BANNER YEAR FOR MESOBLAST WITH FIRST FDA PRODUCT APPROVAL AND SUCCESSFUL COMMERCIAL LAUNCH OF RYONCIL® Financial Results and Operational Update for the Full Year Ended June 30, 2025 Melbourne, Australia; August 29 and New York, USA; August 28, 2025: Mesoblast Limited (ASX:MSB; Nasdaq:MESO), global leader in allogeneic cellular medicines for inflammatory diseases, today provided financial results and an operational update for the period ended June 30, 2025. Mesoblast Chief Executive Dr. Silviu Itescu said, “This has been a banner year for the Company, with achievement of FDA approval for Ryoncil®, the first and only FDA approved mesenchymal stromal cell (MSC) product in the United States and a successful commercial launch of the product for treatment of steroid-refractory acute graft-versus-host disease (SR-aGvHD) in pediatric patients aged 2 months and older (“children”)”. ”We are working tirelessly to transform Mesoblast into a fast-paced commercial biotechnology company with demonstrable sales of our first commercial product Ryoncil®. We intend to deliver additional approved indications for sales of Ryoncil® and to launch a next-generation platform technology into potential blockbuster indications including heart failure and chronic low back pain.” FINANCIAL AND CORPORATE HIGHLIGHTS FOR THE YEAR ENDED JUNE 30, 2025 • Revenue from cell therapy products was US$17.2 million, up 191% on prior year. • Revenue growth was driven by successful launch of Ryoncil® in the final quarter, with US$13.2 million gross sales and US$11.3 million in reported net sales after 14.6% gross to net adjustment. • Net operating cash spend was US$50.0 million, a 3% increase on prior year, inclusive of costs related to commercial team build and product launch. • Cash on hand at June 30, 2025, was US$162 million (A$247 million)1. • Mesoblast was added to the S&P Dow Jones Indices’ S&P/ASX 200 Index effective March 6, 2025, on the Australian Stock Exchange (ASX), and to the Nasdaq Biotechnology Index (Nasdaq: NBI) as part of the annual reconstitution of the 2024 Nasdaq index December 23, 2024. • Board of Directors was strengthened with appointments of Dr. Gregory George MD PhD and Ms Lyn Cobley. INVESTMENT HIGHLIGHTS Mission Mesoblast is committed to bring to market innovative off-the-shelf allogeneic cellular medicines to treat serious and life-threatening inflammatory illnesses. Product Portfolio • Ryoncil® the only FDA-approved MSC therapy for any indication; lifesaving for pediatric SR-aGvHD. • Revascor® has