
SUCCESSFUL COMMERCIAL LAUNCH OF RYONCIL® Activity Report for Quarter Ended June 30, 2025 (Appendix 4C) Melbourne, Australia: July 18 and New York, USA: July 17, 2025: Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today announced gross revenue from sales of Ryoncil® (remestemcel-L-rknd) through the first quarter post product launch. Ryoncil® is the first and only FDA approved mesenchymal stromal cell (MSC) product in the Unites States and became commercially available for purchase on March 28, 2025, within one quarter of receiving FDA approval for treatment of steroid-refractory acute graft-versus-host disease (SR-aGvHD) in children. Mesoblast Chief Executive Dr. Silviu Itescu said, “We are pleased with the commercial launch activities of Ryoncil® in the first quarter post-launch and look forward to updating on the current quarter's progress now that mandatory state CMS coverage has become effective as of July 1, and we complete onboarding of the remaining major U.S. transplant centers.” FINANCIAL HIGHLIGHTS FOR QUARTER ENDED JUNE 30, 2025 • US$13.2 million gross revenue (unaudited) from sales of Ryoncil® post-launch March 28 through to June 30.1 • US$1.6 million revenue from royalties on sales of TEMCELL® HS Inj.2 sold in Japan by our licensee. • US$16.6 million net operating cash spend for the quarter. • US$162 million (A$247 million)3 cash on hand at June 30, 2025. OPERATIONAL HIGHLIGHTS FOR RYONCIL® IN SR-aGvHD • Mesoblast has onboarded more than 25 transplant centers since product launch and expects during this quarter to complete the onboarding process across all 45 priority transplant centers that account for approximately 80% of U.S. pediatric transplants. • Coverage for Ryoncil® continues to expand with over 250 million US lives insured by commercial and government payers. Federal Medicaid coverage by Centers for Medicare and Medicaid (CMS) is in place and mandatory fee-for-service Medicaid coverage for Ryoncil® became effective July 1 in all US states. • To assist patients and institutions with insurance coverage, financial assistance, and access programs, ensuring that no patient is left behind in receiving this potentially life-saving therapy, Mesoblast has established a patient access hub termed MyMesoblast™, where Ryoncil® is available for ordering. Additional information is available on ryoncil.com, where valuable resources for healthcare providers, patients and caregivers can be found. • During the quarter, Ryoncil® received seven years of orphan-drug exclusive approval from U.S. Food and Drug Administration (FDA) for treatment of SR-aGvHD in pediatric