UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of August 2025
Commission File Number: 001-42484
ASCENTAGE PHARMA GROUP INTERNATIONAL
(Translation of Registrant’s name into English)
68 Xinqing Road
Suzhou Industrial Park
Suzhou, Jiangsu
China
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Explanatory Note
On August 20, 2025, Ascentage Pharma Group International (“Ascentage Pharma” or the “Company”) issued a press release entitled, “Ascentage Pharma Reports 2025 Interim Unaudited Six Months Financial Results and Business Updates”. A copy of the press release is furnished as Exhibit 99.1 to this Report. On August 21, 2025, Ascentage Pharma Group International posted an announcement on the Hong Kong Stock Exchange entitled, “Announcement of Unaudited Interim Results for the Six Months Ended June 30, 2025”. A copy of the announcement is furnished as Exhibit 99.2 to this Report.
Approval of New Form of Indemnification Agreement and Interest Payments
As previously disclosed, on July 17, 2025, the Company closed an offshore placement and top-up subscription of new shares pursuant to which Dajun Yang Dynasty Trust, an affiliate of the Company’s Chief Executive Officer, Dajun Yang, M.D., Ph.D. (the “Vendor”), offered and sold 22 million ordinary shares, par value US$0.0001 per share, of the Company at a price of HKD68.60 per share and 22 million new ordinary shares were issued to the Vendor at a price of HKD68.60, resulting in net proceeds to the Company of approximately HKD1,492 million (approximately US$190.1 million based on an exchange rate of 1 USD to 7.85 HKD).
Given the advantages realized by the Company through the use of the above top-up placement, and the Company’s January 2023 top-up placement, the Company’s Board considered and approved certain amendments to the indemnification agreement for its directors and officers (“Indemnification Agreement”). The Indemnification Agreement amends the existing form of indemnification agreement, filed as Exhibit 4.1 to the Company’s most recent annual report on Form 20-F, to provide for indemnification of affiliates of an indemnitee for any action or inaction taken by the indemnitee in the role of an officer or director of the Company, or by such affiliates in their capacity of a shareholder of the Company, for actions taken at the request of the Company (each such affiliate and indemnitee, an “Indemnified Person”) and approved, as necessary, by the Company’s Board. There were no other substantive changes made to the existing form of indemnification agreement. In addition, considering that the underlying structure of these top-up placements represents a de-facto share-loan arrangement, whereby Indemnified Persons loaned ordinary shares for specific period of days, respectively, the Board considered and approved the payment of up to $600,000 in interest payments to those Indemnified Persons that enabled the successful completion of those top-up placements.
The foregoing description of the Indemnification Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the form of indemnification agreement, which is filed as Exhibit 99.3 to this report on Form 6-K and is incorporated herein by reference.
INDEX TO EXHIBITS
| Exhibit Number |
Exhibit Title | |
| 99.1 | Press release dated August 20, 2025 | |
| 99.2 | Announcement dated August 21, 2025 | |
| 99.3 | Indemnification Agreement |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ASCENTAGE PHARMA GROUP INTERNATIONAL | ||
| Date: August 21, 2025 | /s/ Dajun Yang | |
| Name: | Dajun Yang | |
| Title: | Chief Executive Officer | |
Exhibit 99.1
ASCENTAGE PHARMA REPORTS 2025 INTERIM UNAUDITED SIX MONTHS FINANCIAL RESULTS AND BUSINESS UPDATES
| ● | Product sales from Olverembatinib in the first half of 2025 increased 93% year-over-year to US$30.3 million (RMB217.4 million), primarily attributable to the expansion of NRDL coverage |
| ● | Commenced commercial sales of Lisaftoclax in China, following approval on July 10, 2025 by China’s NMPA for the treatment of adult patients with CLL/SLL who have previously received at least one systemic therapy including BTK inhibitors |
| ● | Registrational Phase III trial for 1L HR MDS (GLORA-4) cleared by FDA and EMA, and first patients enrolled in Europe and China |
| ● | Completed a top-up placement in July 2025, resulting in US$190.1 million in net proceeds |
| ● | Nine registrational clinical trials are in progress, including three cleared by FDA |
| ● | Chinese (Mandarin) language investor webcast at 9:00 pm EDT on August 20, 2025 / 9:00 am HKT on August 21, 2025, and English language investor webcast at 8:00 am EDT / 8:00 pm HKT on August 21, 2025 |
ROCKVILLE, MD and SUZHOU, China, August 20, 2025 – Ascentage Pharma Group International (Ascentage Pharma) (NASDAQ: AAPG; HKEX: 6855) (referred hereinto as “Ascentage Pharma,” the “Company,” “we,” “us” or “our”), a global, commercial stage, integrated biopharmaceutical company engaged in the discovery, development and commercialization of novel, differentiated therapies to address unmet medical needs in cancer, today reported its unaudited financial results for the six months ended June 30, 2025, and provided updates on key ongoing clinical programs and commercial activities.
Dr. Dajun Yang, Chairman and Chief Executive Officer of Ascentage Pharma, said, “We reported strong momentum across our business in the first half of 2025, highlighted by the remarkable 93% year-over-year growth in Olverembatinib sales of $30.3 million, driven by expanded National Reimbursement Drug List (NRDL) coverage that has significantly improved patient access in China. In addition, the historic approval of Lisaftoclax in July marks a pivotal milestone as the first Bcl-2 inhibitor to receive conditional approval for chronic lymphocytic leukemia (CLL) / small lymphocytic leukemia (SLL) treatment in China. Our robust pipeline continues to advance with nine registrational clinical trials, all of which are actively ongoing, including three cleared by the FDA, demonstrating our commitment to bringing innovative cancer therapies to patients globally. The successful completion of our financing in July, raising US$190.1 million in net proceeds, strengthens our balance sheet and provides additional resources needed to execute our commercialization strategy and development programs. These achievements highlight our capability to execute globally and our commitment to delivering novel therapies to patients worldwide.”
Key Commercial Product and Pipeline Updates
Olverembatinib (HQP1351) is a novel, next-generation TKI and the first third-generation BCR-ABL1 TKI approved in China for treatment of patients with chronic myeloid leukemia (CML) in chronic-phase (-CP) or CML in accelerated phase (-AP) with T315I mutations, and in CML-CP that is resistant and/or intolerant to first and second-generation TKIs. Since 2021, commercialization of Olverembatinib in China continues to perform well. Additional potential indications for Olverembatinib are being evaluated in ongoing clinical trials.
Commercial progress
| ● | Revenue from sales of Olverembatinib in China increased 93% to US$30.3 million for the six months ended June 30, 2025, compared to US$15.5 million for the six months ended June 30, 2024. |
| ● | All approved indications for Olverembatinib have been covered since January 2025 by the China’s NRDL, which has bolstered the affordability and accessibility of Olverembatinib. |
| ● | The number of direct-to-patient (DTP) pharmacies and hospitals where Olverembatinib is on formulary reached 782 as of June 30, 2025, a 17% increase compared to June 30, 2024. In particular, the number of hospitals where Olverembatinib is on formulary increased 47% over the same period to 295 hospitals as of June 30, 2025 from 201 hospitals as of June 30, 2024. |
Clinical progress
| ● | Enrollment continues in a registrational Phase III clinical trial of Olverembatinib for the treatment of patients with succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumor (GIST) who have not responded to prior systemic treatment (POLARIS-3). |
| ● | Enrollment continues in an FDA-cleared registrational Phase III clinical trial of Olverembatinib for previously treated CML-CP patients, both with and without T315I mutation (POLARIS-2). |
| ● | Enrollment continues in a registrational Phase III clinical trial of Olverembatinib in combination with chemotherapy versus imatinib in combination with chemotherapy in patients with newly diagnosed Philadelphia chromosome-positive ALL (Ph+ ALL) (POLARIS-1). |
Anticipated progress
| ● | Plan to seek clearance from the FDA to initiate a registrational Phase III clinical trial of Olverembatinib in newly diagnosed Ph+ ALL patients. |
Lisaftoclax (APG-2575) is a novel, oral B-cell lymphoma 2 (Bcl-2) inhibitor developed to treat a variety of hematologic malignancies and solid tumors by selectively blocking Bcl-2 to restore the normal apoptosis process in cancer cells. Lisaftoclax is approved in China for the treatment of adult patients with CLL/SLL who have previously received at least one systemic therapy including Bruton’s tyrosine kinase (BTK) inhibitors and is being evaluated for additional potential indications in ongoing clinical trials.
Commercial progress
| ● | Lisaftoclax was approved on July 10, 2025, by China’s National Medical Products Administration (NMPA) for the treatment of adult patients with CLL/SLL who have previously received at least one systemic therapy including BTK inhibitors, which makes Lisaftoclax the first Bcl-2 inhibitor to receive conditional approval and marketing authorization for the treatment of patients with CLL/SLL in China, and the second Bcl-2 inhibitor approved globally. Shortly after the approval, commercial sales of Lisaftoclax commenced in China. |
| ● | Lisaftoclax received its first recommendation in April 2025 in the Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Lymphoid Malignancies as a monotherapy for the treatment of patients with relapsed/refractory (R/R) CLL/SLL. |
Clinical progress
| ● | Enrollment continues in a multicenter, registrational Phase III clinical trial of Lisaftoclax in combination with azacitidine for the treatment of patients who are newly diagnosed with higher-risk (HR) myelodysplastic syndrome (MDS) (GLORA-4). GLORA-4 has been cleared by the FDA and EMA and was originally approved by the China CDE in 2024. Currently, the study is enrolling patients globally, with the first patients dosed in Europe and China. |
| ● | Enrollment continues in a registrational Phase III clinical trial of Lisaftoclax for the treatment of newly diagnosed old or unfit patients with acute myeloid leukemia (AML) (GLORA-3). |
| ● | Enrollment continues in a registrational Phase III clinical trial to evaluate Lisaftoclax in combination with the BTK inhibitor acalabrutinib, versus immunochemotherapy in treatment-naïve patients with CLL/SLL (GLORA-2) to validate a fixed duration combination regimen as a first-line treatment. |
| ● | Enrollment continues in an FDA-cleared registrational Phase III clinical trial of Lisaftoclax in combination with BTK inhibitors in patients with CLL/SLL previously treated with BTK inhibitors (GLORA). |
| ● | Enrollment advancing in the Phase 1b/II clinical trials of Lisaftoclax in combination therapies for the treatment of patients with multiple myeloma (MM) in the United States. |
Recent Developments
| ● | Completed a top-up placement of ordinary shares in July 2025, resulting in US$190.1 million in net proceeds. |
| ● | Appointed Dr. Veet Misra as Chief Financial Officer and Eric Huang as Senior Vice President of Global Corporate Development and Finance. |
Half Year 2025 Unaudited Financial Results
Revenue for the six months ended June 30, 2025 was US$32.6 million, compared to US$113.4 million for the six months ended June 30, 2024, which represented a decrease of US$80.7 million, or 71.6% on a constant currency basis. The decrease in revenue was primarily due to intellectual property revenue of US$93.4 million recorded during the six months ended June 30, 2024. Product sales of Olverembatinib in China increased by US$14.8 million, or 92.5% on a constant currency basis, to US$30.3 million for the first half of 2025 from US$15.5 million for the six months ended June 30, 2024.
Selling and distribution expenses for the six months ended June 30, 2025 were US$19.2 million, compared to US$12.3 million for the six months ended June 30, 2024, which represented an increase of US$6.9 million, or 53.7% on a constant currency basis. The increase was attributable to expansions in commercialization efforts of Olverembatinib and preparation for anticipated launch of Lisaftoclax.
Research and development expenses for the six months ended June 30, 2025 were US$73.8 million, compared to US$61.1 million for the six months ended June 30, 2024, which represented an increase of US$12.7 million, or 19.0% on a constant currency basis. The increase was attributable to increased external research and development expenses related to our ongoing global clinical trials.
Administrative expenses for the six months ended June 30, 2025 were US$13.9 million, compared to US$12.0 million for the six months ended June 30, 2024, which represented an increase of US$1.9 million, or 14.6% on a constant currency basis. The increase was due to the increase in the consulting fees and agency fees.
Financing costs for the six months ended June 30, 2025 were US$3.9 million, compared to US$4.7 million for the six months ended June 30, 2024, which represented a decrease of US$0.8 million, or 18.4% on a constant currency basis. The decrease was due to lower effective interest rates in relation to bank borrowings.
Other expenses for the six months ended June 30, 2025 were US$5.6 million, compared to US$1.0 million for the six months ended June 30, 2024, which represented an increase of US$4.6 million, or 465.6% on a constant currency basis. The increase was primarily attributable to the increase in fair value loss of contingent consideration related to acquisition of Guangzhou Healthquest Pharma Co., Ltd.
Loss for the six months ended June 30, 2025 was US$82.5 million, compared to the profit of US$22.4 million for the six months ended June 30, 2024. The loss per share attributable to ordinary equity holders was $0.24 per ordinary share for the six months ended June 30, 2025, compared to the earnings per share of $0.08 per ordinary share for the six months ended June 30, 2024.
Cash and bank balances as of June 30, 2025, were US$231.9 million, compared to US$172.8 million as of December 31, 2024, which represented an increase of US$59.1 million, or 31.7% on a constant currency basis. The increase was primarily due to the net proceeds of US$132.5 million from the U.S. initial public offering in January 2025.
Following the top-up placement in July 2025, which resulted in US$190.1 million in net proceeds, these net proceeds together with existing cash and cash equivalents, loan facilities and future sales will enable the Company to fund operating expenses and capital expenditure requirements.
Investor Conference Call and Webcast
Ascentage Pharma will be holding investor webcasts to discuss its six months 2025 unaudited interim results.
Ascentage Pharma will host a Chinese (Mandarin) language investor webcast at 9:00 pm EDT on August 20, 2025 / 9:00 am HKT on August 21, 2025. To access the Chinese language investor event or conference call, please register in advance here.
The English language investor conference call and webcast will be held at 8:00 am EDT / 8:00 pm HKT on August 21, 2025. To access the English language webcast, please register in advance here. The webcast replay for English language conference call and presentation will also be available on the News & Events page of the Ascentage Pharma website.
Statement Regarding Unaudited Financial Information
This press release includes unaudited condensed consolidated financial information as of and for the six months ended June 30, 2025, which has not been audited by the Company’s auditors. The unaudited information for the six months ended June 30, 2025, is preliminary, based on the information available at this time and subject to changes in connection with the completion of the review of the Company’s financial statements. As such, the Company’s actual results and financial condition as reflected in the financial statements that will be included in the Company’s Annual Report on Form 6-K, may be adjusted or presented differently from the financial information herein and the variations could be material. The unaudited condensed consolidated financial statements include the accounts of the Company and its subsidiaries. All periods presented have been accounted for in conformity with IFRS accounting standard as issued by the International Accounting Standards Board and pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (the “SEC”).
Currency and Exchange Rate Information
Unless otherwise indicated, translations from RMB to U.S. dollars for the six months ended June 30, 2025 and 2024 and as at December 31, 2024 are made at RMB7.1636 to US$1.00, RMB 7.2672 to US$1.00 and RMB 7.2993 to US$1.00, representing the noon buying rate in the City of New York, as certified by the Federal Reserve Bank of New York, on June 30, 2025, June 28, 2024 and December 31, 2024, respectively. Ascentage Pharma makes no representation that the RMB or U.S. dollar amounts referred to in this press release could have been or could be converted into U.S. dollars or RMB, as the case may be, at any particular rate or at all.
About Ascentage Pharma
Ascentage Pharma Group International (NASDAQ: AAPG; HKEX: 6855) (“Ascentage Pharma” or the “Company”) is a global, commercial stage, integrated biopharmaceutical company engaged in the discovery, development and commercialization of novel, differentiated therapies to address unmet medical needs in cancer. The company has built a rich pipeline of innovative drug products and candidates that include inhibitors targeting key proteins in the apoptotic pathway, such as Bcl-2 and MDM2-p53 and next-generation kinase inhibitors.
The Company’s first approved product, Olverembatinib, is the first novel third-generation BCR-ABL1 inhibitor approved in China for the treatment of patients with CML in chronic phase (CML-CP) with T315I mutations, CML in accelerated phase (CML-AP) with T315I mutations, and CML-CP that is resistant or intolerant to first and second-generation TKIs. It is covered by the China National Reimbursement Drug List (NRDL). Ascentage Pharma is currently conducting an FDA-cleared registrational Phase III trial, called POLARIS-2, of Olverembatinib for CML, as well as registrational Phase III trials for patients with newly diagnosed Ph+ ALL, called POLARIS-1, and SDH-deficient GIST patients, called POLARIS-3.
The Company’s second approved product, Lisaftoclax, is a novel Bcl-2 inhibitor for the treatment of various hematologic malignancies. Lisaftoclax has been approved by China’s National Medical Products Administration (NMPA) for the treatment of adult patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have previously received at least one systemic therapy including Bruton’s tyrosine kinase (BTK) inhibitors. The Company is currently conducting four global registrational Phase III trials: the FDA-cleared GLORA study of Lisaftoclax in combination with BTK inhibitors in patients with CLL/SLL previously treated with BTK inhibitors for more than 12 months with suboptimal response; the GLORA-2 study in patients with newly diagnosed CLL/SLL; the GLORA-3 study in newly diagnosed, elderly and unfit patients with AML; and the FDA-cleared GLORA-4 study in patients with newly diagnosed HR MDS.
Leveraging its robust R&D capabilities, Ascentage Pharma has built a portfolio of global intellectual property rights and entered into global partnerships and other relationships with numerous leading biotechnology and pharmaceutical companies, such as Takeda, AstraZeneca, Merck, Pfizer, and Innovent, in addition to research and development relationships with leading research institutions, such as Dana-Farber Cancer Institute, Mayo Clinic, National Cancer Institute and the University of Michigan.
Cautionary Note Regarding Forward-Looking Statements
For more information, visit https://ascentage.com/ This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical facts, contained in this press release may be forward-looking statements, including statements that express Ascentage Pharma’s opinions, expectations, beliefs, plans, objectives, assumptions or projections regarding future events or future results of operations or financial condition. These forward-looking statements are subject to a number of risks and uncertainties as discussed in Ascentage Pharma’s filings with the SEC, including those set forth in the sections titled “Risk factors” and “Cautionary note regarding forward-looking statements” in its Annual Report on Form 20-F for the year ended December 31, 2024, filed with the SEC on April 16, 2025, the sections headed “Forward-looking Statements” and “Risk Factors” in the prospectus of the Company for its Hong Kong initial public offering dated October 16, 2019, and other filings with the SEC and/or The Stock Exchange of Hong Kong Limited it has made or it makes from time to time that may cause actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements contained in this press release do not constitute profit forecast by the Company’s management.