potential for FDA accelerated approval in end-stage HFrEF. • Rexlemestrocel-L in Phase 3 trial for potential approval in CLBP. • Additional pipeline therapies targeting unmet medical needs. Market Opportunity • Steroid-refractory acute GvHD ~$1 billion annual addressable market for children & adults. • Biologic-refractory inflammatory bowel disease grater than US$5 billion. • Heart failure with reduced ejection fraction (HFrEF) >$10 billion addressable market. Exhibit 99.1

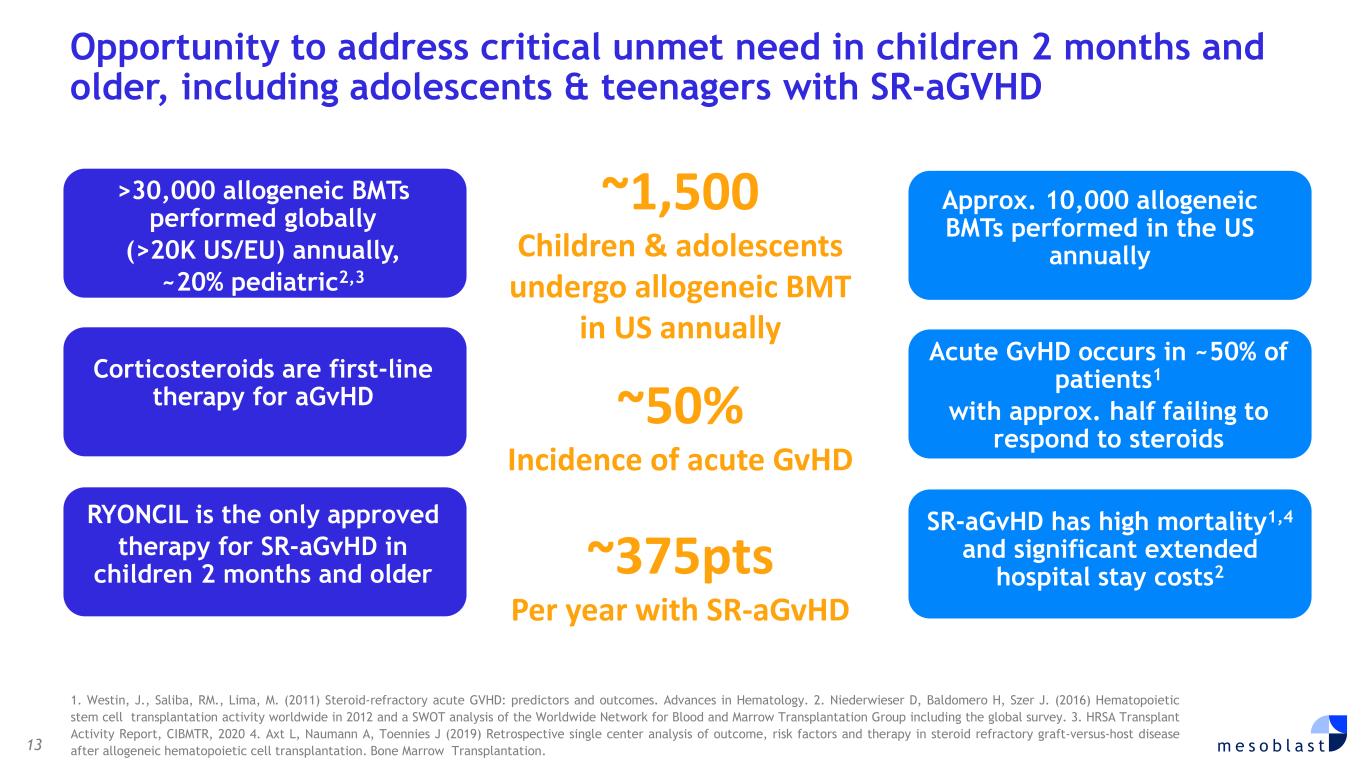
• Chronic low back pain (CLBP) >$10 billion addressable market. • Additional potential multi-billion-dollar opportunities from existing and future product pipeline based on existing technology platforms. Competitive Advantage • Proven scientific approach, with deep understanding of mechanism of action (MOA). • Robust and extensive intellectual property. • Extensive positive clinical trial results across multiple indications. • Demonstrated ability to meet regulatory requirements of FDA. • Scalability of manufacturing processes and proprietary next generation technology. Financials and Projections • Strong balance sheet to support Ryoncil® launch and product portfolio. • Current revenue streams anticipated to expand based on Ryoncil® performance. • Continued prudent cash management strategy. • Long-term revenue potential from the existing and future pipeline. Upcoming Milestones • Commence Ryoncil® registration trial for label expansion in adults with severe SR-aGvHD. • Commence Ryoncil® registration trial for label expansion in adults and children with inflammatory colitis. • Revascor® BLA submission for accelerated approval. • Rexlemestrocel-L CLBP Phase 3 completion and BLA submission. • Expansion into new indications and markets. OPERATIONAL HIGHLIGHTS Ryoncil® (remestemcel-L-rknd) for Steroid-Refractory Acute Graft Versus Host Disease (SR- aGvHD) in Children In December 2024, Ryoncil® became the first and only Food and Drug Administration (FDA) approved mesenchymal stromal cell (MSC) product in the Unites States. Ryoncil® became commercially available for purchase on March 28, 2025, within one quarter of receiving FDA approval for treatment of SR-aGvHD in children. Coverage for Ryoncil® continues to expand with over 250 million US lives insured by commercial and government payers. Federal Medicaid coverage by Centers for Medicare and Medicaid (CMS) is in place and mandatory fee-for-service Medicaid coverage for Ryoncil® became effective July 1, 2025 