UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): October 30, 2023
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 001-36019 | 26-1434750 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
26 Main Street, Chatham, New Jersey 07928
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (862) 904-8182
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | TNXP | The NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On October 30, 2023, Tonix Pharmaceuticals Holding Corp. (the “Company”) announced data from two oral presentations which were delivered recently at the American College of Surgeons Clinical Congress 2023 (“ACS”), and The International Pancreas and Islet Transplant Association (“IPITA”), the International Xenotransplantation Association (IXA), and the Cell Transplant and Regenerative Medicine Society (“CTRMS”) Joint Congress by faculty at the Center for Transplantation Sciences, Massachusetts General Hospital (“MGH”) in October 2023, which involve data from the Company’s TNX-1500 (Fc-modified dimeric anti-CD40L monoclonal antibody [mAb]) product candidate for the prevention of organ transplant rejection. A copy of the press release which discusses this matter is furnished hereto as Exhibit 99.01, and incorporated herein by reference. Copy of the presentations are furnished hereto as Exhibits 99.02 and 99.03, and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.01, 99.02 and 99.03 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01. | Other Events. |
On October 30, 2023, the Company announced data from two oral presentations which were delivered at the ACS, IPITA, IXA and CTRMS which involve data from TNX-1500 for the prevention of organ transplant rejection. The oral presentations entitled, “Pilot Evaluation of a Clinical Xeno Heart Transplant Regimen in a Preclinical Model” and “Extended Survival of 9- and 10-Gene Edited Pig Heart Xenografts with Ischemia Minimization and CD154 Costimulation Blockade-Based Immunosuppression” include data demonstrating the use of TNX-1500 as maintenance therapy after xeno heart transplant in non-human primates. In both studies, genetically engineered pigs in baboon transplants were treated with cold-perfused ischemia minimization and a novel costimulation-based immunosuppressive regimen including TNX-1500. The multi-GE pigs were provided by eGenesis, Inc. and Revivicor, Inc.
Forward- Looking Statements
This Current Report on Form 8-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating to the Company’s product development, clinical trials, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities and other statement that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management’s current beliefs and assumptions.
These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential,” “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the SEC. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this press release. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) |
Exhibit No. |
Description. | ||
|
Press release of the Company, dated October 30, 2023 Pilot Evaluation of a Clinical Xeno Heart Transplant Regimen in a Preclinical Model Extended Survival of 9- and 10-Gene Edited Pig Heart Xenografts with Ischemia Minimization and CD154 Costimulation Blockade-Based Immunosuppression |
||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: October 30, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01
Tonix Pharmaceuticals Announces New Data Involving TNX-1500 (Fc-modified dimeric anti-CD40L mAb) in Heart Xenotransplantation in Animal Models at the ACS Clinical Congress and IPITA-IXA-CTRMS Joint Congress
Research Directed by Faculty of the Center for Transplantation Sciences, Massachusetts General Hospital
TNX-1500 is currently in Phase 1 Clinical Development
Tonix is Developing TNX-1500 for Prevention of Kidney Allograft Rejection as the First Indication; Multiple Other Indications, including Autoimmune Disorders, are Planned
CHATHAM, N.J., October 30, 2023 (GLOBE NEWSWIRE) – Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a biopharmaceutical company with marketed products and a pipeline of development candidates, today announced data from two oral presentations which were delivered recently at the American College of Surgeons (ACS) Clinical Congress 2023, and The International Pancreas and Islet Transplant Association (IPITA), the International Xenotransplantation Association (IXA), and the Cell Transplant and Regenerative Medicine Society (CTRMS) Joint Congress by faculty at the Center for Transplantation Sciences, Massachusetts General Hospital (MGH) in October 2023. The data involve Tonix’s TNX-1500 (Fc-modified dimeric anti-CD40L monoclonal antibody [mAb]) which is currently in Phase 1 development for the prevention of organ transplant rejection. Copies of the presentations are available under the Scientific Presentations tab of the Tonix Pharmaceuticals website at www.tonixpharma.com.
The oral presentations titled, “Pilot Evaluation of a Clinical Xeno Heart Transplant Regimen in a Preclinical Model” and “Extended Survival of 9- and 10-Gene Edited Pig Heart Xenografts with Ischemia Minimization and CD154 Costimulation Blockade-Based Immunosuppression” by Dr. Ikechukwu Ileka et al. include data demonstrating the use of TNX-1500 as maintenance therapy after xeno heart transplant in non-human primates. In both studies, genetically engineered (GE) pigs in baboon transplants were treated with cold-perfused ischemia minimization and a novel costimulation-based immunosuppressive regimen including TNX-1500. The multi-GE pigs were provided by eGenesis and Revivicor.
“The results of these preclinical studies are encouraging and demonstrate the potential of genetically engineered pig hearts in the context of a clinically applicable regimen,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals. “Because anti-CD40L treatment is widely recognized as critical to the success of xeno organ transplant, no animals were transplanted without anti-CD40L treatment.”
“These and other data1,2,3 confirm the rationale for us to pursue development of TNX-1500 to prevent rejection in human transplantation,” said Dr. Lederman. “We are currently enrolling in a Phase 1 trial in healthy volunteers to support the development of TNX-1500 for the prevention of allograft rejection. However, long term we hope to develop TNX-1500 for xenotransplantation in which the donor organ comes from genetically engineered pigs.”
1Lassiter G., et al. Am. J. Transplant. 2023. https://doi.org/10.1016/j.ajt.2023.03.022
2Miura S., et al. Am. J. Transplant. 2023. https://doi.org/10.1016/j.ajt.2023.03.025
3Anand R.P., et al. Nature. 2023. 622, 393–401.
