UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): June 24, 2025
NEKTAR THERAPEUTICS
(Exact Name of Registrant as Specified in Charter)
| Delaware | 0-24006 | 94-3134940 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
455 Mission Bay Boulevard South
San Francisco, California 94158
(Address of Principal Executive Offices and Zip Code)
Registrant’s telephone number, including area code: (415) 482-5300
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value | NKTR | Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD.
On June 24, 2025, Nektar Therapeutics (the “Company”) issued a press release reporting topline results from its Phase 2b REZOLVE-AD (atopic dermatitis) clinical trial. A copy of the press release is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K.
On June 24, 2025, the Company also updated its corporate presentation, which includes, among other updates, additional information regarding its Phase 2b REZOLVE-AD (atopic dermatitis) clinical trial. A copy of the corporate presentation is furnished herewith as Exhibit 99.2 to this Current Report on Form 8-K. The updated corporate presentation is also available on the investor relations section of the Company’s website at https://ir.nektar.com/. Information contained on the Company’s website is not incorporated by reference into this Current Report on Form 8-K, and you should not consider any information on, or that can be accessed from, the Company’s website as part of this Current Report on Form 8-K.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.1 and 99.2 attached hereto, is being furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and shall not be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibits 99.1 and 99.2.
Item 8.01 Other Events.
Phase 2b RESOLVE-AD Topline Results
On June 24, 2025, the Company announced topline results from its Phase 2b REZOLVE-AD (atopic dermatitis) clinical trial.
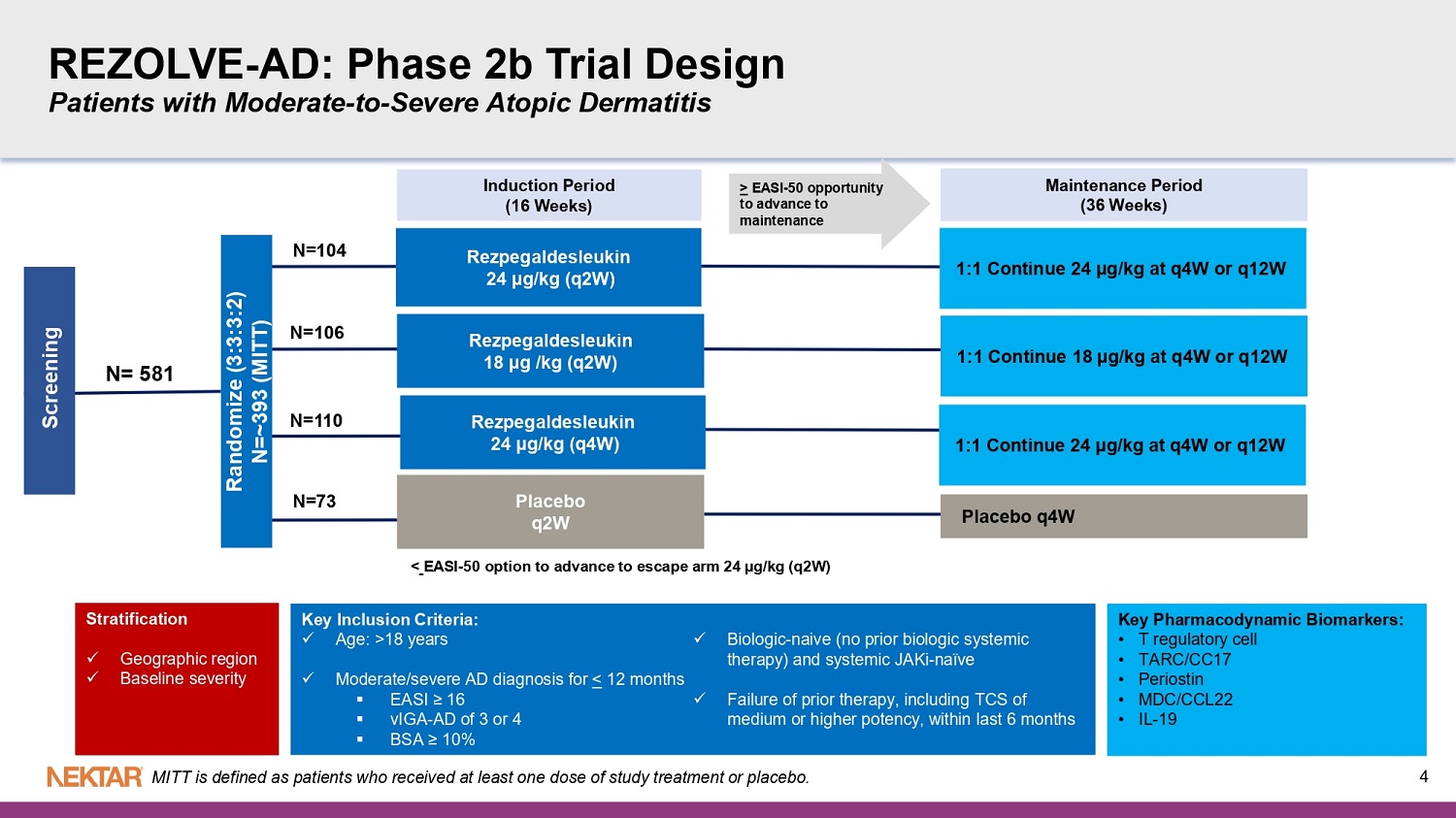
The global Phase 2b study is being conducted in 393 patients with moderate-to-severe atopic dermatitis. Patients were randomized (3:3:3:2) to receive subcutaneous treatment with three doses of rezpegaldesleukin: a high dose of 24 µg/kg every two weeks (q2w), a middle dose of 18 µg/kg every two weeks (q2w), and a low dose of 24 µg/kg every four weeks (q4w), or placebo q2w. The primary endpoint and secondary endpoints were assessed at week 16. Following a 16-week induction period, rezpegaldesleukin-treated patients who achieved Eczema Area and Severity Score (EASI) percent score reductions of >50 were re-randomized (1:1) to continue at the same dose level on a q4w or q12w regimen through week 52 in a blinded maintenance period. Placebo patients with EASI percent score reductions of >50 percent continue to receive placebo q4w.
The trial met its primary endpoint of the mean improvement in EASI from baseline at week 16 for all three dose arms of rezpegaldesleukin versus placebo (p<0.001).
All three dose arms also achieved statistical significance at week 16 for the key secondary endpoints of EASI-75 (percent of patients who achieve ≥75% reduction in EASI from baseline), EASI-50 (percent of patients who achieve ≥50% reduction in EASI from baseline) and BSA (mean percent improvement in Body Surface Area score from baseline).
The q2w arms of rezpegaldesleukin (high and middle doses) achieved statistical significance at week 16 for the key secondary endpoints of vIGA-AD 0/1 (percent of patients achieving a score of 0 or 1 on the validated Investigator’s Global Assessment for Atopic Dermatitis with ≥ 2-point reduction from baseline) and Itch NRS (percent of patients with baseline ≥ 4 who experienced a ≥ 4-point reduction in the Itch Numerical Rating Score from baseline).
In addition, at week 16, the high dose of 24 µg/kg q2w achieved statistical significance on EASI-90 (percent of patients who achieve ≥ 90% reduction in EASI from baseline).
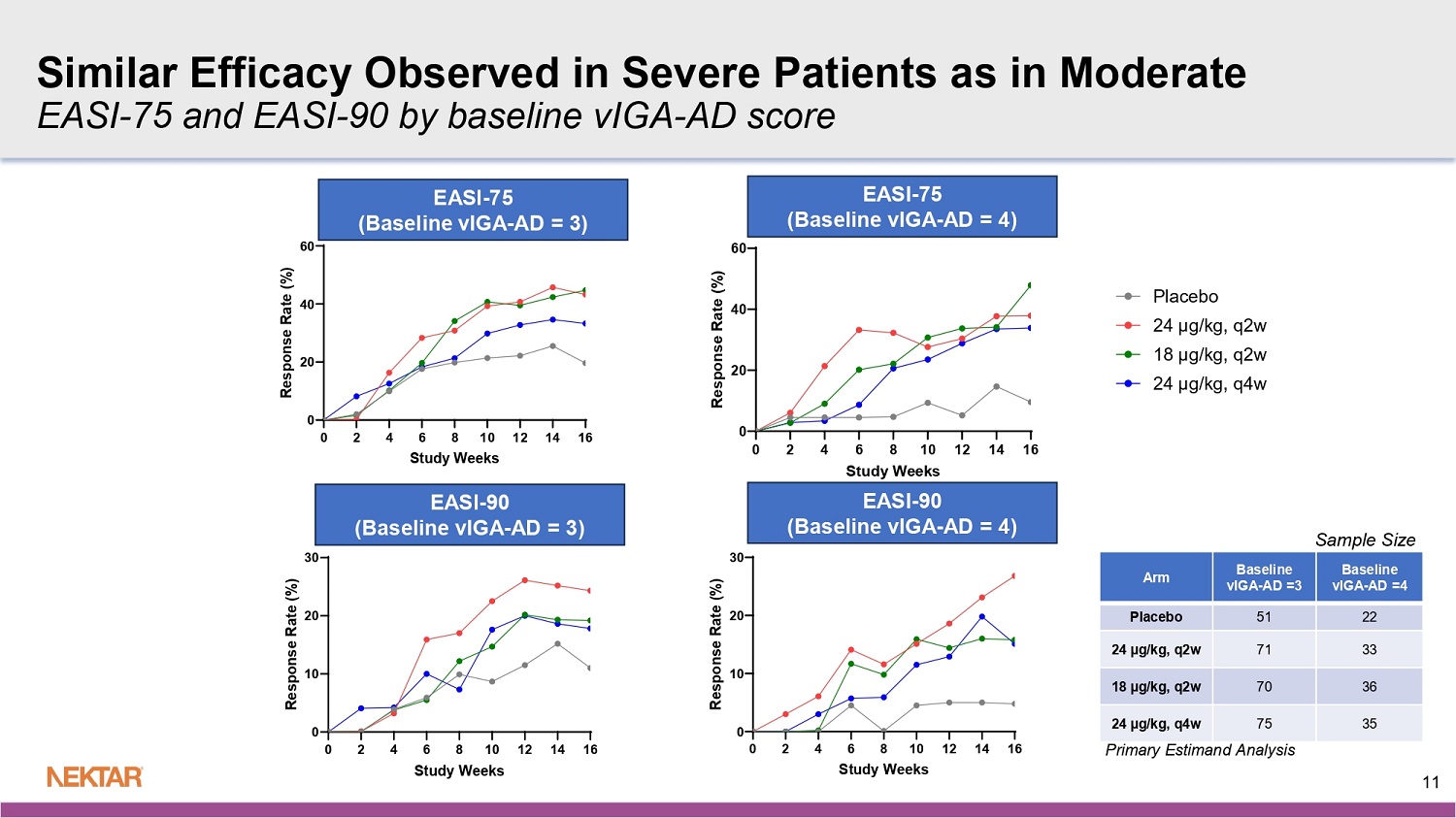
When evaluating EASI-75 and EASI-90 by disease severity using baseline vIGA-AD score, similar responses were observed in severe patients (baseline vIGA-AD of 4) as in moderate patients (baseline vIGA-AD of 3).
Week 16 Efficacy
|
|
24 µg/kg q2w (high dose) |
18 µg/kg q2w (middle dose) |
24 µg/kg q4w (low dose) |
Placebo |
| Primary Endpoint | N=104 | N=106 | N=110 | N=73 |
| Mean improvement in EASI score from baseline |
61% p<0.001 |
58% p<0.001 |
53% p<0.001 |
31% |
| Key Secondary Endpoints | ||||
| EASI-75 |
42% p<0.001 |
46% p<0.001 |
34% p<0.05 |
17% |
| vIGA-AD 0/1 |
20% p<0.05 |
26% p<0.01 |
19% ns |
8% |
| EASI-90 |
25% p<0.05 |
18% ns |
17% ns |
9% |
| Itch NRS* |
42% p<0.01 |
35% p<0.05 |
23% ns |
16% |
| Mean improvement in BSA score from baseline |
54% p<0.001 |
48% p<0.001 |
43% p<0.001 |
17% |
| EASI-50 |
66% p<0.001 |
66% p<0.001 |
55% p<0.01 |
34% |
| * | Patients with baseline Itch NRS ≥ 4 used as denominator for assessing Itch NRS response (N=63, 95, 92, and 102 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w arms); ns=not significant. |
Across all three dose arms, translational blood biomarker data demonstrate robust on-target and dose-dependent pharmacological activity with an increase in total Tregs of up to 6-fold in the high dose arm. Sustained Treg cell proliferation was observed at week 16 as compared to baseline and was correlated with reduction of key T helper 2 (Th2) inflammatory markers: IL-19, TARC/CCL17, periostin, and MDC/CCL22.
The safety profile for the 16-week induction period for rezpegaldesleukin was consistent with previously reported results. The most common treatment-emergent adverse events (TEAEs) were local injection site reactions (ISRs), observed in 69.7% of all rezpegaldesleukin-treated patients, with the largest proportion of these being mild or moderate (99.6%). ISRs were self-resolving and <1% of patients discontinued because of an ISR. Across all rezpegaldesleukin doses administered in the study over the 16-week induction period, 55.9% had no reports of ISRs, 30.1% had mild reports, 13.8% had moderate reports, and only 0.2% were severe. Other TEAEs more commonly observed (>5%) in the study treatment arms (n=320) versus placebo (n=73) include eosinophilia (7.8% vs. 2.7%), pyrexia (6.3% vs 2.7%), headache (6.3% vs. 4.1%) and arthralgia (5.0% vs 1.4%).
In the pooled rezpegaldesleukin arms, TEAEs, excluding ISRs, were reported in 60.3% of patients and in 57.5% of placebo-treated patients.
There was no increased risk of conjunctivitis, oral ulcers, or infections, including oral herpes, in the rezpegaldesleukin arms.
Safety over 16-Week Induction Period
| 24 µg/kg q2w | 18 µg/kg q2w | 24 µg/kg q4w | Pooled drug arms | Placebo | |
| N=104 | N=106 | N=110 | N=320 | N=73 | |
| Patients with any TEAE, excluding ISRs | 69 (66.3%) | 60 (56.6%) | 64 (58.2%) | 193 (60.3%) | 42 (57.5%) |
| Patients with any Serious AE | 1 (1.0%) | 4 (3.8%) | 0 | 5 (1.6%) | 0 |
| Any Drug-Related Serious AE1 | 0 | 2 (1.9%) | 0 | 2 (0.6%) | 0 |
| Patients with Severe AE | 3 (2.9%) | 6 (5.7%) | 1 (0.9%) | 10 (3.1%) | 1 (1.4)% |
| Any Drug-Related Severe AE2 | 3 (2.9%) | 3 (2.8%) | 0 | 6 (1.9%) | 0 |
| TEAEs leading to study drug discontinuation | 8 (7.7%) | 5 (4.7%) | 5 (4.7%) | 18 (5.6%) | 0 |
| 1. | Serious TRAEs: Drug hypersensitivity – severe; Tonsillitis – moderate. Both events resolved. |
| 2. | Severe TRAEs (excluding Serious TRAEs): pyrexia (24 µg/kg q2w); two ISRs (24 µg/kg q2w); ISR, chest pain (18 µg/kg q2w). All five events resolved. |
The Company plans to submit the REZOLVE-AD 16-week induction results for presentation at a medical conference later in 2025.
The Company expects to report full 52-week data from the RESOLVE-AD trial in early 2026 and expects top-line Phase 2b data for rezpegaldesleukin in alopecia areata in the fourth quarter of 2025.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding the Company’s plans, progress, and timing relating to the Company’s rezpegaldesleukin program in atopic dermatitis, including plans to submit the REZOLVE-AD 16-week induction results for presentation at a medical conference later in 2025 and timing for full 52-week data from the REZOLVE-AD trial, and timing for topline results from the Phase 2b REZOLVE-AA (alopecia areata) trial. The Company intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan,” “on track,” or similar expressions or the negative of those terms. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. The express or implied forward-looking statements included in this Current Report on Form 8-K are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: (i) the Company’s statements regarding the therapeutic potential of rezpegaldesleukin are based on preclinical and clinical findings and observations and are subject to change as research and development continue; (ii) rezpegaldesleukin is an investigational agent and continued research and development for this drug candidate is subject to substantial risks, including negative safety and efficacy findings in future clinical studies (notwithstanding positive findings in earlier preclinical and clinical studies); (iii) rezpegaldesleukin is in clinical development and the risk of failure is high and can unexpectedly occur at any stage prior to regulatory approval; (iv) the timing of the commencement or end of clinical trials and the availability of clinical data may be delayed or unsuccessful due to regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, changing standards of care, evolving regulatory requirements, clinical trial design, clinical outcomes, competitive factors, or delay or failure in ultimately obtaining regulatory approval in one or more important markets; (v) a Fast Track designation does not increase the likelihood that rezpegaldesleukin will receive marketing approval in the United States; (vi) patents may not issue from the Company’s patent applications for the Company’s drug candidates, patents that have issued may not be enforceable, or additional intellectual property licenses from third parties may be required; and (vii) certain other risk factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of the Company’s most recent Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and any other filings that Nektar has made or may make with the U.S. Securities and Exchange Commission in the future. They are based on current expectations and projections about future events and are therefore subject to risks and uncertainties, which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. Such statements are qualified in their entirety by the inherent risks and uncertainties surrounding future expectations. Therefore, you should not rely on any of these forward-looking statements. The Company does not assume any obligation to update the forward-looking information contained in this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. | Description | |
| 99.1 | Press release issued by Nektar Therapeutics on June 24, 2025, furnished herewith. | |
| 99.2 | Nektar Therapeutics Corporate Presentation, furnished herewith | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| NEKTAR THERAPEUTICS | ||
| Date: June 24, 2025 | By: | /s/ Mark A. Wilson |
| Mark A. Wilson | ||
| Chief Legal Officer and Secretary | ||
Exhibit 99.1

REZOLVE-AD Phase 2b Study of Rezpegaldesleukin Meets Primary and Key Secondary Endpoints in Patients with Moderate-to-Severe Atopic Dermatitis
Achieved statistical significance on primary endpoint at week 16 for mean percent change in EASI score from baseline for all rezpegaldesleukin arms versus placebo
Achieved statistical significance for key secondary endpoints at week 16 of disease reduction, including EASI-75, EASI-90, Itch NRS, vIGA-AD and BSA
Rapid onset
of EASI reduction and magnitude of itch improvement show potential differentiation of this novel regulatory
T-cell mechanism as a first
and best-in-class immune-modulator
Robust dose-dependent reduction of inflammatory biomarkers in atopic dermatitis including TARC/CCL17, periostin, MDC/CCL22, and IL-19
Safety profile consistent with previously reported results
Data expected in Q1 2026 from continued treatment of patients with atopic dermatitis in long-term maintenance part of REZOLVE-AD study
Top-line Phase 2b data for rezpegaldesleukin in alopecia areata expected in Q4 2025
Conference call and webcast with management and atopic dermatitis experts today at 8:15 am ET / 5:15 am PT
SAN FRANCISCO, June 24, 2025 /PRNewswire/ -- Nektar Therapeutics (Nasdaq: NKTR), a clinical-stage biotechnology company focused on development of novel immunology therapies, today announced statistically significant data from the 16-week induction period of the ongoing Phase 2b REZOLVE-AD study of investigational rezpegaldesleukin, an IL-pathway agonist and regulatory T-cell (Treg) proliferator.
