UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE QUARTERLY PERIOD ENDED JUNE 30, 2025
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File Number: 001-38793
| INMUNE BIO INC. |
| (Exact name of registrant as specified in its charter) |
| Nevada | 47-5205835 | |
| (State of incorporation) | (I.R.S. Employer Identification No.) |
225 NE Mizner Blvd., Suite 640
Boca Raton, FL 33432
(Address of principal executive office) (Zip code)
(561) 710-0512
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per share | INMB | The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period than the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of August 7, 2025, there were 26,585,258 shares of our common stock, par value $0.001 per share, outstanding.
INMUNE BIO INC.
FORM 10-Q
FOR THE SIX MONTHS ENDED JUNE 30, 2025
INDEX
| PART I – FINANCIAL INFORMATION | 1 | |
| Item 1. | Financial Statements | 1 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 18 |
| Item 3. | Quantitative and Qualitative Disclosure About Market Risk | 35 |
| Item 4. | Controls and Procedures | 35 |
| PART II – OTHER INFORMATION | 36 | |
| Item 1. | Legal Proceedings | 36 |
| Item 1A. | Risk Factors | 36 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 36 |
| Item 3. | Defaults Upon Senior Securities | 36 |
| Item 4. | Mine Safety Disclosures | 36 |
| Item 5. | Other Information | 36 |
| Item 6. | Exhibits | 39 |
| Signatures | 40 | |
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements
INMUNE BIO INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
(Unaudited)
| June 30, 2025 |
December 31, 2024 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 33,374 | $ | 20,922 | ||||
| Research and development tax credit receivable | 1,605 | 1,181 | ||||||
| Other tax receivable | 550 | 228 | ||||||
| Prepaid expenses and other current assets | 505 | 331 | ||||||
| TOTAL CURRENT ASSETS | 36,034 | 22,662 | ||||||
| Equipment | 706 | |||||||
| Operating lease – right of use asset | 374 | 307 | ||||||
| Other assets | 570 | 79 | ||||||
| Acquired in-process research and development intangible assets | 16,514 | |||||||
| TOTAL ASSETS | $ | 37,684 | $ | 39,562 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued liabilities | $ | 7,671 | $ | 6,539 | ||||
| Accounts payable and accrued liabilities – related parties | 184 | 25 | ||||||
| Deferred liabilities | 511 | 517 | ||||||
| Operating lease, current liabilities | 220 | 140 | ||||||
| TOTAL CURRENT LIABILITIES | 8,586 | 7,221 | ||||||
| Long-term operating lease liabilities | 231 | 244 | ||||||
| TOTAL LIABILITIES | 8,817 | 7,465 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Preferred stock, $0.001 par value, 10,000,000 shares authorized, 0 shares issued and outstanding | ||||||||
| Common stock, $0.001 par value, 200,000,000 shares authorized, 26,585,258 and 22,280,451 shares issued and outstanding, respectively | 27 | 22 | ||||||
| Additional paid-in capital | 226,904 | 195,754 | ||||||
| Accumulated other comprehensive loss | (763 | ) | (575 | ) | ||||
| Accumulated deficit | (197,301 | ) | (163,104 | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY | 28,867 | 32,097 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 37,684 | $ | 39,562 | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
INMUNE BIO INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share and per share amounts)
(Unaudited)
| For the Three Months Ended June 30, |
For the Six Months Ended June 30, |
|||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| REVENUE | $ | $ | $ | 50 | $ | 14 | ||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| General and administrative | 2,253 | 2,812 | 4,569 | 5,150 | ||||||||||||
| Research and development | 5,804 | 7,053 | 13,443 | 15,746 | ||||||||||||
| Impairment of acquired in-process research and development intangible assets | 16,514 | 16,514 | ||||||||||||||
| Total operating expenses | 24,571 | 9,865 | 34,526 | 20,896 | ||||||||||||
| LOSS FROM OPERATIONS | (24,571 | ) | (9,865 | ) | (34,476 | ) | (20,882 | ) | ||||||||
| OTHER INCOME, NET | 113 | 119 | 279 | 111 | ||||||||||||
| NET LOSS | $ | (24,458 | ) | $ | (9,746 | ) | $ | (34,197 | ) | $ | (20,771 | ) | ||||
| Net loss per common share – basic and diluted | $ | (1.05 | ) | $ | (0.50 | ) | $ | (1.49 | ) | $ | (1.11 | ) | ||||
| Weighted average common shares outstanding – basic and diluted | 23,298,455 | 19,307,323 | 22,899,539 | 18,666,898 | ||||||||||||
| COMPREHENSIVE LOSS | ||||||||||||||||
| Net loss | $ | (24,458 | ) | $ | (9,746 | ) | $ | (34,197 | ) | $ | (20,771 | ) | ||||
| Other comprehensive income (loss) – foreign currency translation | (153 | ) | (44 | ) | (188 | ) | 86 | |||||||||
| Total comprehensive loss | $ | (24,611 | ) | $ | (9,790 | ) | $ | (34,385 | ) | $ | (20,685 | ) | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
INMUNE BIO INC.
CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2025
(In thousands, except share amounts)
(Unaudited)
| Accumulated | ||||||||||||||||||||||||
| Additional | Other | Total | ||||||||||||||||||||||
| Common Stock | Paid-In | Comprehensive | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Loss | Deficit | Equity | |||||||||||||||||||
| Balance as of December 31, 2024 | 22,280,451 | $ | 22 | $ | 195,754 | $ | (575 | ) | $ | (163,104 | ) | $ | 32,097 | |||||||||||
| Stock-based compensation | - | 2,076 | 2,076 | |||||||||||||||||||||
| Sale of common stock for cash | 649,860 | 1 | 5,272 | 5,273 | ||||||||||||||||||||
| Exercise of warrants for cash | 100 | 1 | 1 | |||||||||||||||||||||
| Loss on foreign currency translation | - | (35 | ) | (35 | ) | |||||||||||||||||||
| Net loss | - | (9,739 | ) | (9,739 | ) | |||||||||||||||||||
| Balance as of March 31, 2025 | 22,930,411 | 23 | 203,103 | (610 | ) | (172,843 | ) | 29,673 | ||||||||||||||||
| Stock-based compensation | - | 1,534 | 1,534 | |||||||||||||||||||||
| Sale of common stock for cash | 3,654,847 | 4 | 22,267 | 22,271 | ||||||||||||||||||||
| Loss on foreign currency translation | - | (153 | ) | (153 | ) | |||||||||||||||||||
| Net loss | - | (24,458 | ) | (24,458 | ) | |||||||||||||||||||
| Balance as of June 30, 2025 | 26,585,258 | $ | 27 | $ | 226,904 | $ | (763 | ) | $ | (197,301 | ) | $ | 28,867 | |||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
INMUNE BIO INC.
CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2024
(In thousands, except share amounts)
(Unaudited)
| Accumulated | ||||||||||||||||||||||||
| Additional | Other | Total | ||||||||||||||||||||||
| Common Stock | Paid-In | Comprehensive | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Deficit | Equity | |||||||||||||||||||
| Balance as of December 31, 2023 | 17,950,776 | $ | 18 | $ | 159,143 | $ | (799 | ) | $ | (121,022 | ) | $ | 37,340 | |||||||||||
| Stock-based compensation | - | 1,779 | 1,779 | |||||||||||||||||||||
| Gain on foreign currency translation | - | 130 | 130 | |||||||||||||||||||||
| Net loss | - | (11,025 | ) | (11,025 | ) | |||||||||||||||||||
| Balance as of March 31, 2024 | 17,950,776 | 18 | 160,922 | (669 | ) | (132,047 | ) | 28,224 | ||||||||||||||||
| Stock-based compensation | - | 2,350 | 2,350 | |||||||||||||||||||||
| Common stock issued for cash | 198,364 | 2,032 | 2,032 | |||||||||||||||||||||
| Common stock and warrants issued for cash | 1,557,592 | 2 | 13,463 | 13,465 | ||||||||||||||||||||
| Loss on foreign currency translation | - | (44 | ) | (44 | ) | |||||||||||||||||||
| Net loss | - | (9,746 | ) | (9,746 | ) | |||||||||||||||||||
| Balance as of June 30, 2024 | 19,706,732 | $ | 20 | $ | 178,767 | $ | (713 | ) | $ | (141,793 | ) | $ | 36,281 | |||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
INMUNE BIO INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(Unaudited)
| For the Six Months Ended June 30, |
||||||||
| 2025 | 2024 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (34,197 | ) | $ | (20,771 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation | 3,610 | 4,129 | ||||||
| Accretion of debt discount | 58 | |||||||
| Impairment of acquired in-process research and development intangible assets | 16,514 | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Research and development tax credit receivable | (424 | ) | (1,238 | ) | ||||
| Other tax receivable | (322 | ) | 267 | |||||
| Prepaid expenses | (174 | ) | 497 | |||||
| Prepaid expenses – related party | 142 | |||||||
| Other assets | (491 | ) | 50 | |||||
| Accounts payable and accrued liabilities | 1,132 | 1,381 | ||||||
| Accounts payable and accrued liabilities – related parties | 159 | 104 | ||||||
| Deferred liabilities | (6 | ) | 32 | |||||
| Operating lease liabilities | - | (13 | ) | |||||
| Net cash used in operating activities | (14,199 | ) | (15,362 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of equipment | (706 | ) | ||||||
| Net cash used in investing activities | (706 | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Net proceeds from sale of common stock and warrants | 27,544 | 15,497 | ||||||
| Exercise of warrants for cash | 1 | |||||||
| Repayments of debt | (5,000 | ) | ||||||
| Net cash provided by financing activities | 27,545 | 10,497 | ||||||
| Impact on cash from foreign currency translation | (188 | ) | 86 | |||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 12,452 | (4,779 | ) | |||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 20,922 | 35,848 | ||||||
| CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 33,374 | $ | 31,069 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOWS INFORMATION: | ||||||||
| Cash paid for income taxes | $ | $ | ||||||
| Cash paid for interest expense | $ | $ | 523 | |||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
INMUNE BIO INC.
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
INmune Bio Inc. (the “Company” or “INmune Bio”) was organized in the State of Nevada on September 25, 2015 and is a clinical stage biotechnology pharmaceutical company focused on developing and commercializing its product candidates to treat diseases where the innate immune system is not functioning normally and contributing to the patient’s disease. INmune Bio has three product platforms. The DN-TNF product platform utilizes dominant-negative technology to selectively neutralize soluble TNF, a key driver of innate immune dysfunction and mechanistic target of many diseases and was used for its Alzheimer’s clinical trial (“XPro”). The CORDStrom product platform is a pooled, human umbilical cord mesenchymal stem cell product currently being developed to treat recessive dystrophic epidermolysis bullosa (“RDEB”). The Natural Killer Cell Priming Platform includes INKmune aimed at priming the patient’s NK cells to eliminate minimal residual disease in patients with cancer. INmune Bio’s product platforms utilize a precision medicine approach for the treatment of a wide variety of hematologic malignancies, solid tumors and chronic inflammation.
NOTE 2 – GOING CONCERN
These unaudited condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
The Company has incurred significant losses and negative cash flows from operations since inception and expects to incur additional losses until such time that it can generate significant revenue from the commercialization of its product candidates. During the six months ended June 30, 2025, the Company incurred a net loss of $34.2 million and had net cash flows used in operating activities of $14.2 million. Given the Company’s projected operating requirements and its existing cash and cash equivalents, the Company is projecting insufficient liquidity to sustain its operations through one year following the date that the financial statements are issued. These conditions and events raise substantial doubt about the Company’s ability to continue as a going concern.
In response to these conditions, management is currently evaluating different strategies to obtain the required funding of future operations. Financing strategies may include, but are not limited to, the public or private sale of equity, debt financings or funds from other capital sources, such as government funding, collaborations, strategic alliances, divestment of non-core assets, or licensing arrangements with third parties. There can be no assurances that the Company will be able to secure additional financing, or if available, that it will be sufficient to meet its needs or on favorable terms. Because management’s plans have not yet been finalized and are not within the Company’s control, the implementation of such plans cannot be considered probable. As a result, the Company has concluded that management’s plans do not alleviate substantial doubt about the Company’s ability to continue as a going concern.
The unaudited condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying financial statements are presented in U.S. dollars and have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”), and pursuant to the accounting and disclosure rules and regulations of the U.S. Securities and Exchange Commission (“SEC”). The unaudited condensed consolidated financial statements include the accounts of INmune Bio Inc. and its subsidiaries. Intercompany transactions and balances have been eliminated.
In the opinion of management, the interim financial information includes all normal recurring adjustments necessary for a fair statement of the results for the interim periods. These unaudited condensed consolidated interim financial statements should be read in conjunction with the audited financial statements and notes thereto for the year ended December 31, 2024, included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 27, 2025.
Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological innovations, protection of proprietary technology, dependence on key personnel, compliance with government regulations and the need to obtain additional financing to fund operations. Product candidates currently under development will require significant additional research and development efforts, including extensive preclinical studies, clinical trials and regulatory approval prior to commercialization. These efforts require significant amounts of additional resources, adequate personnel, infrastructure and extensive compliance and reporting.
The Company’s product candidates are still in development and, to date, none of the Company’s product candidates have been approved for sale.
There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection for the Company’s intellectual property will be obtained or maintained, that any products developed will obtain necessary government regulatory approval or that any approved products will be commercially viable. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will generate any revenue from any of its products. The Company operates in an environment of rapid change in technology and substantial competition from other pharmaceutical and biotechnology companies.
The Company relies and expects to continue to rely on a small number of vendors to manufacture supplies and materials for its use in the clinical trial programs. These programs could be adversely affected by a significant interruption in these manufacturing services.
Use of Estimates
Preparing financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. Actual results and outcomes may differ from management’s estimates and assumptions.
Fair Value of Financial Instruments
The Company measures certain assets and liabilities in accordance with authoritative guidance which requires fair value measurements to be classified and disclosed in one of the following three categories:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities.
Level 2: Observable prices that are based on inputs not quoted on active markets but corroborated by market data.
Level 3: Unobservable inputs are used when little or no market data is available.
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. The Company reviews the fair value hierarchy classification on a quarterly basis. Changes in the ability to observe valuation inputs may result in a reclassification of levels for certain assets or liabilities within the fair value hierarchy. The Company did not have any transfers of assets and liabilities between the levels of the fair value measurement hierarchy during the years presented.
The carrying amounts of financial instruments such as cash and cash equivalents, research and development tax credit receivable, other tax receivable, prepaid expenses, and accounts payable and accrued liabilities approximate the related fair values due to the short-term maturities of these instruments.
Cash and Cash Equivalents
The Company considers all short-term, highly liquid investments with an original maturity at the date of purchase of three months or less to be cash equivalents. The Company maintains cash balances that may be uninsured or in deposit accounts that exceed Federal Deposit Insurance Corporation limits. The Company maintains its cash deposits with major financial institutions.
Research and Development Tax Incentive Receivable
The Company, through its wholly owned subsidiary in Australia (“AUS”), participates in the Australian research and development tax incentive program, such that a percentage of our qualifying research and development expenditures are reimbursed by the Australian government, and such incentives are reflected as a reduction of research and development expense. The Australian research and development tax incentive is recognized when there is reasonable assurance that the incentive will be received, the relevant expenditure has been incurred and the amount of the consideration can be reliably measured. At each period end, management estimates the reimbursement available to the Company based on available information at the time.
The Company, through its wholly owned subsidiary in the United Kingdom (“UK”), participates in the research and development program provided by the United Kingdom tax relief program, such that a percentage of our qualifying research and development expenditures are reimbursed by the United Kingdom government, and such incentives are reflected as a reduction of research and development expense. The United Kingdom research and development tax incentive is recognized when there is reasonable assurance that the incentive will be received, the relevant expenditure has been incurred and the amount of the consideration can be reliably measured. At each period end, management estimates the reimbursement available to the Company based on available information at the time.
Equipment
Equipment is recorded at cost and depreciated using the straight-line method over the estimated useful lives of the assets and consist of scientific equipment with a 5 year life. Repairs and maintenance costs are charged to expense as incurred. At June 30, 2025, the Company’s equipment was not yet placed into service.
Intangible Assets
The Company capitalizes costs incurred in connection with in-process research and development purchased from others if the asset has alternative uses and such uses are not restricted under applicable license agreements; patent applications (principally legal fees), patent purchases, and trademarks related to its cell line as intangible assets. Acquired in-process research and development costs that do not have alternative uses are expensed as incurred. When the assets are determined to have a finite life (upon completion of the development of the in-process research and development for its DN-TNF platform), the useful life will be determined and the in-process research and development intangible assets will be amortized.
During the fourth quarter and if business factors indicate more frequently, the Company performs an assessment of the qualitative factors affecting the fair value of our in-process research and development. If the qualitative assessment suggests that impairment is more likely than not, a quantitative analysis is performed. The quantitative analysis involves a comparison of the fair value of the in-process research and development with the carrying amount. If the carrying amount of the in-process research and development exceeds its fair value, an impairment loss is recognized in an amount equal to that excess.
During the six months ended June 30, 2025, the Company released the Phase 2 clinical trial results for our Alzheimer’s drug candidate, XPro, which failed to meet the primary endpoint, though a subgroup showed potential benefits. Due to insufficient resources to fund further trials, the Company has halted immediate plans to develop XPro for Alzheimer’s or other indications and are instead seeking a partner to continue these studies. As part of preparing its interim unaudited condensed consolidated financial statements, the Company determined that the intangible asset’s fair value was likely below its carrying value. Following a quantitative impairment assessment, the Company estimated the asset’s fair value at $0 as of June 30, 2025, resulting in a recorded impairment of $16,514,000.
Basic and Diluted Loss per Share
Basic loss per share is computed by dividing net loss available to common shareholders by the weighted average number of outstanding common shares during the period. Diluted loss per share gives effect to all dilutive potential common shares outstanding during the period. Dilutive loss per share excludes all potential common shares if their effect is anti-dilutive. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding due to the Company’s net loss position.
At June 30, 2025 and 2024, the Company had potentially issuable shares as follows:
| June 30, | ||||||||
| 2025 | 2024 | |||||||
| Stock options | 7,281,307 | 6,291,807 | ||||||
| Warrants | 3,944,138 | 1,602,978 | ||||||
| Total | 11,225,445 | 7,894,785 | ||||||
Revenue Recognition
The Company recognizes revenue when the customer obtains control of promised goods or services, in an amount that reflects the consideration the Company expects to receive in exchange for those goods or services. The Company recognizes revenue following the five-step model prescribed under ASC Topic 606: (1) identify contract(s) with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations in the contract; and (5) recognize revenues when (or as) the Company satisfies the performance obligations. The Company records the expenses related to revenue in research and development expense, in the periods such expenses were incurred.
