UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): December 2, 2025
JASPER THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-39138 | 84-2984849 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
2200 Bridge Pkwy Suite #102
Redwood City, California 94065
(Address of Principal Executive Offices) (Zip Code)
(650) 549-1400
Registrant’s telephone number, including area code
N/A
(Former Name, or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
(Title of each class) |
(Trading Symbol) |
(Name of exchange on which registered) |
||
| Voting Common Stock, par value $0.0001 per share | JSPR | The Nasdaq Stock Market LLC | ||
| Redeemable Warrants, each ten warrants exercisable for one share of Voting Common Stock at an exercise price of $115.00 | JSPRW | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On December 2, 2025, Jasper Therapeutics, Inc. (the “Company”) issued a press release reporting positive preliminary clinical data from the Company’s ETESIAN Phase 1b study of subcutaneous briquilimab in adult participants with allergic asthma and the completion of its internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg /180mg Q8W) and disclosing that the Company will hold a conference call and webinar at 8:00 am Eastern Time on December 2, 2025 to present findings from its investigation into the anomalous results from the BEACON study in CSU reported in July, as well as the preliminary data from the ETESIAN study in asthma.
A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference. A copy of the presentation to be used in connection with the conference call and webinar on December 2, 2025 is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. | Description | |
| 99.1 | Press Release, dated December 2, 2025. | |
| 99.2 | Presentation – Jasper Therapeutics: ETESIAN Data + Beacon Investigation Update, December 2025. | |
| 104 | Cover Page Interactive Data File, formatted in Inline Extensible Business Reporting Language (iXBRL). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: December 2, 2025 | JASPER THERAPEUTICS, INC. | ||
| By: | /s/ Herb Cross | ||
| Name: | Herb Cross | ||
| Title: | Chief Financial Officer | ||
Exhibit 99.1

Jasper Therapeutics Reports Positive Preliminary Data from ETESIAN Study of Briquilimab in Asthma and Findings from BEACON Study Internal Investigation
Reductions in airway hyperresponsiveness
and suppressed eosinophilic response at
both 6 weeks and 12 weeks observed after a single 180mg dose of Briquilimab in the ETESIAN Study
Preliminary data from ETESIAN study supports further development of briquilimab in asthma
Jasper also announces completion of internal BEACON study investigation noting no deviations or issues with drug product utilized
Jasper to host conference call and webinar today at 8:00 a.m. ET
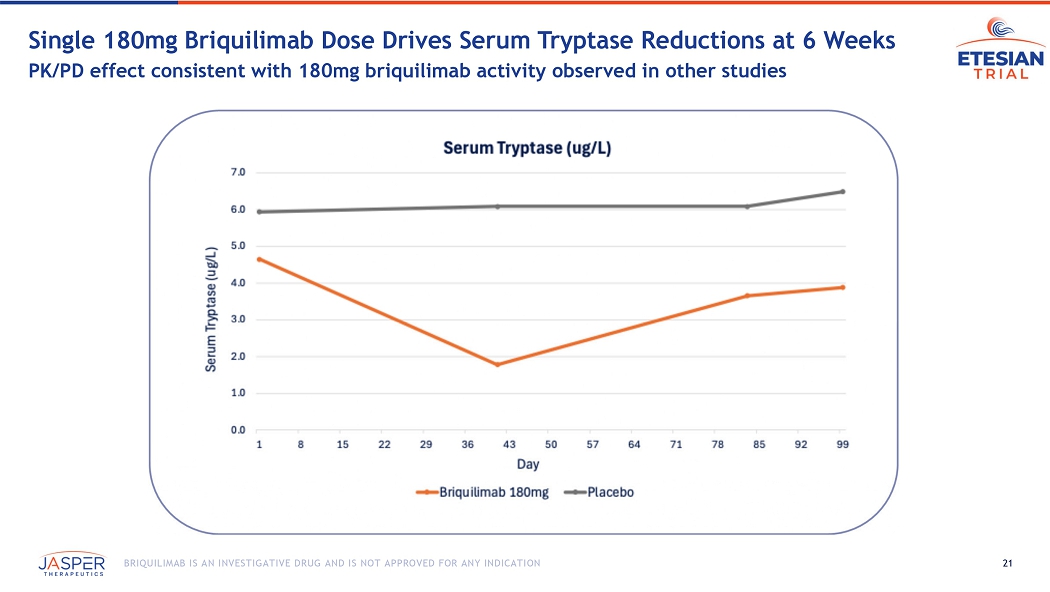
REDWOOD CITY, Calif., December 2, 2025 (GLOBE NEWSWIRE) – Jasper Therapeutics, Inc. (Nasdaq: JSPR) (Jasper), a clinical stage biotechnology company focused on development of briquilimab, a novel antibody therapy targeting KIT (CD117) to address mast cell driven diseases such as chronic spontaneous urticaria (CSU), chronic inducible urticaria (CIndU) and asthma, reported positive preliminary clinical data from Jasper’s ETESIAN Phase 1b study of subcutaneous briquilimab in adult participants with allergic asthma. A single subcutaneous 180mg dose of briquilimab demonstrated substantial reductions in sputum eosinophils at both six and twelve weeks, as well as improvements over baseline in FEV1 in both Early Asthmatic Response (EAR) and Late Asthmatic Response (LAR). Significant reductions in serum tryptase were observed, consistent with reductions observed in other briquilimab studies at the 180mg dose level. Briquilimab was well tolerated in the study, demonstrating a favorable safety profile.
Jasper also announced the completion of its internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg /180mg Q8W), where no US patients (n=10) achieved Complete Response or Well Controlled UAS7 by week 12. Based on the work completed, the additional data from subsequent dosing of the US patients and input from a panel of CSU KOLs, Jasper has concluded that the unexpected efficacy results observed in the US patients was not the result of any issues with the drug product. Rather, it was likely a result of patient selection issues, as it appears that 9 of the 10 patients in question did not have CSU as their symptoms were not mast cell-driven.
“I am pleased to see the initial results of the ETESIAN study, the first clinical study to evaluate an agent specifically targeting mast cells in asthma patients” said Dr. Elliot Israel, Director of Clinical Research in the Pulmonary and Critical Care Division at the Brigham and Women’s Hospital in Boston. “The initial results demonstrate the potential to reduce both airway hyperresponsiveness and the accumulation of eosinophils in the airways, both of which are key factors in managing chronic asthma and reducing exacerbations. Given that a substantial portion of asthma patients remain underserved by currently approved therapies, depleting mast cells through KIT inhibition may represent an intriguing new treatment option for patients with chronic asthma.”
“We are very pleased to present the positive preliminary data from the ETESIAN study, which demonstrates proof of concept for mast cell depletion using briquilimab as a potential therapeutic option for patients with asthma,” said Dr. Daniel Adelman, Acting Chief Medical Officer of Jasper. “Mast cells are believed to be a key driver of the inflammatory cascade underlying chronic asthma, and both the reductions in airway hyperresponsiveness and the significant reduction in sputum eosinophils demonstrated at 6 weeks after a single 180mg dose of briquilimab strongly support the potential of mast cell depletion to drive a therapeutic benefit for asthma patients. These data, combined with the favorable safety profile observed in the ETESIAN study and in other briquilimab clinical studies, provide a strong rationale for further development of briquilimab in asthma. On behalf of the entire Jasper team, I’d like to thank the investigators and the patients who participated in the study, along with their families and caregivers.”
ETESIAN Study Design and Preliminary Data Summary:
The Phase 1b/2a ETESIAN study was a single dose double-blind, placebo-controlled challenge study that enrolled approximately 17 patients across 6 sites in Canada. The primary objective of the study was to demonstrate proof-of-concept for briquilimab in asthma utilizing a potentially therapeutic dose to inform future trials in the broader asthma population. The study was conducted utilizing a single 180mg dose of subcutaneous briquilimab and key assessments included both EAR measured at 6 weeks, and LAR measured at 12 weeks, changes in airway hyperresponsiveness, mast cell depletion and recovery, and safety.
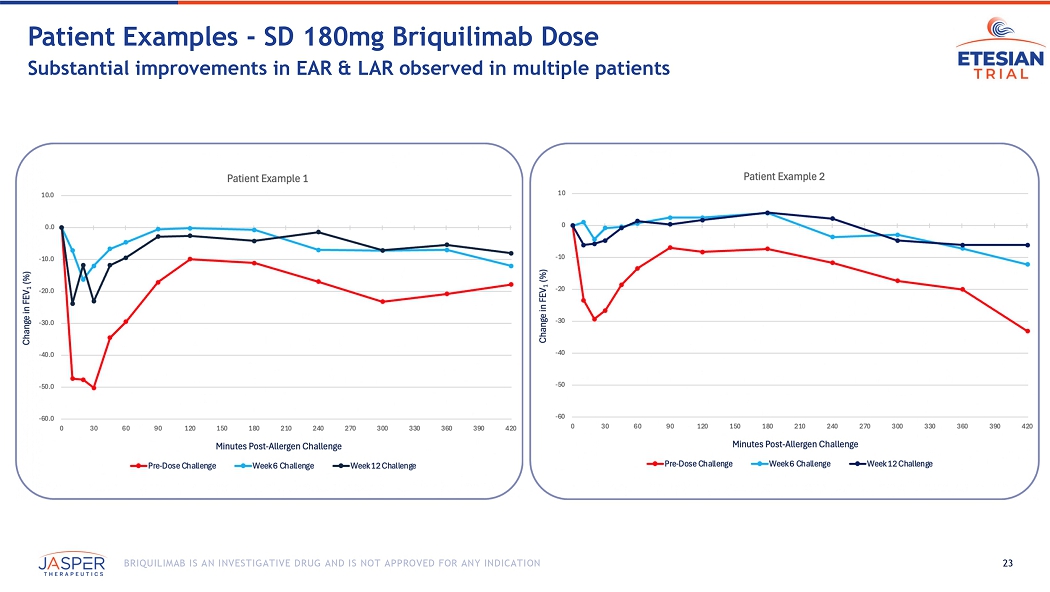
The preliminary data includes the results from 14 participants, 8 receiving a single dose of 180mg briquilimab and 6 receiving placebo, who completed at least 6 weeks of allergen challenge testing following initial dosing with investigational product. Compared to baseline, briquilimab reduced the allergen induced LAR (measured by the mean maximum percentage fall in FEV1 (%Max FEV1) and fall in area under the FEV1 time response curve (AUC)) at both 6 and 12 weeks. Patients who received briquilimab showed an improvement in the LAR %Max FEV1 of 10.4% at 6 weeks and 8.7% at 12 weeks compared to baseline and an improvement in the LAR AUC of 25.4% at 6 weeks and 23.3% at 12 weeks.
