UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): June 30, 2023
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 001-36019 | 26-1434750 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
26 Main Street, Chatham, New Jersey 07928
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (862) 904-8182
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | TNXP | The NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01. Entry into a Material Definitive Agreement.
On June 30, 2023, in connection with the Asset Purchase, as defined below, Tonix Medicines, Inc., a Delaware corporation (“Acquisition Sub”), a wholly-owned subsidiary of Tonix Pharmaceuticals Holding Corp, Inc. (the “Company”, and together with Acquisition Sub, the “Purchaser”), entered into a Transition Services Agreement (the “Transition Services Agreement”) with Upsher Smith Laboratories, LLC, a Minnesota limited liability company (“Seller”). Pursuant to the Transition Services Agreement, Seller will provide certain transition services to Purchaser for base fees equal to $100,000 per month for the first six months, and $150,000 per months for the seventh through ninth months, plus additional monthly fees for each service category totaling up to $150,000 per month.
The foregoing description of the Transition Services Agreement does not purport to be complete and is qualified in its entirety to the full text of the Transition Services Agreement, which is filed hereto as Exhibit 10.01, and is incorporated herein by reference.
Item 2.01 Completion of Acquisition or Disposition of Assets.
As previously disclosed, on June 23, 2023, Purchaser entered into that certain Asset Purchase Agreement (the “Asset Purchase Agreement”) with Seller. On June 30, 2023, pursuant to the Asset Purchase Agreement, Purchaser purchased and acquired Seller’s assets related to Seller’s Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg products (such businesses collectively, the “Business” and the asset purchase, the “Asset Purchase”), inventory related to the Business and assumed certain liabilities of Seller. The closing (“Closing”) occurred on June 30, 2023.
As consideration for the Asset Purchase, Purchaser paid to Seller $15 million in cash, $12 million of which was paid at Closing and $3 million of which is payable on the earlier of March 2024 and the completion of the transition services to be provided by Seller, as described above, and $10 million in cash at Closing to acquire certain Business-related inventories.
The foregoing description of the Asset Purchase Agreement and the Asset Purchase does not purport to be complete and is qualified in its entirety to the full text of the Asset Purchase Agreement, which was filed as Exhibit 1.01 to the Company’s Current Report on Form 8-K filed on June 26, 2023, and is incorporated herein by reference.
Item 7.01 Regulation FD Disclosure.
On July 3, 2023, the Company announced the closing of the Asset Purchase. A copy of the press release which discusses this matter is furnished hereto as Exhibit 99.01, and incorporated herein by reference.
The Company updated its investor presentation, which is used to conduct meetings with investors, stockholders and analysts and at investor conferences, and which the Company intends to place on its website, which may contain nonpublic information. A copy of the presentation is filed as Exhibit 99.02 hereto and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.01 and 99.02 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(a) Financial Statements of Business Acquired.
The financial statements required by Item 9.01(a), with respect to the Asset Purchase described in Item 2.01 herein, will be filed by amendment to this Current Report on Form 8-K as soon as practicable and in any event not later than 71 days after the date on which this Current Report on Form 8-K is required to be filed pursuant to Item 2.01.
(b) Pro Forma Financial Information.
The pro forma financial information required by Item 9.01(b), with respect to the Asset Purchase described in Item 2.01 herein, will be filed by amendment to this Current Report on Form 8-K as soon as practicable and in any event not later than 71 days after the date on which this Current Report on Form 8-K is required to be filed pursuant to Item 2.01.
| (d) |
Exhibit No. |
Description. | ||
|
10.01 †
|
Transition Services Agreement, dated as of June 30, 2023, by and among Upsher-Smith Laboratories, LLC and Tonix Medicines, Inc. |
|||
| 99.01 | Press Release of the Company, dated July 3, 2023 | |||
| 99.02 | Corporate Presentation by the Company for July 2023 | |||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
________
†The annexes, schedules, and certain exhibits to this Exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant hereby agrees to furnish supplementally a copy of any omitted annex, schedule or exhibit to the SEC upon request.
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: July 3, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||
Tonix Pharmaceuticals Holding Corp, Inc. 8-K
Exhibit 10.01
TRANSITION SERVICES AGREEMENT
This Transition Services Agreement (this “Agreement”) is entered into as of June [30], 2023 (the “Effective Date”) by and between Upsher-Smith Laboratories, LLC, a Minnesota limited liability company having its principal place of business located at 6701 Evenstad Drive, Maple Grove, MN 55369 (“Seller”), and Tonix Medicines, Inc. (a wholly-owned subsidiary of Tonix Pharmaceuticals Holding Corp.), a Delaware corporation having its principal place of business located at 26 Main Steet, Suite 101, Chatham, NJ 07928 (“Purchaser”). Seller and Purchaser may each be referred to herein individually as a “Party” and together as the “Parties” to this Agreement. Capitalized terms used herein and not otherwise defined shall have the meanings ascribed to such terms in the Asset Purchase Agreement (as defined below).
RECITALS
WHEREAS, Seller and Purchaser have entered into that certain Asset Purchase Agreement, dated as of June [23], 2023 (the “Asset Purchase Agreement”), pursuant to which, Seller has agreed to sell, transfer and assign to Purchaser, the Purchased Assets, and Purchaser has agreed to purchase the Purchased Assets from Seller; and
WHEREAS, in order to facilitate the transition of the Business to Purchaser and as contemplated in the Asset Purchase Agreement, Seller and Purchaser have agreed to enter into this Agreement as of the Closing, pursuant to which Seller will provide Purchaser with certain services, in each case on a transitional basis and subject to the terms and conditions set forth herein.
NOW, THEREFORE, in consideration of the mutual agreements and covenants hereinafter set forth, Seller and Purchaser hereby agree as follows:
ARTICLE 1 SERVICES
1.1 Provision of Services. Subject to the terms and conditions of this Agreement, Seller shall perform the services for Purchaser set forth on Exhibit A (as such Exhibit A may be amended or supplemented by the Parties in writing from time to time, the “Services”). As designated under each of the Services on Exhibit A, Seller will, among other Services: (a) manage and perform Purchaser’s obligations under the designated Acquired Contracts on behalf of Purchaser, at Purchaser’s expense (subject to Section 1.9), and at the direction of Purchaser, and transition such relationships to Purchaser, and (b) provide certain Services solely with respect to the Products under existing Seller direct contracts with designated third parties (the “Seller Third Party Contracts”), including the sale and distribution of Product under the Customer Contracts identified on Schedule 1 to Exhibit A. The Services provided under Seller Third Party Contracts shall be at Purchaser’s expense (subject to Section 1.9) and shall be subject in all respects to the terms of those contracts, including Seller’s confidentiality obligations to the counterparties and any applicable restrictions under those contracts, and shall continue, as to each Seller Third Party Contract, until such time as the applicable Service expires or terminates, or the earlier date on which Purchaser notifies Seller in writing that Purchaser has entered into its own contract with a counterparty to a Seller Third Party Contract. On the Effective Date, Purchaser will deliver to Seller a letter of authorization, in substantially the form attached as Exhibit B, granting to Seller the authority to manage and perform Purchaser’s obligations under the Acquired Contracts designated on Exhibit B on behalf of Purchaser during the Term of this Agreement until such time as the applicable Service is terminated in accordance with this Agreement.
1.2 Service Coordinators. Each Party shall designate one or more of its employees having sufficient knowledge and background to act as the principal point-of-contact between the Purchaser and Seller in order to coordinate the provision of each of the Services under this Agreement (each, a “Service Coordinator”). Each of the Purchaser and Seller may change one or more of its Service Coordinators upon written notice to the other. The Service Coordinators will meet and discuss the coordination of the Services on a regular basis, or from time to time, as reasonably requested by either Purchaser or Seller, to discuss the Services and any issues with respect thereto. It is expected that, commencing on July 5, 2023, the Service Coordinators will make appropriate members of their respective teams available to participate on routine operations calls, at times to be mutually agreed by the Service Coordinators, to share information and review the general operation of the Services. In no event shall the Service Coordinators or participants on an operations call have the authority to amend this Agreement, including Exhibit A.
1.3 Standard of Service. Seller shall at all times perform or cause the Services to be performed (a) with the use of reasonable care, efforts, skill and judgment and in a manner that that is at least of the same nature, quality standards, timeliness, operational, performance and other service levels and standards as substantially consistent with Seller’s past practices during the six (6) months prior to the Effective Date (subject to the changes described in the Asset Purchase Agreement and the Seller Disclosure Schedules); and (b) in compliance with all applicable Laws. Seller shall be responsible for providing or arranging for the provision of the necessary resources, systems, facilities, equipment, and personnel as are reasonably necessary to provide the Services substantially consistent with Seller’s past practices during the six (6) months prior to the Effective Date (subject to the changes described in the Asset Purchase Agreement and the Seller Disclosure Schedules) and in accordance with this Agreement.
1.4 Information Sharing. Seller will keep Purchaser reasonably informed with regard to the Services. As part of the Services, Seller will generate and maintain the Product-related data and reports described on Exhibit A, and will provide Purchaser with reasonable and regular access (in real-time, where available) to all such data and reports and the readily-available underlying support and internal control information for such data and reports.
1.5 Cooperation. During the Term (as defined below) and for up to six (6) months after any expiration or termination of this Agreement in its entirety, Seller will reasonably cooperate with Purchaser to ensure a smooth transition of the Services from Seller to Purchaser, including by making Seller personnel who are knowledgeable about the Services reasonably available to Purchaser to answer questions and provide requested documents and information.
1.6 Acquired Contracts and Seller Third Party Contracts. The Acquired Contracts were assigned to Purchaser as of the Effective Date. All Services provided by Seller under the Acquired Contracts and Seller Third Party Contracts are on behalf of Purchaser and at Purchaser’s direction. Seller shall not be responsible or liable for the actions and omissions of any third party under any Acquired Contract or Seller Third Party Contract. If the counterparty to a Seller Third Party Contract breaches such Seller Third Party Contract in connection with the Services, then Seller will use reasonable efforts, at Purchaser’s expense, to enforce such Seller Third Party Contract with respect to such breach and will pass through to Purchaser the benefit of any rights and remedies available to Seller with respect to such breach.
1.7 Employees. For such time as any employees of Seller are providing the Services under this Agreement, such employees (a) shall remain employees of Seller, and (b) shall not be deemed to be employees of the Purchaser or any other party receiving such Service for any purpose. The terms of this Agreement are not intended to cause any of the Parties to become a joint employer for any purpose. Seller’s employees shall at all times remain subject to the direction and control of Seller, and Purchaser shall have no responsibility or liability to such individuals whatsoever, whether by virtue of the relationships established between Seller and Purchaser in connection with this Agreement or otherwise. Seller shall be solely responsible for such employees, including, but not limited to, their welfare, salaries, wages, bonuses and commissions, fringe benefits, employee benefits, including severance and worker’s compensation, and legally-required employer contributions and tax obligations, including the withholding and payment of applicable taxes relating to such employment.
1.8 Additional Services. Seller shall consider in good faith any reasonable request by the Purchaser for access to any additional services that are not included in the Services, but which Seller performed for its own account in the operation of the Business prior to the Effective Date (the “Additional Services”), provided that Seller shall be under no obligation to provide any Additional Services in its sole discretion. Any such Additional Services that Seller agrees in writing to perform shall be subject to additional Service Fees as may be mutually agreed in writing by the Parties. Any Additional Services set forth on an amendment to Exhibit A, signed by both Parties, shall constitute Services under this Agreement and be subject in all respects to the provisions of this Agreement for the duration of the Term.
1.9 Service Fees; Expense Reimbursement. As consideration for Seller providing the Services in accordance with this Agreement, Purchaser shall pay to Seller the sum of the monthly service fee amounts specified on Exhibit A until the applicable End Date for each Service, subject to the applicable Base Monthly Service Fee set forth on Exhibit A (the “Service Fees”). In addition to the Service Fees, Purchaser shall reimburse Seller for the amount of all out-of-pocket expenditures made on behalf of Purchaser in the ordinary course of performance of the Services, subject to pre-approval by Purchaser in writing (email sufficient) of expenses over $5,000 in each individual case (the “Expense Reimbursements”); provided, however, that Purchaser will not be responsible for any expenses incurred by Seller for payments to a third party service provider or other subcontractor with respect to any aspect of the Services that Seller performed for itself at any time during the twelve (12) months prior to the Effective Date. Seller will deduct the Service Fees and Expense Reimbursements owed to Seller by Purchaser from any amounts remitted to Purchaser as further described under the heading “Commercial Operations Services” on Exhibit A. If the Commercial Operations Services have been terminated, or if the amount of a remittance is insufficient in any month to cover the amount of the applicable Services Fees and Expense Reimbursements, then Seller shall invoice Purchaser for the remaining amount of Services Fees and Expense Reimbursements due and Purchaser shall pay the invoice within 5 Business Days of receipt of the invoice.
ARTICLE 2
TERM AND TERMINATION
2.1 Term.
(a) Unless terminated earlier in accordance with Section 2.2, the term of this Agreement (the “Term”) shall begin on the Effective Date and shall continue until the End Date (as defined below) for the last-to-expire category of Services.
(b) Seller shall commence providing the Services upon the Effective Date and shall continue to provide each particular category of Services until the earlier of (i) the end date set forth on Exhibit A for such category of Services (as such end date may be extended only by the mutual, written agreement of the Parties prior to the 10th day of the month of the end date set forth on Exhibit A, the “End Date”); and (ii) termination of such category of Services by Purchaser at the end of a calendar month following advance written notice thereof to Seller no later than the 10th day of such month. In the event the Parties mutually agree to extend the End Date for a given category of Services, an amendment to Exhibit A will be executed to reflect the agreed term extension and additional Service Fees to be charged by Seller for the Services during such extended term. Except as set forth in Section 1.5, Seller has no obligation to provide Services following the expiration or earlier termination of this Agreement or any particular Service.
2.2 Termination.
(a) In the event a Party (the “Breaching Party”) fails to perform any of its material obligations under this Agreement and such failure remains uncured for a period of thirty (30) days after written notice of such failure by the other Party (the “Non-Breaching Party”), the Non-Breaching Party may immediately terminate this Agreement upon written notice of such termination to the Breaching Party. If Seller is unable to perform a Service on account of: (i) the action or inaction of a third party engaged by the Purchaser, or (ii) a Force Majeure Event (as defined in Section 2.4), then, in each case, Seller’s inability to perform shall not constitute a breach of the Agreement.
2.3 Effect of Expiration or Termination.
(a) Upon any expiration or termination of this Agreement in its entirety, all Services and other obligations of the Parties under this Agreement shall automatically terminate, except for those provisions set forth in Section 5.9, which shall survive any termination or expiration of this Agreement. Expiration or termination of one Service hereunder shall not terminate any other Services hereunder.
(b) Termination of this Agreement or any of the Services hereunder shall not release either Party from any liability or other obligation incurred prior to the effective date of such termination or expiration.
2.4 Force Majeure Event. If Seller is prevented from or delayed in complying, either totally or in part, with any of the terms or provisions of this Agreement by reason of fire, flood, storm, strike, walkout, lockout, delays by unaffiliated suppliers or carriers, shortages of fuel, power, raw materials or components, any law, order, proclamation, regulation, ordinance, demand, seizure or requirement of any governmental authority, riot, civil commotion, war, rebellion, governmental orders (including executive orders), laws, restrictions, regulations, actions, mandates, ordinances, injunctions or decrees, pandemics, epidemics or quarantines, national, regional, state, or local emergency declarations, acts of terrorism, nuclear accident or other causes beyond the reasonable control of any such Person or other acts of God, or acts, omissions or delays in acting by any governmental or military authority or Purchaser (a “Force Majeure Event”), then upon notice to Purchaser, Seller’s performance of the affected provisions and/or other requirements of this Agreement (excluding the Term) shall be suspended during the period of such Force Majeure Event and Seller shall have no liability to Purchaser or any other Person in connection therewith (provided that, if requested by Purchaser, the Parties will discuss in good faith the potential extension of the Term in light of such suspension). Purchaser’s obligation to pay the applicable Service Fees and expense reimbursement while the Force Majeure Event is continuing shall also be suspended with respect to the categories of Services that are subject to the Force Majeure Event, but Purchaser’s obligation to pay the Base Monthly Service Fee will continue for the duration of the Force Majeure Event. Seller and Purchaser shall make commercially reasonable efforts to remove such Force Majeure Event or mutually terminate the applicable Service if the Force Majeure Event continues for longer than 60 days.
ARTICLE 3
CONFIDENTIALITY AND INTELLECTUAL PROPERTY
3.1 Confidentiality. The provisions set forth in Section 7.05 of the Asset Purchase Agreement are hereby incorporated by reference and shall apply mutatis mutandis. Any and all Confidential Information disclosed under this Agreement, including, but not limited to, information that is developed or generated in connection with the performance of the Services, shall be subject to the terms and conditions of Section 7.05 of the Asset Purchase Agreement.
3.2 Intellectual Property. Except as otherwise expressly provided in this Agreement or any other Transaction Document, each Party shall retain ownership of the Intellectual Property that it owns immediately following the Closing and any derivative works, additions, modifications, translations or enhancements thereof created by a Party pursuant to this Agreement. To the extent that any Intellectual Property arises out of the performance of this Agreement, Seller will own all such Intellectual Property relating to the Services, except that any Intellectual Property related solely and exclusively to the Purchased Assets is hereby assigned to Purchaser. Seller hereby grants Purchaser a non-exclusive, perpetual, irrevocable, royalty-free, transferable, sublicensable license with respect to any other Intellectual Property related to the Purchased Assets and any work product and deliverables generated or provided by Seller in connection with the Services.
ARTICLE 4
REPRESENTATIONS AND WARRANTIES; LIMITATION ON LIABILITY
4.1 Representations and Warranties. Each Party represents and warrants to the other that:
(a) it is a legal entity validly existing and in good standing under the Laws of the jurisdiction of its organization;
(b) it has all necessary power and authority, and has taken all action necessary, to authorize, execute, deliver and perform this Agreement, in accordance with the terms of this Agreement; and
(c) this Agreement has been duly and validly executed and delivered by such Party, and, assuming the due authorization, execution and delivery by the other Party, constitutes a valid, legal and binding agreement of such Party, enforceable against such Party in accordance with its terms, subject to the effect of any applicable Laws relating to bankruptcy, reorganization, insolvency, moratorium, fraudulent conveyance or preferential transfers, or similar Laws relating to or affecting creditors’ rights generally and subject, as to enforceability, to the effect of general principles of equity (regardless of whether such enforceability is considered in a proceeding at equity or at Law).
4.2 Limitation on Liability. Except in the event of a Party’s gross negligence, fraud or willful misconduct, and without limiting either Party’s rights or obligations under Article IX of the Asset Purchase Agreement and Section 4.4 of this Agreement, in no event shall either Party have any liability under any provision of this Agreement for any punitive, incidental, consequential, special or indirect damages (including loss of future revenue or income, loss of business reputation or opportunity, or diminution of value or any damages based on any type of multiple) in each case to the extent relating to the breach or alleged breach of this Agreement and whether based on statute, contract, tort or otherwise, and whether or not arising from sole, joint, or concurrent negligence, strict liability, criminal liability or other fault. Notwithstanding the foregoing, except in the event of a Party’s gross negligence, fraud or willful misconduct, and without limiting either Party’s rights or obligations under Article IX of the Asset Purchase Agreement and Section 4.4 of this Agreement, if either Party suffers Losses arising out of this Agreement, which Losses were caused by the other Party’s breach of this Agreement, the sole liability of such breaching Party shall be (i) if the breaching Party is the Party that performed the Service, to refund the Service Fees for the relevant Service paid for but not properly performed, or (ii) if the breaching Party is not the Party that performed the Service, to pay the Service Fees if payable but not otherwise paid. Except in the event of Seller’s gross negligence, fraud or willful misconduct, and without limiting either Party’s rights or obligations under Article IX of the Asset Purchase Agreement and Section 4.4 of this Agreement, in no event shall Seller have aggregate liability under this Agreement in excess of the total Service Fees received pursuant to this Agreement.
4.3 DISCLAIMER OF WARRANTIES. EXCEPT AS SPECIFICALLY SET FORTH HEREIN, SELLER DISCLAIMS ANY AND ALL WARRANTIES, CONDITIONS OR REPRESENTATIONS (EXPRESS OR IMPLIED, ORAL OR WRITTEN) WITH RESPECT TO THE SERVICES, AND ANY SOFTWARE AND EQUIPMENT PROVIDED AS PART OF THE SERVICES, INCLUDING ANY AND ALL IMPLIED WARRANTIES OR CONDITIONS OF TITLE, NONINFRINGEMENT, ACCURACY OF INFORMATIONAL CONTENT, MERCHANTABILITY, OR FITNESS OR SUITABILITY FOR ANY PURPOSE (WHETHER OR NOT SELLER KNOWS OR HAS REASON TO KNOW ANY SUCH PURPOSE), WHETHER ALLEGED TO ARISE BY LAW, BY REASON OF CUSTOM OR USAGE IN THE TRADE, OR BY COURSE OF DEALING.
4.4 Indemnification. Each Party (the “Indemnitor”) shall indemnify and defend the other Party, its Affiliates and each of their respective employees, managers, directors, officers, equityholders, agents and representatives (each an “Indemnitee”), and shall hold each of them harmless from, any and all Losses incurred or sustained by an Indemnitee in connection with any Third Party Claim to the extent arising out, relating to or resulting from the Indemnitor’s (a) breach of this Agreement or (b) gross negligence, fraud or willful misconduct in connection with the provision or receipt of the Services.
ARTICLE 5
MISCELLANEOUS
5.1 Independent Contractors. The respective rights and obligations of Purchaser and Seller under this Agreement shall be limited to the contractual rights and obligations expressly set forth herein on the terms and conditions set forth herein. It is expressly agreed that Seller, on the one hand, and Purchaser, on the other hand, will be independent contractors and that neither this Agreement nor the relationship between the Parties will be construed as creating a partnership, joint venture or agency. Neither Seller, on the one hand, nor Purchaser, on the other hand, will have the authority to make any statements, representations or commitments of any kind, or to take any action or to incur any liability or obligation which will be binding on the other, without the prior consent of the other Party to do so. All persons employed by a Party will be employees of such Party and not of the other Party and all costs and obligations incurred by reason of any such employment will be for the account and expense of such Party.
5.2 Notices. All notices, requests, consents, claims, demands, waivers, and other communications hereunder shall be in writing and shall be deemed to have been given: (i) when delivered, if delivered personally to the intended recipient; (ii) when received by the addressee, if sent by an internationally recognized overnight courier service; (iii) on the date sent by email (with confirmation of receipt of the email and any attachments); or (iv) on the fifth (5th) day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective Parties at the following addresses (or at such other address for a Party as shall be specified in a notice given in accordance with this Section 5.2):
(a) if to Seller:
Upsher-Smith Laboratories, LLC 6701 Evenstad Drive Maple Grove, MN 55369 Email: Rich.Fisher@upsher-smith.com Attention: President With a copy (which shall not constitute notice) to: Upsher-Smith Laboratories, LLC 6701 Evenstad Drive Maple Grove, MN 55369 Email: USLContracts@upsher-smith.com Attention: General Counsel
With a copy (which shall not constitute notice) to:
Ballard Spahr LLP
80 South Eighth Street, Suite 2000
Minneapolis, MN 55402
Email: rummelb@ballardspahr.com
Attention: Barbara Rummel
(b) if to Purchaser:
Tonix Medicines, Inc.
26 Main Steet, Suite 101
Chatham, NJ 07928
Email: jim.hunter@tonixpharma.com
Attention: James Hunter, Executive VP Commercial Operations
With a copy (which shall not constitute notice) to:
Lowenstein Sandler LLP
One Lowenstein Drive
Roseland, New Jersey 07068
Email: mlerner@lowenstein.com
Attention: Michael J. Lerner, Esq.
5.3 Headings. The headings used in this Agreement are intended for convenience only and will not be considered part of the written understanding among the Parties and will not affect the construction of this Agreement.
5.4 Entire Agreement. This Agreement (including any Exhibits attached hereto and expressly referenced herein), constitutes the entire agreement between the Parties with respect to the subject matter hereof and supersedes all prior agreements and understandings, whether oral or written, with respect to such subject matter. Each Party confirms that no representations, warranties, covenants or understandings of any kind, nature or description whatsoever are being made or relied upon by any Party with respect to this Agreement, except such as are as specifically set forth herein. For clarity, the Asset Purchase Agreement shall not be superseded by this Agreement and shall be construed to have independent force and effect.
5.5 Successors and Assigns; Assignment. This Agreement shall be binding upon and shall inure to the benefit of the Parties hereto and their respective successors and permitted assigns. Neither Party may assign or delegate this Agreement or its rights or obligations hereunder, in whole or in part, without the prior written consent of the other Party, which consent shall not be unreasonably withheld, conditioned or delayed; provided, however, that either Party may assign this Agreement without any obligation to obtain the other Party’s consent in connection with a permitted assignment of the Asset Purchase Agreement or in connection with a merger or sale of substantially all of the assets of such Party, so long as such assignee or successor becomes obligated to perform the obligations of the assigning Party incurred or arising after the effective date of such assignment. No assignment shall relieve the assigning party of any of its obligations or Liabilities hereunder incurred or arising prior to the effective date of such assignment. Any assignment in breach of this Section 5.5 shall be null and void ab initio.
5.6 No Third Party Beneficiaries. This Agreement is for the sole benefit of the Parties hereto and their respective successors and permitted assigns. Nothing in this Agreement, express or implied, is intended to or shall confer on any other Person any legal or equitable right, benefit, or remedy of any nature whatsoever.
5.7 Amendment and Waiver. This Agreement, including any Exhibits attached hereto and expressly referenced herein, may only be amended, modified or otherwise changed, in whole or in part, in a writing specified to be an explicit amendment to this Agreement duly executed by authorized representatives of each of the Parties. No waiver by any Party of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the Party so waiving. No waiver by any Party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any right, remedy, power or privilege arising from this Agreement shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
5.8 Governing Law; Jurisdiction.
(a) The provisions of Sections 11.08, 11.09 and 11.10 of the Asset Purchase Agreement are incorporated herein by reference, mutatis mutandis, except that reference to the Agreement in such provisions shall be deemed to refer to this Agreement.
5.9 Survival. The provisions of Section 1.5, Section 2.3, ARTICLE 3, ARTICLE 4 and ARTICLE 5 shall survive the expiration or earlier termination of this Agreement, as well as any other provisions which by their terms survive such expiration or termination.
5.10 Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Agreement delivered by facsimile, email, or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Agreement.
[signature page follows]
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed as of the date first written above by their respective officers thereunto duly authorized.
SELLER
Upsher-Smith Laboratories, LLC
By:
/s/ Rich Fischer
Name: Rich Fischer
Title: President & COO
PURCHASER
Tonix Medicines, Inc.
By:
/s/ James Hunter
Name: James Hunter
Title: President
[Signature page to Transition Services Agreement]
EXHIBIT A
SERVICES
A-
EXHIBIT B
LETTER OF AUTHORIZATION
B-
EXHIBIT C
BLANKET PURCHASE ORDER
C-
Tonix Pharmaceuticals Holding Corp, Inc. 8-K
Exhibit 99.01
Tonix Pharmaceuticals Completes Acquisition of Two FDA-Approved, Marketed Migraine Products
Strategic Acquisition Helps Build Tonix’s Commercial Capabilities and Infrastructure Ahead of Potential Launch of TNX-102 SL for the Management of Fibromyalgia
Acquisition of Zembrace® SymTouch® (sumatriptan injection) and Tosymra® (sumatriptan nasal spray), Indicated for the Treatment of Acute Migraine in Adults, Complements Tonix’s Current Intranasal Clinical Development Program of TNX-1900 for Migraine Prevention
CHATHAM, N.J., July 3, 2023 – Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix Pharmaceuticals or Tonix), a biopharmaceutical company, and its wholly-owned commercialization subsidiary Tonix Medicines, Inc. (Tonix Medicines), today announced it has completed the acquisition of two currently-marketed products from Upsher-Smith Laboratories, LLC (Upsher-Smith): Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg. Zembrace SymTouch and Tosymra are both indicated for the treatment of acute migraine with or without aura in adults. As previously announced, these products collectively generated product sales of approximately $23 million for the full year 2022.1
“The acquisition of these two products represents an important step in the evolution of Tonix into a fully integrated biopharmaceutical company and builds on our expertise in central nervous systems disorders,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals. “In addition to the potential growth that these two on-market products represent over time, the acquisition helps build Tonix’s commercial capabilities ahead of the potential launch of our TNX-102 SL product candidate for the treatment of fibromyalgia, and aligns strongly with our TNX-1900 (intranasal potentiated oxytocin) product candidate in clinical development for the prevention of headaches in chronic migraine.”
“Migraine headaches and breakthrough migraine headaches remain a significant burden, and we believe both Zembrace SymTouch and Tosymra are well-suited to address the unmet needs of patients currently using traditional or emerging oral acute migraine medications, particularly for rapid-onset treatment,” said James Hunter, Executive Vice President of Commercial Operations at Tonix Pharmaceuticals and President of Tonix Medicines.
Zembrace SymTouch is the only actively promoted brand of sumatriptan autoinjector in the United States (other sumatriptan autoinjector products on the market are Imitrex® and generics to Imitrex®). It has a unique low dose and has demonstrated onset of migraine pain relief in as few as 10 minutes (17% of patients vs. 5% for placebo).2 Zembrace SymTouch also demonstrated migraine pain freedom for 46% of patients (vs 27% for placebo) at 2 hours in a single-attack, double-blind study (N=230).3 Zembrace SymTouch currently has patent protection to 2036. Tosymra employs Intravail® permeation enhancer technology and is pharmacokinetically equivalent to 4 mg subcutaneous sumatriptan.4,5 Tosymra delivers migraine pain relief in as little as 10 minutes with just one spray for some patients (13% vs. 5% for placebo).2,5, 6 Tosymra currently has patent protection to 2031.
About Migraine
Nearly 40 million people in the United States suffer from migraine7 and it has been recognized as the second leading cause of disability in the world.7 Migraine is characterized by debilitating attacks lasting four to 72 hours with multiple symptoms, including pulsating headaches of moderate to severe pain intensity often associated with nausea or vomiting, and/or sensitivity to sound (phonophobia) and sensitivity to light (photophobia).8
References:
| 1. | IQVIA 2022 retail sales from the National Sales Perspectives (NSP) audit within the SMART database estimates Zembrace sales of ~$19.6 M and Tosymra sales of ~$3.5 M |
| 2. | Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatriptan Research Group. Arch Neurol. 1992;49(12):1271-1276. |
| 3. | Landy, S. et al. Efficacy and safety of DFN-11 (sumatriptan injection, 3 mg) in adults with episodic migraine: a multicenter, randomized, double-blind, placebo-controlled study. J Headache Pain. 19, 69 (2018). |
| 4. | Brand-Schieber E, Munjal S, Kumar R, et al. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patients. Med Devices (Auckl). 2016;9:131-137. |
| 5. | Tosymra [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, LLC: Feb 2021. |
| 6. | Wendt J, et al. A randomized, double-blind, placebo-controlled trial of the efficacy and tolerability of a 4-mg dose of subcutaneous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517-526. |
| 7. | GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17(11):954-976. |
| 8. | Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. |
Tonix Pharmaceuticals Holding Corp.*
Tonix is a biopharmaceutical company focused on commercializing, developing, discovering and licensing therapeutics to treat and prevent human disease and alleviate suffering. Tonix markets Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg. Zembrace SymTouch and Tosymra are each indicated for the treatment of acute migraine with or without aura in adults. Tonix’s development portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS development portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia with topline data expected in the first quarter of 2024. TNX-102 SL is also being developed to treat Long COVID, a chronic post-acute COVID-19 condition. Enrollment in a Phase 2 study has been completed, and topline results are expected in the third quarter of 2023. TNX-601 ER (tianeptine hemioxalate extended-release tablets), a once-daily formulation being developed as a treatment for major depressive disorder (MDD), is also currently enrolling with topline results expected in the first quarter of 2024. TNX-4300 (estianeptine) is a small molecule oral therapeutic in preclinical development to treat MDD, Alzheimer’s disease and Parkinson’s disease. TNX-1900 (intranasal potentiated oxytocin), in development for chronic migraine, is currently enrolling with topline data expected in the fourth quarter of 2023. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the third quarter of 2023. Tonix’s rare disease development portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology development portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 is expected to be initiated in the third quarter of 2023. Tonix’s infectious disease pipeline includes TNX-801, a vaccine in development to prevent smallpox and mpox. TNX-801 also serves as the live virus vaccine platform or recombinant pox vaccine platform for other infectious diseases. The infectious disease development portfolio also includes TNX-3900 and TNX-4000, classes of broad-spectrum small molecule oral antivirals.
* Tonix’s product development candidates are investigational new drugs or biologics and have not been approved for any indication.
Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis, Inc. All other marks are the property of their respective owners.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Peter Vozzo
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505
Media Contact
Ben Shannon
ICR Westwicke
ben.shannon@westwicke.com
(919) 360-3039
Zembrace® SymTouch® (sumatriptan Injection): IMPORTANT SAFETY INFORMATION
Zembrace SymTouch (Zembrace) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack:
| · | discomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back |
| · | severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw |
| · | pain or discomfort in your arms, back, neck, jaw or stomach |
| · | shortness of breath with or without chest discomfort |
| · | breaking out in a cold sweat |
| · | nausea or vomiting |
| · | feeling lightheaded |
Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem.
Do not use Zembrace if you have:
| · | history of heart problems |
| · | narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) |
| · | uncontrolled high blood pressure |
| · | hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. |
| · | had a stroke, transient ischemic attacks (TIAs), or problems with blood circulation |
| · | severe liver problems |
| · | taken any of the following medicines in the last 24 hours: almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, ergotamines, dihydroergotamine. |
| · | are taking certain antidepressants, known as monoamine oxidase (MAO)-A inhibitors or it has been 2 weeks or less since you stopped taking a MAO-A inhibitor. Ask your provider for a list of these medicines if you are not sure. |
| · | an allergy to sumatriptan or any of the components of Zembrace |
Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements.
Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.
Zembrace may cause serious side effects including:
| · | changes in color or sensation in your fingers and toes |
| · | sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever |
| · | cramping and pain in your legs or hips; feeling of heaviness or tightness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold feeling or color changes in one or both legs or feet |
| · | increased blood pressure including a sudden severe increase even if you have no history of high blood pressure |
| · | medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider. |
| · | serotonin syndrome, a rare but serious problem that can happen in people using Zembrace, especially when used with anti-depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. |
| · | hives (itchy bumps); swelling of your tongue, mouth, or throat |
| · | seizures even in people who have never had seizures before |
The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or tired.
Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Zembrace. For more information, ask your provider.
This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use. You can also visit www.upsher-smith.com or call 1-888-650-3789.
You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
INDICATION AND USAGE
Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine.
Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.
Tosymra® (sumatriptan nasal spray): IMPORTANT SAFETY INFORMATION
Tosymra can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack:
| · | discomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back |
| · | severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw |
| · | pain or discomfort in your arms, back, neck, jaw, or stomach |
| · | shortness of breath with or without chest discomfort |
| · | breaking out in a cold sweat |
| · | nausea or vomiting |
| · | feeling lightheaded |
Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem.
Do not use Tosymra if you have:
| · | history of heart problems |
| · | narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) |
| · | uncontrolled high blood pressure |
| · | severe liver problems |
| · | hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. |
| · | had a stroke, transient ischemic attacks (TIAs), or problems with blood circulation |
| · | taken any of the following medicines in the last 24 hours: almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, ergotamines, or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above. |
| · | are taking certain antidepressants, known as monoamine oxidase (MAO)-A inhibitors or it has been 2 weeks or less since you stopped taking a MAO-A inhibitor. Ask your provider for a list of these medicines if you are not sure. |
| · | an allergy to sumatriptan or any ingredient in Tosymra |
Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements.
Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.
Tosymra may cause serious side effects including:
| · | changes in color or sensation in your fingers and toes |
| · | sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever |
| · | cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet |
| · | increased blood pressure including a sudden severe increase even if you have no history of high blood pressure |
| · | medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider. |
| · | serotonin syndrome, a rare but serious problem that can happen in people using Tosymra, especially when used with anti-depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. |
| · | hives (itchy bumps); swelling of your tongue, mouth, or throat |
| · | seizures even in people who have never had seizures before |
The most common side effects of Tosymra include: tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation.
Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra. For more information, ask your provider.
This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use. You can also visit www.upsher-smith.com or call 1-888-650-3789.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
INDICATION AND USAGE
Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults.
Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches.
Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age.
Tonix Pharmaceuticals Holding Corp, Inc. 8-K
Exhibit 99.02

