UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 31, 2025
| BiomX Inc. |
| (Exact Name of Registrant as Specified in its Charter) |
| Delaware | 001-38762 | 82-3364020 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (I.R.S. Employer Identification No.) |
|
22 Einstein St., Floor 4 Ness Ziona, Israel |
7414003 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: +972 723942377
| n/a |
| (Former name or former address, if changed since last report) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value | PHGE | NYSE American |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On March 31, 2025, BiomX Inc., or the Company, issued a press release announcing positive results from its Phase 2 Trial evaluating BX211 for the treatment of Diabetic Foot Osteomyelitis, or DFO, a copy of which is furnished as Exhibit 99.1. In addition, on March 31, 2025, the Company posted an updated corporate slide presentation in the “Investors” portion of its website at www.biomx.com. A copy of the slide presentation is furnished as Exhibit 99.2 hereto. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
Item 8.01 Other Events.
As disclosed above, on March 31, 2025, the Company announced positive results from Phase 2 Trial Evaluating BX211 for the treatment of DFO, or the Phase 2 Trial.
The Phase 2 Trial is a randomized, double-blind, placebo-controlled, multi-center study investigating the safety, tolerability, and efficacy of BX211 for individuals with DFO associated with S. aureus. The Phase 2 Trial enrolled a total of 41 patients randomized for treatment at a 2:1 ratio, 26 of whom received intravenous (IV) and topical administration of BX211 on week 1 followed by a topical weekly dose through week 12, while 15 patients were assigned to the placebo arm. Over the 12-week treatment period, all subjects (treatment and placebo) were also treated in accordance with standard of care, including with systemic antibiotic therapy as appropriate. A readout of study results at Week 13 evaluated healing of the wound associated with osteomyelitis. The primary efficacy endpoint was Percent Area Reduction, or PAR, of study ulcer through week 13. Study design was guided in part by experience with numerous compassionate cases using phage therapy for the treatment of DFO and osteomyelitis.
The topline Phase 2 Trial results included:
| ● | BX211 was found to be safe and well-tolerated. |
| ● | BX211 produced sustained and statistically significant1 PAR of ulcer size, (p = 0.046 at week 12; p=0.052 at week 13), with a separation from placebo (standard of care) starting at week 7 and a difference greater than 40% by week 10. |
| ● | BX211 produced statistically significant1 improvements in both ulcer depth at week 13 (in patients with ulcer depth defined as bone at baseline) (p=0.048), and in reducing the expansion of ulcer area (p=0.017), compared to placebo. |
| ● | BX211 demonstrated favorable trends compared to placebo across several additional clinical parameters, including: proportion of visits with no clinical evidence of infection; evidence of resolving DFO by MRI/X-ray at week 12; proportion of patients with abnormal C-Reactive Protein at baseline that achieved a reduction of CRP of at least 50% at any point in the study; and greater Wagner scale improvement2. |
| ● | Through week 13, BX211 demonstrated comparable efficacy against both Methicillin susceptible and resistant strains, as well as against high and low biofilm producers—consistent with the orthogonal mechanism of phage therapy to antibiotics and its inherent anti-biofilm capabilities. |
| 1 | All p-values described in this Form 8-K are non-adjusted. |
| 2 | The Wagner Scale is a clinical grading system used to classify the severity of diabetic foot ulcers, ranging from 0 (intact skin) to 5 (extensive gangrene). |
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit | Description | |
| 99.1 | Press Release dated March 31, 2025, titled “BiomX Announces Positive Results from Phase 2 Trial Evaluating BX211 for the Treatment of Diabetic Foot Osteomyelitis (DFO)” (furnished herewith) | |
| 99.2 | Investor Presentation Deck dated February 26, 2025 (furnished herewith) | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL documents) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| BIOMX INC. | |||
| March 31, 2025 | By: | /s/ Jonathan Solomon | |
| Name: | Jonathan Solomon | ||
| Title: | Chief Executive Officer | ||
Exhibit 99.1
BiomX Announces Positive Topline Results from Phase 2 Trial Evaluating BX211 for the Treatment of Diabetic Foot Osteomyelitis (DFO)
| ● | BX211 was safe and well-tolerated |
| ● | BX211 produced sustained and statistically significant1 Percent Area Reduction (PAR) of ulcer size (p = 0.046 at week 12; p=0.052 at week 13), with a separation from placebo starting at week 7 and a difference greater than 40% by week 10 |
| ● | Compared to placebo, BX211 also produced statistically significant1 improvements in both ulcer depth at week 13 (in patients with ulcer depth defined as bone at baseline) (p=0.048), and in reducing the expansion of ulcer area (p=0.017). |
| ● | BiomX is planning for a Phase 2/3 trial of BX211, pending U.S. Food and Drug Administration (FDA) feedback |
The Company will host a conference call and webcast today at 9:00 AM ET, followed by a Key Opinion Leader (KOL) event on April 3, 2025, at 11:00 AM ET to discuss the results
NESS ZIONA, Israel, March 31, 2025 (GLOBE NEWSWIRE) -- BiomX Inc. (NYSE American: PHGE) (“BiomX” or the “Company”), a clinical-stage company advancing novel natural and engineered phage therapies that target specific pathogenic bacteria, today announced positive, topline safety and efficacy results from the Company’s DFO Adaptive Novel Care Evaluation (DANCE™) Phase 2 trial evaluating its BX211 phage treatment for DFO associated with Staphylococcus aureus (S. aureus). The findings demonstrated BX211 to be safe and well-tolerated and that patients receiving BX211 exhibited statistically significant1 and sustained reduction of ulcer size (PAR)(p = 0.046 at week 12; p=0.052 at week 13), with a separation from placebo starting at week 7 and a difference greater than 40% by week 10. In addition, BX211 also produced statistically significant1 improvements in both ulcer depth at week 13 (in patients with ulcer depth defined as bone at baseline, ulcer depth was classified according to deepest tissue involved as measured by swab) (p=0.048), and in reducing the expansion of ulcer area (p=0.017). Over the 12-week treatment period, all patients (treatment and placebo) were treated in accordance with standard of care, including with systemic antibiotic therapy as appropriate. Following the successful Phase 2 readout of BX211, the Company is planning for a Phase 2/3 trial, pending discussions and feedback from the U.S Food and Drug Administration.
| 1 | All p-values described in this release are non-adjusted |
| 2 | The Wagner Scale is a clinical grading system used to classify the severity of diabetic foot ulcers, ranging from 0 (intact skin) to 5 (extensive gangrene). |
“We believe these data represent one of the strongest demonstrations to date of the therapeutic potential of phage therapy. We are grateful to all the patients who participated, and the treating teams who enrolled patients into the study, as well as the continued and ongoing support from the U.S. Defense Health Agency (DHA) for this program,” said Jonathan Solomon, BiomX’s Chief Executive Officer. “Today, 30-40% of DFO cases lead to lower extremity amputations related to serious bacterial infections, accounting for the majority of the 160,000 lower limb amputations in diabetic patients each year in the United States. Based on the results announced today, we believe BiomX’s novel phage therapy approach has the potential to help address the major unmet need in DFO. Moreover, in an era of modern conflict and rising antibiotic-resistant wounds, the need for innovative wound care solutions underscores the broader relevance of this program beyond DFO. BiomX is dedicated to the advancement of phage therapy, which we believe holds promise in redefining the treatment of chronic infections.”
