UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 30, 2025
| INMUNE BIO INC. |
| (Exact name of registrant as specified in charter) |
| Nevada | 001-38793 | 47-5205835 | ||
| (State or other jurisdiction | (Commission File Number) | (IRS Employer | ||
| of incorporation) | Identification No.) |
225 NE Mizner Blvd., Suite 640, Boca Raton, Florida 33432
(Address of Principal Executive Offices) (Zip Code)
(858) 964 3720
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, If Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per shares | INMB | The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mart if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On June 30, 2025, INmune Bio, Inc. (the “Company”) issued a press release announcing topline results from its Phase 2 MINDFuL trial evaluating XPro™ in patients with early Alzheimer’s disease.
The Company also made available an updated corporate presentation, which includes additional information about the Company and its clinical programs.
Copies of the press release and the corporate presentation are filed as Exhibits 99.1 and 99.2, respectively, to this Current Report on Form 8-K and are incorporated herein by reference.
Item 9.01 Financial statements and Exhibits
(d) Exhibits.
| 99.1 | Press Release, dated June 30, 2025 | |
| 99.2 | Corporate Presentation, dated June 30, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| INMUNE BIO INC. | ||
| Date: June 30, 2025 | By: | /s/ David Moss |
| David Moss | ||
| Chief Financial Officer | ||
Exhibit 99.1
INmune Bio Reports Key Findings from Phase 2 MINDFuL Trial of XPro™ in Early Alzheimer’s Disease
In the Phase 2 MINDFuL trial of XPro™ in patients with early Alzheimer’s Disease (AD) with biomarkers of inflammation, the modified intent-to-treat (mITT) population (n=200) did not meet the primary cognitive endpoint (EMACC), however in a predefined population of amyloid-positive early AD patients with two or more biomarkers of inflammation (n=100), a benefit of XPro™ treatment over placebo was observed in cognitive, behavioral and biological endpoints.
Treatment with XPro™ was well-tolerated and safe, even in the high risk ApoE4+/+ patient group, and ARIA-E or ARIA-H was not observed in any patients. The most common adverse events (AE) were injection site reactions.
Additional analyses will be presented at Alzheimer’s Association International Conference (AAIC) in Toronto, Canada (July 27-31, 2025) and the Company will submit for Breakthrough Therapy designation with the FDA.
Conference call today at 8AM ET, details below.
Boca Raton, June 29, 2025 (GLOBE NEWSWIRE) -- INmune Bio Inc. (NASDAQ: INMB), today announced results from its Phase 2 MINDFuL trial (NCT05318976) evaluating XPro™, a selective soluble TNF inhibitor, in early AD with biomarkers of inflammation. Despite showing no effects in the modified intent-to-treat population (mITT, n=200), predefined analyses demonstrated a cognitive benefit for XPro™ over placebo on the primary endpoint EMACC, a behavioral benefit on the Neuropsychiatric Inventory, and a biological benefit on pTau217 in early Alzheimer’s patients with two or more biomarkers of inflammation at baseline (n=100), marking a key milestone in the development of XPro™ for Early AD.
The MINDFuL trial, a double-blind, placebo-controlled study, enrolled 208 participants with early-stage AD to assess XPro™’s potential in slowing cognitive decline by tackling neuroinflammation. Patients with at least one of four inflammatory biomarkers (elevated CRP, ESR, HbA1c, or at least one ApoE4 allele) were randomized 2:1 (XPro™:placebo) and treated over 24 weeks. The primary endpoint was change in cognition over 6 months on the Early Mild Alzheimer’s Cognitive Composite (EMACC), a cognitive assessment designed specifically to measure change in early AD patients. While the primary endpoint was not met in the mITT group, key changes in clinical measures of cognition, behavior, and an AD-related biomarker demonstrated a benefit in a subpopulation of patients treated with XPro™ over patients treated with placebo. These results identify a clear population of AD patients for which XPro™ might have therapeutic benefit.
Key Findings in the Amyloid positive Early AD participants with two or more biomarkers of inflammation (N=100):
| ● | Cognition: A clinical benefit of XPro™ over placebo was observed on the primary endpoint EMACC (effect size: 0.27) and on the secondary endpoint Neuropsychiatric Inventory (effect size: -0.24). |
| ● | Alzheimer’s Disease Biomarkers: A biological benefit of XPro™ over placebo was observed in blood levels of pTau217 (effect size: -0.20), the gold standard measure of AD pathology in blood. |
| ● | Safety and tolerability: XPro™ treatment was safe and well tolerated, without any occurrences of ARIA-E or ARIA-H. Injection site reactions (ISR) were common (80% of XPro™ group compared to placebo group (<20%). ISRs were short-term redness and/or pain at the injection site in two thirds of cases. Of the 14 patients in the XPro™ arm that discontinued the trial, ISR was the cause in 10 patients. There were no deaths, drug-related hospitalizations or organ system toxicity in the trial. |
| ● | Novel mechanism of action: This study demonstrates that it is possible to safely target neuroinflammation in patients where neuroinflammation is a driver of AD pathology. With the increased interest in inflammation as a disease-modifier in AD and neurodegeneration, these results provide the basis for further development of XPro™ in neurodegeneration. |
“These results highlight the potential of XPro™,” stated RJ Tesi, MD, CEO of INmune Bio. “Our findings indicate that XPro™ may offer benefits to Alzheimer’s patients across all age groups, regardless of comorbidities, additional medications, or ApoE4 status. This evidence lays the foundation for advancing XPro™ as a promising treatment option for Alzheimer’s disease.”
CJ Barnum, PhD, VP of CNS Drug Development, added, “By targeting neuroinflammation, a key driver of Alzheimer’s disease progression, XPro™ offers a novel mechanism to potentially slow disease progression and cognitive symptoms for persons living with Alzheimer’s disease and inflammation. The continued development of this therapeutic, whether as a standalone treatment or in combination with other therapies, holds promise in addressing this critical and growing unmet medical need.”
Additional analyses are underway and will be presented at AAIC in Toronto, Canada (July 27-31, 2025).
Expert Commentary on Use of Effect Size in Clinical Trials
“In early-phase Alzheimer’s disease trials, absolute effect sizes of 0.2 or greater are considered preliminary evidence of potential therapeutic efficacy and are informative for signal detection in early phase studies when sample sizes are small and the unknowns of a novel mechanism are significant,” said Dr. Judith Jaeger, a leading expert in cognitive assessment and consultant to INmune Bio on this trial. “When a therapy consistently meets the 0.2 benchmark across multiple parameters (clinical and biological), confidence in the validity of the observed effects increases, indicating a therapy is worth advancing.”
Next Steps
INmune Bio will present additional analyses from the MINDFuL trial at AAIC® in Toronto, Canada (July 27-31, 2025). Based on the totality of the data, the company intends to:
| ● | File for Breakthrough Therapy Designation with the FDA. |
| ● | Schedule an End-of-Phase 2 meeting with the FDA in Q4 2025 to define the path for a pivotal trial to support XPro™ approval in early AD. |
| ● | Engage regulatory authorities in the UK, EU, and other regions in parallel. |
MINDFuL Results Call Information:
To participate in this event, dial approximately at least 10 minutes before the beginning of the call or use the webcast link below. Please ask for the INmune Bio MINDFuL Conference Call when reaching the operator.
Date: June 30, 2025
Time: 8:00 AM Eastern Time
Participant Dial-in: +1-800-267-6316 Participant Dial-in (international): +1-203-518-9783
Conference ID: INMUNE
NOTE: THIS CONFERENCE ID WILL BE REQUIRED FOR ENTRY
To join by webcast link please click here or copy and past the link below into your browser:
https://viavid.webcasts.com/starthere.jsp?ei=1725659&tp_key=5cf89734df
A transcript will follow approximately 24 hours from the scheduled call. A replay will also be available through July 30, 2025 by dialing 1-844-512-2921 or 1-412-317-6671 (international) and entering pin no. 11159260.
Acknowledgements
INmune Bio wishes to extend their gratitude to the participants, caregivers, trial site staff, vendors, and any others who supported the efforts of this proof-of-concept phase 2 clinical trial.
About MINDFuL (NCT05318976)
The MINDFuL trial is an international, blinded, randomized Phase 2 trial in patients with Early AD with biomarkers of elevated neuroinflammation. Early AD includes patients with MCI (mild cognitive impairment) and mild AD. Patients must have at least one of four biomarkers of inflammation – elevated CRP, HgbA1c, ESR or ApoE4 allele. Patients receive either XPro™ or placebo (2:1 ratio) for 6 months. The cognitive endpoints are EMACC and CDR. XPro™ is given as a once-a-week subcutaneous injection. For more information on the MINDFuL clinical trial please visit www.clinicaltrials.gov or www.inmunebio.com
About Judith Jaeger, PhD
Judith Jaeger, PhD, is the principal developer of the EMACC. Judith Jaeger PhD is founder and President of CognitionMetrics, a prominent neurocognition consulting firm. Dr. Jaeger is an internationally recognized expert in designing cognitive function testing programs to use in clinical trials with more than two decades’ experience.
About EMACC
The EMACC is a validated cognitive composite derived from six standardized neuropsychological tests, empirically developed to provide optimal for sensitivity to decline in early AD. Unlike traditional clinical rating scales, EMACC minimizes variance from informant demographics, providing an objective measure of cognitive performance with no floor or ceiling effects.
About XPro™
XPro™ is an advanced tumor necrosis factor (TNF) inhibitor that targets soluble TNF (sTNF) while preserving trans-membrane TNF (tmTNF) and TNF receptors. By reducing neuroinflammation, XPro™ may offer significant benefits for patients with neurologic disorders, potentially enhancing cognitive function and supporting neuronal communication.
About INmune Bio Inc.
INmune Bio Inc. is a publicly traded (NASDAQ: INMB), clinical-stage biotechnology company focused on developing treatments that target the innate immune system to fight disease. INmune Bio has three product platforms: the Dominant-Negative Tumor Necrosis Factor (DN-TNF) product platform utilizes dominant-negative technology to selectively neutralize soluble TNF, a key driver of innate immune dysfunction and a mechanistic driver of many diseases. DN-TNF product candidates are in clinical trials to determine if they can treat Mild Alzheimer’s disease, Mild Cognitive Impairment and treatment-resistant depression (XPro™). The Natural Killer Cell Priming Platform includes INKmune® developed to prime a patient’s NK cells to eliminate minimal residual disease in patients with cancer and is currently in trials in metastatic castration-resistance prostate cancer. The third program, CORDStrom™, is a proprietary allogeneic, pooled, human umbilical cord-derived mesenchymal Stromal/Stem cell (hucMSCs) platform that recently completed a blinded randomized trial in recessive dystrophic epidermolysis bullosa. INmune Bio’s product platforms utilize a precision medicine approach for diseases driven by chronic inflammation and cancer.
Forward Looking Statements
Clinical trials are in early stages and there is no assurance that any specific outcome will be achieved. Any statements contained in this press release related to the development or commercialization of product candidates and other business and financial matters, including without limitation, trial results and data, including the results of the Phase 2 MINDFuL trial, the timing of key milestones, future plans or expectations for the treatment of XPro™, and the prospects for receiving regulatory approval or commercializing or selling any product or drug candidates may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any forward-looking statements contained herein are based on current expectations but are subject to several risks and uncertainties. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements because of these risks and uncertainties. CORDstrom™, XPro1595 (XPro™, pegipanermin), and INKmune®™ have either finished clinical trials, are still in clinical trials or are preparing to start clinical trials and have not been approved by the US Food and Drug Administration (FDA) or any regulatory body and there cannot be any assurance that they will be approved by the FDA or any regulatory body or that any specific results will be achieved. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company’s ability to produce more drug for clinical trials; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company’s filings with the Securities and Exchange Commission, including the Company’s Annual Report on Form 10-K, the Company’s Quarterly Reports on Form 10-Q and the Company’s Current Reports on Form 8-K. The Company assumes no obligation to update any forward-looking statements to reflect any event or circumstance that may arise after the date of this release.
Company Contact:
David Moss
Chief Financial Officer
(561) 710-0512
info@inmunebio.com
Daniel Carlson
Head of Investor Relations
(415) 509-4590
dcarlson@inmunebio.com
Exhibit 99.2

1 Corporate Presentation June 2025 NASDAQ: INMB 2 FORWARD LOOKING STATEMENTS This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events.

When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements. Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook. Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements. They are neither statements of historical fact nor guarantees of assurance of future performance. We caution you therefore against relying on any of these forward - looking statements. Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations; our ability to protect our intellectual property rights; the impact of any infringement actions or other litigation brought against us; competition from other providers and products; our ability to develop and commercialize products and services; changes in government regulation; our ability to complete capital raising transactions; and other factors (including the risks contained in the section of this prospectus entitled “Risk Factors”) relating to our industry, our operations and results of operations. Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results. Clinical trials are in early stages and there is no assurance that any specific outcome will be achieved. Any statements cont ain ed in this press release related to the development or commercialization of product candidates and other business and financial matters, including without limitation, trial results an d data, including the results of the Phase 2 MINDFuL trial, the timing of key milestones, future plans or expectations for the treatment of XPro , and the prospects for receiving regulatory approval or commercializing or selling any product or drug candidates may constitute forward - looking statements as that term is defined in the Private Securities Litigatio n Reform Act of 1995. Any forward - looking statements contained herein are based on current expectations but are subject to several risks and uncertainties. Actual results and the ti ming of certain events and circumstances may differ materially from those described by the forward - looking statements because of these risks and uncertainties. CORDstrom , XPro1595 (XPro , pegipanermin), and INKmune® have either finished clinical trials, are still in clinical trials or are preparing to start clinical trials and have not been app rov ed by the US Food and Drug Administration (FDA) or any regulatory body and there cannot be any assurance that they will be approved by the FDA or any regulatory body or that any sp eci fic results will be achieved. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncerta int ies relating to the Company’s ability to produce more drug for clinical trials; the availability of substantial additional funding for the Company to continue its operations and t o c onduct research and development, clinical studies and future product commercialization; and, the Company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company’s filings with the Securities and Exchange Commissio n, including the Company’s Annual Report on Form 10 - K, the Company’s Quarterly Reports on Form 10 - Q and the Company’s Current Reports on Form 8 - K. The Company assumes no obligation to update any forward - looking statements to reflect any event or circumstance that may arise after the date of this release.
