UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the Month of November 2025
Commission File Number: 001-41621
RADIOPHARM THERANOSTICS LIMITED
(Name of Registrant)
Level 3, 62 Lygon Street, Carlton South, Victoria, 3053, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ☐ No ☒
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-Radiopharm Theranostics Limited (the “Company”) published two announcements (the “Public Notice”) to the Australian Securities Exchange on November 20, 2025 titled:
RADIOPHARM THERANOSTICS LIMITED
EXPLANATORY NOTE
“Presentation to Annual General Meeting”
“Results of Meeting”
A copy of the Public Notice is attached as an exhibit to this report on Form 6-K.
This report on Form 6-K (including the exhibit hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
EXHIBITS
| Exhibit Number |
Description | |
| 99.1 | Presentation to Annual General Meeting | |
| 99.2 | Results of Meeting |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| RADIOPHARM THERANOSTICS LIMITED | ||
| Date: November 20, 2025 | By: | /s/ Phillip Hains |
| Phillip Hains | ||
| Company Secretary | ||
Exhibit 99.1


NOVEMBER 20 , 202 5 NASDAQ: RADX / ASX: RAD Annual General Meeting Notice & Disclaimer 2 The information in this presentation does not constitute personal investment advice . The presentation is not intended to be comprehensive or provide all information required by investors to make an informed decision on any investment in Radiopharm Theranostics Ltd ACN 647 877 889 (Company) . In preparing this presentation, the Company did not take into account the investment objectives, financial situation and particular needs of any particular investor . Further advice should be obtained from a professional investment adviser before taking any action on any information dealt with in the presentation . Those acting upon any information without advice do so entirely at their own risk . Whilst this presentation is based on information from sources which are considered reliable, no representation or warranty, express or implied, is made or given by or on behalf of the Company, any of its directors, or any other person about the accuracy, completeness or fairness of the information or opinions contained in this presentation . No responsibility or liability is accepted by any of them for that information or those opinions or for any errors, omissions, misstatements (negligent or otherwise) or for any communication written or otherwise, contained or referred to in this presentation . Neither the Company nor any of its directors, officers, employees, advisers, associated persons or subsidiaries are liable for any direct, indirect or consequential loss or damage suffered by any person as a result of relying upon any statement in this presentation or any document supplied with this presentation, or by any future communications in connection with those documents and all of those losses and damages