the Receiving Party.
1.73“Dispute” has the meaning given in the ADR Exhibit.
1.74“Divisional” means, with respect to a Patent, a divisional, continuation, continuation-in-part, reissue, substitution, extension or addition thereof.
1.75“Draft Launch Notice” has the meaning given in Section 16.3.
1.76“Draft Patent Report” has the meaning given in Section 17.3.
1.77“Earlier Product” has the meaning given in Section 15.13.
1.78“[***] Changed Product” means a Changed Product that is Launched before [***] Launch, that has a Device or Consumable of [***] or [***] Changed Product as a [***] Product.
1.79“Effective Date” means the date on which all counterparts of this Agreement are duly executed by the Signatories or, if the counterparts are not executed on the same day, the date on which the last such counterpart is duly executed.
1.80A Patent claim is “Enforceable” for the purposes of this Agreement if, as a matter of the governing law as determined under this Agreement: (1) such Patent claim would not be found to be invalid; (2) such Patent claim would not be found to be unenforceable; (3) the Person seeking to assert such Patent claim is Entitled to such Patent claim; and (4) in relation to [***] Patents, the patent is in full force and effect. A Patent is “Enforceable” with respect to a Product for the purposes of this Agreement if the Product is Within the Scope of an Enforceable claim of that Patent. “Enforceability” shall be interpreted accordingly.
1.81“Enforcing DAC” has the meaning given in Section 37.8(a).
1.82A Person is not “Entitled” to a Patent or Patent claim (or lacks “Entitlement” thereof) for the purposes of this Agreement if the Person does not have, as a matter of the governing law of such Patent or Patent claim, sufficient right, title and interest in such Patent or Patent claim to assert such Patent or Patent claim, including if the Person is not the true owner of the Patent.
1.83“Established” means in relation to a Changed Product of an HNB Product, that at any point [***] after Launch, as proven by the Launching Party to the Patent Owning Party with contemporaneous evidence: (1) (A) the Changed Product has been the subject of a bona fide marketing campaign [***] and (B) the Changed Product is readily available for consumers, who are independent of a Party and its Supply Chain Entities, to purchase from a Party or its Supply Chain Entities,[***] or (2) the Party or its Supply Chain Entities has [***] and (B) the Party has provided, to its Supply Chain Entities which are not its Affiliates, more than: [***]. In the case of each of (1) and (2), the Changed Product must have been available to consumers for a continuous period of [***] months [***], provided that the Changed Product has in fact been Launched in [***]. For the purpose of this definition, a Consumable “unit” is a not a pack or carton; it is a single Consumable. [***] “Establish” and “Establishment” shall be interpreted accordingly.
1.84“Excepted Claims” has the meaning given in Section 4.2(a).
1.85“[***]” has the meaning given in Section 10.1.
1.86“[***]” has the meaning given in Section 10.1.
1.87“Excluded Changes” means: (1) a change resulting in a substantially different [***]; (2) a change which results in the Consumable ([***]); (3) a change from [***] Consumable to [***] Consumable (or vice versa); (4) a change to a Device resulting in [***]; or (5) a change from a Device [***] to Consumable [***] (or vice versa).
1.88“Excluded Claims” has the meaning given in Section 15.11.
1.89“Excluded Patent Families” has the meaning given in Section 15.9.
1.90“Excluded Patent Family Members” has the meaning given in Section 15.10.
1.91“Existing Accessories” means the Accessories for Existing Products: (1) which Accessories have been [***]; and (2) identical to those described in (1) if manufactured, marketed, distributed or sold after [***] (prior to, during or after the Term).
1.92“Existing DAC” means each Designated Associated Company of a Party that is listed in Schedule 1.1.92, as applicable.
1.93“Existing Product [***]” means: (1) in relation to a Consumable that is an Existing Product, that Consumable and all Consumables that have been [***], that are [***] with the same Device or Devices as that Consumable, which Device or Devices have been [***]; and (2) in relation to a Device that is an Existing Product, that Device and all Devices that have been [***], that are [***] with the same Consumable or Consumables as that Device, which Consumable or Consumables have been [***].
1.94“Existing Products” means the Products: (1) that have [***], as listed in Schedule 1.1.94; and (2) identical to those described in (1) if manufactured, marketed, distributed or sold after [***] (prior to, during or after the Term).
1.95“Existing Replacement Parts” means Replacement Parts for Existing Products: (1) which Replacement Parts have [***]; and (2) identical to those described in (1) if manufactured, marketed, distributed or sold after [***] (prior to, during or after the Term).
1.96“Existing Upgrade Parts” means Upgrade Parts for Existing Products: (1) which Upgrade Parts have been [***]; and (2) identical to those described in (1) if manufactured, marketed, distributed or sold after [***] (prior to, during or after the Term).
1.97“Expedited Dispute” has the meaning given in the ADR Exhibit.
1.98“Final Launch Notice” has the meaning given in Section 17.7.
1.99“Final Patent Report” has the meaning given in Section 17.7.
1.100“First Appellate Level” means, in relation to a Validity Challenge: (1) where the first instance hearing is before a national court (including the Unified Patents Court) or a national patent office, the relevant first appellate court, [***], as applicable; or (2) where the first instance hearing is before the European Patent Office, the Technical Boards of Appeal.
1.101“First [***] Sale” has the meaning given in Section 9.4.
1.102“First Instance Level” means, in relation to a Validity Challenge: (1) where before a national court (including the Unified Patents Court) or a national patent office, the first instance court, in particular, the [***] as applicable; or (2) where the first instance hearing is before the European Patent Office, the opposition Division.
1.103“[***] Sublicensable IPR” means, with respect to a Party, that such Party has licensed such Intellectual Property Rights from [***] under such licence or Intellectual Property Rights [***] by such Party under such licence or Intellectual Property Rights for, as a result of, [***] or exercise of any rights granted [***].
1.104“[***]” means [***] and/or [***]Changed Product (including [***]).
1.105“[***] Changed Product” means any [***] Changed Product of BAT.
1.106“[***] Changed Product Field of Use” has the meaning given in Section 11.2.
1.107“[***] Section 18 Products” has the meaning given in Section 15.27(e)(ii).
1.108“[***] Patent Family Number” has the meaning given in Section 10.5(a).
1.109“Governmental Authority” means any national, regional, local or other governmental, or administrative body, instrumentality, department or agency, any court, tribunal, administrative hearing body, commission or other similar dispute-resolving panel or body or any other self-regulatory authority, and any Person acting on behalf of any of the foregoing but excluding the Arbitral Tribunal.
1.110“Gross Revenue” means, in relation to a Product, reported gross revenue from Third Parties in connection with the sale, distribution or any related export or import of that Product.
1.111“[***] Companies” means any Associated Company of a Party so long (1) as more [***] of that Associated Company’s Turnover is generated through [***], from time to time, and (2) that Associated Company [***].
1.112“Health and Wellness Product” means a product anywhere in the Territory [***], one or more of: (1) a medicinal product; (2) a medical device; (3) a product available only by prescription; or (4) a product marketed, distributed and sold to: (A) diagnose, prevent or treat medical conditions; (B) improve health; or (C) maintain or encourage a general state of health or a healthy activity; and (5) ingredients and components for each of (1) to (4).
1.113“HNB Consumable” means a Consumable with a non-liquid, nicotine-containing or non-nicotine-containing substrate (including gels, with or without tobacco plant material) that is: (1) only Usable with an HNB Device, a [***] Device or a [***] Device [***] in which a nicotine-containing or non-nicotine-containing, non-liquid substrate (including gels, with or without tobacco plant material) is heated, and not burned, to generate an aerosol intended for inhalation; or (2) heated and not burned by heat derived from [***] (a “HNB [***] Product”); but in the case of (1) and (2) excluding products that are exclusively [***]; or (3) a co-existing variant of (1) or (2) that is provided as a product line extension of (1) or (2) [***]a nicotine-containing or non-nicotine-containing aerosol[***], even where such variant would otherwise be considered a [***].
1.114“HNB Device” means: (1) a Device that is only Usable with HNB Consumables, excluding products that are exclusively [***] and (2) a co-existing variant of (1) that is provided as a product line extension of (1) [***] a nicotine-containing or non-nicotine-containing aerosol [***] even where such variant would otherwise be considered a [***].