“Phage therapy has a critical role to play in treating infections where antibiotic resistance has emerged or existing treatments have underperformed,” said Dr. Robert T. “Chip” Schooley, M.D., Distinguished Professor of Medicine, Division of Infectious Diseases and Global Public Health and Co-Director, Center for Innovative Phage Applications and Therapeutics at the University of California, San Diego. “The promising topline data in this trial provide an important inflection point for this approach and its potential to address the most challenging infections.”
“Diabetic foot infections are often a complex and difficult-to-treat consequence of diabetes, leading to serious adverse effects on patient quality of life,” said Dr. Benjamin A. Lipsky, M.D., FACP, FIDSA, FRCP (London), FRCPS (Glasgow), Professor of Medicine Emeritus at University of Washington, Seattle. “The most serious and feared complication of DFO is lower extremity amputation, which is associated with a five-year mortality rate of about 50%. With the progress seen so far and given the improved ulcer healing seen in this study, BX211 may have the potential to reduce amputations. BX211 is a program to watch closely as it progresses into more advanced clinical studies.”
Summary of Phase 2 BX211 Results
BiomX’s Phase 2 trial is a randomized, double-blind, placebo-controlled, multi-center study investigating the safety, tolerability, and efficacy of BX211 for individuals with DFO associated with S. aureus. The study enrolled a total of 41 patients randomized for treatment at a 2:1 ratio, 26 of whom received intravenous (IV) and topical administration of BX211 on week 1 followed by a topical weekly dose through week 12, while 15 patients were assigned to the placebo arm. Over the 12-week treatment period, all subjects (treatment and placebo) were also treated in accordance with standard of care, including with systemic antibiotic therapy as appropriate. A readout of study results at week 13 evaluated healing of the wound associated with osteomyelitis. The primary efficacy endpoint was PAR of study ulcer through week 13. Study design was guided in part by experience with numerous compassionate cases using phage therapy for the treatment of DFO and osteomyelitis.
The topline Phase 2 results included:
| ● | BX211 was found to be safe and well-tolerated. |
| ● | BX211 produced sustained and statistically significant1 PAR of ulcer size (p = 0.046 at week 12; p=0.052 at week 13), with a separation from placebo (standard of care) starting at week 7 and a difference greater than 40% by week 10. |
| ● | BX211 produced statistically significant1 improvements in both ulcer depth at week 13 (in patients with ulcer depth defined as bone at baseline) (p=0.048), and in reducing the expansion of ulcer area (p=0.017), compared to placebo. |
| ● | BX211 demonstrated favorable trends compared to placebo across several additional clinical parameters, including: proportion of visits with no clinical evidence of infection; evidence of resolving DFO by MRI/X-ray at week 12; proportion of patients with abnormal C-Reactive Protein at baseline that achieved a reduction of CRP of at least 50% at any point in the study; and greater Wagner scale improvement2. |
| ● | Through week 13, BX211 demonstrated comparable efficacy against both Methicillin-susceptible and resistant strains, as well as against high and low biofilm producers—consistent with the orthogonal mechanism of phage therapy to antibiotics and its inherent anti-biofilm capabilities. |
BiomX expects to present additional data from the Phase 2 study at upcoming scientific conferences.
Today’s Conference Call and Webcast Information
BiomX management will host a conference call and webcast today at 9:00 AM ET to review the topline Phase 2 trial results, accompanied by a slide deck presentation, which will be available on the Company’s website and filed via Form 8-K. To participate in the conference, please dial +877-407-0724 (U.S.), or +1 201-389-0898 (International), or click on the webcast link here.
A live and archived webcast of the call will also be available on the Investors section of the Company’s website at www.biomx.com.
BiomX to Host Virtual KOL Event – April 3, 2025
The Company has scheduled a virtual KOL Event to discuss the topline results from the Phase 2 trial. The event will take place on April 3, 2025, at 11:00 am ET, and will include participation from BiomX senior management and two KOLs, Dr. Robert T. “Chip” Schooley, M.D., Distinguished Professor of Medicine, Division of Infectious Diseases and Global Public Health and Co-Director, Center for Innovative Phage Applications and Therapeutics at the University of California, San Diego, and Dr. Benjamin A. Lipsky, M.D., FIDSA, FRCP (London), FRCPS (Glasgow) Professor of Medicine Emeritus at University of Washington, Seattle. To register for the event, please click here.
About BX211
BX211 is a phage treatment for the treatment of DFO associated with S. aureus. DFO is a bacterial infection of the bone that usually develops from an infected foot ulcer and is a leading cause of amputation in patients with diabetes. Pending feedback from the FDA, BiomX is planning for a Phase 2/3 clinical trial of BX211.
About BiomX
BiomX is a clinical-stage company leading the development of natural and engineered phage cocktails and personalized phage treatments designed to target and destroy harmful bacteria for the treatment of chronic diseases with substantial unmet needs. BiomX discovers and validates proprietary bacterial targets and applies its BOLT (“BacteriOphage Lead to Treatment”) platform to customize phage compositions against these targets. For more information, please visit www.biomx.com, the content of which does not form a part of this press release.
Safe Harbor
This press release contains express or implied “forward-looking statements” within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. For example, when BiomX refers to the potential safety and toleration of BX211, the potential benefits of BX211, future clinical development of BX211 and the relevance and potential of phage therapy in the treatment of chronic infections, it is using forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on BiomX management’s current beliefs, expectations and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of BiomX’s control. These risks and uncertainties include, but are not limited to, changes in applicable laws or regulations; the possibility that BiomX may be adversely affected by other economic, business, and/or competitive factors, including risks inherent in pharmaceutical research and development, such as: adverse results in BiomX’s drug discovery, preclinical and clinical development activities, the risk that the results of preclinical studies and early clinical trials may not be replicated in later clinical trials, BiomX’s ability to enroll patients in its clinical trials, and the risk that any of its clinical trials may not commence, continue or be completed on time, or at all; decisions made by the FDA and other regulatory authorities; investigational review boards at clinical trial sites and publication review bodies with respect to our development candidates; BiomX’s ability to obtain, maintain and enforce intellectual property rights for its platform and development candidates; its potential dependence on collaboration partners; competition; uncertainties as to the sufficiency of BiomX’s cash resources to fund its planned activities for the periods anticipated and BiomX’s ability to manage unplanned cash requirements; and general economic and market conditions. Therefore, investors should not rely on any of these forward-looking statements and should review the risks and uncertainties described under the caption “Risk Factors” in BiomX’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 25, 2025, and additional disclosures BiomX makes in its other filings with the SEC, which are available on the SEC’s website at www.sec.gov. Forward-looking statements are made as of the date of this press release, and except as provided by law BiomX expressly disclaims any obligation or undertaking to update forward-looking statements.
Contacts:
BiomX Inc.
