UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 8, 2025
| BiomX Inc. |
| (Exact Name of Registrant as Specified in its Charter) |
| Delaware | 001-38762 | 82-3364020 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (I.R.S. Employer Identification No.) |
|
22 Einstein St., Floor 4 Ness Ziona, Israel |
7414003 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: +972 723942377
| n/a |
| (Former name or former address, if changed since last report) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value | PHGE | NYSE American |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On July 8, 2025, BiomX Inc., or the Company, issued a press release announcing the publication of a peer-reviewed article in Nature Communications presenting new efficacy data from the Phase 1b/2a trial of BX004 in cystic fibrosis patients with chronic Pseudomonas aeruginosa infections. A copy of the press release is furnished as Exhibit 99.1. The Company also posted an updated corporate slide presentation on its website, furnished as Exhibit 99.2. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit | Description | |
| 99.1 | Press Release dated July 8, 2025, titled “BiomX Announces Publication in Nature Communications of Phage Cocktail BX004 Phase 1b/2a Part 1 Data Demonstrating Strong Activity in Cystic Fibrosis” (furnished herewith) | |
| 99.2 | Corporate Presentation Deck dated July 8, 2025 (furnished herewith) | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL documents) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| BIOMX INC. | |||
| July 8, 2025 | By: | /s/ Jonathan Solomon | |
| Name: | Jonathan Solomon | ||
| Title: | Chief Executive Officer | ||
2
Exhibit 99.1
BiomX Announces Publication in Nature Communications of Phage Cocktail BX004 Phase 1b/2a Part 1 Data Demonstrating Strong Activity in Cystic Fibrosis
Premier research journal article provides validation for BiomX’s phage therapy platform, showcasing first-in-human Phase 1b/2a trial results for antibiotic-resistant P. aeruginosa infections
New, updated data demonstrates a further bacteria reduction of 2.7 log₁₀ (approximately 500-fold) compared to placebo, with no emergent resistance and preservation of a healthy microbiome
BiomX is advancing its Phase 2b trial of BX004 with topline results expected Q1 2026
NESS ZIONA, Israel, July 8, 2025 -- BiomX Inc. (NYSE American: PHGE) (“BiomX” or the “Company”), a clinical-stage company advancing novel natural and engineered phage therapies that target specific pathogenic bacteria, today announced the publication of a peer-reviewed article in Nature Communications titled, “Phage therapy with nebulized cocktail BX004-A for chronic Pseudomonas aeruginosa infections in cystic fibrosis: a randomized first-in-human trial”. The article notably features previously unreported antimicrobial efficacy data from the Phase 1b/2a clinical trial and reinforces the strength of BiomX's innovative approach to developing bacteriophage therapies for chronic disease with substantial unmet needs. The publication is available at: Link.
“The publication of our peer-reviewed results in a preeminent research journal, including new data showing antimicrobial activity of BX004, provides significant third-party validation of our phage therapy platform to treat patients with chronic P. aeruginosa cystic fibrosis (CF) infections,” said Jonathan Solomon, BiomX's Chief Executive Officer. “Building upon the strong scientific rigor of our clinical program and our findings in patients showing meaningful bacterial reduction where antibiotics have failed, we have initiated our Phase 2b trial of BX004, with topline results expected in the first quarter of 2026.”
The peer-reviewed results of Part 1 of BiomX’s BX004 Phase 1b/2a study include new analyses showing that BX004 achieved a substantially greater improvement of approximately 500-fold (additional improvement of 2.7 log₁₀) in bacterial reduction compared with placebo in CF patients. Notably, the data highlights that no bacterial resistance to BX004 emerged during the trial, addressing a critical limitation of traditional antibiotics. Findings from Part 1 of the Phase 1b/2a study were consistent with the results observed in Part 2.
“Drawing on decades of experience in large-scale genomic analysis and bacterial defense mechanisms, the study demonstrates how large-scale data analysis can be used to optimize bacteriophage cocktails for treating chronic infection associated with cystic fibrosis,” said Rotem Sorek, Ph.D., Professor of Genetics, Weizmann Institute of Science. “By combining experimental and computational methods, we've developed a design approach that broadens bacterial strain coverage, lowers the likelihood of resistance, and enhances activity against bacterial biofilms, establishing an effective framework for designing next-generation bacteriophage therapeutics.”
Key Highlights from the Study
Part 1 of BiomX's Phase 1b/2a study evaluated the safety, tolerability, pharmacokinetics, and anti-microbiologic activity of BX004 over a 7-day treatment period in nine CF patients (seven on BX004, two on placebo) with chronic P. aeruginosa pulmonary infection. The Part 1 data demonstrated:
| ● | Strong Safety Profile: BX004 was safe and well-tolerated with no treatment-related safety events across all patients and dose levels tested. |
| ● | Successful bacterial reduction achieved: At day 15, BX004-treated patients showed a negative 1.42 log₁₀ reduction in P. aeruginosa bacteria from baseline, while patients receiving placebo worsened by +1.26 log₁₀ CFU/g. This 2.7 log₁₀ CFU/g treatment effect (which represents approximately a 500-fold, or 99.8%1, greater bacterial reduction with BX004 versus placebo) was achieved on top of standard of care inhaled antibiotics. These findings resulted from an additional post hoc analysis and are being reported for the first time. Results at day 4 during BX004 treatment showed P. aeruginosa burden reduction (1.9 log₁₀ CFU per gram of sputum difference between groups). |
| ● | Therapeutic phages successfully reached and persisted at infection site: Phages were detected in all patients treated with BX004 during the dosing period, including in several patients up to day 15 (one week after end of therapy). As expected, no phages were detected in patients receiving placebo. |
| ● | No bacterial resistance to treatment: There was no emerging treatment-related resistance to BX004 during or after treatment with BX004, addressing efficacy of phage against bacteria where resistance is common amongst traditional antibiotics. |
| ● | Favorable shifts in microbiome composition post treatment: Microbiological signals included a reduction in P. aeruginosa relative abundance and an increase in microbiome alpha diversity in the phage-treated group, in contrast to the placebo group. |
| 1 | A 2.7 log₁₀ reduction represents a 10^2.7 = ~500-fold reduction in bacterial load, which equates to approximately 99.8% reduction. |
The Nature Communications publication describes the full translational path from laboratory discovery to clinical testing. Environmental phages were isolated and screened using P. aeruginosa grown under conditions mimicking the CF lung environment. In silico screening confirmed the absence of known genes associated with antibiotic resistance or virulence.
About BX004
BiomX is developing BX004, a fixed multi-phage cocktail, for the treatment of CF patients with chronic pulmonary infections caused by P. aeruginosa, a main contributor to morbidity and mortality in patients with CF. In February 2023, BiomX announced positive results from Part 1 of the Phase 1b/2a study, showing safety, tolerability, and microbiologic activity. In November 2023, BiomX announced positive topline results from Part 2 of the Phase 1b/2a trial, in which BX004 demonstrated improvement in pulmonary function associated with a reduction in P. aeruginosa burden compared to placebo in a predefined subgroup of patients with reduced lung function (baseline FEV1<70%). BiomX is now enrolling patients in a randomized, placebo-controlled Phase 2b trial of BX004 in CF patients with chronic P. aeruginosa lung infections. The 8-week study will assess lung function, bacterial load, and quality of life metrics. BX004 has received U.S. Food and Drug Administration Fast Track and Orphan Drug Designations.
About BiomX
BiomX is a clinical-stage company leading the development of natural and engineered phage cocktails and personalized phage treatments designed to target and destroy harmful bacteria for the treatment of chronic diseases with substantial unmet needs. BiomX discovers and validates proprietary bacterial targets and applies its BOLT (“BacteriOphage Lead to Treatment”) platform to customize phage compositions against these targets. For more information, please visit www.biomx.com, the content of which does not form a part of this press release.
Safe Harbor
This press release contains express or implied “forward-looking statements” within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. For example, when BiomX refers to its plans to initiate and enroll patients in the Phase 2b trial and timing of topline results thereof, enrollment of patients in a Phase 2b trial of BX004, the Company’s leadership in developing natural and engineered phage cocktails and personalized phage treatments for chronic diseases, the potential safety, efficacy and toleration of BX004, the potential benefits of BX004, future clinical development of BX004, and the potential of its candidates to address the substantial unmet needs of patients with intractable infections, it is using forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on BiomX management’s current beliefs, expectations and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of BiomX’s control. These risks and uncertainties include, but are not limited to, changes in applicable laws or regulations; the possibility that BiomX may be adversely affected by other economic, business, and/or competitive factors, including risks inherent in pharmaceutical research and development, such as: adverse results in BiomX’s drug discovery, preclinical and clinical development activities, the risk that the results of preclinical studies and early clinical trials may not be replicated in later clinical trials, BiomX’s ability to enroll patients in its clinical trials, and the risk that any of its clinical trials may not commence, continue or be completed on time, or at all; decisions made by the FDA and other regulatory authorities; decisions made by investigational review boards at clinical trial sites and publication review bodies with respect to our development candidates; BiomX’s ability to obtain, maintain and enforce intellectual property rights for its platform and development candidates; its potential dependence on collaboration partners; competition; uncertainties as to the sufficiency of BiomX’s cash resources to fund its planned activities for the periods anticipated and BiomX’s ability to manage unplanned cash requirements; and general economic and market conditions. Therefore, investors should not rely on any of these forward-looking statements and should review the risks and uncertainties described under the caption “Risk Factors” in BiomX’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 25, 2025, and additional disclosures BiomX makes in its other filings with the SEC, which are available on the SEC’s website at www.sec.gov. Forward-looking statements are made as of the date of this press release, and except as provided by law, BiomX expressly disclaims any obligation or undertaking to update forward-looking statements.
Contacts:
BiomX
Inc.
Ben Cohen
Head Corporate Communications
benc@biomx.com
Exhibit 99.2


Corporate Presentation July 2025 Clinical Stage Programs Addressing Urgent Need for Overcoming Antibiotic Resistance NYSE American: PHGE SAFE HARBOR STATEMENT About this Presentation The information contained in this presentation has been prepared by BiomX Inc. and its subsidiaries (collectively, the “Company” or “ BiomX ”) and contains information pertaining to the business and operations of the Company. The information contained in this presentation is current only as of the date on its cover. For an y t ime after the cover date of this presentation, the information, including information concerning our business, financial condition, results of operations and prospects, may have changed. The delivery of this presentation shall not, under any circumstances, create any implication that there have been no changes in our affairs after the date of this presentation. We have not authorized any pe rso n to give any information or to make any representations about us in connection with this presentation that is not contained herein. If any information has been or is given or any representation s h ave been or are made to you outside of this presentation, such information or representations should not be relied upon as having been authorized by us. Forward - Looking Statements This presentation contains certain “forward - looking statements” within the meaning of the “safe harbor” provisions of the U.S. P rivate Securities Litigation Reform Act of 1995. Forward - looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would ,” “positioned,” “future,” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. Forward - looking statements are neither historical fact s nor assurances of future performance. Instead, they are based only on BiomX management’s current beliefs, expectations and assumptions. For example, when we discuss future potential clinical trials, in cl uding their design, objectives, costs, endpoints, potential benefits and timing thereof, the potential outcomes of discussions that we may have with the U.S. Food and Drug Administration (“FDA”) an d foreign regulatory agencies, including timing thereof, the use of Real World Evidence and potential to obtain accelerated approval, among others, potential commercial opportunities, the poten tia l to use our product candidates for new indications, our financial needs to fund future clinical trials, forecasted expenses and our ability to protect our intellectual property assets in the fut ure we are making forward - looking statements. In addition, past and current pre - clinical and clinical results, as well as compassionate use, are not indicative and do not guarantee future success of BiomX clinical trials. Because forward - looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of whic h a re outside of our control. Actual results and outcomes may differ materially from those indicated in the forward - looking statements. Therefore, you should not rely on any of these forward - lookin g statements. You should review additional disclosures we make in our filings with the Securities and Exchange Commission (the “SEC”), which are available on the SEC’s website at www.sec.gov. Exc ept as required by law, we are under no duty to (and expressly disclaim any such obligation to) update or revise any of the forward - looking statements, whether as a result of new information, future e vents or otherwise. No Offer or Solicitation This presentation is for informational purposes only. Nothing in this presentation constitutes an offer to buy or sell or a s oli citation of an offer to buy or sell investments, loans, securities, partnership interests, commodities or any other financial instruments. This presentation and any oral statements made in connection with thi s presentation do not constitute and may not be used for or in connection with, an offer or solicitation by anyone in any state or jurisdiction in which such an offer or solicitation is no t a uthorized or permitted, or to any person to whom it is unlawful to make such offer or solicitation. Trademarks and Service Marks The trademarks and service marks included herein are the property of the owners thereof and are used for reference purposes o nly . Such use should not be construed as an endorsement of such products. FDA This presentation concerns certain products that are under clinical investigation and which have not yet been cleared for mar ket ing by the FDA. These products are currently limited by federal law to investigational use, and no representation is made as to the safety or effectiveness of these products for the purposes for w hic h they are being investigated.