The global Phase 2b study is being conducted in 393 patients with moderate-to-severe atopic dermatitis. Patients were randomized (3:3:3:2) to receive subcutaneous treatment with three doses of rezpegaldesleukin: a high dose of 24 µg/kg every two weeks (q2w), a middle dose of 18 µg/kg every two weeks (q2w), and a low dose of 24 µg/kg every four weeks (q4w), or placebo q2w. The primary endpoint and secondary endpoints were assessed at week 16. Following a 16-week induction period, rezpegaldesleukin-treated patients who achieved EASI percent score reductions of >50 were re-randomized (1:1) to continue at the same dose level on a q4w or q12w regimen through week 52 in a blinded maintenance period. Placebo patients with EASI percent score reductions of >50 percent continue to receive placebo q4w.
Rezpegaldesleukin Achieved Primary and Key Secondary Efficacy Endpoints at Week 16
The trial met its primary endpoint of the mean improvement in Eczema Area and Severity Score (EASI) from baseline at week 16 for all three dose arms of rezpegaldesleukin versus placebo (p<0.001).
All three dose arms also achieved statistical significance at week 16 for the key secondary endpoints of EASI-75 (percent of patients who achieve ≥75% reduction in EASI from baseline), EASI-50 (percent of patients who achieve ≥50% reduction in EASI from baseline) and BSA (mean percent improvement in Body Surface Area score from baseline).
The q2w arms of rezpegaldesleukin (high and middle doses) achieved statistical significance at week 16 for the key secondary endpoints of vIGA-AD 0/1 (percent of patients achieving a score of 0 or 1 on the validated Investigator’s Global Assessment for Atopic Dermatitis with ≥ 2-point reduction from baseline) and Itch NRS (percent of patients with baseline ≥ 4 who experienced a ≥ 4-point reduction in the Itch Numerical Rating Score from baseline).
In addition, at week 16, the high dose of 24 µg/kg q2w achieved statistical significance on EASI-90 (percent of patients who achieve ≥ 90% reduction in EASI from baseline).
When evaluating EASI-75 and EASI-90 by disease severity using baseline vIGA-AD score, similar responses were observed in severe patients (baseline vIGA-AD of 4) as in moderate patients (baseline vIGA-AD of 3).
“These data from REZOLVE-AD show a fast onset of both EASI response and itch relief within the first few doses of rezpegaldesleukin treatment, which are important metrics for physicians as they assess treatment options in atopic dermatitis,” Prof. Jonathan Silverberg, MD, PhD, MPH Professor of Dermatology at George Washington University School of Medicine and Health Sciences. “This shows the advantage of a broad-based Treg mechanism over other immune-modulation approaches in development to treat the disease. Additionally, we don’t see any increased risk of incidence of conjunctivitis, oral herpes, or oral ulcers with this mechanism of action as we do with other mechanisms.”
Week 16 Efficacy
| 24 µg/kg q2w (high dose) |
18 µg/kg q2w (middle dose) |
24 µg/kg q4w (low dose) |
Placebo | |
| Primary Endpoint | N=104 | N=106 | N=110 | N=73 |
| Mean improvement in EASI score from baseline | 61% p<0.001 | 58% p<0.001 | 53% p<0.001 | 31% |
| Key Secondary Endpoints | ||||
| EASI-75 | 42% p<0.001 | 46% p<0.001 | 34% p<0.05 | 17% |
| vIGA-AD 0/1 | 20% p<0.05 | 26% p<0.01 | 19% ns | 8% |
| EASI-90 | 25% p<0.05 | 18% ns | 17% ns | 9% |
| Itch NRS* | 42% p<0.01 | 35% p<0.05 | 23% ns | 16% |
| Mean improvement in BSA score from baseline | 54% p<0.001 | 48% p<0.001 | 43% p<0.001 | 17% |
| EASI-50 | 66% p<0.001 | 66% p<0.001 | 55% p<0.01 | 34% |
| * | Patients with baseline Itch NRS ≥ 4 used as denominator for assessing Itch NRS response (N=63, 95, 92, and 102 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w arms); ns=not significant. |
“These REZOLVE-AD results present a new therapeutic hypothesis for treatment of dermatological diseases and the investigators are looking forward to rezpegaldesleukin advancing in development in atopic dermatitis,” said Prof. David Rosmarin M.D., Chair, Department of Dermatology and Associate Professor of Dermatology, Indiana University School of Medicine. “With the establishment of this efficacy profile in the dermatological setting of atopic dermatitis, we are also eager to see the upcoming results from the ongoing REZOLVE-AA study in patients with severe to very-severe alopecia areata.”
Across all three dose arms, translational blood biomarker data demonstrate robust on-target and dose-dependent pharmacological activity with an increase in total Tregs of up to 6-fold in the high dose arm. Sustained Treg cell proliferation was observed at week 16 as compared to baseline and was correlated with reduction of key T helper 2 (Th2) inflammatory markers: IL-19, TARC/CCL17, periostin, and MDC/CCL22.
“We believe that the REZOLVE-AD study results clearly demonstrate that Nektar has established a new biology and harnessed the promise of Tregs as an important potential therapeutic modality to treat inflammatory skin disorders and other autoimmune conditions,” said Howard W. Robin, President and CEO of Nektar Therapeutics. “These compelling efficacy findings are further boosted by the translational data that show, for the first time, that rezpegaldesleukin also reduced key markers of Th2 inflammation in atopic dermatitis. With this validation in atopic dermatitis, we also look forward to reporting results in the fourth quarter of this year for rezpegaldesleukin in alopecia areata.”
Nektar plans to submit these REZOLVE-AD 16-week induction results for presentation at a medical conference later in 2025.
Safety Profile Consistent with Previously Reported Results
The safety profile for the 16-week induction period for rezpegaldesleukin was consistent with previously reported results. The most common treatment-emergent adverse events (TEAEs) were local injection site reactions (ISRs), observed in 69.7% of all rezpegaldesleukin-treated patients, with the largest proportion of these being mild or moderate (99.6%). ISRs were self-resolving and <1% of patients discontinued because of an ISR. Across all rezpegaldesleukin doses administered in the study over the 16-week induction period, 55.9% had no reports of ISRs, 30.1% had mild reports, 13.8% had moderate reports, and only 0.2% were severe. Other TEAEs more commonly observed (>5%) in the study treatment arms (n=320) versus placebo (n=73) include eosinophilia (7.8% vs. 2.7%), pyrexia (6.3% vs 2.7%), headache (6.3% vs. 4.1%) and arthralgia (5.0% vs 1.4%).
In the pooled rezpegaldesleukin arms, TEAEs, excluding ISRs, were reported in 60.3% of patients and in 57.5% of placebo-treated patients.
There was no increased risk of conjunctivitis, oral ulcers, or infections, including oral herpes, in the rezpegaldesleukin arms.
Safety over 16-Week Induction Period
| 24 µg/kg q2w | 18 µg/kg q2w | 24 µg/kg q4w | Pooled drug arms | Placebo | |
| N=104 | N=106 | N=110 | N=320 | N=73 | |
| Patients with any TEAE, excluding ISRs | 69 (66.3%) | 60 (56.6%) | 64 (58.2%) | 193 (60.3%) | 42 (57.5%) |
| Patients with any Serious AE | 1 (1.0%) | 4 (3.8%) | 0 | 5 (1.6%) | 0 |
| Any Drug-Related Serious AE1 | 0 | 2 (1.9%) | 0 | 2 (0.6%) | 0 |
| Patients with Severe AE | 3 (2.9%) | 6 (5.7%) | 1 (0.9%) | 10 (3.1%) | 1 (1.4)% |
| Any Drug-Related Severe AE2 | 3 (2.9%) | 3 (2.8%) | 0 | 6 (1.9%) | 0 |
| TEAEs leading to study drug discontinuation | 8 (7.7%) | 5 (4.7%) | 5 (4.7%) | 18 (5.6%) | 0 |
| 1. | Serious TRAEs: Drug hypersensitivity – severe; Tonsillitis – moderate. Both events resolved. |
| 2. | Severe TRAEs (excluding Serious TRAEs): pyrexia (24 µg/kg q2w); two ISRs (24 µg/kg q2w); ISR, chest pain (18 µg/kg q2w). All five events resolved. |
Conference Call and Webcast to Discuss Results of Phase 2b REZOLVE-AD Trial
Nektar management will host a conference call and live webcast with Drs. Silverberg and Rosmarin today, June 24, 2025, to review the results at 8:15 a.m. Eastern Time / 5:15 a.m. Pacific Time.
The accompanying slides and the webcast of the conference call can be accessed through a link on Nektar’s website on the investor relations page. To access the webcast directly, please click on the following link to register to join the Zoom webcast: https://lifescievents.com/event/sro974rcsq260kbgiw59/
The web broadcast of the conference call will be available for replay through July 25, 2025.
About REZOLVE-AD Phase 2b Study
The REZOLVE-AD trial was initiated in October 2023 and enrolled patients across approximately 110 sites globally with: 68% enrolled and treated in Europe, including Poland, Bulgaria, Germany, Czech Republic, Spain, Croatia and Hungary; 16% enrolled and treated in the United States; 11% enrolled and treated in Canada; and 5% enrolled and treated in Australia. Patient randomization was stratified based on baseline disease severity measured by vIGA-AD and geographic region. Key enrollment criteria in the study included a minimum EASI score of 16.0, a minimum Body Surface Area (BSA) of 10% and a minimum vIGA-AD of 3.
About Rezpegaldesleukin
Autoimmune and inflammatory diseases cause the immune system to mistakenly attack and damage healthy cells in a person’s body. A failure of the body’s self-tolerance mechanisms enables the formation of the pathogenic T lymphocytes that conduct this attack. Rezpegaldesleukin is a potential first-in-class resolution therapeutic that may address this underlying immune system imbalance in people with many autoimmune and inflammatory conditions. It targets the interleukin-2 receptor complex in the body to stimulate proliferation of powerful inhibitory immune cells known as regulatory T cells. By activating these cells, rezpegaldesleukin may act to bring the immune system back into balance.
In February 2025, the U.S. Food and Drug Administration (FDA) granted Fast Track designation for rezpegaldesleukin for the treatment of adult and pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Rezpegaldesleukin is being developed as a self-administered injection for a number of autoimmune and inflammatory diseases. It is wholly owned by Nektar Therapeutics.
About Atopic Dermatitis
Atopic dermatitis is the most common type of eczema, affecting approximately 30 million people in the United States.1 AD is characterized by a defect in the skin barrier, which allows allergens and other irritants to enter the skin, leading to an immune reaction and inflammation.
About Nektar Therapeutics
Nektar Therapeutics is a clinical-stage biotechnology company focused on developing treatments that address the underlying immunological dysfunction in autoimmune and chronic inflammatory diseases. Nektar’s lead product candidate, rezpegaldesleukin (REZPEG, or NKTR-358), is a novel, first-in-class regulatory T cell stimulator being evaluated in two Phase 2b clinical trials, one in atopic dermatitis and one in alopecia areata. Nektar’s pipeline also includes a preclinical bivalent tumor necrosis factor receptor type II (TNFR2) antibody and bispecific programs, NKTR-0165 and NKTR-0166, and a modified hematopoietic colony stimulating factor (CSF) protein, NKTR-422. Nektar, together with various partners, is also evaluating NKTR-255, an investigational IL-15 receptor agonist designed to boost the immune system’s natural ability to fight cancer, in several ongoing clinical trials. Nektar is headquartered in San Francisco, California. For further information, visit www.nektar.com and follow us on LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements which can be identified by words such as: “will,” “expect,” “develop,” “potential,” “plan,” and similar references to future periods. Examples of forward-looking statements include, among others, statements regarding the therapeutic potential and safety profile of, and future development plans for, rezpegaldesleukin, the results and timing for reporting the full 52-week data from REZOLVE-AD, the results and timing for reporting data from REZOLVE-AA, the potential for rezpegaldesleukin to be a first-in-class T regulatory cell therapy, the potential market opportunity in atopic dermatitis and alopecia areata, the advantage of a broad-based Treg mechanism over other immune-modulation approaches in development to treat atopic dermatitis, and the high unmet need for a new mechanism of action in atopic dermatitis and alopecia areata. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results to differ materially from those indicated in the forward-looking statements include, among others: (i) our statements regarding the therapeutic potential of rezpegaldesleukin are based on preclinical and clinical findings and observations and are subject to change as research and development continue; (ii) rezpegaldesleukin is an investigational agent and continued research and development for this drug candidate is subject to substantial risks, including negative safety and efficacy findings in future clinical studies (notwithstanding positive findings in earlier preclinical and clinical studies); (iii) rezpegaldesleukin is in clinical development and the risk of failure is high and can unexpectedly occur at any stage prior to regulatory approval; (iv) the timing of the commencement or end of clinical trials and the availability of clinical data may be delayed or unsuccessful due to regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, changing standards of care, evolving regulatory requirements, clinical trial design, clinical outcomes, competitive factors, or delay or failure in ultimately obtaining regulatory approval in one or more important markets; (v) a Fast Track designation does not increase the likelihood that rezpegaldesleukin will receive marketing approval in the United States; (vi) patents may not issue from our patent applications for our drug candidates, patents that have issued may not be enforceable, or additional intellectual property licenses from third parties may be required; and (vii) certain other important risks and uncertainties set forth in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 9, 2025. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
For Investors:
Corey Davis, Ph.D.
LifeSci Advisors
212-915-2577
cdavis@lifesciadvisors.com
For Media:
Madelin Hawtin
LifeSci Communications
603-714-2638
mhawtin@lifescicomms.com
| 1 | Eczema stats. National Eczema Association (2022, September 27). |
https://nationaleczema.org/research/eczema-facts/
Exhibit 99.2


1 Phase 2b REZOLVE - AD Topline Results from 16 - Week Induction Rezpegaldesleukin in Patients with Moderate - to - Severe Atopic Dermatitis June 24, 2025 Forward - Looking Statements 2 Safe Harbor Statement This presentation and any accompanying oral discussion contains forward - looking statements within the meaning of the Private Sec urities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding Nektar Therapeutics (the “Company” or “Nektar”)’s plans, progress, an d t iming relating to the Company’s rezpegaldesleukin program in atopic dermatitis, including expectations for the end of Phase 2 Meeting with the U.S. Food and Drug Administratio n, timing for topline results from the Phase 2b REZOLVE - AA (alopecia areata) trial, timing for the 52 - week maintenance data and 52 - week off study treatment durability data from the Pha se 2b REZOLVE - AD (atopic dermatitis) trial, and the presentation of data, rezpegaldesleukin’s potential to be a first - in - class T regulatory cell therapy, the potential market opportunity in atopic dermatitis and high unme t need for a new mechanism of action, the Company’s current and future research and development plans or expectations, the structure, timing a nd success of the Company’s planned clinical trials, the potential benefits of any of the Company’s current or future product candidates in treating patients, and the Company’s goals an d strategy. Nektar intends such forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward - looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential, ” “ plan,” “on track,” or similar expressions or the negative of those terms. Such forward - looking statements are based upon current expectations that involve risks, changes in circumstances, assumpt ions, and uncertainties. The express or implied forward - looking statements included in this presentation are only predictions and are subject to a number of risks, uncertaintie s and assumptions, including, without limitation: risks related to the success, cost, and timing of the Company’s development activities and clinical trials, risks related to the Co mpa ny’s dependence on the success of rezpegaldesleukin , the outcomes of competitive immunotherapy clinical trials, significant competition for the Company’s product candidates, the risk th at preliminary and interim data from the Company’s clinical studies are subject to audit and verification procedures that could result in material changes in the final data and ma y change as more patient data become available, risks related to delays in clinical trials, risks related to dependence on third parties to conduct clinical trials, risks regardin g f uture capital requirements, risks related to dependence on the Company’s collaboration agreements, risks related to the Company’s reliance on contract manufacturers and suppliers, risks re lat ed to obtaining regulatory approval for the Company’s drug candidates, risks related to the Company’s ability to protect and maintain its intellectual property position, risks rel ate d to legal proceedings and related litigation costs and liabilities and other risk factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Fina nci al Condition and Results of Operations” sections of Nektar’s most recent Annual Report on Form 10 - K, subsequent Quarterly Reports on Form 10 - Q and any other filings that Nektar has made or may make with the U.S. Securities and Exchange Commission in the future. Any forward - looking statements contained in this presentation and any accompanying oral discu ssion represent Nektar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Nektar e xpl icitly disclaims any obligation to update any forward - looking statements. Certain information contained in this presentation may be derived from information provided by industry sources. The Company bel ieves such information is accurate and that the sources from which it has been obtained are reliable. However, the Company cannot guarantee the accuracy of, and has not inde pen dently verified, such information.

REZOLVE - AD Phase 2b Validates Rezpeg as a First - in - Class Novel T - Regulatory Mechanism in Atopic Dermatitis (AD) Novel T - Reg MOA differentiates from existing and in - development biologics Up to 6 - fold increase in T - regs Clear dose - dependent reduction in multiple AD biomarkers: IL - 19, TARC/CCL17, Periostin , MDC/CCL22 Safety consistent with previously - reported safety profile with no new safety concerns • No increased risk of conjunctivitis, oral ulcers, or infections, including oral herpes, in study treatment arms • Most frequent AEs were mild injection site reactions (ISRs) that were self - resolving (<1% discontinuations due to ISRs) All 3 Dose Arms Met Primary Endpoint: Highest Dose Met all Six Key Secondaries: Other 2 doses also met multiple secondary endpoints EASI - 75 ( p<0.001) vIGA - AD 0/1 ( p<0.05) Itch - NRS (p<0.01) EASI - 90 (p - <0.05) BSA (p<0.001) % improvement in EASI at 16 weeks (p<0.001) Clear dose - dependent response Rapid onset of action (early separation from placebo) Equal efficacy observed in severe patients as in moderate 3 REZOLVE - AD: Phase 2b Trial Design Patients with Moderate - to - Severe Atopic Dermatitis Induction Period (16 Weeks) Randomize (3:3:3:2) N=~393 (MITT) Maintenance Period ( 36 Weeks) 1:1 Continue 24 µg/kg at q4W or q12W Rezpegaldesleukin 24 µg/kg (q2W) Rezpegaldesleukin 24 µg /kg (q4W) P lacebo q 4W Key Inclusion Criteria: x Age: >18 years x Moderate/severe AD diagnosis fo r < 12 months ▪ EASI ≥ 16 ▪ vIGA - AD of 3 or 4 ▪ BSA ≥ 10% x Biologic - naive (no prior biologic systemic therapy) and systemic JAKi - naïve x Failure of prior therapy, including TCS of medium or higher potency, within last 6 months Rezpegaldesleukin 18 µg /kg (q2W) Placebo q 2W Screening Stratification x Geographic region x Baseline severity Key Pharmacodynamic Biomarkers: • T regulatory cell • TARC/CC17 • Periostin • MDC/CCL22 • IL - 19 N= 581 N=106 N=110 N=73 N=104 1:1 Continue 18 µg/kg at q4W or q12W 1:1 Continue 24 µg/kg at q4W or q12W > EASI - 50 opportunity to advance to maintenance < EASI - 50 option to advance to escape arm 24 µg/kg (q2W) 4 MITT is defined as patients who received at least one dose of study treatment or placebo.