1.115“HNB [***] Product” means an Other HNB Changed Product that is within limb (2) of the definition of HNB Consumable.
1.116“HNB Product” means an HNB Device, HNB Consumable (including a HNB [***] Product), [***] Device or [***] Device.
1.117“[***] Device” means: (1) a Device that is only Usable with either (at the election of a consumer on each use) of an HNB Consumable or a Vapour Consumable [***] in which a substrate is heated to generate an aerosol intended for inhalation, excluding products that are exclusively [***]; and (2) a co-existing variant of (1) that is provided as a product line extension of (1) [***] nicotine-containing or non-nicotine-containing aerosol [***] even where such variant would otherwise be considered a [***].
1.118“[***] Device” means: (1) a Device that is only Usable (at the election of the consumer) with either of, or simultaneously both of, a HNB Consumable and/or Vapour Consumable [***] in which a substrate is heated to generate an aerosol intended for inhalation, excluding products that are exclusively [***]; and (2) a co-existing variant of (1) that is provided as a product line extension of (1) [***]such variant to form a nicotine-containing or non-nicotine-containing aerosol [***] even where such variant would otherwise be considered a [***].
1.119“Identified” means identified in Schedule 1.1.94 either: (1) as a stock keeping unit; or (2) as a type of product and brand family together with technical attributes which may be combined in different combinations to form such Existing Product. If identified in accordance with (2), then [***]; and “Identify” shall be interpreted accordingly.
1.120“Identified PAC DACs” means each Existing DAC of a Party that is listed in Schedule 1.1.92 in the case of BAT or Schedule 1.1.92 in the case of PMP, as applicable.
1.121“[***]” means the HNB Products that are (a) Devices identified in the PMI HNB Devices section of Schedule 1.1.94 as [***] and (b) the Consumables identified in the PMI HNB Consumables section of Schedule 1.1.94 as being as being of the ‘[***].
1.122“[***] Changed Product” means any [***] Changed Product of PMP.
1.123“[***] Changed Product” has the meaning given in Section 11.3.
1.124“Incoming Party” has the meaning given in Section 21.8(c).
1.125“Independent Accountant” has the meaning given in Schedule 19.7.
1.126“[***]” means such Devices and Consumables within Notified [***] that have been determined to be Compliant in accordance with this Agreement.
1.127“[***] Consumable” has the meaning given in Section 9.5(h).
1.128“[***] Consumable Set” has the meaning given in Section 9.5(h).
1.129“[***]Covenant not to Sue” has the meaning given in Section 9.2.
1.130“[***] Devices” has the meaning given in Section 9.5.
1.131“[***] Field of Use” has the meaning given in Section 9.3.
1.132“[***] Launch” means, as established by BAT to PMP with contemporaneous evidence: (1) all of[***] is the subject of a bona fide marketing campaign [***]; and (2) all of [***] is readily available for consumers, who are independent of BAT and its Supply Chain Entities, to purchase from BAT or its Supply Chain Entities, [***]; and (3) BAT or its Supply Chain Entities has [***].
1.133“[***] Launch Notice” has the meaning given in Section 16.3.
1.134“[***] Royalty” has the meaning given in Section 10.5(c).
1.135“[***] Family” has the meaning given in Section 15.16.
1.136“Innocent Party” has the meaning given in Section 30.2(a).
1.137“Intellectual Property Rights” means Patents, rights to inventions, copyright and neighbouring and related rights, trade marks and service marks, business names and domain names, rights in get-up and trade dress, goodwill and the right to sue for passing off or unfair competition, rights in designs, rights in computer software, database rights, rights to use, and protect the confidentiality of, confidential information (including know-how and trade secrets) and all other intellectual property rights, in each case whether registered or unregistered and including all applications and rights to apply for and be granted, renewals or extensions of, and rights to claim priority from, such rights and all similar or equivalent rights or forms of protection which subsist or will subsist now or in the future in any part of the Territory.
1.138“International Phase” has the meaning given in Section 15.4.
1.139“[***]” has the same meaning as “[***]” as understood under the law of England and Wales specifically in patent law. Furthermore, where reference is made to the “[***]” (or “[***]”)
of a [***] Patent or [***], then this is the [***] of the independent claim (or the [***] of each of the independent claims) of that [***] Patent or [***]. To the extent there is any disagreement between the Parties as to that meaning, then such dispute is resolved under the ADR Exhibit and by the Arbitral Tribunal considering the relevant [***] Patent in light of the case law of the courts of England and Wales clarifying the meaning of “[***]”.
1.140“Involuntary Transfer” has the meaning given in Section 21.5.
1.141“IPR Obligations” has the meaning given in Section 21.3.
1.142“[***] Devices” means the Devices listed in [***] Schedule 1.1.94 as being the [***].
1.143“ITC” has the meaning given in Section 3.1.
1.144“[***]” means [***], a corporation incorporated under the laws of [***], and its Associated Companies.
1.145“[***] Existing Products” means Products manufactured by or on behalf of [***] and distributed by PMP, as set forth in Schedule 1.1.94, including [***].
1.146 “Later Product” has the meaning given in Section 15.13.
1.147“Launch” means relation to a Changed Product, the point at which, as proven by the Launching Party to the Patent Owning Party with contemporaneous evidence: (1) the Changed Product is the subject of a bona fide marketing campaign [***]; and (2) the Changed Product is readily available for consumers, who are independent of a Party and its Supply Chain Entities, to purchase from a Party or its Supply Chain Entities, [***]; and (3) the Party or its Supply Chain Entities has (A)[***], and (B) the Party has provided, to its Supply Chain Entities which are not its Affiliates, more than [***] in the case of Devices and [***] in the applicable Consumable Family in the case of Consumables. [***]. A Consumable “unit” is a not a pack or carton; it is a single Consumable. References in this definition to a “Changed Product” include any other Changed Product which differs from a Changed Product solely by virtue of being a Non-Notifiable [***]. In relation to [***].
1.148“Launch Notice” means a Draft Launch Notice or a Final Launch Notice.
1.149“Launching Party” has the meaning given in Section 16.3.
1.150“Limitation Right” has the meaning given in Section 30.2(a).
1.151“Limited Territory” means worldwide excluding the [***].
1.152“Measures” means any form of summary, preliminary, provisional or interim relief (however described), seizure or sequestration of property granted by any Governmental Authority, including without limitation, preliminary injunctions and temporary restraining orders.
1.153“Members” has the meaning given in Section 36.2(a).
1.154“[***].
1.155“[***] Consumable” means a Consumable of a Party [***] is Usable with any Device of such Party to enable a consumer to have [***].
1.156“[***]” means, in relation to a Party for an applicable jurisdiction, [***] of the applicable Product where either: (1) the market of legitimate retail sale of the Product is the applicable jurisdiction or; (2) [***] the Product will be or has been sold to consumers in the applicable jurisdiction, [***] in the applicable jurisdictions, less:
(a) [***]; and
(b) [***];
[***]”.
1.157“New DAC” means a Person that becomes a Designated Associated Company after the Effective Date.
1.158“[***]” means a [***] of Replacement [***] that is not a [***] or a [***].
1.159“[***] Patent” means a [***] Patent – HNB that is not a [***] Patent or a [***] Patent – Vapour that is not a [***] Patent.
1.160“[***] Patent” means a [***] Patent – HNB or a [***] Patent – Vapour in each case wherein the [***] is solely implemented by means of [***] of a Device.
1.161“[***] Patent – HNB” means any Patent of a Party claiming a technology relevant to an HNB Product or component thereof that is not a [***] Patent - HNB. [***].
1.162“[***] Patent – Vapour” means any Patent of a Party claiming a technology relevant to a Vapour Product or component thereof that is not a [***] Patent – Vapour. [***].
1.163“[***] Patents” means [***] Patents and [***] Patents.
1.164“Non-Notifiable [***]” has the meaning given in Section 16.7.
1.165“[***] Companies” means any Associated Company of a Party for so long as: (1) that Associated Company is not involved directly or indirectly in [***]; and (2) that Associated Company does not [***], and has no right to [***].
1.166“[***]” is pseudonym for a group of future HNB Products, all as provided in the [***] Launch Notice.
