Document
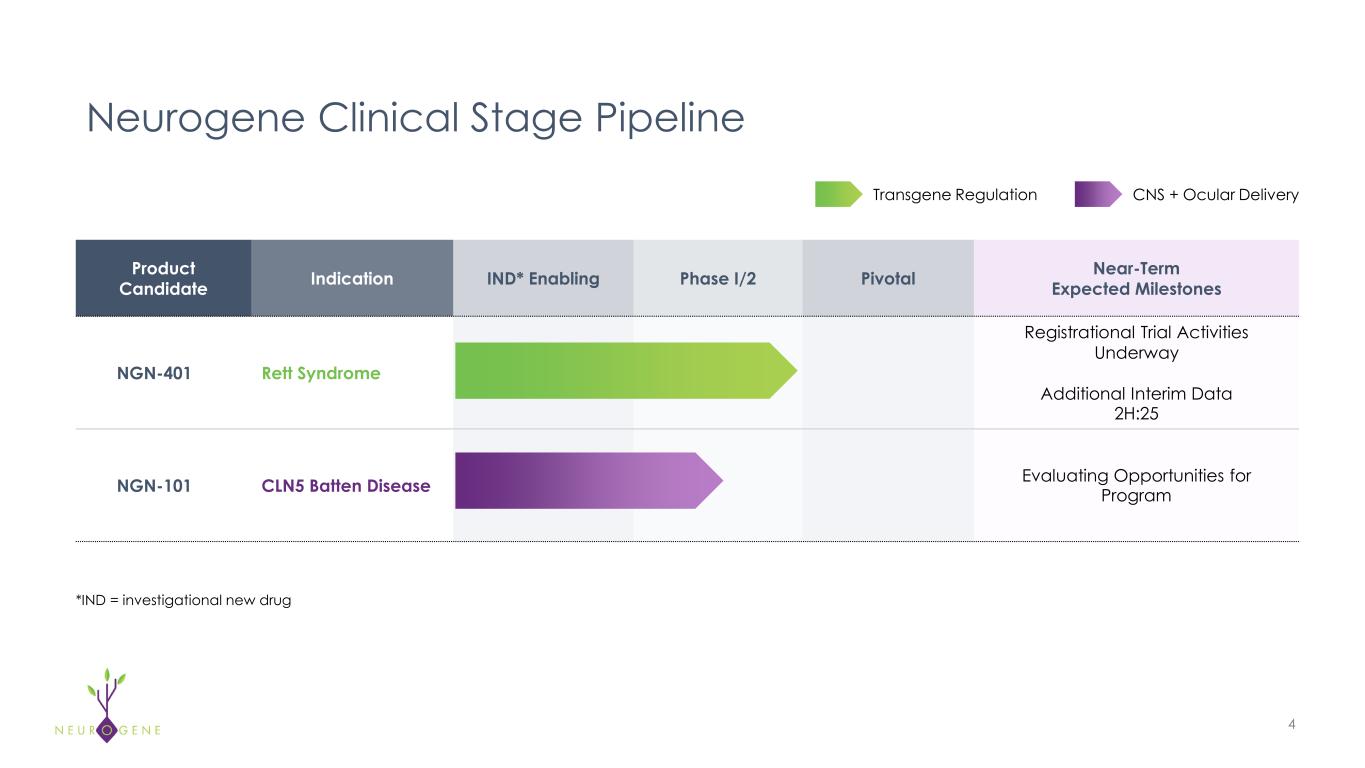
Neurogene Announces Positive Regulatory Update for NGN-401 Gene Therapy in Rett Syndrome with Plans to Initiate Dosing in Embolden™ Registrational Trial in Q4 2025
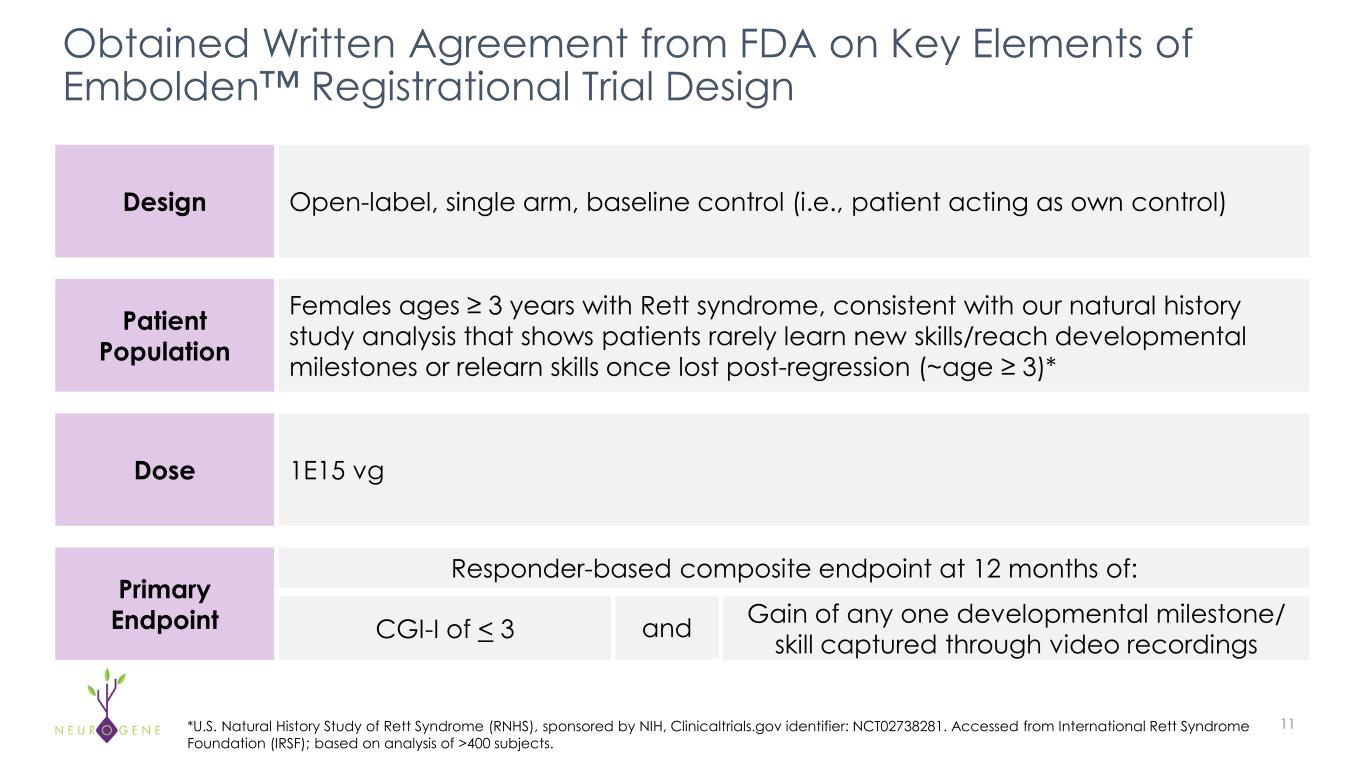
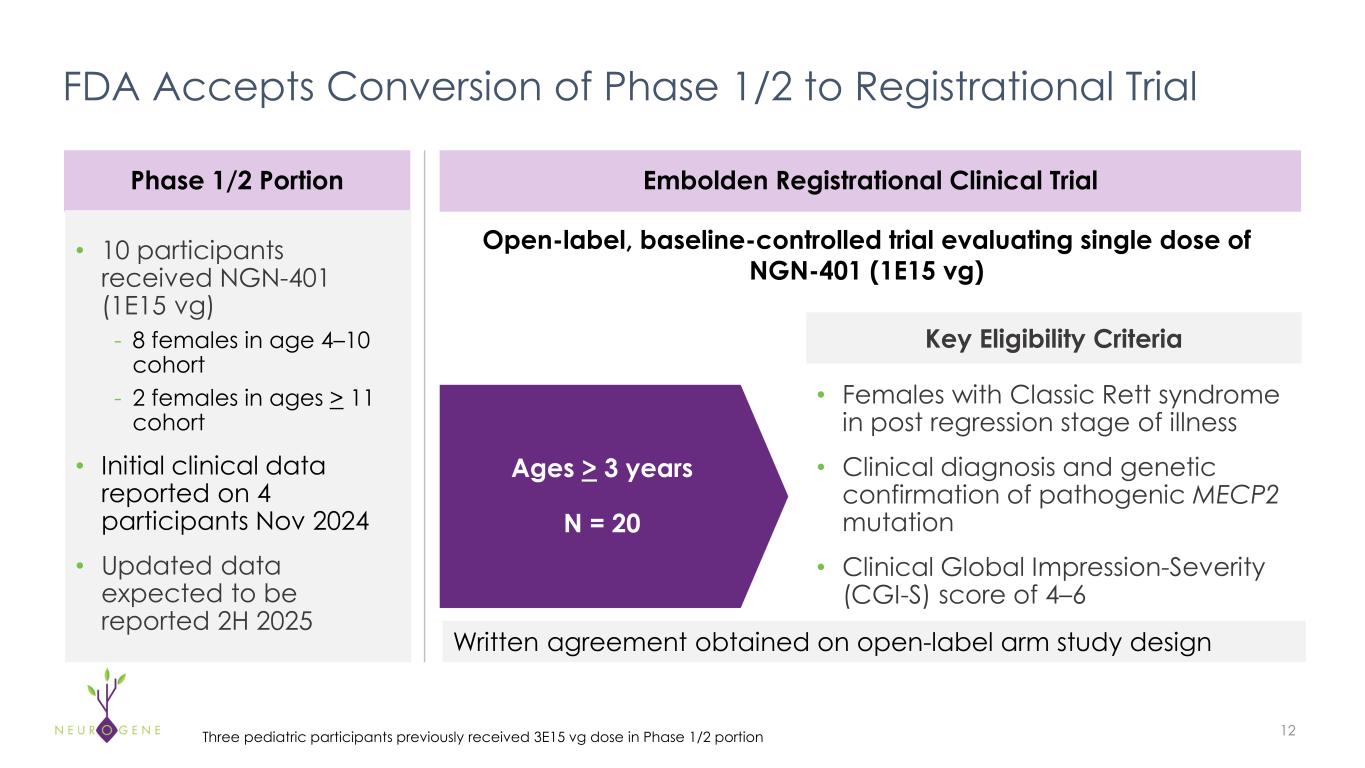
Completed discussions with FDA on registrational protocol; 13 sites to allow for rapid enrollment
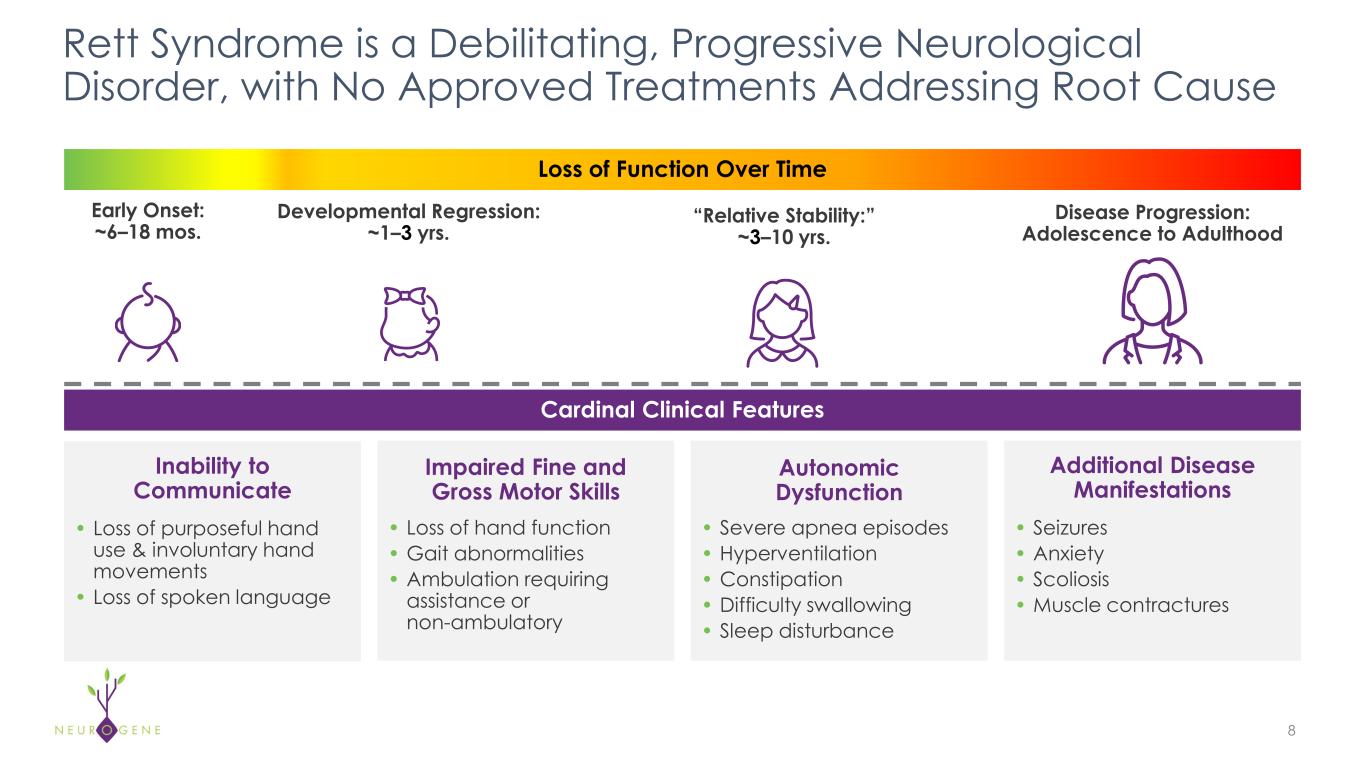
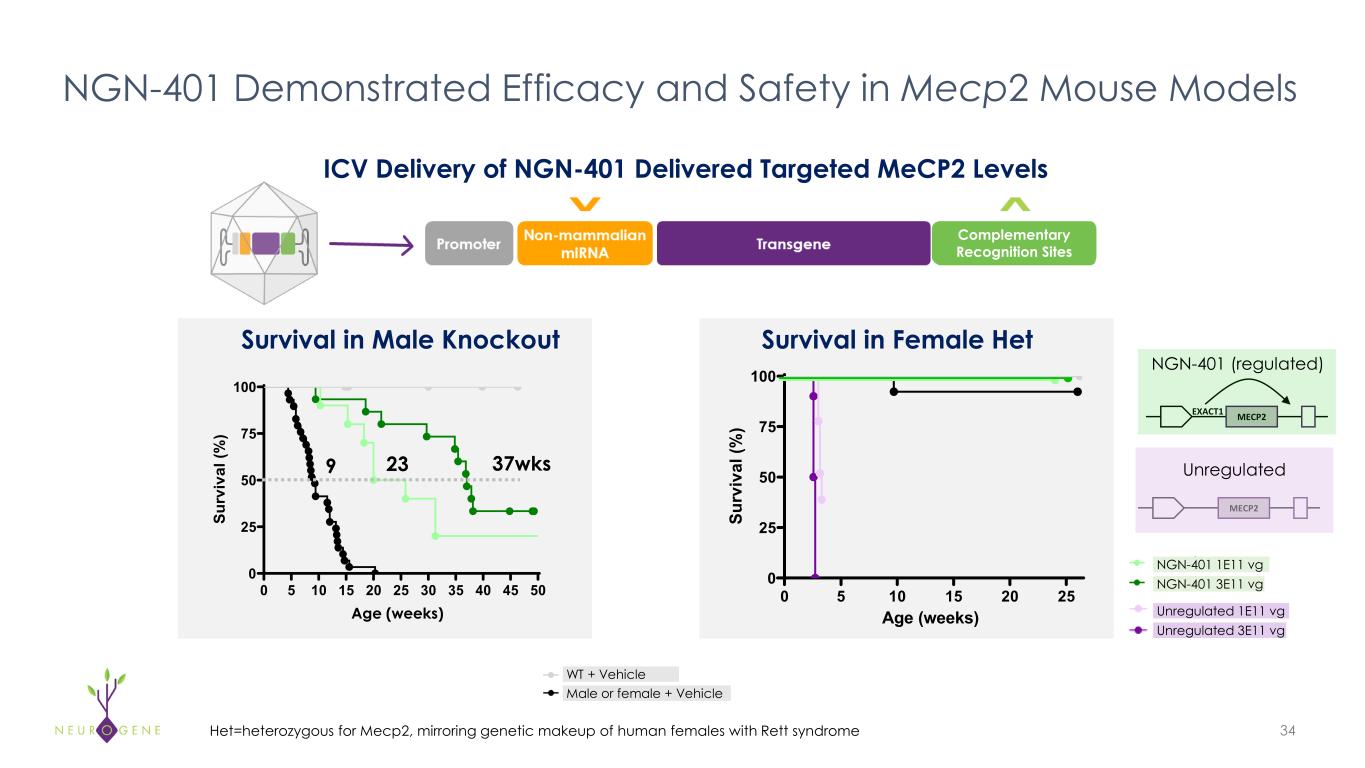
New preclinical data at ESGCT Congress highlight NGN-401 best-in-class potential and add to body of