a 3 XPro » TNF is the master regulator of the innate immune system and causes TNF dysfunction in many neurologic disease. » XPro TM is designed to reestablish normal TNF function to treat disease. CORDStrom » Mesenchymal Stromal cells (MSC) are recognized for their immunomodulatory, anti - inflammatory and wound healing properties with potential to treat a diverse set of diseases. » CORDStrom TM technology is designed to solve the limitations of previous MSC therapies and is currently in development for a rare pediatric disease and other indications. INKmune » Natural Killer cells are responsible for detecting and eliminating cancer cells and become dysfunctional with age. » INKmune TM works within the body to activate the patients own NK cells against multiple forms of cancer. Three Platforms Modulating the Innate Immune System 4 Approaching Alzheimer’s as an Immunologic Disease
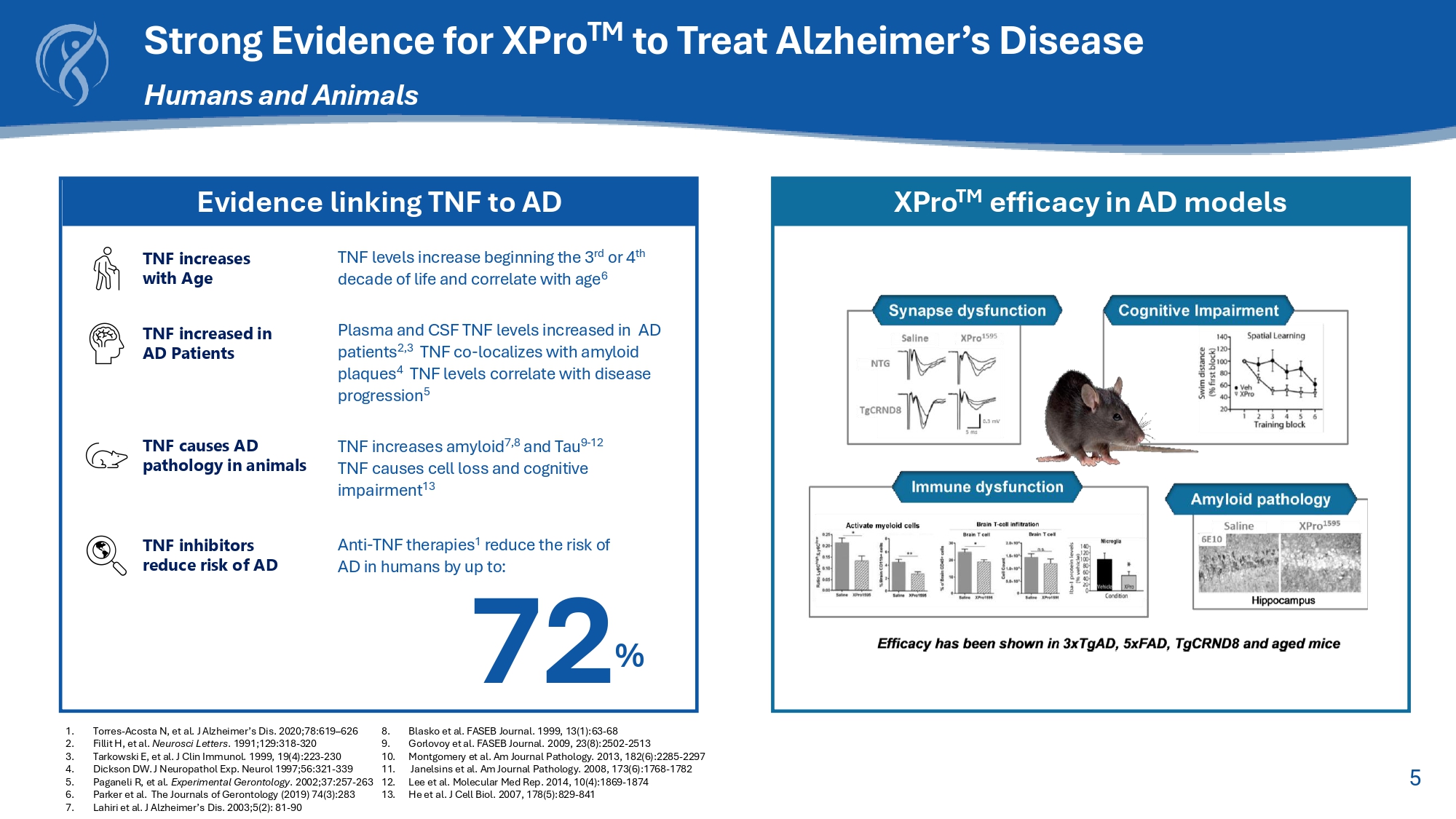
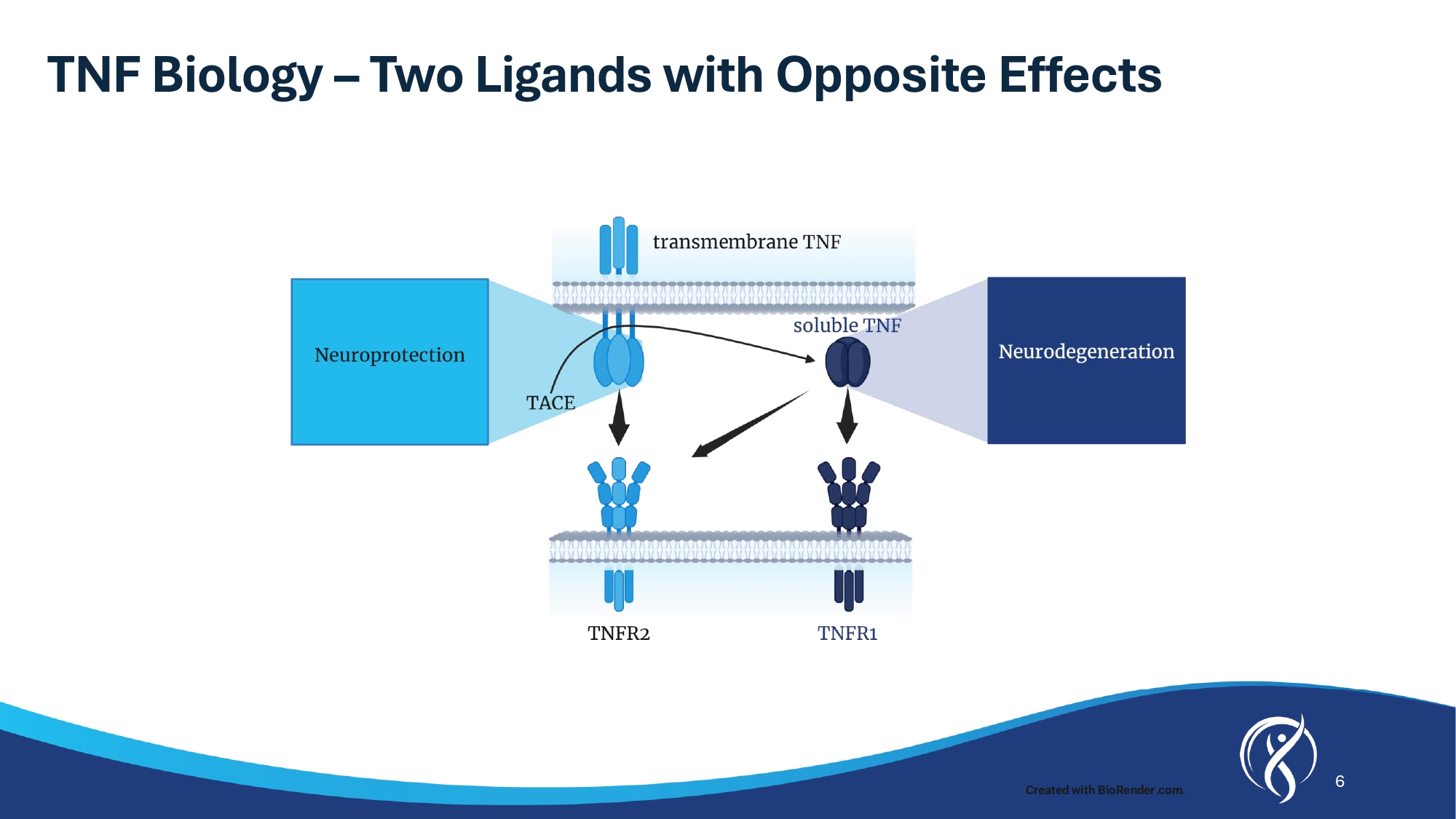
5 Strong Evidence for XPro TM to Treat Alzheimer’s Disease Humans and Animals 1. Torres - Acosta N, et al. J Alzheimer’s Dis. 2020;78:619 – 626 2. Fillit H, et al. Neurosci Letters . 1991;129:318 - 320 3. Tarkowski E, et al. J Clin Immunol. 1999, 19(4):223 - 230 4. Dickson DW. J Neuropathol Exp. Neurol 1997;56:321 - 339 5. Paganeli R, et al . Experimental Gerontology . 2002 ; 37 : 257 - 263 6. Parker et al . The Journals of Gerontology ( 2019 ) 74 ( 3 ) : 283 7. Lahiri et al . J Alzheimer’s Dis . 2003 ; 5 ( 2 ) : 81 - 90 8. Blasko et al . FASEB Journal . 1999 , 13 ( 1 ) : 63 - 68 9. Gorlovoy et al . FASEB Journal . 2009 , 23 ( 8 ) : 2502 - 2513 10. Montgomery et al . Am Journal Pathology . 2013 , 182 ( 6 ) : 2285 - 2297 11. Janelsins et al . Am Journal Pathology . 2008 , 173 ( 6 ) : 1768 - 1782 12. Lee et al . Molecular Med Rep . 2014 , 10 ( 4 ) : 1869 - 1874 13. He et al . J Cell Biol . 2007 , 178 ( 5 ) : 829 - 841 Evidence linking TNF to AD XPro TM efficacy in AD models TNF inhibitors reduce risk of AD Anti - TNF therapies 1 reduce the risk of AD in humans by up to: TNF causes AD pathology in animals TNF increases amyloid 7,8 and Tau 9 - 12 TNF causes cell loss and cognitive impairment 13 TNF increases with Age TNF levels increase beginning the 3 rd or 4 th decade of life and correlate with age 6 72 % TNF increased in AD Patients Plasma and CSF TNF levels increased in AD patients 2,3 TNF co - localizes with amyloid plaques 4 TNF levels correlate with disease progression 5 6 TNF Biology – Two Ligands with Opposite Effects Created with BioRender.com .
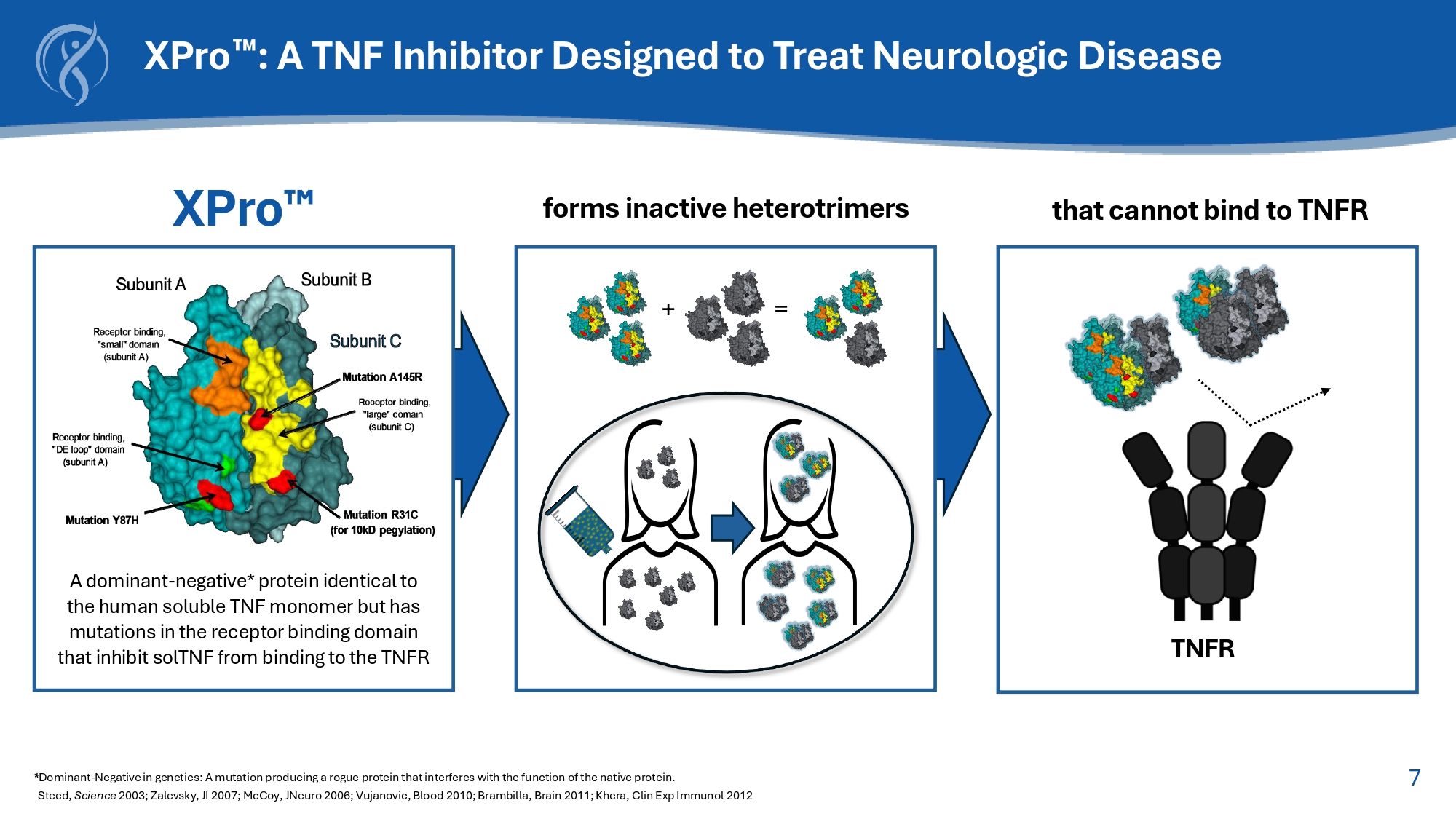
7 XPro : A TNF Inhibitor Designed to Treat Neurologic Disease * Dominant - Negative in genetics: A mutation producing a rogue protein that interferes with the function of the native protein. Steed, Science 2003; Zalevsky , JI 2007; McCoy, JNeuro 2006; Vujanovic, Blood 2010; Brambilla, Brain 2011; Khera, Clin Exp Immunol 2012 that cannot bind to TNFR forms inactive heterotrimers + = XPro A dominant - negative* protein identical to the human soluble TNF monomer but has mutations in the receptor binding domain that inhibit solTNF from binding to the TNFR TNFR 8 Selective Inhibition of Soluble TNF is Necessary to Treat AD Disease Worsening Etanercept Non - selective TNF inhibition Disease (Model of Multiple Sclerosis) Adapted from: Karamita et al., 2017.