patients 2 months of age and older. This period of statutory exclusivity means that the FDA will not approve another MSC product for this indication during the 7-year period from the approval of Ryoncil®. • Separately, Mesoblast has biologic exclusivity preventing another sponsor from referencing the Ryoncil® biologic license application (BLA) until December 2036, twelve years from its first approval which would prevent market entry by a biosimilar. • These statutory exclusivities are in addition to Mesoblast’s strong U.S. intellectual property position on MSC composition of matter, manufacturing and indications, including SR-aGvHD, that provide a commercial barrier to entry against competitors through 2044. Exhibit 99.1

• In July, Mesoblast held a Type B meeting with FDA for Ryoncil® to discuss a pivotal trial in adults with SR-aGvHD. This trial will be conducted with the NIH-funded Bone Marrow Transplant Clinical Trials Network (BMT-CTN), the objective being to extend the product’s label from children to adults with SR-aGvHD. Meeting minutes are expected in the coming weeks. OPERATIONAL HIGHLIGHTS FOR REXLEMESTROCEL IN CHRONIC INFLAMMATORY HEART FAILURE AND LOW BACK PAIN • Mesoblast met with FDA in June in follow-up to the successful Type B meeting in early 2024 where FDA stated that the results of the presented studies with Revascor® in the treatment of patients with ischemic heart failure with reduced ejection fraction (HFrEF) and inflammation could support accelerated approval under the existing Regenerative Medicine Advanced Therapy (RMAT) designation for end-stage HFrEF patients with a left ventricular assist device (LVAD), • Mesoblast and FDA are aligned on items required for filing a biologics license application (BLA) for Revascor® regarding chemistry, manufacturing & controls (CMC), potency assays for commercial product release, and proposed design and primary endpoint for the confirmatory trial post-approval. • The 300-patient randomized controlled confirmatory Phase 3 trial of Mesoblast’s second generation allogeneic, STRO3-immunoselected, and industrially manufactured stromal cell product candidate rexlemestrocel-L is actively enrolling in patients with chronic low back pain (CLBP) due to inflammatory degenerative disc disease (DDD) of less than five years duration at multiple sites across the U.S. Other Fees to Non-Executive Directors were US$71,577, consulting payments to Non-Executive Directors were US$122,767 and salary payments to full-time Executive Directors were US$267,465, detailed in Item 6 of the Appendix 4C cash flow report for the quarter.4 From August 