UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
or
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-39032
PROFOUND MEDICAL CORP.
(Exact name of registrant as specified in its charter)
Ontario, Canada |
Not Applicable |
(State or other jurisdiction |
(I.R.S. Employer Identification No.) |
|
|
|
2400 Skymark Avenue, Unit #6, Mississauga, (Address of principal executive offices) |
L4W 5K5 (Zip Code) |
Registrant’s telephone number, including area code (647) 476-1350
Securities registered pursuant to Section 12(b) of the Exchange Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Shares, No Par Value Per Share |
PROF |
Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
Large accelerated filer ☐ |
|
Accelerated filer ☐ |
Non-accelerated filer ☒ |
|
Smaller reporting company ☒ |
|
|
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the registrant’s voting and non-voting common shares held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common shares were last sold as of the last business day of the registrant’s most recently completed second fiscal quarter was $241,514,836.
As of March 7, 2025, the registrant had 30,039,809 common shares, no par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
EXPLANATORY NOTE
Profound Medical Corp. (“ Profound” or the “Company”), a corporation organized under the laws of Ontario, Canada, qualifies as a “Foreign Private Issuer,” as defined in Rule 3b-4 under the Securities Exchange Act of 1934 (the “Exchange Act”) in the United States. The Company has voluntarily elected to file annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K with the United States Securities and Exchange Commission (the “SEC”) instead of filing on the reporting forms available to foreign private issuers.
Although the Company has voluntarily chosen to file periodic reports and current reports, as well as registration statements, on U.S. domestic issuer forms, the Company will maintain its status as a foreign private issuer. Accordingly, as a foreign private issuer, the Company remains exempt from the U.S. federal proxy rules pursuant to Section 14 of the Exchange Act and Regulations 14A and 14C thereunder, Regulation FD, and its officers, directors, and principal shareholders are not subject to the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
TABLE OF CONTENTS
i
Forward-Looking Statements
This annual report on Form 10-K for the year ended December 31, 2024, or this Annual Report on Form 10-K, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that involve risks and uncertainties, principally in the sections titled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” All statements other than statements of historical fact contained in this Annual Report on Form 10-K, including statements regarding future events, our ability to maximize capture of future milestones payments, our workforce reduction and related restructuring activities, our future financial and operating performance, anticipated timing and amounts of milestone and other payments under collaboration agreements, business strategy and plans, objectives of management for future operations, timing and outcome of legal and other proceedings and our ability to finance our operations are forward-looking statements. We have attempted to identify forward-looking statements by using terms such as including “anticipates,” “approach,” “believes,” “can,” “contemplate,” “continue,” “look forward,” “ongoing,” “could,” “estimates,” “expects,” “intends,” “may,” “appears,” “suggests,” “future,” “likely,” “goal,” “plans,” “potential,” “possibly,” “projects,” “predicts,” “seek,” “should,” “target,” “would” or “will” and other similar words or expressions or the negative of these terms or other comparable terminology. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks and uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements, to differ materially. The description of our Business set forth in Item 1, the Risk Factors set forth in Item 1A and our Management’s Discussion and Analysis of Financial Condition and Results of Operations set forth in Item 7 as well as other sections in this report, discuss some of the factors that could contribute to these differences. These forward-looking statements include, among other things, statements about:
| ● | the accuracy of our estimates regarding expenses, future revenues, uses of cash, capital requirements and the need for additional financing; |
| ● | our workforce reduction and related restructuring activities; |
| ● | our ability to realize the anticipated benefits of our corporate strategy; |
| ● | our cash runway and the sufficiency of our financial resources to fund our operations; |
| ● | the initiation, timing, progress, results, and decisions of our partners’ development activities, preclinical studies and clinical trials with respect to our product candidates; |
| ● | our collaborators’ election to pursue or continue research, development and commercialization activities; |
| ● | our ability to obtain future reimbursement and/or milestone payments from our collaborators; |
| ● | our ability to obtain and maintain intellectual property protection for our product candidates; |
| ● | our partners’ ability to successfully commercialize our partnered product candidates; |
| ● | the size and growth of the markets for our partnered product candidates and our partners’ ability to serve those markets; |
| ● | the rate and degree of market acceptance of any future products; |
| ● | the success of competing products that are or may become available; |
| ● | regulatory developments in the United States and other countries; |
| ● | and any restrictions on our ability to use our net operating loss carryforwards. |
PART I
Item 1. BUSINESS
We are a commercial-stage medical device company focused on the development and marketing of customizable, incision-free therapeutic systems for the ablation of diseased tissue utilizing our platform technologies. Our lead product, the TULSA-PRO system, combines real-time MRI, robotically-driven transurethral sweeping action/thermal ultrasound and closed-loop temperature feedback control to ablate whole gland or physician defined region of malignant or benign prostate tissue. The TULSA-PRO system has been shown in clinical and commercial settings to be an effective tool for physicians who are treating prostate diseases including cancer and other conditions such as benign prostatic hyperplasia (“BPH”).
In August 2019, the TULSA-PRO system received FDA clearance as a Class II device in the United States for thermal ablation of prescribed prostate tissue, using TULSA based on the Company’s TACT whole gland ablation pivotal study. It is also CE Marked in the EU for ablation of targeted prostate tissue (benign or malignant).
1
The TULSA-PRO system was approved by Health Canada in November 2019.
Our Sonalleve system is CE Marked in the EU for ablation of uterine fibroids and adenomyotic tissue, palliative relief of pain associated with bone metastases, treatment of osteoid osteoma, and management of benign desmoid tumors. The Sonalleve system is also approved in China and South Korea for non-invasive treatment of uterine fibroids. In November 2020, the Sonalleve system received HDE approval from the FDA for treatment of osteoid osteoma in the extremities.
Our systems are designed to be used with MRI scanners and are currently compatible with select MRI scanners manufactured by Philips, Siemens and GE Healthcare. We have generated revenues from the commercialization of our systems in the United States, EU and Asia. With the goal of increasing commercial adoption of products, we continue to pursue additional regulatory approvals in international jurisdictions and invest in research and development and in clinical studies designed to increase the body of evidence necessary to support customer coverage and reimbursement, both government and private payors. We may also consider synergistic strategic acquisitions to expand the applications of our platform technology and expand our commercial footprint.
Our business model in the United States is based primarily upon recurring revenues, charging a one-time fee that includes a supply of one-time-use devices, use of the TULSA-PRO and its ‘Profound Genius Services’, which provides comprehensive clinical training and case support focused on workflow efficiency. In other, international markets, and more recently in the United States, we continue to deploy a business model that consists of two components - sales of durable goods and one-time-use devices for each patient treated.
Our financial strategy to date has been to raise sufficient funds through securities offerings and bank financings to fund specific programs within a focused budget, and, following the August 2019 FDA clearance of our TULSA-PRO system, to drive commercial utilization. As our commercialization efforts increase and/or further program development costs increase, we may need to raise additional capital. See Item 4, “Risk Factors” for more information.
Our Technology Platform
Based on the clinical data from the TACT pivotal trial and additional studies conducted in the European Union (EU), we believe physicians may elect to use TULSA-PRO to ablate benign or malignant prostate tissue in patients with a variety of prostate diseases, including prostate cancer and BPH. Prostate cancer is one of the most common types of cancer affecting men. The annual incidence of newly diagnosed cases in 2024 is estimated to reach 299,010 in the United States according to the American Cancer Society, representing 29% of all new cancer diagnoses in men. The American Cancer Society further estimates that there are currently 3.3 million men living with prostate cancer in the United States, increasing to 5.8 million men when also including EU. Although 10-year survival outcomes for localized prostate cancer remain favorable, it remains the second-leading cause of cancer death in American men, behind only lung cancer. BPH is a histologic diagnosis that refers to the proliferation of smooth muscle and epithelial cells within the prostatic transition zone. According to the American Urological Association, BPH is nearly ubiquitous in the aging male population with worldwide autopsy-proven increase in histological prevalence starting at ages 40 to 45 years, reaching 60% at age 60 and 80% at age 80.


Illustration of our TULSA-PRO disposable and how it is utilized during a prostate ablation procedure.
TULSA-PRO delivers ultrasound energy through a transurethral catheter, a one-time-use device that is placed in the patient’s prostate through a natural orifice. Ultrasound energy is then delivered by the catheter in the shape of a plane or focused to a blade.
2
Externally, the catheter is connected to a software-controlled robot that rotates up to 360-degrees in a sweeping action to impart thermal energy and thus ablate tissue. The real-time temperature measurement of the prostate via MRI thermometry is coupled with closed-loop process control. The feedback enables delivery of the appropriate amount of ultrasound energy to gently heat the physician-prescribed region of prostate tissue to the target temperature required for cell kill without boiling or charring the tissue. To preserve the urethra within the prostate, the temperature of the transurethral catheter is maintained at an appropriate level by circulating water inside the catheter. Similarly, a water-cooled, specially designed catheter is placed in the patient’s rectum during the ablation process to keep it protected from thermal damage during the procedure. The TULSA-PRO in conjunction with its Thermal Boost module, enables surgeons to temporarily increase the ablation target temperature in treatment boundary regions which might harbor higher risk cancer features in large prostates where the treatment radius is >15 mm, further increasing user confidence that sufficient margins have been ablated. A study published in the Journal of Urology in March 2021, Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Cancer, found that TULSA-PRO’s incision-free, controlled and gentle heating process may result in lower post procedural pain and complications, faster recovery, and reduced potential for side-effects that diminish quality of life, all the while delivering effective ablation of targeted diseased tissue, and significant, desirable shrinkage of the prostate via resorption of the dead tissue over time, which may provide long-term durable benefit.
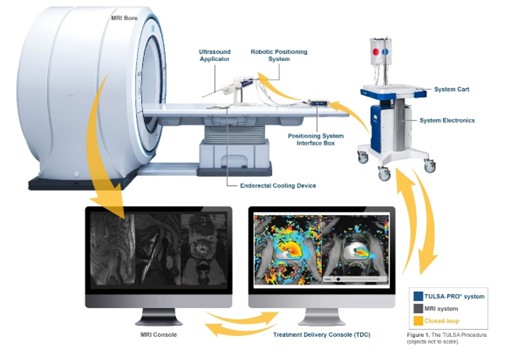
TULSA-PRO system complete workflow with the MRI system.
3
Sonalleve delivers its ultrasound energy via a disc located outside the patient. Its ultrasound energy is focused to create small cylindrical hot spots a certain distance into the patient. Overlapping cylinders create ablation of the physician-prescribed desired tissue. Similar to TULSA-PRO, Sonalleve also provides for controlled temperature increases to achieve cell kill.


Sonalleve system integrated with MRI magnet.
The physician is in charge of using the Profound devices and decides which tissue needs to be ablated to impart therapeutic effect. We believe that in the hands of trained physicians, our systems have the ability to provide customizable, incision-free ablative therapies with the precision of real-time MRI visualization and thermometry, focused ultrasound and closed-loop temperature feedback control as shown below. A study published in the Journal of Urology in March 2021, Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Cancer, found that our technologies offer clinicians and appropriate patients a better alternative to traditional surgical or radiation therapies, with respect to clinical outcomes, side effects and recovery time.

Customizable incision-free ablation of unrivalled variety of prostate indications.
Products
TULSA-PRO
Clinical Studies
In March 2014, we completed enrollment and treatment of 30 patients in the TULSA multi-jurisdictional safety and precision study. Based on the trial results, in April 2016, Profound received a CE Certificate of Conformity for the TULSA-PRO system from its Notified Body in the EU. In the fourth quarter of 2016, Profound initiated a pilot commercial launch of TULSA-PRO in key European markets where the CE Mark is accepted.
4
We received FDA clearance for the TULSA-PRO system in August 2019 for transurethral ultrasound ablation of prostate tissue, based on results from the Company’s TACT Pivotal Clinical Trial. The TACT Pivotal Clinical Trial is a prospective, open-label, single-arm pivotal clinical study, which initially included 115 treatment-naïve localized prostate cancer patients across 13 research sites in the United States, Canada and Europe, enrolled patients between August 2016 and February 2018. Subsequent to FDA clearance in 2019, the TACT trial re-opened enrollment of an additional 35 patients across 3 research sites in the United States (2 sites from the initial TACT recruitment period and 1 new additional site) to increase the proportion of men in the study who are American and with intermediate-risk prostate cancer.
Localized Prostate Cancer, Ablation Safety and Efficacy: TACT Pivotal Study
The TACT Pivotal Clinical Trial is a large, multi-center prospective study in which men with predominately intermediate-risk prostate cancer received whole gland ablation sparing the urethra and apical sphincter. Results demonstrate that MRI-guided TULSA is a minimally invasive procedure for effective prostate cancer ablation with a favorable side effect profile, minimal impact on quality of life and low rates of residual disease. TACT met its primary regulatory endpoint of prostate-specific antigen (“PSA”) reduction in 96% of men to a median nadir of 0.34 ng/ml and 0.5 ng/ml at 12 months. Median decrease in perfused prostate volume as assessed by a central radiology core lab using 12-month MRI was 91%, from a median 37 cc at baseline to 2.8 cc. At 12 months, extensive biopsy sampling of the markedly reduced prostate volume demonstrated histological benefit of elimination of clinically significant prostate cancer for nearly 80% of men. There was no evidence of cancer in 65% of men and 14% had low-volume clinically insignificant disease. The authors noted, however, that thermally fixed non-viable cells can retain their apparently malignant tissue morphology, confounding Gleason grading and potentially introducing false positives. By two and five years, 7% and 21.7%, of men sought additional treatment for their prostate cancer (prostatectomy, radiation, second TULSA not allowed by protocol). Two-thirds of study subjects with clinically significant cancer (ISUP Grade Group [GG] ≥ 2) had extensive disease (either bilateral or ≥5 positive cores), allowing for evaluation of oncologically relevant secondary outcomes including PSA stability, post-treatment biopsy, and salvage treatment. Notwithstanding the limitations of comparisons between ablative and extirpative therapies, the 21.7% five-year rate of salvage treatment and 21% rate of residual clinically significant prostate cancer in intermediate-risk patients are in line with accepted rates of early failure or additional intervention after standard treatments and goals for retreatment after ablative therapies. By five years, the median PSA nadir further reduced to 0.26 ng/ml. PSA reduction was durable over the extended follow-up period, from 0.53 ng/ml at one year to 0.63 ng/ml at five years.

TACT clinical trial PSA outcome to 5 years.
TULSA was associated with a high degree of safety and maintenance of quality-of-life, durable to five years and comparing favorably to radical prostatectomy and other whole-gland ablation techniques. At 12 months, 96% of men returned to baseline urinary continence, and 75% of potent men maintained or returned to erections sufficient for penetration. These rates continued to improve with increasing recovery time, with 97% of patients socially continent and 87% recovering erectile function at five years. A total of 12 attributable grade 3 adverse events occurred in 8% of men, including genitourinary infection (4%), urethral stricture (2%), urinary retention (1.7%), urethral calculus and pain (1%), and urinoma (1%), all of which resolved by 12 months. There were no attributable grade 4 or higher events, rectal injuries, severe incontinence requiring surgical intervention, or severe erectile dysfunction unresponsive to medication.
5
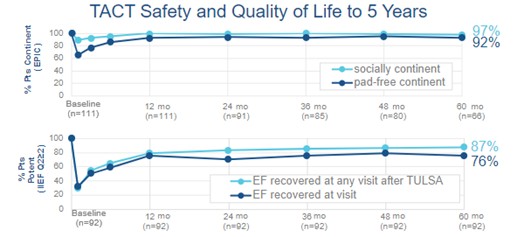
TACT clinical trial urinary continence and erectile function outcomes to five years.
Other TULSA Clinical Studies
Localized Prostate Cancer, Durability of Outcomes: Safety and Precision Study
Our initial multi-jurisdictional clinical trial, which enrolled 30 subjects, demonstrated that MRI-guided TULSA is safe and precise for ablation in patients with localized prostate cancer, providing spatial ablation precision of ± 1.3 mm with a well-tolerated side-effect profile and minor or no impact on urinary, erectile and bowel function at 12 months. Notably, there was no intent to treat in this study which mandated a conservative whole-gland treatment plan less a generous, 3 mm, circumferential safety margin. There was no grade 4 or higher adverse events, one transient attributable grade 3 event (epididymitis), and notably no injury to rectal or periprostatic structures. Functional outcomes measured with the International Prostate Symptom Score (“IPSS”) and International Index of Erectile Function (IIEF-15), both showed a favorable, anticipated trend of initial deterioration with subsequent, gradual improvement toward baseline levels. Intra-operative MRI thermometry measured 90% thermal ablation of the prostate gland, consistent with the wide safety margin which was expected to spare 10% viable prostate at the gland periphery. The median PSA decreased 90% from 5.8 ng/ml pre-treatment to nadir of 0.6 ng/ml, and median prostate volume reduced by 88% on one-year MRI. Even though there was no oncologic intent, and many cancers occur in the intentionally untreated region of the prostate, residual disease was assessed. Prostate biopsy at one year identified decreased cancer burden with 61% reduction in cancer length, clinically significant cancer was found in 9 of 29 men (31%), and any cancer in 16 of 29 (55%).
Follow-up data to three and five years demonstrate durability of the outcomes, with continued treatment safety and stable quality of life, as well as predictable PSA and biopsy oncological outcomes based on treatment-day imaging and early PSA follow-up, without precluding any potential salvage therapy options. Repeat prostate biopsy at three years demonstrated durable histological outcomes, with only one subject upgrading to GG 1 from negative at 12 months, and one subject upgrading to GG 2 from GG 1 at 12 months. Between one and five years, there were no new serious adverse events. By five years, 16 men completed protocol follow-up, three withdrew with PSA <0.4 ng/ml, 10 had salvage therapy without complications (six prostatectomy, three radiation and one laser ablation), and one died of an unrelated cause. Of 16 men with complete follow-up data, five-year median PSA remained at 0.55 ng/ml. Median IPSS of 6 at baseline was stable at 5 by three months, and 6.5 at five years. At baseline, 9 of 16 had erections sufficient for penetration, 11 of 16 at one year, and 7 of 16 at five years. All 16 subjects had leak-free, pad-free continence at one and five years. Predictors of salvage therapy included lower ablation coverage and higher PSA nadir. At five years after TULSA, cancer specific survival was 100%, and overall survival 97%.
6
Clinical Studies of TULSA for Benign Prostatic Hyperplasia (BPH), Relief of Lower Urinary Tract Symptoms (LUTS)
Promising safety and feasibility of the TULSA-PRO system to relieve Lower Urinary Tract Symptoms (“LUTS”) associated with BPH has been demonstrated in two clinical studies showing improvements in IPSS comparable to modern minimally invasive surgical therapies. A retrospective 30 patient prostate cancer Safety and Precision Study analysis from the clinical study of a subgroup of nine patients who also had LUTS concurrent with prostate cancer (baseline IPSS ≥ 12) demonstrated significant IPSS improvement of 58% from 16.1 to 6.3 at 12 months (p=0.003), with at least a moderate (≥ 6 points) symptom reduction in eight of nine patients. IPSS Quality of Life (“QoL”) improved in eight of nine patients. Erectile function (IIEF-EF) remained stable from 14.6 at baseline to 15.7 at 12 months. The proportion of patients with erections sufficient for penetration was unchanged. Full urinary continence (pad-free, leak-free) was achieved at 12 months in all patients. In five men who suffered from more severe symptoms (baseline IPSS ≥ 12 and Qmax < 15 ml/s), peak urine flow rate (“Qmax”) increased from 11.6 ml/s to 22.5 ml/s at 12 months. All adverse events were mild to moderate with no serious events reported.
A prospective clinical study of TULSA-PRO® for BPH has been conducted with early outcomes published in 2022. All measures of urinary function and quality of life improved during the initial twelve-month follow up among the first 10 subjects treated, while no adverse effects were seen on sexual and bowel functions: average IPSS decreased from 17.5 to 4.0, IPSS QoL decreased from 4.0 to 0.5, and Qmax increased from 12.4 ml/s to 21.8 ml/s, among several other improved urinary measures. A single serious adverse event had occurred, abscess of the epididymis requiring drainage at two weeks post therapy. Enrollment of this study has been increased to 30 subjects, with complete study results pending publication.


Select outcomes from clinical trial of TULSA-PRO to relieve lower urinary tract symptoms in men with BPH.
7
Clinical study of TULSA for treatment of radio-recurrent localized prostate cancer, Salvage TULSA (sTULSA)
Salvage ablation of radio-recurrent localized prostate cancer has been evaluated in a prospective clinical study of TULSA-PRO published in 2024. The report includes 39 subjects who were successfully treated. All but one of the subjects were discharged on the first postoperative day; one subject was discharged on the second post-operative day. Median catheterization time was 18 days. Median PSA decreased from 3.3 ng/ml at baseline to 0.05 ng/ml at three months and was 0.17 ng/ml at 12 months. On the 12-month biopsy, 89% of subjects were free of cancer in the treated zone, and 78% were free of cancer in or out of the treated zone. MRI and PSMA PET-CT results were negative for cancer in 92% of subjects within the prostate and 79% overall. Importantly, the population was enriched in more aggressive and high-risk disease at baseline: the distribution of ISUP grade group was 9% GG2, 34% GG3, 25% GG4, and 32% GG5, and two subjects had disease outside the prostate. In contrast with the TACT and other TULSA clinical studies, which restricted to patients with no prior treatment for prostate cancer, the sTULSA population is complex and at significantly increased risk of side effects: before receiving TULSA, all had prior radiation therapy, three subjects had undergone prior salvage therapy after the radiation therapy failed, 16 were receiving hormonal therapy at enrollment, and 12 had a history of transurethral interventions. Serious adverse events were experienced by 28% of subjects, including three patients with puboprostatic fistulas and two patients requiring cystectomy. Still, this is an important study generating evidence of the safety and efficacy of TULSA in an underserved population which faces significant incremental toxicity with standard treatments.
Clinical study of TULSA for palliation of symptomatic locally advanced prostate cancer, Palliative TULSA (pTULSA)
Patients with symptomatic locally advanced prostate cancer can suffer from severe urinary retention due to bladder outlet obstruction, intractable hematuria and frequent hospitalization. While these complications are commonly treated by palliative transurethral resection of the prostate (“TURP”) intended to debulk the tumour, the improvement is often insufficient and TURP may be contraindicated in patients who cannot safely discontinue anticoagulants. The safety and feasibility of MRI-guided TULSA was evaluated as an alternative palliative treatment option for men suffering from symptomatic locally advanced prostate cancer. Ten patients with locally advanced prostate cancer were enrolled, half with clinical stage T4 disease and half with clinical T3. Prior to TULSA, all 10 subjects had continuous indwelling catheterization due to urinary retention, and 90% had history of recurrent and/or ongoing gross hematuria. Three of the subjects had palliative TURP performed six months prior to receiving palliative TULSA, all of which were unsuccessful. One week after palliative TULSA, 50% of the subjects were catheter-free. At last follow-up, 100% of the subjects were free of gross hematuria, and 80% had an improvement in catheterization, with 70% completely catheter-free. Notably, the average hospitalization time from local complications reduced from 7.3 to 1.4 days in the six-month period before and after palliative TULSA. All adverse events were related to urinary tract infections, with two subjects requiring intravenous administration of antibiotics and three subjects resolved with oral antibiotics alone. No other treatment related adverse events were recorded, with no rectal injury or fistula. Further, there was no indication for blood transfusions and there was no perioperative mortality.
8
CAPTAIN trial
CAPTAIN (A Comparison of TULSA Procedure vs. Radical Prostatectomy in Participants with Localized Prostate Cancer) is a prospective, multi-centre randomized controlled trial of 201 subjects aimed at comparing the safety and efficacy of the TULSA procedure (performed with the TULSA-PRO system) with radical prostatectomy (“RP”) in men with organ-confined, intermediate-risk, Gleason Score 7 (Grade Group 2 and 3) prostate cancer. In the CAPTAIN trial, 134 subjects will be randomized to receive one or two TULSA procedures, and 67 subjects will be randomized to receive RP. The trial sites are primarily located in the United States, with the exception of two sites in Canada and one in Europe. Site activation has been completed for 19 sites to date, and those sites are currently recruiting patients.
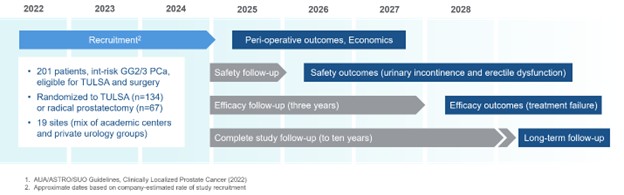
RP is currently the gold-standard surgical treatment for intermediate-risk prostate cancer. RP effectively controls disease but carries risk of significant side effects such as long-term erectile dysfunction and urinary incontinence. The TULSA procedure may reduce the risk of side effects relative to RP, with high spatial, thermal, and anatomic resolution of the target volume enabling precise ablation of prostate tissue while sparing functionally important structures. To achieve precise ablation, the procedure combines transurethral, robotically driven therapeutic ultrasound with real-time visualization of temperature and automated control of heating from magnetic resonance thermometry., potentially.
The goal of the CAPTAIN trial is to demonstrate that the efficacy of the TULSA procedure is not inferior to RP, while demonstrating superior quality of life outcomes in patients receiving the TULSA procedure as compared to those patients receiving RP. The primary safety endpoint is the proportion of subjects who preserve both erectile potency and urinary continence at one year after treatment. The primary efficacy endpoint is the proportion of subjects who are free from any additional treatment for prostate cancer by three years after treatment. Secondary endpoints include comparison of rates of complications, cost effectiveness, and timing of the return to baseline activity. Long-term follow-up will be gathered for up to 10 years after treatment.
Sonalleve
Our Sonalleve system combines real-time MRI and thermometry with focused ultrasound delivered from the outside of the patient to enable precise and incision-free ablation of diseased tissue. We acquired the Sonalleve technology from Philips in 2017.
The Sonalleve system is CE marked in the EU for ablation of uterine fibroids and adenomyotic tissue, palliative pain relief associated with bone metastases, treatment of osteoid osteoma, and management of benign desmoid tumors. The uterine fibroids application is also available for sale in Canada. In 2018, the Sonalleve system was also approved in China and South Korea by the National Medical Products Administration for the non-invasive treatment of uterine fibroids. Philips Oy registered Sonalleve in several Middle East, and Southeast Asian countries. In 2020 Sonalleve also received HDE from the U.S. FDA for treatment of osteoid osteoma in the extremities.
9
Sonalleve Clinical Applications
Uterine Fibroids and Adenomyosis
Uterine fibroids (“UFs”) are the most common non-cancerous tumors in women of childbearing age. Both surgical and medical treatments are available, and the choice depends on number, size, and location of UFs, patient’s age and preferences, and pregnancy expectations. To date, symptomatic UFs have been mostly treated with radical surgery (hysterectomy) in women who have completed childbearing, or conservative surgery (myomectomy and endometrial ablation) in women who wish to preserve fertility. Today, the radiologist also has interventional options available. Minimally or non-invasive interventional radiology procedures include uterine artery embolization.

Uterine fibroid ablation using Sonalleve MR-HIFU
There is currently no ideal treatment for adenomyosis, and new options are needed. Drawing on experience of treatment of uterine fibroids, MR-HIFU has been explored as a potential new conservative treatment and MR-HIFU is an early-stage, non-invasive, therapeutic technology with the potential to improve the quality of life and decrease the cost of care for patients with adenomyosis.
To achieve its current regulatory clearances, the Sonalleve MR-HIFU System has undergone several studies and clinical trials for uterine applications at Sunnybrook Health Sciences Centre (Toronto, Ontario), University Medical Center Utrecht (Utrecht, the Netherlands), University Hospital St. André (Bordeaux, France), Samsung Medical Center (Seoul, Korea), Peking University First Hospital Beijing (Beijing, China), First Affiliated Hospital of Medical College of Xi’an Jiaotong University (Xi’an, China), Turku University Hospital (Turku, Finland), National Institutes of Health (Bethesda, MD, USA), St. Luke’s Episcopal Hospital (Houston, TX, USA), and others.
In addition, a comprehensive literature review provides supportive evidence showcasing the beneficial action of MR-HIFU in uterine fibroid and adenomyosis therapy. These studies include the Verpalen et al. 2020, Nguyen 2020, Yeo et al. 2017, Kim et al. 2017, and Hocquelet et al. 2017 that utilized the Sonalleve MR-HIFU system. Specifically, the studies show impressive performance in terms of ablation efficiency, therapeutic efficacy, symptom reduction, and/or QoL improvement. There were no treatment-related serious adverse events in any of these studies, although Browne et al. 2020 describes a procedure-related major complication in the form of deep vein thrombosis that was noted in one study subject (0.8%) and subsequently and successfully treated with anticoagulation therapy. Minor adverse events, when present, typically include 1st and 2nd degree skin burns, local swelling, cramps, leg pain, abdominal pain, buttock pain, and back pain, which are all known and anticipated adverse events of MR-HIFU therapy.
Palliative Bone Pain Treatment
Pain caused by bone metastases are common in the event of malignancy and are inevitably associated with serious complications that may deteriorate the QoL of patients and become life threatening.
10
For patients with bone metastases, clinical evaluation reports (GCP-10277 Rev. B) were completed in October, 2020 showing significant decrease in pain score, dosage of medication, or quality of life are to be expected with MR-HIFU bone therapy. The randomized controlled Phase III study by Hurwitz et al. published in the Journal of the National Cancer Institute in April 2014 represents some of the most important clinical data that has been reported. In 112 subjects receiving MR-HIFU compared against 35 subjects receiving sham treatment, significant pain reduction at three months (decrease in worst NRS pain ≥ 2 without increase in pain medication) was 64.3% vs. 20.0% (p<0.001), with mean NRS reduction of 3.6 ± 3.1 vs. 0.7 ± 2.4 from an initial median NRS score of 7.0 in both groups. Improvement in average BPI-QoL at three months was 2.4 points superior in the MR-HIFU group (p<0.001), representing a clinically important reduction in impairment caused by bone metastasis pain.
The clinical data show that a statistically significant decrease in pain scores and/or in medication dosage and increase in quality of life are possible with MR-HIFU bone metastasis therapy.
Osteoid Osteoma Treatment
Osteoid osteoma is a relative rare, painful bone tumor that typically occurs in the cortex of long bones, especially in children and adolescents, and accounts for approximately 10% of all benign bone tumors.
Current osteoid osteoma treatment options include surgery and radiofrequency ablation, which is a less invasive option than surgical resection. Although RFA can have a high success rate, the treatment is invasive and can potentially cause minor and major complications. It also exposes patients and operators to ionizing radiation associated with the CT imaging guidance. Sonalleve MR-HIFU provides an alternative therapy choice for osteoid osteoma that is precise, completely non-invasive, and free from ionizing radiation.