1.167“Novation Items” means: (1) Existing Products, [***] (if [***] Launch occurs on or before [***]) and Changed Products (if Launched prior to the novation); (2) Accessories and Upgrade Parts, in each case Sold for Use with Part 1 Products, which Accessories and Upgrade Parts are on the market prior to the novation; and (3) Replacement Parts Sold for Use with Part 1 Product, which Part 1 Products are Launched prior to the novation, regardless of whether the Replacement Parts are on the market prior to the novation.
1.168“Omitted Consumable” has the meaning given in Section 8.2.
1.169“Omitted Device” has the meaning given in Section 8.1.
1.170“[***]” means HNB [***] Products and Vapour [***] Products.
1.171“Other HNB Changed Product” has the meaning given in Section 12.2.
1.172“Other Injunctions” has the meaning given in Section 3.2.
1.173“Outgoing Party” has the meaning given in Section 21.8(c).
1.174“Out-of-Scope Item” means any product which is not a Product or ARU or Component of any of the foregoing.
1.175“Packaging” means, in respect of a Product or ARU or Component of any of the foregoing, the internal and external packaging [***].
1.176“Part 1 Products” means Existing Products, [***] and Changed Products.
1.177“Part 2 Products” means all Products excluding Part 1 Products.
1.178“Party” and “Parties” have the meaning given in Section 1.3.
1.179“Patent Family” means all Patents (including any granted patents issuing from currently pending patent applications) existing from time to time in any jurisdiction in the Territory and that are derived from a single PCT application, or claiming the benefit of priority from a common priority-giving right or any Patents that have one or more common PCT applications or priority claims (including the relevant priority-giving right or PCT application) and “Patent Family Member” means any Patent within a Patent Family.
1.180“Patent Owning Party” has the meaning given in Section 16.3.
1.181“Patent Report” has the meaning given in Section 17.3.
1.182“Patents” means the following, and all national equivalents of the following, in any and all jurisdictions in the Territory: (1) all classes or types of patents, including utility patents, utility models, statutory invention registrations, innovation patents, invention certificates, re-examinations, reissues, extensions, renewals and all other post-grant variations thereof but excluding design patents and registered designs; and (2) all applications (including provisional and non-provisional applications), continuations, divisionals, continuations-in-part, extensions, supplementary protection certificates, renewals and re-examinations, rights to inventions and all other pre-grant variations thereof for which applications may be filed in any country or jurisdiction in the Territory.
1.183“Paying Party” has the meaning given in Section 18.2.
1.184“Person” means any individual, partnership, joint venture, corporate or unincorporated body, firm, trust, association, Governmental Authority or any other entity not specifically listed herein, and includes a Party and its Representatives.
1.185“PMP [***] Patent Family” means those Patent Families [***]
1.186“PMP [***] Patent Family” means those Patent Families [***]
1.187“PMP [***] Patent Families” means all Patent Families which include one or more [***] Patents owned by PMP.
1.188“Post-Exercise Changed Products” has the meaning given in Section 30.2(b)(ii).
1.189“PPD” means a process and/or product description prepared in accordance [***].
1.190“Pre-Arbitration Procedure” has the meaning given in the ADR Exhibit.
1.191“Previous Device” means a Device which is a [***] Product and “Previous Consumable” means a Consumable which is a [***] Product.
1.192“Principles” has the meaning given in Section 25.3.
1.193“Proceedings” means all proceedings documented in Schedule 2.1(A).
1.194“Product Acquisition” has the meaning given in Section 21.6.
1.195“Product Category” means the product category consisting of HNB Products or the product category consisting of Vapour Products. [***]
1.196“Product Claims” means any action directly or indirectly based on, arising out of or related, in whole or in part to: (1) [***] of Products; (2) [***] of Products; or (3) [***] regarding to Products, in each case (1) to (3) regardless whether such action [***]. For the avoidance of doubt, Product Claims shall not include[***] (A) [***]; or (B) [***]
1.197“Product Recall” means a product recall mandated by a regulatory body with jurisdiction over the Product.
1.198“Product Specification” means the Product information [***].
1.199“Products” means HNB Products and Vapour Products.
1.200“Prohibited Assertion Claims” has the meaning given in Section 37.9.
1.201“Proposed [***] Changed Product” has the meaning given in Section 15.27(c).
1.202“Proposed [***] Features” has the meaning given in Section 15.27(c)(iii).
1.203“Proposed [***] Patent Family” has the meaning given in Section 15.27(c)(i).
1.204“Proposed Regulatory Changed Product” has the meaning given in Section 16.2(c).
1.205“Proposed Removed [***] Changed Products” has the meaning given in Section 15.27(c)(iv).
1.206“Proposed Removed [***] Products” has the meaning given in Section 15.27(c)(ii).
1.207“[***]” means [***].
1.208“R&D” has the meaning given in Section 23.1(c).
1.209“Receiving Party” means the Party or its Representatives receiving information of the Disclosing Party.
1.210“[***] Product” means any Device or Consumable of a Part 1 Product of a Party, which is changed by that Party, provided that[***]. For the avoidance of doubt, a Changed Product can be a [***] Product for any later Changed Product [***].
1.211“[***] Product Patents” has the meaning given in Section 16.3(e)(i).
1.212“Refused [***] Patent Families” has the meaning given in Section 17.10(a).
1.213“[***]” has the meaning given in Section 15.3.
1.214“[***] Patent Family Number” has the meaning given in Section 10.5(b).
1.215“Regulatory Change” has the meaning given in Section 16.2(c)(i).
1.216“Regulatory Changed Product” has the meaning given in Section 16.2(d)(i)(E).
1.217“Released Claims” has the meaning given in Section 4.1.
1.218“Released Claims Covenant not to Sue” has the meaning given in Section 5.1.
1.219“Released Party” has the meaning given in Section 4.1.
1.220“Released Patent Families” means all Patent Families which include one or more Released Patents such released Patent Families to be listed in Schedule 1.1.220.
1.221“Released Patents” means all Patents in issue in the Proceedings.
1.222“Released Principal Brands” has the meaning given in Section 4.1(f).
1.223“Released Territorial Brands” has the meaning given in Section 4.1(f).
1.224“Released Territorial Brands Claims” has the meaning given in Section 4.1(f).
1.225“Releasing Party” has the meaning given in Section 4.1.
1.226“Relevant” has the meaning given in Section 15.2. “Relevance” shall be interpreted accordingly.
1.227“Relevant [***] IPR” has the meaning given in Section 21.5.
1.228“Relevant [***] Patents” is determined in accordance with either Section 18.4(c) or 18.5(b), as the case may be.
1.229“Relevant [***] Patents” is determined in accordance with either Section 18.4 or 18.5, as the case may be.
1.230“Relevant HNB Sanctions” has the meaning given in Section 15.21 or 15.23, as applicable.
1.231“Relevant IPR” has the meaning given in Section 21.3.
1.232“Relevant Patent” means, in relation to a Product, a Patent that is Relevant to that Product.
1.233“Relevant Patent Family” means, in relation to a Product, a Patent Family that is Relevant to that Product.
1.234“Relevant [***] IPR” has the meaning given in Section 21.5(a).
1.235“Relevant Vapour Sanctions” has the meaning given in Section 15.22 or Section 15.24, as applicable.
1.236“Remaining Additional Patent Families” has the meaning given in Section 17.11.
1.237“Remaining Party” has the meaning given in Section 21.8(c).
1.238“[***] Changed Products” has the meaning given in Section 15.27(d)(iii).
1.239“[***] Products” has the meaning given in Section 15.27(d)(iii).
1.240“[***]” means a [***] of [***] that is not a [***] where the addition of the [***] to [***] is necessitated by the removal of an [***] in order for a Patent Family to become a [***] Patent Family.
1.241“Replacement Parts” means like-for-like replacement Components of a Product, including batteries and caps but excluding for the avoidance of doubt: (1) entire Devices; (2) entire Consumables; (3) Upgrade Parts; (4) in the case of [***] Replacement Parts, [***]; and (5) any Component of a Product that has a different Relevant Patent Family than the Component it replaces.