As a result of these factors, you should not rely on these forward-looking statements as predictions of future events. The forward-looking statements contained in this press release are based on Ascentage Pharma’s current expectations and beliefs concerning future developments and their potential effects and speak only as of the date of such statements. Ascentage Pharma does not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact Information
Investor Relations:
Hogan Wan, Head of IR and Strategy
Ascentage Pharma
Hogan.Wan@ascentage.com
+86 512 85557777
Stephanie Carrington
ICR Healthcare
AscentageIR@icrhealthcare.com
+1 (646) 277-1282
Media Relations:
Jon Yu
ICR Healthcare
AscentagePR@icrhealthcare.com
+1 (646) 677-1855
Ascentage Pharma Group International
Condensed consolidated statements of profit or loss
(Amounts in thousands of Renminbi (“RMB”) and U.S. dollar (“US$”), except for number of shares and per share data)
| For the Six Months Ended June 30, | ||||||||||||||||
| 2023 | 2024 | 2025 | 2025 | |||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (Unaudited) | (Unaudited) | (Unaudited) | (Unaudited) | |||||||||||||
| REVENUE | ||||||||||||||||
| Intellectual property | - | 678,416 | - | - | ||||||||||||
| Products | 129,533 | 124,823 | 212,874 | 29,716 | ||||||||||||
| Others | 13,168 | 20,507 | 20,825 | 2,907 | ||||||||||||
| Total revenue | 142,701 | 823,746 | 233,699 | 32,623 | ||||||||||||
| Cost of sales | ||||||||||||||||
| Products | (18,154 | ) | (14,158 | ) | (20,659 | ) | (2,884 | ) | ||||||||
| Others | - | (901 | ) | (991 | ) | (138 | ) | |||||||||
| Total cost of sales | (18,154 | ) | (15,059 | ) | (21,650 | ) | (3,022 | ) | ||||||||
| Gross profit | 124,547 | 808,687 | 212,049 | 29,601 | ||||||||||||
| Other income and gains | 17,021 | 17,346 | 36,661 | 5,118 | ||||||||||||
| Selling and distribution expenses | (83,319 | ) | (89,637 | ) | (137,787 | ) | (19,234 | ) | ||||||||
| Administrative expenses | (91,340 | ) | (86,988 | ) | (99,685 | ) | (13,915 | ) | ||||||||
| Research and development expenses | (309,814 | ) | (444,079 | ) | (528,561 | ) | (73,784 | ) | ||||||||
| Other expenses | (4,175 | ) | (7,106 | ) | (40,192 | ) | (5,612 | ) | ||||||||
| Finance costs | (52,719 | ) | (34,076 | ) | (27,798 | ) | (3,880 | ) | ||||||||
| Share of profit/(loss) of a joint venture | 196 | (1,252 | ) | 1 | - | |||||||||||
| (LOSS)/PROFIT BEFORE TAX | (399,603 | ) | 162,895 | (585,312 | ) | (81,706 | ) | |||||||||
| Income tax expense | (2,746 | ) | (69 | ) | (5,512 | ) | (770 | ) | ||||||||
| (LOSS)/PROFIT FOR THE PERIOD | (402,349 | ) | 162,826 | (590,824 | ) | (82,476 | ) | |||||||||
| Attributable to: | ||||||||||||||||
| Ordinary equity holders of the Company | (402,351 | ) | 163,001 | (590,768 | ) | (82,468 | ) | |||||||||
| Non-controlling interests | 2 | (175 | ) | (56 | ) | (8 | ) | |||||||||
| (402,349 | ) | 162,826 | (590,824 | ) | (82,476 | ) | ||||||||||
| (LOSS)/EARNINGS PER SHARE ATTRIBUTABLE TO ORDINARY EQUITY HOLDERS OF THE COMPANY | ||||||||||||||||
| Basic | (1.47 | ) | 0.56 | (1.73 | ) | (0.22 | ) | |||||||||
| Diluted | (1.47 | ) | 0.55 | (1.73 | ) | (0.22 | ) | |||||||||
Ascentage Pharma Group International
Condensed consolidated statements of comprehensive loss
(Amounts in thousands of Renminbi and U.S. dollar, except for number of shares and per share data)
| For the Six Months Ended June 30, | ||||||||||||||||
| 2023 | 2024 | 2025 | 2025 | |||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (Unaudited) | (Unaudited) | (Unaudited) | (Unaudited) | |||||||||||||
| (LOSS)/PROFIT FOR THE PERIOD | (402,349 | ) | 162,826 | (590,824 | ) | (82,476 | ) | |||||||||
| OTHER COMPREHENSIVE INCOME/(LOSS) | ||||||||||||||||
| Other comprehensive income that may be reclassified to profit or loss in subsequent periods: | ||||||||||||||||
| Exchange differences on translation of foreign operations | (699 | ) | 40 | 1,095 | 153 | |||||||||||
| Other comprehensive income that will not be reclassified to profit or loss in subsequent periods: | ||||||||||||||||
| Exchange differences on translation of the Company | 40,479 | 2,229 | (2,035 | ) | (284 | ) | ||||||||||
| OTHER COMPREHENSIVE INCOME/(LOSS) FOR THE PERIOD, NET OF TAX | 39,780 | 2,269 | (940 | ) | (131 | ) | ||||||||||
| TOTAL COMPREHENSIVE (LOSS)/INCOME FOR THE PERIOD | (362,569 | ) | 165,095 | (591,764 | ) | (82,607 | ) | |||||||||
| Attributable to: | ||||||||||||||||
| Ordinary equity holders of the Company | (362,571 | ) | 165,270 | (591,708 | ) | (82,599 | ) | |||||||||
| Non-controlling interests | 2 | (175 | ) | (56 | ) | (8 | ) | |||||||||
| (362,569 | ) | 165,095 | (591,764 | ) | (82,607 | ) | ||||||||||
Ascentage Pharma Group International
Condensed consolidated statements of financial position
(Amounts in thousands of Renminbi and U.S. dollar, except for number of shares and per share data)
| As at | ||||||||||||
| December 31, 2024 |
June 30, 2025 |
June 30, 2025 |
||||||||||
| RMB | RMB | US$ | ||||||||||
| (Audited) | (Unaudited) | (Unaudited) | ||||||||||
| NON-CURRENT ASSETS | ||||||||||||
| Property, plant and equipment | 849,450 | 821,201 | 114,635 | |||||||||
| Right-of-use assets | 56,109 | 50,760 | 7,086 | |||||||||
| Goodwill | 24,694 | 24,694 | 3,447 | |||||||||
| Other intangible assets | 75,998 | 70,994 | 9,910 | |||||||||
| Investment in a joint venture | 32,717 | 32,718 | 4,567 | |||||||||
| Financial assets at fair value through profit or loss (“FVTPL”) | 1,141 | 4,617 | 645 | |||||||||
| Deferred tax assets | 44,236 | 33,385 | 4,660 | |||||||||
| Other non-current assets | 59,303 | 99,055 | 13,828 | |||||||||
| Total non-current assets | 1,143,648 | 1,137,424 | 158,778 | |||||||||
| CURRENT ASSETS | ||||||||||||
| Inventories | 6,597 | 8,591 | 1,199 | |||||||||
| Trade receivables, net | 83,143 | 78,362 | 10,939 | |||||||||
| Prepayments, other receivables and other assets | 123,211 | 160,313 | 22,379 | |||||||||
| Cash and bank balances | 1,261,211 | 1,661,454 | 231,930 | |||||||||
| Total current assets | 1,474,162 | 1,908,720 | 266,447 | |||||||||
| CURRENT LIABILITIES | ||||||||||||
| Trade payables | 91,966 | 118,676 | 16,567 | |||||||||
| Other payables and accruals | 258,098 | 249,358 | 34,808 | |||||||||
| Contract liabilities | 37,485 | 37,485 | 5,233 | |||||||||
| Interest-bearing bank and other borrowings | 779,062 | 833,783 | 116,392 | |||||||||
| Total current liabilities | 1,166,611 | 1,239,302 | 173,000 | |||||||||
| NET CURRENT ASSETS | 307,551 | 669,418 | 93,447 | |||||||||
| TOTAL ASSETS LESS CURRENT LIABILITIES | 1,451,199 | 1,806,842 | 252,225 | |||||||||
Ascentage Pharma Group International
Condensed consolidated statements of financial position
(Amounts in thousands of Renminbi and U.S. dollar, except for number of shares and per share data)
| As at | ||||||||||||
| December 31, 2024 |
June 30, 2025 |
June 30, 2025 |
||||||||||
| RMB | RMB | US$ | ||||||||||
| (Audited) | (Unaudited) | (Unaudited) | ||||||||||
| NON-CURRENT LIABILITIES | ||||||||||||
| Contract liabilities | 248,460 | 229,628 | 32,055 | |||||||||
| Interest-bearing bank and other borrowings | 889,4351 | 882,382 | 123,176 | |||||||||
| Deferred tax liabilities | 5,368 | - | - | |||||||||
| Deferred income | 27,500 | 6,500 | 907 | |||||||||
| Other non-current liabilities | 6,274 | 12,423 | 1,734 | |||||||||
| Total non-current liabilities | 1,177,037 | 1,130,933 | 157,872 | |||||||||
| TOTAL LIABILITIES | 2,343,648 | 2,370,235 | 330,872 | |||||||||
| EQUITY | ||||||||||||
| Equity attributable to ordinary equity holders of the Company | ||||||||||||
| Ordinary shares (par value of US$0.0001 per share as of December 31, 2024 and June 30, 2025; 315,224,993 and 348,999,320 shares authorized, issued and outstanding as of December 31, 2024 and June 30, 2025, respectively) | 214 | 239 | 33 | |||||||||
| Treasury shares | (8 | ) | (2,960 | ) | (413 | ) | ||||||
| Share premium | 6,545,129 | 7,546,108 | 1,053,396 | |||||||||
| Capital and reserves | (384,515 | ) | (389,056 | ) | (54,310 | ) | ||||||
| Exchange fluctuation reserve | (126,071 | ) | (127,011 | ) | (17,730 | ) | ||||||
| Accumulated losses | (5,770,555 | ) | (6,361,323 | ) | (888,006 | ) | ||||||
| 264,194 | 665,997 | 92,970 | ||||||||||
| Non-controlling interests | 9,968 | 9,912 | 1,383 | |||||||||
| Total equity | 274,162 | 675,909 | 94,353 | |||||||||
Exhibit 99.2
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement.

ASCENTAGE PHARMA GROUP INTERNATIONAL
亞盛醫藥集團
(Incorporated in the Cayman Islands with limited liability)
(Stock Code: 6855)
ANNOUNCEMENT OF UNAUDITED INTERIM
RESULTS
FOR THE SIX MONTHS ENDED JUNE 30, 2025
The Board is pleased to announce the unaudited consolidated results of Ascentage Pharma Group International for the six months ended June 30, 2025, together with the comparative figures for the six months ended June 30, 2024.
FINANCIAL HIGHLIGHTS
Revenue for the six months ended June 30, 2025 was RMB233.7 million (US$32.6 million) which represents a decrease of RMB590.0 million (US$80.7 million), or 71.6%, as compared to the six months ended June 30, 2024, primarily because of intellectual property revenue of RMB678.4 million (US$95.3 million) during the six months ended June 30, 2024. Product sales from Olverembatinib in China increased by RMB104.5 million (US$14.9 million), or 93%, to RMB217.4 million (US$30.3 million) for the first half of 2025 compared to RMB112.9 million (US$15.5 million) for the six months ended June 30, 2024.
Total operating expenses for the six months ended June 30, 2025 increased by RMB145.3 million (US$21.5 million), or 23.4% to RMB766.0 million (US$106.9 million), as compared to the same period of 2024. Research and development expenses increased by RMB84.5 million (US$12.7 million), or 19.0%, to RMB528.6 million (US$73.8 million) for the six months ended June 30, 2025 primarily attributable to increased external research and development expenses related to our ongoing global clinical trials. Selling and distribution expenses increased by RMB48.2 million (US$6.9 million), or 53.7%, to RMB137.8 million (US$19.2 million) for the six months ended June 30, 2025, primarily attributable to expansion in commercialization of Olverembatinib and preparation for the launch of Lisaftoclax.
Net loss was RMB590.8 million (US$82.5 million) for the six months ended June 30, 2025, compared to profit of RMB162.8 million (US$22.4 million) for the six months ended June 30, 2024, which was primarily attributable to the decrease in intellectual property revenue as explained above.
As at June 30, 2025, the Group’s cash and bank balances were RMB1,661.5 million (US$231.9 million), or an increase of RMB400.2 million (US$59.1 million), or 31.7% compared with RMB1,261.2 million (US$172.8 million) as at December 31, 2024, which was primarily attributable to the net proceeds of US$132.5 million from its U.S. initial public offering in January 2025. In addition, after the Reporting Period, in July 2025, we have received net proceeds of HK$1,492.5 million (US$190.1 million) arising from the 2025 Placing.
BUSINESS HIGHLIGHTS
Lisaftoclax has been approved in China for CLL/SLL
| 1. | On July 10, 2025, Lisaftoclax was approved by China’s National Medical Products Administration (NMPA) for the treatment of adult patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have previously received at least one systemic therapy, including Bruton’s tyrosine kinase, or BTK, inhibitors. |
| 2. | This approval for Lisaftoclax demonstrates Ascentage Pharma’s exceptional ability to execute its overall strategy in translating clinical development to approved products. Lisaftoclax is the first Bcl-2 inhibitor to receive conditional approval and marketing authorization for the treatment of patients with CLL/SLL in China, and the second Bcl-2 inhibitor approved globally. |
Olverembatinib revenue grew significantly after NRDL coverage expansion
| 1. | Revenue from sales of Olverembatinib in China increased 93% to RMB217.4 million (US$30.3 million) for the six months ended June 30, 2025, compared to RMB112.9 million (US$15.5 million) for the six months ended June 30, 2024. |
| 2. | All approved indications of Olverembatinib are covered since January 2025 by the China’s National Reimbursement Drug List, or NRDL, which bolstered the affordability and accessibility of the drug in China. |
| 3. | The number of hospitals where Olverembatinib are on formulary and Direct-to-Patient, or DTP, pharmacies reached 782 as of June 30, 2025, a 17% increase compared to June 30, 2024. In particular, the number of hospitals where Olverembatinib is on formulary increased approximately 47% over the same period to 295 hospitals as of June 30, 2025 from 201 hospitals as of June 30, 2024. |
MANAGEMENT DISCUSSION & ANALYSIS
OVERVIEW
We are a global, commercial stage, integrated biopharmaceutical company engaged in the discovery, development and commercialization of novel, differentiated therapies to address unmet medical needs in cancer.
Our lead drug products, Olverembatinib and Lisaftoclax, were developed by us to treat multiple major hematological malignancies as well as solid tumors that occur globally. Currently, for hematological malignancies, Olverembatinib is directed towards or intended to address chronic myeloid leukemia, or CML, and acute lymphocytic leukemia, or ALL, and Lisaftoclax is directed towards or intended to address chronic lymphocytic leukemia, or CLL, small lymphocytic leukemia, or SLL, acute myeloid leukemia, or AML, myelodysplastic syndrome, or MDS, and multiple myeloma, or MM. These particular hematological diseases alone are expected to exceed US$160 billion in aggregate market size by 2035, according to an industry report commissioned by us and independently prepared by Frost & Sullivan, or the F&S Report.
Our first product, Olverembatinib, is a novel, next-generation tyrosine kinase inhibitor, or TKI, that was the first BCR-ABL1 TKI approved in China for treatment of patients with CML in chronic phase, or CML-CP, with T315I mutations, CML in accelerated phase, or CML-AP, with T315I mutations, and CML-CP that is resistant and/or intolerant to first and second-generation TKIs. We are currently commercializing Olverembatinib in China. Since January 2025, all commercialized indications of Olverembatinib have been included in the NRDL, which bolstered the affordability and accessibility of the drug in China. We are currently conducting an FDA-cleared, registrational Phase III trial, called POLARIS-2, of Olverembatinib for CML. In addition, we are conducting registrational Phase III trials for patients with newly diagnosed Ph+ ALL and SDH-deficient GIST patients. In June 2024, we entered into an Exclusive Option Agreement with Takeda Pharmaceuticals International AG, or Takeda, pursuant to which we granted Takeda an exclusive option to enter into an exclusive license agreement for Olverembatinib. If exercised, the Option would allow Takeda to license global rights to develop and commercialize Olverembatinib in all territories outside of the PRC, Hong Kong, Macau, Taiwan and Russia.
Our second product, Lisaftoclax, is a novel Bcl-2 inhibitor that, on July 10, 2025, was approved by China’s NMPA for the treatment of adult patients with CLL/SLL, who have previously received at least one systemic therapy including BTK inhibitors. This milestone makes Lisaftoclax the first Bcl-2 inhibitor receiving conditional approval and marketing authorization for the treatment of patients with CLL/SLL in China, and the second Bcl-2 inhibitor approved globally. We are also currently conducting four registrational Phase III trials of Lisaftoclax: (1) the GLORA study of Lisaftoclax in combination with BTK inhibitors in patients with CLL/SLL previously treated with BTK inhibitors for more than 12 months with suboptimal response, (2) the GLORA-2 study in combination with acalabrutinib in patients with newly diagnosed CLL/SLL, (3) the GLORA-3 study in combination with azacitidine, or AZA, in newly diagnosed, elderly and unfit patients with AML; and (4) the GLORA-4 study in combination with AZA in patients with newly diagnosed higher risk, or HR, MDS.
Our central strategy has been to leverage our expertise in chemistry to synthesize inhibitors targeting proteins and pathways that drive the key hallmarks of cancer. Beyond our two products, we have several other clinical-stage assets in U.S., China or international clinical trials. To date, we have utilized our knowledge of small molecule discovery together with our ability to execute clinical trials globally to develop novel treatments to address unmet medical needs in cancer. Backed by our strong scientific foundation, we use state-of-the-art technologies to discover and develop innovative therapeutic agents directed towards our target patient populations.
We are empowered by our technical expertise in structure-based drug design and our innovative drug discovery engine, which allows us to address unmet medical needs by targeting key apoptotic pathways and tyrosine kinases that have been well-known and validated in the field. These core competencies have allowed us to develop small molecule and degrader candidate therapeutics against a range of well-characterized apoptotic targets including Bcl-2, Bcl-2/Bcl-xL, IAP, and MDM2-p53. In addition, we are building next-generation cell signaling inhibitor candidates (i.e., BCR-ABL1, ALK, FAK inhibitors) as well as epigenome-modifying agents (i.e., EED inhibitor). Earlier stage in our pipeline, we are harnessing our deep understanding of protein degraders to develop a wide range of therapeutic candidates, such as proteolysis targeting chimera molecules, or PROTACs, that target traditionally undruggable proteins that are implicated in oncogenesis. We are the only company in the world with active clinical programs targeting all three known classes of key apoptosis regulators, according to the F&S Report.
Leveraging our robust internal research and development capabilities, we have built an intellectual property portfolio with rights that span globally. As of June 30, 2025, we cumulatively have 478 issued patents globally, which includes over 20 new patents issued during the reporting period, while excluding the expiration and abandonment of certain patents unrelated to our core product portfolio. 342 issued patents are issued outside of China as of the end of the Reporting Period.
We have also established collaborations and other relationships with leading biotechnology and pharmaceutical companies around the world, including a collaboration and license agreement with Innovent as well as clinical collaboration agreements with AstraZeneca, Merck & Co., and Pfizer Inc. We also have research and development collaborations with leading research institutions, including, but not limited to, Dana-Farber Cancer Institute, Mayo Clinic, MD Anderson Cancer Center, National Cancer Institute, and the University of Michigan.
BUSINESS OVERVIEW
Product Pipeline
The following table summarizes our clinical-stage pipeline consisting of six small molecule drug candidates, including ongoing trials for Olverembatinib and Lisaftoclax for oncology indications beyond those currently approved in China, along with the development status of each candidate, as of July 31, 2025:

| 1. | The globe icon refers to trials that have received clearance, or for which we expect to obtain clearance, in two or more countries or regions. The U.S. flag refers to trials for which we have received clearance from the FDA to conduct trials in the United States. The China flag refers to trials for which we have conducted, are currently conducting, or plan to conduct only in China. |
| 2. | The globe icon also indicates having global development and commercialization rights. |
| 3. | CLL/SLL patients who have previously received at least one systemic therapy, including BTK inhibitors. |
| 4. | Registrational trials for ongoing CLL/SLL, AML and MDS. Phase 2 trials ongoing for MM. |
| 5. | Two registrational trials ongoing for NSCLC. Phase 2 trials ongoing for ovarian cancer. |
Current Core Products
Olverembatinib (HQP1351)
Our first product, Olverembatinib, is a novel, next-generation TKI. Olverembatinib is the first third generation BCR-ABL1 TKI approved in China for treatment of patients with CML-CP with T315I mutations, CML-AP with T315I mutations and CML-CP that is resistant and/or intolerant to first and second-generation TKIs. Olverembatinib received support from China’s National Major New Drug Discovery and Manufacturing Program. Since January 2025, all approved indications of Olverembatinib are covered by the China’s NRDL, which bolstered the affordability and accessibility of the drug in China.
Olverembatinib was included as an Emerging Treatment Option in the 2024 National Comprehensive Cancer Network USA, or NCCN, guidelines for the management of CML and received recommendation from the Chinese Society of Clinical Oncology, or CSCO, guidelines for the treatment of CML and Ph+ ALL. As of the date of this report, the FDA has granted four Orphan Drug Designations (ODDs) to Olverembatinib, including for CML, ALL, AML and GIST, as well as Fast-Track Designation for treatment of CML in patients with certain genetic markers who have failed to respond to treatments with existing TKIs. Olverembatinib was also granted an Orphan Designation by the European Medicines Agency, or EMA, for the treatment of CML.
The following table summarizes registrational trials that were completed or ongoing worldwide for Olverembatinib:
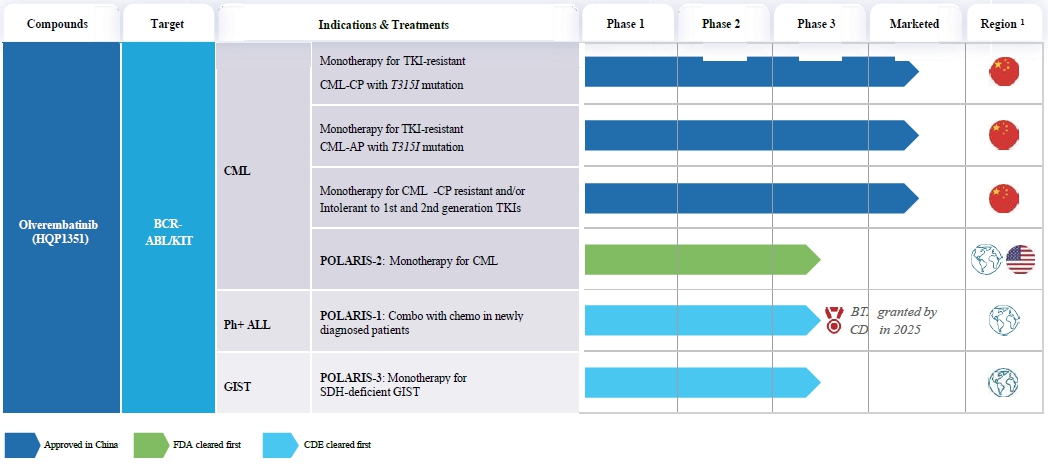
| 1. | The globe icon as used in this table refers to trials that are currently taking place in at least two countries. The US flag refers to trials for which we have received clearance from the FDA to conduct trials in the United States. The China flag refers to trials for which we have conducted only in China. |
The recent progress of Olverembatinib is as follows:
Commercial progress
| 1. | Revenue from sales of Olverembatinib in China increased 93% to RMB217.4 million (US$30.3 million) for the six months ended June 30, 2025, compared to RMB112.9million (US$15.5 million) for the six months ended June 30, 2024. |
| 2. | All approved indications of Olverembatinib are covered since January 2025 by China’s NRDL, which bolstered the affordability and accessibility of the drug in China. |
| 3. | The number of DTP pharmacies and hospitals where Olverembatinib is on formulary reached 782 as of June 30, 2025, a 17% increase compared to June 30, 2024. In particular, the number of hospitals where Olverembatinib is on formulary increased 47% compared to June 30, 2024. |
| 4. | In April 2025, Olverembatinib received an upgraded recommendation in the CSCO Guidelines for the Diagnosis and Treatment of Leukemias in Children and Adolescent and retained its recommendations in the CSCO Guidelines for the Diagnosis and Treatment of Hematological Malignancies. |
Clinical progress
| 1. | We continue enrollment in a registrational Phase III clinical trial of Olverembatinib in combination with chemotherapy versus imatinib in combination with chemotherapy in patients with newly diagnosed Ph+ ALL (POLARIS-1). |
| 2. | We continue enrollment in a FDA-cleared registrational Phase III clinical trial of Olverembatinib for previously treated CML-CP patients, both with and without T315I mutation (POLARIS-2). |
| 3. | We continue enrollment in a registrational Phase III clinical trial of Olverembatinib for the treatment of patients with SDH-deficient GIST who have failed prior systemic treatment (POLARIS-3). |
| 4. | We obtained Breakthrough Therapy Designation (BTD) for Olverembatinib in March 2025 from the CDE of China’s NMPA for combination with low-intensity chemotherapy for the first-line treatment of newly-diagnosed patients with Ph+ ALL. |
Data publications
| 1. | In June 2025, the updated results from multiple studies of Olverembatinib were presented as posters at the 2025 European Hematology Association Hybrid Congress (EHA 2025). The results showed broad therapeutic potential and demonstrated clinical benefit in the treatment of Ph+ ALL. According to the results, Olverembatinib demonstrated high CR and CMR rates, as well as favorable tolerability in first-line treatment of newly diagnosed and relapsed/refractory Ph+ ALL as well as specific subtypes of some hematologic malignancies (e.g., myeloid/lymphoid neoplasm with FGFR1 rearrangement). Furthermore, studies on various combinations of Olverembatinib (with venetoclax plus azacitidine, the VP regimen, blinatumomab, or inotuzumab ozogamicin) have shown encouraging results that revealed Olverembatinib’s potential to offer additional treatment options and improve long-term prognoses for patients with Ph+ ALL. |
| 2. | In April 2025, we released data of Olverembatinib in combination with Lisaftoclax overcoming venetoclax resistance in preclinical models of AML as well as preclinical data of Olverembatinib in combination with Lisaftoclax in T-ALL at the 2025 American Association for Cancer Research (AACR 2025). |
Expected Progress of Olverembatinib
| 1. | We plan to seek clearance from the FDA to initiate a registrational Phase III clinical trial in newly diagnosed Ph+ ALL patients. |
Key Products and Pipeline Candidates
Lisaftoclax (APG-2575)
Lisaftoclax is a novel, oral Bcl-2 inhibitor developed to treat a variety of hematologic malignancies and solid tumors by selectively blocking Bcl-2 to restore the normal apoptosis process in cancer cells. In July 2025, Lisaftoclax was approved by China’s NMPA for the treatment of adult patients with CLL/SLL who have previously received at least one systemic therapy, including BTK inhibitors, which makes Lisaftoclax the first Bcl-2 inhibitor receiving conditional approval and marketing authorization for the treatment of patients with CLL/SLL in China as well as the second Bcl-2 inhibitor approved globally. Currently, Lisaftoclax has received clearances and approvals for clinical studies in China, the United States, Australia, and Europe, with indications including CLL/ SLL, non-Hodgkin’s lymphoma, or NHL, AML, MM, MDS, Waldenström’s macroglobulinemia, or WM, and certain solid tumors. Furthermore, the FDA has granted five ODDs to Lisaftoclax, specifically for the treatment of patients with follicular lymphoma, or FL, WM, CLL, MM, and AML.
