UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES ACT OF 1934
For the transition period from to
Commission file number: 001-12584
THERIVA BIOLOGICS, INC.
(Exact name of registrant as specified in its charter)
Nevada |
|
13-3808303 |
(State or other jurisdiction of incorporation or |
|
(I.R.S. Employer |
9605 Medical Center Drive, Ste. 270 |
|
20850 |
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code:
(301) 417-4364
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
||
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock |
|
TOVX |
|
NYSE American |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the issuer: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer, “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
|
|||
Large Accelerated Filer |
☐ |
|
Accelerated Filer |
☐ |
|
|
|
|
|
Non-accelerated Filer |
☒ |
|
Smaller Reporting Company |
☒ |
|
|
|
|
|
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant as of June 30, 2023, the last business day of the registrant’s recently completed second fiscal quarter, was approximately $16,791,123 million based on $1.00, the closing price of the registrant’s common stock as reported by the NYSE American on that date.
As of March 21, 2024, the registrant had 17,148,049 shares of common stock outstanding.
Documents incorporated by reference: None
THERIVA BIOLOGICS, INC.
FORM 10-K
TABLE OF CONTENTS
Page |
||
3 |
||
|
||
5 |
||
|
||
31 |
||
|
||
59 |
||
|
||
59 |
||
|
|
|
60 |
||
|
||
60 |
||
|
||
60 |
||
|
||
60 |
||
|
||
60 |
||
|
||
61 |
||
|
||
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
61 |
|
|
||
68 |
||
|
||
69 |
||
|
||
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
108 |
|
|
||
108 |
||
|
||
109 |
||
|
||
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
109 |
|
|
|
|
110 |
||
|
||
110 |
||
|
||
113 |
||
|
||
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
123 |
|
|
||
Certain Relationships and Related Transactions, and Director Independence |
124 |
|
|
||
125 |
||
|
||
126 |
||
|
||
126 |
||
|
||
132 |
2
PART I
Special Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K (this “Annual Report”) contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that involve substantial risks and uncertainties. The forward-looking statements are contained principally in Part I, Item 1. “Business,” Part I, Item 1A. “Risk Factors,” and Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” but are also contained elsewhere in this Annual Report. In some cases you can identify forward-looking statements by terminology such as “may,” “should,” “potential,” “continue,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” and similar expressions. These statements are based on our current beliefs, expectations, and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and generally beyond our control, that could cause actual results to differ materially from those expressed, projected or implied in or by the forward-looking statements.
You should refer to Item 1A. “Risk Factors” section of this Annual Report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We do not undertake any obligation to update any forward-looking statements.
Unless the context requires otherwise, references to “we,” “us,” “our,” “Theriva,” and “Theriva Biologics,” refer to Theriva Biologics, Inc. and its subsidiaries.
Summary Risk Factors
The following is a summary of the key risks relating to the Company. A more detailed description of each of the risks can be found below under Item 1A. Risk Factors.
Risks Related to Our Financial Position and Capital Requirements
| ● | Our consolidated financial statements as of December 31, 2023 have been prepared under the assumption that we will continue as a going concern for the next twelve months. |
| ● | We will need to raise additional capital to operate our business. |
| ● | We expect to continue to incur significant operating and capital expenditures and we will need additional funds to support our operations. |
| ● | The actual amount of funds we will need to operate is subject to many risk factors, some of which are beyond our control. |
| ● | We currently have a limited operating history as an oncology company, no products approved for commercial sale, have no significant source of revenue and may never generate significant revenue. |
| ● | We have identified material weaknesses in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated or that additional material weaknesses will not occur in the future. |
| ● | We expect to seek to raise additional capital in the future, which may be dilutive to stockholders or impose operational restrictions. |
| ● | Our operating results may fluctuate significantly, which makes our future operating results difficult to predict. |
| ● | If our acquired intangible assets become impaired, we may be required to record a significant charge to earnings. |
Risks Related to Our Business
| ● | Prior to 2022 we did not conduct any cancer research and development activities and there can be no assurance that we will successfully be able to do so. |
| ● | The development and commercialization of oncolytic viruses have experienced certain challenges. |
| ● | Our research and development efforts may not succeed in developing successful products and technologies. |
| ● | We may not realize the benefits from any strategic alliances we form or licensing arrangements we enter into. |
3
| ● | We may not be able to retain rights licensed to us by others to commercialize key products and may not be able to establish or maintain the relationships we need to develop, manufacture, and market our products. |
| ● | We may incur additional expenses in connection with our licenses and collaboration arrangements and our development of our product candidates. |
| ● | Developments by competitors may render our products or technologies obsolete or non-competitive. |
| ● | We may seek to selectively establish collaborations, and, if we are unable to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans. |
| ● | If the parties we depend on for product manufacturing are unsuccessful in providing adequate drug supply, or if existing drug supply becomes unusable, it may delay or impair our ability to develop, manufacture and market our product candidates. |
| ● | Any problems obtaining the standard of care drugs that we administer with VCN-01, could result in a delay or interruption in our clinical trials. |
| ● | We may fail to retain or recruit necessary personnel, and we may be unable to secure the services of consultants. |
| ● | Global health crises may adversely affect our planned operations. |
| ● | Business disruptions could seriously harm our future revenue and financial condition and increase costs and expenses. |
| ● | Unfavorable economic conditions could adversely affect our business, financial condition or results of operations. |
| ● | We rely extensively on our information technology systems which are vulnerable to risks, including cybersecurity and data leakage risks. |
| ● | Our business and operations would suffer in the event of computer system failures. |
| ● | Any failure to maintain the security of information relating to our patients, customers, employees and suppliers, whether as a result of cybersecurity attacks or otherwise, could expose us to litigation, government enforcement actions and costly response measures, and could disrupt our operations and harm our reputation. |
| ● | We may face particular data protection, data security and privacy risks in connection with the European Union’s Global Data Protection Regulation and other privacy regulations. |
Regulatory Risks
| ● | If we do not obtain the necessary regulatory approvals we may not be able to develop or sell our product candidates. |
| ● | Clinical trials are very expensive, time consuming, and difficult to design and implement. |
| ● | The results of our clinical trials may not support our proposed product candidate claims and the results of preclinical studies and completed clinical trials are not necessarily predictive of future results. |
| ● | Difficulties in enrolling, retaining, or completing patients in our clinical trials or delays in enrollment are expected to result in our clinical development activities being delayed or otherwise adversely affected. |
| ● | Patients who are administered our product candidates may experience unexpected side effects or other safety risks that could cause a halt in clinical development, preclude approval or limit the commercial potential of the product candidate. |
| ● | It is possible that we may not be able to obtain or maintain orphan drug designation or exclusivity for our drug candidates,. |
| ● | Our product candidates, if approved for sale, may not gain acceptance among physicians, patients and the medical community. |
| ● | We depend on third parties, including researchers and sublicensees, who are not under our control. |
| ● | We currently have no marketing, sales or distribution organization and have no experience in marketing products as a company. |
| ● | Reimbursement may not be available for our product candidates, which would impede sales. |
| ● | Healthcare reform measures could hinder or prevent our product candidates’ commercial success. |
| ● | If we fail to comply with state and federal healthcare regulatory laws, we could face substantial penalties, damages, fines, disgorgement, exclusion from participation in governmental healthcare programs, and the curtailment of operations, any of which could harm our business. |
| ● | If we obtain approval to commercialize our clinical product candidates outside of the United States, a variety of risks associated with international operations could harm our business |
| ● | If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. |
Intellectual Property Risks
| ● | We rely on patent applications and various regulatory exclusivities to protect some of our product candidates and our ability to compete may be limited or eliminated if we are not able to protect our products. |
| ● | We may incur substantial costs as a result of litigation or other proceedings relating to protecting our intellectual property rights, as well as costs associated with lawsuits. |
| ● | If we infringe the rights of others, we could be prevented from selling products or forced to pay damages. |
4
| ● | We enjoy restricted geographical protection with respect to certain patents. |
| ● | We may become subject to claims challenging inventorship or ownership of our patents and other intellectual property. |
Risks Related to Our Securities
| ● | We cannot assure you that our common stock will be liquid or that it will remain listed on the NYSE American exchange. |
| ● | We expect to seek to raise additional capital in the future, which may be dilutive to stockholders or impose operational restrictions. |
| ● | The market price of our common stock has been and may continue to be volatile and adversely affected by various factors. |
| ● | Our Articles of Incorporation and bylaws and Nevada law have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline. |
| ● | We do not intend to pay dividends in the foreseeable future on our common stock. |
| ● | Resales of our common stock in the public market by our stockholders may cause the market price of our common stock to fall. |
| ● | The shares of common stock offered under our current Amended and Restated At The Market Issuance Sales Agreement may be sold in “at the market” offerings, and investors who buy shares at different times will likely pay different prices. |
Item 1. Business.
Overview
We are a diversified clinical-stage company developing therapeutics designed ot treat cancer and related diseases in areas of high unmet need. As a result of the acquisition in March 2022 of Theriva Biologics, S.L. (“VCN”, formerly named VCN Biosciences, S.L.), described in more detail below (the “Acquisition”), we began transitioning our strategic focus to oncology, which is now our primary focus, through the development of VCN’s new oncolytic adenovirus platform designed for intravenous and intravitreal delivery to trigger tumor cell death, to improve access of co-administered cancer therapies to the tumor, and to promote a robust and sustained anti-tumor response by the patient’s immune system. Our lead product candidate, VCN-01, a clinical stage oncolytic human adenovirus that is modified to express an enzyme, PH20 hyaluronidase, is currently being administered in a Phase 2 clinical study for the treatment of pancreatic cancer, a Phase 1 clinical study for the treatment of retinalblastoma, a Phase 1 clinical study for the treatment of head and neck squamous cell carcinoma and a Phase 1 clinical study for the treatment of solid tumors.
Prior to the Acquisition, our focus was on developing therapeutics designed to treat gastrointestinal (GI) diseases which included our clinical development candidates: (1) SYN-004 (ribaxamase) which is designed to degrade certain commonly used intravenous (IV) beta-lactam antibiotics within the GI tract to prevent microbiome damage, thereby preventing overgrowth and infection by pathogenic organisms such Clostridioides difficile infection (CDI) and vancomycin resistant Enterococci (VRE), and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients, and (2) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. As part of our strategic transformation into an oncology focused company, we are exploring value creation options for our SYN-004 and SYN-020 assets, including out-licensing or partnering.
5
Our Current Product Pipeline
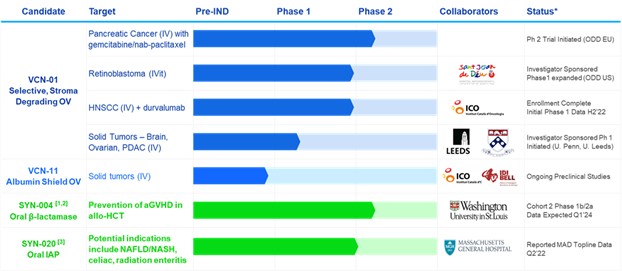
*Based on management’s current beliefs and expectations
allo-HCT allogeneic hematopoietic cell transplant. CPI immune checkpoint inhibitor. HNSCC head and neck squamous cell carcinoma. IV intravenous. IVit intravitreal. ODD Orphan Drug Designation. For other abbreviations see the text.
¹Additional products with preclinical proof-of-concept include SYN-006 (carbapenemase) to prevent aGVHD and infection by carbapenem resistant enterobacteriaceae and SYN-007 (ribaxamase) DR to prevent antibiotic associated diarrhea with oral β-lactam antibiotics.
²Depending on funding/partnership. SYN-004 may enter an FDA-agreed Phase 3 clinical trial for the treatment of CDI.
³We have an option-license agreement with Massachusetts General Hospital to develop SYN-020 in several potential indications related to inflammation and gut barrier dysfunction.
4We have an option agreement with Sant Joan de Déu-Barcelona Children’s Hospital to license their intellectual property rights related to the use of VCN-01 in combination with topoisomerase I inhibitor chemotherapies for the treatment of cancer.
Our Current Oncology-Focused Pipeline
Oncolytic Viruses
Our oncology platform is based on oncolytic virotherapy (“OV therapy”), which exploits the ability of certain viruses to kill tumor cells and trigger an anti-tumor immune response. This novel class of anticancer agents has unique mechanisms of action compared to other cancer drugs. Oncolytic viruses exploit the fact that cancer cells contain mutations that cause them to lose growth control and form tumors. Once inside a tumor cell, oncolytic viruses exploit the tumor cell machinery to generate thousands of additional copies of the virus, which then kill the tumor cell and spread to neighboring cells, causing a chain reaction of cell killing. This infection and tumor cell killing by OVs also alerts the immune system, which can then attack the virus infected tumor cells to help destroy the tumor in some instances.
Our OV product candidates are engineered to efficiently infect and selectively replicate to a high extent in tumor cells versus normal host cells, which enables intravenous delivery. By contrast, many other oncolytic viruses in clinical development today are administered by direct injection into the tumor. Intravenous delivery has the potential to expand the therapeutic effect of OVs because the virus can infect both the primary tumor and tumor metastases throughout the body.
6
Our first product candidate, VCN-01, is a clinical stage oncolytic human adenovirus that is modified to express an enzyme,PH20 hyaluronidase, that degrades hyaluronan in the tumor stroma, which helps the virus and other molecules to penetrate and spread throughout the tumor. VCN-01 can be used alone or in combination with other cancer therapies, such as chemotherapy and immunotherapy, for difficult to treat cancers. An expanding intellectual property portfolio supports our oncology programs, and because our products are characterized as biologics with Orphan Drug designation in our target indications, they will be further protected by data and/or market exclusivity in major markets.
VCN-01 — An oncolytic human type-5 adenovirus engineered for intravenous administration and to express a tumor matrix degrading enzyme (PH20 hyaluronidase) that facilitates the entry of therapeutics and immune cells into tumors
VCN-01 is a genetically modified oncolytic adenovirus that has been engineered to contain four independent genetic modifications on the backbone of the wild-type human adenovirus serotype 5 (HAd5) genome. These modifications have been shown in preclinical and clinical studies to confer tumor selective replication and antitumor activity. VCN-01 was engineered to replicate in and kill virtually all types of solid tumor cells, to expose tumor neoantigens of lysed tumors, to reduce liver tropism, and to express PH20 hyaluronidase to enhance the penetration of virus, chemotherapy, immuno-oncology therapy, and immune cells into the tumor.
Malignant tumors are made up of tumor cells as well as significant supporting tissue known as tumor stroma. The tumor stroma supports the formation and growth of tumors and contains cells and other components that are required for robust tumor growth and metastasis. The stroma also forms an effective barrier to the entry of therapeutic agents such as chemotherapy and immuno-oncology products . A key structural component of the tumor stroma is hyaluronic acid, and tumor levels of hyaluronic acid have been clinically associated with reduced survival in metastatic pancreatic cancer patients. VCN-01 is designed to overcome the stroma barrier problem by expressing the hyaluronan degrading enzyme PH20 hyaluronidase after it infects tumor cells. Expression of PH20 by VCN-01 is designed to degrade the hyaluronic acid within the tumor stroma and improves virus spread throughout the tumor. Based upon the foregoing, we believe our oncolytic virus platform, exemplified by VCN-01, represents a new and potentially powerful form of therapy that combines tumor cell killing, anti-tumor immunity and stroma destruction after intravenous delivery.
The VCN-01 product candidate is provided as a sterile liquid concentrate that is diluted for infusion or injection. The proposed therapeutic indication for VCN-01 is the treatment of solid tumors, as its selectivity mechanism relies on cellular properties shared by virtually all human tumor cells. Our initial indication for clinical development is unresectable metastatic pancreatic cancer, a disease for which there is currently no cure and only limited therapeutic options.
VCN-01 has been administered to 116 patients across multiple Phase 1 clinical trials and Phase 2 VIRAGE trial, including patients with pancreatic cancer, head and neck squamous cell carcinoma, ovarian carcinoma, colorectal cancer, and retinoblastoma.
Pancreatic Ductal Adenocarcinoma
Cancer of the pancreas consists of two main histological types: cancer that arises from the ductal (exocrine) cells of the pancreas or, much less often, cancers may arise from the endocrine compartment of the pancreas. Pancreatic ductal adenocarcinoma (“PDAC”) accounts for more than 90% of all pancreatic tumors. It can be located either in the head of the pancreas or in the body-tail. Pancreatic cancer usually metastasizess to the liver and peritoneum. Other less common metastatic sites are the lungs, brain, kidney and bone. In its early stages, pancreatic cancer does not typically result in any characteristic symptoms. In many instances, progressive abdominal pain is the first symptom. Therefore, in most cases, pancreatic cancer is diagnosed in its late stages (locally advanced non-metastatic or metastatic stage of the disease) when surgical resection and possibly curative treatment is not possible. It is generally assumed that only 10% of cases are resectable at presentation, whereas 30-40% of patients are diagnosed at local advanced/unresectable stage and 50-60% present with distant metastases.
7
PDAC Clinical Unmet Need and Market Opportunity
PDAC is one of the most fatal cancers, accounting for the 4th highest cause of cancer-associated deaths in the US and the European Union. Despite significant research efforts, minimal progress has been achieved to date. The five-year overall survival rate is < 10% and has not substantially improved over the last 30 years. Surgery is the only treatment that offers the prospect of long term-survival; however, the 5-year survival for the limited number of patients in whom resection is possible remains low (20 – 30 %). Patients with advanced disease are managed with chemotherapy. In recent years, the combination of gemcitabine with albumin-bound paclitaxel (GA), and the combination of folic acid, 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) have emerged as the standard of care. More recently, liposomal irinotecan, approved for second line PDAC in combination with fluorouracil and leucovorin, has shown potential as first line therapy when administered along with 5-FU and other chemotherapies. However, the results are still very poor and new therapeutic interventions are needed. The increase is particularly evident in younger people and several studies anticipate that pancreatic cancer is expected to become the second leading cause of cancer-related death in the United States by 2030. The rising incidence of pancreatic cancer and its current economic burden place increased pressure to improve outcomes for patients.
In May 2011, the Committee for Orphan Medicinal Products (“COMP”) from the European Medicines Agency (“EMA”) recommended granting Orphan Medicinal Product Designation to VCN-01 for the treatment of pancreatic cancer and in June 2011, the European Commission confirmed the designation under Regulation (“EC”) No 141/2000 of the European Parliament and of the Council.
In June 2023, the FDA granted Orphan Drug designation to VCN-01 for the treatment of pancreatic cancer.
Phase 1a/Proof of Concept Trial of VCN-01 by intratumoral administration in PDAC
In September 2019, VCN presented a poster at the European Society for Molecular Oncology (“ESMO”) annual meeting describing initial mechanism of action data from a multicenter, Phase 1 dose escalation study of intratumoral (“IT”) VCN-01 administered to pancreatic cancer patients in combination with standard doses/schedules of either gemcitabine or nab-paclitaxel plus gemcitabine (NCT02045589). The study was conducted at three hospitals in Spain and 8 patients with confirmed histologic diagnosis of unresectable PDAC amenable to endoscopic ultrasound guided (“EUS”) injection were treated with 3 injections (coincident with 1st day of the chemotherapy cycles) at two different dose levels of VCN-01 (six patients had metastatic disease and two had locally advanced disease). The treatment regimen was generally well-tolerated; however, one patient died from severe intraabdominal fluid collection that was considered to be related to VCN-01 treatment. Evaluation of virus pharmacokinetics and PH20 levels in serum were consistent with strong virus replication in the tumors. This was supported by the presence of viral particles in tumor cells as assessed in paired tumor biopsies collected before and after treatment. Tumor stiffness was reduced in all VCN-01-injected lesions as measured by elastography. Disease stabilization of injected lesions was observed in 5 out of 6 patients although subsequent tumor progression was observed in most of the patients due to the appearance of new lesions or growth of distant, non-injected, metastatic lesions. This study provided encouraging mechanism of action data for VCN-01; however, intratumoral injection did not appear to deliver sufficiently high VCN-01 levels for effective delivery to non-injected tumors. We believe these results supported the evaluation of the safety/tolerability and potential efficacy of VCN-01 via intravenous administration in combination with chemotherapy and/or immunotherapies for the treatment of advanced PDAC. The results of this study were published in the Journal for Immunotherapy of Cancer. 2021 Nov;9(11):e003254. doi: 10.1136/jitc-2021-003254.
Phase 1 Trial of intravenous VCN-01 with or without nab-paclitaxel plus gemcitabine in patients with solid tumors and PDAC
In March 2022, we announced the peer-reviewed publication of a Phase 1, multicenter, open-label, dose-escalation study investigating the safety, tolerability and biodistribution of intravenous VCN-01 oncolytic adenovirus with or without standard-of-care (SoC) chemotherapy (gemcitabine/nab-paclitaxel) in patients with advanced solid tumors (NCT02045602). The data, published in the Journal for ImmunoTherapy of Cancer, suggests that intravenous treatment with VCN-01 is feasible and has an acceptable safety profile, with encouraging biological and clinical activity. (Journal for Immunotherapy of Cancer 2022;10:e003255. doi:10.1136/jitc-2021-003255).
Data from the publication had previously been presented, in part, in a poster at the ESMO 2019 annual meeting. The published study was a multicenter, open-label, dose-escalation phase I clinical trial of a single dose of intravenous VCN-01 alone (Part I, 16 patients with advanced refractory solid tumors) or in combination with nab-paclitaxel plus gemcitabine (Part II and III; patients with pancreatic adenocarcinoma). In Part II, 12 patients received VCN-01 dose concurrent with chemotherapy on day 1, whereas in Part III 14 additional patients received the dose of VCN-01 seven days before chemotherapy. The recommended Phase 2 doses (RP2D) were determined to be 1x1013 viral particles (vp)/patient in Part I, 3.3x1012 vp/patient in Part II and 1x1013 vp/patient in Part III. Based on its apparent safety profile and the absence of dose-limiting toxicities, 1x1013 vp/patient using sequential dosing schedule was selected for further clinical development.
8
Pharmacokinetic data showed dose linearity, as well as relevant VCN-01 exposure. Analysis of VCN-01 clearance in patients enrolled in Part II did not show significant differences with respect to patients receiving VCN-01 as a single agent. VCN-01 viral genomes were detected in tumor tissue in 5 out of 6 biopsies. A second viral peak in plasma and increased hyaluronidase serum levels suggested replication after intravenous injection in all patients. Increased levels of immune biomarkers (IFNγ, sLAG3, IL-6, IL-10) were found after VCN-01 administration. In patients with pancreatic adenocarcinoma, the overall response rate (ORR) was 50% for Part II and 50% for Part III, as assessed by the investigators. Median progression free survival (PFS) for patients in Part III was 6.7 months, and median overall survival (OS) was 13.5 months. Eight patients (66.7%) survived more than 12 months. In addition, in April 2021, a subgroup analysis of patients at the RP2D (1.x1013 vp/patient followed by nab-paclitaxel plus gemcitabine one week later, n=6) was conducted and showed an ORR of 83%, with a median PFS of 6.3 months and median OS of 20.8 months. Some VCN-01 treated patients appeared to benefit from late-onset responses. This form of delayed anti-tumor activity is not common with chemotherapy but is frequently observed with immunotherapies. We believe an immune mechanism of action associated with the oncolytic activity of VCN-01 may be the underlying explanation. VCN-01 appeared to convert the typically immunosuppressive tumor microenvironment of pancreatic adenocarcinomas into an enhanced inflammatory microenvironment (IDO, CD28, PD-1, CTL signature up-regulation, and collagen formation) after treatment.
Phase 2 Trial of intravenous VCN-01 with or without nab-paclitaxel plus gemcitabine in patients with solid tumors and PDAC
In January 2023, we dosed the first patients in VIRAGE, the Phase 2b randomized, open-label, placebo-controlled, multicenter clinical trial of systemically administered VCN-01 in combination with standard-of-care (SoC) chemotherapy (gemcitabine/nab-paclitaxel) as a first line therapy for patients with newly-diagnosed metastatic pancreatic ductal adenocarcinoma. The study is expected to enroll 92 patients and be conducted at approximately 25 sites in the US and EU. Two doses of VCN-01 are included in the treatment arm: the 1st dose is administered on day 1, then one week later 3 cycles of gemcitabine and nab-paclitaxel as standard of care is administered. The second VCN-01 dose is administered 7 days before the 4th cycle of chemotherapy (approximately 90 days after the first VCN-01 dose), followed by additional cycles of gemcitabine/nab-paclitaxel chemotherapy.
Patient dosing was initiated in the U.S. in July 2023 and the fourteen patients have received their second doses of intravenous VCN-01, which were well tolerated and demonstrated the expected VCN-01 safety profile.
On February 7, 2024, we announced that the Independent Data Monitoring Committee (IDMC) recommended the continuation of enrollment as planned into VIRAGE, a multinational, Phase 2b, randomized, open-label, controlled clinical trial evaluating VCN-01 in combination with standard-of-care chemotherapy (gemcitabine/nab-paclitaxel) as a first-line therapy for patients with metastatic pancreatic ductal adenocarcinoma (PDAC).
According to the IDMC's comprehensive assessment of clinical data from patients enrolled across 6 sites open in the U.S. and 9 sites open in Spain, the ongoing Phase 2b trial will continue without any changes to the protocol. No safety concerns were raised based on the evaluation of data presented at the IDMC meeting. Intravenous VCN-01 has been well tolerated and demonstrated a safety profile consistent with prior clinical trials. Importantly, no additional toxicities were observed in patients receiving a second dose of VCN-01, providing the first clinical evidence of the feasibility of repeated systemic dosing. VIRAGE remains on track to complete enrollment in the first half of 2024.
Retinoblastoma
Retinoblastoma is a tumor that originates in the retina and it is the most common type of eye cancer in children. It occurs in approximately 1/14,000 - 1/18,000 live newborns and accounts for 15% of the tumors in the pediatric population < 1 year old. The average age of pediatric patients at diagnosis is 2, and it rarely occurs in children older than 6. In the US, retinoblastoma shows an incidence rate of 3.3 per 1,000,000 with only about 200 to 300 children diagnosed per year according to the American Cancer Society. Bilateral retinoblastoma (Rb1 germinal mutation) represents 25-35% of the cases while unilateral retinoblastoma (sporadic mutation) accounts for 65-75%. While retinoblastoma is a highly curable disease in the US, with a current disease-free survival rate of >95%, the clinical challenge for those who treat retinoblastoma is to preserve life and to prevent the loss of an eye, blindness and other serious effects of treatment that reduce the patient’s life span or the quality of life. In addition, children with retinoblastoma have been more likely to lose their eye and die of metastatic disease in low-resource countries.
9
Current treatments are not without significant morbidity, which may include visual impairment and severe cosmetic deformity secondary to enucleation and/or irradiation of the orbital region. The use of intravenous chemotherapy and more recently intra-arterial and intravitreal chemotherapy have resulted in a significantly greater number of eyes preserved with fewer long-term effects compared to past treatments such as external radiation therapy. However, allowing patients with advanced intraocular disease to be treated conservatively, led to the appearance of a subgroup of patients with advanced intraocular disease who relapsed after an initial response. Most of these cases include those patients who present gross vitreous or subretinal seeding. Once the aforementioned treatments are exhausted, these patients rarely manage to preserve the eyes and vision and must be enucleated. The ocular preservation rate of these eyes with advanced disease is still less than 50%.
In February 2022, the FDA granted Orphan Drug designation to VCN-01 for the treatment of retinoblastoma.
Phase 1 Trial of intravitreal VCN-01 in patients with retinoblastoma
During the third quarter of 2017, VCN entered into a Clinical Trial Agreement with Hospital Sant Joan de Déu (Barcelona, Spain) to conduct an investigator sponsored Phase 1 clinical study evaluating the safety and tolerability of two intravitreal injections of VCN-01 in patients with intraocular retinoblastoma refractory to systemic, intra-arterial or intravitreal chemotherapy, or radiotherapy, in whom enucleation was the only recommended treatment (NCT03284268). Patients received two doses of VCN-01 injected 14 days apart using a dose escalation regimen. At this time, the dose-escalation phase of the study has already been completed in 6 patients distributed in two cohorts (2 x 109 vp/eye and 2 x 1010 vp/eye). VCN-01 was well tolerated to date after intravitreal administration, although some degree of intravitreal inflammation and associated turbidity were observed. Inflammation has been managed and potential turbidity minimized with local and systemic administration of anti-inflammatory drugs. VCN-01 does not appear to change the retinal function, and selective VCN-01 replication in retinoblastoma cells has been observed by immunohistochemical analysis. Replication within retinoblastoma tumors over time was detected and VCN-01 reduced the number of vitreous seeds in 4 out of 5 patients treated at 2 x 1010 vp/eye (n=5). The investigator has reported that one patient treated with VCN-01 has had a complete regression lasting more than 30 months.
This Phase 1 trial with VCN-01 has now completed enrollment and a total of nine (9) patients been treated. This study is expected to complete patient follow-up in Q1 2024. A pre-IND meeting with the FDA was held on December 19, 2023 to discuss the path forward for VCN-01 as an adjunct to chemotherapy in pediatric patients with advanced retinoblastoma. The FDA provided some guidance on the potential endpoints and patient population for an advanced clinical trial and encouraged submission of a formal protocol under a US IND in order to provide more detailed commentary.
Dr. Angel Montero-Carcaboso presented new data from the study for which he is the lead investigator atan oral presentation entitled “Topotecan enhances oncolytic adenovirus infection, replication and antitumor activity in retinoblastoma,” at Fundació Sant Joan de Déu at the SIOP 2022 Congress of the International Society of Pediatric Oncology, that was held in Barcelona, Spain from September 28-October 1, 2022. The new data from the study for which Dr. Angel Montero-Carcaboso is the lead investigator further support evaluation of VCN-01, an oncolytic adenovirus expressing hyaluronidase, and topotecan for the treatment of refractory retinoblastoma. Key data and conclusions showcased in the SIOP presentation include:
| ● | VCN-01 treatment in combination with topotecan, but not with carboplatin or melphalan, significantly increased VCN-01 infection and replication in retinoblastoma cells (p=0.0007) in vitro. |
| ● | In athymic mice engrafted with human retinoblastomas, topotecan administered systemically after intratumoral VCN-01 increased viral genome replication and the number of VCN-01 infected cells when compared to administration of VCN-01 alone (p = 0.0002). |
| ● | Sequential administration of intratumoral VCN-01 followed by systemic topotecan significantly increased median ocular survival, compared to VCN-01 alone (p =0.0364). |
10
VCN-01 in combination with Immunomodulatory therapeutics
Based on the clinical and pre-clinical data described below, we believe that the administration of VCN-01, can elicit an anti-tumor immune response that could potentiate the effects of VCN-01 and co-administered therapeutics. Biopsies from the Phase 1 trial of PDAC patients administered intravenous VCN-01 demonstrated lymphocyte (CD8+) infiltration and modulated levels of immune markers in tumors, including an induction of the PD1/PD-L1 expression in tumor tissue from some of the patients. Preclinical experiments demonstrated that VCN-01 significantly increased extravasation of an anti-PD-L1 antibody into subcutaneous xenograft tumors compared to non-treated (PBS) tumors and also that PH20 hyaluronidase improves the ingress of T-cells in animal models. Thus, we hypothesize that the administration of VCN-01 into the tumor will help to overcome the observed resistance to PD-L1 checkpoint inhibitors and to mesothelin-directed CAR-T cells.
Phase 1 Trial of intravenous VCN-01 in Combination with Durvalumab in Subjects with Recurrent/ Metastatic SCCHN
In February 2019, VCN entered into a Clinical Trial Agreement with Catalan Institute of Oncology (ICO) (Spain) to conduct an investigator sponsored Phase 1 clinical study to evaluate the safety, tolerability and RP2D of a single intravenous injection of VCN-01 combined with durvalumab in two administration regimens: VCN-01 concomitantly with durvalumab, or sequentially with durvalumab starting two weeks after VCN-01 administration (NCT03799744). The study is also designed to evaluate whether VCN-01 treatment can re-sensitize PD-(l)-1 refractory tumors to subsequent anti-PD-L1 therapy. Durvalumab is a human monoclonal antibody (mAb) of the immunoglobulin G (IgG) 1 kappa subclass that inhibits binding of PD-L1. It is marketed as IMFINZI® by AstraZeneca/MedImmune, who supplied the product for its use in the clinical study. This Phase I trial is a multicenter, open label, dose escalation study in patients with histologically confirmed head and neck squamous cell carcinoma from specific sites: oral cavity, oropharynx, larynx or hypopharynx that is recurrent/metastatic (R/M) and not amenable to curative therapy by surgery or radiation. In addition, all patients should have undergone prior exposure to anti-PD-(L) 1 and progressed. Patients are entered at each dose level, according to a planned dose escalation schedule. The treatment is a single intravenous VCN-01 dose combined with concomitant intravenous durvalumab (MEDI4736) 1500 mg Q4W (Arm I) or durvalumab starting two weeks after VCN-01 administration (“sequential schedule”; Arm II). Patient recruitment into Arm I and Arm II was performed concurrently. Intravenous VCN-01 was administered to each patient only once during the trial at the VCN-01 dose level to which they were randomized. Durvalumab was administered Q4W until disease progression, unacceptable toxicity, withdrawal of consent, or another discontinuation criterion. Patient recruitment into the study was completed in February 2022 with a total of 18 patients enrolled. On September 05, 2022 we announced a presentation of initial data from this study in a poster at the European Society for Medical Oncology (ESMO) Congress. The poster reported that treatment with VCN-01 had an acceptable safety profile when administered with durvalumab in the sequential schedule and the most common treatment-related adverse events were dose-dependent and reversible pyrexia, flu-like symptoms and increases in liver transaminases. Sustained blood levels of VCN-01 viral genomes and increased serum hyaluronidase levels were maintained for over six weeks and analysis of tumor samples showed an increase in CD8 T cells (a marker of tumor inflammation); upregulation of PD-L1; and downregulation of matrix-related pathways after VCN-01 administration. The last patients in this study are currently being followed for overall survival and patent samples are being analyzed to evaluate potential VCN-01 pharmacodynamic effects.
On October 16, 2023, we presented additional data from this study in a poster at the European Society for Medical Oncology (ESMO) 2023 Congress held virtually and in Madrid, Spian from October 20-24, 2023. Key data and conclusions featured in the ESMO presentation include:
| ● | 20 patients were enrolled with a median of 4 prior lines of therapy, from which six in the concomitant (CS) (single dose of VCN-01 in combination with durvalumab on day 1) and 12 in the sequential (SS) (single dose of VCN-01 on day -14 and durvalumab on day 1) were evaluable for response. |
| ● | In the CS cohort at the 3.3×1012 viral particles (vp) dose, overall survival (OS) was 10.4 months. |
| ● | In the SS cohort at the 3.3×1012vp dose OS was 15.5 months, whereas in the SS cohort at the 1×1013 vp dose OS was 17.3 months. |
| ● | 11 patients (61.1%) were alive >12 months (2 in CS; 5 in SS at 3.3×1012vp, 4 in SS at 1×1013 vp). |
| ● | In spite of the advanced stage of the disease, and a global objective response rate for the trial of 5.5%, most of the patients appeared to benefit from subsequent treatment, with 2 patients showing complete responses to palliative chemotherapy and at least one patient still alive 4 years after entering the study. |
| ● | Biological activity: Patients showed VCN-01 replication and increased serum hyaluronidase levels were maintained for over six weeks. |
11
| ● | Observed an increase in CD8 T cells, a marker of tumor inflammation and an upregulation of PD-L1 in tumors. |
| ● | Increase of PDL1-CPS (16/21; p=0.013) and CD8 T-cells (12/21; p=0.007) from baseline were found in tumor biopsies. |
| ● | There was a statistical significant correlation between OS observed in patients and CPS on day 8 (p=0.005). |
Phase 1 Trial evaluating the safety and feasibility of huCART-meso cells when given in combination with VCN-01
In July 2021, VCN entered into a Clinical Trial Agreement with the University of Pennsylvania (Philadelphia) to conduct an investigator sponsored Phase 1 clinical study to evaluate the safety, tolerability and feasibility of intravenous administration of VCN-01 in combination with lentiviral transduced huCART-meso cells (developed by the laboratory of Dr. Carl June) in patients with histologically confirmed unresectable or metastatic pancreatic adenocarcinoma and serous epithelial ovarian cancer (NCT05057715). This is a Phase I study evaluating the combination of VCN-01 when given in combination with huCART-meso cells in a dose-escalation design in two cohorts (N = 3-6), where patients receive VCN-01 as a single IV infusion (at 3.3x1012 or 1x1013 vp) on Day 0, followed by a single dose of 5x107 huCART-meso cells on Day 14 via IV infusion. huCART-meso cells are modified T-cells targeting the mesothelin antigen, which is frequently expressed in multiple tumor types, particularly in pancreatic and ovarian cancers. Dr. June’s previous clinical studies have shown that huCART-meso cells encounter significant challenges in the tumor microenvironment, including immunosuppressive cells and soluble factors as well as metabolic restrictions. Initial VCN-01 clinical data from the studies described above suggest that administration of VCN-01 may increase tumor immunogenicity and improve access of the huCART-meso cells to tumor cells. This Phase I study will evaluate the safety and tolerability of the VCN-01 huCART-meso cell combination and test the hypothesis that administration of VCN-01 may enhance the potential antitumor effects of the co-administered huCART-meso cells.
On July 8, 2022, we were notified that the first patient to be dosed with VCN-01 had passed the safety evaluation period in this study. The study is on-going.
On June 22, 2023, at their Cellicon Valley conference, and again at the Society for Immunotherapy of Cancer (SITC) meeting in San Diego, CA on November 03, 2023, and the International Oncolytic Virotherapy Conference (IOVC2023) in Calgary on November 13 2023, University of Pennsylvania investigators presented preliminary clinical safety and pharmacokinetic data from this study highlighting the feasibility of administering VCN-01 in sequence with huCART-meso cells in pancreatic and ovarian cancer patients. VCN-01 persistence was suggestive of tumor infection and active replication. The peak and duration of huCART-meso T cells in the peripheral blood as well as duration of stable disease in evaluable patients showed encouraging trends.
The study will test higher doses of VCN-01 and will interrogate tumor biopsies to gain further insights. The results will inform and guide optimization of the combination of CAR T cells with oncolytic virus.
Phase 1 Trial evaluating the intravenous administration of VCN-01 in patients prior to surgical resection of high-grade brain tumors
In the second quarter of 2021, VCN entered into a Clinical Trial Agreement with the University of Leeds (UK) to sponsor a proof-of-concept Phase 1 clinical study to evaluate whether intravenously administered VCN-01 can cross the blood-brain barrier and infect the target brain tumor. This is an open-label, non-randomized, single center study of VCN-01 given intravenously at a dose of 1x1013 virus particles to patients prior to planned surgery for recurrent high-grade primary or metastatic brain tumors. We believe that the intravenous delivery of anti-cancer therapy to brain tumors, if effective, may enable the treatment of systemically disseminated brain metastases and may allow for reduction in the need to use neurosurgery to administer the drugs. This study aims to assess the presence of VCN-01 within the resected surgical specimen after systemic VCN-01 delivery and determine the safety of intravenous VCN-01 in patients with recurrent high-grade glioma or brain metastases. By confirming the presence of VCN-01 in high grade brain tumors following intravenous delivery, this study may pave the way for larger trials to study VCN-01 efficacy, both as a monotherapy and in combination with PD-1/PD-L1 blockade. This trial has already received approval from Medicines & Healthcare Products Regulatory Agency (MHRA) from UK Government.
On January 9, 2023, we issued a press release announcing that the first patient was dosed in this study and recruitment is on-going.
12
Our Current Gastrointestinal (GI) and Microbiome-Focused Pipeline
Our SYN-004 (ribaxamase) and SYN-020 clinical programs are focused on the gastrointestinal tract (GI) and the gut microbiome, which is home to billions of microbial species and composed of a natural balance of both “good” beneficial species and potentially “bad” pathogenic species. When the natural balance or normal function of these microbial species is disrupted, a person’s health can be compromised. All of our programs are supported by our growing intellectual property portfolio. We are maintaining and building our patent portfolio through: filing new patent applications; prosecuting existing applications; and licensing and acquiring new patents and patent applications.
SYN-004 (ribaxamase) — Prevention of antibiotic-mediated microbiome damage, thereby preventing overgrowth and infection by pathogenic organisms such as Clostridioides difficile infection (CDI) and vancomycin resistant Enterococci (VRE), and reducing the incidence and severity of acute graft-versus-host disease (aGVHD) in allogeneic HCT recipients
SYN-004 (ribaxamase) is a proprietary oral capsule prophylactic therapy designed to degrade certain IV beta-lactam antibiotics excreted into the GI tract and thereby maintain the natural balance of the gut microbiome. Preventing beta-lactam damage to the gut microbiome has a range of potential therapeutic outcomes, including prevention of CDI, suppression of the overgrowth of pathogenic species (particularly antimicrobial-resistant organisms) and potentially reducing the incidence and/or severity of aGVHD in allogeneic hematopoietic cell transplant (HCT) patients. SYN-004 (ribaxamase) 75 mg capsules are intended to be administered orally while patients are administered certain IV beta-lactam antibiotics. The capsule dosage form is designed to release the SYN-004 (ribaxamase) enzyme into proximal small intestine, where it has been shown to degrade beta-lactam antibiotics in the GI tract without altering systemic antibiotic levels. Beta-lactam antibiotics are a mainstay in hospital infection management and include the commonly used penicillin and cephalosporin classes of antibiotics.
Clostridioides difficile Infection
Clostridioides difficile (formerly known as Clostridium difficile and often called C. difficile or CDI) is a leading type of hospital acquired infection and is frequently associated with IV beta-lactam antibiotic treatment. The Centers for Disease Control and Prevention (CDC) identified C. difficile as an “urgent public health threat,” particularly given its resistance to many drugs used to treat other infections. CDI is a major unintended risk associated with the prophylactic or therapeutic use of IV antibiotics, which may adversely alter the natural balance of microflora that normally protect the GI tract, leading to C. difficile overgrowth and infection. Other risk factors for CDI include hospitalization, prolonged length of stay (estimated at 7 days), underlying illness, and immune-compromising conditions including the administration of chemotherapy and advanced age. According to a paper published in BMC Infectious Diseases (Desai K et al. BMC Infect Dis. 2016; 16: 303) the economic cost of CDI was approximately $5.4 billion in 2016 ($4.7 billion in healthcare settings; $725 million in the community) in the U.S., mostly due to hospitalizations.
Limitations of Current Treatments and Market Opportunity
CDI is a widespread and often drug resistant infectious disease. Approximately 20% of patients who have been diagnosed with CDI experience a recurrence of CDI within one to three months. Furthermore, controlling the spread of CDI has proven challenging, as the C. difficile spores are easily transferred to patients via normal contact with healthcare personnel and with inanimate objects. There is currently no vaccine or approved product for the prevention of primary (incident) CDI. The current standard of care for primary CDI, as outlined by the Infectious Disease Society of America (IDSA), is to treat with powerful antibiotics such as fidaxomicin or vancomycin. Prolonged use of fidaxomicin and vancomycin has been shown to further exacerbate damage to the gut microbiome, leading to increased risk of CDI recurrence as well as the emergence of pathogenic and antimicrobial-resistant (AMR) organisms, such as vancomycin-resistant enterococci (VRE). AMR is a serious global threat and one which world leaders have begun to take action against. According to the European Society of Clinical Microbiology and Infections Disease (ECCMID), failure to address AMR could lead to a potential “antibiotic Armageddon”, resulting in 10 million deaths worldwide by 2050 and may cost as much as $100 trillion in worldwide economic output.
13
According to a paper published in BMC Infectious Diseases,”Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach”. (Desai et.al., BMC Infect Dis 16: 303), it is estimated that approximately 606,000 patients are infected with C. difficile annually in the U.S., and it has been reported that approximately 44,500 deaths are attributable to CDI-associated complications each year. According to IMS Health Incorporated*, in 2016, the potential addressable market for SYN-004 (ribaxamase) included approximately 227 million doses of intravenous Penicillin and Cephalosporin antibiotics which were administered in the United States and which may contribute to the onset of CDI. Additional data derived from IMS Health Incorporated states that in 2016, the worldwide market for SYN-004 (ribaxamase)-addressable intravenous beta-lactam antibiotics was approximately 7.5 billion doses, which may represent a multi-billion-dollar market opportunity for us. If approved, SYN-004 (ribaxamase) would be the first therapeutic intervention indicated to prevent the onset of antibiotic-mediated primary CDI.
Phase 1a and 1b Clinical Trial Pharmacokinetic Data
In March 2015, we reported supportive pharmacokinetic data from a Phase 1a clinical trial, which suggested that SYN-004 (ribaxamase) should have no effect on the IV antibiotic in the bloodstream, allowing the antibiotic to fight the primary infection. In February 2015, we reported supportive topline results from a subsequent Phase 1b clinical trial of escalating doses of oral SYN-004 (ribaxamase), with no safety or tolerability issues reported at dose levels and dosing regimens that were equivalent to or exceeded those expected to be studied in subsequent clinical trials. The Phase 1a (40 participants) and 1b (24 participants) clinical trials of SYN-004 (ribaxamase) were initiated in December 2014.
Two Phase 2a Clinical Trials: Topline Results
In December 2015, we reported supportive topline results from our first Phase 2a clinical trial of SYN-004 (ribaxamase, NCT02419001). The study demonstrated that SYN-004 (ribaxamase) successfully degraded IV ceftriaxone in the chyme of ten participants with ileostomies without affecting the levels of ceftriaxone in the bloodstream. In May 2016, we reported supportive topline results from a second Phase 2a clinical trial of SYN-004 (ribaxamase) in 14 healthy participants with functioning ileostomies administered IV ceftriaxone with and without oral SYN-004 (ribaxamase) (NCT02473640). This second study demonstrated that the 150 mg dose of SYN-004 (ribaxamase), both alone and in the presence of the proton pump inhibitor (PPI), esomeprazole, degraded ceftriaxone excreted into the chyme resulting in ceftriaxone levels that were low or not-detectable. Ceftriaxone plasma concentrations in participants of the second study were not altered by SYN-004 (ribaxamase) in the presence or absence of an oral PPI, suggesting limited drug-drug interactions. The 150 mg dose of SYN-004 (ribaxamase) was well tolerated by all participants in this clinical trial.
Phase 2b Proof of Concept Clinical Trial Design & Results
In September 2015, we initiated a multicenter, randomized, placebo-controlled Phase 2b proof-of-concept clinical study in 412 patients (206 per group; NCT02563106).
On January 5, 2017, we announced positive topline data from our Phase 2b proof-of-concept clinical trial intended to evaluate the ability of SYN-004 (ribaxamase) to prevent CDI, CDAD (C. difficile-associated diarrhea) and AAD (antibiotic-associated diarrhea) in patients hospitalized for a lower respiratory tract infection and receiving IV ceftriaxone. Results from this study demonstrated that SYN-004 (ribaxamase) achieved its primary endpoint of significantly reducing CDI. Preliminary analysis of the data indicated seven confirmed cases of CDI in the placebo group compared to two cases in the SYN-004 (ribaxamase) treatment group. Patients receiving SYN-004 (ribaxamase) achieved a 71.4% relative risk reduction (p-value=0.045) in CDI rates compared to patients receiving placebo. SYN-004 (ribaxamase) treated patients also demonstrated a significant reduction in new colonization by vancomycin-resistant enterococci (VRE) compared to placebo (p-value=0.002). Results from this trial also demonstrated that patients administered ribaxamase in conjunction with IV-ceftriaxone demonstrated comparable cure rates (approximately 94%) for the treatment of primary infection compared to the placebo group. Results from this trial also demonstrated that the percentage of subjects reporting at least one treatment emergent adverse event (TEAE) was similar between SYN-004 (ribaxamase) and placebo treatment groups (40.8% vs 44.2%). Adverse events reported during this trial were comparable between treatment and placebo arms. Serious adverse events (SAEs) in the treatment arm, including fatal AEs, which exceeded those in the placebo arm, were not considered drug-related by investigators at the clinical sites, or by an independent third-party, each of whom determined SAEs were attributable to disparities in the underlying health and comorbidities between the groups.
* |
This information is an estimate derived from the use of information under license from the following IMS Health Incorporated information service: IMS Health Analytics for the full year 2016. IMS expressly reserves all rights, including rights of copying, distribution, and republication. |
14
On October 6, 2016 we were awarded a government contract in the amount of $521,014 by the CDC’s Broad Agency Announcement (BAA) 2016-N-17812 to examine changes in the gut resistome of patients in our Phase 2b clinical study. Data generated under this contract are consistent with SYN-004’s (ribaxamase) mode of action of preserving the normal gut flora by degrading ceftriaxone in the upper GI tract of study participants treated with SYN-004 (ribaxamase). The data further demonstrated that SYN-004 (ribaxamase) significantly reduced the loss of microbial diversity, reduced overgrowth of opportunistically pathogenic species(such as VRE), and reduced the emergence of antimicrobial resistance (AMR) genes caused by ceftriaxone treatment in SYN-004 (ribaxamase) treated patients compared to placebo.
Future Potential Regulatory Strategy for Prevention of Primary CDI
On November 21, 2018, we announced results from our End-of-Phase 2 meeting with the FDA during which key elements of a Phase 3 clinical program were confirmed. Pursuant to the meeting, the FDA proposed criteria for Phase 3 clinical efficacy and safety which, if achieved, may support submission for marketing approval of SYN-004 (ribaxamase) on the basis of a single Phase 3 clinical trial. The proposed SYN-004 (ribaxamase) Phase 3 clinical program entails a single, global, event-driven clinical trial with a fixed maximum number of approximately 4,000 patients for total enrollment and evaluates the potential efficacy and safety of ribaxamase in a broad patient population by enrolling patients with a variety of underlying infections treated with a range of IV beta-lactam antibiotics.
The proposed Phase 3 clinical trial incorporates co-primary safety and efficacy endpoints (mortality and the reduction in the incidence of CDI at one month after the last drug dose in the SYN-004 (ribaxamase) treatment group versus placebo,respectively). We expect the clinical development costs to complete this trial to be in excess of $80 million and anticipate initiating the Phase 3 clinical program only after securing additional potential financing via a strategic partnership.
Acute Graft-Versus-Host-Disease in Allogeneic Hematopoietic Cell Transplant (allogeneic HCT) Recipients & SYN-004 (ribaxamase)
In parallel with our clinical and regulatory efforts, we completed a Health Economics Outcomes Research (HEOR) study, which was conducted to generate key insights on how we can expect Health Care Practitioners, or HCPs, to evaluate patient access for SYN-004 (ribaxamase) while also providing a framework for potential reimbursement strategies. After evaluating findings from the study, we believe that there is significant potential value in exploring the development of SYN-004 (ribaxamase) in a narrower patient population where the incidence of the disease endpoint is high and the clinical development may be less costly.
We believe allogeneic hematopoietic cell transplant (HCT) recipients, who have a very high risk of CDI, VRE colonization and potentially fatal bacteremia, and acute-graft-vs-host disease (aGVHD), represent such a patient population. Published literature has demonstrated a strong association between these adverse outcomes and microbiome damage caused by IV beta-lactam antibiotics in these patients. Approximately 80-90% of HCT recipients receive IV beta-lactam antibiotics to treat febrile neutropenia. Penicillins and cephalosporins are first-line therapies in the USA and EU, whereas carbapenems are first-line in China. Antibiotic-mediated damage to the gut microbiome is strongly associated with GVHD, bloodstream infections, VRE bacteremia, transplant relapse, and increased mortality in HCT recipients, raising concern over the spectrum of antibiotics used during HCT.
CDI occurs in up to 31% of HCT patients and is associated with GVHD and increased mortality. aGVHD occurs in 30-60% of allogeneic HCT recipients and is recognized as a primary contributor to morbidity and mortality in this patient population. The most recent available data indicate approximately 8,000 reported allogeneic HCT procedures each year in the USA, 19,800 procedures in Europe, 12,700 in China, and 3,500 in Japan. First-line treatments for aGVHD fail in more than 50% of patients and 2-year survival in patients with steroid refractory aGVHD is only 20%. At least one U.S. study found allogeneic HCT recipients who developed aGVHD had 3-times higher in-hospital mortality and almost 2-fold higher median hospital costs than patients who did not develop aGVHD. It has been reported that in-patient costs for allogeneic HCT in the USA range from $180,000-$300,000 depending on the disease severity. In 2014, all-cause costs for allogeneic HCT in the USA were greater than $600,000 per patient (up to 12 months post-transplant). VRE infection is a persistent problem in HCT patients and VRE colonization after HCT has been associated with decreased patient survival.
Phase 1b/2a Clinical Study in Allogeneic HCT Recipients
In August 2019, we entered into a Clinical Trial Agreement (CTA) with the Washington University School of Medicine (Washington University) to conduct a Phase 1b/2a clinical trial of SYN-004 (ribaxamase). Under the terms of this agreement, we serve as the sponsor of the study and supply SYN-004 (ribaxamase). Dr. Erik R. Dubberke, Professor of Medicine and Clinical Director, Transplant Infectious Diseases at Washington University and a member of the SYN-004 (ribaxamase) steering committee serves as the principal investigator of the clinical trial in collaboration with his Washington University colleague Dr. Mark A. Schroeder, Associate Professor of Medicine, Division of Oncology, Bone Marrow Transplantation and Leukemia.
15
The Phase 1b/2a clinical trial will comprise a single center, randomized, double-blinded, placebo-controlled clinical trial of oral SYN-004 (ribaxamase) in up to 36 evaluable adult allogeneic HCT recipients. The goal of this study is to evaluate the safety, tolerability and potential absorption into the systemic circulation (if any) of oral SYN-004 (ribaxamase; 150 mg four times daily) administered to allogeneic HCT recipients who receive an IV carbapenem or beta-lactam antibiotic to treat fever. Study participants will be enrolled into three sequential cohorts administered a different study-assigned IV antibiotic. Each cohort seeks to complete eight evaluable participants treated with SYN-004 (ribaxamase) and four evaluable participants treated with placebo. Safety and pharmacokinetic data for each cohort will be reviewed by an independent Data and Safety Monitoring Committee, which will make a recommendation on whether to proceed to the next IV antibiotic cohort. The study will also evaluate potential protective effects of SYN-004 on the gut microbiome as well as generate preliminary information on potential therapeutic benefits and patient outcomes of SYN-004 in allogeneic HCT recipients.
To date, we have completed the first of 3 cohorts (Cohort 1) in this study, which enrolled 19 patients who received at least 1 dose of study drug (SYN-004 or Placebo randomized 2:1). Sixteen patients received at least one dose of intravenous (IV) meropenem and 12 of these patients completed sufficient doses of IV meropenem to be evaluable towards the study endpoints. On September 27, 2022, we issued a press release announcing positive outcomes from the Data and Safety Monitoring Committee (“DSMC”) review of results from the first Cohort and their recommendation that the study may proceed to enroll Cohort 2 in which study drug (SYN-004 or Placebo) is administered in combination with the IV beta-lactam antibiotic piperacillin/tazobactam. On November 3, 2022 we announced the first patient had been dosed in Cohort 2. Patient dosing is on-going and if enrollment proceeds on the current schedule, we may be positioned to announce data readouts for the second cohort during the first half of 2024 and the third cohort during the first half of 2025.
On February 16, 2023 and April 13, 2023 we announced the presentation of safety and pharmacokinetic data from Cohort 1 of the Phase 1b/2a Clinical Trial of SYN-004 (ribaxamase) in allogeneic hematopoietic cell transplant recipients at the 2023 Tandem Meetings: Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR and at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) respectively.
SYN-020 — Oral Intestinal Alkaline Phosphatase (IAP)
SYN 020 is a quality-controlled, recombinant version of bovine Intestinal Alkaline Phosphatase (IAP) produced under cGMP conditions and formulated for oral delivery. The published literature indicates that IAP functions to diminish GI and systemic inflammation, tighten the gut barrier to diminish “leaky gut,” diminish fat absorption, and promote a healthy microbiome. Despite its broad therapeutic potential, a key hurdle to commercialization has been the high cost of IAP manufacture which is commercially available for as much as $10,000 per gram. We believe we have developed technologies to traverse this hurdle and now have the ability to produce more than 3 grams per liter of SYN-020 and anticipate a cost of roughly a few hundred dollars per gram at commercial scale. Based on the known mechanisms as well as our own supporting animal model data, we intended to initially develop SYN-020 to mitigate the intestinal damage caused by radiation therapy that is routinely used to treat pelvic cancers. While we believe SYN-020 may play a pivotal role in addressing acute and long-term complications associated with radiation exposure to the GI tract, we have also begun planning for potential development of SYN-020 in large market indications with significant unmet medical needs. Such indications include celiac disease, non-alcoholic fatty liver disease (“NAFLD”), and indications to treat and prevent metabolic and inflammatory disorders associated with aging, which are supported by our collaboration with Massachusetts General Hospital (“MGH”).
On June 30, 2020, we submitted an IND application to the FDA in support of an initial indication for the treatment of radiation enteropathy secondary to pelvic cancer therapy. On July 30, 2020, we announced that we received a study-may-proceed letter from the FDA to conduct a Phase 1a single-ascending-dose (“SAD”) study in healthy volunteers designed to evaluate SYN-020 for safety, tolerability and pharmacokinetic parameters (NCT04815993). On April 1, 2021, we announced that enrollment had commenced in the Phase 1 SAD clinical trial of SYN-020. On June 29, 2021, we announced that enrollment, patient dosing and observation had been completed in the Phase 1, open-label, SAD study of SYN-020. The SAD study enrolled 6 healthy adult volunteers into each of four cohorts with SYN-020 given orally as single doses ranging from 5 mg to 150 mg. The data demonstrated that SYN-020 maintained a favorable safety profile, was well tolerated at all dose levels, and no adverse events were attributed to the study drug. No serious adverse events were reported.
16
During the third quarter of 2021 we initiated a Phase 1 clinical study evaluating multiple ascending doses (“MAD”) of SYN-020 (NCT05045833). On October 21, 2021 we announced that patient enrollment, dosing, and observation commenced in the Phase 1 MAD study of SYN-020. The placebo-controlled, blinded study enrolled 32 healthy adult volunteers into four cohorts with SYN-020 administered orally in doses ranging from 5 mg to 75 mg twice daily for 14 days with a follow-up evaluation at day 35. Each cohort included six subjects who received SYN-020 and two who received placebo. On May 10, 2022, we announced positive safety data from the Phase 1 MAD study demonstrating that SYN-020 maintained a favorable safety profile and was well-tolerated across all dose levels. There were a few treatment-related adverse events, and all were mild (grade 1) and resolved without medical intervention. The most common adverse event, constipation, occurred in three out of 24 subjects in the treatment arm and in one out of eight subjects in the placebo arm. No adverse event led to discontinuation of the study drug and there were no serious adverse events. Additionally, fecal SYN-020 analyses verified intestinal bioavailability while plasma levels of SYN-020 were below the limit of quantitation in all samples at all timepoints verifying that SYN-020 was not absorbed into the systemic circulation.
During the second quarter of 2020, we announced that we entered into an agreement with Massachusetts General Hospital (“MGH”) granting us an option for an exclusive license to intellectual property and technology related to the use of IAP to maintain GI and microbiome health, diminish systemic inflammation, and treat age-related diseases. During the second quarter of 2021, we announced an amendment to our option for an exclusive license agreement with MGH to include intellectual property and technology related to the use of SYN-020 to inhibit liver fibrosis in select diseases, including NAFLD. Research published by a team of investigators led by Richard Hodin, MD, Chief of the Massachusetts General Hospital Division of General and Gastrointestinal Surgery and Professor of Surgery, Harvard Medical School, evaluated long-term oral supplementation of IAP, including SYN-020, in mice. Dr. Hodin’s research demonstrated that IAP administration, starting at 10 months of age, slowed the microbiome changes, gut-barrier dysfunction, and gastrointestinal and systemic inflammation that normally accompany aging. Additionally, the IAP administration resulted in improved metabolic profiles in the aged mice, diminished frailty, and extended lifespan. Under the terms of the agreement, we are granted exclusive rights to negotiate a worldwide license with MGH to commercially develop SYN-020 to treat and prevent metabolic and inflammatory diseases associated with aging. If executed, we plan to use this license in the advancement of an expanded clinical development program for SYN-020.
The Phase 1 data from our SAD and MAD studies are intended to support the development of SYN-020 in multiple clinical indications including radiation enteritis, NAFLD, celiac disease, and indications supported by our collaboration with Massachusetts General Hospital. With our transition to an oncology focused Company, we are exploring strategic opportunities to enable advancement of this potentially valuable asset.
Future Potential Regulatory Strategy for Prevention of Primary CDI
As part of our strategic transformation into an oncology focused company, we are exploring value creation options for our SYN-004 and SYN-020 assets, including out-licensing or partnering.
Research Programs
VCN-11 Albumin Shield™ Technology
VCN-11 is a novel virus that we believe has the potential to extend our OV platform. VCN-11 has been engineered to contain all of the features of VCN-01 as well as an additional modification to include an albumin binding domain (ABD) in the virus capsid. The virus capsid is the target for neutralizing antibodies (NAbs) that are generated by the host immune system to destroy circulating viruses. The presence of an albumin binding domain, however, blocks the binding of most neutralizing antibodies, which allows the virus to reach the tumor following intravenous administration. This “Albumin Shield” works because human blood contains a large amount of albumin to coat the VCN-11 virus. Importantly, this coating of albumin appears to be displaced after the virus reaches tumor cells to infect them. In pre-clinical mouse studies to test the functionality of the “albumin shield”, mice pre-immunized with virus are able to completely neutralize an unmodified OV because they have a large concentration of neutralizing antibodies in their blood. By contrast, viruses containing the albumin binding domain such as VCN-11 are not neutralized and retain their ability to infect and destroy tumor cells. We believe these results support the further development of VCN-11 for tumors in which rapid multi-dosing may be beneficial.
In the second quarter of 2020, VCN had several interactions with Spanish regulatory authorities (AEMPS) to agree on the design of the non-clinical GLP toxicology and biodistribution studies that are required to support a first-in-human clinical trial for VCN-11.
17
In March 2021, preclinical data obtained with VCN-11 was published (J Control Release. 2021 Apr 10;332:517-528), showing that VCN-11 induced 450 times more cytotoxicity in tumor cells than in normal cells. VCN confirmed VCN-11 hyaluronidase production by measuring the activity of the PH20 enzyme with a hyaluronic acid-degradation assay, and by measuring PH20 activity in VCN-11 infected tumors in vivo. VCN-11 evaded NAbs from different sources and tumor levels of VCN-11 were demonstrated in the presence of high levels of NAbs in vivo, whereas the control virus without ABD was neutralized. VCN-11 showed a low toxicity profile in athymic nude mice and Syrian hamsters, allowing treatments with high doses and fractionated administrations without major toxicities (up to 1.2x1011vp/mouse and 7.5x1011vp/hamster). VCN-11 increased ALT levels on day 3 within an acceptable range that returned to normal levels by day 9. Fractionated intravenous administration of VCN-11 (splitting the dose into two portions administered 4 h apart) appeared to improve VCN-11 circulation kinetics and increase tumor levels. VCN-11 showed antitumor efficacy in the presence of NAbs against Ad5 and itself.
In May 2022, we presented on VCN-11 at the 25th Annual Meeting of the American Society of Gene & Cell Therapy (ASGCT). The presentation included preclinical results showcasing the potential of VCN-11 to balance safety, with no major toxicities observed, and effectively target tumors after intravenous re-administration, even in the presence of high level NAbs. Our internal discovery programs are currently evaluating new oncolytic viruses derived from VCN-11 that may expand the potential efficacy of Albumin Shield viruses.
SYN-006, SYN-007, other oncolytic virus
To date, our Research programs that have been directed to the development of GI acting products have generated preclinical proof-of-concept with two potential pipeline products (SYN-006 and SYN-007) designed to expand the utility of our beta-lactamase strategy. SYN-007 is a specially formulated version of SYN-004 (ribaxamase) designed to be used with orally administered beta-lactam antibiotics to protect the gut microbiome from antibiotic-mediated dysbiosis. SYN-006 is a carbapenemase designed to degrade intravenous (IV) carbapenem antibiotics within the GI tract to maintain the natural balance of the gut microbiome for the prevention of CDI, overgrowth of pathogenic organisms and the emergence of antimicrobial resistance (AMR). The scope of our research is expanding to include development of new oncolytic virus products with alternative modes of action, and may include oncology applications of our existing products such as SYN-006 and SYN-007.
Intellectual Property
All of our programs are supported by growing patent estates. In total, Theriva Biologics has over 130 U.S. and foreign patents and over 65 U.S. and foreign patents pending. VCN, through assignment or exclusive licenses, controls over 40 U.S. and foreign patents and over 15 U.S. and foreign patents pending.
The SYN-004 (ribaxamase) program is supported by IP that is assigned to Theriva Biologics, namely U.S. and foreign patents (in most major markets, e.g. Europe (including Germany, Great Britain and France), Japan, China and Canada, among others) and U.S. and foreign patents pending (in most major markets, e.g. Europe (including Germany, Great Britain and France), Japan, China and Canada, among others). For instance, U.S. Patent Nos. 8,894,994 and 9,587,234, which include claims to compositions of matter and pharmaceutical compositions of beta-lactamases, including SYN-004 (ribaxamase), have patent terms to at least 2031. Further, U.S. Patent 9,301,995 and 9,301,996, both of which will expire in at least 2031, cover various uses of beta-lactamases, including SYN-004 (ribaxamase), in protecting the microbiome, and U.S. Patent Nos. 9,290,754, 9,376,673, 9,404,103, 9,464,280, and 9,695,409 which will expire in at least 2035, covers further beta-lactamase compositions of matter related to SYN-004 (ribaxamase).
The SYN-020 (oral intestinal alkaline phosphatase (IAP)) program is supported by IP that is assigned to Theriva Biologics, namely U.S. and foreign patents and patent applications (in many major markets, e.g. Europe, China, Japan, Korea, Canada, and Australia). These patents and patent applications, which cover various formulations, medical uses and manufacture of SYN-020, are expected to expire in 2038-2040, without taking potential patent term extensions or patent term adjustment into account.
18
The VCN-01 and VCN-11 programs are supported by U.S. and foreign patents and patent applications that are assigned to VCN or exclusively licensed from Fundació Privada Institut d’Investigacio Biomedica de Bellvitge (IDIBELL), Institut Catala d’Oncologia (ICO), and Hospital Sant Joan de Déu in Barcelona. The patents and patent applications include U.S. patents and foreign patents (in most major markets, e.g. Europe, China, Japan, Korea, Canada, Israel, Mexico, Russia, and Australia) and U.S. and foreign patents pending (in most major markets, e.g. Europe, China, Korea, Canada, Mexico, and India). The patents and patent applications cover compositions of matter and pharmaceutical compositions of oncolytic adenoviruses and various medical uses of the same. For instance, U.S. Patent No. 10,316,065, which expires in 2030 without taking potential patent term extensions or patent term adjustment into account, provides composition of matter and pharmaceutical composition coverage for a genus of engineered oncolytic adenovirus suitable for the treatment of solid tumors. Other patents and patent applications, if granted, will provide protection to 2037 without taking potential patent term extensions or patent term adjustment into account.
Our goal is to (i) obtain, maintain, and enforce patent protection for our products, formulations, processes, methods, and other proprietary technologies, (ii) preserve our trade secrets, and (iii) operate without infringing on the proprietary rights of other parties worldwide. We seek, where appropriate, the broadest intellectual property protection for product candidates, proprietary information, and proprietary technology through a combination of contractual arrangements and patents.
Acquisition of VCN Biosciences, S.L. (now known as Theriva Biologics, S.L.)
On March 10, 2022, we completed our acquisition (the “VCN Acquisition”) of all the outstanding shares of VCN (the “VCN Shares”) from the shareholders of VCN pursuant to the terms of the Share Purchase Agreement (“Purchase Agreement”) that we entered into with VCN and the shareholders of VCN Biosciences, S.L. (the “Sellers”) on December 14, 2021. Upon consummation of the Acquisition, VCN became our wholly owned subsidiary. As consideration for the purchase of the VCN Shares of capital stock, we paid $4,700,000 (the “Closing Cash Consideration”) to Grifols Innovation and New Technologies Limited (“Grifols”), the owner of approximately 86% of the equity of VCN, and issued to the remaining Sellers 2,639,530 shares of our common stock, $0.001 par value (the “Closing Shares”), representing 19.99% of the outstanding shares of our common stock on December 14, 2021, the date of the Purchase Agreement. As additional consideration for the purchase of the VCN Shares held by Grifols, we also agreed to make the following milestone payments to Grifols:
Milestone Payments
US$3MM upon VCN-01 US IND Safe to Proceed pancreatic ductal adenocarcinoma (“PDAC”, or other first indication), which payment was made in Q4 2022 upon attaining the milestone
US$2.75MM upon VCN-01 US IND Safe to Proceed – retinoblastoma (“RB”, or other second indication)
US$3.25MM upon VCN-01 US first patient dosed– PDAC (or other first indication) after receipt of VCN-01 US IND Safe to Proceed for PDAC being informed, which payment was made in Q4 2023 upon attaining the milestone
US$3.25MM upon VCN-01 US first patient dosed – RB (or other second indication) after receipt of VCN-01 US IND Safe to Proceed for RB being informed
US$6MM upon VCN-01 US Phase 2 trial meets the primary endpoint or if a Phase 2 trial is not conducted and only a Phase 3 trial is conducted then upon a Phase 3 being initiated – PDAC (or other first indication)
US$8MM upon VCN-01 Pivotal Trial meeting the primary endpoint or upon BLA Submission – RB (or other second indication)
US$12MM upon VCN-01 US Phase 3 trial meeting the primary endpoint or upon BLA Submission – PDAC (or other first indication)
US$16MM upon VCN-01 BLA Approval – PDAC (or other first indication)
US$16MM upon VCN-01 BLA Approval – RB (or other second indication)
Pursuant to the Purchase Agreement, at the Closing we assumed $2,400,000 of liabilities of VCN, which includes certain loans from the Spanish Government and the Catalan Government Agency.
19
The Purchase Agreement contains customary representations, warranties and covenants of the Sellers and us. Subject to certain customary limitations, the Sellers have agreed to indemnify us and our officers and directors against certain losses related to, among other things, breaches of their representations and warranties, certain specified liabilities and the failure to perform covenants or obligations under the Purchase Agreement.
Effective November 15, 2022, as part of our corporate rebranding, VCN changed its name to Theriva Biologics S.L. without other changes to its corporate structure.
Theriva is a clinical-stage biopharmaceutical company developing new oncolytic adenoviruses for the treatment of cancer. Theriva’s lead product candidate, VCN-01, is being studied in clinical trials for pancreatic cancer and retinoblastoma with additional investigator sponsored trials in indications including head and neck squamous cell carcinoma (HNSCC) serous epithelial ovarian cancer and brain tumors. VCN-01 is designed to be administered systemically, intratumorally or intravitreally, either as a monotherapy or in combination with standard of care, to treat a wide variety of cancer indications. VCN-01 is designed to replicate selectively and aggressively within tumor cells, and to degrade the tumor stroma barrier that serves as a significant physical and immunosuppressive barrier to cancer treatment. Degrading the tumor stroma has been shown to improve access to the tumor by the virus and additional therapies such as chemo- and immuno-therapies. Importantly, degrading the stroma exposes tumor antigens, turning “cold” tumors “hot” and enabling a sustained anti-tumor immune response. Theriva has the exclusive rights to four patent families for proprietary technologies, as well as technologies developed in collaboration with the Virotherapy Group of the Catalan Institute of Oncology (ICO-IDIBELL) and with Hospital Sant Joan de Deu (HSJD), with a number of additional patents pending.
Our Current Collaborations
IDIBELL Technology Transfer Agreement
On August 31, 2010, VCN entered into a Technology Transfer Agreement (the “Technology Transfer Agreement”) with the Bellvitge Biomedical Research Institute (“IDIBELL”) for the exclusive license of the right to use a Spanish patent number P200901201 titled “Oncolytic adenoviruses for treating cancer” which is co-owned by IDIBELL and Catalan Oncology Institute (“ICO”) for the term of the patent. The Technology Transfer Agreement provides that IDIBELL is entitled to a low single digit percentage royalty on the income collected by VCN from the utilization of products derived from the licensed technology, prior to applying any value-added tax, if any, and low single digit percentage royalty on other income received by VCN arising from the use of the licensed technology, including income related to sublicenses of the licensed technology to third parties and advance payments or payments made for goals that were met and/or services associated with the licensed technology. The Technology Transfer Agreement terminates upon the expiration of the patent rights and is subject to early termination by either party in the event of a breach by the other party of its obligations thereunder. In addition, IDIBELL has the right to revoke the license if VCN ceases business activities for a continuous year or ceases to utilize the technology subject of the Technology Transfer Agreement, uses the technology in violation of the principals of IDIBELL or ICO or stops maintaining the patent licensed under the Technology Transfer Agreement
ICO Marketing License
On May 16, 2009, VCN entered into a Contract to Grant a Marketing License (the “ICO License Agreement”) with the Catalan Institute of Oncology (the “ICO”) for a manufacturing and marketing license of a patent P200700665 titled “Adenovirus with mutations in the area of endoplasmic retention of protein E3-19k and their use in the treatment of cancer” in connection with a sublicense identified therein. The validity period of the license granted is unlimited with the only applicable limit being the patent’s own validity. The ICO License Agreement provides that the ICO is entitled to a royalty of low double digit percentage of the net value of the income from the concession of the identified sublicense and low double digit percentage on other lump sums received thereunder. VCN and its sublicensees have an obligation to use all diligent and commercially reasonable efforts for the exploitation of the patent, otherwise, ICO may proceed to recover the license. The ICO License terminates upon the expiration of the patent rights and is subject to early termination by either party in the event of a breach by the other party of its obligations thereunder.
20
IDIBELL/ICO License Agreement
On March 4, 2016, VCN entered into a License Agreement (the “IDIBELL/ICO License Agreement”) with IDIBELL and the ICO, for the exclusive license of the right to use a family of patents whose priority application is European patent application EP 14 38 2162.7 titled “Adenovirus comprising an albumin-binding moiety”. The License Agreement provides that IDIBELL and ICO, as licensors, are entitled to share a low single digit percentage royalty on the annual Net Sales (as defined in the IDIBELL/ICO License Agreement)collected by VCN from the utilization of products derived from the licensed technology and a royalty on sublicensing income received from the licensed technology at a rate of: low double digit percentage during the first 3 years following the effective date of the agreement, mid - single digit percentage during the term of 3 to 7 years following the effective date and low single digit percentage thereafter. The IDIBELL/ICO License Agreement also provides for certain fixed payments, including a payment 25 days following the date of concession of the licensed patent in a minimum of three European jurisdictions and a payment 25 days following the date of concession of an American patent derived from the licensed patent. The IDIBELL/ICO License is for an indefinite term subject to early termination (i) by mutual agreement of the parties; (ii) by licensor in the event of at least two successive breaches or three alternate breaches calculated annually of the obligation to pay any consideration; (iii) by VCN at its discretion due to certain patent infringements of rights protected by the patents or due to the absence of protection of the patent in any countries in the territory which is worldwide or (iv) in the event of a breach by the other party of its obligations thereunder which are not remedied within thirty (30) days. In addition, the licensors have the right to revoke the IDIBELL/ICO License Agreement if VCN during a continuous period of two years abandons its research or development activities of the licensed patent or activities aimed at exploitation of the resulting products, VCN has undertaken no marketing whatsoever during the term of the IDIBELL/ICO License Agreement or uses the patent licensed for purposes other those as set forth in the IDIBELL/ICO License Agreement.
