UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) October 17, 2025
Hoth Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 001-38803 | 82-1553794 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (I. R. S. Employer Identification No.) |
1177 Avenue of the Americas, 5th Floor, Suite 5066
New York, NY 10036
(Address of principal executive offices, including ZIP code)
(646) 756-2997
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common stock, $0.0001 par value | HOTH | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
Hoth Therapeutics, Inc. (the “Company”) has prepared presentation materials (the “Presentation Materials”) that management intends to use from time to time on and after October 17, 2025, in presentations about the Company’s operations and performance. The Presentation Materials are filed as Exhibit 99.1 to this Current Report on Form 8-K.
The information contained in the Presentation Materials is summary information that should be considered within the context of the Company’s filings with the Securities and Exchange Commission and other public announcements that the Company may make by press release or otherwise from time to time. The Presentation Materials speak as of the date of this Current Report on Form 8-K. While the Company may elect to update the Presentation Materials in the future or reflect events and circumstances occurring or existing after the date of this Current Report on Form 8-K, the Company specifically disclaims any obligation to do so.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. | Description | |
| 99.1 | Presentation Materials | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: October 17, 2025 | Hoth Therapeutics, Inc. |
| /s/ Robb Knie | |
| Robb Knie | |
| Chief Executive Officer |
2
Exhibit 99.1

October 2025 Innovating for Everyone Clinical - stage biopharmaceutical company focused on next generation therapeutics meeting unmet patient needs.

