UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 10, 2025
| INMUNE BIO INC. |
| (Exact name of registrant as specified in charter) |
| Nevada | 001-38793 | 47-5205835 | ||
| (State or other jurisdiction | (Commission File Number) | (IRS Employer | ||
| of incorporation) | Identification No.) |
225 NE Mizner Blvd., Suite 640, Boca Raton, Florida 33432
(Address of Principal Executive Offices) (Zip Code)
(561) 710-0512
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, If Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per shares | INMB | The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
INmune Bio Inc. (the “Company”) has prepared a slide deck that the Company intends to use in presentations from time to time (the “Presentation”). The Presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 and Exhibit 99.1 of this Current Report on Form 8-K is furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. The information in this Item 7.01 and Exhibit 99.1 of this Current Report on Form 8-K shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this Current Report on Form 8-K, regardless of any general incorporation language in any such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Investor Presentation | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| INMUNE BIO INC. | ||
| Date: November 10, 2025 | By: | /s/ David Moss |
| Name: | David Moss | |
| Title: | Chief Executive Officer | |
Exhibit 99.1

1 Corporate Presentation November 2025 NASDAQ: INMB Modulating the Innate Immune System to Transform Inflammation - Driven Disease 2 FORWARD - LOOKING STATEMENTS This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events.

When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements. Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook. Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements. They are neither statements of historical fact nor guarantees of assurance of future performance. We caution you therefore against relying on any of these forward - looking statements. Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations; our ability to protect our intellectual property rights; the impact of any infringement actions or other litigation brought against us; competition from other providers and products; our ability to develop and commercialize products and services; changes in government regulation; our ability to complete capital raising transactions; and other factors (including the “Risk Factors” contained in our most recent Annual Report on Form 10 - K and any other filings that we have made or may make with the SEC in the future), such as those relating to our industry, our operations and results of operations. Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results. Clinical trials are in early stages and there is no assurance that any specific outcome will be achieved. Any statements contained herein related to the development or commercialization of product candidates and other business and financial matters, including without limitation, trial results and data, including the results of the Phase 2 MINDFuL trial, the timing of key milestones, future plans or expectations for the treatment of XPro , and the prospects for receiving regulatory approval or commercializing or selling any product or drug candidates may constitute forward - looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any forward - looking statements contained herein are based on current expectations but are subject to several risks and uncertainties. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward - looking statements because of these risks and uncertainties. CORDstrom , XPro1595 (XPro , pegipanermin), and INKmune® have either finished clinical trials, are still in clinical trials or are preparing to start clinical trials and have not been approved by the US Food and Drug Administration (FDA) or any regulatory body and there cannot be any assurance that they will be approved by the FDA or any regulatory body or that any specific results will be achieved. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company’s ability to produce more drug for clinical trials; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company’s filings with the Securities and Exchange Commission, including the Company’s Annual Report on Form 10 - K, the Company’s Quarterly Reports on Form 10 - Q and the Company’s Current Reports on Form 8 - K. The Company assumes no obligation to update any forward - looking statements to reflect any event or circumstance that may arise after the date hereof.
