UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): September 18, 2025
NEKTAR THERAPEUTICS
(Exact Name of Registrant as Specified in Charter)
| Delaware | 0-24006 | 94-3134940 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
455 Mission Bay Boulevard South
San Francisco, California 94158
(Address of Principal Executive Offices and Zip Code)
Registrant’s telephone number, including area code: (415) 482-5300
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value | NKTR | Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On September 18, 2025, Nektar Therapeutics (“Nektar”) posted slides on its website (www.nektar.com) containing additional information related to certain new data from the ongoing REZOLVE-AD Phase 2b study of rezpegaldesleukin, as discussed in Item 8.01 below. A copy of the slides is attached hereto as Exhibit 99.1 and is incorporated herein by reference. Information contained on Nektar’s website is not incorporated by reference into this Current Report on Form 8-K, and you should not consider any information on, or that can be accessed from, Nektar’s website as part of this Current Report on Form 8-K.
The information under this Item 7.01, including Exhibit 99.1 hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing. Nektar undertakes no obligation to update, supplement or amend the material attached hereto as Exhibit 99.1.
| Item 8.01 | Other Events. |
On September 18, 2025, Nektar announced certain new data from the ongoing REZOLVE-AD Phase 2b study of rezpegaldesleukin. A copy of the press release issued in connection with the announcement is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| NEKTAR THERAPEUTICS | ||
| Date: September 18, 2025 | By: | /s/ Mark A. Wilson |
| Mark A. Wilson | ||
| Chief Legal Officer and Secretary | ||
Exhibit 99.1

Efficacy and Safety of Rezpegaldesleukin, A Selective Regulatory T - Cell - Inducing Interleukin - 2 Conjugate, in the Treatment of Atopic Dermatitis: Final Results from the 16 - Week Induction of a Randomized Phase 2b Study (REZOLVE - AD) Jonathan I. Silverberg 1 , Thomas Bieber 2 , Richard B. Warren 3 , Raj Chovatiya 4,5 , Melinda Gooderham 6 , Spyridon Gkalpakiotis 7 , Adam Reich 8 , Dedée F. Murrell 9 , Justyna Skibinska 10 , Joanna Renczynska - Matysko 11 , Neil Sadick 12,13 , Iva Blajić 14 , Athanasios Tsianakas 15 , Oliver Weirich 16 , Pablo Fernandez - Penas 17,18 , Johannes S. Kern 19,20 , Trinidad Montero Vilchez 21 , Todor A. Popov 22 , Anil Kurian 23 , Sohail Chaudhry 24 , Zachary Lee 24 , Maria Hannaway 24 , Danni Yu 24 , Yi Liu 24 , Christie Fanton 24 , Meng Zhang 24 , Wang Waltz 24 , Jenny Gilbert 24 , Soratree Charoenthongtrakul 24 , Charleen Jue 24 , Mario Marcondes 24 , Brian Kotzin 24 , Mary Tagliaferri 24 , Jonathan Zalevsky 24 , David Rosmarin 25 1 Department Of Dermatology, George Washington University School Of Medicine, Washington, DC, United States; 2 Bieber Dermatology Consulting, Feldafing, Germany; 3 Dermatology Centre, Northern Care Alliance NHS Foundation Trust & Division of Musculoskeletal and Dermatological Sciences, Manchester NIHR Biomedical Research Centre, Manchester Academic Health Science Centre, University of Manchester, UK; 4 Rosalind Franklin University of Medicine and Science Chicago Medical School, North Chicago, IL, United States; 5 Center for Medical Dermatology + Immunology Research, Chicago, IL, United States; 6 Department of Medicine, Queen’s University, Kingston, ON, Canada; 7 Department of Dermatovenereology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic; 8 Department of Dermatology, Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; 9 School of Medicine, University of Notre Dame, Sydney, NSW, Australia; 10 Klinika Ambroziak, Warsaw, Poland; 11 Synexus Polska, Gdansk, Poland; 12 Department Of Dermatology, Weill Cornell Medicine, New York, NY, United States; 13 Sadick Research Group, New York, NY, United States; 14 Department of Dermatovenereology, Sestre Miilosrdnice University Hospital Center, School of Medicine, Catholic University of Croatia, Zagreb, Croatia; 15 Fachklinik Bad Bentheim, Bad Bentheim, Germany; 16 Rosenpark Research GmbH, Darmstadt, Germany; 17 Department of Dermatology, Westmead Hospital, NSW, Australia; 18 Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, NSW, Australia; 19 The School of Translational Medicine, Monash University, Melbourne, Australia; 20 Department of Dermatology, Alfred Health, Melbourne, Australia; 21 Department of Dermatology at Hospital Universitario Virgen de las Nieves, Granada, Spain; 22 University Hospital Sv.

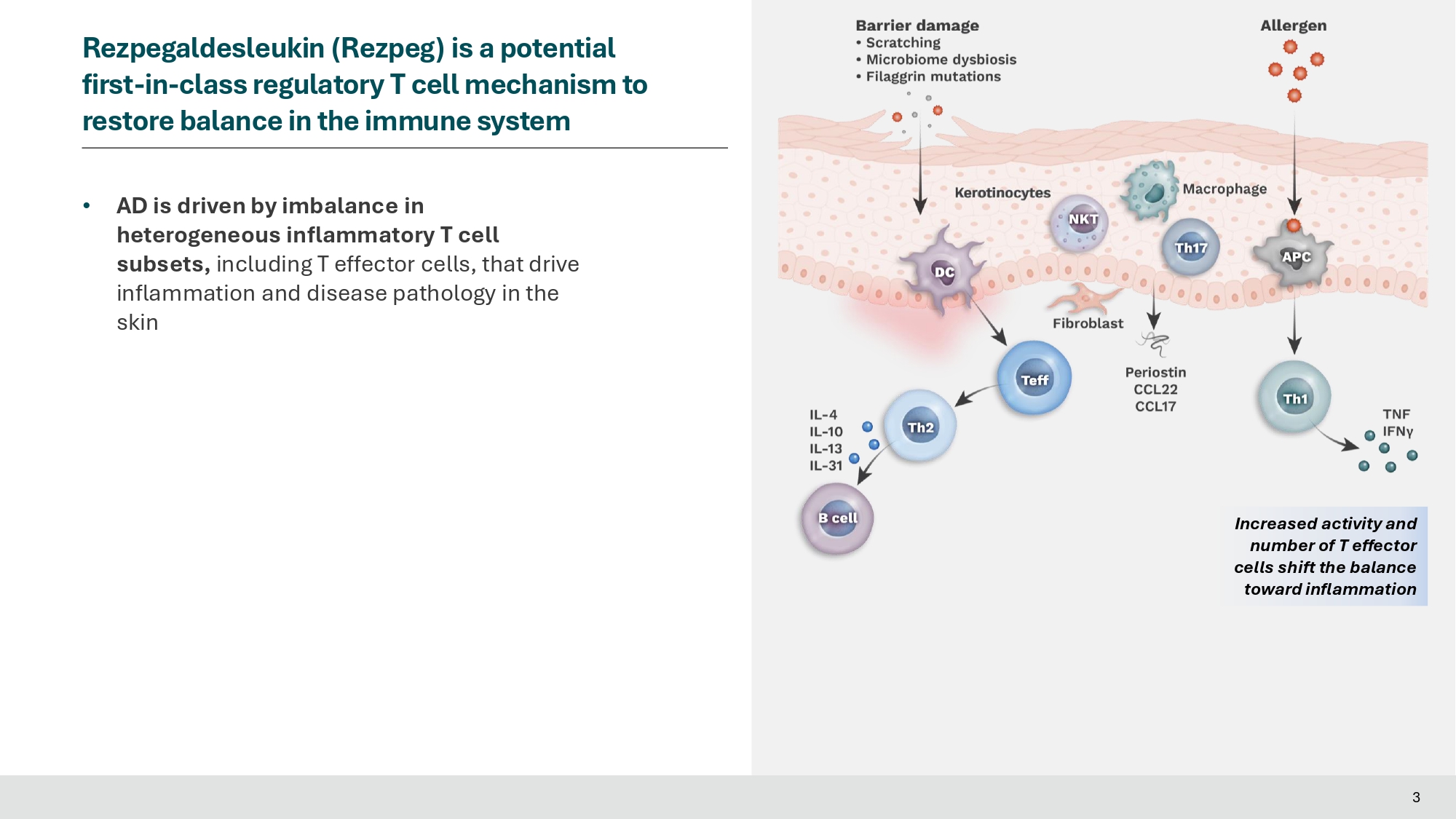
Ivan Rilski Medical Center, Sofia, Bulgaria; 23 Vida Clinical Research, Edmonton, Canada; 24 Nektar Therapeutics, San Francisco, CA, United States; 25 Indiana University School of Medicine, Indianapolis, IN, United States Presenter and Conflicts Jonathan Silverberg, MD, PhD, MPH • Professor of Dermatology at The George Washington University School of Medicine and Health Sciences • Director of Clinical Research and Contact Dermatitis Conflict of Interest Disclosures: Speaker, advisory board member, consultant and/or received institutional grants from Abbvie, Alamar, Aldena, Amgen, AObiome, Arcutis, Arena, Asana, Aslan, BioMX, Biosion, Bodewell, Boehringer - Ingelheim, Bristol Myers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, Corevitas, Dermavant, Dermtech, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, Leo Pharma, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi - Genzyme, Shaperon, TARGET - RWE, Union, UpToDate 2 • AD is driven by imbalance in heterogeneous inflammatory T cell subsets, including T effector cells, that drive inflammation and disease pathology in the skin 3 Rezpegaldesleukin (Rezpeg) is a potential first - in - class regulatory T cell mechanism to restore balance in the immune system Increased activity and number of T effector cells shift the balance toward inflammation
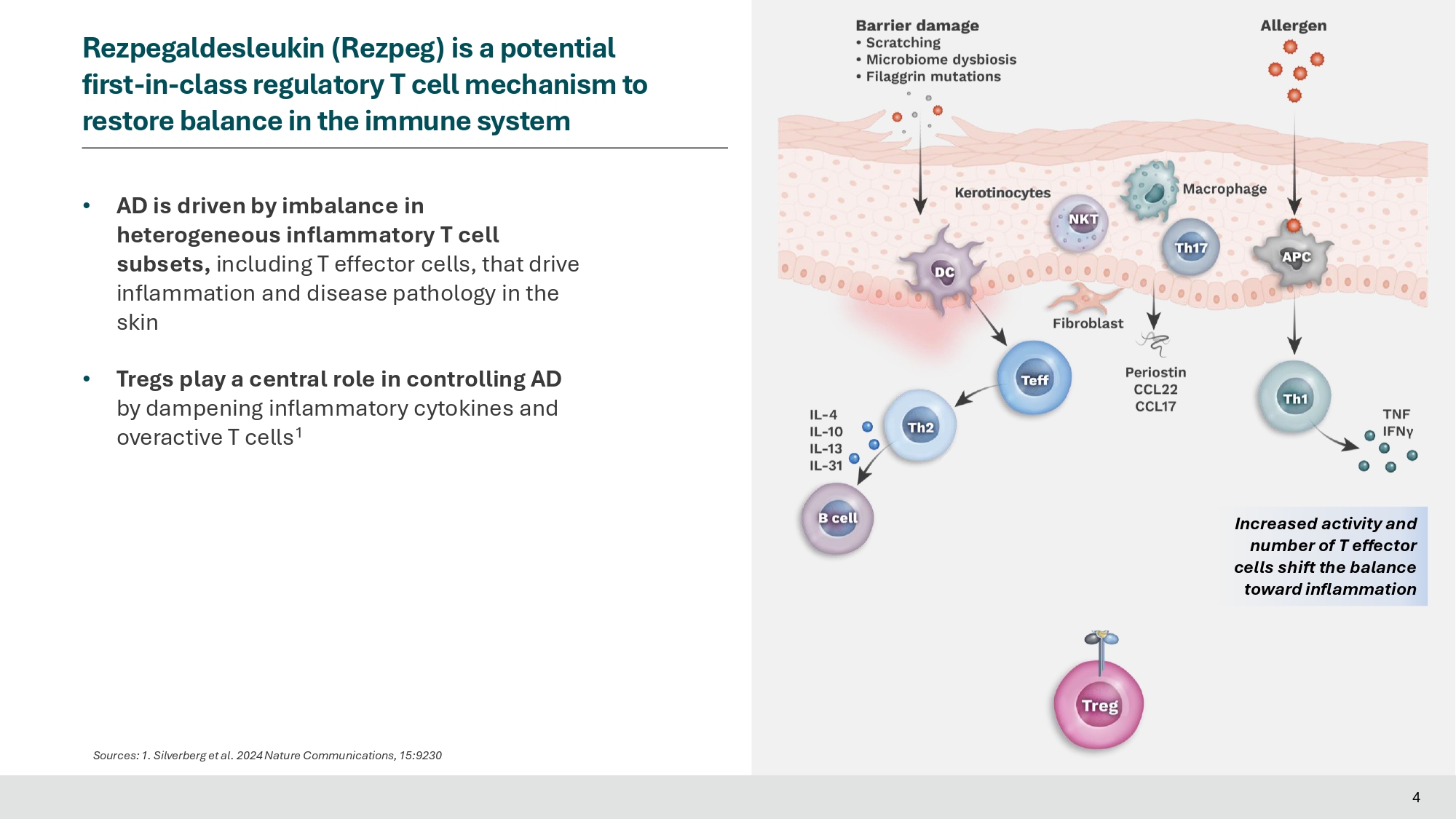
• AD is driven by imbalance in heterogeneous inflammatory T cell subsets, including T effector cells, that drive inflammation and disease pathology in the skin • Tregs play a central role in controlling AD by dampening inflammatory cytokines and overactive T cells 1 4 Rezpegaldesleukin (Rezpeg) is a potential first - in - class regulatory T cell mechanism to restore balance in the immune system Sources: 1. Silverberg et al. 2024 Nature Communications, 15:9230 Increased activity and number of T effector cells shift the balance toward inflammation Rezpegaldesleukin (Rezpeg) is a potential first - in - class regulatory T cell mechanism to restore balance in the immune system Sources: 1.