Ben Cohen
Head Corporate Communications
benc@biomx.com
Exhibit 99.2


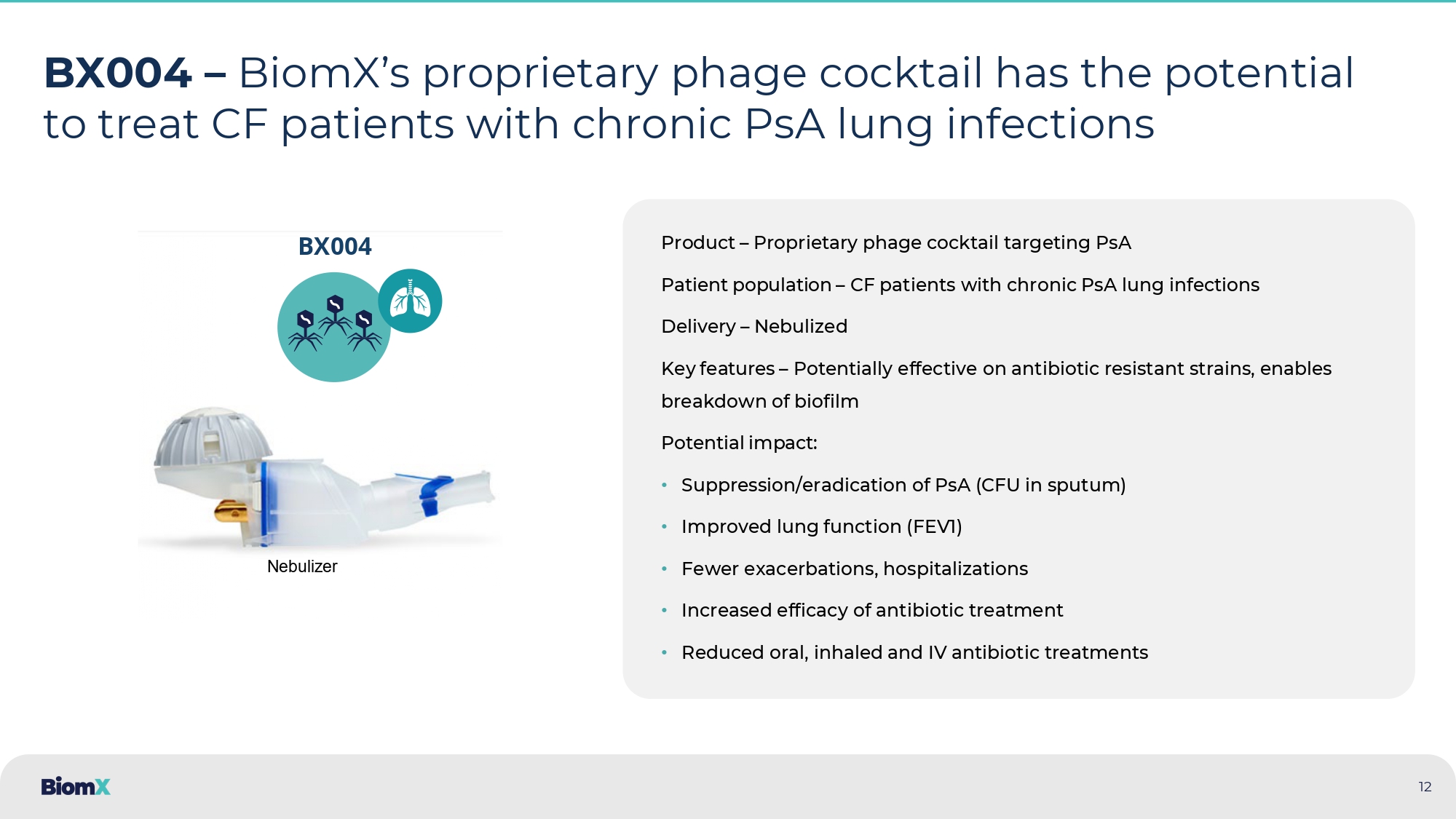
INVESTOR PRESENTATION March 2025 Revolutionizing the Treatment of Infections Associated with Chronic Disease Through Phage Therapy NYSE American: PHGE 1 SAFE HARBOR STATEMENT About this Presentation The information contained in this presentation has been prepared by BiomX Inc. and its subsidiaries (collectively, the “Company” or “ BiomX ”) and contains information pertaining to the business and operations of the Company. The information contained in this presentation is current only as of the date on its cover. For an y t ime after the cover date of this presentation, the information, including information concerning our business, financial condition, results of operations and prospects, may have changed. The delivery of this presentation shall not, under any circumstances, create any implication that there have been no changes in our affairs after the date of this presentation. We have not authorized any pe rso n to give any information or to make any representations about us in connection with this presentation that is not contained herein. If any information has been or is given or any representation s h ave been or are made to you outside of this presentation, such information or representations should not be relied upon as having been authorized by us. Forward - Looking Statements This presentation contains certain “forward - looking statements” within the meaning of the “safe harbor” provisions of the U.S. P rivate Securities Litigation Reform Act of 1995. Forward - looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would ,” “positioned,” “future,” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. Forward - looking statements are neither historical facts nor a ssurances of future performance. Instead, they are based only on BiomX management’s current beliefs, expectations and assumptions. For example, when we discuss future potential clinical trials, in clu ding their design, objectives, costs, endpoints, potential benefits and timing thereof, the potential outcomes of discussions that we may have with the U.S. Food and Drug Administration and foreign re gulatory agencies, potential commercial opportunities, our financial needs to fund future clinical trials, forecasted expenses and our ability to protect our intellectual property assets in the fut ure we are making forward - looking statements. In addition, past and current pre - clinical and clinical results, as well as compassionate use, are not indicative and do not guarantee future success of BiomX clinical trials. Because forward - looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of whic h a re outside of our control. Actual results and outcomes may differ materially from those indicated in the forward - looking statements. Therefore, you should not rely on any of these forward - lookin g statements. You should review additional disclosures we make in our filings with the Securities and Exchange Commission (the “SEC”), which are available on the SEC’s website at www.sec.gov. Exc ept as required by law, we are under no duty to (and expressly disclaim any such obligation to) update or revise any of the forward - looking statements, whether as a result of new information, future e vents or otherwise. No Offer or Solicitation This presentation is for informational purposes only. Nothing in this presentation constitutes an offer to buy or sell or a s oli citation of an offer to buy or sell investments, loans, securities, partnership interests, commodities or any other financial instruments. This presentation and any oral statements made in connection with thi s presentation do not constitute and may not be used for or in connection with, an offer or solicitation by anyone in any state or jurisdiction in which such an offer or solicitation is no t a uthorized or permitted, or to any person to whom it is unlawful to make such offer or solicitation. Trademarks and Service Marks The trademarks and service marks included herein are the property of the owners thereof and are used for reference purposes o nly . Such use should not be construed as an endorsement of such products. FDA This presentation concerns certain products that are under clinical investigation and which have not yet been cleared for mar ket ing by the U.S. Food and Drug Administration. These products are currently limited by federal law to investigational use, and no representation is made as to the safety or effectiveness of t hes e products for the purposes for which they are being investigated. 2 INVESTOR PRESENTATION 3 AT - A - GLANCE Company Pipeline Highlights Unmet need Partners Key Investors Clinical stage biotech harnessing the therapeutic potential of phage therapy • BX004 for CF – Positive results in P1b/2a study.