© 2023 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P0457 July 3, 2023 (Doc 1250 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
3 © 2023 Tonix Pharmaceuticals Holding Corp. Investment Highlights DIVERSE PIPELINE Tonix’s c ore focus is on central nervous system disorders , but we also target unmet needs across multiple therapeutic areas including immunology, infectious disease and rare disease. STRATEGIC PARTNERSHIPS Partnering strategically with other biotech companies , world - class academic and non - profit research organizations to bring innovative therapeutics to market faster. IN - HOUSE CAPABILITIES Investment in domestic , in - house, R&D and manufacturing to accelerate development timelines and improve the ability to respond to pandemics. FINANCIAL POSITION Tonix had approximately $72 M in cash and cash equivalents as of 3/31/23. Tonix has no debt .

4 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Clinical Development Programs Status/Next Milestone Indication Candidates* Mid - Phase 3 - >50% enrolled Phase 2 enrollment complete Fibromyalgia (FM) Long COVID (PASC 2 ) TNX - 102 SL 1 Mid - Phase 2, Targeted 3Q 2023 Start Cocaine Intoxication - FDA Breakthrough Designation TNX - 1300 3 Phase 2 - enrolling 5 Prevention of Chronic Migraine TNX - 1900 4 Phase 2 - enrolling 6 Depression TNX - 601 ER Phase 2 ready Prader - Willi Syndrome - FDA Orphan Drug Designation TNX - 2900 7 Phase 1, Targeted 3Q 2023 Start Organ Transplant Rejection/ Autoimmune Conditions TNX - 1500 8 *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) also has active INDs for Agitation in Alzheimer’s Disease (AAD), Alcohol Use Disorder (AUD), and Posttraumatic Stress Disorder (PTSD). All indications are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 TNX - 1300 (double - mutant cocaine esterase) is licensed from Columbia University . 4 Acquired from Trigemina ; license agreement with Stanford University; Planned investigator - initiated Binge Eating Disorder (BED) study is expected start 2Q 2023. 5 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 6 Phase 1 trial for formulation development was completed outside of the U.S.; Other potential indications include PTSD and neu roc ognitive d ysfunction from steroids 7 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) 8 anti - CD40L humanized monoclonal antibody – IND cleared 5 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO Five Late - Stage CNS Programs to be in the Clinic by 2023 1 Three studies Enrolling Now Active Studies • In Phase 3: ‒ TNX - 102 SL for fibromyalgia (>50% enrolled) Potential Pivotal Study • In Phase 2: ‒ TNX - 102 SL for fibromyalgia - type Long COVID (enrollment complete) ‒ TNX - 1900 for migraine headache (new mechanism for US patients) ‒ TNX - 601 ER for major depressive disorder (new mechanism for US patients) Potential Pivotal Study Entering Phase 2 • In 3Q 2023: ‒ TNX - 1300 for cocaine intoxication (FDA Breakthrough Therapy Designation) Potential Pivotal Study 1 Not approved for any indication © 2023 Tonix Pharmaceuticals Holding Corp.