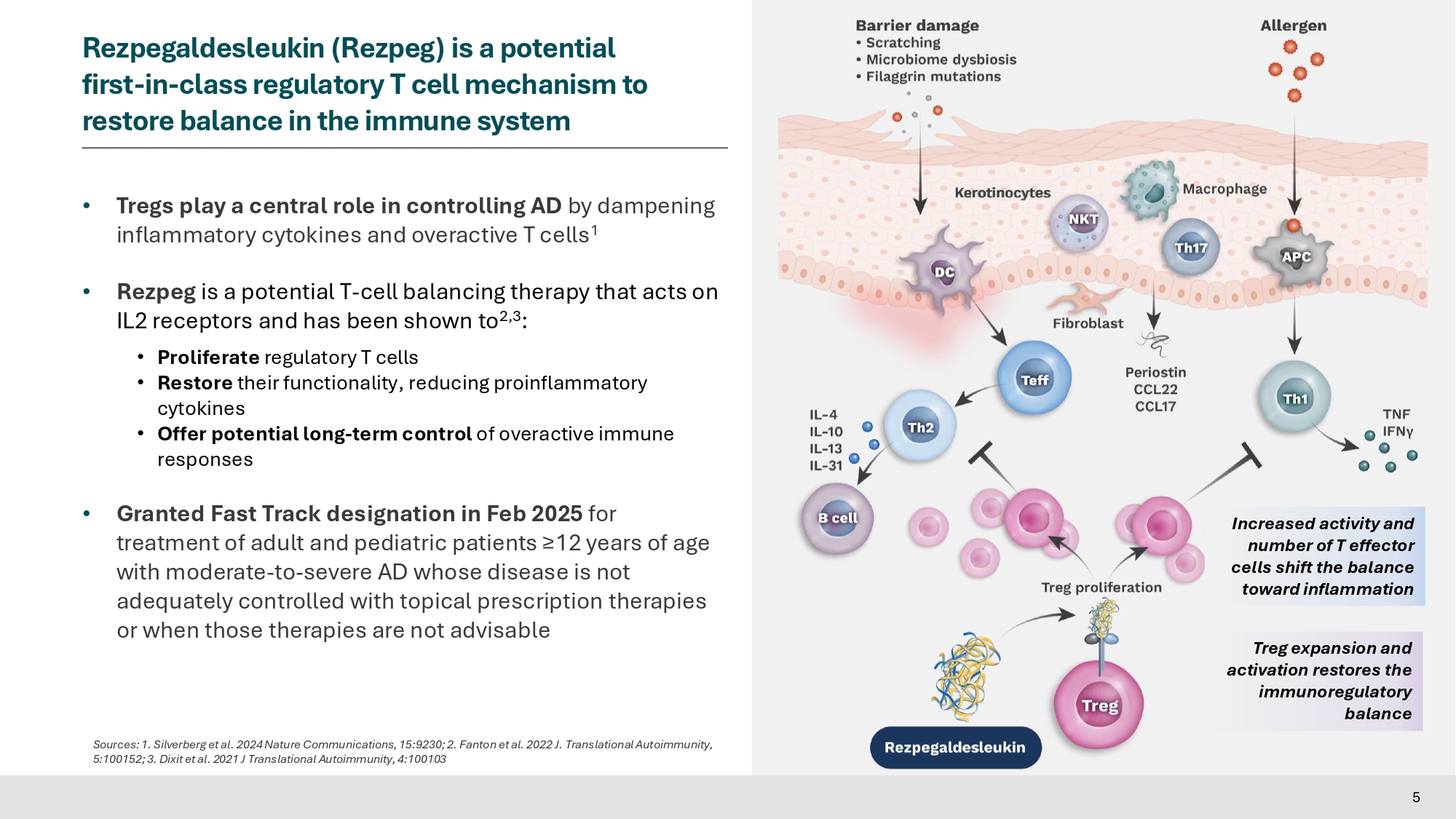
Silverberg et al. 2024 Nature Communications, 15:9230; 2. Fanton et al. 2022 J. Translational Autoimmunity, • Tregs play a central role in controlling AD by dampening inflammatory cytokines and overactive T cells 1 • Rezpeg is a potential T - cell balancing therapy that acts on IL2 receptors and has been shown to 2,3 : • Proliferate regulatory T cells • Restore their functionality, reducing proinflammatory cytokines • Offer potential long - term control of overactive immune responses • Granted Fast Track designation in Feb 2025 for treatment of adult and pediatric patients ≥12 years of age with moderate - to - severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable Treg expansion and activation restores the immunoregulatory balance Increased activity and number of T effector cells shift the balance toward inflammation 5:100152; 3. Dixit et al.
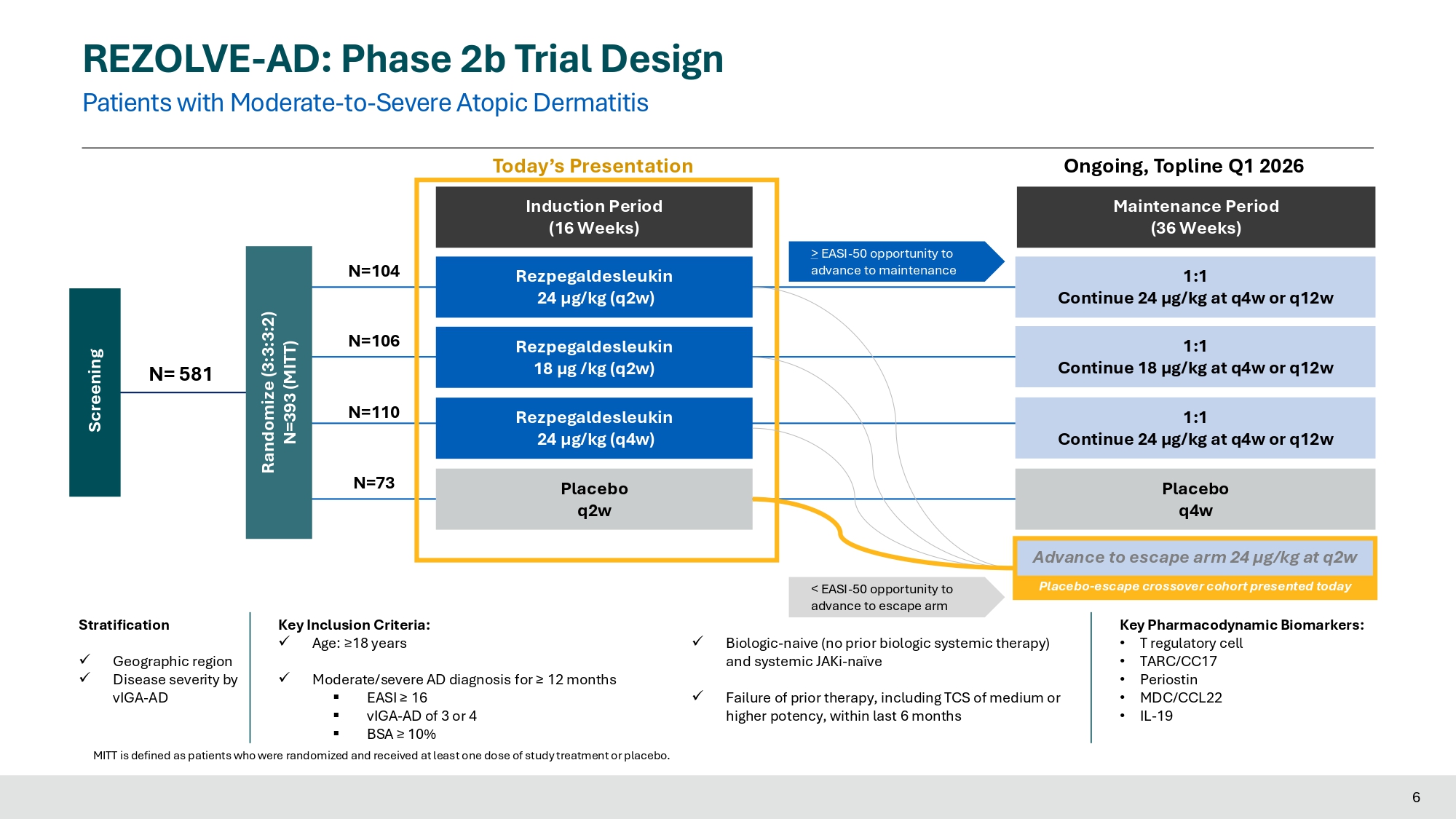
2021 J Translational Autoimmunity, 4:100103 5 REZOLVE - AD: Phase 2b Trial Design Patients with Moderate - to - Severe Atopic Dermatitis Induction Period (16 Weeks) Maintenance Period (36 Weeks) Key Inclusion Criteria: x Age: ≥18 years x Moderate/severe AD diagnosis for ≥ 12 months ▪ EASI ≥ 16 ▪ vIGA - AD of 3 or 4 ▪ BSA ≥ 10% x Biologic - naive (no prior biologic systemic therapy) and systemic JAKi - naïve x Failure of prior therapy, including TCS of medium or higher potency, within last 6 months Stratification x Geographic region x Disease severity by vIGA - AD Key Pharmacodynamic Biomarkers: • T regulatory cell • TARC/CC17 • Periostin • MDC/CCL22 • IL - 19 Randomize (3:3:3:2) N=393 (MITT) 1:1 Continue 24 µg/kg at q4w or q12w Rezpegaldesleukin 24 µg/kg (q2w) Rezpegaldesleukin 24 µg/kg (q4w) Placebo q4w Rezpegaldesleukin 18 µg /kg (q2w) Placebo q2w Screening N= 581 N=106 N=110 N=73 N=104 1:1 Continue 18 µg/kg at q4w or q12w 1:1 Continue 24 µg/kg at q4w or q12w > EASI - 50 opportunity to advance to maintenance Today’s Presentation Ongoing, Topline Q1 2026 MITT is defined as patients who were randomized and received at least one dose of study treatment or placebo.