This presentation contains "forward - looking statements" within the meaning of the “safe - harbor” provisions of the Private Securities Litigation Reform Act of 1995 . These statements are identified by the use of words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential” and similar expressions that are intended to identify forward - looking statements . Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of Hoth Therapeutics, Inc . (“Hoth” or the “Company”) to differ materially from the results expressed or implied by such statements . These forward - looking statements are made on the basis of the current beliefs, expectations and assumptions of management, are not guarantees of performance and are subject to significant risks and uncertainty . These forward - looking statements should, therefore, be considered in light of various important factors, including those set forth in Hoth’s reports that it files from time to time with the Securities and Exchange Commission (the “Commission”) and which you should review, including those statements under “Item 1 A – Risk Factors” in Hoth’s Annual Report on Form 10 - K, as amended by its Quarterly Reports on Form 10 - Q and other reports that Hoth files with the Commission . Important factors that could cause actual results to differ materially from those described in forward - looking statements contained in this presentation include, but are not limited to : the adverse impact on economies around the world of the ongoing COVID - 19 pandemic ; changes to our anticipated sources of revenues ; competitive conditions ; difficulties in obtaining regulatory approvals for the Company’s product candidates ; changes in economic and political conditions ; the success of our research and development initiates ; and other factors . These forward - looking statements should not be relied upon as predictions of future events and Hoth cannot assure you that the events or circumstances discussed or reflected in these statements will be achieved or will occur . If such forward - looking statements prove to be inaccurate, the inaccuracy may be material . You should not regard these statements as representation or warranty by Hoth or any other person that we will achieve our objectives and plans in any specified timeframe, or at all . You are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date of this presentation . The Company disclaims any obligations to publicly update or release any revisions to the forward - looking information contained in this presentation, whether as a result of new information, future events or otherwise, after the date of this presentation or to reflect the occurrence of unanticipated events, except as required by law .

: HOTH Safe Harbor Statement At Hoth Therapeutics, we strive to develop innovative, impactful, and ground - breaking treatments with a goal to improve patient quality of life . We are a catalyst in early - stage pharmaceutical research and development, elevating promising drugs from the bench to pre - clinical and clinical testing . Utilizing a patient - centric approach, we collaborate and partner with a team of scientists, clinicians, and key opinion leaders to seek out and investigate medications that hold immense potential to create breakthroughs and diversify treatment options . Our mission is to bring value to both our shareholders and our patient populations . : HOTH Our Mission Key Investment Highlights : HOTH Clinical Programs Robust Pre - Clinical Development Programs Targeting Unmet Medical Needs to Address Broad Market Experienced Management and Advisory Board
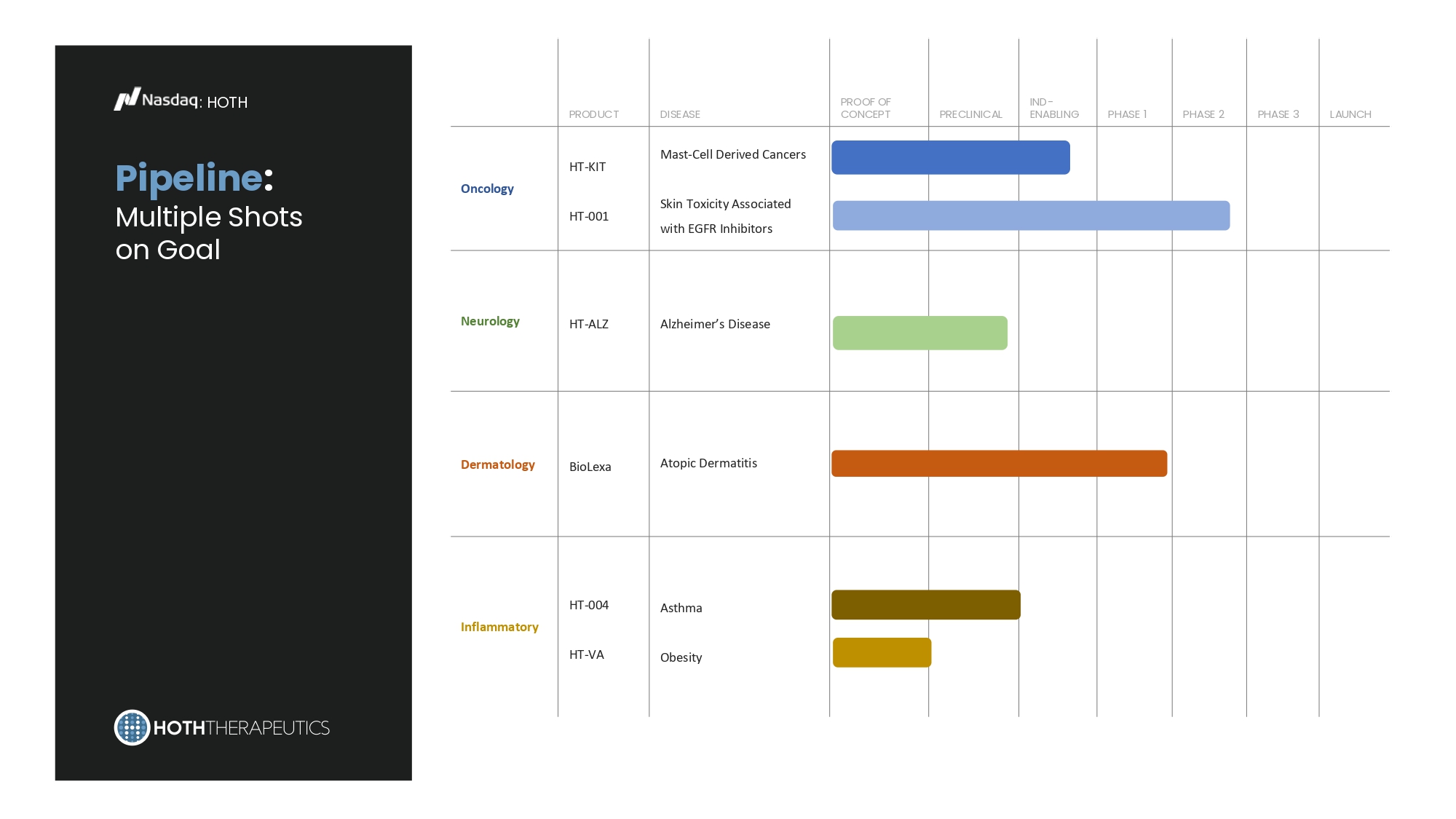
Pipeline : Multiple Shots on Goal : HOTH LAUNCH PHASE 3 PHASE 2 PHASE 1 IND - ENABLING PRECLINICAL PROOF OF CONCEPT DISEASE PRODUCT Mast - Cell Derived Cancers Skin Toxicity Associated with EGFR Inhibitors HT - KIT HT - 001 Oncology Alzheimer’s Disease HT - ALZ Neurology Atopic Dermatitis BioLexa Dermatology Asthma Obesity HT - 004 HT - VA Inflammatory Primary Development : HOTH HT - 001 Topical Gel HT - KIT Injection

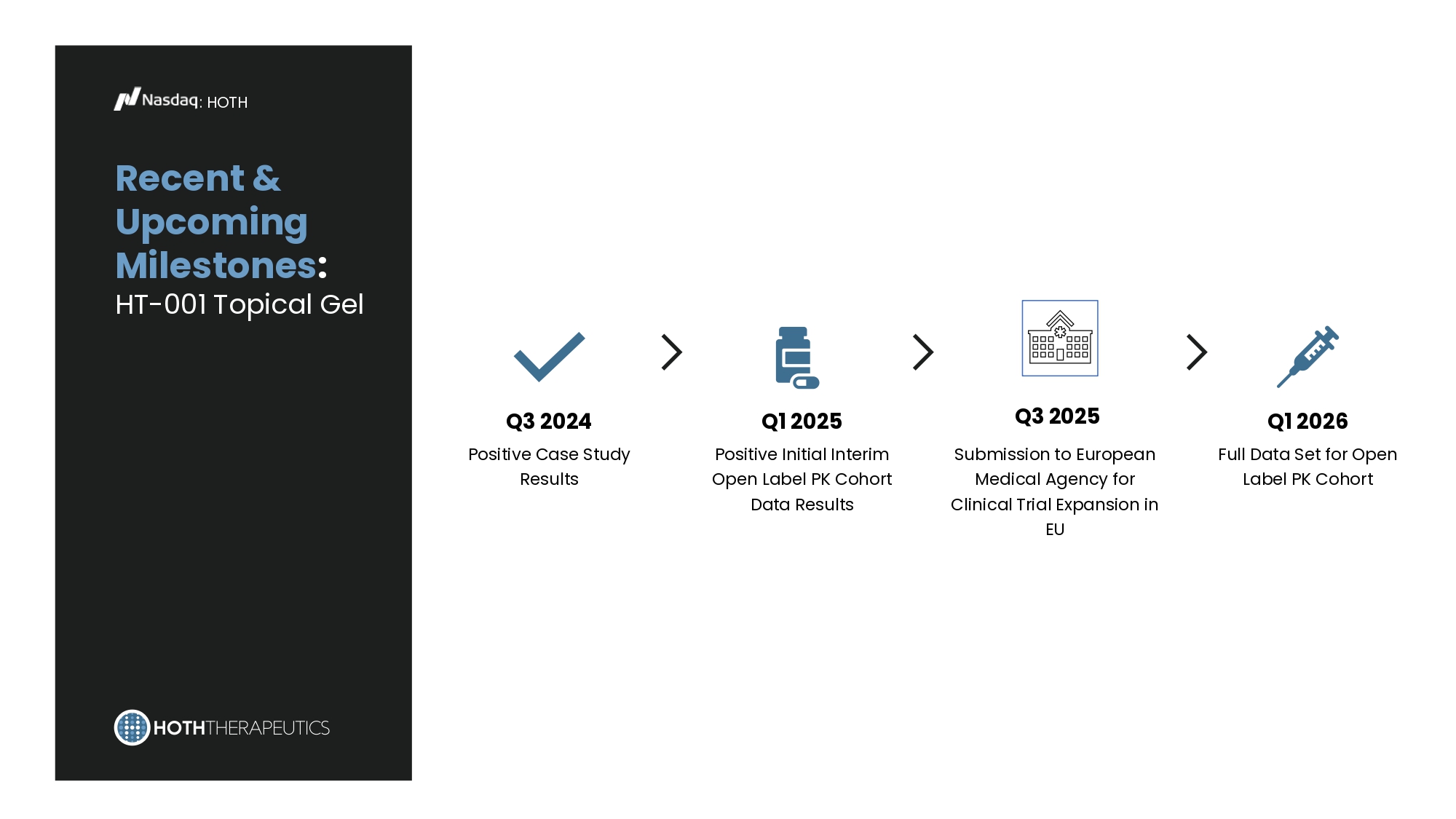