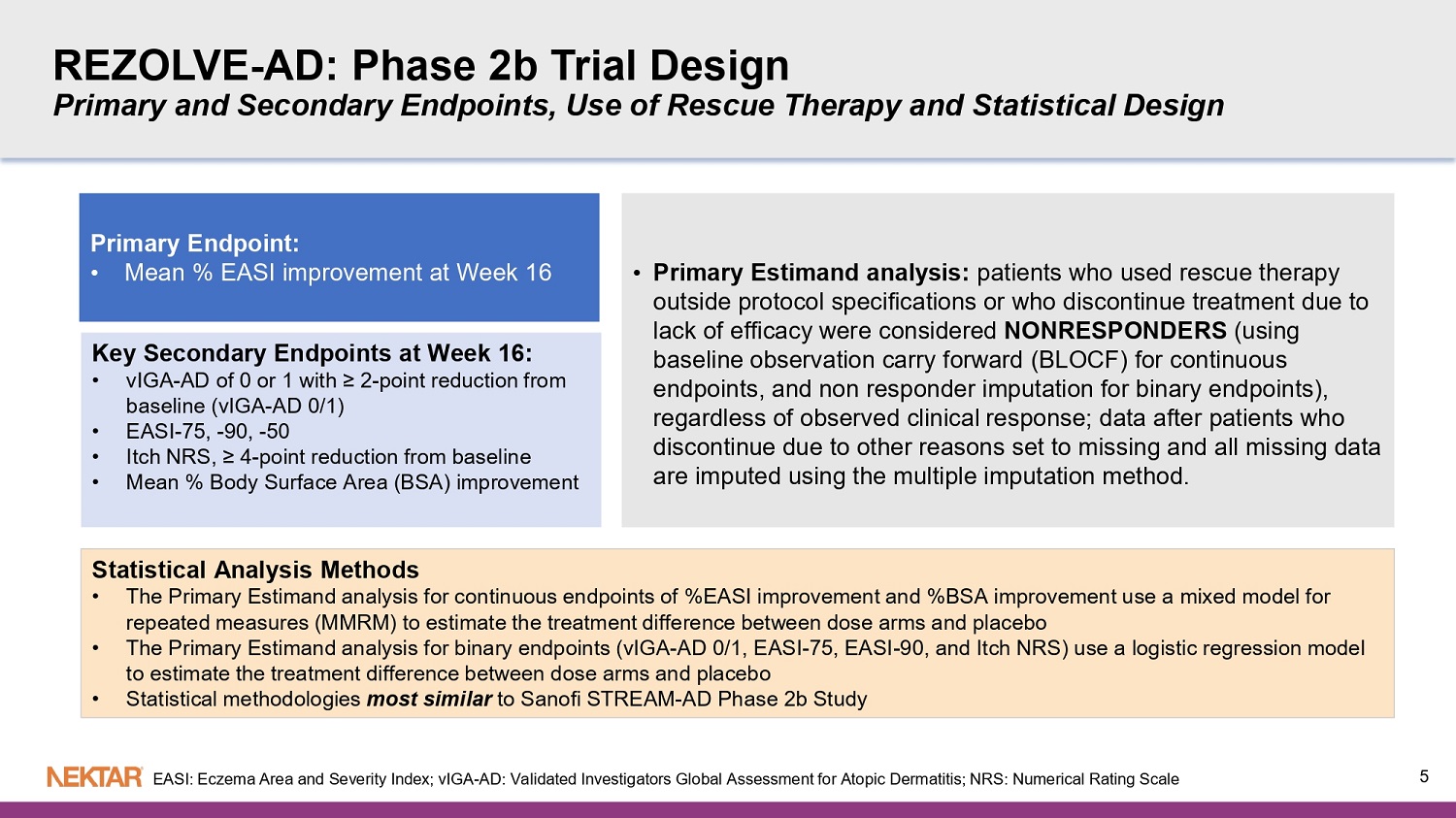
REZOLVE - AD: Phase 2b Trial Design Primary and Secondary Endpoints, Use of Rescue Therapy and Statistical Design Primary Endpoint: • Mean % EASI improvement at Week 16 Key Secondary Endpoints at Week 16: • vIGA - AD of 0 or 1 with ≥ 2 - point reduction from baseline ( vIGA - AD 0/1) • EASI - 75, - 90, - 50 • Itch NRS, ≥ 4 - point reduction from baseline • Mean % Body Surface Area (BSA ) improvement • Primary Estimand analysis: patients who used rescue therapy outside protocol specifications or who discontinue treatment due to lack of efficacy were considered NONRESPONDERS (using baseline observation carry forward (BLOCF) for continuous endpoints, and non responder imputation for binary endpoints), regardless of observed clinical response; data after patients who discontinue due to other reasons set to missing and all missing data are imputed using the multiple imputation method. Statistical Analysis Methods • The Primary Estimand analysis for continuous endpoints of %EASI improvement and %BSA improvement use a mixed model for repeated measures (MMRM) to estimate the treatment difference between dose arms and placebo • The Primary Estimand analysis for binary endpoints ( vIGA - AD 0/1, EASI - 75, EASI - 90, and Itch NRS) use a logistic regression model to estimate the treatment difference between dose arms and placebo • Statistical methodologies most similar to Sanofi STREAM - AD Phase 2b Study 5 EASI: Eczema Area and Severity Index; vIGA - AD: Validated Investigators Global Assessment for Atopic Dermatitis; NRS: Numerical Rating Scale Baseline Demographics and Disease Characteristics ▪ Patient Demographics - Patients were predominantly recruited from Europe, but also from North America and Australia - Stratification Factor: North America (27.5%) vs Rest of World (72.5%) - Majority of patients were under 65 years - old, well balanced among men and women - Majority were White (84.2%) with mean ± SD disease duration of 21.5 ± 14.9 years ▪ Baseline Disease Characteristics, mean ± SD: - EASI was 26.0 ± 9.8 • EASI < 21 (40.7%) vs EASI ≥ 21 (59.3%) - Stratification Factor: Baseline vIGA - AD 3 (67.9%) vs vIGA - AD 4 (32.1%) - BSA was 39.5 ± 20% - Itch NRS was 6.8 ± 1.95 - Well - balanced across arms 6
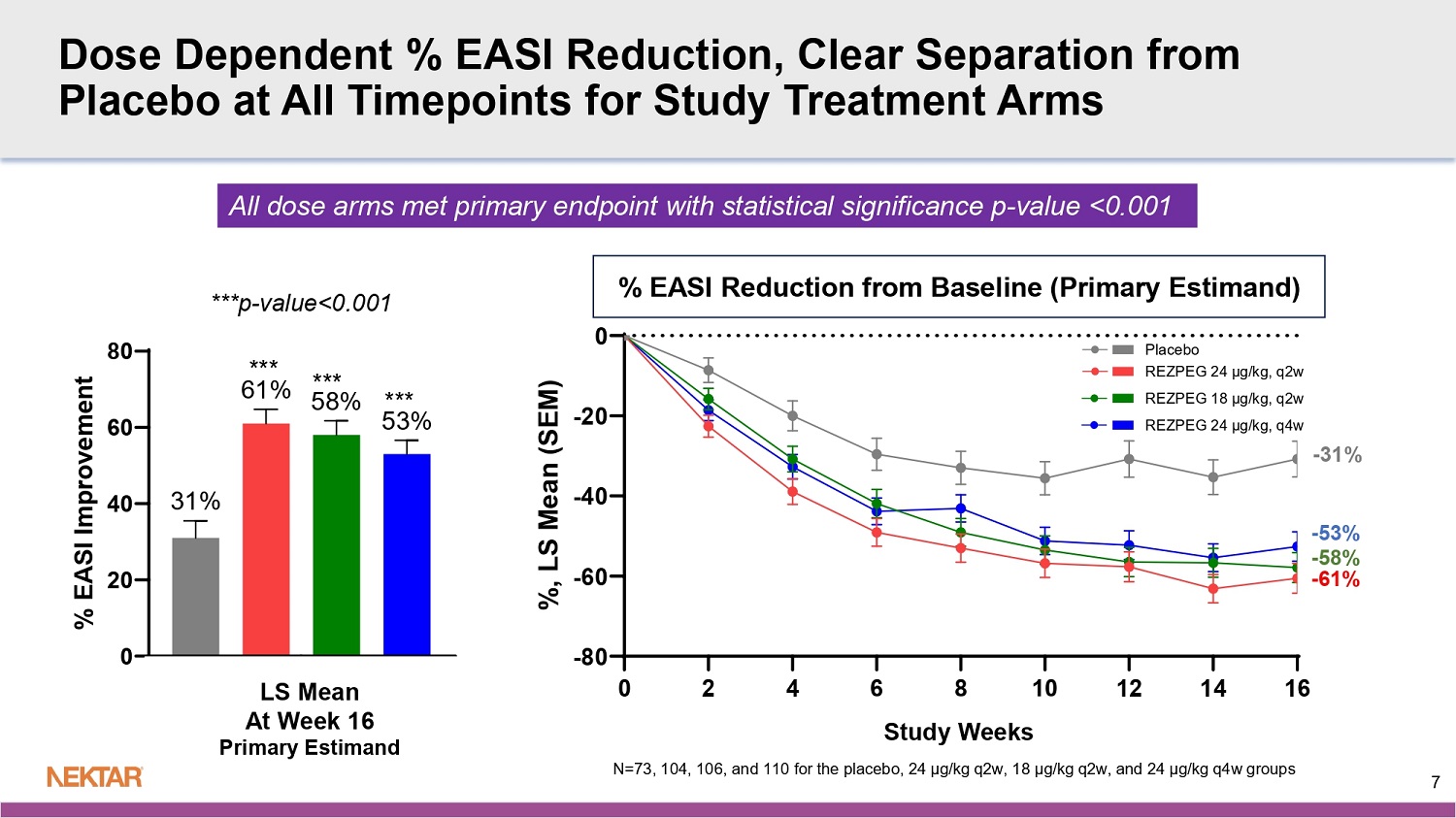
0 2 4 6 8 10 12 14 16 -80 -60 -40 -20 0 % EASI Reduction From Baseline Study Weeks % , L S M e a n ( S E M ) - 61% - 58% - 53% - 31% Study Weeks Dose Dependent % EASI Reduction, Clear Separation from Placebo at All Timepoints for Study Treatment Arms 0 20 40 60 80 53% 58% 61% 31% Week 16 % E A S I I m p r o v e m e n t *** *** *** ***p - value<0.001 % EASI Reduction from Baseline (Primary Estimand ) LS Mean At Week 16 Primary Estimand 7 All dose arms met primary endpoint with statistical significance p - value <0.001 N=73, 104, 106, and 110 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w groups ***p - value<0.001 **p - value<0.01 *p - value<0.05 EASI - 90 EASI - 50 EASI - 75 8 High Dose Met all Key Secondary Endpoints Multiple endpoints met for 2 additional dose arms Primary Estimand : N=73, 104, 106, and 110 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w groups

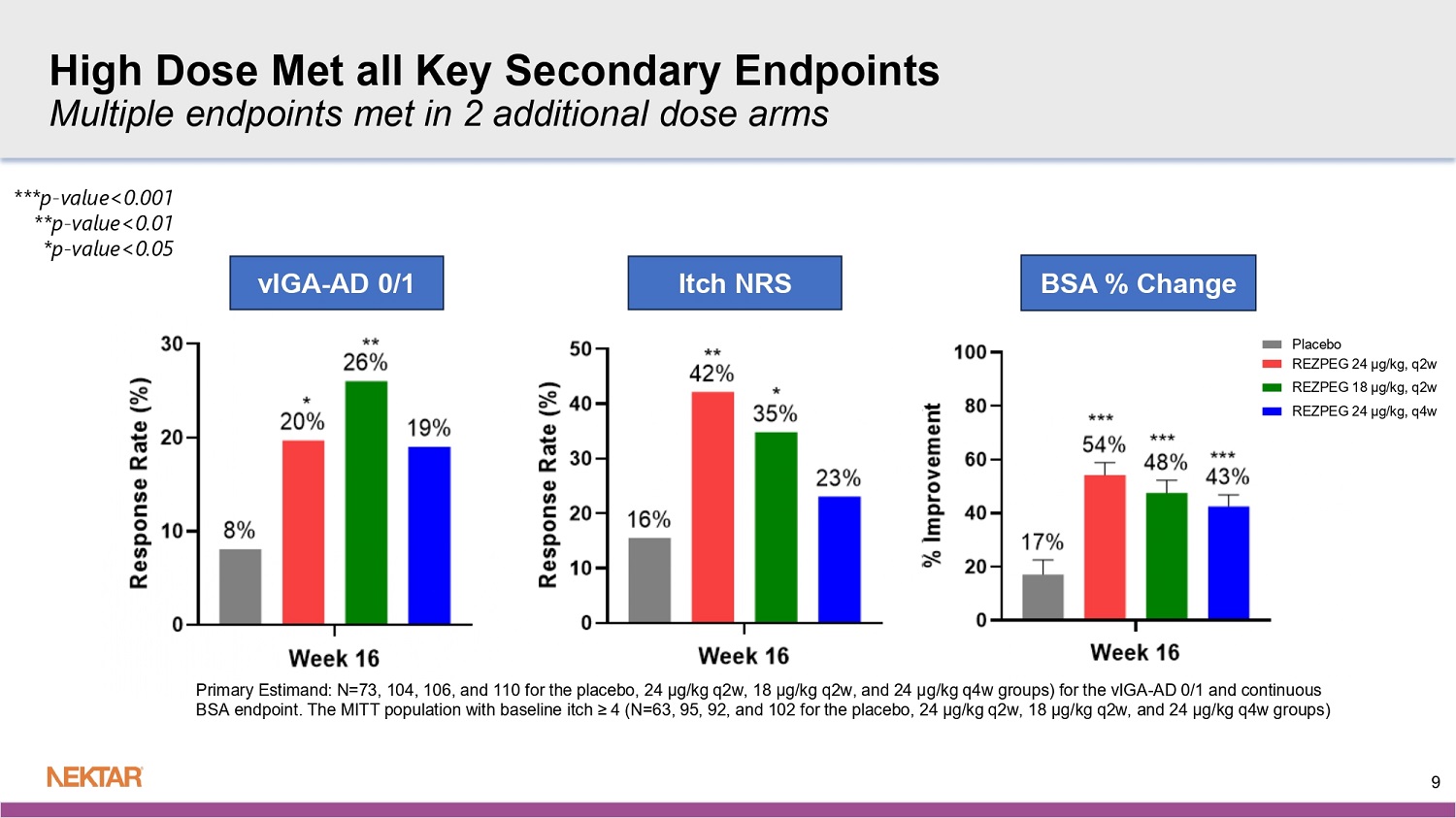
vIGA - AD 0/1 Itch NRS BSA % Change ***p - value<0.001 **p - value<0.01 *p - value<0.05 9 High Dose Met all Key Secondary Endpoints Multiple endpoints met in 2 additional dose arms Primary Estimand : N=73, 104, 106, and 110 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w groups) for the vIGA - AD 0/1 and continuous BSA endpoint. The MITT population with baseline itch ≥ 4 (N=63, 95, 92, and 102 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w groups)
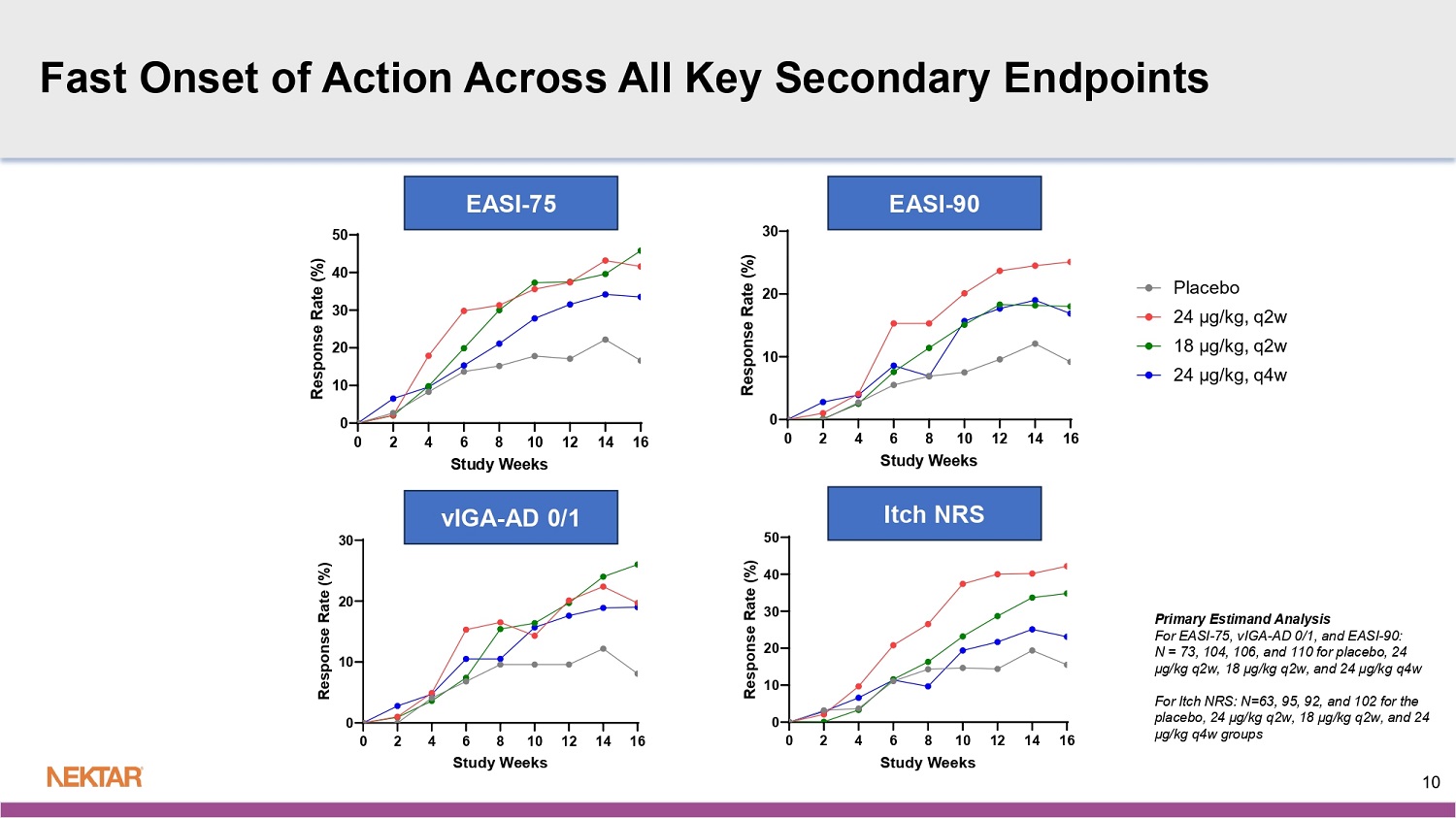
Fast Onset of Action Across All Key Secondary Endpoints 0 2 4 6 8 10 12 14 16 0 10 20 30 vIGA-AD 0/1 Study Weeks R e s p o n s e R a t e ( % ) 0 2 4 6 8 10 12 14 16 0 10 20 30 EASI-90 Study Weeks R e s p o n s e R a t e ( % ) 0 2 4 6 8 10 12 14 16 0 10 20 30 40 50 Itch NRS Study Weeks R e s p o n s e R a t e ( % ) 0 2 4 6 8 10 12 14 16 0 10 20 30 40 50 EASI-75 Study Weeks R e s p o n s e R a t e ( % ) EASI - 75 EASI - 90 vIGA - AD 0/1 Itch NRS 10 Primary Estimand Analysis For EASI - 75, vIGA - AD 0/1, and EASI - 90: N = 73, 104, 106, and 110 for placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w For Itch NRS: N=63, 95, 92, and 102 for the placebo, 24 µg/kg q2w, 18 µg/kg q2w, and 24 µg/kg q4w groups 0 2 4 6 8 10 12 14 16 0 10 20 30 EASI-90 (Baseline vIGA-AD 0/1 = 4) Study Weeks R e s p o n s e R a t e ( % ) Baseline vIGA - AD =4 Baseline vIGA - AD =3 Arm 22 51 Placebo 33 71 24 µg/kg, q2w 36 70 18 µg/kg, q2w 35 75 24 µg/kg, q4w Sample Size 0 2 4 6 8 10 12 14 16 0 20 40 60 EASI-75 (Baseline vIGA-AD 0/1 = 4) Study Weeks R e s p o n s e R a t e ( % ) 0 2 4 6 8 10 12 14 16 0 20 40 60 EASI-75 (Baseline vIGA-AD 0/1 = 3) Study Weeks R e s p o n s e R a t e ( % ) Primary Estimand Analysis EASI - 75 (Baseline vIGA - AD = 4) EASI - 75 (Baseline vIGA - AD = 3) EASI - 90 (Baseline vIGA - AD = 4) 11 Similar Efficacy Observed in Severe Patients as in Moderate EASI - 75 and EASI - 90 by baseline vIGA - AD score 0 2 4 6 8 10 12 14 16 0 10 20 30 EASI-90 (Baseline vIGA-AD 0/1 = 3) Study Weeks R e s p o n s e R a t e ( % ) EASI - 90 (Baseline vIGA - AD = 3)
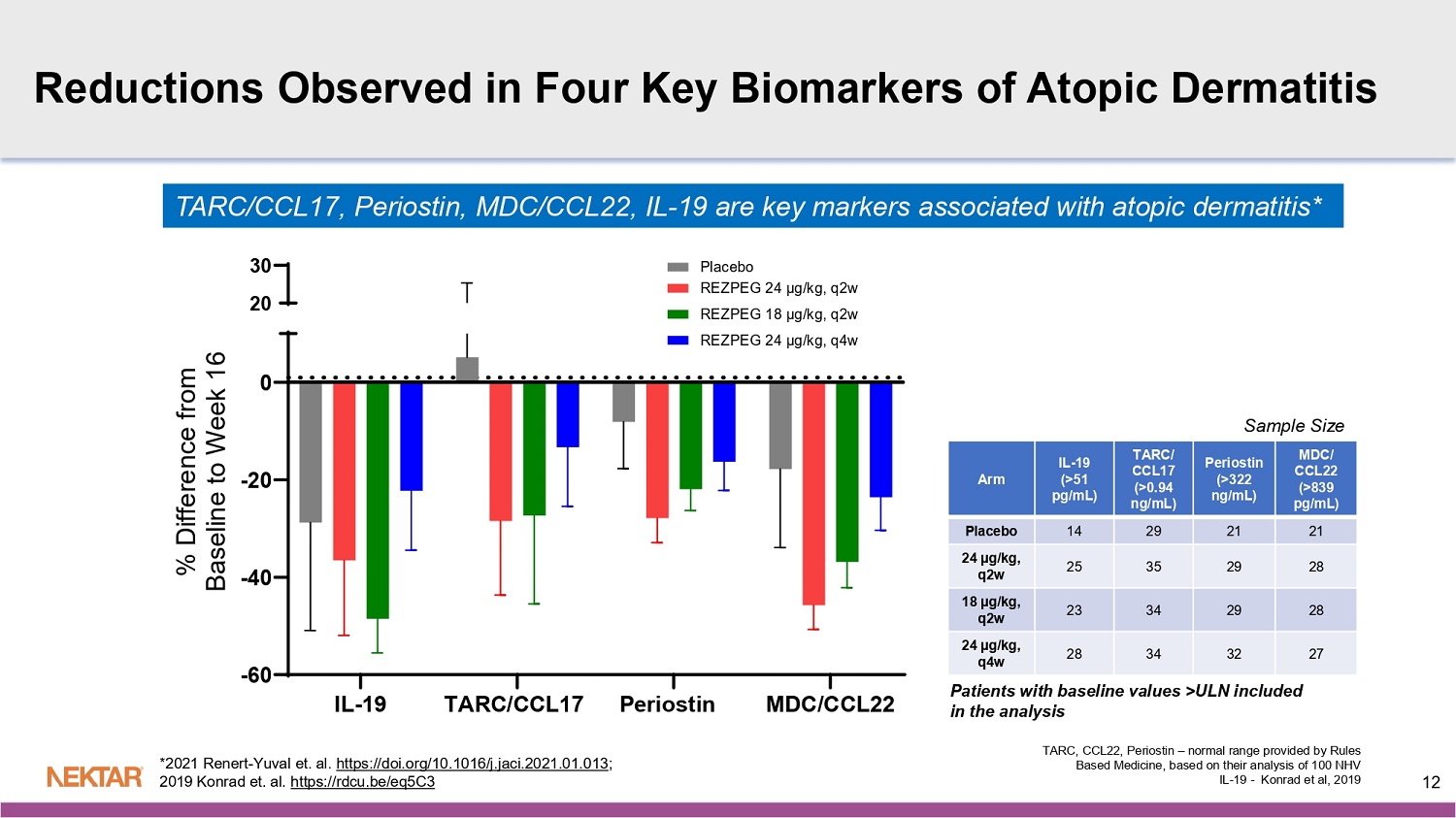
Patients with baseline values >ULN included in the analysis MDC/ CCL22 (>839 pg /mL) Periostin (>322 ng/mL) TARC/ CCL17 (>0.94 ng/mL) IL - 19 (>51 pg /mL) Arm 21 21 29 14 Placebo 28 29 35 25 24 µg/kg, q2w 28 29 34 23 18 µg/kg, q2w 27 32 34 28 24 µg/kg, q4w IL-19 TARC/CCL17 Periostin MDC/CCL22 -60 -40 -20 0 20 30 % D i f f e r e n c e f r o m B a s e l i n e t o W e e k 1 6 TARC, CCL22, Periostin – normal range provided by Rules Based Medicine, based on their analysis of 100 NHV IL - 19 - Konrad et al, 2019 TARC/CCL17, Periostin , MDC/CCL22, IL - 19 are key markers associated with atopic dermatitis* 12 *2021 Renert - Yuval et. al. https://doi.org/10.1016/j.jaci.2021.01.013 ; 2019 Konrad et. al.