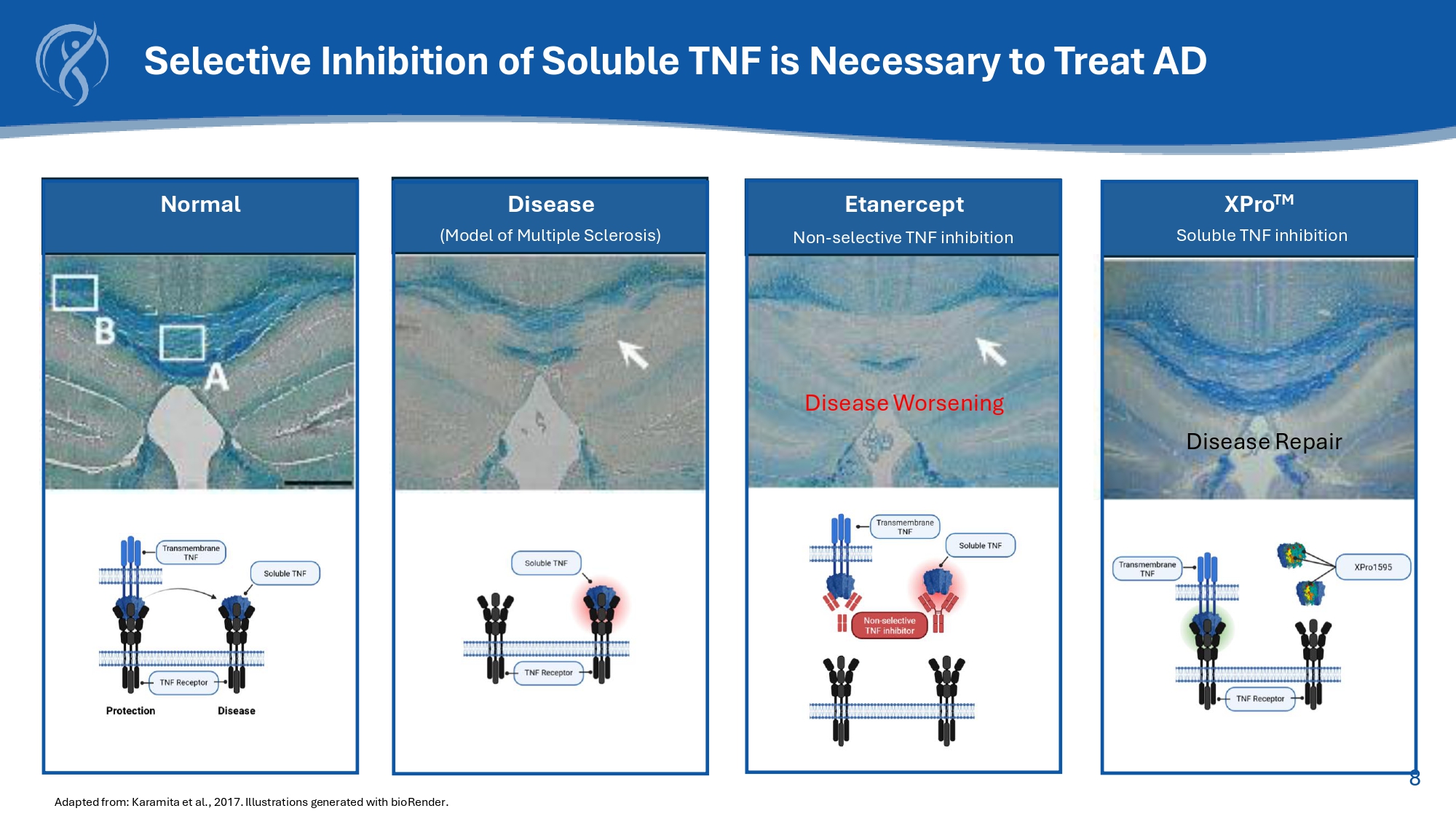
Illustrations generated with bioRender .
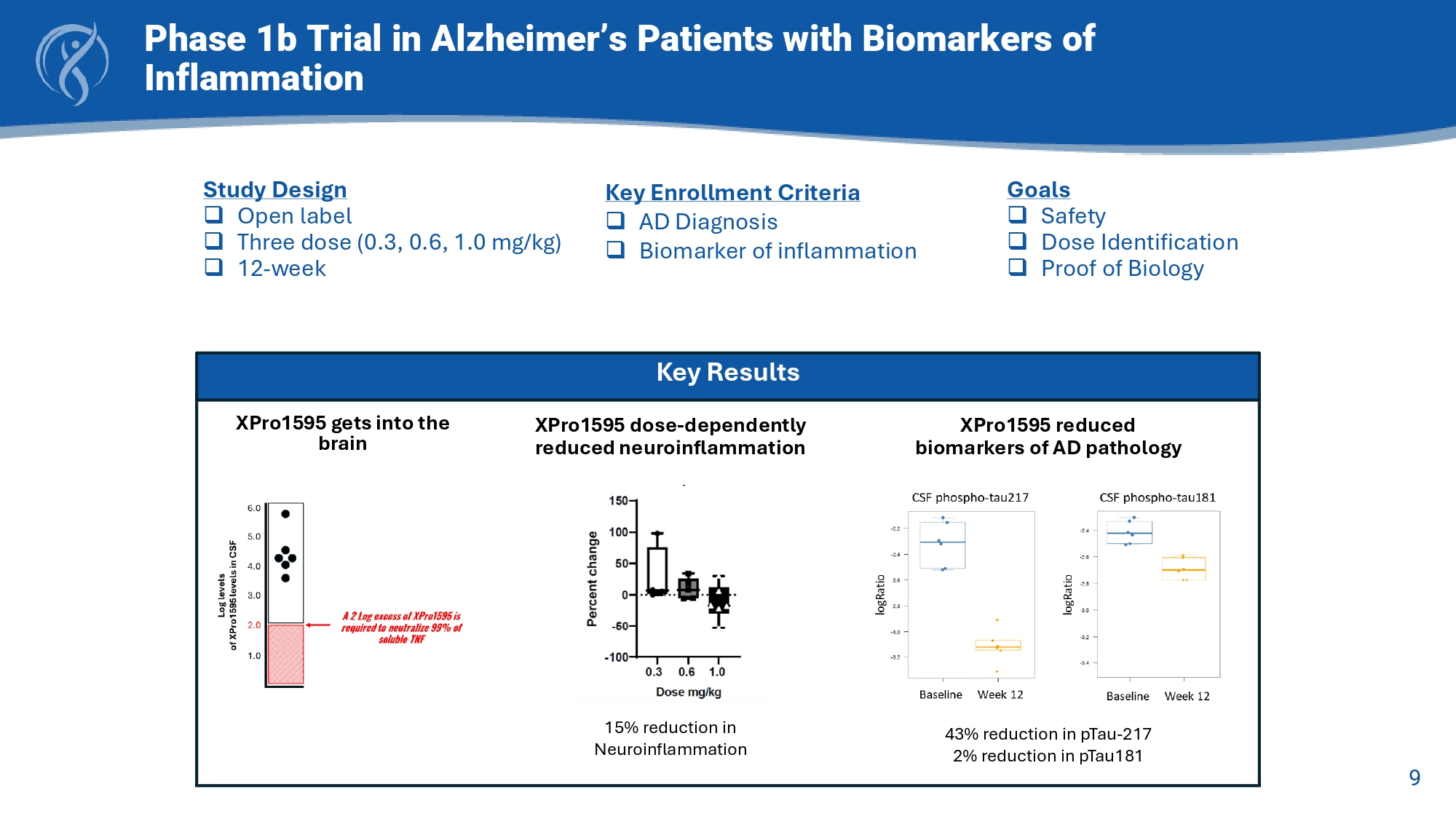
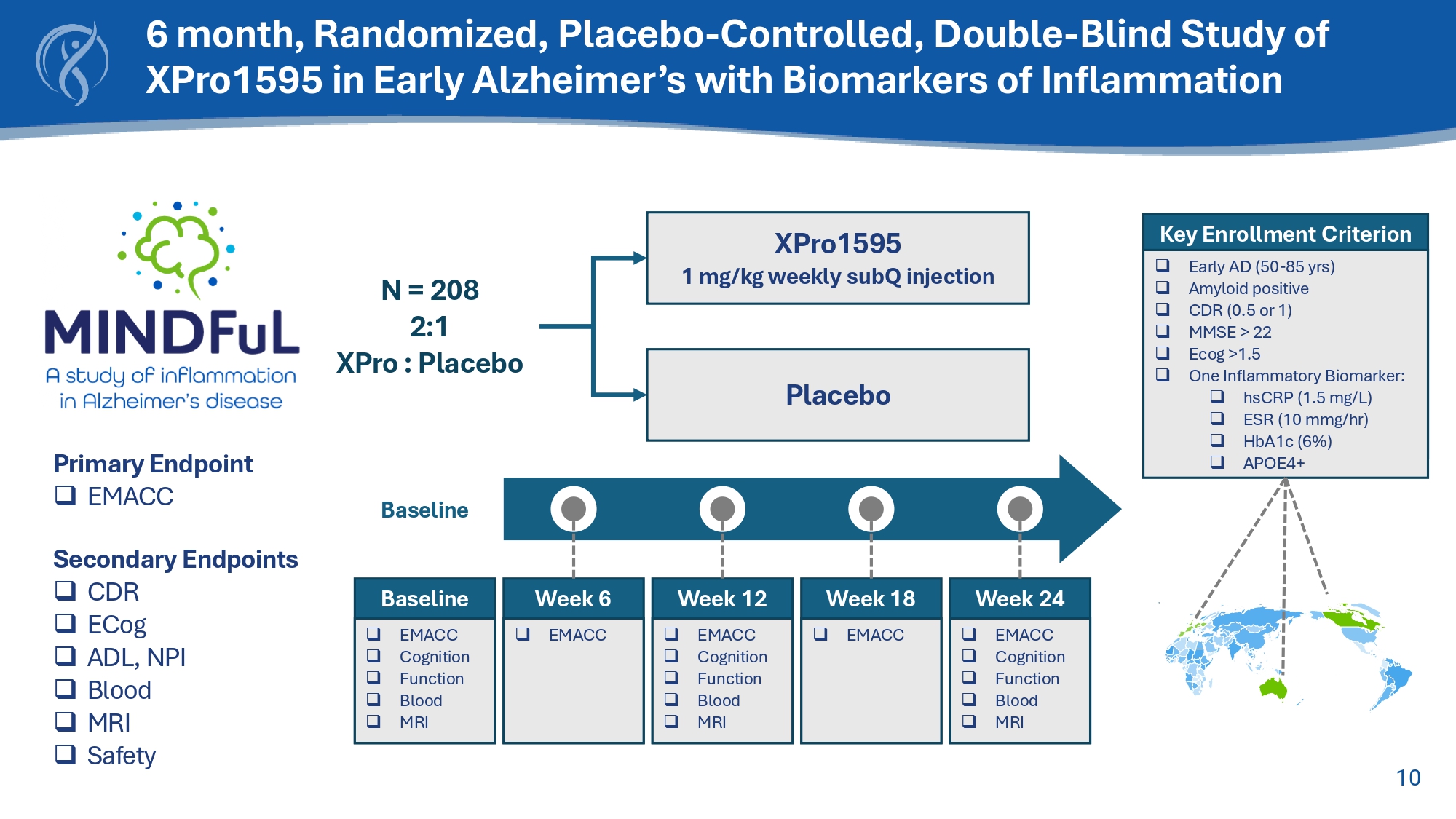
Normal XPro TM Disease Repair Soluble TNF inhibition 9 Phase 1b Trial in Alzheimer’s Patients with Biomarkers of Inflammation Key Results XPro1595 gets into the brain XPro1595 reduced biomarkers of AD pathology 43% reduction in pTau - 217 2% reduction in pTau181 XPro1595 dose - dependently reduced neuroinflammation 15% reduction in Neuroinflammation Study Design □ Open label □ Three dose (0.3, 0.6, 1.0 mg/kg) □ 12 - week Goals □ Safety □ Dose Identification □ Proof of Biology Key Enrollment Criteria □ AD Diagnosis □ Biomarker of inflammation 10 Key Enrollment Criterion □ Early AD (50 - 85 yrs) □ Amyloid positive □ CDR (0.5 or 1) □ MMSE > 22 □ Ecog >1.5 □ One Inflammatory Biomarker: □ hsCRP (1.5 mg/L) □ ESR (10 mmg / hr ) □ HbA1c (6%) □ APOE4+ Baseline □ EMACC □ Cognition □ Function □ Blood □ MRI Baseline Week 6 □ EMACC Week 12 □ EMACC □ Cognition □ Function □ Blood □ MRI Week 18 □ EMACC Week 24 □ EMACC □ Cognition □ Function □ Blood □ MRI Primary Endpoint □ EMACC Secondary Endpoints □ CDR □ ECog □ ADL, NPI □ Blood □ MRI □ Safety 6 month, Randomized, Placebo - Controlled, Double - Blind Study of XPro1595 in Early Alzheimer’s with Biomarkers of Inflammation XPro1595 1 mg/kg weekly subQ injection Placebo N = 208 2:1 XPro : Placebo
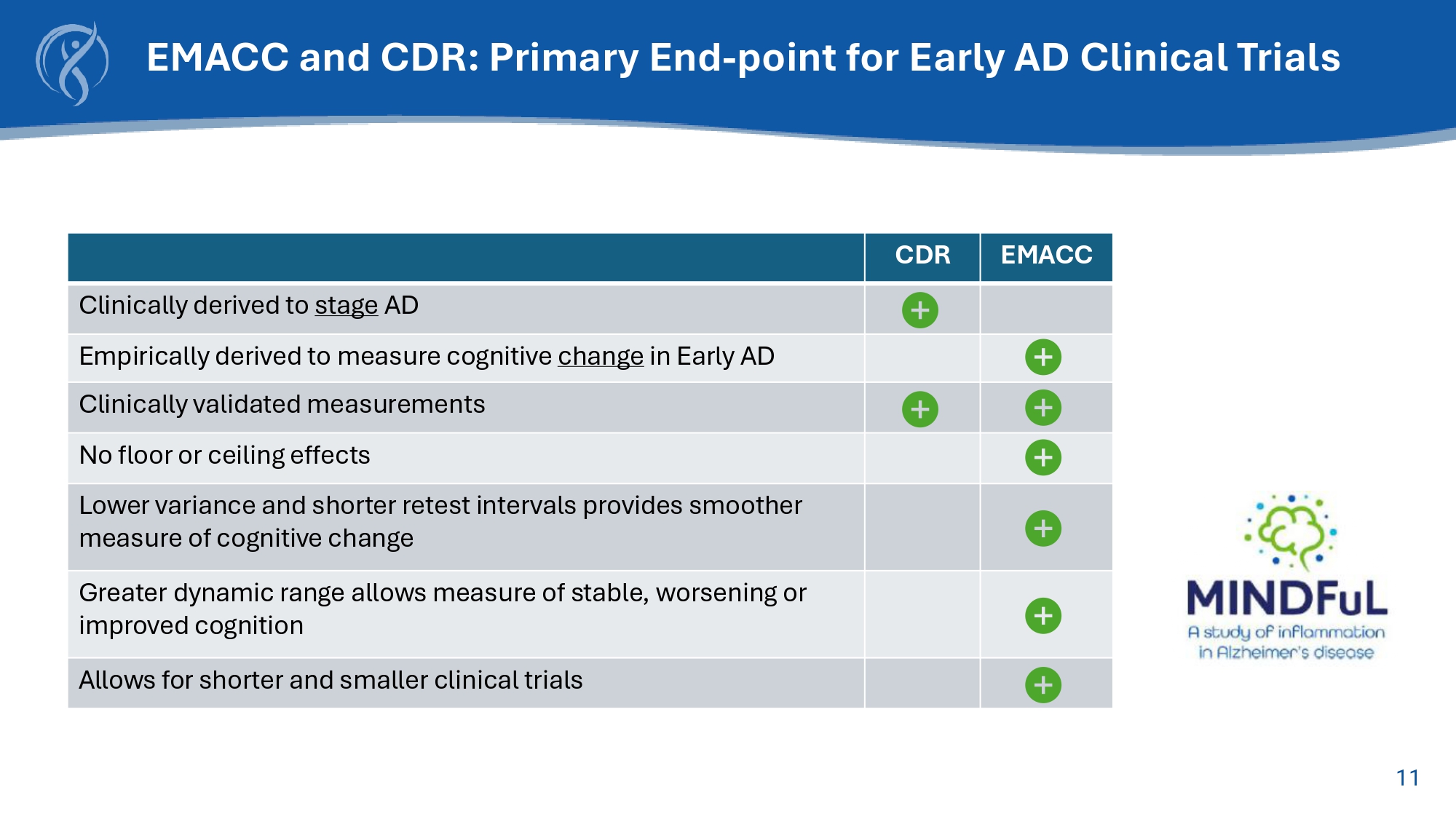
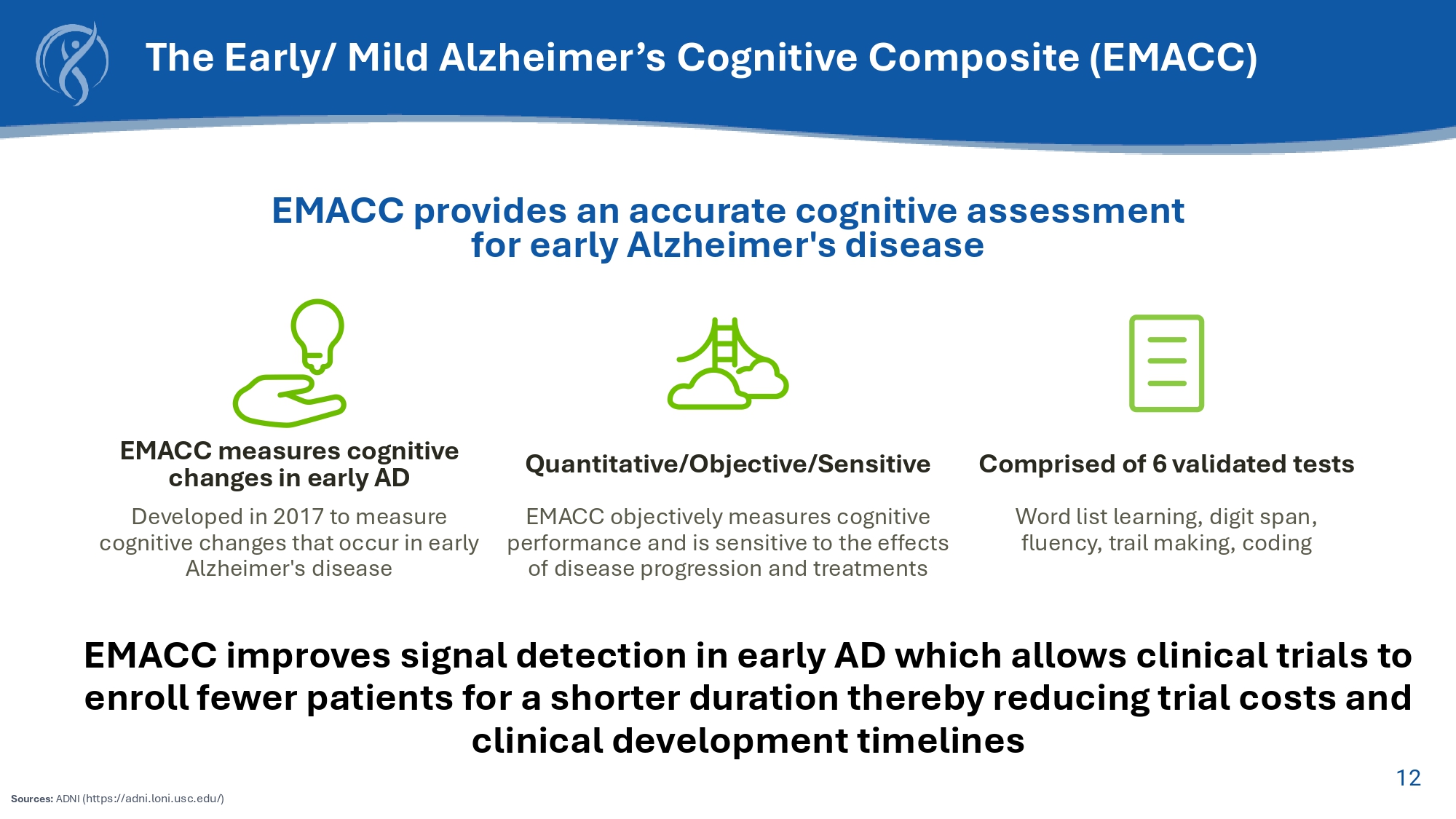
11 EMACC CDR Clinically derived to stage AD Empirically derived to measure cognitive change in Early AD Clinically validated measurements No floor or ceiling effects Lower variance and shorter retest intervals provides smoother measure of cognitive change Greater dynamic range allows measure of stable, worsening or improved cognition Allows for shorter and smaller clinical trials EMACC and CDR: Primary End - point for Early AD Clinical Trials 12 EMACC measures cognitive changes in early AD Developed in 2017 to measure cognitive changes that occur in early Alzheimer's disease Quantitative/Objective/Sensitive EMACC objectively measures cognitive performance and is sensitive to the effects of disease progression and treatments Comprised of 6 validated tests Word list learning, digit span, fluency, trail making, coding EMACC provides an accurate cognitive assessment for early Alzheimer's disease EMACC improves signal detection in early AD which allows clinical trials to enroll fewer patients for a shorter duration thereby reducing trial costs and clinical development timelines The Early/ Mild Alzheimer’s Cognitive Composite (EMACC) Sources: ADNI (https://adni.loni.usc.edu/)

13 Phase 2 Study Results 14 XPro TM for Early AD: Background Neuroinflammation in AD • Recognized contributor to disease progression in AD 1 • Associated with synaptic dysfunction/loss and cognitive impairment across the AD continuum 2 • Implicated in neurotoxic gliosis and tau - related neurodegeneration downstream of amyloid - beta (A β ) in AD 3 XPro TM Mechanism of Action • Selective, brain - penetrant neutralizer of the soluble and proinflammatory form of tumor necrosis factor (solTNF) • Safely and selectively inhibits inflammatory signaling • Does not cause immunosuppression • Does not interfere with immune homeostasis XPro TM in AD: Phase 1 • Demonstrated safety in Phase 1b study in AD (n=20) • Dose dependent reduction in inflammatory cytokines in cerebrospinal fluid (CSF) • Dose dependent modulation of synaptic proteins differentially affected in AD 5 • Signal for target engagement in gray matter cortices most frequently impacted by AD pathology 6 1 Jack CR Jr, et al. Alzheimers Dement. 2024. 2 Taddei RN, et al. JAMA Neurol. 2023. 3 Sánchez - Juan P, et al. Brain. 2024. 5 Pope P, et al. Alzheimer's & Dementia , 2024. 20(S8): p. e095343. 6 Pope, P., et al. Alzheimer's Dement ., 19: e083229.
