UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended September 30, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report:
For the transition period from _________ to _____________.
Commission file number: 001-39805
BGM Group Ltd |
(Exact name of Registrant as Specified in its Charter) |
N/A |
(Translation of Registrant’s name into English)
Cayman Islands |
(Jurisdiction of Incorporation or Organization) |
|
No. 152 Hongliang East 1st Street, No. 1703, Tianfu New District, Chengdu, 610200 People’s Republic of China +86-028-64775180 |
(Address of Principal Executive Offices) |
|
|
Chen Xin, Chief Executive Officer No. 152 Hongliang East 1st Street, No. 1703, Tianfu New District, Chengdu, 610200 People’s Republic of China +86-028-64775180 Email: xinchen@qiliancorp.com |
(Name, Telephone, E-mail and/or Facsimile Number and Address of Company Contact Person) |
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Class A Ordinary Shares |
|
BGM |
|
The Nasdaq Stock Market LLC |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
An aggregate of 7,226,480 ordinary shares, consisting of 6,006,480 Class A ordinary shares, par value US$0.00833335 per share, and 1,220,000 Class B ordinary shares, par value US$0.00833335 per share, as of September 30, 2024.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
Emerging growth company |
☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☒ |
|
International Financial Reporting Standards as issued by the |
|
Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow: Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☐ No ☐
TABLE OF CONTENTS
4 |
||
|
|
|
6 |
||
|
|
|
|
7 |
|
|
|
|
7 |
||
|
|
|
7 |
||
|
|
|
7 |
||
|
|
|
51 |
||
|
|
|
94 |
||
|
|
|
94 |
||
|
|
|
108 |
||
|
|
|
116 |
||
|
|
|
116 |
||
|
|
|
117 |
||
|
|
|
118 |
||
|
|
|
133 |
||
|
|
|
134 |
||
|
|
|
|
135 |
|
|
|
|
135 |
||
|
|
|
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
135 |
|
|
|
|
135 |
||
|
|
|
137 |
||
|
|
|
137 |
||
|
|
|
137 |
||
|
|
|
137 |
||
|
|
|
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
137 |
|
|
|
|
138 |
||
|
|
|
138 |
||
|
|
|
138 |
||
|
|
|
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
138 |
|
|
|
|
138 |
||
|
|
|
139 |
||
|
|
|
|
140 |
|
|
|
|
140 |
||
|
|
|
140 |
||
|
|
|
140 |
||
3
INTRODUCTION
As used in this annual report on Form 20-F, (i) “we,” “us,” “Parent,” “BGM,” “our company,” the “Company,” or “our” refers specifically to BGM Group Ltd (formerly known as Qilian International Holding Group Limited); (ii) “Gansu QLS,” “variable interest entity” or “ VIE” refers to Gansu Qilianshan Pharmaceutical Co., Ltd., a company incorporated in the People’s Republic of China; (iii) “WFOE” or “PRC Subsidiary” are to Qilian International Trading (Chengdu) Co., Ltd., formerly known as Chengdu Qilian Trading Co., Ltd., and Qilian Shan International Trade (Hainan) Co., Ltd., both of which are limited liability company organized under the laws of the PRC and are wholly-owned by Qilian International (Hong Kong) Holdings Limited, a limited liability company organized under the laws of Hong Kong.
It is important to note that BGM is not a Chinese operating company but a Cayman Islands holding company with no material business operations. BGM conducts its operations in China through the variable interest entity-- Gansu Qilianshan Pharmaceutical Co. Ltd. (the “VIE”, “Gansu QLS”) and its subsidiaries. Investors in BGM’s ordinary shares are not purchasing equity interest in its operating entities in China but instead are purchasing equity interest in a Cayman Islands holding company.
BGM receives the economic benefits of Gansu QLS and its subsidiaries’ business operation through a series of contractual arrangements, or the VIE Agreements. As a result of the VIE Agreements, BGM is the primary beneficiary of Gansu QLS for accounting purposes and treats it as a PRC consolidated entity under U.S. GAAP. BGM consolidates the financial results of Gansu QLS and its subsidiaries in its consolidated financial statements in accordance with U.S. GAAP. BGM does not own any equity interest in Gansu QLS and its subsidiaries. For detailed descriptions of each of the VIE Agreement, please refer to disclosures under “Item 4. Information on the Company-A. History and Development of the Company- Our Holding Company Structure and Contractual Arrangements” in this annual report on Form 20-F.
Unless the context otherwise requires, in this annual report on Form 20-F, references to:
| ● | “Affiliated Entities” are to BGM’s two subsidiaries through equity ownership, along with Gansu QLS (the “VIE”) and the VIE’s subsidiaries, which BGM does not own through equity ownership; |
| ● | “Ahan” are to Jiuquan Ahan Biotechnology Co., Ltd., a limited liability company organized under the laws of the PRC, which is 100% owned by Gansu QLS; |
| ● | “Ahan® Antibacterial Paste” are to a disinfection paste made from a mixture of 11 traditional Chinese herbal ingredients used to treat refractory chronic skin diseases; |
| ● | “APIs” are to Active Pharmaceutical Ingredients, which refer to any substance or mixture of substances intended to be used in the manufacture of a drug (medicinal) product and that, when used in the production of a drug, becomes an active ingredient of the drug product; |
| ● | “BGM” are to BGM Group Ltd (formerly known as Qilian International Holding Group Limited), an exempted company with limited liability incorporated under the laws of the Cayman Islands; |
| ● | “Cangmen” are to Tibet Cangmen trading Co., Ltd., a limited liability company organized under the laws of the PRC, which is 100% owned by Gansu QLS; |
| ● | “Chengdu QLS” are to Chengdu Qilianshan Biotechnology Co., Ltd., a limited liability company organized under the laws of the PRC, which is 79.71% owned by Gansu QLS; |
| ● | “China” or the “PRC” are to the People’s Republic of China, excluding Taiwan but including the special administrative regions of Hong Kong and Macau for the purposes of this annual report only; |
| ● | “Class A ordinary shares” are to our Class A ordinary shares, par value of US$0.00833335 each; |
| ● | “Class B ordinary shares” are to our Class B ordinary shares, par value of US$0.00833335 each; |
| ● | “Gan Di Xin®” are to an innovative antitussive and expectorant medicine made from raw licorice materials; |
| ● | “Gansu QLS” are to Gansu Qilianshan Pharmaceutical Co. Ltd., a limited liability company organized under the laws of the PRC, which BGM controls via a series of contractual arrangements between WFOE and Gansu QLS; |
| ● | “Hainan Trade” are to Qilian Shan International Trade (Hainan) Co., Ltd., a limited liability company organized under the laws of the PRC and is wholly-owned by Qilian International (Hong Kong) Holdings Limited, a limited liability company organized under the laws of Hong Kong. |
| ● | “Heparin Sodium Preparation” are to a primary ingredient for pharmaceutical companies to produce medications used in treating cardiovascular diseases, cerebrovascular diseases, and hemodialysis; |
| ● | “Moshangfa” are to Moshangfa (Gansu) Fertilizer Industry Co., Ltd., formerly known as Jiuquan Qiming Biotechnology Co., Ltd., a limited liability company organized under the laws of the PRC, which is 100% owned by Gansu QLS; |
| ● | “Ordinary Shares” are to our Class A ordinary shares and Class B ordinary shares; |
| ● | “Qilian HK” are to BGM’s wholly owned subsidiary, Qilian International (Hong Kong) Holdings Limited, a Hong Kong corporation; |
| ● | “Qilian Shan® Licorice Extract” are to a primary ingredient for pharmaceutical companies to manufacture traditional licorice tablets; |
| ● | “Qilian Shan® Licorice Liquid Extract” are to a primary ingredient for medical preparation companies to produce compound licorice oral solutions; |
| ● | “Qilian Shan® Oxytetracycline APIs” are to an active ingredient used by pharmaceutical companies in the manufacturing of medications that use oxytetracycline; |
4
| ● | “Qilian Shan® Oxytetracycline Tablets” are to tablets used to prevent and treat a wide range of diseases in chickens, turkeys, cattle, swine, and human; |
| ● | “Rugao” are to Rugao Tianlu Animal Products Co., Ltd., a limited liability company organized under the laws of the PRC, which is 100% owned by Chengdu QLS; |
| ● | “Samen” are to Tibet Samen Trading Co., Ltd., a limited liability company organized under the laws of the PRC, which was 100% owned by Gansu QLS. Samen was dissolved in June 2023; |
| ● | “TCM” are to Traditional Chinese Medicine, a style of traditional medicine built on a foundation of more than 2,500 years of Chinese medical practice that includes various forms of herbal medicine, acupuncture, massage (tui na), exercise (qigong), and dietary therapy; |
| ● | “TCMD” are to Traditional Chinese Medicine Derivatives, a type of product derived from TCM that has been prepared through modern medicine manufacturing procedures to be ready for use; |
| ● | “VIE” are to Gansu QLS, the variable interest entity; |
| ● | “VIE Agreements” are to a series of contractual arrangements, including Exclusive Service Agreement, as amended on August 27, 2019 and later terminated and replaced by Hainan Exclusive Service Agreement on December 1, 2022, the Call Option Agreement, the Equity Pledge Agreement, the Shareholders’ Voting Rights Proxy Agreement and Powers of Attorney, and the Spousal Consents; |
| ● | “we,” “us,” “Parent,” or “the Company” are to BGM Group Ltd; |
| ● | “WFOE” or “PRC Subsidiary” are to Qilian International Trading (Chengdu) Co., Ltd., formerly known as Chengdu Qilian Trading Co., Ltd., and Qilian Shan International Trade (Hainan) Co., Ltd., both of which are limited liability company organized under the laws of the PRC and are wholly-owned by Qilian International (Hong Kong) Holdings Limited, a limited liability company organized under the laws of Hong Kong; |
| ● | “Xiongguan® Organic Fertilizer” are to a fertilizer product designed to improve crop yield, increase soil’s chemical properties, and reduce soil compaction; |
| ● | “Xiongguan® Organic-Inorganic Compound Fertilizer” are to a fertilizer product made from both organic materials and traditional chemical fertilizer and is designed to increased plant growth; |
| ● | “Zhongqiao” are to Zhongqiao Youguan (Chengdu) E-Commerce Service Co., Ltd., a limited liability company organized under the laws of the PRC, which is a 51% subsidiary of Hainan Trade; and |
| ● | “Zhu Xiaochang® Sausage Casings” are to an all-natural food product used for culinary purposes. |
This annual report on Form 20-F includes our audited consolidated financial statements for the fiscal years ended September 30, 2024, 2023, and 2022. In this annual report, we refer to assets, obligations, commitments, and liabilities in our consolidated financial statements in United States dollars. These dollar references are based on the exchange rate of RMB to United States dollars, determined as of a specific date or for a specific period. Changes in the exchange rate will affect the amount of our obligations and the value of our assets in terms of United States dollars which may result in an increase or decrease in the amount of our obligations and the value of our assets.
This annual report contains translations of certain RMB amounts into U.S. dollars at specified rates. Unless otherwise stated, the following exchange rates are used in this annual report:
|
|
September 30, |
||||
US$Exchange Rate |
|
2024 |
|
2023 |
|
2022 |
At the end of the year - RMB |
|
RMB 7.0176 to $1.00 |
|
RMB7.2960 to $1.00 |
|
RMB7.1135 to $1.00 |
Average rate for the year - RMB |
|
RMB 7.2043 to $1.00 |
|
RMB7.0533 to $1.00 |
|
RMB6.5532 to $1.00 |
5
FORWARD-LOOKING INFORMATION
This annual report on Form 20-F contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that involve substantial risks and uncertainties. Known and unknown risks, uncertainties and other factors, including those listed under “Item 3. Key Information—D. Risk Factors,” may cause our and the VIE and its subsidiaries’ actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause our and the VIE and its subsidiaries’ actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our, the VIE and its subsidiaries’ financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
| ● | our and the VIE and its subsidiaries’ mission, goals and strategies; |
| ● | our and the VIE and its subsidiaries’ future business development, financial conditions and results of operations; |
| ● | the expected growth of the PRC pharmaceutical and chemical industries in China; |
| ● | our and the VIE and its subsidiaries’ expectations regarding demand for and market acceptance of their products; |
| ● | our and the VIE and its subsidiaries’ expectations regarding their relationships with their suppliers and customers; |
| ● | competition in our and the VIE and its subsidiaries’ industries; and |
| ● | relevant government policies and regulations relating to our and the VIE and its subsidiaries’ industry. |
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our, the VIE and its subsidiaries’ actual results could be materially different from our expectations. Other sections of this annual report include additional factors that could adversely impact BGM and its affiliated entities’ business and financial performance. Moreover, our and the VIE and its subsidiaries’ operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for our management to predict all risk factors and uncertainties, nor can we assess the impact of all factors on our, the VIE and its subsidiaries’ business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. You should read thoroughly this annual report and the documents that we refer to with the understanding that our actual future results may be materially different from, or worse than, what we expect. We qualify all of our forward-looking statements by these cautionary statements.
This annual report contains certain data and information that we obtained from various government and private publications. Statistical data in these publications also include projections based on a number of assumptions. The pharmaceutical industry may not grow at the rate projected by market data, or at all. Failure of this market to grow at the projected rate may have a material and adverse effect on our and the VIE and its subsidiaries’ business and the market price of the Ordinary Shares. In addition, the rapidly evolving nature of this industry results in significant uncertainties for any projections or estimates relating to the growth prospects or future condition of our and the VIE and its subsidiaries’ market. Furthermore, if any one or more of the assumptions underlying the market data are later found to be incorrect, actual results may differ from the projections based on these assumptions. You should not place undue reliance on these forward-looking statements.
The forward-looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this annual report and the documents that we refer to in this annual report and exhibits to this annual report completely and with the understanding that our and the VIE and its subsidiaries’ actual future results may be materially different from what we expect.
6
PART I
Item 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not Applicable.
Item 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not Applicable.
Item 3. KEY INFORMATION
Our Holding Company Structure and Contractual Arrangements with the Consolidated Affiliated Entities
BGM Group Ltd is not a Chinese operating company but a Cayman Islands holding company with no business operations. The business operations are conducted by Gansu Qilianshan Pharmaceutical Co., Ltd. (the “VIE”, “Gansu QLS”) and its subsidiaries established in the PRC. See “Item 4.C. INFORMATION ON THE COMPANY - Our Corporate Structure” for further information regarding our affiliated entities’ names, places of incorporation, and equity ownership. BGM and its affiliated entities are subject to legal and operational risks associated with being mostly based in the PRC and Hong Kong and having all of their operations in the PRC, discussed in greater detail below. BGM is incorporated in the Cayman Islands-- a holding company with no material operations, the Company conducts its operations in China through the variable interest entities-- Gansu QLS and its subsidiaries. Investors in BGM’s ordinary shares are not purchasing equity interest in its operating entities in China but instead are purchasing equity interest in a Cayman Islands holding company.
BGM receives the economic benefits of Gansu QLS and its subsidiaries’ business operation through a series of contractual arrangements, or the VIE Agreements. As a result of the VIE Agreements, BGM is the primary beneficiary of Gansu QLS for accounting purposes and treat it as a PRC consolidated entity under U.S. GAAP. BGM consolidates the financial results of Gansu QLS and its subsidiaries in its consolidated financial statements in accordance with U.S. GAAP. Neither BGM nor its investors own any equity ownership in, direct foreign investment in, or control through such ownership/investment of Gansu QLS. These VIE Agreements have not been tested in a court of law in the PRC. As a result, investors in BGM’s ordinary shares thus are not purchasing equity interest in its operating entities in China but instead are purchasing equity interest in a Cayman Islands holding company. As used in this annual report, (i) “Gansu QLS,” “variable interest entity” or “ VIE” refers to Gansu Qilianshan Pharmaceutical Co., Ltd., a company incorporated in the People’s Republic of China; (ii) “WFOE” or “PRC Subsidiary” refers to Qilian International Trading (Chengdu) Co., Ltd., formerly known as Chengdu Qilian Trading Co., Ltd., and Qilian Shan International Trade (Hainan) Co., Ltd., both of which are limited liability company organized under the laws of the PRC and are wholly-owned by Qilian International (Hong Kong) Holdings Limited, a limited liability company organized under the laws of Hong Kong; and (iii) “BGM”, “the Company” refers to BGM Group Ltd, an exempted company with limited liability incorporated under the laws of the Cayman Islands.
Our corporate structure is subject to risks associated with BGM’s contractual arrangements with the VIE. The Company that investors will own may never have a direct ownership interest in the businesses that are conducted by the VIE. If the PRC government finds that the agreements that establish the structure for operating the VIE and its subsidiaries’ business in China do not comply with PRC laws and regulations, or if these regulations or the interpretation of existing regulations change or are interpreted differently in the future, we could be subject to severe penalties or be forced to relinquish our interests in the operations of the VIE and its subsidiaries. This would result in the VIE being deconsolidated. The majority of our assets, including the necessary licenses to conduct business in China, are held by the VIE and its subsidiaries. A significant part of our revenue is generated by the VIE. An event that results in the deconsolidation of the VIE would have a material effect on the VIE and its subsidiaries’ operations and result in the Ordinary Shares diminishing substantially in value or even becoming worthless. The Company, our Hong Kong entity, the VIE and its subsidiaries, and our investors face uncertainty about potential future actions by the PRC government that could affect the enforceability of the contractual arrangements with the VIE and, consequently, significantly affect the financial performance of the VIE and the Company as a whole. For a detailed description of the risks associated with our corporate structure, please refer to risks disclosed under “Item 3. Key Information-D. Risk Factors-Risks Related to Our Corporate Structure” in this annual report on Form 20-F.
7
In addition, while BGM will take every precaution available to enforce the contractual and corporate relationship of the VIE agreements, these contractual arrangements are less effective than direct ownership and BGM may incur substantial costs to enforce the terms of the arrangements. For example, the VIE, its subsidiaries, and their shareholders could breach their contractual arrangements with BGM by, among other things, failing to conduct their operations in an acceptable manner or taking other actions that are detrimental to BGM’s interests. If BGM had direct ownership of the VIE and its subsidiaries, it would be able to exercise its rights as a shareholder to effect changes in the board of directors of the VIE, which in turn could implement changes, subject to any applicable fiduciary obligations, at the management and operational level. However, under VIE Agreements, BGM relies on the performance by the VIE and its shareholders of their obligations under the contracts to direct the operation of the VIE and its subsidiaries. As such, the shareholders of VIE and its subsidiaries may not act in the best interests of BGM or may not perform their obligations under these contracts. In addition, failure of the VIE shareholders to perform certain obligations could compel BGM to rely on legal remedies available under PRC laws, including seeking specific performance or injunctive relief, and claiming damages, which may not be effective. Further, it is uncertain whether any new PRC laws or regulations relating to variable interest entity structures will be adopted or if adopted, what they would provide. PRC regulatory authorities could disallow this structure, which would materially adversely affect the value of BGM’s ordinary shares, and could cause the value of such securities to significantly decline or become worthless. BGM faces numerous challenges in enforcing these contractual agreements due to uncertainties under Chinese law as well as jurisdictional limits. For a description of the risks related to these contractual arrangements and our corporate structure, see “Risk Factors - Risks Related to Our Corporate Structure.” For detailed descriptions of each of the VIE Agreement, please refer to disclosures under “Item 4. Information on the Company-A. History and Development of the Company- Our Holding Company Structure and Contractual Arrangements” in this annual report on Form 20-F.
BGM faces legal and operational risks associated with having the majority of its operations in China. The Chinese government has significant authority to exert influence on the ability of a China-based company, such as BGM, to conduct its business. Therefore, investors of BGM and its business conducted by the VIE and its subsidiaries face potential uncertainty from the PRC government. Changes in China’s economic, political or social conditions or government policies could materially adversely affect BGM and its affiliated entities’ business and results of operations. For example, BGM faces risks associated with PRC governmental authorities’ significant oversight and discretion over the businesses and financing activities of the VIE, the requirement of regulatory approvals for offerings conducted overseas by and foreign investment in China-based issuers, the use of variable interest entities, the enforcement of anti-monopoly regime, the regulatory oversight on cybersecurity and data privacy as well as the risk of delisting due to if the PCAOB is unable to conduct inspection on our auditors, which may impact our ability to conduct certain businesses, accept foreign investments, or list on a United States or other foreign exchange. These risks could result in a material adverse change in the VIE and its subsidiaries’ operations conducted by the VIE and its subsidiaries and the value of BGM’s ordinary shares, significantly limit or completely hinder BGM’s ability and the ability of any holder of its Ordinary Shares or other securities of BGM to offer or continue to offer such securities to investors, or cause the value of such securities to significantly decline. In particular, recent statements and regulatory actions by China’s government, such as those related to data security or anti-monopoly concerns, as well as the PCAOB’s ability to inspect our auditors, may impact BGM’s ability to conduct its business through the VIE and its subsidiaries, accept foreign investments, or be listed on a U.S. or other foreign stock exchange. See “Item 3. Key Information - D. Risk Factors - Risks Related to Doing Business in China - The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we, the VIE or its subsidiaries were to be subject to such oversight and control, it may result in a material adverse change to the VIE and its subsidiaries’ business operations, significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors, and cause its ordinary shares to significantly decline in value or become worthless” and “Item 3. Key Information - D. Risk Factors - Risks Related to Doing Business in China - Uncertainties with respect to the PRC legal system and the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us, hinder BGM’s ability and the ability of any holder of BGM’s securities to offer or continue to offer such securities, result in a material adverse change to the WFOE and the VIE and its subsidiaries’ business operations, and damage BGM and its subsidiaries’ reputation, which would materially and adversely affect BGM and its affiliates’ financial condition and results of operations and cause the Ordinary Shares to significantly decline in value or become worthless.”
8
BGM has been advised by Gansu Quanyi Law Firm, our PRC counsel, that as of the date of this Annual Report, our listing in the U.S. is not subject to the review, permission or prior approval of PRC authorities including the Cyberspace Administration of China (“CAC”) or the China Securities Regulatory Commission (“CSRC”) because (i) the CSRC currently has not issued any definitive rule or interpretation concerning whether our listing is subject to this regulation; and (ii) our operating entities (the WFOE, the VIE and its subsidiaries) were established and operate in PRC are not included in the categories of industries and companies whose foreign securities offerings are subject to review by the CSRC or the CAC. Uncertainties still exist, however, due to the possibility that laws, regulations, or policies in the PRC could change rapidly in the future. In the event that the PRC government expanded the categories of industries and companies whose foreign securities offerings are subject to review by the CSRC or the CAC, and BGM inadvertently concluded that relevant permissions or approvals were not required or that BGM did not receive or failed to maintain relevant permissions or approvals required and such permissions were subsequently rescinded, any action by the PRC government could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly decline or be worthless.
On December 16, 2021, the PCAOB issued a report on its determination that it is unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in China and in Hong Kong because of positions taken by PRC and Hong Kong authorities in those jurisdictions. The PCAOB has made such determination as mandated under the Holding Foreign Companies Accountable Act. Pursuant to each annual determination by the PCAOB, the SEC will, on an annual basis, identify issuers that have used non-inspected audit firms and thus are at risk of such suspensions in the future. Our auditors, ZH CPA, LLC and Enrome LLP, the independent registered public accounting firms that issue the audit reports included elsewhere in this annual reports, as auditors of companies that are traded publicly in the U.S. and firms registered with the PCAOB, are subject to laws in the U.S., pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. ZH CPA, LLC and Enrome LLP are located in Denver, Colorado and Singapore, and have been inspected by the PCAOB on a regular basis. Our auditors are not subject to the determination issued by the PCAOB on December 16, 2021.
On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”) was signed into law by President Biden, which contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act and amended the HFCA Act by requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period for triggering the prohibition on trading. On August 26, 2022, the CSRC, the Ministry of Finance of the PRC (the “MOF”), and the PCAOB signed a Statement of Protocol (the “Protocol”) governing inspections and investigations of audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. Pursuant to the fact sheet with respect to the Protocol disclosed by the SEC, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. See “—D. Risk Factors—Risks Related to Doing Business in China — Our Ordinary Shares may be delisted and prohibited from being traded under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect auditors who are located in China. The delisting and the cessation of trading of our Ordinary Shares, or the threat of their being delisted and prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections deprives our investors with the benefits of such inspections.”
An investment in our ordinary shares involves a high degree of risk and should be considered speculative. You should carefully consider the following risks set out below and other information before investing in our ordinary shares. If any event arising from these risks occurs, the VIE and its subsidiaries’ business, prospects, financial condition, results of operations or cash flows could be adversely affected, the trading price of our ordinary shares could decline and all or part of your investment may be lost.
9
Transfers of Cash Amongst Our Subsidiaries, the VIE, and the VIE’s Subsidiaries
BGM is permitted under the laws of Cayman Islands to provide funding to its subsidiary in Hong Kong (Qilian HK) through loans or capital contributions without restrictions on the amount of the funds. Qilian HK is permitted under the laws of Hong Kong to provide funding to BGM through dividend distribution without restrictions on the amount of the funds. Any determination related to our dividend policy will be made at the discretion of BGM’s board of directors after considering its financial condition, results of operations, capital requirements, contractual requirements, business prospects and other factors the board of directors deems relevant, and subject to the restrictions contained in any financing instruments. Subject to the Companies Act (Revised) of the Cayman Islands and its memorandum and articles of association (as amended and restated from time to time), BGM’s board of directors may authorize and declare a dividend to shareholders at such time and of such an amount as they think fit if they are satisfied, on reasonable grounds, that immediately following the dividend the value of its assets will exceed its liabilities and BGM will be able to pay its debts as they become due. There is no further restriction under the Companies Act (Revised) of the Cayman Islands on the amount of funds which may be distributed by BGM by dividend.
If BGM determines to pay dividends on any of its Ordinary Shares, as a holding company, it will be dependent on receipt of funds from its Hong Kong subsidiary by way of dividend payments. Under the current practice of the Inland Revenue Department of Hong Kong, no tax is payable in Hong Kong in respect of dividends paid by us. The laws and regulations of the PRC do not currently have any material impact on transfer of cash from BGM to Qilian HK or from Qilian HK to BGM. There are no restrictions or limitation under the laws of Hong Kong imposed on the conversion of HK dollar into foreign currencies and the remittance of currencies out of Hong Kong, nor there is any restriction on foreign exchange to transfer cash between BGM and its affiliated entities, across borders and to U.S investors, nor there is any restrictions and limitations to distribute earnings from BGM’s operating business conducted by its PRC based VIE and its subsidiaries, to BGM and U.S. investors and amounts owed.
Current PRC regulations permit WFOE to pay dividends to our Hong Kong subsidiary only out of its accumulated after-tax profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, WFOE is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. WFOE could further set aside a portion of its after-tax profits to fund a discretionary reserve, although the amount to be set aside, if any, is determined at the discretion of its shareholders. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
While the PRC government imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC, none of the entities affiliated to the Company are on the negative list of domestic and foreign investments explicitly prohibited by the Chinese government. Thus, the Company will not experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from its VIE’s profits. If WFOE incurs debt on its own in the future, the instruments governing the debt may restrict its ability to pay dividends or make other payments. If BGM or its subsidiaries are unable to receive all of the revenues from their operations through the current VIE agreements, it may be unable to pay dividends on its ordinary shares.
Cash dividends, if any, on BGM’s ordinary shares will be paid in U.S. dollars. If BGM is considered a PRC tax resident enterprise for tax purposes, any dividends it pays to its overseas shareholders may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10.0%. In order for BGM to pay dividends to its shareholders, it will rely on payments made from the VIE and its subsidiaries to WFOE, pursuant to VIE agreements between them, and the distribution of such payments to Qilian HK as dividends from WFOE. Certain payments from the VIE and its subsidiaries to WFOE are subject to PRC taxes, including enterprise income taxes, VAT and certain other taxes, as the case maybe.
For the year ended September 30, 2024, cash flow from WFOE to VIE included proceeds from repayment of loan of $702,469 and net proceeds from product sales and purchase of $898,863. For the year ended September 30, 2023, cash flow from VIE to WFOE included payment of $39,508 for net payments for products sales and purchase. For the year ended September 30, 2022, cash flow from WFOE to VIE included proceeds from repayment of loan of $762,986, interest payment of $166,458 and net payment for product sales and purchase of $209,064.
10
See “Dividend Policy”, “Risk Factors — BGM is a holding company and it relies for funding on dividend payments from its affiliated entities by contracts, which are subject to restrictions under PRC laws. Any limitation on the ability of BGM’s affiliated entities to make payments to it could have a material adverse effect on BGM’s ability to maintain its business.”, Summary Consolidated Financial Data and Consolidated Statements of Change in Shareholders’ Equity in the Report of Independent Registered Public Accounting Firm for more information.
PRC Limitation on Overseas Listing and Share Issuances
Currently, BGM and its affiliated entities, are not required to obtain approval from Chinese authorities, including the China Securities Regulatory Commission, or CSRC, or Cybersecurity Administration Committee, or CAC, to operate and list on U.S. exchanges or issue securities to foreign investors. If approval is required in the future and BGM is denied permission from Chinese authorities to list on U.S. exchanges, BGM will not be able to operate or to continue listing on U.S. exchange, which would materially affect the interest of the investors. It is uncertain when and whether the Company will be required to obtain permission from the PRC government to continue to operate or to list on U.S. exchanges in the future, and even when such permission is obtained, whether it will be denied or rescinded. Although BGM and its affiliated entities are currently not required to obtain permission from any of the PRC federal or local government and have not received any denial to list on the U.S. exchange, BGM’s operations and ability to continue to list and issue securities to foreign investors may be adversely affected in the future, directly or indirectly, by existing or future laws and regulations relating to BGM’s PRC business operations. For more detailed information, see “Risks Related to Doing Business in China — The PRC government may intervene and influence the WFOE and the VIE and its subsidiaries’ business operations at any time or may exert more control over offerings conducted overseas and foreign investment in China based issuers, which could result in a material change in the WFOE and the VIE and its subsidiaries’ business operations or the value of BGM’s securities. Additionally, the governmental and regulatory interference could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. BGM and its affiliated entities are also currently not required to obtain approval from Chinese authorities to list on U.S. exchanges, however, if they are required to obtain approval in the future and are denied permission from Chinese authorities to list on U.S. exchanges, BGM will not be able to continue listing on U.S. exchange, which would materially affect the interest of the investors.”
Financial Information Related to the VIE
The following tables provide condensed consolidating schedules depicting the financial position, cash flows, and results of operations for the parent, subsidiaries, WFOE, the consolidated VIE, and any eliminating adjustments and consolidated totals as of and for the years ended September 30, 2024, 2023 and 2022.
Selected Condensed Consolidating Statements of Operations Information
|
|
For the Year ended September 30, 2024 |
||||||||||
|
|
|
|
|
|
|
|
The VIE |
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination(4) |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Total revenues |
|
— |
|
— |
|
698,583 |
|
25,097,953 |
|
(698,585) |
|
25,097,951 |
Including: Service fee revenue (loss absorbed) from the VIE |
|
— |
|
— |
|
698,585 |
|
— |
|
(698,585) |
|
— |
Cost of revenues |
|
— |
|
— |
|
7,807 |
|
20,975,389 |
|
— |
|
20,983,196 |
Total operating expenses |
|
820,606 |
|
(3,694) |
|
358,251 |
|
4,198,253 |
|
(694,890) |
|
4,678,526 |
Including: Service fee expense charged by the WFOE |
|
— |
|
— |
|
— |
|
698,585 |
|
(698,585) |
|
— |
Share of (loss) income of subsidiary (1) |
|
80,506 |
|
76,813 |
|
— |
|
— |
|
(157,319) |
|
— |
Net income (loss) |
|
(1,522,700) |
|
80,506 |
|
76,813 |
|
5,534 |
|
(157,315) |
|
(1,517,161) |
11
|
|
For the Year ended September 30, 2023 |
||||||||||
|
|
|
|
|
|
|
|
The VIE |
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination(4) |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Total revenues |
|
— |
|
— |
|
(1,260,840) |
|
46,471,478 |
|
1,260,840 |
|
46,471,478 |
Including: Service fee revenue (loss absorbed) from the VIE |
|
— |
|
— |
|
(1,333,066) |
|
— |
|
1,333,066 |
|
— |
Cost of revenues |
|
— |
|
— |
|
3,255 |
|
44,716,729 |
|
— |
|
44,719,984 |
Total operating expenses |
|
649,697 |
|
— |
|
307,155 |
|
2,143,901 |
|
1,260,840 |
|
4,361,593 |
Including: Service fee expense charged by the WFOE |
|
— |
|
— |
|
— |
|
(1,333,066) |
|
1,333,066 |
|
— |
Share of (loss) income of subsidiary (1) |
|
(1,602,772) |
|
(1,602,772) |
|
— |
|
— |
|
3,205,544 |
|
— |
Net income (loss) |
|
(7,780,620) |
|
(1,602,772) |
|
(1,602,772) |
|
(341,450) |
|
3,205,544 |
|
(8,122,070) |
|
|
For the Year ended September 30, 2022 |
||||||||||
|
|
|
|
|
|
|
|
The VIE |
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination(4) |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Total revenues |
|
— |
|
— |
|
7,440,476 |
|
64,468,807 |
|
(7,054,258) |
|
64,855,025 |
Including: Service fee revenue from the VIE |
|
— |
|
— |
|
2,730,580 |
|
— |
|
(2,730,580) |
|
— |
Cost of revenues |
|
— |
|
— |
|
4,268,747 |
|
58,682,658 |
|
(4,323,677) |
|
58,627,728 |
Total operating expenses |
|
522,923 |
|
— |
|
318,236 |
|
6,014,715 |
|
(2,730,580) |
|
4,125,294 |
Including: Service fee expense charged by the WFOE |
|
— |
|
— |
|
— |
|
2,730,580 |
|
(2,730,580) |
|
— |
Share of income of subsidiary(1) |
|
2,720,596 |
|
2,720,596 |
|
— |
|
— |
|
(5,441,192) |
|
— |
Net income |
|
3,050,625 |
|
2,720,596 |
|
2,720,596 |
|
21,632 |
|
(7,147,192) |
|
1,366,257 |
Selected Condensed Consolidating Balance Sheets Information
|
|
As of September 30, 2024 |
||||||||||
|
|
|
|
|
|
|
|
The VIE |
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination(4) |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Cash and cash equivalents |
|
1,856,344 |
|
1,058,635 |
|
2,427,472 |
|
4,474,803 |
|
— |
|
9,817,254 |
Amount due from the Parent/WFOE(2) |
|
— |
|
— |
|
— |
|
5,128,511 |
|
(5,128,511) |
|
— |
Total current assets |
|
10,191,931 |
|
1,113,335 |
|
2,464,159 |
|
15,999,790 |
|
(5) |
|
29,769,210 |
Service fee receivable from the VIE |
|
— |
|
— |
|
11,789,750 |
|
— |
|
(11,789,750) |
|
— |
Investment in subsidiary(3) |
|
11,141,678 |
|
11,141,678 |
|
— |
|
— |
|
(22,283,356) |
|
— |
Other non-current assets |
|
— |
|
3,328,215 |
|
6,139,822 |
|
16,016,484 |
|
(2,250,003) |
|
23,234,518 |
Total assets |
|
21,333,609 |
|
15,583,228 |
|
20,393,731 |
|
37,144,785 |
|
(41,451,624) |
|
53,003,728 |
Amounts due to the VIE and its subsidiaries(2) |
|
(3,435,100) |
|
4,436,707 |
|
4,126,905 |
|
— |
|
(5,128,512) |
|
— |
Total current liabilities |
|
— |
|
1,149 |
|
2,853,836 |
|
5,921,003 |
|
(3) |
|
8,775,985 |
Service fee payable to the WFOE |
|
— |
|
— |
|
— |
|
11,789,750 |
|
(11,789,750) |
|
— |
Other non-current liabilities |
|
— |
|
— |
|
— |
|
134,394 |
|
— |
|
134,394 |
Total liabilities |
|
(3,435,100) |
|
4,437,856 |
|
6,980,741 |
|
17,845,147 |
|
(16,918,264) |
|
8,910,379 |
Total shareholders’ equity |
|
24,768,709 |
|
11,145,373 |
|
13,412,990 |
|
19,299,638 |
|
(24,533,361) |
|
44,093,349 |
Total liabilities and shareholders’ equity |
|
21,333,609 |
|
15,583,228 |
|
20,393,731 |
|
37,144,785 |
|
(41,451,625) |
|
53,003,728 |
12
|
|
As of September 30, 2023 |
||||||||||
|
|
|
|
|
|
|
|
The VIE |
|
|
|
|
|
|
|
|
|
|
|
|
and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination(4) |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Cash and cash equivalents |
|
277,218 |
|
— |
|
322,834 |
|
6,876,195 |
|
— |
|
7,476,247 |
Amount due from the Parent/WFOE(2) |
|
— |
|
— |
|
— |
|
4,125,329 |
|
(4,125,329) |
|
— |
Total current assets |
|
15,230,237 |
|
— |
|
369,857 |
|
23,037,856 |
|
(4,125,329) |
|
34,512,621 |
Service fee receivable from the VIE |
|
— |
|
— |
|
11,091,165 |
|
— |
|
(11,091,165) |
|
— |
Investment in subsidiary(3) |
|
10,693,672 |
|
10,693,672 |
|
— |
|
— |
|
(21,387,344) |
|
— |
Other non-current assets |
|
— |
|
— |
|
2,772,206 |
|
13,973,167 |
|
— |
|
16,745,373 |
Total assets |
|
25,923,910 |
|
10,693,672 |
|
14,233,228 |
|
37,011,023 |
|
(36,603,839) |
|
51,257,994 |
Amounts due to the VIE and its subsidiaries(2) |
|
575,793 |
|
— |
|
3,549,536 |
|
— |
|
(4,125,329) |
|
— |
Total current liabilities |
|
575,793 |
|
— |
|
3,539,555 |
|
6,670,120 |
|
(4,125,329) |
|
6,660,139 |
Service fee payable to the WFOE |
|
— |
|
— |
|
— |
|
11,091,165 |
|
(11,091,165) |
|
— |
Other non-current liabilities |
|
— |
|
— |
|
— |
|
246,454 |
|
— |
|
246,454 |
Total liabilities |
|
575,793 |
|
— |
|
3,539,555 |
|
18,007,739 |
|
(15,216,494) |
|
6,906,593 |
Total shareholders’equity |
|
25,348,117 |
|
10,693,672 |
|
10,693,673 |
|
19,003,284 |
|
(21,387,345) |
|
44,351,401 |
Total shareholders’liabilities and equity |
|
25,923,910 |
|
10,693,672 |
|
14,233,228 |
|
37,011,023 |
|
(36,603,839) |
|
51,257,994 |
Selected Condensed Consolidating Cash Flows Information
|
|
For the Year ended September 30, 2024 |
||||||||||
|
|
|
|
|
|
|
|
The VIE and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Net cash (used in) provided by operating activities |
|
(4,220,872) |
|
3,379,370 |
|
1,203,550 |
|
182,190 |
|
— |
|
544,238 |
Net cash (used in) provided by investing activities |
|
5,800,000 |
|
(1,078,215) |
|
(1,404,938) |
|
(2,333,429) |
|
— |
|
983,418 |
Net cash used in financing activities |
|
— |
|
— |
|
— |
|
(491,728) |
|
— |
|
(491,728) |
|
|
For the Year ended September 30, 2023 |
||||||||||
|
|
|
|
|
|
|
|
The VIE and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Net cash (used in) provided by operating activities |
|
(635,467) |
|
— |
|
(268,752) |
|
1,203,435 |
|
12,993 |
|
312,209 |
Net cash used in by investing activities |
|
(1,000,000) |
|
— |
|
(29,347) |
|
(3,700,105) |
|
(12,993) |
|
(4,742,445) |
Net cash provided by (used in) financing activities |
|
(1,787,517) |
|
— |
|
56,711 |
|
(1,190,278) |
|
— |
|
(2,921,084) |
|
|
For the Year ended September 30, 2022 |
||||||||||
|
|
|
|
|
|
|
|
The VIE and |
|
|
|
Consolidated |
|
|
Parent |
|
Qilian HK |
|
WFOE |
|
subsidiaries |
|
Elimination |
|
Total |
|
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
|
US$ |
Net cash (used in) provided operating activities |
|
(527,971) |
|
— |
|
280,889 |
|
12,901,270 |
|
— |
|
12,654,188 |
Net cash used in investing activities |
|
— |
|
— |
|
(1,341,994) |
|
(1,153,972) |
|
(762,986) |
|
(3,258,952) |
Net cash used in financing activities |
|
— |
|
— |
|
(762,986) |
|
(5,937,529) |
|
762,986 |
|
(5,937,529) |
13
The following table represents the roll-forward of the investments in our subsidiaries, the VIE and the VIE’s subsidiaries:
|
|
USD |
As of September 30, 2020 |
|
6,966,081 |
Share of income of subsidiaries, the VIE and the VIE’s subsidiaries |
|
2,974,990 |
Effect of exchange rate |
|
6,858 |
As of September 30, 2021 |
|
9,947,929 |
Share of income of subsidiaries, the VIE and the VIE’s subsidiaries |
|
2,720,596 |
Effect of exchange rate |
|
(22,641) |
As of September 30, 2022 |
|
12,645,883 |
Share of income of subsidiaries, the VIE and the VIE’s subsidiaries |
|
(1,602,772) |
Effect of exchange rate |
|
(349,439) |
As of September 30, 2023 |
|
10,693,672 |
Share of income of subsidiaries, the VIE and the VIE’s subsidiaries |
|
80,506 |
Effect of exchange rate |
|
367,500 |
As of September 30, 2024 |
|
11,141,678 |
Notes
| (1) | It represents the elimination of share of income by BGM from Qilian HK with the net income recognized at Qilian HK level, and share of income by Qilian HK from the WFOE with the net income recognized at the WFOE level, respectively. |
| (2) | It represents the elimination of intercompany balances among BGM, Qilian HK, the Primary WFOE, and the VIEs and their subsidiaries that we consolidate. |
| (3) | As of September 30, 2024, the $4,012,005 intercompany balances included $702,469 loan of WFOE due to the VIE and its subsidiaries, $3,001,210 of receivable of the VIE and its subsidiaries from WFOE originated from purchase made by WFOE from the VIE and its subsidiaries and $232,338 of other payable to the VIE and its subsidiaries from WFOE. |
As of September 30, 2023, the $4,125,329 intercompany balances included $575,793 loan due to the VIE and its subsidiaries from the Parent, $3,316,379 of receivable of the VIE and its subsidiaries from WFOE originated from purchase made by WFOE from the VIE and its subsidiaries and $233,157 of other payable to the VIE and its subsidiaries from WFOE.
| (4) | It represents the elimination of the investments in Qilian HK by BGM, and investments in the WFOE by Qilian HK, respectively. |
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
14
D. Risk Factors
Summary of Risk Factors
Investing in our Ordinary Shares involves significant risks. You should carefully consider all of the information in this annual report before making an investment in our Ordinary Shares. Below please find a summary of the principal risks we, our subsidiaries, the VIE and its subsidiaries face, organized under relevant headings. The legal and operational risks associated with having operations in the PRC also apply to our presence in Hong Kong. As Hong Kong currently operates under a different set of laws from the PRC, the laws, regulations and the discretion of the governmental authorities in the PRC discussed in this annual report are expected to apply to our entities and businesses in the PRC, rather than entities or businesses in Hong Kong. However, there can be no assurance as to whether the government of Hong Kong will enact laws and regulations similar to the PRC, or whether any laws or regulations of the PRC will become applicable to our operations in Hong Kong in the future. These risks are discussed more fully in the section titled “Item 3. Key Information—D. Risk Factors” in this annual report.
Risks Related to our Corporate Structure
We, our subsidiaries, the VIE and its subsidiaries are also subject to risks and uncertainties related to our corporate structure, including, but not limited to, the following:
| ● | PRC laws and regulations governing our subsidiaries, the VIE, and its subsidiaries’ businesses and the validity of certain of our contractual arrangements are uncertain. If we, our subsidiaries, the VIE or its subsidiaries are found to be in violation, we, our subsidiaries, the VIE or its subsidiaries could be subject to sanctions. In addition, changes in PRC laws and regulations or changes in interpretations thereof may materially and adversely affect the WFOE and the VIE and its subsidiaries’ business. |
| ● | We rely on contractual arrangements with the VIE and its subsidiaries in China for the VIE and its subsidiaries’ business operations, which may not be as effective in providing operational control or enabling us to derive economic benefits as through ownership of controlling equity interests, and the VIE’s shareholders may fail to perform their obligations under the contractual arrangements. |
| ● | Gansu QLS’s shareholders may have potential conflicts of interest with us, which may materially and adversely affect BGM and its affiliated entities’ business and financial condition and the value of your investment in our shares. |
Risks Related to Doing Business in China
| ● | The approval and/or other requirements of the China Securities Regulatory Commission, or the CSRC, or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we and our affiliated entities cannot predict whether or how soon we, the VIE or its subsidiaries will be able to obtain such approval. |
| ● | Our Ordinary Shares may be delisted and prohibited from being traded under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect auditors who are located in China. The delisting and the cessation of trading of our Ordinary Shares, or the threat of their being delisted and prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections deprives our investors with the benefits of such inspections. |
| ● | On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden, which contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act and amended the HFCA Act by requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, and thus, would reduce the time before our Ordinary Shares may be prohibited from trading or delisted. |
15
| ● | On December 16, 2021, the PCAOB issued a report on its determination that it is unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in China and in Hong Kong because of positions taken by PRC and Hong Kong authorities in those jurisdictions. The PCAOB has made such determination as mandated under the Holding Foreign Companies Accountable Act. Pursuant to each annual determination by the PCAOB, the SEC will, on an annual basis, identify issuers that have used non-inspected audit firms and thus are at risk of such suspensions in the future. Our auditors, ZH CPA, LLC and Enrome LLP, the independent registered public accounting firms that issue the audit reports included elsewhere in this annual report, as auditors of companies that are traded publicly in the U.S. and firms registered with the PCAOB, are subject to laws in the U.S., pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. ZH CPA, LLC and Enrome LLP are located in Denver, Colorado and Singapore, respectively, and have been inspected by the PCAOB on a regular basis. Our auditors are not subject to the determination issued by the PCAOB on December 16, 2021. |
| ● | The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we or our affiliated entities were to be subject to such oversight and control, it may result in a material adverse change to the WFOE and the VIE and its subsidiaries’ business operations, significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors, and cause Ordinary Shares to significantly decline in value or become worthless. See “-Risks Relating to Doing Business in China -The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we and our affiliated entities were to be subject to such oversight and control, it may result in a material adverse change to our, the VIE or its subsidiaries business operations, significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors, and cause our Ordinary Shares to significantly decline in value or become worthless”; |
| ● | On December 28, 2021, the CAC, the National Development and Reform Commission (“NDRC”), and several other administrations jointly issued the revised Measures for Cybersecurity Review, or the “Revised Review Measures”, which became effective and replaced the existing Measures for Cybersecurity Review on February 15, 2022. According to the Revised Review Measures, if an “online platform operator” that is in possession of personal data of more than one million users intends to list in a foreign country, it must apply for a cybersecurity review. Based on a set of Q&A published on the official website of the State Cipher Code Administration in connection with the issuance of the Revised Review Measures, an official of the said administration indicated that an online platform operator should apply for a cybersecurity review prior to the submission of its listing application with non-PRC securities regulators. Moreover, the CAC released the draft of the Regulations on Network Data Security Management in November 2021 for public consultation, which among other things, stipulates that a data processor listed overseas must conduct an annual data security review by itself or by engaging a data security service provider and submit the annual data security review report for a given year to the municipal cybersecurity department before January 31 of the following year. Given the recency of the issuance of the Revised Review Measures and their pending effectiveness, there is a general lack of guidance and substantial uncertainties exist with respect to their interpretation and implementation. For more information, see page 29 under “The PRC government may intervene or influence the WFOE or the VIE and its subsidiaries’ business operations at any time or may exert more control over offerings conducted overseas and foreign investment in China based issuers, which could result in a material change in the WFOE and the VIE and its subsidiaries’ business operations or the value of BGM’s securities.” Additionally, the governmental and regulatory interference could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. We and our affiliated entities are also currently not required to obtain approval from Chinese authorities to list on U.S. exchanges, however, if we or our affiliated entities are required to obtain approval in the future and are denied permission from Chinese authorities to list on U.S. exchanges, we will not be able to continue listing on U.S. exchange, which would materially affect the interest of the investors. |
16
| ● | Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect BGM and its affiliated entities’ business, financial condition, and results of operations. See “Risks Relating to Doing Business in the PRC-Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect BGM and its affiliated entities’ business, financial condition, and results of operations”. |
| ● | Changes in laws, regulations and policies in the PRC and uncertainties with respect to the interpretation and enforcement of the laws, regulations and policies in the PRC and the fact that rules and regulations in the PRC can change quickly with little advance notice. Rules and regulations in the PRC are subject to changes by the relevant authorities. Sometimes such authorities will publish draft of the revisions to existing rules and regulations for public comments and consultation before enacting such revisions. But such consultations are done on case-by-case basis and we otherwise lack public channels to learn the extents of the revisions beforehand, in which case we might have limited time to ensure timely compliance upon the enactment of such revisions. Uncertainties with respect to the PRC legal system and the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us, hinder BGM’s ability and the ability of any holder of BGM’s securities to offer or continue to offer such securities, result in a material adverse change to the WFOE and the VIE and its subsidiaries’ business operations, and damage our reputation, which would materially and adversely affect BGM and its affiliated entities’ financial condition and results of operations and cause the Ordinary Shares to significantly decline in value or become worthless. |
| ● | A severe or prolonged downturn in the Chinese or global economy could materially and adversely affect BGM and its affiliated entities’ business and financial condition. |
| ● | Substantial uncertainties exist with respect to the interpretation of the PRC Foreign Investment Law and how it may impact the viability of our current corporate structure, corporate governance and business operations. |
Risks Related to the WFOE, the VIE and its Subsidiaries’ Business
Risks and uncertainties related to the WFOE, the VIE and its subsidiaries’ business include, but are not limited to, the following:
| ● | The VIE and its subsidiaries face significant competition in industries experiencing rapid technological change, and there is a possibility that their competitors may achieve regulatory approval and develop new product candidates before the VIE and its subsidiaries, which may harm our and the VIE and its subsidiaries’ financial condition and the ability of the VIE and its subsidiaries to successfully market or commercialize any of their product candidates. |
| ● | The pharmaceutical business of the WFOE, the VIE and its subsidiaries is subject to inherent risks relating to product liability and personal injury claims. |
| ● | The business operations of the WFOE, the VIE and its subsidiaries require a number of permits and licenses. We cannot assure you that the VIE and its subsidiaries can maintain all required licenses, permits and certifications to carry on their business at all times. |
| ● | A significant portion of the VIE and its subsidiaries’ revenue is concentrated in a few large customers, and the WFOE, the VIE and its subsidiaries do not have long-term agreements with their key customers and rely upon their longstanding relationship with these customers. If the WFOE and the VIE and its subsidiaries lose one or more of their customers, BGM and its affiliated entities’ results of operations may be adversely and materially impacted. |
| ● | The WFOE and the VIE and its subsidiaries source raw materials used for manufacturing from a limited number of suppliers. If the WFOE and the VIE and its subsidiaries lose one or more of the suppliers, their operation may be disrupted, and BGM and its affiliated entities’ results of operations may be adversely and materially impacted. |
| ● | If the WFOE and the VIE and its subsidiaries fail to increase their brand name recognition, they may face difficulty in obtaining new customers. |
| ● | Any disruption in the supply chain of raw materials and the products of the WFOE and the VIE and its subsidiaries could adversely impact their ability to produce and deliver products. |
17
Risks Related to Our Ordinary Shares
| ● | Risks and uncertainties related to our Ordinary Shares include, but are not limited to, the following: |
| ● | The trading price of our Ordinary Shares is likely to be volatile, which could result in substantial losses to investors. |
| ● | Since our directors and executive officers hold approximately 23.7% of our Ordinary Shares, representing approximately 96.4% of the aggregate voting power, as of the date of this annual report, they have the ability to elect directors and approve matters requiring shareholder approval by way of resolution of members. |
| ● | As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders. |
| ● | The recent joint statement by the SEC and PCAOB, proposed rule changes submitted by Nasdaq, and the Holding Foreign Companies Accountable Act all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to BGM and its affiliated entities’ performance. For more information, see page 45 under Risks Related to Our Ordinary Shares. |
Risks Related to Our Corporate Structure
PRC laws and regulations governing the VIE and its subsidiaries’ businesses and the validity of certain of our contractual arrangements are uncertain. If we or our affiliated entities are found to be in violation, we could be subject to sanctions. In addition, changes in PRC laws and regulations or changes in interpretations thereof may materially and adversely affect the VIE and its subsidiaries’ business.
Current PRC laws and regulations place certain restrictions and conditions on foreign ownership of certain areas of businesses. In accordance with the Special Administrative Measures on Access of Foreign Investment, promulgated in June 2020 and effective in July 2020, or the Negative List, foreign investors are not prohibited nor restricted from investing in our current operations and production. See “Item 4. Information on the Company—B. Business Overview—Regulation—PRC Laws and Regulations on Foreign Investment.” The VIE and its subsidiaries conducts their business activities in China. We are a holding company and do not conduct any business activities. Qilian International Trading (Chengdu) Co., Ltd., or WFOE, has entered into contractual arrangements with the VIE and its shareholders, and such contractual arrangements enable us to exercise certain control over, receive substantially all of the economic benefits of, and have an exclusive option to purchase all or part of the equity interest and assets in the VIE when and to the extent permitted by PRC law. For a detailed description of these contractual arrangements, see “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements between WFOE and Gansu QLS.” We have evaluated the guidance in FASB ASC 810 and concluded that we are the primary beneficiary of the VIE and its subsidiaries because of these contractual arrangements. Accordingly, under U.S. GAAP, the financial statements of the VIE are consolidated as part of our financial statements. In fiscal years ended September 30, 2024, 2023, and 2022, the VIE and its subsidiaries contributed to 100% of our total revenues.
However, BGM is a Cayman Islands holding company with no equity ownership in the VIE or its subsidiaries. We do not conduct any business. The business operations are instead conducted in China through the VIE and the VIE’s subsidiaries with which we have maintained only contractual arrangements. Investors in our Ordinary Shares thus are not purchasing equity interest in our consolidated affiliated entities in China but instead are purchasing equity interest in a Cayman Islands holding company. If the PRC government deems that our contractual arrangements with the VIE do not comply with PRC regulatory restrictions on foreign investment in the relevant industries, or if these regulations or the interpretation of existing regulations change or are interpreted differently in the future, we and the VIE could be subject to severe penalties or be forced to relinquish our interests in those operations. Our holding company in the Cayman Islands, the VIE, and investors of our Company face uncertainty about potential future actions by the PRC government that could affect the enforceability of the contractual arrangements with the VIE and, consequently, significantly affect the financial performance of the VIE and our Company as a group.
18
There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including, but not limited to, the laws and regulations governing the business operations of the VIE and its subsidiaries, or the enforcement and performance of our contractual arrangements with the VIE and its shareholders. These laws and regulations may be subject to change, and their official interpretation and enforcement may involve substantial uncertainty. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. Due to the uncertainty and complexity of the regulatory environment, we cannot assure you that we, the VIE and its subsidiaries would always be in full compliance with applicable laws and regulations, the violation of which may have adverse effect the business and reputation of the VIE and its subsidiaries.
Our PRC counsel, Gansu Quanyi Law Firm, is of the opinion that (i) the ownership structure of WFOE and the VIE does not violate applicable PRC laws and regulations currently in effect, and (ii) the contractual arrangements are valid, binding and enforceable in accordance with the applicable PRC laws or regulations currently in effect.
Although we believe we and the VIE and its subsidiaries are not in violation of current PRC laws and regulations, we cannot assure you that the PRC government would agree that our contractual arrangements comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. The PRC government has broad discretion in determining rectifiable or punitive measures for non-compliance with or violations of PRC laws and regulations. If the PRC government determines that the VIE or any of the VIE’s subsidiaries do not comply with applicable law, it could revoke their business and operating licenses, require them to discontinue or restrict their operations, restrict their right to collect revenues, block their websites, require them to restructure their operations, impose additional conditions or requirements with which they may not be able to comply, impose restrictions on their business operations or on their customers, or take other regulatory or enforcement actions against them that could be harmful to their business. Any of these or similar occurrences could significantly disrupt the business operations of the VIE and its subsidiaries or restrict the VIE and its subsidiaries from conducting a substantial portion of their business operations, which could materially and adversely affect the business, financial condition and results of operations of BGM and its affiliated entities. If any of these occurrences results in our inability to direct the activities of the VIE or its subsidiaries that most significantly impact its economic performance, and/or our failure to receive the economic benefits from the VIE or its subsidiaries, we may not be able to consolidate these entities in our consolidated financial statements in accordance with U.S. GAAP. In addition, our shares may decline in value or become worthless if we are unable to assert our contractual control rights over the assets of those entities that conduct a significant part of the VIE and its subsidiaries’ operations.
BGM relies on contractual arrangements with the VIE and VIE’s subsidiaries in China for its business operations, which may not be as effective in providing operational control or enabling us to derive economic benefits as through ownership of controlling equity interests, and the VIE’s shareholders may fail to perform their obligations under the contractual arrangements.
BGM relies on and expects to continue to rely on its wholly owned PRC Subsidiary’s contractual arrangements with Gansu QLS and Gansu QLS’s shareholders to operate its business. These contractual arrangements may not be as effective in providing BGM with control over Gansu QLS as ownership of controlling equity interests would be in providing BGM with control over, or enabling BGM to derive economic benefits from the operations of Gansu QLS. Under the current contractual arrangements, as a legal matter, if Gansu QLS or any of its shareholders executing the VIE contractual arrangements fails to perform its, his or her respective obligations under these contractual arrangements, BGM may have to incur substantial costs and resources to enforce such arrangements, and rely on legal remedies available under PRC laws, including seeking specific performance or injunctive relief, and claiming damages, which BGM cannot assure investors will be effective. For example, if shareholders of a variable interest entity were to refuse to transfer their equity interests in such variable interest entity to BGM or its designated persons when it exercises the purchase option pursuant to these contractual arrangements, BGM may have to take a legal action to compel them to fulfill their contractual obligations.
If (i) the applicable PRC authorities invalidate these contractual arrangements for violation of PRC laws, rules and regulations, (ii) the VIE or its shareholders terminate the contractual arrangements or (iii) the VIE or its shareholders fail to perform their obligations under these contractual arrangements, BGM’s business operations in China would be materially and adversely affected, and the value of BGM’s shares would substantially decrease. Further, if BGM fails to renew these contractual arrangements upon their expiration, BGM would not be able to continue its business operations unless the then current PRC law allows BGM to directly operate businesses in China.
19
In addition, if the VIE or all or part of its assets become subject to liens or rights of third-party creditors, BGM may be unable to continue some or all of its business activities, which could materially and adversely affect BGM and its affiliated entities’ business, financial condition and results of operations. If the VIE undergoes a voluntary or involuntary liquidation proceeding, its shareholders or unrelated third-party creditors may claim rights to some or all of these assets, thereby hindering BGM’s ability to operate its business, which could materially and adversely affect BGM and its affiliated entities’ business and its ability to generate revenues.
All of these contractual arrangements are governed by PRC law and provide for the resolution of disputes through arbitration in the PRC. The legal environment in the PRC is not as developed as in some other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could limit BGM’s ability to enforce these contractual arrangements. In the event BGM is unable to enforce these contractual arrangements, BGM may not be able to exert expected control over its operating entities and BGM may be precluded from operating its business, which would have a material adverse effect on its financial condition and results of operations.
If the PRC government deems that BGM’s contractual arrangements with the VIE do not comply with PRC regulatory restrictions on foreign investment in the relevant industries, or if these regulations or the interpretation of existing regulations change in the future, BGM could be subject to severe penalties or be forced to relinquish our interests in those operations.
BGM has entered into, through WFOE, a series of contractual arrangements with the VIE and its shareholders. These contractual arrangements enable BGM to (i) direct the activities that most significantly affect the economic performance of the VIE and its subsidiaries; (ii) receive substantially all of the economic benefits from the VIE and its subsidiaries in consideration for the services provided by the PRC Subsidiary; and (iii) have an exclusive option to purchase all or part of the equity interests in the VIE or to all or part of the assets of the VIE, when and to the extent permitted by PRC law, or request any existing shareholder of the VIE to transfer all or part of the equity interest in the VIE to another PRC person or entity designated by BGM at any time in its discretion.
These agreements make BGM their “primary beneficiary” for accounting purposes under U.S. GAAP. For descriptions of these contractual arrangements, see “Item 4. Information on the Company—C. Organizational Structure—Contractual Agreements with the VIE and its Shareholders.” BGM believes that its corporate structure and contractual arrangements comply with the current applicable PRC laws and regulations. BGM’s PRC legal counsel, based on its understanding of the relevant laws and regulations, is of the opinion that each of the contracts among our wholly owned PRC Subsidiary, the consolidated VIE and their shareholders is valid, binding and enforceable in accordance with its terms. However, BGM’s PRC legal counsel has also advised us that there are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including the Foreign Investment Law (2019), Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules and the Telecommunications Regulations and the relevant regulatory measures concerning the telecommunications industry. Accordingly, the PRC regulatory authorities may take a view that is contrary to the opinion of the PRC legal counsel. There can be no assurance that the PRC government authorities, such as the Ministry of Commerce, or the MOFCOM, the MIIT, or other authorities that regulate BGM’s business and/or other participants in the relevant industry, would agree that BGM’s corporate structure or any of the above contractual arrangements comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. PRC laws and regulations governing the validity of these contractual arrangements are uncertain and the relevant government authorities have broad discretion in interpreting these laws and regulations.
If the PRC government determines that these contractual arrangements do not comply with its restrictions on foreign investment in the internet business, if these regulations or the interpretation of existing regulations change or are interpreted differently in the future, or if the PRC government otherwise finds that BGM, the VIE, or any of its subsidiaries is in violation of PRC laws or regulations or lack the necessary permits or licenses to operate the VIE and its subsidiaries’ business, the relevant PRC regulatory authorities, including but not limited to the MIIT, which regulates internet information service companies, would have broad discretion in dealing with such violations, including:
| ● | revoking Gansu QLS and its subsidiaries’ business and operating licenses; |
| ● | discontinuing or restricting the VIE and its subsidiaries’ operations; |
| ● | imposing fines or confiscating any of our income that they deem to have been obtained through illegal operations; |
| ● | requiring us or our PRC affiliated entities to restructure the relevant ownership structure or operations; |
| ● | placing restrictions on our right to collect revenues; |
20
| ● | restricting or prohibiting BGM’s use of the proceeds from its initial public offering to finance the business and operations of the VIE; and |
| ● | taking other regulatory or enforcement actions that could be harmful to the VIE and its subsidiaries’ business. |
The imposition of any of these penalties could have a material and adverse effect on BGM’s business, financial condition and results of operations. If any of these penalties results in our inability to direct the activities of the VIE that most significantly impact its economic performance, and/or BGM’s failure to receive the economic benefits from the VIE, BGM may not be able to consolidate the financial results of the VIE and its subsidiaries in its consolidated financial statements in accordance with U.S. GAAP. In addition, BGM’s shares may decline in value or become worthless if it is unable to assert its contractual control rights over the assets of its affiliated entities in PRC that conduct all or substantially all of BGM’s operations.
Gansu QLS’s shareholders may have potential conflicts of interest with us, which may materially and adversely affect BGM and its affiliated entities’ business and financial condition and the value of your investment in our shares.
The equity interests of Gansu QLS are held by a total of 151 shareholders. Their interests in the VIE may differ from the interests of our Company as a whole. These shareholders may breach, or cause Gansu QLS to breach, or refuse to renew the existing contractual arrangements we have with Gansu QLS, which would have a material adverse effect on BGM’s ability to control Gansu QLS and its subsidiaries and receive economic benefits from them. For example, the shareholders may be able to cause our agreements with Gansu QLS to be performed in a manner adverse to us by, among other things, failing to remit payments due under the contractual arrangements to us on a timely basis. We cannot assure you that when conflicts of interest arise, any or all of these shareholders will act in the best interests of our Company or such conflicts will be resolved in our favor.
Currently, we do not have any arrangements to address potential conflicts of interest between these shareholders and our Company, except that we could exercise our purchase option under the exclusive option agreement with these shareholders to request them to transfer all of their equity interests in Gansu QLS to a PRC entity or individual designated by us, to the extent permitted by PRC laws. If we cannot resolve any conflict of interest or dispute between us and the shareholders of Gansu QLS, we would have to rely on legal proceedings, which could result in the disruption of the VIE and its subsidiaries’ business and subject us and our affiliated entities to substantial uncertainty as to the outcome of any such legal proceedings.
Contractual arrangements in relation to the VIE may be subject to scrutiny by the PRC tax authorities and they may determine that we or the VIE owe additional taxes, which could negatively affect our results of operations and the value of your investment.
Under applicable PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities within ten years after the taxable year when the transactions are conducted. The Enterprise Income Tax Law of the People’s Republic of China (the “EIT Law”) requires every enterprise in China to submit its annual enterprise income tax return together with a report on transactions with its related parties to the relevant tax authorities. The tax authorities may impose reasonable adjustments on taxation if they have identified any related party transactions that are inconsistent with arm’s length principles. We may face material and adverse tax consequences if the PRC tax authorities determine that the contractual arrangements between our WFOE, Gansu QLS, and the shareholders of Gansu QLS were not entered into on an arm’s length basis in such a way as to result in an impermissible reduction in taxes under applicable PRC laws, rules and regulations, and adjust Gansu QLS’s income in the form of a transfer pricing adjustment. A transfer pricing adjustment could, among other things, result in a reduction of expense deductions recorded by Gansu QLS for PRC tax purposes, which could in turn increase their tax liabilities without reducing WFOE’s tax expenses. In addition, if WFOE were to request that the shareholders of Gansu QLS transfer their equity interests in Gansu QLS at nominal or no value pursuant to these contractual arrangements, such transfer could be viewed as a gift and could subject WFOE to PRC income tax. Furthermore, the PRC tax authorities may impose late payment fees and other penalties on Gansu QLS for the adjusted but unpaid taxes according to the applicable regulations. BGM and its affiliated entities’ results of operations could be materially and adversely affected if Gansu QLS’s tax liabilities increase or if it is required to pay late payment fees and other penalties.
21
If we exercise the option to acquire equity ownership of Gansu QLS, the ownership transfer may subject us to certain limitation and substantial costs.
Pursuant to the VIE contractual arrangements, WFOE has the exclusive right to purchase all or any part of the equity interests in Gansu QLS from Gansu QLS’s shareholders for a nominal price, unless the relevant government authorities or then applicable PRC laws request that a minimum amount be used as the purchase price, in such case the purchase price shall be the lowest amount under such request. The shareholders of Gansu QLS will be subject to PRC individual income tax on the difference between the equity transfer price and the then current registered capital of Gansu QLS. Additionally, if such a transfer takes place, the competent tax authority may require WFOE to pay enterprise income tax for ownership transfer income with reference to the market value, in which case the amount of tax could be substantial.
Risks Related to Doing Business in China
The approval and/or other requirements of the CSRC or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we and our affiliated entities cannot predict whether or how soon we will be able to obtain such approval.
The Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, purport to require offshore special purpose vehicles that are controlled by PRC companies or individuals and that have been formed for the purpose of seeking a public listing on an overseas stock exchange through acquisitions of PRC domestic companies or assets to obtain CSRC approval prior to publicly listing their securities on an overseas stock exchange. The interpretation and application of the regulations remain unclear. If a governmental approval is required, it is uncertain how long it will take for us or our affiliated entities to obtain such approval, and, even if we or our affiliated entities obtain such approval, the approval could be rescinded. Any failure to obtain, or a delay in obtaining, the requisite governmental approval for an offering, or a rescission of such CSRC approval if obtained by us or our affiliated entities, may subject us or our affiliated entities to sanctions imposed by the relevant PRC regulatory authority, which could include fines and penalties on the operations of the WFOE and the VIE and its subsidiaries in China, restrictions or limitations on BGM’s ability to pay dividends outside of China, and other forms of sanctions that may materially and adversely affect BGM and its affiliates’ business, financial condition, and results of operations.
Our PRC counsel has advised us that, based on its understanding of the current PRC laws and regulations, we or our affiliated entities will not be required to submit an application to the CSRC for the approval under the M&A Rules for an offering, because (i) the CSRC currently has not issued any definitive rule or interpretation concerning whether any follow-on offerings are subject to this regulation; and (ii) we did not acquire any equity interests or assets of a “PRC domestic company,” as such terms are defined under the M&A Rules.
22
However, our PRC counsel has further advised us that there remains some uncertainty as to how the M&A Rules will be interpreted or implemented in the context of an overseas offering, and its opinions summarized above are subject to any new laws, rules and regulations or detailed implementations and interpretations in any form relating to the M&A Rules. We cannot assure you that relevant PRC governmental authorities, including the CSRC, would reach the same conclusion as our PRC counsel, and hence, we or our affiliated entities may face regulatory actions or other sanctions from them. Furthermore, relevant PRC governmental authorities promulgated the Opinions on Strictly Cracking Down Illegal Securities Activities, which provided that the administration and supervision of overseas-listed China-based companies will be strengthened, and the special provisions of the State Council on overseas issuance and listing of shares by such companies will be revised, clarifying the responsibilities of domestic industry competent authorities and regulatory authorities. However, the Opinions on Strictly Cracking Down Illegal Securities Activities were only issued recently, leaving uncertainties regarding the interpretation and implementation of these opinions. It is possible that any new rules or regulations may impose additional requirements on us. In addition, on July 10, 2021 and November 14, 2021, the Cyberspace Administration of China, or the CAC, issued a revised draft of the Measures for Cybersecurity Review and a draft of the Regulations on the Network Data Security, respectively, for public comments, according to which, among other things, operators of “critical information infrastructure” or data processors holding over one million users’ personal information shall apply to the Cybersecurity Review Office for a cybersecurity review before any listing on a foreign stock exchange. It is uncertain when the final measures will be issued and take effect, how they will be enacted, interpreted or implemented, and whether they will affect us. If it is determined in the future that CSRC approval or other procedural requirements are required to be met for, and prior to, an offering, it is uncertain whether we or our affiliated entities can or how long it will take us or our affiliated entities to obtain such approval or complete such procedures and any such approval could be rescinded. Any failure to obtain or delay in obtaining such approval or completing such procedures for an offering, or a rescission of any such approval, could subject us or our affiliated entities to sanctions by the relevant PRC governmental authorities. The governmental authorities may impose restrictions and penalties on the WFOE and the VIE and its subsidiaries’ operations in China, such as the suspension of our apps and services, revocation of our licenses, or shutting down part or all of the VIE or its subsidiaries’ operations, limit BGM’s ability to pay dividends outside of China, delay or restrict the repatriation of the proceeds from an offering into China or take other actions that could have a material adverse effect on BGM and its affiliated entities’ business, financial condition, results of operations and prospects, as well as the trading price of the Ordinary Shares. The PRC governmental authorities may also take actions requiring us, or making it advisable for us, to halt an offering before settlement and delivery of the Ordinary Shares. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that settlement and delivery may not occur. In addition, if the PRC governmental authorities later promulgate new rules or explanations requiring that we or our affiliated entities obtain their approvals for filings, registrations or other kinds of authorizations for an offering, we cannot assure you that we or our affiliated entities can obtain the approval, authorizations, or complete required procedures or other requirements in a timely manner, or at all, or obtain a waiver of the requisite requirements if and when procedures are established to obtain such a waiver.
Our Ordinary Shares may be delisted and prohibited from being traded under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect auditors who are located in China. The delisting and the cessation of trading of our Ordinary Shares, or the threat of their being delisted and prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections deprives our investors with the benefits of such inspections.
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. The HFCA Act provides that if the SEC determines that we have filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years, the SEC shall prohibit our shares or ADSs from being traded on a national securities exchange or in the “over-the-counter” trading market in the U.S.
Our auditors, the independent registered public accounting firms that issue the audit reports included elsewhere in this annual report, as auditors of companies that are traded publicly in the United States and firms registered with the PCAOB, are subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. ZH CPA, LLC and Enrome LLP are located in Denver, Colorado and Singapore, and have been inspected by the PCAOB on a regular basis.
23
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the HFCA Act. We will be required to comply with these rules if the SEC identifies us as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCA Act, including the listing and trading prohibition requirements described above. In September 2021, the PCAOB adopted a rule related to the PCAOB’s responsibilities under the HFCA Act, which establishes a framework for the PCAOB determine, as contemplated under the HFCA Act, whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. The rule was approved by the SEC in November 2021 and has become effective.
