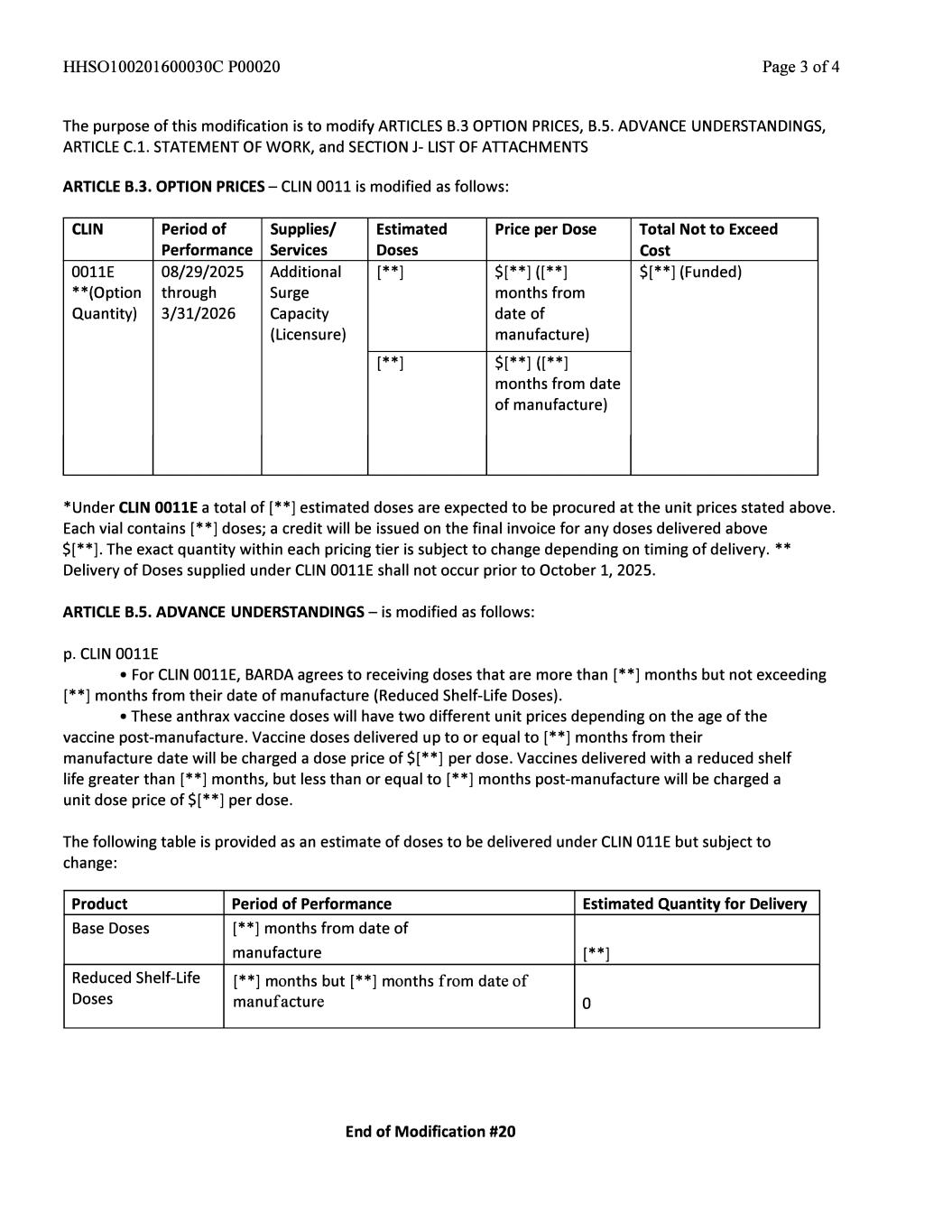
Document
Emergent BioSolutions Awarded $30 Million Contract Modification for CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted), a Two-Dose Anthrax Vaccine for Post-Exposure Prophylaxis Use
GAITHERSBURG, Md., September 2, 2025 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE: EBS) today announced it has received a $30 million contract modification from the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), to supply CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted). Deliveries are expected to begin this calendar year and are scheduled to be completed by March 2026.