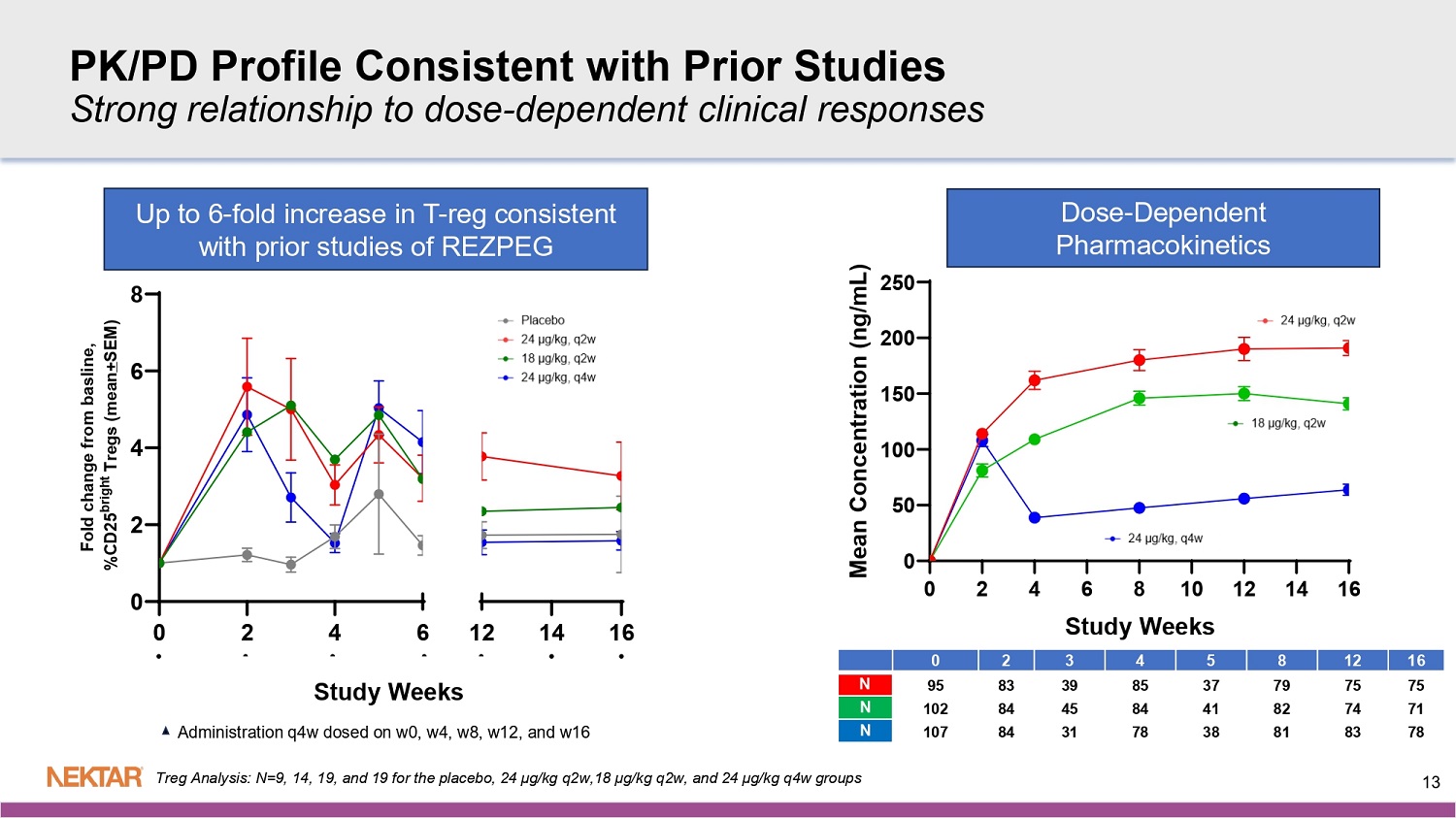
https://rdcu.be/eq5C3 Reductions Observed in Four Key Biomarkers of Atopic Dermatitis Sample Size Study Weeks 13 0 2 4 6 0 2 4 6 8 12 14 16 Study Weeks F o l d c h a n g e f r o m b a s l i n e , % C D 2 5 b r i g h t T r e g s ( m e a n + S E M ) Up to 6 - fold increase in T - reg consistent with prior studies of REZPEG PK/PD Profile Consistent with Prior Studies Strong relationship to dose - dependent clinical responses 16 12 8 5 4 3 2 0 75 75 79 37 85 39 83 95 N 71 74 82 41 84 45 84 102 N 78 83 81 38 78 31 84 107 N Administration q4w dosed on w0, w4, w8, w12, and w16 Treg Analysis: N=9, 14, 19, and 19 for the placebo, 24 µg/kg q2w,18 µg/kg q2w, and 24 µg/kg q4w groups Study Weeks Dose - Dependent Pharmacokinetics 0 2 4 6 8 10 12 14 16 0 50 100 150 200 250 M e a n C o n c e n t r a t i o n ( n g / m L )
REZOLVE - AD: Safety Summary ▪ Safety of rezpeg for 16 - week induction period in this Phase 2b study is consistent with previously observed and reported safety profile - Serious and severe AEs were rare (1.6% and 3.1%, respectively) for rezpeg - exposed patients - Discontinuation rate due to AEs was low (5.6%) for rezpeg - exposed patients and was within the range of rates seen in contemporary Phase 2b studies - No imbalance to suggest an increased risk of infection over placebo • No increased risk of conjunctivitis, oral ulcers, or infections, including oral herpes, in study treatment arms ▪ The most frequently observed adverse event was injection site reactions (ISRs) - Nearly all were mild - moderate in severity and self - resolving - The treatment discontinuation rate due to ISRs was very low (0.6%) for rezpeg exposed patients - Planning ISR mitigation strategy for commercialization 14 ISR Severity Breakdown Across All Dose Administrations By Severity Level Over 16 - Week Induction % of ISRs in each arm across all dose administrations.
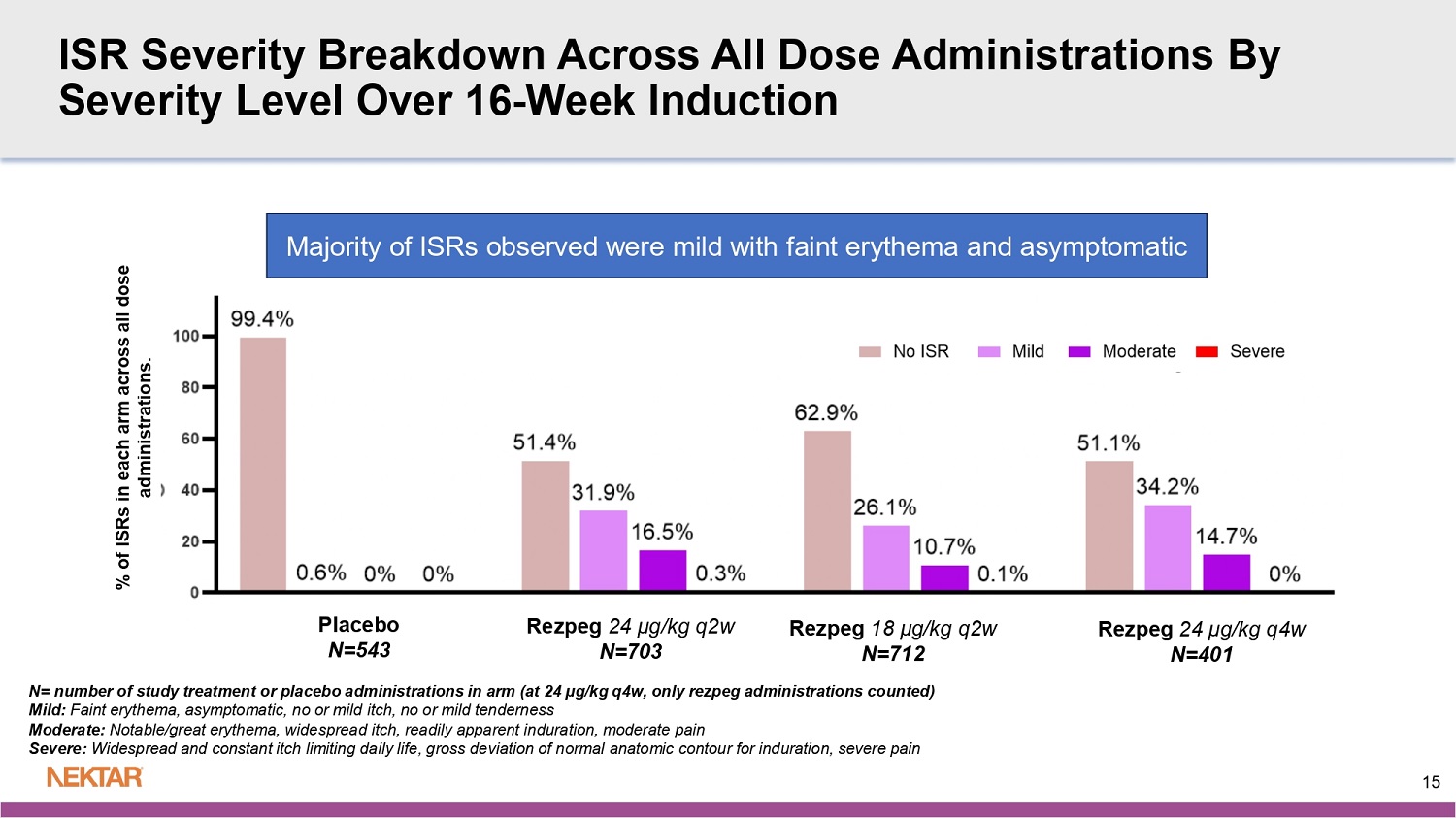
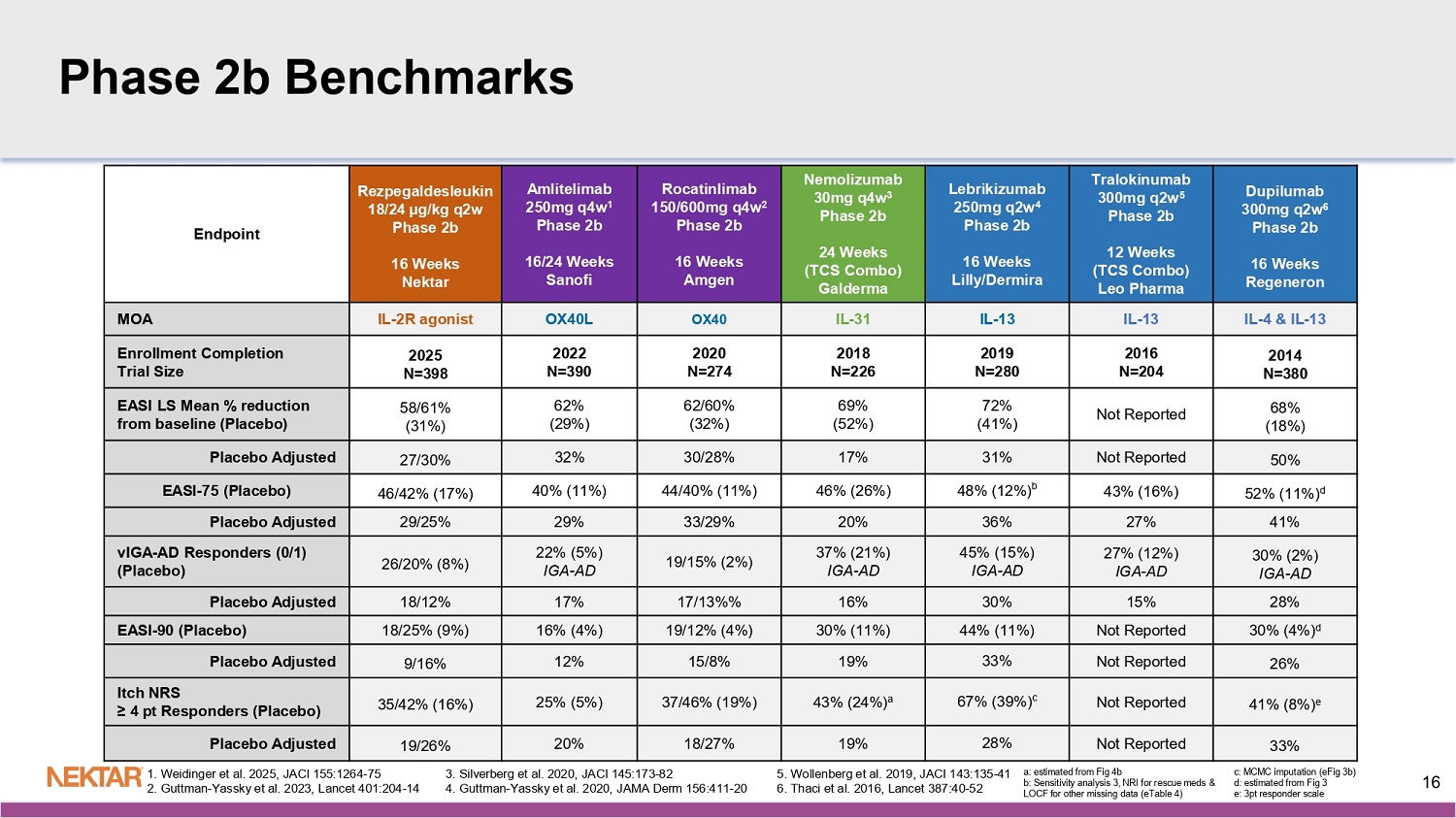
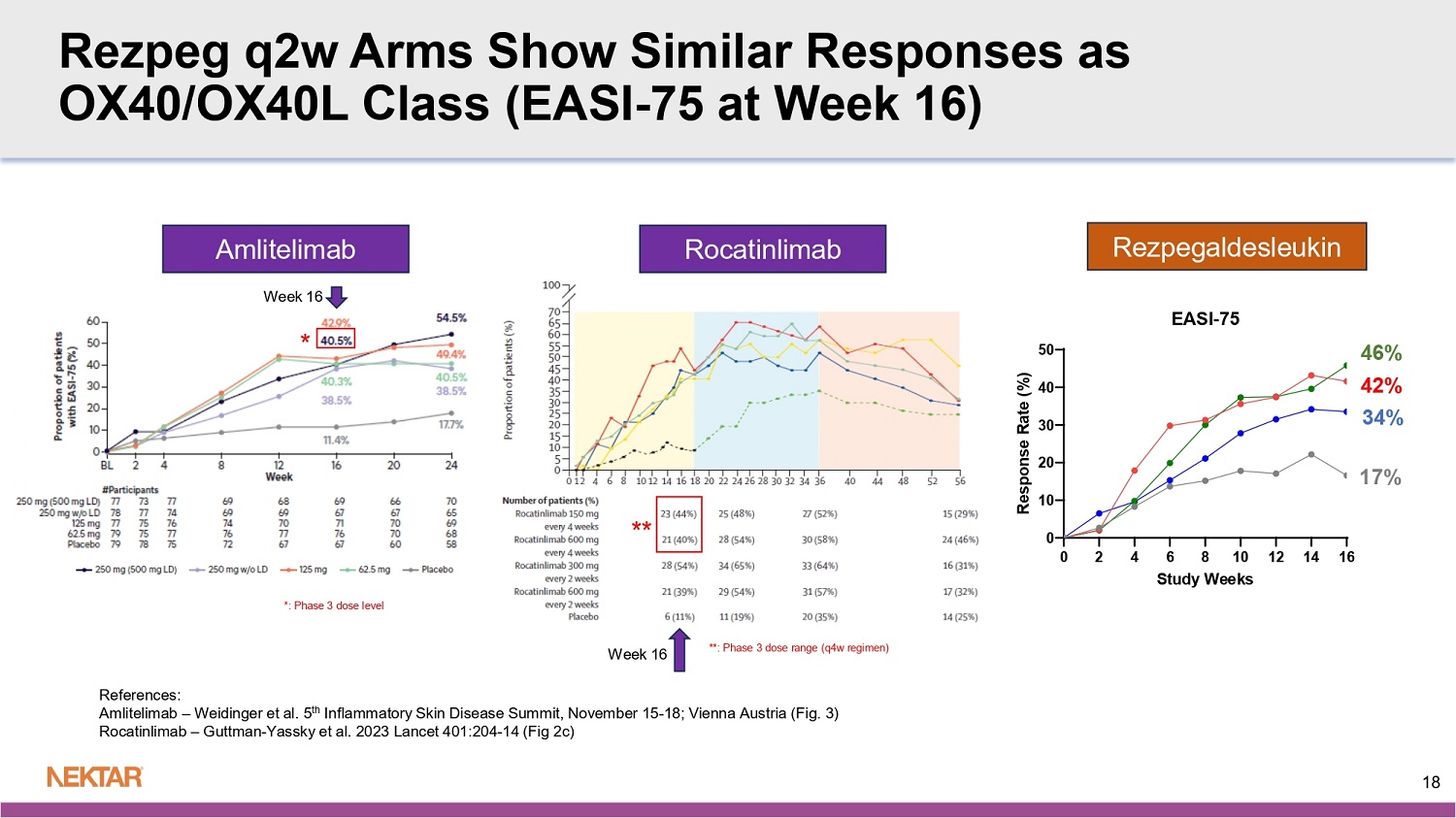
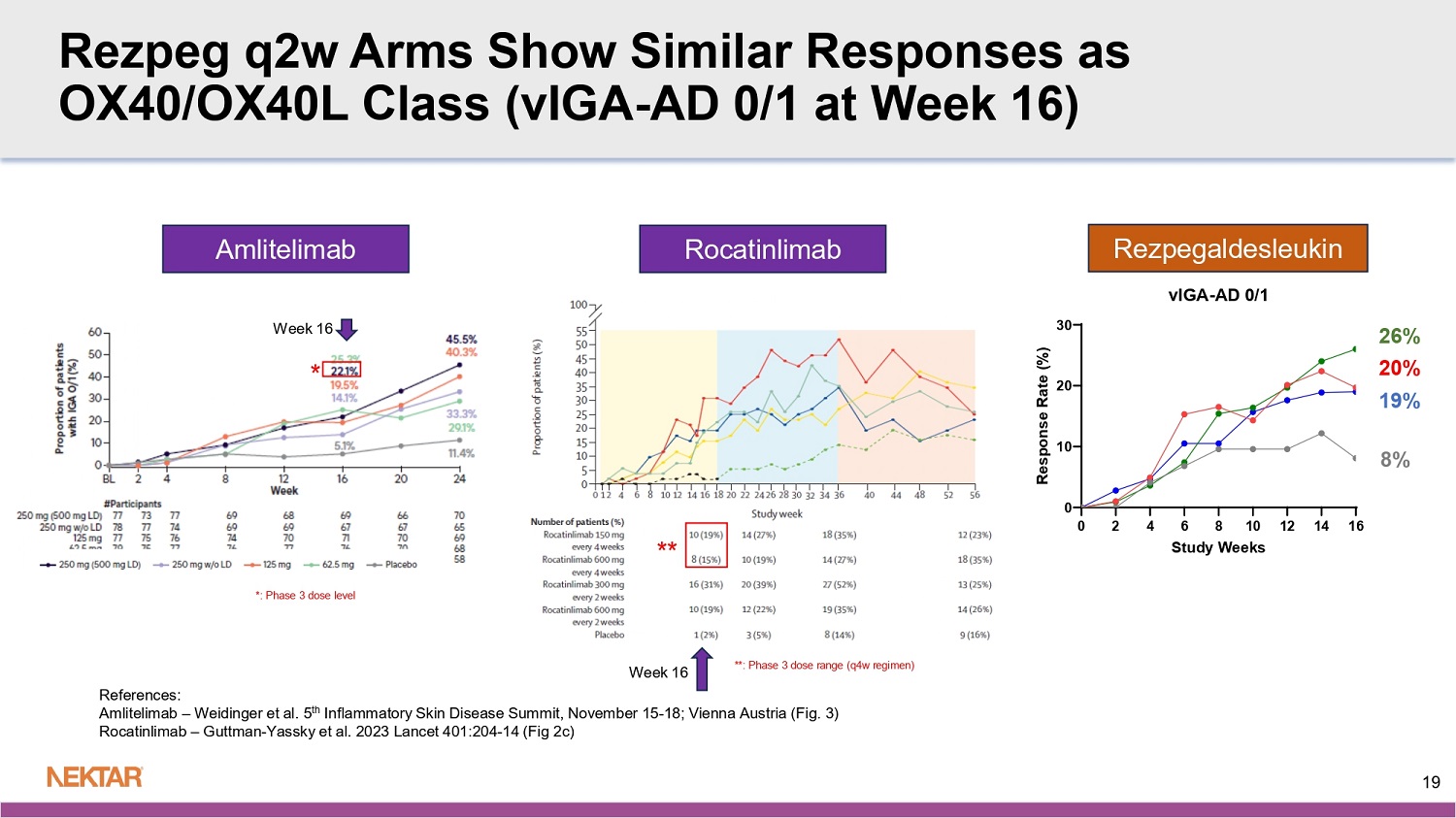
N= number of study treatment or placebo administrations in arm (at 24 µg/kg q4w, only rezpeg administrations counted) Mild: Faint erythema, asymptomatic, no or mild itch, no or mild tenderness Moderate: Notable/great erythema, widespread itch, readily apparent induration, moderate pain Severe: Widespread and constant itch limiting daily life, gross deviation of normal anatomic contour for induration, severe pain Majority of ISRs observed were mild with faint erythema and asymptomatic 15 Rezpeg 24 µg/kg q2w N=703 Rezpeg 18 µg/kg q2w N=712 Rezpeg 24 µg/kg q4w N=401 Placebo N=543 Phase 2b Benchmarks Dupilumab 300mg q2w 6 Phase 2b 16 Weeks Regeneron Tralokinumab 300mg q2w 5 Phase 2b 12 Weeks (TCS Combo) Leo Pharma Lebrikizumab 250mg q2w 4 Phase 2b 16 Weeks Lilly/ Dermira Nemolizumab 30mg q4w 3 Phase 2b 24 Weeks (TCS Combo) Galderma Rocatinlimab 150/600mg q4w 2 Phase 2b 16 Weeks Amgen Amlitelimab 250mg q4w 1 Phase 2b 16/24 Weeks Sanofi Rezpegaldesleukin 18/24 µg/kg q2w Phase 2b 16 Weeks Nektar Endpoint IL - 4 & IL - 13 IL - 13 IL - 13 IL - 31 OX40 OX40L IL - 2R agonist MOA 2014 N=380 2016 N=204 2019 N=280 2018 N=226 2020 N=274 2022 N=390 2025 N=398 Enrollment Completion Trial Size 68% (18%) Not Reported 72% (41%) 69% (52%) 62/60% (32%) 62% (29%) 58/61% (31%) EASI LS Mean % reduction from baseline (Placebo) 50% Not Reported 31% 17% 30/28% 32% 27/30% Placebo Adjusted 52% (11%) d 43% (16%) 48% (12%) b 46% (26%) 44/40% (11%) 40% (11%) 46/42% (17%) EASI - 75 (Placebo) 41% 27% 36% 20% 33/29% 29% 29/25% Placebo Adjusted 30% (2%) IGA - AD 27% (12%) IGA - AD 45% (15%) IGA - AD 37% (21%) IGA - AD 19/15% (2%) 22% (5%) IGA - AD 26/20% (8%) vIGA - AD Responders (0/1) (Placebo) 28% 15% 30% 16% 17/13%% 17% 18/12% Placebo Adjusted 30% (4%) d Not Reported 44% (11%) 30% (11%) 19/12% (4%) 16% (4%) 18/25% (9%) EASI - 90 (Placebo) 26% Not Reported 33% 19% 15/8% 12% 9/16% Placebo Adjusted 41% (8%) e Not Reported 67% (39%) c 43% (24%) a 37/46% (19%) 25% (5%) 35/42% (16%) Itch NRS ≥ 4 pt Responders (Placebo) 33% Not Reported 28% 19% 18/27% 20% 19/26% Placebo Adjusted 1. Weidinger et al. 2025, JACI 155:1264 - 75 2. Guttman - Yassky et al. 2023, Lancet 401:204 - 14 5. Wollenberg et al. 2019, JACI 143:135 - 41 6. Thaci et al. 2016, Lancet 387:40 - 52 16 3. Silverberg et al. 2020, JACI 145:173 - 82 4. Guttman - Yassky et al.