1.242“Representative” means, with respect to any Person, any and all directors, officers, employees, employee consultants and other agents of such Person for such period as they are a director, officer, employee, employee consultant or other agent of such Person.
1.243“[***] Patent” has the meaning given in Section 15.3.
1.244“[***]” has the meaning given in Section 15.3.
1.245“[***] Patent Offices” means: (1) in respect of HNB Products, [***]; and (2) in respect of Vapour Products,[***].
1.246“[***] Sublicensable IPR” means, with respect to a Party that such Party has licensed such Intellectual Property Rights [***].
1.247“[***] Patents” has the meaning given in Section 18.2(b).
1.248“Royalty Rate Records” has the meaning given in Section 18.11.
1.249“Royalty [***]” means the [***] set out in Schedule 1.1.249.
1.250“[***] HNB Market Re-entry” has the meaning given in Section 15.21.
1.251“[***] Vapour Market Re-entry” has the meaning given in Section 15.22.
1.252“Sanctions” means economic, financial and trade sanctions laws, regulations, trade embargoes, export controls, Sanctions Lists and other restrictive measures, including (but not limited to) those enacted, administered, implemented and/or enforced from time to time by the UN, the US, the UK, the EU (including its Member States), Switzerland and any other territory or authority with jurisdiction over a Party’s activities (or the activities of its Associated Companies), to the extent any of the foregoing is applicable to a Party or its Associated Companies, as the case may be.
1.253“Sanctions List” means the “Specially Designated Nationals and Blocked Persons” list maintained by OFAC, the “Consolidated List of Financial Sanctions Targets” maintained by His Majesty’s Treasury, the “Consolidated List of Persons and Entities subject to Financial Sanctions” maintained by the European Commission, the “Consolidated List of Sanctioned Individuals, Entities and Organisations” maintained by the State Secretariat for Economic Affairs and any similar list maintained by, or public announcement of Sanctions designation made by, any relevant sanctions authority.
1.254“Section 6 BAT Patent Families – [***]” has the meaning given in Section 6.2(b).
1.255“Section 6 BAT Patent Families – Other HNB” has the meaning given in Section 6.2(a).
1.256“Section 6 BAT Patent Families – Vapour” has the meaning given in Section 6.2(c).
1.257“Section 6 PMP Patent Families – HNB” has the meaning given in Section 6.1(a).
1.258“Section 6 PMP Patent Families – Vapour” has the meaning given in Section 6.1(b).
1.259“Section 15.21 Addition Date” has the meaning given in Section 15.21.
1.260“Section 15.21 Dispute” has the meaning given in Section 15.21.
1.261“Section 15.21 Sanctions Reimposition Notice” has the meaning given in Section 15.21.
1.262“Section 15.22 Addition Date” has the meaning given in Section 15.22.
1.263“Section 15.22 Dispute” has the meaning given in Section 15.22.
1.264“Section 15.22 Sanctions Reimposition Notice” has the meaning given in Section 15.22.
1.265“Section 15.23 Addition Date” has the meaning given in Section 15.23.
1.266“Section 15.23 Dispute” has the meaning given in Section 15.23.
1.267“Section 15.23 Sanctions Reimposition Notice” has the meaning given in Section 15.23.
1.268“Section 15.24 Addition Date” has the meaning given in Section 15.24.
1.269“Section 15.24 Dispute” has the meaning given in Section 15.24.
1.270“Section 15.24 Sanctions Reimposition Notice” has the meaning given in Section 15.24.
1.271“Signatory” has the meaning given in Section 1.3.
1.272“[***] Consumable” means a single Consumable that is not a [***] Consumable.
1.273“Sold for Use with” means, in relation to any Replacement Parts or Upgrade Parts of a Party on the market in the Territory for use by consumers to replace a Component of that Party’s Product: (1) [***] of the Product, Replacement Part or Upgrade Part [***] with that Party’s Product; (2) [***].
1.274“Specified Breaches” has the meaning given in Section 30.1.
1.275“[***] Family” has the meaning given in Section 18.5(c).
1.276“[***] Device” has the meaning given in Section 18.5(c).
1.277“[***] Patents” has the meaning given in Section 18.5(c).
1.278“Standstill Exhibit” means the standstill agreement between the Parties at Exhibit B.
1.279“Subject Countries” has the meaning given in Section 21.6.
1.280“Supply Chain Entities” means any Third Party whether now or in the future involved in the supply, purchase, distribution, manufacture, assembly, use, marketing, promotion, packaging, transportation, storage, import, export or sale of a Product and Components thereof and related raw materials, on behalf of a Party including farmers, manufacturers, suppliers, both direct and indirect customers, and wholesale and retail distributors and resellers of Products anywhere in the Territory of a Party in each case, to the extent that such Third Party is performing acts in connection with such involvement.
1.281“System” means a combination of a Consumable and a Device that are Usable together.
1.282“Tax Deduction” has the meaning given in Section 19.1.
1.283“Term” has the meaning given in Section 27.1.
1.284“Territory” means worldwide excluding [***].
1.285“Third Party” means a Person other than a Party to this Agreement.
1.286“Turnover” means, in relation to a Party or a Person (in the case of the definition of [***] Companies) for an applicable jurisdiction, Gross Revenue of the applicable Product where either: (1) the market of legitimate retail sale of the Product is the applicable jurisdiction; or (2) [***]the Product will be or has been sold to consumers in the applicable jurisdiction, [***] in the applicable jurisdictions.
1.287“[***]” [***].
1.288“Upgrade Parts” means replacement or add-on Components, Sold for Use with a Product (the “Base Product”) that change such Base Product, including without limitation any Component directly involved in or affecting aerosol production and Components that change the consumer experience, and/or the durability and/or battery life of such Base Product, but excluding: (1) entire Devices; (2) entire Consumables; and (3) for BAT, any product Sold for Use with any other product of BAT that results in a [***].
1.289“Upheld [***] Patent Families” has the meaning given in Section 17.10(a).
1.290“Usable” means can be used together to generate an aerosol, however, the following shall be disregarded in assessing the same: [***].
“Use” and “Used” shall be interpreted accordingly.
1.291“Validity Challenge” means any proceeding (including any appeal) in which the validity, enforceability or Entitlement of any Patent is challenged before any Governmental Authority empowered to decide on the validity, enforceability or Entitlement of that Patent including without limitation (1) invalidity, revocation or nullity challenges; (2) any pre-grant or post-grant oppositions, re-examination procedures, third party observations, or other validity challenges to Patents; or (3) any other proceeding filed before any forum that seeks to challenge the validity, enforceability or Entitlement of any Patent (including post grant review proceedings in the United States) and/or seeks a declaration (other than one of non-infringement) in respect of one or more Patents but excluding US District Court declaratory judgment proceedings. For the avoidance of doubt, Validity Challenges shall not include any proceeding in which infringement of a Patent is asserted, or in which a declaration concerning any Product(s) in respect of one or more Patents, including whether a Product is Within the Scope of any claim of a Patent is sought. For the further avoidance of doubt, the consideration under this Agreement, including the ADR Exhibit of whether a Patent claim is Enforceable by one Party against a Product of the other Party is not a Validity Challenge.
1.292“Vapour Changed Product” has the meaning given in Section 13.2.
1.293“Vapour Consumable” means: (1) a Consumable consisting of a liquid receptacle that is removable by the consumer (“Pod”); (2) an e-liquid; (3) a [***] Product, in each case of (1) and (2), that is only Usable with a Vapour Device or a [***] Device or a [***] Device, excluding products that are exclusively [***], in each case (1) to (3) containing a nicotine-containing liquid substrate; or (4) a co-existing variant of (1), (2) or (3) that is provided as a product line extension of (1) [***] a nicotine-containing or non-nicotine-containing aerosol [***] even where such variant would otherwise be considered a [***].
1.294“Vapour Device” means: (1) a Device that is only Usable with a Vapour Consumable to form a System in which a nicotine-containing liquid substrate is electrically heated to vaporise components of the liquid to form a nicotine-containing aerosol intended for inhalation, excluding products that are exclusively [***]; and (2) a co-existing variant of (1) that is provided as a product line extension of (1)[***] a nicotine-containing or non-nicotine-containing aerosol [***]even where such variant would otherwise be considered a [***]. For the avoidance of doubt, any liquid receptacles that are refillable but not removeable by the consumer (“Tanks”) are Components of a Vapour Device.