CNS: KEY DEVELOPMENT CANDIDATES
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* Cyclobenzaprine ( Protectic ® ) Pipeline in a Product Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) is currently enrolling • >50% enrolled Next Steps: Topline results expected 1Q 2024 Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) has completed enrollment of 60 patients Next Steps: Topline results expected 3Q 2023 Patents Issued *TNX - 102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption Potent binding and antagonist activities at the serotonergic - 5 - HT2A, adrenergic - α1, histaminergic - H1, and muscarinic - M1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled with no recognized abuse potential 8 © 2023 Tonix Pharmaceuticals Holding Corp.
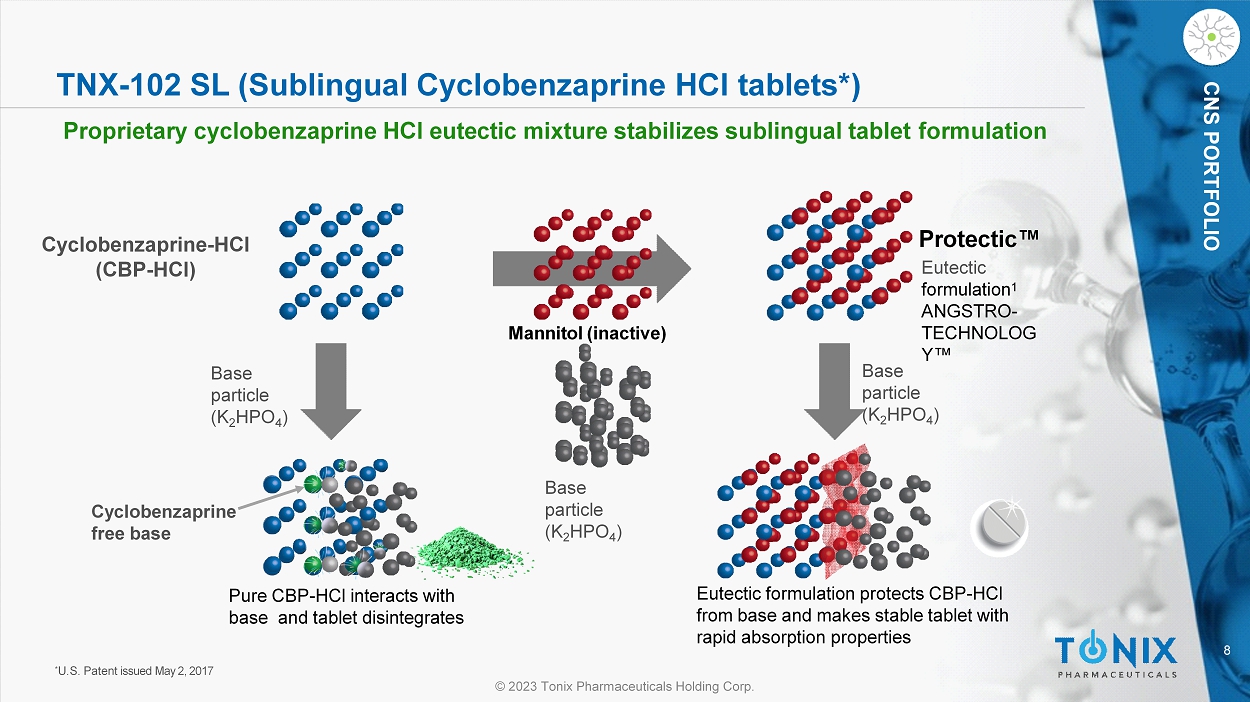
CNS PORTFOLIO TNX - 102 SL (Sublingual Cyclobenzaprine HCl tablets*) Proprietary cyclobenzaprine HCl e utectic m ixture s tabilizes s ublingual t ablet f ormulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) C y cl o be n z a p r i n e - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cy c l ob en zapr ine free base Protectic Œ Eutectic formulation 1 ANGSTRO - T E C H NO L O G Y Œ Mannitol (inactive) * U.S. Patent issued May 2, 2017 9 © 2023 Tonix Pharmaceuticals Holding Corp.
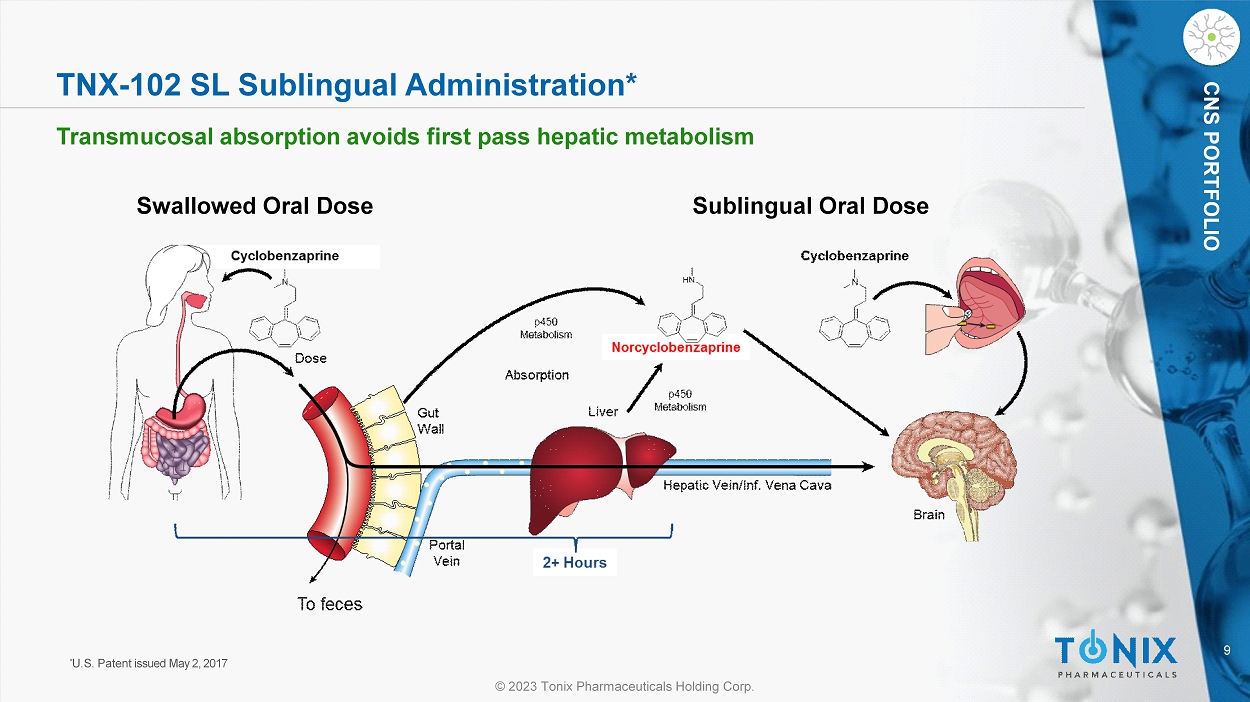
CNS PORTFOLIO TNX - 102 SL Sublingual Administration* Transmucosal absorption a voids first pass hepatic metabolism * U.S. Patent issued May 2, 2017 Swallowed Oral Dose Sublingual Oral Dose 10 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets CNS PORTFOLIO Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • A fflicts an estimated 6 - 12 million adults in the U.S., approximately 90% of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT is currently enrolling Next Steps: Topline results expected 1Q 2024 *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning 11 © 2023 Tonix Pharmaceuticals Holding Corp.
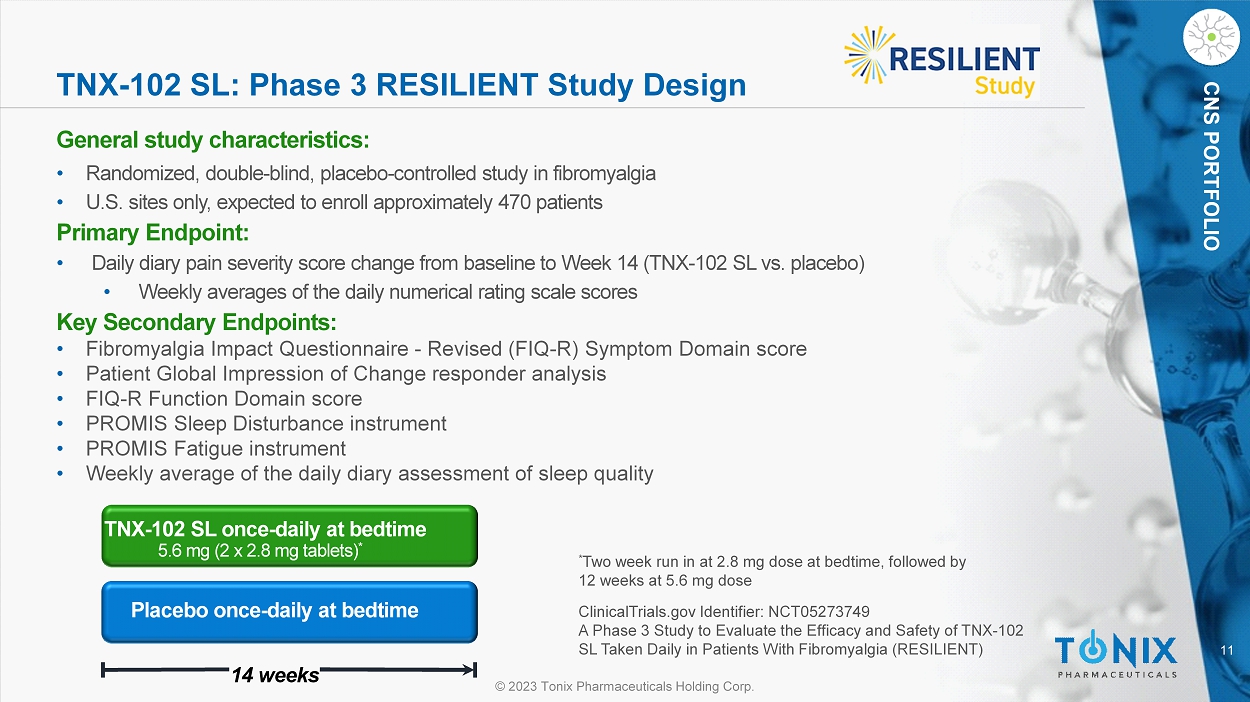
CNS PORTFOLIO TNX - 102 SL: Phase 3 R ESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia • U.S. sites only, expected to enroll approximately 470 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores Key Secondary Endpoints: • Fibromyalgia Impact Questionnaire - Revised (FIQ - R) Symptom Domain score • Patient Global Impression of Change responder analysis • FIQ - R Function Domain score • PROMIS Sleep Disturbance instrument • PROMIS Fatigue instrument • Weekly average of the daily diary assessment of sleep quality Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT)
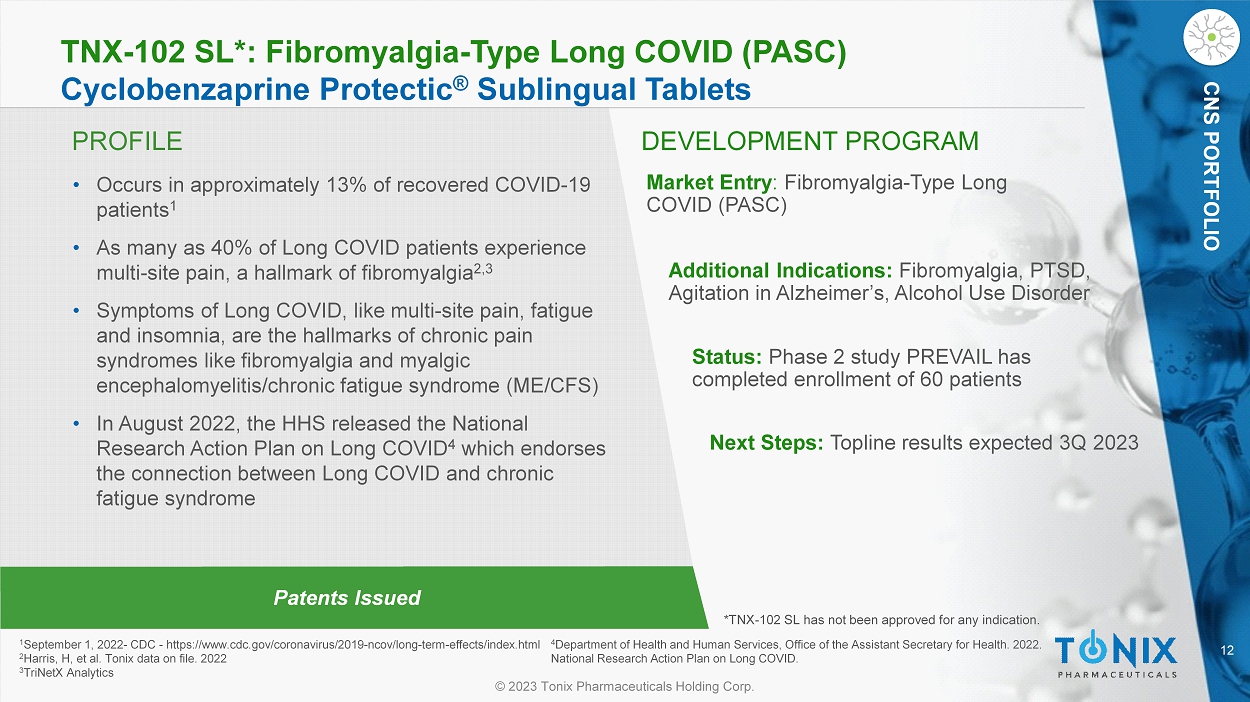
12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia - Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 13% of recovered COVID - 19 patients 1 • As many as 40% of Long COVID patients experience multi - site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and chronic fatigue syndrome Market Entry : Fibromyalgia - Type Long COVID (PASC) Status: Phase 2 study PREVAIL has completed enrollment of 60 patients Next Steps: Topline results expected 3Q 2023 1 September 1, 2022 - CDC - https://www.cdc.gov/coronavirus/2019 - ncov/long - term - effects/index.html 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO Additional Indications: Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Long COVID.
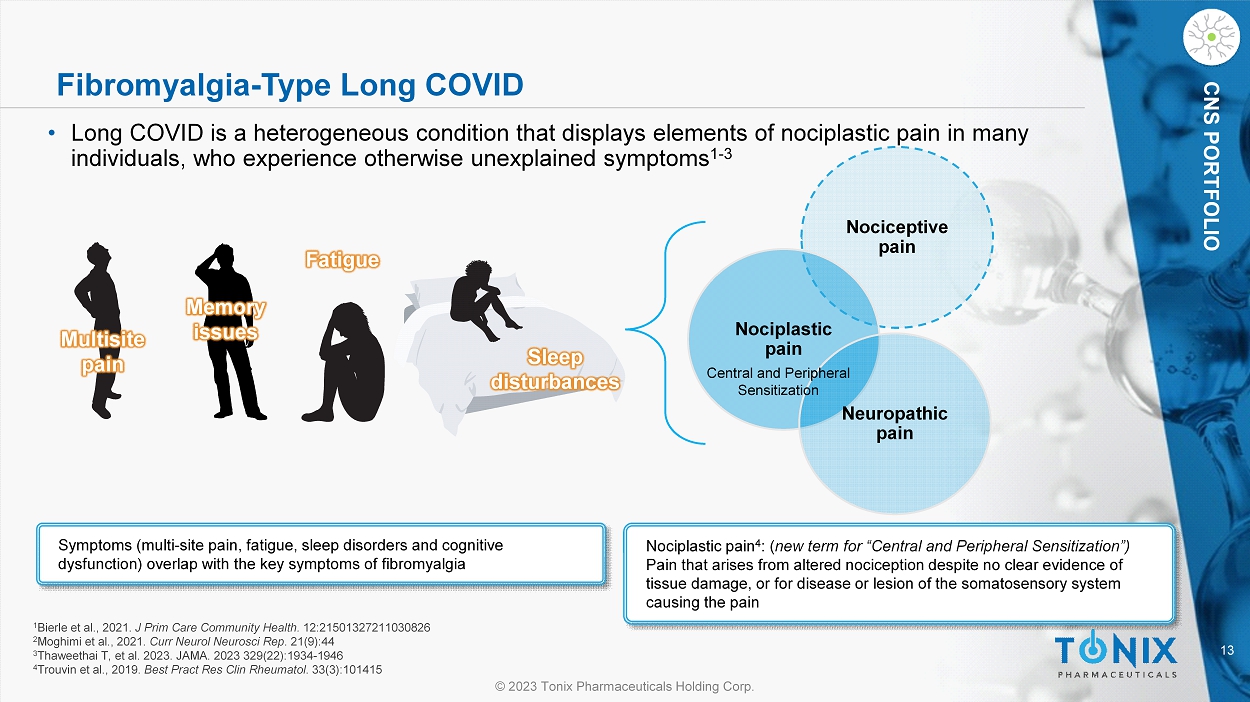
13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia - Type Long COVID • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained symptoms 1 - 3 Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia Multisite pain Memory issues Fatigue Sleep disturbances 1 Bierle et al., 2021. J Prim Care Community Health. 12:21501327211030826 2 Moghimi et al., 2021. Curr Neurol Neurosci Rep . 21(9):44 3 Thaweethai T, et al. 2023. JAMA. 2023 329(22):1934 - 1946 4 Trouvin et al., 2019. Best Pract Res Clin Rheumatol . 33(3):101415 Nociceptive pain Nociplastic pain Neuropathic pain Nociplastic pain 4 : ( new term for “Central and Peripheral Sensitization”) Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Central and Peripheral Sensitization 14 © 2023 Tonix Pharmaceuticals Holding Corp.
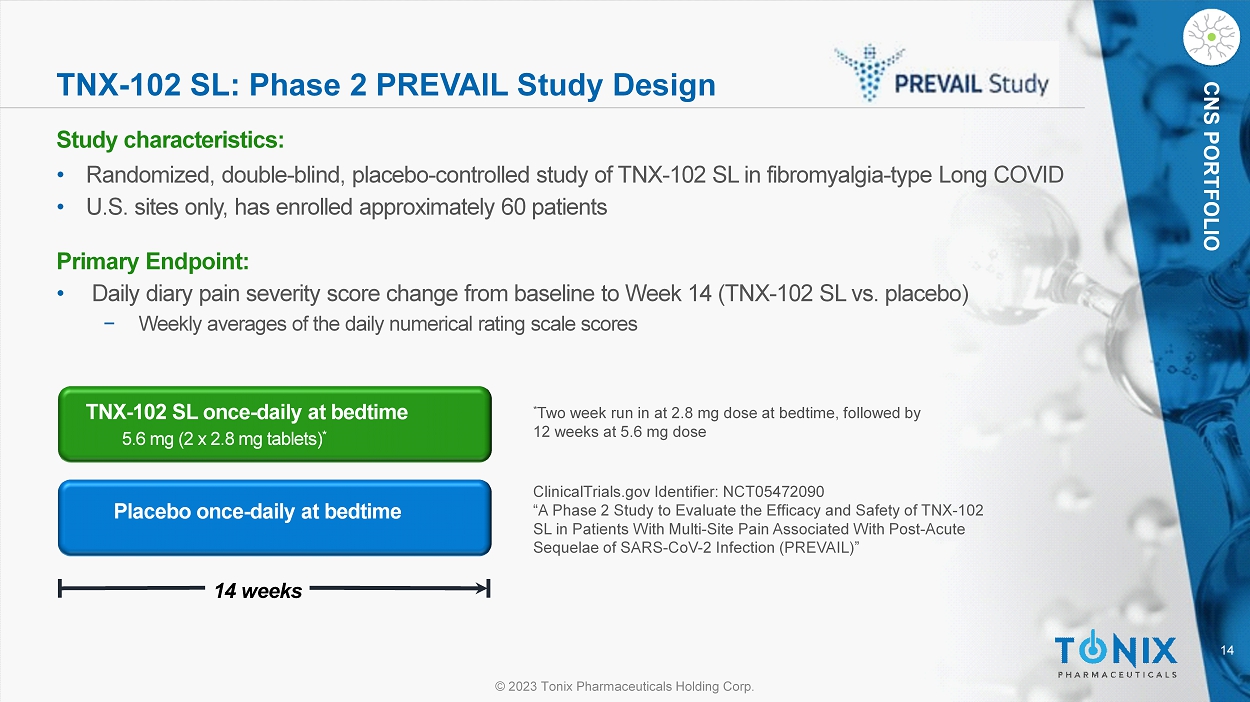
CNS PORTFOLIO TNX - 102 SL: Phase 2 PREVAIL Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, has enrolled approximately 60 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) − Weekly averages of the daily numerical rating scale scores Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL in Patients With Multi - Site Pain Associated With Post - Acute Sequelae of SARS - CoV - 2 Infection (PREVAIL)”
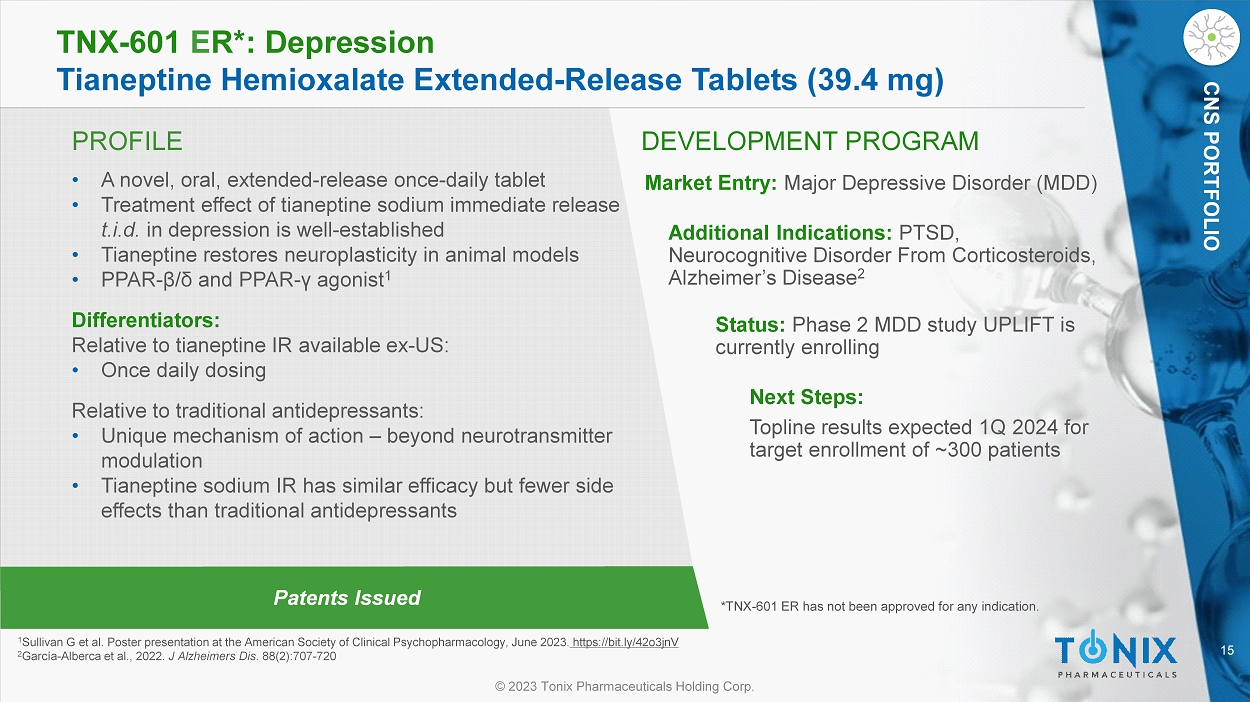
15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 601 E R*: Depression Tianeptine Hemioxalate Extended - Release Tablets (39.4 mg) CNS PORTFOLIO • A novel, oral, extended - release once - daily tablet • Treatment effect of tianeptine sodium immediate release t.i.d. in depression is well - established • Tianeptine restores neuroplasticity in animal models • PPAR - β / δ and PPAR - γ agonist 1 Differentiators: Relative to tianeptine IR available ex - US: • Once daily dosing Relative to traditional antidepressants: • Unique mechanism of action – beyond neurotransmitter modulation • Tianeptine sodium IR has similar efficacy but fewer side effects than traditional antidepressants Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 2 Status: Phase 2 MDD study UPLIFT is currently enrolling Next Steps: Topline results expected 1Q 2024 for target enrollment of ~300 patients 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 *TNX - 601 ER has not been approved for any indication.
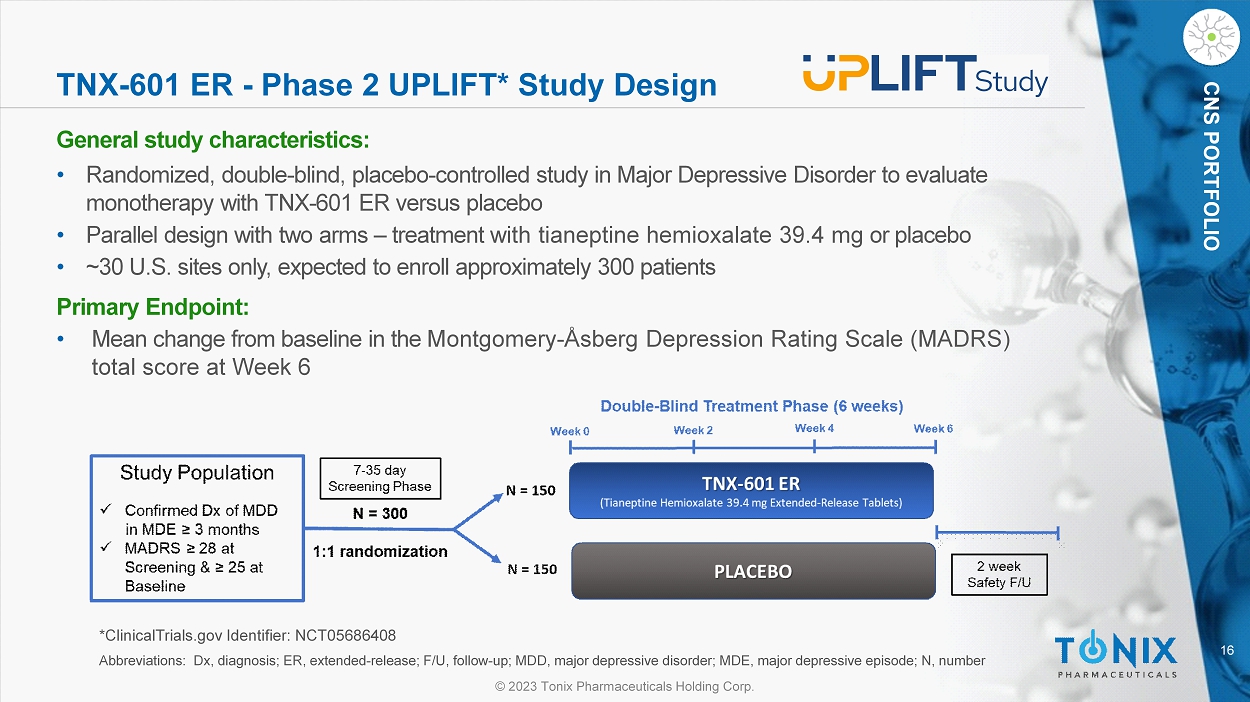