are expressly disclaimed . Certain statements contained in this presentation, including, without limitation, statements containing the words “believes,” “plans,” “expects,” “anticipates,” and words of similar import, constitute “forward - looking statements . ” Such forward - looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of the Company to be materially different from any future results, performance or achievements expressed or implied by such forward - looking statements . Such factors include, among others, the following : the risk that our clinical trials will be delayed and not completed on a timely basis ; the risk that the results from the clinical trials are not as favourable as we anticipate ; the risk that our clinical trials will be more costly than anticipated ; and the risk that applicable regulatory authorities may ask for additional data, information or studies to be completed or provided prior to their approval of our products . Given these uncertainties, undue reliance should not be placed on such forward - looking statements . The Company disclaims any obligation to update any such factors or to publicly announce the results of any revisions to any of the forward - looking statements contained herein to reflect future events or developments except as required by law . This presentation is not a prospectus or other disclosure document under the Corporations Act 2001 ( Cth ) and will not be lodged with the Australian Securities and Investments Commission . This presentation is for information purposes only and is not an invitation or offer of securities for subscription, purchase or sale in any jurisdiction . The distribution of this presentation (including electronically) outside Australia may be restricted by law . If you come into possession of this presentation, you should observe such restrictions as any non - compliance with these restrictions could contravene applicable securities laws (see the section captioned ‘International offer restrictions’) . In particular, this document does not constitute an offer to sell, or a solicitation of an offer to buy, securities in the United States . The New Shares have not been, and will not be, registered under the US Securities Act of 1933 and may not be offered or sold in the United States except in transactions exempt from, or not subject to, the registration requirements of the US Securities Act and applicable US state securities laws . Any opinions expressed reflect the Company’s position at the date of this presentation and are subject to change .
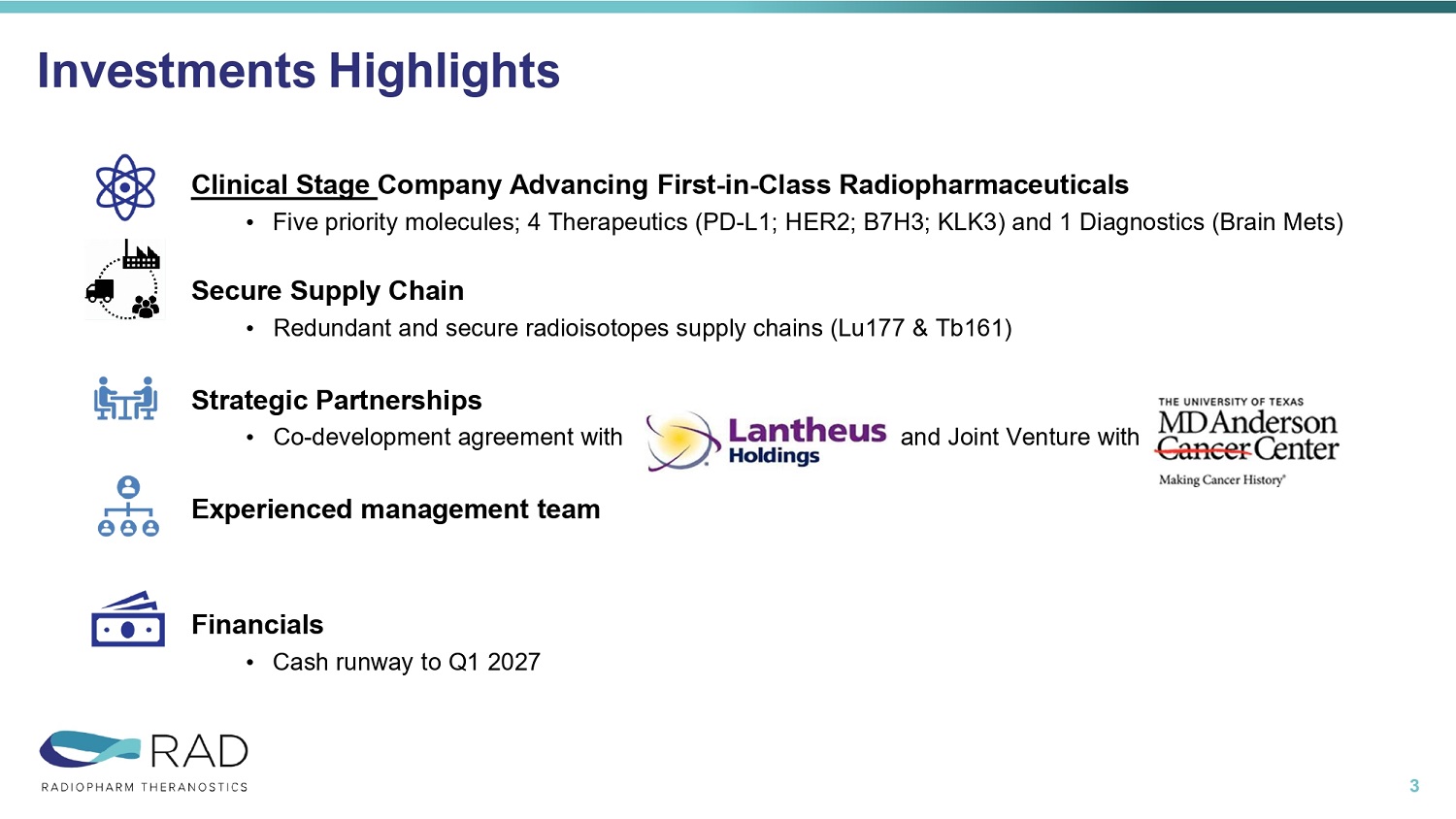
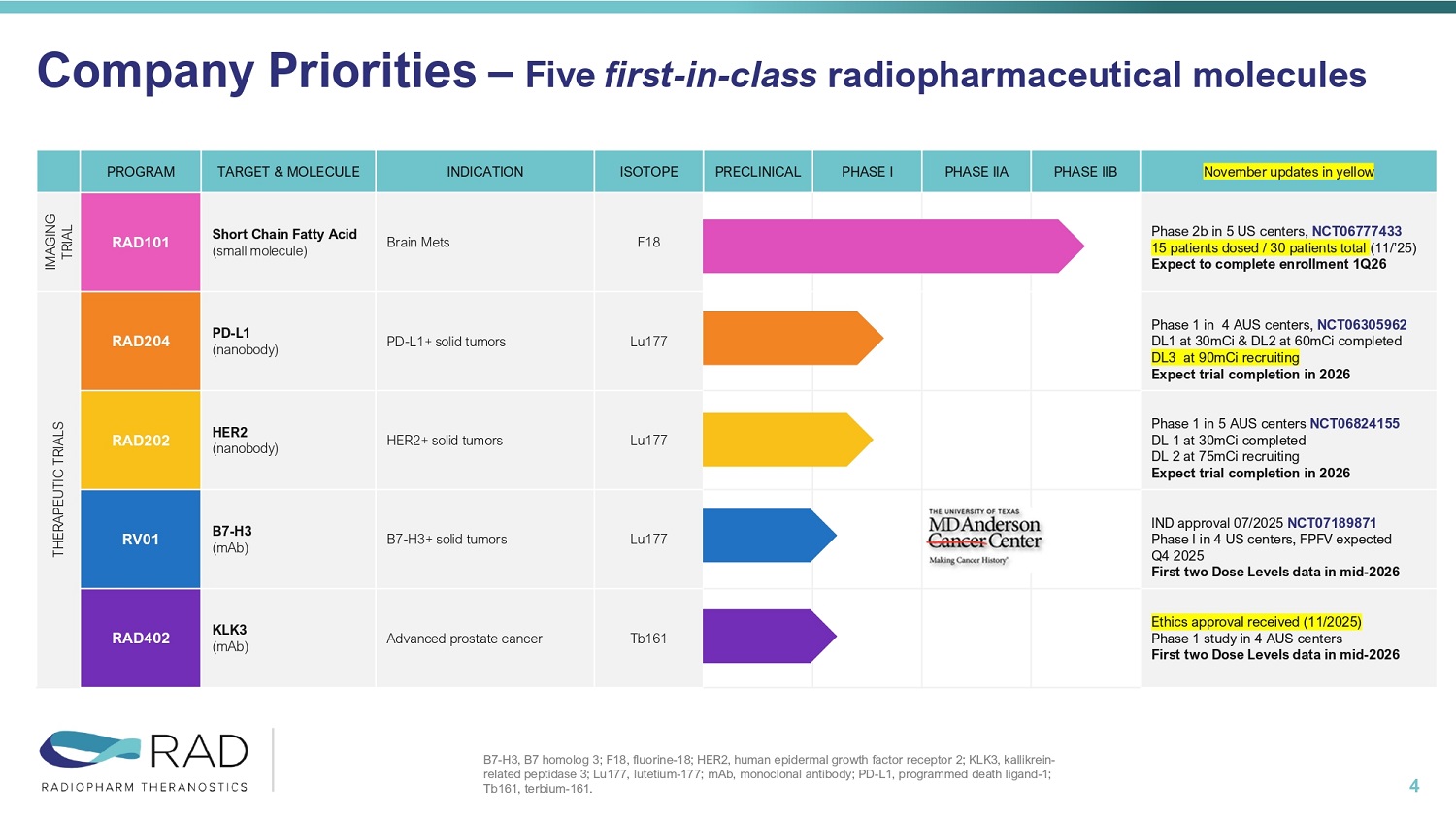