1.295“[***] Licence” means the licence granted by [***] to [***] pursuant to Section 20.
1.296“Vapour [***] Product” means a Vapour Consumable that can be used to generate an aerosol without combination with a separate Device by the consumer and the liquid receptacle of which is not refillable or removable by the consumer (for example, [***]).
1.297“Vapour Product” means a Vapour Device or a Vapour Consumable (including a Vapour [***] Product).
1.298“Viable” has the meaning given in Section 16.2(c)(iii).
1.299A Product is “Within the Scope of” a claim of a Patent for the purposes of this Agreement if that Product, [***] is found pursuant to the ADR Procedure or by agreement between the Parties to fall within the scope of such claim under the applicable governing patent law as determined by this Agreement. A Product is “Within the Scope of” a Patent if that Product, [***] is found pursuant to the ADR Procedure or by agreement between the Parties to fall within the scope of at least one claim of such Patent under the applicable governing patent law as determined by this Agreement. For the avoidance of doubt, if a Party wishes to rely on the [***] to establish that a Product is Within the Scope of a Patent, then the other Party may rely upon any defences available under the applicable law to the application of [***].
1.300“Working Hours” means the hours of 9:30 a.m. to 5:30 p.m. CET on a Business Day.
EX-21
14
pm-ex21_123123xq4.htm
EX-21
Document
List of Subsidiaries
As of December 31, 2023
Listed below are subsidiaries of Philip Morris International Inc. (the “Company”) as of December 31, 2023, and their state or country of organization.
|
|
|
|
|
|
|
|
|
| Name |
|
State or
Country of
Organization
|
|
|
|
| Abal Hermanos S.A. |
|
Uruguay |
| AG Snus Aktieselskab |
|
Denmark |
| Alkapharmics Sarl |
|
Switzerland |
| AO Philip Morris Izhora |
|
Russian Federation |
| C.A. Tabacalera Nacional |
|
Venezuela |
| Claudio HoldCo A/S |
|
Denmark |
| Cogent International Manufacturing Ltd. |
|
Canada |
| Compania Colombiana de Tabaco S.A.S. |
|
Colombia |
| CTPM International S.A. |
|
Switzerland |
| Egypt Investments Holding Ltd. |
|
United Arab Emirates |
| f6 Cigarettenfabrik GmbH & Co.KG |
|
Germany |
| f6 Geschaftsfuhrungs GmbH |
|
Germany |
| Fertin Pharma A/S |
|
Denmark |
| Fertin Pharma Goa India Private Limited |
|
India |
| Fire Up International BV. |
|
Netherlands |
| Fortune Landequities and Resources Inc. |
|
Philippines |
| Gotlands Snus AB |
|
Sweden |
| House of Oliver Twist A/S |
|
Denmark |
| Industrias del Tabaco, Alimentos y Bebidas S.A. ITABSA |
|
Ecuador |
| Innovata Biomed Limited |
|
United Kingdom |
| Innovata HK Ltd |
|
Hong Kong |
| Innovata Limited |
|
United Kingdom |
| IPM India Wholesale Trading Private Limited |
|
India |
| Jagotec AG |
|
Switzerland |
| Latin America and Canada Investments B.V. |
|
Netherlands |
| Leonard Dingler (Proprietary) Limited |
|
South Africa |
| Limited Liability Company Philip Morris Georgia |
|
Georgia |
| Limited Liability Company Philip Morris Sales & Marketing |
|
Republic of Moldova |
| Limited Liability Company Philip Morris Sales and Distribution |
|
Ukraine |
| MacMillan Maxwell & Co. of Zimbabwe (Pvt.) Limited |
|
Zimbabwe |
| Massalin Particulares S.R.L. |
|
Argentina |
| NCP NextGen A/S |
|
Denmark |
|
|
|
|
|
|
|
|
|
| NYZ AB |
|
Sweden |
| Orecla Investments Limited |
|
United Kingdom |
| Orecla Realty, Inc. |
|
Philippines |
| Orecla Sarl |
|
Switzerland |
| Orecla Services SA |
|
Switzerland |
| Pan Africa Entrepreneurs Limited |
|
United Kingdom |
| Pan Africa Entrepreneurs Services Sarl |
|
Senegal |
| Pan Africa Invest Company Limited |
|
United Kingdom |
| Papastratos Cigarettes Manufacturing Company Single Member S.A. |
|
Greece |
| Papastratos International B.V. |
|
Netherlands |
| Park (U.K.) Limited |
|
United Kingdom |
| Park Tobacco Limited |
|
United Kingdom |
| PdB HoldCo LLC |
|
United States |
| Philip Morris (Australia) Limited |
|
Australia |
| Philip Morris (China) Management Co. Ltd. |
|
China |
| Philip Morris (Malaysia) Sdn. Bhd. |
|
Malaysia |
| Philip Morris (New Zealand) Limited |
|
New Zealand |
| Philip Morris (Pakistan) Limited |
|
Pakistan |
| Philip Morris (Thailand) Ltd. |
|
United States |
| Philip Morris Aktiebolag |
|
Sweden |
| Philip Morris Albania Sh.p.k. |
|
Albania |
| Philip Morris Algeria Sarl |
|
Algeria |
| Philip Morris ApS |
|
Denmark |
| Philip Morris Armenia Limited Liability Company |
|
Armenia |
| Philip Morris Asia Limited |
|
Hong Kong |
| Philip Morris Austria GmbH |
|
Austria |
| Philip Morris Bangladesh Limited |
|
Bangladesh |
| Philip Morris Benelux B.V. |
|
Belgium |
| Philip Morris BH d.o.o. za trgovinu Sarajevo |
|
Bosnia and Herzegovina |
| Philip Morris Brands Sarl |
|
Switzerland |
| Philip Morris Brasil Industria e Comercio Ltda. |
|
Brazil |
| Philip Morris Brasil S.A. |
|
United States |
| Philip Morris Brasil Comercio e Distribuicao Ltda |
|
Brazil |
| Philip Morris Bulgaria EOOD |
|
Bulgaria |
| Philip Morris Chile Comercializadora Limitada |
|
Chile |
| Philip Morris Costa Rica, Sociedad Anonima |
|
Costa Rica |
| Philip Morris CR a.s. |
|
Czech Republic |
| Philip Morris Cyprus Ltd |
|
Cyprus |
| Philip Morris Dominicana, S.A. |
|
Dominican Republic |
| Philip Morris Eesti Osauhing |
|
Estonia |
|
|
|
|
|
|
|
|
|
| Philip Morris Egypt Holdings Limited |
|
United Kingdom |
| Philip Morris Egypt Limited Liability Company |
|
Egypt |
| Philip Morris El Salvador Sociedad Anonima de Capital Variable |
|
El Salvador |
| Philip Morris Electronics (Shenzhen) Ltd. |
|
China |
| Philip Morris Finance SA |
|
Switzerland |
| Philip Morris Finland Oy |
|
Finland |
| Philip Morris France S.A.S. |
|
France |
| Philip Morris Global Brands Inc. |
|
United States |
| Philip Morris GmbH |
|
Germany |
| Philip Morris Group Pension Plan Trustees Limited |
|
United Kingdom |
| Philip Morris Holdings Sarl |
|
Switzerland |
| Philip Morris Holland Holdings B.V. |
|
Netherlands |
| Philip Morris Hungary Cigarette Trading Ltd. |
|
Hungary |
| Philip Morris International Holdings B.V. |
|
Netherlands |
| Philip Morris International Inc. |
|
United States |
| Philip Morris International Insurance (Ireland) Designated Activity Company |
|
Ireland |
| Philip Morris International IT Service Center Sarl |
|
Switzerland |
| Vectura Fertin Pharma Research Laboratories Pte. Ltd. |
|
Singapore |
| Philip Morris International Service Center, S.L. Sociedad Unipersonal |
|
Spain |
| Philip Morris International Services Sarl |
|
Switzerland |
| Philip Morris Investments B.V. |
|
Netherlands |
| Philip Morris Investments B.V./Jordan Ltd.Co. |
|
Jordan |
| Philip Morris Italia S.r.l. |
|
Italy |
| Philip Morris Jamaica Limited |
|
Jamaica |
| Philip Morris Japan Godo-Kaisha |
|
Japan |
| Philip Morris Kazakhstan LLP |
|
Kazakhstan |
| Philip Morris Korea Inc. |
|
South Korea |
| Philip Morris KSA Holdings Limited |
|
United Kingdom |
| Philip Morris Latin America Services S.R.L. |
|
Argentina |
| Philip Morris Limited |
|
United Kingdom |
| Philip Morris Limited |
|
Australia |
| Philip Morris Limited |
|
Nigeria |
| Philip Morris Ljubljana, storitveno podjetje, d.o.o. |
|
Slovenia |
| Philip Morris Ltd |
|
Israel |
| Philip Morris Luxembourg S.a.r.l. |