The Company records deferred revenues when cash payments are received or due in advance of performance, including amounts which are refundable.
Stock-Based Compensation
The Company utilizes the Black-Scholes option pricing model to estimate the fair value of stock option awards at the date of grant, which requires the input of highly subjective assumptions, including expected volatility and expected life. Changes in these inputs and assumptions can materially affect the measure of estimated fair value of our share-based compensation. These assumptions are subjective and generally require significant analysis and judgment to develop. When estimating fair value, some of the assumptions will be based on, or determined from, external data and other assumptions may be derived from our historical experience with stock-based payment arrangements. The appropriate weight to place on historical experience is a matter of judgment, based on relevant facts and circumstances. The Company accounts for forfeitures of stock options as they occur.
Research and Development
Research and development (“R&D”) costs are expensed as incurred. Research and development credits are recorded by the Company as a reduction of research and development costs. Major components of research and development costs include cash compensation, stock-based compensation, costs of preclinical studies, clinical trials and related clinical manufacturing, costs of drug development, costs of materials and supplies, facilities cost, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on the Company’s behalf.
The Company recognizes grants as contra research and development expense in the consolidated statement of operations on a systematic basis over the periods in which the entity recognizes as expenses the related costs for which the grants are intended to compensate.
Income Taxes
The Company follows the liability method of accounting for income taxes. Under this method, deferred income tax assets and liabilities are recognized for the estimated tax consequences attributable to differences between the financial statement carrying values and their respective income tax basis (temporary differences). The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
Foreign Currency Translation
The Company’s financial statements are presented in the U.S. dollar (“$”), which is the Company’s reporting currency, while its functional currencies are the U.S. Dollar for its U.S. based operations, British Pound (“GBP”) for its United Kingdom-based operations and Australian Dollars (“AUD”) for its Australian-based operations. All assets and liabilities are translated at the exchange rate on the balance sheet date, stockholders’ equity is translated at historical rates and statement of operations items are translated at the weighted average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income. Gains and losses resulting from the translations of foreign currency transactions and balances are reflected in the statement of operations and comprehensive income (loss).
Segment Information
The Company has one primary business activity and operates in one reportable segment.
The Company’s chief operating decision maker (“CODM”) is its Chief Financial Officer who evaluates performance and makes operating decisions about allocating resources based on financial data presented on a consolidated basis. The measures of profitability and the significant segment expenses reviewed by the CODM are consistent with these financial statements and footnotes.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures (“ASU 2023-09”). The guidance in ASU 2023-09 improves the transparency of income tax disclosures by greater disaggregation of information in the rate reconciliation and income taxes paid disaggregated by jurisdiction. The standard is effective for public companies for fiscal years beginning after December 15, 2024 and for interim periods for fiscal years beginning after December 15, 2025, with early adoption permitted. The Company is currently evaluating the impact that the adoption of ASU 2023-09 may have on its consolidated financial statements.
In November 2024, the FASB issued ASU 2024-03, Income Statement-Reporting Comprehensive Income-Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses (“ASU 2024-03”). ASU 2024-03 requires additional disclosure of specific types of expenses included in the expense captions presented on the face of the income statement as well as disclosures about selling expenses. ASU 2024-03 is effective for fiscal years beginning after December 15, 2026, and interim periods beginning after December 15, 2027, with early adoption permitted. ASU 2024-03 may be applied prospectively with the option for retrospective application for all prior periods presented. The Company is currently evaluating the impact of adopting this guidance on the Company’s current financial position, results of operations or financial statement disclosures.
Subsequent Events
The Company evaluates events that have occurred after the balance sheet date of June 30, 2025, through the date which the financial statements are issued.
NOTE 4 – RESEARCH AND DEVELOPMENT ACTIVITY
According to AUS tax law, the Company is allowed an R&D tax credit that reduces a company’s tax bill in AUS for expenses incurred in R&D subject to certain requirements. The Company’s Australian subsidiary submits R&D tax credit requests annually for research and development expenses incurred. At June 30, 2025 and December 31, 2024, the Company recorded a research and development tax credit receivable of $1,605,000 and $1,181,000, respectively, for R&D expenses incurred in Australia.
Xencor, Inc. License Agreement
On October 3, 2017, the Company entered into a license agreement (“Xencor License Agreement”) with Xencor, Inc. (“Xencor”), which discovered and developed a proprietary biological molecule that inhibits soluble tumor necrosis factor. On June 10, 2021, the Company and Xencor entered into a First Amendment to License Agreement pursuant to which, among other things, Section 3.2 of the Xencor License Agreement was amended to change the due diligence milestones. Pursuant to the Xencor License Agreement, Xencor granted the Company an exclusive worldwide, royalty-bearing license in licensed patent rights, licensed know-how and licensed materials (as defined in the license agreement) to make, develop, use, sell and import any pharmaceutical product that comprises, contains, or incorporates Xencor’s proprietary protein known as “XPro” that inhibits soluble tumor necrosis factor (or all modifications, formulations and variants of the licensed protein that specifically bind soluble tumor necrosis factor) alone or in combination with one or more active ingredients, in any dosage or formulation (“Licensed Products”). The Company believes the protein has numerous medical applications. Such additional alternative applications of the technology are available under the Xencor License Agreement.
The Company also agreed to pay Xencor a 5% royalty on Net Sales of all Licensed Products in a given calendar year, which are payable on a country-by- country and licensed product by licensed product basis until the date that is the later of (a) the expiration of the last to expire valid claim covering such Licensed Product in such country or (b) ten years following the first sale to a third party of the licensed product in such country.
During the six months ended June 30, 2025, the Company released the Phase 2 clinical trial results for our Alzheimer’s drug candidate, XPro, which failed to meet the primary endpoint, though a subgroup showed potential benefits. Due to insufficient resources to fund further trials, the Company has halted immediate plans to develop XPro for Alzheimer’s or other indications and are instead seeking a partner to continue these studies. As part of preparing its interim unaudited condensed consolidated financial statements, the Company determined that the intangible asset’s fair value was likely below its carrying value. Following a quantitative impairment assessment, the Company estimated the asset’s fair value at $0 as of June 30, 2025, resulting in a recorded impairment of $16,514,000.
Cordstrom License Agreement
On February 6, 2025, the Company and Great Ormond Street Hospital for Children NHS Foundation Trust (“GOSH”) entered into a license agreement for the exclusive commercial use to clinical trial data associated with a GOSH study investigating the potential of CORDStrom to treat RDEB in pediatric patients (the “MissionEB study”). The Company owns the intellectual property covering CORDStrom, the investigational medicinal product used in the Mission EB study. In addition, the Company owns intellectual property and maintains trade secret protections covering the manufacturing of CORDStrom. With this license to the clinical trial data, the Company intends to prepare applications seeking marketing authorization of CORDStrom for treatment of pediatric RDEB in each of the FDA, EMA, and MHRA. Terms of the license agreement include an upfront payment of £250,000 (approximately $0.3 million at June 30, 2025) and a single milestone payment of up to £6,000,000 (approximately $8.2 million as of June 30, 2025) due on the first to occur marketing authorization to be granted by the FDA, EMA or MHRA, which had not occurred as of June 30, 2025. At June 30, 2025 and December 31, 2024, the Company recorded $0.3 million and $0, respectively, payable to GOSH within accounts payable and accrued liabilities in the consolidated balance sheets.
Pursuant to the GOSH license agreement, the Company has an obligation to provide CORDStrom to the MissionEB study at no cost. While Part 1 of the study is completed, Part 2 of the MissionEB study is currently uninitiated due to a lack of funding by the National Health Services England (“NHSE”). It is unknown whether funding for the study will be allocated by NHSE or its successor agency in the United Kingdom. The Company has not recorded an estimated obligation for the supply of the MissionEB trial with CORDStrom as it is unknown if the MissionEB trial will resume.
INKmune License Agreement
On October 29, 2015, the Company entered into an exclusive license agreement (the “INKmune License Agreement”) with Immune Ventures, LLC (“Immune Ventures”). Pursuant to the INKmune License Agreement, the Company was granted exclusive worldwide rights to the patents, including rights to incorporate any improvements or additions to the patents that may be developed in the future. In consideration for the patent rights, the Company agreed to the following milestone payments:
| (in thousands) | ||||
| Each Phase I initiation | $ | 25 | ||
| Each Phase II initiation | $ | 250 | ||
| Each Phase III initiation | $ | 350 | ||
| Each NDA/EMA filing | $ | 1,000 | ||
| Each NDA/EMA awarded | $ | 9,000 | ||
In addition, the Company agreed to pay the licensor a royalty of 1% of net sales during the life of each patent granted to the Company. The License is owned by Immune Ventures. RJ Tesi, the Company’s President and a member of our Board of Directors, David Moss, its Chief Financial Officer and Treasurer and Mark Lowdell, its Chief Scientific Officer, are the owners of Immune Ventures. No sales have occurred under this license. During December 2023, the Company initiated a Phase I trial with INKmune in patients with metastatic castration-resistant prostate cancer. At December 31, 2024 and June 30, 2025, the Company recorded $25,000 payable to Immune Ventures within accounts payable and accrued liabilities – related parties in the consolidated balance sheet.
The term of the agreement began on October 29, 2015 and ends on a country-by-country basis on the date of the expiration of the last to expire patent rights where patent rights exists, unless terminated earlier in accordance with the agreement. Upon the termination of the agreement, we shall have a fully paid up, perpetual, royalty-free license without further obligation to Immune Ventures. The agreement can be terminated by Immune Ventures if, after 60 days from the Company’s receipt of notice that the Company has not made a payment under the agreement, and the Company still does not make this payment. On July 20, 2018 and October 30, 2020, the parties amended the agreement under which the Company was required achieve milestones pursuant to the agreement.
On April 17, 2023, the parties executed an additional amendment to the agreement under which the Company removed the due diligence requirements to achieve reasonable commercial efforts to bring INKmune to market. This removed all requirements of clinical trial timelines and the filing timelines of an NDA or equivalent. All other provisions in the INKmune License Agreement shall continue in full force and effect.
University of Pittsburg License Agreement
On October 3, 2017, the Company entered into an Assignment and Assumption Agreement with Immune Ventures related to intellectual property licensed from the University of Pittsburgh. Pursuant to the Assignment and Assumption Agreement (“Assignment Agreement”), Immune Ventures assigned all of its rights, obligations and liabilities under an Exclusive License Agreement between the University of Pittsburgh – Of the Commonwealth System of Higher Education (“Licensor”) and Immune Ventures to INmune Bio (“Licensee”), (the “PITT Agreement”).
Consideration under the PITT Agreement includes: (i) annual maintenance fees, (ii) royalty payments based on the sale of products making use of the licensed technology, and (iii) milestone payments.
The Company owes annual maintenance fees under the PITT Agreement in the amount of $25,000 payable on June 26 of each year until the first commercial sale. At June 30, 2025, the Company owed the University of Pittsburgh $25,000 for annual maintenance fees.
Upon first commercial sale of a product making use of the licensed technology under the PITT Agreement, the Licensee is required to pay royalties equal to 2.5% of Net Sales each calendar quarter.
Moreover, under the PITT Agreement the Licensee is required to make milestone payments as follows:
| (in thousands) | ||||
| Each Phase I initiation | $ | 50 | ||
| Each Phase III initiation | $ | 500 | ||
| First commercial sale of product making use of licensed technology | $ | 1,250 | ||
The PITT Agreement expires upon the earlier of: (i) expiration of the last claim of the Patent Rights (as defined in the PITT Agreement) forming the subject matter of the PITT Agreement; or (ii) the date that is 20 years from the effective date of the agreement (June 26, 2037).
The Licensee may terminate the PITT Agreement upon 3 months prior written notice provided all payments under the license are current. The Licensor may terminate the PITT Agreement upon written notice if: (i) Licensee defaults as to performance of material obligations which have not been cured within 60 days after receiving written notice; or (ii) Licensee ceases to carry out its business, becomes bankrupt or insolvent, applies for or consents to the appointment of a trustee, receiver or liquidator of its assets or seeks relief under any law for the aid of debtors.
NOTE 5 – FAIR VALUE MEASUREMENTS
The following table presents the hierarchy for assets and liabilities measured at fair value on a recurring basis:
| (in thousands) | Total | Quoted Price in Active Market (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| June 30, 2025: | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 32,919 | $ | 32,919 | $ | $ | ||||||||||
| Total cash equivalents | $ | 32,919 | $ | 32,919 | $ | $ | ||||||||||
| (in thousands) | Total | Quoted Price in Active Market (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| December 31, 2024: | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Treasury Bills | $ | 10,260 | $ | 10,260 | $ | $ | ||||||||||
| Money market fund | 10,328 | 10,328 | ||||||||||||||
| Total cash equivalents | $ | 20,588 | $ | 20,588 | $ | $ | ||||||||||
NOTE 6 – COMMITMENTS
In April 2025, the Company wholly owned subsidiary, INmune Bio International Ltd., entered into an agreement whereby the Company leases manufacturing space from a third party in the United Kingdom for 2 years. The lease requires payments of approximately $77,000 each quarter during the first year and $154,000 each quarter during the second year. The lease commencement date is August 2025.
As of June 30, 2025, the maturities of our lease liabilities are as follows:
| (in thousands, except years) | ||||
| 2025 | $ | 136 | ||
| 2026 | 278 | |||
| 2027 | 91 | |||
| Total lease payments | 505 | |||
| Less: imputed interest | (54 | ) | ||
| Present value of future lease payments | 451 | |||
| Less: operating lease, current liabilities | (220 | ) | ||
| Long-term operating lease liabilities | $ | 231 | ||
| Weighted-average remaining lease term | 1.8 years | |||
| Weighted-average discount rate | 12.0 | % |
During the three and six months ended June 30, 2025 the Company recognized $55,000 and $95,000, respectively, in operating lease expense, which is included in general and administrative expenses in the Company’s consolidated statement of operations.
During the three and six months ended June 30, 2024, the Company recognized $41,000 and $80,000, respectively, in operating lease expense, which is included in general and administrative expenses in the Company’s consolidated statement of operations
During April 2025, the Company’s wholly-owned subsidiary, INmune Bio International. Ltd., entered into a 2-year collaboration agreement with a vendor whereby it shall make fixed payments to the vendor in exchange for services pursuant to manufacturing CORDStrom in the United Kingdom. A summary of the commitments payable for these services pursuant to the agreement is as follows as of June 30, 2025:
| (in thousands, except years) | ||||
| 2025 | $ | 532 | ||
| 2026 | 1,285 | |||
| 2027 | 1,152 | |||
| Total | $ | 2,969 | ||
NOTE 7 – RELATED PARTY TRANSACTIONS
UCL
At June 30, 2025 and December 31, 2024, the Company recorded a payable to UCL of $133,000 and $0, respectively, for medical research performed on behalf of the Company. During the six months ended June 30, 2025 and 2024, the Company made no payments to UCL. UCL is a wholly owned subsidiary of the University of London. The Company’s Chief Scientific and Manufacturing Officer is a professor at the University of London.
AmplifyBio
At June 30, 2025 and December 31, 2024, the Company recorded a payable to AmplifyBio of $26,000 and $0, respectively, for medical research performed on behalf of the Company. During the six months ended June 30, 2025 and 2024, the Company paid AmplifyBio $41,000 and $233,000, respectively. During 2025, AmplifyBio ceased operations. Amplify Bio’s former CEO is on the board of directors of the Company.
NOTE 8 – DEBT
During 2021, the Company entered into a Loan and Security Agreement (the “Term Loan”) with Silicon Valley Bank and SVB Innovation Credit Fund VIII, L.P., together (the “Lenders”) in which the Company borrowed $15 million. The Term Loan was secured by the Company’s assets. During December 2024, the Company paid off the Term Loan in full. During February 2025, the Company entered into a letter agreement with the Lenders whereby the Term Loan was terminated.
For the three and six months ended June 30, 2024, the Company recognized interest expense of $250,000 and $607,000, respectively, related to the Term Loan
NOTE 9 – STOCKHOLDERS’ EQUITY
Registered Direct Offerings
During June 2025, the Company entered into securities purchase agreements with investors whereby the Company sold 3,000,000 shares of the common stock in a registered direct offering in exchange for gross proceeds of $18.9 million (net proceeds of approximately $17.4 million).
During April 2024, the Company entered into a securities purchase agreement with an investor whereby the Company sold 986,000 shares of the Company’s common stock and warrants to purchase an additional 986,000 shares of the Company’s common stock in a registered direct offering in exchange for gross proceeds of approximately $9.7 million (net proceeds of approximately $8.9 million). The exercise price of the warrants is $9.84 and the warrants are exercisable until April 29, 2026. The Company determined that the warrants were equity classified. The fair value of the warrants was approximately $5.8 million and was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model include: (1) discount rate of 4.97% based on the applicable US Treasury bill rate (2) expected life of 2.0 years, (3) expected volatility of approximately 77% based on the trading history of the Company, and (4) zero expected dividends.
During April 2024, the Company entered into securities purchase agreements with investors whereby the Company sold 571,592 shares of the Company’s common stock and warrants to purchase an additional 571,592 shares of the Company’s common stock in a registered direct offering in exchange for gross proceeds of approximately $4.8 million (net proceeds of approximately $4.5 million). Directors and officers that participated in the offering paid a combined offering price of $8.445 per share and warrant, and other investors paid $8.32 per share and warrant. The exercise price of the warrants is $9.152, and the warrants are exercisable for two years from the issuance dates. The Company determined the warrants were equity classified. The fair value of the warrants was approximately $3.0 million and was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model include: (1) discount rate of 4.89% based on the applicable US Treasury bill rate (2) expected life of 2.0 years, (3) expected volatility of approximately 78% based on the trading history of the Company, and (4) zero expected dividends.
Common Stock – At the Market Offering
During March 2021, the Company entered into a sales agreement (“Sales Agreement”) with BTIG, LLC (“BTIG”), as sales agent, to establish an At-The-Market (“ATM”) offering program of up to $45 million of common stock, subject to certain limitations on the amount of common stock that may be offered and sold by the Company set forth in the sales agreement. During August 2023, the Company and BTIG entered into Amendment No. 1 to the Sales Agreement. The Company is required to pay BTIG a commission of 3% of the gross proceeds from the sale of shares. During the six months ended June 30, 2024, the Company issued and sold 198,364 shares of common stock at an average price of $10.56 per share under the ATM program. The aggregate net proceeds were approximately $2.0 million after commission expenses.