in all US states. To date, we have onboarded 32 transplant centers and aim to have onboarded by the end of this quarter the top 45 centers that account for 80% of pediatric bone marrow transplants in the United States. Assistance to Patients with SR-aGvHD and their Caregivers for Access to Ryoncil® To assist patients and institutions with insurance coverage, financial assistance, and access programs, ensuring that no patient is left behind in receiving this potentially life-saving therapy, Mesoblast has established a patient access hub termed MyMesoblast™, where Ryoncil® is available for ordering. Additional information is available on ryoncil.com, where valuable resources for healthcare providers, patients and caregivers can be found. Ryoncil® Lifecycle Extension in Adults with Severe SR-aGvHD Most of the sites already onboarded for use of Ryoncil® in children with SR-aGvHD also perform adult bone marrow transplants. We have an active compassionate care program to provide Ryoncil® to adults with SR- aGvHD who have failed other therapies. Adults with severe SR-aGvHD have a high rate of non-responsiveness to second-line agents such as ruxolitinib. Survival in those with SR-aGvHD who have failed at least one additional agent, such as ruxolitinib, remains as low as 20-30% by 100 days.2,3 In contrast, among 25 patients aged 12 and older

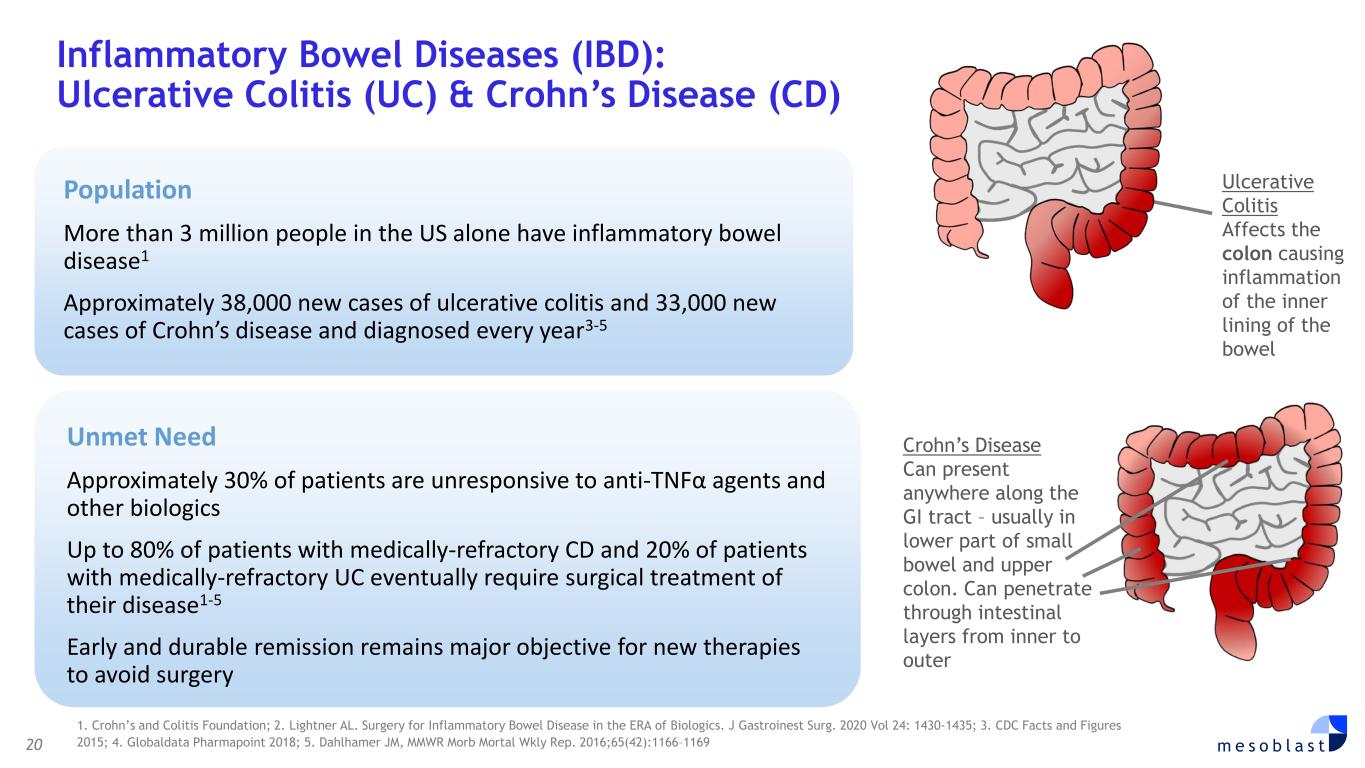
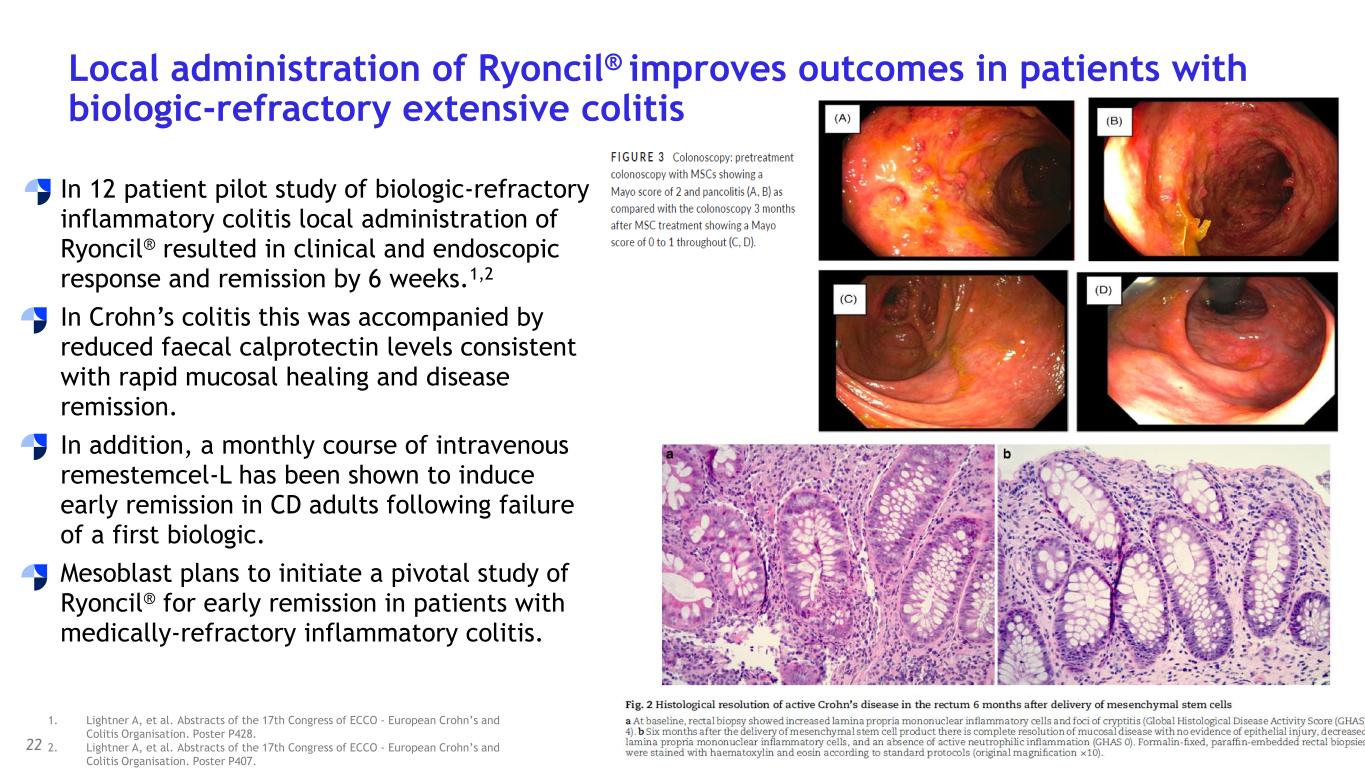
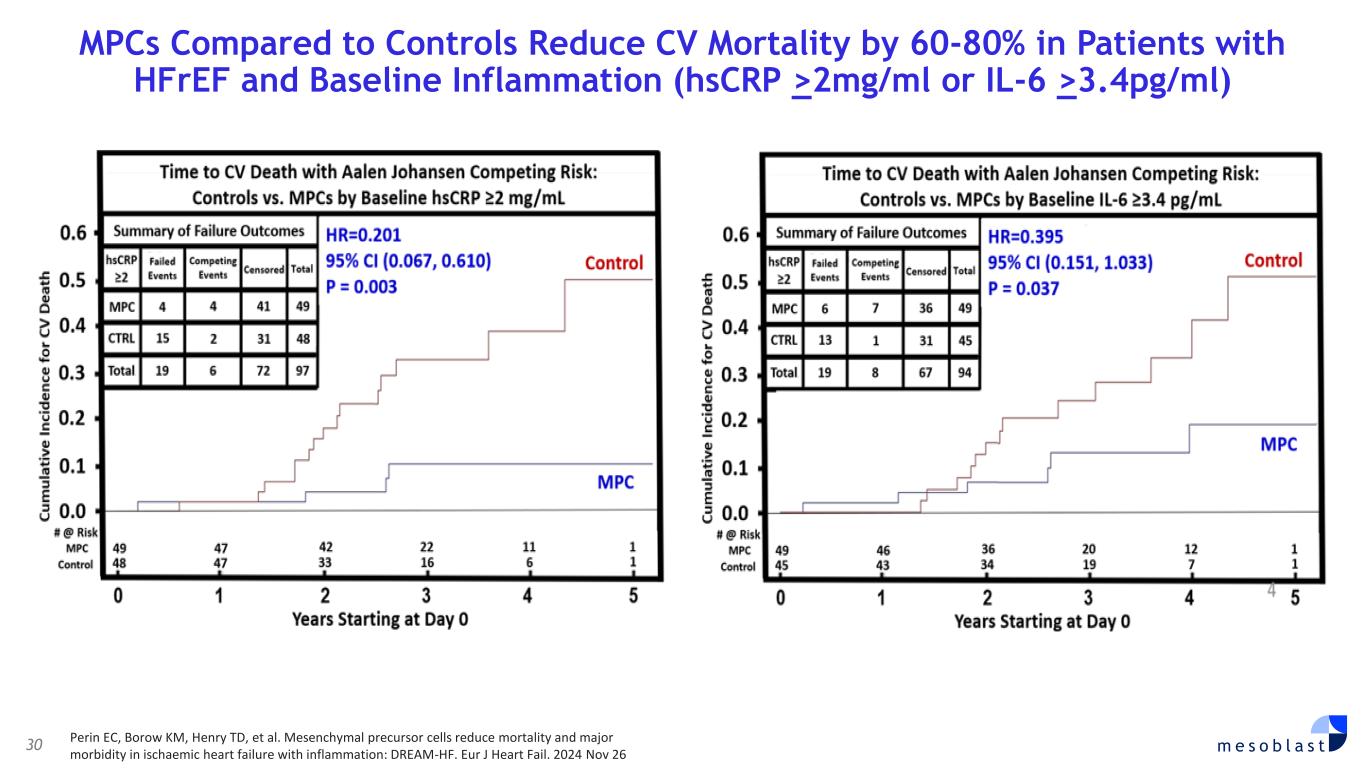
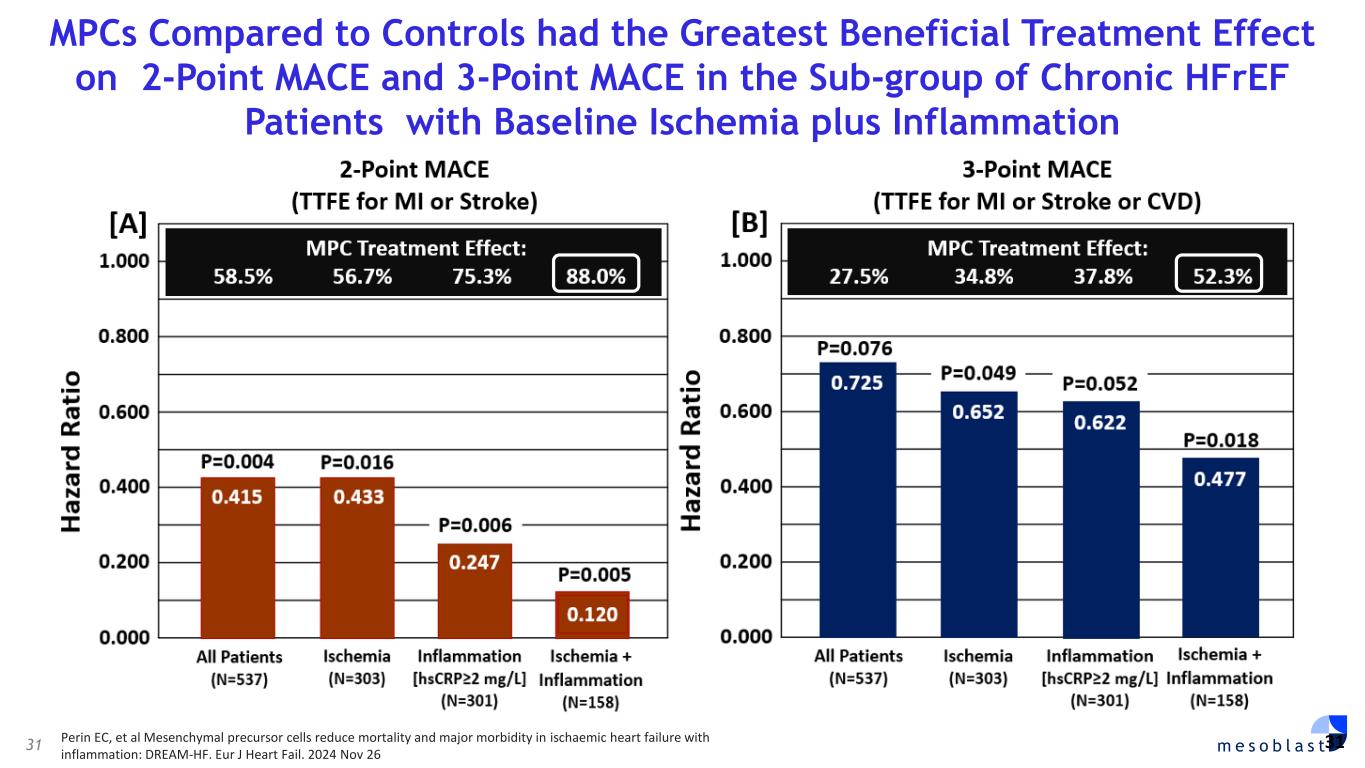
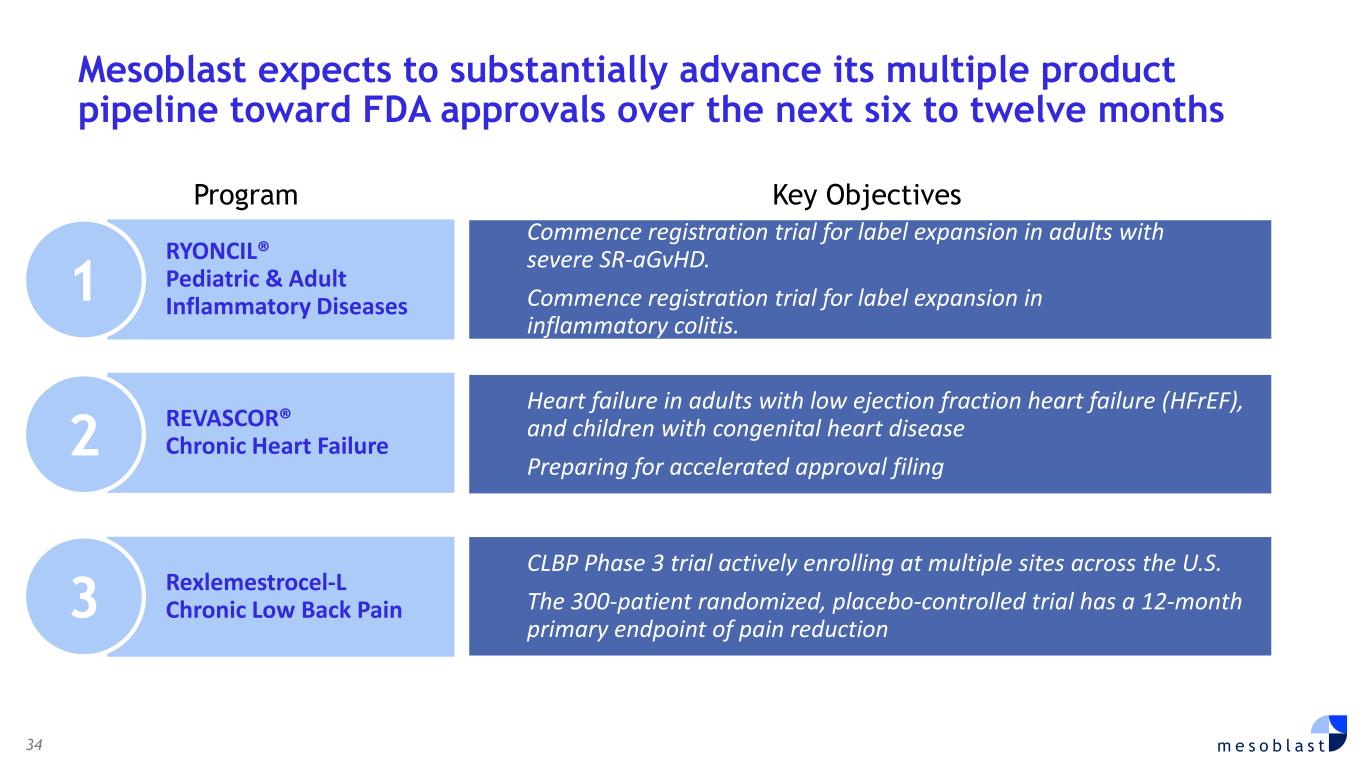
with SR-aGvHD who failed ruxolitinib or other second-line agents, Day-100 survival was 76% after Ryoncil® treatment was used under expanded access. On the basis of these results, Mesoblast intends to seek label extension for Ryoncil® in adults with severe SR-aGvHD. Mesoblast recently met with FDA to discuss a pivotal trial for Ryoncil® in adults with severe SR-aGvHD. Given the continued unmet need in adults with severe SR-aGvHD, Mesoblast intends to conduct a pivotal study of Ryoncil® on top of approved second-line therapy in patients with severe SR-aGvHD. This trial will be conducted with the NIH-funded Bone Marrow Transplant Clinical Trials Network (BMT-CTN), the objective being to extend Ryoncil’s® label from children to adults with SR-aGvHD, a population approximately three times the size of the pediatric SR-aGvHD population. Ryoncil® Lifecycle Extension in Inflammatory Diseases Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD) remains