16 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER - Phase 2 UPLIFT* Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Major Depressive Disorder to evaluate monotherapy with TNX - 601 ER versus placebo • Parallel design with two arms – treatment with tianeptine hemioxalate 39.4 mg or placebo • ~30 U.S. sites only, expected to enroll approximately 300 patients Primary Endpoint: • Mean change from baseline in the Montgomery - Åsberg Depression Rating Scale (MADRS) total score at Week 6 *ClinicalTrials.gov Identifier: NCT05686408 Abbreviations: Dx, diagnosis; ER, extended - release; F/U, follow - up; MDD, major depressive disorder; MDE, major depressive episo de; N, number 17 © 2023 Tonix Pharmaceuticals Holding Corp.
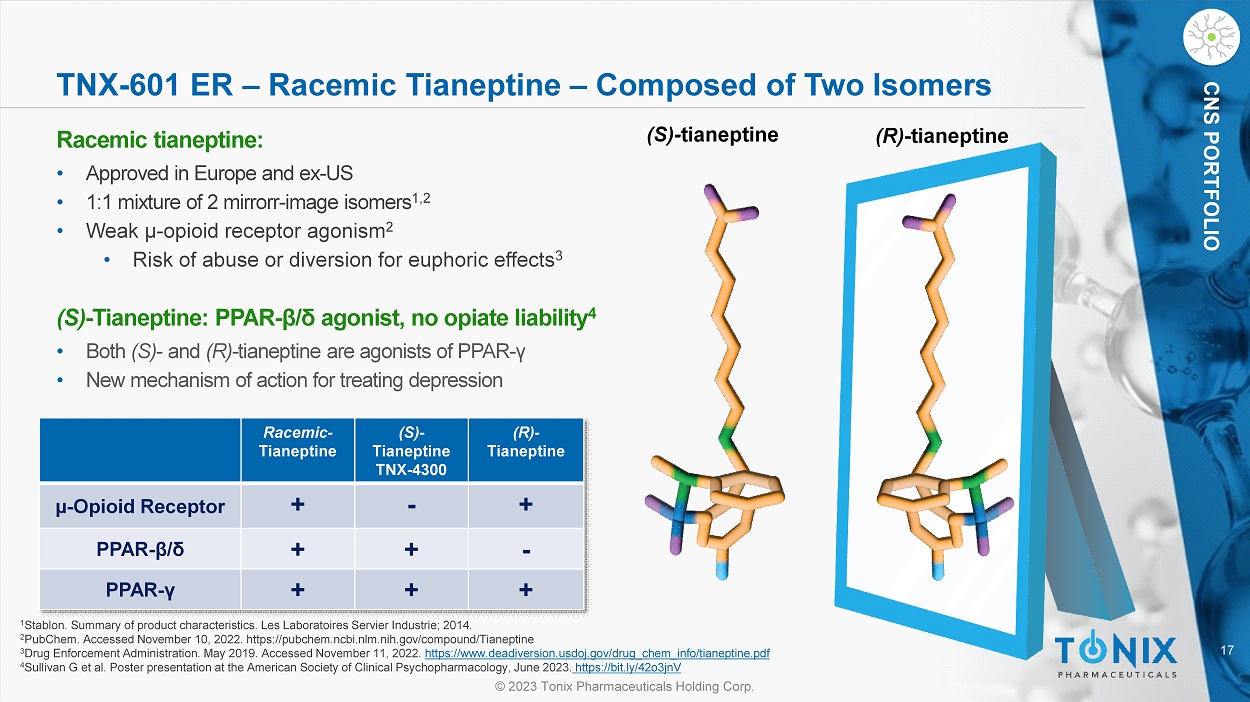
CNS PORTFOLIO TNX - 601 ER – Racemic Tianeptine – Composed of Two Isomers Racemic tianeptine : • Approved in Europe and ex - US • 1:1 mixture of 2 mirror r - image isomers 1,2 • Weak µ - o pioid receptor agonism 2 • Risk of abuse or diversion for euphoric effects 3 (S) - Tianeptine: PPAR - β / δ agonist, no opiate liability 4 • Both (S) - and (R) - tianeptine are agonists of PPAR - γ • New mechanism of action for treating depression (S) - t ianeptine (R) - tianeptine 1 Stablon. Summary of product characteristics. Les Laboratoires Servier Industrie ; 2014. 2 PubChem. Accessed November 10, 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Tianeptine 3 Drug Enforcement Administration. May 2019. Accessed November 11, 2022. https://www.deadiversion.usdoj.gov/drug_chem_info/tianeptine.pdf 4 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV (R) - Tianeptine (S) - Tianeptine TNX - 4300 Racemic - Tianeptine + - + µ - Opioid Receptor - + + PPAR - β / δ + + + PPAR - γ 18 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 4300*: Depression, Alzheimer’s & Parkinson’s diseases Estianeptine (Single (S) - isomer of Tianeptine) CNS PORTFOLIO • Single isomer, oral treatment • Proposed mechanism of action from lab studies indicates estianeptine is the active ingredient of TNX - 601 ER 1 • PPAR - β / δ and PPAR - γ agonist • Free of µ - opioid receptor activity • Estianeptine restores neuroplasticity in tissue culture Differentiators: Relative to racemic tianeptine IR or TNX - 601 ER: • Lack of opioid liability Relative to traditional antidepressants: • Unique mechanism of action – beyond neurotransmitter modulation • Racemic tianeptine sodium IR has similar efficacy but fewer side effects than traditional antidepressants Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 2 Status: Pre - clinical Next Steps: Expect IND can be supported by pre - clinical and clinical data from TNX - 601 (racemic tianeptine) development 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 *TNX - 4300 is in the pre - IND stage of development and has not been approved for any indication 19 © 2023 Tonix Pharmaceuticals Holding Corp.
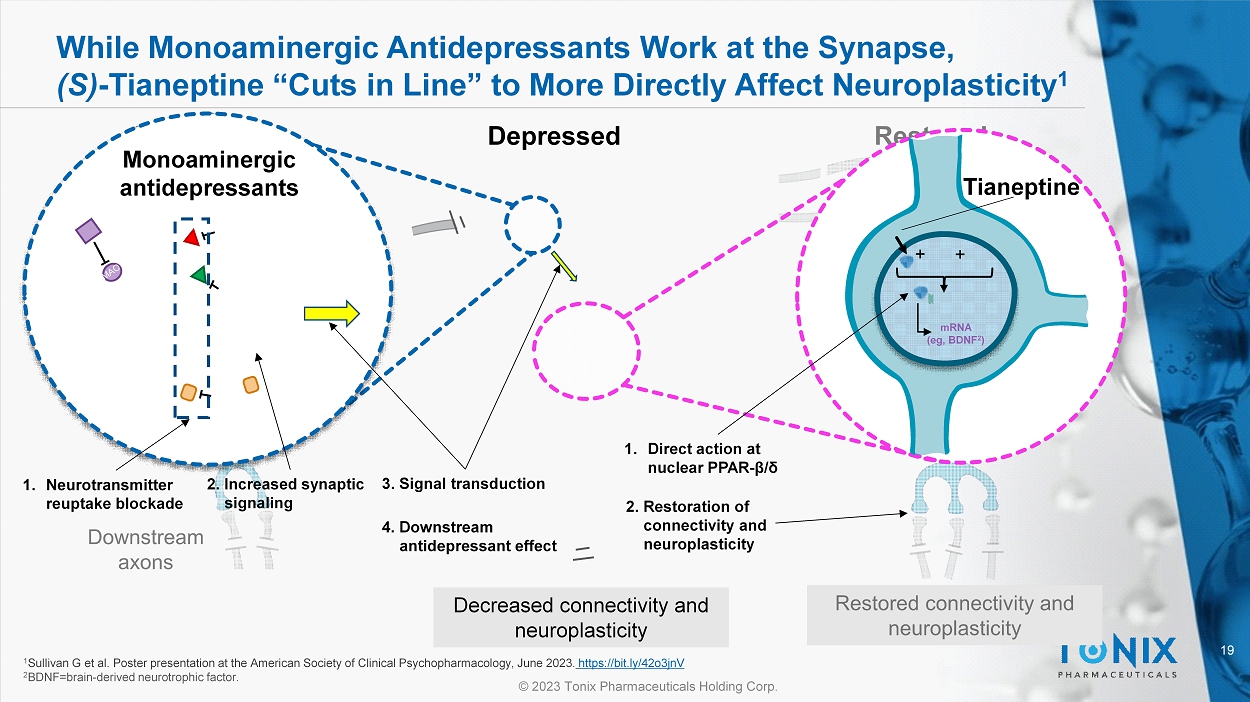
While Monoaminergic Antidepressants Work at the Synapse, (S) - Tianeptine “Cuts in Line” to More Directly Affect Neuroplasticity 1 Depressed Decreased connectivity and neuroplasticity Tianeptine + + mRNA ( eg , BDNF 2 ) 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 BDNF=brain - derived neurotrophic factor. Monoaminergic antidepressants 1. Neurotransmitter reuptake blockade 1. Direct action at nuclear PPAR - β/δ 2. Increased synaptic signaling 3. Signal transduction 4. Downstream antidepressant effect 2. Restoration of connectivity and neuroplasticity 20 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium CNS PORTFOLIO • Intranasal OT has potential utility in treating migraine 1 • Magnesium is known to potentiate the binding of OT to its receptor 2,3 • One billion individuals worldwide suffer from migraines Differentiator: Novel non - CGRP antagonist approach to treatment Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Phase 2 study PREVENTION is currently enrolling 4 Next Steps: Topline results expected 4Q 2023 Investigator initiated Phase 2 t rial in obesity - associated binge e ating disorder 2Q 2023 1 Tzabazis et al., 2017. Headache . 57 Suppl 2:64 - 75 2 Antoni et al., 1989. Biochem J . 257(2):611 - 4 3 Meyerowitz et al., 2022. Nat Struct Mol Biol . (3):274 - 281 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide. Oxytocin receptor Oxytocin 21 © 2023 Tonix Pharmaceuticals Holding Corp.
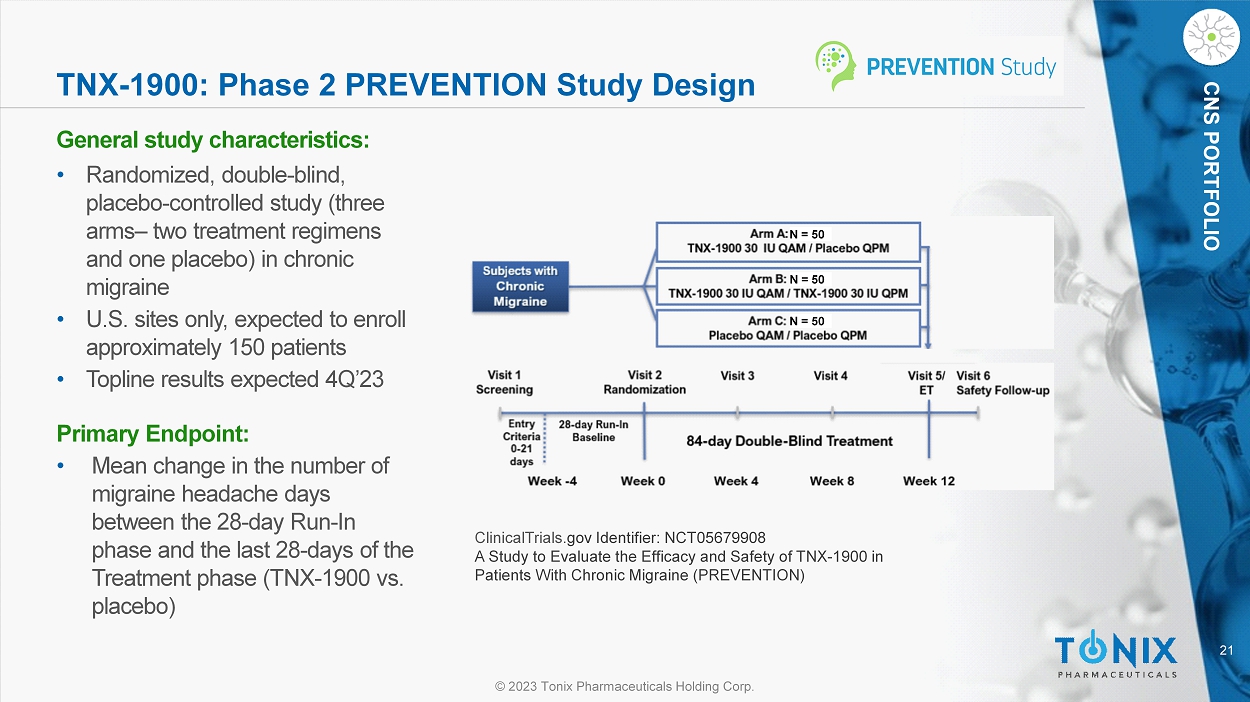
CNS PORTFOLIO TNX - 1900: Phase 2 PREVENTION Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study (three arms – two treatment regimens and one placebo) in chronic migraine • U.S. sites only, expected to enroll approximately 150 patients • Topline results expected 4Q’23 Primary Endpoint: • M ean change in the number of migraine headache days between the 28 - day Run - In phase and the last 28 - days of the Treatment phase (TNX - 1900 vs. placebo) ClinicalTrials. gov Identifier: NCT05679908 A Study to Evaluate the Efficacy and Safety of TNX - 1900 in Patients With Chronic Migraine (PREVENTION) N = 50 N = 50 N = 50 22 © 2023 Tonix Pharmaceuticals Holding Corp.
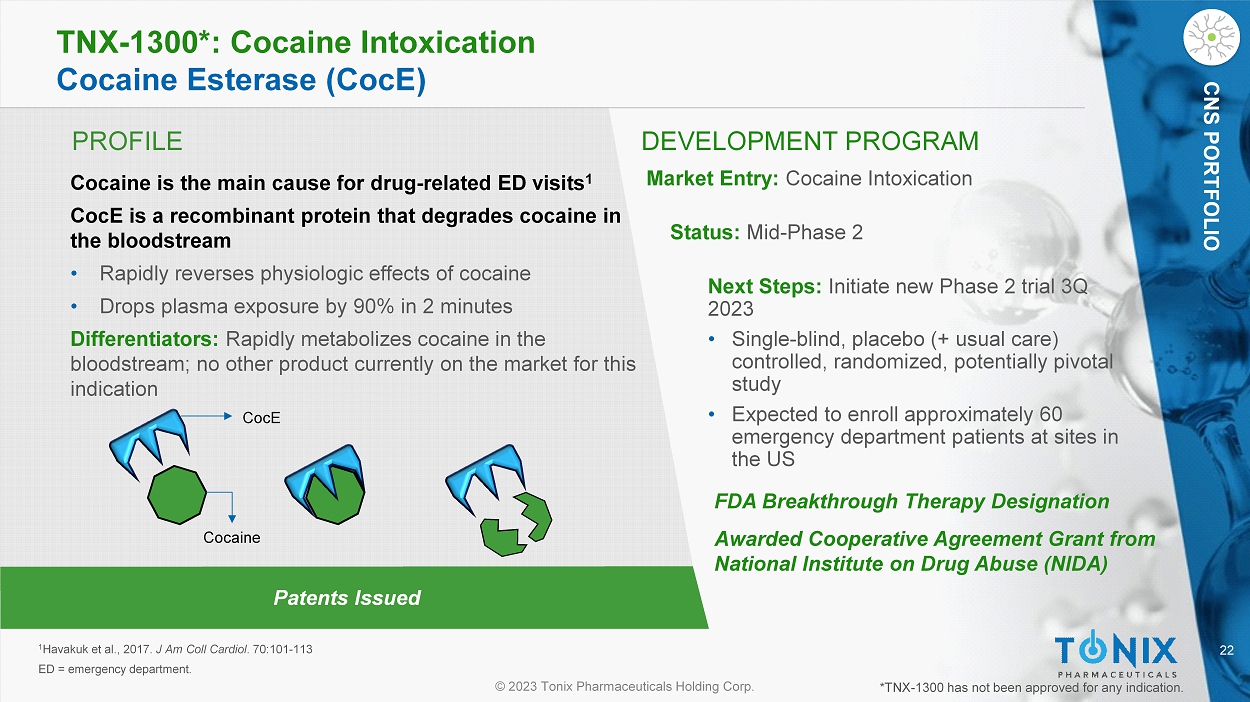
CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1300*: Cocaine Intoxication Cocaine Esterase ( CocE ) Cocaine is the main cause for drug - related ED visits 1 CocE is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes Differentiators: Rapidly metabolizes cocaine in the bloodstream; no other product currently on the market for this indication Market Entry: Cocaine Intoxication Status: Mid - Phase 2 Next Steps: Initiate new Phase 2 trial 3Q 2023 • S ingle - blind, placebo (+ usual care) controlled, randomized, potentially pivotal study • Expected to enroll approximately 60 emergency department patients at sites in the US 1 Havakuk et al., 2017. J Am Coll Cardiol . 70:101 - 113 ED = emergency department. FDA Breakthrough Therapy Designation Awarded Cooperative Agreement Grant from National Institute on Drug Abuse (NIDA) *TNX - 1300 has not been approved for any indication. CNS PORTFOLIO CocE Cocaine © 2023 Tonix Pharmaceuticals Holding Corp.

