UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): September 21, 2023
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 001-36019 | 26-1434750 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
26 Main Street, Chatham, New Jersey 07928
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (862) 904-8182
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | TNXP | The NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On September 21, 2023, Tonix Pharmaceuticals Holding Corp. (the “Company”) announced a poster presentation showing research results for the Company’s mTNX-1700 (murine trefoil factor family member 2- murine serum albumin, or mTFF2-MSA) product candidate (the “Poster”) at the Seventh International Cancer Immunotherapy Conference 2023 (“CICON23”): Translating Science into Survival, being held September 20-23, 2023. A copy of the press release which discusses this matter is furnished hereto as Exhibit 99.01, and incorporated herein by reference. A copy of the Poster is furnished hereto as Exhibit 99.02 and incorporated herein by reference.
The Company updated its TNX-102 SL (cyclobenzaprine HCl sublingual tablet) product candidate presentation, which the Company intends to place on its website, which may contain nonpublic information. A copy of the presentation is filed as Exhibit 99.03 hereto and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.01, 99.02 and 99.03 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01. | Other Events. |
On September 21, 2023, the Company announced the presentation of the Poster CICON23. The Poster, entitled, “mTFF2-MSA (mTNX-1700) Suppresses Tumor Growth and Increases Survival in Anti-PD-1 Treated CT26.WT Subcutaneous and CT26-Luciferase Orthotopic Syngeneic Colorectal Cancer Models by Targeting MDSCs in BALB/C Mice,” includes data demonstrating that mTNX-1700, a novel fusion protein, has single agent activity and can improve on the therapeutic effects of anti-PD-1 in treating syngeneic mouse models of advanced colorectal cancer. In two models, mTNX-1700 increased survival rates and suppresses tumor growth. Additive tumor growth suppression effects were observed when mTNX-1700 and anti-PD-1 were used in combination. The Company believes that the data demonstrate the potential of TNX-1700 in treating cancer both as a single agent and in combination with other immuno-oncology drugs, particularly anti-PD-1, and highlight how mTNX-1700 targets myeloid-derived suppressor cells as a novel mechanism to treat cancer.
Forward- Looking Statements
This Current Report on Form 8-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating to the Company’s product development, clinical trials, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities and other statement that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management’s current beliefs and assumptions.
These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential,” “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the SEC. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this press release. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) |
Exhibit No. |
Description. | ||
|
104 |
Press Release of the Company, dated September 21, 2023 mTFF2-MSA (mTNX-1700) Suppresses Tumor Growth and Increases Survival in Anti-PD-1 Treated CT26.WT Subcutaneous and CT26-Luciferase Orthotopic Syngeneic Colorectal Cancer Models by Targeting MDSCs in BALB/C Mice Corporate Presentation by the Company for September 2023 Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: September 21, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01
Tonix Pharmaceuticals Presents New Preclinical Data at Seventh International Cancer Immunotherapy Conference 2023
mTNX-1700 (mTFF2-MSA) suppresses tumor growth and increases survival rates in preclinical colorectal cancer models
mTNX-1700 shows single agent activity and additive effects in combination with anti-PD-1
mTNX-1700 targets myeloid-derived suppressor cells (MDSCs) in a novel mechanism to treat cancer
CHATHAM, N.J., September 21, 2023 – Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP), a biopharmaceutical company with marketed products and a pipeline of development candidates, today announced a poster presentation showing research results for mTNX-1700 (murine trefoil factor family member 2- murine serum albumin, or mTFF2-MSA) at the Seventh International Cancer Immunotherapy Conference 2023 (CICON23): Translating Science into Survival, being held September 20-23, 2023, in Milan, Italy. The poster is available under the Scientific Presentations tab of the Tonix website at www.tonixpharma.com.
The poster presentation, titled, “mTFF2-MSA (mTNX-1700) Suppresses Tumor Growth and Increases Survival in Anti-PD-1 Treated CT26.WT Subcutaneous and CT26-Luciferase Orthotopic Syngeneic Colorectal Cancer Models by Targeting MDSCs in BALB/C Mice,” includes data demonstrating that the novel fusion protein, mTNX-1700 has single agent activity and can improve on the therapeutic effects of anti-PD-1 in treating syngeneic mouse models of advanced colorectal cancer. In two models, mTNX-1700 increases survival rates and suppresses tumor growth. Additive tumor growth suppression effects were observed when mTNX-1700 and anti-PD-1 were used in combination.
“mTNX-1700 is a novel fusion protein that has single agent activity and augments PD-1 blockade therapy in combination therapy in advanced and metastatic syngeneic mouse models of colorectal and gastric cancer,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals. “Certain cancers are unresponsive to anti-PD-1 therapy. Despite the significance of PD-1 checkpoint blockade in treating many types of cancer, there’s a need to better understand why some cancers don’t respond and how to make them responsive. We believe these data show the potential of TNX-1700 in treating cancer both as a single agent and in combination with other immuno-oncology drugs, particularly anti-PD-1, and highlight how mTNX-1700 targets MDSCs as a novel mechanism to treat cancer.”
About Trefoil Factor Family Member 2 (TFF2)
Human TFF2 is a secreted protein, encoded by the TFF2 gene in humans, that is expressed in gastrointestinal mucosa where it functions to protect and repair mucosa. TFF2 is also expressed at low levels in splenic immune cells and is now appreciated to have intravascular roles in the spleen and in the tumor microenvironment. In gastric cancer, TFF2 is epigenetically silenced, and TFF2 is suggested to be protective against cancer development through several mechanisms. Tonix is developing TNX-1700 (rTFF2-HSA) for the treatment of gastric and colon cancers under a license from Columbia University. The inventor of the core technology at Columbia is Dr. Timothy Wang, who is an expert in the molecular mechanisms of carcinogenesis whose research has focused on the carcinogenic role of inflammation in modulating stem cell functions. Dr. Wang demonstrated that knocking out the mTFF2 gene in mice leads to faster tumor growth and that overexpression of TFF2 markedly suppresses tumor growth by curtailing the homing, differentiation, and expansion of MDSCs to allow activation of cancer-killing CD8+ T cells.1 He went on to show that a novel engineered form of recombinant murine TFF2 (mTFF2-CTP) had an extended half-life in vivo and was able to suppress MDSCs and tumor growth in an animal model of colorectal cancer. Later, he showed in gastric cancer models that suppressing MDSCs using chemotherapy enhances the effectiveness of anti-PD1 therapy and significantly reduces tumor growth.2 Dr. Wang proposed the concept of employing rTFF2 in combination with other therapies in cancer prevention and early treatment. Dr. Wang presented data at the American Association for Cancer Research (AACR) conference as a collaboration between Tonix and Columbia University in 2020 that includes data from a preclinical study which investigated the role of PD-L1 in colorectal tumorigenesis and evaluated the utility of targeting myeloid-derived suppressor cells (MDSCs) in combination with PD-1 blockade in mouse models of colorectal cancer. The data show that anti-PD-1 monotherapy was unable to evoke anti-tumor immunity in this model of colorectal cancer, but mTFF2-CTP augmented the efficacy of anti-PD-1 therapy. Anti-PD-1 in combination with TFF2-CTP showed greater anti-tumor activity in PD-L1-overexpressing mice.
1Dubeykovskaya ZA et al, Nat Commun 2016
2Kim W et al, Gastroenterology 2021
Tonix Pharmaceuticals Holding Corp.*
Tonix is a biopharmaceutical company focused on commercializing, developing, discovering and licensing therapeutics to treat and prevent human disease and alleviate suffering. Tonix Medicines, our commercial subsidiary, markets Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg under a transition services agreement with Upsher-Smith Laboratories, LLC from whom the products were acquired on June 30, 2023. Zembrace SymTouch and Tosymra are each indicated for the treatment of acute migraine with or without aura in adults. Tonix’s development portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS development portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead development CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia, having completed enrollment of a potentially confirmatory Phase 3 study in the third quarter of 2023, with topline data expected in the fourth quarter of 2023. TNX-102 SL is also being developed to treat fibromyalgia-type Long COVID, a chronic post-acute COVID-19 condition. Enrollment in a Phase 2 proof-of-concept study has been completed, and topline results were reported in the third quarter of 2023. TNX-601 ER (tianeptine hemioxalate extended-release tablets) is a once-daily oral formulation being developed as a treatment for major depressive disorder (MDD), that completed enrollment in a Phase 2 proof-of-concept study in the third quarter of 2023, with topline results expected in the fourth quarter of 2023. TNX-4300 (estianeptine) is a single isomer version of TNX-601, small molecule oral therapeutic in preclinical development to treat MDD, Alzheimer’s disease and Parkinson’s disease. Relative to tianeptine, estianeptine lacks activity on the µ-opioid receptor while maintaining activity in the rat Novel Object Recognition test in vivo and the ability to activate PPAR-β/δ and neuroplasticity in tissue culture. TNX-1900 (intranasal potentiated oxytocin), is in development for preventing headaches in chronic migraine, and has completed enrollment in a Phase 2 proof-of-concept study with topline data expected in the fourth quarter of 2023. TNX-1900 is also being studied in binge eating disorder, pediatric obesity and social anxiety disorder by academic collaborators under investigator-initiated INDs. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the fourthquarter of 2023. Tonix’s rare disease development portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology development portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 was initiated in the third quarter of 2023. Tonix’s infectious disease pipeline includes TNX-801, a vaccine in development to prevent smallpox and mpox. TNX-801 also serves as the live virus vaccine platform or recombinant pox vaccine platform for other infectious diseases. The infectious disease development portfolio also includes TNX-3900 and TNX-4000, which are classes of broad-spectrum small molecule oral antivirals.
*Tonix’s product development candidates are investigational new drugs or biologics and have not been approved for any indication.
Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis, Inc. All other marks are property of their respective owners.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Peter Vozzo
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505
Media Contact
Ben Shannon
ICR Westwicke
ben.shannon@westwicke.com
(919) 360-3039
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL FOR LONG COVID RESULTS OF THE TNX - CY - PA201 ‘PREVAIL’ TRIAL BRIEFING DECK Version P0487 September 20, 2023 (Doc 1319 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Clinical Development Programs Status/Next Milestone Indication Candidates* Mid - Phase 3 – enrollment complete Phase 2 – Topline Data Announced 5 Sept 2023 Fibromyalgia (FM) Long COVID (PASC 2 ) TNX - 102 SL 1 Mid - Phase 2, Targeted 3Q 2023 Start Cocaine Intoxication - FDA Breakthrough Designation TNX - 1300 3 Phase 2 – enrollment complete 5 Prevention of Chronic Migraine TNX - 1900 4 Phase 2 – enrollment complete 6 Major Depressive Disorder TNX - 601 ER Phase 2 ready Prader - Willi Syndrome - FDA Orphan Drug Designation TNX - 2900 7 Phase 1 – currently enrolling Organ Transplant Rejection/ Autoimmune Conditions TNX - 1500 8 *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) also has active INDs for Agitation in Alzheimer’s Disease (AAD), Alcohol Use Disorder (AUD), and Posttraumatic Stress Disorder (PTSD). These 3 indications are Phase 2 ready 2 Post - Acute Sequelae of COVID - 19. Completed Phase 2 Proof of Concept Study 3 TNX - 1300 (double - mutant cocaine esterase) is licensed from Columbia University 4 Acquired from Trigemina ; license agreement with Stanford University; Planned investigator - initiated Binge Eating Disorder (BED) study initiated 3 Q 2023 5 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 6 Phase 2 trial for formulation development of TNX - 601 ER (tianeptine hemioxalate extended - release tablets) has completed enrollme nt – topline data in Q4 2023 ; Other potential indications include PTSD and neurocognitive dysfunction from steroids 7 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) 8 anti - CD40L humanized monoclonal antibody – IND cleared and Phase 1 PK/PD trial in healthy volunteers is currently ongoing © 2023 Tonix Pharmaceuticals Holding Corp.