evidence that ICV administration is superior to IT-L in reaching key brain regions underlying Rett syndrome
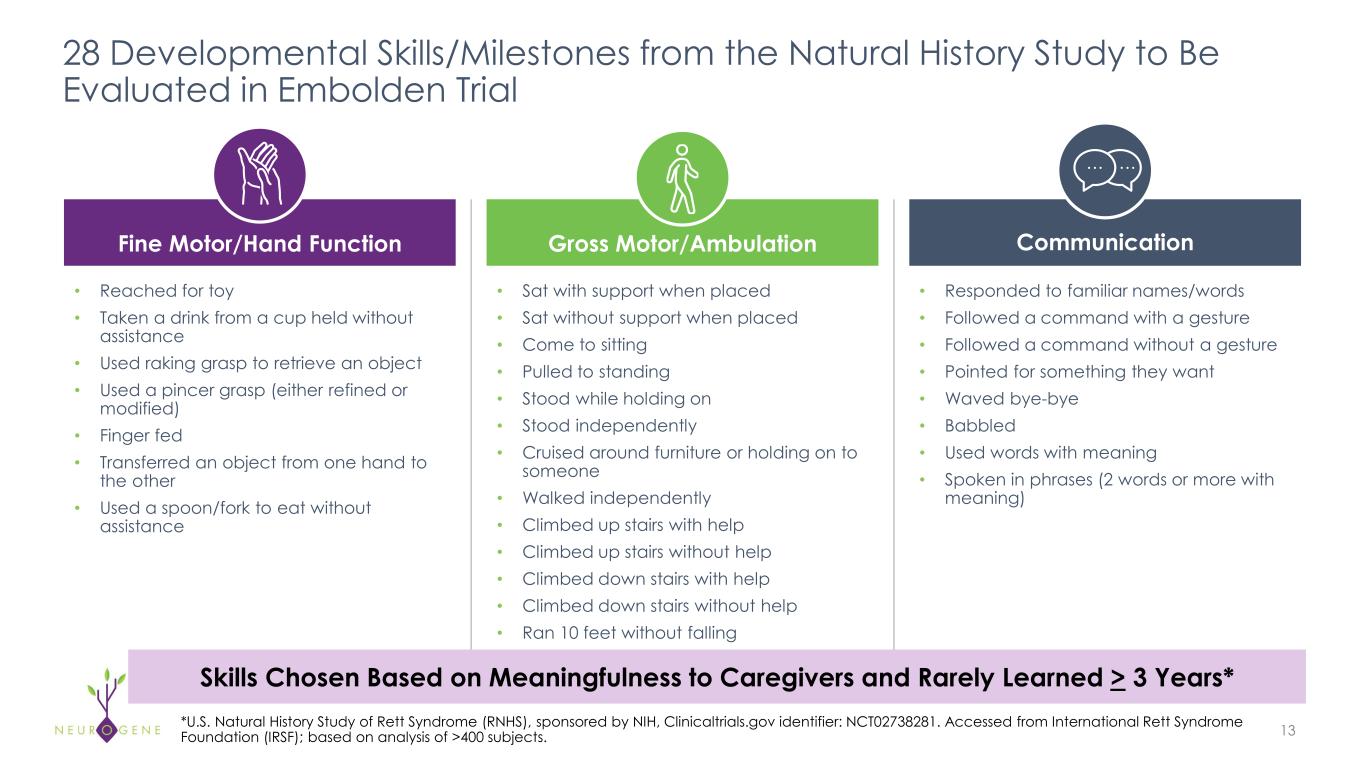
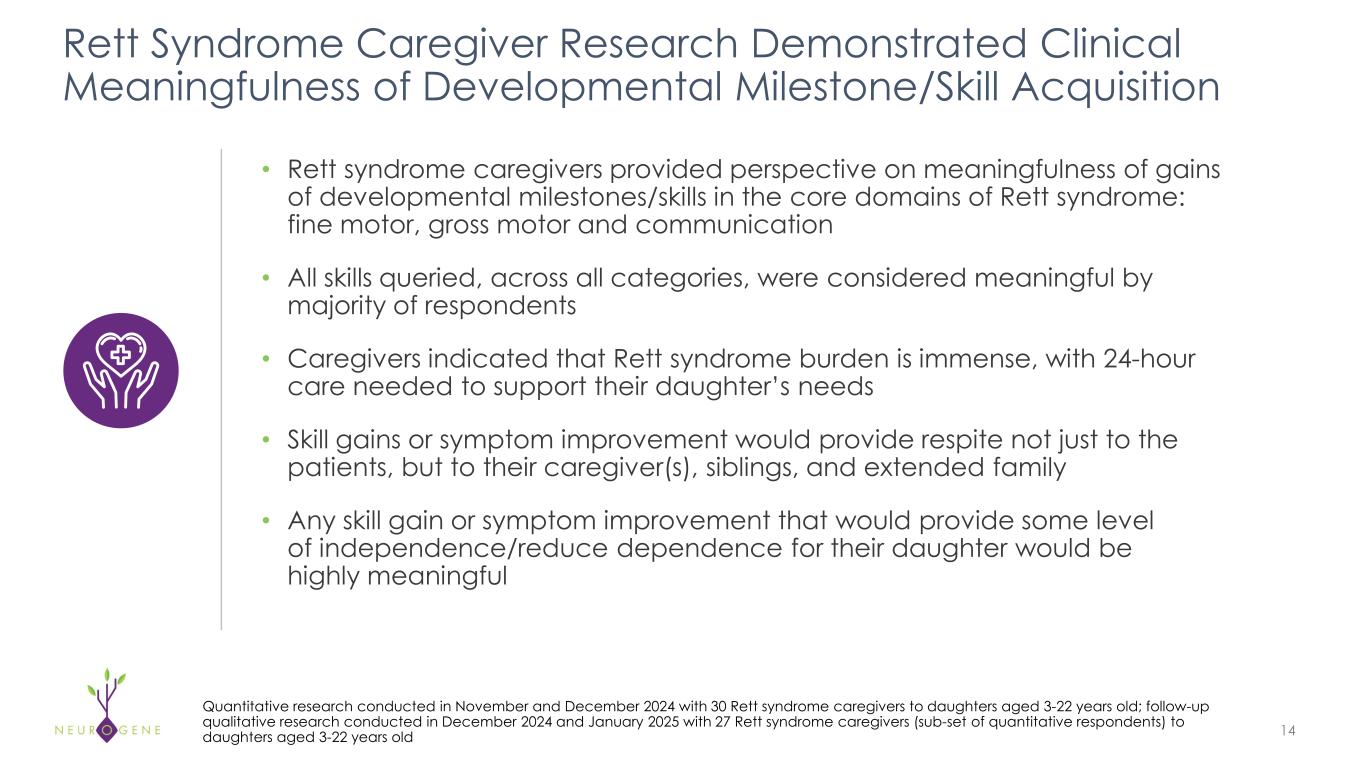
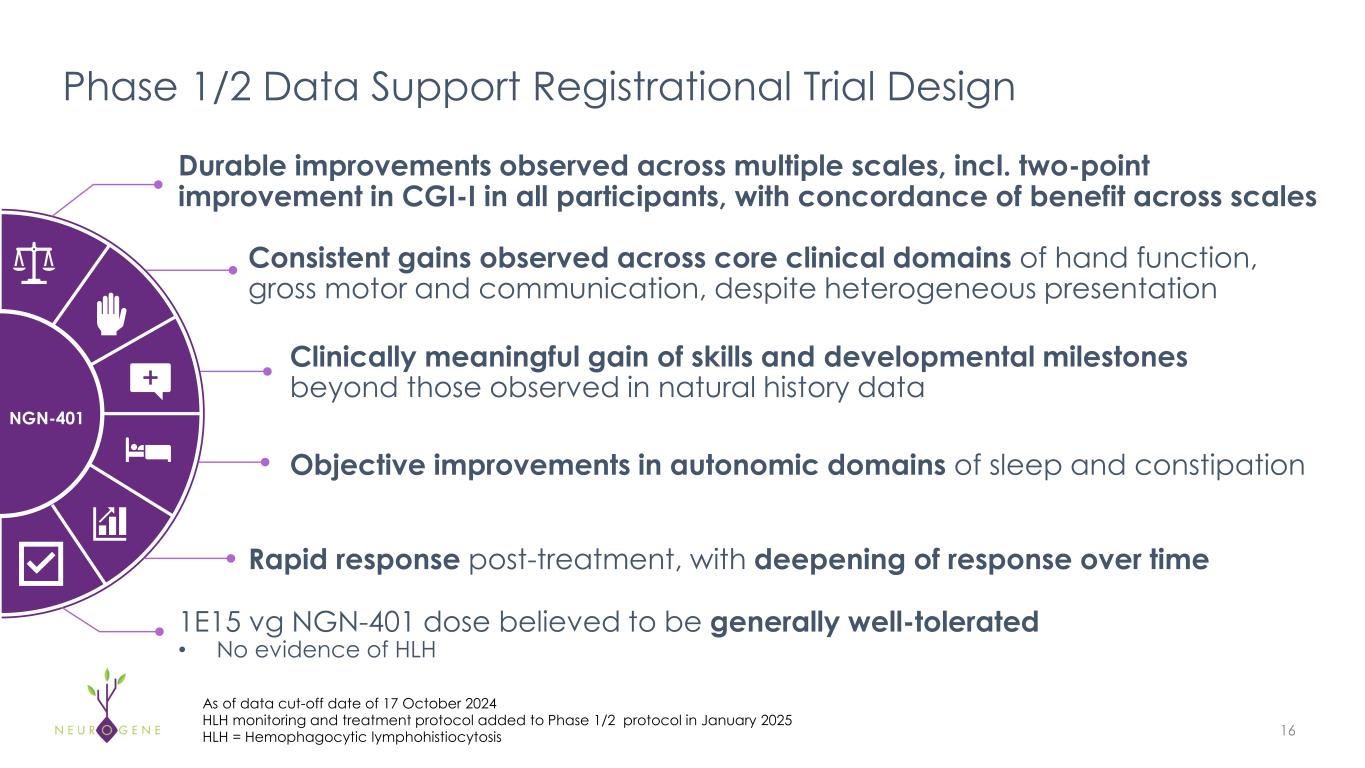
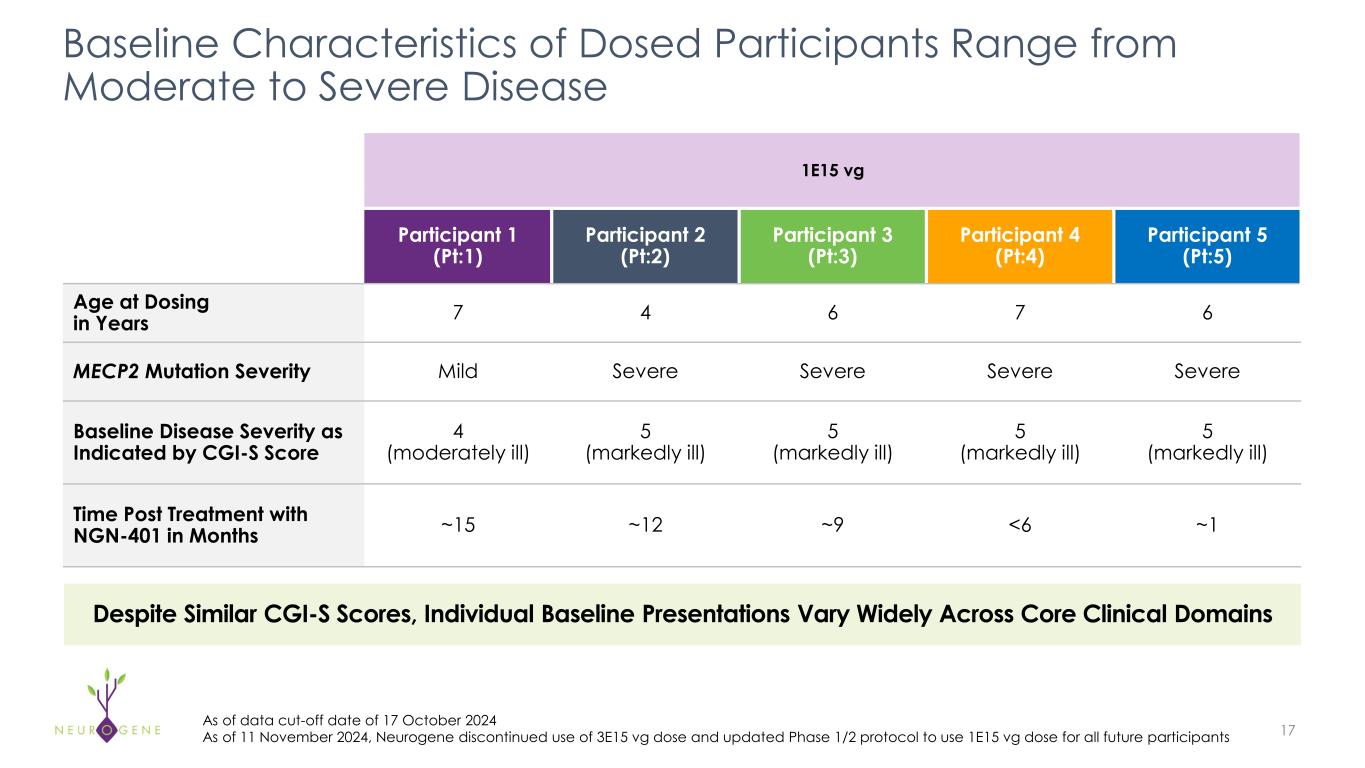
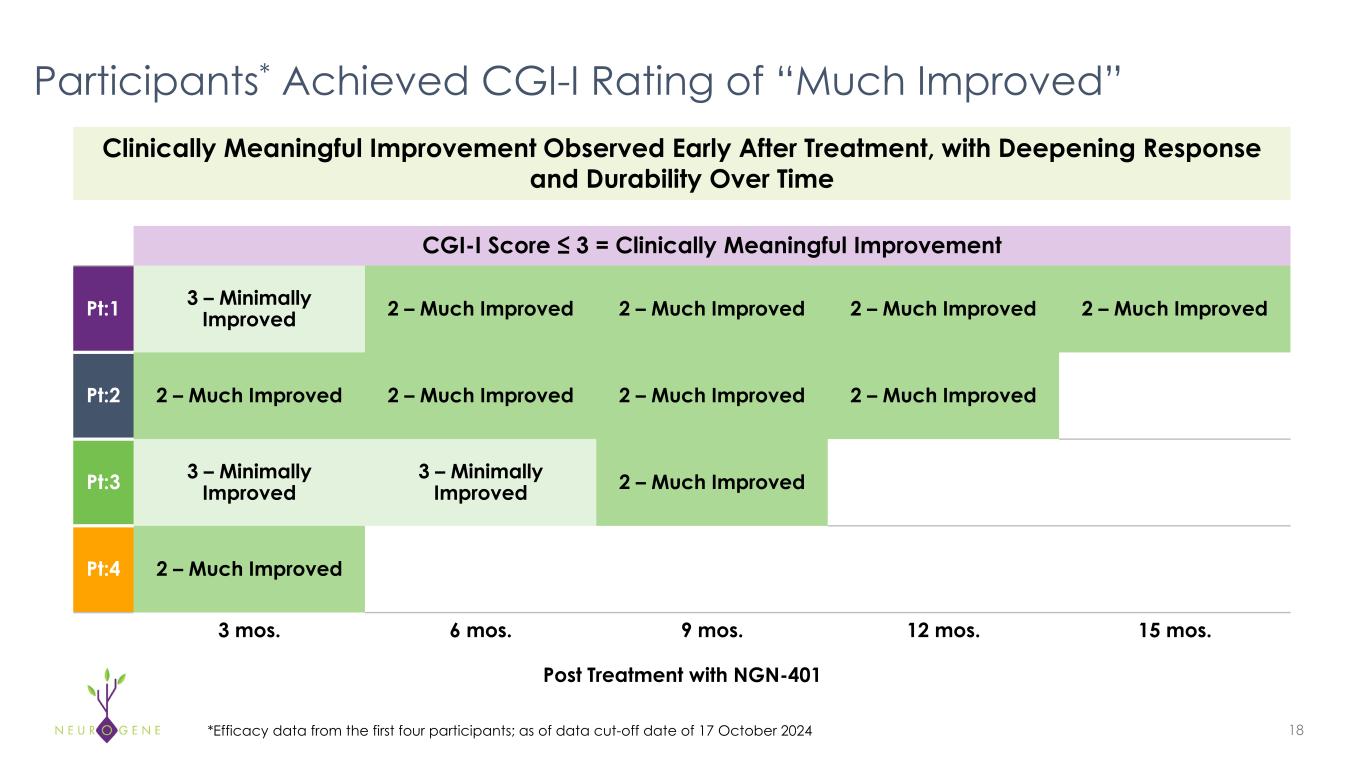
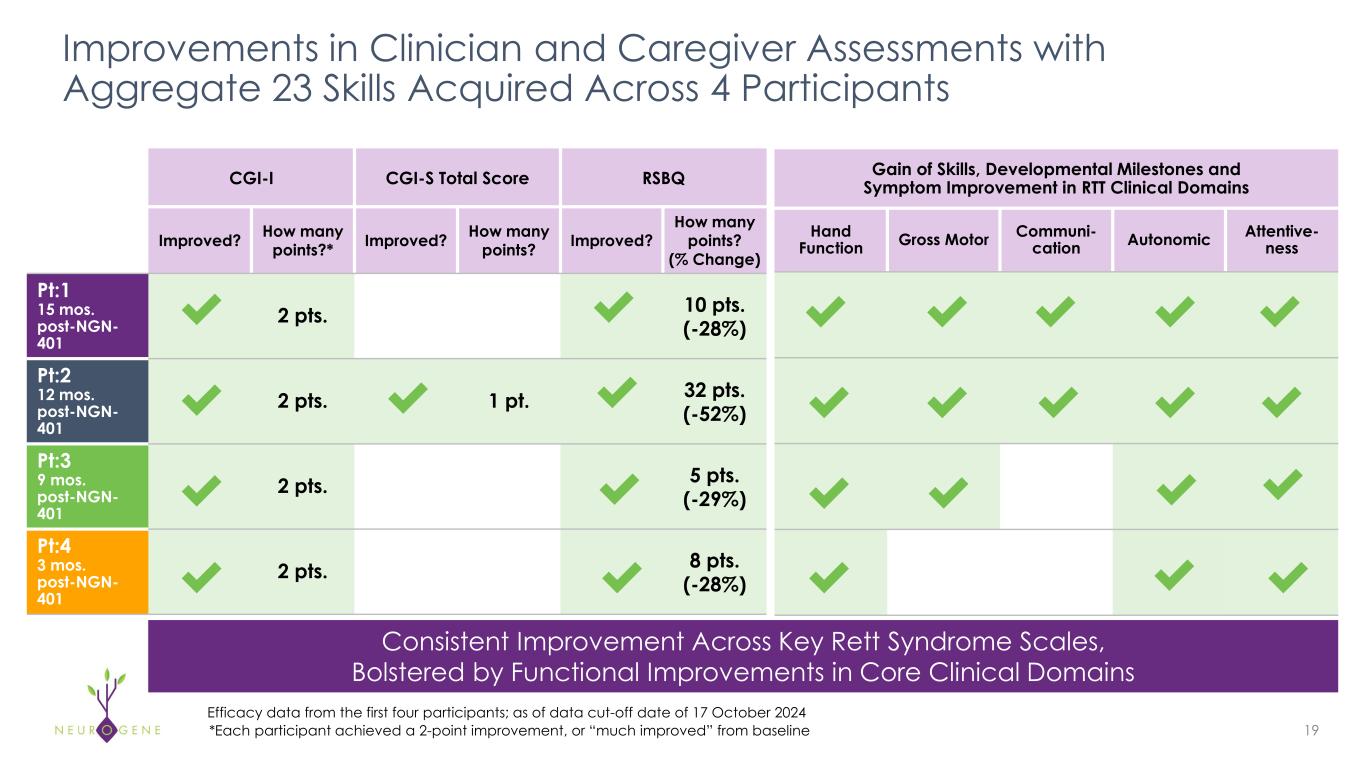
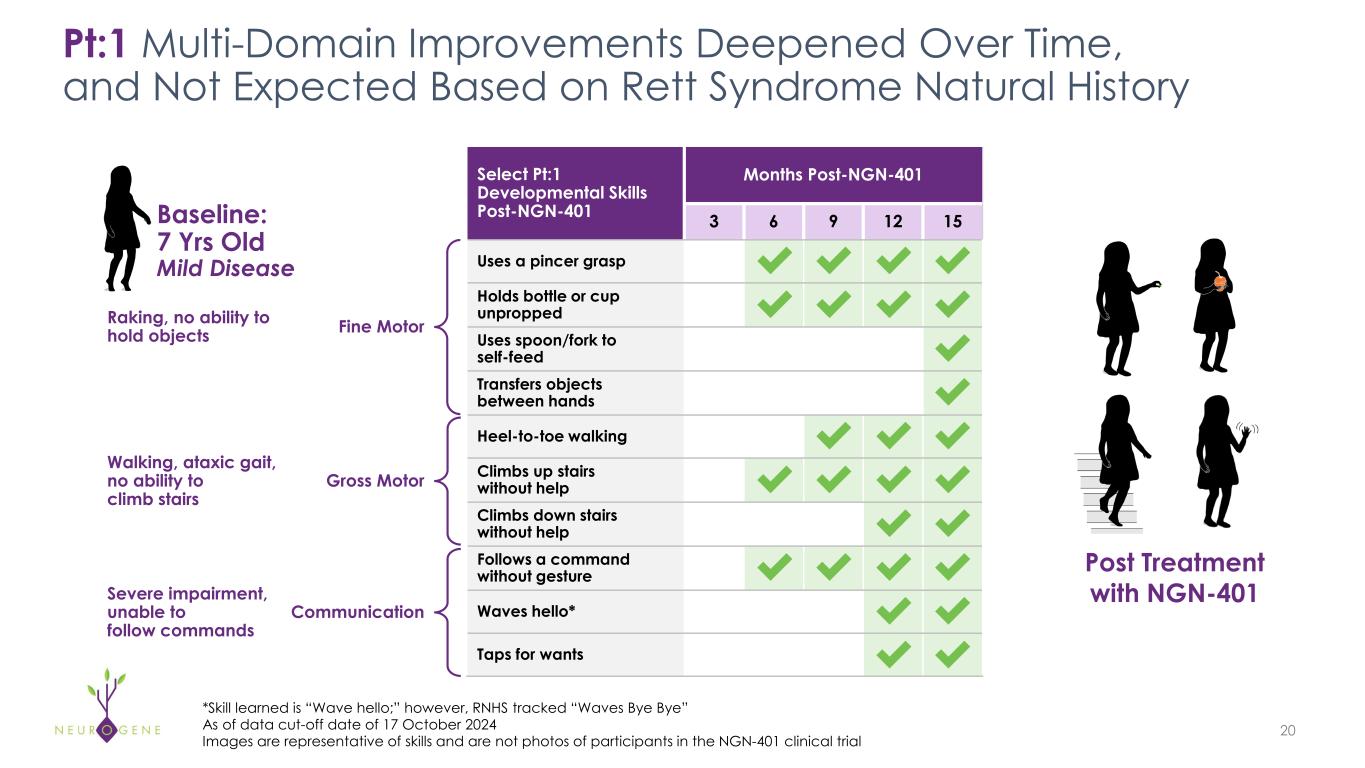
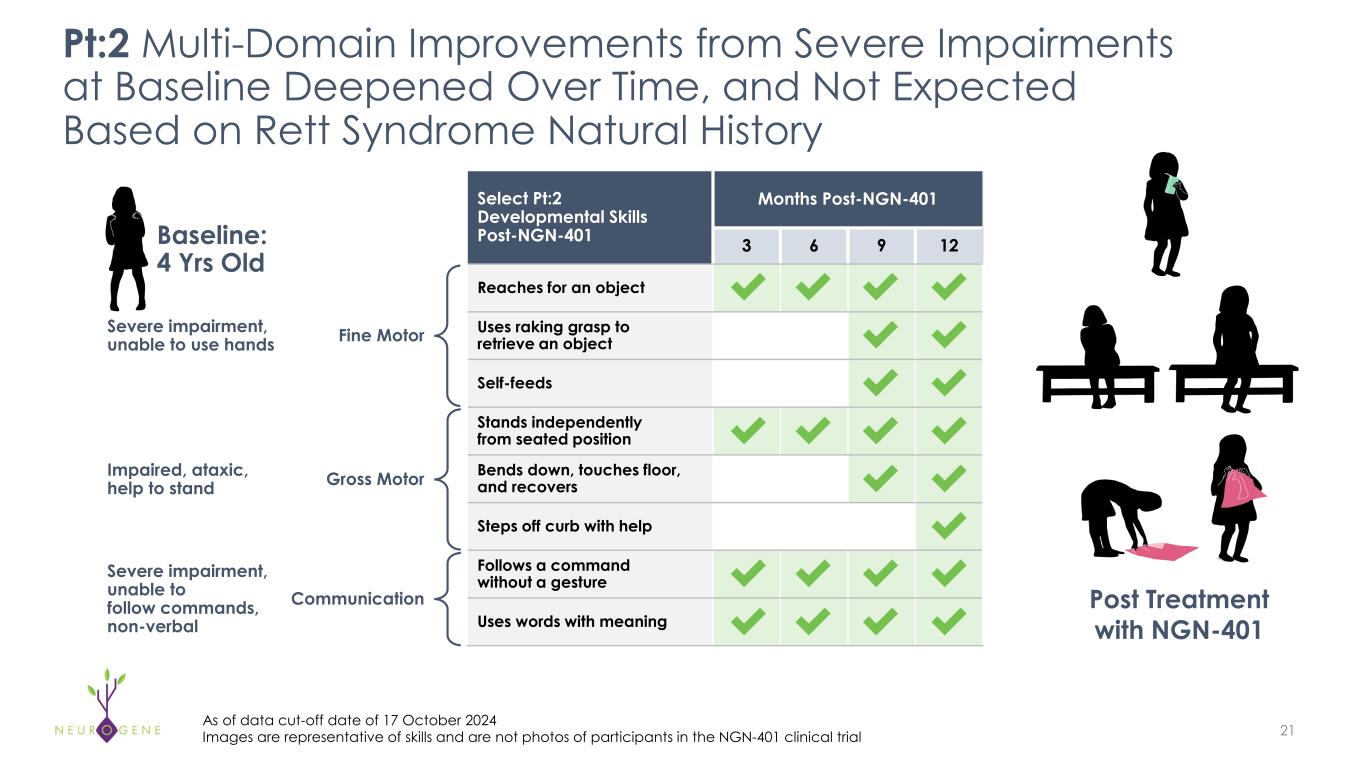
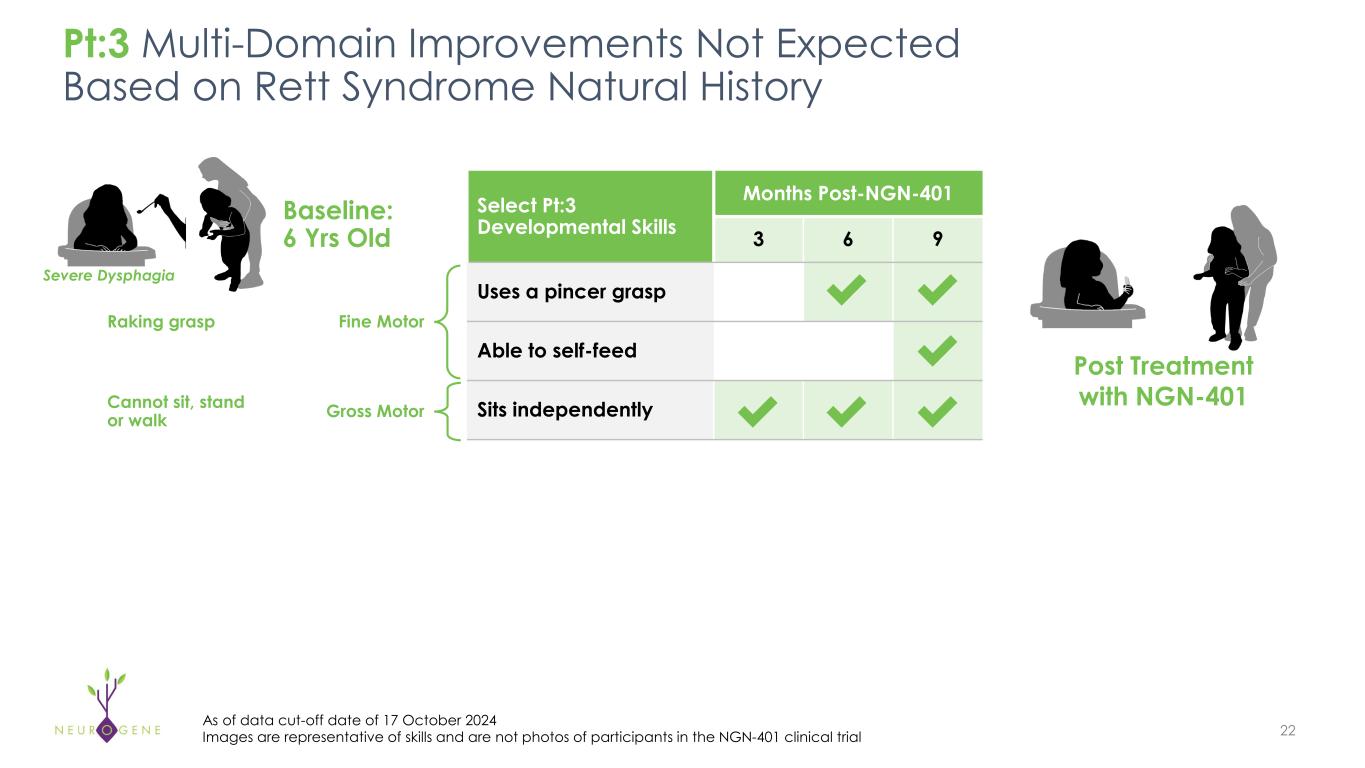
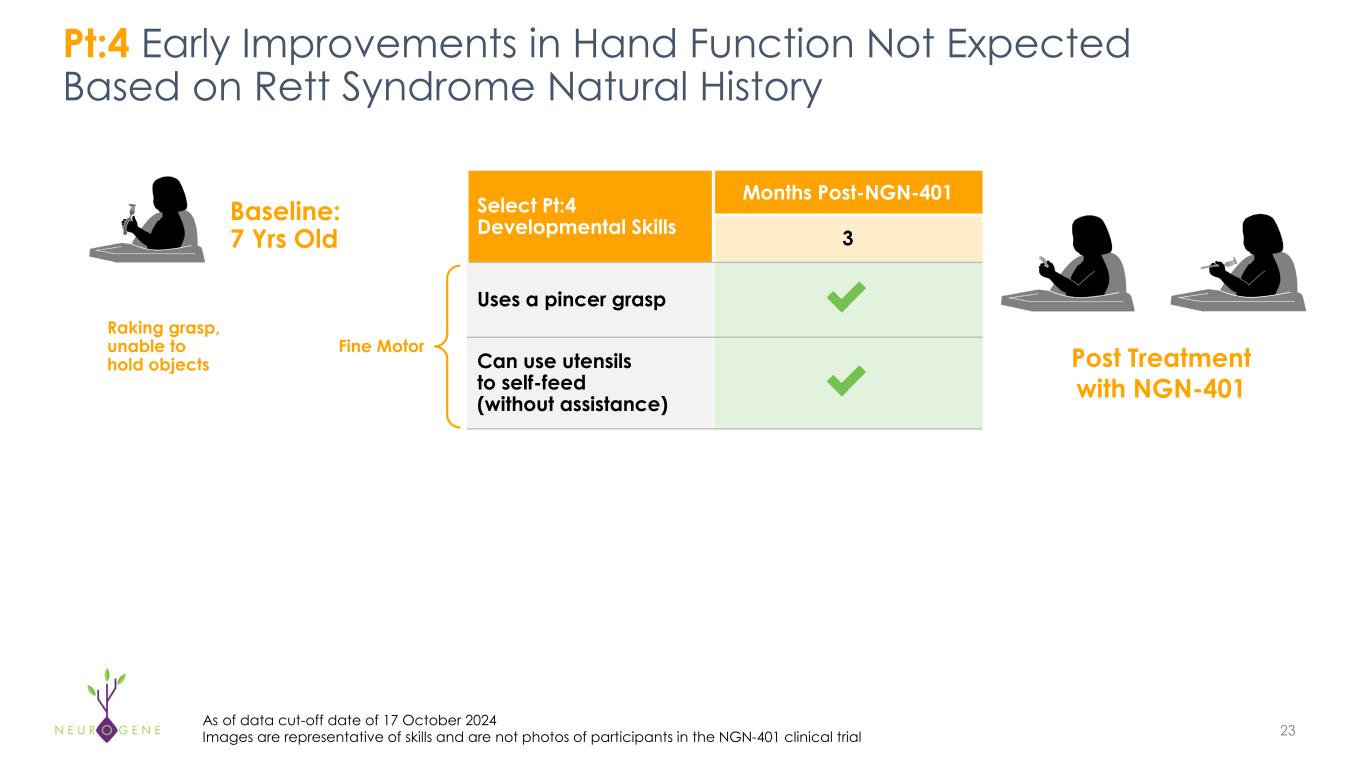
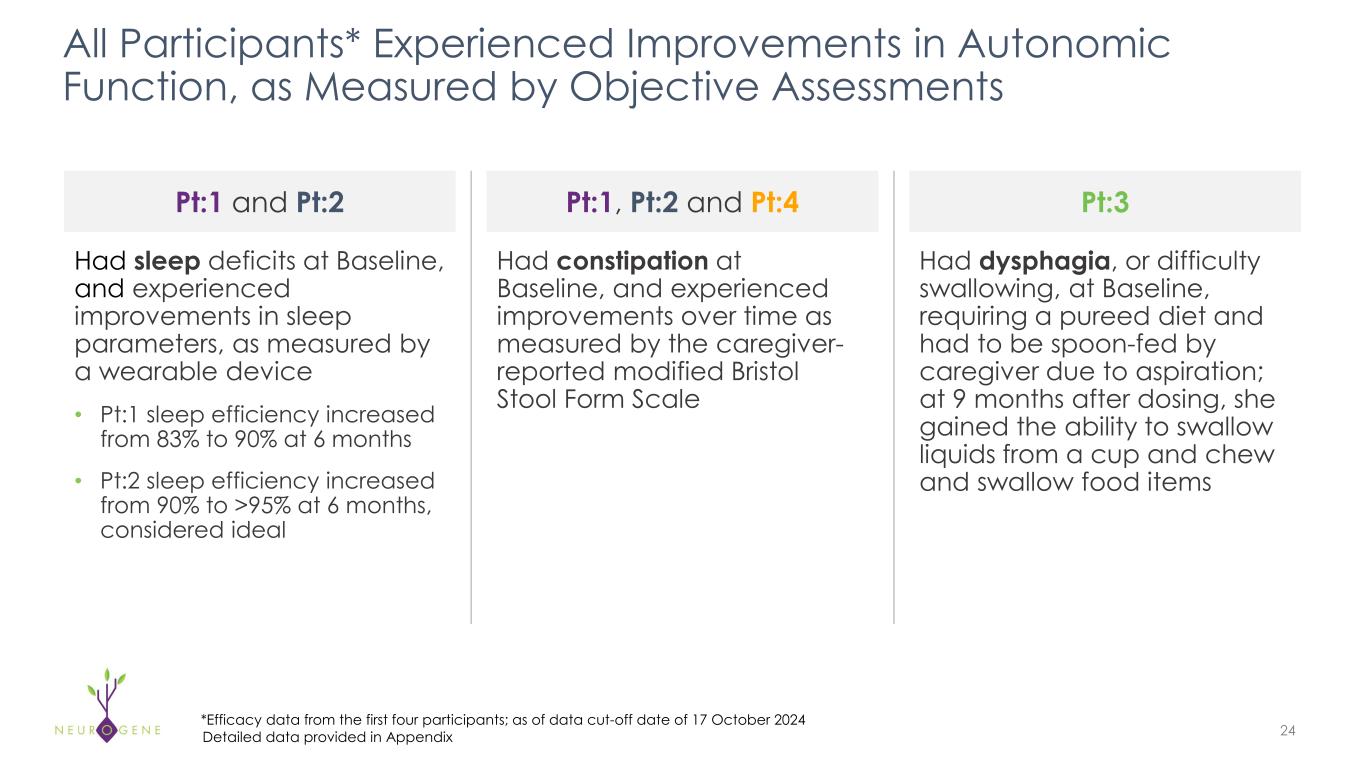