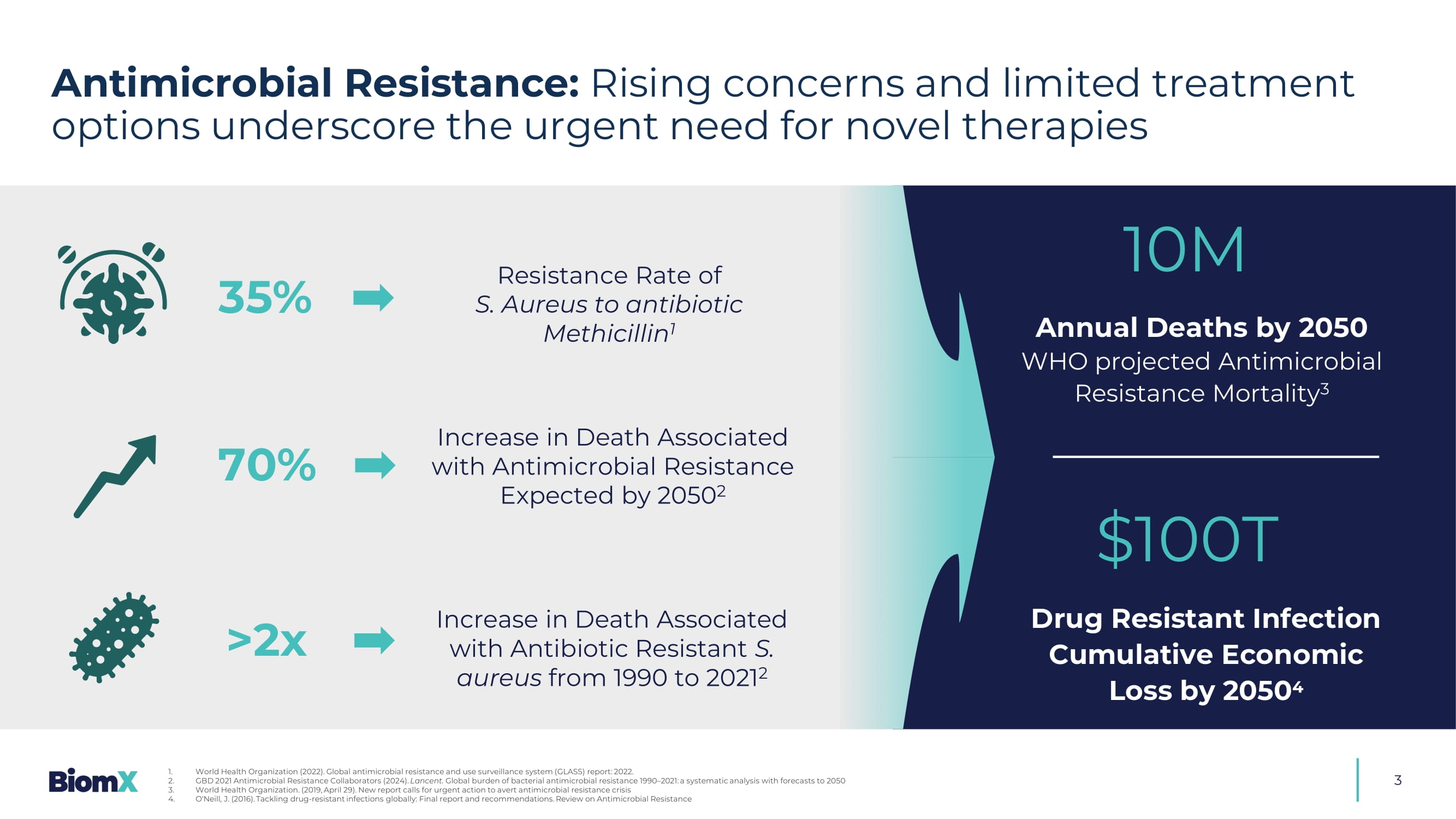
3 10M Annual Deaths by 2050 WHO projected Antimicrobial Resistance Mortality 3 $100T Drug Resistant Infection Cumulative Economic Loss by 2050 4 1. World Health Organization (2022). Global antimicrobial resistance and use surveillance system ( GLASS) report: 2022. 2. GBD 2021 Antimicrobial Resistance Collaborators (2024). Lancent. Global burden of bacterial antimicrobial resistance 1990 – 2021: a systematic analysis with forecasts to 2050 3. World Health Organization. (2019, April 29). New report calls for urgent action to avert antimicrobial resistance crisis 4. O'Neill, J. (2016). Tackling drug - resistant infections globally: Final report and recommendations. Review on Antimicrobial Resis tance &UHDWHGE\0DUWLQV5DWNXV IURPWKH1RXQ3URMHFW 70% >2x Increase in Death Associated with Antimicrobial Resistance Expected by 2050 2 Increase in Death Associated with Antibiotic Resistant S. aureus from 1990 to 2021 2 Resistance Rate of S.
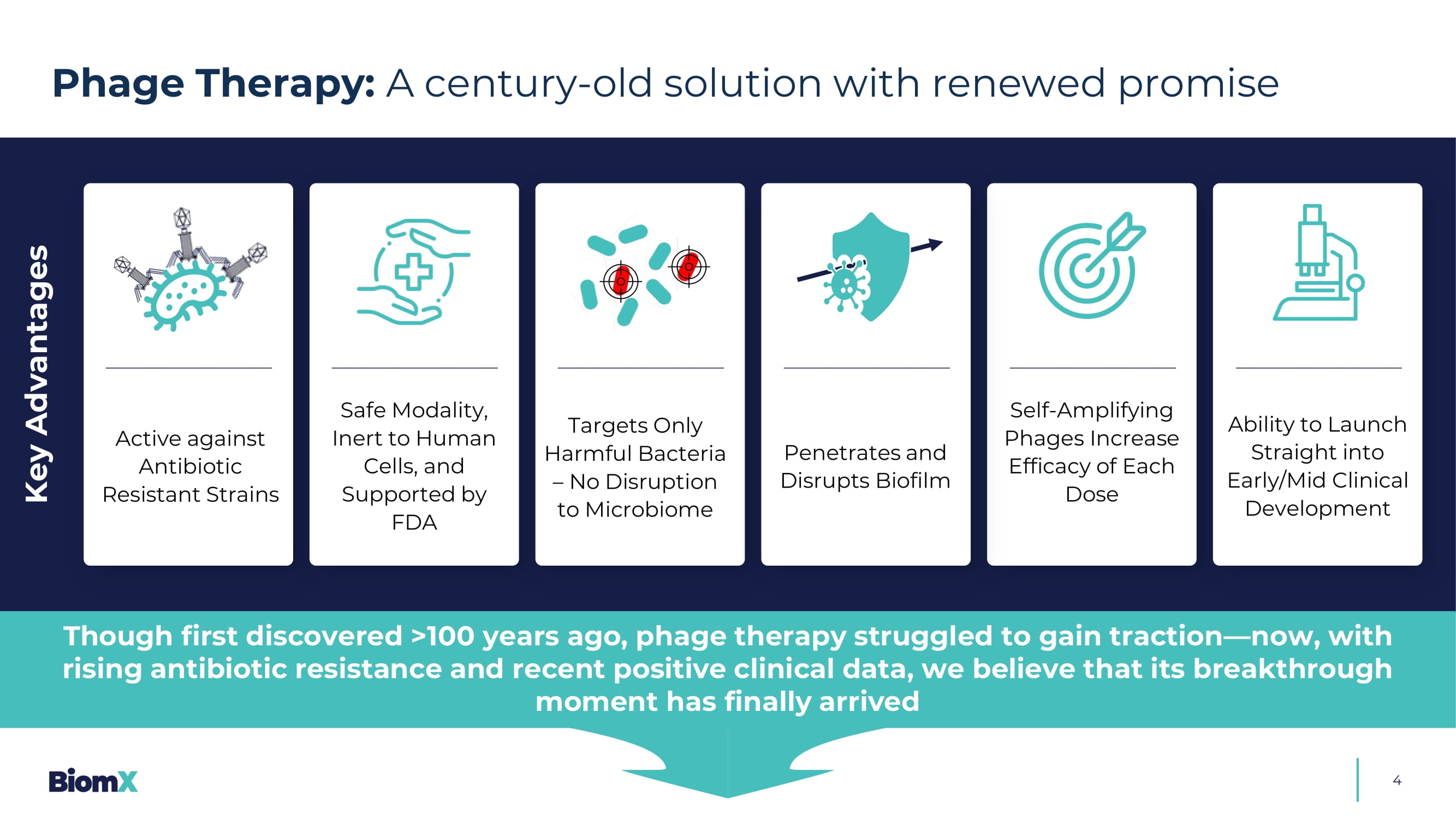
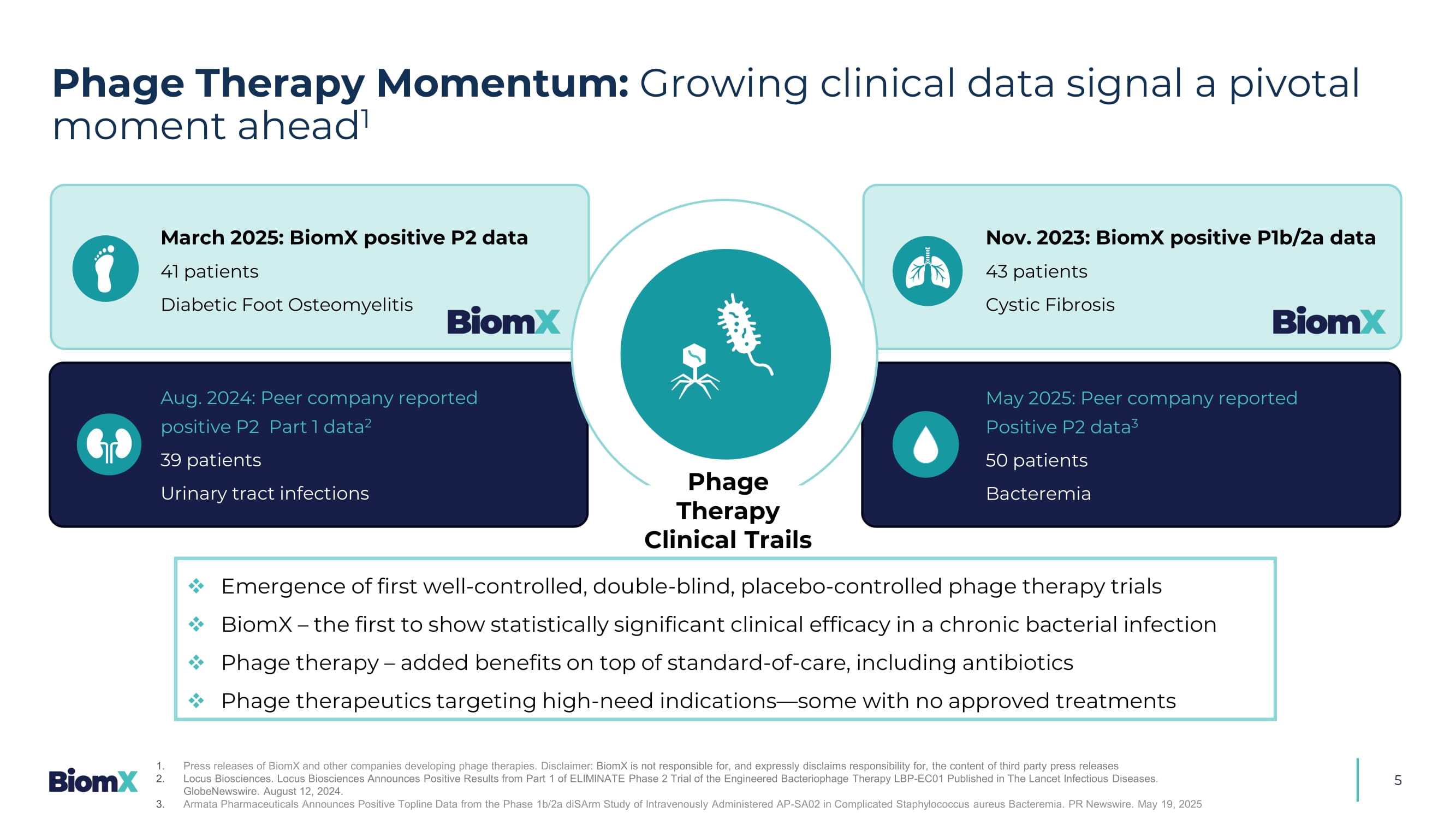
Aureus to antibiotic Methicillin 1 Antimicrobial Resistance: Rising concerns and limited treatment options underscore the urgent need for novel therapies &UHDWHGE\$QGUH%XDQG IURPWKH1RXQ3URMHFW 35% 4 Phage Therapy: A century - old solution with renewed promise Key Advantages Active against Antibiotic Resistant Strains Safe Modality, Inert to Human Cells, and Supported by FDA Targets Only Harmful Bacteria – No Disruption to Microbiome Penetrates and Disrupts Biofilm Self - Amplifying Phages Increase Efficacy of Each Dose Ability to Launch Straight into Early/Mid C linical Development Though first discovered >100 years ago, phage therapy struggled to gain traction — now, with rising antibiotic resistance and recent positive clinical data, we believe that its breakthrough moment has finally arrived Phage Therapy Momentum: Growing clinical data signal a pivotal moment ahead 1 5 □ Emergence of first well - controlled, double - blind, placebo - controlled phage therapy trials □ BiomX – the first to show statistically significant clinical effi c acy in a chronic bacterial infection □ Phage therapy – added benefit s on top of standard - of - care, including antibiotics □ Phage therapeutics targeting high - need indications — some with no approved treatments March 2025: BiomX positive P2 data 41 patients Diabetic Foot Osteomyelitis Aug. 2024: Peer company reported positive P2 Part 1 data 2 39 patients Urinary tract infections May 2025: Peer company reported Positive P2 data 3 50 patients Bacteremia Phage Therapy Clinical Trails Nov. 2023: BiomX positive P1b/2a data 43 patients Cystic Fibrosis 1. Press releases of BiomX and other companies developing phage therapies. Disclaimer: BiomX is not responsible for, and expressly disclaims responsibility for, the content of third party press releases 2. Locus Biosciences. Locus Biosciences Announces Positive Results from Part 1 of ELIMINATE Phase 2 Trial of the Engineered Bact eri ophage Therapy LBP - EC01 Published in The Lancet Infectious Diseases. GlobeNewswire. August 12, 2024. 3. Armata Pharmaceuticals Announces Positive Topline Data from the Phase 1b/2a diSArm Study of Intravenously Administered AP - SA02 in Complicated Staphylococcus aureus Bacteremia. PR Newswire.

May 19, 2025 Company Overview: 6 BiomX’s Phage Therapy Solutions Alarming rise in antimicrobial resistance signals urgent need for better treatment Phage therapy emerging as a powerful solution for persistent infections, supported by growing body of efficacy and safety data BX211 – S. aureus infections P ositive efficacy and safety data (Phase 2) in DFI & DFO with S. aureus infection, showing sustained ulcer reduction and superior recovery outcomes in bone - depth ulcers Planned Phase 2/3 pending regulatory discussions Potential additional Phase 2 ready indications: PJI BX004 – P. aeruginosa infections Positive Phase 1b/2a data in CF patients with ௗ P.