During August 2024, the Company entered into an amended and restated at-the-market sales agreement with RBC Capital Markets LLC and BTIG (together, the “Sales Agents”) relating to the offer and sale of shares of our common stock with an aggregate offering price of up to $75.0 million. The Company is required to pay the Sales Agents a commission of 3% of the gross proceeds from the sale of shares. During the six months ended June 30, 2025, the Company issued and sold 1,304,707 shares of common stock at an average price of $8.01 per share under the ATM program. The aggregate net proceeds were approximately $10.1 million after commission expenses. At June 30, 2025, the Company had $64.5 million of common stock available under the amended and restated at-the-market agreement.
Stock options
The following table summarizes stock option activity during the six months ended June 30, 2025:
| (in thousands, except share and per share amounts) | Number of Shares |
Weighted- average Exercise Price |
Weighted- average Remaining Contractual Term (years) |
Aggregate Intrinsic Value |
||||||||||||
| Outstanding at January 1, 2025 | 7,203,307 | $ | 8.29 | 6.49 | $ | 1,218 | ||||||||||
| Options granted | 100,000 | $ | 7.88 | 10.0 | ||||||||||||
| Options exercised | $ | - | ||||||||||||||
| Options cancelled | (22,000 | ) | $ | 5.05 | - | |||||||||||
| Outstanding at June 30, 2025 | 7,281,307 | $ | 8.29 | 6.03 | $ | |||||||||||
| Exercisable at June 30, 2025 | 5,379,014 | $ | 8.78 | 4.94 | $ | |||||||||||
During the three and six months ended June 30, 2025, the Company recognized stock-based compensation expense of approximately $1.5 million and $3.6 million, respectively, related to the vesting of stock options. During the three and six months ended June 30, 2024, the Company recognized stock-based compensation expense of approximately $2.3 million and $4.1 million, respectively, related to the vesting of stock options. As of June 30, 2025, there was approximately $9.2 million of total unrecognized compensation cost related to non-vested stock options which is expected to be recognized over a weighted-average period of 2.67 years.
Warrants
The Company issued warrants to the Company’s lenders upon obtaining a loan in June 2021. The warrants have a 10-year term and an exercise price of $14.05. At June 30, 2025, respectively, 45,386 of these warrants are outstanding and the intrinsic value of these warrants is $0.
During April 2024, the Company issued 1,557,592 warrants to investors in connection with the sale of common stock. At June 30, 2025, 1,557,592 of these warrants are outstanding and are exercisable for cash at a weighted average price of $9.59 per share. The intrinsic value of these warrants was $0 as of June 30, 2025.
During September 2024, the Company issued 2,341,260 warrants to investors in connection with the sale of common stock. At June 30, 2025, 2,341,160 of these warrants are outstanding and are exercisable for cash at a weighted average price of $6.40 per share. The intrinsic value of these warrants was $0 as of June 30, 2025.
Stock-based Compensation by Class of Expense
The following summarizes the components of stock-based compensation expense in the consolidated statements of operations for the six months ended June 30, 2025 and 2024 respectively:
| (in thousands) | Three Months Ended June 30, 2025 |
Three Months Ended June 30, 2024 |
Six Months Ended June 30, 2025 |
Six Months Ended June 30, 2024 |
||||||||||||
| Research and development | $ | 627 | $ | 996 | $ | 1,457 | $ | 1,698 | ||||||||
| General and administrative | 907 | 1,354 | 2,153 | 2,431 | ||||||||||||
| Total | $ | 1,534 | $ | 2,350 | $ | 3,610 | $ | 4,129 | ||||||||
Shareholder Rights Agreement
On December 30, 2020, the Board of Directors (the “Board”) of the Company approved and adopted a Rights Agreement, dated as of December 30, 2020, by and between the Company and VStock Transfer, LLC, as rights agent, pursuant to which the Board declared a dividend of one preferred share purchase right (each, a “Right”) for each outstanding share of the Company’s common stock held by stockholders as of the close of business on January 11, 2021. When exercisable, each right initially would represent the right to purchase from the Company one one-thousandth of a share of a newly designated series of preferred stock, Series A Junior Participating Preferred Stock, par value $0.001 per share, of the Company, at an exercise price of $300.00 per one one-thousandth of a Series A Junior Participating Preferred Share, subject to adjustment. Subject to various exceptions, the Rights become exercisable in the event any person (excluding certain exempted or grandfathered persons) becomes the beneficial owner of twenty percent or more of the Company’s common stock without the approval of the Board. The Rights Agreement was amended in 2021, 2022, 2023 and 2024 to extend the expiration date and shall expire on December 30, 2025.
NOTE 10 – GOVERNMENT GRANT
The Company has a grant awarded by the National Institutes of Health for approximately $2.0 million to support a Phase 2 study of XPro in patients with treatment resistant depression. The Company has decided it will not initiate a treatment resistant depression study using XPro. As of June 30, 2025, the Company has not received any proceeds pursuant to this grant.
NOTE 11 – LEGAL
Dispute
The Company has an ongoing dispute with a vendor in which the Company believes that the vendor did not properly provide services for which they have invoiced the Company. As of June 30, 2025, the Company has outstanding invoices with the vendor which aggregate approximately $1.6 million, of which the Company has recorded approximately $0.2 million, which is the Company’s estimate of the obligation incurred, and the remaining $1.4 million has not been recorded by the Company as the Company believes the invoices were sent erroneously. The Company and the vendor are still attempting to resolve the dispute and legal proceedings have not been threatened.
Litigation
The Company is subject to claims and suits that arise from time to time in the ordinary course of our business. Although management currently believes that resolving claims against the Company, individually or in aggregate, will not have a material adverse impact in the Company’s consolidated financial statements, these matters are subject to inherent uncertainties and management’s view of these matters may change in the future.
NOTE 12 – SUBSEQUENT EVENTS
On August 4, 2025, Dr. Raymond J. Tesi informed the Company of his intention to retire and resign from his roles as President, Chief Executive Officer, Chief Medical Officer, Chairman of the Board of Directors (the “Board”) and all positions from the Company and its subsidiaries, effective on the Effective Date (as defined below). Dr. Tesi’s resignation is not the result of any dispute or disagreement with the Company or the Board on any matter relating to the Company’s operations, policies or practices.
In connection with Dr. Tesi’s retirement, Dr. Tesi and the Company entered into a Separation Agreement and Mutual Release, dated August 4, 2025 (the “Severance Agreement”), pursuant to which, the Company agreed to pay Dr. Tesi $166,000 of severance within thirty days, pay Dr. Tesi for accrued but unused vacation days, and pay the cost of health insurance coverage for Dr. Tesi and his spouse through December 31, 2025. The Severance Agreement is subject to a seven-day revocation period following execution and shall become effective on August 12, 2025, if not revoked before (the “Effective Date”).
Under the terms of the Severance Agreement, all unvested stock options held by Dr. Tesi will remain outstanding and continue to vest in accordance with their original terms, provided that Dr. Tesi remains in compliance with the Severance Agreement. All vested stock options will remain exercisable for the later of five years following the Effective Date or their original expiration date. The agreement also imposes resale limitations on Dr. Tesi’s beneficial ownership of Company securities, restricting him from selling more than 25% of his beneficially owned shares of common stock in any calendar month during the 18-month period following the Effective Date.
The Company further agreed to maintain directors’ and officers’ liability insurance for Dr. Tesi for a period of at least three years following the Effective Date on terms no less favorable than those applicable to its then-serving officers and directors. The Severance Agreement also reaffirms Dr. Tesi’s right to indemnification under Nevada law, the Company’s articles of incorporation and bylaws, as amended and in effect as of the date hereof, and provides for contribution rights in the event indemnification is unavailable.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
This Form 10-Q contains certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. For this purpose, any statements contained in this Form 10-Q that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, words such as “may,” “will,” “expect,” “believe,” “anticipate,” “estimate” or “continue” or comparable terminology are intended to identify forward-looking statements. These statements by their nature involve substantial risks and uncertainties, and actual results may differ materially depending on a variety of factors, many of which are not within our control. These factors include but are not limited to economic conditions generally and in the industries in which we may participate; competition within our chosen industry, including competition from much larger competitors; technological advances and failure to successfully develop business relationships.
Description of Business
Overview
Our objective is to develop and commercialize our product candidates to treat diseases where the innate immune system is dysfunctional causing or contributing to the patient’s disease. Innate immune dysfunction can occur for a variety of reasons including genetics, lifestyle, and other factors. However, age plays a significant role in the development of immune dysfunction. Innate immune dysfunction can be seen in cancer where Natural Killer (“NK”) cells are impaired and facilitate a tumor’s evasion of the immune system and subsequent disease progression. Chronic inflammation is implicated in various diseases, where it impairs the innate immune system. Our primary focus continues to be treatment of cancer with INKmune, treatment of Alzheimer’s Disease (“AD”) with XPro1595 and treatment of receswsive dystrophic epidermolysis bullosa (RDEB) with CORDStrom, a proprietary, pooled, human umbilical cord mesenchymal stromal cell platform. RDEB is a pediatric orphan disease caused by mutations in the COL7A1 gene which results in highly debilitating skin blistering, dysphagia and failure to thrive with chronic wound problems that often result in fatal squamous cell carcinoma.
XPro for AD has completed Phase I and Phase II trials with enrollment of patients at clinical sites in the United Kingdom, EU, Australia and Canada. In light of the recent Phase 2 results of XPro in AD along with company resources, the treatment resistant depression trial will not be pursued. The INKmune program is in an open label Phase II trial in metastatic castrate resistant prostate cancer (“mCRPC”). CORDStrom for the treatment of children with RDEB has completed a pivotal blinded randomized cross-over trial. The data will be submitted for marketing authorization by filing a Marketing Authorization Application (MAA) in the United Kingdom followed by a Biologics License Application (“BLA”) with the FDA in the US which is anticipated in the first half of 2026.
We believe our DN-TNF platform can be used as a CNS (“central nervous system”) therapy to target glial activation to prevent progression of AD along with other inflammatory diseases. The primary focus of the Company’s development efforts for XPro is AD. In each case, we believe neutralizing sTNF is a cornerstone to the treatment of these diseases.
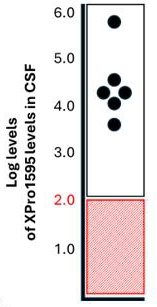
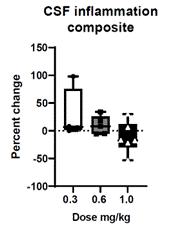
We believe the DN-TNF platform can be used to treat selected neurodegenerative diseases by reducing neuroinflammation without immunosuppression. The Company believes the core pathology of cognitive decline is a combination of neurodegeneration and synaptic dysfunction. Neurodegeneration is nerve cell death that may include demyelination. Synaptic dysfunction means the connections between nerve cells stop working efficiently and may decrease in number. The combination of neurodegeneration and synaptic dysfunction causes cognitive decline and behavioral changes associated with AD. XPro completed a Phase I trial treating patients with Alzheimer’s disease that was partially funded by a Part-the-Clouds Award from the Alzheimer’s Association. We believe XPro targets activated microglia and astrocytes of the brain that produce sTNF that promotes nerve cell loss, synaptic dysfunction and prevents myelin repair - key elements in the development of dementia. In animal models, elimination of sTNF prevents nerve cell dysfunction, reverses synaptic pruning and promotes myelin repair. The Phase I trial in patients with biomarkers of inflammation with AD has been completed. The open label, dose escalation trial was designed to demonstrate that XPro can safely decrease neuroinflammation in patients with AD and biomarkers of inflammation (ADi). The goal of the Phase 1 trial was to demonstrate safety in the target population (patients with AD), demonstrate target engagement by showing XPro got into the brain in therapeutically relevant concentrations and reduced neuroinflammation) and identify the best dose for phase 2. XPro got into the brain (Figure 1a) and dose dependently decreased biomarker of neuroinflammation in the CSF (Figure 1b) with patients treated with the highest dose (1mg/kg/week dose) having the greatest reduction in neuroinflammation. A broad analysis of proteomic changes following treatment of XPro revealed significant changes in CSF proteins related to CNS neuronal function, immune/inflammatory response, Cytoskeletal, metabolic processes, and dendritic spine morphogenesis and synaptic plasticity. Of note, XPro reduced neuronal injury markers Visinin-like protein-1 (91%) and Neurofilament light (84%), improved measures of synaptic function as evinced by a 222% increase in Contactin 2 and a 56% decrease neurogranin. Finally, XPro significantly reduced CSL levels of p-Tau217 (43%) and pTau181 (2%) after 3 months of therapy (Figure 1c).
| A | B | C |
 |
|
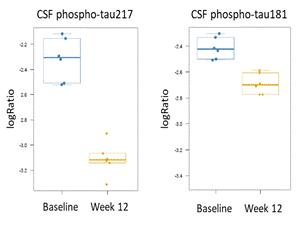 |
Figure 1: (A) XPro gets into the brain at therapeutically relevant concentrations. XPro neutralizes 99.9% of soluble TNF when drug levels exceed two logs. (B) XPro dose dependently reduces CSF inflammation in the brain. CSF composite – a composite score of change of all cytokines measured in the OLINK Target 48 Cytokine panel. (C) XPro (at 1 mg/kg dose) reduces CSF pTau217 and pTau181 as measure by proteomics.
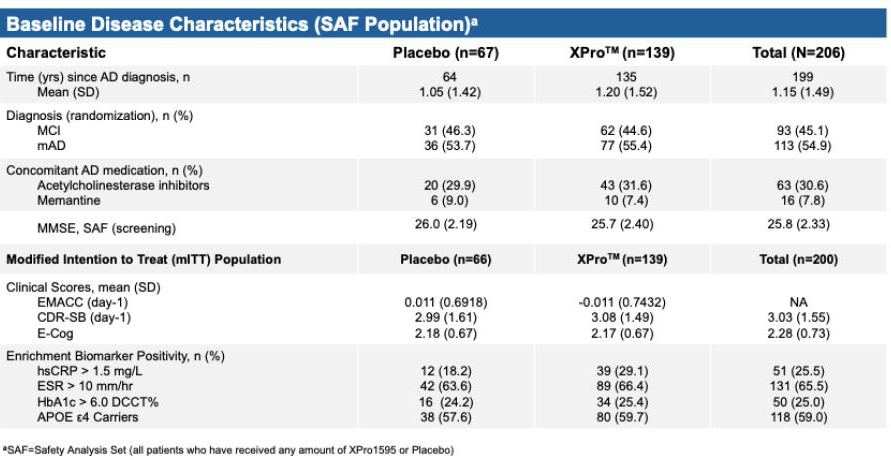
The Phase II study, also known as AD-02 and MINDFuL, was a multicenter, randomized, double-blind, placebo-controlled clinical trial evaluating the safety, tolerability, and efficacy of XPro1595 in individuals with early Alzheimer’s disease with biomarkers of inflammation (ADi). The primary goal of AD02 was to determine if XPro could affect cognition following 6 months of treatment. Participants with a diagnosis of early AD (mild cognitive impairment or mild AD) were randomized in a 2:1 (XPro:Placebo) ratio to receive either 1.0 mg/kg of XPro1595 or placebo via weekly subcutaneous injections for 6 months. An enrichment strategy mirroring to the successful strategy used in the Phase I trial was used to align the mechanism of the drug with the patients AD pathology. Eligibility required the presence of at least one inflammatory biomarker—either high-sensitivity C-reactive protein (hsCRP > 1.5 mg/L), erythrocyte sedimentation rate (ESR > 10 mm/h), hemoglobin A1c (HbA1c > 6.0% DCCT), or at least one APOE4 allele. The primary endpoint was the Early and Mild Alzheimer’s Cognitive Composite (EMACC), with secondary endpoints of Clinical Dementia Rating Scale – Sum of Boxes (CDR-SB), Everyday Cognition Scale (E-Cog), Neuropsychiatric Inventory (NPI-12), ADCS-ADL, and biomarkers such as pTau-217 and GFAP. MRI-based neuroinflammation and brain volumetrics are also evaluated. The AD program had sites in Australia, Canada, the United Kingdom, France, Germany, Spain, Czech Republic and Slovakia.
Full enrollment in the Phase II AD trial occurred in late 2024 with 208 patients enrolled and top-line data was received during June 2025. In the Phase 2 MINDFuL trial of XPro™ in patients with early Alzheimer’s Disease (AD) with biomarkers of inflammation, the modified intent-to-treat (mITT) population (n=200) did not meet the primary and key secondary endpoints (figure 1). Efficacy, Demographics and Safety data are shown below.
Figure 1: Phase 2 Study Results – mITT population Primary and Key Secondary Endpoints, Change From Baseline
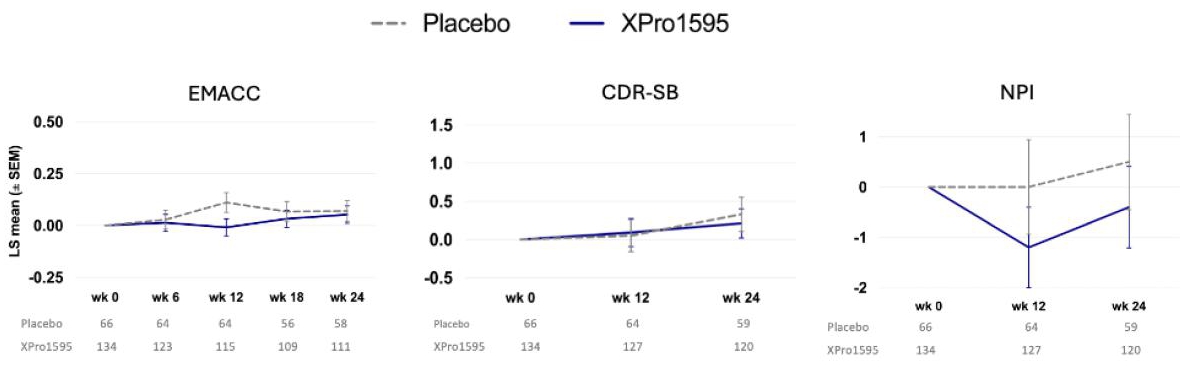
Figure 1: As these graphs depict, the primary and secondary endpoints in this trial were not met as no decline in the placebo groups were observed. A trend was observed in NPI that favored XPro1595 over placebo. For reference, A higher EMACC score =better, A lower CDR and NPI score is better. EMACC: LS Mean Diff (SE): -0.018 (0.0414), 90% CI: -0.0860, 0.0509, p-value: 0.672. CDR-SB: LS Mean Diff (SE): -0.11 (0.185), 90% CI: -0.417, 0.195, p-value: 0.5491. NPI: LS Mean Diff (SE): -0.9 (0.78), 90% CI: -2.18, 0.39, p-value: 0.2499
Prespecified subgroups analyses suggested a signal that favored XPro in a predetermined population of patients that were both amyloid positive and had a higher burden of inflammation defined by 2 or more biomarkers of inflammation (from hereon referred to as enriched group). As shown in figure 2, the mITT placebo group did not decline whereas patients in the enriched group did decline. Decline in the placebo group is required to test the ability of a treatment to prevent or slow decline.