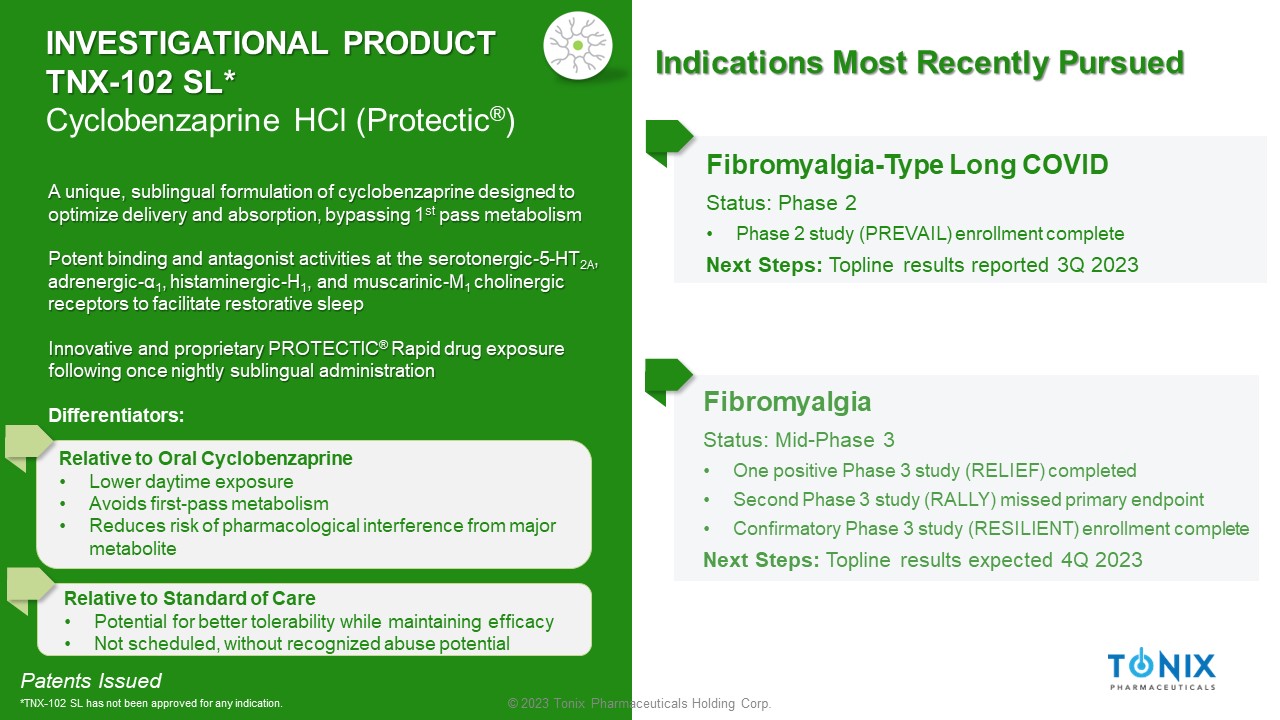
INVESTIGATIONAL PRODUCT TNX - 102 SL* Cyclobenzaprine HCl ( Protectic ® ) Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) enrollment complete Next Steps: Topline results expected 4Q 2023 Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) enrollment complete Next Steps: Topline results reported 3Q 2023 Patents Issued *TNX - 102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption, bypassing 1 st pass metabolism Potent binding and antagonist activities at the serotonergic - 5 - HT 2A , adrenergic - α 1 , histaminergic - H 1 , and muscarinic - M 1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following once nightly sublingual administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled, without recognized abuse potential Indications Most Recently Pursued 4 © 2023 Tonix Pharmaceuticals Holding Corp.
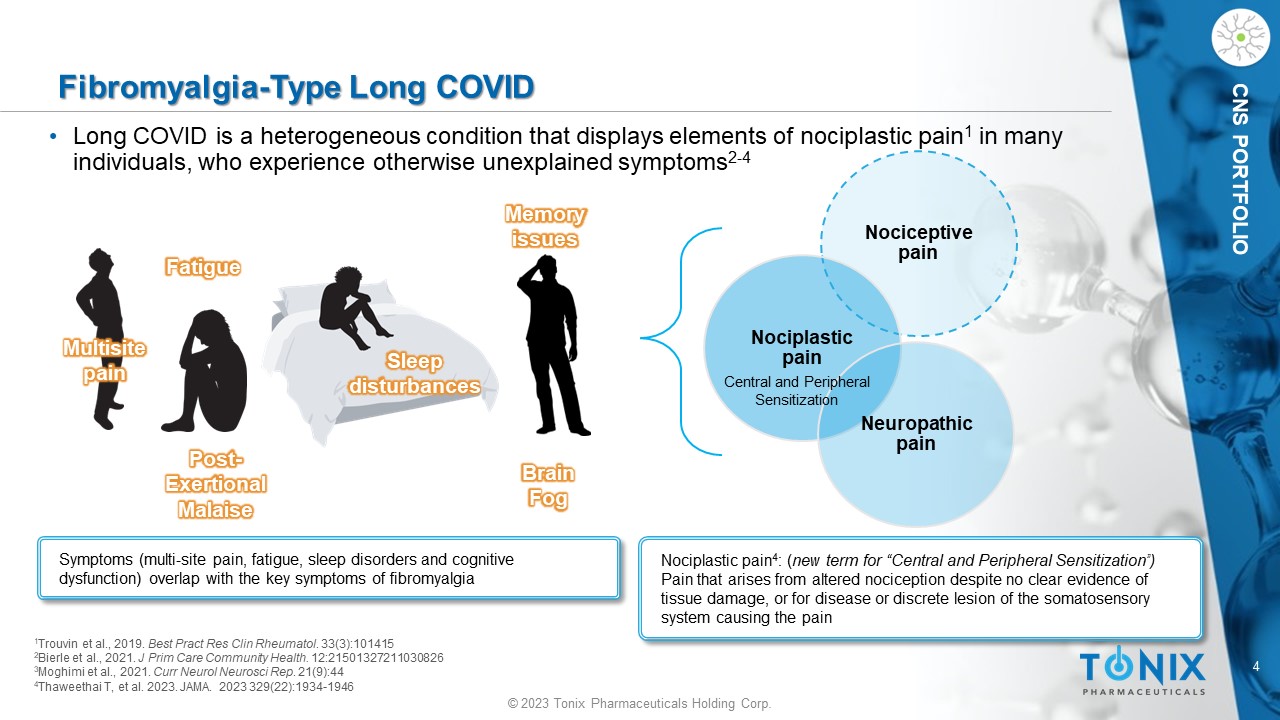
CNS PORTFOLIO Fibromyalgia - Type Long COVID • Long COVID is a heterogeneous condition that displays elements of nociplastic pain 1 in many individuals, who experience otherwise unexplained symptoms 2 - 4 Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia Multisite pain Memory issues Fatigue Sleep disturbances 1 Trouvin et al., 2019. Best Pract Res Clin Rheumatol . 33(3):101415 2 Bierle et al., 2021. J Prim Care Community Health. 12:21501327211030826 3 Moghimi et al., 2021. Curr Neurol Neurosci Rep . 21(9):44 4 Thaweethai T, et al. 2023. JAMA. 2023 329(22):1934 - 1946 Nociceptive pain Nociplastic pain Neuropathic pain Nociplastic pain 4 : ( new term for “Central and Peripheral Sensitization”) Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or discrete lesion of the somatosensory system causing the pain Central and Peripheral Sensitization Post - Exertional Malaise Brain Fog 5 © 2023 Tonix Pharmaceuticals Holding Corp.
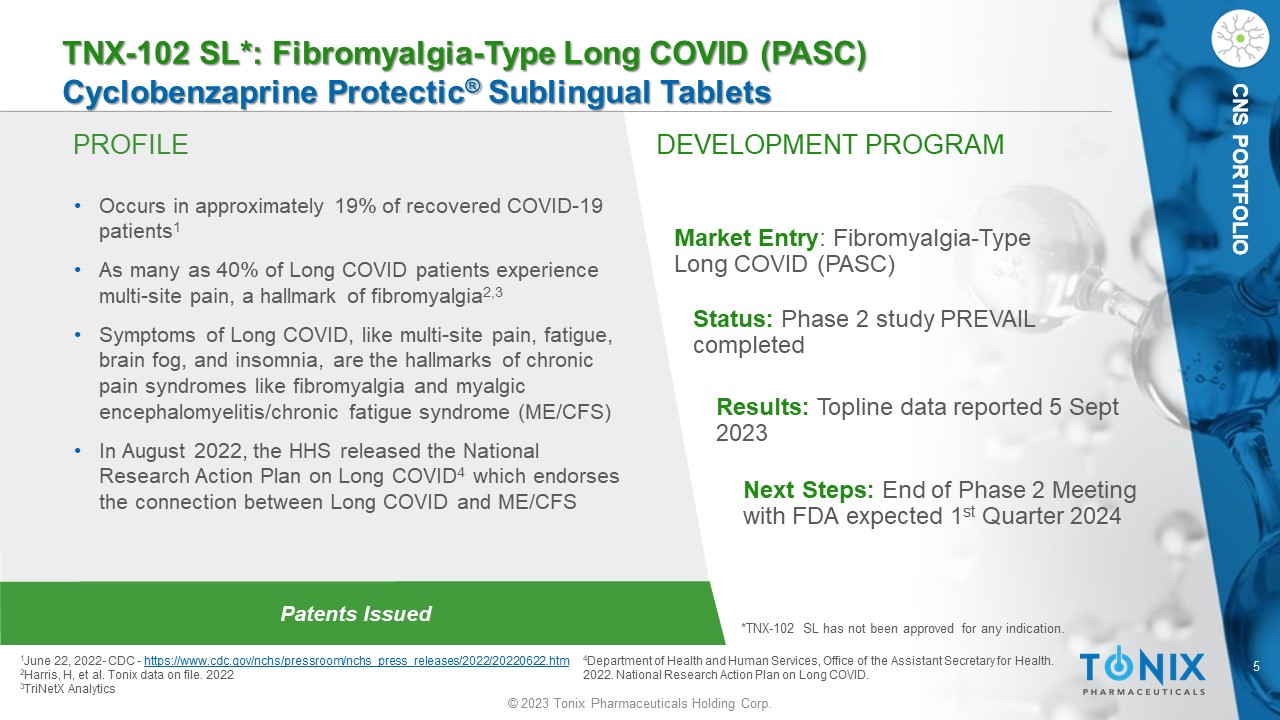
CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia - Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 19% of recovered COVID - 19 patients 1 • As many as 40% of Long COVID patients experience multi - site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi - site pain, fatigue, brain fog, and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and ME/CFS Market Entry : Fibromyalgia - Type Long COVID (PASC) Status: Phase 2 study PREVAIL completed Results : Topline data reported 5 Sept 2023 1 J une 22, 2022 - CDC - https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Long COVID. Next Steps: End of Phase 2 Meeting with FDA expected 1 st Quarter 2024 6 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO TNX - 102 SL: Phase 2 Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, completed enrollment of 63 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) − Weekly averages of the daily numerical rating scale scores Secondary Endpoints: • Change from Baseline in the weekly average of the daily diary NRS assessment of sleep quality at the Week 14 endpoint • Change from Baseline in the PROMIS Fatigue score at the Week 14 endpoint • Change from Baseline in the PROMIS Cognitive Function – Abilities score at the Week 14 endpoint • Change from Baseline in the PROMIS Sleep Dis turbance score at the Week 14 endpoint • Proportion of patients with a Patient Global Impression of Change (PGIC) rating of “much improved” or “very much improved” at th e Week 14 endpoint • Change from Baseline to Week 14 in the Sheehan Disability Scale (SDS) total score Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 (2 x placebo tablets) * Study Characteristics and Select Inclusion Criteria: • Approximately 30 clinical trial sites across the US • Visits: Screening, Baseline, Week 2, Week 6, Week 10, Week 14, Safety F/U Weeks 16 & 18 • Inclusion: age 18 - 65, female or male, confirmed hx SARS - CoV - 2 ≥ 3 months prior to enrollment ( documented PCR, nucleic acid tests, or rapid antigen tests) • New onset or worsening of pain that coincides with index COVID - 19 illness • Multi - site pain as defined by modified Michigan Body Map following COVID - 19 illness • O n - site 7 - day recall NRS average daily Long COVID - r elated pain intensity score ≥ 4 and ≤ 9 • Post - COVID - 19 Functional Status (PCFS) scale score of ≥ at Screening & Baseline • Willing and able to withdraw from: approved FM drugs, opioids, tramadol, tapentadol, tricyclics, trazodone, orexin receptor antagonists, benzodiazepines 7 © 2023 Tonix Pharmaceuticals Holding Corp.