2023 to July 2025, our Non-Executive Directors have voluntarily reduced cash payment of their fees by 50% and Executive Directors (our Chief Executive and Chief Medical Officers) reduced their base salaries by 30%, in lieu of accepting equity-based incentives. A copy of the Appendix 4C – Quarterly Cash Flow Report for the fourth quarter FY2025 is attached. About Mesoblast Mesoblast (the Company) is a world leader in developing allogeneic (off-the-shelf) cellular medicines for the treatment of severe and life-threatening inflammatory conditions. The therapies from the Company’s proprietary mesenchymal lineage cell therapy technology platform respond to severe inflammation by releasing anti-inflammatory factors that counter and modulate multiple effector arms of the immune system, resulting in significant reduction of the damaging inflammatory process. Mesoblast’s RYONCIL® (remestemcel-L) for the treatment of steroid-refractory acute graft versus host disease (SR-aGvHD) in pediatric patients 2 months and older is the first FDA approved mesenchymal stromal cell (MSC) therapy. Please see the full Prescribing Information at www.ryoncil.com. Mesoblast is committed to developing additional cell therapies for distinct indications based on its remestemcel-L and rexlemestrocel-L allogeneic stromal cell technology platforms. RYONCIL is being developed for additional inflammatory diseases including SR-aGvHD in adults and biologic-resistant inflammatory bowel disease. Rexlemestrocel-L is being developed for heart failure and chronic low back pain. The Company has established commercial partnerships in Japan, Europe and China. About Mesoblast intellectual property: Mesoblast has a strong and extensive global intellectual property portfolio, with over 1,000 granted patents or patent applications covering mesenchymal stromal cell compositions of matter, methods of manufacturing and indications. These granted patents and patent applications provide commercial protection extending through to at least 2044 in all major markets. About Mesoblast manufacturing: The Company’s proprietary manufacturing processes yield industrial-scale, cryopreserved, off-the-shelf, cellular medicines. These cell therapies, with defined pharmaceutical release criteria, are planned to be readily available to patients worldwide. Mesoblast has locations in Australia, the United States and Singapore and is listed on the Australian Securities Exchange (MSB) and on the Nasdaq (MESO). For more information, please see www.mesoblast.com, LinkedIn: Mesoblast Limited and Twitter: @Mesoblast