Osteoid osteoma treatment using Sonalleve MR - HIFU
Desmoid Tumor Treatment
The recent studies have assessed the use of Sonalleve MR-HIFU in treatment of osteoid osteoma, showing a high clinical success rate and complete symptom resolution without any serious adverse effects and only few minor adverse effects that promptly resolve. The Sonalleve MR-HIFU device offers a novel, minimally invasive, MRI-guided method to treat osteoid osteoma safely and effectively. A desmoid tumor, also called desmoid fibromatosis or aggressive fibromatosis, is a non-metastasizing but locally aggressive proliferation of myofibroblasts that affects children and adults, with a peak incidence in early adulthood. Traditional management of desmoid tumors includes observation, surgical resection, radiation, and/or chemotherapy. Observation allows assessment of the rate of tumor growth and may be acceptable in small, slow-growing, or asymptomatic lesions. Surgical resection is often a highly morbid procedure and has a high rate of recurrence even with negative margins. Radiotherapy provides somewhat improved local control rates but the morbidity from radiation, including burns, fibrosis, chronic edema, and pathologic fractures, is problematic. In addition, the small but finite risk of a radiation-induced malignancy is particularly troublesome in this young patient population, considering the tumor being treated is benign.
11
Recently, MR-HIFU has been assessed as a non-invasive therapy of desmoid tumors, showing good clinical success and even complete tumor eradication in some cases with low number and relative mild adverse events, which typically promptly resolve. The Sonalleve MR-HIFU device offers a novel, non-invasive, MRI-guided method to treat desmoid tumors.
This technology is ideally suited for the treatment of desmoid tumors in a patient population that is generally young, otherwise healthy, and would like to avoid the morbidity of traditional surgical, radiation, and medical therapies for a benign disease. Magnetic resonance imaging provides visualization of critical neurovascular structures and allows sparing of these structures during therapy. While complete ablation of a desmoid tumor may not be possible in all cases because of involvement of these structures, significant reduction in tumor volume is often obtained with a corresponding improvement in pain and functional impairment. As the natural history of the disease often involves recurrence, the ability to re-treat with MR-HIFU without an upper dose limit is also an advantage.
Business Strategy
We initiated our launch of the TULSA-PRO system in the United States in the fourth quarter of 2019 and the first patient was treated in the United States in a clinical service setting in January 2020. Since then, our business model has evolved to a recurring revenue model that includes durable hardware usage, one-time-use devices and our Genius service, which includes necessary support for a productive start-up of the practice. In 2024, we introduced in the United States a capital sales model in addition to the purely recurring revenue model that we have been using since 2019.
We generate revenues from capital sales, one-time-use devices and related services, in the EU (principally in Germany), United States and Asia. For the year ended December 31, 2024, approximately 78%, 8% and 14% of revenues were generated in the United States, EU and Asia, respectively, compared to approximately 71%, 26% and 3%, respectively for the year ended December 31, 2023. Revenue on a quarter over quarter basis is expected to fluctuate given that we are maintaining a limited European commercial effort and remain primarily focused on the U.S. market.
On January 10, 2020, we announced the signing of our first-ever US multi-site imaging center agreement for TULSA-PRO with RadNet, Inc., the largest owner and operator of outpatient imaging centers in the United States, pursuant to which we will install TULSA-PRO systems at three RadNet imaging centers in the greater Los Angeles.
Our TULSA-PRO system is primarily marketed to early adopter physicians who specialize in treatment of prostate disease including urologists and radiologists at opinion leading hospitals. TULSA-PRO services are available at either independent imaging centers or at hospital-based imaging centers.
Historically treatment of conditions such as localized prostate disease and uterine fibroids have included surgical intervention. Over time, surgery has evolved from an ‘open’ technique, to laparoscopic, to robotic surgery. The motivation of surgeons behind this evolution has been to perform procedures that reduce invasiveness, improve clinical outcomes and reduce recovery times. Profound is now taking this concept to the next level by enabling customizable, incision-free therapies for the MRI-guided ablation of diseased tissue with the TULSA-PRO and Sonalleve systems. These incision-free and radiation-free procedures offer surgeons the option of providing predictable and customizable procedures that eliminate invasiveness, offer the potential to improve clinical outcomes and further reduce hospital stays and patient recovery times.
We are establishing our own direct sales and marketing teams for sales of TULSA-PRO systems and the one-time-use devices related thereto, as well as for Sonalleve systems in the jurisdictions where it is approved. The primary focus of our direct sales team is to cultivate adoption of the TULSA-PRO technology, support clinical customers with the TULSA-PRO procedures and increase the utilization of the systems and one-time-use devices. We expect to generate recurring revenues from the use of the system, one-time-use devices, clinical support and service maintenance.
We also collaborate with our strategic partners Philips and Siemens for lead generation and distribution of durable equipment.
On December 2, 2024, Profound Medical and Siemens Healthineers announced a definitive co-sales and co-marketing agreement of its TULSA-PRO and Free.Max MRI, to offer a complete solution for MRI-guided prostate therapy.
12
On December 21, 2020, we entered into a co-development agreement with GE Healthcare (the “GE Agreement”) whereby we and GE Healthcare agreed to a non-exclusive, worldwide license that will enable us to interface our TULSA-PRO system with certain GE Healthcare MRI scanners. The collaboration with GE Healthcare expands our potential to interface with a significant portion of GE’s new and currently installed MRI scanners globally. In March 2022, we confirmed the TULSA-PRO system’s new compatibility with GE Healthcare’s 3T MRI scanners and signed the first site agreement for a TULSA-PRO system interfaced with a GE scanner.
Competition
TULSA-PRO
The TULSA-PRO system is intended to ablate benign and malignant prostate tissue, however there are other treatment options for prostate disease. There are currently no marketed devices indicated for the treatment of prostate diseases or prostate cancer and our FDA indication and CE Mark in the EU also do not include treatment of any particular disease or condition. However, there are a number of devices indicated for the destruction or removal of prostate tissue and devices indicated for use in performing surgical procedures that physicians and surgeons currently utilize when treating patients with prostate disease, including prostate cancer. Approaches that physicians and surgeons currently use to address prostate disease include: (1) watchful waiting/active surveillance; (2) simple prostatectomy; (3) radical prostatectomy (includes open, laparoscopic and robotic procedures); (4) radiation therapies including, external beam radiation therapy, brachytherapy and high dose radiation; (5) cryoablation; and (6) trans-rectal high intensity focused ultrasound (“HIFU”). In addition, certain adjunct or less common procedures are used or are under development to address prostate disease, such as androgen deprivation therapy and proton beam therapy.
Each of the foregoing competing options have their own limitations and benefits and may only be appropriate for limited patient populations. For example, active surveillance is generally recommended for patients who have been diagnosed with earlier stage, lower risk, disease where the possibility of side effects from intervention may outweigh the expected benefit of the chosen procedure. For clinicians and patients, the gap between active surveillance and the most commonly utilized options of surgery or radiation therapy imposes the possibility of substantial side effects, creating a need for a less invasive methodology to remove diseased prostate tissue that is both radiation- and incision-free and provides a more favorable side-effect profile.
We believe that the flexibility of the TULSA-PRO system may allow the Company to demonstrate its use as a tool for ablating benign and malignant diseased prostate tissue with greater speed and precision than current options while minimizing potential side effects. We believe that the TULSA-PRO system may overcome certain limitations of other devices and methodologies for removing or addressing diseased prostate tissue including HIFU, such as complications associated with trans-rectal delivery and limitations relating to prostate size. We believe that a transurethral (inside out) ablation approach with millimeter accuracy has advantages over HIFU in ablating the whole gland safely.
Watchful Waiting; Active Surveillance
Watchful waiting means no treatment until there is an indication that the cancer has spread. Active surveillance is monitoring of the prostate cancer closely with PSA tests and digital rectal exams. Prostate biopsies may also be done to see if the cancer is becoming more aggressive. Test results will indicate whether a more aggressive treatment option should be considered.
Simple Prostatectomy
Simple prostatectomy is recommended for men with severe urinary symptoms caused by an obstructive prostate gland and whose symptoms are not responsive to other medical or minimally-invasive therapies. Simple prostatectomy involves removing only the obstructive portion of the prostate gland rather than the entire gland and surrounding tissue. A simple prostatectomy can be open or robotic. Open simple prostatectomy can be conducted through retropubic, suprapubic, or perineal routes. Simple prostatectomy has higher morbidity and longer hospitalization in comparison to less invasive therapies such as transurethral resection of the prostate. Simple prostatectomy is contraindicated in the presence of cancer.
13
Radical Prostatectomy
Radical prostatectomy, an open surgical removal of the entire prostate gland and some surrounding tissues, represents a current standard of care, practiced by urologists in North America and Europe, which procedure involves the removal of the localized cancerous tissue. However, the conventional open surgical technique has high post-surgery incidences of impotence and incontinence and long recovery time. Recently, robotic surgery systems have become more common in the market. Cited benefits of robotic technique include improved precision and range of motion. Risks specific to robotic technique include longer operation time, the possible need to convert the procedure to a non-robotic approach, and the need for additional or larger incision sites. Converting the procedure could mean a longer operation time, resulting in a longer time under anesthesia.
External Beam Radiation Therapy (“EBRT”)
EBRT requires multiple weekly clinic visits over a period of six to eight weeks. The procedure directs a beam of radiation from outside the body to cancerous tissue inside the body. Although such procedures are relatively costly with studies showing significant risk of collateral damage and lengthy recovery times, it is non-invasive. It can also be used to irradiate cancer that has spread to other areas.
Brachytherapy and High Dose Radiation
With brachytherapy, radioactive seeds are implanted in the prostate to irradiate the cancerous tissue. The seeds irradiate the prostate over time and decay in place to background levels; they remain implanted and inert afterwards. Side effects of brachytherapy are similar to those of EBRT in terms of urinary, bowel and erectile function. An alternative is HDR, in which highly radioactive seeds are temporarily inserted, then removed during the same procedure, leaving nothing implanted afterward. HDR has the ability to target tissue, but requires hospital stays and usually is accompanied by adjunct EBRT over several weeks.
Cryoablation
Cryoablation freezes cells to death by introducing cooled liquids and gases to an area of cancerous tissue. Studies show cryoablation offers poor precision and has delivered impotence rates that are almost as high as those for conventional radical prostatectomy. The procedure also carries a risk of potential damage to the tissue between the urethra and rectum, potentially resulting in a urinary rectal fistulas.
Trans-rectal High Intensity Focused Ultrasound (“HIFU”)
Trans-rectal HIFU is used increasingly in the European Union, United States and Canada. This technique utilizes focused ultrasound that is delivered through the rectal wall to treat the prostate. Image guidance is generally provided by ultrasound. At an FDA urology panel meeting in 2014, the panel indicated that HIFU can lead to complications such as rectal fistulae and rectal incontinence. Due to the focused treatment zone, this treatment requires approximately three hours to complete. One limitation of HIFU is prostate size; the procedure is limited to patients with prostate volume smaller than 40 cubic centimeters. Patients with larger prostates need a separate surgical procedure, such as TURP or ADT, both described below, to de-bulk or reduce the size of the prostate prior to HIFU. This additional procedure increases costs and the risk of complications. Recent studies have indicated positive survival outcomes and thermal ultrasound appears to be gaining traction in certain settings.
Adjunct and Emerging Therapies
Androgen deprivation therapy (“ADT”) uses hormones to suppress testosterone production and alleviate symptoms, but with the primary side-effect of reduced sexual interest and activity. Although historically used as a last line of defense for the disease (and typically in a palliative setting), it is increasingly used as a first line treatment or in combination with other treatments.
TURP is a surgical procedure that removes portions of the prostate gland through the penis. This procedure is used to relieve moderate to severe urinary symptoms caused by an enlarged prostate, a condition known as BPH. This procedure is also used in adjunct to a HIFU procedure when a prostate gland is larger than 40 cubic centimeters.
14
Proton beam therapy is a way to deliver radiation to tumors using tiny, sub-atomic particles (protons) instead of the photons used in conventional radiation treatment. Proton beam therapy uses new technology to accelerate atoms to approximately 93,000 miles per second, separating the protons from the atom. While moving at this high speed, the particles are “fired” at the patient’s tumor. These charged particles deliver a very high dose of radiation to the cancer but release very little radiation to the normal tissue in their path. In theory, this approach minimizes damage to healthy organs and structures surrounding the cancer. The radiation beams must pass through the skin, the bladder and the rectum on the way to the prostate gland, and once they reach the gland, they encounter normal prostate cells and the nerves that control penile erections. Damage to these tissues can lead to complications, including bladder problems, rectal leakage or bleeding, and erectile dysfunction.
We believe that use of the TULSA-PRO system as a tool to ablate prostate tissue can provide a clinician and his or her patients with the following clinical advantages:
| ● | Clinically shown to have millimeter accuracy designed to ablate prostate tissue while sparing nearby critical structures, and that real time MR thermometry also ensures precision in ablation temperature, minimizing side effects that can occur from overheating; |
| ● | Enables clinician to define the boundaries of the tissue to be ablated, whether the whole prostate or any of its subsections, to ensure customization of the needs of each patient; |
| ● | Transurethral approach allows for ablation of even the largest prostates that may be 120 cubic centimeters or larger in size; |
| ● | Potential to be a single outpatient procedure with a rapid recovery time; and |
| ● | Designed to be compatible with leading MRI platforms and could become part of a continuum of care from MR imaging diagnosis, MR guided biopsy to MR guided treatment. |
We believe that the flexibility of the TULSA-PRO system may allow us to demonstrate its use as a tool for ablating benign and malignant diseased prostate tissue with greater speed and precisions than current options while minimizing potential side effects. We believe that the TULSA-PRO system may overcome certain limitations of other devices and methodologies for removing or addressing disease prostate tissue including HIFU, such as complications associated with trans-rectal delivery and limitations relating to prostate size. We believe that a transurethral (inside out) ablation approach with millimeter accuracy has advantages over HIFU in ablating the whole gland safely.
Sonalleve
The treatment choices for uterine fibroids usually depend on the symptoms of the patient, size of the fibroid, desire for future pregnancy, and preference of the treating gynecologist. Most common treatment options for uterine fibroids include: (1) hormonal medications including gonadotrophin releasing hormone agonists (“Gn-RH”); (2) progesterone releasing intra-uterine devices; (3) surgical procedures such as hysterectomy and myomectomy; and (4) uterine artery embolization.
We believe that the Sonalleve system may provide a treatment option that is more convenient and comfortable with less side effects than surgical procedures, such as hysterectomy or myomectomy.
Hormonal Medications
Fibroids can be treated with hormonal drugs, such as Gn-RH agonists. Gn-RH agonists can treat fibroids by blocking the production of estrogen and progesterone, putting women into a temporary postmenopausal state. As a result, menstruation stops, fibroids shrink, and anemia is often alleviated. Other hormonal medications can also be utilized in patients with uterine fibroids. In many cases, however, medication may provide only temporary relief from the symptoms caused by fibroids. The symptoms often return when the patient stops taking the medication. Moreover, the side effects of some drugs may cause them to be unsuitable for some patients. Gn-RH agonists typically are used for no more than three to six months because long-term use can cause loss of bone.
Progesterone Releasing Intra-Uterine Devices
Progesterone releasing intra-uterine devices can relieve heavy bleeding caused by fibroids. However, these devices can only provide symptom relief and do not impact the fibroid itself.
15
Uterine Artery Embolization
Uterine artery embolization involves injection of embolic agents into the arteries that supply the uterus, thereby cutting off the blood supply to the fibroids. Many women require at least one day of hospitalization and heavy pain medication. The prolonged pain may slow down the recovery period. Complications may occur if the blood supply to the ovaries or other organs is compromised.
Surgery
Surgical options for the treatment of uterine fibroids include hysterectomy and myomectomy. Hysterectomy is a surgical procedure which involves the complete removal of uterus with or without removal of the cervix, ovaries and fallopian tubes. Hysterectomy can be performed abdominally in an open, laparoscopic, robotic-assisted or vaginal method. Surgical options are associated with blood loss, hospital stays, long recovery times, pain and scarring. Post-operative complications can include infections, urinary incontinence, vaginal prolapse, fistula formation and chronic pain. After a hysterectomy, a woman will enter menopause and is infertile. Myomectomy is a surgical procedure to remove uterine fibroids from the wall of the uterus. The procedure can be performed with an abdominal incision, laparoscopic, or hysteroscopic.
Current osteoid osteoma treatment options include surgery and radiofrequency ablation, which is a less invasive option than surgical resection. Although RFA can have a high success rate, the treatment is invasive and can potentially cause minor and major complications. It also exposes patients and operators to ionizing radiation associated with the CT imaging guidance.
We believe that use of the Sonalleve system as a tool to ablate uterine fibroids or osteoid osteoma can provide a clinician and his or her patients with the following clinical advantages:
| ● | Millimeter accuracy designed to ablate uterine fibroid while sparing nearby critical structures; |
| ● | Outpatient procedure with rapid recovery time, not requiring general anesthesia; and |
| ● | Non-invasive approach using thermal ablation designed to heat the uterine fibroid; and guided by real-time MRI with temperature (thermometry) feedback. |
Intellectual Property
Our intellectual property is comprised of a broad and world-wide portfolio of patents, patent applications, trademarks, copyrights, trade secrets and other proprietary assets. Our intellectual property portfolio is both growing and dynamic and includes approximately 40 patent families representing approximately 165 granted or allowed patents and 25 patent applications in various stages of review and prosecution around the world.
Many of our patents and patent applications claim electronic and mechanical aspects of hardware, software and methods related to ultrasonic ablation of tissue. The intellectual property assets are largely directed to (i) using real time MRI imaging as a tool to plan, monitor or control said ultrasonic ablation; (ii) MRI thermometry methods, especially in respect of our ultrasound therapy processes and devices; (iii) the phasing, beam-forming, and control of acoustic arrays and similar energy sources; (iv) computational method to improve filtering, imaging and analyzing the results of MRI-guided thermal therapy processes; and (v) secondary and support systems such as active cooling of near-target tissues. The portfolio covers both the “TULSA” and the “Sonalleve” families of products, as well as generic technologies and applications and extensions of our products.
We believe that the protection of our intellectual property is an essential element of our business and we intend to continue our investment in the development of our intellectual property portfolio. We have worked over the past year to pursue, maintain and expand on the intellectual property portfolio acquired from Philips in 2017. This intellectual property has been strengthened and extended to many jurisdictions around the globe in support of our sales, development and marketing efforts.
We pursue a global intellectual property strategy, registering for patent protection in all jurisdictions where we intend to carry on business, including the United States, Canada, Japan, major European markets (e.g., Germany, France, U.K., Italy, Spain and Turkey) and the emerging markets (e.g., Brazil, Russia, India, and China).
16
We also rely upon trade secrets, know-how and other proprietary, confidential information for the protection of our technology. We require all employees, consultants, scientific advisors and other contractors to enter into confidentiality agreements to protect against the disclosure of such proprietary information. Each inventor is required to execute a formal assignment specific to each invention that he or she has listed, and which is officially recorded in the proper patent office.
In addition to developing our own intellectual property portfolio, we have licensed and acquired intellectual property rights from third parties through exclusive licenses, collaborative research and asset purchase agreements. Material license agreements include an exclusive license with Sunnybrook entered into on May 11, 2010 (the “Sunnybrook License”). Under the Sunnybrook License, Sunnybrook granted us an exclusive worldwide and royalty-free right to use certain defined Sunnybrook technology in connection with, among other things, manufacturing, marketing and selling products such as the TULSA-PRO system, in the field of MRI-guided transurethral ultrasound therapy. Under the license, we are subject to various obligations, including a milestone payment of C$250,000 that was paid in connection with our FDA clearance of TULSA-PRO in August 2019. In addition, we are required to pay legal costs associated with patent application preparation, filing and maintenance. If either party to the Sunnybrook License breaches or fails to perform a material obligation and fails to cure such breach or perform such obligations within a 30-day cure period, the non-breaching party may terminate the agreement. Material obligations include our agreement not to use the technology or intellectual property outside of the license scope, not to use the technology or intellectual property outside the field of MRI-guided transurethral ultrasound therapy (or permitting our customers to do so) and not to breach confidentiality obligations.
Regulatory
On August 15, 2019, we obtained 510(k) clearance for commercial sale of the TULSA-PRO as a class II device in the United States and have previously received a CE Certificate of Conformity for our products in European Union, and we have obtained regulatory approval for Sonalleve in China. On November 20, 2019, the TULSA-PRO was approved as a class III device by Health Canada, which is key to our global expansion strategy that requires a country-of-origin approval for medical devices. Additionally, the TULSA-PRO system has received regulatory clearances or approvals for commercial sale in Saudi Arabia, Singapore, South Korea and Malaysia, while the Sonalleve system has received regulatory clearance or approval for commercial sale in Canada, Saudi Arabia, South Korea and Malaysia. Our long-term goal is to expand our regulatory indications in Asia and other parts of the world where potential profitable business development opportunities warrant such investments.
United States
Regulation of Medical Devices
The FDA strictly regulates medical devices under the authority of the federal Food, Drug, and Cosmetic Act (“FFDCA”) and the regulations promulgated by the FDA under the FFDCA. The FFDCA and the implementing regulations govern, among other things, the following related to our products: preclinical and clinical testing, design, manufacture, safety, efficacy, labeling, packaging, storage, installation, servicing, record keeping, sales and distribution, importation, post-market adverse event reporting, recalls, and advertising and promotion.
The TULSA-PRO system, Sonalleve, and any future medical devices that we may develop, will be classified by the FDA under the statutory framework described in the FFDCA. Medical devices are classified into three classes from lowest risk (class I) to highest risk (class III). Unless an exemption applies, medical devices require FDA clearance or approval prior to commercial sale in the United States depending on the assigned risk class. Most class I devices and some class II devices are exempt from premarket review requirements. Class I devices are subject to “general controls,” which include establishment registration and device listing, requirements of the Quality System Regulation (“QSR”), labeling requirements, medical device reporting, and reporting of corrections and removals.
Most class II devices and some class I devices require FDA clearance of a 510(k) premarket notification prior to marketing; however, the FDA has the authority to exempt a class II device from the premarket notification requirement under certain circumstances. As a result, manufacturers of most class II devices must submit premarket notifications to the FDA under Section 510(k) of the FFDCA (21 U.S.C. § 360(k)) in order to obtain the necessary authorization to market or commercially distribute such devices. To obtain 510(k) clearance, manufacturers must submit to the FDA adequate information demonstrating that the proposed device is “substantially equivalent” to a “predicate device” that is already on the market. A predicate device is a legally marketed device that is not subject to PMA, meaning, (i) a device that was legally marketed prior to May 28, 1976 (a “preamendments device”) and for which a PMA is not required, (ii) a device that has been reclassified from class III to class II or I, or (iii) a device that was found substantially equivalent through the 510(k) process.
17
Following receipt of a premarket notification for a device, the FDA determines whether the submission is sufficiently complete to permit a substantive review. The agency typically issues a decision on a 510(k) application that is accepted for review within 90 days of receipt. However, the FDA may stop the review clock for up to 180 days to request that the applicant respond to the agency’s requests for additional information about the proposed device. If the FDA agrees that the device is substantially equivalent to the predicate device identified by the applicant in a premarket notification submission, the agency will grant 510(k) clearance for the new device, permitting the applicant to commercialize the device. Premarket notifications are subject to user fees, unless a specific exemption applies. In addition to the general controls, Class II devices are subject to “special controls,” such as performance standards, post-market surveillance requirements, patient registries and guidance documents, as identified in the classification regulation for the device type.
If there is no adequate predicate to which a manufacturer can compare its proposed device, the proposed device is automatically classified as a class III device. In such cases, a device manufacturer must then fulfill the more rigorous PMA requirements or can request a risk-based classification determination for its device in accordance with the De Novo classification process.
Devices that are intended to be life sustaining or life supporting, devices that are implantable, devices that present a potential unreasonable risk of harm or are of substantial importance in preventing impairment of health, and devices that are not substantially equivalent to a predicate device and for which safety and effectiveness cannot be assured solely by the general controls and special controls are placed in class III. Such devices require FDA approval of a premarket approval application, or PMA, demonstrating reasonable assurance of safety and effectiveness of the device, prior to commercial distribution, unless the device is a preamendments device not yet subject to a regulation requiring premarket approval. Class III devices are subject to the general controls and any conditions of approval in the PMA approval order, which can include postmarket study requirements. The PMA process requires the manufacturer to demonstrate through extensive data, including data from preclinical studies and one or more clinical studies, that the device is safe and effective for its proposed indication. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review and determine whether the proposed device can be approved for commercialization, although in practice, PMA reviews often take significantly longer, and it can take up to several years for the FDA to issue a final decision. Before approving a PMA, the FDA generally also performs an on-site inspection of manufacturing facilities for the product to ensure compliance with the QSR.
If the FDA’s evaluation of the PMA application and inspection of the manufacturing facility is favorable, the FDA may issue an approval order authorizing commercial marketing of the device, or an “approvable letter,” which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been met to the satisfaction of the FDA, the agency will issue a PMA approval order, subject to the conditions of approval and the limitations established in the approval order. If the FDA’s evaluation of a PMA application or manufacturing facility is not favorable, the FDA will deny approval of the PMA or issue a “not approvable letter.” The FDA may also determine that additional studies are necessary, in which case the PMA approval may be delayed for several months or years while such additional studies are conducted and data is submitted in an amendment to the PMA. The PMA process can be expensive, uncertain and lengthy, and each PMA submission is subject to a substantial user fee unless a specific exemption applies. PMA approval may also be granted with post-approval requirements such as the need for additional patient follow-up or requirements to conduct additional clinical trials.
Novel devices that have not been classified and devices deemed not substantially equivalent to a predicate device are automatically classified into class III. The manufacturer can submit a De Novo classification request to classify such a device into class I or class II based on evidence that the device in fact presents low or moderate risk, instead of following the typical class III device pathway requiring the submission and approval of a PMA application. The FDA typically issues a decision on a De Novo classification request within 150 days of receipt. If the manufacturer seeks reclassification into class II, the classification request must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. If the FDA grants the De Novo request, the device may be legally marketed in the United States. However, the FDA may reject the classification request if the agency identifies a suitable legally marketed predicate device that provides a reasonable basis for review of substantial equivalence or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and adequate special controls cannot be developed. De Novo classification requests are subject to user fees, unless a specific exemption applies.
18
There is also a separate pathway for Humanitarian Use Devices, which are medical devices intended to benefit patients in the treatment or diagnosis of a disease or condition that affects or is manifested in not more than 8,000 individuals in the United States per year. Once a device has received designation as a Humanitarian Use Device, the sponsor may seek marketing authorization for the device under a Humanitarian Device Exemption (“HDE”) application. An HDE application must demonstrate the device will not expose patients to an unreasonable or significant risk of illness or injury and the probable benefit to health outweighs the risk of injury or illness (but is not required to demonstrate reasonable assurance of effectiveness). Devices with an approved HDE may only be used pursuant to the review and authorization of an institutional review board (“IRB”) and are subject to certain profit and use restrictions, in addition to all applicable general controls.
After a device is placed on the market, numerous regulatory requirements apply. Device manufacturers must register their establishments annually, list the devices they manufacture and pay an annual registration fee. Device manufacturers are also subject to the QSR, which includes both design control requirements and good manufacturing practice requirements (such as requirements for purchasing controls, document controls, production and process controls, labeling and packaging controls, control of nonconforming product, complaint handling, corrective and preventative actions, storage, handling, distribution, and servicing). Devices must be labeled in accordance with the FDA’s device labeling regulations, including Unique Device Identification requirements. The FDA also regulates the promotion of medical devices, including a requirement that all device promotion be truthful and non-misleading and a prohibition against the promotion of devices for “off-label” uses, i.e., uncleared or unapproved uses.
Under the medical device reporting regulations, manufacturers must submit a report to the FDA if they become aware of information that reasonably suggests that one of their marketed devices may have caused or contributed to a death or serious injury or malfunctioned and the malfunction would be likely to cause or contribute to a death or serious injury if it were to recur. The medical device reporting requirements also extend to healthcare facilities that use medical devices in providing care to patients, or “device user facilities,” which include hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or outpatient treatment facilities, but not physician offices. A device user facility must report any device-related death to both the FDA and the device manufacturer, or any device-related serious injury to the manufacturer (or, if the manufacturer is unknown, to the FDA) within 10 days of the event. Device user facilities are not required to report device malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur but may voluntarily report such malfunctions through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program.
Manufacturers must also report any corrections or removals, which can include, among other actions, repairs, adjustments, relabeling, or destruction of distributed devices, if the correction or removal was initiated to reduce a risk to health or to remedy a violation of the FFDCA caused by the device which may present a risk to health. The FDA also has the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture. The authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious adverse health consequences or death. A manufacturer may, under its own initiative, recall one or more of its products if any distributed devices fail to meet established specifications, are otherwise misbranded or adulterated under the FDCA, or if any other material deficiency is found. A device manufacturer must report to the FDA any correction, removal or recall of its devices, if such action is taken to reduce a risk to health posed by such devices or to remedy a violation of the FDCA caused by such devices that may present a risk to health, within 10 working days after the recall is initiated.
In addition, any modification to a legally marketed device (regardless of marketing authorization pathway) that could significantly affect the device’s safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) submission or a new PMA or PMA supplement. The FDA requires each manufacturer to make the determination of whether a device modification requires a new 510(k) or PMA submission in the first instance, but the FDA may review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance or PMA for a particular change, the FDA may retroactively require the manufacturer to submit a 510(k) or PMA application. The FDA may also require the manufacturer to cease its marketing activities for the modified device in the United States and/or recall the device until the appropriate marketing authorization for the modification is obtained.
19
The FDA has broad enforcement authority to take action against a failure to comply with the clinical trial, premarket review, or postmarket regulatory requirements discussed above and the agency conducts routine inspections of device manufacturers to determine compliance with these requirements. FDA enforcement typically takes the form of inspectional observations at the close of inspection, a warning letter (a public letter alleging violations of regulatory significance), or an untitled letter (a typically non-public letter alleging violations of lesser significance). However, the FDA has authority to take additional enforcement actions, including: civil monetary penalties, criminal fines and prosecution, injunctions, product seizure, withdrawal of marketing authorizations, mandatory recall, and import detentions.
Medical Device Clinical Studies
Clinical studies are almost always required to support PMA applications and are sometimes required to support 510(k) and De Novo classification submissions. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with, among other laws and regulations governing clinical trials and human subject protections, the FDA’s good clinical practice (“GCP”) regulations, including the investigational device exemption (“IDE”) regulations that govern investigational device labeling, prohibit promotion of investigational devices, and specify recordkeeping, reporting and monitoring responsibilities of trial sponsors and investigators. If the device presents a “significant risk,” as defined by the FDA, the agency requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical studies. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a patient. An IDE application must be supported by appropriate non-clinical data, such as animal and laboratory test results, showing that the device has a safety profile appropriate for human testing and that the trial protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA expressly approves or denies the application in writing or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the trial, the FDA may permit a clinical trial to proceed under a conditional approval or the sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. In addition, the trial must be approved by, and conducted under the oversight of an IRB for each clinical site. If the device presents a non-significant risk to the patient according to criteria established by FDA as part of the IDE regulations, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate authorization from the FDA, but must still comply with abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
As part of its clinical trial oversight responsibilities, an IRB must review and approve, among other things, the trial protocol and informed consent information to be provided to clinical trial subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical studies, including details of the protocol and eventually trial results, also must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on the ClinicalTrials.gov data registry. Information related to the product, patient population, phase of investigation, trial sites and other aspects of the clinical trial are made public as part of the trial registration. Sponsors are also obligated to disclose the results of their clinical studies after completion. Disclosure of the results of these studies can be delayed in some cases for up to two years after the date of completion of the trial. Failure to timely register a covered clinical study or to submit study results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. The NIH Final Rule on ClinicalTrials.gov registration and reporting requirements became effective in 2017, and the government has brought enforcement actions against non-compliant clinical trial sponsors.
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and more frequently if an unanticipated serious adverse event (“SAE”) occurs. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the clinical protocol, GCP, or other IRB requirements or if the investigational product has been associated with unexpected serious harm to patients.
20
In the Consolidated Appropriations Act for 2023, Congress amended the FFDCA to require the sponsor of any pivotal clinical trial that will be used to demonstrate the safety and effectiveness of a medical device marketing authorization submission to develop a diversity action plan for such trial, and if submission of an IDE application is required, to submit such diversity action plan to the FDA. The action plan must include the sponsor’s diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them. The FDA may grant a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect device pivotal clinical trial planning and timing, but if FDA objects to a sponsor’s diversity action plan and requires the sponsor to amend the plan or take other actions, it may delay trial initiation.
Federal Trade Commission Regulatory Oversight
Our advertising for our products is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission, or FTC, as well as comparable state consumer protection laws. Under the Federal Trade Commission Act, or FTC Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
European Union
On April 5, 2017, the EU adopted a new Medical Devices Regulation (EU) 2017/745 (the “New EU MDR”), which repealed and replaced the Medical Devices Directive (MDD) effective May 26, 2021. Under transitional provisions as they currently stand, medical devices with Notified Body certificates issued under the Medical Devices Directive prior to May 26, 2021 will remain valid until December 31, 2027 (for class III and class IIb implantable devices) or until December 31, 2028 (for medium and low risk class IIb devices, as well as class IIa, Im, Is, and Ir devices), except for certificates issued in accordance with Annex IV to the Active Implantable Medical Devices Directive 90/385/EEC or Annex IV to the MDD which became void at the latest on May 27, 2022. To be eligible for the transitional validity period, the device manufacturer must have submitted to an authorized Notified Body an application for conformity assessment to the New EU MDR by May 26, 2024; entered into a written agreement for surveillance by an authorized Notified Body by September 26, 2024; implement a quality management system that complies with applicable MDR requirements; ensure the devices continue to comply with applicable MDD requirements; not make any significant changes to the design or intended use of such devices; and ensure that the devices do not present an unacceptable risk to health and safety. After the expiry of any applicable transitional period, only devices that have been CE marked under the New EU MDR may be placed on the market in the EU.
On the basis that TULSA-PRO and Sonalleve systems benefit from the New EU MDR transition period, these devices can be placed on the market under their MDD certificates provided they, and we, continue to comply with the eligibility criteria for the transitional validity period described above. Under the MDD, legal manufacturers of medical devices, such as the TULSA-PRO and Sonalleve systems, are required to comply with the essential requirements laid down in Annex I of the MDD (the “Essential Requirements”). Active implantable medical devices and in-vitro diagnostic medical devices are regulated in separate EU directives. Compliance with these requirements, in addition to the other eligibility criteria described above, during the transition period to the New EU MDR entitles us to affix the CE Mark to our medical devices, without which they cannot be commercialized in the European Union. To demonstrate compliance with the Essential Requirements and obtain the right to affix the CE Mark to our medical devices, the MDD required the TULSA-PRO and Sonalleve devices to undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. The MDD provides for four different classifications of medical devices based on their potential risks and vulnerability of the human body: Class I, Class IIa, Class IIb and Class III. Except for low-risk medical devices (Class I with no measuring function and which are not sterile), in relation to which the manufacturer may prepare an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the Essential Requirements, a conformity assessment procedure requires the intervention of a Notified Body. A Notified Body is a private entity designated by the competent authorities of a European Union Member State to conduct conformity assessments and to perform their tasks under the MDD (as implemented in the respective national legal system) in the public interest. Depending on the device’s risk category/class, the conformity assessment of the Notified Body extends to the quality assurance system established by the manufacturer and/or the product design, as well as to the Technical Documentation to be compiled by the manufacturer for each device to demonstrate compliance with the relevant Essential Requirements. The Notified Body issues a certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the Essential Requirements. This certificate, which is valid for up to five years, entitles the manufacturer to affix the CE Mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
21
Therefore, when the MDD certificates become void, medical devices need to fully comply with the New EU MDR. The MDR changed several aspects of the regulatory framework for medical device marketing in Europe in order to increase regulatory oversight of all medical devices marketed in the EU (which, in turn, increased the costs, time and requirements to place innovative or high-risk medical devices on the European market). The MDR among other things:
| ● | strengthens the rules on placing devices on the market and reinforces surveillance once they are available; |
| ● | establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| ● | improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| ● | sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the European Union, or EU; and |
| ● | strengthens the rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts before they are placed on the market. |
An overarching requirement under the MDR is that any device must be designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others. In addition, the device must meet the performance specifications intended by the manufacturer and be designed, manufactured and packaged in a suitable manner. To that effect, the European Commission has adopted various standards applicable to medical devices. These include standards governing common requirements, such as sterilization and safety of medical electrical equipment and product standards for certain types of medical devices. There are also harmonized standards relating to design and manufacture. A harmonized standard is a European standard developed by a recognized European Standards Organization. While not mandatory, compliance with harmonized standards is a way for manufacturers to demonstrate that products comply with relevant EU legislation.
To demonstrate compliance with the General Safety and Performance Requirements (“GSPRs”) set forth in the MDR, medical device manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its (risk) classification, similar to the conformity assessment procedure under the MDD. Conformity assessment procedures require an assessment of the technical documentation, including the device description, the design stages, the manufacturing process, available clinical evidence, literature data for the product, and post-market experience in respect of similar products already marketed. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can self-declare the conformity of its products with the GSPRs (except for any parts which relate to sterility or measuring functions), a conformity assessment procedure requires the intervention of a Notified Body. A Notified Body typically audits and examines a product’s technical dossiers and the manufacturer’s quality management system (which must, in particular, comply with ISO 13485 related to Medical Devices Quality Management Systems). If satisfied that the medical device conforms to the relevant GSPRs, the Notified Body issues a certificate of conformity, which is valid for a fixed duration (not to exceed five years) and which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE Mark to the device, allowing the device to be legally marketed throughout the EU.
Throughout the term of the certificate, the manufacturer will be subject to periodic surveillance audits to verify continued compliance with the applicable requirements. In particular, there will be a new audit by the Notified Body before it renews the relevant certificate(s).
As a general rule, demonstration of conformity of medical devices with the GSPRs must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. In order to demonstrate safety and effectiveness for their medical devices, manufacturers must, save limited exceptions, conduct clinical investigations in accordance with the requirements of Annex VII and Annex XV to the MDR. Clinical investigations for medical devices usually require the approval of an ethics committees and approval by the national regulatory authorities. Both regulators and ethics committees also require the submission of periodic safety reports during a study and may request a copy of the final study report.
22
After a device is placed on the market, it remains subject to significant regulatory requirements. For CE marked devices, certain modifications to the device or quality system depending on the conformity assessment procedure used must be submitted to and approved by the Notified Body before placing the modified device on the market. Economic operators, include device manufacturers, must register their establishments and devices in the EUDAMED database starting in January 2026. Additionally, manufacturers and authorized representatives must now appoint a person responsible for regulatory compliance.
In the European Union, we must establish a medical device vigilance system (for reporting incidents) and a post-marketing surveillance system (to monitor data about the device and confirm the benefits of the device continue to outweigh the risks). Under this system, serious incidents occurring in the EU that led, might lead or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health (either temporary or permanent) or that pose a serious public health threat must be reported to the competent authorities of the European Union Member States. Manufacturers are required to take Field Safety Corrective Actions (“FSCAs”), including product recalls and withdrawals, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. Manufacturers must report any FSCAs in respect of devices made available on the market or undertaken in a third country in relation to a device made available on the EU market.
If the requirements for application of the CE Mark are not (or no longer) fulfilled, or in other cases of non-compliance with applicable medical devices law:
| ● | the Notified Body has the power to withdraw, suspend or limit the scope of the applicable certificate of conformity, in accordance with the principle of proportionality; |
| ● | the competent authorities of the EU Member States may require relevant economic operators to take the necessary actions to bring the device into compliance and/or address the risk, which can include withdrawal from the market or recall; and |
| ● | depending on the EU member state, criminal and/or administrative sanctions (e.g., fines) may apply. |
The New EU MDR prohibits making any misleading claims about a device’s intended purpose, safety and/or performance. Therefore, devices can only be marketed for their intended purpose. In addition, the advertising and promotion of our products in the European Union are subject to the provisions of Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other national legislation in the individual European Union Member States governing the advertising and promotion of medical devices. These laws may limit or restrict the advertising and promotion of our products to the public and may impose limitations on our promotional activities with healthcare professionals.
United Kingdom
The United Kingdom left the European Union on January 31, 2020 (commonly referred to as “Brexit”), with a transitional period that expired on December 31, 2020. The United Kingdom and the European Union entered into a trade agreement known as the Trade and Cooperation Agreement (“TCA”), which became effective on January 1, 2021. The TCA does not specifically refer to medical devices. However, as a result of Brexit, the New EU MDR will not be implemented in the United Kingdom (except in Northern Ireland), and previous legislation that mirrored the New EU MDR in UK law has been revoked. The regulatory regime for medical devices in the United Kingdom will continue to be based on the requirements derived from previous EU legislation, and the United Kingdom may choose to retain regulatory flexibility or align with the MDR going forward. CE-Markings will continue to be recognized in the United Kingdom, and certificates issued by EU recognized Notified Bodies will be valid in the United Kingdom, until the earlier of June 30, 2028 or the expiration of the certificate for devices compliant with the MDD or until June 30, 2030 for devices compliant with the New EU MDR. For medical devices placed on the UK market after this period, the UK Conformity Assessed (“UKCA”), marking will be mandatory. In contrast, UKCA marking and certificates issued by UK Notified Bodies will not be recognized on the EU market. The TCA does provide for cooperation and exchange of information in the area of product safety and compliance, including market surveillance, enforcement activities and measures, standardization related activities, exchanges of officials, and coordinated product recalls (or other similar actions). For medical devices that are locally manufactured but use components from other countries, the “rules of origin” criteria will need to be reviewed. Depending on which countries products will ultimately be sold in, manufacturers may start seeking alternative sources for components if this would allow them to benefit from no tariffs. In March 2023, the UK government and the European Commission reached agreement on a regulatory framework, the Windsor Framework, governing the marketing of medical products in Northern Ireland. Under the Windsor Framework, which became effective on January 1, 2025, the New EU MDR and CE-mark requirements will continue to apply to medical devices marketed in Northern Ireland. It remains to be seen how UK rules will impact regulatory requirements for our product candidates and our product in the United Kingdom.
23
We continue to evaluate the potential impacts on our business of the TCA, and any amendments, or other agreements affecting trade between the UK and EU.
Canada
Health Canada’s Therapeutic Products Directorate (“TPD”) is the Canadian authority that regulates medical devices. In general, prior to being given market authorization to sell a Class II, III or IV medical device in Canada, a manufacturer must present and/or attest to substantive scientific evidence of a product’s safety, efficacy and quality as required by the Food and Drugs Act and the Medical Devices Regulations (“Canada MDR”).
The Medical Devices Bureau (“MDB”) of the TPD applies the Canada MDR through a combination of pre-market review, post-approval surveillance and quality systems in the manufacturing process. Medical devices are classified into one of four classes, where Class I represents the lowest risk and Class IV represents the highest risk. In order to perform investigational testing involving human subjects in Canada for a Class II, III or IV medical device, authorization for the testing must be granted by the MDB. A Medical Device License is a pre-market requirement for a Class II, III and IV medical device, including for Class II, III or IV medical devices previously authorized for sale for investigational testing now to be offered for general/commercial sale. A Medical Device License is issued to the device manufacturer, provided the requirements of the Canada MDR are met.
The Canada MDR requires that medical devices be manufactured under a certified QMS that meets the criteria of the international standard, ISO 13485 (“Medical devices–Quality management systems–Requirements for regulatory purposes”). The MDB currently recognizes the Medical Device Single Audit Program, which provides for a single audit procedure recognized by Australia, Brazil, Canada, Japan and the United States demonstrating routine compliance with quality management system requirements. We manufacture the TULSA-PRO and Sonalleve systems under a certified ISO 13485 quality management system.
Regulatory Status
TULSA-PRO
On November 20, 2019, TULSA-PRO received approval as a class III device from Health Canada, which is key to our global expansion strategy that requires a country of origin approval for medical devices. On August 15, 2019, we received 510(k) clearance for commercial sales of the TULSA-PRO as a class II device in the United States for TULSA of prostate tissue, and in April 2016 the TULSA-PRO system was CE marked in the European Union for ablation of targeted prostate tissue (benign or malignant). Outside of these jurisdictions, the TULSA-PRO system will require country-specific pre-market clearance or approval prior to launch.
Upon completion of our safety and feasibility study for TULSA-PRO in April 2016, we were granted CE Mark approval for the commercial sale of the TULSA-PRO system in Europe and in other CE Mark jurisdictions.
In August 2016, we initiated the TACT Pivotal Clinical Trial, which the FDA approved under an IDE application. The TACT Pivotal Clinical Trial was designed to support a 510(k) premarket notification submission in the United States. This submission was made in May 2019 in support of clearance of the TULSA-PRO system by the FDA for use in the ablation of prostate tissue in the United States.
In Canada, we are currently manufacturing the TULSA-PRO system under a certified ISO 13485 quality management system. The Canadian market is considered a lower priority from a commercialization strategy perspective in light of its relatively small size.
Sonalleve
On November 27, 2020, the FDA authorized commercial distribution in the United States of the Sonalleve system for the treatment of osteoid osteoma in the extremities under the HDE program. Osteoid osteoma is a non-cancerous bone tumor that occurs most often in the long bones of the leg, such as the femur and tibia, of young children and adolescents. Osteoid osteoma causes a dull, aching pain that is moderate in intensity, but can worsen and become severe, especially at night. Computed tomography (CT) guided radiofrequency ablation, the most commonly used osteoid osteoma treatment, requires drilling through muscle and soft tissue into bone, and also exposes the patient to radiation from the imaging necessary to guide the probe that is inserted to heat and destroy tumor tissue.
24
The Sonalleve applications for ablation of uterine fibroids and adenomyotic tissue, palliative pain relief associated with bone metastases, treatment of osteoid osteoma, and management of benign desmoid tumors are CE marked and available in the European Union and its Member States. The uterine fibroids application is also available for sale in Canada and South Korea. Sonalleve has been registered in several Middle East, North African, and Southeast Asian countries. We are also in the process of assessing current clinical research network activities and the investigator lead studies in the United States to form regulatory strategies for several potential indications.
In 2018, Sonalleve was also approved in China by the NMPA for the non-invasive treatment of uterine fibroids.
Reimbursement
Our ability to successfully commercialize our products depends in large part on the extent to which coverage and adequate reimbursement for such products and related treatments or procedures will be available from government health administration authorities, government and private health insurers, and other organizations or third-party public or private payors. Pricing and reimbursement procedures and decisions vary from country to country. Many government health authorities and private payors condition payment on the cost-effectiveness of the product. Even if a device has obtained marketing authorization in the relevant jurisdiction, there is no guarantee that third-party payors will reimburse providers or patients for the cost of the device and related procedures or that the amount of such reimbursement will be adequate to cover the cost of the device. The availability of coverage and adequate reimbursement to hospitals and clinicians using our products therefore is important to our ability to generate revenue and we plan to pursue coverage and reimbursement for our products in the key markets where we obtain marketing authorization for such products. Successful commercialization of our authorized products will also depend on the cost of the system and the availability of coverage and adequate reimbursement from payors.
On July 11, 2024, it was announced that U.S. Centers for Medicare and Medicaid Services (“CMS”) has issued its proposed rules establishing, for the first time, a Category 1 CPT code for the TULSA procedure, effective January 1, 2025. On November 1, 2024, CMS announced its final rule, including final payment rates for the new TULSA codes effective in 2025.
According to the final rule, TULSA will have three Category 1 CPT codes to cover how therapy is delivered depending on if there are one or two physicians involved in the procedure: 51721 TULSA Device Management and 55881 TULSA Treatment, when two physicians are involved in the procedure, and 55882 TULSA Complete Procedure, when performed by a single physician. TULSA will have a 0-day global period, indicating that the payment associated with the codes will only cover the work performed on the day TULSA is performed. Physicians will thereby bill for any pre or post patient visit separately using existing codes. This will provide physicians with the most flexibility to assess the appropriate number of visits needed by each patient and enable their safe and fast recovery. TULSA codes have also been assigned to all three relevant sites of service: Hospital Outpatient (“HOPD”), Ambulatory Surgical Center (“ASC”), and Private Office/Non-Facility (“OBL”). The spectrum of the location of service will ensure patients can be treated in whatever setting they and their physician believe appropriate and convenient for each patient.
For Hospital Payment, the Final Rule has established TULSA CPT 55882 as a Level 7 Urology Ambulatory Payment Classification (“APC”) for 2025 with a Medicare National Average payment of $12,992.42. For ASCs, the facility payment for CPT 55882 will be $10,728.00 (Medicare National Average). This represents increases of approximately 41% and 49% for hospitals and ASCs, respectively, over TULSA payments previously set in the Proposed Rule announced in July 2024 and is also higher than the Final Rule for mainstream treatment modalities for prostate cancer, such as robotic radical proctectomy (Laparoscopy Level 2), as well as for benign prostatic hyperplasia (BPH) treatments, such as Aquablation (Urology Level 6).
The Final Rule for the Physician Fee Schedule has set the total Facility (HOPD or ASC) Relative Value Units (“RVU”) at 6.47 for CPT 51721 TULSA Device Management and 14.56 RVU for CPT 55881 TULSA Treatment, when 2 physicians are involved in the TULSA procedure. If one physician performs the complete TULSA procedure, the RVU is 17.91 for CPT 55882.
The Proposed Rule for Physician fee schedule for Non-Facility (OBL or Private Office) has set RVU at 16.25 for CPT 51721 TULSA Device Management and 263.05 RVU for CPT 55881 TULSA Treatment, when 2 physicians are involved in the TULSA procedure. If one physician performs the complete TULSA procedure, the RVU is 272.21 for CPT 55882.
As noted above, the TULSA procedure will have a 0-day Global Period, meaning that all post-operative visits are billed separately. This is distinct from all other comparable prostate treatments which are 90-day Global Period and therefore include bundled payments for all post-operative visits performed in the first 90 days.
25
The typical range of post-operative office visits would be approximately 9-11 total RVUs in the first 90-days.
The below tables summarize the proposed rule Codes, RVUs and Facility Dollar Amounts.
Facility Fee Schedule:
CPT Code |
|
Description |
|
HOPD |
|
ACS |
|
||
55882 |
|
TULSA Complete Procedure |
|
$ |
12,992.42 |
1 |
$ |
10,728.00 |
1 |
1 |
Amounts are exact, not in thousands. |
Physician Fee Schedule:
|
|
|
|
Physician Total RVU |
|
|
|
Physician Total RVU with |
||||
|
|
|
|
Facility |
|
Non- |
|
|
|
typical 90-day Follow-Up |
||
|
|
|
|
(HOPD, |
|
Facility |
|
Typical 90-Day |
|
Facility |
|
Non-Facility |
CPT Code |
|
Description |
|
ASC) |
|
(OBL) |
|
Follow-up |
|
(HOPD, ASC) |
|
(OBL) |
51721 |
|
TULSA Device Management |
|
6.47 |
|
16.25 |
|
9.37 - 11.61 |
|
15.84 - 18.08 |
|
25.62 - 27.86 |
55881 |
|
TULSA Treatment |
|
14.56 |
|
263.05 |
|
n/a |
|
14.56 |
|
263.05 |
51721 & 55881 Total |
|
Procedure Total |
|
21.03 |
|
279.30 |
|
9.37 - 11.61 |
|
30.40 - 32.66 |
|
288.67 - 290.91 |
|
|
(Two Physician) |
|
|
|
|
|
|
|
|
|
|
55882 |
|
TULSA Complete Procedure (One Physician) |
|
17.91 |
|
272.21 |
|
9.37 - 11.61 |
|
27.28 - 29.52 |
|
281.58 - 283.82 |
Employees and Human Capital Resources
As of December 31, 2024, we had 142 employees, all of whom were full-time employees. 19 of our employees are represented by a labor union or covered under a collective bargaining agreement. We consider our relationship with our employees to be in good standing and no issues have been noted.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. We invest in the ongoing development of our workforce through various training programs and leadership development initiatives. Employees have access to continuous learning tools fostering professional growth. We prioritize employee wellbeing through flexible work arrangements and wellness programs. By providing these resources, we aim to create a positive work-life balance, which contributes to employee satisfaction and retention. To attract top talent, we offer competitive compensation and benefits packages, including health insurance, and paid time off. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Corporate Information
Profound is the company resulting from a “three-cornered” amalgamation involving Mira, Mira Subco (a subsidiary formed to complete the amalgamation) and Profound Medical Inc. (“Old PMI”). Old PMI was formed by articles of incorporation under the Business Corporations Act (Ontario) (“OBCA”) on June 13, 2008. Mira was formed by articles of incorporation under the OBCA on July 16, 2014, and following its initial public offering in Canada, was a “capital pool company” listed on the TSX-V. As a capital pool company, Mira had no assets other than cash and did not carry on any operations. On June 3, 2015, in anticipation of the amalgamation, Mira changed its name to “Profound Medical Corp.” (becoming “Profound”) and completed a consolidation of its share capital on the basis of one post-consolidation common share for every 13.6363 pre-consolidation common shares. On June 4, 2015, Mira (now “Profound”), Mira Subco and Old PMI completed the amalgamation, with Profound as our surviving holding company, and Mira Subco and Old PMI amalgamating to form a new OBCA subsidiary, Profound Medical Inc. (“PMI”), to serve as the holding subsidiary of our operating subsidiaries. Upon completion of the amalgamation, Profound commenced trading on the TSX-V. On July 13, 2018, Profound graduated from the TSX-V and commenced trading on the TSX under the symbol “PRN”. On October 29, 2019, Profound commenced trading on the Nasdaq Capital Market under the symbol “PROF”.
26
Our head and registered office is located at 2400 Skymark Avenue, Unit 6, Mississauga, Ontario, L4W 5K5. Our telephone number is (647) 476-1350. Our website address is www.profoundmedical.com. Information contained on, or that can be accessible through, our website is not a part of this Annual Report.
Available Information
Additional information about us is available on our website at www.profoundmedical.com, on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. The aforementioned information is made available in accordance with legal requirements and is not, unless otherwise specifically stated, incorporated by reference into this Annual Report on Form 10-K. We make available free of charge, through our website, annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, as well as proxy statements, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Reports, proxy statements and other information filed with the SEC may also be obtained through the SEC’s website (www.sec.gov).
Our code of ethics, other corporate policies and procedures, and the charters of our Audit Committee, Human Resources and Corporate Governance Committee are available through our Internet website at https://profoundmedical.com/investors/#governance.
Item 1A. RISK FACTORS
Investing in our common shares involves a high degree of risk. You should carefully consider the risks and uncertainties described below, the section of this Annual Report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes appearing elsewhere in this Annual Report, before investing in our common shares. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks occur, our business, operating results and prospects could be materially harmed. In that event, the price of our common shares could decline, and you could lose part or all of your investment. This Annual Report also contains forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.” Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors, including those set forth below.
Summary of Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in this section below, that represent challenges that we face in connection with the successful implementation of our strategy. The occurrence of one or more of the events or circumstances described in more detail in the risk factors below, alone or in combination with other events or circumstances, may have an adverse effect on our business, cash flows, financial condition, and results of operations. Such risks include, but are not limited to:
| ● | We have a limited operating history and history of operating losses. |
| ● | Our business is capital intensive and requires significant investment. |
| ● | We are exposed to foreign currency risk, which exposure will increase as we commercialize our approved products in the United States. |
| ● | We rely on collaborative partners to assist in the sales and marketing and/or distribution of our approved products. |
| ● | We may not achieve our commercialization and future product development goals in the time frames expected, or at all. |
| ● | Our products, including the TULSA-PRO system, may not achieve or maintain expected levels of market acceptance. |
| ● | Successful commercialization of our authorized products will depend on the cost of the system and the availability of coverage and adequate reimbursement coverage from third-party payers. |
| ● | We intend to rely primarily on our in-house sales and marketing capabilities for our commercialization strategy, which will require substantial build-up and commitment of resources. |
| ● | We may experience manufacturing scaling issues in connection with our commercialization strategy. |
| ● | We rely on third parties to manufacture and supply components of our systems. |
| ● | We depend on single-source suppliers for some of the components in our systems. |
| ● | We face significant competition in the markets for our products. |
27
| ● | Data from our clinical trials may not support regulatory approvals or clearances and/or reimbursement coverage for our products. |
| ● | We may rely on third parties to perform clinical trial planning, provide critical advice, conduct our clinical trials and facilitate obtaining regulatory approvals or clearances for our product candidates. |
| ● | We depend on key managerial personnel for our continued success. |
| ● | Research and development carries substantial risk and we may not be able to expand our product portfolio. |
| ● | Rising insurance costs could negatively impact our profitability. |
| ● | If we fail to properly maintain the integrity of our data or we experience a cyber-attack or other breach of these systems, our business could be adversely affected. |
| ● | A portion of our employees are unionized, and our good labor relations may not continue. |
| ● | If our facilities are damaged or destroyed, we may experience delays that could negatively impact our revenues. |
| ● | We face risks associated with acquisition of businesses and technologies. |
| ● | Our products and operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business. |
| ● | We may be unable to obtain, or experience significant delays in obtaining, FDA clearances or other regulatory authorizations for our product candidates and/or enhancements to our approved or cleared products. |
| ● | Attracting patients to perform clinical trials and meeting clinical trial objectives can be more costly and time-consuming than expected and could be adversely affected by another health crisis. |
| ● | We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses or engaged in false or misleading promotion. |
| ● | Compliance with regulations for quality systems for medical device companies is difficult, time consuming and costly. |
| ● | Modifications to our cleared or approved products may require new regulatory clearances or approvals or may require us to recall or cease marketing our products until such additional clearances or approvals are obtained. |
| ● | If our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, and such events can result in voluntary corrective actions or agency enforcement actions. |
| ● | Legislative or regulatory reform of the healthcare systems in which we intend to operate may affect our ability to sell our products profitably and could adversely affect our business. |
| ● | We are subject to “fraud and abuse” laws, anti-bribery laws, environmental laws and privacy and security regulations. Any violation by our employees or other agents could expose us to severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations. |
| ● | We may not be able to protect our intellectual property rights throughout the world. |
| ● | We may incur substantial costs as a result of litigation or other proceedings relating to enforcement of our or our licensors’ patent and other intellectual property rights and we may be unable to protect our rights to, or use of, our technology. |
| ● | Our business, financial condition, cash flows and results of operations are subject to risks arising from our international operations. |
| ● | Future sales or the issuances of our securities may cause the market price of our Common Shares to decline. |
| ● | The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation. |
| ● | We are a smaller reporting company, and the reduced reporting requirements applicable to smaller reporting companies may make our common shares less attractive to investors. |
| ● | If equity research analysts research or reports about our business or if they issue unfavorable commentary or downgrade our Common Shares, the price of our Common Shares could decline. |
| ● | We may be subject to securities litigation, which is expensive and could divert management attention. |
| ● | We have never paid dividends on our Common Shares and we do not anticipate paying any dividends in the foreseeable future. |
| ● | If we are unable to satisfy the requirements of Sarbanes-Oxley, or our internal controls over financial reporting are not effective, the reliability of our financial statements may be questioned. |
| ● | Any default under our existing debt that is not waived by the applicable lender could materially adversely impact our results of operations and financial results and may have a material adverse effect on the trading price of our Common Shares. |
| ● | As a foreign private issuer whose shares are listed on Nasdaq, we intend to follow certain home country corporate governance practices instead of certain Nasdaq requirements. |
28
| ● | We will incur significantly increased costs and devote substantial management time as a result of operating as a U.S. public company. |
| ● | We may lose foreign private issuer status in the future, which could result in significant additional costs and expenses. |
| ● | It may be difficult for United States investors to effect service of process or enforcement of actions against us or certain of our directors and officers under U.S. federal securities laws. |
| ● | We may be a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes, which generally would result in certain adverse U.S. federal income tax consequences to our U.S. shareholders. |
| ● | If we are required to register as an “investment company” under the Investment Company Act, significant compliance costs and applicable restrictions could have a material adverse effect on our business. |
Risk Factors Relating to Our Operating History and Financial Condition
We have a limited operating history and history of operating losses.
We commenced operations in June 2008 and only began generating revenues in 2017. As of December 31, 2024, we had an accumulated deficit of $245,170,000 and had cash and cash equivalents of $54,912,000. Since inception, we have incurred significant losses each year. For the year ended December 31, 2024, we recorded a net loss of $27,816,000, and for the year ended December 31, 2023, we recorded a net loss of $28,323,000. We expect to incur significant operating losses even as we begin to commercialize the TULSA-PRO system in the United States following our FDA clearance, which will require significant expenditures to increase our sales and marketing capabilities and expand our manufacturing and distribution capacity, as well as other expenses related to increasing reimbursement coverage and gaining market acceptance among patients, physicians/clinicians and others in the medical community. In addition, we plan to continue product research and development and clinical trials and may pursue additional regulatory approvals. We expect to have sufficient cash to finance our operations for at least the next 18 months. There is no assurance that we will ever successfully commercialize our systems, generate significant revenues from our approved products or achieve profitability. Even if profitability is achieved, we may not be able to sustain or increase profitability. Our failure to achieve or maintain profitability could negatively impact the value of the Common Shares.
Our business is capital intensive and requires significant investment to increase our commercial capacity for our approved products, and the resources to do so may not be available in amounts or on terms acceptable to us, if at all.
Our business requires substantial capital investment in order to commercialize our approved products, in particular to expand our sales and marketing capabilities and increase our manufacturing capacity, as well as to conduct research and development and to obtain regulatory approvals for existing products and future product candidates. In order to secure financing, if available, it is likely that we would need to sell additional Common Shares and/or securities that are exchangeable for or convertible into Common Shares, incur additional indebtedness and/or enter into development, manufacturing, distribution and/or licensing relationships. Our CIBC Credit Agreement includes covenants which require us to achieve certain financial performance measures and contains restrictions on our ability to incur additional debt. Any future equity financing may be dilutive to existing shareholders. Any future debt financing arrangements we enter into would likely contain restrictive covenants that would impose significant operating and/or financial restrictions on us. The availability of equity or debt financing will be affected by, among other things, our commercial progress and market acceptance in respect of the TULSA-PRO system and other approved products, as well as the results of our research and development, our ability to obtain regulatory approvals, the state of the capital markets generally, strategic alliance agreements, and other relevant considerations.
Any additional financing may not be obtained on favorable terms, if at all. If we cannot obtain adequate funding on reasonable terms, we may not be able advance our business strategy and/or the commercialization of our approved products, and we may need to terminate or delay clinical trials, curtail significant regulatory initiatives, and/or sell, license or assign rights to our technologies, products or product candidates.
Our cash outflows are expected to consist primarily of expenditures to increase our commercial capacity, particularly in sales and marketing, as well as in manufacturing and distribution. In addition, we intend to continue internal and external research and development efforts to develop and expand our product pipeline, as well as incur general and administrative expenditures to support our corporate infrastructure. If we do not obtain sufficient additional capital, there may be substantial doubt about our ability to continue as a going concern and realize assets and pay liabilities as they become due. Depending upon the results of our research and development programs and the availability of financial resources, we could decide to accelerate, terminate or reduce certain projects, or commence new ones.
29
Any failure on our part to raise additional funds on terms favorable to us, or at all, may require us to significantly change or curtail current or planned operations in order to conserve cash until such time, if ever, that sufficient proceeds from operations are generated, and could result in us not taking advantage of business opportunities, in the termination or delay of clinical trials for one or more of our product candidates, in curtailment of our product development programs designed to identify new product candidates, and/or in the sale or assignment of rights to our technologies, products or product candidates. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
We are exposed to foreign currency risk, which exposure will increase as we commercialize our approved products in the United States; to date, we have not hedged against risk associated with foreign exchange rate exposure.
As we commercialize our approved products, in particular our TULSA-PRO system in the United States, we expect that a significant portion of our revenues, expenses, current assets and current liabilities will be denominated in United States dollars, Euros and other foreign currencies. Currently, our financial statements are expressed in United States dollars. A decrease in the value of such foreign currencies relative to the United States dollar could result in decreases in revenues from currency exchange rate fluctuations. To date, we have not hedged against risk associated with foreign exchange rate exposure. Consequently, our results of operations may be negatively affected by foreign currency exchange rate fluctuations, which could have a negative impact on the market price of our Common Shares.
Risks Related to Our Business and Growth Strategy
We currently rely on our collaborative partners, and we may rely on additional collaborative partnerships, to assist in the sales and marketing and/or distribution of our approved products.
We currently rely on our collaborative partnerships for the sales and marketing and/or distribution of our approved products, in particular Philips, Siemens and GE Healthcare, who promote our systems that are compatible with the MRI scanners produced and sold by them to end users, including hospitals and clinics. In the future, we intend to enter into similar arrangements with other producers of MRI scanners to increase the compatibility of our products and to promote and increase market acceptance among hospitals, clinics and other end-users. However, we can provide no assurance that we will be successful in establishing such additional arrangements, which could negatively impact our commercialization strategy and may have a material adverse effect on our business, results of operations and financial condition. See “—We rely on the compatibility of our products with MRI scanners in the successful commercialization of our products” above.
We may also seek out, evaluate and negotiate other third-party marketing and/or distribution arrangements for our products in the jurisdictions where they are approved, which may involve the commitment of substantial time and effort and may not ultimately result in an arrangement that is favorable to our commercialization goals (e.g. if such third-party marketing or distribution partners are not as successful in promoting our products as anticipated). If any of these third-party collaborators are unable or unwilling to promote and/or deliver our products to our customers in an effective manner, then our business, financial condition and operating results could be materially impacted.
Additionally, if any of our relationships with third-party collaborators is terminated, whether by us or the third-party for any reason, there can be no assurance that we will be able to obtain alternative sales and marketing and/or distribution channels rapidly or effectively enough to prevent disruptions in sales generated in those markets or otherwise to ensure the success of our products in those markets. Any such termination may have a material adverse impact on our business, results of operations and financial condition.
We may not achieve our commercialization and future product development goals in the time frames expected, or at all.
We may set goals for and make public statements regarding the timing of the accomplishment of objectives material to our success, such as the timing and extent of product launches in the jurisdictions where they are approved for marketing and sale, in particular our expected commercialization of the TULSA-PRO system following FDA clearance in the United States; third-party reimbursement for our approved products; the timing and terms of any collaborations, partnerships, licenses, acquisitions or other agreements; the commencement and completion of clinical trials, including follow-up data on our TACT Pivotal Clinical Trial and CAPTAIN trial; and anticipated regulatory submission and approval dates for our products in additional jurisdictions, and for future product candidates. The actual timing of these events can vary dramatically due to factors such as the uncertainties inherent in the arrangements sufficient to commercialize our products, including in respect of manufacturing, distribution and marketing, as well as market competition and adverse results from our clinical trials, and other factors and described herein, many of which are beyond our control.
30
There can be no assurance that we will achieve our commercialization goals in respect of the TULSA-PRO system in the United States, or that future efficacy and safety results from our TACT Pivotal Clinical Trial and CAPTAIN trial will be favorable. If we fail to commercialize the TULSA-PRO system in the United States or any other approved products in the time frame and to the extent that we anticipate, our business, results of operations and financial condition may be materially adversely affected, and the price of the Common Shares could decline.
Our products, including the TULSA-PRO system, may not achieve or maintain expected levels of market acceptance.
The commercial success of our approved products, including the TULSA-PRO system which was FDA-cleared in the United States in August 2019, is dependent upon achieving and maintaining market acceptance. New medical devices that appear promising in development may fail to reach the market or may have only limited or no commercial success. Levels of market acceptance for our products could be impacted by several factors, many of which are not within our control, including but not limited to:
| ● | safety, efficacy, convenience and cost-effectiveness of our systems as a method of ablation of prostate tissue, uterine fibroids, bone metastases compared to products of our competitors or other forms of treatment; |
| ● | scope of approved uses and marketing approval or clearance; |
| ● | timing of market entry of our products versus those of our competitors; |
| ● | difficulties in, or excessive costs required in the process of, manufacturing our products; |
| ● | expanding compatibility of our systems to work with MRI scanners other than those made by Philips, Siemens and GE Healthcare, and maintaining our existing relationships with Philips, Siemens and GE Healthcare; |
| ● | infringement or alleged infringement of the patents or intellectual property rights of others; |
| ● | acceptance of the price of our products relative to those of our competitors; |
| ● | acceptance and adoption of our products by patients, physicians/clinicians and the medical community; |
| ● | the availability of training necessary for proficient use of our products, as well as willingness of physicians and technicians to participate in such training; |
| ● | the perceived risks generally associated with the use of new products and procedures; |
| ● | the placement of our products in treatment guidelines published by leading medical organizations; |
| ● | the size and growth rate of the market for our products in the major geographies in which we operate or intend to operate, in particular in the United States; and |
| ● | acceptance of our products by government and third-party payers for adequate reimbursement coverage. |
In addition, the success of any new product will depend on our ability to either successfully build our in-house sales and marketing capabilities or to maintain or secure new, or to realize the benefits of existing or future arrangements with, third-party marketing or distribution partners. See “Risk Factors—We intend to rely primarily on our in-house sales and marketing capabilities for our commercialization strategy, which will require substantial build-up and commitment of resources” and “Risk Factors—We currently rely on our collaborative partners, and we may rely on additional collaborative partnerships, to assist in the sales and marketing and/or distribution of our approved products” below. If we are unable to commercialize new products successfully, whether through a failure to achieve market acceptance, a failure to build our own in-house sales and marketing capabilities, a failure to maintain or secure new or existing marketing partners or to realize the benefits of our arrangements with our marketing and distribution partners, there may be a material adverse effect on our business, financial condition and results of operations and it could cause the market value of our Common Shares to decline.
31
Market acceptance of our approved products also depends on our ability to identify and address the relevant market. For example, our TULSA-PRO system is FDA-cleared in the United States for transurethral ultrasound ablation of prostate tissue and is not specific to any particular condition or disease. For more information, see “We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or ‘off - label’ uses or engaged in false or misleading promotion.” below. Furthermore, our estimates of the number of patients who have received or might have been candidates to use a specific product may not accurately reflect the true market or market prices for such products or the extent to which such products will actually be used by patients. Our failure to successfully introduce and market our approved products could have a material adverse effect on our business, financial condition, and results of operations.
Successful commercialization of our approved products, including the TULSA-PRO system, and future product development depends upon our maintaining strong working relationships with physicians/clinicians.
If we fail to maintain positive working relationships with physicians/clinicians, our approved products, including our TULSA-PRO system, may not achieve the level of market acceptance sufficient for successful commercialization of the products. It is important for us to market our approved systems successfully to physicians/clinicians who we expect will use our approved products, and we depend on our sales and marketing personnel (and those of our collaborative partners, e.g., Philips, Siemens and GE Healthcare) to do so in an effective manner. We can provide no assurance that physicians/clinicians will prescribe or otherwise utilize our TULSA-PRO systems based on our existing clinical data (such as our TACT and CAPTAIN data) or the results of any future clinical trials, or at all. See “Risk Factors—Data from our clinical trials may not support regulatory approvals or clearances and/or reimbursement coverage for our products” below. We also rely on our relationships with physicians/clinicians to further develop our existing products and develop future product candidates in line with the clinical needs and expectations of the professionals who we expect will use and support the devices. These development efforts are similarly dependent upon us and our collaborative partners maintaining working relationships with physicians/clinicians.
In addition, we rely on physicians/clinicians to provide considerable knowledge and experience that assists us in the marketing and sale of our approved products and development of our products and product candidates. Physicians/clinicians assist us as researchers, marketing and product consultants, inventors and public speakers. If we are unable to maintain strong relationships with these professionals and continue to receive their advice and input, the development and marketing of our products could suffer, which could have a material adverse effect on our business, financial condition and operating results.
Physicians/clinicians misuse could result in negative publications, negative sentiment or adverse events, thereby limiting market acceptance and future sales of our products.
There is a risk that physicians/clinicians may misuse our products, such as not following the instructions for use, not using our products on the intended patient population, using our products with unapproved or modified hardware or software, or misuse by inadequately trained staff. Physicians/clinicians may also initiate their own clinical studies which may be poorly designed or controlled, and may result in adverse safety or efficacy results. Any of the foregoing could result in negative publications, negative sentiment or adverse events or regulatory actions in respect of our products, thereby limiting market acceptance and sales of our products, which could have a material adverse effect on our business, financial condition and results of operations.
We rely on the compatibility of our products with MRI scanners in the successful commercialization of our products.
We have designed our TULSA-PRO system to be capable of integration with some of the MRI scanners from three of the major MRI manufacturers (Philips, Siemens and GE Healthcare), and the Sonalleve system with one MRI manufacturer (Philips). Although we believe that our approved products can be used by the vast majority of hospitals and treatment facilities, not all such facilities utilize MRI scanners that are compatible with the TULSA-PRO and Sonalleve systems, and such facilities would be required to acquire (or outsource to other facilities that already have) compatible MRI equipment, which may increase their costs and which could restrict or delay utilization of our systems by such facilities. Accordingly, we intend to expand compatibility of the systems with other MRI scanners in the future, which would require design changes to our systems, collaboration with the manufacturer of the MRI scanner and may require additional regulatory approvals. We may be unsuccessful in making the necessary design changes and, if required, receiving the necessary regulatory approvals for such changes, and the terms of any such arrangements that we may enter into in the future with the MRI scanner manufacturers may not be on as favorable terms.
32
Accordingly, we can provide no assurance that we will be successful in any such expansion of the compatibility of our products to other MRI scanners.
Successful commercialization of our authorized products will also depend on the cost of the system and the availability of coverage and adequate reimbursement coverage from third-party payers.
Successful commercialization of our products for which we obtain marketing authorization, including our TULSA-PRO system, depends largely upon the cost of the system and the availability of coverage and adequate reimbursement for the system, and the medical procedure associated with its use, from third-party payers, such as government healthcare programs, private health insurers and other organizations, such as health maintenance organizations and managed care organizations. We expect that our systems will be purchased by healthcare providers, including clinics and hospitals that use MRI scanners that are compatible with our systems, and that these providers will subsequently bill various third-party payers or will be responsible for covering the costs of the system through the provider’s operating budget. Although we expect there to be an out-of-pocket market for our authorized products, an out-of-pocket market alone is unlikely to be sufficient to support successful commercialization of such products. To date we have not secured significant coverage or reimbursement for any of our products from government or third-party payers in the jurisdictions where we have obtained regulatory authorizations, including our TULSA-PRO system in the United States. We can provide no assurance that third-party payers will provide coverage and adequate reimbursement for our TULSA-PRO system to treat our targeted indications based on our existing clinical data (such as our TACT and CAPTAIN data) or the results of any future clinical trials, or at all. See “Risk Factors—Data from our clinical trials may not support regulatory approvals or clearances and/or coverage and reimbursement for our products” below. Accordingly, we likely will need to conduct additional research and successfully complete additional clinical trials in order to obtain such coverage (e.g., follow-up data from our TACT Pivotal Clinical Trial and CAPTAIN trial). Such additional research and clinical trials may require significant time and resources, and may not be successful, which could result in the postponement of or inability to obtain coverage and reimbursement for our authorized products, which could significantly delay or otherwise negatively affect our commercialization strategy. Any of the foregoing could, in turn, have a material adverse effect on our business, results of operations and financial condition.
Third-party payers carefully review and increasingly challenge the prices charged for medical devices, procedures and services. Government healthcare programs in the United States and the European Union may reimburse certain providers at a pre-determined all-inclusive amount for all the costs associated with a particular procedure performed or course of treatment, based on such factors as the patient’s principal diagnosis, age and severity or complexity. Similarly, the surgeon or physician may be reimbursed at a pre-determined amount based on the procedure performed, and without taking into consideration the actual costs incurred, including the actual cost of the specific devices used.
New products are being increasingly scrutinized with respect to whether or not they will be covered at all by the various health plans and at what level of reimbursement. In some instances, economic research studies are and will be required to demonstrate whether our products and approach are superior from a long-term cost containment standpoint. Third-party payers may determine that use of our products in the treatment of patients is not reasonable or medically necessary, not cost-effective, experimental, or primarily intended for non-approved indications. Such determinations could have a material adverse effect on our business, results of operations and financial condition.
Further, healthcare reform measures that may be adopted in the future may impose more rigorous coverage and reimbursement standards. We are unable to predict what, if any, additional legislation or regulation impacting the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect such legislation or regulation would have on our business.
We intend to rely primarily on our in-house sales and marketing capabilities for our commercialization strategy, which will require substantial build-up and commitment of resources.
We intend to rely primarily on our in-house sales and marketing capabilities in order to advance our commercialization strategy, particularly in the United States in respect of our FDA-cleared TULSA-PRO system. This will require a substantial commitment of time and resources in the near-term, and we may be unsuccessful in executing on this strategy, which could negatively impact our anticipated commercialization. As a result of the COVID-19 pandemic, we remain in the early stages of expanding our U.S. sales and marketing capabilities and can provide no assurance that we will be successful in establishing a marketing presence and sales force sufficient to commercialize the TULSA-PRO system successfully in the United States.
33
In addition, by relying on an in-house sales and marketing function, we may have less visibility in the U.S. market (particularly among hospitals) than we would have if we had significant third-party distribution relationships. Any shortcomings in our in-house sales force may have a material adverse effect on our business, results of operations and financial condition.
We may experience manufacturing scaling issues in connection with our commercialization strategy, as we have limited experience assembling and testing our approved products, including the TULSA-PRO system, at a significant scale.
As we implement our commercialization strategy, in particular in respect of the TULSA-PRO system in the United States, we may not be able to produce sufficient quantities of systems or maintain consistent quality control in the production of our systems. We have limited experience in assembling and testing our approved products, including our TULSA-PRO system, on a commercial scale. To commercialize our approved products successfully and become profitable, we must be able to assemble and test such in commercial quantities in compliance with applicable regulatory requirements, and at an acceptable cost. Increasing our capacity to assemble and test our products on a commercial scale will require us to improve internal efficiencies, including hiring additional experienced personnel, which may result in significant capital expenditures. We may encounter a number of difficulties in increasing our assembly and testing capacity, including:
| ● | managing production yields; |
| ● | maintaining quality control and assurance; |
| ● | providing component and service availability; |
| ● | maintaining adequate control policies and procedures; |
| ● | hiring and retaining qualified personnel; and |
| ● | complying with U.S. and Canadian regulations (including at the state, provincial and/or federal levels) and applicable foreign regulations. |
In particular, our ability to increase our assembly and testing capacity successfully will greatly depend on our ability to hire, train and retain an adequate number of employees, in particular employees with the appropriate level of knowledge, background and skills to assemble and test our products. We compete with several other medical device companies to hire and retain these skilled employees, and we may be unable to hire and retain such employees in numbers sufficient to increase our in-house capabilities.
We currently intend to partner with one or more additional QSR-compliant and FDA-registered contract manufacturers for our TULSA-PRO systems in the United States. However, we may not be successful in establishing or maintaining such partnerships on acceptable terms or in the timeframe necessary to commercialize our products successfully, or at all.
In addition, we may encounter difficulties in scaling our manufacturing operations, whether in-house or through third-party contract manufacturers, as a result of, among other things, quality control and quality assurance issues and availability of components and raw material supplies. Any such quality control issues may negatively affect production and sales of our products, and may require increased repair or re-engineering costs due to product returns, defects and increased expenses due to switching to alternate suppliers, and reputational damage, any of which could negatively affect our business and reputation.
If we are unable to satisfy commercial demand for our products, in particular our TULSA-PRO system in the United States, due to our inability (or the inability of any of our contract manufacturers) to assemble and test such products in sufficient quantities with consistent quality control, and in compliance with applicable regulatory requirements (and in a cost-efficient manner), our ability to commercialize such products successfully, and market acceptance of our products could be adversely affected as our target customers may instead purchase or use our competitors’ products. This, in turn, could have a material adverse effect on our business, results of operations and financial condition.
34
We rely on third parties to manufacture and supply components of our systems.
The TULSA-PRO and Sonalleve systems consists of common electronic components, proprietary capital equipment and proprietary one-time-use devices. We purchase standard electronic components for our systems from a number of third-party vendors. The capital equipment consists of custom system electronics, a treatment delivery console, fluid circuits and an MRI compatible robotic positioning system. Printed circuit boards and assemblies and custom mechanical parts are outsourced from approved suppliers.
We cannot be certain that manufacturing sources for all components will continue to be available or that we can continue to outsource the manufacturing of our components on reasonable or acceptable terms. If we encounter delays or difficulties with contract manufacturers, delivery of our products could be delayed. In addition, we could be forced to secure new or alternative contract manufacturers or suppliers. Securing a replacement contract manufacturer or supplier could be difficult, and we may not be able to do so in a timely manner or without significant expense. Any loss of a manufacturer or any difficulties that could arise in the manufacturing process could significantly affect our ability to supply sufficient amounts of our products to our customers on a timely basis, which may negatively affect our market share and, correspondingly, could have a material adverse effect on our business, results of operations and financial condition.
In addition, not all of our suppliers provide us with guaranteed minimum production levels, and we rely on single-source suppliers for some of our components. See “Risk Factors—We depend on single-source suppliers for some of the components in our systems” below. Furthermore, we do not currently have long-term supply contracts, and accordingly, our suppliers could terminate their services at any time without penalty within agreed notice periods. As a result, there can be no assurance that we will be able to obtain sufficient quantities of components in the future necessary to commercialize our approved products.
Our reliance on third-party manufacturers and suppliers involves a number of additional risks, including, among other things:
| ● | contract manufacturers or suppliers may fail to comply with regulatory requirements or make errors in manufacturing that could negatively affect the efficacy or safety of our products or cause delays in shipments of products; |
| ● | we or our contract manufacturers and suppliers may not be able to respond to unanticipated changes in customer orders, and if orders do not match forecasts, our suppliers may have excess or inadequate inventory of materials and components; |
| ● | we or our contract manufacturers and suppliers may be subject to price fluctuations of raw materials and key components due to a lack of long-term supply arrangements for key components; |
| ● | we or our contract manufacturers and suppliers may lose access to critical services and components, resulting in an interruption in the manufacture, assembly and shipment of our products; |
| ● | fluctuations in demand for products that our contract manufacturers and suppliers manufacture for others may affect their ability or willingness to deliver components in a timely manner; |
| ● | suppliers or contract manufacturers may wish to discontinue supplying components or services for risk management reasons; |
| ● | we may not be able to find new or alternative components or reconfigure our system and manufacturing processes in a timely manner if the necessary components become unavailable; and |
| ● | contract manufacturers and suppliers may encounter financial hardships unrelated to our demand, which could inhibit their ability to fulfill orders and meet our requirements. |
If any of these risks materialize or worsen, it could significantly increase costs and impact our ability to meet demand for our products, in particular in respect of our planned commercialization of TULSA-PRO in the United States. If we are unable to satisfy commercial demand for the TULSA-PRO system or other approved products in a timely manner, our ability to generate revenue could be impaired, market acceptance of our products could be adversely affected, and customers may instead purchase or use competitors’ products. As a result, our business, results of operations and financial condition may be materially adversely affected.
35
We depend on single-source suppliers for some of the components in our systems.
We currently rely on a single source for the manufacture of some of the components of our TULSA-PRO and Sonalleve systems. Although we intend to procure alternative supply sources for our components as our commercialization efforts increase, we can provide no assurance that we will be successful. Establishing additional or replacement suppliers for these components will take a substantial amount of time and could result in increased costs and impair our ability to produce our products. In addition, our products are highly technical and are required to meet exacting specifications, and any quality control problems that we experience from such alternative supply sources could negatively affect our reputation and market acceptance of our products.
We may also have difficulty obtaining similar components from other suppliers that are acceptable to the FDA or foreign regulatory authorities. The failure of our suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action, including warning letters, product recalls, termination of distribution, product seizures, or civil penalties. See “Risk Factors—Risks Relating to the Regulation of the Company and Our Products” below for more information.
If we fail to procure alternative supply sources on acceptable terms or at all, our planned commercialization of TULSA-PRO in the United States could be negatively affected, which could have a material adverse effect on our business, operating results and financial condition.
We face significant competition in the markets for our products, and in particular, there are numerous devices and procedures that compete with our TULSA-PRO system.
Our products face significant competition from currently available and future medical devices or surgical methodologies that are used in the same patient populations as our products. See Item 3.5, “Narrative Description of the Business—Competition”. Some of these available options are well-established, and our competitors have greater financial resources, development, selling and marketing capabilities than we do. We may face further competition from medical equipment/supply companies that focus their efforts on developing and marketing products that are similar in nature to our products, but that in some instances offer improvements over our products. Our competitors may succeed in developing technologies and products that are more effective or less expensive to use than our products. These developments could render our products uncompetitive, which would have a material adverse effect on our business, financial condition and operating results. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products. They may also establish exclusive collaborative or licensing relationships with our competitors.
Further, our industry is also subject to changing industry standards, market trends and customer preferences and to competitive pressures which can, among other things, necessitate revisions in pricing strategies, price reductions and reduced profit margins. Our success will depend, in part, on our ability to achieve technological superiority in our products and operations and maintain such superiority in the face of new technologies. No assurance can be given that further modification of our product offerings will not be required in order to meet demands or to make changes necessitated by developments made by competitors that might render our products less competitive. Our future success will be influenced by our ability to continue to develop, test and market our products and future product candidates, including increasing and/or maintaining their compatibility with MRI scanners. Although we have committed resources to these efforts, there can be no assurance that we will be successful.
Data from our clinical trials may not support regulatory approvals or clearances and/or reimbursement coverage for our products.
Regulatory clearances and approvals for the commercial sale of any of our product candidates require that we demonstrate through clinical trials that the product candidate is safe and effective for its intended use or, to receive 510(k) clearance in the United States, that the product candidate is substantially equivalent to an existing predicate device for its intended use. While we have obtained 510(k) clearance for TULSA-PRO, additional follow-up data from our TACT Pivotal Clinical Trial and CAPTAIN trial may not be consistent with our 12-month data in terms of efficacy and/or safety profile, which in certain circumstances may result in the FDA taking regulatory actions that are adverse to us. In addition, our TACT Pivotal Clinical Trial and CAPTAIN trial involves a relatively small number of subjects. Because of the small sample size, the results may not be indicative of future results.
We believe that third-party payers, in determining reimbursement coverage for our products, including the TULSA-PRO system, generally would rely upon our clinical trial results, such as TACT and CAPTAIN, that were obtained in support of our applications for regulatory authorization; however, we may be required to provide additional data from our existing trials and/or conduct additional clinical trials prior to obtaining reimbursement coverage for the TULSA-PRO system and other authorized products, which would likely involve significant time and expense, and may have a material adverse effect on our business, results of operations and financial condition.
36
In the future, we may also seek regulatory authorization, which may include 510(k) clearance, for other product candidates, which likewise could be adversely affected by insufficient clinical trial results. Obtaining product clearance or approval and conducting the requisite clinical trials is a long, expensive and uncertain process and is subject to delays and failures at any stage. There can be no assurance that clinical trials will be completed successfully within any specified period of time, if at all. In addition, a regulatory authority may disagree with our interpretation of the data from our clinical trials, or may find the clinical trial data inadequate to support clearance or approval, and may require us to extend existing clinical trials and/or pursue additional clinical trials, which would increase costs and could further delay regulatory approval or clearance of our products, or cause such regulatory approvals or clearances to be denied altogether.
The data from a clinical trial may be inadequate to support clearance or approval of an application to applicable regulatory authorities for numerous reasons including, but not limited to:
| ● | prevalence and severity of adverse events and other unforeseen safety issues; |
| ● | changes in regulatory requirements, policies or guidelines; |
| ● | the interim or final results are insufficient (including in respect of the time period for which results were obtained), inconclusive or unfavorable as to the safety or efficacy of the device; |
| ● | the FDA or other regulatory authorities concluding that a clinical trial design is inadequate to demonstrate safety and efficacy for a particular use, or to demonstrate substantial equivalence to a predicate device; and |
| ● | the FDA or other regulatory authorities concluding that the trial was not conducted in compliance with regulatory requirements or lacked controls necessary to ensure the integrity of the trial data. |
We, the FDA or other regulatory authorities may suspend or terminate a clinical trial at any time if it is determined that enrolled subjects may be or are being exposed to unacceptable health risks, including the risk of death, that our devices are not manufactured under acceptable conditions or with acceptable quality, or that the trial is not being conducted according to the protocol and in compliance with Good Clinical Practice and regulatory requirements. Further, success in nonclinical studies and early clinical trials does not mean that future clinical trials will be successful because medical devices and/or treatment options in later stage clinical trials may fail to demonstrate sufficient safety and efficacy to the satisfaction of the FDA and other regulatory authorities despite having progressed through initial clinical trials. We cannot be sure that the later trials will replicate the results of prior trials.
Even if our clinical trials are completed as planned, there can be no certainty that trial results will support our product candidate claims or that the FDA or foreign authorities will agree with our conclusions regarding them or agree that they are adequate to support approval or clearance. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product candidate and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our regulatory submissions and, ultimately, negatively affect our ability to commercialize our systems and generate revenues.
If our products do not prove to be safe and effective, or substantially equivalent to a predicate device, in clinical trials to the satisfaction of the relevant regulatory authorities or third-party payers, if the clinical studies do not support our product candidate claims or if they result in the discovery of adverse side effects, then our regulatory authorizations and reimbursement coverage (as applicable) may be delayed or denied altogether, and our business, financial condition and results of operation could be materially adversely affected.
37
We may rely on third parties to perform clinical trial planning, provide critical advice, conduct our clinical trials and facilitate obtaining regulatory approvals or clearances for our product candidates. Such third parties may not perform satisfactorily, including failing to meet deadlines for the completion of clinical trials.
We may rely on third parties to provide clinical trial planning conduct certain clinical trials, perform data collection and analysis and provide marketing, manufacturing, regulatory advice and other services that are crucial to our business. We may be unable to find suitable partners, external consultants or service providers to provide such services or such arrangements may not be available on commercially reasonable terms. Further, we may engage third parties that may cease to be able to provide these services or may not provide these services in a timely or professional manner. In particular, our technology and product development activities or clinical trials conducted in reliance on third parties may be delayed, suspended, or terminated if the third parties do not devote a sufficient amount of time or effort to our activities or otherwise fail to successfully carry out their contractual duties or to meet regulatory obligations or expected deadlines; if we replace a third party; if the quality or accuracy of the data obtained by third parties is compromised due to their failure to adhere to clinical protocols, regulatory requirements, or for other reasons including the loss of data; or if the third party becomes bankrupt or enters into liquidation.
We may not always have the ability to control the performance of third parties in their conduct of their activities. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, or agreements with such third parties are terminated for any reason, we would be required to find a replacement third party to conduct the required activities. We may be unable to enter into a new agreement with another third party on commercially acceptable terms, if at all. Furthermore, if the quality or accuracy of the data obtained by the third party is compromised, or if data are otherwise lost, we would be required to repeat the affected trial. Third-party performance failures may therefore increase our development costs, delay our ability to obtain regulatory authorization, and delay or prevent the commercialization of our products in target markets. In addition, our third-party agreements usually contain a clause limiting such third party’s liability, such that we may not be able to obtain full compensation for any losses that we may incur in connection with the third party’s performance failures.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA and other regulatory authorities require us to comply with good clinical practice regulations and international standards relating to the conduct, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties, over which we have limited control, to manage those operations does not relieve us of these responsibilities and requirements. Our failure or any failure by these third parties to comply with these regulations or to recruit a sufficient number of patients may require us to repeat clinical trials, which would delay the marketing authorization process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws. We also are required to register ongoing clinical trials and post the results of certain completed clinical trials on certain government-sponsored databases, such as ClinicalTrials.gov in the United States, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties for any reason, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory authorizations for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
If we are unable to establish such arrangements when, and as necessary, we could be required to undertake these activities at our own expense, which would significantly increase capital requirements and may delay the development, approval and future commercialization of our product candidates, which could have a material adverse effect on our business, financial condition and operating results.
We depend on key managerial personnel for our continued success.
We are highly dependent upon our small team of managerial personnel, particularly that of our Chief Executive Officer, Arun Menawat. We do not maintain any “key man” insurance policies on Dr. Menawat or any other members of senior management. Our anticipated growth will require additional expertise and the addition of new qualified personnel. There is intense competition for qualified personnel in the medical device field.
38
Therefore, we may not be able to attract and retain the qualified personnel necessary for the development of our business. We must continue to retain, motivate and recruit executives and other key employees. The failure to motivate, or the loss of the services of, existing personnel, as well as the failure to recruit additional key managerial personnel in a timely manner, would harm our business development programs, and our ability to manage day-to-day operations, attract collaboration partners, attract and retain other employees, generate revenues, and could have a material adverse impact on our business, financial condition and results of operations.
Research and development carries substantial risk and we may not be able to expand our product portfolio.
Future growth may also depend on, among other factors, our ability to successfully develop new product candidates and make product improvements to meet evolving market needs. We may not be able to successfully expand our product portfolio to generate new revenue opportunities in the future. Although we believe we have the scientific and technical resources available to improve our products and develop new products, future products will nevertheless be subject to the risks of failure inherent in the development of products based on innovative technologies. In addition, any such research and development activities may involve significant capital expenditures. There can be no assurance that we will be able to successfully develop future products and tests, which would prevent us from introducing new products in the marketplace and negatively impact our ability to grow revenues and become profitable.
In addition, the identification of new product candidates for development may require that we enter into licensing or other collaborative agreements with others, including medical device and pharmaceutical companies and research institutions. These collaborative agreements may require that we pay license fees, make milestone payments or pay royalties or grant rights, including marketing rights, to one or more parties, and such amounts may be material to our results of operations and financial condition. Moreover, these arrangements may contain covenants restricting our product development or business efforts in the future. Any such arrangements would also increase our reliance on third parties.
We may be subject to product liability claims, which can be expensive, difficult to defend and may result in large judgments or settlements, and/or warranty claims on our products.
The use of medical devices for treatment of humans, whether in clinical trials or after marketing clearance or approval is obtained, can result in product liability claims. Product liability claims can be expensive, difficult to defend and may result in large judgments or settlements against us. In addition, third-party collaborators and licensees may not protect us from product liability claims.
We currently maintain product liability insurance in connection with the use of our products in clinical trials and in commercial use; however, we may not have adequate protection against all potential liabilities under these insurance policies. If we are unable to obtain sufficient levels of insurance at acceptable cost or otherwise protect against potential product liability claims, we will be exposed to product liability claims. A successful product liability claim in excess of our insurance coverage could harm our financial condition, results of operations and prevent or interfere with our commercialization efforts and future product development. In addition, any successful claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable terms. Even if a claim is not successful, defending such a claim may be time-consuming and expensive.
We also bear the risk of warranty claims on our products, generally for one year after sale. We may not be successful in claiming recovery of the relevant components from our suppliers in the event of a successful warranty claim against us by a customer, or that any recovery from such suppliers would be adequate. In addition, warranty claims brought by our customers related to third-party components may arise after the expiration of our corresponding warranty with our third-party suppliers, which would require us to bear the burden of any such warranty claims.
Rising insurance costs could negatively impact our profitability.
The cost of insurance, including director and officer, worker’s compensation, property, product liability and general liability insurance, has risen significantly in recent years and is expected to continue to increase. In particular, our product liability insurance is subject to price increases if we experience product liability claims. In response, we may increase deductibles and/or decrease certain coverages to mitigate these costs. These increases, and our increased risk due to increased deductibles and reduced coverages, could have a negative impact on our business, financial condition and results of operations.
39
We are increasingly dependent on sophisticated information technology systems to operate our business and if we fail to properly maintain the integrity of our data or we experience a cyber-attack or other breach of these systems, our business could be adversely affected.
We are increasingly dependent on sophisticated information technology for our development activities, products and infrastructure. We rely on information technology systems to process, transmit and store electronic information in our day-to-day operations. The complexity of our information technology systems makes them vulnerable to increasingly sophisticated cyber-attacks, malicious intrusion, breakdown, destruction, loss of data privacy, or other significant disruption. Any such event could be prolonged and/or could go undetected for a significant period of time. Our products and their information systems require an ongoing commitment of resources to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information, and changing customer patterns.
In addition, third parties may attempt to hack into our products or systems and may obtain data relating to patients, our products or our proprietary information. If we fail to maintain or protect our information systems and data integrity effectively, we could lose existing customers, have difficulty attracting new customers, have problems in determining product cost estimates and establishing appropriate pricing, have difficulty preventing, detecting, and controlling fraud, have disputes with customers, physicians, and other health care professionals, become subject to litigation, have regulatory sanctions or penalties imposed, experience increases in operating expenses, incur expenses or lose revenues as a result of a data privacy breach, or suffer other adverse consequences. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
A portion of our employees are unionized, and our good labor relations may not continue.
As of December 31, 2024, 19 of our employees in Vantaa, Finland were unionized. Currently, labor relations are in good standing and no issues have been noted; however, the maintenance of a productive and efficient labor environment cannot be assured. If any of our employees at our other manufacturing facilities unionize in the future, or if protracted and extensive work stoppages occur, labor disruptions such as strikes or lockouts could have a material adverse effect on our business, financial condition and results of operations.
If our facilities are damaged or destroyed, we may experience delays that could negatively impact our revenues.
Our facilities may be affected by natural or man-made disasters. If our facilities were affected by a disaster, we would be forced to rely on third-party manufacturers or to set up production at another manufacturing facility. In such an event, we might not be able to find a suitable alternate manufacturer or might face significant delays in manufacturing which would prevent us from being able to sell our products. In addition, our insurance may not be sufficient to cover all of the potential losses and may not continue to be available to us on acceptable terms, or at all.
We face risks associated with acquisition of businesses and technologies.
As part of our growth strategy, we intend to evaluate and may pursue additional acquisitions of, or significant investments in, complementary companies or technologies to increase our technological capabilities and expand our product offerings. For example, in July 2017, we acquired from Philips the technologies and asset underlying our Sonalleve system. Acquisitions and the successful integration of new technologies, products, assets or businesses may require significant attention from our management and could result in a diversion of resources from our existing business, which in turn could have an adverse effect on our business operations. Other risks typically encountered with acquisitions include disruption of our ongoing business; difficulties in integration of the acquired operations and personnel; inability of our management to maximize our financial and strategic position by the successful implementation or integration of the acquired technology into our product offerings; being subject to known or unknown contingent liabilities, including taxes, expenses and litigation costs; and inability to realize expected synergies or other anticipated benefits which may, among other things, also lead to goodwill impairments or other write-offs. For example, our ability to achieve the anticipated benefits of the Sonalleve Transaction depends in part on our ability to realize the anticipated growth opportunities and synergies from the acquired assets and technologies, including our further development of the Sonalleve system.
We cannot assure you that we will be successful in overcoming these risks or any other problems we may encounter in connection with the Sonalleve Transaction or potential future acquisitions. Our inability to successfully integrate the operations of an acquired business, including a successful implementation of the technologies and assets we acquire, and realize anticipated benefits associated with an acquisition, could have a material adverse effect on our business, financial condition, results of operations and cash flows.
40
Acquisitions or other strategic transactions may also result in dilution to our existing shareholders if we issue additional equity securities as consideration or partial consideration as well as in the incurrence of indebtedness if we borrow funds to finance such transactions.
Risks Relating to Regulation of the Company and Our Products
Our products and operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
Our products are regulated as medical devices in the United States and other jurisdictions. We and our products are subject to extensive regulation in the United States and elsewhere, including by the FDA and its foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance, classification and approval; recordkeeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; post-market approval trials; and product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. The FDA enforces its regulatory requirements through, among other means, periodic announced or unannounced inspections. We do not know whether we or our contract manufacturers will be found substantially compliant with applicable regulations in connection with any future FDA inspections. Failure to comply with applicable regulations could jeopardize our ability to sell our authorized products, or obtain marketing authorization for any of our product candidates, and could result in enforcement actions such as: warning letters; fines; injunctions; civil penalties; termination of distribution; recalls or seizures of products; delays in the introduction of products into the market; total or partial suspension of production; refusal to grant future clearances or approvals; withdrawals or suspensions of clearances or approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal penalties.
We may be unable to obtain, or experience significant delays in obtaining, FDA clearances or other regulatory authorizations for our product candidates and/or enhancements to our approved or cleared products.
Our products are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities and Notified Bodies. The process of obtaining FDA clearances or approvals, or equivalent third country authorizations to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all.
In the United States, before we can market a new medical device, or a new use of, or other significant modification to an existing, marketed medical device, we must first receive either clearance under Section 510(k) of the FFDCA, approval of a PMA or grant of a De Novo classification request from the FDA, unless an exemption applies. In the 510(k) clearance process, before a device may be marketed, the FDA must determine that a proposed device is substantially equivalent to a legally marketed predicate device. To be substantially equivalent, the proposed device must have the same or similar intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness from the predicate device. In the process of obtaining PMA approval, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life sustaining, life supporting or implantable devices. In the De Novo classification process, a manufacturer whose novel device under the FDA would otherwise be automatically classified as class III and require the submission and approval of a PMA prior to marketing is able to request initial classification of the device as class I or class II based on evidence that the device in fact presents a low or moderate risk. If the FDA grants the De Novo classification request, the applicant receives authorization to market the device. If the De Novo process results in the classification of a device as class II, the authorized device may be used subsequently as a predicate device for future 510(k) submissions.
The PMA approval, 510(k) clearance and De Novo classification processes can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process can take anywhere from three to 12 months or longer to complete.
41
The process of obtaining a PMA or De Novo classification is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is submitted to the FDA. In addition, PMAs and De Novo classification requests generally require the applicant to have conducted one or more clinical trials. Despite the time, effort and cost expended in seeking a marketing authorization, there is no assurance that the FDA will grant it. Any delay or failure to obtain necessary regulatory marketing authorizations could harm our business. Furthermore, even if we are granted such marketing authorizations, they may include significant limitations on the indicated uses for the device, which may limit the potential commercial market for the device.
The FDA or foreign regulatory authorities can delay, limit or deny marketing authorization or certification of a device for many reasons, including:
| ● | our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory authorities that our products are safe and effective for their intended uses; |
| ● | the disagreement of the FDA or foreign regulatory authorities with the design or implementation of our clinical trials or the interpretation of data from non- clinical studies or clinical trials; |
| ● | serious and unexpected adverse device effects experienced by subjects enrolled in our clinical trials; |
| ● | the data from our nonclinical studies and clinical trials may be insufficient to support marketing authorization, where required; |
| ● | our inability to demonstrate that the clinical and other benefits of the device outweigh the risks; |
| ● | the manufacturing process or facilities we use may not meet applicable requirements; and |
| ● | the potential for approval policies or regulations of the FDA or foreign regulatory authorities to change significantly in a manner rendering our clinical data or regulatory filings insufficient for marketing authorization. |
We expect to generate a significant portion of our revenues from sales of our marketed systems, in particular our FDA-cleared TULSA-PRO system, but may be unable to do so if the systems do not continue to prove to be safe and effective for our intended use in clinical trials to the satisfaction of the relevant regulatory authorities in the United States, EU Member States, China or other countries. In addition, no assurance can be given that our other product candidates will prove to be sufficiently safe and effective in clinical trials or that we will receive regulatory approvals in the jurisdictions where we seek to market the systems. In addition, no assurance can be given that current regulations relating to regulatory approval will not change or become more stringent.
Any delay in, or failure to receive or maintain, regulatory clearance or approval of other products under development would adversely affect our ability to commercialize those products, thereby adversely affecting operations and could prevent us from generating revenue from these products or achieving profitability. Any failure to obtain regulatory clearance or approval would materially adversely affect our business, financial condition and results of operations.
Our ability to continue sales of our product in the EU may be materially impaired if we do not take necessary steps to comply with the certification requirements of the new EU Medical Devices Regulation.
On May 25, 2017, the EU Medical Devices Regulation 2017/745, or the MDR, entered into force, repealing and replacing Council Directive 93/42/EEC, or the Medical Devices Directive, and Council Directive 90/385/EEC, or the AIMD Directive. Unlike directives, which must be implemented into the national laws of the EU member states, regulations are directly applicable (i.e., without the need for adoption of EU member state laws implementing them) in all EU member states from their effective applicability date and are intended to eliminate differences in the regulation of medical devices among EU member states. The New EU MDR, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EU for medical devices and ensure a high level of safety and health while supporting innovation.
The New EU MDR became effective on May 26, 2021. Devices lawfully placed on the market pursuant to the MDD prior to May 26, 2021 could initially continue to be made available on the market or put into service until May 26, 2025. Nevertheless, the European Parliament adopted legislation to extend this transitional period to give manufacturers more time to switch from the previously applicable provisions to the new certification requirements for medical devices as laid down by the New EU MDR. For high risk, class III and class IIb implantable devices the transitional period was extended until December 31, 2027. For medium and low risk, class IIb devices and class IIa, Im, Is and Ir devices the transition period was extended until December 31, 2028. The New EU MDR among other things:
| ● | Strengthens the rules on placing devices on the market and reinforces surveillance once they are available; |
42
| ● | Establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| ● | Improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| ● | Sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and |
| ● | Strengthens the rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts before they are placed on the market. |
These modifications may have an effect on the way we design and manufacture product and products candidates and conduct our business in the EU and EEA. For example, as a result of the transition towards the new regime, Notified Body review times have lengthened, and product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business. Although our TULSA-PRO and Sonalleve systems have been certified under the MDD as class IIb devices, and are therefore eligible to remain on the EU market until the extended deadline of December 31, 2028, we are evaluating the implementation of the new requirements of the New EU MDR. We cannot exclude unexpected regulatory hurdles and possible delays while transitioning towards the new regime.
The EU-UK Trade and Cooperation Agreement, or TCA, came into effect on January 1, 2021. The TCA does not specifically refer to medical devices. However, as a result of Brexit, the New EU MDR will not be implemented in the UK, and previous legislation that mirrored the New EU MDR in UK law has been revoked. The regulatory regime for medical devices in the United Kingdom will continue to be based on the requirements derived from current EU legislation, and the United Kingdom may choose to retain regulatory flexibility or align with the New EU MDR going forward. CE markings will continue to be recognized in the United Kingdom, and certificates issued by EU-recognized Notified Bodies will be valid in the United Kingdom, until the earlier of June 30, 2028 or the expiration of the certificate for devices compliant with the MDD or until June 30, 2030 for devices compliant with the New EU MDR. For medical devices placed on the UK market after this period, the UK Conformity Assessed, or UKCA, marking will be mandatory. In contrast, UKCA marking and certificates issued by UK Notified Bodies will not be recognized on the EU market. The TCA does provide for cooperation and exchange of information in the area of product safety and compliance, including market surveillance, enforcement activities and measures, standardization related activities, exchanges of officials, and coordinated product recalls (or other similar actions). For medical devices that are locally manufactured but use components from other countries, the “rules of origin” criteria will need to be reviewed. Depending on which countries products will be ultimately sold in, manufacturers may start seeking alternative sources for components if this would allow them to benefit from no tariffs. Under the Windsor Framework, an agreement between the UK government and the European Commission, the rules for placing medical devices on the Northern Ireland market differ from those in the United Kingdom. These modifications may have an effect on the way we design and manufacture products and we conduct our business in these countries.
Seeking, obtaining and maintaining certification in the EU under the MDR, with the CE-Mark to be re-certified before December 31, 2027, can be an uncertain process and Notified Bodies have limited resources and may experience backlogs.
Devices such as our TULSA-PRO and Sonalleve systems currently on the market in the EU that have been granted a CE Mark under the MDD, will need to be re-evaluated and re-certified in accordance with the New EU MDR. Any modification to an existing CE Marked medical device will also require review and certification under the New EU MDR. As many CE Mark certifications will become void as part of the transition to the New EU MDR, Notified Bodies must certify medical devices in accordance with the requirements of the New EU MDR.
The New EU MDR requires a re-designation of the Notified Bodies, the organizations designated by the EU member state in which they are based that are responsible for assessing whether medical devices and manufacturers of medical devices meet the applicable regulatory requirements in the EU. To be re-designated, Notified Bodies must demonstrate increased technical expertise in their scope of designation, as well as improved quality management systems. This re-designation process has caused backlogs in the assessment of medical devices and medical device manufacturers during the transition period leading up to May 26, 2021, the effective date of the MDR. In the European Union, currently 50 Notified Bodies have been re-designated, including one for Belgium.
To be able to continue to place our CE Marked devices on the EU market, if we decide to do so, such products must be re-certified under the New EU MDR before the applicable extended deadline of December 31, 2028. The re-certification requires us to present documentation and other evidence demonstrating that the performance and the safety of the system has been maintained and that the system continues to meet existing regulations and standards.
43
Otherwise, the marketing and sale of our TULSA-PRO and Sonalleve systems in EU member states may be temporarily or permanently prohibited. Significant modifications to the our authorized products, if any, will require certification under the New EU MDR and cannot be implemented during the extended transition period.
The overall backlogs experienced by the Notified Bodies having already been re-designated might have a negative impact on the re-certification of our TULSA-PRO and Sonalleve systems. We believe, however, that we are on track to meet the new requirements by the deadlines set forth in the New EU MDR.
Any third-party entities that we rely upon for distribution of our products in the EU must also comply with applicable requirements of the New EU MDR. If a distributor in the EU fails to meet such requirements, on a timely basis or at all, the marketing and sale of our authorized products by such distributor may be temporarily or permanently prohibited.
Any delay or failure to comply with the New EU MDR could result in the sale of our authorized products being temporarily or permanently prohibited in EU member states and may affect our reputation, business, financial condition, results of operations and prospects.
If clinical trials are conducted in a manner that fails to meet all FDA requirements, the FDA may delay our clearances or approvals, or the deficiencies may be so great that the FDA could refuse to accept all or part of our data or trigger enforcement action.
Clinical trials are generally required to support PMA approval and De Novo classification and are sometimes required to support 510(k) clearance. Such trials, if conducted in the United States and involve a significant risk device, require an IDE application, including one or more proposed study protocol as well as the number of subjects and study sites, to be approved in advance by the FDA. Clinical trials involving a non-significant risk device do not require FDA approval of an IDE application but are still subject to abbreviated requirements under the IDE regulation. Further, some device clinical trials are exempted from the IDE regulation. Although we do not expect to submit any additional IDE applications for any further clinical trials involving the TULSA-PRO system, we may need to obtain an IDE application for any clinical trials designed to expand the indications for or support any significant modifications to the TULSA-PRO. In addition, FDA approval of IDE applications may be required in support of clinical trials involving other product candidates.
Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an IRB and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, we must also obtain each subject’s informed consent which must comply with FDA requirements, state and federal privacy regulations and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Additionally, we may decide at any time, for business or other reasons, to terminate a clinical trial. Following completion of a clinical trial, we would need to collect, analyze and present the data in an appropriate submission to the FDA. Even if a study is completed and submitted to the FDA, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device for its intended use, or may be equivocal or otherwise not be sufficient to obtain FDA clearance or approval of our product. In addition, the FDA may perform a bioresearch monitoring inspection of a study, and if it finds deficiencies, we will need to expend resources to correct those deficiencies, which may delay clearance or approval or the deficiencies may be so great that the FDA could refuse to accept all or part of the data or could trigger enforcement action.
44
Attracting patients to perform clinical trials and meeting clinical trial objectives can be more costly and time-consuming than expected and could be adversely affected by another health crisis.
In order conduct our clinical trials, we must recruit, screen and enroll eligible patients. Patients may be identified from the investigator’s own clinical practice or hospital or may be referred by another physician. Potential clinical trial participants must provide informed consent before undergoing certain clinical tests that are used to determine patient eligibility based on inclusion/exclusion criteria. As a result, at the time of informed consent, we do not know if a patient will be eligible to participate in the trial, so we will need to screen many more patients than we intend to enroll in order to meet our enrollment criteria. Not all patients who undergo screening will ultimately be eligible for enrollment in our clinical trials. Moreover, some of the enrolled participants may not comply with the requirements of the trial, thereby leading to poor or unusable data, or some may withdraw from the trial, which may compromise the results of the clinical trial.
We may not be able to initiate, continue and/or complete in a timely manner clinical trials if we are unable to locate and enroll a sufficient number of eligible patients within the planned recruitment period to participate in these trials as required by the applicable regulatory authorities in the United States, Europe and any other applicable jurisdictions.
Delays in subject enrollment or failure of trial subjects to continue to participate in a clinical trial may delay commencement or completion of the clinical trial, cause an increase in the costs of the clinical trial and delays, or result in the failure of the clinical trial. Patient enrollment in our clinical trials may be affected by many factors including:
· |
the use of the investigational device and the nature of the procedures being performed under the clinical trial protocol; |
· |
the existence of a competing device with FDA marketing authorization and long-term data supporting its safety and efficacy; |
· |
clinicians’ and patients’ perceptions as to the potential advantages and risks of our investigational devices in relation to other available therapies, including any new product candidates that may be approved for the indications we are investigating; |
· |
the size and nature of the patient population; |
· |
the severity of the disease under investigation; |
· |
the eligibility criteria for the trial in question; |
· |
subject compliance with the trial protocol; |
· |
the design of the clinical trial; |
· |
the referral practices of physicians; |
· |
limitations placed on enrollment by regulatory authorities or other bodies; |
· |
the ability to monitor trial subjects adequately during and after treatment; |
· |
the proximity and availability of clinical trial sites for prospective subjects; |
· |
efforts to facilitate timely enrollment; and |
· |
other clinical trials competing for the same target patients as those of our clinical trials. |
Any difficulties in enrolling a sufficient number of subjects for any of our clinical trials, or any subjects withdrawing from the clinical trials or not complying with the trial protocols, could result in significant delays and could require us to abandon one or more clinical trials altogether. If our trial sites are restricted or delayed in performing the required procedures or following up with their trial subjects, this may lead to missing information and may potentially impact clinical trial data quality and integrity. Enrollment delays and other issues with our clinical trials may result in increased research and development costs that may exceed the resources available to us and may lead to delays in obtaining marketing authorization in target markets.
If we or our suppliers fail to comply with ongoing FDA or other foreign regulatory authority requirements or if we experience unanticipated problems with our authorized products, we could be subject to restrictions or withdrawal from the market.
Our authorized products, and any other products for which we obtain regulatory clearance or approval, as well as the respective manufacturing processes, postmarket surveillance and reporting, post-approval clinical testing and promotional activities for such products, are subject to continued regulatory review, oversight and periodic inspections by the FDA and other regulatory bodies (and Notified Bodies, as applicable). In particular, we and some of our suppliers are required to comply with the QSR and international standards for the manufacture of products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain regulatory clearance or approval.
45
Regulatory bodies, such as the FDA, enforce good manufacturing practice requirements, such as the QSR in the United States, and other regulations through periodic announced or unannounced inspections. We and our contract manufacturers have been, and anticipate in the future being, subject to such inspections.
Medical device manufacturers must submit periodic reports to the FDA and other regulatory authorities after obtaining marketing authorization in the applicable countries or jurisdictions. Such reports generally include information about failures and certain adverse events or malfunctions associated with the device after its marketing authorization. Failure to submit such reports, or failure to submit the reports in a timely manner, could result in enforcement action by the FDA or other applicable regulatory authority. Following its review of the periodic reports, the FDA or other regulatory authority might ask for additional information or initiate further investigation. Accordingly, we and our contract manufacturers must continuously expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance, and quality control.
In the United States, the FDA and other federal and state agencies, including the U.S. Department of Justice, closely regulate compliance with all requirements governing medical device products, including requirements pertaining to marketing and promotion of devices in accordance with the provisions of the approved labeling and manufacturing of products in accordance with QSR requirements. Violations of such requirements may lead to investigations alleging violations of the FFDCA and other statutes, including the False Claims Act and other federal and state healthcare fraud and abuse laws as well as state consumer protection laws. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, or the later discovery of previously unknown adverse events or other problems with our products could result in, among other things, any of the following enforcement actions:
· |
untitled letters, warning letters, fines, injunctions, consent decrees or civil penalties; |
· |
customer notifications for repair, replacement or refunds; |
· |
recall, withdrawal, detention or seizure of our products; |
· |
operating restrictions or partial suspension or total shutdown of production; |
· |
refusing or delaying our requests for premarket review or marketing authorization of new products or modified products; |
· |
operating restrictions; |
· |
suspension, modification, or withdrawal of marketing authorizations that have already been granted; |
· |
refusals to allow imports and/or to issue documentation necessary to facilitate exports; |
· |
refusal to grant export approval for our products; or |
· |
imposition of administrative or criminal penalties. |
If any of these actions were to occur, we may be required to expend significant time and resources to address or defend such actions, and our reputation may be harmed and our product sales and/or profitability may be negatively affected. Furthermore, key component suppliers may not currently be, or may not continue to be, in compliance with all applicable legal requirements or our supplier control requirements, which could result in our failure to manufacture our products on a timely basis and in the required quantities, if at all.
In addition, the FDA or other regulatory authorities may change their marketing authorization policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay marketing authorization of any product candidate under development or impact our ability to modify any authorized products on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain marketing authorizations, increase the costs of compliance or restrict our ability to maintain any marketing authorizations we have obtained.
46
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses or engaged in false or misleading promotion.
Regulatory clearances and approvals may be subject to limitations on the intended uses for which our products may be marketed and reduce our potential to successfully commercialize our products. While physicians/clinicians, in most jurisdictions, can use our products in ways or circumstances other than those strictly within the scope of the regulatory clearance or approval, we are required, in many jurisdictions, to limit our training and promotion of our products to the cleared or approved intended uses. For example, if the FDA determines that our promotional materials, labeling, training or other marketing constitutes promotion of an uncleared or unapproved, or “off-label” use, it could request that we modify or cease use of those training or promotional materials until we obtain FDA clearance or approval for those uses or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil monetary penalty and/or criminal penalties. Discussions that may be viewed as off-label promotion by FDA include discussions regarding treatment of a specific disease or condition when FDA has cleared or approved a device with a general tool-type indication that does not mention any particular disease or condition. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an uncleared or unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of our products would be impaired.
In addition to promoting our products in a manner consistent with our clearances and approvals, we must have adequate substantiation for the claims we make for our products. If any of our claims are determined to be false, misleading or deceptive, we could be subject to enforcement action. In addition, unsubstantiated claims also present a risk of consumer class action or consumer protection litigation and competitor challenges.
Compliance with regulations for quality systems for medical device companies is difficult, time consuming and costly.
We have developed and maintain a quality management system for medical devices intended to ensure quality of our products and activities. The system is designed to be in compliance with regulations in many different jurisdictions, including the QSR mandated by the FDA in the United States and the requirements of the MDD and New EU MDR in the European Union, including the international standard ISO 13485 required by the member states in Europe that recognize the CE Mark. The FDA issued a final rule on January 31, 2024 describing revisions to the QSR to harmonize it with ISO 13485:2016. The harmonized regulations, which will be called the Quality Management System Regulation, or QMSR, will become effective on February 2, 2026.
Compliance with regulations for quality management systems for medical device companies is time consuming and costly, and there are changes in such regulations from time to time. While management believes that we are compliant with existing quality management system regulations for medical device companies as of the date of this Annual Report, it is possible that we may be found to be noncompliant with new or existing regulations in the future. In addition, we may be found to be noncompliant as a result of future changes in, or interpretation of, the regulations for quality systems. If we do not achieve compliance or subsequently become noncompliant, the regulatory authorities may require that we take appropriate action to address nonconformance issues identified in a regulatory audit, and may, if we do not take such corrective actions in a timely manner, withdraw marketing clearance, or require product recall or take other enforcement action.
Our external vendors must, in general, also comply with the applicable quality system requirements. Any of our external vendors may become noncompliant with the applicable quality system regulations, which could result in enforcement action by regulatory authorities, including, for example a Warning Letter from the FDA or a requirement to withdraw from the market or suspend distribution, or export or use of products manufactured by one or more of our vendors.
If we or our contract manufacturers fail to comply with such laws and regulations where we would intend to market our products, we could be subject to enforcement action including recall of our device, withdrawal of approval, authorization, certification or clearance and civil and criminal penalties. If any of these events occur, it may materially and adversely affect our business, financial condition, results of operations and prospects.
47
Modifications to our cleared or approved products may require new regulatory clearances or approvals or may require us to recall or cease marketing our products until such additional clearances or approvals are obtained.
Certain modifications to our products may require the submission of new 510(k) premarket notifications, PMA supplements, or other regulatory agency approval applications or documents. If a modification is implemented to address a safety concern, we may also need to initiate a recall or cease distribution of the affected device. The FDA can review a manufacturer’s decision not to submit a new 510(k) premarket notification, PMA supplement or PMA for a modification and may disagree. The FDA may also on its own initiative determine that clearance of a new 510(k) or approval of a new PMA submission is required. We may make additional modifications to our products in the future that we believe do not or will not require clearance of a new 510(k) or approval of a new PMA. If we begin manufacture and distribution of the modified devices and the FDA later disagrees with our determination and requires the submission of a new 510(k) or PMA for the modifications, we may also be required to recall the distributed modified devices and to stop distribution of the modified devices until we have received approval or clearance for the modified device, which could have an adverse effect on our business. If the FDA does not clear or approve the modified devices, we may need to redesign the devices, which could also harm our business. When a device is marketed without a required clearance or approval, the FDA has the authority to take informal enforcement actions such as the issuance of a Warning Letter, or bring a formal enforcement action, including injunction, seizure and criminal prosecution. The FDA considers formal enforcement actions generally when there is a serious risk to public health or safety or the company’s corrective and preventive actions are inadequate to address the FDA’s concerns.
Where we determine that modifications to our products require clearance of a new 510(k) or approval of a new PMA or PMA supplement, we may not be able to obtain those additional clearances or approvals for the modifications or additional indications in a timely manner, or at all. For those products sold in the EEA, we must notify an EU Notified Body, if significant changes are made to the products or if there are substantial changes to our quality assurance systems affecting those products. Delays in obtaining required future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm its future growth. Additionally, such changes could mean we would no longer be able to rely on existing MDD CE Marks under the transition periods and would need to obtain a CE Mark under the New EU MDR.
Our contract manufacturers are subject to regulatory compliance by the FDA, Health Canada and regulatory authorities in the EU and other jurisdictions.
Our contract manufacturers must comply with applicable FDA, EU, Health Canada and other applicable foreign regulations, which include quality control and quality assurance requirements, as well as the corresponding maintenance of records and documentation and manufacture of devices according to the specifications contained in the applicable regulatory file. The manufacturing practices of our third-party suppliers are subject to ongoing regulation and periodic inspection. In the United States, the methods used in, and the facilities used for, the manufacture of medical devices must comply with the QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, and servicing of medical devices. Furthermore, we will be required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. Our authorized device products also subject to similar state regulations and various laws and regulations of other countries governing manufacturing. If our contract manufacturers do not or cannot comply with these requirements, our ability to commercialize our approved products may be adversely affected.
The introduction of new or alternative manufacturers or suppliers also may require manufacturing or design changes to our products that are subject to FDA and other regulatory clearances or approvals. Similarly, in the European Union, the introduction of new or alternative manufacturers or suppliers could be considered to constitute a substantial change to our quality system or result in design changes to our products which could affect compliance with the Essential Requirements for the Notified Body’s certificate under the MDD (which continues to be valid during the transition period) and with the General Safety and Performance Requirements once a Notified Body certificate under the New EU MDRs is required.
If a substantial change is made to a device relying on an MDD certificate it will no longer benefit from the transition period set out in the New EU MDR. In this case the product would need to be CE marked under the New EU MDR to be placed on the market. Once CE marked under the New EU MDR these changes must be disclosed to our Notified Body in the EU before implementation. The Notified Body will then assess the changes and verify whether they affect the products’ conformity with the General Safety and Performance Requirements. If the assessment is favorable the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing certificates attesting compliance with the General Safety and Performance Requirements.
48
We may also be required to assess the new manufacturer’s compliance with all applicable regulations and guidelines, which could further impede our ability to manufacture our products in a timely manner. As a result, we could incur increased production costs, experience delays in deliveries of our products, suffer damage to our reputation, and experience a material adverse effect on our business, financial condition, and results of operations.
Our products may in the future be subject to product recalls that could harm our reputation, business and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious adverse health consequences or death. In addition, we may initiate voluntary recalls of our products in the future to the extent we experience safety or other concerns with such products. For voluntary corrections or removals, the FDA requires that manufacturers report to the FDA within 10 working days after the correction or removal is initiated if the action was initiated to reduce a risk to health posed by the device or to remedy a violation of the FFDCA caused by the device which may present a risk to health. Companies are required to maintain certain records of corrections and removals, even if they are not reportable to the FDA. We may determine that any particular voluntary recall that we initiate does not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.
In the European Union, incidents and serious incidents must be reported to the relevant authorities of the European Union Member States, and manufacturers are required to take FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market (such FSCAs must also be reported to relevant authorities). The timing and means of making the report depend on the severity of the incident (for example, serious incidents that resulted in death or serious deterioration require immediate reporting to competent authorities, whereas incidents might be included in the periodic safety update report and/or trend reporting). For purposes of these regulations, an “incident” is defined as any malfunction or deterioration in the characteristics or performance of a device made available on the market, including use-error due to ergonomic features, as well as any inadequacy in the information supplied by the manufacturer and any undesirable side-effect. “Serious incident” is defined as any incident that directly or indirectly led, might have led or might lead to any of the following: (a) the death of a patient, user or other person, (b) the temporary or permanent serious deterioration of a patient’s, user’s or other person’s state of health, (c) a serious public health threat. An FSCA is defined as a corrective action taken by a manufacturer for technical or medical reasons to prevent or reduce the risk of a serious incident in relation to a device made available on the market. A FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. In addition, governmental or other competent bodies or authorities have the authority to require the recall of products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found.
A government-mandated or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of the TULSA-PRO system, Sonalleve system or any future products would divert managerial and financial resources and could have an adverse effect on our financial condition and results of operations.
If our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, and such events can result in voluntary corrective actions or agency enforcement actions.
Under FDA medical device reporting regulations, manufacturers are required to report to the FDA information that reasonably suggests that one of their marketed devices may have caused or contributed to a death or serious injury or has malfunctioned and that the device or a similar device marketed by the manufacturer would likely cause or contribute to death or serious injury if the malfunction were to recur. If we fail to report these events to the FDA within the required timeframes, or at all, the FDA could take enforcement action against us. Similar enforcement action could be taken by the competent authorities in the European Union if we do not comply with our medical devices vigilance obligations. In addition, our EU Notified Body could decide to suspend or withdraw our CE Certificates of Conformity. Any such adverse event involving the TULSA-PRO or Sonalleve systems also could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, audit or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of personnel time and capital, distract management from operating the business and may harm our reputation and could have a material adverse effect on our business, financial condition and operating results.
49
Legislative or regulatory reform of the healthcare systems in which we intend to operate may affect our ability to sell our products profitably and could adversely affect our business.
The governments and regulatory authorities in the United States, the European Commission, Canada and other markets in which we expect to sell our devices may propose and adopt new legislation and regulatory requirements relating to medical product approval criteria, manufacturing and marketing requirements. In addition, regulations and guidance promulgated by the FDA, the European Commission, and other regulatory bodies are often revised or reinterpreted by the agency and other relevant regulatory bodies in ways that may significantly affect our business and products. It is impossible to predict whether legislative changes will be enacted or regulations, guidance or interpretations changed and what the impact of such changes, if any, may be. Such legislation or changes in regulatory requirements, or the failure to comply with such, could adversely impact our operations and could have a material adverse effect on our business, financial condition and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. Future legislative and regulatory proposals to further reform healthcare or reduce healthcare costs may prevent, limit or delay regulatory authorization of our product candidates or coverage or reimbursement for such product candidates, if approved, or even lower reimbursement for the procedures associated with the use of such product candidates. More broadly, such future legislation or regulation may materially impact the ability of the FDA and other regulatory agencies to operate as they have historically operated. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could impact our revenue from the sale of our products. We cannot be sure whether additional legislative changes will be enacted, or whether any of the FDA’s regulations, guidances or interpretations will be changed, or what the impact of such changes on the agency and its scientific review staff, if any, may be.
In December 2022, the U.S. Congress enacted the Consolidated Appropriations Act for 2023, an omnibus appropriations bill, which included amendments to the FDDCA under the Food and Drug Omnibus Reform Act of 2022, or FDORA. In addition to the requirement that sponsors of pivotal trials submit diversity action plans for pivotal trials (see “Government Regulation-Regulatory Landscape in the United States-Device Clinical Studies”), FDORA included new requirements for cyber devices, defined as any medical device that is or includes software that is validated, installed, or authorized by the manufacturer; can connect to the internet; and may be vulnerable to cybersecurity threats. Under the FDORA amendments to the DFDCA, any application for marketing authorization of the cyber device must include a software bill of materials and a cybersecurity plan describing the methods by which the manufacturer will monitor, identify and address cybersecurity vulnerabilities. Any failure by a cyber device manufacturer to comply with applicable cybersecurity requirements is considered a violation of the FDDCA and will subject the manufacturer to enforcement actions and possibly legal sanctions. The growth of overall healthcare costs as a percentage of gross domestic product in many countries means that governments and payers are under intense pressure to control healthcare spending even more tightly. As a result, our businesses and the healthcare industry in general are operating in an ever more challenging environment with very significant pricing pressures. In recent years, national, federal, provincial, state and local officials and legislators have proposed, or are reportedly considering proposing, a variety of price-based reforms to the healthcare systems in the United States, the European Union and other countries. Some proposals include measures that would limit or eliminate payments for certain medical procedures and treatments or subject pricing to government control. In addition, proposed legislation may limit access to healthcare insurance coverage, which could reduce the volume of medical procedures involving our authorized device products. Furthermore, in certain foreign markets, the pricing or profitability of healthcare products is subject to government controls and other measures that have been prepared by legislators and government officials. While we cannot predict whether any such legislative or regulatory proposals or reforms will be adopted, the adoption of any such proposals or reforms could adversely affect the commercial viability of our existing and potential products. In addition, any changes of, or uncertainty with respect to, coverage or reimbursement rates relating to our authorized products, or procedures involving such products, could affect demand for our products, which in turn could impact our ability to successfully commercialize our authorized products and have a material adverse effect on our business, financial condition and results of operations.
Other legislation or regulatory proposals may adversely affect our revenues and profitability.
Existing and proposed changes in the laws and regulations affecting public companies may cause us to incur increased costs as we evaluate the implications of new rules and responds to new requirements. Failure to comply with the new rules and regulations could result in enforcement actions or the assessment of other penalties.
50
The new laws and regulations could make it more difficult to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board, or as executive officers. We may be required to hire additional personnel and utilize additional outside legal, accounting and advisory services, all of which could cause our general and administrative costs to increase beyond what we currently have planned. Although we intend to evaluate and monitor developments with respect to these rules, we cannot predict or estimate the amount of the additional costs we may incur or the timing of such costs.
We are subject to “fraud and abuse” laws, anti-bribery laws, environmental laws and privacy and security regulations. Any violation by our employees or other agents could expose us to severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
Our business is subject to the FCPA, which generally prohibits U.S. companies and their officers, directors and employees from giving, promising, offering or authorizing, directly or indirectly, any payments or anything of value to foreign officials for the purpose of obtaining or retaining business or directing business to any company or person, by securing an improper advantage, influencing any act or decision by a foreign official in their official capacity, or inducing a foreign official to do or omit to do something in violation of their lawful duty. The FCPA also requires issuers to maintain accurate books and records and adequate internal controls. In addition, we are subject to anti-bribery laws of the nations in which we conduct business (e.g., Bribery Act 2010 in the United Kingdom, Articles 299a and 299b of the German Criminal Code specifically addressing bribery in the healthcare sector, the Corruption of Foreign Public Officials Act in Canada and laws adopted pursuant to the Organisation for Economic Co-operation and Development Convention on Combating Bribery of Foreign Public Officials in International Business Transactions). If our employees or our agents are found to have engaged in prohibited conduct under our policies and procedures, or under the FCPA or other anti-bribery laws to which we may be subject, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
Our operations may be directly or indirectly affected by various broad United States or foreign healthcare fraud and abuse laws. For example, healthcare laws and regulations in the United States may constrain the business or financial arrangements and relationships through which we research, market, sell and distribute any authorized products or product candidates. In particular, the United States federal Anti-Kickback Statute prohibits any person from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in return for or to induce the referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of an item or service, for which payment may be made under United States federal healthcare programs, such as the Medicare and Medicaid programs. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. This statute has been interpreted to apply to arrangements between device manufacturers on one hand and prescribers and purchasers on the other. For example, the United States government has sought to apply the Anti-Kickback Statute to device manufacturers’ financial relationships with physician consultants. Among other theories, the United States government has alleged that some such relationships are payments to induce the consultants to arrange for or recommend the ordering, purchasing or leasing of the manufacturers’ products by the hospitals, medical institutions and other entities with whom they are affiliated. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and arrangements that involve remuneration that could induce prescribing, purchases, or recommendations may be subject to government scrutiny if they do not qualify for an exemption or a safe harbor.
The False Claims Act imposes criminal and civil penalties against individuals or entities for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment by a federal government program or making a false statement or record material to payment of a false claim or avoiding, decreasing or concealing an obligation to pay money to the federal government. The government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. Suits filed under the False Claims Act can be brought by the United States government or they can be brought by an individual on behalf of the United States government, as “qui tam” actions, and such individuals, commonly known as “whistleblowers,” may share in any damages paid by the entity to the United States government in fines or settlement. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the United States government, plus civil penalties of up to approximately $25,000 for each separate false claim. Various states have also enacted laws modeled after the False Claims Act.
51
The U.S. federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), imposes criminal and civil liability on any person who knowingly and willfully executes, or attempts to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false, fictitious, or fraudulent statements or representations in connection with the delivery of, or payment for, healthcare benefits, items or services relating to health care matters. In addition, HIPAA and HITECH impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information on certain health care providers, health plans, and health care clearinghouses, known as covered entities, as well as their respective business associates, individuals and entities that perform services on their behalf that involve the use or disclosure of individually identifiable health information and their subcontractors that use, disclose or otherwise process such information.
The U.S. federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-authorized drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Centers for Medicare and Medicaid Services information related to payments and other transfers of value to physicians, certain advanced non-physician healthcare practitioners, and teaching hospitals as well as ownership and investment interests held by physicians and their immediate family members.
Additionally, we are subject to U.S. state and foreign equivalents of each of the healthcare laws and regulations described above, among others, some of which may be broader in scope and may apply regardless of the payor. Many U.S. states have adopted laws similar to the federal Anti-Kickback Statute and False Claims Act, and may apply to our business practices, including, but not limited to, research, distribution, sales or marketing arrangements and claims involving health care items or services reimbursed by non-governmental payors, including private insurers. In addition, certain U.S. states require medical device companies to comply with the device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring device manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices, including our financial arrangements with physicians, some of whom receive compensation in the form of stock options, which could be viewed as influencing the purchase of or use of our products in procedures they perform and may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations.
Any action brought against us for violations of these laws or regulations, even if successfully defended, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. We may be subject to private qui tam actions brought by individual whistleblowers on behalf of the federal or state governments, with potential liability under the federal False Claims Act including mandatory treble damages and significant per-claim penalties. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Any of the foregoing consequences will negatively affect our business, financial condition and results of operations.
We are subject to, and may in the future become subject to, U.S. federal and state, and foreign, stringent privacy laws, information security laws, regulations, policies and contractual obligations related to data privacy and security and changes in such laws, regulations, policies and contractual obligations could adversely affect our business.
We and our current and potential collaborators may be subject to federal, state and foreign data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the United States, numerous federal and state laws and regulations, including federal health information privacy laws (e.g., HIPAA as amended by HITECH), state data breach notification laws, state health information privacy laws and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators.
52
In particular, HIPAA imposes requirements on certain healthcare providers, health plans and healthcare clearinghouses, or “covered entities,” as well as their business associates that perform services for them that involve the use or disclosure of individually identifiable health information, called protected health information (“PHI”), under HIPAA, relating to the privacy and security of PHI, including the use of mandatory contractual terms, or Business Association Agreements, in some circumstances, as well as privacy and security standards and breach notification requirements. We may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA, as amended by HITECH, or other privacy and data security laws. Depending on the facts and circumstances, we could be subject to criminal penalties if we knowingly obtain, use, or disclose PHI maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA. However, determining whether PHI has been handled in compliance with applicable privacy standards and our contractual obligations can be complex and may be subject to changing interpretation.
We are not subject to HIPAA, but our customers, research collaborators and others in the United States with whom we do business are. Accordingly, we must ensure that any business arrangements that we have with covered entities are structured to comply with HIPAA and ensure that we have the authority to obtain any PHI that may be disclosed to us. If we are unable to properly protect the privacy and security of PHI or other personal, sensitive, or confidential information in our possession, we could be found to have breached our contracts. Further, if we fail to comply with applicable privacy laws, including applicable HIPAA privacy and security standards, we could face significant administrative, civil and criminal penalties. Enforcement activity can also result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal and outside resources. Furthermore, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents. In addition to the risks associated with enforcement activities and potential contractual liabilities, our ongoing efforts to comply with evolving laws and regulations at the federal and state level may be costly and require ongoing modifications to our policies, procedures and systems.
Many state laws govern the privacy and security of personal information and data in specified circumstances, many of which differ from each other in significant ways, are often not pre-empted by HIPAA, and may have a more prohibitive effect than HIPAA, thus complicating compliance efforts. For example, the California Confidentiality of Medical Information Act (“CMIA”) imposes restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. California’s patient privacy laws, for example, provide for penalties of up to $250,000 and permit injured parties to sue for damages. In addition to the CMIA, in 2018, California enacted the California Consumer Privacy Act (“CCPA”) which creates new individual privacy rights for California consumers (as defined in the law) and places increased privacy and security obligations on entities handling personal data of consumers or households. The CCPA requires covered companies to provide new disclosure to consumers about such companies’ data collection, use and sharing practices, provide such consumers new ways to opt-out of certain sales or transfers of personal information, and provide consumers with additional causes of action. While there is currently an exception for PHI that is subject to HIPAA and clinical trial regulations, as currently written, the CCPA may impact our business activities. In addition, the California Privacy Rights Act (“CPRA”) was recently enacted to strengthen elements of the CCPA and became effective on January 1, 2023. A number of other states have considered similar privacy proposals, with states like Colorado, Connecticut, Delaware, Florida, Indiana, Iowa, Montana, Oregon, Tennessee, Texas, Utah and Virginia enacting their own privacy laws. These privacy laws may impact our business activities and exemplify the vulnerability of our business to the evolving regulatory environment related to personal data.
In the European Union, we may be subject to the General Data Protection Regulation (“GDPR”) which went into effect in May 2018 and which imposes obligations on companies that operate in our industry with respect to the processing of personal data and the cross-border transfer of such data. The GDPR applies to any company established in the EEA and to companies established outside the EEA that process personal data in connection with the offering of goods or services to data subjects in the EEA or the monitoring of the behavior of data subjects in the EEA. The GDPR establishes stringent requirements applicable to the processing of personal data, including strict requirements relating to the validity of consent of data subjects, expanded disclosures about how personal data is used, requirements to conduct data protection impact assessments for “high risk” processing, limitations on retention of personal data, special provisions affording greater protection to and requiring additional compliance measures for “special categories of personal data” including health and genetic information of data subjects, mandatory data breach notification (in certain circumstances), “privacy by design” requirements, and direct obligations on service providers acting as processors. The GDPR also prohibits the international transfer of personal data from the EEA to countries outside of the EEA unless made to a country deemed to have adequate data privacy laws by the European Commission or a data transfer mechanism has been put in place. If we or our partners’ or service providers’ privacy or data security measures fail to comply with the GDPR requirements, we may be subject to litigation, regulatory investigations, enforcement notices requiring us to change the way we use personal data and/or fines of up to 20 million Euros or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, negative publicity, reputational harm and a potential loss of business and goodwill.
53
The GDPR may also impose additional compliance obligations relating to the transfer of data between us and our affiliates, collaborators, or other business partners. For example, on July 16, 2020, the Court of Justice of the European Union (“CJEU”), issued a landmark opinion in the case Maximilian Schrems vs. Facebook (Case C-311/18), called Schrems II. This decision (a) calls into question commonly relied upon data transfer mechanisms as between the European Union Member States and the United States (such as the Standard Contractual Clauses) and (b) invalidates the European Union-U.S. Privacy Shield on which many companies had relied as an acceptable mechanism for transferring such data from the European Union to the United States.
On July 10, 2023, the European Commission adopted an adequacy decision for a new mechanism for transferring data from the EU to the United States – the EU-US Data Privacy Framework (the “Framework”). The Framework provides EU individuals with several new rights, including the right to obtain access to their data, or obtain correction or deletion of incorrect or unlawfully handled data. The adequacy decision followed the signing of an executive order introducing new binding safeguards to address the points raised in the Schrems II decision. Notably, the new obligations were geared to ensure that data can be accessed by US intelligence agencies only to the extent necessary and proportionate and to establish an independent and impartial redress mechanism to handle complaints from Europeans concerning the collection of their data for national security purposes. The Commission will continually review developments in the US along with its adequacy decision. Adequacy decisions can be adapted or even withdrawn in the event of developments affecting the level of protection in the applicable jurisdiction. Future actions of EU data protection authorities are difficult to predict. Some patients or other service providers may respond to these evolving laws and regulations by asking us to make certain privacy or data-related contractual commitments that we are unable or unwilling to make. This could lead to the loss of current or prospective patients or other business relationships.
Relatedly, following the United Kingdom’s withdrawal from the European Union (i.e., Brexit), and the expiry of the Brexit transition period, which ended on December 31, 2020, the European Union GDPR has been implemented in the United Kingdom (as the “UK GDPR”). The UK GDPR sits alongside the UK Data Protection Act 2018 which implements certain derogations in the European Union GDPR into United Kingdom law. Under the UK GDPR, companies not established in the UK but who process personal data in relation to the offering of goods or services to individuals in the UK, or to monitor their behavior will be subject to the UK GDPR – the requirements of which are (at this time) largely aligned with those under the EU GDPR and as such, may lead to similar compliance and operational costs with potential fines of up to £17.5 million or 4% of global turnover.
Our employees, independent contractors, principal investigators, contract research organizations, consultants or vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, principal investigators, contract research organizations, consultants or vendors may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: FDA regulations, including those laws requiring the reporting of true, complete and accurate information to the FDA; manufacturing standards; federal and state healthcare fraud and abuse laws and regulations; or laws that require the true, complete and accurate reporting of financial information or data. In addition, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use or misrepresentation of information obtained in the course of clinical trials or creating fraudulent data in our nonclinical studies or clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished potential profits and future earnings, and curtailment of our operations, any of which could adversely affect our business, financial condition, results of operations or prospects.
54
Compliance with environmental laws and regulations could be expensive, and failure to comply with these laws and regulations could subject us to significant liability.
We may use hazardous materials in our research and development and manufacturing processes. We are subject to various regulations governing use, storage, handling and disposal of these materials and associated waste products. We will need one or more licenses to handle such materials, but there can be no assurance that it will be able to retain these licenses in the future or obtain licenses under new regulations if and when they are required by governing authorities. We cannot completely eliminate the risk of contamination or injury resulting from hazardous materials, and we may incur liability as a result of any such contamination or injury. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources and any applicable insurance. We would also likely incur expenses related to any such incidents. Such future expenses or liability could have a significant negative impact on its business, financial condition and results of operations. Further, we cannot assure that the cost of compliance with these laws and regulations will not materially increase in the future. We may also be subject to liability in respect of the operations of prior owners or operators of any properties we may own, at manufacturing sites where operations have previously resulted in spills, discharges or other releases of hazardous substances into the environment. We could be held strictly liable under environmental laws for contamination of property that we occupy without regard to fault or whether our actions were in compliance with law at the time. Our liability could also increase if other responsible parties, including prior owners or operators of our facilities, fail to complete their clean-up obligations or satisfy indemnification obligations to us. Similarly, if we fail to ensure compliance with applicable environmental laws in foreign jurisdictions in which we operate, we may not be able to offer our products and may be subject to civil or criminal liabilities.
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent our product candidates from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business rely, which could negatively impact our business.
The ability of the FDA to review and authorize new medical products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA and other agencies may also slow the time necessary for new medical products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times, including from December 22, 2018 through January 25, 2019, and congressional impasses periodically threaten to cause future government shutdowns. Most recently, the U.S. government nearly shutdown at the end of December 2024 due to disagreements in Congress over a continuing resolution package to fund federal government operations. When a shutdown occurs, certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. Moreover, government shutdowns or slowdowns can increase the time needed for an agency to complete its review or make final approvals or other administrative decisions. If a prolonged government shutdown or slowdown occurs, it could significantly affect the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Risk Factors Relating to Intellectual Property
If we breach any of the agreements under which we license rights to our technology from third parties, we could lose license rights that are important to our business. Certain of our license agreements may not provide an adequate remedy for their breach by the licensor.
We license certain development and commercialization rights for certain technologies used in our systems and expect to enter into similar licenses in the future. For instance, we license exclusive intellectual property rights from Sunnybrook that enable us to use, manufacture, distribute and sell the TULSA-PRO system. Under this royalty-free license, we are subject to various obligations, including the milestone payment of C$250,000 we paid upon obtaining FDA clearance of our TULSA-PRO system, and legal costs associated with patent application preparation, filing and maintenance.
55
If we breach or otherwise terminate any of the agreements under which we license rights to our technology from third parties, we could lose intellectual property rights that are important to our business and incur other liabilities. Certain of our license agreements may not provide an adequate remedy for their breach by the licensor. The loss or breach of any of these license agreements could have a material adverse effect on our business, results of operations and financial condition.
Our proprietary rights may not adequately protect our technologies.
Our commercial success will depend on our ability to obtain patents (or exclusive rights thereto) and to maintain adequate protection for our technologies in the United States, Europe, Canada and other countries. We own or have exclusive rights to multiple issued United States patents and several pending United States patent applications in respect of our products. For the TULSA-PRO system, our patent rights include rights licensed to us from Sunnybrook and other intellectual property that we have developed. We acquired the patent rights for the Sonalleve system from Philips. We or our licensors will be able to protect such proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
We apply for patents covering our technologies as we deem appropriate. However, we may fail to apply for patents on important technologies in a timely fashion, or at all. Our existing patent applications and any future patents we may obtain may not be sufficiently broad to prevent others from utilizing our technologies or from developing competing products and technologies. In addition, we cannot guarantee that:
| ● | we or our licensors were the first to make the inventions covered by each of our licensed or issued patents and pending patent applications; |
| ● | we or our licensors were the first to file patent applications for these inventions; |
| ● | others will not independently develop similar or alternative technologies or duplicate any of our or our licensors’ technologies; |
| ● | any of our or our licensors’ pending patent applications will result in issued patents; |
| ● | any of our or our licensors’ patents will be valid or enforceable; |
| ● | any patents issued to us or our licensors and collaboration partners will provide us with any competitive advantages, or will not be challenged by third parties; |
| ● | we will develop or in-license additional proprietary technologies that are patentable; or |
| ● | the patents of others will not have an adverse effect on our business. |
The actual protection afforded by a patent varies on an offering-by-offering basis, from country to country and depends upon many factors, including the type of patent, the scope of our or our licensors’ coverage, the availability of regulatory related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patents. Our or our licensors’ ability to maintain and solidify our or our licensors’ proprietary position for our products will depend on our or our licensors’ success in obtaining effective patent claims and enforcing those claims once granted. Our or our licensors’ issued patents and those that may be issued in the future may be challenged, invalidated or circumvented, and the rights granted under any such issued patents may not provide us with proprietary protection or competitive advantages against competitors with similar products or offerings. Due to the extensive amount of time required for the development, testing and regulatory review of a medical device, it is possible that, before our devices can be commercialized, any relevant patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
56
Protection afforded by patents may be adversely affected by recent or future changes to patent related statutes and administrative procedures, for example, such as in the laws of the United States or to USPTO rules. Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. For example, on September 16, 2011, the Leahy-Smith Act was signed into law in the United States. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. However, it is not fully clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. As such, the Leahy-Smith Act and its implementation, as well as any future changes to patent law in the United States or elsewhere, could increase the uncertainties and costs surrounding the prosecution of our or our licensors’ patent applications and the enforcement or defense of our or our licensors’ issued patents, all of which could have a material adverse effect on our business, financial condition and operating results.
Moreover, we or our licensors may be subject to a third-party preissuance submission of prior art to the USPTO and other patent offices, or become involved in opposition, derivation, re-examination, inter partes review or interference proceedings, or other preissuance or post-grant proceedings in the United States or other jurisdictions, challenging our or our licensors’ patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our or our licensors’ patent rights, allow third parties to commercialize our technology or product and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our or our licensors’ patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future products. Changes to the current patent statutes may adversely affect the protection afforded by our patents and/or open our patents up to third-party attack in non-litigation settings. The costs of patent enforcement or invalidity proceedings could be substantial, result in adverse determinations, and divert management attention from our business.
We also rely on trade secrets to protect some of our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to maintain. While we use reasonable efforts to protect our trade secrets, our or our collaboration partners’ employees, consultants, contractors or scientific and other advisors may unintentionally or willfully disclose our proprietary information to competitors. Enforcement of claims that a third-party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain, and may divert our efforts and attention from other aspects of our business. In addition, non-U.S. courts are sometimes less willing than courts in the United States to protect trade secrets. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secrets against them and our business could be harmed.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates, and products and services, when and if we have any, in every jurisdiction would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we or our licensors have not obtained patent protection to develop competing products. These products may compete with our products, when and if we have any, and may not be covered by any of our or our licensors’ patent claims or other intellectual property rights.
The laws of some countries do not protect intellectual property rights to the same extent as the laws of the United States and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, may not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or pharmaceuticals, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our or our licensors’ patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
The patent protection for our technologies may expire before we are able to maximize our commercial value which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue.
The patents for our technologies have varying expiration dates; although the patents for the technologies we use are not expected to expire in the near term, when these patents expire, we may be subject to increased competition and may not be able to recover our development costs or license fees. In some of the larger economic territories, such as the United States and the European Union, patent term extension/restoration may be available to compensate for time taken during aspects of a product candidate’s regulatory review. However, we cannot be certain that any extension will be granted or, if granted, what the applicable time period or the scope of patent protection afforded during any extended period will be.
57
If we or our licensors are unable to obtain patent term extension/restoration or some other exclusivity, we could be subject to increased competition and our opportunity to establish or maintain product revenue could be substantially reduced or eliminated. Furthermore, we may not have sufficient time to recover our development costs prior to the expiration of our or our licensors’ patents in the United States or elsewhere.
We may incur substantial costs as a result of litigation or other proceedings relating to enforcement of our or our licensors’ patent and other intellectual property rights and we may be unable to protect our rights to, or use of, our technology.
If we choose to go to court to try to stop or prevent a third-party from using the inventions claimed in our or our licensors’ patents, that third-party has the right to ask the court to rule that these patents are invalid and/or should not be enforced against that third-party. Even if we were successful in stopping the infringement of these patents, these lawsuits are expensive and would consume time and other resources and divert attention from other aspects of our business. In addition, there is a risk that the court will decide that these patents are invalid or unenforceable and that we do not have the right to prevent the other party from using the inventions. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to prevent the other party’s activities on the ground that such other party’s activities do not infringe our rights.
We may be subject to lawsuits from, liable for damages to, or be required to enter into license agreements with, a third-party that claims we infringed its patents or otherwise misused its proprietary information.
If we wish to use the technology in issued and unexpired patents owned by others, we will need to obtain a license from the owner, enter into litigation to challenge the validity or enforceability of these patents or incur the risk of litigation in the event that the owner asserts that we infringed these patents. The failure to obtain a license for technology or the failure to challenge an issued patent owned by others that we may require to develop or commercialize our product candidates may have a material adverse impact on us.
In addition, if a third-party asserts that we infringed its patents or other proprietary rights, we could face a number of risks that could seriously harm our results of operations, financial condition and competitive position, including:
| ● | patent infringement and other intellectual property claims, which would be costly and time consuming to defend, whether or not the claims have merit, and which could delay the regulatory approval process and divert management’s attention from our business; |
| ● | substantial damages for past infringement, including possible treble damages in some jurisdictions, which we may have to pay if a court determines that our product candidates, offerings or technologies infringe a competitor’s patent or other proprietary rights; |
| ● | a court prohibiting us from selling or licensing our technologies unless the third-party licenses patents or other proprietary rights to us on commercially reasonable terms, which it is not required to do; and |
| ● | if a license is available from a third-party, we may have to pay substantial royalties or lump sum payments or grant cross licenses to our patents or other proprietary rights to obtain that license. |
The coverage of patents is subject to interpretation by the courts and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Patent laws in the United States as well as the laws of certain other jurisdictions provide for provisional rights in published patent applications beginning on the date of publication, including the right to obtain reasonable royalties, if a patent is subsequently issued and certain other conditions are met. While we believe that there may be multiple grounds on which to challenge the validity of United States patents and the counterparts filed in other jurisdictions possibly relevant to our business, we cannot predict the outcome of any invalidity challenge. Alternatively, it is possible that we may determine it is prudent to seek a license from a patent holder to avoid potential litigation and other potential disputes. We cannot be sure that a license would be available to us on acceptable terms, or at all.
58
Because some patent applications in certain jurisdictions may be maintained in secrecy until the patents are issued, because patent applications in the United States and many other jurisdictions are typically not published until 18 months after filing and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our or our licensors’ issued patents or our pending applications or our licensors’ pending applications, or that we or our licensors were the first to invent the technology.
Patent applications filed by third parties that cover technology similar to ours may have priority over our or our licensors’ patent applications and could further require us to obtain rights to issued patents covering such technologies. If another party files a United States patent application on an invention similar to ours, we may elect to participate in or be drawn into an interference or other proceeding declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our United States patent position with respect to such inventions.
We may also be subject to damages resulting from claims that we or our employees or consultants have wrongfully used or disclosed alleged trade secrets of third parties. Many of our employees were previously employed, and certain of our consultants are currently employed, at universities or medical device companies, including our competitors or potential competitors. Although we have not received any claim to date, we may be subject to claims that we, or these employees or consultants, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of these current or former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. We may be subject to claims that employees of our partners or licensors of technology licensed by us have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. We may become involved in litigation to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel; and even if we are successful in defending such claims, they can be expensive and would consume time and other resources and divert attention from other aspects of our business.
Some of our competitors may be able to sustain the costs of complex patent and other intellectual property litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations. We cannot predict whether third parties will assert these claims against us or against our licensors, or whether those claims will harm our business. If we or our licensors are forced to defend against these claims, whether they are with or without any merit, whether they are resolved in favor of or against us or our licensors, we may face costly litigation and diversion of management’s attention and resources. As a result of these disputes, we may have to develop costly non-infringing technology, or enter into licensing agreements. These agreements, if necessary, may be unavailable on terms acceptable to us, if at all, which could have a material adverse effect on our business, financial conditions and results of operations.
Risks Relating to the International Scope of our Business
Our business, financial condition, cash flows and results of operations are subject to risks arising from our international operations.
We conduct a portion of our business outside Canada and the U.S. and in the future expect to expand our operations into new international jurisdictions, including emerging markets. In addition, the manufacturing facilities for our products sold in the U.S. are located in Canada and other jurisdictions outside of the U.S.
Our foreign operations are subject to risks inherent in conducting business abroad, such as: difficulties in coordinating and managing foreign operations, price and currency exchange controls, political and economic instability, compliance with multiple regulatory regimes, differing degrees of protection for intellectual property, unexpected changes in foreign regulatory requirements and restrictive governmental actions.
On February 1, 2025, the President of the United States issued executive orders directing the United States to impose new tariffs on imports originating from Canada, Mexico and China. These orders call for additional 25% duty on imports into the United States of Canadian origin and Mexican origin products and 10% duty on Chinese origin products, except for Canadian energy resources that are subject to an additional 10% duty. We are assessing the direct and indirect impacts to our business of such tariffs, retaliatory tariffs or other trade protectionist measures implemented as this situation develops, and such impacts could be material.
59
Adverse economic conditions impacting our customers or uncertainty about global economic conditions could cause purchases of our products to decline, which would adversely affect our revenues and operating results. Moreover, our projected revenues and operating results are based on assumptions concerning certain levels of customer spending and ongoing use of our TULSA-PRO system.
Risk Factors Relating to Our Common Shares
Future sales or the issuances of our securities may cause the market price of our Common Shares to decline.
The market price of our Common Shares could decline as a result of issuances of securities (including our Common Shares) by us, exercises of outstanding options or warrants for additional Common Shares or sales by our existing shareholders of Common Shares in the market, or the perception that these issuances or sales could occur. Sales of Common Shares by shareholders may make it more difficult for us to sell equity securities at a time and price that we deem appropriate. As of December 31, 2024, there were a total of 2,291,152 outstanding share options issued under our Share Option Plan, 324,621 Restricted Stock Units (“RSUs”), 91,670 Deferred Stock Units (“DSUs”) issued. In addition, as of December 31, 2024, the maximum number of Common Shares reserved for issuance under this plan is 3,905,175 Common Shares or such other number as may be approved by the holders of the voting shares of the Company.
We expect that the price of our Common Shares may fluctuate significantly.
The market price of securities of many companies, particularly development and early commercial stage medical device companies, experience wide fluctuations in price that are not necessarily related to the operating performance, underlying asset values or prospects of such companies.
The market price of our Common Shares could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
| ● | delays in respect of our commercialization of the TULSA-PRO system in the United States; |
| ● | adverse results or delays in our future planned data collection for the TACT Pivotal Clinical Trial and any future clinical trials that we may conduct; |
| ● | regulatory actions with respect to our products and/or product candidates; |
| ● | changes in laws or regulations applicable to our products or any future product candidates, including but not limited to clinical trial requirements for approvals; |
| ● | actual or anticipated fluctuations in our financial condition and operating results; |
| ● | actual or anticipated changes in our growth rate relative to our competitors; |
| ● | competition from existing products or new products that may emerge; |
| ● | announcements by us, our collaborators or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| ● | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| ● | issuance of new or updated research or reports by securities analysts; |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| ● | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
60
| ● | additions or departures of key management or scientific personnel; |
| ● | disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for its products; |
| ● | announcement or expectation of additional debt or equity financing efforts; |
| ● | sales or issuances of our Common Shares by us, our insiders or our other shareholders, including by exercise of outstanding options or warrants; and |
| ● | general economic and market conditions, including tariffs or trade restrictions. |
These and other market and industry factors may cause the market price and demand for our Common Shares to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their Common Shares and may otherwise negatively affect the liquidity of our Common Shares. In addition, stock markets in general, and the TSX, the Nasdaq and the share prices of biotechnology companies in particular, have experienced price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
We are incorporated under the laws of Ontario, Canada. The rights of holders of our common shares are governed by Ontario and Canadian law, including the provisions of the Securities Act (Ontario), and by our Articles of Incorporation. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations.
We are a smaller reporting company, and the reduced reporting requirements applicable to smaller reporting companies may make our common shares less attractive to investors.
We are a “smaller reporting company” as defined in Section 12 of the Exchange Act. For as long as we continue to be a smaller reporting company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies, including not being required to comply with the auditor attestation requirements of Section 404 of Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our stock price may be more volatile.
If equity research analysts research or reports about our business or if they issue unfavorable commentary or downgrade our Common Shares, the price of our Common Shares could decline.
The trading market for our Common Shares will rely in part on the research and reports that equity research analysts publish about us and our business, over which we have no control. The price of our Common Shares could decline if one or more equity analysts downgrade our Common Shares or if analysts issue other unfavorable commentary or cease publishing reports about us or our business.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our Common Shares may be volatile, and in the past companies that have experienced volatility in the market price of their shares have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could adversely impact our business. Any adverse determination in litigation could also subject us to significant liabilities.
61
We have never paid dividends on our Common Shares and we do not anticipate paying any dividends in the foreseeable future. Consequently, any gains from an investment in our Common Shares will likely depend on whether the price of our Common Shares increases.
We have not paid dividends on our Common Shares to date and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, capital appreciation, if any, of our Common Shares will be your sole source of gain for the foreseeable future. Consequently, in the foreseeable future, you will likely only experience a gain from your investment in our Common Shares if the price of our Common Shares increases. In addition, the terms of the CIBC Credit Agreement restrict our ability and the ability of our subsidiaries to pay dividends and make certain distributions and transfers. As a result, only appreciation of the price of the Common Shares will provide a return to holders of Common Shares.
If we are unable to satisfy the requirements of Sarbanes-Oxley, or our internal controls over financial reporting are not effective, the reliability of our financial statements may be questioned.
We are subject to certain of the requirements of Sarbanes-Oxley. Section 404 of Sarbanes-Oxley (“Section 404”) requires companies subject to the reporting requirements of the U.S. securities laws to complete a comprehensive evaluation of our internal controls over financial reporting. To comply with this statute, we are required to document and test our internal control procedures and our management are required to assess and issue a report concerning our internal controls over financial reporting. As a smaller reporting company, we are exempt from certain reporting requirements, including the independent auditor attestation requirements of Section 404(b) of Sarbanes-Oxley. Under this exemption, our independent auditor is not required to attest to and report on management’s assessment of our internal controls over financial reporting until we no longer qualify for such exemption. We continue to address our compliance with Section 404 by strengthening, assessing and testing our system of internal controls to provide the basis for our report. However, the continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore, we believe that our business will grow both domestically and internationally, in which case our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of our testing, our management has identified and may identify additional material weaknesses or significant deficiencies, which may not be remedied in a timely manner to meet the deadline imposed by Sarbanes-Oxley. As described below, we have identified a material weakness in our internal control over financial reporting for the year ended December 31, 2024. If our management cannot favorably assess the effectiveness of our internal controls over financial reporting, or our independent registered public accounting firm identifies additional material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our securities may suffer.
We have identified a material weakness in our internal control over financial reporting. If we are unable to successfully remediate this material weakness in our internal control over financial reporting, we may not be able to report our financial condition or results of operations accurately or in a timely manner, which may adversely affect investor confidence in us and, as a result, materially and adversely affect our business and the value of our Common Shares.
We have identified a material weakness in our internal control over financial reporting for the year ended December 31, 2024. In conjunction with the preparation of the Company’s financial statements for the year ended December 31, 2024, and specifically in connection with the recognition of revenue under ASC 606, Revenue from contracts with customers, management has determined that the controls over the review of contract terms and arrangements with customers did not operate effectively during 2024. This material weakness resulted in audit adjustments to revenue, trade and other receivables and prepaid expenses, deposits and other assets, which were recorded prior to the issuance of the financial statements as of and for the year ended December 31, 2024.
Our efforts to address the identified material weakness are ongoing. We cannot assure you that these measures will significantly improve or remediate the material weakness described above. We also cannot assure you that we have identified all or that we will not have additional material weaknesses in the future. Accordingly, a material weakness may still exist when we report on the effectiveness of our internal control over financial reporting for purposes of our attestation when required by reporting requirements under the Exchange Act or Section 404 of the Sarbanes-Oxley Act.
We expect to incur additional costs to remediate these control deficiencies, though there can be no assurance that our efforts will be successful or avoid potential future material weaknesses. If we are unable to successfully remediate our existing or any future material weaknesses in our internal control over financial reporting, or if we identify any additional material weaknesses, the accuracy and timing of our financial reporting may be adversely affected, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, investors may lose confidence in our financial reporting, and our stock price may decline as a result.
62
Any default under our existing debt that is not waived by the applicable lender could materially adversely impact our results of operations and financial results and may have a material adverse effect on the trading price of our Common Shares.
We are required to comply with the covenants in the CIBC Credit Agreement and such covenants may create a risk of default on our debt if we cannot satisfy or continue to satisfy these covenants. If we are determined not to have complied or in the future cannot comply with a debt covenant or anticipate that we will be unable to comply with a debt covenant under any debt instrument we are a party to, including the CIBC Loan, management may seek a waiver and/or amendment to the applicable debt instrument in respect of any such covenant in order to avoid any breach or default that might otherwise result therefrom. On March 31, 2024, we were in breach of the covenant in the CIBC Loan that revenue for any fiscal quarter must be 15% greater than revenue for the same fiscal quarter in the prior fiscal year. Prior to such breach, we obtained a waiver from CIBC, pursuant to which CIBC has waived such breach. On September 26, 2023, an amendment to the CIBC Loan changed financial covenants. The revised covenants specify that unrestricted cash must be greater than either (i) negative EBITDA for the most recent nine -month period or (ii) $7,500, reported monthly. Additionally, recurring revenue for any fiscal quarter must be 15% greater than the same quarter in the prior fiscal year, reported quarterly. As of December 31, 2024, we were in compliance with these covenants. Future compliance depends on achieving specific revenue, EBITDA, and cash levels. If we default under a debt instrument, including the CIBC Loan, and the default is not waived by the lender(s), the debt extended pursuant to the CIBC Loan and any other debt instruments could become due and payable prior to its stated due date. If such event were to occur in the future, we cannot give any assurance that (i) CIBC and/or our other lenders will agree to any covenant amendments or waive any covenant breaches or defaults that may occur, and (ii) we could pay this debt if it became due prior to its stated due date. Accordingly, if we are unable to negotiate a covenant waiver or replace or refinance our existing debt on favorable terms or at all, such default could materially adversely impact our results of operations and financial results and may have a material adverse effect on the trading price of our Common Shares. Future compliance with the financial covenants included in the CIBC Loan is dependent upon achieving certain revenue, EBITDA, and anticipated cash levels. Management considers there is a potential for a breach of these covenants in the future due to the volatility and unpredictability of our revenues.
As a foreign private issuer whose shares are listed on Nasdaq, we intend to follow certain home country corporate governance practices instead of certain Nasdaq requirements.
As a foreign private issuer whose shares are listed on Nasdaq, we are permitted to follow certain home country corporate governance practices instead of certain requirements of the Nasdaq rules. We intend to adopt and approve material changes to equity incentive plans in accordance with TSX listing rules, which do not impose a requirement of shareholder approval for such actions. In addition, we intend to follow the TSX listing rules in respect of private placements instead of Nasdaq requirements to obtain shareholder approval for certain dilutive events (such as issuances that will result in a change of control, certain transactions other than a public offering involving issuances of a 20% or greater interest in us and certain acquisitions of the stock or assets of another company) and the minimum quorum requirement for a shareholders meeting. Under Nasdaq listing rules, the required minimum quorum for a shareholders meeting is 33 1/3% of the outstanding Common Shares, and our minimum quorum requirement is only 10% of the total number of voting rights attaching to all outstanding Common Shares. Accordingly, our shareholders may not be afforded the same protection as provided under Nasdaq corporate governance rules for domestic issuers.
We will incur significantly increased costs and devote substantial management time as a result of operating as a U.S. public company.
As a U.S. public company, we have and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company or as a Canadian public company. For example, we are subject to the reporting requirements of the U.S. Exchange Act, and are required to comply with the applicable requirements of Sarbanes-Oxley and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules and regulations subsequently implemented by the SEC and the including the establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Compliance with these requirements has increased and likely will continue to increase our legal and financial compliance costs and will make some activities more time consuming and costly. In addition, management and other personnel have needed to divert attention from operational and other business matters to devote substantial time to these public company requirements. In particular, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404, which involve annual assessments of a company’s internal controls over financial reporting. We may need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge and may need to establish an internal audit function.
63
We cannot predict or estimate the amount of such additional costs we may incur as a result of becoming a U.S. public company or the timing of such costs.
We may lose foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination will be made with respect to us on June 30, 2025. We may in the future lose foreign private issuer status if a majority of our Common Shares are held in the United States and we fail to meet the additional requirements necessary to avoid loss of foreign private issuer status, such as if: (i) a majority of our directors or executive officers are U.S. citizens or residents; (ii) a majority of our assets are located in the United States; or (iii) our business is administered principally in the United States. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer will be significantly more than the costs incurred as an SEC foreign private issuer. If we lose our foreign private issuer status on this determination date, we would have to comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders would become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we would lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq listing rules. As a U.S. listed public company that is not a foreign private issuer, we would incur significant additional legal, accounting and other expenses that we do not currently incur as a foreign private issuer, as well as increased accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors.
It may be difficult for United States investors to effect service of process or enforcement of actions against us or certain of our directors and officers under U.S. federal securities laws.
We are incorporated under the laws of the Province of Ontario, Canada. A majority of our directors and officers are not U.S. citizens or residents. Because all or a substantial portion of our assets and these persons are located outside the United States, it will be difficult for United States investors to effect service of process in the United States upon us or our directors or officers, or to realize in the United States upon judgments of United States courts predicated upon civil liabilities under the U.S. Exchange Act or other United States laws. It may also be difficult to have a judgment rendered in a U.S. court recognized or enforced against us in Canada.
We may be a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes, which generally would result in certain adverse U.S. federal income tax consequences to our U.S. shareholders.
In general, a non-U.S. corporation is a PFIC for any taxable year in which (i) 75% or more of its gross income consists of passive income or (ii) 50% or more of the value of its assets consists of assets that produce, or are held for the production of, passive income. Generally, “passive income” includes interest, dividends, rents, royalties and certain gains, and cash generally is a passive asset for PFIC purposes. We have made no determination as to whether we are classified as a PFIC for U.S. federal income tax purposes. The determination of whether we are a PFIC depends on the particular facts and circumstances (such as the valuation of our assets, including goodwill and other intangible assets) and is also affected by the application of the PFIC rules, which are subject to differing interpretations. The fair market value of our assets is expected to depend, in part, upon (i) the market price of the Common Shares, which is likely to fluctuate, and (ii) the composition of our income and assets, which will be affected by how, and how quickly, we spend any cash that is raised in any financing transaction. If we were a PFIC for any taxable year during which a U.S. shareholder owned the Common Shares, such U.S. shareholder generally will be subject to certain adverse U.S. federal income tax consequences, including increased tax liability on gains from dispositions of the Common Shares and certain distributions and a requirement to file annual reports with the Internal Revenue Service. In light of the foregoing, no assurance can be provided that we are not currently a PFIC or that we will not become a PFIC in any future taxable year. Prospective investors should consult their own tax advisers regarding our PFIC status.
If we are required to register as an “investment company” under the Investment Company Act, significant compliance costs and applicable restrictions could have a material adverse effect on our business.
We do not believe that we are an “investment company” under the Investment Company Act, but we can provide no assurance that we will not be deemed an “investment company” in the future.
64
Section 3(a)(1)(A) of the Investment Company Act defines the term “investment company” to mean any issuer that “is or holds itself out as being engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting, or trading in securities.” Section 3(a)(1)(C) of the Investment Company Act defines “investment company” as any issuer which “is engaged or proposes to engage in the business of investing, reinvesting, owning, holding, or trading in securities, and owns or proposes to acquire investment securities having a value exceeding 40 per centum of the value of such issuer’s total assets (exclusive of government securities and cash items) on an unconsolidated basis.” Generally, any issuer meeting the definition of an investment company is subject to all applicable provisions of the Investment Company Act and must register with the Commission under Section 8 of the Investment Company Act, unless it meets the terms and conditions of various exceptions provided by the Investment Company Act or in rules adopted by the SEC under the Investment Company Act. The term “investment securities” is very broadly defined in the Investment Company Act. We believe that the cash on our balance sheet is held in a manner so that it constitutes “cash items” instead of “investment securities” within the meaning of the Investment Company Act, and accordingly, we do not believe we are required to register as an investment company; however, if we no longer hold our cash in this manner, we may need to find another available exemption from registration under the Investment Company Act.
For example, Rule 3a-2 of the Investment Company Act provides that inadvertent or transient investment companies will not be treated as investment companies subject to the provisions of the Investment Company Act, provided the issuer has the requisite intent to be engaged in a non-investment business, evidenced by the issuer’s business activities and an appropriate resolution of the issuer’s board of directors, within one year from the commencement of the earlier of (1) the date on which the issuer owns securities and/or cash having a value exceeding 50% of the value of such issuer’s total assets on either a consolidated or unconsolidated basis, or (2) the date on which an issuer owns or proposes to acquire investment securities (as defined in section 3(a) of the Investment Company Act) having a value exceeding 40% of the value of such issuer’s total assets (exclusive of government securities and cash items) on an unconsolidated basis. If the Company becomes an inadvertent investment company, and fails to meet the requirements of the transient investment company exemption under Rule 3a-2 of the Investment Company Act, then we will be required to register as an investment company with the SEC.
However, if we were to be deemed an investment company, we would be required to register as an investment company or adjust our business strategy and assets. If we were required to register as an investment company under the Investment Company Act, we would incur substantial expenses associated with such registration, and we would become subject to substantial regulation with respect to our capital structure, management, operations, transactions with affiliated persons, asset composition, including restrictions with respect to diversification and industry concentration, and other matters, which would have a material adverse effect on our business.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
Item 1C. CYBERSECURITY
We recognize the critical importance of maintaining the trust and confidence of our patients, employees, and business partners toward our business and are committed to protecting the confidentiality, integrity and availability of our business operations and systems. Our audit committee and board of directors are involved in oversight of our risk management activities, and cybersecurity represents an important element of our overall approach to risk management. We have taken into account recognized frameworks established by the National Institute of Standards and Technology, or NIST, when developing cybersecurity policies, standards, processes and practices among other considerations. In general, we seek to address cybersecurity risks through a comprehensive, cross-functional approach that is focused on preserving the confidentiality, security and availability of the information that we collect and store by identifying, preventing and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
Cybersecurity Risk Management and Strategy: Effect of Risk
We face risks related to cybersecurity such as unauthorized access, cybersecurity attacks and other security incidents, including as perpetrated by hackers and unintentional damage or disruption to hardware and software systems, loss of data, and misappropriation of confidential information. To identify and assess material risks from cybersecurity threats, we maintain a comprehensive cybersecurity program to ensure our systems are effective and prepared for information security risks, including regular oversight of our programs for security monitoring for internal and external threats to ensure the confidentiality and integrity of our information assets. We consider risks from cybersecurity threats alongside other company risks as part of our overall risk assessment process. We employ a range of tools and services, including phishing training, regular network and endpoint monitoring, audits and vulnerability assessments to inform our risk identification and assessment.
65
We also identify our cybersecurity threat risks by comparing our processes to standards set by NIST. To provide for the availability of critical data and systems, maintain regulatory compliance, manage our material risks from cybersecurity threats, and protect against and respond to cybersecurity incidents, we undertake the following activities:
| ● | monitor emerging data protection laws and implement changes to our processes that are designed to comply with such laws; |
| ● | through our policies, practices and contracts (as applicable), require employees, as well as third parties that provide services on our behalf, to treat confidential information and data with care and enter into confidentiality agreements, and enter into data processing agreements with third parties that are processing personal data we control; |
| ● | employ technical safeguards that are designed to protect our information systems from cybersecurity threats, including physical security measures to prevent access to data processing systems, firewalls, intrusion prevention and detection systems, email security controls, anti-malware functionality and access controls, endpoint detection and response systems, all of which are evaluated and improved through internal and external vulnerability assessments and cybersecurity threat intelligence; |
| ● | provide mandatory training and notifications for our employees and contractors regarding cybersecurity threats as a means to equip them with effective tools to understand, identify and address cybersecurity threats, and to communicate our evolving information security policies, standards, processes and practices; |
| ● | conduct mandatory annual phishing training and regular phishing email simulations for all employees and contractors with access to our email systems to enhance awareness and responsiveness to possible threats; |
| ● | utilize pseudonymized data for patients and use other encryption methods to ensure security of personal data; |
| ● | leverage procedures informed by appropriate incident handling frameworks to help us identify, protect, detect, respond and recover when there is an actual or potential cybersecurity incident; and |
| ● | carry information security risk insurance that provides protection against the potential losses arising from a cybersecurity incident. |
Our incident response plan coordinates the activities we take to prepare for, detect, respond to and recover from cybersecurity incidents, which include processes to triage, assess severity for, escalate, contain, investigate and remediate the incident, as well as to comply with potentially applicable legal obligations and mitigate damage to our business and reputation.
As part of the above processes, we engage with third parties, including annually having a qualified third-party review our incident response plan and our cybersecurity measures to help identify areas for continued focus, improvement and compliance.
Our processes also address cybersecurity threat risks associated with our use of third-party service providers, including those who have access to patient and employee data or our systems. In addition, cybersecurity considerations affect the selection and oversight of our third-party service providers. We perform diligence on third parties that have access to our systems, data or facilities that house such systems or data, and assess cybersecurity threat risks identified through such diligence. Additionally, we generally require those third parties that could introduce significant cybersecurity risk to us to agree by contract to manage their cybersecurity risks in specified ways, for example, by engaging with known, reputable vendors, and requiring they have industry standard safeguards and notification procedures. We may also ask vendors associated with increased cybersecurity risk to complete a periodic questionnaire regarding their security practices for ongoing vendor management purposes.
We have not identified risks from known cybersecurity threats, including as a result of any prior cybersecurity incidents, that have materially affected or are reasonably likely to materially affect us, including our operations, business strategy, results of operations, or financial condition.
Cybersecurity Governance: Management
Cybersecurity is an important part of our risk management processes and an area of focus for our board of directors and management. In general, our board of directors oversees risk management activities designed and implemented by our management, and considers specific risks, including, for example, risks associated with our strategic plan, business operations, and capital structure.
66
The board of directors is responsible for the oversight of risks from cybersecurity threats.
Annually, our audit committee and board of directors receives an update from management of our cybersecurity threat risk management and strategy processes covering topics such as data security posture, results from third-party assessments, progress towards pre-determined risk-mitigation-related goals, our incident response plan, and material cybersecurity threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. In such sessions, our members generally receives information that includes a cybersecurity summary and other information discussing current and emerging material cybersecurity threat risks, and describing our ability to mitigate those risks, as well as recent developments, evolving standards, technological developments and information security considerations arising with respect to our peers and third parties, Our board of directors also receive prompt and timely information regarding any cybersecurity incident that meets establishing reporting thresholds, as well as ongoing updates regarding any such incident until it has been addressed.
Members of our audit committee and board of directors are also encouraged to regularly engage in conversations with management on cybersecurity-related news events and discuss any updates to our cybersecurity risk management and strategy programs. Material cybersecurity threat risks are also considered during separate board meeting discussions of important matters like enterprise risk management, operational budgeting, business continuity planning, and other relevant matters.
Our cybersecurity risk management and strategy processes, which are discussed in greater detail above, are led by our Chief Financial Officer in consultation with our Chief Executive Officer and Legal Counsel. Such individuals and their respective teams have collectively over ten years of prior work experience in various roles involving managing information security, developing cybersecurity strategy, implementing effective information and cybersecurity programs. These management team members are informed about and monitor the prevention, mitigation, detection, and remediation of cybersecurity incidents through their management of, and participation in, the cybersecurity risk management and strategy processes described above, including the operation of our incident response plan. As discussed above, these management team members report to the audit committee and our board of directors annually about cybersecurity threat risks, among other cybersecurity related matters.
Item 2. PROPERTIES
We currently lease office space in and around the Mississauga, Ontario and Vantaa, Finland area.
We lease office space at 2400 Skymark Avenue, Unit 6, Mississauga, Ontario, Canada, containing approximately 38,148 square feet of office and manufacturing space pursuant to a lease agreement that expires in September 2026. This is our corporate headquarters.
We also lease office space at Äyritie 4B, 01510 Vantaa, Finland, pursuant to a lease agreement that expires in December 2025. This lease replaced a lease at the same address which expired in December 2024. The initial lease term for this lease was less than twelve months.
We believe these facilities will be adequate for the foreseeable future and that suitable additional or substitute space will be available as and when needed.
Item 3. LEGAL PROCEEDINGS
From time to time, we may be subject to legal proceedings. We are not currently a party to or aware of any proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
Item 4. MINE SAFETY DISCLOSURES
Not applicable.
67
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common shares are traded on The Nasdaq Capital Market and The Toronto Stock Exchange under the symbol “PROF” and “PRN”, respectively. On October 16, 2019 our common shares began trading on Nasdaq.
Stockholders
As of March 7, 2025, we had 30,039,809 outstanding common shares, no outstanding preferred shares, and approximately 8,364 holders of record of our outstanding common shares.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
None.
Dividend Policy
We have never declared or paid cash dividends on our common shares. We intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors might deem relevant.
Item 6. [RESERVED]
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. You should carefully read the “Risk Factors” section of this Annual Report to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section titled “Special Note Regarding Forward-Looking Statements.” Unless stated otherwise, all references to “$” are to United States dollars in thousands and all references to “C$” are to Canadian dollars in thousands.
Overview
We are a commercial-stage medical device company focused on the development and marketing of customizable, incision-free therapeutic systems for the image guided ablation of diseased tissue utilizing its platform technologies and leveraging the healthcare system’s existing imaging infrastructure. Our lead product (the “TULSA-PRO system”) combines real-time MRI, robotically driven transurethral sweeping-action thermal ultrasound with closed-loop temperature feedback control for the ablation of prostate tissue. The product is comprised of one-time-use devices and durable equipment that are used in conjunction with a customer’s existing MRI scanner.
68
We are commercializing TULSA-PRO, a technology that combines real-time MRI, robotically-driven transurethral ultrasound and closed-loop temperature feedback control. The TULSA procedure, performed using the TULSA-PRO system, has the potential of becoming a mainstream treatment modality across the entire prostate disease spectrum; ranging from low-, intermediate-, or high-risk prostate cancer; to hybrid patients suffering from both prostate cancer and benign prostatic hyperplasia (“BPH”); to men with BPH only; and also, to patients requiring salvage therapy for radio-recurrent localized prostate cancer. TULSA employs real-time MR guidance for pixel-by-pixel precision to preserve prostate disease patients’ urinary continence and sexual function, while killing the targeted prostate tissue via a precise sound absorption technology that gently heats it to kill temperature (55-57°C). TULSA is an incision- and radiation-free “one-and-done” procedure performed in a single session that takes a few hours. Virtually all prostate shapes and sizes can be safely, effectively, and efficiently treated with TULSA. There is no bleeding associated with the procedure; no hospital stay is required; and most TULSA patients report quick recovery to their normal routine. TULSA-PRO is CE marked, Health Canada approved, and 510(k) cleared by the U.S. Food and Drug Administration (“FDA”).
We are also commercializing Sonalleve, an innovative therapeutic platform that is CE marked for the treatment of uterine fibroids and palliative pain treatment of bone metastases. Sonalleve has also been approved by the China National Medical Products Administration for the non-invasive treatment of uterine fibroids and has FDA approval under a Humanitarian Device Exemption for the treatment of osteoid osteoma. We are in the early stages of exploring additional potential treatment markets for Sonalleve where the technology has been shown to have clinical application, such as non-invasive ablation of abdominal cancers and hyperthermia for cancer therapy.
We deploy a hybrid recurring revenue business model in the United States to market TULSA-PRO, i) charging a one-time payment that includes a supply of our one-time-use device, use of the system as well as our Genius services that support each TULSA center with clinical and patient recruitment and ii) a traditional model of charging for the system separately as capital and an additional per patient charge for the one-time-use devices and associated Genius services.. The Sonalleve product is marketed primarily outside North America in European and Asian countries, deploying a capital sales model. Outside of North America, we generate most of our revenues from our system sales in Europe and Asia, where we deploy a more traditional hybrid business model, charging for the system separately as a capital sale and an additional per patient charge for the one-time-use devices and associated Genius services.
Profound’s Technology
TULSA-PRO and Sonalleve share the common technological concept of using MRI to enable visualization by the surgeon of desired tissue in real time. Both products also use thermal ultrasound technology to gently heat and ablate tissue using the real-time thermometry capability of the MRI.
TULSA-PRO delivers its ultrasound energy through a transurethral catheter, a one-time-use device that is placed in the patient’s prostate through a natural orifice. Focused ultrasound energy is then delivered by the catheter in the shape of a blade. Externally the catheter is connected to a software controlled robotic manipulator that rotates up to 360-degree in a sweeping action to impart thermal energy and thus ablation of tissue. The real time temperature measurement of the prostate is coupled with closed loop process control that measures the appropriate amount of ultrasound energy to gently heat the physician-prescribed region of prostate tissue to the target temperature to achieve cell kill without boiling or charring the tissue. As a measure to keep the urethra within the prostate viable, the temperature of the transurethral catheter is maintained at an appropriate level by circulating water inside the catheter. Similarly, a water-cooled specially designed catheter is placed in the patient’s rectum during the ablation process to keep it protected from thermal damage during the procedure. The TULSA-PRO in conjunction with its Thermal Boost module, enables surgeons to temporarily increase the ablation target temperature in prostate regions where advanced stage cancer might reside, further increasing their confidence that aggressive cancer cells have been ablated. We believe that TULSA-PRO’s controlled and relatively gentle heating process may result in lower post procedural pain and complications, reduced potential of life affecting side effects, and in significantly desirable shrinkage of the prostate via resorption of the dead tissue over time, which may provide a longer-term durable benefit.
Sonalleve delivers its ultrasound energy via a disc located outside the patient. Its ultrasound energy is focused to create small cylindrical hot spots a certain distance into the patient. Overlapping cylinders create ablation of the physician-prescribed desired tissue. Similar to TULSA-PRO, Sonalleve also provides for controlled temperature increases to achieve cell kill.
69
The physician is in charge of using the Profound devices and decides which tissue needs to be ablated to impart therapeutic effect. We believe that in the hands of trained physicians, our systems have the ability to provide customizable, incision-free ablative therapies with the precision of real-time MRI visualization and thermometry, focused ultrasound and closed-loop temperature feedback control. We believe that our technology offers clinicians and appropriate patients a better alternative to traditional surgical or radiation therapies, with respect to clinical outcomes, side effects and recovery time.
Results of Operations
The following selected financial information as at and for the years ended December 31, 2024 and 2023 have been derived from the audited consolidated financial statements and should be read in conjunction with those audited consolidated financial statements and related notes.
|
|
For years ended December 31, |
||||
|
|
2024 |
|
2023 |
||
|
|
$ |
|
$ |
||
Revenue |
|
|
10,680 |
|
|
7,199 |
Operating expenses |
|
|
40,099 |
|
|
32,963 |
Other (income) expense |
|
|
(5,244) |
|
|
(200) |
Net loss for the year |
|
|
27,816 |
|
|
28,323 |
Basic and diluted loss per share |
|
|
1.12 |
|
|
1.34 |
|
|
Years ended |
|
||||||
|
|
December 31 |
|
||||||
|
|
2024 |
|
2023 |
|
Change |
|
||
|
|
$ |
|
$ |
|
$ |
|
% |
|
Revenue |
|
10,680 |
|
7,199 |
|
3,481 |
|
48 |
% |
Cost of sales |
|
3,643 |
|
2,887 |
|
756 |
|
26 |
% |
Gross profit |
|
7,037 |
|
4,312 |
|
2,725 |
|
63 |
% |
Gross margin |
|
66 |
% |
60 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses |
|
|
|
|
|
|
|
|
|
Research and development |
|
16,965 |
|
14,424 |
|
2,541 |
|
18 |
% |
Selling, general and administrative |
|
23,134 |
|
18,539 |
|
4,595 |
|
25 |
% |
Total operating expenses |
|
40,099 |
|
32,963 |
|
7,136 |
|
22 |
% |
|
|
|
|
|
|
|
|
|
|
Other (income) expense |
|
|
|
|
|
|
|
|
|
Net finance (income) expense |
|
(1,436) |
|
(775) |
|
(661) |
|
85 |
% |
Net foreign exchange (gain) loss |
|
(3,808) |
|
575 |
|
(4,383) |
|
(762) |
% |
Total other (income) expense |
|
(5,244) |
|
(200) |
|
(5,044) |
|
2,522 |
% |
|
|
|
|
|
|
|
|
|
|
Net loss before income taxes |
|
27,818 |
|
28,451 |
|
(633) |
|
(2) |
% |
|
|
|
|
|
|
|
|
|
|
Income taxes |
|
(2) |
|
(128) |
|
126 |
|
(98) |
% |
|
|
|
|
|
|
|
|
|
|
Net loss attributed to shareholders for the year |
|
27,816 |
|
28,323 |
|
(507) |
|
(2) |
% |
|
|
|
|
|
|
|
|
|
|
Other comprehensive (income) loss |
|
|
|
|
|
|
|
|
|
Item that may be reclassified to profit or loss |
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
2,823 |
|
(644) |
|
3,467 |
|
(538) |
% |
Net loss and comprehensive loss for the year |
|
30,639 |
|
27,679 |
|
2,960 |
|
11 |
% |
|
|
|
|
|
|
|
|
|
|
Loss per share |
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share |
|
1.12 |
|
1.34 |
|
(0.22) |
|
(16) |
% |
Basic and diluted weighted average common share outstanding |
|
24,765,503 |
|
21,182,558 |
|
|
|
|
|
70
Recent Developments
On January 2, 2024, the Company closed a public offering, resulting in the issuance of 2,666,667 common shares at a price of $7.50, for gross proceeds of $20,000.
On January 16, 2024, the Company closed a non-brokered private placement, resulting in the issuance of 391,667 common shares at a price of $7.50, for gross proceeds of $2,938.
On December 10, 2024, we closed a public offering, resulting in the issuance of 5,366,705 common shares at a price of $7.50 per share, for gross proceeds of $40,250.
Key Components of Our Results of Operations
Revenue
We deploy a hybrid recurring revenue business model in the United States to market TULSA-PRO, i) charging a one-time payment that includes a supply of our one-time-use device, use of the system as well as our Genius services that support each TULSA center with clinical and patient recruitment and ii) a traditional model of charging for the system separately as capital and an additional per patient charge for the one-time-use devices and associated Genius services. The Sonalleve product is marketed primarily outside North America in European and Asian countries deploying a one-time capital sales model with limited recurring service revenue. Outside of North America, we generate most of our revenues from our system sales (both TULSA-PRO and Sonalleve) in Europe and Asia where we deploy a more traditional hybrid business model, charging for the system separately as capital and an additional per patient charge for the one-time-use devices and associated Genius services. Revenue is comprised of recurring – non-capital revenue, which consists of the sale of one-time-use devices, lease of medical devices, procedures and services associated with extended warranties and capital equipment, which is the one-time sale of capital equipment.
For the year ended December 31, 2024, we recorded revenue totaling $10,680, with $2,440 from the one-time sale of capital equipment and $8,240 from recurring – non-capital revenue. For the year ended December 31, 2023, we recorded revenue of $7,199, with $393 from the one-time sale of capital equipment and $6,806 from recurring – non-capital revenue. The increase of $3,481 or 48% in revenue for the year ended December 31, 2024, was the result of higher recurring revenue and capital sales in the United States during 2024.
Cost of sales
Cost of sales primarily includes the cost of finished goods, depreciation of equipment under lease, inventory write-downs, royalties, warranty expenses, freight and direct overhead and labor expenses necessary to acquire or manufacture the finished goods.
For the year ended December 31, 2024, we recorded a cost of sales of $3,643, related to the sale of medical devices, capital and non-capital, which reflects a 66% gross profit. For the year ended December 31, 2023, we recorded a cost of sales of $2,887, related to the sale of medical devices, capital and non-capital, which reflects a 60% gross profit. The gross profit was higher in 2024 by $2,725 or 63% due to manufacturing operating at higher efficiency rates based on improvements that have been implemented and the growth in the number of capital systems sold.
Operating Expenses
Operating expenses consist of two components: research and development (“R&D”) and selling, general and administrative (“SG&A”).
R&D Expenses
R&D expenses are comprised of costs incurred in performing R&D activities, including new product development, continuous product improvement, investment in clinical trials and related clinical manufacturing costs, materials and supplies, salaries and benefits, consulting fees, patent procurement costs, and occupancy costs related to R&D activity.
71
For the year ended December 31, 2024, R&D expenses increased by $2,541, or 18% to $16,965 compared to $14,424 for the year ended December 31, 2023. The increase in R&D expenses was largely due to increased headcount and lower reimbursement of workforce costs associated with research projects, increased enrolment for the CAPTAIN trial and recruitment efforts, and higher material expenditures due to spending on R&D initiatives to increase compatibility with MRI scanners, reduce design costs and improve efficiencies. These expenses promote the ongoing development and improvement of the products while further strengthening the commitment to a reliable and customizable product.
SG&A expenses
Selling, general and administrative expenses are comprised of business development costs related to the market development activities and commercialization of our systems, including salaries and benefits, marketing support functions, occupancy costs, insurance, various management and administrative support functions and other miscellaneous marketing and management costs.
SG&A expenses for the year ended December 31, 2024 increased by $4,595, or 25% to $23,134 compared to $18,539 for the year ended December 31, 2023. The increase in SG&A was due to increased sales force and commission payments, the release of commercial segments and marketing advertisement campaigns, increased travel for conferences, bad debt expense and costs associated with hosting our educational event Pro-Talk Live in September 2024. Offsetting these amounts was a decrease to insurance due to lower premium rates.
Net finance (income) expense
Net finance (income) expense is primarily comprised of the following: (i) the CIBC Credit Agreement (as defined herein) accreting to the principal amount repayable and its related interest expense; (ii) interest income from cash and cash equivalents; (iii) the lease liability interest expense; and (iv) the interest income on trade and other receivables.
Net finance (income) expense increased $661 to ($1,436) during the year ended December 31, 2024, compared to ($775) during the year ended December 31, 2024. The increase in net finance (income) expense was due to the change in the amortized cost of trade and other receivables being fully recognized, increase in interest income from cash and cash equivalents and decrease in the CIBC Loan interest and accretion expenses.
Liquidity and Capital Resources
As of December 31, 2024, we had cash of $54,912 compared to $26,213 as of December 31, 2023. Historically, our primary source of cash has been financing activities, e.g., equity offerings as well as the CIBC Loan (as defined below).
Based on our current operating plans, we expect that our existing cash and sales of our products and services will enable us to fund our operating expenses and capital expenditure requirements for at least the next 12 months from the filing date of this Annual Report. During that time, we expect that our expenses will increase, primarily due to the continued commercialization of TULSA-PRO and Sonalleve.
Use of Proceeds
2024 Offering and non-brokered private placement
We received net proceeds of $21,079 from the Public Offering and Private Placement completed in January 2024. We intend to use net proceeds from the Public Offering and Private Placement to fund the continued commercialization of the TULSA-PRO system in the United States, the continued development and commercialization of the TULSA-PRO system and the SONALLEVE system globally and for working capital and general corporate purposes.
72
In addition, there have been no material adjustments to the cost or timing of the business objective previously disclosed in such prospectus supplement.
|
|
Total spending as |
|
|
of December 31, |
|
|
2024 |
|
|
$ |
TULSA-PRO commercialization |
|
11,660 |
Sonalleve development and commercialization |
|
1,951 |
Working capital and general corporate purposes |
|
7,468 |
Total |
|
21,079 |
On December 10, 2024, we received net proceeds of $36,132 from the public offering of 5,366,705 Common Shares at $7.50. We intend to use net proceeds from the public offering to fund the continued commercialization of the TULSA-PRO system in the United States, the continued development and commercialization of the TULSA-PRO system and the SONALLEVE system globally and for working capital and general corporate purposes. As of December 31, 2024, we had yet to use any of the proceeds.
CIBC Loan
We entered into a credit agreement with Canadian Imperial Bank of Commerce (“CIBC”) on November 3, 2022 (the “Original CIBC Credit Agreement”), for gross proceeds of C$10,000, maturing on November 3, 2027, with an interest rate based on CIBC prime plus 2% (the “CIBC Loan”). We were required to make interest-only payments until October 31, 2023, and monthly repayments on the principal of C$208 plus accrued interest commenced on October 31, 2023. All of our obligations under the Original CIBC Credit Agreement are guaranteed by our current and future subsidiaries and include security of first priority interests in our and our subsidiaries’ assets. Initially, we had financial covenants in relation to the CIBC Loan where unrestricted cash is at all times greater than EBITDA for the most recent six-month period, reported on a monthly basis and that revenue for any fiscal quarter must be 15% greater than revenue for the same fiscal quarter in the prior fiscal year, reported on a quarterly basis.
On September 26, 2023 an amendment to the CIBC Loan resulted in a change to the financial covenants. The amended covenants are that unrestricted cash must at all times be greater of: (i) to the extent EBITDA is negative for such period, EBITDA for the most recent nine-month period or (ii) $7,500, reported on a monthly basis; and that recurring revenue for any fiscal quarter must be 15% greater than recurring revenue for the same fiscal quarter in the prior fiscal year, reported on a quarterly basis.
On May 3, 2024, a second amendment to the CIBC Loan resulted in another amendment to the financial covenants. The amended covenants are that the recurring revenue covenant shall not be tested for any fiscal quarter in the 2024 fiscal year so long as unrestricted cash is no less than 2.5 multiplied by the principal amount of outstanding CIBC Loan at all times. We are in compliance with these financial covenants as at December 31, 2024.
On May 3, 2024, a second amendment to the CIBC Loan resulted in another amendment to the financial covenants. The amended covenants are that the recurring revenue covenant shall not be tested for any fiscal quarter in the 2024 fiscal year so long as unrestricted cash is no less than 2.5 multiplied by the outstanding principal amount of the CIBC Loan at all times. We are in compliance with these financial covenants as at December 31, 2024.
On March 3, 2025, we entered into an amended and restated credit agreement with CIBC (the “CIBC Credit Agreement”), which amended the terms of the CIBC Loan and the existing long-term debt provided under the Original CIBC Credit Agreement was repaid with proceeds from a new revolving line of credit provided by CIBC to us. The line of credit bears interest at the Wall Street Journal Prime Rate subject to a floor of 6.25%. The CIBC Credit Agreement contains certain financial covenants, and the obligations thereunder are secured by, inter alia, a general security agreement over our assets and the assets of our subsidiaries. The revolving line of credit matures on March 3, 2027 and provides an option to increase the amount of the revolving commitment by $5,000 within 18 months from March 3, 2025, subject to achieving a minimum trailing 12 month revenue exceeding $15,000. The exercise of the option would result in the size of the revolving commitment increasing from $10,000 to a maximum of $15,000. Additionally, the CIBC Credit Agreement provides that we may request a one-time increase in the principal amount of the revolving line of credit up to a maximum amount of $10,000, which is subject to the approval of CIBC in its sole discretion.
73
Cash Flow
We manage liquidity risk by monitoring actual and projected cash flows. A cash flow forecast is performed regularly to ensure that we have sufficient cash to meet our operational needs while maintaining sufficient liquidity. Our cash requirements depend on numerous factors, including market acceptance of our products, the resources devoted to developing and supporting the products and other factors. We expect to continue to devote substantial resources to expand procedure adoption and acceptance of our products.
We may require additional capital to fund R&D activities and any significant expansion of operations. Potential sources of capital could include equity and/or debt financings, development agreements or marketing agreements, the collection of revenue resulting from future commercialization activities and/or new strategic partnership agreements to fund some or all costs of development. There can be no assurance that we will be able to obtain the capital sufficient to meet any or all of our needs. The availability of equity or debt financing will be affected by, among other things, the results of R&D, our ability to obtain regulatory approvals, the market acceptance of our products, the state of the capital markets generally, strategic alliance agreements and other relevant commercial considerations. In addition, if we raise additional funds by issuing equity securities, existing security holders will likely experience dilution, and any incurring of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict operations. Any failure on our part to raise additional funds on terms favorable to us or at all may require us to significantly change or curtail current or planned operations in order to conserve cash until such time, if ever, that sufficient proceeds from operations are generated, and could result in us not being in a position to take advantage of business opportunities, in the termination or delay of clinical trials for our products, in curtailment of product development programs designed to identify new products, in the sale or assignment of rights to technologies, product and/or an inability to file market approval applications at all or in time to competitively market products.
|
|
Years ended December 31, |
||
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Cash provided by (used in) operating activities |
|
(23,453) |
|
(22,609) |
Cash provided by (used in) financing activities |
|
54,696 |
|
1,756 |
Foreign exchange on cash |
|
(2,544) |
|
549 |
Net increase (decrease) in cash |
|
28,699 |
|
(20,304) |
Operating Activities
Net cash provided by (used in) operating activities for the year ended December 31, 2024 was $(23,453). The principal use of the operating cash flows during the year related to a net loss of $27,816 and an increase in net operating assets and liabilities of $591 and partially offset by non-cash charges of $3,772. The cash used in operating expenses was primarily due to the increased efforts supporting the commercialization and expansion of our products. This resulted in an increase in headcount, travel and marketing fees. Non-cash charges consisted primarily of share-based compensation, amortization and depreciation.
Net cash provided by (used in) operating activities for the year ended December 31, 2023 was $(22,589). The principal use of the operating cash flows during the year related to a net loss of $28,323 and an increase in net operating asset and liabilities of $540 and by non-cash charges of $5,174. The cash used in operating expenses was primarily due to the increased headcount and commission payments, increased sales and marketing efforts in the US and overall consulting and legal fees. Non-cash charges consisted primarily of share-based compensation, amortization and depreciation.
Financing Activities
Net cash provided by (used in) financing activities for the year ended December 31, 2024 was $54,696 primarily from the proceeds of the issuance of common shares of $57,211, net of issuance costs, and proceeds of $45 from the exercise of share options which were offset by the $2,560 repayments of long-term debt.
Net cash provided by (used in) financing activities for the year ended December 31, 2023 was $1,756 primarily of proceeds from the issuance of warrants of $2,423 and proceeds of $245 from the exercise of share options which were offset by the $912 repayments of long-term debt.
74
Foreign Exchange on Cash
Cash was impacted by the change in the foreign exchange rates for the Company’s foreign currency denominated cash (non-USD). The value of our currencies decreased, resulting in a decrease in our cash holdings.
Contractual obligations
The following table summarizes our significant contractual obligations:
|
|
|
|
|
|
December 31, 2024 |
||
|
|
|
|
|
|
|
|
Between 1 |
|
|
Carrying |
|
Future cash |
|
Less than 1 |
|
year and 5 |
|
|
amount |
|
flows |
|
Year |
|
years |
|
|
$ |
|
$ |
|
$ |
|
$ |
Accounts payables and accrued liabilities |
|
1,317 |
|
1,317 |
|
1,317 |
|
— |
Lease liability |
|
460 |
1 |
480 |
|
274 |
|
206 |
Long-term debt |
|
4,661 |
|
5,282 |
|
2,034 |
|
3,248 |
Total |
|
6,438 |
|
7,079 |
|
3,625 |
|
3,454 |
1 |
Present value of the lease payments that are not paid, discounted using the interest rate implicit in the lease. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined under applicable SEC rules.
Critical Accounting Policies and Estimates
The preparation of consolidated financial statements in conformity with US GAAP requires management to make estimates and judgements that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenue and expenses during the year. Actual results could differ from these estimates. As additional information becomes available or actual amounts are determinable, the recorded estimates are revised and reflected in operating results in the year in which they are determined.
Critical accounting policies
Revenue
Revenue is derived primarily from the sale of the TULSA-PRO and Sonalleve systems and one time use devices. All products generally contain a one-year warranty.
The Company recognizes revenue when the customer obtains control of promised goods or services and in an amount that reflects the consideration to which the Company expects to be entitled to receive in exchange for those goods or services. To achieve this core principle, the Company applies the five-step revenue model to contracts within its scope: (i) identify the contract(s) with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
The amount of revenue to be recognized is based on the transaction price the Company expects to receive in exchange for its goods and services. For contracts that contain multiple performance obligations, the Company allocates the transaction price to each performance obligation and recognizes the related revenue when or as control of each individual performance obligation is transferred to customers.
75
Recurring – non-capital
Recurring - non-capital revenue consists of the sale of one-time-use devices and services associated with extended warranties. Revenue from sale of one-time-use devices is recognized when control is transferred to the customers, which generally occurs at the time of shipment. Service revenue related to extended warranties is deferred and recognized on a straight-line basis over the extended warranty period covered by the customer contract.
Capital equipment
Capital equipment revenue consists of the sale of capital equipment including installation and training amounts. Revenue is recognized when the Company transfers control to the customer, which is generally at the time of shipment. The Company’s customer arrangements generally do not provide a right of return.
Contract Assets
Contract assets arise from billed amounts in customer arrangements and the Company’s right to payment is not just subject to the passage of time, typically related to installation of the product. The Company recognizes a receivable at the point in time at which it has an unconditional right to payment.
Sales to distributors
The Company markets and sells its products primarily through its direct sales force, which sells its products to end customers. A portion of the Company’s revenue is generated by sales to distributors primarily in Europe and Asia. When the Company transacts with a distributor, its contractual arrangement is with the distributor and not with the end customer. Whether the Company transacts business with and receives the order from a distributor or directly from an end customer, its revenue recognition policy and resulting pattern of revenue recognition for the order are generally the same.
Critical accounting estimates
Trade and other receivables
The key judgements and estimates are used in determining the allowance for expected credit losses. Trade and other receivables are stated net of an allowance for expected credit losses. The Company grants credit to customers in the normal course of business and maintains an allowance for expected credit losses which reflect the current estimate of expected credit losses expected to be incurred over the life of the receivables. The Company considers various factors in establishing, monitoring, and adjusting its allowance for expected credit losses, including the aging of the accounts and aging trends, the historical level of charge-offs, and specific credit exposures related to particular customers. The Company also monitors other risk factors and forward-looking information, such as country risk, when determining credit limits for customers and establishing adequate allowances. Uncollectible accounts are written-off against the allowance when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, failure to make contractual payments for a period of greater than 180 days past due.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
76
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Financial Statements |
|
Page |
Report of Independent Registered Public Accounting Firm (PCAOB ID: 271) |
|
F-2 |
Consolidated Balance Sheets as of December 31, 2024 and 2023 |
|
F-4 |
|
F-5 |
|
Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2024 and 2023 |
|
F-6 |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2024 and 2023 |
|
F-7 |
|
F-8 |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Profound Medical Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Profound Medical Corp. and its subsidiaries (the Company) as of December 31, 2024 and 2023, and the related consolidated statements of operations and comprehensive loss, of shareholders’ equity and of cash flows for the years then ended, including the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
F-2
Revenue Recognition
As described in Notes 2 and 12 to the consolidated financial statements, the Company’s revenue was $10.7 million for the year ended December 31, 2024. Recurring non-capital revenue consists of revenues from the sale of one-time-use devices and services associated with extended warranties. Capital equipment revenue consists of revenues from the sale of capital equipment including installation and training amounts. The amount of revenue to be recognized is based on the transaction price the Company expects to receive in exchange for its goods and services. For contracts that contain multiple performance obligations the Company allocates the transaction price to each performance obligation and recognizes the related revenue when or as control of each individual performance obligation is transferred to customers. Revenue from sale of one-time-use devices and capital equipment is recognized when control is transferred to the customers, which generally occurs at the time of shipment. Service revenue related to extended warranties is deferred and recognized on a straight-line basis over the extended warranty period covered by the customer contract.
The principal considerations for our determination that performing procedures relating to revenue recognition is a critical audit matter is the high degree of audit effort in performing procedures and evaluating audit evidence related to revenue recognition.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others, evaluating the recognition of revenue on a sample basis by (i) evaluating the customer contract terms; (ii) identifying and assessing performance obligations; and (iii) obtaining and evaluating the underlying purchase orders, shipping documents, invoices and payment support, as applicable.
/s/ PricewaterhouseCoopers LLP
Chartered Professional Accountants, Licensed Public Accountants
Toronto, Canada
March 7, 2025
We have served as the Company’s auditor since 2013.
F-3
Profound Medical Corp.
Consolidated Balance Sheet
As at December 31, 2024 and 2023
In USD (000s)
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
Cash |
|
54,912 |
|
26,213 |
Trade and other receivables, net (note 3) |
|
7,045 |
|
7,288 |
Inventory (note 4) |
|
5,801 |
|
6,989 |
Prepaid expenses and deposits |
|
1,307 |
|
1,406 |
Total current assets |
|
69,065 |
|
41,896 |
|
|
|
|
|
Property and equipment, net (note 5) |
|
425 |
|
909 |
Intangible assets, net (note 6) |
|
261 |
|
490 |
Right-of-use assets, net (note 9) |
|
396 |
|
661 |
Deferred tax assets, net (note 13) |
|
87 |
|
— |
Total assets |
|
70,234 |
|
43,956 |
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
Accounts payable |
|
1,317 |
|
865 |
Accrued expenses and other current liabilities (note 7) |
|
2,835 |
|
2,419 |
Deferred revenue |
|
419 |
|
721 |
Long-term debt (note 8) |
|
1,737 |
|
2,104 |
Lease liabilities (note 9) |
|
257 |
|
259 |
Total current liabilities |
|
6,565 |
|
6,368 |
|
|
|
|
|
Deferred tax liabilities, net (note 13) |
|
— |
|
59 |
Deferred revenue |
|
49 |
|
728 |
Long-term debt (note 8) |
|
2,924 |
|
5,000 |
Lease liabilities (note 9) |
|
203 |
|
504 |
Other non - current liabilities (note 9) |
|
71 |
|
73 |
Total liabilities |
|
9,812 |
|
12,732 |
|
|
|
|
|
Commitments and contingencies (note 15) |
|
|
|
|
|
|
|
|
|
Shareholders’ equity |
|
|
|
|
|
|
|
|
|
Common shares, no par value, unlimited shares authorized, 30,039,809 and 21,370,565 issued and outstanding at December 31, 2024 and 2023, respectively (note 10) |
|
281,552 |
|
222,205 |
Additional paid-in capital |
|
21,298 |
|
20,808 |
Accumulated other comprehensive income |
|
2,742 |
|
5,565 |
Accumulated deficit |
|
(245,170) |
|
(217,354) |
Total shareholders’ equity |
|
60,422 |
|
31,224 |
|
|
|
|
|
Total liabilities and shareholders’ equity |
|
70,234 |
|
43,956 |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Profound Medical Corp.
Consolidated Statements of Operations and Comprehensive Loss
For the year ended December 31, 2024 and 2023
In USD (000s)
|
|
2024 |
|
2023 |
|
|
|
$ |
|
$ |
|
Revenue (note 12) |
|
|
|
|
|
Recurring - non-capital |
|
8,240 |
|
6,806 |
|
Capital equipment |
|
2,440 |
|
393 |
|
|
|
10,680 |
|
7,199 |
|
Cost of sales |
|
3,643 |
|
2,887 |
|
Gross profit |
|
7,037 |
|
4,312 |
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
Research and development |
|
16,965 |
|
14,424 |
|
Selling, general and administrative |
|
23,134 |
|
18,539 |
|
Total operating expenses |
|
40,099 |
|
32,963 |
|
|
|
|
|
|
|
Operating loss |
|
33,062 |
|
28,651 |
|
|
|
|
|
|
|
Other (income) expenses |
|
|
|
|
|
Net finance (income) expense |
|
(1,436) |
|
(775) |
|
Net foreign exchange (gain) loss |
|
(3,808) |
|
575 |
|
Total other (income) expenses |
|
(5,244) |
|
(200) |
|
|
|
|
|
|
|
Net loss before income taxes |
|
27,818 |
|
28,451 |
|
|
|
|
|
|
|
Income tax (recovery) expense (note 13) |
|
144 |
|
(187) |
|
Deferred tax expense (note 13) |
|
(146) |
|
59 |
|
Total income tax (recovery) expense |
|
(2) |
|
(128) |
|
|
|
|
|
|
|
Net loss attributed to shareholders for the year |
|
27,816 |
|
28,323 |
|
|
|
|
|
|
|
Other comprehensive (income) loss |
|
|
|
|
|
Item that may be reclassified to (income) loss |
|
|
|
|
|
Foreign currency translation adjustment |
|
2,823 |
|
(644) |
|
|
|
|
|
|
|
Net loss and other comprehensive loss for the year |
|
30,639 |
|
27,679 |
|
|
|
|
|
|
|
Loss per share (note 14) |
|
|
|
|
|
Basic and diluted net loss per common share |
|
1.12 |
|
1.34 |
|
Basic and diluted weighted average common shares outstanding |
|
24,765,503 |
|
21,182,558 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Profound Medical Corp.
Consolidated Statements of Shareholders’ Equity
For the year ended December 31, 2024 and 2023
In USD (000s)
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
Additional |
|
Other |
|
|
|
|
|
|
|
|
|
|
Paid-in |
|
Comprehensive |
|
Accumulated |
|
|
|
|
Common Shares |
|
Capital |
|
Income |
|
Deficit |
|
Tota1 |
||
|
|
Shares |
|
Amount $ |
|
$ |
|
$ |
|
$ |
|
$ |
Balance – January 1, 2023 |
|
20,879,497 |
|
216,453 |
|
20,254 |
|
4,921 |
|
(189,031) |
|
52,597 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year |
|
— |
|
— |
|
— |
|
— |
|
(28,323) |
|
(28,323) |
Cumulative translation adjustment – net of tax of $nil |
|
— |
|
— |
|
— |
|
644 |
|
— |
|
644 |
Exercise of share options (note 11) |
|
33,799 |
|
403 |
|
(158) |
|
— |
|
— |
|
245 |
Exercise of warrants (note 8) |
|
285,138 |
|
3,705 |
|
(1,264) |
|
— |
|
— |
|
2,441 |
Vesting of RSUs (note 11) |
|
162,131 |
|
1,509 |
|
(1,509) |
|
— |
|
— |
|
— |
Vesting of DSUs (note 11) |
|
10,000 |
|
135 |
|
(135) |
|
— |
|
— |
|
— |
Change in terms of DSUs (note 11) |
|
— |
|
— |
|
203 |
|
— |
|
— |
|
203 |
Share-based compensation (note 11) |
|
— |
|
— |
|
3,417 |
|
— |
|
— |
|
3,417 |
Balance – December 31, 2023 |
|
21,370,565 |
|
222,205 |
|
20,808 |
|
5,565 |
|
(217,354) |
|
31,224 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year |
|
— |
|
— |
|
— |
|
— |
|
(27,816) |
|
(27,816) |
Cumulative translation adjustment – net of tax of $nil |
|
— |
|
— |
|
— |
|
(2,823) |
|
— |
|
(2,823) |
Shares issued in private placement and public offerings (note 10) |
|
8,425,039 |
|
57,211 |
|
— |
|
— |
|
— |
|
57,211 |
Exercise of share options (note 11) |
|
7,101 |
|
76 |
|
(31) |
|
— |
|
— |
|
45 |
Vesting of RSUs (note 11) |
|
228,774 |
|
1,990 |
|
(1,990) |
|
— |
|
— |
|
— |
Vesting of DSUs (note 11) |
|
8,330 |
|
70 |
|
(70) |
|
— |
|
— |
|
— |
Share-based compensation (note 11) |
|
— |
|
— |
|
2,581 |
|
— |
|
— |
|
2,581 |
Balance – December 31, 2024 |
|
30,039,809 |
|
281,552 |
|
21,298 |
|
2,742 |
|
(245,170) |
|
60,422 |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Profound Medical Corp.
Consolidated Statements of Cash Flows
For the year ended December 31, 2024 and 2023
In USD (000s)
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Cash flows from operating activities |
|
|
|
|
Net loss for the year |
|
(27,816) |
|
(28,323) |
Adjustments to reconcile net loss to net cash provided by operating activities: |
|
|
|
|
Depreciation of property and equipment (note 5) |
|
707 |
|
727 |
Amortization of intangible assets (note 6) |
|
229 |
|
202 |
Non-cash lease expense adjustment |
|
(38) |
|
(45) |
Share-based compensation (note 11) |
|
2,581 |
|
3,417 |
Interest and accretion expense (note 8) |
|
600 |
|
727 |
Change in amortized cost of trade and other receivables |
|
(307) |
|
146 |
Changes in operating assets and liabilities: |
|
|
|
|
Trade and other receivables (note 3) |
|
186 |
|
(956) |
Inventory (note 4) |
|
656 |
|
353 |
Prepaid expenses and deposits |
|
31 |
|
(158) |
Accounts payable, accrued expenses and other liabilities (note 7) |
|
815 |
|
1,354 |
Deferred revenue |
|
(948) |
|
187 |
Income taxes payable (note 13) |
|
— |
|
(299) |
Deferred tax liabilities (note 13) |
|
(58) |
|
59 |
Deferred tax assets (note 13) |
|
(91) |
|
— |
Net cash used in operating activities |
|
(23,453) |
|
(22,609) |
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
Issuance of commons shares (note 10) |
|
62,106 |
|
— |
Payments of financing costs (note 10) |
|
(4,895) |
|
— |
Repayments of long-term debt (note 8) |
|
(2,560) |
|
(912) |
Proceeds from the exercise of stock options (note 11) |
|
45 |
|
245 |
Proceeds from the exercise of warrants (note 8) |
|
— |
|
2,423 |
Net cash provided by financing activities |
|
54,696 |
|
1,756 |
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents |
|
31,243 |
|
(20,853) |
Effect of exchange rate changes on cash |
|
(2,544) |
|
549 |
Cash, beginning of year |
|
26,213 |
|
46,517 |
Cash, end of year |
|
54,912 |
|
26,213 |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
1Description of business
Profound Medical Corp. (Profound) and its subsidiaries (together, the Company) were incorporated under the Ontario Business Corporations Act on July 16, 2014. The Company is a commercial-stage medical device company focused on the development and marketing of customizable, incision-free therapeutic systems for the ablation of diseased tissue utilizing platform technologies.
The Company’s registered address is 2400 Skymark Avenue, Unit 6, Mississauga, Ontario, Canada, L4W 5K5.
2 |
Summary of significant accounting policies |
Basis of preparation
The Company prepares its consolidated financial statements in accordance with accounting principles generally accepted in the United States (US GAAP). The consolidated financial statements include the accounts of wholly owned subsidiaries, after elimination of intercompany accounts and transactions. The consolidated financial information presented herein reflects all financial information that, in the opinion of management, is necessary for a fair statement of financial position, results of operations and cash flows for the periods presented.
Use of estimates
The preparation of the Company’s consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenue and expenses during the reporting period. Significant estimates and assumptions reflected in these consolidated financial statements include, but are not limited to, assumptions related to the valuation of inventory, the determination of the amortized cost of trade and other receivables, determination of expected credit loss, and the valuation of stock options and warrants. The Company based its estimates on historical experience, known trends and other market-specific or other relevant factors that it believes to be reasonable under the circumstances. On an ongoing basis, management evaluates its estimates when there are changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates.
Certain of the Company’s revenue is generated from sales to distributors. Where these sales have payment terms based on installation, the Company exercises judgement in determining when to recognize revenue. Once revenue is recognized, the Company records a contract asset until such time as the right to payment is not just subject to the passage of time, typically related to installation of the product.
Consolidation
The financial statements include the accounts of the Company and all its consolidated subsidiaries after elimination of intercompany transactions and balances. The Company consolidates all entities that it controls either through a majority voting interest or as the primary beneficiary of variable interest entities (VIE).
Currently, the Company has no involvement with variable interest entities. All subsidiaries are evaluated under the voting interest entity model. The Company consolidates those entities it controls through a majority voting interest.
The consolidated financial statements of the Company include the following wholly owned subsidiaries: Profound Medical Inc. (Canada), Profound Medical Oy (Finland), Profound Medical GmbH (Germany), Profound Medical (U.S.) Inc. (United States), Profound Medical Technology Services (Beijing) Co., Ltd. (China) and 2753079 Ontario Inc. (Canada).
F-8
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
Segment reporting
Operating segments reflect the way the Company is managed, and for which separate financial information is available and evaluated regularly by the Company’s chief operating decision maker (CODM) in deciding how to allocate resources and assess performance. The chief executive officer, who is the CODM, views the Company’s operations and manages its business in one operating segment, which is medical technology focused on magnetic resonance guided ablation procedures for the treatments to ablate the prostate gland, uterine fibroids, osteoid osteoma and nerves for palliative pain relief for patients with metastatic bone disease.
Foreign currency translation
The consolidated financial statements are presented in US dollars. The functional currency of Profound Medical Corp. is Canadian dollars. The functional currency of each subsidiary is determined based on facts and circumstances in the financial and operational environment relevant for each subsidiary. Where the Company’s presentation currency of US dollars differs from the functional currency of a subsidiary, the assets, liabilities and equity of the subsidiary are translated from the functional currency into the presentation currency at the exchange rates as at the reporting date. The income and expenses of the subsidiaries are translated at rates approximating the exchange rates at the dates of the transactions. Exchange differences arising on the translation of the consolidated financial statements of the Company’s subsidiaries are recognized in other comprehensive (income) loss.
Foreign currency transactions are translated into the functional currency of the Company or its subsidiaries, using the exchange rates prevailing at the dates of these transactions. Foreign exchange gains and losses resulting from the settlement of foreign currency transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in currencies other than an entity’s functional currency are recognized in the consolidated statements of operations and comprehensive loss, within net foreign exchange (gain) loss.
Fair value measurements
Certain assets and liabilities of the Company are carried at fair value under US GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
| ● | Level 1 - Quoted prices in active markets for identical assets or liabilities. |
| ● | Level 2 - Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. |
| ● | Level 3 - Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. |
For assets and liabilities that are recognized in the consolidated financial statements on a recurring basis, the Company determines whether transfers have occurred between levels in the hierarchy by reassessing the categorization at the end of each reporting period. There were no transfers between levels during the period presented. The Company currently does not have any level 3 financial instruments.
F-9
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The Company considers its cash, trade and other receivables, net, prepaid expenses and deposits, accounts payable, accrued expenses and other liabilities and long-term debt to be financial instruments.
Concentrations of credit risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and trade and other receivables, net. The Company maintains its cash balances in various operating accounts including cash deposited at a major financial institution that management believes to be creditworthy. Management has not previously experienced non-performance by any financial institution. Concentrations of credit risk with respect to trade and other receivables, net are limited due to a large number of customers who are widely dispersed. The Company monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business.
Trade and other receivables and allowance for expected credit losses
Trade and other receivables are stated net of an allowance for expected credit losses. The Company grants credit to customers in the normal course of business and maintains an allowance for expected credit losses which reflect the current estimate of credit losses expected to be incurred over the life of the receivables. The Company considers various factors in establishing, monitoring, and adjusting its allowance for expected credit losses, including the aging of the accounts and aging trends, the historical level of charge-offs, and specific credit exposures related to particular customers. The Company also monitors other risk factors, such as country risk, when determining credit limits for customers and establishing adequate allowances. Uncollectible accounts are written-off against the allowance when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, failure to make contractual payments for a period of greater than 180 days past due.
Inventory
Inventories are valued at the lower of cost and net realizable value. Net realizable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale. Cost is determined using the first-in, first-out method for finished goods and weighted average cost for raw materials.
The Company evaluates the carrying value of inventory on a regular basis, taking into account factors such as historical and anticipated future sales compared with quantities on hand, the price the Company expects to obtain for products in their respective markets compared with historical cost, obsolescence due to development of technology.
Property and equipment, net
Property and equipment are stated at cost, less accumulated depreciation and accumulated impairment losses. The initial cost of property and equipment consists of its purchase price and any directly attributable costs of bringing the asset to its working condition and location for its intended use. Expenditures incurred after the assets have been put into operation, such as repairs and maintenance, are charged to the consolidated statements of operations and comprehensive loss during the year in which they are incurred.
The major categories of property and equipment are depreciated on a straight-line basis as follows:
Furniture and fittings |
|
5 years |
|
Equipment under operating lease |
|
2 years |
|
Leasehold improvements |
|
Lesser of the estimated useful life or the lease term |
|
Residual values, methods of depreciation and useful lives of the assets are reviewed annually and adjusted if appropriate.
F-10
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
Intangible assets
The Company’s intangible assets are stated at cost, less accumulated amortization and accumulated impairment losses. Intangible assets are amortized on a straight-line basis in the consolidated statements of operations and comprehensive loss over their estimated useful lives.
The major categories of intangible assets are amortized as follows:
Exclusive licence agreement |
|
20 years |
|
Software |
|
5 years |
|
Impairment of long-lived assets
Property and equipment, net, right-of-use assets, and intangible assets with finite lives are tested for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. These assets are evaluated for impairment on an individual asset or group of assets with similar characteristics basis. If indicators of impairment are present, the asset is tested for recoverability by comparing the carrying value of the asset to the related estimated undiscounted future cash flows expected to be derived from the asset, which include the amount and timing of the projected future cash flows. If the expected undiscounted cash flows are less than the carrying value of the asset, then the asset is considered to be impaired and its carrying value is written down to fair value, based on the related estimated discounted future cash flows.
Accounts payable, accrued expenses and other current liabilities
These amounts represent liabilities for goods and services provided to the Company before the end of the financial year, which are unpaid. Accounts payable, accrued expenses and other current liabilities are presented as current liabilities unless payment is not due within 12 months after the reporting period. They are recognized initially at their fair value and subsequently measured at amortized cost using the effective interest method.
Long-term debt
Long-term debt is initially recognized at fair value, net of transaction costs incurred. Long-term debt is subsequently measured at amortized cost. Any difference between the proceeds (net of transaction costs) and the principal amount is recognized in the consolidated statements of operations and comprehensive loss over the contractual lives of the long-term debt using the effective interest method.
Long-term debt is removed from the consolidated balance sheets when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial liability that has been extinguished and the consideration paid is recognized in the consolidated statements of operations and comprehensive loss, within other (income) expense, net.
Warrants
The Company issued warrants to certain of its debt holder and equity investors and accounts for warrant instruments as either equity-classified or liability-classified instruments based on an assessment of the specific terms of the warrants and applicable authoritative guidance in ASC 480 Distinguishing Liabilities from Equity (ASC 480) and ASC 815, Derivatives and Hedging (ASC 815). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own stock and whether the holders of the warrants could potentially require net cash settlement in a circumstance outside of the Company’s control, among other conditions for equity classification.
F-11
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
Leases
Leases where the Company is the Lessee
The Company accounts for leases in accordance with ASC 842, Leases (ASC 842). At inception of a contract, the Company assesses whether a contract is, or contains, a lease based on whether the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The Company determines the initial classification and measurement of its right-of-use assets and lease liabilities at the lease commencement date. The lease term includes any renewal options and termination options that the Company is reasonably certain to exercise.
Lease liabilities and the corresponding right-of-use assets are recorded based on the present values of lease payments over the terms. The present value of the lease payments is determined using the rate implicit in that lease. If the information necessary to determine the rate implicit in a lease is not available, the Company uses its incremental borrowing rate at the commencement of the lease, which represents the rate of interest that the Company would incur to borrow on a collateralized basis over a similar term.
All leases must be classified as either an operating lease or finance lease. The classification is determined based on whether substantive control has been transferred to the lessee. The classification governs the pattern of lease expense recognition. For leases classified as operating leases, total lease expense over the term of the lease is equal to the undiscounted payments due in accordance with the lease arrangement. Fixed lease expense is recognized on a straight-line basis over the term of each lease and includes: (i) imputed interest during the period on the lease liability determined using the effective interest rate method plus (ii) amortization of the right-of-use asset for that period. Amortization of the right-of-use asset during the period is calculated as the difference between the straight-line expense and the imputed interest on the lease liability for that period. Variable lease expense is recognized in the period in which the obligation for variable lease payments is incurred. All of the Company’s leases are classified as operating leases.
The Company has elected not to record on the consolidated balance sheets a lease for which the term is 12 months or less.
Leases where the Company is the Lessor
Revenue from leasing arrangements is not subject to the revenue standard for contracts with customers and remains separately accounted for under ASC 842. In accordance with ASC 842, lessors should classify and account for a lease as an operating lease or a finance lease. All of the Company’s leases are qualified as operating leases. The Company does not derecognize the leased equipment at the time of the arrangement but depreciates the leased equipment over its useful life.
Revenue
Revenue is derived primarily from the sale of the TULSA-PRO and Sonalleve systems and one time use devices. All products generally contain a one-year warranty.
The Company recognizes revenue when the customer obtains control of promised goods or services and in an amount that reflects the consideration to which the Company expects to be entitled to receive in exchange for those goods or services. To achieve this core principle, the Company applies the five-step revenue model to contracts within its scope: (i) identify the contract(s) with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
F-12
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The amount of revenue to be recognized is based on the transaction price the Company expects to receive in exchange for its goods and services. For contracts that contain multiple performance obligations, the Company allocates the transaction price to each performance obligation and recognizes the related revenue when or as control of each individual performance obligation is transferred to customers.
Recurring – non-capital
Recurring - non-capital revenue consists of the sale of one-time-use devices and services associated with extended warranties. Revenue from sale of one-time-use devices is recognized when control is transferred to the customers, which generally occurs at the time of shipment. Service revenue related to extended warranties is deferred and recognized on a straight-line basis over the extended warranty period covered by the customer contract.
Capital equipment
Capital equipment revenue consists of the sale of capital equipment including installation and training amounts. Revenue is recognized when the Company transfers control to the customer, which is generally at the time of shipment. The Company’s customer arrangements generally do not provide a right of return.
Contract Assets
Contract assets arise from billed amounts in customer arrangements and the Company’s right to payment is not just subject to the passage of time, typically related to installation of the product. The Company recognizes a receivable at the point in time at which it has an unconditional right to payment.
Sales to distributors
The Company markets and sells its products primarily through its direct sales force, which sells its products to end customers. A portion of the Company’s revenue is generated by sales to distributors primarily in Europe and Asia. When the Company transacts with a distributor, its contractual arrangement is with the distributor and not with the end customer. Whether the Company transacts business with and receives the order from a distributor or directly from an end customer, its revenue recognition policy and resulting pattern of revenue recognition for the order are generally the same.
Cost of sales
Cost of sales primarily includes the cost of finished goods, depreciation of equipment under lease, inventory write-downs, royalties, warranty expense, freight and direct overhead and labor expenses necessary to acquire or manufacture the finished goods.
Income taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.
Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
F-13
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained based on the technical merits of the position. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority. The provision for income taxes includes the effects of unrecognized tax benefits, as well as the related interest and penalties.
Share-based compensation
The Company grants share options periodically to certain employees, directors and officers.
Options currently outstanding vest over four years and have a contractual life of ten years. Each tranche in an award is considered a separate award with its own vesting period and grant date fair value. The fair value of each tranche is measured at the date of grant using the Black-Scholes option pricing model. Compensation expense is recognized over the tranche’s vesting period using the graded vesting method by increasing additional paid-in capital based on the number of awards expected to vest.
The Company has a long-term incentive plan (LTIP) with a requisite service period of 3 years. For each Restricted Share Unit (RSU) and Deferred Share Unit (DSU) granted under the long-term incentive plan, the Company recognizes an expense equal to the market value of a Profound common share at the date of grant based on the number of RSUs and DSUs expected to vest, recognized over the term of the vesting period, with a corresponding credit to additional paid-in capital for share-based compensation anticipated to be equity settled or a corresponding credit to a liability for those anticipated to be cash settled. Share-based compensation is adjusted for subsequent changes in management’s estimate of the number of RSUs or DSUs that are expected to vest, for RSUs or DSUs anticipated to be cash settled and changes in the market value of Profound common shares. The effect of these changes is recognized in the period of the change. Vested RSUs and DSUs are settled either in Profound common shares or in cash or a combination thereof at the discretion of the Company.
As of December 31, 2024, the Company authorized for issuance under the share-based compensation a total of 3,089,175 share option, 716,000 RSUs and 100,000 DSUs.
Share-based compensation is recognized in the consolidated statements of operations and comprehensive loss in the same manner as the award recipients’ other compensation costs. Forfeitures are recognized as a reduction of share-based compensation expense as they occur.
Research and development costs
Research and development costs are charged to expense as incurred.
Clinical trial expenses result from obligations under contracts with vendors, consultants and clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided to the Company. These expenses are recorded according to the progress of the clinical trial as measured by patient progression and the timing of various aspects of the clinical trial. Clinical trial accrual estimates are determined through discussions with internal clinical personnel and outside service providers as to the progress or state of completion of clinical trials, or the services completed. Service provider status is then compared to the contractually obligated fees to be paid for such services. During the course of a clinical trial, the Company may adjust the rate of clinical expense recognized if actual results differ from management’s estimates.
F-14
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
Advertising and marketing costs
Advertising and marketing costs are expensed as incurred. These costs are included in selling, general and administrative expenses and were $4,478 and $3,348 for the years ended December 31, 2024 and 2023, respectively.
Loss per share
Basic loss per share is calculated by dividing the net loss by the weighted average number of common shares outstanding during the reporting period. Diluted loss per share is calculated by dividing the applicable net loss by the sum of the weighted average number of shares outstanding during the reporting period and all additional common shares that would have been outstanding if potentially dilutive common shares had been issued during the reporting period, except where the effect of such common shares would be antidilutive.
For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding as inclusion of the potentially dilutive common shares would be antidilutive.
Comprehensive (income) loss
Comprehensive (income) loss comprises of net (income) loss and other comprehensive (income) loss. Other comprehensive (income) loss includes foreign currency translation adjustments. Accumulated other comprehensive (income) loss is recorded as a component of shareholders’ equity.
Contingencies
The Company records a liability in the consolidated financial statements on an undiscounted basis for loss contingencies related to legal actions when a loss is known or considered probable and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed.
Recently adopted accounting pronouncements
In September 2022, the Financial Accounting Standards Board (FASB) issued ASU 2022-04, Liabilities—Supplier Finance Programs (Subtopic 405-50), which requires that a buyer in a supplier finance program disclose sufficient information about the program to allow a user of financial statements to understand the program’s nature, activity during the period, changes from period to period, and potential magnitude. The Company adopted this guidance on January 1, 2024. The adoption of this standard did not have an impact on the Company’s consolidated financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting: Improvements to Reportable Segment Disclosures. This ASU modified the disclosure and presentation requirements primarily through enhanced disclosures of significant segment expenses and clarified that single reportable segment entities must apply ASC 280 in its entirety. This guidance is effective for the Company for the year beginning January 1, 2024, with early adoption permitted. The amendments should be applied retrospectively to all prior periods presented in the financial statement. The Company adopted ASU 2023-07 on January 1, 2024 and the adoption did not have a material effect on the Company’s consolidated financial statements.
F-15
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
In December 2023, the FASB issued ASU 2023-09, Income Taxes (ASC 740): Improvements to Income Tax Disclosures, which includes amendments that further enhance income tax disclosures, primarily through standardization and disaggregation of rate reconciliation categories and income taxes paid by jurisdiction. The amendments are effective for all public entities for fiscal years beginning after December 15, 2024, and early adoption is permitted. The Company elected to early adopt ASU 2023-09 on January 1, 2024 retrospectively and the adoption has an effect on the Company’s disclosures on income taxes (note 13).
Recently issued accounting pronouncements
In October 2023, the FASB issued ASU 2023-06, Disclosure Improvements. The amendments in this update are the result of the FASB’s decision to incorporate into the Codification certain disclosures referred by the SEC that overlap with, but require incremental information to, US GAAP. The amendments in this update represent changes to clarify or improve disclosure and presentation requirements of a variety of topics in the Codification. For entities subject to the SEC’s existing requirements, the effective date for each amendment will be the date on which the SEC’s removal of that related disclosure from Regulation S-X or Regulation S-K becomes effective, with early adoption prohibited. The amendments in this update should be applied prospectively. The Company is currently evaluating the impact of this guidance.
The Company does not believe there are any other recently issued, but not yet effective, accounting standards that would have a significant impact on the Company’s financial position or results of operations.
3 |
Trade and other receivables, net |
Trade receivables and other receivables, net, as of December 31, 2024 and 2023 consist of the following:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Trade receivables, gross |
|
5,245 |
|
3,048 |
Contract assets, gross |
|
1,340 |
|
4,097 |
Trade receivables and contract assets |
|
6,585 |
|
7,145 |
Allowance for expected credit losses |
|
(158) |
|
(76) |
Less amortized cost adjustment |
|
— |
|
(315) |
Trade receivables, net |
|
6,427 |
|
6,754 |
Tax receivables |
|
308 |
|
414 |
Other receivables |
|
310 |
|
120 |
Total trade and other receivables, net |
|
7,045 |
|
7,288 |
During the year ended December 31, 2024, $2,147 (2023 – $nil) of trade receivables were written off.
The activity in the allowance for expected credit losses for trade receivables was as follows:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Balance, beginning of year |
|
76 |
|
— |
Provision for allowance for expected credit losses |
|
82 |
|
76 |
Balance, end of year |
|
158 |
|
76 |
F-16
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The allowance for expected credit losses as at December 31, 2024 and 2023 for trade receivables is as follows:
|
|
2024 |
||||||||||||
|
|
|
|
0–30 |
|
31‑60 |
|
61‑90 |
|
90+ |
|
Contract |
|
|
|
|
Current |
|
days |
|
days |
|
days |
|
days |
|
assets |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected loss rate |
|
0.84 |
% |
3.02 |
% |
3.02 |
% |
5.96 |
% |
6.02 |
% |
6.02 |
% |
|
Gross carrying amount |
|
4,180 |
|
713 |
|
— |
|
— |
|
352 |
|
1,340 |
|
6,585 |
Allowance for expected credit losses |
|
35 |
|
21 |
|
— |
|
— |
|
21 |
|
81 |
|
158 |
|
|
2023 |
||||||||||||
|
|
|
|
0–30 |
|
31‑60 |
|
61‑90 |
|
90+ |
|
Contract |
|
|
|
|
Current |
|
days |
|
days |
|
days |
|
days |
|
assets |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected loss rate |
|
0.84 |
% |
1.42 |
% |
1.35 |
% |
2.46 |
% |
3.62 |
% |
0.84 |
% |
|
Gross carrying amount |
|
2,400 |
|
— |
|
93 |
|
— |
|
555 |
|
4,097 |
|
7,145 |
Allowance for expected credit losses |
|
20 |
|
— |
|
1 |
|
— |
|
20 |
|
35 |
|
76 |
4 |
Inventory |
Inventory as of December 31, 2024 and 2023 consist of the following:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Finished goods |
|
3,837 |
|
4,638 |
Raw materials |
|
1,964 |
|
2,351 |
Inventory |
|
5,801 |
|
6,989 |
During the year ended December 31, 2024, $3,178 (2023 - $2,704) of inventory was recognized in cost of sales. The Company recognized $43 inventory write - downs in cost of sales during the year ended December 31, 2024 (2023 – $3).
5 |
Property and equipment, net |
The major components of property and equipment, net, as of December 31, 2024 and 2023 consist of the following:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Leasehold improvements |
|
542 |
|
542 |
Equipment under operating lease |
|
2,273 |
|
2,583 |
Total |
|
2,815 |
|
3,125 |
Accumulated depreciation |
|
(2,390) |
|
(2,216) |
Property and equipment, net |
|
425 |
|
909 |
Depreciation expense for the year ended December 31, 2024 was $707 (2023 - $727). During the year ended December 31, 2024, the Company sold $532 (2023 - $nil) of equipment under operating lease to various customers.
F-17
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
6 |
Intangible assets |
The major components of intangible assets as of December 31, 2024 and 2023 consist of:
|
|
|
|
2024 |
|
2023 |
||||||||
|
|
|
|
$ |
|
$ |
||||||||
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Remaining |
|
|
|
Accumulated |
|
|
|
|
|
Accumulated |
|
|
|
|
Useful |
|
Gross |
|
Amortization |
|
Net |
|
Gross |
|
Amortization |
|
Net |
|
|
Lives |
|
Carrying |
|
and |
|
Carrying |
|
Carrying |
|
and |
|
Carrying |
|
|
(Years) |
|
Amount |
|
Impairments |
|
Amount |
|
Amount |
|
Impairments |
|
Amount |
Exclusive licence agreement |
|
4.7 |
|
231 |
|
(142) |
|
89 |
|
231 |
|
(114) |
|
117 |
Software |
|
1 |
|
978 |
|
(806) |
|
172 |
|
978 |
|
(605) |
|
373 |
|
|
|
|
1,209 |
|
(948) |
|
261 |
|
1,209 |
|
(719) |
|
490 |
The Company has a licence agreement (the licence) with Sunnybrook Health Sciences Centre (Sunnybrook), pursuant to which Sunnybrook licenses to the Company certain intellectual property and exclusively licenced-in rights that enable the Company to use Sunnybrook’s technology for MRI-guided trans-urethral ultrasound therapy. The Company has the option to acquire rights to improvements to the relevant technology and intellectual property. If the Company fails to comply with any of its obligations or otherwise breaches this agreement, Sunnybrook may have the right to terminate the licence.
Amortization expense for the year ended December 31, 2024 was $229 (2023 - $202). Aggregate amortization expense for each of the five succeeding years related to intangible assets held as of December 31, 2024 is estimated as follows:
2025 |
|
189 |
2026 |
|
21 |
2027 |
|
19 |
2028 |
|
19 |
2029 |
|
13 |
Total |
|
261 |
7 |
Accrued expenses and other current liabilities |
Accrued expenses and other current liabilities, as of December 31, 2024 and 2023 consist of the following:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Accrued employee compensation |
|
706 |
|
752 |
Clinical trails |
|
325 |
|
663 |
Other general accruals |
|
1,804 |
|
1,004 |
Accrued expenses and other current liabilities |
|
2,835 |
|
2,419 |
8 |
Long-term debt |
On November 3, 2022, the Company signed a credit agreement with CIBC (the “Original CIBC Credit Agreement”) to provide a secured loan for total gross proceeds of C$10,000 maturing on November 3, 2027 with an interest rate based on CIBC prime plus 2% (CIBC Loan). The Company was required to make interest only payments until October 31, 2023 and monthly repayments of C$208 plus accrued interest commenced on October 31, 2023. All obligations of the Company under the credit agreement with CIBC are guaranteed by current and future subsidiaries of the Company and include security of first priority interests in the assets of the Company and its subsidiaries. Initially, the Company had financial covenants in relation to the CIBC Loan where unrestricted cash is at all times greater than EBITDA for the most recent six-month period, reported on a monthly basis and that revenue for any fiscal quarter must be 15% greater than revenue for the same fiscal quarter in the prior fiscal year, reported on a quarterly basis.
F-18
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The term loan matures in November 2027.
On September 26, 2023, an amendment to the CIBC Loan resulted in a change to the financial covenants. The amended covenants are that unrestricted cash must at all times be greater of: (i) to the extent EBITDA is negative for such period, EBITDA for the most recent nine-month period and (ii) $7,500, reported on a monthly basis; and that recurring revenue for any fiscal quarter must be 15% greater than recurring revenue for the same fiscal quarter in the prior fiscal year, reported on a quarterly basis.
On May 3, 2024, a second amendment to the CIBC Loan resulted in another change to the financial covenants. The amended covenants are that the recurring revenue covenant shall not be tested for any fiscal quarter in the 2024 fiscal year so long as unrestricted cash is no less than 2.5 multiplied by the principal amount of outstanding CIBC Loan at all times. The Company is in compliance with these financial covenants as at December 31, 2024.
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Balance - Beginning of year |
|
7,104 |
|
7,174 |
Interest and accretion expense |
|
600 |
|
727 |
Foreign exchange |
|
(483) |
|
115 |
Repayment |
|
(2,560) |
|
(912) |
Balance - End of year |
|
4,661 |
|
7,104 |
Less: Current portion |
|
1,737 |
|
2,104 |
Long-term portion |
|
2,924 |
|
5,000 |
Principal payments required on long-term debt outstanding at December 31, 2024 are $1,737 in 2025, $1,738 in 2026, and $1,186 in 2027.
In connection with the CIBC term loan agreements, the Company had issued warrants to CIBC on July 30, 2018 and November 3, 2022, with each warrant entitling the holder to acquire one common share at a price of C$9.70 and C$5.29 per common share, respectively, with a cashless exercise feature (collectively, CIBC Warrants). These warrants were determined to be equity classified and the fair value of the warrants on issuance date was recognized in additional paid-in capital. On June 14, 2023, all of the outstanding CIBC Warrants were exercised resulting in the issuance of common shares of the Company, resulting in a reclassification from additional paid-in capital to share capital. There were no CIBC Warrants outstanding as of December 31, 2024 and 2023.
9 |
Leases |
Leases where the Company is the Lessee
The Company leases certain office premises. Its operating leases have fixed payment structures expiring in 2026. Lease liabilities and corresponding right-of-use assets were recognized based on the present value of future lease payments.
Lease expense for the years 2024 and 2023 include:
|
|
2024 |
|
2023 |
|
|
|
$ |
|
$ |
|
Operating lease costs |
|
243 |
|
247 |
|
Total lease costs |
|
243 |
|
247 |
|
F-19
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
Other information related to operating leases for 2024 and 2023 is as follows:
|
|
2024 |
|
2023 |
|
Cash paid from operating cash flows for amounts included in the measurement of lease liabilities |
|
288 |
|
292 |
|
Weighted average remaining lease term |
|
1.75 years |
|
2.75 years |
|
Weighted average discount rate |
|
5.99 |
% |
5.99 |
% |
Maturities of the operating lease liabilities and minimum payments for operating leases having initial or remaining noncancellable terms in excess of one year as of December 31, 2024 were as follows:
2025 |
|
274 |
2026 |
|
206 |
Total |
|
480 |
Less: Imputed interest |
|
20 |
Present value of remaining lease payments |
|
460 |
Less: Current portion |
|
257 |
Non-current portion |
|
203 |
Leases where the Company is the lessor
Certain medical equipment are leased to customers under contractual arrangements that typically include an operating lease as well as performance obligations for sale of one-time-use devices. Contract terms vary by customer and may include options to terminate the contract or options to extend the contract. Where instruments are provided under operating lease arrangements, some portion or the entire lease revenue may be variable and collected as part of expected sales of certain related goods, which are separate performance obligations from subsequent non-lease component (e.g., sale of one-time-use devices). The allocation of revenue between the lease and non-lease components is based on standalone selling prices.
Assets related to operating leases are reported within property and equipment, net on the consolidated balance sheets. The original cost and the net book value of such assets were $2,273 and $332, respectively, as of December 31, 2024 (2023 - $2,583 and $751, respectively).
10 |
Share capital |
Common shares
The Company is authorized to issue an unlimited number of common shares.
|
|
2024 |
|
2023 |
Issued and outstanding (with no par value) |
|
$ |
|
$ |
30,039,809 (2023 – 21,370,565) common shares |
|
281,552 |
|
222,205 |
On January 2, 2024, the Company closed a public offering, resulting in the issuance of 2,666,667 common shares at a price of $7.50, for gross proceeds of $20,000 ($18,238, net of transaction costs).
On January 16, 2024, the Company closed a non-brokered private placement, resulting in the issuance of 391,667 common shares at a price of $7.50, for gross proceeds of $2,938 ($2,841, net of transaction costs).
On December 10, 2024, the Company closed a public offering, resulting in the issuance of 5,366,705 common shares at a price of $7.50, for gross proceeds of $40,250 ($36,132, net of transaction costs).
F-20
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
Voting Power
Except as otherwise required by law, the holders of common shares possess all voting power for the election of the Company’s directors and all other matters requiring shareholder action. Holders of common shares are entitled to one vote per share on matters to be voted on by shareholders.
Dividends
Holders of common shares will be entitled to receive such dividends, if any, as may be declared from time to time by the Company’s board of directors in its discretion out of funds legally available therefor. In no event will any stock dividends or stock splits or combinations of stock be declared or made on common stock unless the shares of common stock at the time outstanding are treated equally and identically.
Liquidation, Dissolution and Winding Up
In the event of the Company’s voluntary or involuntary liquidation, dissolution, distribution of assets or winding-up, the holders of the common stock will be entitled to receive an equal amount per share of all of the Company’s assets of whatever kind available for distribution to shareholders, after the rights of the creditors have been satisfied.
Warrants
A summary of warrants outstanding is shown below for the year ended December 31, 2023. There are no warrants outstanding at December 31, 2024:
|
|
|
|
|
|
Weighted |
|
|
|
|
Weighted |
|
average |
|
|
|
|
average |
|
remaining |
|
|
|
|
exercise |
|
contractual |
|
|
Number of |
|
price |
|
life |
|
|
warrants |
|
C$ |
|
(years) |
Balance - January 1, 2023 |
|
772,270 |
|
13.29 |
|
0.82 |
Expired |
|
(458,477) |
|
14.00 |
|
— |
Exercised |
|
(313,793) |
|
13.48 |
|
— |
Balance - December 31, 2023 |
|
— |
|
— |
|
— |
11 |
Share-based payments |
Share options
Effective May 20, 2020, the Company adopted amendments to the share option plan (the Share Option Plan). The maximum number of common shares reserved for issuance under the share option plan and the long-term incentive plan is 3,905,175 common shares or such other number as may be approved by the holders of the voting shares of the Company.
As at December 31, 2024, 2,291,152 (2023 – 1,474,809) options are outstanding. Each share option granted allows the holder to purchase one common share, at an exercise price not less than the lesser of the closing trading price of the common shares on the TSX (or other exchange where the common shares are listed), on the date a share option is granted and the volume-weighted average price of the common shares for the five trading days immediately preceding the date the share option is granted. Share options granted under the Share Option Plan generally have a maximum term of ten years and vest over a period of up to four years.
F-21
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
A summary of the share option activity during the year presented and the total number of share options outstanding as at those dates are set forth below:
|
|
|
|
Weighted |
|
Weighted |
|
|
|
|
|
|
average |
|
average |
|
|
|
|
|
|
exercise |
|
remaining |
|
Aggregate |
|
|
Number |
|
price |
|
contractual |
|
intrinsic |
|
|
of options |
|
C$ |
|
term |
|
value |
Balance - January 1, 2023 |
|
1,511,773 |
|
16.07 |
|
|
|
|
Granted |
|
59,300 |
|
15.42 |
|
|
|
|
Exercised |
|
(33,799) |
|
9.86 |
|
|
|
|
Forfeited/expired |
|
(62,465) |
|
16.13 |
|
|
|
|
Balance - December 31, 2023 |
|
1,474,809 |
|
16.19 |
|
6.08 |
|
474 |
Exercisable - December 31, 2023 |
|
1,218,581 |
|
15.46 |
|
5.75 |
|
470 |
Expected to vest -December 31, 2023 |
|
1,474,809 |
|
16.19 |
|
6.08 |
|
474 |
Balance - January 1, 2024 |
|
1,474,809 |
|
16.19 |
|
|
|
|
Granted |
|
946,900 |
|
11.14 |
|
|
|
|
Exercised |
|
(7,101) |
|
8.99 |
|
|
|
|
Forfeited/expired |
|
(123,456) |
|
16.17 |
|
|
|
|
Balance - December 31, 2024 |
|
2,291,152 |
|
14.13 |
|
5.36 |
|
429 |
Exercisable - December 31, 2024 |
|
1,326,573 |
|
15.98 |
|
4.92 |
|
348 |
Expected to vest -December 31, 2024 |
|
2,291,152 |
|
14.13 |
|
5.36 |
|
429 |
The Company estimated the fair value of the share options granted during the year using the Black-Scholes option pricing model with the weighted average assumptions below. The Company estimated the expected future stock price volatility for its common stock by using its historical volatility based on daily price observations for the most recent historical period equal to the length of the instrument’s expected life of options.
|
|
November 18, |
|
March 18, |
|
Grant date |
|
2024 |
|
2024 |
|
Exercise price |
|
C$11.14 |
|
C$11.24 |
|
Expected volatility |
|
70 |
% |
70 |
% |
Expected life of options |
|
6 years |
|
6 years |
|
Risk-free interest rate |
|
3.17 |
% |
3.54 |
% |
Dividend yield |
|
— |
|
— |
|
|
|
March 22, |
|
June 12, |
|
September 8, |
|
November 16, |
|
Grant date |
|
2023 |
|
2023 |
|
2023 |
|
2023 |
|
Exercise price |
|
C$13.39 |
|
C$19.87 |
|
C$12.38 |
|
C$12.41 |
|
Expected volatility |
|
70 |
% |
70 |
% |
69 |
% |
70 |
% |
Expected life of options |
|
6 years |
|
6 years |
|
6 years |
|
6 years |
|
Risk-free interest rate |
|
3.38 |
% |
3.22 |
% |
3.71 |
% |
4.16 |
% |
Dividend yield |
|
— |
|
— |
|
— |
|
— |
|
The weighted average grant date fair values of share options granted for the year ended December 31, 2024 were C$11.14 (2023 - C$10.10). The total remaining unrecognized compensation expense related to non-vested share options for the year ended December 31, 2024 was $4,160, which will be amortized over the weighted-average period of 1.6 years.
Long-term incentive plan
Effective May 17, 2023, the Company adopted the amended long term incentive plan (the LTIP). The LTIP is an incentive-based equity compensation plan that provides for the grant of restricted share units (the RSUs) and deferred share units (the DSUs, together with the RSUs, the Units).
F-22
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The maximum number of units which may be reserved for issuance under this LTIP in respect of grants of RSUs and DSUs shall not exceed 4.9% of the issued and outstanding common shares on a non-diluted basis, provided that, the maximum number of shares which may be reserved for issuance pursuant to all of the Company’s security-based compensation arrangements shall not in the aggregate exceed 13% of the issued and outstanding common shares on a non-diluted basis. The Company may grant Units to officers, directors or employees of the Company. Each Unit represents the right to receive one common share in accordance with the terms of the LTIP. The number of Units granted at any particular time will be calculated by dividing the dollar amount of such grant by the market value of a common share on the applicable grant date, which is equal to the volume weighted average trading price of all common shares traded on the TSX (or other exchange where the Common Shares are listed) for the five trading days immediately preceding such date. RSUs and DSUs granted under the LTIP vest over a period of up to three years.
The following table summarizes RSUs activities:
|
|
|
|
Weighted |
|
|
|
|
average grant |
|
|
|
|
date fair value |
|
|
Number of |
|
per share |
|
|
RSUs |
|
C$ |
Balance - January 1, 2023 |
|
443,861 |
|
12.62 |
Granted |
|
235,500 |
|
12.38 |
Vested |
|
(162,131) |
|
13.82 |
Forfeited |
|
(23,834) |
|
10.15 |
Balance - December 31, 2023 |
|
493,396 |
|
12.23 |
Granted |
|
107,500 |
|
11.02 |
Vested |
|
(228,774) |
|
13.33 |
Forfeited |
|
(47,501) |
|
11.25 |
Balance - December 31, 2024 |
|
324,621 |
|
11.18 |
The total remaining unrecognized compensation expense related to non-vested RSUs for the year ended December 31, 2024 was $2,522, which will be amortized over the weighted-average period of 1.8 years.
Effective May 17, 2023, the Company adopted the approval of revision to the amended LTIP. Previously, vested DSUs were settled either in common shares or in cash or a combination thereof at the discretion of the holder and were classified as a cash-settled liability. Under the amended LTIP, vested DSUs are settled either in common shares or in cash or a combination thereof at the discretion of the Company. The change in terms resulted in the DSUs being classified as equity settled and the effect of this change was recognized in 2023 resulting in a reclassification between accrued expenses and other current liabilities and additional paid-in capital of $203.
F-23
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
A summary of the DSUs changes during the year are set forth below:
|
|
|
|
Weighted |
|
|
|
|
average grant |
|
|
|
|
date fair value |
|
|
Number of |
|
per share |
|
|
DSUs |
|
C$ |
Balance - January 1, 2023 |
|
60,000 |
|
9.41 |
Granted |
|
25,000 |
|
12.38 |
Vested |
|
(10,000) |
|
9.41 |
Balance - December 31, 2023 |
|
75,000 |
|
10.40 |
Granted |
|
25,000 |
|
11.07 |
Vested |
|
(8,330) |
|
12.38 |
Balance - December 31, 2024 |
|
91,670 |
|
10.40 |
The total remaining unrecognized compensation expense related to non-vested DSUs for the year ended December 31, 2024 was $512, which will be amortized over the weighted-average period of 1.7 years.
Share-based compensation expense
The following table presents the components and classification of share-based compensation recognized for share options, RSUs, and DSUs for the years ended December 31, 2024 and 2023:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Share options |
|
635 |
|
1,211 |
RSUs |
|
1,517 |
|
1,914 |
DSUs |
|
429 |
|
292 |
Share-based compensation |
|
2,581 |
|
3,417 |
|
|
|
|
|
Cost of sales |
|
24 |
|
104 |
Research and development |
|
636 |
|
758 |
Selling, general and administrative |
|
1,921 |
|
2,555 |
Share-based compensation |
|
2,581 |
|
3,417 |
12 |
Revenue |
The following table provides information about disaggregated revenue by products and services:
|
|
For the year ended December 31, 2024 |
||||
|
|
Contracts with |
|
|
|
|
|
|
customers |
|
Leasing |
|
Total |
|
|
$ |
|
$ |
|
$ |
Revenue |
|
|
|
|
|
|
Recurring - non-capital |
|
7,300 |
|
940 |
|
8,240 |
Capital equipment |
|
2,440 |
|
— |
|
2,440 |
|
|
9,740 |
|
940 |
|
10,680 |
F-24
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
|
|
For the year ended December 31, 2023 |
||||
|
|
Contracts with |
|
|
|
|
|
|
customers |
|
Leasing |
|
Total |
|
|
$ |
|
$ |
|
$ |
Revenue |
|
|
|
|
|
|
Recurring - non-capital |
|
5,506 |
|
1,300 |
|
6,806 |
Capital equipment |
|
393 |
|
— |
|
393 |
|
|
5,899 |
|
1,300 |
|
7,199 |
13 |
Income taxes |
Taxes on earnings reflect the annual effective rates, including charges for interest and penalties. Deferred income taxes reflect the tax consequences on future years of differences between the tax bases of assets and liabilities and their financial reporting amounts.
The components of loss before income taxes for 2024 and 2023 consist of:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Domestic |
|
(28,286) |
|
(29,351) |
Foreign |
|
468 |
|
900 |
|
|
(27,818) |
|
(28,451) |
The components of (provision for) benefit from income taxes for 2024 and 2023 consist of:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Current |
|
|
|
|
Foreign |
|
144 |
|
(187) |
Total current income tax expense |
|
144 |
|
(187) |
Deferred |
|
|
|
|
Foreign |
|
(146) |
|
59 |
Total deferred tax expense |
|
(146) |
|
59 |
Total income tax (recovery) expense |
|
(2) |
|
(128) |
During the year ended December 31, 2024, the Company has early adopted ASU 2023-09 to enhance the income taxes disclosures regarding income taxes paid and the rate reconciliation disclosure. The income taxes paid by the Company are as follows:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
United States |
|
252 |
|
160 |
|
|
252 |
|
160 |
F-25
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The (provision for) benefit from income taxes differs from the expected amount calculated by applying the Company’s Canadian federal statutory rate to loss before income taxes for 2024 and 2023 as follows:
|
|
2024 |
|
2023 |
||||
|
|
$ |
|
% |
|
$ |
|
% |
Loss before income taxes |
|
(27,818) |
|
— |
|
(28,451) |
|
— |
(Provision for) benefit from income taxes |
|
|
|
|
|
|
|
|
Canadian federal statutory rate of 15% (2023 - 15%) |
|
(4,173) |
|
15.0 |
|
(4,268) |
|
15.0 |
Provincial tax / state tax |
|
(2,998) |
|
10.8 |
|
(3,073) |
|
10.8 |
Foreign tax effects |
|
|
|
|
|
|
|
|
United States |
|
|
|
|
|
|
|
|
Statutory tax rate differences between United States and Canada |
|
16 |
|
— |
|
15 |
|
— |
Finland |
|
|
|
|
|
|
|
|
Statutory tax rate differences between Finland and Canada |
|
10 |
|
— |
|
19 |
|
— |
Germany |
|
|
|
|
|
|
|
|
Statutory tax rate differences between Germany and Canada |
|
(42) |
|
0.1 |
|
— |
|
— |
Changes in valuation allowance |
|
7,085 |
|
(25.5) |
|
6,944 |
|
(24.5) |
Non-taxable or non-deductible items |
|
206 |
|
(0.7) |
|
467 |
|
(1.6) |
True-up and other adjustments |
|
(106) |
|
0.3 |
|
(232) |
|
0.8 |
|
|
(2) |
|
— |
|
(128) |
|
0.5 |
The components of deferred tax assets and liabilities are summarized as follows:
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Deferred tax assets: |
|
|
|
|
Operating loss carry forwards |
|
43,031 |
|
36,067 |
SR&ED expenditure pool |
|
4,344 |
|
4,202 |
Benefit of Investment tax credits |
|
2,078 |
|
2,786 |
Excess of tax value of property and equipment over book value |
|
2,392 |
|
2,112 |
Long term debt |
|
(32) |
|
78 |
Financing fees |
|
1,042 |
|
543 |
Reserves |
|
827 |
|
220 |
Total deferred tax assets |
|
53,682 |
|
46,008 |
Valuation allowance |
|
(53,595) |
|
(46,008) |
Net deferred tax assets |
|
87 |
|
— |
|
|
|
|
|
Deferred tax liabilities: |
|
|
|
|
Excess of accounting value of property, plant and equipment over tax value |
|
— |
|
59 |
Total deferred tax liabilities |
|
— |
|
59 |
Deferred income taxes reflect future tax effects of temporary differences between the tax and financial reporting basis of the Company’s assets and liabilities measured using enacted tax laws and statutory tax rates applicable to the periods when the temporary differences will affect taxable income. When necessary, deferred tax assets are reduced by a valuation allowance, if based on the weight of available positive and negative evidence, it is more likely than not that some portion or all the deferred tax assets will not be realized. The Company has $53,595 in valuation allowance against its deferred tax assets, for the year ended December 31, 2024 (2023 - $46,008).
F-26
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
The Company has non-capital loss carry-forwards in Canada of approximately $157,555 which expires as follows:
|
|
$ |
2028 |
|
128 |
2029 |
|
215 |
2030 |
|
51 |
2031 |
|
446 |
Thereafter |
|
156,715 |
Total |
|
157,555 |
The Company has SR&ED expenditures in Canada of approximately $15,905 as at December 31, 2024, which can be carried forward indefinitely to reduce future years’ taxable income.
The Company has approximately $3,471 of Canadian federal and provincial tax credits that are available to be applied against Canadian federal and provincial taxes otherwise payable in future years and that expire in varying amounts from 2028 to 2044.
14 |
Loss per share |
The following table shows the calculation of basic and diluted loss per share:
|
|
2024 |
|
2023 |
|
||
Net loss for the year |
|
$ |
27,816 |
|
$ |
28,323 |
|
Weighted average number of common shares |
|
|
24,765,503 |
|
|
21,182,558 |
|
Basic and diluted loss per share |
|
$ |
1.12 |
|
$ |
1.34 |
|
The computation of diluted loss per share is equal to the basic loss per share due to the anti-dilutive effect of the share options, RSUs, DSUs and warrants. Of the 2,291,152 (2023 – 1,474,809) share options, 324,621 (2023 – 493,396) RSUs, and 91,670 (2023 – 75,000) DSUs not included in the calculation of diluted loss per share for the year ended December 31, 2024, 1,326,573 (2023 – 1,218,581) were exercisable.
15Commitments and contingencies
All directors and officers of the Company are indemnified by the Company for various items including, but not limited to, all costs to settle lawsuits or actions due to their association with the Company, subject to certain restrictions. The Company has purchased directors’ and officers’ liability insurance to mitigate the cost of any potential future lawsuits or actions. The term of the indemnification is not explicitly defined but is limited to events for the period during which the indemnified party served as a director or officer of the Company. The maximum amount of any potential future payment cannot be reasonably estimated but could have a material adverse effect on the Company.
The Company has also indemnified certain lenders and underwriters in relation to certain debt and equity offerings and their respective affiliates and directors, officers, employees, shareholders, partners, advisers and agents and each other person, if any, controlling any of the underwriters or lenders or their affiliates against certain liabilities.
As of December 31, 2024 and 2023, no material amounts were accrued for the Company’s obligations under these indemnification provisions.
F-27
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
16 |
Segment reporting |
The Company’s operations are categorized into one industry segment, which is medical technology focused on magnetic resonance guided ablation procedures for the treatments to ablate the prostate gland, uterine fibroids, osteoid osteoma and nerves for palliative pain relief for patients with metastatic bone disease. The CODM regularly reviews the operating results of the Company on a consolidated basis as part of making decisions for allocating resources and evaluating performance. Further, the CODM is regularly provided with the consolidated expenses as noted on the consolidated statements of operations and comprehensive loss.
The following tables represent total revenue by geographic area, based on the location of the location of the reporting entity for the years ended December 31, 2024 and 2023, respectively:
|
|
For the year ended December 31, 2024 |
||||||
|
|
Canada |
|
USA |
|
Germany |
|
Total |
|
|
$ |
|
$ |
|
$ |
|
$ |
Revenue |
|
|
|
|
|
|
|
|
Recurring - non-capital |
|
891 |
|
6,458 |
|
891 |
|
8,240 |
Capital equipment |
|
1,548 |
|
892 |
|
— |
|
2,440 |
|
|
2,439 |
|
7,350 |
|
891 |
|
10,680 |
|
|
For the year ended December 31, 2023 |
||||||
|
|
Canada |
|
USA |
|
Germany |
|
Total |
|
|
$ |
|
$ |
|
$ |
|
$ |
Revenue |
|
|
|
|
|
|
|
|
Recurring - non-capital |
|
230 |
|
5,126 |
|
1,450 |
|
6,806 |
Capital equipment |
|
— |
|
— |
|
393 |
|
393 |
|
|
230 |
|
5,126 |
|
1,843 |
|
7,199 |
The following tables represent other geographic information for the years ended December 31, 2024 and 2023, respectively:
|
|
For the year ended December 31, 2024 |
||||||||||
|
|
Canada |
|
USA |
|
Germany |
|
China |
|
Finland |
|
Total |
|
|
$ |
|
$ |
|
$ |
|
$ |
|
$ |
|
$ |
Total assets |
|
58,743 |
|
6,351 |
|
1,661 |
|
92 |
|
3,387 |
|
70,234 |
Intangible assets |
|
261 |
|
— |
|
— |
|
— |
|
— |
|
261 |
Property and equipment |
|
93 |
|
332 |
|
— |
|
— |
|
— |
|
425 |
Right-of-use assets |
|
396 |
|
— |
|
— |
|
— |
|
— |
|
396 |
Amortization of intangible assets |
|
229 |
|
— |
|
— |
|
— |
|
— |
|
229 |
Depreciation of property and equipment |
|
66 |
|
641 |
|
— |
|
— |
|
— |
|
707 |
|
|
For the year ended December 31, 2023 |
||||||||||
|
|
Canada |
|
USA |
|
Germany |
|
China |
|
Finland |
|
Total |
|
|
$ |
|
$ |
|
$ |
|
$ |
|
$ |
|
$ |
Total assets |
|
34,302 |
|
4,067 |
|
1,952 |
|
82 |
|
3,553 |
|
43,956 |
Intangible assets |
|
490 |
|
— |
|
— |
|
— |
|
— |
|
490 |
Property and equipment |
|
158 |
|
751 |
|
— |
|
— |
|
— |
|
909 |
Right-of-use assets |
|
661 |
|
— |
|
— |
|
— |
|
— |
|
661 |
Amortization of intangible assets |
|
202 |
|
— |
|
— |
|
— |
|
— |
|
202 |
Depreciation of property and equipment |
|
57 |
|
670 |
|
— |
|
— |
|
— |
|
727 |
F-28
Profound Medical Corp.
Notes to Consolidated Financial Statements
December 31, 2024 and 2023
In USD (000s)
17 |
Subsequent events |
On February 1, 2025, the President of the United States issued three executive orders directing the United States to impose new tariffs on imports originating from Canada, Mexico and China. These orders call for additional 25% duty on imports into the United States of Canadian-origin and Mexican-origin products and 10% duty on Chinese origin products, except for Canadian energy resources that are subject to an additional 10% duty. The Company is assessing the direct and indirect impacts to its business of such tariffs, retaliatory tariffs or other trade protectionist measures implemented as this situation develops, and such impacts could be material.
On March 3, 2025, the Company entered into an amended and restated credit agreement with CIBC (the “CIBC Credit Agreement”), which amended the terms of the CIBC Loan and the existing long-term debt provided under the Original CIBC Credit Agreement was repaid with proceeds from a new revolving line of credit provided by CIBC to the Company. The line of credit bears interest at the Wall Street Journal Prime Rate subject to a floor of 6.25%. The CIBC Credit Agreement contains certain financial covenants, and the obligations thereunder are secured by, inter alia, a general security agreement over the assets of the Company and its subsidiaries. The revolving line of credit matures on March 3, 2027 and provides an option to increase the amount of the revolving commitment by $5,000 within 18 months from March 3, 2025, subject to achieving a minimum trailing 12 month revenue exceeding $15,000. The exercise of the option would result in the size of the revolving commitment increasing from $10,000 to a maximum of $15,000. Additionally, the CIBC Credit Agreement provides that the Company may request a one-time increase in the principal amount of the revolving line of credit up to a maximum amount of $10,000, which is subject to the approval of CIBC in its sole discretion.
F-29
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
Item 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures. Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this Form 10-K, have concluded that, based on such evaluation, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting. The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, the company’s principal executive and principal financial officers and effected by the company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that:
| ● | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the company’s internal control over financial reporting as of December 31, 2024. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework.
Based on our assessment, management believes that, as of December 31, 2024, the Company’s internal control over financial reporting was not effective based on those criteria as a result of a material weakness in internal control over financial reporting discussed in the paragraphs below.
A material weakness is a deficiency, or a combination of deficiencies, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
In conjunction with the preparation of the Company’s financial statements for the year ended December 31, 2024, and specifically in connection with the recognition of revenue under ASC 606, Revenue from contracts with customers, management has determined that the controls over the review of contract terms and arrangements with customers did not operate effectively during 2024. This material weakness resulted in audit adjustments to revenue, trade and other receivables and prepaid expenses, deposits and other assets, which were recorded prior to the issuance of the financial statements as of and for the year ended December 31, 2024.
77
Management considers these adjustments to constitute a material weakness that requires remediation, and management is in the process of implementing remediation measures to address the identified material weakness.
Management’s Remediation Plan. In an effort to address the identified material weakness and enhance our internal controls related to revenue recognition, management plans to expand the finance team to include more Chartered Professional Accountants (CPAs) with technical expertise and experience in evaluating more complex areas of US GAAP in evaluating contract terms and arrangements with customers, and engage third-party consultants to assist with assessing the accounting for more complex revenue contracts, as necessary. Management’s efforts are ongoing and its remediation plan is expected to be completed during 2025.
If these remedial measures are insufficient to address the material weakness described above, or are not implemented timely, or additional deficiencies arise in the future, a reasonable possibility exists that a material misstatement in our interim or annual financial statements may occur in the future.
Changes in Internal Control Over Financial Reporting. Other than the material weakness described above, there were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during our most recently completed fiscal year and fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Attestation Report of the Registered Public Accounting Firm. We are a smaller reporting company, and therefore our independent registered public accounting firm has not issued a report on the effectiveness of internal control over financial reporting.
Item 9B. OTHER INFORMATION
Rule 10b5-1 Trading Plans
During the fiscal quarter ended December 31, 2024, none of our directors or executive officers adopted, modified or terminated any contract, instruction or written plan for the purchase or sale of our securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) or any “non-Rule 10b5-1 trading arrangement” as defined in Item 408(c) of Regulation S-K.
Item 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
None.
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Executive Officers and Directors
The following table provides information regarding our executive officers and directors, including their ages as of March 7, 2025:
Name |
|
Age |
|
Position |
Arun Menawat |
|
70 |
|
Chief Executive Officer and Director, Chair of the Board (Principle Executive Officer) |
Rashed Dewan |
|
57 |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
Mathieu Burtnyk |
|
43 |
|
President |
Tom Tamberrino |
|
45 |
|
Chief Commercial Officer |
Brian Ellacott(2) |
|
67 |
|
Director, Lead Independent Director |
Cynthia Lavoie(1) |
|
57 |
|
Director |
Murielle Lortie(1)(3) |
|
55 |
|
Director |
Arthur Rosenthal(1)(2)(4) |
|
78 |
|
Director |
Kris Shah(2) |
|
63 |
|
Director |
Notes:
| (1) | Member of the Audit Committee. |
| (2) | Member of the Human Resources and Corporate Governance Committee. |
78
| (3) | Chair of the Audit Committee. |
| (4) | Chair of the Human Resources and Corporate Governance Committee. |
Executive Officers
Arun Menawat – Chief Executive Officer and Director – Dr. Menawat has served as the Chief Executive Officer of Profound since 2016. He has an accomplished history of executive leadership success in the healthcare industry. Prior to joining Profound in August 2016, he served as the Chairman, President and CEO of Novadaq Technologies Inc., a TSX and Nasdaq listed company that marketed medical imaging and therapeutic devices for use in the operating room, from 2003 to 2016. Previously, he was President and Chief Operating Officer and Director of another publicly listed medical imaging software company, Cedara Software. His educational background includes a Bachelor of Science in Biology, University of District of Columbia, Washington, D.C., and a Ph.D. in Chemical Engineering, from the University of Maryland, College Park, MD, including graduate research in Biomedical Engineering from the National Institute of Health, Bethesda, MD. He also earned an Executive M.B.A. from the J.L. Kellogg School of Management, Northwestern University, Evanston, IL.
Rashed Dewan – Chief Financial Officer - Mr. Rashed Dewan has served as Chief Financial Officer of Profound since March 2022. He previously served as Chief Accounting Officer of Profound from May 2021 to March 2022, and as VP Finance of Profound from July 2015 to May 2021. He has over 20 years of finance and accounting experience in public and private companies, with expertise in the medical device sector. Mr. Dewan has extensive experience with systems design and implementation and a strong track record of success in accounting, finance, sales and operations management. Mr. Dewan is a Certified Public Accountant and has a Bachelor of Science Degree with a concentration in Accounting from the University of Southern California.
Mathieu Burtnyk – President - Dr. Mathieu Burtnyk has over 20 years of experience creating and developing imaging technologies and therapeutic ultrasound solutions, from benchtop to bedside, with a focus on prostate disease. He started his career in academia, obtaining his PhD in Medical Biophysics at the University of Toronto and Sunnybrook Health Sciences Center. He is the inventor of the patented closed-loop temperature feedback control algorithm used by the TULSA-PRO today in clinic. Dr. Burtnyk joined Profound in 2011, leading the scientific design and execution of pre-clinical through Phase I and TACT Pivotal clinical studies. Dr. Burtnyk has served as President of Profound since November 2024. He previously served as Chief Operating Officer of Profound from March 2024 to November 2024, as SVP Product Leader for TULSA-PRO of Profound from February 2021 to February 2024, and as VP of Clinical Affairs of Profound from July 2019 to February 2021. Dr. Burtnyk received his Ph.D. in Medical Biophysics from the University of Toronto.
Tom Tamberrino – Chief Commercial Officer - Mr. Tom Tamberrino has served as Chief Commercial Officer of Profound since October 2024. He is a seasoned leader with a distinguished career in sales and marketing leadership, business development, and executive management, with much of his success rooted in the U.S. healthcare industry. He brings a wealth of experience, including a history of entrepreneurial ventures and significant contributions to the medical technology sector. Prior to joining Profound Medical, Mr. Tamberrino served as Managing Partner of AKB LLC from February 2018 to October 2024. Earlier in his career, Mr. Tamberrino held progressive sales management positions at LifeCell Corporation, eventually serving as Area Director. During his time at LifeCell Corporation, he led a 50-person sales team across the Northeastern U.S. and Canada, marketing regenerative tissue matrices. He previously served as Vice President of Sales and Marketing at Novadaq Technologies Inc. for several years.. Mr. Tamberrino holds a Bachelor of Science degree in Marketing with a Minor in Psychology from Georgetown University and a Master of Business Administration from Emory University.
Non-Employee Directors
Brian Ellacott – Director – Mr. Ellacott is an experienced global medical device executive. Mr. Ellacott has served as Chief Executive Officer of Belmont Instrument since December 2017. Belmont Instrument is a Boston based private equity owned medical device company with a leading global position in fluid warming and infusion systems. Prior to Belmont Instrument, Mr. Ellacott was the President and CEO of Laborie. Laborie is a Urology and Gastroenterology medical device company based in Toronto with manufacturing facilities in Toronto, Montreal, Enschede, NL, Attikon, Switzerland and Portsmouth, New Hampshire. Mr. Ellacott joined private equity owned Laborie as President and CEO in July 2013 and in four years completed 14 global acquisitions tripling Laborie’s revenue and increasing EBITDA eight-fold. The company was ranked as one of the fastest growing and most profitable medical device companies in the world. Prior to joining Laborie, Mr. Ellacott served as Executive Vice President and General Manager of Invacare’s (NYSE: IVC) $1 billion North and South American homecare and rehabilitation business. Mr. Ellacott has also held executive positions with Baxter International and American Hospital Supply, with assignments in Canada, Australia and the United States.
79
Mr. Ellacott serves on the board of Belmont Instrument and is the past Chairman of the board of the Canadian Assistive Devices Association. Mr. Ellacott holds a Bachelor of Business Administration Degree from Wilfrid Laurier University, Waterloo, Ontario, Canada and is a dual United States and Canadian citizen.
Cynthia Lavoie – Director – Dr. Lavoie has served as President and Managing Director of AllosteRx Capital Management (“AllosteRx”) since August 2018. She also serves as President and Chief Investment Officer of CCRM Enterprises since August 2020. Prior to co-founding AllosteRx, Cynthia was a General Partner with TVM Life Science Management Inc. (“TVM”), a global venture capital group with main offices in Munich and Montreal. She was recruited to TVM from VG (VenGrowth) Partners Inc., where she was a Partner and co-headed its life sciences fund. Cynthia is currently chair of the board of directors at Fibrocor Therapeutics, a fibrosis company in Toronto and Board Director of Apiary Therapeutics, a cell therapy start-up based in Toronto. A seasoned healthcare investment professional with 20 years of experience in venture capital, Dr. Lavoie’s expertise includes creating companies de novo and leading investments into businesses developing therapeutics, devices, and diagnostic tools. Cynthia has taken active roles on boards of companies located in Canada and the US from start-up to revenue-generating stages. These include Acer Therapeutics (NASDAQ: ACER), Cytochroma (acquired by OPKO Health), VisualSonics (acquired by SonoSite, now FujiFilm SonoSite), and Trillium Therapeutics (NASDAQ: TRIL) (acquired by Pfizer). Before joining the investment community, Dr. Lavoie served in a variety of academic and scientific leadership positions for 10 years, working with research institutes and life science companies. Cynthia earned her MBA with first class honors from Rotman School of Management at the University of Toronto and earned her Ph.D in Molecular Biology with Dean’s honors from McGill University.
Murielle Lortie – Director – Ms. Lortie has an accomplished history of financial leadership success within the global life science industry. She currently serves as Chief Financial Officer of Claridge Inc since September 2021. Prior to joining Claridge Inc., Ms. Lortie was Chief Financial Officer Liminal BioSciences Inc. (“Liminal”), a Nasdaq-listed, clinical-stage biopharmaceutical company from September 2018 to September 2021. Prior to joining Liminal, Ms. Lortie was Vice President & Chief Financial Officer and Advisor to the CEO, Global Strategy, Mergers & Acquisitions at Pharmascience Inc. Previously, she has held senior positions in finance at Bristol Myers Squibb, including Vice-President of Finance for Bristol Myers Squibb Canada Co. and Global Director of Finance supporting BMS Headquarters. Ms. Lortie is a Chartered Professional Accountant and member of the Ordre des comptables professionnels agrées du Québec. She holds a Graduate Diploma in Accountancy from Concordia University and a Bachelor of Business Administration Bishop’s University. She has extensive corporate governance experience, previously serving on the Boards of Bellus Health Inc. and Pharmascience Barbados Ltd. & Pharmascience International Ltd. Ms. Lortie is currently the Chair of the Board at Bishops University.
Arthur L. Rosenthal – Director – Dr. Rosenthal has served as director of Profound since June 2018. Dr. Rosenthal formerly served as director and Chair of Compensation Committee for LivaNova PLC, a UK global medical technology company. Prior, Dr. Rosenthal served on the Cyberonics board of directors as a non-executive director and Chair of the Compensation Committee from January 2007 to October 2015. Since June 2010, Dr. Rosenthal has served as Professor of Practice in the Biomedical Engineering Department at Boston University. Since December 2011, Dr. Rosenthal has also served as CEO of gEyeCue, Ltd., which he co-founded, a development stage medical device company working on a guided biopsy for lower and upper gastrointestinal cancer screening. From June 2011 until July 2012, Dr. Rosenthal served as executive vice chairman of Cappella Medical Devices Ltd. (now ArraVasc Ltd.), a development-stage company focused on novel device solutions for coronary artery disease. From June 2009 until June 2011, Dr. Rosenthal served as President and CEO of Cappella, Inc. Dr. Rosenthal served as chairman, from January 2002, and CEO, commencing in January 2005, of Labcoat, Ltd. until its acquisition by Boston Scientific Corporation in December 2008. From January 1994 to May 2000, Dr. Rosenthal was a Senior Vice President, Corporate Officer, and Chief Development Officer of Boston Scientific, and from May 2000 until his retirement in January 2005, he was a Senior Vice President, Chief Scientific Officer, and Executive Committee Member of Boston Scientific. From 2000 until 2010, Dr. Rosenthal served as a non-executive director, and from 2006 through 2009, as chairman of the Remuneration Committee, of Renovo, Ltd., a U.K. based pharmaceutical company that became publicly traded in 2006. In July 2009, Dr. Rosenthal joined the board of Interface Biologics, Inc., a Toronto-based development stage company focused on drug delivery devices, as a non-executive director. In April 2011, Dr. Rosenthal was elected Chairman at Interface Biologics, Inc. From April 2013 to May 2015, Dr. Rosenthal served as non-executive director and Member of the Compensation Committee of Arch Technologies, Inc. and is currently a member of Arch’s Clinical Advisory Board. In 2015, Dr. Rosenthal was appointed to the Industrial Advisory Committee, CURAM (National University in Galway, Ireland). Since 2003, Dr. Rosenthal has been a Fellow of the American Institute of Medical and Biological Engineering.
Kris Shah – Director – Kris Shah is the president of Baylis Medical Technologies, Inc. (“Baylis”), a leader in the development and commercialization of innovative medical devices in the fields of radiology and neurosurgery. Headquartered in Canada, Baylis also provides contract manufacturing services to some of the world’s leading medical device companies.
80
Kris joined Baylis in 1989 as a co-founder and served as president from 2015 until it was acquired by Boston Scientific in 2022. Baylis is a leading developer, manufacturer, and distributor of specialized medical devices for interventional cardiology. Baylis had previously divested its interventional pain management business to Kimberly Clark Corporation (now Avanos Medical, Inc.) in 2009, and its bone tumor ablation business (OsteoCool) to Medtronic plc in 2016. Kris also co-founded the consulting business OME Group in 1991, which was sold to Ernst and Young in 2011. Kris is an active board member for AdvaMed Accel and Intellijoint Surgical. In the past he has served on the boards of Venture Lab, MEDEC, and the Business Advisory Committee of HTX and Conavi Medical Inc. His list of accomplishments includes numerous patents, the Ernst and Young Entrepreneur Award for Healthcare in Quebec (2011) and the University of Waterloo Alumni Achievement Award (2014). Kris has a B.Sc. in Electrical Engineering from the University of Waterloo.
Board Mandate
The Board has responsibility for the stewardship of the Company. The Board has adopted a written mandate for the Board (the “Mandate”) to confirm and enhance the Board’s ongoing duty and responsibility for stewardship of the Company, a copy of which is available on the Company’s website at www.profoundmedical.com. The Board is ultimately responsible for supervising the management of the business and affairs of the Company and, in doing so, is required to act in the best interests of the Company. The Board generally discharges its responsibilities either directly or through the Audit Committee and the Human Resources and Corporate Governance Committee. Specific responsibilities of the Board set out in the Mandate include:
| (a) | Appointing Management – including approval of the Chief Executive Officer, the compensation of the executive officers and the oversight of succession planning programs; |
| (b) | Board Organization – including responding to recommendations received from the Human Resources and Corporate Governance Committee, but the Board retains the responsibility for managing its own affairs; |
| (c) | Strategic Planning – including the review and approval of the Company’s business, financial and strategic plans on at least an annual basis; |
| (d) | Monitoring of Financial Performance and Other Financial Reporting Matters – including the review of the Company’s ongoing financial performance and results of operations and review and approval of the Company’s audited and interim consolidated financial statements and management’s discussion and analysis of financial conditions and results of operations; |
| (e) | Risk Management – including the identification of the Company’s principal business risks and the implementation of appropriate systems to effectively monitor and manage such risks; |
| (f) | Policies and Procedures – including the approval and monitoring of all policies and procedures including those related to corporate governance, ethics and confidentiality; |
| (g) | Communication and Reporting – including the oversight of the timely and accurate disclosure of financial reports and other material corporate developments; and |
| (h) | Other Responsibilities – including those related to position descriptions, orientation and continuing education, nomination of directors and Board evaluations and matters in respect of any disposition, material commitment or venture, or significant expenditure in either monetary or business terms. |
Role of Board in Risk Oversight
One of the key functions of the Board is to oversee the Company’s risk management process. The Board is responsible for identifying the Company’s principal business risks and the implementation of appropriate systems to effectively monitor and manage such risks. The Audit Committee has the responsibility to review and discuss our major financial risk exposures and the policy steps management will take to monitor and control such exposures. The Audit Committee also monitors compliance with legal and regulatory requirements.
81
Board Composition
Our Board presently has six members. As a foreign private issuer, under the listing requirements and rules of Nasdaq, we are not required to have independent directors on our board of directors, except that our audit committee is required to consist fully of independent directors. However, our board of directors has determined that Brian Ellacott, Cynthia Lavoie, Murielle Lortie, Arthur Rosenthal and Kris Shah are all “independent” as such term is defined by National Instrument 58-101 – Disclosure of Corporate Governance Practices and Nasdaq rules. Arun Menawat is a non-independent director as he is our Chief Executive Officer. Each of the independent directors has no direct or indirect material relationship with the Company, including any business or other relationship, which could reasonably be expected to interfere with the director’s ability to act with a view to the best interests of the Company or which could reasonably be expected to interfere with the exercise of the director’s independent judgment.
If the Chairman is not independent, the independent directors may select one of their members to be appointed lead independent director of the Board (“Lead Independent Director”) for such term as the independent directors may determine. The Lead Independent Director is responsible for chairing meetings of the independent directors and seeking to ensure that the Board is able to carry out its role.
Dr. Arun Menawat acts as Chairman of the Board. Since Dr. Menawat is not independent, Brian Ellacott has been appointed Lead Independent Director of the Board.
Meetings of Independent Directors
The entire complement of independent directors on the Board and each of the committees meet regularly without management present. The Chairman of the Board conducts these sessions at Board meetings and the Chair of each committee conducts them at committee meetings. During the last financial year ended December 31, 2023, there were eight such meetings of the independent directors.
Chairman of the Board
Dr. Arun Menawat is the Chief Executive Officer of the Company and as a result does not meet the Board’s independence standards. The primary functions of the Chairman are to facilitate the operations and deliberations of the Board and the satisfaction of the Board’s responsibilities under its Mandate. The Chairman’s key responsibilities include duties relating to providing overall leadership to the Board, chairing board and Shareholder meetings, acting as a liaison between management, the members of the Board and the Chairs of the various committees of the Board, and communicating with Shareholders and regulators. The responsibilities of the Chairman are reviewed by the Human Resources and Corporate Governance Committee and considered by the Board for approval each year.
Director Term Limits and Other Mechanics of Board Renewal
The Board has not established any term limits for directors, as the Board takes the view that term limits are an arbitrary mechanism for removing directors which can result in valuable, experienced directors being forced to leave the Board solely because of length of service. The Board’s priorities continue to be ensuring the appropriate skill sets are present amongst the Board to optimize the benefit to the Company. The Board conducts annual evaluations of the individual directors, the committees of the Board and the Chairman of the Board, which are overseen by the Human Resources and Corporate Governance Committee, to ensure these objectives are met. See “Board Assessments”.
Board Meetings
The Board holds a minimum of one regular quarterly meeting and a corporate strategy session each year, as well as additional meetings as required. An in-camera session of the directors is held at each regularly scheduled Board and committee meeting, as deemed necessary, so that the independent members of the Board have an opportunity to meet without the presence of management members of the Board.
82
Meeting Attendance
During our fiscal year ended December 31, 2024, there were six (6) meetings of our Board of Directors, four (4) meetings of our Audit Committee and (3) meetings of the Human Resources and Corporate Governance Committee. No director attended fewer than 75% of the total number of meetings of our board of directors and of the committees of our board of directors on which he or she served. Our board of directors has adopted a policy under which each member of our board of directors is strongly encouraged but not required to attend each annual meeting of our stockholders.
Orientation and Continuing Education
Pursuant to the Mandate, it is the responsibility of the Board to provide an orientation program for new directors and ongoing educational opportunities for all directors. New directors are expected to participate in an initial information session on the Company in the presence of its senior executive officers to learn about, among other things, the business of the Company, its financial situation and its strategic planning. All directors will receive a record of public information about the Company, as well as other relevant corporate and business information including corporate governance practices of the Company, the structure of the Board and its standing committees, its corporate organization, the charters of the Board and its standing committees, the Code (as defined herein) and other relevant corporate policies.
Continuing education opportunities are directed at enabling individual directors to maintain or enhance their skills and abilities as directors, as well as ensuring that their knowledge and understanding of the Company’s affairs remains current. Directors are kept informed as to matters which may impact the Company’s operations through regular reports and presentations at Board and committee meetings.
Code of Business Conduct and Ethics
The Company has adopted a written Code of Business Conduct and Ethics (the “Code”) for directors, officers and employees and is available on our website at www.profoundmedical.com. Information contained on, or that can be accessed through, our website does not constitute a part of this report and is not incorporated by reference herein. The objective of the Code is to provide guidelines for maintaining the integrity, reputation, honesty, objectivity and impartiality of the Company and its subsidiaries. The Code addresses compliance with laws, conflicts of interest, corporate opportunity, confidentiality, fair dealing with customers, suppliers, competitors, officers and employees, protection and proper use of company assets and accounting complaints. The Board has the ultimate responsibility for the stewardship of the Code and is responsible for considering any request for waivers from the Code. Any waiver of the Code’s provisions is subject to the disclosure and other provisions of applicable securities laws and the applicable rules of any and all securities exchanges on which the securities of the Company are listed and posted for trading. A copy of the Code is available on the Company’s website at www.profoundmedical.com. If we make any amendment to the Code or grant any waivers, including any implicit waiver, from a provision of the Code, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the SEC. Disclosure regarding any amendments to, or waivers from, provisions of the Code that apply to our directors, principal executive officer or principal financial officer will be included in a Current Report on Form 8-K within four business days following the date of the amendment or waiver, unless website posting or the issuance of a press release of such amendments or waivers is then permitted by the rules of The Nasdaq Stock Market.
The Board monitors compliance with the Code and reviews it on at least an annual basis to determine whether updates are appropriate. Where a director or officer has any interest in or a perceived conflict involving a contract or business relationship with the Company, that director or officer is excluded from all discussions and deliberations regarding the contract or relationship and such director abstains from voting in respect thereof. Directors and executive officers have disclosed to the Company all directorships held by such member and the existence and nature of any interests that could result in a conflict situation with the Company.
The Board has also adopted a whistleblower policy (the “Whistleblower Policy”) relating to the reporting of inappropriate activity to encourage and promote a culture of ethical business conduct. The Whistleblower Policy is intended to encourage and facilitate the reporting of questionable accounting, internal accounting controls or auditing matters.
83
Nomination of Directors
The Human Resources and Corporate Governance Committee has the responsibility for reviewing the composition of the Board by taking into account, among other things, its size and the particular competencies and skills of its members. The Human Resources and Corporate Governance Committee, in consultation with the Chairman of the Board and Chief Executive Officer, will then identify potential Board nominees and recommend such nominees for election as directors based on the competencies and skills each new member possesses in the context of the needs of the Company. The Board as a whole is then responsible for nominating new directors. The Human Resources and Corporate Governance Committee is composed entirely of independent directors.
The Board seeks nominees that have the following characteristics: (i) a track record in general business management; (ii) special expertise in an area of strategic interest to the Company; (iii) the ability to devote time; and (iv) support for the Company’s mission and strategic objectives.
While the Company has not adopted a written policy relating to the identification and nomination of women directors, it recognizes that diversity is an economic driver of competitiveness for companies and it strives to promote an environment and culture conducive to the appointment of well qualified persons so that there is appropriate diversity to maximize the achievement of corporate goals. Gender of a potential candidate is one component in the overall list of factors the Human Resources and Corporate Governance Committee considers when selecting candidates for executive officer and senior manager appointments, and membership on the Board and its committees. The Human Resources and Corporate Governance Committee is of the opinion that if gender was the overriding factor governing the selection of Board nominees, it could unduly restrict the Board’s ability to select the most appropriate nominees and candidates. The Company has not adopted targets regarding women on the Board or women in executive officer and senior management positions as it does not believe that such targets are necessary at this time given the size of the Board and that the director nomination process recognizes the benefits of diversity. There are currently two women on the Board.
Director and Executive Compensation
The Human Resources and Corporate Governance Committee oversees the remuneration policies and practices of the Company. The principal responsibilities of the Human Resources and Corporate Governance Committee include: (i) considering the Company’s overall remuneration strategy and, where information is available, verifying the appropriateness of existing remuneration levels using external sources for comparison; (ii) comparing the nature and amount of the Company’s directors’ and executive officers’ compensation to performance against goals set for the year while considering relevant comparative information, independent expert advice and the financial position of the Company, and (iii) making recommendations to the Board in respect of director and executive officer remuneration matters, with the overall objective of ensuring maximum shareholder benefit from the retention of high quality board and executive team members.
Board Assessments
The Board is responsible for ensuring that there is a process in place for annually evaluating the effectiveness and contribution of the Chief Executive Officer, the Board, the committees of the Board, the Chairman of the Board and the individual directors based on their applicable terms of reference or position description.
The objective of the assessments is to ensure the continued effectiveness of the Board in the execution of its responsibilities and to contribute to a process of continuing improvement. In addition to any other matters the Board deems relevant, the assessments may consider in the case of the Board or a committee, the applicable terms of reference, the applicable position descriptions, as well as the competencies and skills each individual director is expected to bring to the Board.
The Human Resources and Corporate Governance Committee annually reviews and makes recommendations to the Board on the method and content of such evaluations and oversees the evaluation process.
Board Committees
The Board has two standing committees, the Audit Committee and the Human Resources and Corporate Governance Committee. Below is a description of the committees. The Board has adopted a written charter for each of the committees below that is available to shareholders on our website at https://profoundmedical.com/investors/.
84
Audit Committee
The Audit Committee oversees the accounting and financial reporting practices and procedures of the Company’s financial statements. The principal responsibilities of the Audit Committee include: (i) the integrity of the consolidated financial statements of the Company; (ii) the Company’s compliance with legal and regulatory requirements; (iii) the public accountants’ qualifications and independence; and (iv) the performance of the Company’s internal audit function and public accountants. The Audit Committee shall oversee the preparation of and review the report required by the rules of any and all securities regulatory bodies to which the Company is subject to be included in the Company’s annual proxy statement.
Composition of the Audit Committee
The following are the current members of the Audit Committee:
Name |
|
Independence |
|
Financial Literacy |
Cynthia Lavoie |
|
Independent |
|
Financially Literate |
Murielle Lortie |
|
Independent |
|
Financially Literate |
Arthur Rosenthal |
|
Independent |
|
Financially Literate |
The relevant education and experience of each member of the Audit Committee, is provided above, under the heading “Election of Directors”. All of the Audit Committee members are “independent” as required by the TSX and as defined in the listing standards of The Nasdaq Stock Market LLC and under Rule 10A-3 under the Exchange Act and each member is financially literate in that each has the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by the Company’s financial statements. The Company’s board of directors has determined that each of Cynthia Lavoie, Murielle Lortie and Arthur Rosenthal, members of its Audit Committee, is an “audit committee financial expert” as defined by applicable SEC rules.
Audit Committee Oversight
At no time since the commencement of the Company’s most recently completed financial period was a recommendation of the Audit Committee to nominate or compensate an external auditor not adopted by the Board.
Human Resources and Corporate Governance Committee
The Human Resources and Corporate Governance Committee is comprised of Brian Ellacott, Kris Shah and Arthur Rosenthal. All three members are independent directors.
The key responsibilities of the Human Resources and Corporate Governance Committee include:
| (a) | Annually review and approve corporate goals and objectives relevant to compensation of executive officers for whom compensation is required to be individually reported under applicable securities laws, evaluate the NEOs’ performance in light of those goals and objectives, and set the NEOs’ respective compensation levels based on this evaluation. |
| (b) | Annually review the Chief Executive Officer’s evaluation of the performance of the other officers of the Company and such other senior management and key employees of the Company or any subsidiary of the Company as may be identified to the Committee by the Board (collectively, the “Designated Executives”) and review the Chief Executive Officer’s recommendations with respect to the amount of compensation to be paid to the Designated Executives. |
| (c) | Annually review, assess the competitiveness and appropriateness of and approve the compensation package of each of the Designated Executives. |
| (d) | Review and approve any employment contracts or arrangements with each of the Designated Executives, including any retiring allowance arrangements or any similar arrangements to take effect in the event of a termination of employment. |
85
| (e) | Review and recommend to the Board compensation policies and processes and in particular, the compensation policies and processes for the Designated Executives. |
| (f) | In determining the long-term incentive component of the Chief Executive Officer’s compensation and each Designated Executive’s compensation, consider the Company’s performance and relative shareholder return, the value of similar incentive awards to executives at comparable companies, and the awards given to Company executives in past years. |
| (g) | Make recommendations to the Board with respect to incentive compensation and equity-based plans, and review and make recommendations with respect to the performance or operating goals for participants in such plans. |
| (h) | Have the sole authority to retain and terminate any compensation consultant to be used to assist in the evaluation of director, Chief Executive Officer or senior executive compensation and have sole authority to approve the consultant’s fees and other retention terms. |
| (i) | Adopt, administer, approve and ratify awards under incentive compensation and stock plans, including amendments to the awards made under any such plans, and review and monitor awards under such plans. |
| (j) | Review and report to the Board on the appropriateness of the succession planning of the Company, including appointing, training and monitoring senior management. |
| (k) | Review the significant human resources policies, plans and programs of the Company to ensure they are supportive of the Company’s near and long-term strategies. |
| (l) | Undertake on behalf of, and in an advisory capacity to, the Board such other initiatives as may be necessary or desirable to assist the Board in discharging its responsibility to ensure that appropriate human resources development, performance evaluation, compensation and management development programs are in place and operating effectively. |
Position Descriptions
The Board has developed written position descriptions which identify the responsibilities of the Chairman of the Board and the Chief Executive Officer. The Board has not developed written position descriptions for the Chair of each committee of the Board. The Board believes that the charters of the Audit Committee and the Human Resources and Corporate Governance Committee adequately delineate the roles of the Chairs of such committees. Each of the Audit Committee and the Human Resources and Corporate Governance Committee are responsible for reviewing their respective charters on a regular basis and to recommend to the Board any changes as considered appropriate from time to time.
Corporate Governance
We qualify as a “Foreign Private Issuer,” as defined in Rule 3b-4 under the Exchange Act. As a result, in accordance with Nasdaq listing requirements, we may rely on home country governance requirements and certain exemptions thereunder rather than complying with Nasdaq corporate governance standards.
Although we have voluntarily chosen to file registration statements, periodic reports and current reports on U.S. domestic issuer forms, we will maintain our status as a foreign private issuer. While we voluntarily follow most Nasdaq corporate governance rules, we may choose to take advantage of limited exemptions from the following:
| ● | U.S. federal proxy rules pursuant to Section 14 of the Exchange Act and Regulations 14A and 14C thereunder; |
| ● | Regulation FD; |
| ● | Section 16 rules requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades in a short period of time, which will provide less data in this regard than shareholders of U.S. companies that are subject to the Exchange Act; |
86
| ● | the requirement that our board have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| ● | the requirement to have independent director oversight of director nominations. |
We intend to follow Canadian corporate governance practices in lieu of Nasdaq corporate governance requirements as follows:
We intend to adopt and approve material changes to equity incentive plans in accordance with Toronto Stock Exchange (“TSX”) listing rules, which do not impose a requirement of shareholder approval for such actions. In addition, we intend to follow the TSX listing rules in respect of private placements instead of Nasdaq requirements to obtain shareholder approval for certain dilutive events (such as issuances that will result in a change of control, certain transactions other than a public offering involving issuances of a 20% or greater interest in us and certain acquisitions of the stock or assets of another company) and the minimum quorum requirement for a shareholders meeting. Under Nasdaq listing rules, the required minimum quorum for a shareholders meeting is 33 1/3% of the outstanding common shares, and our minimum quorum requirement is only 10% of the total number of voting rights attaching to all outstanding common shares.
Although we may rely on certain home country corporate governance practices, we must comply with Nasdaq’s Notification of Noncompliance requirement (Nasdaq Rule 5625) and the Voting Rights requirement (Nasdaq Rule 5640). Further, we must have an audit committee that satisfies Nasdaq Rule 5605(c)(3), which addresses audit committee responsibilities and authority and requires that the audit committee consist of members who meet the independence requirements of Nasdaq Rule 5605(c)(2)(A)(ii).
We intend to take all actions necessary for us to maintain compliance as a foreign private issuer under the applicable corporate governance requirements of the Sarbanes-Oxley Act, the rules adopted by the SEC and Nasdaq listing rules. Accordingly, our shareholders will not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
Insider Trading Policy
We have adopted the Profound Medical Corp. Corporate Disclosure, Confidentiality and Trading in Securities by Directors, Officers, Employees and Consultants Policy (the “Insider Trading Policy”) governing the purchase, sale and/or other dispositions of our securities by our directors, officers, employees and other covered persons. The Insider Trading Policy prohibits, among other things, insider trading and certain speculative transactions in our securities (including short sales, transacting in call or put options and other hedging transactions in our securities) and establishes a regular black-out period schedule during which directors, executive officers, employees and other covered persons may not trade in the Company’s securities, as well as certain pre-clearance procedures that directors and certain officers, employees and other covered persons must observe prior to effecting any transaction in our securities. We believe the Insider Trading Policy is reasonably designed to promote compliance with applicable insider trading laws, rules and regulations, as well as the exchange listing standards applicable to us. Although we have not adopted an insider trading policy governing our purchase, sale, and/or other disposition of our securities, as part of the oversight of risk, the Board, or one or more of its committees, approves any transaction, plan or arrangement by or with the Company with respect to our securities on a case-by-case basis, and as part of their procedures to review and approve any such transaction, plan or arrangement, the Board or committee consults with legal counsel to ensure compliance with applicable insider trading laws, rules and regulations, and listing standards. The foregoing description of our Insider Trading Policy is qualified in its entirety by reference to the full text of the Insider Trading Policy, filed as Exhibit 19.1 to this Annual Report on Form 10-K.
Shareholder Communications to our Board of Directors
Generally, shareholders who have questions or concerns should contact our Investor Relations team at 647-476-1350. However, any stockholders who wish to address questions regarding our business directly with our Board of Directors, or any individual director, should direct his or her questions in writing to the Chairman of our Board of Directors at 2400 Skymark Ave. Unit #6, Mississauga, Ontario, L4W5K5, Canada. Communications will be distributed to our board of directors, or to any individual director or directors as appropriate, depending on the facts and circumstances outlined in the communications. Items that are unrelated to the duties and responsibilities of our board of directors may be excluded.
87
Item 11. EXECUTIVE COMPENSATION
As a foreign private issuer in the United States, we are deemed to comply with this Item if we provide information required by Items 6.B,and 6.E.2 of Form 20-F, with more detailed information provided if otherwise made publicly available or required to be disclosed in Canada. We have provided information required by Items 6.B and 6.E.2 of Form 20-F below. As a foreign private issuer in the United States, we are not required to disclose executive compensation according to the requirements of Regulation S-K that apply to U.S. domestic issuers, and we are not otherwise required to adhere to the U.S. requirements relative to certain other proxy disclosures and requirements. Our executive compensation disclosure complies with Canadian requirements, which are, in many respects, substantially similar to U.S. rules.
Compensation Philosophy and Objectives of Compensation Programs
The executive compensation program adopted by Profound and applied to its executive officers is designed to:
| (a) | attract and retain qualified and experienced executives who have international business and operations experience and will contribute to the success of Profound; |
| (b) | ensure that the compensation of the executive officers provides a competitive base compensation package, with additional compensation to reward success and create a strong link between corporate performance and compensation; and |
| (c) | motivate executive officers to enhance long term shareholder value, with current compensation being weighted toward at-risk long-term incentives in the form of Options and restricted share units (“RSUs”) so as to foster alignment with the interests of the Shareholders. |
The goals of the compensation program are to attract and retain the most qualified people with relevant experience, to motivate and reward such individuals on a short term and long-term basis, and to create alignment between corporate performance and compensation. The Human Resources and Corporate Governance Committee and the Board intend that the total cash components of compensation (base salary plus annual cash bonus) target the median of a benchmark group in comparable industries with similar market capitalization (the “Compensation Peer Group”).
Aggregate compensation (including annual cash bonus and equity-based compensation) payable to each NEO (as defined below) is based on the achievement of certain performance goals. Performance goals are established annually and designed to align with the Company’s strategic objectives. As described in greater detail below, performance goals affect equity-based compensation grants and annual cash bonuses.
Profound does not believe that its compensation programs encourage excessive or inappropriate risk taking as: (i) employees receive both fixed and variable compensation, and the fixed (salary) portion provides a steady income regardless of Common Share value which allows employees to focus on the business; (ii) the Share Option Plan encourages a long term perspective due to the vesting provisions of the options (see “Share Option Plan” below); and (iii) annual bonus is earned only if short-term objectives of the Company are achieved. Profound believes that the compensation program is appropriately structured and balanced to motivate its executives and reward the achievement of annual performance goals, as well as the achievement of long term growth in shareholder value. NEOs and directors are not permitted to purchase financial instruments, including, for greater certainty, prepaid variable forward contracts, equity swaps, collars, or units of exchange funds, that are designed to hedge or offset a decrease in market value of equity securities granted as compensation or held, directly or indirectly, by the NEO or director.
Aligning Management and Shareholders
The Company’s compensation program seeks to align management interests with Shareholder interests through both short-term and long-term incentives linking compensation to performance. The short-term incentive is an annual cash bonus which is linked to individual performance and the Company’s performance. Further, long-term incentives of Option grants comprise a significant portion of overall compensation for the Company’s NEOs (as defined herein). The Human Resources and Corporate Governance Committee believes this is appropriate because it creates a direct correlation between variations in the Company’s share price (which is based in part on the Company’s financial performance) and the compensation of its NEOs, thereby aligning the interests of the Company’s executives and Shareholders.
88
Clawback Policy
The Company has adopted a clawback policy which applies to cash bonus awards made, and RSUs granted, to the NEOs and any other individuals as determined by the Board from time to time. Under the clawback policy, a clawback may be triggered if an NEO is indicted for or convicted of an act involving gross negligence, fraud, theft, dishonesty or willful misconduct. Among other remedial actions, the enforcement of the clawback policy may involve forfeiture or cancellation of unpaid cash bonus awards or unvested RSUs and recoupment of the value of such awards. The Human Resources and Corporate Governance Committee will continue to keep this policy under review as part of its regular risk review.
Base Salary
Base salary is intended to reflect an executive officer’s position within the corporate structure, his or her years of experience and level of responsibility, and salary norms in the sector and the general marketplace. As such, decisions with respect to base salary levels for executive officers are not based on objective identifiable performance measures but for the most part are determined by reference to competitive market information for similar roles and levels of responsibility, as well as more subjective performance factors such as leadership, commitment, accountability, industry experience and contribution. The Company’s view is that a competitive base salary is a necessary element for retaining qualified executive officers, as it creates a meaningful incentive for individuals to remain at Profound and not be unreasonably susceptible to recruiting efforts by the Company’s competitors.
In determining the base salary of the Named Executive Officers (as defined herein), the Board considered: (i) recruiting and retaining executives critical to the success of Profound and the enhancement of shareholder value; (ii) providing fair and competitive compensation; (iii) balancing the interests of management and Shareholders; and (iv) rewarding performance, both on an individual basis and with respect to operations in general.
Long-term Incentives
Long-term incentives, in the form of Options, are intended to align the interests of Profound’s directors and its executive officers with those of the Shareholders, to provide a long-term incentive that rewards these individuals for their contribution to the creation of shareholder value and to reduce the cash compensation Profound would otherwise have to pay. The Share Option Plan is administered by the Board. In establishing the number of Options to be granted to any particular executive officer, reference was made to the number of Options granted to officers of other companies involved in similar businesses. The Board also considers previous grants of Options and the overall number of Options that are outstanding relative to the number of outstanding Common Shares in determining whether to make any new grants of Options and the size and terms of any such grants, as well as the performance of the executive officer as demonstrated through his or her level of effort, time, responsibility, ability, experience, level of commitment and performance goals in determining the level of incentive share option compensation.
Bonus Awards
The Board will consider whether it is appropriate and in the best interests of the Company to award a discretionary cash bonus to executive officers for the most recently completed financial year and if so, in what amount. A cash bonus may be awarded to reward performance that has led to increased value for Shareholders through property acquisitions or divestitures, the formation of new strategic or joint venture relationships and/or capital raising efforts.
Quantitative performance objectives include the achievement of the Company’s revenue target, departmental and individual goals, which may be quantitative or qualitative in nature. These have been established for each individual executive officer by the Board with alignment of such corporate/individual goals with the CEO and include objectives such as research and product development, company productivity, revenue growth and long-term strategic guidance of the Company. These corporate, departmental and individual goals form the basis for the review of the executive officers and the determination of cash bonuses at the end of each year with the Board. These awards are reviewed yearly to ensure that corporate performance metrics and individual goals are consistent from year to year.
Bonus award payments are based on the following assessment of:
| (a) | whether or not the executive officers have successfully met or exceeded the established corporate, departmental and individual performance metrics and goals; |
89
| (b) | the executive officers’ decisions and actions and whether or not they are aligned with the Company’s long-term growth strategy and have created value for Shareholders; |
| (c) | whether any near-term goals and objectives were not met because the executive officers made decisions in the best long-term interests of the Company or due to factors outside of the executive officers’ control; and/or |
| (d) | additional initiatives undertaken by the executive officers, which were not contemplated in the initial objectives. |
The following targets, as a percentage of base salary, were approved for each NEO for the fiscal year ending December 31, 2024:
Position |
|
Target |
|
CEO |
|
80 |
% |
Other NEOs |
|
20 - 50 |
% |
Benefits Plans
The Named Executive Officers are entitled to life insurance, health and dental benefits.
Performance Graph
The following graph illustrates the cumulative return to Shareholders of a $100 investment in Common Shares from December 31, 2019 to December 31, 2024, as compared to the cumulative total return on the Standard & Poor’s/TSX Index and Standard & Poor’s/Nasdaq Composite Index for the same period, assuming the reinvestment of cash distributions and/or dividends.
|
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
|
2019 |
|
2020 |
|
2021 |
|
2022 |
|
2023 |
|
2024 |
|
|
$ |
|
$ |
|
$ |
|
$ |
|
$ |
|
$ |
Profound Medical |
|
100.00 |
|
177.56 |
|
96.68 |
|
98.17 |
|
75.25 |
|
63.80 |
S&P/TSX Composite Index |
|
100.00 |
|
102.17 |
|
124.38 |
|
113.61 |
|
122.82 |
|
144.92 |
Nasdaq Composite Index |
|
100.00 |
|
143.64 |
|
174.36 |
|
116.64 |
|
167.30 |
|
215.22 |