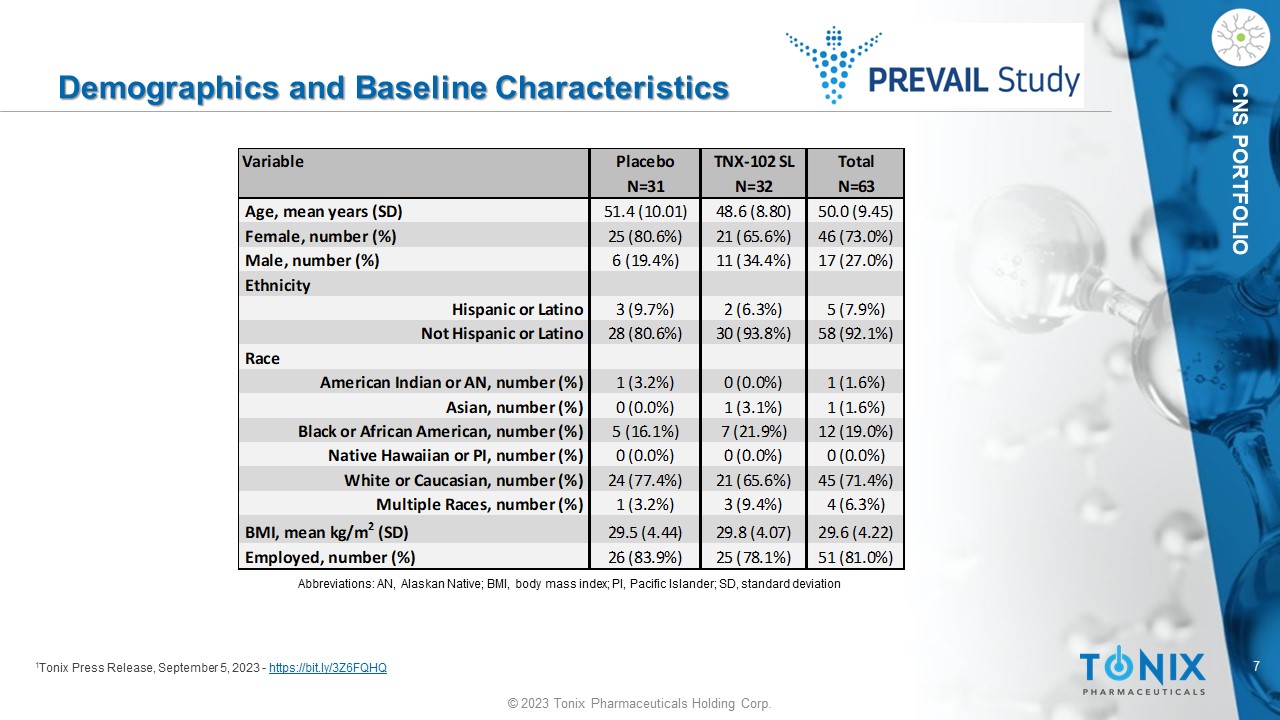
CNS PORTFOLIO Demographics and Baseline Characteristics 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ Variable Placebo TNX-102 SL Total N=31 N=32 N=63 Age, mean years (SD) 51.4 (10.01) 48.6 (8.80) 50.0 (9.45) Female, number (%) 25 (80.6%) 21 (65.6%) 46 (73.0%) Male, number (%) 6 (19.4%) 11 (34.4%) 17 (27.0%) Ethnicity Hispanic or Latino 3 (9.7%) 2 (6.3%) 5 (7.9%) Not Hispanic or Latino 28 (80.6%) 30 (93.8%) 58 (92.1%) Race American Indian or AN, number (%) 1 (3.2%) 0 (0.0%) 1 (1.6%) Asian, number (%) 0 (0.0%) 1 (3.1%) 1 (1.6%) Black or African American, number (%) 5 (16.1%) 7 (21.9%) 12 (19.0%) Native Hawaiian or PI, number (%) 0 (0.0%) 0 (0.0%) 0 (0.0%) White or Caucasian, number (%) 24 (77.4%) 21 (65.6%) 45 (71.4%) Multiple Races, number (%) 1 (3.2%) 3 (9.4%) 4 (6.3%) BMI, mean kg/m 2 (SD) 29.5 (4.44) 29.8 (4.07) 29.6 (4.22) Employed, number (%) 26 (83.9%) 25 (78.1%) 51 (81.0%) Abbreviations: AN, Alaskan Native; BMI, body mass index; PI, Pacific Islander; SD, standard deviation 8 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO Topline Results 1 TNX - 102 SL showed a robust effect size of 0.5 in improving fatigue and showed consistent activity across secondary measures of sleep quality, cognitive function, disability and Patient Global Impression of Change, but did not meet the primary endpoint of multi - site pain reduction at Week 14 ‒ There is currently no drug approved to treat Long COVID TNX - 102 SL was generally well tolerated with an adverse event (AE) profile comparable to prior studies with TNX - 102 SL. ‒ AE - related discontinuations were similar in drug and placebo arms. ‒ No new safety signals were observed Findings fulfill the objectives of this proof - of - concept study, supporting the decision to advance the program based on a proposed primary endpoint using the PROMIS Fatigue scale ‒ Fatigue is the signature symptom of Long COVID and it has been identified as the dominant symptom contributing to disability 2 ‒ In both of our prior Phase 3 studies of TNX - 102 SL 5.6 mg in fibromyalgia, we observed numerical improvement in the PROMIS fatig ue score (in RELIEF p= 0.007 MMRM and in RALLY p= 0.007 MMRM) ‒ Although the validity of PROMIS Fatigue is not yet established in Long COVID, we believe the results of PREVAIL, toge ther with extensive data from studies in other chronic conditions 3 - 5 – including Tonix’s studies in fibromyalgia – make PROMIS Fatigue a solid candidate for the primary endpoint of future Long COVID registrational studies. 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Walker S, et al . BMJ Open 2023;13:e069217. doi:10.1136/ bmjopen - 2022 - 069217 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology , 73, 89 - 102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology , 73, 128 – 134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation , 92(10 Supplement), S20 - S27.
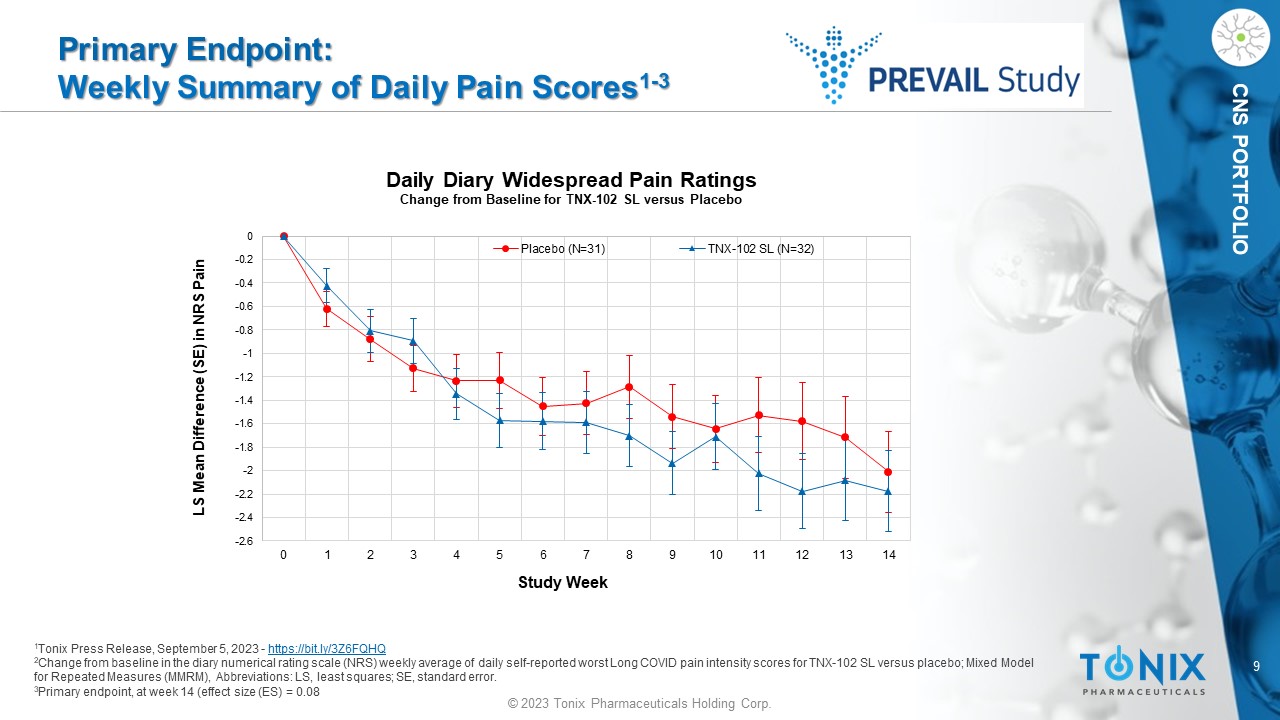
9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Primary Endpoint: Weekly Summary of Daily Pain Scores 1 - 3 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Change from baseline in the diary numerical rating scale (NRS) weekly average of daily self - reported worst Long COVID pain inten sity scores for TNX - 102 SL versus placebo; M ixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error. 3 Primary endpoint, at week 14 (effect size (ES) = 0.08 -2.6 -2.4 -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean Difference (SE) in NRS Pain Study Week Daily Diary Widespread Pain Ratings Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL (N=32)
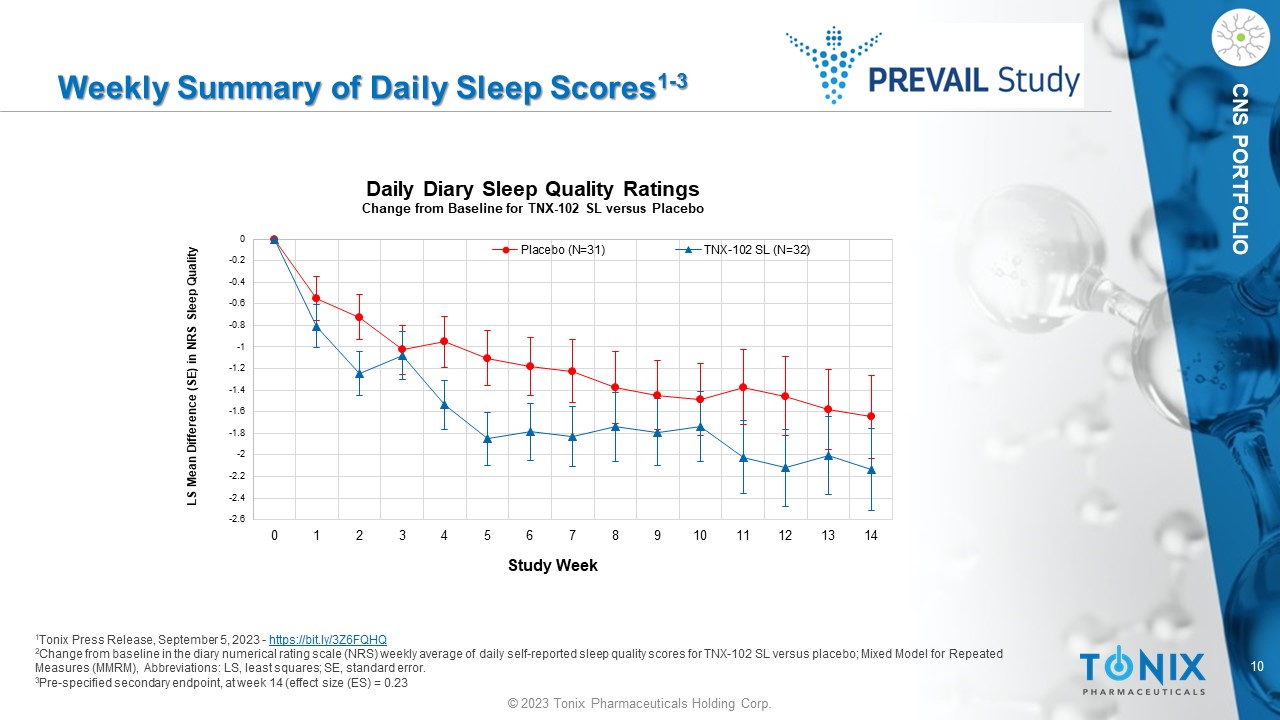
10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Weekly Summary of Daily Sleep Scores 1 - 3 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Change from baseline in the diary numerical rating scale (NRS) weekly average of daily self - reported sleep quality scores for TN X - 102 SL versus placebo; M ixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error. 3 Pre - specified secondary endpoint, at week 14 (effect size (ES) = 0.23 -2.6 -2.4 -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean Difference (SE) in NRS Sleep Quality Study Week Daily Diary Sleep Quality Ratings Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL (N=32)
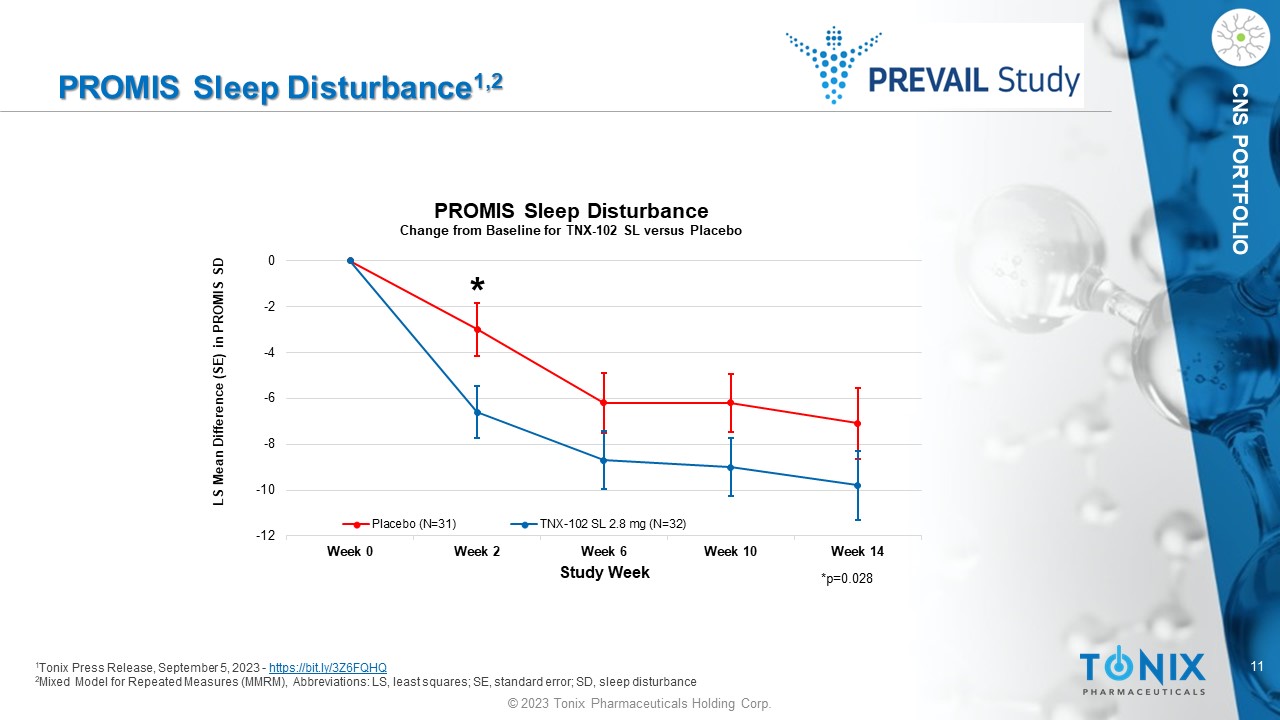
11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROMIS Sleep Disturbance 1,2 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Mixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error; SD, sleep disturbance -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean Difference (SE) in PROMIS SD Study Week PROMIS Sleep Disturbance Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL 2.8 mg (N=32) *p=0.028 * 12 © 2023 Tonix Pharmaceuticals Holding Corp.