On September 22, 2021, the PCAOB adopted a new rule related to its responsibilities under the HFCA Act, which provides a framework for the PCAOB to use when determining, as contemplated under the HFCA Act, whether it is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. The new rule is subject to approval by the SEC.
On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the Holding Foreign Companies Accountable Act. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions.
On December 16, 2021, the PCAOB issued a report on its determination that it is unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in China and in Hong Kong because of positions taken by PRC and Hong Kong authorities in those jurisdictions. The PCAOB has made such determination as mandated under the Holding Foreign Companies Accountable Act. Pursuant to each annual determination by the PCAOB, the SEC will, on an annual basis, identify issuers that have used non-inspected audit firms and thus are at risk of such suspensions in the future. Our auditors, ZH CPA, LLC and Enrome LLP, the independent registered public accounting firms that issue the audit reports included elsewhere in this annual report, as auditors of companies that are traded publicly in the U.S. and a firm registered with the PCAOB, are subject to laws in the U.S., pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. Our auditors are located in Denver, Colorado and Singapore, and have been inspected by the PCAOB on a regular basis. Our auditors are not subject to the determination issued by the PCAOB on December 16, 2021.
On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden, which contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act and amended the HFCA Act by requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period for triggering the prohibition on trading.
On August 26, 2022, the CSRC, the MOF, and the PCAOB signed the Protocol governing inspections and investigations of audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. Pursuant to the fact sheet with respect to the Protocol disclosed by the SEC, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination.
The recent developments would add uncertainties to our and the VIE and its subsidiaries’ operations and we cannot assure you whether Nasdaq or regulatory authorities would apply additional and more stringent criteria to us after considering the effectiveness of our auditor’s audit procedures and quality control procedures, adequacy of personnel and training, or sufficiency of resources, geographic reach or experience as it relates to the audit of our financial statements. In addition, any additional actions, proceedings, or new rules resulting from these efforts to increase U.S. regulatory access to audit information could create some uncertainty for investors, the market price of our ordinary shares could be adversely affected, and we could be delisted if we and our auditor are unable to meet the PCAOB inspection requirement or being if we are required to engage a new audit firm, which would require significant expense and management time.
24
Our Ordinary Shares may be delisted and prohibited from being traded under the HFCA Act if the PCAOB is unable to inspect auditors who are located in China in the furture. The delisting and the cessation of trading of our Ordinary Shares, or the threat of their being delisted and prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections deprives our investors with the benefits of such inspections.
The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we or our affiliated entities were to be subject to such oversight and control, it may result in a material adverse change to BGM and its affiliated entities’ business operations, significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors, and cause Ordinary Shares to significantly decline in value or become worthless.
BGM and its affiliated entities’ business, prospects, financial condition, and results of operations may be influenced to a significant degree by political, economic, and social conditions in China generally. The PRC government has significant authority to intervene or influence the China operations of an offshore holding company at any time, which could result in a material adverse change to BGM and its affiliated entities’ operations and the value of our Ordinary Shares. The PRC government has recently indicated an intent to exert more oversight and control over listings conducted overseas and/or foreign investment in China-based issuers. Any such action may hinder BGM’s ability to offer or continue to offer its securities to investors, result in a material adverse change to BGM and its affiliated entities’ business operations, and damage our reputation, which could cause Ordinary Shares to significantly decline in value or become worthless. See also “Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect BGM and its affiliated entities’ business, financial condition, and results of operations.”
Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect BGM and its affiliated entities’ business, financial condition, and results of operations.
We may be subject to a variety of cybersecurity, data privacy, data protection, and other laws and regulations related to data, including those relating to the collection, use, sharing, retention, security, disclosure, and transfer of confidential and private information, such as personal information and other data. These laws and regulations apply not only to third-party transactions, but also to transfers of information within our organization. These laws and regulations may restrict the WFOE and the VIE and its subsidiaries’ business activities and require us or our affiliated entities to incur increased costs and efforts to comply, and any breach or noncompliance may subject us or our affiliated entities to proceedings against us or our affiliated entities, damage our and the affiliated entities’ reputation, or result in penalties and other significant legal liabilities, and thus may materially and adversely affect our and the affiliated entities’ business, financial condition, and results of operations.
In China, the cybersecurity, data privacy, data protection, or other data-related laws and regulations are relatively new and evolving, and their interpretation and application may be uncertain. For example, on November 14, 2021, the Administration Regulations on Cyber Data Security (Draft for Comments) (the “Draft Regulation”) was proposed by the Cyberspace Administration of China, or the CAC, for public comments until December 13, 2021. The Draft Regulation reiterates that data processors which process the personal information of at least one million users must apply for a cybersecurity review if they plan on listing its securities overseas, and the Draft Regulation further requires the data processors to apply for cybersecurity review in accordance with relevant laws and regulations under the following circumstances: (i) such data processor engages in merger, reorganization or division of internet platform operators that have gathered a large number of data resources related to national security, economic development and public interests affects or may affect national security; (ii) the listing of such data processor overseas affects or may affect national security; and (iii) such data processor engages in other data processing activities that affect or may affect national security. Any failure to comply with such requirements may subject us or our affiliated entities to, among others, suspension of services, fines, revocation of relevant business permits or business licenses, and/or penalties. Since the CAC is still seeking comments on the Draft Regulation from the public as of the date of this annual report, the Draft Regulation (especially its operative provisions) and its anticipated adoption or effective date are subject to further changes with substantial uncertainty.
25
As of the date of this annual report, we or our affiliated entities have not engaged in the relevant businesses provided in the Draft Regulation. As such, we or our affiliated entities currently do not expect that the draft measures by the CAC or other recent regulations will have an impact on ours and our affiliated entities’ business or results of operations, and we believe that we and our affiliated entities are compliant with the regulations and policies that have been issued by the CAC to date. As of the date of this annual report, we or our affiliated entities have not been subjected to any investigation, nor have we or any of our affiliated entities received any notice, warning, or sanction from applicable government authorities (including the CAC) with regard to the WFOE and the VIE and its subsidiaries’ business operations concerning any issues related to cybersecurity and data security. In addition, we or our affiliated entities have not been involved in any review, investigation, enquiry, penalty, or other legal proceedings initiated by applicable governmental or regulatory authorities or third parties in relation to in relation to cyber security or data protection. However, we and our affiliated entities still face uncertainties regarding the interpretation and implementation of these laws and regulations in the future. Cybersecurity review could result in disruption in the WFOE and the VIE and its subsidiaries’ operations, negative publicity with respect to our Company, and diversion of our managerial and financial resources. Furthermore, if we or our affiliated entities were found to be in violation of applicable laws and regulations in China during such review, we or our affiliated entities could be subject to fines or other government sanctions and reputational damage. Therefore, potential cybersecurity review, if applicable to us, could materially and adversely affect BGM and its affiliated entities’ business, financial condition, and results of operations.
In addition, the PRC Data Security Law, which was promulgated by the Standing Committee of the National People’s Congress (the “SCNPC”) on June 10, 2021 and took effect on September 1, 2021, requires data collection to be conducted in a legitimate and proper manner, and stipulates that, for the purpose of data protection, data processing activities must be conducted based on data classification and hierarchical protection system for data security. Furthermore, the Opinions on Strictly Cracking Down Illegal Securities Activities, recently issued jointly by the General Office of the Communist Party of China Central Committee and the General Office of the State Council, require (i) speeding up the revision of the regulatory provisions on strengthening the confidentiality and archives management relating to overseas issuance and listing of securities and (ii) improving the laws and regulations relating to data security, cross-border data flow, and management of confidential information. The PRC Personal Information Protection Law, which was promulgated by the SCNPC on August 20, 2021 and took effect on November 1, 2021, integrates the scattered rules with respect to personal information rights and privacy protection and applies to the processing of personal information within China as well as certain personal information processing activities outside China, including those for the provision of products and services to natural persons within China or for the analysis and assessment of acts of natural persons within China. There remain uncertainties regarding the further interpretation and implementation of those laws and regulations. If they are deemed to be applicable to us, we cannot assure you that we or our affiliated entities will be compliant with such new regulations in all respects, and we or our affiliated entities may be ordered to rectify and terminate any actions that are deemed illegal by the government authorities and become subject to fines and other government sanctions, which may materially and adversely affect BGM and its affiliated entities’ business, financial condition, and results of operations.
The PRC government’s significant oversight over the WFOE and the VIE and its subsidiaries’ business operations could result in a material adverse change in the WFOE, the VIE and its subsidiaries’ operations and the value of our Ordinary Shares.
We conduct business in China primarily through the WFOE and the VIE and its subsidiaries. The WFOE, the VIE and its subsidiaries’ operations in China are governed by PRC laws and regulations. The PRC government has significant oversight over the conduct of the WFOE, the VIE and its subsidiaries’ business, and it regulates and may intervene in the VIE and its subsidiaries’ operations, which could result in a material adverse change in the WFOE, the VIE and its subsidiaries’ business operations and/or the value of our Ordinary Shares. Also, the PRC government has recently indicated an intent to exert more oversight over offerings that are conducted overseas and/or foreign investment in China-based issuers. Any such action could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors. In addition, implementation of industry-wide regulations directly targeting the WFOE and the VIE and its subsidiaries’ operations could cause BGM’s securities to significantly decline in value or become worthless. Therefore, investors of our Company face potential uncertainty from actions taken by the PRC government affecting the WFOE and the VIE and its subsidiaries’ business.
26
Uncertainties with respect to the PRC legal system and the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us, hinder BGM’s ability and the ability of any holder of BGM’s securities to offer or continue to offer such securities, result in a material adverse change to the WFOE and the VIE and its subsidiaries’ business operations, and damage BGM and its subsidiaries’ reputation, which would materially and adversely affect BGM and its affiliates’ financial condition and results of operations and cause the Ordinary Shares to significantly decline in value or become worthless.
The PRC legal system is based on written statutes, and court decisions have limited precedential value. The PRC legal system is evolving rapidly and PRC laws, regulations, and rules may change quickly with little or no advance notice. The interpretations of many PRC laws, regulations, and rules are done inconsistently, subjecting the enforcement of the same to a great deal of uncertainties. From time to time, we or our affiliated entities may have to resort to court and administrative proceedings to enforce legal rights. However, since the administrative authorities in China have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to predict the outcome of a judicial or administrative proceeding in China than in more developed legal systems. Furthermore, the PRC legal system is, in part, based on government policies and internal rules, some of which are not published in a timely manner, or at all, but which may have retroactive effect. As a result, we or our affiliated entities may not always be aware of an instance of violation of these policies and rules even after its occurrence. Such unpredictability surrounding our contractual, property (including intellectual property), and procedural rights could adversely affect the WFOE and the VIE and its subsidiaries’ business and impede BGM’s ability to continue its operations through the WFOE and the VIE and its subsidiaries.
Laws and regulations concerning our industries are also developing and evolving in China and the PRC governmental authorities may further promulgate new laws and regulations regulating our industries and other businesses we or our affiliated entities have already engaged in or may further expand into in the future. Although we and our affiliated entities have taken measures to comply with and to avoid violation of applicable laws and regulations, we cannot assure you that our, the VIE and its subsidiaries’ business practices are and will remain in full compliance with applicable PRC laws and regulations.
In addition, the PRC government may regulate or intervene in the WFOE and the VIE and its subsidiaries’ operations at any time, or may exercise more oversight and control at any time over offerings conducted outside of China and foreign investment in China-based companies. For example, the recently issued Opinions on Strictly Scrutinizing Illegal Securities Activities emphasized the need to strengthen the management over illegal securities activities and the supervision on overseas listings by China-based companies. These opinions propose to take effective measures, such as promoting the establishment of relevant regulatory systems, to deal with the risks and incidents facing China-based overseas-listed companies, and fulfill the demand for cybersecurity and data privacy protection. These opinions and any future related implementation rules may subject us or our affiliated entities to additional compliance requirement in the future. As these opinions were recently issued, official guidance and interpretation of these opinions are absent in several material respects at this time. In addition, the Measures for Cybersecurity Censorship (Revised Draft for Comments) issued by the CAC on July 10, 2021, if enacted in the current form, extends the scope of cybersecurity review to cover data processing operators engaging in data processing activities that affect or may affect national security, including the listed in a foreign country. If the final version of the draft measures mandates clearance of cybersecurity review by companies like us, we may face uncertainties as to whether such clearance can be timely obtained, or at all. Therefore, we cannot assure you that we or our affiliated entities will remain fully compliant with any new regulatory requirements or any future implementation rules on a timely basis, or at all. Any failure of us or our affiliated entities to fully comply with applicable laws and regulations may significantly limit or completely hinder BGM’s ability to offer or continue to offer such securities, cause significant disruption to the WFOE and the VIE and its subsidiaries’ business operations, and severely damage our reputation, which would materially and adversely affect BGM and its affiliates’ financial condition and results of operations and cause the Ordinary Shares to significantly decline in value or become worthless.
27
The PRC government may intervene or influence the WFOE and the VIE and its subsidiaries’ business operations at any time or may exert more control over offerings conducted overseas and foreign investment in China based issuers, which could result in a material change in the WFOE and the VIE and its subsidiaries’ business operations or the value of BGM’s securities. Additionally, the governmental and regulatory interference could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. We and our affiliated entities are also currently not required to obtain approval from Chinese authorities to list on U.S. exchanges. However, if we or our affiliated entities are required to obtain approval in the future and are denied permission from Chinese authorities to list on U.S. exchanges, we will not be able to continue listing on U.S. exchange, which would materially affect the interest of the investors
We do not conduct any business operations. The business operations are conducted through our affiliated entities in PRC, which subject us and our affiliated entities to certain laws and regulations in China. The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those relating to taxation, environmental regulations, land use rights, property and other matters. The central or local governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
For example, the Chinese cybersecurity regulator announced on July 2, 2021 that it had begun an investigation of Didi Global Inc. (NYSE: DIDI) and two days later ordered that the company’s app be removed from smartphone app stores. On July 24, 2021, the General Office of the Communist Party of China Central Committee and the General Office of the State Council jointly released the Guidelines for Further Easing the Burden of Excessive Homework and Off-campus Tutoring for Students at the Stage of Compulsory Education, pursuant to which foreign investment in such firms via mergers and acquisitions, franchise development, and variable interest entities are banned from this sector.
As such, our, the VIE and its entities’ business segments may be subject to various government and regulatory interference in the provinces in which they operate. We or our affiliated entities could be subject to regulations by various political and regulatory entities, including various local and municipal agencies and government sub-divisions, and these regulations may be interpreted and applied inconsistently by different agencies or authorities. We may incur increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure to comply, and such compliance or any associated inquiries or investigations or any other government actions may:
| ● | delay or impede our development; |
| ● | result in negative publicity or increase our operating costs; |
| ● | require significant management time and attention; and |
| ● | subject our Company to remedies, administrative penalties and even criminal liabilities that may harm the WFOE and the VIE and its subsidiaries’ business, including fines assessed for our current or historical operations, or demands or orders that our affiliated entities modify or even cease their business practices. |
28
The regulatory framework for the collection, use, safeguarding, sharing, transfer and other processing of personal information and important data worldwide is rapidly evolving in PRC and is likely to remain uncertain for the foreseeable future. Regulatory authorities in China have implemented and are considering a number of legislative and regulatory proposals concerning data protection. For example, the PRC Cybersecurity Law, which became effective in June 2017, established China’s first national-level data protection for “network operators,” which may include all organizations in China that connect to or provide services over the internet or other information network. The PRC Data Security Law, which was promulgated by the Standing Committee of PRC National People’s Congress, or the SCNPC, on June 10, 2021 and became effective on September 1, 2021, outlines the main system framework of data security protection. As of the date of this Annual Report on Form 20-F, we or our affiliated entities have not been involved in any investigations on data security compliance made in connection with the PRC Data Security Law, and we or our affiliated entities have not received any inquiry, notice, warning, or sanctions in such respect. Based on the foregoing, we do not expect that, as of the date of this annual report, the PRC Data Security Law would have a material adverse impact on our, the VIE or its subsidiaries’ business.
In August 2021, the Standing Committee of the National People’s Congress of China promulgated the Personal Information Protection Law which became effective on November 1, 2021. The Personal Information Protection Law provides a comprehensive set of data privacy and protection requirements that apply to the processing of personal information and expands data protection compliance obligations to cover the processing of personal information of persons by organizations and individuals in China, and the processing of personal information of persons outside of China if such processing is for purposes of providing products and services to, or analyzing and evaluating the behavior of, persons in China. The Personal Information Protection Law also provides that critical information infrastructure operators and personal information processing entities who process personal information meeting a volume threshold to be set by Chinese cyberspace regulators are also required to store in China the personal information generated or collected in China, and to pass a security assessment administered by Chinese cyberspace regulators for any export of such personal information. Moreover, pursuant to the Personal Information Protection Law, persons who seriously violate this law may be fined for up to RMB50 million or 5% of annual revenues generated in the prior year and may also be ordered to suspend any related activity by competent authorities.
Recent statements by the Chinese government have indicated an intent to exert more oversight and control over offerings that are conducted overseas and/or foreign investments in China-based issuers. On July 6, 2021, the General Office of the Communist Party of China Central Committee and the General Office of the State Council jointly issued a document to crack down on illegal activities in the securities market and promote the high-quality development of the capital market, which, among other things, requires the relevant governmental authorities to strengthen cross-border oversight of law-enforcement and judicial cooperation, to enhance supervision over China-based companies listed overseas, and to establish and improve the system of extraterritorial application of the PRC securities laws. On December 24, 2021, the CSRC published the Provisions of the State Council on the Administration of Overseas Securities Offering and Listing by Domestic Companies (the “Administration Provisions”), and the Administrative Measures for the Filing of Overseas Securities Offering and Listing by Domestic Companies (the “Measures”), which are now open for public comment.
Furthermore, on July 10, 2021, the CAC issued a revised draft of the Measures for Cybersecurity Review for public comments, which required that, among others, in addition to “operator of critical information infrastructure”, any “data processor” controlling personal information of no less than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review, and further elaborated the factors to be considered when assessing the national security risks of the relevant activities. On December 28, 2021, the CAC, the National Development and Reform Commission (“NDRC”), and several other administrations jointly issued the revised Measures for Cybersecurity Review, or the “Revised Review Measures”, which became effective and replaced the existing Measures for Cybersecurity Review on February 15, 2022. According to the Revised Review Measures, if an “online platform operator” that is in possession of personal data of more than one million users intends to list in a foreign country, it must apply for a cybersecurity review. Based on a set of Q&A published on the official website of the State Cipher Code Administration in connection with the issuance of the Revised Review Measures, an official of the said administration indicated that an online platform operator should apply for a cybersecurity review prior to the submission of its listing application with non-PRC securities regulators. Moreover, the CAC released the draft of the Regulations on Network Data Security Management in November 2021 for public consultation, which among other things, stipulates that a data processor listed overseas must conduct an annual data security review by itself or by engaging a data security service provider and submit the annual data security review report for a given year to the municipal cybersecurity department before January 31 of the following year. Given the recency of the issuance of the Revised Review Measures and their pending effectiveness, there is a general lack of guidance and substantial uncertainties exist with respect to their interpretation and implementation.
29
BGM has been advised by Gansu Quanyi Law Firm, our PRC counsel, that as of the date of this Annual Report, our listing in the U.S. is not subject to the review, permission or prior approval of any PRC authorities including the Cyberspace Administration of China (“CAC”) or the China Securities Regulatory Commission (“CSRC”) because (i) the CSRC currently has not issued any definitive rule or interpretation concerning whether our listing is subject to this regulation; and (ii) our operating entities affiliated to us were established and operate in PRC are not included in the categories of industries and companies whose foreign securities offerings are subject to review by the CSRC or the CAC. Uncertainties still exist, however, due to the possibility that laws, regulations, or policies in the PRC could change rapidly in the future. In the event that the PRC government expanded the categories of industries and companies whose foreign securities offerings are subject to review by the CSRC or the CAC, and we inadvertently concluded that relevant permissions or approvals were not required or that we or our affiliated entities did not receive or failed to maintain relevant permissions or approvals required and such permissions were subsequently rescinded, any action by the PRC government could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly decline or be worthless.
Further, the promulgation of new laws or regulations, or the new interpretation of existing laws and regulations, in each case that restrict or otherwise unfavorably may impact the ability or the way the VIE or its subsidiaries’ may conduct their business and could require them to change certain aspects of their business to ensure compliance, which could decrease demand for their products or services, reduce revenues, increase costs, require them to obtain more licenses, permits, approvals or certificates, or subject it to additional liabilities. As such, the WFOE and the VIE and its subsidiaries’ operations could be adversely affected, directly or indirectly, by existing or future PRC laws and regulations relating to its business or industry, which could result in a material adverse change in the value of BGM’s securities, potentially rendering it worthless. As a result, both you and us face uncertainty about future actions by the PRC government that could significantly affect BGM’s ability to offer or continue to offer securities to investors and cause the value of BGM’s securities to significantly decline or be worthless.
PRC regulation of loans to, and direct investments in, PRC entities by offshore holding companies may delay or prevent us from using proceeds from future financing activities to make loans or additional capital contributions to our PRC Subsidiary.
As an offshore holding company, we may transfer funds to the PRC Subsidiary or finance our operating entity by means of loans or capital contributions. Any capital contributions or loans that we, as an offshore entity, make to our Company’s PRC Subsidiary, are subject to PRC regulations. Any loans to the PRC Subsidiary, which are foreign-invested enterprises, cannot exceed statutory limits based on the difference between the amount of our investments and registered capital in such subsidiary, and shall be registered with China’s State Administration of Foreign Exchange (“SAFE”), or its local counterparts. Furthermore, any capital increase contributions we make to the PRC Subsidiary, which are foreign-invested enterprises, shall be approved by China’s Ministry of Commerce (“MOFCOM”), or its local counterparts. We or our affiliated entities may not be able to obtain these government registrations or approvals on a timely basis, if at all. If we or our affiliated entities fail to obtain such approvals or make such registration, our ability to make equity contributions or provide loans to the Company’s PRC Subsidiary or to fund its operations may be negatively affected, which may adversely affect its liquidity and ability to fund working capital and expansion projects and meet its obligations and commitments. As a result, our liquidity and our ability to fund and expand the WFOE and the VIE and its subsidiaries’ business may be negatively affected.
A severe or prolonged downturn in the Chinese or global economy could materially and adversely affect the VIE and its subsidiaries’ business and financial condition.
The COVID-19 pandemic had a severe and negative impact on the Chinese and the global economy in 2020. Even before the outbreak of COVID-19, the global macroeconomic environment was facing numerous challenges. The growth rate of the Chinese economy had already been slowing since 2010. There is considerable uncertainty over the long-term effects of the expansionary monetary and fiscal policies which had been adopted by the central banks and financial authorities of some of the world’s leading economies, including the United States and China, even before 2020. Unrest, terrorist threats and the potential for war in the Middle East and elsewhere may increase market volatility across the globe. There have also been concerns about the relationship between China and other countries, including the surrounding Asian countries, which may potentially have economic effects. In particular, there is significant uncertainty about the future relationship between the United States and China with respect to trade policies, treaties, government regulations and tariffs. Economic conditions in China are sensitive to global economic conditions, as well as changes in domestic economic and political policies and the expected or perceived overall economic growth rate in China. Any severe or prolonged slowdown in the global or Chinese economy may materially and adversely affect BGM and its affiliated entities’ business, results of operations and financial condition.
30
Adverse changes in political and economic policies of the PRC government could have a material adverse effect on the overall economic growth of China, which could reduce the demand for the WFOE and the VIE and its subsidiaries’ products and materially and adversely affect their competitive position.
All of the business operations of the VIE and its subsidiaries are conducted in China. Accordingly, BGM and its affiliated entities’ business, results of operations, financial condition and prospects are subject to economic, political and legal developments in China. Although the Chinese economy is no longer a planned economy, the PRC government continues to exercise significant control over China’s economic growth through direct allocation of resources, monetary and tax policies, and a host of other government policies such as those that encourage or restrict investment in certain industries by foreign investors, control the exchange between RMB and foreign currencies, and regulate the growth of the general or specific market. These government involvements have been instrumental in China’s significant growth in the past 30 years. In response to the recent global and Chinese economic downturn, the PRC government has adopted policy measures aimed at stimulating the economic growth in China. If the PRC government’s current or future policies fail to help the Chinese economy achieve further growth or if any aspect of the PRC government’s policies limits the growth of our industry or otherwise negatively affects the WFOE and the VIE and its subsidiaries’ business, their growth rate or strategy, and results of operations could be adversely affected as a result.
Under the EIT Law, we may be classified as a “Resident Enterprise” of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC shareholders.
China passed the EIT Law, which became effective on December 29, 2018, and its implementing rules, which became effective on April 23, 2019. Under the EIT Law, an enterprise established outside of China with “de facto management bodies” within China is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes, which is subject to an EIT rate of 25.0% on its global income. The implementing rules of the EIT Law define de facto management as “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise.
On April 22, 2009, the State Administration of Taxation of China (the “SAT”) issued the Notice Concerning Relevant Issues Regarding Cognizance of Chinese Investment Controlled Enterprises Incorporated Offshore as Resident Enterprises pursuant to Criteria of de facto Management Bodies, or the Notice, further interpreting the application of the EIT Law and its implementation to offshore entities controlled by a Chinese enterprise or group. Pursuant to the Notice, an enterprise incorporated in an offshore jurisdiction and controlled by a Chinese enterprise or group will be classified as a “non-domestically incorporated resident enterprise” if (i) its senior management in charge of daily operations reside or perform their duties mainly in China; (ii) its financial or personnel decisions are made or approved by bodies or persons in China; (iii) its substantial assets and properties, accounting books, corporate stamps, board and shareholder minutes are kept in China; and (iv) over half of its directors with voting rights or senior management reside in China. A resident enterprise would be subject to an enterprise income tax rate of 25% on its worldwide income and must pay a withholding tax at a rate of 10% when paying dividends to its non-PRC shareholders. Because substantially all of the WFOE and the VIE and its subsidiaries’ operations and senior management are located within the PRC and are expected to remain so for the foreseeable future, we may be considered a PRC resident enterprise for enterprise income tax purposes and therefore subject to the PRC enterprise income tax at the rate of 25% on worldwide income. However, it remains unclear as to whether the Notice is applicable to an offshore enterprise controlled by a Chinese natural person. Therefore, it is unclear how tax authorities will determine tax residency based on the facts of each case.
31
If the PRC tax authorities determine that we are a “resident enterprise” for PRC enterprise income tax purposes, a number of unfavorable PRC tax consequences could follow. First, we or our affiliated entities may be subject to the enterprise income tax at a rate of 25% on worldwide taxable income as well as PRC enterprise income tax reporting obligations. In our case, this would mean that income such as non-China source income would be subject to PRC enterprise income tax at a rate of 25%. Currently, the WFOE and the VIE and its subsidiaries do not have any non-China source income, as they conduct their sales in China. However, under the EIT Law and its implementing rules, dividends paid to us from the PRC Subsidiary would be deemed as “qualified investment income between resident enterprises” and therefore qualify as “tax-exempt income” pursuant to clause 26 of the EIT Law. Second, it is possible that future guidance issued with respect to the new “resident enterprise” classification could result in a situation in which the dividends we pay with respect to our Ordinary Shares, or the gain our non-PRC shareholders may realize from the transfer of our Ordinary Shares, may be treated as PRC-sourced income and may therefore be subject to a 10% PRC withholding tax. The EIT Law and its implementing regulations are, however, relatively new and ambiguities exist with respect to the interpretation and identification of PRC-sourced income, and the application and assessment of withholding taxes. If we are required under the EIT Law and its implementing regulations to withhold PRC income tax on dividends payable to our non-PRC shareholders, or if non-PRC shareholders are required to pay PRC income tax on gains on the transfer of their Ordinary Shares, the WFOE and the VIE and its subsidiaries’ business could be negatively impacted and the value of your investment may be materially reduced. Further, if we were to be treated as a “resident enterprise” by PRC tax authorities, we and our affiliated entities would be subject to taxation in both China and such countries in which we have taxable income, and our PRC tax may not be creditable against such other taxes.
We may be exposed to liabilities under the Foreign Corrupt Practices Act and Chinese anti-corruption law.
We are subject to the U.S. Foreign Corrupt Practices Act (the “FCPA”), and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers as defined by the statute for the purpose of obtaining or retaining business. We and our affiliated entities are also subject to Chinese anti-corruption laws, which strictly prohibit the payment of bribes to government officials. We and our affiliated entities have operations, agreements with third parties, and make sales in China, which may experience corruption. Our activities in China create the risk of unauthorized payments or offers of payments by one of the employees, consultants or distributors of our Company, because these parties are not always subject to our control.
Although we believe we and our affiliated entities have complied in all material respects with the provisions of the FCPA and Chinese anti-corruption law as of the date of the annual report on Form 20-F, our existing safeguards and any future improvements may prove to be less than effective, and the employees, consultants or distributors of our Company may engage in conduct for which we or our affiliated entities might be held responsible. Violations of the FCPA or Chinese anti-corruption law may result in severe criminal or civil sanctions, and we or our affiliated entities may be subject to other liabilities, which could negatively affect BGM and its affiliated entities’ business, operating results and financial condition. In addition, the government may seek to hold the Company liable for successor liability FCPA violations committed by companies in which it invests or that it acquires.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of the RMB into foreign currencies and, in certain cases, the remittance of currency out of China. The WFOE and the VIE and its subsidiaries receive substantially all of our revenues in RMB. Under our current corporate structure, our income is primarily derived from dividend payments from the PRC Subsidiary. Shortages in the availability of foreign currency may restrict the ability of the PRC Subsidiary to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency denominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions can be made in foreign currencies without prior approval from SAFE by complying with certain procedural requirements. However, approval from appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of loans denominated in foreign currencies. The PRC government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay dividends in foreign currencies to our security-holders.
32
BGM is a holding company and it relies for funding on dividend payments from its affiliated entities by contracts, which are subject to restrictions under PRC laws. Any limitation on the ability of BGM’s affiliated entities to make payments to it could have a material adverse effect on BGM’s ability to maintain its business.
BGM is a holding company incorporated in the Cayman Islands, and it operates its core businesses through the WFOE and the VIE and its subsidiaries in the PRC. Therefore, the availability of funds for BGM to pay dividends to its shareholders and to service its indebtedness depends upon dividends received from the WFOE, the VIE and its subsidiaries. If the WFOE and the VIE and its subsidiaries incur debt or losses, their ability to pay dividends or other distributions to BGM may be impaired. As a result, BGM’s ability to pay dividends and to repay its indebtedness will be restricted.
Under PRC laws and regulations, BGM’s PRC Subsidiary, as a wholly foreign-owned enterprise in China, may pay dividends only out of its accumulated after-tax profits as determined in accordance with PRC accounting standards and regulations. In addition, a wholly foreign-owned enterprise is required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund certain statutory reserve funds, until the aggregate amount of such funds reaches 50% of its registered capital. At its discretion, a wholly foreign-owned enterprise may allocate a portion of its after-tax profits based on PRC accounting standards to discretional funds. These reserve funds and discretional funds are not distributable as cash dividends.
Under existing PRC foreign exchange regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from the SAFE, by complying with certain procedural requirements. Therefore, our PRC Subsidiary is able to pay dividends in foreign currencies to its non-PRC shareholders without prior approval from the SAFE, subject to the condition that the remittance of such dividends outside of the PRC complies with certain procedures under PRC foreign exchange regulation, such as the overseas investment registrations by the beneficial owners of our Company who are PRC residents. However, approval from, or registration with, appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of loans denominated in foreign currencies.
In response to the persistent capital outflow and RMB’s depreciation against the U.S. dollar in the fourth quarter of 2016, the PBOC and the SAFE have implemented a series of capital control measures, including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments and shareholder loan repayments. The PRC government may continue to strengthen its capital controls and our PRC Subsidiary’s dividends and other distributions may be subjected to tighter scrutiny in the future. Any limitation on the ability of our PRC Subsidiary to pay dividends or make other distributions to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to the VIE and its subsidiaries’ business, pay dividends, or otherwise fund and conduct the VIE and its subsidiaries’ business. See also “— Under the EIT Law, we may be classified as a ‘Resident Enterprise’ of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC shareholders.”
Any limitation on the ability of BGM’s affiliated entities in PRC to pay dividends or make other distributions to it could materially and adversely limit its ability to grow, make investments or acquisitions that could be beneficial to its business, pay dividends, or otherwise fund and conduct its business.
The WFOE and the VIE and its subsidiaries’ business may be materially and adversely affected if any of our PRC Subsidiary, the VIE or the VIE’s subsidiaries declares bankruptcy or becomes subject to a dissolution or liquidation proceeding.
The Enterprise Bankruptcy Law of the PRC, or the Bankruptcy Law, came into effect on June 1, 2007. The Bankruptcy Law provides that an enterprise will be liquidated if the enterprise fails to settle its debts as and when they fall due and if the enterprise’s assets are, or are demonstrably, insufficient to clear such debts.
Our PRC Subsidiary, the VIE and the VIE’s subsidiaries hold substantially all the assets that are important to the WFOE and the VIE and its subsidiaries’ business operations. If any of these entities undergoes a voluntary or involuntary liquidation proceeding, unrelated third-party creditors may claim rights to some or all of these assets, thereby hindering WFOE, the VIE and its subsidiaries’ to operate their business, which could materially and adversely affect BGM and its affiliated entities’ business, financial condition and results of operations.
33
According to SAFE’s Notice of the State Administration of Foreign Exchange on Further Improving and Adjusting Foreign Exchange Administration Policies for Direct Investment, promulgated on November 19, 2012 and amended on May 4, 2015, and the Provisions on the Foreign Exchange Administration of Domestic Direct Investment of Foreign Investors, effective on May 13, 2013, if any of our PRC Subsidiary, the VIE, or the VIE’s subsidiaries undergoes a voluntary or involuntary liquidation proceeding, prior approval from SAFE for remittance of foreign exchange to our shareholders abroad is no longer required, but we still need to conduct a registration process with the SAFE local branch. It is not clear whether “registration” is a mere formality or involves the kind of substantive review process undertaken by SAFE and its relevant branches in the past.
Substantial uncertainties exist with respect to the interpretation of the PRC Foreign Investment Law and how it may impact the viability of our current corporate structure, corporate governance and business operations.
The MOFCOM published a discussion draft of the proposed Foreign Investment Law in January 2015, or the 2015 FIL Draft, which expands the definition of foreign investment and introduces the principle of “actual control” in determining whether a company is considered a foreign-invested enterprise, or an FIE. Under the 2015 FIL Draft, VIEs that are controlled via contractual arrangement would also be deemed as foreign invested enterprises, if they are ultimately “controlled” by foreign investors.
On March 15, 2019, the National People’s Congress approved the Foreign Investment Law of the PRC, or the Foreign Investment Law, which came into effect on January 1, 2020, repealing simultaneously the Law of the PRC on Chinese-foreign Equity Joint Ventures, the Law of the PRC on Wholly Foreign-owned Enterprises and the Law of the PRC on Chinese-foreign Cooperative Joint Ventures, together with their implementation rules and ancillary regulations. Pursuant to the Foreign Investment Law, foreign investment refers to any investment activity within China directly or indirectly carried out by foreign natural persons, enterprises, or other organizations, including investment in new construction project, establishment of foreign funded enterprise or increase of investment within China alone or jointly with any other investor, merger and acquisition, and investment in any other way stipulated under laws, administrative regulations, or provisions of the State Council. Although the Foreign Investment Law has deleted the particular reference to the concept of “actual control” and contractual arrangements, as compared to the 2015 FIL Draft, there is no assurance that foreign investment via contractual arrangement would not be interpreted as a type of indirect foreign investment activity in the future. In addition, the definition of foreign investment activities contains a catch-all provision providing that investments made by foreign investors through other methods specified in laws or administrative regulations or other methods prescribed by the State Council, which leaves leeway for future laws, administrative regulations or provisions promulgated by the State Council to provide for contractual arrangements as a method of foreign investment. Given the foregoing, it is uncertain whether our contractual arrangements will be deemed to be in violation of the market entry clearance requirements for foreign investment under the PRC laws and regulations.
Even if the VIE were to be identified as an FIE in the future, we believe that the WFOE and the VIE and its subsidiaries’ current business would not be adversely affected. However, if they were to engage in any business conduct involving third parties identified as prohibited or restricted on the Negative List, we and our affiliated entities may be subject to laws and regulations on foreign investment. Such might be the case for Gansu QLS’s proposed acquisition of enterprises manufacturing traditional Chinese medicine pieces. In addition, our shareholders would also be prohibited or restricted to invest in certain sectors on the Negative List. However, even if the VIE were to be identified as an FIE, the validity of our contractual arrangements with Gansu QLS and its shareholders, as well as our corporate structure, would not be adversely affected. We would still be able to receive benefits from the VIE in accordance with the contractual arrangements. In addition, as the Chinese government has been updating the Negative List in recent years and reducing the sectors prohibited or restricted for foreign investment, it is possible in the future that, even if the VIE is identified as an FIE, it is still allowed to acquire or hold equity of enterprises in sectors currently prohibited or restricted for foreign investment.
34
It may be difficult for overseas regulators to conduct investigation or collect evidence within China.
Shareholder claims or regulatory investigation that are common in the United States generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to providing information needed for regulatory investigations or litigation initiated outside China. Although the authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such cooperation with the securities regulatory authorities in the Unities States may not be efficient in the absence of mutual and practical cooperation mechanism. Furthermore, according to Article 177 of the PRC Securities Law, which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the PRC territory. While detailed interpretation of or implementation rules under Article 177 have yet to be promulgated, the inability for an overseas securities regulator to directly conduct investigation or evidence collection activities within China may further increase the difficulties you face in protecting your interests. See also “—Risks Related to Our Ordinary Shares— The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States. For instance, you may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts may be limited, because we are incorporated under Cayman Islands law” for risks associated with investing in us as a Cayman Islands holding company.
You may experience difficulties in effecting services of legal process, enforcing foreign judgments or bringing actions in China against us or our management based on foreign laws.
We are an exempted company incorporated under the laws of the Cayman Islands; however, we do not conduct any businesses and all business operations are conducted by the WFOE and the VIE and its subsidiaries in China and most of our assets are located in China. In addition, all of our directors and executive officers are nationals or residents of the PRC and most of their assets are located outside the United States. As a result, it may be difficult for you to effect service of process upon us or our management inside mainland China. It may also be difficult for you to enforce in U.S. courts of the judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors. In addition, there is uncertainty as to whether the courts of the Cayman Islands or the PRC would recognize or enforce judgments of U.S. courts against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other forms of written arrangement with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
The custodians or authorized users of our controlling non-tangible assets, including chops and seals, may fail to fulfill their responsibilities, or misappropriate or misuse these assets.
Under the PRC law, legal documents for corporate transactions, including agreements and contracts are executed using the chop or seal of the signing entity or with the signature of a legal representative whose designation is registered and filed with relevant PRC market regulation administrative authorities.
In order to secure the use of our chops and seals, we and our affiliated entities have established internal control procedures and rules for using these chops and seals. In any event that the chops and seals are intended to be used, the responsible personnel will submit an application and the application will be verified and approved by authorized employees in accordance with our internal control procedures and rules. In addition, in order to maintain the physical security of our chops, we and our affiliated entities generally have them stored in secured locations accessible only to authorized employees. Although we and our affiliated entities monitor such authorized employees, the procedures may not be sufficient to prevent all instances of abuse or negligence. There is a risk that our employees could abuse their authority, for example, by entering into a contract not approved by us or seeking to gain control of one of our affiliated entities or the VIE or its subsidiaries. If any employee obtains, misuses or misappropriates our chops and seals or other controlling non-tangible assets for whatever reason, we and our affiliated entities could experience disruption to our normal business operations. We and our affiliated entities may have to take corporate or legal action in such an event, which could involve significant time and resources to resolve and divert management from the WFOE and the VIE and its subsidiaries’ operations.
35
Fluctuations in exchange rates could adversely affect the VIE and its subsidiaries’ business and the value of BGM’s securities.
Changes in the value of the RMB against the U.S. dollar, Euro and other foreign currencies are affected by, among other things, changes in China’s political and economic conditions. Any significant revaluation of the RMB may have a material adverse effect on our revenues and financial condition, and the value of, and any dividends payable on our shares in U.S. dollar terms. For example, to the extent that we need to convert U.S. dollars we receive from our initial public offering into RMB for our WFOE and VIE and its subsidiaries’ business operations, appreciation of the RMB against the U.S. dollar would have an adverse effect on RMB amount we would receive from the conversion. Conversely, if we decide to convert the RMB into U.S. dollars for the purpose of paying dividends on our Ordinary Shares or for other business purposes, appreciation of the U.S. dollar against the RMB would have a negative effect on the U.S. dollar amount available to us. In addition, fluctuations of the RMB against other currencies may increase or decrease the cost of imports and exports, and thus affect the price-competitiveness of the WFOE and the VIE and its subsidiaries’ products against products of foreign manufacturers or products relying on foreign inputs.
Since July 2005, the RMB is no longer pegged to the U.S. dollar. Although the People’s Bank of China regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future PRC authorities may lift restrictions on fluctuations in the RMB exchange rate and lessen intervention in the foreign exchange market.
Increases in labor costs in the PRC may adversely affect BGM and its affiliated entities’ business and results of operations.
The currently effective PRC Labor Contract Law, or the Labor Contract Law was first adopted on June 29, 2007, later amended on December 28, 2012 and effective on July 1, 2013. The PRC Labor Contract Law has reinforced the protection of employees who, under the Labor Contract Law, have the right, among others, to have written employment contracts, to enter into employment contracts with no fixed term under certain circumstances, to receive overtime wages and to terminate or alter terms in labor contracts. Furthermore, the Labor Contract Law sets forth additional restrictions and increases the costs involved with dismissing employees. To the extent that our affiliated entities need to significantly reduce their workforce, the Labor Contract Law could adversely affect their ability to do so in a timely and cost-effective manner, and their results of operations could be adversely affected. In addition, for employees whose employment contracts include non-competition terms, the Labor Contract Law requires the WFOE and the VIE and its subsidiaries to pay monthly economic compensation after such employment is terminated, which will increase our operating expenses.
We expect that the WFOE and the VIE and its subsidiaries’ labor costs, including wages and employee benefits, will continue to increase. Unless the WFOE and the VIE and its subsidiaries are able to pass on these increased labor costs to their customers by increasing the prices of their products and services, their financial conditions and results of operations could be materially and adversely affected.
Some of our shareholders are not in compliance with the PRC’s regulations relating to offshore investment activities by PRC residents, and as a result, the shareholders may be subject to penalties if we are not able to remediate the non-compliance.
In July 2014, the SAFE promulgated the Circular on Issues Concerning Foreign Exchange Administration over the Overseas Investment and Financing and Roundtrip Investment by Domestic Residents via Special Purpose Vehicles, or “Circular 37”. According to Circular 37, prior registration with the local SAFE branch is required for Chinese residents to contribute domestic assets or interests to offshore companies, known as special purpose vehicles, or SPVs. Circular 37 further requires amendment to a PRC resident’s registration in the event of any significant changes with respect to the SPV, such as an increase or decrease in the capital contributed by PRC individuals, share transfer or exchange, merger, division, or other material event. Further, foreign investment enterprises established by way of round-tripping shall complete the relevant foreign exchange registration formalities pursuant to the prevailing foreign exchange control provisions for direct investments by foreign investors, and disclose the relevant information such as actual controlling party of the shareholders truthfully.
36
There are a total of 151 Gansu QLS shareholders, who are PRC residents. Amongst them, 122 have signed the VIE Agreements, but only 82 have completed the Circular 37 Registration. The remaining 40 shareholders who have yet to complete the Circular 37 Registration hold a total of 4.5% of shares of Gansu QLS. We have asked our shareholders who are Chinese residents to make the necessary applications and filings as required by Circular 37. While we attempt to comply, and attempt to ensure that our shareholders who are subject to these rules comply, with the relevant requirements, we cannot, however, provide any assurances that all of our shareholders who are Chinese residents will comply with our request to make or obtain any applicable registration or comply with other requirements required by Circular 37 or other related rules. The Chinese resident shareholders’ failure to comply with Circular 37 registration would not impose penalties on our Company, but it may result in restrictions being imposed on part of foreign exchange activities of the offshore special purpose vehicles, including restrictions on its ability to receive registered capital as well as additional capital from Chinese resident shareholders who fail to complete Circular 37 registration; and repatriation of profits and dividends derived from special purpose vehicles to China, by the Chinese resident shareholders who fail to complete Circular 37 registration, are also illegal. In addition, the failure of the Chinese resident shareholders to complete Circular 37 registration may subject each of the shareholders to fines less than RMB50,000. We cannot assure you that each of our Chinese resident shareholders will in the future complete the registration process as required by Circular 37.
The VIE is not in compliance with the PRC’s regulations relating to employee’s social insurance and housing funds, and as a result, Gansu QLS and its subsidiaries may be subject to penalties if it is not able to remediate the non-compliance.
Pursuant to the Social Security Law of the PRC, or the Social Security Law, which was promulgated by the SCNPC on October 28, 2010 and amended on December 29, 2018, employers shall pay the basic pension insurance, medical insurance, work-related injury insurance, unemployment insurance and maternity insurance for employees. Gansu QLS has not deposited social security premiums for some of the employees in accordance with the Social Security Law. Although Gansu QLS has failed to deposit social security premiums in full, we believe that no additional amount is required to be paid by Gansu QLS since (i) some of the employees of Gansu QLS are over the age limit to be paid social insurance fees, and some chose to waive receiving social insurance fees deposited by Gansu QLS and decided to participate in their own voluntary social insurance plans instead; and (ii) pursuant to the Emergency Notice on Practicing Principles of the State Council Executive Meeting and Stabilizing Work on Collecting Social Insurance Premiums promulgated by the Ministry of Human Resources and Social Security on September 21, 2018, local authorities are prohibited from recovering the unpaid social insurance premiums from enterprises. Thus, it is unlikely that the overdue social insurance premiums would be ordered to be repaid by Gansu QLS.
In accordance with the Regulation on Management of Housing Provident Fund (the “Regulations of HPF”), which were promulgated by the PRC State Council on April 3, 1999, and last amended on March 24, 2019, employers must register at the designated administrative centers and open bank accounts for employees’ housing fund deposits. Employers and employees are also required to pay and deposit housing funds in an amount no less than 5% of the monthly average salary of each of the employees in the preceding year in full and on time. Gansu QLS had not opened such bank accounts or deposited its employees’ housing funds until August 2019. On the basis that (i) Gansu QLS has opened the account for housing funds and deposited housing funds for staff since August 2019, and (ii) the local authorities had not taken enforceable measures to collect housing funds from local enterprises, we believe it is unlikely that the overdue unpaid housing fund would be ordered to be recovered from Gansu QLS. However, Chengdu QLS has not opened bank accounts for its employees’ housing fund deposits, nor has it deposited employees’ housing funds in accordance with the Regulations of HPF. Thus, Chengdu QLS may be ordered by PRC authorities to open a housing funds account, make the payment, and deposit an amount required by the PRC authorities within a prescribed time limit. If Chengdu QLS fails to comply to PRC authorities’ order within the prescribed time limit, a court ordered compulsory enforcement may be adopted and a fine of no less than RMB10,000 but no more than RMB50,000 shall be imposed.
Since the VIE failed to make adequate social insurance and housing fund contributions, it may be subject to fines and legal sanctions, and BGM and its affiliated entities’ business, financial condition and results of operations may be adversely affected.
37
If we become directly subject to the recent scrutiny, criticism and negative publicity involving U.S.-listed Chinese companies, we may have to expend significant resources to investigate and resolve the matter which could harm the WFOE and the VIE and its subsidiaries’ business operations and our reputation and could result in a loss of your investment in our stock, especially if such matter cannot be addressed and resolved favorably.
Recently, U.S. public companies that have substantially all of their operations in China have been the subject of intense scrutiny, criticism and negative publicity by investors, financial commentators and regulatory agencies, such as the SEC. Much of the scrutiny, criticism and negative publicity has centered around financial and accounting irregularities, a lack of effective internal controls over financial accounting, inadequate corporate governance policies or a lack of adherence thereto and, in many cases, allegations of fraud. As a result of the scrutiny, criticism and negative publicity, the publicly traded stock of many U.S. listed Chinese companies has sharply decreased in value and, in some cases, has become virtually worthless. Many of these companies are now subject to shareholder lawsuits and SEC enforcement actions and are conducting internal and external investigations into the allegations. It is not clear what effect this sector-wide scrutiny, criticism and negative publicity will have on our Company and the VIE and its subsidiaries’ business. If we become the subject of any unfavorable allegations, whether such allegations are proven to be true or untrue, we will have to expend significant resources to investigate such allegations and/or defend the Company. This situation may be a major distraction to our management. If such allegations are not proven to be groundless, our Company and business operations will be severely hampered and your investment in our stock could be rendered worthless.
You may face difficulties in protecting your interests and exercising your rights as a shareholder since we do not conduct any business and substantially all of the business operations are conducted by the WFOE and the VIE and its subsidiaries in China, and almost all of our officers and directors reside outside the U.S.
Although we are incorporated in the Cayman Islands, we do not conduct any business and substantially all of the business operations are conducted by the WFOE and the VIE and its subsidiaries in China. All of our current officers and almost all of our directors reside outside the U.S. and substantially all of the assets of those persons are located outside of the U.S. It may be difficult for you to conduct due diligence on the Company or such directors in your election of the directors and attend shareholders meeting if the meeting is held in China. We plan to have one shareholder meeting each year at a location to be determined, potentially in China. As a result of all of the above, our public shareholders may have more difficulty in protecting their interests through actions against our management, directors or major shareholders than would shareholders of a corporation doing business entirely or predominantly within the U.S.
If we are classified as a passive foreign investment company, United States taxpayers who own our Ordinary Shares may have adverse United States federal income tax consequences.
A non-U.S. corporation such as ourselves will be classified as a passive foreign investment company, which is known as a PFIC, for any taxable year if, for such year, either:
| ● | At least 75% of our gross income for the year is passive income; or |
| ● | The average percentage of our assets (determined at the end of each quarter) during the taxable year which produce passive income or which are held for the production of passive income is at least 50%. |
Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets.
If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. taxpayer who holds our Ordinary Shares, the U.S. taxpayer may be subject to increased U.S. federal income tax liability and may be subject to additional reporting requirements.
Depending on our assets held for the production of passive income, it is possible that, for our 2022 taxable year or for any subsequent year, more than 50% of our assets may be assets which produce passive income, in which case we would be deemed a PFIC, which could have adverse US federal income tax consequences for US taxpayers who are shareholders. We will make this determination following the end of any particular tax year.
38
Although the law in this regard is unclear, we treat our consolidated affiliated entities as being owned by us for United States federal income tax purposes, not only because we exercise certain level of control over the operation of such entities but also because we are entitled to substantially all of their economic benefits, and, as a result, we consolidate their operating results in our consolidated financial statements. For purposes of the PFIC analysis, in general, a non-U.S. corporation is deemed to own its pro rata share of the gross income and assets of any entity in which it is considered to own at least 25% of the equity by value. See “Item 10. Additional Information—E. Taxation—United States Federal Income Tax Considerations—PFIC.”
Risks Related to the WFOE and the VIE and its Subsidiaries’ Business
The WFOE and the VIE and its subsidiaries face significant competition in industries experiencing rapid technological change, and there is a possibility that their competitors may achieve regulatory approval and develop new product candidates before the WFOE and the VIE and its subsidiaries, which may harm our financial condition and the ability of the WFOE and the VIE and its subsidiaries to successfully market or commercialize any of their product candidates.
The pharmaceutical and chemical industries currently are characterized by rapidly changing technologies, significant competition and a strong emphasis on intellectual property. The WFOE and the VIE and its subsidiaries will face competition with respect to their current and future pharmaceutical and fertilizer product candidates from major pharmaceutical and chemical companies in China. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization of pharmaceutical and fertilizer products. For example, competition for improving oxytetracycline strains comes from conventional and advanced breeding techniques. Other potentially competitive sources of improvement in oxytetracycline yields include improvements in specific biotechnology areas and information management.
The WFOE and the VIE and its subsidiaries have competitors in China that manufacture products similar to theirs. These companies sell similar products to ours and some of them may have more assets, resources and a larger market share. We believe the WFOE and the VIE and its subsidiaries are able to compete with these competitors because of their geographical location in Western China, unique combination of products and lower prices of products.
Some of the current or potential competitors of the WFOE and the VIE and its subsidiaries may have significantly greater financial resources and expertise in research and development, manufacturing, product testing, obtaining regulatory approvals and marketing approved products than they do. Mergers and acquisitions in the pharmaceutical, chemical and agricultural industries may result in even more resources being concentrated among a smaller number of competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with the WFOE and the VIE and its subsidiaries in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to, or necessary for, the research and development (“R&D”) projects of the WFOE and the VIE and its subsidiaries. Business opportunities of the WFOE and the VIE and its subsidiaries could be reduced or eliminated if their competitors develop and commercialize products that are more effective, more convenient or are less expensive than any products the WFOE and the VIE and its subsidiaries develop alone or with collaborators or that would render any such products obsolete or non-competitive. The competitors of the WFOE and the VIE and its subsidiaries also may obtain regulatory approval for their products more rapidly than the WFOE and the VIE and its subsidiaries may obtain approval for any that they develop, which could result in the competitors of the WFOE and the VIE and its subsidiaries establishing a strong market position before any new products of the WFOE and the VIE and its subsidiaries are able to enter the market. Additionally, technologies developed by the competitors of the WFOE and the VIE and its subsidiaries may render the product candidates of the WFOE and the VIE and its subsidiaries uneconomical or obsolete, and the WFOE and the VIE and its subsidiaries or their collaborators may not be successful in marketing any product candidates they may develop against competitors. The availability of the competitors’ products could limit the demand, and the price the WFOE and the VIE and its subsidiaries are able to charge, for any products that they develop alone or with collaborators.
39
The pharmaceutical business of the WFOE and the VIE and its subsidiaries is subject to inherent risks relating to product liability and personal injury claims.
We, the VIE, and the VIE’s subsidiaries, as a pharmaceutical group, are exposed to risks inherent in the manufacturing and distribution of pharmaceutical products, such as with respect to improper filling of prescriptions, labeling of prescriptions, adequacy of warnings, and unintentional distribution of counterfeit drugs. In addition, product liability claims may be asserted against us, the VIE, or the VIE’s subsidiaries with respect to any of the products the WFOE and the VIE and its subsidiaries sell and as a distributor, and we, the VIE, and the VIE’s subsidiaries will be required to pay for damages for any successful product liability claim against them, although we, the VIE, and the VIE’s subsidiaries may have the right under applicable PRC laws, rules and regulations to recover from the relevant manufacturer or distributors for compensation they paid to their customers in connection with a product liability claim. The WFOE and the VIE and its subsidiaries may also be obligated to recall affected products. If we, the VIE, or the VIE’s subsidiaries are found liable for product liability claims, they could be required to pay substantial monetary damages. Furthermore, even if we, the VIE, or its subsidiaries successfully defend themselves against this type of claim, they could be required to spend significant management, financial and other resources, which could disrupt their business, their reputation and their brand name. The WFOE and the VIE and its subsidiaries, like many other similar companies in China, do not carry product liability insurance. As a result, any imposition of product liability could materially harm the business, financial condition and results of operations of our Company, the VIE, and the VIE’s subsidiaries. In addition, the WFOE and the VIE and its subsidiaries do not have any business interruption insurance due to the limited coverage of any available business interruption insurance in China, and as a result, any business disruption or natural disaster could severely disrupt the business operations of the WFOE and the VIE and its subsidiaries, and significantly decrease our revenue and profitability.
We and our affiliated entities have limited sources of working capital and will need substantial additional financing.
The working capital required to implement the business plan and build new facilities to expand the production capacity of the WFOE and the VIE and its subsidiaries will most likely be provided by funds obtained through offerings of our equity, debt, debt-linked securities, and/or equity-linked securities, and revenues generated by us. No assurance can be given that we and our affiliated entities will have revenues sufficient to sustain the operations of the WFOE and the VIE and its subsidiaries or that we would be able to obtain equity/debt financing in the current economic environment. If we do not have sufficient working capital and the WFOE and the VIE and its subsidiaries are unable to generate sufficient revenues or raise additional funds, the WFOE and the VIE and its subsidiaries may delay the completion of or significantly reduce the scope of their current business plan; delay some of their development and clinical or marketing efforts; postpone the hiring of new personnel; or, under certain dire financial circumstances, substantially curtail or cease their operations.
The WFOE and the VIE and its subsidiaries need sufficient financing to implement their business plan, which includes expanding the marketing efforts for Gan Di Xin® and increasing the manufacturing capacities for their oxytetracycline products, fertilizer products and Heparin Sodium Preparations. The WFOE and the VIE and its subsidiaries will also need sufficient financing to materialize their future plan of acquiring traditional Chinese medicine enterprises. We estimate that carrying out these business projects will require at least $21.5 million in the next 3 years. Our inability to obtain sufficient additional financing would have a material adverse effect on the ability of the WFOE and the VIE and its subsidiaries to implement their business plans. As of September 30, 2024, we had cash and cash equivalents of approximately $9.8 million, total current assets of $29.8 million, and total current liabilities of $8.8 million. We will need to engage in capital-raising transactions in the near future. Such financing transactions may well cause substantial dilution to our shareholders and could involve the issuance of securities with rights senior to the outstanding shares. Our ability to complete additional financings depends on, among other things, the state of the capital markets at the time of any proposed offering, market reception of the Company and the likelihood of the success of its business model and offering terms. There is no assurance that we will be able to obtain any such additional capital through asset sales, equity or debt financing, or any combination thereof, on satisfactory terms or at all. Additionally, no assurance can be given that any such financing, if obtained, will be adequate to meet our capital needs and to support the WFOE and the VIE and its subsidiaries’ operations. If we do not obtain adequate capital on a timely basis and on satisfactory terms, our revenues and operations and the value of our Ordinary Shares and Ordinary Share equivalents would be materially negatively impacted and the WFOE and the VIE and its subsidiaries’ operations may cease.
40
We, the VIE, and the VIE’s subsidiaries depend on certain key personnel, and loss of these key personnel could have a material adverse effect on the WFOE and the VIE and its subsidiaries’ business, financial condition and results of operations.
The success of our Company, the VIE, and the VIE’s subsidiaries is, to a certain extent, attributable to the management, sales and marketing, and research and development expertise of key personnel. We depend upon the services of Mr. Zhanchang Xin, chairman of the board of directors, for the continued growth and operation of our Company, due to his industry experience, technical expertise, as well as his personal and business contacts in the PRC. We may not be able to retain Mr. Zhanchang Xin for any given period of time. Although we have no reason to believe that Mr. Zhanchang Xin will discontinue his services with us or the VIE, the interruption or loss of his services would adversely affect the ability of us, the WFOE, the VIE, and the VIE’s subsidiaries to effectively run their business and pursue their business strategy as well as our results of operations. We, the VIE and the VIE’s subsidiaries do not carry key man life insurance for any of the key personnel, nor do we foresee purchasing such insurance to protect against the loss of key personnel.
BGM and its affiliated entities’ may not be able to hire and retain qualified personnel to support their growth and if BGM and its affiliated entities’ are unable to retain or hire these personnel in the future, their ability to improve their products and implement their business objectives could be adversely affected.
BGM and its affiliated entities must attract, recruit and retain a sizeable workforce of technically competent employees. Competition for senior management and personnel in the PRC is intense and the pool of qualified candidates in the PRC is limited. BGM and its affiliated entities’ may not be able to retain the services of their senior executives or personnel, or attract and retain high-quality senior executives or personnel in the future. This failure could materially and adversely affect BGM and its affiliated entities’ future growth and financial condition.
A significant portion of our revenue is concentrated in a few large customers, and the WFOE, the VIE and its subsidiaries do not have long-term agreements with their key customers and rely upon their longstanding relationship with these customers. If the WFOE and the VIE and its subsidiaries lose one or more of their customers, BGM and its affiliated entities’ results of operations may be adversely and materially impacted.
The customers of the WFOE and the VIE and its subsidiaries consist of qualified distributors, dealers and corporate customers. The WFOE and the VIE and its subsidiaries have several large customers with whom we generated substantial revenue each year, and the composition of largest customers has changed from year to year. For the fiscal year ended September 30, 2024, two customers represented approximately 16% and 12% of the sales of the WFOE and the VIE and its subsidiaries, respectively. For the fiscal year ended September 30, 2023, two customers represented approximately 15% and 14% of the sales of the WFOE and the VIE and its subsidiaries, respectively. For the fiscal year ended September 30, 2022, two customers represented approximately 11% and 11% of the sales of the WFOE and the VIE and its subsidiaries, respectively. Since the WFOE and the VIE and its subsidiaries do not have long-term customer supply agreements with such large customers and rely primarily upon their goodwill and reputation to sustain the business relationship, BGM and its affiliated entities’ results of operations may be adversely and materially impacted if one or more of these customers stop purchasing from the VIE or its subsidiaries.
The WFOE and the VIE and its subsidiaries source raw materials used for manufacturing from a limited number of suppliers. If the VIE and its subsidiaries lose one or more of the suppliers, their operation may be disrupted, and BGM and its affiliated entities’ results of operations may be adversely and materially impacted.
For the fiscal year ended September 30, 2024, two of the suppliers of the WFOE and the VIE and its subsidiaries accounted for 12% and 10% of the total purchases. For the fiscal year ended September 30, 2023, one vendor of the WFOE and the VIE and its subsidiaries accounted for 11% of the total purchases. For the fiscal year ended September 30, 2022, one vendor accounted for 14% of total purchase. If the WFOE and the VIE and its subsidiaries lose suppliers and are unable to swiftly engage new suppliers, their operations may be disrupted or suspended, and they may not be able to deliver hardware products to their customers on time. The WFOE and the VIE and its subsidiaries may also have to pay a higher price to source from a different supplier on short notice. While the WFOE and the VIE and its subsidiaries are actively searching for and negotiating with new suppliers, there is no guarantee that they will be able to locate appropriate new suppliers or supplier merger targets in their desired timeline. As such, BGM and its affiliated entities’ results of operations may be adversely and materially impacted.
41
If the WFOE and the VIE and its subsidiaries fail to increase their brand name recognition, they may face difficulty in obtaining new customers.
Although the brand name of the WFOE and the VIE and its subsidiaries is well-respected in the Chinese pharmaceutical and chemical industry, they still believe that maintaining and enhancing the brand name recognition in a cost-effective manner is critical to achieving widespread acceptance of the current and future products and services of the WFOE and the VIE and its subsidiaries, and is an important element in the effort of the WFOE and the VIE and its subsidiaries to increase their customer base. Successful promotion of the brand name of the WFOE and the VIE and its subsidiaries will depend largely on their marketing efforts and ability to provide reliable and quality products at competitive prices. Brand promotion activities may not necessarily yield increased revenue, and even if they do, any increased revenue may not offset the expenses the WFOE and the VIE and its subsidiaries will incur in marketing activities. If the WFOE and the VIE and its subsidiaries fail to successfully promote and maintain their brand, or if they incur substantial expenses in an unsuccessful attempt to promote and maintain their brand, the VIE and its subsidiaries may fail to attract new customers or retain their existing customers, in which case BGM and its affiliated entities’ business, operating results and financial condition, would be materially adversely affected.
Any disruption in the supply chain of raw materials and the products of the WFOE and the VIE and its subsidiaries could adversely impact their ability to produce and deliver products.
Some products manufactured by the WFOE and the VIE and its subsidiaries are resource-based products. Thus, the WFOE and the VIE and its subsidiaries must manage their supply chain for raw materials and delivery of their products competently. Even though Chengdu QLS enjoys considerable advantages resulting from its access to high quality, low cost, and abundant local resources, supply chain fragmentation and local protectionism within China may cause disruption risks for the WFOE, the VIE and its other subsidiaries. Local administrative bodies and physical infrastructure built to protect local interests pose transportation challenges for raw material transportation, as well as product delivery throughout China. In addition, profitability and volume could be negatively impacted by limitations inherent within the supply chain, including competitive, governmental, legal, natural disasters, and other events that could affect both supply and price. Any of these occurrences could cause significant disruptions to the supply chain of the WFOE and the VIE and its subsidiaries, manufacturing capability and distribution system that could adversely affect the ability of the WFOE and the VIE and its subsidiaries to produce and deliver some of their products.
Additionally, some of the raw materials the WFOE and the VIE and its subsidiaries use are procured from farmers, who are usually subject to environmental risks outside of their control. Thus, these farmers may not have the ability to supply continuously and stably if environmental and climate changes adversely affect their business.
The success of the WFOE and the VIE and its subsidiaries depends on their ability to protect their intellectual property.
The success of the WFOE and the VIE and its subsidiaries depends on their ability to obtain and maintain patent protection for products developed utilizing their technologies, in the PRC and in other countries, and to enforce these patents. There is no assurance that any of the existing and future patents of the WFOE and the VIE and its subsidiaries will be held valid and enforceable against third-party infringement, or that the products of the WFOE and the VIE and its subsidiaries will not infringe any third-party patent or intellectual property. Although the WFOE and the VIE and its subsidiaries own 31 valid patents and have filed two additional patent applications with the Patent Administration Department of the PRC, there is no assurance that they will be granted.
Any patents relating to the technologies of the WFOE and the VIE and its subsidiaries may not be sufficiently broad to protect their products. In addition, the patents of the WFOE and the VIE and its subsidiaries may be challenged, potentially invalidated or potentially circumvented. The patents of the WFOE and the VIE and its subsidiaries may not afford them protection against competitors with similar technology or permit the commercialization of their products without infringing third-party patents or other intellectual property rights.
The WFOE and the VIE and its subsidiaries also rely on or intend to rely on their trademarks, trade names and brand names to distinguish their products from the products of their competitors, and have registered or will apply to register a number of these trademarks. However, third parties may oppose our trademark applications or otherwise challenge our use of the trademarks. In the event that the trademarks of the WFOE and the VIE or its subsidiaries are successfully challenged, the WFOE and the VIE and its subsidiaries could be forced to rebrand their products, which could result in loss of brand recognition and could require them to devote resources to advertising and marketing these new brands. Further, the competitors of the WFOE and the VIE and its subsidiaries may infringe their trademarks, or they may not have adequate resources to enforce their trademarks.
42
In addition, the WFOE and the VIE and its subsidiaries also have trade secrets, non-patented proprietary expertise and continuing technological innovation that they shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, the trade secrets and proprietary technology of the WFOE and the VIE and its subsidiaries may otherwise become known or be independently developed by their competitors. If patents are not issued with respect to products arising from research, the WFOE and the VIE and its subsidiaries may not be able to maintain the confidentiality of information relating to these products.
Implementation and enforcement of PRC laws relating to intellectual property have historically been deficient and ineffective. Accordingly, protection of intellectual property rights in China may not be as effective as in the United States or other developed countries. Furthermore, policing unauthorized use of proprietary technology is difficult and expensive. The WFOE and the VIE and its subsidiaries rely on a combination of patent, copyright, trademark, and trade secret laws and restrictions on disclosure to protect their intellectual property rights. Despite the efforts of the WFOE and the VIE and its subsidiaries to protect their proprietary rights, third parties may attempt to copy or otherwise obtain and use such intellectual property or seek court declarations that they do not infringe upon the intellectual property rights of the WFOE and the VIE and its subsidiaries. Monitoring unauthorized use of the intellectual property of the WFOE and the VIE and its subsidiaries is difficult and costly, and we cannot assure you that the steps the WFOE and the VIE and its subsidiaries have taken or will take will prevent misappropriation of their intellectual property. From time to time, the WFOE and the VIE and its subsidiaries may have to resort to litigation to enforce their intellectual property rights, which could result in substantial costs and diversion of their resources and a favorable outcome, in such event, is not assured.
The WFOE and the VIE and its subsidiaries face risks related to research and the ability to develop new pharmaceutical and chemical products.
Our growth and survival depend on the ability of the WFOE and the VIE and its subsidiaries to consistently discover, develop and commercialize new products and find new and improved technology. As such, if the WFOE and the VIE and its subsidiaries fail to make sufficient investments in research, be attentive to unmet consumer needs or focus on advancing pharmaceutical and chemical product technology, their current and future products could be surpassed by more effective or advanced products of other companies.
The business operations of the WFOE and the VIE and its subsidiaries require a number of permits and licenses. We cannot assure you that the VIE and its subsidiaries can maintain all required licenses, permits and certifications to carry on their business at all times.
Pharmaceutical companies in China are required to obtain certain permits and licenses from various PRC governmental authorities, including Pharmaceutical Product Permits.
The VIE and its subsidiaries have obtained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in the PRC. The latest amended Drug Administration Law took effect on December 1, 2019 and has vacated the GMP certificate requirements for pharmaceutical companies. As such, the WFOE and the VIE and its subsidiaries do not need to renew their current GMP certificates. However, we cannot assure you that the WFOE and the VIE and its subsidiaries can maintain all the other required licenses, permits and certifications to carry on their business at all times. Moreover, these licenses, permits and certifications are subject to periodic renewal and/or reassessment by the relevant PRC governmental authorities and the standards of such renewal or reassessment may change from time to time. The WFOE and the VIE and its subsidiaries intend to apply for the renewal of these licenses, permits and certifications when required by then applicable laws and regulations. Any failure by the WFOE or the VIE or the VIE’s subsidiaries to obtain and maintain all licenses, permits and certifications necessary to carry on their business at any time could have a material adverse effect on the WFOE and the VIE and its subsidiaries’ business, financial condition and results of operations. In addition, any inability to renew these licenses, permits and certifications could severely disrupt the business of the WFOE and the VIE and its subsidiaries, and prevent them from continuing to carry on their business. Any changes in the standards used by governmental authorities in considering whether to renew or reassess the business licenses, permits and certifications of the WFOE and the VIE and its subsidiaries, as well as any enactment of new regulations that may restrict the conduct of their business, may also decrease our revenue and/or increase our costs and materially reduce our profitability and prospects. Furthermore, if the interpretation or implementation of existing laws and regulations changes or if new regulations come into effect requiring us or our affiliated entities to obtain any additional licenses, permits or certifications that were previously not required for the WFOE and the VIE and its subsidiaries to operate their existing businesses, we cannot assure you that the WFOE and the VIE and its subsidiaries will successfully obtain such licenses, permits or certifications.
43
Gan Di Xin®, exclusively produced by Gansu QLS, is subject to continuing regulation by the National Medical Products Administration (the “NMPA”) in China. The innovative product, Ahan® Antibacterial Paste, is subject to continuing regulation by the National Health and Family Planning Commission. If the labeling or manufacturing process of an approved pharmaceutical product is significantly modified, the NMPA may require that the WFOE and the VIE and its subsidiaries obtain a new pre-market approval.
Adverse publicity associated with the products of the WFOE and the VIE and its subsidiaries, ingredients or network marketing program, or those of similar companies, could harm our financial condition and operating results.
The results of the WFOE and the VIE and its subsidiaries’ operations may be significantly affected by the public’s perception of the WFOE and the products of the WFOE and the VIE and its subsidiaries and similar companies. This perception depends upon opinions concerning:
| ● | the safety and quality of the products and product ingredients of the WFOE and the VIE and its subsidiaries; |
| ● | the safety and quality of similar products and ingredients distributed by other companies; and |
| ● | the downstream distributors and sales forces of the WFOE and the VIE and its subsidiaries. |
Adverse publicity concerning any actual or purported failure to comply with applicable laws and regulations regarding product claims and advertising, good manufacturing practices, or other aspects of the business of the WFOE and the VIE and its subsidiaries, whether or not resulting in enforcement actions or the imposition of penalties, could have an adverse effect on the goodwill of the WFOE and the VIE and its subsidiaries, and could negatively affect their sales and ability to generate revenue. In addition, the consumers’ perception of the safety and quality of products and ingredients of the WFOE and the VIE and its subsidiaries, as well as similar products and ingredients distributed by other companies, can be significantly influenced by media attention, publicized scientific research or findings, widespread product liability claims and other publicity concerning the products or ingredients of the WFOE and the VIE and its subsidiaries or similar products and ingredients distributed by other companies. Adverse publicity, whether or not accurate or resulting from consumers’ use or misuse of the products, that associates consumption of the products or product ingredients of the WFOE and the VIE and its subsidiaries or any similar products or ingredients with illness or other adverse effects, questions the benefits of their or similar products or claims that any such products are ineffective, inappropriately labeled or have inaccurate instructions as to the products’ use, could negatively impact the reputation of the WFOE and the VIE and its subsidiaries or the market demand for their products.