Saint Joan De Déu Collaboration and License Agreement
On February 15, 2016, VCN entered into a Collaboration Agreement to Conduct a Clinical Trial and Grant an Operating License (the “Collaboration and License Agreement”) with the Saint Joan De Déu Hospital (the “Hospital”) and the Saint Joan De Déu Foundation (the “Foundation”, and together with the Hospital, the “Institution”) regarding the conduct of a clinical trial to evaluate the safety and activity of VCN-01 in patients with refractory retinoblastoma. The Collaboration and License Agreement provides that if the trial results are positive and VCN is interested in continuing with the development of VCN-01 for the treatment of retinoblastoma; (a) the parties undertake to apply their best efforts to negotiate and, where appropriate, sign an agreement to collaborate in the development and execution of the following phases of the development of VCN-01 for the treatment of retinoblastoma; (b) the Institution shall grant to VCN an exclusive, worldwide and indefinite license to use and exploit the trial results and their possible patents exclusively for the treatment of retinoblastoma; (c) VCN shall pay the Foundation five hundred thousand Euros (€500,000), subject to reduction for any public and/or private economic aid that third parties may grant to the Institution for the conduct of the trial and/or any advance payments made by VCN before the end of the trial; (d) VCN shall pay the Foundation three hundred twenty thousand Euros (€320,000) once following the trial results of a pivotal study, to be carried out by VCN, has been completed which allows it to obtain the marketing authorization of the product following from the results, which payment must be made within a maximum period of four (4) years from the date on which Institution has delivered the final report of the trial to VCN ; and (e) the parties will use their best efforts to negotiate and, where appropriate, sign a product supply agreement in order that the Hospital can use VCN-01 for compassionate use in the treatment of retinoblastoma. The Collaboration and License Agreement continues in force and effect until all obligations arising from the trial have been fulfilled, subject to early termination for a material breach by a party of any of their contractual and/or legal obligations, or, in the case of any other type of breach, when the breaching party has been asked in writing to remedy the breach and the breach is not cured within thirty (30) days from the date on which the written request was sent.
On November 1, 2023, VCN and Sant Joan de Déu-Barcelona Children’s Hospital entered into an agreement for an exclusive worldwide option to negotiate an exclusive license of certain Sant Joan de Deu intellectual property rights related to the use of VCN-01 in combination with topoisomerase I inhibitor chemotherapies for the treatment of cancer. The collaboration builds on growing data that suggests coadministration of VCN-01 with topoisomerase I inhibitors such as topotecan can enhance VCN-01 replication and antitumor activity in preclinical cancer models. Combination of VCN-01 with a topoisomerase I inhibitor is expected to provide a synergistic antitumor effect wherein a chemotherapy-mediated increase in tumor VCN-01 levels may enable greater degradation of the tumor stroma, significantly increasing chemotherapy access and tumor destruction.
21
Washington University School of Medicine in St. Louis Clinical Trial Agreement
On August 7, 2019, we entered into a clinical trial agreement (“CTA”) with Washington University School of Medicine in St. Louis (“Washington University”) to conduct a Phase 1b/2a single-center, randomized, double-blinded, placebo-controlled clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of oral SYN-004 (ribaxamase) in up to 36 adult allogeneic hematopoietic cell transplant (HCT) recipients (the “Study”). Under the terms of the CTA, we will serve as the sponsor of the Study and supply SYN-004 (ribaxamase), as well as compensate Washington University for all research services to be provided in connection with the Study which is estimated to cost approximately $3,200,000. Dr. Erik R. Dubberke, Professor of Medicine and Clinical Director, Transplant Infectious Diseases at Washington University will serve as the principal investigator of the trial in collaboration with his Washington University colleague Dr. Mark A. Schroeder, Associate Professor of Medicine, Division of Oncology, Bone Marrow Transplantation and Leukemia.
The CTA continues in effect until completion of all obligations under the CTA. Either party may terminate the CTA prior to completion of its obligations (i) if authorization of the study is withdrawn by the FDA; (ii) if the emergence of any adverse reaction or side effect with SYN-004 (ribaxamase) administered in the Study is of such magnitude or incidence in the opinion of either party to support termination; or (iii) upon a breach of the terms of the CTA if the breaching party fails to cure the breach within 30 days after receipt of notice. We have the right to terminate the CTA (i) effective immediately if Washington University fails to perform the study in accordance with the terms of the protocol, the CTA or applicable laws or regulations or if Washington University or the principal investigator become debarred or (ii) upon 14 days written notice and Washington University has the right to terminate the CTA upon 14 days notice if the principal investigator becomes unable to perform or complete the Study and the parties have not, prior to the expiration of such fourteen (14) day period, agreed to an alternative principal investigator.
Massachusetts General Hospital Exclusive Option License Agreement
On May 27, 2020, we entered into an agreement with Massachusetts General Hospital (“MGH”) granting us an option for an exclusive license to intellectual property and technology related to the use of intestinal alkaline phosphatase (“IAP”) to maintain gastrointestinal (GI) and microbiome health, diminish systemic inflammation, and treat age-related diseases. If executed, we plan to use this license in the advancement of an expanded clinical development program for SYN-020, our proprietary recombinant version of bovine IAP currently in pre-clinical development. Under the terms of the agreement, we are granted exclusive rights to negotiate a worldwide license with MGH to commercially develop SYN-020 to treat and prevent metabolic and inflammatory diseases associated with aging. During the second quarter of 2021, we announced an amendment to our option for an exclusive license agreement with MGH to include intellectual property and technology related to the use of SYN-020 to inhibit liver fibrosis in select diseases, including NAFLD. To date, we have not exercised the option.
The University of Texas at Austin License Agreement and Sponsored Research Agreement
On December 19, 2012, we entered into a Patent License Agreement (the “Texas License Agreement”) with UT Austin for the exclusive license of the right to use, develop, manufacture, market and commercialize certain research and patents related to pertussis antibodies developed in the lab of Dr. Jennifer A. Maynard, Associate Professor of Chemical Engineering. In accordance with the terms of the Texas License Agreement we made the following payments to the UT Austin: a payment of past patent expenses, an annual payment of $50,000 per year commencing on the effective date through December 31, 2014 and a $25,000 payment on December 31, 2015. The Texas License Agreement also provides that UT Austin is entitled to milestone payments of $50,000 upon commencement of Phase 1 Clinical Trials, $100,000 upon commencement of Phase 3 Clinical Trials, $250,000 upon NDA submission in the United States, $100,000 upon European Medicines Agency approval and $100,000 upon regulatory approval in an Asian country. In addition, the University is entitled to a running royalty upon Net Product Sales and Net Service Sales (as defined in the Texas License Agreement and currently projected to be 2037 (not accounting for possible extensions)). The License Agreement terminates upon the expiration of the patent rights (as defined in the Texas License Agreement); provided, however that the Texas License Agreement is subject to early termination by us in our discretion and by the University for a breach of the Texas License Agreement by us.
22
In connection with the Texas License Agreement, we also entered into a Sponsored Research Agreement (the “Sponsored Research Agreement”) with the University pursuant to which the University will perform certain research work related to pertussis under the direction of Dr. Jennifer Maynard. All inventions conceived during such research shall be subject to the Texas License Agreement and we will obtain certain rights to patents and technology developed during the course of such research. We paid the University a fixed fee for the first year of $303,287 and the second and third years of $316,438 and $328,758, respectively. The termination date of the Sponsored Research Agreement n was amended multiple times and Sponsored Research Agreement expired on January 17, 2023. Upon a termination or due to a breach by the University, we were only be responsible for all reasonable expenses that do not exceed the fixed annual amount and that are incurred by the University prior to the termination date for services performed prior to the termination date.
We have an issued U.S. patent and patents pending in the U.S. and internationally (e.g. Europe, China, Japan, Australia, and China) on compositions and uses of SYN-005 that are co-owned by UT Austin and ourselves or licensed to us, and we have an issued U.S. patent and patent applications on other pertussis mAbs licensed from UT Austin.
Manufacturing
VCN-01 & VCN-11
Our oncolytic virus platform viruses (e.g. VCN-01, VCN-11) are biologics that can be readily synthesized by processes that we have developed in collaboration with Contract and Development Manufacturing Organizations (CDMOs) such as Thermo Fisher, BioReliance, GenIBET, and others. We do not own or operate manufacturing facilities for the production of our product candidates, VCN-01 and VCN-11, but we do produce and test viruses and virus processes at our facilities in Spain. Our cell and virus seed stocks and master/working cell banks are used for current and future production. Our cells for manufacturing are approved by and licensed from US regulatory authorities. Clinical and commercial supplies will be manufactured in facilities and by processes that comply with the FDA and other regulatory agency requirements. We plan to rely on third parties to manufacture commercial quantities of products that we successfully develop through regulatory approval. We have contracted with GenIbet and ThermoFisher to provide what we believe are adequate clinical supplies for our planned clinical trials.
Our upstream and downstream processes for producing oncolytic viruses are well understood in the industry and use industry standard cell factories and single use bioreactors for manufacturing. All downstream purifications employ single-use columns and filters, and release testing is performed by third-party vendors using qualified or validated assays. Critical quality attributes and other product testing specifications for our clinical supplies are agreed to with regulatory authorities prior to release and use.
We have previously encountered some delays in manufacturing due to the impact of COVID-19 on the supply chain. The potential impact of similar supply chain issues from a COVID-19 resurgence or other pandemic, if any, on our on-going and future clinical trials is currently unknown.
SYN-004 and SYN-020
Our product candidates SYN-004 and SYN-020 are biologics that can be readily synthesized by processes that we have developed; however, the manufacturing for our clinical programs, including SYN-004 and SYN-020 may require long lead times and has in the past been subject to COVID-19 related global supply chain interruptions. We do not own or operate manufacturing facilities for the production of these product candidates for preclinical and clinical activities. We rely on third-party contract manufacturers, and in most cases only one third-party, to manufacture critical raw materials, drug substance and final drug product for our research, preclinical development and clinical trial activities. Commercial quantities of any drugs we seek to develop will have to be manufactured in facilities and by processes that comply with the FDA and other regulations, and we plan to rely on third parties to manufacture commercial quantities of products we successfully develop through FDA approval.
Research and Development
During the years ended December 31, 2023 and 2022, we incurred approximately $14.3 million and $11.7 million, respectively, in research and development expenses.
23
Government Regulation
In the U.S., the formulation, manufacturing, packaging, storing, labeling, promotion, advertising, distribution and sale of our products are subject to regulation by various governmental agencies, including primarily the FDA. Our proposed activities may also be regulated by various agencies of the states, localities and foreign countries in which our proposed products may be manufactured, distributed and sold. The FDA, in particular, regulates the formulation, manufacture and labeling of prescription drugs, such as those that we intend to distribute. FDA regulations require us and our suppliers to meet relevant cGMP regulations for the preparation, packing, labeling, and storage of all drugs.
Any products manufactured or distributed by us pursuant to FDA approvals are subject to pervasive and continuing FDA regulation, including record-keeping requirements, reporting of adverse experiences, submitting periodic reports, drug sampling and distribution requirements, manufacturing or labeling changes, record-keeping requirements, and compliance with FDA promotion and advertising requirements. Drug manufacturers and their subcontractors are required to register their facilities with the FDA and state agencies, and are subject to periodic unannounced inspections for GMP compliance, imposing procedural and documentation requirements upon us and third-party manufacturers. Failure to comply with these regulations could result, among other things, in suspension of regulatory approval, recalls, suspension of production or injunctions, seizures, or civil or criminal sanctions. We cannot be certain that we or our present or future subcontractors will be able to comply with these regulations.
The FDA regulates prescription drug labeling and promotion activities in the United States. The FDA actively enforces regulations prohibiting the marketing of products for unapproved uses. The FDA permits the promotion of drugs for unapproved uses in certain circumstances, subject to stringent requirements. We and our product candidates are subject to a variety of state laws and regulations which may hinder our ability to market our products. Whether or not FDA approval has been obtained, approval by foreign regulatory authorities must be obtained prior to commencing clinical trials, and sales and marketing efforts in those countries. These approval procedures vary in complexity from country to country, and the processes may be longer or shorter than that required for FDA approval. We may incur significant costs to comply with these laws and regulations now or in the future.
The FDA, comparable foreign regulators and state and local pharmaceutical regulators impose substantial requirements upon clinical development, manufacture and marketing of pharmaceutical products. These and other entities regulate research and development and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising, and promotion of our products. The drug approval process required by the FDA under the Food, Drug, and Cosmetic Act and Public Health Service Act (for biologics) generally involves:
| ● | preclinical laboratory and animal tests; |
| ● | submission of an IND, prior to commencing human clinical trials; |
| ● | adequate and well-controlled human clinical trials to establish safety and efficacy for intended use; |
| ● | submission to the FDA of an NDA or BLA; and |
| ● | FDA review and approval of an NDA or BLA. |
The testing and approval process requires substantial time, effort, and financial resources, and we cannot be certain that any approval will be granted on a timely basis, if at all. OVs such as VCN-01 are genetically modified organisms and their import and use are subject to additional review and approval by dedicated agencies in some countries where we propose to run clinical trials, including Spain and other European countries.
Preclinical tests include laboratory evaluation of the product candidate, its chemistry, formulation and stability, and animal studies to assess potential safety and efficacy. Certain preclinical tests must be conducted in compliance with good laboratory practice regulations. Violations of these regulations can, in some cases, lead to invalidation of the studies, requiring them to be replicated. In some cases, long-term preclinical studies are conducted concurrently with clinical studies.
24
We will submit the preclinical test results, together with manufacturing information and analytical data, to the FDA as part of an IND, which must become effective before we begin human clinical trials. The IND automatically becomes effective 30 days after filing, unless the FDA raises questions about conduct of the trials outlined in the IND and imposes a clinical hold, in which case, the IND sponsor and FDA must resolve the matters before clinical trials can begin. It is possible that our submission may not result in FDA authorization to commence clinical trials. The timing and requirements of IND review may differ from the FDA in other countries, potentially delaying study initiation at sites in those countries.
Clinical trials must be supervised by qualified investigators in accordance with current good clinical practice (cGCP) regulations, which include informed consent requirements. Each study must be approved and monitored by the appropriate Institutional Review Boards (IRBs) or ethics committees (ECs) which are periodically informed of the study’s progress, adverse events and changes in research. OVs such as VCN-01 are genetically modified organisms and their use is also subject to review and approval by the Institutional Biosafety Committee (IBC) at each clinical trial site. Annual updates are submitted to the FDA and comparable foreign regulators (if required) with more frequent reporting if certain serious adverse events occur.
Human clinical trials of drug candidates typically have three sequential phases that may overlap:
Phase 1: The drug is initially tested in healthy human subjects or patients for safety, dosage tolerance, absorption, metabolism, distribution, and excretion.
Phase 2: The drug is studied in a limited patient population to identify possible adverse effects and safety risks, determine efficacy for specific diseases and establish dosage tolerance and optimal dosage.
Phase 3: When Phase 2 evaluations demonstrate that a dosage range is effective with an acceptable safety profile, Phase 3 trials to further evaluate dosage, clinical efficacy and safety, are undertaken in an expanded patient population, often at geographically dispersed sites.
We cannot be certain that we will successfully complete Phase 1, Phase 2, or Phase 3 testing of our product candidates within any specific time period, if at all. Furthermore, the FDA or comparable foreign regulator, an IRB/EC or the IND sponsor may suspend clinical trials at any time on various grounds, including a finding that subjects or patients are exposed to unacceptable health risk. Under the Pediatric Research Equity Act, we also must prepare, within 60 days of an End of Phase 2 meeting, a pediatric study plan or request for waiver or deferral of pediatric studies in the indication under development. Concurrent with these trials and studies, we also develop chemistry and physical characteristics data and finalize a manufacturing process in accordance with cGMP requirements. The manufacturing process must conform to consistency and quality standards, and we must develop methods for testing the quality, purity, and potency of the final products. Appropriate packaging is selected and tested, and chemistry stability studies are conducted to demonstrate that the product does not undergo unacceptable deterioration over its shelf-life. Results of the foregoing are submitted to the FDA as part of an NDA (or BLA in case of biologic products) for marketing and commercial shipment approval. The FDA reviews each NDA or BLA submitted and may request additional information. A 60-day period after the sponsor’s submission of an NDA or BLA is used by the FDA to determine whether the application is sufficiently complete to permit substantive review, in which case the application is accepted for filing. The timing and requirements of NDA or BLA review may differ from the FDA in other countries,
Once the FDA accepts the NDA or BLA for filing, it begins its in-depth review. The FDA has substantial discretion in the approval process and may disagree with our interpretation of the data submitted or identify new concerns. The process may be significantly extended by requests for new information or clarification of information already submitted. As part of this review, the FDA may refer the application to an advisory committee, typically a panel of clinicians. Manufacturing establishments often are inspected prior to NDA or BLA approval to assure compliance with GMPs and with manufacturing commitments made in the application.
Submission of an NDA or BLA with clinical data requires payment of a substantial fee. In return, the FDA assigns a goal for review and decision on the application, in which the FDA may approve or deny the NDA or BLA, or issue a complete response letter outlining information needed to support approval, including a potential need for additional clinical data. Even if these data are submitted, the FDA may ultimately decide the NDA or BLA does not satisfy approval criteria. If the FDA approves the NDA or BLA, the product becomes available for marketing. Product approval may be withdrawn if regulatory compliance is not maintained or safety problems occur. The FDA may require post-marketing studies, also known as Phase 4 studies, as a condition of approval, and Risk Evaluation and Mitigation Strategies (REMS) requires surveillance programs to monitor approved products that have been commercialized. The agency has the power to require changes in labeling or prohibit further marketing based on the results of post-marketing surveillance.
25
Satisfaction of these and other regulatory requirements typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures on our activities. We cannot be certain that the FDA or other regulatory agencies will approve any of our products on a timely basis, if at all. Success in preclinical or early-stage clinical trials does not assure success in later-stage clinical trials. Data obtained from preclinical and clinical activities are not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval. Even if a product receives regulatory approval, the approval may be significantly limited to specific indications or uses.
Even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain regulatory approvals would have a material adverse effect on our business.
The FDA’s or comparable foreign regulatory agency may change their policies, and additional government regulations may be enacted which could prevent or delay regulatory approval of our potential products. Increased attention to the containment of health care costs worldwide could result in new government regulations materially adverse to our business. Public perception and sentiment regarding genetically modified organisms and/or viral therapies (including vaccines) can be highly variable and may impact legislation regarding the potential sue of our products. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the U.S. or abroad.
Orphan Drug Act
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the name of the sponsor, identity of the drug or biologic and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not shorten the duration of the regulatory review or approval process, but does provide certain advantages, such as a waiver of Prescription Drug User Fee Act (“PDUFA”) fees, enhanced access to FDA staff and potential waiver of pediatric research requirements.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same drug or biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the application user fee. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Orphan Drug Designation is also available in Europe from the European Medicines Agency (EMA) and provides for 10 years of market exclusivity if granted. The requirements, costs and timing for obtaining and maintaining EMA Orphan Drug Designation differ from the FDA.
In May 2011, the Committee for Orphan Medicinal Products ("COMP") from the EMA recommended granting Orphan Medicinal Product Designation to VCN-01 for the treatment of pancreatic cancer and in June 2011, the European Commission confirmed the designation under Regulation ("EC") No 141/2000 of the European Parliament and of the Council.
In February 2022, the FDA granted Orphan Drug designation to VCN-01 for the treatment of retinoblastoma.
In June 2023, the FDA granted Orphan Drug Designation to VCN-01 for the treatment of pancreatic cancer.
26
Other Healthcare Laws and Compliance Requirements
In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. The federal Anti-Kickback Statute prohibits any person, including a prescription drug manufacturer (or a party acting on its behalf), from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce or reward either the referral of an individual, or the furnishing, recommending or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In addition, various states have enacted anti-kickback statues and false claims laws analogous to the False Claims Act. Also, the Health Insurance Portability and Accountability Act of 1996 (HIPAA) created several federal crimes, including healthcare fraud, and false statements relating to the delivery of or payments for healthcare benefits, items or services. HIPAA and its implementing regulations also established uniform federal standards for certain “covered entities” (healthcare providers, health plans and healthcare clearinghouses) governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of protected health information.
Because of the breadth of these and other laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties, damages, fines, imprisonment, exclusion from participation in government healthcare programs, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, private “qui tam” actions brought by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government contracts, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
In order to market any product outside of the United States, a company also must comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, an applicant will need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can initiate clinical trials or market products in those countries or jurisdictions. Specifically, the process governing approval of medicinal products in the EU generally follows the same lines as in the United States. It entails satisfactory completion of pharmaceutical development, nonclinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. It also requires the submission to relevant competent authorities for clinical trials authorization and to the EMA or to competent authorities in EU Member States for a marketing authorization application, or MAA, and granting of a marketing authorization by competent authorities in EU Member States or the European Commission before the product can be marketed and sold in the EU.
27
Data Privacy
Strict data privacy laws regulating the collection, transmission, storage and use of employee data and consumers’ personally-identifying information are evolving in the European Union, U.S. and other jurisdictions in which we operate. Outside of the United States, the laws, regulations and standards in many jurisdictions apply broadly to the collection, use, and other processing of personal information. For example, in the European Union, the collection and use of personal data are governed by the provisions of the General Data Protection Regulation (the “GDPR”). The GDPR, together with national legislation, regulations and guidelines of the European Union. member states governing the processing of personal data, impose strict obligations on entities subject to the GDPR, including but not limited to: (i) accountability and transparency requirements, and enhanced requirements for obtaining valid consent from data subjects; (ii) obligations to consider data protection as any new products or services are developed and to limit the amount of personal data processed; (iii) obligations to comply with the data protection rights of data subjects; and (iv) obligations to report certain personal data breaches to governmental authorities and individuals. Data protection authorities from the different E.U. member states and other European countries may enforce the GDPR and national data protection laws differently, and introduce additional national regulations and guidelines, which adds to the complexity of processing European personal data. Failure to comply with the requirements of the GDPR and the related national data protection laws may result in significant monetary fines and other administrative penalties (the GDPR authorizes fines for certain violations of up to 4% of global annual revenue or €20 million, whichever is greater) as well as civil liability claims from individuals whose personal data was processed. Additionally, expenses associated with compliance could reduce our operating margins.
The GDPR also prohibits the transfer of personal data from the E.U. to countries outside of the E.U. unless made to a country deemed by the European Commission to provide adequate protection for personal data or accomplished by means of an approved data transfer mechanism (e.g., standard contractual clauses). Data protection authority guidance and enforcement actions that restrict companies’ ability to transfer data may increase risk relating to data transfers or make it more difficult or impossible to transfer E.U. personal data to the U.S.
Competitive Environment
The pharmaceutical and biotechnology industries are characterized by rapidly evolving technology and intense competition. Our competitors include major multi-national pharmaceutical companies and biotechnology companies developing both generic and proprietary therapies to treat serious diseases. Many of these companies are well-established and possess technical, human, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, many of our potential competitors have formed strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that afford these companies potential research and development and commercialization advantages in the therapeutic areas we are currently pursuing.
Academic research centers, governmental agencies and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those being developed by us. In addition, many of these competitors may be able to obtain patent protection, obtain FDA and other regulatory approvals and begin commercial sales of their products before us.
Our oncology product candidates compete with all other oncology products being developed for the indications that we are focusing oncolytic virus (OV) products being developed by third parties.
Only three oncolytic virus (OV) products have been approved in different global markets. Amgen Inc.’s Imlygic® (T-VEC, OncoVEX) for melanoma (USA); Daiichi Sankyo Company, Limited‘s DELYTACT® for malignant glioma (Japan) and Shanghai Sunway Biotech Co., Ltd Oncorine® for patients with late-stage refractory nasopharyngeal cancer (China).
More than 60 companies have publicly identified that they are pursuing clinical development of different forms of OV products. Adenoviruses are the most commonly used viruses in these programs, with modified adenoviruses under development by companies including AdCure Bio LLC, Calidi Biotherapeutics, Inc.,Candel Therapeutics, Inc., CG Oncology, Inc., Elicera Therapeutics AB, EpicentRx, Inc., GeneMedicine, Co Ltd., IconOVir Bio, Inc., Lokon Pharma AB, Memgen, Inc., Multivir, Inc., NewGenPharm Incorporation, Oncolys BioPharma, Inc., Orca Therapeutics B.V., Akamis Bio Ltd (formerly PsiOxus Therapeutics Ltd), Shanghai Sunway Biotech Co., Ltd, Circio Holding ASA (formerly Targovax Oy|Targovax ASA), Teolytics Ltd, Tessa Therapeutics, TILT Biotherapeutics, Ltd., and Valo Therapeutics Oy.
28
OV products have been or are being developed using other virus backbones, including: arenavirus (Hookipa Pharma, Inc.), Coxsackie virus (Viralytics Ltd., Oncorus Inc.); herpes simplex virus (Amgen, Inc., Candel Therapeutics, Inc., Daiichi Sankyo Company Ltd.,ImmVira Co. Ltd, Replimune, Inc., Takara Bio, Inc., Treovir LLC, Virogin Biotech, Inc. Wuhan Binhui Biotechnology Co., Ltd.); Maraba virus (Turnstone Biologics, Inc.); measles virus (Themis Biosciences GmbH, Vyriad, Inc.); myxoma virus (OncoMyx Therapeutics, Inc.); parvovirus (Oryx GmbH & Co. KG), reovirus (Oncolytics Biotech, Inc.); poliovirus (Istari Oncology, Inc.);Seneca Valley virus (Seneca Therapeutics Inc., Oncorus Inc.); vesicular stomatitis virus (Boehringer Ingelheim, Cytonus Therapeutics, Inc., Vyriad, Inc.); and vaccinia viruses (Genelux Corporation, Imugene Ltd, Joint Biosciences Ltd, KaliVir Immunotherapeutics LLC, SillaJen, Inc., Transgene SA, Turnstone Biologics, Corp.).
OV companies that have identified pancreatic cancer or PDAC as a proposed clinical indication include Akamis Bio Ltd, Boehringer Ingelheim GmbH, Candel Therapeutics, Inc., GeneMedicine, Co Ltd., Lokon Pharma AB, Memgen, Inc., NewGenPharm Incorporation, Oncolytics Biotech, Oryx GmbH & Co. KG, Takara Bio, Inc., TILT Biotherapeutics Ltd), V2ACT Therapeutics™ LLC (a Genelux Corporation joint venture), Virogin Biotech, Inc.,and Wuhan Binhui Biotechnology Co., Ltd. OV companies that have identified retinoblastoma as a potential target indication include Seneca Therapeutics Inc. and Shanghai Sunway Biotech Co., Ltd.
Theriva Biologics’ OV products are designed to be systemically, intratumorally or intravitreally injected; selectively replicate only in tumor cells versus normal host cells; have reduced liver tropism compared to wild type adenovirus type 5; and express an enzyme (PH20 hyaluronidase) that degrades the tumor stroma barrier. If confirmed in Phase 2 and later clinical trials, we believe these features significantly differentiate Theriva Biologics’ products from competing OVs and will enable our products to be co-administered with other therapeutic modalities such as chemotherapy and immuno - oncology products to improve cancer treatment outcomes.
Companies that currently sell or are developing proprietary products for the prevention and treatment of C. difficile infection include: Actelion Pharmaceutical Ltd., Artugen Therapeutics, Inc., AzurRx, Inc., Deinove, Pfizer Inc., Merck & Co. Inc., Merus B.V., Pfizer Inc., Rebiotix, Inc., Seres Therapeutics, Inc., Summit Therapeutics plc. and Vedanta Biosciences Inc. Companies that sell or are developing products for the treatment or prevention of acute graft - versus - host - disease (aGVHD) include: Amgen, Inc., Astellas Pharma, Janssen Biotech, Inc., Mallinckrodt plc, Mesoblast, Inc., Novartis International AG, Pfizer, Inc. Roche AG and Takeda Pharmaceutical Company Ltd.
Not only do our product candidates compete with other product candidates being developed for similar or the same indications, we also compete for employees and for clinical trial sites.
Corporate History
Our predecessor, Sheffield Pharmaceuticals, Inc., was incorporated in 1986, and in 2006 engaged in a reverse merger with Pipex Therapeutics, Inc., a publicly-traded Delaware corporation formed in 2001. After the reverse merger, we changed our name to Pipex Pharmaceuticals, Inc., and in October 2008 we changed our name to Adeona Pharmaceuticals, Inc. On October 15, 2009, we engaged in a merger with a wholly owned subsidiary for the purpose of reincorporating in the State of Nevada. On February 15, 2012, we changed our name to Synthetic Biologics, Inc. On August 10, 2018, we effected a one for thirty-five reverse stock split of our authorized, issued and outstanding common stock. On July 15, 2022, we effected a one for ten reverse stock split of our authorized, issued and outstanding common stock. On October 12, 2022, we changed our name to Theriva Biologics, Inc.
29
Human Capital
We believe that our success depends upon our ability to attract, develop and retain key personnel. As of March 25, 2024, we employed 22 individuals, all but one of whom are full-time employees, of which 6 were part of our research and clinical development team in the United States and 9 are part of VCN’s research and clinical development team located in Spain, 2 are part of VCN’s management team located in Spain and 4 are part of our financial reporting and accounting team located in the United States.
A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. None of our employees in the United States are covered by collective bargaining agreements, and management considers relations with our employees to be in good standing. As is the usual situation in Spain, all the employees are currently covered by a collective bargaining system specific for the pharma sector. Although we continually seek to add additional talent to our work force, management believes that it has sufficient human capital to operate its business successfully.
Competitive Pay and Benefits
Our compensation programs are designed to align the compensation of our employees with our performance and to provide the proper incentives to attract, retain and motivate employees to achieve superior results. The structure of our compensation programs balances incentive earnings for both short-term and long-term performance. Specifically:
| ● | we provide employee wages that are competitive and consistent with employee positions, skill levels, experience, knowledge and geographic location; |
| ● | we engage nationally recognized outside compensation and benefits consulting firms to independently evaluate the effectiveness of our executive compensation and benefit programs and to provide benchmarking against our peers within the industry; |
| ● | we align our executives’ long-term equity compensation with our shareholders’ interests by linking realizable pay with stock performance; and |
| ● | all employees are eligible for health insurance, paid and unpaid leaves, a retirement plan and life and disability/accident coverage. We also offer a variety of voluntary benefits that allow employees to select the options that meet their needs, including flexible time-off, telemedicine, and unpaid parental leave. |
Health and Safety
The health and safety of our employees is our highest priority, and this is consistent with our operating philosophy. Accordingly, with the global spread of the ongoing novel coronavirus pandemic, we have implemented plans designed to address and mitigate the impact of the COVID-19 pandemic on the safety of our employees and our business, which include:
| ● | adding work from home flexibility; |
| ● | adjusting attendance policies to encourage those who are sick to stay home; |
| ● | increasing cleaning protocols across all locations; and |
| ● | initiating regular communication regarding impacts of the COVID-19 pandemic, including health and safety protocols and procedures. |
30
Available Information
Additional information about Theriva Biologics is contained at our website, www.therivabio.com. Information contained on our website is not incorporated by reference into, and does not form any part of, this Annual Report. We have included our website address as a factual reference and do not intend it to be an active link to our website. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act are available free of charge through the investor relations page of our internet website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (the “SEC”). The following Corporate Governance documents are also posted on our website: Code of Conduct, Code of Ethics for Financial Management and the Charters for the Audit Committee, Compensation Committee and Nominations Committee of the Board of Directors. Our phone number is (301) 417-4364 and our facsimile number is (301) 417-4367. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Commission. The address of that website is www.sec.gov.
Item 1A. Risk Factors.
Investing in our securities involves a high degree of risk. In addition to the risks related to our business set forth in this Annual Report and the other information included in this Annual Report, you should carefully consider the risks described below before purchasing our securities. Additional risks, uncertainties and other factors not presently known to us or that we currently deem immaterial may also impair our business operations.
RISKS RELATED TO OUR FINANCIAL POSITION AND CAPITAL REQUIREMENTS
Our auditor’s report on our consolidated financial statements contains an explanatory paragraph regarding our ability to continue as a going concern.
Our consolidated financial statements as of December 31, 2023 have been prepared under the assumption that we will continue as a going concern for the next twelve months. In addition, our independent registered public accounting firm has issued a report that includes an explanatory paragraph referring to our recurring losses from operations (anticipated continued losses in the future) and net capital deficiency that raise substantial doubt in our ability to continue as a going concern without additional capital becoming available. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Our consolidated financial statements as of December 31, 2023 did not include any adjustments that might result from the outcome of this uncertainty. We expect that our current cash will be able to fund operations through the fourth quarter of 2024 and into the first quarter of 2025 but will not be sufficient to fund operations for twelve months from the date of the filing of this Annual Report.
We will need to raise additional capital to operate our business and our failure to obtain funding when needed may force us to delay, reduce or eliminate certain of our development programs or commercialization efforts.
During the year ended December 31, 2023, our operating activities used net cash of approximately $19.0 million and as of December 31, 2023 our cash and cash equivalents were $23.2 million. With the exception of the three months ended December 31, 2017 and June 30, 2010, we have experienced significant losses since inception and have a significant accumulated deficit. As of December 31, 2023, our accumulated deficit totaled approximately $309.3 million on a consolidated basis. Pursuant to the VCN Purchase Agreement, we have agreed to use reasonable efforts to commercialize VCN-01 and we agreed as a post- closing covenant to commit to fund VCN's research and development programs, including but not limited to VCN-01 PDAC Phase 2 clinical trial, VCN-01 RB pivotal trial and necessary G&A within a budgetary plan of approximately $27.8 million over the next three years. We expect to incur additional operating losses in the future and therefore expect our cumulative losses to increase. We do not expect to derive revenue from any source in the near future until we or our potential partners successfully commercialize our products. We expect our expenses to increase in connection with our anticipated activities, particularly as we continue research and development, initiate and conduct clinical trials, and seek marketing approval for our product candidates. Until such time as we receive approval from the FDA and other regulatory authorities for our product candidates, we will not be permitted to sell our products and therefore will not have product revenues from the sale of products. For the foreseeable future we will have to fund all of our operations and capital expenditures from equity and debt offerings, cash on hand, licensing and collaboration fees and grants, if any.
31
We expect that our current cash will be able to fund operations through the fourth quarter of 2024 and into the first quarter of 2025 but will not be sufficient to fund operations for twelve months from the date of the filing of this Annual Report. We will need to raise additional capital to fund our operations and meet our current timelines and we cannot be certain that funding will be available on acceptable terms on a timely basis, or at all. Based on our current plans, our cash and cash equivalents will be sufficient to complete our planned clinical trials of VCN-01 (in PDAC and retinoblastoma), Phase 1a/2a clinical trial of SYN-004, but will not be sufficient for additional trials of VCN-01, SYN-020 or SYN-004, which are expected to require significant cash expenditures. In addition, based on the significant anticipated cost of a Phase 3 clinical program in a broad indication for SYN-004, we expect it will not be feasible for us to initiate and complete this trial at this time without a partner given the capital constraints tied to our current market cap and share price. As part of our strategic transformation into an oncology focused company, we are exploring value creation options for our SYN-004 and SYN-020 assets, including out-licensing or partnering. Further development of VCN’s product candidates will require additional funding. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that may impact our ability to conduct our business and also have a dilutive effect on our stockholders. A failure otherwise to secure additional funds when needed in the future whether through an equity or debt financing or a sufficient amount of capital without a strategic partnership could result in us being unable to complete planned preclinical and clinical trials or obtain approval of our product candidates from the FDA and other regulatory authorities. In addition, we could be forced to delay, discontinue or curtail product development, forego sales and marketing efforts, and forego licensing in attractive business opportunities. Our ability to raise capital through the sale of securities may be limited by the rules of the SEC and NYSE American that place limits on the number and dollar amount of securities that may be sold. There can be no assurances that we will be able to raise the funds needed, especially in light of the fact that our ability to sell securities registered on our registration statement on Form S-3 will be limited until such time the market value of our voting securities held by non-affiliates is $75 million or more. We also may be required to seek collaborators for our product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available.
We expect to continue to incur significant operating and capital expenditures and we will need additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all, which would force us to delay, reduce or suspend our research and development programs and other operations or commercialization efforts.
We have a history of losses and we have incurred, and will continue to incur, substantial losses and negative operating cash flow. Even if we succeed in developing and commercializing one or more of our product candidates, we may still incur substantial losses for the foreseeable future and may not sustain profitability. We anticipate a need for additional employees as we undertake later stage clinical trials. We have also incurred certain obligations pursuant to the terms of the VCN Purchase Agreement including the assumption of $2.4 million of liabilities and have agreed to a post-closing covenant to commit to fund research and development of VCN-01 and OV pipeline programs, including but not limited to the VCN-01 PDAC Phase 2 trial, a VCN-01 RB pivotal trial and necessary G&A within a budgetary plan of approximately $27.8 million.
Further development of VCN-01 and pipeline OV product candidates will require additional expenditures. We also expect to continue to incur significant operating and capital expenditures and anticipate that our expenses will substantially increase in the foreseeable future as we do the following:
| ● | continue to undertake preclinical development of our OV pipeline and mid and late-stage clinical trials for our product candidates, including VCN-01; |
| ● | seek regulatory approvals for our product candidates; |
| ● | develop our product candidates for commercialization; |
| ● | implement additional internal systems and infrastructure; |
| ● | license or acquire additional technologies; |
| ● | lease additional or alternative office facilities; |
| ● | manufacture product for clinical trials and commercial use; and |
| ● | hire additional personnel, including members of our management team. |
32
We may experience negative cash flow for the foreseeable future as we fund our development and clinical programs with capital expenditures. As a result, we will need to raise additional capital or generate significant revenues in order to achieve and maintain profitability. We may not be able to generate these revenues or achieve profitability in the future. Our failure to achieve or maintain profitability, which we do not anticipate will occur in the near future, could negatively impact the value of our common stock and underlying securities. There can be no assurance that funding will be available on acceptable terms on a timely basis, or at all. The various ways that we could raise capital carry potential risks. Any additional sources of financing will likely involve the issuance of our equity securities, which will have a dilutive effect on our stockholders. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or tests or grant licenses on terms that are not favorable to us.
The actual amount of funds we will need to operate is subject to many risk factors, some of which are beyond our control.
The actual amount of funds we will need to operate is subject to many factors, some of which are beyond our control. These factors include the following:
| ● | the progress of our research activities and ability to attract patients; |
| ● | the number and scope of our research programs; |
| ● | the progress of our preclinical and clinical development activities; |
| ● | the progress of the development efforts of parties with whom we have entered into research and development agreements and amount of funding received from partners and collaborators; |
| ● | our ability to maintain current research and development licensing arrangements and to establish new research and development and licensing arrangements; |
| ● | our ability to achieve our milestones under licensing arrangements; |
| ● | the costs associated with manufacturing-related services to produce materials for use in our clinical trials; |
| ● | the costs involved in prosecuting and enforcing patent claims and other intellectual property rights; |
| ● | the costs incurred to screen and enroll patients; and |
| ● | The costs and timing of regulatory approvals. |
We have based our estimate on assumptions that may prove to be wrong. We may need to obtain additional funds sooner or in greater amounts than we currently anticipate. Potential sources of financing include strategic relationships, public or private sales of our shares or debt and other sources. Additionally, we may seek to access the public or private equity markets when conditions are favorable due to our long-term capital requirements. We do not have any committed sources of financing at this time, and it is uncertain whether additional funding will be available when we need it on terms that will be acceptable to us, or at all.
We currently have a limited operating history as an oncology company, no products approved for commercial sale, have no significant source of revenue and may never generate significant revenue.
We are a clinical-stage biopharmaceutical company that began to focus on development of oncolytic viruses for treatment of various types of cancer in 2022. We have never generated any product revenue, do not expect to generate revenue in the near future and do not have any products approved for sale. Our operations to date have been primarily focused on developing our product candidates. We have not yet successfully obtained marketing approval, manufactured any product candidate at commercial scale, or conducted sales and marketing activities that will be necessary to successfully commercialize our product candidates. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing product candidates.
33
All of our existing product candidates are in various stages of development and will require extensive additional clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before they could provide us with any revenue. As a result, even if we successfully develop, achieve regulatory approval and commercialize our products, we may be unable to generate revenue for many years, if at all. We do not anticipate that we will generate revenue from product sales for at least several years, if at all. If we are unable to generate revenue from product sales, we will not become profitable, and we may be unable to continue our operations.
Our ability to generate revenue depends heavily on:
| ● | our ability to raise additional capital on a timely basis to continue to fund our clinical trials; |
| ● | demonstration in current and future clinical trials that our lead product candidate, VCN-01, as well as each of our other product candidates, is safe and effective;and |
| ● | our ability to seek and obtain regulatory approvals, including with respect to the indications we are seeking; |
Even if we receive regulatory approval for the sale of any of our product candidates, we do not know when we will begin to generate revenue, if at all. Our ability to generate revenue depends on a number of factors, including our ability to:
| ● | set an acceptable price for our products and obtain coverage and adequate reimbursement from third-party payors; |
| ● | establish sales, marketing, manufacturing and distribution systems; |
| ● | add operational, financial and management information systems and personnel, including personnel to support our clinical, manufacturing and planned future clinical development and commercialization efforts and operations as a public company; |
| ● | develop manufacturing capabilities for bulk materials and manufacture commercial quantities of product candidates at acceptable cost levels; |
| ● | achieve broad market acceptance of our product candidates in the medical community and with third-party payors and consumers; |
| ● | attract and retain an experienced management and advisory team; |
| ● | successfully launch commercial sales of our products, whether alone or in collaboration with others; and |
| ● | maintain, expand and protect our intellectual property portfolio. |
Because of the numerous risks and uncertainties associated with development and manufacturing, we are unable to predict if we will generate revenue. If we cannot successfully execute on any of the factors listed above, our business may not succeed, we may never generate revenue and your investment will be adversely affected.
34
We have identified material weaknesses in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated or that additional material weaknesses will not occur in the future
If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results, prevent fraud, or file our periodic reports in a timely manner, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price. Our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rule 13a- 15(f) under the Exchange Act. Based on our assessment, we have concluded that we did not maintain effective review controls at a sufficient level of precision with certain financial statement areas and over unusual transactions involving complex accounting and related.disclosure requirements. We also did not maintain effective information technology general controls over user access, program change management, and segregation of duties, within certain key information systems supporting the Company’s accounting and financial reporting processes. Additionally, many of our business process controls dependent upon the information derived from these information systems were also ineffective, as we did not design and implement controls to validate the completeness and accuracy of underlying data utilized in the operation of those controls. While we plan to take remedial action to address the material weaknesses, we cannot provide any assurance that such remedial measures, or any other remedial measures we take, will be effective. If we fail to maintain effective internal control over financial reporting, we may not be able to accurately report our financial results, detect or prevent fraud, or file our periodic reports in a timely manner, which may, among other adverse consequences, cause investors to lose confidence in our reported financial information and lead to a decline in our stock price. In addition, a material weakness will not be considered remediated until the applicable controls operate for a sufficient period of time and management has concluded, through testing, that these controls are designed and operating effectively. Although management believes that the material weaknesses will be remediated by the end of the fiscal year there can be no assurance that the deficiencies will be remediated at such time or that the internal control over financial reporting, as modified, will enable us to identify or avoid material weaknesses in the future.
We expect to seek to raise additional capital in the future, which may be dilutive to stockholders or impose operational restrictions.
We expect to seek to raise additional capital in the future to help fund development of our proposed products. If we raise additional capital through the issuance of equity or of debt securities, the percentage ownership of our current stockholders will be reduced. We may also enter into strategic transactions, issue equity as consideration for acquisitions or part of license issue fees to our licensors, compensate consultants or settle outstanding payables using equity that may be dilutive. We are authorized to issue 350,000,000 shares of common stock, of which 17,148,049 shares of common stock were outstanding as of December 31, 2023. At December 31, 2023, we had reserved 6,834,797 shares of common stock for issuance upon exercise of our outstanding options, and preferred shares. In addition, at such date, we had 2,822,845 shares of our common stock reserved for future issuance under our equity incentive plans. If all of these securities were to be exercised, the total number of shares of our common stock that we would be required to issue is 9,657,642, which in addition to the 17,148,049 shares outstanding, would leave 323,194,309 authorized but unissued shares of common stock available to be issued.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share paid by existing stockholders, thereby subjecting such stockholders to dilution. Our stockholders may experience additional dilution in net book value per share and any additional equity securities may have rights, preferences and privileges senior to those of the holders of our common stock.
We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by existing stockholders, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by existing stockholders.
35
Our operating results may fluctuate significantly, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or our guidance.
Our quarterly and annual operating results may fluctuate significantly in the future, which makes it difficult for us to predict our future operating results. The VCN Purchase Agreement requires that we make certain cash payments to Grifols upon attainment of certain milestones, which payments may vary significantly from period to period and any such variance could cause a significant fluctuation in our operating results from one period to the next. From time to time, we may enter into collaboration agreements with other companies that include development funding and significant upfront and milestone payments and/or royalties, which may become an important source of our revenue. Accordingly, our revenue may depend on development funding and the achievement of development and clinical milestones under any potential future collaboration and license agreements and sales of our products, if approved. These upfront and milestone payments may vary significantly from period to period and any such variance could cause a significant fluctuation in our operating results from one period to the next. In addition, our manufacturing and clinical trial expenses, which are anticipated to be significant, may fluctuate significantly quarter to quarter based upon whether or not we are engaged in clinical trials or manufacturing our product candidates, and timing of our process development work. Furthermore, we measure compensation cost for stock-based awards made to employees at the grant date of the award, based on the fair value of the award as determined by our board of directors, and recognize the cost as an expense over the employee's requisite service period. As the variables that we use as a basis for valuing these awards change over time, our underlying stock price and stock price volatility, the magnitude of the expense that we must recognize may vary significantly. Furthermore, our operating results may fluctuate due to a variety of other factors, many of which are outside of our control and may be difficult to predict, including the following:
| ● | the timing and cost of, and level of investment in, research and development activities relating to current product candidates and any future product candidates, which will change from time to time; |
| ● | our ability to enroll patients in clinical trials and the timing of enrollment; |
| ● | the timing and cost of manufacturing our current product candidates and any future product candidates, which may vary depending on FDA guidelines and requirements, the quantity of production and the terms of our agreements with manufacturers; |
| ● | expenditures that we will or may incur to acquire or develop additional product candidates and technologies; |
| ● | the timing and outcomes of clinical studies or competing product candidates; |
| ● | changes in the competitive landscape of our industry, including consolidation among our competitors or partners; |
| ● | any delays in regulatory review or approval of our current product candidates or any of our future product candidates; |
| ● | the level of demand for our current product candidates and any future product candidates, should they receive approval, which may fluctuate significantly and be difficult to predict; |
| ● | the risk/benefit profile, cost and reimbursement policies with respect to our products candidates, if approved, and existing and potential future drugs that compete with our product candidates; |
| ● | competition from existing and potential future drugs that compete with our current product candidates or any of our future product candidates; |
| ● | our ability to commercialize our current product candidates or any future product candidate inside and outside of the United States, either independently or working with third parties; |
| ● | our ability to establish and maintain collaborations, licensing or other arrangements; |
| ● | our ability to adequately support future growth; |
| ● | potential unforeseen business disruptions that increase our costs or expenses; |
36
| ● | future accounting pronouncements or changes in our accounting policies; and |
| ● | the changing and volatile global economic environment. |
The cumulative effects of these factors could result in large fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance. This variability and unpredictability could also result in our failing to meet the expectations of industry or financial analysts or investors for any period. If our revenue or operating results fall below the expectations of analysts or investors or below any forecasts we may provide to the market, or if the forecasts we provide to the market are below the expectations of analysts or investors, the price of our common stock could decline substantially. Such a stock price decline could occur even when we have met any previously publicly stated revenue and/or earnings guidance we may provide.
If our acquired intangible assets become impaired, we may be required to record a significant charge to earnings.
We regularly review acquired intangible assets for impairment when events or changes in circumstances indicate that the carrying value may not be recoverable. We test goodwill and indefinite-lived intangible assets for impairment at least annually. Factors that may be considered a change in circumstances, indicating that the carrying value of the intangible assets may not be recoverable, include: macroeconomic conditions, such as deterioration in general economic conditions; industry and market considerations, such as deterioration in the environment in which we operate; cost factors, such as increases in labor or other costs that have a negative effect on earnings and cash flows; our financial performance, such as negative or declining cash flows or a decline in actual or planned revenue or earnings compared with actual and projected results of relevant prior periods; other relevant entity-specific events, such as changes in management, key personnel, strategy, or customers; and sustained decreases in share price.
RISKS RELATED TO OUR BUSINESS
Prior to 2022 we had not conducted any cancer research and development activities and there can be no assurance that we will successfully be able to do so.
Prior to the VCN Acquisition, our focus was on the microbiome and our research and development was focused primarily on therapeutics for various microbiome related diseases. Upon the VCN Acquisition, our focus has shifted to the use of oncolytic viruses to treat cancer. Although, our members of management and scientific/development teams have experience in this field, we may not be successful as a company with such focus.
In the past Oncolytic Viruses have experienced certain safety and efficacy challenges.
Although current clinical trials of oncolytic virotherapies have supported their role as a potential treatment for cancer, there is the risk of virus-related toxicities in vivo and possible transmission to patients' contacts, such as other patients and health care workers. In recent years, clinical trials to address these concerns have been conducted. Any such transmission by VCN-01 or a competitor would have an adverse impact on our future OV research and development efforts. Likewise, a number of oncolytic virotherapies have previously failed to meet their primary endpoints in advanced clinical trials, potentially reducing investor and partner interest or confidence in the development of new such therapies, however well differentiated they are from previous products.
Our research and development efforts may not result in commercially successful products and technologies, which may limit our ability to achieve profitability.
We must continue to explore opportunities that may lead to new products and technologies. To accomplish this, we must commit substantial efforts, funds, and other resources to research and development. A high rate of failure is inherent in the research and development of new products and technologies. Any such expenditures that we make will be made without any assurance that our efforts will be successful. Failure can occur at any point in the process, including after significant funds have been invested.
37
The success of our business currently depends on our development, approval and commercialization of our lead product candidate, VCN-01. Our ongoing Phase 1b/2a clinical trial of SYN-004 for the prevention of aGVHD in allogeneic HCT recipients, our completed Phase 1 single ascending and multiple ascending dose studies of SYN-020 and ongoing early-stage clinical trials of VCN-01 are not designed as registrational clinical trials and we currently do not have the necessary funding to complete any late stage registrational clinical trials. There are many uncertainties known and unknown that may affect the outcome of future clinical trials. All of our product candidates, including VCN-01, SYN-004 (ribaxamase), and SYN-020 will require additional clinical and non-clinical development, regulatory review and approval in multiple jurisdictions, substantial investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before we can generate any revenue from product sales. Regardless of whether our clinical trials are deemed to be successful, promising new product candidates may fail to reach the market or may only have limited commercial success because of efficacy or safety concerns, failure to achieve positive clinical outcomes, inability to obtain necessary regulatory approvals or satisfy regulatory criteria, limited scope of approved uses, excessive costs to manufacture, the failure to establish or maintain intellectual property rights, or infringement of the intellectual property rights of others. Failure to obtain regulatory approvals of VCN-01, SYN-004 (ribaxamase) or SYN-020 in a timely manner would have a material adverse impact on our business. Even if we successfully develop VCN-01, SYN-004 (ribaxamase), SYN-020 or other new products or enhancements, they may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors’ innovations. Innovations may not be quickly accepted in the marketplace because of, among other things, entrenched patterns of clinical practice or uncertainty over third-party reimbursement. We cannot state with certainty when or whether any of our products under development will be launched, whether we will be able to develop, license, or otherwise acquire drug candidates or products, or whether any products will be commercially successful. Failure to launch successful new products or new indications for existing products may cause our products to become obsolete, which may limit our ability to achieve profitability.
We may form or seek strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We may form or seek strategic alliances, create joint ventures or collaborations or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy. If we license products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture. We cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction. Any delays in entering into new strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates in certain geographies for certain indications, which would harm our business prospects, financial condition and results of operations.
38
We rely on licenses to use various technologies that are material to our business and we may not be able to retain rights licensed to us by others to commercialize key products and may not be able to establish or maintain the relationships we need to develop, manufacture, and market our products.
In addition to our own patent applications, we also currently rely on licensing agreements with third party patent holders/licensors for our products. We have entered license agreements upon which our OV technology is dependent. If we breach the terms of any of our license agreements or collaborations, including any failure to make royalty payments required thereunder or failure to reach certain developmental milestones or fulfill our obligations under the agreements could result in a termination of the agreements. The Technology Transfer Agreement”) between VCN and IDIBELL for the exclusive license of the right to use a Spanish patent number P200901201 titled “Oncolytic adenoviruses for treating cancer” provides IDIBELL with the right to revoke the license if VCN ceases business activities for a continuous year or ceases to utilize the technology subject of the Technology Transfer Agreement, uses the technology in violation of the principals of IDIBELL or ICO or stops maintaining the patent licensed under the Technology Transfer Agreement. The ICO License provides that VCN and its sublicensees have an obligation to use all diligent and commercially reasonable efforts for the exploitation of the patent, otherwise, ICO may proceed to recover the license. The “IDIBELL/ICO License Agreement provides that the licensors have the right to revoke the IDIBELL/ICO License Agreement if VCN during a continuous period of two years abandons its research or development activities of the licensed patent or activities aimed at exploitation of the resulting products. We entered into an option agreement with MGH to enter into an exclusive license to intellectual property and technology related to the use of IAP to maintain GI and microbiome health, diminish systemic inflammation, and treat age-related diseases. There can be no assurance that we will be able to reach agreement on license terms or that the terms will be favorable to us. This license agreement is expected to require us to meet certain diligence requirements and timelines in order to keep the license agreement in effect. In addition, certain license agreements, including the one that may potentially be entered into with MGH, typically contain provisions requiring royalty free non-exclusive licenses to the U.S government if any federal funding was used to invent any of the patents being licensed. In the event we or our sublicensee are not able to meet our diligence requirements contained in the license agreement with MGH or any other license agreement, we may not be able to retain the rights granted under our agreement or renegotiate with our arrangement institution on reasonable terms, or at all. If any license were to terminate and we were to lose the right to commercialize our products, our business opportunity would be adversely affected. Furthermore, we currently have very limited product development capabilities, and limited marketing or sales capabilities. For us to research, develop, and test our product candidates, we would need to contract with outside researchers, in most cases those parties that did the original research and from whom we have licensed the technologies. Our agreement with UT Austin allows UT Austin to terminate its agreement if we fail to comply with the terms of the agreement.
We can give no assurances that any of our issued patents licensed to us or any of our other patent applications will provide us with significant proprietary protection or be of commercial benefit to us. Furthermore, the issuance of a patent is not conclusive as to its validity or enforceability, nor does the issuance of a patent provide the patent holder with freedom to operate without infringing the patent rights of others.
We may incur additional expenses in connection with our licenses and collaboration arrangements and our development of our product candidates.
VCN’s collaboration agreements require that Theriva Biologics S.L. engage in certain research and development activities that require additional expenditures. Our agreements with Washington University and MGH may require that we initiate certain studies and file or have accepted an NDA within a certain amount of time, each of which are costly and will require additional expenditures. Although all manufacturing, preclinical studies and human clinical trials are expensive and difficult to design and implement, costs associated with the manufacturing, research and development of biologic product candidates are generally greater in comparison to small molecule product candidates. Due to our small work force, we expect in future years to require additional personnel to support our later stage research and development efforts. Manufacturing of VCN-01, SYN-004 (ribaxamase) and SYN-020 to support potential future clinical studies will require us to incur additional expenses.
Because development activities in our collaborations are sometimes determined pursuant to joint steering committees, future development costs associated with these programs may be difficult to anticipate and may exceed our expectations. Our actual cash requirements may vary materially from our current expectations for a number of other factors that may include, but are not limited to, unanticipated technical challenges, enrollment challenges, changes in the focus and direction of our development activities or adjustments necessitated by changes in the competitive landscape in which we operate. If we are unable to continue to financially support such collaborations due to our own working capital constraints, we may be forced to delay our activities. If we are unable to obtain additional financing on terms acceptable to us or at all, we may be forced to seek licensing partners or discontinue development.
39
Developments by competitors may render our products or technologies obsolete or non-competitive.
The pharmaceutical and biotechnology industries, including the oncolytic virus industry and the monoclonal antibody industry, are characterized by rapidly evolving technology and intense competition. Our competitors include major multi-national pharmaceutical companies and biotechnology companies developing both generic and proprietary therapies to treat serious diseases. Many of our competitors have drugs that have already been commercialized and therefore benefit from being first to market their products. Many of these companies are well-established and possess technical, human, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, many of our potential competitors have formed strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that afford these companies’ potential research and development and commercialization advantages in the therapeutic areas we are currently pursuing. Academic research centers, governmental agencies and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those being developed by us. In addition, many of these competitors may be able to obtain patent protection, obtain FDA and other regulatory approvals and begin commercial sales of their products before us, including for different indications of the same active ingredients that comprise our pipeline products. These competitors will compete with us in product sales as well as recruitment and retention of qualified scientific and management personnel, establishment of clinical trial sites and patient enrollment for clinical trials, as well as in the acquisition of technologies and technology licenses complementary to our programs or advantageous to our business. Companies pursuing clinical development of modified oncolytic adenoviruses include AdCure Bio LLC, Calidi Biotherapeutics, Inc., Candel Therapeutics, Inc., CG Oncology, Inc., Elicera Therapeutics AB, EpicentRx, Inc., GeneMedicine, Co Ltd., IconOVir Bio, Inc., Lokon Pharma AB, Memgen, Inc., Multivir, Inc., NewGenPharm Incorporation, Oncolys BioPharma, Inc., Orca Therapeutics B.V., Akamis Bio Ltd. (formerly PsiOxus Therapeutics Ltd), Shanghai Sunway Biotech Co., Ltd, Circio Holding ASA (formerly Targovax Oy|Targovax ASA), Tessa Therapeutics, Theolytics Ltd., TILT Biotherapeutics, Ltd., and Valo Therapeutics Oy. OV products have been or are being developed using other virus backbones, including: arenavirus (Hookipa Pharma, Inc.); Coxsackie virus (Viralytics Ltd., Oncorus Inc.); herpes simplex virus (Amgen, Inc., Candel Therapeutics, Inc., Daiichi Sankyo Company Ltd., Replimune, Inc., Takara Bio, Inc., Treovir LLC, Virogin Biotech, Inc., Wuhan Binhui Biotechnology Co., Ltd.); Maraba virus (Turnstone Biologics, Inc.); measles virus (Themis Biosciences GmbH, Vyriad, Inc.); myxoma virus (OncoMyx Therapeutics, Inc.); parvovirus (Oryx GmbH & Co. KG), reovirus (Oncolytics Biotech, Inc.); Seneca Valley virus (Seneca Therapeutics Inc., Oncorus Inc.); vesicular stomatitis virus (Boehringer Ingelheim, Cytonus Therapeutics, Inc., Vyriad, Inc.); and vaccinia viruses (Genelux Corporation, Imugene Ltd, Joint Biosciences Ltd, KaliVir Immunotherapeutics LLC, SillaJen, Inc., Transgene SA, Turnstone Biologics, Corp.). In addition, academic research centers may develop technologies that compete with our VCN-01, SYN-004 and SYN-020, products and our other technologies. Should clinicians or regulatory authorities view alternative therapeutic regiments as more effective than our products, this might delay or prevent us from obtaining regulatory approval for our products, or it might prevent us from obtaining favorable reimbursement rates from payers, such as Medicare, Medicaid, hospitals and private insurers.
Not only do our product candidates compete with other product candidates being developed for similar or the same indications, we also compete for employees and for clinical trial sites.
We may seek to selectively establish collaborations, and, if we are unable to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans.
Our product development programs and the potential commercialization of our clinical product candidates will require substantial additional cash to fund expenses. For some of our product candidates, we may decide to collaborate with governmental entities or additional pharmaceutical and biotechnology companies for the development and potential commercialization of our product candidates.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with our product candidate.
40
If the parties we depend on for supplying substance raw materials for our product candidates and certain manufacturing-related services do not timely supply these products and services in sufficient quality or quantity, or if current drug supply becomes unusable,it may delay or impair our ability to develop, manufacture and market our product candidates.
We rely on suppliers for the substance raw materials of our product candidates and third parties for manufacturing-related services to produce material that meets appropriate content, quality and stability standards and use in clinical trials of our products and, after approval, for commercial distribution. To succeed, clinical trials require adequate supplies of study material, which may be difficult or uneconomical to procure or manufacture and there can be no assurance that we will successfully procure such study material or even if procured, that we can do so in quantities and in a timely manner to allow our clinical trials to proceed as planned. Drug supply, once produced, is stored at clinical trial sites and vendor depots and we rely on these locations to maintain and protect the drug supply appropriately. Moreover, clinical drug supply has a finite shelf-life that may not be fully established prior to initiating early-stage clinical trials. We and our suppliers and vendors may not be able to (i) produce our study material to appropriate standards for use in clinical studies, (ii) perform under any definitive manufacturing, supply or service agreements with us, or (iii) remain in business for a sufficient time to successfully produce and market our product candidates, or (iv) prevent the drug supply from becoming unusable due to damage, loss or shelf-life expiration. If we do not maintain important manufacturing and service relationships, we may fail to find a replacement supplier or required vendor or manufacturer which could delay or impair our ability to conduct clinical trials and obtain regulatory approval for our products and substantially increase our costs or deplete profit margins, if any. If we do find replacement manufacturers and vendors, we may not be able to enter into agreements with them on terms and conditions favorable to us and there could be a substantial delay before a new facility could be qualified and registered with the FDA and foreign regulatory authorities.
The third-party manufacturers of the active pharmaceutical ingredient (API) and drug product for our lead product candidates, VCN-01, SYN-004 (ribaxamase) and SYN-020, are established cGMP manufacturers. For all other therapeutic areas, we have not yet established cGMP manufacturers for our biologic and drug candidates. We have used only one API manufacturer for each of our product candidates (VCN-01, SYN-004 or SYN-020) used in clinical trials to date. Although we believe additional manufacturers are available, if any of our manufacturers were to limit or terminate production or otherwise fail to meet the quality or delivery requirements needed to satisfy the supply commitments, the process of locating and qualifying alternate sources could require up to several months, during which time our production could be delayed. Any curtailment in the availability of VCN-01, SYN-004 (ribaxamase) or SYN-020 could have a material adverse effect on our business, financial position and results of operations. In addition, because regulatory authorities must generally approve raw material sources for pharmaceutical products, changes in raw material suppliers may result in production delays or higher raw material costs.
The manufacture of our product candidates requires significant expertise and manufacturers may encounter difficulties in production, particularly in scaling up production. These problems include difficulties with production costs and yields, quality control, including stability of the product and quality assurance testing, shortages of qualified personnel, as well as compliance with federal, state and foreign regulations. We may experience longer than expected lead times with respect to the manufacture of clinical drug supply, which may result from the increase in manufacturing scale necessary to conduct our anticipated late-stage clinical trials and result in trial delays. Furthermore, during the COVID-19 pandemic, many manufacturers prioritized the manufacture of COVID-19 related products, increasing the manufacturing lead times for non-COVID-19 related products. If a pandemic should occur again and manufacturers prioritized the manufacture of pandemic related products, we may suffer a delay or interruption in the supply of clinical trial supplies.In addition, any could delay the completion of our clinical trials, increase the costs associated with conducting our clinical trials and, depending upon the period of delay, require us to commence new clinical trials at significant additional expense or to terminate a clinical trial.
We are responsible for ensuring that each of our contract manufacturers comply with the cGMP requirements of the FDA and other regulatory authorities from which we seek to obtain product approval. While we oversee compliance, we do not have control over our manufacturers and their compliance with regulatory requirements. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. The approval process for NDAs includes a review of the manufacturer’s compliance with cGMP requirements. We are responsible for regularly assessing a contract manufacturer’s compliance with cGMP requirements through record reviews and periodic audits and for ensuring that the contract manufacturer takes responsibility and corrective action for any identified deviations.
A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. Furthermore, if our manufacturers fail to deliver the required commercial quantities on a timely basis and at commercially reasonable prices, we may be unable to meet demand for any approved products and would lose potential revenues.
41
For our Phase 2 clinical trial of VCN-01 in patients with PDAC, we are administering our clinical product candidate, VCN-01, in combination with other approved standard of care drugs. Any problems obtaining the standard of care drugs could result in a delay or interruption in our clinical trials.
For our planned Phase 2 clinical trial of VCN-01 in patients with PDAC, we are administering VCN-01 in combination with the already approved standard of care drugs, gemcitabine/nab-paclitaxel, for which there has recently been a supply shortage. Therefore, our success will be dependent upon the continued use of and ability to obtain the standard of care drugs. We expect that in any other clinical trials we conduct for additional indications, our clinical product candidate will also be administered in combination with drugs owned by third parties. If any of the standard of care or third-party drugs that are used in our clinical trials are unavailable while the trials are continuing, the timeliness and commercialization costs could be impacted. In addition, if any of these other drugs are determined to have safety or efficacy problems, our clinical trials and commercialization efforts would be adversely affected.
If third-party vendors, upon whom we rely to conduct our preclinical studies or clinical trials, do not perform or fail to comply with strict regulations, these studies or trials may be delayed, terminated, or fail, or we could incur significant additional expenses, which could materially harm our business.
We have limited resources dedicated to designing, conducting and managing our preclinical studies and clinical trials. We rely on, third parties, including CROs, consultants and principal investigators, to assist us in designing, managing, conducting, monitoring and analyzing the data from our preclinical studies and clinical trials. We rely on these vendors and individuals to perform many facets of the clinical development process on our behalf, including conducting preclinical studies, the recruitment of sites and subjects for participation in our clinical trials, maintenance of good relations with the clinical sites, and ensuring that these sites are conducting our trials in compliance with the trial protocol and applicable regulations. If these third parties fail to perform satisfactorily, or do not adequately fulfill their obligations under the terms of our agreements with them, we may not be able to enter into alternative arrangements without undue delay or additional expenditures, and therefore the preclinical studies and clinical trials of our clinical product candidates may be delayed or prove unsuccessful.
Further, the FDA, the EMA, or similar regulatory authorities in other countries, may inspect some of the clinical sites participating in our clinical trials or our third-party vendors’ sites to determine if our clinical trials are being conducted according to good clinical practices, or GCPs, or similar regulations. If we or a regulatory authority determine that our third-party vendors are not in compliance with, or have not conducted our clinical trials according to applicable regulations, we may be forced to exclude certain data from the results of the trial, or delay, repeat or terminate such clinical trials.
We may fail to retain or recruit necessary personnel, and we may be unable to secure the services of consultants.
As of March 30, 2024, we employed 21 full-time employees, including employees located at Theriva Biologics’ offices in Barcelona, Spain. We have also engaged clinical consultants to advise us on our clinical programs and regulatory consultants to advise us on our dealings with the FDA and other foreign regulatory authorities. Due to our small work force, we expect in future years to require additional personnel to support our later stage research and development efforts. We have been and may be required to retain additional consultants and employees in order to fulfill our obligations under our licenses and collaborations for our development of VCN-01, SYN-004 (ribaxamase), SYN-020, and our agreements with Washington University and other collaborators. Our future performance will depend in part on our ability to successfully integrate newly hired officers into our management team and our ability to develop an effective working relationship among senior management.
Certain of our directors, scientific advisors, and consultants serve as officers, directors, scientific advisors, or consultants of other biopharmaceutical or biotechnology companies that might be developing competitive products to ours. Other than corporate opportunities, none of our directors are obligated under any agreement or understanding with us to make any additional products or technologies available to us. Similarly, we can give no assurances, and we do not expect and stockholders should not expect, that any biomedical or pharmaceutical product or technology identified by any of our directors or affiliates in the future would be made available to us other than corporate opportunities. We can give no assurances that any such other companies will not have interests that are in conflict with our interests.
Losing key personnel or failing to recruit necessary additional personnel would impede our ability to attain our development objectives. There is intense competition for qualified personnel in the drug and biologic development areas, and we may not be able to attract and retain the qualified personnel we would need to develop our business.
42
We rely on independent organizations, advisors, and consultants to perform certain services for us, including handling substantially all aspects of regulatory approval, clinical management, manufacturing, marketing, and sales. We expect that this will continue to be the case. Such services may not always be available to us on a timely basis when we need them.
Global health crises may adversely affect our planned operations.
Our business and the business of the supplier of our clinical product candidates and the suppliers of the standard of care drugs that are administered in combination with our product candidates could be materially and adversely affected by the risks, or the public perception of the risks, related to a pandemic or other health crisis, such as the recent outbreak of COVID-19. We have experienced delays in patient enrollment due to the COVID-19 pandemic. To date, we are on track to meet all of our previously announced future clinical milestones; however, if the COVID-19 pandemic increases in severity or we should experience another pandemic, we could once again experience delays in patient enrollment and experience significant disruptions to our clinical development timelines. If we experience delays in patient enrollment or patients drop outs and we deem it necessary or advisable to improve patient recruitment by, among other things, opening additional clinical sites, we could incur increased clinical program expenses. Any such disruptions or delays would, and any such increased clinical program expenses could, adversely affect our business, financial condition, results of operations and growth prospects. In addition to delays or difficulties in enrolling patients in our clinical trials, we could experience the following disruptions that could severely impact our business and clinical trials, including:
| ● | unwillingness of potential study participants to enroll in new clinical trials and/or visit healthcare facilities; |
| ● | postponement of enrollment in our clinical studies; |
| ● | diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials; |
| ● | interruption of key clinical trial activities, such as clinical site visits by study participants and clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others; |
| ● | limitations in employee resources that would otherwise be focused on the conduct of our clinical trials, including because of sickness of employees or their families, the desire of employees to avoid contact with large groups of people, or substantial numbers of resignations; |
| ● | delays in receiving approval from local regulatory authorities to initiate our planned clinical trials; |
| ● | delays in clinical site initiation due to understaffing in departments required for contracting and study start-up; |
| ● | delays in clinical sites receiving the supplies and materials needed to conduct our clinical trials; |
| ● | interruption in global shipping that may affect the manufacture and transport of clinical trial materials, such as investigational drug product used in our clinical trials; |
| ● | changes in local regulations as part of a response to the COVID-19 coronavirus outbreak which may require us to change the ways in which our clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether; |
| ● | delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; and |
| ● | delay in the timing of interactions with the FDA and other regulatory agencies due to absenteeism by employees or by the diversion of their efforts and attention to approval of other therapeutics or other activities related to COVID-19. |
43
In addition, a significant outbreak of contagious diseases in the human population could result in the complete or partial closure of one or more manufacturing facilities which could impact our supply of our product candidates or the standard of care drugs that are administered in combination with our product candidates. In addition, an outbreak near where our clinical trial sites are located, has in the past, and may in the future impact our ability to recruit patients, and would likely delay our clinical trials, and could affect our ability to complete our clinical trials within the planned time periods. In addition, it could impact economies and financial markets, resulting in an economic downturn that could impact our ability to raise capital or slow down potential partnering relationships.
Our business and the business of the suppliers of our clinical product candidates has been and is expected to continue to be materially and adversely affected by the pandemic and post-pandemic workforce and supply-chain issues. While we are currently not experiencing material delays, such events could result in the delay or complete or partial closure of clinical trial sites or one or more manufacturing facilities which could impact our supply of our clinical product candidates. In addition, it could impact economies and financial markets, resulting in an economic downturn that could impact our ability to raise capital or slow down potential partnering relationships.
In addition, the outbreak of a pandemic could disrupt our operations due to absenteeism by infected or ill members of management or other employees, or absenteeism by members of management and other employees who elect not to come to work due to the illness affecting others in our office, or due to quarantines. Pandemics could also impact members of our Board of Directors resulting in absenteeism from meetings of the directors or committees of directors, and making it more difficult to convene the quorums of the full Board of Directors or its committees needed to conduct meetings for the management of our affairs.
The extent to which the virus may continue to impact our business and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions and social distancing in the United States and Spain, business closures or business disruptions and the effectiveness of actions taken in the United States, Spain, and other countries to contain and treat the disease. We do not yet know the full extent of potential delays or impacts on our business, operations, or the global economy as a whole. While the original spread of COVID-19 has been mitigated, the continued emergence of novel virus strains mean there is no guarantee that a future outbreak of this or any other widespread epidemics will not occur, or that the global economy will recover, either of which could seriously harm our business.
Business disruptions could seriously harm our future revenue and financial condition and increase costs and expenses.
Our operations and those of our third-party suppliers and collaborators could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes or other extreme weather conditions, medical epidemics, labor disputes, war or other business interruptions. Any interruption could seriously harm our ability to timely proceed with any clinical programs or to supply product candidates for use in our clinical programs or during commercialization. For example, the COVID-19 pandemic did, at points, cause an interruption in our clinical trial activities. Additionally, supply chain disruptions impacted and may continue to impact our research activities. Moreover, at the end of 2021 and into 2022, tensions between the United States and Russia escalated when Russia amassed large numbers of military ground forces and support personnel on the Ukraine-Russia border and, in February 2022, Russia invaded Ukraine. In response, North Atlantic Treaty Organization, or NATO has deployed additional military forces to Eastern Europe and the Biden administration announced certain sanctions against Russia. The invasion of Ukraine and the retaliatory measures that have been taken, or could be taken in the future, by the United States, NATO, and other countries have created global security concerns that could result in a regional conflict and otherwise have a lasting impact on regional and global economies, any or all of which could disrupt our supply chain, and despite the fact that we currently do not plan any clinical trials in Eastern Europe, may adversely impact the cost and conduct of our international clinical trials of our product candidates.
Unfavorable U.S. or global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and financial markets. A severe or prolonged economic downturn could result in a variety of risks to our business, including weakened demand for our technologies and our ability to raise additional capital when needed on favorable terms, if at all. Recently, the rate of inflation has increased throughout the U.S. economy. Inflation may adversely affect us by increasing the costs associated with performing research and development on internal research initiatives and partnered programs. We may experience significant increases in the prices of labor, consumables, and other costs of doing business. In an inflationary environment, such cost increases may outpace our expectations, causing us to use cash faster than forecasted. A weak or declining economy may also strain our partners, possibly resulting in supply disruption, or cause delays in their payments to us. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
44
In addition, the global macroeconomic environment could be negatively affected by, among other things, COVID-19 or other pandemics or epidemics, instability in global economic markets, increased U.S. trade tariffs and trade disputes with other countries, instability in the global credit markets, supply chain weaknesses, instability in the geopolitical environment as a result of the withdrawal of the United Kingdom from the European Union, the Russian invasion of Ukraine, the war in the Middle East and other political tensions, and foreign governmental debt concerns. Such challenges have caused, and may continue to cause, uncertainty and instability in local economies and in global financial markets.
We rely extensively on our information technology systems, and our systems and infrastructure face certain risks, including cybersecurity and data leakage risks.
We rely on our information technology systems and infrastructure to process transactions, summarize results and manage our business, including maintaining client and supplier information. In the ordinary course of business, we collect, store and transmit large amounts of confidential information, and it is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. Additionally, we utilize third parties, including cloud providers, to store, transfer and process data. Our information technology systems, as well as the systems of our suppliers and other partners, whose systems we do not control, are vulnerable to outages and an increasing risk of continually evolving deliberate intrusions to gain access to company sensitive information. The size and complexity of our information technology systems, and those of our third-party vendors with whom we contract, make such systems potentially vulnerable to service interruptions and security breaches from inadvertent or intentional actions by our employees, partners or vendors, from attacks by malicious third parties, or from intentional or accidental physical damage to our systems infrastructure maintained by us or by third parties. Data security incidents and breaches by employees and others with or without permitted access to our systems pose a risk that sensitive data may be exposed to unauthorized persons or to the public. Maintaining the secrecy of this confidential, proprietary, or trade secret information is important to our competitive business position. While we have taken steps to protect such information and invested in information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches in our systems or the unauthorized or inadvertent wrongful use or disclosure of confidential information that could adversely affect our business operations or result in the loss, dissemination, or misuse of critical or sensitive information. A cyber-attack or other significant disruption involving our information technology systems, or those of our vendors, suppliers and other partners, could also result in disruptions in critical systems, corruption or loss of data and theft of data, funds or intellectual property. A breach of our security measures or the accidental loss, inadvertent disclosure, unapproved dissemination, misappropriation or misuse of trade secrets, proprietary information, or other confidential information, whether as a result of theft, hacking, fraud, trickery or other forms of deception, or for any other reason, could enable others to produce competing products, use our proprietary technology or information, or adversely affect our business or financial condition. We may be unable to prevent outages or security breaches in our systems. We remain potentially vulnerable to additional known or yet unknown threats as, in some instances, we, our suppliers and our other partners may be unaware of an incident or its magnitude and effects. We also face the risk that we expose our vendors or partners to cybersecurity attacks. Any or all of the foregoing could adversely affect our results of operations and our business reputation.
Our business and operations would suffer in the event of computer system failures.
Despite the implementation of security measures, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses, malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber-intrusions, including by computer hackers, foreign governments, and cyber-terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our current or future product development programs. For example, the loss of clinical trial data from completed or any future ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur material legal claims and liability, damage to our reputation, and the further development of our product candidates could be delayed.
45
Any failure to maintain the security of information relating to our patients, customers, employees and suppliers, whether as a result of cybersecurity attacks or otherwise, could expose us to litigation, government enforcement actions and costly response measures, and could disrupt our operations and harm our reputation.