|
Luxembourg |
| Philip Morris Management Services (Middle East) Limited |
|
United Arab Emirates |
| Philip Morris Manufacturing & Technology Bologna S.p.A. |
|
Italy |
| Philip Morris Manufacturing GmbH |
|
Germany |
| Philip Morris Manufacturing Senegal S.A.R.L. |
|
Senegal |
| Philip Morris Maghreb SARL |
|
Morocco |
|
|
|
|
|
|
|
|
|
| Philip Morris Mexico Productos Y Servicios, Sociedad de Responsabilidad Limitada de Capital Variable |
|
Mexico |
| Philip Morris Mexico, Sociedad Anónima de Capital Variable |
|
Mexico |
| Philip Morris Misr Limited Liability Company |
|
Egypt |
| Philip Morris Montenegro DOO |
|
Montenegro |
| Philip Morris Nicaragua Sociedad Anonima |
|
Nicaragua |
| Philip Morris North Africa SARL |
|
Tunisia |
| Philip Morris Norway AS |
|
Norway |
| Philip Morris Operations a.d. Nis |
|
Serbia |
| Philip Morris Panama Sociedad en Comandita por Acciones |
|
Panama |
| Philip Morris Paraguay S.A. |
|
Paraguay |
| Philip Morris Pensionstreuhand GmbH |
|
Germany |
| Philip Morris Peru, Sociedad Anónima |
|
Peru |
| Philip Morris Polska Distribution Społka z ograniczona odpowiedzialnoscia |
|
Poland |
| Philip Morris Polska Społka Akcyjna |
|
Poland |
| Philip Morris Polska Tobacco Społka z ograniczona odpowiedzialnoscia |
|
Poland |
| Philip Morris Products S.A. |
|
Switzerland |
| Philip Morris Research Laboratories GmbH |
|
Germany |
| Philip Morris Reunion S.A.R.L. |
|
Réunion |
| Philip Morris Romania S.R.L. |
|
Romania |
| Philip Morris Pazarlama ve Satis A.S. |
|
Turkey |
| Philip Morris Sales and Marketing Ltd. |
|
Russian Federation |
| Philip Morris Services d.o.o. Beograd |
|
Serbia |
| Philip Morris Services India Sarl |
|
Switzerland |
| Philip Morris Services S.A. |
|
Switzerland |
| Philip Morris Seyahat Perakende Satis Anonim Sirketi |
|
Turkey |
| Philip Morris Singapore Pte. Ltd. |
|
Singapore |
| Philip Morris Slovakia s.r.o. |
|
Slovakia |
| Philip Morris South Africa (Proprietary) Limited |
|
South Africa |
| Philip Morris South Africa Holdings (Proprietary) Limited |
|
South Africa |
| Philip Morris Spain, S.L. |
|
Spain |
| Philip Morris Switzerland Sarl |
|
Switzerland |
| Philip Morris T & T Limited |
|
Trinidad and Tobago |
| Philip Morris Taiwan S.A. |
|
Switzerland |
| Philip Morris Tanzania Limited |
|
Tanzania |
| Philip Morris Trading (Thailand) Company Limited |
|
Thailand |
| Philip Morris Trading S.R.L. |
|
Romania |
| Philip Morris Travel Retail Hong Kong Limited |
|
Hong Kong |
| Philip Morris Tutunski Kombinat Prilep LLC Skopje |
|
Macedonia |
| Philip Morris Vietnam Limited Liability Company |
|
Vietnam |
| Philip Morris World Trade S.a r.l. |
|
Switzerland |
|
|
|
|
|
|
|
|
|
| Philip Morris Yonetim Hizmetleri A.S. |
|
Turkey |
| Philip Morris Zagreb d.o.o. za vanjsku i unutarnju trgovinu |
|
Croatia |
| Philmor Global Business Services (India) Private Limited |
|
India |
| Philip Morris Tutun Mamulleri Sanayi ve Ticaret A.S. |
|
Turkey |
| Pinkerton Tobacco Co. LP. |
|
United States |
| PM Equity Partner Sarl |
|
Switzerland |
| PM Tobakk Norge AS |
|
Norway |
| PMFTC Inc. |
|
Philippines |
| PMI Business Solutions (Philippines) Inc. |
|
Philippines |
| PMI Engineering S.A. |
|
Switzerland |
| PMI Global Services Inc. |
|
United States |
| PMI Global Studio Limited |
|
United Kingdom |
| PMI Service Center Europe spolka z ograniczona odpowiedzialnoscia |
|
Poland |
| PMM-S.G.P.S., S.A. |
|
Portugal |
| PrJSC Philip Morris Ukraine |
|
Ukraine |
| ProfiGen do Brasil Ltda. |
|
Brazil |
| ProfiGen LLC |
|
United States |
| Proveedora Ecuatoriana S.A. PROESA |
|
Ecuador |
| PT Agasam |
|
Indonesia |
| PT Hanjaya Mandala Sampoerna Tbk. |
|
Indonesia |
| PT Persada Makmur Indonesia |
|
Indonesia |
| PT Perusahaan Dagang Dan Industri Panamas |
|
Indonesia |
| PT Philip Morris Indonesia |
|
Indonesia |
| PT Philip Morris Sampoerna International Service Center |
|
Indonesia |
| PT Sampoerna Indonesia Sembilan |
|
Indonesia |
| PT SRC Indonesia Sembilan |
|
Indonesia |
| PT Taman Dayu |
|
Indonesia |
| PT Wahana Sampoerna |
|
Indonesia |
| Reviti International Sarl |
|
Switzerland |
| Rothmans, Benson & Hedges Inc. |
|
Canada |
| Sampoerna International Pte. Ltd. |
|
Singapore |
| SIA Philip Morris Latvia |
|
Latvia |
| SkyePharma Holding AG |
|
Switzerland |
| SMCI Holding Inc. |
|
United States |
| SMINT Holdings Corp. |
|
Philippines |
| Superior Tobacco Co N.V. |
|
Aruba |
| Superior Tobacco Company Curacao N.V. |
|
Curacao |
| Svenska Tändsticksbolaget försaljningsaktiebolag |
|
Sweden |
| Swedish Match AB (GUO) |
|
Sweden |
| Swedish Match Cigars Inc. |
|
United States |
| Swedish Match da Amazonia S.A. |
|
Brazil |
|
|
|
|
|
|
|
|
|
| Swedish Match Denmark A/S |
|
Denmark |
| Swedish Match Deutschland GmbH |
|
Germany |
| Swedish Match Distribution A/S |
|
Norway |
| Swedish Match do Brazil S.A. |
|
Brazil |
| Swedish Match Dominicana S.A. |
|
Dominican Republic |
| Swedish Match Fósforos Portugal, SA |
|
Portugal |
| Swedish Match France SAS |
|
France |
| Swedish Match Holding AB |
|
Sweden |
| Swedish Match Industries AB |
|
Sweden |
| Swedish Match Intellectual Property AB |
|
Sweden |
| Swedish Match Jupiter AB |
|
Sweden |
| Swedish Match Kibrit ve Cakmak Endustri A.S. |
|
Turkey |
| Swedish Match Leaf Tobacco Company |
|
United States |
| Swedish Match Lighters BV |
|
Netherlands |
| Swedish Match Norge AS |
|
Norway |
| Swedish Match North America LLC |
|
United States |
| Swedish Match North Europe AB |
|
Sweden |
| Swedish Match Overseas BV |
|
Netherlands |
| Swedish Match Philippines Inc. |
|
Philippines |
| Swedish Match Philippines Sales Inc. |
|
Philippines |
| Swedish Match Retail AB |
|
Sweden |
| Swedish Match Switzerland AG |
|
Switzerland |
| Swedish Match Treasury Switzerland AG |
|
Switzerland |
| Swedish Match US AB |
|
Sweden |
| Swedish Match USA Inc. |
|
United States |
| Swedmat Corp. |
|
Philippines |
| Tabacalera Centroamericana Sociedad Anonima |
|
Guatemala |
| Tabacontrole S.G.P.S., S.A. |
|
Portugal |
| Tabacos Desvenados, Sociedad Anonima de Capital Variable |
|
Mexico |
| Tabamark S.A. |
|
Uruguay |
| Tabaqueira - Empresa Industrial de Tabacos, S.A. |
|
Portugal |
| Tabaqueira II, S.A. |
|
Portugal |
| TabLabs Inc. |
|
Canada |
| The Pinkerton Tobacco Co. LLC |
|
United States |
| Triaga Inc. |
|
United States |
| UAB Philip Morris Baltic |
|
Lithuania |
| UAB Philip Morris Lietuva |
|
Lithuania |
| United Kingdom Tobacco Company Limited (The) |
|
United Kingdom |
| Vectura Fertin Pharma, Inc. |
|
United States |
| Vectura Fertin Pharma Malaysia SDN. BHD. |
|
Malaysia |
| Vectura Fertin Pharma Private Limited |
|
India |
|
|
|
|
|
|
|
|
|
| Vectura Delivery Devices Limited |
|
United Kingdom |
| Vectura Gmbh |
|
Germany |
| Vectura Group Investments Limited |
|
United Kingdom |
| Vectura Group Limited |
|
United Kingdom |
| Vectura Group Services Limited |
|
United Kingdom |
| Vectura Inc. |
|
United States |
| Vectura Ireland Limited |
|
United Kingdom |
| Vectura Limited |
|
United Kingdom |
EX-23
15
pm-ex23_123123xq4.htm
EX-23
Document
Exhibit 23