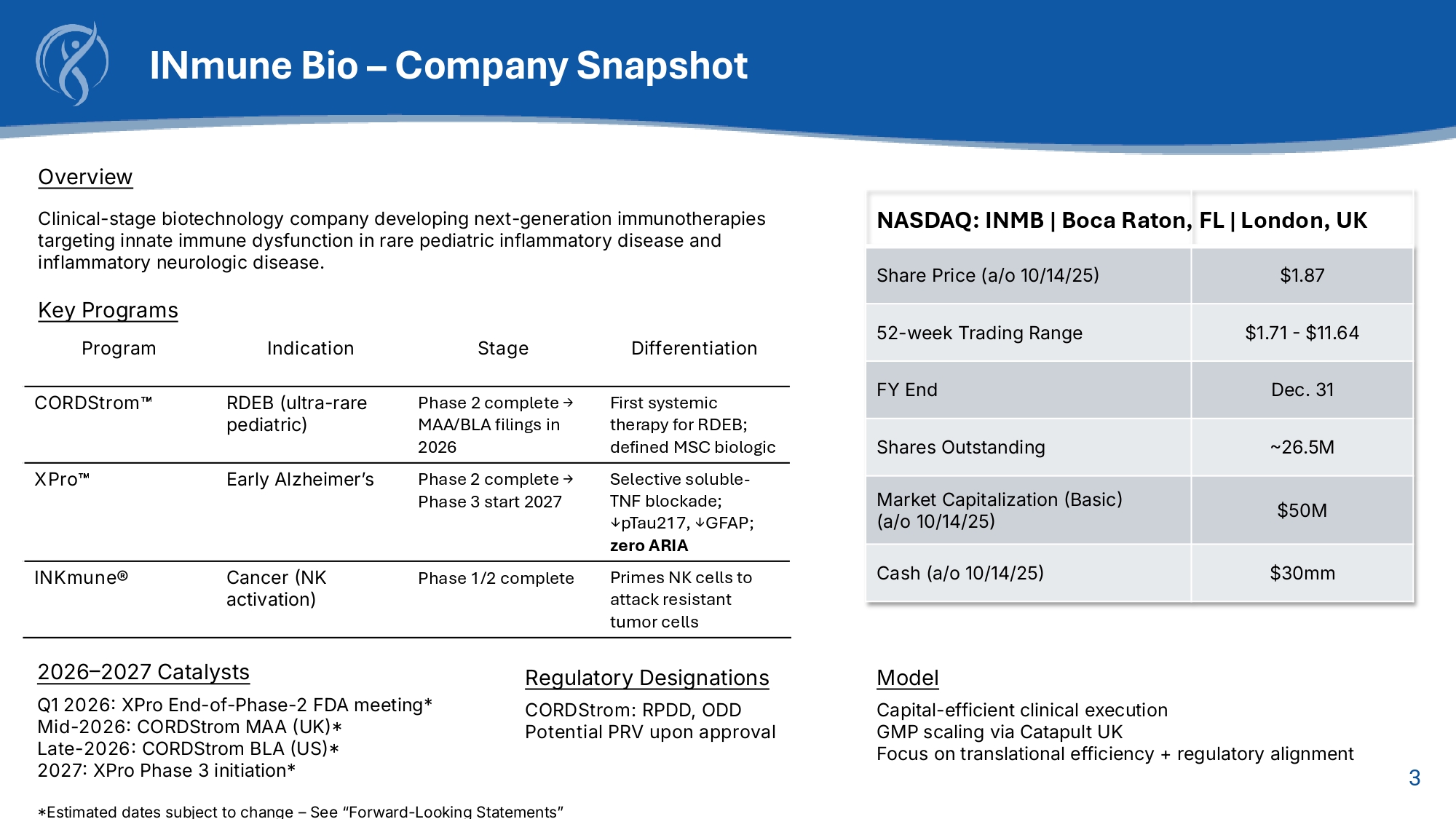
3 Overview Clinical - stage biotechnology company developing next - generation immunotherapies targeting innate immune dysfunction in rare pediatric inflammatory disease and inflammatory neurologic disease. Key Programs INmune Bio – Company Snapshot FL | London, UK NASDAQ: INMB | Boca Raton, $1.87 Share Price (a/o 10/14/25) $1.71 - $11.64 52 - week Trading Range Dec. 31 FY End ~26.5M Shares Outstanding $50M Market Capitalization (Basic) (a/o 10/14/25) $30mm Cash (a/o 10/14/25) Differentiation Stage Indication Program First systemic therapy for RDEB; defined MSC biologic Phase 2 complete → MAA/BLA filings in 2026 RDEB (ultra - rare pediatric) CORDStrom Selective soluble - TNF blockade; ↓pTau217, ↓GFAP; zero ARIA Phase 2 complete → Phase 3 start 2027 Early Alzheimer’s XPro Primes NK cells to attack resistant tumor cells Phase 1/2 complete Cancer (NK activation) INKmune® 2026 – 2027 Catalysts Q1 2026: XPro End - of - Phase - 2 FDA meeting* Mid - 2026: CORDStrom MAA (UK)* Late - 2026: CORDStrom BLA (US)* 2027: XPro Phase 3 initiation* *Estimated dates subject to change – See “Forward - Looking Statements” Regulatory Designations CORDStrom: RPDD, ODD Potential PRV upon approval Model Capital - efficient clinical execution GMP scaling via Catapult UK Focus on translational efficiency + regulatory alignment 4 Late - Stage Pipeline Across Inflammation s Immunology Filing / Phase 3 Phase 2 Phase 1 Pre - clinical Indication / Patient Pop (US&EU) Program/Platform RPDD + ODD Received MAA 2026 → BLA 2026 RDEB (pediatric, ultra - rare) (4K) CORDStrom EOP2 Q1 2026 → Phase 3 2027 Early Alzheimer's Disease with Infl.
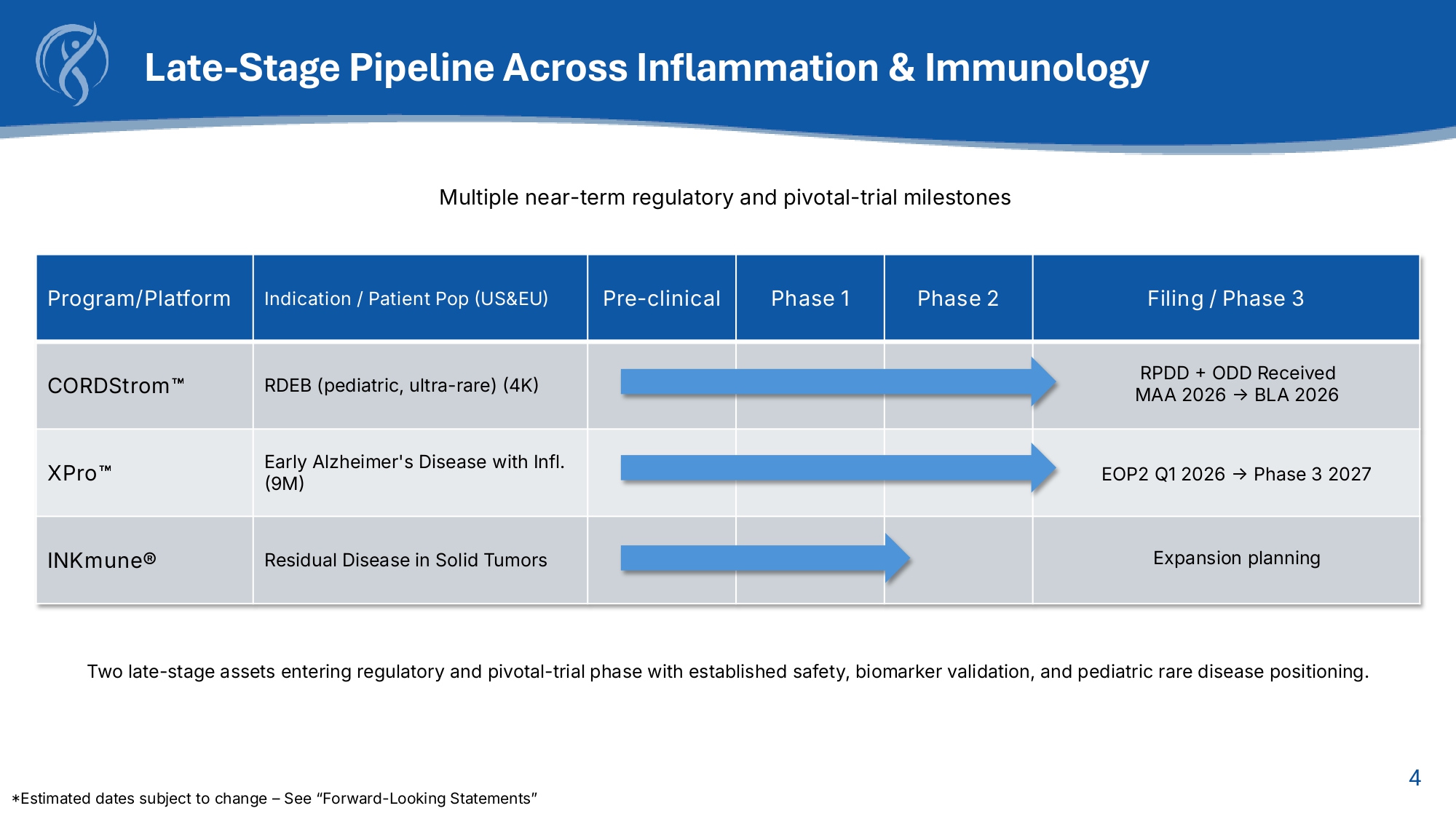
(9M) XPro Expansion planning Residual Disease in Solid Tumors INKmune® Multiple near - term regulatory and pivotal - trial milestones Two late - stage assets entering regulatory and pivotal - trial phase with established safety, biomarker validation, and pediatric rare disease positioning. *Estimated dates subject to change – See “Forward - Looking Statements”
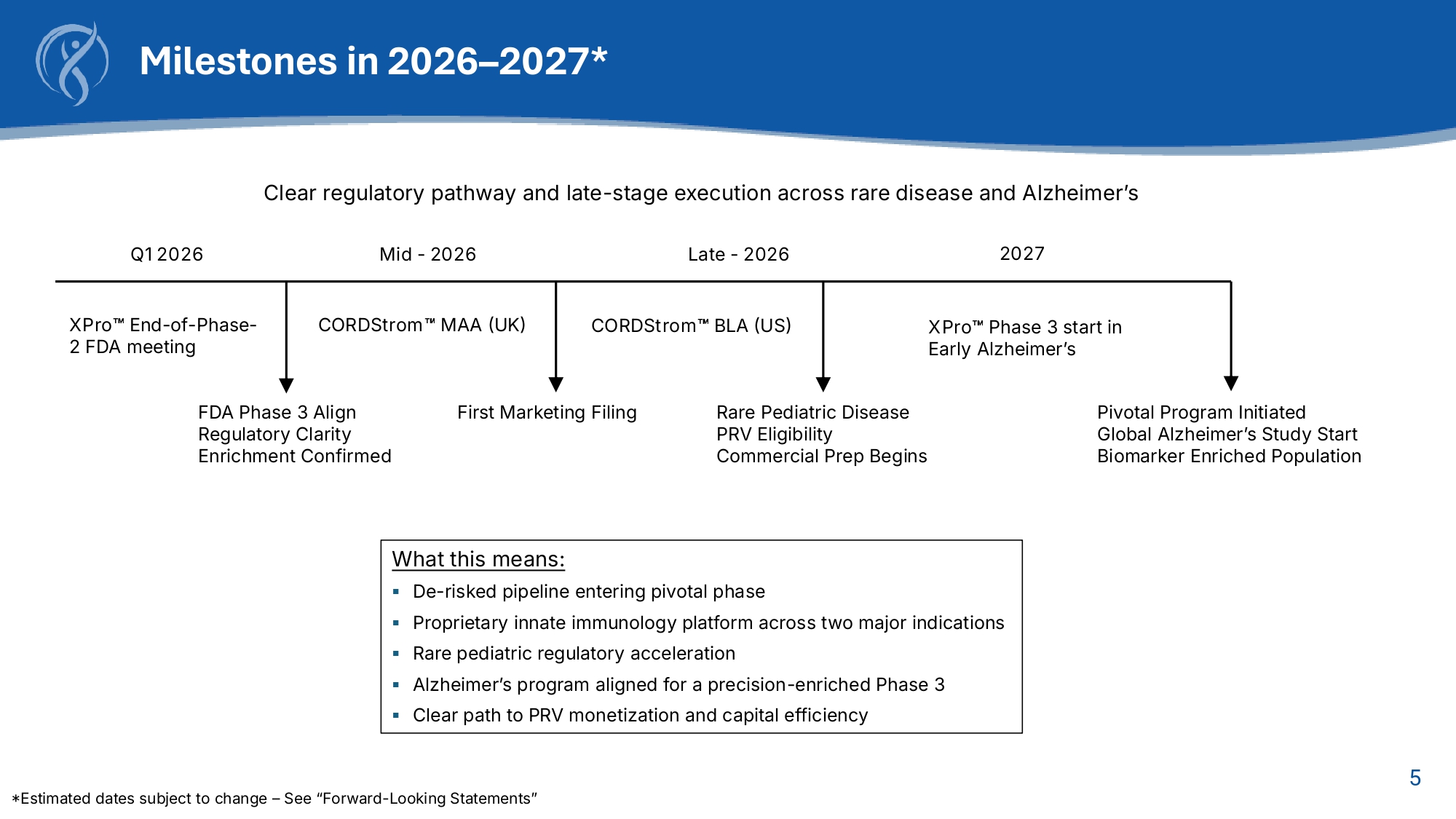
5 Milestones in 2026 – 2027* Clear regulatory pathway and late - stage execution across rare disease and Alzheimer’s Q1 2026 Mid - 2026 Late - 2026 2027 XPro End - of - Phase - 2 FDA meeting CORDStrom MAA (UK) CORDStrom BLA (US) First Marketing Filing FDA Phase 3 Align Regulatory Clarity Enrichment Confirmed XPro Phase 3 start in Early Alzheimer’s Rare Pediatric Disease PRV Eligibility Commercial Prep Begins Pivotal Program Initiated Global Alzheimer’s Study Start Biomarker Enriched Population What this means: ▪ De - risked pipeline entering pivotal phase ▪ Proprietary innate immunology platform across two major indications ▪ Rare pediatric regulatory acceleration ▪ Alzheimer’s program aligned for a precision - enriched Phase 3 ▪ Clear path to PRV monetization and capital efficiency *Estimated dates subject to change – See “Forward - Looking Statements”
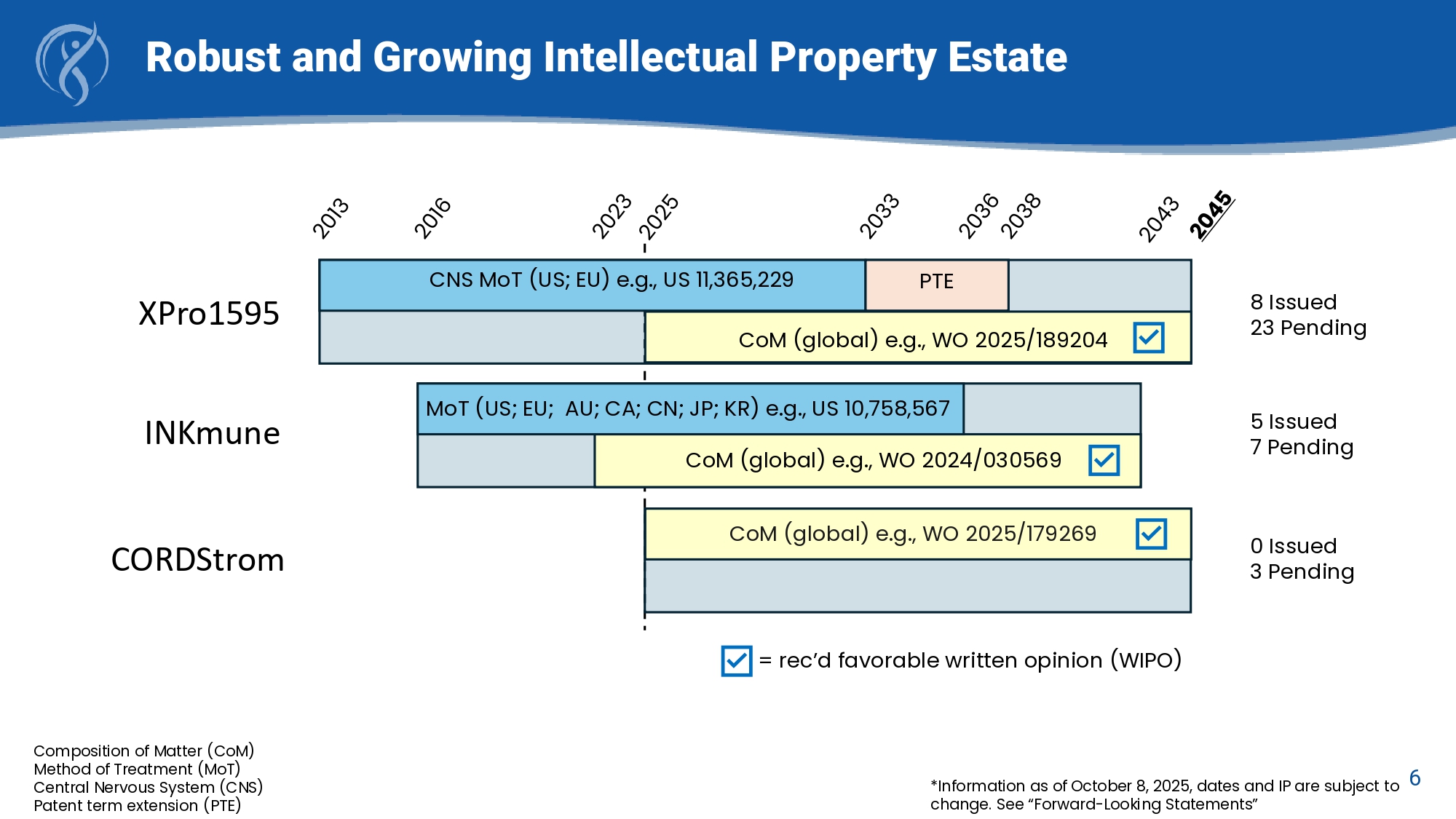
*Information as of October 8, 2025, dates and IP are subject to 6 change. See “Forward - Looking Statements” Robust and Growing Intellectual Property Estate CNS MoT (US; EU) e.g., US 11,365,229 PTE CoM (global) e.g., WO 2024/030569 CoM (global) e.g., WO 2025/189204 MoT (US; EU; AU; CA; CN; JP; KR) e.g., US 10,758,567 CoM (global) e.g., WO 2025/179269 XPro1595 INKmune CORDStrom 8 Issued 23 Pending 5 Issued 7 Pending 0 Issued 3 Pending Composition of Matter (CoM) Method of Treatment (MoT) Central Nervous System (CNS) Patent term extension (PTE) = rec’d favorable written opinion (WIPO)

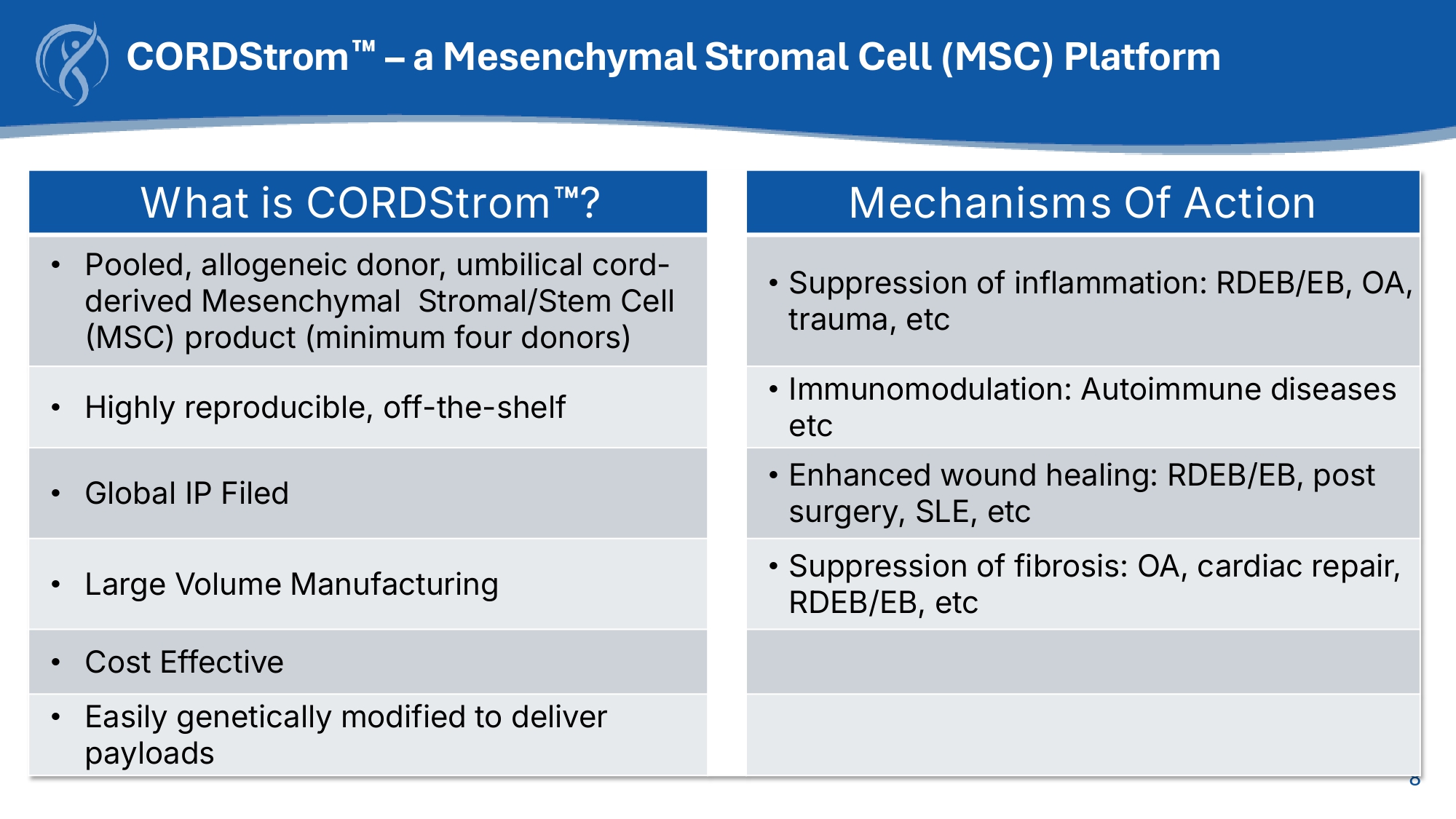
7 CORDStrom for EB Off - the - shelf allogeneic, pooled mesenchymal stromal cell (MSC) platform 8 CORDStrom – a Mesenchymal Stromal Cell (MSC) Platform Mechanisms Of Action What is CORDStrom ? • Suppression of inflammation: RDEB/EB, OA, trauma, etc • Pooled, allogeneic donor, umbilical cord - derived Mesenchymal Stromal/Stem Cell (MSC) product (minimum four donors) • Immunomodulation: Autoimmune diseases etc • Highly reproducible, off - the - shelf • Enhanced wound healing: RDEB/EB, post surgery, SLE, etc • Global IP Filed • Suppression of fibrosis: OA, cardiac repair, RDEB/EB, etc • Large Volume Manufacturing • Cost Effective • Easily genetically modified to deliver payloads • CORDStrom Source: Umbilical Cord Tissues from multiple donors.