RARE DISEASE: KEY CANDIDATES
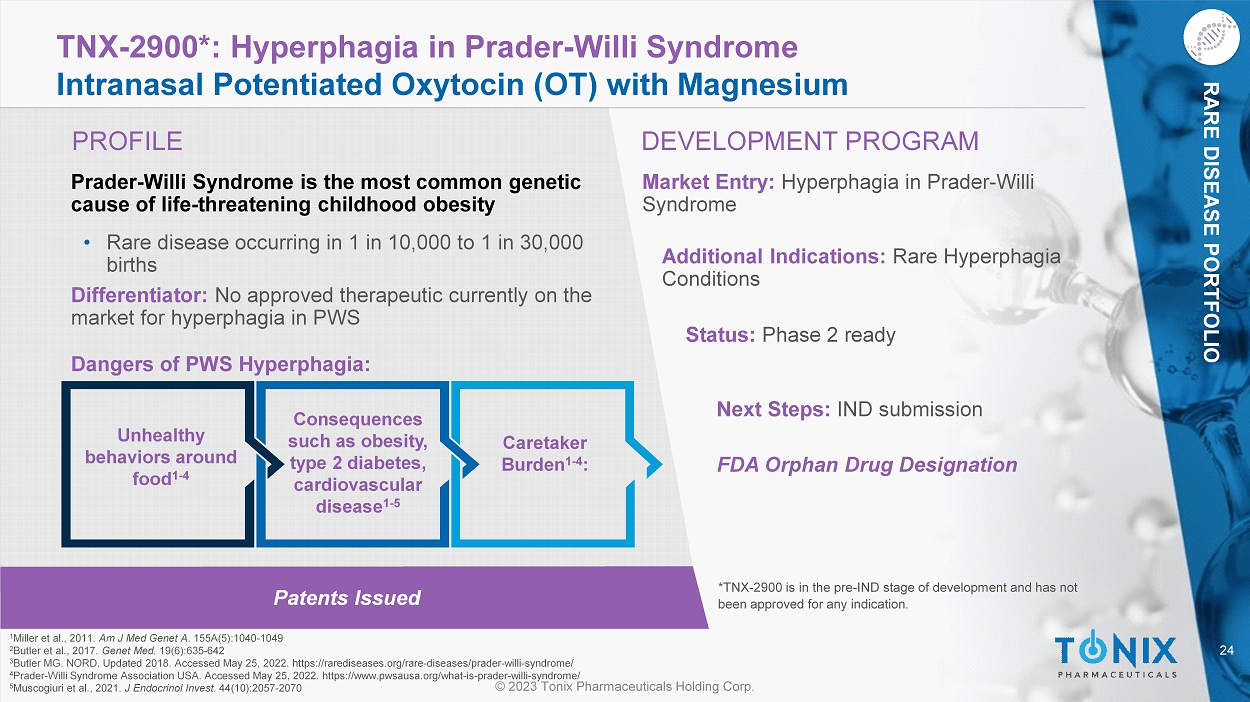
24 © 2023 Tonix Pharmaceuticals Holding Corp. RARE DISEASE PORTFOLIO Patents Issued PROFILE DEVELOPMENT PROGRAM TNX - 2900*: Hyperphagia in Prader - Willi Syndrome Intranasal Potentiated Oxytocin (OT) with Magnesium Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Rare disease occurring in 1 in 10,000 to 1 in 30,000 births Differentiator: No approved therapeutic currently on the market for hyperphagia in PWS Dangers of PWS Hyperphagia: Market Entry: Hyperphagia in Prader - Willi Syndrome Additional Indications: Rare Hyperphagia Conditions Status: Phase 2 ready Next Steps: IND submission *TNX - 2900 is in the pre - IND stage of development and has not been approved for any indication. Caretaker Burden 1 - 4 : Unhealthy behaviors around food 1 - 4 Consequences such as obesity, type 2 diabetes, cardiovascular disease 1 - 5 1 Miller et al., 2011. Am J Med Genet A . 1 55A(5):1040 - 1049 2 Butler et al., 2017. Genet Med. 19(6):635 - 642 3 Butler MG. NORD. Updated 2018. Accessed May 25, 2022. https://rarediseases.org/rare - diseases/prader - willi - syndrome/ 4 Prader - Willi Syndrome Association USA. Accessed May 25, 2022. https://www.pwsausa.org/what - is - prader - willi - syndrome/ 5 Muscogiuri et al., 2021. J Endocrinol Invest . 44(10):2057 - 2070 FDA Orphan Drug Designation © 2023 Tonix Pharmaceuticals Holding Corp.