a major unmet need across the adult and pediatric population where early and durable remission remains especially challenging. According to recent estimates, more than three million people (1.3%) in the US alone have inflammatory bowel disease, with more than 38,000 new cases of ulcerative colitis and 33,000 new cases of Crohn’s disease diagnosed every year.4-6 Despite recent advances, approximately 30% of patients are primarily unresponsive to anti-TNFα agents and even among responders, up to 10% will lose their response to the drug every year7,8. Up to 80% of patients with medically-refractory Crohn’s disease and 20% of patients with medically-refractory ulcerative colitis eventually require surgical treatment of their disease,7,8 which can have a devastating impact on quality of life. A recent pilot study in adults with inflammatory colitis demonstrated positive outcomes (rapid mucosal healing and disease remission) in biologic refractory patients receiving Ryoncil® by direct endoscopic injection to areas of inflammation.9,10 This extends earlier Mesoblast data showing that intravenously delivered remestemcel-L can induce early remission in CD adults who have failed an anti-TNF agent. Given the effectiveness of Ryoncil® in GI-related SR-aGvHD, and the existing data on adult inflammatory colitis, Mesoblast plans to initiate a pivotal study of Ryoncil® for early remission in patients with medically- refractory inflammatory colitis. Ryoncil® Market Exclusivity Ryoncil® received seven years of orphan-drug exclusive approval from FDA for treatment of SR- aGvHD in pediatric patients 2 months of age and older, meaning that the FDA will not approve another MSC product for this indication during the 7-year exclusivity period. Mesoblast also has biologic exclusivity preventing another sponsor from referencing the Ryoncil® biologic license application (BLA) until December 2036, preventing market entry by a biosimilar. Mesoblast’s strong U.S. intellectual property position on MSC composition of matter, manufacturing and indications, including SR-aGvHD, provide even further commercial barriers to entry against competitors through 2044. Revascor® (rexlemestrocel-L) for Chronic Heart Failure with Reduced Ejection Fraction (HFrEF) and Persistent Inflammation In November 2024 a publication in the prestigious peer-reviewed European Journal of Heart Failure (EJHF) reported that a single intramyocardial injection of Revascor® resulted in improved survival in high-risk NYHA Class II/III