References / Footnotes 1. Includes initial inventory purchases by our distributor. 2. TEMCELL® HS Inj. is a registered trademark of JCR Pharmaceuticals Co. Ltd. 3. Translated at 1A$:0.655US$ being the June 30, 2025 rate as reported by the Reserve Bank of Australia. 4. As required by ASX listing rule 4.7 and reported in Item 6 of the Appendix 4C, reported are the aggregated total payments to related parties being Executive Directors and Non-Executive Directors. Forward-Looking Statements This press release includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. Forward-looking statements include, but are not limited to, statements about: the initiation, timing, progress and results of Mesoblast’s preclinical and clinical studies, and Mesoblast’s research and development programs; Mesoblast’s ability to advance product candidates into, enroll and successfully complete, clinical studies, including multi-national clinical trials; Mesoblast’s ability to advance its manufacturing capabilities; the timing or likelihood of regulatory filings and approvals, manufacturing activities and product marketing activities, if any; the commercialization of Mesoblast’s RYONCIL for pediatric SR-aGVHD and any other product candidates, if approved; regulatory or public perceptions and market acceptance surrounding the use of stem-cell based therapies; the potential for Mesoblast’s product candidates, if any are approved, to be withdrawn from the market due to patient adverse events or deaths; the potential benefits of strategic collaboration agreements and Mesoblast’s ability to enter into and maintain established strategic collaborations; Mesoblast’s ability to establish and maintain intellectual property on its product candidates and Mesoblast’s ability to successfully defend these in cases of alleged infringement; the scope of protection Mesoblast is able to establish and maintain for intellectual property rights covering its product candidates and technology; estimates of Mesoblast’s expenses, future revenues, capital requirements and its needs for additional financing; Mesoblast’s financial performance; developments relating to Mesoblast’s competitors and industry; and the pricing and reimbursement of Mesoblast’s product candidates, if approved. You should read this press release together with our risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast’s actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, and accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise. Release authorized by the Chief Executive. For more information, please contact: Corporate Communications / Investors Paul Hughes T: +61 3 9639 6036 Media – Global Allison Worldwide Emma Neal T: +1 603 545 4843 E: emma.neal@allisonworldwide.com Media – Australia BlueDot Media Steve Dabkowski T: +61 419 880 486 E: steve@bluedot.net.au