Figure 2: Phase 2 Study Results – Placebo group decline in the mITT and enriched population
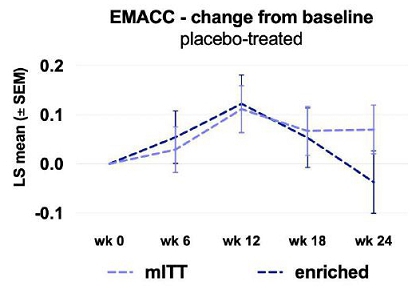
Figure 2: Placebo patients in the mITT did not show decline on the EMACC over the 24 week study. In the enriched group, placebo patients did decline over 24 weeks.
To evaluate a subgroup after missing the primary endpoint, we used effect size as the primary metric due to the smaller sample size (n=100). Effect size, measured by Cohen’s D, is well-suited for small samples and allows comparisons across different measures (e.g., cognitive tests and biomarkers). Unlike p-values, which indicate the likelihood of results being due to chance, effect size reflects clinical relevance and is commonly used for signal detection in Phase 2 studies.
We defined a promising signal as a minimum effect size of 0.2, where XPro outperformed placebo on multiple endpoints aligned with our hypothesis and the drug’s mechanism of action. Results must also be appropriate for the trial’s parameters, meaning the observed effects should align with the trial’s duration and endpoints. For example, if a clinical measure typically requires a longer time to show meaningful change than the trial’s 6-month timeframe, an observed effect on that endpoint would not be considered supportive. Signal detection was based on the effect size difference in LS mean change from baseline (MMRM model) between XPro and placebo at 6 months, ensuring results were meaningful, relevant, and appropriate for the trial’s design and objectives.
Using this method, the enriched population (50% of the total sample, n=100) showed trends toward improvement with XPro on the primary endpoint (EMACC) and a key secondary endpoint (NPI) (Figure 3). With the placebo group showing the expected decline on EMACC over six months, a beneficial effect of XPro became evident. EMACC, which measures cognition (higher scores are better), showed an effect size of 0.27, exceeding the company’s threshold of 0.2, though the p-value of 0.16 fell short of the <0.1 target. For neuropsychiatric symptoms (NPI), the enriched population showed a stronger beneficial effect compared to the overall population, with an effect size of -0.23 and a p-value of 0.2. There was no effect on CDR-SB, which measures cognition and function (lower scores are better). We also evaluated the effect size of additional endpoints (Figure 4). Across most endpoints, XPro showed favorable trends, with effect sizes approaching the 0.2 threshold for clinical relevance.
Figure 3: Phase 2 Study Results – Enriched population primary and key secondary endpoints, change from baseline
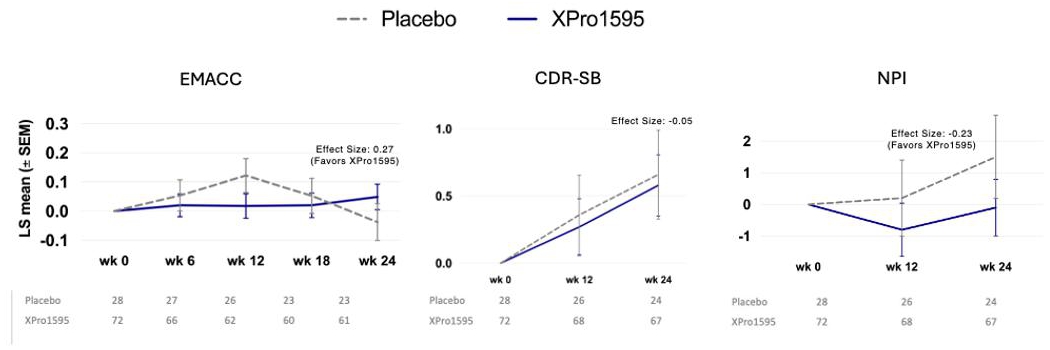
Figure 3: The enriched population show effect size >0.2 favoring XPro1595 on the EMACC and NPI. , A higher EMACC score =better, A lower CDR and NPI score is better. EMACC: LS Mean Diff (SE): 0.086 (0.0603), 90% CI: -0.0146, 0.1857, p-value: 0.1594. CDR-SB: LS Mean Diff (SE): -0.08 (0.307), 90% CI: -0.593, 0.426, p-value: 0.7859. NPI: LS Mean Diff (SE): -1.6 (1.25), 90% CI: -3.71, 0.47, p-value: 0.2003
Figure 4: Phase 2 Study Results – Most endpoints favor treatment with XPro1595.
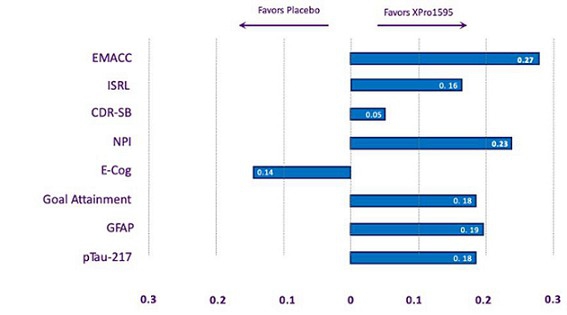
Figure 4: Effect size of XPro across multiple endpoints described as absolute effect sizes (cohen’s D).
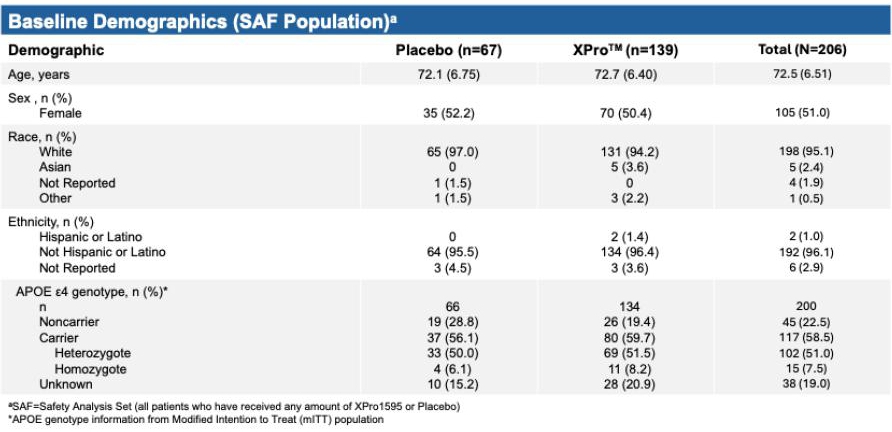
Demographics
Safety
| Safety: Treatment Emergent Adverse Events (TEAEs): Safety Analyses Set | |||
| Event, n (%) | Placebo (n=67) | XPro1595 (n=139) | Total (n=206) |
| Any TEAE | 59 (88.1%) | 131 (94.2%) | 190 (92.2%) |
|
Any TEAE by Maximum Severity Mild Moderate Severe |
34 (50.7%) 22 (32.8%) 3 (4.5%) |
73 (52.5%) 56 (40.3%) 2 (1.4%) |
107 (51.9%) 78 (37.9%) 5 (2.4%) |
| Any Serious TEAE | 5 (7.5%) | 8 (5.8%) | 13 (6.3%) |
| Any Treatment-Related Serious TEAE | 0 | 2 (1.4%) | 2 (1.0%) |
| Any TEAE Leading to Treatment Discontinuation | 2 (3.0%) | 12 (8.6%) | 14 (6.8%) |
| Any TEAE Leading to Study Withdrawal | 2 (3.0%) | 12 (8.6%) | 14 (6.8%) |
| Any TEAE with Fatal Outcome | 0 | 0 | 0 |
| System Organ Class & Preferred Term | Placebo (n=67) | XPro1595 (n=139) | Total (n=206) |
| General disorders and administration site conditions | |||
| Injection site reaction | 2 (3.0%) | 73 (52.5%) | 75 (36.4%) |
| Injection site erythema | 0 | 49 (35.3%) | 49 (23.8%) |
| Fatigue | 9 (13.4%) | 17 (12.2%) | 26 (12.6%) |
| Injection site hypersensitivity | 0 | 14 (10.1%) | 14 (6.8%) |
| Injection site pruritus | 0 | 12 (8.6%) | 12 (5.8%) |
| Infections and infestations | |||
| Upper respiratory tract infection | 11 (16.4%) | 9 (6.5%) | 20 (9.7%) |
| Musculoskeletal and connective tissue disorders | |||
| Arthralgia | 4 (6.0%) | 16 (11.5%) | 20 (9.7%) |
| Nervous system disorders | |||
| Headache | 7 (10.4%) | 14 (10.1%) | 21 (10.2%) |
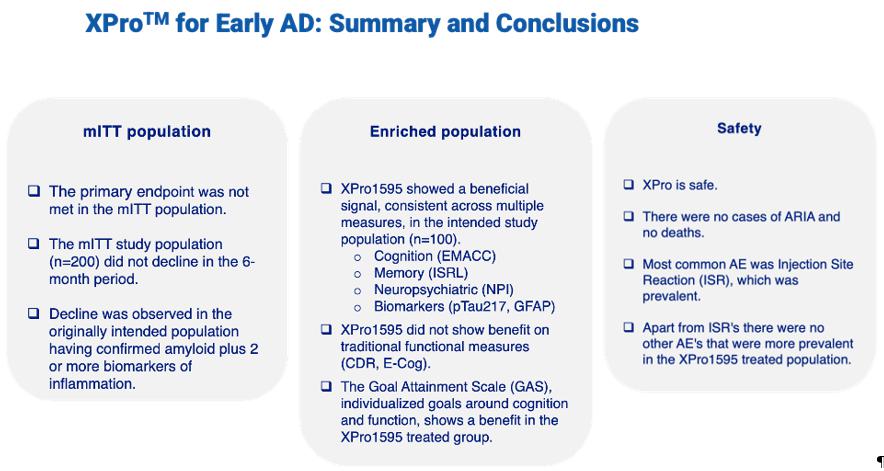
The Company believes these findings from the Phase 2 results indicate that XPro may offer benefits to a readily identified subgroup of Alzheimer’s patients across all ages with biomarker-defined neuroinflammation, regardless of comorbidities or ApoE4 status and potentially lays the foundation for advancing XPro as a promising treatment for AD. The Company is planning an end-of-phase 2 meeting with the FDA, which is expected to occur towards the end of 2025, to determine next steps and expects to be eligible for Break Through status.
CORDStrom, developed by INmune Bio circa 2020, represents a breakthrough in mesenchymal stromal cell technology. The CORDStrom platform leverages, among other things, proprietary screening, pooling and expansion techniques to create off-the-shelf, allogeneic, pooled human umbilical cord -derived mesenchymal stromal cells (HucMSCs) as medicines to treat complex inflammatory diseases. CORDStrom products are designed to provide high-quality, off-the-shelf, batch-to-batch consistent, scalable, cGMP manufactured, potent cellular medicines that can be produced at low cost and with repeatable specification. Initially developed at the INKmune manufacturing facilities utilizing United Kingdom academic grant funding, CORDStrom is a product platform that shows promise as a therapy for RDEB and many other debilitating conditions. While the first generation CORDStrom product is agnostic to indication, the platform enables creation of indication-specific products, which can be tuned for optimization of anti-inflammatory, immunomodulatory, wound healing, and other characteristics.
The CORDStrom product platform shares many similarities, including raw materials, equipment, and procedures, with the Company’s INKmune oncology product, enabling the Company to leverage economies of scale, experienced staff, and other resources to strategically manufacture both products in a rotational campaign with resource and environmental efficiencies.
Children with RDEB have skin that is damaged by even the smallest amount of friction which causes severe blistering, deep wounds, and scars. It is caused by a fault in a gene that makes collagen, a protein that holds the skin layers together. There are limited options available for treatment, none that adequately meet the needs of patients, and the condition gets worse over time with most children reliant on a wheelchair as they move into their teenage years. Many of those with an RDEB diagnosis will also go on to develop aggressive life-threatening skin cancer in adulthood caused by the accumulated damage to their skin. The Company estimates roughly 2,000 people suffer from RDEB in the US, United Kingdom and EU representing a large unmet opportunity to potentially provide routine clinical care to these children.
Since 2020, the Company has supplied CORDStrom HucMSCs as an investigational medical product to the Great Ormond Street Hospital (“GOSH”), London, in connection with the MissionEB study, which was primarily funded by a grant from the National Institute for Health and Care Research (“NIHR”) in the United Kingdom. INmune Bio was compensated for CORDStrom used in the trial and was not a sponsor of the Mission EB study. Investigators recently concluded a double blinded, placebo-controlled arm of the study, which evaluated the safety and efficacy of CORDStrom in 30 pediatric patients (less than 16 years old) in the United Kingdom with intermediate and severe RDEB using a novel cross-over clinical trial design. Patients were randomized to CORDStrom or placebo arms and received 2, intravenous infusions two weeks apart and then followed for 9 months. Each child then crossed over to the other arm and received two doses of placebo or CORDStrom two weeks apart with a further 9-month follow-up.
All patients were treated as day-cases and no CORDStrom related serious adverse events were reported through the study. Top-line results showed the treatment was easily administered, well tolerated and there were beneficial effects across all types of patients receiving CORDStrom with respect to Itch Man Scale, iscorEB clinician score and iscorEB skin involvement. Most notably, CORDStrom significantly reduced itch scores as measured by the Itch Man Scale. In patients with the most severe disease activity, CORDStrom reduced itch at 3 months and led to a sustained reduction of over 27% at 6 months. These results demonstrate a clinically meaningful reduction in itch severity sustained over time. Intermediate group patients showed a broader range of improvements, including reduced skin involvement and less pain as well as large reduction in itch. The younger patients (less than 10 years old) showed improvements in skin score, indicating better skin integrity and reduced disease activity. Interviews with patients and caregivers on completing follow up strongly support the clinical benefits of the therapy; both caregivers and patients were able to correctly identify which treatment had been CORDStrom and which had been placebo. Those who completed the study are asking to continue on therapy, which the Company intends to pursue as an open-label study.
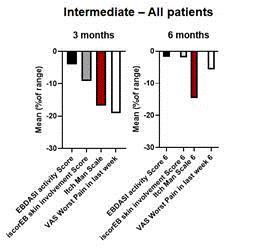
The Mission EB data form the basis of a license that was entered into between INmune Bio and GOSH, whereby the Company gains exclusive access to the clinical study data for commercial uses in exchange for payment of an initiation milestone of £250,000 (approximately $0.3 million at June 30, 2025) and a single development milestone of approximately £6 million (approximately $7.8 million at June 30, 2025) due on receipt of first marketing authorization from the FDA, EMA, or MHRA, which has not occurred yet, and an ongoing commitment to supply CORDStrom to patients enrolled in an open label arm of the Mission EB trial, subject to certain limitations.
After reviewing results of the Mission EB study, the Company initiated a Type C meeting with the FDA to obtain CMC and regulatory feedback and submitted information, data and requests for Rare Pediatric Disease and Orphan Drug Designations (RPDD/ODD).
The FDA granted RPDD to the Company’s CORDStrom product on December 13, 2024, ahead of the sunset period under Section 529(b)(5) of the Federal Food, Drug, and Cosmetic Act. As such, CORDStrom remains eligible to receive a Priority Review Voucher (PRV) if approved by the FDA on or prior to September 30, 2026, assuming the PRV program is not extended. If granted, a PRV can be redeemed to receive priority review for a different product. Alternatively, a PRV may be transferred or sold to another organization.
The FDA granted ODD to the Company’s CORDStrom product on January 6, 2025. Benefits of ODD include certain tax credits and eligibility for select grants, waiver of FDA user fees, including the BLA application fees, access to frequent meetings with the FDA for efficient drug development, and eligibility for seven (7) years of market exclusivity post approval.
The Company plans to prepare for and hold a pre-BLA meeting to discuss particulars of its planned BLA submission, with intent to submit a BLA this year seeking approval of CORDStrom for treatment of RDEB. Concurrently, the Company will also seek to submit MAAs to the EU and United Kingdom in 2026.
We have demonstrated that INKmune improves the ability of the patient’s own NK cells to attack their tumor. INKmune interacts with the patient’s NK cells to convert them from inert resting NK cells into memory-like NK cells that kill the patient’s cancer cells. . INKmune is designed to be given to patients after their immune system has recovered after cytotoxic chemotherapy to target the residual disease that remains after treatment with cytotoxic therapy. We believe INKmune can be used to treat numerous hematologic malignancies and solid tumors including leukemia, multiple myeloma, lymphoma, lung, ovary, breast, renal and prostate cancer. The Company sponsored a Phase I trial using INKmune to treat patients with high risk MDS/AML, a form of leukemia in the UK. Due to Covid restrictions only one patient completed treatment and follow-up in the Phase I trial for MDS; a further three AML patients were treated compassionately. Due to the post-Covid recruitment problems, the Company decided to terminate further enrollment in the MDS/AML trial in March 2024. Nonetheless, from the four patients treated and completing follow-up it was determined that INKmune therapy is safe and promotes development of cancer killing memory-like NK cells that are activated and can kill NK-resistant cancer cells which can be found in the patient’s circulation for up to 4 months after completion of treatment. The Company initiated a separate multicenter Phase I/II trial of INKmune in a metastatic castrate resistant prostate cancer in the US. The open label trial enrolled the first patient in December 2023 and is currently in Phase II across 6 US sites.