180mg Single-Dose Asthmatic Response Assessments at Week 6 and Week 12
| 6-Week Change (Baseline to Day 42) |
12-Week Change (Baseline to Day 84) |
||||
| % Max FEV1 | AUC | % Max FEV1 | AUC | ||
| Early Asthmatic Response, 0-2 Hours (EAR) | |||||
| Briquilimab (n=8) | 12.74 | 18.27 | 8.13 | 10.56 | |
| Placebo (n=6) | 9.88 | 5.93 | 6.16 | 3.89 | |
| Late Asthmatic Response, 2-7 Hours (LAR) | |||||
| Briquilimab (n=7) | 10.37 | 25.35 | 8.69 | 23.34 | |
| Placebo (n=5) | 3.13 | 9.35 | 0.20 | 1.92 | |
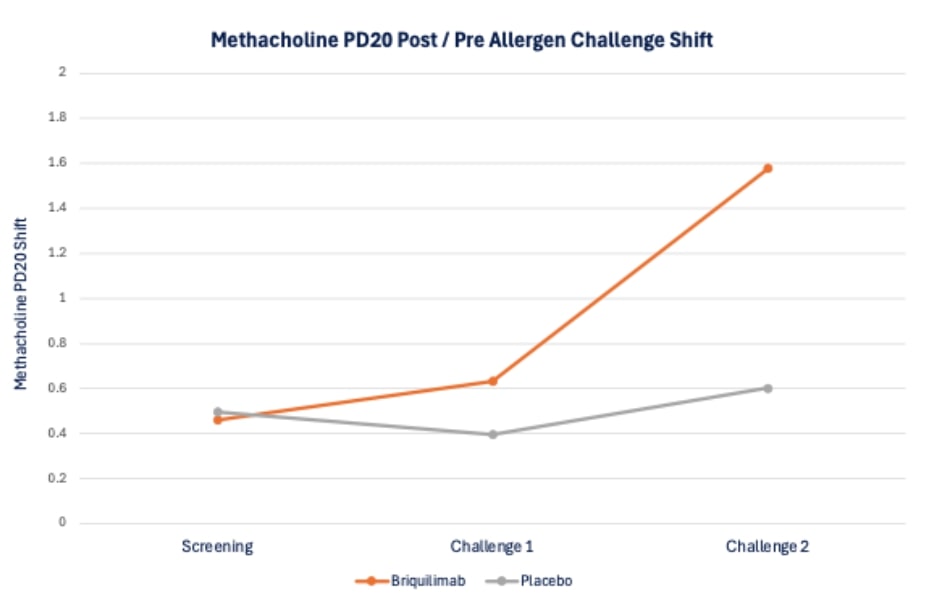
Patient airway hyperresponsiveness was also assessed pre- and post-allergen challenge by methacholine PD20. At baseline, prior to administration of briquilimab, patients randomized to both the placebo group and the briquilimab group had similar drops in the ratio of post- to pre-allergen challenge methacholine PD20 (dose of methacholine required to drive a 20% decrease in FEV1) following allergen challenge of 0.50 and 0.46, respectively. At the week 6 challenge the shift in the methacholine PD20 response was 0.40 for placebo and 0.63 for briquilimab and at the week 12 challenge the shift was 0.60 for placebo and 1.58 for briquilimab indicating an increased resistance to methacholine following allergen in patients dosed with briquilimab.
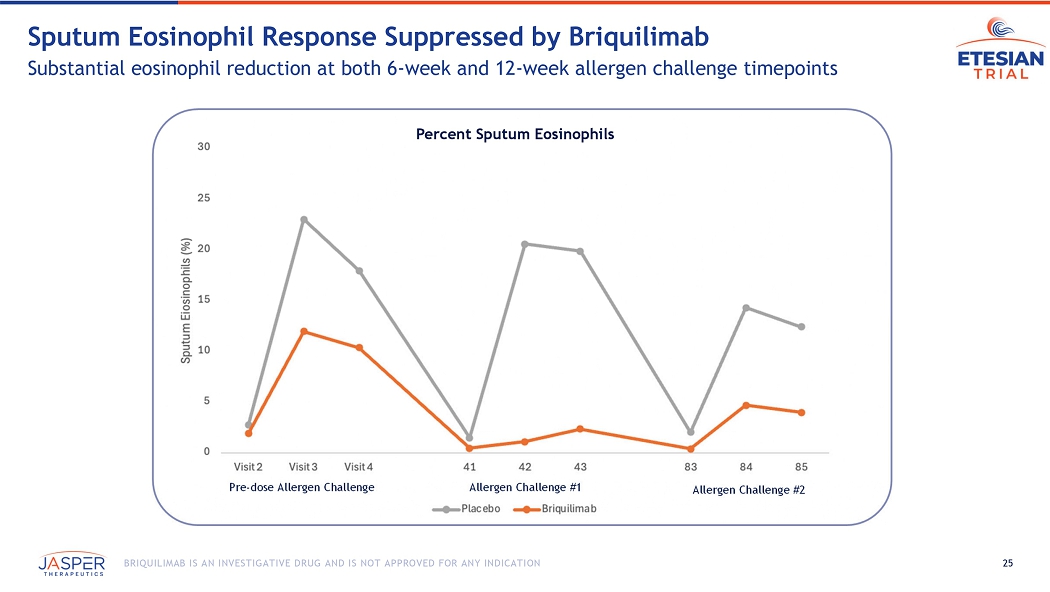
Treatment with briquilimab also decreased levels of sputum eosinophils before and after allergen challenge. Patients in the briquilimab group had reduced mean sputum eosinophil levels at pre allergen challenge timepoints of 1.88% at baseline to 0.44% at day 41 and 0.38% at day 83. Briquilimab also reduced mean sputum eosinophil levels 24 hours following allergen challenge. At baseline, prior to briquilimab dosing, patients randomized to the briquilimab group had a 24-hour post allergen challenge eosinophil level of 10.3%. Following dosing of briquilimab the 24-hour post allergen challenge eosinophil level was 2.32% at 6 weeks and 3.98% at 12 weeks.

Briquilimab was well-tolerated in the study, with no dose limiting toxicities observed. Safety observations potentially related to KIT blockade were infrequent and generally limited to low grade events, none of which resulted in discontinuations or dose delays and the majority of which resolved during repeat dosing.
BEACON Cohort 8 & Cohort 9 Internal Investigation Concluded
Jasper announced the completion of its internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg/180mg Q8W), where no US patients (n=10) achieved Complete Response or Well Controlled UAS7 by week 12. In response, Jasper’s internal investigation included:
| ● | switching all US patients to a new lot of drug product for the remainder of their doses on study to determine if drug product played a role, |
| ● | a comprehensive review of all manufacturing records, drug handling, site training/ logs and data handling, |
| ● | recovery and testing by Jasper and independent labs of drug product samples from across the supply chain, |
| ● | a review of all US sites and all US patients, including protocol adherence patient medical histories, patient screening and all pharmacokinetics, pharmacodynamics and efficacy data, and | |
| ● | assembling a KOL panel to review the internal investigation findings, including full patient dossiers, which provided its input and conclusions from the findings. |
The investigation has been completed, and based on the work completed, the additional data from subsequent dosing of the US patients and input from the KOL panel, Jasper has concluded that the unexpected lack of efficacy observed in the US patients was not the result of any issues with drug product, but rather appears to be the result of patient selection issues, specifically the fact that it appears that 9 of the 10 patients did not have CSU as their disease did not appear to be mast-cell driven. This is specifically evidenced by the fact that 8 of the 9 patients continued to demonstrate consistent pharmacodynamic responses despite no improvement in UAS7 scores.
“I commend the Jasper team for the professional manner in which they managed the anomalous results received in July, by promptly notifying clinical sites and conducting a thorough investigation into the root cause,” said Martin Metz, M.D., Professor of Dermatology and Allergy Charité – Universitätsmedizin Berlin and member of the KOL panel. “While it appears that 9 of the 10 patients enrolled in the US sites likely did not have mast cell-driven CSU, their data still provide valuable insights into the pharmacodynamics and the safety profile of briquilimab. I’m very encouraged with the overall profile of briquilimab to date, and I look forward to seeing additional data in early 2026.”