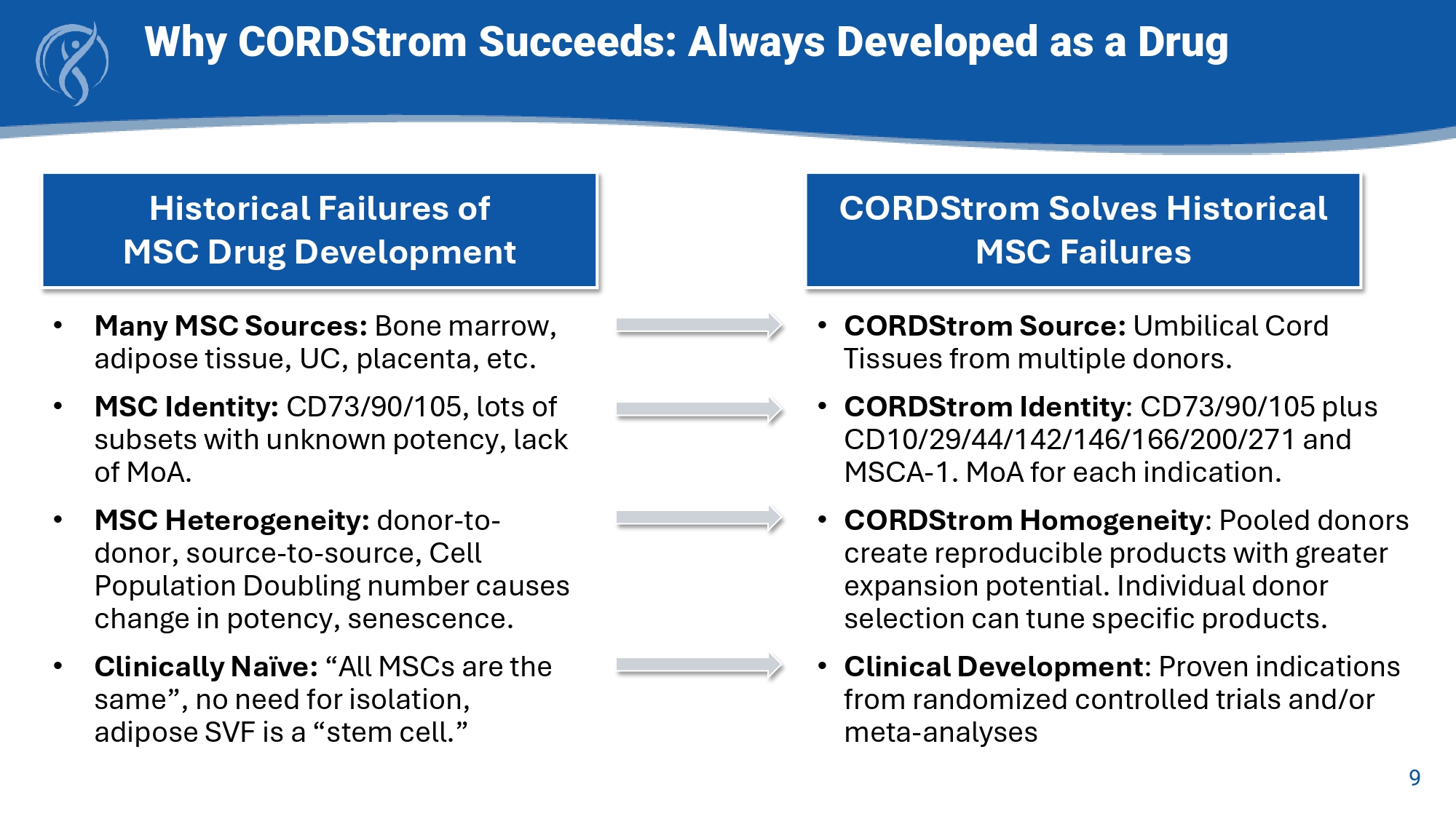
• CORDStrom Identity : CD73/90/105 plus CD10/29/44/142/146/166/200/271 and MSCA - 1. MoA for each indication. • CORDStrom Homogeneity : Pooled donors create reproducible products with greater expansion potential. Individual donor selection can tune specific products. • Clinical Development : Proven indications from randomized controlled trials and/or meta - analyses 9 Why CORDStrom Succeeds: Always Developed as a Drug • Many MSC Sources: Bone marrow, adipose tissue, UC, placenta, etc. • MSC Identity: CD73/90/105, lots of subsets with unknown potency, lack of MoA. • MSC Heterogeneity: donor - to - donor, source - to - source, Cell Population Doubling number causes change in potency, senescence.
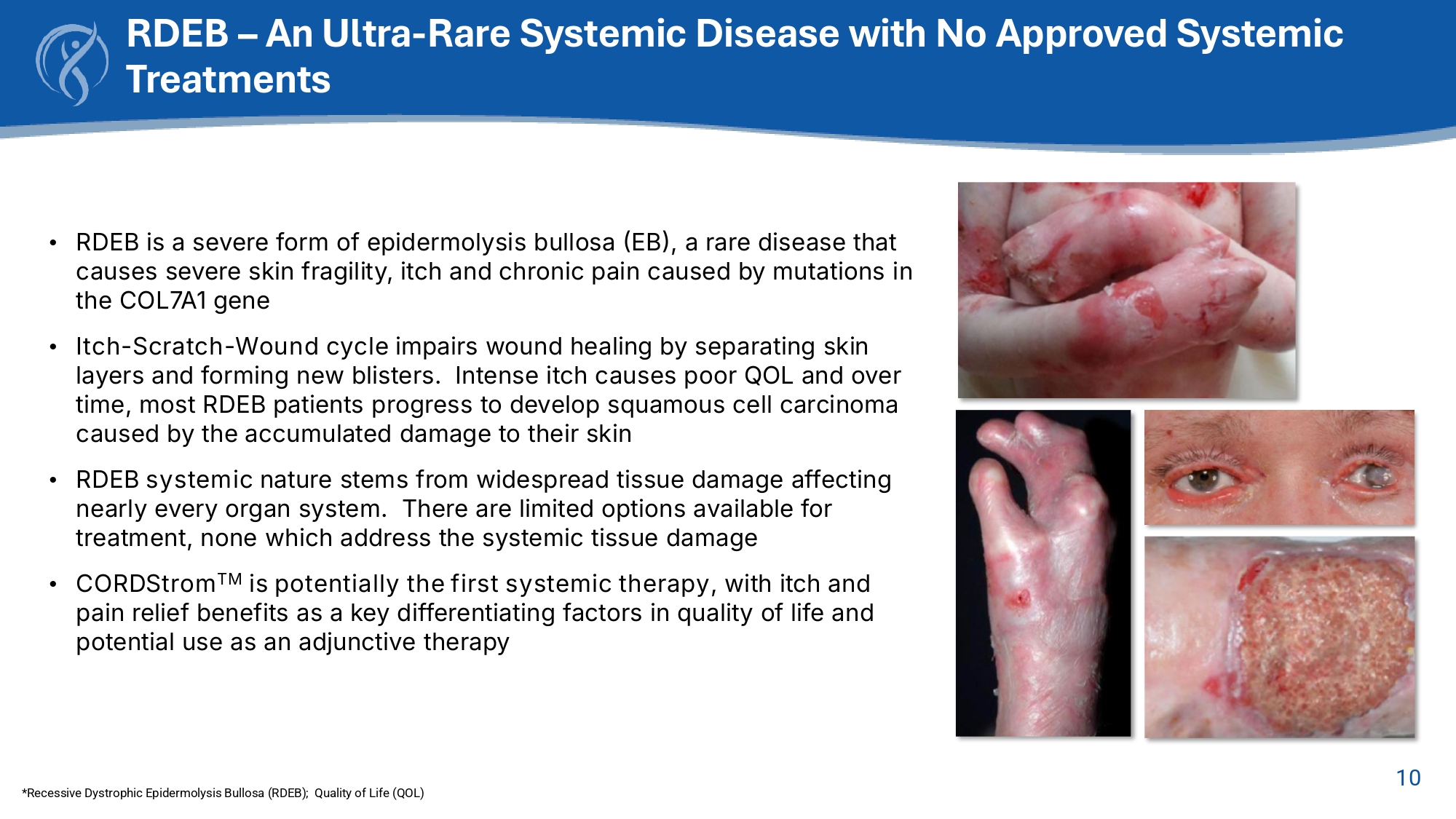
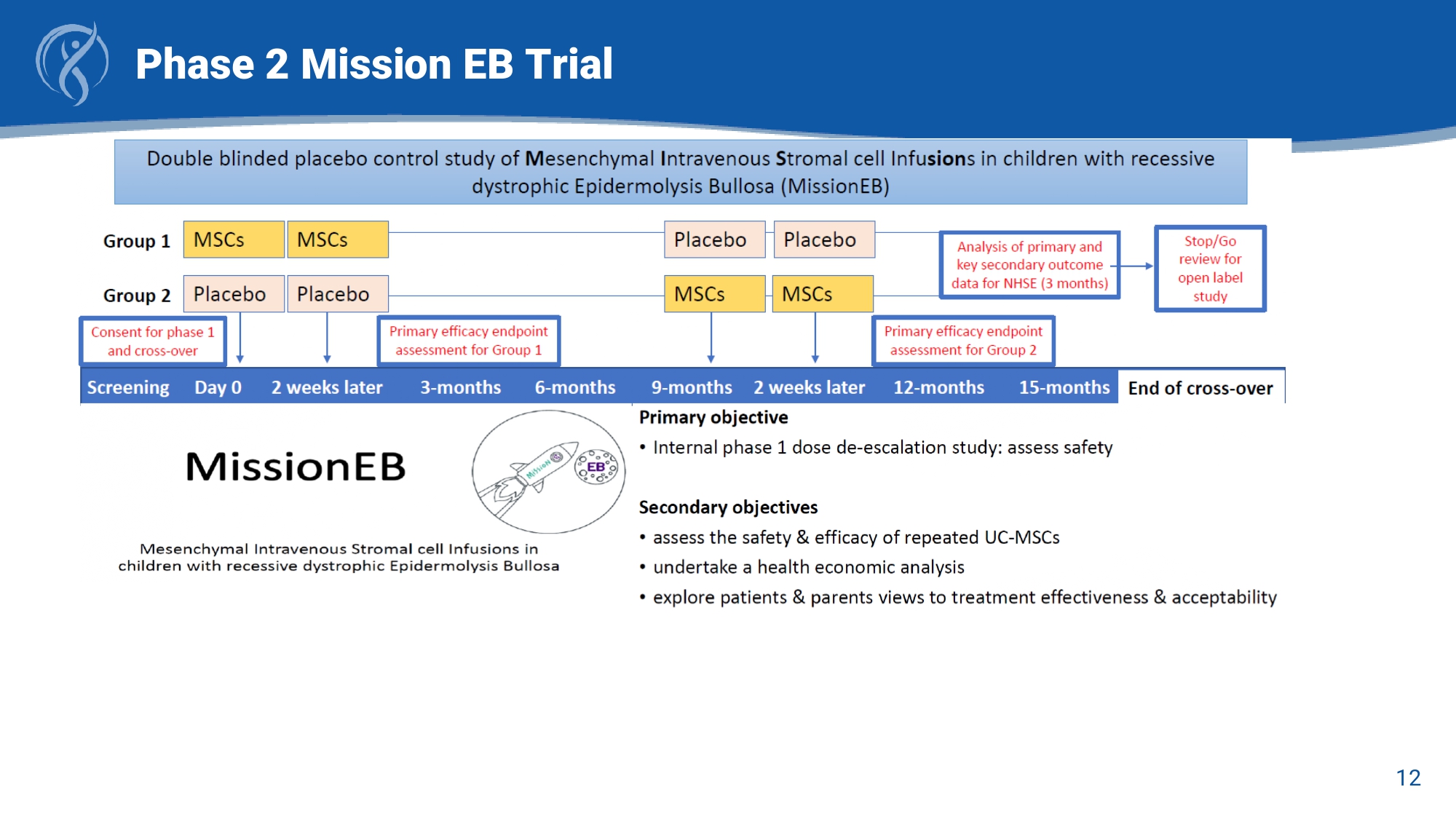
• Clinically Naïve: “All MSCs are the same”, no need for isolation, adipose SVF is a “stem cell.” Historical Failures of MSC Drug Development CORDStrom Solves Historical MSC Failures 10 RDEB – An Ultra - Rare Systemic Disease with No Approved Systemic Treatments *Recessive Dystrophic Epidermolysis Bullosa (RDEB); Quality of Life (QOL) • RDEB is a severe form of epidermolysis bullosa (EB), a rare disease that causes severe skin fragility, itch and chronic pain caused by mutations in the COL7A1 gene • Itch - Scratch - Wound cycle impairs wound healing by separating skin layers and forming new blisters . Intense itch causes poor QOL and over time, most RDEB patients progress to develop squamous cell carcinoma caused by the accumulated damage to their skin • RDEB systemic nature stems from widespread tissue damage affecting nearly every organ system. There are limited options available for treatment, none which address the systemic tissue damage • CORDStrom TM is potentially the first systemic therapy , with itch and pain relief benefits as a key differentiating factors in quality of life and potential use as an adjunctive therapy 12 Phase 2 Mission EB Trial
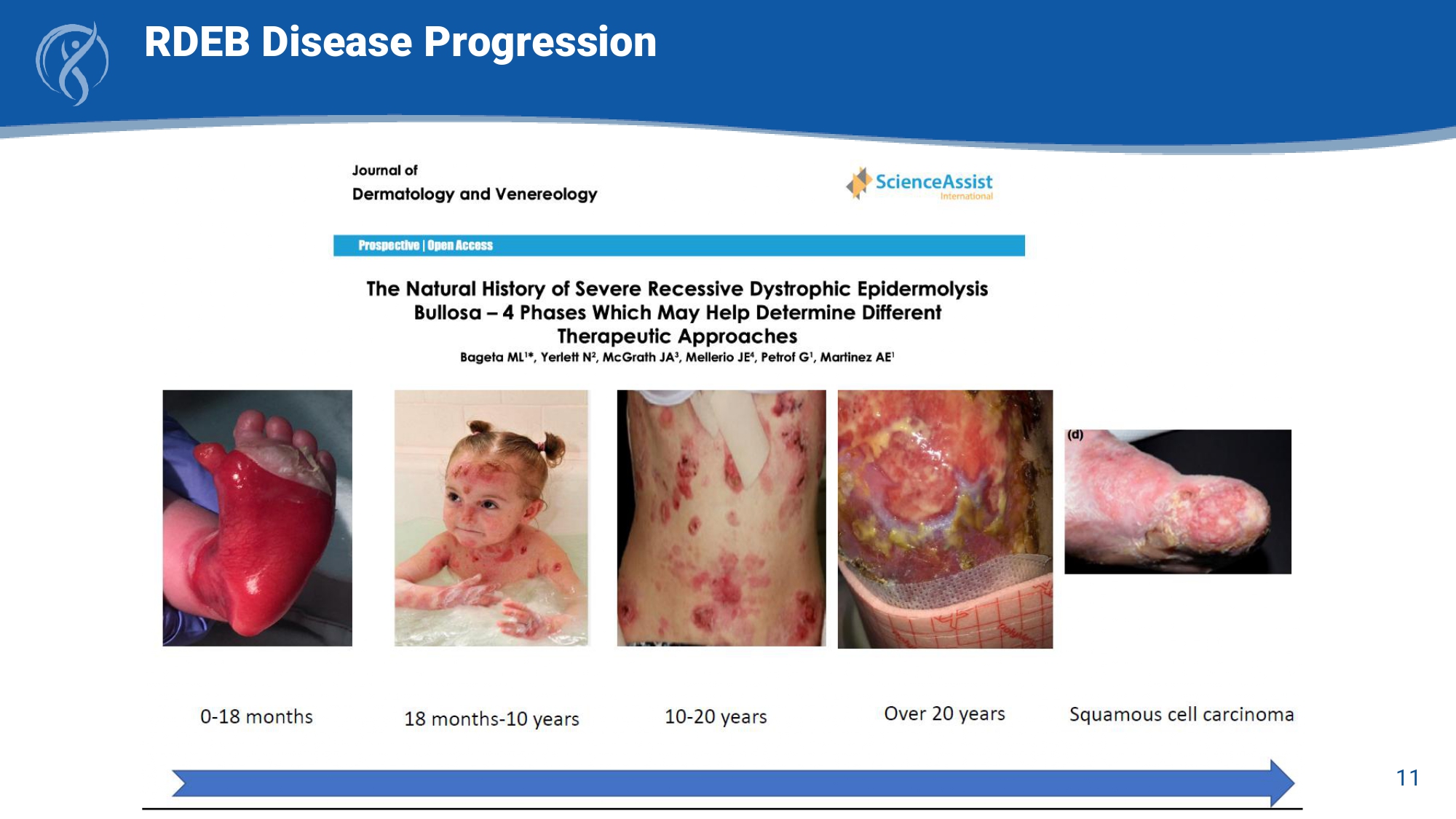
11 RDEB Disease Progression
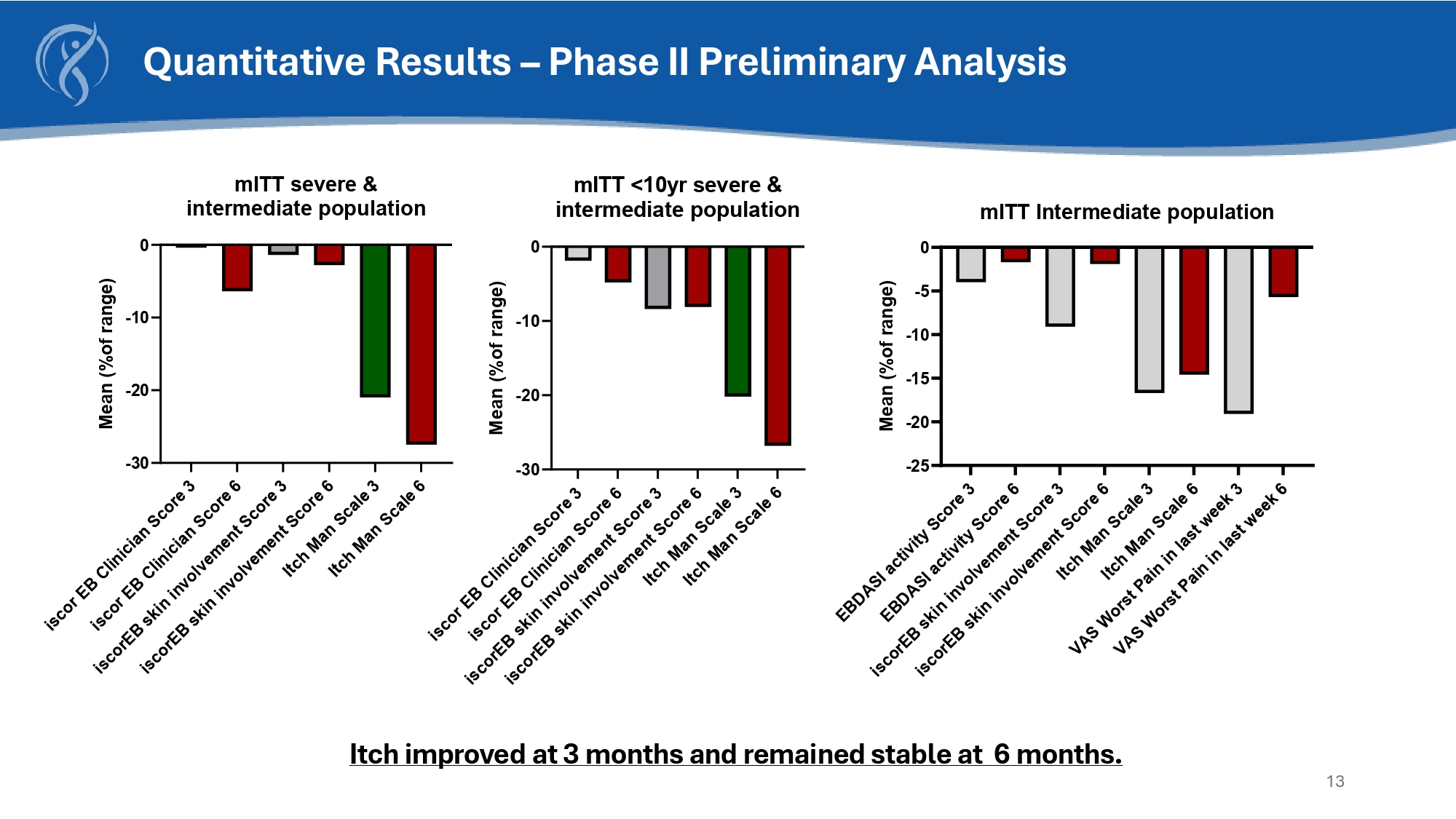
Itch improved at 3 months and remained stable at 6 months. 13 Quantitative Results – Phase II Preliminary Analysis - 30 - 20 - 10 0 mITT severe & intermediate population Mean (%of range) - 30 - 20 - 10 0 mITT <10yr severe & intermediate population Mean (%of range) - 25 - 20 - 15 - 10 - 5 0 mITT Intermediate population Mean (%of range)
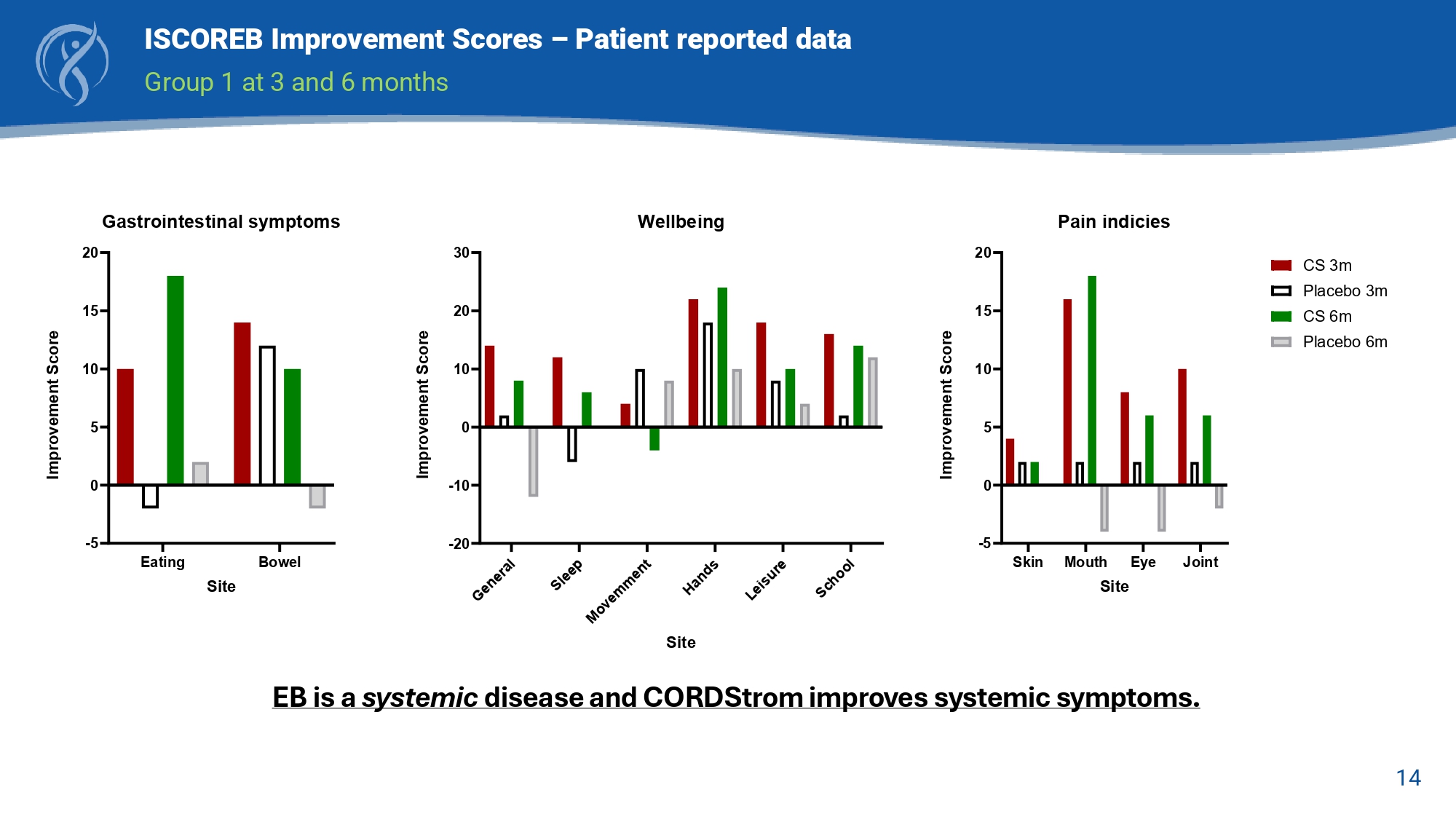
14 ISCOREB Improvement Scores – Patient reported data Group 1 at 3 and 6 months Skin Eye Joint Mouth Site - 5 0 5 10 15 20 Pain indicies CS 3m Placebo 3m CS 6m Placebo 6m Improvement Score Eating Bowel - 5 0 5 10 15 20 Gastrointestinal symptoms Site Improvement Score - 20 - 10 0 10 20 30 Wellbeing Site Improvement Score EB is a systemic disease and CORDStrom improves systemic symptoms.