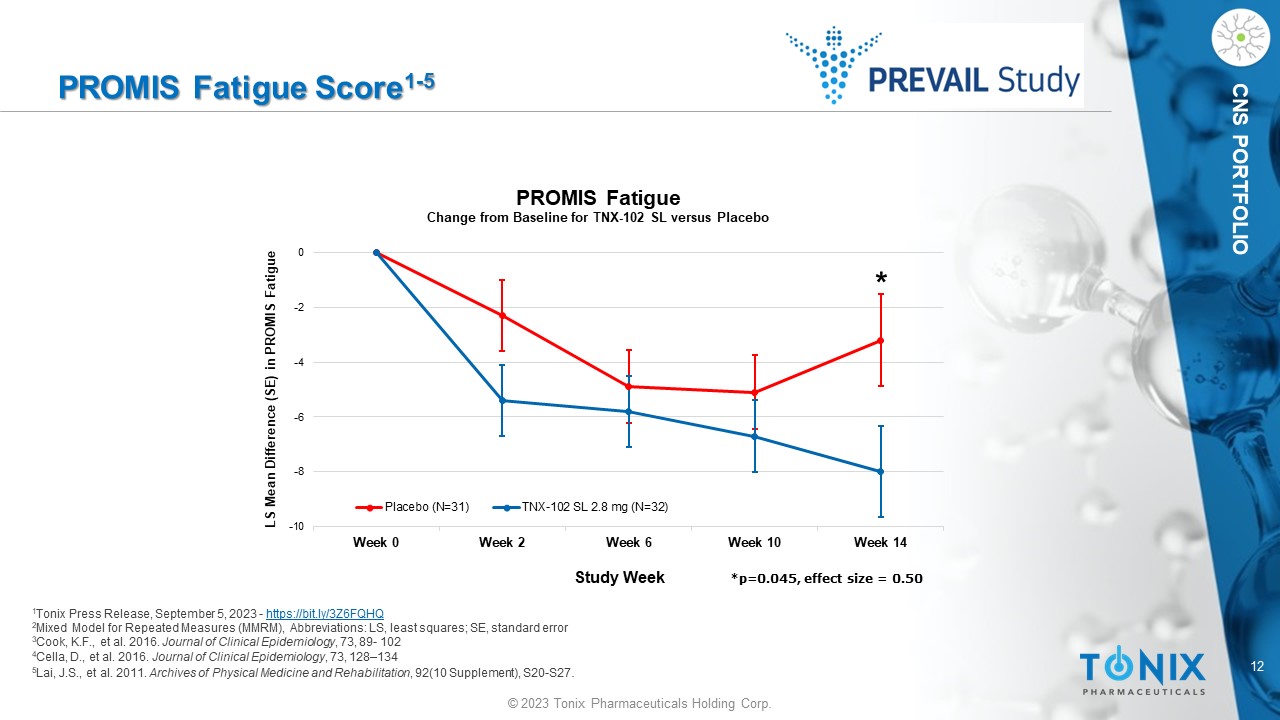
CNS PORTFOLIO PROMIS Fatigue Score 1 - 5 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Mixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology , 73, 89 - 102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology , 73, 128 – 134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation , 92(10 Supplement), S20 - S27 . -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean Difference (SE) in PROMIS Fatigue Study Week PROMIS Fatigue Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL 2.8 mg (N=32) * *p=0.045, effect size = 0.50 13 © 2023 Tonix Pharmaceuticals Holding Corp.
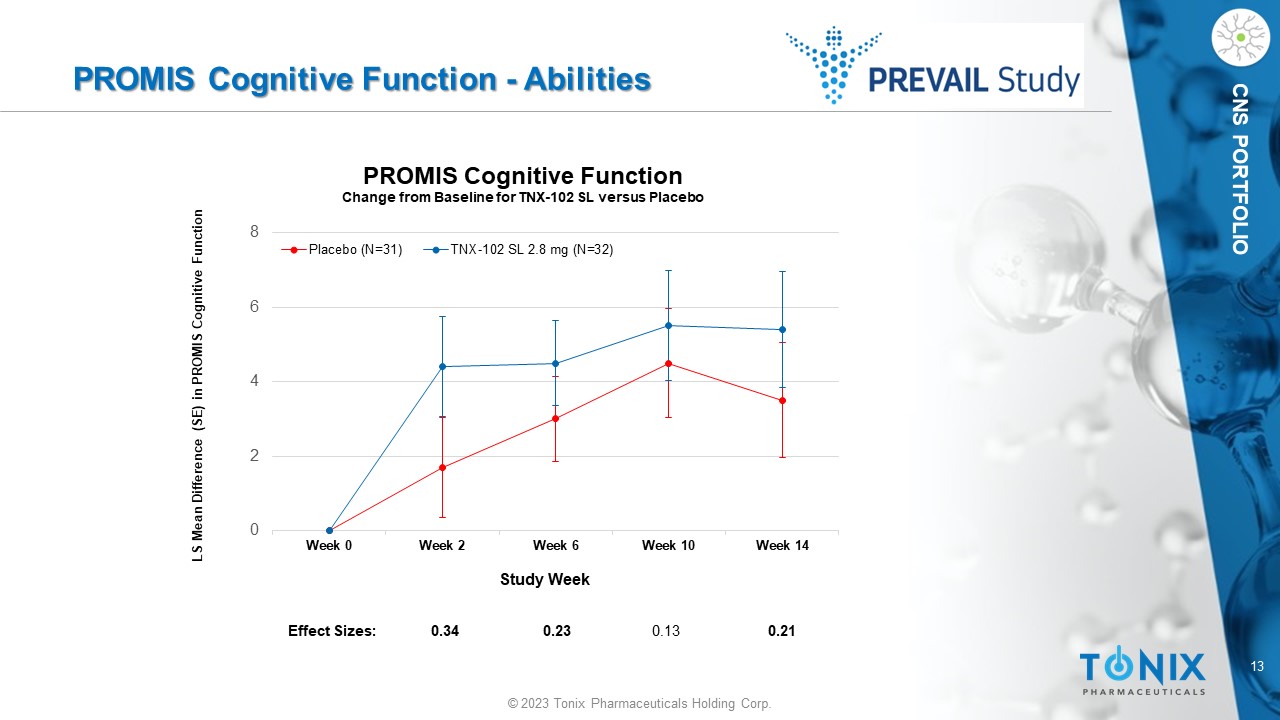
CNS PORTFOLIO PROMIS Cognitive Function - Abilities 0 2 4 6 8 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean Difference (SE) in PROMIS Cognitive Function Study Week PROMIS Cognitive Function Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL 2.8 mg (N=32) Effect Sizes: 0.34 0.23 0.13 0.21 14 © 2023 Tonix Pharmaceuticals Holding Corp.
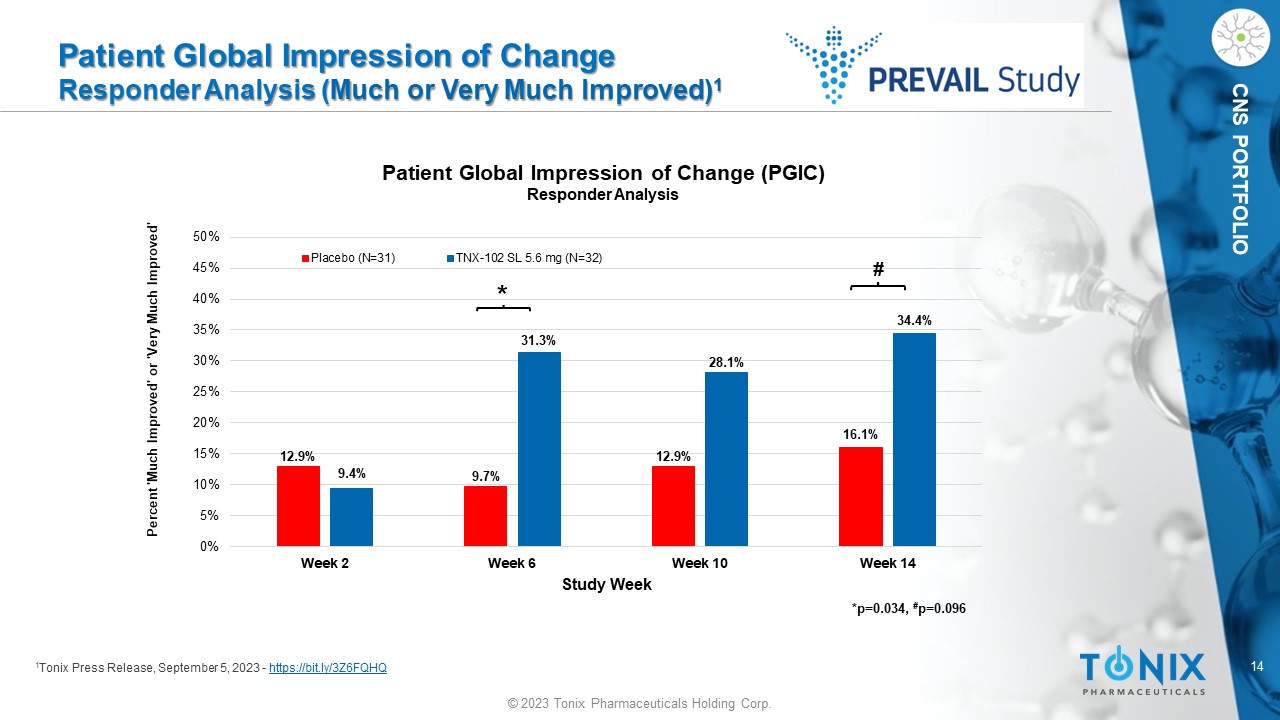
CNS PORTFOLIO Patient Global Impression of Change Responder Analysis ( Much or Very Much Improved) 1 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 12.9% 9.7% 12.9% 16.1% 9.4% 31.3% 28.1% 34.4% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Week 2 Week 6 Week 10 Week 14 Study Week Patient Global Impression of Change (PGIC) Responder Analysis Placebo (N=31) TNX-102 SL 5.6 mg (N=32) Percent 'Much Improved' or 'Very Much Improved' * # *p=0.034, # p= 0.096 15 © 2023 Tonix Pharmaceuticals Holding Corp.