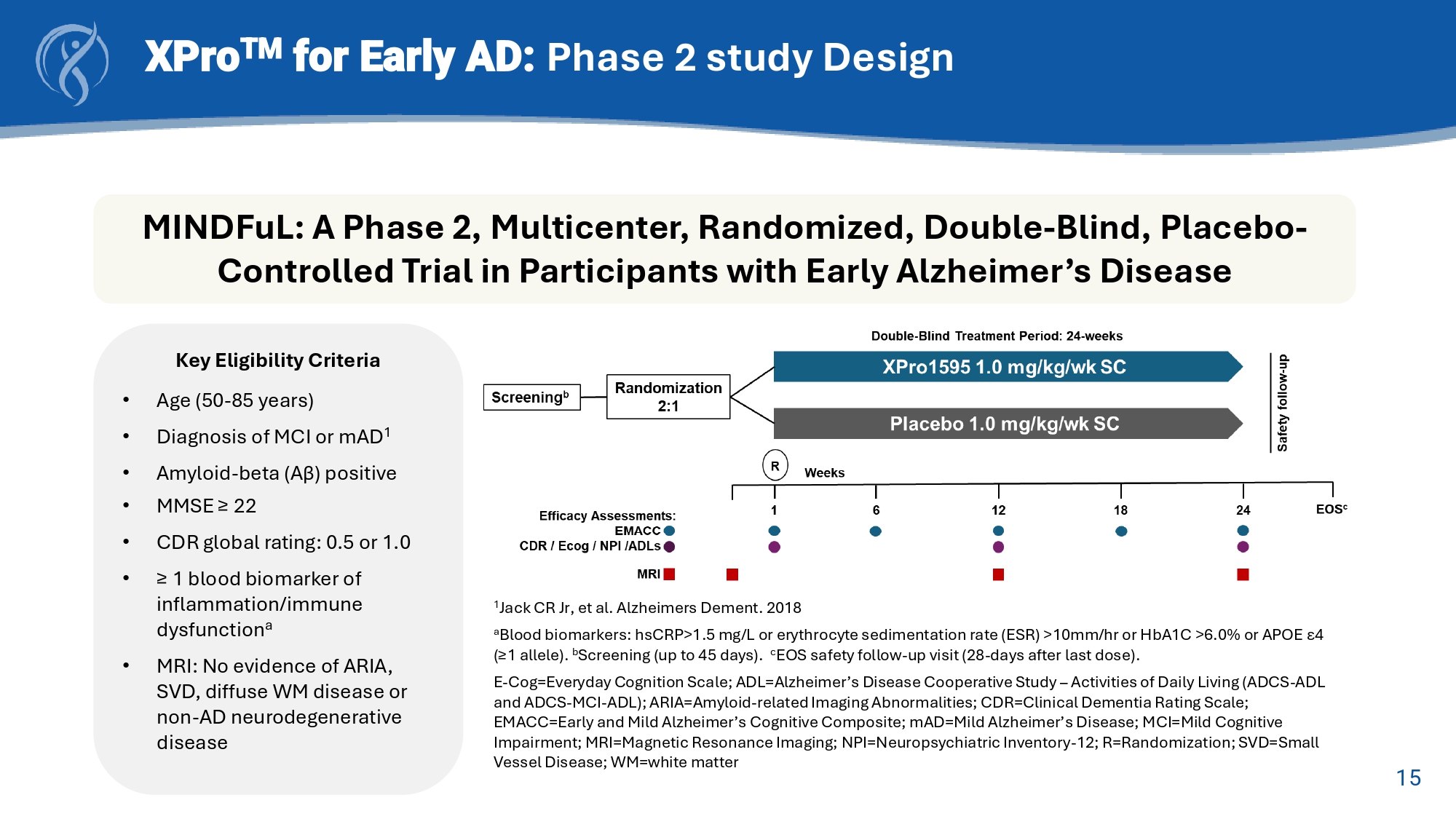
15 XPro TM for Early AD: Phase 2 study Design MINDFuL : A Phase 2, Multicenter, Randomized, Double - Blind, Placebo - Controlled Trial in Participants with Early Alzheimer’s Disease Key Eligibility Criteria • Age (50 - 85 years) • Diagnosis of MCI or mAD 1 • Amyloid - beta (Aβ) positive • MMSE ≥ 22 • CDR global rating: 0.5 or 1.0 • ≥ 1 blood biomarker of inflammation/immune dysfunction a • MRI: No evidence of ARIA, SVD, diffuse WM disease or non - AD neurodegenerative disease 1 Jack CR Jr, et al. Alzheimers Dement. 2018 a Blood biomarkers: hsCRP >1.5 mg/L or erythrocyte sedimentation rate (ESR) >10mm/ hr or HbA1C >6.0% or APOE ε 4 (≥1 allele). b Screening (up to 45 days). c EOS safety follow - up visit (28 - days after last dose). E - Cog=Everyday Cognition Scale; ADL=Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS - ADL and ADCS - MCI - ADL); ARIA=Amyloid - related Imaging Abnormalities; CDR=Clinical Dementia Rating Scale; EMACC=Early and Mild Alzheimer’s Cognitive Composite; mAD =Mild Alzheimer’s Disease; MCI=Mild Cognitive Impairment; MRI=Magnetic Resonance Imaging; NPI=Neuropsychiatric Inventory - 12; R=Randomization; SVD=Small Vessel Disease; WM=white matter 16 XPro TM for Early AD: Phase 2 Study Design Change from Baseline (CFB) to Week - 24 Trial Endpoints • Early and Mild Alzheimer’s Cognitive Composite (EMACC) Primary Endpoint • Clinical Dementia Rating Scale – Sum of Boxes (CDR - SB) Key Secondary Endpoint • Everyday Cognition Scale (E - Cog) Secondary Endpoints • Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS - ADL and ADCS - MCI - ADL) • Blood Biomarkers of AD pathology (pTau - 217) and neuroinflammation (GFAP) • MRI Brain volumetrics
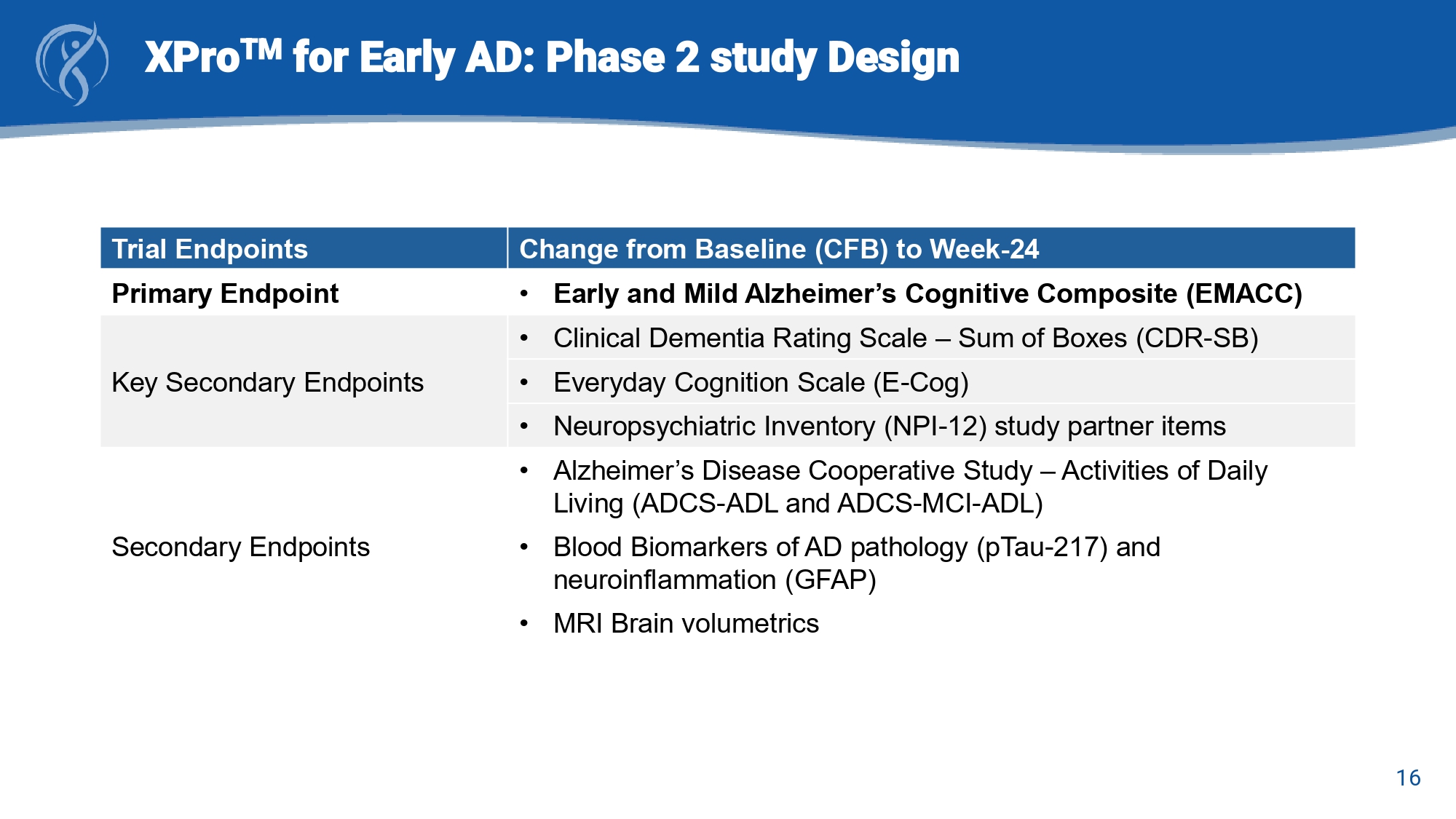
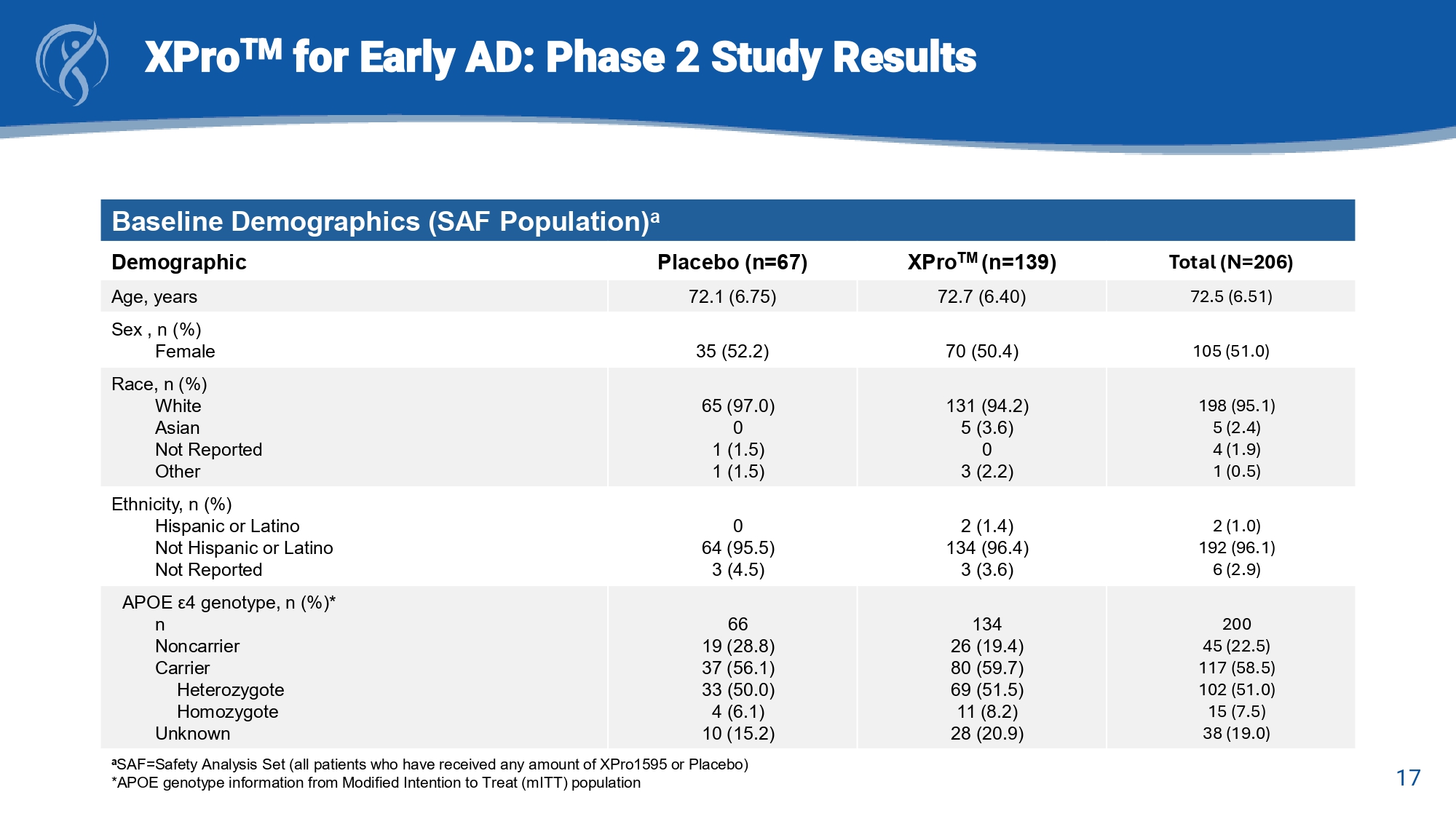
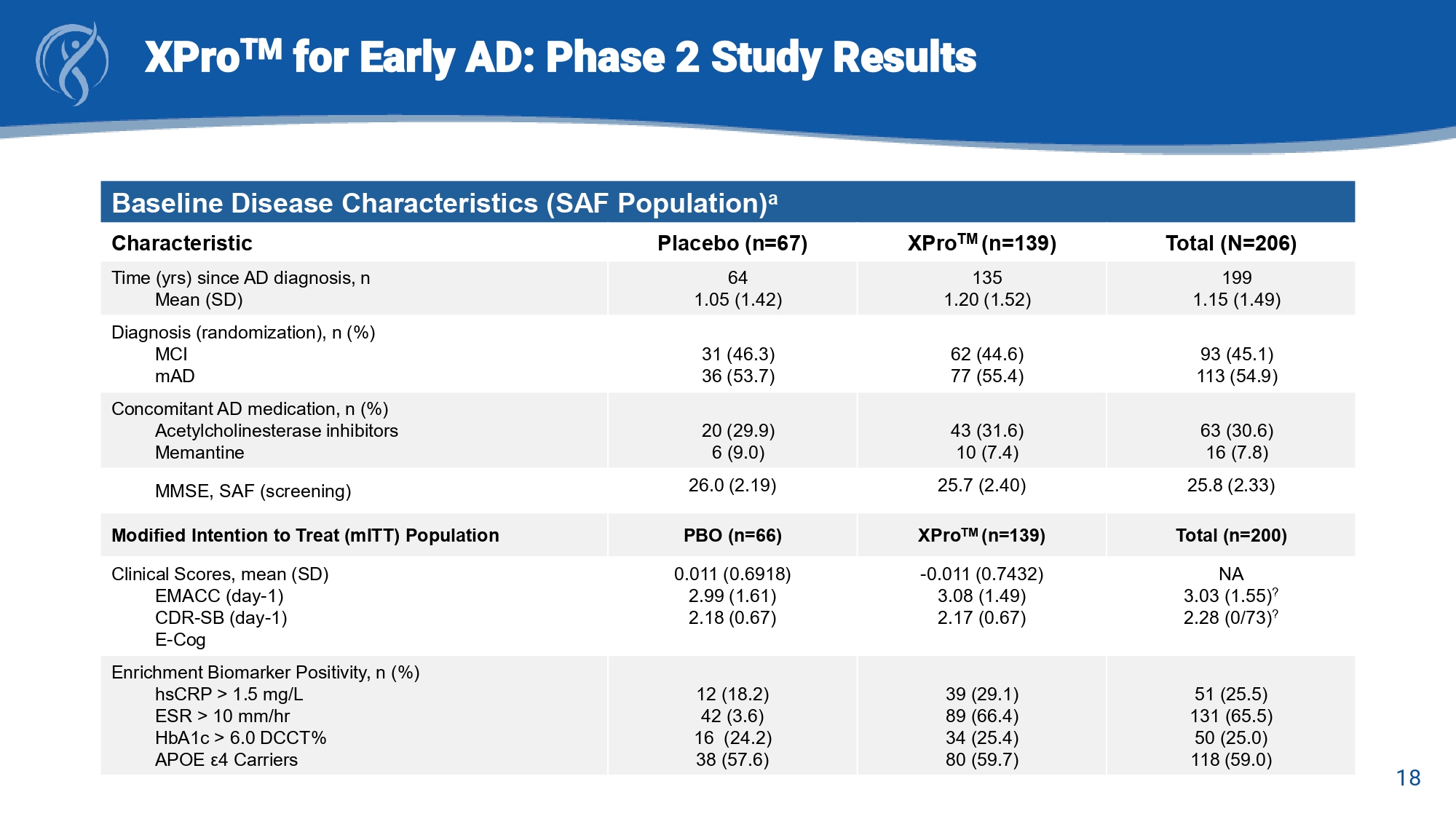
16 XPro TM for Early AD: Phase 2 study Design Change from Baseline (CFB) to Week - 24 Trial Endpoints • Early and Mild Alzheimer’s Cognitive Composite (EMACC) Primary Endpoint • Clinical Dementia Rating Scale – Sum of Boxes (CDR - SB) Key Secondary Endpoints • Everyday Cognition Scale (E - Cog) • Neuropsychiatric Inventory (NPI - 12) study partner items • Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS - ADL and ADCS - MCI - ADL) Secondary Endpoints • Blood Biomarkers of AD pathology (pTau - 217) and neuroinflammation (GFAP) • MRI Brain volumetrics 17 XPro TM for Early AD: Phase 2 Study Results Baseline Demographics (SAF Population) a Total (N=206) XPro TM (n=139) Placebo (n=67) Demographic 72.5 (6.51) 72.7 (6.40) 72.1 (6.75) Age, years 105 (51.0) 70 (50.4) 35 (52.2) Sex , n (%) Female 198 (95.1) 5 (2.4) 4 (1.9) 1 (0.5) 131 (94.2) 5 (3.6) 0 3 (2.2) 65 (97.0) 0 1 (1.5) 1 (1.5) Race, n (%) White Asian Not Reported Other 2 (1.0) 192 (96.1) 6 (2.9) 2 (1.4) 134 (96.4) 3 (3.6) 0 64 (95.5) 3 (4.5) Ethnicity, n (%) Hispanic or Latino Not Hispanic or Latino Not Reported 200 45 (22.5) 117 (58.5) 102 (51.0) 15 (7.5) 38 (19.0) 134 26 (19.4) 80 (59.7) 69 (51.5) 11 (8.2) 28 (20.9) 66 19 (28.8) 37 (56.1) 33 (50.0) 4 (6.1) 10 (15.2) APOE ε 4 genotype, n (%)* n Noncarrier Carrier Heterozygote Homozygote Unknown a SAF=Safety Analysis Set (all patients who have received any amount of XPro1595 or Placebo) *APOE genotype information from Modified Intention to Treat ( mITT ) population 18 XPro TM for Early AD: Phase 2 Study Results Baseline Disease Characteristics (SAF Population) a Total (N=206) XPro TM (n=139) Placebo (n=67) Characteristic 199 1.15 (1.49) 135 1.20 (1.52) 64 1.05 (1.42) Time (yrs) since AD diagnosis, n Mean (SD) 93 (45.1) 113 (54.9) 62 (44.6) 77 (55.4) 31 (46.3) 36 (53.7) Diagnosis (randomization), n (%) MCI mAD 63 (30.6) 16 (7.8) 43 (31.6) 10 (7.4) 20 (29.9) 6 (9.0) Concomitant AD medication, n (%) Acetylcholinesterase inhibitors Memantine 25.8 (2.33) 25.7 (2.40) 26.0 (2.19) MMSE, SAF (screening) Total (n=200) XPro TM (n=139) PBO (n=66) Modified Intention to Treat ( mITT ) Population NA 3.03 (1.55) ? 