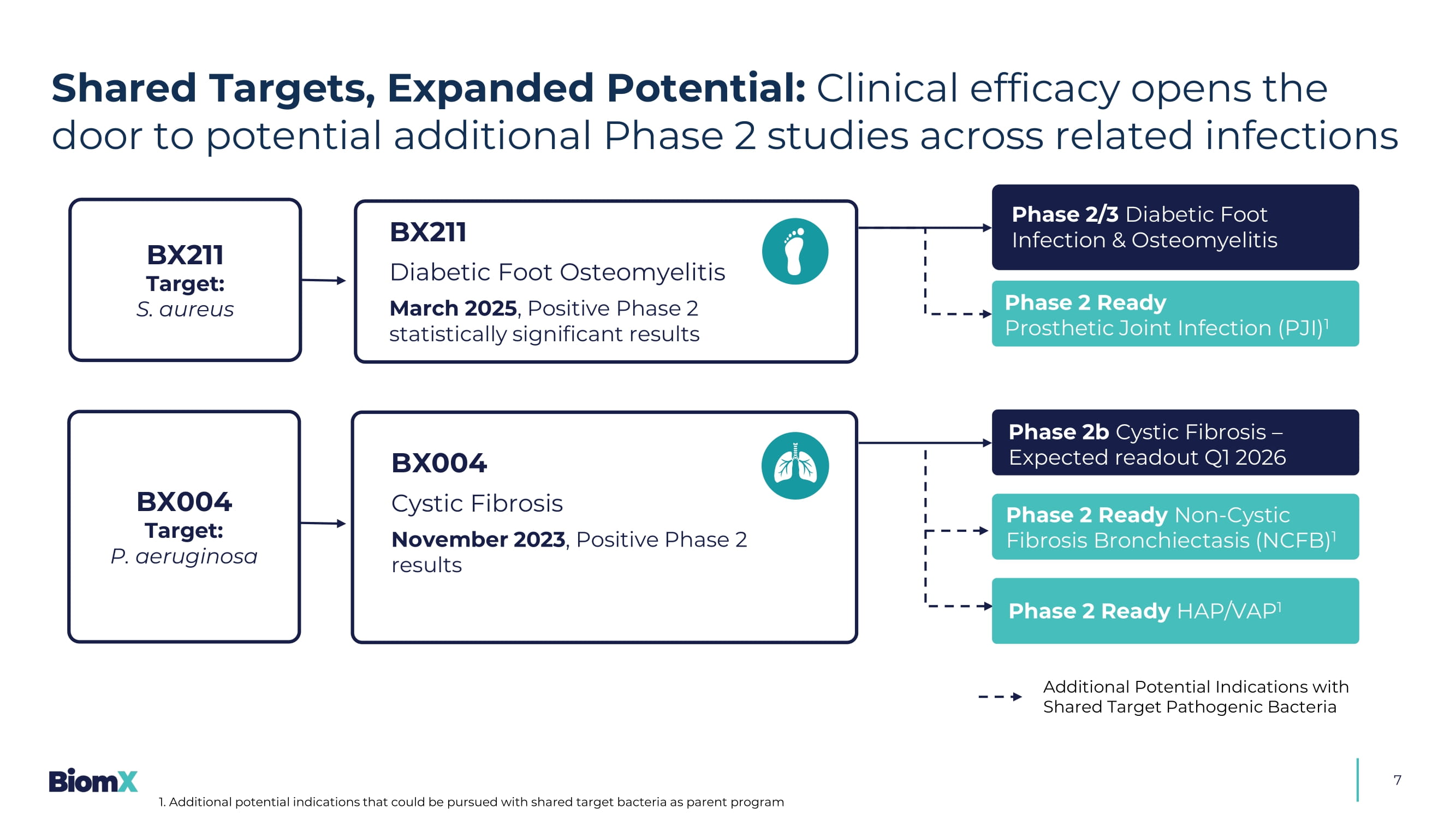
aeruginosa ࣯ infection, demonstrating sputum culture conversion , improved lung function, and bacterial reduction Phase 2b study ongoing Potential a dditional Phase 2 ready indications: NCFB, HAP/VAP DFI: Diabetic Foot Infection; DFO: Diabetic Foot Osteomyelitis; PJI: Prosthetic Joint Infection CF: Cystic Fibrosis; NCFB: Non - cystic Fibrosis Bronchiectasis; HAP/HVP: Hospital - acquired / Ventilator - associated Pneumonia 7 Additional Potential Indications with Shared Target Pathogenic Bacteria BX004 Cystic Fibrosis November 2023 , Positive Phase 2 results BX211 Diabetic Foot Osteomyelitis March 2025 , Positive Phase 2 statistically significant results BX211 Target: S. aureus BX004 Target: P. aeruginosa Phase 2b Cystic Fibrosis – Expected readout Q1 2026 Phase 2 Ready Non - Cystic Fibrosis Bronchiectasis (NCFB) 1 Phase 2 Ready HAP/VAP 1 Phase 2/3 Diabetic Foot Infection & Osteomyelitis Phase 2 Ready Prosthetic Joint Infection (PJI) 1 Shared Targets, Expanded Potential: Clinical efficacy opens the door to potential additional Phase 2 studies across related infections 1.
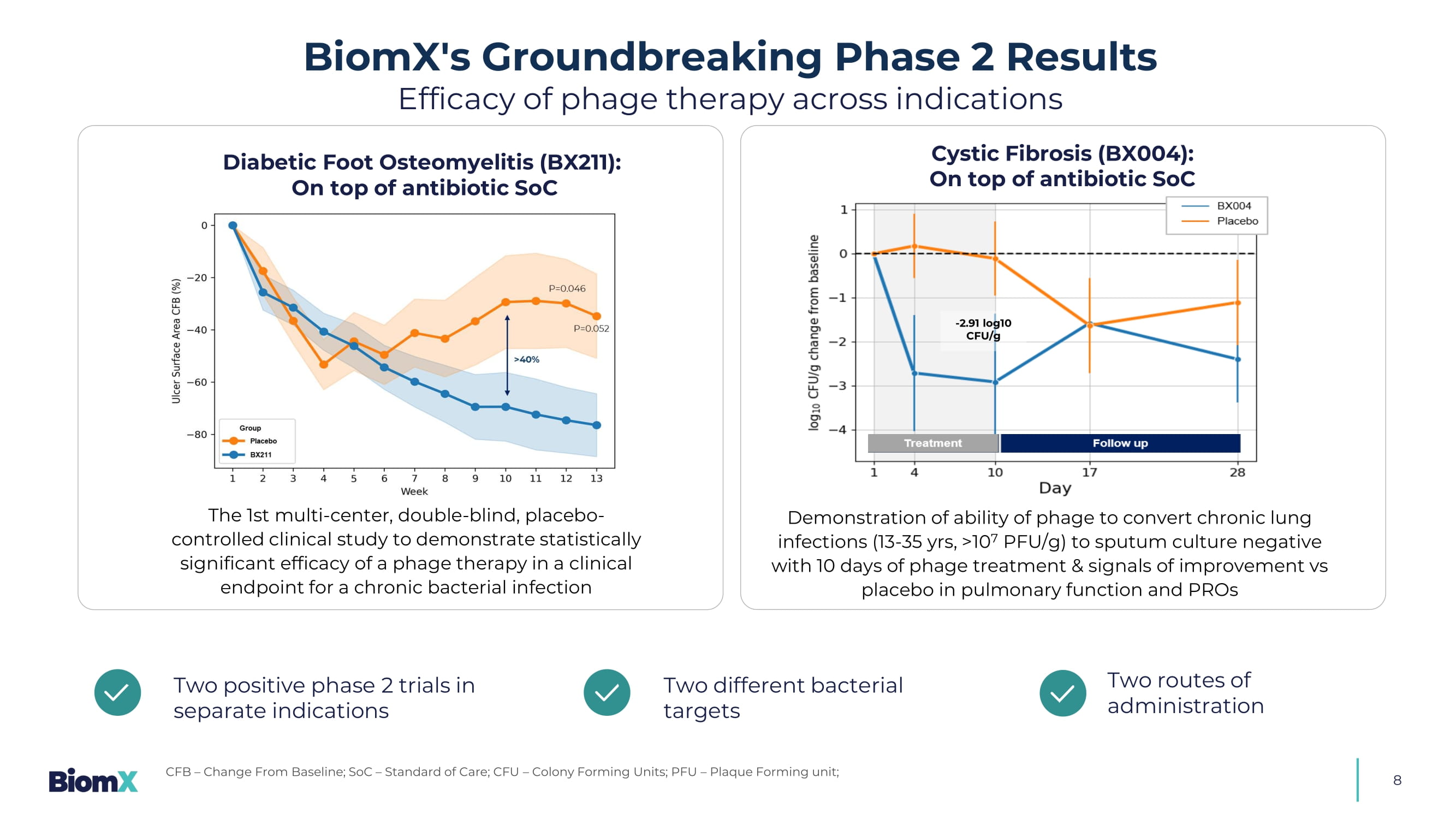
Additional potential indications that could be pursued with shared target bacteria as parent program 8 Diabetic Foot Osteomyelitis (BX211): On top of antibiotic SoC The 1st multi - center, double - blind, placebo - controlled clinical study to demonstrate statistically significant efficacy of a phage therapy in a clinical endpoint for a chronic bacterial infection Two positive phase 2 trials in separate indications Two different bacterial targets Two routes of administration BiomX's Groundbreaking Phase 2 Results Efficacy of phage therapy across indications CFB – Change From Baseline; SoC – Standard of Care; CFU – Colony Forming Units; PFU – Plaque Forming unit; - 2.91 log10 CFU/g Cystic Fibrosis (BX004): On top of antibiotic SoC Demonstration of ability of phage to convert chronic lung infections (13 - 35 yrs, >10 7 PFU/g) to sputum culture negative with 10 days of phage treatment & signals of improvement vs placebo in pulmonary function and PROs 9 Strong Science & Scientific Founders Trusted by top biotech and healthcare investors ≥ $215 M Raised 1 Working with mission - aligned global leaders ~$40 M Non - diluted Funding from U.S. Navy BiomX — Built on a Strong Foundation: Backed by leaders in science, industry, and capital 1. >$200M raised from the investors listed, among others Prof. Rotem Sorek Head of microbial genomics group at Weizmann Institute Phage genomics and CRISPR research Prof. Eran Elinav Principal investigator at Weizmann Institute Immune system and intestinal microbiome interactions Prof. Timothy K. Lu Associate professor leading synthetic biology group, MIT Synthetic biology, biochemical engineering Dr. Carl Merril National Institutes of Health, Adaptative Phage Therapeutics


BX211 Diabetic Foot Infections & Diabetic Foot Osteomyelitis (DFI & DFO)
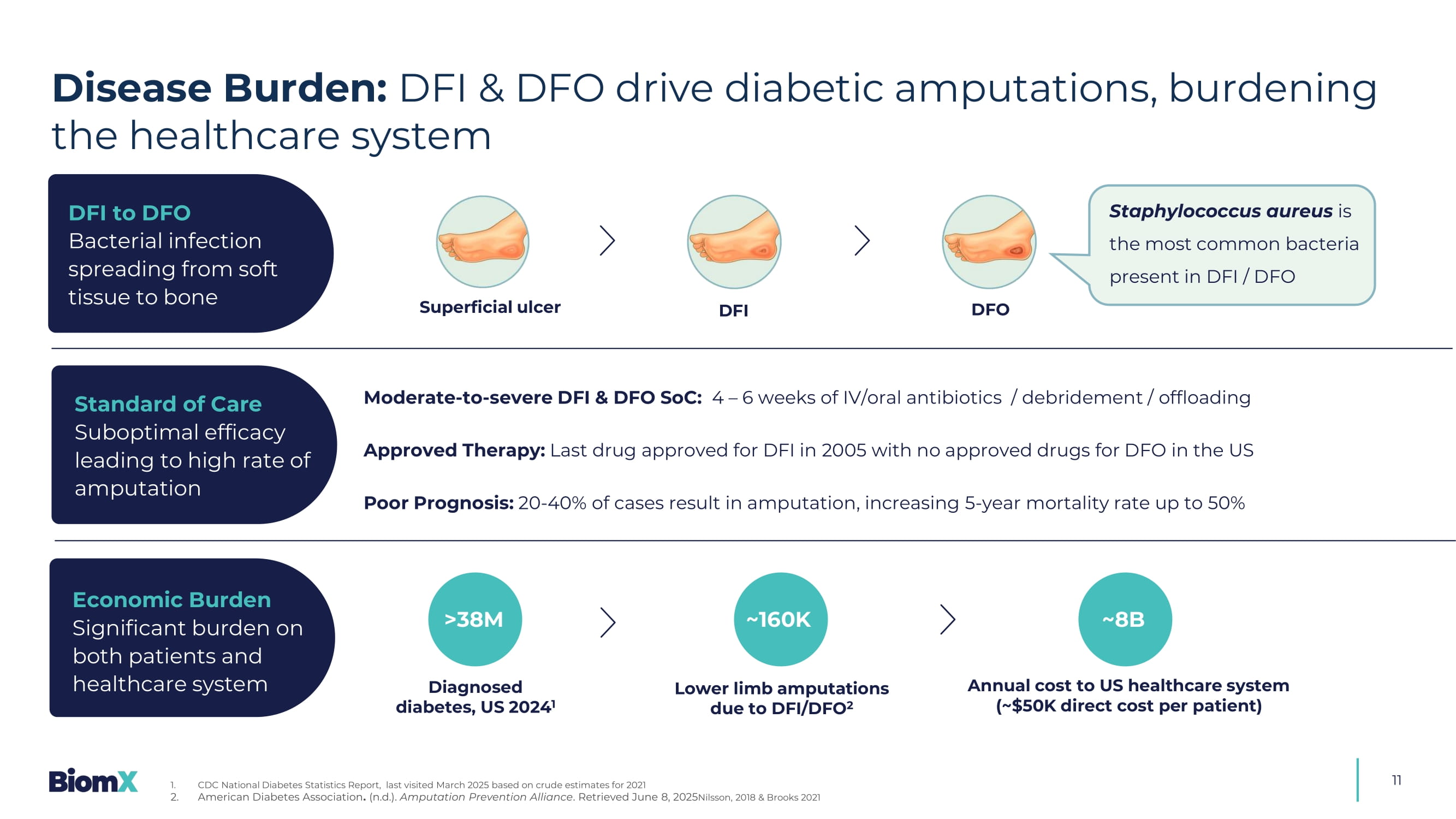
11 Superficial ulcer DFI DFO Staphylococcus aureus is the most common bacteria present in DFI / DFO Moderate - to - severe DFI & DFO SoC: 4 – 6 weeks of IV/oral antibiotics / debridement / offloading Approved Therapy: Last drug approved for DFI in 2005 with no approved drugs for DFO in the US Poor Prognosis: 20 - 40% of cases result in amputation, increasing 5 - year mortality rate up to 50% >38M Diagnosed diabetes, US 2024 1 Lower limb amputations due to DFI/DFO 2 Annual cost to US healthcare system (~$50K direct cost per patient) ~8B DFI to DFO Bacterial infection spreading from soft tissue to bone Standard of Care Suboptimal efficacy leading to high rate of amputation Economic Burden Significant burden on both patients and healthcare system ~160K Disease Burden: DFI & DFO drive diabetic amputations, burdening the healthcare system 1. CDC National Diabetes Statistics Report, last visited March 2025 based on crude estimates for 2021 2. American Diabetes Association . (n.d.). Amputation Prevention Alliance .