“We are very pleased to be able to close out our internal investigation of the anomalous results seen in the BEACON data released in July and very grateful to Dr. Metz and the other KOLs who supported the rapid completion of this effort,” said Ronald Martell, President and Chief Executive Officer of Jasper. “Going forward, we are confident that the learnings from this investigation and the recommendations from our KOLs will help us minimize the enrollment of patients that may not have mast cell-driven disease. Most importantly, we are very pleased that the internal investigation demonstrated that there were no issues with the drug product utilized in the study, and we are looking forward to the last wave of BEACON data expected in Q1 2026 that will enable us to select final doses for the Phase 2b CSU study, planned to commence mid-2026.”
Conference Call / Webinar
Jasper will host a conference call and webinar today at 8:00 a.m. ET, including remarks from Dr. Martin Metz, M.D., Professor of Dermatology and Allergy Charité – Universitätsmedizin Berlin and the principal European investigator on the BEACON study. A live question and answer session with management will follow the formal presentation. A link to the webinar, including presentation slides, can be found here.
The presentation slides and a link to the live and archived webcast will also be available on the Events & News – Events page of Jasper’s Investor Relations website.
About Jasper
Jasper is a clinical-stage biotechnology company focused on developing briquilimab as a therapeutic for chronic mast cell diseases. Briquilimab is a targeted aglycosylated monoclonal antibody that blocks stem cell factor from binding to the cell-surface receptor KIT, thereby inhibiting signaling through the receptor. This inhibition disrupts the critical survival signal, leading to the depletion of the mast cells via apoptosis which removes the underlying source of the inflammatory response in mast cell driven diseases such as chronic urticaria and asthma. Jasper is currently conducting clinical studies of briquilimab as a treatment in patients with CSU, CIndU, and asthma. Briquilimab has a demonstrated efficacy and safety profile in patients and healthy volunteers, with positive clinical outcomes in both CSU, CIndU and asthma. For more information, please visit us at www.jaspertx.com.
Forward-Looking Statements
Certain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are sometimes accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding briquilimab’s potential, including with respect to its potential in mast cell driven diseases such as CSU, CIndU and asthma, its potential to reduce both airway hyperresponsiveness and the accumulation of eosinophils in the airways of asthma patients and its potential as a new treatment option for patients with asthma; briquilimab’s safety profile; Jasper’s internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg /180mg Q8W); the potential of mast cell depletion to drive a therapeutic benefit for asthma patients; the further development of briquilimab in asthma; Jasper’s expectations regarding a registrational program in CSU, including the expected timing of the Phase 2b study and dose selection; Jasper’s expected timing for presenting data from additional BEACON cohorts; and the topics expected to be discussed during the webinar. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of Jasper and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Many actual events and circumstances are beyond the control of Jasper. These forward-looking statements are subject to a number of risks and uncertainties, including general economic, political and business conditions; the risk that the potential product candidates that Jasper develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; the risk that clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this press release; the risk that prior test, study and trial results, including preliminary results for the ETESIAN study reported in this press release, may not be replicated in continuing or future studies and trials; the risk that Jasper may be unable to raise capital to continue its operations and continue the BEACON study; the risk that Jasper will be unable to successfully market or gain market acceptance of its product candidates; the risk that prior study results may not be replicated; the risk that Jasper’s product candidates may not be beneficial to patients or successfully commercialized; patients’ willingness to try new therapies and the willingness of physicians to prescribe these therapies; the effects of competition on Jasper’s business; the risk that third parties on which Jasper depends for laboratory, clinical development, manufacturing and other critical services will fail to perform satisfactorily; the risk that Jasper’s business, operations, clinical development plans and timelines, and supply chain could be adversely affected by the effects of health epidemics; the risk that Jasper will be unable to obtain and maintain sufficient intellectual property protection for its investigational products or will infringe the intellectual property protection of others; and other risks and uncertainties indicated from time to time in Jasper’s filings with the SEC, including its Annual Report on Form 10-K for the year ended December 31, 2024 and subsequent Quarterly Reports on Form 10-Q. If any of these risks materialize or Jasper’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. While Jasper may elect to update these forward-looking statements at some point in the future, Jasper specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Jasper’s assessments of any date subsequent to the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Contacts:
Alex Gray (investors)
Jasper Therapeutics
650-549-1454
agray@jaspertherapeutics.com
Joyce Allaire (investors)
LifeSci Advisors
617-435-6602
jallaire@lifesciadvisors.com
Media:
media@jaspertx.com
Exhibit 99.2

Jasper Therapeutics ETESIAN Data + BEACON Investigation Update December 2025