2.28 (0/73) ? - 0.011 (0.7432) 3.08 (1.49) 2.17 (0.67) 0.011 (0.6918) 2.99 (1.61) 2.18 (0.67) Clinical Scores, mean (SD) EMACC (day - 1) CDR - SB (day - 1) E - Cog 51 (25.5) 131 (65.5) 50 (25.0) 118 (59.0) 39 (29.1) 89 (66.4) 34 (25.4) 80 (59.7) 12 (18.2) 42 (3.6) 16 (24.2) 38 (57.6) Enrichment Biomarker Positivity, n (%) hsCRP > 1.5 mg/L ESR > 10 mm/ hr HbA1c > 6.0 DCCT% APOE ε4 Carriers
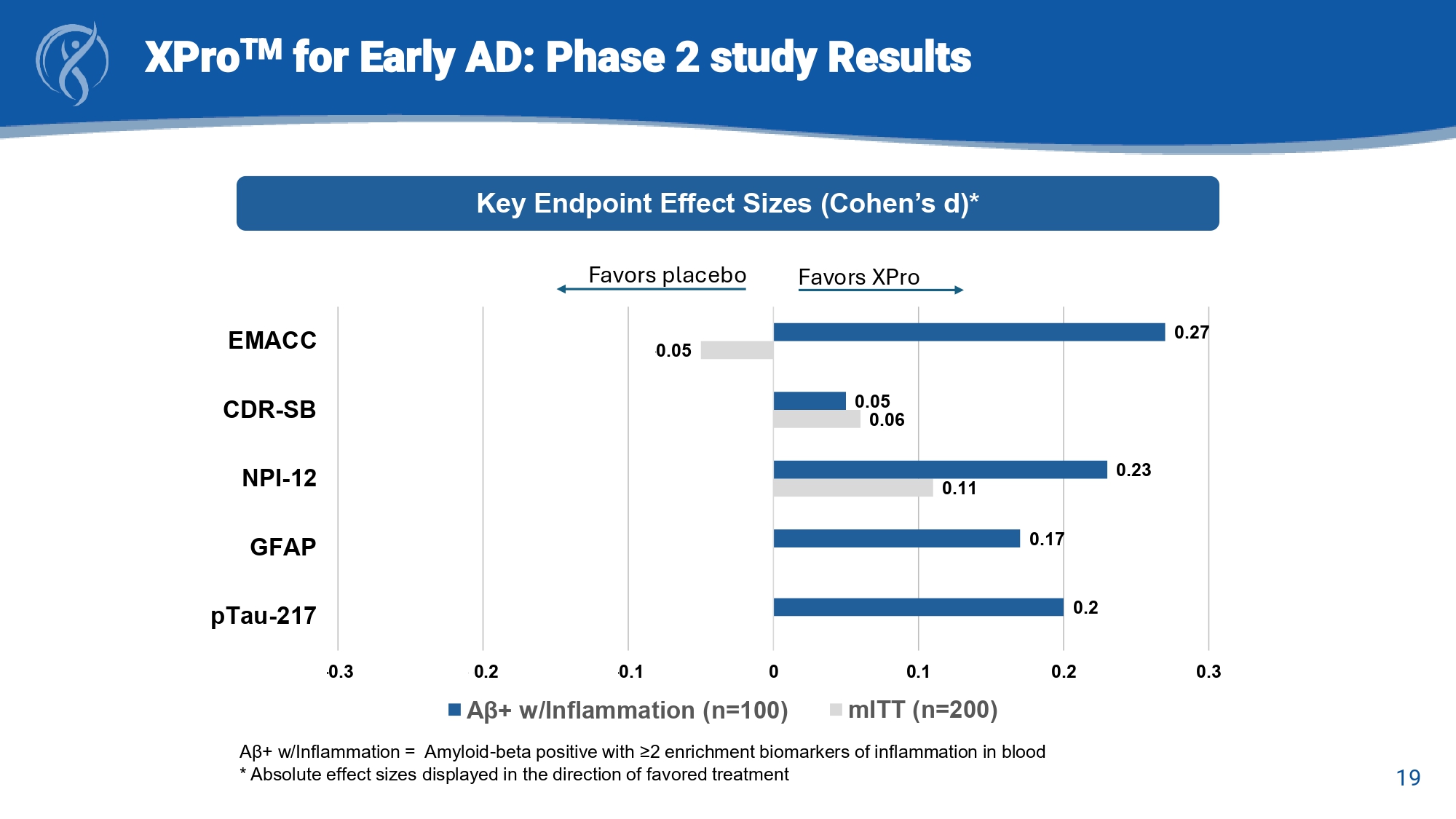
19 XPro TM for Early AD: Phase 2 Study Topline Results a A β + w/Inflammation = Amyloid - beta positive with ≥2 enrichment biomarkers of inflammation in blood Key Endpoint Effect Sizes (Cohen’s d) 0.11 - 0.06 - 0.05 0.2 0.17 0.23 - 0.05 0.27 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 pTau-217 GFAP NPI-12 CDR-SB EMACC A β+ w/Inflammation (n=100) mITT (n=200)
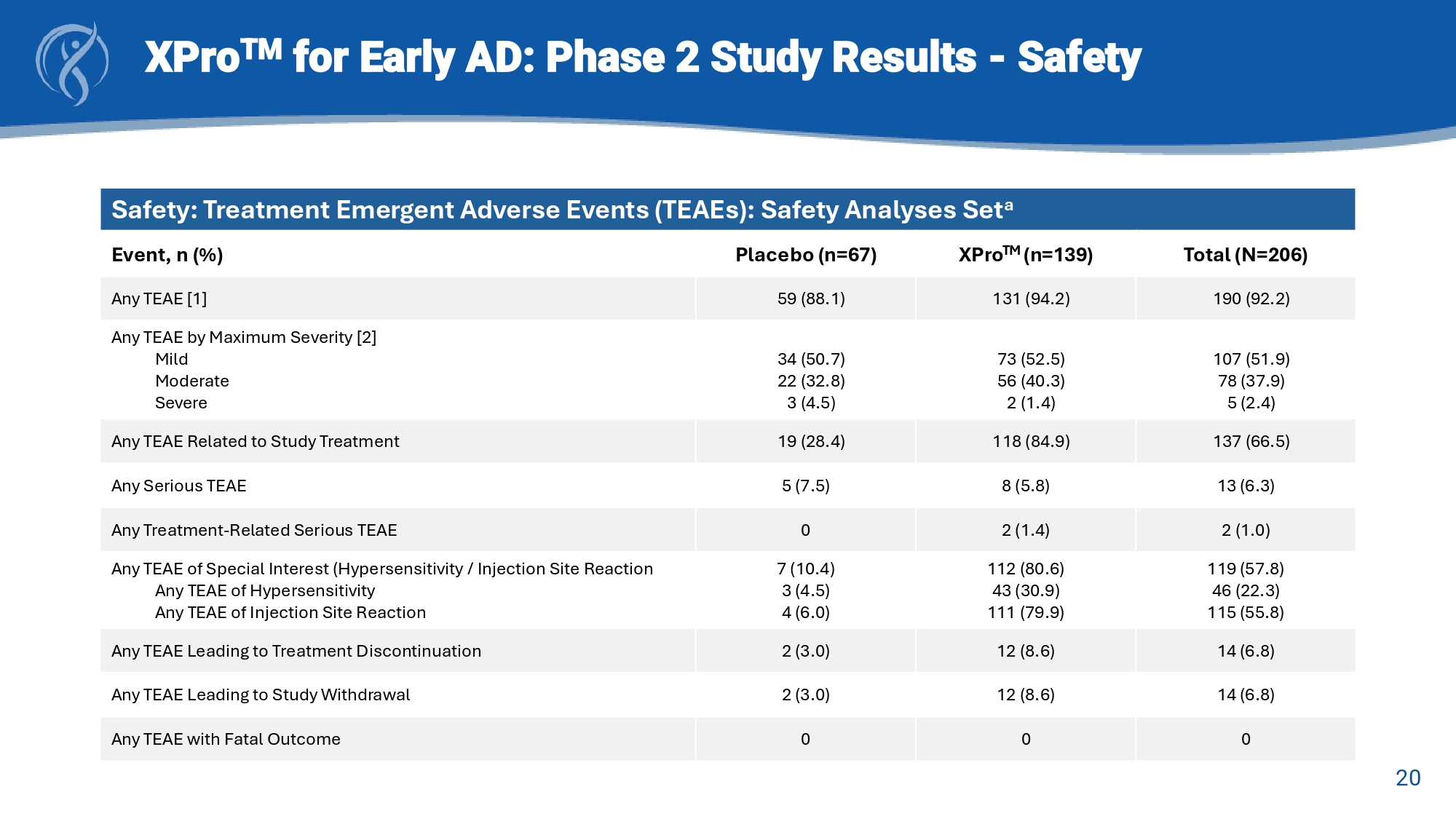
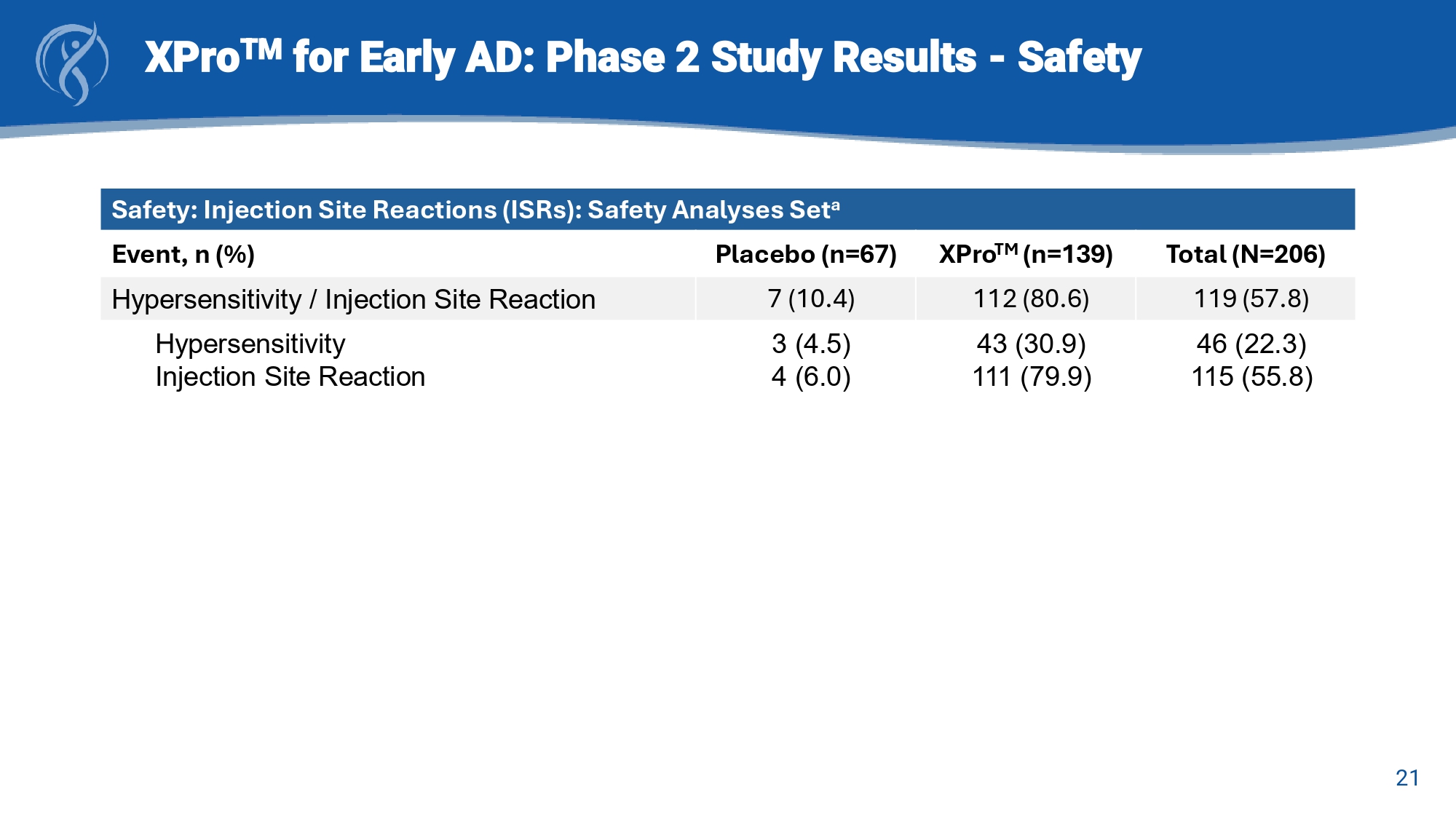
19 XPro TM for Early AD: Phase 2 study Results A β + w/Inflammation = Amyloid - beta positive with ≥2 enrichment biomarkers of inflammation in blood * Absolute effect sizes displayed in the direction of favored treatment Key Endpoint Effect Sizes (Cohen’s d)* 0.11 0.06 - 0.05 0.2 0.17 0.23 0.05 0.27 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 pTau-217 GFAP NPI-12 CDR-SB EMACC A β+ w/Inflammation (n=100) mITT (n=200) Favors XPro Favors placebo 20 XPro TM for Early AD: Phase 2 Study Results - Safety Safety: Treatment Emergent Adverse Events (TEAEs): Safety Analyses Set a Total (N=206) XPro TM (n=139) Placebo (n=67) Event, n (%) 190 (92.2) 131 (94.2) 59 (88.1) Any TEAE [1] 107 (51.9) 78 (37.9) 5 (2.4) 73 (52.5) 56 (40.3) 2 (1.4) 34 (50.7) 22 (32.8) 3 (4.5) Any TEAE by Maximum Severity [2] Mild Moderate Severe 137 (66.5) 118 (84.9) 19 (28.4) Any TEAE Related to Study Treatment 13 (6.3) 8 (5.8) 5 (7.5) Any Serious TEAE 2 (1.0) 2 (1.4) 0 Any Treatment - Related Serious TEAE 119 (57.8) 46 (22.3) 115 (55.8) 112 (80.6) 43 (30.9) 111 (79.9) 7 (10.4) 3 (4.5) 4 (6.0) Any TEAE of Special Interest (Hypersensitivity / Injection Site Reaction Any TEAE of Hypersensitivity Any TEAE of Injection Site Reaction 14 (6.8) 12 (8.6) 2 (3.0) Any TEAE Leading to Treatment Discontinuation 14 (6.8) 12 (8.6) 2 (3.0) Any TEAE Leading to Study Withdrawal 0 0 0 Any TEAE with Fatal Outcome 21 XPro TM for Early AD: Phase 2 Study Results - Safety Safety: Injection Site Reactions (ISRs): Safety Analyses Set a Total (N=206) XPro TM (n=139) Placebo (n=67) Event, n (%) 119 (57.8) 112 (80.6) 7 (10.4) Hypersensitivity / Injection Site Reaction 46 (22.3) 115 (55.8) 43 (30.9) 111 (79.9) 3 (4.5) 4 (6.0) Hypersensitivity Injection Site Reaction
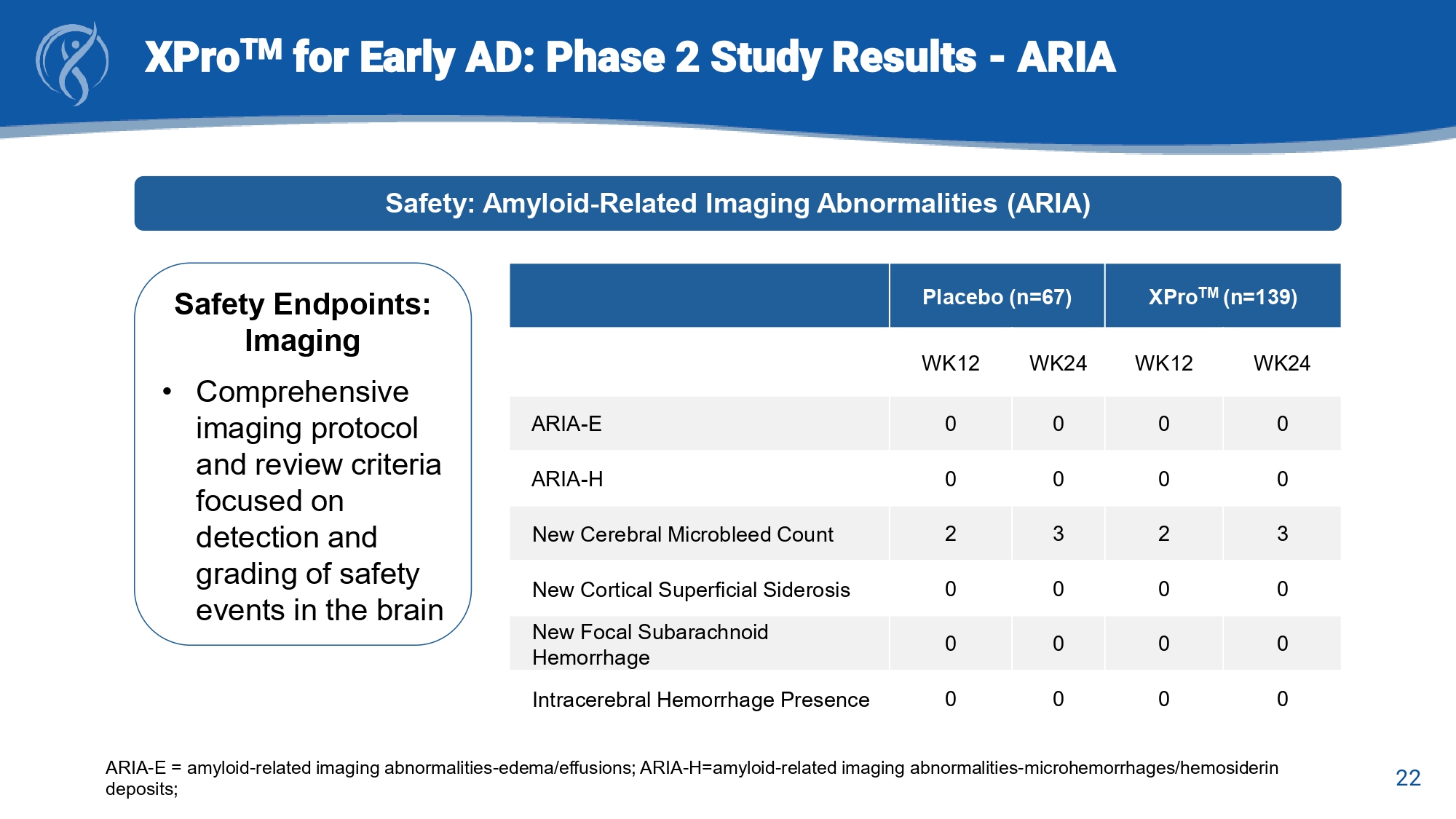