The trend shown in the above graph does not necessarily correspond to the Company’s trend of compensation for the NEOs (as defined herein) for the period disclosed above. The Company considers a number of factors in connection with its determination of appropriate levels of compensation including, but not limited to, the demand for and supply of skilled professionals with experience in the medical device industry, individual performance, the Company’s performance (which is not necessarily tied exclusively to the trading price of the Common Shares on the TSX and Nasdaq) and other factors discussed herein.
90
Named Executive Officers
The following individuals are considered the “Named Executive Officers” or “NEOs” for the purposes of the disclosure:
| (a) | each individual who, during any part of the most recently completed financial year, served as the Company’s Chief Executive Officer or CEO, including an individual performing functions similar to a CEO; |
| (b) | each individual who, during any part of the most recently completed financial year, served as the Company’s Chief Financial Officer or CFO, including an individual performing functions similar to a CFO; |
| (c) | each of the three most highly compensated executive officers of the Company, including its subsidiaries, or the three highly compensated officers acting in a similar capacity, other than the CEO and CFO, at the end of the most recently completed financial year whose total compensation was more than C$150,000 for the fiscal year ended December 31, 2024; and |
| (d) | each individual who would be a Named Executive Officer under paragraph (c) but for the fact the individual was not an executive officer of the Company and was not acting in a similar capacity as of December 31, 2024. |
Summary Compensation Table
The following table sets forth information concerning the total compensation for the three most recently completed financial years paid to the Named Executive Officers as of the most recently completed financial year. Dr. Menawat is the only officer of the Company that also serves as a director of the Company.
|
|
|
|
|
|
|
|
|
|
Non-Equity Incentive |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
Plan Compensation |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
($) |
|
|
|
|
|
|
||
|
|
|
|
|
|
Share- |
|
Option- |
|
Annual |
|
Long Term |
|
|
|
|
|
|
|
|
|
|
|
|
Based |
|
Based |
|
Incentive |
|
Incentive |
|
Pension |
|
All Other |
|
Total |
|
|
|
|
Salary |
|
Awards |
|
Awards |
|
Plan |
|
Plan |
|
Value |
|
Compensation(1) |
|
Compensation |
Name and Principal Position |
|
Year |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
Arun Menawat |
|
2024 |
|
514,439 |
|
595,395 |
(2) |
1,185,443 |
(5) |
167,737 |
|
— |
|
— |
|
— |
|
1,948,575 |
Chief Executive Officer and Director |
|
2023 |
|
370,244 |
|
454,079 |
(3) |
— |
|
65,625 |
|
— |
|
— |
|
— |
|
889,948 |
|
|
2022 |
|
343,750 |
|
364,503 |
(4) |
— |
|
208,125 |
|
— |
|
— |
|
— |
|
916,378 |
Rashed Dewan(6) |
|
2024 |
|
252,003 |
|
267,923 |
(2) |
740,902 |
(5) |
47,963 |
|
— |
|
— |
|
— |
|
1,308,791 |
Chief Financial Officer |
|
2023 |
|
195,173 |
|
454,079 |
(3) |
— |
|
20,004 |
|
— |
|
— |
|
— |
|
669,256 |
|
|
2022 |
|
206,146 |
|
291,602 |
(4) |
— |
|
44,228 |
|
— |
|
— |
|
— |
|
541,976 |
Mathieu Burtnyk(6) |
|
2024 |
|
252,003 |
|
178,615 |
(2) |
938,475 |
(5) |
47,963 |
|
— |
|
— |
|
— |
|
1,417,056 |
Senior Vice-President, Product Leader TULSA-PRO |
|
2023 |
|
195,173 |
|
181,631 |
(3) |
— |
|
20,004 |
|
— |
|
— |
|
— |
|
396,808 |
|
|
2022 |
|
206,146 |
|
291,602 |
(4) |
— |
|
39,955 |
|
— |
|
— |
|
— |
|
537,703 |
Tom Tamberrino(7) |
|
2024 |
|
66,456 |
|
— |
|
987,869 |
(5) |
— |
|
— |
|
— |
|
— |
|
1,054,325 |
Chief Commercial Officer |
|
2023 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
2022 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
Abbey Goodman(8) |
|
2024 |
|
399,153 |
|
119,074 |
(2) |
— |
|
25,000 |
|
— |
|
— |
|
— |
|
543,227 |
Chief Commercial Officer |
|
2023 |
|
324,346 |
|
181,631 |
(3) |
— |
|
50,000 |
|
— |
|
— |
|
— |
|
555,977 |
|
|
2022 |
|
218,750 |
|
291,602 |
(4) |
— |
|
135,000 |
|
— |
|
— |
|
— |
|
645,352 |
Notes:
| (1) | Nil indicates that perquisites and other personal benefits did not exceed C$50,000 or 10% of the total salary of the NEO for the financial year. |
| (2) | The value shown is the product of the number of RSUs granted multiplied by the Common Share TSX closing price on the grant date of C$12.85. |
| (3) | The value shown is the product of the number of RSUs granted multiplied by the Common Share TSX closing price on the grant date of C$12.38. |
| (4) | The value shown is the product of the number of RSUs granted multiplied by the Common Share TSX closing price on the grant date of C$9.41. |
91
| (5) | Option based awards granted utilize the Black-Scholes option pricing model to determine the fair value. The input factors to determine the fair value were volatility 70%, exercise price C$11.14, interest rate 3.17% and expected life of 6 years. This methodology was chosen to be consistent with the accounting fair value used by the Company in its financial statements and since the Black-Scholes option pricing model is a commonly used methodology for valuing options which provides an objective and reasonable estimate of fair value. |
| (6) | Amounts paid in Canadian dollars and converted to United States dollars for reporting purposes. On December 31, 2024, the exchange rate for Canadian dollars expressed in United States dollars (as reported by the Bank of Canada) was C$1.00 = US$0.695. |
| (7) | Mr. Tamberrino joined the Company on October 14, 2024. |
| (8) | Ms. Goodman was terminated from her role as Chief Commercial Officer effective October 24, 2024. |
Outstanding Option-Based and Share-Based Awards
The following table sets forth information with respect to the unexercised Options granted under the Share Option Plan and RSUs granted under the Company’s long-term incentive plan (the “LTIP”) to the NEOs that were outstanding as of December 31, 2024.
|
|
Option-Based Awards |
|
Share-Based Awards |
||||||||||
|
|
Number of |
|
|
|
|
|
Value of |
|
|
|
Market |
|
Market |
|
|
Common |
|
|
|
|
|
Unexercised |
|
Number |
|
Value of |
|
Value of |
|
|
Shares |
|
Option |
|
|
|
In-the- |
|
of RSUs |
|
RSUs that |
|
Vested RSUs |
|
|
Underlying |
|
Exercise |
|
Option |
|
Money |
|
that have |
|
have not |
|
not paid out |
|
|
Unexercised |
|
Price |
|
Expiration |
|
Options |
|
not |
|
Vested |
|
or Distributed |
Name and Principal Position |
|
Options |
|
(C$) |
|
Date |
|
($)(6) |
|
Vested |
|
($)(7) |
|
($) |
Arun Menawat(1) |
|
93,406 |
|
14.60 |
|
Aug 22, 2026 |
|
— |
|
|
|
|
|
|
Chief Executive Officer and Director |
|
1,650 |
|
13.50 |
|
Sep 15, 2026 |
|
— |
|
|
|
|
|
|
|
|
8,345 |
|
11.00 |
|
Nov 24, 2026 |
|
— |
|
|
|
|
|
|
|
|
35,439 |
|
11.00 |
|
Dec 21, 2026 |
|
— |
|
70,001 |
|
525,428 |
|
— |
|
|
167,392 |
|
9.20 |
|
May 16, 2029 |
|
186,140 |
|
|
|
|
|
|
|
|
98,573 |
|
17.44 |
|
May 20, 2030 |
|
— |
|
|
|
|
|
|
|
|
49,287 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
|
|
|
|
|
|
|
240,000 |
|
11.14 |
|
Nov 18, 2034 |
|
— |
|
|
|
|
|
|
Rashed Dewan(2) |
|
3,000 |
|
15.00 |
|
Sept 8, 2025 |
|
— |
|
|
|
|
|
|
Chief Financial Officer |
|
5,000 |
|
13.50 |
|
July 19, 2026 |
|
— |
|
|
|
|
|
|
|
|
7,500 |
|
11.00 |
|
Nov 24, 2026 |
|
— |
|
|
|
|
|
|
|
|
4,500 |
|
8.50 |
|
Nov 16, 2027 |
|
7,193 |
|
56,668 |
|
425,350 |
|
— |
|
|
2,500 |
|
9.20 |
|
May 16, 2029 |
|
2,780 |
|
|
|
|
|
|
|
|
27,667 |
|
17.44 |
|
May 20, 2030 |
|
— |
|
|
|
|
|
|
|
|
51,533 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
|
|
|
|
|
|
|
150,000 |
|
11.14 |
|
Nov 18, 2034 |
|
— |
|
|
|
|
|
|
Mathieu Burtnyk(3) |
|
3,000 |
|
15.00 |
|
Sept 8, 2025 |
|
— |
|
|
|
|
|
|
President |
|
2,500 |
|
8.50 |
|
Nov 16, 2027 |
|
3,996 |
|
|
|
|
|
|
|
|
42,000 |
|
9.20 |
|
May 16, 2029 |
|
46,704 |
|
36,668 |
|
275,230 |
|
— |
|
|
18,333 |
|
17.44 |
|
May 20, 2030 |
|
— |
|
|
|
|
|
|
|
|
30,867 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
|
|
|
|
|
|
|
190,000 |
|
11.14 |
|
Nov 18, 2034 |
|
— |
|
|
|
|
|
|
Tom Tamberrino(4) |
|
200,000 |
|
11.14 |
|
Nov 18, 2034 |
|
— |
|
— |
|
— |
|
— |
Chief Commercial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbey Goodman(5) |
|
15,000 |
|
11.23 |
|
Nov 18, 2029 |
|
— |
|
|
|
|
|
|
Chief Commercial Officer |
|
10,000 |
|
15.15 |
|
Mar 12, 2030 |
|
— |
|
|
|
|
|
|
|
|
15,333 |
|
17.44 |
|
May 20, 2030 |
|
— |
|
— |
|
— |
|
— |
|
|
53,696 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
|
|
|
|
|
Notes:
| (1) | Dr. Menawat holds 694,092 Options, with 448,948 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (2) | Mr. Dewan holds 251,700 Options, with 96,329 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (3) | Dr. Burtnyk holds 286,700 Options, with 93,498 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (4) | Mr. Tamberrino holds 200,000 Options, with nil of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
92
| (5) | As of December 31, 2024, Ms. Goodman held 94,029 Options, with 94,029 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (6) | The value shown is the product of the number of Common Shares underlying the Option multiplied by the difference between the Common Share TSX closing price on December 31, 2024 of C$10.80 and the exercise price. |
| (7) | The value shown is the product of the number of outstanding RSUs multiplied by the Common Share TSX closing price on December 31, 2024 of C$10.80. |
Incentive Plan Awards — Value Vested or Earned During the Year Ended December 31, 2024
The following table sets forth information with respect to the value of Options vested during the year ended December 31, 2024 as well as the cash bonuses granted to the NEOs during the year ended December 31, 2024.
|
|
|
|
|
|
Non-Equity Incentive |
|
|
Option-Based Awards |
|
Share-Based Awards |
|
Plan Compensation |
|
|
Value Vested During |
|
Value Vested During |
|
Value earned during the |
|
|
Year |
|
Year |
|
year |
Name and Principal Position |
|
($)(1) |
|
($)(2) |
|
($) |
Arun Menawat Chief Executive Officer and Director |
|
— |
|
595,395 |
|
167,737 |
Rashed Dewan Chief Financial Officer |
|
— |
|
267,923 |
|
47,963 |
Mathieu Burtnyk President |
|
— |
|
178,615 |
|
47,963 |
Tom Tamberrino Chief Commercial Officer |
|
— |
|
— |
|
— |
Abbey Goodman Chief Commercial Officer |
|
— |
|
119,074 |
|
25,000 |
Notes:
| (1) | The value shown is the product of the number of Common Shares underlying the Options that vested during the year multiplied by the difference between the Common Share TSX closing price on the day the Options vested and the exercise price of the Options that vested. |
| (2) | The value shown is the product of the number of Common Shares underlying the RSUs that vested during the year multiplied by the Common Share TSX closing price on the day the RSUs vested. |
Termination and Change of Control Benefits
Each of Dr. Menawat, Mr. Dewan, Dr. Burtnyk, Mr. Tamberrino and Ms. Goodman are a party to an executive employment agreement (the “Executive Employment Agreements”) with the Company. The Executive Employment Agreements have an indefinite term and contain standard confidentiality and non-solicitation provisions. Profound has agreed pursuant to the Executive Employment Agreements that each of Dr. Menawat, Mr. Dewan, Dr. Burtnyk, Mr. Tamberrino and Ms. Goodman will receive base salaries determined by the Board and may receive discretionary bonuses, grants of Options, grants of RSUs, reimbursement of expenses, benefits and certain perquisites as set forth in the Executive Employment Agreements, with the amounts paid in 2024 with respect to such matters set forth in the Summary Compensation Table.
93
The following table sets forth information with respect to the estimated aggregate dollar amount to which each current NEO would have been entitled if the event resulting in termination of employment occurred on December 31, 2024.
|
|
|
|
|
|
|
Value of |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonus and |
|
Value of |
|
|
|
||
|
|
|
|
Cash |
|
other |
|
Option |
|
|
|
|||
Name |
|
Triggering Event |
|
Payment |
|
Benefits |
|
Awards |
|
Total Payout |
||||
Arun Menawat |
|
Termination with cause/resignation |
|
|
— |
(1) |
|
— |
|
|
— |
(2) |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination without cause/Termination with a change of control |
|
$ |
550,000 |
(4) |
$ |
440,000 |
|
$ |
186,140 |
(2) |
$ |
1,176,140 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rashed Dewan |
|
Termination with cause/resignation |
|
|
— |
(1) |
|
— |
|
|
— |
(2) |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination without cause/Termination with a change of control |
|
$ |
300,000 |
(5) |
$ |
112,195 |
(3) |
$ |
9,973 |
(2) |
$ |
422,168 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mathieu Burtnyk |
|
Termination with cause/resignation |
|
|
— |
(1) |
|
— |
|
|
— |
(2) |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination without cause/Termination with a change of control |
|
$ |
150,000 |
(6) |
$ |
107,922 |
(3) |
$ |
50,700 |
(2) |
$ |
308,622 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tom Tamberrino |
|
Termination with cause/resignation |
|
|
— |
(1) |
|
— |
|
|
— |
(2) |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination without cause/Termination with a change of control |
|
$ |
150,000 |
(7) |
|
— |
(3) |
|
— |
(2) |
$ |
150,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbey Goodman |
|
Termination with cause/resignation |
|
|
— |
(1) |
|
— |
|
|
— |
(2) |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination without cause/Termination with a change of control |
|
$ |
150,000 |
(8) |
|
— |
(3) |
|
— |
(2) |
$ |
150,000 |
Notes:
| (1) | In the event of a termination for just cause or resignation, the Company shall have no further obligation to Dr. Menawat, Mr. Dewan, Dr. Burtnyk, Mr. Tamberrino or Ms. Goodman, as applicable, other than the payment of unpaid base salary, any bonus declared but not yet paid, plus all outstanding vacation pay and expense reimbursement. |
| (2) | The value shown is the product of the number of Common Shares underlying the vested Options multiplied by the difference between the Common Share TSX closing price on December 31, 2024 of C$10.80 and the exercise price. |
| (3) | The value shown is a sum of the semi annual cost of benefits and the average cash bonus paid in respect of the years ended December 31, 2024, 2023 and 2022. |
| (4) | If Dr. Menawat’s employment is terminated without cause, he is entitled to twelve months of pay of base salary in lieu of notice and an amount equal to the then current target annual bonus prorated based on the number of days elapsed in the calendar year until the date of termination as a percentage of the total number of days in such calendar year. |
| (5) | If Mr. Dewan’s employment is terminated without cause, he is entitled to the greater of: (i) twelve months’ notice; or (ii) the minimum notice (or pay in lieu) and minimum severance, if any, to which he would be entitled under employments standards legislation. |
| (6) | If Dr. Burtnyk’s employment is terminated without cause, he is entitled to six months’ notice and minimum severance, if any, to which he would be entitled under employments standards legislation. |
| (7) | If Mr. Tamberrino’s employment is terminated without cause, he is entitled to the greater of: (i) six months’ notice; or (ii) the minimum notice (or pay in lieu) and minimum severance, if any, to which he would be entitled under employments standards legislation. |
| (8) | Ms. Goodman was terminated from her role as Chief Commercial Officer effective October 24, 2024. In connection with her termination, Ms. Goodman received a cash payment of $150,000, equal to six months’ notice. |
94
Director Compensation
The directors of the Company, other than the current CEO, were paid an annual fee of $50,000 for their services in respect of the financial year-ended December 31, 2024. The Chair of the Audit Committee is entitled to an additional annual fee of $5,000 and the Chair of the Human Resources and Corporate Governance Committee is entitled to an additional annual fee of $5,000. Audit Committee members are entitled to an additional annual fee of $2,500 and Human Resources and Corporate Governance Committee members are entitled to an additional annual fee of $2,500. Directors of the Company are also eligible to receive Options and/or deferred share units (“DSUs”) as an initial grant when joining the Board and on an annual basis. Except as set out below, directors are not eligible to receive other compensation.
Summary Compensation Table
The following table sets forth information concerning compensation paid to the non-executive directors for the year ended December 31, 2024.
|
|
|
|
Option-based |
|
Share-based |
|
All Other |
|
|
|
|
Fees Earned |
|
awards |
|
awards |
|
Compensation |
|
Total |
Name |
|
($) |
|
($) |
|
($)(1) |
|
($) |
|
($) |
Brian Ellacott |
|
57,500 |
|
— |
|
38,468 |
|
— |
|
95,968 |
Cynthia Lavoie |
|
52,500 |
|
— |
|
38,468 |
|
— |
|
90,968 |
Murielle Lortie |
|
55,000 |
|
— |
|
38,468 |
|
— |
|
93,468 |
Arthur Rosenthal |
|
57,500 |
|
— |
|
38,468 |
|
— |
|
95,968 |
Kris Shah |
|
52,500 |
|
— |
|
38,468 |
|
— |
|
90,968 |
Notes:
| (1) | The directors were granted 5,000 DSUs each. The value shown is the product of the number of DSUs issued multiplied by the Common Share TSX closing price on the grant date, November 18, 2024, of C$11.07. |
95
Outstanding Option-Based and Share-Based Awards
The following table sets forth information with respect to the unexercised Options granted under the Share Option Plan and DSUs granted under the LTIP to the non-executive directors that were outstanding as of December 31, 2024.
|
|
Option-Based Awards |
|
Share-Based Awards |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Market |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
or payout |
|
Market or |
|
|
Number of |
|
|
|
|
|
Value of |
|
|
|
value of |
|
payout value |
|
|
Common |
|
|
|
|
|
Unexercised |
|
Number |
|
DSUs |
|
of vested |
|
|
Shares |
|
Option |
|
|
|
In-the- |
|
of DSUs |
|
that have |
|
DSUs not |
|
|
Underlying |
|
Exercise |
|
Option |
|
Money |
|
that have |
|
not |
|
paid out or |
|
|
Unexercised |
|
Price |
|
Expiration |
|
Options |
|
not |
|
vested |
|
distributed |
Name |
|
Options |
|
(C$) |
|
Date |
|
($)(5) |
|
Vested |
|
($) |
|
($)(6) |
Brian Ellacott(1) |
|
3,300 |
|
10.20 |
|
June 15, 2028 |
|
1,376 |
|
|
|
|
|
|
|
|
10,000 |
|
11.23 |
|
Nov 18, 2029 |
|
— |
|
|
|
|
|
|
|
|
10,000 |
|
17.44 |
|
May 20, 2030 |
|
— |
|
8,334 |
|
62,555 |
|
75,060 |
|
|
10,000 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
|
|
|
|
|
Cynthia Lavoie(2) |
|
10,000 |
|
28.16 |
|
March 3, 2031 |
|
— |
|
|
|
|
|
|
|
|
10,000 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
8,334 |
|
62,555 |
|
75,060 |
Murielle Lortie(3) |
|
10,000 |
|
23.02 |
|
Dec 15, 2030 |
|
— |
|
|
|
|
|
|
|
|
10,000 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
8,334 |
|
62,555 |
|
75,060 |
Arthur Rosenthal(4) |
|
3,300 |
|
10.20 |
|
June 15, 2028 |
|
1,376 |
|
|
|
|
|
|
|
|
10,000 |
|
11.23 |
|
Nov 18, 2029 |
|
— |
|
|
|
|
|
|
|
|
10,000 |
|
17.44 |
|
May 20, 2030 |
|
— |
|
8,334 |
|
62,555 |
|
75,060 |
|
|
10,000 |
|
22.08 |
|
May 21, 2031 |
|
— |
|
|
|
|
|
|
Kris Shah |
|
— |
|
— |
|
— |
|
— |
|
8,334 |
|
62,555 |
|
75,060 |
Notes:
| (1) | Mr. Ellacott holds 33,300 Options, with 32,264 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (2) | Dr. Lavoie holds 20,000 Options, with 18,345 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (3) | Ms. Lortie holds 20,000 Options, with 18,964 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (4) | Dr. Rosenthal holds 33,300 Options, with 32,264 of these Options vested and exercisable and the remaining balance vesting over a three year period from their respective grant dates. |
| (5) | The value shown is the product of the number of Common Shares underlying the Option multiplied by the difference between the Common Share TSX closing price on December 31, 2024 of C$10.80 and the exercise price. |
| (6) | The value shown is the product of the number of outstanding DSUs multiplied by the Common Share TSX closing price on December 31, 2024 of C$10.80. |
96
Incentive Plan Awards — Value Vested or Earned During the Year Ended December 31, 2024
The following table sets forth information with respect to the value of Options vested during the year ended December 31, 2024 as well as the cash bonuses granted to non-executive directors during the year ended December 31, 2024.
|
|
Option-Based Awards |
|
Share-Based Awards |
|
Non-Equity Incentive Plan |
|
|
Value Vested During |
|
Value Vested During the |
|
Compensation Value |
|
|
Year |
|
Year |
|
earned during the year |
Name |
|
($)(1) |
|
($)(2) |
|
($) |
Brian Ellacott |
|
— |
|
12,899 |
|
— |
Cynthia Lavoie |
|
— |
|
12,899 |
|
— |
Murielle Lortie |
|
— |
|
12,899 |
|
— |
Arthur Rosenthal |
|
— |
|
12,899 |
|
— |
Kris Shah |
|
— |
|
12,899 |
|
— |
Notes:
| (1) | The value shown is the product of the number of Common Shares underlying the Options that vested during the year multiplied by the difference between the Common Share TSX closing price on the day the Options vested and the exercise price of the Options that vested. |
| (2) | The value shown is the product of the number of Common Shares underlying the DSUs that vested during the year multiplied by the Common Share TSX closing price on the day the DSUs vested. |
Share Option Plan
The Company’s amended and restated share option plan (the “Share Option Plan”) is administered by the Board which may, from time to time, delegate to a committee of the Board, all or any of the powers conferred to the Board under the Share Option Plan. The Share Option Plan was originally adopted by the Board on June 4, 2015, and then amended and restated on December 8, 2016 and again on July 13, 2018.
The amendments made on July 13, 2018 were as follows: (i) inclusion of the Insider Participation Limits (as defined herein); (ii) removal of TSX Venture Exchange (“TSXV”) required participation limits since the Company was no longer listed on the TSXV; (iii) clarification to the share reserve since the Company was listed on the TSX and pursuant to the Share Option Plan, the reserve changed from a fixed number to a fixed percentage as described below; (iv) inclusion of an additional amendment to the list of amendments that require Shareholder approval (being removing or exceeding the Insider Participation Limits); and (v) other amendments of a housekeeping nature.
The Share Option Plan provides that the Board may from time to time, in its discretion, grant to directors, officers, employees, consultants and any other person or entity engaged to provide ongoing services to the Company non-transferable Options, provided that the maximum number of Common Shares reserved for issuance under the Share Option Plan is equal to 13% of the issued and outstanding shares in the capital of the Company at the time of any Option grant. If any Option is exercised, cancelled, expired, surrendered or otherwise terminated for any reason, the number of Common Shares in respect of which the Option is exercised, cancelled, expired, surrendered or otherwise terminated, as the case may be, will again be available for purchase pursuant to Options granted under the plan. As at December 31, 2024, 1,326,573 Options have been granted under the Share Option Plan, which represents 4.4% of the issued and outstanding Common Shares of the Company as at December 31, 2024. As at December 31, 2024, 2,578,602 Options are available for grant under the Share Option Plan, which represents 8.6% of the issued and outstanding shares in the capital of the Company as at December 31, 2024.
The aggregate number of Common Shares that may be (i) issued to insiders of the Company within any one-year period, or (ii) issuable to insiders of the Company at any time, in each case, under the Share Option Plan alone or when combined with all other security-based compensation arrangements of the Company, cannot exceed 10% of the outstanding Common Shares (the “Insider Participation Limits”).
The Board shall determine the exercise price of the Options, provided that, it cannot be less than the Market Price of the Common Shares on the date of grant. For the purposes of the Share Option Plan, “Market Price” means the volume-weighted average price of the Common Shares on the stock exchange where the majority of trading volume and value of the Common Shares occurs, for the five trading days immediately preceding the relevant date on which the Market Price is to be determined.
97
The expiry date for an Option shall not be later than the 10th anniversary of the date an Option is granted, subject to the expiry date falling with a corporate blackout period or within 5 business days following the expiry of such a blackout period, in which case the expiry date will be extended to the 10th business day following the expiry of the blackout period.
Unless otherwise specified by the Board, each Option generally vests and becomes exercisable as to 1/4 on the first anniversary of the date of grant and as to 1/36 on the first day of each calendar month thereafter. The Board has the discretion to permit accelerated vesting of Options.
The Company does not provide any financial assistance to optionees to facilitate the purchase of Common Shares issued pursuant to the exercise of Options under the Share Option Plan. Options granted under the Share Option Plan are not transferable or assignable (except to an optionee’s estate) and no Options may be exercised by anyone other than the optionee or his or her legal representative during the lifetime of the optionee.
The Share Option Plan contains the following provisions regarding the exercise and cancellation of Options following a change in the employment status of an optionee. In the event of:
| (a) | an optionee’s retirement, the optionee will continue to participate in the plan and each Option that has vested or that vests within 12 months following the retirement date continues to be exercisable until the earlier of the Option’s expiry date and the date that is 12 months from the retirement date, and any Options that have not been exercised by such time will immediately expire and be cancelled; |
| (b) | an optionee’s death or disability, each vested Option is exercisable until the earlier of the Option’s expiry date and 6 months following the date of death or disability, as applicable, and any Options that have not been exercised by such time will immediately expire and be cancelled; |
| (c) | a termination without cause for an employee optionee, or the termination by the Company or an affiliate of a consulting agreement or arrangement (other than for breach) or the death or disability of a consultant, each vested Option is exercisable until the earlier of the Option’s expiry date and 90 days following the date of termination, death or disability, as applicable, and any Options that have not been exercised by such time will immediately expire and be cancelled; |
| (d) | a termination for cause or resignation of an employee optionee, or the termination by the Company or an affiliate of a consulting agreement or arrangement (for breach) or the voluntary termination by the consultant, all Options (whether vested or unvested) terminate on the date of termination or resignation, as applicable; and |
| (e) | a director (who is not an employee or consultant) ceases to hold office, each vested Option is exercisable until the earlier of the Option’s expiry date and 60 days following the cessation date, and any Options that have not been exercised by such time will immediately expire and be cancelled. |
The Board may from time to time, without notice and without Shareholder approval, amend, modify, change, suspend or terminate the Share Option Plan or any Options granted thereunder as it, in its discretion determines appropriate, provided, however, that no such amendment, modification, change, suspension or termination of the Share Option Plan or any Option granted thereunder may materially impair any rights of an optionee or materially increase any obligations of an optionee under the plan without the consent of the optionee, unless the Board determines such adjustment is required or desirable in order to comply with any applicable securities laws or stock exchange requirements. Amendments that can be made by the Board without Shareholder approval include, but are not limited to, housekeeping amendments, amendments to comply with applicable law or stock exchange rules, amendments necessary for Options to qualify for favorable treatment under applicable tax laws, amendments to the vesting provisions of the Share Option Plan or any Option, amendments to include or modify a cashless exercise feature, amendments to the termination or early termination provisions of the Share Option Plan or any Option, and amendments necessary to suspend or terminate the Share Option Plan.
98
Shareholder approval is required for the following amendments to be made to the Share Option Plan:
| (a) | increase to the number of Common Shares reserved for issuance under the Share Option Plan, except pursuant to the provisions in the plan that permit the Board to make equitable adjustments in the event of transactions affecting the Company or its capital; |
| (b) | reduce the exercise price of an Option, except pursuant to the provisions in the plan that permit the Board to make equitable adjustments in the event of transactions affecting the Company or its capital; |
| (c) | extend the term of an Option beyond the original expiry date, except where an expiry date would have fallen within a blackout period or within 5 business days following the expiry of such a blackout period; |
| (d) | permit an Option to be exercisable beyond 10 years from its date of grant, except where an expiry date would have fallen within a blackout period; |
| (e) | permit Options to be transferred other than for normal estate settlement purposes; |
| (f) | remove or exceeds the Insider Participation Limits; |
| (g) | permit awards, other than the Options, to be granted under the Share Option Plan; or |
| (h) | delete or reduce the range of amendments which require Shareholder approval. |
As required by section 613 of the TSX Company Manual, the Company’s annual burn rate, which represents the number of Options granted under the Share Option Plan divided by the weighted average number of Common Shares outstanding as at the end of a fiscal year, was 7.3% in 2022, 6.9% in 2023 and 5.4% in 2024.
Description of the Company’s LTIP
On May 20, 2020, the Shareholders approved the adoption of the LTIP. The LTIP is an incentive-based equity compensation plan that provides for the grant of RSUs and DSUs.
The RSUs may be granted to any director, officer, employee or consultant of the Company or any of its affiliates and any such person’s personal holding company, as designated by the Board in a resolution (the “RSU Participants”) upon the terms and conditions set forth in a grant agreement. The DSUs may be granted to any director of the Company who has been designated by the Company for participation in the LTIP and who has agreed to participate in the LTIP (the “DSU Participants”, together with the RSU Participants, the “Participants”), upon the terms and conditions set forth in a grant agreement. Subject to Board approval, once each fiscal year, a DSU Participant may elect to be paid up to 100% of this or her annual board retainer in the form of DSUs, with the remaining balance (if any) being paid in cash.
The LTIP was amended and restated by the Board on April 3, 2023, with respect to the revisions to the LTIP’s amendment provision intended to more closely track the TSX amendment provision requirements and governance best practices (as further described below). Other than the revisions to the amendment provision, none of the amendments made to the LTIP required Shareholder approval. The amendments made to the LTIP that did not require Shareholder approval include decreasing the maximum number of Common Shares issuable pursuant to the LTIP from 13% of the outstanding Common Shares to 4.9% of the outstanding Common Shares, provided that, the maximum number of Common Shares which may be reserved for issuance pursuant to all of the Company’s security-based compensation arrangements shall not in the aggregate exceed 13% of the issued and outstanding Common Shares including new non-employee director participation limits, including a new default vesting schedule for DSUs, permitting DSU Participants holding vested DSUs to elect settlement timing (subject to certain restrictions), providing the Company with the ability to elect whether vested DSUs are settled in Common Shares or cash, including a clawback provision, and other amendments of an administrative or “housekeeping” nature. These amendments do not require Shareholder approval because of the LTIP’s amendment provision that allows these amendments to be made without Shareholder approval.
99
The LTIP is intended to advance the interests of the Company by: (i) providing Participants with additional incentives; (ii) rewarding the performance of the Participants through the issuance of the Units; (iii) increasing the proprietary interest of the Participants in the success of the Company; (iv) encouraging the Participants to remain with the Company or its affiliates; and (v) attracting new directors, employees, officers and consultants to the Company or its affiliates.
The LTIP will be administered by the Board and the Human Resources and Corporate Governance Committee. The Board is responsible for, among other things, granting the RSUs to the RSU Participants, granting the DSUs to the DSU Participants, determining the terms of such grants, and interpreting the LTIP and all agreements entered into thereunder. Pursuant to the LTIP, the number of RSUs (including fractional RSUs) granted at any particular time will be calculated by dividing (i) the dollar amount of such grant by (ii) the market value of a Common Share on the applicable grant date, which is equal to the volume weighted average trading price of all Common Shares traded on the TSX (or other exchange where the Common Shares are listed) for the five (5) trading days immediately preceding such date (the “Market Value”). The number of DSUs (including fractional DSUs) granted at any particular time will be calculated by dividing (i) the dollar amount of such grant by (ii) the Market Value of a Common Share on the applicable grant date.
The RSUs will vest 1/3 on each of the first, second and third anniversary dates of the original grant, provided that the RSU Participant is continuously employed by or in service with the Company, or any of its affiliates, until the respective vesting date. The Board would have the option to add any performance-based vesting criteria at its discretion. After the RSUs have vested, a Canadian RSU Participant may deliver a settlement notice to the Company in respect of any or all vested RSUs it desires to settle. U.S. RSU Participants must settle any vested RSUs within 70 days after such RSUs vested unless otherwise specified in the grant agreement. The Company may elect to settle the vested RSUs in cash, in Common Shares issued from treasury, or a combination thereof. Since the form of settlement (i.e. cash and/or Common Shares) is at the option of the Company, all RSUs must settle no later than December 31 in the third calendar year following the year in which the services giving rise to the RSUs were rendered.
DSUs granted prior to April 3, 2023 and any DSUs that a director elects to receive in lieu of annual cash board retainers will vest on the DSU termination date, which is the date on which the DSU Participant ceases to be a director and, if applicable, an employee of the Company for any reason. DSUs granted on or after April 3, 2023 will vest 1/3 on each of the first, second and third anniversary dates of the original grant, provided that the DSU Participant is continuously in service with the Company, or any of its affiliates, until the respective vesting date.
After the DSUs have vested and following a DSU Participant ceasing to hold all positions with the Company, a Participant may elect when to settle the Participant’s vested DSUs (subject to certain restrictions) and the Company will elect to settle such DSUs in such DSU Participant’s notional account for cash, Common Shares issued from treasury, or a combination thereof. U.S. DSU Participant’s shall settle any vested DSUs within 70 days on the date from such DSU Participant incurs a “separation from service” within the meaning of Section 409A of U.S. Internal Revenue Code of 1986.
The maximum number of Common Shares which may be reserved for issuance under the LTIP cannot exceed 4.9% of the issued and outstanding Common Shares from time to time on a non-diluted basis (representing an aggregate of 1,471,951 Common Shares as at December 31, 2024), provided that the Board may make appropriate adjustments in the Common Shares issuable or amounts payable to preclude a dilution or enlargement of the benefits under the LTIP as a result of a consolidation, share split or similar change in the capital structure of the Company, subject to any required approval by any stock exchange or regulatory authority. As at December 31, 2024, an aggregate of 416,291 Units, representing approximately 1.4% of the issued and outstanding Common Shares, are outstanding under the LTIP. As at December 31, 2024, an aggregate of 1,055,660 Units, representing approximately 3.5% of the issued and outstanding Common Shares, are available for grant under the LTIP.
Certain other restrictions on grants apply, including that: (i) the number of Common Shares issuable to insiders (as a group), at any time, under the LTIP and any other security-based compensation arrangements, including the Share Option Plan, shall not exceed 10% of the Company’s issued and outstanding Common Shares; (ii) the number of Common Shares issued to insiders (as a group), within a one-year period, under the LTIP and any other security-based compensation arrangements cannot exceed 10% of the Company’s issued and outstanding Common Shares; and (iii) the Company is prohibited from granting to any individual non-employee director of the Company more than $150,000 worth of awards under any security-based compensation arrangement of the Company (with no more than $100,000 attributable to stock options) annually based on the grant date fair value of the Units, other than in respect of awards granted to non-employee directors in lieu of cash fees on a value for value basis.
100
Under the LTIP, the Company will not provide financial assistance to Participants in connection with the settlement of Units by Participants. Except as the Board may otherwise determine, if a RSU Participant ceases to be a RSU Participant for any reason, including, without limitation, as a result of his or her resignation, voluntary or involuntary termination (including with or without cause), retirement, disability, or death, any unvested RSUs held by such RSU Participant shall expire. Each DSU Participant is entitled to terminate his or her participation in the LTIP by filing a termination notice with the designated officer of the Company. Thereafter, any portion of such DSU Participant’s annual board retainer payable and all subsequent annual board retainers shall be paid in cash.
In no event may the rights or interests of a Participant under the LTIP be assigned, encumbered, pledged, transferred or alienated in any way, except to the extent that certain rights may pass to a beneficiary or legal representative upon death of a Participant, by will or by the laws of succession and distribution.
Any Unit which is subject to recovery, cancellation, forfeiture, revocation or recoupment under applicable laws, stock exchange listing requirements or policies adopted by the Company, including the Company’s clawback policy, will be subject to such deductions, cancellations, forfeitures, revocations, recoupments and clawbacks as may be required pursuant to such laws, stock exchange listing requirements or policies.
In the event of a change of control, merger, amalgamation, arrangement, business combination or other transaction pursuant to which the Common Shares of the Company are converted into, or exchanged for, other property, whether in the form of securities of another entity, cash or otherwise, any surviving or acquiring company must, (i) assume any Unit outstanding under the LTIP on substantially the same economic terms and conditions as the LTIP; or (ii) substitute or replace restricted share units and deferred share units, as applicable for those RSUs and DSUs outstanding under the LTIP on substantially the same economic terms and conditions. In the event any surviving or acquiring company neglects or refuses (as determined by the Board, acting reasonably) to assume any Units or to substitute or replace similar restricted share units and deferred share units, as applicable, for those outstanding RSUs and DSUs in connection with a such an event, then with respect to any Units held by Participants, the vesting of such Units will automatically accelerate and be fully vested. Additionally, the Board may, in its discretion: (a) terminate, conditionally or otherwise and on such terms as it sees fit, the RSUs not settled following successful completion of such event; and (b) accelerate, conditionally or otherwise and on such terms as it sees fit, the vesting of Units or otherwise modify the terms of the Units to assist the Participants to obtain the advantage of holding Common Shares during the event.
In the event of a potential change of control following a take-over bid, the Board may, in its discretion, conditionally or otherwise and on such terms as it sees fit, accelerate the vesting of all of a Participant’s unvested Units to a date prior to the expiry date of such take-over bid or offer, such that all of a Participant’s Units will immediately vest at such time. In such event, all RSUs so vested may be settled conditionally or otherwise, from such date until their respective expiry date so as to permit the Participant to tender the Common Shares received upon such settlement pursuant to the take-over bid or offer.
The Board may make certain amendments to the LTIP or to any Unit outstanding thereunder without seeking shareholder approval, including, but not limited to, housekeeping amendments, amendments to comply with applicable law or stock exchange rules, amendments necessary for Units to qualify for favorable treatment under applicable tax laws, amendments to the vesting provisions of the LTIP or any Unit, amendments to the termination or early termination provisions of the LTIP or any Unit, and amendments necessary to suspend or terminate the LTIP. Only the following types of amendments will not be able to be made without obtaining shareholder approval:
| ● | increasing the number of Common Shares reserved for issuance under the LTIP (other than as a result of a share split or similar change in the capital structure of the Company); |
| ● | permitting awards to be transferred or assigned other than for normal estate settlement purposes; |
| ● | permitting the introduction or reintroduction of non-employee directors as participants on a discretionary basis or increasing limits previously imposed on non-employee director participation; |
| ● | removing or exceeding the participation limits on insiders; |
| ● | amendments which delete or reduce the range of amendments which require approval by the Shareholders; and |
101
| ● | amendments required to be approved by shareholders under applicable laws or the rules, regulations and policies of any stock exchange on which the Common Shares are listed. |
As required by section 613 of the TSX Company Manual, the Company’s annual burn rate, which represents the number of Units granted under the LTIP divided by the weighted average number of Common Shares outstanding as at the end of a fiscal year, was 2.4% in 2022, 2.7% in 2023 and 1.7% in 2024.
Policies and Practices Related to the Grant of Certain Equity Awards
Our equity awards, including stock options, are granted in connection with regularly scheduled meetings of the Human Resources and Corporate Governance Committee and the Board which are scheduled in March and August after the release of our quarterly financial results for the prior quarter. Our trading black-out period normally lifts after two trading days following such release of information. The Human Resources and Corporate Governance Committee may also grant equity awards to individuals upon hire or promotion to executive officer positions, which awards are granted on a quarterly basis. These equity awards are not granted during any trading black-out periods. The Human Resources and Corporate Governance Committee does not grant equity awards in anticipation of the release of material non-public information. Similarly, we do not time the release of material non-public information based on equity award grant dates.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information with respect to the beneficial ownership of our common shares as of March 7, 2025 for:
| ● | each person or group of affiliated persons known by us to be the beneficial owner of more than five percent of our capital stock; |
| ● | each of our directors; |
| ● | each of our named executive officers; and |
| ● | all of our current directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Under those rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. Except as noted by footnote, and subject to community property laws where applicable, we believe, based on the information provided to us, that the persons and entities named in the table below have sole voting and investment power with respect to all common shares shown as beneficially owned by them.
The percentage of beneficial ownership in the table below is based on 30,039,809 common shares outstanding as of March 7, 2025. Options to purchase common shares that are exercisable within 60 days of March 7, 2025 are deemed to be beneficially owned by the persons holding these options for the purpose of computing percentage ownership of that person, but are not treated as outstanding for the purpose of computing any other person’s ownership percentage.
102
Unless otherwise indicated, we believe that each person named in the table below has sole voting and investment power with respect to all common shares beneficially owned by them. Unless otherwise indicated, the business address of each of the following entities or individuals is c/o Profound Medical Corp., 2400 Skymark Avenue, Unit 6, Mississauga, Ontario, Canada, L4W5K5.
|
|
Shares Beneficially |
|
Percentage of Shares |
|
Name of Beneficial Owner |
|
Owned |
|
Beneficially Owned |
|
Greater than 5% Stockholders: |
|
|
|
|
|
Neil Gagnon (Gagnon Securities LLC)(1) |
|
3,346,743 |
|
11.2 |
% |
Named Executive Officers and Directors: |
|
|
|
|
|
Arun Menawat(2) |
|
1,016,502 |
|
3.3 |
% |
Rashed Dewan(3) |
|
147,462 |
|
0.5 |
% |
Mathieu Burtnyk(4) |
|
128,324 |
|
0.4 |
% |
Tom Tamberrino(5) |
|
13,333 |
|
0.0 |
% |
Brian Ellacott(6) |
|
101,300 |
|
0.3 |
% |
Cynthia Lavoie(7) |
|
25,798 |
|
0.1 |
% |
Murielle Lortie(8) |
|
22,948 |
|
0.1 |
% |
Arthur Rosenthal(9) |
|
50,600 |
|
0.2 |
% |
Kris Shah |
|
— |
|
0.0 |
% |
All current executive officers and directors as a group (9 persons) |
|
2,544,421 |
|
8.3 |
% |
Notes:
(1) |
Based on the Schedule 13G/A filed by Gagnon Securities LLC on January 23, 2025. Consists of shares held by Gagnon Securities LLC, Gagnon Advisors, LLC and Neil Gagnon. Mr. Gagnon is the managing member and principal owner of Gagnon Securities LLC and may be deemed to share voting power with respect to 1,771,667 shares and dispositive power with respect to 1,920,060 shares held in customer accounts of Gagnon Securities LLC. Gagnon Securities LLC and Mr. Gagnon expressly disclaim beneficial ownership of all securities held in the accounts. Mr. Gagnon is the Chief Executive Officer of Gagnon Advisors, LLC. Mr. Gagnon and Gagnon Advisors, in its role as investment manager to Gagnon Investment Associates, LLC (“GIA”), a private investment fund, may be deemed to share voting and dispositive power with respect to the 896,671 shares held by GIA. Gagnon Advisors and Mr. Gagnon expressly disclaim beneficial ownership of all securities held by GIA. The business address of each of these entities or individuals 1370 Ave. of Americas, 26th Floor, New York, NY 10019. |
(2) |
Consists of (i) 563,448 common shares held by Dr. Menawat, (ii) options to purchase 453,054 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Dr. Menawat. |
(3) |
Consists of (i) 46,839 common shares held by Mr. Dewan, (ii) options to purchase 100,623 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Mr. Dewan. |
(4) |
Consists of (i) 32,252 common shares held by Dr. Burtnyk, (ii) options to purchase 96,072 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Dr. Burtnyk. |
(5) |
Consists of 13,333 common shares held by Mr. Tamberrino. |
(6) |
Consists of (i) 68,000 common shares held by Mr. Ellacott, (ii) options to purchase 33,300 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Mr. Ellacott. |
(7) |
Consists of (i) 6,000 common shares held by Dr. Lavoie, (ii) options to purchase 19,798 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Dr. Lavoie. |
(8) |
Consists of (i) 3,150 common shares held by Ms. Lortie, (ii) options to purchase 19,798 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Ms. Lortie. |
(9) |
Consists of (i) 17,300 common shares held by Dr. Rosenthal, (ii) options to purchase 33,300 common shares issuable upon the exercise of options to purchase common shares exercisable within 60 days of March 7, 2025 held by Dr. Rosenthal. |
103
Securities Authorized for Issuance under Equity Incentive Plans
Equity Compensation Plan Information
The following table provides certain aggregate information with respect to all of the Company’s equity compensation plans in effect as of December 31, 2024.
|
|
Number of securities to |
|
Weighted-average |
|
Number of securities |
|
|
|
|
be issued upon exercise |
|
exercise price of |
|
remaining available for |
|
|
|
|
of outstanding options, |
|
outstanding options, |
|
future issuance under |
|
|
Plan Category |
|
warrants and rights |
|
warrants and rights |
|
equity compensation plans |
|
|
Equity compensation plans approved by securityholders |
|
2,707,443 |
(1) |
C$ |
14.13 per Common Share |
(2) |
1,197,732 |
(3) |
Equity compensation plans not approved by securityholders |
|
— |
|
|
— |
|
— |
|
Total |
|
2,707,443 |
|
C$ |
14.13 per Common Share |
|
1,197,732 |
(4) |
Notes:
(1) |
Consists of options to purchase 2,291,152 Common Shares outstanding under the Share Option Plan, and 416,291 Common Shares subject to RSUs and DSUs outstanding under the LTIP as of December 31, 2024. |
(2) |
Reflects the weighted-average exercise price of options to purchase Common Shares outstanding as of December 31, 2024. |
(3) |
Consists of 798,023 Common Shares reserved under the Share Option Plan, and 399,709 Common Shares reserved under the LTIP as of December 31, 2024. |
(4) |
The aggregate maximum number of Common Shares that may be issued under the LTIP may not exceed 4.9% of the issued and outstanding Common Shares from time to time on a non-diluted basis. The aggregate maximum number of Common Shares that may be issued under the Share Option Plan, together with all other security-based compensation arrangements of the Company, is limited to 13% of the issued and outstanding Common Shares. |
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Under existing SEC rules, some transactions, commonly referred to as “related party transactions,” are required to be disclosed to shareholders, including any transactions in which we have been a participant, in which the amount involved exceeds the lesser of (i) $120,000 or (ii) 1% of the average of our total assets as of December 31, 2023 and 2024, and in which any of our directors, executive officers or holders of more than 5% of our capital stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest.
We are not aware of any related party transactions or series of similar transactions to which we have been or will be a party since January 1, 2023, other than compensation arrangements, which are described where required under the “Compensation of Named Executive Officers” and “Director Compensation” sections above.
Policies and Procedures for Related Party Transactions
We have adopted a written policy that requires all transactions between us and any director, executive officer, holder of 5% or more of any class of our capital stock or any member of the immediate family of, or entities affiliated with, any of them, or any other related persons, as defined in Item 404 of Regulation S-K, or their affiliates, in which the amount involved is equal to or greater than $120,000, be approved in advance by the Audit Committee. Any request for such a transaction must first be presented to the Audit Committee for review, consideration and approval. In approving or rejecting any such proposal, the Audit Committee is to consider the relevant facts and circumstances available and deemed relevant to the Audit Committee, including, but not limited to, the extent of the related party’s interest in the transaction, and whether the transaction is on terms no less favorable to us than terms we could have generally obtained from an unaffiliated third party under the same or similar circumstances.
104
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table presents fees for professional audit services rendered by PricewaterhouseCoopers LLP (PwC) for the audit of our annual financial statements for the years ended December 31, 2024 and December 31, 2023, and fees billed for other services rendered by PwC during those periods:
|
|
|
|
|
Audit Related |
|
|
|
|
|
|
|
Financial Year Ending |
|
Audit Fees(1) |
|
Fees |
|
Tax Fees(2) |
|
All Other Fees |
||||
December 31, 2023 |
|
$ |
583,000 |
|
$ |
— |
|
$ |
75,000 |
|
$ |
— |
December 31, 2024 |
|
$ |
386,000 |
|
$ |
— |
|
$ |
69,000 |
|
$ |
— |
Notes:
| (1) | Audit fees includes annual audit, quarterly reviews and work performed in relation to offerings. |
| (2) | Tax fees includes fees related to annual tax returns and scientific research credit return along with tax and transfer pricing advice. |
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Public Accountant
Consistent with SEC policies regarding auditor independence, the audit committee has responsibility for appointing, setting compensation and overseeing the work of our independent registered public accounting firm. In recognition of this responsibility, the audit committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
Prior to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of four categories of services to the audit committee for approval.
1. Audit services include audit work performed in the preparation of financial statements, as well as work that generally only an independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and attest services and consultation regarding current financial accounting and/or reporting standards.
2. Audit-Related services are for assurance and related services that are traditionally performed by an independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements.
3. Tax services include all services performed by an independent registered public accounting firm’s tax personnel except those services specifically related to the audit of the financial statements, and include fees in the areas of tax compliance, tax planning, and tax advice.
4. Other Fees are those associated with services not captured in the other categories. We generally do not request such services from our independent registered public accounting firm but these fees may include permitted advisory services and license fees associated with an accounting research tool.
Prior to engagement, the audit committee pre-approves these services by category of service. The fees are budgeted and the audit committee requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the audit committee requires specific pre-approval before engaging our independent registered public accounting firm.
The audit committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the audit committee at its next scheduled meeting.
105
PART IV
Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
Item 15(a). |
The following documents are filed as part of this annual report on Form 10-K: |
Item 15(a)(1) and (2) |
See “Index to Consolidated Financial Statements and Financial Statement Schedules” at Item 8 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto. |
Item 15(a)(3) |
Exhibits |
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
Exhibit Number |
|
Exhibit Description |
|
Filed with this Report |
|
Incorporated by Reference herein from Form or Schedule |
|
Filing Date |
|
SEC File/Reg. Number |
3.1 |
|
|
|
|
Form S-8 (Exhibit 4.1) |
|
11/7/2019 |
|
333-234574 |
|
3.2 |
|
|
|
|
Form S-8 (Exhibit 4.2) |
|
11/7/2019 |
|
333-234574 |
|
3.3 |
|
|
|
|
Form S-8 (Exhibit 4.3) |
|
11/7/2019 |
|
333-234574 |
|
3.4 |
|
|
|
|
Form S-8 (Exhibit 4.4) |
|
11/7/2019 |
|
333-234574 |
|
4.1 |
|
|
X |
|
|
|
|
|
|
|
4.2 |
|
|
X |
|
|
|
|
|
|
|
10.1+ |
|
Employment Agreement, dated January 1, 2020, as amended, by and between the Company and Arun Menawat |
|
X |
|
|
|
|
|
|
10.2+ |
|
Employment Agreement, dated October 14, 2024, by and between the Company and Tom Tamberrino |
|
X |
|
|
|
|
|
|
10.3+ |
|
|
X |
|
|
|
|
|
|
|
10.4+ |
|
|
X |
|
|
|
|
|
|
|
10.5+ |
|
|
|
|
Form S-8 (Exhibit 99.1) |
|
5/20/2020 |
|
333-238528 |
|
10.6+ |
|
|
|
|
Form S-8 (Exhibit 99.1) |
|
11/7/2019 |
|
333-234574 |
|
10.7 |
|
|
X |
|
|
|
||||
10.8 |
|
Siemens Agreement dated January 23, 2019. between the Company and Siemens Healthcare GmbH |
|
X |
|
|
|
|||
10.9* |
|
|
X |
|
|
|
|
|
|
|
19 |
|
|
X |
|
|
|
|
|
|
|
21 |
|
|
X |
|
|
|
|
|
|
106
23.1 |
|
|
X |
|
|
|
|
|
|
|
31.1 |
|
|
X |
|
|
|
|
|
|
|
31.2 |
|
|
X |
|
|
|
|
|
|
|
32† |
|
|
X |
|
|
|
|
|
|
|
97+ |
|
|
|
|
Form 40-F (Exhibit 97.0) |
|
3/7/2024 |
|
001-39032 |
|
101.INS |
|
Inline XBRL Instance Document |
|
X |
|
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
X |
|
|
|
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
X |
|
|
|
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
X |
|
|
|
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
X |
|
|
|
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
X |
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as iXBRL and contained in Exhibit 101). |
|
X |
|
|
|
|
|
|
+ |
Management contract or compensatory plan or arrangement. |
* |
Certain portions of this exhibit have been omitted pursuant to Item 601(b)(10) of Regulation S-K. |
† |
The certifications attached as Exhibit 32 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of such Form 10-K), irrespective of any general incorporation language contained in such filing. |
Item 16. FORM 10-K SUMMARY
Not applicable.
107
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
PROFOUND MEDICAL CORP.
Date: |
March 7, 2025 |
By: |
/s/ Arun Menawat |
|
|
|
Arun Menawat |
||
|
|
Chief Executive Officer |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signatures |
|
Title |
|
Date |
|
|
|
|
|
|
|
By: |
/s/ Arun Menawat |
|
Chief Executive Officer and Director |
|
March 7, 2025 |
|
Arun Menawats |
|
(principal executive officer) |
|
|
|
|
|
|
|
|
By: |
/s/ Rashed Dewan |
|
Chief Financial Officer |
|
March 7, 2025 |
|
Rashed Dewan |
|
(principal financial officer and principal accounting officer) |
|
|
|
|
|
|
|
|
By: |
/s/ Brian Ellacott |
|
Director |
|
March 7, 2025 |
|
Brian Ellacott |
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Cynthia Lavoie |
|
Director |
|
March 7, 2025 |
|
Cynthia Lavoie |
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Murielle Lortie |
|
Director |
|
March 7, 2025 |
|
Murielle Lortie |
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Arthur Rosenthal |
|
Director |
|
March 7, 2025 |
|
Arthur Rosenthal |
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Kris Shah |
|
Director |
|
March 7, 2025 |
|
Kris Shah |
|
|
|
|
108
Exhibit 4.1
DESCRIPTION OF SECURITIES REGISTERED
PURSUANT TO SECTION 12 OF THE
SECURITIES EXCHANGE ACT OF 1934
As of the date of filing of our Annual Report on Form 10-K for the year ended December 31, 2024 (the “Annual Report on Form 10-K”), Profound Medical Corp. (“Profound,” the “Company,” “we,” “us” and “our”) has one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended: our common shares, no par value (“Common Shares”).
The following description of our Common Shares is a summary and does not purport to be complete. It is based on and qualified in its entirety by reference to our Articles of Incorporation, as amended (the “Articles of Incorporation”) and our Bylaws (the “Bylaws”), each of which are incorporated by reference as exhibits to the Annual Report on Form 10-K, of which this Exhibit 4.1 is a part.
Description of Common Shares
Authorized Capital Shares: Our authorized capital shares include an unlimited number of Common Shares. The primary trading markets of exchange for our Common Shares are The Nasdaq Stock Market (“Nasdaq”) and the Toronto Stock Exchange (“TSX”), under the trading symbols “PROF” and “PRN,” respectively.
Voting Rights: Holders of our Common Shares are entitled to receive notice of and to attend all meetings of shareholders to be convened by the Company. Each holder of our Common Shares is entitled to one vote per Common Share held on all matters voted on by the shareholders, either in person or by proxy.
Dividends and Liquidation Rights: Holders of our Common Shares are entitled to receive dividends, if any, as may be declared by our board of directors (the “Board”) in its discretion, out of funds legally available for the payment of dividends. Holders of our Common Shares are entitled to share ratably in all assets of the Company legally available for distribution to holders of Common Shares in the event of liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary.
Other Rights and Preferences: There are no sinking fund, preemptive or redemption rights attached to our Common Shares.
Transfer Agent and Registrar: The transfer agent and registrar for our Common Shares in Canada is TSX Trust Company, 301-100 Adelaide Street West, Toronto, Ontario, Canada M5H 4H1, telephone number is 1-866-600-5869. The transfer agent and registrar for our Common Shares in the United States is Computershare Trust Company, N.A., 118 Fernwood Avenue, Edison, NJ, USA 08837, telephone number 1-732-417-2700.
|
|
Exhibit 10.1
EXECUTION COPY