patients with ischemic heart failure and inflammation.11 This identifies the HFrEF population that is responsive to Revascor® and will be the target of a confirmatory trial after accelerated approval, if received. In follow-up to the successful Type B meeting in early 2024 under the existing Regenerative Medicine Advanced Therapy (RMAT) designation for Revascor® in end-stage HFrEF patients with a left ventricular assist device (LVAD), where FDA stated that the results of the presented studies could support accelerated approval, Mesoblast met with FDA on June 3, 2025 to align on key items for BLA filing. Mesoblast and FDA are aligned on items required for filing a biologics license application (BLA) for Revascor® regarding chemistry, manufacturing & controls (CMC), potency assays for commercial product release, and proposed design and primary endpoint for the confirmatory trial post-approval. Rexlemestrocel-L for Chronic Low Back Pain associated with Degenerative Disc Disease The 300-patient randomized controlled confirmatory Phase 3 trial of Mesoblast’s second generation allogeneic, STRO3-immunoselected, and industrially manufactured stromal cell product candidate

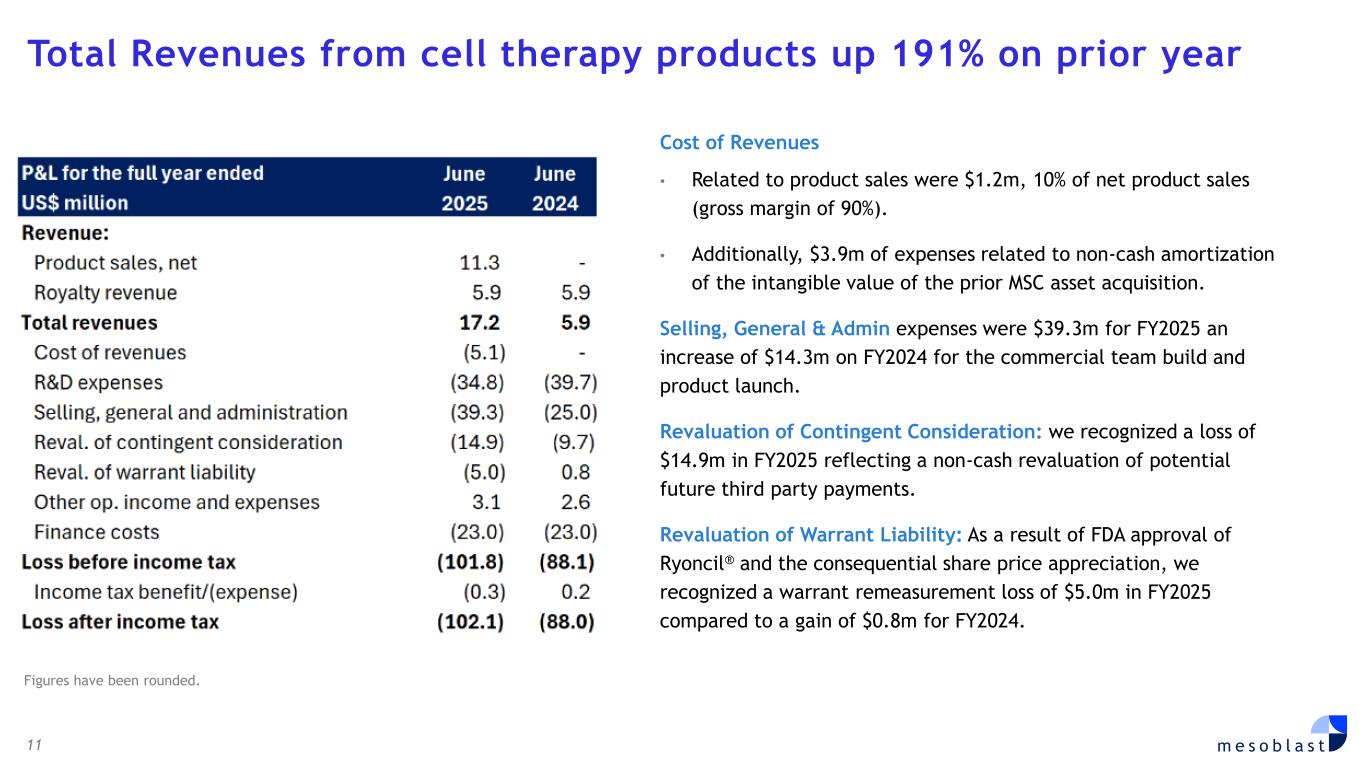
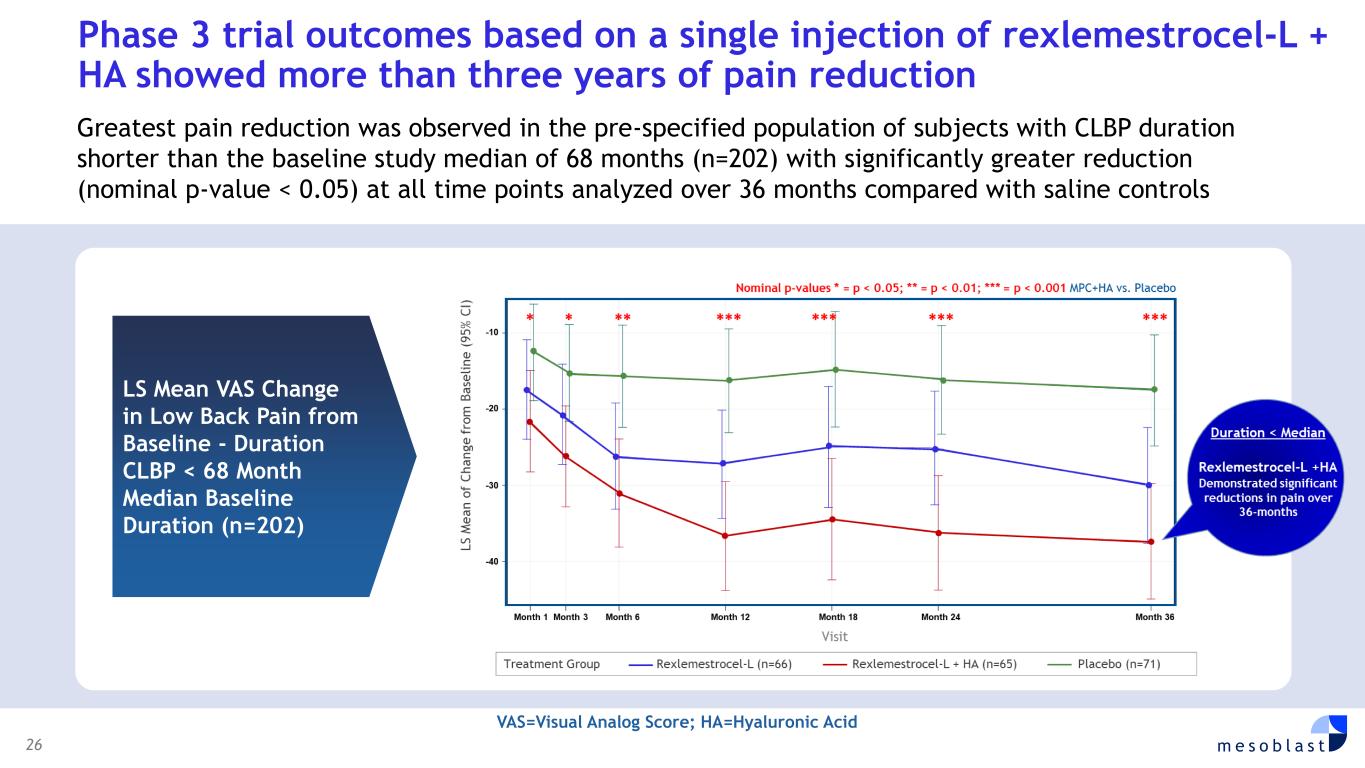
rexlemestrocel-L is actively enrolling in patients with chronic low back pain (CLBP) due to inflammatory degenerative disc disease (DDD) of less than five years duration at multiple sites across the U.S. FDA has previously agreed on the design of this 300-patient randomized, placebo-controlled confirmatory Phase 3 trial, and the 12-month primary endpoint of pain reduction as an approvable indication. This endpoint was successfully met in Mesoblast’s first Phase 3 trial. Key secondary measures include improvement in quality of life and function. A particular focus is on treatment of patients on opioids, since discogenic back pain accounts for approximately 50% of prescription opioid usage in the US. Significant pain reduction and opioid cessation were observed in Mesoblast’s first Phase 3 trial. Rexlemestrocel-L has received RMAT designation from FDA for the treatment of chronic low back pain. CORPORATE GOVERNANCE Board of Directors was strengthened with appointments of Dr. Gregory George MD PhD and Ms Lyn Cobley. Dr. George founded and managed the largest privately owned ambulatory surgical center company in the United States, SurgCenter Development. Dr. George brings to the Board his background as a medical scientist with unique