UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
September 19, 2025
Date of Report (Date of earliest event reported)
Advanced Biomed Inc.
(Exact name of Company as specified in its charter)
| Nevada | 001-42548 | 87-2177170 | ||
| (State or other jurisdiction | (Commission File Number) | (IRS Employer | ||
| of Incorporation) | Identification Number) |
No. 689-85 Xiaodong Road, Yongkang District
Tainan City, Taiwan
(Address of principal executive offices)
886-6-3121716
(Registrant’s telephone number including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the Company under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock | ADVB | The Nasdaq Stock Market LLC |
Item 7.01 Regulation FD Disclosure.
On September 19, 2025, the Company issued a press release regarding the launch of A+PerfusC™ – Integrated Perfusion 3D Cell Culture Platform for Precision Medicine and Drug Discovery. A copy of the press release is attached as Exhibit 99.1 hereto.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), nor shall it be deemed incorporated by reference in any of the Company’s filings under the Securities Act, or the Exchange Act, whether made before or after the date hereof, except as shall be expressly set forth by specific reference to this report in such filing.
Item 9.01 Financial Statement and Exhibits.
| Exhibit No. | Description | |
| 99.1* | Press Release, dated September 19, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| * | Furnished herewith. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Company has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Advanced Biomed Inc. | ||
| Date: September 19, 2025 | By: | /s/ Yi Lu |
| Yi Lu | ||
| Chief Executive Officer | ||
2
Exhibit 99.1
Advanced Biomed Inc. Announces Launch of A+PerfusC™ – Integrated Perfusion 3D Cell Culture Platform for Precision Medicine and Drug Discovery
Tainan City, Taiwan, — September 19, 2025 — Advanced Biomed Inc. (Nasdaq: ADVB) (the “Company”, “Advanced Biomed”), a biotechnology company focused on developing and commercializing innovative biomedical products for precision medicine and advanced diagnostics, today announced the launch of its A+PerfusC™ system, a compact, all-in-one perfusion 3D cell culture incubator designed to replicate human physiological conditions in vitro.
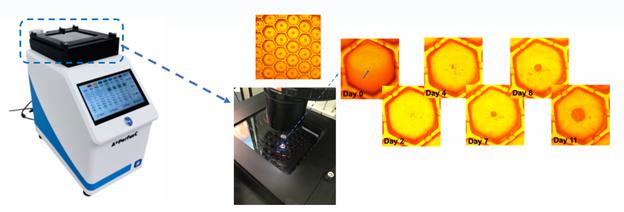
(A+PerfusC™ 3D System-a perfusion-based 3D cell culture in a compact incubator)
The A+PerfusC™ platform integrates automated perfusion with environmental control in a compact format that can be mounted directly onto a microscope for real-time observation. The system supports up to 12 days of continuous, hands-free culture, reducing the risk of human error and contamination. By maintaining uniform nutrient delivery and preventing waste accumulation, the platform promotes spheroid and organoid formation, enhancing cell viability, growth, and drug response predictability. The system is characterized and proved by:
| ● | 8 independent channel/patient source per plate |
| ● | 600 tumor spheroids per row for one patient |
| ● | 4,800 tumor spheroids per plate |
| ● | Easy to transfer into 96 or 384 well plate for high-throughput drug screening |
| ● | Easy to mount on microscopy platforms |
| ● | Expandable- control platform supporting up to four incubators. (undergoing) |
A+PerfusC™ system and its 3D culture plate (consumable) have passed internal testing and validation with design finalized. Currently the Company is advancing its mass-production development. Its commercialization can be achieved following mass-production development finalized and quality control standards established.
Potential applications include personalized oncology research, invitro drug screening, regenerative medicine, organoid studies, and stem cell research. According to Mordor Intelligence, the global 3D cell culture market was valued at USD 2.32 billion in 2025 and is projected to exceed USD 4.71 billion by 2030, representing a compound annual growth rate of over 15.6% (source: https://www.mordorintelligence.com/industry-reports/3d-cell-culture-market).
Dr. Yi Lu, CEO of the Company, commented: “Traditional static cell culture often leads to uneven nutrient delivery and waste buildup, compromising cell growth, viability, and reproducibility. Notably, in certain high-density culture scenarios, particularly within multi-microwell 3D culture devices, metabolic waste can rapidly accumulate inside the micro-grooves, requiring more frequent medium replacement which can be labor-intensive to preserve culture quality.”
“Our 3D culture system evenly delivers nutrients and clears waste, reducing cellular stress, boosting long-term viability, and more accurately mimicking human tissue complexity for improved drug response and disease modeling.”
“The Company plans to scale up the A+PerfusC™ system for high-throughput use and integrate imaging with AI-driven analytics, aiming to accelerate tumor profiling, refine cell therapy protocols, and advance personalized treatment development.”
About Advanced Biomed Inc.
Advanced Biomed Inc. is a Nevada-incorporated holding company specializing in innovative biomedical technologies for cancer detection and precision medicine.
Operating through subsidiaries in Taiwan, Hong Kong, and Mainland China, the Company has developed a proprietary microfluidic platform that integrates semiconductor and biotechnology to enable advanced circulating tumor cell (CTC) detection, enrichment, and analysis. Its portfolio includes devices, biochips, and designed for cancer screening, diagnosis, treatment selection, and prognosis assessment, with regulatory clearances in progress in China and plans for future global expansion.
For more information, please visit: www.advanbiomed.com.
Forward-Looking Statements
This press release contains forward-looking statements. Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other statements that are other than statements of historical facts. When the Company uses words such as “may, “will, “intend,” “should,” “believe,” “expect,” “anticipate,” “project,” “estimate” or similar expressions that do not relate solely to historical matters, it is making forward-looking statements. Forward-looking statements are not guarantees of future performance and involve risks and uncertainties that may cause the actual results to differ materially from the Company's expectations discussed in the forward-looking statements. These statements are subject to uncertainties and risks including, but not limited to, the uncertainties related to market conditions and other factors discussed in the “Risk Factors” section of the registration statement filed with the SEC. For these reasons, among others, investors are cautioned not to place undue reliance upon any forward-looking statements in this press release. Additional factors are discussed in the Company's filings with the SEC, which are available for review at www.sec.gov. The Company undertakes no obligation to publicly revise these forward-looking statements to reflect events or circumstances that arise after the date hereof.
For more information, please contact:
Advanced Biomed Inc.
Steven I-Fang Cheng
| Email: | info@advbiomedicine.com |
| invest@advbiomedicine.com | |