The Phase I/II trial using INKmune to treat patients with metastatic castrate resistant prostate cancer (mCPRC) is an open label trial. Biomarker data from the patients will be visible as patients are treated. The Company will report data from each cohort as it becomes available. Because of the modified Bayesian design, the Company estimates the trial will be completely enrolled Q425 with top-line data available 6 months later. Topline data are divided into immunologic and tumor response variables. The most important immunologic response variable is related to memory-like NK cell persistence. There are 3 important variables to tumor response: i) blood PSA changes; ii) change in PSMA-PET scan and iii) change in circulating tumor DNA (ctDNA). INKmune is not a hormone-targeting treatment and will not directly reduce PSA levels but tumor load measured by PSMA-PET and/or ctDNA are expected to decrease with treatment. We do not expect this 6-month trial to provide survival data.
We continue to incur significant development and other expenses related to our ongoing operations. As a result, we are not and have never been profitable and have incurred losses in each period since our inception, resulting in substantial doubt in our ability to continue as a going concern. We reported a net loss of $34.2 million for the six months ended June 30, 2025. As of June 30, 2025 and December 31, 2024, we had cash and cash equivalents of $33.4 million and $20.9 million, respectively. We expect to continue to incur significant losses for the foreseeable future, and we expect these losses to increase as we continue our research and development of, and seek regulatory approvals for, our product candidates. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues, if any.
Our recurring net losses and negative cash flows from operations raised substantial doubt regarding our ability to continue as a going concern within one year after the issuance of our unaudited condensed consolidated financial statements for the six months ended June 30, 2025. Until we can generate sufficient revenue from the commercialization of our product candidates, we expect to finance our operations through the public or private sale of equity, debt financings or other capital sources, such as government funding, collaborations, strategic alliances, divestment of non-core assets, or licensing arrangements with third parties. To date, the Company has relied on equity and debt financing to fund its operations.
Other Developments
The new U.S. administration has announced or imposed a series of tariffs on U.S. trading partners. In response, several countries have threatened or imposed retaliatory measures. At this time, we do not anticipate the tariffs and changes in trade policies in place as of the filing of this Quarterly Report on Form 10-Q to have a significant adverse effect on our business or operations.
Following recent changes more broadly within the NIH and FDA, we have not noticed any disruption of communications with the NIH and FDA to date and continue to maintain productive interactions. To date, there has been no impact to the Company’s operations due to any changes at the NIH or FDA.
Research and Development
Research and development expense consists of expenses incurred while performing research and development activities to discover and develop our product candidates. This includes conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings for product candidates. We recognize research and development expenses as they are incurred. Our research and development expense primarily consist of:
| ● | clinical trial and regulatory-related costs; |
| ● | expenses incurred under agreements with investigative sites and consultants that conduct our clinical trials; | |
| ● | manufacturing and testing costs and related supplies and materials; and | |
| ● | employee-related expenses, including salaries, benefits, travel and stock-based compensation. |
The following table summarizes our research and development expenses by product candidate for the periods indicated (in thousands):
| Three Months Ended | Six Months Ended | |||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| External Costs | ||||||||||||||||
| DN-TNF - Alzheimer’s disease | $ | 3,249 | $ | 4,776 | $ | 8,101 | $ | 11,130 | ||||||||
| INKmune - High Risk MDS/AML & Prostate cancer and CORDStrom | 1,092 | 1,067 | 2,365 | 2,254 | ||||||||||||
| Preclinical and other programs | 62 | 248 | 62 | 361 | ||||||||||||
| Accrued research and development rebate | (243 | ) | (953 | ) | (336 | ) | (1,262 | ) | ||||||||
| Total external costs | 4,160 | 5,138 | 10,192 | 12,483 | ||||||||||||
| Internal costs | 1,644 | 1,915 | 3,251 | 3,263 | ||||||||||||
| Total | $ | 5,804 | $ | 7,053 | $ | 13,443 | $ | 15,746 | ||||||||
We typically use our employee resources across our development programs. We track outsourced development costs by product candidate or development program, but we do not allocate internal costs personnel costs including salaries and stock-based compensation to specific product candidates or development programs.
We participate, through our wholly owned subsidiary in Australia, in the Australian research and development tax incentive program, such that a percentage of our qualifying research and development expenditures are reimbursed by the Australian government, and such incentives are reflected as a reduction of research and development expense. The Australian research and development tax incentive is recognized when there is reasonable assurance that the incentive will be received, the relevant expenditure has been incurred and the amount of the consideration can be reliably measured.
We participate, through our wholly owned subsidiary in the United Kingdom, in the research and development program provided by the United Kingdom tax relief program, such that a percentage of our qualifying research and development expenditures are reimbursed by the United Kingdom government, and such incentives are reflected as a reduction of research and development expense. The United Kingdom research and development tax incentive is recognized when there is reasonable assurance that the incentive will be received, the relevant expenditure has been incurred and the amount of the consideration can be reliably measured.
Substantially all our research and development expenses to date have been incurred in connection with our current and future product candidates. We expect our research and development expenses to increase significantly for the foreseeable future as we advance an increased number of our product candidates through clinical development, including the conduct of our planned clinical trials and manufacturing drug to be used in those clinical trials. The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. The successful development of product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs required to complete the remaining development of any product candidates. This is due to the numerous risks and uncertainties associated with the development of product candidates.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
| ● | per patient trial costs; | |
| ● | the number of sites included in the clinical trials; | |
| ● | the countries in which the clinical trials are conducted; | |
| ● | the length of time required to enroll eligible patients; | |
| ● | the number of patients that participate in the clinical trials; | |
| ● | the number of doses that patients receive; | |
| ● | the cost of comparative agents used in clinical trials; | |
| ● | the drop-out or discontinuation rates of patients; |
| ● | potential additional safety monitoring or other studies requested by regulatory agencies; | |
| ● | the duration of patient follow-up; | |
| ● | the efficacy and safety profile of the product candidate; and | |
| ● | the cost of manufacturing, finishing, labelling and storage drug used in the clinical trial. |
We do not expect any of our product candidates to be commercially available for at least the next several years, if ever. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, which may fluctuate significantly from quarter-to-quarter and year-to-year. We anticipate that our expenses will increase substantially as we:
| ● | continue research and development, including preclinical and clinical development of our existing product candidates; | |
| ● | potentially seek regulatory approval for our product candidates; | |
| ● | seek to discover and develop additional product candidates; | |
| ● | establish a commercialization infrastructure and scale up our manufacturing and distribution capabilities to commercialize any of our product candidates for which we may obtain regulatory approval; |
| ● | seek to comply with regulatory standards and laws; | |
| ● | maintain, leverage and expand our intellectual property portfolio; | |
| ● | hire clinical, manufacturing, scientific and other personnel to support our product candidates development and future commercialization efforts; | |
| ● | add operational, financial and management information systems and personnel; and | |
| ● | incur additional legal, accounting and other expenses in operating as a public company. |
Results of Operations
Comparison of the Three Months Ended June 30, 2025 and 2024
The following table summarizes our results of operations for the periods indicated:
| Three Months Ended June 30, |
||||||||||||
| (in thousands) | 2025 | 2024 | Change | |||||||||
| Revenues | $ | - | $ | - | $ | - | ||||||
| Operating expenses: | ||||||||||||
| Research and development | 5,804 | 7,053 | (1,249 | ) | ||||||||
| General and administrative | 2,253 | 2,812 | (559 | ) | ||||||||
| Impairment of acquired in-process research and development intangible assets | 16,514 | - | 16,514 | |||||||||
| Total operating expenses | 24,571 | 9,865 | 14,706 | |||||||||
| Loss from operations | (24,571 | ) | (9,865 | ) | (14,706 | ) | ||||||
| Other income, net | 113 | 119 | (6 | ) | ||||||||
| Net loss | $ | (24,458 | ) | $ | (9,746 | ) | $ | (14,712 | ) | |||
Research and Development
Research and development expenses were approximately $5.8 million during the three months ended June 30, 2025, compared to approximately $7.1 million during the three months ended June 30, 2024. The change in research and development expenses during the three months ending June 30, 2025 compared to the three months ending June 30, 2024 is mainly due to the Company incurring $1.5 million less expenses related to our Alzheimer’s clinical program due to the Company completing the Phase 2 clinical trial.
General and Administrative
General and administrative expenses were approximately $2.3 million during the three months ended June 30, 2025 compared to $2.8 million during the three months ended June 30, 2024. The decrease in general and administrative expenses was mainly due to the Company incurring $0.4 million lower stock-based compensation during 2025.
Impairment of acquired in-process research and development intangible assets
During the three months ended June 30, 2025, the Company released the Phase 2 clinical trial results for our Alzheimer’s drug candidate, XPro, which failed to meet the primary endpoint, though a subgroup showed potential benefits. Due to insufficient resources to fund further trials, the Company has halted immediate plans to develop XPro for Alzheimer’s or other indications and are instead seeking a partner to continue these studies. As part of preparing its interim unaudited condensed consolidated financial statements, the Company determined that the intangible asset’s fair value was likely below its carrying value. Following a quantitative impairment assessment, the Company estimated the asset’s fair value at $0 as of June 30, 2025, resulting in a recorded impairment of $16.5 million.
Other Expense, net
During the three months ended June 30, 2025, and 2024, the Company recorded $0.1 million of other income due to the Company earning interest income on its cash investments.
Comparison of the Six Months Ended June 30, 2025 and 2024
The following table summarizes our results of operations for the periods indicated:
| Six Months Ended June 30, |
||||||||||||
| (in thousands) | 2025 | 2024 | Change | |||||||||
| Revenues | $ | 50 | $ | 14 | $ | 36 | ||||||
| Operating expenses: | ||||||||||||
| Research and development | 13,443 | 15,746 | (2,303 | ) | ||||||||
| General and administrative | 4,569 | 5,150 | (581 | ) | ||||||||
| Impairment of acquired in-process research and development intangible assets | 16,514 | - | 16,514 | |||||||||
| Total operating expenses | 34,526 | 20,896 | 13,630 | |||||||||
| Loss from operations | (34,476 | ) | (20,882 | ) | (13,594 | ) | ||||||
| Other income, net | 279 | 111 | 168 | |||||||||
| Net loss | $ | (34,197 | ) | $ | (20,771 | ) | $ | (13,426 | ) | |||
Revenues
During the six months ended June 30, 2025, the Company recognized revenue from a license agreement that was terminated during 2025. During the six months ended June 30, 2024, the Company recognized revenue from the sale of MSC’s.
Research and Development
Research and development expenses were approximately $13.4 million and $15.7 million during the six months ended June 30, 2025 and 2024, respectively. The change in research and development expenses during the six months ending June 30, 2025 compared to the six months ending June 30, 2024 is mainly due to the Company incurring $3.0 million less Alzheimer’s clinical program expenses due to the trial being completed in 2025, partially offset by the Company recording $0.9 million less accrued rebate during the six months ended June 30, 2025.
General and Administrative
General and administrative expenses were approximately $4.6 million and $5.2 million during the six months ended June 30, 2025 and 2024, respectively. The $0.6 million decrease in general and administrative expenses was mainly due to $0.3 million lower stock-based compensation and $0.3 million lower investor relations expense.
Impairment of acquired in-process research and development intangible assets
During the six months ended June 30, 2025, the Company released the Phase 2 clinical trial results for our Alzheimer’s drug candidate, XPro, which failed to meet the primary endpoint, though a subgroup showed potential benefits. Due to insufficient resources to fund further trials, the Company has halted immediate plans to develop XPro for Alzheimer’s or other indications and are instead seeking a partner to continue these studies. As part of preparing its interim unaudited condensed consolidated financial statements, the Company determined that the intangible asset’s fair value was likely below its carrying value. Following a quantitative impairment assessment, the Company estimated the asset’s fair value at $0 as of June 30, 2025, resulting in a recorded impairment of $16.5 million.
Other Income, net
During the six months ended June 30, 2025, the Company recorded $0.3 million of other income due to the Company earning interest income on its cash investments. During the six months ended June 30, 2024, the Company earned $0.1 million of other income consisting of interest income partially offset by interest expense.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis.
We incurred a net loss of $34.2 million and $20.8 million for the six months ended June 30, 2025 and 2024, respectively. Net cash used in operating activities was $14.2 million and $15.4 million for the six months ended June 30, 2025 and 2024, respectively. Since inception, we have funded our operations primarily with proceeds from the sales of our common stock. As of June 30, 2025, we had cash and cash equivalents of $33.4 million. We anticipate that operating losses and net cash used in operating activities will increase over the next few years as we advance our products under development.
During the six months ending June 30, 2025, the Company sold 1,304,707 shares of common stock at an average price of $8.01 for gross proceeds of approximately $10.4 million under the ATM offering.
During June 2025, the Company entered into securities purchase agreements with investors whereby the Company sold 3,000,000 shares of the common stock in a registered direct offering in exchange for gross proceeds of $18.9 million (net proceeds of approximately $17.4 million).
Our primary uses of capital are, and we expect will continue to be, third-party clinical and preclinical research and development services, costs incurred to manufacture our drugs under development, compensation and related expenses, legal, patent and other regulatory expenses and general overhead costs. We believe our use of CROs provides us with flexibility in managing our spending.
The Company incurs significant research and development expenses in Australia and the United Kingdom. Fluctuations in the rate of exchange between the United States dollar and the pound sterling as well as the Australian dollar could adversely affect our financial results, including our expenses as well as assets and liabilities. We currently do not hedge foreign currencies but will continue to assess whether that strategy is appropriate. As of June 30, 2025, the cash balance held by our foreign subsidiaries with currencies other than the United States dollar was approximately $0.2 million.
Our recurring net losses and negative cash flows from operations, as well as forecast of continued losses and negative cash flows from operations, raised substantial doubt regarding our ability to continue as a going concern within one year after the issuance of our unaudited condensed consolidated financial statements for the six months ended June 30, 2025. Until we can generate sufficient revenue from the commercialization of our product candidates, we expect to finance our operations through the public or private sale of equity, debt financing or other capital sources, such as government funding, collaborations, strategic alliances, divestment of non-core assets, or licensing arrangements with third parties. Our cash and cash equivalents were $33.4 million and total current assets were $36.0 million at June 30, 2025, which the Company is projecting will be insufficient to sustain its operations through one year following the date that the financial statements are issued.
Additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development of one or more of our product candidates or cease operations. If we raise additional funds through the issuance of additional debt or equity securities it could result in dilution to our existing stockholders, increased fixed payment obligations and these securities may have rights senior to those of our common stock and could contain covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license our intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects.
Financing strategies we may pursue include, but are not limited to, the public or private sale of equity, debt financing or funds from other capital sources, such as government or grant funding, collaborations, strategic alliances, divestment of non-core assets, or licensing arrangements with third parties. There can be no assurances additional capital will be available to secure additional financing, or if available, that it will be sufficient to meet our needs on favorable terms. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development of one or more of our product candidates. If we raise additional funds through the public or private sale of equity or debt financings, it could result in dilution to our existing stockholders or increased fixed payment obligations and these securities may have rights senior to those of our common stock and could contain covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license our intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects.
Cash Flows
The following table summarizes our cash flows for the periods indicated:
| Six Months Ended June 30, |
||||||||
| (in thousands) | 2025 | 2024 | ||||||
| Net cash and cash equivalents (used in) provided by: | ||||||||
| Operating activities | $ | (14,199 | ) | $ | (15,362 | ) | ||
| Investing activities | (706 | ) | - | |||||
| Financing activities | 27,545 | 10,497 | ||||||
| Change in cash and cash equivalents | 12,640 | (4,865 | ) | |||||
| Impact on cash from foreign currency translation | (188 | ) | 86 | |||||
| Cash and cash equivalents, beginning of period | 20,922 | 35,848 | ||||||
| Cash and cash equivalents, end of period | $ | 33,374 | $ | 31,069 | ||||
Operating Activities
Operating activities used approximately $14.2 million of cash during the six months ended June 30, 2025, resulting mainly from our loss of $34.2 million, partially offset by an intangibles impairment expense of $16.5 million and non-cash stock-based compensation of $3.6 million.
Operating activities used approximately $15.4 million of cash during the six months ended June 30, 2024, resulting from our loss of $20.8 million, partially offset by changes in our net operating assets and liabilities of $1.2 million and non-cash stock-based compensation of $4.1 million. The change in our net operating assets and liabilities was mainly due to an increase in accounts payable and accrued liabilities of $1.4 million, a decrease in prepaid expenses of $0.5 million and a decrease in other tax receivable of $0.3 million, partially offset by an increase in research and development tax receivable of $1.2 million.
Investing Activities
During the six months ended June 30, 2025, the Company acquired $0.7 million of equipment to be used in its CORDStrom clinical program.
Financing Activities
During the six months ended June 30, 2025, the Company sold 1,304,707 shares of common stock under its ATM program for net proceeds of $10.1 million.
During June 2025, the Company sold 3,000,000 shares of its common stock in a registered direct offering in exchange for gross proceeds of $18.9 million (net proceeds of $17.4 million).
During the six months ended June 30, 2024, the Company sold 198,364 shares of its common stock under its ATM program for net proceeds of approximately $2.0 million.
During the six months ended June 30, 2024, the Company sold 1,557,692 shares of its common stock and 1,557,592 warrants to purchase its common stock for net proceeds of $13.5 million.
During the six months ended June 30, 2024, the Company repaid $5.0 million of its debt.
Critical Accounting Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our unaudited condensed consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. Actual results may differ from these estimates. Our critical accounting estimates are discussed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and there have been no material changes during the six months ended June 30, 2025.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Pursuant to Item 305(e) of Regulation S-K (§ 229.305(e)), the Company is not required to provide the information required by this Item as it is a “smaller reporting company,” as defined by Rule 229.10(f)(1).
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Principal Executive Officer and Interim Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) at the end of the period covered by this quarterly report.
Based on this evaluation, we concluded that, as of such date, our disclosure controls and procedures were effective to provide reasonable assurance that the information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management, including our Principal Executive Officer and Interim Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
We recognize that any controls system, no matter how well designed and operated, can provide only reasonable assurance of achieving its objectives, and our management necessarily applies its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the period covered by this quarterly report that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act).
PART II – OTHER INFORMATION
Item 1. Legal Proceedings
We are not currently a party to any pending legal proceedings that we believe will have a material adverse effect on our business or financial conditions. We may, however, be subject to various claims and legal actions arising in the ordinary course of business from time to time.
Item 1A. Risk Factors
Not required for smaller reporting companies.
Item 2. Recent Sales of Unregistered Securities; Use of Proceeds from Registered Securities
None.