operational experience having built a start-up company in the medical field and turning it into a highly efficient multi-billion-dollar commercial organization. Dr George is Mesoblast’s largest shareholder. Ms Cobley has been a global leader in the financial services industry with over 30 years’ experience in senior positions at international and domestic banks. Ms Cobley is currently a director of Commonwealth Bank of Australia, has previously served as CEO of Westpac Institutional Bank, and as Group Treasurer of Commonwealth Bank of Australia, and has held senior roles at Barclays Capital and Citibank Limited. FINANCIAL RESULTS FOR FULL YEAR ENDED JUNE 30, 2025 (FY2025) • Total Revenues from cell therapy products were US$17.2m, up 191% on prior year. Revenue growth driven by successful launch of Ryoncil® in the final quarter. • Net Product Sales were driven by successful launch of Ryoncil® in the final quarter, with US$13.2 million gross sales and US$11.3 million in reported net sales after 14.6% gross to net adjustment. • Royalties from sales of cell therapies by our licensees were US$5.9m for both FY2025 and FY2024. • Cost of Revenues related to product sales were US$1.2m (10% of net product sales). Additionally, US$3.9m of expenses related to non-cash amortization of the intangible value of the prior MSC asset acquisition. • Research & Development expenses were US$34.8m for FY2025, compared with US$39.7m for FY2024, a reduction of 12%. • Selling, General & Administration expenses were US$39.3m for FY2025 an increase of US$14.3m on FY2024 for the commercial team build and product launch. • Contingent Consideration: we recognized a loss of US$14.9m in FY2025 reflecting a non-cash revaluation of potential future third party payments. • Fair value movement of warrants: As a result of FDA approval of Ryoncil® and the consequential share price appreciation, our warrant remeasurement increased by US$5.0m in FY2025 compared to a gain of US$0.8m for FY2024. • Other operating income & expenses: for FY2025 we recognized a gain of US$3.1m compared with a gain of US$2.6m for FY2024 due to increased interest income. • Finance Costs of US$23.0m for FY2025 for borrowing arrangements include US$17.7m of non-cash expenditure comprising accruing interest and other borrowing costs. Loss after tax for FY2025 was US$102.1m compared to US$88.0m for FY2024. The net loss attributable to ordinary shareholders was 8.46 US cents per share for FY2025, compared with 8.91 US cents per share for FY2024.