IMMUNOLOGY: KEY CANDIDATES
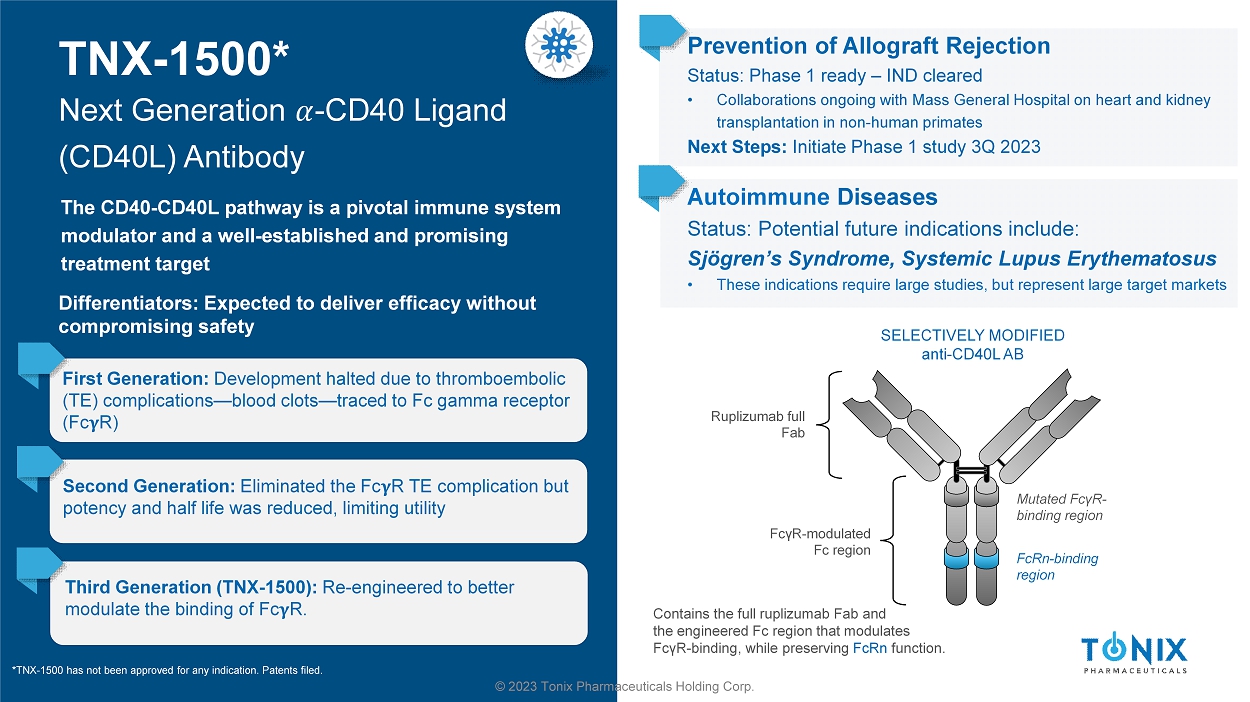
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 1500* Next Generation ߙ - CD40 Ligand (CD40L) Antibody The CD40 - CD40L pathway is a pivotal immune system modulator and a well - established and promising treatment target First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Prevention of Allograft Rejection Status: Phase 1 ready – IND cleared • Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates Next Steps: Initiate Phase 1 study 3 Q 2023 SELECTIVELY MODIFIED anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500): Re - engineered to better modulate the binding of Fc R. *TNX - 1500 has not been approved for any indication. Patents filed. Differentiators: Expected to deliver efficacy without compromising safety Autoimmune Diseases Status: Potential future indications include: Sjögren’s Syndrome, Systemic Lupus Erythematosus • These indications require large studies, but represent large target markets 27 © 2023 Tonix Pharmaceuticals Holding Corp.
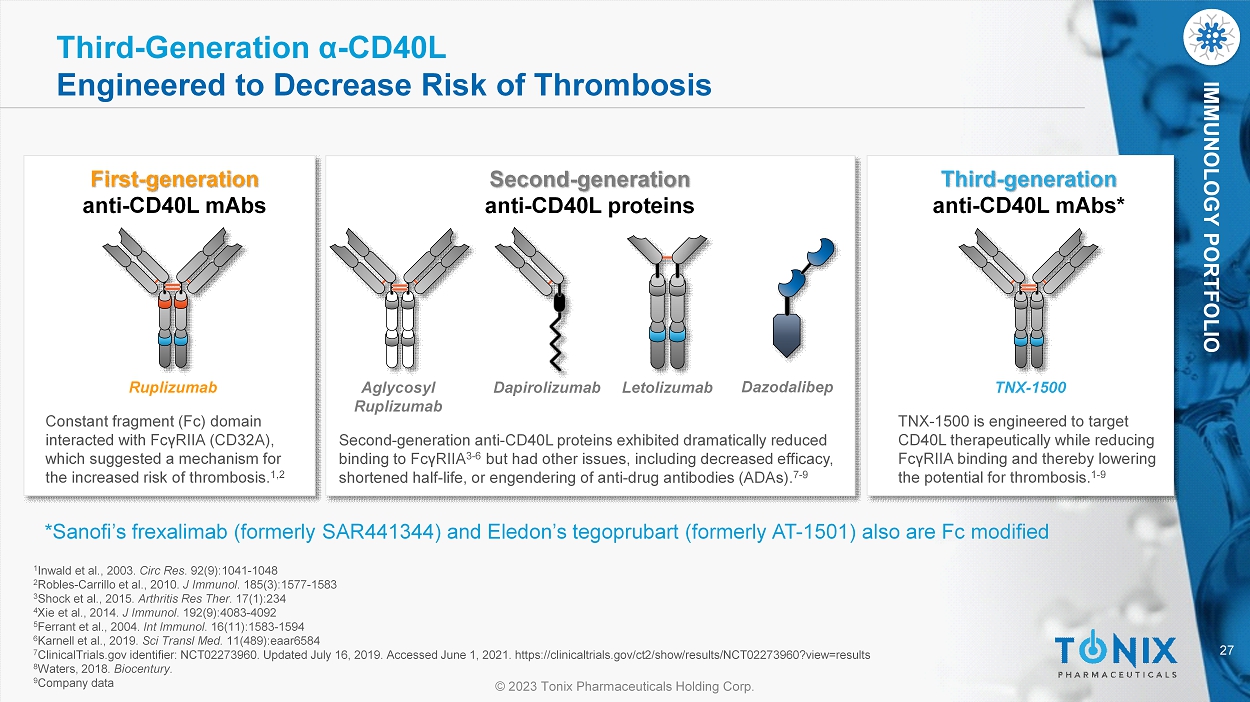
IMMUNOLOGY PORTFOLIO Third - Generation α - CD40L Engineered to Decrease Risk of Thrombosis First - generation anti - CD40L mAbs Constant fragment (Fc) domain interacted with FcγRIIA (CD32A), which suggested a mechanism for the increased risk of thrombosis. 1,2 Ruplizumab Second - generation anti - CD40L proteins Second - generation anti - CD40L proteins exhibited dramatically reduced binding to FcγRIIA 3 - 6 but had other issues, including decreased efficacy, shortened half - life, or engendering of anti - drug antibodies (ADAs). 7 - 9 Dapirolizumab Letolizumab Aglycosyl Ruplizumab Third - generation anti - CD40L mAbs * TNX - 1500 is engineered to target CD40L therapeutically while reducing FcγRIIA binding and thereby lowering the potential for thrombosis. 1 - 9 TNX - 1500 *Sanofi’s frexalimab (formerly SAR441344) and Eledon’s tegoprubart (formerly AT - 1501) also are Fc modified 1 Inwald et al., 2003. Circ Res . 92(9):1041 - 1048 2 Robles - Carrillo et al., 2010. J Immunol . 185(3):1577 - 1583 3 Shock et al., 2015. Arthritis Res Ther . 17(1):234 4 Xie et al., 2014. J Immunol . 192(9):4083 - 4092 5 Ferrant et al., 2004. Int Immunol . 16(11):1583 - 1594 6 Karnell et al., 2019. Sci Transl Med. 11(489):eaar6584 7 ClinicalTrials.gov identifier: NCT02273960. Updated July 16, 2019. Accessed June 1, 2021. https://clinicaltrials.gov/ct2/show /re sults/NCT02273960?view=results 8 Waters, 2018. Biocentury . 9 Company data Dazodalibep 28 © 2023 Tonix Pharmaceuticals Holding Corp.
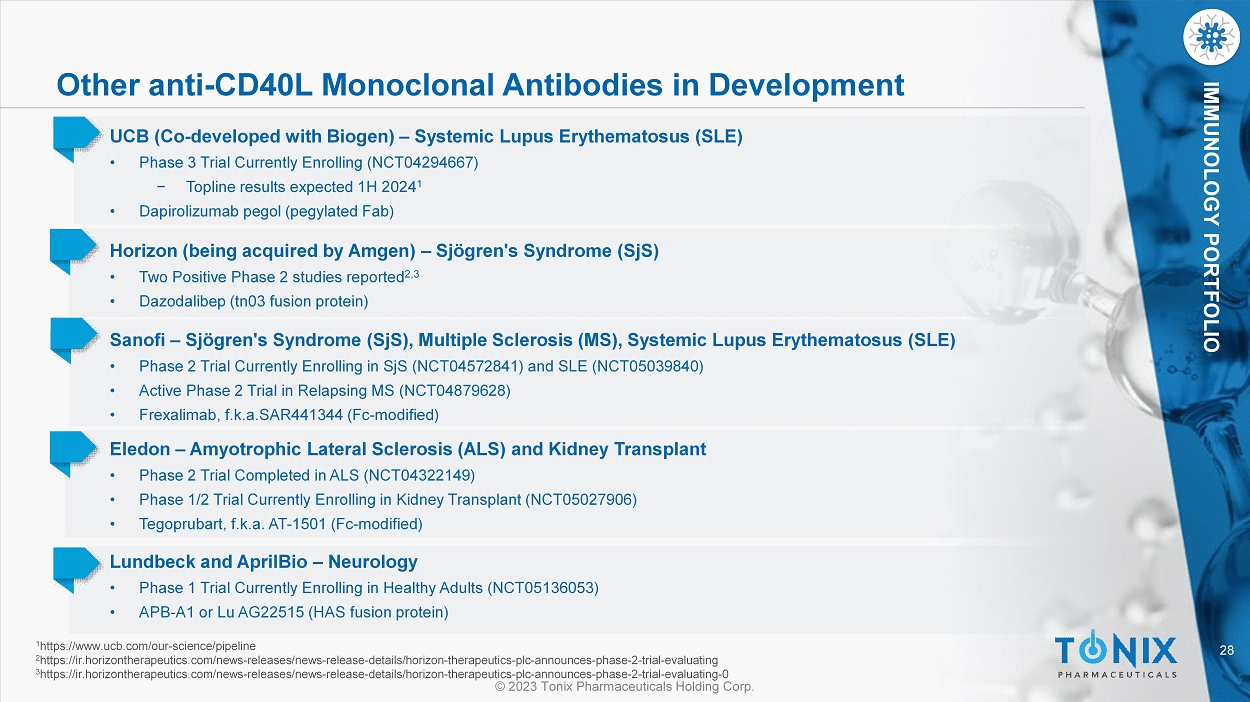
IMMUNOLOGY PORTFOLIO Other anti - CD40L Monoclonal Antibodies in Development UCB (Co - developed with Biogen) – Systemic Lupus Erythematosus (SLE) • Phase 3 Trial Currently Enrolling (NCT04294667) − Topline results expected 1H 2024 1 • Dapirolizumab pegol (pegylated Fab) Horizon (being acquired by Amgen) – Sjögren's Syndrome ( SjS ) • Two Positive Phase 2 studies reported 2,3 • Dazodalibep (tn03 fusion protein) Sanofi – Sjögren's Syndrome ( SjS ), Multiple Sclerosis (MS), Systemic Lupus Erythematosus (SLE) • Phase 2 Trial Currently Enrolling in SjS (NCT04572841) and SLE (NCT05039840) • Active Phase 2 Trial in Relapsing MS (NCT04879628) • Frexalimab , f.k.a . SAR441344 (Fc - modified) Eledon – Amyotrophic Lateral Sclerosis (ALS) and Kidney Transplant • Phase 2 Trial Completed in ALS (NCT04322149) • Phase 1/2 Trial Currently Enrolling in Kidney Transplant (NCT05027906) • T egoprubart , f.k.a . AT - 1501 (Fc - modified) 1 https://www.ucb.com/our - science/pipeline 2 https://ir.horizontherapeutics.com/news - releases/news - release - details/horizon - therapeutics - plc - announces - phase - 2 - trial - evaluatin g 3 https://ir.horizontherapeutics.com/news - releases/news - release - details/horizon - therapeutics - plc - announces - phase - 2 - trial - evaluatin g - 0 Lundbeck and AprilBio – Neurology • Phase 1 Trial Currently Enrolling in Healthy Adults (NCT05136053) • APB - A1 or Lu AG22515 (HAS fusion protein)