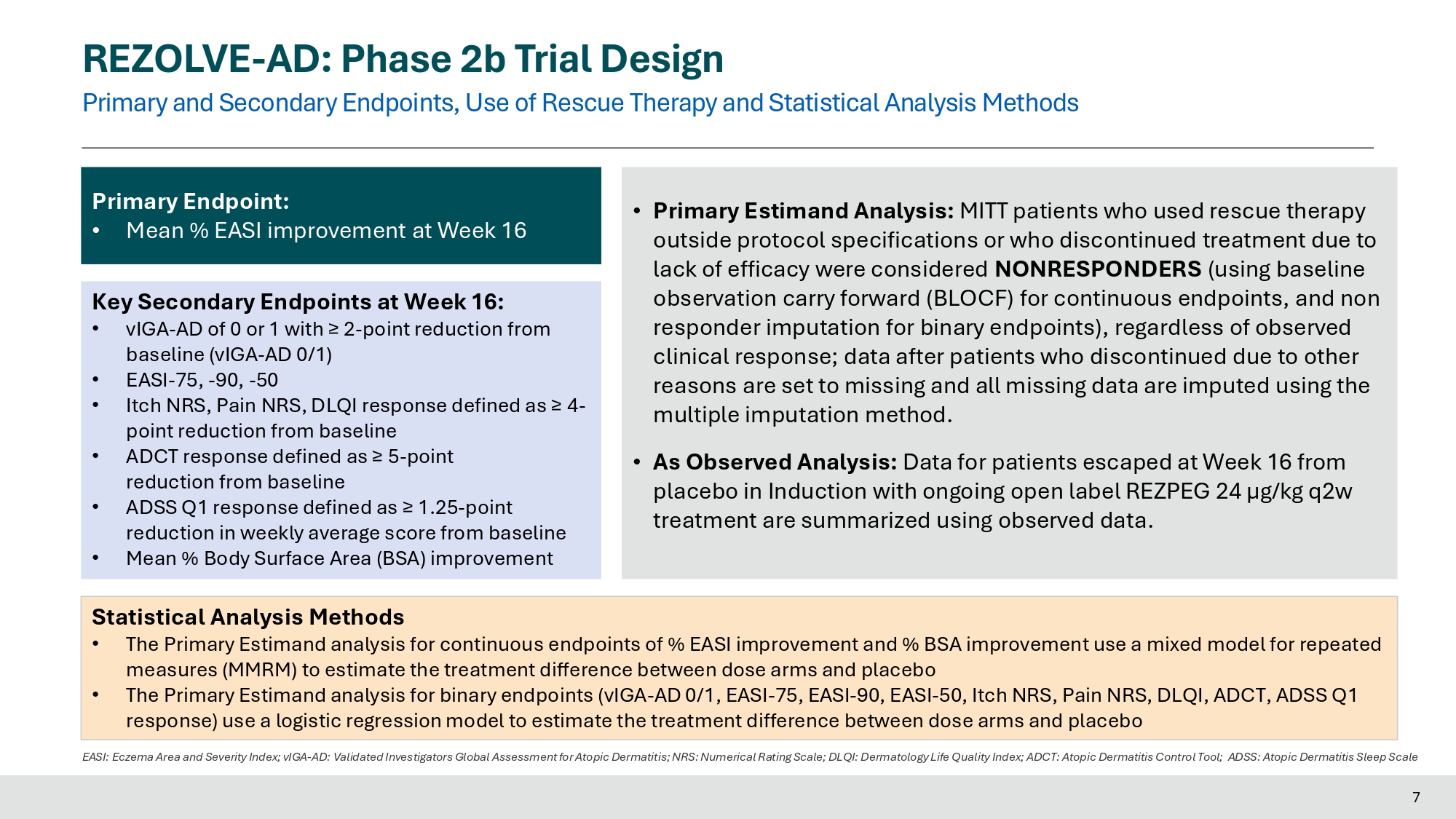
< EASI - 50 opportunity to advance to escape arm Advance to escape arm 24 µg/kg at q2w Placebo - escape crossover cohort presented today 6 REZOLVE - AD: Phase 2b Trial Design Primary and Secondary Endpoints, Use of Rescue Therapy and Statistical Analysis Methods 7 EASI: Eczema Area and Severity Index; vIGA - AD: Validated Investigators Global Assessment for Atopic Dermatitis; NRS: Numerical Rating Scale; DLQI: Dermatology Life Quality Index; ADCT: Atopic Dermatitis Control Tool; ADSS: Atopic Dermatitis Sleep Scale Primary Endpoint: • Mean % EASI improvement at Week 16 Key Secondary Endpoints at Week 16: • vIGA - AD of 0 or 1 with ≥ 2 - point reduction from baseline (vIGA - AD 0/1) • EASI - 75, - 90, - 50 • Itch NRS, Pain NRS, DLQI response defined as ≥ 4 - point reduction from baseline • ADCT response defined as ≥ 5 - point reduction from baseline • ADSS Q1 response defined as ≥ 1.25 - point reduction in weekly average score from baseline • Mean % Body Surface Area (BSA) improvement • Primary Estimand Analysis: MITT patients who used rescue therapy outside protocol specifications or who discontinued treatment due to lack of efficacy were considered NONRESPONDERS (using baseline observation carry forward (BLOCF) for continuous endpoints, and non responder imputation for binary endpoints), regardless of observed clinical response; data after patients who discontinued due to other reasons are set to missing and all missing data are imputed using the multiple imputation method. • As Observed Analysis : Data for patients escaped at Week 16 from placebo in Induction with ongoing open label REZPEG 24 µg/kg q 2 w treatment are summarized using observed data .
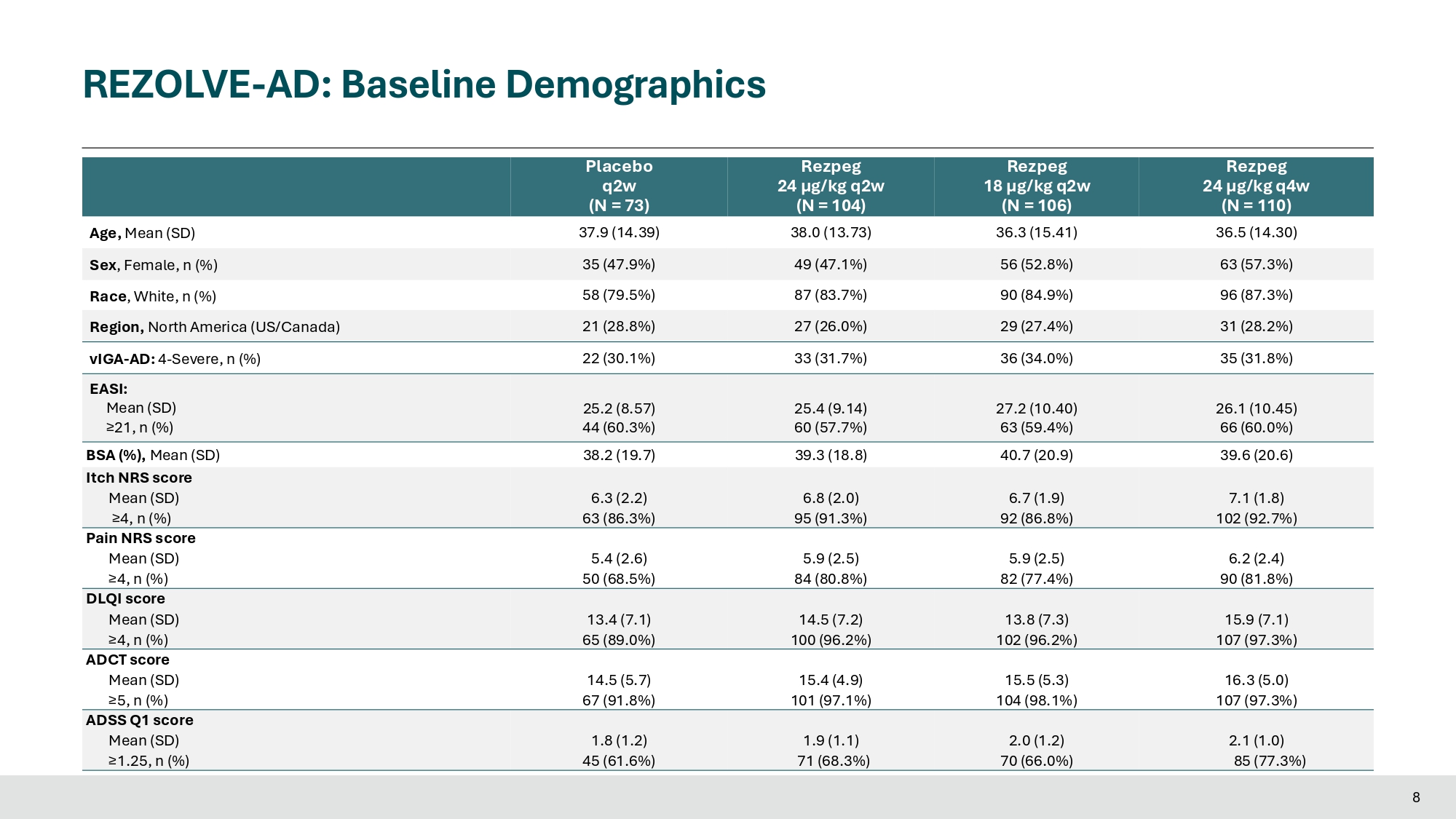
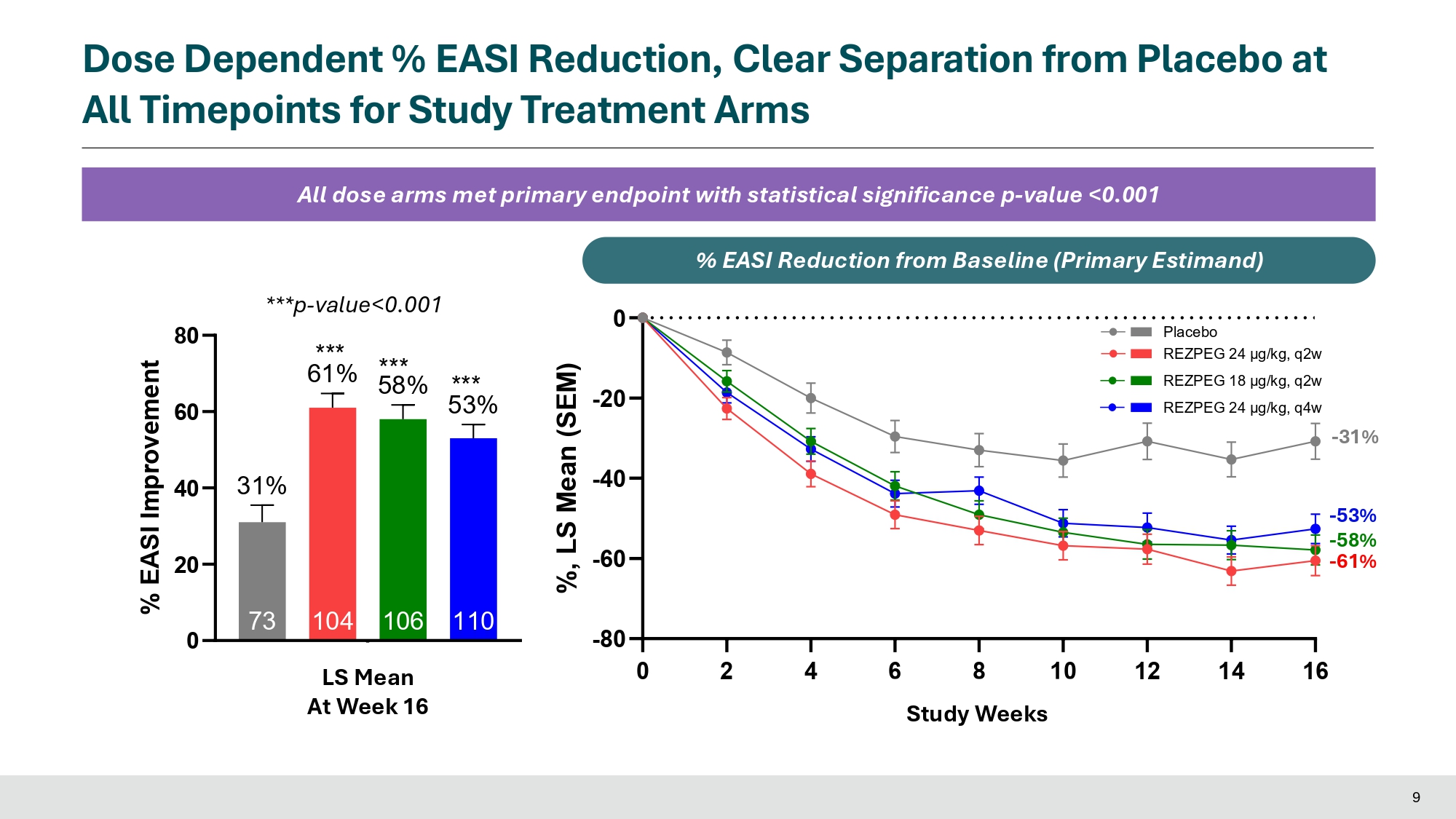
Statistical Analysis Methods • The Primary Estimand analysis for continuous endpoints of % EASI improvement and % BSA improvement use a mixed model for repeated measures (MMRM) to estimate the treatment difference between dose arms and placebo • The Primary Estimand analysis for binary endpoints (vIGA - AD 0/1, EASI - 75, EASI - 90, EASI - 50, Itch NRS, Pain NRS, DLQI, ADCT, ADSS Q1 response) use a logistic regression model to estimate the treatment difference between dose arms and placebo Rezpeg 24 µg/kg q4w (N = 110) Rezpeg (N = 106) Rezpeg (N = 104) Placebo (N = 73) 36.5 (14.30) 36.3 (15.41) 38.0 (13.73) 37.9 (14.39) Age, Mean (SD) 63 (57.3%) 56 (52.8%) 49 (47.1%) 35 (47.9%) Sex , Female, n (%) 96 (87.3%) 90 (84.9%) 87 (83.7%) 58 (79.5%) Race , White, n (%) 31 (28.2%) 29 (27.4%) 27 (26.0%) 21 (28.8%) Region, North America (US/Canada) 35 (31.8%) 36 (34.0%) 33 (31.7%) 22 (30.1%) vIGA - AD: 4 - Severe, n (%) EASI: 26.1 (10.45) 27.2 (10.40) 25.4 (9.14) 25.2 (8.57) Mean (SD) 66 (60.0%) 63 (59.4%) 60 (57.7%) 44 (60.3%) ≥21, n (%) 39.6 (20.6) 40.7 (20.9) 39.3 (18.8) 38.2 (19.7) BSA (%), Mean (SD) Itch NRS score 7.1 (1.8) 6.7 (1.9) 6.8 (2.0) 6.3 (2.2) Mean (SD) 102 (92.7%) 92 (86.8%) 95 (91.3%) 63 (86.3%) ≥4, n (%) Pain NRS score 6.2 (2.4) 5.9 (2.5) 5.9 (2.5) 5.4 (2.6) Mean (SD) 90 (81.8%) 82 (77.4%) 84 (80.8%) 50 (68.5%) ≥4, n (%) DLQI score 15.9 (7.1) 13.8 (7.3) 14.5 (7.2) 13.4 (7.1) Mean (SD) 107 (97.3%) 102 (96.2%) 100 (96.2%) 65 (89.0%) ≥4, n (%) ADCT score 16.3 (5.0) 15.5 (5.3) 15.4 (4.9) 14.5 (5.7) Mean (SD) 107 (97.3%) 104 (98.1%) 101 (97.1%) 67 (91.8%) ≥5, n (%) ADSS Q1 score 2.1 (1.0) 2.0 (1.2) 1.9 (1.1) 1.8 (1.2) Mean (SD) 85 (77.3%) 70 (66.0%) 71 (68.3%) 45 (61.6%) ≥1.25, n (%) 8 REZOLVE - AD: Baseline Demographics Dose Dependent % EASI Reduction, Clear Separation from Placebo at All Timepoints for Study Treatment Arms All dose arms met primary endpoint with statistical significance p - value <0.001 ***p - value<0.001 0 20 40 60 80 110 53% 106 104 73 31% Week 16 *** 61% % EASI Improvement *** 58% *** LS Mean At Week 16 0 2 4 6 8 10 12 14 16 - 80 - 60 - 40 - 20 0 Study Weeks %, LS Mean (SEM) - 53% - 58% - 61% - 31% Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w Study Weeks % E A % S I E R e A d S u c I t R i o e n d f r u o m c t B i o a s n e l F i n r e o ( m P ri m B a a r s y e E s l i t n i m e an d ) 9
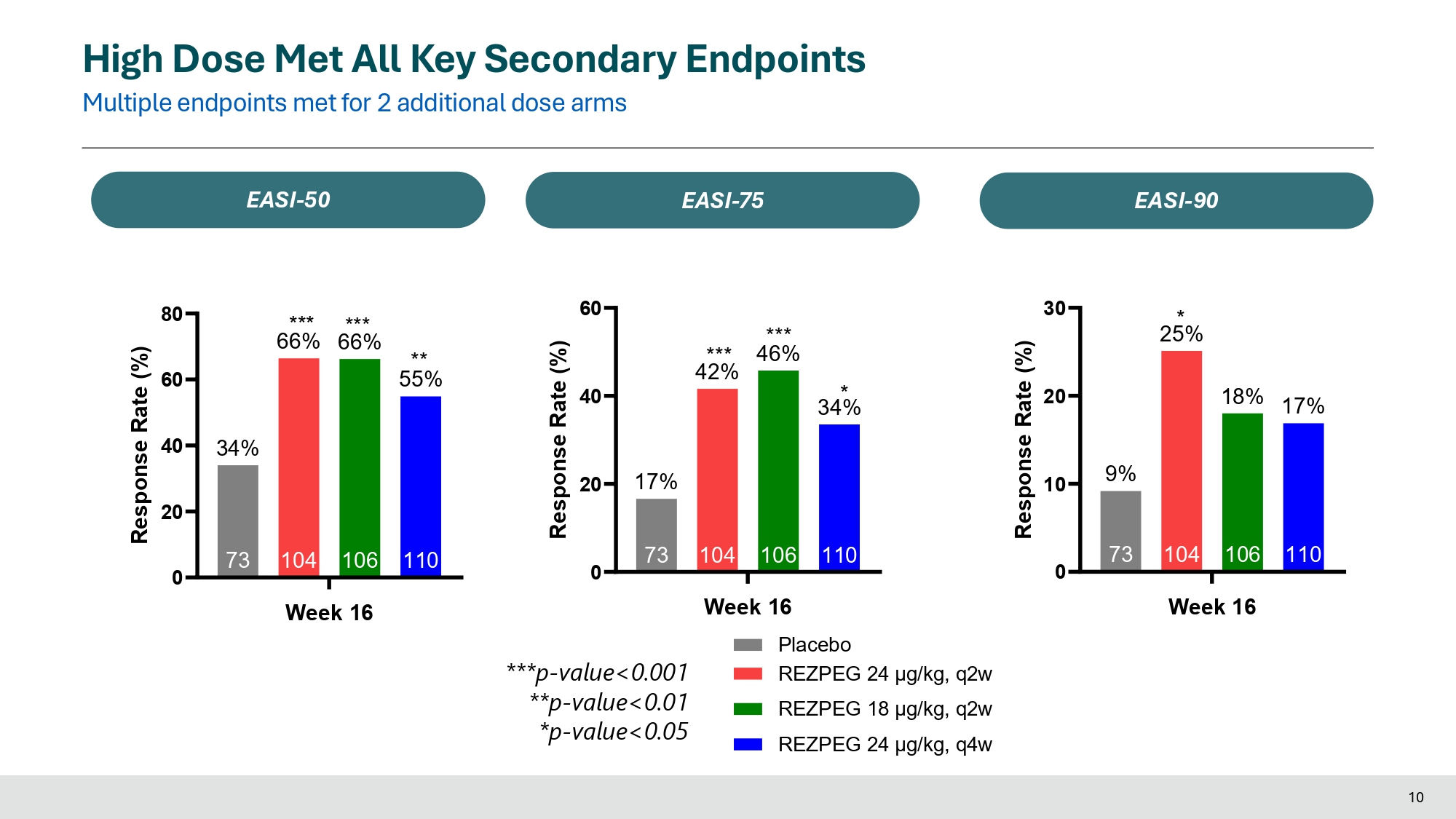
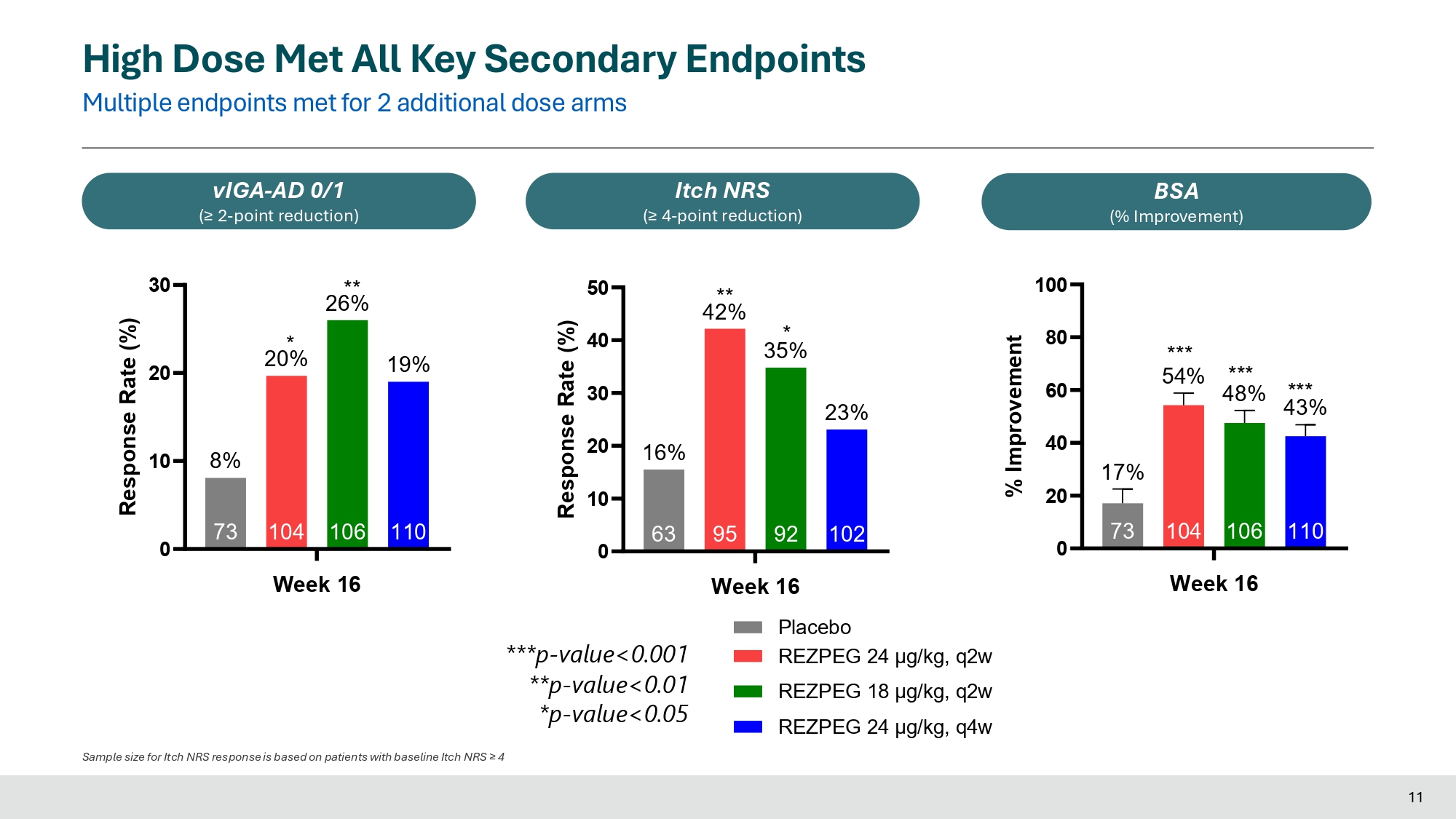
0 20 40 60 80 110 106 104 66% 66% 73 34% Week 16 Response Rate (%) *** *** ** 55% 0 20 40 60 110 34% 106 104 42% 73 17% Response Rate (%) *** *** 46% * 0 10 20 30 110 17% 106 18% 104 25% 73 9% Week 16 Response Rate (%) * High Dose Met All Key Secondary Endpoints Multiple endpoints met for 2 additional dose arms EASI - 50 EASI - 75 EASI - 90 ***p - value<0.001 **p - value<0.01 *p - value<0.05 Week 16 Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w 10 0 20 30 110 19% 106 26% 104 20% 73 10 8% Week 16 Response Rate (%) * ** 0 10 20 30 40 50 102 23% 92 35% 95 42% 63 16% Response Rate (%) ** * 0 20 40 60 80 100 110 43% 106 104 73 17% *** 54% *** 48% Week 16 % Improvement *** High Dose Met All Key Secondary Endpoints Multiple endpoints met for 2 additional dose arms vIGA - AD 0/1 (≥ 2 - point reduction) Itch NRS (≥ 4 - point reduction) BSA (% Improvement) ***p - value<0.001 **p - value<0.01 *p - value<0.05 Week 16 Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w Sample size for Itch NRS response is based on patients with baseline Itch NRS ≥ 4 11
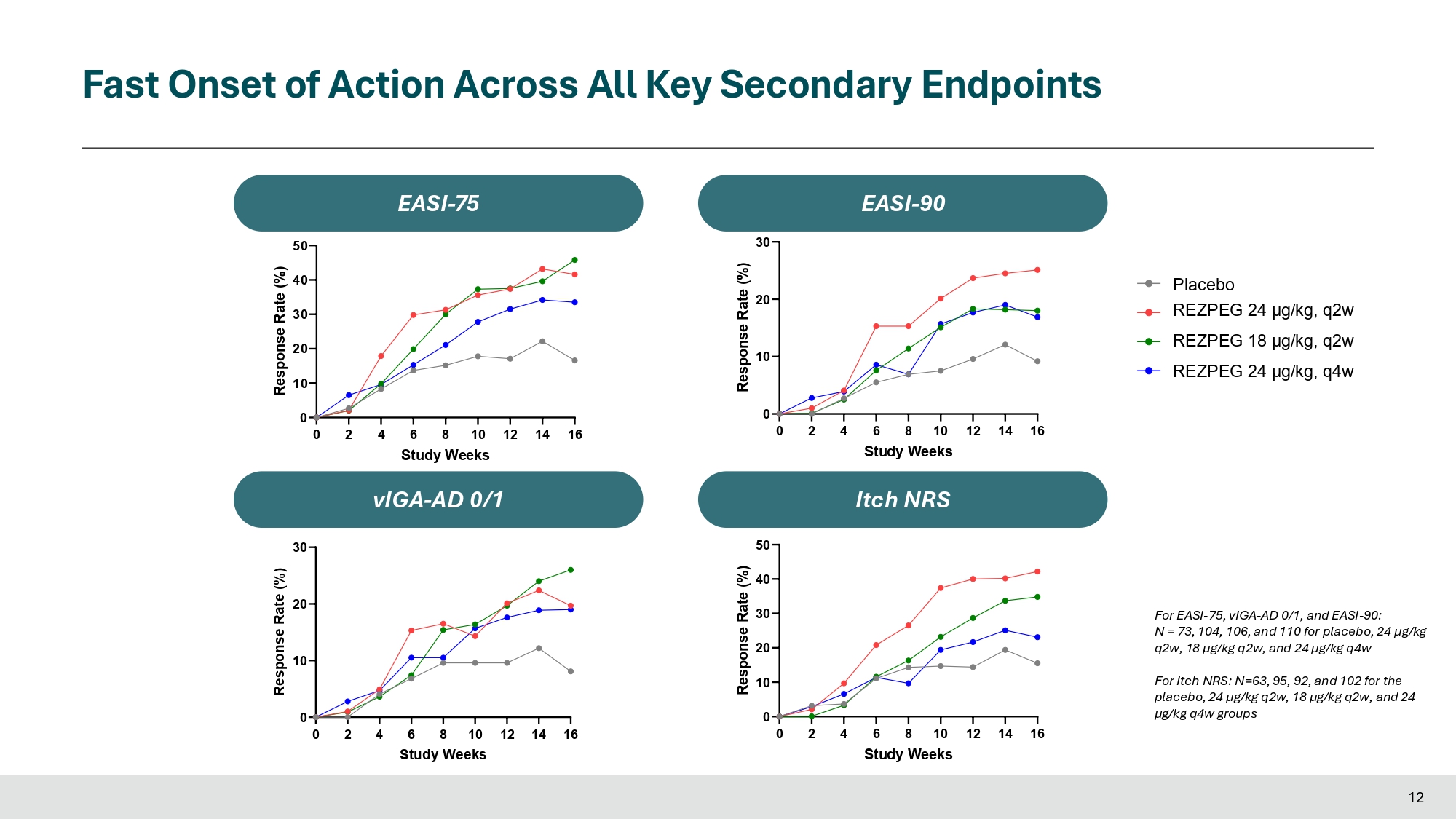