Investments Highlights 3 Clinical Stage Company Advancing First - in - Class Radiopharmaceuticals • Five priority molecules; 4 Therapeutics (PD - L1; HER2; B7H3; KLK3) and 1 Diagnostics (Brain Mets) Secure Supply Chain • Redundant and secure radioisotopes supply chains (Lu177 & Tb161) Strategic Partnerships • Co - development agreement with and Joint Venture with MD Anderson Experienced management team Financials • Cash runway to Q1 2027 November updates in yellow PHASE IIB PHASE IIA PHASE I PRECLINICAL ISOTOPE INDICATION TARGET & MOLECULE PROGRAM Phase 2b in 5 US centers, NCT06777433 15 patients dosed / 30 patients total (11/’25) Expect to complete enrollment 1Q26 F18 Brain Mets Short Chain Fatty Acid (small molecule) RAD101 IMAGING TRIAL Phase 1 in 4 AUS centers, NCT06305962 DL1 at 30mCi & DL2 at 60mCi completed DL3 at 90mCi recruiting Expect trial completion in 2026 Lu177 PD - L1+ solid tumors PD - L1 (nanobody) RAD204 THERAPEUTIC TRIALS Phase 1 in 5 AUS centers NCT06824155 DL 1 at 30mCi completed DL 2 at 75mCi recruiting Expect trial completion in 2026 Lu177 HER2+ solid tumors HER2 (nanobody) RAD202 IND approval 07/2025 NCT07189871 Phase I in 4 US centers, FPFV expected Q4 2025 First two Dose Levels data in mid - 2026 Lu177 B7 - H3+ solid tumors B7 - H3 (mAb) RV01 Ethics approval received (11/2025) Phase 1 study in 4 AUS centers First two Dose Levels data in mid - 2026 Tb161 Advanced prostate cancer KLK3 (mAb) RAD402 Company Priorities – Five first - in - class radiopharmaceutical molecules 4 B7 - H3, B7 homolog 3; F18, fluorine - 18; HER2, human epidermal growth factor receptor 2; KLK3, kallikrein - related peptidase 3; Lu177, lutetium - 177; mAb, monoclonal antibody; PD - L1, programmed death ligand - 1; Tb161, terbium - 161.
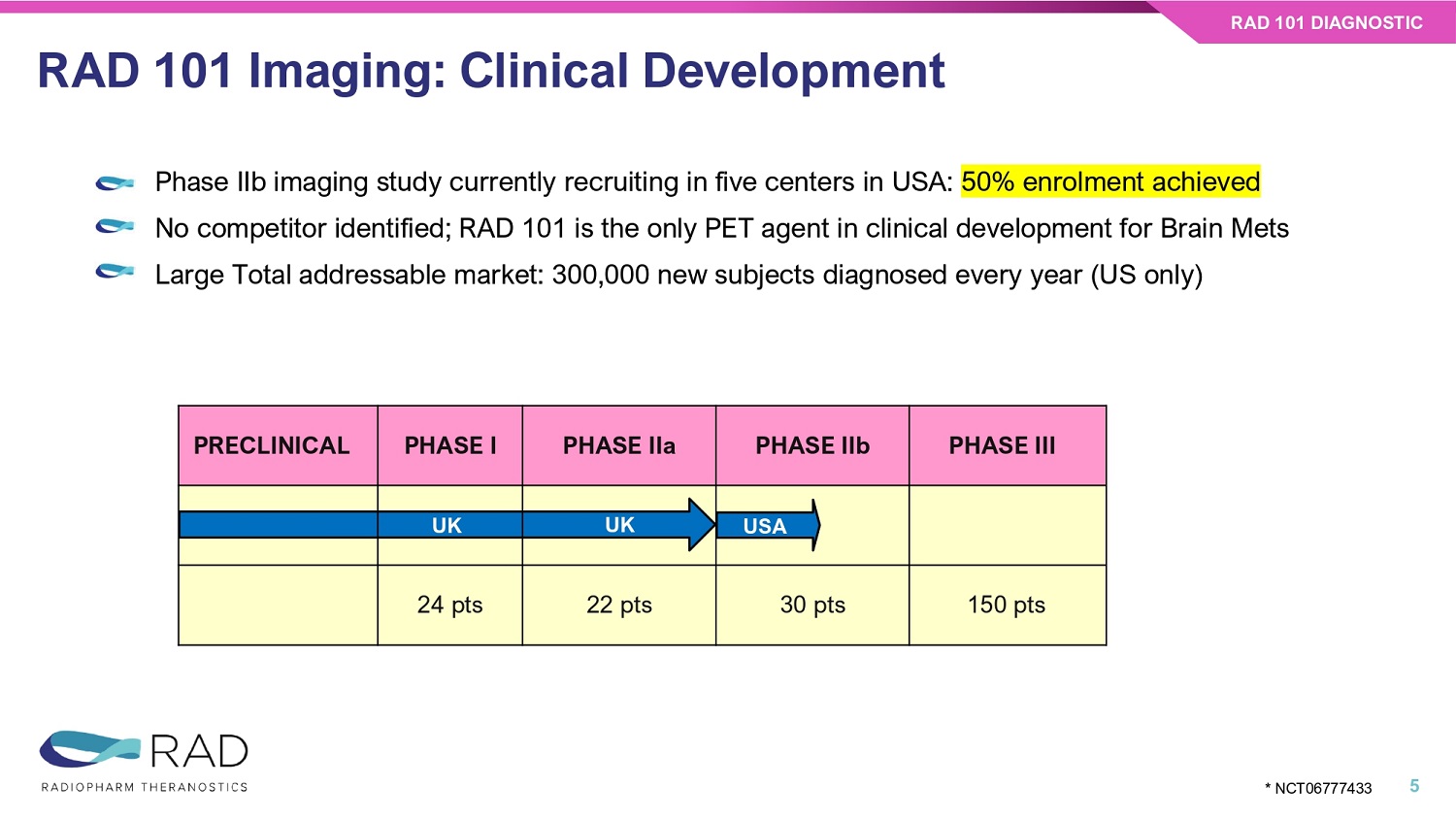
RAD 101 Imaging: Clinical Development RAD 101 DIAGNOSTIC 5 Phase IIb imaging study currently recruiting in five centers in USA : 50% enrolment achieved No competitor identified; RAD 101 is the only PET agent in clinical development for Brain Mets Large Total addressable market: 300,000 new subjects diagnosed every year (US only) USA PHASE III PHASE IIb PHASE IIa PHASE I PRECLINICAL UK UK 150 pts 30 pts 22 pts 24 pts * NCT06777433 • Images from n=3 subjects in the ongoing study released • Showing increased metabolic activity in areas with equivocal MRI findings (suspected relapse) • N=15 subjects dosed as of 11/17/2025 Clinical Data from Phase IIb RAD 101 DIAGNOSTIC 6


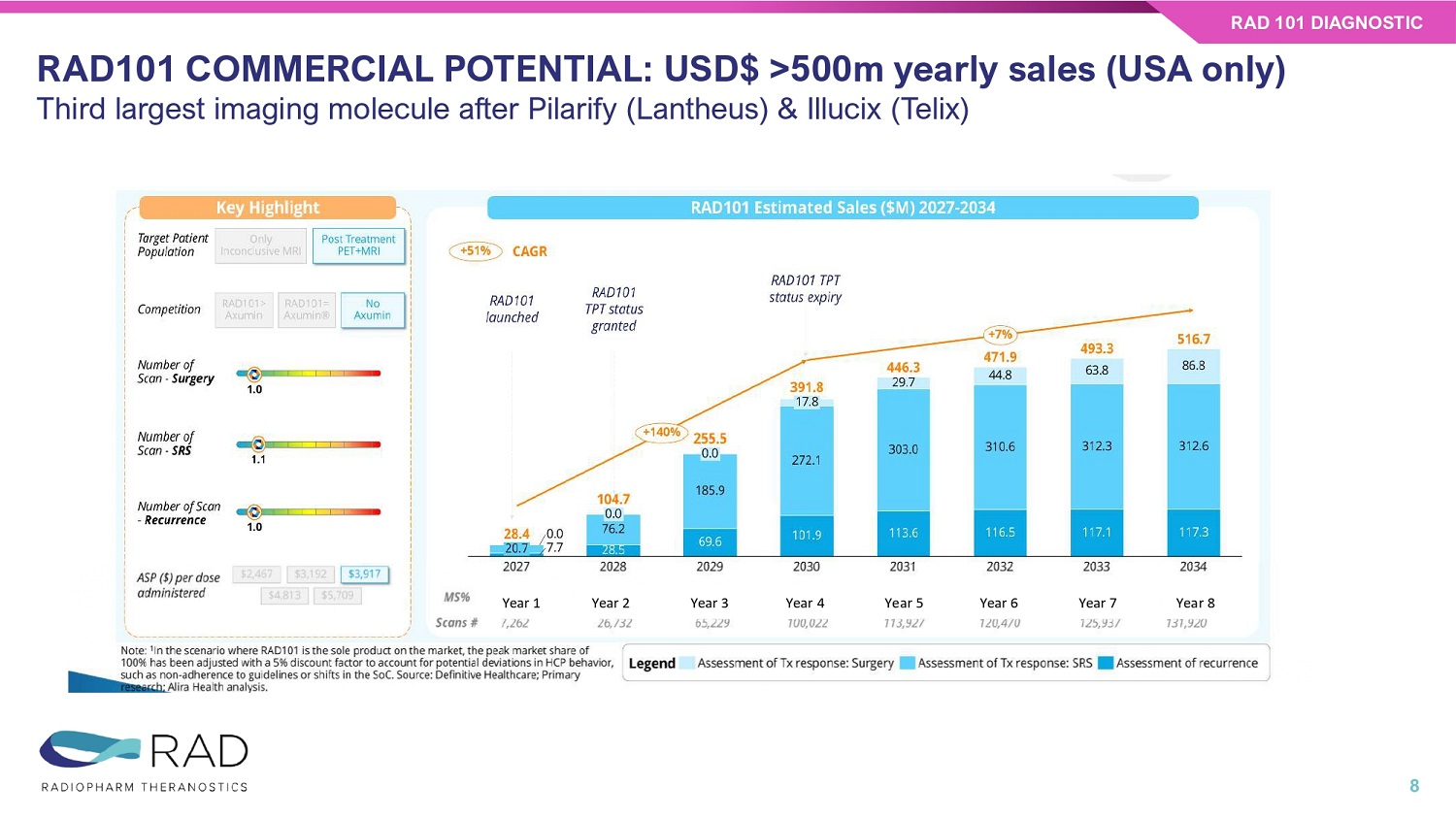
Phase IIb • 11 patient data to be released by Q1 2026 • Trial completion N=30/30 pts by Q1 2026 • Phase 2b readout in the first half of 2026 Phase III • FDA Meeting to align on Phase III - mid 2026 • Phase III start – Q4 2026 Next Steps RAD 101 DIAGNOSTIC 7 RAD101 COMMERCIAL POTENTIAL: USD$ >500m yearly sales (USA only) Third largest imaging molecule after Pilarify (Lantheus) & Illucix (Telix) RAD 101 DIAGNOSTIC 8 Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Year 7 Year 8

RAD 204 THERAPEUTIC 9 Molecule: 177Lu - RAD204 Targeting MoA : PD - L1 Therapeutic for: PD - L1+ TUMORS Dose Dose Level 10 (0.37 GBq) Imaging dose Phase 0 (Imaging Period with 177 Lu - RAD204 im ) 30 mCi (1.1.