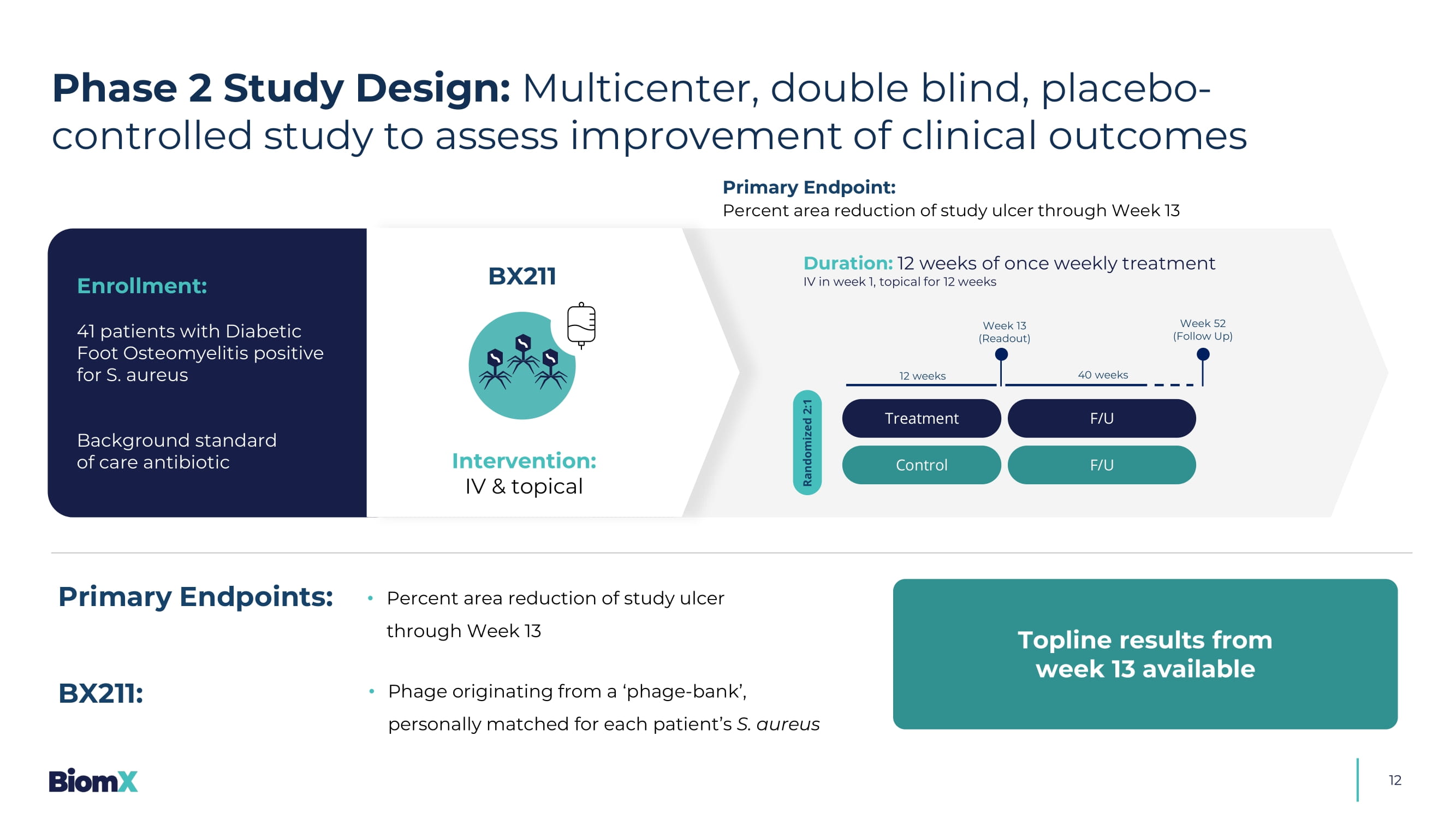
Retrieved June 8, 2025 Nilsson, 2018 & Brooks 2021 Treatment Control Randomized 2:1 Week 13 (R eadout ) Week 52 (Follow Up) 12 weeks 40 weeks F/U F/U 12 Duration: 12 weeks of once weekly treatment IV in week 1, topical for 12 weeks Enrollment: 41 patients with Diabetic Foot Osteomyelitis positive for S. aureus Background standard of care antibiotic Primary Endpoint: Percent area reduction of study ulcer through Week 13 BX211 Intervention: IV & topical • Percent area reduction of study ulcer through Week 13 Primary Endpoints: • Phage originating from a ‘phage - bank’, personally matched for each patient’s S. aureus BX211: Topline results from week 13 available Phase 2 Study Design: Multicenter, double blind, placebo - controlled study to assess improvement of clinical outcomes 13 PAR - Percentage Area Reduction Results Highlight: Phase 2 diabetic foot osteomyelitis 1.
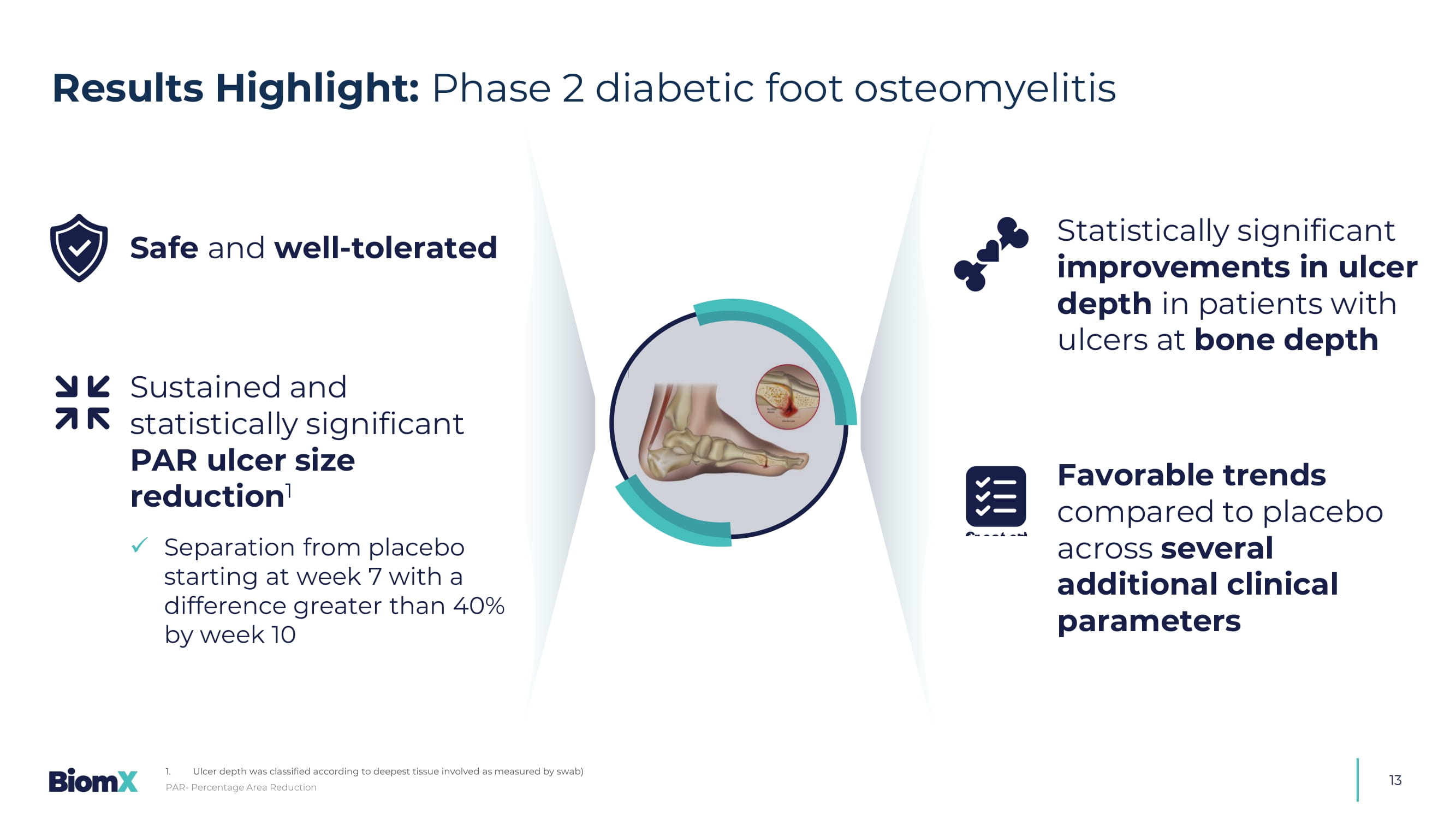
Ulcer depth was classified according to deepest tissue involved as measured by swab) S afe and well - tolerated S ustained and statistically significant PAR ulcer size reduction 1 x S eparation from placebo starting at week 7 with a difference greater than 40% by week 10 &UHDWHGE\,1$ IURP1RXQ3URMHFW Statistically significant improvements in ulcer depth in patients with ulcers at bone depth F avorable trends compared to placebo across several additional clinical parameters 14 Percent Area Reduction (PAR) from baseline of ulcer surface area (LS Mean ± SE ) 2 (See detailed data in next slide) BX211 Group Placebo >40% P=0.052 P=0.046 Efficacy (1): BX211 showed clinically relevant, statistically significant 1 , reduction in ulcer surface area 1.
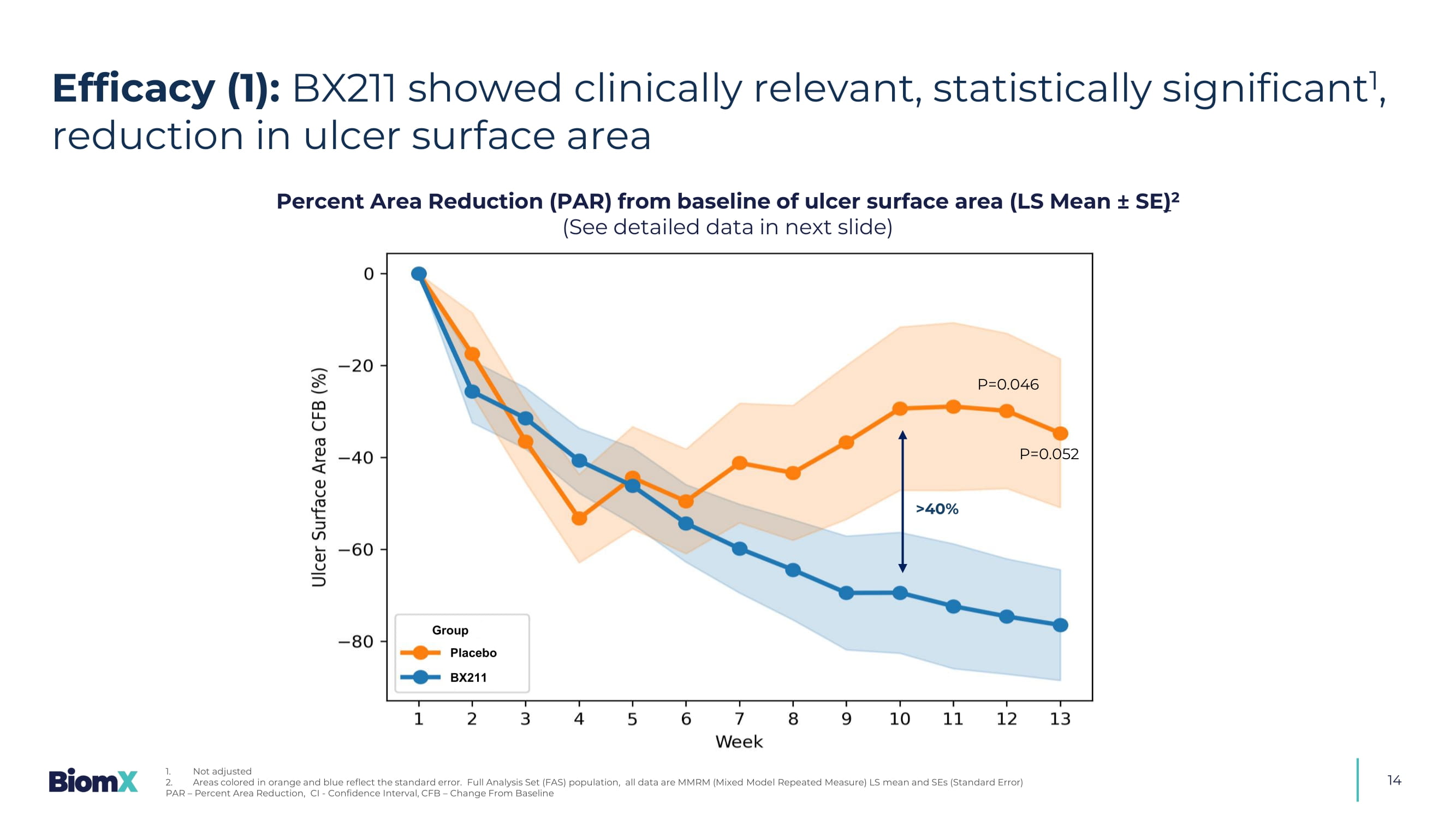
Not adjusted 2. Areas colored in orange and blue reflect the standard error.