Item 3. Defaults Upon Senior Securities
Not applicable.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
Severance Agreement
On August 4, 2025, Dr. Raymond J. Tesi informed the Company of his intention to retire and resign from his roles as President, Chief Executive Officer, Chief Medical Officer, Chairman of the Board of Directors (the “Board”) and all positions from the Company and its subsidiaries, effective on the Effective Date (as defined below). Dr. Tesi’s resignation is not the result of any dispute or disagreement with the Company or the Board on any matter relating to the Company’s operations, policies or practices.
In connection with Dr. Tesi’s retirement, Dr. Tesi and the Company entered into a Separation Agreement and Mutual Release, dated August 4, 2025 (the “Severance Agreement”), pursuant to which, in exchange for a general release of claims and other customary terms, the Company agreed to provide Dr. Tesi with a severance payment of $166,000, less applicable taxes and withholdings, payable within 30 days of execution of the Severance Agreement. The Company also agreed to pay Dr. Tesi $97,128.38 in payment for 48.25 accrued but unused vacation days as of the Effective Date. In addition, the Company will continue to pay the cost of health insurance coverage for Dr. Tesi and his spouse through December 31, 2025, either by continuing existing coverage or reimbursing COBRA premiums, subject to early termination if Dr. Tesi becomes covered under another employer’s plan.
Under the terms of the Severance Agreement, all unvested stock options held by Dr. Tesi as of the Effective Date will remain outstanding and continue to vest in accordance with their original terms, provided that Dr. Tesi remains in compliance with the Severance Agreement. All vested stock options will remain exercisable for the later of five years following the Effective Date or their original expiration date. The agreement also imposes resale limitations on Dr. Tesi’s beneficial ownership of Company securities, restricting him from selling more than 25% of his beneficially owned shares of common stock in any calendar month during the 18-month period following the Effective Date.
The Company further agreed to maintain directors’ and officers’ liability insurance for Dr. Tesi for a period of at least three years following the Effective Date on terms no less favorable than those applicable to its then-serving officers and directors. The Severance Agreement also reaffirms Dr. Tesi’s right to indemnification under Nevada law, the Company’s articles of incorporation and bylaws, as amended and in effect as of the date hereof, and provides for contribution rights in the event indemnification is unavailable.
The Severance Agreement includes mutual releases of claims, as well as customary provisions relating to confidentiality, non-disparagement, cooperation, and return of Company property. The Severance Agreement is subject to a seven-day revocation period following execution and shall become effective on August 12, 2025, if not revoked before (the “Effective Date”).
The foregoing summary of the Severance Agreement is qualified in its entirety by reference to the full text of the Severance Agreement, which is filed as Exhibit 10.4 hereto and incorporated herein by reference.
Appointment of New Chairman
On August 4, 2025, the Board appointed J. Kelly Ganjei, an existing member of the Board, as Chairman of the Board, effective as of the Effective Date. Mr. Ganjei has served on the Board since September 2016 and brings extensive leadership and industry experience to the role of Chairman. Mr. Ganjei will hold office until the election and qualification of a successor or until either individual’s earlier death, resignation or removal.
Appointment of New President, Chief Executive Officer and Member of the Board
On August 4, 2025, the Board appointed David J. Moss, who was then serving as the Company’s Chief Financial Officer, Treasurer and Secretary, as President, Chief Executive Officer and as a member of the Board, effective as of the Effective Date. On the same date, the Board also appointed Mr. Moss as the Company’s Principal Executive Officer, effective immediately. In connection with these appointments, Mr. Moss resigned as Chief Financial Officer, effective immediately. The Principal Executive Officer position will terminate as of the Effective Date, upon the effectiveness of Mr. Moss’s appointment as President, Chief Executive Officer and as a member of the Board.
Mr. Moss, age 55, is a co-founder and has been the Chief Financial Officer since the Company’s formation in September 2015. He also serves as Secretary and Treasurer and from September 15, 2015 until April 2018, Mr. Moss was also a member of the Board. Mr. Moss was audit committee chair for Qilian International Holding Groups LTD. from December 2020 to February 2022 and served as a director and audit committee chair of Xiangtai Food Co from Aug 2019 to Aug 2020 and was a director of Pegasi Energy Resources Corporation from May 2007 to January 2014 and was a founding investor in Reliant Service Group LLC which sold in 2015 to a leading private equity firm. From 1996 until 2001 he served as Managing Partner at a Seattle based venture capital firm, The Phoenix Partners. From November 2010 until October 2011, Mr. Moss was the Chief Executive Officer, sole director and a majority shareholder of Tamandare Explorations Inc. a private specialty pharmaceutical company. In October 2011 Tamandare Explorations engaged in a merger transaction pursuant with Tonix Pharmaceuticals Holding Corp., which at the time had its common stock listed on the OTC Bulletin Board and is currently listed on Nasdaq Capital Market. In connection with the merger transaction Mr. Moss resigned as Tamandare Explorations Chief Executive Officer and a member of its board of directors. From 2001 until the formation of INmune Bio in 2015, Mr. Moss has invested in healthcare technology companies. Mr. Moss holds an MBA from Rice University and a BA in Economics from the University of California, San Diego.
For serving as the Company’s President and Chief Executive Officer, Mr. Moss’s compensation paid by the Company will continue at its current amount pursuant to his previous disclosed employment agreement, between Mr. Moss and the Company, dated January 1, 2021, with an annual base salary of $408,722 and remain eligible for an annual discretionary bonus with a target amount of 40% of his then current base salary as determined by the Board and/or compensation committee of the Board in its discretion based upon the achievement of corporate and/or individual objectives that are determined in the sole discretion of the Board. Mr. Moss will not receive any additional compensation for serving as a member of the Board. The terms of employment of Mr. Moss as the Company’s President and Chief Executive Officer will be revisited by the Compensation Committee and the Board and disclosed to the market in the future.
There are no arrangements or understandings between Mr. Moss and any other persons pursuant to which he was elected as an officer or director. Mr. Moss does not have any family relationships with any of the Company’s directors or executive officers. There are no transactions involving the Company and Mr. Moss that the Company would be required to report pursuant to Item 404(a) of Regulation S-K.
Appointment of Interim Chief Financial Officer
On August 4, 2025, the Board appointed Cory Ellspermann as Interim Chief Financial Officer of the Company, effective immediately.
Mr. Ellspermann, age 53, has served as our Interim Chief Financial Officer since August 2025. Prior to Mr. Ellspermann’s appointment as our Interim Chief Financial Officer, Mr. Ellspermann served as our Controller and VP of Finance since June 2019. Mr. Ellspermann possesses nearly 30 years of financial management experience at public and private companies. Prior to joining us, Mr. Ellspermann was Senior Accounting Manager at Artivest, an alternative investments company. He is a certified public accountant in the State of Texas and previously served as a Senior Audit Manager at Ernst & Young. He holds a BS in Accounting from Purdue University.
Effective upon his appointment as Interim Chief Financial Officer, Mr. Ellspermann will continue to serve pursuant to his employment agreement with the Company dated December 16, 2021, which provides for at-will employment and sets forth his compensation and benefits. Under the employment agreement, Mr. Ellspermann receives an annual base salary of $181,125 and is eligible for a performance-based bonus, subject to criteria established by the Company’s compensation committee.
If the Company terminates Mr. Ellspermann’s employment without Cause (as defined in the employment agreement), he is entitled to nine months of severance pay, subject to the execution and non-revocation of a release of claims. In the event of a termination without Cause in connection with a Change in Control (as defined in the employment agreement), all of Mr. Ellspermann’s time-based equity awards will vest in full, and any options will become fully exercisable.
The employment agreement contains customary provisions regarding confidentiality, ownership of intellectual property, and restrictions on competition, solicitation, and interference with the Company’s business relationships during his employment and for one year thereafter.
There are no arrangements or understandings between Mr. Ellspermann and any other persons pursuant to which he was elected as an officer. Mr. Ellspermann does not have any family relationships with any of the Company’s directors or executive officers. There are no transactions involving the Company and Mr. Ellspermann that the Company would be required to report pursuant to Item 404(a) of Regulation S-K.
The terms of employment of Mr. Ellspermann as the Company’s Interim Chief Financial Officer will be revisited by the Compensation Committee and the Board and disclosed to the market in the future. The foregoing summary of Mr. Ellspermann’s employment agreement is qualified in its entirety by reference to the full text of the employment agreement, which is filed as Exhibit 10.5 hereto and incorporated herein by reference.
Item 6. Exhibits
| * | Filed herewith. |
| ** | Furnished herewith. |
| † | Certain confidential information contained in this agreement has been omitted because it is both not material and is the type that the registrant treats as private or confidential. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| INmune Bio Inc. | ||
| Date: August 7, 2025 | By: | /s/ David Moss |
| David Moss | ||
| Principal Executive Officer | ||
| (Principal Executive Officer) | ||
| Date: August 7, 2025 | By: | /s/ Cory Ellspermann |
| Cory Ellspermann | ||
| Interim Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) |
Exhibit 10.4
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS BOTH NOT MATERIAL AND IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
This SEPARATION AGREEMENT AND MUTUAL RELEASE (“Agreement”) is made and entered into by and between Raymond Joseph Tesi, an individual and having a primary address at [***] (“Executive”), and INmune Bio Inc., a Nevada corporation with its principal place of business at 225 NE Mizner Blvd, Suite 640, Boca Raton, Florida 33432 (the “Company”). Executive and the Company are sometimes referred to individually herein as a “Party,” and together herein as the “Parties.”
RECITALS
WHEREAS, Executive and the Company are parties to an Employment Agreement entered into as of January 6, 2021 (the “Employment Agreement”). Terms that appear in initial capital form in this Agreement and which are not otherwise defined herein have the meaning set forth in the Employment Agreement;
WHEREAS, the Company and Executive agree that Executive’s employment with the Company will terminate as of the Effective Date (as defined herein);
WHEREAS, the Company and Executive also agree that Executive shall terminate his service as a member of the Company’s board of directors (the “Board”) as of the Effective Date; and
WHEREAS, the Parties wish to amicably conclude their relationship.
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants and agreements contained herein, and for other good and valuable consideration, the sufficiency and receipt of which is hereby acknowledged, the Parties hereby agree as follows:
1. Termination of Duties; Termination of Employment Agreement.
(a) Executive hereby resigns as the Company’s Chief Executive Officer, a member of the Board, as of the Effective Date of this Agreement. For avoidance of doubt, as of the Effective Date, the Executive also resigns from all other positions that the Executive may have held with the Company and its subsidiaries including any board memberships with any subsidiary or any officer or other position with any subsidiary of the Company. The Employment Agreement shall be terminated in its entirety as of the Effective Date and shall be of no further force or effect.
(b) The Company will pay Executive his base salary earned through the Effective Date, all benefits accrued or vested through the Effective Date pursuant to all fringe benefit plan documents, and $97,128.38, less applicable taxes and withholdings, in payment for Executive’s 48.25 accrued but unused vacation days as of the Effective Date.
2. Severance Benefits. In exchange for the waiver and release (Section 4) and all other consideration provided under this Agreement and the Employment Agreement:
(a) Severance Payment. Within 30 days from entry into this Agreement, the Company shall pay Executive $166,000, less applicable taxes and withholdings.
(b) Tax Consequences. The Company makes no representations or warranties with respect to the tax consequences of the payments made pursuant to Section 2(a) and any other consideration provided to Executive or made on his behalf under the terms of this Agreement. Executive agrees and understands that, other than the Company’s share of payroll taxes, he is responsible for his payment, if any, of any local, state, and/or federal taxes on the payments and any other consideration provided hereunder by the Company and any penalties or assessments thereon. Executive further agrees to indemnify and hold the Company harmless from any claims, demands, deficiencies, penalties, interest, assessments, executions, judgments, or recoveries by any government agency against the Company for any amounts claimed due on account of Executive’s failure to pay or delayed payment of Executive’s share of any local, federal, and/or state taxes.
(c) Section 409A. The payments made under this Agreement are intended to comply with section 409A of the Internal Revenue Code of 1986, as amended, and applicable guidance issued thereunder (“Section 409A”) or an exemption or exclusion therefrom, provided that the Executive acknowledges and agrees that he shall be solely responsible for any taxes and/or penalties imposed under Section 409A. Payments made under this Agreement will be interpreted and construed, to the extent possible, to be distributed in the short-term deferral period, as defined under Treasury Regulation section 1.409A-1(b)(4), or the separation pay exemption, as provided in Treasury Regulation section 1.409A-1(b)(9). For purposes of this Agreement, July 31, 2025 is the date in which Executive’s “separation from service,” as defined in Treasury Regulation section 1.409A-1(h), occurred. For purposes of this Agreement, each payment made, and benefits provided under this Agreement is hereby designated as a separate payment and will not collectively be treated as a single payment, as provided in Treasury Regulation section 1.409A-2(b)(2)(iii).
(d) Health Insurance; COBRA Premiums. The Company hereby agrees to continue to pay for Executive’s and Executive’s spouse’s health insurance from the Effective Date through December 31, 2025 (the “COBRA Premium Period”), either through the payment of the existing premiums for Executive and Executive’s spouse, if permitted, or through the payment of COBRA premiums for Executive and Executive’s spouse (“COBRA Premiums”). The Executive shall take all reasonable actions in order to timely elect COBRA or otherwise continue health insurance coverage. The Parties agree and acknowledge that the Company’s obligation to remit the COBRA Premiums will immediately cease if, during the COBRA Premium Period, Executive commences participation in an alternate health insurance plan through a new employer. In the event that, during the COBRA Premium Period, Executive so commences participation in an alternate health insurance plan through a new employer, Executive shall promptly notify the Company of same.
(e) Officers and Directors Insurance. For a period of at least three (3) years following the Effective Date, the Company shall maintain directors and officers liability insurance in accordance with the Company’s usual and customary practices, which policy shall include customary coverage for Executive with respect to his service on the Board of Directors and as an officer of the Company prior to the Effective Date. Notwithstanding anything to the contrary set forth herein, the Company and Executive understand and agree that (i) the amount and nature of such insurance coverage shall be as determined by the Board of Directors of the Company, and (ii) the insurance coverage afforded to Executive shall be no less than the coverage maintained for the benefit of the Company’s continuing executive officers and members of the Company’s Board of Directors.
(f) Contribution.
(i) Whether or not the indemnification provided under Nevada law (Nev. Rev. Stat. § 78.751) or pursuant to the Company’s articles of incorporation and/or bylaws is available, in respect of any threatened, pending or completed action, suit or proceeding in which the Company is jointly liable with Executive (or would be if joined in such action, suit or proceeding), the Company shall pay, in the first instance, the entire amount of any judgment or settlement of such action, suit or proceeding without requiring Executive to contribute to such payment and the Company hereby waives and relinquishes any right of contribution it may have against Executive. The Company shall not enter into any settlement of any action, suit or proceeding in which the Company is jointly liable with Indemnitee (or would be if joined in such action, suit or proceeding) unless such settlement provides for a full and final release of all claims asserted against Executive.
(ii) Without diminishing or impairing the obligations of the Company set forth in the preceding subparagraph, if, for any reason, Executive shall elect or be required to pay all or any portion of any judgment or settlement in any threatened, pending or completed action, suit or proceeding in which the Company is jointly liable with Indemnitee (or would be if joined in such action, suit or proceeding), the Company shall contribute to the amount of expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred and paid or payable by Executive in proportion to the relative benefits received by the Company and all officers, directors or employees of the Company, other than Executive, who are jointly liable with Executive (or would be if joined in such action, suit or proceeding), on the one hand, and Executive, on the other hand, from the transaction or events from which such action, suit or proceeding arose; provided, however, that the proportion determined on the basis of relative benefit may, to the extent necessary to conform to law, be further adjusted by reference to the relative fault of the Company and all officers, directors or employees of the Company other than Executive who are jointly liable with Executive (or would be if joined in such action, suit or proceeding), on the one hand, and Executive, on the other hand, in connection with the transaction or events that resulted in such expenses, judgments, fines or settlement amounts, as well as any other equitable considerations which applicable law may require to be considered. The relative fault of the Company and all officers, directors or employees of the Company, other than Executive, who are jointly liable with Indemnitee (or would be if joined in such action, suit or proceeding), on the one hand, and Executive, on the other hand, shall be determined by reference to, among other things, the degree to which their actions were motivated by intent to gain personal profit or advantage, the degree to which their liability is primary or secondary and the degree to which their conduct is active or passive.
(iii) The Company hereby agrees to fully indemnify and hold Executive harmless from any claims of contribution which may be brought by officers, directors or employees of the Company, other than Executive, who may be jointly liable with Executive.
(iv) To the fullest extent permissible under applicable law, if the indemnification provided for under Nevada law or pursuant to the Company’s articles of incorporation and/or bylaws is unavailable to Executive for any reason whatsoever, the Company, in lieu of indemnifying Executive, shall contribute to the amount incurred by Executive, whether for judgments, fines, penalties, excise taxes, amounts paid or to be paid in settlement and/or for expenses, in connection with any claim relating to an indemnifiable event under Nevada law or pursuant to the Company’s articles of incorporation and/or bylaws, in such proportion as is deemed fair and reasonable in light of all of the circumstances of such proceeding in order to reflect (i) the relative benefits received by the Company and Executive as a result of the event(s) and/or transaction(s) giving cause to such proceeding and/or (ii) the relative fault of the Company (and its directors, officers, employees and agents) and Executive in connection with such event(s) and/or transaction(s).
(g) Public Announcement. The Parties agree that, reasonably following the Effective Date, the Parties will mutually agree to the content of the announcement of Executive’s separation from the Company.
3. Options Vesting and Limitation on Sales.
(a) Upon the Effective Date of this Agreement, all stock options previously granted to Executive that are vested as of the Effective Date shall remain exercisable for a period which is the later of (i) five (5) years from the Effective Date, or (ii) the current expiration date of such options. All unvested options granted to Executive under the Company’s 2017 Stock Incentive Plan, 2019 Stock Incentive Plan and 2021 Stock Incentive Plan, as amended, and held by Executive as of the Effective Date shall not terminate, shall continue to remain effective and shall continue to vest pursuant to their respective terms so long as Executive does not violate any of the terms of this Agreement. The Company and Executive shall enter into an amendment, on mutually agreeable written terms, to any option agreement if necessary to reflect the agreements in this Section. To the extent necessary, immediately following the Effective Date, the Company shall take any and all necessary steps in order to effectuate the foregoing vesting of Executive’s unvested options and warrants.