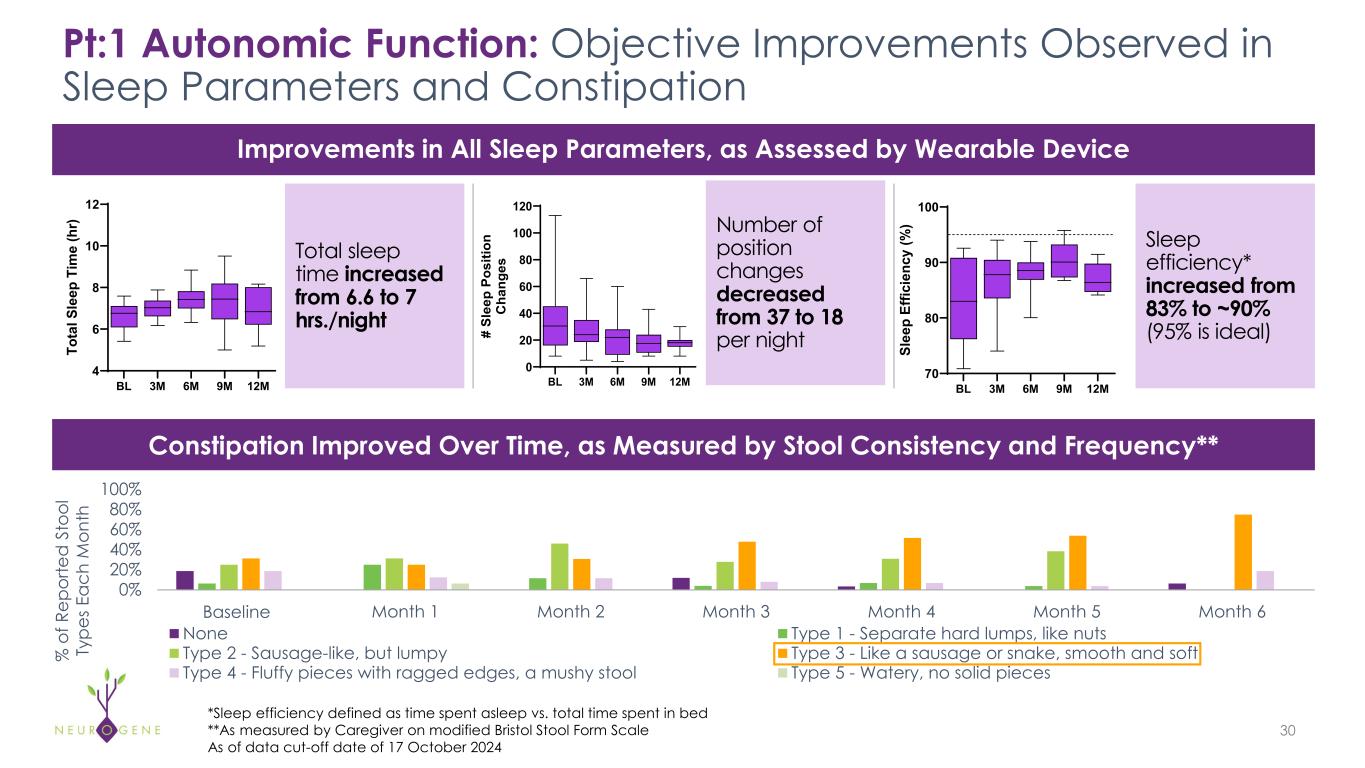
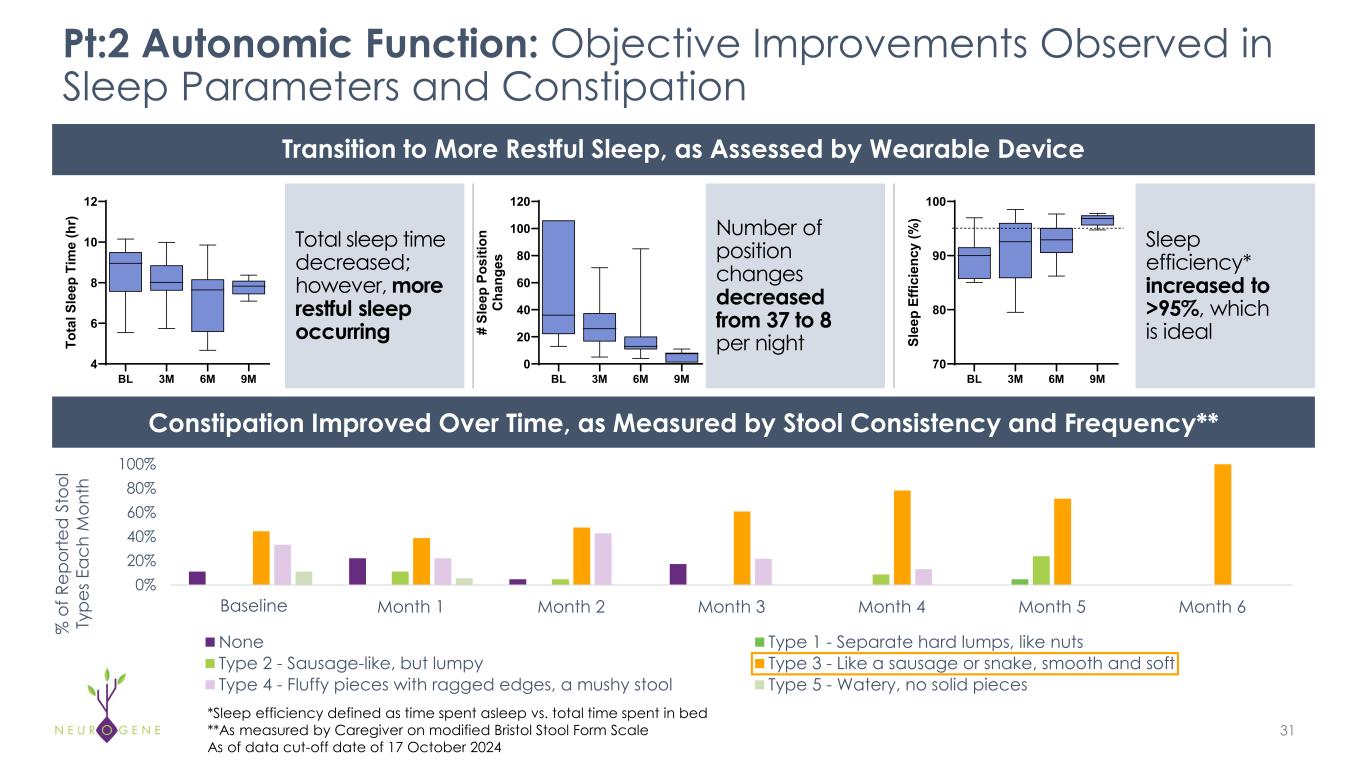
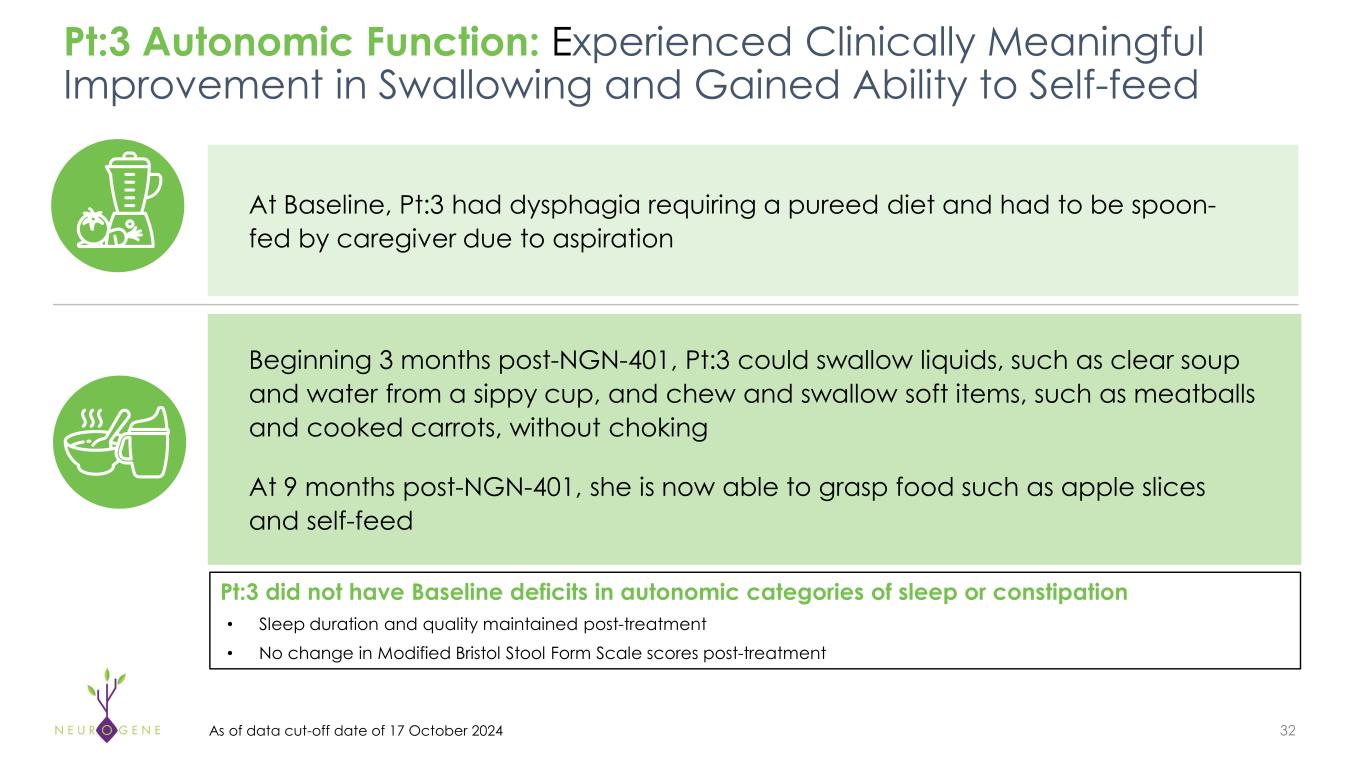
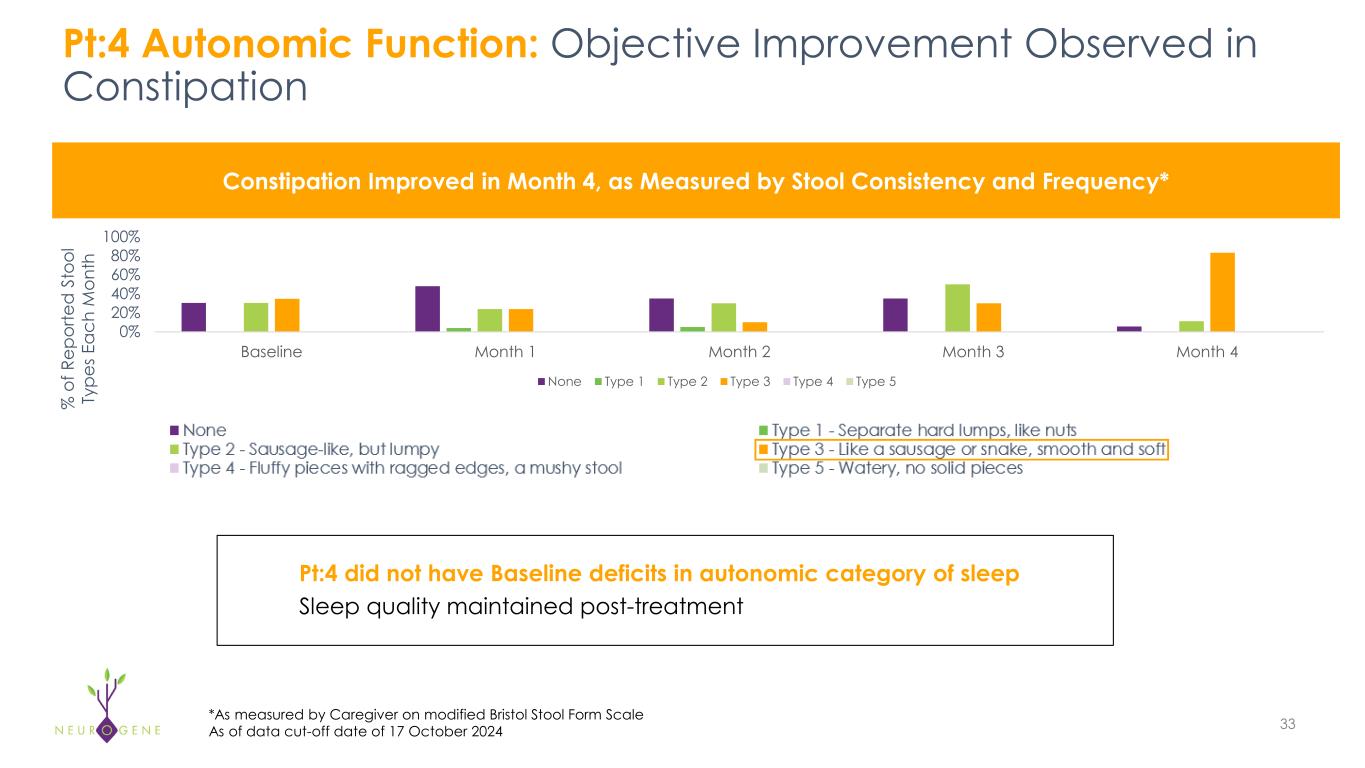
As previously reported, the first four participants in the NGN-401 Phase 1/2 trial gained 23 developmental milestones/skills, representing multi-domain improvement that requires transduction of key brain regions and nervous system
The preclinical data also provide evidence-based confirmation that ICV and IT-L administration result in comparable peripheral organ transduction, showing no liver-sparing benefit to IT-L when compared to ICV