EMPLOYMENT AGREEMENT
THIS EMPLOYMENT AGREEMENT (the “Agreement”) dated as of the 1st day of January, 2020 (the “Effective Date”).
BETWEEN:
PROFOUND MEDICAL (US) INC.
(the “Company”)
AND, for the limited purposes of Sections 1.1, 2.3 and 16.1 hereof:
PROFOUND MEDICAL CORP.
(the “Parent”)
AND:
ARUN MENAWAT
(the “Employee”)
WHEREAS Parent, which owns 100% of the equity of the Company, and the Employee entered into a letter agreement as to employment on August 12, 2016 which was amended and restated as of January 1, 2018 (the “Original Agreement”); and
WHEREAS the Employee has relocated to Florida, and the Company and the Employee wish the Company to employ the Employee on the terms and conditions set forth herein.
NOW THEREFORE in consideration of the covenants and agreements herein, the sufficiency of which is acknowledged by each of the parties, the parties agree as follows:
1. |
EMPLOYMENT |
1.1 |
Effectiveness of the Agreement: The terms and conditions of employment of the Employee by the Parent prior to the Effective Date shall be governed by the Original Agreement and the attachments and exhibits thereto, and as of the Effective Date the Original Agreement will be terminated, provided that the respective rights and obligations of Parent and the Employee thereunder will survive such termination to the extent necessary to carry out the intentions of the parties thereunder. This Agreement will be deemed effective as of the Effective Date. |
1.2 |
Title: The Employee shall serve the Company as its President and shall serve as an officer and/or director of affiliates of the Company as required by the board of directors of the Company (the “Board”) from time to time, including serving as the President and Chief Executive Officer of the Parent. The Employee will report to the Board. The Employee acknowledges the Company may |
re-assign, re-allocate, or re-organize the Employee’s duties and responsibilities if reasonable based on changing circumstances, provided such duties and responsibilities are consistent with the role of a President of the Company and a Chief Executive Officer of the Parent, and do not reflect a material adverse change in the Employee’s status, duties or reporting relationship.
1.3 |
Service: During the term of this Agreement, in addition to the Employee’s common law duties to the Company, the Employee covenants and agrees as follows: |
(a) |
Loyalty to the Company: Throughout the Employee’s employment, the Employee will faithfully serve the Company and use the Employee’s best efforts to promote the business of the Company. The Employee will act honestly and in good faith, in the best interests of the Company. |
(b) |
Service: The Employee’s hours and schedule may change from time to time depending on the Company’s business needs. The Employee shall not engage in any other business, profession or occupation, or become an officer, employee, contractor for service, agent, or representative of any other company, partnership, firm, person, organization, or enterprise during the term of this Agreement, provided that Employee may participate in a non- operating capacity on boards of directors or as an advisor as set on in Exhibit A or other boards or advisory work requiring no more than a comparable commitment in the aggregate to those set forth in Exhibit A, provided in each case that such participation does not interfere with the proper discharge of the Employee’s duties to the Company hereunder. For greater certainty, the Employee shall have no restrictions in his involvements in not for profit organizations, provided in each case that such participation does not interfere with the proper discharge of his duties to the Company hereunder . The Employee shall promptly notify the Board of any new positions undertaken by him from time to time. |
(c) |
No Personal Benefit: The Employee will not receive or accept for the Employee’s own benefit or for any other person or entity’s benefit, either directly or indirectly, any commission, rebate, discount, gratuity or profit from any person or entity having or proposing to have one or more business transactions with the Company, without the prior approval of the Company, except as permitted by the Company policy. |
(d) |
Business Opportunities: During the Employee’s employment with the Company, the Employee will, to the extent reasonably practicable, communicate and channel to the Company all knowledge, business and customer contacts and any other information that could concern or be in any way beneficial to the business of the Company. Any such information communicated to the Company as aforesaid will be and remain the property of the Company notwithstanding any subsequent termination of the Employee’s employment. |
(e) |
Place of Work: The Employee may work from remotely, provided that the Employee will be required to travel (both domestically and internationally) as required by the Company from time to time in order to perform the Employee’s duties, including travel to the Parent’s home office approximately once every six (6) weeks. |
(f) |
Pre-existing Obligations: The Employee is hereby requested and directed by the Company to comply with any existing common law, contractual or statutory obligations to the Employee’s former employer and to any other person or entity. The Company is not |
employing the Employee to obtain the confidential information or business opportunities of the Employee’s former employer or any other person or entity.
1.4 |
Directorship and Offices: Upon the termination of employment with the Company for any reason, the Employee will immediately resign any directorship or office the Employee may hold in the Company, Parent or any other affiliates of the Company and, except as provided expressly in this Agreement, the Employee will not be entitled to receive any written notice of termination or payment in lieu of notice, or to receive any severance pay, damages or compensation for loss of any directorship, office or otherwise. The Employee agrees that failure to tender such resignation(s) will amount to termination for Cause (as defined in Section 3.3(c)), for which the Company may treat the employment as being terminated for after-acquired Cause. Notwithstanding the foregoing, the termination of employment will not automatically disqualify the Employee from serving as a director. |
1.5 |
Exclusion/Debarment: The Employee represents and warrants that the Employee has never been, and as a condition of continued employment shall never be, during the term of this Agreement, excluded from any contracting by any Canadian or United States government agency or authority. The Employee further represents and warrants that the Employee is not subject to any final adverse action, as that term is defined in 42 U.S.C. § 1320a-7e(g), and that no final adverse action has previously occurred or is pending or threatened against the Employee. The Employee represents and warrants that the Employee is not under investigation by the FDA or any other Canadian, United States or foreign regulatory agency nor has the Employee been debarred, suspended, proposed for debarment, declared ineligible, or voluntarily excluded by any Canadian, United States or foreign government department or agency. |
1.6 |
Company Policy: The Employee acknowledges and agrees that the employment relationship will be governed by the standards and terms established by the Company’s policies as they are established from time to time and the Employee agrees to comply with the terms of such policies which may be introduced, amended, deleted or modified in the sole discretion of the Company, provided that doing so does not constitute a material change in the Employee’s terms of employment. |
1.7 |
Professional Conduct: The Employee acknowledges and agrees that effective performance of the Employee’s duties requires integrity and the Company’s confidence in the Employee’s working relationships with other employees of the Company and with all other persons with whom the Employee deals in the course of employment. |
2. |
COMPENSATION |
2.1 |
Base Salary: The Employee will earn an annualized salary of (i) US$500,000 from the Effective Date until December 30, 2019. (ii) US$525,000 from January 1, 2020 until September 30, 2021 and (iii) thereafter shall earn an annualized salary of US$275,000 (as in effect from time to time, the “Base Salary”), less applicable deductions, payable in arrears for all services and work the Employee performs for the Company. Any increases will be in the discretion of the Board. The Base Salary will be payable in accordance with the Company’s normal payroll practices. |
2.2 |
Annual Discretionary Bonus: Provided that through the date of payment the Employee remains an employee of the Company in good standing and the Employee has not received or given notice of termination, for each calendar year, the Employee will be eligible to receive an annual bonus of up to 75% of the Employee’s Base Salary for such year ( and if there were changes in the Base Salary over the course year, the actual amounts due and owing for the entire year as Base Salary in |
effect from time to time) based on the assessment of the Board of the Employee’s and the Company’s achievement of certain milestones and objectives determined by the Board. Such milestones and objectives (including the evaluation of achievement thereof), and the bonus amount, will be at the sole discretion of the Board and will be payable in accordance with the Company’s standard payroll policies, subject to applicable payroll deductions and withholdings. Such bonus shall be paid within ninety (90) days after the end of the applicable calendar year.
2.3 |
Stock Options: The options to purchase common shares of the Parent previously received by the Employee (“Stock Options”) shall continue to vest during the term of this Agreement in accordance with their terms. The Parent shall consider compensation as a whole annually, including the form, terms and size of stock incentives, of chief executive officers of comparable companies when considering compensation changes or the grant of additional Stock Options to the Employee by reference to comparable companies and information and/or reports from independent compensation consultants (which information and reports will be shared with the Employee). Subject to the ultimate terms each applicable Stock Option agreement, the vesting of any Stock Options granted on or after October 1, 2020 shall be contingent upon the Parent achieving a minimum market capitalization, determined based on closing price, for ninety (90) consecutive calendar days, of CDN$1 Billion at any time during the term period of the applicable option, in addition to satisfaction of any time-based or other vesting conditions that may apply. |
2.4 |
Benefits: The Employee will be entitled to participate in the standard insurance plans and other benefit programs (the “Employee Benefits”) which are substantially similar, to the extent practical and commercially reasonable, which the Parent offers to its executive level employees from time- to-time. The Company reserves the right to unilaterally revise the terms of the Employee Benefits, to change carriers, or to eliminate any Employee Benefits altogether. The Employee Benefits will be provided in accordance with the formal plan documents or policies and any issues with respect to entitlement or payment of benefits under any of the Employee Benefits will be governed by the terms of such documents or policies establishing the benefits in issue and will be a matter between the Employee and the insurer. The Company’s liability with respect to the Employee Benefits will be limited to the payment of its share of applicable premiums. |
2.5 |
Car Allowance: The Company shall provide the Employee with a monthly car allowance of CDN$1,000 converted into US dollars in accordance with the Company’s payroll policy, as amended from time to time. |
2.6 |
Expenses: The Company will reimburse the Employee for all reasonable business expenses actually and exclusively incurred in connection with the performance of duties under this Agreement, provided such expenses are incurred and accounted for in accordance with the general policies and procedures of the Company, as established from time to time. |
2.7 |
National Holidays: The Company recognizes the national holidays observed in the United States. |
2.8 |
Vacation: The Employee shall be entitled to four (4) weeks of paid vacation per annum, to be taken at such reasonable times as the Company shall in its discretion permit and in accordance with the Company’s vacation policy in effect from time to time. Vacation entitlement for any partial year of employment shall be pro-rated based on the portion of the year worked by the Employee. |
3. |
TERMINATION OF AGREEMENT AND EMPLOYMENT |
3.1 |
Obligations Upon Termination: Upon termination of the Employee’s employment, for any reason: |
(a) |
the Company shall pay the Employee for (i) all unpaid Base Salary, and any unused vacation that may be required to be paid out by law, earned or accrued up to and including the Employee’s last day of employment (the “Termination Date”), such payment to be made no later than the first regular payroll date of the Company following the Termination Date; (ii) reimbursement of any eligible unreimbursed business expenses incurred by Employee through the Termination Date and timely and properly reported by Employee, such reimbursement to be paid in accordance with Company policy; and (iii) any other payments and benefits to which Employee may be entitled under the terms of any applicable compensation or benefit arrangement of the Company or under applicable law as of the Termination Date; |
(b) |
all benefits coverage and other perquisites of the Employee’s employment shall cease on the later of the Termination Date or as specified in 3.3(a), subject to applicable law and plan terms; and |
(c) |
all files, computer disks, information and documents pertaining to the Company’s business shall remain the property of the Company, and shall promptly be delivered by the Employee to the Company’s office, and no copy, duplication or reproduction of any kind whatsoever shall be made of such files, computer disks, information or documents, or retained by the Employee, without the express written consent of the Company. The Employee agrees to deliver all electronic information to the Company and to destroy any copies held by the Employee belonging to the Company. |
3.2 |
Termination by the Employee: The Employee may terminate this Agreement at any time by providing the Company with ninety (90) days’ prior written notice. Upon receipt of such notice, the Company may at any time terminate the employment of the Employee and pay the Employee the amount of Base Salary and continue all Employee Benefits the Employee would have otherwise received during the balance of the aforementioned notice period, subject to applicable law. |
3.3 |
Termination by the Company: The Company may terminate the Employee’s employment at any time: |
(a) |
without Cause by providing the Employee (subject to Section 3.8) with (A) twelve (12) months of pay of Base Salary in lieu of notice (the twelve (12) month period following the Termination Date, the “Severance Period”) and (B) an amount equal to the then current target annual bonus prorated based on the number of days elapsed in the calendar year until the date of termination as a percentage of the total number of days in such calendar year. The Employee will continue to provide reasonable services to the Company on an as- needed and part-time basis for a period of up to three (3) months following the termination of his employment (the “Transition Period”), including assistance with transition of duties, unless the requirement for active service is expressly waived in whole or in part by the Company, in its sole discretion. The Employee agrees that the Company may, in its sole discretion, limit or discontinue the Executive’s access to business records and Confidential Information during the Transition Period. The Employee agrees that any services provided to the Company during the Transition Period shall be for no additional compensation in excess of the severance payments and benefits contemplated under this Agreement. Regardless of whether or not the Employee provides active service during the Transition Period, the Employee shall continue to abide by all obligations owing under this Agreement. The payments due the Employee hereunder are not subject to mitigation if the Employee receives compensation during the Severance Period from third parties as long as the Employee provides the services during the Transition Period as set forth herein. |
(b) |
for frustration of contract upon the death of the Employee or any incapacitation of the Employee that constitutes an undue hardship for the Company; |
(c) |
at any time for Cause, without notice or pay in lieu of notice or any other form of compensation, severance pay or damages. For the purposes of this Agreement, “Cause” includes: |
i. |
any material breach of the provisions of this Agreement or the Company’s policies by the Employee if the Company has provided the Employee, with written notice of such breach and such breach has not been remedied within thirty (30) days thereafter; |
ii. |
any intentional or grossly negligent disclosure of any Confidential Information by the Employee; |
iii. |
any fraud, misappropriation of the property or funds of the Company or its affiliates, embezzlement, or other similar acts of dishonesty; |
iv. |
the willful allowance by the Employee of the Employee’s duty to the Company or its affiliates and the Employee’s personal interests to come into conflict in a material way in relation to any transaction or matter that is of a substantial nature, other than conflicts which are fully disclosed and approved by the Board; |
v. |
the Employee’s criminal, or other conviction of any criminal summary conviction offence or indictable offence that is detrimental to the business or reputation of the Company or its affiliates; |
vi. |
if the Employee’s misconduct causes the Employee to be subject to a final and non-appealable enforcement order (or regulatory settlement in respect thereof) concerning a securities, financial services, health or other regulatory offence that is detrimental to the business or reputation of the Company or its affiliates; and/or |
vii. |
any and all commissions, omissions or other conduct which would constitute just cause under the laws of Ontario or the laws of Canada applicable therein, in addition to the specified causes noted above. |
3.4 |
Termination in Connection with Change In Control: |
(a) |
“Change in Control” means (i) a reorganization or merger of the Parent with or into any other corporation or corporations or on an acquisition of securities of the Parent (excluding an equity financing of the Parent with the principal purpose of raising capital or a merger with any subsidiary or other affiliate) in which transaction the Parent’s shareholders immediately prior to such transaction own immediately after such transaction, less than 50% of the voting power of the surviving corporation or its parent, and/or (ii) the sale or disposition by the Parent to an unrelated third party of substantially all of its business and assets. Without limiting the foregoing, a transaction will not constitute a Change in Control if (x) its sole purpose is to change the jurisdiction of the Parent’s incorporation, or (y) its sole purpose is to create a holding company or other entity that will be owned in substantially the same proportions by the persons who held the Parent’s securities immediately prior to such transaction. |
(b) |
If Employee’s employment is terminated without Cause within 30 days prior to, upon or within 12 months following the occurrence of a Change in Control, and in accordance with Section 3.8 the Employee signs and returns to the Company without revocation a release prepared by the Company of all legally waivable claims related to or arising from the Employee’s employment with the Company, then (i) the Company shall pay the Employee (A) twenty-four (24) months of the Employee’s then-current annual Base Salary and (B) an amount equal to the then current annual target bonus and (ii) the Stock Options shall vest as set forth in Section 3.4(d) (collectively, the “Change in Control Benefits”). |
(c) |
If, within twelve (12) months following a Change in Control, there is a material diminution of the Employee’s role in the company, change in work location of more than 50 kilometers (except as contemplated pursuant to Section 1.3(c) hereto), diminution of status, any materially detrimental change in compensation (including options and bonus) or benefits (considered as a whole), or diminution of reporting structure, the Employee shall have the right to resign within 30 days after the event and receive the Change in Control Benefits, subject to Section 3.8. |
(d) |
If the Employee is entitled to Change in Control Benefits, then, subject to Section 3.8, any unvested Stock Options shall immediately vest. If any provision of this Agreement conflicts with a provision of the Stock Option agreement(s) or applicable stock option plan, the provision more favorable to the Employee shall govern. |
3.5 |
Timing of Payments: All amounts payable hereunder in respect of Base Salary shall be paid in installments in accordance with the Company’s normal payroll practices. Payments in respect of annual or prorated bonuses shall be paid at the time the annual bonus would otherwise be paid. |
3.6 |
No Implied Entitlement: Other than as expressly provided herein, the Employee will not be entitled to receive any further pay or compensation, severance pay, notice, payment in lieu of notice, incentives, bonuses, benefits or damages of any kind from the Company or from any affiliate of the Company. The Employee will not be entitled to receive any further pay or compensation (except for pay, if any, accrued and owing under this Agreement up to the date of termination of employment) from the Company, and for clarity, without limiting the foregoing, the Employee will not be entitled to any bonus or pro rata bonus payment that has not already been awarded to the Employee by the Company, except as set out in paragraph 3.3(a) or except for that which was specified as part of the Change in Control Benefits. |
3.7 |
Continued Effect: Notwithstanding any changes in the terms and conditions of the Employee’s employment which may occur in the future, including any changes in position, duties or compensation, the termination provisions in this Agreement will continue to be in effect for the duration of the Employee’s employment with the Company unless otherwise amended in writing and signed by the Company. |
3.8 |
Obligations Upon Termination: The Employee agrees that he shall not be entitled to receive any severance fee or other benefits under Section 3.3 or 3.4 of this Agreement if the Employee breaches any of his obligations arising under Sections 4, 5, 6, 7, 8, 9 and 11 hereof. The Employee acknowledges that until a release in the form of Exhibit B hereto (a “Release”) is timely executed and delivered to the Company and becomes effective and irrevocable, the Company will not make any severance payments or benefits due under Section 3.3 or 3.4 of this Agreement following termination of the Employee, and any such severance payments or benefits that would otherwise be provided within 28 days following the Employee’s Termination Date shall instead be paid in a |
lump sum on the Company’s first regular payroll date thereafter (with the remaining payments and benefits provided on the original schedule). The Employee further acknowledges that if the Release is not timely executed and delivered to the Company, or if it is revoked following delivery, the severance payments and other benefits described in Sections 3.3 and 3.4 shall be forfeited.
4. |
CONFIDENTIAL INFORMATION PROTECTIONS |
4.1 |
At all times during and after the Employee’s employment, the Employee will hold in confidence and will not disclose, use, lecture upon, or publish any of the Company’s Confidential Information (defined below), except as may be required in connection with the Employee’s work for the Company, or as expressly authorized by the Board. Unless forming part of the Employee’s customary duties for the Company, the Employee will obtain the written approval of the Board before publishing or submitting for publication any material (written, oral, or otherwise) that relates to the Employee’s work at the Company and/or incorporates any Confidential Information. The Employee hereby assigns to the Company any rights the Employee may have or acquire in any and all Confidential Information and recognize that all Confidential Information shall be the sole and exclusive property of the Company and its assigns. |
4.2 |
The term “Confidential Information” shall mean any and all confidential knowledge, data or information related to the Company’s business or its actual or demonstrably anticipated research or development, including without limitation (a) trade secrets, inventions, ideas, processes, computer source and object code, data, formulae, programs, other works of authorship, know-how, improvements, discoveries, developments, designs, and techniques; (b) information regarding products, services, plans for research and development, marketing and business plans, budgets, financial statements, contracts, prices, suppliers, employees and customers; (c) information regarding the skills and compensation of the Company’s employees, contractors, and any other service providers of the Company and (d) the existence of any business discussions, negotiations, or agreements between the Company and any third party. |
4.3 |
The Employee understands that the Company has received and in the future will receive from third parties confidential or proprietary information (“Third Party Information”) subject to a duty on the Company’s part to maintain the confidentiality of such information and to use it only for certain limited purposes. During and after the term of the Employee’s employment, the Employee will hold Third Party Information in strict confidence and will not disclose to anyone (other than the Company personnel who need to know such information in connection with their work for the Company) or use, Third Party Information, except in connection with the Employee’s work for the Company or unless expressly authorized by an officer of the Company in writing. |
4.4 |
The Employee represents that employment by the Company does not and will not breach any agreement with any former employer, including any non-compete agreement or any agreement to keep in confidence or refrain from using information acquired by the Employee prior to employment by the Company. The Employee further represents that the Employee has not entered into, and will not enter into, any agreement, either written or oral, in conflict with the Employee’s obligations under this Agreement. During employment by the Company, the Employee will not improperly make use of, or disclose, any information or trade secrets of any former employer or other third party, nor will the Employee bring onto the premises of the Company or use any unpublished documents or any property belonging to any former employer or other third party, in violation of any lawful agreements with that former employer or third party. The Employee will use in the performance of the Employee’s duties only information that is generally known and used by persons with training and experience comparable to the Employee’s own, is common knowledge in the industry or otherwise legally in the public domain, or is otherwise provided or developed by |
the Company.
4.5 |
Notwithstanding any other provision of this Agreement: (a) the Employee will not be held criminally or civilly liable under any federal or state trade secret law for any disclosure of a trade secret that: (i) is made (A) in confidence to a federal, state, or local government official, either directly or indirectly, or to an attorney, and (B) solely for the purpose of reporting or investigating a suspected violation of law; or (ii) is made in a complaint or other document filed under seal in a lawsuit or other proceeding; and (b) if the Employee files a lawsuit for retaliation by the Company for reporting a suspected violation of law, the Employee may disclose the Company’s trade secrets to the Employee’s attorney and use the trade secret information in the court proceeding if the Employee: (i) files any document containing trade secrets under seal; and (ii) does not disclose trade secrets, except pursuant to court order. |
4.6 |
The Employee understands that nothing contained in this Agreement limits the Employee’s ability to file a charge or complaint with the Equal Employment Opportunity Commission, the National Labor Relations Board, the Occupational Safety and Health Administration, the Securities and Exchange Commission or any other federal, state or local governmental agency or commission (each, a “Government Agency”). The Employee further understands that this Agreement does not limit the Employee’s ability to communicate with any Government Agencies or otherwise participate in any investigation or proceeding that may be conducted by any Government Agency, including providing documents or other information, without notice to the Company. This Agreement does not limit the Employee’s right to receive an award for information provided to any Government Agencies. |
5. |
INVENTIONS |
5.1 |
As used in this Agreement, the term “Invention” means any ideas, concepts, information, materials, processes, data, programs, know-how, improvements, discoveries, developments, designs, artwork, formulae, other copyrightable works, and techniques and all Intellectual Property Rights in any of the items listed above. The term “Intellectual Property Rights” means all trade secrets, copyrights, trademarks, mask work rights, patents and other intellectual property rights recognized by the laws of any jurisdiction or country. The term “Moral Rights” means all paternity, integrity, disclosure, withdrawal, special and any other similar rights recognized by the laws of any jurisdiction or country. |
5.2 |
The Employee has disclosed in Exhibit C a complete list of all Inventions that (a) the Employee has, or has caused to be, alone or jointly with others, conceived, developed, or reduced to practice prior to the commencement of the Employee’s employment by the Company; (b) in which the Employee has an ownership interest or which the Employee has a license to use; (c) and that the Employee wishes to have excluded from the scope of this Agreement (collectively referred to as “Prior Inventions”). If no Prior Inventions are listed in Exhibit C, the Employee warrants that there are no Prior Inventions. The Employee agrees that the Employee will not incorporate, or permit to be incorporated, Prior Inventions in any of the Company Inventions (defined below) without the Company’s prior written consent. If, in the course of the Employee’s employment with the Company, the Employee incorporates a Prior Invention into a Company process, machine or other work, the Employee hereby grants the Company a non-exclusive, perpetual, fully-paid and royalty-free, irrevocable and worldwide license, with rights to sublicense through multiple levels of sublicensees, to reproduce, make derivative works of, distribute, publicly perform, and publicly display in any form or medium, whether now known or later developed, make, have made, use, sell, import, offer for sale, and exercise any and all present or future rights in, such Prior Invention. |
5.3 |
Inventions assigned to the Company or to a third party as directed by the Company pursuant to this Section 5 are referred to in this Agreement as “Company Inventions”. Subject to subsection 5.5 (including the proviso set forth therein) and the Prior Inventions the Employee has set forth in Exhibit C, the Employee hereby assigns and agrees to assign in the future (when any such Inventions or Intellectual Property Rights are first reduced to practice or first fixed in a tangible medium, as applicable) to the Company all rights, title, and interest in and to any and all Inventions (and all Intellectual Property Rights with respect thereto) made, conceived, reduced to practice, or learned by the Employee, either alone or with others, during the period of the Employee’s employment by the Company. Any assignment of Inventions (and all Intellectual Property Rights with respect thereto) hereunder includes an assignment of all Moral Rights. To the extent such Moral Rights cannot be assigned to the Company, and to the extent the following is allowed by the laws in any country where Moral Rights exist, the Employee hereby unconditionally and irrevocably waives the enforcement of such Moral Rights, and all claims and causes of action of any kind against the Company or related to the Company’s customers, with respect to such rights. The Employee further acknowledges and agrees that neither the Employee’s successors-in-interest nor legal heirs retain any Moral Rights in any Inventions (and any Intellectual Property Rights with respect thereto). |
5.4 |
During the period of employment, the Employee will promptly and fully disclose to the Company in writing (a) all Inventions authored, conceived, or reduced to practice by the Employee, either alone or with others, and (b) all patent applications filed by the Employee or in which the Employee is named as an inventor or co-inventor; provided, however, that such disclosure obligation does not apply to Inventions (or Intellectual Property Rights with respect thereto) which are not directly or indirectly related to the Company’s then current or future business or are not invented with the Company resources or during the Employee’s working hours. The Employee agrees to keep and maintain adequate and current records (in the form of notes, sketches, drawings and in any other form that is required by the Company) of all Inventions made by the Employee during the period of the Employee’s employment by the Company, which records shall be available to, and remain the sole property of, the Company at all times. |
5.5 |
The Employee agrees that, as directed by the Company, the Employee will assign to the Company or a third party, including without limitation, all rights, title, and interest in and to any particular Company Invention; provided, however, it is understood that the Company will not elect to direct such assignment of a Company Invention if it is not directly or indirectly related to the Company’s then current or future business or was not invented with the Company resources or during Employee’s working hours. |
5.6 |
During and after the period of employment and at the Company’s request and expense, the Employee will provide reasonable assistance to the Company, including consenting to and joining in any action, to obtain and enforce Canadian, United States and foreign Intellectual Property Rights and Moral Rights relating to Company Inventions in all countries. If the Company is unable to secure the Employee’s signature on any document needed in connection with such purposes, the Employee hereby irrevocably designates and appoints the Company and its duly authorized officers and agents as the Employee’s agent and attorney in fact, which appointment is coupled with an interest, to act on the Employee’s behalf to execute and file any such documents and to do all other lawfully permitted acts to further such purposes with the same legal force and effect as if executed by the Employee. |
5.7 |
The Employee agrees that the Employee will not incorporate into any Company software or otherwise deliver to the Company any software code licensed under the GNU General Public License or Lesser General Public License or any other license that, by its terms, requires or |
conditions the use or distribution of such code on the disclosure, licensing, or distribution of any source code owned or licensed by the Company.
6. |
RETURN OF COMPANY PROPERTY |
6.1 |
Without limiting the provisions of Section 3.1(c), upon termination of the Employee’s employment or upon the Company’s request at any other time, the Employee will deliver to the Company all of the Company’s property, equipment, and documents, together with all copies thereof, and any other material containing or disclosing any Inventions, Third Party Information or Confidential Information and certify in writing that the Employee has fully complied with the foregoing obligation. The Employee agrees that the Employee will not copy, delete, or alter any information contained upon any Company computer or Company equipment before the Employee returns it to the Company. In addition, if the Employee has used any personal computer, server, or e-mail system to receive, store, review, prepare or transmit any Company information, including but not limited to, Confidential Information, the Employee agrees to provide the Company with a computer-useable copy of all such Confidential Information and then permanently delete and expunge such Confidential Information from those systems; and the Employee agrees to provide the Company access to the Employee’s system as reasonably requested to verify that the necessary copying and/or deletion is completed. |
7. |
NON-COMPETITION/NON-SOLICITATION |
7.1 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (1) year after termination of such employment for any reason, the Employee will not, without the Company’s express written consent, anywhere in the world, be employed by or contribute to any business activity that is competitive with the business of the Company or it’s affiliates or involves the use of real-time magnetic resonance imaging or ultrasound or similar technologies in the delivery of ablative tools. |
7.2 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (1) year after termination of such employment for any reason, the Employee will not, either directly or indirectly, solicit or attempt to solicit any employee, independent contractor, or consultant of the Company or its affiliates to terminate his, her or its relationship with Company or its affiliates in order to become an employee, consultant, or independent contractor to or for any other person or entity. |
8. |
NOTIFICATION OF NEW EMPLOYER |
8.1 |
After the termination of this Agreement, the Employee consents to the notification of any new employer of the Employee’s rights and obligations under this Agreement, by the Company providing a copy of this Agreement. |
9. |
INJUNCTIVE RELIEF |
9.1 |
The Employee acknowledges and agrees that: (a) the services to be rendered by the Employee to the Company are of a special and unique character; (b) the Employee will obtain knowledge and skill relevant to the Company’s industry, methods of doing business and marketing strategies by virtue of the Employee’s employment with the Company; (c) the restrictive covenants and other terms and conditions of this Agreement are reasonable and reasonably necessary to protect the legitimate business interest of the Company; (d) the amount of the Employee’s compensation reflects, in part, the Employee’s obligations and the Company’s rights under the restrictive |
covenants set forth herein; (e) the Employee has no expectation of any additional compensation, royalties or other payment of any kind not otherwise referenced herein in connection herewith; and (f) the Employee will not be subject to undue hardship by reason of full compliance with the terms and conditions of the restrictive covenants set forth herein or the Company’s enforcement thereof. The Employee further acknowledges that, because the Employee’s services are personal and unique and because the Employee will have access to the Confidential Information, any breach of the restrictive covenants set forth in this Agreement would cause irreparable injury to the Company and/or its affiliates for which monetary damages would not be an adequate remedy and, therefore, any such actual or threatened breach will entitle the Company to seek, in addition to other available remedies, temporary or permanent injunctive relief (including specific performance) or other equitable relief, without the necessity of showing any actual damages or that money damages would not afford an adequate remedy, and without the necessity of posting any bond or other security. The rights and remedies provided to each party in this Agreement are cumulative and in addition to any other rights and remedies available to such party at law or in equity. In addition, the Employee acknowledges and agrees that, should the Employee violate any restrictive covenant, the obligation at issue will be tolled for the period of the violation and will resume to run from the first date on which the Employee ceases to be in violation of the obligation. The Employee further acknowledges and agrees that if should breach any restrictive covenant, then, in addition, to the Company’s other legal and equitable remedies, the Company may suspend or cease payment of any Change in Control or other severance payments and benefits to which the Employee might otherwise be entitled.
10. |
PUBLICITY |
10.1 |
Except to the extent required by the Employee’s duties or customarily performed by the Employee, the Employee shall not, without the prior written consent of the Company, make or give any public announcements, press releases or statements to the public or the press regarding the Employee’s work or the Company’s business. |
11. |
NOTICES |
11.1 |
All notices, requests, demands and other communications to be given pursuant to the terms of this Agreement shall be in writing and shall be deemed to have been duly given (i) on the date of delivery if personally delivered or sent by a recognized overnight courier or (ii) on the date of transmission if sent by email (provided notice is then also promptly delivered personally or by a recognized overnight courier), in each case to the parties at the addresses listed below: |
(a) |
If to the Employee, at the Employee’s then current address in the Company’s payroll records; |
(b) |
If to the Company: |
Profound Medical (US) Inc.
Attention:Arthur Rosenthal
E-mail:#################
With a copy (which shall not constitute notice) to:
Torys LLP
1114 Ave of the Americas, 23rd Floor
New York, NY 10036.7703
Attention:Cheryl Reicin
Email:###################
Each party may change its address for receipt of notice by giving notice of the change to the other party.
12. |
SURVIVAL |
12.1 |
The Employee’s obligations under this Agreement shall survive the termination of the Employee’s employment, regardless of the manner or reason for termination, including, without limitation, those set forth in Sections 4 through 7 hereof. |
12.2 |
The assignment of this Agreement by the Company to any successor or other assignee and shall be binding upon the Employee’s heirs and legal representatives. |
13. |
SEVERABILITY |
13.1 |
Should any provision of this Agreement be held by a court of competent jurisdiction to be enforceable only if modified, or if any portion of this Agreement will be held as unenforceable and thus stricken, such holding will not affect the validity of the remainder of this Agreement, the balance of which will continue to be binding upon the parties with any such modification to become a part hereof and treated as though originally set forth in this Agreement. The parties further agree that any such court is expressly authorized to modify any such unenforceable provision of this Agreement in lieu of severing such unenforceable provision from this Agreement in its entirety, whether by rewriting the offending provision, deleting any or all of the offending provision, adding additional language to this Agreement or by making such other modifications as it deems warranted to carry out the intent and agreement of the parties as embodied herein to the maximum extent permitted by applicable law. The parties expressly agree that this Agreement as so modified by the court will be binding upon and enforceable against each of them. In any event, should one or more of the provisions of this Agreement be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability will not affect any other provisions hereof, and if such provision or provisions are not modified as provided above, this Agreement will be construed as if such invalid, illegal or unenforceable provisions had not been set forth herein. |
14. |
WAIVER |
14.1 |
Any waiver or failure to enforce any provision of this Agreement on one occasion will not be deemed a waiver of that provision or any other provision on any other occasion. |
15. |
ASSIGNMENT |
15.1 |
The Company may assign this Agreement to (i) an affiliate, subsidiary, related company or partnership without prior notice or consent of the Employee, or (ii) a new employer in connection with any transaction or reorganization; provided that any such successor or assignee expressly assumes in writing the Company’s obligations under this Agreement and has direct or indirect |
responsibility for managing the underlying business of the Company. This Agreement will inure to the benefit of the Company and its permitted successors and assigns. The Employee may not assign any of his rights nor delegate any of the duties hereunder.
16. |
ENTIRE AGREEMENT |
16.1 |
This Agreement is the final, complete and exclusive agreement of the parties with respect to the subject matter hereof, and supersedes all prior and contemporaneous written and oral communications, understandings, agreements, representations and warranties between the Employee and the Company with respect to such matters. The Original Agreement is terminated as of the Effective Date subject to survival of certain provisions thereunder as set forth in Section 1.1. No modification of or amendment to this Agreement, or any waiver of any rights under this Agreement, will be effective unless in writing and signed by the Employee and the Company. Any subsequent change or changes in duties, salary or compensation will not affect the validity or scope of this Agreement. |
17. |
HEADINGS |
17.1 |
The headings utilized in this Agreement are for convenience only and are not to be construed in any way as additions or limitations of the covenants and agreements contained in this Agreement. |
18. |
INDEPENDENT LEGAL ADVICE |
18.1 |
The Employee acknowledges that the Employee has read and understands this Agreement, and acknowledges that an opportunity was provided to obtain separate legal advice about it. The Company shall reimburse the Employee for the reasonable costs of obtaining legal advice regarding this Agreement. |
19. |
COUNTERPARTS |
19.1 |
This Agreement may be executed in two or more counterparts, each of which will be deemed to be an original and all of which will constitute one Agreement. |
20. |
LAWS |
20.1 |
This Agreement shall be governed by and interpreted in accordance with the laws of the State of Florida without regard to any conflict- or choice-of-law principle that would call for the application of the law of another jurisdiction. |
21. |
COMPANY AND AFFILIATES |
21.1 |
Whenever the context suggests , the term “the Company” shall also include the Parent and the affiliates of the Company and of the Parent, including, without limitation, for purposes of Sections 4-7 and 9. Without limiting the foregoing, Confidential Information of the Company includes information of the Parent and the affiliates of the Company and of the Parent, and the non solicitation and noncompetition clauses relate to the employees and business of the Parent and the and the affiliates of the Company and of the Parent. |
22. |
TAX MATTERS |
22.1 |
The Company will have the right to withhold and deduct from any payments or deemed payments hereunder any amounts of tax withholding that it determines be required by federal, state and local law, whether U.S. or non-U.S. |
22.2 |
This Agreement is intended to comply with or satisfy an exemption from Section 409A of the U.S. Internal Revenue Code of 1986, as amended, and the Treasury Regulations and other administrative guidance promulgated thereunder (collectively, “Section 409A”), and will be construed and administered in accordance with Section 409A. Notwithstanding any other provision of this Agreement, payments provided under this Agreement may only be made upon an event and in a manner that complies with Section 409A or an applicable exemption. Any payments under this Agreement that may be excluded from Section 409A either as separation pay due to an involuntary separation from service or as a short-term deferral will be excluded from Section 409A to the maximum extent possible. If this Agreement provides for a payment to be made within a window of time (e.g., “payment will be made within 30 days”), the date that payment is actually to be made will be within the sole and absolute discretion of the Company. To the extent that any payment or benefit described in this Agreement constitutes “non-qualified deferred compensation” under Section 409A, and to the extent that such payment or benefit is payable upon the Employee’s termination of employment, then such payments or benefits will be payable only upon the Employee’s “separation from service” as determined under Section 409A, and “termination,” “termination of employment” and like terms will be construed accordingly. Furthermore, the parties hereto acknowledge and agree that all or any part of any payment to be made to the Employee within six months following the Employee’s separation from service other than by reason of the Employee’s death will instead be paid in a lump sum without interest on the first business day that is more than six months after the date of the Employee’s separation from service (or, if later, the first business day that is more than 18 months after the applicable date of correction, as required by Internal Revenue Service Notice 2010-6) if the Company determines in good faith that the Employee is a “specified employee” within the meaning of Section 409A and that the earlier payment of such amount would give rise to tax, penalties or interest under Section 409A. All in- kind benefits provided and expenses eligible for reimbursement under this Agreement will be provided by the Company or incurred by the Employee during the time periods set forth in this Agreement. All reimbursements will be paid as soon as administratively practicable, but in no event will any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year will not affect the in-kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year. Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit. Notwithstanding the foregoing, the Company makes no representations that the payments and benefits provided under this Agreement comply with Section 409A, and in no event will the Company be liable for all or any portion of any taxes, penalties, interest or other expenses that may be incurred by the Employee pursuant to Section 409A. |
IN WITNESS WHEREOF the parties have duly executed this Agreement as of the 1st day of January, 2020.
/s/ ARUN MENAWAT |
|
ARUN MENAWAT |
|
|
|
PROFOUND MEDICAL (US) INC.
Per:
Name:Arun Menawat
Title:C.E.O.
PROFOUND MEDICAL CORP., for the limited purposes of Sections 1.1, 2.3 and 16.1 hereof
Per: |
|
|
Name: Arthur Rosenthal |
|
|
Title: Director |
|
|
Exhibit A
CURRENT BOARD POSITIONS AND OTHER BUSINESS ACTIVITIES
APPROVED BY THE COMPANY
Elminda Ltd, Chairman of the Board
Stereotexis Inc., Director
Baylis Medical, Advisor
Sudev Foundation, Advisor
Spartan Bioscience Inc., Director
Amplitude Venture Capital Management Inc., Advisor This Separation and Release Agreement (this “Release Agreement”) is entered into as of [●], by and between Arun Menawat (the “Employee”) and Profound Medical (US) Inc. (the “Company”).
Exhibit B
SEPARATION AND RELEASE AGREEMENT
RECITALS
WHEREAS, the Company and the Employee, together with Profound Medical Corp. for limited purposes, previously entered into an Employment Agreement dated as of January 1, 2020 (the “Employment Agreement”);
WHEREAS, the Employee’s employment with the Company has terminated effective as of [●]; and
WHEREAS, capitalized terms used but not defined herein have the meanings ascribed to them in
the Employment Agreement.
NOW, THEREFORE, in consideration of the Employee’s employment and the mutual covenants, promises and obligations set forth herein and in the Employment Agreement, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. |
General Release and Covenant Not to Sue. |
a.In consideration of the Employee’s right to [severance pursuant to Section 3.3 of the Employment Agreement / Change in Control Benefits (as defined in the Employment Agreement)], the Employee, on behalf of the Employee and the Employee’s heirs, executors, administrators, trustees, legal representatives, successors and assigns (hereinafter collectively referred to for purposes of this Section 1 as the “Employee”), hereby agrees to irrevocably and unconditionally waive, release and forever discharge Parent, the Company and their respective past, present and future affiliates and related entities, parent and subsidiary corporations, divisions, shareholders, predecessors, and current, former and future officers, directors, employees, trustees, fiduciaries, administrators, executives, agents, representatives, investors, successors and assigns (collectively, the “Company Released Parties”) from any and all waivable claims, charges, demands, sums of money, actions, rights, promises, agreements, causes of action, obligations and liabilities of any kind or nature whatsoever, at law or in equity, whether known or unknown, existing or contingent, suspected or unsuspected, apparent or concealed, U.S., Canadian or otherwise (collectively, “Claims”) that the Employee has now or in the future may claim to have against any or all of the Company Released Parties based upon or arising out of any facts, acts, conduct, omissions, transactions, occurrences, contracts, claims, events, cause, matters or things of any conceivable kind or character existing or occurring or claimed to exist or to have occurred prior to the date of the Employee’s execution of this Release Agreement in any way whatsoever relating to or arising out of the Employee’s employment with the Company Released Parties or the termination of such employment. Such Claims include, without limitation, Claims arising under the Civil Rights Act, the Age Discrimination in Employment Act (“ADEA”), the Older Workers Benefit Protection Act (the “OWBPA”), the Americans with Disabilities Act, the Employee Retirement Income Security Act, the Family and Medical Leave Act and any other federal, state or local statutory laws relating to employment, discrimination in employment, termination of employment, wages, benefits or otherwise or any other federal, state or local constitution, statute, rule or regulation, including, without limitation, any ordinance addressing fair employment practices, any Claims for employment or reemployment by the Company Released Parties (and the Employee hereby agrees not to seek such employment or reemployment in future), any common-law Claims, including, without limitation, any actions in tort, defamation and breach of contract, any Claim or damage arising out of the d.The Employee understands and agrees that nothing in this Release Agreement limits or interferes with the Employee’s right, without notice to or authorization of the Company, to communicate in good faith with any Government Agency (as defined below) for the purpose of reporting a possible violation of law, or to participate in any investigation or proceeding that may be conducted by any Government Agency, including by providing documents or other information, or for the purpose of filing a charge or complaint with a Government Agency.
Employee’s employment with or separation from the Company Released Parties (including any Claim for retaliation) under any common-law theory or any federal, state or local statute or ordinance not expressly referenced above, and any and all Claims for attorneys’ fees and costs.
b.The Employee understands that the Employee is releasing the Company Released Parties from Claims that Employee may not know about as of the date of the execution of this Release Agreement and that it is the Employee’s knowing and voluntary intent even though the Employee recognizes that someday the Employee may learn that some or all of the facts the Employee currently believes to be true are untrue and even though the Employee might then regret having signed this Release Agreement. Nevertheless, the Employee understands that the Employee is expressly assuming that risk and agrees that this Release Agreement will remain effective in all respects in any such case. The Employee expressly and completely waives all rights the Employee may have under any law that is intended to protect the Employee from waiving unknown claims, and the Employee understands the significance of doing so.
c.In consideration of the terms set forth in this Release Agreement, the Employee represents that Employee has not filed or permitted to be filed against the Company Released Parties any charges, complaints or lawsuits, and the Employee covenants and agrees that the Employee will not file or permit to be filed any lawsuits at any time hereafter with respect to the subject matter of this Release Agreement and claims released pursuant to this Release Agreement (including, without limitation, any claims relating to the termination of the Employee’s employment) except as may be necessary to enforce this Release Agreement or to seek a determination of the validity of the waiver of the Employee’s rights under ADEA.
As used in this Release Agreement, “Government Agency” means the Equal Employment Opportunity Commission, the National Labor Relations Board, the Occupational Safety and Health Administration, the U.S. Securities and Exchange Commission, the Financial Industry Regulatory Authority, any other self-regulatory organization or any other federal, state or local governmental agency or commission. In the event that the Employee files a charge or complaint with a Government Agency, or a Government Agency asserts a claim on the Employee’s behalf, the Employee agrees that the Employee’s release of claims in this Release Agreement will nevertheless bar the Employee’s right to any monetary or other recovery (including reinstatement), except that the Employee does not waive: (i) the Employee’s right to receive a whistleblower award from a Government Agency for information provided to such Government Agency; (ii) any recovery to which the Employee may be entitled pursuant to workers’ compensation and unemployment insurance laws; and (iii) any other right where a waiver is expressly prohibited by law. Moreover, nothing in this Release Agreement prevents or waives the Employee’s right to challenge the validity of this Release Agreement under ADEA as amended by the OWBPA or otherwise.
e.Nothing in this Section 1, or elsewhere in this Release Agreement, is intended as, or will be deemed or operate as, a release by the Employee of (i) any claims for [severance / Change in Control Benefits] or other payments to which the Employee is entitled under the express language of the Employment Agreement, (ii) any claims for vested benefits under any tax-qualified or other employee benefit plans of the Company and its affiliates, (iii) any rights of the Employee as an equityholder of the Company or any of its affiliates, (iv) any rights that the Employee had immediately prior to the Employee’s termination with respect to indemnification by any Company Released Party or coverage under any directors’ and officers’ insurance policy and any run-off policy thereto, (v) any rights that cannot be waived under applicable law or (vi) any claims arising after the date of this Release Agreement.
2.No Admission of Wrongdoing. Neither by offering to make nor by making this agreement does either party admit any failure of performance, wrongdoing or violation of law.
3. |
Acknowledgments. The Employee acknowledges that: |
a.Before entering into this Release Agreement, the Employee has had the opportunity to consult any attorney or other advisor of the Employee’s choice, and the Employee has been advised to do so if the Employee chooses;
b.The Employee has entered into this Release Agreement of the Employee’s own free will, and no promises or representations have been made to the Employee by any person to induce the Employee to enter into this Release Agreement other than the express terms set forth herein;
c.The Employee has read this Release Agreement and understands all of its terms, including the release of claims and covenant not to sue set forth in Section 1 above;
d.The Severance is in consideration of this release of claims and covenant not to sue and constitute consideration in addition to anything of value to which the Employee is already entitled;
e.The Employee has 21 days within which to consider this Release Agreement (although the Employee may choose voluntarily to sign it sooner);
f.The Employee has seven days following the date the Employee signs this Release Agreement to revoke this Release Agreement, which revocation will be effected by the Employee’s delivery of a written notice of such revocation to [●]; and
g.This Release Agreement will not become effective or enforceable until the first day following the expiration of the seven-day revocation period, provided that the Employee has signed, returned to the Company and not revoked this Release Agreement.
4.Miscellaneous. This Release Agreement, for all purposes, will be construed in accordance with the laws of the State of Florida without regard to any conflict- or choice-of-law principles that would call for the application of the law of another jurisdiction. The provisions of the Employment Agreement that survive its termination will remain in full force and effect in accordance with the terms of the Employment Agreement. There will be no presumption that any ambiguity in this Release Agreement should be resolved in favor of one party hereto and against another party hereto. Any controversy concerning the construction of this Release Agreement will be decided neutrally without regard to authorship. This Release Agreement may be executed in multiple counterparts, each of which will be deemed an original and will have the same effect as if the signatures to each were on the same instrument.
THE UNDERSIGNED HAVE CAREFULLY READ THE FOREGOING RELEASE AGREEMENT, KNOW THE CONTENTS THEREOF, FULLY UNDERSTAND IT AND SIGN THE SAME AS HIS, HER OR ITS OWN FREE ACT.
[remainder of page intentionally left blank; signature page follows]
IN WITNESS WHEREOF, the parties hereto have executed this Separation and Release Agreement as of the date first above written.
|
PROFOUND MEDICAL (US) INC. |
|||
|
|
|
||
|
By: |
|
||
|
|
Name: |
|
|
|
|
Title: |
|
|
|
|
|
||
|
|
|
||
EXECUTIVE |
|
|
||
|
|
|
||
Signature: |
|
|
|
|
Print Name: |
|
|
|
|
EXHIBIT C
INVENTIONS
1.Prior Inventions Disclosure. The following is a complete list of all Prior Inventions (as provided in Subsection 5.2 of the attached Employment Agreement):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
IN WITNESS WHEREOF the parties have duly executed this Agreement as of the 1st day of January, 2020.
/s/ ARUNMENAWAT |
|
ARUNMENAWAT |
|
PROFOUND MEDICAL (US) INC.
Per:
Name: Arun Menawat
Title:C.E.O.
PROFOUND MEDICAL CORP., for the limited purpose of section 1.1, 2.3 and 16.1 hereof
Per: |
/s/ Arthur Rosenthal |
|
Name: Arthur Rosenthal |
|
|
Title: Director |
|
|

Personal & Confidential
August 14, 2024
Arun Menawat
Dear Arun,
I am pleased to confirm that effective retroactively from April 1, 2024, your new annual salary has been adjusted to 550,000 USD. The retroactive amount will be included in the August 31, 2024 pay date.
In addition to the increase of your base salary, your annual discretionary bonus will increase from 75% to 80% of your base salary for 2024. The bonus criteria are as follows:
| ● | 50% will be based on achieving the company's revenue target |
| ● | 25% will be based on completion of reimbursement goals |
| ● | 25% will be based on securing financing to maintain the company's balance sheet, with a minimum raise of $30 million. |
Sincerely,
/s/ Brian Ellacott
Brian Ellacott
Lead Independent Director
Exhibit 10.2

EMPLOYMENT AGREEMENT
THIS EMPLOYMENT AGREEMENT (the “Agreement”) dated as of the 14th day of October 2024 (the “Effective Date”).
BETWEEN:
PROFOUND MEDICAL (US) INC.
(the “Company”)
AND:
THOMAS TAMBERRINO
(the “Employee”)
NOW THEREFORE in consideration of the covenants and agreements herein, the sufficiency of which is acknowledged by each of the parties, the parties agree as follows:
1. |
EMPLOYMENT |
1.1 |
Title: The Employee shall serve the Company as its Chief Commercial Officer. The Employee shall report directly to the Chief Executive Officer (“CEO”) and shall have such duties and responsibilities commensurate with his title. The Employee acknowledges the Company may re- assign, re-allocate, or re-organize the Employee’s duties and responsibilities only if reasonably based on changing circumstances, provided such duties and responsibilities are consistent with the role of a Chief Commercial Officer of a similarly sized Company and do not reflect a material adverse change in the Employee’s status, duties or reporting relationship. |
1.2 |
Service: During the term of this Agreement, in addition to the Employee’s common law duties to the Company, the Employee covenants and agrees as follows: |
(a) |
Loyalty to the Company: Throughout the Employee’s employment, the Employee will faithfully serve the Company and use the Employee’s best efforts to promote the business of the Company. The Employee will act honestly and in good faith, in the best interests of the Company. |
(b) |
Service: The Employee’s hours and schedule may change from time to time depending on the Company’s business needs. The Employee shall not engage in any other business, profession or occupation, or become an officer, employee, contractor for service, agent, or representative of any other company, partnership, firm, person, organization, or enterprise during the term of this Agreement; provided that the Employee may engage in the approved outside activities set forth in Exhibit A. |
(c) |
No Personal Benefit: The Employee will not receive or accept for the Employee’s own benefit or for any other person or entity’s benefit, either directly or indirectly, any commission, rebate, discount, gratuity or profit from any person or entity having or proposing to have one or more business transactions with the Company, without the prior |

approval of the Company, except as permitted by the Company policy.
(d) |
Business Opportunities: During the Employee’s employment with the Company, the Employee will, to the extent reasonably practicable, communicate and channel to the Company all knowledge, business and customer contacts and any other information that could concern or be in any way beneficial to the business of the Company. Any such information communicated to the Company as aforesaid will be and remain the property of the Company notwithstanding any subsequent termination of the Employee’s employment. |
(e) |
Place of Work: The Employee may work remotely within the United States, provided that the Employee will be required to travel (both domestically and internationally) as required by the Company from time to time in order to perform the Employee’s duties. |
(f) |
Pre-existing Obligations: The Employee is hereby requested and directed by the Company to comply with any existing common law, contractual or statutory obligations to the Employee’s former employer and to any other person or entity. The Company is not employing the Employee to obtain the confidential information or business opportunities of the Employee’s former employer or any other person or entity. |
1.3 |
Exclusion/Debarment: The Employee represents and warrants that the Employee has never been, and as a condition of continued employment shall never be, during the term of this Agreement, excluded from any contracting by any Canadian or United States government agency or authority. The Employee further represents and warrants that the Employee is not subject to any final adverse action, as that term is defined in 42 U.S.C. § 1320a-7e(g), and that no final adverse action has previously occurred or is pending or threatened against the Employee. The Employee represents and warrants that the Employee is not under investigation by the FDA or any other Canadian, United States or foreign regulatory agency nor has the Employee been debarred, suspended, proposed for debarment, declared ineligible, or voluntarily excluded by any Canadian, United States or foreign government department or agency. |
1.4 |
Company Policy: The Employee acknowledges and agrees that the employment relationship will be governed by the standards and terms established by the Company’s policies as they are established from time to time and the Employee agrees to comply with the terms of such policies which may be introduced, amended, deleted or modified in the sole discretion of the Company, provided that doing so does not constitute a material change in the Employee’s terms of employment. |
1.5 |
Professional Conduct: The Employee acknowledges and agrees that effective performance of the Employee’s duties requires integrity and the Company’s confidence in the Employee’s working relationships with other employees of the Company and with all other persons with whom the Employee deals in the course of employment. |
2. |
COMPENSATION |
2.1 |
Base Salary: The Employee will earn an annualized salary of (i) US$300,000 from the Effective Date (the “Base Salary”), less applicable deductions, payable in arrears for all services and work the Employee performs for the Company. Any increases will be in the discretion of the Board of Directors of the Company (the “Board”). The Base Salary will be payable in accordance with the Company’s normal payroll practices. |
2.2 |
Variable compensation: Beginning in the fiscal year 2025, the Employee will have the opportunity to earn an annual commission of up to $300,000 (the “Target Commission”), which shall be based on the annual topline global revenue target for Profound Medical Corp. (the “Parent”) and its subsidiaries as determined for each year by the Board and senior management team (the “Revenue |

Target”), and as reported on the Parent’s public financial statements. If seventy percent (70%) of the Revenue Target is achieved, the Employee shall earn seventy percent (70%) of the Target Commission. For each additional percentage point achieved after seventy percent (70%) of the Revenue Target, the Employee shall earn a commensurate increase of the Employee’s Target Revenue, up to a maximum of one hundred percent (100%) of the Target Commission is earned. If at least seventy (70%) of the Revenue Target in a given year is not achieved, the Employee will not earn any commission for that year but the Board, in its sole discretion, may elect to award the Employee a discretionary commission for that year. By way of example only, if 87% of the Revenue Target is achieved, the Employee shall earn 87% of the Employee’s Target Commission. If, however (and again by way of example only), only 46% of the Revenue Target is achieved, the Employee will receive 0% of the Employee’s Target Commission.
For the fiscal year 2024 only, the Employee’s Target Commission shall be $75,000 (e.g. 25% of the maximum Target Commission) and shall be guaranteed by the Company (the “2024 Guaranteed Commission”). The 2024 Guaranteed Commission will be paid on the same schedule as all other similar commissions.
Any earned Target Commission, minus applicable tax deductions, will be paid as soon as practicable after the revenue computations for the applicable year have been made, following the last pay date of the fiscal year. The Employee must be employed with the Company at the time the Target Commission is paid to earn the Target Commission unless the Employee is entitled to Severance Benefits or Change in Control Benefits (as those terms are defined below), the conditions of which are set forth in this Agreement.
The Company will periodically review its compensation programs and commission plans and reserves the right to modify any such programs and plans accordingly.
2.3 |
Stock Options: The management of the Company will request the Board on the Board meeting following the Employee’s start date to grant options for 200,000 shares of common stock (the “Options”) in the Parent. Each option shall vest and become exercisable as to 1/4 on the first anniversary of the Employee’s start date and as to 1/36 on the first day of each calendar month thereafter. Once approved, the Options will be granted as soon as reasonably practicable and shall be subject to the terms and conditions of the Profound Medical Amended and Restated Option Plan (the “Plan”). |
2.4 |
Benefits: The Employee will be entitled to participate in the standard insurance plans and other benefit programs (the “Employee Benefits”) which are substantially similar, to the extent practical and commercially reasonable, which the Parent offers to its executive level employees from time- to-time. The Company reserves the right to unilaterally revise the terms of the Employee Benefits, to change carriers, or to eliminate any Employee Benefits altogether. The Employee Benefits will be provided in accordance with the formal plan documents or policies and any issues with respect to entitlement or payment of benefits under any of the Employee Benefits will be governed by the terms of such documents or policies establishing the benefits in issue and will be a matter between the Employee and the insurer. The Company’s liability with respect to the Employee Benefits will be limited to the payment of its share of applicable premiums. Employee Benefits shall include Directors and Officers liability insurance and Employee shall be covered by and subject to any indemnification policies maintained by the Company. |
2.5 |
Car Allowance: The Company shall provide the Employee with a monthly car allowance of $600.00, subject to applicable taxes and withholdings. |
2.6 |
Expenses: The Company will reimburse the Employee for all reasonable business expenses actually and exclusively incurred in connection with the performance of duties under this Agreement, provided such expenses are incurred and accounted for in accordance with the general |

policies and procedures of the Company, as established from time to time.
2.7 |
National Holidays: The Company recognizes the national holidays observed in the United States. |
2.8 |
Vacation: The Employee shall be entitled to four (4) weeks of paid vacation per annum, to be taken at such reasonable times as the Company shall in its discretion permit and in accordance with the Company’s vacation policy in effect from time to time. Vacation entitlement for any partial year of employment shall be pro-rated based on the portion of the year worked by the Employee. |
3. |
TERMINATION OF AGREEMENT AND EMPLOYMENT |
3.1 |
Obligations Upon Termination: Upon termination of the Employee’s employment, for any reason: |
(a) |
the Company shall pay the Employee for (i) all unpaid Base Salary, and any unused vacation that may be required to be paid out by law, earned or accrued up to and including the Employee’s last day of employment (the “Termination Date”), such payment to be made no later than the first regular payroll date of the Company following the Termination Date; (ii) reimbursement of any eligible unreimbursed business expenses incurred by Employee through the Termination Date and timely and properly reported by Employee, such reimbursement to be paid in accordance with Company policy; and (iii) any other payments and benefits to which Employee may be entitled under the terms of any applicable compensation or benefit arrangement of the Company or under applicable law as of the Termination Date (collectively, Sections 3.1(a)(i) – (iii) are the “Accrued Obligations”); |
(b) |
all benefits coverage and other perquisites of the Employee’s employment shall cease on the later of the Termination Date, subject to applicable law and plan terms; and |
(c) |
all files, computer disks, information and documents pertaining to the Company’s business shall remain the property of the Company, and shall promptly be delivered by the Employee to the Company’s office, and no copy, duplication or reproduction of any kind whatsoever shall be made of such files, computer disks, information or documents, or retained by the Employee, without the express written consent of the Company. The Employee agrees to deliver all electronic information to the Company and to destroy any copies held by the Employee belonging to the Company. |
3.2 |
Termination by the Employee: The Employee may terminate this Agreement at any time: |
(a) |
without the occurrence of a Good Reason Event (as that term is defined below) by providing the Company with ninety (90) days’ prior written notice. Upon receipt of such notice, the Company may at any time terminate the employment of the Employee without further obligation or compensation to the Employee other than the Accrued Obligations. |
(b) |
with the occurrence of a Good Reason Event, in which case the Employee shall be entitled to receive the Accrued Obligations and the Severance Benefits (as defined below) provided that the Employee complies with the Employee’s obligations set forth in Section 3.8. |
(c) |
For the purposes of this Agreement, “Good Reason Event” means the existence of any one or more of the following conditions without the Employee’s consent, provided the Employee submits written notice to the Company within 45 days of when such condition(s) first arose specifying the condition(s): (A) material diminution of the Employee’s role in the Company; (B) material diminution of the Employee’s status in the |

Company; or (C) any materially detrimental change in compensation and benefits (considered as a whole) offered by the Company but will exclude any “across the board” reductions in compensation and benefits of similarly situated Company employees. The Employee’s continued employment subsequent to an event that may constitute a Good Reason Event shall not be deemed to be a waiver of the Employee’s rights under this provision (subject to the 45-day time period specified herein). Upon receipt of written notice from the Employee regarding a condition constituting a Good Reason Event, the Company shall then have 30 days to correct the condition (the “Cure Period”). If such condition is not corrected by the last day of the Cure Period, the Employee’s resignation for Good Reason shall become effective on the 31st day following the Employee’s written notice specifying the events giving rise to a Good Reason Event resignation.
3.3 |
Termination by the Company: The Company may terminate the Employee’s employment at any time: |
(a) |
without Cause by providing the Employee (subject to Section 3.8 of this Agreement) with the Accrued Obligations and the following severance benefits (collectively, the “Severance Benefits”): (i) six (6) months of pay of Base Salary (but without giving effect to any reduction if one or all of the bases for the Employee’s resignation for a Good Reason Event is a reduction in Base Salary) payable to the Employee in installments beginning with the first practicable payroll period occurring upon the later of (A) after the Release (as that term is defined below) becomes irrevocable and effective or (B) the expiration of the Transition Period, and will continue in accordance with the Company’s normal payroll practices; (ii) any earned but unpaid Target Commission for a previous calendar year; (iii) if applicable and not yet paid, the 2024 Guaranteed Commission, which shall be payable in a single lump sum in the first practicable payroll period occurring after the Release becomes irrevocable and effective; and (iv) the pro-rated amount of the Target Commission that the Employee could have earned had the Employee been employed by the Company for the full fiscal year, which shall be payable in a single lump sum in the first practicable payroll period occurring after the Release becomes irrevocable and effective. For purposes of this Section 3.3(a)(iv) only, the topline revenue earned by the Parent and its subsidiaries through the end of the Employee’s last full month of work shall be used to calculate the pro-rated Revenue Target. |
By way of example only, if the Employee is terminated without Cause in May and the total revenue for the calendar year through April was $3,000,000, and the Revenue Target for that year was $12,000,000, for these purposes the projected total revenue for that year would be $9,000,000. Seventy-five percent of the Revenue Target would have been achieved for that year and, therefore we assume the Employee would have received 75% of the Target Commission ($225,000) had the Employee been employed through the expected date of payment of the Target Commission for such year. Under this Section 3.3(a)(iv), and again by way of example only, the Employee would be then be eligible to receive a prorated portion of such amount based on the number of days elapsed in the calendar year through the date of termination ($225,000 multiplied by a ratio, the numerator which is the number of days elapsed in the calendar year through the date of termination and the denominator which is 365).
(b) |
for frustration of contract upon the death of the Employee or if the Employee becomes subject to a Disability. For purposes of this Agreement, “Disability” means the Employee is unable to perform the essential functions of the Employee’s position, with or without a reasonable accommodation, for a period of 90 consecutive calendar days or 180 non- consecutive calendar days within any rolling 12-month period. In the event of a termination pursuant to this Section 3.3(b), the Company shall pay the Employee (or the |

Employee’s estate) the Accrued Obligations and no further compensation.
(c) |
at any time for Cause, without notice or pay in lieu of notice. In the event of a termination for Cause, the Company shall pay the Employee the Accrued Obligations and no further compensation. For the purposes of this Agreement, “Cause” shall be defined as: |
i. |
any material breach of the provisions of this Agreement or the Company’s policies by the Employee if the Company has provided the Employee, with written notice of such breach and such breach has not been remedied within thirty (30) days thereafter; |
ii. |
any intentional or grossly negligent disclosure of any Confidential Information by the Employee; |
iii. |
any fraud, misappropriation of the property or funds of the Company or its affiliates, embezzlement, or other similar acts of dishonesty; |
iv. |
the willful allowance by the Employee of the Employee’s duty to the Company or its affiliates and the Employee’s personal interests to come into conflict in a material way in relation to any transaction or matter that is of a substantial nature, other than conflicts which are fully disclosed and approved by the Board; |
v. |
the Employee’s criminal, or other conviction of any criminal summary conviction offence or indictable offence (other than a traffic citation) that is detrimental to the business or reputation of the Company or its affiliates; or |
vi. |
if the Employee’s willful misconduct or gross negligence causes the Employee to be subject to a final and non-appealable enforcement order (or regulatory settlement in respect thereof) concerning a securities, financial services, health or other regulatory offence that is detrimental to the business or reputation of the Company or its affiliates. |
3.4 |
Termination in Connection with a Change in Control: In the event that either the Company terminates the Employee without Cause or the Employee resigns following a Good Reason Event, in either instance to occur within twelve (12) months following the occurrence of a Change in Control, and provided that the Employee complies with the Employee’s obligations set forth in Section 3.8, the Employee shall be entitled to receive the following benefits (the “Change in Control Severance Benefits”): (i) the Accrued Obligations; (ii) the Severance Benefits; and (iii) any unvested Options shall immediately vest if the Employee was employed for twelve (12) months prior to the Change in Control. If any provision of this Section 3.4 conflicts with any provision of the Plan or any applicable award agreement, the provision more favorable to the Employee shall govern. |
The parties acknowledge and agree that the payments and benefits described in this Section 3.4 are intended to be in lieu of, and not in addition to, the payments and benefits described in Sections 3.2 or 3.3 and, accordingly, in the event that the Employee is eligible for and/or receives any payments or benefits pursuant to this Section 3.4, the Employee shall not be eligible for and/or receive any payments or benefits pursuant to Sections 3.2 or 3.3.
For the purposes of this Agreement, “Change in Control” shall be defined as: (i) a reorganization or merger of the Parent with or into any other corporation or corporations or on an acquisition of securities of the Parent (excluding an equity financing of the Parent with the principal purpose of raising capital or a merger with any subsidiary or other affiliate) in which transaction the Parent’s shareholders immediately prior to such transaction own immediately after such transaction, less than 50% of the voting power of the surviving corporation or its parent, and/or (ii) the sale or disposition by the Parent to an unrelated third party of substantially all of its business and assets.