(b) Executive acknowledges that he currently beneficially owns 1,554,106 shares of the Company’s common stock (the “Executive’s Common Stock”), including vested and unvested options. Executive agrees that for 18 months following the Effective Date, he shall not sell more than 25% of the Executive’s Common Stock beneficially owned as of the Effective Date in any calendar month. Executive agrees that all sales of the Executive’s Common Stock shall be conducted in compliance with all applicable securities laws and regulations as well as any insider trading policies of the Company that apply to Executive. Executive agrees to timely make any required filings with the Securities and Exchange Commission with respect to the sale of any of the Executive’s Common Stock.
4. Mutual Release of All Claims.
(a) Executive Released Claims. Executive, on behalf of Executive and anyone claiming through Executive, hereby fully and completely releases, acquits and forever discharges the Company, its affiliates and related entities, and each of their respective current and former employees, officers, directors, stockholders, agents, employees, insurers, attorneys, joint venture partners, transferees, successors and assigns (each a “Company Released Party” and collectively, the “Company Released Parties”), collectively, separately, and severally, of and from any and all claims, demands, damages, causes of action, debts, liabilities, controversies, judgments, and suits of every kind and nature whatsoever, foreseen, unforeseen, known or unknown, including those that arise out of or relate to Executive’s employment or termination of employment with the Company, Executive’s directorship at the Company or that Executive has had, now has, or may have against the Company Released Parties (or any of them) at any time up to and including the date Executive executes this Agreement (the claims released in this Section are collectively referred to as the “Executive Released Claims”). The Executive Released Claims include all claims arising under any federal, state or local statute or ordinance, constitutional provision, public policy or common law, regarding (i) employment, employment benefits, or employment discrimination and/or retaliation including, without limitation, Title VII of the Civil Rights Act of 1964 and the Civil Rights Act of 1991, 42 U.S.C. 2000e et seq.; Sections 1981 through 1988 of Title 42 of the United States Code; the Employee Retirement Income Security Act of 1974, 29 U.S.C. 100 et seq.; the Workers Adjustment and Retraining Notification Act, 29 U.S.C. Section 2101 et seq.; the National Labor Relations Act, 29 U.S.C. § 151 et seq.; the Fair Labor Standards Act, 29 U.S.C. § 201 et seq.; the Equal Pay Act, 29 U.S.C. § 201 et seq.; the Immigration Reform and Control Act, 8 U.S.C. § 1101 et seq.; the Americans with Disabilities Act of 1990, 42 U.S.C. § 12101 et seq.; the Age Discrimination and Employment Act of 1967 and the Older Workers Benefits Protection Act, 29 U.S.C. § 621 et seq. (“ADEA”); the Genetic Information Non- Discrimination Act; the Occupational Safety and Health Act, 29 U.S.C. § 651 et seq.; the Family and Medical Leave Act of 1993, 29 U.S.C. § 2601 et seq.; and COBRA, 29 U.S.C. § 1161 et seq., all as amended; and all laws relating to family and medical leave, retaliation, discrimination on the basis of race, color, religion, creed, sex, sex harassment, sexual orientation, gender identity, marital status, pregnancy, national origin, genetic carrier status, ancestry, handicap or disability, alienage, present or past history of mental disorders or physical disability, candidacy for or activity in a general assembly or other public office, constitutionally protected acts of speech, membership in any organization engaged in civil defense, veteran’s status, any military service, application for military service, or any other federal human rights law; (ii) claims under any federal, state or local statutes or common law, including, without limitation, wrongful discharge, wrongful termination, breach of any written or oral, express or implied, contract, agreement or understanding between Executive and the Company Released Parties or any of them, promissory estoppel, unjust enrichment, breach of a covenant of good faith and fair dealing, violation of public policy, defamation, interference with contractual relations, intentional or negligent infliction of emotional distress, invasion of privacy, misrepresentation, deceit, fraud, negligence, personal injury, or any claim to attorneys’ fees under any applicable statute or common law theory of recovery; (iii) claims under any federal, state or local statute, regulation or executive order (as amended through the date Executive signs this Agreement) relating to whistleblower protections, violation of public policy, or any other form of retaliation or wrongful termination, including but not limited to the Dodd- Frank Wall Street Reform and Consumer Protection Act of 2010 and the Sarbanes-Oxley Act of 2002; (iv) claims under any Company compensation, benefit, stock option, incentive compensation, bonus, restricted stock, retirement, pension, life, health, sick leave, paid time off, severance, accident, disability insurance and/or equity plan, program, policy, practice or agreement; and (v) any other tort, statutory or common law cause of action. Nothing herein shall constitute a waiver of Executive’s right to seek an award pursuant to Section 21F of the Securities Exchange Act of 1934, as amended.
(b) To the extent permitted by applicable law, this general release also includes any and all wage and hour related claims arising out of or in any way connected with your employment or directorship with the Company, including and any claims for unpaid or delayed payment of wages, overtime, bonuses, commissions, incentive payments or severance, missed or interrupted meal periods, interest, attorneys’ fees, costs, expenses, liquidated damages, treble damages or damages of any kind to the maximum extent permitted by law.
(c) Because Executive is forty (40) years of age or older, Executive is hereby informed that Executive has or might have specific rights and/or claims under ADEA, and Executive agrees and understands that: (i) in consideration of the amounts and benefits described in Section 2 of this Agreement, which are in addition to anything of value to which Executive is already entitled, Executive specifically waives any rights and/or claims under the ADEA to the extent that such rights and/or claims arose prior to the date this Agreement was executed; (ii) Executive does not waive any rights or claims under the ADEA that may arise after the date this Agreement is executed; (iii) Executive acknowledges that Executive has been advised to consult with counsel of his choice prior to executing this Agreement, that Executive was informed and understood when originally presented with this Agreement that Executive has twenty-one (21) days to review it and consider its terms before signing it, and that Executive also has been advised that any revisions to this Agreement will not affect or extend the calculation of the twenty-one (21)-day period; (iv) Executive has not been subject to any undue or improper influence interfering with the exercise of Executive’s free will in deciding whether to execute this Agreement, and Executive agrees that Executive has carefully read and fully understands all of the provisions of this Agreement, Executive knowingly and voluntarily agrees to all of the terms set forth in this Agreement, and Executive acknowledges that in entering into this Agreement, Executive is not relying on any representation, promise or inducement made by the Company or its attorneys with the exception of those promises contained in this Agreement; and (v) Executive may revoke this Agreement for a period of seven (7) days following his execution hereof and all rights and obligations of both parties under this Agreement shall not become effective or enforceable until the seven (7) day revocation period has expired. To be clear, in the event that Executive revokes this Agreement prior to the expiration of the seven (7) day revocation period, this Agreement will be deemed null and void. This Agreement shall become effective as of the eighth (8th) day after Executive shall have executed the Agreement, assuming Executive has not duly revoked the Agreement prior to such date (such eighth day, the “Effective Date”).
(d) Nothing in this Section 4 shall constitute a release or waiver of (i) claims regarding, or a forfeiture of, Executive’s vested benefits under any employee benefit or other plan, including, without limitation, a retirement plan, a pension plan, a life insurance plan, any stock, stock option, or other equity plan and/or associated agreement, if any, or otherwise, (ii) any obligations of the Company to provide defense and/or indemnification for, or advancement of expenses in connection with, claims brought against Executive arising from or related to Executive’s employment with or service as a director of the Company (or the holding of any office, position, appointment, or official capacity with the Company or its affiliates) as may be required by law (including without limitation Nev. Rev. Stat. § 78.751), or pursuant to the Company’s articles of incorporation (including without limitation Article VII thereof) or bylaws (including without limitation Article VIII thereof); (iii) directors’ and officers’ insurance rights that Executive may have in respect of Executive’s service to the Company; and (iv) Executive’s right to file for unemployment benefits. In the event that Executive files for unemployment benefits, the Company shall not challenge such filing.
(e) Company Released Claims. The Company, on behalf of itself, its affiliates, parents, subsidiaries, and related entities, and its and each of their respective current and former officers, directors, and boards of directors, including the transferees, successors, agents, and assigns of each of the foregoing (collectively, the “Company Releasors”), hereby irrevocably and unconditionally release, acquit and forever discharge Executive, including Executive’s heirs, successors, and assigns (collectively, the “Executive Released Parties”), from any and all charges, complaints, claims, liabilities, obligations, promises, agreements, controversies, damages, actions, causes of action, suits, rights, demands, costs, losses, debts and expenses (including attorneys’ fees and costs actually incurred) of any nature whatsoever, known or unknown, suspected or unsuspected, that any or all of the Company Releasors now has, or has ever had, or ever shall have, against any of the Executive Released Parties by reason of any and all acts, omissions, events, circumstances or facts existing or occurring through the date on which this Agreement is executed by the Company (the claims released in this Section 4(e) are collectively referred to as the “Company Released Claims”).
(f) Certain Exclusions. The Company Released Claims and the Executive Released Claims do not include (i) any claims to enforce this Agreement or any terms thereof; (ii) any claims related to acts, omissions or events occurring after the date this Agreement is signed by the Executive or the Company, as applicable; and (iii) any claims that cannot legally be waived by private agreement.
(g) Release of Unknown Claims. It is the intention of Executive and the Company that this Agreement serve as a general release which shall be effective as a bar to each and every claim, demand, or cause of action it releases. Each Party recognizes that it may have some claim, demand, or cause of action against the other Party of which such Party is totally unaware and unsuspecting which such Party is giving up by execution of this Agreement and the general release contained herein. It is the intention of the Parties in executing this Agreement that it will deprive such Party of each such claim, demand or cause of action and prevent such Party from asserting such claim against the other Party.
5. Non-Disclosure of Confidential Information; Trading Acknowledgment.
(a) Non-Disclosure of Confidential Information. During employment with the Company, Executive was given access to the confidential and proprietary information of the Company (“Confidential Information”). Confidential Information shall not be deemed to include any information that becomes generally available to the public other than as a result of any unauthorized disclosure by the Executive, or that becomes available to the Executive on a nonconfidential basis from a source other than the Company (or any Company Released Party) where such disclosing Person is not bound by a duty of confidentiality, or other contractual, legal or fiduciary obligation, to the Company. Executive will not disclose or any Confidential Information for the benefit of Executive or any person or entity other than the Company. Executive understands that, without limiting any other remedies, the Company may seek from a court of competent jurisdiction an injunction to prohibit such unauthorized use or disclosure of Confidential Information. Notwithstanding any provision of this Agreement that prohibits the disclosure of trade secrets or confidential information, Executive understands that he may not be held criminally or civilly liable under any Federal or State trade secret law for the disclosure of a trade secret that (i) is made (A) in confidence to a Federal, State or local government official, either directly or indirectly, or to an attorney, and (B) solely for the purpose of reporting or investigating a suspected violation of law, or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding. In addition, if Executive files a lawsuit or other court proceeding against any of the Company Released Parties for retaliating against him for reporting a suspected violation of law, Executive may disclose the trade secret or confidential information to the attorney representing Executive and use the trade secret or confidential information in the court proceeding, if Executive seeks to file any document containing the trade secret or confidential information under seal and does not disclose the trade secret or confidential information, except pursuant to court order. Notwithstanding the foregoing, Executive acknowledges and agrees that nothing in this Agreement shall limit, curtail or diminish any of the Company’s statutory rights under the Defend Trade Secrets Act, any applicable state law regarding trade secrets, or common law.
(b) Trading Acknowledgment. Executive acknowledges that applicable securities laws require that he refrain from trading in securities of the Company while he is in possession of such material, nonpublic information. The Company covenants that it will not disclose any material, nonpublic information to Executive after the Effective Date that will not be disclosed by the Company on its Quarterly Report on Form 10-Q for the fiscal quarter ending September 30, 2025.
6. Non-Disparagement.
(a) Executive will not (i) make any statements or take any actions that in any way disparage or could harm the reputation and/or goodwill of the Company, or (ii) in any way, directly or indirectly, cause or encourage the making of such statements or the taking of such action by anyone else. This Section does not, in any way, restrict or impede Executive from exercising protected rights to the extent that such rights cannot be waived by agreement or from complying with any applicable law or regulation or a valid order of a court of competent jurisdiction or an authorized government agency, provided that such compliance does not exceed that required by the law, regulation, or order.
(b) The Company, including the Company’s officers and directors, will not (i) make any statements or take any actions that in any way disparage or could harm the reputation and/or goodwill of Executive, or (ii) in any way, directly or indirectly, cause or encourage the making of such statements or the taking of such action by anyone else.
7. Cooperation. In connection with any internal investigation or participation of the Company or any of its affiliated entities in any current or future litigation or governmental investigation or proceeding that relates to events which occurred during Executive’s employment or directorship or about which Executive has information, Executive shall provide reasonable cooperation and devote such time as may be reasonably required in the preparation, prosecution or defense of any action, investigation, inquiry or other proceeding, including but not limited to, the execution of truthful declarations or providing information and/or documents requested by the Company all at the sole expense of the Company, subject, in all instances, to the reasonable availability of Executive. To the extent that the Company determines that Executive’s physical presence is necessary under this Section 7, the Company shall reimburse the Executive for the Executive’s reasonable travel and other out-of-pocket expenses incurred in connection with the foregoing and subject to the delivery of reasonable documentary support for such expenses. Executive shall not be required to cooperate against his own legal interests. If the Executive’s cooperation as a former officer and/or director of the Company is reasonably determined to conflict with or be adverse to the interests of the Company, Executive may retain his own counsel and shall be indemnified by the Company for such reasonable attorneys’ fees. Executive agrees that, in the event he is subpoenaed by any person or entity (including, but not limited to, any governmental entity) to give testimony or provide documents (in a deposition, court proceeding or otherwise) which in any way relates to Executive’s employment by the Company and/or any service as a director of the Company, Executive will give prompt notice of such request to the Company’s Chief Executive Officer and, unless declining to comply with such subpoena would reasonably be expected to subject Executive to liability or other consequences under the law, will make no disclosure until the Company has had a reasonable opportunity to contest the right of the requesting person or entity to such disclosure. Executive acknowledges that he has no right or authority to waive any attorney-client or attorney work product privilege belonging to the Company, and that he shall not provide any information in violation of such privileges. Executive further agrees that, unless required by law or applicable legal process, he shall not meet or otherwise communicate with any counterparty or any representative of any counterparty to any litigation in which the Company (or any of its officers or directors) is a party, whether or not nominal, without the prior written consent of the Company.
8. Return of Company Property. Within five (5) business days after the Effective Date, Executive shall undertake reasonable efforts to return to the Company any Company property in Executive’s possession, custody, or control, at the Company’ expense. In the event that Executive subsequently discovers that he is still in possession of Company property, he will promptly arrange for the return of such property. Notwithstanding the foregoing, Executive shall be permitted to retain his calendar and his contacts, all compensation-related plans and agreements, any documents reasonably needed for personal tax purposes, and his personal notes, journals, diaries and correspondence, including personal emails. In addition, Executive shall retain his mobile phone(s) and computing device(s), including computers, iPads, and the like.
9. Acknowledgements.
(a) This Agreement is not intended to be, and shall not be construed as, any admission that Executive, the Company, any Company Released Party, or any Executive Released Party has violated any law or committed any wrongdoing whatsoever. The Company agrees that the Executive shall be under no restrictions on his ability to seek or accept employment, consultancy, or any other engagement with any other company or entity following the Effective Date, including those in the same industry or competing with the Company.
(b) This Agreement, including the terms, facts, circumstances, statements, negotiations, and documents relating hereto, shall not be admissible or submitted as evidence in any litigation in any forum for any purpose other than to secure enforcement of the terms and conditions of this Agreement or to defend against a claim relating to this Agreement.
(c) Except as is otherwise provided herein, this Agreement contains the entire understanding between Executive and the Company concerning the subject matter hereof and supersedes all prior agreements and understandings (written or oral) relating to the subject matter hereof. Neither party is relying on, or may rely upon, any prior or contemporaneous statements or representations, whether verbal or written, not expressly set forth in this Agreement. This Agreement may not be amended, supplemented, or modified in any manner, except in a writing signed by Executive and an authorized representative of the Company. This Agreement constitutes the duly authorized, valid and binding agreement of each Party enforceable against such Party in accordance with its terms.
(d) The Company shall indemnify Executive as provided in the Company’s Articles of Incorporation and bylaws.
(e) This Agreement shall be governed by, and construed in accordance with and subject to, the laws of the State of Florida applicable to agreements made and to be performed entirely within such state or torts without regard to its conflicts of law rules. The Company and Executive hereby irrevocably and unconditionally submit, for themselves and their property, to the exclusive jurisdiction of any Florida State court or federal court of the United States of America sitting in the State of Florida, Palm Beach County, and any appellate court from any thereof, and the Company and Executive hereby irrevocably and unconditionally agree that all claims in respect of any such action or proceeding may be heard and determined in Florida State court or federal court of the United States of America sitting in the State of Florida, Palm Beach County. The Company and the Executive irrevocably waive, to the fullest extent permitted by law, the defense of an inconvenient forum to the maintenance of such action or proceeding in any such court. The Company and Executive agree that a final judgment in any such action or proceeding shall be conclusive and, may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law. Executive and the Company agree not to commence a claim or proceeding hereunder in a court other than a Florida State court or federal court located in the State of Florida, Palm Beach County, except if such claim or proceeding is first brought in such Florida State court or federal court located in the State of Florida, and such court or courts have denied jurisdiction over such claim or proceeding.
(f) Nothing in this Agreement will (or should be construed to): (i) interfere with Executive’s right and responsibility to give truthful testimony under oath; (ii) restrict Executive’s ability to communicate information regarding wages or terms and conditions of his employment with the Company; (iii) prohibit Executive from disclosing the information contained in this Agreement to the Equal Employment Opportunity Commission (“EEOC”) or any state agency responsible for enforcing anti-discrimination laws; or (iv) preclude Executive from participating in an investigation, filing a charge, or otherwise communicating with the EEOC or any other fair employment agency, but, in connection with any such charge or proceeding, Executive will have no personal right to any monetary recovery of any kind. Consistent with Rule 21F-17 of the Securities Exchange Act of 1934, any confidentiality and non-disclosure provisions in this Agreement or arising from this Agreement do not prohibit or restrict Executive (or his attorneys) from: initiating communications directly with, or responding to any inquiry from, or providing testimony before, the U.S. Securities and Exchange Commission, NASD/FINRA, any other self- regulatory organization, any other state or federal regulatory authority or pursuant to court or administrative proceedings. In broadest terms, nothing herein is intended to impede any governmental investigation, Executive’s ability to report potential violations of the federal and state securities laws or Executive’s participation in any whistleblower rewards program.