The following table summarizes the registrational trials completed or ongoing for Lisaftoclax:
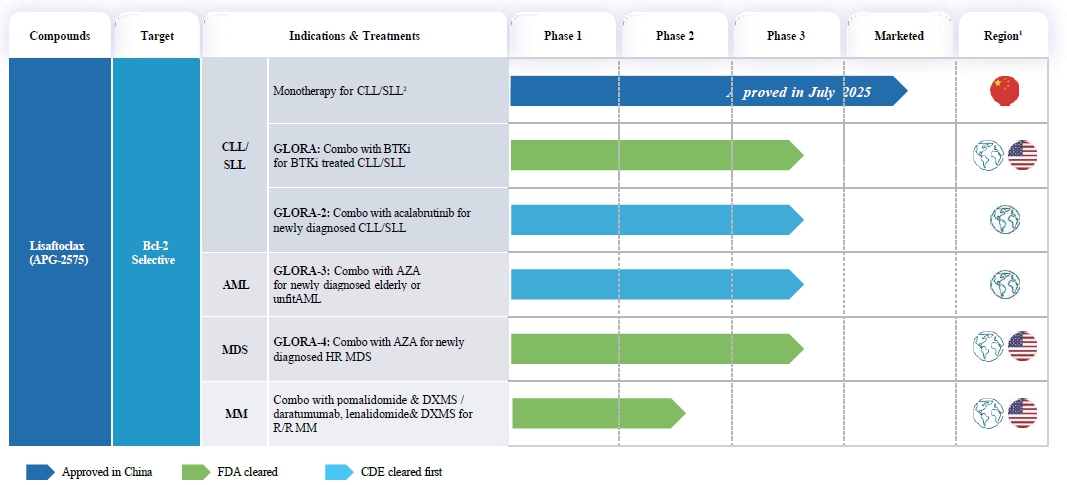
| 1. | The globe icon as used in this table refers to trials that are currently taking place in at least two countries. The U.S. flag refers to trials for which we have received clearance from the FDA to conduct trials in the United States. The China flag refers to trials for which we have conducted or currently conduct only in China. |
| 2. | CLL/SLL patients who have previously received at least one systemic therapy including BTK inhibitors. |
A summary of recent progress of Lisaftoclax is as follows:
Commercial progress
| 1. | On July 10, 2025, Lisaftoclax was approved by China’s NMPA for the treatment of adult patients with CLL/SLL who have previously received at least one systemic therapy including BTK inhibitors, which makes Lisaftoclax the first Bcl-2 inhibitor receiving conditional approval and marketing authorization for the treatment of patients with CLL/SLL in China, and the second Bcl-2 inhibitor approved globally. Shortly after the approval, we have commenced the commercial sales of Lisaftoclax in China. |
| 2. | In April 2025, Lisaftoclax received its first recommendation in the CSCO Guidelines for the Diagnosis and Treatment of Lymphoid Malignancies, as a monotherapy for the treatment of patients with relapsed/refractory (R/R) CLL/SLL. |
Clinical progress
| 1. | We continue enrollment in a global, multi-center, registrational Phase III clinical trial, called GLORA-4, of Lisaftoclax in combination with AZA for the treatment of patients who are newly diagnosed with HR MDS. GLORA-4 trial has been cleared by the FDA and EMA. |
| 2. | We continue enrollment in a registrational Phase III clinical trial, called GLORA-3, of Lisaftoclax in combination with AZA for the treatment of newly diagnosed old or unfit patients with AML. |
| 3. | We continue enrollment in a registrational Phase III clinical trial, called GLORA-2, to evaluate Lisaftoclax in combination with the BTK inhibitor acalabrutinib, versus immunochemotherapy in treatment-naïve patients with CLL/SLL, to validate a fixed duration of combination regimen as a first-line treatment. |
| 4. | We continue enrollment in an FDA-cleared registrational Phase III clinical trial, called GLORA, of Lisaftoclax in combination with BTK inhibitors in patients with CLL/SLL previously treated with BTK inhibitors. |
| 5. | We continue enrollment in the Phase Ib/II clinical trials of Lisaftoclax in combination with other therapies for the treatment of patients with MM in the United States. |
| 6. | Phase Ib/II studies of Lisaftoclax as a single agent or in combination with other therapies for the treatment of patients with AML/MDS are ongoing in China. |
| 7. | Phase Ib/II studies of Lisaftoclax in combination with other therapies for the treatment of patients with AML/MDS are also ongoing in the United States. |
| 8. | A Phase Ib/II study of Lisaftoclax, both as a single agent and in combination with the BTK inhibitor ibrutinib or combination with rituximab for the treatment of patients with WM, is ongoing in the United States, Australia, and China. |
Data publications
| 1. | In June 2025, we presented updated results of Lisaftoclax combined with AZA in patients with myeloid malignancies that are treatment-naïve (TN) or that have had prior venetoclax exposure. We presented this data in an oral presentation at the 61st ASCO Annual Meeting. This is an ongoing multi-country, multi-center Phase Ib/II study of Lisaftoclax, which as of data cutoff in April 2025, had enrolled a total of 103 patients, including patients with TN or R/R AML or MDS. The data of this study once again underscored the promising antitumor activity and manageable tolerability of Lisaftoclax in myeloid malignancies. This study reported that Lisaftoclax was able to achieve tumor responses in patients for the first time that are refractory to venetoclax. Specifically, in efficacy-evaluable venetoclax-refractory patients with R/R AML/Mixed Phenotype Acute Leukemia, or MPAL, the overall response rate (ORR) was 31.8%, suggesting that Lisaftoclax has a favorable antitumor profile and is differentiated from other drugs within the same class. This is also the third consecutive year in which this study of Lisaftoclax was selected for presentations at the ASCO Annual Meeting. |
Expected progress of Lisaftoclax
| 1. | We plan to initiate clinical studies to confirm Lisaftoclax’s potential to overcome venetoclax resistance in patients who have failed venetoclax treatment. |
APG-2449
APG-2449 is a novel, orally active, small-molecule inhibitor of focal adhesion kinase, or FAK, a third generation inhibitor of anaplastic lymphoma kinase, or ALK, and an inhibitor of receptor tyrosine kinase C-ros oncogene 1, or ROS1. It is a triple ligase kinase inhibitor designed and developed by Ascentage Pharma. It is the first FAK inhibitor approved by CDE for clinical studies in China. A first-in-human trial, cerebrospinal fluid pharmacokinetics or PK analyses showed that APG-2449 was brain-penetrant. An updated study of APG-2449 demonstrated preliminary clinical benefit in patients with NSCLC whose disease was TKI naïve and resistant to second-generation ALK inhibitors, especially in those with brain metastases. In addition, high phosphorylated FAK, or pFAK, expression levels in baseline tumor tissue correlated with improved APG-2449 treatment responses in patients with NSCLC resistant to second-generation ALK inhibitors, suggesting that the increase in pFAK levels may be associated with second-generation ALK TKI resistance.
Recent progress of APG-2449 is as follows:
Clinical progress
| 1. | Two CDE-cleared registrational Phase III clinical trials are ongoing that are separately evaluating APG-2449 in patients with NSCLC who are resistant to or intolerant of second- generation ALK TKIs and treatment-naïve patients with ALK-positive advanced or locally advanced NSCLC. |
| 2. | A Phase 1b/2 study of APG-2449 in combination with liposomal doxorubicin hydrochloride in platinum-resistant ovarian cancer is ongoing. |
Data publications
| 1. | In April 2025, we released updated preclinical data of APG-2449 at AACR 2025, demonstrating enhanced antitumor activity with chemotherapy in preclinical models of small- cell lung cancer, or SCLC, with activated FAK. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET APG-2449 SUCCESSFULLY.
Alrizomadlin (APG-115)
Alrizomadlin (APG-115) is a novel, orally bioavailable, small-molecule inhibitor of mouse double minute 2-p53 homolog, or MDM2-p53, designed to be highly specific for disruption of the protein- protein interaction of MDM2 and p53 in order to restore activation of p53 tumor suppressor activity. It is undergoing multiple clinical studies in China, the United States, and Australia as a single agent or in combination with immunotherapy or chemotherapy for treating solid tumors and hematologic malignancies.
The FDA has granted six ODDs for alrizomadlin for the treatment of soft-tissue sarcoma, gastric cancer, AML, retinoblastoma, stage IIB-IV melanoma, and neuroblastoma. In addition, alrizomadlin has been granted two Rare Pediatric Disease Designations, or RPDD designations by the FDA for the treatment of neuroblastoma and retinoblastoma.
Recent progress of alrizomadlin is as follows:
Clinical progress
We are currently advancing the following clinical studies of alrizomadlin in the United States and/ or Australia:
| 1. | A Phase Ib/II study of alrizomadlin monotherapy or in combination with pembrolizumab in patients with unresectable or metastatic melanoma (in collaboration with Merck & Co.) or other advanced solid tumors. |
| 2. | A Phase IIa study evaluating the pharmacokinetics, safety and efficacy of alrizomadlin as a single agent or in combination with Lisaftoclax in subjects with relapsed/refractory T-cell Prolymphocytic Leukemia, or R/R T-PLL, or NHL. |
| 3. | A collaborative research study of alrizomadlin monotherapy or in combination with chemotherapy in a Phase II study for the treatment of salivary gland cancer. |
In addition, the CDE has granted approval for the following clinical trials of alrizomadlin in China:
| 1. | A Phase Ib/II clinical study of alrizomadlin in combination with anti-PD-1 antibody (JS001) toripalimab, for the treatment of patients with advanced liposarcoma (LPS) or other advanced solid tumors. |
| 2. | A Phase Ib study of alrizomadlin as a single agent or in combination with azacitidine or cytarabine in patients with R/R AML and relapsed/progressed high-/very high-risk MDS. |
| 3. | A Phase I clinical study of alrizomadlin alone or in combination with Lisaftoclax in children with recurrent or refractory neuroblastoma or other solid tumors. |
Data publications
| 1. | In June 2025, we released clinical data from our Phase II study of alrizomadlin as a single agent or in combination with PD-1 inhibitor toripalimab in patients with advanced adenoid cystic carcinoma, or ACC, or other solid tumors in a poster presentation at the 61st ASCO Annual Meeting. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET ALRIZOMADLIN (APG-115) SUCCESSFULLY.
Pelcitoclax (APG-1252)
Pelcitoclax is a novel, highly potent, small-molecule drug candidate designed to restore apoptosis through dual inhibition of the Bcl-2/Bcl-xL proteins for the treatment of SCLC, NSCLC, neuroendocrine tumor and NHL. It was granted an ODD by the FDA for the treatment of SCLC.
In various clinical trials conducted in the United States, Australia and China, patients have been treated with pelcitoclax as a monotherapy or in combination with other antitumor agents. Pelcitoclax has been well tolerated in patients to date using either weekly or biweekly intermittent dosing schedules. Preliminary antitumor activity was observed as a single agent in heavily pretreated patients.
Recent progress of pelcitoclax is as follows:
Clinical progress
Pelcitoclax is currently under investigation in a variety of combination trials, including:
| 1. | A Phase Ib study of pelcitoclax plus osimertinib in patients with epidermal growth factor receptor, or EGFR, mutant NSCLC in China; |
| 2. | A Phase Ib/II study of pelcitoclax as a single agent or in combination with other therapeutic agents in patients with R/R NHL in China. |
| 3. | A Phase I study of pelcitolclax in combination with cobimetinib in recurrent ovarian and endometrial cancers. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET PELCITOCLAX (APG-1252) SUCCESSFULLY.
APG-5918
APG-5918 is a potent, orally bioavailable, and highly selective embryonic ectoderm development, or EED, inhibitor. EED is a core subunit of the Polycomb Repressive Complex 2, or PRC2. Preliminary study results from our preclinical models of anemia demonstrated that APG-5918 has the potential to improve hemoglobin or Hb insufficiency induced by chronic kidney disease, or CKD.
We have initiated an FDA-regulated, multi-center, open-label Phase I clinical trial to evaluate the safety, pharmacokinetics, and efficacy of APG-5918 in patients with advanced solid tumors or lymphomas, including NHL, who have progressed while on or are intolerant to approved therapies, or for whom no standard treatments are available.
Recent progress of APG-5918 is as follows:
Clinical progress
| 1. | We continue the ongoing Phase I clinical trial of APG-5918 for the treatment of patients with advanced solid tumors and hematologic malignancies in China and the U.S. |
| 2. | We continue the Phase I clinical trial of APG-5918 for the treatment of patients with anemia- related indications in China. The first part of the single ascending dose, or SAD, study in healthy subjects has been completed, and the second part of multiple ascending dose, or MAD, phase in anemic subjects is ongoing. |
Data publications
| 1. | In June 2025, we released the preclinical results of APG-5918 at EHA 2025, demonstrating that APG-5918 exhibits potent antitumor activity and synergizes with histone deacetylase inhibitor tucidinostat in preclinical T-cell lymphoma, or TCL, models. |
| 2. | In April 2025, we released preclinical data of APG-5918 at AACR 2025, demonstrating that APG-5918 alone or in combination with enzalutamide is a promising therapeutic strategy for the treatment of patients with prostate cancer. |
Cautionary Statement required by Rule 18A.05 of the Listing Rules: WE MAY NOT BE ABLE TO ULTIMATELY DEVELOP AND MARKET APG-5918 SUCCESSFULLY.
Discovery programs
Protein degraders
Our deep understanding of heterobifunctional molecules and ligase biology has allowed us to develop protein degraders targeting traditionally undruggable proteins of interest implicated in key oncologic pathways. We believe we have the ability to develop differentiated degraders with superior pharmacokinetic-pharmacodynamic, or PK/PD, profiles resulting in less off-target effects than other degraders in clinical development. Through our degrader platform, we also believe we can develop cancer therapeutics targeted at resistance mechanisms that have traditionally plagued small molecule inhibitors.
We have identified and nominated our first targeted protein degrader, or TPD, candidate for pre-clinical development. This orally bioavailable degrader is targeting the p53-MDM2 pathway. In the last twenty years, many highly potent and orally active MDM2 inhibitors have been developed as a way to activate the p53 tumor suppressor gene, and several are currently in clinical development, including alrizomadlin. However, inhibition of p53 often leads to upregulation of MDM2, which, in turn, has limited the efficacy of MDM2 inhibitors evaluated by others to date. Therefore, we believe that a degrader approach has the potential to be a transformative new strategy against these key oncology targets.
We have also identified several compounds that are capable of rapidly reducing the levels of the Bcl-xL protein in human cancer cell lines and thereby inhibiting cancer cell growth that is dependent on Bcl-xL. Based on our initial studies, we believe our Bcl-xL protein degrader approach has the potential to demonstrate strong antitumor activity along with low levels of platelet toxicity. We are in the process of selecting and nominating our first Bcl-xL degrader as a candidate for pre-clinical development. The potential candidates exhibit high selectivity for the Bcl-xL target, demonstrating potent cellular and degradation activity, and showing remarkable in vivo efficacy in xenograft mice models.
RESEARCH AND DEVELOPMENT
We have a proven track record of accomplishment in research discovery, global clinical development, and commercialization of novel biopharmaceuticals directed towards cancer. We plan to continue to diversify and expand our product pipeline through both in-house research and development and collaboration with biotechnology and pharmaceutical companies, as well as academic institutions. We have an experienced scientific advisory board, or SAB, chaired by Dr. Shaomeng Wang, our co-founder and non-executive Director. Members of our SAB are physician scientists with expertise in cancer research and drug development. They are not our employees but periodically provide us with assistance and guide our clinical development programs through regularly scheduled SAB meetings.
For the six months ended June 30, 2024 and 2025, our research and development expenses were RMB444.1 million and RMB528.6 million, respectively.
INTELLECTUAL PROPERTY RIGHTS
Intellectual property rights are fundamental to our business. Through our robust research and development, we have strategically developed a global intellectual property portfolio with exclusive rights to issue patents or patent applications worldwide with respect to our products and product candidates. As of June 30, 2025, we cumulatively had 478 issued patents globally, this total includes over 20 new patents issued during the reporting period, while excluding the expiration and abandonment of certain patents unrelated to our core product portfolio. 342 issued patents were issued outside of China as of June 30, 2025.
COMMERCIALIZATION
Ascentage Pharma is executing its dual-engine commercialization strategy.
We achieved robust revenue growth in the first half of 2025, and we are confident of extending the growth in the second half, driven by the expanded coverage of Olverembatinib in the NRDL since the beginning of 2025 and commercial launch of Lisaftoclax in July 2025. As of July 31, 2025, we have a fully operational commercialization team in China consisting of more than 140 staff members and our commercialization effort in China covers over 1,000 hospitals across the country. With Lisaftoclax’s differentiated clinical profile, our established commercial capabilities and market leading pipeline in hematological oncology, we plan to accelerate market penetration for Lisaftoclax, which is the first Bcl-2 inhibitor receiving conditional approval and marketing authorization for the treatment of patients with CLL/SLL in China.
Revenue from sales of Olverembatinib in China was RMB217.4 million for the six months ended June 30, 2025, compared to RMB112.9 million for the six months ended June 30, 2024, which represented an increase of RMB104.5 million, or 93%. The strong revenue growth was primarily driven by the expanded coverage in NRDL, which has begun to cover CML-CP patients who are resistant and/or intolerant to first and second-generation TKIs since the beginning of 2025. Continued acceleration of new patient prescriptions and extension of the duration of treatment will support sustained growth of Olverembatinib in the future.
Our team, together with Innovent Biologics, Inc. (1801.HK), currently cover approximately 867 hospitals and 290 distributors in China. During the six months ended of June 30, 2025, we have entered 782 DTP pharmacies and hospitals, increased approximately 17% at this point compared to June 30, 2024. In particular, the number of hospitals where Olverembatinib is on formulary increased approximately 47% to 295 hospitals as of June 30, 2025 from 201 hospitals as of June 30, 2024. We will continue to collaborate with Innovent to accelerate market penetration, laying a solid foundation for accessibility for newly approved and pipeline products and indications.
Olverembatinib was included in 2025 version of the China Anti-Cancer Association (CACA) guidelines and 2025 version of CSCO guidelines for the treatment of CML and Ph+ ALL. In April 2025, Olverembatinib received an upgraded recommendation in the 2025 version of “CSCO Guidelines for the Diagnosis and Treatment of Leukemias in Children and Adolescent” for children with Ph+ ALL who harbor the T315I BCR-ABL1 kinase domain mutation. Olverembatinib was included as an Emerging Treatment Option in the 2024 NCCN guidelines for the management of CML and included in the updated 2025 European LeukemiaNet Recommendations. Ascentage Pharma is committed to the expansion of commercialization and accessibility of Olverembatinib in the China market and abroad.
Lisaftoclax, our second product, was approved by China NMPA on July 10, 2025. We have commenced commercialization of Lisaftoclax in China with our fully in-house commercialization team. The first prescription in China of Lisaftoclax was issued just 15 days after CDE approval, demonstrating Ascentage Pharma’s speed and efficiency to market. We are committed to accelerating Lisaftoclax’s market entry to obtain a competitive edge and secure market leading advantage for its approved indication.
Lisaftoclax has been recommended in the 2025 CSCO Guidelines for the Diagnosis and Treatment of Lymphoma for the monotherapy of patients with relapsed/refractory CLL/SLL, based on its outstanding clinical data. This marks the first inclusion of Lisaftoclax in the CSCO Guidelines and makes it the only originally developed in China Bcl-2 inhibitor to receive CSCO guidelines recommendation. It represents a landmark step for Ascentage Pharma in advancing this innovative drug to truly benefit patients and a major breakthrough for China drug development innovation in the field of hematological oncology.
CHEMISTRY, MANUFACTURING AND CONTROLS
We have established our own Suzhou facility as our global R&D center and manufacturing facility. The R&D center and the manufacturing center was commissioned into use in the second half of 2021 and the fourth quarter of 2022, respectively.
The Suzhou manufacturing center has more than 200,000 square feet of space, and the manufacturing capacity for both oral solid tablet and capsule formulations is up to 250 million dosage units per year. We also maintain manufacturing capability at the Suzhou center for injectable drug products, including lyophilized formulations. In the fourth quarter of 2022, we obtained a Drug Manufacturing License (Certificate A). In 2024, the Suzhou manufacturing center completed the technical transfer and process validation campaign of Olverembatinib tablets. At the same time, we obtained the updated version of the Drug Manufacturing Licenses (including certificates A, B and C) and passed GMP compliance inspection conducted by Jiangsu Medical Products Administration which allows our facility to manufacture and supply Olverembatinib oral solid tablets for supply for global clinical trials as well as for commercial sales in the China market.
In April 2023, we received a zero-deficiency report from the Good Manufacturing Practices (GMP) compliance audit of Ascentage Pharma’s global manufacturing center by a Qualified Person (QP) of the European Union (EU). We believe this report indicates that our Global Manufacturing Center and quality management system implemented at the site are compliant with the standards of the EU GMP, marking the achievement of a major milestone that will pave the way for our continued global expansion.
In 2023, we completed the technical transfer of the Lisaftoclax tablets, which allows for the in-house production and supply of the drug for our global clinical trials. We completed the drug tablet coating, debossing development, and the GMP production of Olverembatinib tablets, thereby preparing for future applications to the global regulatory authorities including the FDA.
In the first half of 2025, Lisaftoclax pre-approval inspections for both drug substance and drug product were successfully completed through collaboration of Ascentage Pharma and Contract Development and Manufacturing Organizations, or CDMOs, which facilitated Lisaftoclax NDA approval in China.
In addition, we leased a facility with a size of approximately 50,000 square feet for R&D and manufacturing in China Medical City, Taizhou, Jiangsu Province, China, where we produce and supply preclinical test articles and clinical trial materials for some of our drug candidates. We believe that the existing facilities are adequate for our current needs.
BUSINESS DEVELOPMENT
In addition to our strong in-house research and development team, we have established global collaboration and other relationships with leading biotechnology and pharmaceutical companies as well as academic institutions. We will continue to seek partnerships to maximize the value of our pipeline products.
On June 14, 2024, Ascentage Pharma, Ascentage HK, Ascentage GZ, Ascentage SZ and Takeda entered into an Exclusive Option Agreement, pursuant to which we granted Takeda an exclusive option to enter into an exclusive license agreement for Olverembatinib. If exercised, the Option would allow Takeda to license global rights to develop and commercialize Olverembatinib in all territories outside of the PRC, Hong Kong, Macau, Taiwan and Russia. Pursuant to the Exclusive Option Agreement, Ascentage Pharma shall be solely responsible for all clinical development of Olverembatinib before the potential exercise of the Option. The Exclusive Option Agreement calls for Ascentage to receive an option payment of US$100 million related to intellectual property income and option payment under the Exclusive Option Agreement. Additionally, Ascentage Pharma is eligible for an option exercise fee as additional potential milestone payments of up to approximately US$1.2 billion plus a 12%-19% royalty rate based on annual net sales. On July 2, 2024, Ascentage Pharma received the option payment related to intellectual property income and option payment under the Exclusive Option Agreement.
The Exclusive Option Agreement would allow Ascentage Pharma to leverage the global commercial expertise of Takeda with a proven record of accomplishment and global oncology footprint to potentially broaden the impact that Olverembatinib could have on patients worldwide.