P2b results expected in Q1 2026 • BX211 for moderate - severe DFO & DFI – Positive results in P2 study, Preparing for Phase 2/3 Treatment of underlying persistent infections in chronic diseases that become harder to treat as antibiotic - resistant pathogens emerge • Pulmonary infections in Cystic Fibrosis (CF) and Non - Cystic Fibrosis Bronchiectasis (NCFB) patients are the primary causes of death • 20 % - 40 % of Severe to moderate Diabetic Foot Infections (DFI) & Diabetic Foot Osteomyelitis (DFO) cases result in amputation due to bacterial infection 4 OUR SCIENCE PHAGE THERAPIES

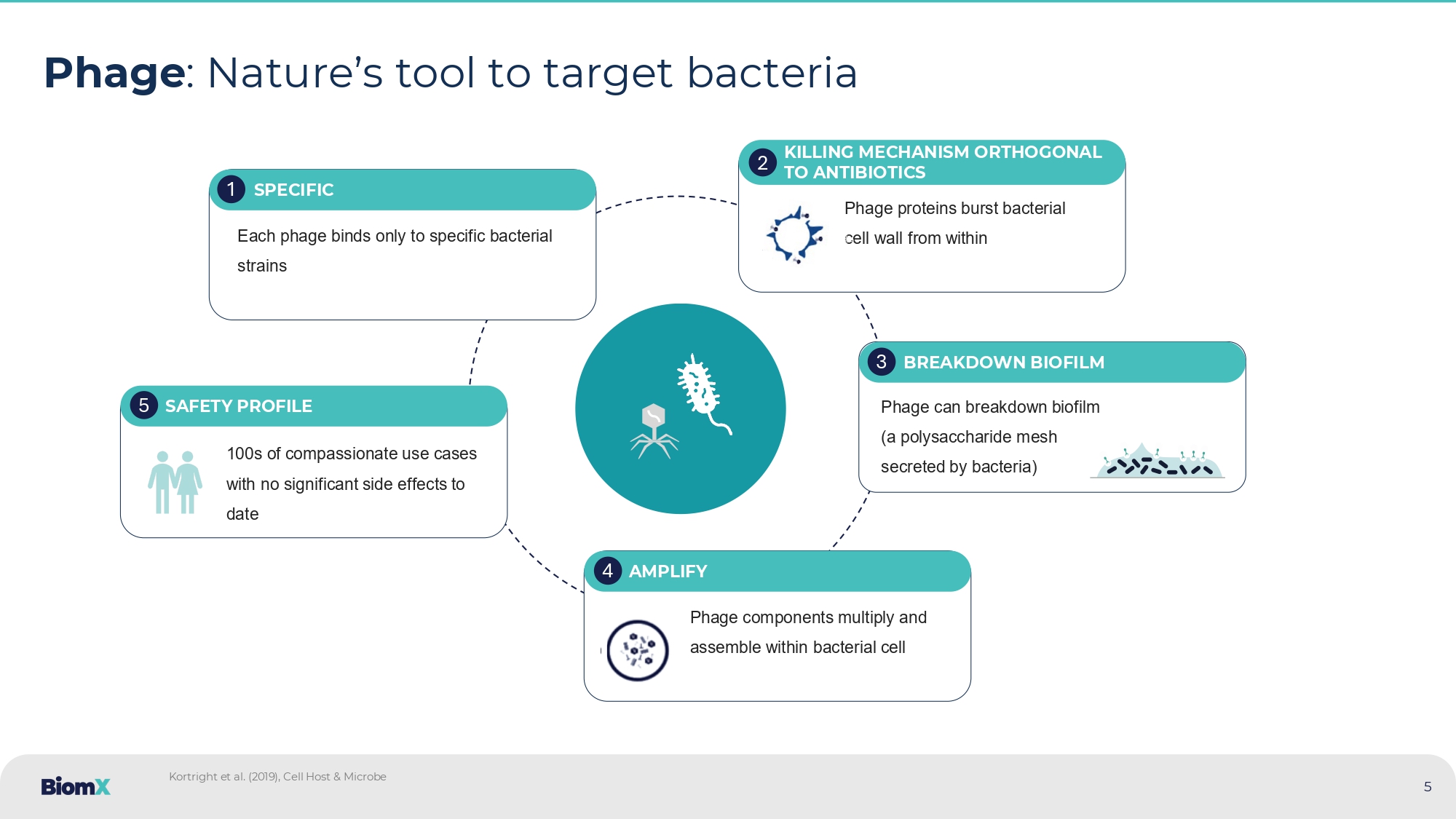
Phage : Nature’s tool to target bacteria Each phage binds only to specific bacterial strains SPECIFIC 1 Phage proteins burst bacterial cell wall from within KILLING MECHANISM ORTHOGONAL TO ANTIBIOTICS 2 Phage can breakdown biofilm (a polysaccharide mesh secreted by bacteria) BREAKDOWN BIOFILM 3 Phage components multiply and assemble within bacterial cell AMPLIFY 4 100 s of compassionate use cases with no significant side effects to date SAFETY PROFILE 5 Kortright et al.
