2023Q3FALSE--12-310001280776P3Yhttp://fasb.org/us-gaap/2023#NonoperatingIncomeExpensehttp://fasb.org/us-gaap/2023#NonoperatingIncomeExpenseP3Y00012807762023-01-012023-09-3000012807762023-10-31xbrli:shares00012807762023-09-30iso4217:USD00012807762022-12-31iso4217:USDxbrli:shares00012807762023-07-012023-09-3000012807762022-07-012022-09-3000012807762022-01-012022-09-300001280776us-gaap:CommonStockMember2022-12-310001280776us-gaap:AdditionalPaidInCapitalMember2022-12-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001280776us-gaap:RetainedEarningsMember2022-12-310001280776us-gaap:RetainedEarningsMember2023-01-012023-03-3100012807762023-01-012023-03-310001280776us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-310001280776us-gaap:CommonStockMember2023-01-012023-03-310001280776us-gaap:CommonStockMember2023-03-310001280776us-gaap:AdditionalPaidInCapitalMember2023-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001280776us-gaap:RetainedEarningsMember2023-03-3100012807762023-03-310001280776us-gaap:RetainedEarningsMember2023-04-012023-06-3000012807762023-04-012023-06-300001280776us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300001280776us-gaap:CommonStockMember2023-04-012023-06-300001280776us-gaap:CommonStockMember2023-06-300001280776us-gaap:AdditionalPaidInCapitalMember2023-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001280776us-gaap:RetainedEarningsMember2023-06-3000012807762023-06-300001280776us-gaap:RetainedEarningsMember2023-07-012023-09-300001280776us-gaap:AdditionalPaidInCapitalMember2023-07-012023-09-300001280776us-gaap:CommonStockMember2023-07-012023-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-07-012023-09-300001280776us-gaap:CommonStockMember2023-09-300001280776us-gaap:AdditionalPaidInCapitalMember2023-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-09-300001280776us-gaap:RetainedEarningsMember2023-09-300001280776us-gaap:CommonStockMember2021-12-310001280776us-gaap:AdditionalPaidInCapitalMember2021-12-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001280776us-gaap:RetainedEarningsMember2021-12-3100012807762021-12-310001280776us-gaap:RetainedEarningsMember2022-01-012022-03-3100012807762022-01-012022-03-310001280776us-gaap:AdditionalPaidInCapitalMember2022-01-012022-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-03-310001280776us-gaap:CommonStockMember2022-01-012022-03-310001280776us-gaap:CommonStockMember2022-03-310001280776us-gaap:AdditionalPaidInCapitalMember2022-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-310001280776us-gaap:RetainedEarningsMember2022-03-3100012807762022-03-310001280776us-gaap:RetainedEarningsMember2022-04-012022-06-3000012807762022-04-012022-06-300001280776us-gaap:AdditionalPaidInCapitalMember2022-04-012022-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012022-06-300001280776us-gaap:CommonStockMember2022-04-012022-06-300001280776us-gaap:CommonStockMember2022-06-300001280776us-gaap:AdditionalPaidInCapitalMember2022-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-300001280776us-gaap:RetainedEarningsMember2022-06-3000012807762022-06-300001280776us-gaap:RetainedEarningsMember2022-07-012022-09-300001280776us-gaap:AdditionalPaidInCapitalMember2022-07-012022-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-07-012022-09-300001280776us-gaap:CommonStockMember2022-09-300001280776us-gaap:AdditionalPaidInCapitalMember2022-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-09-300001280776us-gaap:RetainedEarningsMember2022-09-3000012807762022-09-30vtl:employeevtl:lease0001280776us-gaap:SubsequentEventMember2014-04-172023-10-310001280776country:US2023-09-300001280776country:DE2023-09-300001280776country:AU2023-09-30vtl:financialInstitution0001280776country:USus-gaap:MoneyMarketFundsMember2023-09-30xbrli:pure0001280776vtl:AustraliaAndGermanyMembersrt:MinimumMember2023-09-300001280776srt:MaximumMembervtl:AustraliaAndGermanyMember2023-09-300001280776us-gaap:BankTimeDepositsMember2023-09-300001280776us-gaap:BankTimeDepositsMember2022-12-310001280776srt:MinimumMember2023-09-300001280776srt:MaximumMember2023-09-3000012807762022-10-012022-12-310001280776country:DEsrt:MaximumMember2023-01-012023-09-30iso4217:EUR0001280776vtl:PreFundedWarrantsMember2023-01-310001280776us-gaap:StockOptionMember2023-01-012023-09-300001280776us-gaap:StockOptionMember2022-01-012022-09-300001280776vtl:NewYorkCityMember2023-09-300001280776vtl:GrafelfingGermanyMember2020-04-070001280776vtl:NewYorkCityMember2023-01-012023-09-300001280776vtl:PlaneggGermanyMember2023-02-012023-02-280001280776us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001280776us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001280776us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-3100012807762020-11-3000012807762022-10-310001280776vtl:December2020ATMMember2020-12-310001280776vtl:December2020ATMMember2023-09-30utr:D0001280776vtl:December2020ATMMemberus-gaap:SubsequentEventMember2023-10-310001280776vtl:May2022ATMMember2022-05-100001280776vtl:May2022ATMMember2023-09-300001280776us-gaap:SubsequentEventMembervtl:May2022ATMMember2023-10-310001280776vtl:December2020ATMMember2023-07-012023-09-300001280776vtl:December2020ATMMember2023-01-012023-09-300001280776vtl:December2020ATMMember2022-01-012022-09-300001280776us-gaap:CommonStockMember2022-10-102022-10-100001280776us-gaap:CommonStockMember2022-10-100001280776vtl:PreFundedWarrantsMember2022-10-102022-10-100001280776vtl:PreFundedWarrantsMember2022-10-10vtl:vote0001280776vtl:A2021EmployeeStockPurchasePlanMemberus-gaap:EmployeeStockOptionMember2023-09-300001280776us-gaap:EmployeeStockOptionMember2023-09-300001280776vtl:TwoThousandFourteenEquityIncentivePlanMembervtl:EmployeeStockOptionsforFutureGrantMember2023-09-300001280776vtl:A2017InducementEquityIncentivePlanMembervtl:EmployeeStockOptionsforFutureGrantMember2023-09-300001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMembervtl:EmployeeStockOptionsforFutureGrantMember2023-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2021-04-252021-04-250001280776vtl:A2021EmployeeStockPurchasePlanMember2021-04-250001280776srt:MaximumMembervtl:A2021EmployeeStockPurchasePlanMember2023-09-012023-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2021-08-012021-08-010001280776vtl:A2021EmployeeStockPurchasePlanMember2023-07-012023-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2023-01-012023-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2022-07-012022-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2022-01-012022-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2019-07-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2019-07-012019-07-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2020-01-012023-09-300001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2023-06-282023-06-280001280776vtl:A2019OmnibusEquityIncentivePlanMembersrt:MaximumMember2023-01-012023-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMembervtl:IncentiveEmployeeStockOptionMember2023-01-012023-09-300001280776vtl:NonStatutoryEmployeeStockOptionMembervtl:A2019OmnibusEquityIncentivePlanMembersrt:MinimumMember2023-01-012023-09-300001280776vtl:NonStatutoryEmployeeStockOptionMembervtl:A2019OmnibusEquityIncentivePlanMembersrt:MaximumMember2023-01-012023-09-300001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-12-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2023-01-012023-09-300001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2023-09-300001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2021-12-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-01-012022-09-300001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2023-01-012023-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2022-01-012022-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2023-07-012023-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2022-07-012022-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2023-01-012023-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2022-01-012022-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2023-07-012023-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2022-07-012022-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2023-01-012023-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2022-01-012022-09-300001280776vtl:EmployeeMember2023-07-012023-09-300001280776vtl:EmployeeMember2022-07-012022-09-300001280776vtl:EmployeeMember2023-01-012023-09-300001280776vtl:EmployeeMember2022-01-012022-09-300001280776vtl:TwoThousandFourteenEquityIncentivePlanMember2023-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2017-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2022-01-012022-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2021-07-012021-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2021-01-012021-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2022-07-012022-09-300001280776srt:DirectorMember2023-04-272023-04-270001280776srt:ScenarioForecastMembervtl:DuaneNashMDJDMBAMembersrt:BoardOfDirectorsChairmanMembervtl:ExecutiveChairmanAgreementMembersrt:AffiliatedEntityMember2022-12-282023-12-310001280776srt:ScenarioForecastMembervtl:DuaneNashMDJDMBAMembersrt:BoardOfDirectorsChairmanMembervtl:ExecutiveChairmanAgreementMembersrt:AffiliatedEntityMember2024-01-012024-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
|
|
|
|
|
|
| ☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2023
or
|
|
|
|
|
|
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-36201
Immunic, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
| Delaware |
|
56-2358443 |
| (State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
| 1200 Avenue of the Americas |
|
|
| Suite 200 |
|
|
| New York, |
NY |
10036 |
| (Address of principal executive offices) |
|
(Zip Code) |
(332) 255-9818
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered |
| Common Stock, $0.0001 par value |
IMUX |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
|
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
On October 31, 2023, 45,145,383 shares of common stock, $0.0001 par value, were outstanding.
IMMUNIC, INC.
INDEX
|
|
|
|
|
|
|
|
|
| |
|
Page No. |
|
|
| Item 1. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Item 2. |
|
|
| Item 3. |
|
|
| Item 4. |
|
|
|
|
|
|
|
| Item 1. |
|
|
| Item 1A. |
|
|
| Item 2. |
|
|
| Item 3. |
|
|
| Item 4. |
|
|
| Item 5. |
|
|
| Item 6. |
|
|
IMMUNIC, INC.
Condensed Consolidated Balance Sheets
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
|
(Unaudited) |
|
|
| Assets |
|
|
|
| Current assets: |
|
|
|
| Cash and cash equivalents |
$ |
59,689 |
|
|
$ |
106,745 |
|
| Investments - other |
— |
|
|
9,629 |
|
| Other current assets and prepaid expenses |
5,545 |
|
|
9,490 |
|
| Total current assets |
65,234 |
|
|
125,864 |
|
| Property and equipment, net |
288 |
|
|
294 |
|
| Right-of-use assets, net |
1,412 |
|
|
1,552 |
|
| Other long-term assets |
43 |
|
|
43 |
|
| Total assets |
$ |
66,977 |
|
|
$ |
127,753 |
|
| Liabilities and Stockholders’ Equity |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable |
$ |
3,199 |
|
|
$ |
4,281 |
|
| Accrued expenses |
13,659 |
|
|
7,986 |
|
| Other current liabilities |
923 |
|
|
810 |
|
| Total current liabilities |
17,781 |
|
|
13,077 |
|
| Long term liabilities |
|
|
|
| Operating lease liabilities |
789 |
|
|
992 |
|
| Total long-term liabilities |
789 |
|
|
992 |
|
| Total liabilities |
18,570 |
|
|
14,069 |
|
| Commitments and contingencies (Note 4) |
|
|
|
| Stockholders’ equity: |
|
|
|
Preferred stock, $0.0001 par value; 20,000,000 authorized and no shares issued or outstanding at September 30, 2023 and December 31, 2022 |
— |
|
|
— |
|
Common stock, $0.0001 par value; 130,000,000 shares authorized and 44,595,383 and 39,307,286 shares issued and outstanding as of September 30, 2023 and December 31, 2022, respectively |
4 |
|
|
4 |
|
| Additional paid-in capital |
433,818 |
|
|
427,925 |
|
| Accumulated other comprehensive income |
3,905 |
|
|
3,035 |
|
| Accumulated deficit |
(389,320) |
|
|
(317,280) |
|
| Total stockholders’ equity |
48,407 |
|
|
113,684 |
|
| Total liabilities and stockholders’ equity |
$ |
66,977 |
|
|
$ |
127,753 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Operations
(In thousands, except share and per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Three Months
Ended September 30, |
|
Nine Months
Ended September 30, |
| |
|
|
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
|
|
|
$ |
19,796 |
|
|
$ |
16,537 |
|
|
$ |
63,931 |
|
|
$ |
50,520 |
|
| General and administrative |
|
|
|
|
3,774 |
|
|
3,579 |
|
|
11,911 |
|
|
11,641 |
|
| Total operating expenses |
|
|
|
|
23,570 |
|
|
20,116 |
|
|
75,842 |
|
|
62,161 |
|
| Loss from operations |
|
|
|
|
(23,570) |
|
|
(20,116) |
|
|
(75,842) |
|
|
(62,161) |
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
| Interest income |
|
|
|
|
766 |
|
|
230 |
|
|
2,534 |
|
|
343 |
|
| Other income (expense), net |
|
|
|
|
35 |
|
|
(1,338) |
|
|
1,268 |
|
|
(2,115) |
|
| Total other income (expense) |
|
|
|
|
801 |
|
|
(1,108) |
|
|
3,802 |
|
|
(1,772) |
|
| Net loss |
|
|
|
|
$ |
(22,769) |
|
|
$ |
(21,224) |
|
|
$ |
(72,040) |
|
|
$ |
(63,933) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted |
|
|
|
|
$ |
(0.51) |
|
|
$ |
(0.69) |
|
|
$ |
(1.63) |
|
|
$ |
(2.16) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted-average common shares outstanding, basic and diluted |
|
|
|
|
44,574,377 |
|
|
30,564,995 |
|
|
44,227,264 |
|
|
29,655,946 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Comprehensive Loss
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Three Months
Ended September 30, |
|
Nine Months
Ended September 30, |
| |
|
|
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Net loss |
|
|
|
|
$ |
(22,769) |
|
|
$ |
(21,224) |
|
|
$ |
(72,040) |
|
|
$ |
(63,933) |
|
| Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation |
|
|
|
|
(77) |
|
|
274 |
|
|
870 |
|
|
(134) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive loss |
|
|
|
|
$ |
(22,846) |
|
|
$ |
(20,950) |
|
|
$ |
(71,170) |
|
|
$ |
(64,067) |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Nine Months Ended September 30, 2023 |
| |
Common Stock |
|
Additional
Paid-In
Capital |
|
Accumulated
Other
Comprehensive
Income (Loss) |
|
Accumulated
Deficit |
|
Total
Stockholders’
Equity |
| |
Shares |
|
Amount |
|
| Balance at January 1, 2023 |
39,307,286 |
|
|
$ |
4 |
|
|
$ |
427,925 |
|
|
$ |
3,035 |
|
|
$ |
(317,280) |
|
|
$ |
113,684 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(25,272) |
|
|