2020, JAMA Derm 156:411 - 20 a: estimated from Fig 4b b: Sensitivity analysis 3, NRI for rescue meds & LOCF for other missing data (eTable 4) c: MCMC imputation (eFig 3b) d: estimated from Fig 3 e: 3pt responder scale Benchmark to Phase 2b OX40/OX40L Class Rocatinlimab 150/600mg q4w 2 Phase 2b 16 Weeks Amgen Amlitelimab 250mg q4w 1 Phase 2b 16 Weeks Sanofi Rezpegaldesleukin 18/24 µg/kg q2w Phase 2b 16 Weeks Nektar Endpoint OX40 OX40L IL - 2R agonist MOA 2020 N=274 2022 N=390 2025 N=398 Enrollment Completion Trial Size 62/60% (32%) 62% (29%) 58/61% (31%) EASI LS Mean % reduction from baseline (Placebo) 30/28% 32% 27/30% Placebo Adjusted 44/40% (11%) 40% (11%) 46/42% (17%) EASI - 75 (Placebo) 33/29% 29% 29/25% Placebo Adjusted 19/15% (2%) 22% (5%) IGA 26/20% (8%) vIGA - AD Responders (0/1) (Placebo) 17/13%% 17% 18/12% Placebo Adjusted 19/12% (4%) 16% (4%) 18/25% (9%) EASI - 90 (Placebo) 15/8% 12% 9/16% Placebo Adjusted 37/46% (19%) 25% (5%) 35/42% (16%) Itch NRS ≥ 4 pt Responders (Placebo) 18/27% 20% 19/26% Placebo Adjusted 1. Weidinger et al. 2025, JACI 155:1264 - 75 2. Guttman - Yassky et al. 2023, Lancet 401:204 - 14 17 Rezpeg q2w Arms Show Similar Responses as OX40/OX40L Class (EASI - 75 at Week 16) 42% 46% 34% 17% * ** *: Phase 3 dose level **: Phase 3 dose range (q4w regimen) References: Amlitelimab – Weidinger et al.
5 th Inflammatory Skin Disease Summit, November 15 - 18; Vienna Austria (Fig. 3) Rocatinlimab – Guttman - Yassky et al. 2023 Lancet 401:204 - 14 (Fig 2c) Week 16 Week 16 18 Amlitelimab Rocatinlimab Rezpegaldesleukin Rezpeg q2w Arms Show Similar Responses as OX40/OX40L Class (vIGA - AD 0/1 at Week 16) * *: Phase 3 dose level ** **: Phase 3 dose range (q4w regimen) 20% 26% 19% 8% Week 16 Week 16 19 Amlitelimab Rocatinlimab Rezpegaldesleukin References: Amlitelimab – Weidinger et al.
5 th Inflammatory Skin Disease Summit, November 15 - 18; Vienna Austria (Fig. 3) Rocatinlimab – Guttman - Yassky et al. 2023 Lancet 401:204 - 14 (Fig 2c)

20 David Rosmarin, MD Chair of the Department of Dermatology at Indiana University School of Medicine Kampen - Norins Scholar in Dermatology Jonathan Silverberg, MD, PhD, MPH Professor of Dermatology at The George Washington University School of Medicine and Health Sciences Director of Clinical Research and Contact Dermatitis Dr. Silverberg is Professor of Dermatology at The George Washington University School of Medicine and Health Sciences in Washington, DC. He is the Director of Clinical Research and Contact Dermatitis. Dr. Silverberg's area of clinical subspecialty is inflammatory skin disease, particularly atopic and contact dermatitis. Dr. Silverberg has also been a local, national and/or international principal investigator for numerous clinical trials for novel treatments in atopic dermatitis and other inflammatory disorders. Dr. Silverberg's research interests include drug development, clinical trial design, biomarkers, dermato - epidemiology, health services research, patient - reported outcomes, comorbidities and burden of itch and inflammatory skin disease and evidence - based dermatology. His publications include more than 1000 peer - reviewed articles, abstracts and book chapters. He is an associate editor for the Journal of the American Academy of Dermatology, British Journal of Dermatology and Current Dermatology Reports. Dr. Rosmarin is Chair of the Department of Dermatology at Indiana University and is Kampen - Norins Scholar in Dermatology. He is nationally recognized and serves as a referral for physicians with difficult to manage inflammatory diseases such as atopic dermatitis. Previously, Dr. Rosmarin served as the Director of the Clinical Trials Unit in the Department of Dermatology at Tufts Medical Center. His research interests focus on development of novel therapeutics and investigating novel uses of established therapies, with a particular focus on chronic skin diseases such as atopic dermatitis, vitiligo, discoid lupus, and hidradenitis suppuritiva . For his training, Dr. Rosmarin went to medical school at NYU, dermatology residency at Boston University - Tufts combined training program, and fellowship at Brigham and Women’s Hospital.