Tonix Pharmaceuticals Holding Corp.*
Tonix is a biopharmaceutical company focused on commercializing, developing, discovering and licensing therapeutics to treat and prevent human disease and alleviate suffering. Tonix Medicines, our commercial subsidiary, markets Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg under a transition services agreement with Upsher-Smith Laboratories, LLC from whom the products were acquired on June 30, 2023. Zembrace SymTouch and Tosymra are each indicated for the treatment of acute migraine with or without aura in adults. Tonix’s development portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS development portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead development CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia, having completed enrollment of a potentially confirmatory Phase 3 study in the third quarter of 2023, with topline data expected in late December 2023. TNX-102 SL is also being developed to treat fibromyalgia-type Long COVID, a chronic post-acute COVID-19 condition. Enrollment in a Phase 2 proof-of-concept study has been completed, and topline results were reported in the third quarter of 2023. TNX-601 ER (tianeptine hemioxalate extended-release tablets) is a once-daily oral formulation being developed as a treatment for major depressive disorder (MDD), that completed enrollment in a Phase 2 study in the third quarter of 2023, with topline results expected in early November of 2023. TNX-4300 (estianeptine) is a single isomer version of TNX-601, a small molecule oral therapeutic in preclinical development to treat MDD, Alzheimer’s disease and Parkinson’s disease. Relative to tianeptine, estianeptine lacks activity on the mu-opioid receptor while maintaining activity and the ability to activate PPAR-β/δ and neuroplasticity in tissue culture. TNX-1900 (intranasal potentiated oxytocin), is in development as a preventive treatment in chronic migraine, and the clinical phase of a Phase 2 proof-of-concept study is now completed with topline data expected in early December 2023. TNX-1900 is also being studied in binge eating disorder, pediatric obesity and social anxiety disorder by academic collaborators under investigator-initiated INDs. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the fourth quarter of 2023. Tonix’s rare disease development portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology development portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 was initiated in the third quarter of 2023. Tonix’s infectious disease pipeline includes TNX-801, a vaccine in development to prevent smallpox and mpox. TNX-801 also serves as the live virus vaccine platform or recombinant pox vaccine platform for other infectious diseases, including TNX-1800, in development as a vaccine to protect against COVID-19. The infectious disease development portfolio also includes TNX-3900 and TNX-4000, which are classes of broad-spectrum small molecule oral antivirals.
*Tonix’s product development candidates are investigational new drugs or biologics and have not been approved for any indication.
Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis, Inc. All other marks are property of their respective owners.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID-19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Peter Vozzo
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505
Media Contact
Ben Shannon
ICR Westwicke
ben.shannon@westwicke.com
443-213-0495
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

Pilot Evaluation Of A Clinical Xeno Heart Transplant Regimen In A Preclinical Model I. Ileka 1 , R. Chaban 1 , K. Kinoshita 1 , G. McGrath 1 , Z. Habibabady 1 , A. Calhoun 1 , A. Maenaka 1 , M. Ma 1 , V. Diaz 1 , S. Sanatkar 1 , S. Low 2 , W. Qin 2 , K. Hall 2 , K. Whitworth 3 , D.K.C. Cooper 1 R.N.

Pierson III 1 1 Center for Transplantation Sciences, Massachusetts General Hospital and Harvard Medical School, Boston, MA 2 eGenesis, Inc., Cambridge, MA 3 National Swine Resource and Research Center (NSRRC), Columbia, MO Relevant Disclosures • R. Chaban was supported by the Benjamin Research Fellowship from the German Research Foundation (DFG) • I. Ileka is supported by T32 5T32 AI007529 - 24 • Gene edited pigs were provided by eGenesis and NSRRC (NIH grant U42OD011140) • Tonix Pharmaceuticals provided TNX - 1500*, a humanized, Fc - modified, dimeric anti - CD154 mAb • This work was supported by NIH grants UO1 AI153612 (Pierson), U19 AI090959 (Cooper), and sponsored research agreements with Tonix Pharmaceuticals and eGenesis • D.K.C. Cooper is a consultant to eGenesis * TNX - 1500 is an investigational new biologics and is not approved for any indication Background • Gene - edited (GE) pigs for Xenotransplantation.