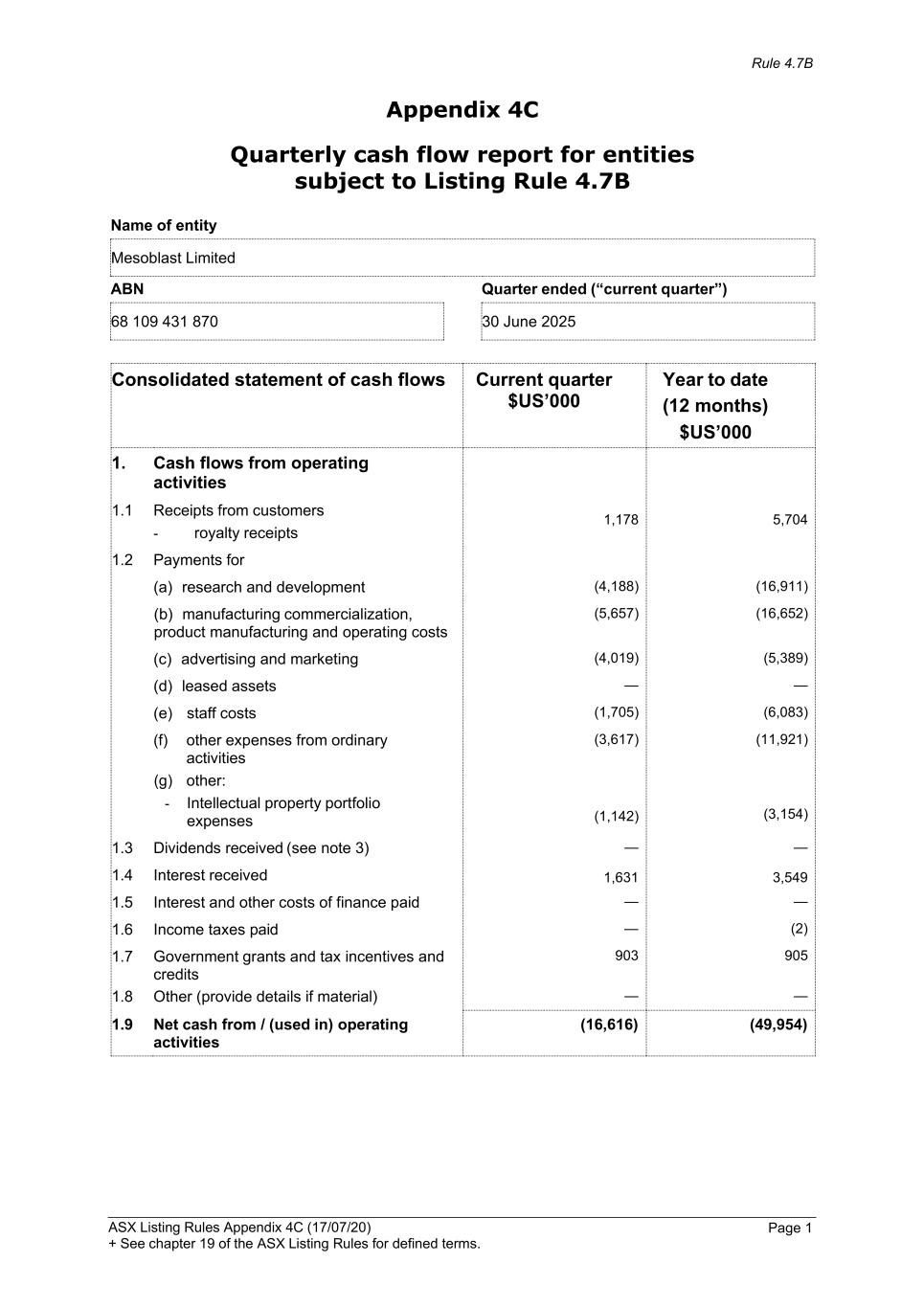
Rule 4.7B ASX Listing Rules Appendix 4C (17/07/20) + See chapter 19 of the ASX Listing Rules for defined terms. Page 1 Appendix 4C Quarterly cash flow report for entities subject to Listing Rule 4.7B Name of entity Mesoblast Limited ABN Quarter ended (“current quarter”) 68 109 431 870 30 June 2025 Consolidated statement of cash flows Current quarter $US’000 Year to date (12 months) $US’000 1. Cash flows from operating activities 1.1 Receipts from customers - royalty receipts 1,178 5,704 1.2 Payments for (a) research and development (4,188) (16,911) (b) manufacturing commercialization, product manufacturing and operating costs (5,657) (16,652) (c) advertising and marketing (4,019) (5,389) (d) leased assets — — (e) staff costs (1,705) (6,083) (f) other expenses from ordinary activities (g) other: - Intellectual property portfolio expenses (3,617) (11,921) (1,142) (3,154) 1.3 Dividends received (see note 3) — — 1.4 Interest received 1,631 3,549 1.5 Interest and other costs of finance paid — — 1.6 Income taxes paid — (2) 1.7 Government grants and tax incentives and credits 903 905 1.8 Other (provide details if material) — — 1.9 Net cash from / (used in) operating activities (16,616) (49,954)
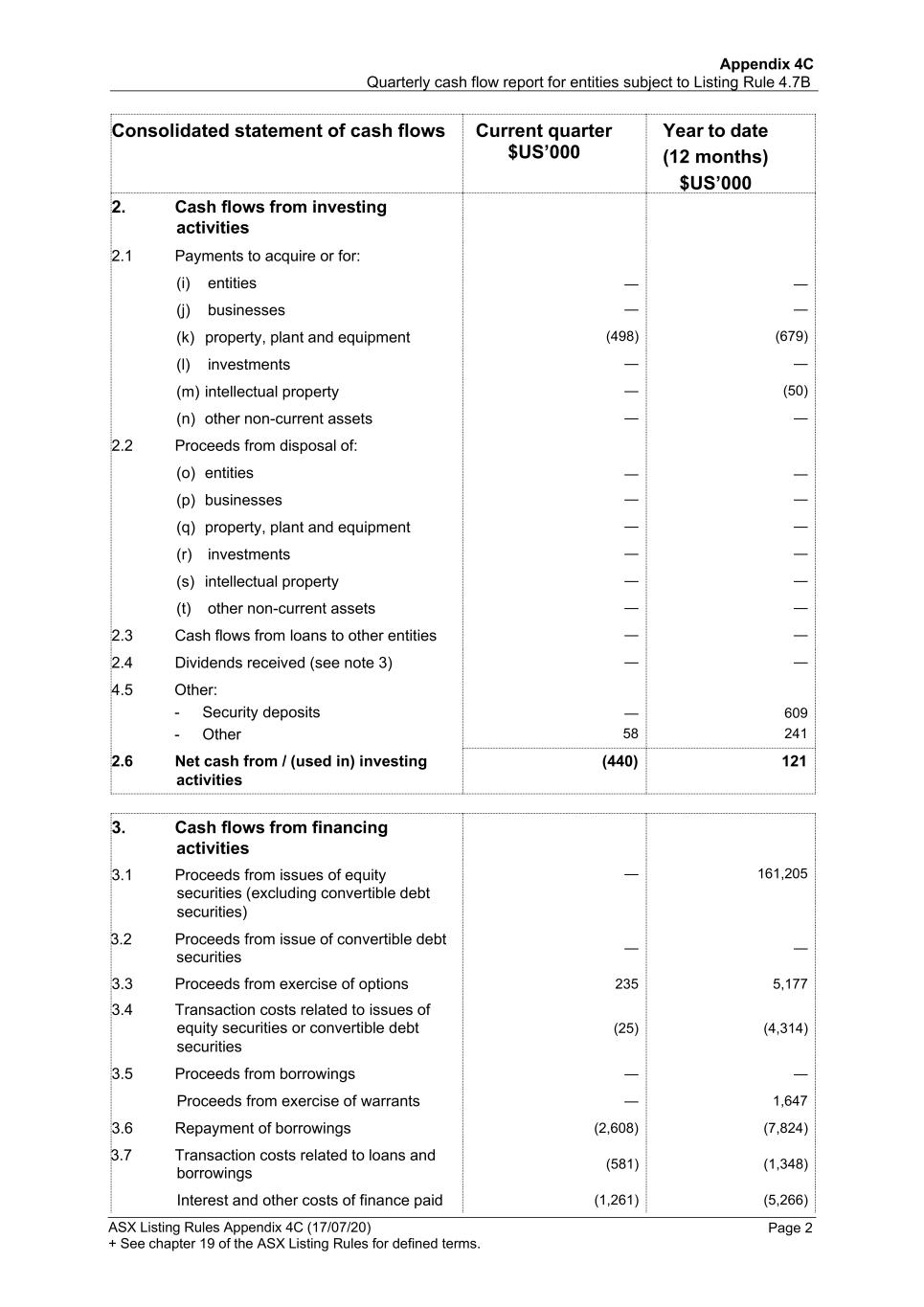
Appendix 4C Quarterly cash flow report for entities subject to Listing Rule 4.7B ASX Listing Rules Appendix 4C (17/07/20) + See chapter 19 of the ASX Listing Rules for defined terms. Page 2 Consolidated statement of cash flows Current quarter $US’000 Year to date (12 months) $US’000 2. Cash flows from investing activities 2.1 Payments to acquire or for: (i) entities — — (j) businesses — — (k) property, plant and equipment (498) (679) (l) investments — — (m) intellectual property — (50) (n) other non-current assets — — 2.2 Proceeds from disposal of: (o) entities — — (p) businesses — — (q) property, plant and equipment — — (r) investments — — (s) intellectual property — — (t) other non-current assets — — 2.3 Cash flows from loans to other entities — — 2.4 Dividends received (see note 3) — — 4.5 Other: - Security deposits — 609 - Other 58 241 2.6 Net cash from / (used in) investing (440) 121 activities 3. Cash flows from financing activities 3.1 Proceeds from issues of equity — 161,205 securities (excluding convertible debt securities) 3.2 Proceeds from issue of convertible debt securities — — 3.3 Proceeds from exercise of options 235 5,177 3.4 Transaction costs related to issues of equity securities or convertible debt (25) (4,314) securities 3.5 Proceeds from borrowings — — Proceeds from exercise of warrants — 1,647 3.6 Repayment of borrowings (2,608) (7,824) 3.7 Transaction costs related to loans and borrowings (581) (1,348) Interest and other costs of finance paid (1,261) (5,266)