The WFOE and the VIE and its subsidiaries face risks related to natural disasters, extreme weather conditions, health epidemics and other catastrophic incidents, which could significantly disrupt their operations and negatively affect our results of operations and financial condition.
In the past, China has experienced significant natural disasters, including earthquakes, extreme weather conditions, as well as health scares related to epidemics, and any similar event could materially impact the business of the WFOE and the VIE and its subsidiaries in the future. If a disaster or other disruption were to occur in the future that affects the regions where the WFOE and the VIE and its subsidiaries operate their business, the business operations of the WFOE and the VIE and its subsidiaries could be materially and adversely affected due to loss of personnel, damages to their manufacturing facilities and volatile Chinese markets. Even if the WFOE and the VIE and its subsidiaries are not directly affected, such a disaster or disruption could affect the operations or financial condition of the ecosystem participants such as suppliers and distributors, which could harm our results of operations.
In general, the business of the WFOE and the VIE and its subsidiaries could be affected by public health epidemics. If any of the employees or staff members of the WFOE and the VIE and its subsidiaries who operates manufacturing facilities or conduct R&D activities is suspected of having contracted a contagious disease, the WFOE and the VIE and its subsidiaries may be required to apply quarantines to their facilities or suspend manufacturing operations entirely. Furthermore, any future outbreak may restrict economic activities in affected regions and beyond, resulting in reduced business volume, temporary closure of factories or other disruptions of the business operations of the WFOE and the VIE and its subsidiaries, and adversely affect BGM and its affiliated entities’ results of operations.
44
For the fiscal years ended September 30, 2023 and 2022, the COVID-19 pandemic negatively impacted the WFOE and the VIE and its subsidiaries’ business operations. Specifically, the WFOE and the VIE and its subsidiaries’ production costs materially increased due to an increase in raw material prices. In addition, the market demand for some of the products of the WFOE and the VIE and VIE’s subsidiaries decreased, resulting in a decrease in market prices of these products. These two factors combined led to a decrease in our profit margin and a decrease in our net income for the fiscal years ended September 30, 2023 and 2022.
Risks Related to Our Ordinary Shares
The trading price of our Ordinary Shares is likely to be volatile, which could result in substantial losses to our investors.
The trading price of our Ordinary Shares has been volatile and is likely to continue to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors, including the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the United States. The securities of some of these companies have experienced significant volatility since their initial public offerings, including, in some cases, substantial price declines in their trading prices. The trading performances of other Chinese companies’ securities after their offerings may affect the attitudes of investors toward Chinese companies listed in the United States in general and consequently may impact the trading performance of our Ordinary Shares, regardless of our actual operating performance.
In addition to market and industry factors, the price and trading volume for our Ordinary Shares may be highly volatile for factors specific to our own operations, including the following:
| ● | our operating and financial performance; |
| ● | quarterly variations in the rate of growth of our financial indicators, such as net income per share, net income and revenues; |
| ● | the public reaction to our press releases, our other public announcements and our filings with the SEC; |
| ● | strategic actions by our competitors; |
| ● | changes in revenue or earnings estimates, or changes in recommendations or withdrawal of research coverage, by equity research analysts; |
| ● | speculation in the press or investment community; |
| ● | the failure of research analysts to cover our Ordinary Shares; |
| ● | sales of our Ordinary Shares by us or other shareholders, or the perception that such sales may occur; |
| ● | changes in accounting principles, policies, guidance, interpretations or standards; |
| ● | additions or departures of key management personnel; |
| ● | actions by our shareholders; |
| ● | domestic and international economic, legal and regulatory factors unrelated to our performance; and |
| ● | the realization of any risks described under this “Risk Factors” section. |
Any of these factors may result in large and sudden changes in the volume and price at which our Ordinary Shares will trade.
45
In the past, shareholders of public companies have often brought securities class action suits against those companies following periods of instability in the market price of their securities. If we were involved in a class action suit, it could divert a significant amount of our management’s attention and other resources from the WFOE and the VIE and its subsidiaries’ business and operations and require us to incur significant expenses to defend the suit, which could harm our results of operations. Any such class action suit, whether or not successful, could harm our reputation and restrict our ability to raise capital in the future. In addition, if a claim is successfully made against us, we may be required to pay significant damages, which could have a material adverse effect on our financial condition and results of operations.
Since our directors and executive officers hold approximately 23.7% of our Ordinary Shares, representing approximately 96.4% of the aggregate voting power, as of the date of this annual report, they have the ability to elect directors and approve matters requiring shareholder approval by way of resolution of members.
Mr. Zhanchang Xin, our chairman of the board of directors, is currently the beneficial owner of 2,767,800 Class A ordinary shares and 10,200,000 Class B ordinary shares, or approximately 13.3% of our outstanding Ordinary Shares, representing approximately 49.2% of the aggregate voting power, of which approximately 10.9% are directly held by Ahanzhai Development Limited, an entity 100% owned by Mr. Xin. Ms. Furong Cao, our director, is currently the beneficial owner of 9,800,000 Class B ordinary shares, or approximately 10.1% of our outstanding Ordinary Shares through LX Management Company Limited, an entity 100% owned by Ms. Cao, representing approximately 47.2% of the aggregate voting power of our total issued and outstanding shares. They have the power to elect all directors and approve all matters requiring shareholder approval without the votes of any other shareholder. They are expected to have significant influence over a decision to enter into any corporate transaction and have the ability to prevent any transaction that requires the approval of shareholders, regardless of whether or not our other shareholders believe that such transaction is in our best interests. Such concentration of voting power could have the effect of delaying, deterring, or preventing a change of control or other business combination, which could, in turn, have an adverse effect on the market price of our Ordinary Shares, or prevent our shareholders from realizing a premium over the then-prevailing market price for their Ordinary Shares.
For as long as we are an emerging growth company, we will not be required to comply with certain reporting requirements, including those relating to accounting standards and disclosure about our executive compensation, that apply to other public companies.
In April 2012, President Obama signed into law the JOBS Act. We are classified as an “emerging growth company” under the JOBS Act. For as long as we are an emerging growth company, which may be up to five full fiscal years, unlike other public companies, we will not be required to, among other things, (i) provide an auditor’s attestation report on management’s assessment of the effectiveness of our system of internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act, (ii) comply with any new requirements adopted by the PCAOB requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer, (iii) provide certain disclosure regarding executive compensation required of larger public companies or (iv) hold nonbinding advisory votes on executive compensation. We will remain an emerging growth company for up to five years, although we will lose that status sooner if we have more than $1.235 billion of revenues in a fiscal year, have more than $700 million in market value of our Ordinary Shares held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period.
To the extent that we rely on any of the exemptions available to emerging growth companies, you will receive less information about our executive compensation and internal control over financial reporting than issuers that are not emerging growth companies. If some investors find our Ordinary Shares to be less attractive as a result, there may be a less active trading market for our Ordinary Shares and our stock price may be more volatile.
46
If we fail to establish and maintain proper internal financial reporting controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
Pursuant to Section 404 of the Sarbanes-Oxley Act, we are required to file a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. The presence of material weaknesses in internal control over financial reporting could result in financial statement errors which, in turn, could lead to errors in our financial reports and/or delays in our financial reporting, which could require us to restate our operating results. We might not identify one or more material weaknesses in our internal controls in connection with evaluating our compliance with Section 404 of the Sarbanes-Oxley Act. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal controls over financial reporting, we need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal control.
If we are unable to conclude that we have effective internal controls over financial reporting, investors may lose confidence in our operating results, the price of the Ordinary Shares could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, the Ordinary Shares may not be able to remain listed on Nasdaq Global Market.
As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders.
Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
| ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q or current reports on Form 8-K; |
| ● | the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; |
| ● | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; |
| ● | the selective disclosure rules by issuers of material nonpublic information under Regulation FD; and |
| ● | certain audit committee independence requirements in Rule 10A-3 of the Exchange Act. |
We are required to file an annual report on Form 20-F within four months of the end of each fiscal year. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer.
47
Because we are a foreign private issuer and are exempt from certain Nasdaq corporate governance standards applicable to U.S. issuers, you will have less protection than you would have if we were a domestic issuer.
The Nasdaq Listing Rules requires listed companies to have, among other things, a majority of its board members be independent. As a foreign private issuer, however, we are permitted to follow home country practice in lieu of the above requirements, or we may choose to comply with the above requirement within one year of listing. The corporate governance practice in our home country, the Cayman Islands, does not require a majority of our board to consist of independent directors. Thus, although a director must act in the best interests of the Company, it is possible that fewer board members will be exercising independent judgment and the level of board oversight on the management of our company may decrease as a result. In addition, the Nasdaq Listing Rules also requires U.S. domestic issuers to have a compensation committee, a nominating/corporate governance committee composed entirely of independent directors, and an audit committee with a minimum of three members. We, as a foreign private issuer, may not be subject to all these requirements. The Nasdaq Listing Rules may require shareholder approval for certain corporate matters, such as requiring that shareholders be given the opportunity to vote on all equity compensation plans and material revisions to those plans, certain ordinary share issuances. We intend to follow home country practice in lieu of the requirements under the Nasdaq Listing Rules with respect to certain corporate governance standards which may afford less protection to investors.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination with respect to our status will be made on March 31, 2025. We would lose our foreign private issuer status if, for example, more than 50% of our Ordinary Shares are directly or indirectly held by residents of the U.S. and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms beginning on March 31, 2025, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq Listing Rules. As a U.S. listed public company that is not a foreign private issuer, we would incur significant additional legal, accounting and other expenses that we would not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange.
The requirements of being a public company may strain our resources and divert management’s attention.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of Nasdaq, and other applicable securities rules and regulations. Despite recent reforms made possible by the JOBS Act, compliance with these rules and regulations will nonetheless increase our legal, accounting, and financial compliance costs and investor relations and public relations costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.” The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our and the VIE and its subsidiaries’ business and operating results as well as proxy statements.
As a result of disclosure of information in this Form 20-F and in filings required of a public company, our and the VIE and its subsidiaries’ business and financial condition are more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our and the VIE and its subsidiaries’ business and operating results could be harmed, and even if the claims do not result in litigation or are resolved in BGM and its affiliated entities’ favor, these claims, and the time and resources necessary to resolve them, could divert the resources of BGM and its affiliated entities’ management and adversely affect BGM and its affiliated entities’ business, brand and reputation and results of operations.
Being a public company and being subject to these new rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members for our board of directors, particularly to serve on our audit committee and compensation committee, and to serve as qualified executive officers, generally.
48
We do not intend to pay dividends for the foreseeable future.
We currently intend to retain any future earnings to finance the operation and expansion of the WFOE and the VIE and its subsidiaries’ business. We do not expect to declare or pay any dividends in the foreseeable future. As a result, you may only receive a return on your investment in our Ordinary Shares if the market price of our Ordinary Shares increases.
A sale or perceived sale of a substantial number of our Ordinary Shares may cause the price of our Ordinary Shares to decline.
Sales of our Ordinary Shares in the public market, or the perception that these sales could occur, could cause the market price of our Ordinary Shares to decline. Our Ordinary Shares outstanding are also available for sale subject to volume and other restrictions as applicable under Rules 144 and 701 under the Securities Act. To the extent these shares are sold into the market, the market price of our Ordinary Shares could decline.
Certain holders of our Ordinary Shares may cause us to register under the Securities Act the sale of their shares. Registration of these shares under the Securities Act would result in Ordinary Shares representing these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration. Sales of these registered shares in the public market could cause the price of our Ordinary Shares to decline.
The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States. For instance, you may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts may be limited, because we are incorporated under Cayman Islands law.
Our corporate affairs are governed by our currently effective memorandum and articles of association, by the Companies Act (Revised) of the Cayman Islands and by the common law of the Cayman Islands. The rights of shareholders to take action against our directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law in the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from the common law of England, the decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedents in the United States. In particular, the Cayman Islands has a less developed body of securities laws relative to the United States. Some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law than the Cayman Islands. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States.
Shareholders of Cayman Islands exempted companies like us have no general rights under Cayman Islands law to inspect corporate records (other than copies of the memorandum and articles of association, the register of mortgages and charges, and any special resolutions passed by the shareholders) or to obtain copies of lists of shareholders of these companies. Our directors have discretion under our articles of association to determine whether or not, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
Certain corporate governance practices in the Cayman Islands, which is our home country, differ significantly from requirements for companies incorporated in other jurisdictions such as the United States. If we choose to follow home country practice in the future, our shareholders may be afforded less protection than they otherwise would under rules and regulations applicable to U.S. domestic issuers.
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a company incorporated in the United States.
The recent joint statement by the SEC and PCAOB, proposed rule changes submitted by Nasdaq, and the Holding Foreign Companies Accountable Act all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to BGM and its affiliated entities’ performance.
49
On April 21, 2020, SEC Chairman Jay Clayton and PCAOB Chairman William D. Duhnke III, along with other senior SEC staff, released a joint statement highlighting the risks associated with investing in companies based in or have substantial operations in emerging markets including China. The joint statement emphasized the risks associated with lack of access for the PCAOB to inspect auditors and audit work papers in China and higher risks of fraud in emerging markets.
On May 18, 2020, Nasdaq filed three proposals with the SEC to (i) apply minimum offering size requirement for companies primarily operating in “Restrictive Market”, (ii) adopt a new requirement relating to the qualification of management or board of director for Restrictive Market companies, and (iii) apply additional and more stringent criteria to an applicant or listed company based on the qualifications of the company’s auditors.
On May 20, 2020, the U.S. Senate passed the Holding Foreign Companies Accountable Act requiring a foreign company to certify it is not owned or controlled by a foreign government if the PCAOB is unable to audit specified reports because the company uses a foreign auditor not subject to PCAOB inspection. If the PCAOB is unable to inspect the company’s auditors for three consecutive years, the issuer’s securities are prohibited to trade on a national securities exchange or in the over the counter trading market in the U.S. On December 2, 2020, the U.S. House of Representatives approved the Holding Foreign Companies Accountable Act. On December 18, 2020, the Holding Foreign Companies Accountable Act was signed into law. In June 2021, the Senate passed the Accelerating Holding Foreign Companies Accountable Act, which, if signed into law, would reduce the time period for the delisting under the HFCA Act to two years, instead of three years.
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements in the HFCA Act. On December 2, 2021, the SEC adopted amendments to finalize rules implementing the submission and disclosure requirements in the HFCA Act. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate. We will be required to comply with these rules if the SEC identifies us as having a “non-inspection” year under a process to be subsequently established by the SEC. The final amendments require any identified registrant to submit documentation to the SEC establishing that the registrant is not owned or controlled by a government entity in the public accounting firm’s foreign jurisdiction, and also require, among other things, disclosure in the registrant’s annual report regarding the audit arrangements of, and government influence on, such registrants. Under the HFCA Act, our securities may be prohibited from trading on the Nasdaq or other U.S. stock exchanges if our auditor is not inspected by the PCAOB for three consecutive years, and this ultimately could result in our Ordinary Shares being delisted.
If any such policies or deliberations were to materialize, the resulting legislation, if it were to apply to us, would likely have a material adverse impact on our and the VIE and its subsidiaries’ business and the price of our ordinary shares. Should the PCAOB determine that it cannot inspect or fully investigate our auditor for three consecutive years, an exchange may determine to delist our securities.
On December 16, 2021, the PCAOB issued a report on its determinations that it was unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in Mainland China and in Hong Kong, because of positions taken by PRC authorities in those jurisdictions. The PCAOB made its determinations pursuant to PCAOB Rule 6100, which provides a framework for how the PCAOB fulfills its responsibilities under the HFCA Act. The report further listed in its Appendix A and Appendix B, Registered Public Accounting Firms Subject to the Mainland China Determination and Registered Public Accounting Firms Subject to the Hong Kong Determination, respectively. Our auditors, ZH CPA, LLC and Enrome LLP, as auditors of companies that are traded publicly in the United States and firms registered with the PCAOB, are subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess our auditors’ compliance with the applicable professional standards. Our auditors did not appear as part of the determination and were not listed under its appendix A or appendix B.
50
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
Our Holding Company Structure and Contractual Arrangements
BGM Group Ltd is not a Chinese operating company but a Cayman Islands holding company with its business operations conducted by Gansu Qilianshan Pharmaceutical Co., Ltd. (the “VIE”, “Gansu QLS”) and its subsidiaries established in the PRC. BGM Group Ltd (formerly known as Qilian International Holding Group Limited) is a Cayman Islands exempted company with limited liability incorporated on February 7, 2019. Qilian International (Hong Kong) Holdings Limited., which we refer to as “Qilian HK”, our wholly-owned subsidiary, was incorporated in Hong Kong on January 30, 2019. Qilian HK’s wholly owned subsidiary, Qilian International Trading (Chengdu) Co., Ltd., formerly known as Chengdu Qilian Trading Co., Ltd., which we refer to as “WFOE”, was organized pursuant to PRC laws on May 15, 2019. Gansu Qilianshan Pharmaceutical Co. Ltd., which we refer to as Gansu QLS, the VIE, was established in August 30, 2006, as a result of restructuring from Gansu State-operated Qilianshan Pharmaceutical Factory, which was incorporated in July 1969 in Jiuquan, Gansu Province, PRC pursuant to PRC laws. Gansu QLS’ shareholders include certain PRC residents and corporate entities controlled by PRC residents.
Pursuant to PRC laws, each entity formed under PRC law shall have certain business scope approved by the Administration of Industry and Commerce or its local counterpart. As such, WFOE’s business scope is to primarily engage in business development, technology service, technology consulting, intellectual property service and business management consulting. Since the sole business of WFOE is to provide Gansu QLS with technical support, consulting services and other management services relating to its day-to-day business operations and management in exchange for a consulting fee, which is at WFOE’s discretion and can be the net income of Gansu QLS, such business scope is necessary and appropriate under the PRC laws. Gansu QLS, on the other hand, has been granted a business scope different from WFOE to enable it to develop, manufacture, market and sell its products.
Since we intend to acquire upstream and downstream companies manufacturing traditional Chinese medicine pieces, in which foreign investors are prohibited from investing, our WFOE cannot hold equity of Gansu QLS. We control Gansu QLS through contractual arrangements. BGM is a holding company with no business operation other than holding the shares in Qilian HK and Qilian HK is a pass-through entity with no business operation. WFOE is exclusively engaged in the business of managing the operation of Gansu QLS and its subsidiaries.
Gansu QLS, the VIE, was established on August 30, 2006, by restructuring from Gansu State-operated Qilianshan Pharmaceutical Factory, which was incorporated in July 1969 in Jiuquan, Gansu Province, PRC pursuant to PRC laws.
On April 17, 2020, Rugao was incorporated under the laws of the People’s Republic of China. Rugao is the 100% owned subsidiary of Chengdu QLS. It was intended to be used as procurement and manufacturing assistance entity for Chengdu QLS and as a point of expansion for the VIE and its subsidiaries’ sausage casings business in Jiangsu Province.
51
On January 12, 2021, our Ordinary Shares commenced trading on the Nasdaq Global Market under the symbol “QLI.” We raised approximately US$23,865,085 in net proceeds from our initial public offering after deducting underwriting commissions and the offering expenses payable by us. As part of BGM Group Ltd’s (the “Company”) efforts to optimize its corporate structure, Qilian International Trading (Chengdu) Co., Ltd. (“Chengdu Trade”) and Gansu Qilianshan Pharmaceutical Co., Ltd. (“Gansu QLS”) executed certain exclusive service termination agreement (the “Service Termination Agreement”) to terminate certain contractual service arrangements between Chengdu Trade and Gansu QLS. As a result of the aforementioned termination, Chengdu Trade will no longer have contractual control over, nor receive the economic benefits of Gansu QLS. In connection with such termination, Hainan Trade, a wholly-owned subsidiary of Qilian International (Hong Kong) Holdings Limited, entered into a certain exclusive service agreement with Gansu QLS (the “Hainan Exclusive Service Agreement”), through which Hainan Trade obtained contractual control over Gansu QLS. The Service Termination Agreement became effective on December 1, 2022. The Hainan Exclusive Service Agreement was signed on December 1, 2022. Pursuant to the Hainan Exclusive Service Agreement between Gansu QLS and Hainan Trade, Hainan Trade provides Gansu QLS with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, business management and information. For services rendered to Gansu QLS by Hainan Trade under this agreement, Hainan Trade is entitled to collect a service fee that shall be equal to 99.214% of the net profits of Gansu QLS. The Hainan Exclusive Service Agreement shall remain in effect for ten years unless earlier terminated upon written confirmation from both Hainan Trade and Gansu QLS before expiration. Otherwise, this agreement shall be extended by another ten years automatically. The Hainan Exclusive Service Agreement does not prohibit related party transactions. Hainan Trade enjoys a favorable income tax rate and individual income tax rate for its employees of 15%. The Company expects change of the structure described above will save income tax expense and attracting talent in long term.
In the opinion of Gansu Quanyi Law Firm, the Company’s PRC legal counsel, the contractual arrangements between Gansu Qilianshan Pharmaceutical Co., Ltd. and Qilian Shan International Trade (Hainan) Co., Ltd are valid, binding and enforceable under current PRC law. However, these contractual arrangements may not be as effective in providing control as direct ownership. There are substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulation regarding such contractual arrangements and their effectiveness.
On August 11, 2023, Zhongqiao was established as a limited liability company organized under the laws of the PRC. Hainan Trade owns 51% equity interests of Zhongqiao. The remaining 49% equity interests are owned by Sichuan Shihua Investment Management Co., Ltd., a PRC company controlled by Yuchang Xin, the brother of Zhanchang Xin, our Chairman of the Board.
On November 27, 2023, we applied to transfer our Ordinary Shares to The Nasdaq Capital Market (the “Capital Market”), as allowed under the Nasdaq Listing Rules. On December 13, 2023, the transfer from The Nasdaq Global Market to the Capital Market was approved. Effective at the opening of business on December 15, 2023, our Ordinary Shares were transferred to the Capital Market and continued to trade under the symbol “QLI”.
Effective at the opening of business on August 11, 2024, the trading symbol of our Class A ordinary shares was changed to “BGM” on the Nasdaq Stock Market.
On October 18, 2024, shareholders approved the change of our company name to BGM Group Ltd at an extraordinary meeting of shareholders. Effective on October 30, 2024, we changed our name to “BGM Group Ltd.”
On November 27, 2024, BGM Group Ltd entered into a transaction agreement with CISG Holdings Ltd, Patriton Limited, a wholly owned subsidiary of CISG Holdings Ltd, and other related parties. Pursuant to the transaction agreement, the Company issued 69,995,661 Class A ordinary shares of par value of US$0.00833335 each to CISG Holdings Ltd, as consideration for 100% of the equity interest of Patriton Limited.
Our principal executive offices are located at No. 152 Hongliang East 1st Street, No. 1703, Tianfu New District, Chengdu, 610200 People’s Republic of China. The VIE and its subsidiaries’ telephone at this address is +86-0937-2689523. We maintain a corporate website at https://www.bgmgroupltd.com/. The information contained in our website is not a part of this annual report.
The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC using its EDGAR system.
See “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Capital Expenditures” for a discussion of our capital expenditures.
52
Our Corporate Structure
The following diagram illustrates our current corporate structure, which includes our significant subsidiaries as of the date of this annual report:
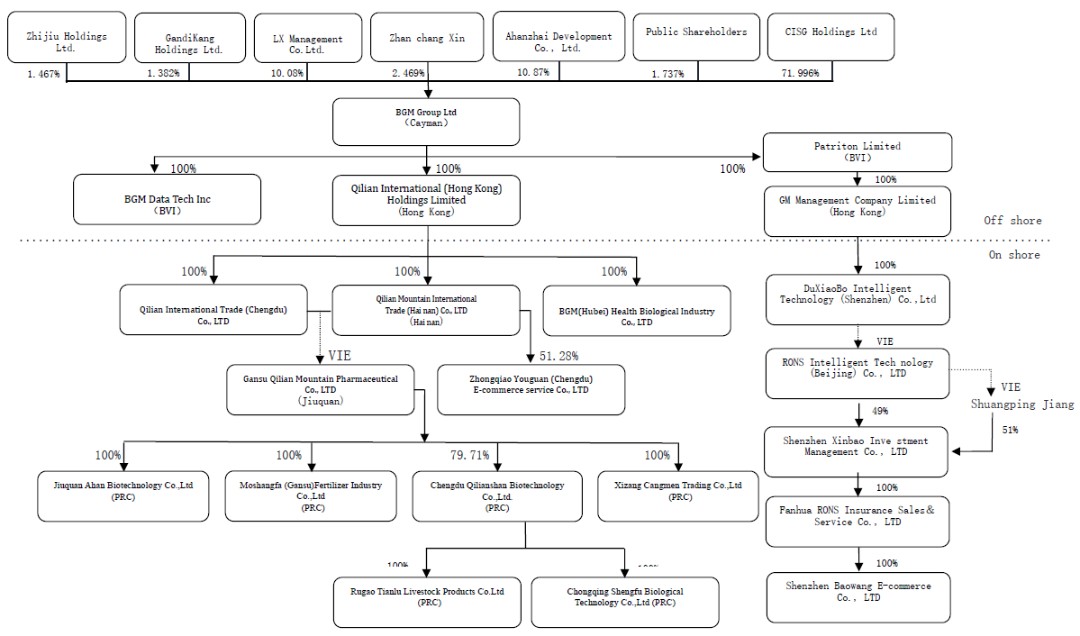
*48.718% equity interests of Zhongqiao Youguan (Chengdu) E-Commerce Service Co., Ltd. are owned by Sichuan Shihua Investment Management Co., Ltd., a PRC entity that is controlled by Yuchang Xin, the brother of Zhanchang Xin, our chairman of the Board.
20.29% equity interests of Chengdu QLS are collectively owned by 49 individual shareholders, none of whom is an affiliate of the Company.
53
The Company is incorporated in the Cayman Islands. As a holding company with no material operations, the Company conducts its operations in China through the variable interest entities, Gansu QLS and its subsidiaries. The Company receives the economic benefits of Gansu QLS and its subsidiaries’ business operation through a series of contractual arrangements, or the VIE Agreements. As a result of the VIE Agreements, we are the primary beneficiary of Gansu QLS for accounting purposes and treat it as a PRC consolidated entity under U.S. GAAP. We consolidate the financial results of Gansu QLS and its subsidiaries in our consolidated financial statements in accordance with U.S. GAAP. Neither we nor our investors own any equity ownership in, direct foreign investment in, or control through such ownership/investment of Gansu QLS. These VIE Agreements have not been tested in a court of law in the PRC. As a result, investors in our ordinary shares thus are not purchasing equity interest in our operating entities in China but instead are purchasing equity interest in a Cayman Islands holding company. As used in this annual report, (i) “Gansu QLS,” “variable interest entity” or “ VIE” refers to Gansu Qilianshan Pharmaceutical Co., Ltd., a company incorporated in the People’s Republic of China; (ii) “WFOE” or “Chengdu Trade” refers to Qilian International Trading (Chengdu) Co., Ltd., formerly known as Chengdu Qilian Trading Co., Ltd., a limited liability company organized under the laws of the PRC, which is wholly-owned by Qilian International (Hong Kong) Holdings Limited, a limited liability company organized under the laws of Hong Kong; and (iii) “BGM” or “the Company” refers to BGM Group Ltd, an exempted company with limited liability incorporated under the laws of the Cayman Islands.
Our corporate structure is subject to risks associated with our contractual arrangements with the VIE. The Company that investors will own may never have a direct ownership interest in the businesses that are conducted by the VIE. If the PRC government finds that the agreements that establish the structure for operating the VIE and its subsidiaries’ business in China do not comply with PRC laws and regulations, or if these regulations or the interpretation of existing regulations change or are interpreted differently in the future, we could be subject to severe penalties or be forced to relinquish our interests in the operations of the VIE. This would result in the VIE being deconsolidated. The majority of our assets, including the necessary licenses to conduct business in China, are held by the VIE and its subsidiaries. A significant part of our revenue is generated by the VIE. An event that results in the deconsolidation of the VIE would have a material effect on the VIE and its subsidiaries’ operations and result in the Ordinary Shares diminishing substantially in value or even becoming worthless. The Company, our Hong Kong entity, the VIE and its subsidiaries, and our investors face uncertainty about potential future actions by the PRC government that could affect the enforceability of the contractual arrangements with the VIE and, consequently, significantly affect the financial performance of the VIE and our company as a whole. For a detailed description of the risks associated with our corporate structure, please refer to risks disclosed under “Item 3. Key Information-D. Risk Factors-Risks Related to Our Corporate Structure” in this annual report on Form 20-F.
In addition, while we will take every precaution available to enforce the contractual and corporate relationship of the VIE agreements, these contractual arrangements are less effective than direct ownership and we may incur substantial costs to enforce the terms of the arrangements. For example, the VIE, its subsidiaries, and their shareholders could breach their contractual arrangements with us by, among other things, failing to conduct their operations in an acceptable manner or taking other actions that are detrimental to our interests. If we had direct ownership of the VIE and its subsidiaries (which we do not), we would be able to exercise our rights as a shareholder to effect changes in the board of directors of the VIE, which in turn could implement changes, subject to any applicable fiduciary obligations, at the management and operational level. However, under VIE Agreements, we will only rely on the performance by the VIE and its shareholders of their obligations under the contracts to direct the operation of the VIE and its subsidiaries. As such, the shareholders of VIE and its subsidiaries may not act in the best interests of our company or may not perform their obligations under these contracts. In addition, failure of the VIE shareholders to perform certain obligations could compel us to rely on legal remedies available under PRC laws, including seeking specific performance or injunctive relief, and claiming damages, which may not be effective. Further, it is uncertain whether any new PRC laws or regulations relating to variable interest entity structures will be adopted or if adopted, what they would provide. PRC regulatory authorities could disallow this structure, which would materially adversely affect the value of BGM’s Ordinary Shares, and could cause the value of such securities to significantly decline or become worthless. BGM faces numerous challenges in enforcing these contractual agreements due to uncertainties under Chinese law as well as jurisdictional limits. For a description of the risks related to these contractual arrangements and our corporate structure, see “Risk Factors - Risks Related to Our Corporate Structure.” For detailed descriptions of each of the VIE Agreement, please refer to disclosures under “Item 4. Information on the Company-A. History and Development of the Company- Our Holding Company Structure and Contractual Arrangements” in this annual report on Form 20-F.
54
Contractual Arrangements between WFOE and Gansu QLS
Due to PRC legal restrictions on foreign ownership in the pharmaceutical sector, neither we nor our subsidiaries own any equity interest in Gansu QLS. Instead, we only control (not as effective as direct ownership) and receive the economic benefits of Gansu QLS’s business operation through a series of contractual arrangements. WFOE, Gansu QLS and its shareholders entered into a series of contractual arrangements, also known as VIE Agreements, on May 20, 2019.
As a result of these contractual arrangements, we have the power to direct activities of the VIE that most significantly impact its economic performance. We are also entitled to receive substantially all of the economic benefits generated by the VIE as primary beneficiary and we bear the obligation to absorb any and all economic losses it incurs. In addition, we have an exclusive option to purchase all or part of the equity interests in the VIE when and to the extent permitted by PRC law. For the reasons above, we are able to consolidate the financial results of the VIE into our financial statements in accordance with U.S. GAAP.
Each of the VIE Agreements is described in detail below:
Exclusive Service Agreement
Pursuant to the original Exclusive Service Agreement between Gansu QLS and WFOE, WFOE provides Gansu QLS with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, business management and information. For services rendered to Gansu QLS by WFOE under this agreement, WFOE is entitled to collect a service fee that shall be equal to 99.214% of the net profits of Gansu QLS, with such percentage determined in accordance with “ARTICLE 3 - SERVICE FEES” of the Amended Exclusive Service Agreement executed on August 27, 2019, as amended on February 25, 2021. This percentage represents the number of shares of Gansu QLS held by shareholders having signed the VIE Agreements over the total number of issued and outstanding shares of Gansu QLS.
On December 1, 2022, Chengdu Trade and Gansu Qilianshan Pharmaceutical Co.,Ltd. executed certain exclusive service termination agreement (the “Service Termination Agreement”) to terminate the previously signed Exclusive Service Agreement, as amended on August 27, 2019. As a result of the aforementioned termination, Chengdu Trade will no longer have contractual control over, nor receive the economic benefits of Gansu QLS. In connection with such termination, Hainan Trade, a wholly-owned subsidiary of Qilian International (Hong Kong) Holdings Limited, entered into a certain exclusive service agreement with Gansu QLS (the “Hainan Exclusive Service Agreement”) on December 1, 2022, through which Hainan Trade obtained contractual control over Gansu QLS. Pursuant to the Hainan Exclusive Service Agreement, Hainan Trade provides Gansu QLS with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, business management and information. For services rendered to Gansu QLS by Hainan Trade under this agreement, Hainan Trade is entitled to collect a service fee that shall be equal to 99.214% of the net profits of Gansu QLS. The Hainan Exclusive Service Agreement shall remain in effect for ten years unless earlier terminated upon written confirmation from both Hainan Trade and Gansu QLS before expiration. Otherwise, this agreement shall be extended by another ten years automatically. The Hainan Exclusive Service Agreement does not prohibit related party transactions.
In the opinion of Gansu Quanyi Law Firm, the Company’s PRC legal counsel, the Hainan Exclusive Service Agreement is valid, binding and enforceable under current PRC law. However, such agreement may not be as effective in providing control as direct ownership. There are substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulation regarding such contractual arrangements and their effectiveness.
WFOE is currently managing Gansu QLS pursuant to the terms of the Exclusive Service Agreement. WFOE has absolute authority relating to the management of Gansu QLS, including but not limited to decisions with regard to expenses, salary raises and bonuses, hiring, firing and other operational functions. The Exclusive Service Agreement does not prohibit related party transactions. The audit committee of the registrant is required to review and approve in advance any related party transactions, including transactions involving WFOE or Gansu QLS.
55
Equity Pledge Agreement
Under the Equity Pledge Agreement between WFOE and certain shareholders of Gansu QLS together holding 76,196,640 shares, or 99.214% of the total issued and outstanding shares, of Gansu QLS (“Gansu QLS Shareholders”), the Gansu QLS Shareholders pledged all of their equity interests in Gansu QLS to WFOE to guarantee the performance of Gansu QLS’ obligations under the Exclusive Service Agreement. Under the terms of the Equity Pledge Agreement, in the event that Gansu QLS breaches its contractual obligations under the Exclusive Service Agreement, WFOE, as pledgee, will be entitled to certain rights, including, but not limited to, the right to collect dividends generated by the pledged equity interests. The Gansu QLS Shareholders also agreed that upon occurrence of any event of default, as set forth in the Equity Pledge Agreement, WFOE is entitled to dispose of the pledged equity interest in accordance with applicable PRC laws. The Gansu QLS Shareholders further agree not to dispose of the pledged equity interests or take any actions that would prejudice WFOE’s interest.
The Equity Pledge Agreement shall be effective until the latest date of the following: (1) the secured debt in the scope of pledge is satisfied (or otherwise discharged); (2) WFOE exercises its pledge rights pursuant to provisions and conditions of the Equity Pledge Agreement; and (3) the Gansu QL Shareholders transfer all the pledged equity interests to WFOE according to the Call Option Agreement, or other entity or individual designated by it.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of Gansu QLS’s obligations under the Exclusive Service Agreement, (2) ensure the Gansu QLS Shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice WFOE’s interests without WFOE’s prior written consent and (3) provide WFOE control over Gansu QLS. Under the Call Option Agreement, WFOE may be able to acquire the equity interests or the assets in Gansu QLS any time to the extent permitted by the PRC Law. In the event Gansu QLS breaches its contractual obligations under the Exclusive Service Agreement, WFOE will be entitled to foreclose on the Gansu QLS Shareholders’ equity interests in Gansu QLS and may (1) exercise its option to purchase or designate third parties to purchase part or all of their equity interests or the assets in Gansu QLS and in this situation, WFOE may terminate the Exclusive Service Agreement, Equity Pledge Agreement and Call Option Agreement after acquisition of all equity interests or assets in Gansu QLS or form new VIE structure with the third parties designated by WFOE; or (2) dispose of the pledged equity interests or assets and be paid in priority out of proceed from the disposal in which case the VIE structure will be terminated.
Call Option Agreement
Under the Call Option Agreement, the Gansu QLS Shareholders irrevocably granted WFOE (or its designee) an exclusive right to purchase, to the extent permitted under PRC law, once or at multiple times, at any time, a portion or whole of the equity interests or assets in Gansu QLS held by the Gansu QLS Shareholders. The purchase price should be no more than $1.00 subject to any appraisal or restrictions required by applicable PRC laws and regulations.
The agreement remains effective until all the transferred equity or transferred asset of Gansu QLS is legally transferred under the name of WFOE and/or other entity or individual designated by it.
Shareholders’ Voting Rights Proxy Agreement and Powers of Attorney
Under the Shareholders’ Voting Rights Proxy Agreement and each Power of Attorney, each Gansu QLS Shareholder authorizes WFOE to act on their behalf as their exclusive agent and attorney with respect to all rights as shareholders, including but not limited to: (a) the attendance of the shareholder’s meeting and the execution of relative Shareholder Resolution(s) of Gansu QLS; (b) exercising all the shareholder’s rights, including voting, that shareholders are entitled to under the laws of China and the Articles of Association, including but not limited to the sale or transfer or pledge or disposition of shares in part or in whole; and (c) designating and appointing on behalf of shareholders the legal representative, the executive director, supervisor, the chief executive officer and other senior management members of Gansu QLS.
Each Power of Attorney is coupled with an interest and shall be irrevocable and continuously valid from the date of its execution, so long as the relevant Gansu QLS Shareholder is a shareholder of Gansu QLS.
Spousal Consent
The spouses of the Gansu QLS Shareholders agreed, via a spousal consent, to the execution of the “Transaction Documents” including: (a) the Call Option Agreement entered into with WFOE and Gansu QLS; (b) the Shareholders’ Voting Rights Proxy Agreement entered into with WFOE and Gansu QLS; (c) the Equity Pledge Agreement entered into with WFOE; and (d) the Power of Attorney executed by each Gansu QLS Shareholder, and the disposal of the equity interests of Gansu QLS held by each Gansu QLS Shareholder and registered in his/her name.
56
The spouses further undertake not to make any assertions in connection with the equity interests of Gansu QLS which are held by the Gansu QLS Shareholders. They confirm that the Gansu QLS Shareholders can perform, amend, or terminate the Transaction Documents without their authorization or consent. They undertake to execute all necessary documents and take all necessary actions to ensure appropriate performance of the agreements.
B. Business Overview
Overview of our Company
BGM Group Ltd (“BGM”) is not an operating company but a Cayman Islands holding company. BGM’s operations are conducted through contractual arrangements with the VIE based in China. PRC laws, regulations, and rules restrict and impose conditions on direct foreign investment in certain types of businesses, and we therefore rely on the VIE to operate these businesses in China. BGM Group Ltd does not own equity interest in the VIE or its subsidiaries. For a summary of these contractual arrangements, see “Item 4. Information on the Company — A. History and Development of the Company — Our Holding Company Structure and Contractual Arrangements — Contractual Arrangements between WFOE and Gansu QLS.” Investors in our Ordinary Shares thus are not acquiring equity interest in our operating entities in China but instead are acquiring interest in a Cayman Islands holding company.
The WFOE and the VIE and its subsidiaries face legal and operational risks associated with having the majority of their operations in China. The Chinese government has significant authority to exert influence on the ability of a China-based company, such as us, to conduct its business. Therefore, investors of BGM and its business conducted by the WFOE and the VIE and its subsidiaries face potential uncertainty from the PRC government. Changes in China’s economic, political or social conditions or government policies could materially adversely affect BGM and its affiliated entities’ business and results of operations. For example, we and our affiliated entities face risks associated with PRC governmental authorities’ significant oversight and discretion over the businesses and financing activities of the VIE, the requirement of regulatory approvals for offerings conducted overseas by and foreign investment in China-based issuers, the use of variable interest entities, the enforcement of anti-monopoly regime, the regulatory oversight on cybersecurity and data privacy as well as the risk of delisting if the PCAOB is unable to conduct inspection on our auditors, which may impact our ability to conduct certain businesses, accept foreign investments, or list on a United States or other foreign exchange. These risks could result in a material adverse change in the WFOE and the VIE and its subsidiaries’ operations and the value of BGM’s Ordinary Shares, significantly limit or completely hinder BGM’s ability and the ability of any holder of its Ordinary Shares or other securities of BGM to offer or continue to offer such securities to investors, or cause the value of such securities to significantly decline. In particular, recent statements and regulatory actions by China’s government, such as those related to data security or anti-monopoly concerns, as well as the PCAOB’s ability to inspect our auditors, may impact BGM’s ability to conduct its business through the WFOE and the VIE and its subsidiaries, accept foreign investments, or be listed on a U.S. or other foreign stock exchange. See “Item 3. Key Information - D. Risk Factors - Risks Related to Doing Business in China - The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we and our affiliated entities were to be subject to such oversight and control, it may result in a material adverse change to the WFOE and the VIE and its subsidiaries’ business operations, significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors, and cause its ordinary shares to significantly decline in value or become worthless” and “Item 3. Key Information - D. Risk Factors - Risks Related to Doing Business in China - Uncertainties with respect to the PRC legal system and the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us, hinder BGM’s ability and the ability of any holder of BGM’s securities to offer or continue to offer such securities, result in a material adverse change to the WFOE and the VIE and its subsidiaries’ business operations, and damage BGM and its subsidiaries’ reputation, which would materially and adversely affect BGM and its affiliates’ financial condition and results of operations and cause the Ordinary Shares to significantly decline in value or become worthless.”
57
BGM has been advised by Gansu Quanyi Law Firm, our PRC counsel, as of the date of this Annual Report, our listing in the U.S. is not subject to the review, permission or prior approval of any PRC authorities including the Cyberspace Administration of China (“CAC”) or the China Securities Regulatory Commission (“CSRC”) because (i) the CSRC currently has not issued any definitive rule or interpretation concerning whether our listing is subject to this regulation; and (ii) our operating entities affiliated to us were established and operate in PRC are not included in the categories of industries and companies whose foreign securities offerings are subject to review by the CSRC or the CAC. Uncertainties still exist, however, due to the possibility that laws, regulations, or policies in the PRC could change rapidly in the future. In the event that the PRC government expanded the categories of industries and companies whose foreign securities offerings are subject to review by the CSRC or the CAC, and we or our affiliated entities inadvertently concluded that relevant permissions or approvals were not required or that we or our affiliated entities did not receive or failed to maintain relevant permissions or approvals required and such permissions were subsequently rescinded, any action by the PRC government could significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly decline or be worthless.
Our corporate structure is subject to risks associated with our contractual arrangements with the VIE. Investors may never directly hold equity interests in the VIE. If the PRC government finds that the contractual arrangements which establish the structure of the VIE and its subsidiaries’ business operations do not comply with PRC laws and regulations, or if these regulations or their interpretations change in the future, we or our affiliated entities could be subject to severe penalties or be forced to relinquish our interests in those operations, which would result in our variable interest entities, being deconsolidated. Substantial all of the VIE and its subsidiaries’ assets, including the necessary licenses to conduct business are held by the VIE and its subsidiaries. Substantial all of our revenue is generated by the VIE and its subsidiaries. The deconsolidation of the VIE would have a material adverse effect on the VIE and its subsidiaries’ operations and substantially diminish the value of our Ordinary Shares. There are uncertainties about potential future actions by the PRC government that could affect the enforceability of our contractual arrangements with our variable interest entities and, consequently, significantly affect our financial performance. The value of the Ordinary Shares may significantly decline or become worthless as a result. For a detailed description of the risks associated with our corporate structure, please refer to risks disclosed under “Risk Factors — Risks Related to Our Corporate Structure.”
In addition, trading in BGM’s securities may be prohibited under the HFCA Act if the PCAOB determines that it cannot inspect the workpapers prepared by our auditor, and that as a result an exchange may determine to delist BGM’s securities. On December 16, 2021, the PCAOB issued a report on its determination that it is unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in China and in Hong Kong because of positions taken by PRC and Hong Kong authorities in those jurisdictions. Our auditors, independent registered public accounting firms that issue the audit reports included elsewhere in this annual report, as auditors of companies that are traded publicly in the U.S. and firms registered with the PCAOB, are subject to laws in the U.S., pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. Our auditors, ZH CPA, LLC and Enrome LLP, are located in Denver, Colorado and Singapore, and have been inspected by the PCAOB on a regular basis. Our auditors are not subject to the determination issued by the PCAOB on December 16, 2021. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden, which contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act and amended the HFCA Act by requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period for triggering the prohibition on trading. On August 26, 2022, the CSRC, the MOF and the PCAOB signed the Protocol governing inspections and investigations of audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. Pursuant to the fact sheet with respect to the Protocol disclosed by the SEC, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. See “Item 3. Key Information — D. Risk Factors — Risks Related to Doing Business in China — Our Ordinary Shares may be delisted and prohibited from being traded under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect auditors who are located in China. The delisting and the cessation of trading of our Ordinary Shares, or the threat of their being delisted and prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections deprives our investors with the benefits of such inspections.”
58
Cash Transfers and Dividend Distribution
BGM conducts its business operations in China through the WFOE and the VIE and the VIE’s subsidiaries. If needed, BGM can transfer cash to our PRC Subsidiary through loans and/or capital contributions, and our PRC subsidiary can transfer cash to BGM through issuing dividends or other distributions. Our PRC Subsidiary can transfer cash to the VIE through intercompany loans and capital contributions, and the VIE can transfer cash to our PRC Subsidiary as service fees under the VIE contractual arrangements.
Current PRC regulations permit our PRC Subsidiary to pay dividends to its shareholders only out of its accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. For details, see “Item 3. Key Information—D. Risk Factors — Risks Related to Doing Business in China — BGM is a holding company and it relies for funding on dividend payments from its affiliated entities by contracts, which are subject to restrictions under PRC laws. Any limitation on the ability of BGM’s affiliated entities to make payments to it could have a material adverse effect on BGM’s ability to maintain its business.” In addition, cash transfers from our holding company are subject to applicable PRC laws and regulations on loans and direct investment. For details, see “Item 3. Key Information—D. Risk Factors — Risks Related to Doing Business in China — PRC regulation of loans to, and direct investments in PRC entities by offshore holding companies may delay or prevent us from using proceeds from future financing activities to make loans or additional capital contributions to the PRC Subsidiary.”
For the year ended September 30, 2024, cash flow from WFOE to VIE included proceeds from repayment of loan of $702,469 and net proceeds from product sales and purchase of $898,863. For the year ended September 30, 2023, cash flow from VIE to WFOE included payment of $39,508 for net payments for products sales and purchase. For the year ended September 30, 2022, cash flow from WFOE to VIE included net proceeds used for product sales and purchase of $272,527.
We have not declared or paid dividends in the year ended September 30, 2024, nor any dividends or distributions were made by a subsidiary or VIE to our holding company. We do not have a fixed dividend policy. Our board of directors have complete discretion on whether to distribute dividends, subject to applicable laws. See “Item 3. Key Information—D. Risk Factors — Risks Related to Our Ordinary Shares — We do not intend to pay dividends for the foreseeable future.”
Recent Regulatory Developments
On July 10, 2021, the CAC published the Measures for Cybersecurity Review (Revised Draft for Comments), which will replace the current Measures for Cybersecurity Review after it is adopted and becomes effective. The draft measures, among others, stipulate that if an operator has personal information of over one million users and intends to be listed in a foreign country, it must be subject to the cybersecurity review. On November 14, 2021, the CAC released the Regulations on the Network Data Security (Draft for Comments) and accepted public comments until December 13, 2021. The draft Regulations provided that data processors refer to individuals or organizations that autonomously determine the purpose and the manner of processing data. If a data processor that processes personal data of more than one million users would like to list overseas, it shall apply for a cybersecurity review according to the draft Regulations. Besides, data processors that are listed overseas shall carry out an annual data security assessment.
As advised by our PRC legal counsel, the draft measures and regulations were released for public comment only, and its provisions and anticipated adoption or effective date may be subject to change and thus its interpretation and implementation remain substantially uncertain. We cannot predict the impact of the draft measures and regulations, if any, at this stage, and we and our affiliated entities will closely monitor and assess the statutory developments in this regard. See “Item 3. Key Information—D. Risk Factors —Risks Related to Doing Business in China— The approval and/or other requirements of the CSRC or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we and our affiliated entities cannot predict whether or how soon we will be able to obtain such approval.”
59
On July 6, 2021, the relevant PRC governmental authorities made public the Opinions on Strictly Cracking Down Illegal Securities Activities in Accordance with the Law. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision on overseas listings by China-based companies and proposed to take effective measures, such as promoting the construction of relevant regulatory systems to deal with the risks and incidents faced by China-based overseas-listed companies. As these opinions are recently issued, official guidance and related implementation rules have not been issued yet and the interpretation of these opinions remains unclear at this stage. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—The approval and/or other requirements of the CSRC or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we and our affiliated entities cannot predict whether or how soon we will be able to obtain such approval.” As of the date of this annual report, we have not received any inquiry, notice, warning, or sanctions regarding offshore offering from the CSRC or any other PRC governmental authorities.
We have been advised by our PRC legal counsel, Gansu Quanyi Law Firm, that in the event that we conduct a follow-on offering of securities, we are required to file with the CSRC in accordance with the Provisions of the State Council on the Administration of Overseas Securities Offering and Listing by Domestic Companies (Draft for Comments) and Administrative Measures for the Filing of Overseas Securities Offering and Listing by Domestic Companies (Draft for Comments), released by the CSRC on December 24, 2021. In the absence of such offering plan, we and our affiliated entities believe that we are currently not required to obtain any permission or approval from the CSRC and the CAC in the PRC to issue securities to foreign investors. However, there is no guarantee that this will continue to be the case in the future in relation to BGM’s future offerings or the continued listing of BGM’s securities on a U.S. securities exchange, or even in the event such permission or approval is required and obtained, it will not be subsequently revoked or rescinded. If we and our affiliated entities do not receive or maintain the approvals, or we or our affiliated entities inadvertently conclude that such approvals are not required, or applicable laws, regulations, or interpretations change such that we and our affiliated entities are required to obtain approval in the future, we and our affiliated entities may be subject to an investigation by competent regulators, fines or penalties, or an order prohibiting us from conducting an offering, and these risks could result in a material adverse change in our and the VIE and its subsidiaries’ operations and the value of BGM’s securities, significantly limit or completely hinder BGM’s ability to offer or continue to offer securities to investors, or cause such securities to significantly decline in value or become worthless.
Business Overview
The WFOE and the VIE and its subsidiaries operate a pharmaceutical and chemical company based in China that focuses on the development, manufacture, marketing, and sale of oxytetracycline products, licorice products, traditional Chinese medicine derivatives (“TCMD”) product, heparin product, sausage casings, and fertilizers. The VIE and its subsidiaries independently developed Gan Di Xin® and Ahan® Antibacterial Paste within their research and development department. The products of the VIE and its subsidiaries are sold in more than 20 provinces in China.
| ● | Licorice products include Gan Di Xin®, Qilian Shan® Licorice Extract, and Qilian Shan® Licorice Liquid Extract. The VIE and its subsidiaries’ Gan Di Xin® is an innovative antitussive and expectorant medicine made from raw licorice materials. The VIE and its subsidiaries’ Qilian Shan® Licorice Extract is a primary ingredient for pharmaceutical companies to manufacture traditional licorice tablets. The VIE and its subsidiaries’ Qilian Shan® Licorice Liquid Extract is the primary ingredient for medical preparation companies to produce compound licorice oral solutions. |
| ● | Oxytetracycline products include Qilian Shan® Oxytetracycline Tablets and Qilian Shan® Oxytetracycline Active Pharmaceutical Ingredients (“API”). The VIE and its subsidiaries’ Qilian Shan® Oxytetracycline Tablets are used to prevent and treat a wide range of diseases in chickens, turkeys, cattle, swine, and human. The VIE and its subsidiaries’ Qilian Shan® Oxytetracycline APIs are used by pharmaceutical companies in the manufacturing of medications that use oxytetracycline as an active ingredient. |
| ● | TCMD product, Ahan® antibacterial paste, is made from a mixture of 11 traditional Chinese herbal ingredients. It is used to treat refractory chronic skin diseases. |
| ● | Heparin product, Heparin Sodium Preparation, is a primary ingredient for pharmaceutical companies to produce medications used in treating cardiovascular diseases, cerebrovascular diseases, and hemodialysis. |
| ● | Sausage casings include Zhu Xiaochang® Sausage Casings, which are all-natural food products used for culinary purposes. |
60
| ● | Fertilizer products include Xiongguan® Organic Fertilizer and Xiongguan® Organic-Inorganic Compound Fertilizer. The VIE and its subsidiaries’ Xiongguan® Organic Fertilizer is designed to improve crop yield, increase soil’s chemical properties, and reduce soil compaction. The VIE and its subsidiaries’ Xiongguan® Organic-Inorganic Compound Fertilizer is made from both organic materials and traditional chemical fertilizer, and is designed to increased plant growth. |
We also have a strategic focus on the technology fields of AI application, intelligent robots, algorithmic computing power, cloud computing, and biopharmaceuticals. In terms of AI application implementation, we rely on big data mining and AI Agent technology, and utilize the two platforms of Du Xiao Bao and Bao Wang to provide comprehensive and professional AI solutions and intelligent robot services for insurance companies, insurance brokers, and consumers. Its services cover multiple key scenarios such as sales and marketing, underwriting assessment, claims processing, and customer service. We are capable of analyzing consumer data, building consumer profiles, accurately predicting insurance needs, and providing highly customized services for consumers. In the field of biopharmaceuticals, we deeply integrate AI-assisted decision-making into every link of production and manufacturing, achieving supply chain optimization, process efficiency improvement, and market trend prediction. This provides scientific decision-making basis for our management and offers high-quality products and precise services for consumers.
Products
The WFOE and the VIE and its subsidiaries currently manufacture ten products. The VIE and its subsidiaries independently developed Gan Di Xin® and Ahan® Antibacterial Paste within the VIE and its subsidiaries’ research and development department. The products of the VIE and its subsidiaries are sold in more than 20 provinces in China. The following list outlines the current products of the VIE and its subsidiaries under six categories— oxytetracycline products, licorice products, TCMD product, heparin product, sausage casings, and fertilizers.
61
Product |
|
Product |
|
|
|
|
Category |
|
Name |
|
Intended Use |
|
Government Agency Approval |
Licorice Products |
|
Gan Di Xin® (1) |
|
Used orally as antitussive and expectorant medicine. |
|
Pharmaceutical Manufacturing Permit approved by Gansu Food and Drug Administration on August 14, 2018. Re-registration approved by the Gansu Provincial Food and Drug Administration on February 7, 2020, May 14, 2015 and April 30, 2004. |
|
|
Qilian Shan® Licorice Exact (1) |
|
Used for treating bronchitis, pharyngitis, bronchial asthma and chronic adrenal insufficiency. |
|
Pharmaceutical Manufacturing Permit approved by Gansu Food and Drug Administration on August 14, 2018. Re-registration approved by Gansu Food and Drug Administration on May 14, 2015. The Company filed for record with Drug-related Information Filing Platform in July 2019, which removed any further need for re-registration according to Article 11 of the Notice of the NMPA on Strengthening the Supervision and Administration of Extracts and Extracts in the Production of Chinese Medicine. |
|
|
Qilian Shan® Licorice Liquid Extract (1) |
|
Used for treating bronchitis, pharyngitis, bronchial asthma and chronic adrenal insufficiency. |
|
Pharmaceutical Manufacturing Permit approved by Gansu Food and Drug Administration on August 14, 2018. Re-registration approved by the Gansu Provincial Food and Drug Administration on May 14, 2015 and April 30, 2004. The Company filed for record with Drug-related Information Filing Platform in July 2019, which removed any further need for re-registration according to Article 11 of the Notice of the NMPA on Strengthening the Supervision and Administration of Extracts and Extracts in the Production of Chinese Medicine. |
Oxytetracycline Products |
|
Qilian Shan® Oxytetracycline API (1) |
|
Used for treating following diseases: Rickettsia, Mycoplasma infection, Chlamydia infection, Regression fever, Brucellosis cholera, Rabbit fever and Plague |
|
Pharmaceutical Manufacturing Permit approved by Gansu Food and Drug Administration on August 14, 2018. Re-registration approved by Gansu Food and Drug Administration on May 14, 2015. The Company filed for record with Drug-related Information Filing Platform in July 2019, which removed any further need for re-registration according to Article 11 of the Notice of the NMPA on Strengthening the Supervision and Administration of Extracts and Extracts in the Production of Chinese Medicine. |
|
|
Qilian Shan® Oxytetracycline Tablets (1) |
|
Used orally for treating the following diseases: Rickettsia, Mycoplasma infection, Chlamydia infection, Regression fever, Brucellosis cholera, Rabbit fever and Plague. |
|
Pharmaceutical Manufacturing Permit approved by Gansu Food and Drug Administration on August 14, 2018. Re-registration approved by Gansu Provincial Food and Drug Administration on February 7, 2020, May 14, 2015 and September 19, 2010. |
TCMD Product |
|
Ahan® Antibacterial Paste (2) |
|
Designed as a rubbing ointment to kill Staphylococcus aureus, Candida albicans and Escherichia coli. It treats psoriasis, various dermatitis and eczema, mites, onychomycosis, and genital itching. |
|
Sanitary License for Manufactures of Disinfectant Products approved by Health and Family Planning Commission of Gansu Province and Shaanxi Provincial Center for Disease Control and Prevention on June 1, 2017. |
Heparin Product |
|
Heparin Sodium Preparations (3) |
|
Designed for the prevention of thrombosis and embolism; treatment of diffuse intravascular coagulation (DIC) caused by various causes; and other anticoagulation purposes. |
|
Business license issued by Chengdu Administration for Industry and Commerce on June 23, 2014. |
Sausage Casings |
|
Zhu Xiaochang® Sausage Casing (3) |
|
Used for culinary purposes. |
|
Business license issued by Chengdu Administration for Industry and Commerce on June 23, 2014. |
Fertilizers |
|
Xiongguan® Organic Fertilizer (4) |
|
Designed as a base application fertilizer. It is used to improve soil quality, increases crop yield and improves agricultural products’ quality. |
|
National Manufacturing License for Industrial Products approved by Gansu Provincial Agriculture and Animal Husbandry on August 5, 2016. Fertilizer Registration Certificate of The People’s Republic of China approved by PRC Ministry of Agriculture on May 19, 2020. |
|
|
Xiongguan® Organic-Inorganic compound Fertilizer (4) |
|
Designed as a base and top application fertilizer. It is used to improve soil structure, prevents soil compaction, increases soil’s water retention capacity, improves crops’ drought/cold weather resistance, and enhances crops’ rooting. |
|
National Manufacturing License for Industrial Products approved by Gansu Provincial Quality Inspection Bureau and Gansu Provincial Agriculture and Animal Husbandry on August 5, 2016. Fertilizer Registration Certificate of The People’s Republic of China approved by PRC Ministry of Agriculture on May 19, 2020. |
| (1) | This product is manufactured by the VIE,Gansu GLS. |
| (2) | This product is manufactured by the VIE’s subsidiary, Ahan. |
| (3) | This product is manufactured by the VIE’s subsidiary, Chengdu QLS. |
| (4) | This product is manufactured by the VIE’s subsidiary, Moshangfa. |
62
The following is a detailed description of the current products of the WFOE and the VIE and its subsidiaries and products in development.
Licorice Products
Gan Di Xin®- As an enhanced type of compound licorice tablet, Gan Di Xin is an antitussive and expectorant medicine made from raw licorice materials. Gansu QLS independently researched and developed Gan Di Xin using their patented purification, thin-film coating and inclusion technology (the “3-in-1 technology”, Patent Number ZL 200410030776.4, issued on October 25, 2006). The effective medical ingredients in compound licorice tablets become active only when they are absorbed by the bloodstream. However, traditional licorice tablets’ efficacy is drastically reduced when the effective medical ingredients are swallowed and enter the gastrointestinal tract. Rather than being absorbed by the bloodstream directly, the effective medical ingredients go through the liver’s metabolism process first, which renders the ingredients ineffective. Such phenomenon is called “first pass effect”. The 3-in-1 technology adopted by the Gansu QLS has helped Gan Di Xin bypass the so called “first pass effect” by allowing Gan Di Xi to be dissolved slowly in patients’ mouths, whereby the active ingredients are absorbed through oral mucosa, enabling them to enter blood circulation directly rather than being metabolized by the liver. In this way, Gan Di Xin’s effectiveness can be preserved.
Gan Di Xin is currently categorized as a chemical medicine that falls under China’s State Category V New Drug. According to the New Drug Approval Methods promulgated in July 1985 and revised in April 1999 by the State Drug Administration of PRC, claims of new indications for marketed chemical drugs shall be categorized as a V Category New Drug in the application for approval. Since Gansu QLS applied to have Gan Di Xin approved as a marketed drug reducing dosages, thus adding new indications to already marketed drugs, when Gan Di Xin was approved, it was approved as a Category V New Drug. The application and approval procedures for Category V New Drugs are divided into two stages: clinical research and production and sale. The application for a Category V New Drug is pre-examined by the provincial branches of the National Medical Products Administration, and re-examined by the National Medical Products Administration. Gan Di Xin was issued the National New Drug Certificate (No. H20040463) on April 30, 2004 with Drug Registration Approval (No. 20040640). As a pharmaceutical manufacturer, Gansu QLS is subject to the national medicine quality standard of WS1-(X-001)-2015Z for product registration and manufacturing.

Gansu QLS’s Gan Di Xin® is innovative in terms of its unconventional administration methods, taste, and efficacy. The unique manufacturing process adopted by Gansu QLS, the abundance of local source materials, and the geographical location of Gansu QLS are crucial elements contributing to the success Gansu QLS’s Gan Di Xin®.
Gansu QLS introduced Gan Di Xin® to the Chinese market in 2004. Gan Di Xin® has enjoyed growing popularity in recent years due to its easy administration method, strong efficacy, and soothing taste. Gansu QLS sold approximately 2.99 million pieces for year ended September 30, 2023. Gan Di Xin® was awarded “Famous Trademark of Gansu Province” in 2011 by the Gansu Famous Brand Strategy Promotion Committee of the Gansu government. Gan Di Xin® was also awarded “China Chemical and Pharmaceutical Industry’s Excellent Product Brand” in 2013 by the China Chemical Pharmaceutical Industry Association, China Pharmaceutical Business Association, China Non-Prescription Drug Association, and China Pharmaceutical Enterprise Development and Promotion Association. Currently, Gansu QLS sells Gan Di Xin® in more than 20 provinces in China.
63
Qilian Shan® Licorice Extract and Qilian Shan® Licorice Liquid Extract — Gansu QLS’s licorice extract is a type of API made from processed high quality licorice. This licorice liquid extract is a type of API made from fluid extract of further processed licorice extract. Gansu QLS’s licorice extract is the primary product for pharmaceutical companies to manufacture traditional licorice tablets. Gansu QLS’s licorice liquid extract is also the primary product for medical preparation companies to produce compound licorice oral solutions. Both the traditional compound licorice tablets and compound licorice oral solutions are prescriptive palliatives that help to relieve the symptoms of mucosa irritations and gastrointestinal smooth muscle spasms; they are also used in treating bronchitis, bronchial asthma, throat inflammation and chronic adrenal insufficiency.
Oxytetracycline Products
Qilian Shan® Oxytetracycline Tablets – Oxytetracycline is a yellow crystalline broad-spectrum antibiotic C22H24N2O9, which is active against a wide variety of bacteria. Oxytetracycline works by interfering with the ability of bacteria to produce essential proteins. Without these proteins, the bacteria cannot grow, multiply and increase in numbers. Oxytetracycline therefore stops the spread of the infection and the remaining bacteria are killed by the immune system or eventually die.
Gansu QLS uses the active ingredient oxytetracycline to manufacture oxytetracycline tablets. Gansu QLS’s Qilian Shan® Oxytetracycline Tablets are used to prevent and treat a wide range of diseases in chickens, turkeys, cattle, swine, and human. Gansu QLS sells its Qilian Shan® Oxytetracycline tablets in more than 20 provinces. Most of the customers who purchase Gansu QLS’s oxytetracycline tablets are pharmaceutical companies.
Qilian Shan® Oxytetracycline APIs— Pharmaceutical companies use Gansu QLS’s oxytetracycline APIs in the manufacture of other medications that use oxytetracycline as an active ingredient in such pharmaceutical products.
Gansu QLS is the only producer in China manufacturing both oxytetracycline tablets and oxytetracycline APIs. Both Qilian Shan® Oxytetracycline tablets and Qilian Shan® Oxytetracycline APIs are certified by the State Food and Drug Administration (“CFDA”), which has been superseded as NMPA. Gansu QLS has obtained the Pharmaceutical Production License and the re-registration approval for the production of the WFOE and the VIE and its subsidiaries’ oxytetracycline products. All registrations and qualifications for production are within their validity period.
While oxytetracycline products manufactured by Gansu QLS’s domestic competitors are certified for veterinary use only by the Chinese Ministry of Agriculture (“MOA”), Gansu QLS’s products are also qualified for human consumption by the CFDA. Gansu QLS relies on an established production system as well as a quality control process for its product manufacturing process. Gansu QLS believes that certain key production indicators such as fermentation unit, fermentation yield, and bacterial infection rate have given Gansu QLS distinctive advantages over its competitors, such as excellent per unit production rate, stable and premium quality of products, or large scale production capability.
TCMD Product
Ahan® Antibacterial Paste— Categorized as a disinfecting product under the Law of the PRC on Prevention and Treatment of Infectious Disease, Ahan® antibacterial paste is made from a mixture of 11 traditional Chinese herbal ingredients including Scutellariae Radix, Phellodendri Chinensis Cortex, Rhei Radix Et Rhizoma, Cnidii Fructus and Dictamni Cortex. It is used to treat refractory chronic skin diseases caused by Staphylococcus aureus, Moniliaalbican, and Escherichia coli. It is also prescribed for people suffering from skin infections such as psoriasis, eczema and onychomycosis.
Heparin Product
Heparin Sodium Preparations- Heparin sodium is a prescription drug that has multiple biological and medical functions such as anticoagulation, antithrombotic, hypolipidemic and anti-atherosclerosis. It is used in treating cardiovascular diseases, cerebrovascular diseases, and hemodialysis. Heparin sodium decreases the risk of coagulation, which is the formation of blood clots in the blood vessels. Heparin sodium is used in preventing blood clotting during open-heart surgery, bypass surgery, kidney dialysis, and blood transfusions. In low doses, it can help prevent and reduce coagulation in certain patients, especially those who underwent surgeries or must remain in bed for a long time. Heparin sodium is also valuable in diagnosing and treating disseminated intravascular coagulation, a serious blood condition in which increased clotting depletes the clotting factors needed to control bleeding, causing excessive bleeding. Heparin sodium has been widely used as an anticoagulant in the world since its first use in 1935.
64
Chengdu QLS, a subsidiary of the VIE, purchases healthy, locally raised pigs and extracts heparin-rich organic materials from their small intestinal mucosa. Chengdu QLS then processes extracted heparin materials into heparin crude products, which are then sent to manufacturers of heparin sodium raw material for further preparations. Chengdu QLS’s crude heparin is intended for use as a component of other drugs in the Chinese biochemical and medical industry such as Enoxaparin Sodium Injection and Nadroparin Calcium Injection.
Sausage Casings
Zhu Xiaochang® Sausage Casings – Chengdu QLS’s sausage casings are soft cylindrical containers made from small intestines of locally raised pigs. They can be used to contain sausage mixes or for certain medical uses. Chengdu QLS’s all-natural sausage casings are strong and flexible enough to resist the pressure produced by filling them with sausage mix and are permeable to water vapor and gases. Chengdu QLS’s sausage casings offer resistance at low or high temperatures and under customary culinary or medical preparations.
Chengdu QLS’s Heparin Sodium Preparations and Zhu Xiaochang® sausage casings are resource-based products. Chengdu QLS enjoys high quality, low cost, and abundant local resources, which enables it to focus on production technologies and quality control procedures.
Fertilizers
Xiongguan® Organic Fertilizer— Moshangfa’s organic fertilizer combines functional microorganisms and composites of organic materials such as animal and plant residues. In addition to its high nutrient efficiency, Moshangfa’s organic fertilizer is designed to improve crop yield, increase soil’s chemical properties, and reduce soil compaction.
Xiongguan® Organic-Inorganic Compound Fertilizer— Primarily sold in six Western Chinese provinces, Moshangfa’s organic-inorganic compound fertilizer contains both organic materials and composites from traditional chemical fertilizer. The organic materials are a mixture of animal feces and peat moss, which are then treated by microbial fermentation process. The organic materials are further mixed with composites from traditional chemical fertilizer, along with humic acid, amino acid and beneficial microbial bacteria. The final product is a granulated nutritious blend designed to increased plant growth.
Products Currently in Development
Microbial Fertilizer — Microbial fertilizer is a type of multi-element fertilizer containing various strains of living microorganisms. It is a mixture of peat, cow dung, sheep manure carefully cultivated with beneficial bacteria such as lactobacillus, photosynthetic bacteria, and Bacillus. It is a compound bacterial fertilizer rich in various antioxidant substances, amino acids, and digestive enzymes. Microbial fertilizer’s bio-mechanism is creating positive influence upon crops and plants through solubilization of phosphorus, nitrogen fixation, production of plant nutrients and phytohormones, protection from pathogens and recovery from stressful environmental conditions. Functionally, microbial fertilizer enhances crops and plants’ resilience against pests, diseases, and harsh environmental conditions, thus reducing yield loss over time. In addition to providing essential nutrients for crops, it stimulates the growth of roots through chemical substances released by living microorganisms, creating a virtuous cycle of nutrients accumulation that increases crop yields.
Bio-organic Fertilizer — bio-organic fertilizer is a combination of functional microorganisms and organic materials mainly composed of animal and plant residues (such as mixtures of livestock manure and straws). Manufactured through environmental-friendly processes, Moshangfa’s product is expected to have the following benefits— high nutrient utilization efficiency, the capability to improve crop yield and quality, and the ability to improve soil’s physical and chemical properties.
The two products in development must be registered with the PRC Ministry of Agriculture before they can be produced, sold, or advertised. Moshangfa obtained the Fertilizer Registration Certificates of The People’s Republic of China for the two products under development from the PRC Ministry of Agriculture on May 19, 2020.
Manufacturing Process
The following is a brief description of the manufacturing process of the current products of the WFOE and the VIE and its subsidiaries.
65
Licorice Products
Gan Di Xin® — Gansu QLS’s facilities produce licorice tablets by combining Licorice Extract, hydrochloric acid, and diluted ammonia. The resulting paste-like mixture is then dried and pulverized into fine powder. That powder is then mixed with camphor extract, star anise oil, and betacyclodextrin. The mixture is further stirred, refrigerated, filtered, dried, combined with more ingredients before final granulation, pressure forming and packaging processes.
Qilian Shan® Licorice Extract and Qilian Shan® Licorice Liquid Extract — To make Licorice Extract, Gansu QLS boils and purifies a mixture of water and licorice raw materials. Gansu QLS then extracts the clear liquid lying above solid licorice residue after precipitation and process the clear liquid into a thick, paste-like solid concentration called Licorice Extract.
To make Licorice Liquid Extract, Gansu QLS first applies heat to a mixture of water and Licorice Extract Power. It then adds ethanol to the heated solutions, stir, let stand overnight, and extracts the clear liquid lying above solid residue. Such procedures are repeated three times before mixing all the clear liquid that was extracted. After removing the residues and ethanol content in the clear liquid, Gansu QLS then adds other chemicals to the clear liquid mixture to ensure that the content of glycyrrhizic acid and alcohol in the clear liquid mixture is in compliance with relevant industry regulations. Gansu QLS’s facilities then purify the clear liquid mixture before packaging.
Oxytetracycline Products
Qilian Shan® Oxytetracycline Tablets — Gansu QLS mixes oxytetracycline and starch evenly, producing a soft material that later goes through granulation and drying procedures. Gansu QLS then adds magnesium stearate to the mixture as a “flow agent”, which prevents the ingredients in each individual tablet from sticking to each other. Then Gansu QLS pressures form, sugar-coats and finally packages the tablets.
Qilian Shan® Oxytetracycline APIs — Gansu QLS carefully cultivates and reproduces Streptomyces Rimosus under specific conditions. Antibiotic materials are produced and accumulated during this fermentation process. Gansu QLS extracts antibiotic materials from the fermentation products. Gansu QLS then purifies and refines the extractions before finally formulating and packaging the product.