Background • We evaluated hearts from multi - GE pigs in baboon transplants treated with a novel costimulation - based immunosuppressive regimen and cold - perfused ‘ischemia minimization’.
– Remove CHO antigen targets of preformed Ab • TKO (Gal - 1,3 - a Gal, Neu5Gc, Sd ) – Add human regulatory molecules • Complement: CD46, CD55, CD59 • Coagulation: TBM, EPCR, TFPI – Add anti - inflammatory ‘transgenes’ • CD47, HLA - E/ b 2, HO - 1, A20, CD39 Methods • Pig Donors – 3 - , 11 - , or 15 - GE pig hearts – novel costimulation - based immunosuppressive regimen – cold - perfused storage technique designed to minimize graft ischemia . • Five baboons' recipients received heterotopic heart transplants using Steen’s cold - perfusion ischemia minimization – 3 Reference pig hearts ( Ntl . Swine Resource & Research Ctr: NSRRC) • 3 - GE pigs: GTKO. β4 GALNT2KO.CD55 – 2 Multi - GE pig hearts ( eGenesis ) (TKO = GTKO.CMAHKO. β4 GALNT2KO) • TKO.hCD46.CD55.TBM.TFPI.HLAE/ β2 M.CD47. CD59.CD39.A20.HO1.PD - 1 ( EGEN - 4737 [15 - GE]) • TKO.hCD46.CD55.TBM.TFPI.HLAE/ β2 M.CD47. EPCR ( EGEN - 4417 [11 - GE])
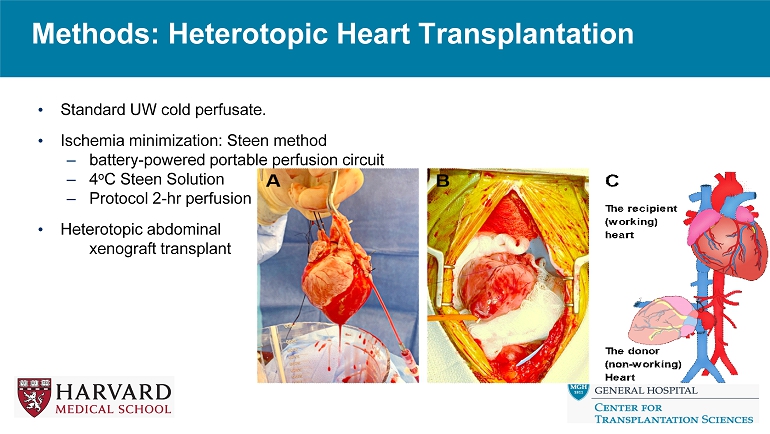
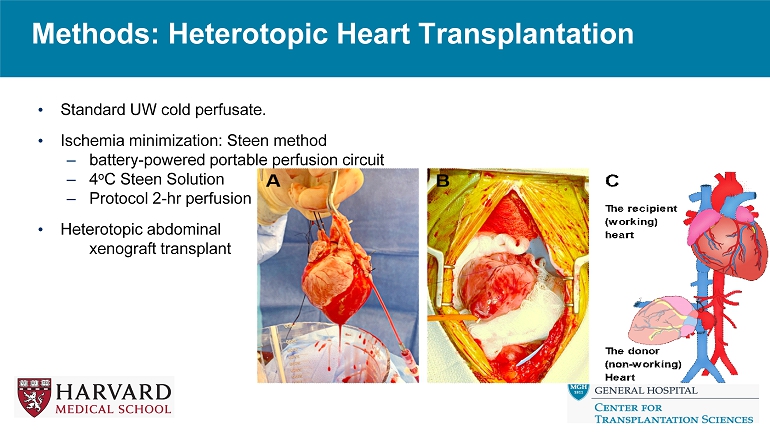
Methods: Heterotopic Heart Transplantation • Standard UW cold perfusate. • Ischemia minimization: Steen method – battery - powered portable perfusion circuit – 4 o C Steen Solution – Protocol 2 - hr perfusion • Heterotopic abdominal xenograft transplant
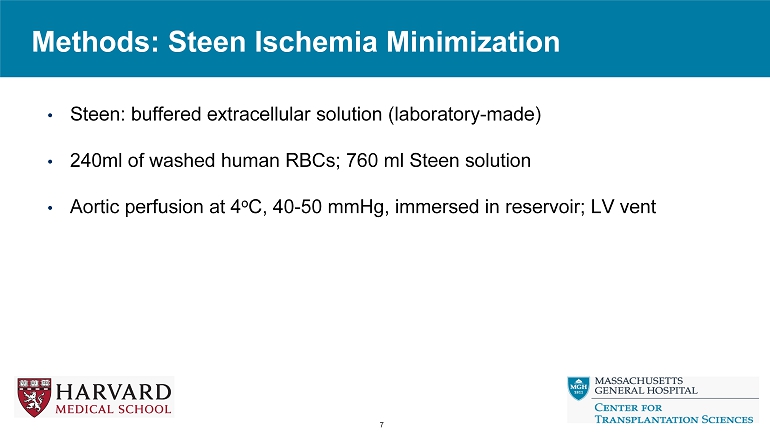
Methods: Steen Ischemia Minimization 7 • Steen: buffered extracellular solution (laboratory - made) • 240ml of washed human RBCs; 760 ml Steen solution • Aortic perfusion at 4 o C, 40 - 50 mmHg, immersed in reservoir; LV vent
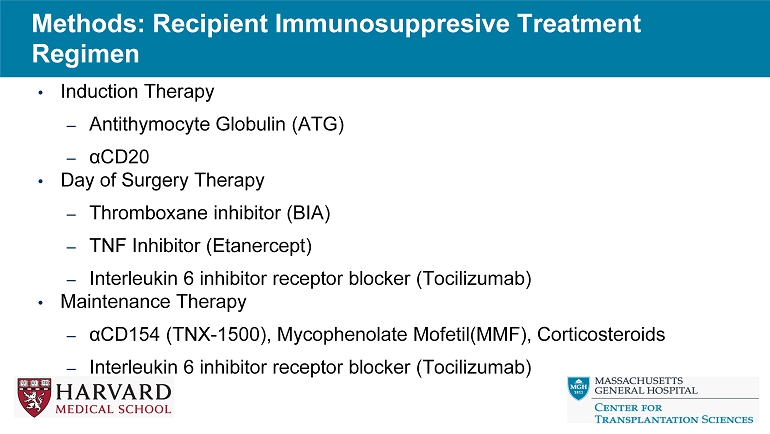