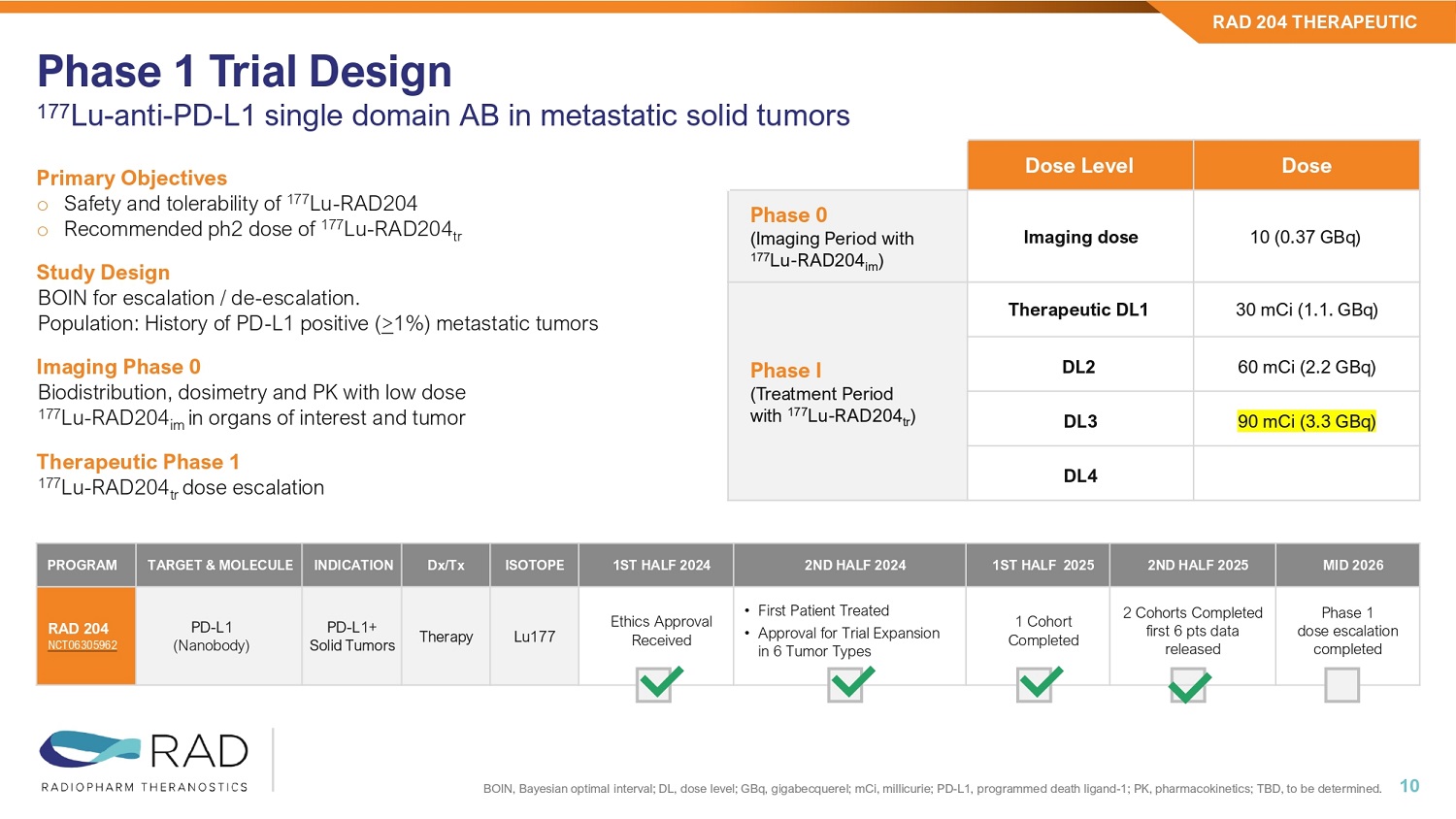
GBq) Therapeutic DL1 Phase I (Treatment Period with 177 Lu - RAD204 tr ) 60 mCi (2.2 GBq) DL2 90 mCi (3.3 GBq) DL3 DL4 Phase 1 Trial Design 177 Lu - anti - PD - L1 single domain AB in metastatic solid tumors 10 Primary Objectives o Safety and tolerability of 177 Lu - RAD204 o Recommended ph2 dose of 177 Lu - RAD204 tr Study Design BOIN for escalation / de - escalation. Population: History of PD - L1 positive ( > 1%) metastatic tumors Imaging Phase 0 Biodistribution, dosimetry and PK with low dose 177 Lu - RAD204 im in organs of interest and tumor Therapeutic Phase 1 177 Lu - RAD204 tr dose escalation RAD 204 THERAPEUTIC BOIN, Bayesian optimal interval; DL, dose level; GBq, gigabecquerel; mCi, millicurie; PD - L1, programmed death ligand - 1; PK, phar macokinetics; TBD, to be determined. MID 2026 2ND HALF 2025 1ST HALF 2025 2ND HALF 2024 1ST HALF 2024 ISOTOPE Dx/Tx INDICATION TARGET & MOLECULE PROGRAM Phase 1 dose escalation completed 2 Cohorts Completed first 6 pts data released 1 Cohort Completed • First Patient Treated • Approval for Trial Expansion in 6 Tumor Types Ethics Approval Received Lu177 Therapy PD - L1+ Solid Tumors PD - L1 (Nanobody) RAD 204 NCT06305962 Clinical data Phase I *One patient (Patient 003 - 009) is not DLT - evaluable (consent withdrawal due to personal reasons) NSCLC, non - small cell lung cancer; PD - 1, programmed death - 1; PDL - 1, programmed death ligand - 1; PFS, progression - free survival.

• First (30mCi) and Second Cohort (60mCi) completed, and 6 patient data released. • Tumor uptake confirmed in all the treated subjects. No tumor reduction above 30% achieved at the first two dose levels • The safety profile has been very favorable, with few adverse events and no related SAEs observed. • Currently recruiting Third Cohort (90mCi).

RAD 204 THERAPEUTIC 11 Tumor Uptake | Significant increase at DL2 vs DL1 RAD 204 THERAPEUTIC 12 Average Absorbed dose at 60 mCi Dose (Gy), with PVC 1 Patients 1.0 #4 0.5 #5 2.8 #6 1.43 Average Absorbed dose at 30 mCi Dose (Gy), with PVC 1 Patients 0.56 #1 0.45 #2 0.21 #3 0.41 30 60 90 0.4 1.4 0 0.5 1 1.5 2 2.5 3 3.5 4 0 20 40 60 80 100 120 140 160 180 Cohort #1 Cohort #2 Cohort #3 Tumor Gy Lu177 mCi Column1 Column2 Lu177 mCi Avg Tumor Gy COHORT#1 COHORT#2 RAD 202 THERAPEUTIC 13 Molecule: 177Lu - RAD202 Targeting MoA : HER2 Therapeutic for: HER2+ TUMORS