Without limiting the foregoing, a transaction will not constitute a Change in Control if (x) its sole purpose is to change the jurisdiction of the Parent’s incorporation, or (y) its sole purpose is to create a holding company or other entity that will be owned in substantially the same proportions by the persons who held the Parent’s securities immediately prior to such transaction.
3.5 |
Timing of Payments: All amounts payable hereunder in respect of Base Salary shall be paid in installments in accordance with the Company’s normal payroll practices. Payments in respect of annual or prorated commissions shall be paid at the time the annual commissions would otherwise be paid unless specifically set forth in this Agreement. All payments hereunder shall be subject to the usual employment withholdings and deductions as required by applicable law. |
3.6 |
No Implied Entitlement: Other than as expressly provided herein, the Employee will not be entitled to receive any further pay or compensation, severance pay, notice, payment in lieu of notice, incentives, bonuses, benefits or damages of any kind from the Company or from any affiliate of the Company. The Employee will not be entitled to receive any further pay or compensation (except for pay, if any, accrued and owing under this Agreement up to the date of termination of employment) from the Company, and for clarity, without limiting the foregoing, the Employee will not be entitled to any bonus or pro rata bonus payment that has not already been awarded to the Employee by the Company, except as set forth in this Agreement. |
3.7 |
Continued Effect: Notwithstanding any changes in the terms and conditions of the Employee’s employment which may occur in the future, including any changes in position, duties or compensation, the termination provisions in this Agreement will continue to be in effect for the duration of the Employee’s employment with the Company unless otherwise amended in writing and signed by the Company. |
3.8 |
Obligations Upon Termination: The Employee agrees that the Employee shall not be entitled to receive any severance fee or other benefits under Sections 3.2, 3.3, or 3.4 of this Agreement if the Employee breaches any of his obligations arising under Sections 4, 5, 6, 7, 8, and 10 hereof. The Employee acknowledges that until a release in the form of Exhibit B hereto (a “Release”) is timely executed and delivered to the Company and becomes effective and irrevocable, the Company will not make any severance payments or benefits due under Sections 3.2, 3.3, or 3.4 of this Agreement following termination of the Employee. The Employee further acknowledges that if the Release is not timely executed and delivered to the Company, or if it is revoked following delivery, the severance payments and other benefits described in Sections 3.2, 3.3, or 3.4 shall be forfeited. |
4. |
CONFIDENTIAL INFORMATION PROTECTIONS |
4.1 |
At all times during and after the Employee’s employment, the Employee will hold in confidence and will not disclose, use, lecture upon, or publish any of the Company’s Confidential Information (defined below), except as may be required in connection with the Employee’s work for the Company, or as expressly authorized by the Board. Unless forming part of the Employee’s customary duties for the Company, the Employee will obtain the written approval of the Board before publishing or submitting for publication any material (written, oral, or otherwise) that relates to the Employee’s work at the Company and/or incorporates any Confidential Information. The Employee hereby assigns to the Company any rights the Employee may have or acquire in any and all Confidential Information and recognize that all Confidential Information shall be the sole and exclusive property of the Company and its assigns. |
4.2 |
The term “Confidential Information” shall mean any and all confidential knowledge, data or information related to the Company’s business or its actual or demonstrably anticipated research or |

development, including without limitation (a) trade secrets, inventions, ideas, processes, computer source and object code, data, formulae, programs, other works of authorship, know-how, improvements, discoveries, developments, designs, and techniques; (b) information regarding products, services, plans for research and development, marketing and business plans, budgets, financial statements, contracts, prices, suppliers, employees and customers; (c) information regarding the skills and compensation of the Company’s employees, contractors, and any other service providers of the Company and (d) the existence of any business discussions, negotiations, or agreements between the Company and any third party. Confidential Information shall not include any information which enters the public domain not as a result of the Employee’s breach of this Agreement or any other obligations owed to the Company or which the Employee had knowledge of prior to the first date of employment with the Company as evidenced through written records.
4.3 |
The Employee understands that the Company has received and in the future will receive from third parties confidential or proprietary information (“Third Party Information”) subject to a duty on the Company’s part to maintain the confidentiality of such information and to use it only for certain limited purposes. During and after the term of the Employee’s employment, the Employee will hold Third Party Information in strict confidence and will not disclose to anyone (other than the Company personnel who need to know such information in connection with their work for the Company) or use, Third Party Information, except in connection with the Employee’s work for the Company or unless expressly authorized by an officer of the Company in writing. |
4.4 |
The Employee represents that employment by the Company does not and will not breach any agreement with any former employer, including any non-compete agreement or any agreement to keep in confidence or refrain from using information acquired by the Employee prior to employment by the Company. The Employee further represents that the Employee has not entered into, and will not enter into, any agreement, either written or oral, in conflict with the Employee’s obligations under this Agreement. During employment by the Company, the Employee will not improperly make use of, or disclose, any information or trade secrets of any former employer or other third party, nor will the Employee bring onto the premises of the Company or use any unpublished documents or any property belonging to any former employer or other third party, in violation of any lawful agreements with that former employer or third party. The Employee will use in the performance of the Employee’s duties only information that is generally known and used by persons with training and experience comparable to the Employee’s own, is common knowledge in the industry or otherwise legally in the public domain, or is otherwise provided or developed by the Company. |
4.5 |
Notwithstanding any other provision of this Agreement: (a) the Employee will not be held criminally or civilly liable under any federal or state trade secret law for any disclosure of a trade secret that: (i) is made (A) in confidence to a federal, state, or local government official, either directly or indirectly, or to an attorney, and (B) solely for the purpose of reporting or investigating a suspected violation of law; or (ii) is made in a complaint or other document filed under seal in a lawsuit or other proceeding; and (b) if the Employee files a lawsuit for retaliation by the Company for reporting a suspected violation of law, the Employee may disclose the Company’s trade secrets to the Employee’s attorney and use the trade secret information in the court proceeding if the Employee: (i) files any document containing trade secrets under seal; and (ii) does not disclose trade secrets, except pursuant to court order. |
4.6 |
The Employee understands that nothing contained in this Agreement limits the Employee’s ability to file a charge or complaint with the Equal Employment Opportunity Commission, the National Labor Relations Board, the Occupational Safety and Health Administration, the Securities and Exchange Commission or any other federal, state or local governmental agency or commission (each, a “Government Agency”). The Employee further understands that this Agreement does not limit the Employee’s ability to communicate with any Government Agencies or otherwise |

participate in any investigation or proceeding that may be conducted by any Government Agency, including providing documents or other information, without notice to the Company. This Agreement does not limit the Employee’s right to receive an award for information provided to any Government Agencies.
5. |
RETURN OF COMPANY PROPERTY |
5.1 |
Without limiting the provisions of Section 3.1(c), upon termination of the Employee’s employment or upon the Company’s request at any other time, the Employee will deliver to the Company all of the Company’s property, equipment, and documents, together with all copies thereof, and any other material containing or disclosing any Inventions, Third Party Information or Confidential Information and certify in writing that the Employee has fully complied with the foregoing obligation. The Employee agrees that the Employee will not copy, delete, or alter any information contained upon any Company computer or Company equipment before the Employee returns it to the Company. In addition, if the Employee has used any personal computer, server, or e-mail system to receive, store, review, prepare or transmit any Company information, including but not limited to, Confidential Information, the Employee agrees to provide the Company with a computer- useable copy of all such Confidential Information and then permanently delete and expunge such Confidential Information from those systems; and the Employee agrees to provide the Company access to the Employee’s system as reasonably requested to verify that the necessary copying and/or deletion is completed. Employee may retain any documents evidencing his terms of employment and compensation without violation hereto. |
6. |
NON-COMPETITION/NON-SOLICITATION |
6.1 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (1) year after termination of such employment for any reason, the Employee will not, without the Company’s express written consent, anywhere in the world, be employed by or contribute to any business activity that is competitive with the business of the Company or its affiliates or involves the use of real-time magnetic resonance imaging or ultrasound or similar technologies in the delivery of ablative tools. |
6.2 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (1) year after termination of such employment for any reason, the Employee will not, either directly or indirectly, solicit or attempt to solicit any employee, independent contractor, or consultant of the Company or its affiliates to terminate his, her or its relationship with Company or its affiliates in order to become an employee, consultant, or independent contractor to or for any other person or entity. |
7. |
NOTIFICATION OF NEW EMPLOYER |
7.1 |
After the termination of this Agreement, the Employee consents to the notification of any new employer of the Employee’s rights and obligations under this Agreement, by the Company providing a copy of this Agreement. |
8. |
INJUNCTIVE RELIEF |
8.1 |
The Employee acknowledges and agrees that: (a) the services to be rendered by the Employee to the Company are of a special and unique character; (b) the Employee will obtain knowledge and skill relevant to the Company’s industry, methods of doing business and marketing strategies by virtue of the Employee’s employment with the Company; (c) the restrictive covenants and other terms and conditions of this Agreement are reasonable and reasonably necessary to protect the legitimate business interest of the Company; (d) the amount of the Employee’s compensation reflects, in part, the Employee’s obligations and the Company’s rights under the restrictive covenants set forth |

herein; (e) the Employee has no expectation of any additional compensation, royalties or other payment of any kind not otherwise referenced herein in connection herewith; and (f) the Employee will not be subject to undue hardship by reason of full compliance with the terms and conditions of the restrictive covenants set forth herein or the Company’s enforcement thereof. The Employee further acknowledges that, because the Employee’s services are personal and unique and because the Employee will have access to the Confidential Information, any breach of the restrictive covenants set forth in this Agreement would cause irreparable injury to the Company and/or its affiliates for which monetary damages would not be an adequate remedy and, therefore, any such actual or threatened breach will entitle the Company to seek, in addition to other available remedies, temporary or permanent injunctive relief (including specific performance) or other equitable relief, without the necessity of showing any actual damages or that money damages would not afford an adequate remedy, and without the necessity of posting any bond or other security. The rights and remedies provided to each party in this Agreement are cumulative and in addition to any other rights and remedies available to such party at law or in equity. In addition, the Employee acknowledges and agrees that, should the Employee violate any restrictive covenant, the obligation at issue will be tolled for the period of the violation and will resume to run from the first date on which the Employee ceases to be in violation of the obligation. The Employee further acknowledges and agrees that if should breach any restrictive covenant, then, in addition, to the Company’s other legal and equitable remedies, the Company may suspend or cease payment of any Severance Benefits or Change in Control Benefits, payments, and benefits to which the Employee might otherwise be entitled.
9. |
PUBLICITY |
9.1 |
Except to the extent required by the Employee’s duties or customarily performed by the Employee, the Employee shall not, without the prior written consent of the Company, make or give any public announcements, press releases or statements to the public or the press regarding the Employee’s work or the Company’s business. Updating the Employee’s social media, including without limitation LinkedIn, to reference the Employee’s position or title with the Company shall not be considered a violation hereto. |
10. |
NOTICES |
10.1 |
All notices, requests, demands and other communications to be given pursuant to the terms of this Agreement shall be in writing and shall be deemed to have been duly given (i) on the date of delivery if personally delivered or sent by a recognized overnight courier or (ii) on the date of transmission if sent by email (provided notice is then also promptly delivered personally or by a recognized overnight courier), in each case to the parties at the addresses listed below: |
(a) |
If to the Employee, at the Employee’s then current address in the Company’s payroll records with a copy (which shall not constitute notice) to Evan Belosa, Esq., |
###############;
(b) |
If to the Company: |
Profound Medical (US) Inc.
Attention:Arun Menawat
E-mail:###################
With a copy (which shall not constitute notice) to:
Mintz LLP
200 Bay Street, South Tower

Suite 2800
Toronto, ON
Attention:Cheryl Reicin
Email:###############
Each party may change its address for receipt of notice by giving notice of the change to the other party.
11. |
SURVIVAL |
11.1 |
The Employee’s obligations under this Agreement shall survive the termination of the Employee’s employment, regardless of the manner or reason for termination, including, without limitation, those set forth in Sections 4 through 6 hereof. |
11.2 |
The assignment of this Agreement by the Company to any successor or other assignee and shall be binding upon the Employee’s heirs and legal representatives. |
12. |
SEVERABILITY |
12.1 |
Should any provision of this Agreement be held by a court of competent jurisdiction to be enforceable only if modified, or if any portion of this Agreement will be held as unenforceable and thus stricken, such holding will not affect the validity of the remainder of this Agreement, the balance of which will continue to be binding upon the parties with any such modification to become a part hereof and treated as though originally set forth in this Agreement. The parties further agree that any such court is expressly authorized to modify any such unenforceable provision of this Agreement in lieu of severing such unenforceable provision from this Agreement in its entirety, whether by rewriting the offending provision, deleting any or all of the offending provision, adding additional language to this Agreement or by making such other modifications as it deems warranted to carry out the intent and agreement of the parties as embodied herein to the maximum extent permitted by applicable law. The parties expressly agree that this Agreement as so modified by the court will be binding upon and enforceable against each of them. In any event, should one or more of the provisions of this Agreement be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability will not affect any other provisions hereof, and if such provision or provisions are not modified as provided above, this Agreement will be construed as if such invalid, illegal or unenforceable provisions had not been set forth herein. |
13. |
WAIVER |
13.1 |
Any waiver or failure to enforce any provision of this Agreement on one occasion will not be deemed a waiver of that provision or any other provision on any other occasion. |
14. |
ASSIGNMENT |
14.1 |
The Company may assign this Agreement to (i) an affiliate, subsidiary, related company or partnership without prior notice or consent of the Employee, or (ii) a new employer in connection with any transaction or reorganization; provided that any such successor or assignee expressly assumes in writing the Company’s obligations under this Agreement and has direct or indirect responsibility for managing the underlying business of the Company. This Agreement will inure to the benefit of the Company and its permitted successors and assigns. The Employee may not assign any of his rights nor delegate any of the duties hereunder. |

15. |
ENTIRE AGREEMENT |
15.1 |
This Agreement is the final, complete and exclusive agreement of the parties with respect to the subject matter hereof, and supersedes all prior and contemporaneous written and oral communications, understandings, agreements, representations and warranties between the Employee and the Company with respect to such matters. No modification of or amendment to this Agreement, or any waiver of any rights under this Agreement, will be effective unless in writing and signed by the Employee and the Company. Any subsequent change or changes in duties, salary or compensation will not affect the validity or scope of this Agreement. |
16. |
HEADINGS |
16.1 |
The headings utilized in this Agreement are for convenience only and are not to be construed in any way as additions or limitations of the covenants and agreements contained in this Agreement. |
17. |
INDEPENDENT LEGAL ADVICE |
17.1 |
The Employee acknowledges that the Employee has read and understands this Agreement, and acknowledges that an opportunity was provided to obtain separate legal advice about it. The Company shall promptly reimburse the Employee for the reasonable costs of obtaining legal advice regarding this Agreement, up to a maximum reimbursement of $5,000. |
18. |
COUNTERPARTS |
18.1 |
This Agreement may be executed in two or more counterparts, each of which will be deemed to be an original and all of which will constitute one Agreement. |
19. |
LAWS |
19.1 |
This Agreement shall be governed by and interpreted in accordance with the laws of the State of Delaware without regard to any conflict- or choice-of-law principle that would call for the application of the law of another jurisdiction. |
20. |
COMPANY AND AFFILIATES |
20.1 |
Whenever the context suggests , the term “the Company” shall also include the Parent and the affiliates of the Company and of the Parent, including, without limitation, for purposes of Sections 4-6 and 8. Without limiting the foregoing, Confidential Information of the Company includes information of the Parent and the affiliates of the Company and of the Parent, and the non solicitation and noncompetition clauses relate to the employees and business of the Parent and the and the affiliates of the Company and of the Parent. |
21. |
TAX MATTERS |
21.1 |
The Company will have the right to withhold and deduct from any payments or deemed payments hereunder any amounts of tax withholding that it determines be required by federal, state and local law, whether U.S. or non-U.S. |
21.2 |
This Agreement is intended to comply with or satisfy an exemption from Section 409A of the |
U.S. Internal Revenue Code of 1986, as amended, and the Treasury Regulations and other administrative guidance promulgated thereunder (collectively, “Section 409A”), and will be construed and administered in accordance with Section 409A. Notwithstanding any other provision of this Agreement, payments provided under this Agreement may only be made upon an event and in a manner that complies with Section 409A or an applicable exemption. Any payments under this Agreement that may be excluded from Section 409A either as separation pay due to an involuntary separation from service or as a short-term deferral will be excluded from Section 409A to the maximum extent possible.

If this Agreement provides for a payment to be made within a window of time (e.g., “payment will be made within 30 days”), the date that payment is actually to be made will be within the sole and absolute discretion of the Company. To the extent that any payment or benefit described in this Agreement constitutes “non-qualified deferred compensation” under Section 409A, and to the extent that such payment or benefit is payable upon the Employee’s termination of employment, then such payments or benefits will be payable only upon the Employee’s “separation from service” as determined under Section 409A, and “termination,” “termination of employment” and like terms will be construed accordingly. Furthermore, the parties hereto acknowledge and agree that all or any part of any payment to be made to the Employee within six months following the Employee’s separation from service other than by reason of the Employee’s death will instead be paid in a lump sum without interest on the first business day that is more than six months after the date of the Employee’s separation from service (or, if later, the first business day that is more than 18 months after the applicable date of correction, as required by Internal Revenue Service Notice 2010-6) if the Company determines in good faith that the Employee is a “specified employee” within the meaning of Section 409A and that the earlier payment of such amount would give rise to tax, penalties or interest under Section 409A. All in- kind benefits provided and expenses eligible for reimbursement under this Agreement will be provided by the Company or incurred by the Employee during the time periods set forth in this Agreement. All reimbursements will be paid as soon as administratively practicable, but in no event will any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year will not affect the in- kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year. Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit. Notwithstanding the foregoing, the Company makes no representations that the payments and benefits provided under this Agreement comply with Section 409A, and in no event will the Company be liable for all or any portion of any taxes, penalties, interest or other expenses that may be incurred by the Employee pursuant to Section 409A.
IN WITNESS WHEREOF the parties have duly executed this Agreement as of the 14th day of October, 2024.
ARUN MENAWAT
PROFOUND MEDICAL (US) INC.
Per:
Name:Arun Menawat
Title:C.E.O.

Signature: /s/ THOMAS TAMBERRINO |
|
|
|
Print Name: THOMAS TAMBERRINO |
|
|
|

Exhibit A
AUTHORIZED OUTSIDE ACTIVITIES
Consistent with the provisions of Section 1.2(b) of the Employment Agreement between Profound Medical (US) Inc. (the “Company”) and Thomas Tamberrino (the “Employee”) dated as of the 14th day of October 2024 (the “Effective Date”), the Employee may engage in the following outside activities but only to the extent that such activities shall, at all times, not interfere with the performance of the Employee’s duties to the Company or create a conflict of interest with the Company:
1. |
The Employee may participate on one (1) board of directors of a charitable organization at a time, which participation must be approved in advance by the Company’s Chief Executive Officer (“CEO”) in the CEO’s sole discretion, acting reasonably. Any board participation approved pursuant to this subsection is limited to a maximum of twelve (12) hours per year; and |
2. |
The Employee may retain any professional licenses that the Employee holds as of the Effective Date and may devote no more than twelve (12) hours every two years to maintaining those professional licenses. |

Exhibit B
SEPARATION AND RELEASE AGREEMENT
This Separation and Release Agreement (this “Release Agreement”) is entered into as of [●], by and between Thomas Tamberrino (the “Employee”) and Profound Medical (US) Inc. (the “Company”).
RECITALS
WHEREAS, the Company and the Employee, for limited purposes, previously entered into an Employment Agreement dated as of October 14, 2024 (the “Employment Agreement”);
WHEREAS, the Employee’s employment with the Company has terminated effective as of [●]; and
WHEREAS, capitalized terms used but not defined herein have the meanings ascribed to them in the Employment Agreement.
NOW, THEREFORE, in consideration of the Employee’s employment and the mutual covenants, promises and obligations set forth herein and in the Employment Agreement, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. |
General Release and Covenant Not to Sue. |
a.In consideration of the Employee’s right to [severance pursuant to the Employment Agreement], the Employee, on behalf of the Employee and the Employee’s heirs, executors, administrators, trustees, legal representatives, successors and assigns (hereinafter collectively referred to for purposes of this Section 1 as the “Employee”), hereby agrees to irrevocably and unconditionally waive, release and forever discharge Parent, the Company and their respective past, present and future affiliates and related entities, parent and subsidiary corporations, divisions, shareholders, predecessors, and current, former and future officers, directors, employees, trustees, fiduciaries, administrators, executives, agents, representatives, investors, successors and assigns (collectively, the “Company Released Parties”) from any and all waivable claims, charges, demands, sums of money, actions, rights, promises, agreements, causes of action, obligations and liabilities of any kind or nature whatsoever, at law or in equity, whether known or unknown, existing or contingent, suspected or unsuspected, apparent or concealed, U.S., Canadian or otherwise (collectively, “Claims”) that the Employee has now or in the future may claim to have against any or all of the Company Released Parties based upon or arising out of any facts, acts, conduct, omissions, transactions, occurrences, contracts, claims, events, cause, matters or things of any conceivable kind or character existing or occurring or claimed to exist or to have occurred prior to the date of the Employee’s execution of this Release Agreement in any way whatsoever relating to or arising out of the Employee’s employment with the Company Released Parties or the termination of such employment. Such Claims include, without limitation, Claims arising under the Civil Rights Act, the Age Discrimination in Employment Act (“ADEA”), the Older Workers Benefit Protection Act (the “OWBPA”), the Americans with Disabilities Act, the Employee Retirement Income Security Act, the Family and Medical Leave Act and any other federal, state or local statutory laws relating to employment, discrimination in employment, termination of employment, wages, benefits or otherwise or any other federal, state or local constitution, statute, rule or regulation, including, without limitation, any ordinance addressing fair employment practices, any Claims for employment or reemployment by the Company Released Parties (and the Employee hereby agrees not to seek such employment or reemployment in future), any common-law Claims, including, without limitation, any actions in tort, defamation and breach of contract, any Claim or damage arising out of the d.In further consideration of the terms set forth in this Release Agreement, the Employee will provide reasonable services to the Company on an as- needed and part-time basis for a period of up to three

Employee’s employment with or separation from the Company Released Parties (including any Claim for retaliation) under any common-law theory or any federal, state or local statute or ordinance not expressly referenced above, and any and all Claims for attorneys’ fees and costs.
b.The Employee understands that the Employee is releasing the Company Released Parties from Claims that Employee may not know about as of the date of the execution of this Release Agreement and that it is the Employee’s knowing and voluntary intent even though the Employee recognizes that someday the Employee may learn that some or all of the facts the Employee currently believes to be true are untrue and even though the Employee might then regret having signed this Release Agreement. Nevertheless, the Employee understands that the Employee is expressly assuming that risk and agrees that this Release Agreement will remain effective in all respects in any such case. The Employee expressly and completely waives all rights the Employee may have under any law that is intended to protect the Employee from waiving unknown claims, and the Employee understands the significance of doing so.
c.In consideration of the terms set forth in this Release Agreement, the Employee represents that Employee has not filed or permitted to be filed against the Company Released Parties any charges, complaints or lawsuits, and the Employee covenants and agrees that the Employee will not file or permit to be filed any lawsuits at any time hereafter with respect to the subject matter of this Release Agreement and claims released pursuant to this Release Agreement (including, without limitation, any claims relating to the termination of the Employee’s employment) except as may be necessary to enforce this Release Agreement or to seek a determination of the validity of the waiver of the Employee’s rights under ADEA.
(3) months following the termination of his employment (the “Transition Period”), including assistance with transition of duties, unless the requirement for active service is expressly waived in whole or in part by the Company, in its sole discretion. The Employee agrees that the Company may, in its sole discretion, limit or discontinue the employee’s access to business records and Confidential Information during the Transition Period. The Employee agrees that any services provided to the Company during the Transition Period shall be for no additional compensation in excess of the severance payments and benefits contemplated under the Agreement. Regardless of whether or not the Employee provides active service during the Transition Period, the Employee shall continue to abide by all obligations owing under the Agreement. The Severance Benefits or the Change in Control Benefits are not subject to mitigation if the Employee receives compensation during the Severance Period from third parties as long as the Employee provides the services during the Transition Period as set forth herein.
e.The Employee understands and agrees that nothing in this Release Agreement limits or interferes with the Employee’s right, without notice to or authorization of the Company, to communicate in good faith with any Government Agency (as defined below) for the purpose of reporting a possible violation of law, or to participate in any investigation or proceeding that may be conducted by any Government Agency, including by providing documents or other information, or for the purpose of filing a charge or complaint with a Government Agency. As used in this Release Agreement, “Government Agency” means the Equal Employment Opportunity Commission, the National Labor Relations Board, the Occupational Safety and Health Administration, the U.S. Securities and Exchange Commission, the Financial Industry Regulatory Authority, any other self-regulatory organization or any other federal, state or local governmental agency or commission. In the event that the Employee files a charge or complaint with a Government Agency, or a Government Agency asserts a claim on the Employee’s behalf, the Employee agrees that the Employee’s release of claims in this Release Agreement will nevertheless bar the Employee’s right to any monetary or other recovery (including reinstatement), except that the Employee does not waive: (i) the Employee’s right to receive a whistleblower award from a Government Agency for information provided to such Government Agency; (ii) any recovery to which the Employee may be entitled pursuant to workers’ compensation and unemployment insurance laws; and (iii) any other right where a waiver is expressly prohibited by law. Moreover, nothing in this Release Agreement prevents or waives the Employee’s right to challenge the validity of this Release Agreement under ADEA as amended by the OWBPA or otherwise.

f.Nothing in this Section 1, or elsewhere in this Release Agreement, is intended as, or will be deemed or operate as, a release by the Employee of (i) any claims for Severance Benefits, Change in Control Benefits, or other payments to which the Employee is entitled under the express language of the Employment Agreement, (ii) any claims for vested benefits under any tax-qualified or other employee benefit plans of the Company and its affiliates, (iii) any rights of the Employee as an equityholder of the Company or any of its affiliates, (iv) any rights that the Employee had immediately prior to the Employee’s termination with respect to indemnification by any Company Released Party or coverage under any directors’ and officers’ insurance policy and any run-off policy thereto, (v) any rights that cannot be waived under applicable law or (vi) any claims arising after the date of this Release Agreement.
2.No Admission of Wrongdoing. Neither by offering to make nor by making this agreement does either party admit any failure of performance, wrongdoing or violation of law.
3. |
Acknowledgments. The Employee acknowledges that: |
a.Before entering into this Release Agreement, the Employee has had the opportunity to consult any attorney or other advisor of the Employee’s choice, and the Employee has been advised to do so if the Employee chooses;
b.The Employee has entered into this Release Agreement of the Employee’s own free will, and no promises or representations have been made to the Employee by any person to induce the Employee to enter into this Release Agreement other than the express terms set forth herein;
c.The Employee has read this Release Agreement and understands all of its terms, including the release of claims and covenant not to sue set forth in Section 1 above;
d.The Severance is in consideration of this release of claims and covenant not to sue and constitute consideration in addition to anything of value to which the Employee is already entitled;
e.The Employee has 21 days within which to consider this Release Agreement (although the Employee may choose voluntarily to sign it sooner);
f.The Employee has seven days following the date the Employee signs this Release Agreement to revoke this Release Agreement, which revocation will be effected by the Employee’s delivery of a written notice of such revocation to [●]; and
g.This Release Agreement will not become effective or enforceable until the first day following the expiration of the seven-day revocation period, provided that the Employee has signed, returned to the Company and not revoked this Release Agreement.
4.Miscellaneous. This Release Agreement, for all purposes, will be construed in accordance with the laws of the State of Florida without regard to any conflict- or choice-of-law principles that would call for the application of the law of another jurisdiction. The provisions of the Employment Agreement that survive its termination will remain in full force and effect in accordance with the terms of the Employment Agreement. There will be no presumption that any ambiguity in this Release Agreement should be resolved in favor of one party hereto and against another party hereto. Any controversy concerning the construction of this Release Agreement will be decided neutrally without regard to authorship. This Release Agreement may be executed in multiple counterparts, each of which will be deemed an original and will have the same effect as if the signatures to each were on the same instrument.
THE UNDERSIGNED HAVE CAREFULLY READ THE FOREGOING RELEASE AGREEMENT,

KNOW THE CONTENTS THEREOF, FULLY UNDERSTAND IT AND SIGN THE SAME AS HIS, HER OR ITS OWN FREE ACT.
[remainder of page intentionally left blank; signature page follows]

IN WITNESS WHEREOF, the parties hereto have executed this Separation and Release Agreement as of the date first above written.
|
PROFOUND MEDICAL (US) INC. |
|||
|
|
|
||
|
By: |
|
||
|
|
Name: |
|
|
|
|
Title: |
|
|
|
|
|
||
|
|
|
||
EMPLOYEE |
|
|
||
|
|
|
||
Signature: |
|
|
|
|
Print Name: |
|
|
|
|
Exhibit 10.3

EMPLOYMENT AGREEMENT
THIS AGREEMENT dated as of March 22, 2022, has an effective date of February 1, 2022 (the “Start date”), and signed acceptance of this agreement date of on or before March 23, 2022, the expiry date of this offer of employment.
BETWEEN:
Profound
(the “Employer” or “Company”)
AND:
Rashed Dewan (the “Employee”)
WHEREAS:
A. |
The Employer has offered to employ the Employee on the terms and conditions described in this Agreement; and |
B. |
The Employee wishes to provide services to the Employer on such terms and conditions. |
NOW THEREFORE in consideration of the covenants and agreements herein, the sufficiency of which is acknowledged by each of the parties, the parties agree as follows:
1. |
EMPLOYMENT |
1.1 |
Start Date: The Employee’s employment with the Company will commence on the “Start date” and will continue until this Agreement is terminated in accordance with section 4 herein. |
1.2 |
Title: The Employer agrees to employ the Employee as Chief Financial Officer (CFO), upon the terms and conditions set out in this Agreement. The Employee will report to the Chief Executive Officer (the ‘CEO’). The Employee acknowledges that he shall undertake such duties and responsibilities for the Company and its affiliates in connection with the Employee’s position as are determined by the CEO from time to time. |
1.3 |
Service: During the term of this Agreement, in addition to the Employee’s common law duties to the Company, the Employee covenants and agrees as follows: |
(a) |
Loyalty to the Company: Throughout the Employee’s employment, the Employee will well and faithfully serve the Company and use the Employee’s best efforts to promote the business of the Company. The Employee will act honestly and in good faith, in the best interests of the Company. |
(b) |
Service: The Employee shall devote his full working time and attention to the affairs of the Company. The Employee shall not engage in any other business, profession or occupation, or become an officer, employee, contractor for service, agent, or representative of any other company, partnership, firm, person, organization, or enterprise during the term of this Agreement, The Employee will not, during the Employee’s employment with the Company, engage in any business, enterprise or |
Page 2
activity that is contrary to or detracts from the due performance of the Employee’s duties or the business of the Company.
(c) |
No Personal Benefit: The Employee will not receive or accept for the Employee’s own benefit or for any other person or entity’s benefit, either directly or indirectly, any commission, rebate, discount, gratuity or profit from any person or entity having or proposing to have one or more business transactions with the Company, without the prior approval of the Company. |
(d) |
Business Opportunities: During the Employee’s employment with the Company, the Employee will communicate and channel to the Company all knowledge, business and customer contacts and any other information that could concern or be in any way beneficial to the business of the Company. Any such information communicated to the Company as aforesaid will be and remain the property of the Company notwithstanding any subsequent termination of the Employee’s employment. |
(e) |
Place of Work: You will work from our Toronto-area offices. You may also be required to undertake reasonable business travel on a periodic basis, both in Canada and internationally. Accordingly, you must be and remain able to travel internationally and must always maintain an up-to-date passport. |
(f) |
Pre-existing Obligations. The Employee is hereby requested and directed by the Company to comply with any existing common law, contractual or statutory obligations to the Employee’s former employer and to any other person or entity. The Company is not employing the Employee to obtain the confidential information or business opportunities of the Employee’s former employer or any other person or entity. |
(g) |
Qualifications, Licences and Permits: The Employee must hold and maintain the appropriate class of licence, certificate, degree, accreditation, qualification, visa and/or permit (“Authorizations”) for the proper performance of the Employee’s duties, and to work in the jurisdictions in which the Employee is performing the Employee’s duties. The Company may require the Employee to provide copies of Authorizations which are necessary for the Employee to perform the Employee’s duties. Should the Employee fail to make reasonable efforts to maintain such Authorizations or if the Employee misrepresents such Authorizations, the Employee’s employment will be deemed frustrated and will terminate without compensation or notice of any kind. All expenses related to the acquisition and maintenance of Authorizations will be borne by the Company. |
1.4 |
Directorship and Offices: Upon the termination of employment with the Company for any reason, the Employee will immediately resign any directorship or office the Employee may hold in the Company or any parent, subsidiary or affiliated companies of the Company and, except as provided expressly in this Agreement, the Employee will not be entitled to receive any written notice of termination or payment in lieu of notice, or to receive any severance pay, damages or compensation for loss of any directorship, office or otherwise. The Employee agrees that failure to tender such resignation(s) will amount to cause for termination, for which the Company may treat the employment as being terminated for after-acquired cause. Notwithstanding the foregoing, the termination of employment will not automatically disqualify the Employee from serving as a director. |
1.5 |
Exclusion/Debarment: The Employee represents and warrants that the Employee has never been, and as a condition of continued employment shall never be, during the term of this Agreement, |
Page 3
excluded from any contracting by any Canadian or United States government agency or authority. The Employee further represents and warrants that the Employee is not subject to any final adverse action, as that term is defined in 42 U.S.C. § 1320a-7e(g), and that no final adverse action has previously occurred or is pending or threatened against the Employee. The Employee represents and warrants that the Employee is not under investigation by the FDA or any other Canadian, United States or foreign regulatory agency nor has the Employee been debarred, suspended, proposed for debarment, declared ineligible, or voluntarily excluded by any Canadian, United States or foreign government department or agency.
1.6 |
Company Policy: The Employee acknowledges and agrees that the employment relationship will be governed by the standards and terms established by the Employer’s policies as they are established from time to time and the Employee agrees to comply with the terms of such policies which may be introduced, amended, deleted or modified in the sole discretion of the Employer. |
1.7 |
Professional Conduct: The Employee acknowledges and agrees that effective performance of the Employee’s duties requires the highest level of integrity and the Employer’s complete confidence in the Employee’s relationship with other employees of the Employer and with all other persons with whom the Employee deals in the course of employment. |
2. |
COMPENSATION |
2.1 |
Base Salary: Effective February 1, 2022, the Employee will earn an annual salary of CAD $260,000.00 (the “Base Salary”), less applicable deductions, payable in arrears for all services and work the Employee performs for the Company. Effective August 1, 2022, the employee base salary will be increase to CAD $270,000.00. Any further increases will be in the discretion of the CEO. |
2.2 |
Annual Discretionary Bonus: Provided the Employee remains an employee of the Company in good standing and the Employee has not received or given notice of termination, the Employee will be eligible to receive an annual bonus of up to 40% of the Employee’s Base Salary based on the assessment of the CEO of the Employee’s and the Company’s achievement of certain milestones and objectives mutually agreed to by Employee and the CEO prior to the start of the bonus period. Such milestones and objectives (including the evaluation of achievement thereof), and the bonus amount, will be at the sole discretion of the CEO and will be payable in accordance with the Company’s standard payroll policies, subject to applicable payroll deductions and withholdings. |
Stock Options: The Employee will be eligible to participate in the Company’s stock option plan, subject to the terms of such plan as may be in place and amended from time to time.
2.3 |
Benefits: The Employee will continue to participate in the standard insurance plans and other benefit programs (the “Employee Benefits”) which the Company may offer to similarly situated employees from time-to-time. The Employer reserves the right to unilaterally revise the terms of the Employee Benefits, to change carriers, or to eliminate any Employee Benefits altogether. The Employee Benefits will be provided in accordance with the formal plan documents or policies and any issues with respect to entitlement or payment of benefits under any of the Employee Benefits will be governed by the terms of such documents or policies establishing the benefits in issue and will be a matter between the Employee and the insurer. The Employer’s liability with respect to the Employee Benefits will be limited to the payment of its share of applicable premiums. |
Expenses: The Company will reimburse the Employee for all reasonable business expenses actually and exclusively incurred in connection with the performance of duties under this
Page 4
Agreement, provided such expenses are incurred in accordance with the general policies and procedures of the Employer, as established from time to time.
Statutory Holidays: The Employer recognizes the statutory holidays observed in Ontario.
Vacation: The Employee shall be entitled to 4 weeks of paid vacation per annum, to be taken at such reasonable times as the Employer shall in its discretion permit and in accordance with the Company’s vacation policy in effect from time to time. Vacation entitlement for any partial year of employment shall be pro-rated based on the portion of the year worked by the Employee.
3. |
TERMINATION OF AGREEMENT AND EMPLOYMENT |
3.1 |
Upon termination of the Employee’s employment, for any reason: |
(a) |
the Employer shall pay the Employee all unpaid Base Salary and vacation pay earned up to and including the Employee’s last day of employment (the “Termination Date”); |
(b) |
all benefits coverage and other perquisites of the Employee’s employment shall cease on the later of the Termination Date or as specified in 3.3 (a); |
(c) |
all files, computer disks, information and documents pertaining to the Employer’s business shall remain the property of the Employer and shall promptly be delivered by the Employee to the Employer’s office, and no copy, duplication or reproduction of any kind whatsoever shall be made of such files, computer disks, information or documents, or retained by the Employee, without the express written consent of the Employer. The Employee agrees to destroy all electronic information belonging to the Employer. |
3.2 |
Termination by Employee: The Employee may terminate this Agreement at any time by providing the Employer with thirty (30) days’ prior written notice. Upon receipt of such notice, the Employer may at any time terminate the employment of the Employee and pay the Employee the amount of Base Salary the Employee would have otherwise received during the balance of the aforementioned notice period. |
3.3 |
Termination by Employer: The Employer may terminate the Employee’s employment at any time: |
a) |
without Cause by providing the Employee with the greater of: (i) working notice or pay in lieu of notice, benefit continuation (and severance pay, if applicable) that is required by the Ontario Employment Standards Act, 2000 as amended or (ii) six (6) months of working notice or pay in lieu of notice if terminated on or before twelve (12) months after the Effective Date, which shall be increased to twelve (12) months of working notice or pay of Base Salary in lieu of notice if terminated after twelve (12) months after the Effective Date (the “Severance Benefit and Severance Period”); |
b) |
for frustration of contract upon the death of the Employee or any incapacitation of the Employee that constitutes an undue hardship for the Employer; |
c) |
at any time for just Cause, without notice or pay in lieu of notice or any other form of compensation, severance pay or damages. For the purposes of this Agreement, “Cause” includes: |
Page 5
I. |
any material breach of the provisions of this Agreement by the Employee; |
II. |
any intentional or grossly negligent disclosure of any confidential information by the Employee; |
III |
conduct on the Employee’s part that is materially detrimental to the business or the financial position of the Employer; |
IV. |
personal conduct on the Employee’s part which is of such a serious and substantial nature that it will injure the reputation of the Employer if the Employee was retained as an employee; or |
V. |
any and all commissions, omissions or other conduct which would constitute just cause at law, in addition to the specified causes noted above. |
3.4 |
Termination in Connection with Change in Control: |
This Agreement terminates if it is not assumed by the successor corporation (or affiliate thereto) upon a Change in Control (as defined below).
(a) |
“Change in Control” means a reorganization or merger of the Company with or into any other corporation or corporations or on an acquisition of securities of the Company (excluding an equity financing of the Company with the principal purpose of raising capital or a merger with any subsidiary or other affiliate) in which transaction the Company’s shareholders immediately prior to such transaction own immediately after such transaction, less than 50% of the voting power of the surviving corporation or its parent, and/or (ii) the sale or disposition by the Company to an unrelated third party of substantially all of its business and assets. Without limiting the foregoing, a transaction will not constitute a Change in Control if (a) its sole purpose is to change the jurisdiction of the Company’s incorporation, or (b) its sole purpose is to create a holding company that will be owned in substantially the same proportions by the persons who held the Company’s securities immediately prior to such transaction. |
(b) |
If the Company terminates this Agreement other than for Cause in advance of the closing, or the Company’s successor terminates this Agreement other than for Cause within 12 months following the occurrence of a Change in Control of the Company, and the Employee signs and returns to the Company without revocation a release prepared by the Company of all legally waivable claims related to or arising from the Employee’s employment with the Company, then (i) the Company shall pay the Employee a sum equal to (A) twelve (12) months of Employee’s then-current annual Base Salary and (B) the then current annual target bonus and (ii) Employee’s Equity Incentive Awards shall vest as set forth in Section 3.4 (d) (collectively, the “Change in Control Benefits”); provided that in the event of a Change of Control that results in the holders of Common Shares receiving consideration for each Common Share of at least three (3) times the thirty (30) day average VWAP for a Common Share for the period ending on the day prior to the Effective Date, the amounts set forth in clauses (i)(A) and (B) above shall be increased from twelve (12) months to eighteen (18) |
Page 6
months of the Employee’s then current annual Base Salary and from 100% to 150% of the Employee’s then current annual target bonus, respectively. Amounts payable hereunder in respect of Base Salary shall be paid in installments in accordance with the Company’s normal payroll practices. Payments in respect of annual bonuses shall be paid at the time the annual bonus would otherwise be paid.
(c) |
If, within 12 months following a Change in Control, there is a material diminution of Employee’s role in the company, material diminution of status, or diminution of reporting structure, Employee shall have the right to resign and receive the Change in Control Benefits. |
(d) |
Effect Of Termination On Equity Incentive Award. |
I. |
If Employee is entitled to Change in Control Benefits, then any unvested Equity Incentive Awards shall immediately vest. |
II. |
If any provision of this Agreement conflicts with a provision of the Award Agreement and/or the Plan, the provision more favorable to the Employee shall govern. |
3.5 |
No Implied Entitlement: |
Other than as expressly provided herein, the Employee will not be entitled to receive any further pay or compensation, severance pay, notice, payment in lieu of notice, incentives, bonuses, benefits, or damages of any kind from the Company or from any affiliate of the Company. Any payment in lieu of the Severance Period provided to the Employee will be inclusive of any entitlements upon termination or severance pay pursuant to the Ontario Employment Standards Act, 2000 as amended, contract, tort, common law or otherwise, and will be subject to statutory withholdings. The Employee will not be entitled to receive any further pay or compensation (except for pay, if any, accrued and owing under this Agreement up to the date of termination of employment) from the Company, and for clarity, without limiting the foregoing, the Employee will not be entitled to any bonus or pro rata bonus payment that has not already been awarded to the Employee by the Company, except for that which was specified as part of the Change in Control Benefits.
3.6 |
Continued Effect: |
Notwithstanding any changes in the terms and conditions of the Employee’s employment which may occur in the future, including any changes in position, duties or compensation, the termination provisions in this Agreement will continue to be in effect for the duration of the Employee’s employment with the Company unless otherwise amended in writing and signed by the Company.
3.7 |
Obligations Upon Termination: |
Employee agrees that he shall not be entitled to receive any severance fee or other benefits under Section 3 of this Agreement if Employee breaches any of his obligations arising under Sections 4, 5, 6, 7, 8, 9 and 11 hereof. Employee acknowledges that until a release in the form of Exhibit B hereto (a “Release”) is timely executed and delivered to the Company, the Company will not be obligated to make any severance payments or provide any other benefits due under this Agreement following termination of Employee. Employee further acknowledges that if the Release is not timely executed and delivered to the Company, the severance payments and other benefits described in this Section 3 shall be forfeited, except as required by statutory minimum standards.
Page 7
4 |
CONFIDENTIAL INFORMATION PROTECTIONS |
4.1 |
At all times during and after the Employee’s employment, the Employee will hold in confidence and will not disclose, use, lecture upon, or publish any of Company’s Confidential Information (defined below), except as may be required in connection with the Employee’s work for Company, or as expressly authorized by the Board. The Employee will obtain the written approval of the Board before publishing or submitting for publication any material (written, oral, or otherwise) that relates to the Employee’s work at Company and/or incorporates any Confidential Information. The Employee hereby assigns to Company any rights Employee may have or acquire in any and all Confidential Information and recognize that all Confidential Information shall be the sole and exclusive property of Company and its assigns. |
4.2 |
The term “Confidential Information” shall mean any and all confidential knowledge, data or information related to Company’s or its affiliates business or its actual or demonstrably anticipated research or development, including without limitation (a) trade secrets, inventions, ideas, processes, computer source and object code, data, formulae, programs, other works of authorship, know-how, improvements, discoveries, developments, designs, and techniques; (b) information regarding products, services, plans for research and development, marketing and business plans, budgets, financial statements, contracts, prices, suppliers, employees and customers; (c) information regarding the skills and compensation of Company’s employees, contractors, and any other service providers of Company; and (d) the existence of any business discussions, negotiations, or agreements between Company and any third party. |
4.3 |
The Employee understands that Company has received and in the future will receive from third parties confidential or proprietary information (“Third Party Information”) subject to a duty on Company’s part to maintain the confidentiality of such information and to use it only for certain limited purposes. During and after the term of the Employee’s employment, the Employee will hold Third Party Information in strict confidence and will not disclose to anyone (other than Company personnel who need to know such information in connection with their work for Company) or use, Third Party Information, except in connection with the Employee’s work for Company or unless expressly authorized by an officer of Company in writing. |
4.4 |
The Employee represents that employment by Company does not and will not breach any agreement with any former employer, including any non-compete agreement or any agreement to keep in confidence or refrain from using information acquired by Employee prior to employment by Company. Employee further represents that Employee has not entered into, and will not enter into, any agreement, either written or oral, in conflict with Employee’s obligations under this Agreement. During employment by Company, Employee will not improperly make use of, or disclose, any information or trade secrets of any former employer or other third party, nor will Employee bring onto the premises of Company or use any unpublished documents or any property belonging to any former employer or other third party, in violation of any lawful agreements with that former employer or third party. Employee will use in the performance of Employee’s duties only information that is generally known and used by persons with training and experience comparable to the Employee’s own, is common knowledge in the industry or otherwise legally in the public domain or is otherwise provided or developed by Company. |
4.5 |
The obligations and rights granted in this section shall survive the termination of this agreement. |
Page 8
5 |
INVENTIONS |
5.1 |
As used in this Agreement, the term “Invention” means any ideas, concepts, information, materials, processes, data, programs, know-how, improvements, discoveries, developments, designs, artwork, formulae, other copyrightable works, and techniques and all Intellectual Property Rights in any of the items listed above. The term “Intellectual Property Rights” means all trade secrets, copyrights, trademarks, mask work rights, patents and other intellectual property rights recognized by the laws of any jurisdiction or country. The term “Moral Rights” means all paternity, integrity, disclosure, withdrawal, special and any other similar rights recognized by the laws of any jurisdiction or country. |
5.2 |
The Employee has disclosed in Exhibit C a complete list of all Inventions that (a) the Employee has, or has caused to be, alone or jointly with others, conceived, developed, or reduced to practice prior to the commencement of the Employee’s employment by Company; (b) in which the Employee has an ownership interest or which the Employee has a license to use; (c) and that the Employee wishes to have excluded from the scope of this Agreement (collectively referred to as “Prior Inventions”). If no Prior Inventions are listed in Exhibit C, the Employee warrants that there are no Prior Inventions. The Employee agrees that the Employee will not incorporate, or permit to be incorporated, Prior Inventions in any Company Inventions (defined below) without Company’s prior written consent. If, in the course of the Employee’s employment with Company, the Employee incorporates a Prior Invention into a Company process, machine or other work, the Employee hereby grants Company a non-exclusive, perpetual, fully-paid and royalty-free, irrevocable and worldwide license, with rights to sublicense through multiple levels of sublicensees, to reproduce, make derivative works of, distribute, publicly perform, and publicly display in any form or medium, whether now known or later developed, make, have made, use, sell, import, offer for sale, and exercise any and all present or future rights in, such Prior Invention. |
5.3 |
Inventions assigned to the Company or to a third party as directed by the Company pursuant to the subsection 5.5 are referred to in this Agreement as “Company Inventions”. Subject to the subsection 5.5 and the Prior Inventions Employee has set forth in Exhibit C, Employee hereby assigns and agrees to assign in the future (when any such Inventions or Intellectual Property Rights are first reduced to practice or first fixed in a tangible medium, as applicable) to the Company all rights, title, and interest in and to any and all Inventions (and all Intellectual Property Rights with respect thereto) made, conceived, reduced to practice, or learned by Employee, either alone or with others, during the period of the Employee’s employment by the Company. Any assignment of Inventions (and all Intellectual Property Rights with respect thereto) hereunder includes an assignment of all Moral Rights. To the extent such Moral Rights cannot be assigned to the Company and to the extent the following is allowed by the laws in any country where Moral Rights exist, the Employee hereby unconditionally and irrevocably waives the enforcement of such Moral Rights, and all claims and causes of action of any kind against the Company or related to the Company’s customers, with respect to such rights. The Employee further acknowledges and agrees that neither the Employee’s successors-in-interest nor legal heirs retain any Moral Rights in any Inventions (and any Intellectual Property Rights with respect thereto). |
5.4 |
During the period of employment, the Employee will promptly and fully disclose to the Company in writing (a) all Inventions authored, conceived, or reduced to practice by the Employee, either alone or with others, and (b) all patent applications filed by the Employee or in which the Employee is named as an inventor or co-inventor. |
5.5 |
The Employee agrees that, as directed by the Company, the Employee will assign to a third party, including without limitation, all rights, title, and interest in and to any particular Company Invention. |
Page 9
5.6 |
During and after the period of employment and at the Company’s request and expense, the Employee will assist the Company in every proper way, including consenting to and joining in any action, to obtain and enforce Canadian, United States and foreign Intellectual Property Rights and Moral Rights relating to Company Inventions in all countries. If the Company is unable to secure the Employee’s signature on any document needed in connection with such purposes, the Employee hereby irrevocably designates and appoints the Company and its duly authorized officers and agents as the Employee’s agent and attorney in fact, which appointment is coupled with an interest, to act on the Employee’s behalf to execute and file any such documents and to do all other lawfully permitted acts to further such purposes with the same legal force and effect as if executed by the Employee. |
5.7 |
The Employee agrees that the Employee will not incorporate into any Company software or otherwise deliver to the Company any software code licensed under the GNU General Public License or Lesser General Public License or any other license that, by its terms, requires or conditions the use or distribution of such code on the disclosure, licensing, or distribution of any source code owned or licensed by the Company. |
5.8 |
The obligations and rights granted in this section shall survive the termination of this agreement. |
6 |
RETURN OF COMPANY PROPERTY |
6.1 |
Upon termination of the Employee’s employment or upon the Company’s request at any other time, the Employee will deliver to the Company all of the Company’s property, equipment, and documents, together with all copies thereof, and any other material containing or disclosing any Inventions, Third Party Information or Confidential Information and certify in writing that the Employee has fully complied with the foregoing obligation. The Employee agrees that the Employee will not copy, delete, or alter any information contained upon any Company computer or Company equipment before the Employee returns it to the Company. In addition, if the Employee has used any personal computer, server, or e-mail system to receive, store, review, prepare or transmit any Company information, including but not limited to, Confidential Information, the Employee agrees to provide the Company with a computer-useable copy of all such Confidential Information and then permanently delete and expunge such Confidential Information from those systems; and the Employee agrees to provide the Company access to the Employee’s system as reasonably requested to verify that the necessary copying and/or deletion is completed. The Employee further agrees that any property situated on the Company’s premises and owned by the Company is subject to inspection by the Company’s personnel at any time with or without notice. Prior to the termination of employment or promptly after termination of employment, the Employee will cooperate with the Company in attending an exit interview and certify in writing that the Employee has complied with the requirements of this section. |
6.2 |
The obligations and rights granted in this section shall survive the termination of this agreement. |
7 |
RECORDS |
7.1 |
The Employee agrees to keep and maintain adequate and current records (in the form of notes, sketches, drawings and in any other form that is required by the Company) of all Inventions made by the Employee during the period of the Employee’s employment by the Company, which records shall be available to, and remain the sole property of, the Company at all times. |
8 |
NON-COMPETITION/NON-SOLICITATION |
8.1 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (1) year after termination of such employment for any reason (except in the event of a |
Page 10
Change of Control pursuant to the proviso at the end of Section 3.4(b), in which event such period shall extend to (12) months after termination of such employment), the Employee will not, without the Company’s express written consent, anywhere in the world, be employed by or contribute to any business activity that is competitive with the business of the Company and involves the use ofreal-time magnetic resonance imaging or ultrasound or similar technologies in the delivery of ablative tools.
8.2 |
The Employee covenants and agrees that for the period of the Employee’ employment by the Company and for one (1) year after termination of such employment for any reason, the Employee will not, either directly or indirectly, solicit or attempt to solicit any employee, independent contractor, or consultant of the Company to terminate his, her or its relationship with Company in order to become an employee, consultant, or independent contractor to or for any other person or entity. |
8.3 |
The obligations and tights granted in this section shall survive the termination of this agreement. |
9 |
NOTIFICATION OF NEW EMPLOYER |
9.1 |
After the termination of this Agreement, the Employee consents to the notification of any new employer of the Employee’s rights and obligations under this Agreement, by the Company providing a copy of this Agreement or otherwise as the Company deems fit. |
10 |
INJUNCTIVE RELIEF |
10.1 The Employee acknowledges that, because the Employee’s services are personal and unique and because Employee will have access to the Confidential Information of the Company, any breach of this Agreement would cause irreparable injury to the Company for which monetary damages would not be an adequate remedy and, therefore, will entitle the Company to injunctive relief (including specific performance). The rights and remedies provided to each party in this Agreement are cumulative and in addition to any other rights and remedies available to such party at law or in equity.
11 |
PUBLICITY |
11.1 The Employee shall not, without the prior written consent of the Employer, make or give any public announcements, press releases or statements to the public or the press regarding the Employee’s work or the Employer’s business.
12 |
NOTICES |
12.1 All notices, requests, demands and other communications to be given pursuant to the terms of this Agreement shall be in writing and shall be deemed to have been duly given on the date of delivery if personally delivered, on the date of transmission if sent by facsimile, one day after being deposited with a recognized overnight courier with postage prepaid, or three days after being mailed first class, postage prepaid, in each case to the parties at the addresses listed below:
12.1.1If to Employee, at the Employee’s then current address in the Company’s payroll records;
12.1.2If to the Company: 2400 Skymark Ave. Unit #6, Mississauga ON L4W SKS 13.1 This Employee’s obligations under this Agreement shall survive the termination of the Employee’s employment, regardless of the manner or reason for termination.
Attention: Arun Menawat
E-mail: #########################
Page 11
Each party may change its address for receipt of notice by giving notice of the change to the other party.
13 |
SURVIVAL |
13.2 The assignment of this Agreement by the Company to any successor or other assignee and shall be binding upon the Employee’s heirs and legal representatives.
14 |
SEVERABILITY |
14.1 If any provision of this Agreement is, for any reason, held to be invalid or unenforceable, the other provisions of this Agreement will remain enforceable, and the invalid or unenforceable provision will be deemed modified so that it is valid and enforceable to the maximum extent permitted by law.
15 |
WAIVER |
15.1 Any waiver or failure to enforce any provision of this Agreement on one occasion will not be deemed a waiver of that provision or any other provision on any other occasion.
16 |
ASSIGNMENT |
16.1 The Company may assign this Agreement to (i) an affiliate, subsidiary, related company or partnership without prior notice or consent of the Employee, or (ii) a new employer in connection with any transaction or reorganization; provided that any such successor or assignee expressly assumes in writing the Company’s obligations under this Agreement and has responsibility for managing the underlying business of the Company. The Employee may not assign any of his rights nor delegate any of the duties hereunder.
17 |
ENTIRE AGREEMENT |
17.1 This Agreement is the final, complete and exclusive agreement of the parties with respect to the subject matter hereof and supersedes and merges all prior communications between us with respect to such matters. No modification of or amendment to this Agreement, or any waiver of any rights under this Agreement, will be effective unless in writing and signed by the Employee and the Company. Any subsequent change or changes in duties, salary or compensation will not affect the validity or scope of this Agreement.
18 |
HEADINGS |
18.1 The headings utilized in this Agreement are for convenience only and are not to be construed in any way as additions or limitations of the covenants and agreements contained in this Agreement.
19 |
INDEPENDENT LEGAL ADVICE |
19.1 The Employee acknowledges that the Employee has read and understands this Agreement, and acknowledges that an opportunity was provided to obtain legal advice about it.
Page 12
20 |
COUNTERPARTS |
20.1 This Agreement may be executed in two or more counterparts, each of which will be deemed to be an original and all of which will constitute one Agreement.
21 |
LAWS |
21.1 This Agreement shall be governed by and interpreted in accordance with the laws of Province and the laws of the Country applicable therein. The Employee and the Company hereby attorn to the exclusive jurisdiction of the superior courts of Ontario to resolve any dispute arising from this Agreement except to the extent the Company seeks injunctive relief outside Ontario where applicable to enforce the Employee’s covenants hereunder.
[Signature Page to Follow]

IN WITNESS WHEREOF the parties have duly executed this Agreement as of the 23 day of March , 2022.
Signed, Sealed and Delivered in the presence of: |
|
) |
|
|
/s/ Magdalena Gorkiewicz |
|
) |
|
|
Name (witness) |
|
) |
|
|
Magdalena Gorkiewicz |
|
) |
|
|
Address |
|
) |
|
|
2400 Skymark Avenue, Unit #6Mississauga, ON L4W 5K5 |
|
) |
|
|
|
|
) |
|
/s/ Rashed Dewan |
HR Operations Manager |
|
) |
|
Rashed Dewan |
Occupation |
|
) |
|
|
PROFOUND
Per: |
/s/ Arun Menawat |
|
|
Arun Menawat, CEO |
|

EXHIBIT A
Pursuant to Section 3.5(a) of the amended and restated share option plan of the Company dated December 8, 2016 (the “Share Option Plan”):
Unless otherwise specified by the Plan Administrator at the time of granting an Option and except as otherwise provided in this Plan, each Option will vest and be exercisable as follows:
Total Number of Option Shares |
|
Vesting Date |
1/4 |
|
On the first anniversary of the Date of Grant. |
|
|
|
1/36 |
|
On the first day of each calendar month following the first anniversary of the Date of Grant. |
Capitalized terms used in this Exhibit A shall have the meaning given in the Share Option Plan.

Exhibit B
R E L E A S E
I, Rashed Dewan of the City of , , for and in consideration of the sum of * ($*) and other good and valuable consideration which is being delivered to me concurrently with the execution and delivery hereof (the receipt and sufficiency of which is hereby acknowledged) do hereby remise, release and forever discharge PROFOUND MEDICAL CORP. (hereinafter called the “Company”), its officers, directors, servants, employees and agents, including any related or associated companies, and their heirs, executors, administrators, successors and assigns, as the case may be, of and from any and all manner of actions, causes of action, suits, contracts, claims, damages, costs and expenses of any nature or kind whatsoever, whether in law or in equity, which, as against the Company or such persons as aforesaid or any of them I have ever had, now have, or at any time hereafter I or my personal representatives can, shall or may have, by reason of or arising out of the termination of my employment with the Company or in any other way connected with my employment with the Company (except as otherwise set forth below), and more specifically, without limiting the generality of the foregoing, any and all claims for damages for termination of my employment, constructive termination of my employment, loss of position, loss of status, loss of future job opportunity, loss of opportunity to enhance my reputation, the timing of the termination and the manner in which it was effected, loss of bonuses, loss of benefits, including life insurance and short and long-term disability benefit coverage, and any other type of damages; provided, however, that notwithstanding anything contained herein, this Release does not apply to any claims related to (i) equity in the Company or its affiliates that I hold, directly or indirectly, including without limitation, the options granted to me in connection with my employment with the Company, or (ii) any unpaid reimbursement of expenses and accrued but unpaid salary or termination payments provided for in the Employment Agreement.
I FURTHER AGREE that this Release includes any and all claims I may have arising under contract, the Ontario Employment Standards Act, 2000, as amended, common law or other applicable law related to my employment with the Company and that the consideration provided includes any amount that I may be entitled to under such legislation and I agree to immediately withdraw any complaint as settled and not to file any complaint pursuant to such legislation with respect to my employment or the termination of my employment.
Page 2
IT IS UNDERSTOOD that the Company has withheld income tax and other statutory deductions from the aforesaid consideration and I agree to indemnify and hold harmless the Company from any further assessment for income tax or other statutory deductions which may be made under statutory authority.
IT IS FURTHER UNDERSTOOD that as a former officer of the Company I owe a fiduciary duty to the Company and I agree that the Company will suffer irreparable harm if confidential information or information unique to the Company is disclosed in any way by me without authorization of the Company and that I have returned to the Company originals or copies of the Company’s corporate records, files, financial documents, client lists, or any other material that is related to the Company’s business, clients, personnel or operations.
IT IS FURTHER UNDERSTOOD AND AGREED that this is not to be construed or considered as an admission of liability on the part of the Company. The terms of this Release set out the entire agreement between myself and the Company and are intended to be contractual and not a mere recital.
IT IS EXPRESSLY ACKNOWLEDGED that the contents, terms and effect of this Release have been explained to me by my lawyer and are fully understood.
IN WITNESS WHEREOF, I, Rashed Dewan, have hereunto set my hand and seal this day of , 20 in the City of ,
Rashed Dewan

EXHIBIT C
INVENTIONS
1.Prior Inventions Disclosure. The following is a complete list of all Prior Inventions (as provided in Subsection 5.2 of the attached Employment Agreement):
☐None
☐See immediately below:
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
____________________________________________________________________________________________________
|
|
Profound Medical Inc. |
Personal & Confidential
August 14, 2024
Rashed Dewan
Dear Rashed,
I am pleased to confirm that effective retroactively from April 1, 2024, your new annual salary has been adjusted to 300,000 USD. The retroactive amount will be included in the August 31, 2024 pay date. Your salary will be converted to and paid in your local currency using the Bank of Canada exchange rates from 1 or 2 business days before the semimonthly payroll submission
In addition to the increase of your base salary, your annual bonus increases from 40% to 50% of your base salary for 2024. The bonus criteria are as follows:
| ● | 50% will be based on achieving the company’s revenue target |
| ● | 25% will be based on your management of the balance sheet and financing support. |
| ● | 25% will be based on preparing the company to become a US domestic form filer on NASDAQ for FY 2024 including conversion of financial reporting to US GAAP. |
Please note that your title remains Chief Financial Officer (CFO), and you continue to report directly to the Chief Executive Officer (CEO).
If you have any questions regarding this adjustment, please feel free to reach out.
Best regards,
/s/ Arun Menawat
Arun Menawat
Chief Executive Officer
Exhibit 10.4