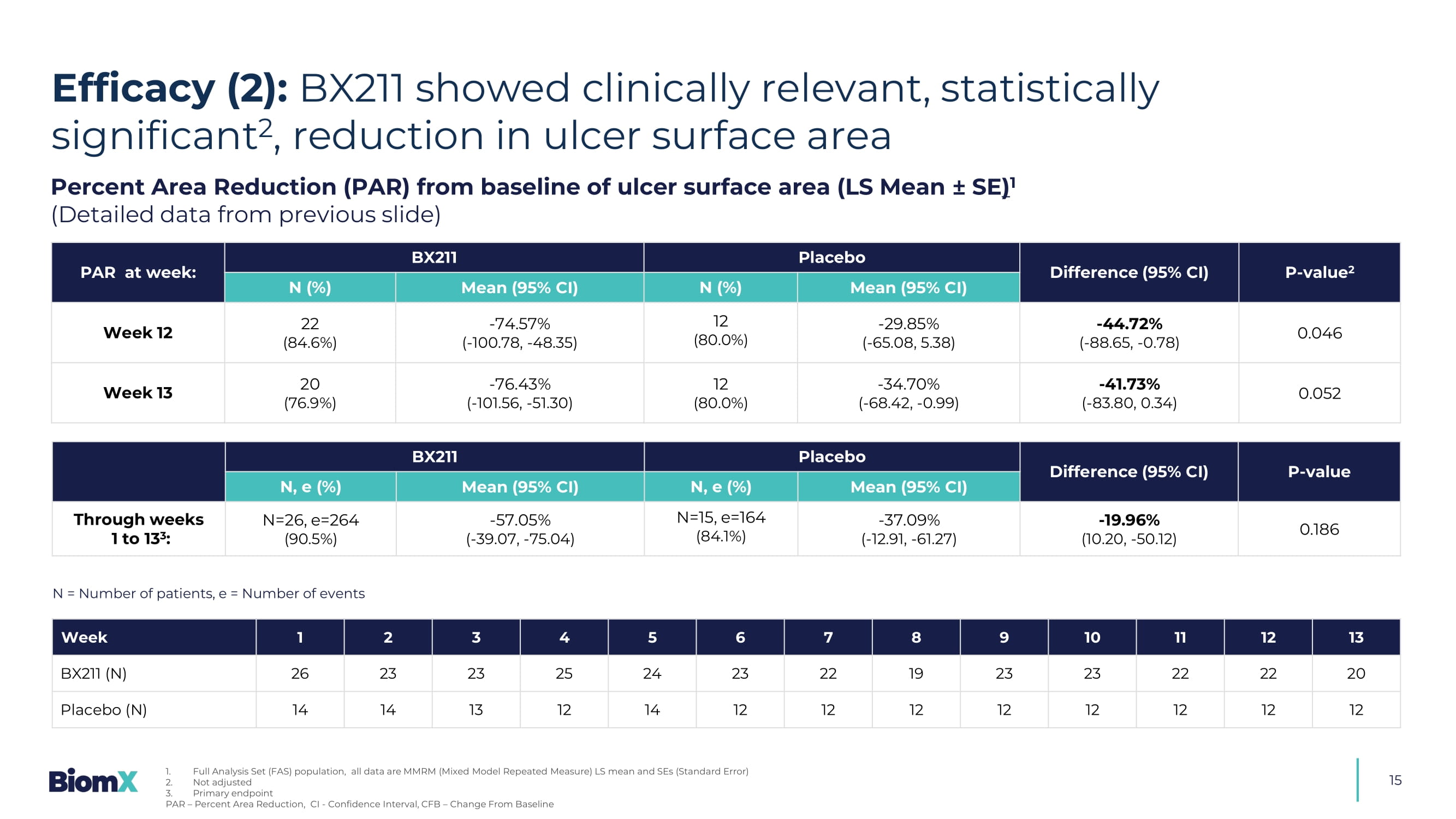
Full Analysis Set (FAS) population, all data are MMRM (Mixed Mode l Repeated Measure) LS mean and SEs (Standard Error) PAR – Percent Area Reduction, CI - Confidence Interval, CFB – Change From Baseline 15 Percent Area Reduction (PAR) from baseline of ulcer surface area (LS Mean ± SE ) 1 (Detailed data from previous slide) P - value 2 Difference (95% CI) Placebo BX211 PAR at week: Mean (95% CI) N (%) Mean (95% CI) N (%) 0.046 - 44.72 % ( - 88.65, - 0.78) - 29.85 % ( - 65.08, 5.38) 12 (80.0%) - 74.57 % ( - 100.78, - 48.35) 22 (84.6%) Week 12 0.052 - 41.73 % ( - 83.80, 0.34) - 34.70 % ( - 68.42, - 0.99) 12 (80.0%) - 76.43 % ( - 101.56, - 51.30) 20 (76.9%) Week 13 P - value Difference (95% CI) Placebo BX211 Mean (95% CI) N, e (%) Mean (95% CI) N, e (%) 0.186 - 19.96 % (10.20, - 50.12) - 37.09 % ( - 12.91, - 61.27) N=15, e=164 (84.1%) - 57.05 % ( - 39.07, - 75.04) N=26, e=264 (90.5%) Through weeks 1 to 13 3 : 13 12 11 10 9 8 7 6 5 4 3 2 1 Week 20 22 22 23 23 19 22 23 24 25 23 23 26 BX211 (N) 12 12 12 12 12 12 12 12 14 12 13 14 14 Placebo (N) N = Number of patients, e = Number of events Efficacy (2): BX211 showed clinically relevant, statistically significant 2 , reduction in ulcer surface area 1. Full Analysis Set (FAS) population, all data are MMRM (Mixed Model Repeated Measure) LS mean and SEs (Standard Error) 2. Not adjusted 3.
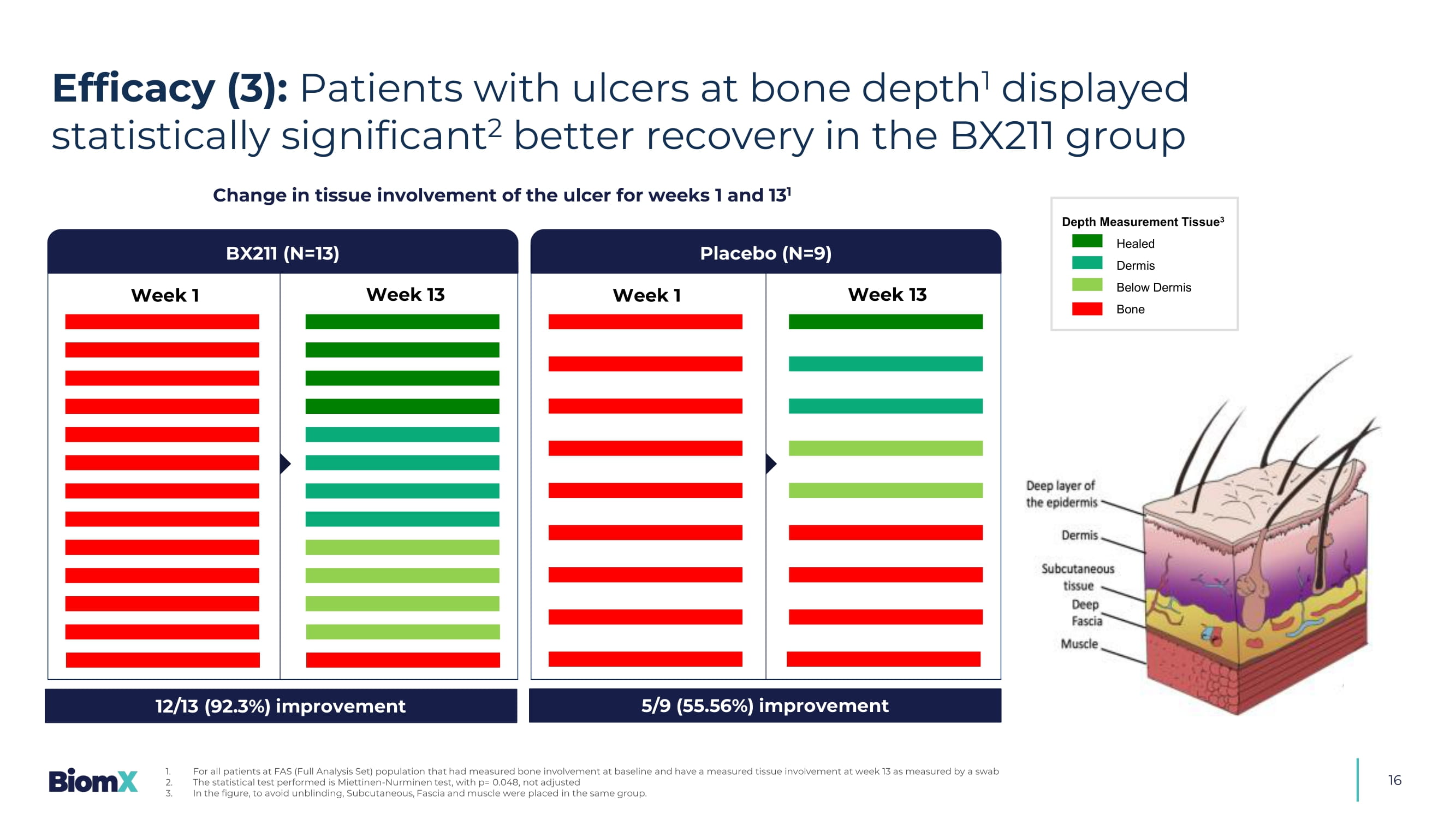
Primary endpoint PAR – Percent Area Reduction, CI - Confidence Interval, CFB – Change From Baseline 16 Change in tissue involvement of the ulcer for weeks 1 and 13 1 Week 1 Week 13 BX211 (N=13) Placebo (N=9) Week 1 Week 13 12/13 (92.3%) improvement 5/9 (55.56%) improvement Depth Measurement Tissue 3 Healed Dermis Below Dermis Bone Efficacy (3): Patients with ulcers at bone depth 1 displayed statistically significant 2 better recovery in the BX211 group 1. For all patients at FAS (Full Analysis Set) population that had measured bone involvement at baseline and have a measured tis sue involvement at week 13 as measured by a swab 2. The statistical test performed is Miettinen - Nurminen test, with p= 0.048, not adjusted 3. In the figure, to avoid unblinding, Subcutaneous, Fascia and muscle were placed in the same group.
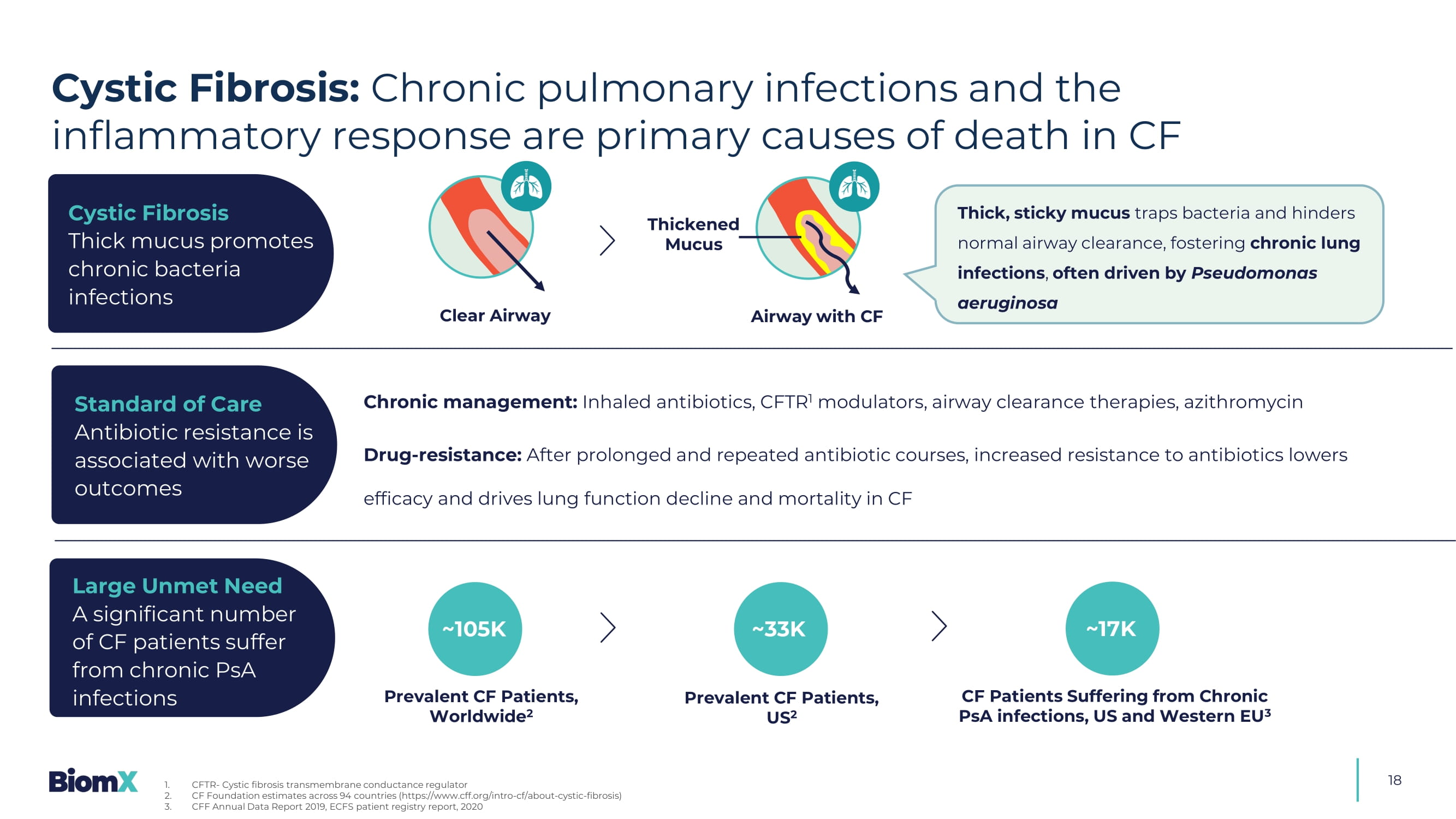
BX004 Cystic Fibrosis and NCFB 18 Clear Airway Thick, sticky mucus traps bacteria and hinders normal airway clearance, fostering chronic lung infections , often driven by Pseudomonas aeruginosa Chronic management: Inhaled antibiotics, CFTR 1 modulators, airway clearance therapies, azithromycin Drug - resistance: After prolonged and repeated antibiotic courses, increased resistance to antibiotics lowers efficacy and drives lung function decline and mortality in CF ~105K Prevalent CF Patients, Worldwide 2 Prevalent CF Patients, US 2 CF Patients Suffering from Chronic PsA infections, US and Western EU 3 ~17K Cystic Fibrosis Thick mucus promotes chronic bacteria infections Standard of Care Antibiotic resistance is associated with worse outcomes Large Unmet Need A significant number of CF patients suffer from chronic PsA infections ~33K Cystic Fibrosis: Chronic pulmonary infections and the inflammatory response are primary causes of death in CF 1. CFTR - Cystic fibrosis transmembrane conductance regulator 2. CF Foundation estimates across 94 countries (https://www.cff.org/intro - cf/about - cystic - fibrosis) 3.