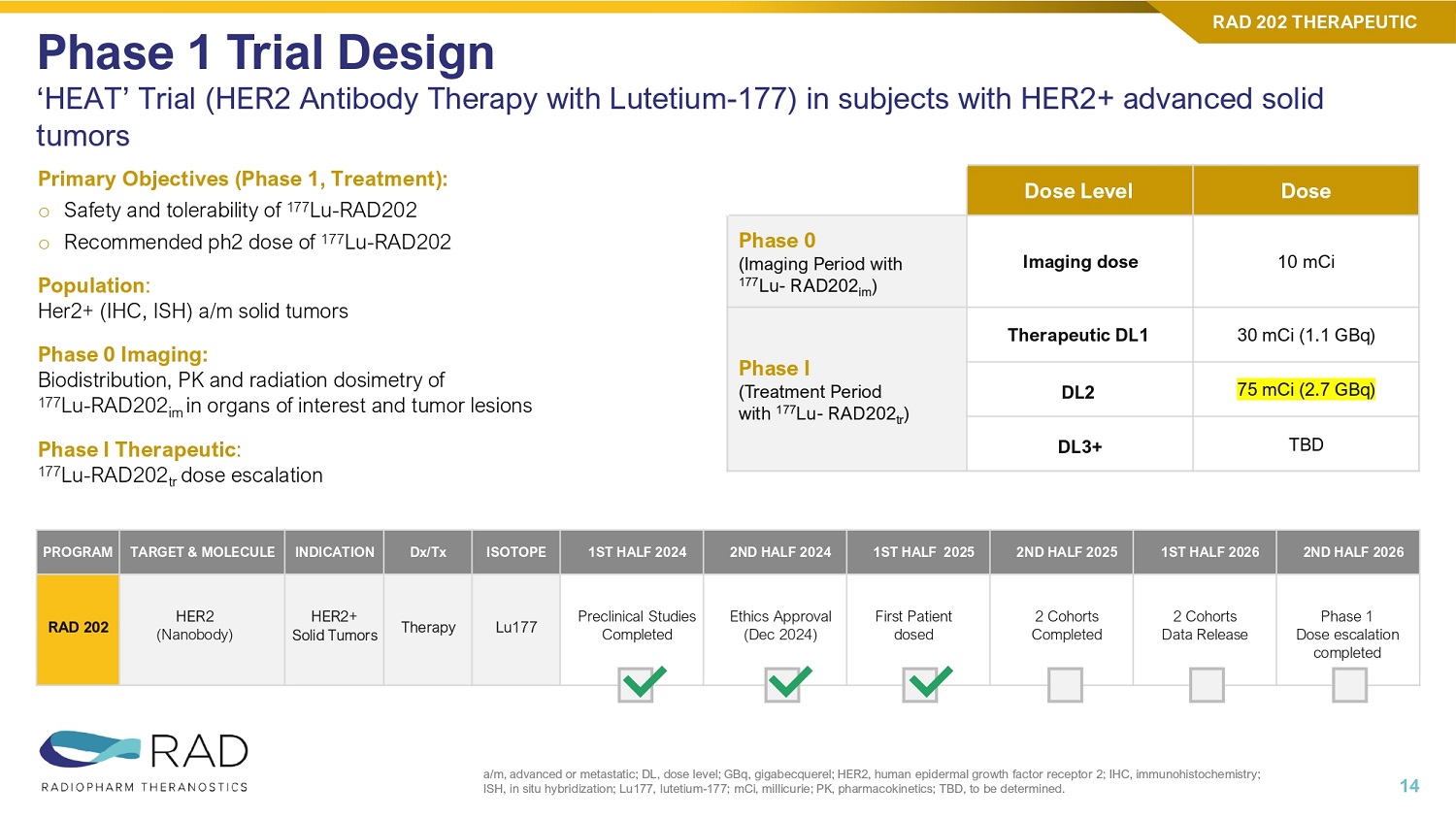
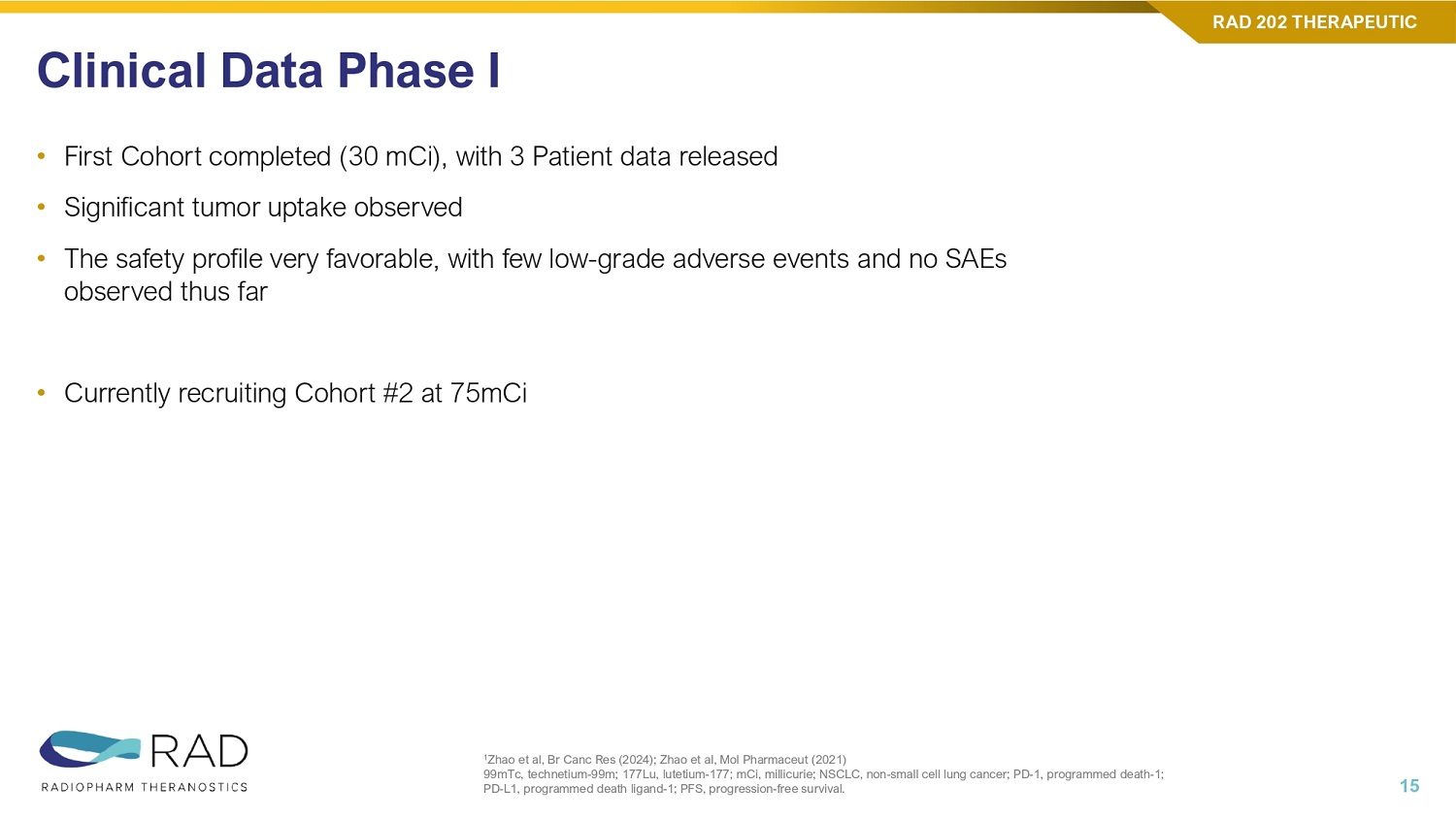
Primary Objectives (Phase 1, Treatment): o Safety and tolerability of 177 Lu - RAD202 o Recommended ph2 dose of 177 Lu - RAD202 Population : Her2+ (IHC, ISH) a/m solid tumors Phase 0 Imaging: Biodistribution, PK and radiation dosimetry of 177 Lu - RAD202 im in organs of interest and tumor lesions Phase I Therapeutic : 177 Lu - RAD202 tr dose escalation Phase 1 Trial Design ‘HEAT’ Trial (HER2 Antibody Therapy with Lutetium - 177) in subjects with HER2+ advanced solid tumors a/m, advanced or metastatic; DL, dose level; GBq, gigabecquerel; HER2, human epidermal growth factor receptor 2; IHC, immunoh ist ochemistry; ISH, in situ hybridization; Lu177, lutetium - 177; mCi, millicurie; PK, pharmacokinetics; TBD, to be determined. 14 RAD 202 THERAPEUTIC Dose Dose Level 10 mCi Imaging dose Phase 0 (Imaging Period with 177 Lu - RAD202 im ) 30 mCi (1.1 GBq) Therapeutic DL1 Phase I (Treatment Period with 177 Lu - RAD202 tr ) 75 mCi (2.7 GBq) DL2 TBD DL3+ 2ND HALF 2026 1ST HALF 2026 2ND HALF 2025 1ST HALF 2025 2ND HALF 2024 1ST HALF 2024 ISOTOPE Dx/Tx INDICATION TARGET & MOLECULE PROGRAM Phase 1 Dose escalation completed 2 Cohorts Data Release 2 Cohorts Completed First Patient dosed Ethics Approval (Dec 2024) Preclinical Studies Completed Lu177 Therapy HER2+ Solid Tumors HER2 (Nanobody) RAD 202 • First Cohort completed (30 mCi), with 3 Patient data released • Significant tumor uptake observed • The safety profile very favorable, with few low - grade adverse events and no SAEs observed thus far • Currently recruiting Cohort #2 at 75mCi Clinical Data Phase I 1 Zhao et al, Br Canc Res (2024); Zhao et al, Mol Pharmaceut (2021) 99mTc, technetium - 99m; 177Lu, lutetium - 177; mCi, millicurie; NSCLC, non - small cell lung cancer; PD - 1, programmed death - 1; PD - L1, programmed death ligand - 1; PFS, progression - free survival.
15 RAD 202 THERAPEUTIC

RAD 202 utilizes an anti - HER2 Nanobody as a targeting moiety RV 01 THERAPEUTIC 16 Molecule: RV01/ BetaBart Targeting MoA : B7H3 Therapeutic for : Multiple Tumor Types RV01 THERAPEUTIC 17 RV01 (Betabart) Key Milestones 1.