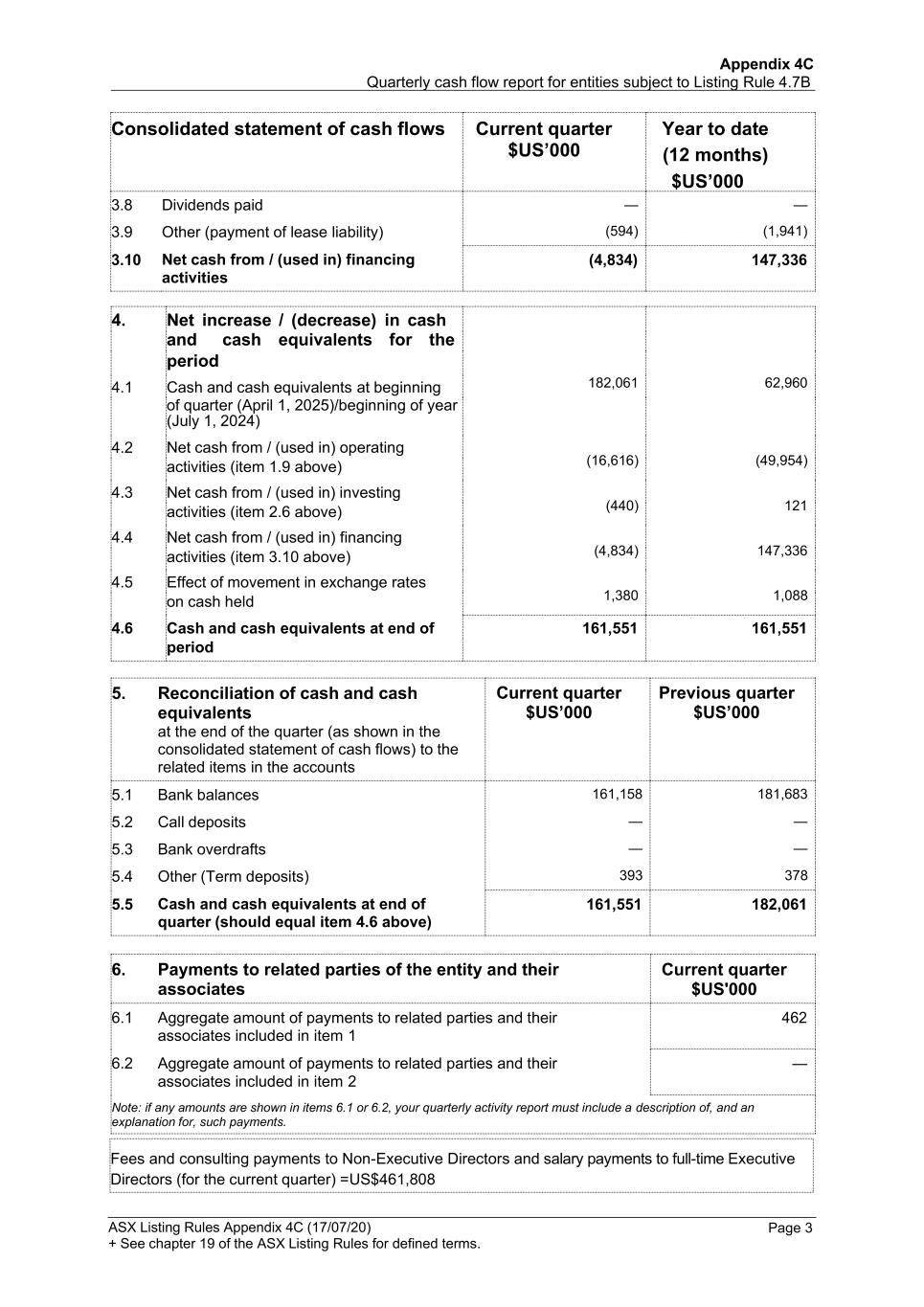
Appendix 4C Quarterly cash flow report for entities subject to Listing Rule 4.7B ASX Listing Rules Appendix 4C (17/07/20) + See chapter 19 of the ASX Listing Rules for defined terms. Page 3 Consolidated statement of cash flows Current quarter $US’000 Year to date (12 months) $US’000 3.8 Dividends paid — — 3.9 Other (payment of lease liability) (594) (1,941) 3.10 Net cash from / (used in) financing activities (4,834) 147,336 4. Net increase / (decrease) in cash 182,061 (16,616) (440) (4,834) 1,380 62,960 (49,954) 121 147,336 1,088 and cash equivalents for the period 4.1 Cash and cash equivalents at beginning of quarter (April 1, 2025)/beginning of year (July 1, 2024) 4.2 Net cash from / (used in) operating activities (item 1.9 above) 4.3 Net cash from / (used in) investing activities (item 2.6 above) 4.4 Net cash from / (used in) financing activities (item 3.10 above) 4.5 Effect of movement in exchange rates on cash held 4.6 Cash and cash equivalents at end of 161,551 161,551 period 5. Reconciliation of cash and cash equivalents at the end of the quarter (as shown in the consolidated statement of cash flows) to the related items in the accounts Current quarter $US’000 Previous quarter $US’000 5.1 Bank balances 161,158 181,683 5.2 Call deposits — — 5.3 Bank overdrafts — — 5.4 Other (Term deposits) 393 378 5.5 Cash and cash equivalents at end of quarter (should equal item 4.6 above) 161,551 182,061 6. Payments to related parties of the entity and their associates Current quarter $US'000 6.1 Aggregate amount of payments to related parties and their associates included in item 1 462 6.2 Aggregate amount of payments to related parties and their associates included in item 2 — Note: if any amounts are shown in items 6.1 or 6.2, your quarterly activity report must include a description of, and an explanation for, such payments. Fees and consulting payments to Non-Executive Directors and salary payments to full-time Executive Directors (for the current quarter) = US$461,808