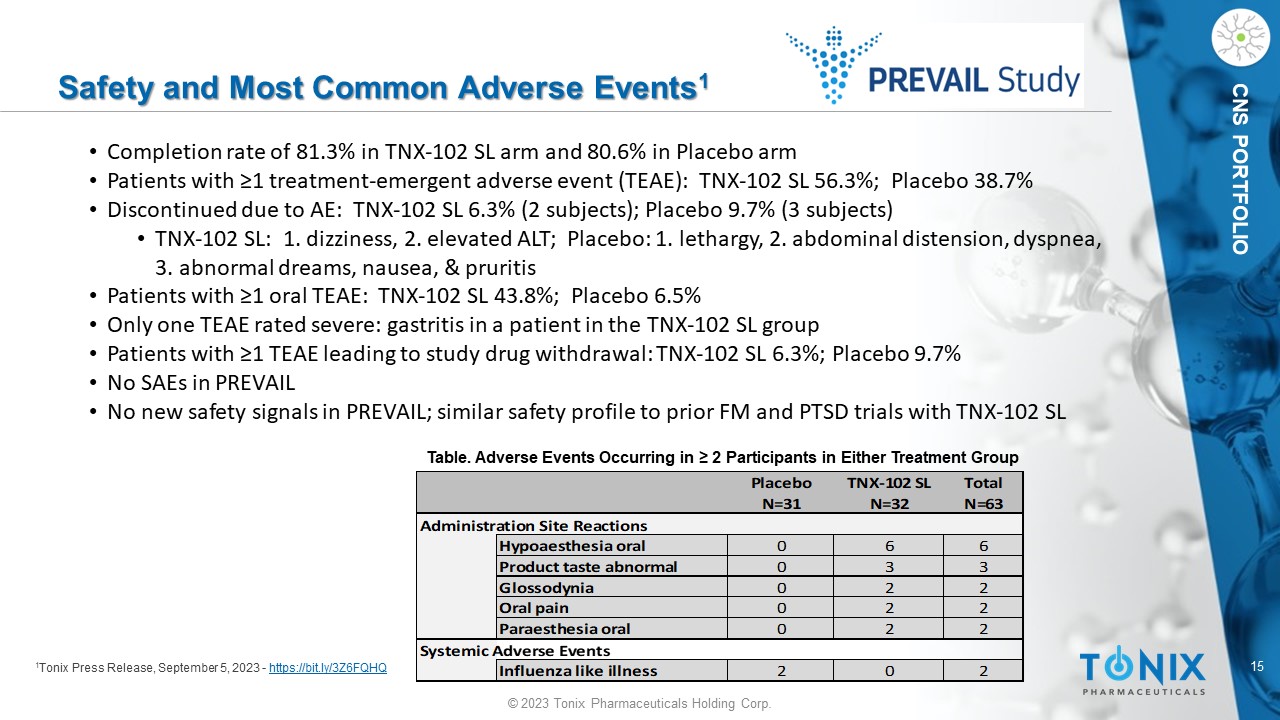
CNS PORTFOLIO Safety and Most Common Adverse Events 1 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ Placebo TNX-102 SL Total N=31 N=32 N=63 Administration Site Reactions Hypoaesthesia oral 0 6 6 Product taste abnormal 0 3 3 Glossodynia 0 2 2 Oral pain 0 2 2 Paraesthesia oral 0 2 2 Systemic Adverse Events Influenza like illness 2 0 2 Table. Adverse Events Occurring in ≥ 2 Participants in Either Treatment Group • Completion rate of 81.3% in TNX - 102 SL arm and 80.6% in Placebo arm • Patients with ≥1 treatment - emergent adverse event (TEAE): TNX - 102 SL 56.3%; Placebo 38.7% • Discontinued due to AE: TNX - 102 SL 6.3% (2 subjects); Placebo 9.7% (3 subjects) • TNX - 102 SL: 1. dizziness, 2. elevated ALT; Placebo: 1. lethargy, 2. abdominal distension, dyspnea, 3. abnormal dreams, nausea, & pruritis • Patients with ≥1 oral TEAE: TNX - 102 SL 43.8%; Placebo 6.5% • Only one TEAE rated severe: gastritis in a patient in the TNX - 102 SL group • Patients with ≥1 TEAE leading to study drug withdrawal: TNX - 102 SL 6.3%; Placebo 9.7% • No SAEs in PREVAIL • No new safety signals in PREVAIL; similar safety profile to prior FM and PTSD trials with TNX - 102 SL 16 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO Next Steps Tonix plans to meet with FDA to discuss a path to registration ‒ Expected date of End of Phase 2 meeting is 1 st Quarter 2024 F atigue is the principal symptom overlapping with chronic fatigue syndrome/ myalgic encephalomyelitis (CFS/ME) and fibromyalgia syndromes ‒ Expected date of fibromyalgia topline is 4 th Quarter 2023 © 2023 Tonix Pharmaceuticals Holding Corp.

THANK YOU

© 2023 Tonix Pharmaceuticals Holding Corp. APPENDIX: ABOUT LONG COVID AND COMMONALITIES WITH FIBROMYALGIA, CFS/ME, AND POST - VIRAL SYNDROMES 18 19 © 2023 Tonix Pharmaceuticals Holding Corp.
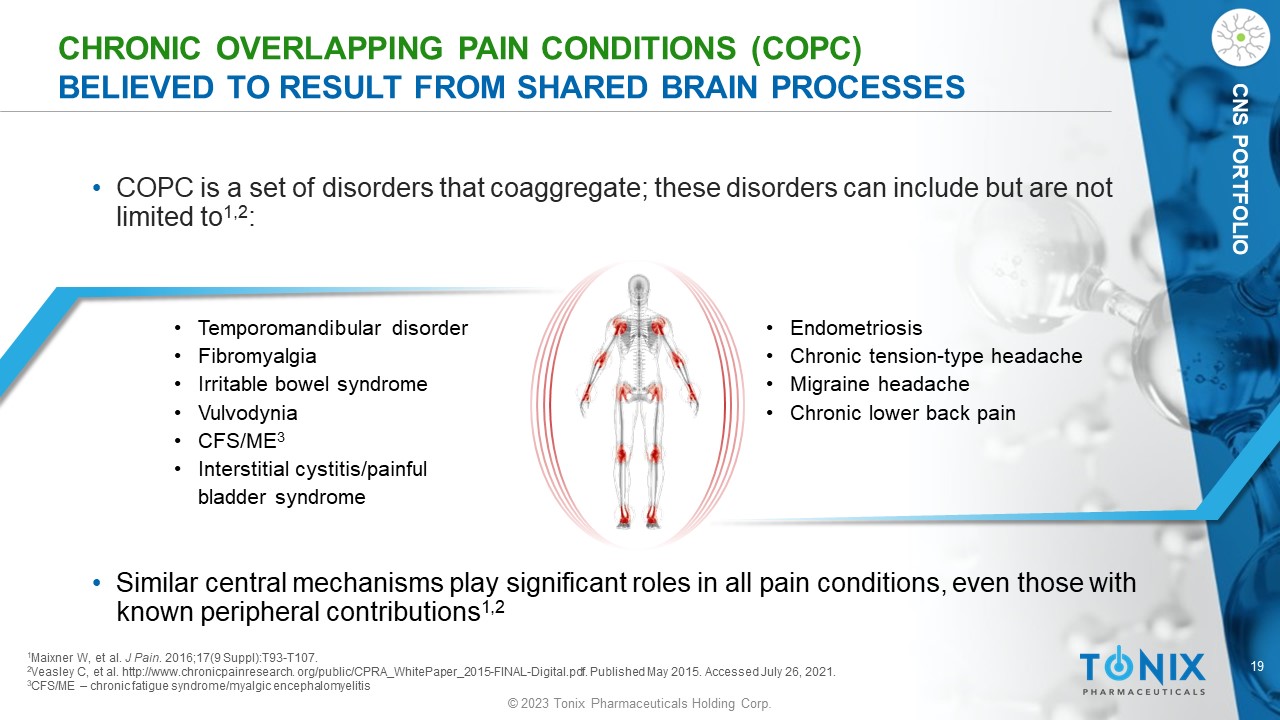
CNS PORTFOLIO CHRONIC OVERLAPPING PAIN CONDITIONS (COPC) BELIEVED TO RESULT FROM SHARED BRAIN PROCESSES • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2 20 © 2023 Tonix Pharmaceuticals Holding Corp.
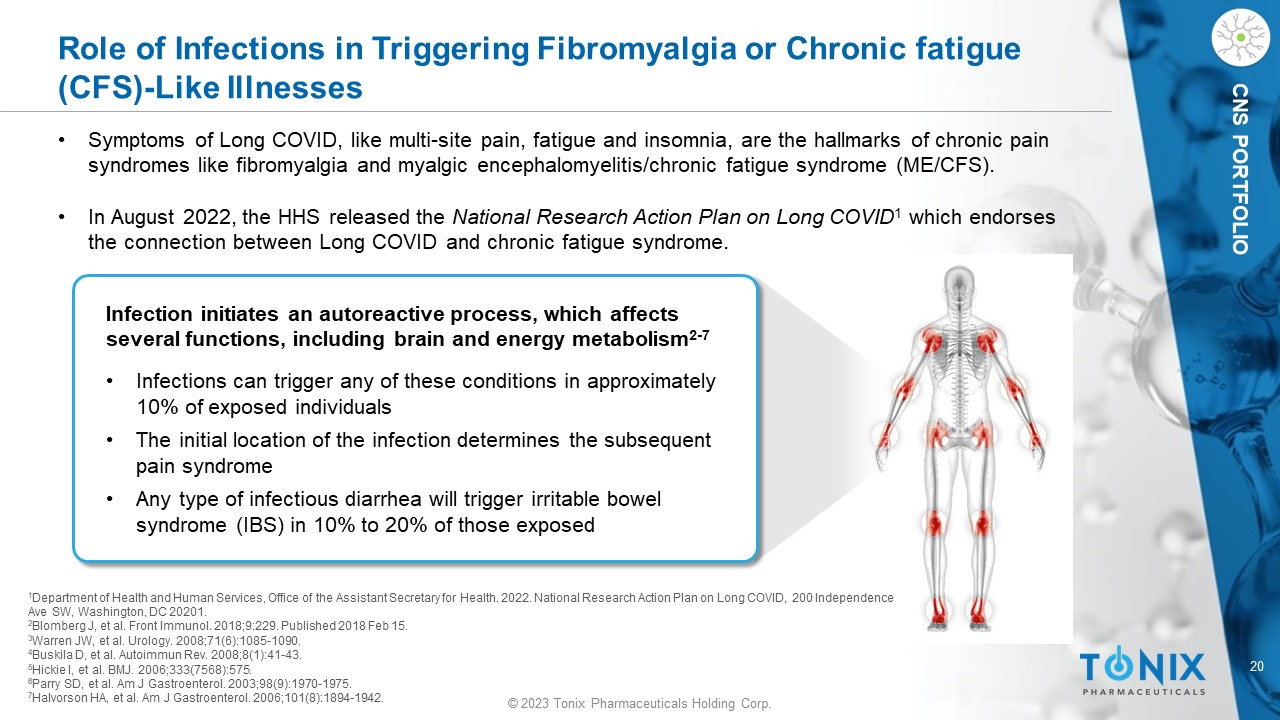
CNS PORTFOLIO Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger irritable bowel syndrome (IBS) in 10% to 20% of those exposed 1 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942. • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • In August 2022, the HHS released the National Research Action Plan on Long COVID 1 which endorses the connection between Long COVID and chronic fatigue syndrome.