TCMD Product
Ahan® Antibacterial Paste — Ahan produces Ahan Antibacterial Paste by mixing water and various Chinese herbal medicine. Ahan then prepares an herbal decoction by heating, purifying and concentrating the mixture. After emulsification, the final products are packaged.
Heparin Product
Heparin Sodium Preparations — Cleaned pigs’ intestines are scraped and intestinal mucosa are collected. The intestinal mucosa is then heated with water and filter the solution, which is then further processed and dried before packaging.
Sausage Casings
Zhu Xiaochang® Sausage Casings — Scraped clean pigs’ intestines are salted, dried and packaged.
Fertilizers
Xiongguan® Organic Fertilizer and Xiongguan® Organic-Inorganic Compound Fertilizer — Moshangfa starts with processing and crushing a mixture of compost and chemical materials. Moshangfa then granulates the crushed composted materials and dries the granulated pellet, uses coating machines to add a protection layer on the surface of the pellets, and finally packages its fertilizer products.
Quality Control and Assurance
In China, each pharmaceutical manufacturer is required to obtain Pharmaceutical Manufacturing Permits granted by the NMPA or its local branches before it engages in any pharmaceutical manufacturing and distribution.
66
Gansu QLS has obtained its Pharmaceutical Manufacturing Permit with the product manufacturing scopes covering the WFOE and the VIE and its subsidiaries’ licorice products and oxytetracycline products. Moshangfa has obtained the National Manufacturing License for Industrial Products that covers the manufacturing of its fertilizer products. Moshangfa also obtained a Fertilizer Registration Certificate of The People’s Republic of China, which was approved by the PRC Ministry of Agriculture on May 19, 2020. Ahan has obtained the Sanitary License for Manufactures of Disinfectant Products that allows it to manufacture its antibacterial paste. The Chinese authorities currently do not require Chengdu QLS to obtain specific qualification or licenses for its sausage casings manufacturing.
The VIE and its subsidiaries have well-qualified and trained professional employees for manufacturing and quality control procedures. The quality control starts with procurement and continues in manufacturing, packaging, storage capabilities, and cost competitiveness to ensure that all of the products of the VIE and VIE’s subsidiaries meet the requirements.
Distribution and Marketing of Products
The products of the WFOE and the VIE and VIE’s subsidiaries are sold in more than 20 provinces nationwide to their qualified distributors, dealers and corporate customers. Currently, there are 5 corporate customers buying Chengdu QLS’s heparin product throughout China, 11 corporate customers buying Chengdu QLS’s sausage casings throughout China, 2 distributors and 32 dealers buying Moshangfa’s fertilizer products throughout China, 30 distributors and two dealers buying Gansu QLS’s oxytetracycline API throughout China, and 189 distributors and one dealer buying Gansu QLS’s oxytetracycline tablets and licorice products throughout China. A qualified distributor is a merchant with a pharmaceutical business qualification certificate, awarded and authorized by the NMPA. The WFOE and the VIE and its subsidiaries intend to engage more qualified distributors and dealers in order to strengthen their distribution network.
We understand the importance of branding and packaging. Packed in unique packaging, the products of the WFOE and the VIE and its subsidiaries bear distinctive trademarks that help them stand out in the market. The VIE and its subsidiaries design packaging for their products and engage third-party manufacturers to produce the packaging.
The WFOE and the VIE and its subsidiaries conduct marketing activities to publicize and enhance their image and brand name. The marketing efforts of the WFOE and the VIE and its subsidiaries are concentrated on attending national meetings, seminars, symposiums, exhibitions for veterinary healthcare and medical industries and other related industries where they can showcase their brand and products.
Customers
The customers of the WFOE and the VIE and its subsidiaries consist of qualified distributors, dealers and corporate customers. The WFOE and the VIE and its subsidiaries have several large customers with whom we generated substantial revenue each year, and the composition of largest customers has changed from year to year. For the fiscal year ended September 30, 2024, two customers represented approximately 16% and 12% of the sales of the WFOE and the VIE and its subsidiaries, respectively. For the fiscal year ended September 30, 2023, two customers represented approximately 15% and 14% of the sales of the WFOE and the VIE and its subsidiaries, respectively. For the fiscal year ended September 30, 2022, two customers represented approximately 11% and 11% of the sales of the WFOE and the VIE and its subsidiaries, respectively. While we believe that one or more of the major customers of the WFOE and the VIE and VIE’s subsidiaries could account for a significant portion of their sales for the foreseeable future, we anticipate that the customer base of the WFOE and the VIE and its subsidiaries will continue to expand and that they will become less dependent on major customers.
67
Suppliers; Sources and Availability of Raw Materials
The WFOE and the VIE and its subsidiaries research, design and manufacture their products at their manufacturing facilities located at Jiuquan City of Gansu Province and Qionglai City of Sichuan Province in China. The principal raw materials used include various chemical and biological materials including, but not limited to, starch, pig intestine, oxalic acid, liquid alkali, liquid ammonia, sodium ferrocyanide, and defoamer agent. None of the current products of the WFOE and the VIE and VIE’s subsidiaries requires any raw materials that are scarce, and the raw materials used in general are readily available from a wide range of local sources. Accordingly, the WFOE and the VIE and its subsidiaries do not have any continuing or long-term supply agreements with any of these suppliers. The WFOE and the VIE and its subsidiaries purchase raw materials from their suppliers on a per purchase order basis. The prices for these raw materials are nevertheless subject to market forces largely beyond the control of the WFOE and the VIE and its subsidiaries, including energy costs, organic chemical feedstock, market demand, and freight costs. The prices for these raw materials have varied significantly in the past and may vary significantly in the future.
For the fiscal year ended September 30, 2024, two of the suppliers of the WFOE and the VIE and its subsidiaries accounted for 12% and 10% of the total purchases. For the fiscal year ended September 30, 2023, one vendor of the WFOE and the VIE and its subsidiaries accounted for 11% of the total purchases. For the fiscal year ended September 30, 2022, one vendor accounted for 14% of total purchase.
Competition
The WFOE and the VIE and its subsidiaries have competitors in China that manufacture products similar to theirs. These companies sell products similar to ours and some of them may have more assets, resources and a larger market share. We believe the WFOE and the VIE and its subsidiaries are able to compete with these competitors because of their geographical location in West China, their unique combination of products, and their products’ lower prices.
Products |
|
Competitors |
Compound Licorice Tablets (a pharmaceutical product that has similar medical efficacy compared to the award winning Gan Di Xin®) |
|
Jiangxi Pharmaceutical Co., Ltd. (the only company in China that manufacture compound licorice tablets) |
Oxytetracycline Tablets |
|
Shanxi Datong Tongxing Antibiotics Co., Ltd.; Chifeng Pharmaceutical Co., Ltd.; Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. |
Oxytetracycline APIs |
|
Yunnan Baiyao Group Co., Ltd.; Kunming Pharmaceutical Group Co., Ltd.; Hunan Jianlang Pharmaceutical Co., Ltd.; Hainan Pharmaceutical Factory Co., Ltd. No. 2 Pharmaceutical Factory; Anhui Fengyuan Pharmaceutical Co., Ltd. |
Licorice Extract and Liquid Extract |
|
Baoji Jinsen Pharmaceutical Co., Ltd.; Xinjiang Tarim Agricultural Comprehensive Development Co., Ltd.; Jiangxi Jin Furong Pharmaceutical Co., Ltd.; Fuzhou Haiwang Jinxiang Chinese Medicine Pharmaceutical Co., Ltd.; Xinjiang Sinopharm Group Co., Ltd. |
Organic Fertilizer |
|
Gansu Shikefeng New Fertilizer Co., Ltd.; Beijing Century Arms Biotechnology Co., Ltd.; Ningxia Yipin Biotechnology Co., Ltd.; Shijiazhuang Golden Sun Bio-organic Fertilizer Co., Ltd.; Ningxia Beite Fertilizer Co., Ltd. |
Organic-Inorganic Compound Fertilizer |
|
Gansu Shikefeng New Fertilizer Co., Ltd.; Gansu Jinhua Group Corporation; Jinzhengda Ecological Engineering Group Co., Ltd.; Stanley Fertilizer Co., Ltd.; Hubei Xinyangfeng Fertilizer Co., Ltd. |
Heparin Sodium Preparations |
|
Chengdu Shenrui Animal Products Co., Ltd.; Guanghan Jinghuang Meat Food Co., Ltd.; Sichuan Xinkang Green Food Co., Ltd.; Yibin Lihao Biotechnology Co., Ltd. |
Sausage Casings |
|
Chengdu Shenrui Animal Products Co., Ltd.; Guanghan Jinghuang Meat Food Co., Ltd.; Sichuan Xinkang Green Food Co., Ltd.; Yibin Lihao Biotechnology Co., Ltd. |
Chinese Herbal Anti-bacterial Paste |
|
Wuhan Laowantong Biotechnology Co., Ltd.; Wuhan Runhe Biomedical Co., Ltd.; Jiangxi Jiarun Biotechnology Co., Ltd.; Jiangxi Cihetang Biotechnology Co., Ltd.; Jiangxi Jianyuantang Biotechnology Co., Ltd. |
68
Honors, Awards, and Qualifications
Honors
Honors |
|
Individual or |
|
Agency |
|
Date |
Vice Presiding Entity of Northwestern Natural Herbal Medicine Technology Innovation Strategical Alliance |
|
Gansu QLS |
|
Northwestern Natural Herbal Medicine Technology Innovation Strategical Alliance |
|
August 2010 |
Vice Presiding Entity for Gansu Province Medical Industry Association |
|
Gansu QLS |
|
Gansu Province Medical Industry Association |
|
May 2013 |
The 3rd Governing Entity of China Narcotics Association |
|
Gansu GLS |
|
China Association of Narcotic Drugs |
|
October 2014 |
Vice Presiding Entity for Jiuquan City Environmental Protection Industrial Association |
|
Gansu QLS |
|
Jiuquan City Environmental Protection Industrial Association |
|
March 2015 |
Gansu Provincial Excellent Engineering Consulting Award (awarded to our chairman of the board of directors) |
|
Zhanchang Xin, our chairman of the board of directors |
|
Gansu Provincial Development and Reform Commission |
|
August 2010 |
Gansu Province’s Famous Brand |
|
Gansu QLS |
|
Gansu Famous Brand Strategy Promotion Committee |
|
December 2011 |
Suzhou District Science and Technology Progress Award |
|
Gansu QLS |
|
Government of Suzhou District, Jiuquan City |
|
August 2012 |
2013 China Chemical and Pharmaceutical Industry’s Excellent Product Brand (awarded to the Gan Di Xin® product) |
|
Gansu QLS |
|
China Chemical Pharmaceutical Industry Association, China Pharmaceutical Business Association, China Non-Prescription Drug Association, China Pharmaceutical Enterprise Development and Promotion Association |
|
November 2013 |
Famous Trademark of Gansu Province (awarded to the VIE’s trademark Qilian Shan®) |
|
Gansu QLS |
|
Gansu Provincial Administration for Industry and Commerce |
|
November 2014 |
Gansu Province Circular Economy Exemplar Enterprise |
|
Gansu QLS |
|
Gansu Provincial Industry and Information Technology Commission |
|
July 2015 |
Nationally Recognized Enterprise Technology Center Status, Provincial Level |
|
Gansu QLS |
|
Gansu Provincial Industry and Information Commission, Gansu Provincial Development and Reform Commission, Gansu Provincial Science and Technology Department, Gansu Provincial Finance Department, Gansu Provincial State Taxation Bureau, Gansu Provincial Local Taxation Bureau |
|
December 2015 |
Famous Trademark of Gansu Province (awarded to the Gan Di Xin® product) |
|
Gansu QLS |
|
Gansu Provincial Administration for Industry and Commerce |
|
December 2015 |
Excellent Entrepreneur Award (awarded to our chairman of the board of directors) |
|
Zhanchang Xin |
|
China Petroleum and Chemical Industry Committee |
|
July 2016 |
Gansu Province “Specialized New Technology” Enterprise |
|
Gansu QLS |
|
Gansu Provincial Industry and Information Technology Commission |
|
November 2017 |
Strategic Emerging Growth Exemplar Enterprise |
|
Gansu QLS |
|
Gansu Provincial Development and Reform Commission |
|
December 2018 |
Little Giant Enterprise of Chengdu City |
|
Chengdu QLS |
|
Sichuan Provincial Economic and Information Commission, Sichuan Provincial SME Bureau, Chengdu City Economic and Information Commission |
|
December 2018 |
Petroleum and Chemical Industry “Specialized and Innovative” Small to Medium Enterprises (the “SME”) Award |
|
Gansu QLS |
|
China Petroleum and Chemical Industry Federation and China SME Development Committee |
|
November 2019 |
Chengdu City’s Unicorn Enterprise Award |
|
Chengdu QLS |
|
Chengdu City Municipal New Economy Commission |
|
June 2019 |
Sichuan Province “Specialized and Innovative” SME Award |
|
Chengdu QLS |
|
Sichuan Province Economic and Information Technology Commission |
|
March 2020 |
Sichuan Province “High-growth” SME Award |
|
Chengdu QLS |
|
Sichuan Province Economic and Information Technology Commission |
|
March 2020 |
Suzhou District “Tax Contribution Award” for 2019 |
|
Gansu QLS |
|
Suzhou District Committee and District Government |
|
March 2020 |
2020 Gansu Provincial Technology Innovation Model Enterprise |
|
Gansu QLS |
|
Gansu Province Industry and Information Technology Commission, Gansu Province Department of Finance |
|
August 2020 |
Jiuquan Leading Talent for Years 2020 to 2022 (awarded to our chairman of the board of directors) |
|
Zhanchang Xin |
|
Government of Jiuquan City and Jiuquan Municipal Committee of the Communist Party of China |
|
November 2020 |
Excellent Entrepreneur Award (awarded to our chairman of the board of directors) |
|
Zhanchang Xin |
|
Government of Economic and Technological Development Zone of Jiuquan City |
|
February 2021 |
Selective Criteria for the Awards
Gansu Provincial Excellent Engineering Consulting Award (awarded to our chairman of the board of directors, Mr. Zhanchang Xin)
The Gansu Provincial Excellent Engineering Consulting Award is awarded by the Gansu Provincial Development and Reform Commission based on the comprehensive evaluation of the engineering consulting achievements accomplished by the applicant and such recognition is only awarded to engineering projects that have reached high level of ingenuity and economic potential within certain industry. Gansu QLS’s “Gan Di Xin Industrialization Project” was recognized as such engineering project and Mr. Zhanchang Xin was recognized as having made outstanding contributions to the project during its establishment, implementation and completion stages.
69
Gansu Province’s Famous Brand
Gansu Province’s Famous Brand is awarded by Gansu Famous Brand Strategy Promotion Committee in accordance with the “Product Quality Law of the People’s Republic of China”, the “Quality Control Guideline of the State Council” and the “Quality Control Implementation Plan of Gansu Province”. In an effort to promote and cultivate excellent local brand of Gansu province, Gansu Famous Brand Strategy Promotion Committee carefully evaluates the applicant’s qualifications based on the following guidelines, which include, but are not limited to: brand-name strategy, products quality control, market share, customer satisfaction, annual profit and tax contribution, production cost and annual profit, applicant’s technological innovation and product development capabilities, and customer service.
Suzhou District Science and Technology Progress Award
In August 2012, the Company’s project “Research and development of oxalic acid extracted from oxytetracycline raw material production waste liquid” was awarded the first prize of “Science and Technology Progress” by the People’s Government of Suzhou County, Jiuquan City. The award is given by the local people’s government after comprehensive evaluation of the VIE project’s key quantitative and qualitative indicators such as technological innovation, project scale, overall technical difficulties involved, economic benefits conferred, and the promotion of scientific and technological progress in related industrial fields.
2013 China Chemical and Pharmaceutical Industry’s Excellent Product Brand (awarded to Gan Di Xin®)
Gansu QLS’s product, Gan Di Xin®, was awarded “2013 China Chemical and Pharmaceutical Industry’s Excellent Product Brand” after a joint review process conducted by the China Chemical Pharmaceutical Industry Association, the China Pharmaceutical Business Association, and the China Pharmaceutical Enterprise Development Promotion Association. The review process was based on the following qualifications, which include but are not limited to, the Company’s R&D capabilities, marketing capabilities, technological innovation, and production scale.
Famous Trademark of Gansu Province (awarded to trademark Qilian Shan®)
According to the “Trademark Law of the People’s Republic of China”, the “Regulations on the Implementation of the Trademark Law of the People’s Republic of China” and other laws and administrative regulations, the Gansu Provincial Administration for Industry and Commerce is authorized to award the “Famous Trademark of Gansu Province” title to eligible applicants based on the following qualifications, which include, but are not limited to: whether the trademark is publicly recognized and legally owned by the applicant, whether the trademark has high reputation/credibility and is well-known to the general public, whether the product behind the trademark is of superior quality than its competitors, and whether the customer service is satisfactory. The Gansu Provincial Administration for Industry and Commerce also evaluates the applicant’s key business indicators such as sales volume, local tax contribution, and annual profit increase in the past three years.
Gansu Province Circular Economy Exemplar Enterprise
According to the “Management Measures for the Identification and Assessment of Key Enterprises in Strategic Emerging Industries in Gansu Province” provided by the Provincial Development and Reform Commission, the Commission has the authority to award the Strategic Emerging Growth Exemplar Enterprise status to local enterprises with the following qualifications, which include, but are not limited to: well-known brand name within certain industry, high business growth, high contribution to local tax revenue, market competitiveness, and future development potential.
Nationally Recognized Enterprise Technology Center Status, Provincial Level
According to the “Gansu Provincial Level Nationally Recognized Enterprise Technology Center Recognition Measures”, Gansu Provincial Department of Industry and Information Technology, together with Gansu Provincial Development and Reform Commission, Gansu Provincial Department of Finance, State Taxation Bureau, and Gansu Provincial Taxation Bureau commissioned certain third-party institutions to conduct a comprehensive review of the applicant’s qualifications based on the following criteria, which include, but are not limited to: annual sales revenue, net profit, capitalization, production scales, competitive strength such as technological innovation, research and development capabilities, and ownership of intellectual property rights.
70
Famous Trademark of Gansu Province (awarded to Gan Di Xin®)
According to the “Trademark Law of the People’s Republic of China”, the “Regulations on the Implementation of the Trademark Law of the People’s Republic of China” and other laws and administrative regulations, the Gansu Provincial Administration for Industry and Commerce is authorized to award the “Famous Trademark of Gansu Province” title to eligible applicants based on the following qualifications, which include, but are not limited to: whether the trademark is publicly recognized and legally owned by the applicant, whether the trademark has high reputation/credibility and is well-known to the general public, whether the product behind the trademark is of superior quality than its competitors, and whether the customer service is satisfactory. The Gansu Provincial Administration for Industry and Commerce also evaluates the applicant’s key business indicators such as sales volume, local tax contribution, and annual profit increase in the past three years.
Excellent Entrepreneur Award (awarded to our chairman of the board of directors, Mr. Zhanchang Xin)
China National Petroleum Corporation and China Chemical Industry Federation jointly reviewed the qualification of Mr. Zhanchang Xin based on the following standards, which include, but are not limited to: the Company’s R&D capacity, the Company’s annual profit increase in the past five years, the Company’s major products, and the Company’s local and national tax contributions.
Gansu Province “Specialized New Technology” Enterprise
According to the “Guiding Opinions on Promoting the Development of Specialized New Technology Enterprises” and “Plans on Promoting the Development of Small and Medium-sized Enterprises” provided by the Ministry of Industry and Information Technology (the “MIIT”), the Gansu Provincial Department of Industry and Information Technology has the authority to award the “Specialized New Technology Enterprise” status to enterprises with the following qualifications, which include, but are not limited to: good operating status, complete and organized financial management system, high-tech industrial products encouraged by the local and central governments, high average annual growth rate of net profit (no less than 10%), low asset-liability ratio (less than 70%), high level of proficiency in business operation and management, high R&D capacity, high product quality, safe manufacturing environment, high financial credit, and high social credit.
Strategic Emerging Growth Exemplar Enterprise
According to the “Management Measures for the Identification and Assessment of Key Enterprises in Strategic Emerging Industries in Gansu Province” provided by the Provincial Development and Reform Commission, the Commission has the authority to award the Strategic Emerging Growth Exemplar Enterprise status to local enterprises with the following qualifications, which include, but are not limited to: well-known brand name within certain industry, high business growth, high contribution to local tax revenue, market competitiveness, and future development potential.
Little Giant Enterprise of Chengdu City Award
According to the “Notice for Carrying out the Cultivation of High-Growth SMEs and Small Giant Enterprises in 2018” (Enterprise Division of Sichuan Provincial Economic and Information Commission [2018] No. 136) issued by the Sichuan Provincial Economic and Information Commission and Sichuan Provincial SME Bureau, the Chengdu City Economic and Information Commission is authorized to award the “Little Giant Enterprise of Chengdu City” Award according to the following qualifications, which include, but are not limited to: sizes of the applicants’ manufacturing capabilities, annual profits, tax contributions, and annual business income.
Petroleum and Chemical Industry “Specialized and Innovative” Small to Medium Enterprises (the “SME”) Award
According to the “Measures for the Recognition of Petroleum and Chemical Industry “Specialized and Innovative” SMEs Award” and “Opinions on Promoting the Healthy Development of SMEs” issued by the General Office of the CPC Central Committee and General Office of the State Council, the China Petroleum and Chemical Industry Federation and China SME Development Committee are authorized to award Petroleum and Chemical Industry “Specialized and Innovative” SME Enterprises Award according to the following qualifications, which include, but are not limited to: well-known brand name within petroleum and chemical industry, strong R&D capacities, specialization and expertise in certain market segments, market competitiveness, ownership of certain highly competitive IPs, and management efficiencies.
71
Chengdu City’s Unicorn Enterprise Award
According to the “Notice of the Chengdu City Municipal New Economy Committee on Cultivating New Economic Enterprise 2018” (Chengdu City Municipal New Economy Development Committee [2018] No. 247) issued by the Chengdu City Municipal New Economy Committee, the Chengdu City Municipal New Economy Committee, with the help of qualified industrial experts and financial experts, is authorized to award the “Chengdu City’s Unicorn Enterprise”. Such award is based on, among other things, investment qualifications, market capital estimation, and annual net income.
Sichuan Province “Specialized and Innovative” SME Award
According to the “Proposal on Cultivating Specialized and Innovative SMEs” and the “Notice on Carrying Out the Proposal of Cultivating Specialized and Innovative SMEs”, Sichuan Province Economic and Information Technology Commission is authorized to award the Sichuan Province “Specialized and Innovative” SME Award according to the following qualifications, which include, but are not limited to: annual business income, annual profits, tax contributions, accounting credibility, social credits, and bank credits.
Sichuan Province “High-growth” SME Award
Sichuan Province Economic and Information Technology Commission is authorized to award the Sichuan Province “High-growth” SME Award based on the applicant’s annual business income in the past two years.
Suzhou District “Tax Contribution Award” for 2019
Suzhou District Committee and District Government of Jiuquan City are authorized to award the Suzhou District “Tax Contribution Award” for 2019 based on the applicant’s overall tax contributions in 2019.
2020 Gansu Provincial Technology Innovation Model Enterprise
Gansu Province Industry and Information Technology Commission and Gansu Province Department of Finance are authorized to award “2020 Gansu Provincial Technology Innovation Model Enterprise” based on the applicant’s annual business income, contribution to innovation evidenced by commercial application, credit worthiness, production scales, and others.
Jiuquan Leading Talent for Years 2020 to 2022
Government of Jiuquan City and Jiuquan Municipal Committee of the Communist Party of China jointly reviewed the qualification of Mr. Zhanchang Xin based on the following standards, which include, but are not limited to: a candidate’s achievement and leadership in their profession, professional ethics, and ability to create economic value and social value through their work.
Excellent Entrepreneur Award (awarded to our chairman of the board of directors)
Government of Economic and Technological Development Zone of Jiuquan City reviewed the qualification of Mr. Zhanchang Xin based on the following standards, which include, but are not limited to: a candidate’s entrepreneurship and ability to lead their company with advanced management philosophy, and the annual income, profits, and status of regulatory compliance of those companies under the candidate’s leadership.
72
Qualifications
Qualifications |
|
Individual |
|
Agency |
|
Issue |
|
Expiration |
National Permit for Industrial Products Manufacturers |
|
Moshangfa |
|
Gansu Provincial Bureau of Quality and Technical Supervision |
|
January 2018 |
|
August 5, 2026 (1) |
Production Permit for Disinfection Product Manufacturers |
|
Ahan |
|
Gansu Provincial Health and Family Planning Commission |
|
June 2017 |
|
June 14, 2025 (2) |
China’s High-tech Enterprise Certificate |
|
Gansu QLS |
|
Gansu Provincial Department of Science and Technology, Gansu Provincial Department of Finance, Gansu Provincial Department of Taxation, Gansu Provincial Local Taxation Bureau |
|
October 2017 |
|
October 2026 (3) |
Pollutant Discharge Permit |
|
Gansu QLS |
|
Jiuquan City Environmental Protection Bureau |
|
December 2017 |
|
December 28, 2025 (4) |
Gansu Province Fertilizer Official Registration Certificate |
|
Moshangfa |
|
Gansu Provincial Agriculture and Animal Husbandry |
|
December 2017 |
|
December 31, 2022 |
Pharmaceutical Production License |
|
Gansu QLS |
|
Gansu Provincial Food and Drug Administration |
|
August 2018 |
|
December 29, 2025 (5) |
Fertilizer Registration Certificate for Xiongguan® Organic Fertilizer |
|
Moshangfa |
|
Ministry of Agriculture and Rural Affairs |
|
May 2020 |
|
May 2025 |
Fertilizer Registration Certificate for Xiongguan® Organic-Inorganic Compound Fertilizer |
|
Moshangfa |
|
Ministry of Agriculture and Rural Affairs |
|
May 2020 |
|
May 2025 |
Notes:
| (1) | The original permit was valid until September 20, 2021. This permit was renewed on September 27, 2021. |
| (2) | The original permit was valid until May 31, 2021. This permit was renewed on June 15, 2021. |
| (3) | The certificate was valid for three years. Gansu QLS’s renewal request was approved in October 2023. |
| (4) | The original permit was valid for three years. Gansu QLS’s renewal request was approved in February 2021. |
| (5) | The original license expired in February 2021. Gansu QLS’s renewal request was approved in February 2021. |
Intellectual Property
Protection of intellectual property is a strategic priority for the WFOE and the VIE and its subsidiaries’ business. The WFOE and the VIE and its subsidiaries rely on a combination of patent, trademark and trade secret laws, as well as confidentiality agreements, to establish and protect the WFOE and the VIE and its subsidiaries’ proprietary rights. The WFOE and the VIE and its subsidiaries do not rely on third-party licenses of intellectual property for use in their business.
Gansu QLS currently holds 21 patents in China. Gansu QLS’s current Chinese issued patents expire at various times from 2026 through 2029. Chengdu QLS currently holds eight patents in China, all of which expire in 2029. Gansu QLS currently has two patent applications pending in China. Moshangfa currently has three patent applications pending in China. Our WFOE and VIE and its subsidiaries have exclusive rights to utilize the processes issued patent rights within the valid term. As for other products of the WFOE and the VIE and its subsidiaries and the related manufacturing processes, since the technology information has been published to public domain by national or local product standard, our WFOE and VIE and its subsidiaries are able to utilize such technology information without need to obtain any patent license. And the WFOE and the VIE and its subsidiaries do not violate existing patent rights of any other party.
73
The following table sets forth a brief description of the Company’s issued and pending patents in China, including their respective publication numbers, application filing date, issue date, expiration date and title.
Patent Number |
|
File Date |
|
Issue Date |
|
Expiration |
|
Title |
|
Status |
ZL 200410030776.4 |
|
April 9, 2004 |
|
October 25, 2006 |
|
October 25, 2026 |
|
Purification, thin-film coating and inclusion technology for the manufacturing of Gan Di Xin®. |
|
Effective |
ZL 201521133480.5 ** |
|
December 30, 2015 |
|
June 22, 2016 |
|
June 22, 2026 |
|
A dust removal process. |
|
Effective |
ZL 201521133504.7 ** |
|
December 30, 2015 |
|
August 24, 2016 |
|
August 24, 2026 |
|
A device for processing oxytetracycline residue. |
|
Effective |
ZL 201521129906.X ** |
|
December 31, 2015 |
|
June 29, 2016 |
|
June 29, 2026 |
|
A treatment system for waste-water residues. |
|
Effective |
ZL 201521133522.5 ** |
|
December 30, 2015 |
|
August 10, 2016 |
|
August 10, 2026 |
|
Double-effect concentrator. |
|
Effective |
ZL 201621459387.8 ** |
|
December 28, 2016 |
|
July 7, 2017 |
|
July 7, 2027 |
|
A new type of oxytetracycline fermenter. |
|
Effective |
ZL 201621454988.X ** |
|
December 28, 2016 |
|
July 7, 2017 |
|
July 7, 2027 |
|
A traditional Chinese medicine extracting device for ulcerative colitis treatments. |
|
Effective |
ZL 201621464545.9 ** |
|
December 28, 2016 |
|
August 25, 2017 |
|
August 25, 2027 |
|
An insecticide spraying device for vegetables. |
|
Effective |
201822114750.8** |
|
December 17, 2018 |
|
December 6, 2019 |
|
December 6, 2028 |
|
An oxytetracycline residue neutralizer. |
|
Effective |
201822114757.X** |
|
December 17, 2018 |
|
December 6, 2019 |
|
December 6, 2028 |
|
An oxytetracycline crystallization mother liquor retriever. |
|
Effective |
ZL 201920537937.0 ** |
|
April 19, 2019 |
|
March 10, 2020 |
|
April 19, 2029 |
|
A Chinese medicine extraction device |
|
Effective |
ZL 201920726585.3 ** |
|
May 12, 2019 |
|
March 10, 2020 |
|
May 21, 2029 |
|
A medicine grinding machine |
|
Effective |
201921243962.4** |
|
August 2, 2019 |
|
April 14, 2020 |
|
August 2, 2029 |
|
An air dying system for oxytetracycline production |
|
Effective |
201921244754.6** |
|
August 2, 2019 |
|
April 14, 2020 |
|
August 2, 2029 |
|
A dryer for organic fertilizers production |
|
Effective |
ZL 201921607930.8** |
|
September 25, 2019 |
|
June 9, 2020 |
|
September 24, 2029 |
|
A centrifuge for extracting heparin sodium with high speed |
|
Effective |
ZL201921596241.1** |
|
September 24, 2019 |
|
July 24, 2020 |
|
September 23, 2029 |
|
A centrifuge for extracting heparin sodium with low speed |
|
Effective |
ZL201921610887.0** |
|
September 26, 2019 |
|
July 7, 2020 |
|
September 25, 2029 |
|
A mixing device for preparing ground pig lung mixture |
|
Effective |
ZL201921595043.3 |
|
September 24, 2019 |
|
June 9, 2020 |
|
September 23, 2029 |
|
A multi-layer separation device for extracting heparin sodium |
|
Effective |
ZL201921596218.2 |
|
September 24, 2019 |
|
June 9, 2020 |
|
September 23, 2029 |
|
A reactor heater for extracting heparin sodium |
|
Effective |
ZL201921606629.5 |
|
September 25, 2019 |
|
June 9, 2020 |
|
September 24, 2029 |
|
A filter and separation device for extracting heparin sodium |
|
Effective |
ZL201921610868.8 |
|
September 26, 2019 |
|
June 9, 2020 |
|
September 25, 2029 |
|
A processing device for extracting heparin sodium from pig intestines with high efficiency |
|
Effective |
ZL201921606671.7 |
|
September 25, 2019 |
|
June 9, 2020 |
|
September 24, 2029 |
|
A separation device for extracting heparin sodium |
|
Effective |
202222729749.2** |
|
October 24, 2022 |
|
February 21, 2023 |
|
October 23, 2032 |
|
An integrated pretreatment system for raw material pharmaceutical production wastewater |
|
Effective |
20222728433.1** |
|
October 24, 2022 |
|
March 10, 2023 |
|
October 23, 2032 |
|
A brine cooling system supporting the liquid tank of the oxytetracycline extraction process |
|
Effective |
20222282670** |
|
February 28, 2023 |
|
April 18, 2023 |
|
February 7, 2033 |
|
A crushing device for the production and preparation of traditional Chinese medicine |
|
Effective |
2022230358383** |
|
February 28, 2023 |
|
April 21, 2023 |
|
February 7, 2033 |
|
A test bench for biopharmaceutical laboratories |
|
Effective |
2022229708935** |
|
February 28, 2023 |
|
April 21, 2023 |
|
February 7, 2033 |
|
A crusher for biopharmaceuticals |
|
Effective |
2022230344003** |
|
February 28, 2023 |
|
April 28, 2023 |
|
February 7, 2033 |
|
An easy-to-clean stirring device for biopharmaceuticals |
|
Effective |
201921244766.9** |
|
August 2, 2019 |
|
August 14, 2020 |
|
August 1, 2029 |
|
A filter for producing liquor rice extract. |
|
Effective |
201822121674.3 |
|
December 18, 2018. |
|
|
PENDING |
|
A sterilization filter for oxytetracycline fermentation liquid |
|
|
|
201910713025.9 |
|
August 2, 2019 |
|
|
PENDING |
|
Processing technology for bio-organic fertilizer. |
|
|
|
201910713063.4 |
|
August 2, 2019 |
|
|
PENDING |
|
Processing technology for potassium humate flush fertilizers |
|
|
|
201910712340.X |
|
August 2, 2019 |
|
|
PENDING |
|
Processing technology for sunflower organic fertilizers. |
|
|
* |
Patent expiration dates are routinely subject to dispute in patent infringement actions. No assurance can be given that third parties infringing the WFOE and the VIE and its subsidiaries’ patents will not dispute the expiration dates of their patents or that they will be successful in defending against such disputes. |
** |
Utility model patents |
Gansu QLS currently has seven trademarks in China. Gansu QLS’s current trademarks in China expire at various times from 2020 through 2030. Moshangfa currently has two trademarks in China. Chengdu QLS currently has one trademark in China. Gansu QLS, Moshangfa and Chengdu QLS currently does not have any trademarks applications pending in China.
74
Trademark Number |
|
Issue |
|
Expiration |
|
Trademark Title |
6084468 |
|
February 14, 2010 |
|
February 13, 2030 |
|
祁连山 (Qilian Shan)** |
3792776 |
|
March 14, 2006 |
|
March 13, 2026 |
|
甘帝欣 (Gan Di Xin) |
13679211 |
|
March 7, 2015 |
|
March 6, 2025 |
|
沙门果 (Shamen Guo) |
13679213 |
|
March 7, 2015 |
|
March 6, 2025 |
|
甘帝康 (Gan Di Kang) |
13679212 |
|
March 7, 2015 |
|
March 6, 2025 |
|
阿含 (Ahan) |
22534753 |
|
April 7, 2018 |
|
April 6, 2028 |
|
阿含斋 (Ahan Zhai) |
20810590 |
|
September 21, 2017 |
|
September 20, 2027 |
|
陌上发 (Moshangfa) |
10336012 |
|
February 28, 2013 |
|
February 27, 2033 |
|
雄关 (Xiongguan)*** |
27770670 |
|
November 14, 2018 |
|
November 13, 2028 |
|
猪小常 (Zhuxiaochang) |
37873604 |
|
March 7, 2020 |
|
March 6, 2030 |
|
祁連國際 (Qilian Guoji) |
* |
Trademark expiration dates are routinely subject to dispute in trademark infringement actions. No assurance can be given that third parties infringing the WFOE and the VIE and its subsidiaries’ trademark will not dispute the expiration dates of their trademarks or that they will be successful in defending against such disputes. |
** |
The original expiration date of this trademark was on February 13, 2020. Gansu QLS submitted a trademark renewal request for 祁连山 (Qilian Shan) to the China National Intellectual Property Administration (“CNIPA”) in July 2019. On February 14, 2020, the CNIPA approved the renewal request for 祁连山 (Qilian Shan) and Gansu QLS obtained a registration certificate for the renewal of such trademark. |
*** |
The original expiration date of this trademark was on February 17, 2023. Moshangfa submitted a trademark renewal request for 雄关 (Xiongguan) to the CNIPA in May 2022. On February 28, 2023, the CNIPA approved the renewal request for 雄关 (Xiongguan) and Moshangfa obtained a registration certificate for the renewal of such trademark. |
Research and Development
The WFOE and the VIE and its subsidiaries established a research and development department in 2015, with its Nationally Recognized Enterprise Technology Center status assessed and approved by the Gansu Provincial Industry and Information Commission, the Gansu Provincial Development and Reform Commission, the Gansu Provincial Science and Technology Department, Gansu Provincial Finance Department, the Gansu Provincial State Taxation Bureau, and the Gansu Provincial Local Taxation Bureau in December 2015. The Nationally Recognized Enterprise Technology Center status is a competitive honor awarded by Chinese government agencies, and such recognition reflects the Company’s comprehensive strength in technological innovation and robust R&D activities. After years of continued development, the R&D department of the WFOE and the VIE and its subsidiaries has become the core of their technological innovation efforts, dramatically improving their R&D capabilities, enhancing their industry competitiveness, and, we believe, improving the Company’s overall business outlook.
75
R&D Achievements
The research and development activities of the WFOE and the VIE and its subsidiaries are project-based and the number of projects they work on varies annually. As of September 30, 2024, the WFOE and the VIE and its subsidiaries had 17 research and development professionals, three of whom have advanced degrees in Medicine and Traditional Medicine. The director of the R&D department, Mr. Zhanchang Xin, is also the chairman and legal representative of Gansu QLS. Under Mr. Zhanchang Xin’s leadership, the R&D department of the WFOE and the VIE and VIE’s subsidiaries contributed to the following recent accomplishments:
At the beginning of October 2016, Ahan established a TCMD research project borrowing ideas from medicines of Chinese Dai ethnicities. This research project created Ahan’s innovative Ahan® antibacterial paste for the treatment of psoriasis, neurodermatitis and other skin ailments. Ahan has completed all necessary filing procedure as required by PRC laws and the Ahan® Antibacterial Paste has been on the Chinese market since November 2017.
Gansu QLS has invested approximately RMB 1,000,000 for its mutational breeding experiment of oxytetracycline-producing bacteria. Gansu QLS has selected and bred superior strains and has successfully increased the average fermentation unit of oxytetracycline from 32000 U/ml to 35000 U/ml and beyond, thereby greatly improving the oxytetracycline product yield while reducing the WFOE and the VIE and its subsidiaries’ production cost.
R&D Development Plan
The WFOE and the VIE and its subsidiaries intend to continue focusing on R&D to improve the quality of their products. The WFOE and the VIE and its subsidiaries also intend to develop new products and exploit unmet market demands in the near future.
With the aid of advanced production technology and manufacturing facilities, Gansu QLS’s production capacity of oxytetracycline has reached its industrial upper limit. After thorough research and investigation, the R&D department of the WFOE and the VIE and VIE’s subsidiaries has concluded that only through improving the quality of oxytetracycline strains can they lead to industrial break-through of oxytetracycline production capacity. Additionally, Gansu QLS has been recently focusing on developing nitrofurantoin enteric-coated tablets and vitacoenzyme as new products to be offered in the future.
Regarding its fertilizer, Moshangfa will continue utilizing its advantage of abundant local raw material sources and expect to develop liquid-flushing fertilizer, crops fertilizer and pharmaceutical fertilizer that fulfills the agricultural production demands of various crops.
The R&D department will further develop Ahan® antibacterial paste to suit different skin types of customers and appeal to customers from different ethnic and cultural regions in China. In addition, Ahan will create more products so as to provide its customers with more choices. Pursuant to the Regulations on Sanitary and Safety Evaluation of Disinfectant Products issued by the National Health and Family Planning Committee on June 27, 2014, Ahan shall file the sanitary and safety evaluation reports of its modified Ahan® antibacterial paste to the provincial health administrative branch before such product can be introduced to the Chinese market. The local authorities shall publish the filing information excluding commercial secrets. Ahan’s filing procedures do not involve approval from the relevant authorities, and enterprises are not required to obtain any certificate in order to complete the filing procedure. Ahan has completed the required filing process for its current version of Ahan® antibacterial paste in May 2017. Ahan will also update the sanitary and safety evaluation report and file the updated report to the competent authorities for any modified Ahan antibacterial paste in the future. Currently, other variants of Ahan® antibacterial paste are also under research and development.
Since the beginning of 2022, Gansu QLS, in an effort to improve the product quality of oxytetracycline raw materials, has implemented the “Quality Improvement Process for Oxytetracycline Injections” project. Gansu QLS has conducted research on various areas based on the aforementioned project, including but not limited to, Vi-zyme Raw Materials, Nitrofurantoin Enteric-Coated Tablets, Pentyverine Citrate Tablets, and others. The Company believes that such research efforts will continue to improve the Company’s overall product quality, reduce production costs, enhance the Company’s independent research and development capabilities, and help develop new products. The Company believes that such research efforts will enhance its market competitiveness.
Facilities
The VIE and its subsidiaries own our premises located at Jiuquan Economic and Technological Development Zone (formerly named No. 2 Dadeli Road, Nanjiao Industrial Park), Jiuquan City, Gansu, China. The VIE and its subsidiaries use the premises not only for corporate and administrative purposes, but also for manufacturing the oxytetracycline products and licorice TCMD products.
76
The VIE and its subsidiaries also currently own the following land use rights and properties for its operations:
|
|
|
|
|
|
Area in |
|
|
Land Use Right |
|
|
|
|
|
Square |
|
|
Holder |
|
Address |
|
Legal Use |
|
Meters |
|
Terms of Use |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No. 71, Jiujindong Road, Suzhou, Jiuquan, Gansu |
|
Industrial |
|
40456.33 |
|
Until June 28, 2057 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No. 71, Jiujindong Road, Suzhou, Jiuquan, Gansu |
|
Industrial |
|
29519.37 |
|
June 28, 2057 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No.2, Da Deli Road, Industrial Park, Jiuquan, Gansu |
|
Industrial |
|
30610.14 |
|
Until January 7, 2043 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No.2, Da Deli Road, Industrial Park, Jiuquan, Gansu |
|
Industrial |
|
24464.59 |
|
Until January 7, 2043 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No.2, Da Deli Road, Industrial Park, Jiuquan, Gansu |
|
Industrial |
|
61972.6 |
|
Until January 7, 2043 |
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu |
|
Industrial |
|
14008.00 |
|
Until January 1, 2059 |
Property Title |
|
|
|
|
|
Area in Square |
Holder |
|
Address |
|
Legal Use |
|
Meters |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No. 71, Jiujin East Road, Suzhou District, Jiuquan City, Gansu |
|
Industrial |
|
20243.26 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No. 71, Jiujin East Road, Suzhou District, Jiuquan City, Gansu |
|
Industrial |
|
11836.27 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No.2, Da Deli Road, Industrial Park, Jiuquan, Gansu |
|
Industrial |
|
1669.33 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No.2, Da Deli Road, Industrial Park, Jiuquan, Gansu |
|
Industrial |
|
63.44 |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
No.2, Da Deli Road, Industrial Park, Jiuquan, Gansu |
|
Industrial |
|
9845.25 |
Chengdu Qilianshan Biotechnology Co.,Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu* |
|
Industrial |
|
1082.84 |
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu* |
|
Industrial |
|
664.08 |
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu* |
|
Industrial |
|
168.34 |
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu* |
|
Industrial |
|
738.09 |
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu* |
|
Industrial |
|
40.77 |
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
No. 8, Yujian Road, Linqiong Town Industrial Park, Qiong Lai City, Chengdu* |
|
Industrial |
|
1130.03 |
* |
Chengdu Qilianshan Biotechnology Co., Ltd. obtained its current property from judicial auctions. It has yet to receive a property ownership certificate for this property. Chengdu Qilianshan Biotechnology Co., Ltd. can still legally use this property even without a property ownership certificate. |
In addition, as of September 30, 2024, the WFOE and the VIE and its subsidiaries leased two properties as employee housing with a total area of approximately 226 square meters, all of which are in Sichuan Province. As of September 30, 2024, we had leased employee dormitories with a total area of approximately 226 square meters, all of which are in Sichuan Province. As of September 30, 2024, Chengdu QLS leases a production facility from a third-party lessor with an aggregate area of approximately 6,000 square meters for an annual rent of RMB200,000 (US$30,969), which Chengdu QLS sublet to Rugao for an annual rent of RMB200,000 (US$30,969). We believe that our, the VIE and its subsidiaries’ current facilities are adequate and suitable for their operations, but we or our affiliated entities may seek additional space as needed to accommodate future growth.
77
Legal Proceedings
We and our affiliated entities are currently not a party to any material legal or administrative proceedings. We and our affiliated entities may from time to time be subject to various legal or administrative claims and proceedings arising in the ordinary course of business. Litigation or any other legal or administrative proceeding, regardless of the outcome, is likely to result in substantial cost and diversion of our resources, including our management’s time and attention.
Regulation
This section sets forth a summary of the most significant rules and regulations that affect the WFOE and the VIE and its subsidiaries’ business activities in China.
PRC Laws and Regulations on Pharmaceutical Manufacture
General Regulations Relating to Pharmaceutical Industry
The pharmaceutical industry in China is highly regulated. The WFOE and the VIE and its subsidiaries operate business in China under a legal regime consisting of the National People’s Congress, which is the country’s highest legislative body, the State Council, which is the highest authority of the executive branch of the PRC central government, and several ministries and agencies under its authority, including the State Administration of Market Regulation (“SAMR”), the NMPA, the MIIT, and their respective local offices.
As a developer and producer of medicinal products, we and our affiliated entities are mainly subject to regulation and oversight by the NMPA and its provincial and local branches. These regulations set forth detailed rules with respect to pharmaceutical companies in China. The Drug Administration Law of the PRC, or the Drug Administration Law, which was first promulgated in 1984 and last amended on August 26, 2019, provides the basic legal framework for the administration of the production and sale of pharmaceutical products in China and covers the manufacturing, distribution, packaging, pricing and advertising of pharmaceutical products. We and our affiliated entities are also subject to other PRC laws and regulations that are applicable to business operators, manufacturers and distributors in general.
Pharmaceutical Marketing Permit Holders
The last amended version of the Drug Administration Law, which was promulgated on August 26, 2019 and took effect on December 1, 2019, adopts the drug marketing authorization holder system and further tightens and expands supervision of drugs to cover the entire processes, including the research and development, production, sale, use and management processes of drugs. Pharmaceutical marketing permit holders shall mean enterprises or pharmaceutical research and development institutes which have obtained a pharmaceutical registration certificate. Pharmaceutical marketing permit holders shall be liable for non-clinical study, clinical trial, manufacturing and business operation, post-market launch study, monitoring, reporting and handling of adverse reactions of the pharmaceuticals. Pharmaceutical marketing permit holders may engage in pharmaceutical manufacturing on their own, and may entrust a pharmaceutical manufacturing enterprise to manufacture. Pharmaceutical marketing permit holders engaging in manufacturing pharmaceutical on their own shall obtain a pharmaceutical manufacturing permit; for entrusted manufacturing, the pharmaceutical marketing permit holder shall entrust a qualified pharmaceutical manufacturing enterprise. The pharmaceutical marketing permit holder and the entrusted manufacturing enterprise shall enter into an entrustment agreement and a quality agreement, and strictly perform the obligations agreed in the agreements. Pharmaceutical marketing permit holders may sell on their own the pharmaceuticals for which they have obtained a pharmaceutical registration certificate, or entrust a pharmaceutical business enterprise to sell. Pharmaceutical marketing permit holders engaging in pharmaceutical retail activities shall obtain a pharmaceutical business permit.
Pharmaceutical Manufacturing Permit
According to the Drug Administration Law and its implementation rules, no pharmaceutical products can be produced in the PRC without a Pharmaceutical Manufacturing Permit. A local pharmaceutical manufacturer must obtain a Pharmaceutical Manufacturing Permit from one of the NMPA’s provincial level branches in order to commence production of pharmaceutical products. Prior to granting such license, the relevant government authority will inspect the manufacturer’s production facilities, and decide whether the sanitary conditions, quality assurance system, management structure and manufacturing equipment have met the standards and criteria. Among other things, such a permit sets forth the permit number, the name, legal representative and registered address of the enterprise, the site and scope of production, issuing institution, date of issuance and effective period.
78
Each Pharmaceutical Manufacturing Permit issued to a pharmaceutical manufacturing enterprise is effective for a period of five years. Any enterprise holding a Pharmaceutical Manufacturing Permit is subject to review by the relevant regulatory authorities on an annual basis. Such enterprise is required to apply for renewal of such permit within six months prior to its expiry and will be subject to re-assessment by the issuing authorities in accordance with the then effective legal and regulatory requirements for the purposes of such renewal.
Our PRC entity Gansu QLS has obtained a Pharmaceutical Manufacturing Permit, which expires on December 29, 2025 and allows it and its subsidiaries to sell all types of pharmaceutical products they are currently selling.
Registration and Approval of Medicine
Pursuant to the PRC Provisions for Drug Registration, a medicine must be registered and approved by the NMPA before it can be manufactured and sold. The registration and approval process requires the manufacturer to submit to the NMPA a registration application containing detailed information concerning the efficacy and quality of the medicine and the manufacturing process and the production facilities the manufacturer expects to use. This process generally takes two to five years and could be longer, depending on the nature of the medicine under review, the quality of the data provided and the workload of the NMPA.
The valid term of a drug approval number is five years. To continue its drug production, the applicant shall submit a re-registration application six months prior to the expiry date. When making re-registration of a drug, the relevant data shall be submitted according to the provisions of the NMPA. If no application for the re-registration of a drug is made upon expiration of the valid term, or the application fails to comply with the provisions on re-registration of the NMPA upon review, the drug approval number shall be withdrawn.
Gansu QLS has obtained the approval numbers for its products and completed the re-registration procedures to ensure each approval number is valid.
National Drug Standard
The national drug standards in China include the Chinese Pharmacopoeia, drug registration standard, and other drug standards published by the NMPA, of which the contents consist of technical requirements, testing methods and manufacturing processes, etc. Pharmaceutical manufacturers shall be subject to the drug registration standard, which refers to the specified specifications of the applied drug approved by the NMPA and shall not be lower than those required by the Chinese Pharmacopoeia.
The Chinese Pharmacopoeia (Latest Version 2015) became effective on December 1, 2015 and has been codified into law with the purpose of providing clear guidance on the pharmaceutical products manufacturing process. The Chinese Pharmacopoeia applies to all aspects of the pharmaceutical products manufacturing process including research and development, production (import), management, use and supervision of pharmaceutical products. It provides standard language that can be used by pharmaceutical companies to draft description, identification, processing, assay, property and flavor, meridian tropism, actions, indications, storage, administration and dosage, precautions and warnings of pharmaceutical products.
Our pharmaceutical products have been issued approval numbers and completed registration procedures, which certifies that the WFOE and the VIE and its subsidiaries’ pharmaceutical products comply with the national drug standards.
Continuing NMPA Regulation
Pharmaceutical manufacturers in China are subject to continuing regulation by the NMPA. If the labeling or manufacturing process of an approved medicine is significantly modified, a new pre-market approval or pre-market approval supplement will be required by the NMPA. Pursuant to the Drug Administration Law, the WFOE and the VIE and its subsidiaries should also be subject to periodic inspection and safety monitoring by the NMPA to determine compliance with regulatory requirements.
If the NMPA approves a medicine, it will issue a new medicine certificate to the manufacturer and impose a monitoring period not more than five years. During the monitoring period, the NMPA will monitor the safety of the new medicine, and will neither accept new medicine certificate applications for an identical medicine by another pharmaceutical company, nor approve the production or import of an identical medicine by other pharmaceutical companies. As a result of these regulations, the holder of a new medicine certificate has the exclusive right to manufacture the new medicine during the monitoring period.
79
The NMPA has a variety of enforcement actions available to enforce its regulations and rules, including fines and injunctions, recall or seizure of products, the imposition of operating restrictions, partial suspension or complete shutdown of production and criminal prosecution.
PRC Laws and Regulations on Pharmaceutical Product Packages
Insert Sheet and Labels of Products
According to the Provisions for the Administration of the Insert Sheets and Labels of Drugs, which became effective on June 1, 2006, the insert sheets and labels of drugs should be reviewed and approved by the NMPA. A drug insert sheet should include the scientific data, conclusions and information concerning drug safety and efficacy in order to direct the safe and rational use of drugs. The inner label of a drug should bear information such as the drug’s name, indication and function, strength, dose and usage, production date, batch number, expiration date and drug manufacturer; and the outer label of a drug should indicate information such as the drug’s name, ingredients, description, indication or function, strength, dose and usage and adverse event. As the WFOE and the VIE and its subsidiaries’ pharmaceutical products have been issued approval numbers and completed registration procedures, the insert sheets and labels of the pharmaceutical products have been reviewed and approved.
Use of Pharmaceutical Product Packages
Pharmaceutical products packages must, in accordance with applicable regulations, be labeled and have an instruction booklet attached to them. The name of the drug, its ingredients, specifications, the manufacturing enterprise, approval number, product batch number, date of production, expiry date, suitability for symptoms or main function, methods of use, dosage, contraindications, side-effects and points to note must be clearly indicated on the label or in the instruction booklet. The labels of narcotic drugs, psychotropic drugs, poisonous drugs, radioactive drugs, drugs for external use only and non-prescription drugs must bear the prescribed mark. Drug packaging must comply with the national and professional standards. If no national or professional standards are available, an enterprise can formulate its own standards and use them in packing after obtaining the approval of the food and drug administration bureau at provincial level. Such enterprise must reapply to the relevant authorities if it needs to change its own packaging standards. Pharmaceuticals that have not developed or received approval for product packages and labels must sell or trade its drugs in China (except for drugs for the military).
Currently, all of our marketed products meet the packaging requirements.
Drug Packaging Manufacturing
On June 18, 2004, the Ministry of Health promulgated the Administration Rules for Packaging Material and Containers Directly Contacting Drugs, which stipulates that enterprises producing packaging material and containers directly containing drugs shall apply for registration after completion of trial work and re-registration 6 months before the expiration of the registration certificate.
Moshangfa has registered for the drug containers it produces and obtained the re-registration certificate.
PRC Laws and Regulations on Advertising of Drug Products
Pursuant to the Measures for the Examination of Drug Advertisements, which came into effect in 2007 and was amended on December 21, 2018, an enterprise seeking to advertise its drugs must apply for an advertisement approval code. The valid term of an advertisement approval code for pharmaceuticals is one year. The content of an approved advertisement may not be altered without prior approval. Where any alteration to the advertisement is needed, a new advertisement approval code shall be obtained. As of the date of this annual report, we and our affiliated entities have not advertised for the WFOE and the VIE and its subsidiaries’ pharmaceutical products, thus not needing to apply for any approval.
80
PRC Laws and Regulations on Disinfectant Products
The SCNPC promulgated the Law of the PRC on Prevention and Treatment of Infectious Disease on February 21, 1989, which took effect on September 1, 1989, and revised it on August 28, 2004 as well as June 29, 2013. Pursuant to the Law of the PRC on Prevention and Treatment of Infectious Disease, disinfectant products used for prevention and treatment of infectious diseases shall measure up to the sanitary standards and specifications of the State. Manufacturers of disinfectant products and disinfectant products to be manufactured for prevention and treatment of infectious diseases shall be subject to examination and approval by the health administration department under the people’s governments at or above the provincial level.
Ahan has obtained the Sanitary License for Manufactures of Disinfectant Products for the Ahan® antibacterial paste it produces.
According to the Regulations on Sanitary and Safety Evaluation of Disinfectant Products issued by the National Health and Family Planning Committee on June 27, 2014, our Ahan® antibacterial paste is categorized as Type II disinfectant, which is a disinfectant product with medium level risks. Type II disinfectant products’ sanitary and safety evaluation reports shall be filed for record with the provincial health administrative branch before the product is introduced to the market. Pursuant to the Regulations on Sanitary and Safety Evaluation of Disinfectant Products issued by the National Health and Family Planning Committee on June 27, 2014, the Company shall file the sanitary and safety evaluation reports of its modified Ahan® antibacterial paste with the provincial health administrative branch before such product can be introduced to the Chinese market. The local competent authorities shall publish the filing information excluding commercial secrets. The filing procedure does not involve approval from the competent authorities, and enterprises are not required to obtain any certificate in order to complete the filing procedure. Ahan has completed the required filing process for its current version of Ahan® antibacterial paste in June 2017.
PRC Laws and Regulations on Fertilizer Production and Registration
Fertilizer usually refers to organic, inorganic and microbial substances and mixture of substances which offer, maintain or improve the nutritional status, output, quality and stress tolerance (abiotic) of crops or the physical, chemical and biological performance of soils or plants, increase the output and quality of agricultural produce or increase stress resistance of plants.
Production License
In China, producers of chemical fertilizers (which are covered by the catalog of industrial products issued by the State Council) are required to obtain a production license from the Market Regulation Departments at the provincial level (the “Provincial MRDs”). An application for license renewal should be made with the applicable Provincial MRD within six months before such license expires. Pursuant to a series of decisions regarding amendments to the product license administration process promulgated by the State Council in September 2018 as well as its Implementation Notifications promulgated by the Administration of Market Regulation (the “State MRD”) on October 16, 2018, the Provincial MRDs are authorized to assess the qualification of such applicant after receiving its renewal application. The assessment process includes appointing staff members from the Provincial MRD to conduct on-site due diligence and reviewing qualification test reports on the applicant’s industrial products issued by qualified inspection institutions within the last year (the “QT Reports”). The Provincial MRD would typically inform the applicant of the on-site due diligence results in writing within 30 days after the due diligence process is completed. Such process may be waived if the applicant makes a representation in writing that its production process and manufacturing conditions have not been changed since the license was last granted or renewed. If the applicant successfully passes the on-site due diligence process (or such requirement is effectively waived), the Provincial MRD will subsequently request the applicant to submit the QT Reports for review. The Provincial MRD will make a final decision on the renewal application within 60 days from the day accepting the application. If the Provincial MRD decides to grant the renewal, then a renewed Production License for Industrial Products will be sent to the applicant within 10 days after the date of the Provincial MRD decision. If the Provincial MRD decides not to grant the renewal, then it will notify the applicant in writing.
The VIE’s subsidiary, Moshangfa, currently owns a valid Production License for Industrial Products, which will expire on August 5, 2026. Its Production License for Industrial Products covers Moshangfa’s organic-inorganic compound fertilizer, which is subject to the regulations for chemical fertilizers.
81
Fertilizer Registration
Fertilizers cannot be imported, produced, sold, or advertised without prior registration with the competent authorities at a ministerial or provincial level. From a registration prospective, fertilizers can be divided into 3 types:
Exempted from Registration — Fertilizers that have been used for many years domestically and have been established with national or industrial product executive standard are exempted from registration: ammonia sulfate, urea, calcium cyanamide, ammonium phosphate (mono and di), phosphor nitrate, superphosphate, potassium chloride; potassium sulfate, potassium nitrate, ammonium chloride, ammonium bicarbonate, calcium magnesium phosphate, potassium dihydrogen phosphate, single microelement fertilizer, and high concentration compound fertilizer;
Registered with provincial agricultural department — compound fertilizer, formula fertilizer (non foliar fertilizer), refined organic fertilizer, and soil acid regulating agents should be registered with provincial agricultural department and can be only sold within the administration area of the province. If the producer or distributor files a provincially registered fertilizer with the department at another province, the fertilizer can be sold in that province too.
Other Fertilizers — Fertilizers that do not comply with the above two criteria should be registered with the MOA. Fertilizer-pesticide mixtures and the homemade organic fertilizer produced by the farmers are also beyond the scope of fertilizer registration management. Homemade organic fertilizers for self-use purpose and fertilizer-pesticide mixtures are controlled under China’s pesticide registration system.
MOA Decree No. 32, the Administrative Measures of Fertilizer Registration in China by MOA, was published on June 23, 2006. This decree specifies China fertilizer registration obligations, product types/registration types and data requirements. Fertilizer products are regulated by the Decree. Companies are required to register fertilizer products in China prior to importing, manufacturing, selling and using in China.
On November 30, 2017, the MOA issued Order 8 to abolish and revise a series of existing ministerial regulations, of which two sections are an amendment to the current fertilizer registration: removal of temporary registration and broadened acceptance scope of field trial report. On December 29, 2017, the MOA released Announcement 2636, the Service Guide to the Administrative Approval of MOA (Batch 2: Fertilizer Registration and Pesticide Registration), which standardizes administrative procedures and further clarifies data requirements including qualification of applicant, timeline, list of required documents, means of submission and administration fee. Depending on the market circulation stage, fertilizer registrations can be classified into registration, registration renewal (each 5 years) and registration amendment (only applicable to crop range and administrative information). Domestic products and imported fertilizer are subject to different assessment criteria and approval procedures.
Moshangfa’s Xiongguan® Organic Fertilizer and Xiongguan® Organic-Inorganic Compound Fertilizer are Category II fertilizers. Moshangfa has registered these two products with the provincial agricultural department. Moshangfa has obtained Gansu Province Fertilizer Official Registration Certificates, which cover the manufacturing of its compound fertilizers and organic fertilizers. Moshangfa has renewed its Gansu Province Fertilizer Official Registration Certificates once in January 2018, and they will expire in December 2022. Pursuant to the Administrative Measures of Fertilizer Registration, Moshangfa will apply for another renewal six months before such certificates expire. Moshangfa has obtained Fertilizer Registration Certificates for both Xiongguan® Organic Fertilizer and Xiongguan® Organic-Inorganic Compound Fertilizer, which were approved by the PRC Ministry of Agriculture on May 19, 2020 and are valid until May 2025.
Data Requirements of Fertilizer Registration Application
The registration application materials consist of an application form, credential documents, test reports, position paper/ evaluation form, safety data, product executive standard, label samples, enterprise information and product samples. Field trials and one quality inspection test are performed prior to the application and another quality inspection and safety tests will be organized by the Secretariat afterward. Domestic applicants are subject to additional preliminary review and product executive standard filing formality at their provincial department. For imported fertilizers, qualification/identity of overseas producers and business relationship with its domestic agent are particularly reviewed. Technical data and sample requirements may vary depending on product nature, as summarized in the table.
82
An enterprise should submit a renewal application of its registration certificate six months before such registration expires. As provided by the Administrative Measures of Fertilizer Registration in China, our Xiongguan® Organic Fertilizer and Xiongguan® Organic-Inorganic Compound Fertilizer should also register their renewal with the Gansu Province Administration of Agriculture. According to the instructions on fertilizer registrations published by the Gansu Government Services (http://www.gszwfw.gov.cn/art/2019/12/20/art_412266_8414.html), the VIE and its subsidiaries are requested by Gansu Province Administration of Agriculture to provide a product quality inspection report issued by a qualified provincial or national inspection institution with China Metrology Accreditation, along with other procedural documents such as application forms. The Gansu Province Administration of Agriculture is responsible for adjudicating the adequacy of our application materials and making final decisions on approval. Moshangfa submitted all required documentation for renewal and was granted new registration certificates in January 2018. Moshangfa’s new registration certificates will expire in May 2025.
PRC Laws and Regulations on Natural Sausage Casings
The production of natural sausage casing must comply with the national standard Natural Sausage Casings (GB/T 7740-2006) promulgated by the General Administration of Quality Supervision, Inspection and Quarantine (the “AQSIQ”) and the Standardization Administration of the PRC, which provides the definitions, categories, manufacturing requirements, quarantine methods, labels, packages, preservation and transportation of natural sausage casings. According to the circular of the AQSIQ, natural sausage casings shall not be categorized as food, and Chengdu QLS does not need to apply for the Food Production License.
PRC Laws and Regulations on Environmental Protection
The Ministry of Ecology and Environment is responsible for the uniform supervision and control of environmental protection in the PRC. It formulates national environmental quality and discharge standards and monitors the PRC’s environmental system. Ecology and Environment bureaus at the county level and above are responsible for environmental protection within their areas of jurisdiction.
Pursuant to the Law on Environmental Impact Evaluation of the PRC promulgated on October 28, 2002 and effective from September 1, 2003, and later amended on July 2, 2016 and December 29, 2018, manufacturers must prepare and file an environmental impact report setting forth the impact that the proposed construction project may have on the environment and the measures to prevent or mitigate the impact for approval by the relevant PRC government authority prior to commencement of construction of the relevant project. Gansu QLS and its subsidiaries have obtained approval for their environmental impact reports as required.
Pursuant to the Environmental Protection Law of the PRC, or the Environmental Protection Law, promulgated on December 26, 1989 with immediate effect and last revised on April 24, 2014, the environmental protection department of the State Council is in charge of promulgating national standards for environmental protection. The Environmental Protection Law requires any facility that produces pollutants or other hazards to incorporate environmental protection measures in its operations and establish an environmental protection responsibility system. Any entity that discharges pollution must obtain the Pollution Discharging License from the relevant environmental protection authority. Remedial measures for breaches of the Environmental Protection Law include a warning, payment of damages or imposition of a fine. Criminal liability may be imposed for a material violation of environmental laws and regulations that causes loss of property, personal injuries or death.
Pursuant to the Law of the People’s Republic of China on the Prevention and Control of Atmospheric Pollution promulgated by the NPC on September 5, 1987, last amended on October 26, 2018 and effective from September 1, 2000, the environmental protection authorities above the county level are in charge of exercising unified supervision and administration of prevention and control of air pollution. Manufacturers discharging polluted air must comply with applicable national and local standards. Manufacturers discharging polluted air must pay polluted air discharging fees. If a manufacturer emits polluted air exceeding national or local standards, it must correct its action during a prescribed period of time and the manufacturer may be subject to penalties.
83
Pursuant to the Water Pollution Prevention Law of the PRC, which was originally promulgated by the NPC on May 11, 1984 and amended on May 15, 1996, February 28, 2008 and June 27, 2017, effective from January 1, 2018, manufacturers must discharge water pollutants in accordance with national and local standards. If the water pollutants discharged exceed national or local standards, the manufacturer would be subject to fines amounting to 0.1-1 million RMB. In addition, the environmental protection authority has the right to order such manufacturer to correct their actions by reducing the amount of discharge during a stipulated period of time by restricting or suspending their operations. If the manufacturer fails to correct its action at the expiration of the stipulated period, the environmental protection authority may, subject to approval by the relevant level of the PRC government, shut down the manufacturer.
Gansu QLS has obtained its Pollutant Discharging Permit valid from December 29, 2020 to December 28, 2025, and Chengdu QLS has obtained the Pollutant Discharging Permit valid from May 30, 2019 to May 29, 2022, as required by the Air Pollution Prevention Law of the PRC as well as the Water Pollution Prevention Law of the PRC.
PRC Laws and Regulations on Foreign Investment
Investment in the PRC by foreign investors and foreign-invested enterprises shall comply with the Catalogue for the Guidance of Foreign Investment Industries (2017 Revision) (the “Catalogue”), which was last amended and issued by MOFCOM and NDRC on June 28, 2017 and became effective since July 28, 2017, and the Negative List, which came into effect on July 23, 2020. The Catalogue and the Negative List contain specific provisions guiding market access for foreign capital and stipulate in detail the industry sectors grouped under the categories of encouraged industries, restricted industries and prohibited industries. Any industry not listed on the Negative List is a permitted industry unless otherwise prohibited or restricted by other PRC laws or regulations. The pharmaceutical industry, except for the production of confidential prescription products of proprietary Chinese medicines and the application of steaming, frying, simmering and calcining and other processing techniques for traditional Chinese medicine pieces production, in which foreign investors are prohibited from investing in, falls within the permitted category in accordance with the Catalogue and the Negative List. As of the date of this annual report, our current production and operation do not fall within any items on the Negative List. However, we may in the future acquire upstream and downstream companies manufacturing traditional Chinese medicine pieces, and as a result it is likely that the WFOE and the VIE and its subsidiaries’ production and operation would be subject to the Negative List. As a result, we would not be able to hold any equity of Gansu QLS and its subsidiaries.
On March 15, 2019, the National People’s Congress approved the Foreign Investment Law, which came into effect on January 1, 2020, repealing simultaneously the Law of the PRC on Sino-foreign Equity Joint Ventures, the Law of the PRC on Wholly Foreign-owned Enterprises and the Law of the PRC on Sino-foreign Cooperative Joint Ventures. The Foreign Investment Law adopts the management system of pre-establishment national treatment and negative list for foreign investment. Policies in support of enterprises shall apply equally to foreign-funded enterprises according to laws and regulations. Foreign investment enterprises shall be guaranteed that they could equally participate in the setting of standards, and the compulsory standards formulated by the State shall be equally applied. Fair competition for foreign investment enterprises to participate in government procurement activities shall be protected. The Foreign Investment Law also stipulates the protection of intellectual property rights and trade secrets. The State also establishes information reporting national security review systems according to the Foreign Investment Law.
PRC Laws and Regulations on Wholly Foreign-owned Enterprises
The establishment, operation and management of corporate entities in China are governed by the PRC Company Law, which was promulgated by the SCNPC on December 29, 1993 and became effective on July 1, 1994. It was last amended on October 26, 2018 and the amendments became effective on October 26, 2018. Under the PRC Company Law, companies are generally classified into two categories, namely, limited liability companies and joint stock limited companies. The PRC Company Law also applies to limited liability companies and joint stock limited companies with foreign investors. Where there are otherwise different provisions in any law on foreign investment, such provisions shall prevail.
The Law of the PRC on Wholly Foreign-invested Enterprises was promulgated and became effective on April 12, 1986, and was last amended and became effective on October 1, 2016. The Implementing Regulations of the PRC Law on Foreign-invested Enterprises were promulgated by the State Council on October 28, 1990. They were last amended on February 19, 2014 and the amendments became effective on March 1, 2014. The Provisional Measures on Administration of Filing for Establishment and Change of Foreign Investment Enterprises were promulgated by MOFCOM and became effective on October 8, 2016, and were last amended on June 30, 2018 with immediate effect. The above-mentioned laws form the legal framework for the PRC Government to regulate Foreign-invested Enterprises. These laws and regulations govern the establishment, modification, including changes to registered capital, shareholders, corporate form, merger and split, dissolution and termination of Foreign-invested Enterprises.
84
According to the above regulations, a Foreign-invested Enterprise should obtain approval from MOFCOM before its establishment and operation. Qilian International Trading (Chengdu) Co., Ltd. is a Foreign-invested Enterprise since established, and has obtained the approval of the local administration of MOFCOM. Its establishment and operation are in compliance with the above-mentioned laws. Gansu QLS is a PRC domestic company, and it is not subject to the record-filling or examination applicable to Foreign-invested Enterprises.
PRC Laws and Regulations on Intellectual Property Rights
Regulations on Trademarks
The Trademark Law of the PRC was adopted at the 24th meeting of the SCNPC on August 23, 1982. Four amendments were made on February 22, 1993, October 27, 2001, August 30, 2013 and April 23, 2019. The last amendment was implemented on November 1, 2019. The Regulations on the Implementation of the Trademark Law of the PRC were promulgated by the State Council of the People’s Republic of China on August 3, 2002, which took effect on September 15, 2002. It was revised on April 29, 2014 and became effective as of May 1, 2014. According to the Trademark Law and the implementing regulations, a trademark which has been approved and registered by the trademark office is a registered trademark, including a trademark of goods, services, collective trademark and certification trademark. The trademark registrant shall enjoy the exclusive right to use the trademark and shall be protected by law. The trademark law also specifies the scope of registered trademarks, procedures for registration of trademarks and the rights and obligations of trademark owners. We and our affiliated entities are currently holding 10 registered trademarks in China and enjoy the corresponding rights.
Regulations on Patents
Pursuant to the Patent Law of the PRC, or the Patent Law, promulgated by the SCNPC on March 12, 1984, as latest amended on December 27, 2008, and effective from October 1, 2009 and the Implementation Rules of the Patent Law of the PRC, promulgated by the State Council on June 15, 2001 and latest amended on January 9, 2010, there are three types of patent in the PRC: invention patent, utility model patent and design patent. The protection period is 20 years for invention patent and 10 years for utility model patent and design patent, commencing from their respective application dates. Any individual or entity that utilizes a patent or conducts any other activity in infringement of a patent without prior authorization of the patentee shall pay compensation to the patentee and is subject to a fine imposed by relevant administrative authorities and, if constituting a crime, shall be held criminally liable in accordance with the law. In the event that a patent is owned by two or more co-owners without an agreement regarding the distribution of revenue generated from the exploitation of any co-owner of the patent, such revenue shall be distributed among all the co-owners.