Appendix 4C Quarterly cash flow report for entities subject to Listing Rule 4.7B ASX Listing Rules Appendix 4C (17/07/20) + See chapter 19 of the ASX Listing Rules for defined terms. Page 4 7. Financing facilities Note: the term “facility’ includes all forms of financing arrangements available to the entity. Add notes as necessary for an understanding of the sources of finance available to the entity. Total facility amount at quarter end $US’000 Amount drawn at quarter end $US’000 7.1 Loan facilities 80,000* 80,000* 7.2 Credit standby arrangements — — 7.3 Other (please specify) — — 7.4 Total financing facilities 80,000* 80,000* 7.5 Unused financing facilities available at quarter end — 7.6 Include in the box below a description of each facility above, including the lender, interest rate, maturity date and whether it is secured or unsecured. If any additional financing facilities have been entered into or are proposed to be entered into after quarter end, include a note providing details of those facilities as well. *Loan facility with Oaktree Capital Management, Inc. Mesoblast refinanced its senior debt facility on November 19, 2021, with a secured five-year credit facility provided by funds managed by Oaktree Capital Management, L.P. (“Oaktree”). The loan had an initial interest only period of three years, at a fixed rate of 9.75% per annum, after which time the principal balance amortizes 5% per quarter beginning December 2024 and a final payment due no later than November 2026. The facility also allowed the Group to make quarterly payments of interest at a rate of 8.0% per annum for the first two years, and the unpaid interest portion (1.75% per annum) has been added to the outstanding loan balance and currently accrues further interest at a fixed rate of 9.75% per annum. The principal balance at the end of the three-year interest only period was $52.2 million, which amortizes at 5% per quarter beginning December 2024. The outstanding loan balance as of June 30, 2025 is $44.3 million. *Loan facility with NovaQuest Capital Management, L.L.C. On June 29, 2018, Mesoblast entered into a secured eight-year term loan with NovaQuest Capital Management, L.L.C. (“NovaQuest”). Mesoblast drew US$30.0 million on closing. The loan term included an interest only period of approximately four years through until July 8, 2022. All interest and principal payments (i.e. the amortization period) have been deferred until after receipt of the first commercial sale of remestemcel-L in the treatment of pediatric patients with SR-aGVHD. Principal is repayable in equal quarterly instalments over the amortization period of the loan based on a percentage of receipts of net sales and are limited by a payment cap. The loan has a fixed interest rate of 15% per annum. The financing is subordinated to the senior creditor, Oaktree.