21 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO POTENTIAL INCREASE IN MYALGIA FOLLOWING THE COVID - 19 PANDEMIC The specific causes may be due to: Chronic pain increase due to COVID - 19 could be nociplastic , neuropathic, or nociceptive Chronic pain newly triggered in individuals without SARS - CoV - 2 infection by exacerbation of risk factors (poor sleep, inactivity, fear, anxiety, and depression) Chronic pain as part of a post viral syndrome or the result of viral - associated organ damage Worsening of chronic pain due to exacerbation of preexisting pain physical or mental complaints Clauw DJ et al. Pain. 2020;161(8):1694 - 1697.
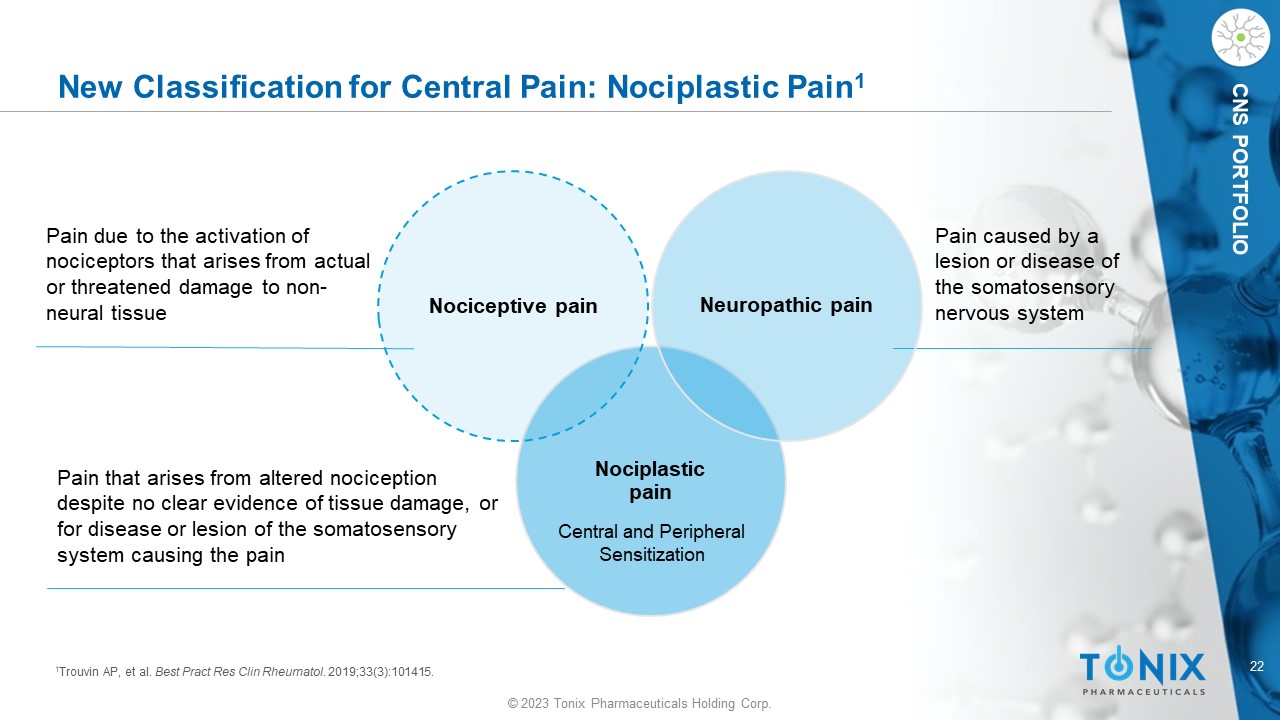
22 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO New Classification for Central Pain: Nociplastic Pain 1 Nociplastic pain Nociceptive pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Central and Peripheral Sensitization 23 © 2023 Tonix Pharmaceuticals Holding Corp.
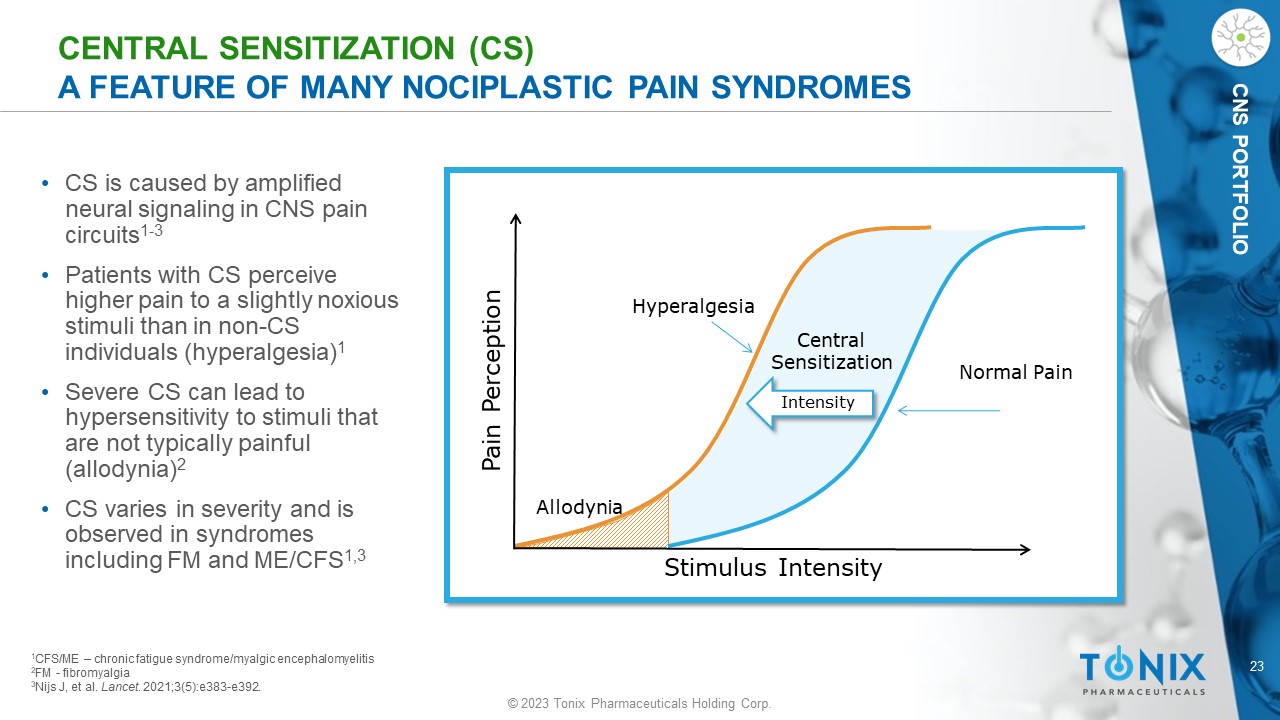
CNS PORTFOLIO CENTRAL SENSITIZATION (CS) A FEATURE OF MANY NOCIPLASTIC PAIN SYNDROMES • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 1 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis 2 FM - fibromyalgia 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
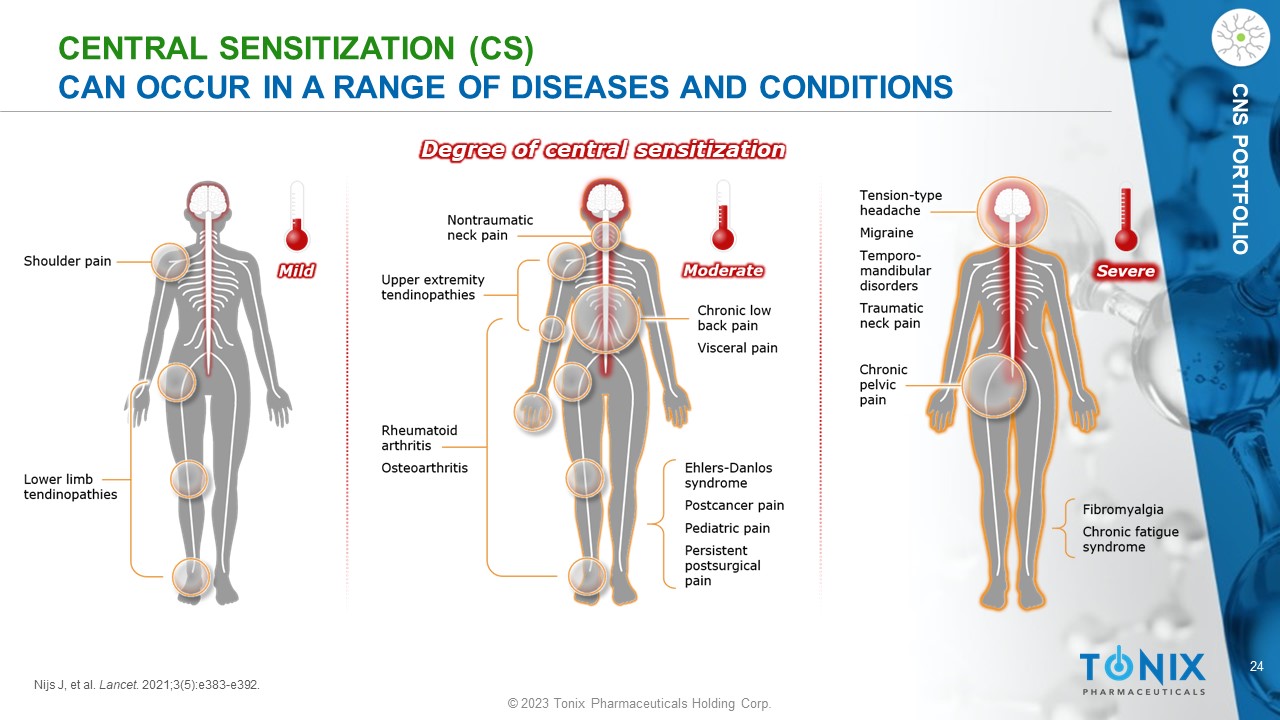
24 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CENTRAL SENSITIZATION (CS) CAN OCCUR IN A RANGE OF DISEASES AND CONDITIONS Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
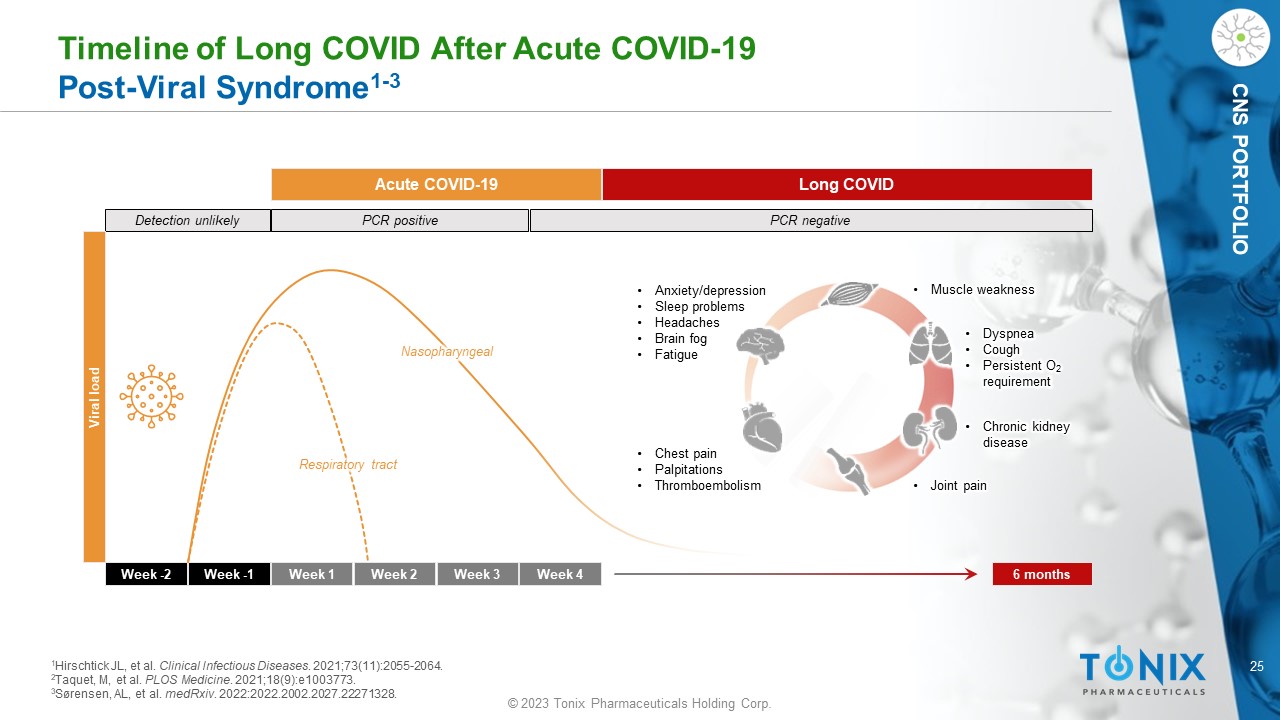
25 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Timeline of Long COVID After Acute COVID - 19 Post - Viral Syndrome 1 - 3 Week - 2 Week - 1 Week 1 Week 2 Week 3 Week 4 6 months Detection unlikely PCR positive PCR negative Acute COVID - 19 Long COVID Viral load Nasopharyngeal Respiratory tract • Chest pain • Palpitations • Thromboembolism • Dyspnea • Cough • Persistent O 2 requirement • Anxiety/depression • Sleep problems • Headaches • Brain fog • Fatigue • Chronic kidney disease • Muscle weakness • Joint pain 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773. 3 Sørensen, AL, et al. medRxiv . 2022:2022.2002.2027.22271328.