Existing patents can become narrowed, invalid or unenforceable due to a variety of grounds, including lack of novelty, creativity, and deficiencies in patent application. In China, a patent must have novelty, creativity and practical applicability. Under the Patent Law, novelty means that before a patent application is filed, no identical invention or utility model has been publicly disclosed in any publication in China or overseas or has been publicly used or made known to the public by any other means, whether in or outside of China, nor has any other person filed with the patent authority an application that describes an identical invention or utility model and is recorded in patent application documents or patent documents published after the filing date. Creativity means that, compared with existing technology, an invention has prominent substantial features and represents notable progress, and a utility model has substantial features and represents any progress. Practical applicability means an invention or utility model can be manufactured or used and may produce positive results. Patents in China are filed with the State Intellectual Property Office, or SIPO. Normally, the SIPO publishes an application for an invention patent within 18 months after the filing date, which may be shortened at the request of applicant. The applicant must apply to the SIPO for a substantive examination within 3 years from the date of application.
Gansu QLS currently holds 21 patents and Chengdu QLS holds 8 patents, respectively, in China and enjoys the corresponding rights. In addition, Gansu QLS, Ahan and Moshangfa have separately filed two, one and three patent applications with the Patent Administration Department of the PRC. We and our affiliated entities have exclusive rights to manufacture the products and utilize the processes issued patent rights within the valid term. As for other products of the WFOE and the VIE and its subsidiaries and the related manufacturing processes, since the technology information has been published to public domain by national or local product standard, we and our affiliated entities are able to utilize such technology information without need to obtain any patent license. To our knowledge, we and our affiliated entities do not violate the existing patent rights of any third party as of the date of this report.
85
Regulations on Domain Names
The MIIT promulgated the Measures on Administration of Internet Domain Names, or the Domain Name Measures, on August 24, 2017, which took effect on November 1, 2017 and replaced the Administrative Measures on China Internet Domain Name promulgated by the MIIT on November 5, 2004. According to the Domain Name Measures, the MIIT is in charge of the administration of PRC internet domain names. The domain name registration follows a first-to-file principle. Applicants for registration of domain names shall provide true, accurate and complete information of their identities to domain name registration service institutions. The applicant will become the holder of such domain names upon completion of the registration procedure. We have completed the filing for recording our domain name of “bgmgroupltd.com” as a provider of non-commercial internet-based information services.
PRC Laws and Regulations on Foreign Exchange
General Administration of Foreign Exchange
The principal regulation governing foreign currency exchange in the PRC is the Administrative Regulations of the PRC on Foreign Exchange (the “Foreign Exchange Regulations”), which were promulgated on January 29, 1996, became effective on April 1, 1996 and were last amended on August 5, 2008. Under these rules, Renminbi is generally freely convertible for payments of current account items, such as trade- and service-related foreign exchange transactions and dividend payments, but not freely convertible for capital account items, such as capital transfer, direct investment, investment in securities, derivative products or loans unless prior approval by competent authorities for the administration of foreign exchange is obtained. Under the Foreign Exchange Regulations, foreign-invested enterprises in the PRC may purchase foreign exchange without the approval of SAFE to pay dividends by providing certain evidentiary documents, including board resolutions, tax certificates, or for trade- and services-related foreign exchange transactions, by providing commercial documents evidencing such transactions.
Registration of Foreign Investment Enterprises
Pursuant to the Notice of State Administration of Foreign Exchange on Promulgation of the Provisions on Foreign Exchange Control on Direct Investments in China by Foreign Investors promulgated by the SAFE, or the Notice, upon establishment of a foreign investment enterprise pursuant to the law, registration formalities shall be completed with the foreign exchange bureau. Upon completion of registration formalities by the entities involved in direct investments in China, the entities may open accounts for direct investments in China such as preliminary expense account, capital fund account and asset realization account, etc. with the bank based on the actual needs. Upon completion of such registration formalities, foreign investment enterprises could also conduct settlement when contributing foreign exchange funds, and remit funds overseas in the event of capital reduction, liquidation, advance recovery of investment, profit distribution, etc.
Our WFOE has completed the foreign exchange registration formalities upon establishment. Consequently, Qilian International (Hong Kong) Holdings Limited, the sole shareholder of WFOE, is able to contribute capital to or receive distributions and dividends from WFOE.
Circular No. 37 and Circular No. 13
Circular 37 was released by SAFE on July 4, 2014 and repealed Circular 75 which had been in effect since November 1, 2005. Pursuant to Circular 37, a PRC resident should apply to SAFE for foreign exchange registration of overseas investments before it makes any capital contribution to a special purpose vehicle, or SPV, using his or her legitimate domestic or offshore assets or interests. SPVs are offshore enterprises directly established or indirectly controlled by domestic residents for the purpose of investment and financing by utilizing domestic or offshore assets or interests they legally hold. Following any significant change in a registered offshore SPV, such as a capital increase, reduction, equity transfer or swap, consolidation or division involving domestic resident individuals, the domestic individuals shall amend the registration with SAFE. Where an SPV intends to repatriate funds raised after completion of offshore financing to the PRC, it shall comply with relevant PRC regulations on foreign investment and foreign debt management. A foreign-invested enterprise established through return investment shall complete relevant foreign exchange registration formalities in accordance with the prevailing foreign exchange administration regulations on foreign direct investment and truthfully disclose information on the actual controller of its shareholders.
86
If any shareholder who is a PRC resident (as determined by Circular No. 37) holds any interest in our SPV and fails to fulfil the required foreign exchange registration with the local SAFE branches, capital contribution to the SPV by the shareholder failing to comply with Circular No. 37, as well as the distribution of profits and dividends derived from the SPV to such shareholder may be prohibited. However, even if such shareholder fails to fulfil the required foreign exchange registration with the local SAFE branches, BGM and Qilian HK are not restricted in their ability to contribute additional capital to WFOE. Since Gansu QLS and its subsidiaries are only controlled by WFOE through contractual arrangements, and since WFOE is not a shareholder of Gansu QLS, neither Gansu QLS nor any of its subsidiaries have any obligations to contribute capital to WFOE, nor have they any rights to receive distributions or dividends from WFOE. Only capital contributions to a special purpose vehicle by its shareholders failing to comply with Circular 37, as well as the repatriation of profits and dividends derived from such special purpose vehicle to China by its shareholders are limited. Our WFOE is not prohibited from distributing its profits and dividends to BGM or Qilian HK or from carrying out other subsequent cross-border foreign exchange activities because WFOE has completed the foreign exchange registration formalities as required upon its establishment. Where a domestic resident fails to complete relevant foreign exchange registration as required, fails to truthfully disclose information on the actual controller of the enterprise involved in the return investment or otherwise makes false statements, the foreign exchange administration authority may, according to Regulation of the People’s Republic of China on Foreign Exchange Administration (2008 Revision) promulgated by the State Council with immediate effect on August 5, 2008, order them to take remedial actions, issue a warning, and impose a fine of less than RMB 300,000 on an institution or less than RMB 50,000 on an individual.
Circular 13 was issued by SAFE on February 13, 2015, became effective on June 1, 2015, and amended on December 30, 2019. Pursuant to Circular 13, a domestic resident who makes a capital contribution to an SPV using his or her legitimate domestic or offshore assets or interests is no longer required to apply to SAFE for foreign exchange registration of his or her overseas investments. Instead, he or she shall register with a bank in the place where the assets or interests of the domestic enterprise in which he or she has interests are located if the domestic resident individually seeks to make a capital contribution to the SPV using his or her legitimate domestic assets or interests; or he or she shall register with a local bank at his or her permanent residence if the domestic resident individually seeks to make a capital contribution to the SPV using his or her legitimate offshore assets or interests.
There are a total of 151 Gansu QLS shareholders, who are PRC residents. Amongst them, 122 have signed the VIE Agreements, but only 82 have completed the Circular 37 Registration. The remaining 40 shareholders who have yet to complete the Circular 37 Registration hold a total of 4.5% of shares of Gansu QLS. The failure of our beneficial shareholders to comply with the registration procedures may subject each of our beneficial shareholders to fines of less than RMB50,000 (approximately US$7,199). Shareholders of offshore SPV who are PRC residents and who have not completed their registrations in accordance with Circular 37 are subject to certain absolute restrictions, under which they cannot contribute any registered or additional capital to such SPV for offshore financing purposes. In addition, these shareholders cannot repatriate any profits and dividends from the SPV to China either.
Shareholders who have completed the Circular 37 registration would not be adversely affected and are allowed to contribute assets into the offshore special purpose vehicle and repatriate profits and dividends from them. Since our WFOE has completed its foreign exchange registration as a foreign investment enterprise, its ability to receive capital contribution, make distributions and pay dividends is not restricted.
Circular 19 and Circular 16
Circular 19 was promulgated by SAFE on March 30, 2015, became effective on June 1, 2015 and last amended on December 30, 2019. According to Circular 19, the foreign exchange capital in the capital account of foreign-invested enterprises, meaning the monetary contribution confirmed by the foreign exchange authorities or the monetary contribution registered for account entry through banks, shall be granted the benefits of Discretional Foreign Exchange Settlement (“Discretional Foreign Exchange Settlement”). With Discretional Foreign Exchange Settlement, foreign capital in the capital account of a foreign-invested enterprise for which the rights and interests of monetary contribution have been confirmed by the local foreign exchange bureau, or for which book-entry registration of monetary contribution has been completed by the bank, can be settled at the bank based on the actual operational needs of the foreign-invested enterprise. The allowed Discretional Foreign Exchange Settlement percentage of the foreign capital of a foreign-invested enterprise has been temporarily set to be 100%. The Renminbi converted from the foreign capital will be kept in a designated account and if a foreign-invested enterprise needs to make any further payment from such account, it will still need to provide supporting documents and to complete the review process with its bank.
87
Furthermore, Circular 19 stipulates that foreign-invested enterprises shall make bona fide use of their capital for their own needs within their business scopes. The capital of a foreign-invested enterprise and the Renminbi it obtained from foreign exchange settlement shall not be used for the following purposes, directly or indirectly:
| ● | expenses beyond its business scope or prohibited by relevant laws or regulations; |
| ● | investment in securities unless otherwise provided by relevant laws or regulations; |
| ● | entrusted loan in Renminbi (unless within its permitted scope of business), repayment of inter-company loans (including advances by a third party) or repayment of bank loans in Renminbi that have been sub-lent to a third party; or |
| ● | expenses related to the purchase of real estate that is not for self-use (except for foreign-invested real estate enterprises). |
Circular 16 was issued by SAFE on June 9, 2016. Pursuant to Circular 16, enterprises registered in the PRC may also convert their foreign debts from foreign currency to Renminbi on a self-discretionary basis. Circular 16 provides an integrated standard for conversion of foreign exchange capital items (including but not limited to foreign currency capital and foreign debts) on a self-discretionary basis applicable to all enterprises registered in the PRC. Circular 16 reiterates the principle that an enterprise’s Renminbi capital converted from foreign currency-denominated capital may not be directly or indirectly used for purposes beyond its business scope or purposes prohibited by PRC laws or regulations, and such converted Renminbi capital shall not be provided as loans to non-affiliated entities.
PRC Laws and Regulations on Taxation
Enterprise Income Tax
The EIT Law was promulgated by the Standing Committee of the National People’s Congress on March 16, 2007, became effective on January 1, 2008, and was last amended on December 29, 2018. The Implementation Rules of the EIT Law were promulgated by the State Council on December 6, 2007, became effective on January 1, 2008 and last amended on April 23, 2019. According to the EIT Law and its implementation rules, enterprises are divided into resident enterprises and non-resident enterprises. Resident enterprises shall pay enterprise income tax on their incomes obtained in and outside the PRC at the rate of 25%. Non-resident enterprises setting up institutions in the PRC shall pay enterprise income tax on the incomes obtained by such institutions in and outside the PRC at the rate of 25%. Non-resident enterprises with no institutions in the PRC, and non-resident enterprises whose incomes having no substantial connection with their institutions in the PRC, shall pay enterprise income tax on their incomes obtained in the PRC at a reduced rate of 10%.
The Arrangement between the PRC and Hong Kong Special Administrative Region for the Avoidance of Double Taxation the Prevention of Fiscal Evasion with respect to Taxes on Income (the “Arrangement”) was promulgated by the SAT on August 21, 2006, with its fifth protocol coming into effect on December 6, 2019. According to the Arrangement, a company incorporated in Hong Kong will be subject to withholding tax at the lower rate of 5% on dividends it receives from a company incorporated in the PRC if it holds a 25% interest or more in the PRC company. The Notice on the Understanding and Identification of the Beneficial Owners in the Tax Treaty (the “Notice”) was promulgated by SAT and became effective on October 27, 2009. According to the Notice, a beneficial ownership analysis will be used based on a substance-over-form principle to determine whether or not to grant tax treaty benefits.
Gansu GLS and its subsidiaries are resident enterprises and pay EIT tax at the statutory rate of 25% in the PRC. It is more likely than not that the Company and its offshore subsidiary would be treated as a non-resident enterprise for PRC tax purposes.
88
Value-added Tax
Pursuant to the Provisional Regulations on Value-added Tax of the PRC, or the VAT Regulations, which were promulgated by the State Council on December 13, 1993, took effect on January 1, 1994, and were amended on November 10, 2008, February 6, 2016, and November 19, 2017, respectively, and the Rules for the Implementation of the Provisional Regulations on Value-added Tax of the PRC, which were promulgated by the MOF on December 25, 1993, and were amended on December 15, 2008, and October 28, 2011, respectively, entities and individuals that sell goods or labor services of processing, repair or replacement, sell services, intangible assets, or immovables, or import goods within the territory of the People’s Republic of China are taxpayers of value-added tax. The VAT rate is 17% for taxpayers selling goods, labor services, or tangible movable property leasing services or importing goods, except otherwise specified; 11% for taxpayers selling services of transportation, postal, basic telecommunications, construction and lease of immovable, selling immovable, transferring land use rights, selling and importing other specified goods including fertilizers; 6% for taxpayers selling services or intangible assets.
According to the Notice on the Adjustment to the Value-added Tax Rates issued by the SAT and the MOF on April 4, 2018, where taxpayers make VAT taxable sales or import goods, the applicable tax rates shall be adjusted from 17% to 16% and from 11% to 10%, respectively. Subsequently, the Notice on Policies for Deepening Reform of Value-added Tax was issued by the SAT, the MOF and the General Administration of Customs on March 20, 2019 and took effective on April 1, 2019, which further adjusted the applicable tax rate for taxpayers making VAT taxable sales or importing goods. The applicable tax rates shall be adjusted from 16% to 13% and from 10% to 9%, respectively.
On April 14, 2008, the PRC Ministry of Science and Technology, the Ministry of Finance and the SAT enacted the Administrative Measures for Accreditation of High and New Technology Enterprises, hereinafter referred to as the “Measures for High-Tech Enterprises”, which was amended on January 29, 2016 and retroactively effective from January 1, 2016. Under the EIT Law and the Measures for High-Tech Enterprises, certain qualified high-tech companies may benefit from a preferential tax rate of 15% if they own core intellectual properties and their business fall into certain industries that are strongly supported by the PRC government and recognized by certain departments of the State Council. On July 11, 2018, the Circular on Extension of the Loss-Covering Carryover Period for High and New Technology Enterprises and Small and Medium-Sized Technological Enterprises was enacted with retroactive effect from January 1, 2018.
Currently, Gansu QLS and its subsidiaries are paying VAT at the rate of 13% for pharmaceutical manufacture and sales, health materials and medical consumable products manufacture, soy products manufacture, selling Heparin Sodium Preparations; 9% for lease of immovable, use of land and second-hand buildings, soy products manufacture, pollution disposing, selling of sausage casing; and 6% for human resource services.
Dividend Withholding Tax
The EIT Law provides that since January 1, 2008, an income tax rate of 20% will normally be applicable to dividends declared to non-PRC resident investors that do not have an establishment or place of business in the PRC, or that have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends are derived from sources within the PRC.
89
Pursuant to an Arrangement Between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Incomes (“Double Tax Avoidance Arrangement”), with its fifth protocol coming into effect on December 6, 2019,and other applicable PRC laws, if a Hong Kong resident enterprise is determined by the competent PRC tax authority to have satisfied the relevant conditions and requirements under such Double Tax Avoidance Arrangement and other applicable laws, the 10% withholding tax on the dividends the Hong Kong resident enterprise receives from a PRC resident enterprise may be reduced to 5%. However, based on the Circular on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties (the “SAT Circular 81”) issued on February 20, 2009 by SAT, if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, such PRC tax authorities may adjust the preferential tax treatment. According to the Circular on Several Questions regarding the “Beneficial Owner” in Tax Treaties, which was issued on February 3, 2018 by the SAT and took effect on April 1, 2018, when determining the applicant’s status of the “beneficial owner” regarding tax treatments in connection with dividends, interests or royalties in the tax treaties, several factors, including without limitation, whether the applicant is obligated to pay more than 50% of his or her income in twelve months to residents in third country or region, whether the business operated by the applicant constitutes the actual business activities, and whether the counterparty country or region to the tax treaties does not levy any tax or grant tax exemption on relevant incomes or levy tax at an extremely low rate, will be taken into account, and it will be analyzed according to the actual circumstances of the specific cases. This circular further provides that applicants who intend to prove his or her status of the “beneficial owner” shall submit the relevant documents to the relevant tax bureau according to the Announcement on Issuing the Measures for the Administration of Non-Resident Taxpayers’ Enjoyment of the Treatment under Tax Agreements.
We and our affiliated entities have not commenced the application process for a Hong Kong tax resident certificate from the relevant Hong Kong tax authority, and there is no assurance that we and our affiliated entities will be granted such a Hong Kong tax resident certificate. We and our affiliated entities have not filed required forms or materials with the relevant PRC tax authorities to prove that we and our affiliated entities should enjoy the 5% PRC withholding tax rate.
PRC Laws and Regulations on Employment and Social Welfare
Labor Law of the PRC
Pursuant to the Labor Law of the PRC, which was promulgated by the Standing Committee of the NPC on July 5, 1994 with an effective date of January 1, 1995 and was last amended on December 29, 2018 and the Labor Contract Law of the PRC, which was promulgated on June 29, 2007, became effective on January 1, 2008 and was last amended on December 28, 2012, with the amendments coming into effect on July 1, 2013, enterprises and institutions shall ensure the safety and hygiene of a workplace, strictly comply with applicable rules and standards on workplace safety and hygiene in China, and educate employees on such rules and standards. Furthermore, employers and employees shall enter into written employment contracts to establish their employment relationships. Employers are required to inform their employees about their job responsibilities, working conditions, occupational hazards, remuneration and other matters with which the employees may be concerned. Employers shall pay remuneration to employees on time and in full accordance with the commitments set forth in their employment contracts and with the relevant PRC laws and regulations. Gansu QLS and its subsidiary companies have entered into written employment contracts with all the employees and performed their obligations under the relevant PRC laws and regulations.
Social Insurance and Housing Fund
Pursuant to the Social Insurance Law of the PRC, which was promulgated by the Standing Committee of the NPC on October 28, 2010, became effective on July 1, 2011, and was last amended on December 29, 2018, employers in the PRC shall provide their employees with welfare schemes covering basic pension insurance, basic medical insurance, unemployment insurance, maternity insurance, and occupational injury insurance. Gansu QLS has not deposited the social insurance fees in full for all the employees in compliance with the relevant regulations. Gansu QLS may be ordered by the social security premium collection agency to make or supplement contributions within a stipulated period, and shall be subject to a late payment fine computed from the due date at the rate of 0.05% per day; where payment is not made within the stipulated period, the relevant administrative authorities shall impose a fine ranging from one to three times the amount of the amount in arrears. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—We are not in compliance with the PRC’s regulations relating to employee’s social insurance and housing funds, and as a result, Gansu QLS and its subsidiaries may be subject to penalties if we are not able to remediate the non-compliance.”
90
In accordance with the Regulations on Management of Housing Provident Fund, which were promulgated by the State Council on April 3, 1999 and last amended on March 24, 2019, employers must register at the designated administrative centers and open bank accounts for depositing employees’ housing funds. Employers and employees are also required to pay and deposit housing funds, with an amount no less than 5% of the monthly average salary of the employee in the preceding year in full and on time. Gansu QLS has opened bank accounts and deposited housing provident funds as required since August 2019. However, Chengdu QLS has not opened bank accounts for its employees’ housing funds deposits, or deposited employees’ housing funds, which may be ordered by the relevant PRC authorities to open the housing funds account, make the payment, and deposit within a prescribed time limit. If Chengdu QLS fails to go through the formalities to open the account within the prescribed time limit, a fine of not less than RMB10,000 nor more than RMB50,000 shall be imposed. If Chengdu QLS fails to make the payment and deposit within the prescribed time limit, an application may be made to the people’s court for compulsory enforcement.
C. Organizational Structure
The following diagram illustrates our current corporate structure, which includes our significant affiliated entities as of the date of this annual report:

*48.718% equity interests of Zhongqiao Youguan (Chengdu) E-Commerce Service Co., Ltd. are owned by Sichuan Shihua Investment Management Co., Ltd., a PRC entity that is controlled by Yuchang Xin, the brother of Zhanchang Xin, our chairman of the Board.
20.29% equity interests of Chengdu QLS are collectively owned by 49 individual shareholders, none of whom is an affiliate of the Company.
91
Contractual Arrangements between WFOE and Gansu QLS
Due to PRC legal restrictions on foreign ownership in the pharmaceutical sector, neither we nor our subsidiaries own any equity interest in Gansu QLS. Instead, we control and receive the economic benefits of Gansu QLS’s business operation through a series of contractual arrangements. WFOE, Gansu QLS and its shareholders entered into a series of contractual arrangements, also known as VIE Agreements, on May 20, 2019.
Each of the VIE Agreements is described in detail below:
Exclusive Service Agreement
Pursuant to the original Exclusive Service Agreement between Gansu QLS and WFOE, WFOE provides Gansu QLS with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, business management and information. For services rendered to Gansu QLS by WFOE under this agreement, WFOE is entitled to collect a service fee that shall be equal to 99.214% of the net profits of Gansu QLS, with such percentage determined in accordance with “ARTICLE 3 - SERVICE FEES” of the Amended Exclusive Service Agreement executed on August 27, 2019, as amended on February 25, 2021. This percentage represents the number of shares of Gansu QLS held by shareholders having signed the VIE Agreements over the total number of issued and outstanding shares of Gansu QLS.
On December 1, 2022, Chengdu Trade and Gansu Qilianshan Pharmaceutical Co.,Ltd. executed certain exclusive service termination agreement (the “Service Termination Agreement”) to terminate the previously signed Exclusive Service Agreement, as amended on August 27, 2019. As a result of the aforementioned termination, Chengdu Trade will no longer have contractual control over, nor receive the economic benefits of Gansu QLS. In connection with such termination, Hainan Trade, a wholly-owned subsidiary of Qilian International (Hong Kong) Holdings Limited, entered into a certain exclusive service agreement with Gansu QLS (the “Hainan Exclusive Service Agreement”) on December 1, 2022, through which Hainan Trade obtained contractual control over Gansu QLS. Pursuant to the Hainan Exclusive Service Agreement, Hainan Trade provides Gansu QLS with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, business management and information. For services rendered to Gansu QLS by Hainan Trade under this agreement, Hainan Trade is entitled to collect a service fee that shall be equal to 99.214% of the net profits of Gansu QLS. The Hainan Exclusive Service Agreement shall remain in effect for ten years unless earlier terminated upon written confirmation from both Hainan Trade and Gansu QLS before expiration. Otherwise, this agreement shall be extended by another ten years automatically. The Hainan Exclusive Service Agreement does not prohibit related party transactions.
In the opinion of Gansu Quanyi Law Firm, the Company’s PRC legal counsel, the Hainan Exclusive Service Agreement is valid, binding and enforceable under current PRC law. However, such agreement may not be as effective in providing control as direct ownership. There are substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulation regarding such contractual arrangements and their effectiveness.
WFOE is currently managing Gansu QLS pursuant to the terms of the Exclusive Service Agreement. WFOE has absolute authority relating to the management of Gansu QLS, including but not limited to decisions with regard to expenses, salary raises and bonuses, hiring, firing and other operational functions. The Exclusive Service Agreement does not prohibit related party transactions. The audit committee of the registrant is required to review and approve in advance any related party transactions, including transactions involving WFOE or Gansu QLS.
Equity Pledge Agreement
Under the Equity Pledge Agreement between WFOE and certain shareholders of Gansu QLS together holding 76,196,640 shares, or 99.214% of the total issued and outstanding shares, of Gansu QLS, the Gansu QLS Shareholders pledged all of their equity interests in Gansu QLS to WFOE to guarantee the performance of Gansu QLS’ obligations under the Exclusive Service Agreement. Under the terms of the Equity Pledge Agreement, in the event that Gansu QLS breaches its contractual obligations under the Exclusive Service Agreement, WFOE, as pledgee, will be entitled to certain rights, including, but not limited to, the right to collect dividends generated by the pledged equity interests. The Gansu QLS Shareholders also agreed that upon occurrence of any event of default, as set forth in the Equity Pledge Agreement, WFOE is entitled to dispose of the pledged equity interest in accordance with applicable PRC laws. The Gansu QLS Shareholders further agree not to dispose of the pledged equity interests or take any actions that would prejudice WFOE’s interest.
92
The Equity Pledge Agreement shall be effective until the latest date of the following: (1) the secured debt in the scope of pledge is cleared off; (2) WFOE exercises its pledge rights pursuant to provisions and conditions of the Equity Pledge Agreement; and (3) the Gansu QL Shareholders transfer all the pledged equity interests to WFOE according to the Call Option Agreement, or other entity or individual designated by it.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of Gansu QLS’s obligations under the Exclusive Service Agreement, (2) ensure the Gansu QLS Shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice WFOE’s interests without WFOE’s prior written consent and (3) provide WFOE control over Gansu QLS. Under the Call Option Agreement, WFOE may be able to acquire the equity interests or the assets in Gansu QLS any time to the extent permitted by the PRC Law. In the event Gansu QLS breaches its contractual obligations under the Exclusive Service Agreement, WFOE will be entitled to foreclose on the Gansu QLS Shareholders’ equity interests in Gansu QLS and may (1) exercise its option to purchase or designate third parties to purchase part or all of their equity interests or the assets in Gansu QLS and in this situation, WFOE may terminate the Exclusive Service Agreement, Equity Pledge Agreement and Call Option Agreement after acquisition of all equity interests or assets in Gansu QLS or form new VIE structure with the third parties designated by WFOE; or (2) dispose the pledged equity interests or assets and be paid in priority out of proceed from the disposal in which case the VIE structure will be terminated.
Call Option Agreement
Under the Call Option Agreement, the Gansu QLS Shareholders irrevocably granted WFOE (or its designee) an exclusive right to purchase, to the extent permitted under PRC law, once or at multiple times, at any time, a portion or whole of the equity interests or assets in Gansu QLS held by the Gansu QLS Shareholders. The purchase price should be no more than $1.00 subject to any appraisal or restrictions required by applicable PRC laws and regulations.
The agreement remains effective until all the transferred equity or transferred asset of Gansu QLS is legally transferred under the name of WFOE and/or other entity or individual designated by it.
Shareholders’ Voting Rights Proxy Agreement and Powers of Attorney
Under the Shareholders’ Voting Rights Proxy Agreement and each Power of Attorney, each Gansu QLS Shareholder authorizes WFOE to act on their behalf as their exclusive agent and attorney with respect to all rights as shareholders, including but not limited to: (a) the attendance of the shareholder’s meeting and the execution of relative Shareholder Resolution(s) of Gansu QLS; (b) exercising all the shareholder’s rights, including voting, that shareholders are entitled to under the laws of China and the Articles of Association, including but not limited to the sale or transfer or pledge or disposition of shares in part or in whole; and (c) designating and appointing on behalf of shareholders the legal representative, the executive director, supervisor, the chief executive officer and other senior management members of Gansu QLS.
Each Power of Attorney is coupled with an interest and shall be irrevocable and continuously valid from the date of its execution, so long as the relevant Gansu QLS Shareholder is a shareholder of Gansu QLS.
Spousal Consent
The spouses of the Gansu QLS Shareholders agreed, via a spousal consent, to the execution of the “Transaction Documents” including: (a) the Call Option Agreement entered into with WFOE and Gansu QLS; (b) the Shareholders’ Voting Rights Proxy Agreement entered into with WFOE and Gansu QLS; (c) the Equity Pledge Agreement entered into with WFOE; and (d) the Power of Attorney executed by each Gansu QLS Shareholder, and the disposal of the equity interests of Gansu QLS held by each Gansu QLS Shareholder and registered in his/her name.
The spouses further undertake not to make any assertions in connection with the equity interests of Gansu QLS which are held by the Gansu QLS Shareholders. They confirm that the Gansu QLS Shareholders can perform, amend, or terminate the Transaction Documents without their authorization or consent. They undertake to execute all necessary documents and take all necessary actions to ensure appropriate performance of the agreements.
D. Property, Plants and Equipment
See “—B. Business Overview—Facilities.”
93
ITEM 4.A. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following discussion of our financial condition and results of operations is based upon and should be read in conjunction with our consolidated financial statements and their related notes included elsewhere in this annual report. This annual report contains forward-looking statements. See “Forward-Looking Information” in this annual report. In evaluating our and the VIE and its subsidiaries’ business, you should carefully consider the information provided under the caption “Item 3. Key Information—D. Risk Factors” in this annual report. We caution you that our and the VIE and its subsidiaries’ business and financial performance are subject to substantial risks and uncertainties.
A. Operating Results
Overview
We are engaged in the research, development, and production of licorice products, oxytetracycline products, TCMD product, heparin product, sausage casings, and fertilizers.
We also have a strategic focus on the technology fields of AI application, intelligent robots, algorithmic computing power, cloud computing, and biopharmaceuticals. In terms of AI application implementation, we rely on big data mining and AI Agent technology, and utilize the two platforms of Du Xiao Bao and Bao Wang to provide comprehensive and professional AI solutions and intelligent robot services for insurance companies, insurance brokers, and consumers. Its services cover multiple key scenarios such as sales and marketing, underwriting assessment, claims processing, and customer service. We are capable of analyzing consumer data, building consumer profiles, accurately predicting insurance needs, and providing highly customized services for consumers. In the field of biopharmaceuticals, we deeply integrate AI-assisted decision-making into every link of production and manufacturing, achieving supply chain optimization, process efficiency improvement, and market trend prediction. This provides scientific decision-making basis for our management and offers high-quality products and precise services for consumers.
We were originally incorporated in the Cayman Islands on February 7, 2019. Our business is mainly conducted by Gansu QLS, the VIE in the PRC, and its subsidiaries, using RMB, the currency of China.
On May 20, 2019 and November 20, 2020, we, through our wholly foreign-owned entity Chengdu Trade, entered into a series of contractual arrangements with Gansu QLS, which include an Exclusive Service Agreement, an Equity Pledge Agreement, a Call Option Agreement, a Shareholders’ Voting Rights Proxy Agreement and Powers of Attorney. Pursuant to the VIE Agreements, WFOE provides Gansu QLS with technical support, consulting services and other management services and is entitled to receive 99.214% of Gansu QLS’ net profits, this percentage being the number of shares of Gansu QLS held by shareholders having signed the VIE Agreements over the total issued and outstanding shares of Gansu QLS. In addition, Gansu QLS’s shareholders have pledged 99.214% of their equity interests in Gansu QLS to WFOE, irrevocably granted WFOE an exclusive option to purchase, to the extent permitted under PRC law, all or part of the equity interests in Gansu QLS, and agreed to entrust all the rights to exercise their voting power to the person(s) appointed by WFOE.
To optimize its corporate structure, Chengdu Trade and Gansu QLS executed certain exclusive service termination agreement (the “Service Termination Agreement”) to terminate certain contractual service arrangements between Chengdu Trade and Gansu QLS. As a result of the aforementioned termination, Chengdu Trade will no longer have contractual control over, nor receive the economic benefits of Gansu QLS. In connection with such termination, Qilian Shan International Trade (Hainan) Co., Ltd (“Hainan Trade”), a wholly-owned subsidiary of Qilian International (Hong Kong) Holdings Limited, entered into a series of VIE Agreements with Gansu QLS. The Service Termination Agreement and the new service agreement with Hainan Trade became effective on December 1, 2022.
Through the VIE Agreements, WFOE is deemed as the primary beneficiary of Gansu QLS for accounting purpose and is able to consolidate the VIE’s financial statements under the U.S. GAAP.
94
Based on the VIE Agreements, Gansu QLS is considered a VIE of Qilian Chengdu/Hainan Trade under U.S. GAAP. As the above entities were under common control before and after the execution of the VIE Agreements, the restructuring was accounted for as a reorganization of entities under common control and consolidated financial statements were prepared as if the reorganization occurred at the beginning of the first period presented. Thus, the financial results presented here include those of the VIE and the VIE’s subsidiaries from the first period presented. Refer to our Risk Factors under “Item 3. Key Information—D. Risk Factors—Risks Related to Our Corporate Structure.”
As of the date of this annual report, there is an aggregate of 97,222,141 ordinary shares, consisting of 77,222,141 Class A ordinary shares, par value of US$0.00833335 each, and 20,000,000 Class B ordinary shares, par value of US$0.00833335 each.
Outlook
We and the VIE and its subsidiaries plan to continue developing their business by expanding their marketing network and investing in pharmaceutical and chemical facilities, which depend heavily on sufficient capital. If we are not able to obtain equity or debt financing, we and our affiliates may not be able to execute the development and expansion plans, which could have material adverse effect on our, the VIE and its subsidiaries’ future business performance and operating results.
Our net revenue for the year ended September 30, 2024 was $25.1 million, representing a decrease of $21.4 million, or 46%, from $46.5 million for the year ended September 30, 2023. Net loss attributable to our shareholders for the year ended September 30, 2024 was $1.4 million, representing a decrease of $6.3 million, or 81.5%, from $7.8 million net loss attributable to our shareholders for the year ended September 30, 2023. Non-GAAP EBITDA (as defined below) for the year ended September 30, 2024 was $(0.3) million, representing an increase of $6.6 million, or (96%), from $(6.9) million for the year ended September 30, 2023. For additional information on EBITDA, please see the subsection “—EBITDA” below.
Key Indicators of the Company’s Performance
In assessing performance, we consider a variety of performance and financial measures, including principal growth in net revenue, gross profit, distribution, general and administrative expenses, net income from operations, and EBITDA (Non-GAAP) (as defined below). The key measures that we use to evaluate the performance of our subsidiaries and VIE and its subsidiaries’ business are set forth below:
Net Revenue
Net revenue is equal to gross sales minus sales returns and sales incentives that the Company offers to our customers, such as discounts that are offset to gross sales. Our net sales are driven by changes in the number of customers, product varieties, selling price, and mix of products sold.
Gross Profit
Gross profit is equal to net sales minus cost of goods sold. Cost of goods sold primarily includes inventory costs (net of supplier consideration), inbound freight, custom clearance fees, and other miscellaneous expenses. Cost of goods sold generally changes as the Company incurs higher or lower costs from suppliers and as the customer and product mix changes.
Selling, General and Administrative, Research and Development Expenses
Selling, general and administrative, research and development expenses primarily consist of salaries and benefits for employees, shipping expense, utilities, maintenance and repairs expenses, insurance expense, depreciation and amortization expenses, research and development expense, selling and marketing expenses, professional fees, and other operating expenses.
95
Non-GAAP Financial Measures-EBITDA
Management uses certain financial measures to evaluate our operating performance which is calculated and presented on the basis of methodologies other than in accordance with GAAP (“Non-GAAP”). These measures should not be considered a substitute for, or superior to, measures of financial performance prepared in accordance with GAAP, and our calculations thereof may not be comparable to similarly entitled measures reported by other companies. We believe that EBITDA is a useful performance measure and can be used to facilitate a comparison of our operating performance on a consistent basis from period to period and to provide for a more complete understanding of factors and trends affecting our subsidiaries and the VIE and its subsidiaries’ business than GAAP measures alone can provide. Our management believes that EBITDA is less susceptible to variances in actual performance resulting from depreciation, amortization and other non-cash charges and more reflective of other factors that affect its operating performance. Our management believes that the use of these Non-GAAP financial measures provides an additional tool for investors to use in evaluating ongoing operating results and trends and in comparing our financial measures with the companies in the same industry, many of which present similar Non-GAAP financial measures to investors. We present EBITDA in order to provide supplemental information that our management considers relevant for the readers of our consolidated financial statements included elsewhere in this annual report, and such information is not meant to replace or supersede U.S. GAAP measures.
Our management defines EBITDA as net income (loss) before interest expense, income taxes, and depreciation and amortization. EBITDA is not defined under U.S. GAAP and is subject to important limitations as analytical tools and, as such, you should not consider them in isolation or as substitutes for analysis of our Company’s financial results as reported under U.S. GAAP. For example, EBITDA:
| ● | excludes certain tax payments that may represent a reduction in cash available to the Company; |
| ● | does not reflect any cash capital expenditure requirements for the assets being depreciated and amortized that may have to be replaced in the future; |
| ● | does not reflect changes in, or cash requirements for, the Company’ working capital needs; and |
| ● | does not reflect the significant interest expense, or the cash requirements, necessary to service the Company’s debt. |
Results of Operations for the years ended September 30, 2024 and 2023
The following table sets forth a summary of our consolidated results of operations for the years ended September 30, 2024 and 2023. The historical results presented below are not necessarily indicative of the results that may be expected for any future period.
|
|
For the years ended |
|
|
|
|
|
|
||||
|
|
September 30, |
|
Changes |
|
|||||||
|
|
2024 |
|
2023 |
|
Amount |
|
% |
|
|||
Net revenue |
|
$ |
25,097,951 |
|
$ |
46,471,478 |
|
$ |
(21,373,527) |
|
(46) |
% |
Cost of revenue |
|
|
20,983,196 |
|
|
44,719,984 |
|
|
(23,736,788) |
|
(53) |
% |
Gross profit |
|
|
4,114,755 |
|
|
1,751,494 |
|
|
2,363,261 |
|
135 |
% |
Selling, general and administrative, research and development expenses |
|
|
4,678,526 |
|
|
4,361,593 |
|
|
316,933 |
|
7 |
% |
Loss from operations |
|
|
(563,771) |
|
|
(2,610,099) |
|
|
2,046,328 |
|
(78) |
% |
Interest income |
|
|
(639,511) |
|
|
99,190 |
|
|
(738,701) |
|
(745) |
% |
Other expense |
|
|
(933,860) |
|
|
(5,391,995) |
|
|
4,458,135 |
|
(83) |
% |
Loss before income tax provision |
|
|
(2,137,142) |
|
|
(7,902,904) |
|
|
5,765,762 |
|
(73) |
% |
Income tax expense/(benefit) |
|
|
(619,981) |
|
|
219,166 |
|
|
(839,147) |
|
(383) |
% |
Net loss |
|
|
(1,517,161) |
|
|
(8,122,070) |
|
|
6,604,909 |
|
(81) |
% |
Less: net loss attributable to non-controlling interest |
|
|
(74,331) |
|
|
(341,450) |
|
|
267,119 |
|
(78) |
% |
Net loss attributable to BGM Group Ltd |
|
$ |
(1,442,830) |
|
$ |
(7,780,620) |
|
$ |
6,337,790 |
|
(81) |
% |
96
Net Revenue
The following table sets forth the breakdown of our net revenue:
|
|
For the years ended |
|
|
|
|
|
|
||||||||
|
|
September 30, |
|
|
|
|
|
|
||||||||
|
|
2024 |
|
2023 |
|
Changes |
|
|||||||||
|
|
Amount |
|
% |
|
Amount |
|
% |
|
Amount |
|
% |
|
|||
Net revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oxytetracycline & licorice products and TCMD |
|
$ |
21,961,282 |
|
87 |
% |
$ |
29,152,228 |
|
63 |
% |
$ |
(7,190,946) |
|
(24) |
% |
Heparin products and sausage casing |
|
$ |
2,230,759 |
|
9 |
% |
$ |
15,318,798 |
|
33 |
% |
$ |
(13,088,039) |
|
(85) |
% |
Fertilizer |
|
$ |
905,910 |
|
4 |
% |
$ |
2,000,452 |
|
4 |
% |
$ |
(1,094,542) |
|
(55) |
% |
Total |
|
$ |
25,097,951 |
|
100 |
% |
$ |
46,471,478 |
|
100 |
% |
$ |
(21,373,527) |
|
(46) |
% |
Compared with net revenue for the year ended September 30, 2023, our net revenue decreased by $21.4 million, or 46%, for the year ended September 30, 2024, which was primarily attributable to a $7.2 million decrease in sales from Oxytetracycline & licorice products and TCMD, a $13.1 million decrease in sales from heparin products and sausage casing, and $1.1 million decreased sales from Fertilizer product.
Oxytetracycline & Licorice Products and TCMD
The sales of oxytetracycline products, licorice products and TCMD accounted for 76%, 21% and 3%, respectively, of this segment’s total sales for the year ended September 30, 2024, and 95%, 5% and 0%, respectively, of such segment’s total sales for the year ended September 30, 2023. For the year ended September 30, 2024, the WFOE and the VIE and its subsidiaries’ sales translated into USD for oxytetracycline products, licorice products and TCMD decreased by approximately $7.2 million, or 24%, from approximately $29.2 million for the year ended September 30, 2023 to approximately $22.0 million for the year ended September 30, 2024. The decrease in sales in this segment is due to a decrease of oxytetracycline product for $10.9 million, offset by 3.3 million of increase from sales of licorice product. The reasons for the decrease in sales of oxytetracycline products are due to that :1) oxytetracycline products has halved, resulting in a decrease in sales from November 2023; 2) the price of the company’s oxytetracycline products is relatively high compared to other companies in the market. The increase in sales of licorice products is due to that: 1) the sales price of licorice products has increased. Because of one or two raw materials of licorice products being monopolized by the market, the price of this material has risen, resulting in an increase in the market sales price of licorice products; 2) the favorable market environment has led to an increase in the sales volume of licorice products; 3) the company signed a general agency agreement for licorice products in May 2024 and implemented an exclusive sales policy; 4) the company developed new products, licorice liquid extract and licorice extract powder in November 2023, which sold well and accounted for 72.8% of licorice revenue.
Heparin Products and Sausage Casings
Sales from heparin products and sausage casing decreased by $13.1 million, or 85%, from $15.3 million for the year ended September 30, 2023, to $2.2 million for the year ended September 30, 2024. The decrease of $10 million in sales of heparin products is mainly due to four reasons: 1) Our country issued a centralized procurement policy for heparin products in hospitals, and the procurement price decreased by 50-70% compared to before. This news has reduced the purchasing volume of heparin products for customers in February 2023. 2) At the same time, the price of heparin products has also entered a downward trend, with the sales unit price dropping from 48,000 yuan in April 2023 to 12,000 yuan in December 2023, and now reaching 15,800 yuan. 3) The customer had a relatively large inventory of heparin products purchased in the early stage, and in order to reduce losses, the customer had to digest the inventory. Therefore, the decrease in customer procurement was the main reason for the company’s sales decline. 4) The company went from a profitable state to a loss state, with more production resulting in greater losses. Therefore, the company made the decision to shut down production to reduce losses. The company has been shut down since September 2023, only entrusted for processing. The sales of sausage casing decreased by $2 million mainly due to the impact of the national centralized procurement policy, resulting in a decrease in the company’s production.
97
Fertilizer
Sales from fertilizer decreased by $1.1 million, or 55%, from $2.0 million for the year ended September 30, 2023 to $0.9 million for the year ended September 30, 2024. The decrease in income is due to that: 1) the sales of organic fertilizers in 2024 are mostly government bidding projects, with very little retail sales; 2) The 2023 bidding projects include organic fertilizers, soil conditioners, and microbial agents, which are no longer included in the 2024 bidding projects.
Cost of Revenue and Gross Profit
The following tables set forth the calculation of gross profit and gross margin for the each of our segments:
|
|
For the years ended |
|
|
|
|
|
|
||||
|
|
September 30, |
|
Changes |
|
|||||||
|
|
2024 |
|
2023 |
|
Amount |
|
% |
|
|||
Oxytetracycline & licorice products and TCMD |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
21,961,282 |
|
$ |
29,152,228 |
|
$ |
(7,190,946) |
|
(25) |
% |
Cost of revenue |
|
|
18,324,619 |
|
|
27,392,225 |
|
|
(9,067,606) |
|
(33) |
% |
Gross profit |
|
$ |
3,636,663 |
|
$ |
1,760,003 |
|
$ |
1,876,660 |
|
107 |
% |
Gross Margin |
|
|
16.6 |
% |
|
6.0 |
% |
|
10.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Heparin products and sausage casing |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
2,230,759 |
|
$ |
15,318,798 |
|
$ |
(13,088,039) |
|
(85) |
% |
Cost of revenue |
|
|
2,105,248 |
|
|
16,267,458 |
|
|
(14,162,210) |
|
(87) |
% |
Gross profit |
|
$ |
125,511 |
|
$ |
(948,660) |
|
$ |
1,074,171 |
|
(113) |
% |
Gross Margin |
|
|
5.6 |
% |
|
(6.2) |
% |
|
11.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fertilizer |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
905,910 |
|
$ |
2,000,453 |
|
$ |
(1,094,543) |
|
(55) |
% |
Cost of revenue |
|
|
553,329 |
|
|
1,060,302 |
|
|
(506,973) |
|
(48) |
% |
Gross profit |
|
$ |
352,581 |
|
$ |
940,151 |
|
$ |
(587,570) |
|
(62) |
% |
Gross Margin |
|
|
38.9 |
% |
|
47.0 |
% |
|
(8.1) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
25,097,951 |
|
$ |
46,471,478 |
|
$ |
(21,373,527) |
|
(46) |
% |
Cost of revenue |
|
|
20,983,196 |
|
|
44,719,984 |
|
|
(23,736,788) |
|
(53) |
% |
Gross profit |
|
$ |
4,114,755 |
|
$ |
1,751,494 |
|
$ |
2,363,261 |
|
135 |
% |
Gross Margin |
|
|
16.4 |
% |
|
3.8 |
% |
|
12.6 |
% |
|
|
Oxytetracycline & Licorice Products and TCMD
Cost of revenue for our oxytetracycline and licorice products and TCMD was $18.3 million for the year ended September 30, 2024, a decrease of $9.1 million, or 33%, from $27.4 million for the year ended September 30, 2023, which was primarily attributable to the decreased sales of the product as described above. Gross margin of our oxytetracycline and licorice products increased from 6.0% to 16.6% primarily due to 1) the newly developed licorice extract products have low costs, and the ratio of dry and wet licorice is constantly adjusted to reduce costs and increase gross margin; 2) as described before, the price of licorice products has increased, resulting in an increase in gross profit Cost of revenue for our heparin products and sausage casings was $2.1 million for the year ended September 30, 2024, a decrease of $14.2 million, or 87%, from $16.3 million for the year ended September 30, 2023.
98
Heparin Products and Sausage Casings
This was primarily attributable to the decreased sales of $13.1 million, or 85%, for the year ended September 30, 2024 compared to the fiscal year ended September 30, 2023. From the negative gross margin of 6.2% in this segment for the fiscal year 2023 to gross margin of 5.6% for the fiscal year 2024, was due to the sales of heparin products and sausage casings in 2024, which is the remaining inventory in 2023. In 2023, the company increased its procurement of fresh sausages, resulting in a 5% increase in yield. Additionally, the average processing fee decreased from 4.51 yuan to 4.09 yuan or 9%.
Fertilizer
Cost of revenue for our fertilizer products was approximately $0.6 million for the year ended September 30, 2024, a decrease of approximately $0.5 million, or 48%, from approximately $1.1 million for the year ended September 30, 2023. This was primarily attributable to the decreased sales of $1.1 million, or 55%, for the year ended September 30, 2024 compared to the fiscal year ended September 30, 2023.
Selling, General and Administrative, Research and Development Expenses
Selling, general and administrative expenses were $4.7 million for the year ended September 30, 2024, representing an increase of approximately $0.3 million, or 7%, from $4.4 million for the year ended September 30, 2023. The increase was mainly attributable to an increase of legal fees of $0.2 million in the parent company.
Interest Income, net
Interest expense, net for the year ended September 30, 2024, increased by approximately $0.7 million. The increase of the balance is due to interest expense of Gansu QLS of prior years were adjusted in 2024.
Other Expense
Other expense was $0.9 million for the year ended September 30, 2024, as compared to $5.4million for the year ended September 30, 2023, which primarily consisted of government grants and investment loss. The decrease is mainly from the $4.7 million increased loss recognized from the fair value change in the investment in trading securities for the year ended September 30, 2023.
Income Tax Expense/(Benefit)Income tax expense decreased by $0.8million, or 383%, from $219,166 for the year ended September 30, 2023 to $ (619,981) for the year ended September 30, 2024. The decreased tax expense for 2024 with a loss before income tax provision is due to the increased for deferred tax asset.
Net Loss Attributable to Non-controlling interest
Net loss attributable to non-controlling interest was approximately $74,331 for the fiscal year ended September 30, 2024, a decrease of $0.3 million, or 78%, from approximately $0.3 million of net loss attributable to non-controlling interest for the year ended September 30, 2023. The decrease was a result of the decrease of net loss of Chengdu QLS, which is partially owned by non-controlling interest holders. Chengdu QLS and its subsidiaries experienced a net loss of approximately $0.3 million for the year ended September 30, 2024 and it experienced a net loss of approximately $1.6 million for the year ended September 30, 2023. Chengdu QLS and its subsidiaries manufacture our heparin products and sausage casings. See “—Net Revenue—Heparin Products and Sausage Casings” and “—Cost of Revenue and Gross Profit—Heparin Products and Sausage Casings.”
Net Loss Attributable to Our Shareholders
As a result of the above, our net loss attributable to our shareholders decreased by $6.3 million, or 81%, from net loss attributable to our shareholders of $7.8 million for the year ended September 30, 2023 to net loss attributable to our shareholders of $1.4 million for the year ended September 30, 2024.
99
EBITDA
The following table sets forth of the calculation of our EBITDA:
|
|
For the years ended |
|
|
|
|
|
|
||||
|
|
September 30, |
|
Changes |
|
|||||||
|
|
2024 |
|
2023 |
|
Amount |
|
% |
|
|||
Net income |
|
$ |
(1,517,161) |
|
$ |
(8,122,070) |
|
$ |
6,604,909 |
|
(81) |
% |
Interest income (expense) |
|
|
639,511 |
|
|
(99,190) |
|
|
738,701 |
|
(745) |
% |
Income tax provision |
|
|
(619,981) |
|
|
219,166 |
|
|
(839,147) |
|
(383) |
% |
Depreciation & Amortization |
|
|
1,237,229 |
|
|
1,143,064 |
|
|
94,165 |
|
8 |
% |
EBITDA |
|
$ |
(260,402) |
|
$ |
(6,859,030) |
|
$ |
6,598,628 |
|
(96) |
% |
Percentage of EBITDA to revenue |
|
|
(1.0) |
% |
|
(14.8) |
% |
|
13.7 |
% |
|
|
Our EBITDA was $(0.3) million for the year ended September 30, 2024, an increase of $6.6 million, or (96)%, compared to $(6.9) million for the year ended September 30, 2023. This was mainly due to the increase in net income resulting from increased the loss from investment due to the fair value change discussed above for the years ended September 30, 2023. The percentage of EBITDA to revenue was (1.0) % and (14.8)% for the years ended September 30, 2024 and 2023, respectively.
Results of Operations for the years ended September 30, 2023 and 2022
The following table sets forth a summary of our consolidated results of operations for the years ended September 30, 2023 and 2022. The historical results presented below are not necessarily indicative of the results that may be expected for any future period.
|
|
For the years ended |
|
|
|
|
|
|
||||
|
|
September 30, |
|
Changes |
|
|||||||
|
|
2023 |
|
2022 |
|
Amount |
|
% |
|
|||
Net revenue |
|
$ |
46,471,478 |
|
$ |
64,855,025 |
|
$ |
(18,383,547) |
|
(28) |
% |
Cost of revenue |
|
|
44,719,984 |
|
|
58,627,728 |
|
|
(13,907,744) |
|
(24) |
% |
Gross profit |
|
|
1,751,494 |
|
|
6,227,297 |
|
|
(4,475,803) |
|
(72) |
% |
Selling, general and administrative, research and development expenses |
|
|
4,361,593 |
|
|
4,125,294 |
|
|
236,299 |
|
6 |
% |
Income from operations |
|
|
(2,610,099) |
|
|
2,102,003 |
|
|
(4,712,102) |
|
(224) |
% |
Interest income |
|
|
99,190 |
|
|
24,860 |
|
|
74,330 |
|
299 |
% |
Other expense |
|
|
(5,391,995) |
|
|
(566,304) |
|
|
(4,825,691) |
|
852 |
% |
Income (loss) before income tax provision |
|
|
(7,902,904) |
|
|
1,560,559 |
|
|
(9,463,463) |
|
(606) |
% |
Income tax expense |
|
|
219,166 |
|
|
194,302 |
|
|
24,864 |
|
13 |
% |
Net income (loss) |
|
|
(8,122,070) |
|
|
1,366,257 |
|
|
(9,488,327) |
|
(694) |
% |
Less: net income (loss) attributable to non-controlling interest |
|
|
(341,450) |
|
|
289,564 |
|
|
(631,014) |
|
(218) |
% |
Net income (loss) attributable to BGM Group Ltd |
|
$ |
(7,780,620) |
|
$ |
1,076,693 |
|
$ |
(8,857,313) |
|
(823) |
% |
Net Revenue
The following table sets forth the breakdown of our net revenue:
|
|
For the years ended |
|
|
|
|
|
|
||||||||
|
|
September 30, |
|
|
|
|
|
|
||||||||
|
|
2023 |
|
2022 |
|
Changes |
|
|||||||||
|
|
Amount |
|
% |
|
Amount |
|
% |
|
Amount |
|
% |
|
|||
Net revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oxytetracycline & licorice products and TCMD |
|
$ |
29,152,228 |
|
63 |
% |
$ |
40,305,988 |
|
62 |
% |
$ |
(11,153,760) |
|
(28) |
% |
Heparin products and sausage casing |
|
$ |
15,318,798 |
|
33 |
% |
$ |
23,460,467 |
|
36 |
% |
$ |
(8,141,669) |
|
(35) |
% |
Fertilizer |
|
$ |
2,000,452 |
|
4 |
% |
$ |
1,088,570 |
|
2 |
% |
$ |
911,882 |
|
84 |
% |
Total |
|
$ |
46,471,478 |
|
100.0 |
% |
$ |
64,855,025 |
|
100.0 |
% |
$ |
(18,383,547) |
|
(28) |
% |
100
Compared with net revenue for the year ended September 30, 2022, our net revenue decreased by $18.4 million, or 28%, for the year ended September 30, 2023, which was primarily attributable to a $11.2 million decrease in sales from Oxytetracycline & licorice products and TCMD, and a $8.1 million decrease in sales from heparin products and sausage casing, offset by $0.9 million increased sales from Fertilizer product.
Oxytetracycline & Licorice Products and TCMD
The sales of oxytetracycline products, licorice products and TCMD accounted for 95%, 5% and 0%, respectively, of this segment’s total sales for the year ended September 30, 2023, and 98%, 2% and 0%, respectively, of such segment’s total sales for the year ended September 30, 2022. For the year ended September 30, 2023, the WFOE and the VIE and its subsidiaries’ sales translated into USD for oxytetracycline products, licorice products and TCMD decreased by approximately $11.2 million, or 28%, from approximately $40.3 million for the year ended September 30, 2022 to approximately $29.2 million for the year ended September 30, 2023. The decrease in sales in this segment is due to a decrease of oxytetracycline product for $11.8 million, offset by 0.7 million of increase from sales of licorice product. Due to the intense economic relation and trade conflicts between China and U.S, we have seen the significant decrease of demand of our oxytetracycline products from customers in the exporting industry. In 2023, the Company had to cease operation of the oxytetracycline manufacturing facility for two months in order to reduce inventory. Sales quantity decreased by 24% compared to 2022 and sales price decreased by 8%. In addition, with the ease of restrictive measures relating to the COVID-19, the Company decreased the sales price of licorice product by 6% in order to take the market shares. The pricing policy stimulated the sales significantly and increased the quantity sold by 155% compared to that of the year ended September 30, 2022.
Heparin Products and Sausage Casings
Sales from heparin products and sausage casing decreased by $8.1 million, or 35%, from $23.5 million for the year ended September 30, 2022, to $15.3 million for the year ended September 30, 2023. Similar to oxytetracycline as described above, we experienced the significant decrease of demand for heparin product and sausage casing products from customers due to the intense trading relation with the U.S and European countries. At the same time, we did not see much increase of domestic consumption post COVID. In addition, commence from February 2023, heparin product became one of the centralized procurement medicine regulated by the PRC government which intensified the competition of the product in the market. Selling price was decreased by 28% and 7% from 2022 to September 2023 for heparin products and sausage casing respectively. Sales quantity increased by 9% for heparin products and decreased 9% for sausage casing from 2022 to 2023 respectively.
Fertilizer
Sales from fertilizer increased by $0.9 million, or 84%, from $1.1 million for the year ended September 30, 2022 to $2.0 million for the year ended September 30, 2023. For the fiscal year ended September 30, 2023, the sales are mainly made to governmental purchase program with higher price and large volume.
101
Cost of Revenue and Gross Profit
The following tables set forth the calculation of gross profit and gross margin for the each of our segments:
|
|
For the years ended |
|
|
|
|
|
|
||||
|
|
September 30, |
|
Changes |
|
|||||||
|
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
% |
|
Oxytetracycline & licorice products and TCMD |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
29,152,228 |
|
$ |
40,305,988 |
|
$ |
(11,153,760) |
|
(28) |
% |
Cost of revenue |
|
|
27,392,225 |
|
|
36,210,950 |
|
|
(8,818,725) |
|
(24) |
% |
Gross profit |
|
$ |
1,760,003 |
|
$ |
4,095,038 |
|
$ |
(2,335,035) |
|
(57) |
% |
Gross Margin |
|
|
6.0 |
% |
|
10.2 |
% |
|
(4.2) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Heparin products and sausage casing |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
15,318,798 |
|
$ |
23,460,467 |
|
$ |
(8,141,669) |
|
(35) |
% |
Cost of revenue |
|
|
16,267,458 |
|
|
21,656,748 |
|
|
(5,389,290) |
|
(25) |
% |
Gross profit |
|
$ |
(948,660) |
|
$ |
1,803,719 |
|
$ |
(2,752,379) |
|
(153) |
% |
Gross Margin |
|
|
(6.2) |
% |
|
7.7 |
% |
|
(13.9) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fertilizer |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
2,000,453 |
|
$ |
1,088,570 |
|
$ |
911,883 |
|
84 |
% |
Cost of revenue |
|
|
1,060,302 |
|
|
760,030 |
|
|
300,272 |
|
40 |
% |
Gross profit |
|
$ |
940,151 |
|
$ |
328,540 |
|
$ |
611,611 |
|
186 |
% |
Gross Margin |
|
|
47.0 |
% |
|
30.2 |
% |
|
16.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
46,471,478 |
|
$ |
64,855,025 |
|
$ |
(18,383,547) |
|
(28) |
% |
Cost of revenue |
|
|
44,719,984 |
|
|
58,627,728 |
|
|
(13,907,743) |
|
(24) |
% |
Gross profit |
|
$ |
1,751,494 |
|
$ |
6,227,297 |
|
$ |
(4,475,803) |
|
(72) |
% |
Gross Margin |
|
|
3.8 |
% |
|
9.6 |
% |
|
(5.8) |
% |
|
|
Oxytetracycline & Licorice Products and TCMD
Cost of revenue for our oxytetracycline and licorice products and TCMD was $27.4 million for the year ended September 30, 2023, a decrease of $8.8 million, or 24%, from $36.2 million for the year ended September 30, 2022, which was primarily attributable to the decreased sales of the product as described above. Gross margin of our oxytetracycline and licorice products decreased from 10.2% to 6% primarily due to 1) selling price decreased because of the weak demand and over supply of product in the market as described in the revenue section above, and 2) cost per unit increased as the production was decreased, and allocation of fixed overhead cost increased. For the year ended September 30, 2023, the production of oxytetracycline decreased by 25%.
Heparin Products and Sausage Casings
Cost of revenue for our heparin products and sausage casings was $16.3 million for the year ended September 30, 2023, a decrease of $5.4 million, or 25%, from $21.7 million for the year ended September 30, 2022. This was primarily attributable to the decreased sales of $8.1 million, or 35%, for the year ended September 30, 2023 compared to the fiscal year ended September 30, 2022. The negative gross margin in this segment for the fiscal year 2023 was due to significant drop of selling price of heparin. As described above, the exporting demand was significant affected by the trading relation between China and the U.S and European countries. The supply exceeded the demand dramatically, which led to the decrease in the selling price. In addition, commence from February 2023, heparin product became one of the centralized procurement medicine by the PRC government which intensified the competition of the product in the market, leading the decrease of selling price. At the same time, heparin product had the first profitable year in 2022 since as the combined effects of the COVID-19 pandemic and African Swine fever have gradually reduced. In addition, the raw material’s price had an increase in 2023 as well.
102
Fertilizer
Cost of revenue for our fertilizer products was approximately $1.1 million for the year ended September 30, 2023, an increase of approximately $300,000, or 40%, from approximately $706,000 for the year ended September 30, 2022. Cost went up as the sales went up in 2023. Gross margin increased by 16.8% of the sales was made primarily to governmental purchase program with a higher price. For the fiscal year ended September 30, 2023, average selling price increased by 13% compared to that of 2022. In addition, unit cost of the fertilizer decreased slightly as the Company increased its manufacturing efficiencies with a lower fixed manufacturing cost allocation to each unit produced.
Selling, General and Administrative, Research and Development Expenses
Selling, general and administrative expenses were $4.4 million for the year ended September 30, 2023, representing an increase of approximately $0.2 million, or 6%, from $4.1 million for the year ended September 30, 2022. The increase was mainly attributable to an increase of $0.7 million due to the maintenance cost during shutdown and bad debt expense, as well as $0.2 million increased sales force payroll and shipping cost due to inflation, offset by a decrease in R&D expenses of $0.7 million due to the decrease of R&D projects.
Interest Income (Expenses), net
Interest income (expense), net for the year ended September 30, 2023 increased by approximately $74,000 compared to $25,000 for the year ended September 30, 2022. The increase of the balance is due to decrease of interest expense from decreased bank loan balance outstanding during 2023 compared to that in 2022.
Other Income (expense)
Other expense was $5.4 million for the year ended September 30, 2023, as compared to $0.6 million for the year ended September 30, 2022, which primarily consisted of government grants and investment loss. The increase is mainly from the $4.7 million increased loss recognized from the fair value change in the investment in trading securities.
Income Taxes Provision
Provision for income taxes increased by $25,000, or 13%, from $194,000 for the year ended September 30, 2022 to $219,000 for the year ended September 30, 2023. The increased tax expense for 2023 with a loss before income tax provision is due to the increased provision made for deferred tax asset. Based on the management’s expectation, the deferred tax asset is probably not able to be realized as a tax benefit for future periods due to the expected loss.
Net Income (loss) Attributable to Non-controlling interest
Net loss attributable to non-controlling interest was approximately $0.3 million for the fiscal year ended September 30, 2023, a decrease of $0.6 million, or 218%, from approximately $0.3 million of net income attributable to non-controlling interest for the year ended September 30, 2022. The decrease was a result of the increase of net loss of Chengdu QLS, which is partially owned by non-controlling interest holders. Chengdu QLS and its subsidiaries experienced a net loss of approximately $1.6 million for the year ended September 30, 2023 and it experienced a net income of approximately $1.3 million for the year ended September 30, 2022. Chengdu QLS and its subsidiaries manufacture our heparin products and sausage casings. See “—Net Revenue—Heparin Products and Sausage Casings” and “—Cost of Revenue and Gross Profit—Heparin Products and Sausage Casings.”
Net Income (loss) Attributable to Our Shareholders
As a result of the above, our net income attributable to our shareholders decreased by $8.9 million, or 823%, from $1.1 million for the year ended September 30, 2022 to net loss attributable to our shareholders of $7.8 million for the year ended September 30, 2023.
103
EBITDA
The following table sets forth of the calculation of our EBITDA:
|
|
For the years ended |
|
|
|
|
|
|
||||
|
|
September 30, |
|
Changes |
|
|||||||
|
|
2023 |
|
2022 |
|
Amount |
|
% |
|
|||
Net income |
|
$ |
(8,122,070) |
|
$ |
1,366,257 |
|
$ |
(9,488,327) |
|
(694) |
% |
Interest expense |
|
|
(99,190) |
|
|
(24,860) |
|
|
(74,330) |
|
299 |
% |
Income tax provision |
|
|
219,166 |
|
|
194,302 |
|
|
24,864 |
|
13 |
% |
Depreciation & Amortization |
|
|
1,143,064 |
|
|
1,224,672 |
|
|
(81,608) |
|
(7) |
% |
EBITDA |
|
$ |
(6,859,030) |
|
$ |
2,760,371 |
|
$ |
(9,619,401) |
|
(348) |
% |
Percentage of EBITDA to revenue |
|
|
(14.8) |
% |
|
4.3 |
% |
|
(19.1) |
% |
|
|
Our EBITDA was $(6.9) million for the year ended September 30, 2023, a decrease of $9.6 million, or 348%, compared to $2.8 million for the year ended September 30, 2022. This was mainly due to the decrease in net income resulting from decreased gross profit as well as the loss from investment due to the fair value change discussed above. The percentage of EBITDA to revenue was (14.8) % and 4.3% for the years ended September 30, 2023 and 2022, respectively.
B.Liquidity and Capital Resources
Liquidity and Capital Resources
As of September 30, 2024, we had cash of approximately $9.8 million. We have funded our working capital and other capital requirements primarily by cash flow from operations, and bank loans. In the year ended September 30, 2021, we received approximately $24 million as proceeds from our initial public offering. Cash was used to pay for the investment in trading securities.
Although our management believes that the cash generated from operations will be sufficient to meet our normal working capital needs for at least the next twelve months, our ability to repay our current obligations will depend on the future realization of our current assets. Our management has considered the historical experience, the economy, trends in the pharmaceutical industry, the expected collectability of accounts receivable and the realization of the inventories as of September 30, 2024. Based on these considerations, our management believes that we have sufficient funds to meet our working capital requirements and debt obligations as they become due for at least the next twelve months from the date of this annual report. However, there is no assurance that management will be successful in their plan. There are a number of factors that could potentially arise and result in shortfalls to our plan, such as the demand for the WFOE and the VIE and its subsidiaries’ products, economic conditions, the competitive pricing in the industry and our banks and suppliers being able to provide continued supports. If the future cash flow from operations and other capital resources are insufficient to fund our liquidity needs, we may be forced to reduce or delay our expected acquisition plan, sell assets, obtain additional debt or equity capital or refinance all or a portion of our and our affiliates’ debt.
The following table summarizes our cash flow data for the years ended September 30, 2024, 2023 and 2022:
|
|
For the Years ended |
||||||
|
|
September 30, |
||||||
|
|
2024 |
|
2023 |
|
2022 |
||
Net cash provided by operating activities |
|
$ |
544,238 |
|
$ |
312,209 |
|
12,654,188 |
Net cash provided by (used) in investing activities |
|
|
983,418 |
|
|
(4,742,445) |
|
(3,258,953) |
Net cash used in financing activities |
|
|
(491,728) |
|
|
(2,921,084) |
|
(5,937,529) |
Effect of exchange rate on cash |
|
|
1,305,079 |
|
|
(151,446) |
|
(1,086,067) |
Net (decrease) increase in cash, cash equivalents and restricted cash |
|
$ |
2,341,007 |
|
$ |
(7,502,766) |
|
2,371,640 |
Operating Activities
Net cash provided by operating activities consists primarily of net income adjusted for non-cash items, including depreciation and amortization, accounts receivable and inventory reserve, deferred tax, unrealized gain(loss) from trading securities and adjusted for the effect of working capital changes.
104
Net cash provided by operating activities was approximately $0.5 million for the year ended September 30, 2024, an increase of $0.2million in cash provided by operating activities, or 74%, compared to net cash provided by operating activities of $0.3 million for the year ended September 30, 2023. The increase of net cash inflow was a result of the following:
1. |
Decrease in net loss of $6.6 million, from net loss of $8.1 million to net loss of $1.5 million. |
2. |
Change in inventory reserve was $0.8 million net cash outflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in inventory reserve was $0.4 million net cash inflow, which led to a $1.2 million decrease in net cash outflow from operating activities. It’s because the inventory reserve was provisioned in previous years was sold in 2024, so the inventory reserve made in previous years was written off in 2024. |
3. |
Change in account receivable was $0.4 million net cash inflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in account receivable was $1.2 million net cash outflow, which led to a $1.6 million increase in net cash inflow from operating activities. |
4. |
Change in bank acceptance note receivable was $0.9 million net cash inflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in bank acceptance note receivable was $1.7 million net cash outflow, which led to a $2.6 million increase in net cash inflow from operating activities. The main reason is that since November 2023, the production volume of oxytetracycline has been halved. As a result, the sales volume has decreased, leading to a corresponding reduction in cash collection. Moreover, 90% of the payments received for oxytetracycline are mainly in the form of six - month bank acceptance drafts. With the decrease in sales volume, the corresponding notes receivable have also declined. |
5. |
Change in inventory was $1.0 million net cash inflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in inventory was $3.4 million net cash inflow, which led to a $2.4 million decrease in net cash outflow from operating activities. This is mainly because the market price of heparin sodium has been continuously dropping since March 2023 under the influence of the national centralized drug procurement policy. The company decided to shut down all heparin sodium production lines to reduce business risks. The production suspension is the main reason. |
6. |
Change in other current assets was $1.5 million net cash outflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in other current assets was $1.4 million net cash inflow, which led to a $2.9 million decrease in net cash outflow from operating activities. This is mainly because the input tax was reclassified to other current assets in 2024. |
7. |
Change in account payable was $0.4 million net cash inflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in account payable was $1.6 million net cash outflow, which led to a $2.0 million increase in net cash inflow from operating activities. |
8. |
Change in contract liabilities was $0.6 million net cash outflow for the year ended September 30, 2024. For the year ended September 30, 2023, the change in contract liabilities was $0.5 million net cash inflow, which led to a $1.1 million decrease in net cash outflow from operating activities. |
9. |
Change in unrealized gain from marketable securities was $0.8 million for the year ended September 30, 2024. For the year ended September 30, 2023, the change in unrealized gain from marketable securities was $5.5 million net cash inflow, which led to a $4.7 million decrease in net cash outflow from operating activities. This is due to the company’s redemption of part of the funds and the conversion of the remaining funds into stocks. |
Net cash provided by operating activities was approximately $0.3 million for the year ended September 30, 2023, a decrease of $12.3 million in cash provided by operating activities, or 98%, compared to net cash provided by operating activities of $12.7 million for the year ended September 30, 2022. The decrease of net cash inflow was a result of the following:
1. |
Decrease in net income of $9.5 million, from $1.4 million to net loss of 8.1 million. Net loss excluding non-cash items were $0.8 million, compared to $3.9 million of net income, which represented a decrease of cash inflow of $4.7 million |
105
2. |
Change in account receivable was $1.2 million net cash outflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in account receivable was $1.0 million net cash inflow, which led to a $2.2 million decrease in net cash inflow from operating activities. |
3. |
Change in bank acceptance note receivable was $1.7 million net cash outflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in bank acceptance note receivable was $8.7 million net cash inflow, which led to a $10.4 million decrease in net cash inflow from operating activities. |
4. |
Change in inventory was $3.4 million net cash inflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in inventory was $2.2 million net cash inflow, which led to a $1.2 million increase in net cash inflow from operating activities. |
5. |
Change in other current assets was $1.4 million net cash inflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in other current assets was $1.2 million net cash outflow, which led to a $2.6 million increase in net cash inflow from operating activities. |
6. |
Change in account payable was $1.6 million net cash outflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in account payable was $0.8 million net cash outflow, which led to a $0.8 million increase in net cash outflow from operating activities. |
7. |
Change in contract liabilities was $0.5 million net cash inflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in contract liabilities was $1.8 million net cash outflow, which led to a $2.3 million increase in net cash inflow from operating activities. |
8. |
Change in tax payable was $0.6 million net cash outflow for the year ended September 30, 2023. For the year ended September 30, 2022, the change in tax payable was $0.6 million net cash inflow, which led to a $1.2 million increase in net cash outflow from operating activities. |
Investing Activities
Net cash provided by investing activities was approximately $1.0 million for the year ended September 30, 2024, an increase of $5.7million, or (121%), compared to $4.7 million net cash used in investing activities for the year ended September 30, 2023. The increase was mainly due to the decreased short term investment of for $1.0 million and, the purchase of intangible assets of $0.8 million, the increase of investment in payments on long term investment of $1.4 million, cash received from disposal of long term investment of $0.5million, the increase of payment made for construction of $0.9 million, and redemption from marketable securities $4.8million.