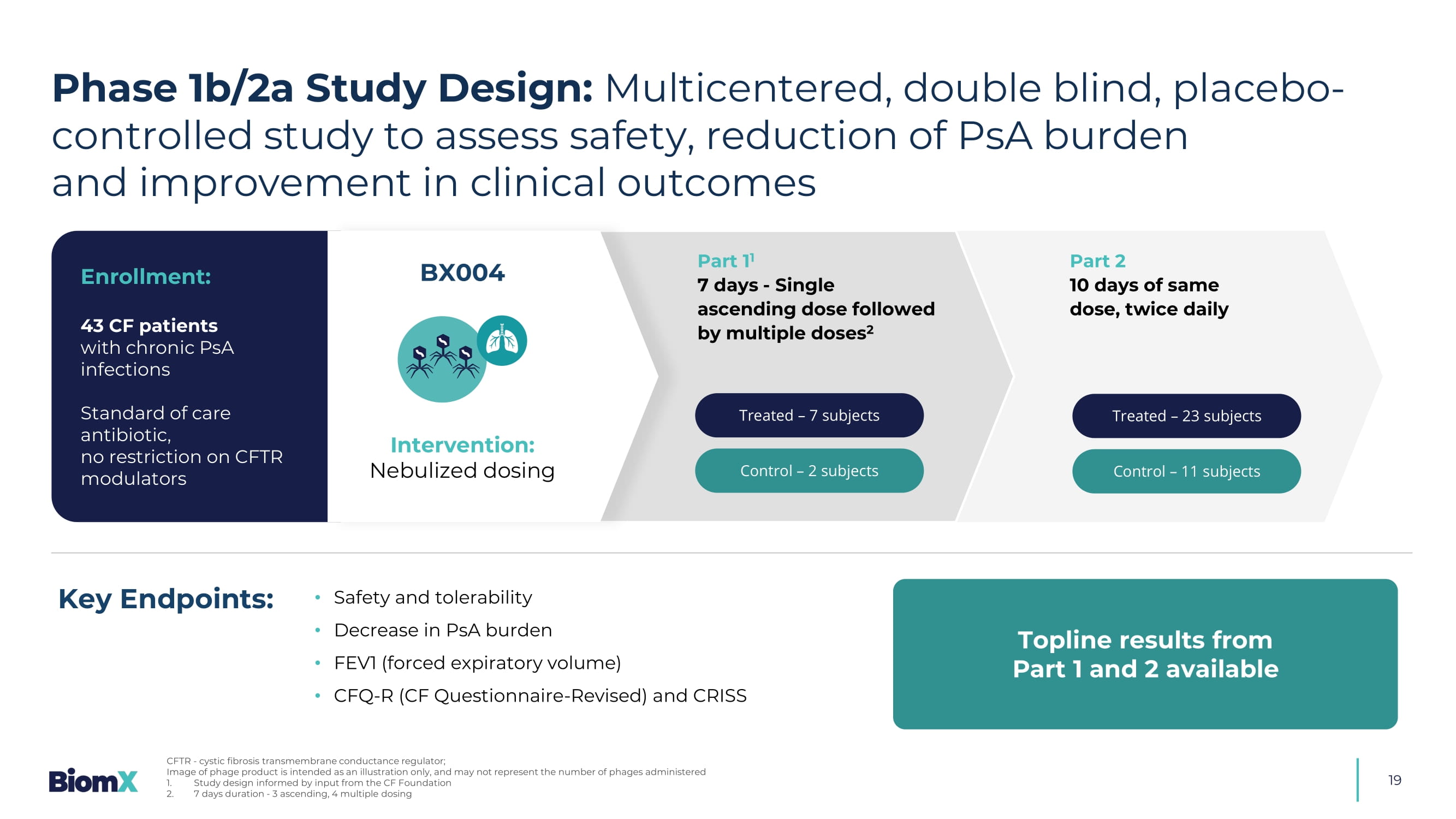
CFF Annual Data Report 2019, ECFS patient registry report, 2020 Airway with CF Thickened Mucus 19 E nrollment : 43 CF patients with chronic PsA infections Standard of care antibiotic, no restriction on CFTR modulators Treated – 7 subjects Control – 2 subjects Treated – 23 subjects Control – 11 subjects Part 1 1 7 days - Single ascending dose followed by multiple doses 2 Part 2 10 days of same dose, twice daily Phase 1b/2a Study Design: Multicentered, double blind, placebo - controlled study to assess safety, reduction of PsA burden and improvement in clinical outcomes CFTR - cystic fibrosis transmembrane conductance regulator; Image of phage product is intended as an illustration only, and may not represent the number of phages administered 1. Study design informed by input from the CF Foundation 2.
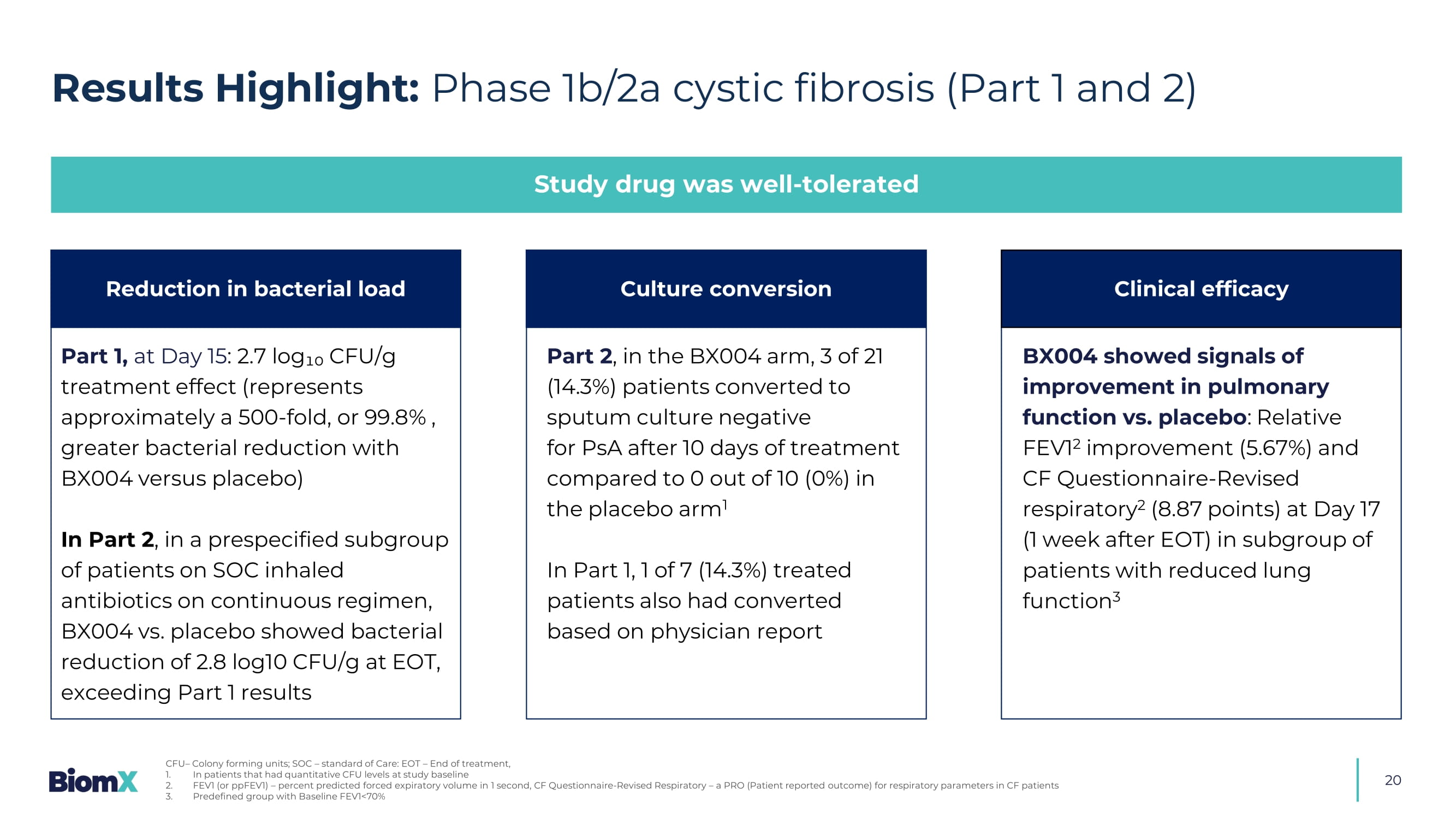
7 days duration - 3 ascending, 4 multiple dosing Topline results from Part 1 and 2 available • Safety and tolerability • Decrease in PsA burden • FEV1 (forced expiratory volume) • CFQ - R (CF Questionnaire - Revised) and CRISS Key Endpoints: BX004 Intervention: Nebulized dosing 20 Results Highlight: Phase 1b/2a cystic fibrosis (Part 1 and 2) Study drug was well - tolerated Part 1, at Day 15 : 2.7 log₁₀ CFU/g treatment effect (represents approximately a 500 - fold, or 99.8% , greater bacterial reduction with BX004 versus placebo) In Part 2 , i n a prespecified subgroup of patients on SOC inhaled antibiotics on continuous regimen, BX004 vs. placebo showed bacterial reduction of 2.8 log10 CFU/g at EOT, exceeding Part 1 results BX004 showed signals of improvement in pulmonary function vs. placebo : Relative FEV1 2 improvement (5.67%) and CF Questionnaire - Revised respiratory 2 (8.87 points) at Day 17 (1 week after EOT) in subgroup of patients with reduced lung function 3 Part 2 , in the BX004 arm, 3 of 21 (14.3%) patients converted to sputum culture negative for PsA after 10 days of treatment compared to 0 out of 10 (0%) in the placebo arm 1 In Part 1, 1 of 7 (14.3%) treated patients also had converted based on physician report Reduction in bacterial load Culture conversion Clinical efficacy CFU – Colony forming units; SOC – standard of Care: EOT – End of treatment, 1. In patients that had quantitative CFU levels at study baseline 2. FEV1 (or ppFEV1) – percent predicted forced expiratory volume in 1 second, CF Questionnaire - Revised Respiratory – a PRO (Patient reported outcome) for respiratory parameters in CF patients 3.