(25,272) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
1,979 |
|
|
— |
|
|
— |
|
|
1,979 |
|
| Foreign exchange translation adjustment |
— |
|
|
— |
|
|
— |
|
|
776 |
|
|
— |
|
|
776 |
|
| Shares issued from exercise of pre-funded warrants |
5,096,552 |
|
|
— |
|
|
51 |
|
|
— |
|
|
— |
|
|
51 |
|
| Balance at March 31, 2023 |
44,403,838 |
|
|
$ |
4 |
|
|
$ |
429,955 |
|
|
$ |
3,811 |
|
|
$ |
(342,552) |
|
|
$ |
91,218 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(23,999) |
|
|
(23,999) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
1,798 |
|
|
— |
|
|
— |
|
|
1,798 |
|
| Foreign exchange translation adjustment |
— |
|
|
— |
|
|
— |
|
|
171 |
|
|
— |
|
|
171 |
|
| Shares issued in connection with the Company's Employee stock purchase plan |
84,533 |
|
|
$ |
— |
|
|
96 |
|
|
$ |
— |
|
|
$ |
— |
|
|
96 |
|
| Balance at June 30, 2023 |
44,488,371 |
|
|
$ |
4 |
|
|
$ |
431,849 |
|
|
$ |
3,982 |
|
|
$ |
(366,551) |
|
|
$ |
69,284 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(22,769) |
|
|
(22,769) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
1,687 |
|
|
— |
|
|
— |
|
|
1,687 |
|
Issuance of common stock - at the market Sales Agreement net of issuance costs of $9 |
107,012 |
|
|
— |
|
|
282 |
|
|
— |
|
|
— |
|
|
282 |
|
| Foreign exchange translation adjustment |
— |
|
|
— |
|
|
— |
|
|
(77) |
|
|
— |
|
|
(77) |
|
| Balance at September 30, 2023 |
44,595,383 |
|
|
$ |
4 |
|
|
$ |
433,818 |
|
|
$ |
3,905 |
|
|
$ |
(389,320) |
|
|
48,407 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Nine Months Ended September 30, 2022 |
|
Common Stock |
|
Additional
Paid-In
Capital |
|
Accumulated
Other
Comprehensive
Income (Loss) |
|
Accumulated
Deficit |
|
Total
Stockholders’
Equity |
|
Shares |
|
Amount |
|
| Balance at January 1, 2022 |
26,335,418 |
|
|
3 |
|
|
324,237 |
|
|
(252) |
|
|
(196,873) |
|
|
127,115 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(20,808) |
|
|
(20,808) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
2,069 |
|
|
— |
|
|
— |
|
|
2,069 |
|
| Foreign exchange translation adjustment |
— |
|
|
— |
|
|
— |
|
|
(58) |
|
|
— |
|
|
(58) |
|
| Shares issued in connection with the Company's stock option plan |
852 |
|
|
— |
|
|
5 |
|
|
— |
|
|
— |
|
|
5 |
|
Issuance of common stock - at the market Sales Agreement net of issuance costs of $918 |
2,904,113 |
|
|
— |
|
|
29,638 |
|
|
— |
|
|
— |
|
|
29,638 |
|
| Balance at March 31, 2022 |
29,240,383 |
|
|
3 |
|
|
$ |
355,949 |
|
|
$ |
(310) |
|
|
$ |
(217,681) |
|
|
$ |
137,961 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(21,901) |
|
|
(21,901) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
2,062 |
|
|
— |
|
|
— |
|
|
2,062 |
|
| Foreign exchange translation adjustment |
— |
|
|
— |
|
|
— |
|
|
(350) |
|
|
— |
|
|
(350) |
|
| Shares issued in connection with the Company's Employee stock purchase plan |
24,612 |
|
|
$ |
— |
|
|
130 |
|
|
$ |
— |
|
|
$ |
— |
|
|
130 |
|
Issuance of common stock - at the market Sales Agreement net of issuance costs of $308 |
1,300,000 |
|
|
$ |
— |
|
|
9,946 |
|
|
$ |
— |
|
|
$ |
— |
|
|
9,946 |
|
| Balance at June 30, 2022 |
30,564,995 |
|
|
$ |
3 |
|
|
$ |
368,087 |
|
|
$ |
(660) |
|
|
$ |
(239,582) |
|
|
$ |
127,848 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(21,224) |
|
|
(21,224) |
|
| Stock-based compensation |
— |
|
|
— |
|
|
1,912 |
|
|
— |
|
|
— |
|
|
1,912 |
|
| Foreign exchange translation adjustment |
— |
|
|
— |
|
|
— |
|
|
274 |
|
|
— |
|
|
274 |
|
| Balance at September 30, 2022 |
30,564,995 |
|
|
$ |
3 |
|
|
$ |
369,999 |
|
|
$ |
(386) |
|
|
$ |
(260,806) |
|
|
108,810 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
| |
Nine Months
Ended September 30, |
| |
2023 |
|
2022 |
| Cash flows from operating activities: |
|
|
|
| Net loss |
$ |
(72,040) |
|
|
$ |
(63,933) |
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
| Depreciation and amortization |
88 |
|
|
58 |
|
| Unrealized foreign currency loss |
712 |
|
|
4,217 |
|
|
|
|
|
| Stock-based compensation |
5,464 |
|
|
6,043 |
|
|
|
|
|
| Changes in operating assets and liabilities: |
|
|
|
| Other current assets and prepaid expenses |
3,881 |
|
|
2,061 |
|
| Accounts payable |
(951) |
|
|
1,151 |
|
| Accrued expenses |
5,961 |
|
|
465 |
|
| Other liabilities |
84 |
|
|
(102) |
|
| Net cash used in operating activities |
(56,801) |
|
|
(50,040) |
|
| Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Purchases of property and equipment |
(169) |
|
|
(113) |
|
| Sale of investments - Other |
9,796 |
|
|
— |
|
| Net cash provided by (used in) investing activities |
9,627 |
|
|
(113) |
|
| Cash flows from financing activities: |
|
|
|
|
|
|
|
Proceeds from public offering of common stock through At The Market offering, net of issuance costs of $9 and $1,226, respectively |
282 |
|
|
39,584 |
|
| Proceeds from exercise of stock options |
— |
|
|
5 |
|
| Proceeds from the exercise of pre-funded warrants |
51 |
|
|
— |
|
| Proceeds from shares issued in connection with the Company's employee stock purchase plan |
96 |
|
|
130 |
|
|
|
|
|
| Net cash provided by financing activities |
429 |
|
|
39,719 |
|
| Effect of exchange rate changes on cash and cash equivalents |
(311) |
|
|
(3,658) |
|
| Net change in cash and cash equivalents |
(47,056) |
|
|
(14,092) |
|
| Cash and cash equivalents, beginning of period |
106,745 |
|
|
86,863 |
|
| Cash and cash equivalents, end of period |
$ |
59,689 |
|
|
$ |
72,771 |
|
|
|
|
|
|
|
|
|
| Supplemental disclosure of noncash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
|
| Operating lease right-of use asset obtained in exchange for lease obligation |
$ |
544 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Description of Business and Basis of Financial Statements
Description of Business
Immunic, Inc. ("Immunic" or the "Company") is a biotechnology company developing a clinical pipeline of selective oral immunology therapies focused on treating chronic inflammatory and autoimmune diseases. The Company is headquartered in New York City with its main operations in Gräfelfing near Munich, Germany. The Company currently has approximately 80 employees.
Immunic is pursuing clinical development of orally administered, small molecule programs, each of which has unique features intended to directly address the unmet needs of patients with serious chronic inflammatory and autoimmune diseases. These include the vidofludimus calcium (IMU-838) program, which is in Phase 3 clinical development for patients with multiple sclerosis (“MS”) and which has shown therapeutic activity in Phase 2 clinical trials in patients suffering from relapsing-remitting MS, progressive MS and moderate-to-severe ulcerative colitis (“UC”); the IMU-856 program, which is targeted to regenerate bowel epithelium and restore intestinal barrier function, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, UC, Crohn’s disease or irritable bowel syndrome with diarrhea; and the IMU-381 program, which is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases.
The Company’s business, operating results, financial condition and growth prospects are subject to significant risks and uncertainties, including the failure of its clinical trials to meet their endpoints, failure to obtain regulatory approval and needing additional funding to complete the development and commercialization of the Company's three development programs.
Liquidity and Financial Condition
Immunic has no products approved for commercial sale and has not generated any revenue from product sales. It has never been profitable and has incurred operating losses in each year since inception in 2016. The Company has an accumulated deficit of approximately $389.3 million as of September 30, 2023 and $317.3 million as of December 31, 2022. Substantially all of Immunic's operating losses resulted from expenses incurred in connection with its research and development programs and from general and administrative costs associated with its operations.
Immunic expects to incur significant expenses and increasing operating losses for the foreseeable future as it initiates and continues the development of its product candidates and adds personnel necessary to advance its pipeline of product candidates. Immunic expects that its operating losses will fluctuate significantly from quarter-to-quarter and year-to-year due to timing of development programs.
From inception through October 31, 2023, Immunic has raised net cash of approximately $355.9 million from private and public offerings of preferred and common stock. As of September 30, 2023, the Company had cash and cash equivalents of approximately $59.7 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these financial statements without raising additional capital and such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Basis of Presentation and Consolidation
The accompanying consolidated financial statements have been prepared in conformity with United States generally accepted accounting principles, ("U.S. GAAP") and include the accounts of Immunic and its wholly-owned subsidiaries, Immunic AG and Immunic Australia Pty Ltd. All intercompany accounts and transactions have been eliminated in consolidation. Immunic manages its operations as a single reportable segment for the purposes of assessing performance and making operating decisions.
Unaudited Interim Financial Information
Immunic has prepared the accompanying interim unaudited condensed consolidated financial statements in accordance with United States generally accepted accounting principles, (“US GAAP”), for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the SEC. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These interim unaudited condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals which, in the opinion of management, are necessary to present fairly Immunic’s consolidated financial position, consolidated results of operations, consolidated statement of stockholders’ equity and consolidated cash flows for the periods and as of the dates presented. The Company’s fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2022 was derived from audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. These condensed consolidated financial statements should be read in conjunction with the annual consolidated financial statements and the notes thereto included on the Company's Annual Report on Form 10-K filed on February 23, 2023. The nature of Immunic’s business is such that the results of any interim period may not be indicative of the results to be expected for the entire year.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires the Company to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and the disclosure of contingent assets and liabilities in the Company’s consolidated financial statements. The most significant estimates in the Company’s financial statements and accompanying notes relate to clinical trial expenses and share-based compensation. Management believes its estimates to be reasonable under the circumstances. Actual results could differ materially from those estimates and assumptions.
Foreign Currency Translation and Presentation
The Company’s reporting currency is United States (“U.S.”) dollars. Immunic AG is located in Germany with the euro being its functional currency. Immunic Australia Pty Ltd.’s functional currency is the Australian dollar. All amounts in the financial statements where the functional currency is not the U.S. dollar are translated into U.S. dollar equivalents at exchange rates as follows:
• assets and liabilities at reporting period-end rates;
• income statement accounts at average exchange rates for the reporting period; and
• components of equity at historical rates.
Gains and losses from translation of the financial statements into U.S. dollars are recorded in stockholders’ equity as a component of accumulated other comprehensive income (loss). Realized and unrealized gains and losses resulting from foreign currency transactions denominated in currencies other than the functional currency are reflected as general and administrative expenses in the Consolidated Statements of Operations. Foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future are recorded in Other Income (Expense). The Consolidated Statements of Cash Flows were prepared by using the average exchange rate in effect during the reporting period which reasonably approximates the timing of the cash flows.
Cash and Cash Equivalents and Investments - other
The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. Time Deposits with an original maturity greater than three months are classified as Investments - other.
Cash and cash equivalents and investments - other consist of cash on hand and deposits in banks located in the U.S. of approximately $35.4 million, Germany of approximately $22.4 million and Australia of approximately $1.9 million as of September 30, 2023. The Company maintains cash and cash equivalent balances denominated in Euro and U.S. dollars with major financial institutions in the U.S. and Germany in excess of the deposit limits insured by the government. Management periodically reviews the credit standing of these financial institutions. The Company currently deposits its cash and cash equivalents with two large financial institutions. Cash and Cash equivalents in the U.S. are held at J.P. Morgan and as of September 30, 2023 are primarily held in a U.S. Government money market fund account earning interest at a rate of 5.0%. Cash and cash equivalents in Germany were earning interest at a rate of 2.00% to 3.25% during the period ended September 30, 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
| Time Deposits |
$0 |
|
$9,629 |
|
$0 |
|
$9,629 |
Fair Value Measurement
Fair value is defined as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants on the measurement date. Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value:
Level 1—Quoted prices in active markets for identical assets or liabilities. Level 1 assets consisted of money market funds for the periods presented. The Company had no Level 1 liabilities for the periods presented.
Level 2—Inputs other than observable quoted prices for the asset or liability, either directly or indirectly; these include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. The Company had no Level 2 assets or liabilities for the periods presented.
Level 3—Unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of assets or liabilities. The Company had no Level 3 assets or liabilities for the periods presented.