2 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Safe Harbor Statements Forward - Looking Statements This investor presentation and any accompanying oral presentation (together, this “Presentation”) contain forward - looking statem ents. All statements other than statements of historical fact contained in this Presentation, including statements regarding the future opportunit ies and prospects of Jasper Therapeutics, Inc. (together with its subsidiary, "Jasper" or the "Company"), including milestones, potential regulatory fili ngs and the anticipated timing thereof, patient enrollment, future timelines, business strategy, and plans and objectives for future operations, are forward - lo oking statements. Jasper has based these forward - looking statements on its estimates and assumptions and its current expectations and projections about futur e events. These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those contained in the "Risk Fa cto rs" section of the Company's Annual Report on Form 10 - K for the year ended December 31, 2024, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K that the Company has subsequently filed or may subsequently file with the SEC. In light of these risks, uncertainties and assumptions, the forward - lo oking events and circumstances discussed in this Presentation are inherently uncertain and may not occur, and actual results could differ materially and adv ers ely from those anticipated or implied in the forward - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of future events. Jasper undertakes no obligation to update publicly or revise any forward - looking statements for any reason after the date of thi s Presentation or to conform these statements to actual results or to changes in Jasper's expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither the Company nor its affiliates, adv ise rs or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or repr ese ntatives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the da te of this Presentation. Such data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company.

3 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION BEACON Trial Investigation Results • Ronald Martell, CEO Jasper Therapeutics • Dr. Daniel Adelman, Acting CMO Jasper Therapeutics BEACON Redosing Data & KOL Feedback • Dr. Martin Metz, Professor of Dermatology and Allergy, Charité – Universitätsmedizin Berlin ETESIAN Trial Interim Results • Dr.
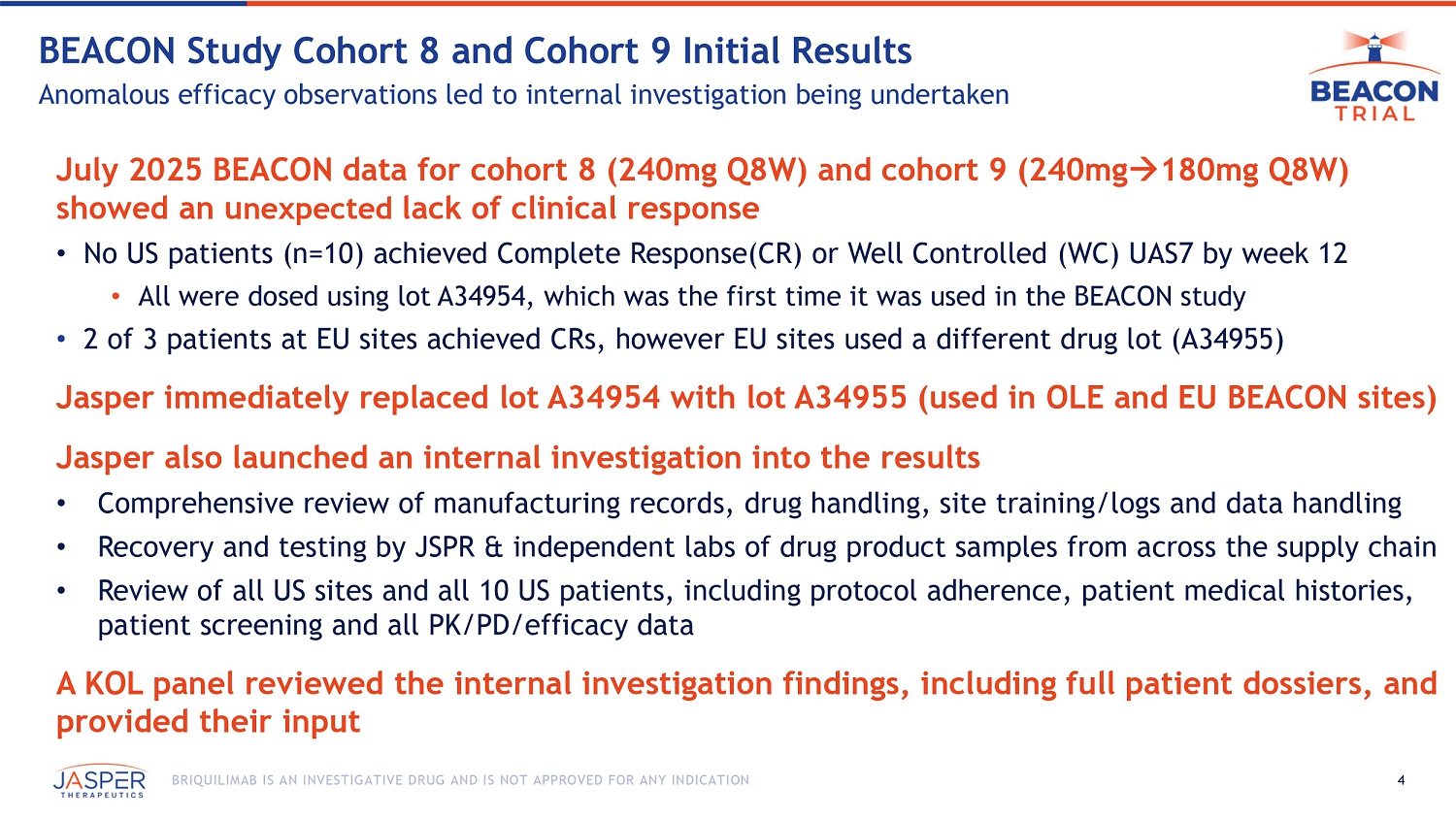
Daniel Adelman, Acting CMO Jasper Therapeutics Q&A Session Agenda 4 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION BEACON Study Cohort 8 and Cohort 9 Initial Results Anomalous efficacy observations led to internal investigation being undertaken July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg 180mg Q8W) showed an u nexpected lack of clinical response • No US patients (n=10) achieved Complete Response(CR) or Well Controlled (WC) UAS7 by week 12 • All were dosed using lot A34954, which was the first time it was used in the BEACON study • 2 of 3 patients at EU sites achieved CRs, however EU sites used a different drug lot (A34955) Jasper immediately replaced lot A34954 with lot A34955 (used in OLE and EU BEACON sites) Jasper also launched an internal investigation into the results • Comprehensive review of manufacturing records, drug handling, site training/logs and data handling • Recovery and testing by JSPR & independent labs of drug product samples from across the supply chain • Review of all US sites and all 10 US patients, including protocol adherence, patient medical histories, patient screening and all PK/PD/efficacy data A KOL panel reviewed the internal investigation findings, including full patient dossiers, and provided their input BEACON Trial Internal Investigation Results Dr. Daniel Adelman, Acting CMO Jasper Therapeutics

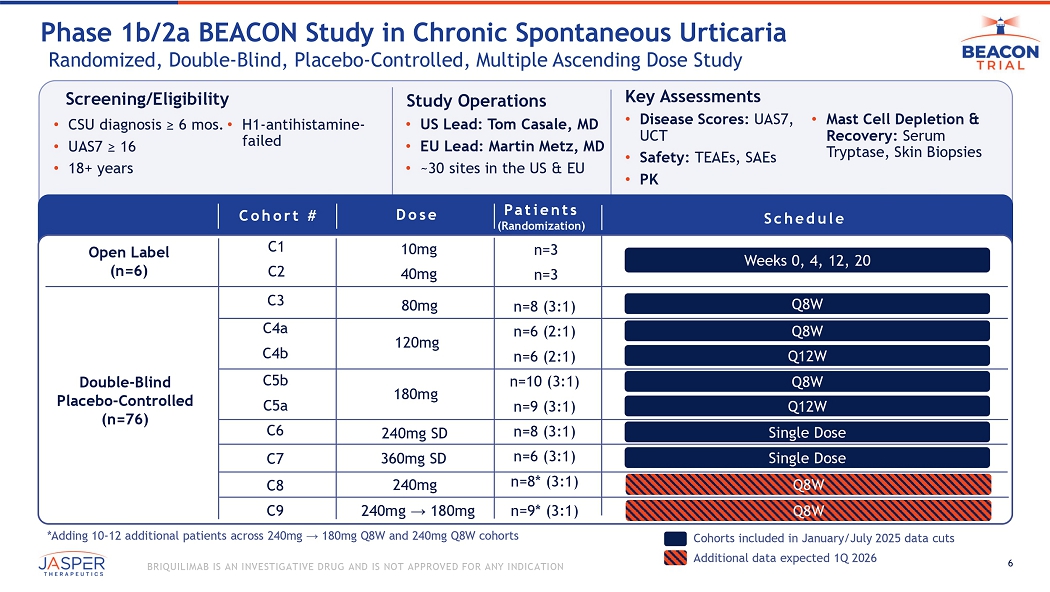
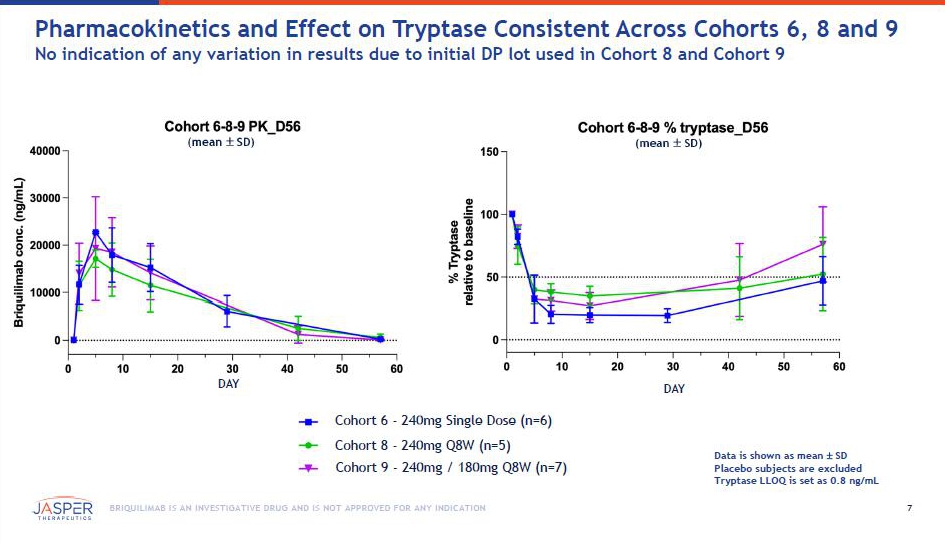
6 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Screening/Eligibility • CSU diagnosis ≥ 6 mos. • UAS7 ≥ 16 • 18+ years • H1 - antihistamine - failed Study Operations • US Lead: Tom Casale, MD • EU Lead: Martin Metz, MD • ~30 sites in the US & EU Key Assessments • Disease Scores: UAS7, UCT • Safety: TEAEs, SAEs • PK • Mast Cell Depletion & Recovery: Serum Tryptase, Skin Biopsies Phase 1b/2a BEACON Study in Chronic Spontaneous Urticaria Randomized, Double - Blind, Placebo - Controlled, Multiple Ascending Dose Study Patients (Randomization) Dose Schedule n=3 n=3 n=8 (3:1) n=6 (2:1) n=6 (2:1) n=10 (3:1) n=9 (3:1) n=8 (3:1) n= 6 (3:1) n=8* (3:1) n=9* (3:1) 10mg 40mg 80mg Open Label ( n =6) Double - Blind Placebo - Controlled ( n = 76 ) Cohorts included in January/July 2025 data cuts Weeks 0, 4, 12, 20 Q8W Q 8W Q12W Q8W Q12W Single Dose Single Dose 120mg 180mg 240mg SD 240mg → 180mg 240mg *Adding 10 - 12 additional patients across 240mg → 180mg Q8W and 240mg Q8W cohorts Additional data expected 1Q 2026 Q8W Q8W Cohort # C1 C2 C4a C4b C5b C5a C8 C3 C6 C7 C9 36 0mg SD 7 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Pharmacokinetics and Effect on Tryptase Consistent Across Cohorts 6, 8 and 9 No indication of any variation in results due to initial DP lot used in Cohort 8 and Cohort 9 (mean SD) DAY Cohort 6 - 240mg Single Dose (n=6) Cohort 8 - 240mg Q8W (n=5) Cohort 9 - 240mg / 180mg Q8W (n=7) (mean SD) DAY Data is shown as mean SD Placebo subjects are excluded Tryptase LLOQ is set as 0.8 ng/mL0
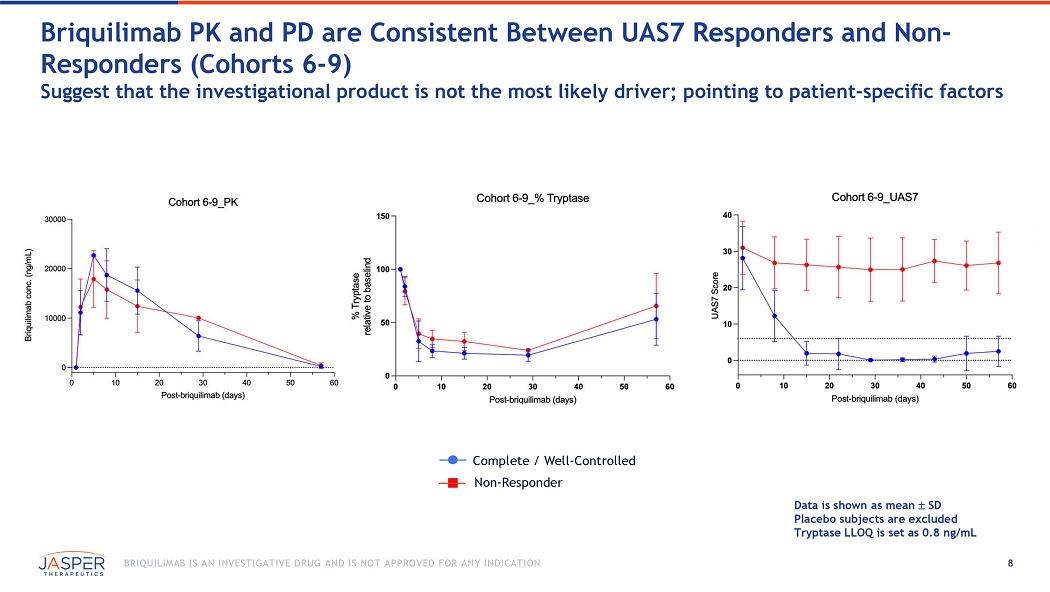
8 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Data is shown as mean SD Placebo subjects are excluded Tryptase LLOQ is set as 0.8 ng/mL Briquilimab PK and PD are Consistent B etween UAS7 Responders and Non - Responders (Cohorts 6 - 9) Suggest that the investigational product is not the most likely driver; pointing to patient - specific factors Complete / Well - Controlled Non - Responder 9 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Physical - Chemical and In - Vitro Cell Line Experiments Show No Difference in Briquilimab Lots or Clinical Samples (internal and external labs) Non - Reduced CGE (% Monomer) SEC (% Main) Sample ID 95.2 96.1 Sample # 1 94.8 96.1 Sample # 2 95.0 96.1 Sample # 3 94.9 96.1 Sample # 4 94.9 96.1 Sample # 5 94.8 96.1 Sample # 6 94.1 96.2 Sample # 7 94.5 96.1 Sample # 8 94.7 96.1 Sample # 9 95.3 97.0 Ref Standard Sample ID Sample ID SEC, size exclusion chromatography.