22 XPro TM for Early AD: Phase 2 Study Results - ARIA Safety: Amyloid - Related Imaging Abnormalities (ARIA) Safety Endpoints: Imaging • Comprehensive imaging protocol and review criteria focused on detection and grading of safety events in the brain XPro TM (n=139) Placebo (n=67) WK24 WK12 WK24 WK12 0 0 0 0 ARIA - E 0 0 0 0 ARIA - H 3 2 3 2 New Cerebral Microbleed Count 0 0 0 0 New Cortical Superficial Siderosis 0 0 0 0 New Focal Subarachnoid Hemorrhage 0 0 0 0 Intracerebral Hemorrhage Presence ARIA - E = amyloid - related imaging abnormalities - edema/effusions; ARIA - H=amyloid - related imaging abnormalities - microhemorrhages/he mosiderin deposits; 23 XPro TM for Early AD: Summary and Conclusions • MINDFuL was a well designed and well executed clinical trial in Early AD x Successful enrollment of a well - balanced cohort of participants with MCI or mAD x Objective measure of cognitive performance with improved sensitivity for detection of discrete changes as the primary outcome • The primary endpoint was not met • Key findings: x Consistent effect sizes of >.20 across multiple measures (EMACC, NPI, pTau217) indicative of positive effects attributable to treatment with XPro TM x Identified a population of AD patients most likely to respond to treatment with XPro TM ( i.e., A β + patients with biomarker evidence of elevated inflammation) • Safety outcomes indicated good safety and tolerability profile in participants with Early AD x Predominantly mild to moderate adverse events (AEs) across both study groups x Most common AE is Injection Site Reactions (ISR) x No ARIA or neuroimaging AEs x No deaths
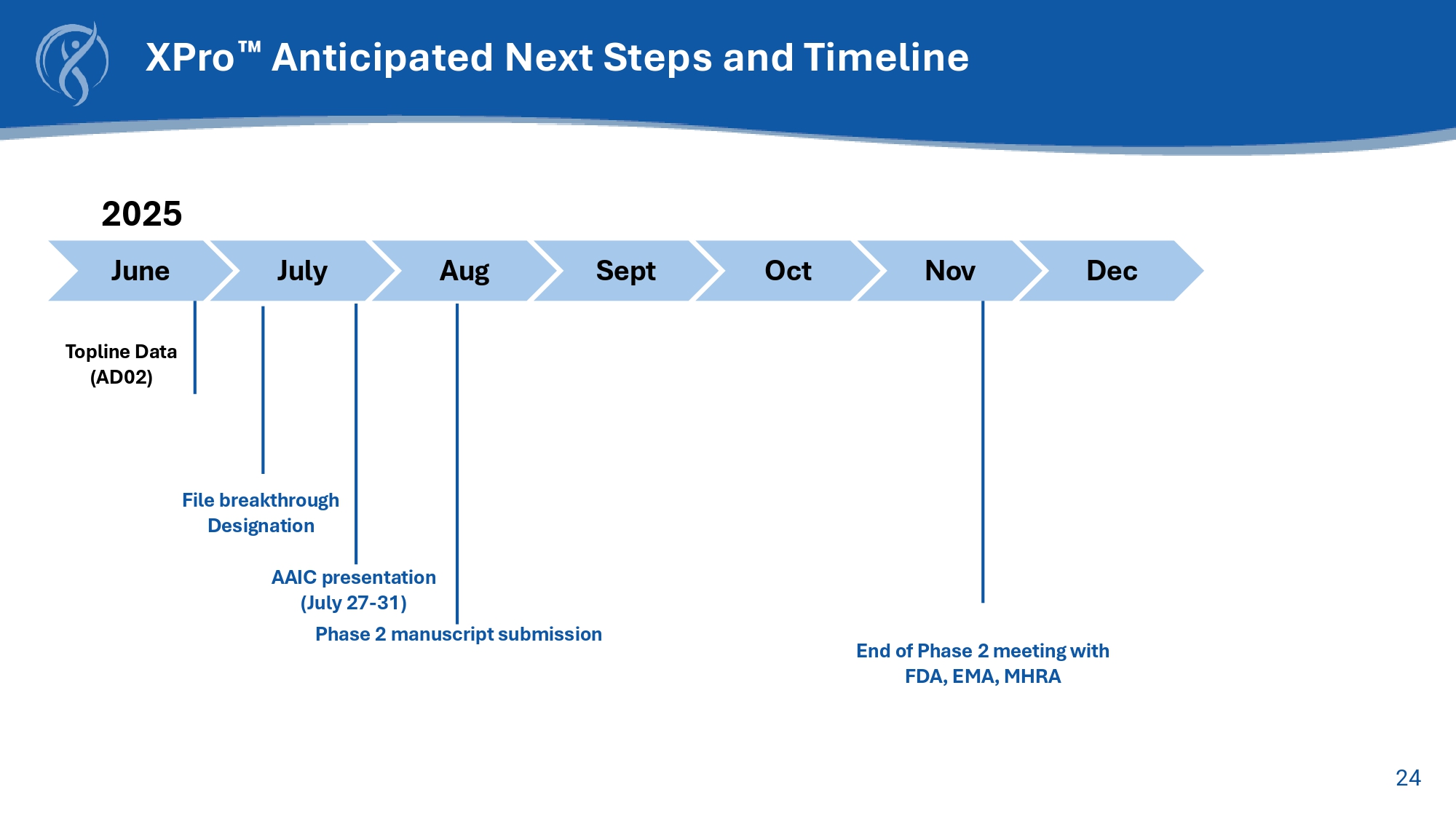
23 XPro TM for Early AD: Summary and Conclusions • MINDFuL was a well designed and well executed clinical trial in Early AD x Successful enrollment of a well - balanced cohort of participants with MCI or mAD x Objective measure of cognitive performance with improved sensitivity for detection of discrete changes as the primary outcome • The primary endpoint was not met • Study results: x Consistent absolute effect sizes indicative of positive effects attributable to treatment with XPro TM x Identified the population of AD patients most likely to respond to treatment with XPro TM ( i.e., A β + patients with biomarker evidence of elevated inflammation) • Safety outcomes indicated good safety and tolerability profile in participants with Early AD x Predominantly mild to moderate adverse events (AEs) across both study groups x No ARIA or neuroimaging AEs x No deaths 24 XPro Anticipated Next Steps and Timeline July Sept Oct Nov Dec 2025 Aug Topline Data (AD02 ) June AAIC presentation (July 27 - 31) Phase 2 manuscript submission End of Phase 2 meeting with FDA, EMA, MHRA File breakthrough Designation

25 for RDEB CORDStrom
26 RDEB – An Ultra - Rare Genetic Disease with Significant Unmet Need *Recessive Dystrophic Epidermolysis Bullosa (RDEB) • RDEB is a severe form of epidermolysis bullosa (EB), a rare disease that causes severe skin fragility, itch and chronic pain • RDEB is caused by mutations in the COL7A1 gene that makes type VII collagen, a protein that holds the layers of skin together • Children with RDEB have skin that is damaged by even the smallest amount of friction which causes severe blistering, deep wounds, and scars • There are limited options available for treatment, none that adequately meet the needs of patients, and the condition gets worse over time, with most children reliant on a wheelchair as they move into their teenage years • Many of those with RDEB will also go on to develop aggressive life - threatening skin cancer in adulthood caused by the accumulated damage to their skin • Krystal Biotech’s VYJUVEK launch in DEB is off to an impressive start (~$84M net revenue in Q3 ’24); CORDStrom is potentially the first systemic therapy, with itch benefit as a key differentiating factor, potential for use as an adjunctive therapy • It is estimated that more than 4000 people suffer from RDEB in the US, UK and EU, representing a > $1B peak sales opportunity 27 CORDStrom Platform Overview Investigational disease - modifying treatment for recessive dystrophic epidermolysis bullosa (RDEB) CORDStrom Overview • CORDStrom is an innovative cellular medicine, comprising allogeneic, pooled human umbilical cord - derived mesenchymal stromal cells ( hucMSCs ) formulated for injection or infusion.