Net cash used in investing activities was approximately $4.7 million for the year ended September 30, 2023, an increase of $1.5 million, or 46%, compared to $3.3 million net cash used in investing activities for the year ended September 30, 2022. The increase was mainly due to the increase of investment in short term investment of $1 million, purchase of intangible assets of $1.8 million, increase of payment made for construction of $0.5 million, offset by the decreased purchase of property and equipment for $1.8 million.
Financing Activities
Net cash used in financing activities was approximately $0.5 million for the year ended September 30, 2024, a decrease of $2.4 million, or 83%, compared to $2.9 million for the year ended September 30, 2023. The decrease was mainly a result of $0.7 million decrease from net cash repaid for bank loan and bank notes payable, and a $1.8 million decrease in dividend paid.
Net cash used in financing activities was approximately $2.9 million for the year ended September 30, 2023, a decrease of $3.0 million, or 51%, compared to $5.9 million for the year ended September 30, 2022. The decrease was mainly a result of $4.8 million decrease from net cash repaid for bank loan and bank notes payable, offset by a $1.8 million increase in dividend paid.
106
Capital Expenditures
Our capital expenditures were $3.9 million, $3.7 million and $3.2 million in fiscal years ended September 30, 2024, 2023 and 2022, respectively. We intend to fund our future capital expenditures with our existing cash balance and cash flow from operating activities. We will continue to make capital expenditures to meet the expected growth of the WFOE and the VIE and its subsidiaries’ business. The capital expenditure for the year ended September 30, 2024 is estimated to be $2.2 million for the new facility to be built in Chongqing city for producing our heparin products, and the construction of the new facility.
Holding Company Structure
BGM Group Ltd (“BGM”) is a holding company with no material operations of its own. BGM conducts its operations primarily through its subsidiaries, the VIE and the VIE’s subsidiaries in China. As a result, BGM’s ability to pay dividends depends upon dividends paid by our PRC Subsidiary. In addition, our PRC Subsidiary is permitted to pay dividends to us only out of its retained earnings, if any, as determined in accordance with the Accounting Standards for Business Enterprise as promulgated by the Ministry of Finance of the PRC, or PRC GAAP. Pursuant to the law applicable to China’s foreign investment enterprise, foreign investment enterprise in the PRC have to make appropriation from their after-tax profit, as determined under PRC GAAP, to reserve funds including (i) general reserve fund, (ii) enterprise expansion fund and (iii) staff bonus and welfare fund. The appropriation to the general reserve fund must be at least 10% of the after-tax profits calculated in accordance with PRC GAAP. Appropriation is not required if the reserve fund has reached 50% of the registered capital of our PRC subsidiary. Appropriation to the other two reserve funds is at our PRC subsidiary’s discretion.
As an offshore holding company, we are permitted under PRC laws and regulations to provide funding from the proceeds of our offshore fund raising activities to our PRC Subsidiary only through loans or capital contributions, subject to the satisfaction of the applicable government registration and approval requirements. See “Item 3. Key Information—3.D. Risk Factors—Risks Related to Doing Business in China— PRC regulation of loans to, and direct investments in, PRC entities by offshore holding companies may delay or prevent us from using proceeds from future financing activities to make loans or additional capital contributions to our PRC Subsidiary.” As a result, there is uncertainty with respect to BGM’s ability to provide prompt financial support to the PRC Subsidiary when needed.
C. Research and Development, Patents and Licenses, etc.
See “Item 4. Information on the Company—B. Business Overview—Intellectual Property.”
D. Trend Information
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the fiscal year ended September 30, 2024 that are reasonably likely to have a material effect on our net revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimates
Our significant accounting policies and their effect on our financial condition and results of operations are fully disclosed in our consolidated financial statements included elsewhere in this annual report. We have prepared our consolidated financial statements in conformity with U.S. GAAP, which requires management to make estimates and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. These estimates are prepared using our best judgment, after considering past and current events and economic conditions. While management believes the factors evaluated provide a meaningful basis for establishing and applying sound accounting policies, management cannot guarantee that the estimates will always be consistent with actual results. In addition, certain information relied upon by us in preparing such estimates includes internally generated financial and operating information and external market information. Actual results may differ from these estimates.
107
We consider an accounting estimate to be critical if: (1) it requires us to make assumptions because the information was not available at the time or it included matters that were highly uncertain at the time we were making our estimate and (2) changes in the estimate could have a material impact on our financial condition or results of operations. We consider the following accounting policies to be both those most important to our financial condition and those that require the most subjective judgment:
Valuation of investment in trading securities: Management relies on estimates of projected cashflows as support for the amounts disclosed in the Company’s financial statements as investments and valuation allowances taken against respective investments. The projections are based on the best estimates available. However, these estimates are subject to potential changes in market conditions, interest rates and market liquidity considerations. Certain inputs involve unobservable inputs and are classified as level 3 of the fair value hierarchy (see Note 2, Summary of significant account policies-Fair Value of Financial Instruments to our consolidated financial statements included elsewhere in this Annual Report). The sensitivity of the fair value calculation to these methods, assumptions, and estimates included could create materially different results under different conditions or using different assumptions.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth information regarding our directors and executive officers as of the date of this annual report.
Name |
|
Age |
|
Position(s) |
Zhanchang Xin |
|
58 |
|
Chairman of the Board of Directors |
Waihua Xu |
|
31 |
|
Independent Director |
Ming Jing |
|
63 |
|
Independent Director |
Maofan Tang |
|
28 |
|
Independent Director |
Furong Cao |
|
54 |
|
Director |
Chen Xin |
|
32 |
|
Chief Executive Officer |
Yaxuan Zhai |
|
31 |
|
Chief Financial Officer |
Mr. Zhanchang Xin has been our Chairman of the Board since our incorporation and had served as the Chief Executive Officer from 2019 to 2024. Since August 2006, Mr. Xin has served as Chairman of the Board of Gansu QLS. Mr. Xin has over 30 years of research and engineering in the pharmaceutical industry. He has worked for Gansu QLS for 34 years and has published pharmaceutical research papers in Chinese medical journals such as “China Medical Industry Journal” and “Gansu Pharmaceutical”. In June 1986, Mr. Xin received a Bachelor Degree in Pharmacy from the School of Medicine at Lanzhou University. Mr. Xin received his Master Degree in Business Administration from Beijing Technology and Business University in December 2004.
Ms. Waihua Xu has served as our independent director since May 2024. Ms. Xu has acquired a wealth of experience in marketing and public relations. Since Auguest 2023, Ms. Xu has been the head of social media and UGC community teams at Shenzhen Geruidi Technology, Ltd., responsible for content operations. From June 2021 to August 2023, Ms. Xu worked at Shenzhen Yiyu Technology, Ltd., as the head of overseas new media operations. From August 2016 to June 2021, Ms. Xu worked as the customer manager at HomilyChart Canada Inc, responsible for developing and implementing marketing plan. Ms. Xu obtained her master’s degree in Leadership from Trinity Western University in 2019 and her bachelor’s degree in English from Hunan Institute of Engineering in 2016.
Mr. Ming Jing has served as our independent director since December 2020. From 2003 to present, Mr. Jing has been a professor, doctorate degree tutor and associate Dean of the School of Pharmacy at Gansu University of Traditional Chinese Medicine and published more than seventy research papers on prominent science journals such as Science Citation Index (SCI) and Chinese Science Citation Database (CSCD). To date, Mr. Jing has also obtained 6 National Patent Certificates as a first inventor. Mr. Jing earned a Bachelor’s Degree from Lanzhou University in 1986.
Mr. Maofan Tang has served as our independent director since June 2023. He has been working as an auditing professional and serving in a middle management position at Beijing Topson LLP since January 2019. Mr. Tang served as an investment manager at Chengdu Shangtou Jinsheng Equity Investment Fund Management Co., Ltd. from March 2018 to December 2018, where he conducted investment analysis, managed investment portfolios, allocated assets, mitigated risks, made investment decisions, oversaw compliance, provided performance reporting, etc. From April 2017 to March 2018, Mr. Tang served as a researcher at Tianfeng Securities Co. Mr. Tang received his postgraduate degree in Finance and Big Data from the University of Sydney in 2019. He received his Bachelor’s Degree in Banking and Finance from Monash University in 2017.
108
Ms. Furong Cao has served as our director since May 2024. Ms. Cao is an experienced professional in business management. She has acquired a wealth of business management experience across a diverse range of industries, such as medical technology, pharmaceuticals, finance, and management consulting. Since July 2021, Ms. Cao has served as the director of operations of Shenzhen Financial Investment Service Co., Ltd., responsible for overseeing the investment strategies. From July 2017 to June 2021, Ms. Cao served as the business director of Shenzhen Beida Sequoia Business Management Co., Ltd., where she was responsible for financial project planning and investment risk assessment. Ms. Cao obtained her bachelor’s degree in Medical Profession from Shanghai Second Medical College in 1993.
Mr. Chen Xin has served as our chief executive officer since May 2024. Mr. Xin worked as an algorithm engineer at Geely Auti Holdings Limited from August 2022 to February 2024, where he led a team in developing perception algorithms for autonomous driving systems. From June 2021 to August 2022, he was an algorithm engineer at Shenzhen DJ Innovatives, where he engineered on image processing algorithms for autonomous driving vehicles. Mr. Xin obtained his bachelor’s degree in Physics from Sichuan University in 2016 and his master’s degree in Physics from National University of Singapore in 2019.
Ms. Yaxuan Zhai has served as our chief financial officer since May 2024 and had served as the finance manager at the Company from October 2023 to May 2024. She worked as an auditor at Baker Tilly China Certified Public Accountants from November 2022 to October 2023. Ms. Zhai obtained her bachelor’s degree in Investment from Fujian Jiangxia University in 2018 and her master’s degree in Finance from The University of Sheffield in 2021.
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as of the date of this annual report.
Board Diversity Matrix | ||||
Country of Principal Executive Offices: |
China |
|||
Foreign Private Issuer |
Yes |
|||
Disclosure Prohibited under Home Country Law |
No |
|||
Total Number of Directors |
5 |
|||
|
Female |
Male |
Non- Binary |
Did Not |
Part I: Gender Identity |
|
|||
Directors |
2 |
3 |
0 |
0 |
Part II: Demographic Background |
|
|||
Underrepresented Individual in Home Country Jurisdiction |
1 |
|||
LGBTQ+ |
0 |
|||
Did Not Disclose Demographic Background |
0 |
|||
Family Relationships
None of our directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K, except that Mr. Zhanchang Xin is the father of Mr. Chen Xin.
109
B. Compensation
Compensation
For the fiscal year ended September 30, 2024, we paid an aggregate of $63,122 in cash to our directors and executive officers, and we paid an aggregate of US$26,650 cash compensation to our non-executive directors. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our directors and executive officers. The PRC Subsidiary, the VIE, and the VIE’s subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance and other statutory benefits and a housing provident fund.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for a specified time period, which will be renewed automatically renewed for an additional one-year term if neither party provides written notice to the other party or proposes to re-negotiate the terms of such agreement three months before the end of the current employment term. We may terminate the employment for cause, at any time, without notice or remuneration, for certain acts of the executive officer, including but not limited to the commitments of any serious or persistent breach or non-observance of the terms and conditions of their employment, conviction of a criminal offense, willful disobedience of a lawful and reasonable order, fraud or dishonesty, severe neglect of his or her duties, violating certain confidentiality and non-disclosure obligations, or breaching his or her non-competition and non-solicitation obligations under such agreements. An executive officer may terminate his or her employment at any time with a one-month prior written notice, subject to certain conditions provided thereunder. Each executive officer has agreed to hold, both during and after the employment agreement expires, in strict confidence and not to use or disclose to any person, corporation or other entity without written consent, any confidential information.
We have also entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we agreed to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our company.
Share Incentive Plan
On April 8, 2024, we adopted an equity incentive plan (the “2024 Plan”), by written resolutions of all the directors of the Company, pursuant to which up to 5,362,500 ordinary shares with par value of US$0.00166667 each of the Company (the “Overall Share Limit”) may be issued. On January 7, 2025, we approved the increase of the Overall Share Limit to 1,072,500 class A ordinary shares of par value of US$0.00833335 each (the “New Overall Share Limit”) by written resolutions of all the directors of the Company to reflect the share consolidation of the Company at a ratio of five-for-one, effective on June 21, 2024. As of the date of this annual report, none of the awards under the 2024 Plan has been granted.
The following paragraphs summarize other key terms of the 2024 Plan:
Types of Awards. The 2024 Plan permits the awards of options.
Plan Administration. Our board of directors or a committee of one or more members of the board of directors will administer the 2024 Plan. The committee or the full board of directors, as applicable, will determine the participants to receive awards, the type and number of awards to be granted to each participant, and the terms and conditions of each award under the 2024 Plan.
Award Agreement. Awards granted under the 2024 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award, which may include the term of the award, the provisions applicable in the event that the grantee’s employment or service terminates, and our authority to unilaterally or bilaterally amend, modify, suspend, cancel or rescind the award.
Eligibility. We may grant awards to employees, directors and consultants of the Company under the 2024 Plan. In addition, under the 2024 Plan, we may grant options that are intended to qualify as incentive share options only to our employees and employees of our subsidiaries.
Vesting Schedule. Under the 2024 Plan, in general, the plan administrator determines the vesting schedule, which is specified in the relevant award agreement.
110
Exercise of Options. Under the 2024 Plan, the option shall be exercisable during its term (prior to the earlier of the expiration date or option termination set forth in the option agreement. The vested portion of option will expire if not exercised prior to the time as the plan administrator determines at the time of its grant.
Transfer Restrictions. Under the 2024 Plan, Unless otherwise determined by the administrator, the option and the rights and privileges conferred hereby shall not be sold, pledged or otherwise transferred (whether by operation of law or otherwise) in any manner otherwise than by will or by the laws of descent or distribution, shall not be subject to sale under execution, attachment, levy or similar process and may be exercised during the lifetime of the optionee only by the optionee. The terms of the 2024 Plan and the awards may not be transferred in any manner by the participant other than in accordance with the exceptions provided in the relevant award agreement or otherwise determined by the plan administrator, such as transfers by will or the laws of descent and distribution.
Termination and Amendment. Unless terminated earlier, the 2024 Plan has a term of ten years. Our board of directors has the authority to amend or terminate the 2024 Plan. Except with respect to amendments made by the plan administrator, no termination, amendment or modification may adversely affect in any material way any awards previously granted pursuant to the 2024 Plan unless agreed by the participant.
C. Board Practices
Board of directors
Our board of directors consists of five directors, including three independent directors. A director is not required to hold any shares in our company to qualify to serve as a director. The Listing Rules of the Nasdaq Stock Market generally require that a majority of an issuer’s board of directors must consist of independent directors. However, the Listing Rules of the Nasdaq Stock Market permit foreign private issuers like us to follow “home country practice” in certain corporate governance matters. Even though we do not currently rely on this “home country practice” exception, we may consider following home country practice in the future.
Committees of the board of directors
We have established the following committees in our board of directors: an audit committee, a compensation committee and a nominating and corporate governance committee. The committees operate in accordance with terms of reference established by our board of directors.
Audit Committee. Our audit committee consists of Maofan Tang, Waihua Xu, and Ming Jing. Maofan Tang is the chairman of our audit committee. We have determined that Maofan Tang, Waihua Xu, and Ming Jing satisfy the “independence” requirements of Section 5605(a)(2) of the Nasdaq Listing Rules and Rule 10A-3 under the Exchange Act. Our board also has determined that Maofan Tang qualifies as an audit committee financial expert within the meaning of the SEC rules or possesses financial sophistication within the meaning of the Nasdaq Listing Rules. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
| ● | appointing the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| ● | reviewing any audit problems or difficulties and management’s response with the independent auditors; |
| ● | discussing the annual audited financial statements with management and the independent auditors; |
| ● | reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any steps taken to monitor and control major financial risk exposures; |
| ● | reviewing and approving all proposed related party transactions; |
| ● | meeting separately and periodically with management and the independent auditors; and |
| ● | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
111
Compensation Committee. Our compensation committee consists of Maofan Tang, Waihua Xu, and Ming Jing. Ming Jing is the chairman of our compensation committee. We have determined that Maofan Tang, Waihua Xu, and Ming Jing satisfy the “independence” requirements of Section 5605(a)(2) of the Nasdaq Listing Rules and Rule 10A-3 under the Exchange Act. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
| ● | reviewing and recommending compensation packages for our most senior executive officers to the board; |
| ● | approving and overseeing compensation packages for our executives other than the most senior executive officers; |
| ● | reviewing and recommending to the board with respect to the compensation of our directors; |
| ● | reviewing periodically and approving any long-term incentive compensation or equity plans; |
| ● | selecting compensation consultants, legal counsel or other advisors after taking into consideration all factors relevant to that person’s independence from management; and |
| ● | reviewing programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans. |
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee currently consists of Ming Jing, Maofan Tang, and Waihua Xu. Waihua Xu is the chairperson of our nominating and corporate governance committee. Ming Jing, Maofan Tang, and Waihua Xu satisfy the “independence” requirements of Section 5605(a)(2) of the Nasdaq Listing Rules and Rule 10A-3 under the Exchange Act. The nominating and corporate governance committee assists the board of directors in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
| ● | identifying and recommending nominees for election or re-election to our board of directors or for appointment to fill any vacancy; |
| ● | reviewing annually with our board of directors its current composition in light of the characteristics of independence, age, skills, experience and availability of service to us; |
| ● | identifying and recommending to our board the directors to serve as members of committees; |
| ● | advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to our board of directors on all matters of corporate governance and on any corrective action to be taken; and |
| ● | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
Duties and Functions of Directors
Under Cayman Islands law, our directors owe fiduciary duties to our company, including a duty of loyalty, a duty to act honestly, and a duty to act in what they consider in good faith to be in our best interests. Our directors must also exercise their powers only for a proper purpose. Our directors also have a duty to exercise the skill they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association, as amended and restated from time to time. We have the right to seek damages if a duty owed by any of our directors is breached.
112
Our board of directors has all the powers necessary for managing, and for directing and supervising, our business affairs. The functions and powers of our board of directors include, among others, (i) convening shareholders’ annual and extraordinary general meetings and reporting its work to shareholders at such meetings, (ii) declaring dividends and other distributions, (iii) appointing officers and determining their terms of offices and responsibilities; (iv) exercising the borrowing powers of our company and mortgaging the property of our company; and (v) approving the transfer of shares in our company, including the registration of such shares in our share register.
Terms of Directors and Officers
Pursuant to our third amended and restated memorandum and articles of association, a director may be appointed by ordinary resolution of the shareholders of our company or by the directors of the Company. Any appointment may be to fill a vacancy or as an additional director of the Company. Without prejudice to the Company’s power to appoint a person to be a director pursuant to the articles of association, the board of directors shall have power at any time to appoint any person who is willing to act as a director, either to fill a vacancy or as an addition to the existing board of directors, subject to the total number of directors not exceeding any maximum number fixed by or in accordance with the articles of association. Any director so appointed shall, if still a director, retire at the next annual general meeting after his appointment and be eligible to stand for election as a director at such meeting. Unless re-appointed or removed from office pursuant to the provisions of the articles of association, each director shall be appointed for a term expiring at the next-following annual general meeting of the Company. At any such annual general meeting, directors will be elected by ordinary resolution of the shareholders of the Company. At each annual general meeting of the Company, each director elected at such meeting shall be elected to hold office for a one-year term and until the election of their respective successors in office or removal pursuant to the articles of association All of executive officers are appointed by and serve at the discretion of our board of directors.
D. Employees
We had 323, 298, and 344 employees in total as of September 30, 2024, 2023 and 2022, respectively. As of September 30, 2024, there were 274 employees in Gansu QLS, 18 employees in Moshangfa, 6 employees in Chengdu QLS, 9 employees in Chongqing Shengfu Biological Technology Co., Ltd (“Chongqing”), 9 employees in Chengdu Trade and 7 employees in Hainan Trading and they work in the following capacities: management, administration, supplement, production, quality control, R&D, strain cultivation, chemical residue cleaning, ingredient combination, disinfection, tablet making, drug preparation, packaging, equipment operator, plate framing, boiler management, bottle making, biochemistry monitoring, powder making, crystalizing, decolorization, docking, product loading, facility repair, air compressor management, water pump management, water treatment, plumbing, welding, hygiene, intestine cleaning, salting, salt disintegration, vehicle management and financial management.
The following table sets forth a breakdown of employees by activity in Jiuquan City and Qionglai City for Gansu QLS, Moshangfa and Chengdu QLS as of September 30, 2024:
|
|
Number of |
Gansu QLS |
|
Employees |
General Management |
|
39 |
Manufacturing Management |
|
27 |
Operators |
|
196 |
Sales Department |
|
12 |
Total |
|
274 |
|
|
Number of |
Moshangfa |
|
Employees |
General Management |
|
4 |
Sales Department |
|
3 |
Drivers |
|
3 |
Operators |
|
8 |
Total |
|
18 |
113
|
|
Number of |
Chengdu QLS |
|
Employees |
General Management |
|
4 |
Financial Department |
|
2 |
Total |
|
6 |
|
|
Number of |
Chongqing |
|
Employees |
General Management |
|
3 |
Production and Quality Control Department |
|
5 |
Sales department |
|
1 |
Total |
|
9 |
|
|
Number of |
Chengdu Trade |
|
Employees |
General Management |
|
3 |
Financial Department |
|
6 |
Total |
|
9 |
Hainan Trade |
|
Employees |
General Management |
|
7 |
Total |
|
7 |
As required by PRC laws and regulations, we and our affiliated entities participate in various employee social security plans that are organized by municipal and provincial governments, including housing, pension, medical insurance and unemployment insurance programs. We and our affiliated entities are required under Chinese law to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time. For the fiscal year ended September 30, 2024, the VIE failed to make full contributions to social insurance and housing funds for part of our employees. Please see “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—We are not in compliance with the PRC’s regulations relating to employee’s social insurance and housing funds, and as a result, Gansu QLS and its subsidiaries may be subject to penalties if we are not able to remediate the non-compliance.” This failure does not constitute any breach of the VIE Agreements, nor will it affect the validity of our VIE Agreements.
We believe that we and the VIE and its subsidiaries maintain a good working relationship with their employees, and we and our affiliates are not in the process of any labor disputes.
E. Share Ownership
Except as specifically noted, the following table sets forth information with respect to the beneficial ownership of our Ordinary Shares as of the date of this annual report by:
| ● | each of our directors and executive officers; and |
| ● | each of our principal shareholders who beneficially own more than 5% of our total outstanding Ordinary Shares. |
The calculations in the table below are based on an aggregate of 97,222,141 ordinary shares, consisting of 77,222,141 Class A ordinary shares, par value of US$0.00833335 each, and 20,000,000 Class B ordinary shares, par value of US$0.00833335 each.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
114
|
|
|
|
|
|
|
|
|
|
|
Class A |
|
Class B |
|
% of Beneficial Ownership |
|
|
|
|
Ordinary |
|
Ordinary |
|
(of total Class A Ordinary Shares and |
|
|
|
|
Shares |
|
Shares |
|
Class B Ordinary Shares) |
|
% of Aggregate Voting Power** |
Directors and Executive Officers†: |
|
|
|
|
|
|
|
|
Zhanchang Xin (1) |
|
2,767,800 |
|
10,200,000 |
|
13.3 |
|
49.2 |
Furong Cao (2) |
|
— |
|
9,800,000 |
|
10.1 |
|
47.2 |
Waihua Xu |
|
— |
|
— |
|
— |
|
— |
Ming Jing |
|
— |
|
— |
|
— |
|
— |
Maofan Tang |
|
— |
|
— |
|
— |
|
— |
Chen Xin |
|
* |
|
— |
|
* |
|
* |
Yaxuan Zhai |
|
— |
|
— |
|
— |
|
— |
All directors and executive officers as a group: |
|
3,028,492 |
|
20,000,000 |
|
23.7 |
|
96.4 |
|
|
|
|
|
|
|
|
|
5% Shareholders: |
|
|
|
|
|
|
|
|
Ahanzhai Development Limited (3) |
|
367,800 |
|
10,200,000 |
|
10.9 |
|
49.1 |
LX Management Company Limited (4) |
|
— |
|
9,800,000 |
|
10.1 |
|
47.2 |
CISG Holdings Ltd (5) |
|
69,995,661 |
|
— |
|
72.0 |
|
3.4 |
Notes:
* |
Less than 1% of our total outstanding Ordinary Shares. |
** |
For each person included in this column, percentage of voting power is calculated by dividing the voting power beneficially owned by such person by the voting power of all of our Ordinary Shares. |
† |
Unless otherwise indicated, the address of our directors and executive officers is No. 152 Hongliang East 1st Street, No. 1703, Tianfu New District, Chengdu, 610200 People’s Republic of China. |
(1) |
Represents 2,400,000 Class A Ordinary Shares directly held by Mr. Zhanchang Xin as well as 367,800 Class A Ordinary Shares and 10,200,000 Class B Ordinary Shares held by Ahanzhai Development Limited, which is 100% owned by Mr. Zhanchang Xin. The registered address of Ahanzhai Development Limited is OMC Chambers, Wickhams Cay 1, Road Town, Tortola, British Virgin Islands. |
(2) |
Represents 9,800,000 Class B Ordinary Shares held by LX Management Company Limited, which is 100% owned by Ms. Furong Cao. The registered address of LX Management Company Limited is Flat 1512, 15/F, Lucky Centre, No.165-171 Wan Chai Road, Wan Chai, 999077, Hong Kong. |
(3) |
Represents 367,800 Class A Ordinary Shares and 10,200,000 Class B Ordinary Shares held by Ahanzhai Development Limited, which is 100% owned by Mr. Zhanchang Xin. The registered address of Ahanzhai Development Limited is OMC Chambers, Wickhams Cay 1, Road Town, Tortola, British Virgin Islands. |
(4) |
Represents 9,800,000 Class B Ordinary Shares held by LX Management Company Limited, which is 100% owned by Ms. Furong Cao. The registered address of LX Management Company Limited is Flat 1512, 15/F, Lucky Centre, No.165-171 Wan Chai Road, Wan Chai, 999077, Hong Kong. |
(5) |
Represents 69,995,661 Class A Ordinary Shares held by CISG Holdings Ltd, which is 100% owned by AIX Inc. AIX Inc. is a company listed on the Nasdaq Global Select Market. The registered address of CISG Holdings Ltd is Vistra Corporate Services Centre, Wickhams Cay II, Road Town, Tortola, VG1110, British Virgin Islands. The principal business address of AIX Inc. is 60F, Pearl River Tower, No. 15 West Zhujiang Road, Zhujiang New Town, Tianhe, Guangzhou, Guangdong Province, People’s Republic of China. |
As of the date of this annual report, we do not have registered holder in the United States.
We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
115
F. Disclosure of Action to Recover Erroneously Awarded Compensation
Not applicable.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
B. Related Party Transactions
Contractual Arrangements between WFOE and Gansu QLS
See “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements between WFOE and Gansu QLS.”
Material Transactions with Related Parties
During the normal course of business, we may make sales to our affiliated companies controlled by our major shareholders or subsidiaries. For the year ended September 30, 2022, we made sales to our affiliated companies in the amount of $122,189. As of September 30, 2022, we had advances from affiliated companies in the amount of $8,740. For the year ended and as of September 30, 2023, there was no related party transaction and related party balance outstanding. For the year ended and as of September 30, 2024, there was no related party transaction and related party balance outstanding.
Terms of Directors and Officers
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Terms of Directors and Officers.”
Employment Agreements and Indemnification Agreements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment Agreements and Indemnification Agreements.”
Share Incentive Plan
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Share Incentive Plan.”
C. Interests of Experts and Counsel
Not applicable.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal Proceedings
We are not currently involved in any material legal or administrative proceedings. From time to time, we may be subject to various legal or administrative claims and proceedings arising in the ordinary course of business. Such legal or administrative claims and proceedings, even if without merit, could result in the expenditure of financial and management resources and potentially result in civil liability for damages.
116
Dividend Policy
We do not have any present plan to pay any cash dividends on our Ordinary Shares in the foreseeable future. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand the WFOE and the VIE and its subsidiaries’ business.
We are a holding company incorporated in the Cayman Islands. We rely principally on dividends from the PRC Subsidiary for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of the PRC Subsidiary to pay dividends to us.
Our board of directors has discretion as to whether to distribute dividends, subject to certain requirements of Cayman Islands law. Subject to Cayman Islands law, the Company may by ordinary resolution of shareholders declare dividends in accordance with the respective rights of the shareholders but no dividend shall exceed the amount recommended by the directors. Under Cayman Islands law, a Cayman Islands company may pay a dividend out of either profit or share premium account, provided that in no circumstances may a dividend be paid if this would result in the company being unable to pay its debts as they fall due in the ordinary course of business. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that our board of directors may deem relevant.
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
ITEM 9. THE OFFER AND LISTING
A. Offering and Listing Details
Our Ordinary Shares have been listed on the Nasdaq Global Market since January 12, 2021 under the symbol “QLI.”
On February 16, 2023, the Board of Directors declared a one-time special cash dividend in the amount of $0.05 per ordinary share. The total amount of cash to be distributed in the divided was $1,787,500 and was paid on March 6, 2023, to all ordinary shareholders of record as of the close of business on February 28, 2023.
On November 27, 2023, we applied to transfer our Ordinary Shares to The Nasdaq Capital Market (the “Capital Market”), as allowed under the Nasdaq Listing Rules. On December 13, 2023, the transfer from The Nasdaq Global Market to the Capital Market was approved. Effective at the opening of business on December 15, 2023, our Ordinary Shares were transferred to the Capital Market and continued to trade under the symbol “QLI”.
Effective at the opening of business on August 11, 2024, the trading symbol of our Class A ordinary shares was changed to “BGM” on the Nasdaq Stock Market.
On October 18, 2024, shareholders approved the change of our company name to BGM Group Ltd at an extraordinary meeting of shareholders. Effective on October 30, 2024, we changed our name to “BGM Group Ltd.”
B. Plan of Distribution
Not applicable.
C. Markets
Our Ordinary Shares are currently listed on the Nasdaq Capital Market under the symbol “BGM.”
D. Selling Shareholders
117
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
We are an exempted company with limited liability incorporated under the laws of the Cayman Islands and our affairs are governed by our memorandum and articles of association, as amended and restated from time to time, and Companies Act (Revised) of the Cayman Islands, which we refer to as the Companies Act below, and the common law of the Cayman Islands.
We incorporate by reference into this annual report our Third Amended and Restated Memorandum and Articles of Association, filed as Exhibit 1.1 of Form 6-K filed on October 22, 2024 with the Securities and Exchange Commission. Our shareholders adopted our third amended and restated memorandum and articles of association by a special resolution of the shareholders on October 18, 2024, which we refer to as the articles below.
The following are summaries of material provisions of our third amended and restated memorandum and articles of association and the Companies Act insofar as they relate to the material terms of our Ordinary Shares.
Registered Office
Our registered office in the Cayman Islands is at Harneys Fiduciary (Cayman) Limited, 4th Floor, Harbour Place, 103 South Church Street, P.O. Box 10240, Grand Cayman KY1-1002, Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees.”
Ordinary Shares
General
Our authorized share capital is US$41,916,750.50, divided into 5,000,000,000 Class A ordinary shares of par value of US$0.00833335 each, 20,000,000 Class B ordinary shares of par value of US$0.00833335 each, and 10,000,000 preferred shares of par value of US$0.00833335 each. All of our issued and outstanding Ordinary Shares are fully paid and non-assessable. Certificates representing the Ordinary Shares are issued in registered form.
Dividends
Subject to the provisions of the Companies Act and any rights attaching to any class or classes of shares under and in accordance with the articles of the Company:
| (a) | the directors may declare dividends or distributions out of our funds which are lawfully available for distribution; and |
118
| (b) | the Company’s shareholders may, by ordinary resolution of the shareholders, declare dividends but no such dividend shall exceed the amount recommended by the directors. |
Subject to the requirements of the Companies Act regarding the application of a company’s share premium account and with the sanction of an ordinary resolution of the shareholders, dividends may also be declared and paid out of any share premium account. The directors when paying dividends to shareholders may make such payment either in cash or in specie.
Unless provided by the rights attached to a share, no dividend shall bear interest.
Voting Rights
A resolution put to the vote of the general meeting shall be decided on a show of hands unless before, or on, the declaration of the result of the show of hands, a poll is duly demanded. Subject to any rights or restrictions as to voting attached to any shares, unless any share carries special voting rights, on a show of hands every shareholder who is present in person and every person representing a shareholder by proxy shall have one vote. On a poll, every shareholder who is present in person and every person representing a shareholder by proxy shall have one vote for each share of which he or the person represented by proxy is the holder, save that each holder of Class B Ordinary Shares shall be entitled to exercise one hundred (100) votes for each Class B Ordinary Share he or she holds on any and all matters. In addition, all shareholders holding shares of a particular class are entitled to vote at a meeting of the holders of that class of shares. Votes may be given either personally or by proxy.
Transfer of Shares
Provided that a transfer of shares complies with applicable rules of Nasdaq Stock Market, a shareholder may transfer shares to another person by completing an instrument of transfer in a common form or in a form prescribed by Nasdaq Stock Market or in any other form approved by the directors, executed:
| (a) | where the shares are fully paid, by or on behalf of that shareholder; and |
| (b) | where the shares are partly paid, by or on behalf of that shareholder and the transferee. |
The transferor shall be deemed to remain the holder of a share until the name of the transferee is entered into the register of members of the Company.
Where the shares are not listed on or subject to the rules of Nasdaq Stock Market, our board of directors may, in its absolute discretion, decline to register any transfer of any share that has not been fully paid up or is subject to a company lien. Our board of directors may also decline to register any transfer of such share unless:
| (a) | the instrument of transfer is lodged with the Company, accompanied by the certificate (if any) for the shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; |
| (b) | the instrument of transfer is in respect of only one class of shares; |
| (c) | the instrument of transfer is properly stamped, if required; |
| (d) | the shares transferred is fully paid and free of any lien in favor of us; |
| (e) | any fee related to the transfer has been paid to us; and |
| (f) | the transfer is not to more than four joint holders. |
If our directors refuse to register a transfer, they are required, within three months after the date on which the instrument of transfer was lodged, to send to each of the transferor and the transferee notice of such refusal.
119
The registration of transfers may, on 14 days’ notice being given by advertisement in such one or more newspapers or by electronic means, be suspended and our register of members closed at such times and for such periods as our board of directors may from time to time determine. The registration of transfers, however, may not be suspended, and the register may not be closed, for more than 30 days in any year.
Liquidation Rights
If we are wound up, the shareholders may, subject to the articles and any other sanction required by the Companies Act, pass a special resolution of the shareholders allowing the liquidator to do either or both of the following:
| (a) | to divide in specie among the shareholders the whole or any part of our assets and, for that purpose, to value any assets and to determine how the division shall be carried out as between the shareholders or different classes of shareholders; and |
| (b) | to vest the whole or any part of the assets in trustees for the benefit of shareholders and those liable to contribute to the winding up. |
The directors have the authority to present a petition for our winding up to the Grand Court of the Cayman Islands on our behalf without the sanction of a resolution passed at a general meeting.
Redemption and Purchase of Own Shares
Subject to the Companies Act and any rights for the time being conferred on the shareholders holding a particular class of shares, we may by action of our directors:
| (a) | issue shares that are to be redeemed or liable to be redeemed, at our option or the shareholder holding those redeemable shares, on the terms and in the manner our directors determine before the issue of those shares; |
| (b) | with the consent by special resolution of the shareholders holding shares of a particular class, vary the rights attaching to that class of shares so as to provide that those shares are to be redeemed or are liable to be redeemed at our option on the terms and in the manner which the directors determine at the time of such variation; and |
| (c) | purchase all or any of our own shares of any class including any redeemable shares on the terms and in the manner which the directors determine at the time of such purchase. |
We may make a payment in respect of the redemption or purchase of its own shares in any manner authorized by the Companies Act, including out of any combination of capital, our profits and the proceeds of a fresh issue of shares.
When making a payment in respect of the redemption or purchase of shares, the directors may make the payment in cash or in specie (or partly in one and partly in the other) if so authorized by the terms of the allotment of those shares or by the terms applying to those shares in accordance with the articles, or otherwise by agreement with the shareholder holding those shares.
Variation of Rights of Shares
If our share capital is divided into different classes of shares, unless the terms on which a class of shares was issued state otherwise, the rights attaching to a class of shares may only be varied if one of the following applies:
| (a) | the shareholders holding not less than two-thirds of the issued shares of that class consent in writing to the variation; or |
| (b) | the variation is made with the sanction of a special resolution passed at a separate general meeting of the shareholders, present in person or by proxy, holding the issued shares of that class. |
Unless the terms on which a class of shares was issued state otherwise, the rights conferred on the shareholder holding shares of any class shall not be deemed to be varied by the creation or issue of further shares ranking pari passu with the existing shares of that class.
120
Alteration of Share Capital
Subject to the Companies Act, our shareholders may, by ordinary resolution:
| (a) | increase our share capital by new shares of the amount fixed by that ordinary resolution and with the attached rights, priorities and privileges set out in that ordinary resolution; |
| (b) | consolidate and divide all or any of our share capital into shares of larger amount than our existing shares; |
| (c) | convert all or any of our paid up shares into stock, and reconvert that stock into paid up shares of any denomination; |
| (d) | sub-divide our shares or any of them into shares of an amount smaller than that fixed, so, however, that in the sub-division, the proportion between the amount paid and the amount, if any, unpaid on each reduced share shall be the same as it was in case of the share from which the reduced share is derived; and |
| (e) | cancel shares which, at the date of the passing of that ordinary resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so cancelled or, in the case of shares without nominal par value, diminish the number of shares into which our capital is divided. |
Subject to the Companies Act and to any rights for the time being conferred on the shareholders holding a particular class of shares, our shareholders may, by special resolution, reduce its share capital in any way.
Calls on Shares and Liens on Shares
Subject to the terms of allotment, the directors may make calls on the shareholders in respect of any monies unpaid on their shares including any premium and each shareholder shall (subject to receiving at least 14 clear days’ notice specifying when and where payment is to be made), pay to us the amount called on his shares. Shareholders registered as the joint holders of a share shall be jointly and severally liable to pay all calls in respect of the share. If a call remains unpaid after it has become due and payable the person from whom it is due and payable shall pay interest on the amount unpaid from the day it became due and payable until it is paid at the rate fixed by the terms of allotment of the share or in the notice of the call or if no rate is fixed, at the rate of ten percent per annum. The directors may, at their discretion, waive payment of the interest wholly or in part.
We have a first and paramount lien on all shares (whether fully paid up or not) registered in the name of a shareholder (whether solely or jointly with others). The lien is for all monies payable to us by the shareholder or the shareholder’s estate:
| (a) | either alone or jointly with any other person, whether or not that other person is a shareholder; and |
| (b) | whether or not those monies are presently payable. |
At any time the directors may declare any share to be wholly or partly exempt from the lien on shares provisions of the articles.
We may sell, in such manner as the directors may determine, any share on which the sum in respect of which the lien exists is presently payable, if due notice that such sum is payable has been given notice to the shareholders holding the shares (or to the person entitled to it in consequence of the death or bankruptcy of that shareholder) demanding payment and stating that if the notice is not complied with the shares may be sold, and that sum is not paid within 14 clear days after the date on which the notice is deemed to be given under the articles.
Unclaimed Dividend
A dividend that remains unclaimed for a period of six years after it became due for payment shall be forfeited to, and shall cease to remain owing by, the company.
121
Forfeiture or Surrender of Shares
If a shareholder fails to pay any capital call, the directors may give to such shareholder not less than 14 clear days’ notice requiring payment and specifying the amount unpaid including any interest which may have accrued, any expenses which have been incurred by us due to that person’s default and the place where payment is to be made. The notice shall also contain a warning that if the notice is not complied with, the shares in respect of which the call is made will be liable to be forfeited.
If such notice is not complied with, the directors may, before the payment required by the notice has been received, resolve that any share the subject of that notice be forfeited (which forfeiture shall include all dividends or other monies payable in respect of the forfeited share and not paid before such forfeiture).
A forfeited share may be sold, re-allotted or otherwise disposed of on such terms and in such manner as the directors determine either to the former shareholder who held that share or to any other person and at any time before a sale, re-allotment or disposition the forfeiture may be cancelled on such terms as the directors think fit.
A person whose shares have been forfeited shall cease to be a shareholder in respect of the forfeited shares, but shall, notwithstanding such forfeiture, remain liable to pay to us all monies which at the date of forfeiture were payable by him to us in respect of the shares, together with all expenses and interest from the date of forfeiture or surrender until payment, but his liability shall cease if and when we receive payment in full of the unpaid amount.
A declaration, whether statutory or under oath, made by a director or the secretary shall be conclusive evidence that the person making the declaration is our director or secretary and that the particular shares have been forfeited or surrendered on a particular date.
Subject to the execution of an instrument of transfer, if necessary, the declaration shall constitute good title to the shares.
Share Premium Account
The directors shall establish a share premium account in accordance with the Companies Act and shall carry the credit of such account from time to time to a sum equal to the amount or value of the premium paid on the issue of any share or capital contributed or such other amounts required by the Companies Act.
Inspection of Books and Records
Holders of our Ordinary Shares will have no general right under the Companies Act to inspect or obtain copies of our register of members or our corporate records.
General Meetings
As a Cayman Islands exempted company, we are not obligated by the Companies Act to call shareholders’ annual general meetings; accordingly, we may, but shall not (unless required by the Nasdaq Stock Market) be obliged to, in each year hold a general meeting as an annual general meeting. Any annual general meeting held shall be convened by our board of directors in accordance with the articles. All general meetings other than annual general meetings shall be called extraordinary general meetings.
The directors may call a general meeting at any time. General meetings shall also be convened on the written requisition of one or more of the shareholders entitled to attend and vote at our general meetings who (together) hold not less than ten percent of the rights to vote at such general meeting in accordance with the notice provisions in the articles, specifying the purpose of the meeting and signed by each of the shareholders making the requisition (and for this purpose each joint holder shall be obliged to sign). If the directors do not convene such meeting within 21 clear days’ after the date of receipt of the written requisition, those shareholders who requested the meeting may convene the general meeting themselves within three months after the end of such period of 21 clear days in which case reasonable expenses incurred by them as a result of the directors failing to convene a meeting shall be reimbursed by us.
122
At least 14 clear days’ notice of an extraordinary general meeting and 21 clear days’ notice of an annual general meeting shall be given to shareholders entitled to attend and vote at such meeting. The notice shall specify the place, the day and the hour of the meeting, if the meeting is to be held in two or more places, the technology that will be used to facilitate the meeting, the general nature of that business subject to the requirements of the Nasdaq Stock Market. In addition, if a resolution is proposed as a special resolution, the text of that resolution shall be given to all shareholders. Notice of every general meeting shall also be given to the directors and our auditors.
Subject to the Companies Act and with the consent of the shareholders who, individually or collectively, hold at least 90 percent of the voting rights of all those who have a right to vote at a general meeting, a general meeting may be convened on shorter notice.
A quorum shall consist of the presence (whether in person or represented by proxy) of one or more shareholders holding shares that represent not less than one-third of the outstanding shares carrying the right to vote at such general meeting.
If, within 15 minutes from the time appointed for the general meeting, or at any time during the meeting, a quorum is not present, the meeting, if convened upon the requisition of shareholders, shall be cancelled. In any other case it shall stand adjourned to the same time and place seven days or to such other time or place as is determined by the directors. If a quorum is not present within 15 minutes of the time appointed for the adjourned meeting, then the shareholders present in person or by proxy shall constitute a quorum.
The chairman may, with the consent of a meeting at which a quorum is present, adjourn the meeting. When a meeting is adjourned for seven days or more, notice of the adjourned meeting shall be given in accordance with the articles.
At any general meeting a resolution put to the vote of the meeting shall be decided on a show of hands, unless a poll is (before, or on, the declaration of the result of the show of hands) demanded by the chairman of the meeting or by at least two shareholders having the right to vote on the resolutions or one or more shareholders present who together hold not less than ten percent of the voting rights of all those who are entitled to vote on the resolution. Unless a poll is so demanded, a declaration by the chairman as to the result of a resolution and an entry to that effect in the minutes of the meeting, shall be conclusive evidence of the outcome of a show of hands, without proof of the number or proportion of the votes recorded in favor of, or against, that resolution.
If a poll is duly demanded it shall be taken in such manner as the chairman directs, not being more than thirty clear days after the poll was demanded.
In the case of an equality of votes, whether on a show of hands or on a poll, the chairman of the meeting at which the show of hands takes place or at which the poll is demanded, shall not be entitled to a second or casting vote.
Directors
We may by ordinary resolution, from time to time, fix the maximum and minimum number of directors to be appointed. Under the articles, we are required to have a minimum of one director and the maximum number of directors shall be unlimited.
A director may be appointed by ordinary resolution or by the directors. Any appointment may be to fill a vacancy or as an additional director.
Unless the remuneration of the directors is determined by the shareholders by ordinary resolution, the directors (other than alternate directors) shall be entitled to such remuneration by way of fees for their services in the office of director as the directors may determine.
The shareholding qualification for directors may be fixed by our shareholders by ordinary resolution and unless and until so fixed no director shall be required to own shares as a condition of his appointment.
Unless removed or re-appointed, each director shall be appointed for a term expiring at the next-following annual general meeting, if one is held. At any annual general meeting held, each director will be elected by an ordinary resolution of our shareholders. At each annual general meeting, each director so elected shall hold office for a one-year term and until the election of their respective successors in office or removed.
A director may be removed by ordinary resolution.
123
A director may at any time resign or retire from office by giving us notice in writing. Unless the notice specifies a different date, the director shall be deemed to have resigned on the date that the notice is delivered to us.
Subject to the provisions of the articles, the office of a director may be terminated forthwith if:
| (a) | he is prohibited by the law of the Cayman Islands from acting as a director; |
| (b) | he is made bankrupt or makes an arrangement or composition with his creditors generally; |
| (c) | he resigns his office by notice to us; |
| (d) | he only held office as a director for a fixed term and such term expires; |
| (e) | in the opinion of a registered medical practitioner by whom he is being treated he becomes physically or mentally incapable of acting as a director; |
| (f) | he is given notice by the majority of the other directors (not being less than two in number) to vacate office (without prejudice to any claim for damages for breach of any agreement relating to the provision of the services of such director); |
| (g) | he is made subject to any law relating to mental health or incompetence, whether by court order or otherwise; or |
| (h) | without the consent of the other directors, he is absent from meetings of directors for continuous period of six months. |
Powers and Duties of Directors
Subject to the provisions of the Companies Act and our articles, our business shall be managed by the directors, who may exercise all our powers. No prior act of the directors shall be invalidated by any subsequent alteration of our memorandum or articles of association. To the extent allowed by the Companies Act, however, shareholders may by special resolution validate any prior or future act of the directors which would otherwise be in breach of their duties.
The directors may delegate any of their powers to any committee consisting of one or more persons who need not be shareholders and may include non-directors so long as the majority of those persons are directors; any committee so formed shall in the exercise of the powers so delegated conform to any regulations that may be imposed on it by the directors. Our board of directors have established an audit committee, compensation committee, and nomination and corporate governance committee.
The board of directors may establish any local or divisional board of directors or agency and delegate to it its powers and authorities (with power to sub-delegate) for managing any of our affairs whether in the Cayman Islands or elsewhere and may appoint any persons to be members of a local or divisional board of directors, or to be managers or agents, and may fix their remuneration.
The directors may from time to time and at any time by power of attorney or in any other manner they determine appoint any person, either generally or in respect of any specific matter, to be our agent with or without authority for that person to delegate all or any of that person’s powers.
The directors may from time to time and at any time by power of attorney or in any other manner they determine appoint any person, whether nominated directly or indirectly by the directors, to be our attorney or our authorized signatory and for such period and subject to such conditions as they may think fit. The powers, authorities and discretions, however, must not exceed those vested in, or exercisable, by the directors under the articles.
The board of directors may remove any person so appointed and may revoke or vary the delegation.
The directors may exercise all of our powers to borrow money and to mortgage or charge its undertaking, property and assets both present and future and uncalled capital or any part thereof, to issue debentures and other securities whether outright or as collateral security for any debt, liability or obligation of ours or our parent undertaking (if any) or any subsidiary undertaking of us or of any third party.
124
A director shall not, as a director, vote in respect of any contract, transaction, arrangement or proposal in which he has an interest which (together with any interest of any person connected with him) is a material interest (otherwise than by virtue of his interests, direct or indirect, in shares or debentures or other securities of, or otherwise in or through, us) and if he shall do so his vote shall not be counted, nor in relation thereto shall he be counted in the quorum present at the meeting, but (in the absence of some other material interest than is mentioned below) none of these prohibitions shall apply to:
| (a) | the giving of any security, guarantee or indemnity in respect of: |
| (i) | money lent or obligations incurred by him or by any other person for our benefit or any of our subsidiaries; or |
| (ii) | a debt or obligation of ours or any of our subsidiaries for which the director himself has assumed responsibility in whole or in part and whether alone or jointly with others under a guarantee or indemnity or by the giving of security; |
| (b) | where we or any of our subsidiaries is offering securities in which offer the director is or may be entitled to participate as a holder of securities or in the underwriting or sub-underwriting of which the director is to or may participate; |
| (c) | any contract, transaction, arrangement or proposal affecting any other body corporate in which he is interested, directly or indirectly and whether as an officer, shareholder, creditor or otherwise howsoever, provided that he (together with persons connected with him) does not to his knowledge hold an interest representing one percent or more of any class of the equity share capital of such body corporate (or of any third body corporate through which his interest is derived) or of the voting rights available to shareholders of the relevant body corporate; |
| (d) | any act or thing done or to be done in respect of any arrangement for the benefit of the employees of us or any of our subsidiaries under which he is not accorded as a director any privilege or advantage not generally accorded to the employees to whom such arrangement relates; or |
| (e) | any matter connected with the purchase or maintenance for any director of insurance against any liability or (to the extent permitted by the Companies Act) indemnities in favor of directors, the funding of expenditure by one or more directors in defending proceedings against him or them or the doing of anything to enable such director or directors to avoid incurring such expenditure. |
A director may, as a director, vote (and be counted in the quorum) in respect of any contract, transaction, arrangement or proposal in which he has an interest which is not a material interest or as described above.
Capitalization of Profits
The directors may resolve to capitalize:
| (a) | any part of our profits not required for paying any preferential dividend (whether or not those profits are available for distribution); or |
| (b) | any sum standing to the credit of our share premium account or capital redemption reserve, if any. |
The amount resolved to be capitalized must be appropriated to the shareholders who would have been entitled to it had it been distributed by way of dividend and in the same proportions.
Register of Members
Under the Companies Act, we must keep a register of members and there should be entered therein:
| ● | the names and addresses of the members, with the addition of, in the case of a company having a capital divided into shares a statement of the shares held by each member, which; |
| ● | distinguishes each share by its number (so long as the share has a number); |
125
| ● | confirms the amount paid or agreed to be considered as paid, on the shares of each member; |
| ● | confirms the number and category of shares held by each member; and |
| ● | confirms whether each relevant category of shares held by a member carries voting rights under the articles of association of the company, and if so, whether such voting rights are conditional; |
| ● | the date on which the name of any person was entered on the register as a member; and |
| ● | the date on which any person ceased to be a member. |
For these purposes, “voting rights” means rights conferred on shareholders in respect of their shares to vote at general meetings of the company on all or substantially all matters. A voting right is conditional where the voting right arises only in certain circumstances.
Under the Companies Act, the register of members of our company is prima facie evidence of the matters set out therein (that is, the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a shareholder registered in the register of members is deemed as a matter of the Companies Act to have legal title to the shares as set against its name in the register of members.
If the name of any person is, without sufficient cause, entered in or omitted from our register of members, or if default is made or unnecessary delay takes place in entering on the register the fact of any person having ceased to be a shareholder of our company, the person or shareholder aggrieved (or any shareholder of our company or our company itself) may, by motion to the Grand Court of the Cayman Islands, to apply for an order that the register be rectified, and the Grand Court of the Cayman Islands may either refuse such application with or without costs to be paid by the applicant or it may, if satisfied of the justice of the case, make an order for the rectification of the register, and may direct the company to pay all the costs of such motion, application or petition, and any damages the party aggrieved may have sustained.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company,” “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions” or elsewhere in this annual report.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—Regulation—PRC Laws and Regulations on Foreign Exchange.”
E. Taxation
The following summary of the Cayman Islands, PRC and U.S. federal income tax considerations of an investment in the Ordinary Shares is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change. This summary does not deal with all possible tax considerations relating to an investment in the Ordinary Shares, such as the tax considerations under U.S. state and local tax laws or under the tax laws of jurisdictions other than the Cayman Islands, the People’s Republic of China and the United States.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation, and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us or holders of our Ordinary Shares levied by the government of the Cayman Islands, except for stamp duties which may be applicable on instruments executed in, or after execution, brought to or produced before a court of the Cayman Islands. The Cayman Islands are not party to any double tax treaties that are applicable to any payments made to or by our company. There are no exchange control regulations or currency restrictions in the Cayman Islands.
126
Payments of dividends and capital in respect of Ordinary Shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of Ordinary Shares, nor will gains derived from the disposal of Ordinary Shares be subject to Cayman Islands income or corporation tax.
People’s Republic of China Taxation
Unless otherwise noted in the following discussion, this section is the opinion of Gansu Quanyi Law Firm, our PRC counsel, insofar as it relates to legal conclusions with respect to matters of People’s Republic of China Enterprise Taxation below.
The following brief description of Chinese enterprise laws is designed to highlight the enterprise-level taxation on our earnings, which will affect the amount of dividends, if any, we are ultimately able to pay to our shareholders. See “Item 8. Financial Information—A. Consolidated Statements and Other Financial Information—Dividend Policy.”
We are a holding company incorporated in Cayman Islands and we gain income by way of dividends paid to us from the PRC Subsidiary. The EIT Law and its implementation rules provide that China-sourced income of foreign enterprises, such as dividends paid by a PRC Subsidiary to its equity holders that are non-resident enterprises, will normally be subject to PRC withholding tax at a rate of 10%, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a preferential tax rate or a tax exemption.
Under the EIT Law, an enterprise established outside of China with a “de facto management body” within China is considered a “resident enterprise,” which means that it is treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. Although the implementation rules of the EIT Law define “de facto management body” as a managing body that actually, comprehensively manage and control the production and operation, staff, accounting, property and other aspects of an enterprise, the only official guidance for this definition currently available is set forth in SAT Notice 82, which provides guidance on the determination of the tax residence status of a Chinese-controlled offshore incorporated enterprise, defined as an enterprise that is incorporated under the laws of a foreign country or territory and that has a PRC enterprise or enterprise group as its primary controlling shareholder. Although BGM Group Ltd does not have a PRC enterprise or enterprise group as our primary controlling shareholder and is therefore not a Chinese-controlled offshore incorporated enterprise within the meaning of SAT Notice 82, in the absence of guidance specifically applicable to us, we have applied the guidance set forth in SAT Notice 82 to evaluate the tax residence status of BGM Group Ltd and its affiliated entities organized outside the PRC.
According to SAT Notice 82, a Chinese-controlled offshore incorporated enterprise will be regarded as a PRC tax resident by virtue of having a “de facto management body” in China and will be subject to PRC enterprise income tax on its worldwide income only if all of the following criteria are met: (i) the places where senior management and senior management departments that are responsible for daily production, operation and management of the enterprise perform their duties are mainly located within the territory of China; (ii) financial decisions (such as money borrowing, lending, financing and financial risk management) and personnel decisions (such as appointment, dismissal and salary and wages) are decided or need to be decided by organizations or persons located within the territory of China; (iii) main property, accounting books, corporate seal, the board of directors and files of the minutes of shareholders’ meetings of the enterprise are located or preserved within the territory of China; and (iv) one half (or more) of the directors or senior management staff having the right to vote habitually reside within the territory of China.
Currently, we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities. Accordingly, we believe that BGM Group Ltd and its offshore subsidiary should not be treated as a “resident enterprise” for PRC tax purposes if the criteria for “de facto management body” as set forth in SAT Notice 82 were deemed applicable to us. However, as the tax residency status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body” as applicable to our offshore entities, we will continue to monitor our tax status.
127
The implementation rules of the EIT Law provide that, (i) if the enterprise that distributes dividends is domiciled in the PRC or (ii) if gains are realized from transferring equity interests of enterprises domiciled in the PRC, then such dividends or gains are treated as China-sourced income. It is not clear how “domicile” may be interpreted under the EIT Law, and it may be interpreted as the jurisdiction where the enterprise is a tax resident. Therefore, if we are considered as a PRC tax resident enterprise for PRC tax purposes, any dividends we pay to our overseas shareholders which are non-resident enterprises as well as gains realized by such shareholders from the transfer of our shares may be regarded as China-sourced income and as a result become subject to PRC withholding tax at a rate of up to 10%. We are unable to provide a “will” opinion because Gansu Quanyi Law Firm, our PRC counsel, believes that it is more likely than not that the Company and its offshore subsidiary would be treated as a non-resident enterprise for PRC tax purposes because we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities as of the date of the prospectus. Therefore, we believe that it is possible but highly unlikely that the income received by our overseas shareholders will be regarded as China-sourced income.
See “Item 3. Key Information—D. Risk Factors— Risks Related to Doing Business in China — Under the EIT Law, we may be classified as a ‘resident enterprise’ of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC shareholders.”
Our company pays an statutory EIT rate of 25% for WFOE and its affiliated entities. The EIT is calculated based on the entity’s global income as determined under PRC tax laws and accounting standards. If the PRC tax authorities determine that we are a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% withholding tax from dividends we pay to our shareholders that are non-resident enterprises. In addition, non-resident enterprise shareholders may be subject to a 10% PRC withholding tax on gains realized on the sale or other disposition of our Ordinary Shares, if such income is treated as sourced from within the PRC. It is unclear whether our non-PRC individual shareholders would be subject to any PRC tax on dividends or gains obtained by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to dividends or gains realized by non-PRC individuals, it would generally apply at a rate of 20% unless a reduced rate is available under an applicable tax treaty. However, it is also unclear whether non-PRC shareholders of the Company would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that the Company is treated as a PRC resident enterprise. There is no guidance from the PRC government to indicate whether or not any tax treaties between the PRC and other countries would apply in circumstances where a non-PRC company was deemed to be a PRC tax resident, and thus there is no basis for expecting how tax treaty between the PRC and other countries may impact non-resident enterprises.
United States Federal Income Tax Considerations
The following does not address the tax consequences to any particular investor or to persons in special tax situations such as:
| ● | banks; |
| ● | financial institutions; |
| ● | insurance companies; |
| ● | regulated investment companies; |
| ● | real estate investment trusts; |
| ● | broker-dealers; |
| ● | persons that elect to mark their securities to market; |
| ● | U.S. expatriates or former long-term residents of the U.S.; |
| ● | governments or agencies or instrumentalities thereof; |
| ● | tax-exempt entities; |
| ● | persons liable for alternative minimum tax; |
128
| ● | persons holding our Ordinary Shares as part of a straddle, hedging, conversion or integrated transaction; |
| ● | persons that actually or constructively own 10% or more of our voting power or value (including by reason of owning our Ordinary Shares); |
| ● | persons who acquired our Ordinary Shares pursuant to the exercise of any employee share option or otherwise as compensation; |
| ● | persons holding our Ordinary Shares through partnerships or other pass-through entities; |
| ● | beneficiaries of a Trust holding our Ordinary Shares; or |
| ● | persons holding our Ordinary Shares through a Trust. |
The discussion set forth below is addressed only to U.S. Holders that purchase Ordinary Shares. Prospective purchasers are urged to consult their own tax advisors about the application of the U.S. federal income tax rules to their particular circumstances as well as the state, local, foreign, and other tax consequences to them of the purchase, ownership, and disposition of our Ordinary Shares.
Material Tax Consequences Applicable to U.S. Holders of Our Ordinary Shares
The following sets forth the material U.S. federal income tax consequences related to the ownership and disposition of our Ordinary Shares. It is directed to U.S. Holders (as defined below) of our Ordinary Shares and is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change. This description does not deal with all possible tax consequences relating to ownership and disposition of our Ordinary Shares or U.S. tax laws, other than the U.S. federal income tax laws, such as the tax consequences under non-U.S. tax laws, state, local, and other tax laws.
The following brief description applies only to U.S. Holders that hold Ordinary Shares as capital assets and that have the U.S. dollar as their functional currency. This brief description is based on the federal income tax laws of the United States in effect as of the date of this annual report and on U.S. Treasury regulations in effect or, in some cases, proposed, as of the date of this annual report, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing authorities are subject to change, which change could apply retroactively and could affect the tax consequences described below.
The brief description below of the U.S. federal income tax consequences to “U.S. Holders” will apply to you if you are a beneficial owner of Ordinary Shares and you are, for U.S. federal income tax purposes,
| ● | an individual who is a citizen or resident of the United States; |
| ● | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia; |
| ● | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| ● | a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
Taxation of Dividends and Other Distributions on Our Ordinary Shares
Subject to the PFIC rules discussed below, the gross amount of distributions made by us to you with respect to the Ordinary Shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). With respect to corporate U.S. Holders, the dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
129
With respect to non-corporate U.S. Holders, including individual U.S. Holders, dividends will be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the Ordinary Shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a PFIC for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Because there is no income tax treaty between the United States and the Cayman Islands, clause (1) above can be satisfied only if the Ordinary Shares are readily tradable on an established securities market in the United States. Under U.S. Internal Revenue Service authority, Ordinary Shares are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on certain exchanges, which presently includes the NYSE and the Nasdaq Stock Market. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our Ordinary Shares, including the effects of any change in law after the date of this annual report.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our Ordinary Shares will constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.”
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your Ordinary Shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
Taxation of Dispositions of Ordinary Shares
Subject to the PFIC rules discussed below, you will recognize taxable gain or loss on any sale, exchange, or other taxable disposition of a share equal to the difference between the amount realized (in U.S. dollars) for the share and your tax basis (in U.S. dollars) in the Ordinary Shares. The gain or loss will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the Ordinary Shares for more than one year, you will generally be eligible for reduced tax rates. The deductibility of capital losses is subject to limitations. Any such gain or loss that you recognize will generally be treated as United States source income or loss for foreign tax credit limitation purposes which will generally limit the availability of foreign tax credits.
PFIC
A non-U.S. corporation is considered a PFIC, as defined in Section 1297(a) of the U.S. Internal Revenue Code, for any taxable year if either:
| ● | at least 75% of its gross income for such taxable year is passive income; or |
| ● | at least 50% of the value of its assets (based on an average of the quarterly values of the assets during a taxable year) is attributable to assets that produce or are held for the production of passive income (the “asset test”). |
Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets. We will be treated as owning our proportionate share of the assets and earning our proportionate share of the income of any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock. In determining the value and composition of our assets for purposes of the PFIC asset test, the value of our assets must be determined based on the market value of our Ordinary Shares from time to time, which could cause the value of our non-passive assets to be less than 50% of the value of all of our assets on any particular quarterly testing date for purposes of the asset test.
130
Based on the operations and the composition of our assets we do not expect to be treated as a PFIC under the current PFIC rules. We must make a separate determination each year as to whether we are a PFIC, however, and there can be no assurance with respect to our status as a PFIC for our current taxable year or any future taxable year. Depending on the amount of assets held for the production of passive income, it is possible that, for our current taxable year or for any subsequent taxable year, more than 50% of our assets may be assets held for the production of passive income. We will make this determination following the end of any particular tax year. In addition, because the value of our assets for purposes of the asset test will generally be determined based on the market price of our Ordinary Shares, our PFIC status will depend in large part on the market price of our Ordinary Shares. Accordingly, fluctuations in the market price of the Ordinary Shares may cause us to become a PFIC. In addition, the application of the PFIC rules is subject to uncertainty in several respects and the composition of our income and assets will be affected by how, and how quickly, we spend our liquid assets. We are under no obligation to take steps to reduce the risk of our being classified as a PFIC, and as stated above, the determination of the value of our assets will depend upon material facts (including the market price of our Ordinary Shares from time to time) that may not be within our control. If we are a PFIC for any year during which you hold Ordinary Shares, we will continue to be treated as a PFIC for all succeeding years during which you hold Ordinary Shares. If we cease to be a PFIC and you did not previously make a timely “mark-to-market” election as described below, however, you may avoid some of the adverse effects of the PFIC regime by making a “purging election” (as described below) with respect to the Ordinary Shares.
If we are a PFIC for your taxable year(s) during which you hold Ordinary Shares, you will be subject to special tax rules with respect to any “excess distribution” that you receive and any gain you realize from a sale or other disposition (including a pledge) of the Ordinary Shares, unless you make a “mark-to-market” election as discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the Ordinary Shares will be treated as an excess distribution. Under these special tax rules:
| ● | the excess distribution or gain will be allocated ratably over your holding period for the Ordinary Shares; |
| ● | the amount allocated to your current taxable year, and any amount allocated to any of your taxable year(s) prior to the first taxable year in which we were a PFIC, will be treated as ordinary income, and |
| ● | the amount allocated to each of your other taxable year(s) will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to years prior to the year of disposition or “excess distribution” cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the sale of the Ordinary Shares cannot be treated as capital, even if you hold the Ordinary Shares as capital assets.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election under Section 1296 of the US Internal Revenue Code for such stock to elect out of the tax treatment discussed above. If you make a mark-to-market election for first taxable year which you hold (or are deemed to hold) Ordinary Shares and for which we are determined to be a PFIC, you will include in your income each year an amount equal to the excess, if any, of the fair market value of the Ordinary Shares as of the close of such taxable year over your adjusted basis in such Ordinary Shares, which excess will be treated as ordinary income and not capital gain. You are allowed an ordinary loss for the excess, if any, of the adjusted basis of the Ordinary Shares over their fair market value as of the close of the taxable year. Such ordinary loss, however, is allowable only to the extent of any net mark-to-market gains on the Ordinary Shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as gain on the actual sale or other disposition of the Ordinary Shares, are treated as ordinary income. Ordinary loss treatment also applies to any loss realized on the actual sale or disposition of the Ordinary Shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such Ordinary Shares. Your basis in the Ordinary Shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, the tax rules that apply to distributions by corporations which are not PFICs would apply to distributions by us, except that the lower applicable capital gains rate for qualified dividend income discussed above under “—Taxation of Dividends and Other Distributions on our Ordinary Shares” generally would not apply.
The mark-to-market election is available only for “marketable stock,” which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a qualified exchange or other market (as defined in applicable U.S. Treasury regulations), including the Nasdaq Capital Market. If the Ordinary Shares are regularly traded on the Nasdaq Capital Market and if you are a holder of Ordinary Shares, the mark-to-market election would be available to you were we to be or become a PFIC.
131
Alternatively, a U.S. Holder of stock in a PFIC may make a “qualified electing fund” election under Section 1295(b) of the US Internal Revenue Code with respect to such PFIC to elect out of the tax treatment discussed above. A U.S. Holder who makes a valid qualified electing fund election with respect to a PFIC will generally include in gross income for a taxable year such holder’s pro rata share of the corporation’s earnings and profits for the taxable year. The qualified electing fund election, however, is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. We do not currently intend to prepare or provide the information that would enable you to make a qualified electing fund election. If you hold Ordinary Shares in any taxable year in which we are a PFIC, you will be required to file U.S. Internal Revenue Service Form 8621 in each such year and provide certain annual information regarding such Ordinary Shares, including regarding distributions received on the Ordinary Shares and any gain realized on the disposition of the Ordinary Shares.
If you do not make a timely “mark-to-market” election (as described above), and if we were a PFIC at any time during the period you hold our Ordinary Shares, then such Ordinary Shares will continue to be treated as stock of a PFIC with respect to you even if we cease to be a PFIC in a future year, unless you make a “purging election” for the year we cease to be a PFIC. A “purging election” creates a deemed sale of such Ordinary Shares at their fair market value on the last day of the last year in which we are treated as a PFIC. The gain recognized by the purging election will be subject to the special tax and interest charge rules treating the gain as an excess distribution, as described above. As a result of the purging election, you will have a new basis (equal to the fair market value of the Ordinary Shares on the last day of the last year in which we are treated as a PFIC) and holding period (which new holding period will begin the day after such last day) in your Ordinary Shares for tax purposes.
IRC Section 1014(a) provides for a step-up in basis to the fair market value for our Ordinary Shares when inherited from a decedent that was previously a holder of our Ordinary Shares. However, if we are determined to be a PFIC and a decedent that was a U.S. Holder did not make either a timely qualified electing fund election for our first taxable year as a PFIC in which the U.S. Holder held (or was deemed to hold) our Ordinary Shares, or a mark-to-market election and ownership of those Ordinary Shares are inherited, a special provision in IRC Section 1291(e) provides that the new U.S. Holder’s basis should be reduced by an amount equal to the Section 1014 basis minus the decedent’s adjusted basis just before death. As such if we are determined to be a PFIC at any time prior to a decedent’s passing, the PFIC rules will cause any new U.S. Holder that inherits our Ordinary Shares from a U.S. Holder to not get a step-up in basis under Section 1014 and instead will receive a carryover basis in those Ordinary Shares.
You are urged to consult your tax advisors regarding the application of the PFIC rules to your investment in our Ordinary Shares and the elections discussed above.
Information Reporting and Backup Withholding
Dividend payments with respect to our Ordinary Shares and proceeds from the sale, exchange, or redemption of our Ordinary Shares may be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding under Section 3406 of the U.S. Internal Revenue Code with at a current flat rate of 24%. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or who is otherwise exempt from backup withholding. U.S. Holders who are required to establish their exempt status generally must provide such certification on U.S. Internal Revenue Service Form W-9. U.S. Holders are urged to consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information. We do not intend to withhold taxes for individual shareholders. Transactions effected through certain brokers or other intermediaries, however, may be subject to withholding taxes (including backup withholding), and such brokers or intermediaries may be required by law to withhold such taxes.
Under the Hiring Incentives to Restore Employment Act of 2010, certain U.S. Holders are required to report information relating to our Ordinary Shares, subject to certain exceptions (including an exception for Ordinary Shares held in accounts maintained by certain financial institutions), by attaching a complete Internal Revenue Service Form 8938, Statement of Specified Foreign Financial Assets, with their tax return for each year in which they hold Ordinary Shares.
F. Dividends and Paying Agents
Not applicable.
132
G. Statement by Experts
Not applicable.
H. Documents on Display
We previously filed with the SEC registration statement on Form F-1 (File Number 333-234460), as amended, to register our Ordinary Shares in relation to our initial public offering.
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. All information filed with the SEC can be obtained over the internet at the SEC’s website at www.sec.gov. The public may obtain information regarding the Washington, D.C. Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a web site at www.sec.gov that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Foreign Exchange Risk
The WFOE and the VIE and its subsidiaries’ business is conducted in the PRC, and almost all of our consolidated revenues and consolidated costs and expenses are denominated in RMB. All of our assets are denominated in RMB. The financial statements that we file with the SEC and provide to our shareholders are presented in U.S. dollars. As a result, we are exposed to foreign exchange risk, as our revenues and results of operations may be affected by fluctuations in the exchange rate between the U.S. dollar and RMB. If the RMB depreciates against the U.S. dollar, the value of our RMB revenues, earnings and assets as expressed in our U.S. dollar financial statements will decline. We have not entered into any hedging transactions in an effort to reduce our exposure to foreign exchange risk.
Interest Rate Risk
Interest rates fluctuate, mainly due to uncertain future market behavior. The Group’s entities are all located in China, and the main raw materials required for the production of each company are procured within China, except for soybeans. Domestic soybeans are mainly sourced from foreign suppliers, the price of which will be affected by exchange rate fluctuations, but soybeans account for a very small proportion of the raw materials, so the interest rate risk associated with exchange rate fluctuations is negligible to the enterprise. The Group’s production of hygromycin APIs, pharmaceutical preparations, organic fertilizers, etc., and its main sales market are in China. Products that can be exported include hygromycin APIs, heparin sodium, and fine enteric coating. BGM did not directly export such products, which were sold by the downstream trade customers. Therefore, the sales of such products may be affected by foreign exchange price changes, but such income accounted for a small proportion of the overall income. We believe we have not been exposed to material risks due to changes in market interest rates.