CGE, capillary gel electrophoresis.

SCF, stem cell factor 10 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Clinical Operations, Data Management and Site Investigations No deviations in drug management, clinical conduct or data entry/handling noted • On - site investigations • Drug substance and drug product manufacturing • Drug kitting, shipment and storage • Injection preparation, timing and storage, types of syringes, injection volume and site • Patient and site data entry, data management and analysis Site investigations • Site specific screening and dosing data reviewed for each patient • Comprehensive patient folios prepared for each patient and reviewed by CSU KOL panel • One new site enrolled 5 patients into active arms of Cohort 8 & 9 with no CR or WC responses • Community - based, clinical research center • All patients had minimal documented medical history of CSU or past treatments for CSU Internal investigation indicates anomalous results are due to patient specific factors • No deviations or issues with Drug Product or Drug Substance were noted BEACON Trial Investigation KOL Review & Redosing Data Dr. Martin Metz, Professor of Dermatology and Allergy Charité – Universitätsmedizin Berlin

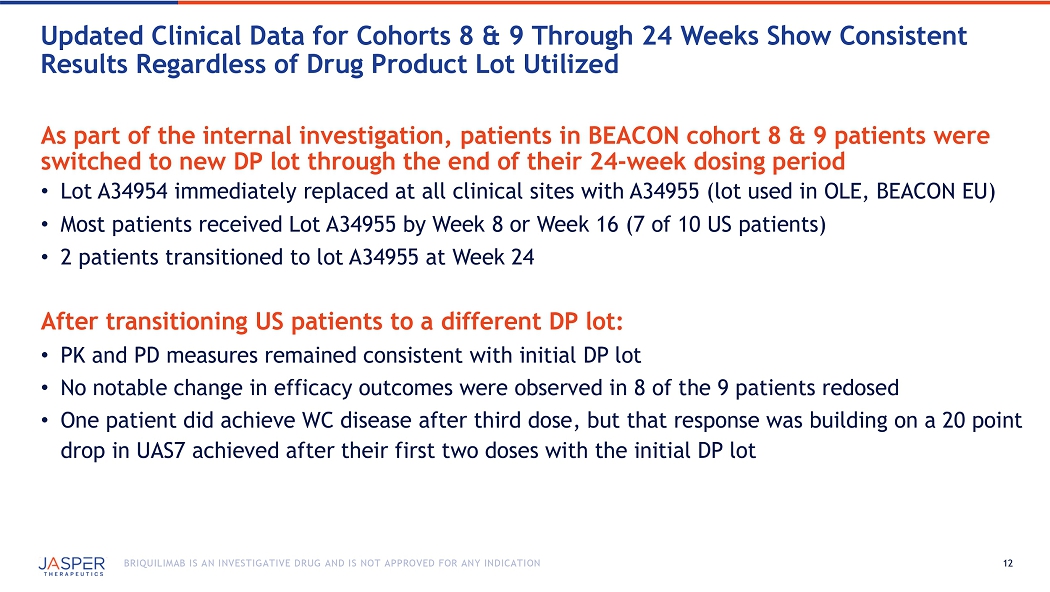
12 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION As part of the internal investigation, patients in BEACON cohort 8 & 9 patients were switched to new DP lot through the end of their 24 - week dosing period • Lot A34954 immediately replaced at all clinical sites with A34955 ( lot used in OLE , BEACON EU ) • Most patients received Lot A34955 by Week 8 or Week 16 (7 of 10 US patients) • 2 patients transitioned to lot A34955 at Week 24 After transitioning US patients to a different DP lot: • PK and PD measures remained consistent with initial DP lot • No notable change in efficacy outcomes were observed in 8 of the 9 patients redosed • One patient did achieve WC disease after third dose, but that response was building on a 20 point drop in UAS7 achieved after their first two doses with the initial DP lot Updated Clinical Data for Cohorts 8 & 9 Through 24 Weeks Show Consistent Results Regardless of Drug Product Lot Utilized 13 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 0 25 50 75 100 125 150 175 Cohort 8 & 9 Cohorts 8 & 9 – Serum Tryptase Deep reductions in tryptase levels maintained after transition to different DP lot 7 of 10 US Patients switched to Lot A34955 Days Serum Tryptase (ng/ml) Cohort 8 (n=6) – 240mg Q8W Cohort 9 (n=7) – 240mg 180mg Q8W Note: per protocol, larger intervals for blood sampling for PK and tryptase measurements following week 8 on study Placebo (n=19)

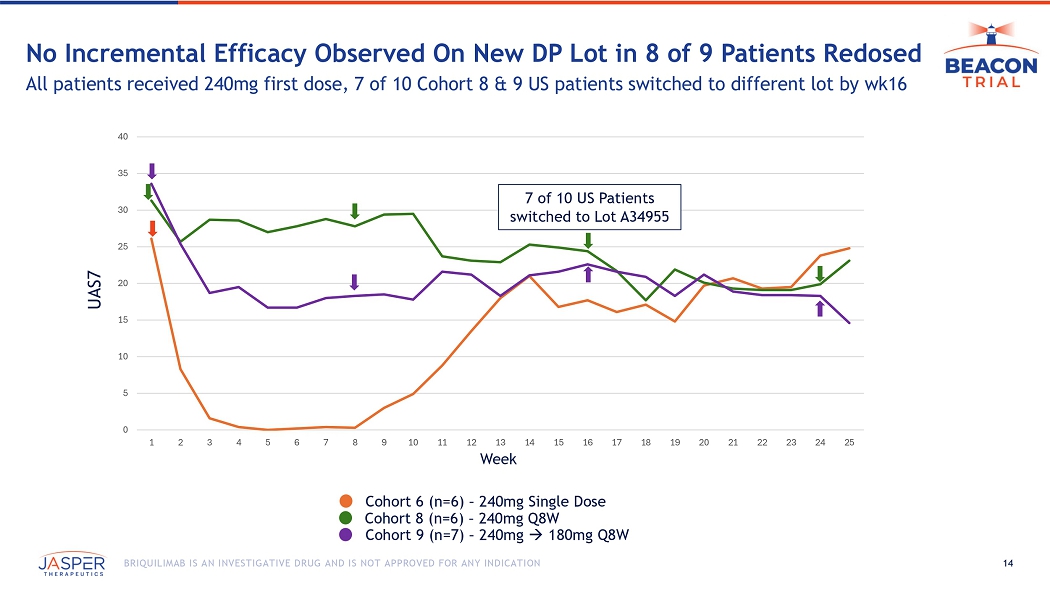
14 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION No Incremental Efficacy Observed On New DP Lot in 8 of 9 Patients Redosed All patients received 240mg first dose, 7 of 10 Cohort 8 & 9 US patients switched to different lot by wk16 0 5 10 15 20 25 30 35 40 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Week UAS7 7 of 10 US Patients switched to Lot A34955 Cohort 8 (n=6) – 240mg Q8W Cohort 9 (n=7) – 240mg 180mg Q8W Cohort 6 (n=6) – 240mg Single Dose 15 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Cohort 9: Single Late Responder Achieved WC Disease After 3 rd dose 20 - point reduction in UAS7 achieved with initial DP Lot 0 5 10 15 20 25 30 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29