NEW YORK – October 9, 2025 – Neurogene Inc. (Nasdaq: NGNE), a clinical-stage company founded to bring life-changing genetic medicines to patients and families affected by rare neurological diseases, today announced the Company has completed discussions with the U.S. Food and Drug Administration (FDA) on its Embolden™ registrational trial protocol and plans to initiate dosing in the fourth quarter of 2025. In response to strong interest from the Rett syndrome community, the trial is expected to enroll across 13 sites.
“In preparation for the Embolden registrational trial, we expanded our clinical trial footprint and have more than doubled our presence in the United States and plan to initiate dosing this quarter,” said Rachel McMinn, Ph.D., Founder and Chief Executive Officer of Neurogene. “Being part of the FDA’s START pilot program and having RMAT designation, we are well-positioned to engage in early discussions with the FDA with the goal of an expedited BLA submission.”
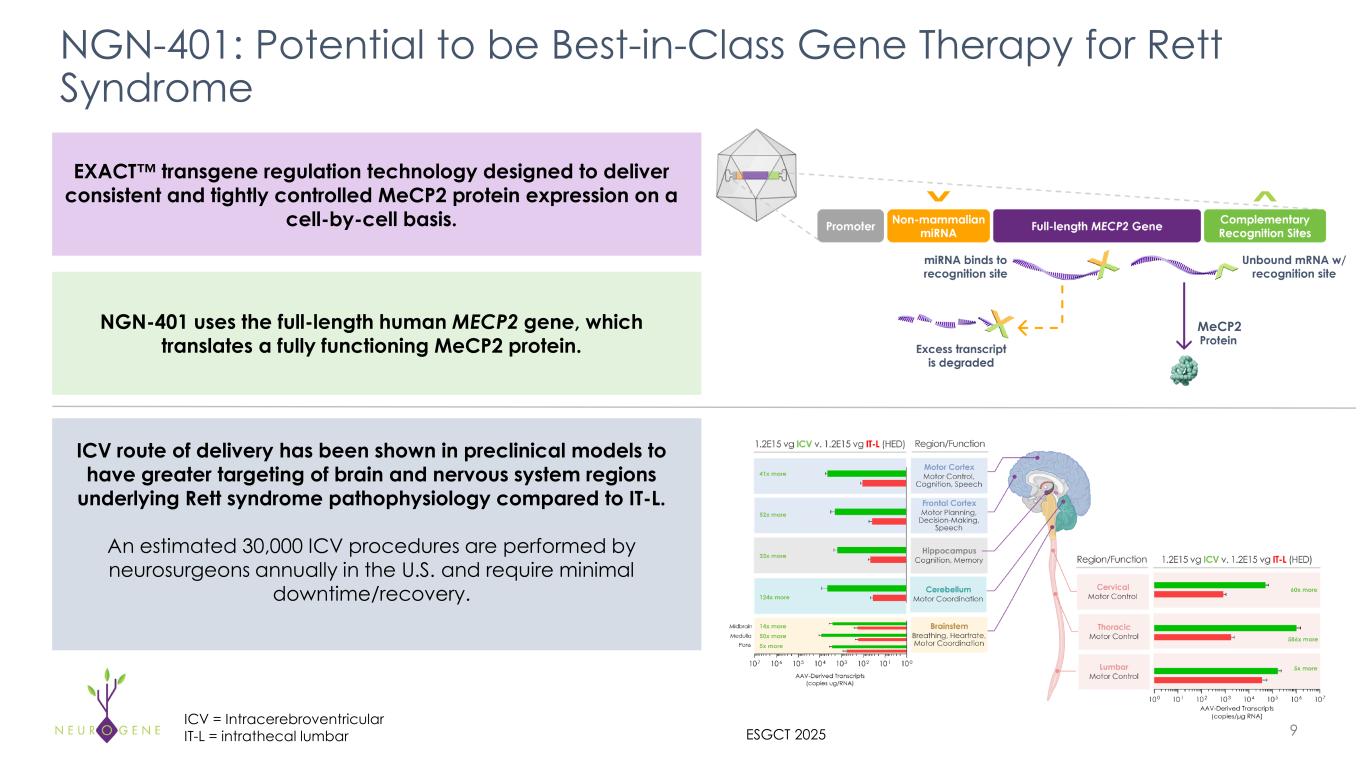
Neurogene also announced new preclinical data from nonhuman primates (NHPs) demonstrating that intracerebroventricular (ICV) delivery of NGN-401 achieves superior AAV biodistribution across brain regions relevant to Rett syndrome, compared to intrathecal lumbar (IT-L) delivery. These findings are consistent with preclinical data generated by other independent laboratories, reinforcing a growing body of evidence that supports ICV delivery for achieving greater expression in the brain. Neurogene’s results further validate and recapitulate this consensus, underscoring the robustness of the approach.
Dr. McMinn continued, “Feedback from the Rett syndrome community and KOLs reinforce that the decision to undergo gene therapy treatment is driven by a single critical factor: the potential for meaningful improvement. The route of administration data presented at the ESGCT Congress this week provide a potential rationale for the global improvement and multi-domain skill acquisition observed in our previously reported1 NGN-401 Phase 1/2 efficacy data. Importantly, the comparable systemic exposure between ICV and IT-L confirms there is no liver-sparing benefit to IT-L administration, consistent with clinical data from intra-CSF administered products.”