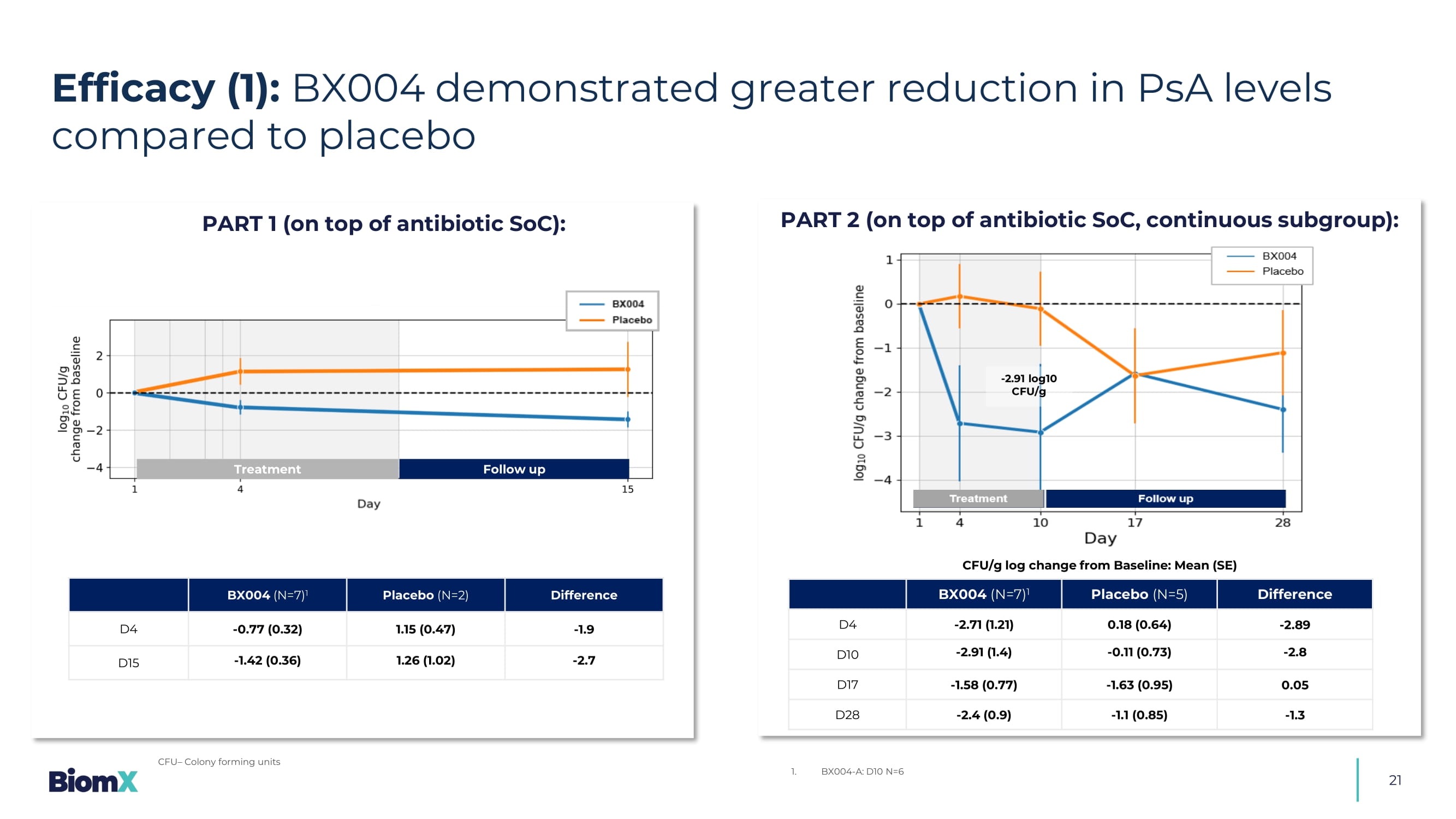
Predefined group with Baseline FEV1<70% 21 Efficacy (1): BX004 demonstrated greater reduction in PsA levels compared to placebo CFU – Colony forming units - 2.91 log10 CFU/g PART 1 (on top of antibiotic SoC): PART 2 (on top of antibiotic SoC, continuous subgroup): Difference Placebo (N=5) BX004 (N=7) 1 - 2.89 0.18 (0.64) - 2.71 (1.21) D4 - 2.8 - 0.11 (0.73) - 2.91 (1.4) D10 0.05 - 1.63 (0.95) - 1.58 (0.77) D17 - 1.3 - 1.1 (0.85) - 2.4 (0.9) D28 1. BX004 - A: D10 N=6 CFU/g log change from Baseline: Mean (SE) Difference Placebo (N=2) BX004 (N=7) 1 - 1.9 1.15 ( 0.47 ) - 0.77 ( 0.32 ) D4 - 2. 7 1.26 ( 1.02 ) - 1.42 ( 0.36 ) D15 Treatment Follow up 22 Baseline PsA 1 in sputum (CFU/g) Duration of PsA infection (years) Patient 2.40x10 3 18 1 5.60x10 7 13 2 1.09x10 7 35 3 1.
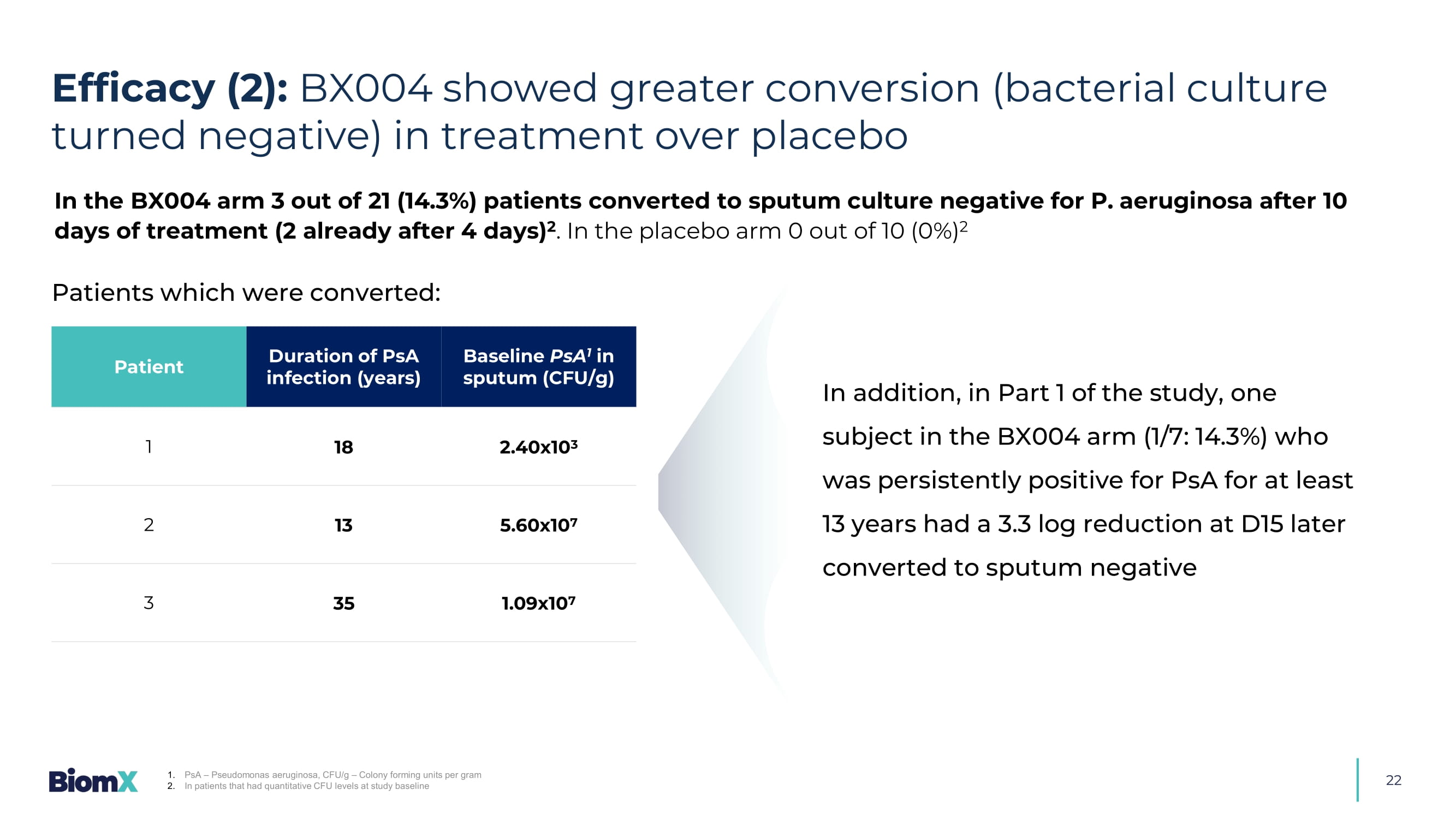
PsA – Pseudomonas aeruginosa, CFU/g – Colony forming units per gram 2. In patients that had quantitative CFU levels at study baseline Patients which were converted: In the BX004 arm 3 out of 21 (14.3%) patients converted to sputum culture negative for P. aeruginosa after 10 days of treatment (2 already after 4 days) 2 .
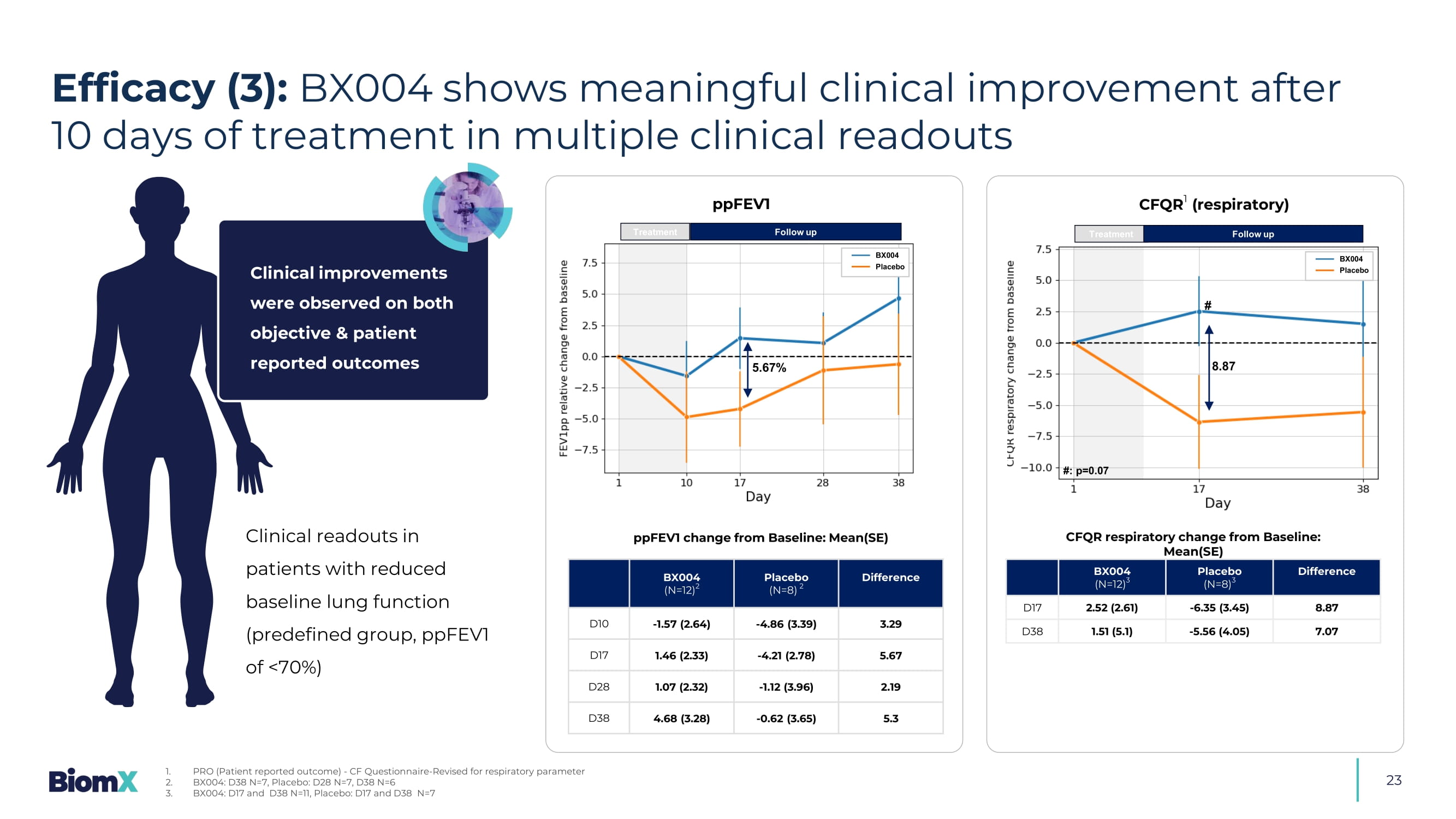
In the placebo arm 0 out of 10 (0%) 2 In addition, in Part 1 of the study, one subject in the BX004 arm (1/7: 14.3%) who was persistently positive for PsA for at least 13 years had a 3.3 log reduction at D15 later converted to sputum negative Efficacy (2): BX004 showed greater conversion (bacterial culture turned negative) in treatment over placebo Clinical readouts in patients with reduced baseline lung function (predefined group, ppFEV1 of <70%) Difference Placebo (N=8) 2 BX004 (N=12) 2 3.29 - 4.86 (3.39) - 1.57 (2.64) D10 5.67 - 4.21 (2.78) 1.46 (2.33) D17 2.19 - 1.12 (3.96) 1.07 (2.32) D28 5.3 - 0.62 (3.65) 4.68 (3.28) D38 Difference Placebo (N=8) 3 BX004 (N=12) 3 8.87 - 6.35 (3.45) 2.52 (2.61) D17 7.07 - 5.56 (4.05) 1.51 (5.1) D38 ppFEV1 change from Baseline: Mean(SE) CFQR respiratory change from Baseline: Mean(SE) Treatment 5.67% Follow up ppFEV1 BX004 Placebo Treatment Follow up CFQR 1 (respiratory) 8.87 BX004 Placebo # #: p=0.07 23 Efficacy (3): BX004 shows meaningful clinical improvement after 10 days of treatment in multiple clinical readouts 1. PRO (Patient reported outcome) - CF Questionnaire - Revised for respiratory parameter 2. BX004: D38 N=7, Placebo: D28 N=7, D38 N=6 3. BX004: D17 and D38 N=11, Placebo: D17 and D38 N=7 Clinical improvements were observed on both objective & patient reported outcomes 24 • Decrease in PsA burden (incl.