Fast Onset of Action Across All Key Secondary Endpoints 0 2 4 6 8 10 12 14 16 0 10 20 30 Study Weeks Response Rate (%) 0 2 4 6 8 10 12 14 16 Study Weeks 0 10 20 30 Response Rate (%) 0 2 4 6 8 10 12 14 16 Study Weeks 0 10 20 30 40 50 Itch NRS Response Rate (%) 0 2 4 6 8 10 12 14 16 Study Weeks 0 10 20 30 40 50 Response Rate (%) E A E A S S I - I - 7 7 5 5 E A E A S S I I - - 9 9 0 0 vIGA - AD 0/1 vIGA - AD 0/1 Itch NRS For EASI - 75, vIGA - AD 0/1, and EASI - 90: N = 73, 104, 106, and 110 for placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w 12 For Itch NRS: N=63, 95, 92, and 102 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w groups Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w High Dose Met All Key Patient - Reported Outcomes Multiple endpoints met in 2 additional dose arms 0 20 40 60 80 100 107 73% 102 64% 100 72% 65 54% Week 16 Response Rate (%) * * DLQI (≥ 4 - point reduction) 0 20 40 60 80 100 107 104 101 67 35% *** 67% ** ** 61% 61% Week 16 Response Rate (%) ADCT (≥ 5 - point reduction) 0 20 40 60 90 23% 82 35% 84 45% 50 22% Week 16 Response Rate (%) * Pain NRS (≥ 4 - point reduction) 0 20 40 60 80 85 46% 70 41% 71 45 30% ** 57% Week 16 Response Rate (%) ADSS Q1 (≥ 1.25 - point reduction) ***p - value<0.001 **p - value<0.01 *p - value<0.05 Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w Sample size for ADCT response is based on patients with baseline ADCT ≥ 5; Sample size for DLQI response is based on patients with baseline DLQI ≥ 4; Sample size for ADSS Q1 response is based on patients with baseline ADSS Q1 ≥ 1.25; Sample size for Pain NRS response is based on patients with baseline Pain NRS ≥ 4 13 Dose Dependent Increase in Tregs and Reduction in Th2 Inflammation 14 Patients with baseline values >ULN included in the analysis IL - 19 TARC/CCL17 Periostin MDC/CCL22 - 60 - 40 - 20 0 30 20 % Difference from Baseline to Week 16 Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w 0 2 0 2 4 6 8 4 6 12 14 16 Study Weeks Fold change from basline, %CD25 bright Tregs (mean + SEM) Up to 6 - fold increase in T - reg consistent with prior studies of REZPEG.
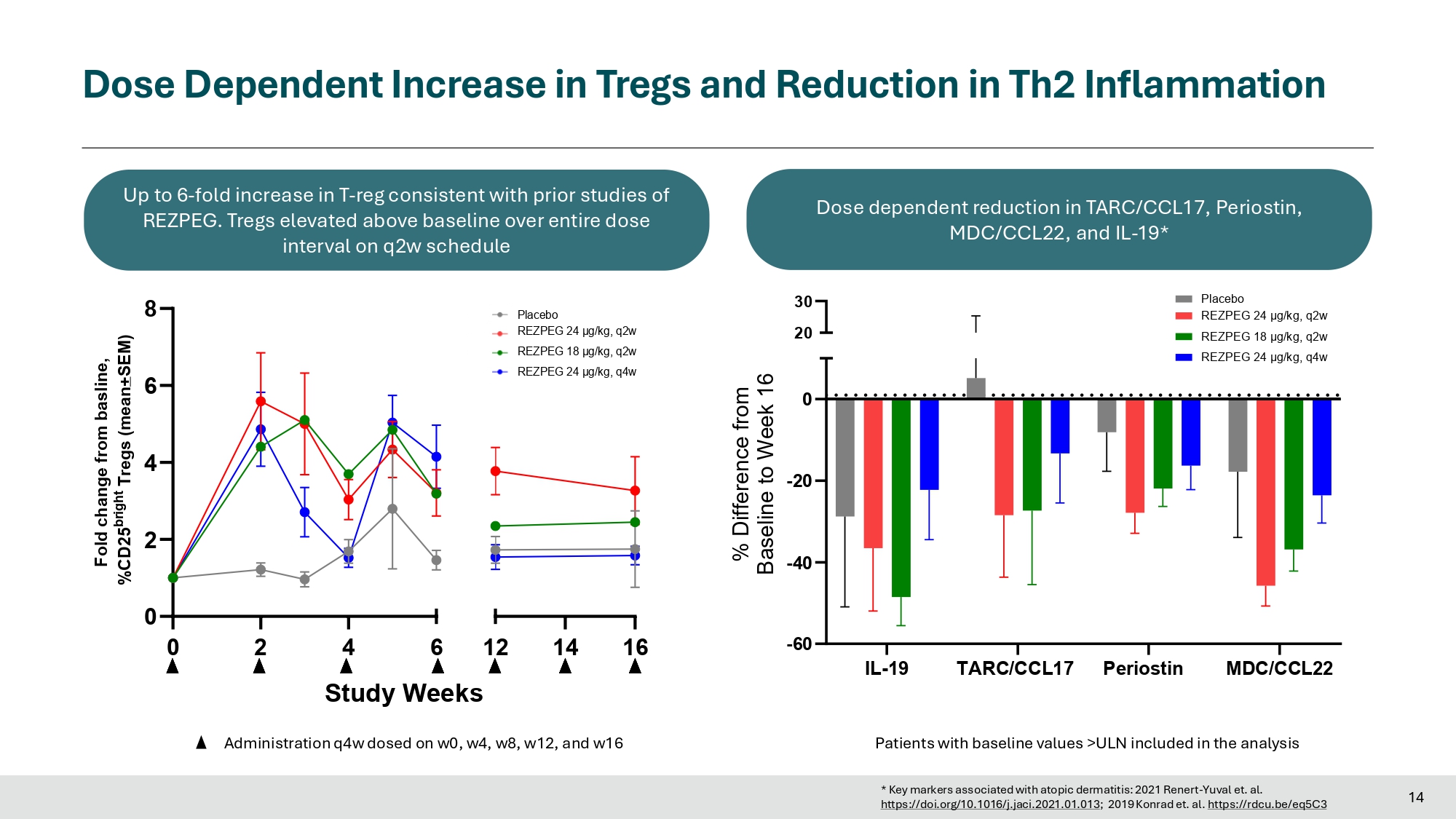
Tregs elevated above baseline over entire dose interval on q2w schedule Administration q4w dosed on w0, w4, w8, w12, and w16 Dose dependent reduction in TARC/CCL17, Periostin, MDC/CCL22, and IL - 19* Placebo REZPEG 24 μg/kg, q2w REZPEG 18 μg/kg, q2w REZPEG 24 μg/kg, q4w https://doi.org/10.1016/j.jaci.2021.01.013 ; 2019 Konrad et. al. https://rdcu.be/eq5C3 * Key markers associated with atopic dermatitis: 2021 Renert - Yuval et. al.
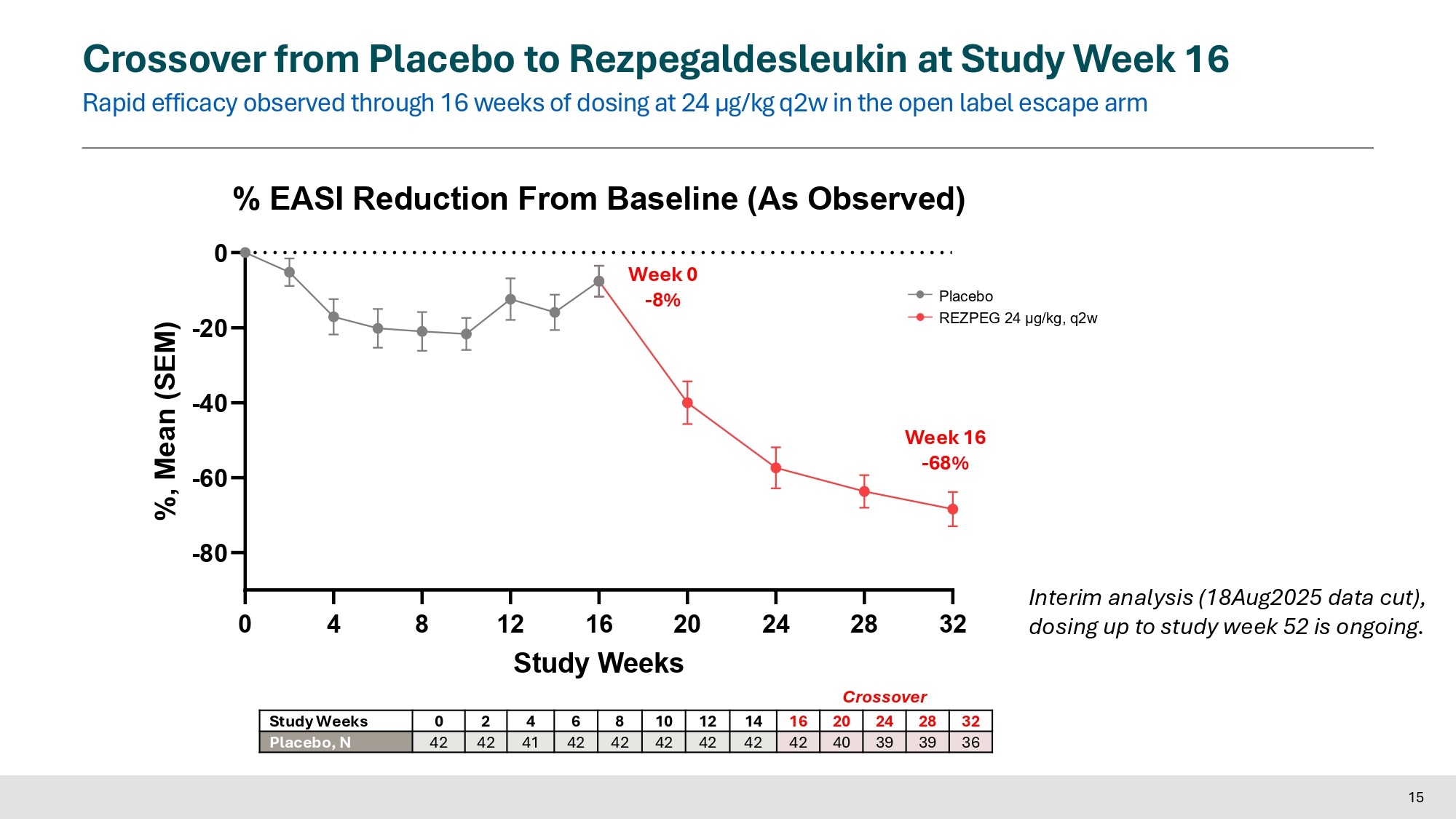
Crossover from Placebo to Rezpegaldesleukin at Study Week 16 Rapid efficacy observed through 16 weeks of dosing at 24 µg/kg q2w in the open label escape arm Placebo REZPEG 24 μg/kg, q2w 0 15 4 8 24 28 32 - 80 - 60 - 40 - 20 % EASI Reduction From Baseline (As Observed) 0 12 16 20 Study Weeks %, Mean (SEM) Week 16 - 68% Week 0 - 8% 32 28 24 20 16 14 12 10 8 6 4 2 0 Study Weeks 36 39 39 40 42 42 42 42 42 42 41 42 42 Placebo, N Interim analysis (18Aug2025 data cut), dosing up to study week 52 is ongoing.