Significant disruptions to our information technology systems or breaches of information security could adversely affect our business. In connection with the pre-clinical and clinical development, sales and marketing of our products and services, we may from time to time transmit confidential information. We also have access to, collect or maintain private or confidential information regarding our clinical trials and the patients enrolled therein, employees, and suppliers, as well as our business. Although we have instituted security measures, there can be no assurance that these security measures will be able to protect against cyberattacks. Cyberattacks are rapidly evolving and becoming increasingly sophisticated. It is possible that computer hackers and others might compromise our security measures, or security measures of those parties that we do business with now or in the future, and obtain the personal information of patients in our clinical trials, vendors, employees and suppliers or our business information. A security breach of any kind, including physical or electronic break-ins, computer viruses and attacks by hackers, employees or others, could expose us to risks of data loss, litigation, government enforcement actions, regulatory penalties and costly response measures, and could seriously disrupt our operations. Any resulting negative publicity could significantly harm our reputation, which could cause us to lose market share and have an adverse effect on our results of operations.
We may face particular data protection, data security and privacy risks in connection with the European Union’s Global Data Protection Regulation and other privacy regulations.
Outside of the United States, the laws, regulations and standards in many jurisdictions apply broadly to the collection, use, and other processing of personal information. For example, in the European Union, the collection and use of personal data are governed by the provisions of the General Data Protection Regulation (the “GDPR”). The GDPR, together with national legislation, regulations and guidelines of the European Union. member states governing the processing of personal data, impose strict obligations on entities subject to the GDPR, including but not limited to: (i) accountability and transparency requirements, and enhanced requirements for obtaining valid consent from data subjects; (ii) obligations to consider data protection as any new products or services are developed and to limit the amount of personal data processed; (iii) obligations to comply with the data protection rights of data subjects; and (iv) obligations to report certain personal data breaches to governmental authorities and individuals. Data protection authorities from the different E.U. member states and other European countries may enforce the GDPR and national data protection laws differently, and introduce additional national regulations and guidelines, which adds to the complexity of processing European personal data. Failure to comply with the requirements of the GDPR and the related national data protection laws may result in significant monetary fines and other administrative penalties (the GDPR authorizes fines for certain violations of up to 4% of global annual revenue or €20 million, whichever is greater) as well as civil liability claims from individuals whose personal data was processed. Additionally, expenses associated with compliance could reduce our operating margins.
The GDPR also prohibits the transfer of personal data from the E.U. to countries outside of the E.U. unless made to a country deemed by the European Commission to provide adequate protection for personal data or accomplished by means of an approved data transfer mechanism (e.g., standard contractual clauses). Data protection authority guidance and enforcement actions that restrict companies’ ability to transfer data may increase risk relating to data transfers or make it more difficult or impossible to transfer E.U. personal data to the U.S.
46
REGULATORY RISKS
If we do not obtain the necessary regulatory approvals in the U.S. and/or other countries we will not be able to develop or sell our product candidates.
We cannot assure you that we will receive the approvals necessary to commercialize any of our product candidates or any product candidates we acquire or develop in the future. We will need FDA approval to commercialize our product candidates in the U.S. and approvals from the FDA-equivalent regulatory authorities in foreign jurisdictions to commercialize our product candidates in those jurisdictions. We will be required to conduct clinical trials that will be costly and we currently do not have the funding to complete any registrational clinical trials. We cannot predict whether our clinical trials will demonstrate the safety and efficacy of our product candidates or if the results of any clinical trials will be sufficient to advance to the next phase of development or for approval from the FDA (or equivalent foreign regulatory authorities). We also cannot predict whether our research and clinical approaches will result in drugs or therapeutics that the FDA considers safe and effective for the proposed indications. The FDA has substantial discretion in the drug approval process. The approval process may be delayed by changes in government regulation, future legislation or administrative action or changes in FDA policy that occur prior to or during our regulatory review. Delays in obtaining regulatory approvals may prevent or delay commercialization of, and our ability to derive product revenues from our product candidates; and diminish any competitive advantages that we may otherwise believe that we hold.
Even if we comply with all FDA (or equivalent foreign regulatory authorities) requests, the FDA may ultimately reject one or more of our NDAs or BLAs. We may never obtain regulatory clearance for any of our product candidates. Failure to obtain FDA approval of any of our product candidates will severely undermine our business by leaving us without a saleable product, and therefore without any source of revenues, until another product candidate can be developed. There is no guarantee that we will ever be able to develop or acquire another product candidate.
In addition, the FDA (or equivalent foreign regulatory authorities) may require us to conduct additional pre-clinical and clinical testing or to perform post-marketing studies, as a condition to granting marketing approval of a product. The results generated after approval could result in loss of marketing approval, changes in product labeling, and/or new or increased concerns about the side effects or efficacy of a product. The FDA has significant post-market authority, including the explicit authority to require post-market studies and clinical trials, labeling changes based on new safety information, and compliance with FDA-approved risk evaluation and mitigation strategies. The FDA’s exercise of its authority has in some cases resulted, and in the future could result, in delays or increased costs during product development, clinical trials and regulatory review, increased costs to comply with additional post-approval regulatory requirements and potential restrictions on sales of approved products.
In foreign jurisdictions, we must also receive approval from the appropriate regulatory authorities before we can commercialize any products, which can be time consuming and costly. Foreign regulatory approval processes generally include all of the risks associated with the FDA approval procedures described above but processes, requirements and timelines for approval by these agencies may differ significantly from the FDA. There can be no assurance that we will receive the approvals necessary to commercialize our product candidate for sale outside the United States.
If the FDA approves any of our product candidates, the labeling, manufacturing, packaging, adverse event reporting, storage, advertising, promotion and record-keeping for our products will be subject to ongoing FDA requirements and continued regulatory oversight and review. Our drug manufacturers and subcontractors that we retain will be required to comply with FDA and other regulations. We may also be subject to additional FDA post-marketing obligations. If we are not able to maintain regulatory compliance, we may not be permitted to market our product candidates and/or may be subject to product recalls, seizures, suspension of regulatory approval, suspension of production, injunctions or civil or criminal sanctions. The subsequent discovery of previously unknown problems with any marketed product, including adverse events of unanticipated severity or frequency, may result in restrictions on the marketing of the product, and could include withdrawal of the product from the market.
Clinical trials are very expensive, time-consuming, and difficult to design and implement.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time-consuming. We estimate that clinical trials for our product candidates would take at least several years to complete. Furthermore, failure can occur at any stage of the trials, and we could encounter problems that cause us to abandon or repeat clinical trials. Commencement and completion of clinical trials may be delayed by several factors, including:
| ● | obtaining an IND application with the FDA or foreign equivalent to commence clinical trials; |
47
| ● | identification of, and acceptable arrangements with, one or more clinical sites; |
| ● | obtaining IRB or EC approval to commence clinical trials; |
| ● | obtaining IBC approval for use of a genetically modified organism; |
| ● | unforeseen safety issues; |
| ● | determination of dosing; |
| ● | lack of effectiveness during clinical trials; |
| ● | slower than expected rates of patient recruitment; |
| ● | inability to monitor patients adequately during or after treatment; |
| ● | lower than expected rates of patient completion of clinical trials; |
| ● | inability to obtain supply of our drug candidate in a timely manner; |
| ● | inability or unwillingness of medical investigators to follow our clinical protocols; and |
| ● | unwillingness of the FDA or foreign equivalent, IRBs/ECs, or IBCs to permit the clinical trials to be initiated. |
In addition, we, IRBs/ECs or the FDA or foreign equivalent may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if IRBs/ECs or the FDA or foreign equivalent finds deficiencies in our submissions or conduct of our trials.
The results of our clinical trials may not support our product candidate claims and the results of preclinical studies and completed clinical trials are not necessarily predictive of future results.
To date, long-term safety and efficacy have not yet been demonstrated in clinical trials for any of our product candidates. Favorable results in our early studies or trials may not be repeated in later studies or trials as was the case with SYN-010. Even if our clinical trials are initiated and completed as planned, we cannot be certain that the results will support our product candidate claims. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful. Success in Phase 1 studies of VCN-01 in PDAC or retinoblastoma does not ensure success of VCN-01, especially in light of the small number of patients treated in those trials. Success of our predecessor P1A clinical product or positive topline data from our previous SYN-004 (ribaxamase) Phase 1 and Phase 2 clinical trials, does not ensure success of SYN-004 (ribaxamase). Furthermore, the FDA could determine that VCN-01 or SYN-004 (ribaxamase) have not demonstrated appropriate safety and thus require additional clinical trials and safety data, despite prior positive clinical trial results. We cannot be sure that the results of later clinical trials would replicate the results of prior clinical trials and preclinical testing nor that they would satisfy the requirements of the FDA or other regulatory agencies. Clinical trials may fail to demonstrate that our product candidates are safe for humans and effective for indicated uses. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that commence clinical trials are never approved as products. Any such failure could cause us or our sublicensee to abandon a product candidate and might delay development of other product candidates. Preclinical and clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals or commercialization. Any delay in, or termination of, our clinical trials would delay our obtaining FDA approval for the affected product candidate and, ultimately, our ability to commercialize that product candidate.
48
Difficulties enrolling patients in our clinical trials or delays in enrollment are expected to result in our clinical development activities being delayed or otherwise adversely affected.
Delays in patient enrollment may result in increased costs or may adversely affect timing or outcome of planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates. This can lead to delays in completion of clinical trials as well as additional expense for recruitment of patients. In addition, any pandemic may result in fewer clinical study personnel being available to conduct clinical testing for patients currently enrolled in our clinical trials.
Patients who are administered our product candidates may experience unexpected side effects or other safety risks that could cause a halt in their clinical development, preclude approval of our product candidates or limit their commercial potential.
Our clinical trials may be suspended at any time for a number of reasons. We may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial subjects. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial subjects. For example, the FDA or foreign equivalents could determine that VCN-01 or SYN-004 has not demonstrated appropriate safety, that adverse events are drug related and require additional clinical trials and safety data, despite positive results from Phase 1 clinical trials of VCN-01 or our SYN-004 Phase 2b clinical trial.
Administering any product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidates for any or all targeted indications. Ultimately, some or all of our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials. Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and could substantially increase commercialization costs.
It is possible that we may not be able to obtain or maintain orphan drug designation or exclusivity for our drug candidates, which could limit the potential profitability of our product candidates.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for the treatment or prevention of rare diseases or conditions with relatively small patient populations as orphan drugs. Under the Orphan Drug Act of 1983, the (“Orphan Drug Act”), the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is defined as a patient population of fewer than 200,000 individuals in the United States. We received orphan drug designation from the FDA for VCN-01 for the treatment of retinoblastoma and from the EMA for the treatment of pancreatic cancer. If a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a seven-year period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for the same indication during that time period with some exceptions. A similar provision in the European Union allows 10 years of exclusivity in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that marketing exclusivity is no longer justified. Orphan drug exclusivity may be lost in Europe under certain situations, such as the inability of the holder of the orphan drug designation to produce sufficient quantities of the drug to meet the needs of patients with the rare disease or condition or for certain other reasons.
Our product candidates, if approved for sale, may not gain acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenues.
If one of our product candidates is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product by physicians, healthcare professionals and third-party payors and our profitability and growth will depend on a number of factors, including:
| ● | demonstration of safety and efficacy; |
| ● | changes in the practice guidelines and the standard of care for the targeted indication; |
| ● | relative convenience and ease of administration; |
| ● | the prevalence and severity of any adverse side effects; |
49
| ● | budget impact of adoption of our product on relevant drug formularies; |
| ● | the availability, cost and potential advantages of alternative treatments, including less expensive generic drugs; |
| ● | pricing, reimbursement and cost effectiveness, which may be subject to regulatory control; |
| ● | effectiveness of our or any of our partners’ sales and marketing strategies; |
| ● | the product labeling or product insert required by the FDA or regulatory authority in other countries; and |
| ● | the availability of adequate third-party insurance coverage or reimbursement. |
If any product candidate that we develop does not provide a treatment regimen that is as beneficial as, or is perceived as being as beneficial as, the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance. Our ability to effectively promote and sell any approved products will also depend on pricing and cost-effectiveness, including our ability to produce a product at a competitive price and our ability to obtain sufficient third-party coverage or reimbursement. If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, patients and third-party payors, our ability to generate revenues from that product would be substantially reduced. In addition, our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources, may be constrained by FDA rules and policies on product promotion, and may never be successful.
We depend on third parties, including researchers and sublicensees, who are not under our control. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to seek or obtain regulatory approval for or commercialize our product candidates.
We depend on independent investigators and scientific collaborators, such as universities and medical institutions or private physician scientists, to advise us and to conduct our preclinical and clinical trials under agreements with us. These collaborators are not our employees and we cannot control the amount or timing of resources that they devote to our programs or the timing of their procurement of clinical-trial data or their compliance with applicable regulatory guidelines. Should any of these scientific inventors/advisors or those of our sublicensee become disabled or die unexpectedly, or should they fail to comply with applicable regulatory guidelines, we or our sublicensee may be forced to scale back or terminate development of that program. They may not assign as great a priority to our programs or pursue them as diligently as we would if we were undertaking those programs ourselves. Failing to devote sufficient time and resources to our drug-development programs, or substandard performance and failure to comply with regulatory guidelines, could result in delay of any FDA applications and our commercialization of the drug candidate involved.
These collaborators may also have relationships with other commercial entities, some of which may compete with us. Our collaborators assisting our competitors could harm our competitive position.
We have in the past, and expect to have in the future, agreements with third-party contract research organizations (CROs) under which we have delegated to the CROs the responsibility to coordinate and monitor the conduct of our VCN-01, SYN-004 and SYN-020 clinical trials and to manage data for our clinical programs. We also rely upon CROs to monitor and manage data for our clinical programs, as well as the execution of future nonclinical studies. We expect to control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
50
Our Phase 1b/2a clinical trial of SYN-004, and Phase 1 and Phase 2 clinical trials for VCN-01 are being conducted by clinical sites over which we have little direct control. We, our CROs and our clinical sites are required to comply with current Good Clinical Practices, or cGCPs, regulations and guidelines issued by the FDA and by similar governmental authorities in other countries where we are conducting clinical trials. We have an ongoing obligation to monitor the activities conducted by our CROs and at our clinical sites to confirm compliance with these requirements. In the future, if we, our CROs or our clinical sites fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving our marketing applications. In addition, our clinical trials must be conducted with product produced under cGMP regulations and will require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. Our CROs are not our employees, and we do not control whether or not they devote sufficient time and resources to our future clinical and nonclinical programs. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities which could harm our competitive position. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our CROs or investigator-sponsored clinical sites do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If our relationship with these CROs terminate, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our CROs, there can be no assurance that it will not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business, financial condition and prospects.
We currently have no marketing, sales or distribution organization and have no experience in marketing products as a company. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to generate product revenue.
We currently have no marketing, sales or distribution capabilities and have no experience in marketing products. We may develop an in-house marketing organization and sales force, which will require significant capital expenditures, management resources and time. We will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel.
If we are unable or decide not to establish internal sales, marketing and distribution capabilities, we will pursue collaborative arrangements regarding the sales and marketing of our products; however, there can be no assurance that we will be able to establish or maintain such collaborative arrangements. Any revenue we receive will depend upon the efforts of such third parties, which may not be successful. We may have little or no control over the marketing and sales efforts of such third parties and our revenue from product sales may be lower than if we had commercialized our product candidates ourselves. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our product candidates.
There can be no assurance that we will be able to develop in-house sales and distribution capabilities or establish or maintain relationships with third-party collaborators to commercialize any product in the United States or overseas.
Reimbursement may not be available for our product candidates, which would impede sales.
Market acceptance and sales of our product candidates may depend on coverage and reimbursement policies and health care reform measures. Decisions about formulary coverage as well as levels at which government authorities and third-party payers, such as private health insurers and health maintenance organizations, reimburse patients for the price they pay for our products as well as levels at which these payors pay directly for our products, where applicable, could affect whether we are able to commercialize these products. We cannot be sure that reimbursement will be available for any of our products. Also, we cannot be sure that coverage or reimbursement amounts will not reduce the demand for, or the price of, our products. If coverage and reimbursement are not available or are available only at limited levels, we may not be able to commercialize our products.
51
In recent years, officials have made numerous proposals to change the health care system in the United States. These proposals include measures that would limit or prohibit payments for certain medical treatments or subject the pricing of drugs to government control. In addition, in many foreign countries, particularly the countries of the European Union, the pricing of prescription drugs is subject to government control. If our products are or become subject to government regulation that limits or prohibits payment for our products, or that subjects the price of our products to governmental control, we may not be able to generate revenue, attain profitability or commercialize our products.
As a result of legislative proposals and the trend towards managed health care in the United States, third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drugs. They may also impose strict prior authorization requirements and/or refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA or foreign equivalent has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly-approved drugs, which in turn will put pressure on the pricing of drugs.
Healthcare reform measures could hinder or prevent our product candidates’ commercial success.
The U.S. government and other governments have shown significant interest in pursuing continued healthcare reform. Any government-adopted reform measures could adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third-party payors. The continuing efforts of the U.S. and foreign governments, insurance companies, managed care organizations and other payors of health care services to contain or reduce health care costs may adversely affect our ability to set prices for our products which we believe are fair, and our ability to generate revenues and achieve and maintain profitability.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, that relate to healthcare availability, methods of delivery or payment for products and services, or sales, marketing or pricing, may limit our potential revenue, and we may need to revise our research and development programs. The pricing and reimbursement environment may change in the future and become more challenging due to several reasons, including policies advanced by the current executive administration in the United States, new healthcare legislation or fiscal challenges faced by government health administration authorities. Specifically, in both the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the health care system in ways that could affect our ability to sell our products profitably.
If we fail to comply with state and federal healthcare regulatory laws, we could face substantial penalties, damages, fines, disgorgement, exclusion from participation in governmental healthcare programs, and the curtailment of operations, any of which could harm our business.
Although we do not provide healthcare services or submit claims for third party reimbursement, we are subject to healthcare fraud and abuse regulation and enforcement by federal and state governments which could significantly impact our business. The laws that may affect our ability to operate include, but are not limited to:
| ● | the federal anti-kickback statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of this statute or specific intent to violate it; |
| ● | the civil FCA, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; knowingly making using, or causing to be made or used, a false record or statement to get a false or fraudulent claim paid or approved by the government; or knowingly making, using, or causing to be made or used, a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| ● | the criminal FCA, which imposes criminal fines or imprisonment against individuals or entities who make or present a claim to the government knowing such claim to be false, fictitious or fraudulent; |
| ● | HIPAA, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
52
| ● | the federal civil monetary penalties statute, which prohibits, among other things, the offering or giving of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a Federal or state governmental program; |
| ● | the federal physician sunshine requirements under the ACA, which require certain manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members; and |
| ● | state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require pharmaceutical companies to comply with the device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; and state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. |
Further, the ACA, among other things, amended the intent requirements of the federal anti-kickback statute and certain criminal statutes governing healthcare fraud. A person or entity can now be found guilty of violating the statute without actual knowledge of the statute or specific intent to violate it. In addition, the ACA provided that the government may assert that a claim including items or services resulting from a violation of the federal Anti- Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. If a government authority were to conclude that we provide improper advice to our customers or encouraged the submission of false claims for reimbursement, we could face action against us by government authorities. Any violations of these laws, or any action against us for violation of these laws, even if we successfully defend against it, could result in a material adverse effect on our reputation, business, results of operations and financial condition.
We have entered into consulting and scientific advisory board arrangements with physicians and other healthcare providers. Compensation for some of these arrangements includes the provision of stock options. While we have worked to structure our arrangements to comply with applicable laws, because of the complex and far-reaching nature of these laws, regulatory agencies may view these transactions as prohibited arrangements that must be restructured, or discontinued, or for which we could be subject to other significant penalties. We could be adversely affected if regulatory agencies interpret our financial relationships with providers who influence the ordering of and use our products to be in violation of applicable laws.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry.
Responding to investigations can be time- and resource-consuming and can divert management’s attention from the business. Additionally, as a result of these investigations, healthcare providers and entities may have to agree to additional onerous compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business.
If we obtain approval to commercialize our clinical product candidates outside of the United States, a variety of risks associated with international operations could harm our business.
If our clinical product candidate is approved for commercialization, we intend to enter into agreements with third parties to market them in certain jurisdictions outside the United States. We expect that we will be subject to additional risks related to international operations or entering into international business relationships, including:
| ● | different regulatory requirements for drug approvals and rules governing drug commercialization in foreign countries; |
| ● | reduced protection for intellectual property rights; |
| ● | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
53
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| ● | foreign reimbursement, pricing and insurance regimes; |
| ● | foreign taxes; |
| ● | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| ● | potential noncompliance with the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.K. Bribery Act 2010 and similar anti-bribery and anticorruption laws in other jurisdictions; |
| ● | product shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| ● | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
We have no prior experience in these areas. In addition, there are complex regulatory, tax, labor and other legal requirements imposed by both the European Union and many of the individual countries in Europe with which we will need to comply.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability lawsuits related to the testing of our product candidates and will face an even greater risk if we sell our product candidates commercially. Currently, we are not aware of any anticipated product liability claims with respect to our product candidates. In the future, an individual may bring a liability claim against us if one of our product candidates causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against the product liability claim, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| ● | decreased demand for our product candidates; |
| ● | injury to our reputation; |
| ● | withdrawal of clinical trial participants; |
| ● | costs of related litigation; |
| ● | initiation of investigations by regulators; |
| ● | substantial monetary awards to patients or other claimants; |
| ● | distraction of management’s attention from our primary business; |
| ● | product recalls; |
| ● | loss of revenue; and |
| ● | the inability to commercialize our product candidates. |
We have clinical trial liability insurance. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for our product candidates. Our current insurance coverage may prove insufficient to cover any liability claims brought against us. In addition, because of the increasing costs of insurance coverage, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy liabilities that may arise.
54
INTELLECTUAL PROPERTY RISKS
We rely on patents, patent applications, trade secrets and various regulatory exclusivities to protect some of our product candidates and our ability to compete may be limited or eliminated if we are not able to protect our products.
The patent positions of pharmaceutical companies are uncertain and may involve complex legal and factual questions. The issuance, scope, validity, enforceability, and commercial value of our current or future patent rights are highly uncertain. We cannot be sure that patent coverage will issue, or will be maintained, to protect our products, in some or all relevant jurisdictions. Our patents may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. Even if patents do successfully issue and even if such patents cover our product candidates and extend for a commercially relevant time, third parties may initiate invalidity, non-infringement, opposition, interference, re-examination, post-grant review, inter partes review, nullification, or derivation actions in court or before patent offices, or similar proceedings challenging the validity, inventorship, ownership, enforceability, or scope of such patents, which may result in the patent claims being narrowed, invalidated, or held unenforceable or circumvented. Additionally, some countries, including China and India, have compulsory licensing laws under which we may be compelled to grant licenses to others.
We may incur significant expenses in protecting our intellectual property and defending or assessing claims with respect to intellectual property owned by others. Any patent or other infringement litigation by or against us could cause us to incur significant expenses and divert the attention of our management. Even for our issued patents, we do not have a guarantee of patent term restoration and marketing exclusivity of the ingredients for our drugs under the Hatch-Waxman Amendments, even if we are granted FDA approval of our products.
Furthermore, others may file patent applications or obtain patents on similar technologies or compounds that compete with our products. We cannot predict how broad the claims in any such patents or applications will be, and whether they will be allowed. Once claims have been issued, we cannot predict how they will be construed or enforced. We may infringe intellectual property rights of others without being aware of it. If another party claims we are infringing their technology, we could have to defend an expensive and time consuming lawsuit, pay a large sum if we are found to be infringing, or be prohibited from selling or licensing our products unless we obtain a license or redesign our product, which may not be possible.
We also rely on trade secrets and proprietary know-how to develop and maintain our competitive position. We cannot be sure our measures to protect our trade secrets will be sufficient. Some of our current or former employees, consultants, scientific advisors, current or prospective corporate collaborators, may unintentionally or willfully disclose our confidential information to competitors or use our proprietary technology for their own benefit. Furthermore, enforcing a claim alleging the infringement of our trade secrets would be expensive and difficult to prove, making the outcome uncertain. Our competitors may also independently develop similar knowledge, methods, and know-how or gain access to our proprietary information through some other means.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights, as well as costs associated with lawsuits.
If any other person files patent applications, or is issued patents, claiming technology also claimed by us in pending applications, we may be required to participate in interference proceedings in the U.S. Patent and Trademark Office to determine priority of invention. We, or our licensors, may also need to participate in interference proceedings involving our issued patents and pending applications of another entity. The European Patent Office and some national patent authorities have formal patent opposition processes where the validity of issued patents may be challenged. If a patent opposition is filed, we, or our licensors, may also need to participate in opposition proceedings involving our issued patents
The intellectual property environment in the oncolytic viruses field is particularly complex, constantly evolving and highly fragmented. We have not conducted freedom-to-use patent searches on all aspects of our product candidates or potential product candidates, and we may be unaware of relevant patents and patent applications of third parties. In addition, the freedom-to-use patent searches that have been conducted may not have identified all relevant issued patents or pending patents. We cannot provide assurance that our proposed products in this area will not ultimately be held to infringe one or more valid claims owned by third parties which may exist or come to exist in the future or that in such case we will be able to obtain a license from such parties on acceptable terms.
We cannot guarantee that the practice of our technologies will not conflict with the rights of others. In some foreign jurisdictions, we could become involved in opposition proceedings, either by opposing the validity of another’s foreign patent or by persons opposing the validity of our foreign patents.
55
We may also face frivolous litigation or lawsuits from various competitors or from litigious securities attorneys. The cost to us of any litigation or other proceeding relating to these areas, even if deemed frivolous or resolved in our favor, could be substantial and could distract management from our business. Uncertainties resulting from initiation and continuation of any litigation could have a material adverse effect on our ability to continue our operations.
If we infringe the rights of others, we could be prevented from selling products or forced to pay damages.
If our products, methods, processes, and other technologies are found to infringe the proprietary rights of other parties, we could be required to pay damages, or we may be required to cease using the technology or to license rights from the prevailing party. Any prevailing party may be unwilling to offer us a license on commercially acceptable terms.
We enjoy restricted geographical protection with respect to certain patents.
Patents are of national or regional effect. While we will try to protect our technologies, products and product candidates with intellectual property rights such as patents throughout the world in major markets, the process of obtaining patents is time-consuming, expensive, and sometimes unpredictable in other countries. We may not pursue or obtain patent protection in all markets. Filing, prosecuting, and defending patents on all of our research programs and product candidates in all countries throughout the world would be prohibitively expensive, and, therefore, the scope and strength of our intellectual property rights will vary from jurisdiction to jurisdiction.
We may become subject to claims challenging inventorship or ownership of our patents and other intellectual property.
We generally enter into confidentiality and intellectual property assignment agreements with our employees, consultants, and contractors. These agreements generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, those agreements may not be honored and may not effectively assign intellectual property rights to us. Moreover, there may be some circumstances where we are unable to negotiate for such ownership rights. Disputes regarding ownership or inventorship of intellectual property can also arise in other contexts, such as collaborations and sponsored research. If we are subject to a dispute challenging our rights in or to patents or other intellectual property, such a dispute could be expensive and time consuming. If we were unsuccessful, we could lose valuable rights in intellectual property that we regard as our own.
RISKS RELATING TO OUR SECURITIES
We cannot assure you that our common stock will be liquid or that it will remain listed on the NYSE American.
Our common stock is listed on the NYSE American. The NYSE American’s listing standards generally mandate that we meet certain requirements relating to stockholders’ equity, stock price, market capitalization, aggregate market value of publicly held shares and distribution requirements. We cannot assure you that we will be able to maintain the continued listing standards of the NYSE American. The NYSE American requires companies to meet certain continued listing criteria including a minimum stockholders’ equity of $6.0 million if an issuer has sustained losses from continuing operations and/or net losses in its five most recent years, as outlined in the NYSE American Company Guide. The NYSE American Company Guide also states that the NYSE normally will not consider removing from listing securities of an issuer if it is in compliance with all of the following:a total value of market capitalization of at least $50.0 million; 1,100,000 publicly-held shares; a market value of publicly held shares of at least $15.0 million; and 400 round lot shareholders.
If our common stock falls below $0.20 per share on a 30-trading-day average it will become subject to the continued listing evaluation and follow-up procedures set forth in Section 1009 of the NYSE American Company Guide which could, among other things, result in initiation of immediate delisting procedures. In the event that we were to fail to meet the requirements of NYSE American per share price requirement or stockholders’ equity requirement and we could not timely cure such deficiency, our listing could become subject to NYSE American continued listing evaluation and follow-up procedures, which could result in delisting procedures.
We previously received notification from the NYSE American citing failure to comply with the minimum stockholders’ equity continued listing standard as set forth in Part 10, Section 1003 of the Company Guide. Although in the past we have been able to cure previously cited deficiencies, there can be no assurance that we will continue to meet the NYSE American continued listing requirements.
56
In addition, in the future we may not be able to maintain minimum stockholders’ equity and/or issue additional equity securities in exchange for cash or other assets, if available, to maintain certain minimum stockholders’ equity required by the NYSE American. If we are delisted from the NYSE American then our common stock will trade, if at all, only on the over-the-counter market, such as the OTC Bulletin Board securities market, and then only if one or more registered broker-dealer market makers comply with quotation requirements. In addition, delisting of our common stock could depress our stock price, substantially limit liquidity of our common stock and materially adversely affect our ability to raise capital on terms acceptable to us, or at all. Delisting from the NYSE American could also have other negative results, including the potential loss of confidence by suppliers and employees, the loss of institutional investor interest and fewer business development opportunities. We cannot assure you that our common stock will be liquid or that it will remain listed on the NYSE American. A failure to regain compliance with the NYSE American stockholders’ equity requirements or failure to continue to meet the other listing requirements could result in a de-listing of our common stock.
The market price of our common stock has been and may continue to be volatile and adversely affected by various factors.
Our stock price has fluctuated in the past, has recently been volatile and may be volatile in the future. By way of example, on February 14, 2023, the price of our common stock closed at $1.13 per share while on November 14, 2023, our stock price closed at $0.39 per share with no discernable announcements or developments by the company or third parties. We may incur rapid and substantial decreases in our stock price in the foreseeable future that are unrelated to our operating performance or prospects. In addition, the outbreak of the novel strain of coronavirus (COVID-19) has caused broad stock market and industry fluctuations. The stock market in general and the market for biotechnology and pharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may experience losses on their investment in our common stock. The market price of our common stock could fluctuate significantly in response to various factors and events, including:
| ● | investor reaction to our business strategy; |
| ● | the success of competitive products or technologies; |
| ● | our continued compliance with the listing standards of the NYSE American; |
| ● | regulatory or legal developments in the United States and other countries, especially changes in laws or regulations applicable to our products; |
| ● | results of our clinical trials; |
| ● | actions taken by regulatory agencies with respect to our products, clinical studies, manufacturing process or sales and marketing terms; |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; |
| ● | the success of our efforts to acquire or in-license additional products or product candidates; |
| ● | developments concerning our collaborations or partners; |
| ● | developments or disputes concerning patents or other proprietary rights, litigation matters and our ability to obtain patent protection for our products; |
| ● | our ability or inability to raise additional capital and the terms on which we raise it; |
| ● | declines in the market prices of stocks generally; |
| ● | trading volume of our common stock; |
| ● | sales of our common stock by us or our stockholders; |
| ● | general economic, industry and market conditions; and |
57
| ● | other events or factors, including those resulting from such events, or the prospect of such events, including war, terrorism and other international conflicts, such as the recent Russian invasion of Ukraine as well as continued and new sanctions against Russia, which restrict a wide range of trade and financial dealings with Russia and Russian persons, public health issues including health epidemics or pandemics, such as the outbreak of the novel coronavirus (COVID-19), and natural disasters such as fire, hurricanes, earthquakes, tornados or other adverse weather and climate conditions, whether occurring in the United States or elsewhere, could disrupt our operations, disrupt the operations of our suppliers or result in political or economic instability. |
These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. Further, recent increases are significantly inconsistent with any improvements in actual or expected operating performance, financial condition or other indicators of value. Since the stock price of our common stock has fluctuated in the past, has been recently volatile and may be volatile in the future, investors in our common stock could incur substantial losses. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business, financial condition, results of operations and growth prospects. There can be no guarantee that our stock price will remain at current prices or that future sales of our common stock will not be at prices lower than those sold to investors.
Additionally, recently, securities of certain companies have experienced significant and extreme volatility in stock price due to short sellers of shares of common stock, known as a “short squeeze.” These short squeezes have caused extreme volatility in those companies and in the market and have led the price per share of those companies to trade at a significantly inflated rate that is disconnected from the underlying value of the company. Many investors who have purchased shares in those companies at an inflated rate face the risk of losing a significant portion of their original investment as the price per share has declined steadily as interest in those stocks have abated. While we have no reason to believe our shares would be the target of a short squeeze, there can be no assurance that we won’t be in the future, and you may lose a significant portion or all of your investment if you purchase our shares at a rate that is significantly disconnected from our underlying value.
Our articles of incorporation and bylaws and Nevada law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our articles of incorporation, as amended, our second amended and restated bylaws and Nevada law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. The Board of Directors could authorize the issuance of an additional series of preferred stock that would grant holders preferred rights to our assets upon liquidation, special voting rights, the right to receive dividends before dividends would be declared to common stockholders, and the right to the redemption of such shares, possibly together with a premium, prior to the redemption of the common stock. To the extent that we do issue additional preferred stock, the rights of holders of common stock could be impaired thereby, including without limitation, with respect to liquidation.
Provisions of our articles of incorporation, as amended, and our second amended and restated bylaws may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, our articles of incorporation, as amended, and second amended and restated bylaws, among other things:
| ● | provide the board of directors with the ability to alter the bylaws without stockholder approval; and |
| ● | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
We do not intend to pay dividends in the foreseeable future on our common stock.
We have never paid cash dividends on our common stock. We currently intend to retain our future earnings, if any, to finance the operation and growth of our business and currently do not plan to pay any cash dividends in the foreseeable future. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if the market price of our common stock price appreciates.
58
Resales of our common stock in the public market by our stockholders may cause the market price of our common stock to fall.
We may issue common stock from time to time in connection with future offerings. Any issuance from time to time of new shares of our common stock, or our ability to issue shares of common stock in future offerings, could result in resales of our common stock by our current stockholders concerned about the potential dilution of their holdings. In turn, these resales could have the effect of depressing the market price for our common stock.
The shares of common stock offered under our current Amended and Restated At The Market Issuance Sales Agreement may be sold in “at the market” offerings, and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares that are sold under our current Amended and Restated At The Market Issuance Sales Agreement at different times will likely pay different prices, and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold, and there is no minimum or maximum sales price.
Investors may experience declines in the value of their shares as a result of share sales made at prices lower than the prices they paid.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity
We maintain a cyber risk management program designed to identify, assess, manage, mitigate, and respond to cybersecurity threats.
Theriva has not, to date, been subject to any cybersecurity incidents resulting in a material adverse effect on the Company; however, future cyber incidents may occur and the Company has implemented processes to manage, mitigate, and respond to cybersecurity threats. We maintain policies and controls over areas such as information security, access on/offboarding, and access and account management, to help govern the processes put in place by management designed to protect our IT assets, data, and services from threats and vulnerabilities. We partner with third-party information technology (“IT”) providers leveraging third-party technology and expertise. These partners, including consultants and other third-party service providers, are a key part of Theriva’s cybersecurity risk management strategy and infrastructure and provide services including, maintenance of an IT assets inventory, periodic vulnerability scanning, identity access management controls including restricted access of privileged accounts, network integrity safeguarded by employing web-based software, including endpoint protection, endpoint detection and response, and remote monitoring management on all devices, industry-standard encryption protocols, critical data backups, infrastructure maintenance, incident response,, and cyber risk advisory, assessment and remediation.
As part of its review of the adequacy of our system of internal controls over financial reporting and disclosure controls and procedures, management and the Audit Committee oversees cybersecurity risk exposures and the steps taken by management to monitor and mitigate cybersecurity risks. Theriva’s management team is responsible for oversight and administration of cybersecurity risk management strategies, and for informing the Board and other relevant stakeholders regarding the prevention, detection, mitigation, and remediation of cybersecurity incidents. In addition, cybersecurity risks are reviewed by our Board of Directors at least annually, as part of the Company’s corporate risk oversight processes.
We face risks from cybersecurity threats that could have a material adverse effect on our business, financial condition, results of operations, cash flows or reputation. Theriva acknowledges that the risk of cyber incident is prevalent in the current threat landscape and that a future cyber incident may occur in the normal course of its business. We proactively seek to detect and investigate unauthorized attempts and attacks against our IT assets, data, and services, and to prevent their occurrence and recurrence where practicable through changes or updates to internal processes and tools and changes or updates to service delivery; however, potential vulnerabilities to known or unknown threats will remain. Further, there is increasing regulation regarding responses to cybersecurity incidents, including reporting to regulators, investors, and additional stakeholders, which could subject us to additional liability and reputational harm. In response to such risks, we have implemented initiatives such as implementation of the cybersecurity risk assessment process and development of an incident response plan. See Item 1A. "Risk Factors" for more information on cybersecurity risks.
59
Item 2. Properties.
Our corporate headquarters are located at 9605 Medical Center Drive, Suite 270, Rockville, Maryland, where we occupy approximately 10,363 square feet of office space under a lease agreement expiring December 31, 2027, with monthly rent of $27,187. Our Spanish subsidiary Theriva Biologics S.L. (formerly VCN Biosciences S.L.) currently leases approximately 8,611 square feet of office and laboratory space office space from Grifols in Parets de Vallès, Barcelona, Spain with a rent of 26,425 Euros per month. This lease was executed for an initial term beginning in January 2023 until October 2026, with an option to renew for an additional five years.
We do not own any real property. We believe that we have adequate space for our anticipated needs and that suitable additional space will be available at commercially reasonable prices as needed.
Item 3. Legal Proceedings.
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, operating results, financial condition or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock has traded on the NYSE American under the symbol “TOVX’ since October 13, 2022. Prior to October 13, 2022 our common stock traded under the symbol “SYN” since February 16, 2012. Prior to February 16, 2012, our common stock traded under the symbol “AEN” since October 16, 2008. The last price of our common stock as reported on the NYSE American on March 21, 2024 was $0.50 per share.
Dividend Policy
We have never paid or declared any cash dividends on our common stock to date, and do not anticipate paying such cash dividends on our common stock in the foreseeable future. Whether we declare and pay dividends is determined by our Board of Directors at their discretion, subject to certain limitations imposed under Nevada corporate law. The timing, amount and form of dividends, if any, will depend on, among other things, our results of operations, financial condition, cash requirements and other factors deemed relevant by our Board of Directors.
The Series A Preferred Stock, none of which remains outstanding, ranked senior to the shares of our common stock and shares of our Series B Preferred Stock with respect to dividend rights and holders of Series A Preferred Stock, none of which remains outstanding, were entitled to a cumulative dividend at the rate of 2.0% per annum, payable quarterly in arrears, as set forth in the Certificate of Designation of Series A Convertible Preferred Stock. The Series C Preferred Stock and Series D Preferred Stock is entitled to receive dividends on an as-if-converted-to-common-Stock basis to and in the same form as dividends actually paid on shares of the Common Stock when, as and if such dividends are paid on shares of the common stock.
Holders
As of March 21, 2024, we had approximately 50 stockholders of record of our common stock. This number does not include stockholders for whom shares are held in a “nominee” or “street” name.
60
Stock Performance Graph
The Company is a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and is not required to provide the information required under this item.
Equity Compensation Plan Information
See Part III–Item 12 under the heading “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters—Equity Compensation Plan Information” of this Annual Report for equity compensation plan information.
Recent Sales of Unregistered Securities
We did not sell any equity securities during the year ended December 31, 2023 in transactions that were not registered under the Securities Act, other than as previously disclosed in our filings with the SEC.
Issuer Purchases of Equity Securities
We did not repurchase any equity securities during the fourth quarter or year ended December 31, 2023.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion of our financial condition and results of operations should be read in conjunction with our audited financial statements and notes thereto for the years ended December 31, 2023 and 2022 included elsewhere in this Annual Report. In addition to historical information, the following discussion contains certain forward-looking statements that involve risks, uncertainties and assumptions. Where possible, we have tried to identify these forward-looking statements by using words such as “anticipate,” “believe,” “intends,” or similar expressions. Our actual results could differ materially from those expressed or implied by the forward-looking statements due to important factors and risks including, but not limited to, those set forth under “Risk Factors” in Part I, Item 1A of this Annual Report.
Overview
We are a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need. As a result of the acquisition in March 2022 of Theriva Biologics, S.L. (“VCN”, formerly named VCN Biosciences, S.L.), described in more detail below (the “Acquisition”), we began transitioning our strategic focus to oncology, which is now our primary focus, through the development of VCN’s new oncolytic adenovirus platform designed for intravenous and intravitreal delivery to trigger tumor cell death, to improve access of co-administered cancer therapies to the tumor, and to promote a robust and sustained anti-tumor response by the patient’s immune system. Our lead product candidate, VCN-01, a clinical stage oncolytic human adenovirus that is modified to express an enzyme, PH20 hyaluronidase, is currently being administered in a Phase 2 clinical study for the treatment of pancreatic cancer, a Phase 1 clinical study for the treatment of retinalblastoma, a Phase 1 clinical study for the treatment of head and neck squamous cell carcinoma and a Phase 1 clinical study for the treatment of solid tumors.
Prior to the Acquisition, our focus was on developing therapeutics designed to treat gastrointestinal (GI) diseases which included our clinical development candidates: (1) SYN-004 (ribaxamase) which is designed to degrade certain commonly used intravenous (IV) beta-lactam antibiotics within the GI tract to prevent microbiome damage, thereby preventing overgrowth and infection by pathogenic organisms such Clostridioides difficile infection (CDI) and vancomycin resistant Enterococci (VRE), and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients, and (2) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. As part of our strategic transformation into an oncology focused company, we are exploring value creation options for our SYN-004 and SYN-020 assets, including out-licensing or partnering.
61
Financial Developments
Tax Credit Receivable
During the year ended December 31, 2023, we recognized a $1.8 million tax credit receivable and offsetting deferred R&D tax credit. We participate in a research and development program sponsored by the Spanish government. The program provides for reimbursement of certain expenses incurred in research and development efforts we incur in Spain. The reimbursements can be through either tax credits or direct refunds. The program provides for certain limits on the types and amounts of expenses and requires participants to complete a certification and apply for the refund annually. Subsequent to the period in which expenses are incurred, the program requires participants to maintain certain workforce levels and research and development expenditures over a 24-month period. In the quarter ended June 30, 2023, we completed the certification and applied for direct reimbursement, for our qualifying research and development expenses incurred in the year ended December 31, 2022. We received approvals from the Spanish government in September and October 2023. The credit will be amortized as a contra-expense over the two-year period 2024 and 2025.
B Riley and AGP Securities Sales Agreement
During the year ended December 31, 2023, we sold an aggregate of 2.0 million shares of our common stock and received net proceeds of approximately $2.2 million before deducting issuance expenses. During the quarter ended December 31, 2023, we sold an aggregate of 105,284 shares of our common stock and received net proceeds of approximately $62,000.
Our Current Product Pipeline
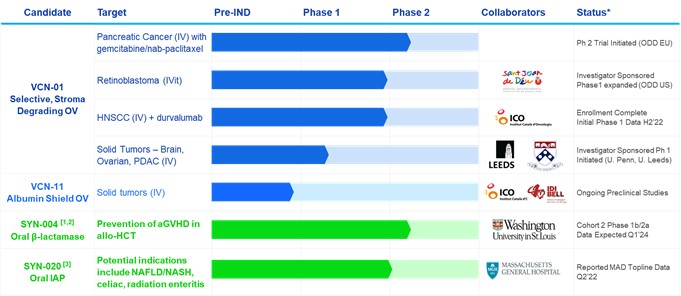
*Based on management’s current beliefs and expectations
allo-HCT allogeneic hematopoietic cell transplant. CPI immune checkpoint inhibitor. HNSCC head and neck squamous cell carcinoma. IV intravenous. IVit intravitreal. ODD Orphan Drug Designation. For other abbreviations see the text.
¹Additional products with preclinical proof-of-concept include SYN-006 (carbapenemase) to prevent aGVHD and infection by carbapenem resistant enterococci and SYN-007 (ribaxamase) DR to prevent antibiotic associated diarrhea with oral β-lactam antibiotics.
²Depending on funding/partnership. SYN-004 may enter an FDA-agreed Phase 3 clinical trial for the treatment of CDI.
³We have an option-license agreement with Massachusetts General Hospital to develop SYN-020 in several potential indications related to inflammation and gut barrier dysfunction.
62
Critical Accounting Estimates
The preparation of our consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP) which requires the use of estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements, and the reported amounts of expenses in the periods presented. We believe that the accounting estimates employed are appropriate and resulting balances are reasonable; however, due to inherent uncertainties in making estimates, actual results may differ from the original estimates, requiring adjustments to these balances in future periods.
There are accounting policies, each of which requires significant judgments and estimates on the part of management, that we believe are significant to the presentation of our consolidated financial statements. The most significant accounting estimates relate to research and development costs, business combinations, contingent consideration, and impairment of long-lived assets.
Business Combination
We account for acquisitions using the acquisition method of accounting, which requires that all identifiable assets acquired, and liabilities assumed be recorded at their estimated fair values. The excess of the fair value of purchase consideration over the fair values of identifiable assets and liabilities is recorded as goodwill. When determining the fair values of assets acquired and liabilities assumed, management makes significant estimates and assumptions. Critical estimates in valuing certain intangible assets include but are not limited to future expected cash flows from acquired patented technology. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
As a result of the acquisition of VCN (see Note 5), we recorded two intangible assets: in-process research and development (“IPR&D”) and goodwill. The IPR&D and goodwill are deemed to have indefinite lives and therefore not amortized.
Goodwill and IPR&D
We classify intangible assets into two categories: (1) intangible assets with indefinite lives not subject to amortization and (2) goodwill. Intangible assets that are deemed to have indefinite lives, including goodwill, are reviewed for impairment annually, or more frequently if events or changes in circumstances indicate that the asset might be impaired. The impairment test for indefinite-lived intangibles, other than goodwill, consists of a comparison of the fair value of the intangible asset with their carrying amount. If the carrying amount exceeds the fair value, an impairment charge is recognized in an amount equal to that excess. Indefinite-lived intangible assets, such as goodwill, are not amortized. We test the carrying amounts of goodwill for recoverability on an annual basis or when events or changes in circumstances indicate evidence a potential impairment exists, using a fair value-based test. If a reporting unit’s carrying value exceeds its fair value, then we will record a goodwill impairment charge for the excess amount.
IPR&D assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development projects. IPR&D assets represent the fair value assigned to technologies that we acquire, which at the time of acquisition have not reached technological feasibility and have no alternative future use. During the period that the assets are considered indefinite-lived, they are tested for impairment on an annual basis, or more frequently if we become aware of any events occurring or changes in circumstances that indicate that the fair value of the IPR&D assets are less than their carrying amounts. If and when development is complete, which generally occurs upon regulatory approval and the ability to commercialize products associated with the IPR&D assets, these assets are then deemed definite-lived and are amortized based on their estimated useful lives at that point in time. If development is terminated or abandoned, we may have a full or partial impairment charge related to the IPR&D assets, calculated as the excess of carrying value of the IPR&D assets over fair value.
Goodwill represents the excess of the purchase price paid when we acquired VCN in March 2022, over the fair values of the acquired tangible or intangible assets and assumed liabilities. We will conduct an impairment test of goodwill on an annual basis as of October 1 of each year and will also conduct tests if events occur or circumstances change that would, more likely than not, reduce our fair value below our net equity value. During the quarters ended September 30, 2023 and December 31, 2022, we experienced a sustained decline in the quoted market price of our common stock and we deemed this to be a triggering event. We performed an impairment analysis and concluded that the Goodwill and IPR&D was not impaired. There were no impairment changes recorded in 2023 or 2022.
63
Contingent Consideration
Consideration paid in a business combination may include potential future payments that are contingent upon the acquired business achieving certain milestones in the future (“contingent consideration”). Contingent consideration liabilities are measured at their estimated fair value as of the date of acquisition, with subsequent changes in fair value recorded in the consolidated statements of operations. We estimate the fair value of the contingent consideration as of the acquisition date using the estimated future cash outflows based on the probability of meeting future milestones. The milestone payments will be made upon the achievement of clinical and commercialization milestones. Subsequent to the date of acquisition, we reassess the actual consideration earned and the probability-weighted future earn-out payments at each balance sheet date. Any adjustment to the contingent consideration liability will be recorded in the consolidated statements of operations. Contingent consideration liabilities expected to be settled within 12 months after the balance sheet date are presented in current liabilities, with the non-current portion recorded under long term liabilities in the consolidated balance sheets.
Long-Lived Assets
Property and equipment is reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. Recoverability measurement and estimating of undiscounted cash flows is done at the lowest possible level for which we can identify assets. If such assets are considered to be impaired, impairment is recognized as the amount by which the carrying amount of assets exceeds the fair value of the assets.
Acquired In-Process Research & Development represents the fair value assigned to those research and development projects that were acquired in a business combination for which the related products have not received regulatory approval and have no alternative future use. IPR&D is capitalized at its fair value as an indefinite-lived intangible asset, and any development costs incurred after the acquisition are expensed as incurred. Upon achieving regulatory approval or commercial viability for the related product, the indefinite-lived intangible asset is accounted for as a finite-lived asset and is amortized on a straight-line basis over the estimated useful life. If the project is not completed or is terminated or abandoned, we may have an impairment related to the IPR&D which is charged to expense. Indefinite-lived intangible assets are tested for impairment annually and whenever events or changes in circumstances indicate that the carrying amount may be impaired. Impairment is calculated as the excess of the asset’s carrying value over its fair value. During the quarters ended September 30, 2023 and December 31, 2022, we experienced a sustained decline in the quoted market price of our common stock and we deemed this to be a triggering event. We performed an impairment analysis and concluded that the Goodwill and IPR&D was not impaired.
Research and Development Costs
We expense research and development costs associated with developmental products not yet approved by the FDA to research and development expense as incurred. Research and development costs consist primarily of license fees (including upfront payments), milestone payments, manufacturing costs, salaries, stock-based compensation and related employee costs, fees paid to consultants and outside service providers for laboratory development, legal expenses resulting from intellectual property prosecution and other expenses relating to the design, development, testing and enhancement of our product candidates. Research and development expenses include external contract research organization (“CRO”) services. We make payments to the CROs based on agreed upon terms and may include payments in advance of study services. We review and accrue CRO expenses based on services performed and rely on estimates of those costs applicable to the stage of completion of study as provided by the CRO. Accrued CRO costs are subject to revisions as such studies progress to completion. At December 31, 2023 and 2022, we have accrued CRO expenses of $1.7 million and $0.8 million, respectively, that are included in accrued expenses. As of December 31, 2023, and 2022, we have prepaid CRO costs of $1.1 million and $2.3 million, respectively, that are included in prepaid expenses.
64
Results of Operations
Years Ended December 31, 2023 and 2022
General and Administrative Expenses
General and administrative expenses decreased to $7.1 million for the year ended December 31, 2023, from $9.9 million for the year ended December 31, 2022. This decrease of 28% is primarily comprised of the decrease in the expense related to the fair value of the contingent consideration of $2.8 million, along with lower salary, investor relations, legal costs, consulting related to the VCN acquisition, and director and officer insurance offset by higher audit fees, and other consulting fees. The charge relating to stock-based compensation expense was $0.4 million for the year ended December 31, 2023, compared to $0.4 million for the year ended December 31, 2022.
Research and Development Expenses
Research and development expenses increased to $14.3 million for the year ended December 31, 2023, from $11.7 million for the year ended December 31, 2022. This increase of 22% is primarily the result of higher clinical trial expenses related to our VIRAGE Phase 2 clinical trial of VCN-01 in PDAC, offset by lower expenses related to our Phase 1b/2a clinical trial of SYN-004 (ribaxamase) in allogeneic HCT recipients, the completed Phase 1a clinical trial of SYN-020, decreased manufacturing expenses related to our Phase 1a clinical trial of SYN-020 and lower other indirect costs. We anticipate research and development expense to increase as we continue enrollment in our VIRAGE Phase 2 clinical trial of VCN-01 in PDAC and finalize our Phase 1 clinical trial in retinoblastoma, expand GMP manufacturing activities for VCN-01, and continue supporting our VCN-11 and other preclinical and discovery initiatives. Research and development expenses also include a charge relating to non-cash stock-based compensation expense of $165,000 for the year ended December 31, 2023, compared to $112,000 for the year ended December 31, 2022. In addition, we expect research and development expenses to increase as we incur higher clinical program costs for our VCN product candidates.
The following table sets forth our research and development expenses directly related to our therapeutic areas for the years ended December 31, 2023 and 2022. These direct expenses were external costs associated with preclinical studies and clinical trials. Indirect research and development costs related to employee costs, facilities, manufacturing, stock-based compensation and research and development support services are not directly allocated to specific drug candidates.
|
|
December 31, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
Therapeutic Areas |
|
(in thousands) |
|
(in thousands) |
||
VCN-01 |
|
$ |
8,153 |
|
$ |
4,344 |
SYN-004 |
|
|
1,095 |
|
|
1,201 |
SYN-020 |
|
|
252 |
|
|
1,069 |
Other therapeutic areas |
|
|
347 |
|
|
528 |
Total direct costs |
|
|
9,847 |
|
|
7,142 |
Total indirect costs |
|
|
4,464 |
|
|
4,581 |
Total research and development |
|
$ |
14,311 |
|
$ |
11,723 |
Total Other Income
Other income was $1,442,000 for the year ended December 31, 2023, compared to other income of $471,000 for the year ended December 31, 2022. Other income for the year ended December 31, 2023 is primarily comprised of interest income of $1,439,000 and an exchange gain of $3,000. Other income for the year ended December 31, 2022 is primarily comprised of interest income of $512,000 offset by an exchange loss of $41,000
Income Tax Benefit
Our income tax benefit was $1.6 million and $1.4 million for the years ended December 31, 2023 and 2022 respectively, solely as a result of net operating losses incurred by our VCN subsidiary acquired in 2022. Our effective tax rate for the years ended December 31, 2023 and 2022 was 8.2% and 6.75%, respectively, which differs from the federal statutory rate of 21% primarily due to the change in our valuation allowance, VCN’s foreign tax benefit, the fair market value adjustment for the contingent consideration, along with the effects of other nondeductible permanent differences.
65
Net Loss
Our net loss for the year ended December 31, 2023 was $18.3 million, or ($1.14) per common share, compared to $19.7 million, or ($1.31) per common share for the year ended December 31, 2022. Net loss attributable to common stockholders for the year ended December 31, 2022 was $20 million and includes the deemed dividend for the effect of the Series C Preferred Stock and Series D Preferred Stock price adjustment of $0.3 million. There was no deemed dividend adjustment in 2023.
Liquidity and Capital Resources
As of December 31, 2023, the Company has a significant accumulated deficit, and with the exception of the three months ended June 30, 2010 and the three months ended December 31, 2017, the Company has experienced significant losses and incurred negative cash flows since inception. We have incurred an accumulated deficit of $309.3 million as of December 31, 2023, and expect to continue to incur losses in the foreseeable future with the recognition of revenue being contingent on successful phase 3 clinical trials and requisite approvals by the FDA or foreign equivalents.
Our cash and cash equivalents totaled $23.2 million as of December 31, 2023, a decrease of $18.6 million from December 31, 2022. During the year ended December 31, 2023, the primary use of cash was for working capital requirements and operating activities which resulted in a net loss of $18.3 million for the year ended December 31, 2023.
With our cash position of $19.0 million in early March 2024, we believe we will be able to fund our operations through the fourth quarter of 2024 and into the first quarter of 2025. Following the anticipated completion of our ongoing Phase 1b/2a clinical study of SYN-004 (ribaxamase) in allogeneic HCT recipients, our ongoing Phase 1 and Phase 2 clinical trials for VCN-01, and the preclinical studies of VCN-11, and related discovery initiatives, we will need to obtain additional funds for future clinical trials. We anticipate that our future clinical trials will be much larger in size and require larger cash expenditures than the aforementioned clinical programs. We do not have any committed sources of financing for future clinical trials at this time, and it is uncertain whether additional funding will be available when we need it on terms that will be acceptable to us, or at all. Management believes its plan, which includes the advancement of VCN-01 and the additional testing of SYN-004 (ribaxamase) will allow us to meet our financial obligations, further advance key products, and maintain our planned operations. However, the amount of additional capital needed by us will also depend upon the costs to advance our VCN-01 clinical programs and whether we continue to develop SYN-004 internally, or out-license or partner such development. If necessary, we may attempt to utilize the ATM or seek to raise additional capital in other financing transactions, neither of which is guaranteed. Use of the ATM is limited by certain restrictions and management’s plan does not rely on additional capital from either of these sources. If we are not able to obtain additional capital (which is not assured at this time), our long-term business plan may not be accomplished, and we may be forced to cease certain development activities. More specifically, the completion of any later stage clinical trial will require significant financing or a significant partnership.
Historically, we have financed our operations primarily through public and private sales of our securities, and we expect to continue to seek and obtain additional capital in a similar manner. During the year ended December 31, 2023, our only source of cash was from sales of our common stock through the Amended and Restated ATM Sales Agreement in which we sold 2.0 million shares of our stock for net proceeds of $2.2 million.
During the year ended December 31, 2022, our only source of cash was from the sales of our Series C Preferred Stock and Series D Preferred Stock.
There can be no assurance that we will be able to continue to raise funds through the sale of shares of common stock through the Amended and Restated ATM Sales Agreement or other equity financings. If we raise funds by selling additional shares of common stock or other securities convertible into common stock, the ownership interest of our existing stockholders will be diluted. If we are not able to obtain funding for future clinical trials when needed, we will be unable to carry out our business plan and we will be forced to delay the initiation of future clinical trials until such time as we obtain adequate financing.
We have committed, and expect to continue to commit, substantial capital in order to implement our business strategy, including our planned product development efforts, preparation for our planned clinical trials, and performance of clinical trials and our research and discovery efforts. We believe our cash position of $19.0 million in early March 2024 is sufficient to fund our operations through at least the end of the fourth quarter of 2024 and into the first quarter 2025, including continuation of our ongoing Phase 1b/2a clinical study of SYN-004 (ribaxamase) in allogeneic HCT recipients for the prevention of aGVHD, our ongoing Phase 1 and Phase 2 clinical trials for VCN-01, preclinical studies of VCN-11 and related discovery initiatives, and to fund our committed obligations under the VCN Purchase Agreement for the VCN Acquisition.
66
We have spent, and expect to continue to spend, a substantial amount of funds in connection with implementing our business strategy, including our planned product development efforts, preparation for our planned clinical trials, performance of clinical trials and our research and discovery efforts. Based on our current plans, our cash and cash equivalents will not be sufficient to enable us to meet our near term or long-term expected plans as it is anticipated that we will not have enough cash to continue our operations for the next twelve months from the date of the filing of this Annual Report. We will be required to obtain additional funding in order to continue the development of certain product candidates within the anticipated time periods (including initiation of planned clinical trials), if at all, and to continue to fund operations at the current cash expenditure levels. We do anticipate that our current cash will allow us to cover overhead costs, manufacturing costs for clinical supply, commercial scale up costs and limited research efforts, including completing our funding requirements for our ongoing Phase 1b/2a clinical study of SYN-004 (ribaxamase) in allogeneic HCT recipients for the prevention of aGVHD, our ongoing Phase 1 and Phase 2 clinical trials for VCN-01, preclinical studies of VCN-11 and related discovery initiatives, and to fund our committed obligations under the VCN Purchase Agreement for the VCN Acquisition.. Our independent registered public accounting firm has issued a report that includes an explanatory paragraph referring to our recurring losses from operations (anticipated continued losses in the future) and net capital deficiency that raise substantial doubt in our ability to continue as a going concern without additional capital becoming available. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Our notes to the consolidated financial statements contain an explanatory paragraph referring to our recurring and continuing losses from operations and expressing substantial doubt in our ability to continue as a going concern without additional capital becoming available. We cannot provide any assurance that we will be able to obtain the required funding to achieve our current business plan, obtain the required regulatory approvals for our product candidates or complete additional corporate partnering or acquisition transactions in order to commercialize such product candidates once regulatory approval is received. If we fail to obtain additional funding for its clinical trials, whether through the sale of securities or a partner or collaborator, and otherwise when needed, we will not be able to execute our business plan as planned and will be forced to cease certain development activities (including initiation of planned clinical trials) until funding is received and our business will suffer, which would have a material adverse effect on our financial position, results of operations and cash flows.
Our ability to continue as a going concern is dependent upon our ability to raise additional capital. Our cash and cash equivalents will not be sufficient to enable us to meet our long-term expected plans, including initiation or completion of future registrational studies for VCN-01, any potential future trials of SYN-004 including Phase 3 clinical programs of SYN-004 (ribaxamase) for prevention of CDI and/or the prevention of aGVHD in allogeneic HCT recipients, or later-stage clinical trials of SYN-020. Therefore, we do not intend to commence future new studies of VCN-01, SYN-004 (ribaxamase) or SYN-020 until we are confident that we have funding necessary to complete such trials. We are actively pursuing additional equity or debt financing, in the form of either a private placement or a public offering and have been in ongoing discussions with strategic institutional investors and investment banks with respect to such possible offerings. However, we do not currently have commitments from any third parties to provide us with capital. Potential sources of financing that we are pursuing include strategic relationships, public or private sales of our equity (including through the FBR Sales Agreement) or debt and other sources. Such additional financing opportunities might not be available to the Company when and if needed, on acceptable terms or at all. We cannot assure that we will meet the requirements for use of the FBR Sales Agreement especially in light of the fact that we are currently limited by rules of the SEC as to the number of shares of common stock that we can sell pursuant to the FBR Sales Agreement due to the market value of our common stock held by non-affiliates. Even if we meet the requirements for use of the FBR Sales Agreement, there can be no assurance that we will be able to continue to raise funds through the sale of shares of common stock through the FBR Sales Agreement. Additionally, we may seek to access the public or private equity markets when conditions are favorable due to our long-term capital requirements. If we are unable to obtain additional capital (which is not assured at this time), our long-term business plan may not be accomplished and we may be forced to cease certain development activities. More specifically, the completion of future Phase 3 and/or registrational clinical studies will require significant financing or a significant partnership. If we raise funds by selling additional shares of common stock or other securities convertible into common stock, the ownership interest of our existing stockholders will be diluted. If we are not able to obtain funding for future clinical trials when needed, we will be unable to carry out our business plan and we will be forced to delay the initiation of future clinical trials until such time as we obtain adequate financing and our operating results and prospects will be adversely affected.
67
Cash Flows
Cash Used in Operating Activities
Net cash used in operating activities was $19.0 million and $19.1 million during the years ended December 31, 2023 and 2022, respectively, which was primarily due to the use of funds in our operations related to the development of VCN-01 our product candidate. Cash used in operating activities for the years ended December 31, 2023 decreased compared to the same period in 2022 due primarily to the payment of contingent consideration to Grifols of $1.7 million and an increase in interest income, which led to a decrease in net loss.
Cash Used In Investing Activities
Cash used in investing activities during the years ended December 31, 2023 was $202,000 for equipment purchases as compared to $4.4 million during the same period in the prior year, which were primarily related to the cash payment for the acquisition of VCN and a pre-acquisition loan to VCN.
Cash Used In Financing Activities
Cash used in financing activities during the year ended December 31, 2023 included at the market offering proceeds of $2.2 million from sales of 2.0 million shares of our common stock which was offset by payment of contingent consideration to Grifols of $1.5 million and $75,000 of payments of debt that we incurred when we acquired VCN. Cash used in financing activities during the year ended December 31, 2022 related to the proceeds received from the issuance of Series C and D preferred stock offset by the payments of contingent consideration of $3.0 million and $1.4 million of debt payments related to loans extended by certain Spanish institutions.
License and Contractual Agreement Obligations
We have entered into several license and collaborative agreements for the right to use research, technology and patents. Some of these license and collaborative agreements may contain milestones. The specific timing of such milestones cannot be predicted and are dependent on future developments as well as regulatory actions which cannot be predicted with certainty (including actions which may never occur). Further, under the terms of certain licensing agreements, we may have the obligation to pay certain milestones contingent upon the achievement of specific levels of sales.
Off-Balance Sheet Arrangements
During the years ended December 31, 2023 and 2022, we did not have, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
Consulting Fees
In November 2017, we engaged a regulatory consultant to assist in our efforts to prepare, file and obtain FDA approval for ribaxamase. The term of the engagement was on a monthly basis, provided that either party may terminate the agreement at any time by providing the other party a six-month notice period. We were obligated to pay the consultant a monthly retainer in addition to the success fee payments of up to an aggregate of $4,500,000 for attainment of certain regulatory milestones. We do not deem the contingent fee is probable at this time.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
The Company is a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and is not required to provide the information required under this item.
68
Item 8. Financial Statements and Supplementary Data.
|
Page |
Report of Independent Registered Public Accounting Firm (BDO USA, P.C.; Raleigh, NC; PCAOB ID# 243) |
70 |
73 |
|
Consolidated Statements of Operations and Comprehensive Loss |
74 |
75 |
|
76 |
|
77 |
69
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
Theriva Biologics, Inc.
Rockville, Maryland
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Theriva Biologics, Inc. (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of operations and comprehensive income, stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations and has not generated positive cash flows from operations which raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Contingent Consideration – Fair value measurement
As discussed in Notes 3 and 5 to the consolidated financial statements, during 2022 the Company completed the acquisition of VCN Biosciences (“VCN”). The purchase consideration transferred included contingent consideration of up to $70.25 million based on the achievement of certain clinical and commercialization milestones of an acquired product, VCN-01, and was initially recorded at its estimated fair value as of the date of acquisition.
70
Subsequent to the date of acquisition, the Company reassesses the fair value at each balance sheet date and the contingent consideration liability was recorded at an estimated fair value of $6.3 million as of December 31, 2023 utilizing the discounted cash flow method.
We identified the determination of the fair value of the contingent consideration liability as a critical audit matter. Under the discounted cash flow method, the key estimates and assumptions used in the valuation of the contingent consideration liability included management’s determination of the probability weighted estimates of future earn-out payments based on successful achievement of certain clinical and commercialization milestones, and the estimated discount rate applicable to the future payment periods. Changes to these key estimates and assumptions could have a significant impact on the fair value of the contingent consideration liability. Auditing management’s valuation methods and these assumptions involve especially challenging and subjective auditor judgment due to the nature and extent of auditor effort required to address these matters, including the specialized knowledge and skill needed.
The primary procedures we performed to address this critical audit matter included:
| - | Assessing the reasonableness of management’s probability weighted estimates of future earn-out payments based on successful achievement of certain clinical and commercialization milestones by comparing to relevant industry studies. |
| - | Utilizing personnel with specialized knowledge and skills in valuation to assist in: |
| o | evaluating the appropriateness of the valuation method; |
| o | testing the mathematical accuracy of the Company’s calculations; and |
| o | evaluating the discount rate applied to future milestone payment periods. |
In-Process Research and Development and Goodwill Impairment Assessment
As described in Notes 3 and 6 to the consolidated financial statements, the Company’s consolidated balances of In-process Research and Development (“IPR&D”) indefinite-lived intangible asset and Goodwill were $19.8 million and $5.7 million, respectively, as of December 31, 3023. The Company reviews goodwill for impairment at least annually and or more frequently if events or circumstances indicate the carrying value at the reporting unit level might exceed its fair value. The IPR&D indefinite-lived intangibles are tested annually for impairment, or more frequently if events or circumstances indicate it is more likely than not the fair value is less than their carrying value. The Company estimates the fair value of its reporting unit and certain IPR&D using an income approach. The Company identified a triggering event during 2023 and performed an impairment analysis for Goodwill and certain IPR&D resulting in no impairment charges being recorded.
We identified the determination of the fair value of the Company’s reporting unit and certain IPR&D as a critical audit matter. Under the income approach, the key assumptions used in the determination of the fair value of the reporting unit include estimates of future cash flows, the discount rate applicable to those future cash flow periods, and the implied control premium. The key assumptions used in the determination of the fair value of certain IPR&D assets using the income approach include estimates of future cash flows and the discount rate applicable to those future cash flow periods. Changes to these key assumptions could have a significant impact on the measurement of the fair value of the reporting unit and certain IPR&D. Auditing management’s valuation methods and these assumptions involve especially challenging and subjective auditor judgment due to the nature and extent of auditor effort required to address these matters, including the specialized knowledge and skill needed.
The primary procedures we performed to address this critical audit matter included:
| - | Evaluating reasonableness of estimated future cash flows by comparing forecasts to historical results. |
| - | Utilizing personnel with specialized knowledge and skills in valuation to assist in: |
| o | evaluating the reasonableness of valuation methods; |
| o | testing the mathematical accuracy of the Company’s calculations; |
71
| o | evaluating the reasonableness of assumptions for goodwill, including the discount rate applied to future cash flows, and implied control premium; |
| o | evaluating the reasonableness of assumptions for IPR&D, including the discount rate applied to future cash flow assumptions; |
| o | evaluating the reasonableness of the implied control premium by comparing the market capitalization of the Company to the fair value determined for the Company’s reporting unit. |
/s/ BDO USA, P.C.
Raleigh, North Carolina
March 25, 2024
We have served as the Company's auditor since 2012.
72
Theriva Biologics, Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands except share and par value amounts)
|
|
December 31, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
23,177 |
|
$ |
41,786 |
Tax credit receivable |
|
|
1,812 |
|
|
— |
Prepaid expenses and other current assets |
|
|
2,414 |
|
|
3,734 |
Total Current Assets |
|
|
27,403 |
|
|
45,520 |
|
|
|
|
|
|
|
Non-Current Assets |
|
|
|
|
|
|
Property and equipment, net |
|
|
422 |
|
|
345 |
Restricted cash |
|
|
102 |
|
|
99 |
Right of use asset |
|
|
1,759 |
|
|
1,199 |
In-process research and development |
|
|
19,755 |
|
|
19,150 |
Goodwill |
|
|
5,700 |
|
|
5,525 |
Deposits and other assets |
|
|
78 |
|
|
23 |
Total Assets |
|
$ |
55,219 |
|
$ |
71,861 |
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
770 |
|
$ |
915 |
Accrued expenses |
|
|
2,995 |
|
|
1,496 |
Accrued employee benefits |
|
|
1,517 |
|
|
1,403 |
Contingent consideration, current portion |
|
|
— |
|
|
2,973 |
Deferred research and development tax credit-current portion |
|
|
906 |
|
|
— |
Loans payable-current |
|
|
63 |
|
|
57 |
Operating lease liability-current portion |
|
|
487 |
|
|
216 |
Total Current Liabilities |
|
|
6,738 |
|
|
7,060 |
|
|
|
|
|
|
|
Non-current Liabilities |
|
|
|
|
|
|
Non-current contingent consideration |
|
|
6,274 |
|
|
7,211 |
Loan Payable - non-current |
|
|
162 |
|
|
221 |
Deferred tax liabilities, net |
|
|
— |
|
|
1,618 |
Non-current deferred research and development tax credit |
|
|
906 |
|
|
— |
Non-current operating lease liability |
|
|
1,442 |
|
|
1,187 |
Total Liabilities |
|
|
15,522 |
|
|
17,297 |
|
|
|
|
|
|
|
Commitments and Contingencies |
|
|
— |
|
|
— |
Temporary Equity |
|
|
|
|
|
|
Series C convertible preferred stock, $0.001 par value; 10,000,000 authorized;275,000 issued and outstanding |
|
|
2,006 |
|
|
2,006 |
Series D convertible preferred stock, $0.001 par value; 10,000,000 authorized;100,000 issued and outstanding |
|
|
728 |
|
|
728 |
Stockholders’ Equity: |
|
|
|
|
|
|
Common stock, $0.001 par value; 350,000,000 shares authorized, 17,868,282 issued and 17,148,049 outstanding at December 31, 2023 and 15,844,061 issued and 15,123,828 outstanding at December 31, 2022 |
|
|
18 |
|
|
16 |
Additional paid-in capital |
|
|
346,519 |
|
|
343,750 |
Treasury stock at cost, 720,233 shares at December 31, 2023 and at December 31, 2022 |
|
|
(288) |
|
|
(288) |
Accumulated other comprehensive income (loss) |
|
|
32 |
|
|
(679) |
Accumulated deficit |
|
|
(309,318) |
|
|
(290,969) |
Total Stockholders‘ Equity |
|
|
36,963 |
|
|
51,830 |
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
|
$ |
55,219 |
|
$ |
71,861 |
See accompanying notes to consolidated financial statements
73
Theriva Biologics, Inc. and Subsidiaries
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
|
|
For the year ended |
||||
|
|
December 31, |
||||
|
|
2023 |
|
2022 |
||
Operating Costs and Expenses: |
|
|
|
|
|
|
General and administrative |
|
$ |
7,120 |
|
$ |
9,858 |
Research and development |
|
|
14,311 |
|
|
11,723 |
Total Operating Costs and Expenses |
|
|
21,431 |
|
|
21,581 |
Loss from Operations |
|
|
(21,431) |
|
|
(21,581) |
Other Income: |
|
|
|
|
|
|
Foreign currency exhange gain (loss) |
|
|
3 |
|
|
(41) |
Interest income |
|
|
1,439 |
|
|
512 |
Total Other Income |
|
|
1,442 |
|
|
471 |
|
|
|
|
|
|
|
Net Loss before income taxes |
|
|
(19,989) |
|
|
(21,110) |
Income tax benefit |
|
|
1,640 |
|
|
1,425 |
|
|
|
|
|
|
|
Net Loss Attributable to Theriva Biologics, Inc. and Subsidiaries |
|
$ |
(18,349) |
|
$ |
(19,685) |
|
|
|
|
|
|
|
Effect of Warrant exercise price adjustment |
|
|
— |
|
|
(340) |
|
|
|
|
|
|
|
Net Loss Attributable to Common Stockholders |
|
$ |
(18,349) |
|
$ |
(20,025) |
|
|
|
|
|
|
|
Net Loss Per Share - Basic and Dilutive |
|
$ |
(1.14) |
|
$ |
(1.31) |
|
|
|
|
|
|
|
Weighted average number of shares outstanding during the period - basic and dilutive |
|
|
16,107,014 |
|
|
15,327,328 |
|
|
|
|
|
|
|
Net Loss |
|
|
(18,349) |
|
|
(19,685) |
Gain (loss) on foreign currency translation |
|
|
711 |
|
|
(679) |
Total comprehensive loss |
|
|
(17,638) |
|
|
(20,364) |
See accompanying notes to consolidated financial statements
74
Theriva Biologics, Inc. and Subsidiaries
Consolidated Statements of Stockholder’s Equity
(In thousands, except share and par value amounts)
|
|
Common Stock $0.001 Par Value |
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
||||
|
|
|
|
|
|
|
Additional |
|
|
|
Other |
|
|
|
Total |
||||
|
|
|
|
|
|
|
Paid-in |
|
Accumulated |
|
Comprehensive |
|
|
|
Stockholders’ |
||||
|
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
Loss |
|
Treasury Stock |
|
Equity |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2021 |
|
13,204,531 |
|
$ |
13 |
|
$ |
336,679 |
|
$ |
(271,284) |
|
$ |
— |
|
— |
|
$ |
65,408 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
— |
|
|
— |
|
|
475 |
|
|
— |
|
|
— |
|
— |
|
|
475 |
Issuance of Common Stock for VCN Acquisition |
|
2,639,530 |
|
|
3 |
|
|
6,596 |
|
|
— |
|
|
— |
|
— |
|
|
6,599 |
Foreign currency exhange loss |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(679) |
|
— |
|
|
(679) |
Treasury Stock |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
(288) |
|
|
(288) |
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(19,685) |
|
|
— |
|
— |
|
|
(19,685) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2022 |
|
15,844,061 |
|
$ |
16 |
|
$ |
343,750 |
|
$ |
(290,969) |
|
$ |
(679) |
|
(288) |
|
$ |
51,830 |
|
|
Common Stock $0.001 Par Value |
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
||||
|
|
|
|
|
|
|
Additional |
|
|
|
|
Other |
|
|
|
Total |
|||
|
|
|
|
|
|
|
Paid-in |
|
Accumulated |
|
Comprehensive |
|
|
|
Stockholders’ |
||||
|
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
Income |
|
Treasury Stock |
|
Equity |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2022 |
|
15,844,061 |
|
$ |
16 |
|
$ |
343,750 |
|
$ |
(290,969) |
|
$ |
(679) |
|
(288) |
|
$ |
51,830 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
— |
|
|
— |
|
|
552 |
|
|
— |
|
|
— |
|
— |
|
|
552 |
Stock issued under “at-the-market” offering |
|
2,024,221 |
|
|
2 |
|
|
2,217 |
|
|
— |
|
|
— |
|
— |
|
|
2,219 |
Foreign currency exhange gains |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
711 |
|
— |
|
|
711 |
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(18,349) |
|
|
— |
|
— |
|
|
(18,349) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2023 |
|
17,868,282 |
|
$ |
18 |
|
$ |
346,519 |
|
$ |
(309,318) |
|
$ |
32 |
|
(288) |
|
$ |
36,963 |
See accompanying notes to consolidated financial statements
75
Theriva Biologics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
|
|
For the year ended |
||||
|
|
2023 |
|
2022 |
||
Cash Flows From Operating Activities: |
|
|
|
|
|
|
Net loss |
|
$ |
(18,349) |
|
$ |
(19,685) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Stock-based compensation |
|
|
552 |
|
|
475 |
Income tax benefit |
|
|
(1,640) |
|
|
(1,425) |
Change in fair value of contingent consideration |
|
|
(660) |
|
|
2,091 |
Payment of contingent consideration |
|
|
(1,731) |
|
|
— |
Non - cash lease expense |
|
|
388 |
|
|
183 |
Depreciation |
|
|
135 |
|
|
85 |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
1,368 |
|
|
(363) |
Deposits and other assets |
|
|
(54) |
|
|
— |
Accounts payable |
|
|
(157) |
|
|
(385) |
Accrued expenses |
|
|
1,470 |
|
|
(267) |
Accrued employee benefits |
|
|
104 |
|
|
333 |
Operating lease liability |
|
|
(422) |
|
|
(124) |
Net Cash Used In Operating Activities |
|
|
(18,996) |
|
|
(19,082) |
|
|
|
|
|
|
|
Cash Flows From Investing Activities: |
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(202) |
|
|
(116) |
Cash paid for business combination; net of cash acquired |
|
|
— |
|
|
(3,863) |
Pre-acquisition loan to VCN |
|
|
— |
|
|
(417) |
Net Cash Used In Investing Activities |
|
|
(202) |
|
|
(4,396) |
|
|
|
|
|
|
|
Cash Flows From Financing Activities: |
|
|
|
|
|
|
Payment of loans payable |
|
$ |
(75) |
|
|
(1,376) |
Proceeds from issuance under at - the - market offering, net of issuance cost |
|
|
2,219 |
|
|
— |
Proceeds from sale of Series C Preferred Stock, net of issuance cost |
|
|
— |
|
|
2,006 |
Proceeds from sale of Series D Preferred Stock, net of issuance cost |
|
|
— |
|
|
728 |
Payment of contingent consideration |
|
|
(1,519) |
|
|
(3,000) |
Purchase of treasury stock |
|
|
— |
|
|
(288) |
Net Cash Provided By (Used In) Financing Activities |
|
|
625 |
|
|
(1,930) |
|
|
|
|
|
|
|
Effects of foreign currency on cash |
|
|
(33) |
|
|
(32) |
Net decrease in cash and cash equivalents and restricted cash |
|
|
(18,606) |
|
|
(25,408) |
Cash and cash equivalents and restricted at the beginning of this period |
|
|
41,885 |
|
|
67,325 |
Cash and cash equivalents and restricted cash at the end of this period |
|
$ |
23,279 |
|
$ |
41,885 |
|
|
|
|
|
|
|
Reconciliation of cash, cash equivalents, and restricted cash reported in the statement of financial position |
|
|
|
|
|
|
Cash and cash equivalents |
|
|
23,177 |
|
|
41,786 |
Restricted cash included in other long-term assets |
|
|
102 |
|
|
99 |
Total cash, cash equivalents, and restricted cash shown in the statement of cash flows |
|
$ |
23,279 |
|
$ |
41,885 |
|
|
|
|
|
|
|
Supplemental non-cash investing and financing activities: |
|
|
|
|
|
|
Right of use assets obtained in exchange for lease liabilities |
|
$ |
937 |
|
$ |
— |
Fair value of contingent consideration issued in a business combination |
|
$ |
— |
|
$ |
11,093 |
Fair value of equity issued as consideration in a business combination |
|
$ |
— |
|
$ |
6,599 |
Effective settlement of pre-closing VCN financing |
|
$ |
— |
|
$ |
417 |
Goodwill measurement period adjustment |
|
$ |
— |
|
$ |
(1,061) |
In-process R&D measurement period adjustment |
|
$ |
— |
|
$ |
810 |
Deferred tax liability measurement period adjustment |
|
$ |
— |
|
$ |
202 |
Effect of Warrant exercise price adjustment |
|
$ |
— |
|
$ |
340 |
See accompanying notes to consolidated financial statements
76
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Organization and Nature of Operations and Basis of Presentation
Description of Business
Theriva Biologics, Inc. (the “Company” or “Theriva Biologics”) is a diversified clinical-stage company developing therapeutics in areas of high unmet need. As a result of the acquisition of Theriva Biologics S.L. (“VCN”, formerly known as VCN Biosciences, S.L.) (the “Acquisition”), described in more detail below, the Company transitioned its strategic focus to oncology through the development of VCN’s new oncolytic adenovirus platform designed for intravenous and intravitreal delivery to trigger tumor cell death, to improve access of co-administered cancer therapies to the tumor, and to promote a robust and sustained anti-tumor response by the patient’s immune system. Prior to the Acquisition, the Company’s focus was on developing therapeutics designed to treat gastrointestinal (GI) diseases in areas which included its clinical development candidates: (1) SYN-004 (ribaxamase) which is designed to degrade certain commonly used intravenous (IV) beta-lactam antibiotics within the GI tract to prevent microbiome damage thereby preventing overgrowth and infection by pathogenic organisms such as Clostridioides difficile infection (CDI), and vancomycin resistant Enterococci (VRE), and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients, and (2) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. On October 12, 2022, the Company changed its name to Theriva Biologics, Inc. In connection with the name change, its common stock began trading on the NYSE American LLC under the new ticker symbol “TOVX” effective as of the opening of trading hours on October 13, 2022. Effective November 15, 2022, the Company’s acquired subsidiary VCN Biosciences, S.L. rebranded to Theriva Biologics, S.L. without other changes to its corporate structure.