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (File No. 333-269690) and Form S-8 (File Nos. 333-149822, 333-149821, 333-181298, 333-217651, 333-229603, 333-265339) of Philip Morris International Inc. of our report dated February 8, 2024 relating to the financial statements and the effectiveness of internal control over financial reporting, which appears in this Form 10-K.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ PRICEWATERHOUSECOOPERS SA |
|
|
PricewaterhouseCoopers SA |
|
|
|
|
|
|
|
|
|
|
|
Lausanne, Switzerland
February 8, 2024
EX-31.1
16
pm-ex311_123123xq4.htm
EX-31.1
Document
Exhibit 31.1
Certifications
I, Jacek Olczak, certify that:
1.I have reviewed this annual report on Form 10-K of Philip Morris International Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: February 8, 2024
|
|
|
| /s/ JACEK OLCZAK |
| Jacek Olczak |
| Chief Executive Officer |
EX-31.2
17
pm-ex312_123123xq4.htm
EX-31.2
Document
Exhibit 31.2
Certifications
I, Emmanuel Babeau, certify that:
1.I have reviewed this annual report on Form 10-K of Philip Morris International Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: February 8, 2024
|
|
|
| /s/ EMMANUEL BABEAU |
| Emmanuel Babeau |
| Chief Financial Officer |
EX-32.1
18
pm-ex321_123123xq4.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Philip Morris International Inc. (the “Company”) on Form 10-K for the period ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Jacek Olczak, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
/s/ JACEK OLCZAK |
| Jacek Olczak |
| Chief Executive Officer |
| February 8, 2024 |
EX-32.2
19
pm-ex322_123123xq4.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Philip Morris International Inc. (the “Company”) on Form 10-K for the period ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Emmanuel Babeau, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
/s/ EMMANUEL BABEAU |
| Emmanuel Babeau |
| Chief Financial Officer |
| February 8, 2024 |
EX-97
20
exhibit97.htm
EX-97
Document
PHILIP MORRIS INTERNATIONAL INC.
POLICY FOR RECOVERY OF ERRONEOUSLY AWARDED INCENTIVE COMPENSATION
(Effective Date: October 2, 2023)
1. INTRODUCTION
Philip Morris International Inc. (the “Company”) is adopting this policy (this “Policy”) to provide for the Company’s recovery of certain Incentive Compensation (as defined below) erroneously awarded to Affected Officers (as defined below) under certain circumstances.
This Policy is administered by the Compensation and Leadership Development Committee (the “Committee”) of the Company’s Board of Directors (the “Board”). The Committee shall have full and final authority to make any and all determinations required or permitted under this Policy. Any determination by the Committee with respect to this Policy shall be final, conclusive and binding on all parties. The Board may amend or terminate this Policy at any time.
This Policy is intended to comply with Section 10D of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), Rule 10D-1 thereunder and the applicable rules of the New York Stock Exchange (the “Exchange”) and will be interpreted and administered consistent with that intent.
Each Affected Officer subject to this Policy must execute the Acknowledgment and Agreement attached hereto as Exhibit A before such Affected Officer will be entitled to receive any cash- or equity-based incentive compensation that is approved, granted or awarded on or after the Effective Date.
2. EFFECTIVE DATE
This Policy shall apply to all Incentive Compensation received by an Affected Officer on or after the Effective Date of this Policy, and to the extent permitted or required by applicable law.
3. DEFINITIONS
For purposes of this Policy, the following terms shall have the meanings set forth below:
“Affected Officer” means any current or former “officer” as defined in Exchange Act Rule 16a-1.
“Erroneously Awarded Compensation” means the amount of Incentive Compensation received that exceeds the amount of Incentive Compensation that otherwise would have been received had it been determined based on the Restatement, computed without regard to any taxes paid by the Affected Officer or withheld by the Company on the Affected Officer’s behalf. In the case of Incentive Compensation based on stock price or total shareholder return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in the Restatement, the amount shall reflect a reasonable estimate of the effect of the Restatement on the stock price or total shareholder return upon which the Incentive Compensation was received, as determined by the Committee in its sole discretion. The Committee may determine the form and amount of Erroneously Awarded Compensation in its sole discretion.
“Financial Reporting Measure” means any measure that is determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures that are derived wholly or in part from such measures, whether or not such measure is presented within the Company’s financial statements or included in a filing with the Securities and Exchange Commission.
Stock price and total shareholder return are Financial Reporting Measures.
“Incentive Compensation” means any compensation that is granted, earned or vested based in whole or in part on the attainment of a Financial Reporting Measure. For purposes of clarity, base salaries, bonuses or equity awards paid solely upon satisfying one or more subjective standards, strategic or operational measures, or continued employment are not considered Incentive Compensation, unless such awards were granted, paid or vested based in part on a Financial Reporting Measure.
“Restatement” means an accounting restatement due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements (i.e., a “Big R” restatement), or that would result in a material misstatement if the error was corrected in the current period or left uncorrected in the current period (i.e., a “little r” restatement).
4. RECOVERY
If the Company is required to prepare a Restatement, the Company shall seek to reasonably promptly recover and claw back from any Affected Officer the Erroneously Awarded Compensation received by the Affected Officer:
(i)on or after the Effective Date;
(ii)after the person begins service as an Affected Officer;
(iii)who serves as an Affected Officer at any time during the performance period for that Incentive Compensation;
(iv)while the Company has a class of securities listed on the Exchange; and
(v)during the three completed fiscal years immediately preceding the date on which the Company was required to prepare the Restatement.
For purposes of this Policy:
•Erroneously Awarded Compensation is deemed to be received in the Company’s fiscal year during which the Financial Reporting Measure specified in the Incentive Compensation is attained, even if the payment or grant of the Incentive Compensation occurs after the end of that period; and
•the date the Company is required to prepare a Restatement is the earlier of (x) the date the Board, the Committee or any officer of the Company authorized to take such action concludes, or reasonably should have concluded, that the Company is required to prepare the Restatement, or (y) the date a court, regulator, or other legally authorized body directs the Company to prepare the Restatement.
To the extent required by applicable law or the rules of the Exchange, any profits realized from the sale of securities of the Company are subject to recoupment under this Policy.