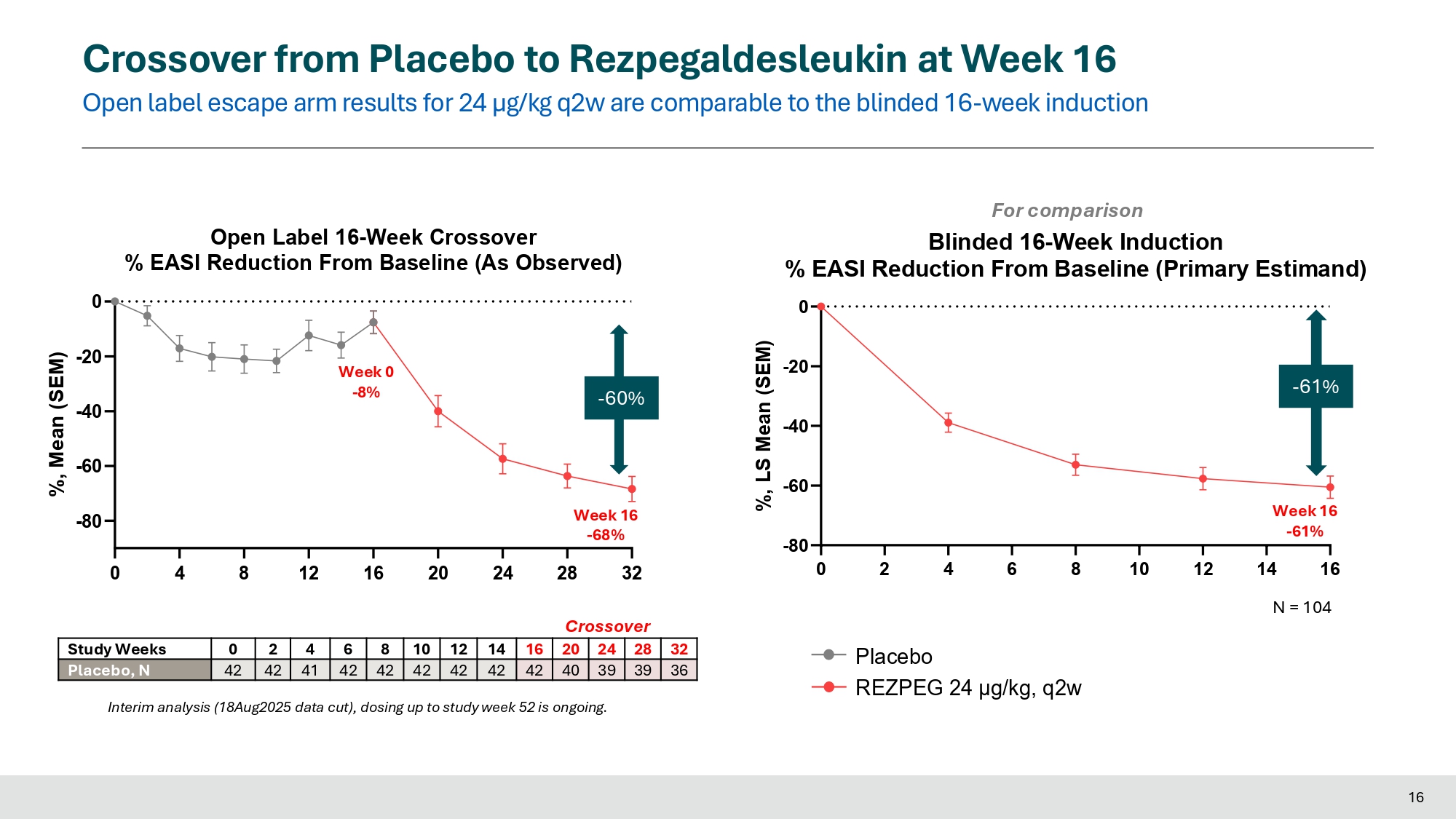
Crossover Crossover from Placebo to Rezpegaldesleukin at Week 16 Open label escape arm results for 24 µg/kg q2w are comparable to the blinded 16 - week induction Placebo REZPEG 24 μg/kg, q2w 0 4 8 12 16 20 24 - 80 - 60 - 40 - 20 0 Open Label 16 - Week Crossover % EASI Reduction From Baseline (As Observed) %, Mean (SEM) 32 28 24 20 16 14 12 10 8 6 4 2 0 Study Weeks 36 39 39 40 42 42 42 42 42 42 41 42 42 Placebo, N Interim analysis (18Aug2025 data cut), dosing up to study week 52 is ongoing.
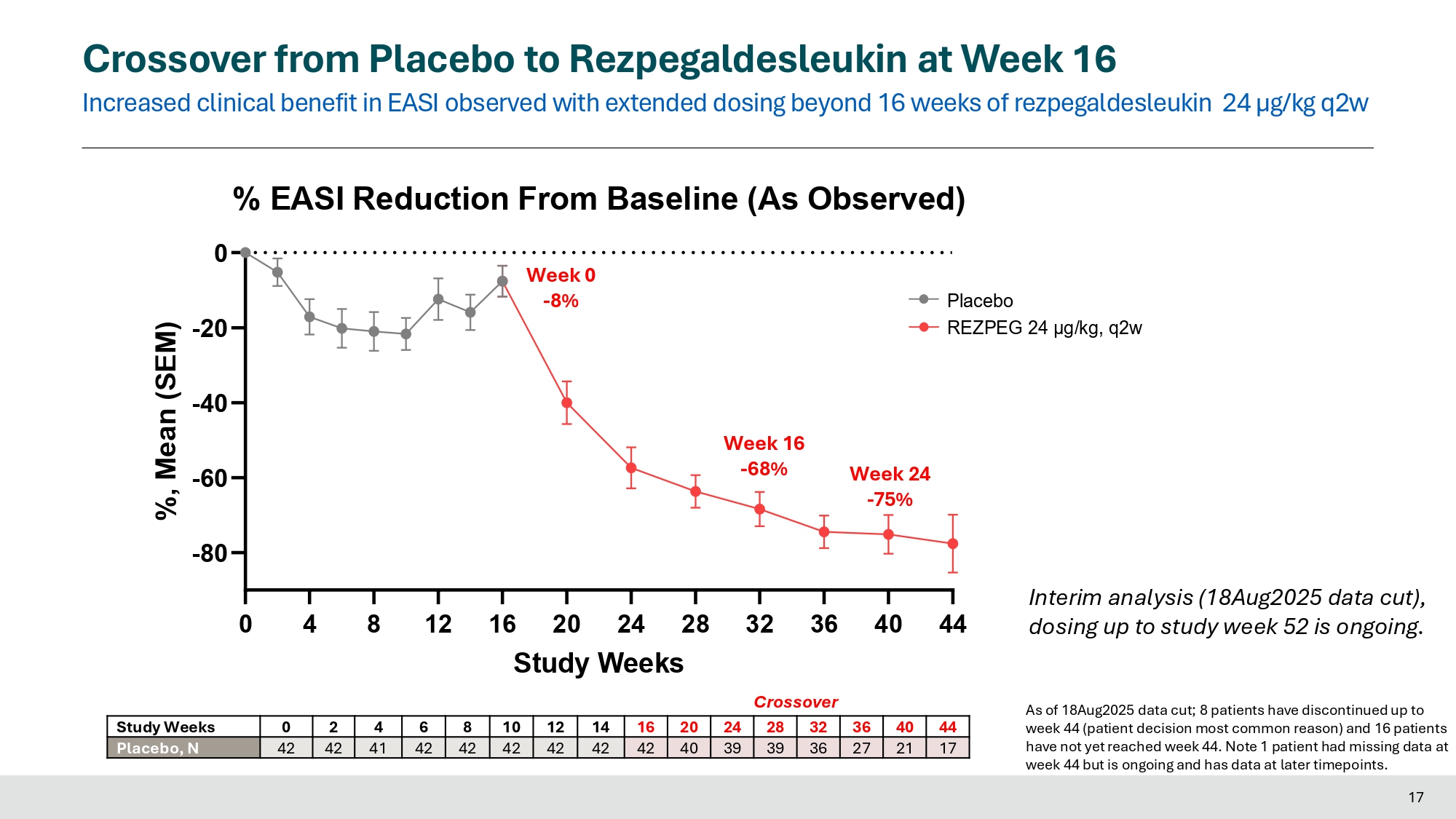
Week 0 - 8% 0 2 4 6 8 10 12 - 80 - 60 - 40 - 20 0 For comparison Blinded 16 - Week Induction % EASI Reduction From Baseline (Primary Estimand) %, LS Mean (SEM) - 60% 16 Week 16 - 68% 28 32 Crossover - 61% Week 16 - 61% 14 16 N = 104 Crossover from Placebo to Rezpegaldesleukin at Week 16 Increased clinical benefit in EASI observed with extended dosing beyond 16 weeks of rezpegaldesleukin 24 µg/kg q2w Placebo REZPEG 24 μg/kg, q2w 0 4 8 12 16 20 24 28 32 36 40 44 17 - 80 - 60 - 40 - 20 % EASI Reduction From Baseline (As Observed) 0 Study Weeks %, Mean (SEM) Week 16 - 68% Week 0 - 8% Week 24 - 75% 44 40 36 32 28 24 20 16 14 12 10 8 6 4 2 0 Study Weeks 17 21 27 36 39 39 40 42 42 42 42 42 42 41 42 42 Placebo, N As of 18Aug2025 data cut; 8 patients have discontinued up to week 44 (patient decision most common reason) and 16 patients have not yet reached week 44. Note 1 patient had missing data at week 44 but is ongoing and has data at later timepoints. Interim analysis (18Aug2025 data cut), dosing up to study week 52 is ongoing.
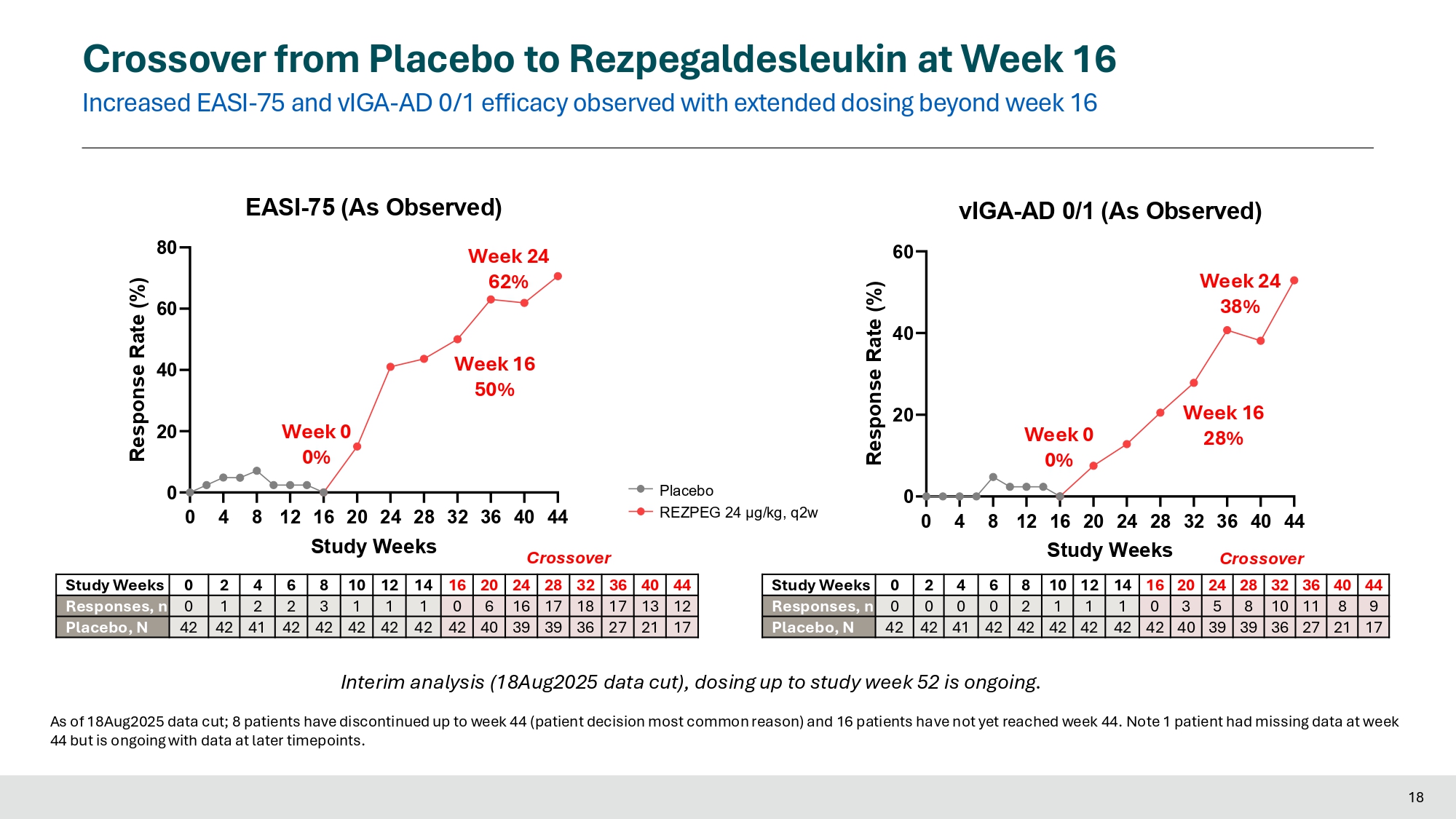
Crossover Crossover from Placebo to Rezpegaldesleukin at Week 16 Increased EASI - 75 and vIGA - AD 0/1 efficacy observed with extended dosing beyond week 16 44 40 36 32 28 24 20 16 14 12 10 8 6 4 2 0 Study Weeks 12 13 17 18 17 16 6 0 1 1 1 3 2 2 1 0 Responses, n 17 21 27 36 39 39 40 42 42 42 42 42 42 41 42 42 Placebo, N 44 40 36 32 28 24 20 16 14 12 10 8 6 4 2 0 Study Weeks 9 8 11 10 8 5 3 0 1 1 1 2 0 0 0 0 Responses, n 17 21 27 36 39 39 40 42 42 42 42 42 42 41 42 42 Placebo, N 0 20 40 60 80 Response Rate (%) Week 16 50% Week 0 0% EASI - 75 (As Observed) Week 24 62% 0 4 8 12 16 20 24 28 32 36 40 44 Study Weeks Crossover 0 4 8 12 16 20 24 28 32 36 40 44 0 20 40 60 vIGA - AD 0/1 (As Observed) Study Weeks Response Rate (%) Week 16 28% Week 0 0% Week 24 38% Interim analysis (18Aug2025 data cut), dosing up to study week 52 is ongoing. As of 18Aug2025 data cut; 8 patients have discontinued up to week 44 (patient decision most common reason) and 16 patients have not yet reached week 44. Note 1 patient had missing data at week 44 but is ongoing with data at later timepoints.