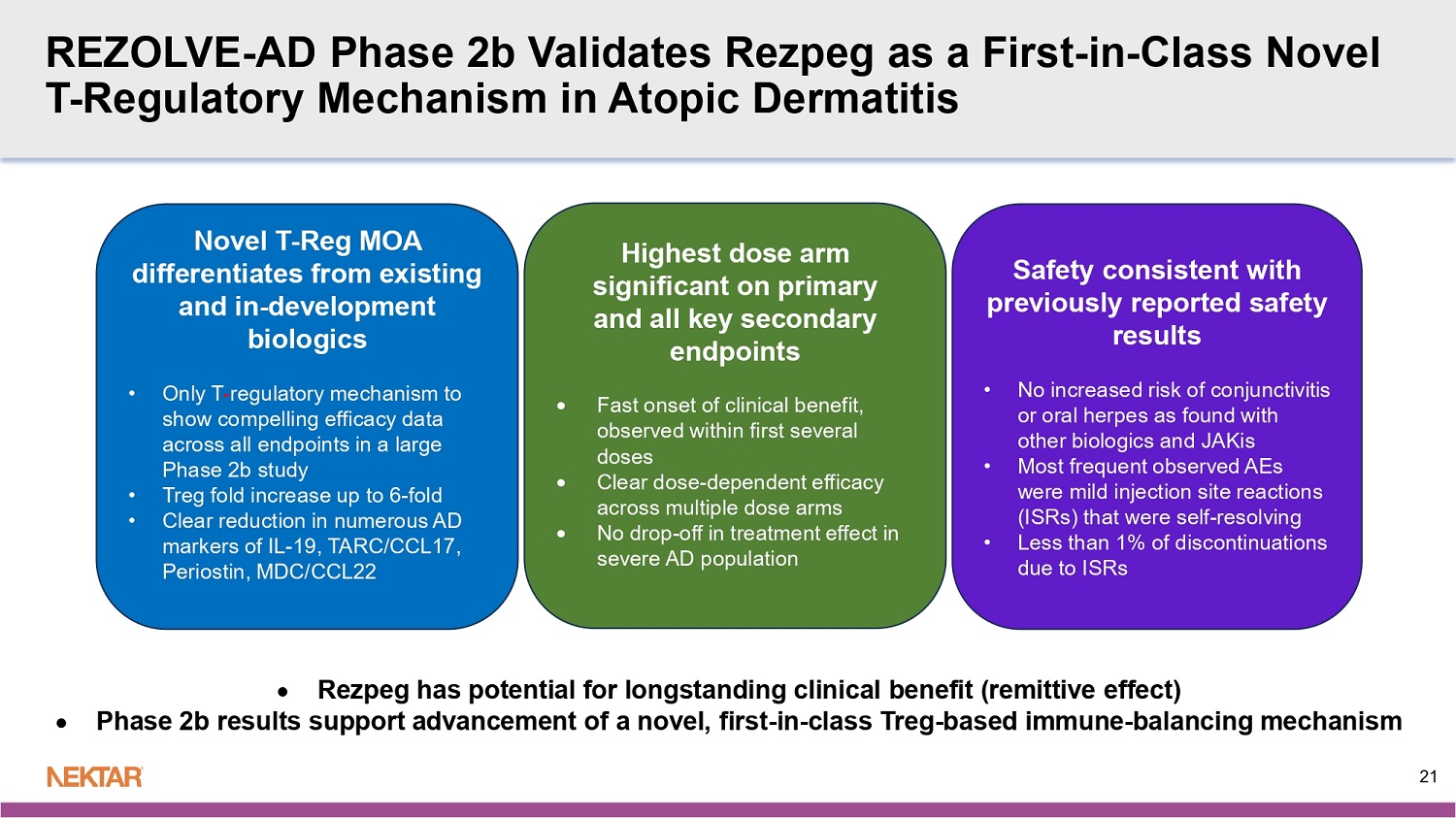
Rezpeg has potential for longstanding clinical benefit ( remittive effect) Phase 2b results support advancement of a novel, first - in - class Treg - based immune - balancing mechanism Novel T - Reg MOA differentiates from existing and in - development biologics • Only T - regulatory mechanism to show compelling efficacy data across all endpoints in a large Phase 2b study • Treg fold increase up to 6 - fold • Clear reduction in numerous AD markers of IL - 19, TARC/CCL17, Periostin , MDC/CCL22 Highest dose arm significant on primary and all key secondary endpoints Fast onset of clinical benefit, observed within first several doses Clear dose - dependent efficacy across multiple dose arms No drop - off in treatment effect in severe AD population Safety consistent with previously reported safety results • No increased risk of conjunctivitis or oral herpes as found with other biologics and JAKis • Most frequent observed AEs were mild injection site reactions (ISRs) that were self - resolving • Less than 1% of discontinuations due to ISRs 21 REZOLVE - AD Phase 2b Validates Rezpeg as a First - in - Class Novel T - Regulatory Mechanism in Atopic Dermatitis Rezpeg Program: Next Steps • End of Phase 2 Meeting with FDA to review Phase 3 development plan • Full presentation of data to be submitted for presentation at a medical meeting in 2025 • Topline results from Phase 2b REZOLVE - AA (alopecia areata) in December 2025 • 52 - week maintenance data from Phase 2b REZOLVE - AD (atopic dermatitis) in early 2026 • 52 - week off - study treatment durability data from Phase 2b REZOLVE - AD in early 2027 22

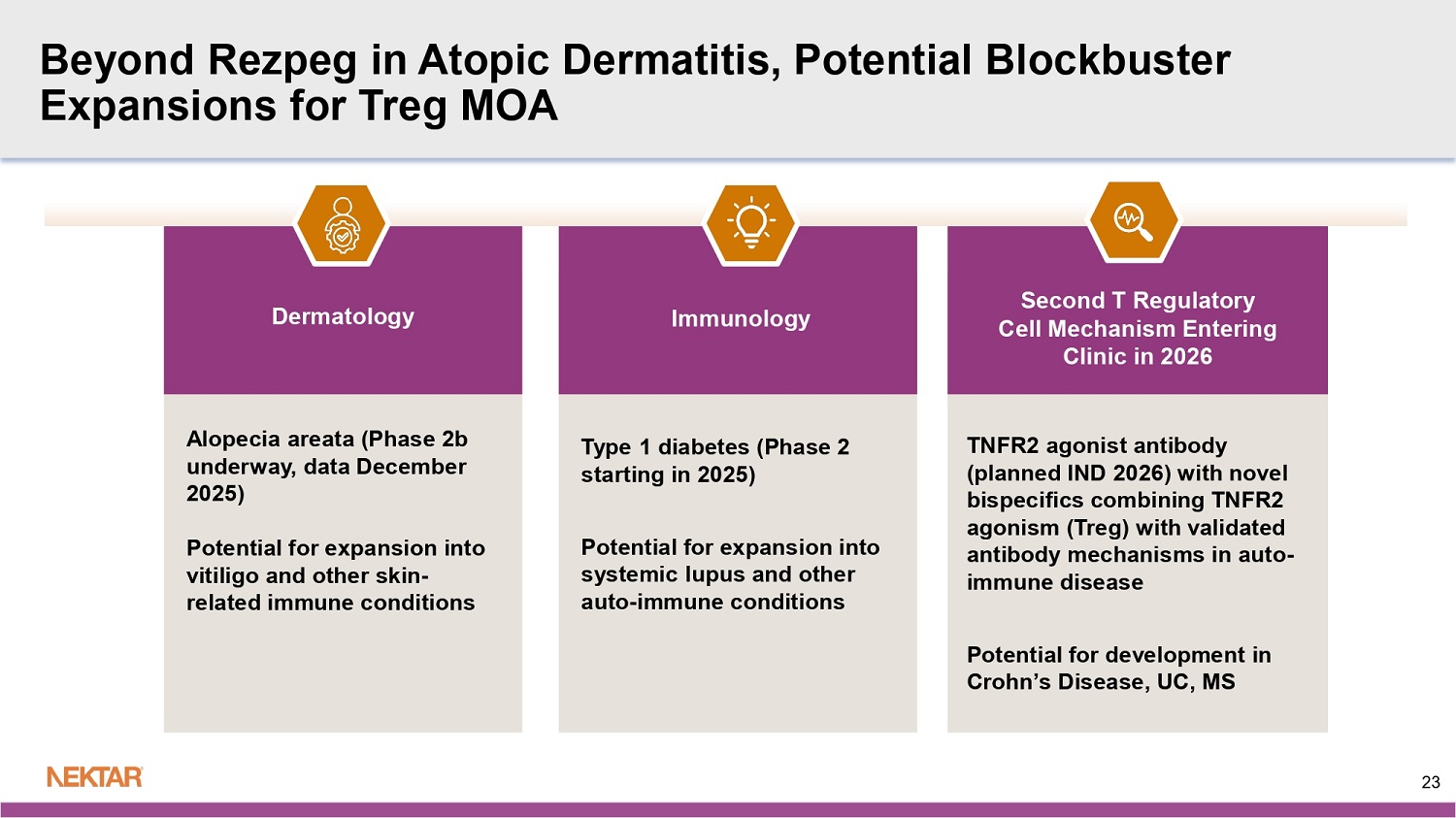
Beyond Rezpeg in Atopic Dermatitis, Potential Blockbuster Expansions for Treg MOA Dermatology Alopecia areata (Phase 2b underway, data December 2025) Potential for expansion into vitiligo and other skin - related immune conditions Immunology Type 1 diabetes (Phase 2 starting in 2025) Potential for expansion into systemic lupus and other auto - immune conditions Second T Regulatory Cell Mechanism Entering Clinic in 2026 TNFR2 agonist antibody (planned IND 2026) with novel bispecifics combining TNFR2 agonism (Treg) with validated antibody mechanisms in auto - immune disease Potential for development in Crohn’s Disease, UC, MS 23 Appendix (data tables, additional materials) 24

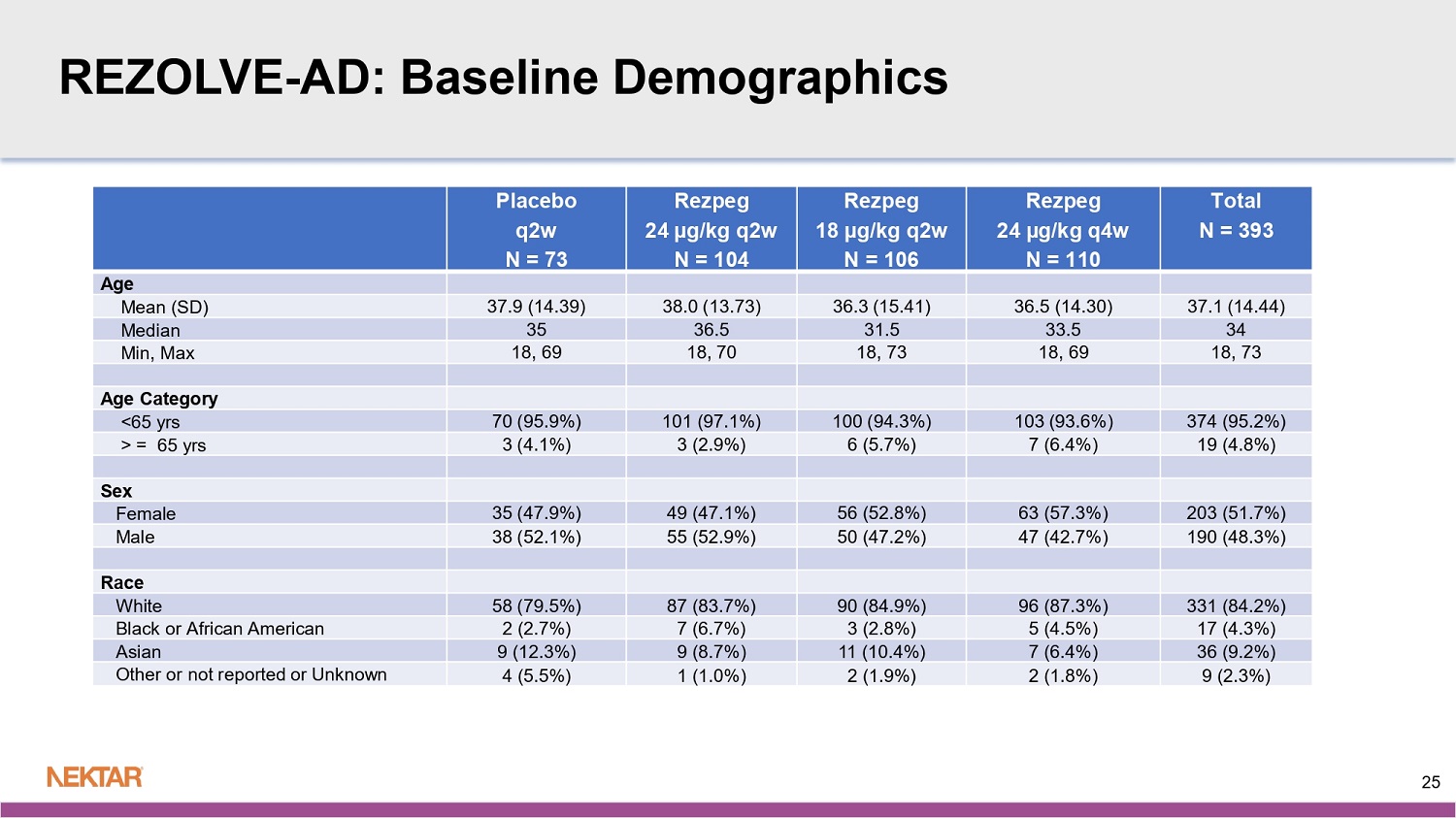
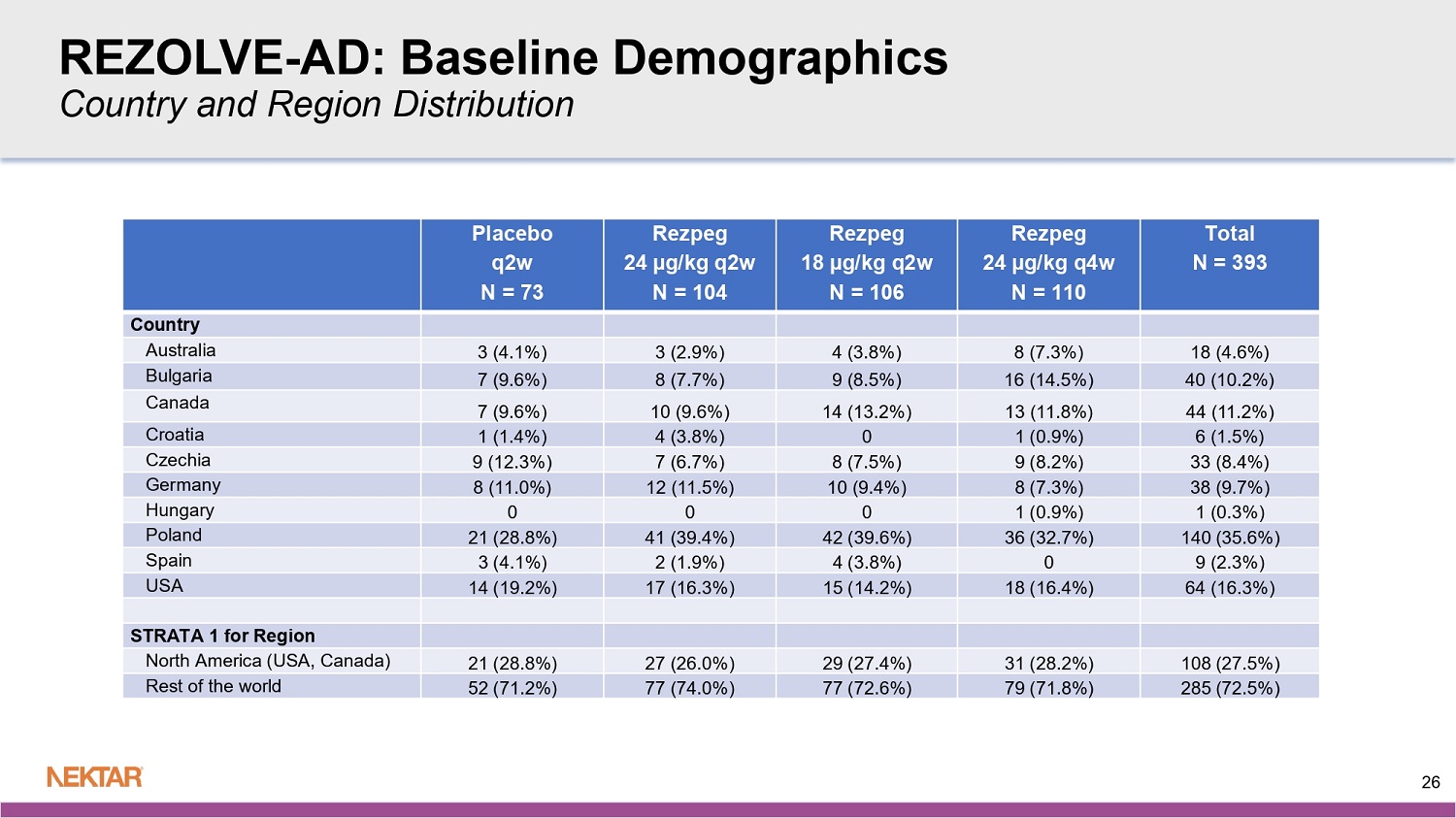
REZOLVE - AD: Baseline Demographics Total N = 393 Rezpeg 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 Age 37.1 (14.44) 36.5 (14.30) 36.3 (15.41) 38.0 (13.73) 37.9 (14.39) Mean (SD) 34 33.5 31.5 36.5 35 Median 18, 73 18, 69 18, 73 18, 70 18, 69 Min, Max Age Category 374 (95.2%) 103 (93.6%) 100 (94.3%) 101 (97.1%) 70 (95.9%) <65 yrs 19 (4.8%) 7 (6.4%) 6 (5.7%) 3 (2.9%) 3 (4.1%) > = 65 yrs Sex 203 (51.7%) 63 (57.3%) 56 (52.8%) 49 (47.1%) 35 (47.9%) Female 190 (48.3%) 47 (42.7%) 50 (47.2%) 55 (52.9%) 38 (52.1%) Male Race 331 (84.2%) 96 (87.3%) 90 (84.9%) 87 (83.7%) 58 (79.5%) White 17 (4.3%) 5 (4.5%) 3 (2.8%) 7 (6.7%) 2 (2.7%) Black or African American 36 (9.2%) 7 (6.4%) 11 (10.4%) 9 (8.7%) 9 (12.3%) Asian 9 (2.3%) 2 (1.8%) 2 (1.9%) 1 (1.0%) 4 (5.5%) Other or not reported or Unknown 25 REZOLVE - AD: Baseline Demographics Country and Region Distribution Total N = 393 Rezpeg 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 Country 18 (4.6%) 8 (7.3%) 4 (3.8%) 3 (2.9%) 3 (4.1%) Australia 40 (10.2%) 16 (14.5%) 9 (8.5%) 8 (7.7%) 7 (9.6%) Bulgaria 44 (11.2%) 13 (11.8%) 14 (13.2%) 10 (9.6%) 7 (9.6%) Canada 6 (1.5%) 1 (0.9%) 0 4 (3.8%) 1 (1.4%) Croatia 33 (8.4%) 9 (8.2%) 8 (7.5%) 7 (6.7%) 9 (12.3%) Czechia 38 (9.7%) 8 (7.3%) 10 (9.4%) 12 (11.5%) 8 (11.0%) Germany 1 (0.3%) 1 (0.9%) 0 0 0 Hungary 140 (35.6%) 36 (32.7%) 42 (39.6%) 41 (39.4%) 21 (28.8%) Poland 9 (2.3%) 0 4 (3.8%) 2 (1.9%) 3 (4.1%) Spain 64 (16.3%) 18 (16.4%) 15 (14.2%) 17 (16.3%) 14 (19.2%) USA STRATA 1 for Region 108 (27.5%) 31 (28.2%) 29 (27.4%) 27 (26.0%) 21 (28.8%) North America (USA, Canada) 285 (72.5%) 79 (71.8%) 77 (72.6%) 77 (74.0%) 52 (71.2%) Rest of the world 26
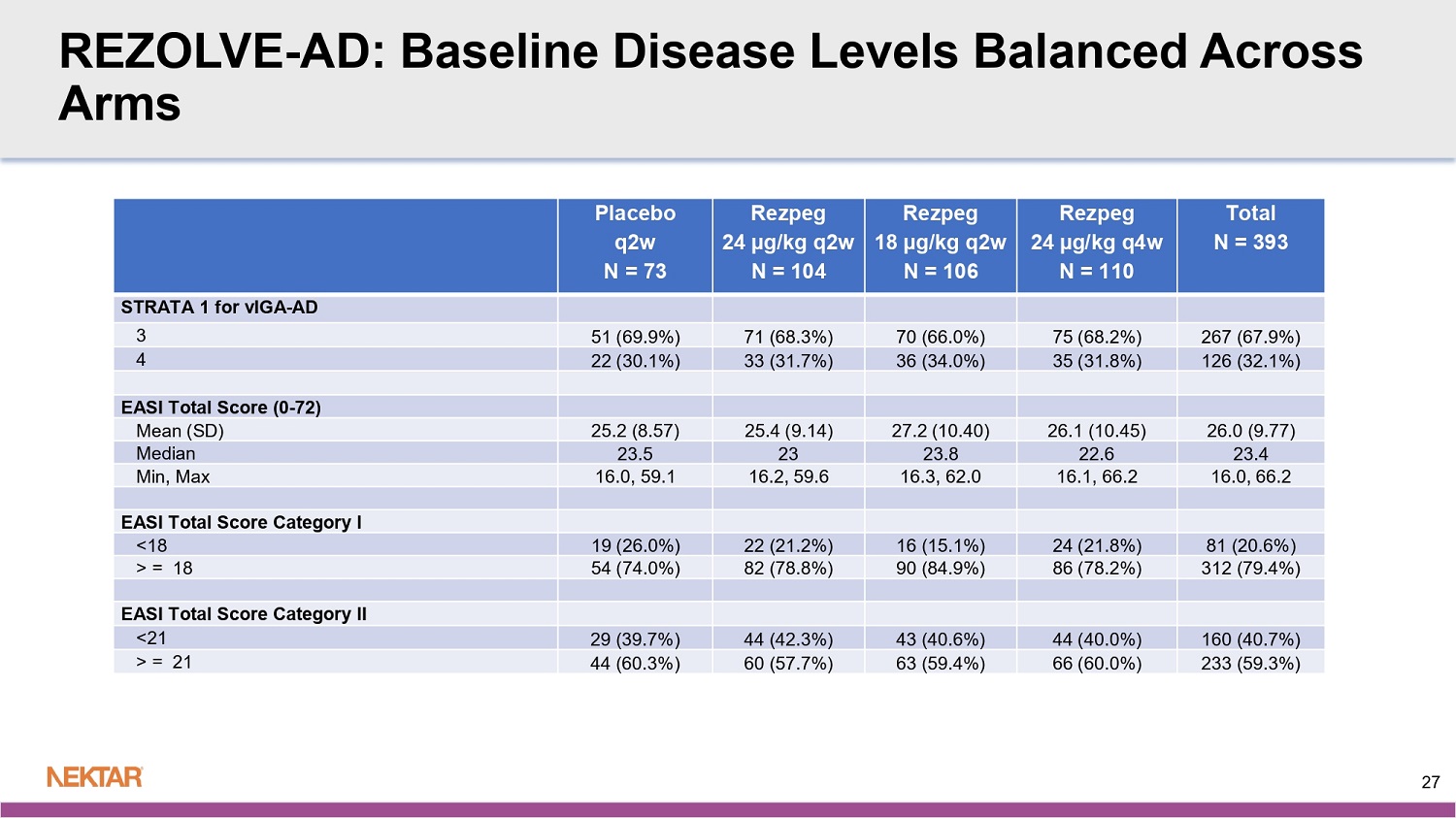
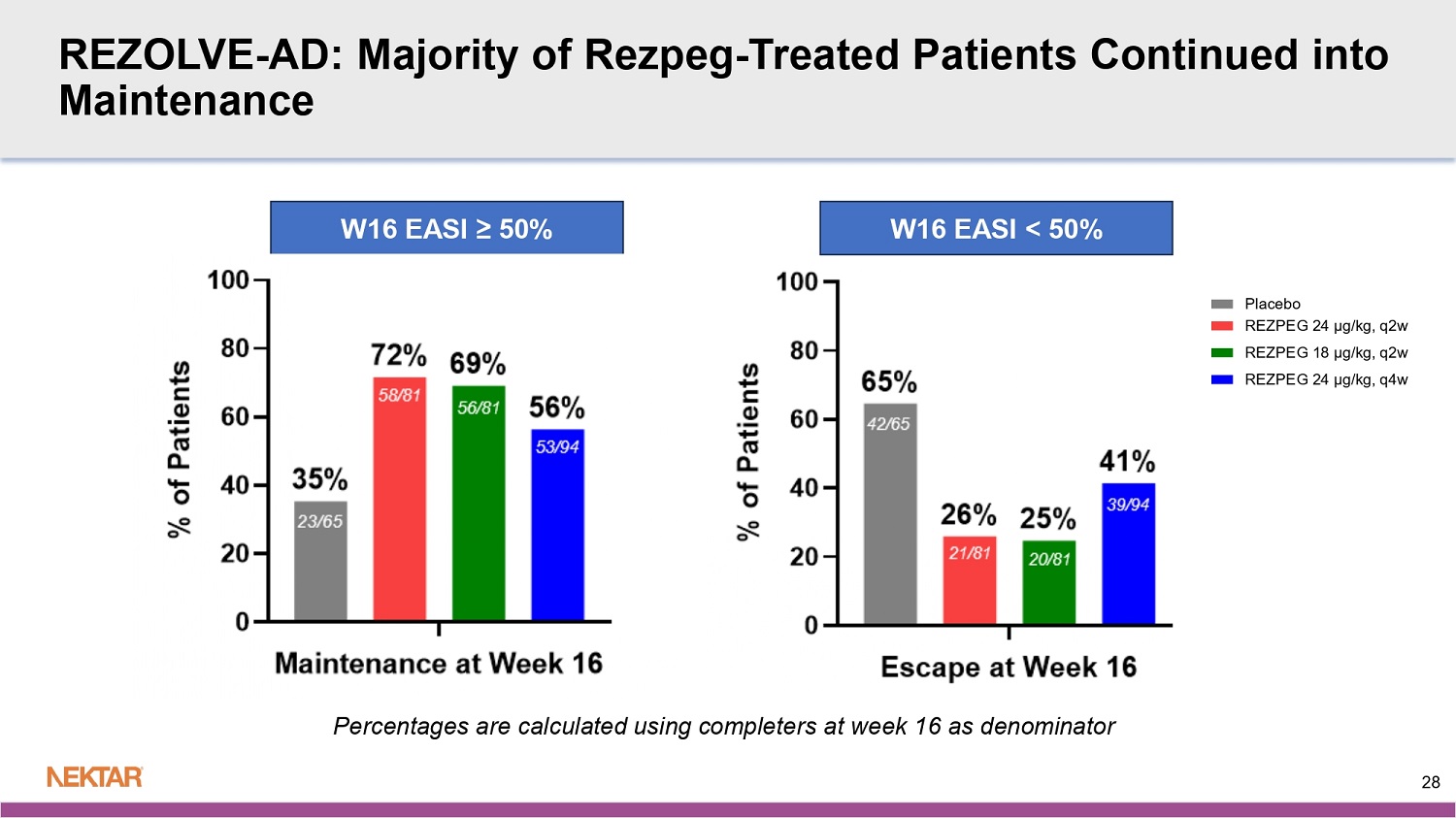
REZOLVE - AD: Baseline Disease Levels Balanced Across Arms Total N = 393 Rezpeg 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 STRATA 1 for vIGA - AD 267 (67.9%) 75 (68.2%) 70 (66.0%) 71 (68.3%) 51 (69.9%) 3 126 (32.1%) 35 (31.8%) 36 (34.0%) 33 (31.7%) 22 (30.1%) 4 EASI Total Score (0 - 72) 26.0 (9.77) 26.1 (10.45) 27.2 (10.40) 25.4 (9.14) 25.2 (8.57) Mean (SD) 23.4 22.6 23.8 23 23.5 Median 16.0, 66.2 16.1, 66.2 16.3, 62.0 16.2, 59.6 16.0, 59.1 Min, Max EASI Total Score Category I 81 (20.6%) 24 (21.8%) 16 (15.1%) 22 (21.2%) 19 (26.0%) <18 312 (79.4%) 86 (78.2%) 90 (84.9%) 82 (78.8%) 54 (74.0%) > = 18 EASI Total Score Category II 160 (40.7%) 44 (40.0%) 43 (40.6%) 44 (42.3%) 29 (39.7%) <21 233 (59.3%) 66 (60.0%) 63 (59.4%) 60 (57.7%) 44 (60.3%) > = 21 27 REZOLVE - AD: Majority of Rezpeg - Treated Patients Continued into Maintenance Percentages are calculated using completers at week 16 as denominator W16 EASI < 50% W16 EASI ≥ 50% 28
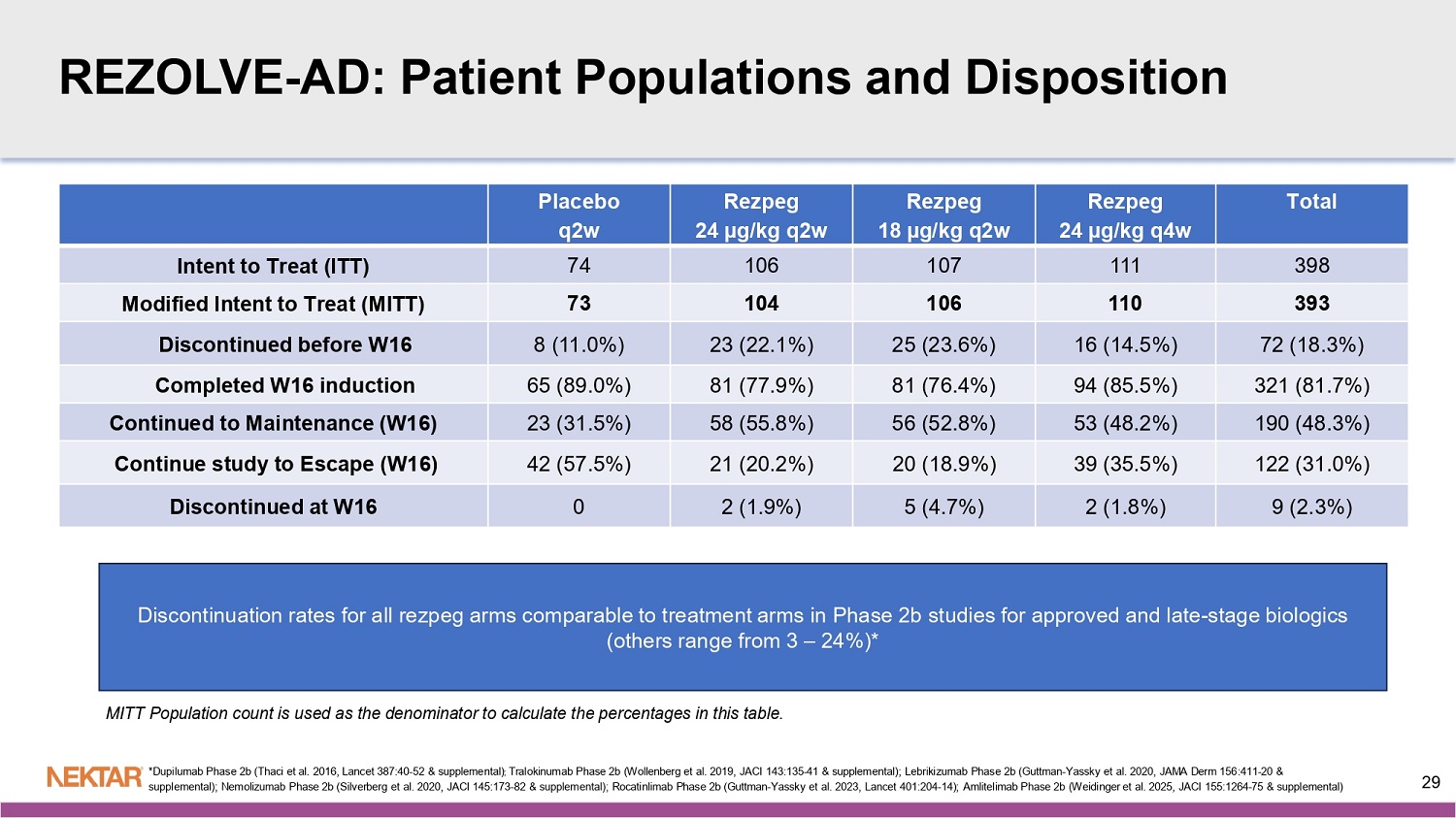