AMENDED AND RESTATED EMPLOYMENT AGREEMENT
THIS AMENDED AND RESTATED EMPLOYMENT AGREEMENT (the “Agreement”) dated as of August 20, 2019 (the“Effective Date”)
BETWEEN:
PROFOUND MEDICAL CORP.
(the “Company”)
AND:
MATHIEU BURTNYK (the “Employee”)
WHEREAS the Company and the Employee entered into a letter agreement as to employment on June 3, 2011 (the “Original Agreement”); and
WHEREAS the Company and the Employee wish to amend and restate the Original Agreement on the terms and conditions set forth herein.
NOW THEREFORE in consideration of the covenants and agreements herein, the sufficiency of which is acknowledged by each of the parties, the parties agree as follows:
1.EMPLOYMENT
1.1 |
Effectiveness of the Agreement: The terms and conditions of employment of the Employee by the Company prior to the Effective Date shall be governed by the Original Agreement and the attachments and exhibits thereto, and as of the Effective Date will be governed by the Agreement. The Employee’s employment with the Company commenced on July 7, 2011 and will continue until this Agreement is terminated in accordance with section 4 herein. |
I .2 |
Title: The Company agrees to continue to employ the Employee as VP Clinical Affairs upon the terms and conditions set out in this Agreement. The Employee will report to the Chief Executive Officer of the Company (the “CEO”) or such person the Company advises from time to time. The Employee acknowledges that he shall undertake such duties and responsibilities for the Company and its affiliates in connection with and commensurate with the Employee’s position as are detennined by the CEO or to anyone else the CEO designates from time to time. The Employee further acknowledges the Company may re-assign, re-allocate, or re-organize the Employee’s duties and responsibilities as circumstances change, provided such duties and responsibilities are consistent with the role of a VP Clinical Affairs. |
1.3 |
Service: During the term of this Agreement, in addition to the Employee’s common law duties to the Company, the Employee covenants and agrees as follows: |
(a) |
Loyalty to the Company: Throughout the Employee’s employment, the Employee will |
Page 2
faithfully serve the Company and use the Employee’s best efforts to promote the business of the Company. The Employee will act honestly and in good faith, in the best interests of the Company.
(b) |
Service: The Employee shall devote his full working time and attention to the affairs of the Company. The Employee shall not engage in any other business, profession or occupation, or become an officer, employee, contractor for service, agent, or representative of any other company, partnership, firm, person, organization, or enterprise during the term of this Agreement, provided that the Employee may participate on boards of directors of other organizations (to a maximum of one (I) at any one time) and in business associations, charitable organizations or other similar organizations, as may be approved by the CEO in its discretion, acting reasonably, and provided that such participation does not interfere with the proper discharge of his duties to the Company. The Employee currently has disclosed that he is not currently a director of a board. |
(c) |
No Personal Benefit: The Employee will not receive or accept for the Employee’s own benefit or for any other person or entity’s benefit, either directly or indirectly, any commission, rebate, discount, gratuity or profit from any person or entity having or proposing to have one or more business transactions with the Company, without the prior approval of the Company. |
(d) |
Business Opportunities: During the Employee’s employment with the Company, the Employee will communicate and channel to the Company all knowledge, business and customer contacts and any other information that could concern or be in any way beneficial to the business of the Company. Any such information communicated to the Company as aforesaid will be and remain the property of the Company notwithstandingany subsequent termination of the Employee’s employment. |
(e) |
Place of Work: The Employee will work from the Company’s premises located in 2400 Skymark Avenue, Unit #6, Mississauga, Ontario L4W 5K5 and it is anticipated that the Employee will be required to travel extensively (both domestically and internationally) in the course of performing the Employee’s duties. In addition, it is anticipated that the Company will increase its focus on sales in the U.S. |
(f) |
Pre-existing Obligations. The Employee is hereby requested and directed by the Company to comply with any existing common law, contractual or statutory obligations to the Employee’s former employer and to any other person or entity. The Company is not employing the Employee to obtain the confidential information or business opportunities of the Employee’s former employer or any other person or entity. |
(g) |
Qualifications, Licences and Permits: The Employee must hold and maintain the appropriate class of licence, certificate, degree, accreditation, qualification, visa and/or permit (“Authorizations”) for the proper performance of the Employee’s duties, and to work in the jurisdictions in which the Employee is performing the Employee’s duties. The Company may require the Employee to provide copies of Authorizations which are necessary for the Employee to perform the Employee’s duties. Should the Employee fail to make reasonable efforts to maintain such Authorizations or if the Employee misrepresents such Authorizations, the Employee’s employment will be deemed frustrated and will terminate without compensation or notice of any kind. All expenses related to the acquisition and maintenance of Authorizations will be borne by the Company. |
Page 3
1.4Exclusion/Debarment: The Employee represents and warrants that the Employee has never been, and as a condition of continued employment shall never be, during the term of this Agreement, excluded from any contracting by any Canadian or United States government agency or authority. The Employee further represents and warrants that the Employee is not subject to any final adverse action, as that term is defined in 42 U.S.C. § 1320a-7e(g), and that no final adverse action has previously occurred or is pending or threatened against the Employee. The Employee represents and warrants that the Employee is not under investigation by the FDA or any other Canadian, United States or foreign regulatory agency nor has the Employee been debarred, suspended, proposed for debarment, declared ineligible, or voluntarily excluded by any Canadian, United States or foreign government department or agency.
1.5 |
Company Policy: The Employee acknowledges and agrees that the employment relationship will be governed by the standards and terms established by the Company’s policies as they are established from time to time and the Employee agrees to comply with the terms of such policies which may be introduced, amended, deleted or modified in the sole discretion of the Company. |
1.6 |
Professional Conduct: The Employee acknowledges and agrees that effective performance of the Employee’s duties requires the highest level of integrity and the Company’s complete confidence in the Employee’s relationship with other employees of the Company and with all other persons with whom the Employee deals in the course of employment. |
2. |
COMPENSATION |
2.1 |
Base Salary: The Employee will earn an annual salary equivalent to CDN$160,500.00 (the “Base Salary”), less applicable deductions, payable in arrears for all services and work the Employee performs for the Company. Any increases will be in the discretion of the CEO. The Base Salary will be payable in accordance with the Company’s normal payroll practices and will be payable in Canadian dollars on the applicable payroll date. The Employee’s hours and schedule may change from time to time depending on the Company’s business needs. |
2.2 |
Annual Discretionary Bonus: Provided the Employee remains an employee of the Company in good standing and the Employee has not received or given notice of termination, for each calendar year, the Employee will be eligible to receive an annual bonus of up to 20% of the Employee’s Base Salary based on the assessment of the Board of the Employee’s and the Company’s achievement of certain milestones and objectives determined by the Board. Such milestones and objectives (including the evaluation of achievement thereof), and the bonus amount, will be at the sole discretion of the Board and will be payable in accordance with the Company’s standard payroll policies, subject to applicable payroll deductions and withholdings. Such bonus shall be paid within ninety (90) days after the end of the applicable calendar year. |
2.3 |
Stock Options: The options to purchase common shares of the Company previously received by the Employee (“Stock Options”) shall continue to vest during the term of this Agreement in accordance with their terms. The Company shall consider compensation asa whole, including stock incentives, of Vice President Clinical Affairs or similar roles of comparable companies when considering compensation or the grant of stock options for the Employee. |
2.4 |
Benefits: The Employee will be entitled to participate in the standard insurance plans and other benefit programs (the “Employee Benefits”) which the Company may offer to management level employees from time-to-time. The Company reserves the right to unilaterally revise the terms of the Employee Benefits, to change carriers, or to eliminate any Employee Benefits altogether. The Employee Benefits will be provided in accordance with the formal plan documents or policies and |
Page 4
any issues with respect to entitlement or payment of benefits under any of the Employee Benefits will be governed by the tenns of such documents or policies establishing the benefits in issue and will be a matter between the Employee and the insurer. The Company’s liability with respect to the Employee Benefits will be limited to the payment of its share of applicable premiums.
2.5 |
Expenses: The Company will reimburse the Employee for all reasonable business expenses actually and exclusively incurred in connection with the performance of duties under this Agreement, provided such expenses are incurred and accounted for in accordance with the general policies and procedures of the Company, as established from time to time. |
2.6 |
Statutory Holidays: The Company recognizes the statutory holidays observed in Ontario. |
2.7 |
Vacation: The Employee shall be entitled to 4 weeks of paid vacation per annum, to be taken at such reasonable times as the Company shall in its discretion permit and in accordance with the Company’s vacation policy in effect from time to time. Vacation entitlement for any partial year of employment shall be pro-rated based on the portion of the year worked by the Employee. |
3. |
TERMINATION OF AGREEMENT AND EMPLOYMENT |
3.1Obligations Upon Termination: Upon termination of the Employee’s employment, for any reason:
(a) |
the Company shall pay the Employee all unpaid Base Salary and vacation pay earned up to and including the Employee’s last day of employment (the “Termination Date”); |
(b) |
all benefits coverage and other perquisites of the Employee’s employment shall cease on the later of the Termination Date or as specified in 3.3(a); |
(c) |
all files, computer disks, information and documents pertaining to the Company’s business shall remain the property of the Company, and shall promptly be delivered by the Employee to the Company’s office, and no copy, duplication or reproduction of any kind whatsoever shall be made of such files, computer disks, information or documents, or retained by the Employee, without the express written consent of the Company. The Employee agrees to deliver all electronic information to the Company and to destroy any copies held by the Employee belonging to the Company. |
3.2 |
Termination by the Employee: The Employee may terminate this Agreement at any time by providing the Company with ninety (90) days’ prior written notice. Upon receipt of such notice, the Company may at any time terminate the employment of the Employee and pay the Employee the amount of Base Salary the Employee would have otherwise received during the balance of the aforementioned notice period. |
3.3Termination by the Company: The Company may terminate the Employee’s employment at any time:
(a) |
without Cause by providing the Employee with the greater of: (i) pay in lieu of notice, benefit continuation (and severance pay, if applicable) that is required by the Ontario Employment Standards Act, 2000 as amended or (ii) (A) a minimum of six (6) months of pay of Base Salary in lieu of notice (the “Severance Period”) and (8) an amount equal to the then current target annual bonus prorated based on the number of days elapsed in the calendar year until the date of termination as a percentage of the total number of days in |
Page 5
such calendar year. The Employee will continue to provide services to the Company on an as-needed basis for a period of up to I month following the termination of his employment (the “Transition Period”), including assistance with transition of duties, unless the requirement for active service is expressly waived in whole or in part by the Company, in its sole discretion. The Employee agrees that the Company may, in its sole discretion, limit or discontinue the Executive’s access to business records and Confidential Information during the Transition Period. The Employee agrees that any services provided to the Company during the Transition Period shall be for no additional compensation in excess of the severance payments and benefits contemplated under this Agreement. Regardless of whether or not the Employee provides active service during the Transition Period, the Employee shall continue to abide by all obligations owing under this Agreement;
(b) |
for frustration of contract upon the death of the Employee or any incapacitation of the Employee that constitutes an undue hardship for the Company; |
(c) |
at any time for just Cause, without notice or pay in lieu of notice or any other fonn of compensation, severance pay or damages. For the purposes of this Agreement., “Cause” includes: |
i. |
any material breach of the provisions of this Agreement by the Employee; |
ii. |
any intentional or grossly negligent disclosure of any confidential information by the Employee; |
iii. |
conduct on the Employee’s part that is materially detrimental to the business or the financial position of the Company; |
iv. |
personal conduct on the Employee’s part which is of such a serious and substantial nature that it will injure the reputation of the Company if the Employee was retained as an employee; or |
v. |
any and all commissions, omissions or other conduct which would constitute just cause at law, in addition to the specified causes noted above. |
3.4 |
Timing of Payments: All amounts payable hereunder in respect of Base Salary or with respect to payments made under the Ontario Employment Standards Act, 2000, as amended, shall be paid in installments in accordance with the Company’s normal payroll practices. Payments in respect of annual or prorated bonuses shall be paid at the time the annual bonus would otherwise be paid. |
3.5 |
No Implied Entitlement: Other than as expressly provided herein, the Employee will not be entitled to receive any further pay or compensation, severance pay, notice, payment in lieu of notice, incentives, bonuses, benefits or damages of any kind from the Company or from any affiliate of the Company. Any payment in lieu of the Severance Period payment provided to the Employee will be inclusive of any entitlements upon termination or severance pay pursuant to the Ontario Employment Standards Act, 2000 as amended, contract, tort, common law or otherwise, and will be subject to statutory withholdings. The Employee will not be entitled to receive any further pay or compensation (except for pay, if any, accrued and owing under this Agreement up to the date of termination of employment) from the Company, and for clarity, without limiting the foregoing, the Employee will not be entitled to any bonus or pro rata bonus payment that has not already been awarded to the Employee by the Company. |
Page 6
3.6 |
Continued Effect: Notwithstanding any changes in the terms and conditions of the Employee’s employment which may occur in the future, including any changes in position, duties or compensation, the termination provisions in this Agreement will continue to be in effect for the duration of the Employee’s employment with the Company unless otherwise amended in writing and signed by the Company. |
3.7 |
Obligations Upon Termination. The Employee agrees that he shall not be entitled to receive any severance fee or other benefits under Section 3 of this Agreement if the Employee breaches any of his obligations arising under Sections 4, 5, 6, Error! Reference source not found., 7, 8 and I 0 hereof. The Employee acknowledges that until a release in the form of Exhibit B hereto (a “Release”) is timely executed and delivered to the Company, the Company will not be obligated to make any severance payments or provide any other benefits due under this Agreement following termination of the Employee. The Employee further acknowledges that if the Release is not timely executed and delivered to the Company, the severance payments and other benefits described in this Section 3 shall be forfeited, except as required by statutory minimum standards. |
4. |
CONFIDENTIAL INFORMATION PROTECTIONS |
4.1 |
At all times during and after the Employee’s employment, the Employee will hold in confidence and will not disclose, use, lecture upon, or publish any of Company’s Confidential Information (defined below), except asmay be required in connection with the Employee’s work for Company, or as expressly authorized by the Board. The Employee will obtain the written approval of the Board before publishing or submitting for publication any material (written, oral, or otherwise) that relates to the Employee’s work at Company and/or incorporates any Confidential Information. The Employee hereby assigns to Company any rights the Employee may have or acquire in any and all Confidential Information and recognize that all Confidential Information shall be the sole and exclusive property of Company and its assigns. |
4.2 |
The tenn “Confidential Information” shall mean any and all confidential knowledge, data or information related to Company’s or its affiliates business or its actual or demonstrably anticipated research or development, including without limitation (a) trade secrets, inventions, ideas, processes, computer source and object code, data, formulae, programs, other works of authorship, know-how, improvements, discoveries, developments, designs, and techniques; (b) information regarding products, services, plans for research and development, marketing and business plans, budgets, financial statements, contracts, prices, suppliers, employees and customers; (c) information regarding the skills and compensation of Company’s employees, contractors, and any other service providers of Company; and (d) the existence of any business discussions, negotiations, or agreement between Company and any third party.. |
4.3 |
The Employee understands that Company has received and in the future will receive from third parties confidential or proprietary information (“Third Party Information”) subject to a duty on Company’s part to maintain the confidentiality of such information and to use it only for certain limited purposes. During and after the term of the Employee’s employment, the Employee will hold Third Party Information in strict confidence and will not disclose to anyone (other than Company personnel who need to know such information in connection with their work for Company) or use, Third Party Information, except in connection with the Employee’s work for Company or unless expressly authorized by an officer of Company in writing. |
4.4 |
The Employee represents that employment by Company does not and will not breach any agreement with any former employer, including any non-compete agreement or any agreement to keep in confidence or refrain from using information acquired by the Employee prior to |
Page 7
employment by Company. The Employee further represents that the Employee has not entered into, and will not enter into, any agreement, either written or oral, in conflict with the Employee’s obligations under this Agreement. During employment by Company, the Employee will not improperly make use of, or disclose, any information or trade secrets of any former employer or other third party, nor will the Employee bring onto the premises of Company or use any unpublished documents or any property belonging to any former employer or other third party, in violation of any lawful agreements with that former employer or third party. The Employee will use in the perfonnance of the Employee’s duties only information that is generally known and used by persons with training and experience comparable to the Employee’s own, is common knowledge in the industry or otherwise legally in the public domain, or is otherwise provided or developed by Company.
5. |
INVENTIONS |
5.1 |
As used in this Agreement, the term “Invention” means any ideas, concepts, information, materials, processes, data, programs, know-how, improvements, discoveries, developments, designs, artwork, formulae, other copyrightable works, and techniques and al I Intellectual Property Rights in any of the items listed above. The term “Intellectual Property Rights” means all trade secrets, copyrights, trademarks, mask work rights, patents and other intellectual property rights recognized by the laws of any jurisdiction or country. The term “Moral Rights” means all paternity, integrity, disclosure, withdrawal, special and any other similar rights recognized by the laws of any jurisdiction or country. |
5.2 |
The Employee has disclosed in Exhibit Ca complete list of all Inventions that (a) the Employee has, or has caused to be, alone or jointly with others, conceived, developed, or reduced to practice prior to the commencement of the Employee’s employment by Company; (b) in which the Employee has an ownership interest or which the Employee has a license to use; (c) and that the Employee wishes to have excluded from the scope of this Agreement (collectively referred to as “Prior Inventions”). If no Prior Inventions are listed in Exhibit C, the Employee warrants that there are no Prior Inventions. The Employee agrees that the Employee will not incorporate, or permit to be incorporated, Prior Inventions in any Company Inventions (defined below) without Company’s prior written consent. If, in the course of the Employee’s employment with Company, the Employee incorporates a Prior Invention into a Company process, machine or other work, the Employee hereby grants Company a non-exclusive, perpetual, fully-paid and royalty-free, irrevocable and worldwide license, with rights to sublicense through multiple levels of sublicensees, to reproduce, make derivative works of, distribute, publicly perfonn, and publicly display in any form or medium, whether now known or later developed, make, have made, use, sell, import, offer for sale, and exercise any and all present or future rights in, such Prior Invention. |
5.3 |
Inventions assigned to the Company or to a third party as directed by the Company pursuant to the subsection 5.5 are referred to in this Agreement as “Company Inventions”. Subject to the subsection 5.5 and the Prior Inventions Employee has set forth in Exhibit C, the Employee hereby assigns and agrees to assign in the future (when any such Inventions or Intellectual Property Rights are first reduced to practice or first fixed in a tangible medium, as applicable) to the Company all rights, title, and interest in and to any and all Inventions (and all Intellectual Property Rights with respect thereto) made, conceived, reduced to practice, or learned by the Employee, either alone or with others, during the period of the Employee’s employment by the Company. Any assignment of Inventions (and all Intellectual Property Rights with respect thereto) hereunder includes an assignment of all Moral Rights. To the extent such Moral Rights cannot be assigned to the Company and to the extent the following is allowed by the laws in any country where Moral Rights exist, the Employee hereby unconditionally and irrevocably waives the enforcement of such Moral Rights, |
Page 8
and all claims and causes of action of any kind against the Company or related to the Company’s customers, with respect to such rights. The Employee further acknowledges and agrees that neither the Employee’s successors-in-interest nor legal heirs retain any Moral Rights in any Inventions (and any Intellectual Property Rights with respect thereto).
5.4 |
During the period of employment, the Employee will promptly and fully disclose to the Company in writing (a) all Inventions authored, conceived, or reduced to practice by the Employee, either alone or with others, and (b) all patent applications filed by the Employee or in which the Employee is named as an inventor or co-inventor. The Employee agrees to keep and maintain adequate and current records (in the form of notes, sketches, drawings and in any other form that is required by the Company) of all Inventions made by the Employee during the period of the Employee’s employment by the Company, which records shall be available to, and remain the sole property of, the Company at all times. |
5.5The Employee agrees that, as directed by the Company, the Employee will assign to the Company or a third party, including without limitation, all rights, title, and interest in and to any particular Company Invention.
5.6 |
During and after the period of employment and at the Company’s request and expense, the Employee will assist the Company in every proper way, including consenting to and joining in any action, to obtain and enforce Canadian, United States and foreign Intellectual Property Rights and Moral Rights relating to Company Inventions in all countries. If the Company is unable to secure the Employee’s signature on any document needed in connection with such purposes, the Employee hereby irrevocably designates and appoints the Company and its duly authorized officers and agents as the Employee’s agent and attorney in fact, which appointment is coupled with an interest, to act on the Employee’s behalf to execute and file any such documents and to do all other lawfully permitted acts to further such purposes with the same legal force and effect as if executed by the Employee. |
5.7 |
The Employee agrees that the Employee will not incorporate into any Company software or otherwise deliver to the Company any software code licensed under the GNU General Public License or Lesser General Public License or any other license that, by its terms, requires or conditions the use or distribution of such code on the disclosure, licensing, or distribution of any source code owned or I icensed by the Company. |
6. |
RETURN OF COMPANY PROPERTY |
6.1 |
Without limiting the provisions of Section 3. l(c), upon termination of the Employee’s employment or upon the Company’s request at any other time, the Employee will deliver to the Company all of the Company’s property, equipment, and documents, together with all copies thereof, and any other material containing or disclosing any Inventions, Third Party Information or Confidential Information and certify in writing that the Employee has fully complied with the foregoing obligation. The Employee agrees that the Employee will not copy, delete, or alter any information contained upon any Company computer or Company equipment before the Employee returns it to the Company. In addition, if the Employee has used any personal computer, server, or e-mail system to receive, store, review, prepare or transmit any Company information, including but not limited to. Confidential Information, the Employee agrees to provide the Company with a computer-useable copy of all such Confidential Information and then permanently delete and expunge such Confidential Information from those systems; and the Employee agrees to provide the Company access to the Employee’s system as reasonably requested to verify that the necessary copying and/or deletion is completed. The Employee further agrees that any property situated on |
Page 9
the Company’s premises and owned by the Company is subject to inspection by the Company’s personnel at any time with or without notice. Prior to the termination of employment or promptly after termination of employment, the Employee will cooperate with the Company in attending an exit interview and certify in writing that the Employee has complied with the requirements of this section.
7. |
NON-COMPETITION/NON-SOLICITATION |
7.1 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (I) year after termination of such employment for any reason (except in the event of a Change of Control pursuant to the proviso at the end of Section 3.4(b), in which event such period shall extend to eighteen (I 8) months after termination of such employment), the Employee will not, without the Company’s express written consent, anywhere in the world, be employed by or contribute to any business activity that is competitive with the business of the Company or involves the use of real-time magnetic resonance imaging or ultrasound or similar technologies in the delivery of ablative tools. |
7.2 |
The Employee covenants and agrees that for the period of the Employee’s employment by the Company and for one (I) year after termination of such employment for any reason (except in the event of a Change of Control pursuant to the proviso at the end of Section 3.4(b), in which event such period shall extend to eighteen (18) months after termination of such employment), the Employee will not, either directly or indirectly, solicit or attempt to solicit any employee, independent contractor, or consultant of the Company to terminate his, her or its relationship with Company in order to become an employee, consultant, or independent contractor to or for any other person or entity. |
8. |
NOTIFICATION OF NEW EMPLOYER |
8.1 |
After the termination of this Agreement, the Employee consents to the notification of any new employer of the Employee’s rights and obligations under this Agreement, by the Company providing a copy of this Agreement or otherwise as the Company deems fit. |
9. |
INJUNCTIVE RELIEF |
9.1 |
The Employee acknowledges that, because the Employee’s services are personal and unique and because the Employee will have access to the Confidential Information of the Company, any breach of this Agreement would cause irreparable injury to the Company for which monetary damages would not be an adequate remedy and, therefore, will entitle the Company to injunctive relief (including specific performance). The rights and remedies provided to each party in this Agreement are cumulative and in addition to any other rights and remedies available to such party at law or in equity. |
10. |
PUBLICITY |
10.1 |
The Employee shall not, without the prior written consent of the Company, make or give any public announcements, press releases or statements to the public or the press regarding the Employee’s work or the Company’s business. |
11.NOTICES
Page 10
11.IAll notices, requests, demands and other communications to be given pursuant to the terms of this Agreement shall be in writing and shall be deemed to have been duly given (i) on the date of delivery if personally delivered or sent by a recognized overnight courier or (ii) on the date of transmission if sent by email (provided notice is then also promptly delivered personally or by a recognized overnight courier), in each case to the parties at the addresses listed below:
(a) |
If to the Employee, at the Employee’s then current address in the Company’s payroll records; |
(b) |
If to the Company: Profound Medical Corp. |
2400 Skymark Avenue, Unit 6
Mississauga, ON L4W SKS
Attention: Chair of the Board of Directors
E-mail:
Each party may change its address for receipt of notice by giving notice of the change to the other party.
12. |
SURVIVAL |
I 2.1 |
The Employee’s obligations under this Agreement shall survive the termination of the Employee’s employment, regardless of the manner or reason for termination, including, without limitation, those set forth in Sections 4 through 7 hereof. |
12.2 |
The assignment of this Agreement by the Company to any successor or other assignee and shall be binding upon the Employee’s heirs and legal representatives. |
I 3.SEVERABILITY
13.1 |
If any provision of this Agreement is, for any reason, held to be invalid or unenforceable, the other provisions of this Agreement will remain enforceable and the invalid or unenforceable provision will be deemed modified so that it is valid and enforceable to the maximum extent permitted by law. |
14. |
WAIVER |
14.1 |
Any waiver or failure to enforce any provision of this Agreement on one occasion will not be deemed a waiver of that provision or any other provision on any other occasion. |
15. |
ASSIGNMENT |
15.1 |
The Company may assign this Agreement to (i) an affiliate, subsidiary, related company or partnership without prior notice or consent of the Employee, or (ii) anew employer in connection with any transaction or reorganization; provided that any such successor or assignee expressly assumes in writing the Company’s obligations under this Agreement and has responsibility for managing the underlying business of the Company. The Employee may not assign any of hisrights nor delegate any of the duties hereunder. |
Page 11
16. |
ENTIRE AGREEMENT |
16.1 |
This Agreement is the final, complete and exclusive agreement of the parties with respect to the subject matter hereof and supersedes and merges all prior communications between us with respect to such matters. No modification of or amendment to this Agreement, or any waiver of any rights under this Agreement, will be effective unless in writing and signed by the Employee and the Company. Any subsequent change or changes in duties, salary or compensation will not affect the validity or scope of this Agreement. |
17.HEADINGS
17.1 |
The headings utilized in this Agreement are for convenience only and are not to be construed in any way as additions or limitations of the covenants and agreements contained in this Agreement. |
18. |
COUNTERPARTS |
18.1This Agreement may be executed in two or more counterparts, each of which will be deemed to be an original and all of which will constitute one Agreement.
19. |
LAWS |
19.I |
This Agreement shall be governed by and interpreted in accordance with the laws of Ontario and the laws of Canada applicable therein. The Employee and the Company hereby attom to the exclusive jurisdiction of the superior courts of Ontario to resolve any dispute arising from this Agreement except to the extent the Company seeks injunctive relief outside Ontario where applicable to enforce the Employee’s covenants hereunder. |
[Signature Page to Follow]
IN WITNESS WHEREOF the parties have duly executed this Agreement as of the 26 day of August , 2019.
|
Signed, Sealed and Delivered in the presence of: |
|
) ) |
|
/s/ ARUN MENAWAT |
|
) |
|
Name (witness) |
|
) |
|
2400 SKYMARK UNIT#6 |
|
) |
|
Address |
|
) |
|
MISS. ON |
|
) |
/s/ MATHIEU BURTNYK |
CEO |
|
) |
MATHIEU BURTNYK |
Occupation |
|
) |
|
PROFOUND MEDICAL CORP
Per: |
/s/ Arun Menawat |
|
|
Arun Menawat |
|
Exhibit B
RELEASE
I, Mathieu Burtnyk of the City of ,in the Province of Ontario, for and in consideration of the sum of($*) and other good and valuable consideration which is being delivered to me concurrently with the execution and delivery hereof (the receipt and sufficiency of which is hereby acknowledged) do hereby remise, release and forever discharge PROFOUND MEDICAL CORP. (hereinafter called the “Company”), its officers, directors, servants, employees and agents, including any related or associated companies, and their heirs, executors, administrators, successors and assigns, as the case may be, of and from any and all manner of actions, causes of action, suits, contracts, claims, damages, costs and expenses of any nature or kind whatsoever, whether in law or in equity, which, as against the Company or such persons as aforesaid or any of them I have ever had, now have, or at any time hereafter I or my personal representatives can, shall or may have, by reason of or arising out of the termination of my employment with the Company or in any other way connected with my employment with the Company (except as otherwise set forth below), and more specifically, without limiting the generality of the foregoing, any and all claims for damages for termination of my employment, constructive termination of my employment, loss of position, loss of status, loss of future job opportunity, loss of oppo11unity to enhance my reputation, the timing of the termination and the manner in which it was effected, loss of bonuses, loss of benefits, including life insurance and short and long-term disability benefit coverage, and any other type of damages; provided, however, that notwithstanding anything contained herein, this Release does not apply to any claims related to (i) equity in the Company or its affiliates that I hold, directly or indirectly, including without limitation, the options granted to me in connection with my employment with the Company, or (ii) any unpaid reimbursement of expenses and accrued but unpaid salary or termination payments provided for in the Employment Agreement.
I FURTHER AGREE that this Release includes any and all claims I may have arising under contract, the Ontario Employment Standards Act, 2000, as amended, common law or other applicable law related to my employment with the Company and that the consideration provided includes any amount that I may be entitled to under such legislation and I agree to immediately withdraw any complaint as settled and not to file any complaint pursuant to such legislation with respect to my employment or the termination of my employment.
Page 2
IT IS UNDERSTOOD that the Company has withheld income tax and other statutory deductions from the aforesaid consideration and I agree to indemnify and hold harmless the Company from any further assessment for income tax or other statutory deductions which may be made under statutory authority.
IT IS FURTHER UNDERSTOOD that as a former officer of the Company I owe a fiduciary duty to the Company and I agree that the Company will suffer irreparable harm if confidential information or information unique to the Company is disclosed in any way by me without authorization of the Company and that I have returned to the Company originals or copies of the Company’s corporate records, files, financial documents, client lists, or any other material that is related to the Company’s business, clients, personnel or operations.
IT IS FURTHER UNDERSTOOD AND AGREED that this is not to be construed or considered as an admission of liability on the part of the Company. The terms of this Release set out the entire agreement between myself and the Company and are intended to be contractual and not a mere recital.
Page 3
IT IS EXPRESSLY ACKNOWLEDGED that the contents, tenns and effect of this Release have been explained to me by my lawyer and are fully understood.
IN WITNESS WHEREOF I, Mathieu Burtnyk, have hereunto set my hand and seal this day of ,20 in the City of , Province of Ontario.
Mathieu Burtnyk
EXBIBIT C
INVENTIONS
1.Prior Inventions Disclosure. The following is a complete list of all Prior Inventions (as provided in Subsection 5.2 of the attached Employment Agreement):
☐None
☒See immediately below:
PATENT # : 8989838 – SYSTEM FOR TREATMENT OF |
DISEASED TISSUE USING CONTROLLED ULTRASONIC HEATING. |
FILED JULY 6, 2010 – GRANTED MARCH 24, 2015 |
PATENT # : 7771418 – TREATMENT OF DISEASED TISSUE |
USING CONTROLLED ULTRASONIC HEATING |
FILED MARCH 9, 2005 – GRANTED AUGUST 10, 2010 |
|
|
|
|
Profound Medical Inc. |
Personal and Confidential
March 7, 2024
Mathieu Burtnyk
Delivered via e-mail: #################
Mathieu,
Congratulations! We are pleased to confirm your promotion to Chief Operating Officer (COO), effective March 7, 2024. Your contributions to the company have been recognized, and we have full confidence that you will excel in this role.
All other terms and conditions outlined in your employment contract, dated August 20, 2019, will remain unchanged. By signing this agreement, you confirm your acceptance of these terms and conditions, as well as the Profound Medical Inc. Restrictions and Non-Disclosure/Intellectual Property Agreements.
We are excited to see the positive impact you will have on our team and organization.
Sincerely,
/s/ Arun Menawat
Arun Menawat, CEO
Please sign and date this letter in the space provided below and return it to Magdalena Gorkiewicz,
##############################
I, the undersigned, hereby accept my promotion to Chief Operating Officer.
Signed: |
/s/ Mathieu Burtnyk |
|
Dated |
19-Mar-2024 |
|
|
Mathieu Burtnyk |
|
|
|
|
|
|
Profound Medical Inc. |
Personal & Confidential
August 14, 2024
Mathieu Burtnyk
Dear Mathieu,
I am pleased to confirm that effective retroactively from April 1, 2024, your new annual salary has been adjusted to 300,000 USD. The retroactive amount will be included in the August 31, 2024 pay date. Your salary will be converted to and paid in your local currency using the Bank of Canada exchange rates from 1 or 2 business days before the semimonthly payroll submission.
In addition to the increase of your base salary, your annual bonus increases from 40% to 50% of your base salary for 2024. The bonus criteria are as follows:
| ● | 50% will be based on achieving the company’s revenue target. |
| ● | 25% will be based on completing the 2024 reimbursement goals to Level 7 |
| ● | 12.5% will be based on completing the TULSA-AI for BPH. |
| ● | 12.5% will be based on supporting the balance sheet through management of COGS as well as supporting financing. |
Please note that your title remains Chief Operating Officer, and you continue to report directly to the Chief Executive Officer (CEO).
If you have any questions regarding this adjustment, please feel free to reach out.
Best regards,
/s/ Arun Menawat
Arun Menawat
Chief Executive Officer
Exhibit 10.7
AMENDED AND RESTATED TECHNOLOGY LICENCE AGREEMENT
THIS AMENDED AND RESTATED TECHNOLOGY LICENCE AGREEMENT is made as of May 16, 2011
BETWEEN:
SUNNYBROOK HEALTH SCIENCES CENTRE, with offices
located at 2075 Bayview Avenue in Toronto, Ontario (the
“Licensor”)
- and-
PROFOUND MEDICAL INC., a company governed by the laws
of the Province of Ontario (the “Licensee”)
RECITALS:
| A. | The Inventors, while employed by Licensor, have made inventions, as described in the patent applications and disclosures listed on Schedules B and C hereto (collectively, the “Inventions”); |
| B. | Each of the Inventors has assigned all of his respective right, title and interest in the Inventions to Licensor; |
| C. | Licensor has filed or is filing patent applications for such Inventions, as listed and described in Schedules B and C; |
| D. | Licensee wishes to commercialize, develop, manufacture, market, distribute and sell Products which may be derived in whole or in part from the practice of the Technology and, therefore, desires to obtain a licence from Licensor for rights to the Technology in the Field of Use; |
| E. | Licensor is willing to grant Licensee such licence upon the terms and conditions in this Agreement; |
| F. | The Licensor and Licensee entered into a technology licence agreement dated June 16, 2008, as amended March 22, 2010 (the “Original Technology Licence Agreement”); |
| G. | Licensee wishes to complete a financing through the issuance of preferred shares (the “Financing”); |
| H. | A shareholders meeting has been called to amend the constating documents of Licensee in satisfaction of a condition of the Financing and Licensor has agreed to support and vote in favour of the amendment to those constating documents and otherwise to support that preferred share financing transaction; and |
| I. | In connection with the Financing the parties have agreed to amend and restate the. Original Technology Licence Agreement as set forth in this Agreement, the terms of |
-2-
which shall supersede any and all licence agreements between the Licensor and Licensee, including the Original Technology Licence Agreement.
NOW THEREFORE, in consideration of the mutual premises set forth herein (the receipt and sufficiency of which are hereby acknowledged), the Parties agree as follows:
ARTICLE 1
DEFINITIONS
1.1 |
The following capitalized terms, and other capitalized terms defined elsewhere in this Agreement, will have the meanings ascribed thereto wherever used in this Agreement: |
(a) |
“Acceptance Period” has the meaning set forth in Section 4.1. |
(b) |
“Affiliate” means any legal entity (such as a corporation, partnership or limited liability company) that controls, is controlled by or is under common control with a Party hereto. The term “control” means the ability to direct the management and policies of said entity, whether through ownership of equity, by contract or otherwise. |
(c) |
“Agreement” means this agreement and all schedules attached hereto, as the same may be amended from time to time. |
(d) |
“Business Day” means any day other than a Saturday, Sunday or a day which is a statutory or civic holiday in Toronto, Ontario. |
(e) |
“Buy Out IP”has the meaning set forth in Section 8.3. |
(t) |
“Buy Out Milestones” means the milestones set out in Part 1 of Schedule D. |
(g) |
“Calendar Quarter” means the following periods of time for each year that this Agreement is in effect: January 1st through March 31st (1st Calendar Quarter); April 1st through June 30th (2nd Calendar Quarter); July 1st through September 30th (3rd Calendar Quarter); and October 1st through December 31st (4th Calendar Quarter). |
(h) |
“Confidential Information” means any business, marketing, technical, scientific or other information disclosed by either Party which, at the time of disclosure, is designated as confidential (or like designation), is disclosed in circumstances of confidence, or would be understood by the Parties, upon exercising reasonable business judgment, to be confidential. Confidential Information includes, without limitation, all technical information, all design documentation and all implementation details relating to the Technology, and all Licensee Updates, and all Licensor Updates and all Trade Secrets. |
(i) |
“Copyright” means the rights prescribed in the Copyright Act (Canada), including those copyright registrations and applications relating to the Technology identified in Schedules B and C. |
(j) |
“Effective Date” means June 16, 2008. |
-3-
(k) |
“End User” means an individual, body corporate, unincorporated organization or any other entity recognized by law to whom or to which, as the case may be, Products are sold or otherwise transferred. |
(1) |
“Fees” means all amounts payable to Licensor under this Agreement for the sale of Products and Services. |
(m) |
“Field of Use” means MRI-guided trans-urethral ultrasound therapy throughout the world. |
(n) |
“Intellectual Property Rights” means all Patents, Copyrights, trade-names and other intellectual property rights relating to the Technology, whether registered or not, owned by or licensed to Licensor, including any and all proprietary rights provided under patent law, copyright law, trademark law or any other statutory provision or applicable common law principles which may provide a right in either ideas, formulae, algorithms, concepts, inventions or know-how relating to the Technology, including Trade Secrets, or the expression or use thereof. |
(o) |
“Inventors” means, collectively, Michael Bronskill, Rajiv Chopra, Kee Tang and Mathieu Burtynk and “Inventor” means any one of them. |
(p) |
”Know-how” means any and all trade secrets, technical expertise, knowledge, confidential information and know-how, whether patentable or unpatentable, relating to the Technology and/or the Inventions, whether in written, machine readable, drawing or oral form, including, without limitation, all technical information, raw material data, product specifications, processes and designs, operating and production data, calculations, computer programs, instructions and techniques, quality control, and other standards and drawings relating thereto developed by an Inventor and which exists as at the Effective Date. |
(q) |
“Licensee Updates” means any update, correction, improvement, enhancement or modification of the Technology created, produced or developed by the Licensee (without the active participation of Licensor personnel) in the Field of Use during the Term. |
(r) |
“Licensor Updates” means any update, correction, improvement, enhancement or modification of the Technology created, produced or developed by the Inventors (or any of them) or Licensor in the Field of Use during the Term. |
(s) |
“Milestones” means the milestones set out in Part 2 of Schedule D. |
(t) |
“Notice of Offer” has the meaning set forth in Section 4.1. |
(u) |
“Party” means either Licensee or Licensor, and “Parties” means both of them, collectively. |
(v) |
“Patents” means the patents, patent applications and patent disclosures listed on Schedules B and C and any continuations, continuations in part, divisionals, reissues, re-examinations, renewals or extensions of such foregoing patents and |
-4-
patent applications and any patents which may issue on, from or as a result of any of the foregoing. For greater clarity, Patents does not include patented Licensor Updates or patented Licensee Updates.
(w) |
“Person” means any individual, sole proprietorship, partnership, unincorporated association, unincorporated syndicate, unincorporated organization, trust, body corporate and a natural person in his or her capacity as trustee, executor, administrator or other legal representative. |
(x) |
“Prime Rate” means the published prime rate of interest per annum that the Royal Bank of Canada offers to its most credit-worthy commercial customers for Canadian dollar loans, adjusted monthly on the last business day of each month that any amount remains outstanding hereunder. |
(y) |
“Product” means any product derived from the Technology or that results from practising or using the Technology, in whole or in part. |
(z) |
“Service” means any service to Third Parties, not provided for free, that is provided by using the Technology, by using a Product or that relates to a Product. |
(aa) |
“Sponsored Research Agreement” means any sponsored research agreement entered into between Licensor and Licensee with respect to the Technology, including the Sponsored Research Agreement entered into between the Parties as of the Effective Date. |
(bb) |
“Technology” means: (i) all existing technology relating to the Field of Use that is described and claimed in the Patents and possessed as at the Effective Date by Licensor that is not (other than as contained in any patent application or patent disclosure) presently available to the public by publication, and (ii) all Know- How and all Intellectual Property Rights relating to such existing technology. |
(cc) |
“Term” has the meaning set forth in Section 13.1. |
(dd) |
“Third Party” means a Person who deals with a Party to this Agreement at arm’s length, which for these purposes has the meaning that it has for purposes of the Income Tax Act (Canada), as in effect on the Effective Date. |
(ee) |
“Trade Secrets” means information and/or knowledge which derives economic value, actual or potential, from not being generally known to, or readily ascertainable by proper means by, other persons who can obtain economic value from its disclosure or use, and which is the subject of efforts that are reasonable under the circumstances to maintain its secrecy. |
(ff) |
“Update Interest” has the meaning set forth in Section 4.1. |
-5-
ARTICLE 2
LICENCE
2.1 |
Grant of Licences. Subject to the terms and conditions of this Agreement, Licensor hereby grants to Licensee and Licensee hereby accepts an exclusive, royalty-free, worldwide right and licence under the Intellectual Property Rights to use the Technology in the Field of Use, with no right to sublicense except as specifically provided in this Section 2.1: |
(a) |
to use, copy, display, create derivative works of and integrate the Technology and any such derivative works into the Products and otherwise to use, copy and practice the Technology (including making Licensee Updates and other improvements to the Technology); |
(b) |
to engage contractors and collaborators to (i) market and sell the Products and (ii) reproduce, develop and make components and any parts thereof incorporated into . any Products; |
(c)to make any Licensee Updates and derivative works thereof, and incorporate Licensee Updates and any such derivative works into the Products, provided that: (i)Licensee provides notification to Licensor of such Licensee Updates, and (ii) Licensee grants Licensor the licence described in Section 3.2(b);
(d) |
to make, have made, use, lease, sell, offer for sale and import Products and to make, have made, use, copy, display, sell and distribute Technology (solely for the purpose to be incorporated into Products) to End Users as components of other products intended for sale to End Users; |
(e) |
to sell any Service(s); and |
(f) |
to provide technical support to End Users; and |
(g) |
to grant sub-licences of all rights set out above and to disclose Confidential Information to sub-licensees, provided that sub-licensees are: (i), with respect to Licensee, a Third Party (ii) bound by tenns and conditions no less onerous than set forth in this Agreement (it being understood that no further amount shall be payable to Licensor or the Inventors in connection with any such sub-licence), provided that Licensee shall provide at least five (5) days prior written notice to Licensor of Licensee’s intent to enter into any such sub-license. |
2.2 |
Prohibited Uses. Licensee may not provide the Technology to any Third Party without Licensor’s prior written permission, except as expressly provided in this Agreement. |
2.3 |
Validity. Licensee agrees that, during the Tenn, it will not, either directly or indirectly, challenge the validity of the Technology and any Intellectual Property Rights associated therewith, including any patents issuing in relation to Licensor Updates, or the ownership rights of Licensor in the Technology or Licensor Updates, and shall not assist any person or entity to do so, provided such obligations are not contrary to law, in which case this |
-6-
Section shall be read down only as necessary to make the obligation valid and enforceable.
2.4 |
Retained Rights. Subject to the provisions set forth in Section 2.5, Licensor expressly reserves from the licence rights granted above: |
(a) |
the right of Licensor to publish or disclose research results arising from perfonnance of the Technology; and |
(b) |
the right to use any and all of the Patents and Technology for the purpose of non commercial research and teaching, educational and administrative purposes, |
provided that Licensor Updates that result from such research are subject to the right of first refusal set out in Section 4.1 and the obligation to disclose set out in Section 3.2(b).
2.5 |
Publications. |
(a) |
Each Party (the “First Party”) will provide a copy of any proposed publication or disclosure related to the Technology to the other Party for its review at least thirty (30) days before submission for publication or disclosure. |
(b) |
Upon the other Party’s written request, to be delivered to the First Party within twenty (20) days of the other Party’s receipt of such proposed publication or disclosure, the First Party will, at the other Party’s sole option: (i) delay publication up to sixty (60) additional days to enable the other Party to secure intellectual property protection of any intellectual property that would be publicly disclosed by such publication, and/or (ii) delete any Confidential Information provided by the other Party from the manuscript or proposed disclosure. |
(c) |
Licensor agrees to acknowledge the support of Licensee in all publications and disclosure related to the Technology that have been funded by Licensee under the Sponsored Research Agreement, unless otherwise agreed in writing by the Parties. |
2.6 |
Know-how. |
Licensor shall give to Licensee a copy of all relevant Know-how that has been reduced to written form, including all Patents, together with all documentation that is reasonably necessary for understanding the Technology, to the extent that it exists and is within the control of Licensor or any of the Inventors, along with oral explanations of the Know how and of wiwritten Confidential Information.
ARTICLE 3
OWNERSHIP
3.1Ownership of Technology.
(a)Except for the rights expressly granted under this Agreement, all right, title and interest in and to the Technology and Licensor Updates, including the Technology incorporated in the Products, are and shall at all times remain the property of Licensor, the entity from whom the Licensor has licensed such Technology or any permitted transferee to whom the Technology has been transferred.
-7-
(b) |
Except for the rights expressly granted under this Agreement, all right, title and interest in and to the Licensee Updates are and shall at all times remain the property of Licensee. |
3.2 |
Ownership of Improvements. |
(a) |
If Licensor receives from any third party (the “Third Party”) a bona.fide written offer (the “Third Party Offer”) with respect to a Licensor Update or if Licensor (or any Inventor) proposes to in any other way take any steps to commercialise any Licensor Update, then Licensor shall, except as provided otherwise in Section 3.2(e), promptly inform Licensee and Licensee shall have a right of first refusal to obtain an exclusive licence to use such Licensor Update in accordance with Article 4. Any disclosure of Licensor Updates shall constitute Licensor’s Confidential Information and be treated in strict confidence by Licensee in accordance with Article 11. For greater clarity, “steps to commercialise” does not include actions to obtain intellectual property rights with respect to any Licensor Update in the absence of any Third Party Offer with respect to such Licensor Update. |
(b) |
Within 10 days of the end of each calendar year during the Term, Licensor shall disclose (on a confidential basis) to Licensee all Licensor Updates (if any) that were generated during the twelve (12) months preceding the end of such calendar year. |
(c) |
Within 10 days of the end of each year during the Term, Licensee shall disclose (on a confidential basis) to Licensor all Licensee Updates (if any) that were generated during the twelve (12) months preceding the end of such calendar year and Licensee shall provide to Licensor a licence, without cost, to use all such Licensee Updates exclusively for the purposes set out in Section 2.4(b), on the same terms and conditions as set out therein, and Licensee Updates shall be deemed to form part of the Technology for the purposes of Section 2.4(b). |
(d) |
Where Licensee exercises its Right of First Refusal in accordance with Article 4, Licensee shall have the right (and Licensor, for greater certainty, shall have no obligation) to file patent applications on any such Licensor Updates, provided that Licensee agrees to pay for all expenses associated with the filing and prosecution of such patent applications. If Licensee elects not to file patent applications for such additions or changes, Licensor shall have the right to apply for patents at its own expense. It is understood that Licensor shall not be obligated to undertake responsibility with respect to any such additions or changes unless it elects to do so. |
(e) |
Notwithstanding Subsections 3.2(a), (b), (c) and (d), any Licensee Updates or Licensor Updates to the Technology which qualify as New Intellectual Property under the terms of the Sponsored Research Agreement shall be treated in accordance with the provisions of such research agreement. |
-8-
3.3 |
Reservation of Rights. Licensor reserves all rights not expressly granted in this Agreement, and nothing in this Agreement, including any grant or reservation of rights set forth herein, shall be construed as implying or giving rise to any implied grant or licence of any right, or as implying any limitation of any reservation of rights, except as expressly set forth in this Agreement. |
ARTICLE 4
RIGHT OF FIRST REFUSAL
4.1 |
Right of First Refusal. |
(a) |
Licensor may not license, convey, transfer or otherwise grant an interest in or to a Licensor Update (an “Update Interest”) to any person or entity and shall not take any action or proceed in any manner with a view to commercialising any Licensor Update except in accordance with the provisions of this Section 4.1 and pursuant to a Third Party Offer that is subject to the right of first refusal set out in this Section 4.1. For greater clarity, “view to commercialising” does not include actions to obtain intellectual property rights with respect to any Licensor Update in the absence of a Third Party Offer with respect to such Licensor Update. |
(b) |
If Licensor receives from any Third Party (including without limitation (i) any entity in which Licensor, any Inventor or any member of Licensor’s faculty or staff has any equity or similar interest and (ii) any entity proposed to receive any rights or interest in or to a Licensor Update in connection with a spin-out or similar transaction with respect to a Licensor Update) a Third Party Offer with respect to an Update Interest, Licensor shall only accept such Third Party Offer subject to compliance with the provisions of this Section 4.1. Upon such conditional acceptance, Licensor shall deliver a written notice (the “Notice of Offer”) to Licensee irrevocably offering the Update Interest at the same price and in all other material respects on the same terms and conditions as provided in the Third Party Offer, provided that if the applicable Licensor Update was developed pursuant to a sponsored research or similar agreement, the price in the Notice of Offer shall not include or account for any funding (whether made in the form of cash or otherwise) previously advanced or otherwise made available to Licensor or any Inventor. Licensor shall deliver, with the Notice of Offer, a true copy of the Third Party Offer. The offer contained in the Notice of Offer shall be irrevocable except with the Licensee’s consent and shall be open for acceptance for a period of 30 Business Days after the date upon which the Notice of Offer is received by the Licensee (the “Acceptance Period”). |
(c) |
Within the Acceptance Period, Licensee may accept the offer contained in the Notice of Offer by delivering to Licensor a written notice to such effect (an “Acceptance Notice”). To permit the practical implementation of this Section 4.11, no Update interest may be provided by Licensor to any Third Party for consideration other than cash or publicly traded common shares of a corporation with a public float in excess of $250 million, the common shares of which are listed and posted for trading on a recognised North American stock exchange. Where the Third Party Offer contemplates consideration in the form of such publicly traded common shares, then Licensee may, in its Acceptance Notice, |
-9-
specify that it shall pay, on the same terms and timelines, the cash equivalent of the market price of such shares. For the purposes of this Section 4.1, “market price” shall have the meaning ascribed to such term in Section 1.3 of OSC Rule 62-504 as in effect on May 16, 2011 and where the relevant date for determination of such market price is the date of the Third Party Offer.
(d) |
If Licensor does not receive an Acceptance Notice from Licensee within the Acceptance Period confirming its acceptance of the offer contained in the Notice of Offer, Licensee’s right to acquire the Update Interest under this Section 4.1 shall cease as at the end of the Acceptance Period and Licensor may thereafter provide the Update Interest to the Third Party at the price and upon the terms and conditions specified in the Third Party Offer, provided that in any event the agreement with the Third Party is on the same terms as contained in the Third Party Offer. |
(e) |
Any provision of an Update Interest to a Third Party pursuant to this Section 4.1 must be completed within 60 days following the expiry of the Acceptance Period, failing which the provisions of this Section 4.1 shall again apply to any proposed provision of an Update Interest, and so on from time to time. |
ARTICLES 5
PAYMENTS; LEASED PREMISES
5.1 |
Execution Payments; Leased Premises. Licensee has paid to Licensor $37,500 ($25,000 on December 30, 2009 and 12,500 in February 2010) and agrees to pay to Licensor a further $62,500 (collectively, the “Execution Payments”) on the following dates: $12,500 on April 1, 2010 and $50,000 on June 1, 2010. The Parties acknowledge the lease of Suite 4040, 3080 Yonge Street, Toronto, ON, M4N 3Nl from September 1, 2008 to August 31, 2009 and further acknowledge that there are no outstanding payment obligations in respect thereof. If the Sponsored Research Agreement executed on June 17, 2008, between the Parties, continues to a mutually agreed-upon level beyond June 30, 2010, Licensor will consider extending the payment dates of the unpaid Execution Payments, with any extension to be agreed to by Licensor in writing at least forty-five (45) days prior to the due date. The Lease Term shall not be less than the aggregate Execution Payments divided by the Lease Rate. For greater certainty, Licensor agrees that the location of the rental space described in the Lease Agreement will not change during the Lease Tenn without Licensee’s prior written consent, such consent not to be unreasonably withheld or delayed. Licensor will provide to Licensee a draft of the lease agreement with respect to such premises (the “Lease Agreement”) within sixty (60) days of the Effective Date. The Execution Payments shall be credited against any payments owing by Licensee to Licensor under the Lease Agreement. If Licensee vacates the rental space provided by Licensor prior to expiry of the Lease Term (in circumstances where Licensor is in full compliance with its obligations under the Lease Agreement), all remaining Execution Payments shall accelerate and immediately become due and payable and Licensor, subject to compliance with the terms of the Lease Agreement, may re-let such premises without deduction against the Execution Payments. |
5.2 |
Milestone Payment. Licensee shall pay to Licensor the amount of Cdn.$250,000 (the “Milestone Payment”) within fifteen (15) days of notification of clearance by the United |
-10-
States Food and Drug Administration (“FDA”) of Licensee’s first Product for sale for human use, including any U.S. 510k or pre-market approval (“PMA”).
5.3 |
Payment. |
The Parties acknowledge that Fees payable within Canada are subject to Hannonized Sales Tax (“HST”). Licensee shall provide to Licensor a statement as to Licensee’s HST status and shall remit such amounts to Licensor as are required under Canadian HST legislation. Licensor is registered to collect HST under registration number 10809 1281 RTOOOl.
(a) |
Licensee shall pay promptly all Fees to Licensor, free and clear of all taxes or other charges, including income taxes, except such taxes as Licensee shall be required by law to withhold. Any withholding taxes or like charges which Licensee shall be required by law to withhold on remittance of the Fees shall be deducted from Fees paid to Licensor. Licensee shall furnish Licensor with copies of official receipts for such taxes or other charges. If any currency conversion shall be required in connection with the payment of Fees hereunder, such conversion shall be made quarterly by using the published closing exchange rate (as published by the Bank of Canada) for the purchase of Canadian Dollars for the day preceding the date of payment. |
(b) |
If Licensee does not pay any amount owing to Licensor within thirty (30) days of such amount becoming due, it shall pay such amount, plus interest calculated at the Prime Rate plus three percent, compounded monthly. The payment of interest shall not be deemed an alternative to the payment of amounts owing on the due date, which payment shall be deemed to be in default and Licensor may terminate this Agreement as provided in Section 13.4 if Licensee fails to remedy the default within the time period set out therein. |
ARTICLE 6
LICENSOR REPRESENTATIONS AND WARRANTIES
6.1 |
Right and Authority Warranty. Licensor represents and warrants that it has the right and authority to grant the rights and licences granted herein, that it has not previously granted any rights with respect to the Technology or the Intellectual Property Rights that would conflict with the rights granted hereunder and further that each of the Inventors has assigned his respective right, title and interest in and to the Inventions and the Technology to Licensor. |
6.2 |
No Lien on Intellectual Property Rights. Licensor represents and warrants that, to its knowledge, the Technology is not subject to any outstanding order, ruling, decree, court judgment or stipulation by or with any court, arbitrator or administrative agency. Licensor further represents and warrants that it has proceeded with reasonable due diligence prior to filing for the Patents and that, to its actual knowledge, the Technology does not infringe on any patent or any other intellectual property rights of any Third Party. |
-11-
6.3 |
Validity. Nothing in this Agreement shall constitute a representation or warranty by Licensor that any Patent pertaining to the Technology is valid or enforceable in any country. |
6.4 |
Exclusion. Except as expressly otherwise set forth herein or provided by applicable law, any liability of the Parties and their respective directors, officers, employees, agents or advisers for any misrepresentation or other act or omission made or committed in the preparation of this Agreement, but not included in this Agreement (including any liability in tort, for breach of pre-contractual obligations or under any implied representations and warranties), shall be excluded. |
6.5 |
Inventors. Licensor confirms that, other than as provided below in this Section 6.5, Nicolas Yak and each of the Inventors is currently, and has been since June 17, 2008, a member of Licensor’s faculty and/or staff. Licensor and Licensee confirm and acknowledge that Kee Tang ceased to be a member of Licensor’s faculty and/or staff on October 31, 2008 and that Dr. Bronskill ceased to be a member of Licensor’s faculty and/or staff on June 30, 2009. Each of the Inventors and Nicolas Yak, to the extent that he contributed to the development of Licensor Updates (including Licensor Updates which qualify as New Intellectual Property under the terms of the Sponsored Research Agreement) while a member of Licensor’s faculty and/or staff, assigned to Licensor all of his respective right, title and interest in and to all such Licensor Updates and New Intellectual Property. |
ARTICLE 7
LICENSEE WARRANTIES
7.1 |
Development and Use of Technology. |
(a) |
Licensee shall diligently proceed with the integration of the Technology into, and the development and manufacture of, Products in order to make them readily available to End Users for sale and use as soon as is commercially reasonable. |
(b) |
Licensee shall market the Products. All marketing and promotion of Products and decisions with respect thereto shall be made by Licensee in the exercise of its absolute discretion. |
(c) |
Licensee shall spend sufficient funds on the development and sale of Products to reasonably carry out its obligations hereunder. |
(d) |
Licensee shall use its commercially reasonable efforts, consistent with reasonable business practices, to perform the Milestones within the established timelines set forth on Schedule D. |
(e) |
The Parties hereby agree that, upon the written request of either of them, they will enter into good faith discussions with respect to the Milestones to be achieved after January 1, 2014 and, in particular, whether the Milestones remain appropriate in the context of Licensee’s progress and the state of development of the Technology, it being understood that there shall be no change to the Milestones unless each of the Parties agree to such change. |
-12-
7.2 |
Reports and Other Deliveries. |
(a) |
Within forty-five (45) days of each calendar year end, Licensee shall provide Licensor with a written report with respect to the Technology that sets out: (i) the amount of money spent by Licensee on research and development, (ii) the amount of money spent by Licensee on operations, (iii) a summary of technological and clinical progress achieved in the previous calendar year, and (iv) the regulatory status of any Products under development. |
(b) |
Within forty-five (45) days of the calendar year end in which Products are first sold to an End User, and within forty-five (45) days of each subsequent calendar year end thereafter, Licensee shall provide Licensor with a written report with respect to the Technology that sets out: (i) a summary of all sales and uses, if any, made of Products during the preceding Calendar Quarter, and (ii) the number and dollar amount of sales made during such Calendar Quarter. |
(c) |
Each written report delivered to Licensor shall include at least the following: |
(i) |
the number of Products manufactured; |
(ii) |
the number of Products sold by Licensee; |
(iii) |
an accounting for all Products used or sold by Licensee. |
(d) |
Unless otherwise provided under the Sponsored Research Agreement, Licensee shall provide Licensor with two copies of software and documentation for all major releases of Products developed throughout the Term, which deliverables shall constitute Licensee’s Confidential Information and shall be treated in strict confidence by Licensor in accordance with Article 11. |
(e) |
Within forty-five (45) days of the date of this Amended and Restated Technology Licence Agreement, Licensor will complete filing all remaining disclosure(s) contemplated pursuant to the terms of the Original Technology Licence Agreement, including disclosing all Updates generated since the Effective Date and prior to May 16, 2011, together with such assistance, advice and correspondence contemplated under Sections 8.2(a) and 8.2(b) of the Original Technology License Agreement. |
7.3 |
Books and Records. Licensee shall keep complete, true and accurate books of account containing the particulars that may be necessary for the purpose of showing the amounts payable to Licensor hereunder, which books of account shall be kept at Licensee’s principal place of business or the principal place of business of the appropriate division of Licensee to which this Agreement relates. Upon at least 24 hours advance notice to Licensee and during regular working hours, all applicable books and the supporting data shall be available for inspection by Licensor or its agents, on a confidential basis, not more than once during any calendar year during the term of this Agreement, unless there is a discrepancy of greater than two percent (2%) from the amounts reported under Section 7.2, for the purpose of verifying compliance with this Agreement, provided that |
-13-
any such inspection shall be conducted in a manner that does not unreasonably interfere with Licensee’s nonnal operations.
7.4 |
Good Faith. Licensee shall act reasonably and in good faith in all respects regarding the Fees and Milestones, and Licensee shall not participate in any scheme, alliance, partnership or other business arrangement or activity that has the effect of evading or reducing the Fees that would otherwise be payable. |
ARTICLE 8
INTELLECTUAL PROPERTY PROSECUTION AND MAINTENANCE
8.1 |
Patent Fees. Licensee shall pay all future costs and legal fees incurred in the preparation, filing and maintenance of United States patent applications US 2007/0239062 and US 2006/0206105, United States patent US 6,589,174; or any issued patents thereof and any United States patents or patent applications derived from confidential disclosure forms as listed in Schedule C, including any taxes payable with respect thereto. The parties hereby confirm and agree that Licensee has paid to Licensor the amount of Cdn.$50,000 as reimbursement for Licensor’s work and expenses incurred in connection with the Patents. |
8.2 |
Filings. |
(a) |
Licensee shall, as between Licensor and Licensee, be responsible for filing, prosecuting and maintaining the Patents and any subsequent patent applications relating to the Technology under this Agreement. Licensee shall use reasonable efforts to obtain such patents and shall not allow such patents to lapse for failure to comply with maintenance obligations. Licensor shall provide to Licensee all such assistance, at Licensee’s cost and expense, as Licensee may reasonably request in connection with Licensee’s efforts to obtain and maintain such patents. Licensee shall consult with Licensor (whose consent shall not be required) in order to determine in which countries other than the United States to pursue any patent applications relating to the Technology, provided that, if Licensee elects not to pursue a patent application in any country, Licensor shall be entitled to do so and (i) Licensee’s license for the Technology for such jurisdiction shall promptly tenninate; (ii) Licensor may license the Technology to a Third Party in such jurisdiction; and (iii) Licensee shall provide such assistance to Licensor, at Licensor’s cost and expense, in this regard as Licensor may reasonably request. |
(b) |
Licensee will keep Licensor reasonably advised of the progress of prosecution and of any actions Licensee proposes to take or has taken in connection with the prosecution or maintenance of the Patents or any patent application included in the definition of Patents. Licensee will provide Licensor with copies of correspondence and all actions issued by patent authorities and shall consider, but is not obligated to incorporate, any comments, remarks or suggestions Licensor may promptly provide to Licensee in writing at least (30) days prior to any patent office due dates of which it becomes aware. |
-14-
(c) |
Unless otherwise agreed, for all Technology and any Licensor Updates thereof, the Parties agree that, if necessary, inventorship shall be defined according to Title 35 of the United States Code. |
8.3 |
Assignment of Rights. |
(a) |
Licensor hereby grants to Licensee an option (the “Buy Out”) to acquire ownership of the Technology, including, without limitation, the Patents (the “Buy Out IP”) upon Licensee having satisfied or otherwise achieved the Buy Out Milestones and paying to Licensor the amount of Cdn $200,000, which amount shall be in addition to any Fees owing or already paid. |
(b) |
Licensor shall assign to Licensee all of its (and of the Inventors’) right, title and interest in and to the Buy Out IP, all pursuant to a written agreement prepared by Licensee and to be entered into by the Parties, which agreement shall contain terms reasonable and customary in similar transactions (it being understood and agreed that no consideration or amount other than the Cdn $200,000 amount noted in Section 8.3(a) plus reimbursement by Licensee for all reasonable costs incurred by Licensor in connection with necessary third party consents or assistance (as notified to Licensee in advance of the Buy Out becoming final) incurred in connection with meeting the Licensor’s obligations under this Section 8.3(a) shall be payable in connection with such assignment). To the extent any rights to the Buy Out IP, including any moral rights, are not capable of assignment under applicable law, Licensor hereby irrevocably and unconditionally waives all enforcement of those rights to the maximum extent permitted under applicable law and agrees to obtain such a waiver in writing from each Licensor personnel, each Inventor and any other personnel involved in the development or creation of any part of the Buy Out IP. Upon Licensee’s request and at Licensee’s expense, Licensor shall execute and deliver to Licensee all instruments and other documents, and shall take such other actions as may be necessary or reasonably requested by Licensee, so that Licensee may protect and defend its rights in and to such Buy Out IP. |
(c) |
Licensee shall, in connection with any Buy Out, provide to Licensor, upon completion of such Buy Out transaction, a license to use the Buy Out IP, without cost, for the purposes set out in Section 2.4(b), on the same terms and conditions as set out therein. |
8.4 |
Infringement. |
(a) |
Each Party will notify the other Party promptly upon becoming aware of any claim or assertion by a Third Party that the Intellectual Property Rights infringe or otherwise are in violation of a Third Party’s patents or intellectual property. The Parties shall promptly enter into discussions with the Third Party to determine the existence and extent of the infringement and the Parties’ mutually agreed course of action. Each Party will absorb its own costs of the discussions. |
(b) |
Licensee and Licensor may agree to jointly defend or pursue litigation, but neither of them shall bind or commit the other to any course of action which involves |
-15-
liability for legal costs, expenses or damages. If Licensee and Licensor fail to agree, within a reasonable time, having regard to the normal progress of litigation, as to any course of action which might be jointly taken, then either of them may take or defend proceedings alone, if legally entitled to act alone, and shall be entitled to retain anything awarded to it by a court or paid to it as a settlement by the Third Party. Where either Licensee or Licensor wishes to act alone or is unable to agree on a joint defence, but formalities require participation of the other, then the other shall join in the proceeding to the extent necessary for formalities. Each will cooperate with the other in making available all necessary documents and witnesses for any legal proceedings. The Party acting alone will reimburse the other for its time and all costs associated with participation in the proceedings, and making available of documents. If either Licensee or Licensor proceeds alone (or with the other as a formality), it shall indemnify the other for reasonable legal costs for representation that is reasonably necessary and for any court-ordered payments. Neither Licensee nor Licensor shall settle any such claim without the consent of the other, Such consent not to be unreasonably withheld.
(c) |
The Parties may negotiate with a Third Party to obtain any additional rights required such as may arise if a Third Party’s patent emerges. Each Party shall absorb its own costs of negotiation. |
ARTICLE 9
PATENT MARKING
9.1 |
Technology Patent. Licensor covenants to provide issued patent numbers pertaining to the Technology to Licensee and such information will be set forth on Schedule B, as may be amended from time to time. |
9.2 |
Patent Marking. Licensee shall mark all Products made, used or sold pursuant to the terms of this Agreement, in accordance with all applicable patent marking laws and, prior to marketing the Product, will obtain written confirmation from Licensor that all applicable patent numbers are set forth on Schedule B. Thereafter, Licensor shall keep Licensee apprised of further Patents it wishes to have marked on Products. |
ARTICLE 10
INDEMNIFICATION AND INSURANCE
10.1 |
Disclaimer. Except as otherwise set forth in this Agreement, all Technology, Licensor Updates and Intellectual Property Rights are provided on an “as is” basis. |
10.2 |
Indemnification by Licensee. Licensee shall save, defend and hold Licensor and its officers, directors, employees, consultants and agents harmless from and against any and all suits, claims, actions, demands, liabilities, expenses and losses, including reasonable legal expenses and attorneys’ fees, resulting directly or indirectly from this Agreement or the development or sale of Products (save and except for any and all suits, claims, actions, demands, liabilities, expenses and losses to the extent caused by the gross negligence or wilful misconduct of Licensor or arising directly or indirectly out of or in connection with the breach of this Agreement by Licensor). This provision shall survive expiration or other termination of the Agreement. |
-16-
10.3 |
Insurance. |
(a) |
Licensee, at its own expense, shall maintain in full force and effect throughout the term of this Agreement (and following termination thereof to cover any claims arising from the perfonnance of this Agreement), a policy of insurance at levels sufficient for the conduct of Licensee’s business and the services contemplated by this Agreement, including the indemnification obligations hereunder, in such amounts and on terms as are customary in the industry for the activities to be conducted by the License under this Agreement. |
(b) |
Upon Licensor’s reasonable request, the amount of insurance coverage shall be increased as may be reasonably necessary. |
(c) |
The coming into force of this Agreement is conditional upon Licensor having reviewed a certificate of insurance from the relevant insurance company, in a form acceptable to Licensor, acting reasonably, evidencing Licensee’s compliance with the provisions of this Section 10.3. Such policy shall contain an endorsement by the insurer providing that it shall not be cancelled or amended without thirty (30) days’ prior notice to Licensor. Licensee shall provide any further certificates of such insurance to Licensor upon Licensor’s written request. |
10.4 |
No Consequential Damages. Except with respect to each Party’s obligations under Article 11, Licensor’s representations under Section 6.2 and Licensee’s obligations under Sections 10.2, neither Party shall be liable for consequential damages or indirect loss of whatever nature hereunder including, but not limited to, lost profits, unless such damage was caused by the gross negligence or wilful misconduct of the breaching party or others for whom such Party is responsible. |
ARTICLE 11
CONFIDENTIALITY
11.1 |
Confidential Information. Any Confidential Information received by one Party (the “Recipient”) from the other Party (the “Disclosing Party”) pursuant to this Agreement shall be used, disclosed or copied only for the purposes of, and only in accordance with, this Agreement. The Recipient shall use, at a minimum, the same degree of care as it uses to protect its own Confidential Information of a similar nature, but no less than reasonable care, to prevent the unauthorized use, disclosure or publication of the Disclosing Party’s Confidential Information. Without limiting the generality of the foregoing: |
(a) |
Recipient shall only disclose the Disclosing Party’s Confidential Information to Recipient’s employees or any individual or entity which (i) has entered into a written agreement with Recipient containing obligations of confidence substantially similar to (but no less protective of the Confidential Information than) those contained in this Agreementand (b) needs access to the Disclosing Party’s Confidential Information consistent with the Recipient’s rights under this Agreement; |
-17-
(c) |
Recipient shall not make or have made any copies of the Disclosing Party’s Confidential Information, except those copies which are reasonable for the purposes of this Agreement; and |
(d) |
Recipient shall affix to any copies it makes of the Disclosing Party’s Confidential Information, to the extent reasonably practicable, all proprietary notices or legends affixed to the Confidential Information as they appear on the copies of the Confidential Information originally received from the Disclosing Party. |
11.2 |
Exclusions. Recipient shall not be bound by obligations restricting disclosure set forth in this Agreement with respect to any Confidential Information which: |
(a) |
was known by the Recipient prior to disclosure, as evidenced by its written records; |
(b) |
was lawfully in the public domain prior to its disclosure, or lawfully becomes publicly available other than through a breach of this Agreement or any other confidentiality obligation on behalf of any Third Party; |
(c) |
was disclosed to the Recipient by a Third Party provided such Third Party, or any . other party from whom such Third Party receives such information, is not in breach of any confidentiality obligation in respect of such information; |
(d) |
is independently developed by the Recipient, as evidenced by its written records; or |
(e) |
is required to be disclosed for patient safety reasons. |
In addition, Disclosing Party’s Confidential Information may be disclosed by a Recipient when such disclosure is compelled pursuant to legal, judicial or administrative proceedings, or otherwise required by law, provided that Recipient advises the Disclosing Party of any such compelled disclosure in a timely manner prior to making any such disclosure (so that Disclosing Party can apply for such legal protection as may be available with respect to the confidentiality of the information which is to be disclosed), and provided that Recipient shall apply for such legal protection as may be available with respect to the confidentiality of the Disclosing Party’s Confidential Information which is required to be disclosed.
11.3 |
Notice of Disclosures. Except for disclosures authorized under the terms of this Agreement, each Party shall notify the other immediately upon learning of any unauthorized disclosure of the other Party’s Confidential Information. |
ARTICLE 12
ASSIGNMENT
12.1 |
Assignment. This Agreement may not be assigned by a Party except that a Party may assign this Agreement in connection with the sale of all or substantially all of the assets of such Party provided that such Party provides at least sixty (60) days’ prior written notice to the other Party of the intent to assign this Agreement and executes an instrument |
-18-
agreeing to remain bound by the terms of this Agreement and further provided that, where such sale is by Licensee, such sale is a bona fide transaction entered into with a third party that deals at arm’s length with Licensee and also with each of the holders of preferred shares in the capital of Licensee.
ARTICLE 13
TERM AND TERMINATION
13.1 |
Term. Subject to Sections 13.2, 13.3 and 13.6, the term of this Agreement (the “Term”) shall commence on the Effective Date and will continue until the earlier of: (i) expiration of the last of the Patents to expire and (ii) the effective date of the Buy Out. |
13.2 |
Breach. Subject to Section 13.4, if a Party is in breach of, or fails to perform, a material obligation under this Agreement, and fails to cure such breach or perform such obligation to the non-breaching Party’s satisfaction, acting reasonably, within thirty (30) days after the date of written notice thereof, the non-breaching Party may, upon thirty (30) days’ additional written notice, terminate this Agreement. For the purposes of this Agreement, breach of a “material obligation” shall include, but not be limited to: (a) use of the Technology and/or Intellectual Property Rights outside of, or otherwise committing or causing to be committed a material act that is outside of the scope of, the licences set forth in Article 2 of this Agreement, (b) authorizing or knowingly acquiescing in an End User’s use of the Products for a purpose other than specified in Section 2.1 of this Agreement, or (c) committing or causing to be committed an act that is a material breach of Article 11 (Confidentiality). |
13.3 |
Termination on Bankruptcy, Insolvency. In the event that (a) a Party ceases to operate as a going concern, or (b) a decree or order of a competent court is entered finally (i) winding up all the affairs of such Party and its Affiliates under the Companies Creditors Arrangement Act (Canada), the Bankruptcy and Insolvency Act (Canada) or the Winding- Up and Restructuring Act (Canada), United States Bankruptcy Code, or any other bankruptcy, insolvency or analogous law, or (ii) ordering the winding-up or liquidation of such Party, and any such decree or order continues and is in effect for a period of thirty (30) days from its issuance and is not withdrawn or discharged within such thirty (30) days of issuance, the other Party shall have the right to terminate this Agreement after the relevant cure period, if any. |
13.4 |
Non-Performance Termination. Licensor may terminate this Agreement, effective immediately, if Licensee is determined to be in non-performance (as set out in Schedule D) of any of the Milestones or if it defaults in any of its payment obligations hereunder and does not: (i) in the case of achievement of Milestones, cure such non-performance within thirty (30) days of receipt of written notice from Licensor as to such non- performance, all as stipulated and in accordance with the description of the Milestones in Schedule D; and (ii) in the case of a payment obligation, cure such default within thirty (30) days of receipt of written notice from Licensor as to such default. |
13.5 |
Conduct on Termination of this Agreement. On termination of this Agreement other than in connection with the Buy Out or expiry of the Term: |
-19-
(a) |
Licensee shall cease to initiate any new sale of Products. Licensee may complete the sale of all Products with respect to (i) written purchase orders that requir.e delivery within ninety (90) days of such termination, or (ii) outstanding quotations to actual and prospective customers, provided these quotations expire within thirty (30) days of such termination; |
(b) |
Licensee shall, within thirty (30) days of such termination, return to Licensor or, if so requested by Licensor, certify in writing to Licensor that all copies of the Technology, Licensor Updates and Confidential Information in the possession or under the legal control of Licensee have been destroyed; and |
(c) |
all existing obligations that have accrued to the date of such termination shall be honoured. |
13.6 |
Survival. The following provisions shall survive tennination or expiry of this Agreement: Article 1 (Definitions), Article 3 (Ownership) (except in the case of termination in connection with the Buy Out, in which case Article 3 shall not survive), Article 10 (Indemnification), Article 11 (Confidentiality), Section 13.5 (Conduct on Termination of this Agreement), Section 13.6 (Survival), Article. 14 (Arbitration) and Article 15 (General). |
ARTICLE 14
ARBITRATION
14.1 |
Arbitration. Subject to either Party’s right to seek injunctive or other temporary relief in any court or other tribunal of competent jurisdiction, any controversy, dispute, difference of opinion or failure to agree which shall arise between the Parties concerning this Agreement or its construction or application, or the rights, duties and obligations of any Party to this Agreement, shall be resolved in the following listed order as needed: |
(a) |
Direct negotiation between Licensee and Licensor with specific regard to the disputed item and reconciliation thereof in good faith, to continue for a maximum period of fifteen (15) days from the “date of initial dispute”, which shall mean the date of notice under Section 14.1 of a dispute initiated by a Party; |
(b) |
Mediation between Licensee and Licensor through an independent individual (“Mediator”), selected upon mutual agreement by the Parties, who has at least ten (10) years experience in the practice of law relating to the subject matter of the dispute, is familiar with details of the disputed item and can assist in reconciliation thereof in good faith, to continue for a maximum period of thirty (30) days from the date of initial dispute; and |
(c) |
Arbitration before a sole arbitrator (“Arbitrator”), selected upon mutual agreement by the Parties, the award and determination for which shall be final and binding upon the Parties. If the Parties do not agree upon the Arbitrator, each Party may apply to a judge of the Ontario Court, General Division under the Arbitration Act, 1991 (Ontario) (the “Arbitration Act”) for the appointment of the Arbitrator. The rules and procedures of the Arbitration Act shall apply to any arbitration conducted hereunder. Unless otherwise agreed to by the Parties in |
-20-
writing, the arbitration shall take place in Toronto in the English language. The Parties shall jointly pay and be responsible for the costs of the arbitration, provided that the Arbitrator may make an award of costs upon the conclusion of the arbitration making one of the Parties liable to pay the costs of the other Party.
14.2 |
Notwithstanding Section 14.1, the individual selected as Mediator or Arbitrator shall be (a) qualified by education and experience to decide the matter in dispute and, in the case of any dispute regarding the performance of the Milestones, an expert in the Field of Use; and (b) at arm’s length from the Parties and shall not be a member of the audit or legal firm or firms who advise the Parties, nor shall the Mediator or Arbitrator be an individual who is, or is a member of a firm, otherwise regularly retained by each of the Parties. |
ARTICLE 15
GENERAL
15.1 |
Notices. All notices shall be in writing and sent by certified or registered mail, return receipt requested, or by hand delivery to the other Party at the following address or fax number: |
(a) |
If to Licensor: |
Sunnybrook Health Sciences Centre
2075 Bayview Avenue
Toronto, Ontario M4N 3M5
Attention: |
Leslie Boehm, Director of Operations and Business Development |
Fax: |
(416) 480-5814 |
(b) |
If to Licensee: |
Profound Medical Inc.
c/o Genesys Ventures II
200 Front Street West, Ste 3004
Toronto, Ontario M5V 3K2
Attention: |
Paul Chipperton, Chief Operating Officer |
Fax: |
(416) 598-3328 |
Such notices shall be deemed to have been received five (5) Business Days after mailing if forwarded by mail or on the day of receipt if delivered by hand or fax. The address of either Party may be changed by giving notice to the other Party in accordance with the foregoing.
15.2 |
Independent Contractors. No agency, partnership, joint venture or employment relationship is or shall be created by virtue of this Agreement. |
15.3 |
Publicity. Neither Party shall use the name of the other Party or make any reference to the terms of this Agreement in any advertising, public relations, promotional materials or |
-21-
media releases without the prior written consent of such other Party, except that Licensee may state that it has licensed Technology from Licensor.
15.4 |
Entire Agreement. The attached Schedules form part of this Agreement and are incorporated by reference herein. This Agreement and the Sponsored Research Agreement, entered into between the Parties as of the same effective date constitute the entire agreement between the Parties as to the subject matter contained herein and supersedes all other agreements between the Parties concerning such subject matter. This Agreement may only be modified by an instrument in writing executed by each Party’s duly authorized representative. |
15.5 |
Headings. The headings, sections and articles are inserted for convenience of reference only and are not intended to be a part of or to affect the meaning or interpretation of this Agreement. |
15.6 |
Waiver. Any waiver of, or consent to depart from, the requirements of any provision of this Agreement shall be effective only if it is in writing and signed by the Party giving it, and only in the specific instance and for the specific purpose for which it has been given. No failure on the part of any Party to exercise, and no delay in exercising, any right under this Agreement shall operate as a waiver of such right. No single or partial exercise of any such right shall preclude any other or further exercise of such right or the exercise of any other right. |
15.7 |
Governing Law. This Agreement shall be governed by, and interpreted and enforced in accordance with, the laws in force in the Province of Ontario (excluding any conflict of laws rule or principle which might refer such questions to the laws of another jurisdiction). Each Party irrevocably attorns to and submits to the non-exclusive jurisdiction of the courts of Ontario with respect to any matter arising hereunder or related hereto. |
15.8 |
Severability. If any one gr more of the provisions contained in this Agreement shall, for any reason, be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provisions of this Agreement. All other provisions shall remain in full force and effect. |
15.9 |
Due Authority. The persons executing this Agreement on behalf of Licensor and Licensee represent and warrant that they are duly authorized to legally bind Licensor and Licensee, respectively, to the terms and conditions of this Agreement. |
15.10 |
Gender and Number. Any reference in this Agreement to gender includes all genders and words importing the singular number only shall include the plural and vice versa. |
15.11 |
Currency. All dollar amounts referred to herein are in Canadian currency, unless otherwise indicated. |
15.12 |
Further Assurances. Each Party shall from time to time promptly execute and deliver and take all further action as may be reasonably necessary or appropriate to give effect to the provisions and intent of this Agreement and to complete the transactions contemplated by this Agreement. |
-22-
15.13 |
Counterparts; Facsimile. This Agreement may be executed in counterparts and transmitted by facsimile, each of which shall be deemed an original and all of which, taken together, shall constitute one and the same instrument. |
[REMAINDER OF THIS PAGE INTENTIONALLY LEFT BLANK]
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the date first written above.
|
SUNNYBROOK HEALTH SCIENCES CENTRE |
||
|
|
||
|
By: |
“Michael Julius” |
|
|
|
Authorized Signature |
|
|
|
|
|
|
Date: |
May 18, 2011 |
|
|
|
||
|
By: |
“Ahsan Welch” |
|
|
|
Authorized Signature |
|
|
|
||
|
Date: |
May 18, 2011 |
|
|
|
|
|
|
PROFOUND MEDICAL INC. |
||
|
|
|
|
|
By: |
“Paul Chipperton” |
|
|
|
Authorized Signature |
|
|
|
|
|
|
Date: |
May 25, 2011 |
|
|
|
|
|
SCHEDULE A
TECHNOLOGY DESCRIPTION
MRI-guided transurethral ultrasound therapy is an image-guided, minimally-inyasive treatment for localized prostate cancer performed within a standard clinical MR imager. High-intensity ultrasound energy is delivered from a device in the urethra using rotational control to generate a precise region of thermal coagulation in the prostate gland. Real-time temperature maps acquired with MRI during heating are used as active feedback to ensure a precise region of thermal damage is generated within the prostate gland. The nature of the heating applicator enables a high degree of spatial control over tissue heating which improves targeting within the prostate gland while reducing damage to surrounding tissues, the main limitation of existing treatments for prostate cancer. The hardware, software and medical devices that make up this technology have been developed within the Imaging Research group at Sunnybrook Health Sciences Centre over the past eight years through peer-reviewed grant support.
The technology is designed to treat low- to moderate-risk localized prostate cancer which is the most rapidly growing segment in this patient population due to widespread use of PSA testing and ultrasound-guided transrectal biopsy. In this population, a minimally invasive treatment that can be delivered efficiently with high precision and minimal recovery time would be highly desirable from the perspective of patients, clinicians and healthcare providers due to the significant complications and lengthy recovery times that accompany conventional radical treatments.
The potential features of this technology are:
l. |
the minimally-invasive transurethral approach; |
2. |
the incorporation of real-time quantitative MR temperature imaging for precise control of treatment |
3. |
short treatment time (~30 minutes); |
4. |
rapid recovery; and |
5. |
the capability to perform multiple treatments in the case of recurrent disease or deliberate focal therapy. |
SCHEDULE B
PATENT APPLICATIONS; PATENTS; AND COPYRIGHTS
Chopra and Bronskill: US 6589174 Technique and apparatus for ultrasound therapy.
Chopra, Bronskill & Burtnyk: US20060206105 Treatment of diseased tissue using controlled ultrasonic heating.
Chopra, Bronskill & Tang: US20070239062 Method and apparatus for obtaining quantitative temperature measurements in prostate and other tissue undergoing thermal therapy treatment.
SCHEDULE C
CONFIDENTIAL INVENTION FORMS
Sunnybrook Research Institute Confidential Invention Disclosures:
Chopra, Bronskill & Burtnyk: 3D algorithm to control transurethral ultrasound heating including temperature noise reduction and extrapolation techniques.
Chopra & Bronskill: Rectal cooling device for use with transurethral thermal therapy.
SCHEDULE D
Part 1. |
BUY OUT MILESTONES |
Event |
Sunset Date |
Treatment of first human patient using the Technology |
December 31, 2010 |
Total direct Third Party investment in Licensee (whether through equity or debt financing, government grants, refundable tax credits or other sources of non-dilutive financing) of at least Cdn $7.5 million or such lesser amount as the Board of Directors of Licensee determines (acting in good faith) is sufficient to fund Licensee’s business plan and to enable Licensee to develop a Product. |
December 31, 2012 |
PMA or 510k regulatory approval |
December 31, 2018 |
Part 2. |
MILESTONES |
Licensee will use its commercially reasonable efforts to develop and commercialize the Technology as it extends to all other intellectual property and technology under its control. Formal petformance reviews may be performed by Licensor every six (6) months from the Effective Date to the termination or expiry of this Agreement. At each review, Licensee will provide a full description of the research and development being performed and to be petformed with the Technology by the Licensee, including copies of relevant internal disclosures, reports and memos.
Non-performance will be regarded as two (2) consecutive reviews in which Licensor, acting reasonably, determines that the progress of the research and development that the Licensee has performed up to the relevant date, in the context of Licensee’s goal to achieve 510k regulatory approval from the U.S. Food and Orug Administration or a PMA from it or any similar regulatory authority by the Sunset Date noted above, is not satisfactory, provided that Licensor shall provide written notice to Licensee of the results of each such investigation. Any conclusion as to non-performance shall be conclusive, subject only to the dispute resolution provisions of this Agreement.
SCHEDULE E
LEASED PREMISES
Up to 1000 square feet at 3080 Yonge St. Toronto, Ontario.
Space can be increased by mutual consent, such consent not to be unreasonably withheld.
Exhibit 10.8
SIEMENS ID
Agreement
by and between
Profound Medical Inc.
(- hereinafter referred to as “the Receiver” -)
and
SIEMENS Healthcare GmbH
(- hereinafter referred to as “SIEMENS” -)
- the Receiver and SIEMENS hereinafter referred to individually
as “PARTY” or collectively as “PARTIES” -
on the provision of information regarding SIEMENS’ interface
CONFIDENTIAL
2 / 26
Contents
Preamble |
3 |
Article 1 - Definitions |
3 |
Article 2 - License |
5 |
Article 3 - Obligations of the PARTIES |
5 |
Article 4 - INTERFACE |
7 |
Article 5 - Provision of DEVELOPER SERVICES, INFORMATION and the SDK |
9 |
Article 6 - Pricing |
12 |
Article 7 - Limitations: Permits, Regulatory Approvals and Standards |
13 |
Article 8 - Billing |
16 |
Article 9 - Marketing |
17 |
Article 10 - Monitoring of Usage Data |
17 |
Article 11 - Revocation of the AUTHORIZATION KEY and LICENSE KEY |
17 |
Article 12 - Notification Page |
18 |
Article 13 - Support |
18 |
Article 14 - Liability |
19 |
Article 15 - Confidentiality |
20 |
Article 16 - Agreement Term, Notification and CHANGE-OF-CONTROL |
21 |
Article 17 - Force Majeure |
22 |
Article 18 - Miscellaneous |
23 |
Article 19 - Arbitration and Substantive Law |
24 |
Annex 1 – Specification of INFORMATION |
Annex 2 – Specification of PRODUCTS |
Annex 3 – Agreement for risks exported in using Access-i |
CONFIDENTIAL
3 / 26
Preamble
The PARTIES entered in a co-marketing and co-selling agreement on February 26, 2016 (the “Original Agreement”).
The PARTIES now wish to substitute and replace the Original Agreement with this Agreement in connection with all sales and other activities as of the Effective Date, such that all prior financial commitments and obligations are released and replaced with the financial commitments and obligations of this Agreement.
SIEMENS is prepared to supply certain interface information and/or other documentation to the Receiver to enable the Receiver to use the Access-I interface for interfacing PRODUCTS to certain SIEMENS MAGNETOM Scanners pursuant to the terms of this Agreement.
The PARTIES therefore agree as follows:
Article 1 - Definitions
1.1 |
INFORMATION hereinafter refers to know-how, information and/or documentation supplied by SIEMENS to the Receiver as detailed in Annex 1. |
1.2 |
PRODUCTS hereinafter refer to Receiver’s products developed for the ablation of soft tissue in conjunction with MRI scanners. |
1.3 |
SIEMENS MAGNETOM SCANNERS hereinafter refer to certain SIEMENS MAGNETOM Scanners which the Receiver intends to interface to its PRODUCTS using the INFORMATION and which are equipped with the INTERFACE. |
1.4 |
CHANGE OF CONTROL hereinafter means, with respect to the Receiver, one of the following events or series of related events: a) a sale of all or substantially all of the Receiver’s assets, voting stock or securities or business relating to this Agreement; b) a merger, reorganization or consolidation involving the Receiver in which the stockholders of the Receiver immediately prior to such transaction cease to own collectively a majority of the voting equity securities of the successor entity; or c) a person or group of persons acting in concert acquire fifty percent (50%) or more of the voting equity securities of the Receiver. |
1.5 |
AFFILIATE shall mean, with respect to a PARTY, a subsidiary, meaning a company in which such PARTY owns or controls, directly or indirectly, more than fifty percent (50%) of the stock or voting rights, or a parent company, meaning a company which owns or controls, directly or indirectly, more than fifty percent (50%) of the stock or voting rights of the relevant PARTY, or a company which is a subsidiary of such PARTY’s parent company. |
CONFIDENTIAL
4 / 26
1.6 |
COMPETITOR hereinafter refers to any entity which has a product line that competes with SIEMENS MAGNETOM SCANNERS. |
1.7 |
END USER hereinafter refers to an individual, company, institution or other legal entity other than the PARTIES or an AFFILIATE of SIEMENS that uses or intends to use the PRODUCTS in combination with a SIEMENS MAGNETOM SCANNER to treat patients or to conduct any form of research. |
1.8 |
OTHERS hereinafter refers to any individual, company, institution or other legal entity other than the PARTIES or any AFFILIATE of either of the PARTIES or END USERS. |
1.9 |
IP hereinafter refers to intellectual property, which is patents, rights to inventions, copyright and related rights, trademarks, trade names and domain names, rights to sue for passing off, rights in designs, rights in computer software, database rights, rights in confidential information (including know-how and trade secrets) and any other equivalent intellectual property rights, in each case whether registered or un- registered and including all applications (or rights to apply) for, and renewals or ex- tensions of, such rights or and all similar or equivalent rights or forms of protection which subsist now or in the future, in any part of the world. |
1.10 |
INTERFACE hereinafter refers to the Access-i (Scanner Remote Control) Interface for 3rd party device integration developed and provided through SIEMENS. |
1.11 |
DEVELOPER SERVICES hereinafter refers to the activities conducted by SIEMENS to support the development efforts of the Receiver to achieve compatibility of the Receiver’s PRODUCTS with the INTERFACE; this does not include any other development efforts in the Receiver’s PRODUCTS. |
1.12 |
SOFTWARE DEVELOPER KIT (SDK) hereinafter refers to a software tool developed and provided through SIEMENS to support the development efforts of the Receiver to achieve compatibility of the Receiver’s PRODUCTS with the INTERFACE. |
1.13 |
AUTHORIZATION KEY hereinafter refers to an authorization key file installed on the Receiver’s PRODUCTS enabling the use of the PRODUCTS in connection with the INTERFACE by End Users. The AUTHORIZATION KEY is time limited and unique to each individual MAGNETOM SCANNER. |
1.14 |
LICENSE KEY hereinafter refers to a software license and license key file installed on the SIEMENS MAGNETOM SCANNERS enabling the INTERFACE on SIEMENS MAGNETOM SCANNERS. The LICENSE KEY has to be purchased by the END USER (at no additional cost to Receiver) from Siemens as a one-time purchase. The provision and the cost of this LICENSE KEY are not covered by this agreement. |
1.15 |
EFFECTIVE DATE hereinafter means January 21st, 2019. |
CONFIDENTIAL
5 / 26
Article 2 - License
2.1 |
Subject to the terms and conditions set out in this Agreement, SIEMENS shall make available and provide to the Receiver (and its personnel and subcontractors) the IN- FORMATION, DEVELOPER SERVICES, the SDK and the AUTHORIZATION KEY and herewith grants to the Receiver under this Agreement the following rights: |
2.2 |
A non-exclusive, non-transferable, worldwide right and license to use the INFORMATION, DEVELOPER SERVICES, the SDK and the AUTHORIZATION KEY for the sole purpose of interfacing PRODUCTS to certain SIEMENS MAGNETOM SCAN- NERS and to make available and deliver such adapted PRODUCTS for use by END USERS for use in connection with SIEMENS MAGNETOM SCANNERS for research and/or development purposes and/or for medical treatment. The license granted herein includes the right to grant sublicenses to Receiver’s AFFILIATES and service providers to Profound carrying out Profounds business and shall exclusively apply to the Receiver’s PRODUCTS and any successor product to the Receiver’s PROD- UCTS which replaces the PRODUCT in question, but not cover any other product of the Receiver. Receiver will not reverse engineer the SDK, the INTERFACE or the AUTHORIZATION KEY for the purpose of the development of adaptations of the Receiver’s PRODUCTS to any other customer other than SIEMENS customers. |
Notwithstanding the above, nothing in this Agreement shall limit the Receiver from developing PRODUCTS that can interface with scanners from multiple suppliers (i.e. that can interface with both SIEMENS MAGNETOM SCANNERS and scanners from other manufacturers) as long as Receiver does not use or disclose to such other manufacturers any CONFIDENTIAL INFORMATION provided to the Receiver under this Agreement in the development of interfaces for such other manufacturers’ scanners.
Article 3 - Obligations of the PARTIES
3.1 |
Obligations of SIEMENS: |
3.1.1 |
SIEMENS will provide the Receiver with information on the related product risks as referred to in Annex 3 and inform the Receiver of any updates during the lifetime of the INTERFACE. |
3.1.2 |
SIEMENS will provide the Receiver with AUTHORIZATION KEYS on a perpetual basis for each individual SIEMENS MAGNETOM SCANNER enabling the use of the INTER- FACE according to the INTERFACE description. |
3.1.3 |
SIEMENS will provide the END USER with the LICENSE KEY to enable use of the SDK with the INTERFACE. |
CONFIDENTIAL
6 / 26
3.1.4 |
SIEMENS will use commercially reasonable efforts to notify the Receiver of any actual or planned change regarding the INTERFACE that might compromise the compatible use of the Receivers PRODUCTS with the SIEMENS INTERFACE, and to provide the Receiver with such INFORMATION and/or collaborative testing as may be reasonably required in order for the Receiver to sufficiently maintain or update the Receiver’s PRODUCTS and, if applicable, the Technical Documentation and/or clinical dossier of such PRODUCTS in accordance with Directive 93/42/EEC or Regulation (EU) 2017/745 on medical devices, as applicable, to ensure continued compatible use with the INTERFACE in view of such change no less than 120 days in advance of the time at which the change becomes effective for End Users SIEMENS MAGNETOM SCANNERs. More generally Siemens will aid reasonable requests to support the attainment of data for validation runs, compatibility testing and other such assistance; however the determination of reasonable will be at Siemens discreation. |
3.1.5 |
SIEMENS will use commercially reasonable efforts to provide the DEVELOPER SER- VICES and the SDK to the Receiver. |
3.1.6 |
SIEMENS shall not have any other obligations than those expressly described in this Agreement. For the sake of clarity, SIEMENS shall not be obligated to disclose any further information than the INFORMATION described in Annex 1. |
3.2 |
Obligations of the Receiver: |
3.2.1 |
The Receiver acknowledges and agrees that the AUTHORIZATION KEY enables the use of the INTERFACE according to the INTERFACE description and that its improper use could cause damage to the system and patient injury. It is the responsibility of the Receiver to comply with all applicable legal requirements related to the PRODUCTS, to understand all consequences of using the PRODUCTS or the INTERFACE on the basis of the INFORMATION provided by SIEMENS in Annex 1 and the product risk information provided in Annex 3, and to refrain from such use if there is any reasonable question of legality, hardware or other property damage, or patient injury. |
3.2.2 |
If the use of the INTERFACE is for the purpose of research conducted or sponsored by the Receiver involving human subjects, it requires appropriate documentation, review and approval by an appropriate Ethics Committee or by the Receiver’s Institutional Re- view Board (“IRB”) according to applicable medical device law and compliance with all Ethics Committee/IRB recommendations and requirements. No human subject re- search may be commenced by Receiver or a party sponsored by Receiver before Ethics Committee/IRB approval has been granted. |
3.2.3 |
The Receiver accepts the exported product risks described in Annex 3 to this contract and any risks as updated by SIEMENS according to Article 3.1.1. It is the responsibility of the Receiver to assess the measures defined in Annex 3 within their processes and |
CONFIDENTIAL
7 / 26
to ensure that reasonable measures are in place to mitigate these risks in using the INTERFACE’s functionality.
3.2.4 |
For the issuance of an AUTHORIZATION KEY, the Receiver has to provide to SIEMENS the serial number of the SIEMENS MAGNETOM SCANNER the PRODUCT will be interfaced with and the duration the PRODUCT is intended to be compatible with the SIEMENS MAGNETOM SCANNER, whereas AUTHORIZATION KEYS can be deactivated on request |
3.2.5 |
The AUTHORIZATION KEY mentioned in Article 3.1.2 (and any other information pro- vided by SIEMENS) shall be (i) used by the Receiver exclusively to use the INTER- FACE for interfacing PRODUCTS to certain SIEMENS MAGNETOM SCANNERS, un- less otherwise expressly agreed to in writing by SIEMENS and (ii) kept strictly confidential and encrypted or in some secure fashion by the Receiver with the same degree of care as is used with respect to the Receiver’s own equally important confidential information to avoid disclosure to any third party, but at least with reasonable care. Without limiting use of the PRODUCTS by END USERS the AUTHORIZATION KEY shall not be made available to OTHERs and shall be made available to AFFILIATES, Receiver’s personnel, contractors for the sole purpose of interfacing and using the PRODUCTS with the dedicated SIEMENS MAGNETOM SCANNER according to the terms and conditions of this Agreement. |
3.2.6 |
The Receiver has the sole responsibility for the combined products and it is the Receiver’s responsibility to ensure that all legal and country specific requirements for such a combination are adhered to, following the obligations of the country in which the com- bination is used. This includes, for example, that no unacceptable risks can be caused by the combination to patients or users. SIEMENS takes no responsibility regarding the combination of the products even though SIEMENS supports the connectivity to the INTERFACE; provided that any damage occurred, if any, was not caused by a gross negligent act or omission of SIEMENS. |
Article 4 - INTERFACE
4.1 |
Update Cycles |
SIEMENS is prepared to continuously develop new functionalities and features for the INTERFACE at no additional cost to Receiver. Such new features and functionalities are intended to be released in up to two application updates per year in accordance with Section 3.1.4. Security related updates and patches can be introduced whenever deemed necessary by SIEMENS. SIEMENS will use commercially reason-able efforts to maintain the compatibility of the PRODUCT with the SIEMENS MAGNETOM SCANNER and provide all necessary information and assistance to Receiver as outlined in Section 3.1.4.
CONFIDENTIAL
8 / 26
4.2 |
Changes to Requirements |
SIEMENS may change and/or issue additional technical requirements for the combi- nation of the PRODUCT with the SIEMENS MAGNETOM SCANNER using the INTERFACE at any time by using commercially reasonable efforts to notifying the Receiver at SIEMENS’ earliest convenience, but no less than 120 days before the changed technical requirements become effective, provided that SIEMENS will endeavor to maintain compatibility of the INTERFACE with any of the Receiver’s PROD- UCTS compliant with the requirements at the time of their submission during next two (2) update cycles of the INTERFACE following the submission of the respective PRODUCT at minimum. Notwithstanding the foregoing, the Receiver has to ensure that the combination of its PRODUCTS to the SIEMENS MAGNETOM SCANNER is still working with the changes in the requirement and SIEMENS shall provide all necessary information and assistance in this respect, as outlined in Section 3.1.4. Such notice period may be shortened by SIEMENS, and the requirements regarding maintenance of compatibility will not apply only to the extent that, based on SIEMENS’ reasonable judgment, SIEMENS believes that a shorter notice period or in- compatibility is necessary in order to avoid any: (i) threat to the security or functionality of the INTERFACE or the DEVELOPER SERVICES; (ii) adverse impact on the Receiver, SIEMENS, the Receiver’s or SIEMENS’ AFFILIATES, END USERS, or any third party, including, without limitation, any risk of personal injury; and/or (iii) subject the Receiver, SIEMENS, the Receiver’s or SIEMENS’ AFFILIATES, END USERS, or any third party to material liability for personal injury or IP infringement; in each case, so long as SIEMENS takes commercially reasonable efforts to maintain the compatibility of the PRODUCT with the SIEMENS MAGNETOM SCANNER. To the extent the Receiver is, due to such changes, materially deprived of the benefits of the INTERFACE or the SDK, the Receiver is entitled to terminate the Agreement in writing with effect upon effectiveness of the change at the earliest and the Receiver will be entitled to a refund of all amounts paid with regards to the affected AUTHORIZATION KEYs by Receiver to SIEMENS over the period of incompatability.
4.3 |
Changes to the INTERFACE |
SIEMENS updates and further develops the technology, features and functionalities of the INTERFACE and is under no obligation to maintain prior versions thereof. Upon the provision of a modified or new version of the INTERFACE, the Receiver is no longer entitled to use previous versions. Should material changes to the INTERFACE be implemented which have an impact on the Receiver’s use, whereas these impacts are known to SIEMENS, or should agreed functionalities be restricted or disabled, SIEMENS will use commercially reasonable efforts to notify the Receiver at SIEMENS’ earliest convenience, but no less than 120 days before the changes become effective.
CONFIDENTIAL
9 / 26
Such notice period may be shortened by SIEMENS only to the extent that, based on SIEMENS’ reasonable judgment, SIEMENS believes that a shorter notice period is necessary in order to avoid any: (i) threat to the security or functionality of the INTERFACE; (ii) adverse impact on the Receiver, SIEMENS, the Receiver’s or SIEMENS’ Affiliates, END USERS, or any third party, including, without limitation, any risk of personal injury; and/or (iii) subject the Receiver, SIEMENS, the Receiver’s or SIEMENS’ Affiliates, END USERS, or any third party to material liability for personal injury or IP infringement; in each case, so long as SIEMENS takes commercially rea- sonable efforts to maintain the compatibility of the PRODUCT with the SIEMENS MAGNETOM SCANNER. To the extent the Receiver is, due to such changes, materially deprived of the benefits of the INTERFACE, the Receiver is entitled to terminate the Agreement in writing with effect upon effectiveness of the change at the earliest and Receiver will be entitled to a refund of all amounts with regards to the affected AUTHORIZATION KEYs paid by Receiver to SIEMENS over the period of incompatability.
4.4 |
Freedom from Defects in Title |
SIEMENS represents, warrants and covenants that during the term of this Agreement, the Receiver’s use of the INTERFACE as permitted under the Agreement does not infringe upon a patent, copyright, trade secret, or other intellectual property right in or to such INTERFACE in the country where SIEMENS has its seat or in countries where SIEMENS have put SIEMENS MAGNETOM SCANNERS on the market. The Receiver acknowledges that SIEMENS and/or its AFFILIATES is not responsible for an infringement of any such intellectual property rights to the extent it is due to: (i) the Receiver’s failure to use the most current version of or a defect correction or patch supplied by SIEMENS; (ii) the combination, operation, or use of the INTERFACE in conjunction with any non-SIEMENS information or with any third-party software, equipment, materials, or products other than as contemplated by the INFORMATION; or (iii) an adjustment or configuration of the INTERFACE not made by SIEMENS (or on its behalf). Notwithstanding any deviating and/or additional obligations expressly set forth in the Agreement, SIEMENS will neither assume any additional obligation nor responsibility that Receiver’s use of the INTERFACE complies with applicable law.
Article 5 - Provision of DEVELOPER SERVICES, INFORMATION and the SDK
5.1 |
Service Standards |
During the term of the Agreement and subject to Article 5.2 to Article 5.4, SIEMENS will provide DEVELOPER SERVICES as a service in conformance with the features and functionalities as described in the Agreement and the INFORMATION. SIEMENS will provide the Receiver with information and make the SDK available in a manner suitable for adapting the Receiver’s PRODUCTS to the INTERFACE.
CONFIDENTIAL
10 / 26
This service will include providing:
| ● | Technical descriptions |
| ● | SDK including |
| o | INTERFACE Simulator |
| o | Test Client for the INTERFACE |
| ● | Library of examples to demonstrate programming and/ or usage of certain functionalities |
| ● | Introductory webinar support/ conference call (up to one hour) |
| ● | Email support to answer basic questions |
| ● | Up to two follow-up conference calls to discuss questions (up to one hour each) |
| ● | One introduction call for each regular update (up to one hour) |
The service does not include code check or any development work, neither software nor hardware related.
5.2 |
Limitations |
Except as set forth in the Agreement:
(a)SIEMENS assumes no obligations to the Receiver in connection with, and any statements about the INTERFACE, the DEVELOPER SERVICES, the SDK and the INFORMATION. It does constitute merely technical information and not obligations of SIEMENS.
(b)None of the PARTIES’ obligations under the Agreement shall be deemed to constitute a guaranteed quality (zugesicherte Eigenschaft) or other guarantee (Garantie); and
(c)Each of the PARTIES disclaims any strict liability (verschuldensunabhängige Haftung) for defects and non-conformance already existing when the Agreement was concluded.
5.3 |
Changes to DEVELOPER SERVICES |
Unless expressly agreed otherwise, SIEMENS provides the DEVELOPER SER- VICES and the SDK as standard services and enables the Receiver to use the agreed DEVELOPER SERVICES and the SDK made generally available by SIEMENS to its other receivers. SIEMENS will update and further develop the technology, features and functionalities of the DEVELOPER SERVICES and the SDK and is under no obligation to maintain prior versions thereof. Upon the provision of a modified or new version of the DEVELOPER SERVICES and the SDK or any other content, the Receiver is no longer entitled to use previous versions.
CONFIDENTIAL
11 / 26
Should material changes to the DEVELOPER SERVICES or the SDK be implemented which have an impact on the Receivers use or should agreed DEVELOPER SERVICES or SDK functionalities be restricted or disabled, SIEMENS will notify the Receiver at least one hundred eighty (180) days before the changes become effective. Such notice period may be shortened only to the extent that, based on SIEMENS reasonable judgment, SIEMENS believes that changes are necessary in order to avoid any: (i) threat to the security or functionality of the INTERFACE or the DEVELOPER SERVICES or the SDK; (ii) ad- verse impact on the Receiver, SIEMENS, the Receiver’s or SIEMENS’ Affiliates, END USERS, or any third party, including, without limitation, any risk of personal injury; and/or (iii) subject the Receiver, SIEMENS, the Receiver’s or SIEMENS’ Affiliates, END USERS, or any third party to material liability for personal injury or IP infringement; in each case, so long as SIEMENS takes commercially reasonable efforts to maintain the compatibility of the PRODUCT with the SIEMENS MAGNETOM SCAN- NER. To the extent the Receiver is, due to such changes, materially deprived of the benefits of the DEVELOPER SERVICES or SDK, the Receiver is entitled to terminate the Agreement in writing with effect upon effectiveness of the change at the earliest and Receiver will be entitled to a refund of all amounts paid by Receiver with regards to the affected AUTHORIZATION KEYs to SIEMENS over the period of incompatability.
5.4 |
Discontinuation of Services |
SIEMENS may discontinue the provision of DEVELOPER SERVICES or the SDK in relation to a PRODUCT for use by an END USER at any time if the respective AUTHORIZATION KEY or LICENSE KEY has been revoked by SIEMENS according to Article 11 - Suspension of the AUTHORIZATION KEY and LICENSE KEY or the Agreement has been terminated according to Article16.2.
5.5 |
Place of Performance |
SIEMENS provides the Receiver access to the DEVELOPER SERVICES and the SDK to be provided over the internet at the WAN exit of the data center used by SIEMENS. Unless otherwise agreed, SIEMENS will use a data center in Germany to provide the DEVELOPER SERVICES and the SDK. SIEMENS reserves the right to change the data center location and use additional data centers at any time and will inform the Receiver of this in due time, provided that any such data centers will be within the European Union. SIEMENS will ensure that AUTHORIZATION KEYS are generated, transferred, and/or revoked from the SIEMENS Headquarter only.
5.6 |
Freedom from Defects in Title |
CONFIDENTIAL
12 / 26
SIEMENS represents, warrants and covenants that during the term of this Agreement, the Receiver’s use of the DEVELOPER SERVICES and SDK as permitted under the Agreement does not infringe upon a patent, copyright, trade secret, or other intellectual property right in or to such DEVELOPER SERVICES and SDK in the country where SIEMENS has its seat or in countries where SIEMENS have put SIEMENS MAGNETOM SCANNERS on the market. The Receiver acknowledges that SIEMENS and/or its AFFILIATES is not responsible for an infringement of any such intellectual property rights to the extent it is due to: (i) the Receiver’s failure to use the most cur- rent version of or a defect correction or patch supplied by SIEMENS for DEVELOPER SERVICES or the SDK; (ii) the combination, operation, or use of the DEVELOPER SERVICES or the SDK in conjunction with any non-SIEMENS information or with any third-party software, equipment, materials, or products other than as contemplated by the INFORMATION; or (iii) an adjustment or configuration of the DEVELOPER SER- VICES or SDK not made by SIEMENS (or on its behalf). Notwithstanding any deviating and/or additional obligations expressly set forth in the Agreement, SIEMENS will neither assume any additional obligation nor responsibility that Receiver’s use of the DEVELOPER SERVICES or the SDK comply with applicable law.
Article 6 - Pricing
6.1 |
As consideration that SIEMENS fulfills the described obligations and provides the described support the PARTIES agree upon a one-time fixed license fee of $100,000 USD to be paid to SIEMENS. |
6.2 |
SIEMENS shall be entitled to invoice the Receiver the one-time fixed license fee ten (10) days after the effective date. |
6.3 |
As consideration for the licenses granted under this Agreement the PARTIES agree upon a yearly license fee calculated based on the number of SIEMENS MAGNETOM SCANNERS being used for patient treatment: |
| ● | [pricing information redacted] USD per scanner per year for the first 10 connected SIEMENS MAGNETOM SCANNERS (Scanner 1 – 10) |
| ● | [pricing information redacted] USD per scanner per year for additional 20 connected SIEMENS MAGNETOM SCANNERS (Scanner 11 – 30) |
| ● | [pricing information redacted] USD per scanner per year for additional 70 connected SIEMENS MAGNETOM SCANNERS (Scanner 31 – 100) |
| ● | [pricing information redacted] USD per scanner for additional SIEMENS MAGNETOM SCANNERS exceeding 100. |
The yearly license fee shall be prorated based on the number of days that elapsed during the year during which the SIEMENS MAGNETOM SCANNERS are in use during the applicable year.
CONFIDENTIAL
13 / 26
There shall be no yearly license fees due in respect of SIEMENS MAGNETOM SCANNERS which are already in use as of the Effective Date until the beginning of the second year of this Agreement.
Any SIEMENS MAGNETOM SCANNERS which are in any year used solely for the purpose of pre-clinical or clinical research, whereas the Receiver is not getting any revenue from the END USER, will not be subject to any license fees. Receiver will advise SIEMENS of the specific SIEMENS MAGNETOM SCANNERS being used solely for pre-clinical or clinical research and, upon request, Receiver will provide an annual attestation as to the number and location of any such SIEMENS MAGNETOM SCANNERS.
Article 7 - Limitations: Permits, Regulatory Approvals and Standards
7.1 |
The Receiver acknowledges and agrees that: |
7.1.1 |
Operation of SIEMENS MAGNETOM SCANNERS demands observance of the applicable statutory provisions (e.g., in the EU, Directive 93/42/EEC on medical devices (“MDD”) and Regulation (EU) 2017/745 on medical devices (“MDR”), to become fully applicable on 26 May 2020) or any future revisions / successor requirements and is limited to particular indicated uses. |
7.1.2 |
Any changes to SIEMENS MAGNETOM SCANNERS by the Receiver, its AFFILIATES or OTHERS are not covered by CE labeling or regulatory approval or other applicable statutory provisions and can be relevant for patient safety. |
7.1.3 |
As part of the INFORMATION, SIEMENS shall only provide the documentation outlined in Annex 1 and does not foresee providing customized documentation, in particular not any part of the END USER documentation for the PRODUCTS and the combination of the PRODUCTS to the SIEMENS MAGNETOM SCANNER. Upon Receiver’s request, SIEMENS shall however provide reasonable assistance and information needed for testing and documenting such combination as related to the INTERFACE only and not for MR compatability in general. However, more generally Siemens will aid reasonable requests to support the attainment of data for validation runs, compatibility testing and other such assistance; however the determination of reasonable will be at Siemens discreation. |
7.1.4 |
SIEMENS does not assume any responsibility for the proper or safe functioning of the Receiver’s PRODUCTS and/or its combination with SIEMENS MAGNETOM SCAN- NERS. The Receiver will remain fully responsible for their PRODUCTS including the combination with the SIEMENS MAGNETOM SCANNER, and the manufacture, sale, marketing and service of the PRODUCTS. SIEMENS however endeavors to provide complete and accurate INFORMATION. |
CONFIDENTIAL
14 / 26
7.1.5 |
SIEMENS is not responsible for an infringement of any third party intellectual property rights due to: (i) The Receiver’s failure to use the most current version of or a defect correction or patch supplied by SIEMENS for the DEVELOPER SERVICES, the SDK or the INTERFACE; (ii) the combination, operation, or use of the DEVELOPER SER- VICES; the SDK or the INTERFACE in conjunction with any Receiver content or with any third-party software, equipment, materials, or products; or (iii) an adjustment or configuration of the DEVELOPER SERVICES; the SDK or the INTERFACE not made by, or on behalf of, SIEMENS or its AFFILIATES (or as contemplated by Section 7.1.7); provided always that the DEVELOPER SERVICES, SDK or the INTERFACE as sup- plied by SIEMENS are not the root cause for such infringement. Notwithstanding any deviating and/or additional obligations expressly set forth in the Agreement, SIEMENS will neither assume any additional obligation nor responsibility that the DEVELOPER SERVICES, the SDK or the INTERFACE and/or the Receiver’s use of the DEVELOPER SERVICES, the SDK or the INTERFACE comply with applicable law. |
7.1.6 |
This Agreement does not cover service related issues for either party at the Receiver’s site or at the END USER’s site and SIEMENS is not responsible for any service or support related to the Receiver’s PRODUCTS and the combination of the PRODUCTS to the SIEMENS MAGNETOM SCANNER unless otherwise agreed upon in writing. |
7.1.7 |
SIEMENS may engage any of its AFFILIATES and any other business partners for and in connection with the provision of DEVELOPER SERVICES, the AUTHORIZATION KEY, INTERFACE, billing or payment. The Receiver agrees to provide all reasonable cooperation required by SIEMENS should it become necessary or desirable, in SIEMENS’ sole discretion, for SIEMENS to use a new or different service providers. SIEMENS will be responsible for any of its obligations hereunder which are performed by its AFFILIATES, business partners and other service providers. |
7.1.8 |
The Receiver may, without accounting to SIEMENS, use and modify information and documentation in order to generate its END USER documentation of the PRODUCTS and the combination of the PRODUCTS to the SIEMENS MAGNETOM SCANNER, provided this is done: |
| ● | at the Receiver’s own cost, responsibility and risk, |
| ● | ensuring that the documentation conforms to the regulations applicable in the target countries and continuously updating it to the newest revision level, in particular as such information and documentation provided by SIEMENS may be country or region-specific and subject to change, |
| ● | without in any way portraying SIEMENS as responsible for the content of the END USER documentation, and clearly identifying the Receiver as responsible for marketing, sale, market-observation and service, subject to customary disclaimers and limitation on liability. |
7.2 |
The Receiver agrees to: |
CONFIDENTIAL
15 / 26
7.2.1 |
Generate and provide all END USER documentation. |
7.2.2 |
Complete risk assessments, risk mitigation, integration and system testing, compatibility testing, etc. for their PRODUCTS and comply with all applicable statutory provisions and regulations (e.g. those stipulated by the FDA or the European Commission or Japanese equivalent regulatory bodies) of the target country in which the combined product will be operated before releasing the PRODUCT into the market. Upon SIEMENS discretion which will not be unreasonably withheld, SIEMENS will use commercially rea- sonable efforts to provide standard documentation and reasonable support in connection with such activities upon request. |
7.2.3 |
Ensure that the Receiver’s END-USER’s are provided with all reasonably relevant instructions, warnings and, if required, training for the Receiver’s PRODUCTS and its combination with the SIEMENS MAGNETOM SCANNERS, particularly if these are safety-relevant. |
7.2.4 |
The Receiver is responsible for post market surveillance (as required by applicable laws and regulations where Receiver’s PRODUCTS are approved and in use) of their PRODUCTS and the combination of their PRODUCTS with SIEMENS MAGENTOM SCANNERS. |
Furthermore, the Receiver has the responsibility for the proper handling and, in case of adverse events, reporting to regulatory bodies customer complaints related to the combination of their PRODUCTS to the SIEMENS MAGNETOM SCANNERS (as re- quired by applicable laws and regulations where Receiver’s PRODUCTS are approved and in use).
The Receiver has the obligation to inform SIEMENS without undue delay about material adverse events in relation to the combination of their PRODUCTS and the SIEMENS MAGNETOM SCANNER to the best of the Receiver’s knowledge.
The Receiver has the responsibility to perform field updates and recalls, if necessary conduct reporting to reduce a risk to health and bring back the combination into specification.
SIEMENS will not take any responsibility related to post market surveillance / vigilance of the combination of the Receiver’s PRODUCTS with the SIEMENS MAGNETOM SCANNERS other than as set out in Section 4.
7.3 |
The Receiver shall indemnify, defend and hold harmless SIEMENS and its AFFILIATES from any and all proceedings, costs, expenses, damages, fees, penalties, and losses (including reasonable attorney fees), in each case arising from a third party claim, that result from: |
CONFIDENTIAL
16 / 26
7.3.1 |
A culpable breach of an obligation under this Agreement by the Receiver, Receiver’s AFFILIATES and their respective employees and agents; and |
7.3.2 |
failures of the SIEMENS MAGNETOM SCANNERS to function in accordance with their INFORMATION to the extent caused by the combination of the Receiver’s PRODUCTS with SIEMENS MAGNETOM SCANNERS. |
7.4 |
SIEMENS shall indemnify, defend and hold harmless Receiver and its AFFILIATES from any and all proceedings, costs, expenses, damages, fees, penalties, and losses (including reasonable attorney fees), in each case arising from a third party claim, that result from: |
7.4.1 |
A non-fulfillment, wrongful act or omission or a culpable breach of an obligation under this Agreement by SIEMENS, SIEMENS AFFILIATES and their respective employees and agents; and |
7.4.2 |
failures of the SIEMENS MAGNETOM SCANNERS, the INTERFACE, or the SDK to function in accordance with their INFORMATION to the extent they are not caused by the combination of the Receiver’s PRODUCTS with SIEMENS MAGNETOM SCANNERS, the INTERFACE, or the SDK; and |
7.4.3 |
a claim that the SIEMENS MAGNETOM SCANNERS, the INTERFACE, or the SDK infringe or misappropriate an OTHER’s IP other than where such infringement or misappropriation would not have occurred but for the combination of the SIEMENS MAGNETOM SCANNERS, the INTERFACE, or the SDK with the PRODUCT. |
Article 8 - Billing
The services and responsibilities described under this Agreement make SIEMENS eligible to receive a yearly license fee calculated as outlined in Article 6.3. The invoices will refer to an applicable timeframe. Receiver will pay all properly invoiced amounts within 30 days of its receipt of the applicable invoice.
The license fee will be paid in US currency to SIEMENS or its respective AFFILIATES. Each PARTY will ensure that its AFFILIATES adopt and comply with the provisions of this Agreement applicable to such transactions to the extent allowed under local law.
Within thirty (30) business days after the end of each calendar quarter, SIEMENS will invoice the Receiver the license fee for the applicable quarter as defined in Article 6 - Pricing based on all connected SIEMENS MAGNETOM SCANNERS and based on the analysis of the received customer data; the number of issued AUTHORIZATION KEYS and the resulting license fee and prorated for the applicable portion of the quarter.
If the Receiver is required by law to make any deduction or to withhold from any sum payable directly to SIEMENS or any of its AFFILIATES hereunder, the Receiver shall promptly effect payment thereof to the applicable tax authorities, and shall also promptly provide SIEMENS or any of its AFFILIATES with official tax receipts or other evidence sufficient to establish that the taxes have been paid and to enable SIEMENS or any of its AFFILIATES to support a claim for income tax credits for such payments.
CONFIDENTIAL
17 / 26
Failure to provide official tax receipts or other evidence of payment shall result in the Receiver paying directly to SIEMENS or any of its AFFILIATES such amounts deducted or withheld from the original payment. The Receiver shall also assist SIEMENS or any of its AFFILIATES in minimizing the withholding rate available under an applicable tax treaty, or otherwise, by supplying SIEMENS or any of its AFFILIATES with any appropriate documentation upon reasonable request.
Article 9 - Marketing
SIEMENS may, but is not obligated to, advertise the PRODUCTS offered by the Receiver. For this purpose, the Receiver grants SIEMENS a non-exclusive, non-transferable, sub-licensable, and royalty-free worldwide license to use all trademarks or trade names of the Receiver’s PRODUCTS.
RECEIVER may, but is not obligated to, advertise the combination of SIEMENS MAGNETOM SCANNERS and the Receiver’s PRODUCTS.
All such advertising materials created by either PARTY using any trademark or trade names of the other PARTY will be subject to prior approval, not to be unreasonably withheld or delayed.
The PARTIES will discuss in good faith the possibilities for a joint marketing collaboration be- tween the PARTIES, aiming towards each of the PARTIES being able to market a joint offering of the PRODUCTS together with SIEMENS MAGNETOM SCANNERS. Any such expanded collaboration shall be agreed upon separately between the PARTIES.
Article 10 - Monitoring of Usage Data
SIEMENS may and will use reasonable efforts to monitor the usage of the INTERFACE by END USERS, e.g., the number of users, for SIEMENS’ internal business purposes, in particular: (i) for security and availability reasons; (ii) to the extent required to ensure compliance of the END USER with the applicable END USER agreement and of the Receiver with this Agreement; and (iii) to detect, prevent, and suspend any use of the INTERFACE exceeding the permitted use under the END USER agreement as well as this Agreement, to charge for such excess use, and otherwise as necessary for payment and billing related tasks.
Article 11 - Suspension of the AUTHORIZATION KEY and LICENSE KEY
SIEMENS is entitled to suspend the AUTHORIZATION KEY for the INTERFACE issued to the Receiver at any time, resulting in the END USER no longer being able to use the Receiver’s PRODUCTS, if in SIEMENS’ reasonable judgment there is a risk that the use of the Receiver’s PRODUCT by the END USER will: (i) constitute a threat to the security or functionality of the END USER’S systems;(ii) adversely impact the END USER, the Receiver, SIEMENS, the Receiver’s or SIEMENS’ AFFILIATES, or any third party, including, without limitation, any risk of personal injury; [or (iii) subject the END USER, the Receiver, SIEMENS, the Receiver’s or SIEMENS’ AFFILIATES, or any third party to liability for personal injury or IP infringement].
CONFIDENTIAL
18 / 26
Furthermore, SIEMENS is entitled to suspend the LICENSE KEY if such revocation is required by law, a court decision, or a request from a governmental body; and (iii) SIEMENS is entitled to revoke the END USER LICENSE KEY for the relevant END USER if, in each case for security or compliance reasons or requests from a governmental body, its access to the INTER- FACE has been suspended or the agreement between SIEMENS and the END USER has been terminated by SIEMENS or the END USER. SIEMENS will, whenever reasonably possible, consult with Receiver in advance of the revocation of a LICENSE KEY. SIEMENS will inform the Receiver about any revocation of a LICENSE KEY as soon as possible. SIEMENS will reinstate any suspended AUTHORIZATION KEY or LICENSE KEY once the circum- stances giving rise to such suspension have been remedied.
Article 12 - Notification Page
SIEMENS is entitled to notify the Receiver, END USERS, and authorities in a suitable form at any time, if: (i) in SIEMENS’ reasonable judgment there is a risk that the use of the Receiver’s PRODUCTS by the END USER will: (a) threaten the security or functionality of the END USER’s systems; (b) adversely impact the END USER or any third party, including, without limitation, any risk of personal injury; or (c) subject the END USER or any third party to liability for personal injury or IP infringement; or (ii) such notification is required by law, a court decision, or a request from a governmental body; provided that SIEMENS will notify Receiver prior to providing any such notice to END USERS or authorities.
The Receiver acknowledges that a revocation of the AUTHORIZATION KEY or the LICENSE KEY pursuant to and in compliance with the conditions of Article 11 - or a notification pursuant to and in compliance with this Article by SIEMENS does not lead to any responsibility of SIEMENS.
Article 13 - Support
Where END USERS contact SIEMENS for support queries, SIEMENS will: (i) process queries concerning the SIEMENS MAGNETOM SCANNERS and provide all support with respect to the SIEMENS MAGNETOM SCANNERS; and (ii) forward queries concerning the Receiver’s PRODUCT to the Receiver within a reasonable period of time and the Receiver (and not SIEMENS) will have responsibility for providing the support. Upon receipt, the Receiver will for- ward support queries from END USERS concerning the SIEMENS MAGNETOM SCANNER to SIEMENS within a reasonable period of time. Each PARTY may notify the other PARTY of changes to its point of contact at least two (2) weeks in advance, e-mail being sufficient.
CONFIDENTIAL
19 / 26
Article 14 - Liability
14.1 |
The PARTIES agree that other than as set out in this Agreement: any information is made available “as is” and no warranties are given or liabilities of any kind are assumed with respect to the quality of such information, including, but not limited, to its fitness for the purpose, non-infringement of third-party rights, its correctness or completeness. Furthermore, none of the Parties’ obligations under this Agreement shall be deemed to constitute a guaranteed quality or other guarantee. In addition, other than as expressly set out in this agreement, each of the Parties disclaims any strict liability for defects and non-conformance already existing when this contract was concluded. |
14.2 |
This clause 14 sets out the entire financial liability of each of the PARTIES (including any liability for the acts or omissions of its AFFILIATES, employees, agents, consult- ants and subcontractors) under this Agreement, including in respect of any breach and any representation, statement or tortious act or omission (including negligence), indemnity, warranty, or strict liability arising under or in connection with this Agreement. |
14.3 |
Subject to clause 14.4 the liability of each PARTY under this Agreement shall be limited to direct losses and only to an amount of EUR 250,000- for each single dam- age event and subject to a maximum of EUR 1,000,000- for all such events. Claims for other indirect losses, including but not limited to claims for business stoppage, indirect lost profit, loss of information or data as well as consequential, special or incidental losses shall be excluded regardless of their legal grounds. |
14.4 |
This disclaimer of liability shall not apply in cases involving intent or gross negligence, or where liability is mandatory by law. |
14.5 |
The Receiver will, in its sole responsibility, decide on the use of the INTERFACE, the SDK, the DEVELOPER SERVICES, the INFORMATION or any other material pro- vided through SIEMENS. SIEMENS does not assume any liability for defects of or damages to the END USER’S systems resulting from the use of the INTERFACE, the SDK, the DEVELOPER SERVICES, the INFORMATION or any other material pro- vided through SIEMENS and hereby waives and excludes any and all liability arising out of or in connection with the use of the INTERFACE, the SDK, the DEVELOPER SERVICES, the INFORMATION or any other material provided through SIEMENS beyond the intended use whether based on contract, tort, negligence, under an indemnity, warranty, strict liability or otherwise except to the extent such defect or dam- age is caused or contributed to by a negligent act or negligent omission of SIEMENS, or a breach of this Agreement. |
CONFIDENTIAL
20 / 26
14.6 |
Subject to of the INTERFACE, the SDK, the DEVELOPER SERVICES, the INFORMATION or any other material provided through SIEMENS or its AFFILIATES is the responsibility of the Receiver. |
Article 15 - Confidentiality
15.1 |
“CONFIDENTIAL INFORMATION” shall mean any information and data, including without limitation, any kind of business, commercial or technical information and data disclosed by the Receiver to SIEMENS or SIEMENS to the Receiver in connection with the execution or performance of this Agreement, irrespective of the medium in which such information or data is embedded, which is: |
| ● | when disclosed in tangible form – marked “Confidential” by the disclosing PARTY |
| ● | or when disclosed orally or visually – identified as such prior to disclosure and summarized in writing by the disclosing PARTY and said summary is given to the receiving PARTY within thirty (30) days after such disclosure marked “Confidential”. In case of disagreement regarding orally or visually disclosed information, the receiving PARTY must present its objections to the summary in writing within thirty (30) days of receipt |
CONFIDENTIAL INFORMATION shall also include any copies or abstracts made thereof as well as any apparatus, data, modules, samples, prototypes or parts thereof.
For the avoidance of doubt, the pricing information described in Article 6.1 and Article 6.3, the library of examples, the SDK, the INTERFACE description shall deemed to be SIEMENS CONFIDENTIAL INFORMATION irrespective of the fact if they are marked or not. All information about the PRODUCTS or Receiver’s business practices which is not made generally available to the public and the pricing information de- scribed in Article 6.1 and Article 6.3 shall be deemed Receiver CONFIDENTIAL IN- FORMATION.
15.2 |
The receiving PARTY shall maintain CONFIDENTIAL INFORMATION received from the disclosing PARTY in confidence and will use such CONFIDENTIAL INFORMATION solely for the purposes of this Agreement, and shall not distribute or disclose it, provided, however, that the receiving PARTY may disclose such information to its officers, AFFILIATES, and those of its employees and subcontractors who need to know it for the purposes of this Agreement. Each PARTY shall impose on its officers, AFFILIATES, and its employees and subcontractors confidentiality obligations no less stringent than its own confidentiality obligations under this Agreement, and the receiving PARTY will be responsible for any violation of the confidentiality obligations under this Agreement by any of its officers, AFFILIATES, employees or subcontractors. |
15.3 |
The obligations of Article 15.2 shall remain in force for a period of five (5) years after the termination of this Agreement. CONFIDENTIAL INFORMATION (as defined in |
CONFIDENTIAL
21 / 26
the Original Agreement) disclosed by either PARTY under the Original Agreement shall be deemed CONFIDENTIAL INFORMATION disclosed under this Agreement and shall be subject to the provisions of this Article 15.
15.4 |
The obligations of Article 15.2 shall not apply to information which is or becomes generally known to the public or which has been obtained independently as evidenced by a PARTY’S business records or acquired legitimately from OTHERS who are to the knowledge of the receiving PARTY free to lawfully disclose the information. |
15.5 |
The provisions of Article 15.2 hereof shall apply to copies of electronically exchanged CONFIDENTIAL INFORMATION made as a matter of routine information technology backup and to CONFIDENTIAL INFORMATION or copies thereof which must be stored by the receiving PARTY or its advisers according to provisions of mandatory law or as required by regulatory bodies such as the FDA, and such CONFIDENTIAL INFORMATION or copies thereof shall be subject to an indefinite confidentiality obligation according to the terms and conditions set forth herein. |
15.6 |
Save as set out in Article 15.5 above, within ninety (90) calendar days after termination of this Agreement, the receiving PARTY shall destroy or, as may be requested by the disclosing PARTY, return all CONFIDENTIAL INFORMATION received and stored electronically and/or on record-bearing media as well as any copies thereof. The receiving PARTY shall confirm in writing such destruction to the disclosing PARTY. Excluded from the obligations set out in this Article 15.6 is information that the Receiver is required to retain in his technical files relating to the PRODUCTS or information that: (a) is required to enforce or comply with the terms of this Agreement or Receiver’s agreements with End Users; or (b) is stored in accordance with a Party’s back-up and disaster recovery systems. |
Article 16 - Agreement Term, Notification and CHANGE-OF-CONTROL
16.1 |
This Agreement shall come into force when signed by both PARTIES and shall have a term for sixty (60) months. Unless earlier terminated, the Agreement shall be ex- tended for successive terms of one (1) year each unless written notice to terminate is given by either PARTY at least three (3) months before the end of the respective contract term. |
16.2 |
Each PARTY shall be entitled to terminate this Agreement prematurely by means of written notice, if the other PARTY breaches a material obligation and fails to rectify the aforesaid breach within ninety (90) calendar days of the non-breaching PARTY’s notice. |
16.3 |
If there is a CHANGE-OF-CONTROL to a COMPETITOR of SIEMENS: |
| ● | the Receiver shall inform SIEMENS in writing within fifteen (15) calendar days, but under no circumstance later than any public announcement by the Receiver, |
CONFIDENTIAL
22 / 26
| ● | The PARTIES shall discuss in good faith within thirty (30) calendar days after SIEMENS has been informed in writing by the Receiver, |
| ● | In particular, it shall be discussed how such CHANGE OF CONTROL would impact the relationship contemplated by this Agreement, including whether the Receiver or such COMPETITOR will terminate this Agreement after the closing of such CHANGE OF CONTROL transaction, and |
| ● | SIEMENS shall be entitled to terminate with immediate effect this Agreement within sixty (60) calendar days after SIEMENS has been informed in writing by the Receiver of such CHANGE OF CONTROL involving a COMPETITOR of SIEMENS, provided that SIEMENS, acting reasonably, shall only terminate this Agreement if such CHANGE OF CONTROL transaction involving a COMPETITOR of SIEMENS will have a detrimental impact on the PARTIES collaboration hereunder relating to the PROD- UCTS and SIEMENS MAGNETOM SCANNERS. |
16.4 |
The provisions in Article 7 - , Article 10 - Article 11 - Article 12 - Article 14 - this Article 16.4 and Article 19 - shall survive the expiration or termination of this Agreement. With regards to the PRODUCTS which have already been connected to SIEMENS MAGNETOM SCANNERS before expiration or termination of this agreement the li- censes granted for the AUTHORIZATION KEY shall be unaffected as well as the corresponding obligation to pay the license fee as set forth in Article 2, Article 6 and Article 8 for all granted AUTHORIZATION KEYs., unless and until such time as Re- ceiver specifies which individual or all such AUTHORIZATION KEYS are no longer in use. Then the Receiver only has the obligation to pay the license fee for all the AUTHORIZATION KEYs still in use. |
Article 17 - Force Majeure
17.1 |
A PARTY shall not be in breach of this Agreement, nor liable for any failure or delay in the performance of any of its obligations under this Agreement as a result of force majeure event provided that: |
17.1.1 |
it promptly notifies the other PARTY in writing of the nature and extent of the force majeure event causing its failure or delay in performance; and |
17.1.2 |
it has used reasonable endeavors to mitigate the effect of the force majeure event, to carry out its obligations under this Agreement in any way that is reasonably practicable and to resume the performance of its obligations as soon as reasonably possible. |
17.2 |
‘Force majeure event’ in the sense of this Agreement shall be one of the following events which is outside the control of a PARTY and its suppliers and contractors and prevents that PARTY from meeting its contractual obligations: |
17.2.1 |
acts of God, fires, floods, earthquakes or other natural disasters; |
CONFIDENTIAL
23 / 26
17.2.2 |
war, embargo, terrorist attack, riots; |
17.2.3 |
explosion or building collapse; |
17.2.4 |
failure of plant machinery, interruption or failure of utility service, including but not limited to electric power, gas or water. |
17.3 |
If either PARTY is prevented from meeting its obligations under this Agreement for a continuous period of more than six (6) months as a result of a ‘force majeure event’, the other PARTY shall be entitled, at its sole discretion, to terminate the Agreement with one (1) month’s written notice. |
Article 18 - Miscellaneous
18.1 |
Neither PARTY shall be entitled, without the prior written consent of the other, to transfer or assign this Agreement or any rights and obligations arising from it to OTHERS, except a transfer or assignment to an AFFILIATE or a purchaser of substantially all of the assets of a PARTY, provided that the transferring or assigning PARTY shall remain responsible for the fulfilment of the obligations it originally incurred under the Agreement. Consent hereto shall not be unreasonably withheld. |
18.2 |
SIEMENS’ obligation to fulfill this Agreement is subject to the proviso that the fulfillment is not prevented by any impediments arising out of national and international foreign trade and customs requirements or any embargos or other sanctions. |
18.3 |
The PARTIES shall comply with all applicable export control, customs and foreign trade regulations (“Foreign Trade Regulations”) and shall obtain all necessary export licenses, if any. |
18.4 |
Any amendments as well as supplements to this Agreement must be in writing and signed by the PARTY to be bound in order to be effective. A waiver of form shall be effective only if agreed upon in writing and signed by the PARTY to be bound. |
18.5 |
If this Agreement requires a notice or a document to be „in writing“ or „in written form“, such notice or document shall be duly signed by the sender and the signed notice or document shall be delivered, sent or transmitted to the other PARTY in its original form or as a telefax copy or a PDF. For the avoidance of doubt, electronic communication shall not qualify as a written notice or document unless confirmed as received by the receiving PARTY. |
18.6 |
Any general terms and conditions of the PARTIES in addition to this Agreement shall be excluded. This shall particularly apply if there is any reference to such terms in |
CONFIDENTIAL
24 / 26
correspondence or other documentation of the PARTIES (e.g., printed, stamped and electronically reproduced).
18.7 |
If any provisions of this Agreement should be held to be illegal, invalid or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby. The PARTIES shall substitute the illegal, invalid, or unenforceable provision by a legal, valid or enforceable one, approximating as closely as possible the original commercial intent of the PARTIES. |
18.8 |
If provisions of this Agreement conflict with the Annexes or other agreements, the provisions of this Agreement shall have precedence. |
18.9 |
The Original Agreement and the PARTIES’ obligations thereunder are hereby terminated and replaced by this Agreement. [Article 7.2 of the Original Agreement shall continue to apply after the Effective Date of this Agreement; provided that Art. 8 of the Original Agreement shall not survive termination of the Original Agreement ac- cording to this section 18.9. |
Article 19 - Arbitration and Substantive Law
19.1 |
If a dispute arises in connection with this Agreement, the responsible representatives of the PARTIES shall attempt, in fair dealing and good faith, to settle such dispute. Upon request of a PARTY a senior management representative of each PARTY shall participate in the negotiations. Each PARTY shall be entitled to terminate these negotiations by written notification to the other PARTY at any time. |
19.2 |
The PARTIES shall attempt to agree on a procedure for Alternative Dispute Resolution (ADR) and the applicable procedural rules (including time limits) within fourteen days after a termination notice under Article 19.1 has been received by the other side. If the PARTIES fail to agree on such procedure within this time limit each PARTY shall be entitled to refer the dispute to arbitration pursuant to Article 19.3. |
19.3 |
All disputes arising in connection with this Agreement which are not resolved pursuant to Article 19.1 or an ADR procedure, including any question regarding the termination or any subsequent amendment of the Agreement, shall be finally settled in accordance with the Rules of Arbitration (“Rules”) of the International Chamber of Commerce (“ICC”). The seat of arbitration shall be Zurich Switzerland. The language to be used in the ADR and the arbitration proceeding shall be English. Any production of documents shall be limited to the documents on which each PARTY specifically relies in its submission(s). Consolidation of arbitrations pending under the Rules into a single arbitration shall only be possible if the PARTIES have agreed to consolidation. The unsuccessful PARTY shall bear the costs of the arbitral proceedings. How- ever, the arbitral tribunal may take into account the extent to which each PARTY has conducted the arbitration in an expeditious and cost-effective manner. |
CONFIDENTIAL
25 / 26
19.4 |
This Agreement shall be subject to the substantive law in force in Germany without reference to any of its conflict of law rules. |
CONFIDENTIAL
26 / 26
Place, Date: |
|
|
|
|
|
Mississauga, Ontario, February 11, 2019 |
|
|
|
|
|
“Aaron Davidson” |
|
“Arun Menawat” |
|
|
|
|
|
|
Name (Print): |
|
Name (Print): |
Aaron Davidson |
|
Arun Menawat |
|
|
|
Title: |
|
Title: |
Chief Financial Officer |
|
Chief Executive Officer |
SIEMENS Healthcare GmbH
Place, Date: |
|
|
|
|
|
Erlangen, Germany, January 23, 2019 |
|
|
|
|
|
“Dr. Robert Krieg” |
|
“Peter Horn” |
|
|
|
|
|
|
Name (Print): |
|
Name (Print): |
Dr. Robert Krieg |
|
Peter Horn |
|
|
|
Title: |
|
Title: |
VP MR Advanced Solutions & Therapy |
|
Senior Vice President Finance |
|
|
Magnetic Resonance |
CONFIDENTIAL
Annex 1
Specification of INFORMATION
Preamble
SIEMENS provides the INFORMATION to the Receiver for the sole purpose of interfacing the PRODUCTS to certain SIEMENS MAGNETOM SCANNERS and to deliver such adapted PRODUCTS to END USERS for interworking with SIEMENS MAGNETOM SCANNERS. The INFORMATION in scope are described in this Annex.
Interface description
Access-I developer guide with comprehensive documentation of the interface, intended for a developer to integrate against this interface. Includes descriptions of the setup, configuration, methods of the interface with examples.
Software Development Kit (SDK)
A test environment is provided to ease the development, integration and testing of remote clients with Access-i. It comprises an Access-I Simulator and a Test Client, which are designed for a Windows PC environment.
The Access-i Simulator enables the remote client implementation and testing without the need for physical access to an MR scanner. It mimics the Access-I related functionality of the MR host
The Access-i Test Client provides an example implementation of a remote client, and also serves as a reference to verify the functionality of the Access-i interface.
Source Code toolbox
As part of the SDK, some source code toolboxes are included to provide an example implementation of a remote client. These can be used in the development of the remote client to enable faster development SIEMENS provides the INFORMATION to the Receiver for the sole purpose of interfacing the PRODUCTS to certain SIEMENS MAGNETOM SCANNERS and to deliver such adapted PRODUCTS to END USERS for interworking with SIEMENS MAGNETOM SCANNERS.
Annex 2
Specification of PRODUCTS
Preamble
The PRODUCTS in scope are described in this Annex.
Product Name
TULSA-PRO
Sonalleve
Product Description
TULSA-PRO: MRI guided directional ultrasound ablation of soft tissue
Sonalleve: MRI guided high-intensity focused ultrasound of soft tissue, bone and nerves
Intended Use
TULSA-PRO: MRI guided directional ultrasound ablation of soft tissue
Sonalleve: MRI guided high-intensity focused ultrasound of soft tissue, bone and nerves
Regulatory Status and Regulatory Roadmap
TULSA-PRO: Approved in EU and soon to be in registration in the US
Sonalleve: Approved in the EU, and several Asian countries including China and soon to be in regi_stration in the US
MR-Compatibility Status
TULSA-PRO: Intention is to be compatible with all Siemens scanners upon which Access I can be operational
Sonalleve: Intention is to be compatible with all Siemens scanners upon which Access I can be operational Siemens Healthcare GmbH has created a risk analysis according to their risk management process fulfilling the requirements given by ISO14971:2012.
Annex 3
Agreement for risks exported in using Access-i
Preamble
Listed below are the residual risks related to the use of “Access-i” interface that cannot be further mitigated by Siemens Healthcare GmbH and which the Receiver accepts in using this interface functionality. It is the responsibility of the Receiver to ensure that the appropriate measures are in place to mitigate these risks in using this’ interface functionality.
Risks exported to the Receiver using the Access-i interface:
NO |
Function |
Error |
Cause |
Hazard |
Measure(s) |
|
|
|
|
|
|
1 |
Remote PC- control session active |
NUMARIS/4 product SW crash or instability, loss in performance. |
SW hang-up / Side effects I secondary effects on NUMARIS/4 product SW |
Delayed diagnosis |
It must be ensured, that the status of the MR system always corresponds to the required status (e.g. sequence is running) during safety relevant interventions |
2 |
No fast way to stop the MR measurement and/or patient table movement in an emergency situation. Visual and audio contact to the patient (window / video /Intercom system (cf. above)) not possible. |
The patient intercom system I video supervision remains at the MR AWP and is not available at the remote PC. |
Various! |
Patients must be monitored, always, and it needs to be ensured that MR measurement and patient table movement can be stopped in emergency situations. |
|
3 |
Connection between MR AWP and remote PC gets lost. |
Technical defect; Forced control take over at the MR AWP (e.g. by User); |
Burns up to 2nd degree; misdiagnosis; Jamming or injection/ infusion needles pulled out- minor or moderate injury |
A termination or a break of the connection between the MR AWP and the remote PC must be recognized. In this case the procedure (e.g. intervention) must be handled appropriately (e.g. stopped). |
|
4 |
Parameters are accidently changed on the MR AWP |
Access to MR System by MR AWP between two remote control sessions possible. |
Delayed diagnosis |
It must be ensured that the protocol parameters are not changed unintentionally before each procedure (e.g. intervention). Only Personnel that are trained must use the MR-Device and |
|
|
|
|
|
the remote PC. It is recommended to have at least two persons for the workflow. |
5 |
Remote PC- Untrained Personnel |
MR device not used correctly |
Untrained personnel are using the MRDevice (via remote control) |
Various! |
Only Personnel which are trained must use the MR-Device and the remote PC. It is recommended to have two persons for the workflow. |
6 |
Remote PC- Popup Issue Service |
Popup confirmed (automatically) by remote PC SW |
Malfunction of remote PC software or intended automated popup confirmation. |
Misdiagnosis |
The remote PC SW must consider all popup related information provided by MR AWP via the issue service for safe and proper use of the MR Device; e.g. automatic table movement. Popups can be handled automatically by SW, if the consequences are known and measures are implemented prevent resulting hazards. |
7 |
Remote PC- Debugging service |
Start of savelog generation shortly before a sequence is started |
Savelog generation can take up to 5 minutes and can lead to a longer reconstruction time |
Delayed diagnosis |
If update rate of data is not sufficient for the required application the procedure (e.g. intervention) must be handled appropriately (e.g. stopped). |
8 |
Remote PC- Image Service |
Image service and/or projection service not available |
Automatic receive of images via websocket functionality |
Delayed diagnosis |
If update rate of data is not sufficient for the required application the procedure (e.g. intervention) must be handled appropriately (e.g. stopped). |
Exhibit 10.9
[***] Certain information has been excluded from this exhibit because it is both not material and the type that the Registrant treats as private or confidential.
AMENDED AND RESTATED CREDIT AGREEMENT
between
PROFOUND MEDICAL INC.
as Borrower
AND
PROFOUND MEDICAL CORP.
2753079 ONTARIO INC.
PROFOUND MEDICAL (U.S.) INC. and
PROFOUND MEDICAL GMBH
as Guarantors
AND
CANADIAN IMPERIAL BANK OF COMMERCE
as Lender
dated as of March 3, 2025
GOODMANS LLP
TABLE OF CONTENTS
ARTICLE I. INTERPRETATION |
1 |
|
Section 1.01 |
Definitions |
1 |
Section 1.02 |
PPSA. |
22 |
Section 1.03 |
Headings. |
22 |
Section 1.04 |
References to Sections. |
22 |
Section 1.05 |
Currency. |
22 |
Section 1.06 |
Gender and Number. |
23 |
Section 1.07 |
Invalidity of Provisions. |
23 |
Section 1.08 |
Transition from GAAP to US GAAP. |
23 |
Section 1.09 |
Amendment or Waiver. |
23 |
Section 1.10 |
Non-Business Days. |
23 |
Section 1.11 |
Currency Equivalents |
23 |
Section 1.12 |
German Terms. |
24 |
ARTICLE II. CREDIT FACILITIES |
25 |
|
Section 2.01 |
The Commitments. |
25 |
Section 2.02 |
Reborrowing |
25 |
Section 2.03 |
Fees. |
25 |
Section 2.04 |
Payment; Computation. |
26 |
Section 2.05 |
Loan Account. |
26 |
Section 2.06 |
Termination or Reduction of the Revolving Credit Facility Commitment |
26 |
Section 2.07 |
Accordion Feature. |
27 |
ARTICLE III. PAYMENTS AND PREPAYMENTS |
27 |
|
Section 3.01 |
Repayment of Advances. |
27 |
Section 3.02 |
Interest. |
28 |
Section 3.03 |
[Intentionally Deleted] |
29 |
Section 3.04 |
Voluntary Prepayment |
29 |
Section 3.05 |
Mandatory Prepayments. |
30 |
Section 3.06 |
Application of Prepayments. |
30 |
Section 3.07 |
[Intentionally Deleted] |
30 |
Section 3.08 |
Payments Generally. |
30 |
Section 3.09 |
Change in Law and Increased Costs |
31 |
Section 3.10 |
Taxes |
32 |
Section 3.11 |
Illegality |
34 |
ARTICLE IV. SECURITY |
34 |
|
Section 4.01 |
Security Documents. |
34 |
Section 4.02 |
Control of Collateral; Control Agreements. |
35 |
Section 4.03 |
Guarantors |
35 |
Section 4.04 |
Real Estate |
36 |
- i -
Section 4.05 |
Delivery of Additional Documentation Required. |
36 |
Section 4.06 |
Right to Inspect. |
36 |
ARTICLE V. REPRESENTATIONS AND WARRANTIES |
37 |
|
Section 5.01 |
Incorporation and Status. |
37 |
Section 5.02 |
Corporate Power and Due Authorization. |
37 |
Section 5.03 |
Business of the Loan Parties. |
37 |
Section 5.04 |
No Contravention. |
37 |
Section 5.05 |
Not Insolvent. |
38 |
Section 5.06 |
Approvals and Consents. |
38 |
Section 5.07 |
Welfare and Pension Plans. |
38 |
Section 5.08 |
Changes Since Date of Financial Statements. |
38 |
Section 5.09 |
No Default Under Agreements. |
39 |
Section 5.10 |
Title to Assets. |
40 |
Section 5.11 |
Financial Matters. |
40 |
Section 5.12 |
No Material Adverse Change |
40 |
Section 5.13 |
Environmental Matters. |
40 |
Section 5.14 |
Assets in Good Condition. |
41 |
Section 5.15 |
Licenses and Agreements. |
41 |
Section 5.16 |
Tax Matters. |
41 |
Section 5.17 |
Insurance. |
41 |
Section 5.18 |
Intellectual Property. |
41 |
Section 5.19 |
Permits. |
42 |
Section 5.20 |
Regulatory Required Permits. |
42 |
Section 5.21 |
Compliance with Laws and Litigation. |
43 |
Section 5.22 |
Material Facts Disclosed. |
43 |
Section 5.23 |
No Rights to Acquire Assets. |
43 |
Section 5.24 |
No Rights to Provide Financial Advisory Services. |
43 |
Section 5.25 |
Chief Executive Office and Location of Assets. |
44 |
Section 5.26 |
Minute Books. |
44 |
Section 5.27 |
Use of Proceeds; Margin Stock. |
44 |
Section 5.28 |
Investment Company Act. |
44 |
Section 5.29 |
Bank Accounts. |
44 |
Section 5.30 |
Shares and Corporate Structure. |
45 |
Section 5.31 |
Liabilities. |
45 |
Section 5.32 |
Employee Matters. |
45 |
Section 5.33 |
Non-Arm’s Length Transactions |
46 |
Section 5.34 |
Description of Real Property |
46 |
Section 5.35 |
Sanctions |
46 |
Section 5.36 |
Anti-Terrorism and Anti-Money Laundering Laws |
47 |
ARTICLE VI. COVENANTS |
48 |
|
Section 6.01 |
Use of Proceeds. |
48 |
Section 6.02 |
Payment of Principal and Interest; Secured Obligations. |
48 |
Section 6.03 |
Lender Expenses. |
48 |
- ii -
Section 6.04 |
Compliance with Laws; Permits; Corporate Existence. |
48 |
Section 6.05 |
Delivery of Collateral and Perfection. |
48 |
Section 6.06 |
Collateral. |
48 |
Section 6.07 |
Operating Leases. |
48 |
Section 6.08 |
Insurance. |
49 |
Section 6.09 |
Transactions with Affiliates. |
49 |
Section 6.10 |
Material Agreement. |
49 |
Section 6.11 |
Reporting. |
49 |
Section 6.12 |
Healthcare Regulatory Matters. |
50 |
Section 6.13 |
Accounts |
51 |
Section 6.14 |
Sanctions |
51 |
Section 6.15 |
Anti-Terrorist Financing and Anti-Money Laundering Laws |
51 |
Section 6.16 |
Negative Covenants. |
52 |
Section 6.17 |
Maintenance of Records. |
53 |
Section 6.18 |
Notices. |
53 |
Section 6.19 |
Limitations on Modifications, Waivers, Extensions. |
54 |
Section 6.20 |
Financial Covenants |
54 |
Section 6.21 |
Intellectual Property. |
54 |
Section 6.22 |
Pension Plans |
55 |
Section 6.23 |
Material Subsidiaries |
55 |
Section 6.24 |
Centre of Main Interests |
56 |
Section 6.25 |
Limitation on Restrictive Agreements |
56 |
Section 6.26 |
Limitation on Hedge Arrangements |
56 |
ARTICLE VII. CONDITIONS |
56 |
|
Section 7.01 |
Conditions Precedent to Amended and Restated Credit Agreement. |
56 |
Section 7.02 |
Conditions Precedent to all Extensions of Credit. |
59 |
ARTICLE VIII. EVENTS OF DEFAULT |
59 |
|
Section 8.01 |
Events of Default. |
59 |
Section 8.02 |
Lender’s Remedies Upon Default. |
62 |
Section 8.03 |
Lender Protective Payments. |
63 |
Section 8.04 |
Remedies Cumulative. |
63 |
Section 8.05 |
Power of Attorney. |
63 |
Section 8.06 |
Notice of Event of Default. |
64 |
Section 8.07 |
Default under Other Encumbrances. |
64 |
Section 8.08 |
Judgment. |
64 |
Section 8.09 |
Application of Proceeds. |
64 |
Section 8.10 |
Limitation of Liability. |
64 |
Section 8.11 |
Borrower Liable |
65 |
Section 8.12 |
Termination of Lender’s Obligations |
65 |
ARTICLE IX. DETAILS REGARDING ADVANCES AND PAYMENTS |
65 |
|
Section 9.01 |
Drawdown Notices |
65 |
- iii -
Section 9.02 |
Minimum Amounts |
66 |
Section 9.03 |
Payments by the Borrower |
66 |
ARTICLE X. GENERAL |
66 |
|
Section 10.01 |
Releases. |
66 |
Section 10.02 |
Governing Law; Jurisdiction; Jury Trial Waiver and Judicial Reference. |
66 |
Section 10.03 |
Whole Agreement. |
67 |
Section 10.04 |
Time. |
67 |
Section 10.05 |
Notices. |
67 |
Section 10.06 |
Successors and Assigns. |
69 |
Section 10.07 |
Indemnification. |
69 |
Section 10.08 |
No Set-Off. |
69 |
Section 10.09 |
Permitted Encumbrance. |
69 |
Section 10.10 |
Press Releases. |
69 |
Section 10.11 |
Confidentiality. |
70 |
Section 10.12 |
Judgment Currency |
70 |
Section 10.13 |
Anti-Money Laundering Legislation |
71 |
Section 10.14 |
Counterparts. |
71 |
Section 10.15 |
Keepwell |
71 |
Section 10.16 |
Acknowledgement Regarding Any Supported QFCs |
72 |
Section 10.17 |
Amendment and Restatement; No Novation. |
73 |
- iv -
AMENDED AND RESTATED CREDIT AGREEMENT
This AMENDED AND RESTATED CREDIT AGREEMENT (as amended, restated, supplemented or otherwise modified from time to time, the “Agreement”) dated as of March 3, 2025, by and among Profound Medical Inc., an Ontario corporation, as borrower (“Borrower”), Profound Medical Corp., an Ontario corporation, as a guarantor (“Parent”), 2753079 Ontario Inc., an Ontario corporation, as a guarantor (“275 Ontario”), Profound Medical GmbH, a German limited liability company (Gesellschaft mit beschränkter Haftung), as a guarantor (“PM Germany”) Profound Medical (U.S.) Inc., a Delaware corporation, as a guarantor (“PM USA”), and Canadian Imperial Bank of Commerce, as lender (“Lender”).
WHEREAS the Borrower, the Guarantors and the Lender entered into a credit agreement dated as of November 3, 2022 (as amended by a first amending agreement dated as of September 26, 2023 and a second amending agreement dated as of May 3, 2024, collectively, the “Original Credit Agreement”)
WHEREAS the Borrower, the Guarantors and the Lender have agreed to amend and restate the Original Credit Agreement to, among other things, provide increased commitments on the terms set forth herein.
AND WHEREAS the Lender is willing to extend such credit on and subject to the terms and conditions hereof.
NOW THEREFORE in consideration of the covenants and agreements herein the parties hereto agree as follows:
ARTICLE I.
INTERPRETATION
Section 1.01Definitions
In this Agreement:
“275 Ontario” has the meaning set forth in the preamble to this Agreement.
“Accordion Request” has the meaning set forth in Section 2.07.
“Account Debtor” means a person obligated on an Account.
“Accounts” has the meaning given to such term in the PPSA.
“Acquisition” means, with respect to any Person, any purchase or other acquisition by such Person, regardless of how accomplished or effected (including any such purchase or other acquisition effected by way of amalgamation, merger, arrangement, business combination or other form of corporate reorganization or by way of purchase, lease or other acquisition arrangements), of (a) any other Person (including any purchase or acquisition of such number of the issued and outstanding securities of, or such portion of an Equity Interest in, such other Person so that such other Person becomes a Subsidiary of the purchaser or of any of its Affiliates) or of all or a material portion of the Property of any other Person, or (b) any division, business, operation or undertaking of any other Person or of all or a material portion of the Property of any division, business, operation or undertaking of any other Person or (c) any material real estate asset.
“Additional Documents” has the meaning set forth in Section 4.05.
“Advance” means an extension of credit by the Lender under this Agreement, including by way of US Base Rate Advances.
“Affiliate” means, with respect to any Person, another Person that directly, or indirectly through one or more intermediaries, Controls or is Controlled by or is under common Control with the Person specified.
“Agreement,” “hereto,” “herein,” “hereof,” “hereby,” “hereunder,” and any similar expressions refer to this Agreement and the schedules attached hereto and not to any particular article, section or other portion hereof, and include any and every instrument supplemental hereto or amending or replacing any part hereof.
“AML Legislation” has the meaning set forth in Section 6.15.
“Anti-Terrorist Financing and Anti-Money Laundering Laws” means all Applicable Law concerning or related to money laundering or financing terrorism and which are applicable to the Lender, any Loan Party or any Affiliate thereof, including the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada) and all similar laws, rules, regulations and other Applicable Laws in place in any foreign country, including the United States.
“Applicable Law” means, in respect of any Person, property, transaction, event or other matter, as applicable, all Laws relating or applicable to such Person, property, transaction, event or matter.
“Board” means a Person’s board of directors, board of managers, board of members or similar governing body, provided that, unless otherwise specified, “Board” shall refer to the applicable Loan Party’s Board.
“Borrower” has the meaning set forth in the preamble to this Agreement.
“Borrowing Base” means the lesser of (A) the Revolving Credit Facility Commitment and (B) the Borrowing Formula.
“Borrowing Base Certificate” means a certificate in the form of Exhibit C that is signed by a Responsible Officer of the Borrower and delivered to the Lender pursuant to this Agreement.
“Borrowing Formula” means four (4) multiplied by the most recent reported trailing 3 month Recurring Revenue less Priority Payables.
- 2 -
“Budget” means the consolidated and consolidating projections and budget, prepared annually on a monthly basis, for Parent and its Subsidiaries adopted and approved by Parent’s Board for the given period.
“Business Day” means a day other than a Saturday, Sunday or any other day on which banks located in the City of Toronto, Province of Ontario are not open for business.
“Canadian Dollars” and “CAD” mean lawful money of Canada.
“Canadian Guarantee” has the meaning set forth in Section 4.01(b).
“Canadian IP Security Agreements” has the meaning set forth in Section 4.01(h).
“Canadian Pension Plan” means any plan, program or arrangement that is a “registered pension plan” as that term is defined in the ITA or that is subject to the funding requirements of the Pension Benefits Act (Ontario) or applicable pension benefits legislation in any other Canadian federal or provincial jurisdiction that is established, registered, sponsored, contributed to or maintained by any Loan Party for the benefit of employees or former employees of such Loan Party, or any dependants or beneficiaries of such person, or under which any Loan Party has any actual or contingent liability, provided that a Canadian Pension Plan shall not include a Non-Canadian Pension Plan or a Statutory Plan.
“Canadian Security Agreement” has the meaning set forth in Section 4.01(a).
“Capital Lease” means any lease that would be considered to be a capital lease in accordance with GAAP; provided, that any lease that would be considered to be an operating lease in accordance with GAAP on the date of this agreement shall not be considered to be a Capital Lease notwithstanding any change in GAAP after such date that may reclassify it as a capital lease.
“Cash Equivalents” means (a) marketable direct obligations issued or unconditionally guaranteed by the United States, the Government of Canada or any agency or any state or province, as applicable, thereof having maturities of not more than one (1) year from the date of acquisition; (b) commercial paper maturing no more than one (1) year after its creation and having the highest rating from either Standard & Poor’s Ratings Group or Moody’s Investors Service, Inc.; (c) certificates of deposit issued maturing no more than one (1) year after issue; (d) money market funds at least ninety-five percent (95%) of the assets of which constitute Cash Equivalents of the kinds described in clauses (a) through (c) of this definition; and (e) comparable instruments as those described in clauses (a) through (d) for any Loan Party that is not formed under the federal or provincial laws of Canada and operates in a jurisdiction outside of Canada.
“Casualty Event” means, with respect to any Property of any Person, any loss of or damage to, or any condemnation or other taking of, such Property for which such Person or any of its Subsidiaries receives insurance proceeds, or proceeds of a condemnation award or other compensation.
- 3 -
“Centre of Main Interests” means the “centre of main interests” of a Loan Party incorporated in a member state of the European Union as that term is used in Article 3(1) of Regulation (EU) 2015/848 of the European Parliament and of the Council of 20 May 2015 on insolvency proceedings (recast).
“Change in Law” means the occurrence, after the date of this Agreement, of any of the following: (a) the adoption or taking effect of any Law, (b) any change in any Law or in the administration, interpretation, implementation or application thereof by any Governmental Authority or (c) the making or issuance of any request, rule, guideline or directive (whether or not having the force of law, but in the case of a request, rule, guideline or directive not having the force of law, being a request, rule, guideline or directive with which Persons customarily, and are expected by the relevant Governmental Authority to, comply and nevertheless are considered to be binding on such Person or its property) by any Governmental Authority; provided that notwithstanding anything herein to the contrary, (i) the Dodd-Frank Wall Street Reform and Consumer Protection Act and all requests, rules, guidelines or directives thereunder or issued in connection therewith and (ii) all requests, rules, guidelines or directives promulgated by the Bank for International Settlements, the Basel Committee on Banking Supervision (or any successor or similar authority) or the United States, European or foreign regulatory authorities, in each case pursuant to Basel III, shall in each case be deemed to be a “Change in Law”, regardless of the date enacted, adopted or issued.
“Change of Control” means, unless otherwise waived in writing by Lender, (a) the consummation of any transaction (including, without limitation, any plan of arrangement, merger, amalgamation or consolidation) the result of which is that any Person or group of Persons acquires or otherwise beneficially owns, directly or indirectly, or acquires or has the voting power and/or investment power in respect of, 50% or more of the Shares of Parent; (b) the first day on which a majority of the members of the Board of the Parent are not Continuing Directors; or (c) if any Loan Party that is a Subsidiary of Parent ceases to be a wholly-owned Subsidiary of Parent or any Subsidiary thereof, or any other Subsidiary ceases to be Controlled by Parent, other than as a result of an amalgamation with a Loan Party or any other Subsidiary permitted hereunder.
“Closing Date” means the date of this Agreement.
“Collateral” means all assets, property (both real and personal) and undertaking that is subject to grant of security pursuant to a Security Document.
“Collateral Access Agreement” means an agreement, in form and substance reasonably satisfactory to Lender, executed and delivered by a Loan Party and the landlord with respect to any applicable leased facility, which agreement is sufficient to give Lender access to any Collateral located at such leased facility.
“Commitment Fee” has the meaning set forth in Section 2.03.
“Compliance Certificate” means a certificate in the form of Exhibit A hereto.
- 4 -
“Continuing Directors” means, as of any date of determination, any member of the Board of any Loan Party or any of their respective Subsidiaries (1) who was a member of such Board on the Closing Date or (2) whose election or nomination for election to such Board has been approved by a majority of the Continuing Directors who were at the time of such nomination or election members of such Board.
“Control” means the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of a Person, whether through the ability to exercise voting power by contract or otherwise; “Controlling”, “Controlled” and “Controls” have meanings correlative thereto.
“Control Agreement” means an agreement, in form and substance reasonably satisfactory to Lender, executed and delivered by a Loan Party and the applicable securities intermediary or bank, which agreement is sufficient to give Lender “control” over the subject Securities Account, Deposit Account or Investment Property under the PPSA or applicable foreign Law, as applicable, and in the case of a Deposit Account governed by Canadian laws, shall include a blocked account agreement the effect of which would be similar to a control agreement even though it may not be required for “control” under Law.
“Default” means any event specified in Section 8.01 hereof, whether or not any requirement in connection with such event for the giving of notice, lapse of time or happening of any further condition has been satisfied.
“Deposit Account” means any checking account, demand deposit account or other deposit or bank account maintained by a Loan Party.
“Disposition” means any sale, assignment, transfer or other disposition of any Property (whether now owned or hereafter acquired) by a Loan Party or any of its Subsidiaries to any other Person excluding any sale, assignment, transfer or other disposition of any Property sold or disposed of in the ordinary course of business and on ordinary business terms.
“Dollars” and “$” means US Dollars.
“Drawdown” means a US Base Rate Advance by the Lender at the request of the Borrower.
“Drawdown Request” means a notice substantially in the form of Exhibit B from the Borrower to the Lender requesting a Drawdown.
“EBITDA” mean, in respect of the Parent on a consolidated basis, at any date of determination for the applicable period, the net income for such period in accordance with GAAP plus: (a) the interest expense for such period; (b) net income tax expense for such period; (c) depreciation and amortization for such period; and (d) any stock-based compensation for such period; minus (x) capitalized software development costs for such period; (y) capitalized research and development costs for such period; and (z) capitalized lease payments for such period that would otherwise have been operating expenses prior to IFRS 16.
- 5 -
“Environmental Laws” means any and all present and future federal, provincial, state, local and foreign laws, rules or regulations, and any orders or decrees, in each case as now or hereafter in effect, relating to the regulation or protection of human health, safety or the environment or to emissions, discharges, releases or threatened releases of pollutants, contaminants, chemicals or toxic or hazardous substances or wastes into the indoor or outdoor environment, including, without limitation, ambient air, soil, surface water, ground water, wetlands, land or subsurface strata, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of pollutants, contaminants, chemicals or toxic or hazardous substances or wastes.
“Equipment” has the meaning given to such term in the PPSA.
“Equity Interests” means (a) in the case of any corporation or company, all shares or capital stock and any securities exchangeable for or convertible into shares or capital stock, (b) in the case of an association or business entity, any and all shares, interests, participation rights or other equivalents of corporate stock (however designated) in or to such association or entity, (c) in the case of a partnership, limited liability company or unlimited liability company, partnership or membership interests (whether general or limited), as applicable, and (d) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distribution of assets of, the issuing Person, and including, in all of the foregoing cases described in clauses (a), (b), (c) or (d), any warrants, rights or other options to purchase or otherwise acquire any of the interests described in any of the foregoing cases.
“Establishment” has the meaning as used in Article 2(10) of Regulation (EU) 2015/848 of the European Parliament and of the Council of 20 May 2015 on insolvency proceedings (recast).
“Event of Default” has the meaning set forth in Section 8.01.
“Exchange Rate” means, in relation to the conversion of one currency into another currency, the spot rate of exchange quoted for by the Lender in accordance with its usual practice at 11:00am Toronto time on the Business Day such conversion is to be made in accordance with its normal practice.
“Excluded Accounts” means collectively (i) Deposit Accounts of a Loan Party in Canada or the United States that collectively hold an aggregate of no more than $750,000, calculated as a daily average amount over a one-month period (provided that such accounts comply with Section 4.02), and (ii) Deposit Accounts of a Loan Party outside of Canada or the United States that collectively hold an aggregate of no more than $500,000, calculated as a daily average amount over a one-month period.
- 6 -
“Excluded Taxes” means, any of the following Taxes imposed on or with respect to the Lender or required to be withheld or deducted from a payment to the Lender, (a) Taxes imposed on or measured by net income (however denominated), franchise or capital Taxes, and branch profits Taxes, in each case, imposed as a result of the Lender being organized under the laws of, or having its principal office or its applicable lending office located in, the jurisdiction imposing such Tax (or any political subdivision thereof), (b) withholding Taxes of the United States imposed on amounts payable to or for the account of the Lender with respect to an applicable interest in an Advance pursuant to a Law in effect on the date on which (i) the Lender acquires by assignment such interest in an Advance or (ii) the Lender changes its lending office, except in each case to the extent that, pursuant to Section 3.10(c), amounts with respect to such Taxes were payable either to the Lender’s assignor immediately before the Lender became a party hereto or to the Lender immediately before it changed its lending office, and (c) Taxes imposed under FATCA.
“FATCA” means sections 1471 through 1474 of the Internal Revenue Code (U.S.), as of the date of this Agreement (or any amended or successor version that is substantively comparable and not materially more onerous to comply with), any current or future regulations or official interpretations thereof, any intergovernmental agreement or foreign legislation (including official administrative rules or practices) implemented to give effect to any intergovernmental agreements entered into thereunder and any agreements entered into pursuant to section 1471(b) of the Internal Revenue Code (U.S.).
“Financing Documents” means this Agreement, the Canadian Guarantee, any other Guarantee, the Security Documents, all Additional Documents and any other document, instrument or agreement now or hereafter entered into in connection with the Secured Obligations or the Collateral.
“Fiscal Quarter” means each of the four (4) fiscal quarters ending March 31, June 30, September 30 and December 31 of the Parent and together comprising a Fiscal Year.
“Fiscal Year” means the twelve-month accounting period of the Parent ending December 31 of each calendar year.
“GAAP” means generally accepted accounting principles which are in effect from time to time in Canada, and which on the Closing Date encompass IRFS provided that if the Loan Parties convert its consolidated financial reporting to US GAAP, in accordance with Section 1.08, then GAAP shall mean US GAAP.
“German Security Agreements” has the meaning set forth in Section 4.01(f).
“Governing Documents” means, for any Person, such Person’s formation documents, as certified by the applicable governmental agency of such Person’s jurisdiction of organization (provided that in case of any later updates to such certified documents a print-out from the electronic commercial register will be sufficient), and, (a) if such Person is a corporation, its certificate of incorporation or formation and bylaws, (b) if such Person is a limited liability company, its certificate of incorporation and/or limited liability company agreement (or similar agreement), and (c) if such Person is a partnership or limited partnership, its partnership agreement (or similar agreement), in each case, including all amendments or modifications thereto.
“Governmental Authority” means any nation or government, any provincial, state or other political subdivision thereof, any agency, authority, instrumentality, regulatory body, court, central bank or other entity exercising executive, legislative, judicial, taxing, regulatory or administrative functions of or pertaining to government, any securities exchange and any self-regulatory organization.
- 7 -
“Guarantee” means any guarantee of the Secured Obligations entered into on or after the Closing Date by any Person required to provide a Guarantee in accordance with the terms of this Agreement (including, without limitation, pursuant to Section 4.01 and Section 4.03 hereof) and which for greater certainty shall include the Canadian Guarantee, which shall be in form and substance satisfactory to the Lender.
“Guarantor” means, collectively, (i) Parent, 275 Ontario, PM Germany, and PM USA, and (ii) any person providing a Guarantee in favor of Lender under the terms of Section 4.03.
“Hazardous Materials” has the meaning set forth in Section 5.13.
“Healthcare Laws” means, collectively, any and all federal, state, provincial or local laws, rules, regulations and administrative manuals, orders, guidelines and requirements issued under or in connection with any government payment program or any law governing the establishment, certification, licensure of or regulating healthcare providers, professionals, facilities (including nursing facilities), medical devices, medications or payors or otherwise governing or regulating the provision of, or payment for, medical and healthcare services.
“Hedge Arrangement” means, for any period, for any Person, any arrangement or transaction between such Person and any other Person which is an interest rate swap transaction, basis swap, forward interest rate transaction, commodity swap, interest rate option, forward foreign exchange transaction, cap transaction, floor transaction, collar transaction, currency swap transaction, cross-currency interest rate swap transaction, currency option or any other similar transaction (including any option with respect to any of such transactions or arrangements) designed to protect or mitigate against risks in interest, currency exchange or commodity price fluctuations.
“IFRS” means the International Financial Reporting Standards issued by the International Accounting Standards Board and as adopted by the Canadian Institute of Chartered Accountants as in effect from time to time.
“Indebtedness” of a Loan Party or any of its Subsidiaries, as the case may be, means, without duplication:
| (a) | all of its indebtedness for or in respect of borrowed money, credit or other financial accommodation, including liabilities and obligations with respect to letters of credit, letters of guarantee, or similar instruments issued or accepted by banks and other financial institutions for the account of such Loan Party or any of its Subsidiaries and all obligations evidenced by notes, bonds, debentures or other similar instruments; |
| (b) | all obligations for or in respect of the deferred purchase or acquisition price of Property or services, whether or not recourse is limited to the repossession and sale of any such Property; |
- 8 -
| (c) | all obligations under any lease entered into as lessee which would be classified as a capital lease in accordance with GAAP; |
| (d) | all obligations of it to purchase, redeem, retract or otherwise acquire any securities issued by such Loan Party or any of its Subsidiaries where such obligation has been exercised or otherwise become payable; |
| (e) | all obligations under Hedge Arrangements; and |
| (f) | all guarantees by such Loan Party or any of its Subsidiaries or any of its Subsidiaries of any of the foregoing, or obligations of such Loan Party or any of its Subsidiaries to purchase or acquire any of the foregoing, or any other assurance given by such Loan Party or any of its Subsidiaries to a creditor of another Person against loss in connection with any of the foregoing incurred by such other Person; |
but “Indebtedness” shall not include unsecured trade debt incurred in the ordinary course of business, issued share capital or surplus, reserves (including, for certainty, reserves in connection with purchase orders) for deferred taxes or general contingencies, minority interests in Subsidiaries, operating leases, nor any contingent liabilities in connection with contracts entered into in the ordinary course of business.
“Indemnified Claim” has the meaning set forth in Section 10.05.
“Indemnified Person” has the meaning set forth in Section 10.07.
“Indemnified Taxes” means Taxes, other than Excluded Taxes imposed on or with respect to any payment made by or on account of any obligation of Borrower.
“Insolvency Legislation” means legislation in any applicable jurisdiction relating to reorganization, arrangement, compromise or re-adjustment of debt, dissolution or winding-up, or any similar legislation, and specifically includes for greater certainty the Bankruptcy and Insolvency Act (Canada), the Companies’ Creditors Arrangement Act (Canada), the German Insolvency Code (Insolvenzordnung) (Germany), the German Act on the Stabilisation and Restructuring Framework for Businesses (Unternehmensstabilisierungs- und restrukturierungsgesetz, StaRUG) (Germany), the Winding-Up and Restructuring Act (Canada) and the Bankruptcy Code (United States), together with any other similar statutes (including corporate statutes) in Canada or any other applicable jurisdiction in which any Loan Party operates.
“Insolvency Proceeding” is any proceeding by or against any Person under any Insolvency Legislation, or any other bankruptcy or insolvency law, including assignments for the benefit of creditors, compositions, extensions generally with its creditors, or proceedings seeking reorganization, arrangement, or other relief.
“Instruments” has the meaning given to such term in the PPSA.
- 9 -
“Intellectual Property” means the following properties and assets owned or held or in which the applicable Person otherwise has any interest, now existing or hereafter acquired or arising:
| (a) | all patents and patent applications, domestic or foreign, all licenses relating to any of the foregoing and all income and royalties with respect to any licenses, all rights to sue for past, present or future infringement thereof, all rights arising therefrom and pertaining thereto and all reissues, divisions, continuations, renewals, extensions and continuations-in-part thereof; |
| (b) | all copyrights and applications for copyright, domestic or foreign, together with the underlying works of authorship (including titles), whether or not the underlying works of authorship have been published and whether said copyrights are statutory or arise under the common law, and all other rights and works of authorship, all computer programs, computer databases, computer program flow diagrams, source codes, object codes and all tangible Property embodying or incorporating any copyrights, all licenses relating to any of the foregoing and all income and royalties with respect to any licenses, and all other rights, claims and demands in any way relating to any such copyrights or works, including royalties and rights to sue for past, present or future infringement, and all rights of renewal and extension of copyright; |
| (c) | all state and provincial (including common law), federal and foreign trade-marks, service marks and trade names, and applications for registration of such trademarks, service marks and tradenames, all licenses relating to any of the foregoing and all income and royalties with respect to any licenses, whether registered or unregistered and wherever registered, all rights to sue for past, present or future infringement or unconsented use thereof, all rights arising therefrom and pertaining thereto and all reissues, extensions and renewals thereof; |
| (d) | all technology created, developed or acquired by such Person, trade secrets, trade dress, trade styles, logos, other sources of business identifiers, mask-works, mask-work registrations, mask-work applications, software, proprietary or confidential information, customer lists, license rights, advertising materials, operating manuals, methods, processes, know-how, techniques, research, studies, algorithms, formulae, databases, quality control procedures, product, service and technical specifications and data, operating, production and quality control manuals, sales literature, drawings, specifications, blue prints, descriptions, inventions, name plates and catalogs; |
| (e) | all domain names, internet protocol addresses and uniform resource locators used in the business and all applications, registrations and rights therein and thereto; |
| (f) | the entire goodwill of or associated with the businesses now or hereafter conducted connected with and symbolized by any of the aforementioned properties and assets; and |
- 10 -
| (g) | all accounts, all intangible intellectual or other similar Property and other general intangibles associated with or arising out of any of the aforementioned properties and assets and not otherwise described above. |
“Intellectual Property Security Agreements” means (i) the Canadian IP Security Agreements, the (ii) US IP Security Agreements, and (iii) any other security agreement creating a security interest in the Intellectual Property of the Loan Parties, in each case as amended, restated, supplemented or otherwise modified from time to time.
“Investment” means any direct or indirect:
| (a) | purchase or other acquisition of Equity Interest or other securities of any other Person or any beneficial interest therein; |
| (b) | purchase or other acquisition of bonds, notes, debentures or other debt securities of any other Person or beneficial interest therein; |
| (c) | loan or advance to any other Person; and |
| (d) | capital contribution to any other Person. |
“Investment Property” has the meaning given to such term in the PPSA.
“ITA” means the Income Tax Act (Canada), as amended from time to time.
“Law” means any statute, law, ordinance, regulation, rule, order, writ, injunction, policies, practices, directives, guidelines or decree of any Tribunal, and which for greater certainty shall include Healthcare Laws and any policies, practices, directives or guidelines of the TSX and NASDAQ.
“Lender” has the meaning set forth in the preamble to this Agreement.
“Lender Expenses” means all reasonable fees, costs, expenses (including, without limitation, reasonable attorneys’ and other legal fees, costs and expenses and audit fees), or any other reasonable charges incurred by Lender in the negotiation, preparation, amendment, administration, monitoring, defense, enforcement and restructuring of the Financing Documents (including without limitation, those incurred after appeal or in Insolvency Proceedings) or the exercise of remedies pursuant thereto, or otherwise incurred by Lender with respect to any Loan Party or the Financing Documents.
“Lien” means any lien, mortgage, charge, hypothecation, pledge, security interest, prior assignment, option, warrant, lease, sublease, right to possession, right of distress, encumbrance, claim, right or restriction which affects, by way of a conflicting ownership interest or otherwise, the right, title or interest in or to any particular property. For purposes of the Financing Documents, a Person shall be deemed to own subject to a Lien any property that it has acquired or holds subject to the interest of a vendor or lessor under any conditional sale agreement, capital lease or other title retention agreement relating to such property.
- 11 -
“Liquidity” means, as of any date of determination, the sum of (a) Revolver Availability as of such date plus (b) Unrestricted Cash as of such date.
“Loan Account” has the meaning set forth in Section 2.05.
“Loan Parties” means Borrower, Parent and each Guarantor from time to time party hereto or any other Financing Document, collectively, and “Loan Party” means any one of such parties.
“Material Adverse Change” means a material adverse change in the business, assets, operations, prospects or condition, financial or otherwise, of the Borrower or any other Loan Party.
“Material Adverse Effect” means a material adverse effect on (a) the business, assets, liabilities (actual or contingent), financial condition, operations, performance or properties of the Loan Parties and their Subsidiaries taken as a whole, (b) the ability of the Loan Parties to perform their obligations under the Financing Documents, (c) the validity or enforceability of the Financing Documents or the Lien granted to Lender thereunder, or (d) the rights and remedies of Lender under the Financing Documents.
“Material Agreements” any contract, agreement, license, sublicense, supply agreement, permit, lease or other instrument, the termination of which would reasonably be expected to have a Material Adverse Effect, including, without limitation, the agreements set forth on Schedule 5.09.
“Material Intellectual Property” means all Intellectual Property and license or sublicense agreements or other agreements with respect to rights in Intellectual Property, of any Loan Party or any of its Subsidiaries, that is material to the business or operations of the Loan Parties on a consolidated basis.
“Material Subsidiary” means (i) each present and future Subsidiary of Parent that at any time individually accounts for more than 5% of either the revenue or assets of Parent on a consolidated basis, (ii) each present and future Subsidiary of Parent that at any time, when aggregated with all other Subsidiaries that are not Loan Parties, accounts for more than 10% of either the revenue or assets of Parent on a consolidated basis and (iii) each other present and future Subsidiary of Parent that is a party to a Material Agreement or holds any Intellectual Property that is material to Parent’s or Borrower’s business. Notwithstanding the foregoing, Profound Medical OY shall not be subject to the test in clause (i) above so long as it complies with the tests in clause (ii) and (iii) above.
“Maturity Date” means March 3, 2027, the date which is two years from the Closing Date.
“Milestone” the time at which Revenue for the most recent reported 12-month period exceeds $15,000,000 at the most recent month ended.
“NASDAQ” means the Nasdaq Stock Market.
- 12 -
“Net Cash Payments” means, with respect to any Disposition, the aggregate amount of all cash payments, and the fair market value of any non-cash consideration, received by a Loan Party or any of its Subsidiaries directly or indirectly in connection with such Disposition; provided that Net Cash Payments shall be net of (i) the amount of any legal, title and recording tax expenses, commissions and other fees and expenses paid by a Loan Party or any of its Subsidiaries in connection with such Disposition and (ii) any federal, provincial, state and local income or other taxes estimated to be payable by a Loan Party or any of its Subsidiaries as a result of such Disposition.
“Non-Canadian Pension Plan” means any plan, program or arrangement that is a pension plan, scheme, fund (including any superannuation fund) or other similar plan, program or arrangement that is established, registered, sponsored, contributed to or maintained outside of Canada by any Loan Party for the benefit of employees or former employees of such Loan Party, or any dependants or beneficiaries of such person, or under which any Loan Party has any actual or contingent liability, which is subject to statutory funding requirements in respect of the payment of pension or retirement benefits thereunder, provided that a Non-Canadian Pension Plan shall not include a Canadian Pension Plan or Statutory Plan. For clarity Non-Canadian Pension Plan shall include, without limitation, any such plan established, registered, sponsored, contributed to, or maintained in Germany.
“Original Credit Agreement” has the meaning set forth in the recitals to this Agreement.
“Parent” has the meaning set forth in the preamble to this Agreement.
“Pension Plan” means a Canadian Pension Plan or a Non-Canadian Pension Plan.
“Pension Plan Event” means, (i) the institution of any steps by any Loan Party or any applicable regulatory authority to terminate a Pension Plan if, as a result of such termination, any such Loan Party shall be required to make an additional contribution to such Pension Plan or to incur an additional liability or obligation to such Pension Plan equal to or in excess of $250,000; (ii) the failure of any Loan Party to make required contributions when due to any Pension Plan in accordance with Applicable Laws, including minimum funding standards (whether or not a funding waiver has been granted); (iii) the withdrawal of any Loan Party from a Pension Plan, if, as a result of such withdrawal, any such Loan Party shall be required to make an additional contribution to such Pension Plan or to incur an additional liability or obligation to such Pension Plan equal to or in excess of $250,000; or (iv) the taking of any steps to establish, acquire, adopt, fund or otherwise contribute to or have any liability under, a Pension Plan that contains a “defined benefit provision” as such term is defined under the ITA or otherwise provides a defined benefit pension.
“Permit” has the meaning set forth in Section 5.19.
“Permitted Acquisition” means any Acquisition that complies with the following criteria:
| (a) | no Event of Default exists at the time of such Acquisition or would exist after giving effect to such Acquisition; |
- 13 -
| (b) | the Acquisition is non-hostile in nature; |
| (c) | the person, division, product line of business acquired in such Acquisition shall be in the same or substantially similar line of business as Borrower or in an adjacent line of business; |
| (d) | the total aggregate consideration for the Acquisition, together with all other Acquisitions hereunder, is not greater than $10,000,000; |
| (e) | if the Acquisition is of a Material Subsidiary, it shall provide a Guarantee and deliver such Security Documents and certificates as are consistent with the documentation provided to the Lender by a Loan Party and comply with the requirements of Section 4.01 and Section 4.03, and if it is an Acquisition of assets it shall otherwise comply with Article IV; and |
| (f) | the Loan Parties (i) demonstrate pro forma compliance with the financial covenants set out in Section 6.20 for the trailing three month period ending immediately prior to the date of the proposed Acquisition before giving effect to such Acquisition and, (ii) reasonably project that the Loan Parties will remain in compliance for the following two (2) fiscal quarters with such financial covenant after giving effect to such Acquisition. |
“Permitted Distribution” means any of the following:
| (a) | the conversion of any convertible securities into other securities pursuant to the terms of such convertible securities as long as the conversion does not involve any payment of cash; |
| (b) | the payment of dividends solely in equity securities a Loan Party; |
| (c) | the payment of dividends or other distributions in cash from any Loan Party to any other Loan Party; |
| (d) | payment of dividends by the Parent pursuant to the then most recent Budget in an amount up to 115% of the amount set forth in such Budget provided that no Event of Default exists and is continuing; |
| (e) | the repurchase of stock, with the exception of preferred stock, of former employees, independent directors or consultants pursuant to stock repurchase agreements up to an aggregate of $250,000 per fiscal year or in connection with a normal course issuer bid. |
“Permitted Encumbrances” means any of the following:
| (a) | Liens set forth on Schedule 1.01 hereto existing as of the Closing Date; |
| (b) | Liens for Taxes not at the time due unless contested in good faith by all necessary proceedings and reserves satisfactory to Lender acting reasonably have been taken; |
- 14 -
| (c) | rights reserved to or vested in any municipality or governmental or other public authority by the terms of any lease, license, franchise, grant or permit, or by any statutory provision, to terminate the same or to require annual or other periodic payments as a condition to the continuance thereof; |
| (d) | reservations, limitations, provisos and conditions expressed in any original grants from the Crown or other grants of real or immovable property, or interests therein, which do not materially affect the use of the affected land for the purpose for which it is used by that Person, and title defects, encroachments or irregularities or other matters relating to title which are of a minor nature and which in the aggregate do not materially impair the use of the affected property for the purpose for which it is used by that Person; |
| (e) | a security interest in cash or governmental obligations deposited in the ordinary course of business to secure worker’s compensation, unemployment insurance, public and statutory obligations, mechanics’, warehousemen’s, carriers’ and other similar liens arising by operation of law in the ordinary course of business and which are not registered or enforceable against any property of any Loan Party; |
| (f) | Lien created by a judgment of a court of competent jurisdiction, as long as the judgment is being contested diligently and in good faith by appropriate proceedings or is promptly satisfied by that Person and does not result in an Event of Default; |
| (g) | Liens on Equipment securing the unpaid purchase price thereof and/or Liens securing Capital Leases; provided that the Indebtedness secured thereby (plus any Indebtedness for equipment financings and Capital Leases as may be set forth on Schedule 1.01 hereto) does not exceed $500,000 and such security interest is limited to the Equipment so leased or acquired; |
| (h) | Liens granted to Lender to secure the Secured Obligations; |
| (i) | inchoate liens or any rights of distress reserved in or exercisable under any real property lease or sublease to which any Loan Party is a lessee which secure the payment of rent or compliance with the terms of such lease or sublease, provided that such rent is not then overdue and such Loan Party is then in compliance in all material respects with such terms; |
| (j) | any Lien which does not secure Indebtedness, the satisfaction of which has been provided for by deposit with the Lender of cash or a surety bond or other security satisfactory to the Lender in an amount sufficient to pay the liability in respect of such Lien in full; and |
| (k) | any other encumbrances Lender declares in writing to be Permitted Encumbrance. |
“Permitted Indebtedness” means any of the following:
| (a) | Indebtedness related to a corporate credit card facility provided that such Indebtedness does not exceed an aggregate principal amount equal to the sum of: |
- 15 -
| (i) | CAD$375,000 of unsecured credit card indebtedness owing to JPMorgan Chase Bank, N.A., Toronto Branch; |
| (ii) | USD$375,000 of unsecured credit card indebtedness owing to JPMorgan Chase Bank, N.A.; and |
| (iii) | EUR€50,000 of unsecured credit card indebtedness owing to American Express Europe S.A. (Germany branch); |
| (b) | the Secured Obligations; |
| (c) | Indebtedness in connection with Capital Leases in an amount not to exceed $500,000; and |
| (d) | unsecured Indebtedness not otherwise permitted in an aggregate amount not to exceed $500,000 outstanding in the aggregate at any given time. |
“Permitted Investments” means:
| (a) | Investments set forth on the Schedule 1.01, existing as of the Closing Date; |
| (b) | Investments consisting of Cash Equivalents; |
| (c) | Investments in a Loan Party or any Subsidiary, provided that Investments of the Loan Parties in a Subsidiary that is not a Loan Party shall not exceed $500,000 per fiscal year; |