(g) Should any provision in this Agreement or any provision of any agreement incorporated or referenced herein be declared or determined by any court to be illegal or invalid, the validity of the remaining parts, terms, or provisions shall not be affected, and the illegal or invalid part, term, or provision shall not be a part of this Agreement. The failure of any party to insist upon strict adherence to any term of this Agreement on any occasion shall not be considered to be a waiver thereof or deprive that party of the right thereafter to insist upon strict adherence to that term or any other term of this Agreement (h) The headings of the several sections in this Agreement are inserted solely for the convenience of the Parties and are not a part of and are not intended to govern, limit or aid in the construction of any term or provision hereof.
The language in all parts of this Agreement shall in all cases be construed simply, according to its fair meaning, and not strictly for or against any of the Parties hereto. Without limitation, there shall be no presumption against any Party on the ground that such Party was responsible for drafting this Agreement or any part thereof.
(i) The failure of either Party hereto at any time to enforce performance by the other Party of any provision of this Agreement shall in no way affect such Party’s rights thereafter to enforce the same, nor shall the waiver by either Party of any breach of any provision hereof be deemed to be a waiver by such Party of any other breach of the same or any other provision hereof.
(j) Except as set forth in this Agreement, the Parties shall each bear their own costs, attorneys’ fees, and other fees incurred in connection with the preparation of this Agreement.
(k) This Agreement inures to the benefit of and binds (i) the Company, each of their respective parents, subsidiaries, and affiliated entities, and their respective successors and assigns, and, for all such entities, each of their employees, agents, directors, officers, servants, representatives, attorneys, partners, joint venturers, shareholders, members, and all persons or entities acting in concert with them, and (ii) Executive and his estate, heirs, and representatives, and his and their successors and assigns. The Company may, without Executive’s consent, assign this Agreement to any affiliate or any successor to its respective business.
(l) Any notices or transmissions to be given hereunder by either Party to the other shall be effected by electronic mail and either (i) personal delivery in writing, or (ii) nationally-recognized overnight delivery service. Notices shall be addressed to the Parties at the following addresses:
(i) If to the Company, to:
David Moss
Chief Financial Officer
INmune Bio Inc.
225 NE Mizner Blvd., Suite 640
Boca Raton, Florida
dmoss@inmunebio.com
with a copy to:
Thomas Rose.
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas, 31st Floor
New York, NY 10036
trose@srfc.law
(ii) If to Executive, to:
Raymond J. Tesi
[***]
[***]
with a copy to:
Sarah Clay Leyshock
Taft Stettinius & Hollister LLP
425 Walnut Street, Suite 1800
Cincinnati, Ohio 45202
sleyshock@taftlaw.com
Either Party may change their above-noted contact information by written notice in accordance with this Section 9(l). Notice delivered personally will be deemed communicated as of actual receipt; and notices sent by overnight delivery service and/or electronic mail will be deemed communicated as of the business day after sending.
BY SIGNING THIS AGREEMENT, EXECUTIVE ACKNOWLEDGES AND AGREES THAT EXECUTIVE HAS BEEN GIVEN TWENTY-ONE (21) DAYS TO CONSIDER ITS TERMS; THAT EXECUTIVE HAS CAREFULLY READ AND UNDERSTANDS ALL OF ITS TERMS, WHICH INCLUDE THE WAIVER OF IMPORTANT RIGHTS; THAT EXECUTIVE AGREES TO ITS TERMS; THAT EXECUTIVE HAS BEEN ADVISED IN WRITING TO CONSULT WITH AN ATTORNEY BEFORE SIGNING AND DID SO TO THE EXTENT EXECUTIVE DEEMED APPROPRIATE; AND THAT EXECUTIVE IS SIGNING IT VOLUNTARILY AND OF EXECUTIVE’S OWN FREE WILL.
| REVIEWED, AGREED AND ACCEPTED: | |
| /s/ Raymond Joseph Tesi | |
| Raymond Joseph Tesi | |
| Date: August 4, 2025 | |
| REVIEWED, AGREED AND ACCEPTED: | |
| INmune Bio Inc. | |
| /s/ David Moss | |
| By: David Moss | |
| Its: Chief Financial Officer | |
| Date: August 4, 2025 |
13
Exhibit 10.5
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS BOTH NOT MATERIAL AND IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
EMPLOYMENT AGREEMENT
This Employment Agreement (the “Agreement”) dated November 15, 2021 by and between INmune Bio, Inc., a corporation duly organized under the laws of the State of Nevada (the “Company”) and Cory Ellspermann, residing at *** (hereinafter referred to as the “Employee”).
WHEREAS, the Company desires to retain the services of Employee as Cory Ellspermann; and
WHEREAS, the Company and the Employee desire to enter into this Agreement to set forth the terms and conditions of the employment relationship between the Company and the Employee;
NOW, THEREFORE, in consideration of the mutual covenants and agreements contained herein, and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
1. Employment. Company hereby agrees to employ Employee as its Controller and Employee hereby accepts such employment in accordance with the terms of this Agreement, and the terms of employment applicable to regular Employees of Company.
2. Duties of Employee. The duties of Employee shall include the performance of all of the duties incident to the position of Controller as may be assigned by the CFO, the CEO, and the Board of Directors of the Company. Employee shall perform all duties in a professional, ethical and businesslike manner and agrees to abide by all bylaws, policies, procedures, or rules of the Company. Employee agrees to devote his best efforts, energies, and skill to the discharge of the duties and responsibilities attributable to his position, and to this end will devote his full time and attention exclusively to the business and affairs of the Company. Employee understands the position is a global, as such is subject to such reasonable travel will be required.
3. Compensation. Employee shall be paid compensation during the term of this Agreement as follows:
a) Cash Compensation. A base salary of one hundred and fifty-four thousand dollars ($154,000) per year, payable in installments according to the Company’s regular payroll schedule. The Company may withhold from any salary or benefits payable to Employee all federal, state, local and other taxes and other amounts as permitted or required pursuant to law, rule, or regulation. The base salary shall be reviewed from time to time and adjusted by the Company’s Board of Directors at its sole discretion.
b) Performance Bonus. Employee shall be entitled to a yearly $20,000 bonus compensation as the Compensation Committee deems appropriate. Such bonus compensation shall be based, in part, on the achievement of performance criteria established by the Compensation Committee, including criteria relating to clinical milestones.
4. Benefits.
a) Vacation. Employee shall be entitled to three (3) weeks paid vacation days each year. The paid vacation days can accrue up to fifty (50) days.
-
b) Sick Leave. Employee shall be entitled to sick leave and emergency leave according to the regular policies and procedures of Company. Additional sick leave or emergency leave over and above paid leave provided by the Company, if any, shall be unpaid and shall be granted at the discretion of the board of directors.
c) Medical and Group Life Insurance. In the event the Company offers such a plan, Company agrees to include Employee, at the Employee’s option, in a group medical and hospital insurance plan the Company may offer during the term of this Agreement. Employee shall be responsible for payment of any federal or state income tax imposed upon these benefits. The offering of a group medical and hospital insurance plan is at the discretion of the Company and NOT a condition of employment by the Employee. The Company reserves the right to amend or terminate any group medical and hospital insurance plan at any time in its sole discretion, subject to the terms of such plan and applicable law.
d) Expense Reimbursement. Employee shall be entitled to reimbursement for all reasonable expenses, including travel and entertainment, incurred by Employee in the performance of Employee’s duties. Employee will maintain records and written receipts as required by the Company policy or as reasonably requested by the Board of Directors to substantiate such expenses.
5. Term.
a) Employee’s employment with the Company is “at will” and may be terminated by the Company or Employee at any time.
b) Termination Without Cause. In the event Company terminates your employment without “Cause”, provided you execute, deliver to the Company and do not revoke a separation agreement and general release within 60 days following your last date of employment, the Company will pay you severance pay at a rate equal to 100% of your base salary, (less lawful deductions), for a period of nine months from the date of such termination, to be paid periodically in accordance with the Company’s normal payroll practices. Payments will commence on the next payroll period following the date the separation agreement becomes enforceable, provided that if the 60-day period to sign the separation agreement extends into the following calendar year, the payments will begin in the new calendar year. The first payment will include all amounts due to you under this paragraph through that date.
c) Termination Without Cause in Connection with a Change in Control. In the event that a Change in Control occurs during your employment with us, and the Company terminates your employment without Cause, provided you execute, deliver to the Company and do not revoke a separation agreement and general release within 60 days following your last date of employment, the Company will accelerate your vesting in all Company time-based equity awards that you then hold. All stock options then held by you shall immediately become exercisable in full and any other stock awards held by you will become free of restrictions.
-
d) Cause. For purposes of this Agreement, “Cause” for termination means: (a) commission of any felony or crime involving dishonesty; (b) participation in any fraud against the Company; (c) material breach of your duties to the Company; (d) persistent unsatisfactory performance of job duties after written notice from the CEO and an opportunity to cure (if deemed curable by the Company in its sole discretion); (e) intentional damage to any material property of the Company; (f) misconduct, or other violation of a Company policy that causes harm; (g) breach of this Agreement, the Confidentiality Agreement (as defined below), or any other written agreement with the Company; or (h) conduct by you which in the good faith and reasonable determination of the CEO demonstrates gross unfitness to serve.
e) Change in Control. For purposes of this Agreement, “Change of Control” shall mean the occurrence of any of the following events: (i) an acquisition of the Company by another entity by means of any transaction or series of related transactions (including, without limitation, any reorganization, merger or consolidation but excluding any merger effected exclusively for the purpose of changing the domicile of the Company), or (ii) a sale of all or substantially all of the assets of the Company (collectively, a “Merger”), so long as in either case the Company’s stockholders of record immediately prior to such Merger will, immediately after such Merger, hold less than fifty percent (50%) of the voting power of the surviving or acquiring entity.
6. Intellectual Property
(a) The Employee hereby agrees that any writing, invention, design, system, process, development, or discovery conceived, developed, created, or made by Employee, alone or with others, during the period of his employment hereunder and applicable to the business of the Company, whether or not patentable, registerable, or copyrightable shall become the sole and exclusive property of the Company (collectively “Employee Developed IP”).
(b) Employee shall disclose the same promptly and completely to the Company and shall, during the period of his employment hereunder and at any time and from time to time hereafter, (i) execute all documents requested by the Company for vesting in the Company the entire right, title, and interest in and to any Employee Developed IP, (ii) execute all documents requested by the Company for filing such applications for and procuring patents, trademarks, service marks, or copyrights as the Company, in its sole discretion, may desire to prosecute with respect to any Employee Developed IP, and (iii) give the Company all assistance it may reasonably require, including the giving of testimony in any suit, action, investigation, or other proceeding, to obtain, maintain, and protect the Company’s right therein and thereto with respect to any Employee Developed IP.
7. Confidentiality, Non-Competition and Non-Solicitation.
Employee acknowledges that his position with the Company is special, unique, and intellectual in character and his position in the Company will place him in a position of confidence and trust with regards to the Company’s business and intellectual property.
(a) Confidentiality. Employee acknowledges that Employee will have access to certain proprietary and confidential information of the Company and its associates or collaborators (the “Confidential Information”). The Employee acknowledges that such information is of great value to the Company, is the sole property of the Company, and has been and will be acquired by his in confidence. In consideration of the obligations undertaken by the Company herein, the Employee will not, at any time, during or after his employment hereunder, reveal, divulge or make known to any person, any information acquired by the Employee during the course of his employment, which is treated as confidential by the Company, and not otherwise in the public domain. Employee agrees not to use or disclose any Confidential Information during the term of this Agreement or thereafter other than in connection with performing Employee’s service for the Company in accordance with this Agreement. The provisions of this Section 7(a) shall survive the termination of the Employee’s employment hereunder.
-
(b) Noncompetition and Non-Solicitation. The Employee agrees and acknowledges that the Confidential Information that the Employee has already received and will receive is valuable to the Company and that its protection and maintenance constitutes a legitimate business interest of the Company, to be protected by the non- competition restrictions set forth herein. The Employee agrees and acknowledges that the non-competition restrictions set forth herein are reasonable and necessary and do not impose undue hardship or burdens on the Employee.
The Employee hereby agrees and covenants that he shall not, after establishing full time employment with the company, without the prior written consent of the Company, directly or indirectly, in any capacity whatsoever, including, without limitation, as an employee, employer, consultant, principal, partner, shareholder, collaborator, officer, director or any other individual or representative capacity, or whether on the Employee’s own behalf or on behalf of any other person or entity or otherwise howsoever, during the term and thereafter to the extent described below, such which shall apply globally:
(i) Engage, own, manage, operate, control, be employed by, consult for, participate in, or be connected in any manner with the ownership, management, operation or control of any business in competition with the Business of the Company, as defined in the next sentence. For purposes hereof, the Company’s “Business” shall mean the development of drugs and/or therapies for the treatment of human disease using anti-TNF strategies as a therapeutic strategy.
(ii) Recruit, solicit or hire, or attempt to recruit, solicit or hire, any employee, collaborator or independent contractor of the Company to leave the employment (or collaborator or independent contractor relationship) thereof, whether or not any such employee, collaborator or independent contractor is party to an employment agreement, for the purpose of competing with the Business of the Company;
(iii) Attempt in any manner to solicit or accept from any customer of the Company, business of the kind or competitive with the business done by the Company with such customer or to persuade or attempt to persuade any such customer to cease to do business or to reduce the amount of business which such customer has customarily done or might do with the Company, or if any such customer elects to move its business to a person other than the Company, provide any services of the kind or competitive with the business of the Company for such customer, or have any discussions regarding any such service with such customer, on behalf of such other person for the purpose of competing with the Business of the Company; or (iv) Interfere with any relationship, contractual or otherwise, between the Company and any other party, including, without limitation, any client, supplier, distributor, collaborator, co-venturer or joint venturer of the Company, for the purpose of soliciting such other party to discontinue or reduce its business with the Company for the purpose of competing with the Business of the Company.
-
With respect to the activities described in Paragraphs (i), (ii), (iii) and (iv) above, the restrictions of this Section 7(b) shall continue during the term of this Agreement and for a period of one (1) year thereafter.
8. Representations and Warranties of Employee. Employee hereby represents and warrants to the Company as follows: (i) Employee has the legal capacity and unrestricted right to execute and deliver this Agreement and to perform all of his or her obligations hereunder; (ii) the execution and delivery of this Agreement by Employee and the performance of his or her obligations hereunder will not violate or be in conflict with any fiduciary or other duty, instrument, agreement, document, arrangement, or other understanding to which Employee is a party or by which he or she is or may be bound or subject; (iii) Employee is not a party to any instrument, agreement, document, arrangement, or other understanding with any person (other than the Company) requiring or restricting the use or disclosure of any confidential information or the provision of any employment, consulting, or other services; and (iv) Employee has reviewed this Agreement with his own legal counsel.
9. Notices. Any notice required by this Agreement or given in connection with it, shall be in writing and shall be given to the appropriate party by personal delivery or by certified mail, postage prepaid, or recognized overnight delivery services;
If to Company:
INmune Bio, Inc.
225 NE Mizner Blvd., Suite 640
Boca Raton, FL 33432
Attn: David J. Moss
Email: dmoss@inmunebio.com
If to Employee:
[***]
10. Miscellaneous.
(a) The Employee may not assign or delegate any of his rights or duties under this Agreement without the express written consent of the Company.
-
(b) This Agreement constitutes and embodies the full and complete understanding and agreement of the parties with respect to the Employee’s employment by the Company, supersedes all prior understandings and agreements, whether oral or written, between the Employee and the Company, and shall not be amended, modified or changed except by an instrument in writing executed by the party to be charged. If any provision of this Agreement, or the application thereof, shall for any reason and to any extent be invalid or unenforceable, then the remainder of this Agreement and the application of such provision to other persons or circumstances shall be interpreted so as reasonably to effect the intent of the parties hereto. The parties further agree to replace such void or unenforceable provision of this Agreement with a valid and enforceable provision that shall achieve, to the extent possible, the economic, business and other purposes of the void or unenforceable provision. No waiver by either party of any provision or condition to be performed shall be deemed a waiver of similar or dissimilar provisions or conditions at the same time or any prior or subsequent time.
(c) This Agreement shall inure to the benefit of, be binding upon and enforceable against, the parties hereto and their respective successors, heirs, beneficiaries and permitted assigns.
(d) The headings contained in this Agreement are for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
(e) This Agreement shall be governed by and construed in accordance with the internal laws of the State of California, and each of the parties hereto irrevocably consents to the jurisdiction and venue of the federal and state courts located in the State of New York, County of New York, for any disputes arising out of this Agreement, or the Employee’s employment with the Company. The prevailing party in any dispute arising out of this Agreement shall be entitled to his or its reasonable attorney’s fees and costs.
(f) This Agreement may be executed simultaneously in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one of the same instrument. The parties hereto have executed this Agreement as of the date set forth above.
-
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first referenced above.
| Employee | Company | |
| /s/ Cory Ellspermann | /s/ R.J. Tesi | |
| Cory Ellspermann | R.J. Tesi, CEO |
-
Exhibit 31.1
Certifications
I, David J. Moss, certify that:
| 1. | I have reviewed this quarterly report on Form 10-Q of INmune Bio Inc. |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; | |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; | |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and | |
| (d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and | |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 7, 2025
| /s/ David Moss | |
| David Moss | |
| Principal Executive Officer | |
| (Principal executive officer) |
Exhibit 31.2
Certifications
I, Cory Ellspermann, certify that:
| 1. | I have reviewed this quarterly report on Form 10-Q of INmune Bio Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; | |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; | |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and | |
| (d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and | |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 7, 2025
| /s/ Cory Ellspermann | |
| Cory Ellspermann | |
|
Interim Chief Financial Officer (Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of INmune Bio Inc. (the “Company”) on Form 10-Q for the period ended June 30, 2025, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, David J. Moss, Principal Executive Officer of the Company, certify to my knowledge and in my capacity, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
| (1) | the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| (2) | the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Dated: August 7, 2025
| /s/ David Moss | |
| David Moss | |
| Principal Executive Officer | |
| (Principal Executive Officer) |
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of INmune Bio Inc. (the “Company”) on Form 10-Q for the period ended June 30, 2025, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Cory Ellspermann, Interim Chief Financial Officer of the Company, certify to my knowledge and in my capacity, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
| (1) | the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| (2) | the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Dated: August 7, 2025
| /s/ Cory Ellspermann | |
| Cory Ellspermann | |
| Interim Chief Financial Officer | |
| (Principal Financial Officer) |