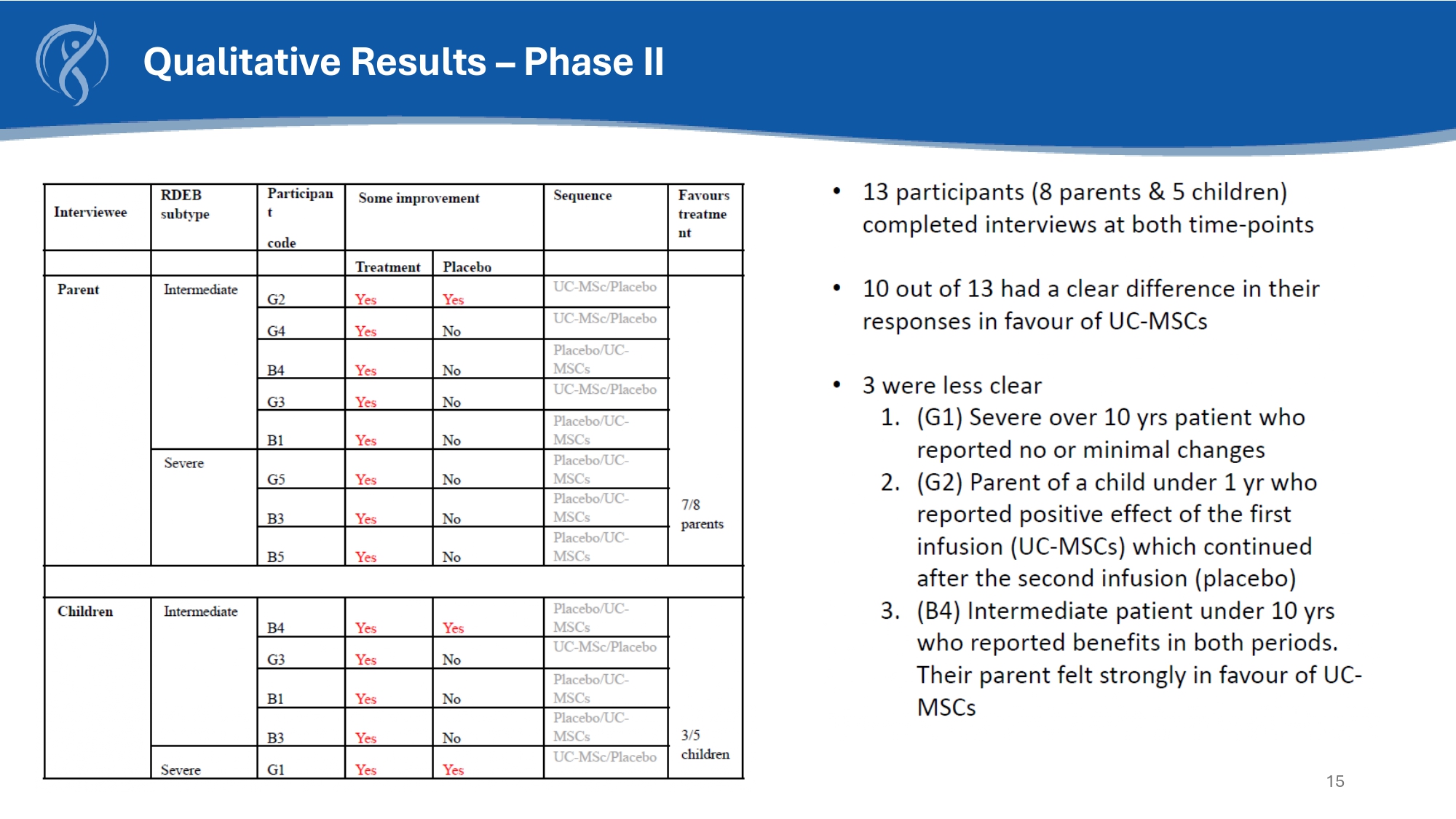


15 Qualitative Results – Phase II 16 Conclusion • With itch reduction over time IscorEB scores have also improved in both RDEB severe cohort and intermediate • There is large improvement in iscorEB scores in RDEB - S cohort at month - 6 with improvement in overall score, patient C clinician scores and skin score • The observed skin improvement and wound closure is likely to be disease modifying, improving pain and quality of life and reducing the long - term risk of squamous cell carcinoma • CORDStrom has proven beneficial treatment with no safety signals in patients with RDEB from age 6 months • Intermediate and younger patients under the age of 10 saw the greater effect with improvement of their skin, pain and itch • Severe and older patients over 10 did not show changes in their skin in the timeframe given however itch and pain improved greatly • Results of the blinded qualitative analysis and clinical photographs were also consistent with these findings • Early treatment with CORDStrom infusion in RDEB patients is disease - modifying particularly showing benefits in younger patients with milder disease • Sever patients saw a clinically significant improvement in itch.
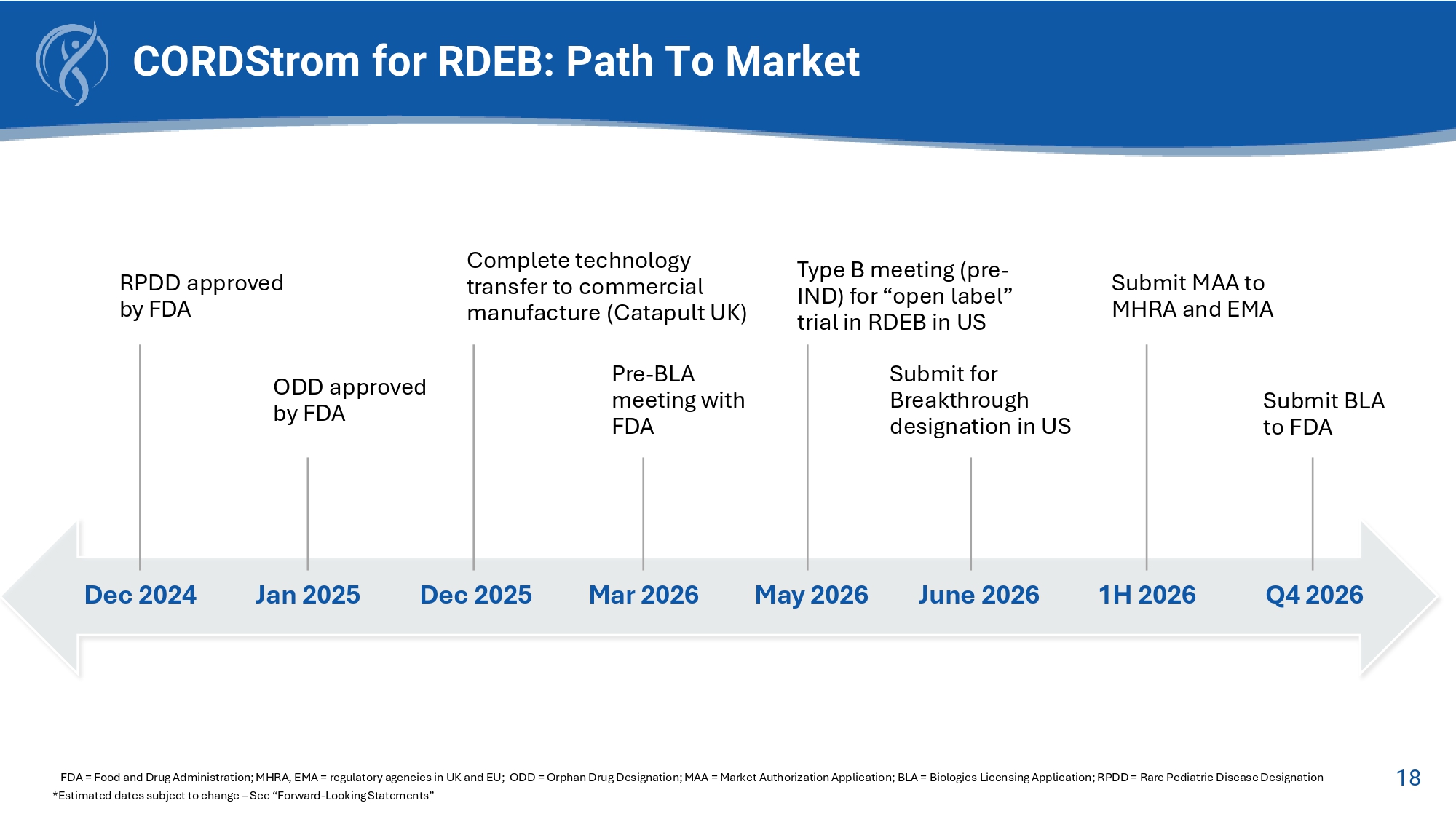
This reduction in itch over time will likely lead to an improvement in wound closure and therefore reduce risk of squamous cell carcinoma later in life 17 Final Statements CORDStrom for RDEB: Path To Market FDA = Food and Drug Administration; MHRA, EMA = regulatory agencies in UK and EU; ODD = Orphan Drug Designation; MAA = Market Authorization Application; BLA = Biologics Licensing Application; RPDD = Rare Pediatric Disease Designation *Estimated dates subject to change – See “Forward - Looking Statements” Dec 2024 Jan 2025 Dec 2025 Mar 2026 May 2026 June 2026 1H 2026 Q4 2026 RPDD approved by FDA ODD approved by FDA Complete technology transfer to commercial manufacture (Catapult UK) Pre - BLA meeting with FDA Type B meeting (pre - IND) for “open label” tria l i n RDEB i n US Submit for Breakthrough designation in US Submit MAA to MHRA and EMA Submit BLA to FDA 18 CORDStrom : Rare Disease Therapy with Systemic Treatment Needs TAM = Total addressable market; Vyjuvek = Product of Krystal Biotech, Inc.; Filsuvez = Product of Chiesi Farmaceutici S.p.A.; Zevaskyn = Product of Abeona Therapeutics Inc. Anticipated treatment schedule • One treatment (2 doses over 14 days) every 4 months: 3 Rx (6 doses)/yr • Anticipated Annual COG per treatment: $120 - 180K (scale dependent) Anticipated reimbursement per treatment: • CORDStrom as the only systemic treatment estimated ~$700,000 per year – Potential TAM $USD: – 1000 RDEB cases - $0.7b – 3500 DEB cases - $2.4b – 12500 EB cases - $8.7b Competitive Positioning • VYJUVEK topical skin cream $750,000 per year • ZEVASKYN Coll7a gene - modified topical skin graft >$3m per treatment / per lesion • FILSUVEZ topical cream for skin lesions $27,000 per 15 tubes – 1 tube per lesion/Rx 19