The head-to-head NHP study compared ICV and IT-L administration of NGN-401 at a dose that approximates the human dose being evaluated in the NGN-401 Phase 1/2 and Embolden clinical trials (1E15 vg) alongside a dose approximately four times higher than the clinically relevant dose administered via IT-L. Key results presented at the European Society of Gene & Cell Therapy (ESGCT) Annual Congress in Seville, Spain, included:
•ICV administration of NGN-401 showed greater expression of the full-length therapeutic MECP2 transgene mRNA in key brain regions underlying Rett syndrome pathophysiology when compared to the same IT-L administered dose; more similar levels of RNA expression were observed in the lumbar spinal cord
oHigher RNA expression in key areas of the brain was also observed when ICV was compared to the approximately four times higher dose IT-L cohort
•Comparable peripheral exposure of vector genome biodistribution was observed in peripheral organs, including the liver, between equivalent ICV and IT-L doses, consistent with clinical data from intra-CSF administered products
Neurogene’s ESGCT Congress Poster Presentation Details
•Title: Route matters: intracerebroventricular (ICV) delivery of NGN-401 drives superior transgene expression to key areas of the brain when compared to intrathecal (IT-L) delivery at clinically relevant dose — implications for a one-time gene therapy for Rett syndrome
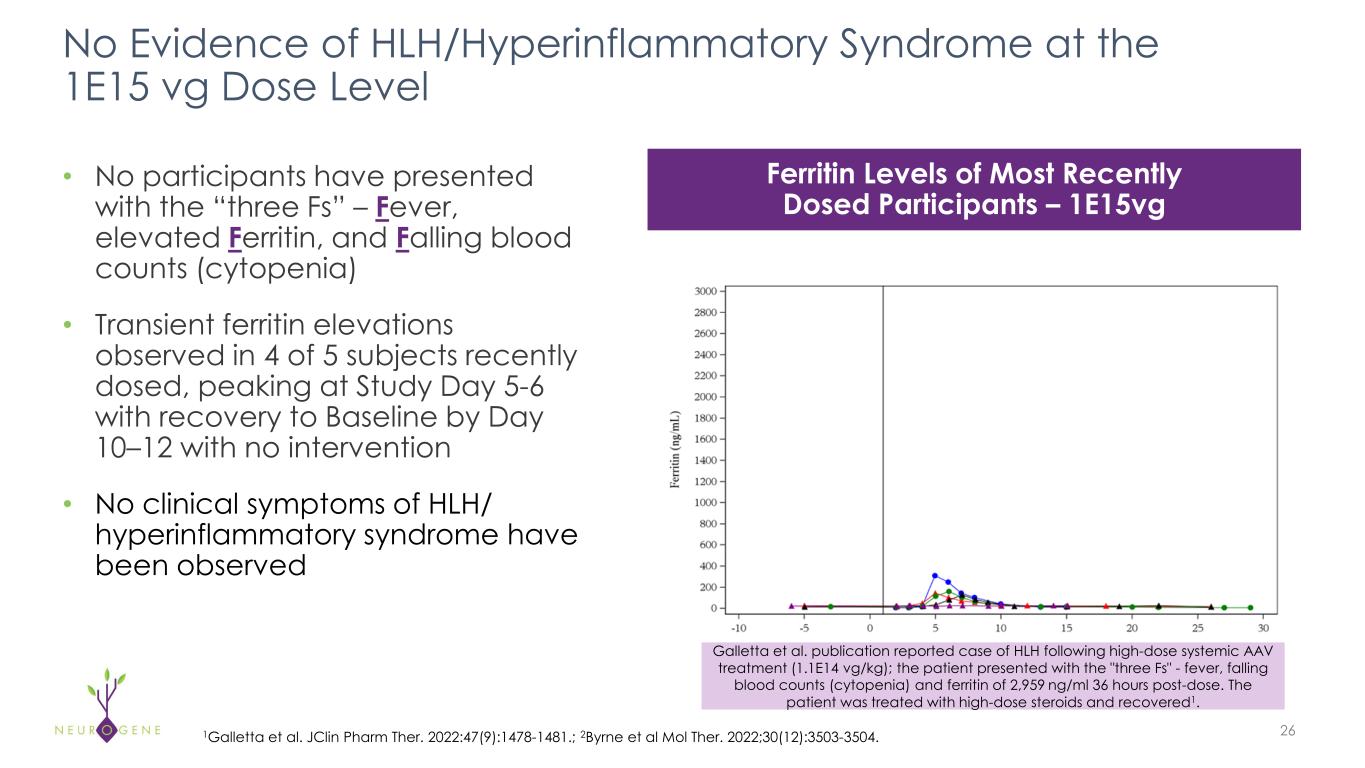
•Title: Hemophagocytic lymphohistiocytosis (HLH)/hyperinflammatory syndrome following high dose AAV9 therapy (Encore from 2025 IRSF Rett Syndrome Scientific Meeting)
Both posters will be presented on October 9, 2025, at 2 p.m. CEST and will be available on Neurogene’s website.
1As of data cut-off date of October 17, 2024
About Neurogene
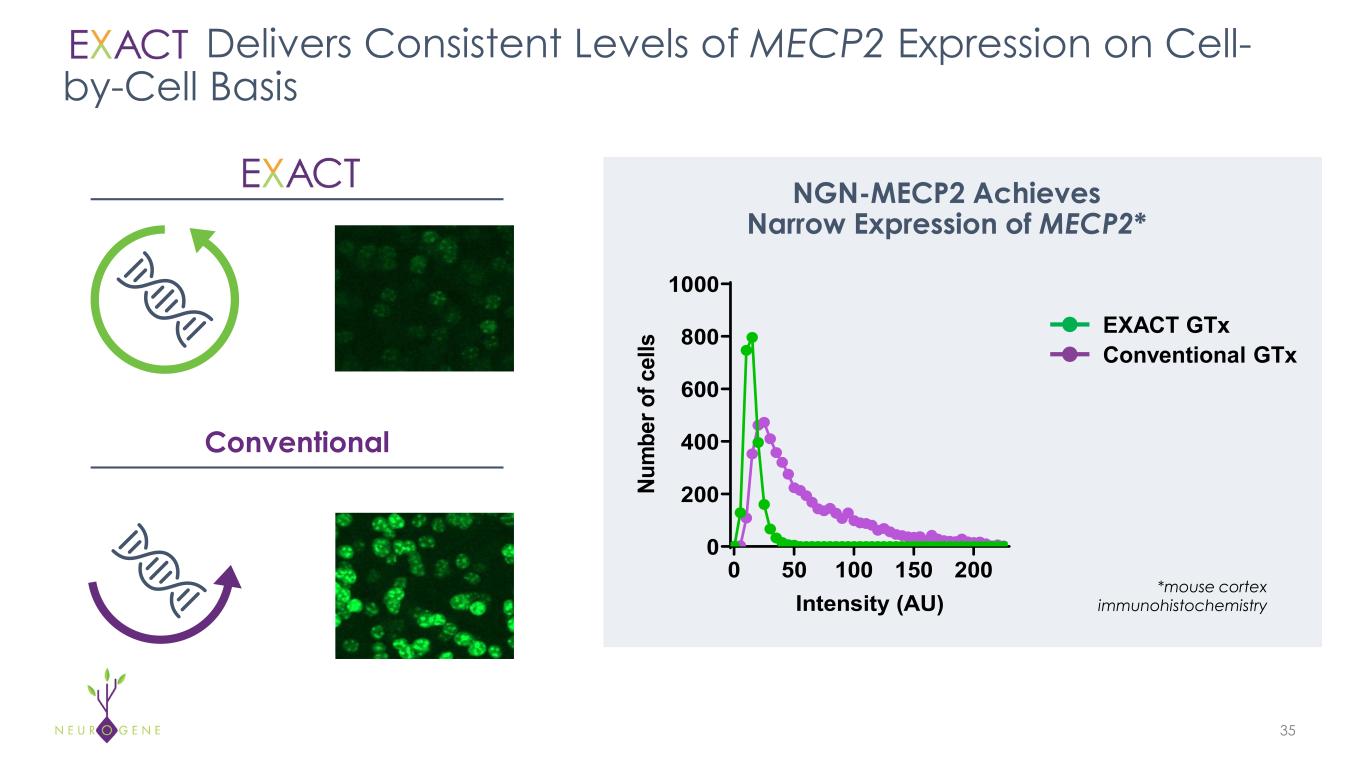
The mission of Neurogene is to treat devastating neurological diseases to improve the lives of patients and families impacted by these rare diseases. Neurogene is developing novel approaches and treatments to address the limitations of conventional gene therapy in central nervous system disorders. This includes selecting a delivery approach to maximize distribution to target tissues and designing products to maximize potency and purity for an optimized efficacy and safety profile. The Company’s novel and proprietary EXACT™ transgene regulation platform technology allows for the delivery of therapeutic levels while limiting transgene toxicity associated with conventional gene therapy. Neurogene has constructed a state-of-the-art gene therapy manufacturing facility in Houston, Texas. CGMP production of NGN-401 was conducted in this facility and will support pivotal clinical development activities. For more information, visit www.neurogene.com.
About NGN-401
NGN-401 is an investigational AAV9 gene therapy being developed as a one-time treatment for Rett syndrome. It is the first clinical candidate to deliver the full-length human MECP2 gene under the control of Neurogene’s EXACT™ transgene regulation technology. EXACT technology is an important advancement in gene therapy for Rett syndrome, specifically because the disorder requires a treatment approach that enables targeted levels of MECP2 transgene expression without causing overexpression-related toxic effects associated with conventional gene therapy.
NGN-401 was selected by the U.S. Food and Drug Administration (FDA) for its START Pilot Program and has also received Regenerative Medicine Advance Therapy (RMAT) designation, orphan drug designation, Fast Track designation and rare pediatric designation from the FDA. Neurogene was previously granted an INTERACT meeting with the FDA regarding the EXACT technology. NGN-401 also received Priority Medicines (PRIME) designation, orphan designation and advanced therapy medicinal product designation from the European Medicines Agency (EMA) and the Innovative Licensing and Application Pathway (ILAP) designation from the United Kingdom (UK) Medicines and Healthcare products Regulatory Agency (MHRA).
Cautionary Note Regarding Forward-Looking Statements
Statements in this press release which are not historical in nature are intended to be, and hereby are identified as, forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current expectations and beliefs of the management of Neurogene, as well as assumptions made by, and information currently available to, management of Neurogene, including, but not limited to, statements regarding: our proposed trial design for the EmboldenTM registrational clinical trial, including details related to the timing and anticipated results of our registrational clinical trial such as anticipated dates for initial enrollment, the number of trial sites participating in the clinical trial, the anticipated date of 12 month endpoint data availability, and the ability to expedite the submission of a BLA application for NGN-401; the potential for improved efficacy of NGN-401 as a treatment for Rett syndrome when compared to other treatment candidates using a different route of administration; patient and KOL sentiment relating to priorities on selecting potential gene therapy treatments; expected future interactions with or positions of the FDA; the safety, tolerability and efficacy of NGN-401; the potential for success of the Embolden registrational clinical trial for NGN-401 for the treatment of Rett syndrome; expected timing for additional interim data from the Company’s NGN-401 Phase 1/2 trial for Rett syndrome; the potential superiority of ICV administration as compared to IT-L administration, based in part on preclinical NHP data; and the effectiveness of the monitoring and treatment protocol for HLH in Neurogene’s Phase 1/2 clinical trial of NGN-401.
Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” “on track,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements contain these words. Forward-looking statements are based on current beliefs and assumptions that are subject to risks, uncertainties and assumptions that are difficult to predict with regard to timing, extent, likelihood, and degree of occurrence, which could cause actual results to differ materially from anticipated results and many of which are outside of Neurogene’s control. Such risks, uncertainties and assumptions include, among other things, the risks and uncertainties identified under the heading "Risk Factors" included in Neurogene’s Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission (SEC) on March 24, 2025, Neurogene’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, filed with the SEC on August 11, 2025, and other filings that the Company has made and may make with the SEC in the future. Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that the contemplated results of any such forward-looking statements will be achieved. Forward-looking statements in this communication speak only as of the day they are made and are qualified in their entirety by reference to the cautionary statements herein. Except as required by applicable law, Neurogene undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
# # #
Company Contact:
Mike Devine
Executive Director, Corporate Communications
michael.devine@neurogene.com
Investor Contact:
Melissa Forst
Argot Partners
Neurogene@argotpartners.com