Phase I therapeutic trial IND received in July 2025 2. Basket Phase I therapeutic trial to start by the end of 2025 1ST HALF 2026 2ND HALF 2025 1ST HALF 2025 2ND HALF 2024 ISOTOPE Dx/Tx INDICATION TARGET & MOLECULE PROGRAM First two cohorts Data release IND approval First Patient Dosed IND submission PRE - IND FDA meeting Lu177 Therapy Solid Tumors B7 - H3 (mAb) RV01 ACHIEVED Future Milestone RAD 202 utilizes an anti - HER2 Nanobody as a targeting moiety RAD402 THERAPEUTIC 18 Molecule: RAD 402 – 161Tb Targeting MoA : KLK3 Therapeutic for : PROSTATE CANCER

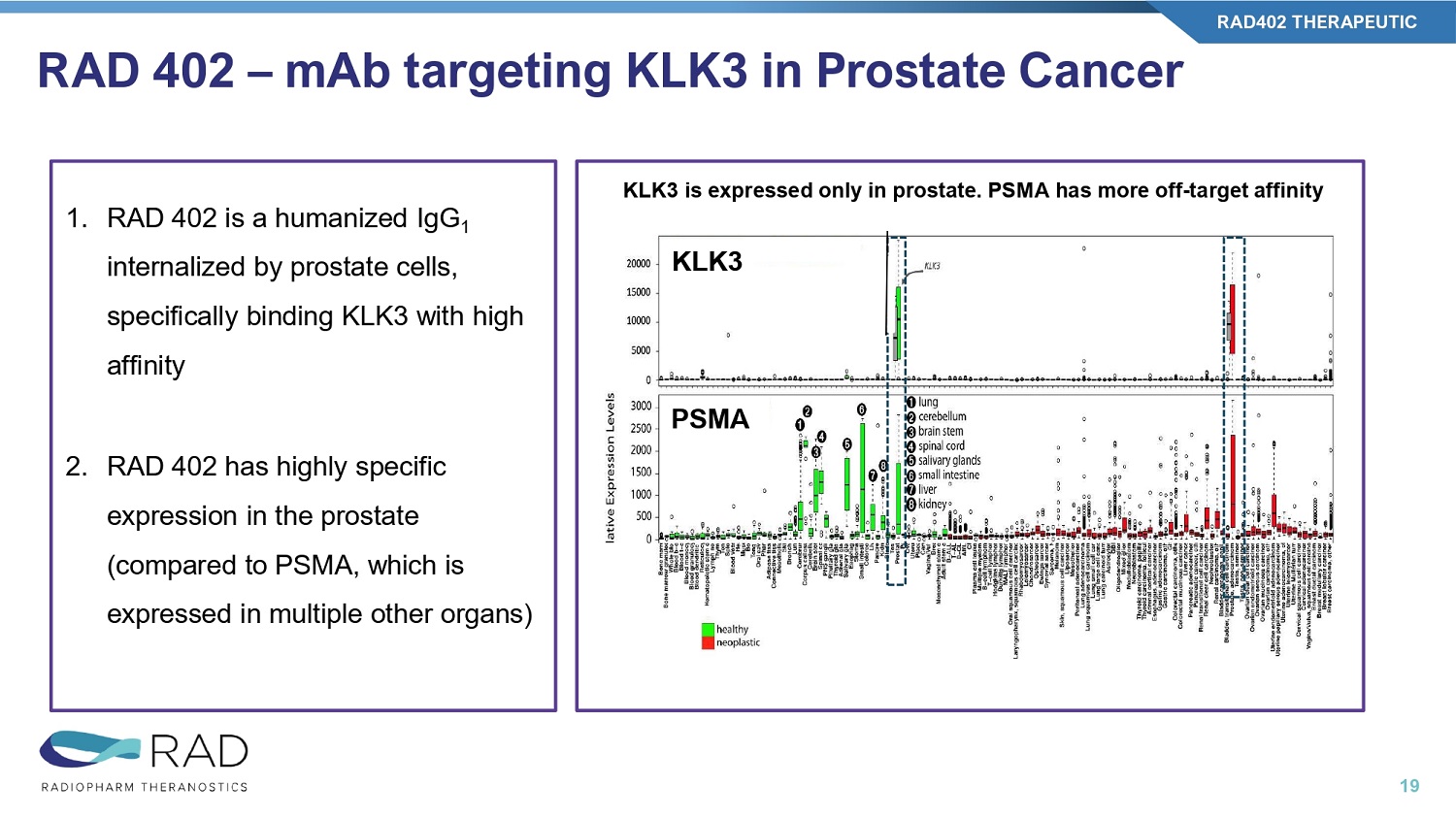
RAD 402 – mAb targeting KLK3 in Prostate Cancer RAD402 THERAPEUTIC 19 1. RAD 402 is a humanized IgG 1 internalized by prostate cells, specifically binding KLK3 with high affinity 2. RAD 402 has highly specific expression in the prostate (compared to PSMA, which is expressed in multiple other organs) KLK3 is expressed only in prostate.
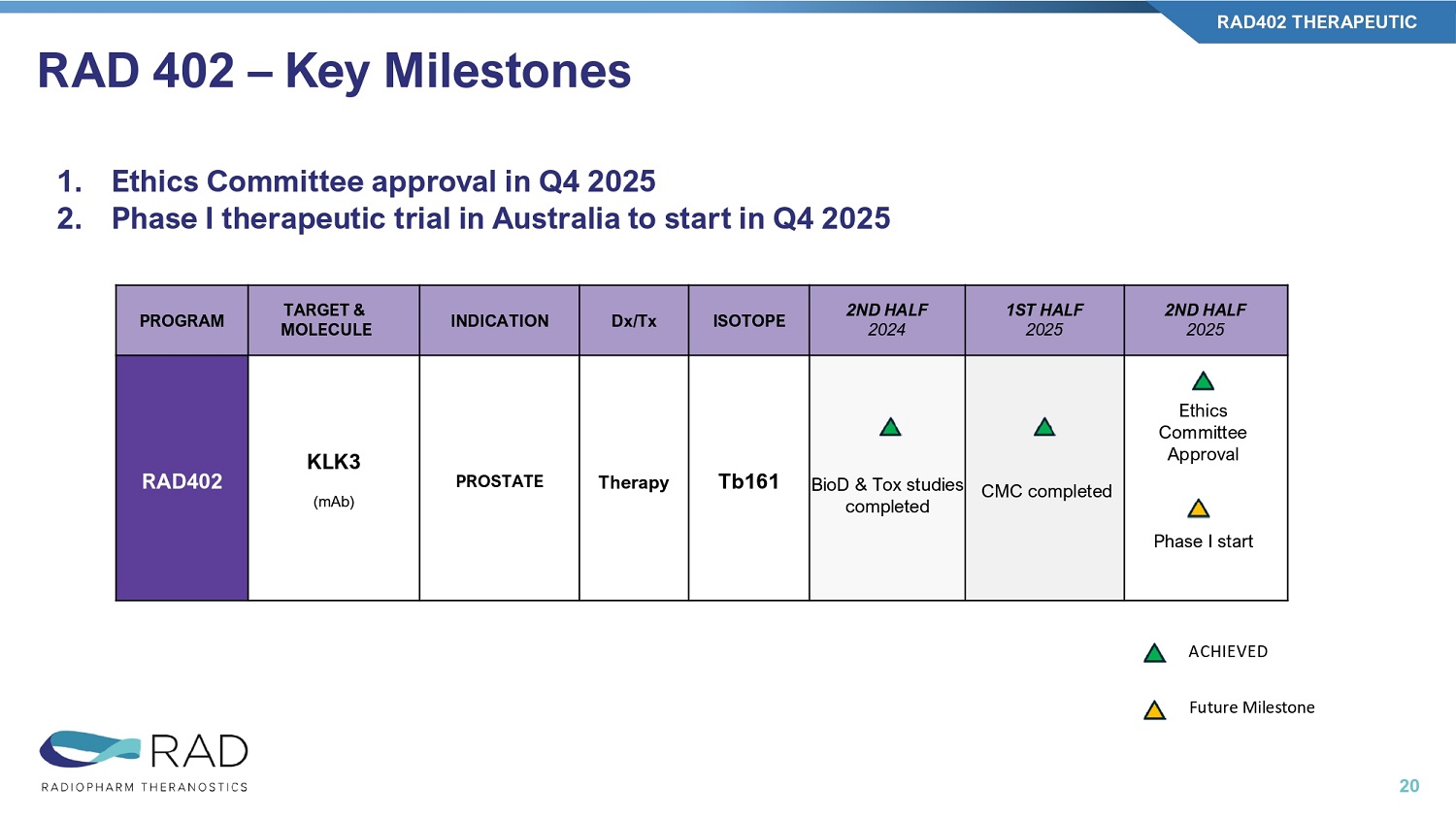
PSMA has more off - target affinity KLK3 PSMA RAD 402 – Key Milestones RAD402 THERAPEUTIC 20 2ND HALF 2025 1ST HALF 2025 2ND HALF 2024 ISOTOPE Dx/Tx INDICATION TARGET & MOLECULE PROGRAM Ethics Committee Approval Phase I start CMC completed BioD & Tox studies completed Tb161 Therapy PROSTATE KLK3 (mAb) RAD402 ACHIEVED Future Milestone 1. Ethics Committee approval in Q4 2025 2.