Methods: Recipient Immunosuppresive Treatment Regimen • Induction Therapy – Antithymocyte Globulin (ATG) – α CD20 • Day of Surgery Therapy – Thromboxane inhibitor (BIA) – TNF Inhibitor (Etanercept) – Interleukin 6 inhibitor receptor blocker (Tocilizumab) • Maintenance Therapy – α CD154 (TNX - 1500), Mycophenolate Mofetil(MMF), Corticosteroids – Interleukin 6 inhibitor receptor blocker (Tocilizumab)
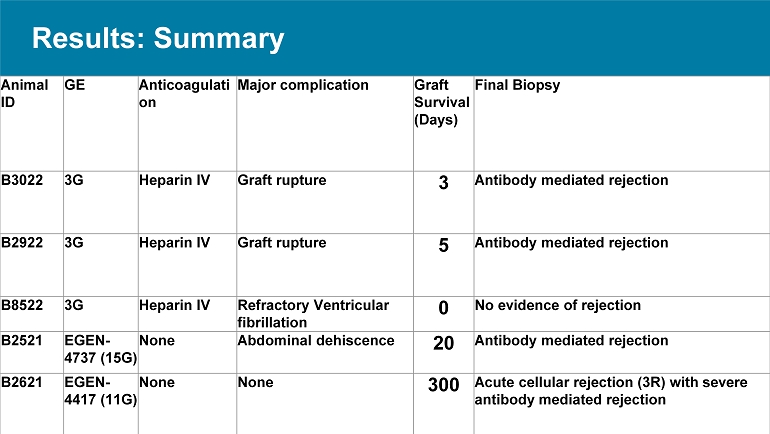
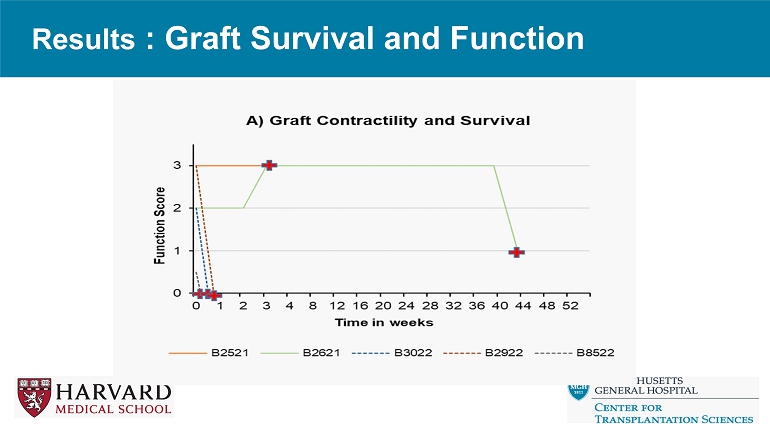
Results: Summary Final Biopsy Graft Survival (Days) Major complication Anticoagulati on GE Animal ID Antibody mediated rejection 3 Graft rupture Heparin IV 3G B3022 Antibody mediated rejection 5 Graft rupture Heparin IV 3G B2922 No evidence of rejection 0 Refractory Ventricular fibrillation Heparin IV 3G B8522 Antibody mediated rejection 20 Abdominal dehiscence None EGEN - 4737 (15G) B2521 Acute cellular rejection (3R) with severe antibody mediated rejection 300 None None EGEN - 4417 (11G) B2621 Results : Graft Survival and Function
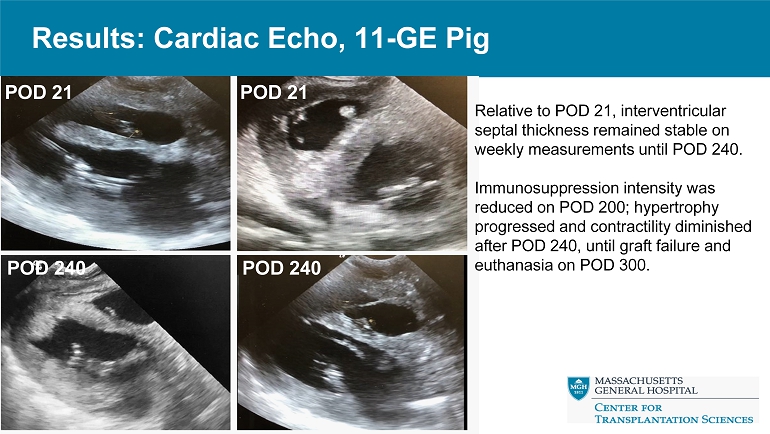
Results: Cardiac Echo, 11 - GE Pig POD 21 POD 21 POD 240 POD 240 Relative to POD 21, interventricular septal thickness remained stable on weekly measurements until POD 240. Immunosuppression intensity was reduced on POD 200; hypertrophy progressed and contractility diminished after POD 240, until graft failure and euthanasia on POD 300.
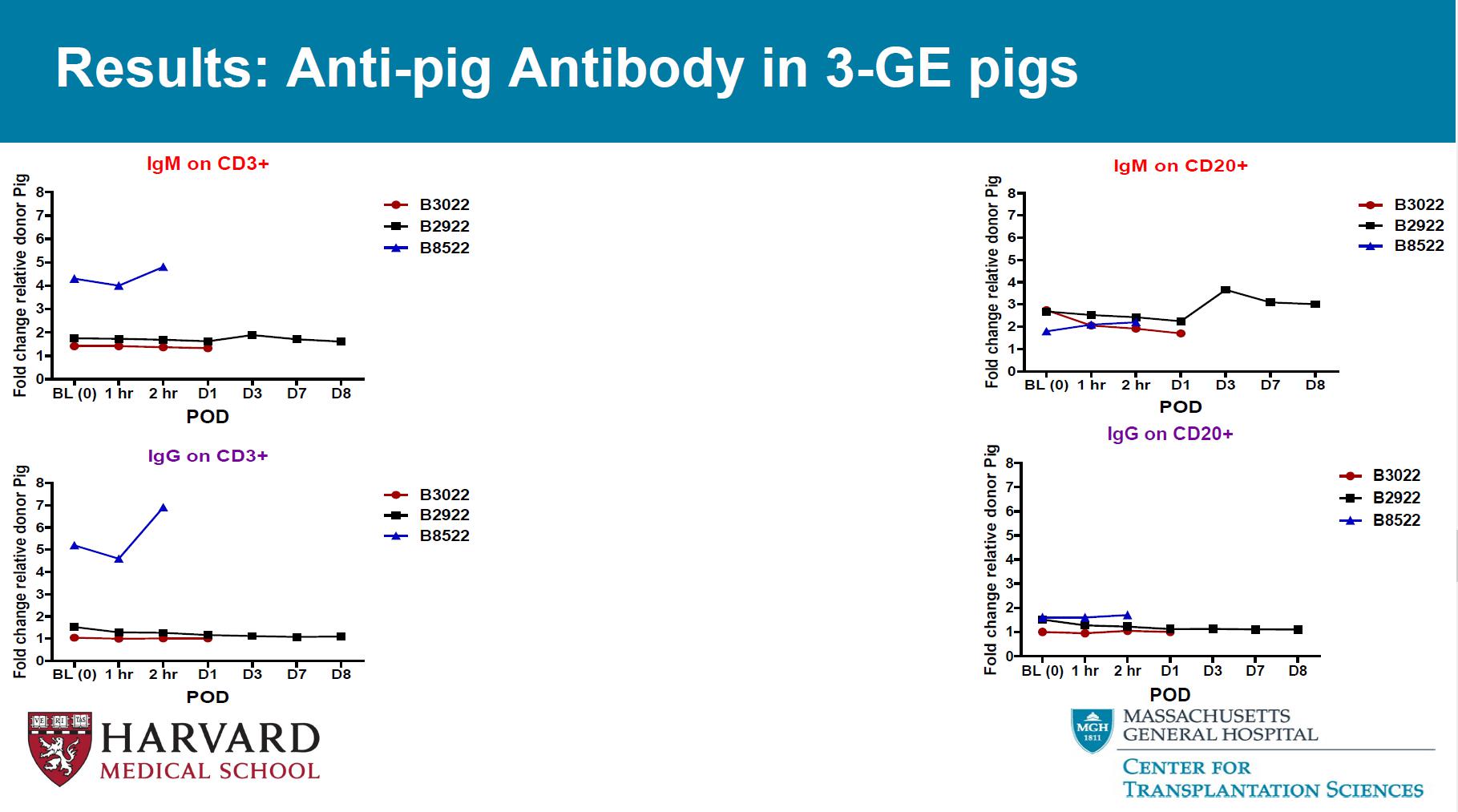
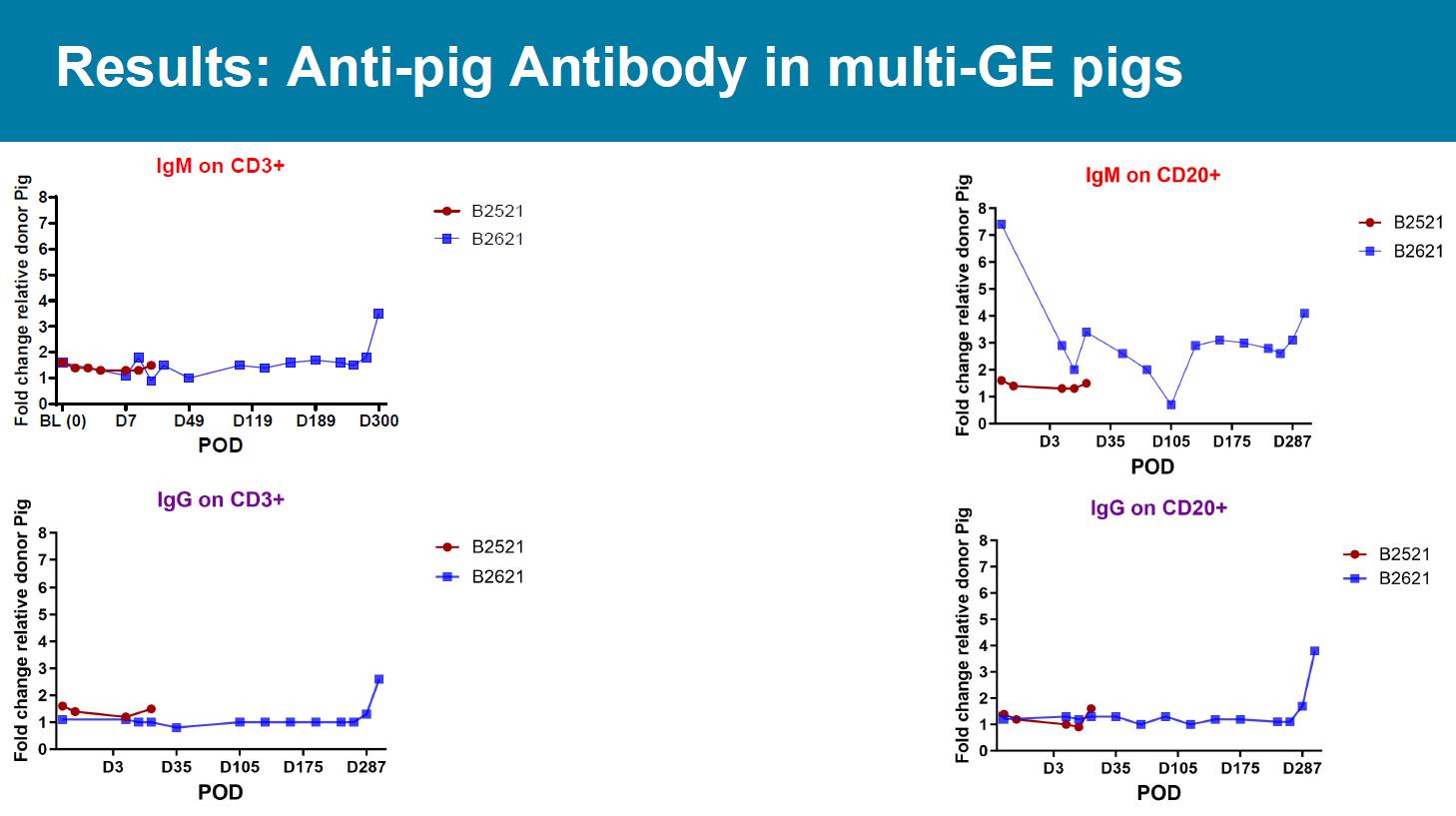
Results: Anti - pig Antibody in 3 - GE pigs IgM on CD3+ POD Fold change relative donor Pig BL (0)1 hr 2 hr D1 D3 D7 D8 0 1 2 3 4 5 6 7 8 B3022 B2922 B8522 IgG on CD3+ POD Fold change relative donor Pig BL (0)1 hr 2 hr D1 D3 D7 D8 0 1 2 3 4 5 6 7 8 B3022 B2922 B8522 Results: Anti - pig Antibody in multi - GE pigs IgM on CD3+ POD F o l d c h a n g e r e l a t i v e d o n o r P i g BL (0) D7 D49 D119 D189 D300 0 1 2 3 4 5 6 7 8 B2521 B2621
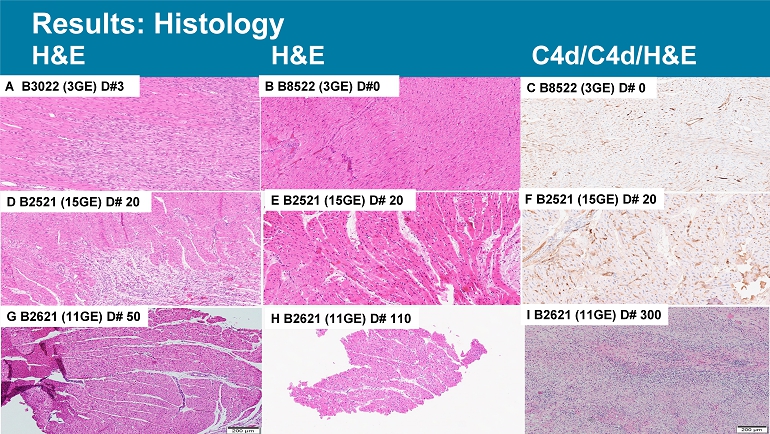
Results: Histology H&E H&E C4d/C4d/H&E A B3022 (3GE) D#3 B B8522 (3GE) D#0 H B2621 (11GE) D# 110 G B2621 (11GE) D# 50 F B2521 (15GE) D# 20 E B2521 (15GE) D# 20 D B2521 (15GE) D# 20 C B8522 (3GE) D# 0 I B2621 (11GE) D# 300 Conclusion • This pilot experience with multi - GE porcine hearts in the context of a clinically applicable ischemia minimization and immunosuppressive regimen supports our working hypothesis that is effective to prolong heart xenograft survival.