Corporate Structure and Basis of Presentation
On July 11, 2022, the Board of Directors of the Company approved a reverse stock split of the Company’s authorized, issued and outstanding shares of common stock, par value $0.001 per share, at a ratio of one (1) share of common stock for every ten (10) shares of common stock (the “Reverse Stock Split”). The Reverse Stock Split was effective on July 25, 2022 (the “Effective Time).
As a result of the Reverse Stock Split, each ten (10) pre-split shares of common stock outstanding automatically combined into one (1) new share of common stock without any action on the part of the holders, and the number of outstanding shares of common stock was reduced from 158,437,840 shares to 15,844,061 shares (subject to rounding of fractional shares) and the number of authorized shares of common stock was reduced from 200,000,000 share to 20,000,000 shares and then increased to 350,000,000 after obtaining approval of the Company’s shareolders at the 2022 annual meeting of stockholders. Stockholders who otherwise were entitled to receive fractional shares because they held a number of pre-reverse stock split shares of the Company’s common stock not evenly divisible by 10, received, in lieu of a fractional share, that number of shares rounded up to the nearest whole share. The Reverse Stock Split did not alter the par value of the Company’s common stock or modify any voting rights or other terms of the common stock. In addition, pursuant to their terms, a proportionate adjustment was made to the per share conversion exercise price and number of shares issuable under all of the Company’s outstanding shares of convertible preferred stock and stock options and warrants to purchase shares of common stock, and the number of shares authorized and reserved for issuance pursuant to the Company’s equity incentive plans was reduced proportionately.
All affected share amounts and exercise/conversion prices in the condensed consolidated financial statements and footnotes below have been adjusted retrospectively for the Reverse Stock Split.
As of December 31, 2023, the Company had nine subsidiaries, Theriva Biologics, S.L., Pipex Therapeutics, Inc. (“Pipex Therapeutics”), Effective Pharmaceuticals, Inc. (“EPI”), Solovax, Inc. (“Solovax”), CD4 Biosciences, Inc. (“CD4”), Epitope Pharmaceuticals, Inc. (“Epitope”), Healthmine, Inc. (“Healthmine”), Putney Drug Corp. (“Putney”) and Synthetic Biomics, Inc. (“SYN Biomics”). Theriva Biologics, S.L.,Pipex Therapeutics, EPI, Healthmine, Putney and SYN Biomics are wholly owned, and Solovax, CD4, and Epitope are majority-owned.
77
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Organization and Nature of Operations and Basis of Presentation – (continued)
For financial reporting purposes, the outstanding common stock of the Company is that of Theriva Biologics, Inc. All statements of operations, equity and cash flows for each of the entities are presented as consolidated. All subsidiaries were formed under the laws of the State of Delaware on January 8, 2001, except for EPI, which was incorporated in Delaware on December 12, 2000, Epitope which was incorporated in Delaware in January 2002, Putney which was incorporated in Delaware in November 2006, Healthmine which was incorporated in Delaware in December 2007 and SYN Biomics which was incorporated in Nevada in December 2013.
Liquidity
As of December 31, 2023, the Company had a significant accumulated deficit of $309,318, and the Company has experienced significant losses and incurred negative cash flows since inception. The Company expects to continue incurring losses for the foreseeable future, with the recognition of revenue being contingent on successful phase 3 clinical trials and requisite approvals by the FDA or foreign equivalents. Historically, the Company has financed its operations primarily through public and private sales of its common stock and a private placement of its preferred stock, and it expects to continue to seek to obtain required capital in a similar manner. The Company has spent, and expects to continue to spend, a substantial amount of funds in connection with implementing its business strategy, including planned product development efforts, clinical trials and research and discovery efforts.
The Company’s cash and cash equivalents totaled $23.2 million as of December 31, 2023, a decrease of $18.6 million from December 31, 2022. During the year ended December 31, 2023, the primary use of cash was for working capital requirements and operating activities which resulted in a net loss of $18.3 million. The Company believes it will be able to fund its operations through the fourth quarter of 2024 and into the first quarter of 2025. However, the actual amount of additional capital needed by the Company will also depend upon the costs to advance its VCN-01 clinical programs and whether it continues to develop SYN-004 internally, or out-licenses or partners such development. If necessary, the Company may attempt to utilize the at-the-market offering facility (“ATM”) or seek to raise additional capital in other financing transactions, neither of which is guaranteed. Use of the ATM is limited by certain restrictions and management’s plan does not rely on additional capital from either of these sources. If the Company is not able to obtain additional capital (which is not assured at this time), its business plan may not be accomplished, and it may be forced to cease certain development activities. More specifically, the completion of any later stage clinical trial will require significant financing or a significant partnership.
2. Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company continues to incur losses and, as of December 31, 2023, the Company had an accumulated deficit of approximately $309.3 million. Since inception, the Company has financed its activities principally from the proceeds from the issuance of equity securities.
The Company’s ability to continue as a going concern is dependent upon the Company’s ability to raise additional debt and equity capital. There can be no assurance that such capital will be available in sufficient amounts or on terms acceptable to the Company. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability of the recorded assets or the classification of liabilities that may be necessary should the Company be unable to continue as a going concern.
The Company does not have sufficient capital to fund its operations beyond the next twelve months. In order to address the Company’s capital needs, including its planned clinical trials, the Company is actively pursuing additional equity or debt financing in the form of either a private placement or a public offering. The Company has been in ongoing discussions with strategic institutional investors and investment banks with respect to such possible offerings. Such additional financing opportunities might not be available to the Company when and if needed, on acceptable terms or at all. If the Company is unable to obtain additional financing in sufficient amounts or on acceptable terms under such circumstances, the Company’s operating results and prospects will be adversely affected.
78
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
2. Going Concern – (continued)
At December 31, 2023 the Company had cash and cash equivalents of approximately $23.2 million. Based upon the Company’s current business plans, management believes that the Company’s current cash on hand will be sufficient to fully execute its plans through December 31, 2024. Commencement of planned future clinical trials is subject to the Company’s successful pursuit of opportunities that will allow it to establish the clinical infrastructure and financial resources necessary to successfully initiate and complete its plan. The Company anticipates its current cash will allow it to cover overhead costs, manufacturing costs for clinical supply, commercial scale up costs and limited research efforts, including completing its funding requirements for its ongoing current trials for VCN-01 and the on-going testing of SYN-004 (ribaxamase). The Company will be required to obtain additional funding in order to continue the development of its current product candidates within the anticipated time periods (including initiation of its planned future clinical trials), if at all, and to continue to fund operations at the current cash expenditure levels. Currently, the Company does not have commitments from any third parties to provide it with capital. Potential sources of financing include strategic relationships, public or private sales of equity (including through the ATM sales agreement) or debt and other sources. The Company cannot assure that it will meet the requirements for use of the ATM Sales Agreement or that additional funding will be available on favorable terms, or at all. Current cash is expected to cover overhead costs, manufacturing costs for clinical supply, commercial scale up costs and limited research efforts. If the Company fails to obtain additional funding for its clinical trials, whether through the sale of securities or a partner or collaborator, and otherwise when needed, it will not be able to execute its business plan as planned and will be forced to cease certain development activities (including initiation of planned clinical trials) until funding is received and its business will suffer, which would have a material adverse effect on its financial position, results of operations and cash flows.
The actual amount of funds the Company will need to operate is subject to many factors, some of which are beyond its control. These factors include the following:
| ● | the progress of its research activities; |
| ● | the number and scope of its research programs; |
| ● | the ability to recruit patients for clinical studies in a timely manner; |
| ● | the progress of its preclinical and clinical development activities; |
| ● | the progress of the development efforts of parties with whom the Company has entered into research and development agreements and amount of funding received from partners and collaborators; |
| ● | its ability to maintain current research and development licensing arrangements and to establish new research and development and licensing arrangements; |
| ● | the Company’s ability to achieve its milestones under licensing arrangements; |
| ● | the costs associated with manufacturing-related services to produce material for use in its clinical trials; |
| ● | the costs involved in prosecuting and enforcing patent claims and other intellectual property rights; and |
| ● | the costs and timing of regulatory approvals. |
The Company has based its estimates of funding requirements on assumptions that may prove to be wrong. The Company may need to obtain additional funds sooner or in greater amounts than it currently anticipates.
If the Company raises funds by selling additional shares of common stock or other securities convertible into common stock, the ownership interest of the existing stockholders will be diluted. If the Company is not able to obtain financing when needed, it may be unable to carry out its business plan. As a result, the Company may have to significantly limit its operations and its business, financial condition and results of operations would be materially harmed.
79
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies
Principles of Consolidation
All intercompany transactions and accounts have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Such estimates and assumptions impact, among others, the following: the estimated useful lives for property and equipment, research and development costs, business combinations, contingent consideration, fair value of long-lived assets, warrants, preferred stock and stock options granted for services or compensation, respectively, and the valuation allowance for deferred tax assets due to continuing and expected future operating losses.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of consolidated financial statements, which management considered in formulating its estimate could change in the near term due to one or more future confirming events. Accordingly, actual results could differ from those estimates.
Risks and Uncertainties
The Company’s operations could be subject to significant risks and uncertainties including financial, operational and regulatory risks and the potential risk of business failure. These conditions may not only limit the Company’s access to capital, but also make it difficult for its customers, its vendors and its ability to accurately forecast and plan future business activities.
Cash and Cash Equivalents
Cash and cash equivalents include cash and highly liquid short-term investments with original maturities of three months or less. All interest bearing and non-interest bearing accounts are guaranteed by the Federal Deposit Insurance Corporation (“FDIC”) up to $250 thousand. The majority of the Company’s cash balances are in excess of FDIC coverage. The Company considers this to be a normal business risk.
Property and Equipment
Property and equipment is recorded at cost and depreciated or amortized using the straight-line method over the estimated useful life of the asset or the underlying lease term for leasehold improvements, whichever is shorter. The estimated useful life by asset description is noted in the following table.
Asset Description |
|
Estimated Useful Life |
Computer, office equipment, furniture and software |
|
3 – 5 years |
Leasehold improvements and fixtures |
|
Lesser of estimated useful life or lease term |
Depreciation and amortization expense was approximately $135,000 and $85,000 for the years ended December 31, 2023 and 2022, respectively. When assets are disposed of, the cost and accumulated depreciation are removed from the accounts with any gain or loss reported in the consolidated statement of operations. Repairs and maintenance are charged to expense as incurred.
The Company reviews property and equipment for impairment to determine if assets are impaired due to obsolescence. As a result of this review, there was no impairment recognized for the years ended December 31, 2023 and 2022.
80
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies – (continued)
Business Combination
The Company accounts for acquisitions using the acquisition method of accounting, which requires that all identifiable assets acquired, and liabilities assumed be recorded at their estimated fair values. The excess of the fair value of purchase consideration over the fair values of identifiable assets and liabilities is recorded as goodwill. When determining the fair values of assets acquired and liabilities assumed, management makes significant estimates and assumptions. Critical estimates in valuing certain intangible assets include but are not limited to future expected cash flows from acquired patented technology. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
As a result of the acquisition of VCN (see Note 5), the Company recorded two intangible assets: in-process research and development (“IPR&D”) and goodwill. The IPR&D and goodwill are deemed to have indefinite lives and therefore not amortized.
IPR&D
IPR&D assets represent the fair value assigned to technologies that the Company acquired, which at the time of acquisition have not reached technological feasibility and have no alternative future use. IPR&D assets are considered to have indefinite-lives until the completion or abandonment of the associated research and development projects. If and when development is complete, which generally occurs upon regulatory approval and the ability to commercialize products associated with the IPR&D assets, these assets are then deemed to have definite lives and are amortized based on their estimated useful lives at that point in time. If development is terminated or abandoned, the Company may have a full or partial impairment charge related to the IPR&D assets, calculated as the excess of carrying value of the IPR&D assets over fair value.
During the period that the assets are considered indefinite-lived, they are tested for impairment on an annual basis on October 1, or more frequently if the Company becomes aware of any events occurring or changes in circumstances that could indicate an impairment. The impairment test consists of a comparison of the estimated fair value of the IPR&D with its carrying amount. If the carrying amount exceeds the fair value, an impairment charge is recognized in an amount equal to that excess. The key assumptions used to value IPR&D include estimates of future cash flows and to the discount rate applicable to the future cash flow periods.
During the quarter ended September 30, 2023, the Company experienced a sustained decline in the quoted market price of the Company’s common stock and the Company deemed this to be a triggering event for impairment. As a result the Company performed an impairment analysis and concluded that there was no impairment as of September 30, 2023. This interim analysis satisfied the requirements of the annual impairment test as the same information would be required for both measurement dates. There were no impairment charges recorded during 2023 and 2022.
Goodwill
The Company tests the carrying amounts of goodwill for recoverability on an annual basis on October 1 or more frequently if events or changes in circumstances indicate that the asset might be impaired. The Company performs a one-step test in its evaluation of the carrying value of goodwill if qualitative factors determine it is necessary to complete a goodwill impairment test. In the evaluation, the fair value of the relevant reporting unit is determined and compared to its carrying value. If the fair value is greater than the carrying value, then the carrying value is deemed to be recoverable, and no further action is required. If the fair value estimate is less than the carrying value, goodwill is considered impaired for the amount by which the carrying amount exceeds the reporting unit’s fair value, and a charge is reported in impairment of goodwill in the Company’s consolidated statements of operations. The key assumptions used to value the reporting unit include estimates of future cash flows, the discount rate applicable and those future cash flow periods, and the implied control premium.
81
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies – (continued)
During the quarter ended September 30, 2023, the Company experienced a sustained decline in the quoted market price of the Company’s common stock and the Company deemed this to be a triggering event for impairment. As a result the Company performed an impairment analysis and concluded that there was no impairment as of September 30, 2023. This interim analysis satisfied the requirements of the annual impairment test as the same information would be required for both measurement dates. There were no impairment charges as of December 31, 2023 and 2022.
Contingent Consideration
Consideration paid in a business combination may include potential future payments that are contingent upon the acquired business achieving certain milestones in the future (“contingent consideration”). Contingent consideration liabilities are measured at their estimated fair value as of the date of acquisition, with subsequent changes in fair value recorded in the consolidated statements of operations. The Company estimates the fair value of the contingent consideration as of the acquisition date using the estimated future cash outflows based on the probability of meeting future milestones. Payments for amounts not in excess of original fair values established at acquisition date (including measurement period adjustments), and not paid within a period considered to be close to the transaction date, are reflected as financing activities in the statement of cash flows. Subsequent to the date of acquisition, the Company reassesses the actual consideration earned and the probability-weighted future earn-out payments at each balance sheet date. The discounted cash flow is method used to value the contingent consideration which includes inputs of not readily observable market data, which are level 3 inputs. Any adjustment to the contingent consideration liability will be recorded in the consolidated statements of operations. Contingent consideration liabilities expected to be settled within 12 months after the balance sheet date are presented in current liabilities, with the non-current portion recorded under long-term liabilities in the consolidated balance sheets. See Fair Value of Financial Instruments below.
Long-Lived Assets Impairment
Long-lived assets include property, equipment, and right of use assets. Management reviews the Company’s long-lived assets for impairment annually or whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be fully recoverable. The judgments made related to the expected useful lives of long-lived assets, definitions of lease terms and the Company’s ability to realize undiscounted cash flows in excess of the carrying amounts of these assets are affected by factors such as the ongoing maintenance and improvements of the assets, changes in economic conditions, changes in usage or operating performance and other factors. The Company determines the extent to which an asset may be impaired based upon its expectation of the asset’s future usability as well as whether there is reasonable assurance that the future cash flows associated with the asset will be in excess of its carrying amount. If the total of the expected undiscounted future cash flows is less than the carrying amount of the asset, a loss is recognized for the difference between the fair value and the carrying value of the asset. No impairment charges were recorded during the year ended December 31, 2023 and 2022.
Loss per Share
Basic net loss per share is computed by dividing net loss attributable to common shareholders by the weighted average number of common shares outstanding. Diluted net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding including the effect of common share equivalents. Diluted net loss per share assumes the issuance of potential dilutive common shares outstanding for the period and adjusts for any changes in income and the repurchase of common shares that would have occurred from the assumed issuance, unless such effect is anti-dilutive. Net loss attributable to common stockholders for the year ended December 31, 2022 includes the effect of the Series C and D preferred stock price adjustment of $0.3 million. The number of shares of common stock underlying Series C and D Preferred shares convertible to common stock that were excluded from the computation of the net loss per common share for the year ended December 31, 2023 and 2022 was 2,459,016. The number of eligible options and warrants for the purchase of common stock that were excluded from the computations of net loss per common share for the year ended December 31, 2023 were 4,375,781 and zero, respectively, and for the year ended December 31, 2022 were 2,295,898 and 634,426, respectively, because their effect is anti-dilutive.
82
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies – (continued)
Research and Development Costs
The Company expenses research and development costs associated with developmental products not yet approved by the FDA to research and development expense as incurred. Research and development costs consist primarily of license fees (including upfront payments), milestone payments, manufacturing costs, salaries, stock-based compensation and related employee costs, fees paid to consultants and outside service providers for laboratory development, legal expenses resulting from intellectual property prosecution and other expenses relating to the design, development, testing and enhancement of the Company’s product candidates. Research and development expenses include external contract research organization (“CRO”) services. The Company makes payments to the CROs based on agreed upon terms and may include payments in advance of study services. The Company reviews and accrues CRO expenses based on services performed and relies on estimates of those costs applicable to the stage of completion of a study as provided by the CRO. Accrued CRO costs are subject to revisions as such studies progress to completion. At December 31, 2023 and 2022, the Company has accrued CRO expenses of $1.7 million and $0.8 million, respectively, that are included in accrued expenses. As of December 31, 2023, and 2022, the Company has prepaid CRO costs of $1.1 million and $2.3 million, respectively, that are included in prepaid expenses.
Leases
The Company assesses all contracts at inception to determine whether a lease exists. The Company’s leases are all classified as operating leases per ASC 842. The Company leases office space under operating leases that typically provide for the payment of minimum annual rentals and may include scheduled rent increases. The Company made an accounting policy election to use the practical expedient that allows lessees to treat the lease and non-lease components of leases as a single lease component. Leases with an initial term of 12 months or less are not recorded on the Company's consolidated balance sheets and to recognize those lease payments on a straightline basis in its consolidated statements of operations and comprehensive loss. Operating lease ROU assets and lease liabilities are recognized at commencement date based on the present value of lease payments over the lease term. The Company used the incremental borrowing rate for all of its leases, as the implicit interest rate was not readily determinable. In determining the Company’s incremental borrowing rate of each lease, the Company considered recent observable credit spreads correlating to the Company's creditworthiness and the term of each of the Company's lease agreements.
Research and Development Tax Credits
The Company, through its Theriva S.L. subsidiary, participates in a Research and Development program sponsored by the Spanish government. The program provides for reimbursement of certain expenses incurred in research and development efforts the Company incurs in Spain. The program provides for certain limits on the types and amounts of expenses and requires participants to complete a certification and apply for the refund annually. Subsequent to the period in which expenses are incurred, the program requires participants to maintain certain workforce levels and research and development expenditures over a 24-month period. The Company accounts for the reimbursement as a tax credit receivable related to amounts that had been approved by the Spanish government and a corresponding deferred research and development tax credit as it was determined that amounts became probable of being received upon the receipt of the approval. Additionally, the Company has elected to account for the tax credit as a contra-expense as this most appropriately reflects the nature of the transaction and will reduce future research and development expenditures as the Company continues to incur expenses in the upcoming 24-month period.
Stock Warrants
The Company’s Warrants are exercisable at any time and from time to time, in whole or in part, following the date of issuance and ending five years from the date of the execution of the Warrant Agreement. The Warrants were measured at fair value at the date of issuance, which was recorded in additional paid-in capital as a reduction of the gross proceeds raised in the public offering.
83
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies – (continued)
Preferred Stock
The Company’s Series C and D Preferred Stock is classified as temporary equity on the accompanying consolidated balance sheet in accordance with authoritative guidance for the classification and measurement of convertible securities.
Fair Value of Financial Instruments
Accounting Standards Codification (“ASC”) 820, Fair Value Measurement, defines fair value as the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is determined based upon assumptions that market participants would use in pricing an asset or liability. Fair value measurements are classified on a three-tier hierarchy as follows:
| ● | Level 1 inputs: Quoted prices (unadjusted) for identical assets or liabilities in active markets; |
| ● | Level 2 inputs: Inputs, other than quoted prices, that are observable either directly or indirectly; and |
| ● | Level 3 inputs: Unobservable inputs for which there is little or no market data, which require the reporting entity to develop its own assumptions. |
In many cases, a valuation technique used to measure fair value includes inputs from multiple levels of the fair value hierarchy described above. The lowest level of significant input determines the placement of the entire fair value measurement in the hierarchy.
The carrying amounts of the Company’s short-term financial instruments, including cash and cash equivalents, accounts payable and accrued liabilities, approximate fair value due to the relatively short period to maturity for these level 1 instruments.
As a result of the acquisition of VCN the Company acquired interest-free or below-market interest rate loans extended by Spanish government. The carrying value of the loans payable approximate fair value and are classified under level 2.
In connection with the Acquisition of VCN, the Company was required to pay up to $70.2 million in additional consideration upon the achievement of certain milestones, including regulatory filings completed noted in Note 5. In September 2022, the Company received approval from the FDA to proceed with the Phase 2 clinical trial of VCN-01 in PDAC. Due to this approval the Company paid Grifols Innovation and New Technologies Limited (“Grifols”), $3.0 million in the fourth quarter 2022. In August 2023, the Company initiated patient dosing in the U.S. in its Phase 2 clinical trial of VCN-01 in PDAC. As a result, payment was made subsequent to September 30, 2023 in the amount of $3.25 million. The discounted cash flow method used to value this contingent consideration includes inputs of not readily observable market data, which are Level 3 inputs. The fair value of the contingent consideration was $6.3 million as of December 31, 2023 and is all reflected as non-current contingent consideration liability. There were no transfers in or out of the level 3 liabilities during the years ended December 31, 2023 and 2022 , with the exception of the reclassification of $3.25 million related to the milestone that was met in the current year and reclassified to accrued expenses and paid prior to year end.
84
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies – (continued)
The following table summarizes the change in the fair value as determined by Level 3 inputs for the contingent consideration liabilities for the year ended December 31, 2023 and 2022:
|
|
(in thousands) |
|
Balance at March 10, 2022 |
|
$ |
11,093 |
Payment of contingent consideration |
|
|
(3,000) |
Change in fair value |
|
|
2,091 |
Balance at December 31, 2022 |
|
$ |
10,184 |
|
|
|
|
Contingent consideration, current portion |
|
$ |
2,973 |
Contingent consideration, net of current portion |
|
|
7,211 |
Balance at December 31, 2022 |
|
$ |
10,184 |
|
|
(in thousands) |
|
Balance at December 31, 2022 |
|
$ |
10,184 |
Payment of contingent consideration |
|
|
(3,250) |
Change in fair value |
|
|
(660) |
Balance at December 31, 2023 |
|
$ |
6,274 |
|
|
|
|
Contingent consideration, current portion |
|
$ |
— |
Contingent consideration, net of current portion |
|
|
6,274 |
Balance at December 31, 2023 |
|
$ |
6,274 |
The fair value of financial instruments measured on a recurring basis is as follows:
|
|
As of December 31, 2023 |
||||||||||
Description |
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
6,274 |
|
$ |
— |
|
$ |
— |
|
$ |
6,274 |
Total liabilities |
|
$ |
6,274 |
|
$ |
— |
|
$ |
— |
|
$ |
6,274 |
|
|
As of December 31, 2022 |
||||||||||
Description |
|
Total |
|
Level 1 |
|
Level 2 |
|
Level 3 |
||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
10,184 |
|
$ |
— |
|
$ |
— |
|
$ |
10,184 |
Total liabilities |
|
$ |
10,184 |
|
$ |
— |
|
$ |
— |
|
$ |
10,184 |
85
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
3. Summary of Significant Accounting Policies – (continued)
The recurring Level 3 fair value measurements of contingent consideration for which a liability is recorded include the following significant unobservable inputs:
|
|
As of December 31, 2023 |
|
||||
|
|
Valuation |
|
Significant |
|
Weighted Average |
|
|
|
Methodology |
|
Unobservable Input |
|
(range, if applicable) |
|
Contingent Consideration |
|
Discounted Cash Flows |
|
Milestone dates |
|
2025-2028 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Discount rate |
|
12.9% to 13.6 |
% |
|
|
|
|
Weighted Average Discount rate |
|
13.16 |
% |
|
|
|
|
Probability of Occurrence (periodic for each Milestone) |
|
11.7% to 92.0 |
% |
|
|
|
|
Probability of occurrence (cumulative through each Milestone) |
|
5.3% to 48.8 |
% |
|
|
As of December 31, 2022 |
|
||||
|
|
Valuation |
|
Significant |
|
Weighted Average |
|
|
|
Methodology |
|
Unobservable Input |
|
(range, if applicable) |
|
Contingent Consideration |
|
Discounted Cash Flows |
|
Milestone dates |
|
2023-2028 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Discount rate |
|
13.4% to 14.1 |
% |
|
|
|
|
Weighted Average Discount rate |
|
13.6 |
% |
|
|
|
|
Probability of Occurrence (periodic for each Milestone) |
|
11.7% to 95.0 |
% |
|
|
|
|
Probability of occurrence (cumulative through each Milestone) |
|
6.9% to 95.0 |
% |
Stock-Based Payment Arrangements
Generally, all forms of stock-based payments, including stock option grants, warrants, restricted stock grants and stock appreciation rights are measured at their fair value on the awards’ grant date typically using the Black-Scholes option pricing model. Forfeitures are recognized in the period they occur. Stock-based compensation awards issued to non-employees for services rendered are recorded at either the fair value of the services rendered or the fair value of the stock-based payment, whichever is more readily determinable. The expense resulting from stock-based payments is recorded in research and development expense or general and administrative expense in the Consolidated Statements of Operations, depending on the nature of the services provided.
Segment information
86
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company operates in one operating segment engaged in the research, development and commercialization of therapeutic drugs in which revenues are derived from product, license, and contract revenues. Operating segments are defined as components of an enterprise where separate financial information is evaluated regularly by the chief operating decision maker (CODM), the chief executive officer, in deciding how to allocate resources and assessing performance. The Company’s CODM allocates resources and assesses performance based upon discrete financial information at the consolidated level.
Foreign Currencies
The functional currency of the Company’s Theriva S.L. subsidiary is the Euro. VCN’s Assets and liabilities are translated to U.S. dollars based on exchange rates at the end of each reporting period. Income and expense items are translated at weighted average exchange rates prevailing during the reporting period. Translation adjustments are accumulated in a separate component of stockholders’ equity in the accompanying consolidated balance sheets. Transaction gains and losses are classified as other income (expense) net in the accompanying consolidated statements of operations.
3. Summary of Significant Accounting Policies – (continued)
Income Taxes
The Company accounts for income taxes under the liability method; under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Realization of deferred tax assets is dependent upon future earnings, the timing and amount of which are uncertain. The portion of any deferred tax asset for which it is more likely than not that a tax benefit will not be realized must then be offset by recording a valuation allowance.
The Company utilizes a two-step approach to recognize and measure uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon tax authority examination, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
Recent Accounting Pronouncements and Developments
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity's Own Equity (subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. This ASU amends the guidance on convertible instruments and the derivatives scope exception for contracts in an entity's own equity and improves and amends the related earnings per share guidance for both Subtopics. The ASU is effective for annual reporting periods after December 15, 2023 and interim periods within those annual periods and early adoption is permitted in annual reporting periods ending after December 15, 2020. The Company has adopted ASU 2020-06 on January 1, 2022. The ASU impacted the analysis of the accounting treatment for the issuance of Convertible Preferred Series C & D stock during the third quarter, specifically the cash conversion and beneficial conversion features.
In December 2023, the FASB issued final guidance in ASU No. 2023-09, Income Taxes (ASC 740): Improvements to Income Tax Disclosures requiring entities to provide additional information in the rate reconciliation and disclosures about income taxes paid. For public business entities, the guidance is effective for annual periods beginning after December 15, 2024. The Company is not early adopting, and therefore, this ASU is not adopted in the current period. The Company does not expect this ASU to have a material impact on the consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures which requires public entities to disclose significant segment expenses regularly provided to the chief operating decision-maker. Public entities with a single reporting segment have to provide all disclosures required by ASC 280, including the significant segment expense disclosures. For public business entities, the guidance is effective for annual periods beginning after December 15, 2024. The Company is not early adopting, and therefore is not adopted in the current period. The Company does not expect this ASU to have a material impact on the consolidated financial statements.
87
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
4. Research and Development Tax Credits
The Company, through its Theriva S.L. subsidiary, participates in a Research and Development program sponsored by the Spanish government. The program provides for reimbursement of certain expenses incurred in research and development efforts the Company incurs in Spain. The reimbursements can be through either tax credits or direct refunds. The program provides for certain limits on the types and amounts of expenses and requires participants to complete a certification and apply for the refund annually. Subsequent to the period in which expenses are incurred, the program requires participants to maintain certain workforce levels and research and development expenditures over a 24-month period.
In the quarter ended June 30, 2023, the Company completed the certification and applied for direct reimbursement, as opposed to a tax credit, for its qualifying research and development expenses incurred in the year ended December 31, 2022. The Company received approvals from the Spanish government in September and October 2023.
4. Research and Development Tax Credits – (continued)
The Company evaluated the program and concluded that it qualified to be accounted for as government assistance. Accordingly, the Company, as allowed by U.S. GAAP, elected to account for the grant by analogizing to the guidance provided by International Accounting Standards (“IAS”) 20, Accounting for Government Grants and Disclosure of Government Assistance. Accordingly, the Company recognized a tax credit receivable related to amounts that had been approved by the Spanish government prior to September 30, 2023 and a corresponding deferred research and development tax credit as it was determined that amounts became probable of being recognized in future periods. Additionally, the Company has elected to account for the tax credit as a contra-expense as this most appropriately reflects the nature of the transaction and will reduce future research and development expenditures over a 24-month period beginning January 1, 2024
5. Business Combination
Summary
On March 10, 2022 (the “Closing”), the Company completed the acquisition of all the outstanding shares of Theriva Biologics, S.L, which at the time was known as VCN Biosciences, S.L. (the “VCN Shares”) from the shareholders of VCN. VCN is a clinical-stage biopharmaceutical company developing new oncolytic adenoviruses for the treatment of cancer. The Company’s lead product candidate, VCN-01, is being studied in a Company sponsored Phase 2 clinical trial for pancreatic cancer with additional investigator sponsored trials in indications including head and neck squamous cell carcinoma (HNSCC), retinoblastoma, brain tumors and ovarian cancers. VCN-01 is designed to be administered systemically, intratumorally or intravitreally, either as a monotherapy or in combination with standard of care chemotherapies or immunotherapies, to treat a wide variety of cancer indications. VCN-01 is designed to replicate selectively and aggressively within tumor cells, and to degrade the tumor stroma barrier that serves as a significant physical and immunosuppressive barrier to cancer treatment. Degrading the tumor stroma has been shown to improve access to the tumor by the virus and additional therapies such as chemo and immunotherapies. Importantly, degrading the stroma exposes tumor antigens, turning “cold” tumors “hot” and enabling a sustained anti-tumor immune response. VCN has the exclusive rights to four patent families for proprietary technologies, as well as technologies developed in collaboration with the Virotherapy Group of the Catalan Institute of Oncology (ICO-IDIBELL) and with Hospital Sant Joan de Deu (HSJD), with a number of additional patents pending. As consideration for the purchase of the VCN Shares and pursuant to the terms of a purchase agreement that the parties entered into (the “Purchase Agreement”), the Company paid $4,700,000 to Grifols Innovation and New Technologies Limited (“Grifols”), the owner of approximately 86% of the equity of VCN, and issued to the remaining sellers and certain key VCN employees and consultants of VCN an aggregate of 2,639,530 shares of its common stock, $0.001 par value per share (the “Common Stock”). In addition to the consideration described above, under the terms of the purchase agreement that the parties entered into, the Company assumed up to $2,390,000 of existing liabilities of VCN and has agreed to make cash payments of up to $70.2 million to Grifols upon the achievement of certain clinical and commercialization milestones. In September 2022, the Company received approval from the FDA to proceed with the Phase 2 clinical trial of VCN-01 in metastatic pancreatic ductal adenocarcinoma (“PDAC”). Due to this approval, the Company paid Grifols $3.0 million in the fourth quarter of 2022. In August 2023, the Company initiated patient dosing in the U.S. in its Phase 2 clinical trial of VCN-01 in PDAC. As a result, the Company paid Grifols $3.25 million in the fourth quarter of 2023.
88
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
In anticipation of the Acquisition, prior to the Closing, the Company loaned VCN $417,000 to help finance the costs of certain of VCN’s research and development activities. At the Closing, VCN and Grifols entered into a sublease agreement for laboratory and office space which didn’t commence until January 2023 as well as a transitional services agreement. As a post-Closing covenant, the Company has agreed to commit to fund VCN’s research and development programs, including, but not limited to, VCN-01 in a pancreatic ductal adenocarcinoma PDAC Phase 2 trial, VCN-01 in a retinoblastoma (RB) Phase 2/3 trial and necessary general and administrative expenses within a budgetary plan of approximately $27.8 million.
5. Business Combination – (continued)
Total purchase consideration including cash, shares of common stock and contingent consideration was valued at approximately $22.8 million, as follows (in thousands):
Cash paid at Closing |
|
$ |
4,700 |
Receivable from VCN “effectively settled“ |
|
|
417 |
Fair value of common shares issued |
|
|
6,599 |
Fair value of contingent consideration |
|
|
11,093 |
|
|
$ |
22,809 |
As of December 31, 2023 and December 31, 2022, the fair value of the contingent consideration was approximately $6.3 million and $10.2 million, respectively. During the year ended December 31, 2023, the Company recognized in operating expense a $0.7 million, decrease in the fair value of the contingent consideration. Upon initiation of patient dosing in the U.S. during the three months ended September 30, 2023, $3.25 million that had previously been included as contingent consideration, was paid to Grifols during the quarter ending December 31, 2023. During the year ended December 31, 2022, the Company recognized in operating expense a $2.1 million increase in the fair value of the contingent consideration.
The allocation of the fair value of the VCN Acquisition updated for measurement period and other adjustments is shown in the table below.
|
|
Estimated fair value |
|
|
|
($in thousands) |
|
Cash and cash equivalents |
|
$ |
837 |
Receivables |
|
|
1,889 |
Property and equipment |
|
|
216 |
In-process research and development intangible asset |
|
|
19,742 |
Goodwill |
|
|
5,696 |
Deferred tax liabilities, net |
|
|
(3,209) |
Accounts payable |
|
|
(522) |
Accrued expenses |
|
|
(113) |
Accrued employee benefits |
|
|
(90) |
Loans payable-current |
|
|
(67) |
Other long-term liabilities |
|
|
(1,570) |
|
|
|
|
Total purchase consideration |
|
$ |
22,809 |
The net assets were recorded at their estimated fair value. In valuing acquired assets and liabilities, fair value estimates were based primarily on future expected cash flows, market rate assumptions for contractual obligations, and appropriate discount rates. In connection with the Acquisition, the Company recognized $19.7 million of indefinite-lived in-process research and development intangible assets. Working capital balances were recorded at their carrying value as they approximated fair value due to nature of the assets and short term duration of the liabilities.
89
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Goodwill is considered an indefinite-lived asset and relates primarily to intangible assets that do not qualify for separate recognition, such as the assembled workforce and synergies between the entities. Goodwill of $5.7 million was established as a result of the Acquisition and is not tax deductible.
5. Business Combination – (continued)
VCN operations recorded a net loss of $5.8 million from the date of Acquisition through December 31, 2022 and $11.4 million for the year ended December 31, 2023.
During the year ended December 31, 2022, the Company recognized the following measurement period adjustments:
| ● | estimate of acquired liabilities resulting in a $277,000 reduction in accrued expenses and goodwill, |
| ● | estimate in the receivable from the prior owner resulting in a $176,000 increase in other receivables and reduction in goodwill. |
| ● | estimated fair value of its in-process R&D resulting in a $810,000 increase in in-process R&D, an increase of $202,000 in deferred tax liabilities and a decrease of $607,000 in goodwill. |
The cumulative impact of the re-measurements during the measurement period, was a reduction in accrued liabilities of $277,000, an increase in other receivables of $176,000, an increase in in-process R&D of $810,000; an increase in deferred tax liabilities of $202,000 and a decrease in goodwill of $1,061,000.
Pro Forma Consolidated Financial Information (unaudited)
The following unaudited pro forma consolidated financial information summarizes the results of operations for the periods indicated as if the VCN Acquisition had been completed as of January 1, 2022 (in thousands):
(in thousands) |
|
2022 |
|
Net revenues |
|
$ |
— |
Net loss |
|
$ |
(20,546) |
Transaction Costs
In conjunction with the Acquisition, the Company incurred approximately $0.2 million in transaction costs during the year ended December 31, 2022, which were expensed as general, and administrative expense in the consolidated statements of operations. There were no acquisition costs incurred during the year ended December 31, 2023.
6. Goodwill and Intangibles
The following table provides the Company’s Goodwill as of December 31, 2023.
|
|
Goodwill (in thousands) |
|
Balance at December 31, 2022 |
|
$ |
5,525 |
Effects of exchange rates |
|
|
175 |
Balance at December 31, 2023 |
|
$ |
5,700 |
The following table provides the Company’s in-process R&D as of December 31, 2023.
|
|
In-process |
|
|
|
R&D (in thousands) |
|
Balance at December 31, 2022 |
|
$ |
19,150 |
Effects of exchange rates |
|
|
605 |
Balance at December 31, 2023 |
|
$ |
19,755 |
90
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
During the quarters ended September 30, 2023 and December 31, 2022, the Company experienced a sustained decline in the quoted market price of the Company’s common stock and the Company deemed this to be a triggering event for impairment. The Company performed an interim impairment analysis and concluded that the Goodwill and IPR&D were not impaired as of September 30, 2023. This interim analysis also satisfied the requirements of the annual impairment test. There were no impairment charges recorded during 2023 and 2022.
7. Selected Balance Sheet Information
PREPAID EXPENSES AND OTHER CURRENT ASSETS (in thousands):
|
|
December 31, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
Prepaid clinical research organizations |
|
$ |
1,119 |
|
$ |
2,293 |
Prepaid insurance |
|
|
496 |
|
|
637 |
Prepaid manufacturing expenses |
|
|
491 |
|
|
418 |
Prepaid consulting, subscriptions and other expenses |
|
|
180 |
|
|
155 |
VAT receivable |
|
|
128 |
|
|
87 |
Receivable from Grifols |
|
|
— |
|
|
144 |
|
|
|
|
|
|
|
Total prepaid expsnese and other current assets |
|
$ |
2,414 |
|
$ |
3,734 |
Prepaid clinical research organizations (CROs) expense is classified as a current asset. The Company makes payments to the CROs based on agreed upon terms that include payments in advance of study services. Receivable from Grifols includes amounts due related to research and development tax rebates, VAT and corporate taxes.
PROPERTY AND EQUIPMENT (in thousands)
|
|
December 31, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
Computers and office equipment |
|
$ |
902 |
|
$ |
897 |
Other property, plant and equipment |
|
|
417 |
|
|
208 |
Leasehold improvements |
|
|
94 |
|
|
94 |
Software |
|
|
11 |
|
|
11 |
|
|
|
1,424 |
|
|
1,210 |
Less: accumulated depreciation and amortization |
|
|
(1,002) |
|
|
(865) |
|
|
|
|
|
|
|
Total property and equipment, net |
|
$ |
422 |
|
$ |
345 |
During the years ended December 31, 2023 and 2022 the Company recognized depreciation expense of $135,000 and 85,000 respectively.
ACCRUED EXPENSES (in thousands)
|
|
December 31, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
Accrued clinical consulting services |
|
$ |
1,700 |
|
$ |
807 |
Accrued manufacturing costs |
|
|
843 |
|
|
197 |
Accrued vendor payments |
|
|
452 |
|
|
492 |
|
|
|
|
|
|
|
Total accrued expesnes |
|
$ |
2,995 |
|
$ |
1,496 |
91
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
7. Selected Balance Sheet Information – (continued)
ACCRUED EMPLOYEE BENEFITS (in thousands)
|
|
December 31, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
Accrued bonus expense |
|
$ |
1,307 |
|
$ |
1,216 |
Accrued compensation expense |
|
|
127 |
|
|
87 |
Accrued vacation expense |
|
|
83 |
|
|
100 |
|
|
|
|
|
|
|
Total accrued employee benefits |
|
$ |
1,517 |
|
$ |
1,403 |
8. Stock-Based Compensation
Stock Incentive Plan
On March 20, 2007, the Company’s Board of Directors approved the 2007 Stock Incentive Plan (the “2007 Stock Plan”) for the issuance of up to 7,143 shares of common stock to be granted through incentive stock options, nonqualified stock options, stock appreciation rights, dividend equivalent rights, restricted stock, restricted stock units and other stock-based awards to officers, other employees, directors and consultants of the Company and its subsidiaries. This plan was approved by the stockholders on November 2, 2007. The exercise price of stock options under the 2007 Stock Plan was determined by the compensation committee of the Board of Directors and could be equal to or greater than the fair market value of the Company’s common stock on the date the option is granted. As of December 31, 2023, there were 86 options issued and outstanding under the 2007 Stock Plan. Only options were issued under the plan.
On November 2, 2010, the Board of Directors and stockholders adopted the 2010 Stock Incentive Plan (“2010 Stock Plan”) for the issuance of up to 8,572 shares of common stock to be granted through incentive stock options, nonqualified stock options, stock appreciation rights, dividend equivalent rights, restricted stock, restricted stock units and other stock-based awards to officers, other employees, directors and consultants of the Company and its subsidiaries. From time to time the number of shares authorized for options was increased such that 400,000 were authorized as of September 5, 2019. The exercise price of stock options under the 2010 Stock Plan is determined by the compensation committee of the Board of Directors and may be equal to or greater than the fair market value of the Company’s common stock on the date the option is granted. Options become exercisable over various periods from the date of grant and expire between five and ten years after the grant date. As of December 31, 2023, there were 198,540 options issued and outstanding under the 2010 Stock Plan. There are no shares available to be issued under this plan. Only options were issued under the plan.
On September 17, 2020, the stockholders approved and adopted the 2020 Stock Incentive Plan ("2020 Stock Plan") for the issuance of up to 400,000 shares of common stock to be granted through incentive stock options, nonqualified stock options, stock appreciation rights, dividend equivalent rights, restricted stock, restricted stock units and other stock-based awards to officers, other employees, directors and consultants of the Company and its subsidiaries. The number of shares authorized for options was increased such that 7,000,000 were authorized as of December 31, 2022. As of December 31, 2023, there were 4,177,155 options issued and outstanding under the 2020 Stock Plan. Only options were issued under the plan.
In the event of an employee’s termination, the Company will cease to recognize compensation expense for that employee. Stock option forfeitures are recognized as incurred. The fair value of the stock-based payment is recognized over the stated vesting period.
92
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
8. Stock-Based Compensation – (continued)
The Company has applied fair value accounting for all stock-based payment awards since inception. The fair value of each option granted is estimated on the date of grant using the Black-Scholes option pricing model. The assumptions used for the years ended December 31, 2023 and 2022 are as follows:
|
|
Year ended December 31, |
|
||||
|
|
2023 |
|
2022 |
|
||
Exercise price |
|
$ |
0.59 |
|
$ |
0.58-2.61 |
|
Expected dividends |
|
|
0 |
% |
|
0 |
% |
Expected volatility |
|
|
90 |
% |
|
95 |
% |
Risk free interest rate |
|
|
4.02 |
% |
|
2.65-3.77 |
% |
Expected life of option (years) |
|
|
4.25 |
|
|
4.3 |
|
Expected dividends —The Company has never declared or paid dividends on its common stock and has no plans to do so in the foreseeable future.
Expected volatility—Volatility is a measure of the amount by which a financial variable such as a share price has fluctuated (historical volatility) or is expected to fluctuate (expected volatility) during a period. The expected volatility assumption is derived from the historical volatility of the Company’s common stock over a period approximately equal to the expected term.
Risk-free interest rate—The assumed risk-free rate used is a zero coupon U.S. Treasury security with a maturity that approximates the expected term of the option.
Expected life of the option—The period of time that the options granted are expected to remain unexercised. Options granted during the years ended 2022 and 2023 have a maximum term of seven years. The Company estimates the expected life of the option term based on the weighted average life between the dates that options become fully vested and the maximum life of options granted.
The Company records stock-based compensation based upon the stated vesting provisions in the related agreements. The vesting provisions for these agreements have various terms as follows:
| ● | immediate vesting, |
| ● | in full on one-year anniversary date of grant date, |
| ● | half vesting immediately and remaining over three years, |
| ● | quarterly over three years, |
| ● | annually over three years, |
| ● | one-third immediate vesting and remaining annually over two years, |
| ● | one-half immediate vesting and remaining over nine months, |
| ● | one-quarter immediate vesting and remaining over three years, |
| ● | one-quarter immediate vesting and remaining over 33 months, |
| ● | monthly over one year, and |
| ● | monthly over three years. |
93
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
8. Stock-Based Compensation – (continued)
During the years ended December 31, 2023 and 2022, the Company granted 2,195,000 and 1,728,000 options to employees and directors having an approximate fair value of $0.9 million and $0.7 million based upon the Black-Scholes option pricing model, respectively.
Stock-based compensation expense included in general and administrative expenses and research and development expenses relating to stock options issued to employees for the years ended December 31, 2023 and 2022 was $373,000 and $260,000, respectively. Stock-based compensation expense included in general and administrative expenses and research and development expenses relating to stock options issued to consultants for the years ended December 31, 2023 and 2022 was $179,000 and $215,000, respectively.
A summary of stock option activity for the years ended December 31, 2023 and 2022 is as follows:
|
|
|
|
Weighted |
|
Weighted Average |
|
Aggregate |
||
|
|
|
|
Average Exercise |
|
Remaining |
|
Intrinsic |
||
|
|
Options |
|
Price |
|
Contractual Life |
|
Value |
||
Balance - December 31, 2021 |
|
625,565 |
|
$ |
16.12 |
|
5.58 years |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
1,728,000 |
|
|
0.58 |
|
|
|
|
|
Exercised |
|
— |
|
|
— |
|
|
|
|
|
Expired |
|
(43,126) |
|
|
67.81 |
|
|
|
|
|
Forfeited |
|
(14,541) |
|
|
3.61 |
|
|
|
|
|
Balance - December 31, 2022 |
|
2,295,898 |
|
|
3.53 |
|
6.44 years |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
2,195,000 |
|
|
0.59 |
|
|
|
|
|
Exercised |
|
— |
|
|
— |
|
|
|
|
|
Expired |
|
(104,270) |
|
|
14.73 |
|
|
|
|
|
Forfeited |
|
(10,847) |
|
|
1.11 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance -December 31, 2023 - outstanding |
|
4,375,781 |
|
$ |
1.80 |
|
7.70 years |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2023 - exercisable |
|
1,251,477 |
|
$ |
4.70 |
|
5.05 years |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
Grant date fair value of options granted - December 31, 2023 |
|
|
|
$ |
873,140 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average grant date fair value - December 31, 2023 |
|
|
|
$ |
0.40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant date fair value of options granted - December 31, 2022 |
|
|
|
$ |
706,264 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average grant date fair value - December 31, 2022 |
|
|
|
$ |
.41 |
|
|
|
|
|
The options outstanding and exercisable at December 31, 2023 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding |
|
Options Exercisable |
|||||||||||||
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
Weighted |
|
|
|
|
|
Weighted |
|
Average |
|
|
|
Weighted |
|
Average |
||
|
|
|
|
|
Average |
|
Remaining |
|
|
|
Average |
|
Remaining |
||
Range of |
|
|
|
Exercise |
|
Contractual |
|
|
|
Exercise |
|
Contractual |
|||
Exercise Price |
|
Options |
|
Price |
|
Life |
|
Options |
|
Price |
|
Life |
|||
$ |
0.00 – $350.00 |
|
4,372,338 |
|
$ |
1.20 |
|
8 years |
|
1,248,004 |
|
$ |
2.62 |
|
5 years |
|
351.00 – $700.00 |
|
270 |
|
|
511.00 |
|
1 years |
|
270 |
|
|
511 |
|
1 years |
|
701.00 – $1000.00 |
|
3,173 |
|
|
779.83 |
|
2 years |
|
3,173 |
|
|
779.83 |
|
2 years |
94
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
8. Stock-Based Compensation – (continued)
As of December 31, 2023, total unrecognized stock-based compensation expense related to stock options was $1.3 million which is expected to be expensed through December 2026.
The FASB’s guidance for stock-based payments requires cash flows from excess tax benefits to be classified as a part of cash flows from operating activities. Excess tax benefits are realized tax benefits from tax deductions for exercised options in excess of the deferred tax asset attributable to stock compensation costs for such options. The Company did not record any excess tax benefits in 2023 or 2022. Cash received from option exercises under the Company’s stock-based compensation plans for the years ended December 31, 2023 and 2022 was zero.
9. Stock Warrants
On October 15, 2018, the Company closed its underwritten public offering pursuant to which it received gross proceeds of approximately $18.6 million before deducting underwriting discounts, commissions and other offering expenses payable by the Company and sold (i)Class A Units (the “Class A Units”), consisting of an aggregate of 252,000 shares of the Common Stock, warrants to purchase an aggregate of 252,000 shares of Common Stock at an exercise price of $13.80 per share, which subsequently was reduced to $6.90 per share and then again to $1.22 (each a “Warrant” and collectively, the “Warrants”) and (ii) Class B Units (the “Class B Units”, and together with the Class A Units, the “Units”), consisting of an aggregate of 15,723 shares of the Company’s Series B Convertible Preferred Stock (the “Series B Preferred Stock”), with a stated value of $1,000 and convertible into shares of Common Stock at the stated value divided by a conversion price of $11.50 per share, with all shares of Series B Preferred Stock convertible into an aggregate of 1,367,218 shares of Common Stock, and issued with a warrant to purchase an aggregate of 1,367,218 shares of Common Stock.
On November 16, 2020, the exercise price of the Warrants was reduced from $13.80 per Warrant per full share of the Company’s Common Stock, to $6.90 per Warrant per full share of Common Stock in accordance with the antidilution terms of the Warrant. The reduction was the result of the issuance of shares of Common Stock by the Company through its ATM facility. The effect of the change in the exercise price of the Warrants as a result of the triggering of the down round protection clause in the Warrants was recorded as a deemed dividend of $0.9 million during the year ended December 31, 2020, which reduces the income available to common stockholders. In addition, pursuant to the underwriting agreement that the Company had entered into with A.G.P./Alliance Global Partners (the “Underwriters”), as representative of the underwriters, the Company granted the Underwriters a 45 day option (the “Over-allotment Option”) to purchase up to an additional 242,883 shares of Common Stock and/or additional Warrants to purchase an additional 242,883 shares of Common Stock. The Underwriters partially exercised the Over-allotment Option by electing to purchase from the Company additional Warrants to purchase 180,783 shares of Common Stock.
If, at the time of exercise, there is no effective registration statement registering, or no current prospectus available for the issuance of the shares of Common Stock to the holder, then the Warrants may only be exercised through a cashless exercise. No fractional shares of Common Stock will be issued in connection with the exercise of a Warrant. In lieu of fractional shares, the holder will receive an amount in cash equal to the fractional amount multiplied by the fair market value of any such fractional shares. The Company has concluded that the Warrants are required to be equity classified. The Warrants were valued on the date of grant using Monte Carlo simulations. During the three months ended March 31, 2021, 1,165,575 Warrants were exercised for cash proceeds of $8.0 million. There were no Warrants exercised during the years ended December 31, 2023 and 2022. The Warrants have expired in October 2023 and are no longer outstanding. Upon expiration, the balance in additional paid-in capital related to the warrants was transferred to the additional paid-in capital balance related to common stock with no effect on additional paid-in capital.
On August 3, 2022, the Company announced the exercise price of Warrants issued by the Company in October 2018 was reduced from $6.90 per Warrant per full share of the Company’s common stock, $0.001 par value per share to $1.22 per Warrant per full share of Common Stock. The reduction was the result of the issuance of shares of Preferred Stock by the Company in a private placement. The effect of the change in the exercise price of the Warrants as a result of the triggering of the down round protection clause in the Warrants was recorded as a deemed dividend of $340,000 during the year ended December 31, 2022, which reduces the income available to common stockholders and had no impact to the Stockholders equity.
95
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
9. Stock Warrants – (continued)
A summary of all warrant activity for the Company the year ended December 31, 2023 and December 31, 2022 is as follows:
|
|
|
|
|
|
|
Weighted Average |
|
|
Number of |
|
Weighted Average |
|
Remaining |
|
|
|
Warrants |
|
Exercise Price |
|
Contractual Life |
|
Balance at December 31, 2021 |
|
634,497 |
|
|
1.24 |
|
1.78 years |
Granted |
|
— |
|
|
— |
|
|
Exercised |
|
— |
|
|
— |
|
|
Forfeited |
|
(71) |
|
|
182 |
|
|
Balance at December 31, 2022 |
|
634,426 |
|
$ |
1.22 |
|
0.78 years |
Granted |
|
— |
|
|
— |
|
|
Exercised |
|
— |
|
|
— |
|
|
Forfeited |
|
(634,426) |
|
|
1.22 |
|
|
Balance at December 31, 2023 |
|
— |
|
$ |
— |
|
— |
10. Stockholders’ Equity
Series C and D Preferred Stock
On July 29, 2022, the Company closed a private placement offering pursuant to the terms of a Securities Purchase Agreement dated as of July 28, 2022 entered into with MSD Credit Opportunity Master Fund, L.P. (the “Securities Purchase Agreement”), pursuant to which the Company issued and sold 275,000 shares of the Company's Series C Convertible Preferred Stock, par value $0.001 per share (the "Series C Preferred Stock"), and 100,000 shares of the Company's Series D Convertible Preferred Stock, par value $0.001 per share (the "Series D Preferred Stock," and together with the Series C Preferred Stock, the "Preferred Stock"), at an offering price of $8.00 per share, for gross proceeds of approximately $3.0 million in the aggregate, before the deduction of discounts, fees and offering expenses. The shares of Preferred Stock are convertible, at a conversion price (the "Conversion Price") of $1.22 per share (subject in certain circumstances to adjustments), into an aggregate of 2,459,016 shares of the Company's Common Stock, at the option of the holders of the Preferred Stock and, in certain circumstances, by the Company. The Securities Purchase Agreement contains customary representations, warranties and agreements by the Company and customary conditions to closing.
The Company included certain proposals at its 2022 annual meeting of stockholders, including (i) an amendment to the Company’s Articles of Incorporation, as amended (the “Charter”), to change the name of the Company to “Theriva Biologics, Inc.” (the “Name Change”), (ii) an amendment to the Articles of Incorporation, as amended to increase the number of authorized shares of Common Stock from 20,000,000 to 350,000,000 (the “Authorized Common Stock Increase”) and (iii) to adjourn any meeting of stockholders called for the purpose of voting on the Authorized Common Stock Increase (collectively, the “Stockholder Items”). The purchaser of the Preferred Stock agreed in the Purchase Agreement to (i) not transfer, offer, sell, contract to sell, hypothecate, pledge or otherwise dispose of the shares of the Preferred Stock until the earlier of the date that the Authorized Common Stock Increase is effected or October 26, 2022 and (ii) vote the shares of the Series C Preferred Stock purchased in the Offering in favor of the Stockholder Items.
96
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
10. Stockholders’ Equity – (continued)
Pursuant to the Securities Purchase Agreement, the Company filed certificates of designation (the "Certificates of Designation") with the Secretary of the State of Nevada designating the rights, preferences and limitations of the shares of Series C Preferred Stock and Series D Preferred Stock. The Certificate of Designation for the Series C Preferred Stock provides, in particular, that the Series C Preferred Stock will have no voting rights other than the right to vote as a class on the Stockholder Items and the right to cast votes on an as converted to Common Stock basis on the Stockholder Items. The Certificate of Designation for the Series D Preferred Stock provides, in particular, that the Series D Preferred Stock will have no voting rights other than the right to vote as a class on the Stockholder Items and the right to cast 20,000 votes per share of Series D Preferred Stock on the Stockholder Items and to vote the shares of the Series D Preferred Stock purchased in the Offering in the same proportion as shares of Common Stock and any other shares of capital stock of the Company that are entitled to vote thereon (excluding any shares of Common Stock that are not voted) on the Stockholder Items.
The holders of Preferred Stock will be entitled to dividends, on an as-if converted basis, equal to dividends actually paid, if any, on shares of Common Stock. The Conversion Price may be adjusted pursuant to the Certificates of Designation for stock dividends and stock splits, subsequent rights offering, pro rata distributions of dividends or the occurrence of a fundamental transaction (as defined in the applicable Certificate of Designation).
The Series C Preferred Stock and Series D Preferred Stock are classified as temporary equity as a result of the deemed liquidation provision. Transaction expenses paid to third parties will be charged to temporary equity and will not be accreted as deemed dividends until redemption becomes probable.
In order to comply with Section 122 of the NYSE American Company Guide, on August 9, 2022 the Company and the holder of the Company's Series C preferred stock and Series D preferred stock amended the Securities Purchase Agreement entered into between them on July 28, 2022 to provide that the holder may only submit 1,549,295 of the votes relating to the Series C Preferred Stock that it would otherwise be entitled to vote.
Stock Repurchase
On December 22, 2022, the Company repurchased an aggregate of approximately 720,000 shares of its common stock, par value $0.001 from three founders of its subsidiary Theriva Biologics S.L. (formerly known as VCN Biosciences S.L.) in a privately negotiated transaction pursuant to the terms of a Share Repurchase Agreement entered into on December 20, 2022 with each of the Selling Stockholders. The price per share was $0.4001, which was the closing price of the Common Stock on the day prior to the closing for an aggregate purchase price was $288,072. The closing was subject to fulfillment of certain conditions, including delivery of certain closing documents. The Share Repurchase Agreement contains customary representations, warranties and covenants of the parties. The repurchase was funded from the Company’s cash on hand and the shares to be repurchased will be held as treasury stock. The Selling Stockholders acquired the shares of the Company’s Common Stock as consideration for the sale of their shares of the subsidiary to the Company in March 2022.
B. Riley Securities and Alliance Global Partners Sales Agreement
On August 5, 2016, the Company entered into the Sales Agreement (the “Original Sales Agreement”) with FBR Capital Markets & Co. (now known as B. Riley Securities) to act as a sales agent, which agreement was amended and restated on February 9, 2021 to add Alliance Global Partners as a sale agent. The amended and restated Sales Agreement (the “Amended and Restated Sales Agreement”) enables the Company to offer and sell shares of common stock from time to time through B. Riley Securities, Inc. and A.G.P./Alliance Global Partners as the Company’s sales agent. Sales of common stock under the Sales Agreement are made in sales deemed to be “at-the-market” equity offerings as defined in Rule 415 promulgated under the Securities Act. The sales agents are entitled to receive a commission rate of up to 3.0% of gross sales in connection with the sale of the Common Stock sold on the Company’s behalf. During the year ended December 31, 2023, the Company sold through the Amended and Restated Sales Agreement approximately 2.0 million shares of the Company’s common stock and received net proceeds of approximately $2.2 million. During the year ended December 31, 2022, there were no sales of the Company’s common stock through the At Market Issuance Sales Agreement and the Amended and Restated Sales Agreement.
97
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
11. Loans payable
As a result of the acquisition of VCN the Company acquired interest-free or below-market interest rates loans (0%-1%) extended by Spanish governmental institutions of Ministerio de Ciencia, Innovacion y Universidades and ACC10 Generalitat de Catalunya (CDIT loans). The maturities of these loans are between 2024 and 2028. As a result of the VCN Acquisition, the Company maintains a restricted cash collateral account of $102,000 relating to the RETOS loan, which is reflected as a non-current asset on the balance sheet.
|
|
December 31, 2023 |
|
December 31, 2023 |
|
December 31, 2022 |
|
December 31, 2022 |
||||
|
|
Current |
|
Non-current |
|
Current |
|
Non-current |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
NEBT Loan |
|
|
8 |
|
$ |
24 |
|
|
13 |
|
|
31 |
RETOS 2015 |
|
|
55 |
|
|
138 |
|
|
44 |
|
|
190 |
|
|
$ |
63 |
|
$ |
162 |
|
$ |
57 |
|
$ |
221 |
A maturity analysis of the debt as of December 31, 2023 is as follows (amounts in thousands of dollars):
2024 |
|
$ |
63 |
2025 |
|
|
65 |
2026 |
|
|
54 |
2027 |
|
|
33 |
2028 |
|
|
10 |
Total |
|
$ |
225 |
12. Related Party
On December 15, 2022, the Company approved the retention of MaryAnn Shallcross, the wife of Steven Shallcross, as director of Clinical Operations, for compensation of $145,000 and the grant of an option to purchase 50,000 shares of common stock having a value of $20,000. On December 14, 2023 the Company approved the retention of MaryAnn Shallcross for compensation of $152,000, a bonus of $70,000 and the grant of an option to purchase 75,000 shares of common stock having a value of $30,000. During the year ended December 31, 2023, Ms. Shallcross had $145,000 in compensation expense. Ms. Shallcross had been performing services for the Company during 2022 for total compensation of less than $120,000.
13. License, Collaborative and Employment Agreements and Commitments
License and Collaborative Agreements
As described below, the Company has entered into several license and collaborative agreements for the right to use research, technology and patents. Some of these license and collaborative agreements may contain milestones. The specific timing of such milestones cannot be predicted and is dependent on future developments as well as regulatory actions which cannot be predicted with certainty (including actions which may never occur). Further, under the terms of certain licensing agreements, the Company may have the obligation to pay certain milestones contingent upon the achievement of specific levels of sales. Due to the long-range nature of such commercial milestone liability amounts, they are neither probable at this time nor predictable and consequently are not recorded in the financial statements or included in this disclosure.
98
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
On August 31, 2010, VCN entered into a Technology Transfer Agreement (the “Technology Transfer Agreement”) with the Bellvitge Biomedical Research Institute (“IDIBELL”) for the exclusive license of the right to use a Spanish patent number P200901201 titled “Oncolytic adenoviruses for treating cancer” which is co-owned by IDIBELL and Catalan Oncology Institute (“ICO”) for the term of the patent. The Technology Transfer Agreement provides that IDIBELL is entitled to a low single digit percentage royalty on the income collected by VCN from the utilization of products derived from the licensed technology, prior to applying any value-added tax, if any, and low single digit percentage royalty on other income received by VCN arising from the use of the licensed technology, including income related to sublicenses of the licensed technology to third parties and advance payments or payments made for goals that were met and/or services associated with the licensed technology. The Technology Transfer Agreement terminates upon the expiration of the patent rights and is subject to early termination by either party in the event of a breach by the other party of its obligations thereunder. In addition, IDIBELL has the right to revoke the license if VCN ceases business activities for a continuous year or ceases to utilize the technology subject of the Technology Transfer Agreement, uses the technology in violation of the principals of IDIBELL or ICO or stops maintaining the patent licensed under the Technology Transfer Agreement. No amounts incurred in 2023 and 2022.
ICO Marketing License
On May 16, 2009, VCN entered into a Contract to Grant a Marketing License (the “ICO License Agreement”) with the Catalan Institute of Oncology (the “ICO”) for a manufacturing and marketing license of a patent P200700665 titled “Adenovirus with mutations in the area of endoplasmic retention of protein E3-19k and their use in the treatment of cancer” in connection with a sublicense identified therein. The validity period of the license granted is unlimited with the only applicable limit being the patent’s own validity. The ICO License Agreement provides that the ICO is entitled to a royalty of low double digit percentage of the net value of the income from the concession of the identified sublicense and low double digit precentage on other lump sums received thereunder. VCN and its sublicensees have an obligation to use all diligent and commercially reasonable efforts for the exploitation of the patent, otherwise, ICO may proceed to recover the license. The ICO License terminates upon the expiration of the patent rights and is subject to early termination by either party in the event of a breach by the other party of its obligations thereunder. No amounts incurred in 2023 and 2022.
IDIBELL/ICO License Agreement
On March 4, 2016, VCN entered into a License Agreement (the “IDIBELL/ICO License Agreement”) with IDIBELL and the ICO, for the exclusive license of the right to use a family of patents whose priority application is European patent application EP 14 38 2162.7 titled “Adenovirus comprising an albumin-binding molety”. The License Agreement provides that IDIBELL and ICO, as licensors, are entitled to share a low single digit percentage royalty on the annual Net Sales (as defined in the IDIBELL/ICO License Agreement)collected by VCN from the utilization of products derived from the licensed technology and a royalty on sublicensing income received from the licensed technology at a rate of: low double digit percentage during the first 3 years following the effective date of the agreement, mid single digit percentage during the term of 3 to 7 years following the effective date and low single digit percentage thereafter. The IDIBELL/ICO License Agreement also provides for certain fixed payments, including a payment 25 days following the date of concession of the licensed patent in a minimum of three European jurisdictions and a payment 25 days following the date of concession of an American patent derived from the licensed patent. The IDIBELL/ICO License is for an indefinite term subject to early termination (i) by mutual agreement of the parties; (ii) by licensor in the event of at least two successive breaches or three alternate breaches calculated annually of the obligation to pay any consideration; (iii) by VCN at its discretion due to certain patent infringements of rights protected by the patents or due to the absence of protection of the patent in any countries in the territory which is worldwide or (iv) in the event of a breach by the other party of its obligations thereunder which are not remedied within thirty (30) days. In addition, the licensors have the right to revoke the IDIBELL/ICO License Agreement if VCN during a continuous period of two years abandons its research or development activities of the licensed patent or activities aimed at exploitation of the resulting products, VCN has undertaken no marketing whatsoever during the term of the IDIBELL/ICO License Agreement or uses the patent licensed for purposes other those as set forth in the IDIBELL/ICO License Agreement. No amounts incurred in 2023 and 2022.
99
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
Saint Joan De Déu Collaboration and License Agreement
On February 15, 2016, VCN entered into a Collaboration Agreement to Conduct a Clinical Trial and Grant an Operating License (the “Collaboration and License Agreement”) with the Saint Joan De Déu Hospital (the “Hospital”) and the Saint Joan De Déu Foundation (the “Foundation”, and together with the Hospital, the “Institution”) regarding the conduct of a clinical trial to evaluate the safety and activity of VCN-01 in patients with refractory retinoblastoma. The Collaboration and License Agreement provides that if the trial results are positive and VCN is interested in continuing with the development of VCN-01 for the treatment of retinoblastoma; (a) the parties undertake to apply their best efforts to negotiate and, where appropriate, sign an agreement to collaborate in the development and execution of the following phases of the development of VCN-01 for the treatment of retinoblastoma; (b) the Institution shall grant to VCN an exclusive, worldwide and indefinite license to use and exploit the trial results and their possible patents exclusively for the treatment of retinoblastoma; (c) VCN shall pay the Foundation five hundred thousand Euros (€500,000), subject to reduction for any public and/or private economic aid that third parties may grant to the Institution for the conduct of the trial and/or any advance payments made by VCN before the end of the trial; (d) VCN shall pay the Foundation three hundred twenty thousand Euros (€320,000) once following the trial results of a pivotal study, to be carried out by VCN, has been completed which allows it to obtain the marketing authorization of the product following from the results, which payment must be made within a maximum period of four (4) years from the date on which Institution has delivered the final report of the trial to VCN ; and (e) the parties will use their best efforts to negotiate and, where appropriate, sign a product supply agreement in order that the Hospital can use VCN-01 for compassionate use in the treatment of retinoblastoma. The Collaboration and License Agreement continues in force and effect until all obligations arising from the trial have been fulfilled, subject to early termination for a material breach by a party of any of their contractual and/or legal obligations, or, in the case of any other type of breach, when the breaching party has been asked in writing to remedy the breach and the breach is not cured within thirty (30) days from the date on which the written request was sent.
On November 2, 2023, VCN and Sant Joan de Déu-Barcelona Children’s Hospital announced an agreement for an exclusive worldwide option to negotiate an exclusive license of certain Sant Joan de Deu intellectual property rights related to the use of VCN-01 in combination with topoisomerase I inhibitor chemotherapies for the treatment of cancer. During the year ended December 31, 2023 the Company paid a Euros (€25,000) option fee.
Washington University School of Medicine in St. Louis Clinical Trial Agreement
On August 7, 2019, the Company entered into a clinical trial agreement (“CTA”) with Washington University School of Medicine in St. Louis (“Washington University”) to conduct a Phase 1b/2a single-center, randomized, double-blinded, placebo-controlled clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of oral SYN-004 (ribaxamase) in up to 36 adult allogeneic hematopoietic cell transplant (HCT) recipients (the “Study”). Under the terms of the CTA, the Company will serve as the sponsor of the Study and supply SYN-004 (ribaxamase), as well as compensate Washington University for all research services to be provided in connection with the Study which is estimated to cost approximately $3,200,000. Dr. Erik R. Dubberke, Professor of Medicine and Clinical Director, Transplant Infectious Diseases at Washington University will serve as the principal investigator of the trial in collaboration with his Washington University colleague Dr. Mark A. Schroeder, Associate Professor of Medicine, Division of Oncology, Bone Marrow Transplantation and Leukemia.
100
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
The CTA continues in effect until completion of all obligations under the CTA. Either party may terminate the CTA prior to completion of its obligations (i) if authorization of the study is withdrawn by the FDA; (ii) if the emergence of any adverse reaction or side effect with SYN-004 (ribaxamase) administered in the Study is of such magnitude or incidence in the opinion of either party to support termination; or (iii) upon a breach of the terms of the CTA if the breaching party fails to cure the breach within 30 days after receipt of notice. The Company has the right to terminate the CTA (i) effective immediately if Washington University fails to perform the study in accordance with the terms of the protocol, the CTA or applicable laws or regulations or if Washington University or the principal investigator become debarred or (ii) upon 14 days written notice and Washington University has the right to terminate the CTA upon 14 days notice if the principal investigator becomes unable to perform or complete the Study and the parties have not, prior to the expiration of such fourteen (14) day period, agreed to an alternative principal investigator. The Company paid $1.1 million related to this agreement during the year ended 2022. There we no payments during 2023.