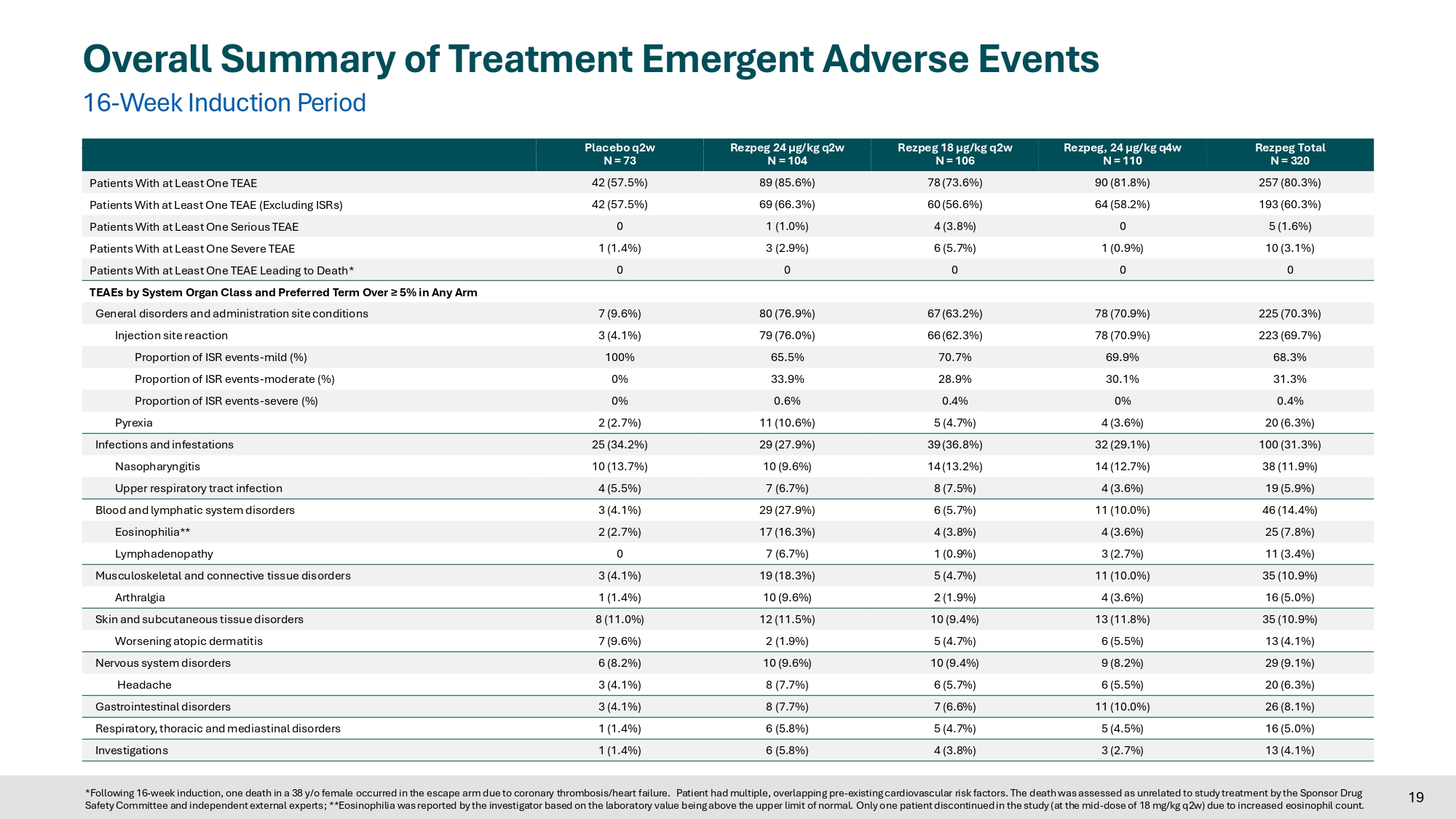
Placebo REZPEG 24 μg/kg, q2w Crossover 18 Rezpeg Total N = 320 Rezpeg, 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 257 (80.3%) 90 (81.8%) 78 (73.6%) 89 (85.6%) 42 (57.5%) Patients With at Least One TEAE 193 (60.3%) 64 (58.2%) 60 (56.6%) 69 (66.3%) 42 (57.5%) Patients With at Least One TEAE (Excluding ISRs) 5 (1.6%) 0 4 (3.8%) 1 (1.0%) 0 Patients With at Least One Serious TEAE 10 (3.1%) 1 (0.9%) 6 (5.7%) 3 (2.9%) 1 (1.4%) Patients With at Least One Severe TEAE 0 0 0 0 0 Patients With at Least One TEAE Leading to Death* TEAEs by System Organ Class and Preferred Term Over ≥ 5% in Any Arm 225 (70.3%) 78 (70.9%) 67 (63.2%) 80 (76.9%) 7 (9.6%) General disorders and administration site conditions 223 (69.7%) 78 (70.9%) 66 (62.3%) 79 (76.0%) 3 (4.1%) Injection site reaction 68.3% 69.9% 70.7% 65.5% 100% Proportion of ISR events - mild (%) 31.3% 30.1% 28.9% 33.9% 0% Proportion of ISR events - moderate (%) 0.4% 0% 0.4% 0.6% 0% Proportion of ISR events - severe (%) 20 (6.3%) 4 (3.6%) 5 (4.7%) 11 (10.6%) 2 (2.7%) Pyrexia 100 (31.3%) 32 (29.1%) 39 (36.8%) 29 (27.9%) 25 (34.2%) Infections and infestations 38 (11.9%) 14 (12.7%) 14 (13.2%) 10 (9.6%) 10 (13.7%) Nasopharyngitis 19 (5.9%) 4 (3.6%) 8 (7.5%) 7 (6.7%) 4 (5.5%) Upper respiratory tract infection 46 (14.4%) 11 (10.0%) 6 (5.7%) 29 (27.9%) 3 (4.1%) Blood and lymphatic system disorders 25 (7.8%) 4 (3.6%) 4 (3.8%) 17 (16.3%) 2 (2.7%) Eosinophilia** 11 (3.4%) 3 (2.7%) 1 (0.9%) 7 (6.7%) 0 Lymphadenopathy 35 (10.9%) 11 (10.0%) 5 (4.7%) 19 (18.3%) 3 (4.1%) Musculoskeletal and connective tissue disorders 16 (5.0%) 4 (3.6%) 2 (1.9%) 10 (9.6%) 1 (1.4%) Arthralgia 35 (10.9%) 13 (11.8%) 10 (9.4%) 12 (11.5%) 8 (11.0%) Skin and subcutaneous tissue disorders 13 (4.1%) 6 (5.5%) 5 (4.7%) 2 (1.9%) 7 (9.6%) Worsening atopic dermatitis 29 (9.1%) 9 (8.2%) 10 (9.4%) 10 (9.6%) 6 (8.2%) Nervous system disorders 20 (6.3%) 6 (5.5%) 6 (5.7%) 8 (7.7%) 3 (4.1%) Headache 26 (8.1%) 11 (10.0%) 7 (6.6%) 8 (7.7%) 3 (4.1%) Gastrointestinal disorders 16 (5.0%) 5 (4.5%) 5 (4.7%) 6 (5.8%) 1 (1.4%) Respiratory, thoracic and mediastinal disorders 13 (4.1%) 3 (2.7%) 4 (3.8%) 6 (5.8%) 1 (1.4%) Investigations Overall Summary of Treatment Emergent Adverse Events 16 - Week Induction Period 19 *Following 16 - week induction, one death in a 38 y/o female occurred in the escape arm due to coronary thrombosis/heart failure. Patient had multiple, overlapping pre - existing cardiovascular risk factors. The death was assessed as unrelated to study treatment by the Sponsor Drug Safety Committee and independent external experts; **Eosinophilia was reported by the investigator based on the laboratory value being above the upper limit of normal. Only one patient discontinued in the study (at the mid - dose of 18 mg/kg q2w) due to increased eosinophil count.
Novel Mechanism of Action with Differentiated Safety Profile 16 - Week Induction Period No observed safety signal for: □ Conjunctivitis □ Facial swelling or erythema □ Oral (aphthous) ulcers □ Asthma □ Myocardial infarction □ Pulmonary embolus (PE) □ Deep venous thrombosis (DVT) □ Malignancy □ Depression / suicidality No increased risk of conjunctivitis, oral ulcers, asthma, infections or MACE 20 Summary 21 • High dose rezpegaldesleukin demonstrated significant improvement over placebo during the 16 - Week induction in: • Primary: EASI LS Mean Percent Change (p<0.001) • Key Secondary: EASI - 75 (p<0.001), vIGA - AD 0/1 (p<0.05), Itch NRS (p<0.01), EASI - 90 (p<0.05), BSA (p<0.001) • Additional PROs: ADCT response (p<0.001), DLQI response (p<0.05), ADSS Q1 response (p<0.01), Pain NRS response (p<0.05) • Other dose levels demonstrated significant improvement in multiple endpoints • Substantial improvement in primary and key secondary endpoints with 24 - weeks of open label escape therapy, as compared to 16 - weeks • Safety consistent with previously - reported safety profile with no new safety concerns in study treatment arms • No increased risk of conjunctivitis, oral ulcers, or infections, including oral herpes • Most frequent AEs were mild injection site reactions (ISRs) that were self - resolving (<1% discontinuations due to ISRs)

Conclusions 22 • First large study to validate the Treg MOA and therapeutic potential of rezpegaldesleukin, an IL - 2 agonist, in moderate - to - severe atopic dermatitis • Upcoming data readouts from this ongoing AD study: • Maintenance data (comparing q4w vs. q12w regimens) is expected 1Q2026 • 1 - year off - treatment data is expected 1Q2027 • Additional data readouts from the rezpegaldesleukin clinical program: • Phase 2b 36 - week treatment data in severe alopecia areata expected in December 2025 • Next steps: Phase 3 planning for moderate to severe atopic dermatitis is underway Acknowledgements 23 • Thank you to: • The patients and their families • The investigators and their study teams • This study was sponsored by Nektar Therapeutics • Copies of these slides may be obtained through Quick Response (QR) code for personal use only and may not be reproduced without written permission of the authors

Exhibit 99.2

Nektar Presents New Data from REZOLVE-AD Phase 2b Study for Rezpegaldesleukin in
Late-Breaker Oral Presentation at EADV 2025
Study met primary and key secondary endpoints at week 16 in patients with moderate-to-severe atopic dermatitis
High dose rezpegaldesleukin achieved statistical significance on multiple patient-reported outcome assessments at completion of 16-week induction period
Interim data presented for patients who received placebo during induction period and crossed over to receive 24 weeks of treatment with high dose rezpegaldesleukin show deepening of EASI-75 response to 62% and deepening of vIGA-AD 0/1 response to 38%
SAN FRANCISCO, Sept. 18, 2025 /PRNewswire/ -- Nektar Therapeutics (Nasdaq: NKTR) today announced new data from the ongoing REZOLVE-AD Phase 2b study of rezpegaldesleukin, an IL-2 pathway agonist and regulatory T-cell (Treg) proliferator, at the 2025 European Academy of Dermatology and Venereology (EADV) Congress in Paris, France. These data were presented by Dr. Jonathan Silverberg, Professor of Dermatology at the George Washington University School of Medicine and Health Sciences and Director of Clinical Research and Contact Dermatitis in a late-breaking oral presentation.