| (d) | Investments consisting of (a) travel advances and employee relocation loans and other employee loans and advances in the ordinary course of business, and (a) loans to employees, officers or directors relating to the purchase of equity securities of any Loan Party pursuant to employee stock purchase plans or agreements approved by such Loan Party’s Board, up to a maximum of $500,000 per annum; and |
| (e) | Investments consisting of Securities Accounts (and the Investment Property therein) in which Lender has a Control Agreement covering such Securities Account. |
“Person” is to be interpreted broadly and includes any individual, partnership, limited partnership, joint venture, syndicate, sole proprietorship, company, limited liability company or corporation with or without share capital, unincorporated association, trust, trustee, executor, administrator or other legal personal representative, regulatory body or agency, government or governmental agency, authority or entity however designated or constituted.
“Plan” means an employee benefit or other plan established or maintained by a Loan Party.
“PM Germany” has the meaning set forth in the preamble to this Agreement.
- 16 -
“PM USA” has the meaning set forth in the preamble to this Agreement.
“Post-Default Rate” has the meaning set forth in Section 3.02(b).
“PPSA” means the Personal Property Security Act (Ontario) as in effect from time to time and the regulations and orders made thereunder, or, in respect of Collateral located in a Canadian province other than Ontario, the personal property security act, regulations and orders in effect in such other province from time to time.
“Priority Payables” means, with respect to any Person, any amount payable by such Person which is secured by a lien which ranks or is capable of ranking prior to or pari passu with the liens created by the Security Documents, including unpaid amounts owing for wages, vacation pay, severance pay (to the extent capable of ranking prior to the liens under the Security Documents under Applicable Law), employee deductions, Taxes, income tax, workers compensation, government royalties, pension fund obligations, Canadian Pension Plan and Non-Canadian Pension Plan obligations, including the amounts of any unfunded liability or solvency deficit, and other statutory or other claims that have or may have priority over, or rank pari passu with, such liens created by the Security Documents.
“Proceedings” has the meaning set forth in Section 5.21.
“Product” means each product, process or service under development, developed, manufactured, licensed, distributed, marketed or sold by any Loan Party and any other similar products or services in which any Loan Party has any proprietary rights or beneficial interests, which for greater certainty shall include the TULSA-PRO and Sonalleve.
“Property” means any interest in any kind of property or asset, whether real, personal or mixed, or tangible or intangible.
“Qualified ECP Guarantor” means in respect of any Hedge Arrangement any Guarantor that (i) constitutes an “eligible contract participant” under the Commodity Exchange Act or any regulations promulgated thereunder, or (ii) can cause another Person (including, for the avoidance of doubt, any other Guarantor not then constituting a “Qualified ECP Guarantor”) to qualify as an “eligible contract participant” at such time by entering into a “keepwell, support, or other agreement” as contemplated by Section 1a(18)(A)(v)(II) or the Commodity Exchange Act.
“Recurring Revenue” means recurring revenue that consists of the sale of TULSA-PRO® consumables, lease of medical devices, procedures and services associated with extended warranties.
“Regulatory Authority” means any Governmental Authority that has responsibility in any country or group of countries over the development, manufacture or commercialization of a Product, including the U.S. Food and Drug Administration, Health Canada and the European Medicines Agency, and any successor agency thereof.
- 17 -
“Regulatory Reporting Event” has the meaning set forth in Section 6.12.
“Regulatory Required Permit” means any and all licenses, approvals and permits issues by any Regulatory Authority or any other Governmental Authority necessary for the testing, manufacture, marketing or sale of any Product by any Loan Party and their respective Subsidiaries.
“Required Liquidity Amount” means, as of any date of determination, the greater of (i) to the extent that EBITDA (calculated in accordance with the definition thereof) is a negative number or loss for the most recent nine-month period, the amount of such loss as reported in a Compliance Certificate, or (ii) $7,500,000.00.
“Responsible Officer” means a Loan Party’s chief executive officer or chief financial officer. Any document delivered hereunder that is signed by a Responsible Officer shall be conclusively presumed to have been authorized by all necessary corporate or other action on the part of such Loan Party and such Responsible Officer shall be conclusively presumed to have acted on behalf of such Loan Party.
“Revenue” means revenue generated from the Parent’s consolidated business and classified as such in accordance with GAAP in the consolidated financial statements of the Parent.
“Revolver Availability” means an amount equal to (a) the Revolving Credit Facility Limit minus (b) the sum of all outstanding Advances under the Revolving Credit Facility.
“Revolving Credit Facility” is defined in Section 2.01(a).
“Revolving Credit Facility Commitment” means, the obligation of the Lender to make Advances to the Borrower under the Revolving Credit Facility up to an aggregate principal amount of $10,000,000 as the same may be increased from time to time pursuant to the Revolving Credit Facility Increase and the other terms hereof.
“Revolving Credit Facility Commitment Period” means the period from and including the Closing Date to the Revolving Credit Facility Termination Date.
“Revolving Credit Facility Increase” is defined in Section 2.01(b).
“Revolving Credit Facility Limit” is defined in Section 2.01(a).
“Revolving Credit Facility Termination Date” means the earliest to occur of: (a) the Maturity Date; and (b) the termination of the Revolving Credit Facility Commitments pursuant to Section 2.06, Section 3.06 or Section 8.12.
“Run-Rate Revenue” means the trailing 3-month Revenue multiplied by four (4).
- 18 -
“Sanctioned Person” means any Person that is: (a) designated under, listed on, or owned or controlled by a Person designated under or listed on, or acting on behalf of a Person designated under or listed on, any list of Persons who are subject to Sanctions under Applicable Law that is binding on the Lender or any Loan Party; (b) located in, incorporated under the laws of, or owned or controlled (directly or indirectly) by, or acting on behalf of a Person located in or organized under the laws of a country or territory that is the target of country-wide or territory-wide Sanctions; or (c) with whom the Lender would not be permitted to make a loan, continue to make a loan or provide financial accommodation to pursuant to any Sanctions.
“Sanctions” means any Applicable Law governing transactions in controlled goods or technologies or dealings with countries, entities, organizations, or individuals subject to economic sanctions and similar measures, including the Special Economic Measures Act (Canada), the United Nations Act (Canada), the Freezing Assets of Corrupt Foreign Officials Act (Canada), Part II.1 of the Criminal Code (Canada), and the Export and Import Permits Act (Canada), and any regulations thereunder, and any similar law, rule, regulation or other Applicable Law in place in any foreign country including those in place in the United States.
“Sanctions Authority” means any of: (a) the Government of Canada; (b) the government of the United States of America; (c) the United Nations; (d) the European Union; (e) the United Kingdom; or (f) the respective departments and agencies of any of the foregoing, including Foreign Affairs, Trade and Development Canada, Global Affairs Canada, Public Safety Canada, the Office of Foreign Assets Control of the U.S. Department of the Treasury and the U.S. Department of State.
“Secured Obligations” means all advances to, and debts, liabilities, obligations, covenants and duties of each Loan Party arising under this Agreement or any other Financing Document or otherwise with respect to any Advance, including, without limitation, Lender Expenses, whether direct or indirect (including those acquired by assumption), absolute or contingent, due or to become due, now existing or hereafter arising and including interest and fees that accrue after the commencement by or against any Loan Party of any Insolvency Proceeding.
“Securities Account” has the meaning given to such term in the PPSA.
“Security Agreements” means the Canadian Security Agreement, the German Security Agreements and the USA Security Agreement.
“Security Documents” means each Security Agreement, any Collateral Access Agreements, any Control Agreement, the Intellectual Property Security Agreements, all PPSA and applicable foreign Law financing statements required thereby to be filed with respect to the security interests in personal Property and fixtures created pursuant thereto, and any and all other agreements or instruments now or hereafter executed and delivered to Lender by any Loan Party or any other Person as security for the Secured Obligations.
“Shares” means the issued and outstanding capital stock, membership units or other securities owned or held of record at any time by any Loan Party in any Subsidiary of such Loan Party.
- 19 -
“Sonalleve” means the innovative therapeutic platform combining real time MR imaging and thermometry with thermal ultrasound to enable precise and incision-free ablation of diseased tissue, CE marked for the treatment of uterine fibroids and palliative pain treatment of bone metastases.
“Standby Fee” is defined in Section 2.03(b).
“Standby Fee Rate” a rate equal to [***].
“Statutory Plan” means the benefit plans sponsored and/or administered by a Governmental Authority and that a Loan Party is required by any domestic or foreign statute to participate in or contribute to with respect to any current or former employee, independent contractor, director or officer of such Loan Party or any beneficiary or dependant thereof, including the Canada Pension Plan, the Quebec Pension Plan, and plans administered pursuant to applicable pension, health Tax, workplace safety insurance and employment insurance legislation.
“Subsidiary” means, with respect to any Person, any corporation, limited corporation, limited liability company, partnership, limited partnership or other entity of which at least a majority of the securities or other ownership interests having by the terms thereof ordinary voting power to elect a majority of the board of directors or other persons performing similar functions of such corporation, limited corporation, limited liability company, partnership, limited partnership or other entity (irrespective of whether or not at the time securities or other ownership interests of any other class or classes of such corporation, limited corporation, limited liability company, partnership, limited partnership or other entity shall have or might have voting power by reason of the happening of any contingency) is at the time directly or indirectly owned or Controlled by such Person or one or more Subsidiaries of such Person or by such Person and one or more Subsidiaries of such Person. Unless otherwise specified herein, “Subsidiary” means a direct or indirect Subsidiary of a Loan Party.
“Taxes” or “Tax” means all present and future taxes, charges, fees, levies, imposts, surtaxes, duties and other assessments, including all income, sales, use, goods and services, value added, capital, capital gains, alternative, net worth, transfer, profits, withholding, payroll, employer health, excise, real property and personal property taxes, and any other taxes, customs duties, fees, assessments, or similar charges of any nature, including Canada Pension Plan or similar plan in any other jurisdiction, and provincial pension plan contributions, unemployment insurance payments and workers’ compensation premiums and including all present or future stamp or documentary taxes or any other excise or property taxes, charges or similar levies arising from any payment made hereunder or under any other Financing Document or from the execution, delivery of enforcement of, or otherwise with respect to, any Financing Document, together with any instalments with respect thereto, and any interest, fines and penalties with respect thereto, imposed by any Governmental Authority (including federal, state, provincial, municipal and foreign Governmental Authorities), and whether disputed or not.
“Term Loan” has the meaning set forth in the Original Credit Agreement.
- 20 -
“Term Sheet” means the term debt financing term sheet issued on November 29, 2024 by Lender and accepted on December 10, 2024 by Borrower.
“Term SOFR Administrator” means the CME Group Benchmark Administration Limited (CBA) (or a successor administrator of forward-looking term rates based on SOFR selected by the Lender in its sole discretion).
“Term SOFR Rate” means, for any calculation with respect to a US Base Rate Advance, the rate per annum determined by the Lender as the Term SOFR Reference Rate for a tenor comparable to the applicable interest period on the day (such day, the “TS Determination Day”) that is two (2) Business Days prior to the first day of such applicable interest period, (a) in the case of a one (1) month interest period or such other tenors as may be published from time to time, as such rate is published by the Term SOFR Administrator; provided, however, that if as of 5:00 p.m. (New York City time) on any TS Determination Day, a Term SOFR Reference Rate for the applicable tenor has not been published by the Term SOFR Administrator, then the Term SOFR Rate will be the Term SOFR Reference Rate for the applicable tenor as published by the Term SOFR Administrator on the first Business Day preceding the TS Determination Day for which a Term SOFR Reference Rate was published by the Term SOFR Administrator for the applicable tenor so long as such first preceding Business Day is not more than three (3) Business Days prior to such TS Determination Day; and (b) in the case of any Term SOFR Reference Rate for any tenor that is not regularly published by the Term SOFR Administrator, as determined by the Lender pursuant to its standard or customary practice.
“Term SOFR Reference Rate” means, for any applicable tenor, the rate per annum determined by the Lender as the forward-looking term rate based on SOFR for such tenor.
“Tribunal” means any state, commonwealth, federal, foreign, territorial or other court or government body, subdivision, agency, department, commission, board, bureau or instrumentality of a governmental body.
“TSX” means the Toronto Stock Exchange.
“TULSA-PRO” is a therapy that combines real-time Magnetic Resonance Imaging with transurethral, robotically-driven therapeutic ultrasound and closed-loop thermal feedback control to provide precise ablation of the prostate while simultaneously actively protecting critical surrounding anatomy from potential side effects.
“Unrestricted Cash” means cash and Cash Equivalents of Borrower that do not appear on Borrower’s balance sheet as “restricted cash” and that (i) are deposited in Canada, Germany or the United States of America in a Deposit Account with the Lender or with an Affiliate of the Lender or (ii) otherwise deposited in Deposit Accounts or Securities Accounts subject to a Control Agreement.
“US Base Rate” for any day, means a rate per annum equal to the greatest of (a) the “prime rate” announced by the Wall Street Journal (“WSJ”) used for determining interest charged on US Dollar commercial loans in effect on such day (or, if no longer quoted by the WSJ, such other national publication selected by the Lender, acting reasonably); (b) the Federal Funds Effective Rate in effect on such day plus 0.50%; and (c) Term SOFR Rate for a one month interest period as published on such day (or if such day is not a Business Day, the immediately preceding Business Day) plus 1.00%; provided that, if at any time the US Base Rate determined as above would be less than the US Base Rate Floor, the US Base Rate shall be deemed to be equal to the US Base Rate Floor.
- 21 -
Any change in the US Base Rate due to a change in the WSJ’s quoted rates, the Federal Funds Effective Rate or Term SOFR Rate shall be effective as of the opening of business on the day such quoted rates become effective.
“US Base Rate Advance” means a Drawdown in U.S. Dollars made by the Lender to the Borrower hereunder which accrues interest at the US Base Rate.
“US Base Rate Floor” means a rate of interest equal to 6.25%.
“US Dollars” and “USD” mean the lawful currency of the United States of America.
“US GAAP” means generally accepted accounting principles that are in effect from time to time in the United States of America.
“US IP Security Agreements” has the meaning set forth in Section 4.01(i).
“Welfare Plan” means, other than a Statutory Plan, any medical, health, hospitalization, insurance or other employee benefit or welfare plan or arrangement of any nature or kind whatsoever, whether oral or written, formal or informal, funded or unfunded, insured or uninsured, applicable to employees or former employees of a Loan Party or a Subsidiary who are resident in Canada, Germany or any other jurisdiction in which any Loan Party operates.
Section 1.02PPSA.
Any terms used in this Agreement that are defined in the PPSA shall be construed and defined as set forth from time to time in the PPSA unless otherwise defined herein.
Section 1.03Headings.
The inclusion of headings in this Agreement is for convenience of reference only and shall not affect the construction or interpretation hereof.
Section 1.04References to Sections.
Whenever in this Agreement a particular article, section or other portion thereof is referred to, such reference pertains to the particular article, section or portion thereof contained herein, unless otherwise indicated.
Section 1.05Currency.
Except where otherwise expressly provided, all amounts in this Agreement are stated and shall be paid in Dollars.
- 22 -
Section 1.06Gender and Number.
In this Agreement, unless the context otherwise requires, words importing the singular include the plural and vice versa and words importing gender include all genders.
Section 1.07Invalidity of Provisions.
Each of the provisions contained in this Agreement is distinct and severable and a declaration of invalidity or unenforceability of any such provision or part thereof by a court of competent jurisdiction shall not affect the validity or enforceability of any other provision hereof.
Section 1.08Transition from GAAP to US GAAP.
If the Loan Parties shall elect as of the end of financial reporting period to prepare their financial statements in accordance with US GAAP, rather than GAAP, then, following delivery to the Lender of a completed Compliance Certificate attaching the information required to be delivered for such financial reporting period, the parties hereto shall use their best efforts to amend (in a manner mutually satisfactory to Lender and Loan Parties) the thresholds or methods of calculation required by Section 6.20 (including any definitions or components applicable thereto) such that compliance therewith is neither more nor less burdensome (as determined by the Lender in its sole discretion) to Loan Parties as a result of such conversion to US GAAP and, thereafter, all references in the Financing Documents to GAAP shall be deemed references to US GAAP.
Section 1.09Amendment or Waiver.
No amendment or waiver of this Agreement shall be binding unless executed in writing by the party to be bound thereby. No waiver of any provision of this Agreement shall constitute a waiver of any other provision nor shall any waiver of any provision of this Agreement constitute a continuing waiver unless otherwise expressly provided.
Section 1.10Non-Business Days.
If any date on which any payment is due or any action is required to be taken is not a Business Day, the date for payment or taking such action shall be the next Business Day following the date specified for such payment or action.
Section 1.11Currency Equivalents
For purposes of calculation and compliance with any limit set forth in Section 6.16 with respect to the amount of Indebtedness, Investment or any other amount subject to a basket that is incurred or made in a currency other than US Dollars, the equivalent amount in US Dollars shall be calculated based on the rate of exchange quoted by the Bank of Canada at the close of business on the Business Day preceding the date the transaction was completed. For purposes of calculation and compliance with the financial covenant set forth in Section 6.20 with respect to any Unrestricted Cash denominated in a currency other than US Dollars, the equivalent amount in US Dollars shall be calculated as of the date of determination based on the rate of exchange used in preparing the applicable financials statements delivered hereunder (or if there is no such rate, the rate of exchange quoted by the Bank of Canada at the close of business on the Business Day preceding the date of calculation).
- 23 -
Section 1.12German Terms.
In this Agreement, where it relates to a company incorporated or established under the laws of Germany or a Financing Document governed by German law, a reference to:
| (a) | “receiver”, “manager”, “conservator”, “trustee”, “agent”, “custodian”, “monitor”, “liquidator” or other similar officer includes a reference to an Insolvenzverwalter, vorläufiger Insolvenzverwalter, Sachwalter, vorläufiger Sachwalter or Restrukturierungsbeauftragter; |
| (b) | seeking to adjudicate a Loan Party or Subsidiary bankrupt or insolvent or seeking the “liquidation”, “dissolution”, “winding-up”, “reorganization”, “arrangement”, “protection”, “relief” or “composition” or initiating or taking steps to initiate any “Insolvency Proceedings” includes an application or filing for, or an institution of, insolvency proceedings (Antrag auf Eröffnung eines Insolvenzverfahrens) (including, without limitation, by way of (vorläufige) Eigenverwaltung or Schutzschirmverfahren), an ordering of provisional measures according to section 21 of the German Insolvency Code (Insolvenzordnung) (Anordnung vorläufiger Maßnahmen) or a rejection of insolvency proceedings on grounds of insufficiency of assets (Abweisung mangels Masse) or any notification (Anzeige) or application (Antrag) in accordance with the German Act on the Stabilisation and Restructuring Framework for Businesses (Unternehmensstabilisierungs- und restrukturierungsgesetz, StaRUG); |
| (c) | a person being “unable to pay its debts” or “unable to pay its debts as they fall due”, includes that person being in a state of Zahlungsunfähigkeit within the meaning of section 17 of the German Insolvency Code (Insolvenzordnung) and a person’s “assets being less than the aggregate sum of its liabilities” includes that person being in a state of Überschuldung within the meaning of section 19 of the German Insolvency Code (Insolvenzordnung); |
| (d) | “management” of a person includes reference to its Geschäftsführung or Vorstand (as applicable); and |
| (e) | the “Governing Documents” includes an excerpt from the competent commercial register (Ausdruck aus dem Handelsregister) of most recent date (not older than 14 days), an up-to-date copy of the articles of association (Satzung) (as filed with the competent commercial register) or partnership agreement (Gesellschaftsvertrag) and, as applicable, an up-to-date copy of the list of shareholders (Gesellschafterliste) as filed with the competent commercial register. |
- 24 -
ARTICLE II.CREDIT FACILITIES
Section 2.01The Commitments.
| (a) | Revolving Credit Facility |
| (i) | The Lender hereby establishes a revolving credit facility (the “Revolving Credit Facility”) in favour of the Borrower in the aggregate amount not to exceed the lesser of $10,000,000 and the Borrowing Base (the “Revolving Credit Facility Limit”). The obligation of the Lender shall be limited to, as of any date of determination, (i) the Revolving Credit Facility Limit minus (ii) the aggregate amount of all Advances under the Revolving Credit Facility then outstanding. |
| (ii) | Subject to the terms and conditions of this Agreement, the Borrower may obtain Advances under the Revolving Credit Facility by way of US Base Rate Advances. |
| (iii) | The Borrower shall have the option to determine which combinations and proportions of Advances shall be drawn down under the Revolving Credit Facility, provided that, (i) the aggregate amount of all Advances under the Revolving Credit Facility shall not exceed the Revolving Credit Facility Limit at any one time and; (ii) the aggregate amount of all advances shall not exceed the Borrowing Base at any one time. |
| (b) | Revolving Credit Facility Increase |
| (i) | Provided that (i) no Default or Event of Default has occurred and is continuing and (ii) the Borrower has achieved the Milestone, the Borrower may, one time, after the Closing Date until September 3, 2026, request that the Lender increase the Revolving Facility Commitment by an amount not to exceed $5,000,000 (the “Revolving Credit Facility Increase”). |
| (ii) | The Borrower shall not be entitled to the Revolving Credit Facility Increase if a Default or Event of Default has occurred and is continuing. |
Section 2.02Reborrowing
Subject to the terms and conditions of this Agreement, during the Revolving Credit Facility Commitment Period, the Borrower may increase or decrease the amount of Advances outstanding under the Revolving Credit Facility by obtaining Advances, making repayments and obtaining further Advances of amounts that have been repaid.
Section 2.03Fees.
| (a) | Commitment Fees. Borrower shall pay to Lender a commitment fee with respect to the Revolving Credit Facility of [***] which is equal to [***] of the Revolving |
- 25 -
Credit Facility Commitment, which fee is fully earned and payable on the Closing Date (the “Commitment Fee”).
| (b) | Standby Fee. Commencing on the Closing Date, the Borrower agrees to pay a standby fee (the “Standby Fee”) to the Lender, calculated at the Standby Fee Rate on the daily unadvanced portion of the Revolving Credit Facility Commitment. The Standby Fee shall be calculated and accrue daily from the date hereof and shall be payable monthly in arrears on the first Business Day of the next succeeding month and on the Revolving Credit Facility Termination Date. |
Section 2.04Payment; Computation.
All fees payable under this Agreement shall be paid on the dates due, in immediately available Canadian Dollars, to Lender. Fees shall be fully earned on the dates set forth in this Agreement and shall not be refundable under any circumstances.
Section 2.05Loan Account.
Lender shall maintain, in accordance with its usual practice, an account on its books in the name of Borrower (the “Loan Account”) on which Borrower will be charged with all Advances made by Lender to Borrower or for Borrower’s account and with all other Secured Obligations hereunder or under the other Financing Documents, including, the date, amount and interest rate of each Advance made to Borrower, accrued interest, fees and expenses, Lender Expenses, and the amount of any sum received by Lender on account of the Secured Obligations. The entries made in lender’s records with respect to the Loan Account pursuant to this Section 2.05 shall be conclusive evidence (absent manifest error) of the existence and amounts of the obligations recorded therein; provided that the failure of Lender to maintain such records or any error therein shall not affect in any manner the obligations of the Borrower to repay the Advances and other Secured Obligations in accordance with this Agreement.
Section 2.06Termination or Reduction of the Revolving Credit Facility Commitment
| (a) | Upon not less than three (3) Business Days’ notice to the Lender, the Borrower shall have the right to terminate the Revolving Credit Facility Commitments or, from time to time, to reduce the aggregate amount of the Revolving Credit Facility Commitments; provided, that, no such termination or reduction of Revolving Credit Facility Commitments shall be permitted if, after giving effect thereto and to any prepayments of Advances under the Revolving Credit Facility made on the effective date thereof, the aggregate principal amount then outstanding of all such Advances would exceed the Revolving Credit Facility Limit. |
| (b) | Any partial reduction of the Revolving Credit Facility Commitments shall be in a minimum amount of $1,000,000 and reductions in excess thereof shall be in integral multiples of $500,000, and shall permanently reduce the Revolving Credit Facility Commitments and the Revolving Credit Facility Limit then in effect. |
- 26 -
Section 2.07Accordion Feature.
| (a) | The Borrower may request a one-time increase in the principal amount of the Revolving Credit Facility Commitment (the “Accordion Request”); provided that: |
| (i) | the Accordion Request shall be submitted by the Borrower to the Lender and shall specify the amount of the requested increase; |
| (ii) | the Accordion Request shall not exceed the sum of $10,000,000; and |
| (iii) | no Default or Event of Default shall have occurred and be continuing or would result therefrom. |
| (b) | The Lender shall not have any obligation, express or implied, to offer to increase or accept the Borrower’s offer to increase the Revolving Credit Facility Commitment. The Lender shall, in its sole discretion, have the ability to syndicate all or any portion of the increase in the Revolving Credit Facility Commitment set out in the Accordion Request if it accepts. |
| (c) | If offers made pursuant to this Section have been accepted and all required consents obtained, then: |
| (i) | the Loan Parties shall execute and deliver such documentation as the Lender, in its sole discretion, deems necessary in order to give effect to the increase, including without limitation an amendment to this Agreement, confirmation that all necessary approvals, acknowledgments of the increase from each Guarantor, authorizations and consents (including board resolutions) have been obtained, opinions of counsel for the Loan Parties, and confirmation that all representations and warranties of the Loan Parties in this Agreement are true and correct as of the day of the increase (except for those provided as at a specific date); and |
| (ii) | upon satisfaction of the conditions in (i) above and payment of all fees and expenses of the Lender associated with the Accordion Request, the Revolving Credit Facility Limit shall be increased by the amount set out in the Accordion Request. |
ARTICLE III.PAYMENTS AND PREPAYMENTS
Section 3.01Repayment of Advances.
The Borrower shall repay or pay, as the case may be, all Secured Obligations under or in connection with the Revolving Credit Facility in full on the Maturity Date, including all outstanding Advances under the Revolving Credit Facility and all accrued interest, fees and other amounts then unpaid with respect to such Advances and the Revolving Credit Facility and the Revolving Credit Facility Commitment thereunder shall be automatically terminated on the Maturity Date.
- 27 -
Section 3.02Interest.
| (a) | Revolving Credit Facility. The Borrower hereby unconditionally promises to pay to Lender interest on each Advance at a rate per annum equal to the US Base Rate. |
| (b) | Post-Default Rate. Notwithstanding the foregoing, the Borrower acknowledges and agrees that if an Event of Default shall have occurred and be continuing, then at the option of Lender for as long as such Event of Default exists, the unpaid balance of all Advances shall bear interest, to the fullest extent permitted by law, at a rate equal to 12% per annum (the “Post-Default Rate”), from the date of the Event of Default has occurred until such Event of Default is cured or waived. The Borrower hereby unconditionally agrees to pay to Lender interest at the Post-Default Rate, as specified in this paragraph from time to time on demand. |
| (c) | Payment. Interest on US Base Rate Advances shall be calculated daily and payable monthly on the first Business Day of the next succeeding month while such Advance is outstanding, or otherwise on the Maturity Date. Notwithstanding the foregoing, (i) accrued interest on the principal amount of any Advance repaid or prepaid shall be payable on the date of the payment or prepayment thereof, and (ii) interest payable at the Post-Default Rate shall be payable on demand. |
| (d) | All computations of interest or fees “per annum” for US Base Rate Advances shall be made on the basis of a year of 365 or 366 days, as the case may be, using the actual number of days elapsed, and the nominal rate method of calculation, and will not be calculated using the effective rate method of calculation or on any other basis that gives effect to the principle of deemed re-investment of interest. Interest shall accrue on each Advance for the day on which the Advance is made, and shall not accrue on an Advance, or any portion thereof, for the day on which the Advance or such portion is paid. |
| (e) | For the purposes of the Interest Act (Canada) and disclosure thereunder, whenever any interest or any fee to be paid hereunder or in connection herewith is to be calculated on the basis of any period of time that is less than a calendar year, the yearly rate of interest to which the rate used in such calculation is equivalent is the rate so used multiplied by the actual number of days in the calendar year in which the same is to be ascertained and divided by 360 or 365, as applicable. The rates of interest under this Agreement are nominal rates, and not effective rates or yields. The principle of deemed reinvestment of interest does not apply to any interest calculation under this Agreement. |
| (f) | If any provision of this Agreement would oblige Borrower to make any payment of interest or other amount payable to the Lender in an amount or calculated at a rate which would be prohibited by law or would result in a receipt by that Lender of “interest” at a “criminal rate” (as such terms are construed under the Criminal Code (Canada)), then, notwithstanding such provision, such amount or rate shall be deemed to have been adjusted with retroactive effect to the maximum amount or rate of interest, as the case may be, as would not be so prohibited by Law or so |
- 28 -
result in a receipt by that Lender of “interest” at a “criminal rate”, such adjustment to be effected, to the extent necessary (but only to the extent necessary), as follows:
| (i) | first, by reducing the amount or rate of interest required to be paid to the Lender; and |
| (ii) | thereafter, by reducing any fees, commissions, premiums and other amounts required to be paid to the Lender which would constitute interest for purposes of section 347 of the Criminal Code (Canada). |
| (g) | Calculation of Interest Rate. EACH OF THE LOAN PARTIES CONFIRMS THAT IT FULLY UNDERSTANDS AND IS ABLE TO CALCULATE THE RATE OF INTEREST APPLICABLE TO EACH OF THE LOANS AND OTHER SECURED OBLIGATIONS BASED ON THE METHODOLOGY FOR CALCULATING PER ANNUM RATES PROVIDED FOR IN THIS AGREEMENT. Lender agrees that if requested in writing by a Loan Party it shall calculate the nominal and effective per annum rate of interest on any Advance or other Secured Obligations outstanding at any time and provide such information to such Loan Party promptly following such request; provided that any error in any such calculation, or any failure to provide such information on request, shall not relieve such Loan Party of any of its obligations under this Agreement or any other Financing Document, nor result in any liability to the Lender. EACH LOAN PARTY HEREBY IRREVOCABLY AGREES NOT TO PLEAD OR ASSERT, WHETHER BY WAY OF DEFENCE OR OTHERWISE, IN ANY PROCEEDING RELATING TO THE FINANCING DOCUMENTS, THAT THE INTEREST PAYABLE UNDER THE FINANCING DOCUMENTS AND THE CALCULATION THEREOF HAS NOT BEEN ADEQUATELY DISCLOSED TO THE LOAN PARTIES, WHETHER PURSUANT TO SECTION 4 OF THE INTEREST ACT (CANADA) OR ANY OTHER APPLICABLE LAW OR LEGAL PRINCIPLE. |
Section 3.03[Intentionally Deleted]
Section 3.04Voluntary Prepayment
| (a) | Voluntary Prepayment of Credit Facilities. The Borrower may at any time and from time to time repay, without penalty, to the Lender the whole or any part of any Advance owing by it, together with accrued interest thereon to the date of prepayment; provided that: |
| (i) | the Borrower shall deliver notice to the Lender not later than 3:00 p.m. (Toronto time) three (3) Business Days prior to the proposed prepayment date; |
| (ii) | the proposed prepayment date is a Business Day; and |
| (iii) | voluntary prepayments shall be in minimum amount equal to $100,000 and in integral multiples of $50,000. |
- 29 -
Section 3.05Mandatory Prepayments.
| (a) | Change of Control. Upon the occurrence of a Change of Control, Borrower shall prepay the Advances in full, together with accrued and unpaid interest thereon, together with all other amounts payable under this Agreement, and Lender shall cease to have any commitment to make Advances under this Agreement. |
| (b) | Borrowing Base Excess. If the Lender determines that, on any day, Advances under the Revolving Credit Facility exceed the Borrowing Base as reported on pursuant to Section 6.11, the Borrower shall repay such excess amount within one (1) Business Day of notice by the Lender. |
Section 3.06Application of Prepayments.
Except as otherwise provided in this Agreement, prepayments pursuant to this Article III shall be applied as follows:
| (a) | First, to pay outstanding fees or Lender Expenses; |
| (b) | Second, to prepay accrued and unpaid interest accrued on the Revolving Credit Facility; |
| (c) | Third, to prepay outstanding principal outstanding in respect of the Revolving Credit Facility but without a permanent reduction thereof; |
| (d) | Fourth, to the payment in full in cash of all other Secured Obligations; and |
| (e) | Fifth, to whomsoever is legally entitled thereto or as a court of competent jurisdiction may direct. |
Section 3.07[Intentionally Deleted]
Section 3.08Payments Generally.
All payments to be made by any Loan Party shall be made without condition or deduction for any counterclaim, defense, recoupment or setoff. Except to the extent otherwise provided herein or in any other Financing Document, the Borrower shall make all payments of principal, interest and other amounts to be made by any Loan Party under the Financing Documents in immediately available funds, without deduction, set-off or counterclaim, to Lender at Lender’s Account (except as otherwise expressly provided in this Agreement), prior to 3:00 p.m. Toronto, Ontario time on the date when due. Any amounts received after such time on any date may, in the discretion of Lender, be deemed to have been received on the next succeeding Business Day for purposes of calculating interest thereon. All amounts owing under any Financing Document are payable in Canadian Dollars unless otherwise specified herein. Except to the extent otherwise provided herein, if any payment under any Financing Document shall be due on a day that is not a Business Day, the date for payment shall be extended to the next succeeding Business Day and interest thereon shall be payable for the period of such extension.
- 30 -
Section 3.09Change in Law and Increased Costs
| (a) | If any Change in Law shall: |
| (i) | impose, modify or deem applicable any reserve, special deposit, additional capital, compulsory loan, insurance charge or similar requirement against assets of, deposits with or for the account of, or credit extended by, the Lender; |
| (ii) | subject the Lender to any Taxes (other than (A) Indemnified Taxes or (B) Taxes described in clauses (b) through (d) of the definition of Excluded Taxes) on its loans, loan principal, letters of credit, commitments, or other obligations, or its deposits, reserves or other liabilities or capital attributable thereto; or |
| (iii) | impose on the Lender any other condition, cost or charge (other than Taxes) affecting this Agreement or any Advance hereunder; |
and the result of any of the foregoing shall be to increase the cost to the Lender of making, continuing, converting to or maintaining any Advance (or of maintaining its obligation to make any such Advance) or to reduce the amount of any sum received or receivable by the Lender hereunder (whether of principal, interest or otherwise), then from time to time Borrower will pay to the Lender such additional amount or amounts as will compensate the Lender for such additional costs incurred or reduction suffered.
| (b) | If the Lender determines that any Change in Law affecting the Lender or any of its lending offices or its holding company, if any, regarding capital or liquidity requirements, has or would have the effect of reducing the rate of return on the Lender’s capital or on the capital of its holding company, if any, as a consequence of this Agreement, the Revolving Credit Facility, its commitment hereunder or the Advances made by the Lender, to a level below that which the Lender or its holding company would have achieved but for such Change in Law (taking into consideration the Lender’s policies and the policies of its holding company with respect to capital adequacy or liquidity, as applicable), then from time to time the Borrower will pay to the Lender in Canadian Dollars such additional amount or amounts as will compensate the Lender or its holding company for any such reduction suffered. |
| (c) | A certificate of the Lender setting forth the amount or amounts necessary to compensate the Lender as specified in clause (a) or (b), as applicable, shall be delivered to Borrower, and any such certificate shall include a brief description of the Change in Law and a calculation of the amount or amounts necessary to compensate the Lender and shall, absent manifest error, be prima facie evidence of the amount of such compensation. In determining such amount, the Lender may use any reasonable method of averaging and attribution that it shall deem applicable. |
- 31 -
Borrower shall pay the Lender the amount shown as due on any such certificate within 30 days after receipt thereof.
| (d) | Failure or delay on the part of the Lender to demand compensation pursuant to this section shall not constitute a waiver of the Lender’s right to demand such compensation. |
Section 3.10Taxes
| (a) | Any and all payments by or on account of any obligation of a Loan Party under any Financing Document shall be made without deduction or withholding for any Taxes, except as required by Applicable Law. If any Applicable Law (as determined in the good faith discretion of the applicable Loan Party) requires the deduction or withholding of any Tax from any such payment by such Loan Party, then the applicable Loan Party shall be entitled to make such deduction or withholding and shall timely pay the full amount deducted or withheld to the relevant Governmental Authority in accordance with Applicable Law and, if such Tax is an Indemnified Tax, then the sum payable by such Loan Party shall be increased as necessary so that after such deduction or withholding has been made (including such deductions and withholdings applicable to additional sums payable under this Section 3.10) the Lender receives an amount equal to the sum it would have received had no such deduction or withholding been made. |
| (b) | Payment of Other Taxes by the Borrower: The Loan Parties shall pay to the relevant Governmental Authority in accordance with Applicable Law, or at the option of the Lender timely reimburse it for the payment of, any present or future stamp, court or documentary, intangible, recording, filing or similar Taxes that arise from any payment made under, from the execution, delivery, performance, enforcement or registration of, from the receipt or perfection of a security interest under, or otherwise with respect to, any Financing Document, except any such Taxes imposed as a result of a present or former connection between an assignee and the jurisdiction imposing such Taxes (other than a connection arising from an assignee having executed, delivered, become a party to, performed its obligations under, received payments under, received or perfected a security interest under, engaged in any transaction pursuant to or enforced any Financing Document) with respect to an assignment of this Agreement (other than an assignment requested by a Loan Party). |
| (c) | Indemnification by the Loan Parties. The Loan Parties shall, jointly and severally, indemnify the Lender, within 10 days after written demand therefor, for the full amount of any Indemnified Taxes (including Indemnified Taxes imposed or asserted on or attributable to amounts payable under this Section 3.10) payable or paid by the Lender or required to be withheld or deducted from a payment to the Lender and any reasonable expenses arising therefrom or with respect thereto, whether or not such Indemnified Taxes were correctly or legally imposed or asserted by the relevant Governmental Authority. A certificate as to the amount of |
- 32 -
such payment or liability delivered to the applicable Loan Party by the Lender shall be conclusive, absent manifest error.
| (d) | Evidence of Payments. As soon as practicable after any payment of Taxes by any Loan Party to a Governmental Authority pursuant to this Section 3.10, such Loan Party shall deliver to the Lender the original or certified copy of a receipt issued by such Governmental Authority evidencing such payment or other evidence of such payment reasonably satisfactory to the Lender. |
| (e) | Status of Lender. If the Lender is entitled to an exemption from or reduction of withholding Tax with respect to payments made under any Financing Document, it shall deliver to the applicable Loan Party, at the time or times reasonably requested in writing by such Loan Party, such properly completed and executed documentation so requested as will permit such payments to be made without withholding or at a reduced rate of withholding. In addition, the Lender, if reasonably requested in writing by any Loan Party, shall deliver such other documentation prescribed by Applicable Law or so requested by such Loan Party as will enable such Loan Party to determine whether or not the Lender is subject to backup withholding or information reporting requirements. Notwithstanding anything to the contrary in the preceding two sentences, the completion, execution and submission of such documentation shall not be required if in the Lender’s reasonable judgment such completion, execution or submission would subject such Lender to any material unreimbursed cost or expense or would materially prejudice the legal or commercial position of the Lender. |
| (f) | Treatment of Certain Refunds. If, following the imposition of any Taxes on any payment by any Loan Party to the Lender in respect of which any Loan Party is required to make an additional payment pursuant to this Section 3.10, the Lender receives a refund of Taxes as to which it has been reimbursed, made whole or indemnified pursuant to this Section 3.10, the Lender will reimburse the Loan Parties an amount equal to such refund, but only to the extent of indemnity payments made or additional amounts paid by the Loan Parties under this Section 3.10 with respect to Taxes giving rise to such refund, net of all out of pocket expenses (including Taxes) of the Lender and without interest (other than any net after-tax interest paid by the relevant Governmental Authority with respect to such refund). Notwithstanding anything to the contrary in this Section 3.10, in no event will the Lender be required to pay any amount to the Loan Parties pursuant to this Section 3.10, the payment of which would place the Lender in a less favorable net after-tax position than the Lender would have been in if the indemnification payments or additional amounts giving rise to such refund had never been paid. Each Loan Party, upon the request of the Lender, agrees to repay to the Lender the amount paid over to the Loan Parties (plus any penalties, interest or other charges imposed by the relevant Governmental Authority) if the Lender is required to repay such refund or reduction to such Governmental Authority. This Section 3.10 shall not be construed to require the Lender to make available its tax returns (or any other information relating to its taxes which it deems confidential) to the Loan Parties or any other Person. |
- 33 -
Section 3.11Illegality
If Lender determines that any Law has made it unlawful, or that any Governmental Authority has asserted that it is unlawful, for Lender or its applicable lending office to make or maintain any Advance (or to maintain its obligation to make any Advance), or to determine or charge interest rates based upon any particular rate, then, on notice thereof by such Lender to Borrower, any obligation of such Lender with respect to the activity that is unlawful shall be suspended until Lender notifies Borrower that the circumstances giving rise to such determination no longer exist. Upon receipt of such notice, Borrower shall, upon demand from Lender, prepay in order to avoid the activity that is unlawful. Upon any such prepayment, Borrower shall also pay accrued interest on the amount so prepaid or converted. Lender agrees to designate a different lending office if such designation will avoid the need for such notice and will not, in the good faith judgment of Lender, otherwise be materially disadvantageous to Lender.
ARTICLE IV.SECURITY
Section 4.01Security Documents.
As general and continuing security for the due payment and performance of the Secured Obligations, the security described below was (or shall be) granted to the Lender under this Agreement:
| (a) | a pledge and general security agreement from Borrower, Parent and 275 Ontario governed by the laws of Ontario and granting security over all of Borrower’s and Parent’s personal property and undertaking in Canada dated as of November 3, 2022 (the “Canadian Security Agreement”); |
| (b) | a Guarantee by Parent, and the other Guarantors governed by the laws of Ontario guaranteeing the Secured Obligations dated as of November 3, 2022 (the “Canadian Guarantee”); |
| (c) | a global assignment agreement from PM Germany governed by the laws of Germany and granting security over PM Germany’s intragroup receivables and trade receivables dated as of December 2, 2022 (the “German Global Assignment Agreement”); |
| (d) | a pledge agreement from PM Germany governed by the laws of Germany and granting security over PM Germany’s accounts balances dated as of December 6, 2022 (the “German Account Pledge Agreement”); |
| (e) | a security confirmation agreement from PM Germany governed by the laws of Germany regarding the German Global Assignment Agreement dated as of the Closing Date (the “German Security Confirmation Agreement”); |
| (f) | a junior ranking pledge agreement (the “German Junior Pledge Agreement” and collectively with the German Account Pledge Agreement, the German Global Assignment Agreement and German Security Confirmation Agreement, the “German Security Agreements”) dated as of the Closing Date from PM Germany |
- 34 -
governed by the laws of Germany and granting security over PM Germany’s accounts balances;
| (g) | a pledge and security agreement from the Borrower and PM USA governed by the laws of New York and granting security over (i) all of the Borrower’s shares in PM USA and (ii) PM USA’s personal property and undertaking in the United States of America dated as of November 3, 2022 (the “USA Security Agreement”); |
| (h) | intellectual property security agreement for all Canadian Intellectual Property dated as of November 3, 2022 and dated as of the Closing Date (collectively, the “Canadian IP Security Agreements”); |
| (i) | intellectual property security agreements for all United States Intellectual Property dated as of November 3, 2022 and dated as of the Closing Date (collectively, the “US IP Security Agreements”); |
| (j) | a Collateral Access Agreement for the Borrower’s Mississauga, Ontario location dated as of November 3, 2022; |
| (k) | a Control Agreement for the Deposit Account with JPMorgan Chase Bank, N.A. dated December 15, 2022; and |
| (l) | any other Security Documents reasonably requested by the Lender. |
Section 4.02Control of Collateral; Control Agreements.
Each Loan Party shall maintain all Deposit Accounts (i) within Canada and the United States in accordance with Section 6.13, and if within the United States, subject to a Control Agreement and (ii) outside of Canada and the United States with financial institutions that are acceptable to the Lender and subject to a Control Agreement (or, if required, similar security instruments) unless such Deposit Accounts are Excluded Accounts. For clarity, all accounts in the United States shall require a Control Agreement unless they are held with the same legal entity as the Lender (and not an Affiliate thereof). If from time to time any Collateral consists of property or rights of any Loan Party in which the perfection or priority of Lender’s security interest is dependent upon Lender’s gaining control of such Collateral (including Securities Accounts or other Investment Property), Borrower shall notify Lender and deliver the appropriate Control Agreements, and each Loan Party shall do the same with respect to any property securing the applicable Guarantee. No Control Agreement in respect of any Deposit Account, Securities Accounts or other Investment Property shall be modified by any Loan Party without the prior written consent of Lender.
Section 4.03Guarantors
If at any time Borrower owns, establishes or acquires a wholly-owned Material Subsidiary (including without limitation any wholly-owned Subsidiary that exists on the Closing Date and becomes a Material Subsidiary), Borrower shall immediately cause such Subsidiary to become a Guarantor hereunder, and deliver such Guarantee and other Security Documents so as to reflect and replicate the security delivered by Borrower on the Closing Date and the intent of Section 4.05(b).
- 35 -
The Borrower shall also deliver or cause the delivery of a pledge of all of the issued and outstanding equity of such new Guarantor concurrently therewith, together with any certificates representing such equity and corresponding powers of attorney in respect of such certificates.
Section 4.04Real Estate
Borrower shall:
| (a) | notify the Lender within ten Business Days of any Loan Party acquiring any freehold, leasehold, licence or other similar interest in real property after the Closing Date; |
| (b) | in the case of any freehold interest in real property located in Canada, Europe or the United States with a value in excess of $350,000, grant a fixed charge to the Lender on any such real property interest (together with any title opinions or title insurance relating thereto in form and substance acceptable to the Lender); and |
| (c) | in the case of any leasehold interest in real property, use commercially reasonable efforts to obtain a Collateral Access Agreement if requested by the Lender. |
Section 4.05Delivery of Additional Documentation Required.
| (a) | At any time upon the written request of Lender, each Loan Party shall execute and deliver to Lender, any and all security agreements, pledges, assignments, endorsements of certificates of title, bailee acknowledgments and all other documents (the “Additional Documents”) that Lender may request in its sole discretion, each in form and substance satisfactory to Lender, to maintain perfection and protect the priority of Lender’s Liens in the Collateral (whether now owned or hereafter arising or acquired), to create and perfect Liens in favor of Lender in any real property acquired after the Closing Date, and in order to fully consummate all of the transactions contemplated hereby and under the other Financing Documents. Each Loan Party authorizes Lender to execute any such Additional Documents in such Loan Party’s name and authorize Lender to file such executed Additional Documents in any appropriate filing office. |
| (b) | It is the intention of the parties hereunder that Borrower and each Material Subsidiary shall grant security over all present and after-acquired personal property and material real estate described in Section 4.05 in the applicable jurisdiction(s) as determined by the Lender, and agree to promptly deliver all to the Lender all Additional Documents that may be required from time to time to effect such intention. |
Section 4.06Right to Inspect.
Lender (through any of its officers, employees, or agents) shall have the right, from time to time hereafter (but, provided no Event of Default has occurred and is continuing, not more than once per fiscal year), at reasonable times and upon reasonable notice, during business hours, to inspect the books and records of each Loan Party and to check, test, and appraise the Collateral in order to verify the Loan Parties’ financial condition or the amount, quality, value, condition of, or any other matter relating to, the Collateral.
- 36 -
ARTICLE V.REPRESENTATIONS AND WARRANTIES
Each Loan Party represents and warrants to Lender, on its own behalf and on behalf of each of its Subsidiaries, that:
Section 5.01Incorporation and Status.
Each Loan Party and each of its respective Subsidiaries is duly incorporated and organized, and is validly existing under its jurisdiction of organization. Such Loan Party and/or Subsidiary is duly registered, licensed or qualified as a foreign corporation, and is up to date in the filing of all corporate and similar returns and is in good standing under the laws of those jurisdictions where it operates and where failure to be so registered, licensed, qualified or up to date would have a Material Adverse Effect on it. For the purposes of Regulation (EU) 2015/848 of the European Parliament and of the Council of 20 May 2015 on insolvency proceedings (recast), the Centre of Main Interests of each Loan Party incorporated in a member state of the European Union is situated in its jurisdiction of organization and each Loan Party incorporated in a member state of the European Union has, and at all times during the three (3) month period prior, had, no Establishment in any other jurisdiction.
Section 5.02Corporate Power and Due Authorization.
Each Loan Party has the corporate power and capacity to enter into, and to perform its obligations under this Agreement and the Financing Documents, to which it is a party, as applicable. Each of this Agreement and the Financing Documents to which it is a party has been duly authorized, executed and delivered by such Loan Party (including, without limitation, receipt of all requisite board, stockholder and member approvals) and is a valid and binding obligation of such Loan Party enforceable in accordance with its terms, subject to the usual exceptions as to bankruptcy and the availability of equitable remedies.
Section 5.03Business of the Loan Parties.
Each Loan Party and each of its respective Subsidiaries has the corporate or other applicable power and capacity to own, lease or license its assets and to carry on its business.
Section 5.04No Contravention.
Neither the entering into and performing under this Agreement or the Financing Documents or the performance by each Loan Party of any of its respective obligations hereunder or thereunder will contravene, breach, result in any default or result in any acceleration, bonus or benefit to any other party under Governing Documents of such Loan Party or under any mortgage, lease, license agreement, supply agreement, contract, agreement, other legally binding instrument, license, permit, Regulatory Required Permit, statute, regulation, order, judgment, decree or law to which it is a party or by which it may be bound.
- 37 -
Section 5.05Not Insolvent.
No Loan Party, no Subsidiary nor any of their predecessors where applicable (a) has committed any act of bankruptcy or initiated or taken steps to initiate any Insolvency Proceeding, (b) (i) is insolvent, (ii) is unable for any reason to unable to meet its obligations as they generally become due, (iii) has ceased paying its current obligations or (iv) is a Person the aggregate of whose property is not, at fair valuation, sufficient, or, if disposed of at a fairly conducted sale under legal process, would not be sufficient to pay all its obligations due and accruing due, (c) has proposed, or given notice of its intention to propose, a compromise or arrangement to its creditors generally, including pursuant to any Insolvency Legislation, or (d) has any petition for a receiving order in bankruptcy filed against it, made a voluntary assignment in bankruptcy, taken any proceeding with respect to any compromise or arrangement, taken any proceeding to have itself declared bankrupt or wound-up, taken any proceeding to have a receiver appointed of any part of its assets, has had any encumbrancer take possession of any of its property.
Section 5.06Approvals and Consents.
No authorization, consent or approval of, or filing with or notice to, any governmental agency, regulatory body, court or other person is required in connection with the execution, delivery or performance and compliance with the terms of this Agreement and the Financing Documents to which any Loan Party is a party nor will such performance and compliance contravene any statute, rule or regulation binding on any Loan Party.
Section 5.07Welfare and Pension Plans.
Each Loan Party and each Subsidiary has adopted all Welfare Plans required by Law and each of such plans has been administered, registered, funded, invested and maintained in compliance with its terms and such Laws in all material respects including, without limitation, all requirements relating to employee participation, funding, investment of funds, benefits and transactions with Loan Parties and persons related to them. No Loan Party nor any Subsidiary has any liability with respect to any post-retirement benefit (a) to any current or former employee, or (b) under a Welfare Plan (except, in each case, as set forth on Schedule 5.07). No Loan Party nor any Subsidiary maintains or contributes to, is required to maintain or contribute to, is a party to, is bound by, or has any liability or contingent liability under, a Pension Plan. There are no actions, claims or proceedings existing, pending or threatened against any Pension Plan or the assets of any such plan which could be reasonably expected to have a Material Adverse Effect.
Section 5.08Changes Since Date of Financial Statements.
No Loan Party nor any Subsidiary is a party to nor is bound by any contract, agreement or commitment that materially adversely affects or could reasonably be expected to materially adversely affect such Loan Party’s business or its financial condition or any of its assets. Additionally, since December 31, 2023, no event has (or would reasonably be expected to) give rise to a Material Adverse Effect, and no Loan Party nor any Subsidiary has (except as set forth on Schedule 5.08):
| (a) | other than in the ordinary course and consistent with past practices of the Loan Parties, sold, transferred or otherwise disposed of, or created, assumed or permitted |
- 38 -
any encumbrance on or in respect of, its property or assets or any part thereof other than Permitted Encumbrances;
| (b) | incurred, assumed or become subject to any material liability except in the ordinary course of business; |
| (c) | suffered a change in assets, liabilities, financial condition, prospects or operations of the Loan Parties, from that reflected in the financial statements as of the date set forth above, other than changes in the ordinary course, which individually or in the aggregate has had or could reasonably be expected to have a Material Adverse Effect; |
| (d) | suffered any damage, destruction or loss, whether or not covered by insurance, which would materially and adversely affecting the properties, business or prospects or financial condition of the Loan Parties; |
| (e) | cancelled or released any debts or claims or waived or surrendered any rights which, in the aggregate, are material; |
| (f) | suffered the resignation or termination of any officer, key employee or group of employees thereof; |
| (g) | made any material change to any material compensation agreement or other Material Agreement with any employee, officer, director or stockholder; |
| (h) | assigned or granted any exclusive license or transferred any Material Intellectual Property or material Regulatory Required Permit; |
| (i) | made any change to any Material Agreement to which any Loan Party is a party or by which it is bound which materially and adversely affects the business, assets, liabilities, financial condition, operations or prospects of the Loan Parties; or |
| (j) | except as described in the Loan Parties’ consolidated financial statements, made any change in its accounting principles and practices as theretofore applied including, without limitation, the basis upon which its assets and liabilities are recorded on its books and its earnings, profits and losses are ascertained. |
Section 5.09No Default Under Agreements.
No Loan Party nor any Subsidiary is in default or breach of any Material Agreement to which it is a party which default or breach has had or could reasonably be expected to have a Material Adverse Effect, no event has occurred that after notice or the passage of time, or both, would constitute such a default or breach, and all Material Agreements are in good standing in all material respects. A list of all Material Agreements, including all material supply agreements, is set forth on Schedule 5.09.
- 39 -
Section 5.10Title to Assets.
The Loan Parties and their respective Subsidiaries are the absolute beneficial owners of and have good and marketable title, free of all charges except for Permitted Encumbrances, to all the material assets owned by them and used in connection with the Loan Parties’ business.
Section 5.11Financial Matters.
The audited consolidated financial statements of the Parent for the fiscal year ended 2023 and the unaudited consolidated financial statements of the Parent, for the periods through December 31, 2024 present fairly in all material respects the consolidated financial position of the Parent, on a consolidated basis as at the dates indicated and the results of their operations and changes in their financial position for the periods specified and reflect all material liabilities (absolute, accrued, contingent or otherwise) of the Parent, as of the dates thereof and such financial statements have been prepared in conformity with GAAP applied, except as otherwise stated therein, on a consistent basis.
Section 5.12No Material Adverse Change
Since December 31, 2023, there has been no Material Adverse Change.
Section 5.13Environmental Matters.
(a) The assets of each Loan Party, and its respective Subsidiaries, have not been used by such Loan Party or Subsidiary in the disposal of, or to produce, store, handle, treat, release, or transport, any Hazardous Materials, where such production, storage, handling, treatment, release or transport was in violation, in any material respect, of applicable Environmental Law, (b) to each Loan Party’s knowledge, no properties or assets of any Loan Party, or any of their respective Subsidiaries, has ever been designated or identified in any manner pursuant to any environmental protection statute as a Hazardous Materials disposal site, (c) no Loan Party, nor its respective Subsidiaries, has received notice that a Lien arising under any Environmental Law has attached to any revenues or to any Property owned or operated by a Loan Party or its respective Subsidiaries, and (d) no Loan Party, nor its respective Subsidiaries, has received a summons, citation, notice, or directive from any federal or state governmental agency concerning any action or omission by any Loan Party, or their respective Subsidiaries, resulting in the releasing or disposing of Hazardous Materials into the environment. As used herein, “Hazardous Materials” means (A) substances that are defined or listed in, or otherwise classified pursuant to, any Applicable Laws or regulations as “hazardous substances,” “hazardous materials,” “hazardous wastes,” “toxic substances,” or any other formulation intended to define, list, or classify substances by reason of deleterious properties such as ignitability, corrosivity, reactivity, carcinogenicity, reproductive toxicity, or “EP toxicity”, (B) oil, petroleum, or petroleum derived substances, natural gas, natural gas liquids, synthetic gas, drilling fluids, produced waters, and other wastes associated with the exploration, development, or production of crude oil, natural gas, or geothermal resources, (C) any flammable substances or explosives or any radioactive materials, and (D) asbestos in any form or electrical equipment that contains any oil or dielectric fluid containing levels of polychlorinated biphenyls in excess of 50 parts per million.
- 40 -
Section 5.14Assets in Good Condition.
All the material physical assets of each Loan Party and its respective Subsidiaries are in good operating condition and in a state of good maintenance and repair subject to the usual wear and tear for assets of that age.
Section 5.15Licenses and Agreements.
Each of the material licenses and agreements to which any Loan Party or any Subsidiary is a party is in good standing and in full force and effect, and neither a Loan Party nor a Subsidiary, nor to the best of the knowledge, information and belief of the Loan Parties, after due inquiry, any other party thereto, is in breach of any material covenants, conditions or obligations contained therein.
Section 5.16Tax Matters.
Each Loan Party and its respective Subsidiaries has filed all federal, provincial, state and other (including foreign) Tax returns which are required to be filed, and have paid all Taxes as shown on said returns, as well as all other Taxes to the extent that they have become due, unless being contested in good faith with appropriate reserves. All Tax liabilities of each Loan Party and each Subsidiary are adequately provided for on such Loan Party’s or Subsidiary’s books, including interest and penalties. No Tax liability has been asserted by taxing authorities for Taxes in excess of those already paid, and no taxing authority has notified any Loan Party, or any of their respective Subsidiaries, of any material deficiency in any federal, state and other Tax returns. Notwithstanding the foregoing, each of the representations set forth in this Section 5.16 shall be deemed to be satisfied where the potential liability does not exceed, and could not reasonably be expected to exceed, $15,000.00; provided that any such liability under such threshold known to the Loan Parties as of the Closing Date is set forth on Schedule 5.16 hereto.
Section 5.17Insurance.
Each Loan Party and its respective Subsidiaries maintains insurance for risks and in amounts customary for prudent companies in the Loan Parties’ or Subsidiary’s industry and owners of comparable assets with responsible insurers against such risks. No Loan Party, nor any of its respective Subsidiaries, is in default with respect to any of the material provisions contained in any current insurance policy or has failed to give any notice or pay any premium or present any unsettled claim under any current insurance policy in a due and timely fashion.
Section 5.18Intellectual Property.
The Loan Parties and their respective Subsidiaries own or possess all their Material Intellectual Property without any known conflict with, or infringement of, the rights of others. To the knowledge of the Loan Parties and their respective Subsidiaries, no Product or service marketed or sold (or proposed to be marketed or sold) by any Loan Party, or any of their respective Subsidiaries, violates or will violate any license or infringes or will infringe in any material manner any intellectual property rights of any other party. Schedule 5.18 lists (i) all applications and registrations of Intellectual Property of the Loan Parties and their Subsidiaries as of the date hereof and identifies which of such Intellectual Property is Material Intellectual Property as of the date hereof, (ii) all in-bound license or sublicense agreements for Material Intellectual Property to which a Loan Party is a party as of the date hereof, and (iii) all material out-bound license or sublicense agreements to which a Loan Party is a party as of the date hereof (in the case of (ii) and (iii) except for licenses of commercially available software or information technology services).
- 41 -
Other than as set forth on Schedule 5.18, or with respect to commercially available software products or information technology services, as of the date hereof there are no outstanding options, licenses, agreements, claims, encumbrances or shared ownership interests of any kind relating to the Material Intellectual Property owned by any Loan Party or Subsidiary, nor is any Loan Party or Subsidiary, bound by or a party to any options, licenses or agreements of any kind with respect to any patents, trademarks, service marks, trade names, copyrights, trade secrets, licenses, information, proprietary rights and processes of any other Person. With respect to any Material Intellectual Property that is a license agreement, Schedule 5.18 indicates as of the date hereof (A) the name of the licensor, (B) the name and date of the agreement pursuant to which such item of Material Intellectual Property is licensed, and (C) whether or not such license agreement grants an exclusive license to any Loan Party or Subsidiary. No Loan Party or Subsidiary has received any written communications alleging that such Loan Party or Subsidiary has violated or, by conducting its business, would violate any of the patents, trademarks, service marks, tradenames, copyrights, trade secrets, mask works or other proprietary rights or processes of any other Person. It will not be necessary to use any inventions of any of such Loan Party’s, or their respective Subsidiaries’, employees or consultants (or Persons it currently intends to hire) made prior to their employment by such Loan Party or Subsidiary. Each employee or consultant engaged in research and development has entered into an invention assignment agreement or similar agreement with such Loan Party or Subsidiary, with respect to all intellectual property rights he or she develops pursuant to his or her employment or engagement to the applicable Loan Party or Subsidiary. No Subsidiary that is not a Loan Party owns any Material Intellectual Property.
Section 5.19Permits.
The Loan Parties, and their respective Subsidiaries, hold all permits (including Regulatory Required Permits), licenses, approvals, consents, authorizations, registrations, certificates and franchises of governmental agencies or regulatory bodies required to own its properties and assets and to carry on its business, except for any of the foregoing where the failure to hold the same would not have a Material Adverse Effect (the “Permits”), and a list of all such Permits is set forth on Schedule 5.19. All the Permits are in full force and effect; each Loan Party, and their respective Subsidiaries, is in compliance in all material respects with all the terms and conditions relating to the Permits; and there are no proceedings in progress, pending or, to such Loan Party’s or Subsidiary’s knowledge, threatened, that may result in revocation, cancellation, suspension, rescission or any adverse modification of any of the Permits nor, to the best of the knowledge, information and belief of such Loan Party or Subsidiary, after due inquiry, are there any facts upon which proceedings could reasonably be based.
Section 5.20Regulatory Required Permits.
With respect to any Product or service of any Loan Party or its respective Subsidiaries, (i) each Loan Party or Subsidiary has received, and such Product or service is the subject of, all Regulatory Required Permits needed in connection with the testing, manufacture, marketing or sale of such Product or conduct of such service as currently being conducted by or on behalf of such Loan Party or Subsidiary, and (ii) such Product is being tested, manufactured, marketed or sold, as the case may be, in material compliance with all Applicable Laws and Regulatory Required Permits, and such Loan Party or Subsidiary has not received any notice from any applicable Regulatory Authority or any other Governmental Authority that such Regulatory Authority or Governmental Authority is conducting a material investigation or review of such Loan Party or Subsidiary.
- 42 -
Section 5.21Compliance with Laws and Litigation.
(a) |
Each Loan Party and its respective Subsidiaries is each conducting its business in compliance with all Applicable Laws, including for greater certainty all Healthcare Laws, of each jurisdiction in which its business is carried on, except where the failure to be in such compliance would not have a Material Adverse Effect; and |
(b) |
There is no court, administrative, regulatory or similar proceeding (whether civil, quasi-criminal or criminal); arbitration or other dispute settlement procedure; investigation or enquiry by any governmental, administrative, regulatory or similar body; or any similar matter or proceeding (collectively, “Proceedings”) against or involving any Loan Party or its Subsidiaries or any of their officers or directors (whether in progress, pending or, to the best of the knowledge, information and belief of such Loan Party or Subsidiary, after due inquiry, threatened); no event has occurred that might give rise to any Proceedings and such Loan Party or Subsidiary is not aware of any existing grounds on which such Proceedings might be commenced and there is no judgment, decree, injunction, rule, award or order of any court, government department, board, commission, agency, arbitrator or similar body outstanding against such Loan Party or Subsidiary or its officers or directors. |
Section 5.22Material Facts Disclosed.
None of the statements, documents, certificates or other items prepared or supplied by any Loan Party, or their respective Subsidiaries, with respect to the transactions contemplated hereby contains an untrue statement of a material fact, or fails to disclose a fact that is necessary to be made in order for any material statement not to be misleading.
Section 5.23No Rights to Acquire Assets.
No person has any agreement or option or right or privilege (whether pre-emptive or contractual) capable of becoming an agreement for the purchase of any assets of any Loan Party, or Subsidiary.
Section 5.24No Rights to Provide Financial Advisory Services.
No person has any agreement or option, or right or privilege (whether pre-emptive or contractual) capable of becoming an agreement for the provision of financial advisory services to any Loan Party or Subsidiary.
- 43 -
Section 5.25Chief Executive Office and Location of Assets.
There is no location at which any Loan Party has any assets (except for Products in transit) other than those locations listed on Schedule 5.25. The chief executive office, registered office, principal place of business and the place where each Loan Party keeps its books and records is located and specifically identified on Schedule 5.25. The locations of any assets stored with a bailee or similar entity are located on Schedule 5.25.
Section 5.26Minute Books.
The minute books, stock certificate books and stock transfer ledgers of each Loan Party are true, correct, complete and up-to-date and contain the minutes of all meetings, and all resolutions, of the board of directors thereof (and all committees thereof) and shareholders thereof, except that minutes of the most recent meeting of the directors may not be included or may be included in such minute books in draft form.
Section 5.27Use of Proceeds; Margin Stock.
The proceeds of any of the Advances will be used solely as provided in this Agreement, and none of such proceeds will be used (i) for the purpose of purchasing or carrying any “margin stock” as defined in Regulations T, U or X of the Board of Governors of the Federal Reserve System (12 C.F.R. Parts 220, 221 and 224), (ii) for the purpose of maintaining, reducing or retiring any indebtedness which was originally incurred to purchase or carry a “margin stock,” or (iii) for any other purpose which might constitute this transaction a “purpose credit” within the meaning of Regulations T, U or X. No Loan Party, nor their respective Subsidiaries, is engaged in the business of extending credit for the purpose, whether immediate, incidental or ultimate, of buying or carrying “margin stock.” No Loan Party nor any Person acting on behalf of any Loan Party has taken or will take any action which might cause any of the Financing Documents to violate Regulations T, U or X, or any other regulations of the Board of Governors of the Federal Reserve System or to violate the Securities Exchange Act of 1934, as amended, or any rule or regulation thereunder, in each case as now in effect or as the same may hereafter be in effect.
Section 5.28Investment Company Act.
No Loan Party or Subsidiary is or is required to be registered as an “investment company” under the Investment Company Act of 1940.
Section 5.29Bank Accounts.
Schedule 5.29 lists, as of the Closing Date, all banks and other financial institutions at which any Loan Party maintains Deposit Account or Securities Accounts, and such Schedule correctly identifies the name and address of each depository or intermediary, the name in which such account is held, a description of the purpose of the account and the complete account number of such account.
- 44 -
Section 5.30Shares and Corporate Structure.
| (a) | The corporate structure of the Loan Parties and their Subsidiaries and shareholders is, as at the Closing Date, as set out in Schedule 5.30, which Schedule contains the authorized capital of the Parent, Borrower and their respective Subsidiaries and the number of issued and outstanding shares and the beneficial owners thereof at such time. Each of the Subsidiaries is, directly or indirectly, wholly-owned by one or more a Loan Party or a Subsidiary. No Loan Party, nor their respective Subsidiaries, is a participant in any joint venture, partnership, limited liability company or similar arrangement. Except as set forth on Schedule 5.30, since its inception, no Loan Party has consolidated or merged with, or acquired assets outside the ordinary course of, or acquired the stock of or any interest in any Person. |
| (b) | No Person (other than the Lender) will have an agreement or option or any other right or privilege (whether by law, pre-emptive or contractual) capable of becoming an agreement or option, including convertible securities, warrants or convertible obligations of any nature, for the purchase, subscription, allotment or issuance of any unissued shares in the capital of the Borrower except as described in Schedule 5.30. |
| (c) | To the extent applicable and subject to satisfaction of all obligations pursuant to Section 6.25, each Loan Party has full power and authority to create a lien on the Shares and no disability or contractual obligation or restriction exists, that would prohibit such Loan Party from pledging the Shares pursuant to this Agreement or the Security Agreements. There are no subscriptions, warrants, rights of first refusal or other restrictions on transfer relative to, or options exercisable with respect to the Shares. The Shares have been and will be duly authorized and (to the extent such concepts are applicable under the laws under which the applicable entity was formed) validly issued, fully paid and non-assessable. The Shares are not the subject of any present or threatened (in writing) suit, action, arbitration, administrative or other proceeding, and no Loan Party knows of any reasonable grounds for the institution of any such proceedings. |
Section 5.31Liabilities.
No Loan Party, nor their respective Subsidiaries, has any material liabilities and, to the best of its knowledge no material contingent liabilities, not disclosed in the financial statements of the Parent, except (i) current liabilities incurred in the ordinary course of business to the date of the financial statements, which have not been, either in any individual case or in the aggregate, materially adverse and (ii) liabilities of a type not required by GAAP to be reflected in financial statements.
Section 5.32Employee Matters.
No Loan Party nor any Subsidiary is a party to or bound by any currently effective employment contract, deferred compensation arrangement, bonus plan, incentive plan, profit sharing plan, retirement agreement or other employee compensation plan or agreement, except as set forth on Schedule 5.32.
- 45 -
No employee or former employee of a Loan Party or Subsidiary has been granted the right to continued employment by such Loan Party or Subsidiary or to any material compensation following termination of employment with such Loan Party or Subsidiary, except as set forth on Schedule 5.32. To each Loan Party’s knowledge, no employee of any Loan Party, or their respective Subsidiaries, nor any consultant with whom such Loan Party, or their respective Subsidiaries, has contracted, is in violation of any term of any employment contract, proprietary information agreement or any other agreement relating to the right of any such individual to be employed by, or to contract with, such Loan Party or Subsidiary; and to each Loan Party’s knowledge the continued employment by such Loan Party or Subsidiary of its respective present employees, and the performance of such Loan Party’s or Subsidiary’s contracts with its independent contractors, will not result in any such violation, and no such Loan Party or Subsidiary has received any notice alleging that such violation has occurred. No Loan Party, nor any of its Subsidiaries, is aware that any officer, key employee or group of employees of such Loan Party or Subsidiary intends to terminate his, her or their employment with any Loan Party, or any of their respective Subsidiaries, as applicable, nor does any Loan Party, or any of their respective Subsidiaries, have a present intention to terminate the employment of any such officer, key employee or group of employees. Each former employee of each Loan Party, and their respective Subsidiaries, whose employment was terminated by such Loan Party or Subsidiary has entered into an agreement with such Loan Party or Subsidiary providing for the full release of any claims against such Loan Party or Subsidiary, or any related party arising out of such employment. There are no actions pending, or to such Loan Party’s or Subsidiary’s knowledge, threatened, by any former or current employee concerning such person’s employment by such Loan Party or Subsidiary. Each of the Loan Parties and each of their respective Subsidiaries is in compliance in all material respects with all Laws respecting labour and employment terms, conditions and practices.
Section 5.33Non-Arm’s Length Transactions
All agreements, arrangements or transactions between any Loan Party or their respective Subsidiaries, on the one hand, and any Affiliate of or other Person not dealing at arm’s length with the Borrower (other than ordinary course arrangements with any employee, management or director of the Borrower), on the other hand, in existence as of the Closing Date are set forth on Schedule 5.33.
Section 5.34Description of Real Property
Schedule 5.34 contains a description as of the Closing Date of (a) all real property owned by the Loan Parties (including municipal addresses, legal description (to the extent available), a brief description of such property and its use), and (b) all real property leased by the Loan Parties (including municipal addresses, legal description (to the extent available), the name of the landlord, the term and any renewal rights under the applicable lease and a brief description of such property and its use).
Section 5.35Sanctions
| (a) | No part of the proceeds of any Drawdown will be used directly or, to the knowledge of the Borrower, any other Loan Party, or any of their respective Subsidiaries, |
- 46 -
indirectly, to fund any operations in, finance or facilitate any investments, activities, business or transaction with, or make any payments to, a Sanctioned Person or in any country or territory, that, at the time of such funding, is, or whose government is, the subject of Sanctions in any manner that would result in any violation by any Person (including the Lender) of (i) any Sanctions; or (ii) any applicable regulations, rules or executive orders issued or administered by any Sanctions Authority.
| (b) | None of the Loan Parties, any of their respective Subsidiaries, or any director, officer or employee of the Loan Parties (i) is or will become a Sanctioned Person; or (ii) knowingly engages or will engage in any dealings or transactions, or is or will be otherwise knowingly associated, with any Sanctioned Person that would result in any violation of (A) any Sanctions, or (B) applicable regulations, rules or executive orders issued or administered by any Sanctions Authority. |
| (c) | Each of the Loan Parties and their respective Subsidiaries has implemented, and maintains in effect, policies and procedures designed to ensure compliance by the Loan Parties, their respective Subsidiaries, their respective directors, officers, employees and agents with all Sanctions and all applicable regulations, rules or executive orders issued or administered by any Sanctions Authority. |
Section 5.36Anti-Terrorism and Anti-Money Laundering Laws
| (a) | Each of the Loan Parties, their Subsidiaries and their respective directors, officers, employees and agents is, and has conducted its business, in compliance with Anti-Terrorist Financing and Anti-Money Laundering Laws. |
| (b) | No part of the proceeds of the Drawdowns, use of proceeds, or other transactions contemplated by this Agreement will violate Anti-Terrorist Financing and Anti-Money Laundering Laws. |
| (c) | None of the Loan Parties, their Subsidiaries or their respective directors, officers, employees or agents, is the subject to any investigation, inquiry or enforcement proceedings by any Governmental Authority regarding any offence or alleged offence under any Anti-Terrorist Financing and Anti-Money Laundering Laws, and no such investigation, inquiry or proceeding is pending or has been threatened. |
| (d) | Each of the Loan Parties and their respective Subsidiaries has implemented, and maintains in effect, policies and procedures designed to ensure compliance by the Loan Parties, their respective Subsidiaries, their respective directors, officers, employees and agents with Anti-Terrorist Financing and Anti-Money Laundering Laws. |
- 47 -
ARTICLE VI.COVENANTS
For as long as the Secured Obligations remain outstanding or Lender has any commitment to extend credit, each Loan Party agrees as follows:
Section 6.01Use of Proceeds.
The Borrower shall only use the proceeds of the Advances (i) on the Closing Date, to repay the Term Loan and (ii) thereafter, for general corporate and working capital purposes.
Section 6.02Payment of Principal and Interest; Secured Obligations.
Each of the Loan Parties will duly and punctually pay the Secured Obligations, including, without limitation, the principal and interest accrued on the Advances at the time and in the manner specified herein.
Section 6.03Lender Expenses.
Each of the Loan Parties shall pay all Lender Expenses on demand.
Section 6.04Compliance with Laws; Permits; Corporate Existence.
Each of the Loan Parties shall (and shall cause its Subsidiaries, if any, to) maintain its corporate existence (other than in accordance with Section 6.16(d) or Section 6.16(e)), carry on and conduct its business in a proper and business-like manner, take all reasonable action to maintain all rights, privileges and franchises necessary or desirable in the normal conduct of its business, including all Permits, and comply with all Applicable Laws, in each case in all material respects. Each of the Loan Parties shall also forthwith notify Lender in writing of the dissolution or wind-up of any existing Loan Party or Subsidiary or the creation or acquisition of any new Subsidiary after the date hereof (and whether such Subsidiary is a Material Subsidiary).
Section 6.05Delivery of Collateral and Perfection.
Each Loan Party shall take such actions at the sole cost and expense of such Loan Party, as may be required or desirable and as requested by Lender, to preserve, protect or perfect the security interests of Lender with respect to the Collateral.
Section 6.06Collateral.
Each Loan Party will defend the Collateral against, and will take such other action as is necessary to remove, any and all security interests on and claims in respect of the Collateral other than the security interests created by this Agreement and Permitted Encumbrances.
Section 6.07Operating Leases.
Neither a Loan Party nor any of its Subsidiaries will enter into or maintain operating leases (other than real property leases) such that the aggregate annual consolidated expenditure on such operating leases would be greater than $350,000.
- 48 -
Section 6.08Insurance.
Each Loan Party shall (and shall cause each of its Subsidiaries to) maintain insurance for risks and in amounts customary for prudent companies in the Loan Parties’ industry and owners of comparable assets with responsible insurers against such risks, which insurance shall be satisfactory to Lender, including but not limited to, business interruption, with Lender being named as a lender first loss payee and mortgagee with respect to any commercial property insurance of each Loan Party and as additional insured with respect to any general liability insurance of each Loan Party, and shall provide appropriate endorsements. Borrower shall promptly notify Lender of the occurrence of any event which would negatively affect any insurance coverage. If a Loan Party does not maintain insurance as required under this Section 6.08 or pay any amount or furnish required proof of payment to Lender, Lender may make all or part of such payment or obtain such insurance policies required in this Section 6.08.
Section 6.09Transactions with Affiliates.
No Loan Party shall or shall permit any of its Subsidiaries to enter into any transaction with any officer, director, employee, shareholder or any Person not dealing at arm’s length or any Affiliate of any of the foregoing (specifically excluding any employment or option agreement or intercompany indebtedness or transactions between a Loan Party and any of its Subsidiaries) unless such transaction is on terms no less favorable to such Loan Party than would be obtainable in an arm’s length transaction. The Loan Parties shall provide the Lender with evidence of compliance with this covenant upon request.
Section 6.10Material Agreement.
Each Loan Party shall observe each term, covenant and agreement contained in the Material Agreements, except to the extent that any non-compliance could not reasonably be expected to result in Material Adverse Effect. At Lender’s request, each Loan Party shall provide copies of any Material Agreements entered into after the Closing Date.
Section 6.11Reporting.
| (a) | Parent shall prepare and provide to Lender with respect to Parent and its Subsidiaries (a) monthly unaudited consolidated financial statements within 30 days of the end of each month accompanied by a Compliance Certificate as referred to in Section 6.20, (b) quarterly unaudited consolidated financial statements within the earlier of forty-five (45) days of the end of each fiscal quarter (including, without limitation, the last fiscal quarter) or the date that such financial statements are filed publicly, (c) audited consolidated financial statements within the earlier of one hundred and twenty (120) days of the Parent’s fiscal year end or the date such financial statements are filed publicly, such audited financial statements to be accompanied by a report and opinion of an independent certified public accountant acceptable to Lender, which report and opinion shall be prepared in accordance with GAAP and shall not be subject to any qualification or exception, (d) as soon as available, but in any event not later the last day of each fiscal year, the Budget for the next fiscal year, and (e) a schedule setting out the particulars of each Deposit |
- 49 -
Account maintained by any Loan Party, along with the applicable cash balance held in such Deposit Account, within 30 days of the end of each month, to be set out in a Compliance Certificate as referred to in Section 6.20. Parent shall provide, upon request by the Lender, general ledger accounts and any board and board subcommittee materials and shall provide online access to the Lender to quarterly board materials.
| (b) | On the same day as delivery of the monthly financial statements above, a Borrowing Base Certificate signed by a Responsible Officer of the Borrower calculating the Borrowing Base. Unless otherwise prescribed by the Lender, a Borrowing Base Certificate shall be substantially in the form attached as Exhibit C. |
Section 6.12Healthcare Regulatory Matters.
Each Loan Party:
| (a) | shall notify Lender promptly, and in any event within ten (10) Business Days, after receiving a written notice of or otherwise becoming aware of or determining that any of the following has occurred and would reasonably be expected to cause a Material Adverse Effect (each, a “Regulatory Reporting Event” and collectively, the “Regulatory Reporting Events”): (i) any Governmental Authority or Regulatory Authority is conducting or has conducted (A) if applicable, an investigation of any of the Loan Parties’ or their respective Subsidiaries’ manufacturing facilities and processes for any Product which investigation has disclosed any material deficiencies or violations of Laws and/or the Regulatory Required Permits related thereto or (B) an investigation or review of any Regulatory Required Permit (other than routine reviews in the ordinary course of business associated with the renewal of a Regulatory Required Permit), (ii) development, testing, and/or manufacturing of any Product that is material to the Loan Parties business (taken as a whole) should cease, (iii) if a Product that is material to the Loan Parties business (taken as a whole) has been approved for marketing and sale, any marketing or sales of such Product should cease or such Product should be withdrawn from the marketplace, or (iv) any Regulatory Required Permit has been revoked or withdrawn. Each Loan Party shall provide to Lender such further information (including copies of such documentation) as Lender may reasonably request with respect to any such Regulatory Reporting Event; and |
| (b) | shall and shall cause each other Loan Party to, obtain all Regulatory Required Permits necessary for compliance in all material respects with Laws with respect to testing, manufacturing, developing, selling or marketing of Products and shall, and shall cause each Loan Party to, maintain and comply in all material respects with all such Regulatory Required Permits, the noncompliance with which could have a Material Adverse Effect. |
- 50 -
Section 6.13Accounts
The Borrower shall cause all proceeds of the Revolving Credit Facility to be funded into a Deposit Account with the Lender or an Affiliate of the Lender in the United States.
Each Loan Party shall cause all of its Deposit Accounts, except for any Excluded Accounts, in Canada and the United States to be held with the Lender as of the Closing Date, provided that, for certainty, all banking services offered to the Borrower will be subject to fees comparable with customers similar to the Borrower and in accordance with the Lender’s standard practice in effect from time to time.
Section 6.14Sanctions
Each Loan Party, and their respective Subsidiaries, shall:
| (a) | comply with all Sanctions; and |
| (b) | conduct its business in such a way and adopt and maintain adequate policies, procedures and controls to ensure that it and each of its directors, officers and employees is in compliance with all Sanctions and that the representations and warranties set out in Section 5.35 are true and correct at all times (and not just at, and as of, the times such representations and warranties are made or deemed to be made). |
Section 6.15Anti-Terrorist Financing and Anti-Money Laundering Laws
| (a) | Each Loan Party and its respective Subsidiaries shall comply with all Anti-Terrorist Financing and Anti-Money Laundering Laws and “know your client” Applicable Laws (collectively, “AML Legislation”). |
| (b) | Each Loan Party acknowledges that, pursuant to AML Legislation, the Lender may be required to obtain, verify and record information regarding each Loan Party, each of their respective Subsidiaries and each of their respective directors, authorized signing officers, direct or indirect shareholders or other Persons in control of any of them, and the transactions contemplated herein. Each Loan Party shall, promptly provide all such information as may be reasonably required by the Lender, or any assignee or participant of the Lender, in order to comply with AML Legislation. |
| (c) | Each Loan Party and its respective Subsidiaries, shall adopt and maintain adequate policies, procedures and controls to ensure that it and each of its directors, officers, employees and agents is in compliance with all AML Legislation. |
- 51 -
Section 6.16Negative Covenants.
Without the prior written consent of Lender, no Loan Party shall (or shall permit any of its Subsidiaries, if any, to):
| (a) | incur, issue or make any request for or permit to exist Indebtedness, except for the Permitted Indebtedness; |
| (b) | grant or permit the existence of any Lien other than Permitted Encumbrances, or enter into any agreement which prohibits the granting of any Lien or assignment by way of security in the assets of any Loan Party or any Subsidiary (including a Lien in such instrument, contract, document or agreement); |
| (c) | make any Disposition of Property (including Intellectual Property) except for: |
(i) |
Dispositions of obsolete or worn-out Equipment; |
(ii) |
Dispositions that comply with Section 3.05(a); and |
(iii) |
Dispositions among Loan Parties; |
| (d) | wind-up or liquidate any Loan Party unless the assets thereof are transferred to another Loan Party and all documents reasonably requested by Lender are delivered in order to preserve the Liens under the Security Documents; |
| (e) | merge, amalgamate or enter into another form of business combination or reorganization (including any joint venture or partnership), except that (i) any Loan Party may merge, amalgamation or consolidated with any other Loan Party, and (ii) any Subsidiary may merge, amalgamate or consolidate into a Loan Party or another Subsidiary, provided that such merger, amalgamation or consolidation does not result in assets being transferred from a Loan Party to a Subsidiary that is not a Loan Party, in each case so long as all documents reasonably requested by the Lender are delivered in order to preserve the Liens under the Security Documents, |
| (f) | make any payment of any dividend, distribution, redemption or other payment (either cash or non-cash) in respect of any Loan Party’s Equity Interests, other than Permitted Distributions; |
| (g) | make any Investment other than Permitted Investments; |
| (h) | make any Acquisition other than Permitted Acquisitions; |
| (i) | change the jurisdiction of organization of any Loan Party, or amend any Loan Party’s Governing Documents in a manner which could reasonably be expected to have a Material Adverse Effect; |
| (j) | permit any assets or property of a Loan Party with a value in excess of $700,000 (including without limitation Collateral within a Deposit Account) to be located in |
- 52 -
a jurisdiction or otherwise established in a way that is not subject to perfected security in favour of the Lender pursuant to the Security Documents (which for clarity shall include any assets in Germany that are not subject to the German Security Agreements);
| (k) | engage in any business other than the business engaged in as of the Closing Date or substantially any substantially similar line of business as Borrower or in an adjacent line of business; |
| (l) | permit a Change of Control, except if Lender will be paid in full in cash at the closing of such Change of Control in accordance with Section 3.05(a); |
| (m) | make or incur any capital expenditures in any fiscal year in the aggregate in an amount which exceeds the amount set forth in the Budget for such a period by more than 15%; |
| (n) | change its Fiscal Year; and |
| (o) | agree or otherwise commit to take any action described in paragraphs (a) through (n) above. |
Section 6.17Maintenance of Records.
The Loan Parties will keep and maintain accurate and complete records of the Collateral.
Section 6.18Notices.
Borrower shall provide written notice to Lender at least 30 days prior to:
| (a) | any change in legal name, or jurisdiction of organization, or chief executive office of any Loan Party, or commencing operations in a new province in Canada or other jurisdiction that is not listed on Schedule 5.25; or |
| (b) | any amendment to the Governing Documents of any Loan Party; and |
Promptly upon becoming aware of same, Borrower shall provide prompt written notice to Lender, of:
| (a) | any Lien (other than Permitted Encumbrances) on, or claim asserted against, any of the Collateral; |
| (b) | the occurrence of any event, claim or occurrence that is or could reasonably be expected to have a Material Adverse Effect, including without limitation, any notice of default pursuant to any license, Permit or other agreement pursuant to which a Loan Party or any of its Subsidiaries licenses, Material Intellectual Property or the commencement of any litigation; |
- 53 -
| (c) | any change in the location of Collateral of a Loan Party (including additional locations), together with an estimate of the value of such Collateral at such new locations; |
| (d) | any material loss of or damage to any of the Collateral; |
| (e) | any material communication with or notice from the TSX or NASDAQ; and |
| (f) | any Material Adverse Change. |
Section 6.19Limitations on Modifications, Waivers, Extensions.
Other than in the ordinary course of business, no Loan Party will (or will permit any of its Subsidiaries, if any, to): (i) amend, modify, terminate or waive any provision of any Permit, contract, license or any agreement giving rise to a material Account except in the ordinary course of business and which would not result in a Material Adverse Effect; or (ii) fail to exercise promptly and diligently its rights under each Permit, contract and agreement giving rise to an Account except in the ordinary course of business and if such failure is or could not reasonably be expected to have a Material Adverse Effect.
Section 6.20Financial Covenants
The Loan Parties shall ensure that:
| (a) | Unrestricted Cash is at all times greater than the Required Liquidity Amount, to be tested as of the last day of each month based on the most recently reported consolidated financial statements delivered pursuant to Section 6.11(a) of this Agreement and reported in the most recent Compliance Certificate (for greater certainty, the Revolver Availability shall not be included for the purpose of calculating Unrestricted Cash); and |
| (b) | Revenue for the most recent reported trailing 12-month period must be 15% greater than Revenue for the same time period in the prior Fiscal Year, to be tested as of the last day of each Fiscal Quarter based on the most recently reported consolidated financial statements delivered pursuant to Section 6.11(a) of this Agreement and reported on in the most recent Compliance Certificate. |
Section 6.21Intellectual Property.
| (a) | Each of the Loan Parties shall protect, defend and maintain the validity and enforceability of the Material Intellectual Property and shall not allow any Material Intellectual Property to be abandoned, forfeited or dedicated to the public without Lender’s consent. No Subsidiary that is not a Loan Party shall own any Material Intellectual Property. |
| (b) | No Loan Party shall, or shall permit any of its Subsidiaries to, enter into a license with respect to Material Intellectual Property with respect to which any Loan Party or such Subsidiary is the licensee that prohibits or otherwise restricts a Loan Party |
- 54 -
or any Subsidiary from granting a security interest in a Loan Party’s or any Subsidiary’s interest in such license or agreement or would adversely affect Lender’s ability to exercise remedies pursuant to any Financing Documents with respect to Collateral without prior notice to Lender and obtaining such modifications to such license or agreement to ensure Lender’s rights and remedies pursuant to the Financing Documents are not materially impaired as Lender may request. The Loan Parties shall not be in material breach of any licenses and agreements pursuant to which any Loan Party licenses Material Intellectual Property.
| (c) | Together with each Compliance Certificate for the months ending March, June, September and December, Borrower shall provide Lender with a report of patents, patent applications, copyright registrations, copyright applications, trademark registrations and trademark applications acquired by any Loan Party or any of its Subsidiaries since the last such report delivered including a report regarding any new in-bound licenses or sublicense agreements pertaining to Material Intellectual Property or new material out-bound license or sublicense agreements to which a Loan Party is a party (in each case except for licenses of commercially available software or information technology services), together with all copies of such applications, licenses, agreements or registrations as Lender may request. With respect to any Intellectual Property registered in Canada, the United States or any other applicable jurisdiction and acquired by any Loan Party or changed after the Closing Date, each of the Loan Parties hereby agrees to give notice of same to the Lender and upon Lender’s request, to deliver, or cause any other Guarantor to deliver, to Lender an Intellectual Property Security Agreements or other documents as Lender may reasonably require to perfect its Lien therein or protect its interest with respect thereto, and authorizes Lender to amend any previously delivered Intellectual Property Security Agreement to amend any schedule thereto to reflect such addition or change. Borrower shall promptly notify Lender of the institution of any action, suit, litigation or proceeding pertaining to the Material Intellectual Property of any Loan Party or a claim of infringement by any Loan Party or any of its Subsidiaries on the intellectual property rights of a third party. |
Section 6.22Pension Plans
The Loan Parties, and their respective Subsidiaries, shall administer, fund, invest and maintain all Pension Plans and Welfare Plans, if any, in compliance with their terms and all Laws.
Section 6.23Material Subsidiaries
The Loan Parties shall cause each Material Subsidiary to promptly comply with Section 4.03. The Loan Parties shall ensure that all Material Agreements are entered into by and all Material Intellectual Property is held through entities that are Loan Parties.
- 55 -
Section 6.24Centre of Main Interests
Each Loan Party incorporated in a member state of the European Union must not cause or allow its registered office or Centre of Main Interests to be in, or maintain an Establishment in, any jurisdiction other than its jurisdiction of incorporation.
Section 6.25Limitation on Restrictive Agreements
The Borrower shall not (and shall cause any Subsidiary of the Borrow to note) create, incur, assume, permit to exist any consensual limitation or restriction on the ability of the Borrower or any Subsidiary of the Borrower to:
(a) |
make any payment to the Lender, provide the Security Documents to the Lender, or perform or observe any of its other covenants or agreements under any of the Financing Documents; or |
(b) |
in the case of Subsidiaries, to make any Distributions to the Borrower or any of its Subsidiaries. |
Section 6.26Limitation on Hedge Arrangements
Each Loan Party shall not enter into Hedge Arrangements of any kind after the date of this Agreement, except for Hedge Arrangements with the Lender in the ordinary course of a Loan Party’s business (but not for speculative purposes) to hedge or mitigate bona fide interest rate, currency or commodity risks to which the Loan Parties are exposed in the conduct of their business or the management of their liabilities.
ARTICLE VII.CONDITIONS
Section 7.01Conditions Precedent to Amended and Restated Credit Agreement.
The Borrower acknowledges that (i) certain conditions precedent have previously been satisfied in connection with the effectiveness of the Original Credit Agreement, and (ii) without limiting the right of the Lender with regard to making further advances, the effectiveness of this Agreement is subject to the following conditions precedent having been satisfied:
(a) |
Lender shall have received this Agreement and each of the other Financing Documents, in form and substance satisfactory to Lender, duly executed by each Person party thereto, and each such document shall be in full force and effect, and no Default or Event of Default shall exist as of the execution of such documents; |
(b) |
Lender shall have received confirmation that the Security Documents (to the extent applicable) have been registered in the applicable jurisdictions so as to result in perfected of security in all jurisdictions required by the Lender on the Closing Date; |
(c) |
Lender shall have received the applicable fees outlined in Section 2.03 together with any other fees contemplated by this Agreement and payable on the Closing Date; |
- 56 -
(d) |
Lender shall have received evidence that Run-Rate Revenue is greater than or equal to $8,000,000 as of the Closing Date; |
(e) |
Lender shall have received evidence that Liquidity is greater than or equal to $40,000,000 (net of any costs and expenses payable hereunder and repayment of the Term Loan) as of the Closing Date; |
(f) |
Lender shall have received a Drawdown Request within the time prescribed for the delivery thereof in Section 9.01 and a payment direction to the Lender, which authorizes and directs the Lender to repay the Term Loan with the proceeds of such Drawdown; |
(g) |
The Term Loan shall be repaid in full concurrently with the Drawdown set out in Section 7.01(f) above; |
(h) |
Lender shall have received a certificate from an officer of each Loan Party attesting to (i) the resolutions of such Loan Party’s applicable Board authorizing its execution, delivery, and performance of this Agreement and the other Financing Documents to which it is a party, (ii) the resolutions of such Loan Party’s stockholders, shareholders or members, as applicable, to the extent required pursuant to such Loan Party’s Governing Documents, (iii) such Loan Party’s Governing Documents, certified by the applicable jurisdiction of organization, and (iv) to the extent applicable in the relevant jurisdiction of incorporation, incumbency of the officers of such Loan Party; |
(i) |
Lender shall have received (i) duly executed copies of the Security Documents and all other Financing Documents and deliveries in connection therewith (in each case to the extent not delivered previously), and (ii) confirmations from the Loan Parties of all Security Documents previously delivered (including, for the avoidance of doubt, a confirmation agreement governed by the laws of Germany), and all such Financing Documents will have been duly registered, filed and recorded in all relevant jurisdictions where required by Applicable Law or where the Lender considers it necessary, in its sole discretion, to do so; |
(j) |
Lender shall have received a duly executed copy of the Junior Pledge Agreement; |
(k) |
Lender shall have received results of a recent searches in each of the jurisdictions where the Loan Parties and the assets of the Loan Parties are located, and such searches reveal no encumbrances on any of the assets of the Loan Parties, except for Permitted Encumbrances or Liens discharged or subordinated on or prior to the Closing Date pursuant to documentation in form and substance satisfactory to the Lender; |
(l) |
to the extent applicable in the relevant jurisdiction of incorporation, Lender shall have received a certificate of existence or good standing (or equivalent) with respect to each Loan Party, dated within 10 days of the Closing Date, which certificate shall indicate the good standing in the applicable jurisdiction of organization; |
- 57 -
(m) |
Lender shall have received a legal opinion letter of Borrower’s counsel in Canada, the United States of America and Germany, the form and substance of which shall be satisfactory to Lender; |
(n) |
Lender shall have received one or more legal opinion letter of Lender’s counsel in Germany regarding validity and enforceability matters with respect to the German Security Agreements, the form and substance of which shall be satisfactory to Lender; |
(o) |
the Borrower shall have delivered a duly completed Compliance Certificate showing pro-forma compliance with Section 6.20 after giving effect to the Advances made on the Closing Date; |
(p) |
the Borrower shall have delivered a duly completed Borrowing Base Certificate; |
(q) |
Each of the Loan Parties shall have received all licenses, approvals, consents or evidence of other actions required by any Person in connection with the execution and delivery by such Loan Party of this Agreement or any other Financing Document or with the consummation of the transactions contemplated hereby and thereby; |
(r) |
Lender shall have received one or more certificates of insurance, together with the endorsements thereto, as are required by Section 6.08, the form and substance of which shall be satisfactory to Lender; |
(s) |
No action, suit, investigation, litigation or proceeding before any arbitrator or Tribunal that could reasonably be expected to have a Material Adverse Effect if adversely determined shall be pending or threatened against any Loan Party or any Subsidiary; |
(t) |
Lender shall have completed its business, legal, and Collateral due diligence, including receipt of the latest Board approved Budget and forecast for 2025 Fiscal Year, management and investor interviews, and customer verification, to be coordinated with the Borrower, the results of which shall be satisfactory to Lender in its sole discretion; |
(u) |
Lender shall have received all documentation and other information required with respect to the Loan Parties under applicable “know-your-customer” and anti-money laundering rules and regulation; |
(v) |
No Material Adverse Effect shall have occurred; |
(w) |
No Material Adverse Change shall have occurred since the date of the Term Sheet. |
(x) |
The Loan Parties shall have paid all Lender Expenses incurred in connection with the transactions evidenced by this Agreement; and |
- 58 -
(y) |
All other documents and legal matters in connection with the transactions contemplated by this Agreement shall have been delivered, executed, or recorded and shall be in form and substance satisfactory to Lender. |
Section 7.02Conditions Precedent to all Extensions of Credit.
The obligation of Lender to make any Advances or other credit extensions under this Agreement shall be subject to the following conditions precedent:
(a) |
the Lender shall have received a Drawdown Request, within the time prescribed for the delivery thereof in Section 9.01; |
(b) |
the representations and warranties contained in this Agreement and the other Financing Documents shall be true and correct in all material respects (unless such representations and warranties are qualified by materiality, in which case such representations and warranties shall be true and correct in all respects) on and as of the date of such extension of credit, as though made on and as of such date (except to the extent that such representations and warranties relate solely to an earlier date); |
(c) |
(i) no Default or Event of Default shall have occurred and be continuing on the date of such extension of credit, nor shall either result from the making thereof, (ii) no Material Adverse Effect shall have occurred, and (iii) there has been no material deterioration in the consolidated financial condition of the Loan Parties since the date of the most recent financial statements submitted to Lender; |
(d) |
no injunction, writ, restraining order, or other order of any nature prohibiting, directly or indirectly, the extending of such credit shall have been issued and remain in force by any Tribunal against any Loan Party, Lender, or any of their Affiliates; and |
(e) |
the Borrower will be in pro-forma compliance with the financial covenants set out in Section 6.20 after giving effect to the Advances requested and has provided satisfactory evidence of same as requested by the Lender. |
ARTICLE VIII.EVENTS OF DEFAULT
Section 8.01Events of Default.
Any of the following shall constitute an Event of Default under this Agreement:
(a) |
failure by any Loan Party to pay in cash all or any part of the Secured Obligations (including, without limitation, principal and interest payments due hereunder) when due and payable hereunder, and in the case of any amount other than principal (which shall not be subject to a cure period) such default shall remain unremedied for a period of 2 Business Days after the due date; |
- 59 -
(b) |
Any Loan Party ceases or threatens to cease to carry on business in the ordinary course or any material part of its business; |
(c) |
Any Loan Party or any Subsidiary becomes unable to satisfy its liabilities as they become due, or at any time the realizable value of the Loan Parties’ assets is less than the aggregate sum of its liabilities, or any of them otherwise commit an act of bankruptcy or admits that it is “insolvent;” |
(d) |
Any Loan Party or any Subsidiary, any creditor of such a party, or any other Person institutes any proceeding or takes any action or executes any agreement in connection with the commencement of any proceeding: |
(i) |
seeking to adjudicate any Loan Party or any Subsidiary bankrupt or insolvent; |
(ii) |
seeking liquidation, dissolution, winding-up, reorganization, arrangement, protection, relief or composition of any Loan Party or Subsidiary or any material part of its property or debt, or making a proposal with respect to any Loan Party or Subsidiary under any law relating to bankruptcy, insolvency, reorganization or compromise of debts or other similar laws including, without limitation, all Insolvency Legislation, unless any such proceeding or other action is successfully contested by the applicable Loan Party or Subsidiary in good faith and is dismissed or stayed within 45 days; |
(iii) |
seeking appointment of a receiver, receiver and manager, trustee, agent, custodian, monitor, liquidator or similar official for any Loan Party or Subsidiary or for any part of their properties and assets or for any part of the Collateral; or |
(iv) |
takes any other action or steps to institute an Insolvency Proceeding; |
(e) |
a receiver, conservator, trustee, custodian, monitor, liquidator or similar official is appointed in respect of any Loan Party or Subsidiary or any of the Collateral; |
(f) |
a failure by any Loan Party or Subsidiary to make payments of principal, interest or other amounts when due with respect to any other Indebtedness, or the occurrence of any default, or any event or condition which, with the giving of notice or passage of time, or both, would constitute a default or event of default by any Loan Party or Subsidiary under the terms of any Indebtedness in excess of $175,000; |
(g) |
if any representation or warranty made by any Loan Party in any of the Financing Documents or any other document, instrument or agreement executed and delivered by any Loan Party at any time to or in favor of Lender is untrue or incorrect in any material respect as of the date on which it is made; |
(h) |
any Loan Party breaches or fails to duly perform or observe any covenant contained in Section 6.20 or Section 6.25; |
- 60 -
(i) |
any Loan Party fails to observe the covenants set forth in Article VI (other than those contained in Section 6.20 and Section 6.25) of this Agreement or any other term, covenant or agreement contained herein or in any of the Financing Documents or any other document, instrument or agreement executed and delivered by any Loan Party at any time to or in favor of Lender and such failure is not cured to the satisfaction of the Lender, acting reasonably, within 10 Business Days after the earlier of (i) written notice thereof by the Lender to the Borrower and (ii) actual knowledge of the Borrower of the same; |
(j) |
any Loan Party challenges or threatens to challenge the validity or enforceability of any of the Financing Documents or terminates or repudiates any of them or attempts to do so, or such Financing Documents or any other document, instrument, agreement or certificate executed and delivered by any Loan Party to Lender shall cease to be in full force and effect or fail, in whole or in part, to constitute a legal, valid, binding and enforceable obligation of any Loan Party, as the case may be; |
(k) |
any Security Document shall cease to create a valid Lien in favor of Lender or Lender’s Lien ceases to be a perfected, first priority Lien in the Collateral (subject to Permitted Encumbrances), or any occurrence, development or change shall occur, which has resulted or is reasonably expected to result in impairment in the perfection or priority of the Liens granted to Lender other than pursuant to Permitted Encumbrances, or there is a material impairment of the Collateral; |
(l) |
any part of the Collateral is attached, seized, levied on or becomes subject to any similar process, or any Governmental Authority (including an administrative body or taxation authority) issues a demand in respect of any Loan Party on any part of the Collateral, or any creditor enforces a Lien on the Collateral or otherwise takes possession of property of any Loan Party, or gives written notice of its intent to do any of the foregoing (unless such notice contested in good faith by appropriate legal proceedings and appropriate reserves satisfactory to Lender have been taken); |
(m) |
any resolution is passed for the winding up, dissolution or liquidation or amalgamation of any Loan Party other than as permitted by Section 6.16(d) or Section 6.16(e) or if any Loan Party loses its charter by expiration, cancellation, forfeiture or otherwise; |
(n) |
there is entered into against any Loan Party or Subsidiary a judgment or order for the payment of money in an aggregate amount in excess of $175,000 or for equitable relief to enjoin, restrain or prevent any Loan Party or Subsidiary from conducting any material part of its business, or which would otherwise reasonably be expected to have a Material Adverse Effect; |
(o) |
a Change of Control occurs; |
(p) |
any Permit (including any Regulatory Required Permit) shall have been revoked, rescinded, suspended, modified in an adverse manner or not renewed for a full term, |
- 61 -
and such revocation, rescission, suspension, modification or non-renewal has, or could reasonably be expected to have, a Material Adverse Effect.;
(q) |
a Pension Plan Event occurs; |
(r) |
if the common shares of the Parent cease to be listed for trading on the TSX or NASDAQ, the Parent ceases to be a reporting issuer in good standing in the Province of Ontario, a cease trade order is issued by a Governmental Authority with respect to the common shares of the Parent which order is in effect for more than twenty (20) Business Days or if the Parent appears on the list of defaulting issuers maintained by the Ontario Securities Commission available for consultation on its website; |
(s) |
the occurrence of a Material Adverse Change; or |
(t) |
any other event or circumstance occurs that could reasonably give rise to a Material Adverse Effect. |
Section 8.02Lender’s Remedies Upon Default.
(a) |
Upon the occurrence, and during the continuation, of an Event of Default, Lender may exercise any of the rights and remedies of a secured party under the PPSA or other similar applicable foreign Law, and any other rights and remedies provided for in this Agreement or any other Financing Document or otherwise available to it at law or in equity, such rights and remedies to include, without limitation, the following, all of which are authorized by each Loan Party: |
(i) |
Declare all Secured Obligations, whether evidenced by this Agreement, by any of the other Financing Documents, or otherwise, immediately due and payable; |
(ii) |
Cease advancing money or extending credit to or for the benefit of Borrower under this Agreement, under any of the Financing Documents, or under any other agreement between any Loan Party, and Lender; |
(iii) |
Terminate this Agreement and any of the other Financing Documents as to any future liability or obligation of Lender, but without affecting any of Lender’s Liens in the Collateral and without affecting the Secured Obligations; |
(iv) |
Settle or adjust disputes and claims directly with Account Debtors for amounts and upon terms which Lender in its sole discretion considers advisable, and in such cases, Lender will credit Borrower’s Loan Account with only the net amounts received by Lender in payment of such Accounts after deducting all Lender Expenses incurred or expended in connection therewith; |
- 62 -
(v) |
Without notice to any Loan Party (such notice being expressly waived), and without constituting a retention of any collateral in satisfaction of an obligation (within the meaning of the PPSA or other similar applicable foreign Law), set off and apply to the Secured Obligations any and all (i) funds coming into possession of Lender, or (ii) Indebtedness at any time owing to or for the credit or the account of any Loan Party held by Lender; |
(vi) |
Deliver a notice of exclusive control, any entitlement order, or other directions or instructions pursuant to any Control Agreement or similar agreements providing control of any Collateral; |
(vii) |
take any and all other remedies at Law or provided for under the applicable Security Documents. |
Section 8.03Lender Protective Payments.
Without notice to or demand upon any Loan Party, make such payments and do such acts as Lender considers necessary or reasonable to protect its security interests in the Collateral, enhance the likelihood of repayment of the Secured Obligations, or pay any other amount payable by or chargeable to any Loan Party pursuant to the terms of this Agreement, including costs, fees, and expenses, and Lender Expenses. All such amounts shall be added to the principal outstanding of the Revolving Credit Facility outstanding hereunder, and be repayable on demand and secured by the Collateral.
Section 8.04Remedies Cumulative.
The rights and remedies of Lender under this Agreement, the other Financing Documents, and all other agreements shall be cumulative. Lender shall have all other rights and remedies not inconsistent herewith as provided under the PPSA or other similar applicable foreign Law, by law, or in equity. No exercise by Lender of one right or remedy shall be deemed an election, and no waiver by Lender of any Event of Default shall be deemed a continuing waiver. No delay by Lender shall constitute a waiver, election, or acquiescence by it.
Section 8.05Power of Attorney.
Each Loan Party hereby irrevocably makes, constitutes, and appoints Lender (and any of Lender’s officers, employees, or agents designated by Lender) as such Loan Party’s true and lawful attorney, during the existence of an Event of Default, with power to (a) sign the name of such Loan Party on any Additional Documents, (b) sign such Loan Party’s name on any invoice or bill of lading relating to the Collateral, drafts against Account Debtors, or notices to Account Debtors, (c) send requests for verification of Accounts, (d) endorse such Loan Party’s name on any collection item that may come into Lender’s possession, (e) make, settle, and adjust all claims under such Loan Party’s policies of insurance and make all determinations and decisions with respect to such policies of insurance, and (f) pay, contest or settle any Lien or other encumbrance or adverse claim in or to the Collateral or any judgment based thereon. The appointment of Lender as each such Loan Party’s attorney, and each and every one of its rights and powers, being coupled with an interest, is irrevocable until all of the Secured Obligations have been fully and finally repaid and performed and any obligation of Lender to extend credit hereunder is terminated.
- 63 -
Each Loan Party hereby releases Lender (and any of Lender’s officers, employees, or agents designated by Lender) from the restrictions (to the extent that such restrictions would otherwise apply) on self-dealing and multi-representation pursuant to section 181 of the German Civil Code (Bürgerliches Gesetzbuch) and similar restrictions (if any) applicable to it pursuant to any other applicable laws, in each case to the extent legally possible to such Loan Party. For the avoidance of doubt, if and to the extent that Lender is authorised to sub-delegate (by power of attorney or otherwise) any powers granted to it under the Financing Documents (or any of them), this shall extend to include such release. Each Loan Party which is barred by its constitutional documents, by-laws or otherwise from validly granting such release will inform Lender accordingly.
Section 8.06Notice of Event of Default.
Each Loan Party shall promptly give notice in writing to Lender of the occurrence of any Event of Default or other event which, with the lapse of time or giving of notice or otherwise, would be an Event of Default, forthwith upon becoming aware thereof. Such written notice shall specify the nature of such default or Event of Default and the steps taken to remedy the same.
Section 8.07Default under Other Encumbrances.
Any amount paid by Lender before or after the occurrence of an Event of Default on account of monies payable under any Lien upon the Collateral or any part thereof shall be repaid by the Loan Parties to Lender on demand and shall:
(a) |
be added to the Secured Obligations and constitute a charge upon the Collateral; and |
(b) |
bear interest at the rate equal to the Post-Default Rate as a reasonable and genuine pre-estimate of damages and not as a penalty. |
Section 8.08Judgment.
Neither the taking of any judgment nor the exercise of any power of seizure or sale shall operate to extinguish the liability of the Loan Parties to perform the Secured Obligations nor shall such operate as a merger of any covenant or affect the right of Lender to receive interest at the specified rate, and any judgment shall bear interest at such rate.
Section 8.09Application of Proceeds.
During the existence of an Event of Default, Lender may apply in any order all payments received or funds coming into its possession.
Section 8.10Limitation of Liability.
Lender shall not be liable by reason of any entry into or taking possession of any of the Collateral charged or intended to be charged by the Security Documents or any part thereof, to account as mortgagee in possession or for anything except actual receipts or be liable for any loss on realization or any act or omission for which a secured party in possession might be liable.
- 64 -
Lender shall not, by virtue of these presents, be deemed to be a mortgagee in possession of the Collateral. Lender shall not be liable or accountable for any failure to exercise its remedies, take possession of, seize, collect, realize, sell, lease or otherwise dispose of or obtain payment for the Collateral and shall not be bound to institute proceedings for such purposes or for the purpose of preserving any rights, remedies or powers of Lender, any Loan Party or any other person in respect of same. Each Loan Party hereby releases and discharges Lender from every claim of every nature, whether sounding in damages or not, which may arise or be caused to any Loan Party or any person claiming through any Loan Party by reason or as a result of anything done or omitted to be done, as the case may be, by Lender or any successor or assign claiming through or under Lender under the provisions of this Agreement, unless such claim is the result of Lender’s gross negligence or willful misconduct as determined by a final, non-appealable judgment. The guarantee limitations set forth in clause 4.10 of the Canadian Guarantee shall apply mutatis mutandis to any indemnity granted by or other secondary liability of a Loan Party incorporated as limited liability company (GmbH) in Germany to the Lender pursuant to this Section 8.10.
Section 8.11Borrower Liable
Borrower hereby waives any requirement that the Lender protect, secure, perfect or insure any security interest or Lien, or any property subject thereto, or exhaust any right or take any action against any other Loan Party or other entity. Borrower agrees not to seek payment directly or indirectly from another Loan Party through a claim of indemnity, contribution, or otherwise with respect to the Secured Obligations, until the Secured Obligations have been repaid in full and this Agreement has terminated. Any agreement providing for indemnification, reimbursement or any other arrangement prohibited under this Section shall be null and void. If any payment is made to a Loan Party in contravention of this Section, such Loan Party to hold such payment in trust for Lender and such payment shall be promptly delivered to Lender for application to the Secured Obligations, whether matured or unmatured.
Section 8.12Termination of Lender’s Obligations
The occurrence of an Event of Default shall relieve the Lender of all obligations to provide any further Drawdowns to the Borrower hereunder and the Lender may give notice to the Borrower declaring the Lender’s obligations to make Advances to be terminated, in which case such obligations shall be immediately terminated.
ARTICLE IX.DETAILS REGARDING ADVANCES AND PAYMENTS
Section 9.01Drawdown Notices
(a) |
The Borrower shall give the Lender a Drawdown Request two (2) Business Days prior to each proposed Drawdown. |
(b) |
Each Drawdown Request shall be delivered by the Borrower to the Lender on a Business Day on or prior to 1:00 p.m. Toronto time. Notices that are received by the Lender after such time shall be deemed to have been given on the next Business Day, unless the Lender agrees, in their sole discretion, to accept such notice at a later time as being effective on the date it is given. |
- 65 -
(c) |
Once delivered to the Lender, a Drawdown Request is irrevocable and shall oblige the Borrower to take the action contemplated therein on the date specified in the Drawdown Request. The Borrower shall indemnify and hold harmless the Lender for all costs incurred by them as a result of the Borrower failing to make any payment or satisfy any conditions precedent to an Advance described in any Drawdown Request on or before the date specified therein for such action to occur. |
Section 9.02Minimum Amounts
Each Drawdown made under this Agreement shall be made in minimum principal amounts of $100,000 and in integral multiples of $50,000.
Section 9.03Payments by the Borrower
(a) |
Except when specifically provided otherwise in this Agreement, all payments of principal, interest, fees and all other amounts to be made by the Borrower under this Agreement shall be paid to the Lender, in the currency in which it is due for value at or before 3:00 p.m. (Toronto time) on the day such payment is due at the Lender’s Office. If any such day is not a Business Day, such amount shall be deemed for purposes of this Agreement to be due on the next Business Day following such day, and any such extension of time shall be included in the computation of any interest of fees payable under this Agreement. |
(b) |
The Lender may debit accounts, credits and other balances maintained by the Borrower from time to time with the Lender, or any of its Affiliates, to facilitate or otherwise obtain payment on accounts of any of the Secured Obligations. |
ARTICLE X.GENERAL
Section 10.01Releases.
Lender may in its discretion from time to time release any part of the Collateral or any other security either with or without any sufficient consideration therefor, without responsibility therefor and without thereby releasing any other part of the Collateral or any other security or any Person from the security created by this Agreement or any of the Financing Documents or from any of the covenants herein contained. Each and every portion into which the Collateral is or may hereafter be divided does and shall stay subject to the Lien pursuant to this Agreement. No Person shall have the right to require the Secured Obligations to be apportioned and Lender shall not be accountable to any Loan Party for any moneys except those actually received by Lender.
Section 10.02Governing Law; Jurisdiction; Jury Trial Waiver and Judicial Reference.
All Financing Documents, except if expressly provided otherwise therein, shall be exclusively (without regard to any rules or principles relating to conflicts of laws) governed by, enforced and construed in accordance with the laws of the Province of Ontario and the federal laws of Canada applicable therein. Each Loan Party hereby irrevocably and unconditionally consents, for itself and its property, to the exclusive jurisdiction of the courts of the Province of Ontario with respect to any matter arising under or relating to this Agreement or any of the other Financing Documents, except if expressly provided otherwise therein.
- 66 -
Each Loan Party expressly submits and consents in advance to such jurisdiction in any action or suit commenced in any such court, and such Loan Party hereby waives any objection that it may have based upon lack of personal jurisdiction, improper venue, or forum non conveniens and hereby consents to the granting of such legal or equitable relief as is deemed appropriate by such court. Nothing in this Agreement shall affect any right that Lender may otherwise have to bring any action or proceeding relating to any Financing Document against a Loan Party or its properties in the courts of any jurisdiction. Each Loan Party hereby waives personal service of the summons, complaints and other process issued in such action or suit and agrees that service of such summons, complains and other process may be made by registered or certified mail addressed to Borrower as its duly appointed process agent at the address set forth in, or subsequently provided to Lender in accordance with this Agreement, and that service so made shall be deemed completed upon the earlier to occur of Borrower’s actual receipt thereof or three (3) days after deposit in the U.S. mails, proper postage paid. TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, EACH LOAN PARTY AND LENDER WAIVES THEIR RIGHT TO A JURY TRIAL OF ANY CLAIMS OR CAUSE OF ACTION ARISING OUT OF OR BASED UPON THIS AGREEMENT, THE FINANCING DOCUMENTS OR ANY CONTEMPLATED TRANSACTION, INCLUDING CONTRACT, TORT, BREACH OF DUTY AND ALL OTHER CLAIMS. THIS WAIVER IS A MATERIAL INDUCEMENT FOR BOTH PARTIES TO ENTER INTO THIS AGREEMENT. EACH PARTY HAS REVIEWED THIS WAIVER WITH ITS COUNSEL.
Section 10.03Whole Agreement.
This Agreement and other Financing Documents and any and all other documents ancillary thereto and executed and delivered in connection therewith, constitute the entire agreement between the parties hereto with respect to the subject matter hereof.
Section 10.04Time.
Time shall be of the essence of all provisions of this Agreement and the other Financing Documents.
Section 10.05Notices.
Any notice or other communication required or permitted to be given hereunder shall be in writing and shall be given by prepaid first-class mail, e-mail, facsimile, or by other electronic communication or by hand-delivery as hereinafter provided. Any such notice or other communication, if mailed by prepaid first-class mail at any time other than during, or within three (3) Business Days prior to, a general discontinuance of postal service due to strike, lockout or otherwise, shall be deemed to have been received on the fourth Business Day after the postmarked date thereof, or if sent or by means of electronic communication, shall be deemed to have been received on the Business Day of the sending (provided it was sent before 4:30 p.m. Toronto, Ontario time), or if delivered by hand shall be deemed to have been received at the time it is delivered to the applicable address noted below either to the individual designated below or to an employee of the addressee at such address with responsibility for matters to which the information relates. Notice of change of address shall also be governed by this Section 10.05.
- 67 -
In the event of a general discontinuance of postal service due to strike, lock-out or otherwise, notices or other communications shall be delivered by hand or any means of electronic communication and shall be deemed to have been received in accordance with the foregoing. Notices and other communications shall be addressed as follows:
(a) |
if to the Loan Parties: |
Profound Medical Inc.
2400 Skymark Ave. Unit #6
Mississauga, Ontario
Attention: Rashed Dewan, Chief Financial Officer
Email: [***]
(b) |
if to Lender: |
for any borrowing request:
Canadian Imperial Bank of Commerce
[***]
e-mail: [***]
Attention: [***]
for all other notices:
Canadian Imperial Bank of Commerce
[***]
e-mail: [***]
Attention: [***]
Canadian Imperial Bank of Commerce
[***]
e-mail: [***]
Fax: [***]
Attention: [***]
- 68 -
Section 10.06Successors and Assigns.
This Agreement shall be binding on each Loan Party and its successors and assigns and shall inure to the benefit of Lender and its successors and permitted assigns. Each Loan Party agrees not to assign, transfer or delegate any of its rights or obligations under this Agreement without the prior written consent of Lender which it may exercise in its sole and absolute discretion. Lender shall be entitled to assign this Agreement to any other party (with, so long as no Default or Event of Default has occurred, the consent of Borrower); provided, that so long as no Default or Event has occurred, such assignment shall not increase the relevant Loan Party’s obligation to reimburse, indemnify or make whole the Lender pursuant to Section 3.09 or Section 3.10 as a result of such assignment. Notwithstanding the foregoing, an assignment to an Affiliate of Lender may be made at any time without consent and an assignment pursuant to a synthetic risk transfer or by way of a sale of participations by the Lender to any Person, may be made at any time without consent or notice to the Loan Parties.
Section 10.07Indemnification.
Each Loan Party hereby agrees to indemnify, defend and hold Lender and its principals, employees, agents, attorneys, or any other Person affiliated with or representing Lender (each, an “Indemnified Person”) harmless against: (i) all obligations, demands, claims, and liabilities (collectively, “Indemnified Claims”) claimed or asserted by any other party in connection with the transactions contemplated by the Financing Documents; and (ii) all losses or expenses (including Lender Expenses) in any way suffered, incurred, or paid by such Indemnified Person as a result of, following from, consequential to, or arising from transactions between Lender and any Loan Party (including attorneys’ and other legal fees and expenses), except for Indemnified Claims or losses finally determined by a court of competent jurisdiction to have been directly caused by such Indemnified Person’s gross negligence or willful misconduct. This provision shall survive the termination of this Agreement and the repayment of the Secured Obligations. The guarantee limitations set forth in clause 4.10 of the Canadian Guarantee shall apply mutatis mutandis to any indemnity granted by or other secondary liability of a Loan Party incorporated as limited liability company (GmbH) in Germany to the Lender pursuant to this Section 10.07.
Section 10.08No Set-Off.
The Secured Obligations shall be paid by the Loan Parties without regard to any set-off, withholding, counterclaim or equities between any Loan Party and Lender.
Section 10.09Permitted Encumbrance.
Notwithstanding any other provision in this Agreement, the parties confirm their intent that the references to Permitted Encumbrances herein are not intended to imply the subordination by Lender to any Person whatsoever.
Section 10.10Press Releases.
Each Loan Party agrees that Lender may issue press releases announcing the fact and extension of the financing under this Agreement and further agrees that Lender may display any Loan Party’s logo on its website and collateral material consistent with its other portfolio companies.
- 69 -
Lender’s use of such logo shall be subject to such Loan Party’s approval, which shall not be unreasonably withheld.
Section 10.11Confidentiality.
Lender agrees to use the same degree of care that it exercises with respect to its own proprietary information to maintain the confidentiality of any and all proprietary, trade secret or confidential information provided to or received by Lender from or on behalf of the Loan Parties, which indicates that it is confidential or would reasonably be understood to be confidential, including business plans and forecasts, non-public financial information, confidential or secret processes, formulae, devices and contractual information, customer lists, and employee relation matters, provided that Lender may disclose such information to its officers, directors, employees, attorneys, accountants and affiliates, and such other Persons to whom Lender shall at any time be required to make such disclosure in accordance with Applicable Law and in connection with the exercise of remedies, and provided that the foregoing shall not apply to information that is in the public domain or becomes part of the public domain (other than as a result of its disclosure by Lender in violation of this Agreement) after disclosure to Lender. The confidentiality agreement in this Section supersedes any prior confidentiality agreement of Lender relating to the Loan Parties.
Section 10.12Judgment Currency
(a) |
If for the purpose of obtaining or enforcing judgment against any Loan Party in any court in any jurisdiction, it becomes necessary to convert into any other currency (such other currency being hereinafter in this Section 10.12 referred to as the “Judgement Currency”) an amount due in into another currency (referred to as the “Required Currency”), the conversion shall be made at the Exchange Rate prevailing on the Business Day immediately preceding: |
(i) |
the date of actual payment of the amount due, in the case of any proceeding in the courts of the Province of Ontario or in the courts of any other jurisdiction that will give effect to such conversion being made on such date; or |
(ii) |
the date on which the judgment is given, in the case of any proceeding in the courts of any other jurisdiction (the date as of which such conversion is made pursuant to this Section 10.12(a)(ii) being hereinafter in this Section referred to as the “Judgement Conversion Date”). |
(b) |
If, in the case of any proceeding in the court of any jurisdiction referred to in clause (a)(ii) of this section, there is a change in the rate of exchange prevailing between the Judgement Conversion Date and the date of actual payment of the amount due, the Loan Parties, jointly and severally, shall pay such additional or lesser amount as may be necessary to ensure that the amount paid in the Judgement Currency, when converted at the rate of exchange prevailing on the date of payment, will produce the amount of the Required Currency, which could have been purchased with the amount of Judgement Currency stipulated in the judgment |
- 70 -
or judicial order at the rate of exchange prevailing on the Judgement Conversion Date.
(c) |
Any amount due from any Loan Party under the provisions of Section (b) shall be due as a separate debt and shall not be affected by judgment being obtained for any other amounts due under or in respect of this Agreement. |
Section 10.13Anti-Money Laundering Legislation
(a) |
Each Loan Party acknowledges that, pursuant to the AML Legislation, Lender may be required to obtain, verify and record information regarding the Loan Parties, their directors, authorized signing officers, direct or indirect shareholders or other Persons in control of such Loan Party, and the transactions contemplated hereby. Each Loan Party shall promptly provide all such information, including supporting documentation and other evidence, as may be reasonably requested by Lender, or any prospective assignee or participant of Lender, in order to comply with any applicable AML Legislation, whether now or hereafter in existence. |
(b) |
Each Loan Party acknowledges and agrees that pursuant to the provisions of the USA Patriot Act (Title III of the Pub. L. 107-56) signed into law October 26, 2001 (the “Patriot Act”), Lender may be required to obtain, verify and record information with respect to the Loan Parties, and each Loan Party hereby agrees to cooperate with Lender and provide them with all information that may be required in order to fulfil its obligations under the Patriot Act. |
Section 10.14Counterparts.
This Agreement may be executed in several counterparts, each of which, when so executed, shall be deemed to be an original and which counterparts taken together shall constitute one and the same Agreement. This Agreement may be executed by facsimile or pdf, and any signature contained hereon by facsimile or pdf shall be deemed to be equivalent to an original signature for all purposes.
Section 10.15Keepwell
Each Qualified ECP Guarantor hereby jointly and severally absolutely, unconditionally and irrevocably undertakes to provide such funds or other support as may be needed from time to time by each other Loan Party to honor all of its obligations under this guaranty in respect of a Hedge Arrangement (provided, however, that each Qualified ECP Guarantor shall only be liable under this Section 10.15for the maximum amount of such liability that can be hereby incurred without rendering its obligations under this Section 10.15 or otherwise under this guaranty voidable under applicable law relating to fraudulent conveyance or fraudulent transfer, and not for any greater amount). Except as otherwise provided herein, the obligations of each Qualified ECP Guarantor of each other Loan Party for all purposes of Section 1a(18)(A)(v)(II) of the Commodity Exchange Act under this Section 10.15 shall remain in full force and effect until the termination of all Hedge Arrangements. Each Qualified ECP Guarantor intends that this Section 10.15constitute, and this Section 10.15 shall be deemed to constitute, a “keepwell, support, or other agreement” for the benefit of each other Loan Party for all purposes of Section 1a(18)(A)(v)(II) of the Commodity Exchange Act.
- 71 -
Section 10.16Acknowledgement Regarding Any Supported QFCs
To the extent that the Financing Documents provide support, through a guarantee or otherwise, for Hedge Arrangements or any other agreement or instrument that is a QFC (such support, “QFC Credit Support” and each such QFC a “Supported QFC”), the parties acknowledge and agree as follows with respect to the resolution power of the Federal Deposit Insurance Corporation under the Federal Deposit Insurance Act and Title II of the Dodd-Frank Wall Street Reform and Consumer Protection Act (together with the regulations promulgated thereunder, the “U.S. Special Resolution Regimes”) in respect of such Supported QFC and QFC Credit Support (with the provisions below applicable notwithstanding that the Financing Documents and any Supported QFC may in fact be stated to be governed by the laws of the State of New York and/or of the United States or any other state of the United States):
(a) |
In the event a Covered Entity that is party to a Supported QFC (each, a “Covered Party”) becomes subject to a proceeding under a U.S. Special Resolution Regime, the transfer of such Supported QFC and the benefit of such QFC Credit Support (and any interest and obligation in or under such Supported QFC and such QFC Credit Support, and any rights in property securing such Supported QFC or such QFC Credit Support) from such Covered Party will be effective to the same extent as the transfer would be effective under the U.S. Special Resolution Regime if the Supported QFC and such QFC Credit Support (and any such interest, obligation and rights in property) were governed by the laws of the United States or a state of the United States. In the event a Covered Party or a BHC Act Affiliate of a Covered Party becomes subject to a proceeding under a U.S. Special Resolution Regime, Default Rights under the Financing Documents that might otherwise apply to such Supported QFC or any QFC Credit Support that may be exercised against such Covered Party are permitted to be exercised to no greater extent than such Default Rights could be exercised under the U.S. Special Resolution Regime if the supported QFC and the Financing Documents were governed by the laws of the United States or a state of the United States. |
(b) |
As used in this Section, the following terms have the following meanings: |
“BHC Act Affiliate” of a party means an “affiliate” (as such term is defined under, and interpreted in accordance with, 12 U.S.C. 1841(k)) of such party.
“Covered Entity” means any of the following:
(i) |
a “covered entity” as that term is defined in, and interpreted in accordance with, 12 C.F.R. §252.82(b); |
(ii) |
a “covered bank” as that term is defined in, and interpreted in accordance with, 12 C.F.R. §47.3(b); or |
- 72 -
(iii) |
a “covered FSI” as that term is defined in, and interpreted in accordance with, 12 C.F.R. §382.2(b). |
“Default Right” has the meaning assigned to that term in, and shall be interpreted in accordance with, 12 C.F.R. §§ 252.81, 47.2 or 382.1, as applicable.
“QFC” has the meaning assigned to the term “qualified financial contract” in, and shall be interpreted in accordance with, 12 U.S.C. 5390(c)(8)(D).
Section 10.17Amendment and Restatement; No Novation.
This Agreement constitutes an amendment and restatement of the Original Credit Agreement, as amended, effective from and after the Closing Date. It is the express intent of the parties to this Agreement that (A) the execution and delivery of this Agreement not constitute a novation or extinguishment of any indebtedness or other obligations owing to the Lender under the Original Credit Agreement but that such indebtedness and other obligations under the Original Credit Agreement shall continue, uninterrupted, but on the amended and restated terms set forth in this Agreement and, as applicable, the other Financing Documents; (B) this Agreement does not supersede the Original Credit Agreement but, instead, amends and restates the Original Credit Agreement on the terms set forth herein; (C) the execution and delivery of any amendment to, or amendment and restatement of, any Security Document executed or delivered in connection with the Original Credit Agreement not constitute a novation or extinguishment of any security interest or Lien created under such Security Document; and (D) all security interests in and Liens on the Collateral granted under any Security Document executed or delivered in connection with the Original Credit Agreement shall, upon the execution and delivery of this Agreement, continue, uninterrupted, to secure the Loan Parties’ indebtedness and obligations under the Financing Documents (as applicable) on the terms set forth in the such Security Document or, as applicable, any amendment to or amendment and restatement of such Security Document executed or delivered in connection with this Agreement. On the Closing Date, the credit facilities described in the Original Credit Agreement, as amended, shall be amended, supplemented, modified and restated in their entirety by the corresponding credit facilities described herein, and all loans and other obligations of the Borrowers and the obligations of the other Loan Parties outstanding or existing as of such date under the Original Credit Agreement are and shall be deemed to be loans and obligations outstanding under the corresponding facilities described herein, without any further action by any Person. In furtherance of (but not limited to) the foregoing, all interest and fees of the Loan Parties under the Original Credit Agreement shall accrue at the rates therefor under the Original Credit Agreement and shall, on and after the Closing Date, accrue at the rates set forth in this Agreement and be payable on the dates set forth in this Agreement.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
- 73 -
IN WITNESS WHEREOF, each of the parties hereto have duly executed this Agreement as of date first written above.
|
PROFOUND MEDICAL INC., as Borrower |
||
|
|
||
|
Per: |
/s/ Arun Swarup Menawat |
|
|
|
Name: |
Arun Swarup Menawat |
|
|
Title: |
Authorized Signing Officer |
|
PROFOUND MEDICAL CORP., as a Guarantor |
||
|
|
||
|
Per: |
/s/ Arun Swarup Menawat |
|
|
|
Name: |
Arun Swarup Menawat |
|
|
Title: |
Authorized Signing Officer |
|
2753079 ONTARIO INC., as a Guarantor |
||
|
|
||
|
Per: |
/s/ Arun Swarup Menawat |
|
|
|
Name: |
Arun Swarup Menawat |
|
|
Title: |
Authorized Signing Officer |
Signature Page – Credit Agreement
|
PROFOUND MEDICAL GMBH, as a Guarantor |
||
|
|
||
|
Per: |
/s/ Arun Swarup Menawat |
|
|
|
Name: |
Arun Swarup Menawat |
|
|
Title: |
Authorized Signing Officer |
|
PROFOUND MEDICAL (U.S.) INC., as a Guarantor |
||
|
|
||
|
Per: |
/s/ Arun Swarup Menawat |
|
|
|
Name: |
Arun Swarup Menawat |
|
|
Title: |
Authorized Signing Officer |
Signature Page – Credit Agreement
|
CANADIAN IMPERIAL BANK OF |
||
|
|
||
|
Per: |
/s/ Julie Silva |
|
|
|
Name: |
Julie Silva |
|
|
Title: |
Assistant General Manager |
|
|
||
|
|
||
|
Per: |
/s/ Graham Quisenberry |
|
|
|
Name: |
Graham Quisenberry |
|
|
Title: |
Assistant General Manager |
Signature Page – Credit Agreement
Exhibit A
COMPLIANCE CERTIFICATE
____________ __, 202__
CIBC Innovation Banking
81 Bay Street, 10th Floor
Toronto, Ontario
M5J 1E7
Attention: [***]
Re:Amended and Restated Credit Agreement, dated as of March 3, 2025 (as amended, restated, supplemented or otherwise modified from time to time, the “Credit Agreement”), among Profound Medical Inc., an Ontario corporation (the “Borrower”), the other Loan Parties from time to time party thereto, and CIBC (“Lender”).
Ladies and Gentlemen:
Concurrently herewith Borrower delivers to Lender the [monthly/quarterly/annual] financial statements for the period ending _________________. Any capitalized terms used herein but not defined shall have the meaning set forth in the Credit Agreement.
This letter shall serve as certification by or on behalf of the Borrower to Lender of the following:
(b)In accordance with Section 6.20 of the Credit Agreement, the Loan Parties are in compliance with the following financial covenants:
For the month ended ____________________________:
(A) Unrestricted Cash |
$____________________________ |
|
|
(B) Required Liquidity Amount (the greater of 9 months EBITDA losses or $7,500,000) |
$____________________________ |
|
|
Required: (A) must be greater than (B) |
|
|
|
Complies: ☐ Yes ☐ No |
|
|
|
[For the quarter ended : | |
| |
(A) Revenue in Most Recent Reported Trailing 12-month Period |
$____________________________ |
|
|
(B) Revenue in the same period in the prior Fiscal Year |
$____________________________ |
|
|
Required: (A) must be at least 15% greater than (B) | |
|
|
Complies: ☐Yes ☐No] [Only applicable Quarterly] | |
|
|
(c) In accordance with Section 6.11 of the Credit Agreement, the Loan Parties maintain the following Deposit Accounts, each of which contains the following cash balance as of the date hereof: | |
Account Holder |
Depository / |
Address |
Account |
Cash Balance |
|
|
|
|
|
(d) Additional confirmations: |
|
||||
|
|
||||
|
(A) |
Any change in the legal name, jurisdiction of organization or location of chief executive office or registered office of any Loan Party or new Canadian Provinces in which they operate? |
|||
|
|
||||
☐Yes, details listed below ☐ No |
|
||||
|
|
||||
|
|
|
|
||
|
(B) |
Are there any new Deposit Accounts or Securities Accounts opened by any Loan Party (not previously disclosed on a Compliance Certificate)? |
|||
- 2 -
☐Yes, details listed below ☐ No |
|
Account Holder |
Depository / Intermediary |
Address |
Account Number |
Restricted |
Control Agreement |
|
|
|
|
☐ |
☐ |
|
(C) |
Have any Subsidiaries been formed, acquired other than Subsidiaries set forth on Section 5.30 to the Agreement or as previously disclosed on a Compliance Certificate? Are any of them Material Subsidiaries? |
☐Yes, details listed below, together with updated organizational chart |
|
☐No |
Name |
Jurisdiction of Organization |
Shares |
Ownership of Subsidiary |
Purpose |
|
|
|
|
|
|
|
|
|
|
(e) Attached hereto are any updates to the schedules to the Agreement to give effect to the statement in clause (a)(iii)(B) above. |
|
(f) [For the certificate for March, June, September and December only to comply with the statement in Section 6.21(c) of the Credit Agreement]: Attached is a list of new Intellectual Property acquired and not previously disclosed. |
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
- 3 -
[SIGNATURE PAGE TO COMPLIANCE CERTIFICATE]
This Compliance Certificate is a Financing Document. Such certification is made as of ____________ __, 202__.
|
BORROWER: |
|
|
[_______][[_______]] |
|
|
|
|
|
By: |
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
|
Exhibit B
DRAWDOWN REQUEST
Date: [●]
To: Canadian Imperial Bank of Commerce (the “Lender”)
Ladies and Gentlemen:
1. |
This Drawdown Request is delivered to you pursuant to that certain amended and restated credit agreement dated as of March 3, 2025 among Profound Medical Inc., an Ontario corporation (the “Borrower”), the other Loan Parties from time to time party thereto, and the Lender (as amended, amended and restated, renewed, extended or supplemented, the “Credit Agreement”). Capitalized terms used and not expressly defined in this Drawdown Request shall have the respective meanings given to them in the Credit Agreement. |
2.The undersigned hereby requests a Drawdown under the Credit Agreement as follows:
(a)Drawdown Date: [●], which day is a Business Day.
(b)Amount of Drawdown: $[●].
(c)Currency: US Dollars.
(d)Credit Facility: Revolving Credit Facility.
(e)Type of Advance: US Base Rate Advance.
3.Payment Instructions. Please remit funds to: [●]1.
4.The undersigned hereby certifies that:
(a)All representations and warranties made in Article V of the Credit Agreement or in the other Financing Documents shall be true and correct in all material respects (unless such representations and warranties are qualified by materiality, in which case such representations and warranties shall be true and correct in all respects) with the same effect as though such representations and warranties had been made on and as of the Drawdown Date (except where such representations and warranties expressly relate to an earlier date, in which case such representations and warranties were true and correct as of such earlier date);
(b)No development or event has occurred that has had or could reasonably be expected to have a Material Adverse Effect and there has been no material deterioration in the
1 |
NTD: Insert Remittance Instructions |
consolidated financial condition of the Loan Parties since the date of the most recent financial statements submitted to Lender;
(c)All conditions precedent to a Drawdown set out in Section 7.02 of the Credit Agreement have been satisfied as of the date of this request;
(d)No Default or Event of Default has occurred and is continuing, or would result from the borrowing requested herein or from the application of the proceeds therefrom;
(e)No injunction, writ, restraining order, or other order of any nature prohibiting, directly or indirectly, the extending of such credit has been issued and remains in force by any Tribunal against any Loan Party or any of their Affiliates; and
(f)The Borrower shall be in pro-forma compliance with the financial covenants set out in Section 6.20 of the Credit Agreement after giving effect to the Drawdown requested and has provided satisfactory evidence of same as requested by the Lender.
|
PROFOUND MEDICAL INC. |
||
|
|
||
|
|
||
|
Per: |
|
|
|
|
Name: |
[●] |
|
|
Title: |
[●] |
- 2 -
Exhibit C
BORROWING BASE CERTIFICATE
TO: |
Canadian Imperial Bank of Commerce Emails: [***] Attention: [***] |
RE: |
Amended and restated credit agreement dated as of March 3, 2025 among Profound Medical Inc., an Ontario Corporation (the “Borrower”), the other Loan Parties from time to time party thereto, and the Lender (as amended, amended and restated, renewed, extended or supplemented, the “Credit Agreement”) |
1. |
This Borrowing Base Certificate is delivered to you pursuant to the provisions of the Credit Agreement. All capitalized terms used in this Borrowing Base Certificate which are defined or given extended meanings in the Credit Agreement have the respective meanings attributed to them in the Credit Agreement. |
2. |
The Borrower hereby certifies that as of [●] the Borrowing Base was determined in accordance with the Credit Agreement as follows: |
(1) |
Recurring Revenue: |
(a) |
Recurring Revenue for the most recently reported trailing 3 months: |
$____________ |
(b) |
(1)(a) multiplied by 4: |
$____________ |
(2) |
Priority Payables: |
(a) |
unpaid amounts owing for wages, vacation pay and severance pay: |
$____________ |
(b) |
employee deductions: |
$____________ |
(c) |
Taxes: |
$____________ |
(d) |
income tax: |
$____________ |
(e) |
workers compensation: |
$____________ |
(f) |
government royalites: |
$____________ |
(g) |
pension fund obligations, Canadian Pension Plan and Non-Canadian Pension Plan obligations, including the amounts of any unfunded liability or solvency deficit: |
$____________ |
(h) |
any amount payable which is secured by a lien which ranks or is capable of ranking prior to or pari passu with the liens created by the Security Documents: |
$____________ |
(i) |
Total Priority Payables [2(a)-2(h)]: |
$____________ |
(3) Borrowing Base = (1)(b) less (2)(i): |
|
$____________ |
3. |
Attached hereto as Exhibit 1 and set forth in reasonable detail are the calculations used to determine the amounts contained in Section 2 above and, for greater certainty, taking into account the appropriate sections of the Credit Agreement, including but not limited to the definition of Borrowing Base. |
DATED this [●] day of [●], [●].
|
PROFOUND MEDICAL INC. |
||
|
|
||
|
|
||
|
Per: |
|
|
|
|
Name: |
[●] |
|
|
Title: |
[●] |
|
|
|
|
EXHIBIT 1
BORROWING BASE CALCULATIONS
[To be completed by Borrower]
Exhibit 19
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 1 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
PROFOUND MEDICAL CORP.
CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES BY DIRECTORS, OFFICERS, EMPLOYEES AND CONSULTANTS POLICY
Corporate Disclosure and Confidentiality
Profound is committed to providing timely, accurate and balanced disclosure of material information about Profound Medical Corp. and its subsidiaries (collectively referred to as "Profound" or the "Company") and their respective businesses, operations, assets and liabilities and commitments, on a consolidated basis, consistent with statutory and regulatory requirements.
A fundamental principle of Canadian and U.S. securities laws is that everyone investing in securities should have equal access to information that may affect their decision as to whether to buy or sell securities. Directors, officers, employees and consultants of a corporation sometimes acquire knowledge of material information concerning the business and affairs of the corporation (or a related entity or an entity with whom the corporation does business) which has not yet been disclosed to the public. If that is the case, they have an unfair advantage in buying or selling securities because the seller or buyer on the other side of the transaction may have made a different investment decision had they been aware of that information.
Similarly, if such a person informs another person of material non-public information, and such person buys or sells securities on the basis of that information, the seller or buyer on the other side of the transaction is, once again, at a disadvantage.
Certain securities laws have been enacted so as to prevent and deter such inequitable trading in securities by providing that:
1. |
Corporations whose shares trade publicly must promptly disclose material information relating to the corporation, and must do so publicly (i.e., no selective disclosure); |
2. |
Employees, consultants, directors, officers and persons receiving material non-public information are prohibited from buying or selling securities of the corporation or from recommending trades or encouraging others to trade in securities of the corporation while in possession of such material non-public information and prior to dissemination of such information to the public; |
3. |
Employees, consultants, directors and officers are prohibited from disclosing material non-public information relating to the corporation to third parties, other than when it is necessary to do so in the course of business of the corporation and the third party has agreed to (or otherwise has a duty to) maintain the confidentiality of such information; and |
4. |
Significant shareholders, officers and directors must report their trades in securities of the corporation. |
In addition, securities laws create a cause of action for investors in the secondary market against corporations and their directors and officers with respect to material misstatements or omissions in their public disclosures.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 2 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
The Company has formulated this policy to assist the Company and its employees, consultants, directors and officers in complying with the foregoing statutory and regulatory requirements. The purpose of this policy is to promote compliance with these requirements by establishing procedures and policies for timely and accurate corporate disclosure, maintaining confidentiality of material information relating to the Company and trading by employees, consultants, directors and officers of the Company in securities of the Company (or the securities of other companies about which such persons may possess material non-public information in connection with their relationship with Profound).
This policy extends to all employees, directors, consultants and officers of Profound and covers disclosures of material information about the Company in all mediums, including without limitation documents filed with applicable securities commissions and stock exchanges, written statements made in Profound's annual and quarterly reports, news releases, letters to shareholders, speeches by management or employees, information contained on Profound's website and other electronic communications and public verbal statements made in meetings and telephone conversations with analysts and investors, interviews with the media as well as press conferences and conference calls.
No disclosure of any material non-public information in respect of the Company is to be made by any employee, consultant, officer or director of the Company, whether by way of news release, public oral statements or filings with securities regulatory authorities or otherwise, EXCEPT in strict compliance with this policy.
Material Information
Securities laws and this policy make frequent reference to material information. In this policy, material information means any information relating to the business, operations, capital and affairs of the Company that when released would have, or would reasonably be expected to have, a significant effect on the market price or value of any of Profound's securities (or the securities of other companies with whom Profound may be conducting business) or to which there is a substantial likelihood that a reasonable investor would attach importance in determining whether to buy or sell such securities. Material information consists of both material facts and material changes relating to Profound's business, operations, capital and affairs and includes developments in Profound's business, operations, capital and affairs. Examples of information which may be material information include but are not limited to:
Changes in Corporate Structure
| ● | changes in share ownership |
| ● | reorganizations, amalgamations, mergers, joint ventures, divestitures or other significant changes in assets |
| ● | takeover bids in respect of Profound's securities or securities of another company or bids by Profound for its own securities |
Changes in Capital Structure
| ● | public or private sales of securities |
| ● | repurchases or redemptions of securities |
| ● | consolidations, subdivisions, stock splits, stock dividends, rights offerings or share exchanges |
| ● | changes in the Company's dividend payments or policies |
| ● | possible initiation of a proxy fight |
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 3 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
| ● | modifications to the rights of security holders |
Financial Results
| ● | quarterly or annual earnings results or changes in financial results |
| ● | projections of, or guidance regarding, future financial or operational performance by the Company (including without limitation reaffirmation of any previously provided projections or guidance on earnings or anticipated financial or operational performance). |
| ● | shifts in financial circumstances, such as cash flow reductions, major asset write-offs or write- downs |
| ● | changes in the value or composition of Profound’s assets |
| ● | changes in Profound’s accounting policies |
| ● | changes in or disagreements with auditors, or a notification that the auditor’s reports may no longer be relied upon |
Business and Operations
| ● | news or developments in the Company’s business or commercialization or product development efforts |
| ● | any development that significantly affects Profound’s resources, technology, intellectual property, products or markets |
| ● | a significant change in capital investment plans or corporate objectives |
| ● | licenses of significant technologies |
| ● | entry into, modification or termination of significant strategic collaborations |
| ● | major labour disputes or disputes with major contractors or suppliers |
| ● | significant new contracts, products, patents, or services or significant losses of (or amendments to) contracts or business |
| ● | pre-clinical, clinical, scientific or research developments |
| ● | developments involving health regulatory authorities or pricing authorities or bodies, such as significant filings or approvals |
| ● | changes to the Board of Directors or executive management, including the departure of Profound’s Chief Executive Officer, Chief Financial Officer or other executive officers (or persons in equivalent positions) |
| ● | the commencement of, or developments in, legal proceedings or regulatory matters |
| ● | waivers of corporate ethics and conduct rules for officers, directors, and other key employees |
| ● | any notice that reliance on a prior audit is no longer permissible |
| ● | de-listing of Profound’s securities or their movement from one quotation system or exchange to another |
| ● | significant transactions with officers, directors or greater than 5% shareholders |
Acquisitions and Dispositions
| ● | acquisitions or dispositions of assets, property (including intellectual property) or joint venture interests |
| ● | acquisitions of other companies, including a take-over bid for, or merger with, another company |
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 4 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
Credit Arrangements
| ● | the borrowing or lending of a significant amount of money in the context of Profound’s business and operations |
| ● | any mortgaging or encumbering of Profound’s assets |
| ● | defaults under debt obligations, agreements to restructure debt, or planned enforcement procedures by a bank or any other creditors |
| ● | changes in rating agency decisions or other deterioration in the Company’s credit status |
| ● | new or amended credit arrangements |
Information is considered nonpublic if it has not previously been publicly disseminated such that investors have had the opportunity to evaluate it, or it has not been filed with a governmental agency as a matter of public record. Information that is public would include information included in broadly disseminated press releases and publicly available documents filed with the securities regulatory authorities.
The Chief Executive Officer and Chief Financial Officer will monitor developments and other matters within the Company that may necessitate disclosure to the public. Whenever questions arise about whether information constitutes material non-public information, they will be determined by the Chief Executive Officer and the Chief Financial Officer, who may elect to consult with outside legal counsel, auditors or other advisors if necessary. The Chief Executive Officer and the Chief Financial Officer will then ensure all material information is released publicly in accordance with the procedures outlined in this policy.
Any director, officer, employee or consultant shall alert the Chief Executive Officer and the Chief Financial Officer if they become aware of any information that may be material if they do not believe the information will otherwise be communicated to them. It is essential that the Chief Executive Officer and the Chief Financial Officer be fully apprised of all material information in order to evaluate and discuss that information to determine the appropriateness and timing for public release of such information or whether the information should remain confidential and, if so, how that material information will be controlled so as to ensure its confidentiality. If a director, consultant, officer or employee does not believe that the information is being dealt with as required by this policy, he or she shall alert the Lead Director of the Company.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 5 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
Policy on Disclosure of Material Information
1.Disclosure Within the Company. Material information relating to the Company should only be disclosed to those officers, employees or consultants who need to know the information to perform their duties. Officers, employees or consultants to whom material information is disclosed shall be advised of the confidential nature of such information and shall be reminded of their obligation to take all reasonable precautions to ensure inadvertent disclosure of material non-public information does not occur. Such precautions may include using code names, not leaving documents where they can be seen or accessed by other persons, not discussing the information in public places or on cellular telephones, storing confidential information in locked cabinets or drawers, securing or coding communications sent by telecommunications, not storing information on computers in a manner that gives rise to a risk that unauthorized operators can gain access to it and shredding confidential documents. The officer or manager responsible for an activity or negotiation involving material non-public information shall be responsible for instituting necessary controls to provide adequate security and to monitor the observance of such controls and, in particular, he or she shall provide the Chief Executive Officer with a list of all such officers, employees or consultants who have access to material non-public information relating directly or indirectly to the Company so that the Insider List (defined below) can be properly maintained. Without limiting the foregoing, necessary controls may include limitations on persons to whom any disclosure of material non-public information may be made (even if in the necessary course of business or authorized by law) without the advance permission of the responsible officer or manager.
2.Disclosure to Third Parties. No material non-public information shall be disclosed by directors, officers, employees or consultants to third parties except in the necessary course of business where the third party has agreed to maintain such information in confidence through a confidentiality agreement, or where otherwise authorized or required by law. Where information is disclosed to a third party in the necessary course of business the third party should be advised that they must not disclose the information to anyone else, other than in the necessary course of business, and they may not trade in securities of the Company until the information has been publicly disclosed by means of a press release or other public filing with the securities regulatory authorities. The execution of a confidentiality agreement does not, however, permit disclosure of confidential information to third parties where this disclosure is not in the necessary course of business. The director, officer or manager responsible for an activity or negotiation involving disclosure of material non-public information shall be responsible for instituting necessary controls to provide adequate security and to monitor the observance of such controls. Without limiting the foregoing, necessary controls may include limitations on persons to whom any disclosure of material non-public information may be made (even if in the necessary course of business or authorized by law) without the advance permission of the responsible officer or manager.
3.Insider Lists. The Company will draw up a list (an "Insider List") of (a) those directors, officers, employees and consultants of the Company who have access to material non-public information relating directly or indirectly to the Company and (b) its principal contacts at any other firm or company acting on its behalf or on its account with whom it has had direct contact and who also have access to material non-public information about the Company, whether on a regular or occasional basis. The Company will maintain any Insider List for the amount of time required by applicable securities regulations.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 6 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
Responsibility for Disclosure. “Disclosure Controls and Procedures” means controls and other procedures of the Company that are designed to ensure that information required to be disclosed by the Company in the reports that it files or submits to Regulators (as defined below) is recorded, processed, summarized and reported, within the time periods specified in the Regulator’s rules and forms. Management has ultimate responsibility for the Company's Disclosure Controls and Procedures. Accordingly, the Chief Executive Officer and the Chief Financial Officer are responsible for the following tasks:
(a) |
Determine when events, developments, changes or other facts constitute material information or a material change in the affairs of Profound. In making such determination, the Chief Executive Officer and Chief Financial Officer will assess the impact of any such event, development or change on: |
(i) |
the financial statements and overall business of Profound; |
(ii) |
the reputation and operations of Profound; |
(iii) |
the strategic direction of Profound; and |
(iv) |
the market price or value of any of Profound's securities. |
(b) |
Review and, as necessary, help revise Profound's Disclosure Controls and Procedures to ensure that: |
(i) |
information required by Profound to be disclosed to securities regulators and stock exchanges (the “Regulators”), and other written and oral information that Profound will disclose to the public is recorded, processed, summarized and reported accurately and on a timely basis; and |
(ii) |
such information is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure. |
(c) |
Assist in documenting, and monitoring the integrity and evaluating the effectiveness of the Disclosure Controls and Procedures, where appropriate. |
(d) |
Review and supervise the preparation of Profound's: |
(i) |
Annual Information Form, Annual Report, Proxy Circular, Financial Statements and any other information filed with the Regulators (collectively, the "Reports"); |
(ii) |
press releases containing financial information, earnings guidance, forward-looking statements, and information about operations; |
(iii) |
correspondence broadly disseminated to Profound’s security holders; and |
(iv) |
other relevant written and oral communications or presentations (items (i) to (iii) above are collectively referred to as, the "Disclosure Statements"). |
(e) |
Discuss with each other and senior management information relative to their responsibilities, including: |
(i) |
the preparation of the Disclosure Statements; and |
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 7 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
(ii) |
the evaluation of the effectiveness of the Disclosure Controls and Procedures. |
(f) |
Maintenance of a record of the Disclosure Statements of Profound. |
(g) |
Review risk factors, underlying assumptions and forward-looking statement language for written and oral communications which contain forward-looking information and review that there is a reasonable basis for any conclusions, forecasts or projections contained in such information. |
(h) |
Review and approve disclosure of information displayed on Profound's web site or other electronic media. |
The Chief Executive Officer and the Chief Financial Officer will meet regularly to carry out their responsibilities hereunder.
4.Prompt Disclosure. To the extent required by law, the Company shall make prompt disclosure of material non-public information to stock exchanges, securities commissions and to the public in accordance with its obligations under applicable securities laws and stock exchange rules. Generally speaking, material information shall be disclosed by news release and posted on SEDAR+ (in Canada) and on EDGAR (in the United States), and, in certain circumstances, the Designated Corporate Spokesperson (as defined below) will conduct a conference call, open to the public, to discuss the information contained in the news release.
Once a determination that material non-public information exists has been made in accordance with this policy, the Chief Executive Officer and Chief Financial Officer will authorize the issuance of a news release, unless such material information is required or permitted to remain confidential.
Should non-public material information be inadvertently disclosed in a selective forum, a news release will be issued immediately in order to publicly disclose that information. Any director, officer, consultant or employee of the Company who becomes aware of disclosure of any material non-public information in violation of this policy is required to report such disclosure to the Chief Executive Officer and the Chief Financial Officer immediately. As discussed in Section 11 below, such disclosures to analysts and investors (e.g., at an “analyst day” or an investor conference) should be preceded by a widely disseminated public announcement, which avoids selective disclosure issues.
News releases will be disseminated through a newswire service that provides broad simultaneous public disclosure in Canada and the United States. News releases will be transmitted to all stock exchange members, relevant regulatory bodies, major national financial media and local media in areas where the head office and operations are located.
Regardless of when an announcement involving material information is released, the market surveillance department of each exchange upon which securities of the Company are listed (each, an "Exchange") must be advised of the content of the release and supplied with a copy in advance of its release in accordance with the rules of the applicable Exchange.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 8 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
5.Principles of Disclosure of Material Information. In complying with the requirement under applicable laws and stock exchange rules to disclose material information forthwith upon the information becoming known to management or, in the case of information previously known, forthwith upon it becoming apparent that the information is material, the content of such disclosure shall be determined by the Chief Executive Officer and the Chief Financial Officer. In making such determination, the following basic disclosure guidelines will be observed:
(a) |
Material information will generally be publicly disclosed as soon as practicable, except under certain limited circumstances, such as: |
(i) |
when such information relates to a transaction or series of transactions that remain subject to further negotiations, and may be abandoned or otherwise not agreed to; |
(ii) |
when disclosure of the information would provide competitors with confidential information that would be of significant benefit to them (for which confidential treatment must be requested and approved by applicable securities regulators); or |
(iii) |
when disclosure of the information would involve disclosure of personally sensitive information (for which confidential treatment must be requested and approved by applicable securities regulators). |
If it is determined that the disclosure of material non-public information will be delayed or omitted for the foregoing or other reasons, complete confidentiality of the material information must be maintained.
(b) |
Announcements of material information should be factual and balanced. |
(c) |
Unfavourable material information must be disclosed as promptly and completely as favourable material information. |
(d)Disclosure must include all relevant information to ensure that no aspect of the disclosure is materiallymisleading.
(e) |
Material non-public information must not be disclosed selectively. If such information has been inadvertently disclosed to an analyst or any other person, it must be publicly disclosed immediately by news release. |
(f) |
To the extent required by law, disclosure will be updated if earlier disclosure has become materially misleading as a result of intervening events. |
The Chief Executive Officer and the Chief Financial Officer shall conduct a reasonable investigation of the subject matter of the disclosure prior to any disclosure being made, including ascertaining all relevant facts from officers, employees, consultants and others whose duties would in the ordinary course give them knowledge of such facts.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 9 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
6.Designated Corporate Spokespersons. Communications with analysts, news media, investors, stock exchanges and securities regulatory authorities or the making of any other statement of material information with respect to the Company in circumstances where a reasonable person would believe that the information will become generally disclosed (“public oral statements”) shall be made only by the Chief Executive Officer or the Chief Financial Officer, or officers designated by such persons (each a "Designated Corporate Spokesperson"). All other directors, officers, employees and consultants are prohibited from publicly communicating information about the Company (unless specifically authorized by the Chief Executive Officer or the Chief Financial Officer) and if approached should refer the enquiry to one of such designated officers. For greater certainty, all public speaking engagements by directors, officers, employees or consultants in connection with the Company must be pre-authorized by the Chief Executive Officer and the Chief Financial Officer.
To the extent possible, any presentation, speech or other public oral statement shall be fully scripted prior to the making thereof and the Chief Executive Officer and the Chief Financial Officer shall be provided with an opportunity to review the information to be disclosed to ensure such information does not include any material non-public information.
Where possible, more than one representative of the Company will be present at all presentations where public oral statements may be made, including individual and group meetings with analysts, media and investors and quarterly conference calls, and every effort shall be made to retain electronic or other records of any public oral statements made in respect of the Company.
A review will be conducted after all such presentations to ensure that selective disclosure of material non- public information has not been made. If selective disclosure of material non-public information has been made, such information shall be immediately disclosed by a widely disseminated news release in accordance with this policy.
7.Release of Forward-Looking Information. Forward-looking information means management’s expectations of future events, conditions or results that is based on assumptions about future economic conditions and courses of action. Forward-looking information includes, among other things, the Company’s financial outlook and other financial information with respect to future results of operations, financial position or cash flows, and may be presented as either a forecast or a projection, and expectations about the development and commercialization of the Company’s products. Forward-looking information is subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from the forward-looking information. The Company will not release financial projections without prior approval of the Audit Committee. It may, from time to time, release other forward-looking information to enable the investment community to better evaluate the Company and its operations. The Company will not disclose significant data or other material information to analysts or investors unless such data or information has been publicly disclosed.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 10 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
If forward-looking information is provided in a document filed with a Regulator, press release or other public disclosure document, the document must contain proximate to that disclosure: (a) reasonable cautionary language identifying the forward-looking information as such, stating that actual results may vary from the forward-looking information and identifying material risk factors that could cause actual results to differ materially from the forward-looking information; (b) a statement of the material factors or assumptions used to develop the forward- looking information; and (c) the Company's policy for updating forward-looking information. In the case of a public oral forward-looking statement, the person making such statement shall: make a cautionary statement that the oral statement contains forward-looking information; and state that (i) actual results could differ materially from a conclusion, forecast or projection in the forward-looking information, (ii) certain material factors or assumptions were applied in drawing a conclusion or making a forecast or projection as reflected in the forward- looking information; (iii) additional information about the material factors that could cause actual results to differ materially from the conclusion or making a forecast or projection as reflected in the forward-looking information, and (iv) the forward-looking statement speaks only as of the date when made and will not be updated or altered as a result of new information, future events or otherwise, except to the extent required by law.
All disclosure of forward-looking information will be reviewed and approved by the Chief Executive Officer and the Chief Financial Officer.
8.Disclosure Record. The Company shall maintain a file of all public information relating to the Company including news releases, research reports, press reports and debriefing notes for a period of five years. The Company’s policy is not to review analyst reports to confirm, correct, clarify or comment on information provided therein.
9.Use of Electronic Media and the Website. Officers responsible for written public disclosures shall also be responsible for electronic communications. The Chief Executive Officer is responsible for ensuring that the Company's website is current and up to date and for monitoring all material information placed on the website to ensure that it is not misleading. Material information is misleading if it is incomplete, incorrect or omits a fact so as to make another statement misleading. Information may also be misleading if it is out of date. Any misstatements of material information on the Company’s website must be addressed immediately.
The Company's website should include all publicly disclosed material information and such other investor relations information as may be determined appropriate; provided that no document relating to an offering of securities shall be posted on the Company's website without first consulting legal counsel. Information should be posted to the Company's website as soon as possible following its public disclosure. Disclosure of material non-public information on the Company's website alone typically does not constitute adequate disclosure. Therefore, any disclosures of material information on the Company's website will be in conjunction with a news release or a public filing on SEDAR+ and EDGAR.
The Chief Executive Officer shall also be responsible for responses to electronic inquiries. Only public information or information which could otherwise be disclosed in accordance with this policy shall be communicated in responding to electronic inquiries.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 11 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
The Chief Executive Officer may designate specific individuals who are authorized to communicate with the public on specified social media platforms. The public should be alerted, including on the Company's website, of any social media accounts that may be used by the Company for disclosure purposes which accounts shall be accounts of the Company and not the personal social media accounts of officers, directors, or employees of the Company. Like all other corporate disclosures, communications made on social media platforms must not be misleading. The Chief Executive Officer is responsible for monitoring communications made on social media platforms. Disclosure of material non-public information on social media platforms alone typically does not constitute adequate disclosure. As such, any disclosures of material information on social media platforms will only be made in conjunction with a news release or a public filing on SEDAR+ and EDGAR. Certain corporate disclosures require the inclusion of additional statements, explanatory language and cautionary language, such as non-IFRS disclosures and forward-looking information which may be difficult or impossible to include in some forms of social media communications. Therefore, disclosures of such information must be avoided in such communications. If the Company is in the course of distributing securities, legal counsel should be consulted before making any communications on social media platforms.
Other than those individuals specifically authorized to communicate with the public on specified social media platforms, employees, consultants, directors and officers are prohibited from discussing matters relating to the Company in any chat rooms or posting any information relating to the Company on any electronic bulletin boards, social media platforms or other electronic forums, including responding to rumours about the Company. If you become aware of any discussion or rumours pertaining to Profound, you should advise the Chief Executive Officer or Chief Financial Officer.
10.Rumours. Provided it is clear that the Company and related entities are not the source of the market rumour, the Designated Corporate Spokespersons will consistently respond to questions about market rumours with a statement to the effect that “it is our policy not to comment on market rumours or speculation”. Should the Exchange request a definitive statement be issued in response to a market rumour that is causing significant volatility in the securities of the Company, the Chief Executive Officer and the Chief Financial Officer will consider the matter and decide on an appropriate response.
11.Contacts with Analysts, Media and Investors. The Company recognizes that analysts and news media are important for disseminating corporate information to the investing public and play a key role in interpreting and clarifying information that has been publicly disclosed by the Company. Only the Designated Corporate Spokespersons may meet or communicate with analysts, media and investors and will initiate contacts or respond to analyst, media and investor calls in accordance with this policy. The Company will provide the same information that has been provided to analysts to individual investors or reporters who request it. Where practicable, analyst conference calls should be held in a public, open manner, following adequate public notice, to allow all interested parties access to such calls.
It is recognized that disclosure of material non-public information to analysts, investors or the media does not constitute adequate disclosure for the purposes of applicable securities laws. Accordingly, if material information is to be announced at an analyst or shareholder meeting or press conference, its announcement must be preceded by a widely disseminated public announcement of such information by news release.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 12 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
12.Quiet Periods. In order to avoid the potential perception or appearance of selective disclosure, the Company will observe a quarterly quiet period, during which no meetings or telephone contacts with analysts and investors will be initiated The quiet period will be in effect from ten (10) business days ahead of the release of the Company of quarterly or annual financial results (or, if the quarterly or annual earnings information is substantially complete (as determined by the Chief Financial Officer) at an earlier date, then from such earlier date) until after (i) the release by the Company of quarterly or annual financial results and (ii) the completion of the quarterly or annual earnings call, if any.
13.Reviewing Analyst Draft Reports and Models. The Company's policy is not to review analysts' research reports or models to confirm, correct, clarify or otherwise comment on information provided therein. Analyst's reports are proprietary information belonging to and expressing the views of the analyst's firm. Re-circulating an analyst's report may be viewed as an endorsement of the report and, therefore, analyst's reports are not to be included in investor packages of the Company. If an analyst's report is provided to any employees of the Company or persons outside of the Company, every effort will be made to ensure that it is evident who authored such report and that the Company not comment on the report.
14.Quarterly Conference Calls. A quarterly conference call may be held with members of the investment community to discuss financial and operating results following the widespread dissemination of the news release announcing such results. The date and time of the call, the subject matter of the call and the means for accessing it shall be included in a news release (such news release to be disseminated in advance of the news release announcing the financial and operating results to be discussed) and may also be announced on the Company's website. Such quarterly conference calls shall be held in an open manner allowing members of the investment community and any other interested party to listen either by telephone and/or through a webcast.
15.Expert Reports and Opinions. Prior to using any report, statement or opinion of an expert in any disclosure document of the Company or in any public oral statement made by a Designated Corporate Spokesperson, the written consent of such expert shall be obtained authorizing such use.
TRADING IN SECURITIES OF THE COMPANY
1.Prohibition on Trading with Material Non-public Information. Trading by directors, officers, employees or consultants in securities of the Company (which includes shares, options, warrants, rights, debentures and puts and calls relating to the Company's securities) is prohibited while in possession of material non-public information. Recommending or influencing trades or encouraging or influencing others to trade in securities of the Company while in possession of material non-public information is also prohibited. The appropriate officer with executive responsibility for a project or particular area should remind each employee or consultant who may have access to the material non-public information that they must not trade in, recommend or influence trades in or encourage or influence others to trade in the Company's securities until public disclosure of the material information has been made. It should be noted that this prohibition on trading extends not only to securities which the director, officer, employee or consultant owns but also to those over which direction or control is exercised (for example, as a trustee or executor of an estate or on behalf of minor children) and also to securities that are indirectly owned (for example, by a corporation controlled by the employee). The prohibition also applies to spouses and children who live in the director, officer or employee's household. The prohibition on recommending or influencing trades or encouraging or influencing others to trade in securities of the Company extends to all persons.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 13 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
2.When Public Disclosure has Been Made. As noted above in “Policy on Disclosure of Material Information,” the Chief Executive Officer/Chief Financial Officer is responsible for determining when disclosure should be made and the nature of the disclosure. Under the securities laws, trading may not commence until public disclosure has been made by the Company and the market has had a period to "absorb" such information. Furthermore, it should be noted that trading is not made permissible by the fact that rumours exist in the market- place or in the media - the disclosure must be made by the Company before trading can commence. It is the policy of the Company that no trading by directors, officers, employees or consultant may be made until two clear trading days have passed following the public disclosure by the Company of the material information.
3.Clearance of Trades. Every director and officer of Profound and every Financial Team Member and Designated Individual listed on Appendix B hereto, is required to pre-clear transactions in Profound’s securities through the Chief Executive Officer or Chief Financial Officer before placing a buy or sell order in the case of a purchase or sale, or otherwise making or committing to complete any other transaction. Persons who request pre- clearance for a trade in Profound’s securities in respect of which there is material non-public information, will be advised by the Chief Executive Officer or Chief Financial Officer that trading in such securities is currently prohibited. No further explanation as to the reason for the trading prohibition will be provided. The Chief Executive Officer or Chief Financial Officer will use his or her reasonable best efforts to provide approval or disapproval within two business days. The person seeking pre-clearance must wait until receiving pre-clearance to execute the transaction. Neither the Company nor the Chief Executive Officer or Chief Financial Officer shall be liable for any delays that may occur due to the pre-clearance process. If the transaction is pre-cleared by the Chief Executive Officer or Chief Financial Officer, it must be executed by the end of the second business day after receipt of pre-clearance. Notwithstanding receipt of pre-clearance of a transaction, if the employee, consultant, director or officer becomes aware of material non-public information about the Company after receiving the pre-clearance but prior to the execution of the transaction, they may not execute the transaction. The responsibility for determining whether such employee, consultant, director or officer is in possession of material non-public information rests with such person.
4.Black-out Periods. Directors, officers, employees and consultants are precluded from trading in securities of the Company for a period beginning on the first day immediately following the end of each fiscal quarter or year and will continue until two (2) clear trading days have passed following the release by the Company of its quarterly or annual financial statements, as applicable, for such fiscal quarter or year. In addition, black-out periods may be imposed by management on notice to the directors, officers, employees and consultants when developments (e.g., developments related to clinical results, product development or regulatory approvals) warrant such trading restrictions. Employees, consultants, directors and officers will be advised in writing of the imposition of any black-out period(s). During black-out periods, directors, officers, employees and consultants are restricted from trading in securities of the Company, and will be notified when the black-out period is lifted. The Company will not, except in exceptional circumstances, during a blackout period (i) grant or establish the exercise price of stock options, or (ii) grant or determine the number of initial grant units of other equity incentive compensation. However, the existence of a black-out period will not prohibit the exercise of any previously- granted, outstanding stock options (provided that the shares are not sold during the black-out period).
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 14 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
5.Trading In Securities of Other Corporations. If directors, officers, employees or consultants in the course of performing their duties become aware of material non-public information about another public company, they may not trade in securities of that other public company or recommend trades or encourage others to trade in such securities until the material information has been publicly disclosed.
6.Compliance with Insider Reporting Obligations. All "reporting insiders" (as defined in applicable Canadian securities legislation) are required to file reports of their trades in securities of the Company and shall file such reports within the time periods prescribed in applicable securities legislation. Failure to report in a timely fashion, will subject reporting insiders to late filing fees in certain jurisdictions. The System for Electronic Disclosure by Insiders ("SEDI") is the insider trade reporting system available over the Internet at www.sedi.ca. SEDI requires reporting insiders to file electronically their insider reports, and issuers to file electronically certain issuer information, over the Internet, using the SEDI web site. SEDI is intended to allow the public to search for and look at public information filed on SEDI over the same web site.
A person is a "reporting insider" if they are:
| ● | A director, Chief Executive Officer, Chief Financial Officer or Chief Operating Officer of the Company, a "major subsidiary" of the Company, a shareholder of the Company that controls 10% or more of the common shares of the Company or a securityholder of the Company that would control 10% or more of the common shares of the Company on a post-conversion basis. A "major subsidiary" means a subsidiary of the Company if the assets (based on the most recent annual audited or interim statement of financial position) are 30% or more of the consolidated assets of the Company on that balance sheet or statement of financial position OR the revenue of the subsidiary (based on the most recent annual audited or interim statement of comprehensive income) is 30% or more of the consolidated revenue of the Company on that statement. |
| ● | A person responsible for a principal business unit, division or function of the Company. |
| ● | A shareholder of the Company that controls 10% or more of the common shares of the Company. |
| ● | An individual performing functions similar to those insiders listed above. |
| ● | Any other insider that in the ordinary course receives or has access to information as to material facts or material changes concerning the Company before the material facts or material changes are generally disclosed AND directly or indirectly, exercises, or has the ability to exercise significant power or influence over the business, operations, capital or development or the Company, regardless of whether that person holds the title of an officer or director. |
As the Company is a reporting issuer in the United States:
(a) |
For so long as Profound is a “foreign private issuer” (as defined in Rule 405 under the U.S. Securities Act of 1933, as amended), directors, executive officers and “beneficial owners” (within the meaning of Rule 13d-3 under the U.S. Securities Exchange Act of 1934, as amended (the “U.S. Exchange Act”)) of 10% or more of the common shares of the Company are not |
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 15 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
required to file beneficial ownership reports (Forms 3, 4 and 5) under Section 16 of the U.S. Exchange Act.
(b) |
However, beneficial owners of 5% or more of the common shares of the Company are subject to Section 13 of the U.S. Exchange Act and accordingly are required to file reports on Schedule 13D or 13G, as applicable. |
7.Hedging. The Board of Directors of the Company believes that it is inappropriate for directors, officers, employees and consultants of the Company to hedge or monetize transactions to lock in the value of holdings in the securities of the Company or to sell “short” securities of the Company. Such transactions, while allowing the holder to own the Company’s securities without the full risks and rewards of ownership, potentially separate the holder’s interests from those of other stakeholders (particularly, in the case of equity securities, the public shareholders of the Company). Therefore, unless otherwise approved by the Company’s Board of Directors, no director, officer, employee or consultant of Profound may, at any time, purchase financial instruments, including prepaid variable forward contracts, instruments for the short sale or purchase or sale of call or put options, equity swaps, collars, or units of exchangeable funds, that are designed to or that may reasonably be expected to have the effect of hedging or offsetting a decrease in the market value of any equity securities granted to any such person as compensation or any other securities of the Company held directly or indirectly thereby.
8.Contact Person. Any director, office, employee or consultant having any questions with respect to this policy or whether material information exists which has not been publicly disclosed or whether or not they may trade in a given circumstance should contact the Chief Executive Officer or the Chief Financial Officer.
ENFORCEMENT OF POLICIES
Violations of these policies may be a violation of securities laws, may constitute a criminal offence and in addition may result in embarrassment or loss to the Company. Under Canadian securities laws persons who make unauthorized disclosure of material information or trade, recommend trades or encourage others to trade while in possession of undisclosed material information are subject to, at the time of implementation of this policy:
| ● | fines of up to $5 million or three times the profit made or loss avoided; plus administrative penalties of up to $1 million for each failure to comply and disgorgement of any amounts obtained as a result of non- compliance; |
| ● | imprisonment for up to 5 years less one day; |
| ● | civil liability to the Company for any profit made; |
| ● | civil liability to the other party to the trade for the loss incurred by such other party (employees may have civil liability to persons who trade with persons to whom the employee "tips" material information). |
Such persons may also be subject to imprisonment for a term not to exceed 10 years under the Canadian Criminal Code.
Such persons may also be subject to criminal and civil penalties under U.S. securities laws.
If the Company discovers that an employee or consultant has violated securities laws, it may refer the matter to the appropriate regulatory authorities.
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 16 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
Employees or consultants who breach this policy are subject to disciplinary action including termination of employment.
REPORT OF COMPLIANCE
The Chief Executive Officer or Chief Financial Officer will provide a report, at least annually, to the Board of Directors summarizing:
After receiving the report of the Chief Executive Officer or Chief Financial Officer, the Board of Directors will review this policy to insure that the administration of the policy is adequate and identify any amendments which may be necessary in light of legal and business developments and the Company’s experience in administering the Policy.
APPLICATION AND INTERPRETATION
Any questions regarding the application or interpretation of this policy should be addressed to the Chief Executive Officer or Chief Financial Officer.
Adopted on June 14, 2018, and Amended on March 7, 2024
Attachments:
Appendix A - Policy Acknowledgment and Sign-off Form
Appendix B – Financial Team Members and Designated Individuals as of March 7, 2024
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 17 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
Appendix A
PROFOUND MEDICAL CORP.
CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES BY DIRECTORS, OFFICERS, EMPLOYEES AND CONSULTANTS POLICY
Acknowledgement and Sign-off
I acknowledge receipt of the Corporate Disclosure, Confidentiality and Trading in Securities By Directors, Officers, Employees and Consultants Policy. I confirm that I have read and fully understand the contents of the policy and my responsibilities as an employee, consultant, or contractor as applicable of the Company.
By my signature below as an employee, consultant or contractor as applicable I agree to comply with the policy as a condition of my employment and my continuing employment at Profound Medical Corp.
I understand that if I have questions, at any time, regarding this policy that I will consult with the Chief Financial Officer or Chief Executive Officer of the Company.
Signature: |
|
|
|
|
|
Printed Name: |
|
|
|
|
|
Date: |
|
|
CONFIDENTIAL
 |
Title: CORPORATE DISCLOSURE, CONFIDENTIALITY AND TRADING IN SECURITIES POLICY |
PAGE 18 of 18 |
|
|
Document Number HR-SP-POLICY019 |
Revision 3 |
|
|
Appendix B
As of March 7, 2024:
“Financial Team Members”
All members of the Company’s financial team, including:
1. |
Matthew Sobczyk, Corporate Controller |
2. |
Daisy Chen, Senior Accountant |
3. |
Dolon Sen, Accountant |
4. |
Luz Perea, General Accountant |
5. |
Elena Seredinina, Account Payable Specialist |
“Designated Individuals”
1. |
Arun Menawat, Chief Executive Officer |
2. |
Rashed Dewan, Chief Financial Officer |
3. |
Mathieu Burtnyk, Chief Operating Officer |
4. |
Abbey Goodman, Chief Commercial Officer U.S. |
CONFIDENTIAL
Exhibit 21
SUBSIDIARIES OF THE COMPANY
Name Subsidiary |
Jurisdiction |
Profound Medical Inc. |
Canada |
2753079 Ontario Inc. |
Canada |
Profound Medical Oy |
Finland |
Profound Medical Technology Services (Beijing) Co., Ltd. |
China |
Profound Medical GmbH |
Germany |
Profound Medical (U.S.) Inc. |
United States |
Exhibit 23.1