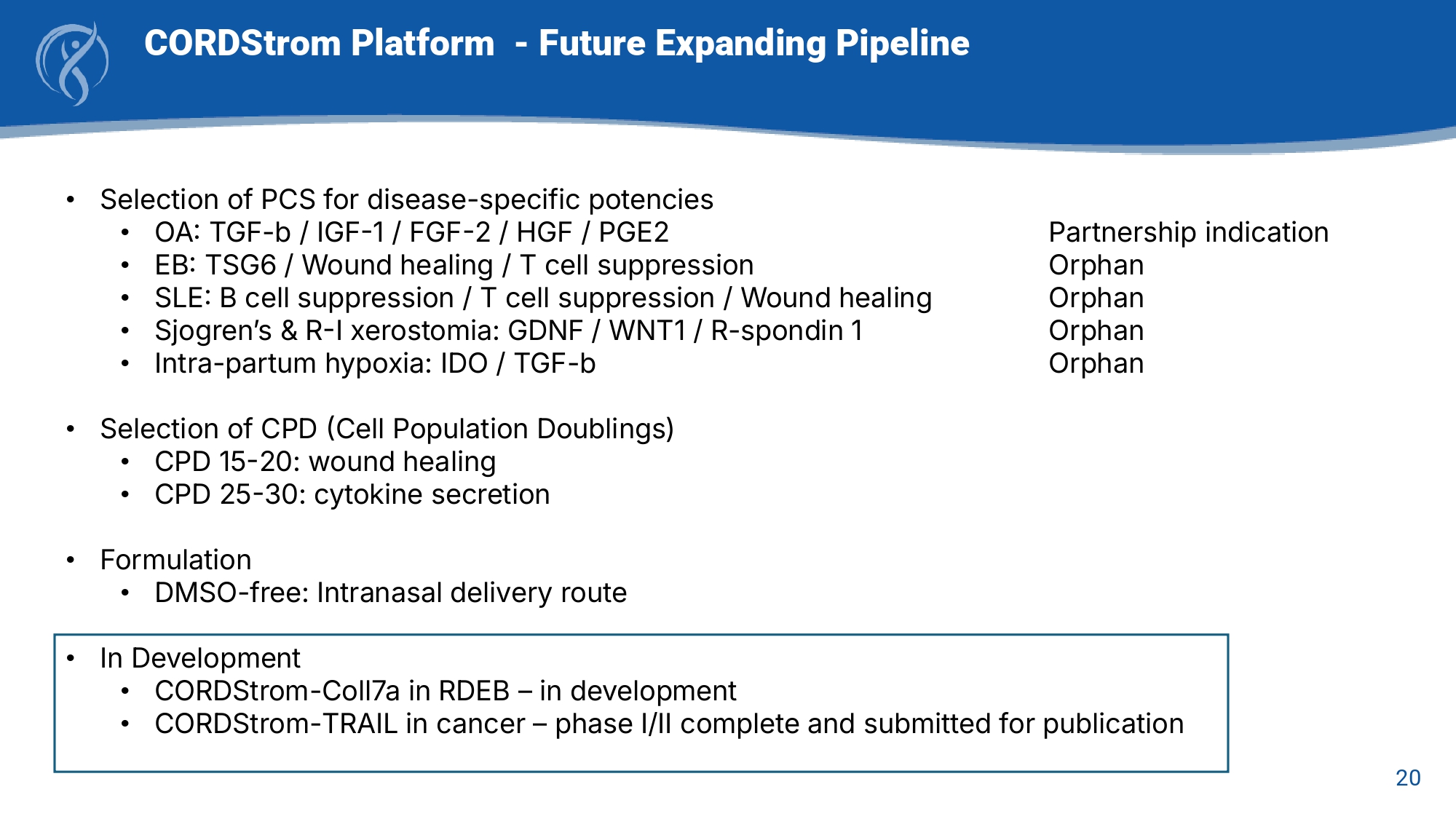
20 CORDStrom Platform - Future Expanding Pipeline • Selection of PCS for disease - specific potencies • OA: TGF - b / IGF - 1 / FGF - 2 / HGF / PGE2 • EB: TSG6 / Wound healing / T cell suppression • SLE: B cell suppression / T cell suppression / Wound healing • Sjogren’s & R - I xerostomia: GDNF / WNT1 / R - spondin 1 • Intra - partum hypoxia: IDO / TGF - b Partnership indication Orphan Orphan Orphan Orphan • Selection of CPD (Cell Population Doublings) • CPD 15 - 20: wound healing • CPD 25 - 30: cytokine secretion • Formulation • DMSO - free: Intranasal delivery route • In Development • CORDStrom - Coll7a in RDEB – in development • CORDStrom - TRAIL in cancer – phase I/II complete and submitted for publication 21 Approaching Alzheimer’s as an Immunologic Disease

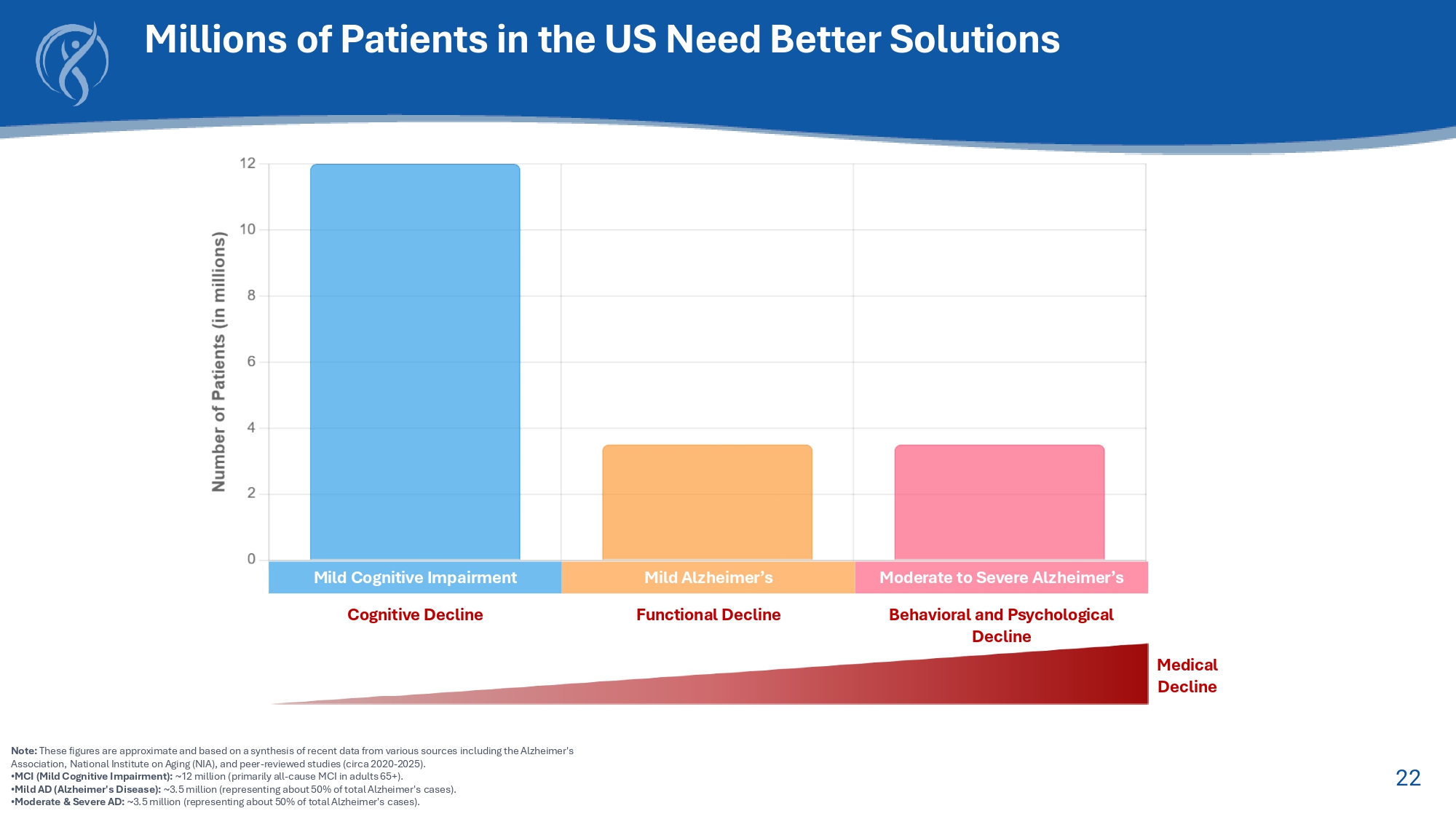
22 Note: These figures are approximate and based on a synthesis of recent data from various sources including the Alzheimer's Association, National Institute on Aging (NIA), and peer - reviewed studies (circa 2020 - 2025). • MCI (Mild Cognitive Impairment): ~12 million (primarily all - cause MCI in adults 65+). • Mild AD (Alzheimer's Disease): ~3.5 million (representing about 50% of total Alzheimer's cases). • Moderate s Severe AD: ~3.5 million (representing about 50% of total Alzheimer's cases). Millions of Patients in the US Need Better Solutions Cognitive Decline Mild Cognitive Impairment Mild Alzheimer’s Moderate to Severe Alzheimer’s Functional Decline Behavioral and Psychological Decline Medical Decline 23 Strong Evidence for anti - TNF to Treat Alzheimer’s Disease 1.
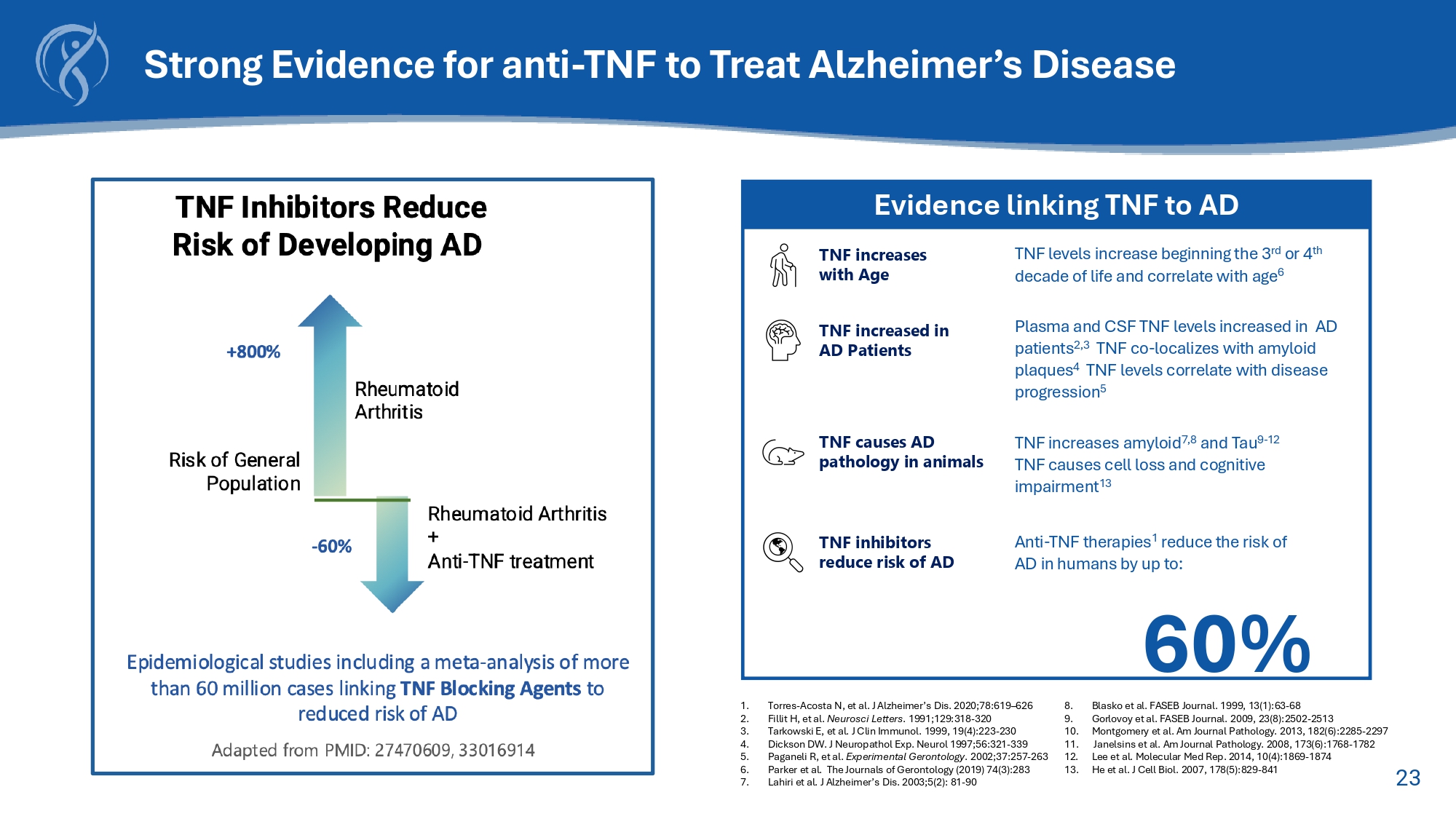
Torres - Acosta N, et al. J Alzheimer’s Dis. 2020;78:619 – 626 8. Blasko et al. FASEB Journal. 1999, 13(1):63 - 68 2. Fillit H, et al. Neurosci Letters . 1991;129:318 - 320 9. Gorlovoy et al. FASEB Journal. 2009, 23(8):2502 - 2513 3. Tarkowski E, et al. J Clin Immunol. 1999, 19(4):223 - 230 10. Montgomery et al. Am Journal Pathology. 2013, 182(6):2285 - 2297 4. Dickson DW. J Neuropathol Exp. Neurol 1997;56:321 - 339 11. Janelsins et al. Am Journal Pathology. 2008, 173(6):1768 - 1782 5. Paganeli R, et al. Experimental Gerontology . 2002;37:257 - 263 12. Lee et al. Molecular Med Rep. 2014, 10(4):1869 - 1874 6. Parker et al. The Journals of Gerontology (2019) 74(3):283 13. He et al. J Cell Biol. 2007, 178(5):829 - 841 7. Lahiri et al. J Alzheimer’s Dis.