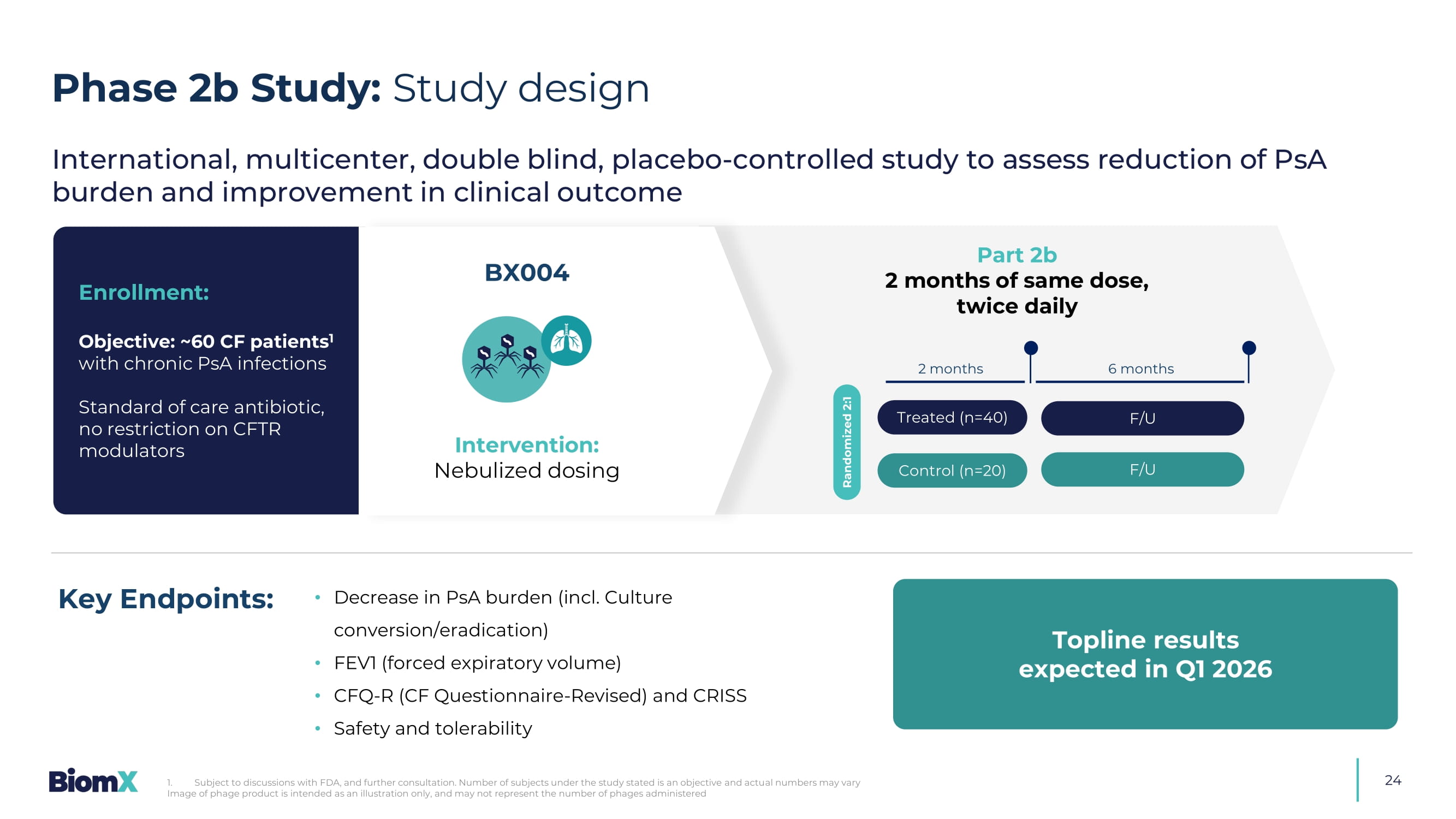
Culture conversion/eradication) • FEV1 (forced expiratory volume) • CFQ - R (CF Questionnaire - Revised) and CRISS • Safety and tolerability Key Endpoints: E nrollment : Objective: ~60 CF patients 1 with chronic PsA infections Standard of care antibiotic, no restriction on CFTR modulators BX004 Treated (n=40) Control (n=20) Randomized 2:1 2 months 6 months F/U F/U International, multicenter, double blind, placebo - controlled study to assess reduction of PsA burden and improvement in clinical outcome Intervention: Nebulized dosing Part 2b 2 months of same dose, twice daily Topline results expected in Q1 2026 Phase 2b Study: Study design 1. Subject to discussions with FDA, and further consultation.
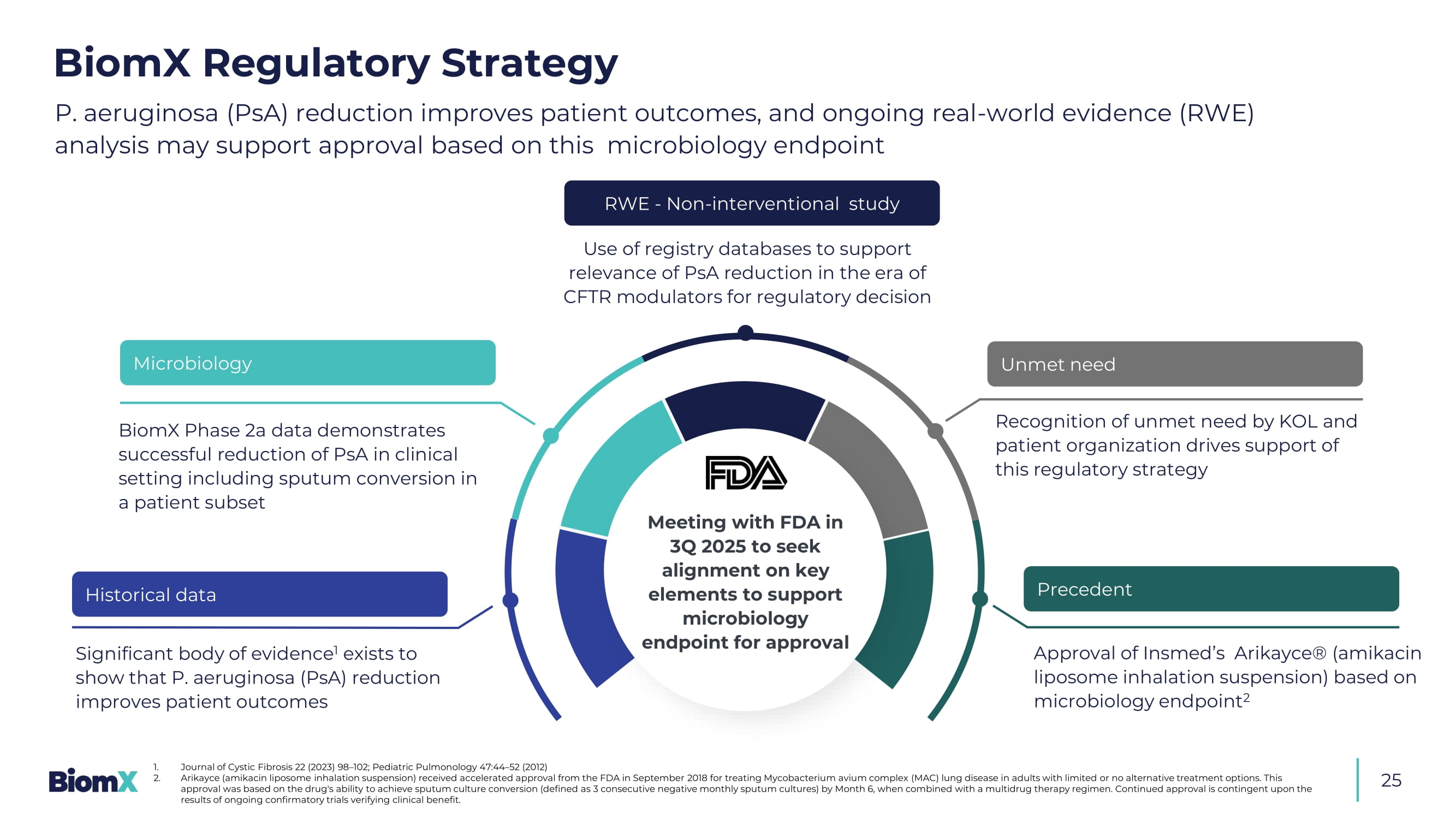
Number of subjects under the study stated is an objective and actu al numbers may vary Image of phage product is intended as an illustration only, and may not represent the number of phages administered Precedent P. aeruginosa (PsA) reduction improves patient outcomes, and ongoing real - world evidence (RWE) analysis may support approval based on this microbiology endpoint 1. Journal of Cystic Fibrosis 22 (2023) 98 – 102; Pediatric Pulmonology 47:44 – 52 (2012) 2. Arikayce (amikacin liposome inhalation suspension) received accelerated approval from the FDA in September 2018 for treating Mycobacte ri um avium complex (MAC) lung disease in adults with limited or no alternative treatment options. This approval was based on the drug's ability to achieve sputum culture conversion (defined as 3 consecutive negative monthly sput um cultures) by Month 6, when combined with a multidrug therapy regimen. Continued approval is contingent upon the results of ongoing confirmatory trials verifying clinical benefit. Recognition of unmet need by KOL and patient organization drives support of this regulatory strategy Significant body of evidence 1 exists to show that P. aeruginosa (PsA) reduction improves patient outcomes Meeting with FDA in 3Q 2025 to seek alignment on key elements to support microbiology endpoint for approval Historical data Microbiology BiomX Phase 2a data demonstrates successful reduction of PsA in clinical setting including sputum conversion in a patient subset RWE - Non - interventional study Use of registry databases to support relevance of PsA reduction in the era of CFTR modulators for regulatory decision Unmet need Approval of Insmed’s Arikayce ® (amikacin liposome inhalation suspension) based on microbiology endpoint 2 BiomX Regulatory Strategy 25 26 NCFB: A promising next candidate given its medical similarity to cystic fibrosis 1.
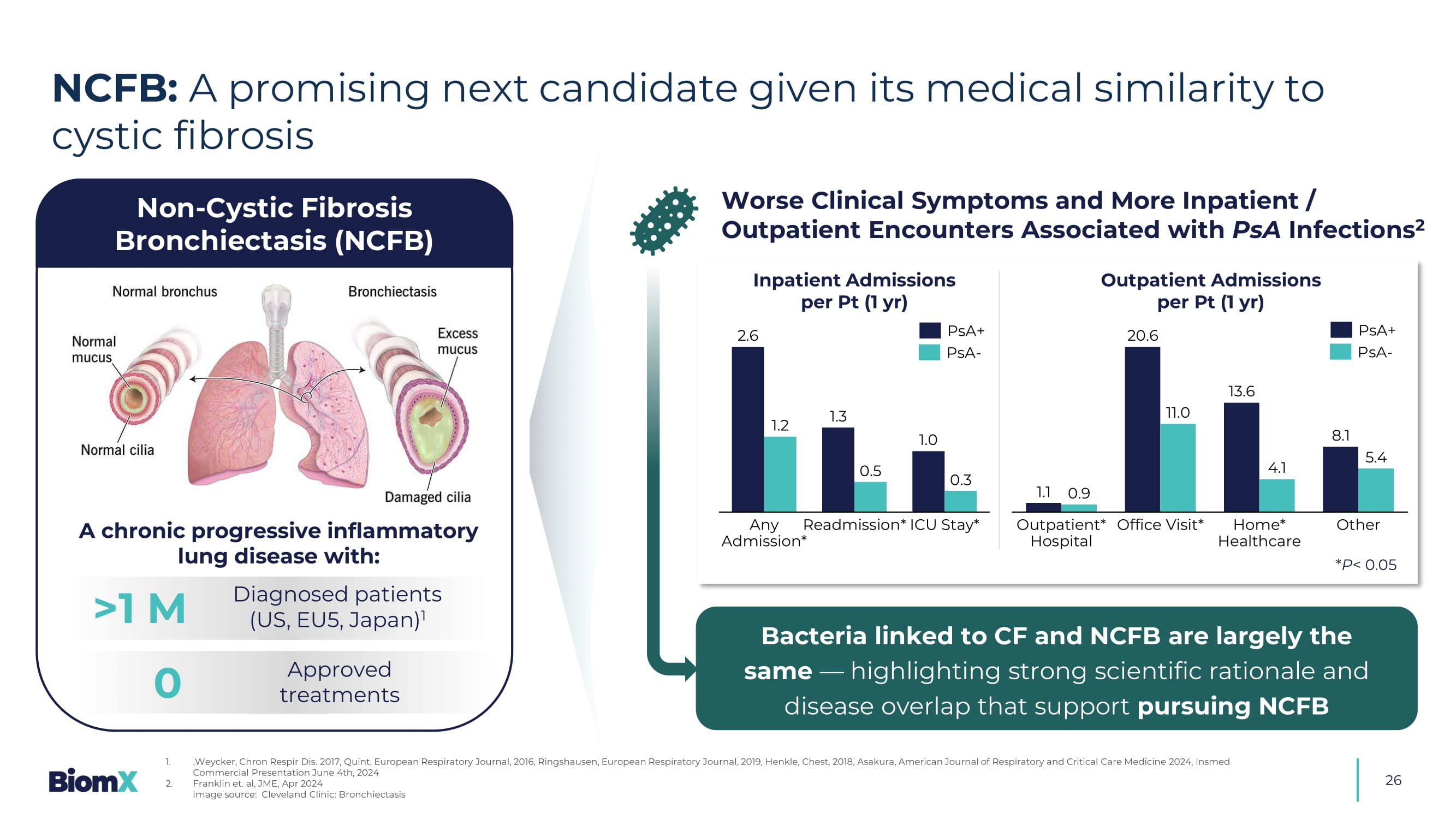
.Weycker, Chron Respir Dis. 2017, Quint, European Respiratory Journal, 2016, Ringshausen , European Respiratory Journal, 2019, Henkle, Chest, 2018, Asakura, American Journal of Respiratory and Critical Care Medicin e 2 024, Insmed Commercial Presentation June 4th, 2024 2. Franklin et. al, JME, Apr 2024 Image source: Cleveland Clinic: Bronchiectasis >1 M A chronic progressive inflammatory lung disease with: 0 Diagnosed patients (US, EU5, Japan) 1 Approved treatments Non - Cystic Fibrosis Bronchiectasis (NCFB) Worse Clinical Symptoms and More Inpatient / Outpatient Encounters Associated with PsA Infections 2 2.6 1.3 1.0 1.2 0.5 0.3 Any Admission* Readmission* ICU Stay* 1.1 20.6 13.6 8.1 0.9 11.0 4.1 5.4 Outpatient* Hospital Office Visit* Home* Healthcare Other Inpatient Admissions per Pt (1 yr) Out patient Admissions per Pt (1 yr) Bacteria linked to CF and NCFB are largely the same — highlighting strong scientific rationale and disease overlap that support pursuing NCFB * P < 0.05 PsA+ PsA - PsA+ PsA -28 2 Phage Therapy Programs 2 Phase 2 Studies ≥ $4 B Addressable Market World - wide ≥ $215 M Raised 3 Patent Families Strong Manufacturing Capabilities Reputable Investors & Partners ≥ 50% PhDs in Leadership &UHDWHGE\$ITRK IURP1RXQ3URMHFW Robust Scientific Expertise ~$40M Non - Dilutive

Summary