133
Credit Risk
Financial instruments that potentially subject us to significant concentrations of credit risk consist primarily of cash, As of September 30, 2024 and 2023, $6,902,275 and $6,197,461 of our cash was on deposit at financial institutions in the PRC, where there currently is no rule or regulation requiring such financial institutions to maintain insurance to cover bank deposits in the event of bank failure. As of September 30, 2024 and 2023, $1,856,344 and $277,218 of our cash was on deposit at financial institutions in the U.S. which were insured by the Federal Deposit Insurance Corporation subject to certain limitations. While management believes that these financial institutions are of high credit quality, it also continually monitors their credit worthiness.
Accounts receivable are typically unsecured and derived from revenue earned from customers, thereby exposed to credit risk, The risk is mitigated by our assessment of its customers’ creditworthiness and its ongoing monitoring of outstanding balances.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Not applicable.
134
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Material Modifications to the Rights of Security Holders
See “Item 10. Additional Information” for a description of the rights of securities holders.
Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1, as amended (File Number: 333-234460) in relation to the initial public offering of 5,000,000 Ordinary Shares at an initial public offering price of $5.00 per Ordinary Share. Our initial public offering closed on January 14, 2021. Univest Securities, LLC was the representative of the underwriters for our initial public offering. On January 15, 2021, Univest Securities, LLC exercised the over-allotment option in full to purchase an additional 750,000 Ordinary Shares.
We received net proceeds of approximately $25.7 million, after deducting underwriting discounts and estimated offering expenses payable by us. The registration statement was declared effective by the SEC on December 30, 2020. The total expense incurred for our Company’s account in connection with our initial public offering was approximately $3.02 million, which included approximately $2.01 million in underwriting discounts for the initial public offering and approximately $1.01 million in other costs and expenses for our initial public offering. None of the transaction expenses included payments to directors or officers of our Company or their associates, persons owning more than 10% or more of our equity securities or our affiliates. None of the net proceeds we received from the initial public offering were paid, directly or indirectly, to any of our directors or officers or their associates, persons owning 10% or more of our equity securities or our affiliates. As of the date of this annual report, we have yet to spend the proceeds from our initial public offering. We still intend to use the proceeds from our initial public offering as disclosed in our registration statement on Form F-1.
ITEM 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon that evaluation, our management has concluded that, due to the material weaknesses identified below, as of September 30, 2024, our disclosure controls and procedures were not effective in ensuring that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act was recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
135
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with Generally Accepted Accounting Principles (GAAP) in the United States of America and includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of our company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with GAAP, and that receipts and expenditures of our company are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of the unauthorized acquisition, use or disposition of our company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness of our internal control over financial reporting to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As required by Rule 13a-15(c) of the Exchange Act, our management conducted an evaluation of our company’s internal control over financial reporting as of September 30, 2024 based on the framework in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that our internal control over financial reporting was not effective as of September 30, 2024.
In accordance with reporting requirements set forth by the SEC, a “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our company’s annual consolidated financial statements will not be prevented or detected on a timely basis. The material weakness identified relate to the deficiency in the ability of our in-house accounting professionals to generate financial statements and related disclosures that comply with USGAAP and in the form required by applicable SEC requirements.
To remedy our identified material weakness identified to date, we have implemented and plan to implement a number of measures to strengthen our internal control over financial reporting, including (i) recruiting more financial reporting and accounting personnel who have adequate U.S. GAAP knowledge; and (ii) implementing regular and continuous U.S. GAAP accounting and financial reporting training programs for our accounting and financial personnel. However, we cannot assure you that we will remediate our material weakness in a timely manner, or at all. See “Item 3. Key Information—Risk Factors—Risks Related to WFOE and The VIE and its Subsidiaries’ Business—If we fail to establish and maintain proper internal financial reporting controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.”
As a company with less than US$1235 billion in revenue for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002, in the assessment of the emerging growth company’s internal control over financial reporting.
Attestation Report of the Registered Public Accounting Firm
This annual report on Form 20-F does not include an attestation report of our registered public accounting firm because we qualify as an “emerging growth company” under section 3(a) of the Exchange Act, and are therefore exempt from the attestation requirement.
Changes in Internal Control over Financial Reporting
Other than as described above, there were no changes in our internal controls over financial reporting that occurred during the period covered by this annual report on Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
136
Item 16. [RESERVED]
ITEM 16.A. AUDIT COMMITTEE FINANCIAL EXPERT
The Board has determined that Mr. Maofan Tang, chairman of the audit committee of our board of directors, qualifies as an “audit committee financial expert” within the meaning of the SEC rules and possesses financial sophistication within the meaning of Listing Rules of the Nasdaq Stock Market. Mr. Maofan Tang satisfies the “independence” requirements of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market and Rule 10A-3 under the Securities Exchange Act of 1934, as amended.
ITEM 16.B. CODE OF ETHICS
Our board of directors has adopted a code of business conduct and ethics that applies to all of our directors, officers, employees, including certain provisions that specifically apply to our principal executive officer, principal financial officer or controller and any other persons who perform similar functions for us. We have filed our code of business conduct and ethics as Exhibit 14.1 of our registration statement on Form F-1 (File Number: 333-234460), as amended, initially filed with the SEC on November 4, 2019.
ITEM 16.C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by ZH CPA, LLC, Marcum Asia CPAs LLP and Enrome LLP, our independent registered public accounting firms, for the periods indicated.
|
|
|
ZH CPA, LLC |
|
Marcum Asia |
|
Enrome LLP |
|
Total |
2024 |
|
US$ |
30,000 |
|
— |
|
300,000 |
|
330,000 |
|
|
|
|
|
|
|
|
|
|
2023 |
|
US$ |
280,000 |
|
— |
|
— |
|
305,000 |
|
|
|
|
|
|
|
|
|
|
2022 |
|
US$ |
300,000 |
|
57,500 |
|
— |
|
387,500 |
Note:
| (1) | “Audit fees” means the aggregate fees billed for professional services rendered by our principal accounting firm for the audit of our annual financial statements and the review of our comparative interim financial statements. |
| (2) | “Audit-related fees” means the aggregate fees billed for professional services rendered by our principal accounting firm for the assurance and related services, which mainly included the audit and review of financial statements and are not reported under “Audit fees” above. |
| (3) | “Tax fees” means the aggregate fees billed for professional services rendered by our principal accounting firm for tax compliance, tax advice and tax planning. |
| (4) | “Other fees” means the aggregate fees incurred in each of the fiscal years listed for the professional tax services rendered by our principal accounting firm other than services reported under “Audit fees,” “Audit-related fees” and “Tax fees.” |
The policy of our audit committee is to pre-approve all audit and non-audit services provided by our principal external auditors, including audit services, audit-related services, tax services, and other services as described above.
ITEM 16.D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16.E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
137
ITEM 16.F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16.G. CORPORATE GOVERNANCE
As a Cayman Islands company listed on the Nasdaq Capital Market, we are subject to the Nasdaq corporate governance listing standards. Nasdaq rules, however, permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the Cayman Islands, which is our home country, may differ significantly from the Nasdaq corporate governance listing standards.
Nasdaq Listing Rule 5635 generally provides that shareholder approval is required of U.S. domestic companies listed on Nasdaq prior to issuance (or potential issuance) of securities (i) equaling 20% or more of the company’s common stock or voting power for less than the greater of market or book value (ii) resulting in a change of control of the company; and (iii) which is being issued pursuant to a stock option or purchase plan to be established or materially amended or other equity compensation arrangement made or materially amended. Notwithstanding this general requirement, Nasdaq Listing Rule 5615(a)(3)(A) permits foreign private issuers to follow their home country practice rather than these shareholder approval requirements. The Cayman Islands do not require shareholder approval prior to any of the foregoing types of issuances. We, therefore, are not required to obtain such shareholder approval prior to entering into a transaction with the potential to issue securities as described above. We currently intend to follow home country practice in lieu of such requirements under Nasdaq listing rules which may afford less protection to investors.
Nasdaq Listing Rule 5605(b)(1) requires listed companies to have, among other things, a majority of its board members be independent. As a foreign private issuer, however, we are permitted to, and we may follow home country practice in lieu of the above requirements. The corporate governance practice in our home country, the Cayman Islands, does not require a majority of our board to consist of independent directors. Currently, a majority of our board members are independent. However, if we change our board composition such that independent directors do not constitute a majority of our board of directors, our shareholders may be afforded less protection than they would otherwise enjoy under Nasdaq’s corporate governance requirements applicable to U.S. domestic issuers. See “Item 3. Key Information—D. Risk Factors—Risks Related to our Ordinary Shares—Because we are a foreign private issuer and are exempt from certain Nasdaq corporate governance standards applicable to U.S. issuers, you will have less protection than you would have if we were a domestic issuer.”
ITEM 16.H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16.I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J. INSIDER TRADING POLICIES
We have adopted insider trading policies and procedures governing the purchase, sale, and other dispositions of the Company’s securities by directors, senior management, and employees that are reasonably designed to promote compliance with applicable insider trading laws, rules and regulations, and any listing standards applicable to the Company. A copy of the Company’s insider trading policy is filed as Exhibit 11.2 to this annual report.
138
ITEM 16K. CYBERSECURITY
Cybersecurity Risk Management and Strategy
We have implemented comprehensive cybersecurity risk assessment procedures to ensure effectiveness in cybersecurity management, strategy and governance and reporting cybersecurity risks. We have also integrated cybersecurity risk management into our overall enterprise risk management system.
We have developed a comprehensive cybersecurity threat defense system to address both internal and external threats. We strive to manage cybersecurity risks and protect sensitive information through various means, such as technical safeguards, procedural requirements, an intensive program of monitoring on our corporate network, frequent testing of aspects of our security posture internally and with outside vendors, a robust incident response program and regular cybersecurity awareness training for employees. Our IT department regularly monitors the performance of our platforms, apps and infrastructure to enable us to respond quickly to potential problems, including potential cybersecurity threats.
As of the date of this annual report, we have not experienced any material cybersecurity incidents or identified any material cybersecurity threats that have affected or are reasonably likely to materially affect us, our business strategy, results of operations or financial condition.
Cybersecurity Governance
Our board of directors considers cybersecurity risk as part of its risk oversight function and undertakes overall risk management, including oversight of cybersecurity and other information technology risks.
Our board of directors receives quarterly reports from management on our cybersecurity risks. In addition, management updates our board of directors, as necessary, regarding any significant cybersecurity incidents. Our board of directors also receives briefings from management on our cyber risk management program.
Our management has primary responsibility for our overall cybersecurity risk management program and supervises our internal cybersecurity personnel. Our management and the IT department, including our chief financial officer and chief executive officer, is responsible for assessing and managing our material risks from cybersecurity threats. Our team’s experience includes computing, management of OA system, management of ERP system, basic database management and network engineering.
Our management oversees efforts to prevent, detect, mitigate, and remediate cybersecurity risks and incidents through various means, which may include briefings from internal security personnel; information obtained from governmental, public or private sources; and alerts and reports produced by security tools deployed in our IT Systems environment.
139
PART III
ITEM 17. FINANCIAL STATEMENTS
We have elected to provide financial statements pursuant to Item 18.
ITEM 18. FINANCIAL STATEMENTS
The consolidated financial statements of BGM Group Ltd are included at the end of this annual report.
ITEM 19. EXHIBITS
Exhibit |
|
Description |
1.1* |
|
|
|
|
|
2.2 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3* |
|
|
|
|
|
8.1* |
|
|
|
|
|
11.1 |
|
|
|
|
|
11.2* |
|
|
|
|
|
12.1* |
|
|
|
|
|
12.2* |
|
|
|
|
|
13.1** |
|
|
|
|
|
13.2** |
|
|
|
|
|
15.1* |
|
|
|
|
|
15.2* |
|
|
|
|
|
15.3* |
|
|
|
|
|
140
97.1* |
|
|
|
|
|
101.INS* |
|
Inline XBRL Instance Document-this instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
|
|
|
101.SCH* |
|
Inline XBRL Taxonomy Extension Scheme Document |
|
|
|
101.CAL* |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF* |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB* |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
101.PRE* |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
104* |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
* |
Filed herewith. |
** |
Furnished herewith. |
141
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
BGM Group Ltd |
|
|
|
|
|
By: |
/s/ Chen Xin |
|
Name: |
Chen Xin |
|
Title: |
Chief Executive Officer (Principal Executive Officer) |
|
|
|
Date: January 27, 2025 |
|
|
142
BGM Group Ltd
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
CONTENTS |
Pages |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (ENROME LLP PCAOB ID: 6907) |
F-2 |
REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (ZH CPA, LLC PCAOB ID : 6413) |
F-3 |
CONSOLIDATED BALANCE SHEETS AS OF SEPTEMBER 30, 2024 AND 2023 |
F-4 |
F-5 |
|
F-6 |
|
CONSOLIDATED STATEMENTS OF CASH FLOWS FOR THE YEARS ENDED SEPTEMBER 30, 2024, 2023 AND 2022 |
F-7 |
F-8 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of BGM Group Ltd
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BGM Group Ltd and its subsidiaries (the “Company”) as of September 30, 2024 and the related consolidated statements of operations and comprehensive income (loss), changes in shareholders’ equity and its cash flows for the year ended September 30, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2024 and the results of its operations and its cash flows for the year ended September 2024, in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatements of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements presentation. We believe that our audits provide a reasonable basis for our opinion.
Emphasis of Matters
Restatement adjustments for changes in share consolidation
The consolidated financial statements of the Company as of September 30, 2023 and 2022 were audited by other auditor. As described in Note 14, the Company adjusted all shares and per share data periods presented for the shares consolidation. We audited the adjustments that were applied to restate the disclosure for share consolidation reflected in the September 30, 2023 and 2022 consolidated financial statements to retrospectively apply the effects of the share consolidation that occurred subsequent to the year ended September 30, 2023 and 2022. However, we were not engaged to audit, review, or apply any procedures to the September 30, 2023 and 2022 consolidated financial statements of the Company other than with respect to such adjustments and, accordingly, we do not express an opinion or any other form of assurance on the September 30, 2023 and 2022 consolidated financial statements taken as a whole.
/s/ Enrome LLP
We have served as the Company’s auditor since 2024
Singapore
January 27, 2025
F-2

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of BGM
Group Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of BGM Group Limited (formerly known as Qilian International Holding Group Ltd.) and its affiliated entities (collectively, the “Company”) as of September 30, 2023, and the related consolidated statements of operations and comprehensive (loss) income, changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended September 30, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2023, and the results of its operations and its cash flows for each of the years in the two-year period ended September 30, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Other Matter
As discussed in Note 2, Note 11 and Note 14 to the consolidated financial statements, subsequent to the date of our report, the Company had a share consolidation at a ratio of five-for-one, effective on June 21, 2024. The related number of shares authorized, shares issued and outstanding as of September 30, 2023 and earnings per share for the years ending September 30, 2023 and 2022 presented on the Company’s consolidated financial statements that were incorporated in the Company’s Form 20-F for the fiscal year ended September 30, 2024 filed on January 27, 2025 with SEC were retroactively adjusted to reflect the share consolidation. These changes have not been audited by us and our report relating to the consolidated financial statements of the Company for the year ending September 30, 2023 will not be revised as a result.
/s/ZH CPA, LLC |
|
|
|
We have served as the Company’s auditor since 2023. | |
999 18th Street, Suite 3000, Denver, CO, 80202 USA Phone: 1.303.386.7224 Fax: 1.303.386.7101 Email: admin@zhcpa.us
F-3
BGM Group Ltd and Subsidiaries
Consolidated Balance Sheets
(Expressed in U.S. Dollars, except for the number of shares)
|
|
As of September 30 |
|
As of September 30 |
||
|
|
2024 |
|
2023 |
||
ASSETS |
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
Cash and cash equivalent |
|
$ |
9,817,254 |
|
$ |
7,476,247 |
Short term investment |
|
|
— |
|
|
1,000,000 |
Accounts receivable, net |
|
|
1,543,160 |
|
|
1,975,716 |
Bank acceptance notes receivable |
|
|
3,337,137 |
|
|
4,131,392 |
Inventories, net |
|
|
5,049,688 |
|
|
4,991,435 |
Prepayment to suppliers, net |
|
|
803,924 |
|
|
708,248 |
Investment in trading securities |
|
|
8,323,587 |
|
|
13,943,019 |
Other current assets |
|
|
894,460 |
|
|
286,564 |
TOTAL CURRENT ASSETS |
|
|
29,769,210 |
|
|
34,512,621 |
|
|
|
|
|
|
|
Property and equipment, net |
|
|
8,610,279 |
|
|
9,143,583 |
Construction in progress |
|
|
5,640,063 |
|
|
2,867,683 |
Intangible assets, net |
|
|
4,539,347 |
|
|
3,423,582 |
Long term investment |
|
|
3,359,786 |
|
|
606,005 |
Operating lease right of use assets |
|
|
— |
|
|
59,300 |
Deferred tax assets |
|
|
424,474 |
|
|
10,778 |
Prepayments for property and equipment |
|
|
660,569 |
|
|
634,442 |
TOTAL ASSETS |
|
|
53,003,728 |
|
|
51,257,994 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
Bank loans |
|
|
— |
|
|
479,715 |
Accounts payable |
|
|
4,125,597 |
|
|
3,592,687 |
Contract liabilities |
|
|
489,784 |
|
|
1,028,318 |
Deferred government grants-current |
|
|
78,718 |
|
|
76,812 |
Taxes payable |
|
|
315,328 |
|
|
203,498 |
Operating lease liabilities, current |
|
|
— |
|
|
73,560 |
Due to related party |
|
|
2,851,526 |
|
|
— |
Accrued expenses and other payables |
|
|
915,032 |
|
|
1,205,549 |
TOTAL CURRENT LIABILITIES |
|
|
8,775,985 |
|
|
6,660,139 |
|
|
|
|
|
|
|
LONG TERM LIABILITIES |
|
|
|
|
|
|
Operating lease liabilities, noncurrent |
|
|
— |
|
|
24,575 |
Deferred government grants - noncurrent |
|
|
134,394 |
|
|
221,879 |
TOTAL LIABILITIES |
|
|
8,910,379 |
|
|
6,906,593 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS’ EQUITY: |
|
|
|
|
|
|
Ordinary Shares, $0.00833335 par value, 100,000,000 and 20,000,000 shares authorized, 7,226,480 and 7,226,480 Ordinary Shares issued and outstanding as of September 30, 2024 and 2023 respectively* |
|
|
59,583 |
|
|
59,583 |
Additional paid-in capital |
|
|
36,410,931 |
|
|
36,410,931 |
Statutory Reserve |
|
|
3,266,081 |
|
|
3,162,333 |
Retained earnings |
|
|
4,349,377 |
|
|
5,896,373 |
Accumulated other comprehensive loss |
|
|
(1,342,128) |
|
|
(2,737,087) |
Total shareholders’ equity attributable to BGM Group Ltd |
|
|
42,743,844 |
|
|
42,792,133 |
Noncontrolling interests |
|
|
1,349,505 |
|
|
1,559,268 |
TOTAL SHAREHOLDERS’ EQUITY |
|
|
44,093,349 |
|
|
44,351,401 |
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
53,003,728 |
|
|
51,257,994 |
*The shares and per share data are presented on a retroactive basis to reflect the Company’s Share Consolidation.
The accompanying notes are an integral part of these consolidated financial statements.
F-4
BGM Group Ltd and Subsidiaries
Consolidated Statements of Operations and Comprehensive Income (Loss)
(Expressed in U.S. Dollars, except for the number of shares)
|
|
For the years ended September 30 |
|
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|
|||
NET REVENUE |
|
$ |
25,097,951 |
|
$ |
46,471,478 |
|
$ |
64,855,025 |
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
|
20,983,196 |
|
|
44,719,984 |
|
|
58,627,728 |
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
|
4,114,755 |
|
|
1,751,494 |
|
|
6,227,297 |
|
|
|
|
|
|
|
|
|
|
|
|
SELLING, GENERAL AND ADMINISTRATIVE, RESEARCH AND DEVELOPMENT EXPENSES |
|
|
4,678,526 |
|
|
4,361,593 |
|
|
4,125,294 |
|
|
|
|
|
|
|
|
|
|
|
|
INCOME (LOSS) FROM OPERATIONS |
|
|
(563,771) |
|
|
(2,610,099) |
|
|
2,102,003 |
|
|
|
|
|
|
|
|
|
|
|
|
Interest income (expense), net |
|
|
(639,511) |
|
|
99,190 |
|
|
24,860 |
|
Investment loss |
|
|
(819,432) |
|
|
(5,523,365) |
|
|
(812,804) |
|
Loss on disposal of long term investment |
|
|
(101,354) |
|
|
— |
|
|
— |
|
Share of results of associates |
|
|
(204,648) |
|
|
— |
|
|
— |
|
Grant income |
|
|
206,415 |
|
|
192,375 |
|
|
413,717 |
|
Other expenses |
|
|
(14,841) |
|
|
(61,005) |
|
|
(167,217) |
|
Total other expense |
|
|
(1,573,371) |
|
|
(5,292,805) |
|
|
(541,444) |
|
|
|
|
|
|
|
|
|
|
|
|
INCOME (LOSS) BEFORE INCOME TAX PROVISION |
|
|
(2,137,142) |
|
|
(7,902,904) |
|
|
1,560,559 |
|
|
|
|
|
|
|
|
|
|
|
|
INCOME TAX EXPENSE/(BENEFIT) |
|
|
(619,981) |
|
|
219,166 |
|
|
194,302 |
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME (LOSS) |
|
|
(1,517,161) |
|
|
(8,122,070) |
|
|
1,366,257 |
|
|
|
|
|
|
|
|
|
|
|
|
Less: net income (loss) attributable to non-controlling interest |
|
|
(74,331) |
|
|
(341,450) |
|
|
289,564 |
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME (LOSS) ATTRIBUTABLE TO BGM Group Ltd |
|
$ |
(1,442,830) |
|
$ |
(7,780,620) |
|
$ |
1,076,693 |
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME (LOSS) |
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
|
1,259,109 |
|
|
(730,903) |
|
|
(3,091,179) |
|
COMPREHENSIVE INCOME (LOSS) |
|
|
(258,052) |
|
|
(8,852,973) |
|
|
(1,724,922) |
|
Less: comprehensive income (loss) attributable to non - controlling interests |
|
|
(210,181) |
|
|
(381,357) |
|
|
101,542 |
|
COMPREHENSIVE INCOME (LOSS) ATTRIBUTABLE TO BGM Group Ltd |
|
|
(47,871) |
|
|
(8,471,616) |
|
|
(1,826,464) |
|
|
|
|
|
|
|
|
|
|
|
|
Earnings (loss) per common share - basic and diluted |
|
$ |
(0.20) |
|
$ |
(1.08) |
* |
$ |
0.15 |
* |
Weighted average shares - basic and diluted |
|
|
7,226,480 |
|
|
7,226,480 |
* |
|
7,226,480 |
* |
*The shares and per share data are presented on a retroactive basis to reflect the Company’s Share Consolidation.
The accompanying notes are an integral part of these consolidated financial statements.
F-5
BGM Group Ltd and Subsidiaries
Consolidated Statements of Changes in Shareholders’ Equity
(Expressed in U.S. Dollars, except for the number of shares)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
Shareholders’ |
|
|
|
|
|
|
||||
|
|
Ordinary Shares |
|
Additional |
|
|
|
|
|
|
|
Comprehensive |
|
|
Equity Attributable |
|
Non-controlling |
|
Total |
|||||||
|
|
Shares* |
|
Amount |
|
Paid-in Capital |
|
Retained Earnings |
|
Statutory Reserve |
|
Loss |
|
to BGM Group Ltd |
|
Interests |
|
Shareholders’ Equity |
||||||||
Balance as of September 30, 2022 |
|
7,226,480 |
|
$ |
59,583 |
|
$ |
36,410,931 |
|
$ |
15,509,177 |
|
$ |
3,118,542 |
|
$ |
(2,046,091) |
|
$ |
53,052,142 |
|
$ |
1,911,394 |
|
$ |
54,963,536 |
Net loss for the year |
|
— |
|
|
— |
|
|
— |
|
|
(7,780,620) |
|
|
— |
|
|
— |
|
|
(7,780,620) |
|
|
(341,450) |
|
|
(8,122,070) |
Acquisition of equity interest from unrelated third party shareholders |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(28,356) |
|
|
(28,356) |
Contribution from non controlling interest |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
56,711 |
|
|
56,711 |
Appropriation for statutory reserve |
|
— |
|
|
— |
|
|
— |
|
|
(44,667) |
|
|
43,791 |
|
|
— |
|
|
(876) |
|
|
876 |
|
|
— |
Dividend |
|
— |
|
|
— |
|
|
— |
|
|
(1,787,517) |
|
|
— |
|
|
— |
|
|
(1,787,517) |
|
|
— |
|
|
(1,787,517) |
Foreign currency translation adjustment |
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
— |
|
|
(690,996) |
|
|
(690,996) |
|
|
(39,907) |
|
|
(730,903) |
Balance as of September 30, 2023 |
|
7,226,480 |
|
$ |
59,583 |
|
$ |
36,410,931 |
|
$ |
5,896,373 |
|
$ |
3,162,333 |
|
$ |
(2,737,087) |
|
$ |
42,792,133 |
|
$ |
1,559,268 |
|
$ |
44,351,401 |
Net loss for the year |
|
— |
|
|
— |
|
|
— |
|
|
(1,442,830) |
|
|
— |
|
|
— |
|
|
(1,442,830) |
|
|
(74,331) |
|
|
(1,517,161) |
Appropriation for statutory reserve |
|
— |
|
|
— |
|
|
— |
|
|
(104,166) |
|
|
103,748 |
|
|
— |
|
|
(418) |
|
|
418 |
|
|
— |
Foreign currency translation adjustment |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
1,394,959 |
|
|
1,394,959 |
|
|
(135,850) |
|
|
1,259,109 |
Balance as of September 30, 2024 |
|
7,226,480 |
|
$ |
59,583 |
|
$ |
36,410,931 |
|
$ |
4,349,377 |
|
$ |
3,266,081 |
|
$ |
(1,342,128) |
|
$ |
42,743,844 |
|
$ |
1,349,505 |
|
$ |
44,093,349 |
*The shares and per share data are presented on a retroactive basis to reflect the Company’s Share Consolidation.
The accompanying notes are an integral part of these consolidated financial statements.
F-6
BGM Group Ltd and Subsidiaries
Consolidated Statements of Cash flows
(Expressed in U.S. Dollars, except for the number of shares)
|
|
For the years ended September 30 |
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|||
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net Income (loss) |
|
$ |
(1,517,161) |
|
|
(8,122,070) |
|
|
1,366,257 |
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
|
|
|
|
|
|
|
|
|
Non-cash operating lease expenses |
|
|
60,785 |
|
|
25,982 |
|
|
22,451 |
Stock based compensation |
|
|
— |
|
|
— |
|
|
20,000 |
Depreciation and amortization |
|
|
1,237,229 |
|
|
1,143,064 |
|
|
1,224,672 |
Provision (reverse) for accounts receivable |
|
|
127,568 |
|
|
37,885 |
|
|
(186,814) |
Reverse for other receivables |
|
|
(55,382) |
|
|
— |
|
|
— |
Inventories provision (reserve) |
|
|
(813,619) |
|
|
388,253 |
|
|
444,894 |
Deferred tax expense (benefit) |
|
|
(406,845) |
|
|
203,544 |
|
|
189,838 |
Unrealized loss from investment in securities |
|
|
819,432 |
|
|
5,527,381 |
|
|
853,000 |
Investment (income) |
|
|
— |
|
|
(4,016) |
|
|
(40,196) |
Share of results of associates |
|
|
204,648 |
|
|
— |
|
|
— |
Loss on disposal of Long term investment |
|
|
101,354 |
|
|
— |
|
|
— |
Property and equipment written off |
|
|
526 |
|
|
— |
|
|
8,755 |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
378,389 |
|
|
(1,223,035) |
|
|
1,027,671 |
Bank acceptance notes receivable |
|
|
949,455 |
|
|
(1,665,594) |
|
|
8,744,826 |
Inventories |
|
|
958,655 |
|
|
3,403,831 |
|
|
2,230,723 |
Prepayment to suppliers |
|
|
(65,476) |
|
|
492,858 |
|
|
41,869 |
Other current assets |
|
|
(1,506,472) |
|
|
1,414,305 |
|
|
(1,198,646) |
Accounts payable |
|
|
378,976 |
|
|
(1,618,317) |
|
|
(805,443) |
Contract liabilities |
|
|
(571,880) |
|
|
502,535 |
|
|
(1,827,461) |
Contract liabilities - related parties |
|
|
— |
|
|
— |
|
|
(17,066) |
Deferred government grants |
|
|
(96,364) |
|
|
(126,198) |
|
|
(275,963) |
Taxes payable |
|
|
101,845 |
|
|
(612,274) |
|
|
584,693 |
Accrued expenses and other payables |
|
|
359,167 |
|
|
539,782 |
|
|
301,165 |
Operating lease liabilities |
|
|
(100,592) |
|
|
4,293 |
|
|
(55,036) |
Net cash provided by operating activities |
|
|
544,238 |
|
|
312,209 |
|
|
12,654,188 |
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(240,133) |
|
|
(179,759) |
|
|
(2,033,510) |
Payment for construction in progress |
|
|
(2,613,123) |
|
|
(1,668,924) |
|
|
(1,198,759) |
Purchase of intangible assets |
|
|
(1,078,215) |
|
|
(1,865,406) |
|
|
(26,683) |
Cash received from disposal of Long term investment |
|
|
463,629 |
|
|
— |
|
|
— |
Dividend received |
|
|
56,198 |
|
|
— |
|
|
— |
Payment for short term investment |
|
|
— |
|
|
(1,000,000) |
|
|
— |
Proceeds from short term investment |
|
|
1,000,000 |
|
|
— |
|
|
— |
Payments on long term investment |
|
|
(1,404,938) |
|
|
— |
|
|
— |
Redemption from marketable securities |
|
|
4,800,000 |
|
|
— |
|
|
— |
Purchase of non controlling interest |
|
|
— |
|
|
(28,356) |
|
|
— |
Net cash provided by (used in) investing activities |
|
|
983,418 |
|
|
(4,742,445) |
|
|
(3,258,952) |
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Proceeds from bank loans |
|
|
— |
|
|
496,222 |
|
|
3,204,541 |
Repayment of bank loans |
|
|
(491,728) |
|
|
(141,778) |
|
|
(3,051,944) |
Repayment of bank notes payable |
|
|
— |
|
|
(1,544,722) |
|
|
(6,090,126) |
Non controlling interest contribution |
|
|
— |
|
|
56,711 |
|
|
— |
Dividend paid |
|
|
— |
|
|
(1,787,517) |
|
|
— |
Net cash used in financing activities |
|
|
(491,728) |
|
|
(2,921,084) |
|
|
(5,937,529) |
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate change on Cash, cash equivalents and restricted cash |
|
|
1,305,079 |
|
|
(151,446) |
|
|
(1,086,067) |
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in Cash, cash equivalents and restricted cash |
|
|
2,341,007 |
|
|
(7,502,766) |
|
|
2,371,640 |
Cash, cash equivalents and restricted cash at beginning of year |
|
|
7,476,247 |
|
|
14,979,013 |
|
|
12,607,373 |
Cash, cash equivalents and restricted cash at end of year |
|
$ |
9,817,254 |
|
|
7,476,247 |
|
|
14,979,013 |
|
|
|
|
|
|
|
|
|
|
Supplemental cash flow information |
|
|
|
|
|
|
|
|
|
Cash paid for interest |
|
$ |
4,262 |
|
$ |
3,656 |
|
$ |
122,237 |
Cash paid for income taxes |
|
$ |
21,910 |
|
$ |
27,440 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Supplemental non-cash activities |
|
|
— |
|
|
— |
|
|
— |
Unpaid RMB 15,000,000($2,107,407) of 25% euqity investment (total considerarion of RMB 25,000,000) to Caihou Capital (Shenzhen) Group |
|
$ |
2,107,407 |
|
$ |
— |
|
$ |
— |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
BGM Group Ltd AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
Qilian International Holding Group Limited (“Qilian International”, or “the Company”) is a Cayman Islands exempted company incorporated on February 7, 2019 as a holding company to develop business opportunities in the People’s Republic of China (“PRC” or “China”).
On October 18, 2024, shareholders approved the change of our company name to BGM Group Ltd at an extraordinary meeting of shareholders. Effective on October 30, 2024, the Company changed our name to “BGM Group Ltd.”
BGM Group Ltd has a strategic focus on the technology fields of AI application, intelligent robots, algorithmic computing power, cloud computing, and biopharmaceuticals.
In terms of AI application implementation, the group relies on big data mining and AI Agent technology, and utilizes the two platforms of Du Xiao Bao and Bao Wang to provide comprehensive and professional AI solutions and intelligent robot services for insurance companies, insurance brokers, and consumers. Its services cover multiple key scenarios such as sales and marketing, underwriting assessment, claims processing, and customer service. The group is capable of analyzing consumer data, building consumer profiles, accurately predicting insurance needs, and providing highly customized services for consumers.
In the field of biopharmaceuticals, the group’s biopharmaceutical division mainly produces oxytetracycline API, crude heparin sodium, and licorice preparations, which are widely supplied to the global animal husbandry, pharmaceutical, and drug retail markets. The group deeply integrates AI-assisted decision-making into every link of production and manufacturing, achieving supply chain optimization, process efficiency improvement, and market trend prediction. This provides scientific decision-making basis for the management and offers high-quality products and precise services for consumers.
Qilian International (Hong Kong) Holdings Ltd (“Qilian HK”) is a wholly-owned subsidiary of Qilian International formed in accordance with the laws and regulations of Hong Kong on January 30, 2019.
Qilian International is a holding company whose only asset is 100% of the equity interest in Qilian HK. Qilian HK is a holding company whose only asset is 100% of the equity interest in Qilian International Trading (Chengdu) Co., Ltd. (“Qilian Chengdu”) and Qilian Shan International Trade (Hainan) Co., Ltd. (“Hainan Trading”), and 51% ownership in Zhongqiao Youguan E-Commerce service Co., Ltd (“Zhongqiao”), collectively the “WFOE”), which are wholly foreign-owned entities organized under the laws of the PRC. Qilian International and Qilian HK do not have any substantive operations of their own but conduct their primary business operations through Qilian Chengdu and Hainan Trading’s variable interest entity, Gansu Qilianshan Pharmaceutical Co., Ltd (“Gansu QLS”, or the “VIE”).
BGM (Hubei) Health Biological industry Co, LTD is a wholly-owned subsidiary of Qilian HK formed on September 12, 2024.
F-8
Gansu QLS was established in August 2006 under the laws of the PRC with initial capital of approximately $0.27 million. After several registered capital increases and capital contributions, the registered and paid capital of Gansu QLS was approximately $12 million as of September 30, 2024 and 2023. Over the years, Gansu QLS has established seven subsidiaries:
|
|
Ownership as of |
|
Ownership as of |
|
|
|
September 30, |
|
September 30, |
|
|
|
2024 |
|
2023 |
|
Moshangfa (Gansu) Fertilizer Industry Co., Ltd (formerly Jiuquan Qiming Biotechnology Co., Ltd, “Moshangfa”) |
|
100 |
% |
100 |
% |
Chengdu Qilianshan Biotechnology Co., Ltd (“Chengdu QLS”) |
|
79.71 |
% |
79.51 |
% |
Jiuquan Ahan Biotechnology Co., Ltd. (“Ahan”) |
|
100 |
% |
100 |
% |
Tibet Samen Trading Co., Ltd (“Samen”) (1) |
|
— |
% |
— |
% |
Tibet Cangmen Trading Co., Ltd (“Cangmen”) |
|
100 |
% |
100 |
% |
Rugao Tianlu Animal Products Co., Ltd (“Rugao”) |
|
79.71 |
% |
79.51 |
% |
Chongqing Shengfu Biological Technology Co., Ltd (“Chongqing”) |
|
79.71 |
% |
79.51 |
% |
(1)Samen was dissolved in June 2023, the business of which continues via the operation of the Company’s other subsidiaries.
On May 20, 2019, Qilian International, through its WFOE, Qilian Chengdu, entered into a series of agreements with Gansu QLS and its shareholders, including an Exclusive Services Agreement, Call Option Agreement, Shareholders’ Voting Rights Proxy and Equity Pledge Agreement, Powers of Attorney, and the Spousal Consents (collectively “VIE agreements”). These contractual arrangements oblige Qilian Chengdu to absorb a majority of the risk of loss from Gansu QLS’s activities and entitle Qilian Chengdu to receive a majority of their residual returns. In essence, Qilian Chengdu has gained certain level of control over Gansu QLS. In addition, 99.214% of Gansu QLS’s shareholders have pledged their equity interest in Gansu QLS to Qilian Chengdu on September 30, 2022 and 2021, irrevocably granted Qilian Chengdu an exclusive option to purchase, to the extent permitted under PRC law, all or part of the equity interests in Gansu QLS, and agreed to entrust all the rights to exercise their voting power to the person(s) appointed by Qilian Chengdu. Through these contractual arrangements, Qilian Chengdu holds 99.214% of the variable interests of Gansu QLS on September 30, 2022 and 2021.
To optimize its corporate structure, Chengdu Trading and Gansu QLS executed certain exclusive service termination agreement (the “Service Termination Agreement”) to terminate certain contractual service arrangements between Chengdu Trade and Gansu QLS. As a result of the aforementioned termination, Chengdu Trade will no longer have contractual control over, nor receive the economic benefits of Gansu QLS. In connection with such termination, Qilian Shan International Trade (Hainan) Co., Ltd (“Hainan Trading”), a wholly-owned subsidiary of Qilian International (Hong Kong) Holdings Limited, entered into a certain exclusive service agreement with Gansu QLS, through which Hainan Trade obtained contractual control over Gansu QLS. The terms of these agreement are identical to the VIE agreement. The Service Termination Agreement and the new service agreement with Hainan Trading became effective on December 1, 2022.
Based on these contractual arrangements, Gansu QLS is considered as a VIE of Qilian Chengdu and Hainan Trading under Financial Accounting Standards Board (“FASB”) Accounting Standards Codification Topic 810 (“ASC 810”), “Consolidation of Variable Interest Entities, an Interpretation of ARB No.51”, because the equity investors in Gansu QLS do not have the characteristics of a controlling financial interest. In addition, Qilian Chengdu and Hainan Trading are the primary beneficiary of Gansu QLS, and, as such, Gansu QLS’s books and records are consolidated into those of WFOE. Risks in relation to the VIE structure are discussed under “Risks and Uncertainties” below.
As the above entities were under common control before and after the consummation of the VIE agreements, the restructuring was accounted for as a reorganization of entities under common control and the consolidation of Qilian International and its subsidiaries, the VIE and its subsidiaries has been accounted for at historical cost and prepared on the basis as if the aforementioned transactions had become effective as of the beginning of the first period presented in the accompanying consolidated financial statements.
Qilian International, its subsidiaries, the VIE and VIE’s subsidiaries are principally engaged in the development, manufacture, marketing, and sale of licorice products, oxytetracycline products, traditional Chinese medicine derivatives (“TCMD”) product, heparin product, sausage casings, and fertilizers.
F-9
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation
The Company, its subsidiaries, the VIE and VIE’s subsidiaries consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The consolidated financial statements include the financial statements of Qilian International, and its subsidiaries, the VIE and VIE’s subsidiaries. All material intercompany accounts and transactions have been eliminated in consolidation. See Risks and Uncertainties disclosure for VIE structures in China.
The carrying amounts of the assets, liabilities, the results of operations and cash flows of the VIE and VIE’s subsidiaries included in the Company, its subsidiaries, the VIE and VIE subsidiaries’ consolidated financial statements after the elimination of intercompany balances and transactions among the VIE and VIE’s subsidiaries, and the Company and its subsidiaries are as follows:
|
|
September 30, |
|
September 30, |
||
|
|
2024 |
|
2023 |
||
ASSETS |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
4,474,803 |
|
$ |
6,876,195 |
Accounts receivable, net |
|
|
1,542,956 |
|
|
1,975,422 |
Bank acceptance receivable |
|
|
3,337,137 |
|
|
4,131,392 |
Inventories, net |
|
|
5,049,688 |
|
|
4,991,435 |
Prepayment to suppliers, net |
|
|
803,767 |
|
|
708,097 |
Other current assets |
|
|
791,439 |
|
|
229,992 |
Total current assets |
|
|
15,999,790 |
|
|
18,912,533 |
Property and equipment, net |
|
|
12,165,110 |
|
|
9,873,502 |
Intangible assets, net |
|
|
3,461,132 |
|
|
3,423,582 |
Long-term investment |
|
|
— |
|
|
606,005 |
Operating lease right of use assets |
|
|
— |
|
|
59,300 |
Deferred tax assets |
|
|
390,242 |
|
|
10,778 |
Total assets |
|
$ |
32,016,274 |
|
$ |
32,885,700 |
LIABILITIES |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Bank loans |
|
$ |
— |
|
$ |
479,715 |
Accounts payable |
|
|
4,120,956 |
|
|
3,578,494 |
Contract liabilities |
|
|
489,784 |
|
|
1,028,318 |
Deferred government grants - current |
|
|
78,718 |
|
|
76,812 |
Taxes payable |
|
|
316,789 |
|
|
225,683 |
Operating lease liabilities, current |
|
|
— |
|
|
73,560 |
Accrued expenses and other payables |
|
|
914,756 |
|
|
1,207,536 |
Total current liabilities |
|
|
5,921,003 |
|
|
6,670,118 |
Operating lease liabilities, long term |
|
|
— |
|
|
24,575 |
Deferred government grants - noncurrent |
|
|
134,394 |
|
|
221,879 |
Total liabilities |
|
|
6,055,397 |
|
|
6,916,574 |
|
|
For the Years ended |
|||||||
|
|
September 30, |
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|||
Net revenue |
|
$ |
25,097,953 |
|
$ |
46,471,478 |
|
$ |
64,468,807 |
Income (loss) from operations |
|
$ |
622,896 |
|
$ |
(1,722,218) |
|
$ |
2,502,014 |
Net income (loss) |
|
$ |
704,119 |
|
$ |
(1,674,516) |
|
$ |
2,752,212 |
F-10
|
|
For the Years Ended |
|||||||
|
|
September 30, |
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|||
Net cash provided by operating activities |
|
$ |
182,190 |
|
$ |
1,203,386 |
|
$ |
12,901,270 |
Net cash used in investing activities |
|
|
(2,333,429) |
|
|
(3,700,105) |
|
|
(1,153,972) |
Net cash used in financing activities |
|
|
(491,728) |
|
|
(1,190,278) |
|
|
(5,937,529) |
Effect of exchange rate on cash |
|
|
241,575 |
|
|
(123,754) |
|
|
(1,018,698) |
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
$ |
(2,401,392) |
|
$ |
(3,810,751) |
|
$ |
4,791,071 |
Retroactivity
On May 29, 2024, the board of directors of the Company approved a share consolidation at a ratio of five-for-one (5:1), effective on June 21, 2024. The related number of shares, shares authorized, shares issued and outstanding and earnings per share presented on the Company’s consolidated financial statements were retroactively adjusted to reflect the share consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions. Such estimates and assumptions affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company, its subsidiaries, the VIE and VIE’s subsidiaries’ accounting estimates included, but are not limited to: allowance for estimated uncollectible receivables, inventory valuations, impairment of long-lived assets, useful lives of property and equipment and intangible assets, fair value of investment in trading securities, impairment of intangible assets, realization of deferred tax assets and uncertain tax position, and income taxes. Actual results could differ from those estimates.
Risks and Uncertainties
Risks of Operation in China
The main operation of the Company, through the WFOE, the VIE and VIE’s subsidiaries, is located in the PRC. Accordingly, the Company, its subsidiaries, the VIE and VIE’s subsidiaries’ business, financial condition, and results of operations may be influenced by political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy. The Company, its subsidiaries, the VIE and VIE’s subsidiaries’ results may be adversely affected by changes in the political, regulatory and social conditions in the PRC. Although the Company, its subsidiaries, the VIE and VIE’s subsidiaries’ have not experienced losses from these situations and believes that it is in compliance with existing laws and regulations including its organization and structure disclosed in Note 1, this may not be indicative of future results.
Risks in relation to the VIE structure
The Company is incorporated in the Cayman Islands. As a holding company with no material operations, the Company conducts its operations in China through the variable interest entities, Gansu QLS and its subsidiaries. The Company receives the economic benefits of Gansu QLS and its subsidiaries’ business operation through a series of contractual arrangements, or the VIE Agreements, which have not been tested in court. As a result of the Company’s indirect ownership in the Qilian Chengdu and Hainan Trading and the VIE Agreements, the Company is regarded as the primary beneficiary of its VIE. The VIE structure is used to replicate foreign investment in Chinese-based companies where Chinese law prohibits direct foreign investment in the operating companies, and that investors may never directly hold equity interests in the Chinese operating entities. The Company relies on contractual arrangements with the VIE and its subsidiaries in China for the business operations, which may not be as effective in providing operational control or enabling the Company to derive economic benefits as through ownership of controlling equity interests, and the VIE’s shareholders may fail to perform their obligations under the contractual arrangements. If the PRC government deems that the VIE Agreements in relation to the VIE do not comply with PRC regulatory restrictions on foreign investment in the relevant industries, or if these regulations or the interpretation of existing regulations change in the future, the Company may have difficulty in enforcing any rights the Company may have under the VIE Agreements in PRC and the Company could be subject to severe penalties or be forced to relinquish the Company’s interests in those operations.
F-11
Technology Innovation and Commodity Risks
The Company, its subsidiaries, the VIE and VIE’s subsidiaries’ business faces rapid technological change, and there is a possibility that the competitors may achieve regulatory approval and develop new product candidates before the Company, its subsidiaries, the VIE and VIE’s subsidiaries, which may harm the financial condition and the ability to successfully market or commercialize any of the product candidates.
The development and commercialization of new pharmaceutical products and fertilizers is highly competitive, and both industries currently are characterized by rapidly changing technologies, significant competition and a strong emphasis on intellectual property. The Company, its subsidiaries, the VIE and VIE’s subsidiaries will face competition with respect to the current and future pharmaceutical and fertilizer product candidates from major pharmaceutical and chemical companies in China. The Heparin and sausage casing products are made from livestock products, which are subjected to significant risks of the market supply of the raw materials.
Exchange Rate Risks
The WFOE, the VIE and VIE’s subsidiaries operate in China, which may give rise to significant foreign currency risks from fluctuations and the degree of volatility of foreign exchange rates between the US$ and the RMB. As of September 30, 2024 and September 30, 2023, cash and restricted cash of $6,902,275 (RMB 48,366,999) and $6,197,461 (RMB 45,216,675), respectively, is denominated in RMB and is held in PRC.
Currency Convertibility Risks
Substantially all of the WFOE, the VIE and VIE’s subsidiaries’ operating activities are transacted in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with other information such as suppliers’ invoices, shipping documents and signed contracts.
Cash and Cash Equivalents
The Company considers all highly liquid investment instruments with an original maturity of three months or less from the date of purchase to be cash equivalents. The cash and cash equivalent don’t do not have withdrawal restrictions.
Short-term Investment
The Company’s short-term investment include a time deposit which has maturity less than 12 months.
Accounts Receivable, net
Accounts receivable are recognized and carried at original invoiced amount less an estimated allowance for uncollectible accounts. The WFOE, the VIE and VIE’s subsidiaries usually grant credit to customers with good credit standing with a maximum of 90 days and determines the adequacy of reserves for doubtful accounts based on individual account analysis and historical collection trends. The Company evaluates the creditworthiness of its customers. Delinquent account balances are written-off against the allowance for doubtful accounts after management has determined that the likelihood of collection is not probable.
Bank acceptance notes receivable
Bank acceptance notes receivable generally due within six months and with specific payment terms and definitive due dates, are comprised of the notes issued by some customers to pay certain outstanding receivable balances to the Company. Bank acceptance notes do not bear interest. From time to time, the Company endorse bank notes receivable to its suppliers as the payment of material purchase. The bank notes receivable is considered sold and derecognized from balance sheets when they are transferred beyond the reach of the Company and its creditors, the purchaser has the right to pledge or exchange the note receivables, and the Company has surrendered control over the transferred note receivable. If the Company does not surrender control, the cash received from the purchaser is account for as a secured borrowing.
F-12
As of September 30, 2024 and 2023, bank acceptance notes receivable from customers were $3,337,137 and $4,131,392, respectively. There was $5,147,192 bank acceptance notes receivable endorsed by the companies to make payments that were unmatured as of September 30, 2024 and derecognized from balance sheet.
Inventories, net
Inventories are stated at the lower of cost or net realizable value. Costs include the cost of raw materials, freight, direct labor and related production overhead. The cost of inventories is calculated using the weighted average method. Any excess of the cost over the net realizable value of each item of inventories is recognized as a provision for diminution in the value of inventories. Net realizable value is the estimated selling price in the normal course of business less any costs to complete and sell products. Allowances for obsolescence are also assessed based on expiration dates, as applicable, taking into consideration historical and expected future product sales.
Property and Equipment, net Property and equipment are stated at cost less accumulated depreciation and impairment charge. The straight-line depreciation method is used to compute depreciation over the estimated useful lives of the assets, as follows:
Items |
|
Useful life |
Property and buildings |
|
20–40 years |
Machinery and equipment |
|
3–10 years |
Automobiles |
|
3–5 years |
Office and electric equipment |
|
3–5 years |
Expenditures for maintenance and repairs, which do not materially extend the useful lives of the assets, are charged to expense as incurred. Expenditures for major renewals and betterments which substantially extend the useful life of assets are capitalized. The cost and related accumulated depreciation of assets retired or sold are removed from the respective accounts, and any gain or loss is recognized in the statements of operations in other income and expenses.
Construction in Progress
Construction in progress is comprised of costs related to the capital projects that are not completed and is not depreciated until such time as the subject asset is ready for its intended use. Construction in progress as of September 30, 2024 and 2023 represents costs of construction incurred for Chongqing’s new manufacturing facilities for heparin products.
Intangible Assets
Intangible assets consist primarily of land use rights, software and license for drug manufacturing (See Note 7). Under the PRC law, all land in the PRC is owned by the government and cannot be sold to an individual or company. The government grants individuals and companies the right to use parcels of land for specified periods of time. Land use rights are stated at cost less accumulated amortization. Intangible assets are amortized using the straight-line method with the following estimated useful lives:
Items |
|
Useful life |
Land use rights |
|
50 years |
Software |
|
10 years |
License for drug manufacturing |
|
10 years |
Leases
On October 1, 2019 the Company adopted Accounting Standards Update (“ASU”) 2016-02. For all leases that were entered into prior to the effective date of ASC 842, we elected to apply the package of practical expedients. Based on this guidance we will not reassess the following: (1) whether any expired or existing contracts are or contain leases; (2) the lease classification for any expired or existing leases; and (3) initial direct costs for any existing leases. The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, current portion of obligations under operating leases, and obligations under operating leases, non-current on the Company’s consolidated balance sheets. Finance leases are included in property and equipment, net, current portion of obligations under finance leases, and obligations under finance leases, non-current on our consolidated balance sheets.
F-13
Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date, adjusted by the deferred rent liabilities at the adoption date. As most of the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made. The Company’s terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Operating lease expense is recognized on a straight-line basis over the lease term.
We have made an accounting policy election to not include leases with an initial term of 12 months or less on the balance sheets and the short term lease expense recognized for the years presented are immaterial.
Investment in Securities
The Company entered into an investment with a iFactors SPC related to shares participating in the Golden Bridge Global Income Opportunities SP (the Fund), an exempted segregated Portfolio Company incorporated in the Cayman Islands and managed by Golden Bridge Capital Management Limited. The Fund primarily invests in bonds offered by private entities (debt securities), globally and also invests in convertible debt securities, publicly traded debt and stock, and governmental fixed income securities. The redemption of such shares for cash can be made with ninety days advance written notice (such written notice period can be extended by the investment manager), except during the lock up period which is initially 24 months and then extended to 36 months, from the initial investment date.
The Company determines the appropriate classification of its investments in debt and equity securities at the time of purchase and reevaluates such determinations at each balance sheet date. Debt securities are classified as held-to-maturity when the Company has the positive intent and ability to hold the securities to maturity. Held-to-maturity securities are recorded as either short term or long term on the Balance Sheet, based on contractual maturity date and are stated at amortized cost. Investment securities that are bought and held principally for the purpose of selling them in the near term are classified as trading securities and are reported at fair value. Investment securities not classified as trading securities or as held-to-maturity securities shall be classified as available-for-sale securities.
As of September 30, 2024 and 2023, the investment consisted of 20,000 units of the Fund. Such securities have been classified as trading securities. The private equity fund is measured at fair value with gains and losses recognized in earnings. For the years ended September 30, 2022 and 2021, as a practical expedient, the Company uses Net Asset Value (“NAV”) or its equivalent to measure the fair value of the Fund. NAV is primarily determined based on information provided by external fund administrators. As of September 30, 2023, the management had intention to redeem the investment and it is probable that the investment will be redeemed for an amount different from the NAV. Thus, the fair value of the investment was measured using discounted cash flow method. The fair value of the Fund was $13,943,019 as of September 30 2023. See Fair Value of Financial Instruments disclosure in this footnote.
As of September 20, 2024, the Company has redeemed $4,800,000 from the Fund Management, with the remaining redemption assets in the Fund amounting to $14,770,000.
The Company agrees to redeem the remaining balance of the agreed redemption assets in the form of securities. The Fund Management shall deliver 18,621,000 shares of Highest Performances Holdings (NASDAQ: HPH) to the Company. Based on the average stock price between September 16 and September 20 in 2024, which is $0.712 per share, the total transfer value amounts to $13,258,152. The fair value of the stock is $9,143,019. Due to the significant fluctuations in the stock price after September 30, 2024, the average stock price from October 1, 2024, to January 21, 2025, is selected as the fair value to adjust the carrying amount.
F-14
Long-Term Investment
Investments in entity in which the Company, its subsidiaries, the VIE and VIE’s subsidiaries can exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting. Under the equity method, the Company, its subsidiaries, the VIE and VIE’s subsidiaries initially record its investment at cost. The Company’s share of investee earnings or losses is recorded in our Consolidated Statements of Operations within Other income (expense). The Company’s interest in the net assets of the investees is included in the equity method investment on the consolidated balance sheets. The Company, its subsidiaries, the VIE and VIE’s subsidiaries evaluate the equity method investments for impairment under ASC 323. An impairment loss on the equity method investments is recognized in earnings when the decline in value is determined to be other-than-temporary. The Company, its subsidiaries, the VIE and VIE’s subsidiaries subsequently adjust the carrying amount of the investment to recognize their proportionate share of each equity investee’s net income or loss into earnings after the date of investment, the adjustment of basis difference initially recognized and the other comprehensive income allocated to the Company from the investees.
Impairment of Long-lived Assets
The Company, its subsidiaries, the VIE and VIE’s subsidiaries review long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the estimated undiscounted cash flows from the use of the asset and its eventual disposition are below the asset’s carrying value, then the asset is deemed to be impaired and written down to its fair value. There were no indicators of impairment of long-lived assets as of September 30, 2024 and September 30, 2023.
Prepayments for property and equipment
The company purchased apartments in Tianxi Center from Chengdu Shuangfa Jundi Real Estate Co., Ltd. on June 15, 2021. The property has not been delivered yet. It is expected that the property will be received in May 2025.
Transactions with Non-controlling Interests of Subsidiaries
The Company, its subsidiaries, the VIE and VIE’s subsidiaries account for a change in ownership interests in its subsidiaries that does not result in a change of control of the subsidiary under the provisions of ASC 810-10-45-23, Consolidation – Other Presentation Matters, which prescribes the accounting for changes in ownership interest that do not result in a change in control of the subsidiary, as defined by GAAP, before and after the transaction. Under this guidance, changes in a controlling shareholder’s ownership interest that do not result in a change of control, as defined by GAAP, in the subsidiary are accounted for as equity transactions. Accordingly, if the controlling shareholder retains control, no gain or loss is recognized in the statements of operations of the controlling shareholder. Similarly, the controlling shareholder will not record any additional acquisition adjustments to reflect its subsequent purchases of additional shares in the subsidiary if there is no change of control. Only a proportional and immediate transfer of carrying value between the controlling and the noncontrolling shareholders occurs based on the respective ownership percentages. For the year ended September 30, 2021, the VIE, Gansu QLS acquired 7.76% of equity interest in Chengdu QLS and its subsidiaries from its shareholders. The equity interest Gansu QLS has in Chengdu QLS increased from 71.75% as of September 30, 2020 to 79.51% as of September 30, 2021.
In the year ended September 30, 2023, the Company made 200,000 RMB (equivalent to $28,356) additional investment to acquire 0.2% ownership of Gansu QLS from third party shareholders and the Company’s ownership in VIE increased to 79.71% as of September 30, 2023.
Non-controlling Interests
Non-controlling interests are recognized to reflect the portion of their equity that is not attributable, directly or indirectly, to the Company as the controlling shareholder. For the Company’s consolidated subsidiaries, VIE and VIE’s subsidiaries, non-controlling interests represent a minority shareholder’s 48.72% ownership interest in Zhongqiao E Commerce Limited (“Zhongqiao”), as well as 0.786% ownership interest in Gansu QLS, 20.29% ownership interest in Chengdu QLS and in subsidiaries including Rugao and Chongqing.
F-15
The following table summarizes the shareholders’ equity for the non-controlling interest from each subsidiary that is not 100% owned by the Company:
|
|
As of |
||||
|
|
September 30, |
|
September 30, |
||
|
|
2024 |
|
2023 |
||
Gansu QLS |
|
$ |
1,146,121 |
|
$ |
169,574 |
Chengdu QLS and subsidiaries |
|
|
181,315 |
|
|
1,332,983 |
Zhongqiao |
|
|
22,069 |
|
|
56,711 |
Total |
|
$ |
1,349,505 |
|
$ |
1,559,268 |
Non-controlling interest in the equity of a subsidiary is reported in equity in the consolidated balance sheets. Net income and losses attributable to the non-controlling interest is reported as described above in the consolidated statements of operations and comprehensive income.
Revenue Recognition
The Company, its subsidiaries, the VIE and VIE’s subsidiaries recognize revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration which the Company expects to receive in exchange for those goods or services. To perform revenue recognition for arrangements within the scope of ASC 606, the Company, its subsidiaries, the VIE and VIE’s subsidiaries perform the following five steps:
| (i) | identification of the promised goods or services in the contract; |
| (ii) | determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; |
| (iii) | measurement of the transaction price, including the constraint on variable consideration; |
| (iv) | allocation of the transaction price to the performance obligations based on estimated selling prices; and |
| (v) | recognition of revenue when (or as) we satisfy each performance obligation. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer, and is the unit of account in ASC 606. |
The majority of the WFOE, the VIE and VIE’s subsidiaries’ contracts have one single performance obligation as the promise to transfer the individual goods is not separately identifiable from other promises in the contracts and are, therefore, not distinct. The revenue streams are recognized at a point in time when title and risk of loss passes and the customer accepts the goods, which generally occurs at delivery. The WFOE, the VIE and VIE’s subsidiaries’ products are sold with no right of return and the WFOE, the VIE and VIE’s subsidiaries do not provide other credits or sales incentives, which would be accounted for as variable consideration. Sales taxes invoiced to customers and remitted to government authorities are excluded from net sales.
The contract liabilities of the Company consist of advance payments from customers. The contract liabilities are reported in a net position on a customer-by-customer basis at the end of each reporting period. Contract liabilities were recognized when the Company receives prepayment from customers resulting from sales contracts. Contract liabilities will be recognized as revenue when the products are delivered. As of September 30, 2024 and 2023, the Company record advance from customers of $489,784 and $1,028,318, respectively, which will be recognized as revenue upon delivery of the products sold.
Refer to Note 15 for disaggregated revenue information.
F-16
Government Grants
Government grants are recognized when there is reasonable assurance that the attached conditions will be complied with. When the grant relates to an expense item, it is net against the expense and recognized in the consolidated statements of operations and comprehensive income over the period necessary to match the grant on a systematic basis to the related costs. Where the grant relates to an asset acquisition, it is recognized in the consolidated statements of operations and comprehensive income in proportion to the useful life of the related assets. Government grants received for the years ended September 30, 2024, 2023 and 2022 were $92,883, $66,177 and $137,754, respectively. As of September 30, 2024 and 2023, the deferred government grants were $106,901 and $298,691, respectively.
Selling, General and Administrative, Research and Development Expenses
Selling, general and administrative, research and development expenses primarily consist of salaries and benefits for employees, shipping expense, utilities, maintenance and repairs expenses, insurance expense, depreciation and amortization expenses, research and development expense, selling and marketing expenses, professional fees, and other operating expenses.
The Company, its subsidiaries, the VIE and VIE’s subsidiaries expense all internal research costs as incurred, which primarily comprise employee costs, internal and external costs related to execution of studies, including manufacturing costs, facility costs of the research center, and amortization, depreciation of intangible assets and property and equipment used in the research and development activities. For the years ended September 30, 2024, 2023 and 2022, total selling, general and administrative, research and development expense were as follows:
|
|
For the Years Ended |
|||||||
|
|
September 30, |
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|||
Selling expense |
|
$ |
592,839 |
|
$ |
961,679 |
|
$ |
751,428 |
General and administrative expense |
|
|
2,724,188 |
|
|
2,831,444 |
|
|
2,149,522 |
Research and development expense |
|
|
1,361,499 |
|
|
568,470 |
|
|
1,224,344 |
Total |
|
$ |
4,678,526 |
|
$ |
4,361,593 |
|
$ |
4,125,294 |
Advertising Cost
Advertising costs are expensed when incurred and are included in selling, general and administrative, research and development expense on the accompanying consolidated statements of operations. The Company incurred $92,904, $145,916 and $166,064 of advertising costs during the years ended September 30, 2024, 2023 and 2022, respectively. Advertising costs consist primarily of online marketing costs, such as advertising on social networking sites and e-mail marketing campaigns.
Income Taxes
The Company, its subsidiaries, the VIE and VIE’s subsidiaries account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, the Company, its subsidiaries, the VIE and VIE’s subsidiaries determine deferred tax assets and liabilities on the basis of the differences between the financial statement and tax bases of assets and liabilities by using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
F-17
The Company, its subsidiaries, the VIE and VIE’s subsidiaries recognize deferred tax assets to the extent that we believe that these assets are more likely than not to be realized. In making such a determination, the Company, its subsidiaries, the VIE and VIE’s subsidiaries consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If the Company, its subsidiaries, the VIE and VIE’s subsidiaries determine that they would be able to realize the deferred tax assets in the future in excess of their net recorded amount, they would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
The Company, its subsidiaries, the VIE and VIE’s subsidiaries record uncertain tax positions in accordance with ASC 740 on the basis of a two-step process in which (1) the Company, its subsidiaries, the VIE and VIE’s subsidiaries determine whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold, the Company, its subsidiaries, the VIE and VIE’s subsidiaries recognize the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority. The Company does not believe that there were any uncertain tax positions as of September 30, 2024 and 2023.
Earnings per Share
The Company computes earnings per share (“EPS”) in accordance with ASC 260, “Earnings per Share” (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted presents the dilutive effect on a per share basis of potential common shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. For the years ended September 30, 2023 and 2022, 300,000 underwriter warrants were considered in the diluted EPS calculation using treasury stock method.
On April 19, 2024, the shareholders of the Company passed resolutions to increase, re-designate and reclassify the Company’s authorized share capital to US$833,335 divided into 350,000,000 Class A ordinary shares of par value US$0.00166667 each, 100,000,000 Class B ordinary shares of par value US$0.00166667 each and 50,000,000 preferred shares of par value US$0.00166667 each. On May 29, 2024, the board of directors of the Company approved a share consolidation at a ratio of five-for-one (5:1), effective on June 21, 2024.
As a result, pursuant to Section 6.1.2 and 8.3 of the Warrants, Univest is hereby notified, and the Company hereby certifies, that the Exercise Price of the Warrant has, pursuant to the terms of the Warrant, been proportionately adjusted from $5.50 per Ordinary Shares to $27.50 per Class A Ordinary Shares, and the number of Class A Ordinary Shares purchasable under the Warrants shall been adjusted from 300,000 to 60,000, in proportion to the decrease in outstanding shares of the Company. Thereafter, the amount of authorized ordinary shares, is 100,000,000 shares, the amount of ordinary shares issued and outstanding is 7,226,480 as of September 30, 2024. There were no other diluted shares for the years ended September 30, 2024, 2023 and 2022.
The following table sets forth the computation of basic and diluted earnings (loss) per share for the years ended September 30, 2024, 2023 and 2022:
|
|
For the Years ended September 30, |
|
|||||||
|
|
2024 |
|
2023* |
|
2022 * |
|
|||
Numerator: |
|
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to ordinary shareholders |
|
$ |
(1,442,830) |
|
$ |
(7,780,620) |
|
$ |
1,076,693 |
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
Weighted-average number of ordinary shares outstanding – basic |
|
|
7,226,480 |
|
|
7,226,480 |
* |
|
7,226,480 |
* |
Weighted-average number of ordinary shares outstanding – diluted |
|
|
7,226,480 |
|
|
7,226,480 |
* |
|
7,226,480 |
* |
Earnings per share – basic |
|
$ |
(0.20) |
|
$ |
(1.08) |
|
$ |
0.15 |
|
Earnings per share – diluted |
|
$ |
(0.20) |
|
$ |
(1.08) |
|
$ |
0.15 |
|
* The shares and per share data are presented on a retroactive basis to reflect the Company’s Share Consolidation.
F-18
The impact of this retroactive adjustment to the applicable reporting periods for the financial statement line items impacted is as follows:
|
|
For the Year ended September 30,2023 |
|||||||
|
|
As Previously Reported |
|
Retroactive Adjustment |
|
As Retroacted |
|||
Numerator: |
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to ordinary shareholders |
|
$ |
(7,780,620) |
|
$ |
— |
|
$ |
(7,780,620) |
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted-average number of ordinary shares outstanding – basic |
|
|
35,750,000 |
|
|
(28,523,520) |
|
|
7,226,480 |
Weighted-average number of ordinary shares outstanding – diluted |
|
|
35,750,000 |
|
|
(28,523,520) |
|
|
7,226,480 |
Earnings per share – basic |
|
$ |
(0.22) |
|
$ |
— |
|
$ |
(1.08) |
Earnings per share – diluted |
|
$ |
(0.22) |
|
$ |
— |
|
$ |
(1.08) |
|
|
For the Year ended September 30,2022 |
|||||||
|
|
As Previously Reported |
|
Retroactive Adjustment |
|
As Retroacted |
|||
Numerator: |
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to ordinary shareholders |
|
$ |
1,076,693 |
|
$ |
— |
|
$ |
1,076,693 |
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted-average number of ordinary shares outstanding – basic |
|
|
35,750,000 |
|
|
(28,523,520) |
|
|
7,226,480 |
Weighted-average number of ordinary shares outstanding – diluted |
|
|
35,750,000 |
|
|
(28,523,520) |
|
|
7,226,480 |
Earnings per share – basic |
|
$ |
0.03 |
|
$ |
— |
|
$ |
0.15 |
Earnings per share – diluted |
|
$ |
0.03 |
|
$ |
— |
|
$ |
0.15 |
Stock Based Compensation
The Company issued shares for its independent director for the service rendered. Stock-based compensation is estimated at the grant date based on the fair value of the shares and is recognized as expense over the requisite service period of the award. The Company recognizes compensation cost on a straight-line basis over the requisite service period of the award, which is generally the award vesting term. The Company has elected to recognize forfeitures as incurred.
Foreign Currency Translation
The Company’s principal country of operations is the PRC. The financial position and results of its operations are determined using RMB, the local currency, as the functional currency. Our financial statements are reported using U.S. Dollars. The results of operations and the statement of cash flows denominated in currency other than U.S. Dollars are translated at the average rate of exchange during the reporting period. Assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the applicable rates of exchange in effect at that date. The equity denominated in the functional currency is translated at the historical rate of exchange at the time of capital contribution. Because cash flows are translated based on the average translation rate, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet. Translation adjustments arising from the use of different exchange rates from period to period are included as a separate component of accumulated other comprehensive income included in statement of changes in equity. Gains and losses from foreign currency transactions are included in the consolidated statements of operations and comprehensive income.
F-19
The value of RMB against US$ and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of RMB may materially affect the Company’s financial condition in terms of US$ reporting. The following table outlines the currency exchange rates that were used in creating the consolidated financial statements in this report:
|
|
September 30, 2024 |
|
September 30, 2023 |
|
September 30, 2022 |
Year-end spot rate |
|
US$1=RMB 7.0074 |
|
US$1=RMB 7.2960 |
|
US$1=RMB 7.1135 |
|
|
|
|
|
|
|
Average rate |
|
US$1=RMB 7.1178 |
|
US$1=RMB 7.0533 |
|
US$1=RMB 6.5532 |
Fair Value of Financial Instruments
The Company records its financial assets and liabilities in accordance with the framework for measuring fair value in accordance with U.S GAAP. This framework establishes a fair value hierarchy that prioritizes the inputs used to measure fair value:
Level 1: Quoted prices for identical instruments in active markets.
Level 2: Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets.
Level 3: Valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
For the year ended September 30, 2022, as a practical expedient, the Company uses Net Asset Value (“NAV”) or its equivalent to measure the fair value of its certain fund investment. NAV is primarily determined based on information provided by external fund administrators. The Company’s investments valued at NAV as a practical expedient are private equity funds, which represent the investment in trading securities on the balance sheet. For the year ended September 30, 2023, the Company planned to sell the investment and fair value measurement using NAV as practical expedient is not permitted. The investment is measured using discounted cash flow method and classified as Level 3 in the fair value hierarchy. The discount rate used for the valuation of trading securities was 28% as of September 30, 2023.
The Company agrees to redeem the remaining balance of the agreed redemption assets in the form of securities. The Fund Management shall deliver 18,621,000 shares of Highest Performances Holdings (NASDAQ: HPH) to the Company. Based on the average stock price between September 16 and September 20 in 2024, which is $0.712 per share, the total transfer value amounts to $13,258,152. The fair value of the stock is $9,143,019. Due to redemptions and conversions into stocks, the value of the stocks was referenced based on the share price as of September 30, 2024. Due to the significant fluctuations in the stock price after September 30, 2024, the average stock price from October 1, 2024, to January 21, 2025, is selected as the fair value to adjust the carrying amount.
Cash and cash equivalents, restricted cash, accounts receivable, bank notes receivable, short term investment, advances to suppliers, other current assets, accounts payable, and accrued expenses and other payables approximate fair value because of the short maturity of those instruments. Based on comparable open market transactions, the fair value of the bank loans, lease liabilities, bank notes payable and other liabilities, including current maturities, approximated their carrying value as of September 30, 2024 and September 30, 2023, respectively.
The Company noted no transfers between levels during any of the periods presented.
The following is a reconciliation of the beginning and ending balance of the investment in securities measured at fair value on a recurring basis for the years ended September 30, 2024 and 2023:
|
|
As of |
|
As of |
||
|
|
September 30, |
|
September 30, |
||
|
|
2024 |
|
2023 |
||
Beginning balance |
|
$ |
13,943,019 |
|
$ |
19,470,400 |
Redemption |
|
|
(4,800,000) |
|
|
— |
Change in fair value |
|
|
(819,432) |
|
|
(5,527,381) |
Ending balance |
|
$ |
8,323,587 |
|
$ |
13,943,019 |
F-20
Concentrations and Credit Risk
A majority of the Company, its subsidiaries, the VIE and VIE’s subsidiaries’ expense transactions are denominated in RMB and a significant portion of the Company and its subsidiaries, the VIE and VIE’s subsidiaries’ assets and liabilities are denominated in RMB. RMB is not freely convertible into foreign currencies. In the PRC, certain foreign exchange transactions are required by law to be transacted only by authorized financial institutions at exchange rates set by the People’s Bank of China (“PBOC”). Remittances in currencies other than RMB by the Company, its subsidiaries, the VIE and VIE’s subsidiaries in China must be processed through the PBOC or other China foreign exchange regulatory bodies which require certain supporting documentation in order to affect the remittance.