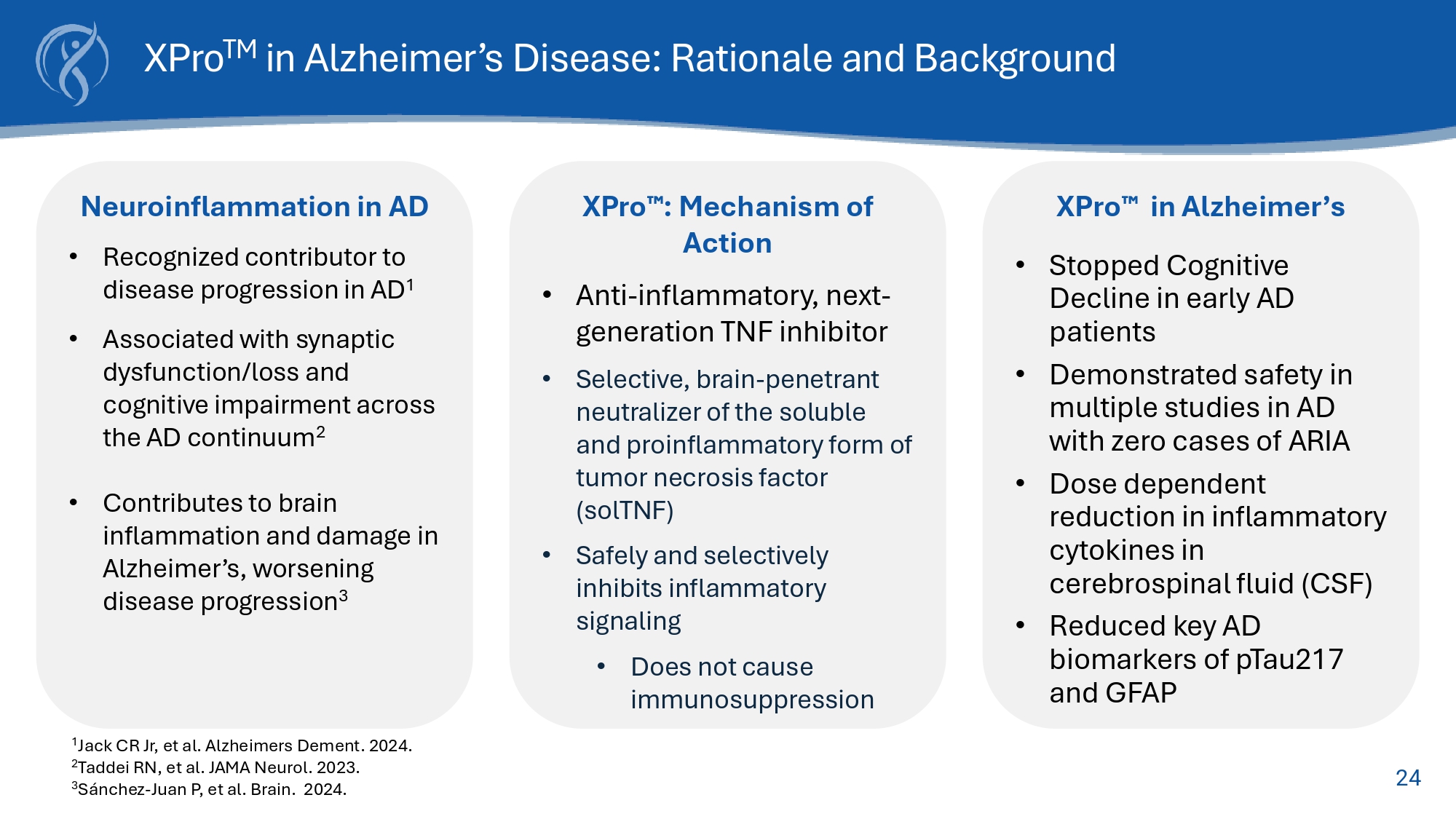
2003;5(2): 81 - 90 Evidence linking TNF to AD TNF inhibitors reduce risk of AD TNF causes AD pathology in animals TNF increases amyloid 7,8 and Tau 6 - 12 TNF causes cell loss and cognitive impairment 13 TNF increases with Age TNF levels increase beginning the 3 rd or 4 th decade of life and correlate with age 6 Anti - TNF therapies 1 reduce the risk of AD in humans by up to: 60% TNF increased in AD Patients Plasma and CSF TNF levels increased in AD patients 2,3 TNF co - localizes with amyloid plaques 4 TNF levels correlate with disease progression 5 24 XPro TM in Alzheimer’s Disease: Rationale and Background Neuroinflammation in AD • Recognized contributor to disease progression in AD 1 • Associated with synaptic dysfunction/loss and cognitive impairment across the AD continuum 2 • Contributes to brain inflammation and damage in Alzheimer’s, worsening disease progression 3 XPro : Mechanism of Action • Anti - inflammatory, next - generation TNF inhibitor • Selective, brain - penetrant neutralizer of the soluble and proinflammatory form of tumor necrosis factor (solTNF) • Safely and selectively inhibits inflammatory signaling • Does not cause immunosuppression XPro in Alzheimer’s • Stopped Cognitive Decline in early AD patients • Demonstrated safety in multiple studies in AD with zero cases of ARIA • Dose dependent reduction in inflammatory cytokines in cerebrospinal fluid (CSF) • Reduced key AD biomarkers of pTau217 and GFAP 1 Jack CR Jr, et al. Alzheimers Dement. 2024. 2 Taddei RN, et al. JAMA Neurol. 2023. 3 Sánchez - Juan P, et al. Brain. 2024.
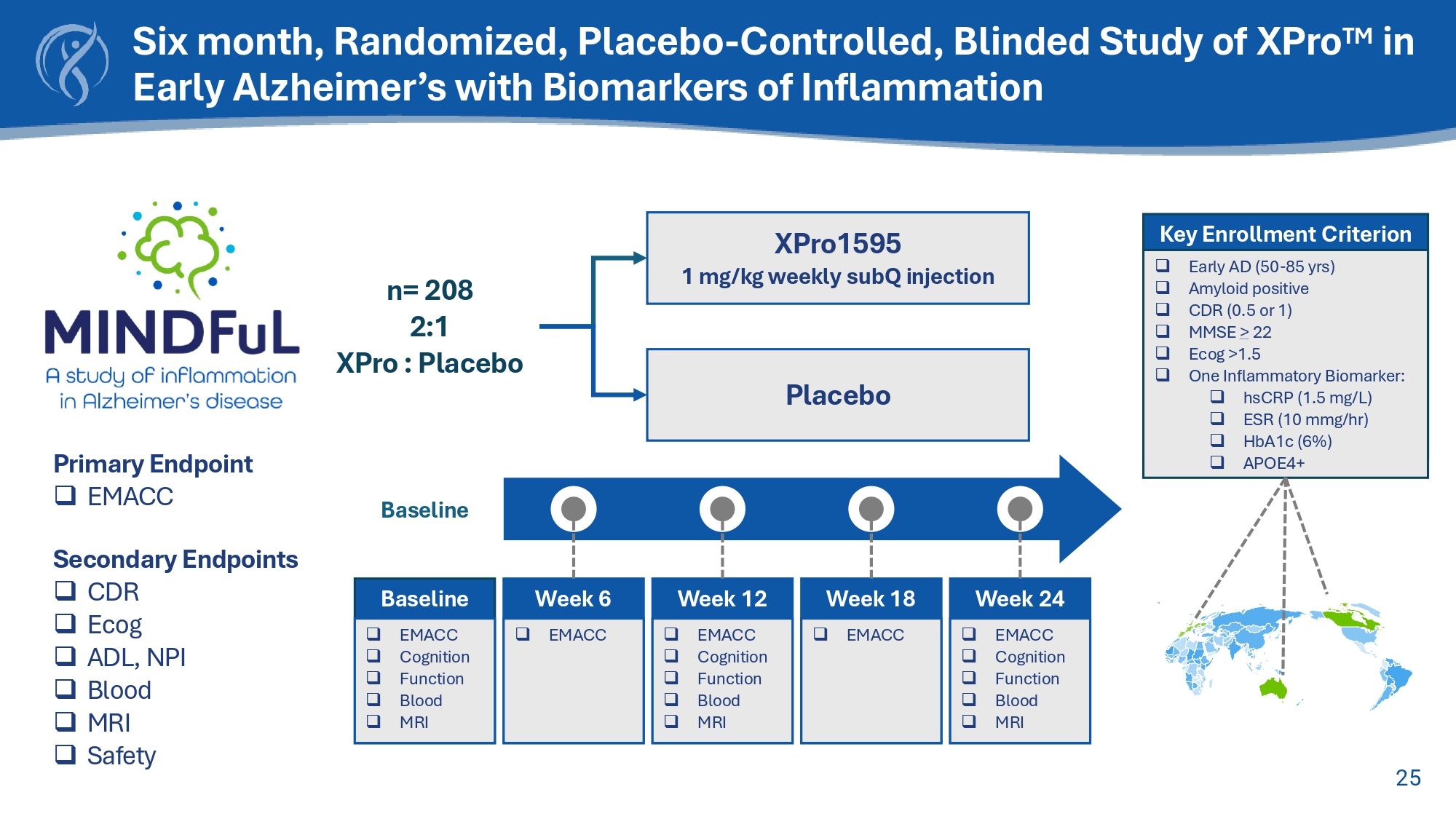
25 Key Enrollment Criterion □ Early AD (50 - 85 yrs) □ Amyloid positive □ CDR (0.5 or 1) □ MMSE > 22 □ Ecog >1.5 □ One Inflammatory Biomarker: □ hsCRP (1.5 mg/L) □ ESR (10 mmg/hr) □ HbA1c (6%) □ APOE4+ Baseline □ EMACC □ Cognition □ Function □ Blood □ MRI Baseline Week 6 □ EMACC Week 12 □ EMACC □ Cognition □ Function □ Blood □ MRI Week 18 □ EMACC Week 24 □ EMACC □ Cognition □ Function □ Blood □ MRI Primary Endpoint □ EMACC Secondary Endpoints □ CDR □ Ecog □ ADL, NPI □ Blood □ MRI □ Safety Six month, Randomized, Placebo - Controlled, Blinded Study of XPro TM in Early Alzheimer’s with Biomarkers of Inflammation XPro15G5 1 mg/kg weekly subQ injection Placebo n= 208 2:1 XPro : Placebo 26 XPro TM Halted Cognitive Decline* in Highly Inflamed Alzheimer’s Patients Change From Baseline Placebo XPro1595 EMACC: LS Mean Diff (SE): 0.086 (0.0603), 90% CI: - 0.0146, 0.1857 , p - value: 0.1594.