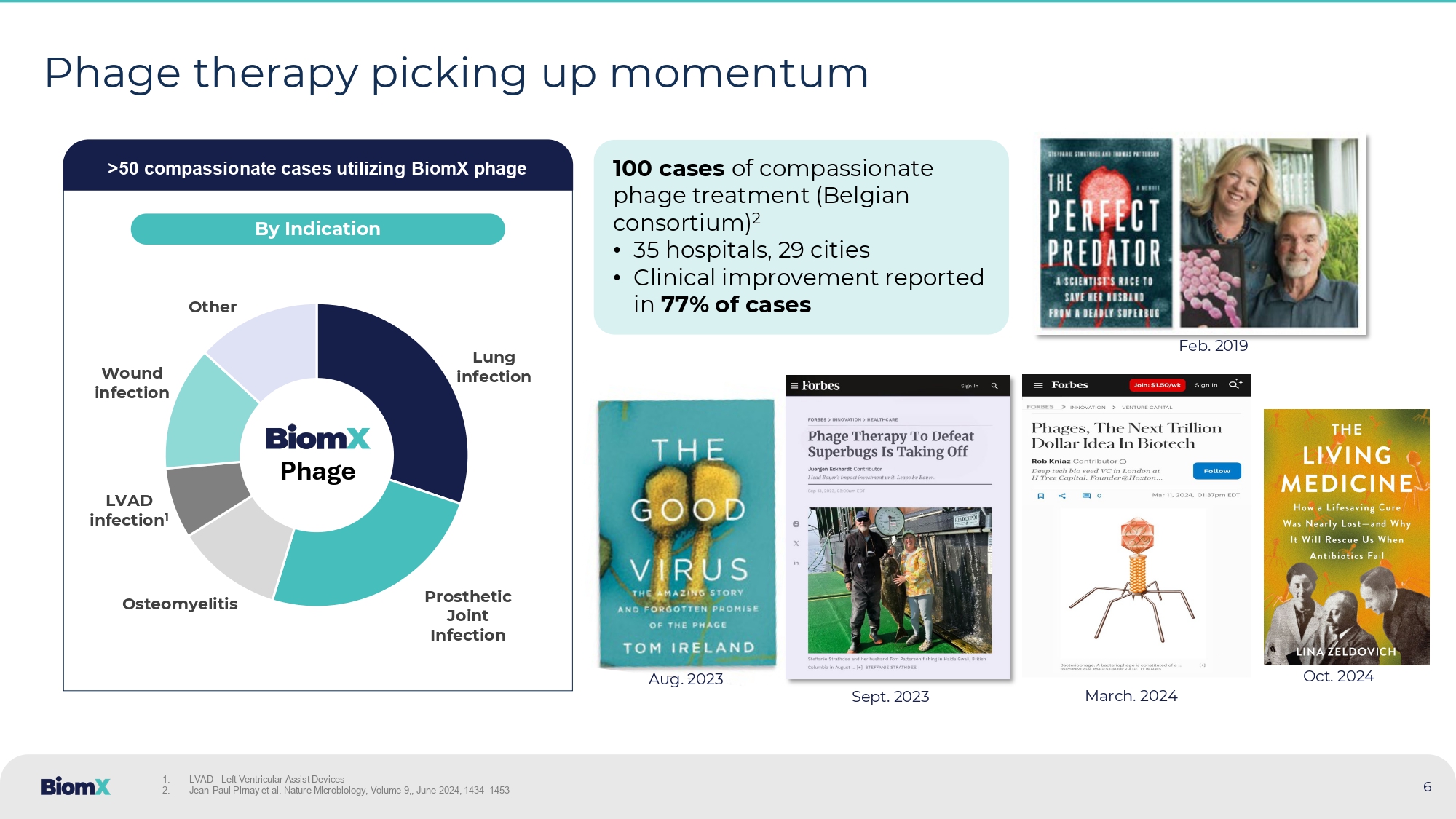
(2019), Cell Host & Microbe 5 6 Phage therapy picking up momentum >50 compassionate cases utilizing BiomX phage Lung infection Prosthetic Joint Infection Osteomyelitis LVAD infection 1 Wound infection Other Phage By Indication Sept. 2023 Aug. 2023 Feb. 2019 1. LVAD - Left Ventricular Assist Devices 2. Jean - Paul Pirnay et al. Nature Microbiology, Volume 9,, June 2024, 1434 – 1453 100 cases of compassionate phage treatment (Belgian consortium) 2 • 35 hospitals, 29 cities • Clinical improvement reported in 77% of cases Oct. 2024 March.
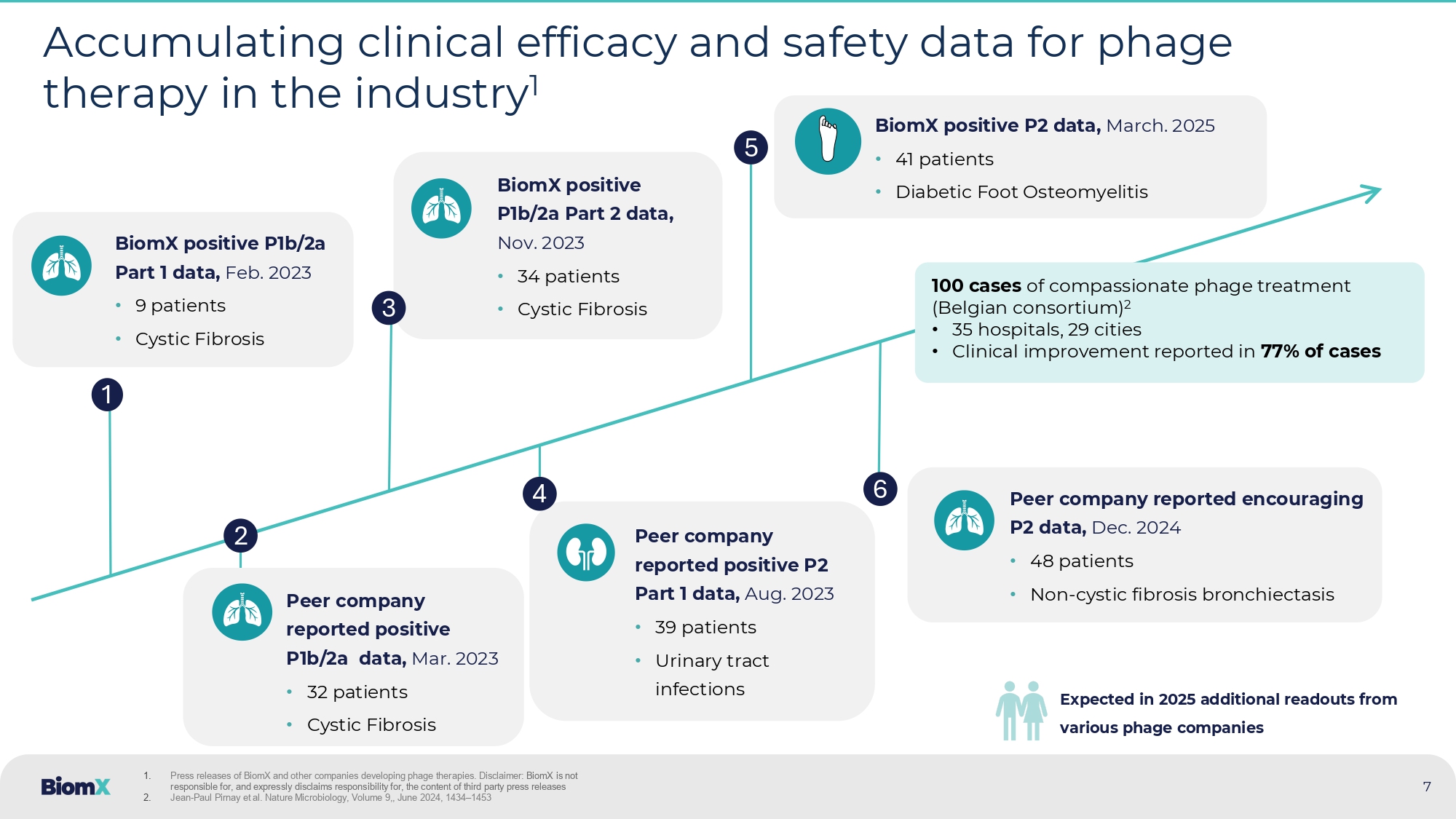
2024 BiomX positive P1b/2a Part 2 data, Nov. 2023 • 34 patients • Cystic Fibrosis Peer company reported positive P2 Part 1 data, Aug. 2023 • 39 patients • Urinary tract infections 7 Accumulating clinical efficacy and safety data for phage therapy in the industry 1 1 2 BiomX positive P1b/2a Part 1 data, Feb. 2023 • 9 patients • Cystic Fibrosis Peer company reported positive P1b/2a data, Mar. 2023 • 32 patients • Cystic Fibrosis 3 4 5 1. Press releases of BiomX and other companies developing phage therapies. Disclaimer: BiomX is not responsible for, and expressly disclaims responsibility for, the content of third party press releases 2. Jean - Paul Pirnay et al. Nature Microbiology, Volume 9 ,, June 2024 , 1434 – 1453 6 100 cases of compassionate phage treatment (Belgian consortium) 2 • 35 hospitals, 29 cities • Clinical improvement reported in 77% of cases Peer company reported encouraging P2 data, Dec. 2024 • 48 patients • Non - cystic fibrosis bronchiectasis BiomX positive P 2 data, March.