As of September 30, 2024 and 2023, $6,902,275 and $6,197,461 of the Company’s cash and cash equivalents and restricted cash were on deposit at financial institutions in the PRC which are protected under Deposit Protection Scheme in accordance with the Deposit Protection Scheme Ordinance. The maximum protection is up to RMB500,000 per depositor per Scheme member, including both principal and interest. Cash and cash equivalent of $1,058,635 and $1,001,568 were deposited at financial institutions in Hong Kong as of September 30, 2024 and 2023, which are insured by Hong Kong Deposit Board and subject to a certain limitation of HKD 500,000 (approximately $ 65,000). As of September 30, 2024 and 2023, $1,856,344 and $277,218 of the Company’s cash were on deposit at financial institutions in the U.S. which were insured by the FDIC subject to certain limitations. The Company has not experienced any losses in such accounts.
Substantially all of the Company’s sales are made to customers that are located in China. The Company has a concentration of its revenues and receivables with specific customers.
For the year ended September 30, 2024, two customers accounted for 16% and 12% of total revenue, respectively and two major vendors accounted for 12% and 10% of the total purchase, respectively. As of September 30, 2024, four major customer’s accounts receivable accounted for 38%, 25%, 16% and 14% of the total account receivable, respectively, and no vendor accounted for more than 10% of the total accounts payable outstanding.
For the year ended September 30, 2023, two customers accounted for 15% and 14% of total revenue, respectively and no vendor accounted for more than 10% of total purchase. As of September 30, 2023, four major customer’s accounts receivable accounted for 31%, 19%, 11%and 10% of the total account receivable, respectively, and no vendor accounted for more than 10% of the total accounts payable outstanding.
For the year ended September 30, 2022, two customers accounted for 11% and 11% of total revenue, respectively and one vendor accounted for 14% of total purchase. As of September 30, 2022, three major customer’s account receivables accounted for 61%, 13% and 11% of the total account receivable, respectively, and one vendor accounted for 18% of the total accounts payable outstanding.
A loss of any of these customers or suppliers could adversely affect the operating results or cash flows of the Company.
Recent Accounting Pronouncements
In January 2025, the FASB issued ASU 2025-01 Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40) The Board issued Update 2024-03 on November 4, 2024. Update 2024-03 states that the amendments are effective for public business entities for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027. Following the issuance of Update 2024-03, the Board was asked to clarify the initial effective date for entities that do not have an annual reporting period that ends on December 31 (referred to as non-calendar year-end entities). Because of how the effective date guidance was written, a non-calendar year-end entity may have concluded that it would be required to initially adopt the disclosure requirements in Update 2024-03 in an interim reporting period, rather than in an annual reporting period. The Board’s intent in the basis for conclusions of Update 2024-03 is clear that all public business entities should initially adopt the disclosure requirements in the first annual reporting period beginning after December 15, 2026, and interim reporting periods within annual reporting periods beginning after December 15, 2027. However, the Board acknowledges that there was ambiguity between the intent in the basis for conclusions in Update 2024-03 and the transition guidance that was included in the Codification when Update 2024-03 was issued In November 2024, the FASB issued ASU 2024-03, “Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses”.
F-21
The amendments in this ASU are intended to improve financial reporting by requiring that public business entities disclose additional information about specific expense categories in the notes to financial statements at interim and annual reporting periods. For interim and annual reporting periods, an entity shall disaggregate, in a tabular format disclosure in the notes to financial statements, all relevant expense captions presented on the face of the income statement in continuing operations into the purchases of inventory, employee compensation, depreciation, amortization, and depletion. This ASU is effective for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027.Early adoption is permitted. The amendments in this Update should be applied either (1) prospectively to financial statements issued for reporting periods after the effective date of this Update or (2) retrospectively to any or all prior periods presented in the financial statements We are currently evaluating the impact the adoption of ASU 2024-03 will have on its consolidated financial statements and related disclosures.
In September 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The Board is issuing the amendments in this Update to enhance the transparency and decision usefulness of income tax disclosures. Investors currently rely on the rate reconciliation table and other disclosures, including total income taxes paid, to evaluate income tax risks and opportunities. While investors find these disclosures helpful, they suggested possible enhancements to better (1) understand an entity’s exposure to potential changes in jurisdictional tax legislation and the ensuing risks and opportunities, (2) assess income tax information that affects cash flow forecasts and capital allocation decisions, and (3) identify potential opportunities to increase future cash flows. The Board decided that the amendments should be effective for public business entities for annual periods beginning after December 15, 2024.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This ASU improves reportable segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses and do not change how a public entity identifies its operating segments, aggregates those operating segments, or applies the quantitative thresholds to determine its reportable segments. The amendments in this Update are effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company does not expect that the adoption of this guidance will have a material impact on the financial position, results of operations and cash flows.
In June 2022, the FASB issued ASU 2022-03, “Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”, which clarifies that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments also clarify that an entity cannot, as a separate unit of account, recognize and measure a contractual sale restriction. This guidance also requires certain disclosures for equity securities subject to contractual sale restrictions. The new guidance is required to be applied prospectively with any adjustments from the adoption of the amendments recognized in earnings and disclosed on the date of adoption. This guidance is effective for the Company beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted. The Company does not expect that the adoption of this guidance will have a material impact on the financial position, results of operations and cash flows.
The Company does not expect the adoption will have material impact on its consolidated financial statements.
The Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the consolidated financial position, statements of operations and cash flows.
NOTE 3 – ACCOUNTS RECEIVABLE, NET
Accounts receivable consisted of the following:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Trade accounts receivable |
|
$ |
1,678,806 |
|
$ |
1,981,545 |
Less: allowances for doubtful accounts |
|
|
(135,646) |
|
|
(5,829) |
Accounts receivable, net |
|
$ |
1,543,160 |
|
$ |
1,975,716 |
F-22
The change of the allowance for doubtful accounts are as follow:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Beginning balance |
|
$ |
5,829 |
|
$ |
4,373 |
Addition |
|
|
127,568 |
|
|
1,618 |
Exchange rate difference |
|
|
2,249 |
|
|
(162) |
Ending balance |
|
$ |
135,646 |
|
$ |
5,829 |
NOTE 4 – INVENTORIES, NET
Inventories consisted of the following:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Raw materials |
|
$ |
2,327,285 |
|
$ |
2,497,298 |
Low value consumables |
|
|
— |
|
|
254,828 |
Work-in-progress |
|
|
400,253 |
|
|
237,987 |
Finished goods |
|
|
2,417,906 |
|
|
2,887,031 |
Inventory provision |
|
|
(95,756) |
|
|
(885,709) |
Total inventories |
|
$ |
5,049,688 |
|
$ |
4,991,435 |
The change of inventories provision are as follow:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Beginning balance |
|
$ |
885,709 |
|
$ |
523,465 |
Addition (reduction) |
|
|
(813,619) |
|
|
388,253 |
Exchange rate difference |
|
|
23,666 |
|
|
(26,009) |
Ending balance |
|
$ |
95,756 |
|
$ |
885,709 |
For the years ended September 30, 2024, 2023 and 2022, the inventories provision expenses were $(813,619), $388,253 and $444,894, respectively.
NOTE 5 – OTHER CURRENT ASSETS
Other current assets consisted of the following:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Prepaid expense |
|
$ |
— |
|
$ |
39,083 |
Other receivables |
|
|
192,387 |
|
|
247,481 |
Input VAT |
|
|
702,073 |
|
|
— |
Total other current assets |
|
$ |
894,460 |
|
$ |
286,564 |
F-23
NOTE 6 – PROPERTY AND EQUIPMENT, NET
Property equipment, net consisted of the following:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Property and Buildings |
|
$ |
13,518,768 |
|
$ |
12,889,450 |
Machinery and equipment |
|
|
18,774,867 |
|
|
17,833,560 |
Automobiles |
|
|
235,360 |
|
|
285,747 |
Office and electric equipment |
|
|
193,294 |
|
|
195,174 |
Subtotal |
|
|
32,722,289 |
|
|
31,203,931 |
Less: accumulated depreciation |
|
|
(24,112,010) |
|
|
(22,060,348) |
Property and equipment, net |
|
$ |
8,610,279 |
|
$ |
9,143,583 |
Depreciation expense was $1,135,383, $1,077,376 and $1,172,644 for the years ended September 30, 2024, 2023 and 2022 respectively. Certain properties and equipment have been pledged as collateral under the bank loan agreement as discussed in Note 9.
As of September, 30, 2024 and 2023, Qilian Chengdu made advance payments for property and buildings acquisition for $660,569 and $634,442, respectively, which was recorded in prepayments for property and equipment on the consolidated balance sheets.
NOTE 7 – INTANGIBLE ASSETS, NET
Intangible assets, net consisted of the following:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Land use rights |
|
$ |
4,226,520 |
|
$ |
4,059,336 |
Software |
|
|
1,118,650 |
|
|
38,836 |
License for drug manufacturing |
|
|
57,082 |
|
|
54,825 |
Total |
|
|
5,402,252 |
|
|
4,152,997 |
Less: accumulated amortization |
|
|
(862,905) |
|
|
(729,415) |
Intangible assets, net |
|
$ |
4,539,347 |
|
$ |
3,423,582 |
Amortization expense was $101,846, $65,688, and $52,028 for the years ended September 30, 2024, 2023 and 2022, respectively. The land use right was pledged for the bank loans. Refer to Note 9.
Estimated future amortization expense for intangible assets is as follows:
|
|
Amortization |
|
Year ending September 30, |
|
expense |
|
2025 |
|
|
86,333 |
2026 |
|
|
86,333 |
2027 |
|
|
85,872 |
2028 |
|
|
85,641 |
2029 |
|
|
85,641 |
Thereafter |
|
|
4,109,527 |
|
|
$ |
4,539,347 |
F-24
NOTE 8 – LONG-TERM INVESTMENT
In July 2017, Moshangfa acquired 40% ownership interest of JiuQuan Funong Biotech Co., Ltd (“Funong”) with a total investment amount of RMB3,300,000, which have been paid in the amount of RMB1,200,000 ($176,121 equivalent) in 2017, RMB1,658,750 ($253,596 equivalent) in 2018, and RMB441,250 ($64,165 equivalent) in 2019, respectively. The investment was accounted for using equity method. In December 2023, Mo Shangfa sold its 40% ownership interest of JiuQuan Funong Biotech Co., Ltd with a total amount of RMB3,300,000.
In July 2024, Qilian International acquired 25% ownership interest of Caihou Capital (Shenzhen) Group Co., Ltd (“Caihou”) with a total investment amount of RMB25,000,000, which have been paid in the amount of RMB10,000,000 ($1,402,584 equivalent) in 2024.The investment was accounted for using equity method. The remaining RMB 15 million was fully paid on October 28, 2024.
Equity method investment consisted of the following:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Equity method investment: |
|
|
|
|
|
|
Cost of equity method investment |
|
|
4,038,588 |
|
|
452,303 |
Share of results of associates |
|
|
(204,648) |
|
|
— |
Loss on disposal of Long term investment |
|
|
(101,354) |
|
|
— |
Profit from equity method investment |
|
|
160,032 |
|
|
208,527 |
Dividend Distribution received |
|
|
(57,083) |
|
|
(54,825) |
Investment disposed |
|
|
(470,931) |
|
|
— |
Exchange rate difference |
|
|
(4,818) |
|
|
— |
Total long-term investment |
|
$ |
3,359,786 |
|
$ |
606,005 |
The investment income attributable to the equity investment of $(306,002), $4,016 and $40,196 for the years ended September 30, 2024, 2023 and 2022, respectively, were included in other income (expense) on the consolidated statements of operations and comprehensive income (loss).
NOTE 9 – BANK LOANS
In June 2023, Chengdu QLS entered into loan agreement with Chengdu Agriculture and Commericial Bank for RMB 3,500,000 (approximately $ 0.5 million). The loans bear fixed interest rates of 3.9% per annum and will mature in June 2024. The credit is secured by Chengdu QLS’s land use right of approximately $637,000.
NOTE 10 – BANK NOTES PAYABLE
Bank notes payable are lines of credit extended by banks that can be endorsed and assigned to vendors as payments for purchases. The notes payable are generally payable within six months. These short-term notes payable are guaranteed for payment and payable by the bank for their full face value. In addition, the banks usually require Gansu QLS to deposit a certain amount of cash (usually in the range of 30% to 50% of the face value of the notes) at the bank as a guarantee deposit, which is classified on the balance sheet as restricted cash.
Gansu QLS had bank notes payable of Nil and $1,531,649 to China Zheshang Bank (“CZB”) as of September 30, 2023 and 2022, respectively. The notes had due date from November 2022 to March 2023. The notes outstanding as of September 30, 2022 have been fully repaid on the due date.
NOTE 11 –TAXES
(a)Corporate Income Taxes
The Company, its subsidiaries, the VIE and VIE’s subsidiaries are subject to income taxes on an entity basis on income arising in or derived from the tax jurisdiction in which each entity is domiciled.
F-25
Cayman Islands
Under the current tax laws of the Cayman Islands, the Company is not subject to tax on its income or capital gains. In addition, no Cayman Islands withholding tax will be imposed upon the payment of dividends by the Company to its shareholders.
Hong Kong
In accordance with the relevant tax laws and regulations of Hong Kong, a company registered in Hong Kong is subject to income taxes within Hong Kong at the applicable tax rate on taxable income. From year of assessment of 2018/2019 onwards, Hong Kong profit tax rates are 8.25% on assessable profits up to HK$2,000,000, and 16.5% on any part of assessable profits over HK$2,000,000. However, the Company’s HK subsidiary did not generate any assessable profits arising in or derived from Hong Kong for the fiscal years ended September 30, 2024, 2023 and 2022, and accordingly no provision for Hong Kong profits tax has been made in these periods.
China
The WFOE, the VIE and VIE’s subsidiaries are all incorporated in the PRC and are subject to PRC income tax, which is computed according to the relevant laws and regulations in the PRC. Under the Corporate Income Tax Law of PRC, current corporate income tax rate of 25% is applicable to all companies, including both domestic and foreign-invested companies. However, according to Tax Preferential Policies for the Development of the Western Region and Chengdu QLS are eligible for a favorable income tax rate of 15% for the years ended September 30, 2024, 2023 and 2022. In accordance with the implementation rules of Corporate Income Tax Law of PRC, a qualified “High and New Technology Enterprise” (“HNTE”) is eligible for a preferential tax rate of 15% with HNTE certificate, subject to a requirement that they re-apply for HNTE status every three years. Gansu QLS is eligible for a favorable income tax rate of 15% for the years ended September 30, 2024, 2023 and 2022.
F-26
On January 17, 2019, the State Taxation Administration issued the notice on the scope of small-scale and low-profit corporate income tax preferential policies of the Ministry of Finance and the State Administration of Taxation, [2019] No. 13 for small-scale and low-profit enterprises whose annual taxable income is less than RMB1,000,000 (including RMB1,000,000), approximately $154,000, for the period from January 1, 2019 to December 31, 2020, the income before tax is reduced to 25% as their taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 5%. While for the portion of annual taxable income exceeding RMB1,000,000, approximately $154,000, but not more than RMB3,000,000, approximately $465,000, the income is reduced to 50% as their taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 10%. On April 2, 2021, the State Taxation Administration further reduced the tax for small-scale and low-profit enterprises for the periods from Jan 1, 2021 to December 31, 2023 as following: for entities whose annual taxable income is less than RMB1,000,000 (including RMB1,000,000), approximately $154,000, the income before tax is reduced to 12.5% as its taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 2.5%. While for the portion of annual taxable income exceeding RMB1,000,000, approximately $154,000, but not more than RMB3,000,000, approximately $465,000, the income is reduced to 50% as their taxable income, which is further reduced to 25% starting from January 2022 and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 10%, or 5% under the further reduced rate starting from January 2022. From January 1, 2023 to December 31, 2024, for small and micro profit enterprises with an annual taxable income not exceeding RMB1,000,000, the income before tax is reduced to 25% as its taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 5%. From January 1, 2022 to December 31, 2024, for small and micro profit enterprises with an annual taxable income exceeding RMB1,000,000 but not exceeding RMB3,000,000, the income before tax is reduced to 25% as its taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 5%. The qualifications of small-scale and low-profit enterprises were examined annually by the Tax Bureau. All of the Company’s affiliated entities other than Gansu QLS met the criteria of small-scale and low-profit enterprises.
Income (loss) before income taxes is derived from the following jurisdiction:
|
|
For the Years ended |
|||||||
|
|
September 30, |
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|||
China |
|
$ |
(537,630) |
|
$ |
(1,725,034) |
|
$ |
2,936,530 |
Hong Kong |
|
|
3,694 |
|
|
— |
|
|
— |
Cayman Islands |
|
|
(1,603,206) |
|
|
(6,177,870) |
|
|
(1,375,971) |
Total |
|
$ |
(2,137,142) |
|
$ |
(7,902,904) |
|
$ |
1,560,559 |
Significant components of the income tax expense/(benefit) were as follows:
|
|
For the Years ended |
|||||||
|
|
September 30, |
|||||||
|
|
2024 |
|
2023 |
|
2022 |
|||
Current income taxes |
|
$ |
(211,402) |
|
$ |
15,622 |
|
$ |
4,464 |
Deferred income taxes |
|
|
(408,579) |
|
|
203,544 |
|
|
189,838 |
Total |
|
$ |
(619,981) |
|
$ |
219,166 |
|
$ |
194,302 |
The impact of these tax holidays decreased our taxes by $86,408, $90,876 and $171,217 for the years ended September 30, 2024, 2023 and 2022, respectively. The benefit of the tax holidays on net income per share was $0.01, $0.01* and $0.02* for the years ended September 30, 2024, 2023 and 2022, respectively. The benefit of the tax holidays on net income per share was $0.003 and $0.006 originally for the years ended September 30, 2023 and 2022, respectively.
*The shares and per share data are presented on a retroactive basis to reflect the Company’s Share Consolidation.
Deferred income taxes reflect the net effects of temporary difference between the carrying amounts of assets and liabilities for financial statement purposes and the amounts used for income tax purposes.
F-27
Temporary differences and carryforwards of the Company, its subsidiaries, the VIE and VIE’s subsidiaries that created significant deferred tax assets and liabilities are as follows:
|
|
As of |
|
As of |
||
|
|
September 30, 2024 |
|
September 30, 2023 |
||
Deferred tax assets: |
|
|
|
|
|
|
Allowance for doubtful accounts and inventories provision |
|
$ |
17,496 |
|
$ |
100,797 |
NOL Carryforwards |
|
|
392,296 |
|
|
344,468 |
Deferred government grants |
|
|
14,682 |
|
|
44,804 |
Deferred tax asset allowance |
|
|
— |
|
|
(479,291) |
Total deferred tax assets |
|
$ |
424,474 |
|
$ |
10,778 |
The Company, its subsidiaries, the VIE and VIE’s subsidiaries periodically evaluates the likelihood of the realization of deferred tax assets, and reduces the carrying amount of the deferred tax assets by a valuation allowance to the extent it believes a portion will not be realized. Based upon management’s assessment of all available evidence, the valuation allowance provided as of September 30, 2024 and 2023 were Nil and $479,291, respectively. The Company’s NOL carryforwards will begin to expire in 2028 and fully expire in 2029.
All of the tax returns of WFOE, VIE and VIE’s subsidiaries remain open for statutory examination by PRC tax authorities for five years from the date of filing. The eligibility of favorable income tax rate is also subject to review by tax authority.
The following table reconciles the statutory rates to the Company, its subsidiaries, the VIE and VIE’s subsidiaries’ effective tax rate:
|
|
For the Years ended |
|
||||
|
|
September 30, |
|
||||
|
|
2024 |
|
2023 |
|
2022 |
|
China Statutory income tax rate |
|
25.0 |
% |
25.0 |
% |
25.0 |
% |
Effect of favorable income tax rate in the PRC |
|
7.4 |
% |
(2.6) |
% |
(10.2) |
% |
Tax rate difference in jurisdictions other than PRC |
|
(18.7) |
% |
(19.5) |
% |
24.1 |
|
R&D credit |
|
(9.7) |
% |
1.8 |
% |
(19.6) |
|
Effect of NOL carryforward |
|
— |
% |
— |
% |
(20.7) |
|
Deferred tax provision |
|
26.8 |
% |
— |
% |
12.2 |
|
Deferred tax allowance |
|
— |
% |
(6.1) |
|
— |
|
Permanent difference |
|
(1.8) |
% |
(1.4) |
% |
1.7 |
% |
Effective tax rate |
|
29.0 |
% |
(2.8) |
% |
12.5 |
% |
(b)Taxes Payable
The Company, its subsidiaries, the VIE and VIE’s subsidiaries’ taxes payable consists of the following:
|
|
September 30, |
|
September 30, |
||
|
|
2024 |
|
2023 |
||
VAT tax payable |
|
$ |
295,769 |
|
$ |
69,805 |
Corporate income tax payable |
|
|
1,166 |
|
|
127,885 |
Business and other taxes payable |
|
|
18,393 |
|
|
5,808 |
Total |
|
$ |
315,328 |
|
$ |
203,498 |
NOTE 12 – RELATED PARTY TRANSACTIONS
During the normal course of business, the VIE and VIE’s subsidiaries may make sales to affiliated companies controlled by its major shareholders or subsidiaries. For the years ended September 30, 2024, 2023 and 2022, the VIE and VIE’s subsidiaries made sales to affiliated companies in the amount of $26,508, Nil, and $122,189 respectively. As of September 30, 2024 and 2023, the VIE and VIE’s subsidiaries had advance from affiliated company for $1,601,332, and Nil, respectively, which is due on demand.
F-28
NOTE 13 – LEASE
As of September 30, 2023, the VIE and VIE’s subsidiaries have one factory lease with expiration date through December 2025. For the years ended September 30, 2024, 2023 and 2022, the lease expenses were Nil, $30,275 and $63,480, respectively. Balance sheet information related to the VIE and VIE’s subsidiaries’ operating leases as of September 30, 2023 and 2022 was as follows:
We terminated the lease agreement due to risk of loss in production of heparin products, Rugao suspends production and terminates housing lease in 2024.
|
|
As of |
|
As of |
|
||
|
|
September 30, |
|
September 30, |
|
||
|
|
2024 |
|
2023 |
|
||
Operating Lease Assets: |
|
|
|
|
|
|
|
Operating Lease right of use asset |
|
$ |
— |
|
$ |
59,300 |
|
Total operating lease assets |
|
|
— |
|
|
59,300 |
|
Operating lease obligations: |
|
|
|
|
|
|
|
Current operating lease liabilities |
|
|
— |
|
|
73,560 |
|
Non-current operating lease liabilities |
|
|
— |
|
|
24,575 |
|
Total Lease liabilities |
|
$ |
— |
|
$ |
98,135 |
|
|
|
|
|
|
|
|
|
Remaining Lease Term Operating Lease |
|
|
— |
|
|
2.25 years |
|
Discount rate |
|
|
— |
% |
|
5.5 |
% |
NOTE 14 –SHAREHOLDERS’ EQUITY
Ordinary Shares
Qilian International was incorporated on February 7, 2019, with 50,000,000 ordinary shares, $0.001 par value, authorized and issued.
On October 16, 2019, the Company’s shareholders approved a reverse split of our outstanding ordinary shares at a ratio of 1-for-1.66667 shares, which resulted in 30,000,000 ordinary shares issued and outstanding. In addition, on the same day, our shareholders approved an increase of the Company’s authorized shares from 50,000,000 ordinary shares at par value of $0.001 per share to 100,000,000 ordinary shares at par value of $0.00166667 per share.
The above actions are collectively referred to as the “reserve split.” As a result of this reverse split, the maximum number of shares that the Company is authorized to issue is 100,000,000 ordinary shares, of $0.00166667 par value per share, of which 30,000,000 ordinary shares are issued and outstanding.
All share information included in the consolidated financial statements and notes thereto have been retroactively adjusted as if the stock reserve split occurred on the first day of the first period presented.
On January 14, 2021, the Company closed its initial public offering (“IPO”) of 5,000,000 ordinary shares, par value $0.00166667 per share, priced at $5.00 per share. The Company completed the IPO pursuant to its registration statement on Form F-1 (File No. 333-234460), originally filed with the Securities and Exchange Commission (the “SEC”) on November 4, 2019 (as amended, the “Registration Statement”). The Registration Statement was declared effective by the SEC on December 30, 2020. On January 15, 2021, the underwriter exercised its over-allotment option to purchase additional 750,000 Ordinary Shares at the price of $5 per share. Total net proceeds the Company received from the IPO were $25,728,401.50. The Ordinary Shares were previously approved for listing on The Nasdaq Global Market and commenced trading under the ticker symbol “QLI” on January 12, 2021.
F-29
On April 19, 2024, the company’s shareholders held a general meeting and passed a resolution on share consolidation. The consolidation of the Company’s authorized share capital, at a ratio of five-for-one on June 21, 2024.Our authorized share capital is US$41,916,750.50, divided into 5,000,000,000 Class A ordinary shares of par value of US$0.00833335 each, 20,000,000 Class B ordinary shares of par value of US$0.00833335 each, and 10,000,000 preferred shares of par value of US$0.00833335 each. All of our issued and outstanding Ordinary Shares are fully paid and non-assessable. Certificates representing the Ordinary Shares are issued in registered form. The related number of shares, shares authorized, shares issued and outstanding and earnings per share presented on the Company’s consolidated financial statements were retroactively adjusted to reflect the share consolidation.
|
|
For the Year ended September 30,2023 |
|||||||
|
|
As Previously Reported |
|
Retroactive Adjustment |
|
As Retroacted |
|||
Par value |
|
$ |
0.00166667 |
|
$ |
— |
|
$ |
0.00833335 |
Shares authorized |
|
|
100,000,000 |
|
|
(80,000,000) |
|
|
20,000,000 |
Shares issued and outstanding |
|
$ |
35,750,000 |
|
$ |
(28,523,520) |
|
$ |
7,226,480 |
|
|
For the Year ended September 30,2022 |
|||||||
|
|
As Previously Reported |
|
Retroactive Adjustment |
|
As Retroacted |
|||
Par value |
|
$ |
0.00166667 |
|
$ |
— |
|
$ |
0.00833335 |
Shares authorized |
|
|
100,000,000 |
|
|
(80,000,000) |
|
|
20,000,000 |
Shares issued and outstanding |
|
$ |
35,750,000 |
|
$ |
(28,523,520) |
|
$ |
7,226,480 |
Stock Based Compensation
As of September 30, 2022, the Company was obligated to issue shares with $20,000 to its former independent directors. The expense Hwas recorded as selling, general and administrative, research and development expense.
Underwriter Warrants
In connection with the Company’s IPO, the Company also agreed to issue to the underwriters and to register herein warrants to purchase up to a total of 300,000 ordinary shares of the Company (equal to 6% of the total number of Ordinary Shares sold in the IPO).
These warrants have warrant term of five years, with an exercise price of $5.50 per share (equal to 110% of the Company’s IPO offering price of $5.00 per share).
The warrants are exercisable at any time, and from time to time, in whole or in part, commencing July 10, 2021 and expiring on January 10, 2026. Management determined that these warrants meet the requirements for equity classification under ASC 815-40 because they are indexed to its own stock. As of September 30 2023, 300,000 underwriter warrants were issued and outstanding (none of the warrants has been exercised as of the date).
On April 19, 2024, the shareholders of the Company passed resolutions to increase, re-designate and reclassify the Company’s authorized share capital to US$833,335 divided into 350,000,000 Class A ordinary shares of par value US$0.00166667 each, 100,000,000 Class B ordinary shares of par value US$0.00166667 each and 50,000,000 preferred shares of par value US$0.00166667 each. On May 29, 2024, the board of directors of the Company approved a share consolidation at a ratio of five-for-one (5:1), effective on June 21, 2024.
As a result, pursuant to Section 6.1.2 and 8.3 of the Warrants, Univest is hereby notified, and the Company hereby certifies, that the Exercise Price of the Warrant has, pursuant to the terms of the Warrant, been proportionately adjusted from $5.50 per Ordinary Shares to $27.50 per Class A Ordinary Shares, and the number of Class A Ordinary Shares purchasable under the Warrants shall been adjusted from 300,000 to 60,000, in proportion to the decrease in outstanding shares of the Company.
F-30
Statutory Reserve
WFOE, VIE and VIE’s subsidiaries are required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations to the voluntary surplus reserve are made at the discretion of the Board of Directors. As of September 30, 2024 and September 30, 2023, the balance of statutory reserve was $3,266,081 and $3,162,333, respectively.
NOTE 15 – SEGMENT REPORTING
ASC 280, “Segment Reporting”, establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organizational structure as well as information about geographical areas, business segments and major customers in financial statements for details on the Company’s business segments. The Company uses the “management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. Management, including the chief operating decision maker, reviews operation results by the revenue of different products. Based on management’s assessment, the Company has determined that it has three operating segments as defined by ASC 280.
The Company, its subsidiaries, the VIE and VIE’s subsidiaries mainly manufactures and distributes active pharmaceutical ingredients and TCMD products as well as other by-products in China. Currently no revenue is derived from international markets. The following table presents segment information for years ended September 30, 2024, 2023 and 2022, respectively:
|
|
For the Years ended September 30, 2024 |
||||||||||
|
|
Oxytetracycline |
|
|
|
|
|
|
|
|
|
|
|
|
& Licorice |
|
|
|
|
Heparin |
|
|
|
||
|
|
products and |
|
|
|
|
products and |
|
|
|
||
|
|
TCMD |
|
Fertilizer |
|
Sausage casing |
|
Total |
||||
Revenue |
|
$ |
21,961,282 |
|
$ |
905,910 |
|
$ |
2,230,759 |
|
$ |
25,097,951 |
Cost of revenue |
|
|
18,324,619 |
|
|
553,329 |
|
|
2,105,248 |
|
|
20,983,196 |
Gross profit |
|
$ |
3,636,663 |
|
$ |
352,581 |
|
$ |
125,511 |
|
$ |
4,114,755 |
Depreciation and amortization |
|
$ |
1,023,783 |
|
$ |
46,684 |
|
$ |
166,763 |
|
$ |
1,237,230 |
Capital expenditures |
|
$ |
3,812,386 |
|
$ |
34,443 |
|
$ |
84,642 |
|
$ |
3,931,471 |
|
|
For the Years ended September 30, 2023 |
||||||||||
|
|
Oxytetracycline |
|
|
|
|
|
|
|
|
||
|
|
& Licorice |
|
|
|
|
Heparin |
|
|
|
||
|
|
products and |
|
|
|
|
products and |
|
|
|
||
|
|
TCMD |
|
Fertilizer |
|
Sausage casing |
|
Total |
||||
Revenue |
|
$ |
29,152,228 |
|
$ |
2,000,452 |
|
$ |
15,318,798 |
|
$ |
46,471,478 |
Cost of revenue |
|
|
27,392,224 |
|
|
1,060,302 |
|
|
16,267,458 |
|
|
44,719,984 |
Gross profit |
|
$ |
1,760,004 |
|
$ |
940,150 |
|
$ |
(948,660) |
|
$ |
1,751,494 |
Depreciation and amortization |
|
$ |
886,360 |
|
$ |
44,943 |
|
$ |
211,761 |
|
$ |
1,143,064 |
Capital expenditures |
|
$ |
8,171 |
|
$ |
30,653 |
|
$ |
3,675,265 |
|
$ |
3,714,089 |
|
|
For the Years ended September 30, 2022 |
||||||||||
|
|
Oxytetracycline |
|
|
|
|
|
|
|
|
|
|
|
|
& Licorice |
|
|
|
|
Heparin |
|
|
|
||
|
|
products and |
|
|
|
|
products and |
|
|
|
||
|
|
TCMD |
|
Fertilizer |
|
Sausage casing |
|
Total |
||||
Revenue |
|
$ |
40,305,988 |
|
$ |
1,088,570 |
|
$ |
23,460,467 |
|
$ |
64,855,025 |
Cost of revenue |
|
|
36,210,950 |
|
|
760,030 |
|
|
21,656,748 |
|
|
58,627,728 |
Gross profit |
|
$ |
4,095,038 |
|
$ |
328,540 |
|
$ |
1,803,719 |
|
$ |
6,227,297 |
Depreciation and amortization |
|
$ |
963,457 |
|
$ |
48,804 |
|
$ |
212,412 |
|
$ |
1,224,673 |
Capital expenditures |
|
$ |
1,908,881 |
|
$ |
91,029 |
|
$ |
1,259,042 |
|
$ |
3,258,952 |
F-31
|
|
September 30, |
|
September 30, |
||
|
|
2024 |
|
2023 |
||
Total Assets |
|
|
|
|
|
|
Oxytetracycline & Licorice products and TCMD |
|
$ |
40,928,941 |
|
$ |
38,382,322 |
Fertilizer |
|
$ |
3,197,285 |
|
$ |
3,291,960 |
Heparin products and Sausage casing |
|
$ |
8,877,502 |
|
$ |
9,583,712 |
Total |
|
$ |
53,003,728 |
|
$ |
51,257,994 |
NOTE 16 – CAPITAL COMMITMENTS
On July 5, 2021, The Company entered into an investment agreement with Chongqing Jintong Industrial Construction Investment Co., Ltd (“Chongqing Jintong”). The Company agreed to invest for the construction of a factory for manufacturing pig by-products in Chongqing Tongnan High Tech Industrial Zone. There are still $1.9 million (RMB 14 million) and $3.2 million (RMB 22.6 million) unpaid as of September 30, 2024 and 2023, respectively. It is expected to be completed in 2025.
NOTE 17 – SUBSEQUENT EVENTS
The Company agrees to redeem the remaining balance of the agreed redemption assets in the form of securities. The Fund Management shall deliver 18,621,000 shares of Highest Performances Holdings (NASDAQ: HPH) to the Company. Based on the average stock price between September 16 and September 20 in 2024, which is $0.712 per share, the total transfer value amounts to $13,258,152. The company anticipates that it can receive the stocks on October 9, 2024.
On November 1, 2024, BGM Group Ltd (the “Company”), entered into share subscription agreements (the “Share Subscription Agreements”) separately with each of Ahanzhai Development Co., Ltd, a British Virgin Islands company (“Ahanzhai Development”), and LX Management Company Limited, a Hong Kong company (“LX Management”). Pursuant to the Share Subscription Agreements, the Company agreed to issue to Ahanzhai Development and LX Management 10,200,000 and 9,800,000 Class B ordinary shares of par value of US$0.00833335 each of the Company (the “Subscription Shares”, each “a Subscription Share”), respectively. The purchase price per Subscription Share is US$0.05 and Ahanzhai Development and LX Management agreed to pay to the Company a total consideration of US$510,000 and US$490,000, respectively. The Subscription Shares were distributed to LX Management Company Limited and Ahanzhai Development Co., Ltd on November 27,2024 and December 16,2024, respectively. A total amount of US$1 million proceeds was received by the Company on December 19, 2024.
On November 27, 2024, BGM Group Ltd (the “Company”), entered into a transaction agreement (the “Transaction Agreement”) with CISG Holdings Ltd, a company incorporated under the laws of the British Virgin Islands and wholly owned by AIX Inc. (NASDAQ: AIFU) (the “Seller”), Patriton Limited, a company incorporated under the laws of British Virgin Islands (the “Target Company”), GM Management Company Limited, a company incorporated under the laws of Hong Kong, DuXiaoBao Intelligent Technology (Shenzhen) Co., Ltd., RONS Intelligent Technology (Beijing) Co., Ltd. (“RONS Intelligent”), Shenzhen Xinbao Investment Management Co., Ltd. (“Shenzhen Xinbao”), Fanhua RONS Insurance Sales & Service Co., Ltd. (“RONS Sales”) and Shenzhen Baowang E-commerce Co., Ltd. (“Shenzhen Baowang”), all of which are companies with limited liability incorporated under the laws of the People’s Rublic of China.
Pursuant to the Transaction Agreement, BGM Group Ltd agreed to purchase from the Seller, 100% of the equity interest of the Target Company, for a consideration of 69,995,661 Class A ordinary shares with a par value of US$0.00833335 per share of the Company (the “Consideration Shares”), at a purchase price of US$2.0 per share of the Consideration Shares. Under the Transaction Agreement, the Seller undertook to conduct a series of restructuring and reorganization arrangements (the “Reorganization”) and upon the completion of such Reorganization and immediately prior to the closing, each of RONS Intelligent, Shenzhen Xinbao, RONS Sales and Shenzhen Baowang will become a wholly owned subsidiary of the Target Company.
The issuance of 69,995,661 Class A ordinary shares was completed on December 27, 2024 and the transaction has been completed.
The Company’s management reviewed all material events that have occurred after the balance sheet date through January 27, 2025 on which these financial statements were available to be issued. Based upon this review, the Company did not identify any subsequent events except disclosed in above that would have required adjustment or disclosure in the consolidated financial statements.
F-32
Exhibit 8.1
BGM Group Ltd
Subsidiaries of the Registrant
Name |
|
Jurisdiction of Incorporation or Organization |
Qilian International (Hong Kong) Holdings Limited |
|
Hong Kong |
|
|
|
Qilian International Trading (Chengdu) Co., Ltd. |
|
People’s Republic of China |
|
|
|
Qilian Shan International Trade (Hainan) Co., Ltd. |
|
People’s Republic of China |
VIE and its subsidiaries
Name |
|
Jurisdiction of Incorporation or Organization |
Gansu Qilianshan Pharmaceutical Co., Ltd. |
|
People’s Republic of China |
|
|
|
Chengdu Qilianshan Biotechnology Co., Ltd. |
|
People’s Republic of China |
|
|
|
Jiuquan Ahan Biotechnology Co., Ltd. |
|
People’s Republic of China |
|
|
|
Moshangfa (Gansu) Fertilizer Industry Co., Ltd. |
|
People’s Republic of China |
|
|
|
Tibet Cangmen Trading Co., Ltd. |
|
People’s Republic of China |
|
|
|
Zhongqiao Youguan (Chengdu) E-Commerce Service Co., Ltd. |
|
People’s Republic of China |
|
|
|
Rugao Tianlu Animal Products Co., Ltd. |
|
People’s Republic of China |
|
|
|
Chongqing Chengfu Biological Technology Co., Ltd. |
|
People’s Republic of China |
|
|
|
BGM (Hubei) Health Bioindustry Co., Ltd. |
|
People’s Republic of China |
Exhibit 11.2
BGM GROUP LTD
POLICY GOVERNING MATERIAL, NON-PUBLIC
INFORMATION AND PREVENTION OF INSIDER TRADING
I.OVERVIEW
This Statement of Policy Governing Material, Non-Public Information and the Prevention of Insider Trading (this “Statement”) of the Company consists of three sections: Section I provides an overview; Section II sets forth the Company’s policies prohibiting insider trading; and Section III explains the scope of insider trading.
The ordinary shares of BGM Group Ltd (the “Company”) are currently trading on the Nasdaq Stock Market LLC (the “Nasdaq”). “Insider trading” occurs when you purchase or sell securities while in possession of inside information relating to such securities. As explained in Section III below, “inside information” is information which is considered to be both “material” and “non-public.” Preventing insider trading is necessary to comply with the United States securities law and to preserve the reputation and integrity of the Company as well as that of all persons affiliated with it.
The Company considers strict compliance with the policies (the “Policy”) as set forth in this statement to be a matter of utmost importance. Violation of this Policy could cause extreme embarrassment and result in possible legal liability to you and the Company. Knowing or willful violations of this Statement or its spirit will be grounds for immediate dismissal from the Company. Violation of the Policy might expose the violator to severe criminal penalties and civil liabilities. The monetary damages flowing from a violation could be three times the profit realized by the violator, as well as the attorney’s fees of the persons being harmed.
This Statement applies to all the officers, directors, employees and consultants of the Company and its subsidiaries or any consolidated entities or any other person or entity (a) over which an individual mentioned above exercises influence upon or exert control of its investment decisions; or (b) which effects a transaction in the Company’s securities, which securities are in fact beneficially owned by any of the individuals mentioned above (“Insider(s)”). Every Insider must review this Statement, and execute and return the Certificate of Compliance attached hereto to the Compliance Officer within seven (7) days after you receive this Statement.
Questions regarding the Statement should be directed to the Compliance Officer.
II.POLICIES PROHIBITING INSIDER TRADING
For purposes of this Statement, while the terms “purchase” and “sell” of securities exclude the acceptance of options granted by the Company thereof and the exercise of options that does not involve the sale of securities, the cashless exercise of options does involve the sale of securities and therefore is subject to the policies as set forth below.
A. |
No Trading with Material Insider Information – no Insider shall purchase or sell any securities of the Company or enter into a binding security trading plan in compliance with Rule 10b5-1 under the U.S. Securities Exchange Act of 1934, as amended and pursuant to the guidelines included in Exhibit A (Rule 10b5-1 Trading Plan Guidelines) (a “Trading Plan”) while in possession of material, non-public information relating to the Company, its ordinary shares or other securities (the “Material Insider Information”) or during certain periods. |
If you possess Material Insider Information, you must wait for the later of (i) forty-eight (48) hours after public disclosure of the Material Insider Information by the Company; or (ii) one full Trading Day on the Nasdaq following such public disclosure before trading the Company’s ordinary shares or other securities. The term “Trading Day” is defined as a day on which the Nasdaq is open for trading. Nasdaq’s regular trading hours are from 9:30 a.m. to 4:00 p.m., New York City time, Monday through Friday.
In addition, no Insider shall purchase or sell any securities of the Company or enter into a Trading Plan, regardless of whether such Insider possesses any Material Insider Information, (1) during any period commencing on the 1st day of each fiscal quarter and ending at the close of trading on the second Trading Day following the date of the Company’s public disclosure of its financial results for that fiscal quarter; or (2) without the prior clearance by the Compliance Officer, during any period designated as a “limited trading period.” The Compliance Officer may declare limited trading periods at times that he deems appropriate, and need not provide any reason for making a declaration.
Furthermore, beginning on the 1st day of each fiscal year, no Insider shall purchase or sell any security of the Company or enter into a Trading Plan until the close of trading on the second Trading Day following the date of the Company’s public disclosure of its financial results for the fiscal year ended on December 31 of the prior year.
Please see Section III below for an explanation of the Material Insider Information.
B. |
No Trading Outside of the Trading Window for Insiders – assuming none of the “no trading” restrictions set forth in Section II.A above applies, insiders may only purchase or sell any securities of the Company or enter into a Trading Plan during the “Trading Window.” |
Generally, there will be four Trading Windows per year, each commencing with the close of trading on the second Trading Day following the date upon which the Company’s financial results for the prior fiscal quarter is released to the public and closing on the last Trading Day of each fiscal quarter.
Furthermore, all transactions in the Company’s securities (including without limitation, acquisitions and dispositions of the ordinary shares and the sale of ordinary shares issued upon exercise of stock options and the execution of a Trading Plan, but excluding the acceptance of options granted by the Company and the exercise of options that does not involve the sale of securities) by officers, directors and key employees designated by the Company from time to time must be pre-approved by the Compliance Officer.
If the Company’s public disclosure of its financial results for a fiscal quarter or fiscal year is released on a Trading Day more than four hours before the Nasdaq closes, then such date of disclosure shall be considered the first Trading Day following such public disclosure.
Please note that trading in Company’s securities during the Trading Window is not a “safe harbor,” and all Insiders should strictly comply with all other policies set forth in this Statement. When in doubt, please do not trade and check with the Compliance Officer first.
C.No Tipping
No Insider shall directly or indirectly disclose any Material Insider Information to anyone who trades in securities (i.e. “tipping”).
D.Confidentiality
No Insider shall communicate any Material Insider Information to anyone outside the Company under any circumstances unless approved by the Compliance Officer in advance, or to anyone within the Company other than on a need-to-know basis.
E.No Comment
No Insider shall discuss any internal matters or developments of the Company with anyone outside of the Company, except as required in the performance of regular corporate duties. Unless you are expressly authorized to the contrary, if you receive any inquiries about the Company or its securities by the financial press, investment analysts or others, or any requests for comments or interviews, you should decline to comment and direct the inquiry or request to the Compliance Officer.
F.Corrective Action
If any potentially Material Insider Information is inadvertently disclosed, any Insider should notify the Compliance Officer immediately so that the Company can determine whether or not corrective actions, such as general disclosure to the public, is warranted.
III.EXPLANATION OF INSIDER TRADING
As noted above, “insider trading” refers to the purchase or sale of securities while in possession of “material” and “non-public” information relating to such securities. “Securities” include not only stocks, bonds, notes and debentures, but also options, warrants and similar instruments. “Purchase” and “sale” are defined broadly under the federal securities law. “Purchase” includes not only the actual purchase of securities, but any contract to purchase or otherwise acquire securities. “Sale” includes not only the actual sale of securities, but any contract to sell or otherwise dispose of securities. These definitions extend to a broad range of transactions including conventional cash-for-stock transactions, the grant and exercise of stock options and acquisitions and exercises of warrants or puts, calls or other options related to the securities. It is generally understood that insider trading includes the following:
| ● | Trading by Insiders while in possession of material, non-public information; |
| ● | Trading by persons other than Insiders while in possession of material, non-public information where the |
information either was given in breach of an Insider’s fiduciary duty to keep it confidential or was misappropriated; or
| ● | Communicating or tipping material, non-public information to others, including recommending the purchase or sale of the securities while in possession of such information. |
As noted above, for the purposes of this Statement, the terms “purchase” and “sell” of securities exclude the acceptance of options granted by the Company thereof and the exercise of options that does not involve the sale of securities. Among other things, the cashless exercise of options does involve the sale of securities and therefore is subject to the policies as set forth in this Statement.
A. |
What Facts are Material? |
The materiality of a fact depends upon the circumstances. A fact is considered to be “material” if it could reasonably be expected to affect the decision of a reasonable investor to buy, sell or hold the Company’s securities or where the fact is likely to have a significant effect on the market price of the Company’s securities. Material Insider Information can be positive or negative and can relate to virtually any aspect of a company’s business or to any type of securities, debt or equity.
Examples of Material Insider Information including, but are not limited to information concerning:
| ● | dividends; |
| ● | corporate earnings or earnings forecasts; |
| ● | changes in financial condition or asset value; |
| ● | negotiations for the mergers or acquisitions or dispositions of significant subsidiaries or assets; |
| ● | significant new contracts or the loss of a significant contract; |
| ● | significant new products or services; |
| ● | significant marketing plans or changes in such plans; |
| ● | capital investment plans or changes in such plans; |
| ● | material litigations, administrative actions or governmental investigations or inquiries about the Company or any of its affiliated companies, officers or directors; |
| ● | significant borrowings or financings; |
| ● | defaults on borrowings; |
| ● | new equity or debt offerings; |
| ● | significant personnel changes; |
| ● | changes in accounting methods and write-offs; and |
| ● | any substantial change in industry circumstances or competitive conditions which could significantly affect the Company’s earnings or prospects for expansion. |
A good general rule of thumb: when in doubt, do not trade. One convenient rule of thumb in making this determination is to ask yourself, “Would the person on the other side of this transaction still want to complete the trade at this price if he or she knew what I know about the Company?” If the answer is “no,” you probably possess material, non-public information.
B.What is Non-public?
Information is “non-public” if it has not been disclosed in a manner that allows it to be widely disseminated. In order for information to be considered public, it must be widely disseminated in a manner making it generally available to investors and confirmed by a reasonably reliable source. Wide dissemination generally occurs through a press release or in the Company’s filing with the United States Security and Exchange Commission (the “SEC”), or through such media as Dow Jones, Reuters Economic Services, The Wall Street Journal, Bloomberg, Associated Press, or United Press International. Reasonable confirmation generally includes confirmation by officers, directors and key employees who have been authorized by the Company to speak on its behalf. The circulation of rumors, even if accurate and reported in the media, does not constitute effective public dissemination.
In addition, even after a public announcement, a reasonable period of time must lapse in order for the market to react to the information. Generally, one should allow approximately forty eight (48) hours following publication as a reasonable waiting period before such information is deemed to be public.
C.Who is an Insider?
Insiders include all officers, directors, employees, consultants and advisors (e.g. accountants, attorneys, investment bankers and consultants) of the Company and its subsidiaries or consolidated entities or any other person or entity (a) over which an individual mentioned above exercises influence or exert control of its investment decisions; or (b) which effects a transaction in the Company’s securities, which securities are in fact beneficially owned by any of the individuals mentioned above. Insiders have independent fiduciary duties to their company and its shareholders not to trade on material non-public information relating to the Company’s securities. In addition, family members and friends of Insiders as well as professional advisors of the Company (such as accountants, attorneys, investment bankers and consultants) who receive material, non-public information about the Company may also fall under the definition of Insiders of the Company.
It should be noted that trading by members of an Insider’s family members can be the responsibility of such Insider under certain circumstances and could give rise to legal and Company- imposed sanctions.
D.Trading by Persons Other than Insiders
Insiders are also prohibited from disclosing material non-public information, or making a recommendation or expressing an opinion regarding the Company’s securities based on such information, to others who might use the information to trade in the Company’s securities. Both the Insider who communicated the material non-public information and the person who receives and uses such information (the “Tippee”) may be liable under the United States securities laws.
Persons other than Insiders also can be liable for insider trading, including Tippees who trade on material, non-public information tipped to them or individuals who trade on material, non-public information which has been misappropriated. Tippees inherit an Insider’s duties and are liable for trading on material, non-public information illegally tipped to them by an Insider. Similarly, just as Insiders are liable for the insider trading of their Tippees, so are Tippees who pass the information along to others who trade. In other words, a Tippee’s liability for insider trading is no different from that of an Insider. Tippees can obtain material, non-public information by receiving overt tips from others or through, among other things, conversations at social, business, or other gatherings.
E.Penalties for Engaging in Insider Trading
Penalties for trading on or tipping material, non-public information can extend significantly beyond any profits made or losses avoided, both for individuals engaging in such unlawful conduct and their employers. The SEC and the United States Department of Justice have made the civil and criminal prosecution of insider trading violations a top priority. Enforcement remedies available to the government or private plaintiffs under the federal securities laws including but not limited to:
| ● | SEC administrative sanctions; |
| ● | securities industry self-regulatory organization sanctions; |
| ● | civil injunctions; |
| ● | damage awards to private plaintiffs; |
| ● | disgorgement of all profits; |
| ● | civil fines for the violator of up to three times the amount of profit gained or loss avoided; |
| ● | civil fines for the employer or other controlling person of a violator (i.e., where the violator is an employee or other controlled person) of up to the greater of US$1,000,000 or three times the amount of profit gained or loss avoided by the violator; |
| ● | criminal fines for individual violators of up to US$1,000,000 (US$2,500,000 for an entity); and |
| ● | jail sentences of up to 10 years. |
In addition, insider trading could result in serious sanctions by the Company, including immediate dismissal. Insider trading violations are not limited to violations of the federal securities laws, other federal and state civil or criminal laws, such as the laws prohibiting mail and wire fraud and the United States Racketeer Influenced and Corrupt Organizations Act (RICO), may also be violated upon the occurrence of insider trading.
Exhibit A
Rule 10b5-1 Trading Plan Guidelines
(1) |
The following guidelines apply for any Trading Plan relating to the securities of the Company. All Trading Plans entered into by any Insider (as defined below) and any amendment, suspension or termination must comply with Rule 10b5-1 of the Exchange Act, the Statement and must meet the following conditions. Capitalized terms not defined herein shall have the meanings given to them under the Statement. |
Overview of 10b5-1 Plans
(2) |
Under Rule 10b5-1, an insider who regularly possesses material nonpublic information (“MNPI”) but who nonetheless wish to buy or sell the issuer’s securities may establish an affirmative defense to an illegal insider trading charge by adopting a written plan to buy or sell at a time when they are not in possession of MNPI, i.e., a Trading Plan. A Trading Plan typically takes the form of a contract between the insider and his or her broker. |
Participants
(3) |
Company directors, officers and employees (each, an “Insider,” and collectively, “Insiders”) are eligible to adopt a Trading Plan. |
Plan and Approval
(4) |
The Trading Plan must be in writing and signed by the Insider, and the Insider must provide a copy to the Compliance Officer. The Company will keep a copy of each Trading Plan in its files. The form of each Trading Plan and any subsequent amendment must be consistent with these guidelines. Each Trading Plan must be approved in writing by the Compliance Officer prior to the adoption, amendment, suspension or termination of such plan. A Trading Plan must not permit an Insider to exercise any subsequent influence over how, when or whether to effect purchases or sales. Sales under a Trading Plan must be via a selected broker. The Insider must act in good faith with respect to a Trading Plan when the plan is adopted and for the duration of the Plan and must not enter into a Trading Plan as part of a plan or scheme to evade the prohibitions of Rule 10b-5. In addition, each Trading Plan must include a representation by the Insider certifying that (a) such person is not in possession of MNPI about the Company or its securities, and (b) the Trading Plan is being adopted in good faith and not as part of a plan to evade the prohibitions of Rule 10b-5. |
Timing and Term of Plan; Cooling-Off Period
(5) |
Each Trading Plan must be adopted (a) during an open Trading Window under the Statement, and (b) when the Insider does not otherwise possess MNPI about the Company. Each Trading Plan must provide for delayed effectiveness after adoption or amendment (a “Cooling-Off Period”). For Insiders who are directors or officers, each Trading Plan must specify that trades may not execute under the Trading Plan until the later of (a) 90 days after the date of adoption or amendment of the Trading Plan; and (b) two (2) business days following the Company’s filing of a quarterly or annual report covering the financial reporting period in which the Trading Plan was adopted or amended, but in no event later than 120 days after the date of adoption or amendment of the Trading Plan. For all other Insiders, each Trading Plan must specify that trades may not execute under the Trading Plan for a period of at least 30 days after the date of adoption or amendment of the Trading Plan. |
Plan Specifications
(6) |
A Trading Plan must be entered into at a time when the Insider has no MNPI about the issuer or its securities (even if no trades will occur until after the release of MNPI). The plan must: (a) specify the amount, price (which may include a limit price) and specific dates of purchases or sales; (b) include a formula or similar method for determining amount, price and date; or (c) give the broker the exclusive right to determine whether, how and when to make purchases and sales, as long as the broker does so without being aware of MNPI at the time the trades are made. |
(7) |
Under the first two alternatives, the Trading Plan cannot give the broker any discretion as to trade dates. As a result, a plan that requests the broker to sell 1,000 shares per week would have to meet the requirements under the third alternative. On the other hand, under the second alternative, the date may be specified by indicating that trades should be made on any date on which the limit price is hit. The affirmative defense is only available if the trade is in fact made pursuant to the preset terms of the Trading Plan (unless the terms are revised at a time when the insider is not aware of any MNPI and could therefore enter into a new plan). Trades are deemed not to have been made pursuant to the plan if the Insider later enters into or alters a corresponding or hedging transaction or position with respect to the securities covered by the plan (although hedging transactions could be part of the plan itself). |
Amendment, Suspension and Termination
(8) |
Amendments, suspensions, and terminations of Trading Plans must be approved in advance in writing by the Compliance |
Officer. In addition, an Insider may voluntarily amend a Trading Plan only (a) during an open Trading Window under the Statement and (b) when such Insider does not otherwise possess MNPI. Insiders may make amendments to a Trading Plan without triggering a Cooling-Off Period so long as the amendment does not change the pricing provisions of the Trading Plan, the amount of securities covered under the Trading Plan or the timing of trades under the Trading Plan, or where a broker executing trades on behalf of the Insiders is substituted by a different broker (so long as the purchase or sales instructions remain the same).
Mandatory Suspension
(9) |
Each Trading Plan must provide for suspension of trades under such plan if legal, regulatory or contractual restrictions are imposed on the Insiders, or if these guidelines are amended, or other events occur, that would prohibit sales under such Trading Plan. |
Sales to Cover
(10) |
An Insider may have only one Trading Plan in effect at any time, except that a written, irrevocable election (an “Election”) by such Insider to sell a portion of the securities of the Company as necessary to satisfy statutory tax withholding obligations arising solely from the vesting of compensatory awards (not including options) (“Sales to Cover”) is permitted even if not included in the directions in the Insider’s Trading Plan, provided that (a) the Election is made during an open Trading Window under the Statement, (b) at the time of the Election, the Insider is not aware of any MNPI, (c) the Sales to Cover are made in good faith and not as part of a plan or scheme to evade the prohibitions of Rule 10b-5, (d) the Insider does not have, and will not attempt to exercise, authority, influence or control over any such Sales to Cover, and (e) the Election contains appropriate representations as to clauses (b)-(d). |
No Overlapping Plans
(11) |
An Insider may adopt a new Trading Plan to replace an existing Trading Plan before the scheduled termination date of such existing Trading Plan, so long as the first scheduled trade under the new Trading Plan does not occur until after all trades under the existing Trading Plan are completed or expire without execution (subject to any Cooling-Off Periods). |
(12) |
However, where the first trade under a later-commencing plan is scheduled during what would have been the Cooling-off Period for that plan assuming the termination date of the earlier-commencing plan were deemed to be the date of adoption of the later-commencing plan, then Rule 10b5-1 would not be available for the later-commencing plan. For example, an Insider who is not an officer or director has in place an existing Trading Plan with a scheduled date for the latest authorized trade of May 31, 2023. On May 1, 2023, that Insider adopts a later-commencing plan, intended to qualify for the affirmative defense under Rule 10b5-1, with a scheduled date for the first authorized trade of June 1, 2023. If that Insider terminates the earlier-commencing plan on May 15, the later-commencing plan will not receive the benefit of the affirmative defense, because June 1 is within 30 days of May 15, the date of termination of the earlier-commencing plan, and thus June 1 is during the “effective cooling-off period.” However, if the later-commencing plan were scheduled to begin trading on July 1, 2023, it could still receive the benefit of the affirmative defense because July 1, 2023 is more than 30 days after May 15 and thus is outside the “effective cooling-off period.” |
(13) |
A series of separate contracts with different brokers to execute trades under a Trading Plan may be treated as a single plan, provided the contracts as a whole meet the conditions under Rule 10b5-1, and provided further that any amendment of one contract is treated as an amendment of all of the contracts under the plan. |
Limitation on Single-Trade Arrangements
(14) |
In any 12-month period, an Insider is limited to one “single-trade plan” — one designed to effect the open market purchase or sale of the total amount of the securities subject to the plan as a single transaction. The following do not constitute single-trade plans: (a) a Trading Plan that gives discretion to an agent over whether to execute the Trading Plan as a single transaction or that provides the agent’s future acts depend on facts not known at the time the Trading Plan’s adoption and might reasonably result in multiple transactions and (b) Sales to Cover. |
No Hedging
(15) |
As described in the Statement, individuals subject to the Statement are prohibited from engaging in any hedging or similar transactions designed to decrease the risks associated with holding securities of the Company. Further to this end, an Insider adopting a Trading Plan may not have entered into or altered a corresponding or hedging transaction or position with respect to the securities subject to the Trading Plan and must agree not to enter into any such transaction while the Trading Plan is in effect. |
Exhibit 12.1
Certification by the Principal Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and
15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Chen Xin, certify that:
| 1. | I have reviewed this annual report on Form 20-F of BGM Group Ltd; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| a. | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b. | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c. | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d. | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
| 5. | The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
| a. | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| b. | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
|
Date: |
January 27, 2025 |
|
|
|
|
|
/s/ Chen Xin |
|
Name: |
Chen Xin |
|
Title: |
Chief Executive Officer |
|
|
(Principal Executive Officer) |
Exhibit 12.2
Certification by the Principal Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-
14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Yaxuan Zhai, certify that:
| 1. | I have reviewed this annual report on Form 20-F of BGM Group Ltd; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| a. | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b. | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c. | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d. | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
| 5. | The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
| a. | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| b. | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
|
Date: |
January 27, 2025 |
|
|
|
|
|
/s/ Yaxuan Zhai |
|
Name: |
Yaxuan Zhai |
|
Title: |
Chief Financial Officer |
|
|
(Principal Financial Officer and Principal Accounting Officer) |
Exhibit 13.1
Certification by the Principal Executive Officer
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
Pursuant to U.S.C. Section 1350 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of Section 1350, Chapter 63 of Title 18, United States Code), I, Chen Xin, Chief Executive Officer of BGM Group Ltd (the “Company”), hereby certify to my knowledge that:
The Annual Report on Form 20-F for the year ended September 30, 2024 of the Company fully complies, in all material respects, with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and information contained in the Form 20-F fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: January 27, 2025
|
/s/ Chen Xin |
|
Chen Xin |
|
Chief Executive Officer |
|
(Principal Executive Officer) |
Exhibit 13.2
Certification by the Principal Financial Officer
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
Pursuant to U.S.C. Section 1350 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of Section 1350, Chapter 63 of Title 18, United States Code), I, Yaxuan Zhai, Chief Financial Officer of BGM Group Ltd (the “Company”), hereby certify to my knowledge that:
The Annual Report on Form 20-F for the year ended September 30, 2024 of the Company fully complies, in all material respects, with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and information contained in the Form 20-F fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: January 27, 2025
|
/s/ Yaxuan Zhai |
|
Yaxuan Zhai |
|
Chief Financial Officer |
|
(Principal Financial Officer and Principal Accounting Officer) |
Exhibit 15.1
January 27, 2025
To: BGM Group Ltd
No. 152 Hongliang East 1st Street, No. 1703,
Tianfu New District, Chengdu, 610200
People’s Republic of China
Dear Sir/Madam,
We hereby consent to the references to our firm’s name under the headings Item 3 Key Information—D. Risk Factors—Risks Related to Our Corporate Structure”, “Item 4 Information on the Company—B. Business Overview—Recent Regulatory Developments” and “Item 10. Additional Information—E. Taxation—People’s Republic of China Taxation” in BGM Group Ltd’s annual report on Form 20-F for the year ended September 30, 2024 (the “Annual Report”), which will be filed with the Securities and Exchange Commission (the “SEC”) on the date hereof. We also consent to the filing of this consent letter with the SEC as an exhibit to the Annual Report.
In giving such consent, we do not thereby admit that we come within the category of persons whose consent is required under Section 7 of the Securities Act of 1933, or under the Securities Exchange Act of 1934, in each case, as amended, or the regulations promulgated thereunder.
Yours sincerely, |
|
|
/s/ Gansu Quanyi Law Firm |
|
|
Gansu Quanyi Law Firm |
|
|
Exhibit 15.2

CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
BGM Group Limited:
We hereby consent to the incorporation by reference in the Registration Statement on Form F-3 (File No. 333-282998), Form F-3 (File No. 333-278591) and Form S-8 (File No. 333-278592), and consent to the inclusion in foregoing Form 20-F (File No. 001-38773) with SEC of BGM Group Limited (formerly known as Qilian International Holding Group Ltd.) and its affiliated entities (collectively, the “Company”) of our report dated February 15, 2024, relating to our audits of the consolidated balance sheet as of September 30, 2023, and the related consolidated statements of operations and comprehensive (loss) income, changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended September 30, 2023, and the related notes (collectively referred to as the “consolidated financial statements”).
We also consent to the reference to us under the heading “Experts” in such Registration Statement.
/s/ ZH CPA, LLC
Denver, Colorado
January 27, 2025
999 18th Street, Suite 3000, Denver, CO, 80202, USA. Phone: 1.303.386.7224 Fax: 1.303.386.7101 Email: admin@zhcpa.us |
Exhibit 15.3

CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form F-3 (File No. 333-278591), Form F-3 (File No. 333-282998) and Form S-8 (File No. 333-278592) of BGM Group Ltd. (formerly known as Qilian International Holding Group Ltd.) and its affiliated entities (collectively, the “Company”) of our report dated on January 27, 2025, relating to the consolidated financial statements, which appears in this annual report on Form 20-F of the Company for the year ended September 30, 2024.

Enrome LLP
Singapore
January 27, 2025
Enrome LLP |
143 Cecil Street #19-03/04 |
admin@enrome-group.com |
|
GB Building, Singapore 069542 |
www.enrome-group.com |
Exhibit 97.1
BGM GROUP LTD
POLICY FOR THE
RECOVERY OF ERRONEOUSLY AWARDED COMPENSATION
A.OVERVIEW
In accordance with the Listing Rule 5608(b) of Nasdaq Stock Market LLC (the “Nasdaq Rules”), Section 10D and Rule 10D-1 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) (“Rule 10D-1”), the Board of Directors (the “Board”) of BGM Group Ltd (the “Company”) has adopted this Policy (the “Policy”) to provide for the recovery of erroneously awarded Incentive-based Compensation from the Executive Officers. All capitalized terms used and not otherwise defined herein shall have the meanings set forth in Section H below.
B.RECOVERY OF ERRONEOUSLY AWARDED COMPENSATION
(1) |
In the event of an Accounting Restatement, the Company will reasonably promptly recover the Erroneously Awarded Compensation Received in accordance with the Nasdaq Rules and Rule 10D-1 as follows: |
(i) |
After an Accounting Restatement, the Compensation Committee (if composed entirely of independent Directors, or in the absence of such a committee, a majority of independent Directors serving on the Board) (the “Committee”) shall determine the amount of any Erroneously Awarded Compensation Received by each Executive Officer and shall promptly notify each Executive Officer with a written notice containing the amount of any Erroneously Awarded Compensation and a demand for repayment or return of such compensation, as applicable. |
(a) |
For Incentive-based Compensation based on (or derived from) the Company’s stock price or total shareholders’ return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in the applicable Accounting Restatement: |
i. |
The amount to be repaid or returned shall be determined by the Committee based on a reasonable estimate of the effect of the Accounting Restatement on the Company’s stock price or total shareholders’ return upon which the Incentive-based Compensation was Received; and |
ii. |
The Company shall maintain documentation of the determination of such reasonable estimates and provide the relevant documentation as required to the Nasdaq. |
(ii) |
The Committee shall have discretion to determine the appropriate means of recovering Erroneously Awarded Compensation based on the particular facts and circumstances. Notwithstanding the foregoing, except as set forth in Section B(2) below, in no event may the Company accept an amount that is less than the amount of Erroneously Awarded Compensation in satisfaction of an Executive Officer’s obligations hereunder. |
(iii) |
To the extent that the Executive Officer has already reimbursed the Company for any Erroneously Awarded Compensation Received under any duplicative recovery obligations established by the Company or applicable law, it shall be appropriate for any such reimbursed amount to be credited to the amount of Erroneously Awarded Compensation that is subject to recovery under this Policy. |
(iv) |
To the extent that an Executive Officer fails to repay all Erroneously Awarded Compensation to the Company when due, the Company shall take all actions reasonable and appropriate to recover such Erroneously Awarded Compensation from the applicable Executive Officer. The applicable Executive Officer shall be required to reimburse the Company for any and all expenses reasonably incurred (including legal fees) by the Company in recovering such Erroneously Awarded Compensation in accordance with the immediately preceding sentence. |
(2) |
Notwithstanding anything herein to the contrary, the Company shall not be required to take the actions contemplated by Section B(1) above if the Committee (which, as specified above, is composed entirely of independent Directors or in the absence of such a committee, a majority of the independent Directors |
serving on the Board) determines that recovery would be impracticable and any of the following three conditions are met:
(i) |
The Committee has determined that the direct expenses paid to a third party to assist in enforcing the Policy would exceed the amount to be recovered. Before making this determination, the Company must make a reasonable attempt to recover the Erroneously Awarded Compensation, documented such attempt(s) and provided such documentation to the Nasdaq; |
(ii) |
Recovery would violate home country law where that law was adopted prior to November 28, 2022, provided that, before determining that it would be impracticable to recover any amount of Erroneously Awarded Compensation based on violation of home country law, the Company has obtained an opinion from the home country counsel, acceptable to the Nasdaq, that recovery would result in such a violation and a copy of the opinion is provided to the Nasdaq; or |
(iii) |
Recovery would likely to cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to the employees of the Company, to fail to meet the requirements of Section 401(a)(13) or Section 411(a) of the Internal Revenue Code of 1986, as amended, and regulations thereunder. |
C.DISCLOSURE REQUIREMENTS
The Company shall file all disclosures with respect to this Policy required by the applicable U.S. Securities and Exchange Commission (“SEC”) filings and rules.
D.PROHIBITION OF INDEMNIFICATION
The Company shall not be permitted to insure or indemnify any Executive Officer against (i) the loss of any Erroneously Awarded Compensation that is repaid, returned or recovered pursuant to the terms of this Policy; or (ii) any claims relating to the Company’s enforcement of its rights under this Policy. Further, the Company shall not enter into any agreement that exempts any Incentive-based Compensation that is granted, paid or awarded to an Executive Officer from the application of this Policy or that waives the Company’s right to recovery of any Erroneously Awarded Compensation, and this Policy shall supersede any such agreement (whether entered into before, on or after the effective date of this Policy).
E.ADMINISTRATION AND INTERPRETATION
This Policy shall be administered by the Committee, and any determinations made by the Committee shall be final and binding on all affected individuals.
The Committee is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Policy and for the Company’s compliance with the Nasdaq Rules, Section 10D, Rule 10D-1 and any other applicable laws, regulations, rules or interpretations of the SEC or the Nasdaq promulgated or issued in connection therewith.
F.AMENDMENT; TERMINATION
The Committee may amend this Policy from time to time in its discretion and shall amend this Policy as it deems necessary. Notwithstanding anything in this Section F to the contrary, no amendment or termination of this Policy shall be effective if such amendment or termination would (after taking into account any actions taken by the Company contemporaneously with such amendment or termination) cause the Company to violate any federal securities laws, SEC rules or the applicable Nasdaq rules.
G.OTHER RECOVERY RIGHTS
This Policy shall be binding and enforceable against all Executive Officers and, to the extent required by the applicable laws or guidance from the SEC or the Nasdaq, their beneficiaries, heirs, executors, administrators or other legal representatives. The Committee intends that this Policy will be applied to the fullest extent required by the applicable laws. Any employment agreement, equity award agreement, compensatory plan or any other agreement or arrangement with an Executive Officer shall be deemed to include, as a condition to the grant of any benefit thereunder, an agreement by the Executive Officer to abide by the terms of this Policy. Any right of recovery under this Policy is in addition to, and not in lieu of, any other remedies or rights of recovery that may be available to the Company under the applicable laws, regulations or rules or pursuant to the terms of any policy of the Company or any provision in any employment agreement, equity award agreement, compensatory plan, agreement or other arrangement.
H.DEFINITIONS
For purposes of this Policy, the following capitalized terms shall have the meanings set forth below.
(1) |
“Accounting Restatement” means an accounting restatement due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements (a “Big R” restatement), or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (a “little r” restatement). |
(2) |
“Clawback Eligible Incentive Compensation” means all Incentive-based Compensation Received by an Executive Officer (i) on or after the effective date of the applicable Nasdaq rules; (ii) after commencing service as an Executive Officer; (iii) who served as an Executive Officer at any time during the applicable performance period relating to any Incentive-based Compensation (whether or not such Executive Officer is serving at the time the Erroneously Awarded Compensation is required to be repaid to the Company); (iv) while the Company has a class of securities listed on a national securities exchange or a national securities association; and (v) during the applicable Clawback Period (as defined below). |
(3) |
“Clawback Period” means, with respect to any Accounting Restatement, the three completed fiscal years of the Company immediately preceding the Restatement Date (as defined below), and if the Company changes its fiscal year, any transition period of less than nine months within or immediately following those three completed fiscal years. |
(4) |
“Erroneously Awarded Compensation” means, with respect to each Executive Officer in connection with an Accounting Restatement, the amount of Clawback Eligible Incentive Compensation that exceeds the amount of Incentive-based Compensation that otherwise would have been Received had it been determined based on the restated amounts, computed without regard to any taxes paid. |
(5) |
“Executive Officer” means each individual who is currently or was previously designated as an “officer” of the Company as defined in Rule 16a-1(f) under the Exchange Act. For the avoidance of doubt, the identification of an executive officer for purposes of this Policy shall include each Executive Officer who is or was identified pursuant to Item 401(b) of Regulation S-K or Item 6.A of Form 20-F, as applicable, as well as the principal financial officer and principal accounting officer (or, if there is no principal accounting officer, the controller). |
(6) |
“Financial Reporting Measures” means measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and all other measures that are derived wholly or in part from such measures. Stock price and total shareholders’ return (and any measures that are derived wholly or in part from stock price or total shareholders’ return) shall, for purposes of this Policy, be considered Financial Reporting Measures. For the avoidance of doubt, a Financial Reporting Measure need not be presented in the Company’s financial statements or included in a filing with the SEC. |
(7) |
“Incentive-based Compensation” means any compensation that is granted, earned or vested based wholly or in part upon the attainment of a Financial Reporting Measure. |
(8) |
“Nasdaq” means the Nasdaq Stock Market LLC. |
(9) |
“Received” means, with respect to any Incentive-based Compensation, actual or deemed receipt, and Incentive- based Compensation shall be deemed received in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-based Compensation award is attained, even if the payment or grant of the Incentive-based Compensation to the Executive Officer occurs after the end of that period. |
(10) |
“Restatement Date” means the earlier to occur of (i) the date which the Board, a committee of the Board or the officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement; or (ii) the date that a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement. |
Effective as of December 1, 2023