The carrying value of cash and cash equivalents, other current assets and prepaid expenses, accounts payable, accrued expenses, and other current liabilities approximates fair value due to the short period of time to maturity.
Property and Equipment
Property and equipment is stated at cost. Depreciation is computed using the straight-line method based on the estimated service lives of the assets, which range from three to thirteen years. Depreciation expense was $34,000 and $17,000 for the three months ended September 30, 2023 and 2022, respectively. Depreciation expense was $88,000 and $58,000 for the nine months ended September 30, 2023 and 2022, respectively.
Impairment of Long-Lived Assets
The Company records impairment losses on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. Impaired assets are then recorded at their estimated fair value. There were no impairment losses during the three and nine months ended September 30, 2023 and 2022.
Goodwill
Business combinations are accounted for under the acquisition method. The total purchase price of an acquisition is allocated to the underlying identifiable net assets, based on their respective estimated fair values as of the acquisition date. Determining the fair value of assets acquired and liabilities assumed requires management’s judgment and often involves the use of significant estimates and assumptions, including assumptions with respect to future cash inflows and outflows, probabilities of success, discount rates, and asset lives, among other items. Assets acquired and liabilities assumed are recorded at their estimated fair values. The excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill.
Goodwill is tested for impairment at the reporting unit level annually in the fourth quarter, or more frequently when events or changes in circumstances indicate that the asset might be impaired. Examples of such events or circumstances include, but are not limited to, an unfavorable clinical trial result, a significant adverse change in legal or business climate, industry market conditions, an adverse regulatory action, sustained decrease in stock price or unanticipated competition.
On October 20, 2022, the Company announced the outcome of a significant interim analysis of its Phase 1b clinical trial of izumerogant (IMU-935) in patients with moderate-to-severe psoriasis that were not deemed positive progress. On October 21, 2022, the Company experienced a significant decrease in the Company's market capitalization. The Company considered this to be a triggering event indicating that it is more likely than not that goodwill was impaired.
The Company performed an analysis of the fair value compared to the Company's book value, utilizing the Company's traded stock price (a level 1 fair value input). As a result of that analysis, the Company recorded an approximately $33.0 million non-cash goodwill impairment charge in the fourth quarter of 2022, which represents a full write down of its previous goodwill balance.
Research and Development Expenses
These costs primarily include external development expenses and internal personnel expenses for its development programs, vidofludimus calcium, izumerogant and IMU-856. Immunic has spent the majority of its research and development resources on vidofludimus calcium, the Company's lead development program, for clinical trials in MS and UC.
Research and development expenses consist of expenses incurred in research and development activities, which include clinical trials, contract research services, certain milestone payments, salaries and related employee benefits, allocated facility costs and other outsourced services. Research and development expenses are charged to operations as incurred.
The Company enters into agreements with contract research organizations (“CROs”) to provide clinical trial services for individual studies and projects by executing individual work orders governed by a Master Service Arrangement (“MSA”). The MSAs and associated work orders provide for regular recurrent payments and payments upon the completion of certain milestones. The Company regularly assesses the timing of payments against actual costs incurred to ensure a proper accrual of related expenses in the appropriate accounting period.
Collaboration Arrangements
Certain collaboration and license agreements may include payments to or from the Company of one or more of the following: non-refundable or partially refundable upfront or license fees; development, regulatory and commercial milestone payments; payment for manufacturing supply services; partial or complete reimbursement of research and development costs; and royalties on net sales of licensed products. The Company assesses whether such contracts are within the scope of Financial Accounting Standards Board (FASB) Accounting Standards Update (“ASU”) 2014-09 “Revenue from Contracts with Customers” and ASU No. 2018-18, “Collaborative Arrangements” ("ASU 2018-18"). ASU 2018-18, clarifies that certain elements of collaborative arrangements could qualify as transactions with customers in the scope of ASC 606.
In October 2018, the Company entered into an option and license agreement (the "Daiichi Sankyo Agreement") with Daiichi Sankyo Co., Ltd. ("Daiichi Sankyo") which granted the Company the right to license a group of compounds, designated by the Company as IMU-856, as a potential new oral treatment option for gastrointestinal diseases such as celiac disease, inflammatory bowel disease, irritable bowel syndrome with diarrhea and other barrier function associated diseases. During the option period, the Company performed agreed upon research and development activities for which it was reimbursed by Daiichi Sankyo up to a maximum agreed-upon limit. Such reimbursement was recorded as other income. There are no additional research and development reimbursements expected under this agreement.
On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856.
Government assistance
Government assistance relating to research and development performed by Immunic Australia is recorded as a component of other (income) expense. This government assistance is recognized at a rate of 43.5% of the qualified research and development expenditures which are incurred. We also receive government assistance from the German Government for reimbursement of research and development expenses up to one million Euros per year. We recognized $0.2 million and $2.3 million of other income related to research activities performed during the three and nine months ended September 30, 2023, respectively and $0.6 million and $2.0 million related to research activities performed during the three and nine months ended September 30, 2022, respectively.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel in executive, finance, business development and other support functions. Other general and administrative expenses include, but are not limited to, stock-based compensation, insurance costs, professional fees for legal, accounting and tax services, consulting, related facility costs and travel.
Stock-Based Compensation
The Company measures the cost of employee and non-employee services received in exchange for equity awards based on the grant-date fair value of the award recognized generally as an expense (i) on a straight-line basis over the requisite service period for those awards whose vesting is based upon a service condition, and (ii) on an accelerated method for awards whose vesting is based upon a performance condition, but only to the extent it is probable that the performance condition will be met. Stock-based compensation is (i) estimated at the date of grant based on the award’s fair value for equity classified awards and (ii) final measurement date for liability classified awards. Forfeitures are recorded in the period in which they occur.
The Company estimates the fair value of stock options using the Black-Scholes-Merton option-pricing model ("BSM"), which requires the use of estimates and subjective assumptions, including the risk-free interest rate, the fair value of the underlying common stock, the expected dividend yield of the Company’s common stock, the expected volatility of the price of the Company’s common stock, and the expected term of the option. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, the Company’s stock-based compensation expense could be materially different in the future.
Leases
The Company leases office space and office equipment. The underlying lease agreements have lease terms of less than 12 months and up to 60 months. Leases with terms of 12 months or less at inception are not included in the operating lease right of use asset and operating lease liability.
The Company has three existing leases for office and laboratory space. At inception of a lease agreement, the Company determines whether an agreement represents a lease and at commencement each lease agreement is assessed as to classification as an operating or financing lease. The Company's leases have been classified as operating leases and an operating lease right-of-use asset and an operating lease liability have been recorded on the Company’s balance sheet. A right-of-use lease asset represents the Company’s right to use the underlying asset for the lease term and the lease obligation represents its commitment to make the lease payments arising from the lease. Right-of-use lease assets and obligations are recognized at the commencement date based on the present value of remaining lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company has used an estimated incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The right-of-use lease asset includes any lease payments made prior to commencement and excludes any lease incentives. The lease term used in estimating future lease payments may include options to extend when it is reasonably certain that the Company will exercise that option. Operating lease expense is recognized on a straight-line basis over the lease term, subject to any changes in the lease or changes in expectations regarding the lease term. Variable lease costs such as common area costs and property taxes are expensed as incurred. Leases with an initial term of twelve months or less are not recorded on the balance sheet.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income (loss) has been reflected as a separate component of stockholders’ equity in the accompanying Consolidated Balance Sheets and consists of foreign currency translation adjustments (net of tax).
Income Taxes
The Company is subject to corporate income tax laws and regulations in the U.S., Germany and Australia. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment in their application.
The Company utilizes the asset and liability method of accounting for income taxes which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the audited consolidated financial statements. Deferred income tax assets and liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of changes in tax rates on deferred tax assets and liabilities is recognized in operations in the period that includes the enactment date. Deferred taxes are reduced by a valuation allowance when, in the opinion of management, it is more likely than not some portion or the entire deferred tax asset will not be realized. As of September 30, 2023 and 2022, respectively, the Company maintained a full valuation allowance against the balance of deferred tax assets.
It is the Company’s policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. The Company recognizes interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. The Company is subject to U.S. federal, New York, California, Texas, German and Australian income taxes. The Company is subject to U.S. federal or state income tax examination by tax authorities for tax returns filed for the years 2003 and forward due to the carryforward of NOLs. Tax years 2016 through 2022 are subject to audit by German and Australian tax authorities. The Company is not currently under examination by any tax jurisdictions.
Warrants
The Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity (“ASC 480-10”) or ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock (“ASC 815-40”). Under ASC 480-10, warrants are considered a liability if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable U.S. GAAP standard. Equity-classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date. All of the Company's 5,096,552 pre-funded warrants were exercised in January 2023.
Net Loss Per Share
Basic net loss per share attributable to common stockholders is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share attributable to common stockholders is computed by dividing the net loss by the weighted-average number of common shares and, if dilutive, common stock equivalents outstanding for the period determined using the treasury-stock method. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding due to the Company’s net loss position.
Potentially dilutive securities, not included in the calculation of diluted net loss per share attributable to common stockholders because to do so would be anti-dilutive, are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
As of September 30, |
|
2023 |
|
2022 |
| Options to purchase common stock |
6,263,910 |
|
|
3,799,573 |
|
|
|
|
|
Recently Issued and/or Adopted Accounting Standards
There are no recently issued accounting standards that would have a significant impact on the company's consolidated financial statements.
3. Balance Sheet Details
Other Current Assets and Prepaid Expenses
Other Current Assets and Prepaid Expenses consist of (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
| Prepaid clinical and related costs |
$ |
2,304 |
|
|
$ |
5,608 |
|
| VAT receivable |
1,187 |
|
|
296 |
|
| Australian research and development tax incentive |
607 |
|
|
2,361 |
|
| Other |
1,447 |
|
|
1,225 |
|
| Total |
$ |
5,545 |
|
|
$ |
9,490 |
|
Accounts Payable
Accounts Payable consist of (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
| Clinical costs |
$ |
2,846 |
|
|
$ |
3,749 |
|
| Legal and audit costs |
71 |
|
|
288 |
|
|
|
|
|
| Other |
282 |
|
|
244 |
|
| Total |
$ |
3,199 |
|
|
$ |
4,281 |
|
Accrued Expenses
Accrued expenses consist of (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
| Accrued clinical and related costs |
$ |
12,415 |
|
|
$ |
6,807 |
|
| Accrued legal and audit costs |
78 |
|
|
169 |
|
| Accrued compensation |
1,049 |
|
|
890 |
|
| Accrued other |
117 |
|
|
120 |
|
| Total |
$ |
13,659 |
|
|
$ |
7,986 |
|
Other Current Liabilities
Other Current Liabilities consist of (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
|
| Lease liabilities |
$ |
662 |
|
|
$ |
571 |
|
|
| Other |
261 |
|
|
239 |
|
|
| Total |
923 |
|
|
810 |
|
|
4. Commitments and Contingencies
Operating Leases
The Company leases certain office space under non-cancelable operating leases. The leases terminate on July 31, 2025 for the New York City office, June 30, 2025 for the Gräfelfing, Germany office and November 30, 2028 related to the new lease of a research laboratory in Planegg, Germany. These agreements include both lease (e.g., fixed rent) and non-lease components (e.g., common-area and other maintenance costs). The non-lease components are deemed to be executory costs and are therefore excluded from the minimum lease payments used to determine the present value of the operating lease obligation and related right-of-use asset. The New York City lease was extended on December 22, 2022 for an additional 27 months resulting in the new lease termination date of July 31, 2025. The New York City lease has a renewal option, but this was not included in calculating the right of use asset and liabilities. On April 7, 2020, the Company signed a five year lease for its facility in Gräfelfing, Germany. On March 1, 2021 and August 1, 2022 the Company added additional lease space at the Gräfelfing, Germany office. Renewal options were not included in calculating the right of use asset and liabilities for this facility. In February 2023, the Company leased space in Germany for a research laboratory. The leases do not have concessions, leasehold improvement incentives or other build-out clauses. Further, the leases do not contain contingent rent provisions. The New York City lease had a six month rent holiday at the beginning of the lease as well as a three month rent holiday upon the 27 month extension starting May 2023. There were net additions of $544,000 related to the addition of new laboratory space in Planegg, Germany in February 2023.
The leases do not provide an implicit rate and, due to the lack of a commercially salable product, the Company is generally considered unable to obtain commercial credit. Therefore, the Company estimated its incremental interest rate to be 6% for the original leases and 8% for the New York City extension and German laboratory, considering the quoted rates for the lowest investment-grade debt and the interest rates implicit in recent financing leases. Immunic used its estimated incremental borrowing rate and other information available at the lease commencement date in determining the present value of the lease payments.
Immunic’s operating lease costs and variable lease costs were $209,000 and $232,000 for the three months ended September 30, 2023 and 2022, respectively and $642,000 and $498,000 for the nine months ended September 30, 2023 and 2022, respectively. Variable lease costs consist primarily of common area maintenance costs, insurance and taxes which are paid based upon actual costs incurred by the lessor.
Maturities of the operating lease obligation are as follows as of September 30, 2023 (in thousands):
|
|
|
|
|
|
|
|
|
| 2023 |
$ |
189 |
|
| 2024 |
|
758 |
|
| 2025 |
|
433 |
|
| 2026 |
|
78 |
|
| 2027 |
|
82 |
|
| Thereafter |
|
55 |
|
| Total |
|
1,595 |
|
| Interest |
|
144 |
|
| PV of obligation |
$ |
1,451 |
|
Contractual Obligations
As of September 30, 2023, the Company has non-cancelable contractual obligations under certain agreements related to its development programs for vidofludimus calcium and IMU-856 totaling approximately $5.1 million, all of which is expected to be paid in the next twelve months.
Other Commitments and Obligations
Daiichi Sankyo Agreement
On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856.