Massachusetts General Hospital Exclusive Option License Agreement
On May 27, 2020, the Company entered into an agreement with Massachusetts General Hospital (“MGH”) granting us an option for an exclusive license to intellectual property and technology related to the use of intestinal alkaline phosphatase (“IAP”) to maintain gastrointestinal (GI) and microbiome health, diminish systemic inflammation, and treat age-related diseases. If executed, the Company plans to use this license in the advancement of an expanded clinical development program for SYN-020, its proprietary recombinant version of bovine IAP currently in pre-clinical development. Under the terms of the agreement, the Company is granted exclusive rights to negotiate a worldwide license with MGH to commercially develop SYN-020 to treat and prevent metabolic and inflammatory diseases associated with aging. During the second quarter of 2021, the Company announced an amendment to its option for an exclusive license agreement with MGH to include intellectual property and technology related to the use of SYN-020 to inhibit liver fibrosis in select diseases, including NAFLD. In January 2023, the company paid $7,500 to extend the option period until July 2024.
University of Texas Austin Agreement
On December 19, 2012, the Company entered into a License Agreement with University of Texas Austin (“UT”) for the exclusive license of the right to use, develop, manufacture, market and commercialize certain research and patents related to pertussis antibodies. The License Agreement provides that UT Austin is entitled to payment of past patent expenses, an annual payment of $50,000 per year commencing on the effective date through December 31, 2014, a $25,000 payment on December 31, 2015 and milestone payments of $50,000 upon commencement of Phase 1 clinical trials, $100,000 upon commencement of Phase 3 clinical trials, $250,000 upon NDA submission in the U.S., $100,000 upon European Medicines Agency approval and $100,000 upon regulatory approval in an Asian country. In addition, UT Austin is entitled to a running royalty upon net sales. The License Agreement terminates upon the expiration of the patent rights; provided, however that the License Agreement is subject to early termination by the Company in its discretion and by UT Austin for a breach of the License Agreement by the Company. No amounts incurred in 2023 and 2022.
In connection with the License Agreement, the Company and UT Austin also entered into a Sponsored Research Agreement pursuant to which UT Austin will perform certain research work related to pertussis. The Sponsored Research Agreement may be renewed annually, in the sole discretion of the Company, after the first year for two additional one year terms with a fixed fee for the first year of $303,000. The Sponsored Research Agreement was renewed for the second and third years for a fixed fee of $316,000 and $329,000 respectively, all payable in quarterly installments. The Sponsored Research Agreement expired January 17, 2023.
101
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
Prev ABR LLC (“Prev”) Agreement
On November 28, 2012, the Company entered into an agreement (“Prev Agreement”) to acquire the C. diff program assets of Prev, including the pre-Investigational New Drug (IND) package, Phase 1 and Phase 2 clinical data, manufacturing process data and all issued and pending U.S. and international patents. Upon execution and closing of the Prev Agreement, the Company paid Prev cash payments of $235,000 and issued 17,858 unregistered shares of its common stock to Prev. As set forth in the Prev Agreement, Prev may be entitled to receive additional consideration upon the achievement of certain milestones, including: (i) commencement of an IND; (ii) commencement of a Phase 1 clinical trial; (iii) commencement of a Phase 2 clinical trial; (iv) commencement of a Phase 3 clinical trial; (v) filing a Biologic License Application (BLA) in the U.S. and for territories outside of the U.S. (as defined in the Prev Agreement); and (vi) approval of a BLA in the U.S. and for territories outside the U.S. With exception of the first milestone payment, the remaining milestones are payable 50% in cash and 50% in the Company’s stock, however, at Prev’s option the entire milestone may be payable in shares of the Company’s stock. As of December 31, 2015, the first three milestones had been met, and at Prev’s option, Prev elected to receive 18,724 shares of the Company’s common stock. Currently, assets licensed under this agreement are used in the Company’s Phase 1b/2a Clinical Study in Allogeneic HCT Recipients. No milestones were achieved or such payments were made subsequent to 2015.
Employment Agreements
On January 3, 2022, the Company entered into a three-year employment agreement with Steven A. Shallcross, (the “2022 Shallcross Employment Agreement”), to serve as the Chief Executive Officer and to continue to serve as the Chief Financial Officer of the Company.
The Employment Agreement has a stated term of three years but may be terminated earlier pursuant to its terms. If Mr. Shallcross’ employment is terminated for any reason, he or his estate as the case may be, will be entitled to receive the accrued base salary, vacation pay, expense reimbursement and any other entitlements accrued by him to the extent not previously paid (the “Accrued Obligations”); provided, however, that if his employment is terminated (i) by the Company without Cause or by Mr. Shallcross for Good Reason (as each is defined in the Employment Agreement) then in addition to paying the Accrued Obligations, (a) the Company will continue to pay his then current base salary and continue to provide benefits at least equal to those that were provided at the time of termination for a period of twelve (12) months and (b) he shall have the right to exercise any vested equity awards until the earlier of six (6) months after termination or the remaining term of the awards; or (ii) by reason of his death or Disability (as defined in the Employment Agreement), then in addition to paying the Accrued Obligations, Mr. Shallcross would have the right to exercise any vested options until the earlier of six (6) months after termination or the remaining term of the awards. In such event, if Mr. Shallcross commenced employment with another employer and becomes eligible to receive medical or other welfare benefits under another employer-provided plan, the medical and other welfare benefits to be provided by the Company as described herein would terminate.
On December 15, 2022, the Board of Directors of the Company awarded Steven A. Shallcross: (i) a cash bonus equal to $385,000, and (ii) an option to purchase 475,000 shares of the Company's common stock. In addition, on December 15, 2022, the Company entered into an Amendment to Mr. Shallcross's Employment Agreement to increase his base salary to $614,250.
On December 14, 2023, the Board of Directors of the Company awarded Steven A. Shallcross: (i) a cash bonus equal to $350,000, and (ii) an option to purchase 700,000 shares of the Company's common stock. In addition, on December 14, 2023, the Company increased his base salary to $644,963 due to a merit increase.
102
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
On March 22, 2022, Synthetic Biologics, Inc. (the “Company”) entered into an employment agreement with Frank Tufaro (the “Employment Agreement”) to serve as the Chief Operating Officer of the Company. Pursuant to the Employment Agreement, Dr. Tufaro had received an annual base salary of $375,000 and was eligible to earn an annual performance bonus of up to forty percent (40%) of his annual base salary. The annual bonus was based upon the assessment of the Company’s Board of Directors (the “Board”) of Dr. Tufaro’s performance and the Company’s attainment of targeted goals set by the Board. In addition, Dr. Tufaro was also be eligible to receive annual equity awards pursuant to the Company’s incentive equity plans, such awards (including the number and type of awards), if any, was to be in the sole discretion of the Board. The Employment Agreement also included confidentiality obligations and inventions assignments by Dr. Tufaro and non-solicitation and non-competition provisions.
The employment agreement had a stated term of three (3) years but may be terminated earlier pursuant to its terms. The employment agreement provided that if Dr. Tufaro’s employment was terminated for any reason, he or his estate as the case may be, would be entitled to receive the accrued base salary, any unpaid annual bonus earned with respect to any calendar year ending on or preceding the date of termination, vacation pay, expense reimbursement and any other entitlements accrued by him to the extent not previously paid (the “Accrued Obligations”); provided, however, that if his employment was terminated (i) by the Company without Cause or by Dr. Tufaro for Good Reason (as each is defined in the Employment Agreement) then in addition to paying the Accrued Obligations, (a) the Company would continue to pay his then current base salary and continue to provide benefits at least equal to those that were provided at the time of termination for a period of six (6) months and (b) all unvested stock options and other equity awards would immediately vest and he would be entitled to exercise any vested equity awards until the earlier of six (6) months after termination or the remaining term of the awards; or (ii) by reason of his death or Disability (as defined in the employment agreement), then in addition to paying the Accrued Obligations, Dr. Tufaro, or his estate as the case may be, would have the right to exercise any vested options until the earlier of six (6) months after termination or the remaining term of the awards. If Dr. Tufaro commenced employment with another employer and became eligible to receive medical or other welfare benefits under another employer-provided plan, the medical and other welfare benefits to be provided by the Company as described herein would terminate.
On December 15, 2022, the Board awarded Frank Tufaro, the Company's Chief Operating Officer: (i) a cash bonus equal to approximately 23% of his current base salary, and (ii) an option to purchase 100,000 shares of the Company's Common Stock. In addition, on December 15, 2022, the Company entered into an Amendment to Dr. Tufaro's Employment Agreement to increase his base salary to $393,750.
Effective May 10, 2023, the Company entered into a Separation Agreement and Release with Frank Tufaro (the “Separation Agreement”) and a consulting agreement with Mr. Tufaro.
In accordance with the terms of the Employment Agreement, the Separation Agreement provides for (i) the payment to Mr. Tufaro of a total of $196,875, paid in bi-monthly installments, less applicable withholding, for a period of six months, (ii) reimbursement of COBRA coverage for himself, his spouse and other eligible dependents for the lesser of: six months or until he commences new employment or substantial self-employment, and (iii) acceleration of the vesting of his outstanding stock options (the “Option Awards”)and (iv) the extension of the period of time for which Mr. Tufaro has the right to exercise any vested shares subject to options until the earlier of (i) the expiration date of the Option Awards, or (ii) six (6) months from the separation date. The Company recorded $22,000 of stock option expense due to the acceleration of the vesting. The Separation Agreement contains mutual general releases of claims and non-disparagement provisions.
The Consulting Agreement has a term of six months unless sooner terminated. Either party may terminate the Consulting Agreement without cause at any time upon thirty (days’ prior written notice or with cause immediately. Mr. Tufaro will be compensated a set daily rate for each full day that he provides consulting services, pro-rated for any days services are provided less than eight hours. There were no amounts paid under this conlsuting agreement during the year ended December 31, 2023.
103
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
Operating Lease
The Company’s existing leases as of December 31, 2023 for its U.S. and Spanish facilities are classified as operating leases. During the quarter ended June 30, 2021, the Company renewed its Rockville, MD facility lease by entering into a Second Lease Amendment which extends the lease term for 63 months beginning on September 1, 2022 and ending on December 31, 2027 at stated rental rates and including a 3-month rent abatement. The Second Amendment also has options for a Tenant Improvement Allowance and a Second Extension Term. The Second Amendment also gives the Company the right to expand its space by giving notice to the landlord before December 31, 2021. The Company did not give notice to expand the space during 2021. The Second Extension Term is offered at market rates and there is no economic incentive for the lessee, therefore the Company has determined that it is not part of the original lease term. There is an option in this Second Amendment to Lease for the Company to borrow funds for tenant improvements subject to an 8.5% interest rate.
The Company also leases research and office facilities in Barcelona, Spain for its 100 percent owned Theriva S.L. subsidiary. The lease that was in existence from December 2021 to December 2022 was a short term agreement with a 90-day termination notice provision that can be exercised by either party. On the closing date of the Theriva S.L. acquisition, a sublease was executed for Theriva S.L. to lease research and office facilities at a new location in Parets del Valles (Barcelona) from the former owner of Theriva S.L.. This lease was executed for an initial term to begin in January 2023 until October 2026, with an option to renew for an additional five years. On January 15, 2023, Theriva S.L. moved into the facilities and the new lease commenced and the prior lease terminated.
Operating lease costs are presented as part of general and administrative expenses in the condensed consolidated statements of operations, and for the year ended December 31, 2023 and 2022 approximated $624,000 and $569,000, respectively. For the Barcelona lease, the day one non-cash addition of right of use assets due to adoption of ASC 842 was $937,000.
A maturity analysis of the Company’s operating leases as of December 31, 2023 is as follows (amounts in thousands of dollars):
Future undiscounted cash flow for the years ending December 31, |
|
|
|
2024 |
|
|
654 |
2025 |
|
|
664 |
2026 |
|
|
582 |
2027 |
|
|
368 |
Total |
|
|
2,268 |
|
|
|
|
Discount factor |
|
|
(339) |
Operating lease liability |
|
|
1,929 |
Operating lease liability - current |
|
|
(487) |
Operating lease liability - long term |
|
$ |
1,442 |
Consulting Fees
In November 2017, the Company engaged a regulatory consultant to assist in the Company’s efforts to prepare, file and obtain FDA approval for ribaxamase. The term of the engagement is on a monthly basis, provided that either party may terminate the agreement at any time by providing the other party a six-month notice period. The Company was obligated to pay the consultant a monthly retainer in addition to success fee payments of up to an aggregate of $4,500,000 for attainment of certain regulatory milestones. The achievement of the milestones is not probable at this time. No amounts incurred in 2023 and 2022.
104
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. License, Collaborative and Employment Agreements and Commitments – (continued)
Risks and Uncertainties
The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values could impact the Company’s business in the future. The Company and its third-party contract manufacturers, contract research organizations, and clinical sites may also face disruptions in procuring items that are essential to the Company’s research and development activities, including, for example, medical and laboratory supplies used in its clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak. Further, although the Company has not experienced any material adverse effects on business due to increasing inflation, it has raised operating costs for many businesses and, in the future, could impact demand or pricing manufacturing of its drug candidates or services providers, foreign exchange rates or employee wages. The Company is actively monitoring the effects that these disruptions and increasing inflation could have on its operations.
Through the VCN Acquisition, the Company has operations in Spain related to conducting research and development, manufacturing, and clinical trials in Western European countries. The invasion of Ukraine by Russia, the war in the Middle East, and the retaliatory measures that have been taken, or could be taken in the future, by the United States, NATO, and other countries have created global security concerns that could result in a regional conflict and otherwise have a lasting impact on regional and global economies, any or all of which could disrupt the Company’s supply chain, and despite the fact that it currently does not plan any clinical trials in Eastern Europe, may adversely impact the cost and conduct of R&D, manufacturing, and international clinical trials of its product candidates.
14. Income Taxes
Losses before income taxes for the years ended December 31, 2023 and 2022 was as follows:
|
|
Year Ended December 31, |
||||
|
|
2023 |
|
2022 |
||
|
|
|
|
|
|
|
Domestic |
|
$ |
(8,568) |
|
$ |
(15,325) |
Foreign |
|
|
(11,421) |
|
|
(5,785) |
|
|
|
|
|
|
|
Income/(Loss) before Income Taxes |
|
$ |
(19,989) |
|
$ |
(21,110) |
The components of income tax benefit consisted of the following for the years ended December 31, 2023 and 2022:
|
|
Year Ended December 31, |
||||
|
|
2023 |
|
2022 |
||
Current: |
|
|
|
|
|
|
Federal |
|
$ |
— |
|
$ |
— |
State |
|
|
— |
|
|
2 |
Foreign |
|
|
— |
|
|
— |
Total Current |
|
|
— |
|
|
2 |
|
|
|
|
|
|
|
Deferred: |
|
|
|
|
|
|
Federal |
|
$ |
— |
|
$ |
— |
State |
|
|
— |
|
|
— |
Foreign |
|
|
(1,640) |
|
|
(1,427) |
Total Deferred |
|
|
(1,640) |
|
|
(1,427) |
Provision (Benefit) for income taxes |
|
|
(1,640) |
|
|
(1,425) |
105
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
14. Income Taxes – (continued)
Income tax (benefit) provision related to continuing operations differ from the amounts computed by applying the statutory income tax rate of 21% to pretax loss as follows (in thousands):
|
|
Year Ended December 31, 2023 |
|
Year Ended December 31, 2022 |
|
|||||
|
|
Amount |
|
Rate |
|
Amount |
|
Rate |
|
|
US Federal Statutory Tax Rate |
|
$ |
(4,198) |
|
21.00 |
% |
(4,433) |
|
21.00 |
% |
State and Local Income Taxes, Net of Federal Income Tax Effect |
|
|
(532) |
|
2.66 |
% |
(678) |
|
3.22 |
% |
Foreign Tax Effects-Spain |
|
|
|
|
|
|
|
|
|
|
Statutory tax rate difference between Spain and United States |
|
|
(457) |
|
2.29 |
% |
(231) |
|
1.10 |
% |
Changes in Valuation Allowances |
|
|
1,332 |
|
(6.66) |
% |
— |
|
0.00 |
% |
Changes in Valuation Allowances |
|
|
2,291 |
|
(11.46) |
% |
2,901 |
|
(13.74) |
% |
Nontaxable or Nondeductible Items |
|
|
(187) |
|
0.93 |
% |
575 |
|
(2.72) |
% |
Other Adjustments |
|
|
111 |
|
(0.56) |
% |
441 |
|
(2.11) |
% |
Effective Tax Rate |
|
$ |
(1,640) |
|
8.20 |
% |
(1,425) |
|
6.75 |
% |
Deferred Tax Assets and Liabilities
Deferred income taxes reflect the net tax effects of loss and credit carryforwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets for federal and state income taxes are as follows (in thousands):
|
|
Year Ended December 31, |
||||
|
|
2023 |
|
2022 |
||
Deferred Tax Assets: |
|
|
|
|
|
|
Federal, State and Foreign NOL Carryforward |
|
$ |
27,356 |
|
$ |
22,235 |
Accrued Compensation |
|
|
24 |
|
|
29 |
Stock Issued For Services |
|
|
957 |
|
|
1,053 |
Stock Issued for Acquisition of Program |
|
|
1,457 |
|
|
1,456 |
Stock Issued for License Agreement |
|
|
1,124 |
|
|
1,362 |
Amortizable License Fee |
|
|
3 |
|
|
4 |
Capitalized Research & Development costs |
|
|
2,422 |
|
|
1,592 |
|
|
|
|
|
|
|
Total Gross DTA |
|
|
33,343 |
|
|
27,731 |
Less: Valuation Allowance |
|
|
(28,351) |
|
|
(24,562) |
Total Deferred Tax Assets |
|
|
4,992 |
|
|
3,169 |
Deferred Tax Liabilities: |
|
|
|
|
|
|
IPR&D |
|
|
(4,939) |
|
|
(4,787) |
ASC 842 Net ROU Assets |
|
|
(53) |
|
|
— |
Total Gross DTL |
|
|
(4,992) |
|
|
(4,787) |
|
|
|
|
|
|
|
Net Deferred Tax Asset (Liability) |
|
$ |
— |
|
$ |
(1,618) |
106
Theriva Biologics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
14. Income Taxes – (continued)
On March 10, 2022, the Company acquired VCN, a Spanish Company in a tax-free stock acquisition. Due to this acquisition, VCN is a wholly owned subsidiary of the company. As a result of the acquisition, a deferred tax liability was established with purchase accounting related to acquired In Process Research and Development. A deferred tax asset was also established with purchase accounting related to VCN’s unlimited life net operating loss carryover.
At December 31, 2023, the Company has a gross Federal net operating loss carry-forward of approximately $72.7 million available to offset future United States taxable income. In addition, it was determined that the utilization of gross Federal net operating losses of approximately $228.3 million was limited by $155.6. million as a result of change of control ownership changes that occurred under Section 382 of the Internal Revenue Code. State NOL’s are also limited by Section 382 of the Internal Revenue Code and were limited accordingly. At December 31, 2023, the Company has a gross Foreign net operating loss carry forward of approximately $25.2 million USD. The foreign net operating loss carries forward indefinitely.
In 2020, the Company completed an Internal Revenue Code Section 382 analysis of its historical net operating loss carry-forward amount. As a result, the prior year net operating loss carry-forward was limited by $155.6 million. The decrease in the prior year net operating loss is attributable to control ownership changes which were determined for the years 2013 and 2018 which caused the reduction in the value of the historical net operating loss carry-forward amounts. Updated section 382 analysis were performed in 2021, 2022, and 2023 to identify if any additional ownership shifts occurred in these years. The result of the updated Section 382 analysis produced an IRC 382 limit due to the 2021 ownership change. There was no ownership change determined for 2022 or 2023. All previously limited net operating losses remain available for use in future periods.
The Company’s pre-2018 net operating losses expire on various dates through 2037 while the net operating loss carry-forward originating in the 2018 year and later carryforward indefinitely and are subject to additional limitations based on taxable income.
At December 31, 2023, the Company has a gross Foreign net operating loss carryforward of approximately $25.2 million related to its newly acquired Spanish subsidiary, VCN. The net operating loss does not expire and is available to offset future Spanish taxable income.
The Company’s valuation allowance at December 31, 2023 was approximately $28.4 million. The net change in valuation allowance during the year ended December 31, 2023, was an increase of approximately $3.8 million primarily due to increases in gross federal and state deferred tax assets in 2023. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The ultimate realization of deferred income tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred income tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. As of December 31, 2023 and 2022, management has established a full valuation allowance against its net deferred tax assets in all US tax jurisdictions. The Company has also established a valuation allowance in its Spanish tax jurisdictions as it is no longer in a net deferred tax liability position in Spain.
Undistributed earnings of the Company’s foreign subsidiary, VCN, are considered to be permanently reinvested and, accordingly, no deferred U.S. income taxes have been provided thereon. Upon distribution of any earnings in the form of dividends or otherwise, those earnings would be subject to U.S. income tax. At the present time, VCN does not have any earnings and thus it is not necessary to estimate the amount of U.S. income taxes that might be payable if these earnings were repatriated.
The Company continually evaluates expiring statutes of limitation, audits, proposed settlements, changes in tax law, and new authoritative rulings. Due to the existence of net operating carryforwards since inception, all of the Company’s income tax filings remain open.
We have incurred net operating losses since inception, and we do not have any significant unrecognized tax benefits.
107
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We adopted and maintaindisclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in the reports filed under the Exchange Act, such as this Annual Report on Form 10-K, is collected, recorded, processed, summarized and reported within the time periods specified under the rules of the SEC. Our disclosure controls and procedures are also designed to ensure that such information is accumulated and communicated to management to allow timely decisions regarding required disclosure. As required under Exchange Act Rule 13a-15, our management, including the Chief Executive Officer who also serves as our Chief Financial Officer, evaluated the effectiveness of disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this Annual Report on Form 10-K. Due to the material weaknesses in internal control over financial reporting as described below, our Chief Executive Officer who also serves as our Chief Financial Officer concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were not effective.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15. Internal control over financial reporting is defined in Rule 13a-15(f) and 15(d)-15(f) under the Exchange Act as a process designed to provide reasonable assurance to our management and Board of Directors regarding the preparation and fair presentation of published financial statements. Management conducted an assessment of our internal control over financial reporting as of December 31, 2023 based on the framework and criteria established by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Based on the assessment, management concluded that, as of December 31, 2023, our internal control over financial reporting was not effective.
Our management, including our Chief Executive Officer who is also our Chief Financial Officer, does not expect that our disclosure controls and procedures and our internal control processes will prevent all errors or fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of error or fraud, if any, within our Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
108
Based on its assessment, management has concluded that the Company did not maintain effective internal control over financial reporting as of December 31, 2023, due to the following previously reported material weaknesses that continued to exist:
| ● | Management did not design and maintain effective review controls at a sufficient level of precision with certain financial statement areas and over unusual transactions involving complex accounting and related disclosure requirements. |
| ● | Management did not maintain effective information technology general controls over user access, program change management, and segregation of duties, within certain key information systems supporting the Company’s accounting and financial reporting processes. Additionally, many of the Company’s business process controls dependent upon the information derived from these information systems were also ineffective, as management did not design and implement controls to validate the completeness and accuracy of underlying data utilized in the operation of those controls. |
Management’s Plan for Remediation
In response to the material weaknesses, management, with oversight of the Audit Committee of the Board of Directors, has identified and begun to implement steps to remediate the material weaknesses. The company hired an third party consultant during 2023 to assist with the remediation efforts. While the Company has made progress during 2023, the remediation efforts are ongoing, as additional time is needed to complete the remediation and allow for the internal controls to be tested by management. Our continued internal control remediation efforts include the following:
| ● | Enhancing existing policies and procedures to facilitate more efficient operations and improve the timely execution of key controls by company personnel. |
| ● | Enhancing program change management, user access provisioning, and monitoring controls to ensure changes to key applications are appropriately reviewed and approved and to enforce appropriate system access and segregation of duties. |
| ● | Improving the design of key controls to ensure reports used in the performance of such controls are complete and accurate as part of the controls execution. |
We are committed to ensuring that our internal controls over financial reporting are designed and operating effectively. Management believes the efforts taken to date and the planned remediation will improve the effectiveness of our internal control over financial reporting. While these remediation efforts are ongoing, the controls must be operating effectively for a sufficient period of time and be tested by management in order to consider them remediated and conclude that the design is effective to address the risks of material misstatement.
Changes in Internal Control Over Financial Reporting
Except for the material weaknesses described above, there has been no change in the Company’s internal control over financial reporting during the Company’s most recent quarter that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
Item 9B. Other Information.
During the three months ended December 31, 2023, no director or officer of the Company adopted or terminated a “Rule 10b5-1 trading arrangement” or “nonRule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not Applicable
109
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Below is certain information regarding our directors and executive officers.
Name |
|
Age |
|
Position |
Steven A. Shallcross |
|
62 |
|
Chief Executive Officer, Chief Financial Officer and Director |
Jeffrey J. Kraws |
|
58 |
|
Chairman |
John Monahan |
|
76 |
|
Director |
Jeffrey Wolf, J.D. |
|
59 |
|
Director |
Steven A. Shallcross. Mr. Shallcross has been a member of our Board of Directors since December 6, 2018 and currently serves as our Chief Executive Officer, a position he was appointed to on December 6, 2018, and our Chief Financial Officer. Mr. Shallcross was appointed as our Interim Chief Executive Officer on December 5, 2017 and has served as our Chief Financial Officer since joining us in June 2015. Mr. Shallcross brings to our company operational, financial and international biotech industry experience, as well as an established track record at leading the financial development and strategy for several publicly traded biotech companies. From May 2013 through May 2015, Mr. Shallcross served as Executive Vice President and Chief Financial Officer of Nuo Therapeutics, Inc. (formerly Cytomedix, Inc.). In January 2016, Nuo Therapeutics, Inc. filed a voluntary petition for relief under Chapter 11 of the U.S. Bankruptcy Code in the United States Bankruptcy Court for the District of Delaware and on April 25, 2016, the Bankruptcy Court entered an order granting approval of Nuo’s plan of reorganization. From July 2012 to May 2013, Mr. Shallcross held the offices of Executive Vice President, Chief Financial Officer and Treasurer of Empire Petroleum Partners, LLC, a motor fuel distribution company. From July 2011 to March 2012, Mr. Shallcross was Acting Chief Financial Officer of Senseonics, a privately-held medical device company located in Germantown, MD. From January 2009 to March 2011, he served as Executive Vice President and Chief Financial Officer of Innocoll AG (formerly privately held Innocoll Holdings, Inc.), a global, commercial-stage biopharmaceutical company specializing in the development and commercialization of collagen-based products. He also served for four years as the Chief Financial Officer and Treasurer of Vanda Pharmaceuticals, Inc., leading the company through its successful IPO and follow-on offering and previously served as the Senior Vice President and Chief Financial Officer of Middlebrook Pharmaceuticals, Inc. (formerly Advancis Pharmaceutical Corporation). In addition, Mr. Shallcross also served as the Chief Financial Officer of Bering Truck Corporation. Since June 2019, Mr. Shallcross has served on the board of directors of Elys Game Technology, Corp. a Nasdaq listed international, vertically integrated commercial-stage company engaged in various aspects of the leisure gaming industry and from April 2021 until June 2022, he served on the board of directors of TwinVee Powercats, Co. (Nasdaq: VEEE), a designer, manufacturer and marketer of recreational and commercial power catamaran boats. He holds an MBA from the University of Chicago’s Booth School of Business, a Bachelor of Science degree in Accounting from the University of Illinois, Chicago, and is a Certified Public Accountant in the State of Illinois.
Mr. Shallcross brings to the Board of Directors significant strategic, business and financial experience related to the business and financial issues facing biotechnology companies. Mr. Shallcross has a broad understanding of the financial markets, financial statements as well as generally accepted accounting principles. Through his services as our Chief Executive Officer and Chief Financial Officer, he has developed extensive knowledge of our business.
110
Jeffrey J. Kraws. Mr. Kraws has been a member of the Company’s Board of Directors since January of 2006, and was appointed independent, non-executive Chairman of the Board in May 2012. Since 2003, Mr. Kraws has served as Chief Executive Officer and co-founder of Crystal Research Associates and CRA Advisors, and since February 2012, he has served as partner and co-founder of TopHat Capital, LLC. Since November 9, 2021, Mr. Kraws serves as the Chief Executive Officer of GridIron Bionutrients, Inc. From August 2016 through January 2021, Mr. Kraws served as the Co-President of Ra Medical Systems Inc. (NYSE: RMED), a medical device company. Mr. Kraws is a partner at Grannus Securities Pty Ltd. (an Australian based private equity fund) since November 2015. Mr. Kraws is a partner of PDK Healthcare Innovations LLC. Mr. Kraws also serves as Chief Financial Officer of Syncromune, Inc. He also consults and assists in management of private companies through his private practice. Mr. Kraws has received some of the most prestigious awards in the industry. Among other awards, he was given a “5-Star Rating” in 2001 by Zacks and was ranked the number one analyst among all pharmaceutical analysts for stock performance in 2001 by Starmine.com. Prior to founding Crystal Research Associates, Mr. Kraws served as co-president of The Investor Relations Group (IRG), a firm representing primarily under-followed, small-capitalization companies. Previously, Mr. Kraws served as a managing director of healthcare research for Ryan Beck & Co. and as director of research/senior pharmaceutical analyst and managing director at Gruntal & Co., LLC (prior to its merger with Ryan Beck & Company). Mr. Kraws served as managing director of the healthcare research group and senior pharmaceutical analyst at First Union Securities (formerly EVEREN Securities); as senior U.S. pharmaceutical analyst for the Swedish-Swiss conglomerate Asea Brown Boveri; and as managing director and president of the Brokerage/Investment Banking operation of ABB Aros Securities, Inc. He also served as senior pharmaceutical analyst at Nationsbanc Montgomery Securities, BT Alex Brown & Sons, and Buckingham Research. Mr. Kraws also has industry experience, having been responsible for competitive analysis within the treasury group at Bristol-Myers-Squibb Company. During 2006 through February of 2007, Mr. Kraws served as our Vice President of Business Development, on a part-time basis. Since December 2013 until April 2023, Mr. Kraws served on the board of directors of Avivagen Inc. (TSX:VIV) and from 2013 until 2020 served on the board of directors of Saleen Automotive, Inc. (OTC Pink: SLNN). He holds an M.B.A. from Cornell University and a B.S. degree from State University of New York — Buffalo. Mr. Kraws brings a strong business background to us, having worked as a pharmaceutical analyst for over 22 years.
Mr. Kraws brings to the Board of Directors significant strategic, business and financial experience related to the business and financial issues facing pharmaceutical companies. Mr. Kraws has a broad understanding of the operational, financial and strategic issues facing pharmaceutical companies. His healthcare experience, executive and leadership experience further qualify him as a member of the Board.
John Monahan. Dr. Monahan has been a member of the Company's Board of Directors since November 11, 2020. Dr. Monahan has served on the board of directors of Scorpius Holdings, Inc. (formerly known as NightHawk Biosciences, Inc. and), a contract development and manufacturing organization since November 2009, and from August 2016 until May 2021 also served on the board of directors of Anixa Biosciences, Inc. (formerly known as ITUS Corporation), a biotechnology company focused on using the body's immune system to diagnose, treat and prevent cancer. He is also a board member of Cellix Ltd. (Ireland) and has served on a number of other public and private boards over the years. Dr. Monahan co-founded Avigen Inc. in 1992, a company which has become a leader in its sector for the development of novel pharmaceutical products for the treatment of serious human diseases. Over a 12-year period as Chief Executive Officer of Avigen he raised over $235 million in several private and public financings including its initial public offering. From 1989-1992, he was Vice President of Research & Development at Somatix Therapy Corp., Alameda, CA and from 1985-1989 he was Director of Molecular & Cell Biology at Triton Biosciences Inc., Alameda, CA. Prior to that from 1982-1985, he was Research Group Chief, Department of Molecular Genetics, Hoffmann-LaRoche, Inc. Nutley, NJ, and from 1975 to 1977 he was an Instructor at Baylor College of Medicine, Houston TX. Dr. Monahan served as a scientific advisory consultant to the Company from 2015 to November 10, 2020 and from 2010 through 2015 he was the Company's Senior Executive Vice President of Research & Development. Dr. Monahan was also a Scientific Advisory Board member of Agilis Biotherapeutics (recently merged into PTC Therapeutics), from 2014 to 2019. Dr. Monahan received his Ph.D. in Biochemistry from McMaster University, Canada and his B.Sc. from University College Dublin, Ireland.
Dr. Monahan brings to our Board of Directors significant knowledge of and experience in the pharmaceutical and medical industries. He has extensive business, managerial, executive and leadership experience that further qualify him to serve as a member of the Board and a valuable understanding of biochemistry and our product candidates.
111
Jeffrey Wolf, J.D. Mr. Wolf, who has been a member of the Company’s Board of Directors since 2006, has substantial experience in creating, financing, nurturing and biomedical ventures based upon breakthrough research and technology. In August 2008, Mr. Wolf founded Scorpius Holdings, Inc. (formerly known as NightHawk Biosciences, Inc.), a publicly traded contract development and manufacturing organization company. Since April 2010, Mr. Wolf has served as the Chief Executive Officer and Chairman of the Board of NightHawk Biosciences, Inc. Prior to founding NightHawk, from June 1997 to March 2011, Mr. Wolf served as managing director at Seed-One Ventures, LLC a venture firm focused on launching and growing exceptional healthcare companies from the ground up. Mr. Wolf has also founded and run several biomedical companies. Mr. Wolf’s start-ups include Avigen, a San Francisco-based gene therapy company where he was a co-founder and director; TyRx Pharma, a company focused on the development of bio-compatible polymers where he was a co-founder and Chairman; and Elusys Therapeutics, a company focused on the development of ANTHIM, an FDA approved antitoxin against anthrax, which is currently a subsidiary of NightHawk. Mr. Wolf received his MBA from Stanford Business School, his J.D. from New York University School of Law and his B.A. from the University of Chicago, where he graduated with honors in Economics.
Mr. Wolf has extensive knowledge of the industry and in particular research and development. His legal and business background provide him with a broad understanding of the legal, operational, financial and strategic issues facing our company. Having served as a board member on other public company boards, Mr. Wolf has an extensive understanding of the operational, financial and strategic issues facing public companies.
Directors’ Term of Office
Directors will hold office until the next annual meeting of stockholders and the election and qualification of their successors. Officers are elected annually by our Board of Directors and serve at the discretion of the Board of Directors.
Audit Committee
The Audit Committee is comprised of Mr. Wolf (Chairman), Mr. Kraws and Dr. Monahan. The Audit Committee is responsible for recommending our independent public accounting firm and reviewing management’s actions in matters relating to audit functions. The Committee reviews with our independent public accountants the scope and results of the audit engagement and the system of internal controls and procedures. The Committee also reviews the effectiveness of procedures intended to prevent violations of laws. The Committee also reviews, prior to publication, our reports on Form 10-K and Form 10-Q. Our Board has determined that all audit committee members are independent under applicable SEC regulations and NYSE American rules. Our Board of Directors has determined that each of Mr. Wolf and Mr. Kraws qualify as “audit committee financial experts” as that term is used in Section 407 of Regulation S-K. Our Audit Committee charter is located on our website www.therivabio.com.
Compensation Committee
Our Compensation Committee consists of Mr. Kraws (Chairman), Dr. Monahan and Mr. Wolf. This committee performs several functions, including reviewing all forms of compensation provided to our executive officers, directors, consultants and employees, including stock compensation. Our Board has determined that all compensation committee members are independent under applicable SEC regulations and NYSE American rules. Our Compensation Committee charter is located on our website www.therivabio.com.
Nominations Committee
Our Nominations Committee consists of Dr. Monahan (Chairman), Mr. Kraws and Mr. Wolf. This committee performs several functions, including identifying qualified individuals to become members of the Board and recommending appointments to the Board and appointment of executive officers. The committee seeks individuals who have an inquisitive and objective perspective, practical wisdom and mature judgment, and the talent and expertise to understand and provide sound and prudent guidance with respect to our activities, operations and interests. Candidates must also be individuals who have the highest personal and professional integrity, who have demonstrated exceptional ability and judgment, and who are likely to be the most effective, in conjunction with the other members of the Board, in collectively serving the long-term interests of stockholders. Our Board has determined that all nominations committee members are independent under applicable SEC regulations and NYSE American rules. Our Nominations Committee charter is located on our website www.therivabio.com.
112
Code of Ethics
We have long maintained a Code of Conduct which is applicable to all of our directors, officers and employees. In addition, we have adopted a Code of Ethics for Financial Management which applies to our Chief Executive Officer, Chief Financial Officer, Treasurer and Controller. Each of these codes is posted on our website at www.therivabio.com.
Insider Trading Policy
We have adopted an insider trading policy (the “Trading Policy”) that is designed to promote compliance with federal securities laws, rules and regulations, as well as the rules and regulations of NYSE American. The Trading Policy was implemented to assure compliance with the securities laws prohibiting insider trading in our securities and disclosure of material, non-public information to outsiders. It prohibits the purchase and sale of our securities by our directors, officers, and employees, as well as members of their households, while in possession of material, non-public information until the third business day after such information is made available to the public. Additionally, our Trading Policy imposes special additional trading restrictions, including requiring pre-clearance of any transaction and prohibiting the purchase or sale of options to sell or buy our securities and short sales. The Trading Policy is annexed to this Annual Report as an exhibit.
Item 11. Executive Compensation.
We are a “smaller reporting company” and the following compensation disclosure is intended to comply with the requirements applicable to smaller reporting companies. Although the rules allow us to provide less detail about our executive compensation program, the Compensation Committee is committed to providing the information necessary to help stockholders understand its executive compensation-related decisions. Accordingly, this section includes supplemental narratives that describe the 2023 executive compensation program for our Named Executive Officers.
The following table summarizes all compensation awarded to, earned by or paid to our Named Executive Officers, Steven A. Shallcross and Frank Tufaro, during the fiscal years presented below.
|
|
|
|
|
|
|
|
|
|
|
|
|
All Other |
|
|
|
|
|
Name and Principal |
|
|
|
|
|
|
|
|
|
Options |
|
Compensation |
|
|
|
|
||
Position |
|
Year |
|
Salary ($)(1) |
|
Bonus ($)(2) |
|
Awards ($)(3) |
|
($)(4) |
|
Total ($) |
|
|||||
Steven Shallcross |
|
2023 |
|
$ |
614,250 |
|
$ |
350,000 |
|
$ |
278,450 |
|
$ |
29,213 |
|
$ |
1,271,913 |
(5) |
Chief Executive Officer |
|
2022 |
|
$ |
585,000 |
|
$ |
385,000 |
|
$ |
192,989 |
|
$ |
27,674 |
|
$ |
1,190,663 |
|
and Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frank Tufaro |
|
2023 |
|
$ |
143,182 |
|
$ |
— |
|
$ |
— |
|
$ |
236,249 |
|
$ |
379,431 |
|
Chief Operating Officer(6) |
|
2022 |
|
$ |
291,667 |
|
$ |
85,000 |
|
$ |
40,629 |
|
$ |
23,963 |
|
$ |
441,259 |
|
| (1) | Mr. Shallcross' annual salary was $585,000 commencing January 1, 2022 and $614,250 commencing January 1, 2023. Dr. Tufaro was appointed our Chief Operating Officer on March 22, 2022 and separated from the Company on May 10, 2023. Dr. Tufaro’s annual salary was $375,000 commencing March 22, 2022. |
| (2) | Amounts represent annual cash bonuses earned for the applicable fiscal year. The annual cash bonuses are paid in the first quarter of the calendar year following the year to which the cash bonus relates. |
| (3) | Amount reflects the grant date fair value of the Named Executive Officer’s stock options, calculated in accordance with FASB ASC Topic 718. For a discussion of the assumptions used in calculating these values, see Note 5 to our consolidated financial statements. In December 2022 and December 2023, Mr. Shallcross was issued options to purchase 475,000 and 700,000 shares of common stock, respectively, Dr. Tufaro was issued 100,000 options in December 2022, and each of these awards vest monthly over 36 months. |
113
| (4) | The all other compensation column is comprised of vacation accrual paid, and the portion of medical, dental and vision premiums paid by us on behalf of our Named Executive Officers. These benefits are offered to all Theriva Biologics’ employees who work at least 17.5 hours per week. All other compensation for Mr. Tufaro includes pursuant to his separation agreement the payment of a total of $196,875 for a period of six months, and reimbursement of COBRA coverage for himself, his spouse and other eligible dependents for six months. |
| (5) | Amount excludes compensation paid to the wife of Mr. Shallcross disclosed under the "Related Party Transactions" |
| (6) | Dr. Tufaro was appointed as our Chief Operating officer on March 22, 2022 and separated from the Company on May 10, 2023. |
Narrative Disclosure to Summary Compensation Table
Overview of Our Compensation Program
A. Philosophy and Objectives
The Compensation Committee seeks to attract and retain superior executive talent by offering competitive base salaries, bonuses and long-term incentives. The Compensation Committee’s philosophy is to deliver higher rewards for superior performance and consequences for underperformance. It is also the Compensation Committee’s practice to provide a balanced mix of cash and equity-based compensation that aligns both the short and long-term interests of our executives with that of our stockholders. Our executive compensation program is based on the following philosophies and objectives:
| ● | Compensation Should Align with Stockholders’ Interests — The Compensation Committee believes that executives’ interests should be aligned with those of the stockholders. Executives are granted stock options so that their total compensation is tied directly to value realized by our stockholders. Executive bonuses are tied directly to the achievement of performance goals that the Compensation Committee believes will ultimately drive stockholder value creation. |
| ● | Compensation is Competitive — The Compensation Committee seeks to provide a total compensation package that attracts, motivates and retains the executive talent that we need in order to maximize our return to stockholders. To accomplish this objective, executive compensation is reviewed annually to ensure that compensation levels are competitive and reasonable relative to our level of performance and to the compensation opportunities provided by comparable companies with which we compete for talent. |
| ● | Compensation Motivates and Rewards the Achievement of Goals — Our executive compensation program is designed to appropriately reward both individual and collective performance that meets and exceeds our annual, long-term and strategic goals. To accomplish this objective, a substantial percentage of total compensation is variable and “at risk”, both through annual incentive compensation in the form of cash bonuses and the granting of long-term incentive awards. |
B. Oversight of Executive Compensation
Role of the Compensation Committee
Pursuant to the terms of its charter, the Compensation Committee is responsible for the review of all aspects of our executive compensation program and makes decisions regarding the compensation of the Named Executive Officers. Our Named Executive Officers for the year ended December 31, 2023 were Steven Shallcross, our Chief Executive Officer who also serves as our Chief Financial Officer, and Frank Tufaro, who served as our Chief Operating Officer until his separation on May 10, 2023.
The Compensation Committee’s responsibilities include but are not limited to the following:
| ● | Establishing on an annual basis the performance goals and objectives for purposes of determining the compensation of our Chief Executive Officer and other senior executive officers. |
114
| ● | Evaluating the Chief Executive Officer’s and other senior executive officers’ performance at least annually in light of those goals and objectives, and based upon these evaluations setting the compensation level for those officers. |
| ● | Reviewing the competitive position of, and making recommendations to, the Board of Directors with respect to the cash-based and equity-based compensation plans and our programs relating to compensation and benefits. |
| ● | Overseeing administration of our stock option plan and incentive compensation plans, making recommendations to the Board of Directors regarding the granting of options and incentives and otherwise assisting the Board of Directors in administering awards under these plans. |
| ● | Reviewing the financial performance and operations of our major benefit plans. |
Additional information regarding the Compensation Committee’s responsibilities is set forth in its charter, which is posted on our website at www.therivabio.com.
Role of the Chief Executive Officer
Our Chief Executive Officer makes recommendations to the Compensation Committee regarding the compensation of our other Named Executive Officers. The Chief Executive Officer does not participate in any discussions or processes concerning his own compensation and participates in a non-voting capacity in discussions or processes concerning the compensation of our other members of management. In addition to our Chief Executive Officer, other members of our management and consultants also attend Compensation Committee meetings from time to time and may take part in discussions of executive compensation.
C. Program Design
The Compensation Committee uses a simple and straightforward approach in compensating our Named Executive Officers in which base salary, annual incentives and stock options are the principal components. In addition, executive officers generally participate in the same benefit programs as other full-time employees.
Our executive compensation program is designed to provide executives with a reasonable level of fixed compensation through base salary and benefits, and an opportunity to earn incentive compensation through the annual and long-term incentive programs based on a mix of individual and corporate performance, individual performance and the value of our stock. We do not currently have formal policies for allocating compensation among base salary, performance-based bonus and equity awards. Instead our Compensation Committee uses its judgment to establish a total direct compensation opportunity for each Named Executive Officer that is a mix of current, short-term and long-term incentive compensation and cash and non-cash compensation that it believes appropriate to achieve the goals of our executive compensation program and corporate objectives. Our target pay mix places a significant emphasis on performance based variable compensation. The incentive plans are designed to pay well when performance meets or exceeds expectations and pay little or no incentive if performance is below expectations.
In designing and implementing our executive compensation program, our Compensation Committee considers our company’s operating and financial objectives, including our risk profile, and the effect that its executive compensation decisions will have on encouraging our executive officers to take an appropriate level of business risk consistent with our overall goal of enhancing long-term stockholder value. In particular, the Compensation Committee considers those business risks identified in our risk factors and the known trends and uncertainties identified in our management discussion and analysis and considers how our executive compensation program serves to achieve our operating and financial objectives while at the same time mitigating any incentives for our executive officers to engage in excessive risk-taking to achieve short-term results that may not be sustainable in the long-term.
Target compensation comprises base salary and performance based variable compensation, including targeted cash bonus amounts and equity-based compensation. As an executive’s level of responsibility increases, the Compensation Committee generally targets a greater portion of the executive’s compensation to be contingent upon performance in the form of variable compensation. For example, historically our Named Executive Officers have a higher percentage of compensation at risk (and thus greater upside and downside potential) relative to our other employees. The Compensation Committee believes this is appropriate because our Named Executive Officers have the greatest influence on our performance.
115
During 2023, the salary for our Chief Executive Officer who also serves as our Chief Financial Officer was 51% of his target compensation package and performance based variable compensation comprised 49% of his target compensation. Of the performance based variable compensation 52% was equity-based compensation and 49% was his target cash bonus. During 2023, our Chief Operating Officer Officer’s salary was 71% of his target annual compensation package and performance based variable compensation comprised 29% of his target annual compensation. Because he served only until May 10, 2023, our Chief Operating Officer received only a pro-rated amount of his base salary, was not paid a cash bonus and did not receive equity-based compensation in 2023.
D. Compensation Review Process
The Compensation Committee annually reviews compensation for our Named Executive Officers. The Compensation Committee considers the executive’s role and responsibilities, corporate and individual performance, and industry-wide compensation practices and trends for other companies of similar size. This approach is used to set base salaries, bonuses, stock option award levels and the mix of compensation elements.
We strive to attract and retain the most highly qualified executive officers in an extremely competitive market. Our Compensation Committee believes that it is important when making its compensation decisions to be informed as to the competitive market for executive talent, including the current practices of comparable public companies with which we compete for such talent. Consequently, our Compensation Committee reviewed an executive compensation benchmarking report prepared by Meridian Compensation Partners, LLC (“Meridian”) at the Compensation Committee’s request. With respect to its analysis of the compensation of the Chief Executive Officer, the Compensation Committee took into account that our Chief Executive Officer also serves as our Chief Financial Officer, which is not typical for most companies.
While the Compensation Committee does take into consideration the data it reviewed, the Committee does not attempt to benchmark our executive compensation against any specific level, range, or percentile of compensation paid at any other companies, does not apply any specific measures of internal or external pay equity in reaching its conclusions, and does not employ tally sheets, wealth accumulation, or similar tools in its analysis. Rather, the Compensation Committee reviews compensation data from the report mentioned above as reference points in making executive compensation decisions especially in light of the fact that our Chief Executive Officer is also performing the role of Chief Financial Officer. The Compensation Committee’s general aim is for our compensation to remain competitive with the market, falling above or below the median of the market data as appropriate based on corporate and individual executive performance, and other factors deemed to be appropriate. Competitive market positioning is only one of several factors, as described below, that the Compensation Committee considers in making compensation decisions, and therefore individual Named Executive Officer compensation may fall at varying levels as compared to the market data.
Our Compensation Committee values the opinion of our stockholders. At our 2022 Annual Meeting of Stockholders approximately 97% of the shares voted (excluding broker non-votes) were cast in support of our fiscal 2022 executive compensation and related disclosures. At that time, our Compensation Committee viewed those voting results as broad stockholder support for our executive compensation program and consequently made no material changes to the program or to our compensation policies. Our Compensation Committee will continue to consider input from stockholders, including through advisory votes on executive compensation, in making compensation decisions and reviewing executive compensation programs and policies.
We currently hold our advisory vote to approve the compensation of our named executive officers (“Say-on-Pay vote”) every three years. Stockholders have an opportunity to cast an advisory vote on the frequency of the Say-on-Pay vote at least every six years, and the next advisory vote on the frequency of the Say-on-Pay vote will be at our 2025 Annual Meeting of Stockholders.
E. Components of Compensation
We provide four compensation components to Named Executive Officers:
| ● | base salary; |
| ● | bonuses based on the achievement of specified goals and objectives; |
| ● | long-term incentives; and |
116
| ● | benefits |
1. Base Salaries
We provide our Named Executive Officers a base salary commensurate with their position, responsibilities and experience. In setting the base salary, the Compensation Committee considers the scope and accountability associated with each Named Executive Officer’s position and such factors as performance and experience of each Named Executive Officer. We design base pay to provide the essential reward for an employee’s work that is required to be competitive in attracting talent. Once base pay levels are initially determined, increases in base pay may be provided to recognize an employee’s specific performance achievements or expansion of responsibilities. The base salaries are targeted to be competitive with other similar biotechnology companies. Base salaries for the Named Executive Officers are set by their respective employment contracts and are reviewed annually by the Compensation Committee referencing an executive compensation benchmarking report provided by Meridian. Mr. Shallcross’ base salary was $585,000 for the year ended December 31, 2022. Mr. Shallcross received a 5% merit increase to $614,250 for the year ended December 31, 2023, and on December 14, 2023 received a 5% merit increase to $644,963.
Our former Chief Operating Officer, Dr. Tufaro, received a base salary of $375,000 for the year ended December 31, 2022. Dr. Tufaro received a 5% merit increase to $393,750 for the year ended December 31, 2023.
The table below shows the 2023 annualized base salary levels for our our Named Executive Officers, including a comparison with 2022.
|
|
2022 |
|
2023 |
||
Named Executive Officer |
|
Base Salary |
|
Base Salary |
||
Steven A. Shallcross, Chief Executive Officer and Chief Financial Officer |
|
$ |
585,000 |
|
$ |
614,250 |
Frank Tufaro, Chief Operating Officer |
|
$ |
375,000 |
|
$ |
393,750 |
2. Bonuses
The Compensation Committee believes that the granting of a bonus is appropriate to motivate the Named Executive Officers. The bonuses are to be rewarded in the discretion of the Compensation Committee and the Board of Directors, based on a review of achievements for the year. The Compensation Committee focuses on individual performance, which enables the Compensation Committee to differentiate among executives and emphasize the link between personal performance and compensation. The Compensation Committee also used information from the Meridian executive compensation benchmarking report in determining bonus amounts. Although the Compensation Committee does not use any fixed formula in determining bonuses, it does link bonuses to objectives the Compensation Committee deems important such as for 2023, effective M&A strategy and implementation, financings, and achievement of clinical milestones.
| ● | Mr. Shallcross’ employment agreement provided that he was eligible for a target bonus of up to fifty percent (50%) of his base salary in cash. After considering Mr. Shallcross’ achievement relative to performance goals in 2023, the Compensation Committee approved a $350,000 cash bonus, or 114% of target. |
| ● | Dr. Tufaro’s employment agreement provided that he was eligible for a target bonus of up to forty percent (40%) of his base salary in cash. Because he served only until May 10, 2023, Dr. Tufaro did not receive a cash bonus for 2023. |
3. Long-Term Incentives
The Compensation Committee believes that a substantial portion of the Named Executive Officer’s compensation should be awarded in equity-based compensation since equity-based compensation is directly linked to the interests of stockholders. The Compensation Committee has elected to grant stock options to the Named Executive Officers and other key employees as the primary long-term incentive vehicle. In making this determination, the Compensation Committee considered a number of factors including: the accounting impact, potential value of stock option grants versus other equity instruments and cash incentives, and the alignment of equity participants with stockholders. The Compensation Committee determined to grant stock options to:
| ● | enhance the link between the creation of stockholder value and executive compensation; |
117
| ● | provide an opportunity for equity ownership; |
| ● | act as a retention tool; and |
| ● | provide competitive levels of total compensation. |
In 2023, the Compensation Committee approved grants of options exercisable for 700,000 shares to Mr. Shallcross. The options had a grant date of December 14, 2023, an exercise price of $0.59, vest pro rata on a monthly basis over 36 months and expire seven years from date of grant. Because he served only until May 10, 2023, Dr. Tufaro did not receive any equity-based compensation in 2023.
The Compensation Committee reviews the performance, potential burn rates and dilution levels to create an option pool that may be awarded to employee participants. Grants to the Named Executive Officers are determined by the Compensation Committee after reviewing market data, including the reports and analysis discussed above and after considering each executive’s performance, role and responsibilities.
The Compensation Committee does not seek to time equity grants to take advantage of information, either positive or negative, about our company that has not been publicly disclosed. Option grants are effective on the date the award determination is made by the Compensation Committee and the exercise price of options is the closing market price of our common stock on the business day of the grant or, if the grant is made on a weekend or holiday, on the prior business day.
4. Benefits
Named Executive Officers are eligible to participate in our standard medical, dental, vision, disability insurance, life insurance plans and other health and welfare plans provided to other full-time employees.
Each of our Named Executive Officers are entitled to participate in our 401(k) contributory defined contribution plan.
Pension Benefits
We do not currently provide pension arrangements or post-retirement health coverage for our employees, although we may consider such benefits in the future.
Retirement Benefits
Each of our Named Executive Officers are eligible to participate in our 401(k) contributory defined contribution plan. Pursuant to our 401(k) plan, all eligible employees, including our Named Executive Officers, are provided with a means of saving for their retirement. We currently match all participating employee contributions up to maximum of 4 percent of compensation which vest immediately.
Nonqualified Deferred Compensation
We do not provide any nonqualified deferred compensation plans to our employees, although we may consider such benefits in the future.
Conclusion
Attracting and retaining talented and motivated management and key employees is essential to creating long-term stockholder value. Offering a competitive, performance-based compensation program with a substantial equity component helps to achieve this objective by aligning the interests of the executive officers and other key employees with those of stockholders. We believe that our compensation program met these objectives and that our 2023 compensation program was appropriate in light of the challenges we and our employees face.
118
Risk Analysis of Our Compensation Program
Our Compensation Committee has reviewed our compensation policies as generally applicable to our employees and believes that our policies do not encourage excessive or inappropriate risk taking and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on us. As part of its assessment, the Compensation Committee considered, among other factors, the allocation of compensation among base salary and short- and long-term compensation, and our approach to establishing company-wide and individual financial, operational and other performance goals.
Outstanding Equity Awards at Fiscal Year End
The table below reflects all outstanding equity awards made to each of the Named Executive Officers that are outstanding at December 31, 2023. We currently grant stock-based awards pursuant to our 2020 Stock Incentive Plan (the “2020 Stock Plan”) and have outstanding awards to Mr. Shallcross under our 2010 Stock Incentive Plan (the “2010 Stock Plan”).
|
|
|
|
Number of |
|
Number of |
|
|
|
|
|
|
|
|
|
Securities |
|
Securities |
|
|
|
|
|
|
|
|
|
Underlying |
|
Underlying |
|
|
|
|
|
|
|
|
|
Unexercised |
|
Unexercised |
|
Option |
|
|
|
|
|
|
|
Options |
|
Options |
|
Exercise |
|
Option |
|
Name |
|
Grant Date(1) |
|
Exercisable |
|
Unexercisable |
|
Price ($) |
|
Expiration Date |
|
Steven Shallcross |
|
12/14/23 |
|
— |
|
700,000 |
|
$ |
0.59 |
|
12/15/30 |
|
|
12/15/22 |
|
158,333 |
|
316,667 |
|
|
0.58 |
|
12/15/29 |
|
|
12/23/21 |
|
43,334 |
|
21,666 |
|
$ |
3.31 |
|
12/23/28 |
|
|
12/30/20 |
|
45,000 |
|
— |
|
$ |
4.17 |
|
12/30/27 |
|
|
12/04/19 |
|
45,000 |
|
— |
|
$ |
4.18 |
|
12/04/26 |
|
|
12/06/18 |
|
20,000 |
|
— |
|
$ |
6.89 |
|
12/06/25 |
|
|
12/20/17 |
|
1,572 |
|
— |
|
$ |
182.00 |
|
12/20/24 |
|
|
06/01/15 |
|
2,573 |
|
— |
|
|
756.00 |
|
06/01/25 |
| (1) | Options will vest pro rata, on a monthly basis, over 36 months. |
Dr. Tufaro resigned from the Company effective May 10, 2023. All or Dr Tufaro’s option awards expired before December 31, 2023.
Employment Agreements
Steven A. Shallcross, Chief Executive Officer, Chief Financial Officer
On January 3, 2022, we entered into a three-year employment agreement with Mr. Shallcross (the “2022 Shallcross Employment Agreement”), to serve as our Chief Executive Officer and to continue to serve as our Chief Financial Officer. The 2022 Employment Agreement replaced the prior employment agreement with us that Mr. Shallcross entered into on December 6, 2018, as amended December 5, 2019 (the “Amended Employment Agreement”). Mr. Shallcross has served as our Chief Financial Officer since June 1, 2015, initially pursuant to the terms of a two year employment agreement that we entered with him on April 28, 2015 (the “Initial Shallcross Employment Agreement”) and then pursuant to an employment agreement we entered into with him on December 6, 2018, which replaced the Initial Shallcross Agreement (the “Amended Shallcross Employment Agreement”). Mr. Shallcross does not receive additional compensation for service as our director. The material terms of the 2022 Shallcross Employment Agreement are set forth below.
Pursuant to the 2022 Employment Agreement, Mr. Shallcross was initially entitled to an annual base salary of $585,000 which was increased to $614,250 for the year ended December 31, 2023 and increased on December 14, 2023 to $644,963 to reflect a 5% merit increase. Mr. Shallcross is also eligible to recieve an annual cash performance bonus targeted at fifty percent (50%) of his annual base salary as well as discretionary annual equity awards pursuant to the Company’s incentive plans. The annual bonus will be based upon the assessment of the Board of Mr. Shallcross’s performance. The 2022 Employment Agreement also includes confidentiality obligations and inventions assignments by Mr. Shallcross and non-solicitation and non-competition provisions.
119
The 2022 Employment Agreement has a stated term of three years but may be terminated earlier pursuant to its terms. If Mr. Shallcross’s employment is terminated for any reason, he or his estate as the case may be, will be entitled to receive the unpaid base salary through the date of termination and accrued vacation, any unpaid annual bonus earned with respect to any calendar year ending on or preceding the date of termination, expense reimbursement and any other entitlements accrued by him to the extent not previously paid (the “Accrued Obligations”); provided, however, that if his employment is terminated (i) by the us without Cause or by Mr. Shallcross for Good Reason (as each is defined in the Employment Agreement) then, subject to him executing a general release in form acceptable to the us that becomes effective, in addition to paying the Accrued Obligations, (a) we will continue to pay his then current base salary and if the Executive timely elects continued coverage under COBRA, the Company will continue to provide benefits at least equal to those that were provided at the time of termination for a period of twelve (12) months and (b) all unvested equity awards will vest and he shall have the right to exercise any such vested equity awards until the earlier of eighteen (18) months after termination or the remaining term of the awards; or (ii) by reason of his death or Disability (as defined in the Employment Agreement), then in addition to paying the Accrued Obligations, Mr. Shallcross or his estate would have the right to exercise any vested options until the earlier of six (6) months after termination or the remaining term of the awards. In such event, if Mr. Shallcross commenced employment with another employer and becomes eligible to receive medical or other welfare benefits under another employer-provided plan, the medical and other welfare benefits to be provided by the Company as described herein would terminate.
The 2022 Employment Agreement provides that upon the closing of a “Change in Control” (as defined in the 2022 Employment Agreement), all unvested options shall immediately vest and the time period that Mr. Shallcross will have to exercise all vested stock options and other awards that Mr. Shallcross may have will be equal to the shorter of: (i) eighteen (18) months after termination, or (ii) the remaining term of the award(s). If within one (1) year after the occurrence of a Change in Control, Mr. Shallcross terminates his employment for “Good Reason” or the Company terminates Mr. Shallcross’s employment for any reason other than death, disability or Cause, Mr. Shallcross will be entitled to receive: (i) the portion of his base salary for periods prior to the effective date of termination accrued but unpaid (if any); (ii) all unreimbursed expenses (if any); (iii) an aggregate amount (the “Change in Control Severance Amount”) equal to two (2) times the sum of his base salary plus an amount equal to the bonus that would be payable if the “target” level performance were achieved under the Company’s annual bonus plan (if any) in respect of the fiscal year during which the termination occurs (or the prior fiscal year if bonus levels have not yet been established for the year of termination) subject to him executing a general release in form acceptable to the Company that becomes effective. If within two (2) years after the occurrence of a Change in Control, Mr. Shallcross terminates his employment for “Good Reason” or the Company terminates Mr. Shallcross’s employment for any reason other than death, disability or Cause, Mr. Shallcross will be entitled to also receive for the period of two (2) consecutive years commencing on the date of such termination of his employment, medical, dental, life and disability insurance coverage for him and the members of his family that are not less favorable to him than the group medical, dental, life and disability insurance coverage carried by the Company for him subject to him executing a general release in form acceptable to the Company that becomes effective. The Change in Control Severance Amount is to be paid in a lump sum if the Change in Control event constitutes a “change in the ownership” or a “change in the effective control” of the Company or a “change in the ownership of a substantial portion of a corporation’s assets” (each within the meaning of Section 409A of the Internal Revenue Code (“Rule 409A”)), or in 48 substantially equal payments, if the Change in Control event does not so comply with Section 409A.
Frank Tufaro, Former Chief Operating Officer,
On March 22, 2022, we entered into an employment agreement with Dr. Tufaro, as amended on December 15, 2022 (the “Employment Agreement”) to serve as the Chief Operating Officer of the Company, which agreement terminated on May 10, 2023. The material terms of the Employment Agreement are set forth below.
Pursuant to the Employment Agreement, as amended Dr. Tufaro was to receive an annual base salary of $393,750 and was eligible to earn an annual performance bonus targeted at forty percent (40%) of his annual base salary. The annual bonus was to be based upon the assessment of the Company’s Board of Dr. Tufaro’s performance and the Company’s attainment of targeted goals set by the Board. In addition, Dr. Tufaro was eligible to receive annual equity awards pursuant to the Company’s incentive equity plans: such awards (including the number and type of awards), if any, were at the sole discretion of the Board. The Employment Agreement also included confidentiality obligations and inventions assignments by Dr. Tufaro and non-solicitation and non-competition provisions.
120
The Employment Agreement had a stated term of three (3) years but could be terminated earlier pursuant to its terms. If Dr. Tufaro’s employment was terminated for any reason, he or his estate as the case may be, would be entitled to receive the accrued base salary, any unpaid annual bonus earned with respect to any calendar year ending on or preceding the date of termination, vacation pay, expense reimbursement and any other entitlements accrued by him to the extent not previously paid (the “Accrued Obligations”); provided, however, that if his employment was terminated (i) by the Company without Cause or by Dr. Tufaro for Good Reason (as each was defined in the Employment Agreement) then in addition to paying the Accrued Obligations, (a) the Company would continue to pay his then current base salary and continue to provide benefits at least equal to those that were provided at the time of termination for a period of six (6) months and (b) all unvested stock options and other equity awards would immediately vest and he would be entitled to exercise any vested equity awards until the earlier of six (6) months after termination or the remaining term of the awards; or (ii) by reason of his death or Disability (as defined in the Employment Agreement), then in addition to paying the Accrued Obligations, Dr. Tufaro, or his estate as the case may be, would have the right to exercise any vested options until the earlier of six (6) months after termination or the remaining term of the awards. If Dr. Tufaro commenced employment with another employer and became eligible to receive medical or other welfare benefits under another employer-provided plan, the medical and other welfare benefits to be provided by the Company as described herein would terminate.
Effective May 10, 2023, the Company entered into a Separation Agreement and Release (the “Separation Agreement”) and a consulting agreement (the “Consulting Agreement”) with Dr. Tufaro. Dr. Tufaro had entered into the Employment Agreement with the Company on March 22, 2022 to serve as our Chief Operating Officer.
In accordance with the terms of the Employment Agreement, the Separation Agreement provides for (i) the payment to Mr. Tufaro of a total of $196,875, paid in bi-monthly installments, less applicable withholding, for a period of six months, (ii) reimbursement of COBRA coverage for himself, his spouse and other eligible dependents for the lesser of: six months or until he commences new employment or substantial self-employment, (iii) acceleration of the vesting of his outstanding stock options (the “Option Awards”) and (iv) the extension of the period of time for which Mr. Tufaro has the right to exercise any vested shares subject to options until the earlier of (a) the expiration date of the Option Awards, or (b) six (6) months from the separation date. The Separation Agreement contains mutual general releases of claims and non-disparagement provisions.
The Consulting Agreement has a term of six months unless sooner terminated. Either party may terminate the Consulting Agreement without cause at any time upon thirty days’ prior written notice or with cause immediately. Mr. Tufaro will be compensated a set daily rate for each full day that he provides consulting services, pro-rated for any days services are provided less than eight hours.
Clawback Policy
The Board has adopted a clawback policy which allows us to recover performance-based compensation, whether cash or equity, from a current or former executive officer in the event of an Accounting Restatement. The clawback policy defines an Accounting Restatement as an accounting restatement of our financial statements due to our material noncompliance with any financial reporting requirement under the securities laws. Under such policy, we may recoup incentive-based compensation previously received by an executive officer that exceeds the amount of incentive-based compensation that otherwise would have been received had it been determined based on the restated amounts in the Accounting Restatement.
The Board has the sole discretion to determine the form and timing of the recovery, which may include repayment, forfeiture and/or an adjustment to future performance-based compensation payouts or awards. The remedies under the clawback policy are in addition to, and not in lieu of, any legal and equitable claims available to the Company. The clawback policy is annexed to this Annual Report as an exhibit.
121
Compensation of Directors
The following table sets forth information for the fiscal year ended December 31, 2023 regarding the compensation of our directors who at December 31, 2023 were not also our Named Executive Officers.
|
|
Fees Earned |
|
|
|
|
|
|
|
|||
|
|
or |
|
Option |
|
Other |
|
|
|
|||
Name |
|
Paid in Cash |
|
Awards(1)(2) |
|
Compensation |
|
Total |
||||
Jeffrey J. Kraws |
|
$ |
176,250 |
|
$ |
43,756 |
|
$ |
— |
|
$ |
220,006 |
|
|
|
|
|
|
|
|
|
|
|
|
|
John Monahan |
|
$ |
66,750 |
|
$ |
43,756 |
|
$ |
— |
|
$ |
110,506 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Jeffrey Wolf |
|
$ |
73,750 |
|
$ |
43,756 |
|
$ |
— |
|
$ |
117,506 |
| (1) | The amounts in the “Option Awards” column reflect the dollar amounts of the grant date fair value for the financial statement reporting purposes for stock options for the fiscal year ended December 31, 2023 in accordance with ASC 718. The fair value of the options was determined using the Black-Scholes model. For a discussion of the assumptions used in computing this valuation, see “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” and Note 5 of the Notes to Consolidated Financial Statements in our Annual Report for the fiscal year ended December 31, 2023. |
| (2) | As of December 31, 2023, the following are the outstanding aggregate number of option awards held by each of our directors who were not also Named Executive Officers: |
|
|
Option |
|
|
Awards |
Name |
|
(#) |
Jeffrey J. Kraws |
|
278,822 |
|
|
|
John Monahan |
|
247,508 |
|
|
|
Jeffrey Wolf |
|
278,822 |
During 2023, our independent, non-executive Chairman of the Board of Directors received an annual cash retainer of $150,000, each other non-employee member of the Board of Directors received an annual cash retainer of $43,000, all non-employee directors receive an annual cash fee of $7,500, $5,000 and $3,750 for service as a member of the Audit, Compensation and Nominations Committees, respectively, or an additional annual cash fee of $15,000, $10,000 and $7,500 for service as Chairman of the Audit, Compensation and Nominations Committees, respectively. In addition, each non-employee member of the Board of Directors was issued an option exercisable for 110,000 shares of our common stock, for a term of seven years, vesting monthly over one year of the date of grant. In setting 2023 compensation for directors, the Compensation Committee relied on a report from Meridian Compensation Partners, LLC.
Compensation Committee Interlocks
During the last fiscal year ended December 31, 2023, none of our executive officers served on the Board of Directors or Compensation Committee of any other entity whose officers served either on our Board of Directors or Compensation Committee.
122
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth information, as of March 25, 2024, or as otherwise set forth below, with respect to the beneficial ownership of our common stock (i) all persons known to us to be the beneficial owners of more than 5% of the outstanding shares of our common stock; (ii) each of our directors and our named executive officers named in the Summary Compensation Table; and (iii) all of our directors and our current executive officer as a group.
|
|
Shares Owned (1) |
|
||
|
|
Number of |
|
|
|
|
|
Shares |
|
Percentages |
|
Name and Address of Beneficial Ownership (2) |
|
Owned |
|
of Shares (3) |
|
Jeffrey J. Kraws (4) |
|
215,309 |
|
1.2 |
|
Steven Shallcross (5) |
|
774,148 |
|
4.4 |
|
Jeffrey Wolf (6) |
|
214,655 |
|
1.2 |
|
John Monahan (7) |
|
183,341 |
|
1.1 |
|
All current officers and directors as a group (4 persons) |
|
1,387,453 |
|
7.6 |
% |
Frank Tufaro(8) |
|
80,645 |
|
* |
|
* |
represents less than 1% of our common stock |
| (1) | The address for each officer and directors is 9605 Medical Center, Suite 270, Rockville, Maryland 20850. |
| (2) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. Except as indicated in the footnotes to the table, to the knowledge of the Company, the persons named in the table have sole voting and investment power with respect to all shares of common stock, prefrred stock, options and/or warrants shown as beneficially owned by them, subject to community property laws, where applicable. Pursuant to the rules of the SEC, the number of shares of our common stock deemed outstanding includes shares issuable pursuant to options held by the respective person or group that are currently exercisable or may be exercised within 60 days of March 25, 2023. We currently have outstanding 275,000 shares of Series C Preferred Stock and 100,000 shares of Series D Preferred Stock convertible, at a conversion price (the “Conversion Price”) of $1.22 per share (subject in certain circumstances to adjustments and to a 4.99% limit on beneficial ownership), into an aggregate of 2,459,016 shares of the common stock. The shares of Series C Preferred Stock and Series D Preferred Stock had voting rights exclusively with respect to certain corporate actions (name change, increase in authorized shares and adjournment with respect to such proposals), which corporate actions were approved by our stockholders and therefore the Series C Preferred Stock and Series D Preferred Stock no longer have voting rights with respect to such corporate matters and only have voting rights with certain limited actions directly impacting the Series C Preferred Stock and Series D Preferred Stock. All of the shares of Series C Preferred Stock and Series D Preferred Stock are owned by MSD Credit Opportunity Master Fund, L.P. MSD Partners, L.P. (“MSD Partners”) is the investment manager of MSD Credit Opportunity Master Fund, L.P. MSD Partners (GP), LLC (“MSD GP”), a Delaware limited liability company, is the general partner of MSD Partners. Each of Gregg R. Lemkau, Marc R. Lisker and Brendan Rogers is a manager of, and may be deemed to beneficially own securities beneficially owned by, MSD GP. The business address of MSD Credit Opportunity Master Fund, L.P. is One Vanderbilt Avenue, 26th Floor, New York, New York 10017. |
| (3) | As of March 25, 2024, the Company had 17,148,049 shares of common stock outstanding. |
| (4) | Includes 214,655 shares issuable upon exercise of options held by Mr. Kraws that are exercisable within the 60-day period following March 25, 2024. Does not include an additional 64,167 shares issuable upon exercise of options held by Mr. Kraws that are not exercisable within the 60-day period following March 25, 2024. |
| (5) | Includes 488,037 shares issuable upon exercise of options held by Mr. Shallcross and 36,111 shares of Common Stock issuable upon exercise of options held by Mrs. Shallcross (Mr. Shallcross’s wife) that are exercisable within the 60-day period following March 25, 2024. Does not include an additional 866,112 shares issuable upon exercise of options held by Mr. Shallcross and 91,889 issuable upon exercise of options held by Mrs. Shallcross that are not exercisable within the 60-day period following March 25, 2024. |
123
| (6) | Includes 214,655 shares issuable upon exercise of options held by Mr. Wolf that are exercisable within the 60-day period following March 25, 2024. Does not include an additional 64,167 shares issuable upon exercise of options held by Mr. Wolf that are not exercisable within the 60-day period following March 25, 2024. |
| (7) | Includes 183,341 shares issuable upon exercise of options held by Dr. Monahan that are exercisable within the 60-day period following March 25, 2024. Does not include an additional 64,167 shares issuable upon exercise of options held by Dr. Monahan that are not exercisable within the 60-day period following March 25, 2024. |
| (8) | Includes 80,645 shares of Common Stock owned by Dr. Tufaro. Dr. Tufaro was appointed as our Chief Operating officer on March 22, 2022 and resigned from the Company effective May 10, 2023. |
Equity Compensation Plan Information
The following table sets forth information about the securities authorized for issuance under our equity compensation plans for the fiscal year ended December 31, 2023.
|
|
|
|
|
|
|
Number of |
|
|
|
|
|
|
|
Securities |
|
|
Number of |
|
|
|
Remaining |
|
|
|
Securities |
|
|
|
Available for |
|
|
|
to be Issued Upon |
|
Weighted-Average |
|
Future Issuance |
|
|
|
Exercise of |
|
Exercise Price of |
|
Under Equity |
|
|
|
Outstanding |
|
Outstanding |
|
Compensation |
|
Plan Category |
|
Options |
|
Options |
|
Plans |
|
Equity compensation plans approved by stockholders: |
|
|
|
|
|
|
|
2001 Stock Incentive Plan |
|
— |
|
$ |
— |
|
— |
2007 Stock Incentive Plan |
|
86 |
|
$ |
304 |
|
— |
2010 Stock Incentive Plan |
|
198,540 |
|
$ |
21.49 |
|
— |
2020 Stock Incentive Plan |
|
4,177,155 |
|
|
0.85 |
|
2,822,845 |
Equity compensation plans not approved by stockholders |
|
N/A |
|
|
N/A |
|
N/A |
Total |
|
4,375,781 |
|
$ |
1.80 |
|
2,822,845 |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Pursuant to our charter, our Audit Committee shall review on an on-going basis for potential conflicts of interest, and approve if appropriate, all our “Related Party Transactions” as required by Section 120 of the NYSE American Company Guide. For purposes of the Audit Committee Charter, “Related Party Transactions” shall mean those transactions required to be disclosed pursuant to SEC Regulation S-K, Item 404.
Except as disclosed under “Executive Compensation,” and below there were no related party transactions during the two years ended December 31, 2023 or the current year.
On December 15, 2022, we approved the retention of MaryAnn Shallcross, the wife of Steven Shallcross, as director of Clinical Operations, for compensation of $145,000 and the grant of an option to purchase 50,000 shares of common stock having a value of $20,000. Ms. Shallcross had been performing services for us during 2022 for total compensation of less than $120,000. On December 14, 2023, Ms. Shallcross’ salary was increased to $152,000, earned a bonus of $70,000 and was granted 75,000 option to purchase Common Stock with a value of $30,000.
124
Director Independence
The Board of Directors undertook a review of the independence of the members of the Board of Directors and considered whether any director has a material relationship with our company that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based upon information requested from and provided by each director concerning their background, employment and affiliations, including family relationships, the Board of Directors has determined that Mr. Kraws, Dr. Monahan and Mr. Wolf are “independent” as that term is defined under the rules of NYSE American. See Part III–Item 10 under the heading “Directors, Executive Officers and Corporate Governance” of this Annual Report for additional information related to director independence.