Additionally, on June 20, 2024, pursuant to the securities purchase agreement dated June 14, 2024 between us and Takeda, Ascentage Pharma issued and allotted to Takeda 24,307,322 Shares (Takeda Shares) at a price per share equal to HK$24.09850 per Share (equivalent to approximately US$3.08549), and with the aggregate purchase price of US$75 million (equivalent to approximately HK$585.77 million). The Share Purchase Price represents a 25.12% premium to the 20-day average closing price of the Shares prior to the date of the Securities Purchase Agreement (being HK$19.26 per Share). Pursuant to the Securities Purchase Agreement, Takeda agreed to certain lock-up arrangements in connection with the Shares until June 20, 2025. Specifically, under the lock-up arrangement, Takeda agreed that, subject to certain exceptions, for a period of 180 days after January 23, 2025, they will not, sell or otherwise transfer or dispose of any Takeda Shares or any securities convertible into or exchangeable for our ordinary shares.
For further details on the Exclusive Option Agreement, the Securities Purchase Agreement and the transactions contemplated thereunder, please refer to our relevant announcements dated June 14, 2024, June 21, 2024 and July 4, 2024.
FINANCING ACTIVITIES
Completion of the U.S. Initial Public Offering
On January 28, 2025, we completed our U.S. initial public offering in which we offered and sold an aggregate 7,325,000 ADSs at an offer price of US$17.25 per ADS, representing 29,300,000 ordinary shares of the Company for gross proceeds of approximately US$126.4 million (equivalent to approximately HK$983.8 million). On February 13, 2025, in connection with the underwriters’ exercise of their over-allotment option, we issued an additional 935,144 ADSs at an offer price of US$17.25 per ADS, representing 3,740,576 ordinary shares of the Company for gross proceeds of approximately US$16.13 million (equivalent to approximately HK$125.6 million). Each ADS represents 4 ordinary shares. Our ADSs are listed on the Nasdaq Global Market, or the Nasdaq, under the symbol “AAPG.”
Therefore, we issued a total of 8,260,144 ADSs (representing 33,040,576 ordinary shares). After the issuance, the total number of our issued and outstanding ordinary shares increased from 315,226,005 shares to 348,266,581 shares. The aggregate gross proceeds raised under the offering were approximately US$142.5 million (equivalent to approximately HK$1,109.4 million). The net proceeds under the offering were approximately US$132.5 million (equivalent to approximately HK$1,031.8 million) after deduction of the underwriting discounts and commissions of approximately US$10.0 million (equivalent to approximately HK$77.7 million).
For details, please refer to the announcements issued by the Company on December 29, 2024, January 21, 2025, January 24, 2025, February 2, 2025, and February 13, 2025.
The 2025 Placing
On July 25, 2025, we issued and sold 22,000,000 subscription Shares (being the same number as the sale Shares) to the Vendor at HK$68.60 per subscription Share (being the same as the placing price). The net proceeds from the subscription amount were approximately HK$1,492.5 million (approximately US$190.1 million based on an exchange rate of 1 USD to 7.85 HKD).
We expect to use the net proceeds from the 2025 Placing in the following manner:
| (i) | approximately 40% will be used for commercialization efforts, including expanding coverage and improving patient access; |
| (ii) | approximately 35% will be used for global clinical development to advance the core pipeline candidates of the Company; and |
| (iii) | approximately 25% will be used for infrastructure and working capital to strengthen global operations. |
For details, please refer to the announcements issued by the Company on July 15, 2025 and July 25, 2025.
Save as disclosed above, there was no fund raising activity carried out by the Company during the Reporting Period.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF PROFIT OR LOSS
For the six months ended June 30, 2025
| Notes | 2025 (Unaudited) RMB’000 |
2024 (Unaudited) RMB’000 |
||||||||
| REVENUE | 5 | 233,699 | 823,746 | |||||||
| Cost of sales | (21,650 | ) | (15,059 | ) | ||||||
| Gross profit | 212,049 | 808,687 | ||||||||
| Other income and gains | 6 | 36,661 | 17,346 | |||||||
| Selling and distribution expenses | (137,787 | ) | (89,637 | ) | ||||||
| Administrative expenses | (99,685 | ) | (86,988 | ) | ||||||
| Research and development expenses | (528,561 | ) | (444,079 | ) | ||||||
| Other expenses | (40,192 | ) | (7,106 | ) | ||||||
| Finance costs | (27,798 | ) | (34,076 | ) | ||||||
| Share of profit/(loss) of a joint venture | 1 | (1,252 | ) | |||||||
| (LOSS)/PROFIT BEFORE TAX | 7 | (585,312 | ) | 162,895 | ||||||
| Income tax expense | 8 | (5,512 | ) | (69 | ) | |||||
| (LOSS)/PROFIT FOR THE PERIOD | (590,824 | ) | 162,826 | |||||||
| Attributable to: | ||||||||||
| Ordinary equity holders of the Company | (590,768 | ) | 163,001 | |||||||
| Non-controlling interests | (56 | ) | (175 | ) | ||||||
| (590,824 | ) | 162,826 | ||||||||
| (LOSS)/EARNINGS PER SHARE ATTRIBUTABLE TO ORDINARY EQUITY HOLDERS OF THE COMPANY | 10 | |||||||||
| Basic | (1.73 | ) | 0.56 | |||||||
| Diluted | (1.73 | ) | 0.55 | |||||||
INTERIM CONDENSED CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME OR LOSS
For the six months ended June 30, 2025
| 2025 (Unaudited) RMB’000 |
2024 (Unaudited) RMB’000 |
|||||||
| (LOSS)/PROFIT FOR THE PERIOD | (590,824 | ) | 162,826 | |||||
| OTHER COMPREHENSIVE (LOSS)/INCOME | ||||||||
| Other comprehensive income that may be reclassified to profit or loss in subsequent periods: | ||||||||
| Exchange differences on translation of foreign operations | 1,095 | 40 | ||||||
| Other comprehensive income that will not be reclassified to profit or loss in subsequent periods: | ||||||||
| Exchange differences on translation of the Company | (2,035 | ) | 2,229 | |||||
| OTHER COMPREHENSIVE (LOSS)/INCOME FOR THE PERIOD, NET OF TAX | (940 | ) | 2,269 | |||||
| TOTAL COMPREHENSIVE (LOSS)/INCOME FOR THE PERIOD | (591,764 | ) | 165,095 | |||||
| Attributable to: | ||||||||
| Ordinary equity holders of the Company | (591,708 | ) | 165,270 | |||||
| Non-controlling interests | (56 | ) | (175 | ) | ||||
| (591,764 | ) | 165,095 | ||||||
INTERIM CONDENSED CONSOLIDATED STATEMENT OF FINANCIAL POSITION
June 30, 2025
| Notes | 30 June 2025 (Unaudited) RMB’000 |
31 December
2024 (Audited) RMB’000 |
||||||||
| NON-CURRENT ASSETS | ||||||||||
| Property, plant and equipment | 11 | 821,201 | 849,450 | |||||||
| Right-of-use assets | 50,760 | 56,109 | ||||||||
| Goodwill | 24,694 | 24,694 | ||||||||
| Other intangible assets | 70,994 | 75,998 | ||||||||
| Investment in a joint venture | 32,718 | 32,717 | ||||||||
| Financial assets at fair value through profit or loss (“FVTPL”) | 4,617 | 1,141 | ||||||||
| Deferred tax assets | 33,385 | 44,236 | ||||||||
| Other non-current assets | 99,055 | 59,303 | ||||||||
| Total non-current assets | 1,137,424 | 1,143,648 | ||||||||
| CURRENT ASSETS | ||||||||||
| Inventories | 8,591 | 6,597 | ||||||||
| Trade receivables, net | 12 | 78,362 | 83,143 | |||||||
| Prepayments, other receivables and other assets | 160,313 | 123,211 | ||||||||
| Cash and bank balances | 1,661,454 | 1,261,211 | ||||||||
| Total current assets | 1,908,720 | 1,474,162 | ||||||||
| CURRENT LIABILITIES | ||||||||||
| Trade payables | 13 | 118,676 | 91,966 | |||||||
| Other payables and accruals | 249,358 | 258,098 | ||||||||
| Contract liabilities | 37,485 | 37,485 | ||||||||
| Interest-bearing bank and other borrowings | 833,783 | 779,062 | ||||||||
| Total current liabilities | 1,239,302 | 1,166,611 | ||||||||
| NET CURRENT ASSETS | 669,418 | 307,551 | ||||||||
| TOTAL ASSETS LESS CURRENT LIABILITIES | 1,806,842 | 1,451,199 | ||||||||
| Notes | 30 June 2025 (Unaudited) RMB’000 |
31 December 2024 (Audited) RMB’000 |
||||||||
| NON-CURRENT LIABILITIES | ||||||||||
| Contract liabilities | 229,628 | 248,460 | ||||||||
| Interest-bearing bank and other borrowings | 882,382 | 889,435 | ||||||||
| Deferred tax liabilities | – | 5,368 | ||||||||
| Deferred income | 6,500 | 27,500 | ||||||||
| Other non-current liabilities | 12,423 | 6,274 | ||||||||
| Total non-current liabilities | 1,130,933 | 1,177,037 | ||||||||
| Net assets | 675,909 | 274,162 | ||||||||
| EQUITY | ||||||||||
| Equity attributable to ordinary equity holders of the Company | ||||||||||
| Share capital | 14 | 239 | 214 | |||||||
| Treasury shares | (2,960 | ) | (8 | ) | ||||||
| Reserves | 668,718 | 263,988 | ||||||||
| 665,997 | 264,194 | |||||||||
| Non-controlling interests | 9,912 | 9,968 | ||||||||
| Total equity | 675,909 | 274,162 | ||||||||
NOTES TO INTERIM CONDENSED CONSOLIDATED FINANCIAL INFORMATION
June 30, 2025
| 1. | CORPORATE AND GROUP INFORMATION |
The Company is a limited liability company incorporated in the Cayman Islands on November 17, 2017. The registered office of the Company is located at the office of Walkers Corporate Limited, with the registered address of 190 Elgin Avenue, George Town, Grand Cayman KY1-9008, Cayman Islands.
The Company is a global biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global medical needs primarily in hematological malignancies.
| 2. | BASIS OF PREPARATION |
The interim condensed consolidated financial information for the six months ended June 30, 2025 has been prepared in accordance with IAS 34 Interim Financial Reporting. The interim condensed consolidated financial information does not include all the information and disclosures required in the annual financial statements, and should be read in conjunction with the Group’s annual consolidated financial statements for the year ended December 31, 2024.
| 3. | CHANGES IN ACCOUNTING POLICIES |
The accounting policies adopted in the preparation of the interim condensed consolidated financial statements are consistent with those applied in the preparation of the Group’s annual consolidated financial statements for the year ended December 31, 2024, except for the adoption of the following amended IFRS Accounting Standards for the first time for the current period’s financial information.
Amendments to HKAS 21 Lack of Exchangeability
Amendments to HKAS 21 specify how an entity shall assess whether a currency is exchangeable into another currency and how it shall estimate a spot exchange rate at a measurement date when exchangeability is lacking. The amendments require disclosures of information that enable users of financial statements to understand the impact of a currency not being exchangeable. As the currencies that the Group had transacted with and the functional currencies of group entities for translation into the Group’s presentation currency were exchangeable, the amendments did not have any impact on the interim condensed consolidated financial information.
| 4. | OPERATING SEGMENT INFORMATION |
For management purposes, the Group has only one reportable operating segment, which is the development and sales of novel small-scale molecule therapies for cancers, hepatitis B virus, or HBV, and certain age-related diseases. Management monitors the operating results of the Group’s operating segment as a whole for the purpose of making decisions about resource allocation and performance assessment. Therefore, no analysis by operating segment is presented.
Geographical information
| (a) | Revenue from external customers |
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 (Unaudited) |
2024 RMB’000 (Unaudited) |
|||||||
| Mainland China | 233,699 | 145,331 | ||||||
| Switzerland | – | 678,415 | ||||||
| Total | 233,699 | 823,746 | ||||||
The revenue information above is based on the locations of the customers.
| (b) | Non-current assets |
| June 30, | December 31, | |||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| (Unaudited) | (Audited) | |||||||
| Mainland China | 1,095,036 | 1,090,914 | ||||||
| United States | 3,796 | 4,474 | ||||||
| Others | 42 | 444 | ||||||
| Total non-current assets | 1,098,874 | 1,095,832 | ||||||
The non-current asset information above is based on the locations of the assets and excludes financial instruments and deferred tax assets.
Information about major customers
Revenue from a customer amounting to over 10% of the total revenue of the Group for the reporting period is as follows:
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 (Unaudited) |
2024 RMB’000 (Unaudited) |
|||||||
| Customer A | – | 678,415 | ||||||
| Customer B | 219,866 | 110,086 | ||||||
| 5. | REVENUE |
An analysis of revenue is as follows:
Disaggregated revenue information for revenue from contracts with customers
| For the six months ended June 30 |
||||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| (Unaudited) | (Unaudited) | |||||||
| Types of goods or services | ||||||||
| Intellectual property revenue | – | 678,415 | ||||||
| Sales of products | 212,874 | 124,824 | ||||||
| Commercialization rights income | 18,691 | 18,691 | ||||||
| Others | 2,134 | 1,816 | ||||||
| Total | 233,699 | 823,746 | ||||||
| Timing of revenue recognition | ||||||||
| At a point in time | ||||||||
| Intellectual property revenue | – | 678,415 | ||||||
| Sales of products | 212,874 | 124,824 | ||||||
| Over time | ||||||||
| Commercialization rights income | 18,691 | 18,691 | ||||||
| Others | 2,134 | 1,816 | ||||||
| Total | 233,699 | 823,746 | ||||||
The following table shows the amounts of revenue recognized in the current reporting period that was included in the contract liabilities at the beginning of the reporting period:
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| (Unaudited) | (Unaudited) | |||||||
| Type of goods and services | ||||||||
| Commercialization rights income | 18,691 | 18,691 | ||||||
| 6. | OTHER INCOME AND GAINS |
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| (Unaudited) | (Unaudited) | |||||||
| Bank interest income | 31,410 | 9,352 | ||||||
| Government grants related to income | 1,001 | 6,705 | ||||||
| Others | 4,250 | 1,289 | ||||||
| Total | 36,661 | 17,346 | ||||||
| 7. | (LOSS)/PROFIT BEFORE TAX |
The Group’s (loss)/profit before tax is arrived at after charging/(crediting):
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 (Unaudited) |
2024 RMB’000 (Unaudited) |
|||||||
| Cost of inventories sold | 16,479 | 14,158 | ||||||
| Cost of inventory write-down | 4,180 | – | ||||||
| Cost of service provided | 991 | 901 | ||||||
| Depreciation of property, plant and equipment * | 33,272 | 35,936 | ||||||
| Depreciation of right-of-use assets* | 5,515 | 5,709 | ||||||
| Amortization of intangible assets* | 5,003 | 5,667 | ||||||
| Research and development costs | 528,561 | 444,079 | ||||||
| Fair value losses on financial assets at FVTPL | 521 | 504 | ||||||
| Fair value losses on financial liabilities at FVTPL | 29,322 | – | ||||||
| Foreign exchange loss, net | 2,676 | 430 | ||||||
| Equity-settled share-based payment expenses* | 13,048 | 8,730 | ||||||
| Loss on disposal of items of property,plant and equipment | – | 17 | ||||||
| Bank interest income | (31,410 | ) | (9,352 | ) | ||||
| Government grants related to income | (1,001 | ) | (6,705 | ) | ||||
| Donations | 7,653 | 5,104 | ||||||
| * | The depreciation of property, plant and equipment, the depreciation of right-of-use assets, the amortization of intangible assets and the equity-settled share-based payment expenses for the period are included in “Cost of sales”, “Research and development expenses”, “Selling and distribution expenses” and “Administrative expenses” in the unaudited interim condensed consolidated statement of profit or loss. |
| 8. | INCOME TAX |
The Group is subject to income tax on an entity basis on profits arising in or derived from the jurisdictions in which members of the Group are domiciled and operate.
Cayman Islands
Under the current laws of the Cayman Islands, the Company, Ascentage Pharma Group International, is not subject to tax on income or capital gain arising in the Cayman Islands. Additionally, upon payments of dividends by the Company to its shareholders, no Cayman Islands withholding tax will be imposed.
Hong Kong
The subsidiaries incorporated in Hong Kong are subject to income tax at the rate of 16.5% on the estimated assessable profits arising in Hong Kong. For the six months ended June 30, 2024 and 2025, the Company did not make any provisions for Hong Kong profits tax as there were no assessable profits derived from or earned in Hong Kong for any of the periods presented.
Mainland China
The Company’s subsidiaries domiciled in the PRC are subject to tax at the statutory rate of 25%, in accordance with the Enterprise Income Tax law (the “EIT Law”), which was effective since January 1, 2008, except for the following entity which is eligible for a preferential tax rate.
Guangzhou Healthquest Pharma Co., Ltd. and Suzhou Yasheng Pharma Co., Ltd. were qualified as High and New Technology Enterprise (“HNTE”) and were subject to tax at a preferential rate of 15% since 2022 and 2023, respectively.
Dividends, interest, rent or royalties payable by the Company’s PRC subsidiaries, to non-PRC resident enterprises, and proceeds from any such non-resident enterprise investor’s disposal of assets (after deducting the net value of such assets) shall be subject to 10% withholding tax, unless the respective non-PRC resident enterprise’s jurisdiction of incorporation has a tax treaty or arrangements with China that provides for a reduced withholding tax rate or an exemption from withholding tax.
United States
The subsidiary operating in the United States is subject to tax at a maximum of 21% for the six months ended June 30, 2024 and 2025. No provision for income tax has been made as the Group had no assessable profits earned in the United States during the reporting period.
A new requirement to capitalize and amortize previously deductible research and experimental expenses resulting from a change in Section 174 made by the Tax Cuts and Jobs Act of 2017 (the “TCJA”) became effective on January 1, 2022. Under the TCJA, the Company is required to capitalize, and subsequently amortize R&D expenses over five years for research activities conducted within the United States and fifteen years for research activities conducted outside of the United States.
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 (Unaudited) |
2024 RMB’000 (Unaudited) |
|||||||
| Current | 29 | – | ||||||
| Deferred | 5,483 | 69 | ||||||
| Total | 5,512 | 69 | ||||||
| 9. | DIVIDENDS |
The board of directors resolved not to declare any interim dividend for the six months ended June 30, 2025 (six months ended June 30, 2024: Nil).
No dividends were paid during the six months ended June 30, 2025 (six months ended June 30, 2024: Nil).
| 10. | (LOSS)/EARNINGS PER SHARE ATTRIBUTABLE TO ORDINARY EQUITY HOLDERS OF THE PARENT |
The calculation of the basic earnings per share amount is based on the profit for the six months ended June 30, 2025 attributable to ordinary equity holders of the parent, and the weighted average number of ordinary shares of 341,591,027 (six months ended June 30, 2024: 291,752,282) outstanding during the period.
The calculation of the diluted earnings per share amount is based on the profit for the period attributable to ordinary equity holders of the Company. The weighted average number of ordinary shares used in the calculation is the number of ordinary shares outstanding during the period, as used in the basic earnings per share calculation, and the weighted average number of ordinary shares assumed to have been issued at no consideration on the deemed conversion of all dilutive potential ordinary shares into ordinary shares.
The calculation of basic and diluted (loss)/earnings per share is based on:
| 2025 | 2024 | |||||||
| RMB’000 | RMB’000 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| (LOSS)/EARNINGS | ||||||||
| (Loss)/profit attributable to ordinary equity holders of the Company,used in the basic and diluted (loss)/earnings per share calculation | (590,768 | ) | 163,001 | |||||
| Number of shares | ||||||||
| 2025 | 2024 | |||||||
| Shares | ||||||||
| Weighted average number of ordinary shares outstanding during the period used in the basic (loss)/earnings per share calculation | 341,591,027 | 291,752,282 | ||||||
| Effect of dilution – weighted average number of ordinary shares: | ||||||||
| RSU | – | 994,365 | ||||||
| Share options | – | 3,277,849 | ||||||
| Total | 341,591,027 | 296,024,496 | ||||||
No adjustment has been made to the basic loss per share amounts presented for the period ended June 30, 2025 in respect of a dilution as the impact of the options and RSU had an anti-dilutive effect on the basic loss per share amount presented.
The weighted average number of shares was after taking into account the effect of treasury shares held.
| 11. | PROPERTY, PLANT AND EQUIPMENT |
| RMB’000 (Unaudited) |
||||
| Carrying value at January 1, 2025 | 849,450 | |||
| Additions | 5,024 | |||
| Depreciation charge for the period | (33,272 | ) | ||
| Exchange realignment | (1 | ) | ||
| Carrying value at June 30, 2025 | 821,201 | |||
| RMB’000 | ||||
| (Unaudited) | ||||
| Carrying value at January 1, 2024 | 905,815 | |||
| Additions | 12,336 | |||
| Disposals | (17 | ) | ||
| Depreciation charge for the period | (35,936 | ) | ||
| Carrying value at June 30, 2024 | 882,198 | |||
During the six months ended June 30, 2025, no impairment loss (six months ended June 30, 2024: Nil) was recognized for property, plant and equipment.
| 12. | TRADE RECEIVABLES |
An ageing analysis of the trade receivables as at the end of the reporting period, based on the invoice date, is as follows:
| June 30, 2025 RMB’000 (Unaudited) |
December 31, 2024 RMB’000 (Audited) |
|||||||
| Within 45 days | 70,300 | 54,484 | ||||||
| 45 to 120 days | – | 28,659 | ||||||
| 120 days to 1 year | 8,062 | – | ||||||
| Total | 78,362 | 83,143 | ||||||
| 13. | TRADE PAYABLES |
An ageing analysis of the trade payables as at the end of the reporting period, based on the invoice date, is as follows:
| June 30, 2025 RMB’000 (Unaudited) |
December 31, 2024 RMB’000 (Audited) |
|||||||
| Within 1 month | 74,137 | 72,506 | ||||||
| 1 to 3 months | 21,972 | 6,288 | ||||||
| 3 to 6 months | 22,567 | 13,172 | ||||||
| Total | 118,676 | 91,966 | ||||||
| 14. | SHARE CAPITAL |
In January 2025, the Company has been listed globally on Nasdaq which offered 8,260,144 American depositary shares or ADSs. The initial public offering price of the ADSs is US$17.25 per ADS and each ADS represents four of the Company’s ordinary shares, and an amount of RMB25,000 was credited as share capital.
During the six months ended June 30, 2025, the Company issued ordinary shares with respect to the share options under the pre-IPO share option scheme exercised by certain grantees of the Company. In connection with the exercised share options, 422,447 new shares of the Company were issued with the weighted average exercise price of HK$0.01, and an amount of RMB300 was credited as share capital.
In June 2025, the Company issued ordinary shares with respect to the restricted share units under the 2021 RSU Scheme and 2022 RSU exercised by certain selected persons of the Company before June 30, 2025 to those selected persons. In connection with the exercised restricted share units, 311,304 new shares of the Company were issued, and an amount of RMB220 was credited as share capital.