For purposes of clarity, in no event shall the Company be required to award any Affected Officers an additional payment or other compensation if the Restatement would have resulted in the grant, payment or vesting of Incentive Compensation that is greater than the Incentive Compensation actually received by the Affected Officer. The recovery of Erroneously Awarded Compensation is not dependent on if or when the Restatement is filed.
5. SOURCES OF RECOUPMENT
To the extent permitted by applicable law, the Committee may, in its discretion, seek recoupment as a debt immediately due and payable from the Affected Officer(s) through any means it determines, which may include any of the following sources: (i) prior Incentive Compensation payments; (ii) future payments of Incentive Compensation; (iii) cancellation of outstanding Incentive Compensation; (iv) direct repayment; and (v) non-Incentive Compensation or securities held by the Affected Officer; and (vi) refunds of excess taxes paid on the gross amount of the Erroneously Awarded Compensation. To the extent permitted by applicable law, the Company may offset such amount against any compensation or other amounts owed by the Company to the Affected Officer.
6. LIMITED EXCEPTIONS TO RECOVERY
Notwithstanding the foregoing, the Committee, in its discretion, may choose to forgo recovery of Erroneously Awarded Compensation under the following circumstances, provided that the Committee (or a majority of the independent members of the Board) has made a determination that recovery would be impracticable because:
(i) The direct expense paid to a third party to assist in enforcing this Policy would exceed the recoverable amounts; provided that the Company has made a reasonable attempt to recover such Erroneously Awarded Compensation, has documented such attempt and has (to the extent required) provided that documentation to the Exchange;
(ii) Recovery would violate home country law where the law was adopted prior to November 28, 2022, and the Company provides an opinion of home country counsel to that effect to the Exchange that is acceptable to the Exchange; or
(iii) Recovery would likely cause an otherwise tax-qualified retirement plan to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder, as amended.
7. NO INDEMNIFICATION OR INSURANCE
The Company will not indemnify, insure or otherwise reimburse any Affected Officer against the recovery of Erroneously Awarded Compensation.
8. NO IMPAIRMENT OF OTHER REMEDIES
This Policy does not preclude the Company from taking any other action to enforce an Affected Officer’s obligations to the Company, including termination of employment, institution of civil proceedings, or reporting of any misconduct to appropriate government authorities. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company under applicable law, regulation or rule or pursuant to the terms of any similar policy in any employment agreement, equity award agreement, or similar agreement and any other legal remedies available to the Company.
9. SUCCESSORS
This Policy shall be binding and enforceable against all Affected Officers and their beneficiaries, heirs, executors, administrators or other legal representatives.
PHILIP MORRIS INTERNATIONAL INC.
POLICY FOR RECOVERY OF ERRONEOUSLY AWARDED INCENTIVE COMPENSATION
ACKNOWLEDGMENT AND AGREEMENT
This Acknowledgment and Agreement (the “Acknowledgment”) is entered into between Philip Morris International Inc. (the “Company”), [insert name of employing entity] (the “Employer”) and the individual named below (the “Undersigned”) as of the date set forth below. The Undersigned is an Affected Officer (as defined in the Policy for Recovery of Erroneously Awarded Incentive Compensation (the “Policy”) to which the form of this Acknowledgment is attached as Exhibit A) and an employee of the Employer.
The Board of Directors of the Company adopted the Policy to establish the conditions under which the Company and/or the Employer may seek to recoup certain compensation from Affected Officers, including the Undersigned, in the event that the Company is required to prepare a Restatement (as defined in the Policy).
The Undersigned has received or may receive compensation, including cash-based incentive compensation and equity-based incentive compensation from the Company and/or the Employer to which the Policy applies.
In consideration of the continued benefits to be received from the Company and/or the Employer and the right to participate in, and receive future benefits, compensation, payments and/or awards under, the cash- and/or equity-based incentive programs of the Company and/or the Employer, the Undersigned hereby acknowledges and agrees that:
1. The Undersigned has read and understands the Policy and has accepted the terms and conditions of the Policy.
2. References to “Company” in section 5 of the Policy shall be construed as also covering the Employer.
3. The cash-based incentive compensation and equity-based incentive compensation that the Undersigned has received or may receive from the Company and/or the Employer will be covered under the definition of Incentive Compensation in the Policy to the extent the conditions therein are met, and in case of any dispute in this regard, the decision of the Company and the Employer will be final.
4. To the extent provided in the Policy, the Policy shall apply to Incentive Compensation (as defined in the Policy) established before or after the date of this Acknowledgment, and the programs and agreements under which such compensation may have been or will be issued in the future shall be deemed to incorporate the terms of the Policy even if the Policy is not explicitly referenced therein and that provision of any benefits, compensation, payments and/or awards under such programs and agreements (regardless of whether they are contractual or discretionary) are deemed to be subject to the terms of the Policy. Nothing in this Acknowledgment shall be construed to expand the scope or terms of the Policy, and the Undersigned is not waiving any defenses in the event of an action for recoupment of compensation under the Policy, other than (i) waiving any defense regarding the retroactive application of the Policy to existing awards and (ii) waiving any claim that the integration clause of any agreement excludes the application of the Policy.
5. The Undersigned is contractually bound by the provisions in the Policy which are of contractual effect.
6. The Undersigned agrees to the Company or Employer deducting the gross amount of Erroneously Awarded Compensation (as defined in the Policy) from any amounts due to him/her and if there are insufficient funds to do so, such amounts shall be immediately recoverable as a debt immediately due to the Company, and the Undersigned agrees to pay back such amounts to the Company.
7. The Undersigned acknowledges and agrees that the Company will not indemnify, insure or otherwise reimburse him/her against the recovery of Erroneously Awarded Compensation.
8. The Company and the Employer, may at its/their sole discretion, assign their respective rights under this Acknowledgment and the Policy to each other and/or any of their respective successors or associated companies.
9. In the event that the Company recovers Erroneously Awarded Compensation from the Affected Officer under the Policy, the Affected Officer could be required to amend personal tax returns or declarations to account for the change in employment income subject to taxation during the given year, to the extent that and in accordance with the laws of the Affected Officer’s current and former Home or Host Country(ies) that require it.
The parties agree that this Acknowledgment and any dispute or claim arising out of or in connection with it or the Policy or their respective subject matters or formation, including non-contractual disputes or claims, as well as any disputes regarding the issue of arbitrability of such claims, shall be submitted, to the fullest extent permitted by applicable law, exclusively to mandatory, binding arbitration before Judicial Arbitration and Mediation Services (“JAMS”), under JAMS’s rules applicable to disputes of the sort asserted. Any such arbitration, including all evidence and testimony exchanged therein, shall be strictly confidential. Except as otherwise provided by the Federal Arbitration Act, the arbitrator, and not any federal or state court, shall have exclusive authority to resolve any dispute relating to the interpretation, applicability, enforceability, and/or formation of the Policy and/or this Acknowledgment. The arbitrator shall issue a written decision and shall have the authority to issue any relief permitted under law or equity, and further shall have the discretion to award the prevailing party its reasonable attorneys’ fees in the event it prevails in any such dispute. The parties further agree that any arbitration shall proceed on an individual basis, and hereby waive any right to proceed on a class, collective or other multi-party basis against the other party, and further waive any right to proceed before a jury. Any arbitration shall take place in the Commonwealth of Virginia, United States of America. Notwithstanding the foregoing, either party may institute an action for injunctive relief in aid of arbitration in the United States District Courts for the Commonwealth of Virginia, United States of America. This Acknowledgment and the Policy shall be construed in accordance with the laws of the Commonwealth of Virginia, United States of America, excluding any conflicts or choice of law rule or principle that might otherwise refer construction or interpretation of the Acknowledgment or Policy to the substantive law of another jurisdiction.
For and on behalf of For and on behalf of
Philip Morris International Inc. [insert name of employing entity]
|
|
|
|
|
|
|
|
|
|
Date:
Signature:
Print Name: ___________________________
|
|
Date:
Signature:
Print Name:
|
Position: ______________________________ Position: ______________________________
Undersigned
|
|
|
|
|
|
|
|
|
|
|
Date:
Signature:
Print Name:
|