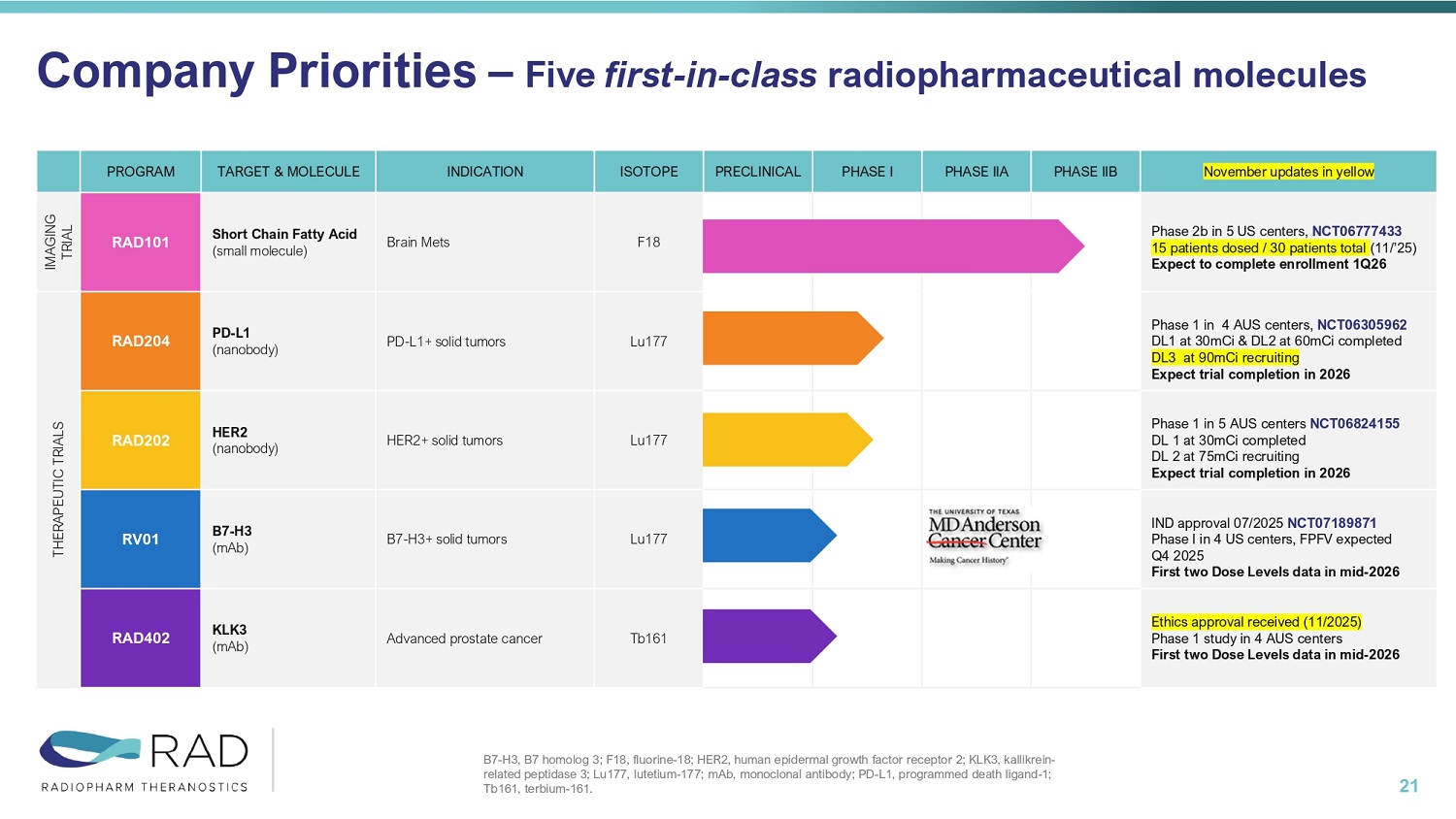
Phase I therapeutic trial in Australia to start in Q4 2025 November updates in yellow PHASE IIB PHASE IIA PHASE I PRECLINICAL ISOTOPE INDICATION TARGET & MOLECULE PROGRAM Phase 2b in 5 US centers, NCT06777433 15 patients dosed / 30 patients total (11/’25) Expect to complete enrollment 1Q26 F18 Brain Mets Short Chain Fatty Acid (small molecule) RAD101 IMAGING TRIAL Phase 1 in 4 AUS centers, NCT06305962 DL1 at 30mCi & DL2 at 60mCi completed DL3 at 90mCi recruiting Expect trial completion in 2026 Lu177 PD - L1+ solid tumors PD - L1 (nanobody) RAD204 THERAPEUTIC TRIALS Phase 1 in 5 AUS centers NCT06824155 DL 1 at 30mCi completed DL 2 at 75mCi recruiting Expect trial completion in 2026 Lu177 HER2+ solid tumors HER2 (nanobody) RAD202 IND approval 07/2025 NCT07189871 Phase I in 4 US centers, FPFV expected Q4 2025 First two Dose Levels data in mid - 2026 Lu177 B7 - H3+ solid tumors B7 - H3 (mAb) RV01 Ethics approval received (11/2025) Phase 1 study in 4 AUS centers First two Dose Levels data in mid - 2026 Tb161 Advanced prostate cancer KLK3 (mAb) RAD402 Company Priorities – Five first - in - class radiopharmaceutical molecules 21 B7 - H3, B7 homolog 3; F18, fluorine - 18; HER2, human epidermal growth factor receptor 2; KLK3, kallikrein - related peptidase 3; Lu177, lutetium - 177; mAb, monoclonal antibody; PD - L1, programmed death ligand - 1; Tb161, terbium - 161.

Thank You www.radiopharmtheranostics.com 22
Exhibit 99.2

ASX ANNOUNCEMENT 20 NOVEMBER 2025 Radiopharm Theranostics Limited Suite 1, Level 3, 62 Lygon Street, Carlton South VIC 3053 Australia ABN: 57 647 877 889 Results of Annual General Meeting Sydney, Australia – 20 November 2025 – Radiopharm Theranostics (ASX:RAD) advises that all resolutions considered at the Annual General Meeting held today were carried by poll. In accordance with ASX Listing rule 3.13.2 and Section 251AA(1) of the Corporations Act 2001, details of the proxies received and votes cast in respect of each resolution are attached. As more than 75% of votes received were cast in favour of Resolution 1, Resolution 10 was withdrawn. Further to the announcement on 19 November 2025 regarding the appointment of Mr Bruce Goodwin as a Non - Executive Director, the Company confirms the re - appointment of Mr Bruce Goodwin as a Non - Executive Director under section 201H(1) of the Corporations Act 2001 following the end of the Annual General Meeting.
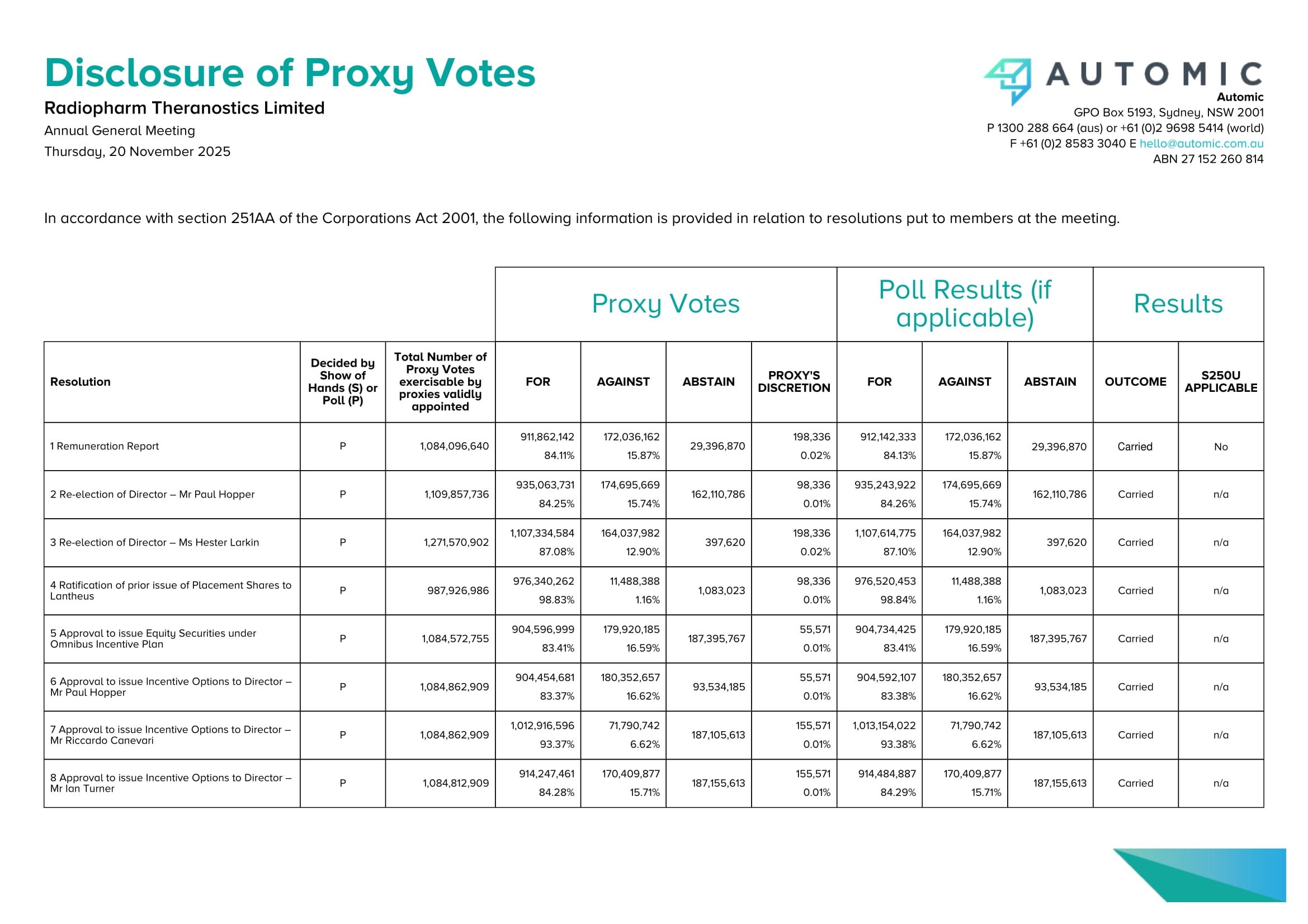