HT - 001 : Value Proposition : HOTH Market Growth: EGFR Inhibitor Skin Toxicity market predicted to grow from $52M in 2018 to $391M by end of 2030* Mechanism of Action: HT - 001’s active ingredient is a neurokinin 1 receptor agonist (NK1RA) that has been shown to have anti - inflammatory properties. The NK1RA mitigates the dermatological side effects by reducing the inflammation caused by inhibition of EGFR from oncology treatments. Addresses Unmet Need: No current approved product on the market that specifically treats EGFR inhibitor cutaneous toxicities, which occur in up to 90% of patients undergoing EFGR inhibitor therapy.** *EGFR Inhibitors - Induced Skin Disorders - Market Insights, Epidemiology, and Market Forecast - 2030 **https://jamanetwork.com/journals/jamadermatology/article - abstract/2767656 Recent & Upcoming Milestones : HT - 001 Topical Gel : HOTH Q3 2024 Positive Case Study Results Q1 2025 Positive Initial Interim Open Label PK Cohort Data Results Q3 2025 Submission to European Medical Agency for Clinical Trial Expansion in EU Q1 2026 Full Data Set for Open Label PK Cohort
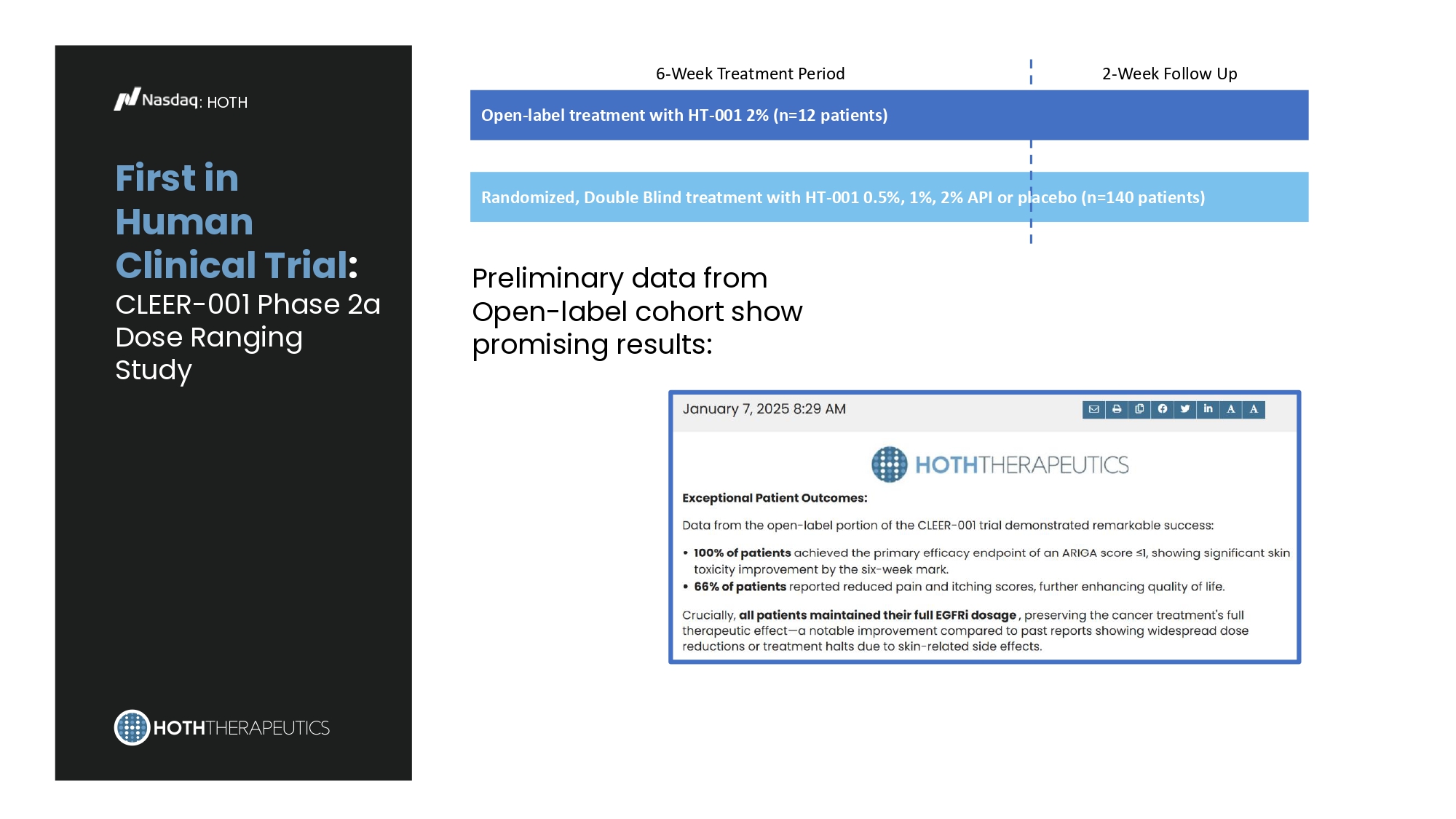
First in Human Clinical Trial : CLEER - 001 Phase 2a Dose Ranging Study : HOTH 6 - Week Treatment Period 2 - Week Follow Up Open - label treatment with HT - 001 2% (n=12 patients) Randomized, Double Blind treatment with HT - 001 0.5%, 1%, 2% API or placebo (n=140 patients) Preliminary data from Open - label cohort show promising results:
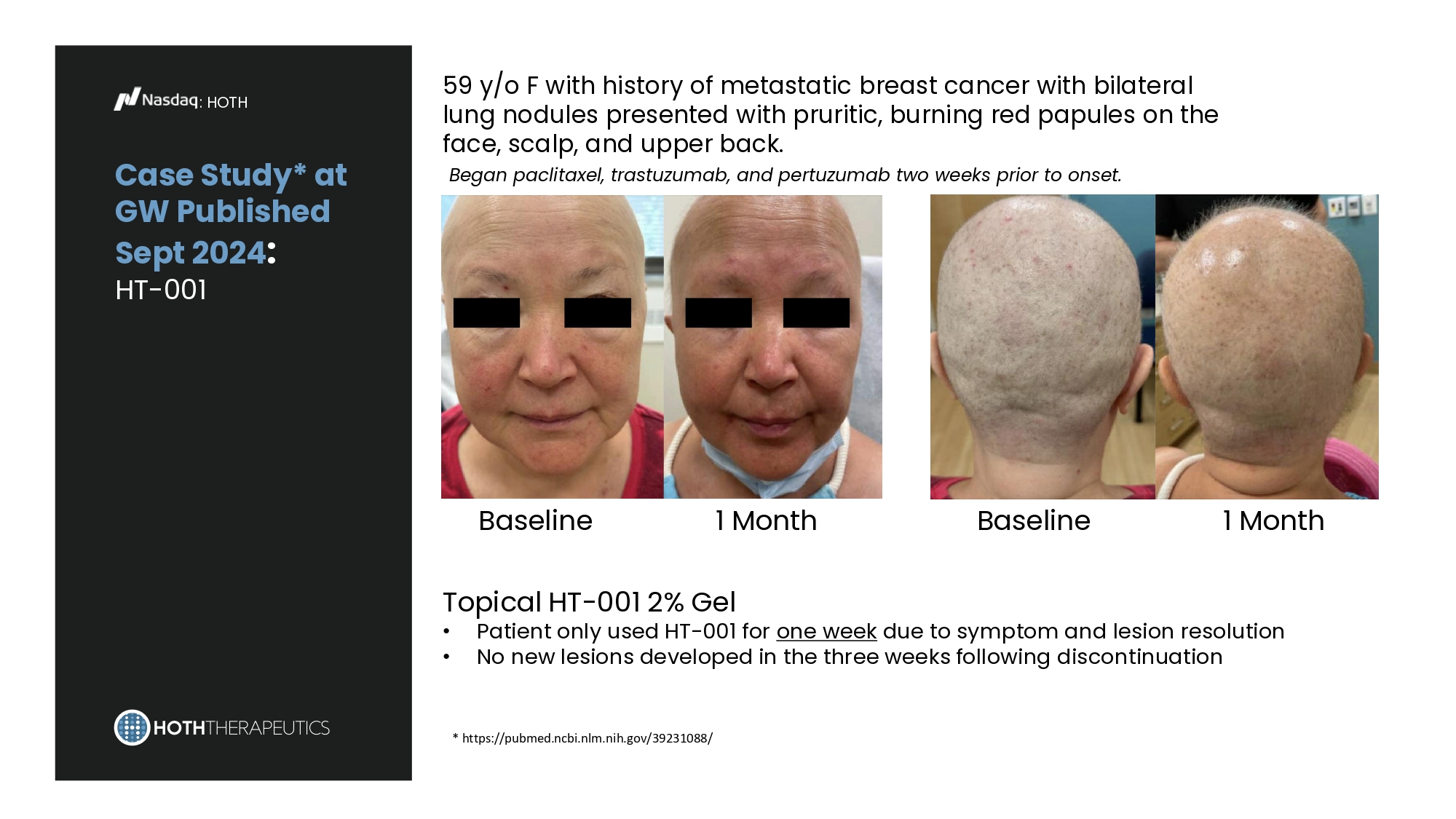
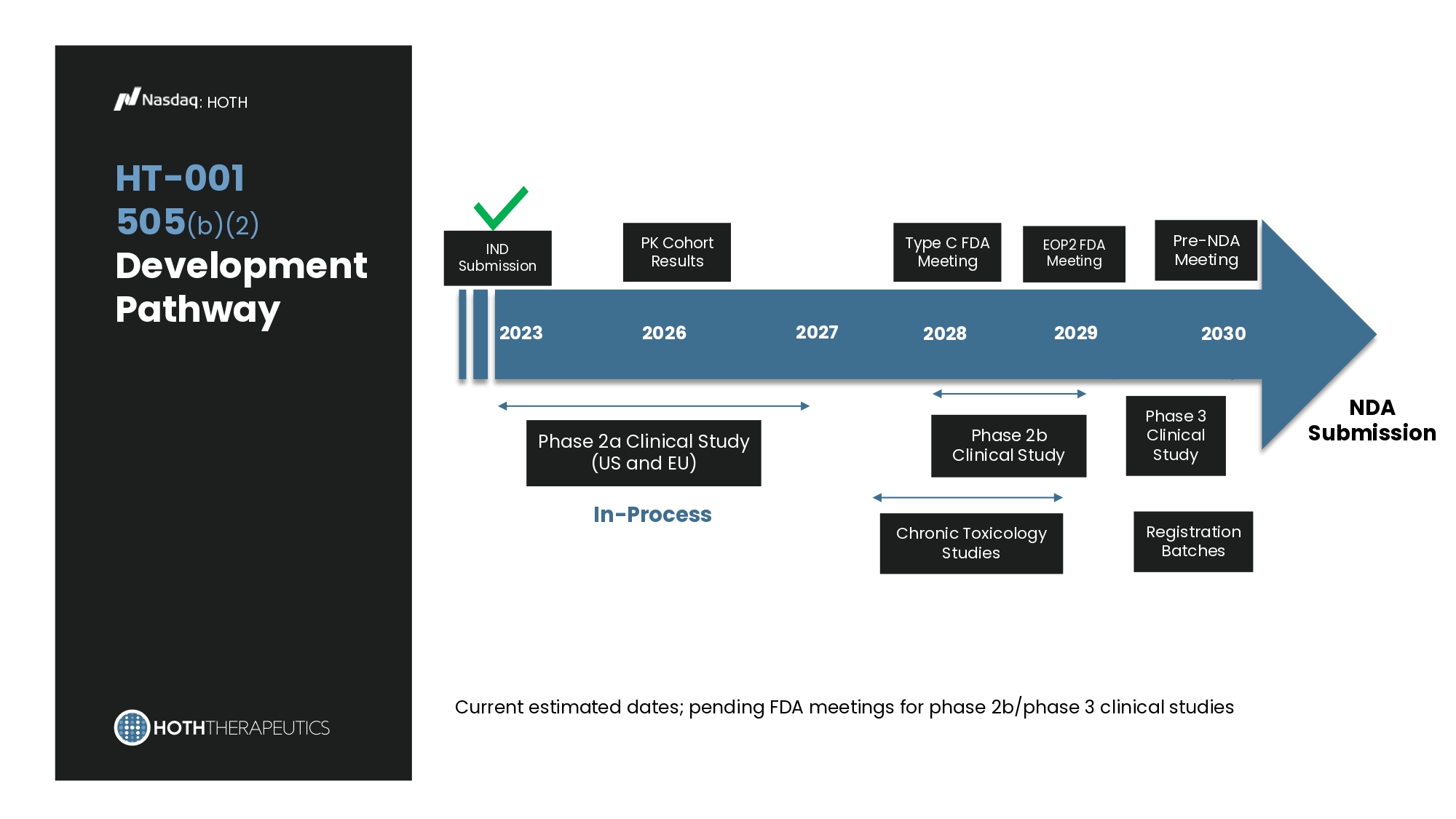
Case Study* at GW Published Sept 2024 : HT - 001 : HOTH 59 y/o F with history of metastatic breast cancer with bilateral lung nodules presented with pruritic, burning red papules on the face, scalp, and upper back. Began paclitaxel, trastuzumab, and pertuzumab two weeks prior to onset. Baseline 1 Month Baseline 1 Month Topical HT - 001 2% Gel • Patient only used HT - 001 for one week due to symptom and lesion resolution • No new lesions developed in the three weeks following discontinuation * https://pubmed.ncbi.nlm.nih.gov/39231088/ : HOTH HT - 001 505 (b)(2) Development Pathway Current estimated dates; pending FDA meetings for phase 2b/phase 3 clinical studies NDA Submission IND Submission 2023 Phase 2a Clinical Study (US and EU) EOP2 FDA Meeting 2030 2026 Phase 2b Clinical Study 2027 Phase 3 Clinical Study Chronic Toxicology Studies Registration Batches Type C FDA Meeting In - Process 2028 2029 Pre - NDA Meeting PK Cohort Results
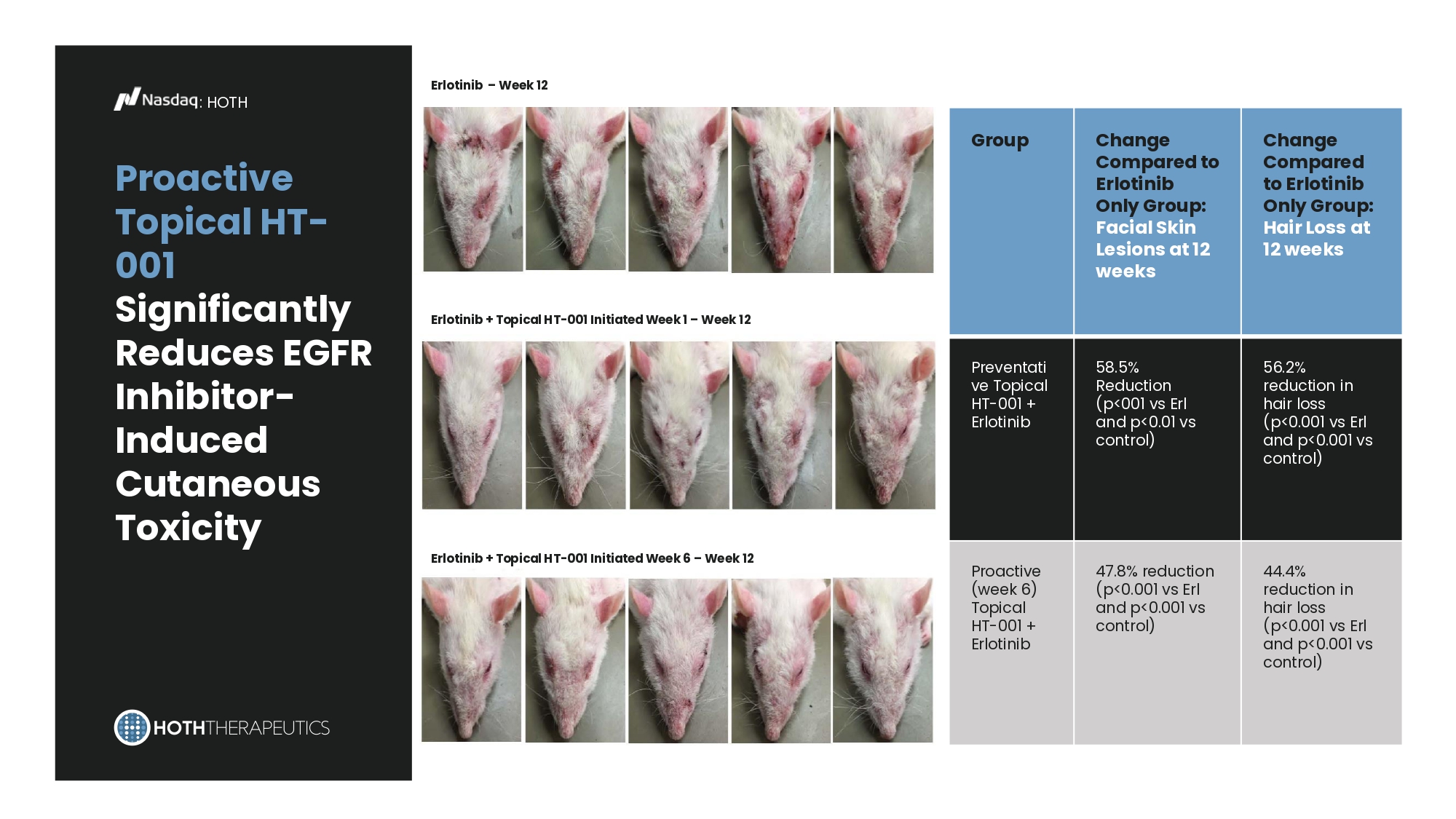
Erlotinib – Week 12 Erlotinib + Topical HT - 001 Initiated Week 1 – Week 12 Erlotinib + Topical HT - 001 Initiated Week 6 – Week 12 : HOTH Proactive Topical HT - 001 Significantly Reduces EGFR Inhibitor - Induced Cutaneous Toxicity Change Compared to Erlotinib Only Group: Hair Loss at 12 weeks Change Compared to Erlotinib Only Group: Facial Skin Lesions at 12 weeks Group 56.2% reduction in hair loss (p<0.001 vs Erl and p<0.001 vs control) 58.5% Reduction (p<001 vs Erl and p<0.01 vs control) Preventati ve Topical HT - 001 + Erlotinib 44.4% reduction in hair loss (p<0.001 vs Erl and p<0.001 vs control) 47.8% reduction (p<0.001 vs Erl and p<0.001 vs control) Proactive (week 6) Topical HT - 001 + Erlotinib HT - KIT : Value Proposition : HOTH Market Growth: Global systemic mastocytosis treatment revenue is $128M and projected to grow at 5.8% CAGR through 2031* Mechanism of Action: HT - KIT is an antisense oligonucleotide that results in non - functional cKIT via mRNA frameshift.** Addresses Unmet Need: KIT D816V mutation found in >80% of adult systemic mastocytosis cases results in confirmational changes that make some tyrosine kinase inhibitor drugs ineffective.** cKIT is also implicated in gastrointestinal stromal tumors, acute myeloid leukemia, and other rare cancers *Global Systemic Mastocytosis Treatment Market Research Report, January 2022, Market.US **Snider et al., Targeting KIT by frameshifting mRNA transcripts as a therapeutic strategy for aggressive mast cell neoplasms, Molecular Therapy (2021), https://doi.org/10.1016/j.ymthe.2021.08.009