• CORDStrom addresses challenges related to the source, identity, heterogeneity, and manufacturing costs of MSCs, positioning them as a viable drug platform. • The FDA has awarded CORDStrom both the Rare Pediatric Disease and Orphan Drug Designation, qualifying it for a Priority Review Voucher post - FDA approval. FDA = Food and Drug Administration; GOSH = Great Ormond Street Hospital; NIHR = National Institute for Health and Care Resear ch; ODD = Orphan Drug Designation; PRV = Priority Review Voucher; RPDD = Rare Pediatric Disease Designation Mission EB Phase 2 Trial • Phase 2 Trial completed by investigators at Great Ormond Street Hospital for Children & Birmingham Children’s Hospital in the UK and primarily funded by grant from NIHR (National Institute of Health and Care Research). • Double - blind, randomized, placebo - controlled, cross - over Phase 2 trial to evaluate the safety and efficacy of CORDStrom in 30 pediatric patients in the UK with intermediate and severe RDEB .
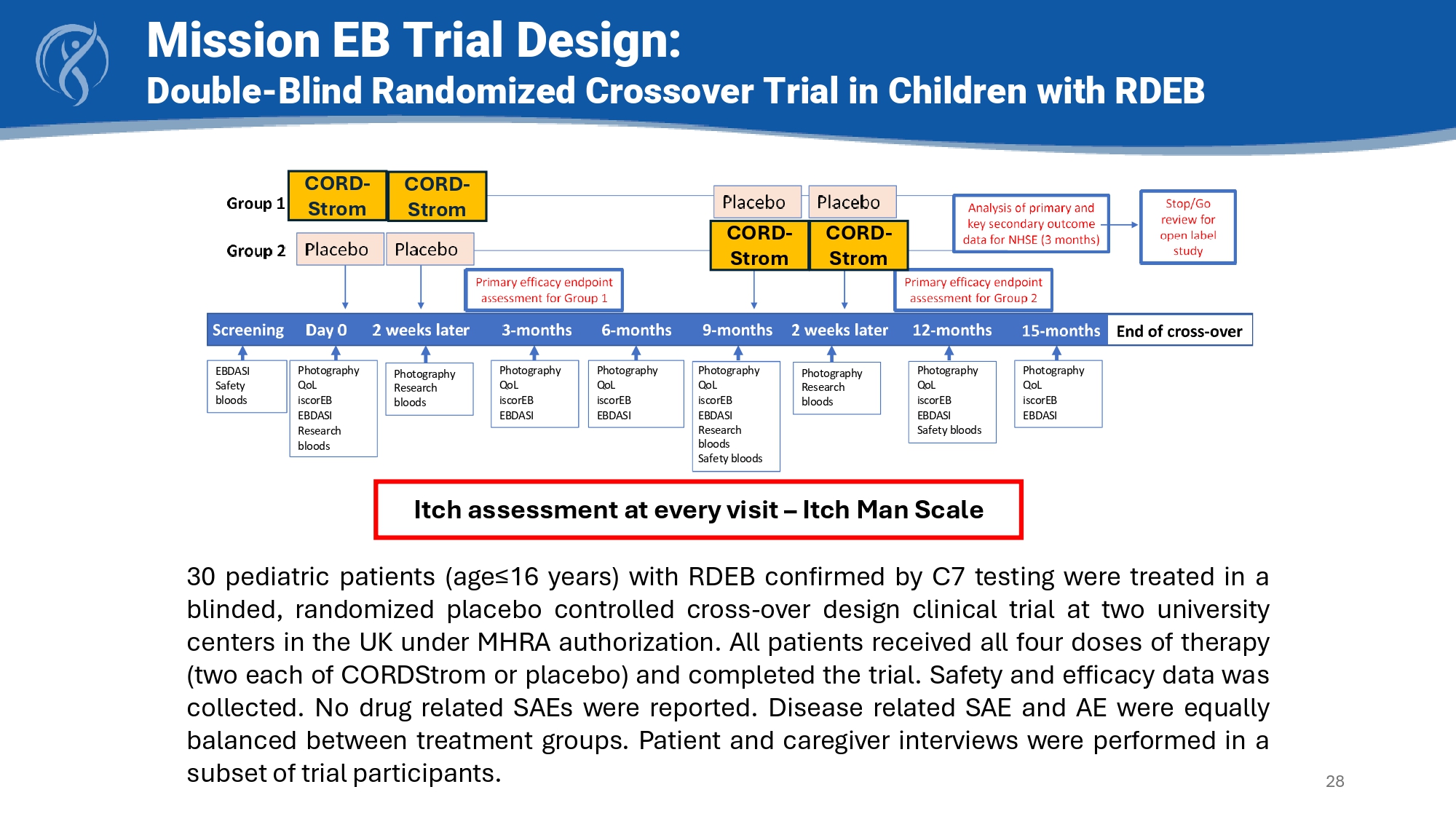
• Patients received two intravenous infusions of placebo or CORDStrom two weeks apart and then followed for nine months; each child then crossed over to the other arm and received two doses of the alternate arm two weeks apart with a further nine - month follow - up • Topline results showed CORDStrom was easily administered, well tolerated and there were beneficial effects with respect to Itch Man Scale, iscorEB clinician score and skin score and QOL • Safety profile - no CORDStrom - related serious adverse events were reported Photography QoL iscorEB EBDASI Research bloods Photography QoL iscorEB EBDASI Photography Research bloods Photography QoL iscorEB EBDASI Safety bloods Photography Research bloods Photography QoL iscorEB EBDASI Photography QoL iscorEB EBDASI EBDASI Safety bloods Photography QoL iscorEB EBDASI Research bloods Safety bloods 30 pediatric patients (age≤ 16 years) with RDEB confirmed by C 7 testing were treated in a blinded, randomized placebo controlled cross - over design clinical trial at two university centers in the UK under MHRA authorization . All patients received all four doses of therapy (two each of CORDStrom or placebo) and completed the trial . Safety and efficacy data was collected . No drug related SAEs were reported . Disease related SAE and AE were equally balanced between treatment groups . Patient and caregiver interviews were performed in a subset of trial participants .
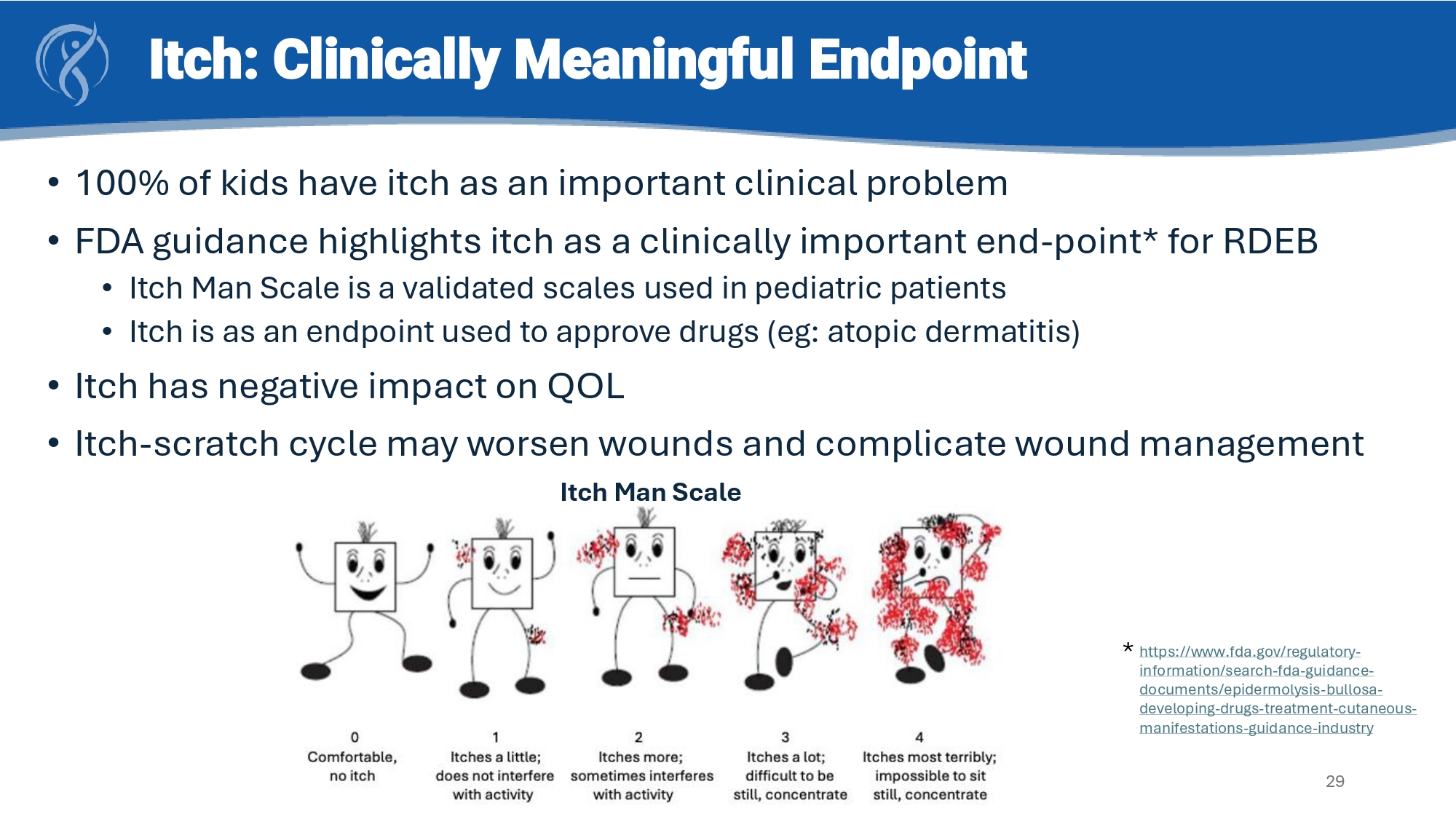
Mission EB Trial Design: Double - Blind Randomized Crossover Trial in Children with RDEB 28 CORD - Strom CORD - Strom CORD - Strom CORD - Strom Itch assessment at every visit – Itch Man Scale Itch: Clinically Meaningful Endpoint • 100% of kids have itch as an important clinical problem • FDA guidance highlights itch as a clinically important end - point* for RDEB • Itch Man Scale is a validated scales used in pediatric patients • Itch is as an endpoint used to approve drugs ( eg : atopic dermatitis) • Itch has negative impact on QOL • Itch - scratch cycle may worsen wounds and complicate wound management 29 https://www.fda.gov/regulatory - information/search - fda - guidance - documents/epidermolysis - bullosa - developing - drugs - treatment - cutaneous - manifestations - guidance - industry * Itch Man Scale Itch improved at 3 months and remained stable at 6 months.