© 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: KEY CANDIDATES © 2023 Tonix Pharmaceuticals Holding Corp.
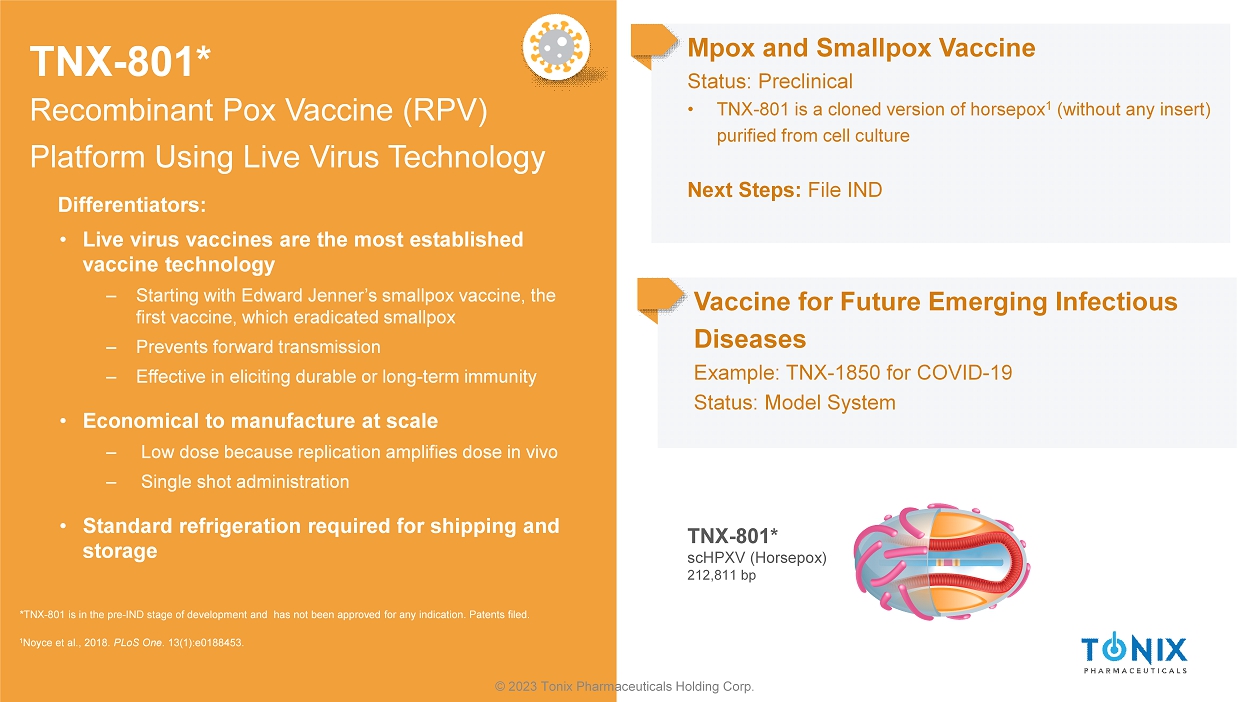
TNX - 801* Recombinant Pox Vaccine (RPV) Platform Using Live Virus Technology Mpox and Smallpox Vaccine Status: Preclinical • TNX - 801 is a cloned version of horsepox 1 (without any insert) purified from cell culture Next Steps: File IND Vaccine for Future Emerging Infectious Diseases Example: TNX - 1850 for COVID - 19 Status: Model System *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication. Patents filed. • Live virus vaccines are the most established vaccine technology ‒ Starting with Edward Jenner’s smallpox vaccine, the first vaccine, which eradicated smallpox ‒ Prevents forward transmission ‒ Effective in eliciting durable or long - term immunity • Economical to manufacture at scale ‒ Low dose because replication amplifies dose in vivo ‒ Single shot administration • Standard refrigeration required for shipping and storage 1 Noyce et al., 2018. PLoS One . 13(1):e0188453. Differentiators: TNX - 801* scHPXV (Horsepox) 212,811 bp 31 © 2023 Tonix Pharmaceuticals Holding Corp.
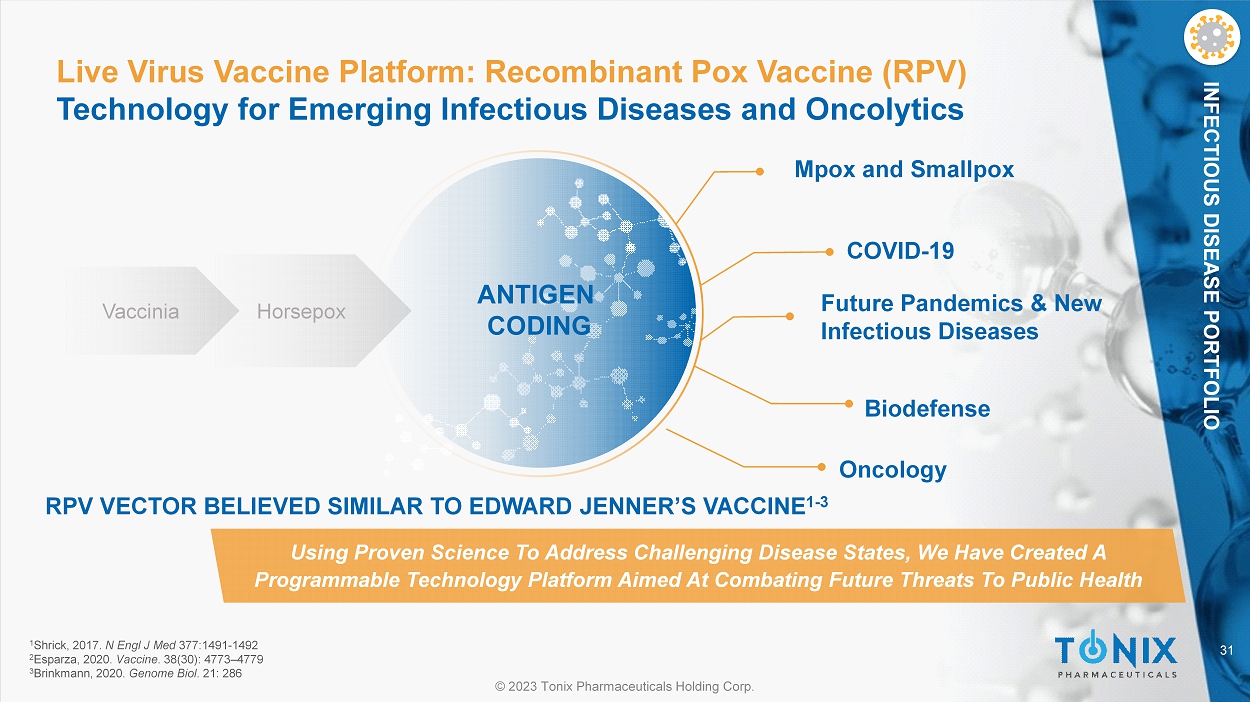
INFECTIOUS DISEASE PORTFOLIO Live Virus Vaccine Platform: Recombinant Pox Vaccine (RPV) Technology for Emerging Infectious Diseases and Oncolytics Mpox and Smallpox Future Pandemics & New Infectious Diseases COVID - 19 Biodefense Using Proven Science To Address Challenging Disease States, We Have Created A Programmable Technology Platform Aimed At Combating Future Threats To Public Health Vaccinia Horsepox ANTIGEN CODING Oncology RPV VECTOR BELIEVED SIMILAR TO EDWARD JENNER’S VACCINE 1 - 3 1 Shrick, 2017. N Engl J Med 377:1491 - 1492 2 Esparza, 2020. Vaccine . 38(30): 4773 – 4779 3 Brinkmann, 2020. Genome Biol . 21: 286 32 © 2023 Tonix Pharmaceuticals Holding Corp.

INFECTIOUS DISEASE PORTFOLIO Internal Development & Manufacturing Capabilities R&D Center (RDC) – Frederick, MD • Functions: ‒ Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases ‒ Research advancing CNS and immunology drugs • Description: ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 • Status: Operational Advanced Development Center (ADC) – North Dartmouth, MA • Function: Development and clinical scale manufacturing of biologics • Description: ~45,000 square feet, BSL - 2 • Status: Operational © 2023 Tonix Pharmaceuticals Holding Corp.

TONIX MEDICINES: MARKETED PRODUCTS

34 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonix Medicines is our Commercial Subsidiary Marketed products Zembrace ® SymTouch ® (sumatriptan injection) 3 mg 1 Tosymra ® (sumatriptan nasal spray) 10 mg 2 Headed by President Jim Hunter ‒ Industry veteran – experience in CNS products ‒ Built Validus Pharmaceuticals Combined 2020 US retail sales ~$23 M ( Zembrace ~$19.6 M and Tosymra ~$3.5 M) 3 ‒ Projecting net sales approximately ~50% of gross sales Upsher Smith Laboratories has managed care contracts covering ~200 M lives ‒ Deal includes a transition period during which Tonix expects to secure its own contracts 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 - For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021 . For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 3 IQVIA, 2022 sales from the National Sales Perspectives (NSP) audit within the SMART database estimates Zembrace sales of ~$19.6 M and Tosymra sales of ~$3.5 M 35 © 2023 Tonix Pharmaceuticals Holding Corp.
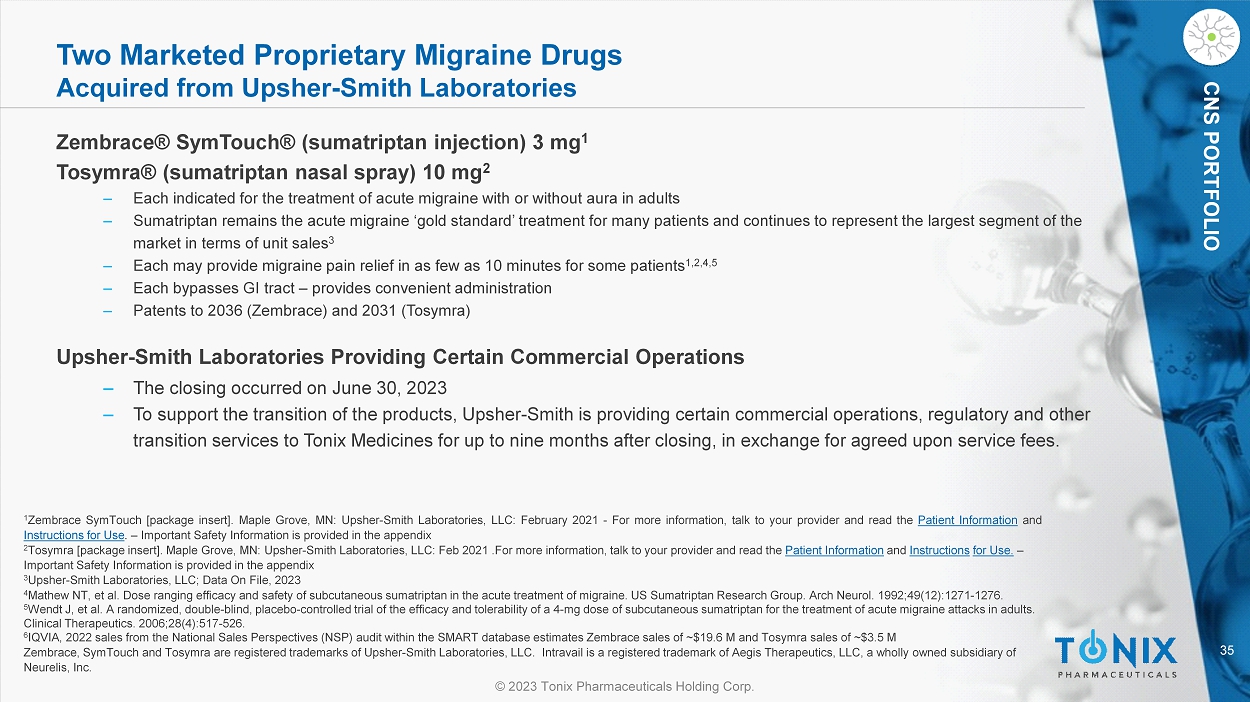
CNS PORTFOLIO Two Marketed Proprietary Migraine Drugs Acquired from Upsher - Smith Laboratories Zembrace ® SymTouch ® (sumatriptan injection) 3 mg 1 Tosymra ® (sumatriptan nasal spray) 10 mg 2 ‒ Each indicated for the treatment of acute migraine with or without aura in adults ‒ Sumatriptan remains the acute migraine ‘gold standard’ treatment for many patients and continues to represent the largest seg men t of the market in terms of unit sales 3 ‒ Each may provide migraine pain relief in as few as 10 minutes for some patients 1,2,4,5 ‒ Each bypasses GI tract – provides c onvenient administration ‒ Patents to 2036 ( Zembrace ) and 2031 ( Tosymra ) Upsher - Smith Laboratories Providing Certain Commercial Operations ‒ The closing occurred on June 30, 2023 ‒ To support the transition of the products, Upsher - Smith is providing certain commercial operations, regulatory and other transition services to Tonix Medicines for up to nine months after closing, in exchange for agreed upon service fees. 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 - For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021 . For more information, talk to your provider and read the Patient Information and Instructions for Use. – Important Safety Information is provided in the appendix 3 Upsher - Smith Laboratories, LLC; Data On File, 2023 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 5 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. 6 IQVIA, 2022 sales from the National Sales Perspectives (NSP) audit within the SMART database estimates Zembrace sales of ~$19.6 M and Tosymra sales of ~$3.5 M Zembrace , SymTouch and Tosymra are registered trademarks of Upsher - Smith Laboratories, LLC. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.
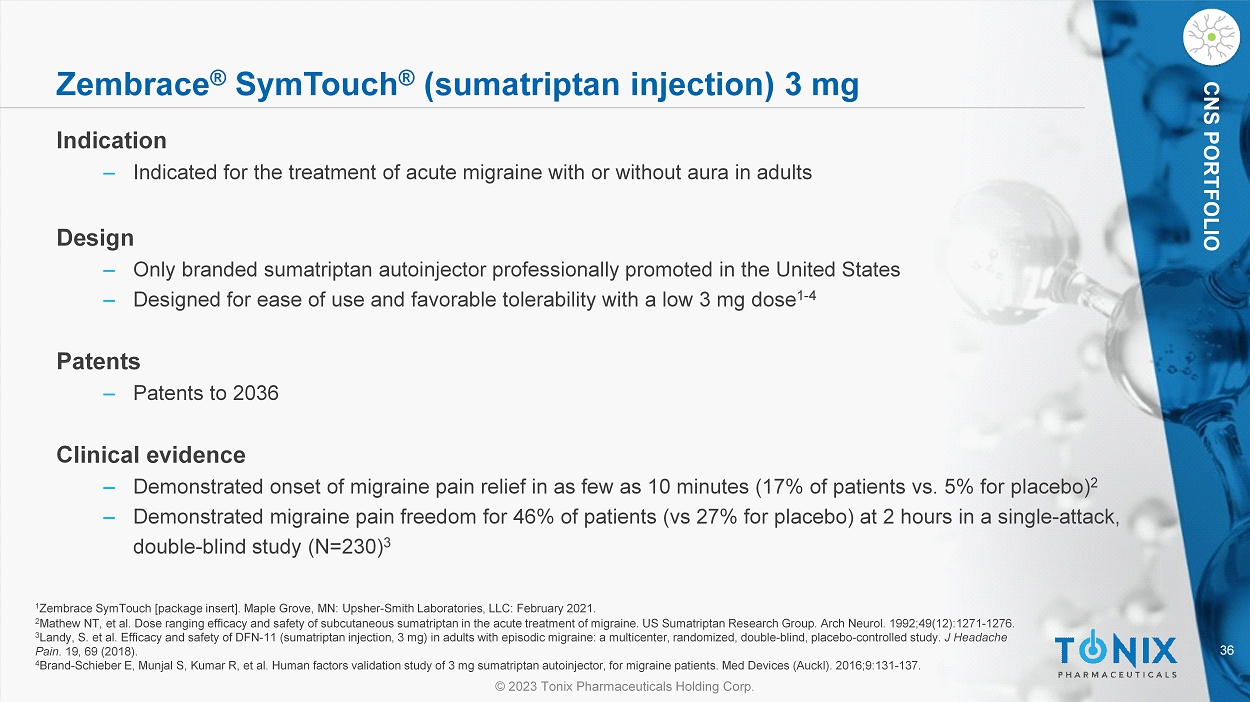
36 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® SymTouch ® (sumatriptan injection) 3 mg Indication ‒ Indicated for the treatment of acute migraine with or without aura in adults Design ‒ O nly branded sumatriptan autoinjector professionally promoted in the United States ‒ Designed for ease of use and favorable tolerability with a low 3 mg dose 1 - 4 Patents ‒ Patents to 2036 Clinical evidence ‒ D emonstrated onset of migraine pain relief in as few as 10 minutes (17% of patients vs. 5% for placebo) 2 ‒ Demonstrated migraine pain freedom for 46% of patients (vs 27% for placebo) at 2 hours in a single - attack, double - blind study (N=230) 3 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 . 2 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 3 Landy, S. et al. Efficacy and safety of DFN - 11 (sumatriptan injection, 3 mg) in adults with episodic migraine: a multicenter, ra ndomized, double - blind, placebo - controlled study. J Headache Pain. 19, 69 (2018). 4 Brand - Schieber E, Munjal S, Kumar R, et al. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patien ts. Med Devices ( Auckl ). 2016;9:131 - 137.