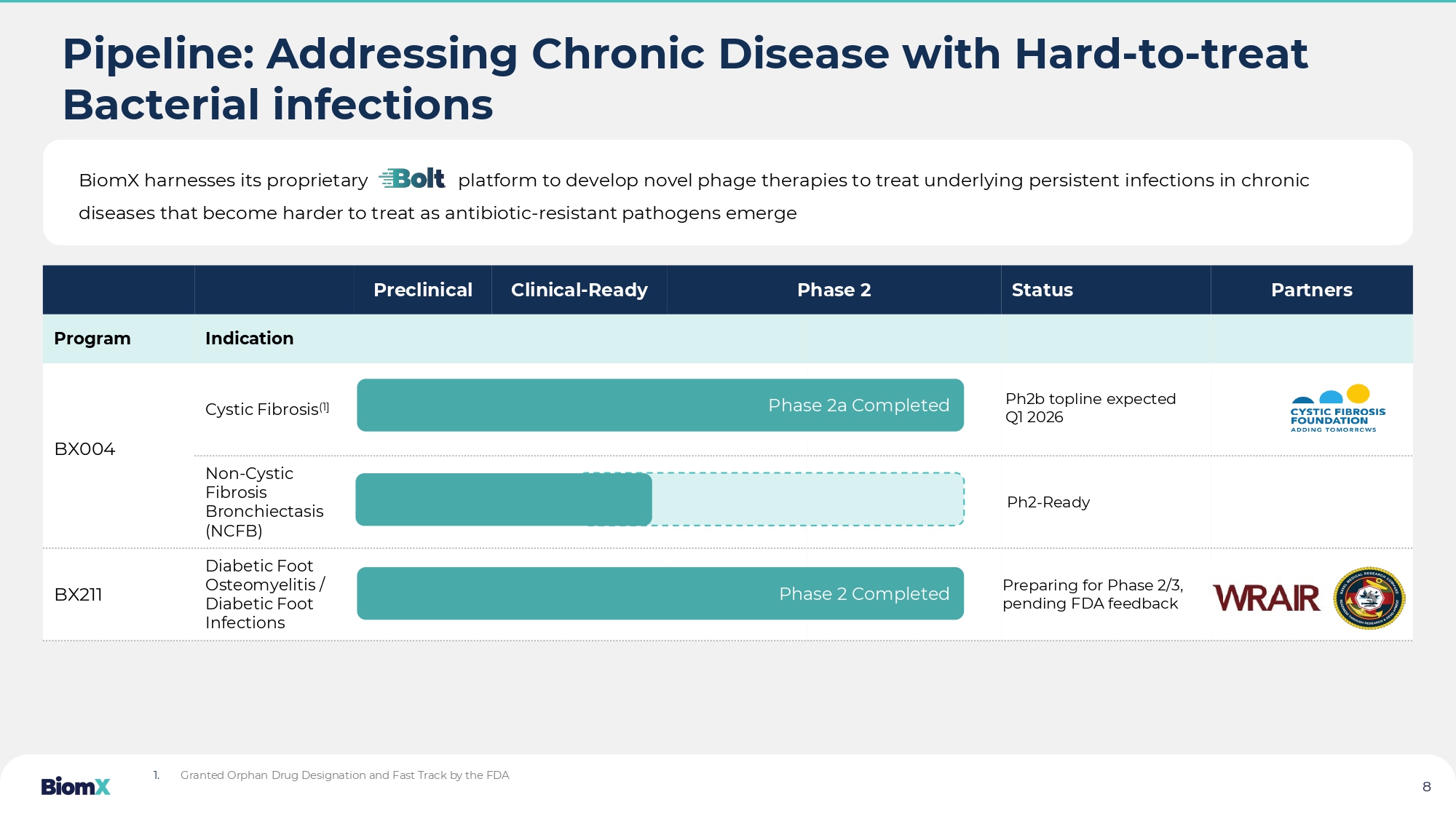
2025 • 41 patients • Diabetic Foot Osteomyelitis Expected in 2025 additional readouts from various phage companies Pipeline: Addressing Chronic Disease with Hard - to - treat Bacterial infections Partners Status Phase 2 Clinical - Ready Preclinical Indication Program Cystic Fibrosis (1] BX004 Non - Cystic Fibrosis Bronchiectasis (NCFB) Diabetic Foot Osteomyelitis / Diabetic Foot Infections BX211 Ph2b topline expected Q1 2026 Preparing for Phase 2/3, pending FDA feedback 1. Granted Orphan Drug Designation and Fast Track by the FDA BiomX harnesses its proprietary platform to develop novel phage therapies to treat underlying persistent in fec tions in chronic diseases that become harder to treat as antibiotic - resistant pathogens emerge 8 Phase 2a Completed Phase 2 Completed Ph2 - Ready BX004 CYSTIC FIBROSIS and NCFB 9
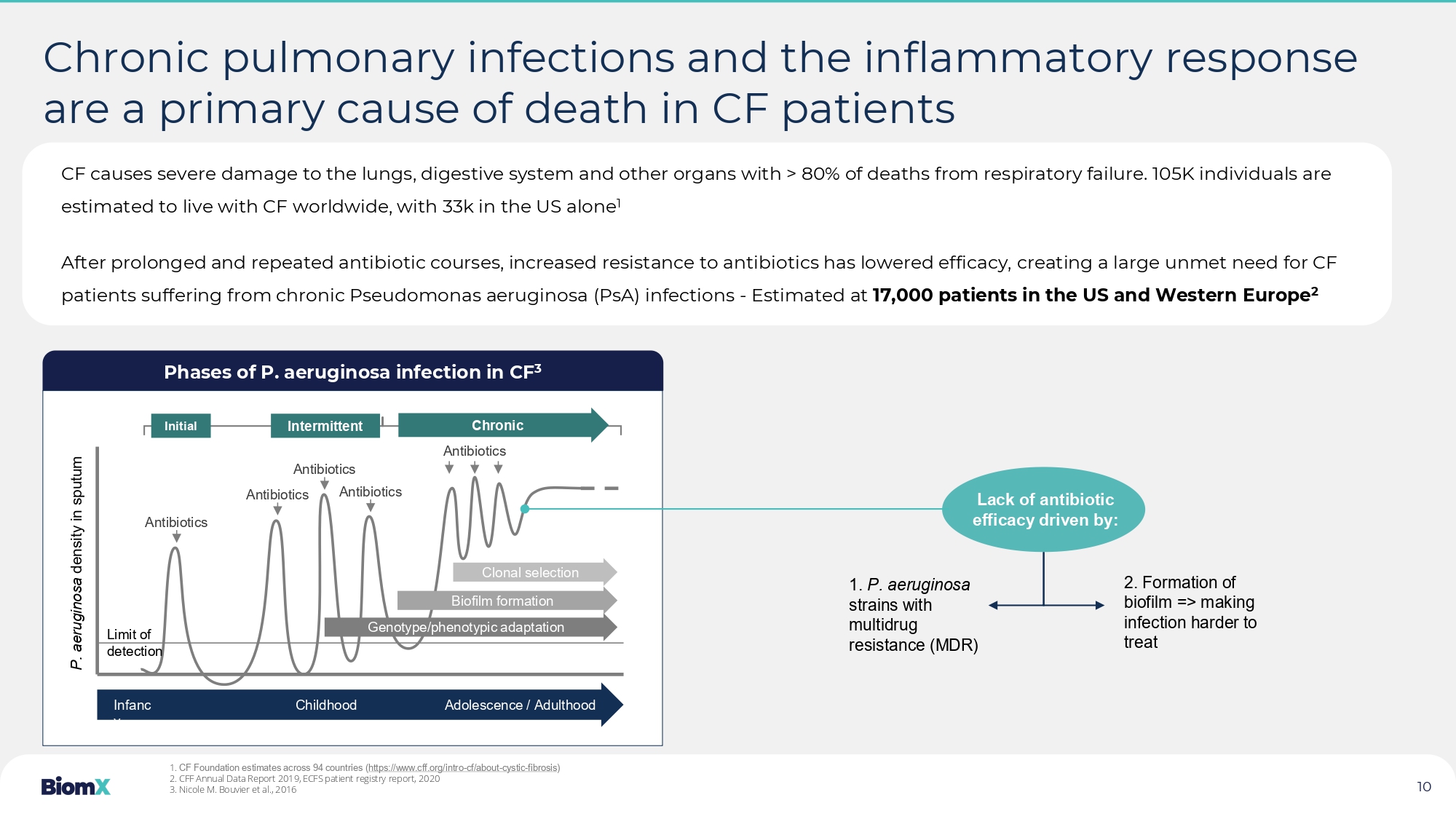
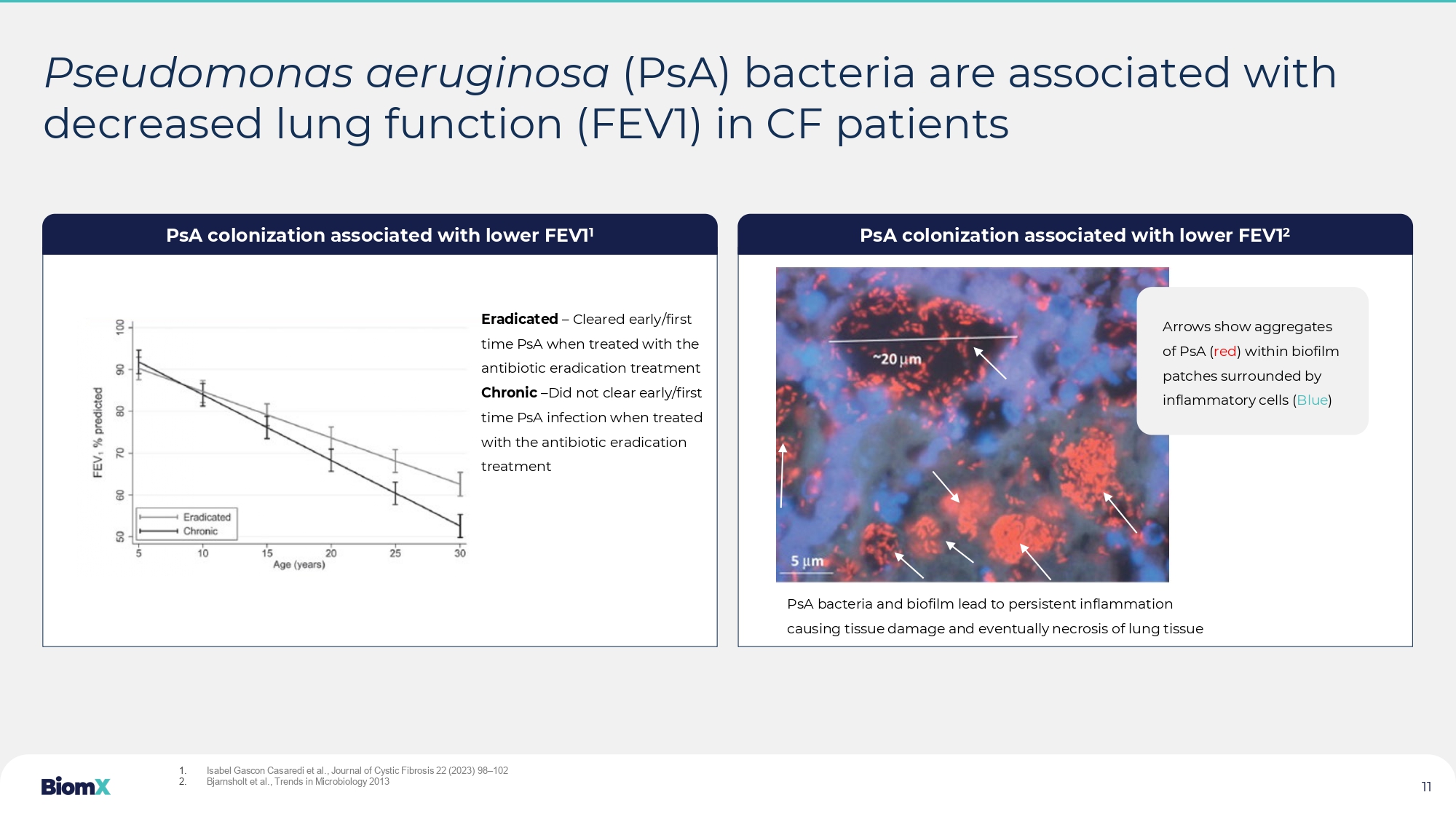
Phases of P. aeruginosa infection in CF 3 Chronic pulmonary infections and the inflammatory response are a primary cause of death in CF patients Antibiotics Antibiotics Antibiotics Antibiotics Antibiotics Initial Intermittent Chronic Clonal selection Biofilm formation Genotype/phenotypic adaptation Infanc y Childhood Adolescence / Adulthood Limit of detection P. aeruginosa density in sputum Lack of antibiotic