Appendix 4C Quarterly cash flow report for entities subject to Listing Rule 4.7B ASX Listing Rules Appendix 4C (17/07/20) + See chapter 19 of the ASX Listing Rules for defined terms. Page 5 8. Estimated cash available for future operating activities $US’000 8.1 Net cash from / (used in) operating activities (item 1.9) (16,616) 8.2 Cash and cash equivalents at quarter end (item 4.6) 161,551 8.3 Unused finance facilities available at quarter end (item 7.5) — 8.4 Total available funding (item 8.2 + item 8.3) 161,551 8.5 Estimated quarters of funding available (item 8.4 divided by item 8.1) 9.7 Note: if the entity has reported positive net operating cash flows in item 1.9, answer item 8.5 as “N/A”. Otherwise, a figure for the estimated quarters of funding available must be included in item 8.5. 8.6 If item 8.5 is less than 2 quarters, please provide answers to the following questions: 8.6.1 Does the entity expect that it will continue to have the current level of net operating cash flows for the time being and, if not, why not? Answer: Not applicable 8.6.2 Has the entity taken any steps, or does it propose to take any steps, to raise further cash to fund its operations and, if so, what are those steps and how likely does it believe that they will be successful? Answer: Not applicable 8.6.3 Does the entity expect to be able to continue its operations and to meet its business objectives and, if so, on what basis? Answer: Not applicable Note: where item 8.5 is less than 2 quarters, all of questions 8.6.1, 8.6.2 and 8.6.3 above must be answered. Compliance statement 1 This statement has been prepared in accordance with accounting standards and policies which comply with Listing Rule 19.11A. 2 This statement gives a true and fair view of the matters disclosed. Date: .......18 July 2025............................................................................. Authorised by: ......Chief Executive............................................................................. (Name of body or officer authorising release – see note 4)

Appendix 4C Quarterly cash flow report for entities subject to Listing Rule 4.7B ASX Listing Rules Appendix 4C (17/07/20) + See chapter 19 of the ASX Listing Rules for defined terms. Page 6 Notes 1. This quarterly cash flow report and the accompanying activity report provide a basis for informing the market about the entity’s activities for the past quarter, how they have been financed and the effect this has had on its cash position. An entity that wishes to disclose additional information over and above the minimum required under the Listing Rules is encouraged to do so. 2. If this quarterly cash flow report has been prepared in accordance with Australian Accounting Standards, the definitions in, and provisions of, AASB 107: Statement of Cash Flows apply to this report. If this quarterly cash flow report has been prepared in accordance with other accounting standards agreed by ASX pursuant to Listing Rule 19.11A, the corresponding equivalent standard applies to this report. 3. Dividends received may be classified either as cash flows from operating activities or cash flows from investing activities, depending on the accounting policy of the entity. 4. If this report has been authorised for release to the market by your board of directors, you can insert here: “By the board”. If it has been authorised for release to the market by a committee of your board of directors, you can insert here: “By the [name of board committee – eg Audit and Risk Committee]”. If it has been authorised for release to the market by a disclosure committee, you can insert here: “By the Disclosure Committee”. 5. If this report has been authorised for release to the market by your board of directors and you wish to hold yourself out as complying with recommendation 4.2 of the ASX Corporate Governance Council’s Corporate Governance Principles and Recommendations, the board should have received a declaration from its CEO and CFO that, in their opinion, the financial records of the entity have been properly maintained, that this report complies with the appropriate accounting standards and gives a true and fair view of the cash flows of the entity, and that their opinion has been formed on the basis of a sound system of risk management and internal control which is operating effectively.