• We plan to further test this hypothesis in an orthotopic heart xenograft model.

Thank you! Key Contributors: R Chaban A Calhoun V Diaz I Ileka RN Pierson III
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

Extended Survival of 9 - and 10 - Gene Edited Pig Heart Xenografts with Ischemia Minimization and CD154 Costimulation Blockade – Based Immunosuppression I. Ileka 1 , R. Chaban 1 , K. Kinoshita 1 , G. McGrath 1 , Z. Habibabady 1 , A. Calhoun 1 , A. Maenaka 1 , M. Ma 1 , V. Diaz 1 ,M. Dufault 1 , L. Burdorf 2 , W. Eyestone 2 , K. Whitworth 3 , D.K.C. Cooper 1 R.N.

Pierson III 1 1 Center for Transplantation Sciences, Massachusetts General Hospital and Harvard Medical School, Boston, MA 2 Revivicor, Inc., Blacksburg, VA 3 National Swine Resource and Research Center (NSRRC), Columbia, MO Relevant Disclosures • R. Chaban was supported by the Benjamin Research Fellowship from the German Research Foundation (DFG) • I. Ileka is supported by T32 5T32 AI007529 - 24 • Gene edited pigs were provided by Revivicor and NSRRC (NIH grant U42OD011140) • Tonix Pharmaceuticals provided TNX - 1500*, a humanized, Fc - modified, dimeric anti - CD154 mAb • This work was supported by NIH grants UO1 AI153612 (Pierson), U19 AI090959 (Cooper), and sponsored research agreements with Tonix Pharmaceuticals * TNX - 1500 is an investigational new biologics and is not approved for any indication Background • Gene - edited (GE) pigs for Xenotransplantation.
Background • We evaluated hearts from multi - GE pigs in baboon transplants treated with a novel costimulation - based immunosuppressive regimen and cold - perfused ‘ischemia minimization’.

– Remove CHO antigen targets of preformed Ab • TKO (Gal - 1,3 - a Gal, Neu5Gc, b 4Gal) – Add human regulatory molecules • Complement: CD46, CD55, CD59 • Coagulation: TBM, EPCR, TFPI – Add human anti - inflammatory ‘transgenes’ • CD47, HLA - E/ b 2 m g, HO - 1, A20, CD39 Methods • Current study: – 3 - , 9 - , or 10 - GE pig hearts – novel costimulation - based immunosuppressive regimen – cold - perfused storage technique designed to minimize graft ischemia . • Eight baboons recipients received heterotopic heart transplants using Steen’s cold - perfusion ischemia minimization – 3 Reference pig hearts ( Ntl . Swine Resource & Research Ctr: NSRRC) • 3 - GE pigs (n=3): GTKO. β4 GALNT2KO.hCD55 – 5 Multi - GE pig hearts ( Revivicor ) • 9 - GE pigs (n=3): GalKO . β4 GalNT2KO. GHRKO. hCD46.hCD55 . hTBM.hEPCR . hCD47 .hHO - 1 • 10 - GE pigs (n=2): GalKO . β4 GalNT2KO. GHRKO. CD46.CD55 . hTBM.hEPCR . hCD47.hHO - 1 . CMAHKO
Methods: Heterotopic Heart Transplantation • Standard UW cold perfusate. • Ischemia minimization: Steen method – battery - powered portable perfusion circuit – 4 o C Steen Solution – Protocol 2 - hr perfusion • Heterotopic abdominal xenograft transplant
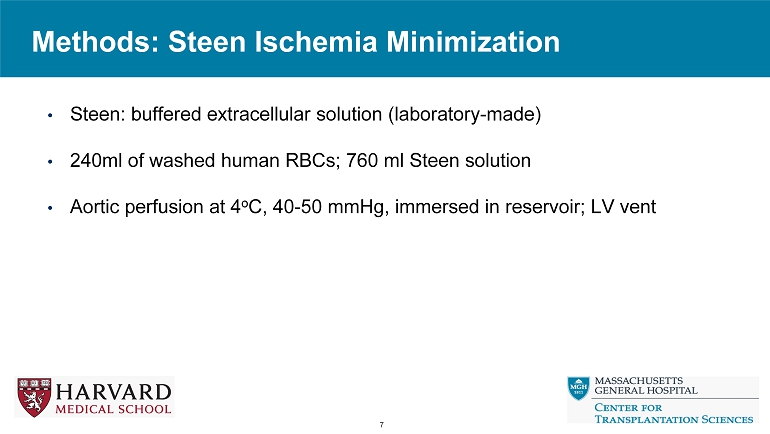
Methods: Steen Ischemia Minimization 7 • Steen: buffered extracellular solution (laboratory - made) • 240ml of washed human RBCs; 760 ml Steen solution • Aortic perfusion at 4 o C, 40 - 50 mmHg, immersed in reservoir; LV vent
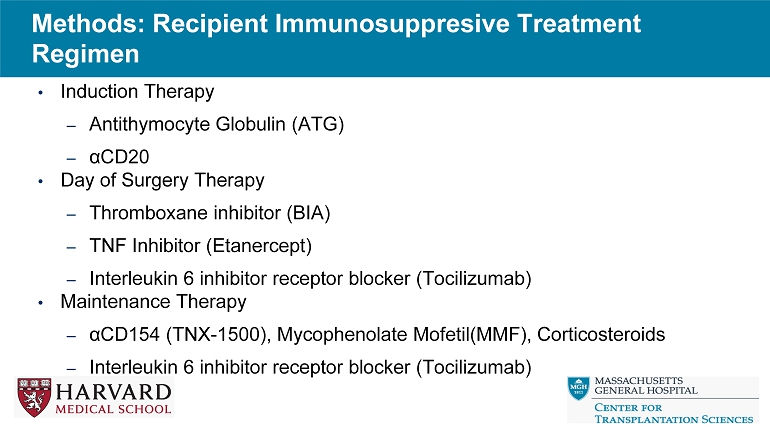
Methods: Recipient Immunosuppresive Treatment Regimen • Induction Therapy – Antithymocyte Globulin (ATG) – α CD20 • Day of Surgery Therapy – Thromboxane inhibitor (BIA) – TNF Inhibitor (Etanercept) – Interleukin 6 inhibitor receptor blocker (Tocilizumab) • Maintenance Therapy – α CD154 (TNX - 1500), Mycophenolate Mofetil(MMF), Corticosteroids – Interleukin 6 inhibitor receptor blocker (Tocilizumab)
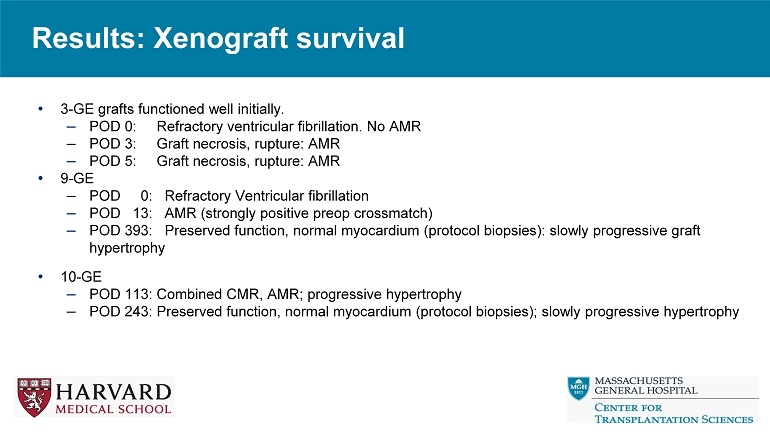
Results: Xenograft survival • 3 - GE grafts functioned well initially. – POD 0: Refractory ventricular fibrillation.
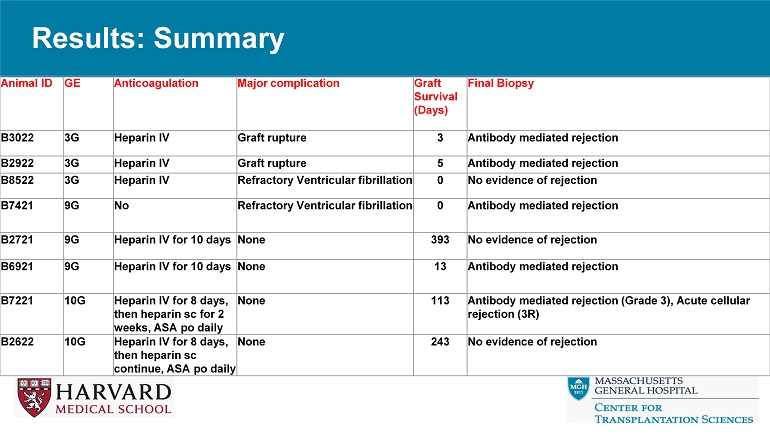
No AMR – POD 3: Graft necrosis, rupture: AMR – POD 5: Graft necrosis, rupture: AMR • 9 - GE – POD 0: Refractory Ventricular fibrillation – POD 13: AMR (strongly positive preop crossmatch) – POD 393: Preserved function, normal myocardium (protocol biopsies): slowly progressive graft hypertrophy • 10 - GE – POD 113: Combined CMR, AMR; progressive hypertrophy – POD 243: Preserved function, normal myocardium (protocol biopsies); slowly progressive hypertrophy Results: Summary Final