FINANCIAL REVIEW
Six Months Ended June 30, 2025 Compared to Six Months Ended June 30, 2024
| For the six months ended June 30, |
||||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| Revenue | 233,699 | 823,746 | ||||||
| Other income and gains | 36,661 | 17,346 | ||||||
| Selling and distribution expenses | (137,787 | ) | (89,637 | ) | ||||
| Research and development expenses | (528,561 | ) | (444,079 | ) | ||||
| Administrative expenses | (99,685 | ) | (86,988 | ) | ||||
| Finance costs | (27,798 | ) | (34,076 | ) | ||||
| Other expenses | (40,192 | ) | (7,106 | ) | ||||
| (Loss)/profit for the period | (590,824 | ) | 162,826 | |||||
| Total comprehensive (loss)/income for the period | (591,764 | ) | 165,095 | |||||
| 1. | Overview |
For the six months ended June 30, 2025, the Group recorded revenue of RMB233.7 million, as compared with RMB823.7 million for the six months ended June 30, 2024, and the total comprehensive loss of RMB591.8 million, as compared with the total comprehensive income of RMB165.1 million for the six months ended June 30, 2024. The loss of the Group was RMB590.8 million for the six months ended June 30, 2025, as compared with the profit of RMB162.8 million for the six months ended June 30, 2024. The selling and distribution expenses of the Group was RMB137.8 million for the six months ended June 30, 2025, as compared with RMB89.6 million for the six months ended June 30, 2024. The research and development expenses of the Group was RMB528.6 million for the six months ended June 30, 2025, as compared with RMB444.1 million for the six months ended June 30, 2024. The administrative expenses of the Group was RMB99.7 million for the six months ended June 30, 2025, as compared with RMB87.0 million for the six months ended June 30, 2024.
| 2. | Revenue |
For the six months ended June 30, 2025, the Group generated revenue of RMB233.7 million from sales of pharmaceutical products, commercialization rights income from Innovent Suzhou and service income, as compared to RMB823.7 million for the six months ended June 30, 2024 representing an decrease of RMB590.0 million, or 71.6%.
| 3. | Other Income and Gains |
The Group’s other income and gains primarily consist of (i) interest income on time deposit at banks; and (ii) government grants related to income. Government grants related to income mainly represent the subsidies received from local governments for the purpose of compensation for expenses arising from research activities and clinical trials, and awards for new drugs development. These government grants related to income were recognized in profit or loss when related costs were subsequently incurred and upon receipt of the acknowledgment of compliance from the government.
Other income and gains for the six months ended June 30, 2025 was RMB36.7 million, as compared to RMB17.3 million for the six months ended June 30, 2024, representing an increase of RMB19.4 million, or 111.4%, which was primarily attributable to (i) the increase in bank interest income to RMB31.4 million for the six months ended June 30, 2025, as compared with RMB9.4 million for the six months ended June 30, 2024; and (ii) the increase in rental income to RMB3.2 million for the six months ended June 30, 2025, as compared with RMB0.4 million for the six months ended June 30, 2024.
| 4. | Selling and Distribution Expenses |
The Group’s selling and distribution expenses primarily consist of marketing expenses, staff costs and travel and meeting expenses.
For the six months ended June 30, 2025, the selling and distribution expenses of the Group increased by RMB48.2 million, or 53.7%, to RMB137.8 million, as compared to RMB89.6 million for the six months ended June 30, 2024. The increase was attributable to the increase in selling and distribution expenses incurred in the commercialization of Olverembatinib and other products.
| 5. | Research and Development Expenses |
The Group’s research and development expenses primarily consist of internal research and development expenses, external research and development expenses, staff costs, IP expenses, materials, depreciation and amortization and RSU expenses of research and development staff.
For the six months ended June 30, 2025, the research and development expenses of the Group increased by RMB84.5 million, or 19.0% to RMB528.6 million from RMB444.1 million for the six months ended June 30, 2024. The increase was primarily attributable to increased external research and development expenses.
The following table sets forth the components of our research and development expenses by nature for the periods indicated.
| For
the six months ended June 30, |
||||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| Internal research and development expenses | 217,376 | 185,729 | ||||||
| External research and development expenses | 79,297 | 43,622 | ||||||
| Staff costs | 173,472 | 156,345 | ||||||
| IP expenses | 4,417 | 4,100 | ||||||
| Materials | 12,826 | 12,860 | ||||||
| Depreciation and amortization | 13,553 | 17,304 | ||||||
| Share option and RSU expenses of R&D staff | 7,928 | 7,287 | ||||||
| Others | 19,692 | 16,832 | ||||||
| Total | 528,561 | 444,079 | ||||||
| 6. | Administrative Expenses |
For the six months ended June 30, 2025, the administrative expenses of the Group increased by RMB12.7 million, or 14.6% to RMB99.7 million from RMB87.0 million for the six months ended June 30, 2024. The increase was primarily attributable to the increased consulting fee and agency fees.
The following table sets forth the components of our administrative expenses for the periods indicated.
| For
the six months ended June 30, |
||||||||
| 2025 RMB’000 |
2024 RMB’000 |
|||||||
| Share option and RSU expenses | 1,201 | 1,161 | ||||||
| Staff costs | 35,431 | 32,502 | ||||||
| Depreciation and amortization | 24,949 | 25,645 | ||||||
| Others | 38,104 | 27,680 | ||||||
| Total | 99,685 | 86,988 | ||||||
| 7. | Finance Costs |
Finance costs represented mainly interest expenses from bank borrowings and lease liabilities.
For the six months ended June 30, 2025, the finance costs of the Group decreased by RMB6.3 million, or 18.4% to RMB27.8 million from RMB34.1 million for the six months ended June 30, 2024. The decrease was primarily attributable to lower interest rate incurred in relation to bank borrowings.
| 8. | Other Expenses |
The Group’s other expenses mainly consisted of donations and fair value adjustment.
For the six months ended June 30, 2025, the Group reported other expenses of RMB40.2 million, as compared to other expenses of RMB7.1 million for the six months ended June 30, 2024, which represented an increase of RMB33.1 million, or 465.6%. The increase was primarily attributable to (i) the increase in fair value loss of contingent consideration related to acquisition of Guangzhou Healthquest Pharma Co., Ltd. to RMB29.3 million, and (ii) the increase in donations to RMB7.7 million for the six months ended June 30, 2025, as compared to RMB5.1 million for the six months ended June 30, 2024.
| 9. | (Loss)/profit for the Reporting Period |
As a result of the foregoing, the loss of the Company increased by RMB753.7 million, to RMB590.8 million for the six months ended June 30, 2025 from the profit of RMB162.8 million for the six months ended June 30, 2024.
| 10. | Cash Flows |
For the six months ended June 30, 2025, net cash outflows used in operating activities of the Group amounted to RMB432.1 million, as compared to that of RMB354.4 million for the six months ended June 30, 2024, mainly due to the decrease of intellectual property income and option payment.
For the six months ended June 30, 2025, net cash outflows used in investing activities of the Group amounted to RMB704.5 million, which consisted of (i) the net increase in property, plant and equipment and other intangible assets of RMB20.0 million; (ii) the net increase in time deposits of RMB637.1 million; (iii) the net increase in purchase of equity investments of RMB4.0 million; and (iv) net increase in contingent consideration related to Guangzhou Healthquest Pharma Co., Ltd of RMB43.4 million. For the six months ended June 30, 2024, net cash outflow from investing activities amounted to RMB131.3 million, which consisted of (i) the net increase in property, plant and equipment and other intangible assets of RMB16.5 million; (ii) the net increase in investment in a joint ventures of RMB16.0 million; and (iii) the net increase in time deposits of RMB98.8 million.
For the six months ended June 30, 2025, net cash inflows from financing activities of the Group amounted to RMB950.7 million, which mainly consisted of (i) net proceeds arising from the initial public offering on Nasdaq of RMB950.2 million; (ii) net proceeds of bank loans which amounted to RMB50.3 million; and (iii) interest paid which amounted to RMB26.2 million. For the six months ended June 30, 2024, net cash inflows from financing activities amounted to RMB396.9 million, which mainly consisted of (i) net proceeds arising from the 2024 Share Subscription of RMB533.9 million; (ii) net repayment of bank loans which amounted to RMB93.7 million; and (iii) interest paid which amounted to RMB33.2 million.
| 11. | Key Financial Ratios |
The following table sets forth the key financial ratios for the periods indicated:
| As
at June 30, |
As
at December 31, |
|||||||
| 2025 | 2024 | |||||||
| Current ratio(1) | 1.5 | 1.3 | ||||||
| Quick ratio(2) | 1.5 | 1.3 | ||||||
| Gearing ratio(3) | 8.2 | % | 154.2 | % | ||||
Notes:
| (1) | Current ratio is calculated using current assets divided by current liabilities as at the same date. |
| (2) | Quick ratio is calculated using current assets less inventories and divided by current liabilities as at the same date. |
| (3) | Gearing ratio is calculated using interest-bearing borrowings less cash and cash equivalents divided by total equity and multiplied by 100%. The decrease was primarily attributable to (i). the increase of cash and bank equivalent from RMB1,261.2 million for the year ended December 31, 2024 to RMB1,661.5 million for the six months ended June 30, 2025; and (ii) the increase of total equity from RMB264.2 million for for the year ended December 31, 2024 to RMB666.0 million for the six months ended June 30, 2025. |
| 12. | Significant Investments |
During the Reporting Period, there were no significant investments held by the Group.
| 13. | Foreign Exchange Risk |
Our financial statements are expressed in RMB, but certain of our cash and bank balances, other receivables and other assets, other investments classified as financial assets measured at FVTPL and trade and other payables are denominated in foreign currencies, and are exposed to foreign currency risk. We currently do not have a foreign currency hedging policy. However, the management monitors foreign exchange exposure and will consider hedging significant foreign currency exposure should the need arise.
| 14. | Material Acquisitions and Disposals |
The Group did not have any material acquisitions or disposals of subsidiaries, consolidated affiliated entities, associated companies or joint ventures for the six months ended June 30, 2025.
| 15. | Bank Loans and Other Borrowings |
As at June 30, 2025, we had bank loans of RMB1,689.7 million denominated in RMB and lease liabilities of RMB26.4 million.
As at June 30, 2025, RMB142.5 million of the Group’s borrowings were at fixed interest rates.
June 30, 2025
| Effective interest rate per annum (%) | Maturity | RMB’000 | ||||||
| Current | ||||||||
| Short-term borrowing – unsecured | 2.20-2.70 or 1 year LPR-0.30 to 0.75 | 2025-2026 | 442,000 | |||||
| Current portion of long term bank loans – unsecured | 2.80-4.50 | 2025-2026 | 97,500 | |||||
| Current portion of long term bank loans – unsecured | 1 year LPR-0.15 to 0.75 or 1 year LPR+0.65 to 0.85 |
2025-2026 | 266,665 | |||||
| Current portion of long term bank loans – secured* | 5 year-LPR-0.85 | 2025-2026 | 16,440 | |||||
| Lease liabilities | 4.00-4.35 | 2025-2026 | 11,178 | |||||
| Subtotal | 833,783 | |||||||
| Non-current | ||||||||
| Bank loans – unsecured | 1 year LPR-0.25 to 0.75 or 1 year LPR+0.70 to 0.85 | 2026-2028 | 228,670 | |||||
| Bank loans – unsecured | 2.80 | 2026-2027 | 45,000 | |||||
| Bank loans – secured* | 5 year-LPR-0.85 | 2026-2038 | 593,441 | |||||
| Lease liabilities | 4.00-4.35 | 2026-2028 | 15,271 | |||||
| Subtotal | 882,382 | |||||||
| Total | 1,716,165 | |||||||
| Note: | LPR stands for the Loan Prime Rate. |
| * | The bank loans amounting to RMB609,881,000 (December 31, 2024: RMB599,745,000) were secured by the pledge of the Group’s buildings with a net carrying amount of approximately RMB711,482,000 (December 31, 2024: RMB731,282,000) and right-of-use assets with a net carrying amount of approximately RMB25,903,000 (December 31, 2024: RMB26,468,000) as at June 30, 2025. Such loans were also guaranteed by two of the Group’s subsidiaries. |
The unsecured bank loans amounting to RMB207,935,000 (December 31, 2024: RMB278,070,000) were guaranteed by the Group’s subsidiaries as at June 30, 2025.
The following table sets forth the maturity analysis of the Group’s interest-bearing bank and other borrowings:
| June
30, 2025 |
December 31, 2024 |
|||||||
| RMB’000 | RMB’000 | |||||||
| Analysed into: | ||||||||
| Within one year | 833,783 | 779,062 | ||||||
| In the second year | 248,714 | 242,473 | ||||||
| In the third to fifth years, inclusive | 152,534 | 159,355 | ||||||
| Beyond five years | 481,134 | 487,607 | ||||||
| Total | 1,716,165 | 1,668,497 | ||||||
| 16. | Charges on Group Assets |
As at June 30, 2025, the Group had pledged the Group’s right-of-use assets with a carrying amount of approximately RMB25.9 million, the buildings with a carrying amount of approximately RMB711.5 million.
| 17. | Contingent Liabilities |
As at June 30, 2025, the Group did not have any material contingent liabilities.
| 18. | Liquidity and Financial Resources |
The Group adopts a conservative approach for cash management and investment on uncommitted funds. We place cash and cash equivalents (which are mostly held in U.S. dollars, Hong Kong dollars and RMB) in short time deposits with authorized institutions in Hong Kong and China.
As at June 30, 2025, the Group’s cash and bank balances was RMB1,661.5 million, which remained relatively constant when compared with RMB1,261.2 million as at December 31, 2024.
As at June 30, 2025, the Group’s cash and bank balances were held mainly in U.S. dollars, Hong Kong dollars and RMB.
As at June 30, 2025, the Group had not used any financial instruments for hedging purposes.
| 19. | Employees and Remuneration Policies |
The following table sets forth a breakdown of our employees as at June 30, 2025 by function:
| Function | Number | % | ||||||
| Research and Development | 421 | 69.6 | ||||||
| Commercial | 112 | 18.5 | ||||||
| Administrative and others | 72 | 11.9 | ||||||
| Total | 605 | 100.0 | ||||||
As at June 30, 2025, we had 605 full-time employees, including a total of 85 employees with M.D. or Ph.D. degrees. Of these, 421 are engaged in full-time research and development and laboratory operations and 184 are engaged in full-time general and administrative and commercial functions, and business development function. Our research and development personnel includes 81 employees with M.D. or Ph.D. degrees, and many of them have experience working in research institutions and hospitals and in the FDA drug approval process.
Our senior management team has extensive experience and expertise in the biotechnology industry and has been contributive in driving the success of our business. As at June 30, 2025, we had 98 senior employees who have an average of 15 to 20 years of experience in relevant fields.
We have also enjoyed more than 82% retention rate of employee over the last two years, which facilitates the growth of our institutional knowledge base. We are actively recruiting talents globally by offering a collaborative work environment, competitive compensation, effective incentive plans, and the opportunity to work on cutting-edge science projects.
Our employees’ remuneration comprises salaries, bonuses, employee provident fund and social security contributions and other welfare payments. In accordance with applicable Chinese laws, we have made contributions to social security insurance funds (including pension plans, medical insurance, work-related injury insurance, unemployment insurance and maternity insurance) and housing funds for our PRC-based employees. For the six months ended June 30, 2024 and 2025, employee benefit expense amounted to RMB218.9 million and RMB234.6 million, respectively.
The Company has also adopted the Pre-IPO Share Option Scheme, the Post IPO Share Option Scheme, the 2018 RSU Scheme, the 2021 RSU Scheme and the 2022 RSU Scheme.
On June 27, 2025, an aggregate of 824,124 RSUs, representing 824,124 Shares, have been further granted under the 2021 RSU Scheme to 439 selected persons (the “2021 Selected Persons”) of the 2021 RSU Scheme (the “2021 Further Grant”), who are employees of the Group. None of the 2021 Selected Persons is a Director, chief executive or substantial shareholder of the Company or an associate of any of them. The 2021 Further Grant would not result in the options and awards granted and to be granted to each individual grantee in the 12-month period up to and including the date of such grant in aggregate to exceed 1% of the Shares in issue (excluding treasury Shares). As such, the 2021 Further Grant will not be subject to approval by the Shareholders in accordance with Rule 17.03D(1) of the Listing Rules.
On June 27, 2025, 816,922 RSUs (the “2022 Awards”), representing 816,922 Shares, have been further granted under the 2022 RSU Scheme to 78 selected persons (the “2022 Selected Persons”) of the 2022 RSU Scheme (the “2022 Further Grant”), among which 176,278 RSUs, representing 176,278 Shares, were granted to Dr. Zhai Yifan (“Dr. Zhai”), who is the chief medical officer and a substantial shareholder of the Company. Pursuant to Rule 17.04(1) of the Listing Rules, the grant of 2022 Awards to Dr. Zhai under the 2022 Further Grant had been approved by the independent non-executive Directors. The grant of 2022 Awards to Dr. Zhai under the 2022 Further Grant would not result in the Shares issued and to be issued in respect of all options and awards granted to Dr. Zhai (excluding any options and awards lapsed in accordance with the terms of the applicable scheme) in the 12-month period up to and including the date of such grant representing in aggregate over 0.1% of the issued Shares (excluding treasury Shares). As such, the grant of 2022 Awards to Dr. Zhai under the 2022 Further Grant will not be subject to approval by the Shareholders pursuant to Rule 17.04(4) of the Listing Rules. Save as disclosed above, none of the 2022 Selected Persons is a Director, chief executive or substantial shareholder of the Company or an associate of any of them. The 2022 Further Grant would not result in the options and awards granted and to be granted to each individual grantee in the 12-month period up to and including the date of such grant in aggregate to exceed 1% of the Shares in issue (excluding treasury Shares). As such, the grant of 2022 Awards to the 2022 Selected Persons other than Dr. Zhai under the 2022 Further Grant will also not be subject to approval by the Shareholders in accordance with Rule 17.03D(1) of the Listing Rules.
For further details of the Pre-IPO Share Option Scheme and the Post-IPO Share Option Scheme, please refer to the section headed “Statutory and General Information – D. Employee Incentive Schemes” in Appendix IV to the Prospectus. For further details of the 2018 RSU Scheme and the grant of RSUs thereunder, please refer to the prospectus of the Company dated October 16, 2019 and the relevant announcements of the Company dated February 2, 2021, May 29, 2023 and October 24, 2024. For further details of the 2021 RSU Scheme and the grant of RSUs thereunder, please refer to the relevant announcements of the Company dated February 2, 2021, May 21, 2021, June 18, 2021, June 25, 2021, July 14, 2021, July 23, 2021, May 29, 2023 and June 27, 2025 as well as the circulars of the Company dated August 31, 2021 and April 30, 2025 and the poll results announcements of the Company dated September 20, 2021 and May 19, 2025. For further details of the 2022 RSU Scheme and the grant of RSUs thereunder, please refer to the relevant announcements of the Company dated June 23, 2022, July 14, 2022, May 8, 2023, May 29, 2023, October 24, 2024 and June 27, 2025.
FUTURE AND OUTLOOK
Leveraging our extensive experience in the global biotechnology industry, we will continue to accelerate our development of six drug candidates in our highly differentiated novel clinical pipeline to next phases and apply for NDAs across the globe.
We will invest more resources to support our key product development through accelerating clinical trial sites development, boosting clinical trial recruitment and increasing material communications with competent authorities. Meanwhile, we also expect to report significant near-term milestones for several key products in global academic conferences on our encouraging preclinical or clinical data, so as to increase our awareness and seek global collaboration opportunities.
We intend to become a fully integrated global biopharmaceutical company with a comprehensive set of capabilities focusing on business development and commercialization beyond our core competency in research and development. In anticipation of the potential commercialization of our drug candidates, we plan to capture additional commercialization opportunities in global pharmaceutical markets through actively pursuing strategic partnerships with global biotechnology and pharmaceutical companies of cooperation over our pipeline assets.
Additionally, we expect to expand our intellectual property portfolio by actively seeking patent rights for our product candidates. We will further enhance our comprehensive and growing global intellectual property portfolio in the future.
Looking forward, we will constantly extend our capability to develop the innovative therapies with better efficacy and affordable costs for patients to address the unmet medical needs, improve patient health and bring benefits to the society globally. At the same time, we will constantly strive to consolidate our position as a leading biotechnology company and maintain good financial health to protect the interests of our Shareholders.
CORPORATE GOVERNANCE AND OTHER INFORMATION
Corporate Governance Practices
We apply the principles and code provisions as set out in the CG Code contained in Appendix C1 to the Listing Rules. Save for the deviation disclosed below, in the opinion of the Directors, the Company has complied with all the code provisions as set out in the CG Code during the Reporting Period.
Pursuant to code provision C.2.1 of the CG Code, companies listed on the Stock Exchange are expected to comply with, but may choose to deviate from the requirement that the responsibilities between the chairman and the chief executive officer should be segregated and should not be performed by the same individual. The Company does not have a separate chairman and chief executive officer, and Dr. Yang currently performs these two roles. The Board believes that such arrangement will not impair the balance of power and authority between the Board and the management of the Company, because (a) decisions to be made by the Board require approval by at least a majority of the Directors and that the Board comprises three independent non-executive Directors, which represents at least one third of the Board composition and satisfies the relevant requirement under the Listing Rules, and we believe that there is sufficient check and balance in the Board; (b) Dr. Yang and other Directors are aware of and undertake to fulfil their fiduciary duties as Directors, which require, among other things, that he acts for the benefit and in the best interests of the Company and will make decisions for the Group accordingly; (c) the balance of power and authority is ensured by the operations of the Board which comprises experienced and high caliber individuals who meet regularly to discuss issues affecting the operations of the Company; and (d) strategic decisions and other key business, financial, and operational policies of the Group are formalized collectively after thorough discussion at both Board and senior management levels.
The Board will continue to review the effectiveness of the corporate governance structure of the Group in order to assess whether separation of the roles of chairman of the Board and chief executive officer is necessary.
Model Code
We have also adopted our own code of conduct regarding securities transactions, namely the policy on management of securities transactions by directors (the “Securities Transactions Code”), which applies to all Directors on terms not less exacting than the required standard indicated by the Model Code.
Upon specific enquiry, all Directors confirmed that they have complied with the Model Code and the Securities Transactions Code during the Reporting Period. In addition, the Company is not aware of any non-compliance of the Model Code and the Securities Transactions Code by the senior management of the Group during the Reporting Period.
Purchase, Sale or Redemption of Listed Securities
During the Reporting Period, neither the Company nor any of its subsidiaries purchased, sold or redeemed any listed securities (including sale of treasury shares (as defined under the Listing Rules)) of the Company. As at June 30, 2025, the Company did not hold any treasury shares.
Use of Net Proceeds
Details of use of net proceeds of fund raising activities carried out by the Company on or before June 30, 2025 are set out below.