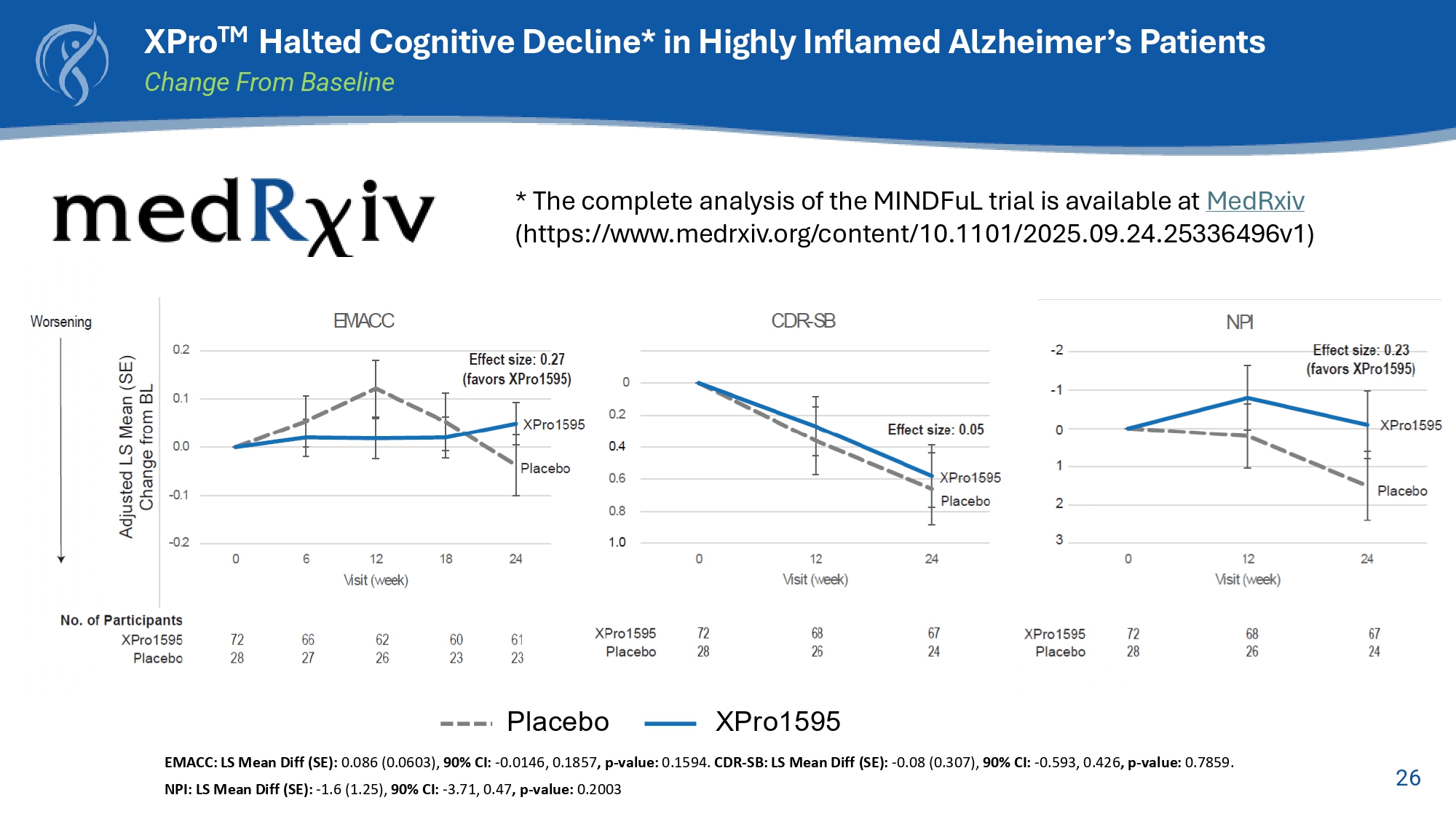
CDR - SB: LS Mean Diff (SE): - 0.08 (0.307), 90% CI: - 0.593, 0.426 , p - value: 0.7859. NPI: LS Mean Diff (SE): - 1.6 (1.25), 90% CI: - 3.71, 0.47 , p - value: 0.2003 * The complete analysis of the MINDFuL trial is available at MedRxiv (https:// www.medrxiv.org/content/10.1101/2025.09.24.25336496v1)
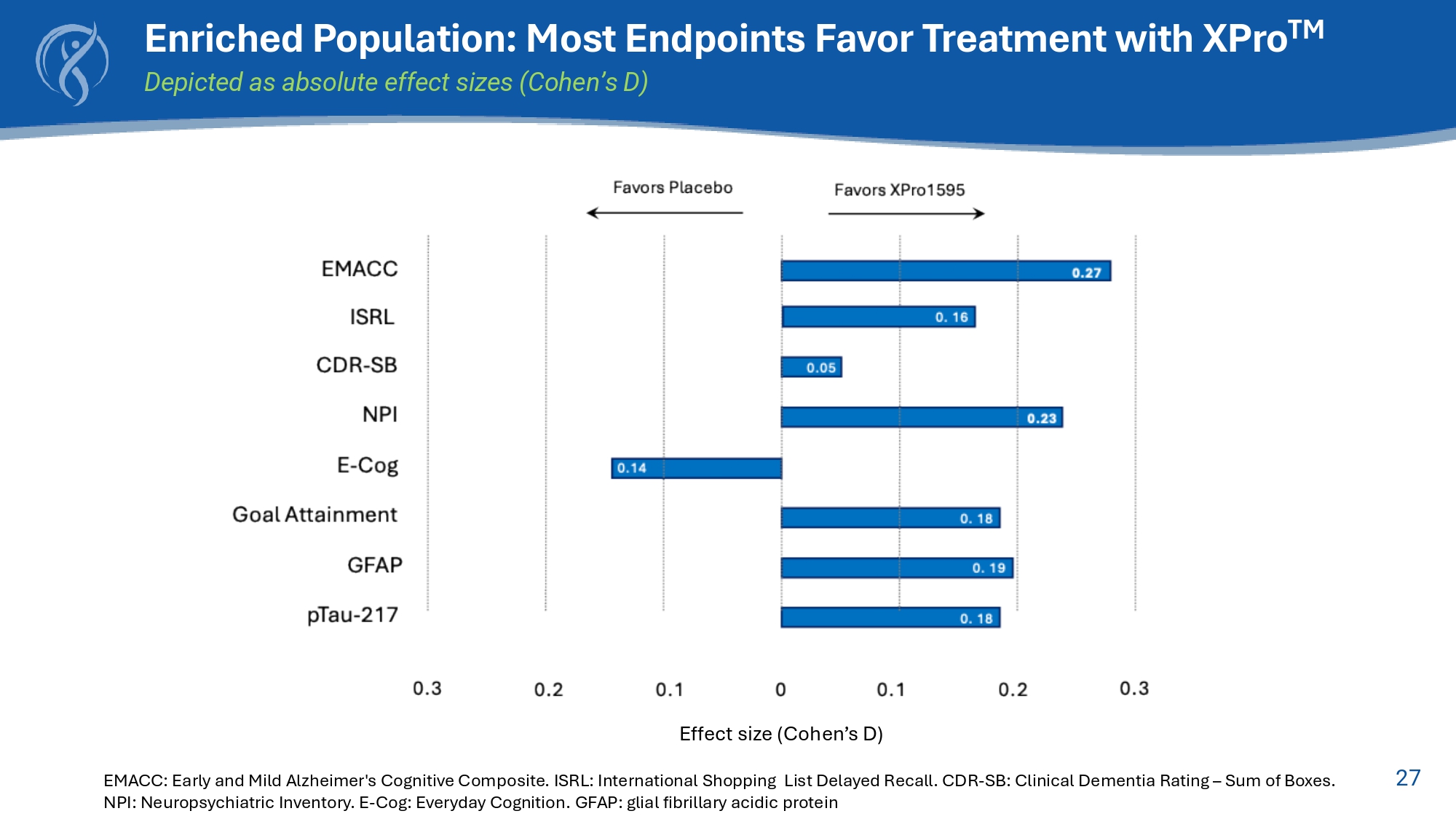
27 Enriched Population: Most Endpoints Favor Treatment with XPro TM Depicted as absolute effect sizes (Cohen’s D) Effect size (Cohen’s D) EMACC: Early and Mild Alzheimer's Cognitive Composite. ISRL: International Shopping List Delayed Recall. CDR - SB: Clinical Dementia Rating – Sum of Boxes. NPI: Neuropsychiatric Inventory. E - Cog: Everyday Cognition.
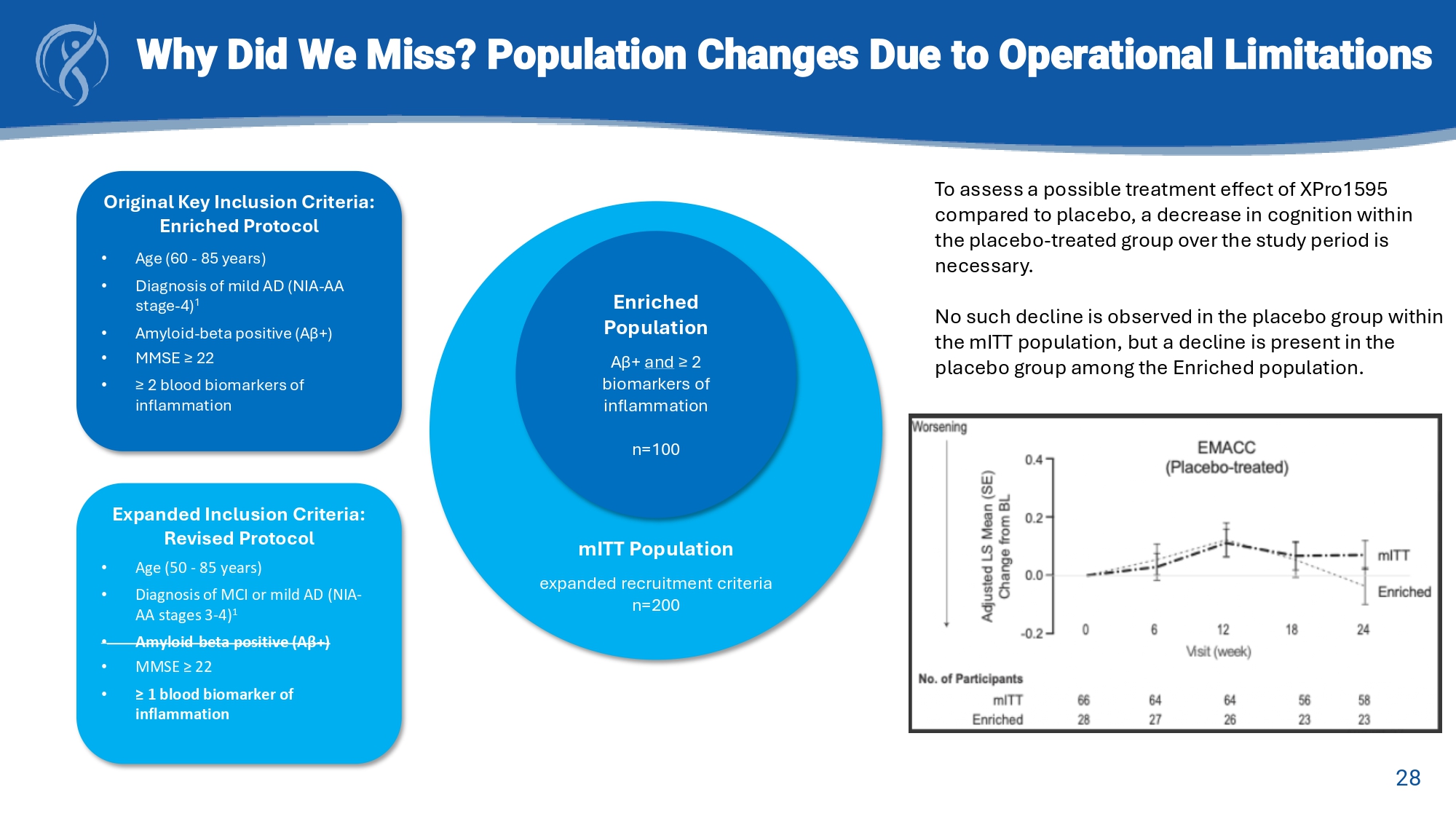
GFAP: glial fibrillary acidic protein 28 Why Did We Miss? Population Changes Due to Operational Limitations Original Key Inclusion Criteria: Enriched Protocol • Age (60 - 85 years) • Diagnosis of mild AD (NIA - AA stage - 4) 1 • Amyloid - beta positive (Aβ+) • MMSE ≥ 22 • ≥ 2 blood biomarkers of inflammation Expanded Inclusion Criteria: Revised Protocol • Age (50 - 85 years) • Diagnosis of MCI or mild AD (NIA - AA stages 3 - 4) 1 • Amyloid - beta positive (Aβ+) • MMSE ≥ 22 • ≥ 1 blood biomarker of inflammation mITT Population expanded recruitment criteria n=200 Enriched Population Aβ+ and ≥ 2 biomarkers of inflammation n=100 To assess a possible treatment effect of XPro1595 compared to placebo, a decrease in cognition within the placebo - treated group over the study period is necessary. No such decline is observed in the placebo group within the mITT population, but a decline is present in the placebo group among the Enriched population.
29 ARIA - E = amyloid - related imaging abnormalities - edema/effusions; ARIA - H=amyloid - related imaging abnormalities - microhemorrhages/hemosiderin deposits; SAF = Safety Analysis Set XPro : Safe, Effective, and Poised for Targeted Phase 3 Success ARIA - E Safety: • Zero cases of ARIA (a major issue with amyloid drugs) despite high - risk patients • No serious treatment - related adverse events Efficacy: • Phase 2 showed preserved cognition and improved biomarkers in patients with neuroinflammatory markers • Broader population analysis diluted results due to low placebo decline Path Forward: • Phase 3 will focus on biomarker - confirmed, inflammation - enriched Alzheimer’s patients — where XPro delivers its strongest benefit. Next steps: End of Phase 2 meeting with FDA for regulatory alignment to validate the enriched population in a fully powered trial.

30 Execution Roadmap: Regulatory s Clinical Milestones CORDStrom (RDEB, rare pediatric) ✓ Phase 2 Pediatric Trial Completed ✓ RPDD / ODD Granted → MAA Submission (UK) – Mid 2026 → BLA Submission (US) – Late 2026 → PRV Eligibility & Launch Readiness – 2027 XPro (Early Alzheimer's, biomarker - enriched) ✓ Phase 2 Study Completed ✓ Biomarker Validation Achieved (pTau217, GFAP) ✓ Patient Population Defined → End - of - Phase - 2 Meeting – Q1 2026 → CMC Phase 3 Optimization → Phase 3 Initiation – 2027 Manufacturing & CMC ▪ Commercial tech transfer nearly complete (Catapult UK) ▪ Scalable GMP infrastructure in place *Estimated dates subject to change – See “Forward - Looking Statements”

31 x Two late - stage programs with strong safety and efficacy signals x First - in - class systemic therapy for RDEB and novel Alzheimer’s mechanism x Multiple near - term regulatory milestones driving value x Capital - efficient development model with strong partnerships Investment Highlights 32 For More Information: INmune Bio Inc. 225 NE Mizner Blvd. Ste. 640 Boca Raton, FL 33432 (561) 710 - 0512 info@inmunebio.com INMB (Nasdaq)