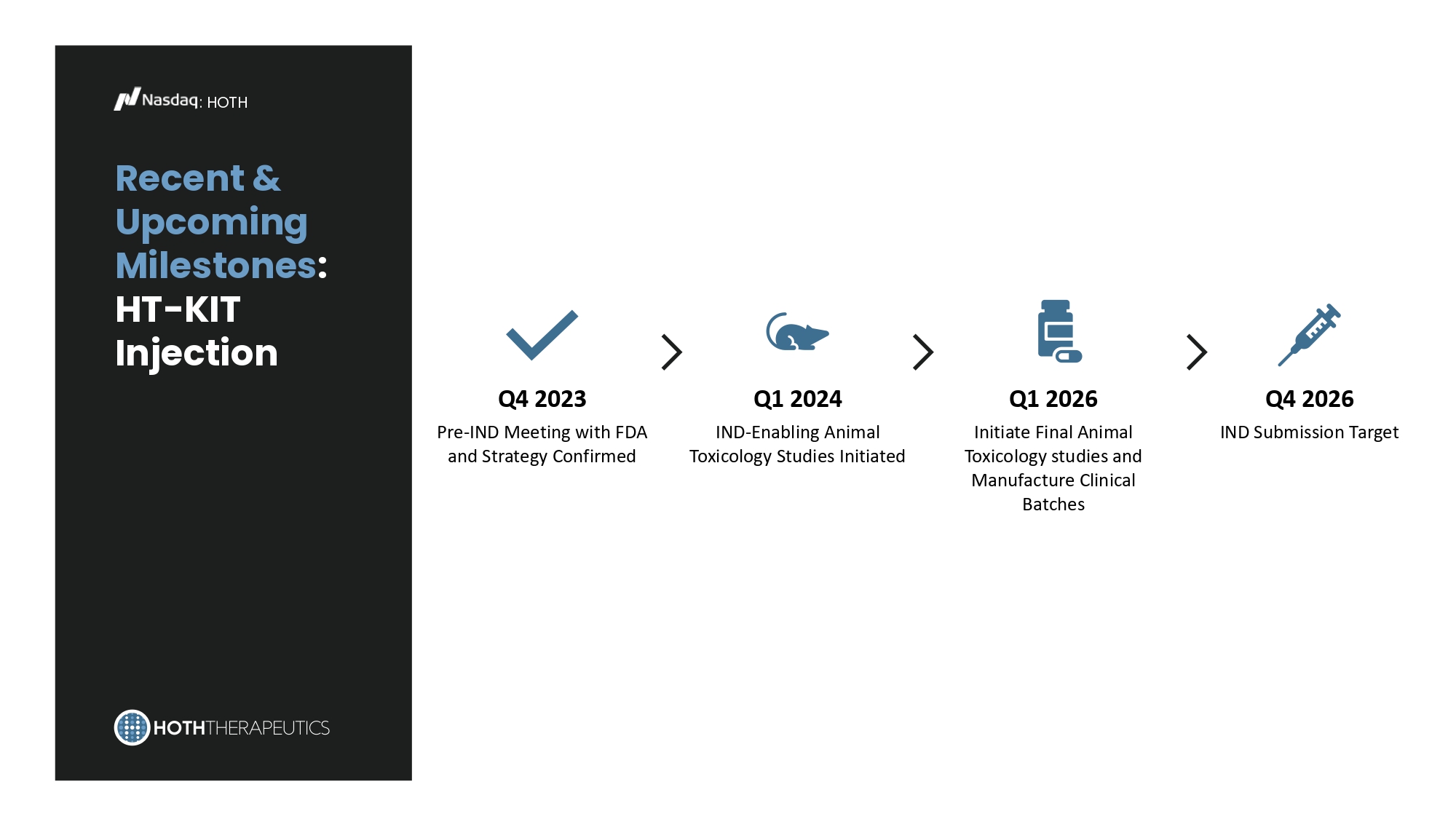
Recent & Upcoming Milestones : HT - KIT Injection : HOTH Q4 2023 Pre - IND Meeting with FDA and Strategy Confirmed Q1 2024 IND - Enabling Animal Toxicology Studies Initiated Q1 2026 Initiate Final Animal Toxicology studies and Manufacture Clinical Batches Q4 2026 IND Submission Target HT - KIT Orphan Drug Development Pathway : HOTH Pivotal Clinical Studies (TBD) Current estimated dates; pending FDA meetings for clinical studies.
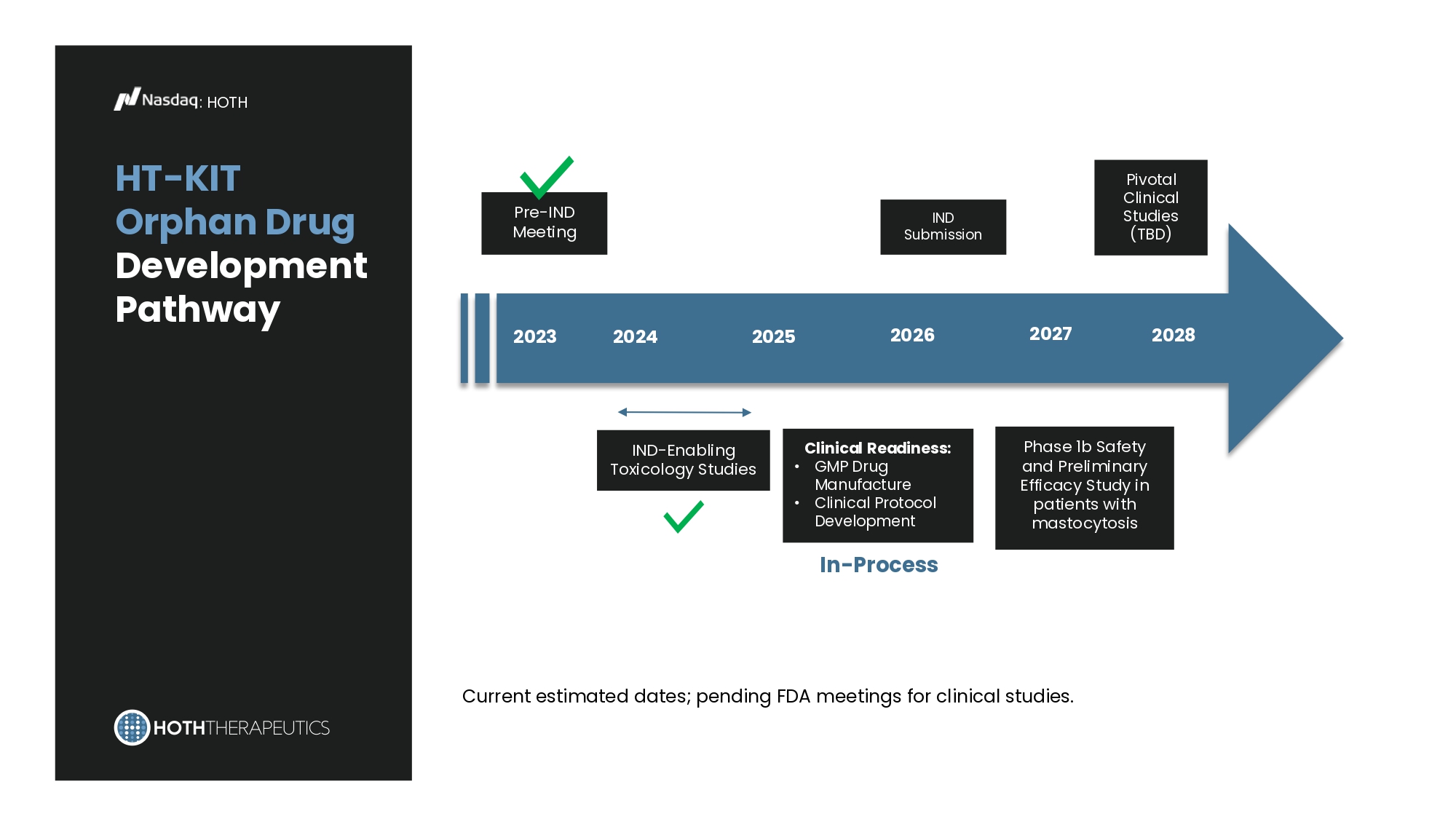
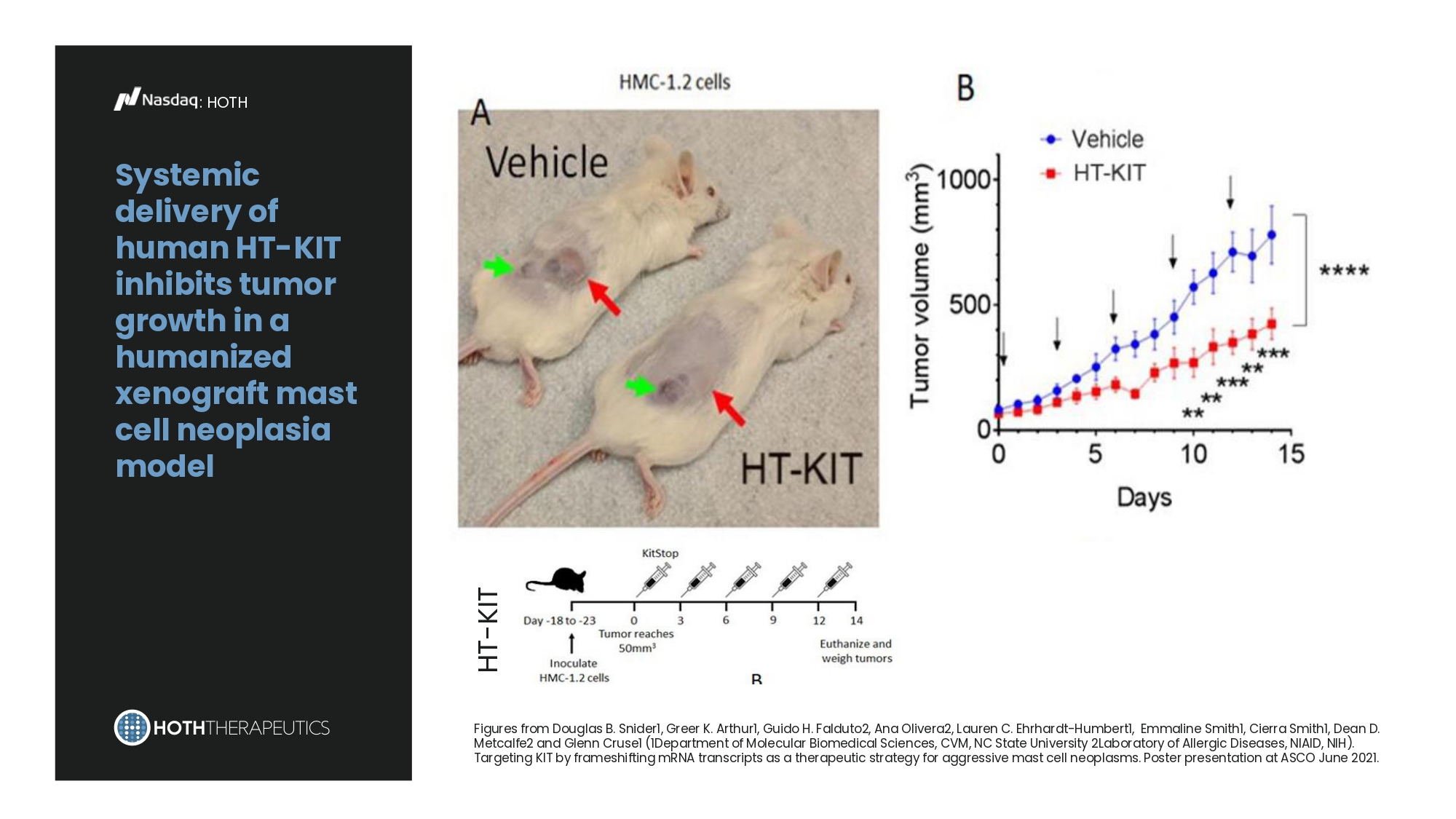
Pre - IND Meeting 2023 2024 IND - Enabling Toxicology Studies 2025 Phase 1b Safety and Preliminary Efficacy Study in patients with mastocytosis IND Submission 2026 Clinical Readiness: • GMP Drug Manufacture • Clinical Protocol Development In - Process 2027 2028 : HOTH Systemic delivery of human HT - KIT inhibits tumor growth in a humanized xenograft mast cell neoplasia model Figures from Douglas B. Snider1, Greer K. Arthur1, Guido H. Falduto2, Ana Olivera2, Lauren C. Ehrhardt - Humbert1, Emmaline Smith1, Cierra Smith1, Dean D. Metcalfe2 and Glenn Cruse1 (1Department of Molecular Biomedical Sciences, CVM, NC State University 2Laboratory of Allergic Diseases, NIAID, NIH). Targeting KIT by frameshifting mRNA transcripts as a therapeutic strategy for aggressive mast cell neoplasms. Poster presentation at ASCO June 2021.
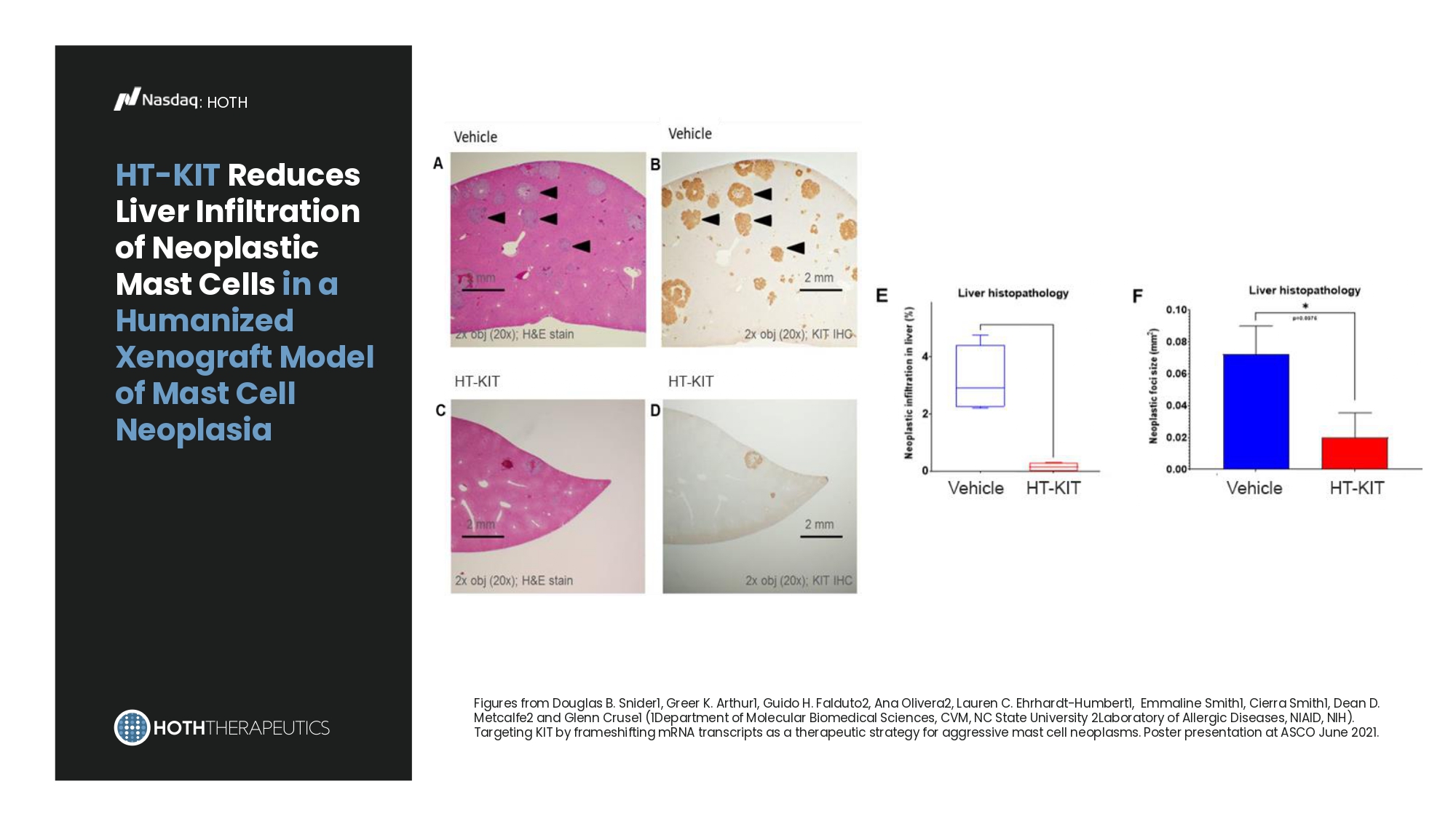
HT - KIT : HOTH HT - KIT Reduces Liver Infiltration of Neoplastic Mast Cells in a Humanized Xenograft Model of Mast Cell Neoplasia Figures from Douglas B. Snider1, Greer K. Arthur1, Guido H. Falduto2, Ana Olivera2, Lauren C. Ehrhardt - Humbert1, Emmaline Smith1, Cierra Smith1, Dean D. Metcalfe2 and Glenn Cruse1 (1Department of Molecular Biomedical Sciences, CVM, NC State University 2Laboratory of Allergic Diseases, NIAID, NIH). Targeting KIT by frameshifting mRNA transcripts as a therapeutic strategy for aggressive mast cell neoplasms. Poster presentation at ASCO June 2021.