REZOLVE - AD: Patient Populations and Disposition Total Rezpeg 24 µg/kg q4w Rezpeg 18 µg/kg q2w Rezpeg 24 µg/kg q2w Placebo q2w 398 111 107 106 74 Intent to Treat (ITT) 393 110 106 104 73 Modified Intent to Treat (MITT) 72 (18.3%) 16 (14.5%) 25 (23.6%) 23 (22.1%) 8 (11.0%) Discontinued before W16 321 (81.7%) 94 (85.5%) 81 (76.4%) 81 (77.9%) 65 (89.0%) Completed W16 induction 190 (48.3%) 53 (48.2%) 56 (52.8%) 58 (55.8%) 23 (31.5%) Continued to Maintenance (W16) 122 (31.0%) 39 (35.5%) 20 (18.9%) 21 (20.2%) 42 (57.5%) Continue study to Escape (W16) 9 (2.3%) 2 (1.8%) 5 (4.7%) 2 (1.9%) 0 Discontinued at W16 MITT Population count is used as the denominator to calculate the percentages in this table. Discontinuation rates for all rezpeg arms comparable to treatment arms in Phase 2b studies for approved and late - stage biologics (others range from 3 – 24%)* *Dupilumab Phase 2b (Thaci et al. 2016, Lancet 387:40 - 52 & supplemental) ; Tralokinumab Phase 2b (Wollenberg et al. 2019, JACI 143:135 - 41 & supplemental); Lebrikizumab Phase 2b (Guttman - Yassky et al. 202 0, JAMA Derm 156:411 - 20 & supplemental); Nemolizumab Phase 2b (Silverberg et al. 2020, JACI 145:173 - 82 & supplemental); Rocatinlimab Phase 2b (Guttman - Yassky et al. 2023, Lancet 401:204 - 14); Amlitelimab Phase 2b (Weidinger et al.
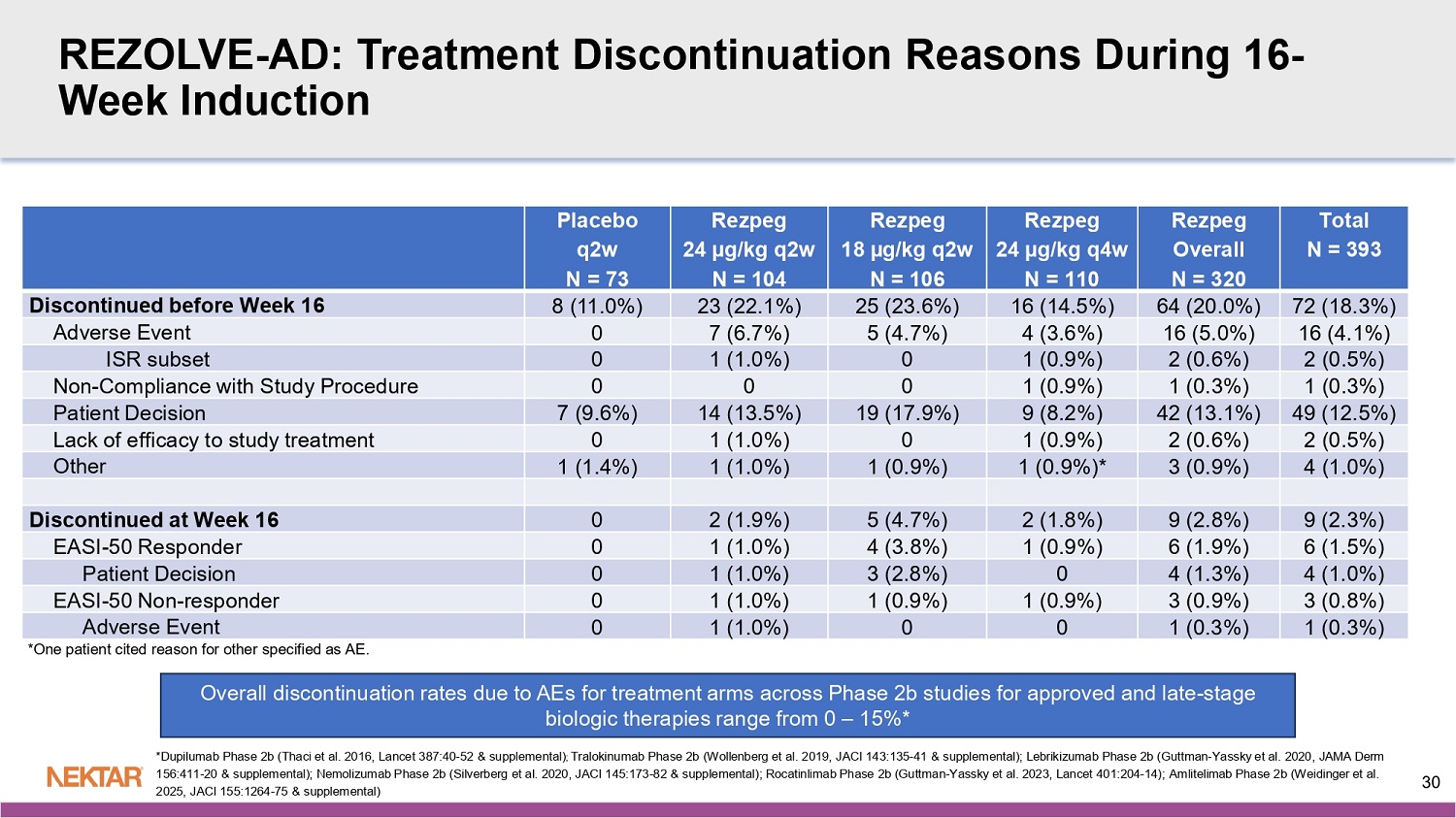
2025, JACI 155:1264 - 75 & suppl emental) 29 REZOLVE - AD: Treatment Discontinuation Reasons During 16 - Week Induction Total N = 393 Rezpeg Overall N = 320 Rezpeg 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 72 (18.3%) 64 (20.0%) 16 (14.5%) 25 (23.6%) 23 (22.1%) 8 (11.0%) Discontinued before Week 16 16 (4.1%) 16 (5.0%) 4 (3.6%) 5 (4.7%) 7 (6.7%) 0 Adverse Event 2 (0.5%) 2 (0.6%) 1 (0.9%) 0 1 (1.0%) 0 ISR subset 1 (0.3%) 1 (0.3%) 1 (0.9%) 0 0 0 Non - Compliance with Study Procedure 49 (12.5%) 42 (13.1%) 9 (8.2%) 19 (17.9%) 14 (13.5%) 7 (9.6%) Patient Decision 2 (0.5%) 2 (0.6%) 1 (0.9%) 0 1 (1.0%) 0 Lack of efficacy to study treatment 4 (1.0%) 3 (0.9%) 1 (0.9%)* 1 (0.9%) 1 (1.0%) 1 (1.4%) Other 9 (2.3%) 9 (2.8%) 2 (1.8%) 5 (4.7%) 2 (1.9%) 0 Discontinued at Week 16 6 (1.5%) 6 (1.9%) 1 (0.9%) 4 (3.8%) 1 (1.0%) 0 EASI - 50 Responder 4 (1.0%) 4 (1.3%) 0 3 (2.8%) 1 (1.0%) 0 Patient Decision 3 (0.8%) 3 (0.9%) 1 (0.9%) 1 (0.9%) 1 (1.0%) 0 EASI - 50 Non - responder 1 (0.3%) 1 (0.3%) 0 0 1 (1.0%) 0 Adverse Event Overall discontinuation rates due to AEs for treatment arms across Phase 2b studies for approved and late - stage biologic therapies range from 0 – 15%* *Dupilumab Phase 2b (Thaci et al. 2016, Lancet 387:40 - 52 & supplemental) ; Tralokinumab Phase 2b (Wollenberg et al. 2019, JACI 143:135 - 41 & supplemental); Lebrikizumab Phase 2b (Guttman - Yassky et al. 202 0, JAMA Derm 156:411 - 20 & supplemental); Nemolizumab Phase 2b (Silverberg et al. 2020, JACI 145:173 - 82 & supplemental); Rocatinlimab Phase 2b (Guttman - Yassky et al. 2023, Lancet 401:204 - 14); Amlitelimab Phase 2b (Weidinger et al. 2025, JACI 155:1264 - 75 & supplemental) 30 *One patient cited reason for other specified as AE.

Overall Summary of Treatment Emergent Adverse Events 16 - Week Induction Period Rezpeg Total N = 320 Rezpeg , 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 257 (80.3%) 90 (81.8%) 78 (73.6%) 89 (85.6%) 42 (57.5%) Patients With at Least One TEAE 193 (60.3%) 64 (58.2%) 60 (56.6%) 69 (66.3%) 42 (57.5%) Patients With at Least One TEAE (Excluding ISRs) 5 (1.6%) 0 4 (3.8%) 1 (1.0%) 0 Patients With at Least One Serious TEAE 10 (3.1%) 1 (0.9%) 6 (5.7%) 3 (2.9%) 1 (1.4%) Patients With at Least One Severe TEAE 0 0 0 0 0 Patients With at Least One TEAE Leading to Death* TEAEs by System Organ Class and Preferred Term Over ≥ 5% in Any Arm 225 (70.3%) 78 (70.9%) 67 (63.2%) 80 (76.9%) 7 (9.6%) General disorders and administration site conditions 223 (69.7%) 78 (70.9%) 66 (62.3%) 79 (76.0%) 3 (4.1%) Injection site reaction 68.3% 69.9% 70.7% 65.5% 100% Proportion of ISR events - mild (%) 31.3% 30.1% 28.9% 33.9% 0% Proportion of ISR events - moderate (%) 0.4% 0% 0.4% 0.6% 0% Proportion of ISR events - severe (%) 20 (6.3%) 4 (3.6%) 5 (4.7%) 11 (10.6%) 2 (2.7%) Pyrexia 100 (31.3%) 32 (29.1%) 39 (36.8%) 29 (27.9%) 25 (34.2%) Infections and infestations 38 (11.9%) 14 (12.7%) 14 (13.2%) 10 (9.6%) 10 (13.7%) Nasopharyngitis 19 (5.9%) 4 (3.6%) 8 (7.5%) 7 (6.7%) 4 (5.5%) Upper respiratory tract infection 46 (14.4%) 11 (10.0%) 6 (5.7%) 29 (27.9%) 3 (4.1%) Blood and lymphatic system disorders 25 (7.8%) 4 (3.6%) 4 (3.8%) 17 (16.3%) 2 (2.7%) Eosinophilia 11 (3.4%) 3 (2.7%) 1 (0.9%) 7 (6.7%) 0 Lymphadenopathy 35 (10.9%) 11 (10.0%) 5 (4.7%) 19 (18.3%) 3 (4.1%) Musculoskeletal and connective tissue disorders 16 (5.0%) 4 (3.6%) 2 (1.9%) 10 (9.6%) 1 (1.4%) Arthralgia 35 (10.9%) 13 (11.8%) 10 (9.4%) 12 (11.5%) 8 (11.0%) Skin and subcutaneous tissue disorders 13 (4.1%) 6 (5.5%) 5 (4.7%) 2 (1.9%) 7 (9.6%) Worsening atopic dermatitis 29 (9.1%) 9 (8.2%) 10 (9.4%) 10 (9.6%) 6 (8.2%) Nervous system disorders 20 (6.3%) 6 (5.5%) 6 (5.7%) 8 (7.7%) 3 (4.1%) Headache 26 (8.1%) 11 (10.0%) 7 (6.6%) 8 (7.7%) 3 (4.1%) Gastrointestinal disorders 16 (5.0%) 5 (4.5%) 5 (4.7%) 6 (5.8%) 1 (1.4%) Respiratory, thoracic and mediastinal disorders 13 (4.1%) 3 (2.7%) 4 (3.8%) 6 (5.8%) 1 (1.4%) Investigations 31 *Following 16 - week induction, one death in a 38 y/o female occurred in the escape arm due to coronary thrombosis/heart failure. Patient had multiple, overlapping pre - existing cardiovascular risk factors. The death was assessed as unrelated to study treatment by the Sponsor Drug Safety Committee and independent external experts.