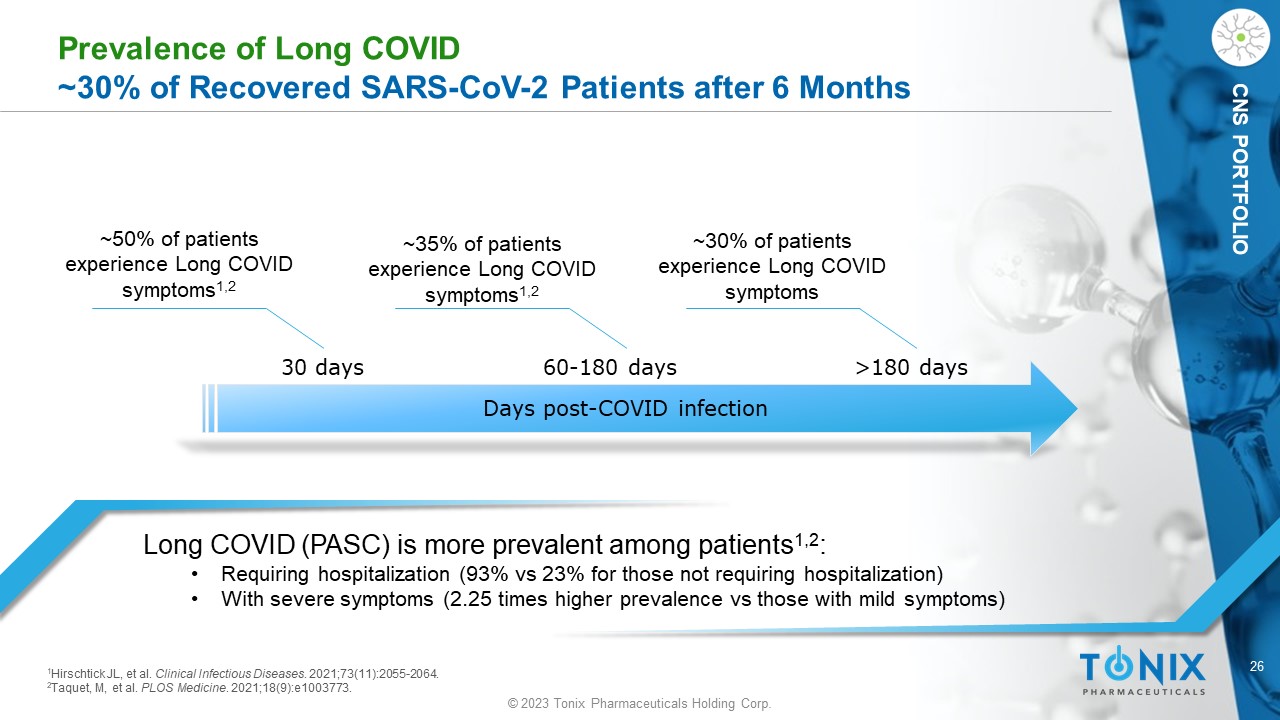
26 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Prevalence of Long COVID ~30% of Recovered SARS - CoV - 2 Patients after 6 Months Long COVID (PASC) is more prevalent among patients 1,2 : • Requiring hospitalization (93% vs 23% for those not requiring hospitalization) • With severe symptoms (2.25 times higher prevalence vs those with mild symptoms) ~50% of patients experience L ong COVID symptoms 1,2 Days post - COVID infection 30 days 60 - 180 days >180 days ~35% of patients experience Long COVID symptoms 1,2 ~30% of patients experience Long COVID symptoms 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773.
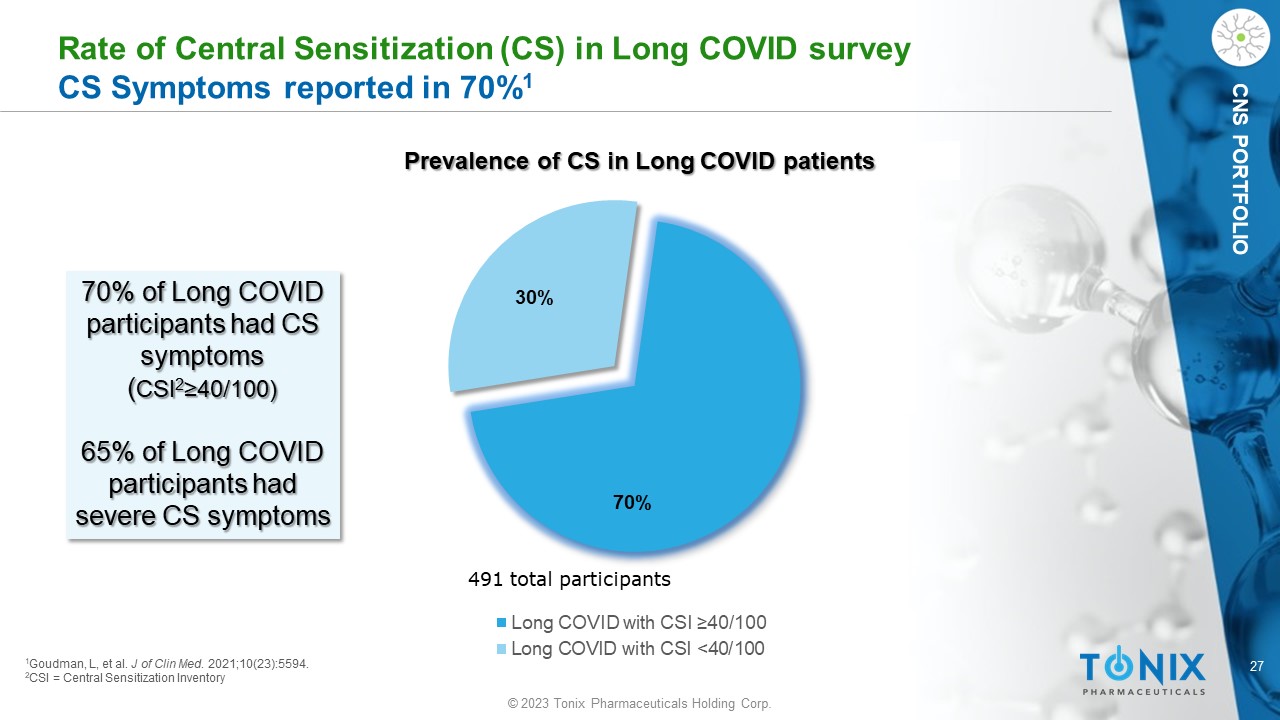
27 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Rate of Central Sensitization (CS) in Long COVID survey CS Symptoms reported in 70% 1 70% 30% Long COVID with CSI ≥40/100 Long COVID with CSI <40/100 491 total participants 1 Goudman , L, et al. J of Clin Med . 2021;10(23):5594. 2 CSI = Central Sensitization Inventory 70% of Long COVID participants had CS symptoms ( CSI 2 ≥40/100) 65% of Long COVID participants had severe CS symptoms Prevalence of CS in Long COVID patients 28 © 2023 Tonix Pharmaceuticals Holding Corp.
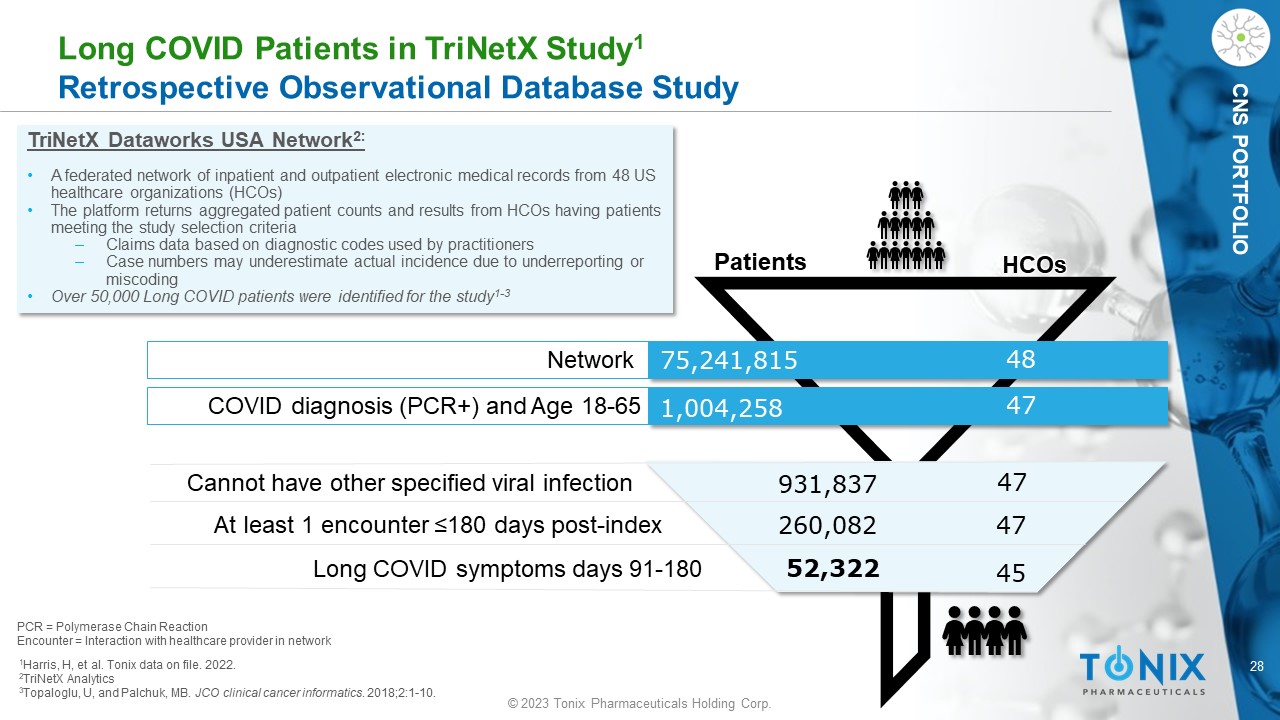
CNS PORTFOLIO Long COVID Patients in TriNetX Study 1 Retrospective Observational Database Study 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 3 Topaloglu, U, and Palchuk , MB. JCO clinical cancer informatics . 2018;2:1 - 10. TriNetX Dataworks USA Network 2: • A federated network of inpatient and outpatient electronic medical records from 48 US healthcare organizations (HCOs) • The platform returns aggregated patient counts and results from HCOs having patients meeting the study selection criteria ‒ Claims data based on diagnostic codes used by practitioners ‒ Case numbers may underestimate actual incidence due to underreporting or miscoding • Over 50,000 Long COVID patients were identified for the study 1 - 3 52,322 260,082 931,837 47 47 45 48 47 75,241,815 1,004,258 HCOs Patients Network COVID diagnosis (PCR+) and Age 18 - 65 Cannot have other specified viral infection At least 1 encounter ≤180 days post - index Long COVID symptoms days 91 - 180 PCR = Polymerase Chain Reaction Encounter = Interaction with healthcare provider in network 29 © 2023 Tonix Pharmaceuticals Holding Corp.