Item 14. Principal Accountant Fees and Services.
Independent Registered Public Accounting Firm Fees and Services
The following table sets forth the aggregate fees including expenses billed to us for the years ended December 31, 2023 and 2022 by BDO USA, P.C.
|
|
December 31, |
||||
|
|
2023 |
|
2022 |
||
Audit Fees |
|
$ |
472,500 |
|
$ |
598,500 |
Tax Fees |
|
|
— |
|
|
19,500 |
Total Fees (1) |
|
$ |
472,500 |
|
$ |
618,000 |
| (1) | Audit fees and expenses were for professional services rendered for the audit and reviews of the consolidated financial statements of the Company, professional services rendered for issuance of consents and assistance with review of documents filed with the SEC. |
Audit Committee Pre-Approval Policy
The Audit Committee has adopted procedures for pre-approving all audit and non-audit services provided by the independent registered public accounting firm, including the fees and terms of such services. These procedures include reviewing detailed back-up documentation for audit and permitted non-audit services. The documentation includes a description of, and a budgeted amount for, particular categories of non-audit services that are recurring in nature and therefore anticipated at the time that the budget is submitted. Audit Committee approval is required to exceed the pre-approved amount for a particular category of non-audit services and to engage the independent registered public accounting firm for any non-audit services not included in those pre-approved amounts. For both types of pre-approval, the Audit Committee considers whether such services are consistent with the rules on auditor independence promulgated by the SEC and the Public Company Accounting Oversight Board (PCAOB). The Audit Committee also considers whether the independent registered public accounting firm is best positioned to provide the most effective and efficient service, based on such reasons as the auditor’s familiarity with our business, people, culture, accounting systems, risk profile, and whether the services enhance our ability to manage or control risks and improve audit quality. The Audit Committee may form and delegate pre-approval authority to subcommittees consisting of one or more members of the Audit Committee, and such subcommittees must report any pre-approval decisions to the Audit Committee at its next scheduled meeting. All of the services provided by the independent registered public accounting firm were pre-approved by the Audit Committee.
125
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a)(1) |
The following financial statements are included in this Annual Report on Form 10-K for the fiscal years ended December 31, 2023 and 2022. |
| 1. | Independent Registered Public Accounting Firm |
| 2. | Consolidated Balance Sheets as of December 31, 2023 and 2022 |
| 3. | Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 |
| 4. | Consolidated Statements of (Deficit) Equity for the years ended December 31, 2023 and 2022 |
| 5. | Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 |
| 6. | Notes to Consolidated Financial Statements |
(a)(2) |
All financial statement schedules have been omitted as the required information is either inapplicable or included in the Consolidated Financial Statements or related notes. |
(a)(3) |
Exhibits |
126
EXHIBIT INDEX
The following exhibits are either filed as part of this report or are incorporated herein by reference:
1.1 |
|
|
|
|
|
1.2 |
|
|
|
|
|
2.1 |
|
|
|
|
|
2.2 |
|
|
|
|
|
3.1 |
|
Certificate of Incorporation, as amended (Incorporated by reference to (i) Exhibit 3.1 of the Registrant’s Current Report on Form 8-K filed October 16, 2008, File No. 001-12584, (ii) Exhibit 3.1 of the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2001 filed August 14, 2001, File No. 001-12584; and (iii) Exhibits 3.1, 4.1 and 4.2 of the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 1998 filed August 14, 1998, File No. 001-12584.) |
|
|
|
3.2 |
|
|
|
|
|
3.3 |
|
|
|
|
|
3.4 |
|
|
|
|
|
3.5 |
|
|
|
|
|
3.6 |
|
|
|
|
|
3.7 |
|
|
|
|
|
3.8 |
|
|
|
|
|
3.9 |
|
|
|
|
|
3.10 |
|
|
|
|
127
3.11 |
|
|
|
|
|
3.12 |
|
|
|
|
|
3.13 |
|
|
|
|
|
3.14 |
|
|
|
|
|
3.15 |
|
|
|
|
|
3.16 |
|
|
|
|
|
3.17 |
|
|
|
|
|
3.18 |
|
|
|
|
|
3.19 |
|
|
|
|
|
3.20 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
|
|
|
10.1* |
|
|
|
|
|
10.2* |
|
|
|
|
|
10.3* |
|
|
|
|
|
10.4 |
|
|
|
|
|
128
10.5+ |
|
|
|
|
|
10.6* |
|
|
|
|
|
10.7* |
|
|
|
|
|
10.8 |
|
Lease dated April 14, 2015 between Registrant. and MCC3, LLC (1) |
|
|
|
10.9* |
|
|
|
|
|
10.10* |
|
|
|
|
|
10.11* |
|
|
|
|
|
10.12* |
|
|
|
|
|
10.13* |
|
|
|
|
|
10.14+ |
|
|
|
|
|
10.15* |
|
|
|
|
|
10.16* |
|
|
|
|
|
10.17* |
|
|
|
|
|
10.18* |
|
|
|
|
|
10.19+ |
|
|
|
|
|
10.20* |
|
|
|
|
|
10.21+ |
|
|
|
|
|
129
10.22+ |
|
|
|
|
|
10.23+ |
|
|
|
|
|
10.24+ |
|
|
|
|
|
10.25* |
|
|
|
|
|
10.26* |
|
Employment Agreement with Mary Ann Shallcross dated April 8, 2022 (1) |
|
|
|
10.27 |
|
|
|
|
|
10.28 |
|
|
|
|
|
10.29* |
|
|
|
|
|
10.30* |
|
|
|
|
|
10.31 |
|
|
|
|
|
10.32* |
|
|
|
|
|
10.33* |
|
|
|
|
|
19.1 |
|
|
|
|
|
21.1 |
|
|
|
|
|
23.1 |
|
Consent of Independent Registered Public Accounting Firm (BDO USA, P.C.) (1) |
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
|
|
|
130
32.1 |
|
|
|
|
|
32.2 |
|
|
|
|
|
97.1 |
|
|
101.INS |
|
Inline XBRL Instance Document (1) |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document (1) |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document (1) |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document (1) |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document (1) |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document (1) |
104 |
|
Cover Page Interactive Data File (formatted in XBRL in Exhibit 101) |
(1)Filed herewith.
* |
Management contract or compensatory plan or arrangement required to be identified pursuant to Item 15(a)(3) of this report. |
+ |
The Company the submitted certain portions of these agreements in accordance with Item 601 (b)(10) of Regulation S-K. The Company agrees to furnish unredacted copies of these exhibits to the SEC upon request. |
131
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned.
THERIVA BIOLOGICS, INC. |
||
By: |
/s/ Steven A. Shallcross |
|
Steven A. Shallcross |
||
Chief Executive Officer, Chief Financial Officer and Director |
||
(Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
||
Date: March 25, 2024 |
||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Steven A. Shallcross, his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Date: March 25, 2024 |
By : |
/s/ Steven A. Shallcross |
Steven A. Shallcross |
||
Chief Executive Officer, Chief Financial Officer and Director |
||
(Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
||
Date: March 25, 2024 |
By : |
/s/ Jeffrey J. Kraws |
Jeffrey J. Kraws |
||
Chairman |
||
Date: March 25, 2024 |
By: |
/s/ John J. Monahan |
John J. Monahan |
||
Director |
||
Date: March 25, 2024 |
By: |
/s/ Jeffrey Wolf |
Jeffrey Wolf |
||
Director |
133
Exhibit 4.3
DESCRIPTION OF SECURITIES
REGISTERED PURSUANT TO SECTION 12 OF THE
SECURITIES EXCHANGE ACT OF 1934, AS AMENDED
Theriva Biologics, Inc. (the “Registrant,” “we,” “us,” and “our”) has one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which is the Registrant’s common stock, par value $0.001 per share (the “Common Stock”).
General
The following description of the Common Stock is a summary and does not purport to be complete. It is subject to and qualified in its entirety by reference to the Registrant’s Articles of Incorporation, as amended (the “Articles of Incorporation”), and Second Amended and Restated Bylaws (the “Bylaws”), each of which are incorporated by reference as an exhibit to our Annual Report on Form 10-K. We encourage you to read our Articles of Incorporation, our Bylaws and the applicable provisions of Nevada Revised Statutes (the “NRS”), for additional information.
Description of Common Stock
Authorized Shares of Common Stock
We currently have authorized 350,000,000 shares of Common Stock.
Voting Rights
The holders of the Common Stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and do not have cumulative voting rights. Accordingly, the holders of a majority of the shares of the Common Stock entitled to vote in any election of directors can elect all of the directors standing for election.
Dividend Rights
Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of Common Stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation Rights
In the event of our liquidation, dissolution or winding up, holders of the Common Stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Other Rights and Preferences
The holders of the Common Stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to the Common Stock. The rights, preferences and privileges of the holders of the Common Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Fully Paid and Nonassessable
All of our outstanding shares of Common Stock are fully paid and nonassessable.
Transfer Agent and Registrar
The transfer agent and registrar for the Common Stock is Equiniti Trust Company (f/k/a Corporate Stock Transfer, Inc.). The transfer agent’s address is 3200 Cherry Creek South Drive, Suite 430, Denver, Colorado 80209.
Listing on the NYSE American
The Common Stock is listed on the NYSE American under the symbol “TOVX.”
Stockholder Registration Rights
Pursuant to the terms of the registration rights agreement that we entered into with Intrexon and an affiliated entity, we were required to file a registration statement with respect to securities issued and are required to maintain the effectiveness of such registration statement. The failure to do so could result in the payment of damages by us. The registration statement was declared effective on April 29, 2013.
Anti-Takeover Effects of Certain Provisions of our Articles of Incorporation and Bylaws
Our Articles of Incorporation and Bylaws contain certain provisions that may have anti-takeover effects, making it more difficult for or preventing a third party from acquiring control of the Registrant or changing our board of directors and management. According to our Articles of Incorporation and Bylaws, the holders of the Common Stock do not have cumulative voting rights in the election of our directors. The lack of cumulative voting makes it more difficult for other stockholders to replace our board of directors or for a third party to obtain control of our company by replacing its board of directors.
Authorized but Unissued Shares
Our authorized but unissued shares of Common Stock will be available for future issuance without stockholder approval. We may use additional shares of Common Stock for a variety of purposes, including future public offerings to raise additional capital, to fund acquisitions and as employee compensation. The existence of authorized but unissued shares of Common Stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Anti-Takeover Effects of Nevada Law
Business Combinations
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the NRS generally prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder for a period of two years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the board of directors prior to the date the interested stockholder obtained such status or the combination is approved by the board of directors and thereafter is approved at a meeting of the stockholders by the affirmative vote of stockholders representing at least 60% of the outstanding voting power held by disinterested stockholders, and extends beyond the expiration of the two-year period, unless:
· |
the combination was approved by the board of directors prior to the person becoming an interested stockholder or the transaction by which the person first became an interested stockholder was approved by the board of directors before the person became an interested stockholder or the combination is later approved by a majority of the voting power held by disinterested stockholders; or |
· |
if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the two years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of Common Stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is generally defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer, or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, (c) 10% or more of the earning power or net income of the corporation, and (d) certain other transactions with an interested stockholder or an affiliate or associate of an interested stockholder.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within two years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Control Share Acquisitions
The “control share” provisions of Sections 78.378 to 78.3793, inclusive, of the NRS apply to “issuing corporations” that are Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents, and that conduct business directly or indirectly in Nevada. The control share statute prohibits an acquirer, under certain circumstances, from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Generally, once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures established for dissenters’ rights.
A corporation may elect to not be governed by, or “opt out” of, the control share provisions by making an election in its articles of incorporation or bylaws, provided that the opt-out election must be in place on the 10th day following the date an acquiring person has acquired a controlling interest, that is, crossing any of the three thresholds described above. We have not opted out of the control share statutes, and will be subject to these statutes if we are an “issuing corporation” as defined in such statutes.
The effect of the Nevada control share statutes is that the acquiring person, and those acting in association with the acquiring person, will obtain only such voting rights in the control shares as are conferred by a resolution of the stockholders at an annual or special meeting. The Nevada control share law, if applicable, could have the effect of discouraging takeovers of our company.
EXHIBIT 10.8
DATE OF LEASE EXECUTION: April 14, 2015
(To be completed by Landlord)
ARTICLE I - REFERENCE DATA
A.SUBJECTS REFERRED TO:
Each reference in this Lease to any of the following subjects shall be construed to incorporate the data stated for that subject in this Section 1.1:
LANDLORD: |
|
MCC3, LLC, a Delaware limited liability company |
|
|
|
MANAGING AGENT: |
|
Jones Lang LaSalle Americas, Inc. |
|
|
|
LANDLORD’S ADDRESS: |
|
c/o New Boston Fund, Inc. |
|
|
|
LANDLORD’S REPRESENTATIVE: |
|
David Langol |
|
|
|
TENANT: |
|
Synthetic Biologics, Inc., a Nevada corporation |
|
|
|
TENANT’S ADDRESS (FOR NOTICE AND BILLING): | ||
|
|
9605 Medical Center Drive, Suite 270 |
|
|
|
TENANT’S REPRESENTATIVE: |
|
Scheer Partners, Inc. |
BUILDING: The building on the parcel of land described in Exhibit A hereto. References in the Lease to “Base Building” mean the Building shell including base Building mechanical, electrical and plumbing systems (other than the horizontal distribution lines, ducts or equipment); main lobby and elevator lobbies on the first floor, but excluding all leased or leasable areas within the Building.
BUILDING ADDRESS: 9605 Medical Center Drive, Rockville, MD 20850
RENTABLE FLOOR AREA OF TENANT’S SPACE: Approximately 5,365 square feet, measured in accordance with Section 10.24 hereof.
TOTAL RENTABLE FLOOR AREA OF THE BUILDING: Approximately 1 15,691 square feet measured in accordance with Section 10.24 hereof.
TENANT’S DESIGN COMPLETION DATE: December 31, 2014
SCHEDULED TERM COMMENCEMENT DATE: May 1, 2015
TERM EXPIRATION DATE: October 31, 2020
APPROXIMATE TERM: Five (5) Years and Six (6) Months
BASE YEAR: Calendar year 2015
ANNUAL RENT: $142,172.50 (full service) for the first Lease Year (subject to an annual adjustment as provided in Section 4.1), computed as follows: $26.50 p.r.s.f. X 5,365 rentable square feet = $142,1 72.50 (full service) Annual Rent.
SECURITY DEPOSIT: $1 1,847 77
 TENANT ALLOWANCE: In accordance with Section 3.5, Tenant is allotted an allowance by the Landlord not to exceed $45.00 p.r.s.f. leased for design and construction of the Leasehold Improvements, as
TENANT ALLOWANCE: In accordance with Section 3.5, Tenant is allotted an allowance by the Landlord not to exceed $45.00 p.r.s.f. leased for design and construction of the Leasehold Improvements, as  provided in Article Ill hereof (the “Tenant Allowance”).
provided in Article Ill hereof (the “Tenant Allowance”).
TENANT IMPROVEMENT REIMBURSEMENT TO LANDLORD: As defined in Section 3.1.
TENANT’S SHARE: Tenant’s Share shall be equal to a fraction, the numerator of which shall be the Rentable Floor Area of Tenant’s Space (as the same may be increased or decreased in accordance with this Lease) and the denominator of which shall be the Total Rentable Floor Area of the Building.
PERMITTED USES: Office only and no other use.
COMMERCIAL GENERAL LIABILITY INSURANCE: |
SINGLE OCCURRENCE: $5,000,000 |
|
AGGREGATE: $10,000,000 |
LEASE YEAR: For all purposes of this Lease, the term [‘Lease Year” shall mean any period of twelve consecutive calendar months during the Term of this Lease which begins on the Commencement Date or an anniversary thereof, provided, however, that if the Commencement Date does not occur on the first day of a month, then the term “Lease Year” shall mean any period of twelve consecutive calendar months during the Term of this Lease beginning on the first day of the next calendar month following the Commencement Date, and the first Lease Year shall also include the period between the Commencement Date and the first day of such succeeding calendar month and provided further that the last Lease Year shall terminate on the Term Expiration Date or such earlier date on which the Lease may be terminated.
SPECIAL PROVISIONS: The first monthly installment of Annual Rent in the amount of $11,847.77 shall be due upon the execution of this Lease by Tenant (the “Rent Deposit”). Provided Tenant is not in default hereunder, the monthly installments of Annual Rent due for the first six (6) months of the Term shall be abated upon the Commencement Date, and the Rent Deposit paid by Tenant pursuant to the immediately preceding sentence shall be applied by Landlord to the seventh (7th) monthly installment of Annual Rent due hereunder. The Security Deposit shall also be due upon the execution of this Lease by Tenant.
B.EXHIBITS.
The exhibits listed below in this section are incorporated in this Lease by reference and are to be construed as part of this Lease:
EXHIBIT A |
Legal Description of Lot |
EXHIBIT B |
Plan Showing Tenant’s Space |
EXHIBIT C |
Conceptual Plan |
EXHIBIT D |
Landlord’s Services |
EXHIBIT E |
Rules and Regulations |
EXHIBIT F |
First Offer Space |
EXHIBIT G |
Form of Estoppel Certificate |
EXHIBIT H |
Form of Lease Commencement Date Agreement |
EXHIBIT I |
Intentionally deleted |
EXHIBIT J |
Form of Mortgagee Subordination and Nondisturbance Agreement |
EXHIBIT K |
Form of Ground Lessor Nondisturbance and Attornment Agreement |

 ARTICLE II - PREMISES AND TERM
ARTICLE II - PREMISES AND TERM
2.1PREMISES.
Subject to and with the benefit of the provisions of this Lease and the Ground Lease (as hereinafter defined) relating to the parcel of land described in Exhibit A attached hereto and made a part hereof (the “Lot”), Landlord hereby leases to Tenant, and Tenant leases from Landlord, Tenant’s Space shown in Exhibit B attached hereto and made a part hereof and commonly known as Suite 270, on the second floor of the Building on the Lot, excluding exterior faces of exterior Walls, the common facilities areas and building service fixtures and equipment serving exclusively or in common other parts of the Building. Tenant’s Space, with such exclusions, is hereinafter referred to as the “Premises”
 Tenant shall have, as appurtenant to the Premises, the right to use in common with others entitled thereto: (a) the common facilities included in the Building or on the Lot to the extent and in the location from time to time designated by Landlord and the common facilities, if any, on the Johns Hopkins University Montgomery County Campus (the “Campus”) of which the Building and Lot are a part to the extent such facilities are available for use by tenants of the Building, and (b) the building service fixtures and equipment serving the Premises.
Tenant shall have, as appurtenant to the Premises, the right to use in common with others entitled thereto: (a) the common facilities included in the Building or on the Lot to the extent and in the location from time to time designated by Landlord and the common facilities, if any, on the Johns Hopkins University Montgomery County Campus (the “Campus”) of which the Building and Lot are a part to the extent such facilities are available for use by tenants of the Building, and (b) the building service fixtures and equipment serving the Premises.
Landlord reserves the right from time to time, without notice or liability to Tenant, (a) to install, repair, replace, use, maintain and relocate for service to the Premises and to other parts of the Building or either, building service fixtures and equipment wherever located in the Building provided that after any repairs or replacements Tenant has substantially the same fixtures and equipment in the Premises and (b) to alter or relocate any common facilities provided that Tenant has access to all of the common facilities . Landlord shall use reasonable efforts to minimize any disruption to Tenant’s enjoyment of the Premises from other construction and repair activity within the Building. Tenant acknowledges that Johns Hopkins University (“JHU”) will conduct evening and weekend classes in the Building and that to accommodate such users, the Building common areas will be open from 7 a.m. to 1 1 p.m., Monday through Friday, and from 8 a.m. to 6 p.m., Saturday, Sunday and Holidays (subject to adjustment from time to time).
References to the “Ground Lease” shall mean and refer to that certain Ground Lease (Phase Ill) by and between Landlord and Johns Hopkins University, dated as of July 16, 2003.
2.2TERM.
To have and to hold for a period (the “Term”) commencing on the earlier of (a) the Scheduled Term Commencement Date or (b) the date on which the Premises are deemed ready for occupancy as provided in Section 3.2, and (c) in any event, the date on which Tenant occupies all or any part of the Premises (whichever of said dates is appropriate being hereafter referred to as the “Commencement Date”), and continuing until the Term Expiration Date, unless sooner terminated as provided in this Lease. Promptly following Landlord’s request, Landlord and Tenant agree to execute a Lease Commencement Date Agreement in the form of Exhibit H which shall establish the Commencement Date and become part of the Lease.
 It is understood and agreed that if Landlord’s Work (as defined in Article III) is not substantially completed by Landlord in accordance with Article Ill on or before the Scheduled Term Commencement Date, or if for any other reason Landlord is unable to deliver possession of the Premises to Tenant as and when herein required, this Lease shall not be void or voidable except as may otherwise herein be expressly provided, nor shall Landlord or Landlord’s agents and employees be liable to Tenant for any loss or damage resulting therefrom. In such event the Commencement Date shall be extended as provided in Article III.
It is understood and agreed that if Landlord’s Work (as defined in Article III) is not substantially completed by Landlord in accordance with Article Ill on or before the Scheduled Term Commencement Date, or if for any other reason Landlord is unable to deliver possession of the Premises to Tenant as and when herein required, this Lease shall not be void or voidable except as may otherwise herein be expressly provided, nor shall Landlord or Landlord’s agents and employees be liable to Tenant for any loss or damage resulting therefrom. In such event the Commencement Date shall be extended as provided in Article III.
2.3OPTION TO RENEW.
Tenant shall have the right to renew the Lease in its entirety for one consecutive additional term of five (5) years (a “Renewal Term”), upon written notice delivered to Landlord (the “Renewal Notice”) not less than 6 months nor more than 16 months prior to the Term Expiration Date; provided that at the time Tenant gives the Renewal Notice to Landlord and for the remainder of the initial Term, (i) Tenant is not in default hereunder, and (ii) Tenant has not assigned this Lease and is not subleasing in the aggregate more than fifty percent (50%) of the Premises (whether or not such assignment or sublease(s) were permitted in accordance with this Lease). In no event shall any sublessee or assignee be entitled to exercise the right to renew set forth in this paragraph.
exercise the right to renew set forth in this paragraph.
If this right to renew is exercised by Tenant, all terms, covenants, conditions and provisions of the Lease (including, without limitation, those related to additional rent, Operating Cost Escalations and Real Estate Tax Escalations) shall apply during the Renewal Term, and the Lease shall continue in full force and effect except that: (a) Tenant shall occupy the Premises in its then “as-is” condition and there shall be no abatement of Annual Rent nor shall there be any credit or allowance given to Tenant and Landlord shall have no obligation to make any improvements or alterations in or to the Premises or the Building; (b) the Annual Rent for the Premises shall be adjusted as hereinafter provided; and (c) Tenant shall have no further right to renew or extend this Lease. Annual Rent for the first year of the Renewal Term will be equal to the then-projected market rate of rent for space comparable to the Premises in the Building as of the expiration of the initial Term and shall thereafter escalate, in accordance with market terms as determined in accordance herewith provided, however, in no event shall Annual Rent be more than one hundred five percent (105%) of the then current Annual Rent (the “Maximum Renewal Rent”).
 If Landlord and Tenant have not agreed in writing as to the Annual Rent to be paid by Tenant during the Renewal Term as provided above within 30 days following delivery of Tenant’s Renewal Notice, then this Renewal Option shall immediately terminate and be of no further force and effect, and the Lease shall terminate on the scheduled Term Expiration Date.
If Landlord and Tenant have not agreed in writing as to the Annual Rent to be paid by Tenant during the Renewal Term as provided above within 30 days following delivery of Tenant’s Renewal Notice, then this Renewal Option shall immediately terminate and be of no further force and effect, and the Lease shall terminate on the scheduled Term Expiration Date.
2.5RIGHT OF FIRST OFFER.
Subject to the conditions subsequently set forth in this Section 2.5, Tenant shall, during the remainder of the initial Term of this Lease, have a one-time right of first offer with respect to the lease of the space identified as the “First Offer Space” shown on Exhibit F hereto and contiguous to the Premises (herein referred to as the “First Offer Space”), when such rentable space becomes Available for Leasing (as hereinafter defined), provided that (a) at the time the First Offer Space becomes Available for Leasing, (i) Tenant is not in default hereunder, (ii) Tenant has not assigned this Lease and is not subleasing in the aggregate more than fifty percent (50%) of the Premises (whether or not such assignment or sublease(s) were permitted in accordance with this Lease), (iii) at least thirty-six (36) months remain in the term of this Lease; provided, however that if the term of this Lease has been extended for the Renewal Term, then such thirty-six (36) month requirement shall not apply , and (b) in no event shall any assignee or sublessee be entitled to exercise the right of first offer set forth in this paragraph. Landlord has not offered a right of first offer to the First Offer Space to any other tenant under lease in the Building entered into prior to the Date of Lease Execution set forth at the beginning of this Lease and will not offer any such right to any future tenants under leases in the Building (unless and to the extent that the same shall be subject and subordinate to this instant Right of First Offer). As a result, Tenant’s rights hereunder in the First Offer Space shall arise only upon the expiration of early termination of the term of any lease of such space entered into prior to the Date of Lease Execution or pursuant to an option, right of refusal, offer or negotiation granted to a tenant under a lease executed prior to the Date of Lease Execution, including all permitted extensions or expansions thereof,. For purposes hereof, First Offer Space shall be “Available for Leasing” if such space is then or will within 12 months thereafter be vacant and unencumbered by any lease, option or right in favor of another tenant or tenants in the Building under leases executed prior the Date of Lease Execution.
If and when the First Offer Space or any portion thereof (herein referred to as the “Offered Space”) becomes Available for Leasing in accordance with the preceding paragraph, prior to entering into a lease of such area with another person or entity, Landlord, by written notice to Tenant (“Landlord’s Offer Notice”), shaft first offer to lease to Tenant the Offered Space provided that no other tenant has a prior claim to such space as described in the preceding paragraph and provided further that the conditions set forth herein are satisfied. Upon the receipt of Landlord’s Offer Notice, Tenant shall have
a lease of such area with another person or entity, Landlord, by written notice to Tenant (“Landlord’s Offer Notice”), shaft first offer to lease to Tenant the Offered Space provided that no other tenant has a prior claim to such space as described in the preceding paragraph and provided further that the conditions set forth herein are satisfied. Upon the receipt of Landlord’s Offer Notice, Tenant shall have  thirty (30) days after receipt of Landlord’s Offer Notice to advise Landlord in writing of Tenant’s election to lease the entire Offered Space (the “Tenant’s Election Notice”), failing which Tenant’s rights as to the Offered Space under this Section 2.5 shall terminate and shall be null and void and Landlord shall be free to lease the Offered Space in its sole discretion to another party or parties or to otherwise encumber the same in favor of other new or existing tenant(s) in the Building on such terms as Landlord may elect.
thirty (30) days after receipt of Landlord’s Offer Notice to advise Landlord in writing of Tenant’s election to lease the entire Offered Space (the “Tenant’s Election Notice”), failing which Tenant’s rights as to the Offered Space under this Section 2.5 shall terminate and shall be null and void and Landlord shall be free to lease the Offered Space in its sole discretion to another party or parties or to otherwise encumber the same in favor of other new or existing tenant(s) in the Building on such terms as Landlord may elect.
Any space taken by Tenant pursuant to this Section 2.5 shall; except as expressly provided in this Section 2.5, be taken by Tenant in its “as is” and “where is” condition and added to the Premises under the terms of this Lease, provided that Landlord shall have no obligation to incur or provide Tenant with any allowance or rent abatement provided under this Lease, and Annual Rent for the Offered Space shall be determined as hereinafter provided. The Annual Rent payable by Tenant for Offered Space leased by Tenant in accordance with this Section 2.5 shall be equal to the then-prevailing market rate of rent with market concessions for comparable space in the market area and shall escalate annually in accordance with market terms, provided however, that in no event shall rent per square foot for the Offered Space be less than the current per square foot rent payable by Tenant hereunder.. In the event Landlord and Tenant are unable to reach agreement upon an amendment to this Lease adding the Offered Space to the Premises, including the rate of rent for such space, within thirty (30) days following the date Landlord received Tenant’s Election Notice, either Landlord or Tenant may elect that the fair market rent and escalations be determined in accordance with the Three Broker Method. If neither Landlord nor Tenant  delivers notice to the other electing to use the Three Broker Method within seven (7) business days following the aforesaid thirty (30) day period, Tenant’s exercise of the Right of First Offer hereunder shall be void, and Landlord shall be free to lease the Offered Space to others on such terms as Landlord may elect.
delivers notice to the other electing to use the Three Broker Method within seven (7) business days following the aforesaid thirty (30) day period, Tenant’s exercise of the Right of First Offer hereunder shall be void, and Landlord shall be free to lease the Offered Space to others on such terms as Landlord may elect.
ARTICLE III- CONSTRUCTION
3.1INITIAL CONSTRUCTION.
Landlord shall cause to be installed in the Premises, at Landlord’s sole cost and expense,(a) all fire and life safety equipment required pursuant to the Completed Plans, (b) all demising partitions required by the Complete Plans, (c) building standard window coverings, and (d) electrical wiring, circuits and panels and plumbing as shown on the Completed Plans; and shall cause the HVAC system serving the Premises to be cleaned and connected to the Base Building HVAC System (“Landlord’s Work”), subject to Force Majeure (as defined in Section 10.12 hereof),
Tenant shall provide approval of plans provided by Landlord’s architect in accordance with Exhibit C hereto drawings and specifications for construction of leasehold improvements in the Premises (such plans and specifications once complete and approved by Landlord, are referred to herein as the “Complete Plans”) all of which shall be prepared at Landlord’s expense (but out of the Tenant Allowance) by an architect selected by Landlord and approved by Landlord (herein referred to as “Landlord’s Architect”) and Landlord’s engineer, including but not limited to:
a.Furniture and Equipment Layout Plans c.Dimensioned Electrical and Telephone Outlet Plans
b.Dimensioned Partition Plans
d.Reflected Ceiling Plans
e.Door and Hardware Schedules
f.Room Finish Schedules including wall, carpet and floor tile colors 
g.Electrical, mechanical and structural engineering plans
h.All necessary construction details and specifications.
Landlord and Tenant shall initial the Complete Plans after the same have been submitted by the Landlord’s Architect and approved by the Tenant and Landlord. Tenant shall not amend or supplement the Complete Plans, including by change order, without Landlord’s approval.
All of Tenant’s construction, installation of furnishings, and later changes or additions shall be coordinated with any work being performed by Landlord in such manner as to maintain harmonious labor relations and not to damage the Building or Lot or interfere with construction of the Building or related improvements or Building operations. Tenant shall have the right to install, at its sole cost and expense, up to two (2) electronic access points to the Premises, so long as the same are complementary and conform to the Building access entry system. Except for installation of furnishings and the installation of telephone and computer and data processing cables and wiring which are to be performed by a specialty contractor at Tenant’s direction and expense (“Tenant’s Contractor”), all work described in the Complete Plans (the “Leasehold Improvements”) shall be performed by Jones Lang LaSalle Construction LP (the “Landlord’s Contractor”).
The subcontractors for building the Leasehold Improvements shall be selected according to the following process. Landlord’s Contractor shall (i) solicit bids from at least three subcontractors for each trade, if applicable, and at the time of submission of final bid and pricing drawings pursuant to Milestone 4 set forth in Exhibit C hereto, Tenant may designate the name of at least one qualified subcontractor to be included in Landlord’s Contractor’s list; (ii) allow Tenant to review the results of bids received for the Leasehold Improvements prior to awarding subcontracts for the work, provided that any delay occasioned by Tenant’s review shall be deemed a Tenant Delay (as hereinafter defined); and (iii) select the lowest qualified bid timely received in accordance with the foregoing process, provided that in no event shall Landlord’s Contractor be required to accept any bid which Landlord’s Contractor believes to be in error, or any bid from a subcontractor to which Landlord’s Contractor may have reasonable objection. If Tenant elects that a trade not go through the three bid process described above, Landlord’s Contractor will obtain prices from a subcontractor in such trade and present the same to Tenant. The Tenant shall then have the right to approve such subcontractor and its prices or reject such subcontractor in which case the Landlord’s Contractor will choose a subcontractor pursuant to the three bid process described above, and any delay occasioned by Tenant’s rejection of any subcontractor shall be deemed a Tenant Delay (as hereinafter defined).
The Landlord’s Contractor shall perform the Leasehold Improvements for a guaranteed cost (including a 5% contractor’s fee and the landlord’s Contractor’s general conditions costs) established in accordance with procedure set forth above. Landlord shall not charge Tenant any fee for construction management. Landlord’s Contractor shall obtain all necessary building permits for constructing the Leasehold Improvements and the initial certificate of occupancy for the Premises.
Landlord will not approve any proposed construction, alterations or additions requiring unusual expense to readapt the Premises to normal office use on lease termination or increasing the cost of construction, insurance or taxes on the Building or of Landlord’s services called for by Section 5.1 unless Tenant first gives assurances acceptable to Landlord that such readaptation will be made prior to such termination without expense to Landlord and makes provisions acceptable to Landlord for payment of such increased cost. Landlord will also disapprove any alterations or additions requested by Tenant which will delay completion of the Premises or the Building. All changes and additions shall be part of the Building except such items as by writing at the time of approval the parties agree either shall be removed by Tenant on termination of this Lease or shall be removed at Tenant’s cost or left at Tenant’s election.
Tenant covenants to pay for all work performed by Tenant or Tenants Contractor, and Tenant shall apply for all permits and licenses required in connection with such work and shall pay all fees due in connection therewith. Tenant shall provide to Landlord the originals of ail such permits and licenses,  and upon substantial completion of such work shall deliver to Landlord a certificate of occupancy for the Premises, if required. Alt such improvements, whether or not paid for by Landlord, and any other improvements which are affixed to the Premises shall be and remain the property of Landlord. During the performance of any work, Tenant must provide Landlord evidence that Tenant or its contractor has in place (i) a policy insuring against “ail risks of physical loss” on a builder’s risk non-reporting form, having replacement cost and agreed amount endorsements, and (ii) commercia% general liability with underlying coverage totaling not less than Five Million Dollars ($5,000,000), each such policy to name Landlord and Landlord’s lenders as an additional insured (and as loss payee on policies other than commercial general liability insurance) and to be in a form reasonably acceptable to Landlord). Such contractor also must provide evidence that it has in place workmen’s compensation insurance in amounts and in form statutorily required. Without in any manner limiting Landlord’s rights and Tenant’s obligations under any other indemnity set forth in this Lease, Tenant shall defend, with counsel reasonably acceptable to Landlord, save harmless and indemnify Landlord from (a) claims or demands of Tenants Contractor or anyone claiming by, through or under Tenant’s Contractor, and (b) liability for injury, loss, accident, or damage to any person or property, including, without limitation, bodily injury and/or death, and from any claims, actions, proceedings and expenses and costs in connection therewith (including, without limitati01m reasonable counsel fees) arising from the acts or omissions of Tenant, its agents, employees, contractor or subcontractors, in performance of any construction, remodeling or redecoration.
and upon substantial completion of such work shall deliver to Landlord a certificate of occupancy for the Premises, if required. Alt such improvements, whether or not paid for by Landlord, and any other improvements which are affixed to the Premises shall be and remain the property of Landlord. During the performance of any work, Tenant must provide Landlord evidence that Tenant or its contractor has in place (i) a policy insuring against “ail risks of physical loss” on a builder’s risk non-reporting form, having replacement cost and agreed amount endorsements, and (ii) commercia% general liability with underlying coverage totaling not less than Five Million Dollars ($5,000,000), each such policy to name Landlord and Landlord’s lenders as an additional insured (and as loss payee on policies other than commercial general liability insurance) and to be in a form reasonably acceptable to Landlord). Such contractor also must provide evidence that it has in place workmen’s compensation insurance in amounts and in form statutorily required. Without in any manner limiting Landlord’s rights and Tenant’s obligations under any other indemnity set forth in this Lease, Tenant shall defend, with counsel reasonably acceptable to Landlord, save harmless and indemnify Landlord from (a) claims or demands of Tenants Contractor or anyone claiming by, through or under Tenant’s Contractor, and (b) liability for injury, loss, accident, or damage to any person or property, including, without limitation, bodily injury and/or death, and from any claims, actions, proceedings and expenses and costs in connection therewith (including, without limitati01m reasonable counsel fees) arising from the acts or omissions of Tenant, its agents, employees, contractor or subcontractors, in performance of any construction, remodeling or redecoration.
Landlord agrees to provide a turnkey buildout per the Landlord’s Architect’s Complete Plan, as developed based upon Exhibit C. Tenant shall pay to Landlord as additional rent the Tenant Improvement Reimbursement to Landlord (also herein referred to as “TIR”). If additional work is requested by the

 Tenant outside of or in addition to the scope of the Completed Plan, with a cost in excess of the allotted Tenant Allowance per Article l, the cost of such additional work shall be approved in writing by the Tenant before construction by the Landlord’s contractor. Tenant Improvement Reimbursement to Landlord shall be the amount equal to the excess of (a) all costs incurred by Landlord on account of the Leasehold Improvements including in the costs so incurred any fees and charges of Tenants Architect or landlord’s engineer paid by Landlord and the cost and fees charged to Landlord by Landlord’s Contractor over (b) the Tenant Allowance, if any, set forth in Section 1 .1 hereof. Tenant shall pay to Landlord one-third (1/3) of the TIR prior to commencement of construction of the Leasehold Improvements, one-third (1/3) of the TIR when construction of Leasehold improvements is 50% completed, and the remainder upon substantial completion of Leasehold Improvements; in each case, on submission by Landlord to Tenant of a statement therefor. Tenant shall pay to Landlord a sum equal to the unpaid balance of TIR, if any, within 30 days of receiving Landlord’s statement therefor. In addition to paying TIR as above provided, Tenant shall pay an amount equal to all costs incurred by Landlord as a result of any change orders signed by Tenant and Landlord affecting the Complete Plans, including the cost and fees charged to Landlord by Landlord’s Contractor with respect to such change orders. Amounts due and payable on account of such change orders shall be included in the statements relating to TIR provided for above, and Tenant shall pay therefor in accordance with each such statement within thirty (30) days, and in all events by the Commencement Date.
Tenant outside of or in addition to the scope of the Completed Plan, with a cost in excess of the allotted Tenant Allowance per Article l, the cost of such additional work shall be approved in writing by the Tenant before construction by the Landlord’s contractor. Tenant Improvement Reimbursement to Landlord shall be the amount equal to the excess of (a) all costs incurred by Landlord on account of the Leasehold Improvements including in the costs so incurred any fees and charges of Tenants Architect or landlord’s engineer paid by Landlord and the cost and fees charged to Landlord by Landlord’s Contractor over (b) the Tenant Allowance, if any, set forth in Section 1 .1 hereof. Tenant shall pay to Landlord one-third (1/3) of the TIR prior to commencement of construction of the Leasehold Improvements, one-third (1/3) of the TIR when construction of Leasehold improvements is 50% completed, and the remainder upon substantial completion of Leasehold Improvements; in each case, on submission by Landlord to Tenant of a statement therefor. Tenant shall pay to Landlord a sum equal to the unpaid balance of TIR, if any, within 30 days of receiving Landlord’s statement therefor. In addition to paying TIR as above provided, Tenant shall pay an amount equal to all costs incurred by Landlord as a result of any change orders signed by Tenant and Landlord affecting the Complete Plans, including the cost and fees charged to Landlord by Landlord’s Contractor with respect to such change orders. Amounts due and payable on account of such change orders shall be included in the statements relating to TIR provided for above, and Tenant shall pay therefor in accordance with each such statement within thirty (30) days, and in all events by the Commencement Date.
 3.2PREPARATION OF PREMISES FOR OCCUPANCY.
3.2PREPARATION OF PREMISES FOR OCCUPANCY.
Landlord agrees to use reasonable efforts to substantially complete the Leasehold Improvements by the Scheduled Term Commencement Date, which date shall, however, be extended for a period equal to that of any delays incurred by Landlord due to Force Majeure (as defined in Section 10.12) or Tenant Delay.
The Premises shall be deemed ready for occupancy on the date on which the Leasehold Improvements, if any, to be constructed by Landlord’s Contractor (collectively “Landlord’s Work”) are substantially complete as certified in writing to Tenant by Landlord’s architect with the exception of minor items which can be fully completed within sixty (60) days without material interference with Tenant and other items which because of the season or weather or the nature of the item are not practicable to do at the time, provided that none of said items is necessary to make the Premises tenantable for the Permitted Uses; provided further, however, that if Landlord is unable to complete construction of Landlord’s Work due to long lead items (as hereinafter defined) included in the Complete Plans or any act or omission of Tenant or its agents, employees, contractors, invitees or licensees or due to delay in Tenant’s compliance with the provisions of Section 3.1 of this Lease (herein sometimes referred to as “Tenant Delays”), then the Premises shall be deemed ready for occupancy no later than the date the Landlord’s Work and Leasehold Improvements would have been substantially completed but for such Tenant Delays. For purposes hereof, “long lead item” shall mean any item(s) of work included in the Complete Plans, or in any change order requested by Tenant, that cannot be completed in accordance with the Landlord’s construction schedule (without overtime or acceleration of the work), provided that Landlord’s Contractor advises Tenant that the item is a long lead item before commencement of construction but not later than the Scheduled Term Commencement Date.
Landlord shall permit Tenant access for installing tele/data equipment and systems furnishings in the Premises prior to the Term if it can be done without material interference with completion of the Building or remaining portions of the Leasehold Improvements.
In the event of Tenant’s failure to comply with the provisions of Section 3. 1 of this Lease or to submit information or to deliver final bidding and pricing documents or the Complete Plans approved by Landlord as herein required and if Tenant does not cure such failure within thirty (30) days of written notice thereof from Landlord, Landlord may, at Landlord’s option, exercisable by notice to Tenant, terminate this Lease on the date specified in said notice to Tenant, and upon such termination Landlord shall have all the rights provided in Article IX of this Lease in the event of Tenant’s default.
3.3GENERAL PROVISIONS APPLICABLE TO CONSTRUCTION.
All construction work required or permitted by this Lease, shall be done in a good and workmanlike manner and in compliance with all applicable laws and all lawful ordinances, regulations, orders, permits and approvals of governmental authority and insurers of the Building. Either party may inspect the work of the other at reasonable times and promptly shall give notice of observed defects, and prior to Tenant taking occupancy of the Premises, Tenant and Landlord shall agree on a punch list of incomplete Leasehold Improvements; provided however, nothing contained herein shall be deemed or construed to preclude Tenant from reporting latent defects in Leasehold Improvements subsequent to taking occupancy.. Landlord’s obligations under Section 3. 1 shall be deemed to have been performed when Tenant commences to occupy any portion of the Premises for the Permitted Uses except for items relating to the Leasehold Improvements which are incomplete or do not conform with the requirements of Section 3.1 and as to which Tenant shall in either case have given written notice to Landlord prior to such commencement. If Tenant shall not have commenced to occupy the Premises for the Permitted Uses within 30 days after they are deemed ready for occupancy as provided in Section 3.2, a certificate of completion by a licensed architect or registered engineer shall be conclusive evidence that Landlord has performed all such obligations except for items stated in such certificate to be incomplete or not in conformity with such requirements.
3.4REPRESENTATIVES.
Each party authorizes the other to rely in connection with their respective rights and obligations under this Article Ill upon approval and other actions on the party’s behalf by Landlord’s Representative in the case of Landlord or Tenant’s Representative in the case of Tenant or by any person designated in substitution or addition by notice to the party relying.
3.5TENANT ALLOWANCE.
Landlord shall provide the allotted Tenant Allowance in the amount specified in Section 1 .1 to be applied toward Landlord’s construction of the Leasehold Improvements, which represent a turnkey build out of the Premises using Building standard materials and finishes based on the mutually agreed upon space plan attached hereto as Exhibit B. The Tenant Allowance shall be applied by Landlord to pay all costs incurred by Landlord in connection with such work, including architectural and engineering fees, permitting fees, general contractor costs, construction management and any other costs associated with the construction of the Premises.
3.6LEASE COSTS.
If Tenant (i) defaults under the Lease prior to Commencement Date and fails to cure such default after any applicable notice and cure periods, Date, or (ii) fails to pay any Annual Rent or Additional Rent due upon Commencement Date, if applicable, or upon the date that the first monthly installment of Annual Rent and Additional Rent are not abated pursuant to Article I hereto, then in any such event, in addition to and not in lieu of any other rights and remedies Landlord may have pursuant to this Lease or at law or in equity, Tenant shall forthwith repay to Landlord upon demand, the Tenant Allowance together with interest thereon at the Default Rate from the date of delivery of the Premises by Landlord in accordance with this Lease until such amount is paid by Tenant (the “Pre-Term Costs”).
default after any applicable notice and cure periods, Date, or (ii) fails to pay any Annual Rent or Additional Rent due upon Commencement Date, if applicable, or upon the date that the first monthly installment of Annual Rent and Additional Rent are not abated pursuant to Article I hereto, then in any such event, in addition to and not in lieu of any other rights and remedies Landlord may have pursuant to this Lease or at law or in equity, Tenant shall forthwith repay to Landlord upon demand, the Tenant Allowance together with interest thereon at the Default Rate from the date of delivery of the Premises by Landlord in accordance with this Lease until such amount is paid by Tenant (the “Pre-Term Costs”).
ARTICLE IV - RENT
4.1RENT.
Commencing on the Commencement Date and continuing for the remainder of the Term, Tenant agrees to pay rent to Landlord, without any offset or reduction whatever, in an amount equal to 1/ 12th of the Annual Rent in equal installments in advance on the first day of each calendar month included in the Term; and for any portion of a calendar month at the beginning or end of the Term, at the proportionate rate payable for such portion, in advance, provided that the first installment of Annual Rent shall be due and payable pursuant to the Special Provisions set forth in Article I hereof Notwithstanding the foregoing, provided Tenant is not in default hereunder, three installments of Annual Rent shall be abated after the Commencement in accordance with the Special Provisions of Section 1 .1 hereof.
As used herein, “Annual Rent” shall mean the sum set forth in Section 1.1. On the first day of the Second Lease Year and on the first day of each subsequent Lease Year or portion thereof during the initial Term, the amount of Annual Rent shall be increased as follows:
Second Lease Year |
|
|
|
|
|
|
|
|
|
|
|
|
|
$27.30 |
x |
5,365 |
|
$146,464.50 |
|
|
Annual Rent |
|
Rentable Square |
= |
Annual Rent |
|
|
p.r.s.f. |
|
Feet |
|
|
|
|
|
|
|
|
|
|
|
Third Lease Year: |
|
|
|
|
|
|
|
|
|
|
|
|
|
$28.12 |
|
5,365 |
|
$150,858.44 |
|
|
Annual Rent |
|
Rentable Square |
= |
Annual Rent |
|
|
p.r.s.f. |
|
Feet |
|
|
|
|
|
|
|
|
|
|
|
Fourth Lease Year: |
|
|
|
|
|
|
|
|
|
|
|
|
|
$28.96 |
x |
5,365 |
|
$155,384.19 |
|
|
Annual Rent |
|
Rentable Square |
= |
Annual Rent |
|
|
p.r.s.f. |
|
Feet |
|
|
|
|
|
|
|
|
|
|
|
Fifth Lease Year: |
|
|
|
|
|
|
|
|
|
|
|
|
|
$29.83 |
|
5,365 |
|
$160,045.71 |
|
|
Annual Rent |
|
Rentable Square |
= |
Annual Rent |
|
|
p.r.s.f. |
|
Feet |
|
|
|
|
|
x |
|
|
|
|
|
Sixth Lease Year: |
|
|
|
|
|
|
|
|
|
|
|
|
|
$30.73 |
|
5,365 |
|
$164,847.09 |
|
|
Annual Rent |
|
Rentable Square |
= |
Annual Rent |
|
|
p.r.s.f. |
|
Feet |
|
|
|
|
The term “additional rent” shall mean Tenant’s Share of Operating Cost Escalations (as defined below), Tenant’s Share of Real Estate Tax Escalations (as defined below), and all other costs, charges and impositions, in addition to Annual Rent, payable by Tenant in accordance with the terms of this Lease.
4.2OPERATING COSTS.
Commencing on the Commencement Date and continuing for the remainder of the Term, Tenant shall pay to Landlord, as additional rent, Tenant’s Share of (as defined below) on or before the thirtieth day following receipt by Tenant of Landlord’s Operating Cost Statement (as defined below). Within one hundred eighty (180) days after the end of each fiscal year ending during the Term and within one hundred twenty (120) days after Lease termination, Landlord shall render a statement (“Landlord’s Operating Cost Statement”) in reasonable detail and according to usual accounting practices, certified by Landlord, and showing for the preceding fiscal year or fraction thereof, as the case may be, Landlord’s operating costs (“Operating Costs”) which shall:
(a) |
exclude, notwithstanding any other provisions in this Section 4.2 to the contrary, the following: |
(i)Ground rent or other rental payments made under any ground lease or underlying lease or payments of principal, interest, late charges, penalties or other charges made on account of any loan;
(ii)Costs of improvements or replacements to the Building which under generally accepted accounting principles are capitalized except as expressly included in Operating Costs under part (b) of this (iii)Costs of leasing commissions, legal, space planning, construction and other expenses incurred in procuring tenants for the Building;
Section 4.2 (“Capital Improvements”);
(iv)Costs of painting, redecorating or other work performed solely for the benefit of another tenant, prospective tenant or occupant;
(v)Salaries, wages, or other compensation paid to officers or executives of Landlord or its property manager at the level of senior vice president and above;
(vi)Salaries, wages, or other compensation or benefits paid to employees of Landlord or its property manager who are not assigned to the operation, management, maintenance, or repair of the Building or Campus; and in the case of any offsite or other employees who are not assigned full time to the operation, management, maintenance or repair of the Building or Campus, Landlord shall reasonably allocate the compensation paid for the wages, salary, or other compensation or benefits paid to such employees among the properties to which such employees are assigned and Operating Costs shall exclude the portion of such compensation not reasonably allocated to the Building or Campus;
(vii)Any fines or penalties incurred due to the violation by Landlord of any governmental rule or authority;
(viii)Any costs for which Landlord actually receives reimbursement from insurance, condemnation awards, or any other source, including other tenants of the Building if charged to such tenants specially and not as an Operating Cost, Real Estate Tax or Electrical Cost;
(ix)Costs in excess of the deductible on applicable insurance policies for repairs, restoration, replacements or other work occasioned by (a) fire, windstorm or other casualty which Landlord is hereunder required to insure against (whether such destruction be total or partial) and (b) the exercise by governmental authorities of the right of eminent domain;
(x)Attorneys’ fees and expenses and costs of litigation in connection with disputes with tenants, other occupants or prospective tenants, or with consultants, management agents, leasing agents, purchasers or mortgagees of the Building; and costs incurred by Landlord due to Landlord’s violation of the terms of this Lease which would not have been incurred by Landlord but for such violation;
(xi)Costs incurred in connection with the original construction of the Building or other improvements constructed with the Building;
(xii)Costs relating to another tenant’s or occupant’s space which (A) were incurred in rendering any service or benefit to such tenant that Landlord was not required to provide, or were for a service in excess of the service that the Landlord was required to provide to Tenant hereunder or (B) were otherwise in excess of the Building standard services then being provided by Landlord to all tenants or other occupants in the Building, whether or not such other tenant or occupant is actually charged therefor by Landlord;
(xiv)Costs incurred in connection with the acquisition of the Lot or sale, financing, refinancing, mortgaging, selling or change of ownership of the Building and/or the Lot, including, but not limited to, attorneys’ fees, title insurance premiums, and recording costs; v Fines, interest, penalties, legal fees or costs of litigation incurred due to the late payments of loan payments, taxes and utility bills;
(xv)Landlord’s or its property manager’s general home office overhead expenses;
(xvi)Costs incurred for any items to the extent covered by a manufacturer’s, materialman’s, vendor’s or contractor’s warranty;
(xvii)Non-cash items, such as deductions for depreciation and amortization of the Building and the Building equipment (except as expressly included in Operating Costs under part (b) of this Section 4.2); interest on capital invested; bad debt losses; rent losses and reserves for such losses;
(xviii) Costs incurred in connection with the operation of any lobby shop or cafeteria owned, operated or subsidized by Landlord (except the non-capital costs of any cafeteria expressly included in Operating Costs under part (b) of this Section 4.2);
(xix)Costs incurred by Landlord which are associated with the operation of the business of the legal entity which constitutes Landlord as the same is separate and apart from the costs of the operation of the Building, including legal entity formation and maintenance charges;
(xx)All amounts which would otherwise be included in Operating Costs which are paid to any affiliate or subsidiary of Landlord to the extent the cost of such goods or services exceed the market rate for similar services under arm’s length contracts in the Washington, D.C. metropolitan area;
(xxi)Rentals and other related expenses incurred in leasing elevators or other equipment ordinarily considered to be of a capital nature;
(b)but include, without limitation: costs of maintenance and repair of any cafeteria or fitness room generally available to all tenants of the Building; expenses of any proceedings for abatement of Real Estate Taxes and assessments with respect to any fiscal year or fraction of a fiscal year; premiums for insurance; fees payable to third parties for audits of Operating Costs, provided that such costs are not incurred due to Landlord’s overcharging tenants for Operating Costs; reasonable legal fees and costs payable in seeking a reduction of Real Estate Taxes and in connection with Building service contracts; compensation and all fringe benefits, worker’s compensation insurance premiums and payroll taxes paid by Landlord to, for or with respect to all persons engaged in the operating, maintaining or cleaning of the Building and Lot so long as such payments are in amounts that are usual and customary for such services for comparable Buildings and properties; all electricity, water and other utility charges not billed directly to tenants by Landlord or the utility; payments to Landlord’s property manager and other independent contractors (including, without limitation, affiliates of Landlord, if applicable) under service contracts for cleaning, operating, managing, maintaining, repairing or testing the Building and Lot so long as such payments are in amounts that are usual and customary for such services for comparable Buildings and properties; ; rent paid by the managing agent or imputed cost equal to the loss of market rent by Landlord for making available to the managing agent space for an office in the Building (not exceeding 1,000 rentable square feet) on the ground floor or above; and all other costs and expenses incurred in connection with owning, administering, cleaning, operating, managing, maintaining and repairing the Building and Lot, or either, and the Building’s pro-rata share of all such costs and expenses applicable to owning, administering, cleaning, operating, managing, maintaining and repairing common areas on the Campus, including, without limitation, common area rent and other charges applicable to the Shady Grove Life Sciences Center, If Landlord installs or constructs a new or replacement capital item for the purpose of reducing Operating Costs or complying with requirements of law not in effect upon the execution date of this Lease, the cost thereof, amortized in accordance with generally accepted accounting principles, shall be included in Landlord’s Operating Costs.
“Tenant’s Share of Operating Cost Escalations” shall be equal to the product of (a) Operating Costs as indicated in Landlord’s Operating Cost Statement, less Operating Costs for the Base Year multiplied by (b) “Tenant’s Share”
Landlord’s Operating Cost Statement shall also show the average number of square feet of the Building which were occupied for the preceding fiscal year or fraction thereof. If less than ninety-five percent (95%) of the Net Rentable Area of the Building is occupied during any full or fractional year of the Term (including the Base Year), the actual Operating Costs shall be adjusted for such year to an amount which Landlord estimates would have been incurred in Landlord’s reasonable judgment had ninety-five (95%) of the Net Rentable Area of the Building been occupied, provided, however, that Operating Costs as so adjusted shall not exceed the actual costs Landlord would have incurred had the Building been ninety-five percent (95%) occupied.
In case of special services which are provided to Tenant but are not rendered to all areas on a comparable basis, the proportion allocable to the Premises shall be the same proportion which the Rentable Floor Area of Tenant’s Space (as the same may be increased or decreased in accordance with this Lease) bears to the total rentable floor area to which such service is so rendered (such latter area to be determined in the same manner as the Total Rentable Floor Area of the Building).
Notwithstanding any other provision of this Section 4.2, if the Term expires or is terminated as of a date other than the last day of a fiscal year, then for such fraction of a fiscal year at the end of the Term, Tenant’s last payment to Landlord under this Section 4.2 shall be prorated and made on the basis of Landlord’s reasonable best estimate of the items otherwise includable in Landlord’s Operating Cost Statement which shall be delivered to Tenant within one-hundred twenty (120) days following expiration or termination of the Term, and shall be made on or before the date 10 days after Landlord delivers such estimate to Tenant.
Tenant shall pay, as additional rent, on the first day of each month of such fiscal year and each ensuing fiscal year thereafter, “Estimated Monthly Payments” equal to 1/12th of Tenant’s Share of the estimated Operating Cost Escalations for the respective fiscal year, with an appropriate additional payment or credit to be made after Landlord’s Operating Cost Statement is delivered to Tenant. If the amount paid by Tenant for Tenant’s Share of estimated Operating Cost Escalations is less than Tenant’s Share of the actual Operating Cost Escalation, Tenant agrees to pay, as additional rent, to Landlord the amount of the differential. If the amount paid by Tenant for Tenant’s Share of estimated Operating Costs is more than Tenant’s Share of the actual Operating Cost Escalations, then Landlord shall credit such excess against Tenant’s subsequent monthly payments for Tenant’s Share of Operating Cost Escalations, as appropriate, until such excess is exhausted. Landlord may adjust such Estimated Monthly Payment from time to time and at any time during a fiscal year (but not more often than twice per fiscal year), and Tenant shall pay, as additional rent, on the first day of each month following receipt of Landlord’s notice thereof, the adjusted Estimated Monthly Payment. Each of Landlord’s Operating Cost Statements given by Landlord pursuant to the Lease shall be conclusive and binding upon Tenant unless, within ninety (90) days after the receipt of the statement, Tenant notifies Landlord that it wishes to audit Landlord’s Operating Costs for the preceding year. If Tenant gives such notice timely requesting the right  to review or audit Landlord’s books and records pertaining to Operating Costs for the preceding year, Landlord shall make available to Tenant for inspection or auditing during normal business hours not more than six (6) months following delivery to Tenant of the Operating Cost Statement to which such review related, at the offices of Landlord’s managing agent where such records are kept, the books and records within Landlord’s managing agent’s possession with respect to Landlord’s Operating Costs for the fiscal year in question. Pending the resolution of any such dispute as to Landlord’s Operating Cost Statement, Tenant shall pay the adjustments, including any underpayment of Tenant’s Share of Operating Cost Escalations, as specified in accordance with Landlord’s Operating Cost Statement, without prejudice to Tenant’s position, as herein provided. If the dispute shall be resolved in Tenant’s favor, Landlord shall credit or pay to Tenant (as hereinabove provided) the amount of Tenant’s overpayment.
to review or audit Landlord’s books and records pertaining to Operating Costs for the preceding year, Landlord shall make available to Tenant for inspection or auditing during normal business hours not more than six (6) months following delivery to Tenant of the Operating Cost Statement to which such review related, at the offices of Landlord’s managing agent where such records are kept, the books and records within Landlord’s managing agent’s possession with respect to Landlord’s Operating Costs for the fiscal year in question. Pending the resolution of any such dispute as to Landlord’s Operating Cost Statement, Tenant shall pay the adjustments, including any underpayment of Tenant’s Share of Operating Cost Escalations, as specified in accordance with Landlord’s Operating Cost Statement, without prejudice to Tenant’s position, as herein provided. If the dispute shall be resolved in Tenant’s favor, Landlord shall credit or pay to Tenant (as hereinabove provided) the amount of Tenant’s overpayment.
If Landlord fails to furnish Tenant any statement of Landlord’s estimate of Tenant’s Share of Operating Cost Escalations for any fiscal year or if Landlord shall furnish such estimate for any fiscal year subsequent to the commencement thereof, then until the first day of the month following the month in which such estimate is furnished to Tenant, Tenant shall pay to Landlord on the first day of each month an amount equal to the monthly sum payable by Tenant to Landlord under this Section 4.2 in respect of the last month of the preceding fiscal year.
4.3REAL ESTATE TAXES.
Commencing on the Commencement Date and continuing for the remainder of the Term, Tenant shall pay to Landlord, as additional rent, Tenant’s Share of Real Estate Tax Escalations (as defined below), if any, on or before the thirtieth day following receipt by Tenant of Landlord’s Real Estate Tax Statement (as defined below). Within one-hundred eighty (180) days after the end of each Fiscal Year ending during the Term and after Lease termination, Landlord shall render a statement (“Landlord’s Real Estate Tax Statement”) certified by Landlord, and showing for the preceding fiscal year or fraction thereof, as the case may be, Real Estate Taxes for the Building and Lot, accompanied by copies of the tax bills relating thereto.
“Tenant’s Share of Real Estate Tax Escalations” shall be equal to the product of (a) the Real Estate Taxes as indicated in Landlord’s Real Estate Tax Statement less Real Estate Taxes for the Base Year multiplied by (b) Tenant’s Share.
The term “Real Estate Taxes” as used above shall mean all taxes of every kind and nature assessed by any governmental authority on the Lot, the Building and improvements, or both, or on any easement benefiting the same, which the Landlord shall become obligated to pay because of or in connection with the ownership, leasing and operation of the Lot, the Building and improvements, or both; installments and interest on assessments for public betterments or public improvements; special assessments, fees, charges, levies, penalties, service payments, excises, assessments, charges, and costs for transit, transit encouragement, traffic reduction programs, or any .other purpose; impositions or taxes of every kind or nature whatsoever assessed or levied or imposed by any governmental entity, governmental authority or any improvement or assessment district of any kind having the direct or indirect power to tax, whether or not consented to or joined in by Landlord, against the Building or Lot or any legal or equitable interest of Landlord therein, whether now or hereafter imposed, and whether or not customary in the contemplation of the parties on the date of this Lease provided that such Real Estate Taxes were not the result of the negligence of Landlord; subject to the following: there shall be excluded from Real Estate Taxes ail federal state or local taxes based upon the net income of Landlord, , excise taxes, franchise taxes, and estate, succession, provided, however, that if at any time during the Term the present system of ad valorem taxation of real property shall be changed so that in lieu of the whole or any part of the ad valorem tax on real property, there shall be assessed on Landlord a capital levy or other tax on the rents received with respect to the Lot, Building and improvements, or both, or a federal, state, county, municipal, or other local income, franchise, excise, sales, profit or similar tax, assessment, levy or charge (distinct from any now in effect) measured by or based, in whole or in part upon any such rents, then any and all of such taxes, assessments, levies or charges, to the extent so measured or based, shall be deemed to be included within the term “Real Estate Taxes.” Tenant acknowledges that in the event that Real Estate Taxes are reduced because nonprofit entities occupy the Building, the share of Real Estate Taxes paid by such tenants shall be structured so that the tax exempt tenant(s) for which such reduction is given receives(s) the full benefit of the property tax exemption.
Notwithstanding any other provision of this Section 4.3, if the Term expires or is terminated as of a date other than the last day of a fiscal year, then for such fraction of a fiscal year at the end of the Term, Tenant’s last payment to Landlord under this Section 4.3 shall be prorated and made on the basis of Landlord’s reasonable best estimate of the items otherwise includable in Landlord’s Real Estate Tax Statement which shall be delivered to Tenant within one-hundred twenty (120) days after the Term expires or is terminated and shall be made on or before 10 days after Landlord delivers such estimate to Tenant. Within thirty (30) days following the date Real Estate Taxes are finally determined for the period for which Tenant made estimated payments, Tenant or Landlord, as the case may be, shall pay to the other the balance of any underpayment or overpayment, respectively, of Tenant’s Share of Real Estate Tax Escalations. This paragraph shall survive the expiration or earlier termination of this Lease.
Tenant shall pay, as additional rent, on the first day of each month of such fiscal year and each ensuing fiscal year thereafter, Estimated Monthly Real Estate Taxes equal to 1/ 12th of Tenant’s Share of the estimated Real Estate Tax Escalations for the respective fiscal year, with an appropriate additional payment or credit to be made after Landlord’s Real Estate Tax Statement is delivered to Tenant. If the amount paid by Tenant for Tenant’s Share of estimated Real Estate Tax Escalations is less than Tenant’s Share of the actual Real Estate Tax Escalations, Tenant agrees to pay, as additional rent, to Landlord the amount of the differential. If the amount paid by Tenant for Tenant’s Share of estimated Real Estate Tax Escalations is more than Tenant’s Share of the actual Real Estate Tax Escalations, then Landlord shall credit such excess against Tenant’s subsequent monthly payments for Tenant’s Share of Real Estate Tax Escalations, as appropriate, until such excess is exhausted. Landlord may adjust such Estimated Monthly Real Estate Tax Payment from time to time and at any time during a fiscal year (but not more often than twice per fiscal year), and Tenant shall pay, as additional rent, on the first day of each month following receipt of Landlord’s notice thereof, the adjusted Estimated Monthly Real Estate Tax Payment. Each of Landlord’s Real Estate Tax Statements given by Landlord pursuant to the Lease shall be conclusive and binding upon Tenant unless, within ninety (90) days after the receipt of such statement, tenant notifies Landlord that it wishes to audit Landlord’s books and records pertaining to Landlord’s Real Estate Taxes for the preceding year. If Tenant gives such notice timely requesting the right to review or audit Landlord’s books and records pertaining to Landlord’s Real Estate Taxes, Landlord shall make available to Tenant for inspection or auditing during normal business hours, at the offices of Landlord’s managing agent where such records are kept, the books and records within Landlord’s managing agent’s possession with respect to Landlord’s Real Estate Taxes for the fiscal year in question.
Pending the resolution of any such dispute as to Landlord’s Real Estate Tax Statement, Tenant shell pay the adjustments, including any underpayment of Tenant’s Share of Real Estate Tax Escalations, as specified in accordance with Landlord’s Real Estate Tax Statement, without prejudice to Tenant’s position, as herein provided. If the dispute shall be resolved in Tenant’s favor, Landlord shall credit or pay to Tenant (as hereinabove provided) the amount of Tenant’s overpayment.
In the event that the method currently used for the computation of the assessed market value of the Building and/or the Lot is discontinued or revised by the State of Maryland, the determination of the increase in Real Estate Taxes under this Section 4.3 shall thereafter be determined by Landlord according to a formula and procedure which, in Landlord’s reasonable judgment, most nearly approximates the method of determination hereinabove set forth, In the event that any business, rent or other taxes which are now or hereafter levied upon Tenant’s use or occupancy of the Premises, on Tenant’s leasehold improvements, on Tenant’s business at the Premises or on Landlord by virtue of Tenant’s occupancy of the Premises, are enacted, changed or altered so that any of such taxes are levied against Landlord or in the event that the mode of collection of such taxes is changed so that Landlord is responsible for collection or payment of such taxes, any and all such taxes shall be deemed to be a part of the Real Estate Taxes and Tenant shall pay to Landlord the full amount of all of such taxes or if they relate to all tenants in the Building, then Tenant’s Share thereof.
If Landlord fails to furnish Tenant any statement of Landlord’s estimate of Tenant’s Share of Real Estate Tax Escalations for any fiscal year or if Landlord shall furnish such estimate for any fiscal year subsequent to the commencement hereof, then until the first day of the month following the month in which such estimate is furnished to Tenant, Tenant shall pay to Landlord on the first day of each month an amount equal to the monthly sum payable by Tenant to Landlord under this Section 4.3 in respect of the last month of the preceding fiscal year.
4.4REFUNDS.
Tenant shall be entitled to its pro rata share of any refund of Real Estate Taxes received by Landlord, net of reasonable costs incurred by Landlord in obtaining such refund not to exceed Real Estate Taxes paid by Tenant with respect to the tax year to which such refund relates (it being understood that the Real Estate Taxes paid by Tenant for any tax year may fall into two separate fiscal years so long as the tax year is not the same as the fiscal year).
4.5INTENTIONALLY OMITTED.
4.6CHANGE OF FISCAL YEAR.
Landlord shall have the right from time to time to change the periods of accounting under Sections 4.2 and 4.3 to any annual period other than a calendar year, and upon any such change all items referred to in Section 4.2 and 4.3 shall be appropriately apportioned. In all Landlord’s Statements (including Operating Cost Statements or Real Estate Tax Statements) rendered under Section 4.2 or 4.3 amounts for periods partially within and partially without the accounting periods shall be appropriately apportioned, and any items which are not determinable at the time of a Landlord’s Statement shall be included therein on the basis of Landlord’s estimate, and with respect thereto Landlord shall render promptly after determination a supplemental Landlord’s Statement, and appropriate adjustment shall be made according thereto. All Landlord’s Statements shall be prepared on an accrual basis of accounting.
4.7PAYMENTS.
All payments of Annual Rent and additional rent shall be made to Managing Agent, or to such other person or place as Landlord may from time to time designate. If any installment of Annual Rent or additional rent or on account of TIR is not paid within five (5) days following the due date thereof, at Landlord’s election, (a) it shall bear interest at a rate equal to the average prime commercial rate from time to time established by the three largest national banks in Washington, D.C. plus two percent (2%) per annum (the “Default Rate”) from such due date, which interest shall be immediately due and payable as further additional rent, and (b) Tenant shall also pay, as additional rent, a late fee equal to three percent (3%) of the late installment.
ARTICLE V · LANDLORD’S COVENANTS
5.1LANDLORD’S COVENANTS DURING THE TERM.
Landlord covenants during the Term:
5.1.1Building Services. - Landlord shall furnish, through Landlord’s employees or independent contractors, the services listed in Exhibit D and throughout this Lease.
5.1.2Additional Building Services. - Landlord shall furnish, through Landlord’s employees or independent contractors, reasonable additional Building operation services upon reasonable advance request of Tenant at equitable rates from time to time established by Landlord to be paid by Tenant. As of the Commencement Date, Landlord’s charges for supplemental heat and air conditioning at other than normal business hours is $25.00 per hour per zone with two (2) zones per floor. Landlord reserves the right to increase this charge from time to time throughout the term, upon prior written notice to Tenant.
5.1.3 Repairs. -- Except as otherwise provided in Article VII, Landlord shaft make, at its sole expense, but subject to recovery if and to the extent permitted by Section 4.2, such repairs to the Building’s roof, exterior walls, floor slabs, other structural components and common facilities of the Building as may be necessary to keep them in serviceable condition.
5.1.4Quiet Enjoyment.Landlord covenants that Landlord has the right to make this Lease and that Tenant on paying the rent and performing its obligations hereunder shall peacefully and quietly have, hold and enjoy the Premises throughout the Term without any manner of hindrance or molestation from Landlord or anyone claiming under Landlord, subject however to all the terms and provisions hereof.
5.1.5Access/Security. — Tenant shall have access to the Premises 24 hours per day, 7 days of the week; subject, however, to a key card access system in the Building at the main entry to the Building and at the secondary Building door (the Tenant shall receive twenty (20) key cards at no cost, provided that the Building main entry door and secondary entry door may remain unlocked 7 a.m. to 1 1 p.m. Monday through Friday and 8 a.m. to 6 p.m. on Saturday); provided further, however, that no representation or warranty is made by Landlord as to the adequacy, completeness or integrity of said access control system and failure of such access control system shall not modify or affect the limitations on Landlord’s liability under this Lease,
5.1.6Intentionally Omitted.
5.1.7Intentionally Omitted.
5.1.8Tenant’s Costs. - Landlord shall pay all costs including, without limitation, reasonable attorneys’ fees incurred by Tenant in connection with the successful enforcement by Tenant of any obligations of Landlord or remedies of Tenant under this Lease.
5.1.9Lobby Directory. — Landlord shall install, at Landlord’s expense, Tenant’s name and suite number on the Building directory in the Building lobby. Building standard suite signage shall be provided at Landlord’s expense, but any changes to the signage may only be made in accordance with the terms of this Lease and such changes shall be at the expense of the Tenant.
5.1.10Compliance. Landlord shall be responsible for the Building’s overall compliance with the Americans with Disabilities Act as it relates to the common areas of the Building (including, without limitation, core area bathrooms) except to the extent that any improvement or renovation is required due to Tenant’s special use of the Premises (other than general office use) . As used in this Section, the Americans with Disabilities Act shall mean the Americans with Disabilities Act of 1991, 42 U.S.C.
S 12.1 01 et seq. and all regulations applicable thereto promulgated as of the date hereof (collectively, “ADA”). Following the Commencement Date, Tenant shall have the responsibility to comply with the requirements of the ADA in the P remises.
5.1.11Insurance. — Landlord shall procure and maintain throughout the Term of this Lease a policy or policies of insurance, at its sole cost and expense (but subject to Section 4.2), causing the Building and any other improvements on the Lot to be insured in the amounts and coverages required under the Ground Lease, a policy of commercial general liability insurance satisfying the terms of the Ground Lease, and such other insurance or higher limits as may be required under the Ground Lease or by the holder of any mortgage on the Building. Any insurance provided for in this Section 5.1 .1 1 may be maintained by means of a policy or policies of blanket insurance, covering additional items or locations or insureds, provided, however, that the coverage afforded Landlord and any such other parties in interest will not be reduced or diminished by reason of the use of such blanket policy of insurance.
5.2INTERRUPTIONS.
Landlord shall not be liable to Tenant for any compensation or reduction of rent by reason of inconvenience or annoyance or for loss of business arising from power losses or shortages or from the necessity of Landlord’s entering the Premises for any of the purposes in this Lease authorized, or for repairing the Premises or any portion of the Building or Lot, or for interruption or termination (by reason of any cause reasonably beyond Landlord’s control, including without limitation, loss of any applicable license or governmental approval), of the services provided by Landlord pursuant to Section 5.1, n case Landlord is prevented or delayed from making any repairs, alterations or improvements, or furnishing any service or performing any other covenant or duty to be performed on Landlord’s part, by reason of any cause reasonably beyond Landlord’s control, Landlord shall not be liable to Tenant therefor, nor, except as expressly otherwise provided in Article VII, shall Tenant be entitled to any abatement or reduction of rent by reason thereof, nor shall the same give rise to a claim in Tenant’s favor that such failure constitutes actual or constructive, total or partial, eviction from the Premises.
Landlord reserves the right to stop any service or utility system when necessary, by reason of accident or emergency or until necessary repairs have been completed, Except in case of emergency repairs, Landlord will give Tenant reasonable advance notice of any contemplated stoppage and will use reasonable efforts to avoid unnecessary inconvenience to Tenant by reason thereof.
Landlord also reserves the right to institute such policies, programs and measures as may be necessary, required or expedient for the conservation or preservation of energy or energy services or as may be necessary or required to comply with applicable codes, rules, regulations or standards.
Notwithstanding the foregoing provisions of this Section 5.2 and any other provision of this Lease to the contrary, in the event that any interruption of service(s) results from a cause arising on the Building or Lot not reasonably beyond Landlord’s control or results from any negligent or willful act or omission of Landlord or its agents or their employees and as a result of such interruption the Premises or material portion thereof is made untenantable for the conduct of Tenant’s business for a period of ten (10) consecutive business days and during such period Tenant does not use or occupy the affected space, the Annual Rent and the additional rent payable by Tenant hereunder for the portion of the Premises which is so untenantable and unused by Tenant shall abate for the period commencing on the day after such tenth (10) consecutive business day and ending on the day upon which the interrupted service(s) is(are) restored.
ARTICLE VI · TENANT’S COVENANTS
6.1TENANT’S COVENANTS DURING THE TERM.
Tenant covenants:
6.1.1Tenant’s Payments. Tenant shall pay when due (a) all Annual Rent and additional rent, (b) all taxes which may be imposed on Tenant’s personal property in the Premises (including, without limitation, Tenant’s fixtures and equipment) regardless to whomever assessed, (c) all charges by public utilities for telephone and other utility services (including service inspections therefor) rendered to the Premises not otherwise required hereunder to be furnished by Landlord without charge and not consumed in connection with any services required to be furnished by Landlord without charge, and (d) as additional rent, all charges to Landlord for services rendered pursuant to Section 5.1.2 hereof.
6.1.2Repairs and Yielding Up. Except as otherwise provided in Article VII, Section 5.1.3 and Section 10.1 1, Tenant shall keep the Premises in good order, repair and condition, reasonable wear and tear and casualty damage required hereunder to be covered by Landlord’s insurance and damage due to condemnation only excepted; and at the expiration or termination of this Lease peaceably to yield up the Premises and all changes and additions therein in such order, repair and condition and free of any and all Hazardous Materials (as hereinafter defined), first removing all goods and effects of Tenant and any items, the removal of which is required by agreement or specified herein to be removed at Tenant’s election and which Tenant elects to remove, and repairing all damage caused by such removal and, if required hereunder, restoring the Premises as and to the extent required and leaving them clean and neat.