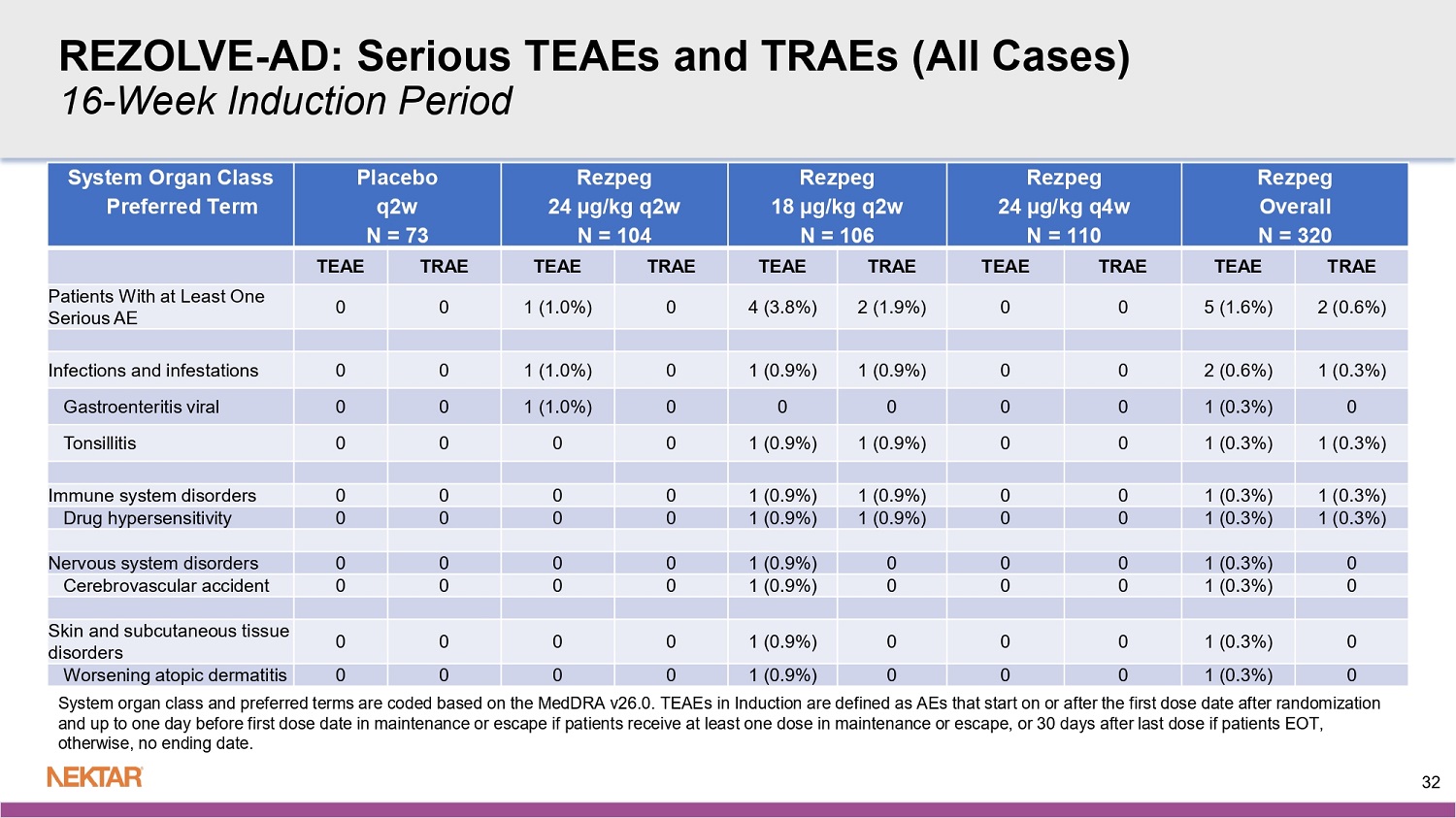
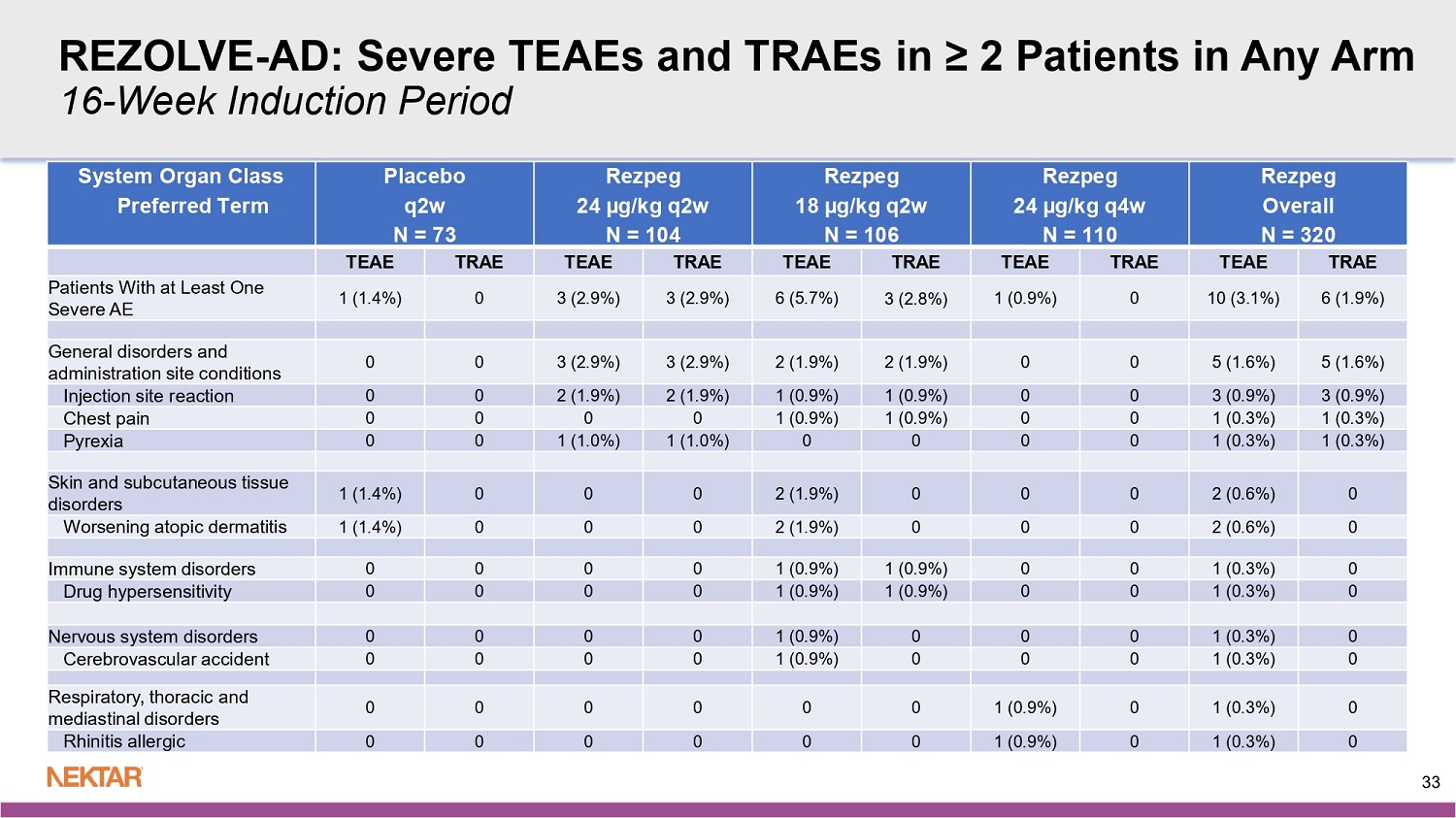
REZOLVE - AD: Serious TEAEs and TRAEs (All Cases) 16 - Week Induction Period Rezpeg Overall N = 320 Rezpeg 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 System Organ Class Preferred Term TRAE TEAE TRAE TEAE TRAE TEAE TRAE TEAE TRAE TEAE 2 (0.6%) 5 (1.6%) 0 0 2 (1.9%) 4 (3.8%) 0 1 (1.0%) 0 0 Patients With at Least One Serious AE 1 (0.3%) 2 (0.6%) 0 0 1 (0.9%) 1 (0.9%) 0 1 (1.0%) 0 0 Infections and infestations 0 1 (0.3%) 0 0 0 0 0 1 (1.0%) 0 0 Gastroenteritis viral 1 (0.3%) 1 (0.3%) 0 0 1 (0.9%) 1 (0.9%) 0 0 0 0 Tonsillitis 1 (0.3%) 1 (0.3%) 0 0 1 (0.9%) 1 (0.9%) 0 0 0 0 Immune system disorders 1 (0.3%) 1 (0.3%) 0 0 1 (0.9%) 1 (0.9%) 0 0 0 0 Drug hypersensitivity 0 1 (0.3%) 0 0 0 1 (0.9%) 0 0 0 0 Nervous system disorders 0 1 (0.3%) 0 0 0 1 (0.9%) 0 0 0 0 Cerebrovascular accident 0 1 (0.3%) 0 0 0 1 (0.9%) 0 0 0 0 Skin and subcutaneous tissue disorders 0 1 (0.3%) 0 0 0 1 (0.9%) 0 0 0 0 Worsening atopic dermatitis System organ class and preferred terms are coded based on the MedDRA v26.0. TEAEs in Induction are defined as AEs that start on or after the first dose date after randomization and up to one day before first dose date in maintenance or escape if patients receive at least one dose in maintenance or esc ape , or 30 days after last dose if patients EOT, otherwise, no ending date. 32 REZOLVE - AD: Severe TEAEs and TRAEs in ≥ 2 Patients in Any Arm 16 - Week Induction Period Rezpeg Overall N = 320 Rezpeg 24 µg/kg q4w N = 110 Rezpeg 18 µg/kg q2w N = 106 Rezpeg 24 µg/kg q2w N = 104 Placebo q2w N = 73 System Organ Class Preferred Term TRAE TEAE TRAE TEAE TRAE TEAE TRAE TEAE TRAE TEAE 6 (1.9%) 10 (3.1%) 0 1 (0.9%) 3 (2.8%) 6 (5.7%) 3 (2.9%) 3 (2.9%) 0 1 (1.4%) Patients With at Least One Severe AE 5 (1.6%) 5 (1.6%) 0 0 2 (1.9%) 2 (1.9%) 3 (2.9%) 3 (2.9%) 0 0 General disorders and administration site conditions 3 (0.9%) 3 (0.9%) 0 0 1 (0.9%) 1 (0.9%) 2 (1.9%) 2 (1.9%) 0 0 Injection site reaction 1 (0.3%) 1 (0.3%) 0 0 1 (0.9%) 1 (0.9%) 0 0 0 0 Chest pain 1 (0.3%) 1 (0.3%) 0 0 0 0 1 (1.0%) 1 (1.0%) 0 0 Pyrexia 0 2 (0.6%) 0 0 0 2 (1.9%) 0 0 0 1 (1.4%) Skin and subcutaneous tissue disorders 0 2 (0.6%) 0 0 0 2 (1.9%) 0 0 0 1 (1.4%) Worsening atopic dermatitis 0 1 (0.3%) 0 0 1 (0.9%) 1 (0.9%) 0 0 0 0 Immune system disorders 0 1 (0.3%) 0 0 1 (0.9%) 1 (0.9%) 0 0 0 0 Drug hypersensitivity 0 1 (0.3%) 0 0 0 1 (0.9%) 0 0 0 0 Nervous system disorders 0 1 (0.3%) 0 0 0 1 (0.9%) 0 0 0 0 Cerebrovascular accident 0 1 (0.3%) 0 1 (0.9%) 0 0 0 0 0 0 Respiratory, thoracic and mediastinal disorders 0 1 (0.3%) 0 1 (0.9%) 0 0 0 0 0 0 Rhinitis allergic 33
Atopic Dermatitis Presents Potential Multi - Billion Dollar Market Opportunity Still High Unmet Need, Especially For New Therapies With Potential for Remittive Effect ~30 million 1 Adults with AD in U.S. ~220 million 2 Adults with AD globally ~50% 3 Adults with AD have moderate - to - severe disease DUPIXENT® is a registered trademarks of Sanofi Biotechnology. Source: 1 Eczema stats. National Eczema Association. (2022, September 27). https://nationaleczema.org/research/eczema - facts/ ; 2 Eczema council. (n.d.). https://www.eczemacouncil.org/assets/docs/global - report - on - atopic - dermatitis - 2022.pdf ; 3 Clarivate TM DRG Mature Markets Data 2023.; 4 DRG Epidemiology; 5 N Engl J Med 2016; 375:2335 - 2348 DOI: 10.1056/NEJMoa1610020; 6 Evaluate Ltd (accessed: 1/6/2025) Atopic dermatitis (AD) is a chronic autoimmune condition that causes inflammation, redness and irritation of the skin. Moderate - to - severe AD is associated with unbearable itching that can result in significant negative impact to quality of life.
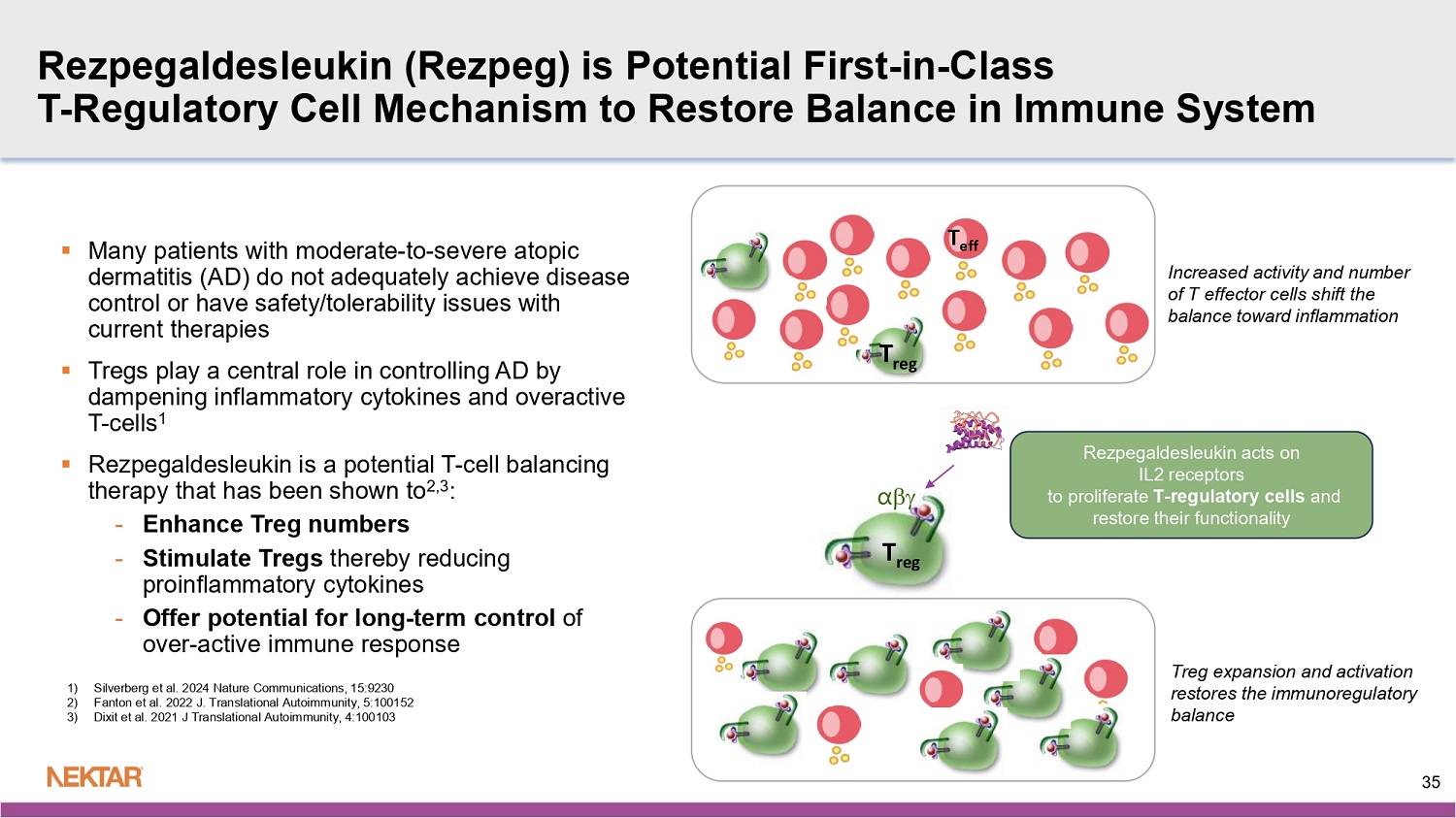
Dupixent ® : current market leader in atopic dermatitis exceeding $10.5B in annual sales, but 50% of patients fail on therapy 5, 6 We believe ther e is a h igh unmet need for new mechanism of action with potential to : • Offer dosing schedules without rebound effect • Induce deep and potentially therapy - free remission • Favorable safety and tolerability profile for ease of use ~8% 4 Patients with moderate/severe AD are treated with a biologic 34 Rezpegaldesleukin ( Rezpeg ) is Potential First - in - Class T - Regulatory Cell Mechanism to Restore Balance in Immune System 35 Increased activity and number of T effector cells shift the balance toward inflammation T reg Treg expansion and activation restores the immunoregulatory balance T reg T eff ▪ Many patients with moderate - to - severe atopic dermatitis (AD) do not adequately achieve disease control or have safety/tolerability issues with current therapies ▪ Tregs play a central role in controlling AD by dampening inflammatory cytokines and overactive T - cells 1 ▪ Rezpegaldesleukin is a potential T - cell balancing therapy that has been shown to 2,3 : - Enhance Treg numbers - Stimulate Tregs thereby reducing proinflammatory cytokines - Offer potential for long - term control of over - active immune response α 1) Silverberg et al. 2024 Nature Communications, 15:9230 2) Fanton et al. 2022 J. Translational Autoimmunity, 5:100152 3) Dixit et al. 2021 J Translational Autoimmunity, 4:100103 Rezpegaldesleukin acts on IL2 receptors to proliferate T - regulatory cells and restore their functionality