Biopsy Graft Survival (Days) Major complication Anticoagulation GE Animal ID Antibody mediated rejection 3 Graft rupture Heparin IV 3G B3022 Antibody mediated rejection 5 Graft rupture Heparin IV 3G B2922 No evidence of rejection 0 Refractory Ventricular fibrillation Heparin IV 3G B8522 Antibody mediated rejection 0 Refractory Ventricular fibrillation No 9G B7421 No evidence of rejection 393 None Heparin IV for 10 days 9G B2721 Antibody mediated rejection 13 None Heparin IV for 10 days 9G B6921 Antibody mediated rejection (Grade 3), Acute cellular rejection (3R) 113 None Heparin IV for 8 days, then heparin sc for 2 weeks, ASA po daily 10G B7221 No evidence of rejection 243 None Heparin IV for 8 days, then heparin sc continue, ASA po daily 10G B2622 Results: Graft Survival, Function, and Morphology
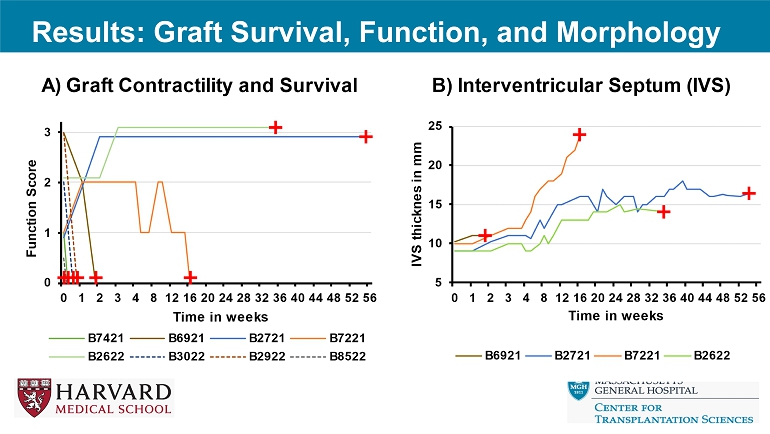
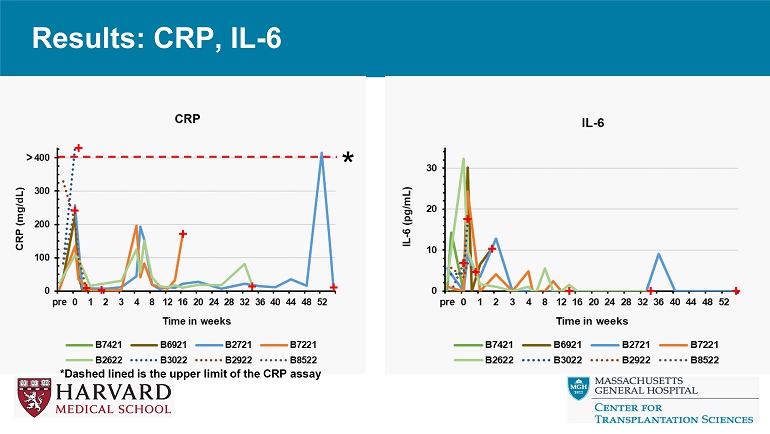
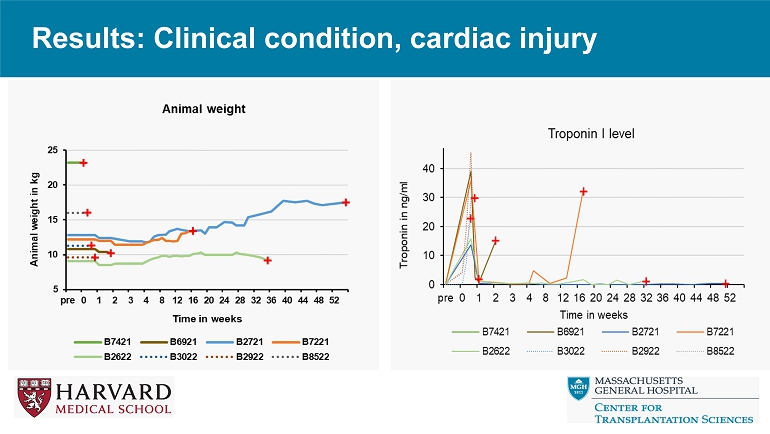
Results: CRP, IL - 6 *Dashed lined is the upper limit of the CRP assay * Results: Clinical condition, cardiac injury
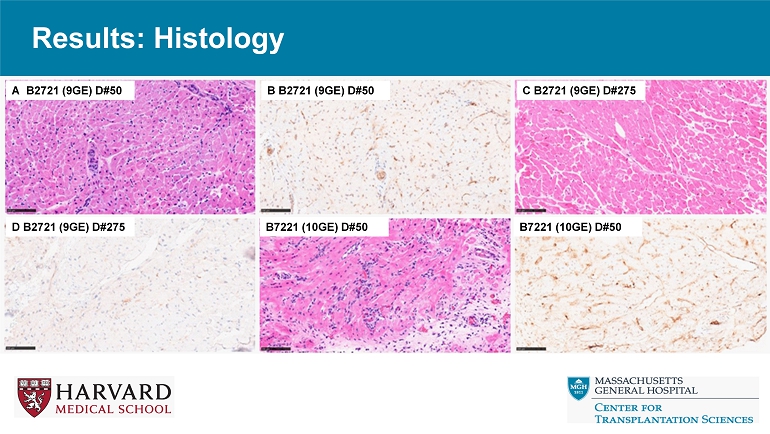
Results: Histology A B2721 (9GE) D#50 B B2721 (9GE) D#50 C B2721 (9GE) D#275 D B2721 (9GE) D#275 B7221 (10GE) D#50 B7221 (10GE) D#50 Results: Histology G B7221 (10GE) D#113 B7221 (10GE) D#113 I B2622 (10GE) D#120 J B2622 (10GE) D#120 K B3022 (3GE) D#3 L B3022 (3GE) D#3
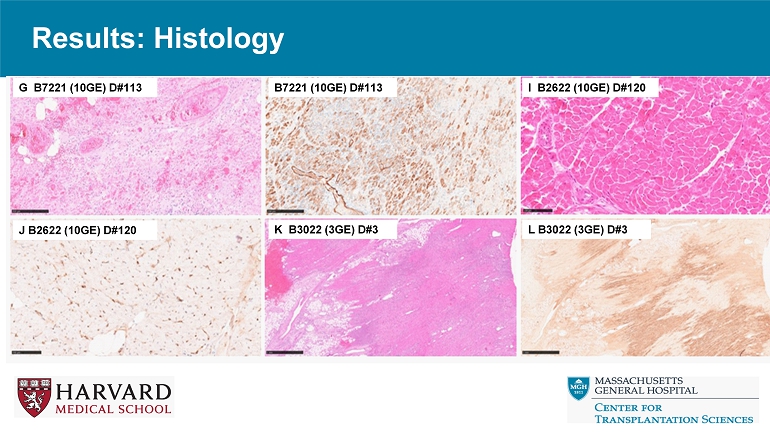
Conclusions • Relative to reference genetics without thrombo - regulatory and anti - inflammatory gene expression, 9 - or 10 - GE pig hearts exhibit promising performance in the context of a clinically applicable regimen including ischemia minimization and αCD154 - based immunosuppression. • Despite GE modifications and use of ischemia minimization, peri - transplant myocardial injury (Troponin leak) and systemic inflammation (CRP, IL - 6 elaboration) occur consistently, but do not prevent graft recovery and long - term survival. • These encouraging results justify further evaluation in an orthotopic heart xenotransplant model.

Thank you! Key Contributors: R Chaban A Calhoun V Diaz I Ileka RN Pierson III