efficacy driven by: 1. P. aeruginosa strains with multidrug resistance (MDR) 2. Formation of biofilm => making infection harder to treat 1. CF Foundation estimates across 94 countries ( https://www.cff.org/intro - cf/about - cystic - fibrosis ) 2. CFF Annual Data Report 2019, ECFS patient registry report, 2020 3. Nicole M. Bouvier et al., 2016 CF causes severe damage to the lungs, digestive system and other organs with > 80% of deaths from respiratory failure. 105K i ndi viduals are estimated to live with CF worldwide, with 33k in the US alone 1 After prolonged and repeated antibiotic courses, increased resistance to antibiotics has lowered efficacy, creating a large u nme t need for CF patients suffering from chronic Pseudomonas aeruginosa (PsA) infections - Estimated at 17,000 patients in the US and Western Europe 2 10 Pseudomonas aeruginosa (PsA) bacteria are associated with decreased lung function (FEV1) in CF patients PsA colonization associated with lower FEV1 1 PsA colonization associated with lower FEV1 2 Eradicated – Cleared early/first time PsA when treated with the antibiotic eradication treatment Chronic – Did not clear early/first time PsA infection when treated with the antibiotic eradication treatment PsA bacteria and biofilm lead to persistent inflammation causing tissue damage and eventually necrosis of lung tissue 1.