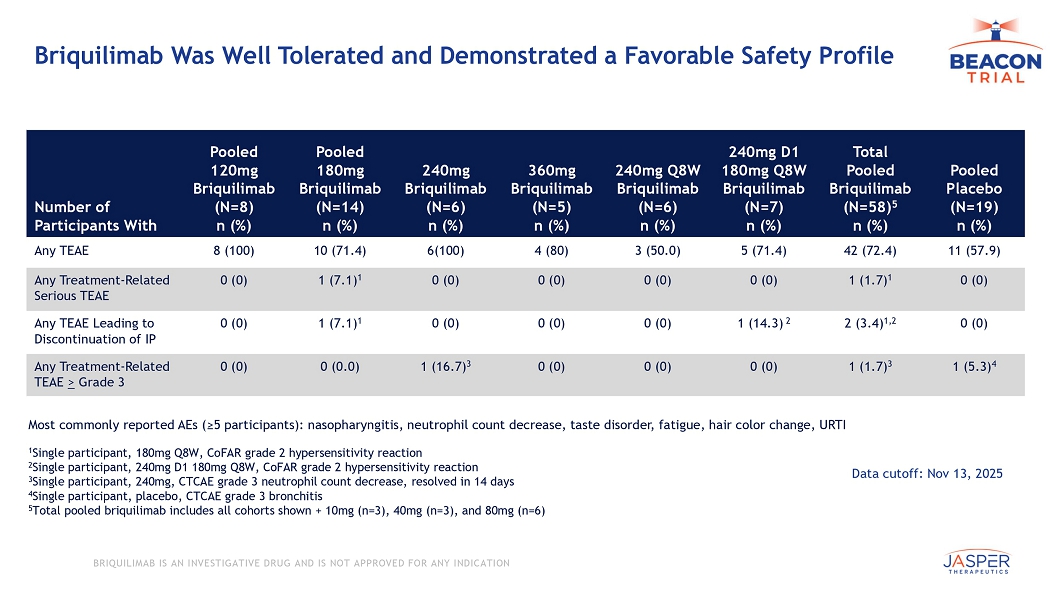
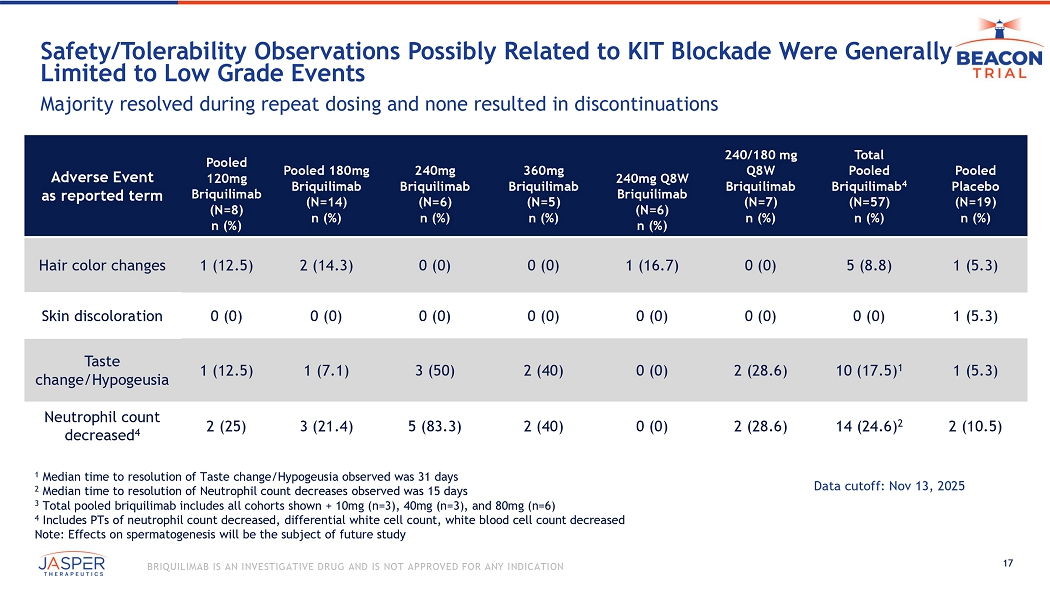
CONFIDENTIAL BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab Was Well Tolerated and Demonstrated a Favorable Safety Profile Pooled Placebo (N=19) n (%) Total Pooled Briquilimab (N=58) 5 n (%) 240mg D1 180mg Q8W Briquilimab (N=7) n (%) 240mg Q8W Briquilimab (N=6) n (%) 360mg Briquilimab (N=5) n (%) 240mg Briquilimab (N=6) n (%) Pooled 180mg Briquilimab (N=14) n (%) Pooled 120mg Briquilimab (N=8) n (%) Number of Participants With 11 (57.9) 42 (72.4) 5 (71.4) 3 (50.0) 4 (80) 6(100) 10 (71.4) 8 (100) Any TEAE 0 (0) 1 (1.7) 1 0 (0) 0 (0) 0 (0) 0 (0) 1 (7.1) 1 0 (0) Any Treatment - Related Serious TEAE 0 (0) 2 (3.4) 1,2 1 (14.3) 2 0 (0) 0 (0) 0 (0) 1 (7.1) 1 0 (0) Any TEAE Leading to Discontinuation of IP 1 (5.3) 4 1 (1.7) 3 0 (0) 0 (0) 0 (0) 1 (16.7) 3 0 (0.0) 0 (0) Any Treatment - Related TEAE > Grade 3 1 Single participant, 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 2 Single participant, 240mg D1 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 3 Single participant, 240 mg, CTCAE grade 3 neutrophil count decrease, resolved in 14 days 4 Single participant, placebo, CTCAE grade 3 bronchitis 5 Total pooled briquilimab includes all cohorts shown + 10mg (n=3), 40mg (n=3), and 80mg (n=6) Most commonly reported AEs (≥5 participants): nasopharyngitis, neutrophil count decrease, taste disorder, fatigue, hair color ch ange, URTI Data cutoff: Nov 13, 2025 17 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Safety/Tolerability O bservations P ossibly R elated to KIT Blockade W ere G enerally L imited to Low G rade E vents Majority resolved during repeat dosing and none resulted in discontinuations Pooled Placebo (N=19) n (%) Total Pooled Briquilimab 4 (N=57) n (%) 240/180 mg Q8W Briquilimab (N=7) n (%) 240mg Q8W Briquilimab (N=6) n (%) 360mg Briquilimab (N=5) n (%) 240mg Briquilimab (N=6) n (%) Pooled 180mg Briquilimab (N=14) n (%) Pooled 120mg Briquilimab (N=8) n (%) Adverse Event as reported term 1 (5.3) 5 (8.8) 0 (0) 1 (16.7) 0 (0) 0 (0) 2 (14.3) 1 (12.5) Hair color changes 1 (5.3) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Skin discoloration 1 (5.3) 10 (17.5) 1 2 (28.6) 0 (0) 2 (40) 3 (50) 1 (7.1) 1 (12.5) Taste change/Hypogeusia 2 (10.5) 14 (24.6) 2 2 (28.6) 0 (0) 2 (40) 5 (83.3) 3 (21.4) 2 (25) Neutrophil count decreased 4 1 Median time to resolution of Taste change/Hypogeusia observed was 31 days 2 Median time to resolution of Neutrophil count decreases observed was 15 days 3 Total pooled briquilimab includes all cohorts shown + 10mg (n=3), 40mg (n=3), and 80mg (n=6) 4 Includes PTs of neutrophil count decreased, differential white cell count, white blood cell count decreased Note: Effects on spermatogenesis will be the subject of future study Data cutoff: Nov 13, 2025
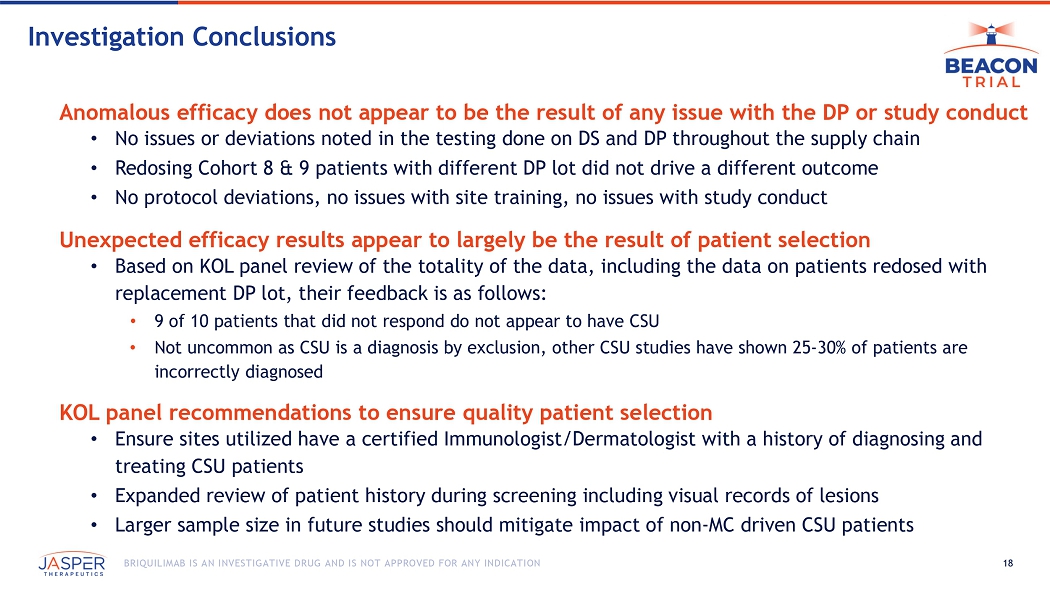
18 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Investigation Conclusions Anomalous efficacy does not appear to be the result of any issue with the DP or study conduct • No issues or deviations noted in the testing done on DS and DP throughout the supply chain • Redosing Cohort 8 & 9 patients with different DP lot did not drive a different outcome • No protocol deviations, no issues with site training, no issues with study conduct Unexpected efficacy results appear to largely be the result of patient selection • Based on KOL panel review of the totality of the data, including the data on patients redosed