Legal Proceedings
The Company is not currently a party to any litigation, nor is it aware of any pending or threatened litigation, that it believes would materially affect its business, operating results, financial condition or cash flows. However, its industry is characterized by frequent claims and litigation including securities litigation, claims regarding patent and other intellectual property rights and claims for product liability. As a result, in the future, the Company may be involved in various legal proceedings from time to time.
5. Fair Value
The following fair value hierarchy tables present information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Fair Value Measurement at September 30, 2023 |
| |
Fair Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
| Assets |
|
|
|
|
|
|
|
| Money market funds |
$ |
35,031 |
|
|
$ |
35,031 |
|
|
$ |
— |
|
|
$ |
— |
|
| Total assets at fair value |
$ |
35,031 |
|
|
$ |
35,031 |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurement at December 31, 2022 |
|
Fair Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
| Assets |
|
|
|
|
|
|
|
| Money market funds |
$ |
85,521 |
|
|
$ |
85,521 |
|
|
$ |
— |
|
|
$ |
— |
|
| Total assets |
$ |
85,521 |
|
|
$ |
85,521 |
|
|
$ |
— |
|
|
$ |
— |
|
There were no transfers between Level 1, Level 2 or Level 3 assets during the periods presented.
For the Company’s money market funds which are included as a component of cash and cash equivalents on the consolidated balance sheet, realized gains and losses are included in interest income (expense) on the consolidated statements of operations.
Our money market fund account is held in our bank in the U.S. and was earning interest at a rate of 5.0% in a U.S. Government money market fund.
The Company has cash balances in banks in excess of the maximum amount insured by the FDIC and other international agencies as of September 30, 2023. The Company has not historically experienced any credit losses with balances in excess of FDIC limits.
The carrying amounts of other current assets and prepaid expenses, accounts payable, accrued expenses, and other current liabilities approximate their fair values due to their short-term nature. The fair value and book value of the money market funds presented in the table above are the same.
6. Common Stock
In November 2020, Immunic filed a shelf registration statement on Form S-3. The 2020 Shelf Registration Statement permits the offering, issuance and sale of up to $250.0 million of common stock, preferred stock, warrants, debt securities, and/or units in one or more offerings and in any combination of the foregoing. As of October 31, 2023, there is $75.0 million remaining on this shelf registration statement. This 2020 Shelf Registration Statement will expire on November 24, 2023. The Company plans to file prior to that expiration date, a new shelf registration statement to replace the expiring Form S-3, which would permit the Company to: (i) continue to sell, subject to applicable SEC requirements, unsold securities remaining on the expiring Form S-3; and (ii) offer and sell additional securities to be registered on the new Form S-3.
In December 2020, the Company filed a Prospectus Supplement for the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of common stock that may be issued and sold under an at-the-market sales agreement ("December 2020 ATM") with SVB Leerink LLC (now Leerink Partners LLC) as agent. The Company has used, and intends to continue to use the net proceeds from the offering to continue to fund the ongoing clinical development of its product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The December 2020 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the December 2020 ATM or (ii) termination of the December 2020 ATM as otherwise permitted thereby. The December 2020 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Prtners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on the Company. As of October 31, 2023, $8.1 million in capacity remains under the December 2020 ATM.
In May 2022, the Company filed a Prospectus Supplement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock that may be issued and sold under another at-the-market sales agreement ("May 2022 ATM") with SVB Leerink LLC (now Leerink Partners LLC) as agent. The Company intends to use the net proceeds from the offering to continue to fund the ongoing clinical development of its product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The May 2022 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the May 2022 ATM or (ii) termination of the May 2022 ATM as otherwise permitted thereby. The May 2022 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Partners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on the Company. As of October 31, 2023, $80.0 million in capacity remains under the May 2022 ATM.
The Company has agreed to pay Leerink Partners LLC a commission equal to 3.0% of the gross proceeds from the sales of common shares pursuant to both ATM's and has agreed to provide Leerink Partners LLC with customary indemnification and contribution rights.
In the three and nine months ended September 30, 2023, the Company raised gross proceeds of $0.3 million pursuant to the December 2020 ATM through the sale of 107,012 shares of common stock at a weighted average price of $2.72 per share. The net proceeds from the December 2020 ATM were $0.3 million after deducting underwriter commissions of $9,000.
In the three months ended September 30, 2022, the Company did not raise any proceeds under its ATM facilities. In the nine months ended September 30, 2022, the Company raised gross proceeds of $40.9 million pursuant to the December 2020 ATM through the sale of 4,204,113 shares of common stock at a weighted average price of $9.72 per share. The net proceeds from the December 2020 ATM were $39.6 million after deducting underwriter commissions of $1.2 million.
Equity Offerings
$60 Million Private Placement Equity Financing
On October 10, 2022, Immunic entered into a Securities Purchase Agreement (the “Purchase Agreement”) for a private placement (the “Private Placement”) with select accredited investors and certain existing investors (each, a “Purchaser” and collectively, the “Purchasers”). Pursuant to the Purchase Agreement, the Company agreed to sell to the Purchasers (i) 8,696,552 shares of the Company’s common stock, par value $0.0001 per share (the “Shares”), at a purchase price of $4.35 per Share, and (ii) 5,096,552 pre-funded warrants (the “Pre-Funded Warrants”) to purchase Common Stock (the “Warrant Shares” and together with the Shares and the Pre-Funded Warrants, the “Securities”), at a purchase price of $4.34 per Pre-Funded Warrant. The Pre-Funded Warrants are classified as a component of permanent equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the shares of common stock with which they were issued, are immediately exercisable, do not embody an obligation for the Company to repurchase its shares, and permit the holders to receive a fixed number of shares of common stock upon exercise.
In addition, the Pre-Funded Warrants do not provide any guarantee of value or return. All of the pre-funded warrants were exercised in January of 2023.
Common Stock
As of September 30, 2023, the Company’s certificate of incorporation, as amended and restated, authorized the Company to issue 130,000,000 shares of common stock, par value of $0.0001 per share. The voting, dividend and liquidation rights of the holders of the Company’s common stock are subject to and qualified by the rights, powers and preferences of any holders of preferred stock.
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are entitled to receive dividends, as may be declared by the Board of Directors, if any. Through September 30, 2023, no cash dividends had been declared or paid.
Preferred Stock
The Company’s certificate of incorporation, as amended and restated, authorizes the Company to issue 20 million shares of $0.0001 par value preferred stock, having rights and preferences to be set by the Board of Directors. No preferred shares were outstanding as of September 30, 2023.
Stock Reserved for Future Issuance
Shares reserved for future issuance at September 30, 2023 are as follows:
|
|
|
|
|
|
| |
Number of
Shares |
| Common stock reserved for issuance for: |
|
| 2021 Employee stock Purchase Plan |
32,358 |
|
| Outstanding stock options |
6,263,910 |
|
| Common stock options available for future grant: |
|
| 2014 Equity Incentive Plan |
43,311 |
|
| 2017 Inducement Equity Incentive Plan |
46,250 |
|
| 2019 Omnibus Equity Incentive Plan |
4,085,919 |
|
| Total common shares reserved for future issuance |
10,471,748 |
|
7. Stock-Based Compensation Plans
2021 Employee Stock Purchase Plan
On April 25, 2021, the Company adopted the 2021 Employee Stock Purchase Plan ("ESPP"), which was approved by stockholder vote at the 2021 Annual Meeting of Stockholders held on June 10, 2021. The ESPP provides eligible employees of the Company with an opportunity to purchase common stock of the Company through accumulated payroll deductions, which are included in other current liabilities until they are used to purchase Company shares. Eligible employees participating in the bi-annual offering period can choose to have up to the lesser of 15% of their annual base earnings or the IRS annual share purchase limit of $25,000 in aggregate market value to purchase shares of the Company’s common stock. The purchase price of the stock is the lesser of (i) 85% of the closing market price on the date of purchase and (ii) the closing market price at the beginning of the bi-annual offering period. The maximum number of shares reserved for delivery under the plan is 200,000 shares. This maximum number was increased by 1 million shares in September 2023, subject to approval by stockholders of the Company at the Company's Annual Stockholders meeting to be held in 2024.
The first enrollment period under the plan commenced on August 1, 2021 and the Company has issued 167,642 shares life-to-date under the ESPP. The Company recognized $19,000 and $102,000 of expense related to the plan during the three and nine months ended September 30, 2023, respectively. The Company recognized $21,000 and $74,000 of expense related to the plan during the three and nine months ended September 30, 2022, respectively.
Stock Option Programs
In July 2019, the Company’s stockholders approved the 2019 Omnibus Equity Incentive Plan (the “2019 Plan”) which was adopted by the Board of Directors (the "Board") with an effective date of June 14, 2019. The 2019 Plan allows for the grant of equity awards to employees, consultants and non-employee directors. An initial maximum of 1,500,000 shares of the Company’s common stock were available for grant under the 2019 Plan. The 2019 Plan included an evergreen provision that allowed for the annual addition of up to 4% of the Company’s fully-diluted outstanding stock, with a maximum allowable increase of 4,900,000 shares over the term of the 2019 Plan. In accordance with this provision, the shares available for grant were increased in 2020 through 2023 by a total of 4,408,871 shares. At the Company's Annual Stockholders meeting on June 28, 2023, stockholders voted to increase the allowable shares under the 2019 plan by 4,440,000 shares as well as to eliminate the evergreen provision. The 2019 Plan is currently administered by the Board, or, at the discretion of the Board, by a committee of the Board, which determines the exercise prices, vesting schedules and other restrictions of awards under the 2019 Plan at its discretion. Options to purchase stock may not have an exercise price that is less than the fair market value of underlying shares on the date of grant, and may not have a term greater than ten years. Incentive stock options granted to employees typically vest over four years. Non-statutory options granted to employees, officers, members of the Board, advisors, and consultants of the Company typically vest over three or four years.
Shares that are expired, terminated, surrendered or canceled under the 2019 Plan without having been fully exercised will be available for future awards.
Movements during the year
The following table summarizes stock option activity for the nine months ended September 30, 2023 and 2022, respectively, for the 2019 Plan:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options |
|
Weighted-
Average
Exercise
Price |
|
Weighted-
Average
Remaining
Contractual
Term (Years) |
|
Aggregate
Intrinsic
Value |
| Outstanding as of January 1, 2023 |
3,791,688 |
|
|
$ |
11.33 |
|
|
|
|
|
| Granted |
2,740,564 |
|
|
$ |
1.71 |
|
|
|
|
|
| Exercised |
— |
|
|
$ |
— |
|
|
— |
|
|
|
| Forfeited or expired |
(268,342) |
|
|
$ |
7.80 |
|
|
|
|
|
| Outstanding as of September 30, 2023 |
6,263,910 |
|
|
$ |
7.27 |
|
|
8.27 |
|
$ |
119,691 |
|
| Options vested and expected to vest as of September 30, 2023 |
6,263,910 |
|
|
$ |
7.27 |
|
|
8.27 |
|
$ |
119,691 |
|
| Options exercisable as of September 30, 2023 |
2,393,294 |
|
|
$ |
11.67 |
|
|
7.12 |
|
$ |
6,160 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options |
|
Weighted-
Average
Exercise
Price |
|
Weighted-
Average
Remaining
Contractual
Term (Years) |
|
Aggregate
Intrinsic
Value |
| Outstanding as of January 1, 2022 |
2,157,460 |
|
|
$ |
13.54 |
|
|
|
|
|
| Granted |
1,837,513 |
|
|
$ |
8.90 |
|
|
|
|
|
| Exercised |
(852) |
|
|
$ |
5.67 |
|
|
|
|
|
| Forfeited or expired |
(194,548) |
|
|
$ |
11.62 |
|
|
|
|
|
| Outstanding as of September 30, 2022 |
3,799,573 |
|
|
$ |
11.40 |
|
|
8.59 |
|
$ |
2,400 |
|
| Options vested and expected to vest as of September 30, 2022 |
3,799,573 |
|
|
$ |
11.40 |
|
|
8.59 |
|
$ |
2,400 |
|
| Options exercisable as of September 30, 2022 |
1,248,875 |
|
|
$ |
13.63 |
|
|
7.64 |
|
$ |
— |
|
Measurement
The weighted-average assumptions used in the BSM option pricing model to determine the fair value of the employee and non-employee stock option grants relating to the 2019 Plan were as follows:
Risk-Free Interest Rate
The risk-free rate assumption is based on U.S. Treasury instruments with maturities similar to the expected term of the stock options.
Expected Dividend Yield
The Company has not issued any dividends and does not expect to issue dividends over the life of the options. As a result, the Company has estimated the dividend yield to be zero.
Expected Volatility
Due to the Company’s limited operating history and a lack of company specific historical and implied volatility data, the Company estimates expected volatility based on the historical volatility of its own stock combined with a group of comparable companies that are publicly traded. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards.
Expected Term
The expected term of options is estimated considering the vesting period at the grant date, the life of the option and the average length of time similar grants have remained outstanding in the past.
The weighted-average grant date fair value of stock options granted under the 2019 Plan during the nine months ended September 30, 2023 and 2022 was $1.32 and $6.94, respectively. The following are the underlying assumptions used in the Black-Scholes-Merton option pricing model to determine the fair value of stock options granted to employees and to non-employees under this stock plan:
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
2023 |
2022 |
| Risk-free interest rate |
4.00% |
2.04% |
| Expected dividend yield |
0% |
0% |
| Expected volatility |
96.0% |
97.8% |
| Expected term of options (years) |
6.01 |
6.00 |
Stock-Based Compensation Expense
Total stock-based compensation expense for all stock awards recognized in the accompanying unaudited condensed consolidated statements of operations is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Three Months
Ended September 30, |
|
Nine Months
Ended September 30, |
| |
|
|
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Research and development |
|
|
|
|
$ |
815,000 |
|
|
$ |
831,000 |
|
|
$ |
2,607,000 |
|
|
$ |
2,392,000 |
|
| General and administrative |
|
|
|
|
872,000 |
|
|
1,081,000 |
|
|
2,857,000 |
|
|
3,651,000 |
|
| Total |
|
|
|
|
$ |
1,687,000 |
|
|
$ |
1,912,000 |
|
|
$ |
5,464,000 |
|
|
$ |
6,043,000 |
|
As of September 30, 2023, there was $12.7 million in total unrecognized compensation expense relating to the 2019 Plan to be recognized over a weighted average period of 2.90 years.