Use of Net Proceeds from the Global Offering
With the Shares of the Company listed on the Stock Exchange on October 28, 2019, the net proceeds from the Global Offering (including shares issued as a result of the full exercise of the over-allotment option) were approximately HK$369.8 million. There was no change in the intended use of net proceeds as previously disclosed in the Prospectus and as at June 30, 2025, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the Global Offering and the actual usage up to June 30, 2025.
| Use of proceeds | Planned allocation of net proceeds | Planned allocation of net proceeds | Planned allocation of net proceeds | Utilized amount (as at June 30, 2025) | ||||||||||||
| (HKD million) | (RMB million) | (RMB million) | ||||||||||||||
| Research and development to bring our Core Product, HQP1351, to commercialization | 42 | % | 155.2 | 138.2 | 138.2 | |||||||||||
| Ongoing and planned clinical trials of APG-1252 | 13 | % | 48.1 | 42.8 | 42.8 | |||||||||||
| Ongoing and planned clinical trials of Lisaftoclax (APG-2575) | 19 | % | 70.3 | 62.5 | 62.5 | |||||||||||
| Ongoing and planned clinical trials of APG-115 | 19 | % | 70.3 | 62.5 | 62.5 | |||||||||||
| Ongoing and planned clinical trials for the rest of the clinical programs of the Company, APG-1387 and APG-2449 | 6 | % | 22.2 | 19.7 | 19.7 | |||||||||||
| Working capital and general corporate purposes | 1 | % | 3.7 | 3.3 | 3.3 | |||||||||||
| Total | 100.0 | % | 369.8 | 329.1 | 329.1 | |||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the Global Offering were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the Global Offering. |
Use of Net Proceeds From the 2020 Placing
The closing of the 2020 Placing of 15,000,000 Shares took place on July 15, 2020. The net proceeds (after the deduction of all applicable costs and expenses) raised from the 2020 Placing were approximately HK$689.5 million. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated July 8, 2020 and as at June 30, 2025, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the 2020 Placing and the actual usage up to June 30, 2025.
| Use of proceeds | Planned allocation of net proceeds | Planned allocation of net proceeds | Planned allocation of net proceeds | Utilized
amount (as at June 30, 2025) |
||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | ||||||||||||||
| Clinical development for other pipeline products, such as Lisaftoclax (APG-2575), APG-115, APG-1387 and APG-1252 | 60 | % | 413.5 | 345.0 | 345.0 | |||||||||||
| Registration, trial production and marketing of the Core Product, HQP1351 | 20 | % | 138.0 | 115.0 | 115.0 | |||||||||||
| Ongoing and planned clinical trials of Lisaftoclax (APG-2575) | 20 | % | 138.0 | 115.0 | 115.0 | |||||||||||
| Total | 100 | % | 689.5 | 575.0 | 575.0 | |||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the 2020 Placing were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2020 Placing. |
Use of Net Proceeds From the 2021 Placing
On February 3, 2021, the Company entered into the 2021 Placing and subscription agreement with Ascentage Limited (the “Vendor”) and J.P. Morgan Securities (Asia Pacific) Limited and China International Capital Corporation Hong Kong Securities Limited (the “2021 Placing Agents”), pursuant to which (i) the Vendor agreed to appoint the 2021 Placing Agents, and the 2021 Placing Agents agreed to act as agents of the Vendor to procure not less than six placees (the “2021 Placees”), on a best effort basis, to purchase up to 26,500,000 shares of the Company (the “2021 Placing Shares”) at the price of HK$44.2 per 2021 Placing Share; and (ii) the Vendor agreed to subscribe for, and the Company agreed to issue to the Vendor up to 26,500,000 new shares of the Company at the price of HK$44.2 per subscription Share (the “2021 Subscription”). The closing of the 2021 Placing took place on February 8, 2021 and the closing of the 2021 Subscription took place on February 11, 2021. A total of 26,500,000 placing Shares have been successfully placed by the 2021 Placing Agents to the 2021 Placees. A total of 26,500,000 subscription Shares had been allotted and issued to the Vendor pursuant to the general mandate granted to the Directors at the AGM held on June 19, 2020. The net proceeds (after the deduction of all applicable costs and expenses) raised from the 2021 Placing were approximately HK$1,153.64 million. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated February 3, 2021 and as at June 30, 2025, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the 2021 Placing and the actual usage up to June 30, 2025.
| Use of proceeds | Planned
allocation of net proceeds |
Planned
allocation of net proceeds |
Planned
allocation of net proceeds |
Utilized
amount (as at June 30, 2025) |
||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | ||||||||||||||
| Clinical development of the key product candidate, APG-2575 | 50 | % | 576.8 | 480.6 | 480.6 | |||||||||||
| Registrational trials for full approval and the commercialization of the Core Product, HQP1351 | 20 | % | 230.7 | 192.2 | 192.2 | |||||||||||
| Clinical development for other pipeline products such as APG-115 (MDM2-p53 inhibitors currently in Phase Ib/II clinical trial), APG-1387 (pan-IAP inhibitor currently in Phase Ib/II clinical trial) and APG-1252 (Bcl-2/Bcl-xL dual inhibitor currently in Phase I clinical trial) | 20 | % | 230.7 | 192.2 | 192.2 | |||||||||||
| General corporate purposes | 10 | % | 115.4 | 96.1 | 96.1 | |||||||||||
| Total | 100 | % | 1,153.6 | 961.1 | 961.1 | |||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the 2021 Placing were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2021 Placing. |
Use of Net Proceeds From the 2023 Placing
On January 18, 2023, the Company entered into the 2023 Placing and subscription agreement with Ascentage Limited (the “Vendor”) and J.P. Morgan Securities (Asia Pacific) Limited, China International Capital Corporation Hong Kong Securities Limited and Citigroup Global Markets Asia Limited (the “2023 Placing Agents”), pursuant to which (i) the Vendor agreed to appoint the 2023 Placing Agents, and the 2023 Placing Agents agreed to act as agents of the Vendor, to procure not less than six placees (the “2023 Placees”), on a best effort basis, to purchase up to 22,500,000 shares of the Company (the “2023 Placing Shares”) at the price of HK$24.45 per 2023 Placing Share; and (ii) the Vendor agreed to subscribe for, and the Company agreed to issue to the Vendor up to 22,500,000 new shares of the Company at the price of HK$24.45 per subscription Share (the “2023 Subscription”). The closing of the 2023 Placing took place on January 20, 2023 and the closing of the 2023 Subscription took place on February 1, 2023. A total of 22,500,000 placing Shares have been successfully placed by the 2023 Placing Agents to the 2023 Placees. A total of 22,500,000 subscription Shares have been allotted and issued to the Vendor pursuant to the generate mandate granted to the Directors by the Shareholders at the annual general meeting of the Company held on May 19, 2022. The net proceeds (after the deduction of all applicable costs and expenses) raised from the 2023 Placing were approximately HK$543.9 million. There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated January 18, 2023 and the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the 2023 Placing and the actual usage up to June 30, 2025.
| Use of proceeds | Planned
allocation of net proceeds |
Planned
allocation of net proceeds |
Planned
allocation of net proceeds |
Utilized
amount (as at June 30, 2025) |
Unutilized
amount (as at June 30, 2025) |
||||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | (RMB million) | ||||||||||||||||
| Clinical trials of the key product candidate APG-2575 | 50 | % | 272.0 | 235.1 | 235.1 | 0 | |||||||||||||
| Clinical trials of the core product HQP1351 | 20 | % | 108.8 | 94.0 | 94.0 | 0 | |||||||||||||
| Clinical development of other key product candidates | 20 | % | 108.8 | 94.0 | 94.0 | 0 | |||||||||||||
| General corporate purposes | 10 | % | 54.4 | 47.0 | 47.0 | 0 | |||||||||||||
| Total | 100 | % | 544.0 | 470.1 | 470.1 | 0 | |||||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the 2023 Placing were received in Hong Kong dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2023 Placing. |
Use of Net Proceeds From the Subscription of Shares by Innovent
Innovent has subscribed for 8,823,863 Shares at a total consideration of HK$388.25 million (being approximately US$50 million) and at the subscription price of HK$44.0 per Share. The completion of the subscription of Shares by Innovent took place on July 23, 2021. The net proceeds (after the deduction of all applicable costs and expenses) raised from the subscription of Shares by Innovent were approximately HK$388.06 million (being approximately US$49.98 million). There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated July 14, 2021 and as at June 30, 2025, the Company has fully utilized the net proceeds in accordance with such intended purposes.
The table below sets out the planned applications of the net proceeds from the subscription of Shares by Innovent and the actual usage up to June 30, 2025.
| Use of proceeds | Planned
allocation of net proceeds |
Planned
allocation of net proceeds |
Planned
allocation of net proceeds |
Utilized
amount (as at June 30, 2025) |
Unutilized
amount (as at June 30, 2025) |
||||||||||||||
| (HK$ million) | (RMB million) | (RMB million) | (RMB million) | ||||||||||||||||
| Development and commercialization of the Company’s Core Product, HQP1351 | 30 | % | 116.42 | 97.10 | 97.10 | 0 | |||||||||||||
| Development of the Company’s key product candidate, APG-2575 | 70 | % | 271.64 | 226.40 | 226.40 | 0 | |||||||||||||
| Total | 100 | % | 388.06 | 323.50 | 323.50 | 0 | |||||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | Net proceeds from the subscription of Shares by Innovent were received in Hong Kong dollars and translated to RMB for application planning. |
Use of Net Proceeds from the 2024 Share Subscription
On June 20, 2024, pursuant to the Securities Purchase Agreement with Takeda, we issued and sold to Takeda 24,307,322 of our ordinary shares, or the Takeda Shares, at a price per share equal to HK$24.09850 (equivalent to approximately US$3.08549), for an aggregate consideration of US$75,000,000 (equivalent to approximately HK$585.77 million). The purchase price per shares in the 2024 Share Subscription is HK$24.09850. The closing price of the Shares on June 14, 2024, being the date on which the terms of the Securities Purchase Agreement was fixed, was HK$23.05.
The number of shares in the 2024 Share Subscription represents approximately 8.37% of the then existing issued share capital of the Company and approximately 7.73% of the then enlarged issued share capital of the Company.
All the Share Subscription Conditions Precedent have been satisfied and the Closing took place on June 20, 2024 (after trading hours). An aggregate of 24,307,322 subscription Shares have been successfully allotted and issued by the Company to Takeda at the Share Purchase Price of HK$24.09850 (equivalent to approximately US$3.08549) per subscription Share pursuant to the terms and conditions of the Securities Purchase Agreement.
The gross proceeds raised from the 2024 Share Subscription is US$75,000,000 (equivalent to approximately HK$585.77 million) and the net proceeds (after deducting all applicable costs and expenses) arising from the 2024 Share Subscription amount to approximately US$75,000,000 (equivalent to approximately HK$585.77 million). There was no change in the intended use of the net proceeds as previously disclosed in the relevant announcement of the Company dated June 14, 2024 and the Company will gradually utilize the net proceeds in accordance with such intended purposes.
The strategic equity investment in the Company by Takeda by way of the 2024 Share Subscription is expected to provide further financial support to the Company’s global clinical development programs.
The table below sets out the planned applications of the net proceeds from the 2024 Share Subscription and the actual usage up to June 30, 2025.
| Use of proceeds | Planned allocation of net proceed |
Planned allocation of net proceed |
Balance of the unutilized amount (as at December 31, 2024) |
Utilized |
Utilized amount (as at June 30, 2025) |
Unutilized amount (as at June 30, 2025) |
Expected timeline for utilizing the remaining balance of net proceeds from the 2024 Share Subscription |
|||||||||||||||||||||||
| (US$ million) | (RMB million) | (RMB million) | (RMB million) | (RMB million) | (RMB million) | |||||||||||||||||||||||||
| Development of the Company’s Core Product, HQP1351 and the Company’s key product candidate, APG-2575 | 90 | % | 67.5 | 480.3 | 115.5 | 70.2 | 422.2 | 45.3 | December 31, 2025 | |||||||||||||||||||||
| Development of the Company’s other key product candidates | 10 | % | 7.5 | 53.3 | 12.8 | 7.8 | 46.9 | 5.0 | December 31, 2025 | |||||||||||||||||||||
| Total | 100 | % | 75 | 533.6 | 128.3 | 78.0 | 469.1 | 50.2 | ||||||||||||||||||||||
Notes:
| (1) | The sum of the data may not add up to the total due to rounding. |
| (2) | The expected timeline for utilizing the remaining balance of net proceeds is based on the best estimation of the market conditions made by the Group and it is subject to the research and development progress of the Group. |
| (3) | Net proceeds from the 2024 Share Subscription were received in U.S. dollars and translated to RMB for application planning. The plan was adjusted slightly due to the fluctuation of the exchange rate since the 2024 Share Subscription. |
Use of Net Proceeds from the U.S. Initial Public Offering
On January 28, 2025, we completed our U.S. initial public offering in which we offered and sold an aggregate 7,325,000 ADSs at an offer price of US$17.25 per ADS, representing 29,300,000 ordinary shares of the Company for gross proceeds of approximately US$126.4 million (equivalent to approximately HK$983.8 million). On February 13, 2025, in connection with the underwriters’ exercise of their over-allotment option, we issued an additional 935,144 ADSs at an offer price of US$17.25 per ADS, representing 3,740,576 ordinary shares of the Company for gross proceeds of approximately US$16.13 million (equivalent to approximately HK$125.6 million). Each ADS represents 4 ordinary shares. Our ADSs are listed on the Nasdaq under the symbol “AAPG.”
Therefore, we issued a total of 8,260,144 ADSs (representing 33,040,576 ordinary shares). After the issuance, the total number of our issued and outstanding ordinary shares increased from 315,226,005 shares to 348,266,581 shares. The aggregate gross proceeds raised under the offering were approximately US$142.5 million (equivalent to approximately HK$1,109.4 million). The net proceeds under the Offering were approximately US$132.5 million (equivalent to approximately HK$1,031.8 million) after deduction of the underwriting discounts and commissions of approximately US$10.0 million (equivalent to approximately HK$77.7 million).
There is no change in our intended use of the net proceeds from our U.S. initial public offering as previously disclosed in our announcements dated February 2, 2025 and February 13, 2025.
For details, please refer to the announcements issued by the Company on December 29, 2024, January 21, 2025, January 24, 2025, February 2, 2025, and February 13, 2025.
The table below sets out the planned applications of the net proceeds from the offering and the actual usage up to June 30, 2025.
| Use of proceeds | Planned allocation of net proceed |
Planned allocation of net proceed |
Utilized amount during the Reporting Period |
Utilized amount (as at June 30, 2025) |
Unutilized amount (as at June 30, 2025 |
Expected
timeline for utilizing the remaining balance of net proceeds from the offering |
||||||||||||||||||
| (US$ million) | (RMB million) | (RMB million) | (RMB million) | (RMB million) | ||||||||||||||||||||
| To pursue NDA approval of Lisaftoclax for R/R CLL in China and to prepare for commercial launch in China, advance the clinical development of Lisaftoclax in the United States and other countries, including completing enrollment for GLORA and pursuing clearance with regulatory authorities to add new trial sites in multiple countries and to pursue additional indications for Lisaftoclax | 50.0-60.0 | 398.4 | 92.2 | 92.2 | 306.2 | June 30, 2026 | ||||||||||||||||||
| To advance the clinical development of Olverembatinib in the United States and other countries, including completing enrollment for POLARIS-2 and pursuing clearance with regulatory authorities to add new trial sites in multiple countries, and to expand the label of Olverembatinib into earlier lines and other indications | 30.0-40.0 | 253.5 | 58.7 | 58.7 | 194.9 | June 30, 2026 | ||||||||||||||||||
| To fund the research and development of our other product candidates, including completing the Phase 1 clinical trial for APG-5918 in anemia and pursuing clearance to initiate a registrational trial for alrizomadlin | 10.0-20.0 | 181.1 | 41.9 | 41.9 | 139.2 | June 30, 2026 | ||||||||||||||||||
| For the development of our future pipeline programs and for working capital and general corporate purposes | 17.5 | 126.8 | 29.3 | 29.3 | 97.4 | June 30, 2026 | ||||||||||||||||||
| Total | 132.5 | 959.8 | 222.1 | 222.1 | 737.7 | |||||||||||||||||||
Note: The sum of the data may not add up to the total due to rounding.
2021 WARRANTS
On July 14, 2021, the Company and Innovent entered into a warrant subscription deed, pursuant to which the Company agreed to issue to Innovent 6,787,587 warrants. The initial subscription price of each warrant share upon exercise of the warrants is HK$57.20. The subscription rights attaching to the warrants may be exercised during the period commencing on the date of issuance of the warrants and ending on the date that is 24 months after the date of issuance of the warrants. The warrants have expired in July 2023 and not been exercised.
Audit Committee
The Company has established the Audit Committee with written terms of reference in accordance with the Listing Rules. The Audit Committee comprises two independent non-executive Directors, namely, Mr. Ye Changqing and Ms. Marina S. Bozilenko, and one non-executive Director Dr. Lu Simon Dazhong. Mr. Ye Changqing is the chairman of the Audit Committee.
The unaudited condensed consolidated financial statements of the Group for the six months ended June 30, 2025 and this announcement have been reviewed by the Group’s external auditor, Ernst & Young, in accordance with the Hong Kong Standard on Review Engagements 2410, “Review of Interim Financial Information Performed by the Independent Auditor of the Entity” issued by the Hong Kong Institute of Certified Public Accountants, and by the Audit Committee. The Audit Committee concluded that such financial statements and this announcement had been prepared in accordance with applicable accounting standards and relevant requirements, and had made adequate disclosure. The Audit Committee has also discussed matters with respect to the accounting policies and practices adopted by the Company and internal control with senior management members of the Company.
Future Plans for Material Investments and Capital Assets
Save as disclosed in this announcement, as at the date of this announcement, there were no future plans regarding material investment or capital assets.
EVENTS AFTER THE REPORTING PERIOD
Save for the 2025 Placing as disclosed under the section headed “Financing Activities” above, subsequent to the six months ended June 30, 2025 and up to the date of this announcement, no important events affecting the Company has taken place that is required to be disclosed.
INTERIM DIVIDEND
The Board does not recommend the distribution of an interim dividend for the six months ended June 30, 2025 (six months ended June 30, 2024: Nil).
PUBLICATION OF INTERIM RESULTS ANNOUNCEMENT AND INTERIM REPORT
This announcement is published on the websites of the Stock Exchange (www.hkexnews.hk) and the Company (www.ascentage.com).
The interim report for the six months ended June 30, 2025 containing all the information required by Appendix D2 to the Listing Rules will be provided to the Shareholders and published on the websites of the Stock Exchange and the Company.
APPRECIATION
The Board would like to express its sincere gratitude to the Shareholders, management team, employees, business partners and customers of the Group for their support and contribution to the Group.