with replacement DP lot, their feedback is as follows: • 9 of 10 patients that did not respond do not appear to have CSU • Not uncommon as CSU is a diagnosis by exclusion, other CSU studies have shown 25 - 30% of patients are incorrectly diagnosed KOL panel recommendations to ensure quality patient selection • Ensure sites utilized have a certified Immunologist/Dermatologist with a history of diagnosing and treating CSU patients • Expanded review of patient history during screening including visual records of lesions • Larger sample size in future studies should mitigate impact of non - MC driven CSU patients Briquilimab in Allergic Asthma Dr. Daniel Adelman, Acting CMO Jasper Therapeutics

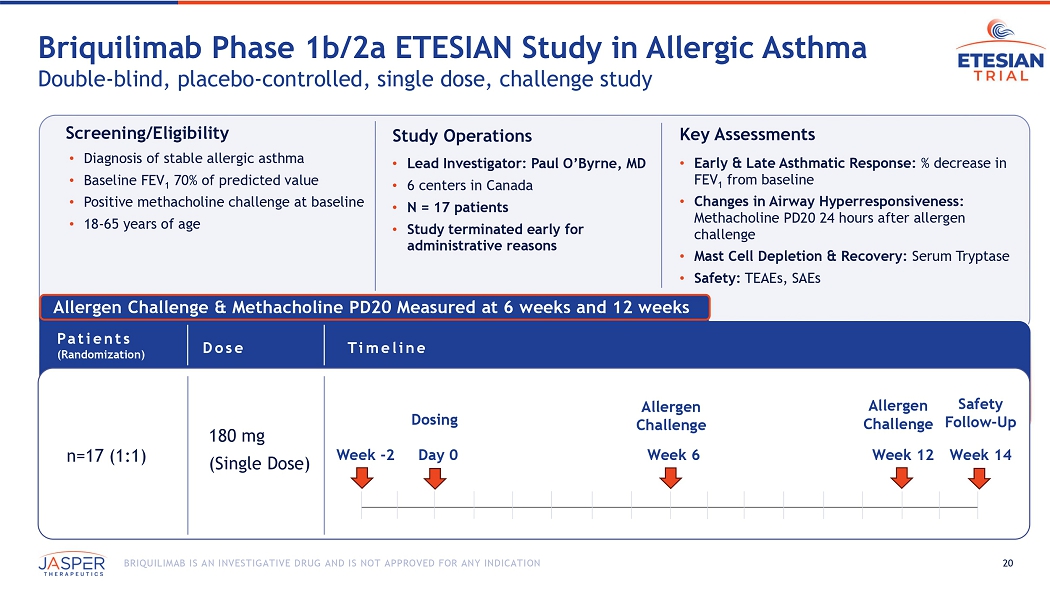
20 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Allergen Challenge & Methacholine PD20 Measured at 6 weeks and 12 weeks Briquilimab Phase 1b/2a ETESIAN Study in Allergic A sthma Double - blind, placebo - controlled, single dose, challenge study n=17 (1:1) 180 mg (Single Dose) Dose Timeline Patients (Randomization) Day 0 Week 12 Week 6 Allergen Challenge Allergen Challenge Week 14 Safety Follow - Up Week - 2 Dosing Screening/Eligibility • Diagnosis of stable allergic asthma • Baseline FEV 1 70% of predicted value • Positive methacholine challenge at baseline • 18 - 65 years of age Study Operations • Lead Investigator: Paul O’Byrne, MD • 6 centers in Canada • N = 17 patients • Study terminated early for administrative reasons Key Assessments • Early & Late Asthmatic Response: % decrease in FEV 1 from baseline • Changes in Airway Hyperresponsiveness: Methacholine PD20 24 hours after allergen challenge • Mast Cell Depletion & Recovery: Serum Tryptase • Safety: TEAEs, SAEs 21 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Single 180mg Briquilimab Dose Drives Serum Tryptase Reductions at 6 Weeks PK/PD effect consistent with 180mg briquilimab activity observed in other studies
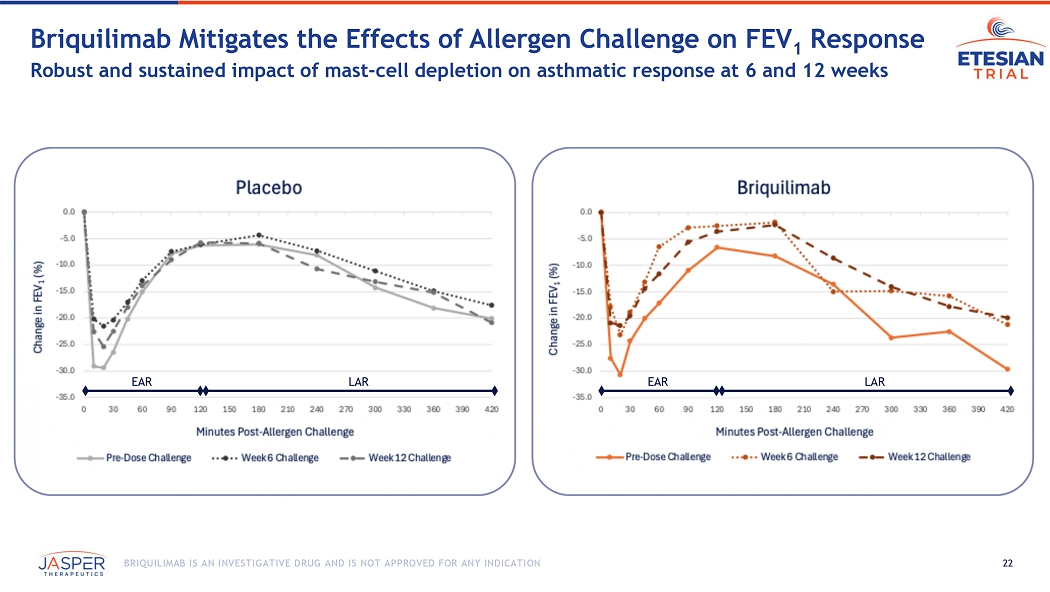
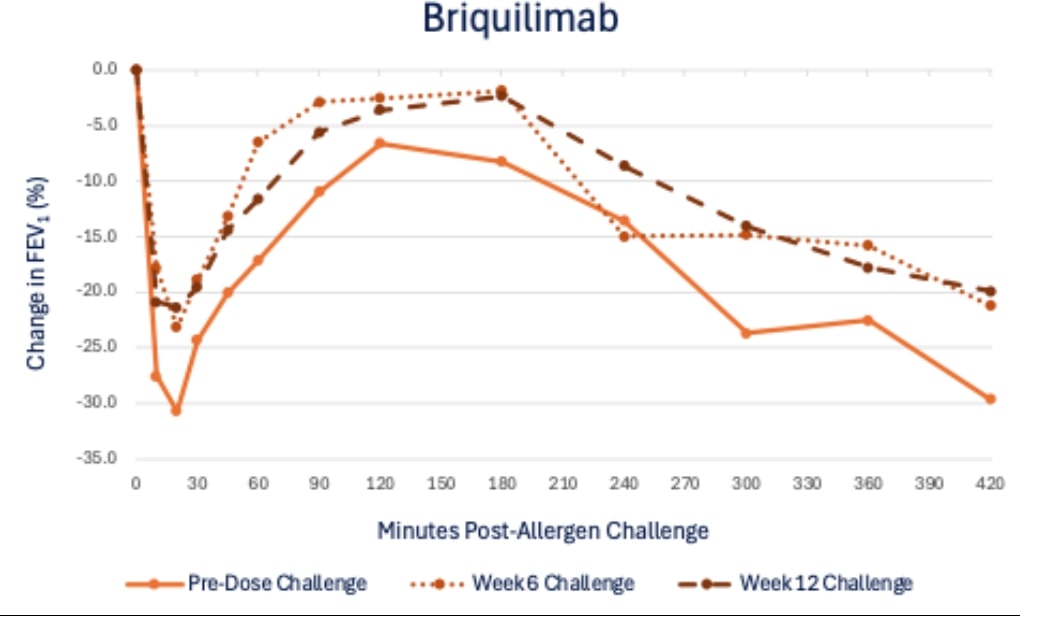
22 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab M itigates the Effects of Allergen Challenge on FEV 1 Response Robust and sustained impact of mast - cell depletion on asthmatic response at 6 and 12 weeks EAR LAR EAR LAR 23 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Patient Examples - SD 180mg Briquilimab Dose Substantial improvements in EAR & LAR observed in multiple patients
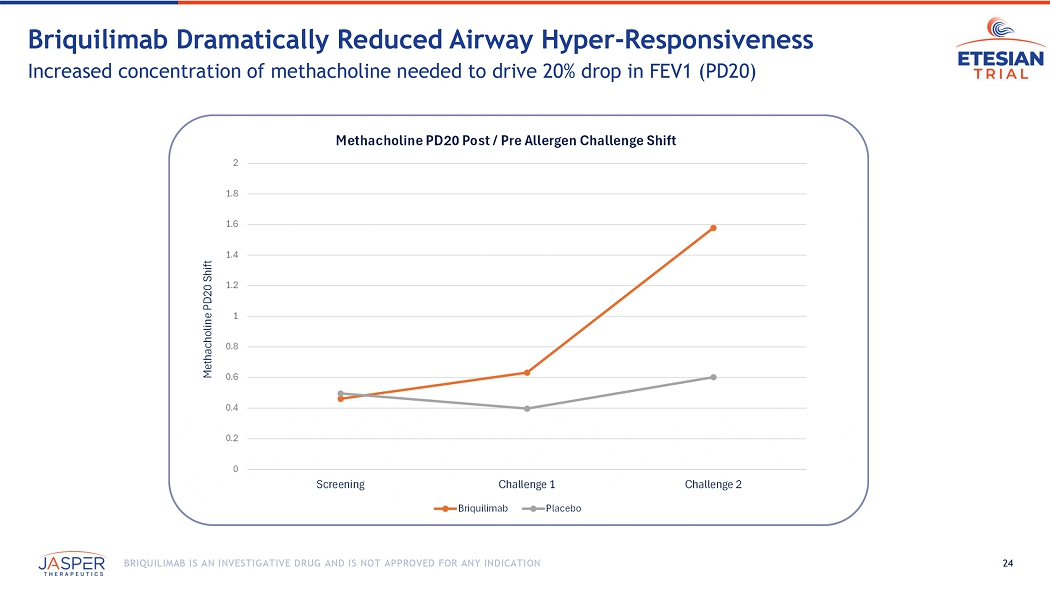
24 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab Dramatically Reduced Airway Hyper - Responsiveness Increased concentration of methacholine needed to drive 20% drop in FEV1 (PD20) 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 2 Screening Challenge 1 Challenge 2 Methacholine PD20 Shift Methacholine PD20 Post / Pre Allergen Challenge Shift Briquilimab Placebo 25 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Sputum Eosinophil Response Suppressed by Briquilimab Substantial eosinophil reduction at both 6 - week and 12 - week allergen challenge timepoints Pre - dose Allergen Challenge Allergen Challenge #1 Allergen Challenge #2 Percent Sputum Eosinophils
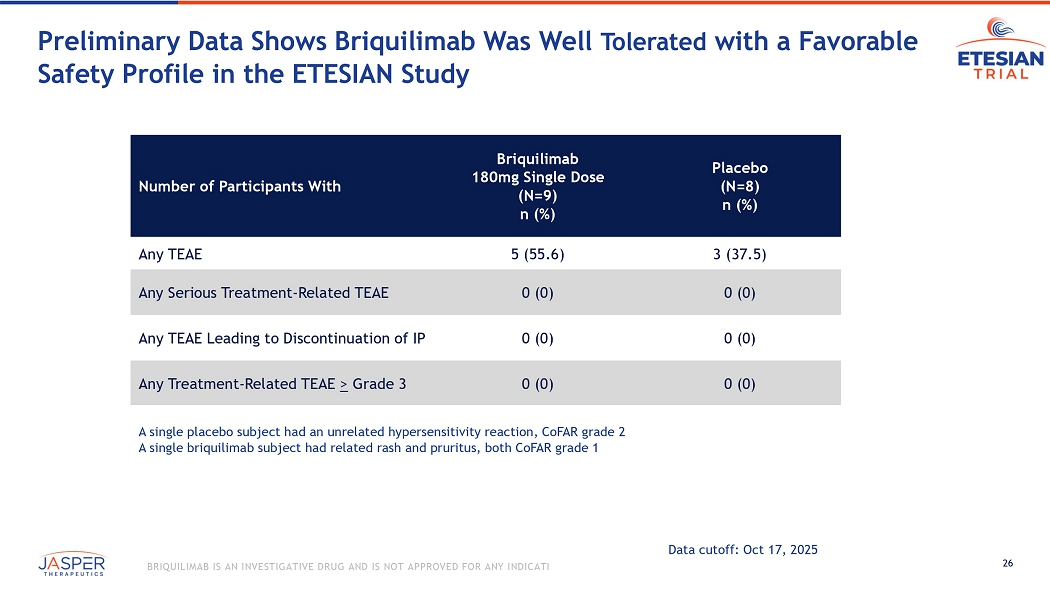