Summary of Equity Incentive Plans Assumed from Vital
Upon completion of the Transaction with Vital Therapies ("Vital") on April 12, 2019, Vital’s 2012 Stock Option Plan (the “2012 Plan”), Vital’s 2014 Equity Incentive Plan (the “2014 Plan”) and Vital’s 2017 Inducement Equity Incentive Plan (the “Inducement Plan”), were assumed by the Company. All awards granted under these plans have either been forfeited or expired.
There remain 43,311 shares available for grant under the 2014 Plan as of September 30, 2023.
On September 2017, Vital’s board of directors approved the Inducement Plan, which was amended and restated in November 2017. Under the Inducement Plan 46,250 shares of Vital’s common stock were reserved to be used exclusively for non-qualified grants to individuals who were not previously employees or directors as an inducement material to a grantee's entry into employment within the meaning of Rule 5635(c)(4) of the Nasdaq Listing Rules.
No expense was recorded for the plans assumed from Vital during the three and nine months ended September 30, 2023 and 2022, respectively.
8. Changes in Board of Directors
Appointment of Richard Rudick, M.D. to Board of Directors
On April 27, 2023, the Company announced the appointment of Dr. Richard Rudick as a member of the Board of Directors, effective as of April 26, 2023. As a Class III director, Dr. Rudick’s initial term lasted until the 2023 Annual Meeting of Stockholders held on June 28, 2023, at which meeting he was elected to a three year term expiring at the 2026 Annual Meeting of Stockholders.
Dr. Richard Rudick, age 72, has over 35 years of experience in the biopharmaceutical industry and academic medicine. Since January 2023, Dr. Rudick has been the President and CEO of Astoria Biologic, a private biotechnology company developing novel therapies for MS. Previously, Dr. Rudick served as the Vice President of Development Science at Biogen, Inc., a biotechnology company which engages in discovering, developing, and delivering therapies for neurological and neurodegenerative diseases, from May 2014 until September 2020. Dr. Rudick also served as a staff neurologist and director of the Mellen Center for the Cleveland Clinic from January 1987 until May 2014. Dr. Rudick holds an M.D. from Case Western Reserve University School of Medicine. The Nominating and Corporate Governance Committee and the Board believe that Dr. Rudick‘s extensive leadership in clinical research and development of MS treatments provides valuable clinical, strategy and management skills to the Board.
Director Resignation
Dr. Vincent Ossipow retired from the Board of Directors on June 28, 2023. Dr. Ossipow’s decision not to stand for re-election was not the result of any disagreement with the Company or its management on any matter relating to the Company’s operations, policies or practices.
9. Related Party Transactions
Executive Chairman Agreement with Duane Nash
On April 15, 2020, the compensation committee of the Board of Directors of the Company independently reviewed and approved entering into an employment agreement with the Executive Chairman of the Board, Duane Nash, MD, JD, MBA (the “Executive Chairman Agreement”) and pursuant to such approval, on April 17, 2020, the Company and Dr. Nash entered into the Executive Chairman Agreement. The Executive Chairman Agreement establishes an “at will” employment relationship. On December 28, 2022, the Company and Dr. Nash entered into Addendum No. Four, which extended the term of employment from December 31, 2022 to December 31, 2023 with a base salary of $30,250 per month. On October 17, 2023, Immunic, Inc. and Dr. Duane Nash entered into Addendum Number 5 to the Employment Agreement dated April 17, 2020, as amended as of October 15, 2020, April 15, 2021, March 15, 2022, and December 28, 2022, to extend the term of Dr. Nash’s employment as Executive Chairman of the Board of Directors of the Company to December 31, 2024. In connection with the Addendum, the Company increased Dr. Nash’s monthly base salary to $32,368 from $30,250 (which includes the cash retainer payable for serving on the Company’s Board or for acting as the Chairman of the Board). All other terms of the Executive Chairman Agreement remain the same.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of financial condition and results of operations should be read in conjunction with our unaudited interim condensed consolidated financial statements and notes thereto included in Item 1 “Financial Statements” in this Quarterly Report and audited Consolidated Financial Statements for the years ended December 31, 2022 and 2021 of Immunic, Inc. filed with the Securities and Exchange Commission ("SEC"), in our Annual Report on Form 10-K on February 23, 2023. As used in this report, unless the context suggests otherwise, “we,” “us,” “our,” “the Company” or “Immunic” refer to Immunic, Inc. and its subsidiaries.
Forward-Looking Statements
In addition to historical information, this Quarterly Report includes forward-looking statements within the meaning of federal securities laws. Forward-looking statements are subject to certain risks and uncertainties, many of which are beyond our control. Such statements include, but are not limited to, statements preceded by, followed by or that otherwise include the words, “believe,” “may,” “might,” “can,” “could,” “will,” “would,” “should,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “project,” “expect,” “potential,” “predicts,” or similar expressions and the negatives of those terms.
Forward-looking statements discuss matters that are not historical facts. Our forward-looking statements involve assumptions that, if they ever materialize or prove correct, could cause our results to differ materially from those expressed or implied by such forward-looking statements. In this Quarterly Report, for example, we make forward-looking statements, among others, regarding potential strategic options; financial estimates and projections; and the sufficiency of our capital resources to fund our operations.
The inclusion of any forward-looking statements in this Quarterly Report should not be regarded as a representation that any of our plans will be achieved. Our actual results may differ from those anticipated in our forward-looking statements as a result of various factors, including those noted below under the caption “Part II, Item 1A-Risk Factors” and in the section headed “Risk Factors” in our Annual Report on Form 10-K filed with the SEC on February 23, 2023, and the differences may be material. These risk factors include, but are not limited to statements relating to our three development programs and the targeted diseases; the potential for vidofludimus calcium, IMU-856 and IMU-381 to safely and effectively target diseases; preclinical and clinical data for the Company’s development programs; the timing of current and future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the Company; expectations regarding our capitalization and financial resources; the development and commercial potential of any product candidates of the Company; the Company’s expected cash runway; and our ability to retain certain personnel important to our ongoing operations and to maintain effective internal control over financial reporting.
Although our forward-looking statements reflect the good faith judgment of our management, these statements are based only on facts and factors currently known by us. As a result, investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update such statements to reflect events or circumstances after the date hereof, except as required by law.
Overview
Immunic, Inc. ("Immunic," “we,” “us,” “our” or the "Company") is a biotechnology company developing a clinical pipeline of selective oral immunology therapies focused on treating chronic inflammatory and autoimmune diseases. We are headquartered in New York City with our main operations in Gräfelfing near Munich, Germany. We currently have approximately 80 employees.
We are pursuing clinical development of orally administered, small molecule programs, each of which has unique features intended to directly address the unmet needs of patients with serious chronic inflammatory and autoimmune diseases. These include the vidofludimus calcium (IMU-838) program, which is in Phase 3 clinical development for patients with multiple sclerosis (“MS”) and which has shown therapeutic activity in Phase 2 clinical trials in patients suffering from relapsing-remitting MS, progressive MS and moderate-to-severe ulcerative colitis (“UC”); the IMU-856 program, which is targeted to regenerate bowel epithelium and restore intestinal barrier function, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, UC, Crohn’s disease or irritable bowel syndrome with diarrhea; and the IMU-381 program, which is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases.
The following table summarizes the potential indications, clinical targets and clinical development status of our three product candidates:
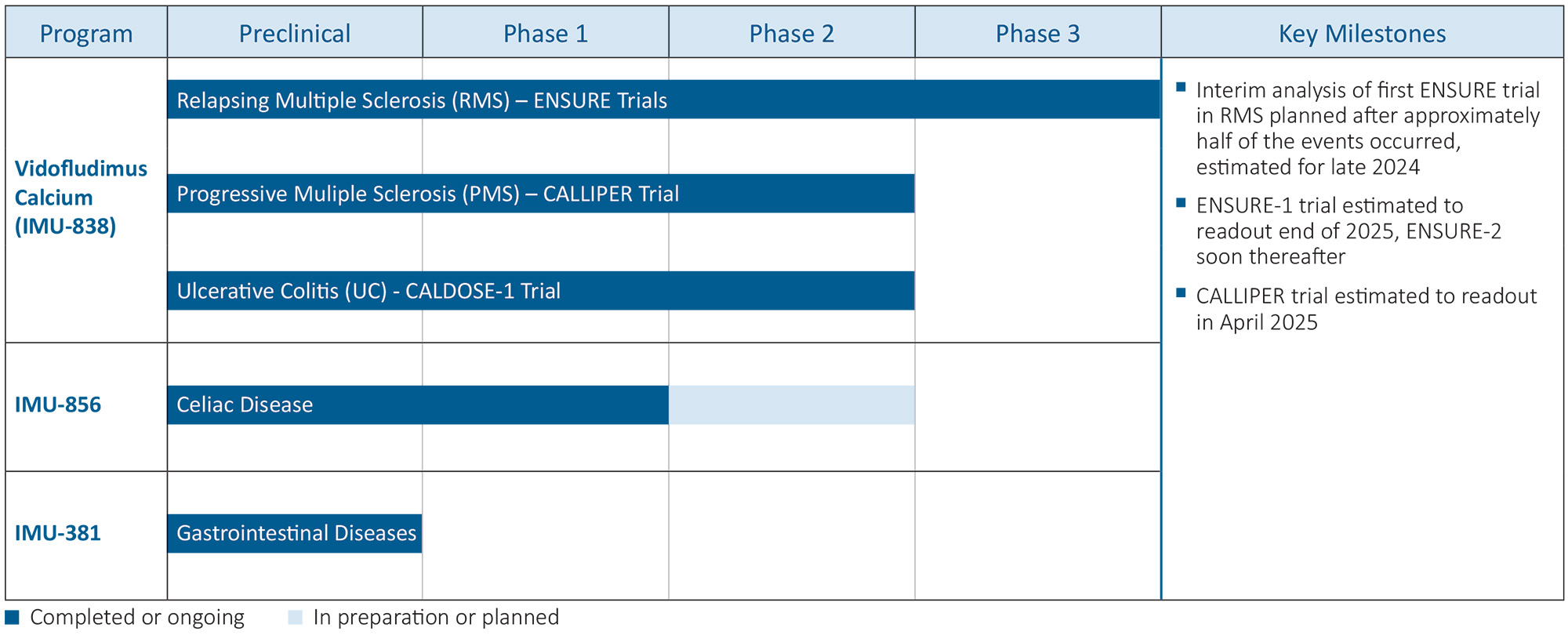
Our most advanced drug candidate, vidofludimus calcium (IMU-838), is being tested in several ongoing MS trials as part of its overall clinical program in order to support a potential approval for patients with MS in major markets. The Phase 3 ENSURE program of vidofludimus calcium in relapsing multiple sclerosis (“RMS”), comprising twin studies evaluating efficacy, safety, and tolerability of vidofludimus calcium versus placebo, and the supportive Phase 2 CALLIPER trial of vidofludimus calcium in progressive multiple sclerosis (“PMS”) are ongoing. On October 9, 2023, we announced positive interim data from the CALLIPER trial, showing biomarker evidence that vidofludimus calcium’s activity extends beyond the previously observed anti-inflammatory effects, thereby further reinforcing its neuroprotective potential. Top-line data from the CALLIPER trial, for which the recruitment of in total 467 patients was completed in August 2023, is expected to be available in April of 2025. Moreover, we currently expect to report an interim futility analysis of the ENSURE program in late 2024 and to read-out the first of the ENSURE trials at the end of 2025. Although we currently believe that each of these goals is achievable, they are each dependent on numerous factors, most of which are not under our direct control and can be difficult to predict. We plan to periodically review this assessment and provide updates of material changes as appropriate.
If approved, we believe that vidofludimus calcium, with combined anti-inflammatory, antiviral, and neuroprotective effects, has the potential to be a unique treatment option targeted to the complex pathophysiology of MS. Recently published preclinical data showed that vidofludimus calcium activates the neuroprotective transcription factor nuclear receptor related 1 (“Nurr1”), which is associated with direct neuroprotective properties and may enhance the potential benefit for patients. Additionally, vidofludimus calcium is a known inhibitor of the enzyme dihydroorotate dehydrogenase (“DHODH”), which is a key enzyme in the metabolism of overactive immune cells and virus-infected cells. This mechanism is associated with the anti-inflammatory and antiviral effects of vidofludimus calcium. We believe that the combined mechanisms of vidofludimus calcium are unique in the MS space and support the therapeutic performance shown in our Phase 2 EMPhASIS trial in relapsing-remitting MS patients, in particular, via data illustrating the potential to reduce magnetic resonance imaging lesions, prevent relapses, reduce the rate of disability progression, and reduce levels of serum neurofilament light chain (“NfL”), an important biomarker of neuronal death. Vidofludimus calcium has shown in clinical trials reported to date a consistent pharmacokinetic, safety and tolerability profile and has already been exposed to more than 1,400 human subjects and patients in either of the drug’s formulations.
IMU-856 is an orally available and systemically acting small molecule modulator that targets Sirtuin 6 (“SIRT6”), a protein which serves as a transcriptional regulator of intestinal barrier function and regeneration of bowel epithelium. Based on preclinical data, we believe this compound may represent a unique treatment approach, as the mechanism of action targets the restoration of the intestinal barrier function and bowel wall architecture in patients suffering from gastrointestinal diseases such as celiac disease, inflammatory bowel disease, irritable bowel syndrome with diarrhea and other intestinal barrier function associated diseases. We believe that, because IMU-856 has been shown in preclinical investigations to avoid suppression of immune cells, it may therefore have the potential to maintain immune surveillance for patients during therapy, which would be an important advantage versus immunosuppressive medications and may allow the potential for combination with available treatments in gastroenterological diseases.