DEFINITIONS
Unless the context requires otherwise, the expressions used in this announcement shall have the meanings as follows:
| “2018 RSU Scheme” | the restricted share unit scheme approved by the Board on July 6, 2018 (as amended from time to time) |
| “2020 Placing” | the placing of 15,000,000 Shares at a price of HK$46.80 each pursuant to the terms and conditions of the 2020 Placing Agreement |
| “2020 Placing Agreement” | the placing agreement entered into among the Company, Citigroup Global Markets Limited and J.P. Morgan Securities (Asia Pacific) Limited dated July 8, 2020 in relation to the 2020 Placing |
| “2021 Placing” | the placing and subscription of 26,500,000 Shares at a price of HK$44.20 each pursuant to the terms and conditions of the 2021 Placing Agreement |
| “2021 Placing Agreement” | the placing and subscription agreement entered into among the Company, the Founders SPV, J.P. Morgan Securities (Asia Pacific) Limited and China International Capital Corporation Hong Kong Securities Limited dated February 3, 2021 in relation to the 2021 Placing |
| “2021 RSU Scheme” | the restricted share unit scheme approved by the Board on February 2, 2021 (as amended from time to time) |
| “2022 RSU Scheme” | the restricted share unit scheme approved by the Board on June 23, 2022 (as amended from time to time) |
| “2023 Placing” | the placing and subscription of 22,500,000 Shares at a price of HK$24.45 each pursuant to the terms and conditions of the 2023 Placing Agreement |
| “2023 Placing Agreement” | the placing and subscription agreement entered into among the Company, the Founders SPV, J.P. Morgan Securities (Asia Pacific) Limited, China International Capital Corporation Hong Kong Securities Limited and Citigroup Global Markets Limited dated January 18, 2023 in relation to the 2023 Placing |
| “2024 Share Subscription” | the purchase of the 24,307,322 new Shares issued by the Company under the general mandate by Takeda pursuant to the Securities Purchase Agreement |
| “2025 Placing” | the placing and subscription of 22,000,000 Shares at a price of HK$68.60 each pursuant to the terms and conditions of the 2025 Placing Agreement |
| “2025 Placing Agreement” | the placing and subscription agreement entered into among the Company, Dajun Yang Dynasty Trust, J.P. Morgan Securities (Asia Pacific) Limited and Citigroup Global Markets Limited dated July 14, 2025 in relation to the 2025 Placing |
| “AACR” | American Association for Cancer Research |
| “ADS(s)” | American depositary share(s), each ADS represents 4 Ordinary Shares |
| “AGM” | annual general meeting of the Company |
| “ALK” | anaplastic lymphoma kinase |
| “ALL” | acute lymphoblastic leukemia |
| “AML” | acute myelogenous leukemia |
| “APG-115” | Alrizomadlin, our novel, orally active small molecule MDM2-p53 inhibitor |
| “APG-1252” | Pelcitoclax, our novel, highly potent, small molecule drug designed to restore apoptosis, or programmed cell death, through selective inhibition of the Bcl-2/Bcl-xL proteins |
| “APG-1387” | our novel, small molecule inhibitor of the IAP |
| “APG-2449” | our third-generation inhibitor of the FAK, ROS1 and ALK kinases |
| “APG-2575” | Lisaftoclax (APG-2575), our novel, orally administered Bcl-2 inhibitor |
| “APG-5918” | our potent, orally available, and selective EED inhibitor |
| “ASCO” | American Society of Clinical Oncology |
| “Ascentage” | collectively, Ascentage Pharma, Ascentage HK, Ascentage GZ, Ascentage SZ |
| “Ascentage GZ” or “Guangzhou Healthquest” | Guangzhou Healthquest Pharma Co. Ltd.* (廣州順健生物醫藥科技有限公司), a company established under the laws of the PRC with limited liability and an indirect wholly-owned subsidiary of the Company |
| “Ascentage HK” | Ascentage Pharma Group Corp Limited (亞盛醫藥集團(香港)有限公司), a limited liability company incorporated under the laws of Hong Kong and a wholly-owned subsidiary of the Company |
| “Ascentage SZ” | Suzhou Ascentage Pharma Co., Ltd.* (蘇州亞盛藥業有限公司), a company established under the laws of the PRC with limited liability and an indirect wholly-owned subsidiary of the Company |
| “AstraZeneca” | AstraZeneca PLC, a UK-Swedish multinational pharmaceutical and biopharmaceutical company headquartered in the United Kingdom, an Independent Third Party |
| “Audit Committee” | the audit committee of the Board |
| “AZA” | Azacitidine |
| “Bcl-2” | B-cell lymphoma 2 |
| “Bcl-2/Bcl-xL” | B-cell lymphoma 2/B-cell lymphoma extra-large; a member of the Bcl-2 family proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately, programmed cell death |
| “BCR” | breakpoint cluster region |
| “BCR-ABL” | a fusion gene formed by the ABL gene from chromosome 9 joining to the BCR gene on chromosome 22, which is found in most patients with chronic myelogenous leukemia, or CML, and in some patients with acute lymphoblastic leukemia, or ALL, or acute myelogenous leukemia or AML |
| “Board” | the board of directors of the Company |
| “BTK” | Bruton’s tyrosine kinase inhibitor |
| “BVI” | the British Virgin Islands |
| “CDE” | the center of drug evaluation of China |
| “CG Code” | the “Corporate Governance Code” as contained in Appendix C1 to the Listing Rules |
| “CLL” | chronic lymphocytic leukemia; a slowly progressing, liquid form of tumor that causes an excess of white blood cells in the bone marrow, blood, liver, and spleen |
| “Closing” | closing under the Securities Purchase Agreement |
| “CML” | chronic myeloid/myelogenous leukemia; a type of cancer that affects the blood and bone marrow |
| “CML-CP” | chronic-phase chronic myeloid leukemia |
| “Company” or “Ascentage Pharma” | Ascentage Pharma Group International (亞盛醫藥集團), an exempted company incorporated in the Cayman Islands with limited liability on November 17, 2017 |
| “Core Product” | has the meaning ascribed to it in Chapter 18A of the Listing Rules |
| “Director(s)” | the director(s) of the Company, including all executive, non- executive and independent non-executive directors |
| “Dr. Guo” | Dr. Guo Edward Ming, a Substantial Shareholder |
| “Dr. Wang” | Dr. Wang Shaomeng, our non-executive director and a Substantial Shareholder |
| “Dr. Yang” | Dr. Yang Dajun, our chairman, chief executive officer, a Substantial Shareholder, and spouse of Dr. Zhai |
| “Dr. Zhai” | Dr. Zhai Yifan, our chief medical officer, a Substantial Shareholder, and spouse of Dr. Yang |
| “EED” | Embryonic Ectoderm Development |
| “EGFR” | epidermal growth factor receptor |
| “Exclusive Option Agreement” | the exclusive option agreement dated June 14, 2024 entered into among Ascentage and Takeda in relation to, among other things, research, development, import, export, manufacture, usage, commercialization and exploitation of Olverembatinib |
| “FAK” | focal adhesion kinase; an enzyme involved in cellular adhesion (how cells stick to each other and their surroundings) and spreading processes (how cells move around) |
| “FDA” | U.S. Food and Drug Administration |
| “FL” | follicular lymphoma |
| “Founders SPV” | Ascentage Limited (now dissolved), a company incorporated in BVI with limited liability which is owned by Dr. Yang (for himself and as settlor of the Yang Family Trust) as to 45.53%, Dr. Guo (for himself and as settlor of the Guo Family Trust) as to 27.69% and Dr. Wang (for himself and as settlor of the Wang Family Trust) as to 26.78%, a Substantial Shareholder |
| “FVTPL” | fair value through profit or loss |
| “GC” | gastric cancer |
| “GIST” | gastrointestinal stromal tumor |
| “Global Offering” | the Hong Kong public offering and the international offering as defined in the Prospectus |
| “GMP” | Good Manufacturing Practices |
| “Group”, “we”, “our” or “us” | the Company and its subsidiaries from time to time |
| “Guo Family Trust” | Ming Edward Guo Dynasty Trust, a discretionary family trust established by Dr. Guo as settlor for the benefits of Dr. Guo’s family members, of which South Dakota Trust is a trustee |
| “HBV” | hepatitis B virus |
| “HK$” or “Hong Kong dollars” or “HKD” | Hong Kong dollars, the lawful currency of Hong Kong |
| “Hong Kong” | the Hong Kong Special Administrative Region of the PRC |
| “HQP1351” | formerly known as D824, or GZD824; Olverembatinib, our third- generation BCR-ABL inhibitor, which was designed to overcome drug resistance caused by BCR-ABL kinase mutants such as T315I mutants |
| “IAP” | inhibitors of apoptosis protein |
| “IFRS” | International Financial Reporting Standards, as issued from time to time by the International Accounting Standards Board |
| “IND” | investigational new drug, an application and approval process required before drug candidates may commence clinical trials |
| “Innovent” | Innovent Biologics, Inc. (信達生物製藥), an exempted company incorporated in the Cayman Islands with limited liability, the shares of which are listed on the Main Board of the Stock Exchange (stock code: 1801) |
| “Innovent Suzhou” | Innovent Biologics (Suzhou) Co., Ltd. (信達生物製藥(蘇州)有限公司), a company with limited liability established under the laws of the PRC and controlled by Innovent |
| “IP” | intellectual property |
| “Listing Rules” | the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited, as amended, supplemented or otherwise modified from time to time |
| “Main Board” | the stock exchange (excluding the option market) operated by the Stock Exchange which is independent from and operates in parallel with the Growth Enterprise Market of the Stock Exchange |
| “MDM2” | Murine Double Minute 2 |
| “MDS” | myelodysplastic syndrome; group of cancers in which immature blood cells in the bone marrow do not mature and therefore do not become healthy blood cells |
| “MM” | multiple myeloma |
| “Model Code” | the “Model Code for Securities Transactions by Directors of Listed Issuers” set out in Appendix C3 to the Listing Rules |
| “NASDAQ” | National Association of Securities Dealers Automated Quotations |
| “NCCN” | National Comprehensive Cancer Network |
| “NDA” | New Drug Application |
| “NHL” | non-Hodgkin’s lymphoma |
| “NMPA” | National Medical Products Administration of the PRC, formerly known as the China National Drug Administration, or CNDA, and the China Food and Drug Administration, or CFDA |
| “NRDL” | National Reimbursement Drug List |
| “NSCLC” | non-small cell lung cancer |
| “ODD” | Orphan Drug Designations |
| “Option” | the exclusive option granted by Ascentage to Takeda to enter into an exclusive license agreement, pursuant to the terms of the Exclusive Option Agreement |
| “PD-1” | Programmed cell death protein 1, a cell surface receptor that belongs to the immunoglobulin superfamily and is expressed on T cells and pro-B cells |
| “Ph+ ALL” | Philadelphia-positive acute lymphoblastic leukemia |
| “Post-IPO Share Option Scheme” | the post-IPO share option scheme approved by the Board on September 28, 2019 as amended from time to time |
| “PRC” or “China” or “Mainland China” | the People’s Republic of China and for the purposes of this announcement only, except where the context requires otherwise, references to China or the PRC exclude Hong Kong, Macau and Taiwan |
| “Pre-IPO Share Option Scheme” | the pre-IPO share option scheme approved by the Board on July 13, 2018 as amended from time to time |
| “Prospectus” | the prospectus of the Company dated October 16, 2019 |
| “R&D” | research and development |
| “relapsed/refractory” or “R/R” | disease or condition which become progressive after treatment (relapsed) or does not respond to the initial treatment (refractory) |
| “Reporting Period” | the six-month period from January 1, 2025 to June 30, 2025 |
| “RMB” | Renminbi, the lawful currency of the PRC |
| “ROS1” | receptor tyrosine kinase with structural similarity to the ALK protein |
| “RSU(s)” | restricted share unit(s) |
| “SCLC” | small cell lung cancer |
| “SDH-” | succinate dehydrogenase- |
| “Securities Purchase Agreement” | the securities purchase agreement dated June 14, 2024 entered into between the Company and Takeda in relation to the 2024 Share Subscription |
| “Shareholders” | holder(s) of the Share(s) |
| “Share(s)” | ordinary share(s) of US$0.0001 par value each in the share capital of the Company |
| “Share Purchase Price” | HK$24.09850 (equivalent to approximately US$3.08549), which is the share purchase price for each Subscription Share under the Securities Purchase Agreement |
| “Share Subscription Conditions Precedent” | the conditions precedent to the 2024 Share Subscription |
| “Stock Exchange” | The Stock Exchange of Hong Kong Limited, a wholly-owned subsidiary of Hong Kong Exchanges and Clearing Limited |
| “SLL” | small lymphocytic leukemia |
| “Subscription Share(s)” | the 24,307,322 shares which the Company agreed to issue and allot, and Takeda agreed to subscribe pursuant to the Securities Purchase Agreement |
| “Substantial Shareholder(s)” | has the meaning ascribed to it under the Listing Rules and unless the context otherwise requires refers to Dr. Yang, Dr. Guo, Dr. Wang, the Founders SPV, Dr. Zhai and HealthQuest Pharma Limited |
| “T315I” | a type of mutation that sometimes results in the failure of tyrosine kinase inhibitor (TKI) treatment |
| “Takeda” | Takeda Pharmaceuticals International AG, a company established under the laws of Switzerland |
| “TKI(s)” | tyrosine kinase inhibitor; a type of pharmaceutical drug that inhibits tyrosine kinases |
| “United States” or “U.S.” | the United States of America, its territories, its possessions and all areas subject to its jurisdiction |
| “US$”, “USD” or “U.S. dollars” | United States dollars, the lawful currency of the United States |
| “Wang Family Trust” | Shaomeng Wang Dynasty Trust, a discretionary family trust established by Dr. Wang as settlor for the benefits of Dr. Wang’s family members, of which South Dakota Trust is a trustee |
| “WM” | waldenström macroglobulinemia |
| “Yang Family Trust” | Dajun Yang Dynasty Trust, a discretionary family trust established by Dr. Yang as settlor for the benefits of Dr. Yang’s family members, of which South Dakota Trust is a trustee |
| “%” | per cent |
| By order of the Board | |
| Ascentage
Pharma Group International Dr. Yang Dajun |
|
| Chairman and Executive Director |
Suzhou, the PRC, August 21, 2025
As at the date of this announcement, the Board is comprised of Dr. Yang Dajun, as chairman and executive Director, Dr. Wang Shaomeng and Dr. Lu Simon DazhongNote as non-executive Directors, and Mr. Ye Changqing, Mr. Ren Wei, Dr. David Sidransky, Ms. Marina S. Bozilenko, Dr. Debra Yu and Dr. Marc E. Lippman, MD as independent non-executive Directors.
Note: Dr. Lu Simon Dazhong satisfy the independence requirements of the U.S. Securities and Exchange Commission and Nasdaq corporate governance requirements.
Exhibit 99.3
ASCENTAGE PHARMA GROUP INTERNATIONAL
INDEMNIFICATION AGREEMENT
This Indemnification Agreement (this “Agreement”) is dated [insert date], and is between Ascentage Pharma Group International, an exempted company incorporated under the laws of the Cayman Islands (the “Company”), and [insert name of indemnitee] (“Indemnitee”).
RECITALS
A. Indemnitee’s service to the Company substantially benefits the Company.
B. Individuals are reluctant to serve as directors or officers of corporations or in certain other capacities unless they are provided with adequate protection through insurance or indemnification against the risks of claims and actions against them arising out of such service.
C. Indemnitee does not regard the protection currently provided by applicable law, the Company’s governing documents and any insurance as adequate under the present circumstances, and Indemnitee may not be willing to serve as a director or officer without additional protection. The Indemnitee is willing to serve, continue to serve and to take an additional service for or on behalf of the Company on the condition that the Indemnitee be so indemnified.
D. In order to induce Indemnitee to continue to provide services to the Company, it is reasonable, prudent and necessary for the Company to contractually obligate itself to indemnify, and to advance expenses on behalf of, Indemnitee and his affiliates as permitted by applicable law.
E. This Agreement is a supplement to and in furtherance of the indemnification provided in the Company’s Articles of Association (the “Articles”), and any resolutions adopted pursuant thereto, and this Agreement shall not be deemed a substitute therefor, nor shall this Agreement be deemed to limit, diminish or abrogate any rights of Indemnified Person (as defined herein) thereunder.
The parties therefore agree as follows:
1. Definitions.
(a) “affiliates” for the purpose of this Agreement, has the meaning defined in Rule 405 under the Securities Act of 1933, as amended.
(b) “Articles” has the meaning ascribed to such term in the Recitals.
(c) A “Change in Control” shall be deemed to occur upon the earliest to occur after the date of this Agreement of any of the following events:
(i) Acquisition of Shares by Third Party. Any Person (as defined below) becomes the Beneficial Owner (as defined below), directly or indirectly, of securities of the Company representing fifteen percent (15%) or more of the combined voting power of the Company’s then outstanding securities; (ii) Change in Board Composition.
During any period of two consecutive years (not including any period prior to the execution of this Agreement), individuals who at the beginning of such period constitute the Company’s board of directors, and any new directors (other than a director designated by a person who has entered into an agreement with the Company to effect a transaction described in Sections 1(a)(i), 1(a)(iii) or 1(a)(iv)) whose election by the board of directors or nomination for election by the Company’s shareholders was approved by a vote of at least two-thirds of the directors then still in office who either were directors at the beginning of the period or whose election or nomination for election was previously so approved, cease for any reason to constitute at least a majority of the members of the Company’s board of directors;
(iii) Corporate Transactions. The effective date of a merger or consolidation of the Company with any other entity, other than a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior to such merger or consolidation continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity) more than 50% of the combined voting power of the voting securities of the surviving entity outstanding immediately after such merger or consolidation and with the power to elect at least a majority of the board of directors or other governing body of such surviving entity;
(iv) Liquidation. The approval by the shareholders of the Company of a complete liquidation of the Company or an agreement for the sale or disposition by the Company of all or substantially all of the Company’s assets; and
(v) Other Events. Any other event of a nature that would be required to be reported in response to Item 6(e) of Schedule 14A of Regulation 14A (or in response to any similar item on any similar schedule or form) promulgated under the Securities Exchange Act of 1934, as amended, whether or not the Company is then subject to such reporting requirement.
(d) “Person” shall have the meaning as set forth in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended; provided, however, that “Person” shall exclude (i) the Company, (ii) any trustee or other fiduciary holding securities under an employee benefit plan of the Company, (iii) any corporation owned, directly or indirectly, by the shareholders of the Company in substantially the same proportions as their ownership of shares of the Company.
(e) “Beneficial Owner” shall have the meaning given to such term in Rule 13d-3 under the Securities Exchange Act of 1934, as amended; provided, however, that “Beneficial Owner” shall exclude any Person otherwise becoming a Beneficial Owner by reason of (i) the shareholders of the Company approving a merger of the Company with another entity or (ii) the Company’s board of directors approving a sale of securities by the Company to such Person.
(f) “Companies Act” means the Companies Act (as revised) of the Cayman Islands.
(g) “Corporate Status” describes the status of a Person who is or was a director, trustee, settlor, general partner, managing member, officer, employee, agent or fiduciary of the Company or of any other corporation, partnership, joint venture, trust, affiliate, employee benefit plan or other enterprise that such Person is or was serving at the request or consent of the Company.
(h) “Disinterested Director” means a director of the Company who is not and was not a party to the Proceeding in respect of which indemnification is sought by Indemnitee.
(i) “Enterprise” means the Company and any other corporation, partnership, limited liability company, joint venture, trust, employee benefit plan or other enterprise of which Indemnitee is or was serving at the request of the Company as a director, trustee, general partner, managing member, officer, employee, agent or fiduciary.
-
(j) “Expenses” include all reasonable and actually incurred attorneys’ fees, retainers, court costs, transcript costs, fees and costs of experts, witness fees, travel expenses, duplicating costs, printing and binding costs, telephone charges, postage, delivery service fees, and all other disbursements or expenses of the types customarily incurred in connection with prosecuting, defending, preparing to prosecute or defend, investigating, being or preparing to be a witness in, or otherwise participating in, a Proceeding. Expenses also include (i) Expenses incurred in connection with any appeal resulting from any Proceeding, including without limitation the premium, security for, and other costs relating to any cost bond, supersedeas bond or other appeal bond or their equivalent, and (ii) for purposes of Section 12(d), Expenses incurred by Indemnified Person in connection with the interpretation, enforcement or defense of Indemnified Person’s rights under this Agreement or the Indemnitee’s rights under any directors’ and officers’ liability insurance policies maintained by the Company. Expenses, however, shall not include amounts paid in settlement by Indemnified Person or the amount of judgments or fines against Indemnified Person.
(k) “Indemnified Person” has the meaning given in clause (m) below.
(l) “Independent Counsel” means a law firm, or a partner or member of a law firm, that is experienced in matters of corporation law and neither presently is, nor in the past five years has been, retained to represent (i) the Company or Indemnified Person in any matter material to either such party (other than as Independent Counsel with respect to matters concerning Indemnified Person under this Agreement, or other indemnitee under similar indemnification agreements), or (ii) any other party to the Proceeding giving rise to a claim for indemnification hereunder. Notwithstanding the foregoing, the term “Independent Counsel” shall not include any person who, under the applicable standards of professional conduct then prevailing, would have a conflict of interest in representing either the Company or Indemnified Person in an action to determine Indemnified Person’s rights under this Agreement.
(m) “Proceeding” means any threatened, pending or completed action, suit, arbitration, mediation, alternative dispute resolution mechanism, investigation, inquiry, administrative hearing or proceeding, whether brought in the right of the Company or otherwise and whether of a civil, criminal, administrative or investigative nature, including any appeal therefrom and including without limitation any such Proceeding pending as of the date of this Agreement, in which Indemnified Person (as defined herein) was, is or will be involved as a party, a potential party, a non-party witness or otherwise by reason of, with or without limitation,: the fact that the Indemnitee is or was an officer or director of the Company, by reason of any action taken (x) by him and/or (y) by him or his affiliate in a capacity as a direct or indirect shareholder of the Company for actions taken at the request of the Company (each such affiliate and Indemnitee, an “Indemnified Person”), approved as necessary by the Company’s board of directors; or of any inaction in his role as an officer or director of the Company and/or as an Indemnified Person for actions taken at the request of the Company, approved as necessary by the Company’s board of directors; or by reason of the fact that Indemnitee is or was serving at the request of the Company as a director, officer, employee, agent or fiduciary of another corporation, partnership, joint venture, trust, affiliate or other Enterprise.
(n) Reference to “other enterprises” shall include employee benefit plans; references to “fines” shall include any excise taxes assessed on a person with respect to any employee benefit plan; references to “serving at the request of the Company” shall include any service as a director, officer, employee or agent of the Company which imposes duties on, or involves services with respect to the Company or any of the Company’s subsidiaries, affiliates, employee benefit or welfare plans, such plan’s participants or beneficiaries or any other enterprise, foreign or domestic.
-
2. Indemnity in Third-Party Proceedings. The Company shall indemnify the Indemnified Person in accordance with the provisions of this Section 2 if any Indemnified Person is, or is threatened to be made, a party to or a participant in any Proceeding, other than a Proceeding by or in the right of the Company to procure a judgment in its favor. Pursuant to this Section 2, Indemnified Person shall be indemnified to the fullest extent permitted by applicable law against all Expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred by Indemnified Person in connection with such Proceeding or any claim, issue or matter therein, if Indemnified Person acted in good faith and in a manner the Indemnified Person reasonably believed to be in or not opposed to the best interests of the Company and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful.
3. Indemnity in Proceedings by or in the Right of the Company. The Company shall indemnify Indemnified Person in accordance with the provisions of this Section 3 if Indemnified Person is, or is threatened to be made, a party to or a participant in any Proceeding by or in the right of the Company to procure a judgment in its favor. Pursuant to this Section 3, Indemnified Person shall be indemnified to the fullest extent permitted by applicable law against all Expenses actually and reasonably incurred by Indemnified Person in connection with such Proceeding or any claim, issue or matter therein, provided, however, that this provision shall not apply to liability arising from dishonesty, willful default or fraud of the Indemnitee. The Indemnitee shall not be found to have been dishonest or committed willful default or fraud under this section except as provided by the Articles.
4. Indemnification for Expenses of a Party Who is Wholly or Partly Successful. To the extent that Indemnified Person is a party to or a participant in and is successful (on the merits or otherwise) in defense of any Proceeding or any claim, issue or matter therein, the Company shall indemnify Indemnified Person against all Expenses actually and reasonably incurred by Indemnified Person in connection therewith. For purposes of this section, the termination of any claim, issue or matter in such a Proceeding by dismissal, with or without prejudice, shall be deemed to be a successful result as to such claim, issue or matter.
5. Indemnification for Expenses of a Witness. To the extent that Indemnitee is, by reason of his or her Corporate Status, a witness in any Proceeding to which Indemnitee is not a party, Indemnitee shall be indemnified to the extent permitted by applicable law against all Expenses actually and reasonably incurred by Indemnitee or on Indemnitee’s behalf in connection therewith.
6. Additional Indemnification.
(a) Notwithstanding any limitation in Sections 2, 3, 4 or 5, the Company shall indemnify Indemnified Person to the fullest extent permitted by applicable law if Indemnified Person is, or is threatened to be made, a party to or a participant in any Proceeding (including a Proceeding by or in the right of the Company to procure a judgment in its favor), including, without limitation, all liability arising out of the negligence or active or passive wrongdoing of the Indemnified Person, against all Expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred by Indemnified Person in connection with the Proceeding or any claim, issue or matter therein.
(b) For purposes of Section 6(a), the meaning of the phrase “to the fullest extent permitted by applicable law” shall include, but not be limited to:
(i) the fullest extent permitted by the provision of the Articles and the Companies Act that authorizes or contemplates additional indemnification by agreement, or the corresponding provision of any amendment to or replacement of the Articles and the Companies Act; and
(ii) the fullest extent authorized or permitted by any amendments to or replacements of the Articles and the Companies Act adopted after the date of this Agreement that increase the extent to which a corporation may indemnify its officers and directors.
-
7. Exclusions. Notwithstanding any provision in this Agreement, the Company shall not be obligated under this Agreement to provide indemnification or otherwise advance Expenses in connection with any Proceeding (or any part of any Proceeding):
(a) for which payment has actually been made to or on behalf of Indemnified Person under any statute, insurance policy, indemnity provision, vote or otherwise, except with respect to any excess beyond the amount paid;
(b) for an accounting or disgorgement of profits pursuant to Section 16(b) of the Securities Exchange Act of 1934, as amended, or similar provisions of federal, state or local statutory law or common law, if Indemnified Person is held liable therefor (including pursuant to any settlement arrangements);
(c) for any reimbursement of the Company by Indemnitee of any bonus or other incentive-based or equity-based compensation or of any profits realized by Indemnitee from the sale of securities of the Company, in either case as required under any clawback or compensation recovery policy adopted by the Company, applicable securities exchange and association listing requirements, including, without limitation, those adopted in accordance with Rule 10D-1 under the Securities Exchange Act of 1934, as amended, and/or the Securities Exchange Act of 1934, as amended (including, without limitation, any such reimbursements that arise from an accounting restatement of the Company pursuant to Section 304 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), or the payment to the Company of profits arising from the purchase and sale by Indemnified Person of securities in violation of Section 306 of the Sarbanes-Oxley Act), if Indemnitee is held liable therefor (including pursuant to any settlement arrangements);
(d) initiated by Indemnified Person, including any Proceeding (or any part of any Proceeding) initiated by Indemnified Person against the Company or its directors, officers, employees, agents, affiliates or other indemnitee, unless (i) the Company’s board of directors authorized the Proceeding (or the relevant part of the Proceeding) prior to its initiation, (ii) the Company provides the indemnification, in its sole discretion, pursuant to the powers vested in the Company under applicable law, (iii) otherwise authorized in Section 12(d) or (iv) otherwise required by applicable law; or
(e) if prohibited by applicable law.
8. Advances of Expenses. The Company shall advance the Expenses incurred by Indemnified Person in connection with any Proceeding prior to its final disposition, and such advancement shall be made as soon as reasonably practicable, but in any event no later than 90 days, after the receipt by the Company of a written statement or statements requesting such advances from time to time (which shall include invoices received by Indemnified Person in connection with such Expenses but, in the case of invoices in connection with legal services, any references to legal work performed or to expenditure made that would cause Indemnified Person to waive any privilege accorded by applicable law shall not be included with the invoice). Advances shall be unsecured and interest free and made without regard to Indemnified Person’s ability to repay such advances. Indemnified Person hereby undertakes to repay any advance to the extent that it is ultimately determined that Indemnified Person is not entitled to be indemnified by the Company. This Section 8 shall not apply to the extent advancement is prohibited by law and shall not apply to any Proceeding (or any part of any Proceeding) for which indemnification is not permitted under this Agreement, but shall apply to any Proceeding (or any part of any Proceeding) referenced in Section 7(b) (in the case of Indemnified Person) or 7(c) (in the case of Indemnitee) prior to a determination that Indemnified Person or Indemnitee, as applicable, is not entitled to be indemnified by the Company pursuant to Section 7(b) or 7(c).