26 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Preliminary Data Shows Briquilimab Was Well Tolerated with a Favorable Safety Profile in the ETESIAN Study Placebo (N=8) n (%) Briquilimab 180mg Single Dose (N=9) n (%) Number of Participants With 3 (37.5) 5 (55.6) Any TEAE 0 (0) 0 (0) Any Serious Treatment - Related TEAE 0 (0) 0 (0) Any TEAE Leading to Discontinuation of IP 0 (0) 0 (0) Any Treatment - Related TEAE > Grade 3 A single placebo subject had an unrelated hypersensitivity reaction, CoFAR grade 2 A single briquilimab subject had related rash and pruritus, both CoFAR grade 1 Data cutoff: Oct 17, 2025 27 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Placebo (N=8) n (%) Total Pooled Briquilimab (N=9) n (%) Adverse Event as reported term 0 (0) 0 (0) Hair color changes 0 (0) 0 (0) Skin discoloration 0 (0) 1 (11.1) 1 Taste change/Hypogeusia 0 (0) 1 (11.1) 2 Neutrophil count decreased 1 CTCAE grade 1 dysgeusia resolved after 55 days 2 CTCAE grade 1 white blood cell count decreased, resolving at time of study completion Data cutoff: Oct 17, 2025 Safety Observations Possibly Related to KIT Blockade Were Limited to Grade 1 Events
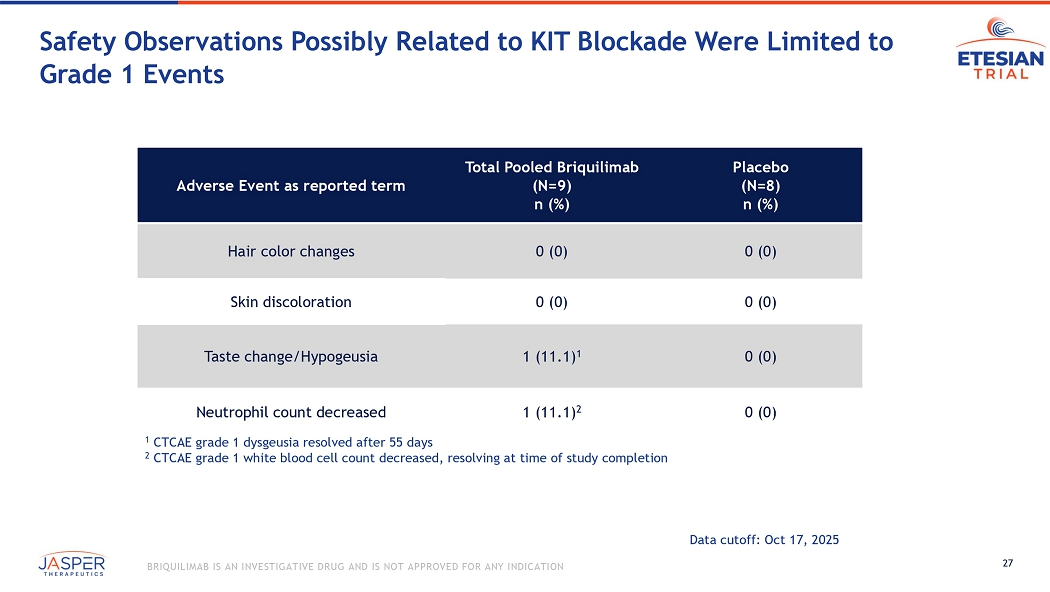
28 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION ETESIAN Study Summary First time a potent KIT - specific therapeutic targeting mast cells has demonstrated potential for the treatment of asthma PK/PD demonstrated deep & sustained biologic activity in key biomarkers, including: • Serum tryptase • Sputum eosinophils Robust improvements observed in both aspects of the challenge study: • Improvement in mean FEV1 response seen at week 6 and week 12 in allergen challenge • Reductions in airway hyper - responsiveness observed in the methacoline challenge Data suggest that mast cells play a central role in airway inflammation • Given that the mast cell may be a central actor in both T2 high and T2 low disease, further developme nt in the broader asthma population is warranted • Next steps being evaluated, including potential dose - ranging/repeat dose studies in asthma Next Steps Ron Martell, CEO

30 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Program Status and Next Steps Briquilimab development in mast - cell driven diseases continues to advance in multiple indications CSU - Briquilimab continues to demonstrate rapid onset, deep clinical response and favorable safety profile • More than 24pt drop in UAS7 with 82% CR and 91% WC disease by week 4 with single dose (240mg SD & 360mg SD, n=11) • Highly effective in OLE study at 180mg Q8W with 73% CR and 82% WC disease at 12 weeks (n=11) • Continued favorable safety profile, r epeat dose of 240mg Q8W and 240mg/180mg Q8W were generally well tolerated CSU - Additional BEACON and OLE data expected in 1H Q1 2026 will enable Phase 2b dose selection • Efficacy and safety data on additional patients enrolled in C8 (240mg Q8W) & C9 (240mg 180mg Q8W) • 24+ weeks of safety data on original patients enrolled in C8 and C9 • 20+ weeks of efficacy and safety data on ~40 CSU patients in OLE study (180mg Q8W) • Consistent PK/PD profile will enable rapid and robust population exposure analysis and dose selection CindU – Multi - dose data in CIndU expected in Q1 2026 data update • 15+ weeks of efficacy and safety data on ~15 CIndU patients in OLE study (180mg Q8W) Asthma – ETESIAN data provide strong proof of concept for briquilimab MOA in asthma • The initial results demonstrate the potential to reduce both airway hypersensitivity and the release of eosinophils • Both of which are key factors in managing chronic asthma and reducing exacerbations. • Jasper evaluating next steps to advance briquilimab in chronic asthma Jasper Therapeutics NASDAQ: JSPR December 2025