Data from the final portion of a Phase 1 clinical trial in celiac disease patients during periods of gluten-free diet and gluten challenge demonstrated positive effects for IMU-856 over placebo in four key dimensions of celiac disease pathophysiology: protection of the gut architecture, improvement of patients’ symptoms, biomarker response, and enhancement of nutrient absorption. IMU-856 was also observed to be safe and well-tolerated in this trial. We are currently preparing clinical Phase 2 testing of IMU-856 in ongoing active celiac disease, while also considering further potential clinical applications in other gastrointestinal disorders.
Immunic has selected IMU-381 as a development candidate to specifically address the needs of gastrointestinal diseases. IMU-381 is a next generation molecule with improved overall properties, supported by a series of chemical derivatives. IMU-381 is currently in preclinical testing.
Additional antiviral-directed development activities remain ongoing through preclinical research examining the potential to treat a broad set of viral indications with new antiviral molecules. We are exploring several options to possibly support further development of our antiviral portfolio, including a potential spin-off into a new or existing company and potential licensing transactions.
We expect to continue to lead most of our research and development activities from our Gräfelfing, Germany location, where dedicated scientific, regulatory, clinical and medical teams conduct their activities. Due to these teams' key relationships with local and international service providers, we anticipate that this will result in more timely and cost-effective execution of our development programs. In addition, we are using our subsidiary in Melbourne, Australia to perform research and development activities in the Australasia region. We also conduct preclinical work in Halle/Saale, Germany through a collaboration with the Fraunhofer Institute.
Our business, operating results, financial condition and growth prospects are subject to significant risks and uncertainties, including the failure of our clinical trials to meet their endpoints, failure to obtain regulatory approval and failure to obtain needed additional funding on acceptable terms, if at all, to complete the development and commercialization of our three development programs.
Liquidity and Financial Condition
We have no products approved for commercial sale and have not generated any revenue from product sales. We have never been profitable and have incurred operating losses in each year since our inception in 2016. We have an accumulated deficit of approximately $389.3 million as of September 30, 2023 and $317.3 million as of December 31, 2022. Substantially all of our operating losses resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
We expect to incur significant expenses and increasing operating losses for the foreseeable future as we initiate and continue the preclinical and clinical development of our product candidates and add personnel necessary to advance our clinical pipeline of product candidates. We expect that our operating losses will fluctuate significantly from quarter-to-quarter and year-to-year due to timing of clinical development programs.
From inception through October 31, 2023, Immunic has raised net cash of approximately $355.9 million from private and public offerings of preferred and common stock. As of September 30, 2023, the Company had cash and cash equivalents of approximately $59.7 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these financial statements without raising additional capital and such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Strategy
We are focused on the development of new molecules that maximize the therapeutic benefits for patients by uniquely addressing biologically relevant immunological targets. We take advantage of our established research and development infrastructure and operations in Germany and Australia to more efficiently develop our product candidates in indications of high unmet need and where the product candidates have the potential to elevate the standard of care for the benefit of patients. Given the mechanisms of action and the data generated for our product candidates, to date, we continue to execute on the clinical development of our programs for established indications as well as explore additional indications where patients could potentially benefit from the unique profiles of each product candidate.
We are currently focused on maximizing the potential of our development programs through the following strategic initiatives:
•Executing the ongoing Phase 3 ENSURE and Phase 2 CALLIPER clinical trial programs of vidofludimus calcium in MS.
•Executing the IMU-856 development program, including preparation of a Phase 2 clinical trial, in patients with ongoing active celiac disease.
•Continuing preclinical research to complement the existing clinical activities, explore additional indications for future development, and where appropriate, generate additional molecules for future development.
•Facilitating readiness for potential commercial launch of our product candidates through targeted and stage-appropriate pre-commercial activities.
•Evaluating potential strategic collaborations for each product candidate in order to complement our existing research and development capabilities and to facilitate potential commercialization of these product candidates by taking advantage of the resources and capabilities of strategic collaborators in order to enhance the potential and value of each product candidate.
Recent Events
Notice of Allowance for United States Patent Protecting the Treatment of Relapsing Multiple Sclerosis with Vidofludimus and Its Salts
On November 2, 2023, we announced that we have received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for patent application 17/391,442, entitled, “Treatment of Multiple Sclerosis Comprising DHODH Inhibitors,” covering a daily dose of about 10 mg to 45 mg of vidofludimus calcium and other salt as well as free acid forms for the treatment of RMS. The claims are expected to provide protection into 2041, unless extended further.
Positive Interim Data from Phase 2 CALLIPER Trial of Vidofludimus Calcium in Progressive Multiple Sclerosis
On October 9, 2023, we announced positive interim data from our phase 2 CALLIPER trial of vidofludimus calcium in patients with PMS. The predefined interim analysis examined the change from baseline to 24 weeks in serum NfL and glial fibrillar acidic protein ("GFAP") levels among approximately the first half of patients enrolled in this trial. We believe that this data showed biomarker evidence that vidofludimus calcium's activity extends beyond the previously observed anti-inflammatory effects, thereby further reinforcing its neuroprotective potential.
Serum NfL responses were consistently observed for vidofludimus calcium across progressive MS disease and all subpopulations. In the overall PMS population at 24 weeks (N=203), vidofludimus calcium was associated with a 6.7% reduction from baseline in serum NfL, compared to a 15.8% increase over baseline in placebo (p=0.01, post hoc). At 48 weeks (N=79), vidofludimus calcium reduced serum NfL by 10.4% from baseline, compared to a 6.4% increase in placebo. Substantial reductions were also seen across all PMS subtypes, as well as in patients that show or do not show disease and/or magnetic resonance imaging ("MRI") activity.
Although early, interim GFAP data also showed a promising signal: at 24 weeks (N=203), GFAP increased by 3.7% for vidofludimus calcium, and 4.4% for placebo. At 48 weeks (N=79), the change was only 2.7% for vidofludimus calcium, with a 6.4% increase for placebo. Progression of GFAP response is generally thought to evolve more slowly than NfL, and we believe that a longer follow-up may further strengthen this signal.
Completion of Enrollment of Phase 2 CALLIPER Trial of Vidofludimus Calcium in Progressive Multiple Sclerosis
On August 17, 2023, we announced completion of enrollment of our phase 2 CALLIPER trial of vidofludimus calcium in patients with PMS. In total, 467 patients with primary PMS, or active or non-active secondary PMS have been randomized to either 45mg of vidofludimus calcium or placebo. A top-line data readout of the full 467 patients is expected in April of 2025.
Vidofludimus Calcium Acts as Potent Nurr1 Activator, Reinforcing Neuroprotective Potential in MS
On May 17, 2023, we announced the publication of preclinical data showing that vidofludimus calcium acts as a potent Nurr1 activator, in addition to its known mode of action as a DHODH inhibitor. Activation of Nurr1 could be responsible for the drug’s postulated neuroprotective effects and may contribute to the previously reported reduction of confirmed disability worsening events in MS patients.
Specifically, preclinical data shows potent Nurr1 activation by vidofludimus calcium at low concentrations in several test systems. The data was published in the peer-reviewed, high impact Journal of Medicinal Chemistry, in a paper entitled, “Development of a potent Nurr1 agonist tool for in vivo applications.”
Presentation of Clinical and Preclinical Data for IMU-856 at Digestive Disease Week 2023, Including Its Molecular Mode of Action
On May 6, 2023, we announced the presentation of clinical and preclinical data for IMU-856 as a virtual e-poster at Digestive Disease Week 2023. Included in this presentation were new data on IMU-856’s mode of action as a potent modulator of SIRT6, a protein which serves as a transcriptional regulator of intestinal barrier function and regeneration of bowel epithelium.
Positive Results From Phase 1b Clinical Trial of IMU-856 in Celiac Disease
On May 4, 2023, we announced positive results from the part C portion of our Phase 1 clinical trial of IMU-856 in patients with celiac disease. The data demonstrated positive effects for IMU-856 over placebo in four key dimensions of celiac disease pathophysiology: protection of the gut architecture, improvement of patients’ symptoms, biomarker response, and enhancement of nutrient absorption. IMU-856 was also observed to be safe and well-tolerated in this trial.
We believe that this data set provides initial clinical proof-of-concept for an entirely new therapeutic approach to gastrointestinal disorders by promoting regeneration of bowel architecture. The data provides first clinical evidence that IMU-856’s ability, observed in preclinical studies, to re-establish proper gut cell renewal translates into clinical benefits for patients with celiac disease. Most importantly, the observed protection of intestinal villi from gluten-induced destruction, independent of targeting immune mechanisms involved specifically in celiac disease, appears to be unique among proposed therapeutic approaches and may be applicable to other gastrointestinal diseases such as UC, Crohn’s disease or irritable bowel syndrome with diarrhea.
Appointment of Richard Rudick, M.D. to Board of Directors
On April 27, 2023, we announced the appointment of Dr. Richard Rudick as a member of our Board of Directors, effective as of April 26, 2023. As a Class III director, Dr. Rudick’s initial term lasted until our 2023 Annual Meeting of Stockholders held on June 28, 2023, at which meeting he was elected to a three year term expiring at the 2026 Annual Meeting of Stockholders.
Dr. Richard Rudick, age 72, has over 35 years of experience in the biopharmaceutical industry and academic medicine. Since January 2023, Dr. Rudick has been the President and CEO of Astoria Biologic, a private biotechnology company developing novel therapies for MS. Previously, Dr. Rudick served as the Vice President of Development Science at Biogen, Inc., a biotechnology company which engages in discovering, developing, and delivering therapies for neurological and neurodegenerative diseases, from May 2014 until September 2020. Dr. Rudick also served as a staff neurologist and director of the Mellen Center for the Cleveland Clinic from January 1987 until May 2014. Dr. Rudick holds an M.D. from Case Western Reserve University School of Medicine. The Nominating and Corporate Governance Committee and the Board believe that Dr. Rudick‘s extensive leadership in clinical research and development of MS treatments provides valuable clinical, strategy and management skills to the Board.
Director Resignation
Dr. Vincent Ossipow retired from the Board of Directors on June 28, 2023. Dr. Ossipow’s decision not to stand for re-election was not the result of any disagreement with the Company or its management on any matter relating to the Company’s operations, policies or practices.
Positive Data from Maintenance Phase of Phase 2 CALDOSE-1 Trial of Vidofludimus Calcium in Moderate-to-Severe UC
On April 5, 2023, we reported positive data from the maintenance phase of our Phase 2b CALDOSE-1 trial of vidofludimus calcium in patients with moderate-to-severe UC. The data showed a dose-linear increase in clinical remission as compared to placebo at week 50. Moreover, an exploratory statistical analysis confirmed the 30 mg dose of vidofludimus calcium to be statistically superior (p=0.0358) in achieving clinical remission at week 50, with a 33.7% absolute improvement over placebo.
A similar effect on clinical remission rates at week 50 was also found among those patients who received corticosteroids during the induction phase. Finally, a dose-linear increase in endoscopic healing was observed, with the 30 mg dose of vidofludimus calcium being associated with a 37.8% absolute improvement over placebo and also achieving statistical significance in an exploratory statistical analysis (p=0.0259).
We believe that the maintenance phase data of CALDOSE-1 confirms vidofludimus calcium's activity in the absence of chronic corticosteroid co-administration. Consistent with prior data sets in other patient populations, administration of vidofludimus calcium in the maintenance phase of this trial was observed to be safe and well-tolerated.
Deprioritization of Izumerogant (IMU-935) Development Program
In order to focus on the rapidly advancing vidofludimus calcium and IMU-856 programs, and considering the totality of available data for izumerogant, including changes in expected time to market and increased complexity of potential further development in this competitive field, we announced on April 5, 2023 to focus our resources and, therefore, deprioritized the clinical portion of our izumerogant development program in psoriasis and castration-resistant prostate cancer.
Components of Results of Operations
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from the sale of products in the foreseeable future. If our development efforts for our product candidates are successful and result in regulatory approval, we may generate revenue in the future from product sales. We cannot predict if, when, or to what extent we will generate revenue from the commercialization and sale of our product candidates. We may never succeed in obtaining regulatory approval for any of our product candidates or achieving market acceptance and commercial success for any product that does receive regulatory approval.
Research and Development Expenses
Research and development expenses consist of costs associated with our research activities, including our product discovery efforts and the development of our product candidates. Our research and development expenses include:
•external research and development expenses and milestone payments incurred under arrangements with third parties, such as CROs, contract manufacturing organizations, collaborations with partners, consultants, and our scientific advisors; and
•internal personnel expenses.
We expense research and development costs as incurred. Non-refundable advance payments for goods and services that will be used in future research and development activities are capitalized as prepaid expenses and expensed when the service has been performed or when the goods have been received.
Since our inception in March 2016, we have spent a total of approximately $278.8 million in research and development expenses through September 30, 2023.
These costs primarily include external development expenses and internal personnel expenses for the three development programs, vidofludimus calcium, izumerogant and IMU-856. We have spent the majority of our research and development resources on vidofludimus calcium, our lead development program, for clinical trials in MS, UC and COVID-19.
In August 2019, Immunic AG received a grant of up to approximately $726,000 from the German Federal Ministry of Education and Research, in support of the InnoMuNiCH (Innovations through Munich-Nippon Cooperation in Healthcare) project. The grant funds have been used to fund a three-year research project relating to autoimmune diseases by us and our three project partners. Since the inception of the grant, we have recorded $726,000 of income in total of which $67,000 and $100,000 were recorded in the nine months ended September 30, 2023 and 2022, respectively, and were classified in Other Income in the accompanying consolidated statement of operations. The funding of this grant is now completed.