Conference Call There will be a webcast today, beginning at 8.30am AEST (Friday, August 29); 6.30pm EDT (Thursday, August 28). It can be accessed via: https://webcast.openbriefing.com/msb-fyr-2025/ The archived webcast will be available on the Investor page of the Company’s website: www.mesoblast.com About Mesoblast Mesoblast (the Company) is a world leader in developing allogeneic (off-the-shelf) cellular medicines for the treatment of severe and life-threatening inflammatory conditions. The therapies from the Company’s proprietary mesenchymal lineage cell therapy technology platform respond to severe inflammation by releasing anti-inflammatory factors that counter and modulate multiple effector arms of the immune system, resulting in significant reduction of the damaging inflammatory process. Mesoblast’s Ryoncil® (remestemcel-L-rknd) for the treatment of steroid-refractory acute graft versus host disease (SR-aGvHD) in pediatric patients 2 months and older is the first FDA-approved mesenchymal stromal cell (MSC) therapy. Please see the full Prescribing Information at www.ryoncil.com. Mesoblast is committed to developing additional cell therapies for distinct indications based on its remestemcel-L and rexlemestrocel-L allogeneic stromal cell technology platforms. Ryoncil® is being developed for additional inflammatory diseases including SR-aGvHD in adults and biologic-resistant inflammatory bowel disease. Rexlemestrocel-L is being developed for heart failure and chronic low back pain. The Company has established commercial partnerships in Japan, Europe and China. About Mesoblast intellectual property: Mesoblast has a strong and extensive global intellectual property portfolio, with over 1,000 granted patents or patent applications covering mesenchymal stromal cell compositions of matter, methods of manufacturing and indications. These granted patents and patent applications are expected to provide commercial protection extending through to at least 2041 in major markets. About Mesoblast manufacturing: The Company’s proprietary manufacturing processes yield industrial- scale, cryopreserved, off-the-shelf, cellular medicines. These cell therapies, with defined pharmaceutical release criteria, are planned to be readily available to patients worldwide. Mesoblast has locations in Australia, the United States and Singapore and is listed on the Australian Securities Exchange (MSB) and on the Nasdaq (MESO). For more information, please see www.mesoblast.com, LinkedIn: Mesoblast Limited and Twitter: @Mesoblast References / Footnotes 1. Translated at 1A$:0.655US$ being the June 30, 2025 rate as reported by the Reserve Bank of Australia. 2. Jagasia M et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020 May 14; 135(20): 1739–1749 3. Abedin S, et al. Ruxolitinib resistance or intolerance in steroid-refractory acute graft versus-host disease — a real-world outcomes analysis. British Journal of Haematology, 2021;195:429–43 4. CDC Facts and Figures 2015 5. Globaldata Pharmapoint 2018 6. Dahlhamer JM, MMWR Morb Mortal Wkly Rep. 2016;65(42):1166–1169 7. Crohn’s and Colitis Foundation 8. Lightner AL. Surgery for Inflammatory Bowel Disease in the ERA of Biologics. J Gastroinest Surg. 2020 Vol 24: 1430-1435 9. Lightner A., et al. A Phase IB/IIA study of remestemcel-L, an allogeneic bone marrow derived mesenchymal stem cell product, for the treatment of medically refractory ulcerative colitis: An interim analysis. Journal of Crohn's and Colitis, Volume 16, Issue Supplement_1, January 2022, Pages i398–i399, https://doi.org/10.1093/ecco-jcc/jjab232.534 10. Lightner A., et al. A Phase IB/IIA study of remestemcel-L, an allogeneic bone marrow derived mesenchymal stem cell product, for the treatment of medically refractory Crohn’s colitis: A preliminary analysis. Journal of Crohn's and Colitis, Volume 16, Issue Supplement_1, January 2022, Pages i412–i413, https://doi.org/10.1093/ecco-jcc/jjab232.555 11. Perin EC. Et al. Mesenchymal precursor cells reduce mortality and major morbidity in ischaemic heart failure with inflammation: DREAM-HF. Eur J Heart Fail 2024. https://doi.org/10.1002/ejhf.3522

Forward-Looking Statements This press release includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. Forward-looking statements include, but are not limited to, statements about: the initiation, timing, progress and results of Mesoblast’s preclinical and clinical studies, and Mesoblast’s research and development programs; Mesoblast’s ability to advance product candidates into, enroll and successfully complete, clinical studies, including multi-national clinical trials; Mesoblast’s ability to advance its manufacturing capabilities; the timing or likelihood of regulatory filings and approvals, manufacturing activities and product marketing activities, if any; the commercialization of Mesoblast’s Ryoncil® for pediatric SR-aGVHD and any other product candidates, if approved; regulatory or public perceptions and market acceptance surrounding the use of stem-cell based therapies; the potential for Mesoblast’s product candidates, if any are approved, to be withdrawn from the market due to patient adverse events or deaths; the potential benefits of strategic collaboration agreements and Mesoblast’s ability to enter into and maintain established strategic collaborations; Mesoblast’s ability to establish and maintain intellectual property on its product