"This new contract modification is the next step in our ongoing engagement with the U.S. government, with the shared goal to help ensure medical countermeasures, like CYFENDUS® vaccine, are readily available to safeguard civilian populations against the potential threat of anthrax,” said Paul Williams, senior vice president, head of products business, global government & public affairs at Emergent. “We're proud to leverage a U.S.-based supply chain for our anthrax vaccines, as we believe this is critical to Emergent’s leadership and continued support of the U.S. government’s national security priorities.”
Anthrax is a Tier 1 biological threat due to its potential to be used for a bioterrorist incident and threat to public health and national security. CYFENDUS® vaccine was approved by the U.S. Food and Drug Administration in July 2023 as a two-dose anthrax vaccine for post-exposure prophylaxis use in individuals 18 through 65 years of age when given with recommended antibacterial drugs.
This follows a previously announced contract modification of $50 million to supply CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) in December 2024. This project has been supported in whole or in part with federal funds from the U.S. Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract HHSO100201600030C.
About CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted)
Indication
CYFENDUS® (Anthrax Vaccine Absorbed, Adjuvanted) is a vaccine indicated for post-exposure prophylaxis of anthrax disease following suspected or confirmed exposure to Bacillus anthracis in persons 18 through 65 years of age when given with recommended antibacterial drugs. The efficacy of CYFENDUS® vaccine for post-exposure prophylaxis (PEP) is based solely on studies in animal models of inhalational anthrax.
Important Safety Information
Contraindication: Do not administer CYFENDUS® to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) following a previous dose of CYFENDUS®, BioThrax® (a licensed anthrax vaccine with the same active ingredient as CYFENDUS®) or any component of the vaccine.
Warnings and Precautions: Management of Acute Allergic Reactions: Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of CYFENDUS®. Pregnancy: CYFENDUS® can cause fetal harm when administered to a pregnant individual. In an observational study, there were more birth defects in infants born to individuals vaccinated with BioThrax® (a licensed anthrax vaccine with the same active ingredient as CYFENDUS®) in the first trimester compared to infants born to individuals vaccinated post pregnancy or individuals never vaccinated with BioThrax®.
Adverse Reactions: The most common (≥10%) injection-site adverse reactions reported were tenderness, pain, arm motion limitation, warmth, induration, itching, swelling, and erythema/redness. The most common systemic adverse reactions were muscle aches, tiredness, and headache.
To report Suspected Adverse Reactions, contact Emergent BioSolutions at 1-800-768-2304 or medicalinformation@ebsi.com; or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Please see the Prescribing Information for CYFENDUS® for full safety information.
About Emergent BioSolutions
At Emergent, our mission is to protect and save lives. For over 25 years, we’ve been at work preparing those entrusted with protecting public health. We deliver protective and life-saving solutions for health threats like smallpox, mpox, botulism, Ebola, anthrax and opioid overdose emergencies. To learn more about how we help prepare communities around the world for today’s health challenges and tomorrow’s threats, visit our website and follow us on LinkedIn, X, Instagram, Apple Podcasts and Spotify.
Safe Harbor Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the expected timing for delivery of the CYFENDUS® vaccine and Emergent’s ability to increase inventories of CYFENDUS ® vaccine to meet requested levels within specified time frames, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “should,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs, and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events, or circumstances.
Readers should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the U.S. Securities and Exchange Commission, when evaluating our forward-looking statements.
Investor Contact:
Richard S. Lindahl
Executive Vice President, CFO
lindahlr@ebsi.com
Media Contact:
Assal Hellmer
Vice President, Communications
mediarelations@ebsi.com