-
9. Procedures for Notification and Defense of Claim.
(a) Indemnitee shall notify the Company in writing of any matter with respect to which Indemnified Person intends to seek indemnification or advancement of Expenses as soon as reasonably practicable following the receipt by Indemnitee of notice thereof. The written notification to the Company shall include, in reasonable detail, a description of the nature of the Proceeding and the facts underlying the Proceeding. The failure by Indemnitee to notify the Company will not relieve the Company from any liability which it may have to Indemnified Person hereunder or otherwise than under this Agreement, and any delay in so notifying the Company shall not constitute a waiver by Indemnified Person of any rights, except to the extent that such failure or delay materially prejudices the Company.
(b) If, at the time of the receipt of a notice of a Proceeding pursuant to the terms hereof, the Company has directors’ and officers’ liability insurance in effect that may be applicable to the Proceeding, the Company shall give prompt notice of the commencement of the Proceeding to the insurers in accordance with the procedures set forth in the applicable policies. The Company shall thereafter take all commercially-reasonable action to cause such insurers to pay, on behalf of Indemnified Person, all amounts payable as a result of such Proceeding in accordance with the terms of such policies.
(c) In the event the Company may be obligated to provide indemnification in connection with a Proceeding, the Company shall be entitled to assume the defense of such Proceeding with counsel approved by Indemnified Person, which approval shall not be unreasonably withheld, conditioned or delayed, upon the delivery to Indemnified Person of written notice of its election to do so. After delivery of such notice, approval of such counsel by Indemnified Person and the retention of such counsel by the Company, the Company will not be liable to Indemnified Person for any fees or expenses of counsel subsequently incurred by Indemnified Person with respect to the same Proceeding. The Company may be obligated to pay the fees and expenses of Indemnified Person’s separate counsel to the extent (i) the employment of separate counsel by Indemnified Person is authorized by the Company, (ii) counsel for the Company or Indemnified Person shall have reasonably concluded that there is a conflict of interest between the Company and Indemnified Person in the conduct of any such defense such that Indemnified Person needs to be separately represented, or (iii) the Company shall not have retained, or shall not continue to retain, counsel to defend such Proceeding. The Company shall have the right to conduct such defense as it sees fit in its sole discretion. Regardless of any provision in this Agreement, Indemnified Person shall have the right to employ counsel in any Proceeding at Indemnified Person’s own expense. The Company shall not be entitled, without the consent of Indemnitee, to assume the defense of any claim brought by or in the right of the Company.
(d) Indemnified Person shall give the Company such information and cooperation in connection with the Proceeding as may be reasonably appropriate.
(e) The Company shall not be liable to indemnify Indemnified Person for any settlement of any Proceeding (or any part thereof) without the Company’s prior written consent, which shall not be unreasonably withheld, conditioned or delayed.
(f) The Company shall not settle any Proceeding (or any part thereof) in a manner that imposes any penalty or liability on Indemnified Person without Indemnified Person’s prior written consent, which shall not be unreasonably withheld, conditioned or delayed.
-
10. Procedures upon Application for Indemnification.
(a) To obtain indemnification, Indemnified Person shall submit to the Company a written request, including therein or therewith such documentation and information as is reasonably available to Indemnified Person and as is reasonably necessary to determine whether and to what extent Indemnified Person is entitled to indemnification following the final disposition of the Proceeding. Any delay in providing the request will not relieve the Company from its obligations under this Agreement, except to the extent such failure is prejudicial.
(b) Upon written request by Indemnified Person for indemnification pursuant to Section 10 (a), a determination with respect to Indemnified Person’s entitlement thereto shall be made in the specific case (i) if a Change in Control shall have occurred, by Independent Counsel in a written opinion to the Company’s board of directors, a copy of which shall be delivered to Indemnified Person or (ii) if a Change in Control shall not have occurred, (A) by a majority vote of the Disinterested Directors, even though less than a quorum of the Company’s board of directors, (B) by a committee of Disinterested Directors designated by a majority vote of the Disinterested Directors, even though less than a quorum of the Company’s board of directors, (C) if there are no such Disinterested Directors or, if such Disinterested Directors so direct, by Independent Counsel in a written opinion to the Company’s board of directors, a copy of which shall be delivered to Indemnified Person or (D) if so directed by the Company’s board of directors, by the shareholders of the Company. If it is determined that Indemnified Person is entitled to indemnification, payment to Indemnified Person shall be made within ten days after such determination. Indemnitee shall cooperate with the person, persons or entity making the determination with respect to Indemnified Person’s entitlement to indemnification, including providing to such person, persons or entity upon reasonable advance request any documentation or information that is not privileged or otherwise protected from disclosure and that is reasonably available to Indemnified Person and reasonably necessary to such determination. Any costs or expenses (including attorneys’ fees and disbursements) actually and reasonably incurred by Indemnitee in so cooperating with the person, persons or entity making such determination shall be borne by the Company, to the extent permitted by applicable law.
(c) In the event the determination of entitlement to indemnification is to be made by Independent Counsel pursuant to Section 10(b), the Independent Counsel shall be selected as provided in this Section 10(c). If a Change in Control shall not have occurred, the Independent Counsel shall be selected by the Company’s board of directors, and the Company shall give written notice to Indemnified Person advising him or her of the identity of the Independent Counsel so selected. If a Change in Control shall have occurred, the Independent Counsel shall be selected by Indemnified Person (unless Indemnified Person shall request that such selection be made by the Company’s board of directors, in which event the preceding sentence shall apply), and Indemnified Person shall give written notice to the Company advising it of the identity of the Independent Counsel so selected. In either event, Indemnified Person or the Company, as the case may be, may, within ten days after such written notice of selection shall have been given, deliver to the Company or to Indemnified Person, as the case may be, a written objection to such selection; provided, however, that such objection may be asserted only on the ground that the Independent Counsel so selected does not meet the requirements of “Independent Counsel” as defined in Section 1 of this Agreement, and the objection shall set forth with particularity the factual basis of such assertion. Absent a proper and timely objection, the person so selected shall act as Independent Counsel. If such written objection is so made and substantiated, the Independent Counsel so selected may not serve as Independent Counsel unless and until such objection is withdrawn or a court has determined that such objection is without merit. If, within 20 days after the later of (i) submission by Indemnified Person of a written request for indemnification pursuant to Section 10(a) hereof and (ii) the final disposition of the Proceeding, the parties have not agreed upon an Independent Counsel, either the Company or Indemnified Person may petition a court of competent jurisdiction for resolution of any objection which shall have been made by the Company or Indemnified Person to the other’s selection of Independent Counsel and for the appointment as Independent Counsel of a person selected by the court or by such other person as the court shall designate, and the person with respect to whom all objections are so resolved or the person so appointed shall act as Independent Counsel under Section 10(b) hereof. Upon the due commencement of any judicial proceeding or arbitration pursuant to Section 12(a) of this Agreement, the Independent Counsel shall be discharged and relieved of any further responsibility in such capacity (subject to the applicable standards of professional conduct then prevailing).
-
(d) The Company agrees to pay the reasonable fees and expenses of any Independent Counsel.
11. Presumptions and Effect of Certain Proceedings.
(a) In making a determination with respect to entitlement to indemnification hereunder, the person, persons or entity making such determination shall, to the fullest extent not prohibited by law, presume that Indemnified Person is entitled to indemnification under this Agreement, and the Company shall, to the fullest extent not prohibited by law, have the burden of proof to overcome that presumption.
(b) The termination of any Proceeding or of any claim, issue or matter therein, by judgment, order, settlement or conviction, or upon a plea of nolo contendere or its equivalent, shall not (except as otherwise expressly provided in this Agreement) of itself create a presumption that Indemnitee acted dishonestly or committed willful default or fraud in accordance with the provisions of the Articles.
(c) Neither the knowledge, actions nor failure to act of any other director, officer, agent or employee of the Enterprise shall be imputed to Indemnified Person for purposes of determining the right to indemnification under this Agreement.
12. Remedies of Indemnified Person.
(a) Subject to Section 12(e), in the event that (i) a determination is made pursuant to Section 10 of this Agreement that Indemnified Person is not entitled to indemnification under this Agreement, (ii) advancement of Expenses is not timely made pursuant to Section 8 or 12(d) of this Agreement, (iii) no determination of entitlement to indemnification shall have been made pursuant to Section 10 of this Agreement within 90 days after the later of the receipt by the Company of the request for indemnification or the final disposition of the Proceeding, (iv) payment of indemnification pursuant to this Agreement is not made (A) within ten days after a determination has been made that Indemnified Person is entitled to indemnification or (B) with respect to indemnification pursuant to Sections 4, 5 and 12(d) of this Agreement, within 30 days after receipt by the Company of a written request therefor, or (v) the Company or any other person or entity takes or threatens to take any action to declare this Agreement void or unenforceable, or institutes any litigation or other action or proceeding designed to deny, or to recover from, Indemnified Person the benefits provided or intended to be provided to Indemnified Person hereunder, Indemnified Person shall be entitled to an adjudication by a court of competent jurisdiction of its, his or her entitlement to such indemnification or advancement of Expenses. Alternatively, Indemnified Person, at its, his or her option, may seek an award in arbitration with respect to its, his or her entitlement to such indemnification or advancement of Expenses, to be conducted by a single arbitrator pursuant to the Commercial Arbitration Rules of the American Arbitration Association. Indemnified Person shall commence such proceeding seeking an adjudication or an award in arbitration within 180 days following the date on which Indemnified Person first has the right to commence such proceeding pursuant to this Section 12(a); provided, however, that the foregoing clause shall not apply in respect of a proceeding brought by Indemnified Person to enforce his or her rights under Section 4 of this Agreement. The Company shall not oppose Indemnified Person’s right to seek any such adjudication or award in arbitration in accordance with this Agreement.
-
(b) Neither (i) the failure of the Company, its board of directors, any committee or subgroup of the board of directors, Independent Counsel or shareholders to have made a determination that indemnification of Indemnitee is proper in the circumstances because Indemnitee has met the applicable standard of conduct, nor (ii) an actual determination by the Company, its board of directors, any committee or subgroup of the board of directors, Independent Counsel or shareholders that Indemnitee has not met the applicable standard of conduct, shall create a presumption that Indemnitee has or has not met the applicable standard of conduct. In the event that a determination shall have been made pursuant to Section 10 of this Agreement that Indemnitee is not entitled to indemnification, any judicial proceeding or arbitration commenced pursuant to this Section 12 shall be conducted in all respects as a de novo trial, or arbitration, on the merits, and Indemnitee shall not be prejudiced by reason of that adverse determination. In any judicial proceeding or arbitration commenced pursuant to this Section 12, the Company shall, to the fullest extent not prohibited by law, have the burden of proving Indemnitee is not entitled to indemnification or advancement of Expenses, as the case may be.
(c) To the fullest extent not prohibited by law, the Company shall be precluded from asserting in any judicial proceeding or arbitration commenced pursuant to this Section 12 that the procedures and presumptions of this Agreement are not valid, binding and enforceable and shall stipulate in any such court or before any such arbitrator that the Company is bound by all the provisions of this Agreement. If a determination shall have been made pursuant to Section 10 of this Agreement that Indemnified Person is entitled to indemnification, the Company shall be bound by such determination in any judicial proceeding or arbitration commenced pursuant to this Section 12, absent (i) a misstatement by Indemnified Person of a material fact, or an omission of a material fact necessary to make Indemnified Person’s statements not materially misleading, in connection with the request for indemnification, or (ii) a prohibition of such indemnification under applicable law.
(d) To the extent not prohibited by law, the Company shall indemnify (x) Indemnified Person against all Expenses that are incurred by Indemnified Person in connection with any action for indemnification or advancement of Expenses from the Company under this Agreement or (y) Indemnitee against all Expenses that are incurred by Indemnitee in connection with any action for indemnification or advancement of Expenses under any directors’ and officers’ liability insurance policies maintained by the Company to the extent Indemnified Person of Indemnitee, as applicable, is successful in such action, and, if requested by Indemnified Person or Indemnitee, as applicable, shall (as soon as reasonably practicable, but in any event no later than 90 days, after receipt by the Company of a written request therefor) advance such Expenses, subject to the provisions of Section 8.
(e) Notwithstanding anything in this Agreement to the contrary, no determination as to entitlement to indemnification shall be required to be made prior to the final disposition of the Proceeding.
13. Contribution. To the fullest extent permissible under applicable law, if the indemnification provided for in this Agreement is unavailable to Indemnified Person, the Company, in lieu of indemnifying Indemnified Person, shall contribute to the amounts incurred by Indemnified Person, whether for Expenses, judgments, fines or amounts paid or to be paid in settlement, in connection with any claim relating to an indemnifiable event under this Agreement, in such proportion as is deemed fair and reasonable in light of all of the circumstances of such Proceeding in order to reflect (i) the relative benefits received by the Company and Indemnified Person as a result of the events and transactions giving rise to such Proceeding; and (ii) the relative fault of Indemnified Person and the Company (and its other directors, officers, employees and agents) in connection with such events and transactions.
-
14. Non-exclusivity. The rights of indemnification and to receive advancement of Expenses as provided by this Agreement shall not be deemed exclusive of any other rights to which Indemnified Person may at any time be entitled under applicable law, the Company’s Articles, any agreement, a vote of shareholders or a resolution of directors, or otherwise. To the extent that a change in Cayman Islands law, whether by statute or judicial decision, permits greater indemnification or advancement of Expenses than would be afforded currently under the Company’s Articles and this Agreement, it is the intent of the parties hereto that Indemnified Person shall enjoy by this Agreement the greater benefits so afforded by such change, subject to the restrictions expressly set forth herein or therein. Except as expressly set forth herein, no right or remedy herein conferred is intended to be exclusive of any other right or remedy, and every other right and remedy shall be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at law or in equity or otherwise. Except as expressly set forth herein, the assertion or employment of any right or remedy hereunder, or otherwise, shall not prevent the concurrent assertion or employment of any other right or remedy.
15. No Duplication of Payments. The Company shall not be liable under this Agreement to make any payment of amounts otherwise indemnifiable hereunder (or for which advancement is provided hereunder) if and to the extent that Indemnified Person has otherwise actually received payment for such amounts under any insurance policy, contract, agreement or otherwise.
16. Insurance. To the extent that the Company maintains an insurance policy or policies providing liability insurance for directors, trustees, general partners, managing members, officers, employees, agents or fiduciaries of the Company or any other Enterprise, Indemnitee shall be covered by such policy or policies to the same extent as the most favorably-insured persons under such policy or policies in a comparable position.
17. Subrogation. In the event of any payment under this Agreement, the Company shall be subrogated to the extent of such payment to all of the rights of recovery of Indemnified Person, who shall execute all papers required and take all action necessary to secure such rights, including execution of such documents as are necessary to enable the Company to bring suit to enforce such rights.
18. Services to the Company. Indemnitee agrees to serve as a director or officer of the Company or, at the request of the Company, as a director, trustee, general partner, managing member, officer, employee, agent or fiduciary of another Enterprise, for so long as Indemnitee is duly elected or appointed or until Indemnitee tenders his or her resignation or is removed from such position. Indemnitee may at any time and for any reason resign from such position (subject to any other contractual obligation or any obligation imposed by operation of law), in which event the Company shall have no obligation under this Agreement to continue Indemnitee in such position. This Agreement shall not be deemed an employment contract between the Company (or any of its subsidiaries or any Enterprise) and Indemnitee. Indemnitee specifically acknowledges that any employment with the Company (or any of its subsidiaries or any Enterprise) is at will, and Indemnitee may be discharged at any time for any reason, with or without cause, with or without notice, except as may be otherwise expressly provided in any executed, written employment contract between Indemnitee and the Company (or any of its subsidiaries or any Enterprise), any existing formal severance policies adopted by the Company’s board of directors or, with respect to service as a director or officer of the Company, the Company’s Articles or the Companies Act. No such document shall be subject to any oral modification thereof.
-
19. Duration. This Agreement shall continue until and terminate upon the later of (a) twelve years after the date that Indemnitee shall have ceased to serve as a director or officer of the Company or as a director, trustee, general partner, managing member, officer, employee, agent or fiduciary of any other Enterprise, as applicable1; or (b) one year after the final termination of any Proceeding, including any appeal, then pending in respect of which Indemnified Person is granted rights of indemnification or advancement of Expenses hereunder and of any proceeding commenced by Indemnified Person pursuant to Section 12 of this Agreement relating thereto.
20. Successors. This Agreement shall be binding upon the Company and its successors and assigns, including any direct or indirect successor, by purchase, merger, consolidation or otherwise, to all or substantially all of the business or assets of the Company, and shall inure to the benefit of Indemnitee and Indemnitee’s heirs, executors and administrators.
21. Severability. Nothing in this Agreement is intended to require or shall be construed as requiring the Company to do or fail to do any act in violation of applicable law. The Company’s inability, pursuant to court order or other applicable law, to perform its obligations under this Agreement shall not constitute a breach of this Agreement. If any provision or provisions of this Agreement shall be held to be invalid, illegal or unenforceable for any reason whatsoever: (i) the validity, legality and enforceability of the remaining provisions of this Agreement (including without limitation, each portion of any section of this Agreement containing any such provision held to be invalid, illegal or unenforceable, that is not itself invalid, illegal or unenforceable) shall not in any way be affected or impaired thereby and shall remain enforceable to the fullest extent permitted by law; (ii) such provision or provisions shall be deemed reformed to the extent necessary to conform to applicable law and to give the maximum effect to the intent of the parties hereto; and (iii) to the fullest extent possible, the provisions of this Agreement (including, without limitation, each portion of any section of this Agreement containing any such provision held to be invalid, illegal or unenforceable, that is not itself invalid, illegal or unenforceable) shall be construed so as to give effect to the intent manifested thereby.
22. Enforcement. The Company expressly confirms and agrees that it has entered into this Agreement and assumed the obligations imposed on it hereby in order to induce Indemnitee to serve as a director or officer of the Company, and the Company acknowledges that Indemnitee is relying upon this Agreement in serving as a director or officer of the Company.
23. Entire Agreement. This Agreement constitutes the entire agreement between the parties hereto with respect to the subject matter hereof and supersedes all prior agreements and understandings, oral, written and implied, between the parties hereto with respect to the subject matter hereof; provided, however, that this Agreement is a supplement to and in furtherance of the Company’s Articles and applicable law.
24. Modification and Waiver. No supplement, modification or amendment to this Agreement shall be binding unless executed in writing by the parties hereto. No amendment, alteration or repeal of this Agreement shall adversely affect any right of Indemnified Person under this Agreement in respect of any action taken or omitted by such Indemnitee in his or her Corporate Status prior to such amendment, alteration or repeal. No waiver of any of the provisions of this Agreement shall constitute or be deemed a waiver of any other provision of this Agreement nor shall any waiver constitute a continuing waiver.
| 1 | Walkers Note: Statute of Limitations is 6 years for tortious and contractual matters and 12 years for matters relating to deeds, trusts etc. |
-
25. Notices. All notices and other communications required or permitted hereunder shall be in writing and shall be mailed by registered or certified mail, postage prepaid, sent by facsimile or electronic mail or otherwise delivered by hand, messenger or courier service addressed:
(a) if to Indemnified Person, to Indemnitee’s address, facsimile number or electronic mail address as shown on the signature page of this Agreement or in the Company’s records, as may be updated in accordance with the provisions hereof; or
(b) if to the Company, to the attention of the Chief Executive Officer or Chief Financial Officer of the Company at 1218 Massachusetts Ave, Suite 200, Cambridge, Massachusetts 02138, or at such other current address as the Company shall have furnished to Indemnified Person, with a copy (which shall not constitute notice) to Megan Baier, Wilson Sonsini Goodrich & Rosati, P.C., 1301 Avenue of the Americas, 40th Floor, New York, NY 10019.
Each such notice or other communication shall for all purposes of this Agreement be treated as effective or having been given (i) if delivered by hand, messenger or courier service, when delivered (or if sent via a nationally-recognized overnight courier service, freight prepaid, specifying next-business-day delivery, one business day after deposit with the courier), (ii) if sent via mail, at the earlier of its receipt or five days after the same has been deposited in a regularly-maintained receptacle for the deposit of the United States mail, addressed and mailed as aforesaid, or (iii) if sent via facsimile, upon confirmation of facsimile transfer or, if sent via electronic mail, upon confirmation of delivery when directed to the relevant electronic mail address, if sent during normal business hours of the recipient, or if not sent during normal business hours of the recipient, then on the recipient’s next business day.
26. Applicable Law and Consent to Jurisdiction. This Agreement and the legal relations among the parties shall be governed by, and construed and enforced in accordance with, the laws of the Cayman Islands, without regard to its conflict of laws rules. Except with respect to any arbitration commenced by Indemnified Person pursuant to Section 12(a) of this Agreement, the Company and each of the Indemnified Person hereby irrevocably and unconditionally (i) agree that any action or proceeding arising out of or in connection with this Agreement shall be brought only in the jurisdiction of the courts of Cayman Islands, and not in any other state or federal court in the United States of America or any court in any other country, (ii) consent to submit to the exclusive jurisdiction of the Cayman Islands court for purposes of any action or proceeding arising out of or in connection with this Agreement, (iii) appoint, to the extent such party is not otherwise subject to service of process in the Cayman Islands courts, Walkers Corporate Limited, 190 Elgin Avenue, George Town, Grand Cayman KY1-9001, Cayman Islands, as its agent in the Cayman Islands courts as such party’s agent for acceptance of legal process in connection with any such action or proceeding against such party with the same legal force and validity as if served upon such party personally within Cayman Islands, (iv) waive any objection to the laying of venue of any such action or proceeding in the Cayman Islands courts, and (v) waive, and agree not to plead or to make, any claim that any such action or proceeding brought in the Cayman Islands courts has been brought in an improper or inconvenient forum.
27. Counterparts. This Agreement may be executed in one or more counterparts, each of which shall for all purposes be deemed to be an original but all of which together shall constitute one and the same Agreement. This Agreement may also be executed and delivered by facsimile signature and in counterparts, each of which shall for all purposes be deemed to be an original but all of which together shall constitute one and the same Agreement. Only one such counterpart signed by the party against whom enforceability is sought needs to be produced to evidence the existence of this Agreement.
28. Captions. The headings of the paragraphs of this Agreement are inserted for convenience only and shall not be deemed to constitute part of this Agreement or to affect the construction thereof.
29. Third Party Rights.
(a) Any Indemnified Person not being a party to this Agreement, may enforce any rights granted to it pursuant to this Agreement in its own right as if it were a party to this Agreement.
(b) Except as expressly provided in paragraph (a) above, a person who is not a party to this Agreement shall not have any rights under the Contracts (Rights of Third Parties) Act, 2014 to enforce any term of this Agreement.
(c) Notwithstanding any term of this Agreement, the consent of or notice to any person who is not a party to this Agreement shall not be required for any termination, rescission or agreement to any variation, waiver, assignment, novation, release or settlement under this Agreement at any time.
(signature page follows)
-
The parties are signing this Indemnification Agreement on the date stated in the introductory sentence.
| ASCENTAGE PHARMA GROUP INTERNATIONAL | |
| (Signature) | |
| (Print name) | |
| (Title) | |
| [INSERT INDEMNITEE NAME] | |
| (Signature) | |
| (Print name) | |
| (Street address) | |
| (City, State and ZIP) |