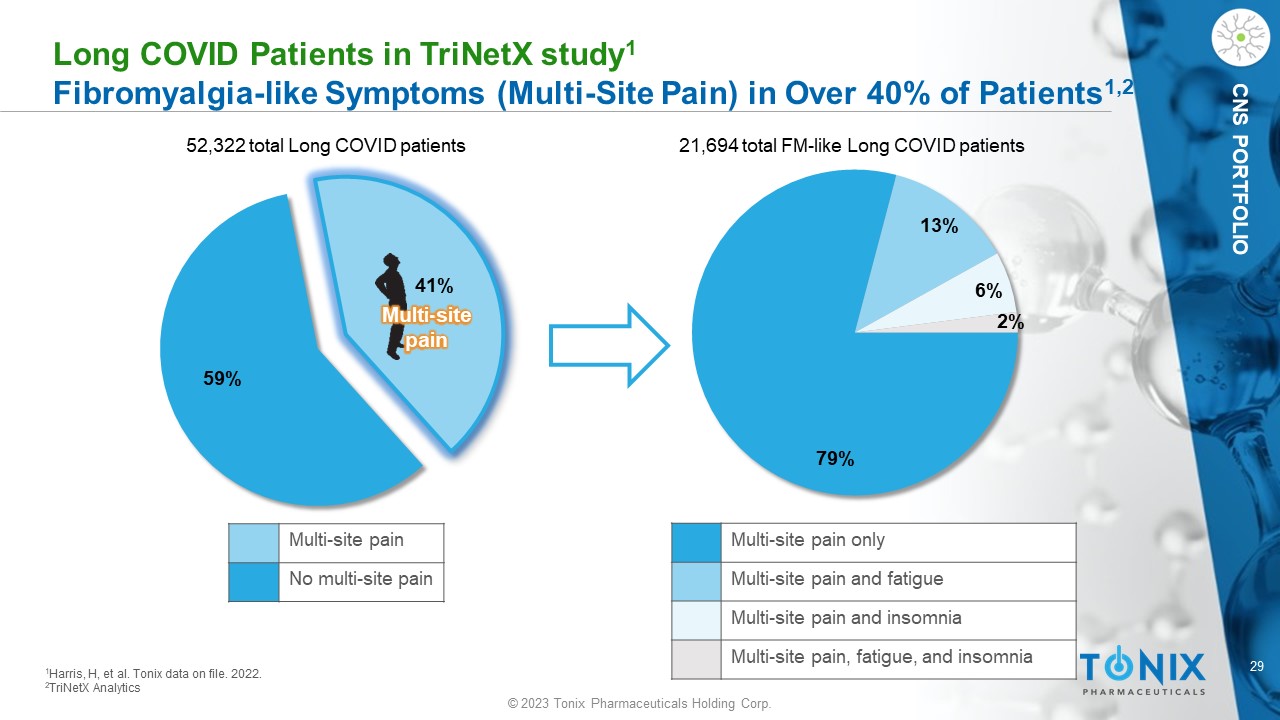
CNS PORTFOLIO L ong COVID P atients in T riNetX study 1 F ibromyalgia - like Symptoms (Multi - Site Pain) in Over 40% of Patients 1,2 79% 13% 6% 2% 52,322 total Long COVID patients 21,694 total FM - like Long COVID patients 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 59% 41% Multi - site pain Multi - site pain No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Multi - site pain, fatigue, and insomnia 30 © 2023 Tonix Pharmaceuticals Holding Corp.
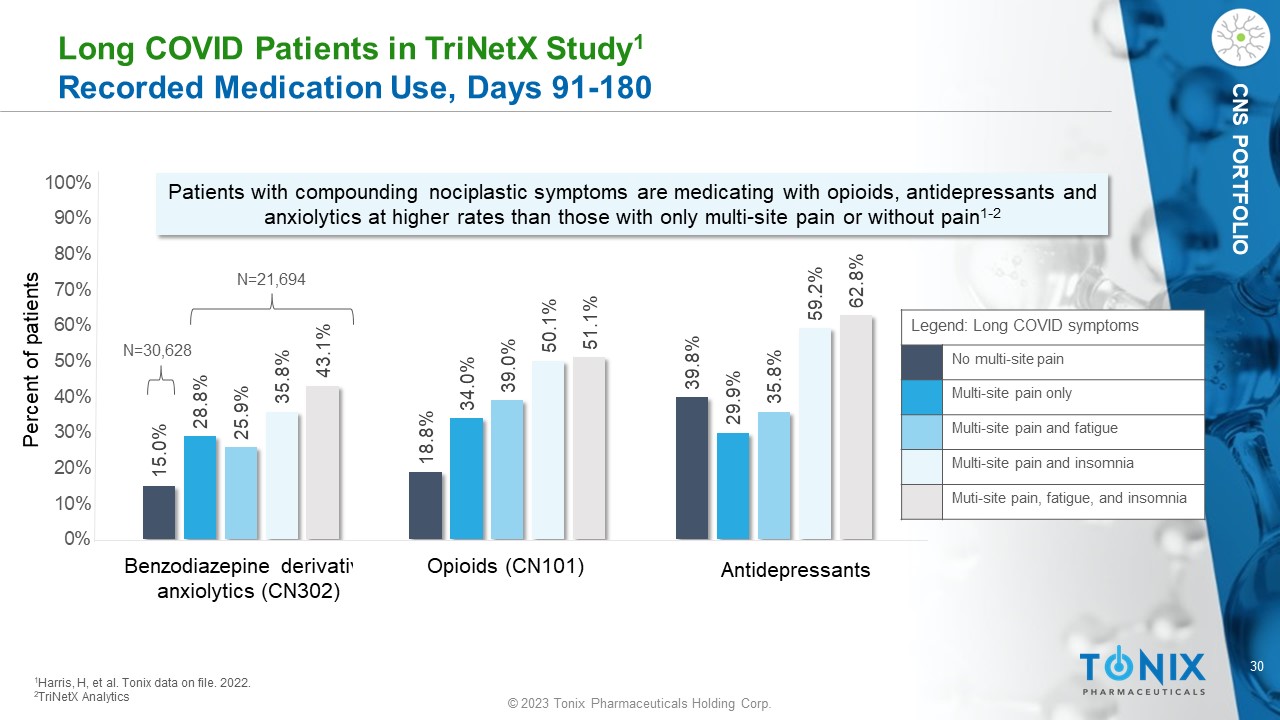
CNS PORTFOLIO L ong COVID Patients in TriNetX Study 1 Recorded Medication Use, Days 91 - 180 15.0% 18.8% 39.8% 28.8% 34.0% 29.9% 25.9% 39.0% 35.8% 35.8% 50.1% 59.2% 43.1% 51.1% 62.8% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Patients with compounding nociplastic symptoms are medicating with opioids, antidepressants and anxiolytics at higher rates than those with only multi - site pain or without pain 1 - 2 1 Harris, H, et al. Tonix dat a on file. 2022. 2 TriNetX Analytics Percent of patients Benzodiazepine derivative anxiolytics (CN302) Opioids (CN101) Antidepressants Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia N=21,694 N=30,628 31 © 2023 Tonix Pharmaceuticals Holding Corp.
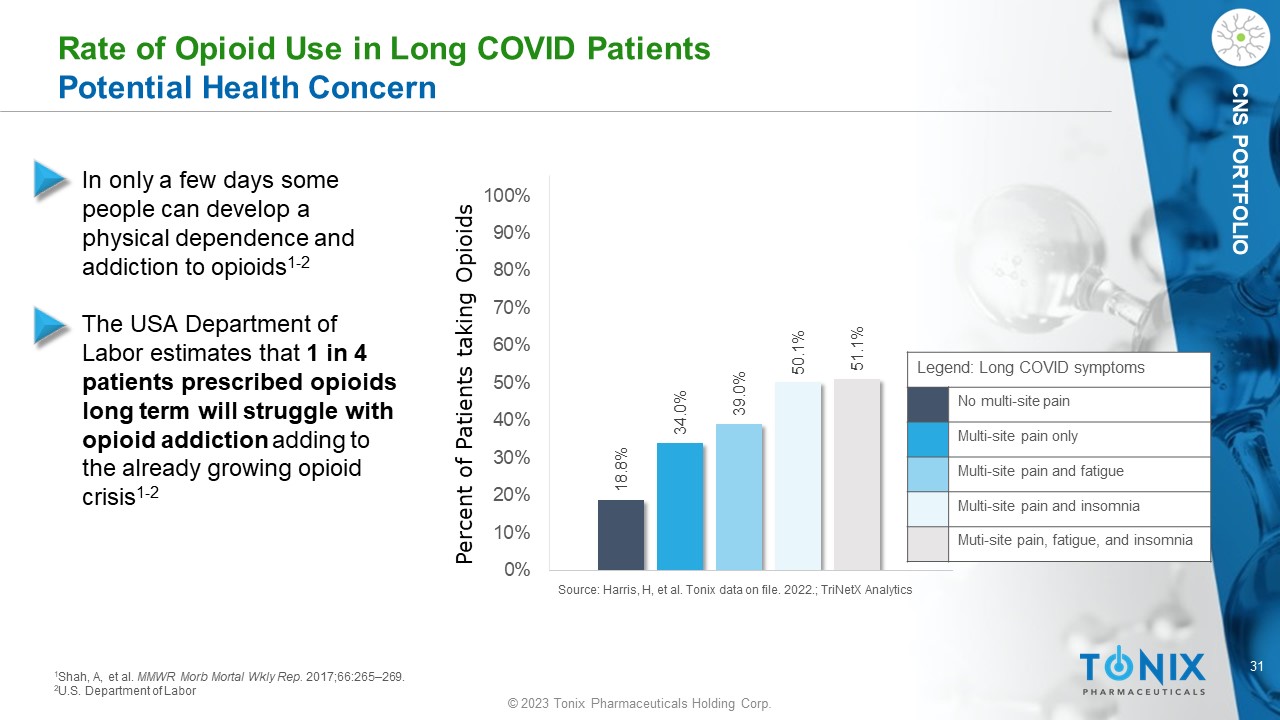
CNS PORTFOLIO 18.8% 34.0% 39.0% 50.1% 51.1% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Percent of Patients taking Opioids Rate of Opioid Use in Long COVID Patients Potential Health Concern • In only a few days some people can develop a physical dependence and addiction to opioids 1 - 2 • The USA Department of Labor estimates that 1 in 4 patients prescribed opioids long term will struggle with opioid addiction adding to the already growing opioid crisis 1 - 2 1 Shah, A, et al. MMWR Morb Mortal Wkly Rep. 2017;66:265 – 269. 2 U.S. Department of Labor Source: Harris, H, et al. Tonix data on file. 2022.; TriNetX Analytics Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia 32 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO Significant Financial Impact of Long COVID for Households and Economies 25% of Long COVID patients are unable to return to work 1 Over 250,000 Quality Adjusted Life - Years (QUALYS) will be lost due to Long COVID in the UK 2 $23.3 billion is estimated to be paid by the UK government to avoid QUALY losses due to Long COVID 2 1 Davis, HE, et al. eClinicalMedicine . 2021;38. 2 Martin, C, et al. PloS one . 2021;16(12):e0260843 - e0260843.
33 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Long COVID Presidential Memorandum President Biden – April 5, 2022 1 Policy • Commits to redoubling efforts to address the long - term effects of COVID - 19 Organizing Government Wide Response • Harnesses the full potential of the Federal Government, in coordination with public - and private - sector partners, to mount a full and effective response National Research Action Plane • Coordinates efforts across the public and private sectors • Orders establishment of the first - ever interagency national research agenda to, among other things, foster development of new treatments based on a better understanding of the pathophysiological mechanisms of the SARS - CoV - 2 virus Previously, Congress awarded NIH $1.15 billion to study Long COVID. 2 • Funded among other things the RECOVER Initiative implemented by the National Institutes of Health. 1 April 5, 2022 President Biden. “Memorandum on Addressing the Long - Term Effects of COVID - 19 - www.whitehouse.gov/briefing - room/pr esidential - actions/2022/04/05/memorandum - on - addressing - the - long - term - effects - of - covid - 19/ 2 The NIH provision of Title III Health and Human Services, Division M -- Coronavirus Response and Relief Supplemental Appropriation s Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260.