In the Phase 2b study, rezpegaldesleukin achieved statistical significance on the primary endpoint of mean improvement in Eczema Area and Severity Index (EASI) at week 16 over baseline for all rezpegaldesleukin arms versus placebo. Statistical significance at week 16 was also achieved for key secondary endpoints measuring disease reduction in patients with moderate to severe atopic dermatitis, including EASI-75, EASI-90, Itch Numerical Rating Scale (NRS), Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) and Body Surface Area (BSA).
“These data from REZOLVE-AD presented today show a rapid onset of treatment effect for both clinician-assessed and patient-reported outcomes following the first few doses of rezpegaldesleukin,” Prof. Jonathan Silverberg, MD, PhD, MPH. “In addition, for the first time, we observe a deepening of clinical effect for patients with extended dosing of investigational therapy beyond 16 weeks, with a strengthening of absolute EASI reduction, along with higher EASI-75 and vIGA 0/1 response rates following 24 weeks of treatment. These results build on the body of data generated to-date for rezpegaldesleukin that show the advantage of this novel, broad-based Treg mechanism over other novel mechanisms in development to treat atopic dermatitis.”
Highlights of the REZOLVE-AD Phase 2b Study:
Week 16 Efficacy
|
24 µg/kg q2w (high dose) |
18 µg/kg q2w (middle dose) |
24 µg/kg q4w (low dose) |
Placebo | |
| Primary Endpoint | N=104 | N=106 | N=110 | N=73 |
| Mean improvement in EASI score from baseline |
61% p<0.001 |
58% p<0.001 |
53% p<0.001 |
31 % |
| Key Secondary Endpoints |
N=73 | N=104 | N=106 | N=110 |
| EASI-75 |
42% p<0.001 |
46% p<0.001 |
34% p<0.05 |
17 % |
| vIGA-AD 0/1 |
20% p<0.05 |
26% p<0.01 |
19% ns |
8 % |
| EASI-90 |
25% p<0.05 |
18% ns |
17% ns |
9 % |
|
Itch NRS* (> 4-point reduction) |
42% p<0.01 |
35% p<0.05 |
23% ns |
16 % |
| Mean improvement in BSA score from baseline |
54% p<0.001 |
48% p<0.001 |
43% p<0.001 |
17 % |
| EASI-50 |
66% p<0.001 |
66% p<0.001 |
55% p<0.01 |
34 % |
| * | N=63, 95, 92, and 102 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w arms; ns=not significant. |
Key Patient-Reported Outcome Assessments
|
Endpoint |
24 µg/kg q2w (high dose) |
18 µg/kg q2w (middle dose) |
24 µg/kg q4w (low dose) |
Placebo |
|
Daily Life Quality Index (> 4-point reduction) |
72% p<0.05 |
64% ns |
73% p<0.05 |
54 % |
|
Atopic Dermatitis (> 5-point reduction) |
67% p<0.001 |
61% p<0.01 |
61% p<0.01 |
35 % |
|
Pain Numeric Rating (> 4-point reduction) |
45% p<0.05 |
35% ns |
23% ns |
22 % |
|
Atopic Dermatitis Sleep (> 1.25-point reduction) |
57% p<0.01 |
41% ns |
46% ns |
30 % |
| * | N=65, 100, 102, and 107 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w arms for DLQI; N=67, 101, 104 and 107 for ADCT; N=45, 71, 70 and 85 for ADSS Q1; and N=50, 84, 82 and 90 for Pain NRS; ns=not significant. |
The global Phase 2b REZOLVE-AD study randomized 393 patients with moderate-to-severe atopic dermatitis to receive subcutaneous treatment with three doses of rezpegaldesleukin: a high dose of 24 µg/kg every two weeks (q2w), a middle dose of 18 µg/kg every two weeks (q2w), and a low dose of 24 µg/kg every four weeks (q4w), or placebo q2w. Primary and secondary endpoints were assessed at week 16. Following week 16, rezpegaldesleukin-treated patients who achieved EASI percent score reductions of >50 (EASI-50) were re-randomized (1:1) to continue at the same dose level on a q4w or q12w regimen through study week 52 in a blinded maintenance period. Placebo patients with EASI percent score reductions of ≥ 50 percent continue to receive placebo q4w.
The REZOLVE-AD study design allowed for patients who originally received placebo in the initial induction period and achieved less than EASI-50 at Week 16 to enter into an open-label treatment escape arm to receive the high-dose rezpegaldesleukin regimen for a treatment period of up to 36 weeks.
Results presented today at EADV included interim data for 42 placebo patients who crossed over into the treatment escape arm. At the time of the data cut (August 18, 2025), 21 patients had reached 24 weeks of treatment with high dose rezpegaldesleukin (24 µg/kg q2w). Continuous treatment with rezpegaldesleukin demonstrated deepening of responses. For these patients, mean percent reduction in EASI at crossover week 16 and at crossover week 24 were 68% and 75%, respectively. EASI-75 responses at crossover week 16 and crossover week 24 were 50% and 62%, respectively. Percent of patients with a vIGA-AD 0/1 response at crossover week 16 and crossover week 24 were 28% and 38%, respectively.
“These results from REZOLVE-AD, including the improved responses observed with duration of dosing beyond 16 weeks, demonstrate the potential of this new biology and the promise of Tregs as a therapeutic modality to treat inflammatory skin disorders,” said Jonathan Zalevsky, Ph.D., Chief Research and Development Officer of Nektar. “With this important validation of a novel Treg mechanism in atopic dermatitis, we look forward to reporting the results in December of this year for rezpegaldesleukin in patients with alopecia areata.”
Safety Over 16-Week Induction Period
| 24 µg/kg q2w |
18 µg/kg q2w |
24 µg/kg q4w |
Pooled drug arms |
Placebo | |
| N=104 | N=106 | N=110 | N=320 | N=73 | |
| Patients with any TEAE, excluding ISRs |
69 (66.3 %) | 60 (56.6 %) | 64 (58.2 %) | 193(60.3 %) | 42 (57.5 %) |
| Patients with any Serious AE | 1 (1.0 %) | 4 (3.8 %) | 0 | 5 (1.6 %) | 0 |
| Any Drug-Related Serious AE1 | 0 | 2 (1.9 %) | 0 | 2 (0.6 %) | 0 |
| Patients with Severe AE | 3 (2.9 %) | 6 (5.7 %) | 1 (0.9 %) | 10 (3.1 %) | 1 (1.4) % |
| Any Drug-Related Severe AE2 | 3 (2.9 %) | 3 (2.8 %) | 0 | 6 (1.9 %) | 0 |
| TEAEs leading to study drug discontinuation |
8 (7.7 %) | 5 (4.7 %) | 5 (4.5 %) | 18 (5.6 %) | 0 |
| 1. | Serious TRAEs: Drug hypersensitivity – severe; Tonsillitis – moderate. Both events resolved. |
| 2. | Severe TRAEs (excluding Serious TRAEs): pyrexia (24 µg/kg q2w); two ISRs (24 µg/kg q2w); ISR, chest pain (18 µg/kg q2w). All five events resolved. |
Details of the presentation at EADV are as follows:
| ● | Abstract ID: LBA-108 |
| ● | Oral Presentation: “Efficacy and Safety of Rezpegaldesleukin, A Selective Regulatory T-Cell-Inducing Interleukin-2 Conjugate, in the Treatment of Atopic Dermatitis: Final Results from the 16-Week Induction of a Randomized Phase 2b Study (REZOLVE AD)” |
| ● | Presenter: Dr. Jonathan Silverberg |
| ● | Session Title: D2T01.3C |
| ● | Presentation Date and Time: Thursday, September 18th 14:45 – 15:00 pm |
| ● | Location: Paris Nord |
The presentation is available on Nektar’s website at http://www.nektar.com under Scientific Publications.
About REZOLVE-AD Phase 2b Study
The REZOLVE-AD trial (NCT06136741) was initiated in October 2023 and enrolled patients across approximately 110 sites globally with: 68% enrolled and treated in Europe, including Poland, Bulgaria, Germany, Czech Republic, Spain, Croatia and Hungary; 16% enrolled and treated in the United States; 11% enrolled and treated in Canada; and 5% enrolled and treated in Australia. Patient randomization was stratified based on baseline disease severity measured by vIGA-AD and geographic region. Key enrollment criteria in the study included a minimum EASI score of 16.0, a minimum Body Surface Area (BSA) of 10% and a minimum vIGA-AD of 3.
About Rezpegaldesleukin
Autoimmune and inflammatory diseases cause the immune system to mistakenly attack and damage healthy cells in a person’s body. A failure of the body’s self-tolerance mechanisms enables the formation of the pathogenic T lymphocytes that conduct this attack. Rezpegaldesleukin is a potential first-in-class resolution therapeutic that may address this underlying immune system imbalance in people with many autoimmune and inflammatory conditions. It targets the interleukin-2 receptor complex in the body to stimulate proliferation of powerful inhibitory immune cells known as regulatory T cells. By activating these cells, rezpegaldesleukin may act to bring the immune system back into balance.
In February 2025, the U.S. Food and Drug Administration (FDA) granted Fast Track designation for rezpegaldesleukin for the treatment of adult and pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. In July 2025, the FDA granted Fast Track designation for rezpegaldesleukin for the treatment of severe alopecia areata (AA) in adults and pediatric patients 12 years of age and older who weigh at least 40 kg.
Rezpegaldesleukin is being developed as a self-administered injection for a number of autoimmune and inflammatory diseases. It is wholly owned by Nektar Therapeutics.
About Atopic Dermatitis
Atopic dermatitis is the most common type of eczema, affecting approximately 30 million people in the United States.1 AD is characterized by a defect in the skin barrier, which allows allergens and other irritants to enter the skin, leading to an immune reaction and inflammation.
About Nektar Therapeutics
Nektar Therapeutics is a clinical-stage biotechnology company focused on developing treatments that address the underlying immunological dysfunction in autoimmune and chronic inflammatory diseases. Nektar’s lead product candidate, rezpegaldesleukin (REZPEG, or NKTR-358), is a novel, first-in-class regulatory T cell stimulator being evaluated in two Phase 2b clinical trials, one in atopic dermatitis and one in alopecia areata. Nektar’s pipeline also includes a preclinical bivalent tumor necrosis factor receptor type II (TNFR2) antibody and bispecific programs, NKTR-0165 and NKTR-0166, and a modified hematopoietic colony stimulating factor (CSF) protein, NKTR-422. Nektar, together with various partners, is also evaluating NKTR-255, an investigational IL-15 receptor agonist designed to boost the immune system’s natural ability to fight cancer, in several ongoing clinical trials.
Nektar is headquartered in San Francisco, California. For further information, visit http://www.nektar.com and follow us on LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements which can be identified by words such as: “will,” “develop,” “potential,” “target,” “promise,” “address,” and similar references to future periods. Examples of forward-looking statements include, among others, statements regarding the therapeutic potential of, and future development plans for, rezpegaldesleukin, NKTR-0165, NKTR-0166, NKTR-422, and NKTR-255. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results to differ materially from those indicated in the forward-looking statements include, among others: (i) our statements regarding the therapeutic potential of rezpegaldesleukin, NKTR-0165, NKTR-0166, NKTR-422 and NKTR-255 are based on preclinical and clinical findings and observations and are subject to change as research and development continue; (ii) rezpegaldesleukin, NKTR-0165, NKTR-0166, NKTR-422 and NKTR-255 are investigational agents and continued research and development for these drug candidates is subject to substantial risks, including negative safety and efficacy findings in future clinical studies (notwithstanding positive findings in earlier preclinical and clinical studies); (iii) rezpegaldesleukin, NKTR-0165, NKTR-0166, NKTR-422 and NKTR-255 are in clinical development and the risk of failure is high and can unexpectedly occur at any stage prior to regulatory approval; (iv) data reported from ongoing clinical trials are necessarily interim data only and the final results will change based on continuing observations; (v) the timing of the commencement or end of clinical trials and the availability of clinical data may be delayed or unsuccessful due to regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, changing standards of care, evolving regulatory requirements, clinical trial design, clinical outcomes, competitive factors, or delay or failure in ultimately obtaining regulatory approval in one or more important markets; (vi) a Fast Track designation does not increase the likelihood that rezpegaldesleukin will receive marketing approval in the United States; (vii) patents may not issue from our patent applications for our drug candidates, patents that have issued may not be enforceable, or additional intellectual property licenses from third parties may be required; and (viii) certain other important risks and uncertainties set forth in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 8, 2025. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Contacts:
For Investors:
Vivian Wu
VWu@nektar.com
Corey Davis, Ph.D.
LifeSci Advisors, LLC
cdavis@lifesciadvisors.com
212-915-2577
Ahu Demir, Ph.D.
LifeSci Advisors, LLC
ademir@lifesciadvisors.com
212-915-3820
For Media:
Jonathan Pappas
LifeSci Communications
857-205-4403
jpappas@lifescicomms.com
1. Eczema stats. National Eczema Association (2022, September 27).
https://nationaleczema.org/research/eczema-facts/
5