Consent of Independent Registered Public Accounting Firm
We hereby consent to the incorporation by reference in the Registration Statements on Form F-10 (No. 333-280236) and Form S-8 (Nos. 333-282050, 333-238528 and 333-234574) of Profound Medical Corp. of our report dated March 7, 2025 relating to the financial statements which appear in this Form 10-K.
/s/ PricewaterhouseCoopers LLP
Chartered Professional Accountants, Licensed Public Accountants
Toronto, Canada
March 7, 2025
Exhibit 31.1
CERTIFICATIONS UNDER SECTION 302
I, Arun Menawat, certify that:
1.I have reviewed this annual report on Form 10-K of Profound Medical Corp.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: March 7, 2025 |
|
|
|
/s/ Arun Menawat |
|
Arun Menawat |
|
Principal Executive Officer |
|
Exhibit 31.2
CERTIFICATIONS UNDER SECTION 302
I, Rashed Dewan, certify that:
1.I have reviewed this annual report on Form 10-K of Profound Medical Corp.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: March 7, 2025 |
|
|
|
/s/ Rashed Dewan |
|
Rashed Dewan |
|
Principal Financial Officer |
|
Exhibit 32
CERTIFICATIONS UNDER SECTION 906
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), each of the undersigned officers of Profound Medical Corp., an Ontario, Canada corporation (the “Company”), does hereby certify, to such officer’s knowledge, that:
The Annual Report for the year ended December 31, 2024 (the “Form 10-K”) of the Company fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of the Company.
Dated: March 7, 2025 |
|
/s/ Arun Menawat |
|
|
Arun Menawat |
|
|
Principal Executive Officer |
|
|
|
Dated: March 7, 2025 |
|
/s/ Rashed Dewan |
|
|
Rashed Dewan |
|
|
Principal Financial Officer |