Our research and development expenses are expected to increase in the foreseeable future as we continue to conduct ongoing research and development activities, initiate new preclinical and clinical trials and build our pipeline of product candidates. Our research and development expenses may also increase in the foreseeable future due to the current inflationary environment as well as supply chain shortages, which result in increased costs.
The process of conducting clinical trials and preclinical studies necessary to obtain regulatory approval is costly and time consuming. We may never succeed in achieving regulatory approval for any of our product candidates.
Successful development of product candidates is highly uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each product candidate and are difficult to predict. We anticipate that we will make determinations as to which programs to pursue and how much funding to direct to each program on an ongoing basis in response to the development and regulatory success of each product candidate, and ongoing assessments as to each product candidate’s commercial potential.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel expenses, professional fees for legal, accounting, tax and business consulting services, insurance premiums and stock-based compensation.
Other Income (Expense), Net
Interest Income
Interest income consists of interest earned on our money market funds and bank accounts which are a portion of our cash and cash equivalents balance. Our interest income has been increasing throughout 2022 and 2023 as global interest rates have been increasing.
Other Income (Expense), Net
Other income (expense) consists primarily of a research and development tax incentive related to clinical trials performed in Australia, a German Government research and development grant and foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future. The intercompany loan between Immunic, Inc. and Immunic AG was settled on September 28, 2022 through an equity infusion from Immunic, Inc. to Immunic AG.
Results of Operations
Comparison of the Three Months Ended September 30, 2023 and 2022
The following table summarizes our operating expenses for the three months ended September 30, 2023 and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Change |
| |
2023 |
|
2022 |
|
$ |
|
% |
| (dollars in thousands) |
(unaudited) |
| Operating expenses: |
|
|
|
| Research and development |
$ |
19,796 |
|
|
$ |
16,537 |
|
|
$ |
3,259 |
|
|
20 |
% |
| General and administrative |
3,774 |
|
|
3,579 |
|
|
195 |
|
|
5 |
% |
|
|
|
|
|
|
|
|
| Total operating expenses |
23,570 |
|
|
20,116 |
|
|
3,454 |
|
|
17 |
% |
| Loss from operations |
(23,570) |
|
|
(20,116) |
|
|
(3,454) |
|
|
17 |
% |
| Total other income (expense) |
801 |
|
|
(1,108) |
|
|
1,909 |
|
|
(172) |
% |
| Net loss |
$ |
(22,769) |
|
|
$ |
(21,224) |
|
|
$ |
(1,545) |
|
|
7 |
% |
Research and development expenses increased by $3.3 million during the three months ended September 30, 2023, as compared to the three months ended September 30, 2022. The increase reflects (i) a $2.8 million increase in external development costs related to the Phase 3 clinical program of vidofludimus calcium in relapsing multiple sclerosis, (ii) a $1.2 million increase in drug supply costs for vidofludimus calcium to support our ongoing trials, (iii) a $1.0 million increase in external development costs related to the Phase 2 clinical trial of vidofludimus calcium in progressive multiple sclerosis, (iv) a $0.5 million increase in personnel expense in research and development related to an increase in headcount and (v) a $0.9 million increase in related costs across numerous categories. The increases were partially offset by (i) a decrease of $2.5 million resulting from deprioritizing the izumerogant program in psoriasis and castration-resistant prostate cancer and (ii) a $0.6 million decrease in external development costs related to the Phase 1 clinical trial of IMU-856.
General and administrative expenses increased by $0.2 million during the three months ended September 30, 2023, as compared to the three months ended September 30, 2022. This increase was spread across numerous categories.
Other income increased by $1.9 million during the three months ended September 30, 2023, as compared to the three months ended September 30, 2022. The increase was primarily attributable to (i) a $1.8 million decrease in foreign exchange losses and (ii) a $0.5 million increase in interest income as a result of higher interest rates. The increase was partially offset by a $0.4 million decrease in research and development tax incentives for clinical trials in Australia as a result of decreased spending on clinical trials in Australia primarily for IMU-856.
Comparison of the Nine Months Ended September 30, 2023 and 2022
The following table summarizes our operating expenses for the nine months ended September 30, 2023 and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Nine Months Ended September 30, |
|
Change |
| |
2023 |
|
2022 |
|
$ |
|
% |
| (dollars in thousands) |
(unaudited) |
| Operating expenses: |
|
|
|
| Research and development |
$ |
63,931 |
|
|
$ |
50,520 |
|
|
$ |
13,411 |
|
|
27 |
% |
| General and administrative |
11,911 |
|
|
11,641 |
|
|
270 |
|
|
2 |
% |
|
|
|
|
|
|
|
|
| Total operating expenses |
$ |
75,842 |
|
|
$ |
62,161 |
|
|
$ |
13,681 |
|
|
22 |
% |
| Loss from operations |
(75,842) |
|
|
(62,161) |
|
|
(13,681) |
|
|
22 |
% |
| Total other income (expense) |
3,802 |
|
|
(1,772) |
|
|
5,574 |
|
|
(315) |
% |
| Net loss |
(72,040) |
|
|
$ |
(63,933) |
|
|
$ |
(8,107) |
|
|
13 |
% |
Research and development expenses increased by $13.4 million during the nine months ended September 30, 2023, as compared to the nine months ended September 30, 2022. The increase reflects (i) a $10.1 million increase in external development costs related to the Phase 3 clinical program of vidofludimus calcium in relapsing multiple sclerosis, (ii) a $2.9 million increase in external development costs related to the Phase 2 clinical trial of vidofludimus calcium in progressive multiple sclerosis, (iii) a $2.3 million increase in drug supply costs for vidofludimus calcium to support our ongoing trials, (iv) a $1.8 million increase in external development costs related to the Phase 1 clinical trial of IMU-856, (v) a $1.6 million increase in personnel expense in research and development related to an increase in headcount, $0.2 million of which was due to non-cash stock based compensation and (vi) a $0.5 million increase in related costs across numerous categories. The increases were partially offset by (i) a decrease of $4.0 million from deprioritizing the izumerogant program in psoriasis and castration-resistant prostate cancer and (ii) a decrease of $1.8 million in external development costs related to the Phase 2 clinical trial of vidofludimus calcium in ulcerative colitis.
General and administrative expenses increased by $0.3 million during the nine months ended September 30, 2023, as compared to the nine months ended September 30, 2022. The increase was primarily due to (i) a $0.2 million increase in travel expense, (ii) a $0.2 million increase in legal and consultancy expense and (iii) a $0.5 million increase across numerous categories. The increases were partially offset by a decrease of $0.6 million in personnel expense in general and administrative which was primarily due to non-cash stock based compensation decrease.
Other income increased by $5.6 million during the nine months ended September 30, 2023, as compared to the nine months ended September 30, 2022. The increase was primarily attributable to (i) a $2.2 million increase in interest income as a result of higher interest rates, (ii) a $3.2 million decrease in foreign exchange losses and (iii) a $1.1 million research allowance attributable to tax year 2021 from the German Federal Ministry of Finance. The increase was partially offset by a $0.9 million decrease in research and development tax incentives for clinical trials in Australia as a result of decreased spending on clinical trials in Australia.
Liquidity and Capital Resources
Financial Condition
We have no products approved for commercial sale and have not generated any revenue from product sales. We have never been profitable and have incurred operating losses in each year since our inception in 2016. We have an accumulated deficit of approximately $389.3 million at September 30, 2023 and $317.3 million as of December 31, 2022. Substantially all of our operating losses resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future as we initiate and continue the preclinical and clinical development of our product candidates and add personnel necessary to operate as a company with an advanced clinical pipeline of product candidates. To the extent additional funds are necessary to meet long-term liquidity needs as we continue to execute our business strategy, we anticipate that they will be obtained through the incurrence of indebtedness, additional equity financings or a combination of these potential sources of funds, although we can provide no assurance that these sources of funding will be available on reasonable terms.
From inception through October 31, 2023, Immunic has raised net cash of approximately $355.9 million from private and public offerings of preferred and common stock. As of September 30, 2023, the Company had cash and cash equivalents of approximately $59.7 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these financial statements without raising additional capital and such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
In November 2020, we filed a shelf registration statement on Form S-3 (the "2020 Shelf Registration Statement"). The 2020 Shelf Registration Statement permits the offering, issuance and sale of up to $250.0 million of common stock, preferred stock, warrants, debt securities, and/or units in one or more offerings and in any combination of the foregoing. As of October 31, 2023, there is $75.0 million remaining on this shelf registration statement. The 2020 Shelf Registration Statement expires on November 24, 2023. The Company plans to file prior to that expiration date, a new shelf registration statement on Form S-3 to replace the expiring 2020 Shelf Registration Statement, which would permit the Company to: (i) continue to sell, subject to applicable SEC requirements, unsold securities remaining on the expiring 2020 Shelf Registration Statement; and (ii) offer and sell additional securities to be registered on the new Form S-3
In December 2020, we filed a Prospectus Supplement for the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of common stock that may be issued and sold under an at-the-market sales agreement with SVB Leerink LLC (now Leerink Partners LLC) as agent ("December 2020 ATM"). We have used and intend to continue to use the net proceeds from the December 2020 ATM to continue to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The December 2020 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the December 2020 ATM or (ii) termination of the December 2020 ATM as otherwise permitted thereby. The December 2020 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Partners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on us. As of October 31, 2023, $8.1 million in capacity remains under the December 2020 ATM.
In May 2022, we filed a Prospectus Supplement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock that may be issued and sold under another at-the-market sales agreement ("May 2022 ATM") with SVB Securities LLC (now Leerink Partners LLC) as agent. We intend to use the net proceeds from the offering to continue to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The May 2022 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the May 2022 ATM or (ii) termination of the May 2022 ATM as otherwise permitted thereby. The May 2022 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Partners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on the Company. As of October 31, 2023, $80.0 million in capacity remains under the May 2022 ATM.
In the three and nine months ended September 30, 2023, we raised gross proceeds of $0.3 million pursuant to the December 2020 ATM through the sale of 107,012 shares of common stock at a weighted average price of $2.72 per share. The net proceeds from the December 2020 ATM were $0.3 million after deducting underwriter commissions of $9,000.
In the three months ended September 30, 2022, we did not raise any proceeds under our ATM facilities. In the nine months ended September 30, 2022, we raised gross proceeds of $40.9 million pursuant to the December 2020 ATM through the sale of 4,204,113 shares of common stock at a weighted average price of $9.72 per share. The net proceeds from the December 2020 ATM were $39.6 million after deducting underwriter commissions of $1.2 million.
Future Capital Requirements
As noted above, we have not generated any revenue from product sales and we do not know when, or if, we will generate any revenue from product sales. We do not expect to generate any revenue from product sales unless and until we obtain regulatory approval for and commercialize any of our product candidates. We expect our expenses to continue to increase as we continue the ongoing research, development, manufacture and clinical trials of, and seek regulatory approval for, our product candidates. We also incur additional costs associated with operating as a public company. In addition, subject to obtaining regulatory approval of any of our product candidates, we anticipate that we will need substantial additional funding in connection with our continuing operations.
Our future expenses and capital requirements are difficult to forecast and will depend on many factors, including, but not limited to:
•the timing and structure of any strategic options and transactions, if any;
•personnel-related expenses, including salaries, benefits, stock-based compensation expense and other compensation expenses related to retention and termination of personnel;
•the scope, progress, duration, results and costs of research and development and ongoing clinical trials;
•the cost and timing of future regulatory submissions;
•the cost and timing of developing and validating the manufacturing processes for any potential product candidates;
•the cost and timing of any commercialization activities, including reimbursement, marketing, sales and distribution costs;
•our ability to establish new collaborations, licensing or other arrangements and the financial terms of such agreements;
•the number and characteristics of any future product candidates we pursue;
•the costs involved with being a public company;
•the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including litigation costs and the outcome of such litigation; and
•the cost, timing and outcome of any future litigation;
•the timing, receipt and amount from the sales of, or royalties on, any future products.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of stock offerings, debt financings, strategic alliances, collaborations and licensing arrangements. Recent developments, however, will make it more difficult and costly for us to obtain funding for our cash needs. We do not expect to achieve revenue from product sales prior to the use of all the net proceeds from our public and private offerings to date. We do not have any committed external source of funds. Additional funds may not be available on acceptable terms, if at all. To the extent that we raise additional capital through the sale of equity securities, the ownership interest of our stockholders will be diluted and it may be on terms that are not favorable to us or our stockholders. Sales of equity securities will also be more difficult for at least the foreseeable future because of general volatility in the equity markets for companies like us, as well as the significant decline in the trading price of our stock following our announcement on October 21, 2022 of the Phase 1b interim analysis of izumerogant in moderate-to-severe psoriasis. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt or other terms that are not favorable to us or our stockholders. Also, the cost of debt financing has increased due to the rise in interest rates beginning in March 2022. If we raise additional funds through collaborations and licensing arrangements with third parties, we would expect to relinquish substantial rights to our technologies or our future products, or grant licenses on terms that may not be favorable to us. If we were to complete a merger, we may relinquish all control over the organization and could experience detrimental tax effects. If we are unable to raise adequate funds, we may have to curtail our product development programs and liquidate some or all of our assets. Any of these factors could harm our operating results and could result in substantial declines in the trading price of our common stock.
As of September 30, 2023, we had cash and cash equivalents of approximately $59.7 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of the financial statements in this Form 10-Q without raising additional capital, which the Company believes could be very challenging, as well as highly-dilutive. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Cash Flows
The following table shows a summary of our cash flows for the nine months ended September 30, 2023 and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
| |
Nine Months Ended September 30, |
| |
2023 |
|
2022 |
| (in thousands) |
(unaudited) |
| Cash (used in) provided by: |
|
|
|
| Operating activities |
$ |
(56,801) |
|
|
$ |
(50,040) |
|
| Investing activities |
9,627 |
|
|
(113) |
|
| Financing activities |
429 |
|
|
39,719 |
|
Operating activities
During the nine months ended September 30, 2023, operating activities used $56.8 million of cash. The use of cash primarily resulted from (i) our net loss of $72.0 million adjusted for non-cash charges of $6.3 million related to $0.7 million for an unrealized foreign currency loss and $5.6 million related to stock-based compensation and depreciation and amortization and a $9.0 million net increase in operating assets and liabilities. Changes in our operating assets and liabilities during the nine months ended September 30, 2023 consisted primarily of (i) a $3.9 million increase in our other current assets and prepaid expenses and (ii) an increase of $5.1 million in our other current liabilities.