Phillip Hains Joint Company Secretary Disclosure of Proxy Votes Radiopharm Theranostics Limited Annual General Meeting Thursday, 20 November 2025 In accordance with section 251AA of the Corporations Act 2001, the following information is provided in relation to resolutio ns put to members at the meeting. Automic GPO Box 5193, Sydney, NSW 2001 P 1300 288 664 (aus) or +61 (0)2 9698 5414 (world) F +61 (0)2 8583 3040 E hello@automic.com.au ABN 27 152 260 814 Proxy Votes Poll Results (if applicable) Results Resolution Decided by Show of Hands (S) or Poll (P) Total Number of Proxy Votes exercisable by proxies validly appointed FOR AGAINST ABSTAIN PROXY'S DISCRETION FOR AGAINST ABSTAIN OUTCOME S250U APPLICABLE 1 Remuneration Report P 1,084,096,640 911,862,142 84.11% 172,036,162 15.87% 29,396,870 198,336 0.02% 912,142,333 84.13% 172,036,162 15.87% 2 Re - election of Director – Mr Paul Hopper P 1,109,857,736 935,063,731 84.25% 174,695,669 15.74% 162,110,786 98,336 0.01% 935,243,922 84.26% 174,695,669 15.74% 162,110,786 Carried n/a 3 Re - election of Director – Ms Hester Larkin P 1,271,570,902 1,107,334,584 87.08% 164,037,982 12.90% 397,620 198,336 0.02% 1,107,614,775 87.10% 164,037,982 12.90% 397,620 Carried n/a 4 Ratification of prior issue of Placement Shares to Lantheus P 987,926,986 976,340,262 98.83% 11,488,388 1.16% 1,083,023 98,336 0.01% 976,520,453 98.84% 11,488,388 1.16% 1,083,023 Carried n/a 5 Approval to issue Equity Securities under Omnibus Incentive Plan P 1,084,572,755 904,596,999 83.41% 179,920,185 16.59% 187,395,767 55,571 0.01% 904,734,425 83.41% 179,920,185 16.59% 187,395,767 Carried n/a 6 Approval to issue Incentive Options to Director – Mr Paul Hopper P 1,084,862,909 904,454,681 83.37% 180,352,657 16.62% 93,534,185 55,571 0.01% 904,592,107 83.38% 180,352,657 16.62% 93,534,185 Carried n/a 7 Approval to issue Incentive Options to Director – Mr Riccardo Canevari P 1,084,862,909 1,012,916,596 93.37% 71,790,742 6.62% 187,105,613 155,571 0.01% 1,013,154,022 93.38% 71,790,742 6.62% 187,105,613 Carried n/a 8 Approval to issue Incentive Options to Director – Mr Ian Turner P 1,084,812,909 914,247,461 84.28% 170,409,877 15.71% 187,155,613 155,571 0.01% 914,484,887 84.29% 170,409,877 15.71% 187,155,613 Carried n/a 29,396,870 Carried No Proxy Votes Poll Results (if applicable) Results Resolution Decided by Show of Hands (S) or Poll (P) Total Number of Proxy Votes exercisable by proxies validly appointed FOR AGAINST ABSTAIN PROXY'S DISCRETION FOR AGAINST ABSTAIN OUTCOME S250U APPLICABLE 9 Approval of 10% capacity under Listing Rule 7.1A P 1,228,497,414 1,160,228,155 94.44% 68,113,688 5.54% 43,471,108 155,571 0.01% 1,160,465,581 94.46% 68,113,688 5.54% 43,471,108 Carried n/a 10 Contingent business - Board Spill Meeting (conditional item) If less than 25% of the votes cast on Resolution 1 are voted against adoption of the Remuneration Report, the Chair will withdraw Resolution 10.
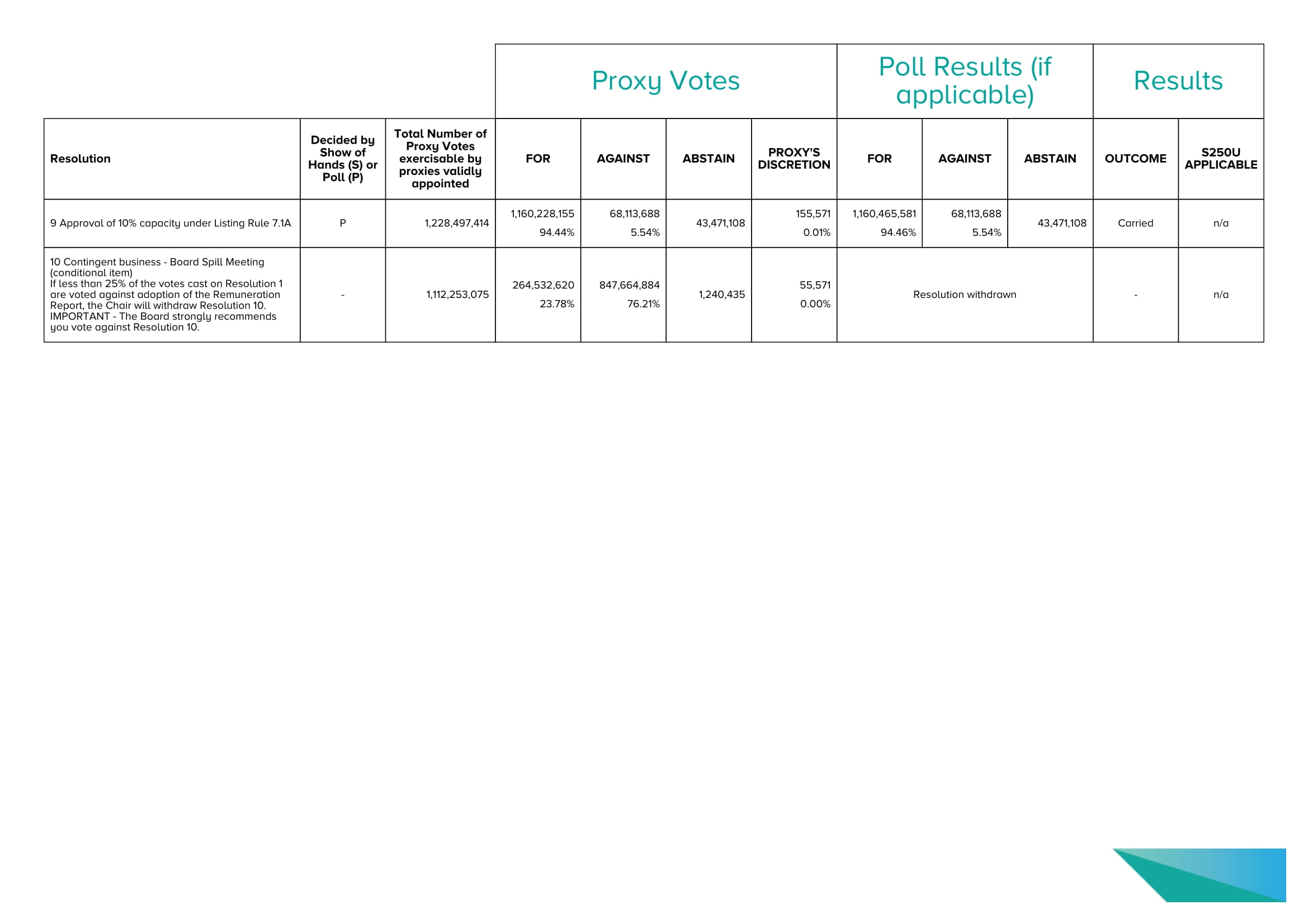
IMPORTANT - The Board strongly recommends you vote against Resolution 10. - 1,112,253,075 264,532,620 23.78% 847,664,884 76.21% 1,240,435 55,571 0.00% Resolution withdrawn - n/a