candidates and Mesoblast’s ability to successfully defend these in cases of alleged infringement; the scope of protection Mesoblast is able to establish and maintain for intellectual property rights covering its product candidates and technology; estimates of Mesoblast’s expenses, future revenues, capital requirements and its needs for additional financing; Mesoblast’s financial performance; developments relating to Mesoblast’s competitors and industry; and the pricing and reimbursement of Mesoblast’s product candidates, if approved. You should read this press release together with our risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast’s actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, and accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise. Release authorized by the Chief Executive. For more information, please contact: Corporate Communications / Investors Paul Hughes T: +61 3 9639 6036 Media – Global Allison Worldwide Emma Neal T: +1 603 545 4843 E: emma.neal@allisonworldwide.com Media – Australia BlueDot Media Steve Dabkowski T: +61 419 880 486 E: steve@bluedot.net.au

Consolidated Income Statement Year Ended June 30, (in U.S. dollars, in thousands, except per share amount) 2025 2024 Revenue: Product sales, net 11,263 — Royalty revenue 5,935 5,902 Total revenues 17,198 5,902 Cost of revenues (including amortization of currently marketed intangible assets, 2025: $3.937 million, 2024: $Nil) (5,130) — Research & development (34,807) (39,716) Selling, general and administration (39,309) (24,980) Fair value remeasurement of contingent consideration (14,887) (9,693) Fair value remeasurement of warrant liability (4,962) 779 Other operating income and expenses 3,053 2,570 Finance costs (22,968) (23,009) Loss before income tax (101,812) (88,147) Income tax benefit/(expense) (330) 191 Loss attributable to the owners of Mesoblast Limited (102,142) (87,956) Losses per share from continuing operations attributable to the ordinary equity holders of the Group: Cents Cents Basic - losses per share (8.46) (8.91) Diluted - losses per share (8.46) (8.91) Consolidated Statement of Comprehensive Income Year Ended June 30, (in U.S. dollars, in thousands) 2025 2024 Loss for the period (102,142) (87,956) Other comprehensive (loss)/income Items that may be reclassified to profit and loss Exchange differences on translation of foreign operations 1,160 51 Items that will not be reclassified to profit and loss Financial assets at fair value through other comprehensive income 374 (743) Other comprehensive income/(loss) for the period, net of tax 1,534 (692) Total comprehensive losses attributable to the owners of Mesoblast Limited (100,608) (88,648)
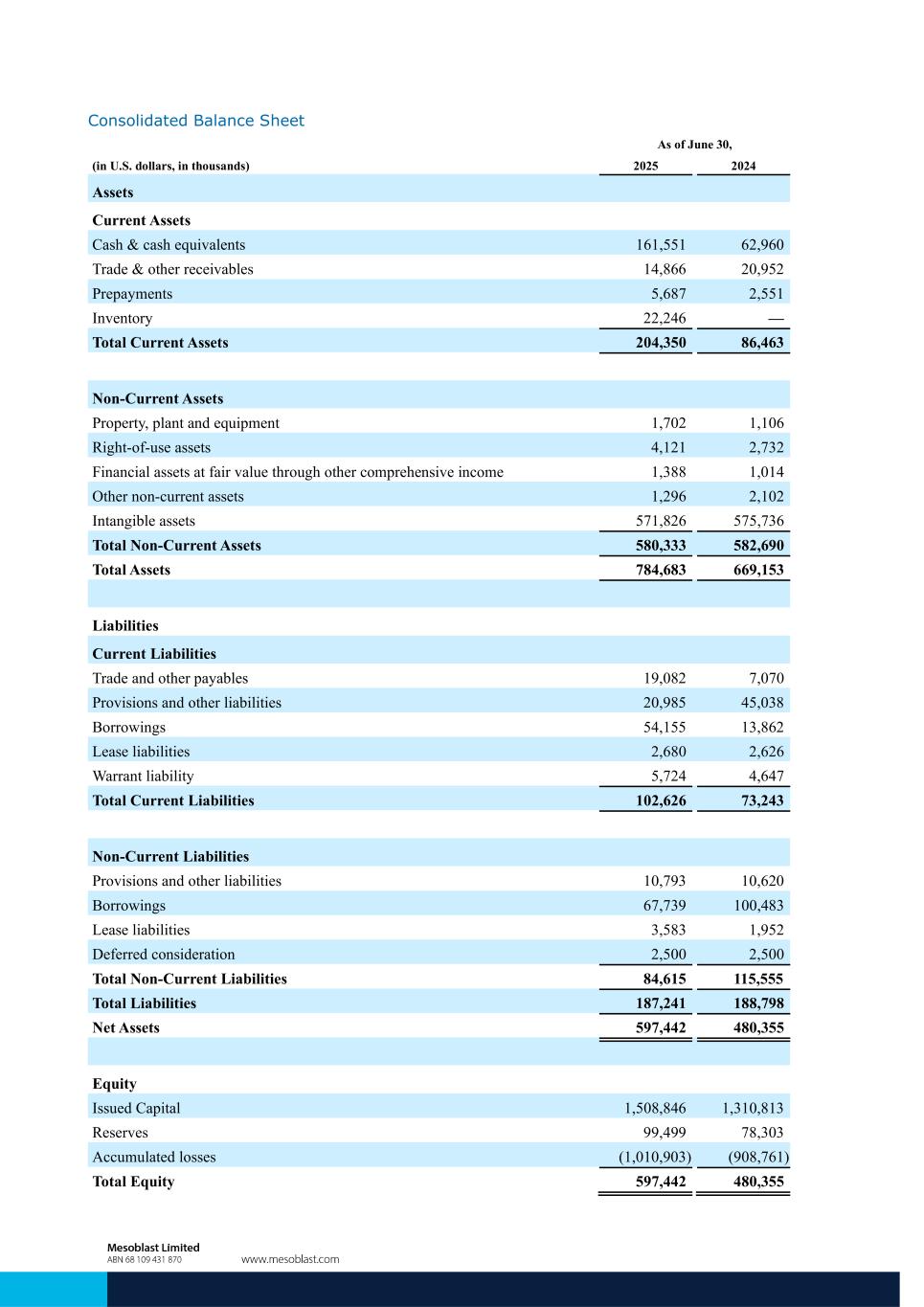
Consolidated Balance Sheet As of June 30, (in U.S. dollars, in thousands) 2025 2024 Assets Current Assets Cash & cash equivalents 161,551 62,960 Trade & other receivables 14,866 20,952 Prepayments 5,687 2,551 Inventory 22,246 — Total Current Assets 204,350 86,463 Non-Current Assets Property, plant and equipment 1,702 1,106 Right-of-use assets 4,121 2,732 Financial assets at fair value through other comprehensive income 1,388 1,014 Other non-current assets 1,296 2,102 Intangible assets 571,826 575,736 Total Non-Current Assets 580,333 582,690 Total Assets 784,683 669,153 Liabilities Current Liabilities Trade and other payables 19,082 7,070 Provisions and other liabilities 20,985 45,038 Borrowings 54,155 13,862 Lease liabilities 2,680 2,626 Warrant liability 5,724 4,647 Total Current Liabilities 102,626 73,243 Non-Current Liabilities Provisions and other liabilities 10,793 10,620 Borrowings 67,739 100,483 Lease liabilities 3,583 1,952 Deferred consideration 2,500 2,500 Total Non-Current Liabilities 84,615 115,555 Total Liabilities 187,241 188,798 Net Assets 597,442 480,355 Equity Issued Capital 1,508,846 1,310,813 Reserves 99,499 78,303 Accumulated losses (1,010,903) (908,761) Total Equity 597,442 480,355

Consolidated Statement of Cash Flow Year Ended June 30, (in U.S. dollars, in thousands) 2025 2024 Cash flows from operating activities Royalty revenue received 5,704 6,776 Government grants and tax incentives and credits received 905 3,819 Payments to suppliers and employees (inclusive of goods and services tax) (60,110) (60,835) Interest received 3,549 1,778 Income taxes (paid)/received (2) 4 Net cash (outflows) in operating activities (49,954) (48,458) Cash flows from investing activities Payments for property, plant and equipment (680) (271) Receipts from investment in sublease 241 234 Receipt of security deposits 609 — Payments for licenses (50) (60) Net cash inflows/(outflows) in investing activities 120 (97) Cash flows from financing activities Repayment of borrowings (7,824) (10,000) Payment of transaction costs from borrowings (1,348) (1,559) Interest and other costs of finance paid (5,266) (5,717) Proceeds from issue of shares 161,205 65,406 Proceeds from exercise of options 5,177 — Proceeds from issue of warrants 1,647 — Payments for share issue costs (4,314) (4,356) Payments for lease liabilities (1,941) (3,522) Net cash inflows by financing activities 147,336 40,252 Net increase/(decrease) in cash and cash equivalents 97,502 (8,303) Cash and cash equivalents at beginning of period 62,960 71,318 Foreign exchange gain/(loss) on the translation of foreign bank accounts 1,089 (55) Cash and cash equivalents at end of period 161,551 62,960