During the nine months ended September 30, 2022, operating activities used $50.0 million of cash. The use of cash primarily resulted from (i) our net loss of $63.9 million adjusted for non-cash charges of $10.3 million related to $4.2 million for an unrealized foreign currency loss and $6.1 million related to stock-based compensation and depreciation and amortization and a $3.6 million net increase in operating assets and liabilities. Changes in our operating assets and liabilities during the nine months ended September 30, 2022 consisted primarily of (i) a $2.0 million increase in our other current assets and prepaid expenses and (ii) an increase of $1.6 million in our other current liabilities.
Investing activities
During the nine months ended September 30, 2023, net investing activities provided $9.6 million of cash, primarily due to the sale of $9.8 million of time deposits partially offset by the purchase of $169,000 of property and equipment.
Net cash used in investing activities was $113,000 during the nine months ended September 30, 2022, which was related to the purchase of property and equipment.
Financing Activities
Net cash provided by financing activities was $0.4 million during the nine months ended September 30, 2023 consisting primarily of net cash proceeds from the sale of common stock under our 2020 ATM facility.
Net cash provided by financing activities was $39.7 million during the nine months ended September 30, 2022 consisting primarily of net cash proceeds from the sale of common stock under our 2020 ATM facility.
Off-Balance Sheet Arrangements
Through September 30, 2023, we have not entered into and did not have any relationships with unconsolidated entities or financial collaborations, such as entities often referred to as structured finance or special purpose entities, established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purpose.
Contractual Obligations
Maturities of the operating lease obligation are as follows as of September 30, 2023:
|
|
|
|
|
|
|
|
|
| 2023 |
$ |
189,000 |
|
| 2024 |
|
758,000 |
|
| 2025 |
|
433,000 |
|
| 2026 |
|
78,000 |
|
| 2027 |
|
82,000 |
|
| Thereafter |
|
55,000 |
|
| Total |
|
1,595,000 |
|
| Interest |
|
144,000 |
|
| PV of obligation |
$ |
1,451,000 |
|
As of September 30, 2023, we have non-cancelable contractual obligations under certain agreements related to our development programs IMU-838, IMU-935 and IMU-856 totaling approximately $5.1 million, all of which is expected to be paid over the next twelve months.
Critical Accounting Policies and Estimates
Our unaudited condensed consolidated financial statements are prepared in conformity with U.S. GAAP. The preparation of our unaudited condensed consolidated financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions. We have reviewed these critical accounting policies and related disclosures with the Audit Committee of our Board.
During the first nine months of 2023, there were no significant changes in our critical accounting policies or in the methodology used for estimates. Our significant accounting policies are described in more detail in (i) Note 2 to our unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report and (ii) our audited consolidated financial statements for the years ended December 31, 2022 and 2021 filed in our Annual Report on Form 10-K on February 23, 2023.
Recently Issued Accounting Standards
There are no recently issued accounting standards that would have a significant impact on the company's consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Sensitivity
We had cash and cash equivalents of $59.7 million as of September 30, 2023, which were held for working capital purposes. We do not enter into investments for trading or speculative purposes. We do not believe that we have any material exposure to changes in the fair value of these investments as a result of changes in interest rates due to their short-term nature. However, $22.4 million of these funds are held in German bank accounts that were earning between 2.00%-3.25% interest as of September 30, 2023. Decreases or increases in interest rates, however, will reduce or increase future investment income, respectively, to the extent we have funds available for investment.
Foreign Currency Exchange Risk
Our primary research and development operations are conducted in our facilities in Germany. We have entered into and may continue to enter into international agreements, primarily related to our clinical studies. Accordingly, we have exposure to foreign currency exchange rates and fluctuations between the U.S. dollar and foreign currencies, primarily the euro and the Australian dollar, which could adversely affect our financial results, including income and losses as well as assets and liabilities. To date, we have not entered into, and do not have any current plans to enter into, any foreign currency hedging transactions or derivative financial transactions. Our exposure to foreign currency risk will fluctuate in future periods as our research and clinical development activities in Europe and Australia change. We currently maintain a significant amount of our assets outside of the U.S.
The functional currencies of our foreign subsidiaries are the applicable local currencies. Accordingly, the effects of exchange rate fluctuations on the net assets of these operations are accounted for as translation gains or losses in accumulated other comprehensive income (loss) within stockholders’ equity. Foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future are recorded in Other Income (Expense). Our German subsidiary is currently a significant portion of our business and, accordingly, a change of 10% in the currency exchange rates, primarily the euro, could have a material impact on their financial position or results of operations.
Although operating in local currencies may limit the impact of currency rate fluctuations on the results of operations of our German and Australian subsidiaries, rate fluctuations may impact the consolidated financial position as the assets and liabilities of our foreign operations are translated into U.S. dollars in preparing our condensed consolidated balance sheets. As of September 30, 2023, our German and Australian subsidiaries had net current assets (defined as current assets less current liabilities), subject to foreign currency translation risk, of $12.8 million. A decrease of approximately $1.3 million in net current assets would result as of September 30, 2023, from a hypothetical 10% adverse change in quoted foreign currency exchange rates, primarily due to the euro. In addition, a 10% change in the foreign currency exchange rates for the nine months ended September 30, 2023, would have impacted our net loss by approximately $6.3 million, primarily due to the euro.
Effects of Inflation
We have experienced a general increase in costs as a result of global inflation, however, we do not believe that inflation and changing prices had a material impact on our results of operations for any periods presented herein.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
An evaluation was carried out, under the supervision of and with the participation of our management, including our Chief Executive Officer and our Principal Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15 (e)) under the Securities Exchange Act of 1934 ("the Exchange Act"), as of the end of the period covered by this report. Based on such evaluation, our Chief Executive Officer and our Principal Financial Officer have concluded that our disclosure controls and procedures are effective to ensure that the information required to be disclosed by us in the reports we file or submit under the Exchange Act was recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the nine months ended September 30, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Part II - OTHER INFORMATION
Item 1. Legal Proceedings
We are not currently a party to any litigation, nor are we aware of any pending or threatened litigation against us, that we believe would materially affect our business, operating results, financial condition or cash flows. Our industry is characterized by frequent claims and litigation including securities litigation, claims regarding patent and other intellectual property rights and claims for product liability. It is not uncommon for lawsuits to be filed alleging lack of process or breach of fiduciary duties by directors, and we may face such suits in the future. As a result, in the future, we may be involved in various legal proceedings from time to time.
Item 1A. Risk Factors
Our business is subject to various risks, including those described in Item 1A of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which we strongly encourage you to review. Other than as set forth below, there have been no material changes to the risk factors described in our Annual Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on February 23, 2023.
Adverse developments affecting the financial services industry, including events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our business, financial condition, or results of operations.
Events involving limited liquidity, defaults, non-performance, or other adverse developments that affect financial institutions, transactional counterparties, or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have recently led to market-wide liquidity problems. In March 2023, Silicon Valley Bank, Signature Bank and Silvergate Capital Corp. were each swept into receivership. Although financial regulators have taken measures to prevent further bank closures, these risks remain. If we were to borrow money in the future and if any of our lenders or counterparties to any such instruments were to be placed into receivership, or if we had cash deposits at any such banks, we may be unable to access our funds.
Although we assess our banking and financing relationships as we believe necessary or appropriate, our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations, clinical programs and product development could be significantly impaired by factors that affect us, the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit, or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and stricter financial and operating covenants, or limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws if, for example, we were unable to obtain access to deposits used for payroll. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
Our clinical trials have been delayed as a result of the ongoing military action by Russia in Ukraine and may also be delayed by the recent outbreak of war in the Middle East. The continuation of these conflicts could have further adverse effects on our business.
Our clinical trials of vidofludimus calcium were originally planned to be conducted at more than 60 sites in Ukraine and Russia, but most had to be relocated to other countries because of the invasion of Ukraine by Russia in February 2022 and resulting sanctions imposed on Russia by the United States and other countries.
These disruptions delayed our clinical development program, increased our costs and may disrupt future planned clinical development activities in these two countries. This military action has continued for more than a year and its future course and effects on our Company are highly unpredictable. We currently have 19 active sites in western Ukraine and the ongoing conflict could put the data associated with these patients in jeopardy as well as extend patient recruitment timelines. We anticipate that Ukraine will make up approximately 15%-20% of our ENSURE- 1 and ENSURE-2 phase 3 patient population. In addition, no clinical sites in Russia have been activated or are intended to be used in the future. Alternative sites to fully and timely compensate for our clinical trial activities in Ukraine may not continue to be available.
The recent outbreak of war between Israel and Hamas may also result in delays or suspensions of our clinical trials in the Middle East. We have already and plan to open in the next few months additional clinical trial sites in Lebanon and Jordan.. Attacks on northern Israel by Hamas allies from Lebanon have resulted in retaliatory strikes in Lebanon by Israel. Hostilities could escalate further and involve other countries in the Middle East where we have or plan to have clinical trial sites.
If our clinical trials are further interrupted, our clinical development program could experience further delays and increased costs and we may have insufficient data to support regulatory approvals of vidofludimus calcium, and any commercialization may be delayed or not approved, which could limit our potential revenue and hurt the competitive position of our potential products.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
Not applicable.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
Item 6. Exhibits
EXHIBITS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Incorporated by Reference |
|
Exhibit
Number
|
|
Exhibit Title |
|
Form |
|
Exhibit |
|
Filing Date |
| 3.1 |
|
|
|
8-K |
|
3.1 |
|
|
July 17, 2019 |
| 3.2 |
|
|
|
8-K |
|
3.1 |
|
|
July 17, 2019 |
| 4.1 |
|
|
|
S-8 |
|
4.2 |
|
|
August 21, 2023 |
| 10.1 |
|
|
|
8-K |
|
10.1 |
|
January 16, 2023 |
| 10.2 |
|
|
|
8-K |
|
10.2 |
|
January 16, 2023 |
| 10.3 |
|
|
|
8-K |
|
10.3 |
|
January 16, 2023 |
| 23.1* |
|
|
|
|
|
|
|
|
| 31.1* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 31.2* |
|
|
|
|
|
|
|
|
| 32.1** |
|
|
|
|
|
|
|
|
| 32.2** |
|
|
|
|
|
|
|
|
| 101.INS* |
|
XBRL Instance Document |
|
|
|
|
|
|
| 101.SCH* |
|
XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
|
| 101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
|
| 101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Database. |
|
|
|
|
|
|
| 101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase Document. |
|
|
|
|
|
|
| 101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
|
| 104* |
|
Cover Page Interactive Data File |
|
|
|
|
|
|
|
|
|
|
|
|
| + |
Indicates a management contract or compensatory plan or arrangement. |
| * |
Filed herewith |
| ** |
In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release No. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Exchange Act. Such certifications will not be deemed to be incorporated by reference into any filings under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates it by reference. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
IMMUNIC, INC.
Date: November 14, 2023 By: /s/ Daniel Vitt
Daniel Vitt
Chief Executive Officer and President
EX-23.1
2
exhibit231202311.htm
EX-23.1
Document
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in the Registration Statements on Form S‑3 (File No. 333‑225230, 333-250083, 333-255303, and 333-268737), Form S‑4 (File No. 333‑229510), and Form S-8 (File No. 333‑233864 and 333-258235) of Immunic, Inc. of our report dated February 23, 2023, relating to the consolidated financial statements of Immunic, Inc., which appears in this annual report on Form 10-K for the year ended December 31, 2022.
/s/ Baker Tilly US, LLP
Minneapolis, Minnesota
February 23, 2023
EX-31.1
3
imux-93023xexhibit311.htm
EX-31.1
Document
Exhibit 31.1
CERTIFICATIONS
I, Daniel Vitt, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q of Immunic, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
|
|
|
| Date: November 14, 2023 |
By: |
/s/ Daniel Vitt |
|
|
Daniel Vitt |
|
|
Chief Executive Officer and President |
|
|
(Principal Executive Officer) |
EX-31.2
4
imux-9x3023xexhibit312.htm
EX-31.2
Document
Exhibit 31.2
CERTIFICATIONS
I, Glenn Whaley, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q of Immunic, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of and for the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
|
|
|
| Date: November 14, 2023 |
By: |
/s/ Glenn Whaley |
|
|
Glenn Whaley |
|
|
Chief Financial Officer |
|
|
(Principal Financial Officer) |
EX-32.1
5
imux-9x30x23xexhibit321.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of Immunic, Inc. (the “Company”) for the period ended September 30, 2023, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Daniel Vitt, as Chief Executive Officer and President of the Company, hereby certifies, pursuant to 18 U.S.C. Section 1350, that to my knowledge:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
|
|
| Date: November 14, 2023 |
By: |
/s/ Daniel Vitt |
|
|
Daniel Vitt |
|
|
Chief Executive Officer and President |
|
|
(Principal Executive Officer) |
EX-32.2
6
imux-93023xexhibit322.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-K of Immunic, Inc. (the “Company”) for the period ended September 30, 2023, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Glenn Whaley as Chief Financial Officer of the Company, hereby certifies, pursuant to 18 U.S.C. Section 1350, that to my knowledge::
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
|
|
| Date: November 14, 2023 |
By: |
/s/ Glenn Whaley |
|
|
Glenn Whaley |
|
|
Chief Financial Officer |
|
|
(Principal Financial Officer) |