6.1.3Occupancy and Use. Tenant shall use and occupy the Premises only for the Permitted Uses; Tenant shall not use or permit the Premises or any portion thereof to be used for any Prohibited Use (as hereinafter defined) or by any Prohibited Person (as defined in  Section 14.4 of the Ground Lease as set forth in Section 10.26 hereof). Tenant shall not injure or deface the Premises, Building, or Lot or permit animal, laboratory or other odors, noises or emissions to emanate from the Premises; and shall not permit in the Premises any use thereof which is improper, offensive, contrary to law or ordinances, or liable to create a nuisance or to invalidate or increase the premiums for any insurance on the Building or its contents or liable to render necessary any alteration or addition to the Building. For purposes hereof: (a) “Prohibited Use” shall mean (i) the manufacture or sale of consumer products (such as, without limitation, alcoholic beverages, tobacco products, or weapons but not including drugs sold over the counter or by medical prescription) recognized as hazardous to human health by federal or Maryland state governmental authorities, (ii) the publication, manufacture, sale, distribution, promotion or purveyance of pornographic material, or (iii) gambling; (b) a “Controlled Affiliate” of any person shall mean any person controlling, controlled by or under common control with such person; and (c) for purposes of the definition of “Controlled Affiliate” the term “controlled” (including the terms, “controlled,” “controlling,” “controlled by,” and “under common control” with) means the possession, direct or indirect, of the power to: (y) vote ten percent (10%) or more of the outstanding voting securities of, or other ownership interests in, such person if the person is a company whose stock or other ownership interests are publicly traded and, if not, to vote more than fifty percent (50%) of the outstanding voting securities of, or other ownership interests in, such person, or (z) otherwise direct the management policies of such person by contract or otherwise. As used herein, a “person” shall mean any individual, partnership, corporation, limited liability company, unincorporated association, trust, estate, or other legal entity. Notwithstanding the foregoing, no federal, state, or local governmental entity, agency or authority (other than a college or university) shall be a “Prohibited Person” for the purposes of this Lease.
Section 14.4 of the Ground Lease as set forth in Section 10.26 hereof). Tenant shall not injure or deface the Premises, Building, or Lot or permit animal, laboratory or other odors, noises or emissions to emanate from the Premises; and shall not permit in the Premises any use thereof which is improper, offensive, contrary to law or ordinances, or liable to create a nuisance or to invalidate or increase the premiums for any insurance on the Building or its contents or liable to render necessary any alteration or addition to the Building. For purposes hereof: (a) “Prohibited Use” shall mean (i) the manufacture or sale of consumer products (such as, without limitation, alcoholic beverages, tobacco products, or weapons but not including drugs sold over the counter or by medical prescription) recognized as hazardous to human health by federal or Maryland state governmental authorities, (ii) the publication, manufacture, sale, distribution, promotion or purveyance of pornographic material, or (iii) gambling; (b) a “Controlled Affiliate” of any person shall mean any person controlling, controlled by or under common control with such person; and (c) for purposes of the definition of “Controlled Affiliate” the term “controlled” (including the terms, “controlled,” “controlling,” “controlled by,” and “under common control” with) means the possession, direct or indirect, of the power to: (y) vote ten percent (10%) or more of the outstanding voting securities of, or other ownership interests in, such person if the person is a company whose stock or other ownership interests are publicly traded and, if not, to vote more than fifty percent (50%) of the outstanding voting securities of, or other ownership interests in, such person, or (z) otherwise direct the management policies of such person by contract or otherwise. As used herein, a “person” shall mean any individual, partnership, corporation, limited liability company, unincorporated association, trust, estate, or other legal entity. Notwithstanding the foregoing, no federal, state, or local governmental entity, agency or authority (other than a college or university) shall be a “Prohibited Person” for the purposes of this Lease.
6.1.4Rules and Regulations, - Tenant shall comply with the Rules and Regulations set forth in Exhibit E and alt other reasonable Rules and Regulations hereafter made by Landlord (not inconsistent with the terms of this Lease), of which Tenant has been given notice, for the care and use of the Building and Lot and their facilities and approaches, it being understood that Landlord shall not be liable to Tenant for the failure of other tenants of the Building to conform to such Rules and Regulations, 6.1.5Safety Appliances and Licenses.Tenant shall keep the Premises equipped with all safety appliances required by law or ordinance or any other regulation of any public authority because of any use made by Tenant and shall procure all licenses and permits so required because of such use and, if requested by Landlord, Tenant shall do any work so required because of such use, it being understood that the foregoing provisions shall not be construed to broaden in any way Tenant’s Permitted Uses.
6.1.6 Assignment and Subletting.Tenant shall not without the prior written consent of Landlord assign this Lease, make any sublease, or permit occupancy of the Premises or any part thereof by anyone other than Tenant, voluntarily or by operation of law, which consent shall not be unreasonably withheld, conditioned or delayed, so long as Tenant is not then in default of this Lease. As additional rent, Tenant shall reimburse Landlord promptly for reasonable legal and other expenses incurred by Landlord in connection with any request by Tenant for consent to assignment or subletting (not to exceed $1 1000 in the aggregate in connection with each such request). No assignment or subletting shall affect the continuing primary liability of Tenant (which, following assignment, shall be joint and several with the assignee). No consent to any of the foregoing in a specific instance shall operate as a waiver in any subsequent instance. Landlord’s consent to any proposed assignment or subletting is required both as to the terms and conditions thereof, and as to the creditworthiness of the proposed assignee or subtenant and the consistency of the proposed assignee’s or subtenant’s business with other uses and tenants in the Building. In the event that any assignee or subtenant pays to Tenant any amounts which (after deducting therefrom costs to Tenant of reasonable legal fees, brokerage fees, improvements, allowances or rent concessions made by Tenant in connection with such sublease or assignment) exceed the Annual Rent and additional rent then payable hereunder, or pro rata portion thereof on a square footage basis for any portion of the Premises, Tenant shall promptly pay fifty percent (50%) of said excess to Landlord as and when received by Tenant. If Tenant requests Landlord’s consent to assign this Lease or sublet a portion of the Premises, Landlord shall have the option, exercisable by written notice to Tenant given within 20 days after receipt of such request, to terminate this Lease (a) entirely in the case of an assignment or (b) with respect to the portion of the Premises desired to be sublet, as the case may be, as of a date specified in such notice which shall be not less than 30 or more than 60 days after the date of such notice.
If, at any time during the Term of this Lease, Tenant is: (i) a corporation or a trust (whether or not having shares of beneficial interest) and there shall occur any direct or indirect change in the identity of any of the persons then having power to participate in the election or appointment of the directors, trustees or other persons exercising like functions and managing the affairs of tenant; or (ii) a partnership or association or otherwise not a natural person (and is not a corporation or a trust), and there shall occur any direct or indirect change in the identity of any of the persons who then are members of such partnership or association or who comprise Tenant, Tenant shall so notify Landlord and Landlord may terminate this Lease by  notice to Tenant given within 90 days thereafter if, in Landlord’s reasonable judgment, the credit of Tenant is thereby impaired.
notice to Tenant given within 90 days thereafter if, in Landlord’s reasonable judgment, the credit of Tenant is thereby impaired.
If Tenant shall at any time or times during the term of this Lease desire to assign this Lease or sublet all or a portion of the Premises, Tenant shall give written notice thereof to Landlord, which notice shall be accompanied by: (a) a conformed or photostatic copy of the proposed assignment or sublease, the effective or commencement date of which shall be at least sixty (60) days after the giving of such notice; (b) a statement setting forth in reasonable detail the identity of the proposed assignee or subtenant, the nature of its business and its proposed use of the Premises; (c) current financial information with respect to the proposed assignee or subtenant, including, without limitation, its most recent financial report and any contract of sale if Tenant is selling its business, and (d) a written confirmation by such subtenant or assignee stating that it is not a “Prohibited Person” (as defined herein). Such notice shall be deemed an offer from Tenant to Landlord whereby Landlord (or Landlord’s designee) may, at its option: (i) consent to such assignment or sublease; (ii) sublease such space from Tenant upon the same terms and conditions therein set forth; (iii) if for more than 50% of the Premises, terminate this Lease and enter into a new lease directly with the proposed sublessee or assignee, without any liability to Tenant; or, (iv) deny consent. Said options may be exercised by Landlord by notice to Tenant at any time within 20 days after such notice has been given by Tenant to Landlord, and during such 20-day period Tenant shall not assign this Lease or sublet such space to any person.
Landlord agrees that subleases of all or a portion of the Premises to an Affiliate of Tenant, as that term is hereinafter defined, whose financial condition is equal to or better than that of Tenant as of the date of such assignment will not require the prior approval of Landlord hereunder, provided Tenant provides Landlord written confirmation of the financial condition of such Affiliate of Tenant, as that term is hereinafter defined, and complies with all terms and conditions of the immediately preceding paragraph, and provided further that any such subtenant uses the Premises for the Permitted Use and is not a Prohibited Person. For purposes hereof, the term ‘Affiliate of Tenant” shall mean (i) any entity that is controlled by Tenant, is a subsidiary of Tenant, is under common control with Tenant or controls Tenant, (ii) the successor to Tenant by consolidation or merger, provided the successor has a net Worth not less than tenant’s net worth as of the date of such merger. Except in the case of the term “Controlled Affiliate” (as used in Section 6.1.3), the term “Control” (including the terms “controlled,” “controlling,” “controlled by,” and “under common control” with) as used in this Lease shall mean ownership of more than 50% of all partnership interests (including more than 50% of all general partnership interests) in a partnership or more than 50% of all classes of stock (including more than 50% of all voting stock) in a corporation or more than 50% of all voting equity interests in a mutual life insurance company. Any suite entry door signage for subtenant(s) and assignee(s) and any change in suite entry door, signage for subtenant(s) or assignee(s) of Tenant shall be at Tenant’s sole expense and subject to Landlord’s prior approval.
6.1.7Indemnity. Tenant shall defend, with counsel approved by Landlord, all actions against Landlord, any partner, member, trustee, stockholder, officer, manager, director, employee or beneficiary of Landlord, holders of mortgages secured by the Premises or the Building and Lot and any other party having an interest in the Premises (“Indemnified Parties”) with respect to, and pay, protect, indemnify and save harmless, to the extent permitted by law, all indemnified Parties from and against, any and all liabilities, losses, damages, costs, expenses (including reasonable attorneys’ fees and expenses), causes of action, suits, claims, demands or judgments of any nature (a) to which any Indemnified party is subject because of its estate or interest in the Building, Lot or Premises or (b) arising from (i) injury to or death of any person, or  damage to or loss of property, on the Premises or connected with the use, condition or occupancy thereof unless caused by the negligence or willful misconduct of Landlord, (ii) violation of this Lease by Tenant, or (iii) any act, omission, fault, negligence or misconduct of Tenant or its agents, employees, contractors, licensees, sublessees or invitees (sometimes herein referred to as “Tenant’s Invitees”).
damage to or loss of property, on the Premises or connected with the use, condition or occupancy thereof unless caused by the negligence or willful misconduct of Landlord, (ii) violation of this Lease by Tenant, or (iii) any act, omission, fault, negligence or misconduct of Tenant or its agents, employees, contractors, licensees, sublessees or invitees (sometimes herein referred to as “Tenant’s Invitees”).
6.1.8Tenant’s Insurance. Tenant shall maintain commercial general liability insurance on the Premises, containing a broad form contractual liability endorsement, insuring Tenant and naming Landlord, its Mortgagee and property managing agent as an additional named insureds against all claims and demands for (i) injury to or death of any person or damage to or loss of property, on the Premises or adjoining walks, streets or ways, or connected with the use, condition or occupancy of any thereof by Tenant or Tenant’s Invitees unless caused by the negligence of Landlord, (ii) violation of this Lease by Tenant, (iii) any act, fault or omission, or other misconduct of Tenant Tenant’s Invitees, (iv) any and all indemnification obligations of Tenant under this Lease including, without limitation, Section 6.1 .7 and Section 10.19 hereof, in amounts which shall, at the beginning of the Term, be at least equal to the limits set forth in Section 1 .1 , and from time to time during the Term, shall be for such higher limits, if any, as are customarily carried in the area in which the Premises are located on property similar to the Premises and used for similar purposes, and shall be written on the “Occurrence Basis” and include Host Liquor liability insurance, and to furnish Landlord with certificates thereof and boiler and machinery insurance in adequate amounts on all fired objects and other fired pressure vessels and systems serving the Premises (if any). If fired objects and other pressure vessels and the damage that may be caused by them or result from them are not covered by Tenant’s extended coverage insurance, then such insurance shall be in an amount not less than $250,000 and be issued on a replacement cost basis. Tenant shall also maintain insurance covering all of the items included in Tenant’s Leasehold Improvements, heating, ventilating and air conditioning equipment maintained by Tenant, trade fixtures, merchandise and personal property from time to time in, on or upon the Premises, and alterations, additions or changes made by Tenant in an amount not less than one hundred percent (100%) of their full depreciated value from time to time during the Term, providing protection against perils included within the standard form of “all risks” fire and casualty insurance policy, together with insurance against sprinkler damage, vandalism and malicious mischief.
Any policy proceeds from such insurance shall be held in trust by Tenant’s insurance company for the repair, construction, and restoration or replacement of the property damaged or destroyed unless this Lease shall cease and terminate under the provisions of this Lease. The insurance policies required to be obtained by Tenant under this Section shall be issued by an insurance company of recognized responsibility with a rating of “A-X” or better in the current “Best’s Insurance Reports” and reasonably acceptable to Landlord, licensed to do business in the jurisdiction in which the Building is located, and shall be written as primary policy coverage and not contributing with or in excess of any coverage that Landlord or any Mortgagee or management agent may carry. All policies that Tenant is required to maintain under this Lease shall contain appropriate clauses or endorsements under which (i) such policies may not be materially changed, amended, cancelled or allowed to lapse without thirty (30) days’ prior notice to Landlord, (ii) no act or omission of Tenant shall effect or limit the obligations of the insurer with respect to the Landlord, (iji) Tenant shall be solely responsible for the payment of all premiums notwithstanding the fact that Landlord is an additional insured under any such policy, and (iv) Landlord, its Mortgagee and property management agent are named an additional insureds under such policy. Any insurance provided for in this Section 6.1.8 may be maintained by means of a policy or policies of blanket insurance, covering additional items or locations or insureds, provided, however, that the coverage afforded Landlord and any such other parties in interest will not be reduced or diminished by reason of the use of such blanket policy of insurance. Neither the issuance of any insurance policy required under this Lease, nor the minimum limits specified herein with respect to Tenant’s insurance coverage, shall be deemed to limit or restrict in any way Tenant’s liability arising under or with respect to this Lease. On or before occupancy by Tenant of any portion of the Premises, for any reason, including the installation of cabling or systems furniture, and in all events prior to the Commencement Date, and at least thirty (30) days prior to the expiration of any policy or certificate furnished by Tenant under this Section, Tenant shall deliver to Landlord copies of all insurance policies required by Tenant to be maintained under the lease or appropriate certificates evidencing the issuance of such policies, together with evidence of payment of all applicable premiums.
6.1.9Tenant’s Worker’s Compensation Insurance. Tenant shall keep all of Tenant’s employees working in the Premises covered by worker’s compensation insurance in statutory amounts not less than $500,000, and to furnish Landlord with certificates thereof.
6.1.10Landlord’s Right of Entry. — Tenant shall permit Landlord and Landlord’s agents entry: to examine the Premises at reasonable times upon prior notice (provided however no notice shall be required in the event of an emergency) and if Landlord shall so elect, to make repairs or replacements (it being understood, however, that Landlord shall use commercially reasonable efforts to minimize disruption of Tenant’s business); to remove, at Tenant’s expense, any changes, additions, signs, curtains, blinds, shades, awnings, aerials, flagpoles, or the like not consented to in writing by Landlord; and to show the Premises to prospective tenants during the 12 months preceding expiration of the Term and to prospective purchasers and mortgagees at all reasonable times.
6.1.11Loading. - Tenant shall not place Tenant’s Property, as defined in Section 6.1.13, upon the Premises so as to exceed a rate of 100 pounds of live load and 20  pounds of dead load per square foot and not to move any safe, vault or other heavy equipment in, about or out of the Premises except in such manner and at such times as Landlord shall in each instance approve; Tenant’s business machines and mechanical equipment which cause vibration or noise that may be transmitted to the Building structure or to any other leased space in the Building shall be placed and maintained by Tenant in settings of cork, rubber, spring or other types of vibration eliminators sufficient to eliminate such vibration or noise.
pounds of dead load per square foot and not to move any safe, vault or other heavy equipment in, about or out of the Premises except in such manner and at such times as Landlord shall in each instance approve; Tenant’s business machines and mechanical equipment which cause vibration or noise that may be transmitted to the Building structure or to any other leased space in the Building shall be placed and maintained by Tenant in settings of cork, rubber, spring or other types of vibration eliminators sufficient to eliminate such vibration or noise.
6.1.12 Landlord’s Costs. — In case Landlord shall be made party to any litigation commenced by or against Tenant or by or against any parties in possession of the Premises or any part thereof claiming under Tenant, Tenant shall pay, as additional rent, all costs including, without implied limitation, reasonable counsel fees incurred by or imposed upon Landlord in connection with such litigation and, as additional rent, also to pay all such costs and fees incurred by Landlord in connection with the successful enforcement by Landlord of any obligations of Tenant or remedies of Landlord under this Lease.
6.1.13 Tenant’s Property. - All the furnishings, fixtures, equipment, effects and property of every kind, nature and description of Tenant and of all persons claiming by, through or under Tenant which, during the continuance of this Lease or any occupancy of the Premises by Tenant or anyone claiming under Tenant, may be on the Premises or elsewhere in the Building or on the Lot shall be at the sole risk and hazard of Tenant, and if the whole or any part thereof shall be destroyed or damaged by fire, water or otherwise, or by the leakage or bursting of water pipes, steam pipes, or other pipes, by theft, or from any other cause, no part of said loss or damage is to be charged to or to be borne by Landlord unless due to the gross negligence of Landlord or its agents or their employees.
6.1.14Labor or Materialmen’s Liens. - Tenant shall pay promptly when due the entire cost of any work done or materials installed in the Premises by Tenant, its agents, employees or contractors; and shall not cause or permit any liens for labor or materials performed or furnished in connection therewith to attach to the Premises; Building or the Lot; and shall bond over any such liens which may so attach or cause the same to be discharged within thirty (30) days.
6.1.15 Changes or Additions. — Tenant shall not make any changes or additions to the  Premises without Landlord’s prior written consent, provided that Tenant shall reimburse Landlord for all engineering fees and expenses incurred by Landlord in connection therewith if Landlord reasonably believes review of such changes or improvements by engineer(s) is appropriate, and provided further that, Tenant shall be solely responsible for assuring that all such changes or additions comply with applicable laws and regulations and in order to protect the functional integrity of the Building, all such changes and additions shall be performed by contractors selected from a list of approved contractors prepared by Landlord and approved by Tenant, such approval not to be unreasonably withheld or delayed.
Premises without Landlord’s prior written consent, provided that Tenant shall reimburse Landlord for all engineering fees and expenses incurred by Landlord in connection therewith if Landlord reasonably believes review of such changes or improvements by engineer(s) is appropriate, and provided further that, Tenant shall be solely responsible for assuring that all such changes or additions comply with applicable laws and regulations and in order to protect the functional integrity of the Building, all such changes and additions shall be performed by contractors selected from a list of approved contractors prepared by Landlord and approved by Tenant, such approval not to be unreasonably withheld or delayed.
6. 1..16Holdover; — Tenant shall pay to Landlord the greater of one and a halftimes(a) the then fair market rent as conclusively determined by Landlord or (b) the total of the Annual Rent and additional rent payable by Tenant during the 12 month period prior to the termination of the Lease, as escalated in accordance with Section 4.1 hereof, for each month or portion thereof, Tenant shall retain possession of the Premises or any part thereof after the termination of this Lease, whether by lapse of time or otherwise, and shall also pay all direct damages sustained by Landlord on account thereof. The provisions of this subsection shall not operate as a waiver by Landlord of the right of re-entry provided in this Lease. At the option of Landlord exercised by a written notice given to Tenant while such holding over continues, such holding over shall constitute an extension of this Lease for a period of one year on the economic terms set forth in the first sentence of this Section 6.1.16.
6.1.17Financial Statements. Tenant shall deliver to Landlord within ninety (90) days following the close of each of Tenant’s fiscal years during the Term, Tenant’s then current financial statement audited by an independent certified public accountant, if such a current audited statement has not been filed with the Securities and Exchange Commission and is available, and otherwise certified as true, correct and complete by Tenant’s Chief Financial Officer.
6.1.18Compliance. Tenant shall, at Tenant’s sole expense, (i) comply with all laws, orders, ordinances, and regulations of federal, state, county, and municipal authorities having jurisdiction over the Premises (collectively, “Laws”) and with all licenses and permits applicable to Tenant’s use thereof (collectively, “Permits”), (ii) comply with the Declaration of Covenants, Restrictions and Easements dated July 18, 2003 by JHU as Declarant (the “Campus CCRs”), the Estoppel Certificate Waiver and Consent Assignment dated July 18, 2003 between JHU and Montgomery County, Maryland (the “Estoppel”), and such other protective covenants, restrictions applicable to the Lot or the Building of which Tenant has prior written notice, and with all requirements of the Ground Lease, as they may be amended from time to time, after notice thereof to Tenant of any such amendment, (iii) comply with any directive, order or citation made pursuant to law by any public officer requiring abatement of any nuisance or which imposes upon Landlord or Tenant any duty or obligation arising from Tenant’s occupancy or use of the Premises or from conditions which have been created by or at the request or insistence of Tenant, or required by reason of a breach of any Tenant’s obligations hereunder or by or through other fault of Tenant (iv) comply with all insurance requirements applicable to the Premises, and (v) furnish all data and information to governmental authorities, with a copy to Landlord, required by Laws or Permits.
If Tenant receives notice of any such directive, order, citation or of any violation of any law, order, ordinance, regulation or any insurance requirement or of any of the protective covenants or restrictions, Tenant shall promptly notify Landlord in writing of such alleged violation and furnish Landlord with a copy of such notice. If Landlord receives notice of any such directive, order, citation, violation of any law, ordinance, regulation, insurance requirement or protective covenants or restrictions pertaining to Tenant or the Premises, Landlord shall promptly notify Tenant in writing thereof and will furnish Tenant with a copy of such notice.
6.1.19Laboratories. - If Tenant uses the Premises or any portion thereof for a laboratory, Tenant shall be responsible, at its sole cost and expense, for routine janitorial and trash removal services for the laboratory areas and other biohazard disposal services for the laboratory areas, if any, in the Premises or other space within the Premises used in connection  with the generation, use, storage, treatment, release or disposal of Hazardous Material as permitted in accordance with Section 10.18 or the housing, care, storage or handling of animals (collectively, “HazMat Facilities”). Such services shall be performed by contractors reasonably acceptable to Landlord and on a sufficient basis to ensure that the laboratory and other HazMat Facilities of the Premises are at all times kept neat, clean, free of trash, Hazardous Material, and biohazards except in appropriate, specially marked containers reasonably approved by Landlord and in compliance with applicable Laws. Tenant shall not cause or permit animals, animal waste, food or supplies relating to animals to be used, housed or stored in the Premises or to be transported within the Building. Prior to the Commencement and from time to time during the Term upon written request by Landlord, Tenant, at its sole cost and expense, shall retain a qualified environmental engineer or consultant, reasonably acceptable to Landlord, to inspect all laboratory and other HazMat Facilities in the Premises and confirm in a written report to Landlord that ail such laboratories and HazMat Facilities comply with applicable Laws. If such engineer or consultant cannot issue such report because the laboratories and HazMat Facilities do not comply with applicable Laws, Tenant shall forthwith, at its sole cost and expense, take any and all actions necessary to bring such laboratories and HazMat Facilities into compliance with such Laws.
with the generation, use, storage, treatment, release or disposal of Hazardous Material as permitted in accordance with Section 10.18 or the housing, care, storage or handling of animals (collectively, “HazMat Facilities”). Such services shall be performed by contractors reasonably acceptable to Landlord and on a sufficient basis to ensure that the laboratory and other HazMat Facilities of the Premises are at all times kept neat, clean, free of trash, Hazardous Material, and biohazards except in appropriate, specially marked containers reasonably approved by Landlord and in compliance with applicable Laws. Tenant shall not cause or permit animals, animal waste, food or supplies relating to animals to be used, housed or stored in the Premises or to be transported within the Building. Prior to the Commencement and from time to time during the Term upon written request by Landlord, Tenant, at its sole cost and expense, shall retain a qualified environmental engineer or consultant, reasonably acceptable to Landlord, to inspect all laboratory and other HazMat Facilities in the Premises and confirm in a written report to Landlord that ail such laboratories and HazMat Facilities comply with applicable Laws. If such engineer or consultant cannot issue such report because the laboratories and HazMat Facilities do not comply with applicable Laws, Tenant shall forthwith, at its sole cost and expense, take any and all actions necessary to bring such laboratories and HazMat Facilities into compliance with such Laws.
6.2BANKRUPTCY.
Tenant acknowledges that this Lease is a lease of nonresidential real property and, therefore agrees that Tenant, as the debtor in possession, or the trustee for Tenant (collectively “the Trustee”) in any proceeding under Title 1 1 of the United States Bankruptcy Code relating to Bankruptcy, as amended, or under any other similar Federal or state statute (collectively, the “Bankruptcy Code”), shall not seek or request any extension of time to assume or reject this Lease or to perform any obligations of this Lease which arise from or after the order of relief.
If the Trustee proposes to assume or to assign this Lease or sublet the Premises (or any portion thereof) to any person who shall have made a bona fide offer to accept an assignment of this Lease or a subletting on terms acceptable to the Trustee, the Trustee shall give Landlord and lessors and mortgagees, of Landlord of which Tenant has notice, written notice setting forth the name and address of such person and the terms and conditions of such offer, no later than 20 days after receipt of such offer, but in any event no later than 10 days prior to the date on which the Trustee makes application to the Bankruptcy Court for authority and approval to enter into such assumption and assignment or subletting. Landlord shall have the prior right and option, to be exercised by written notice to the Trustee given at any time prior to the effective date of such proposed assignment or subletting, to accept an assignment of this Lease or subletting of the Premises upon the same terms and conditions and for the same consideration, if any, as the bona fide offer made by such person, less any brokerage commissions which may be payable out of the consideration to be paid by such person for the assignment or subletting of this Lease.
The Trustee shall have the right to assume Tenant’s rights and obligations under this Lease only if the Trustee: (i) promptly cures, or provides adequate assurance that the Trustee will promptly cure, any default under this Lease; (ii) compensates, or provides adequate assurance that the Trustee will promptly compensate, Landlord for any actual pecuniary loss incurred by Landlord as a result of Tenant’s default under this Lease; and (iii) provides adequate assurance of future performance under this Lease. Adequate assurance of future performance by the proposed assignee shall include, as a minimum, that: (a) the Trustee or any proposed assignee of this Lease shall deliver to Landlord a security deposit in an amount equal to at least 3 months’ Rent accruing under this Lease; (b) any proposed assignee of this Lease shall provide to Landlord an audited financial statement, dated no later than 6 months prior to the effective date to such proposed assignment or sublease with no material change therein as of the effective date, which financial statement shall show the proposed assignee to have a net worth equal to at least 12 months’ Rent accruing under this Lease, or, in the alternative, the proposed assignee shall provide a guarantor of such proposed assignee’s obligations under this Lease, which guarantor shall provide an audited financial statement meeting the above requirements of this clause (b) and execute and deliver to Landlord a guaranty agreement in form and substance acceptable to Landlord; and (c) any proposed assignee shall grant to Landlord a security interest in favor of Landlord in all furniture, fixtures, and other personal property to be used by such proposed assignee in the Premises. All payments of Rent required of Tenant under this Lease, whether or not expressly denominated as such in this Lease, shall constitute rent for the purpose of Title 1 1 of the Bankruptcy Code.
The parties agree that for the purposes of the Bankruptcy Code relating to (i) the obligation of the Trustee to provide adequate assurance that Trustee will “promptly” cure defaults and compensate Landlord for actual pecuniary loss, the word “promptly” shall mean that cure of defaults and compensation will occur no later than 60 days following the filing of any motion or application to assume this Lease; and (ii) the obligation of the Trustee to compensate or to provide adequate assurance that the Trustee will promptly compensate Landlord for “actual pecuniary loss,” the term “actual pecuniary loss” shall mean, in addition to any other provisions contained herein relating to Landlord’s damages upon default, payments of Rent, including interest at the rate of 4% per annum in excess of the Prime Rate on all unpaid Rent, all attorneys’ fees and related costs of Landlord incurred in connection with any default of Tenant and in connection with Tenant’s bankruptcy proceedings.
Any person or entity to which this Lease is assigned pursuant to the provisions of the Bankruptcy Code shall be deemed, without further act or deed, to have assumed all of the obligations arising under this Lease and each of the conditions and provisions hereof on and after the date of such assignment. Any such assignee shall, upon the request of Landlord, forthwith execute and deliver to Landlord an instrument, in form and substance acceptable to Landlord, confirming such assumption.
ARTICLE VII - CASUALTY AND TAKING
7.1CASUALTY AND TAKING.
A.Repair of Damages. If the Building or Premises are damaged by fire, casualty or other destruction thereby rendering the Building or Premises totally or partially inaccessible or unusable, Landlord shall, within thirty (30) days after the date of such damage, at its sole expense reasonably determine (taking into account the time needed for effecting a satisfactory settlement with any insurance company involved, removal of debris, preparation of plans and issuance of all required government permits) how long it will take to restore the damaged area to substantially its condition prior to the fire or other casualty (the ‘Casualty Opinion”). If, in Landlord’s Casualty Opinion, restoration of the Building and Premises substantially to the condition of the Building and Premises prior to the date of such casualty and in compliance with applicable zoning and other applicable laws, permits, approvals and regulations within one hundred eighty (180) days from the date of the damage is not reasonably possible, this Lease shall terminate at Landlord’s election by written notice to Tenant within thirty (30) days after the delivery of Landlord’s Casualty Opinion, which notice shall state the effective date of termination which shall not be more than sixty (60) days nor less than 30 days after the date of such notice. If in any such case the Premises are rendered totally or partially inaccessible or unusable, and Landlord does not so elect to terminate this Lease, Landlord shall, at Landlord’s expense to the extent permitted by the net award of insurance available to Landlord, repair such damage; provided.
however, that Landlord shall have no obligation to repair any damage to, or to replace, Tenant’s personal property. Except as otherwise provided herein, if all or a substantial part of the entire Premises is rendered untenantable by reason of the insured fire, casualty or other destruction, then installments of Annual Rent and additional rent shall abate for such period in the proportion which the area of the Premises so rendered untenantable bears to the total area of the Premises; and provided, further, if, prior to the date when such repairs have been completed, any portion of the Premises as damaged shall be rendered tenantable and shell be used or occupied by Tenant or any person claiming through or under Tenant, then the amount by which the Annual Rent and additional rent shall abate shall be equitably apportioned for the period from the date of any such use or occupancy to the date when such repairs are completed. In addition, if the Premises or the Building is damaged or destroyed within the last year of the Term and either such damage renders at least fifty percent (50%) of the Premises unusable or inaccessible or, in Landlord’s Casualty Opinion, the time necessary to substantially repair and restore such damage exceeds one-sixth of the remaining Term (calculated from date of the damage), then this Lease shall terminate as of the day Landlord’s Casualty Opinion is issued, and the then-applicable Annual Rent and additional rent shall be apportioned as of such date, including any rent abatement as provided above.
B.Termination by Tenant. Notwithstanding the foregoing, it prior to or during the Term of this Lease the Premises is damaged by fire, casualty or other destruction rendering the Premises totally or partially inaccessible or unusable, and either (1) according to the Landlord’s Casualty Opinion, restoration to substantially its condition prior to the fire or other casualty within one hundred eighty (180) days from the date of damage is not reasonably possible, or (2) this Lease is not terminated by either Landlord or Tenant, and restoration of the Premises is not substantially completed within one (1) month following expiration of the period of time within which Landlord estimated that such restoration could be completed (as stated in Landlord’s Casualty Opinion), as such period is extended due to Force Majeure (in accordance with Section 10.12 hereof), then Tenant may give to Landlord (within thirty (30) days after receipt of notice from Landlord that the restoration will require more than one hundred eighty (180) days or within thirty (30) days after the estimated completion date set forth in Landlord’s Casualty Opinion, as the case may be) ten (10) days’ notice of termination of this Lease, and, in the event such notice is given, this Lease shall terminate (whether or not the Term shall have commenced) upon the expiration of such ten (10) days (provided Landlord has not sooner substantially completed restoration as herein required) and the then-applicable Annual Rent shall be apportioned as of such date, including any rent abatement as provided above.
7.2TAKING.
This Lease shall terminate on the date title thereto vests in a governmental or quasigovernmental authority pursuant to a condemnation (as hereinafter defined), and rent shall be apportioned as of such date (a) if the entire Building or the entire Premises or the occupancy of either the entire Building or the entire Premises shall be taken or condemned by any governmental or quasigovernmental authority or sold to a governmental or quasi-governmental authority under threat of such a taking or condemnation (collectively, “condemned” or “condemnation”), (b) at Landlord’s election, by written notice to Tenant, if more than 30% of the Premises is rendered wholly unusable or inaccessible or otherwise untenantable as a result of any condemnation, or (c) at Landlord’s election, by written notice to Tenant, if such condemnation results in a taking to more than 20% of the Building or a reduction by more than 20% of the fair market value of the Building. In addition, on the date title vests in a governmental or quasi-governmental authority pursuant to condemnation described in clause (b) or (c) of this Section 7.2 or pursuant to a condemnation which results in more than 30% of the floor area of the Premises being condemned, this Lease may also be terminated, at Tenant’s election by written notice to Landlord within thirty (30) days following such taking, unless, within such thirty (30) days, Landlord offers to relocate the affected portion of the Premises to another location in the Building comparable to and substantially the same size as the area condemned. Landlord or Tenant may exercise any option given to it hereunder to terminate this Lease provided that Tenant or Landlord, as the case may be, delivers written notice to other no later than thirty (30) days prior to the date title vests in the governmental or quasi-governmental authority.
If a condemnation occurs which does not automatically result in a termination of this Lease in accordance with this Section 7.2 and neither Landlord nor Tenant has the option or timely exercises its option to terminate this Lease as herein provided, then this Lease shall continue in full force and effect as to the part of the Premises not condemned, except that, as of the date title vests in such authority, Tenant shall not be required to pay rent with respect to the part of the Premises condemned (including, without limitation, any part of the Premises condemned temporarily, in which case Tenant shall not be required to pay installments of Annual Rent, additional rent or any other sums with respect to such part of the Premises during such period of time.
7.3RESERVATION OF AWARD.
Landlord reserves to itself any and all rights to receive awards made for damages to the Premises, Building or Lot and the leasehold hereby created, or any one or more of them, accruing by reason of exercise of eminent domain or by reason of anything lawfully done in pursuance of public or other authority. Tenant hereby releases and assigns to Landlord all Tenant’s rights to such awards, and covenants to deliver such further assignments and assurances thereof as Landlord may from time to time request, and hereby irrevocably designates and appoints Landlord its attorney-in-fact to execute and deliver in Tenant’s name and behalf all such further assignments thereof. It is agreed and understood, however, that Landlord does not reserve to itself, and Tenant does not assign to Landlord, any damages payable for (i) movable trade fixtures installed by Tenant or anybody claiming under Tenant, (it) other personal property of Tenant, (iii) loss of customer good will, or (iv) moving and relocation expenses recoverable by Tenant from such authority in a separate action.
ARTICLE VIII - RIGHTS OF MORTGAGEE
8.1PRIORITY OF LEASE.
This Lease is and shall continue to be subject and subordinate to any mortgage now or hereafter of record covering the Lot or Building or both (the “mortgaged premises”) created by Landlord upon acquisition of title to the Lot or thereafter and to any and all advances hereafter made thereunder and to the interest of the holder or holders thereof in the Lot or the Building, or both of them. This Lease shall also be subject and subordinate to the Ground Lease (and all modifications and extensions thereof) under  which Landlord acquires its interest in the Lot. Any such mortgage or ground lease to which this Lease shall be subordinated may contain such terms, provisions and conditions as the holder or ground lessor deems usual or customary.
which Landlord acquires its interest in the Lot. Any such mortgage or ground lease to which this Lease shall be subordinated may contain such terms, provisions and conditions as the holder or ground lessor deems usual or customary.
Notwithstanding the foregoing, Landlord agrees to obtain a non-disturbance and attornment agreement from the current holder of any mortgage substantially in the form attached hereto as Exhibit J, or such other commercially reasonable form required by said mortgagee, which confirms the subordination of this Lease as aforesaid and whereby such holder will agree, so long as Tenant is not then in default hereunder, to recognize the rights of Tenant under this Lease and to accept Tenant as tenant of the Premises under the terms and conditions of this Lease in the event of acquisition of title by such holder (or a third party) through foreclosure proceedings or otherwise and Tenant will agree to recognize the holder of such mortgage or such purchaser as Landlord in such event, which agreement shall be made to expressly bind and inure to the benefit of the successors and assigns of Tenant and of the holder and upon anyone purchasing Landlord’s interest in the mortgaged premises at any foreclosure sale or otherwise. The holder of such mortgage shall have the election to subordinate the same to the rights and interests of Tenant under this Lease exercisable by filing with the appropriate recording office a notice of such election, whereupon the Tenant’s rights and interests hereunder shall have priority over such mortgage or deed of trust. In addition, Landlord agrees to use commercially reasonable efforts to obtain a non disturbance and attornment agreement from the ground lessor under the Ground Lease substantially in the form attached hereto as Exhibit K.
The word “mortgage” as used in this Lease includes mortgages, deeds of trust or other similar instruments evidencing other voluntary liens or encumbrances, and modifications, consolidations, extensions, renewals, replacements and substitutes thereof. The word “holder” shall mean a mortgagee,  and any subsequent holder or holders of a mortgage.
and any subsequent holder or holders of a mortgage.
8.2RIGHTS OF MORTGAGE HOLDERS: LIMITATION OF MORTGAGEE’S LIABILITY.
Until the holder of a mortgage shall enter and take possession of the Premises for the purpose of foreclosure and until a ground lessor shall enter and take possession of the Premises upon termination of the Ground Lease, such holder or ground lessor shall have only such rights of Landlord as are necessary to preserve the integrity of this Lease as security. Upon entry and taking possession of the Premises for the purpose of foreclosing or following termination of the Ground Lease, such holder or ground lessor  which takes possession of the Premises, as the case may be, shall have all rights of Landlord. Notwithstanding any other provision of this Lease to the contrary, including without limitation Section 10.4 no such holder of a mortgage or ground lessor shall be liable, either as mortgagee, ground lessor or as assignee, to perform or be liable in damages for failure to perform, any of the obligations of Landlord unless and until such holder or ground lessor shall enter and take possession of the Premises upon termination of the Ground Lease or for the purpose of foreclosure. Upon any such entry, the holder or ground lessor shall be liable to perform all of the obligations of Landlord accruing from and after such entry, subject to and with the benefit of the provisions of Section 10.4, provided that a discontinuance of any foreclosure proceeding or creation of a new or replacement ground lease shall be deemed a conveyance under said provisions to the owner of the equity or ground lessee of the Premises. For the purpose of this Lease, the word “foreclosure” shall include any trustee’s sale pursuant to the terms of a deed of trust and sale in execution of any other voluntary lien or encumbrance.
which takes possession of the Premises, as the case may be, shall have all rights of Landlord. Notwithstanding any other provision of this Lease to the contrary, including without limitation Section 10.4 no such holder of a mortgage or ground lessor shall be liable, either as mortgagee, ground lessor or as assignee, to perform or be liable in damages for failure to perform, any of the obligations of Landlord unless and until such holder or ground lessor shall enter and take possession of the Premises upon termination of the Ground Lease or for the purpose of foreclosure. Upon any such entry, the holder or ground lessor shall be liable to perform all of the obligations of Landlord accruing from and after such entry, subject to and with the benefit of the provisions of Section 10.4, provided that a discontinuance of any foreclosure proceeding or creation of a new or replacement ground lease shall be deemed a conveyance under said provisions to the owner of the equity or ground lessee of the Premises. For the purpose of this Lease, the word “foreclosure” shall include any trustee’s sale pursuant to the terms of a deed of trust and sale in execution of any other voluntary lien or encumbrance.
8.3INTENTIONALLY OMITTED.
8.4NO PREPAYMENT OR MODIFICATION, ETC.
Tenant shall not pay Annual Rent, Estimated Monthly Payments, Estimated Monthly Real Estate tax Payments, Tenant’s Annual Electrical Cost, other additional rent or any other charge more than ten (10) days prior to the due date thereof, No prepayment of Annual Rent, additional rent or other charge, no assignment of this Lease and no agreement to modify so as to reduce the rent, change the Term, or otherwise materially change the rights of Landlord under this Lease, or to relieve Tenant of any obligations or liability under this Lease shall be valid unless consented to in writing by Landlord’s mortgagees of record, if any.
8.5NO RELEASE OR TERMINATION.

 No act or failure to act on the part of Landlord which would entitle Tenant under the terms of this Lease, or by law, to be relieved of Tenant’s obligations hereunder or to terminate this Lease shall result in a release or termination of such obligations or a termination of this Lease unless (i) Tenant shall have first given written notice of Landlord’s act or failure to act to Landlord’s mortgagees of record, if any, specifying the act or failure to act on the part of Landlord which could or would give basis to Tenant’s
No act or failure to act on the part of Landlord which would entitle Tenant under the terms of this Lease, or by law, to be relieved of Tenant’s obligations hereunder or to terminate this Lease shall result in a release or termination of such obligations or a termination of this Lease unless (i) Tenant shall have first given written notice of Landlord’s act or failure to act to Landlord’s mortgagees of record, if any, specifying the act or failure to act on the part of Landlord which could or would give basis to Tenant’s  rights and (ii) such mortgagees, after receipt of such notice, have failed or refused to correct or cure the condition complained of within a reasonable time thereafter, but nothing contained in this Section 8.5 shall be deemed to impose any obligation on any such mortgagee to correct or cure any such condition. “Reasonable time” as used above means and includes a reasonable time to obtain possession of the mortgaged premises, if the mortgagee elects to do so, and a reasonable time to correct or cure the condition if such condition is determined to exist, not exceeding one hundred twenty (120) days in the aggregate.
rights and (ii) such mortgagees, after receipt of such notice, have failed or refused to correct or cure the condition complained of within a reasonable time thereafter, but nothing contained in this Section 8.5 shall be deemed to impose any obligation on any such mortgagee to correct or cure any such condition. “Reasonable time” as used above means and includes a reasonable time to obtain possession of the mortgaged premises, if the mortgagee elects to do so, and a reasonable time to correct or cure the condition if such condition is determined to exist, not exceeding one hundred twenty (120) days in the aggregate.
8.6CONTINUING OFFER.
The covenants and agreements contained in this Lease with respect to the rights, powers and benefits of a holder of a mortgage (particularly, without limitation thereby, the covenants and agreements contained in this Article VII) constitute a continuing offer to any person, corporation or other entity, which by accepting or requiring an assignment of this Lease or by entry or foreclosure assumes the obligations herein set forth with respect to such holder; such holder is hereby constituted a party to this Lease as an obligee hereunder to the same extent as though its name were written hereon as such; and such holder shall be entitled to enforce such provisions in its own name. Tenant agrees on request of Landlord to execute and deliver from time to time any agreement which may reasonably be deemed necessary to implement the provisions of this Article VI.
8.7MORTGAGEE’S APPROVAL.
If the holder of any mortgage created by Landlord as security for financing of Landlord’s  acquisition of the Lot or construction of the Base Building shall require a modification of the terms and provisions of this Lease as a condition to providing its financing for the said acquisition or construction or any lender shall require such modification as a condition to granting a new mortgage loan for the Building, Landlord shall have the right to cancel this Lease if Tenant refuses to execute and deliver to Landlord, within 30 days after Landlord’s request therefor, a written agreement incorporating such modifications into this Lease; provided, however, that the modifications required do not result in an increase in the rent or additional rent payable by Tenant hereunder or otherwise materially increase Tenant’s obligations or materially reduce Tenant’s rights hereunder.
acquisition of the Lot or construction of the Base Building shall require a modification of the terms and provisions of this Lease as a condition to providing its financing for the said acquisition or construction or any lender shall require such modification as a condition to granting a new mortgage loan for the Building, Landlord shall have the right to cancel this Lease if Tenant refuses to execute and deliver to Landlord, within 30 days after Landlord’s request therefor, a written agreement incorporating such modifications into this Lease; provided, however, that the modifications required do not result in an increase in the rent or additional rent payable by Tenant hereunder or otherwise materially increase Tenant’s obligations or materially reduce Tenant’s rights hereunder.
ARTICLE IX - DEFAULT
9.1EVENTS OF DEFAULT.

 If Tenant shall fail to pay Annual Rent, additional rent, Tenant Improvement Reimbursement to Landlord or any installment thereof or any other monetary obligation including, but not limited to the failure to deliver any amount or letter of credit due as a rent or security deposit arising hereunder to Landlord as and when herein provided and such failure continues after written notice thereof for more than 5 business days; or if Tenant attempts or purports to transfer, assign or encumber this Lease or to sublease or grant a right of use or occupancy in the Premises without complying with Section 6.1 .6 hereof; or if any other default by Tenant shall occur and continue after notice thereof for more than 30 days and such additional timer if any, (not exceeding 90 days in the aggregate) as is reasonably necessary to cure the default if the default is of such a nature that it cannot reasonably be cured in 30 days and Tenant diligently endeavors to cure such default and does within 90 days following such notice; or if Tenant becomes insolvent, fails to pay its debts as they fall due, files a petition under any chapter of the U.S. Bankruptcy Code, 1 1 U.S.C. 101 et seq., as it may be amended (or any similar petition under any insolvency law of any jurisdiction), or if such petition is filed against Tenant and is not dismissed within sixty (60) days; or if Tenant proposes any dissolution, liquidation, composition, financial reorganization or recapitalization with creditors, makes an assignment or trust mortgage for the benefit of creditors, or if a receiver, trustee, custodian or similar agent is appointed or takes possession with respect to any property of Tenant; or if the leasehold hereby created is taken on execution or other process of law in any action against Tenant; then, and in any such case (herein sometimes referred to as an “Event of Default”), Landlord and the agents and servants of Landlord may, in addition to and not in derogation of any remedies for any preceding breach of covenant, immediately or at any time thereafter and without further notice exercise any and all remedies permitted by state or federal law. In the event of any such
If Tenant shall fail to pay Annual Rent, additional rent, Tenant Improvement Reimbursement to Landlord or any installment thereof or any other monetary obligation including, but not limited to the failure to deliver any amount or letter of credit due as a rent or security deposit arising hereunder to Landlord as and when herein provided and such failure continues after written notice thereof for more than 5 business days; or if Tenant attempts or purports to transfer, assign or encumber this Lease or to sublease or grant a right of use or occupancy in the Premises without complying with Section 6.1 .6 hereof; or if any other default by Tenant shall occur and continue after notice thereof for more than 30 days and such additional timer if any, (not exceeding 90 days in the aggregate) as is reasonably necessary to cure the default if the default is of such a nature that it cannot reasonably be cured in 30 days and Tenant diligently endeavors to cure such default and does within 90 days following such notice; or if Tenant becomes insolvent, fails to pay its debts as they fall due, files a petition under any chapter of the U.S. Bankruptcy Code, 1 1 U.S.C. 101 et seq., as it may be amended (or any similar petition under any insolvency law of any jurisdiction), or if such petition is filed against Tenant and is not dismissed within sixty (60) days; or if Tenant proposes any dissolution, liquidation, composition, financial reorganization or recapitalization with creditors, makes an assignment or trust mortgage for the benefit of creditors, or if a receiver, trustee, custodian or similar agent is appointed or takes possession with respect to any property of Tenant; or if the leasehold hereby created is taken on execution or other process of law in any action against Tenant; then, and in any such case (herein sometimes referred to as an “Event of Default”), Landlord and the agents and servants of Landlord may, in addition to and not in derogation of any remedies for any preceding breach of covenant, immediately or at any time thereafter and without further notice exercise any and all remedies permitted by state or federal law. In the event of any such  default, Landlord shall also have the right to re-enter and take possession of all or any portion of the Premises and expel Tenant and those claiming through or under Tenant, either by summary proceedings or by any other action at law, in equity, or otherwise, with or without terminating this Lease, without being deemed guilty of trespass and without prejudice to any other remedies of Landlord for breach of this Lease. Tenant hereby waives all rights of redemption, if any, to the extent such rights may be lawfully waived, and Landlord, without notice to Tenant, may store Tenant’s effects and those of any person claiming through or under Tenant at the expense and risk of Tenant and, if Landlord so elects, may sell such effects at public auction or private sale and apply the net proceeds to the payment of all sums due to Landlord from Tenant, if any, and pay over the balance, if any, to Tenant.
default, Landlord shall also have the right to re-enter and take possession of all or any portion of the Premises and expel Tenant and those claiming through or under Tenant, either by summary proceedings or by any other action at law, in equity, or otherwise, with or without terminating this Lease, without being deemed guilty of trespass and without prejudice to any other remedies of Landlord for breach of this Lease. Tenant hereby waives all rights of redemption, if any, to the extent such rights may be lawfully waived, and Landlord, without notice to Tenant, may store Tenant’s effects and those of any person claiming through or under Tenant at the expense and risk of Tenant and, if Landlord so elects, may sell such effects at public auction or private sale and apply the net proceeds to the payment of all sums due to Landlord from Tenant, if any, and pay over the balance, if any, to Tenant.
9.2TENANT’S OBLIGATIONS AFTER TERMINATION.
In the event that this Lease is terminated under any of the provisions contained in Section 9.1 or shall be otherwise terminated for breach of any obligation of Tenant. Tenant covenants to pay forthwith to Landlord, as compensation, the excess of the total rent reserved for the residue of the Term over the rental value of the Premises for said residue of the Term. In calculating the rent reserved, there shall be included, in addition to the Annual Rent and all additional rent, the value of all other consideration agreed to be paid or performed by Tenant for said residue.
Tenant further covenants as an additional and cumulative obligation after any such termination to pay punctually to Landlord all the sums and perform all the obligations which Tenant covenants in this Lease to pay and to perform in the same manner and to the same extent and at the same time as if this Lease had not been terminated. In calculating the amounts to be paid by Tenant under the next foregoing covenant, Tenant shall be credited with any amount paid to Landlord as compensation as provided in the first sentence of this Section 9.2 and also with the net proceeds of any rents obtained by Landlord by reletting the Premises, after deducting all Landlord’s expenses in connection with such reletting, including, without implied limitation, all repossession costs, brokerage commissions, fees for legal services and expenses of preparing the Premises for such reletting, it being agreed by Tenant that Landlord may (i) relet the Premises or any part or parts thereof for a term or terms which may at Landlord’s option be equal to or less than or exceed the period which would otherwise have constituted the balance of the Term and may grant such concessions and free rent as Landlord in its sole judgment considers advisable or necessary to relet the same, (ii) make such alterations, repairs and decorations in the Premises as Landlord in its sole judgment considers advisable or necessary to relet the same, and (iii) keep the Premises vacant unless and until Landlord is able to rent the Premises to a tenant which is at least as desirable and financially responsible as Tenant is on the date of this Lease, on terms not less favorable to Landlord than those of this Lease. No action of Landlord in accordance with the foregoing or failure to relet or to collect rent under reletting shall operate or be construed to release or reduce Tenant’s liability as aforesaid.
Nothing contained in this Lease shall, however, limit or prejudice the right of Landlord to prove and obtain in proceedings for bankruptcy or insolvency by reason of the termination of this Lease an amount equal to the maximum allowed by any statute or rule of law in effect at the time when, and governing the proceedings in which, the damages are to be proved, whether or not the amount be  greater, equal to or less than the amount of the loss or damages referred to above.
greater, equal to or less than the amount of the loss or damages referred to above.
ARTICLE X - MISCELLANEOUS
10.1NOTICE OF LEASE.
Tenant agrees that it will not record this Lease. Upon request of either party, both parties shall execute and deliver, after the Term begins, a short form of this Lease in form appropriate for recording or registration, and if this Lease is terminated before the Term expires, an instrument in such form acknowledging the date of termination. All fees and recordation and transfer taxes in connection with any such recording of a short form of this Lease shall be paid by the party recording such instrument.
10.2RELOCATION.

 Landlord reserves the right to relocate the Premises to comparable space within the Building or elsewhere within the project in which the Building is located by giving Tenant prior written notice of such intention to relocate. If within 90 days after receipt of such notice Landlord and Tenant have not agreed on the space to which the Premises are to be relocated and the timing of such relocation, Landlord may, without further liability or obligation, by written notice terminate this Lease on that date which is 90 days after delivery of such notice. If Landlord and Tenant do so agree, then effective on the date of such relocation, this Lease shall be amended by deleting the description of the original Premises and substituting therefor a description of such comparable space. Landlord agrees to pay the reasonable costs of moving Tenant to such other space.
Landlord reserves the right to relocate the Premises to comparable space within the Building or elsewhere within the project in which the Building is located by giving Tenant prior written notice of such intention to relocate. If within 90 days after receipt of such notice Landlord and Tenant have not agreed on the space to which the Premises are to be relocated and the timing of such relocation, Landlord may, without further liability or obligation, by written notice terminate this Lease on that date which is 90 days after delivery of such notice. If Landlord and Tenant do so agree, then effective on the date of such relocation, this Lease shall be amended by deleting the description of the original Premises and substituting therefor a description of such comparable space. Landlord agrees to pay the reasonable costs of moving Tenant to such other space.
10.3NOTICES FROM ONE PARTY TO THE OTHER.
All notices, consents, approvals and other communications required or permitted hereunder shall be in writing and addressed, if to the Tenant, at Tenants Address or such other address as Tenant shall have last designated by notice in writing to Landlord and, if to Landlord, at Landlord’s Address or such other address as Landlord shall have last designated by notice in writing to Tenant. Any notice shall have been deemed duly given when personally delivered to such address by hand or when delivered overnight by a nationally recognized air courier.
10.4BIND AND INURE.
The obligations of this Lease shall run with the land, and this Lease shall be binding upon and inure to the benefit of the parties hereto and their respective successors and assigns, except that the Landlord named herein and each successive owner or ground lessee of the Building and Lot shall be liable only for the obligations accruing during the period of its ownership of the Building and Lot or a ground leasehold estate therein. The obligations of Landlord shall be binding upon, and recourse shall be limited solely to Landlord’s leasehold interest in the Building and Lot. No individual partner, member, trustee, stockholder, officer, director, employee or beneficiary of Landlord shall be personally liable under this Lease, and Tenant shall look solely to Landlord’s interest in the Building and Lot as aforesaid in pursuit of its remedies upon an event of default by Landlord hereunder, and the assets of the individual partners, members, trustees, stockholders, officers, employees or beneficiaries of Landlord shall not be subject to levy, execution or other enforcement procedure for the satisfaction of the remedies of Tenant. Notwithstanding anything herein to the contrary, if any provision of this Lease obligates Landlord not to unreasonably withhold or delay its consent or approval, an action for declaratory judgment or specific performance shall be Tenant’s sole right and remedy in any dispute as to whether Landlord has violated  such obligation.
such obligation.
10.5NO SURRENDER.
The delivery of keys to any employee of Landlord or to Landlord’s agent or any employee thereof shall not operate as a termination of this Lease or a surrender of the Premises.
10.6NO WAIVER, ETC.
The failure of Landlord or of Tenant to seek redress for violation of, or to insist upon the strict performance of, any covenant or condition of this Lease or, with respect to such failure of Landlord, any of the Rules and Regulations referred to in Section 6.1.4., whether heretofore or hereafter adopted by Landlord, shall not be deemed a waiver of such violation nor prevent a subsequent act, which would have originally constituted a violation, from having all the force and effect of an original violation, nor shall the failure of Landlord to enforce any of said Rules and Regulations against any other tenant in the Building be deemed a waiver of any such Rules or Regulations. The receipt by Landlord of Annual Rent or additional rent with knowledge of the breach of any covenant of this Lease shall not be deemed a waiver of such breach by Landlord, unless such waiver be in writing and signed by Landlord. No consent or waiver, express or implied, by Landlord or Tenant to or of any breach of any agreement or duty shall be construed as a waiver or consent to or of any other breach of the same or any other agreement or duty.
10.7NO ACCORD AND SATISFACTION.
 No acceptance by Landlord of a lesser sum than the Annual Rent and additional rent then due shall be deemed to be other than on account of the earliest installment of such rent due, nor shall any endorsement or statement on any check or any letter accompanying any check or payment as rent be deemed as accord and satisfaction, and Landlord may accept such check or payment without prejudice to Landlord’s right to recover the balance of such installment or pursue any other remedy in this Lease provided.
No acceptance by Landlord of a lesser sum than the Annual Rent and additional rent then due shall be deemed to be other than on account of the earliest installment of such rent due, nor shall any endorsement or statement on any check or any letter accompanying any check or payment as rent be deemed as accord and satisfaction, and Landlord may accept such check or payment without prejudice to Landlord’s right to recover the balance of such installment or pursue any other remedy in this Lease provided.
10.8CUMULATIVE REMEDIES.
The specific remedies to which Landlord may resort under the terms of this Lease are cumulative and are not intended to be exclusive of any other remedies or means of redress to which it may be lawfully entitled in case of any breach or threatened breach by Tenant of any provisions of this Lease. In addition to the other remedies provided in this Lease, Landlord and Tenant shall each be entitled to the restraint by injunction of the violation or attempted or threatened violation of any of the covenants, conditions or provisions of this Lease or to a decree compelling specific performance of any such covenants, conditions or provisions.
10.9RIGHT TO CURE.
If Tenant shall at any time default beyond the applicable notice and cure period in the performance of any obligation under this Lease, Landlord shall have the right, but shall not be obligated, to enter upon the Premises and to perform such obligation, notwithstanding the fact that no specific provision for such substituted performance by Landlord is made in this Lease with respect to such default. In performing such obligation, Landlord may make any payment of money or perform any other act. All sums so paid by Landlord (together with interest at the Default Rate, and all necessary incidental costs and expenses in connection with the performance of any such act by Landlord) shall be deemed to be additional rent under this Lease and shall be payable to Landlord immediately on demand. Landlord may exercise the foregoing rights without waiving any other of its rights or releasing Tenant from any of its obligations under this Lease.
10.10ESTOPPEL CERTIFICATE.
Tenant agrees, from time to time, upon not less than 15 days’ prior written request by Landlord,  to execute, acknowledge and deliver to Landlord a statement in writing certifying: that this Lease is unmodified and in full force and effect; that, Tenant has no defenses, offsets or counterclaims against its obligations to pay the Annual Rent and additional rent and no defenses against Tenant’s obligation to perform its other covenants under this Lease; that, there are no uncured defaults of Landlord or Tenant under this Lease (or, if there have been modifications, that this Lease is in full force and effect as modified and stating the modifications, and, if there are any defenses, offsets, counterclaims or defaults, setting them forth in reasonable detail); and the dates to which the Annual Rent, additional rent and other charges have been paid and such other matters or facts as reasonably requested by Landlord. Any such statement delivered pursuant to this Section 10.10 shall be in the form attached hereto as Exhibit G or such other form as may reasonably be requested by Landlord or any prospective purchaser of Landlord’s interests under this Lease or holder of a mortgage upon the-premises which include the Premises or any prospective assignees of any such holder and may be relied upon by any such prospective purchaser, holder of a mortgage or assignee thereof.
to execute, acknowledge and deliver to Landlord a statement in writing certifying: that this Lease is unmodified and in full force and effect; that, Tenant has no defenses, offsets or counterclaims against its obligations to pay the Annual Rent and additional rent and no defenses against Tenant’s obligation to perform its other covenants under this Lease; that, there are no uncured defaults of Landlord or Tenant under this Lease (or, if there have been modifications, that this Lease is in full force and effect as modified and stating the modifications, and, if there are any defenses, offsets, counterclaims or defaults, setting them forth in reasonable detail); and the dates to which the Annual Rent, additional rent and other charges have been paid and such other matters or facts as reasonably requested by Landlord. Any such statement delivered pursuant to this Section 10.10 shall be in the form attached hereto as Exhibit G or such other form as may reasonably be requested by Landlord or any prospective purchaser of Landlord’s interests under this Lease or holder of a mortgage upon the-premises which include the Premises or any prospective assignees of any such holder and may be relied upon by any such prospective purchaser, holder of a mortgage or assignee thereof.
10.11WAIVER OF SUBROGATION.
Any insurance carried by either party with respect to the Premises and property therein or occurrences thereon shall include a clause or endorsement denying to the insurer rights of subrogation against the other party to the extent rights have been waived by the insured prior to occurrences of injury or loss. Each party, notwithstanding any provisions of this Lease to the contrary, hereby waives any rights of recovery against the other for _injury or loss due to hazards covered by insurance containing such clause or endorsement to the extent of the indemnification received thereunder or to the extent of the indemnification such party would have received under such insurance if such party had maintained all insurance that it is required to maintain hereunder.
10.12

 INTENTIONALLY OMITTED.
INTENTIONALLY OMITTED.
10.13FORCE MAJEURE.
If either party shall be delayed in performing any obligation under this Lease for any of the reasons set forth below, except any obligation to pay Annual Rent or additional rent or any other sums of money payable hereunder, the time for such performance shall be extended by a period of time equal to such delay, and the party shall not be deemed to be in default where such delays or defaults are due to any one or more of the following (herein referred to as “Force Majeure”): war; insurrection; civil commotion; unusually severe weather; terrorism; strikes; lock-outs; riots; floods; earthquakes; fires; casualties; acts of God; epidemics; quarantine restrictions; freight embargoes; reasonably unforeseeable labor, equipment or material shortages; acts or failure to act of Montgomery County or any other public or governmental agencies or entity; continued illegal occupancy of persons in possession of the Lot or Building or any other reasonable cause relating to this Lease beyond the control or without the fault of the party claiming an extension of time to perform.
10.14BROKERAGE
Tenant represents and warrants that it has dealt with no broker in connection with this transaction other than Cushman & Wakefield of Maryland, Inc. and Scheer Partners, Inc., and agrees to defend, with counsel approved by Landlord, indemnify and save Landlord harmless from and against any and all cost, expense or liability for any compensation, commissions or charges claimed by a broker or agent, other than Cushman & Wakefield of Maryland, Inc. and Scheer Partners, Inc., with respect to Tenant’s dealings in connection with this Lease.
 10.15SUBMISSION NOT AN OFFER.
10.15SUBMISSION NOT AN OFFER.
The submission of a draft of this Lease or a summary of some or all of its provisions does not constitute an offer to lease or demise the Premises, it being understood and agreed that neither Landlord nor Tenant shall be legally bound with respect to the leasing of the Premises unless and until this Lease has been executed by both Landlord and Tenant and a fully executed copy has been delivered to each of them.
10.16 |
REPRESENTATIONS OF LANDLORD. |
Neither Landlord nor Landlord’s agents or brokers have made any representations or promises with respect to the Premises, the Building, the parking facilities, the Lot, or any other portions of the project except as herein expressly set forth. No representation is made regarding the suitability of the premises for Tenant’s particular use or the compliance of Tenants use with applicable Laws, regulations and restrictions set forth in the applicable land records. All reliance with respect to any representations or promises is based solely on those contained herein. No rights, easements, or licenses are acquired by Tenant under this Lease by implication or otherwise except as, and unless, expressly set forth in this Lease.
10.17NO MERGER OF TITLE.
 There shall be no merger of the leasehold estate created by this Lease with the fee estate in the Lot by reason of the fact that the same person or entity may own or hold (i) the leasehold estate created by this Lease or any interest in such leasehold estate and (ii) the fee estate in the Lot or any interest in such fee estate; and no such merger shall occur unless and until al’ persons, including Tenant, having any interest in (i) the leasehold estate created by this Lease or (ii) the fee estate in the Lot, shall join in a written instrument affecting such merger and shall duly record the same.
There shall be no merger of the leasehold estate created by this Lease with the fee estate in the Lot by reason of the fact that the same person or entity may own or hold (i) the leasehold estate created by this Lease or any interest in such leasehold estate and (ii) the fee estate in the Lot or any interest in such fee estate; and no such merger shall occur unless and until al’ persons, including Tenant, having any interest in (i) the leasehold estate created by this Lease or (ii) the fee estate in the Lot, shall join in a written instrument affecting such merger and shall duly record the same.
10.18WAIVER OF TREAL BY JURY.
THE PARTIES HERETO SHALL, AND THEY HEREBY DO, WAIVE TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM BROUGHT BY EITHER OF THEM AGAINST THE OTHER INVOLVING ANY MATTERS WHATSOEVER ARISING OUT OF OR IN ANY WAY CONNECTED WITH THIS LEASE, THE RELATIONSHIP OF LANDLORD AND TENANT, TENANT’S USE AND OCCUPANCY OF THE PREMISES AND/OR ANY CLAIM OF INJURY OR DAMAGE. IN THE EVENT LANDLORD COMMENCES ANY PROCEEDINGS FOR NONPAYMENT OF ANY AMOUNT DUE PURSUANT TO THIS LEASE, TENANT AGREES NOT TO INTERPOSE ANY COUNTER CLAIM (OTHER THAN MANDATORY COUNTERCLAIMS) OF WHATEVER NATURE OR DESCRIPTION IN ANY SUCH PROCEEDINGS. THIS SHALL NOT, HOWEVER, BE CONSTRUED AS A WAIVER OF TENANT’S RIGHT TO ASSERT ANY SUCH CLAIM IN ANY SEPARATE ACTION OR ACTIONS BROUGHT BY TENANT.
10.19HAZARDOUS MATERIALS.
As used in this Lease, the term “Hazardous Materials” means hazardous wastes,” as defined by the Resource Conservation and Recovery Act of 1976, 42 U.S.C.
SS 6901 et seq., as amended from time to time and the regulations adopted and publications promulgated pursuant to said Act; (ii) “hazardous substances,” as defined by the Comprehensive Environmental Response, Compensation and Liability Act of 1 980, 42 U.S.C. SS 9061 et seq., as amended from time to time and the regulations adopted and publications promulgated pursuant to said Act; (iii) “toxic substances,” as defined by the Toxic Substances Control Act, 15 U.S.C. SS 2601 et seq., as amended from time to time and the regulations adopted and publications promulgated pursuant to said Act; (iv) “Hazardous Materials,” as defined by the Hazardous Materials Transportation Act, 49 U.S.C. SS 1802, et seq., as amended from time to time and the regulations adopted and publications promulgated pursuant to said Act; (v) oil or other petroleum products; (vi) any material, waste or substance which is designated as a “hazardous substance” pursuant to Section 31 1 of the Clean Water Act, 33 U.S.C. SS 1251, et seq., or listed pursuant to Section 307 of the Clean Water Act, 33 U.S.C. SS 1317, et seq.; (vii) chlorofluorocarbons, radioactive materials, asbestos in any form that is or could become friable, urea formaldehyde, polychlorinated biphenyls and radon gas; (viii) any other chemicals, biological agents, microbes, viruses, bacteria, materials or substances defined as or included in the definitions of “hazardous substances, “ “hazardous wastes,” “restricted hazardous wastes,” “toxic substances,” “toxic pollutants,” “bio-hazard,” “biological waste,” “medical waste, “ or words of similar import, under any applicable federal, state, or local law or regulation; or (ix) any other flammable, combustible, or explosive liquid or material. “Hazardous Materials” does not include products normally used in operating and maintaining office and classroom space, including cleaning compounds and the like provided such products are sold and used for their intended purpose in accordance with applicable Laws and regulations.
Tenant shall not cause or permit any Hazardous Materials to be generated, produced, brought upon, used, stored, treated, discharged, released, spilled, or disposed of in, on, under or about the Premises, the Building or the Lot by Tenant or Tenant’s Invitees; provided, that Tenant may use and store in the Premises reasonable quantities of standard office supply products and products of the type and quantities identified on Exhibit I hereto used in the conduct of the Permitted Uses provided that (i) Tenant conducts its business according to prudent industry practices, (ii) the use and presence of Hazardous Materials is strictly and property monitored and reported according to all Laws then applicable to the generation, use, storage, treatment, release, spill, discharge or disposal of Hazardous Materials (sometimes herein referred to as ‘Environmental Laws”), and (iii) Tenant complies with all applicable Laws pertaining to the generation, use, storage, treatment, release, spill, discharge or disposal thereof including, without limitation, all such Environmental Laws. As a material inducement to Landlord to allow Tenant to use hazardous material in connection with its business on the Premises, Tenant has delivered to Landlord the list attached hereto as Exhibit I identifying each type and quantities of hazardous material to be brought upon, kept, used, stored, handled, treated, generated on, or released, discharged or disposed of from the Premises and setting forth any and all governmental approvals or permits required in connection with the use, storage, treatment, generation, release or disposal of such Hazardous Materials on or from the Premises (“Hazardous Materials List”). Tenant shall deliver to Landlord an updated Hazardous Materials List at least once a year and shall also deliver an updated list before any new hazardous material is brought onto, kept, used, stored, treated, generated on, or released or disposed of from, the Premises. Tenant shall deliver to Landlord true and correct copies of the following documents (the “HazMat Documents”) relating to the use, storage, treatment, generation, release or disposal of Hazardous Materials prior to the Commencement Date, or if unavailable at that time, concurrent with the receipt from or submission to a governmental authority: permits; approvals; reports and correspondence; storage and management plans, notice of violations of any Law; plans relating to the installation of any storage tanks to be installed in the Premises (provided, said installation of tanks shall only be permitted after Landlord has given Tenant its written consent to do so, which consent may be withheld in Landlord’s sole and absolute discretion); all closure plans or any other documents required by any and all federal, state and local governmental authorities for any storage tanks installed in or on the Premises for the closure of any such tanks; and a Surrender Plan (to the extent surrender in accordance with Section 6.1 .2 cannot be accomplished in 3 months). Tenant is not required, however, to provide Landlord with any portion(s) of the HazMat Documents containing information of a proprietary nature which, in and of themselves, do not contain a reference to any Hazardous Materials or hazardous activities.
Without in any manner limiting Landlord’s rights and Tenant’s obligations under any other indemnity set forth in this Lease, Tenant shall indemnify, defend, with counsel reasonably approved by Landlord, and hold Landlord harmless from and against any and all actions (including, without limitation, remedial or enforcement actions of any kind, administrative or judicial proceedings, and orders or judgments arising out of or resulting costs, claims, damages (including, without limitation, punitive damages), expenses (including, without limitation, reasonable attorneys’, consultants’ and experts’ fees, court costs and amounts paid in settlement of any claims or actions), fines, forfeitures or other civil, administrative or criminal penalties, injunctive or other relief (whether or not based upon personal injury, property damage, contamination of, or adverse effects upon, the environment, water tables or natural resources), liabilities or losses arising from (i) a breach of this Section 10.18 or Section 6, 1.2 by Tenant or Tenant’s Invitees, or (ii) the generation, use, storage, treatment, discharge, release, spill or disposal of Hazardous Materials by Tenant or Tenant’s Invitees.
Landlord shall have the right to conduct annual tests of the Premises to determine whether any contamination of the Premises, Building or the Lot has occurred as a result of Tenant’s use. Tenant shall be required to pay the cost of such annual test; provided, however, that if Tenant conducts its own tests of the Building or the Lot using third party contractors and test procedures acceptable to Landlord which  tests are certified to Landlord, Landlord shall accept such tests in lieu of the annual tests to be paid for by Tenant. In addition, at any time, and from time to time, prior to the expiration or earlier termination of the Term, Landlord shall have the right to conduct appropriate tests of the Premises, the Building and Lot to determine if contamination has occurred as a result of Tenant’s use of the Premises. In connection with such testing, upon the request of Landlord, Tenant shaft deliver to Landlord or its consultant such nonproprietary information concerning the use of Hazardous Materials in or about the Premises by Tenant or any Tenant Invitee. If contamination has occurred for which Tenant is liable under this Section, Tenant shall pay all costs to conduct such tests. If no such contamination is found, Landlord shall pay the costs of such tests, subject to Section 4.2. Landlord shall provide Tenant with a copy of all third party, nonconfidential reports and tests of the Premises made by or on behalf of Landlord during the Term without representation or warranty and subject to a confidentiality agreement. Tenant shall, at its sole cost and expense, promptly and satisfactorily remediate any environmental conditions identified by such testing in accordance with all Environmental Laws. Landlord’s receipt of or satisfaction with any environmental assessment in no way waives any fights which Landlord may have against Tenant.
tests are certified to Landlord, Landlord shall accept such tests in lieu of the annual tests to be paid for by Tenant. In addition, at any time, and from time to time, prior to the expiration or earlier termination of the Term, Landlord shall have the right to conduct appropriate tests of the Premises, the Building and Lot to determine if contamination has occurred as a result of Tenant’s use of the Premises. In connection with such testing, upon the request of Landlord, Tenant shaft deliver to Landlord or its consultant such nonproprietary information concerning the use of Hazardous Materials in or about the Premises by Tenant or any Tenant Invitee. If contamination has occurred for which Tenant is liable under this Section, Tenant shall pay all costs to conduct such tests. If no such contamination is found, Landlord shall pay the costs of such tests, subject to Section 4.2. Landlord shall provide Tenant with a copy of all third party, nonconfidential reports and tests of the Premises made by or on behalf of Landlord during the Term without representation or warranty and subject to a confidentiality agreement. Tenant shall, at its sole cost and expense, promptly and satisfactorily remediate any environmental conditions identified by such testing in accordance with all Environmental Laws. Landlord’s receipt of or satisfaction with any environmental assessment in no way waives any fights which Landlord may have against Tenant.
If tanks storing Hazardous Materials located on the Premises are used by Tenant or are hereafter placed on the Premises by Tenant, Tenant shall install, use, monitor, operate, maintain, upgrade and manage such storage tanks, maintain appropriate records, obtain and maintain appropriate insurance, implement reporting procedures, properly close any storage tanks, and take or cause to be taken all other actions necessary or required under applicable Laws, as such now exist or may hereafter be adopted or amended in connection with the installation, use, maintenance, management, operation, upgrading and closure of such storage tanks.
During any period of time after the expiration or earlier termination of this Lease required by Tenant or Landlord to complete the removal from the Premises, Building or Lot of any Hazardous Materials (including, without limitation, the release and termination of any licenses or permits restricting the use of the Premises and the completion of the approved Surrender Plan), Tenant shall continue to pay the full Annual Rent and Additional Rent in accordance with this Lease for any portion of the Premises not relet by Landlord in Landlord’s sole discretion, which Annual Rent and Additional Rent shall be prorated daily. In the event that Hazardous Materials are discovered upon, in, or under the Premises, and any governmental agency or entity having jurisdiction over the Premises requires the removal of such Hazardous Materials, Tenant shall be responsible for removing those Hazardous Materials arising out of or related to the use or occupancy of the Premises by Tenant or the actions or omissions of Tenant or Tenant’s Invitees. Notwithstanding the foregoing, Tenant shall not take any remedial action in or about the Premises, the Building or the land on which the Building is located without first notifying Landlord of Tenant’s intention to do so and affording Landlord the opportunity to protect Landlord’s interest with respect thereto.
Tenant immediately shall notify Landlord in writing of: (i) any spill, release, discharge or disposal of any Hazardous Material in, on or under the Premises, the Building, or the Lot or any portion thereof, (ii) any enforcement, cleanup, removal or other governmental or regulatory action instituted, contemplated, or threatened (if Tenant has notice thereof) pursuant to any applicable laws, rules or regulations relating to Hazardous Materials; (iii) any claim made or threatened by any person against Tenant, the Premises, the Building or the land on which the Building is located relating to damage, contribution, cost, recovery, compensation, loss or injury resulting from or claimed to result from any Hazardous Materials; and (iv) any reports made to any governmental agency or entity arising out of or in connection with any Hazardous Materials in, on, under, or about or removed from the Premises, the Building or the land on which the Building is located including any complaints, notices, warnings, reports or asserted violations in connection therewith. Tenant also shall supply to Landlord as promptly as possible, and in any event within 5 business days after Tenant first receives or sends the same, copies of all claims, reports, complaints, notices, warnings or asserted violations relating in any way to the Building, the Lot or the Premises or Tenant’s use or occupancy thereof.
10.20PARKING.