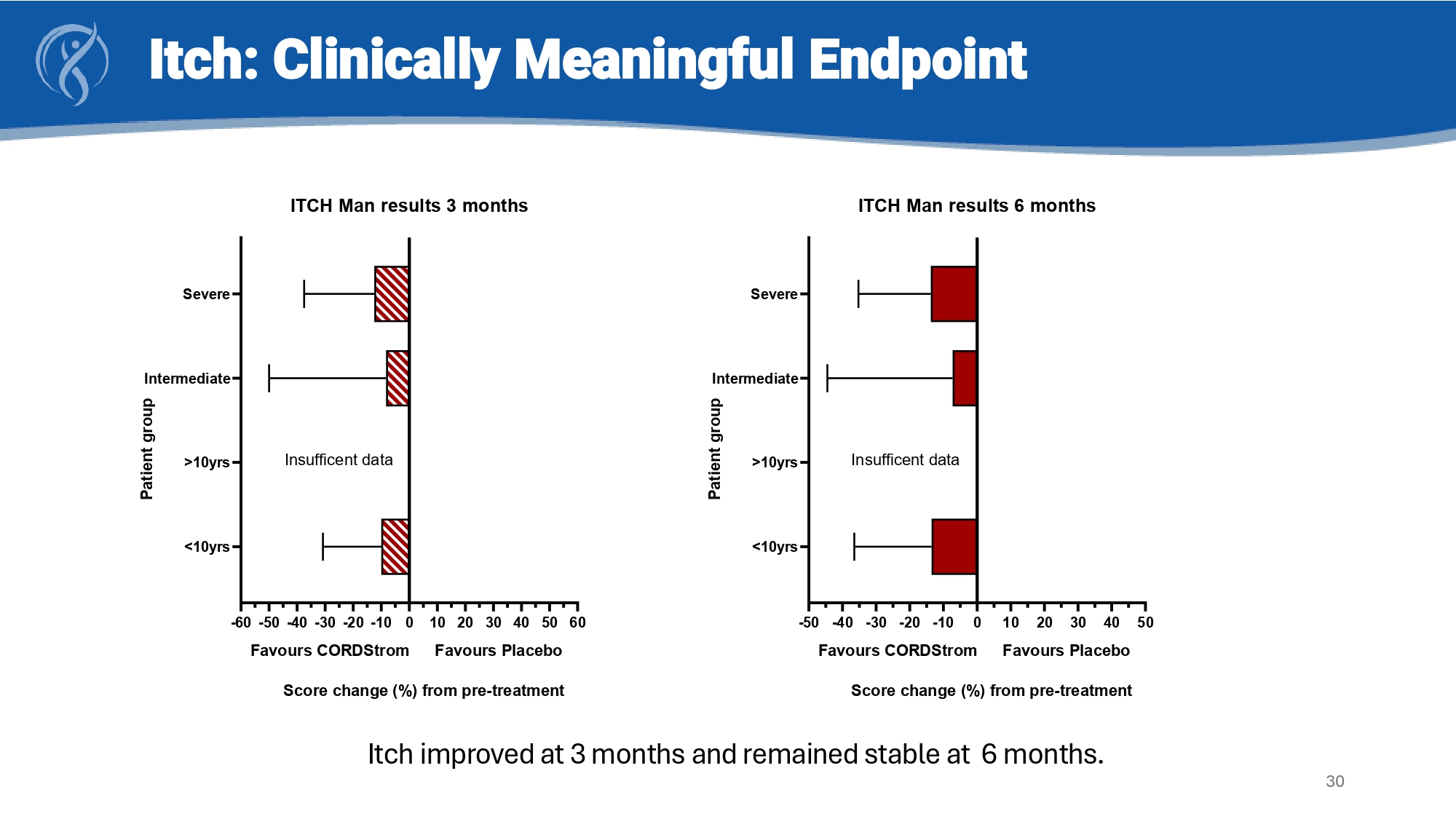
30 Itch: Clinically Meaningful Endpoint -50 -40 -30 -20 -10 0 10 20 30 40 50 <10yrs >10yrs Intermediate Severe ITCH Man results 6 months P a t i e n t g r o u p Favours CORDStrom Favours Placebo Insufficent data Score change (%) from pre-treatment -60-50-40-30 -20-10 0 10 20 30 40 50 60 <10yrs >10yrs Intermediate Severe ITCH Man results 3 months P a t i e n t g r o u p Favours CORDStrom Favours Placebo Insufficent data Score change (%) from pre-treatment CORDStrom for RDEB: Clinical and Qualitative Summary Clinical Benefits • Improvement in itch in all patient groups – the most common and complained of symptom in RDEB • In some patient groups • Less pain • Better iSCOREB wound score • Durable benefit of CORDStrom therapy for 6 months Qualitative Benefits • 10 of 13 respondents confirm benefit of therapy on clinical problems of itch, wound care and quality of life • All patient/caregivers want to remain on therapy • Favoravble safety profile and a formulation which fits conventional drug delivery makes treatment easy 31 NEXT STEPS: Compile and file BLA in US & MAA in UK/EU in 1H 2026 Goals of future open label trial post BLA: i ) correlate decrease in itch with improved wound healing; ii) demonstrate systemic benefits on extra - cutaneous manifestations of disease (e.g.: dysphagia, corneal blisters and scaring)

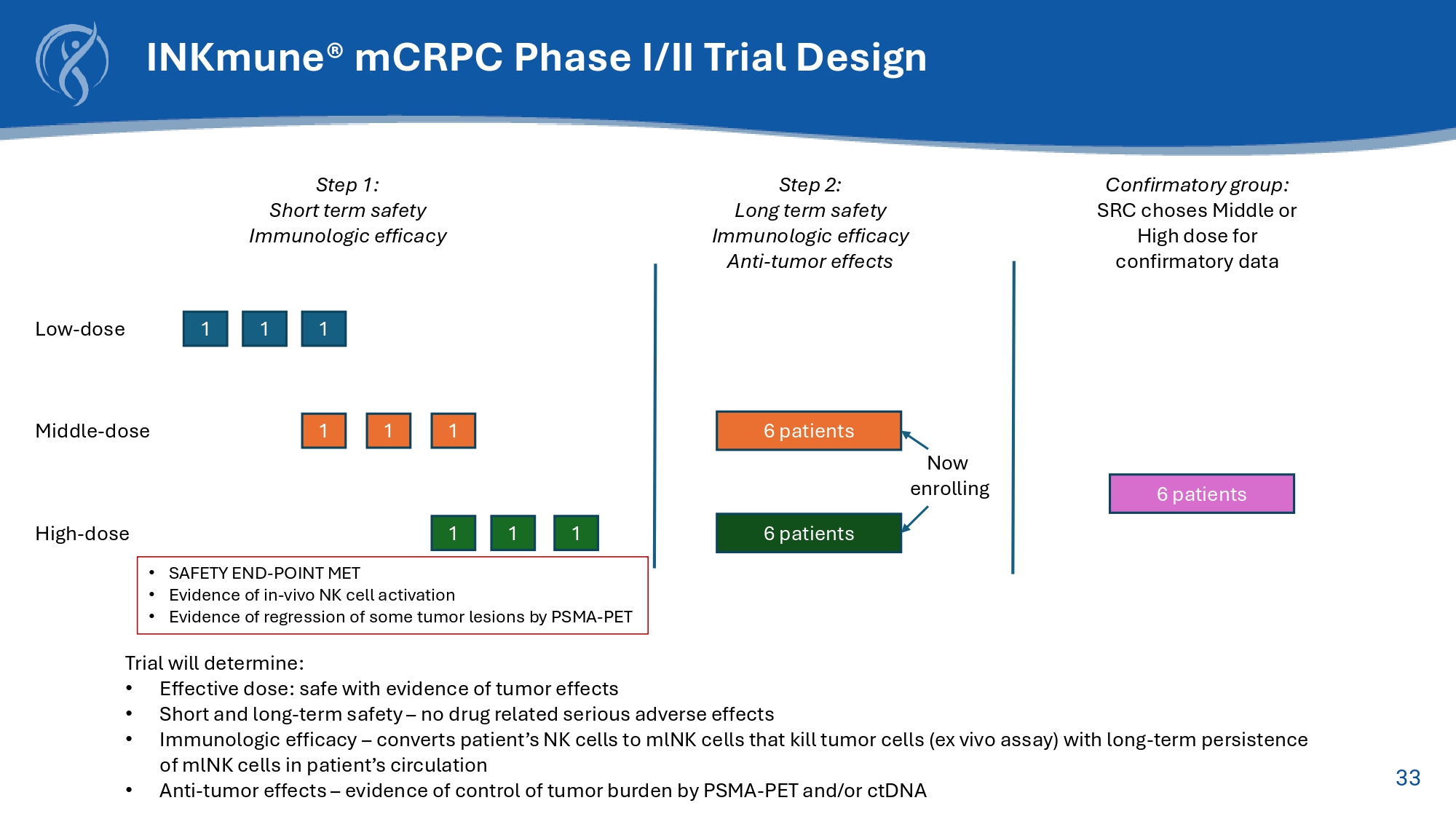
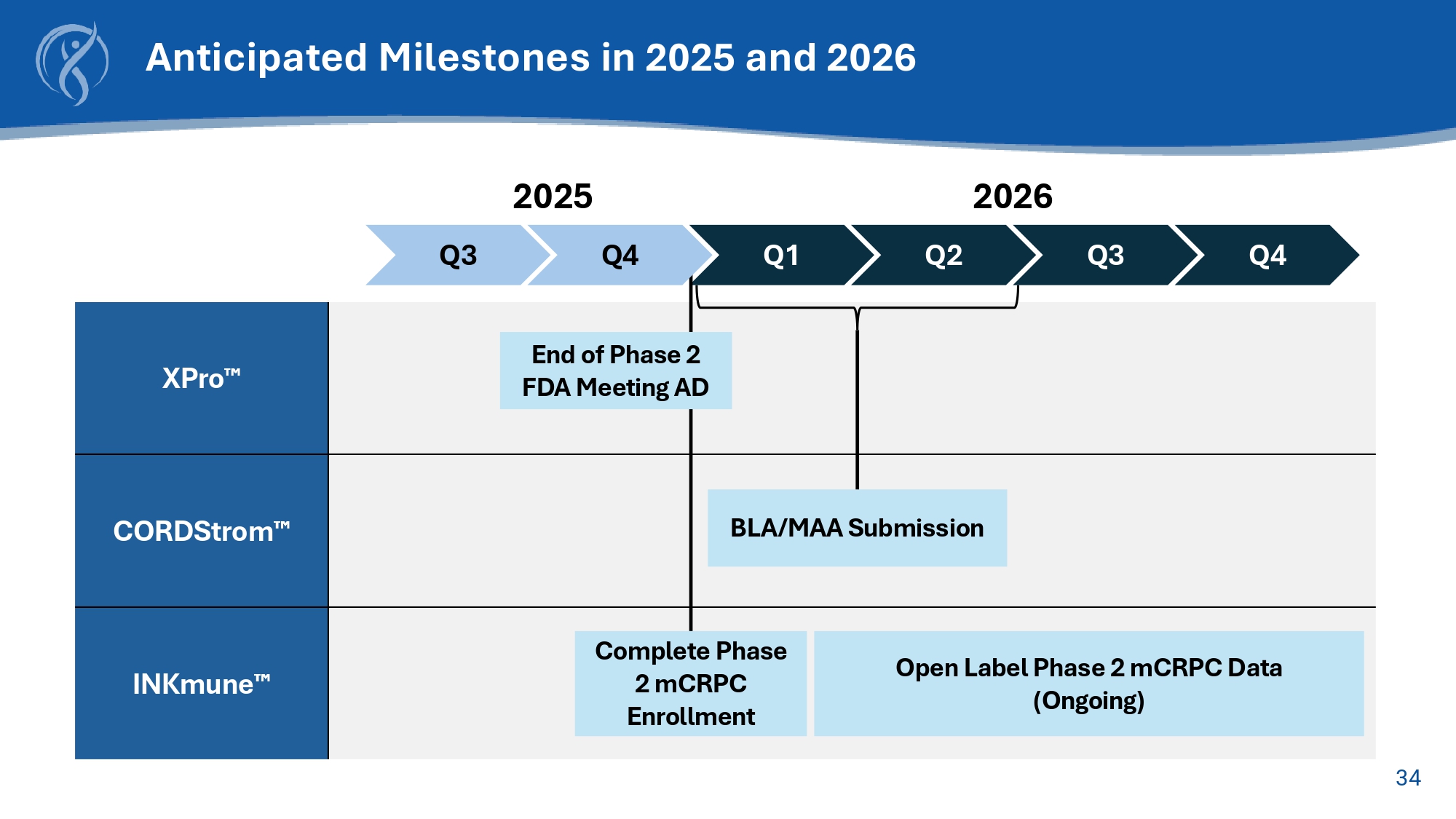
32 Off - the - Shelf NK Therapic Candidate Converts Patient’s Resting NK cells into Cancer Killing Memory like NK cells 33 Step 1: Short term safety Immunologic efficacy Middle - dose 1 1 1 6 patients High - dose 1 1 1 6 patients Step 2: Long term safety Immunologic efficacy Anti - tumor effects 6 patients Confirmatory group: SRC choses Middle or High dose for confirmatory data Low - dose 1 1 1 Trial will determine: • Effective dose: safe with evidence of tumor effects • Short and long - term safety – no drug related serious adverse effects • Immunologic efficacy – converts patient’s NK cells to mlNK cells that kill tumor cells (ex vivo assay) with long - term persistence of mlNK cells in patient’s circulation • Anti - tumor effects – evidence of control of tumor burden by PSMA - PET and/or ctDNA Now enrolling INKmune ® mCRPC Phase I/II Trial Design • SAFETY END - POINT MET • Evidence of in - vivo NK cell activation • Evidence of regression of some tumor lesions by PSMA - PET 34 XPro CORDStrom INKmune Anticipated Milestones in 2025 and 2026 Q3 Q1 Q2 Q3 Q4 2025 2026 BLA/MAA Submission Open Label Phase 2 mCRPC Data (Ongoing) Q4 End of Phase 2 FDA Meeting AD Complete Phase 2 mCRPC Enrollment

35 Contact Us: INmune Bio Inc. 225 NE Mizner Blvd.Suite 640 Boca Raton, FL 33432 (561) 710 - 0512 info@inmunebio.com INMB (Nasdaq)