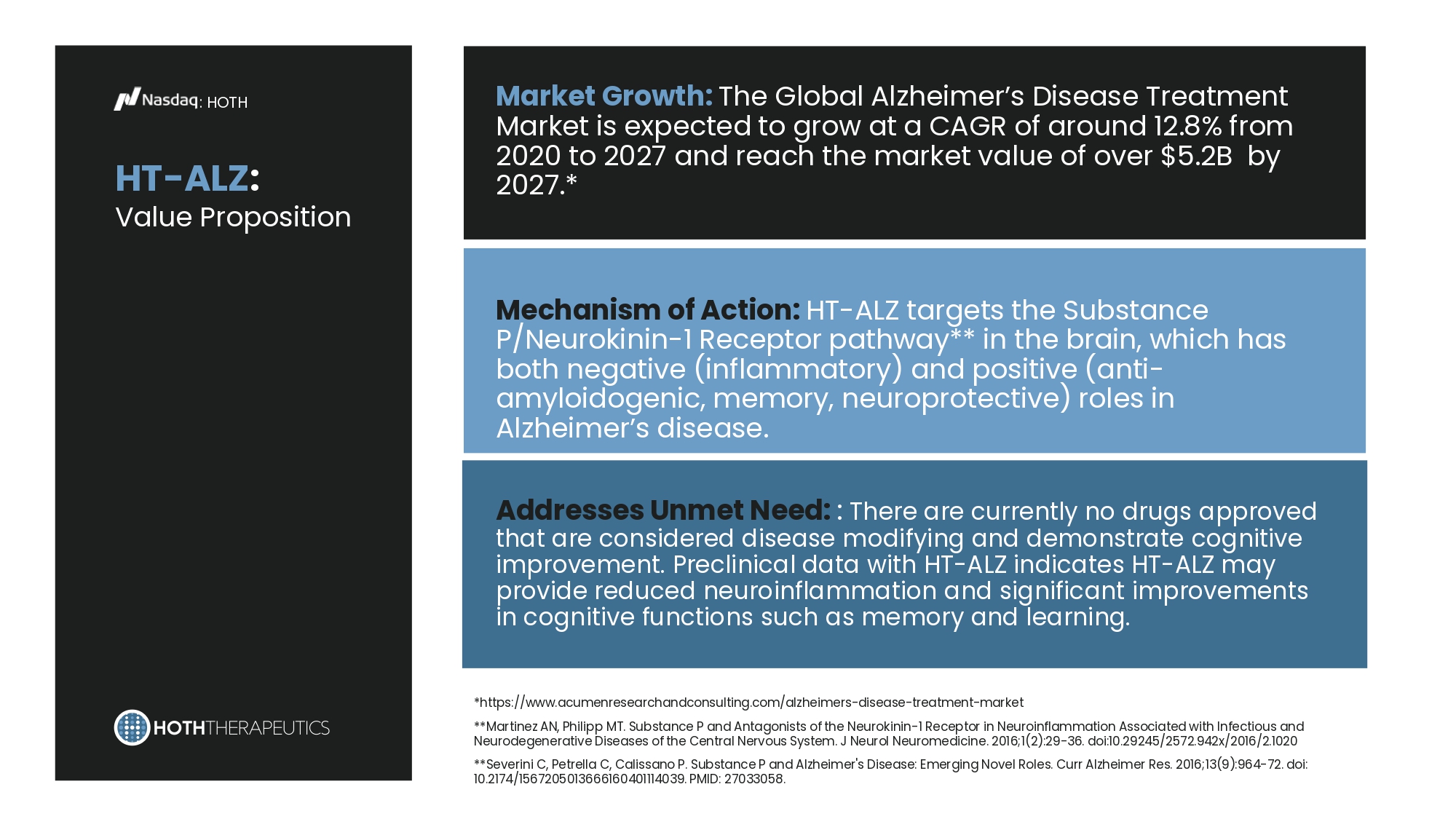
Preclinical Development : HOTH HT - ALZ Oral Film HT - VA HT - ALZ : Value Proposition : HOTH *https:// www.acumenresearchandconsulting.com/alzheimers - disease - treatment - market **Martinez AN, Philipp MT. Substance P and Antagonists of the Neurokinin - 1 Receptor in Neuroinflammation Associated with Infectious and Neurodegenerative Diseases of the Central Nervous System. J Neurol Neuromedicine. 2016;1(2):29 - 36. doi:10.29245/2572.942x/2016/2.1020 **Severini C, Petrella C, Calissano P. Substance P and Alzheimer's Disease: Emerging Novel Roles. Curr Alzheimer Res. 2016;13(9):964 - 72. doi: 10.2174/1567205013666160401114039. PMID: 27033058. Market Growth: The Global Alzheimer’s Disease Treatment Market is expected to grow at a CAGR of around 12.8% from 2020 to 2027 and reach the market value of over $5.2B by 2027.* Mechanism of Action: HT - ALZ targets the Substance P/Neurokinin - 1 Receptor pathway** in the brain, which has both negative (inflammatory) and positive (anti - amyloidogenic, memory, neuroprotective) roles in Alzheimer’s disease. Addresses Unmet Need: : There are currently no drugs approved that are considered disease modifying and demonstrate cognitive improvement. Preclinical data with HT - ALZ indicates HT - ALZ may provide reduced neuroinflammation and significant improvements in cognitive functions such as memory and learning.

Recent & Upcoming Milestones : HT - ALZ Oral Soluble Film : HOTH Q2 2024 HT - ALZ Formulation Work initiated Q3 2024 US Patent Office Awarded HT - ALZ Patent Q3 2024 Preclinical Studies completed at WashU 2025 Pre - IND Meeting Submission HT - VA : Value Proposition : HOTH Market Growth: The global obesity treatment market size was valued at USD 15.92 billion in 2024 and is projected to reach USD 60.53 billion by 2030, growing at a CAGR of 22.31% from 2025 to 2030.* Mechanism of Action: A GDNF family receptor alpha - 1 (GFR α - 1) agonist, important for the differentiation and survival of neurons, GDNF signals through its receptors, including Ret and GFR - 1 α , by activation of the PI3K and MAPK pathways. HT - VA is being formulated to treat or prevent obesity, metabolic syndrome, or insulin resistance by administering an effective amount of a pharmaceutical composition comprising of one or more GDNF receptor agonists.