37 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra ® (sumatriptan nasal spray) 10 mg Indication ‒ Indicated for the treatment of acute migraine with or without aura in adults Design ‒ Novel intranasal sumatriptan product formulated with a permeation enhancer ( Intravail ® technology ) that provides rapid and efficient absorption of sumatriptan 1,2 ‒ Pharmacokinetically equivalent to 4 mg subcutaneous ( s.c. ) sumatriptan 1 Patents ‒ Patents to 2031 Clinical evidence ‒ Tosymra ® delivers migraine pain relief in as little as 10 minutes with just one spray for some patients (13% vs. 5% for placebo) 1 - 3 1 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: 2019. 2 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 3 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. Intravail is a trademark of Aegis, a subsidiary of Neurelis 38 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO Potential for Zembrace and Tosymra in Evolving Migraine Market Documented efficacy of Zembrace 1,2 and Tosymra 3 - 5 as abortive treatments for acute migraine ‒ M igraine pain relief possible in as few as 10 minutes for some patients and convenient administration ‒ Potential to address the unmet needs of patients using traditional or emerging oral acute migraine medications, particularly for rapid - onset treatment Zembrace and Tosymra have potential as first - line or rescue medications ‒ Depending on patient need, prescribers may utilize to treat acute migraine either first - line or in the rescue position in a comprehensive migraine toolbox ‒ Fast onset of action, high pain - relief rates ‒ For certain patients who present to ERs, Zembrace and Tosymra also provide ER staff a straightforward treatment option 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : 2019 . 2 Landy, S. et al. Efficacy and safety of DFN - 11 (sumatriptan injection, 3 mg) in adults with episodic migraine: a multicenter, ra ndomized, double - blind, placebo - controlled study. J Headache Pain. 19, 69 (2018). 3 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021. 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 5 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.

39 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Strategic Fit We expect commercial business of Zembrace and Tosymra will be under our control in 4Q: and projected fibromyalgia topline for TNX - 102 SL is expected 1Q24 ‒ With success in F307 trial, commercial business is expected to speed TNX - 102 SL launch ‒ Commercial business has potential to expand ▪ Potential for “Growth Equity” investors to fund subsequent product acquisitions ▪ Debt can be part of financing strategy for subsequent acquisitions Acquiring subsequent commercial products is easier than buying the first products ‒ Licenses, accounting, managed care relationships facilitate acquisitions Commercial sales is an established business strategy ‒ Historically recession - proof ‒ Opportunities for new products as big pharma focuses on cell - and gene - therapies ‒ Room for innovation in evolving reimbursement market ▪ Constant evolution in Managed care, Medicare/Medicaid, specialty pharmacies, etc.
40 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Value to Tonix of Marketed Proprietary Migraine Drugs Prepare for the launch of TNX - 102 SL for fibromyalgia ‒ Commercial capabilities prior to expected launch of TNX - 102 SL may speed market uptake ‒ Potential to facilitate launch of TNX - 1900 for prevention of chronic migraine once approved ▪ Overlap of prescribers and patients between acute migraine and chronic migraine indications Grow commercial CNS sales capability ‒ Improve sales and margins of these migraine products ▪ Targeting sampling to potential users ▪ Decreasing certain costs ‒ Explore specialty pharmacy channel Build a specialty pharma business ‒ Further product acquisitions ‒ Several companies have bought or built commercial capabilities prior to the launch of their internally - developed products 41 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO Administration of Zembrace and Tosymra Bypass the GI Tract Bypassing the gastrointestinal (GI) tract is a potential advantage for treating acute migraine ‒ P otential to provide a treatment option for migraines complicated by severe nausea and vomiting Need for acute non - oral treatments ‒ GI absorption may be inconsistent in migraineurs due to gastric stasis (also called “gastroparesis”) 1 - 4 ‒ Nausea and vomiting are symptoms of migraine 5 1 Arca KN, et al. 2022. Curr Neurol Neurosci Rep . 22(12):813 - 821 2 Tfelt - Hansen PC. 2017. Cephalalgia . 37(9):892 - 901 3 Parkman HP. 2013. Headache . 53 Suppl 1:4 - 10. 4 Aurora SK, et al. 2013. Cephalalgia . 33(6):408 - 15 5 Pierce M. 2013. Headache . 53 Suppl 1:17 - 20.

© 2023 Tonix Pharmaceuticals Holding Corp. PRECLINICAL PIPELINE 43 © 2023 Tonix Pharmaceuticals Holding Corp.
Pipeline: Key Pre - Clinical Programs Preclinical COVID - 19 (horsepox - based live virus vaccine platform) TNX - 1850 3 Preclinical Smallpox and mpox vaccine TNX - 801 4 Preclinical Filoviruses (broad spectrum antiviral) TNX - 3900 Preclinical Filoviruses (broad spectrum antiviral) TNX - 4000 Preclinical Depression ( estianeptine ) TNX - 4300 Preclinical Attention Deficit Hyperactivity Disorder (ADHD) TNX - 1610 1 Preclinical Gastric and colorectal cancers TNX - 1700 2 1 Acquired from TRImaran Pharma; license agreement with Wayne State University 2 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University 3 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. TNX - 1850 is based on the BA.2 varian t spike protein. 4 Live attenuated vaccine based on horsepox virus Status/Next Milestone Indication Candidates* 44 © 2023 Tonix Pharmaceuticals Holding Corp.
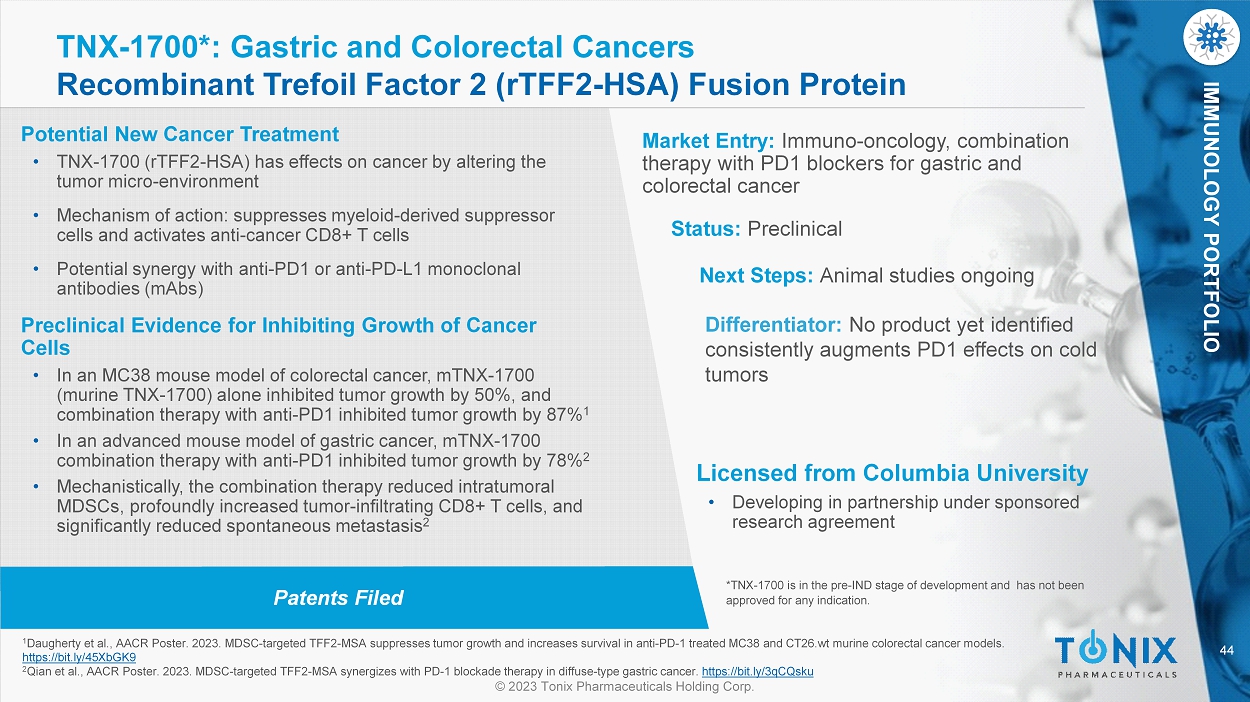
IMMUNOLOGY PORTFOLIO Patents Filed TNX - 1700*: Gastric and Colorectal Cancers Recombinant Trefoil Factor 2 (rTFF2 - HSA) Fusion Protein Potential New Cancer Treatment • TNX - 1700 (rTFF2 - HSA) has effects on cancer by altering the tumor micro - environment • Mechanism of action: suppresses myeloid - derived suppressor cells and activates anti - cancer CD8+ T cells • Potential synergy with anti - PD1 or anti - PD - L1 monoclonal antibodies ( mAbs ) Preclinical Evidence for Inhibiting Growth of Cancer Cells • In an MC38 mouse model of colorectal cancer, mTNX - 1700 (murine TNX - 1700) alone inhibited tumor growth by 50%, and combination therapy with anti - PD1 inhibited tumor growth by 87% 1 • In an advanced mouse model of gastric cancer, mTNX - 1700 combination therapy with anti - PD1 inhibited tumor growth by 78% 2 • Mechanistically, the combination therapy reduced intratumoral MDSCs, profoundly increased tumor - infiltrating CD8+ T cells, and significantly reduced spontaneous metastasis 2 Market Entry: Immuno - oncology, combination therapy with PD1 blockers for gastric and colorectal cancer Status: Preclinical Next Steps: Animal studies ongoing *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication. Differentiator: No product yet identified consistently augments PD1 effects on cold tumors Licensed from Columbia University • Developing in partnership under sponsored research agreement 1 Daugherty et al., AACR Poster. 2023. MDSC - targeted TFF2 - MSA suppresses tumor growth and increases survival in anti - PD - 1 treated MC38 and CT26.wt murine colorectal cancer models. https://bit.ly/45XbGK9 2 Qian et al., AACR Poster. 2023. MDSC - targeted TFF2 - MSA synergizes with PD - 1 blockade therapy in diffuse - type gastric cancer. https://bit.ly/3qCQsku © 2023 Tonix Pharmaceuticals Holding Corp.

TONIX TEAM, NETWORK AND FUTURE OUTLOOK 46 © 2023 Tonix Pharmaceuticals Holding Corp.

TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND COLORECTAL CANCERS Key Development Partners TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 801: SMALLPOX AND MONKEYPOX VACCINE TNX - 1850: COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME TNX - 3700 : COVID - 19 VACCINE (ZINC NANOPARTICLE mRNA TECHNOLOGY ) TNX - 2300 : BOVINE PARAINFLUEZNA VIRUS 47 © 2023 Tonix Pharmaceuticals Holding Corp.

Milestones: Recently Completed and Upcoming Expected Clinical Trial Initiations □ 3 rd Quarter 2023 Phase 1 study start of TNX - 1500 for prevention of allograft rejection □ 3 rd Quarter 2023 Phase 2 study start of TNX - 1300 for the treatment of cocaine intoxication □ 2 nd Quarter 2022 Phase 3 RESILIENT study start of TNX - 102 SL for the management of fibromyalgia □ 3rd Quarter 2022 Phase 2 PREVAIL study start of TNX - 102 SL for the treatment of fibromyalgia - type Long COVID □ 1 st Quarter 2023 Phase 2 PREVENTION study start of TNX - 1900 for the treatment of migraine □ 1st Quarter 2023 Phase 2 UPLIFT study start of TNX - 601 ER for major depressive disorder □ 2 nd Quarter 2023 Acquisition of marketed migraine products x x Expected Data □ 3 rd Quarter 2023 Topline results of Phase 2 PREVAIL study of TNX - 102 SL for fibromyalgia - type Long COVID □ 4 th Quarter 2023 Topline results of Phase 2 PREVENTION study of TNX - 1900 for chronic migraine □ 1 st Quarter 2024 Topline results of Phase 3 RESILIENT study of TNX - 102 SL for fibromyalgia □ 1 st Quarter 2024 Topline results of Phase 2 UPLIFT study of TNX - 601 ER for major depressive disorder* x x *For target enrollment of ~300 patients x © 2023 Tonix Pharmaceuticals Holding Corp.

THANK YOU

49 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® IMPORTANT SAFETY INFORMATION (1 of 2) Zembrace SymTouch ( Zembrace ) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack: D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw; pain or discomfort in your arms, back, neck, jaw or stomach ; shortness of breath with or without chest discomfort ; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem. Do not use Zembrace if you have: H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; severe liver problems ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , dihydroergotamine ; are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor. Ask your provider for a list of these medicines if you are not sure. A n allergy to sumatriptan or any of the components of Zembrace Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

50 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® IMPORTANT SAFETY INFORMATION (2 of 2) Zembrace may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, na usea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips; feeling of heaviness or t igh tness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold fe eli ng or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches g et worse, call your provider. • Serotonin syndrome, a rare but serious problem that can happen in people using Zembrace , especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not th ere (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or tro ubl e walking. • Hives (itchy bumps); swelling of your tongue, mouth, or throat • Seizures even in people who have never had seizures before The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or ti red. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effe cts of Zembrace . For more information, ask your provider. This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e5b104f - 2b9e - 416e - 92fb - ef1bdaea867d You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1 - 800 - FDA - 1088. Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine. Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.

51 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra® IMPORTANT SAFETY INFORMATION (1 of 2) Tosymra® can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack: • D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back ; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw ; pain or discomfort in your arms, back, neck, jaw, or stomach ; shortness of breath with or without chest discomfort; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem. Do not use Tosymra if you have: • H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; severe liver problems ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. • H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above • are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor . A sk your provider for a list of these medicines if you are not sure • A n allergy to sumatriptan or any ingredient in Tosymra Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

52 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider . • Serotonin syndrome, a rare but serious problem that can happen in people using Tosymra, especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have : mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. • Seizures even in people who have never had seizures before The most common side effects of Tosymra include : tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra. For more information, ask your provider. This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=015a5cf9 - f246 - 48bc - b91e - cd730a53d8aa You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch , or call 1 - 800 - FDA - 1088. Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults. Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches. Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age. Tosymra ® IMPORTANT SAFETY INFORMATION (2 of 2)