Tenant may under this Lease and the Campus CCRs use no more than 3.5 parking spaces for each 1,000 rentable square feet of floor area in the Premises in such location or locations upon the Campus as may be designated from time to time by Landlord in accordance With the Ground Lease and Campus CCRs, which, shall be in common with other tenants and subject to the rights of other tenants, on a first-come, first-serve basis; provided all such parking shall be subject to the terms of the Ground Lease and Campus CCRs and Tenant shall not incur any charge for such parking. Use of all such parking spaces shall be in compliance with all rules, regulations, and restrictions set forth by (a) Landlord, (b) all governmental authorities, and (c) the Campus CCRs. Tenant shall reimburse Landlord for the expense of towing any vehicle parked in violation of such rules, regulations, and restrictions where the violation has resulted from the improper use of such space by an employee, licensee, invitee or guest of Tenant; and shall pay to Landlord, upon written demand therefor, a penalty equal to twice the penalty imposed in accordance with the Campus CCRs (currently $25) per violation of such rules, regulations, and restrictions by any identified employee, invitee, licensee or guest of the Tenant. Landlord reserves the right, from time to time, without notice or liability to Tenant to (i) install gates to control access to the parking facility or (ii) to alter or relocate the parking facility, including the right to relocate the parking facility on or off the Lot. Landlord shall not have the right to charge market rates for parking in the event a parking structure is erected for the benefit of the Building and all parking for Tenant shall remain free of charge.
10.21SURVIVAL.
Tenant’s liability for amounts which become due under this Lease and the terms and conditions of the covenants and indemnities set forth in Sections 3.1, 6.1 7, 10.13 and 10.18 and the provisions of Landlord’s covenants and indemnities under Sections 5.1.7 shall remain effective after the expiration or sooner termination of this Lease, for the period of any applicable statutes of limitations.
10.22APPLICABLE LAW AND CONSTRUCTION.
This Lease shall be governed by and construed in accordance with the laws of the State of Maryland (not including its choice of law rules). If any term, covenant, condition or provision of this Lease or the application thereof to any person or circumstances shall be declared invalid or unenforceable by the final ruling of a court of competent jurisdiction having final review, the remaining terms, covenants, conditions and provisions of this Lease and their application to persons or circumstances shall not be affected thereby and shall continue to be enforced and recognized as valid agreements of the parties, and in the place of such invalid or unenforceable provision, there shall be substituted a like, but valid and enforceable, provision which comports to the findings of the aforesaid court and most nearly accomplishes the original intention of the parties.
This Lease, including the exhibits and riders hereto, contains the entire agreement of the parties hereto.
There are no oral or written agreements between Landlord and Tenant affecting this Lease. This Lease may be amended, and the provisions hereof may be waived or modified, only by instruments in writing executed by Landlord and Tenant.
The titles of the several Articles and Sections contained herein are for convenience only and shall not be considered in construing this Lease.
Unless repugnant to the context, the words “Landlord” and “Tenant” appearing in this Lease shall be construed to mean those named above and their respective heirs, executors, administrators, successors and assigns, and those claiming through or under them respectively. If there be more than one tenant, the obligations imposed by this Lease upon Tenant shall be joint and several,
The term “Business Day” or “business day” shall mean any day other than a Saturday, Sunday or a day on which the United States Government offices in the State of Maryland are closed on account of holiday (“Legal Holiday”). Any expression of a time period measured by days which does not expressly provide that it refers to business days shall be deemed to be calendar days. Any time a time period will expire on a day which is a Saturday, Sunday, or Legal Holiday, or if such time period is measured by business days, not a business day, such time period shall be deemed to extend to the next day which is not such a day. 
10.23CONFIDENTIALITY.
The terms of this Lease are privileged and confidential, intended only for the use of Landlord and Tenant. Both parties shall keep the terms of this Lease and any negotiations or agreements related hereto strictly confidential and shall not disclose the same to any other persons or entities excepting only (i) attorneys agents or employees involved in this transaction who have agreed to keep all such information confidential as herein provided (ii) as mutually and specifically agreed otherwise, (iii) as required by order of any court or governmental authority with jurisdiction or by any regulatory or governmental agencies, including the Tenant’s filings with the Securities and Exchange Commission, or (iv) attorneys, agents or employees of the ground lessor under the Ground Lease and all lenders, investors or purchasers involved in any transaction relating to the sale or financing of the Land and Building or any interest therein or any interest in Landlord.
10.24METHOD OF MEASUREMENT.
Rentable Floor Area of the Premises and the Building was measured in accordance with a modified Standard Method of Measuring Floor Area in Office Buildings, Building Owners and Managers Association International (1 996), and its guidelines (“BOMA”) provided that the Rentable Floor Area of the Premises shall be equal to the product of (1) the “useable square footage of the Premises” measured in accordance with BOMA and (2) 1.15. If Landlord or Tenant so elects by written notice to the other delivered not later than the -Commencement Date, Landlord’s Architect shall prepare (at the expense of the party requesting such measurement) a measurement of the Premises, and if the useable square footage of the Premises is determined to be more or less than the useable square footage that was used by Landlord in determining the Rentable Floor Area of Tenant’s Space as set forth in Section 1 .1 hereof, then in either such case this Lease shall be amended in writing, adjusting for all purposes hereunder the specified Rentable Floor Area of Tenant’s Space and the calculation of Annual Rent in accordance with Section 1 .1 and Section 4.1 of this Lease, and such amendment shall be reflected in the lease Commencement Date Agreement. Notwithstanding anything herein to the contrary, except in the case of a remeasurement described above timely exercised by Landlord or Tenant and the amendment of this Lease to reflect the results of such remeasurement as herein provided, Landlord and Tenant agree that for all purposes under this Lease the Rentable Floor Area of the Premises is 5,365 square feet.
10.25APPROVALS.
If Landlord approves plans, specifications, contracts, contractors, sublessees or assignees, subleases or assignments for any purpose under this Lease, such approval shall be solely for the purpose of confirming that the plans, specifications, contract, contractor, sublease, assignment, sublessee or assignee complies or fails to comply with the terms and conditions of this Lease and the Rules and Regulations of Landlord applicable thereto (and, in the case of contractors, assignees or sublessees, that their business, reputation, credit history and financial condition are or are not satisfactory to Landlord).
In no event shall such approval constitute approval of the plans, specifications, contractors, contract, sublessees or assignees, subleases or assignments for any other reason or purpose and in no event shall Landlord have any liability or obligation for or under the same.
10.26GROUND LEASE PROVISIONS.
Tenant acknowledges that the Ground Lease contains the following provisions which are incorporated herein by reference (as used in the following three paragraphs only, “Landlord” shall mean JHU as ground landlord under the Ground Lease, “Tenant” shall mean MCC3, LLC, as ground tenant under the Ground Lease, “Subtenants” shall mean tenants of the Building, “this Lease” shall mean the Ground Lease, “Sublease” shall mean a lease of space in the Building, “MCC Land” shall mean the Montgomery County Campus of JHU, and the “Memorandum of Lease” shall mean the memorandum of ground lease relating to the Ground Lease, which is to be filed among the land records of Montgomery County, Maryland):
“14.2 Permitted Subleases. Tenant shall give Landlord ten (10) days’ advance written notice of the identity of any proposed Subtenant before entering into a Sublease with such Subtenant. Tenant shall have the right to sublease all or any portions of the Improvements without Landlord’s prior approval to one or more Subtenants provided that (i) the Subtenant is not a Prohibited Person and (ii) the uses permitted under the Sublease to such Subtenant comply with the requirements of applicable zoning and land use ordinances and regulations, covenants, conditions, and restrictions of record, including, without limitation, the· Estoppel and the Campus CCRs (collectively, “Land Use Restrictions”) as such Land Use Restrictions are in effect as of the date hereof, unless subsequent land use ordinances and regulations are more restrictive, in which case the more restrictive land use ordinances and regulations shall become applicable to the uses permitted under this Lease, subject to permitted exemptions and grandfather provisions allowing the continuation of existing uses. ‘f after the date hereof, land use ordinances and regulations become more permissive as to permitted uses, such more permissive land use ordinances and regulations shall not be applicable to the uses permitted under this Lease to the extent they are more permissive until Landlord and Tenant shall have specifically agreed in writing that the more permissive land use ordinances and regulations will be applicable. Tenant shall deliver a photocopy of each executed Sublease to Landlord within thirty (30) business days following execution of the same.
14.3Landlord Approval. If Tenant desires to determine whether Landlord would object  to a Subtenant because Landlord believes that the Subtenant is a Prohibited Person, Tenant may, if it so elects, give Landlord prior written notice of the identity of the Person with which Tenant contemplates entering into a Sublease together with comprehensive information regarding such Person in reasonable detail sufficient to assess whether such Person is a Prohibited Person in accordance with this Article XIV. Tenant’s notice to Landlord shall, on the face of the envelope and n the top of the first page of the notice, state the following in all capital letters:
to a Subtenant because Landlord believes that the Subtenant is a Prohibited Person, Tenant may, if it so elects, give Landlord prior written notice of the identity of the Person with which Tenant contemplates entering into a Sublease together with comprehensive information regarding such Person in reasonable detail sufficient to assess whether such Person is a Prohibited Person in accordance with this Article XIV. Tenant’s notice to Landlord shall, on the face of the envelope and n the top of the first page of the notice, state the following in all capital letters:
“PROHIBITED PERSON NOTICE UNDER SECTION 14.3 OF THE PHASE GROUND LEASE, MONTGOMERY COUNTY CAMPUS. APPROVAL DEEMED GIVEN IF OBJECTION IS NOT MADE IN WRITING WITHIN TEN (10) BUSINESS DAYS OF RECEIPT OF THIS NOTICE. Landlord will then advise Tenant in writing within ten (10) business days as to whether it objects to the proposed Subtenant in question, stating in reasonable detail the grounds for Landlord’s objection. Each Subtenant under a proposed Sublease delivered to Landlord shall be deemed approved unless Landlord advises Tenant of Landlord’s objection to the Subtenant under such Sublease within the aforesaid period following receipt of the same. If Landlord timely objects in writing to any proposed Subtenant as herein provided, and Tenant disagrees with Landlord’s objection, then representatives of Landlord and Tenant shall meet to discuss the reasons for Landlord’s objection and why Tenant believes such objection is not justified.
14.4Prohibited Persons. For purposes hereof, “Prohibited Person” shall mean a Person: (a) that is
generally known to the public to engage directly in: (i) the manufacture or sale of consumer products (such as, without limitation, alcoholic beverages, tobacco products, or weapons, but not including drugs sold over the counter or by medical prescription) recognized as hazardous to human health by federal or Maryland state governmental authorities, (ii) the publication, manufacture, sate, distribution, promotion or purveyance of pornographic material, or (iii) gambling or (b) who or which (including any member of an entity’s executive management in the course of employment) has been convicted within the ten (10) year period preceding the date of this Lease of any violation of law that constitutes a felony; or (c) which is a university or college (or any Affiliate thereof) other than Landlord or a college or university which is an Affiliate of Landlord; or (d) engaging in a mission likely to involve activities on the MCC Land which are themselves disruptive or a direct risk to the physical security of people or property on the MCC Land. Notwithstanding the foregoing, no federal, state or local governmental entity, agency or authority (other than a college or university) shall be a “Prohibited Person” for the purposes of this Lease. Sections 14.1, 14.2, 14.3, and 14.4 shall constitute covenants that run with the Land and shall be included in the Memorandum of Lease. Sections 14.2, 14.3 and 14.4 shall be incorporated into each and every Sublease and No disturbance and Attornment Agreement with respect to such Sublease. Each and every Sublease shall state that the use of the premises subject to such Sublease for any of the proscribed activities shall be a default under the Sublease and cause for eviction.
and cause for eviction.
ARTICLE - SECURITY DEPOSIT
Tenant’s Security Deposit in the amount set forth in Section 1 .1 shall, following receipt thereof by Landlord, be held by Landlord, as security, without interest, for and during the Term, which deposit shall be returned to Tenant following the termination of this Lease upon delivery of Landlord’s Statement for the final fiscal year or portion thereof within the Term, provided that all Annual Rent and additional rent due from Tenant has been paid and there exists no breach of any undertaking of Tenant. If a” or any part of the Security Deposit is applied to an obligation of Tenant hereunder, Tenant shall immediately upon request by Landlord restore the Security Deposit to its original amount. Tenant shall not have the right to call upon Landlord to apply all or any part of the Security Deposit to cure any default or fulfill any obligation of Tenant, but such use shall be solely in the discretion of Landlord. Upon any conveyance by Landlord of its interest under this Lease, the Security Deposit may be delivered by Landlord to Landlord’s grantee or transferee. Upon any such delivery, Tenant hereby releases Landlord herein named of any and all liability with respect to the Security Deposit, its application and return, and Tenant agrees to look solely to such grantee or transferee for the return of such security. It is further understood that this provision shall also apply to subsequent grantees and transferees.
Tenant shall have the right to deliver to Landlord an unconditional, irrevocable letter of credit (the “Letter of Credit”) in substitution for the cash Security Deposit, subject to the following terms and conditions. Such letter of credit shall be (a) in form and substance satisfactory to Landlord in its sole discretion; (b) at all times in the amount of the Security Deposit, and shall permit multiple draws; (c) issued by a commercial bank reasonably acceptable to Landlord from time to time and located in the Washington, D.C. metropolitan area; (d) made payable to, and expressly transferable and assignable at no charge by, the owner from time to time of the Building or, at Landlord’s option, the holder of any mortgage (which transfer/assignment shall be conditioned only upon the execution of a written document in connection therewith); (e) payable at sight upon presentment to a local branch of the issuer of a simple sight draft; (f) of a term not less than one year; and (g) at least 60 days prior to the then-current expiration date of such letter of credit either (1) renewed (or automatically and unconditionally extended) from time to time through the ninetieth (90th) day after the expiration of the Lease Term, or (2) replaced with cash in the amount of the Security Deposit. Notwithstanding anything in this Lease to the contrary, any cure or grace periods set forth in this Lease shall not apply to any of the foregoing, and, specifically, if Tenant fails to timely comply with the requirements of subsection (g) above, then Landlord shall have the right to immediately draw upon the letter of credit without notice to Tenant. Landlord may draw upon the whole or any part of the Letter of Credit in the event of any default by Tenant under the Lease and if any part of the amount so drawn is applied to an obligation of Tenant herein, Tenant shall forthwith restore the Letter of Credit to its original amount. Each Letter of Credit shall be issued by a commercial bank that has a credit rating with respect to certificates of deposit, short term deposits or commercial paper of at least P-2 (or equivalent) by Moody’s Investor Service, Inc., or at least A-2 (or equivalent) by Standard & Poor’s Corporation, and shall be otherwise acceptable to Landlord in its sole and absolute discretion.
If the issuer’s credit rating is reduced below P-2 (or equivalent) by Moody’s Investor Service, Inc., or below A-2 (or equivalent) by Standard & Poor’s Corporation, or if the financial condition of such issuer changes in any other materially adverse way, then Landlord shall have the right to require that Tenant obtain from a different issuer a substitute letter of credit that complies in all respects with the requirements of this Section, and Tenant’s failure to obtain such substitute letter of credit within 10 days following Landlord’s written demand therefor (with no other notice or cure or grace period being applicable thereto, notwithstanding anything in this Lease to the contrary) shall entitle Landlord to immediately draw upon the then existing Letter of Credit in whole or in part, without notice to Tenant. In the event the issuer of any Letter of Credit held by Landlord is placed into receivership or conservatorship by the Federal Deposit Insurance Corporation or any successor or similar entity, then, effective as of the date such receivership or conservatorship occurs, said Letter of Credit shall be deemed to not meet the requirements of this Section, and, within 10 days thereof, Tenant shall replace such Letter of Credit with other collateral acceptable to Landlord in its sole and absolute discretion (and Tenant’s failure to do so shall, notwithstanding anything in this Lease to the contrary, constitute an Event of Default for which there shall be no notice or grace or cure periods being applicable thereto other than the aforesaid ten (10) day period). Any failure or refusal of the issuer to honor the Letter of Credit shall be at Tenant’s sole risk and shall not relieve Tenant of its obligations hereunder with respect to the Security Deposit.
EXECUTED as a sealed instrument in two or more counterparts on the day and year first above written.
ATTEST: |
|
LANDLORD: |
|
|
|
|
|
|
|
MCC3,LLC |
|
|
|
By: |
Spaulding and Slye MCC3 LLC, Manager |
|
|
By: |
Spaulding and Slye Holdings LLC, Manager |
|
|
|
|
|
|
By: |
/s/ Marshall Durston |
|
|
Name: |
|
|
|
Title: |
|
|
|
|
|
ATTEST: |
|
TENANT: |
|
|
|
|
|
|
|
SYNTHETIC BIOLOGICS, INC. |
|
|
|
By: |
/s/ Jeffrey Riley |
|
|
Title: CEO |
|
Exhibit 10.19
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [*****], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
SECOND AMENDMENT TO LEASE
THIS SECOND AMENDMENT TO LEASE (“this Second Amendment”) is dated as of May 6, 2021 (“Effective Date”), by and between ARE-MARYLAND NO. 50, LLC, a Delaware limited liability company, having an address at 26 North Euclid Avenue, Pasadena, California 91101 (“Landlord”), and SYNTHETIC BIOLOGICS, INC., a Nevada corporation, having an address at Suite 270, 9605 Medical Center Drive, Rockville, Maryland 20850 (“Tenant”).
RECITALS
A.Landlord and Tenant have entered into a Lease dated April 14, 2015 (“Original Lease”), wherein Landlord’s indirect predecessor in interest, MCC3, LLC, a Delaware limited liability company, leased to Tenant premises containing approximately 5,365 rentable square feet located on the second floor of the Building at Suite 250, 9605 Medical Center Drive, Rockville, Maryland 20850, as more particularly described in the Original Lease (“Original Premises”).
B.Landlord and Tenant have entered into that certain First Amendment to Lease dated March 22, 2016 (“First Amendment”; together with the Original Lease, the “Lease”), wherein Landlord’s direct predecessor in interest, IPX Medical Center Drive Investors, LLC, a Delaware limited liability company (“Immediate Prior Landlord”), leased to Tenant an additional 4,998 rentable square feet of space located on the second floor of the Building (“Expansion Premises”; together with the Original Premises, the “Premises”). The Premises thus consists of a total of 10,363 rentable square feet.
C.By that certain Deed and Assignment and Assumption of Ground Lease dated February 19, 2020, Immediate Prior Landlord assigned to Landlord all of Immediate Prior Landlord’s right, title, and interest in, among other things, the Ground Lease (as defined therein).
D.Landlord and Tenant desire to amend the Lease, among other things, to extend the Term, adjust the Base Year, provide certain tenant improvement allowances, grant an option to expand the Premises, and grant the right to Tenant to extend the Term.
AGREEMENT
Now, therefore, in consideration of the foregoing Recitals, the mutual promises and conditions contained herein, and for other good and valuable consideration, the receipt and legal sufficiency of which are hereby acknowledged, Landlord and Tenant hereby agree that the Lease is amended as follows:
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 2 |
Suite 270, 9605 Medical Center Drive |
|
1.Definitions; Recitals. Terms used in this Second Amendment but not otherwise defined shall have the meanings set forth in the Lease. The Recitals form an integral part of this Second Amendment and are hereby incorporated by reference.
2.Extension of Term. The Term is scheduled to expire, unless earlier terminated in accordance with the terms and conditions of the Lease, on August 31, 2022. The Term of the Lease is hereby extended for a period of 63 months, beginning on September 1, 2022 and expiring, unless earlier terminated or extended in accordance with the terms and conditions of the Lease, on December 31, 2027 (“First Extension Term”).
a.Annual Rent for First Extension Term. The Annual Rent for the First Extension Term is set forth in the table below:
Period |
Annual Rent |
Monthly Installment |
[*****] |
ABATED |
ABATED |
12.1.2022 to 11.30.2023 |
$326,227.24 |
$27,185.60 |
12.1.2023 to 11.30.2024 |
$336,014.06 |
$28,001.17 |
12.1.2024 to 11.30.2025 |
$346,094.48 |
$28,841.21 |
12.1.2025 to 11.30.2026 |
$356,477.31 |
$29,706.44 |
12.1.2026 to 11.30.2027 |
$367,171,63 |
$30,597.64 |
12.1.2027 to 12.31.2027 |
$378,186.78! |
$31,515.56 |
…As long as no Event of Default then exists, Landlord shall abate the Annual Rent for the period [*****]. In the event of any termination of this Lease by Landlord based on an Event of Default by Tenant, the then unamortized portion of the Annual Rent that would have otherwise been due and payable during such period of abatement in the absence of such abatement shall immediately become due and payable and any remaining abatement of Annual Rent shall be of no further force or effect (it being understood that the abated Annual Rent shall be amortized over the First Extension Term, and that as of the date of termination of this Lease under Article IX hereof, any then unamortized portion of such abated Annual Rent shall be included in the calculation of the amounts due and payable by Tenant to Landlord under Article IX after such termination of this Lease.
…The Annual Rent figure is used only to illustrate the annualized amount for the designated time period.
b.Increase in Annual Rent Based on Use of Additional Tenant Improvement Allowance. Annual Rent shall be increased as of the date or dates on which Tenant uses the Additional Tenant Improvement Allowance pursuant to Section 5(bL2 of the Work Letter attached hereto as Exhibit B (such increase to be calculated based on the amount of the Additional Tenant Improvement Allowance used by Tenant, such amount to be amortized over the First Extension Term based on an interest rate of 8.5% per annum; the resulting amount so amortized shall be added to the monthly installments of Annual Rent). For the avoidance of doubt, the Additional Tenant Improvement Allowance shall not be subject to the adjustment in Annual Rent shown in the table above.
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 3 |
Suite 270, 9605 Medical Center Drive |
|
3.Base Year Adjustment. Effective as of January 1, 2022, the Base Year (as defined in Section 1.1 of the Lease) shall be changed from “Calendar year 2015” to “Calendar year 2022.” For the period [*****], Tenant shall not be obligated to pay to Landlord (a) Tenant’s Estimated Monthly Payments of Tenant’s Share of Operating Costs Escalations and (b) Tenant’s Estimated Monthly Real Estate Taxes of Tenant’s Share of Real Estate Tax Escalations. Beginning on [*****], Tenant shall recommence payment to Landlord of (i) Tenant’s Estimated Monthly Payments of Tenant’s Share of Operating Costs Escalations and (ii) Tenant’s Estimated Monthly Real Estate Taxes of Tenant’s Share of Real Estate Tax Escalations. Tenant shall make such payments in accordance with the terms and conditions of Sections 4.2 and 4.3 of the Lease.
4.Landlord’s Repair Work. Within 30 days after the Effective Date, Landlord shall at its expense perform the following work (“Repair Work”) in the Premises in a good and workmanlike manner:
a.Landlord shall engage an HVAC technician to professionally balance the HVAC system serving the Premises and repair or replace the thermostats that control the HVAC system. b. Landlord shall repair or replace the hot water heater serving the Premises (including the dishwasher). If replaced, the replacement shall be of like kind and quality.
Tenant shall provide Landlord and its agents and contractors access to the Premises within such 30 day period so as to allow them to complete the Repair Work in a timely and efficient manner. Tenant recognizes that portions of the Repair Work may entail noise and dust, and Tenant accepts any resulting inconvenience or disruptions to Tenant’s normal business activities at the Premises. Landlord will, however, use commercially reasonable efforts to minimize any such inconvenience or disruptions. The 30 day period within which to complete the Repair Work is subject in all cases to any delays resulting from Landlord’s inability to obtain replacement parts on a timely basis, delays in Landlord gaining access to the Premises that are attributable to Tenant, and any Force Majeure events.
5.Second Extension Term (Section 2.3). Section 2.3 of the Lease is hereby amended by deleting that Section in its entirety and replacing it with the following new Section 2.3:
2.3Option to Extend Term. Tenant shall have the right to extend the First Extension Term of the Lease upon the following terms and conditions:
2.3.1Extension Right. Tenant shall have the right (“Extension Right”) to extend the First Extension Term of the Lease for 5 years (“Second Extension Term”) on the same terms and conditions as the Lease (other than Annual Rent) by giving Landlord written notice of Tenant’s election to exercise the Extension Right at least 9 months prior, and no earlier than 12 months prior, to the expiration of the First Extension Term. If Tenant exercises the Extension Right, the Second Extension Term shall begin on January 1,2028 and, unless earlier terminated in accordance with the terms and conditions of this Lease, end on December 31, 2032.
2.3.2Annual Rent. Upon the commencement of the Second Extension Term, Annual Rent shall be payable at the Market Rate (as defined below).
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 4 |
Suite 270, 9605 Medical Center Drive |
|
Annual Rent shall thereafter be adjusted on each anniversary of the commencement of the Second Extension Term by a percentage as determined by Landlord and agreed to by Tenant at the time the Market Rate is determined. As used herein, “Market Rate” shall mean the annual base rental rate per square foot that a lessor of a leasehold premises comparable in location, size, design and improvements to the Premises would accept in comparable transactions taking into account all relevant factors, including all market concessions (such as allowances, commissions, and free rent) and the specific provisions of this Lease that will remain constant (with the exception of the Tl Allowance). If, on or before the date that is 60 days before the expiration of the First Extension Term, as applicable, the parties have not agreed in writing on the Market Rate and the rent escalations during such Second Extension Term after negotiating in good faith, Tenant may elect arbitration as described in Section 2.3.3 below. If Tenant does not elect such arbitration, Tenant shall be deemed to have waived any right to further extend the Term of the Lease and the Extension Right.
2.3.3 |
Arbitration. |
2.3.3.1Within 10 days of Tenant’s notice to Landlord of its election to arbitrate the Market Rate and escalations, each party shall deliver to the other a proposal containing the Market Rate and escalations that the submitting party believes to be correct (“Extension Proposal”). If either party fails to timely submit an Extension Proposal, the other party’s submitted proposal shall determine the Base Rent and escalations for the Second Extension Term. If both parties submit Extension Proposals, then Landlord and Tenant shall meet within 7 days after delivery of the last Extension Proposal and make a good faith attempt to mutually appoint a single Arbitrator (as defined below) to determine the Market Rate and escalations. If Landlord and Tenant are unable to agree upon a single Arbitrator, then each shall, by written notice delivered to the other within 10 days after the meeting, select an Arbitrator. If either party fails to timely give notice of its selection for an Arbitrator, the other party’s submitted proposal shall determine the Annual Rent for the Second Extension Term. The 2 Arbitrators so appointed shall, within 5 business days after their appointment, appoint a third Arbitrator. If the 2 Arbitrators so selected cannot agree on the selection of the third Arbitrator within the time above specified, then either party, on behalf of both parties, may request such appointment of such third Arbitrator by application to any state court of general jurisdiction in the jurisdiction in which the Premises are located, upon 10 days prior written notice to the other party of such intent.
2.3.3.2The decision of the Arbitrator(s) shall be made within 30 days after the appointment of a single Arbitrator or the third Arbitrator, as applicable. The decision of the single Arbitrator shall be final and binding upon the parties. The average of the two closest Arbitrators in a three Arbitrator panel shall be final and binding upon the parties. Each party shall pay the fees and expenses of the Arbitrator appointed by or on behalf of such party and the fees and expenses of the third Arbitrator shall be borne equally by both parties. If the Market Rate and escalations are not determined by the first day of the Second Extension Term, then Tenant shall pay Landlord Annual Rent in an amount equal to the Annual Rent in effect immediately prior to the Second Extension Term and increased by 3% until such determination is made.
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 5 |
Suite 270, 9605 Medical Center Drive |
|
After the determination of the Market Rate and escalations, the parties shall make any necessary adjustments to such payments made by Tenant. Landlord and Tenant shall then execute and deliver an amendment recognizing the Market Rate and escalations for the Second Extension Term.
2.3.3.3“Arbitrator” shall be any person appointed by or on behalf of either party or appointed pursuant to the provisions hereof and: (i) shall be (A) a member of the American Institute of Real Estate Appraisers with not less than 10 years of experience in the appraisal of improved office and high tech industrial real estate in the greater Rockville, Maryland metropolitan area, or (B) a licensed commercial real estate broker with not less than 15 years’ experience representing landlords and/or tenants in the leasing of high tech or life sciences space in the greater Rockville, Maryland metropolitan area, (ii) devoting substantially all of their time to professional appraisal or brokerage work, as applicable, at the time of appointment and (iii) be in all respects impartial and disinterested.
2.3.4Right Personal. The Extension Right is personal to Tenant and is not assignable without Landlord’s consent, which may be granted or withheld in Landlord’s sole discretion separate and apart from any consent by Landlord to an assignment of Tenant’s interest in the Lease, except that the Extension Right may be transferred without Landlord’s consent to an Affiliate of Tenant.
2.3.5Exceptions. Notwithstanding anything set forth above to the contrary, the Extension Right shall not be in effect and Tenant may not exercise the Extension Right: (i) during any period of time that Tenant is in an Event of Default under any provision of this Lease; or (ii) if Tenant has been in an Event of Default under any provision of this Lease 3 or more times, regardless of whether the Events of Default are cured, during the 12 month period immediately before the date that Tenant intends to exercise the Extension Right, regardless of whether the Events of Default are cured.
2.3.6No Extensions. The period of time within which the Extension Right may be exercised shall not be extended or enlarged by reason of Tenant’s inability to exercise the Extension Right.
2.3.7Termination. The Extension Right shall terminate and be of no further force or effect even after Tenant’s due and timely exercise of the Extension Right, if, after such exercise, but prior to the commencement date of the Second Extension Term, (i) Tenant fails to timely cure any default by Tenant under this Lease; or (ii) Tenant has been in an Event of Default 3 or more times during the period from the date of the exercise of the Extension Right to the date of the commencement of the Second Extension Term, regardless of whether such Events of Default are cured.
6.Tenant Improvement Allowances. Landlord shall provide certain tenant improvement allowances to Tenant for the purpose of improving the Premises as set forth in the Existing Premises Work Letter attached hereto as a part hereof as Exhibit B. For the avoidance of doubt, such allowances relate solely to the Premises leased by Tenant as of the Effective Date and not to the Available Space (as defined below) or the First Offer Space.
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 6 |
Suite 270, 9605 Medical Center Drive |
|
7.[*****]
3. |
Miscellaneous. |
a.Entire Agreement. The Lease, as amended by this Second Amendment, is the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior and contemporaneous oral and written agreements and discussions. The Lease, as so amended by this Second Amendment, may be amended only by an agreement in writing, signed and delivered by the parties hereto.
b.Binding Effect. This Second Amendment is binding upon and shall inure to the benefit of the parties hereto, their respective agents, employees, members, representatives, officers, directors, divisions, subsidiaries, affiliates, assigns, heirs, successors in interest and shareholders.
c.Counterparts/Electronic Signatures. This Second Amendment may be executed in 2 or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature process complying with the U.S. federal ESIGN Act of 2000, such as DocuSign) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes. Electronic signatures shall be deemed original signatures for purposes of this Second Amendment and all matters related thereto, with such electronic signatures having the same legal effect as original signatures.
d.Broker. Landlord and Tenant each represents and warrants that it has not dealt with any broker, agent, or other person (collectively, “Broker”) in connection with this Second Amendment and that no Broker brought about this transaction, other than Jones Lang LaSalle (“JLL”). JLL, which represents Tenant, shall be paid by Landlord pursuant to a separate agreement between Landlord and JLL. Landlord and Tenant each hereby agree to indemnify and hold the other harmless from and against any claims by any Broker, other than JLL, claiming a commission or other form of compensation by virtue of having dealt with Tenant or Landlord, as applicable, with regard to this Second Amendment.
e.Ratification; Conflicts. Except as amended and/or modified by this Second Amendment, the Lease is hereby ratified and confirmed and all other terms of the Lease shall remain in full force and effect, unaltered and unchanged by this Second Amendment. In the event of any conflict between the provisions of this Second Amendment and the provisions of the Lease, the provisions of this Second Amendment shall prevail.
f.SEC filing.
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 7 |
Suite 270, 9605 Medical Center Drive |
|
Landlord and Tenant acknowledge that intends to include this fully executed Second Amendment as an Exhibit to Tenant's SEC filings (but Tenant shall redact the following provisions in the Second Amendment when so filed· (a} Annual Rent and Monthly Installment amounts (b} amount of the Increased Security Deposit (c} duration of any abatement of Annual Rent including the duration of the Available Space Annual Rent Abatement and (d) the amount of the TI Allowances, Including the amount of the Tenant Improvement Allowance and Additional Tenant Improvement Allowance), Landlord acknowledges that Tenant is a publicly traded organization and is required to make this Second Amendment available to the public in accordance with the applicable SEC requirements
[SIGNATURES APPEAR ON NEXT PAGE]
Second Amendment to Lease-- Synthetic Biologics, Inc. |
Page 8 |
Suite 270, 9605 Medical Center Drive |
|
IN WITNESS WHEREOF, the parties hereto have executed this Second Amendment under seal as of the day and year first above written.
|
|
TENANT: |
|
|
|
|
|
|
|
SYNTHETIC BIOLOGICS, INC., |
|
|
|
a Nevada corporation |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Steven A. Shallcross |
|
|
Name: |
Steven A. Shallcross |
|
|
Title: |
Chief Executive Officer |
|
LANDLORD: |
|||
|
|
|
||
|
ARE-MARYLAND NO. 50, LLC, |
|||
|
a Delaware limited liability company |
|||
|
|
|
||
|
By: |
Alexandria Real Estate Equities, L.P., a Delaware |
||
|
|
limited partnership, as its managing member |
||
|
|
|
||
|
|
By: |
ARE-QRS CORP., |
|
|
|
|
a Maryland corporation, |
|
|
|
|
general partner |
|
|
|
|
|
|
|
|
|
By: |
/s/Gregory Kay |
|
|
|
Name: |
Gregory Kay |
|
|
|
Title: |
Vice President RE Legal Affairs |
|
Notary Public |
|
|
|
|
Montgomery County, Maryland |
|
|
|
|
/s/ Mechelle Kulesza |
|
|
|
|
Name: |
Mechelle Kulesza, Notary Public |
|
|
EXHIBIT 10.26
EMPLOYMENT AGREEMENT
This Employment Agreement (the “Agreement”), dated April 8, 2022 by and between Synthetic Biologics, Inc., a corporation organized under the laws of the State of Nevada (the “Corporation”) and Mary Ann Shallcross, an individual (the “Employee”).
1. |
EMPLOYMENT: DUTIES |
(a)General Duties. The Employee shall serve as the Director of Clinical Operations of the Corporation with duties and responsibilities that are customary for such position, including, without limitation, review of the Corporation’s existing programs and any potential new programs. The Employee shall also perform services for such subsidiaries, if any, as may be necessary. The Employee shall perform her duties and discharge her responsibilities pursuant to this Agreement competently, carefully and faithfully. It is expected that the Employee will report directly to the Chief Operating Officer on clinical development matters and to the Head of Corporate & Product Development of the Corporation on strategy and business development matters. Employee shall have such duties, authorities and responsibilities commensurate with the duties, authorities and responsibilities of persons in similar capacities in similarly sized companies.
(b)Devotion of Time. The Employee shall devote at least two days per week to completely and adequately perform her duties to the Corporation.
2.TERM
The term of the Employee’s employment shall be on an at-will basis from the execution date of this Agreement unless terminated earlier under Section 7 of this Agreement. The start date for of the Employee shall be April 1 2022.
3.COMPENSATION
(a)As compensation for the performance of her duties on behalf of the Corporation, Employee shall receive the following:
(i)Base Salary. In consideration of the work to be performed for the Corporation, Employee shall receive a base salary of One Hundred Three Thousand Two Hundred Seventy Five Dollars ($103,275) per year (the “Base Salary”), payable semi-monthly.
(ii)Bonus. The Employee may be entitled to receive a discretionary performance bonus in cash or equity in the sole and absolute discretion of both the Compensation Committee and the Board of Directors of the Corporation.
(iii)Stock Options. The Employee shall receive an option in accordance with the Corporation’s 2020 Stock Incentive Plan to purchase the Corporation’s publicly traded common stock equal to Thirty Thousand (30,000) shares exercisable at the market price per share on the grant date, subject to board approval vesting monthly over thirty-six (36) months on a pro rata basis, commencing on the grant date while employed by the Corporation and such options will remain exercisable for a period of seven years from the date of grant, subject to the terms of the Corporation’s existing stock option plan in case of a termination of employment. Other terms of the option shall be according to the Corporation’s 2020 Stock Incentive Plan.
(b)The Corporation shall reimburse Employee for all normal, usual and necessary expenses incurred by Employee, including all travel, lodging and entertainment, against receipt by the Corporation, as the case may be, of appropriate vouchers or other proof of Employee’s expenditures and otherwise in accordance with such Expense Reimbursement Policy as may from time to time be adopted by the Corporation. Expenses which in the aggregate exceed $1,000 per month will require advance approval in writing. All expense reimbursements are to be paid monthly.
(c)The Corporation shall provide Employee with full advance indemnification to the extent permitted by Nevada law and the certificate of incorporation and bylaws of the Corporation, including indemnification for activities of all subsidiaries.
4.REPRESENTATIONS AND WARRANTIES BY EMPLOYEE
(a)Employee hereby represents and warrants to the Corporation as follows:
(i)Neither the execution and delivery of this Agreement nor the performance by Employee of her duties and other obligations hereunder violates or will violate any statute, law, determination or award, or conflict with or constitute a default under (whether immediately, upon the giving of notice or lapse of time or both) any prior employment agreement, contract, or other instrument to which Employee is a party or by which she is bound.
(ii)Employee has the full right, power and legal capacity to enter into and deliver this Agreement and to perform her duties and other obligations hereunder. This Agreement constitutes the legal, valid and binding obligation of Employee enforceable against her in accordance with its terms. No approvals or consents of any persons or entities are required for Employee to execute and deliver this Agreement or perform her duties and other obligations hereunder.
(iii)Employee understands that some or all of the stock received by Employee pursuant to Section 3(a)(iii) hereof will not be registered under the United States Securities Act of 1933 (the “1933 Act”), and acknowledges that she will be obligated to agree, as a condition to the issuance thereof, that she will acquire such stock for her own account for investment and not with a view to, or for resale in connection with a distribution thereof, and will bear the economic risk of her investment in such stock for an indefinite period of time.
5.CONFIDENTIAL INFORMATION
(a)Employee agrees that during the course of her employment or at any time thereafter, she will not disclose or make accessible to any other person, the Corporation’s products, services and technology, both current and under development, promotion and marketing programs, lists, trade secrets and other confidential and proprietary business information of the Corporation or any affiliates or any of their clients. Employee agrees: (i) not to use any such information for herself or others, and (ii) not to take any such material or reproductions thereof from the Corporation’s facilities at any time during her employment by the Corporation. Employee agrees immediately to return all such material and reproductions thereof in her possession to the Corporation upon request and in any event upon termination of employment.
(b)Except with prior written authorization by the Corporation, Employee agrees not to disclose or publish any of the confidential, technical or business information or material of the Corporation, its clients or any other party to whom the Corporation owes an obligation of confidence, at any time during or after her employment with the Corporation.
2
(c)In the event that Employee breaches any provisions of this Section 5 or there is a threatened breach, then, in addition to any other rights which the Corporation may have, the Corporation shall be entitled, without the posting of a bond or other security, to injunctive relief to enforce the restrictions contained herein. In the event that an actual proceeding is brought in equity to enforce the provisions of this Section 5, Employee shall not urge as a defense that there is an adequate remedy at law, nor shall the Corporation be prevented from seeking any other remedies which may be available. In addition, Employee agrees that in event that she breaches the covenants in this Section 5, in addition to any other rights that the Corporation may have, Employee shall be required to pay to the Corporation any amounts she receives in connection with such breach.
(d)Employee recognizes that in the course of her duties hereunder, she may receive from the Corporation or others information which may be considered “material, non-public information” concerning a public company that is subject to the reporting requirements of the United States Securities and Exchange Act of 1934, as amended. Employee agrees not to:
(i)Buy or sell any security, option, bond or warrant while in possession of relevant material, non-public information received from the Corporation or others in connection herewith;
(ii)Provide the Corporation with information with respect to any public company that may be considered material, non-public information, unless first specifically agreed to in writing by the Corporation; and
(iii)Buy or sell any security, option, bond or warrant other than in accordance with the terms of the Corporation’s trading policy.
6INVENTIONS DISCOVERED BY THE EMPLOYEE
The Employee shall promptly disclose to the Corporation any invention, improvement, discovery, process, formula, or method or other intellectual property, whether or not patentable or copyrightable (collectively, "Inventions"), conceived or first reduced to practice by the Employee, either alone or jointly with others, while performing services hereunder (or, if based on any Confidential Information, within one (1) year after the Term): (a) which pertain to any line of business activity of the Corporation, whether then conducted or then being actively planned by the Corporation, with which the Employee was or is involved, (b) which is developed using time, material or facilities of the Corporation, whether or not during working hours or on the Corporation premises, or (c) which directly relates to any of the Employee's work during the Term, whether or not during normal working hours. The Employee hereby assigns to the Corporation all of the Employee's right, title and interest in and to any such Inventions. During and after the Term, the Employee shall execute any documents necessary to perfect the assignment of such Inventions to the Corporation and to enable the Corporation to apply for, obtain and enforce patents, trademarks and copyrights in any and all countries on such Inventions, including, without limitation, the execution of any instruments and the giving of evidence and testimony, without further compensation beyond the Employee's agreed compensation during the course of the Employee's employment. All such acts shall be done without cost or expense to Employee. Employee shall be compensated for the giving of evidence or testimony after the term of Employee's employment at the rate of $650/day. Without limiting the foregoing, the Employee further acknowledges that all original works of authorship by the Employee, whether created alone or jointly with others, related to the Employee's employment with the Corporation and which are protectable by copyright, are "works made for hire" within the meaning of the United States Copyright Act, 17 U.S.C. (S) 101, as amended, and the copyright of which shall be owned solely, completely and exclusively by the Corporation. If any Invention is considered to be work not included in the categories of work covered by the United States Copyright Act, 17 U. S. C. (S) 101, as amended, such work is hereby assigned or transferred completely and exclusively to the Corporation.
3
The Employee hereby irrevocably designates counsel to the Corporation as the Employee's agent and attorney-in-fact to do all lawful acts necessary to apply for and obtain patents and copyrights and to enforce the Corporation's rights under this Section. This Section 6 shall survive the termination of this Agreement. Any assignment of copyright hereunder includes all rights of paternity, integrity, disclosure and withdrawal and any other rights that may be known as or referred to as "moral rights" (collectively "Moral Rights"). To the extent such Moral Rights cannot be assigned under applicable law and to the extent the following is allowed by the laws in the various countries where Moral Rights exist, the Employee hereby waives such Moral Rights and consents to any action of the Corporation that would violate such Moral Rights in the absence of such consent. The Employee agrees to confirm any such waivers and consents from time to time as requested by the Corporation.
7.TERMINATION
Employee’s employment hereunder shall continue as set forth in Section 2 hereof unless terminated upon the first to occur of the following events:
(a)Employee’s death.
(b)Employee’s “Disability”, meaning Employee’s incapacity, due to physical or mental illness, which results in Employee having been absent from fully performing his duties with the Corporation for a continuous period of more than thirty (30) days or more than sixty (60) days in any period of three hundred sixty-five (365) consecutive days. In the event that the Corporation intends to terminate the employment of Employee by reason of Disability, the Corporation shall give Employee no less than thirty (30) days’ prior written notice of the Corporation’s intention to terminate Employee’s employment. The Employee agrees, in the event of any dispute hereunder as to whether a Disability exists, and if requested by the Corporation, to submit to a physical examination in the state of the Corporation’s Employee offices by a licensed physician selected by mutual agreement between the Corporation and the Employee, the cost of such examination to be paid by the Corporation. The written medical opinion of such physician shall be conclusive and binding upon each of the parties hereto as to whether a Disability exists and the date when such Disability arose. If Employee refuses to submit to appropriate examinations by such physician at the request of the Corporation, the determination of the Employee’s Disability by the Corporation in good faith will be conclusive as to whether such Disability exists. This Agreement shall be interpreted and applied so as to comply with the provisions of the Americans with Disabilities Act (to the extent that it is applicable) and any other applicable laws regarding disability.
(c)Just Cause”, meaning the Employee’s:
(i)acts of embezzlement or misappropriation of funds; or fraud;
(ii)conviction of a felony or other crime involving moral turpitude, dishonesty or
theft;
(iii)willful unauthorized disclosure of confidential information belonging to the Corporation or entrusted to the Corporation by a client;
(iv)material violation of any provision of the Agreement, which is not cured by Employee within thirty (30) days of receiving written notice of such violation by the Corporation; (v)being under the influence of drugs (other than prescription medicine or other medically-related drugs to the extent that they are taken in accordance with their directions) during the performance of Employee’s duties under this Agreement;
4
(vi)engaging in behavior that would constitute grounds for liability for harassment (as proscribed by the U.S. Equal Employment Opportunity Commission Guidelines or any other applicable state or local regulatory body) or other egregious conduct that violates laws governing the workplace; or
(vii)willful failure to perform his written assigned tasks, where such failure is attributable to the fault of Employee, gross insubordination, or dereliction of fiduciary obligations which are not cured by Employee within thirty (30) days of receiving written notice of such violation by the Corporation.
In the event that the Corporation intends to terminate the employment of Employee by reason of Just Cause, the Corporation shall give Employee written notice of the Corporation’s intention to terminate Employee’s employment, and such termination may be effective immediately, unless a cure period applies, in which case the termination date may not precede the expiration date of the applicable cure period.
(d)“Without Just Cause”, meaning written notice by the Corporation to Employee of a termination without Just Cause and other than due to death or Disability.
(e)“Good Reason”, for the purposes of this Agreement can mean:
(i)a material breach by the Corporation of the terms of this Agreement, which breach is not cured within thirty (30) days after notice thereof from Executive; or
(ii)a change in control which shall mean (a) any person becomes the beneficial owner (as term is defined in the Securities Exchange Act of 1934) directly or indirectly, of securities representing more than fifty percent (50%) of the total voting power of Company’s shares; or (b) a change in the composition of the Board of Directors as a result of which fewer than a majority of the directors are Incumbent Directors. Incumbent Directors shall mean directors who are either directors of the Company on the date hereof or are elected by the Board of Directors with the affirmative vote of a majority of the Incumbent Directors at the time of election; or (c) the Company merges with another corporation after which a majority of the shares of the resulting entity are not held by shareholders of the Company prior to the merger; or
In the event that Employee intends to terminate his employment for Good Reason, Employee shall give the Corporation written notice of his intention to terminate his employment, and such termination may be effective immediately, unless a cure period applies, in which case the termination date may not precede the expiration date of the applicable cure period.
(f)Without Good Reason, meaning written notice by Employee to the Corporation of a termination without Good Reason.
(g)If Employee’s employment hereunder is terminated for any reason, Employee or his estate as the case may be, will only be entitled to receive the accrued base salary, vacation pay, expense reimbursement and any other entitlements accrued by Employee under Section 3, to the extent not previously paid (the sum of the amounts described in this subsection shall be hereinafter referred to as the “Accrued Obligations”).
5
In addition to the payment of the Accrued Obligations (as defined in Section 8 below), subject to the Employee’s execution of a release in the form acceptable to the Corporation that Employee allows to become effective and return to the Corporation of all property of the Corporation, if the Employee terminates her employment with the Corporation for Good Reason or the Corporation terminates the Employee's employment for any reason other than Death, Disability or Just Cause and such termination is not within one year after the closing date of a Change in Control, the Corporation shall pay to the Employee as a severance benefit, an amount equal to six months’ base salary. The amount shall be paid to the Employee in accordance with the Corporation’s standard salary payments to its employees but only after the release has become effective and is not subject to revocation. For purposes of this Agreement a Change in Control shall mean (a) any person becomes the beneficial owner (as term is defined in the Securities Exchange Act of 1934) directly or indirectly, of securities representing more than fifty percent (50%) of the total voting power of Corporation’s shares; or (b) a change in the composition of the Board of Directors as a result of which fewer than a majority of the directors are Incumbent Directors. Incumbent Directors shall mean directors who are either directors of the Corporation on the date hereof or are elected by the Board of Directors with the affirmative vote of a majority of the Incumbent Directors at the time of election; or (c) the Corporation merges or consolidates with another corporation and immediately after such merger or consolidation a majority of the shares of the resulting entity are not held by shareholders of the Corporation immediately prior to the merger or consolidation.”
(h)In addition to the payment of the Accrued Obligations, if within one year after the closing date of a Change in Control (as defined below) (subject to the Employee’s execution of a release in the form acceptable to the Corporation that Employee allows to become effective and return to the Corporation of all property of the Corporation, the Employee terminates his employment with the Corporation for Good Reason or the Corporation (or the then former Corporation subsidiary employing the Employee, or the consolidated, surviving or transferee person in the event of a Change in Control pursuant to a consolidation, merger or sale of assets) the Corporation terminates the Employee's employment for any reason other than Death, Disability or Just Cause, then the Employee shall be entitled to receive from the Corporation the following severance payments and benefits upon such termination: (i) a lump sum cash payment, within thirty (30) days after termination but only after the release has become effective and is not subject to revocation, equal to six (6) months of the Employee’s then-current base salary; (ii) immediate vesting of 100% of the then-unvested portion of any outstanding equity awards held by the Executive and the Corporation will extend the exercise period for all equity award(s) granted to the Executive to a period equal to the shorter of: (x) twelve (12) months after termination, or (y) the remaining term of the award(s). In each case such amounts shall be less payroll taxes and withholding required by any federal, state or local law.
Employee shall not be entitled to receive severance payments set forth under clause 7(g) if Employee receives the severance payments set forth under this clause (h).
(i)The severance payments set forth in Section 7(g) and 7(h) shall terminate if Employee willfully breaches any obligations of confidentiality, non-solicitation, non-disparagement, no conflicts or non-competition provision set forth in any other agreement between the Corporation and Employee or under applicable law.”
Upon any such termination under this Section 7, the Employee shall be entitled to receive any unpaid expenses or earned compensation due the Employee up to and including the date of termination and any unexercised option shall vest or terminate in accordance with the terms of the 2020 Stock Incentive Plan and any Option Agreement executed by the Employee.
6
8.NOTICES
Any notice or other communication under this Agreement shall be in person or in writing and shall be deemed to have been given: (i) when delivered personally against receipt therefor; (ii) one (1) day after being sent by Federal Express or similar overnight delivery; (iii) three (3) days after being mailed registered or certified mail, postage prepaid, return receipt requested, to either party at the address set forth above, or to such other address as such party shall give by notice hereunder to the other party; or (iv) when sent by facsimile, followed by oral confirmation and with a hard copy sent as in (ii) or (iii) above.
9.SEVERABILITY OF PROVISIONS
If any provision of this Agreement shall be declared by a court of competent jurisdiction to be invalid, illegal or incapable of being enforced in whole or in part, such provision shall be interpreted so as to remain enforceable to the maximum extent permissible consistent with applicable law and the remaining conditions and provisions or portions thereof shall nevertheless remain in full force and effect and enforceable to the extent they are valid, legal and enforceable, and no provision shall be deemed dependent upon any other covenant or provision unless so expressed herein.
10.ENTIRE AGREEMENT MODIFICATION
This Agreement contains the entire agreement of the parties relating to the subject matter hereof, and the parties hereto have made no agreements, representations or warranties relating to the subject matter of this Agreement which are not set forth herein. No modification of this Agreement shall be valid unless made in writing and signed by the parties hereto.
11.BINDING EFFECT
The rights, benefits, duties and obligations under this Agreement shall inure to, and be binding upon, the Corporation, its successors and assigns, and upon Employee and her legal representatives. This Agreement constitutes a personal service agreement, and the performance of the Employee’s obligations hereunder may not be transferred or assigned by the Employee.
12.NON-WAIVER
The failure of either party to insist upon the strict performance of any of the terms, conditions and provisions of this Agreement shall not be construed as a waiver or relinquishment of future compliance therewith, and said terms; conditions and provisions shall remain in full force and effect. No waiver of any term or condition of this Agreement on the part of either party shall be effective for any purpose whatsoever unless such waiver is in writing and signed by such party.
13.GOVERNING LAW, DISPUTE RESOLUTION
This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of Maryland of the United States of America without regard to principles of conflict of laws.
7
14.HEADING
The headings of paragraphs are inserted for convenience and shall not affect any interpretation of this Agreement.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the day and year first above written.
SYNTHETIC BIOLOGICS, INC.
By: /s/ Frank Tufaro |
|
|
Name: Frank Tufaro |
|
|
Title: Chief Operating Officer |
|
|
Signed and Agreed to:
/s/ Mary Ann Shallcross
Mary Ann Shallcross
8
Exhibit 19.1
AMENDED AND RESTATED
CORPORATE TRADING POLICY
March 4, 2024
THERIVA PHARMACEUTICALS, INC.
CORPORATE TRADING POLICY
I. |
PREAMBLE |
A. |
This document (this “Policy”) contains the policy of Theriva Biologics, Inc., a Nevada corporation, and its subsidiaries (the “Company,”“Theriva” or “we”), concerning the Trading of Theriva Securities, as defined below. This Policy is intended to preserve the reputation and integrity of Theriva as well as that of all persons affiliated with it. |
B. |
This Policy applies to all directors, employees and agents of Theriva (Theriva’s agents include its independent contractors, consultants, accountants and attorneys) located in and outside the United States alike. When we refer to “you” or to “directors, employees, or agents” in this Policy, in addition to you, we also mean members of your immediate family or other persons with whom you share a household, persons that are your economic dependents, and any person over whom, or entity over which, you have control. We will regard trades made at your direction or at the direction of those named in the preceding sentence as trades made by you. |
C. |
The directors, employees and agents of Theriva must act in a manner that does not misuse material financial or other information that has not been publicly disclosed. A failure to do so breaches Theriva’s Code of Conduct and Ethics and will result in sanctions, which may include dismissal for cause. |
D. |
Insider trading in Theriva’s Securities, and disclosure, or “tipping”, of material, non-public information regarding Theriva to outsiders violates laws that impose strict penalties upon both companies and individuals, including both financial sanctions and prison. This Policy is intended to assure compliance with these laws, and has been adopted by Theriva’s board of directors. The ultimate responsibility for complying with this Policy and applicable laws and regulations, however, rests with you. You should use your best judgment and consult with your legal and financial advisors, as needed. |
E. |
The penalties for trading on material non-public information under the Securities Exchange Act of 1934, as amended, include: (i) imprisonment for up to 20 years; (ii) criminal fines of up to $5 million; (iii) civil penalties of up to three times the profits gained or losses avoided; (iv) prejudgment interest; and (v) private party damages. |
F. |
Any sanctions, expenses or losses imposed upon a director, employee or agent of Theriva for violation of insider trading laws will be the sole responsibility of the individual. Theriva will not advance expenses or indemnify the individual for these costs. Furthermore, such costs, as well as attorney’s fees incurred in the defense of claims for such costs, are excluded from coverage under Theriva’s directors and officers liability insurance policy. |
G. |
This Policy applies to any and all transactions in Theriva Securities, except for the exercise of Theriva stock options, including cashless exercises. |
H. |
You are permitted to enter into Rule 10b5-1 plans for trading in Theriva Securities. Rule 10b5-1 of the General Rules and Regulations under the Securities Exchange Act of 1934, as amended, provides an affirmative defense from insider trading liability. To be eligible for this affirmative defense, the Rule 10b5-1 plan must be pre-approved by Theriva’s Chief Financial Officer and outside securities counsel and the plan must meet all of the requirements of Rule 10b5-1. |
A Rule 10b5-1 plan may only be entered into at a time when you do not know of any material non-public information. Additionally, the Rule 10b5-1 plan must either specify the amount, pricing and timing of the trades in advance, or must delegate discretion on these matters to an independent third party. Once entered into, you may not exercise any influence over the amount of Theriva Securities to be traded, the price at which they are to be traded or the date of the trade.
Any Rule 10b5-1 Plan must be submitted for approval five days prior to the entry into the Rule 10b5-1 Plan. No further pre-approval of transactions conducted pursuant to the Rule 10b5-1 Plan will be required. You may not adopt a Rule 10b5-1 Plan outside of a trading window or during any special blackout periods that the Chief Financial Officer and outside securities counsel may designate, or at a time when you are aware of material non-public information. The following requirements apply to all Rule 10b5-1 Plans:
i. |
directors and Section 16 Officers may not commence sales under a Rule 10b5-1 plan until the later of (i) 90 days following the date of adoption or modification of such plan; or (ii) two business days following the disclosure of the Company's financial results in a Form 10-K or Form 10-Q relating to the fiscal quarter in which the Rule 10b5-1 plan was adopted or modified (but not to exceed 120 days following plan adoption or modification); |
2
ii. |
all persons other than directors and Section 16 Officers, may not commence sales under a Rule 10b5-1 plan until 30 days following the date of adoption or modification of such plan; |
iii. |
directors and Section 16 Officers must provide a representation in the Rule 10b5-1 plan certifying that, on the date of adoption or modification of the plan, they (i) are not aware of material nonpublic information about the Company or its securities; and (ii) are adopting or modifying the plan in good faith and not as part of a plan or scheme to evade the prohibitions of Rule 10b-5; |
iv. |
subject to the limited exceptions set forth in Rule 10b5-1, you may not maintain multiple, overlapping plans; |
v. |
subject to the limited exceptions set forth in Rule 10b5-1, you can utilize only one single-trade plan (i.e. a plan designed to effect only a single transaction) during any 12 month period; and |
vi. |
you must act in good faith with respect to the Rule 10b5-1 plan, not just in connection with entering into the plan. |
Theriva may impose additional restrictions on Rule 10b5-1 Plans, including without limitation:
· |
requiring that all plans be managed by an administrator selected by the Company; |
· |
restrictions on termination or modification of plans; |
· |
prohibition on entry into new plans for extended periods following termination of an existing plan; and |
· |
prescribed periods during which persons may enter into plans. |
Modification or termination of Rule 10b5-1 Plans are generally discouraged absent compelling circumstances. Any modification to any Rule 10b5-1 Plan is treated as the entry into a new plan and must comply with all of the above requirements.
I. |
This Policy supersedes any previous policy of Theriva concerning insider trading. In the event of any conflict or inconsistency between this Policy and any other materials previously distributed by the Company concerning insider trading, this Policy shall govern. |
J. |
Theriva reserves the right to amend and interpret this Policy from time to time. |
K. |
Directors, officers and employees should keep certain information concerning the operation of this Policy in strict confidence, since knowledge of certain decisions made pursuant to this Policy could itself constitute material, non-public information. |
3
II. |
DEFINITIONS |
A. |
“Theriva Insiders” include all Theriva employees who, by virtue of their position, are more likely to have access to Material, Non-Public Information. Theriva Insiders include, but are not limited to: |
(i) |
all of Theriva’s executive officers as determined from time to time by Theriva’s Chief Financial Officer and outside securities counsel; and |
(ii) |
all other Theriva employees that are directly involved in the preparation of Theriva’s consolidated financial statements or that have access to information from those financial statements while they are being prepared. |
B. |
“Theriva Securities” include Theriva common stock, preferred stock and options on Theriva stock, including puts and calls, as well as Theriva debt securities such as bonds and promissory notes, if any. |
C. |
“Full Trading Day” - A Full Trading Day has elapsed when after the public disclosure, trading in the security has opened for trading and closed. |
D. |
“Material Non-Public Information” is any non-public information that a reasonable investor would consider important in a decision to buy, sell or hold securities. Information is non-public if it has not yet become publicly available. For purposes of this Policy, information is not considered publicly available until two Full Trading Days have elapsed after such information has either been filed by Theriva with the United States Securities and Exchange Commission, or included in a Theriva press release that has been broadly disseminated to the investing public. |
Any information that could reasonably be expected to affect the price of securities is likely to be considered material. Examples of material information include financial results, proposed mergers and acquisitions, a sale of major assets, changes in dividends, an extraordinary item for accounting purposes, and important business developments or major litigation. The information may be positive or negative.
E. |
“Section 16 Officers” are those employees of Theriva who have received notification from Theriva’s Corporate Secretary that they are obligated to file security ownership reports with the United States Securities and Exchange Commission pursuant to Section 16(a) of the Securities Exchange Act of 1934, as amended. |
F. |
“Trade” or “Trading” includes buying or selling, as well as writing options, including puts and calls. Trading does not include making a bona fide gift. |
4
III. |
PROHIBITIONS FOR ALL DIRECTORS, EMPLOYEES AND AGENTS OF THERIVA |
All directors, employees and agents of Theriva must not:
A. |
Enter into a short sale of Theriva Securities (meaning a sale of securities which are not then owned), including a sale against the box (meaning a sale with delayed delivery). |
B. |
Use standing orders (except standing orders under qualified Rule 10b5-1 plans that has been pre-approved by Theriva’s Chief Financial Officer and outside securities counsel), except for only a very brief period of time (intraday standing orders) by Theriva employees. Standing orders pose the risk that your broker could execute a transaction on your behalf when you are in possession of Material Non-Public Information. |
C. |
Purchase or sell Theriva Securities while they possess Material Non-Public Information. |
D. |
Disclose Material Non-Public Information regarding Theriva to another Theriva employee (except on a need-to-know basis), family members or any other third party. This is intended to assure that no Theriva employee becomes a “tipper”, liable for the trading activities of his or her “tippee”. |
E. |
Participate in any social media and/or chat room discussions about Theriva. |
F. |
Trade Theriva Securities without first obtaining prior clearance from Theriva’s Chief Financial Officer and outside securities counsel or his/her designee. Each proposed transaction will be evaluated to determine if it raises insider trading concerns or other concerns under the federal or state securities laws and regulations. Any advice will relate solely to the restraints imposed by law and will not constitute advice regarding the investment aspects of any transaction. Clearance of a transaction is valid only for a 48- hour period. If the transaction order is not placed within that 48-hour period, clearance of the transaction must be re-requested. If clearance is denied, the fact of such denial must be kept confidential by the person requesting such clearance. |
G. |
Purchase or sell securities of any other company while they possess Material Non-Public Information about the other company learned while performing their job at Theriva. |
H. |
May not pledge Theriva Securities as security for a margin loan in a brokerage account and may not borrow from a brokerage firm, bank or other entity in order to buy Theriva Securities (other than in connection with a so-called “cashless” exercise of options under the Company’s stock plans). |
5
I. |
May not engage in hedging or monetization transactions, including the use of financial instruments such as prepaid variable forwards, equity swaps, collars and exchange funds, and may permit a holder to continue to own Theriva Securities but without the full risks and rewards of ownership. |
J. |
May not at any time sell or buy any publicly traded options to sell or buy Theriva Securities (warrants, puts, calls, etc.). |
IV. |
ADDITIONAL PROHIBITIONS FOR THERIVA INSIDERS |
To avoid even the appearance of impropriety, Theriva Insiders are subject to additional prohibitions. Specifically, Theriva Insiders:
A. |
Are subject to all the restrictions set forth in paragraph III above. |
B. |
May not Trade in Theriva Securities during the following Black-Out Periods: (i) The three week period before the Securities and Exchange Commission filing deadline for any quarterly or annual report and ending when two Full Trading Days have elapsed after the public release of earnings by Theriva for each of Theriva’s four fiscal quarters. |
V. |
ADDITIONAL PROHIBITIONS FOR DIRECTORS AND SECTION 16 OFFICERS OF THERIVA |
To avoid even the appearance of impropriety, directors and Section 16 Officers of Theriva are subject to even further prohibitions. Specifically, all directors and Section 16 Officers of Theriva:
A. |
Are subject to all the restrictions set forth in paragraphs III and IV above. |
B. |
May not Trade in Theriva Securities during the following Black-Out Periods: The three week period before the Securities and Exchange Commission filing deadline for any quarterly or annual report and ending when two Full Trading Days have elapsed after the public release of earnings by Theriva for each of Theriva’s four fiscal quarters. |
C. |
May not buy and sell, or sell and buy, any Theriva equity security within a period of less than six months except that all transactions involving Theriva’s employee benefit plans including transactions involving stock options, restricted stock awards and stock appreciation rights are exempt as long as all the requirements of Rule 16b-3 of the General Rules and Regulations under the Securities Exchange Act of 1934, as amended, are satisfied, including the requirement that the employee benefit plan be in writing and approved by the affirmative votes of a majority of Theriva’s common stock represented at a meeting of Theriva’s shareholders. The short-swing profits realized from any such prohibited transaction must be disgorged to Theriva pursuant to Section 16(b) of the Securities Exchange Act of 1934, as amended. |
6
D. |
May not Trade Theriva Securities without complying with Rule 144 of the General Rules and Regulations under the Securities Act of 1933, as amended. |
* * * * * * *
7
AMENDED AND RESTATED
CORPORATE TRADING POLICY
Policy Receipt Acknowledgement
I hereby acknowledge receipt of the Amended and Restated Corporate Trading Policy for Theriva Biologics, Inc. I have read the policy in its entirety and understand the guidelines for trading of Theriva Biologics, Inc. securities as set forth in the policy.
|
|
Date |
|
|
|
|
|
Print Name |
|
|
|
|
|
Signature |
|
8
Exhibit 21.1
THERIVA BIOLOGICS, INC. SUBSIDIARIES
The following table lists all of the subsidiaries of Theriva Biologics, Inc. and the jurisdiction of incorporation of each subsidiary. Each subsidiary does business under its corporate name indicated in the table.
Subsidiary Name |
|
Ownership |
|
Jurisdiction of Incorporation |
Pipex Therapeutics, Inc. |
|
Wholly owned |
|
Delaware |
Effective Pharmaceuticals, Inc. |
|
Wholly owned |
|
Delaware |
Solovax, Inc. |
|
Majority-owned |
|
Delaware |
CD4 Biosciences, Inc. |
|
Majority-owned |
|
Delaware |
Epitope Pharmaceuticals, Inc. |
|
Majority-owned |
|
Delaware |
Healthmine, Inc. |
|
Wholly owned |
|
Delaware |
Putney Drug Corp. |
|
Wholly owned |
|
Delaware |
Synthetic Biomics, Inc. |
|
Wholly owned |
|
Nevada |
Theriva Biologics, S.L. |
|
Wholly owned |
|
Spain |
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
Theriva Biologics, Inc.
Rockville, MD
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (Nos. 333-180562, 333-188219, 333-156973, 333-206267, 333-207327, 333-203323, 333-226500, 333-224728, 333-255726, 333-260449 and 333-267294), Form S-1 (No. 333-227400), and Form S-8 (Nos. 333-192355, 333-170858, 333-148764, 333-206268, 333-213388, 333-220401, 333-227668, 333-233959, 333-249712 and 333-267910) of Theriva Biologics, Inc. of our report dated March 25, 2024 relating to the consolidated financial statements, which appears in this Annual Report on Form 10-K. Our report on the contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
/s/ BDO USA, P.C. |
|
|
|
Raleigh, North Carolina |
|
March 25, 2024 |
|
Exhibits 31.1 and 31.2
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER AND PRINCIPAL FINANCIAL OFFICER
PURSUANT TO RULE 13a-14(a) OR RULE 15d-14(a) OF THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Steven A. Shallcross, certify that:
1. |
I have reviewed this Annual Report on Form 10-K of Theriva Biologics, Inc.; |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. |
I am responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c. |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d. |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. |
I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: March 25, 2024 |
|
|
|
|
|
|
By: |
/s/ Steven A. Shallcross |
|
Name: |
Steven A. Shallcross |
|
Title: |
Chief Executive Officer and Chief Financial Officer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
Exhibits 32.1 and 32.2
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER AND PRINCIPAL FINANCIAL OFFICER
PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to 18 U.S.C. §1350, as created by Section 906 of the Sarbanes-Oxley Act of 2002, the undersigned officer of Theriva Biologics, Inc. (the “Registrant”) hereby certifies, to such officer’s knowledge, that:
(1) |
the accompanying Annual Report on Form 10-K of the Registrant for the year ended December 31, 2023 (the “Report”) fully complies with the requirements of Section 13(a) or Section 15(d), as applicable, of the Securities Exchange Act of 1934, as amended; and |
(2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Registrant. |
Date: March 25, 2024 |
|
||
|
|
||
|
By: |
/s/ Steven A. Shallcross |
|
|
Name: |
Steven A. Shallcross |
|
|
Title: |
Chief Executive Officer and Chief Financial Officer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
|
Exhibit 97.1
THERIVA BIOLOGICS, INC.
CLAWBACK POLICY
The Board of Directors (the “Board”) of Theriva Biologics, Inc. (the “Company”) has determined that it is in the best interests of the Company to adopt this Clawback Policy (this “Policy”), which provides for the recovery of certain incentive compensation in the event of an Accounting Restatement (as defined below). This Policy is designed to comply with, and shall be interpreted to be consistent with, Section 10D of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), Rule 10D-1 promulgated under the Exchange Act (“Rule 10D-1”) and Section 811 of the NYSE American LLC (the “NYSE American”) Company Guide.
1.Definitions
For purposes of this Policy, the following capitalized terms shall have the meanings set forth below.
“Accounting Restatement” means an accounting restatement of the Company’s financial statements due to the Company’s material noncompliance with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
“Clawback Period” means the three completed fiscal years immediately preceding the date on which the Company is required to prepare an Accounting Restatement, as well as any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three completed fiscal years (except that a transition period that comprises a period of at least nine months shall count as a completed fiscal year). The“date on which the Company is required to prepare an Accounting Restatement” is the earlier to occur of (a) the date the Board, a committee of the Board, or the officer or officers of the Company authorized to take such action if the Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement; or (b) the date a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement.
“Erroneously Awarded Compensation” means, in the event of an Accounting Restatement, the amount of Incentive-Based Compensation previously received that exceeds the amount of Incentive-Based Compensation that otherwise would have been received had it been determined based on the restated amounts in such Accounting Restatement, and must be computed without regard to any taxes paid by the relevant Executive Officer; provided, however, that for Incentive-Based Compensation based on stock price or total stockholder return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement: (i) the amount of Erroneously Awarded Compensation must be based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total stockholder return upon which the Incentive-Based Compensation was received; and (ii) the Company must maintain documentation of the determination of that reasonable estimate and provide such documentation to the NYSE American.
“Executive Officer” means the Company’s president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice-president of the Company in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, or any other person who performs similar policy-making functions for the Company. An executive officer of the Company’s parent or subsidiary is deemed an “Executive Officer” if the executive officer performs such policy making functions for the Company.
“Financial Reporting Measure” means any measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures that are derived wholly or in part from such measures; provided, however, that a Financial Reporting Measure is not required to be presented within the Company’s financial statements or included in a filing with the U.S. Securities and Exchange Commission (the “SEC”) to qualify as a “Financial Reporting Measure.” For purposes of this Policy, Financial Reporting Measures include, but are not limited to, stock price and total stockholder return.
“Incentive-Based Compensation” means any compensation that is granted, earned or vested based wholly or in part upon the attainment of a Financial Reporting Measure. Incentive-Based Compensation is “received” for purposes of this Policy in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-Based Compensation award is attained, even if the payment or grant of such Incentive-Based Compensation occurs after the end of that period.
2.Policy Application.
This Policy applies to Incentive-Based Compensation received by an Executive Officer (a) after beginning services as an Executive Officer; (b) if that person served as an Executive Officer at any time during the performance period for such Incentive-Based Compensation; and (c) while the Company had a listed class of securities on a national securities exchange.
3. Policy Recovery Requirement.
In the event the Company is required to prepare an Accounting Restatement, the Company shall reasonably promptly recoup the amount of any Erroneously Awarded Compensation received by any Executive Officer during the Clawback Period. In the event of an Accounting Restatement, the Board shall determine, in its sole discretion, the amount of any Erroneously Awarded Compensation for each Executive Officer in connection with such Accounting Restatement.
4.Method of Recoupment.
The Board shall determine, in its sole discretion, the timing and method for promptly recouping such Erroneously Awarded Compensation, which may include without limitation: (a) seeking reimbursement of all or part of any cash or equity-based award, (b) cancelling prior cash or equity-based awards, whether vested or unvested or paid or unpaid, (c) cancelling or offsetting against any planned future cash or equity-based awards, (d) forfeiture of deferred compensation, subject to compliance with Section 409A of the Internal Revenue Code and the regulations promulgated thereunder and (e) any other method authorized by applicable law or contract. Subject to compliance with any applicable law, the Board may affect recovery under this Policy from any amount otherwise payable to the Executive Officer, including amounts payable to such individual under any otherwise applicable Company plan or program, including base salary, bonuses or commissions and compensation previously deferred by the Executive Officer.
The Company is authorized and directed pursuant to this Policy to recoup Erroneously Awarded Compensation in compliance with this Policy except to the extent the Compensation Committee of the Board has determined recovery would be impracticable solely if one (1) of the following limited reasons are met, and subject to the following procedural and disclosure requirements:
2
· The direct expense paid to a third party to assist in enforcing the Policy would exceed the amount to be recovered. Before concluding that it would be impracticable to recover any amount of Erroneously Awarded Compensation based on expense of enforcement, the Company must make a reasonable attempt to recover such Erroneously Awarded Compensation, document such reasonable attempt(s) to recover and provide that documentation to the NYSE American;
· Recovery would violate home country law of the Company where that law was adopted prior to November 28, 2022. Before concluding that it would be impracticable to recover any amount of Erroneously Awarded Compensation based on violation of home country law of the Company, the Company must obtain an opinion of home country counsel, acceptable to the NYSE American, that recovery would result in such a violation, and must provide such opinion to the NYSE American; or
· Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder.
5.No Indemnification of Executives Officers.
Notwithstanding the terms of any indemnification or insurance policy or any contractual arrangement with any Executive Officer that may be interpreted to the contrary, the Company shall not indemnify any Executive Officers against the loss of any Erroneously Awarded Compensation, including any payment or reimbursement for the cost of third-party insurance purchased by any Executive Officers to fund potential clawback obligations under this Policy.
6. Required Policy-Related Filings.
The Company shall file all disclosures with respect to this Policy in accordance with the requirements of the federal securities laws, including disclosures required by SEC filings.
7.Acknowledgement.
Each Executive Officer shall sign and return to the Company within thirty (30) calendar days following the later of (i) the effective date of this Policy set forth below or (ii) the date such individual becomes an Executive Officer, the Acknowledgement Form attached hereto as Exhibit A, pursuant to which the Executive Officer agrees to be bound by, and to comply with, the terms and conditions of this Policy.
8.Administration
This Policy shall be administered by the Board or, if so designated by the Board, the Compensation Committee, in which case references herein to the Board shall be deemed references to the Compensation Committee. Any determinations made by the Board shall be final and binding on all affected individuals.
9.Policy Not in Limitation
The Board intends that this Policy shall be applied to the fullest extent of the law. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company under applicable law or pursuant to the terms of any similar policy in any employment agreement, equity award agreement, or similar agreement and any other legal remedies available to the Company.
3
Nothing contained in this Policy, and no recoupment or recovery as contemplated by this Policy, shall limit any claims, damages or other legal remedies the Company or any of its affiliates may have against an Executive Officer arising out of or resulting from any actions or omissions by the Executive Officer.
10.Amendment; Termination.
The Board may amend, modify, supplement, rescind or replace all or any portion of this Policy at any time and from time to time in its discretion, and shall amend this Policy as it deems necessary to comply with applicable law or any rules or standards adopted by a national securities exchange on which the Company’s securities are listed.
11.Successors.
This Policy is binding and enforceable against all Executive Officers and their beneficiaries, heirs, executors, administrators or other legal representatives.
12.Effective Date.
This Policy shall be effective as of November 9, 2023. The terms of this Policy shall apply to any Incentive-Based Compensation that is received by Executive Officers on or after October 2, 2023, even if such Incentive-Based Compensation was approved, awarded or granted to Executive Officers prior to such date.
Approved and adopted: November 9, 2023
4
EXHIBIT A
THERIVA BIOLOGICS, INC. CLAWBACK POLICY
ACKNOWLEDGEMENT FORM
By signing below, the undersigned acknowledges and confirms that the undersigned has received and reviewed a copy of the Theriva Biologics, Inc. (the “Company”) Clawback Policy (the “Policy”).
By signing this Acknowledgement Form, the undersigned acknowledges and agrees that the undersigned is and will continue to be subject to the Policy and that the Policy will apply both during and after the undersigned’s employment or service with the Company. Further, by signing below, the undersigned agrees to abide by the terms of the Policy, including, without limitation, by returning any Erroneously Awarded Compensation (as defined in the Policy) to the Company to the extent required by, and in a manner consistent with, the Policy.
|
EXECUTIVE OFFICER |
|
|
|
|
|
Signature |
|
|
|
|
|
Print Name |
|
|
|
|
|
Date |