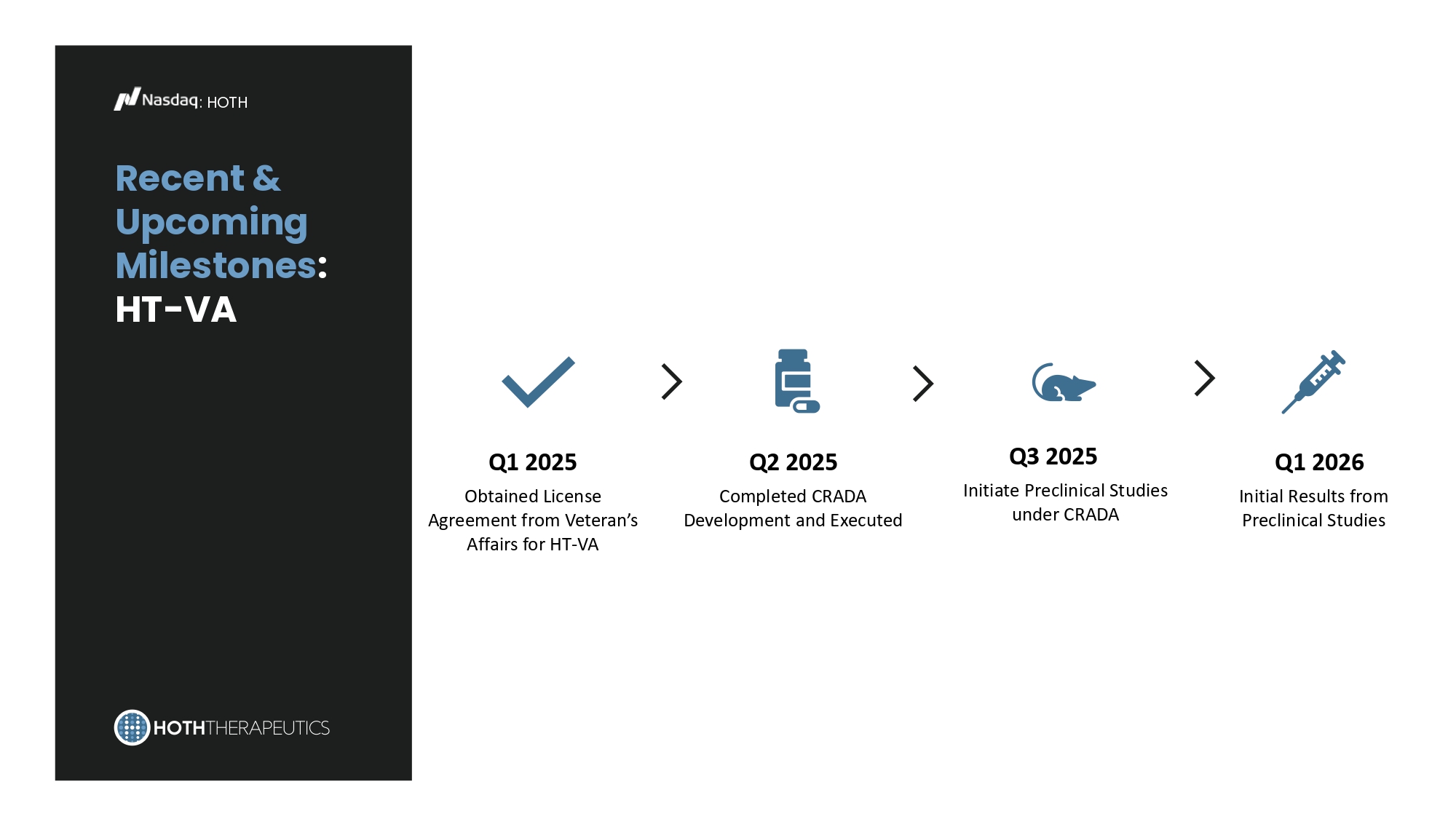
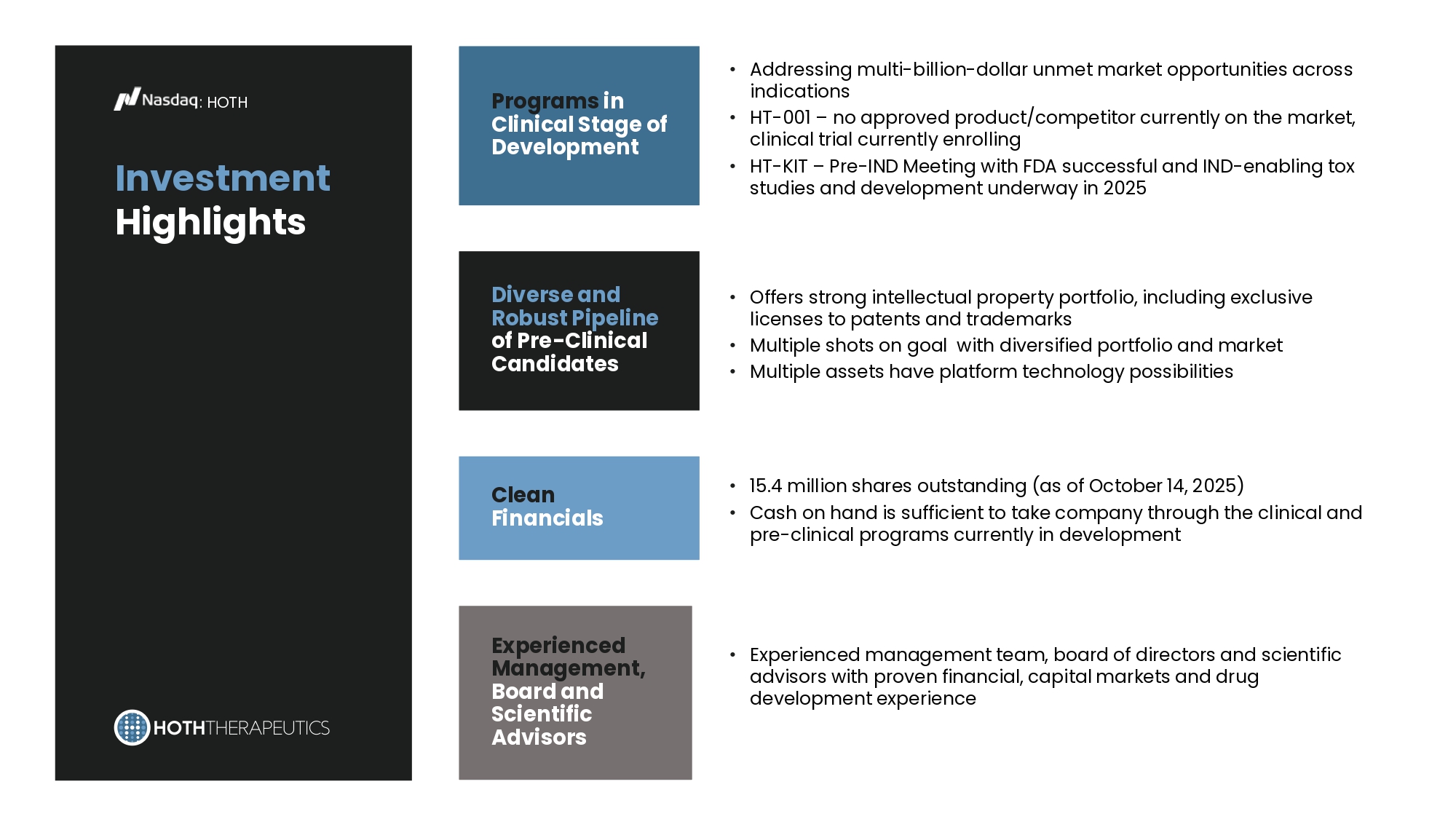
* Obesity Global Market Reference Recent & Upcoming Milestones : HT - VA : HOTH Q1 2025 Obtained License Agreement from Veteran’s Affairs for HT - VA Q2 2025 Completed CRADA Development and Executed Q3 2025 Initiate Preclinical Studies under CRADA Q1 2026 Initial Results from Preclinical Studies Investment Highlights : HOTH Programs in Clinical Stage of Development Diverse and Robust Pipeline of Pre - Clinical Candidates Clean Financials Experienced Management, Board and Scientific Advisors • Addressing multi - billion - dollar unmet market opportunities across indications • HT - 001 – no approved product/competitor currently on the market, clinical trial currently enrolling • HT - KIT – Pre - IND Meeting with FDA successful and IND - enabling tox studies and development underway in 2025 • Offers strong intellectual property portfolio, including exclusive licenses to patents and trademarks • Multiple shots on goal with diversified portfolio and market • Multiple assets have platform technology possibilities • 15.4 million shares outstanding (as of October 14, 2025) • Cash on hand is sufficient to take company through the clinical and pre - clinical programs currently in development • Experienced management team, board of directors and scientific advisors with proven financial, capital markets and drug development experience Thank You.

: HOTH : HOTH Contact Information Investor Relations: Hoth Therapeutics, Inc. investorrelations@hoththerapeutics.com