Isabel Gascon Casaredi et al., Journal of Cystic Fibrosis 22 (2023) 98 – 102 2.
Bjarnsholt et al., Trends in Microbiology 2013 Maya - Add image of biofilm formation by PsA in the lungs Check biofilm pathogenicity Arrows show aggregates of PsA ( red ) within biofilm patches surrounded by inflammatory cells ( Blue ) 11 Product – Proprietary phage cocktail targeting PsA Patient population – CF patients with chronic PsA lung infections Delivery – Nebulized Key features – Potentially effective on antibiotic resistant strains, enables breakdown of biofilm Potential impact : • Suppression/eradication of PsA (CFU in sputum) • Improved lung function (FEV1) • Fewer exacerbations, hospitalizations • Increased efficacy of antibiotic treatment • Reduced oral, inhaled and IV antibiotic treatments 12 BX004 – BiomX’s proprietary phage cocktail has the potential to treat CF patients with chronic PsA lung infections BX004 Nebulizer Multicentered , double blind, placebo controlled study to asses safety , re duction of PsA burden and improvement in clinical outcomes 13 PHASE 1B/2A STUDY – Study design 1 • Safety and tolerability • Decrease in PsA burden • FEV1 ( forced expiratory volume) • CFQ - R (CF Questionnaire - Revised ) and CRISS Key Endpoints: Total enrollment: 43 CF patients with chronic PsA infections Standard of care antibiotic, no restriction on CFTR modulators Intervention: Nebulized dose BX004 Part 1 7 days - Single ascending dose followed by multiple doses 2 Treated – 7 subjects Control – 2 subjects Part 2 10 days of same dose, twice daily Treated – 23 subjects Control – 11 subjects 1.
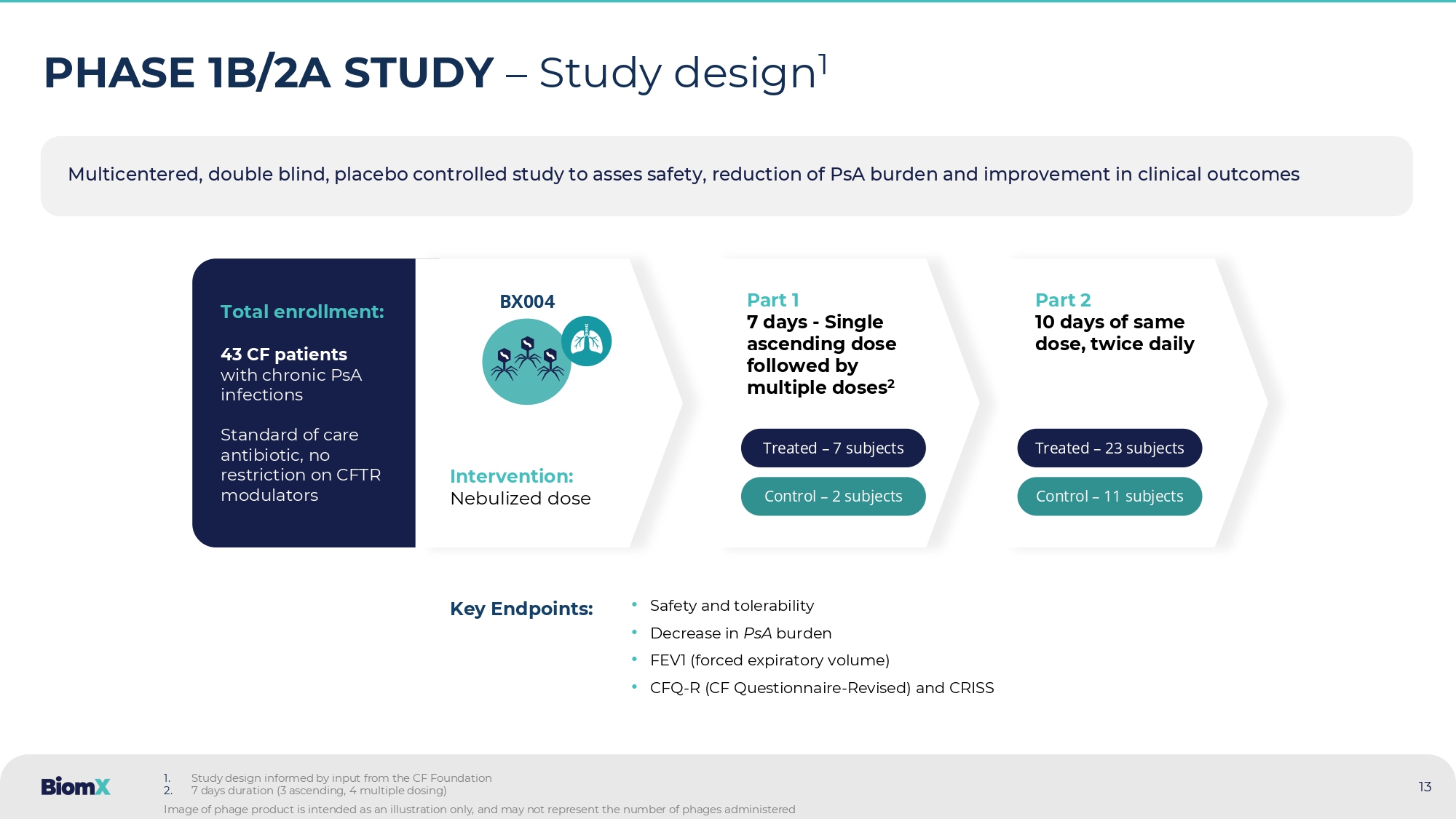
Study design informed by input from the CF Foundation 2.

7 days duration (3 ascending, 4 multiple dosing) Image of phage product is intended as an illustration only, and may not represent the number of phages administered 14 • Study drug was well - tolerated • In Part 1, Mean PsA CFU/g 1 reduction at Day 15: - 1.42 log10 CFU/g (BX004) compared to - 0.28 log10 CFU/g (placebo) • Part 2: BX004 showed signals of improvement in pulmonary function vs. placebo : Relative FEV1 3 improvement (5.67%) and CF Questionnaire - Revised respiratory 3 (8.87 points) at Day 17 (1 week after EOT 3 ) in subgroup of patients with reduced lung function 4 • Culture conversion: Part 2, in the BX004 arm, 3 of 21 (14.3%) patients converted to sputum culture negative for PsA after 10 days of treatment compared to 0 out of 10 (0%) in the placebo arm 2 . In Part 1, 1 of 7 (14.3%) treated patients also had converted based on physician report • Part 2: In full population, BX004 vs. placebo PsA levels were more variable. In a prespecified subgroup of patients on SOC 3 inhaled antibiotics on continuous regimen, BX004 vs. placebo showed bacterial reduction of 2.8 log10 CFU/g at EOT 3 , exceeding Part 1 results Phase 1b/2a – Result highlights (Parts 1 and 2) 1. CFU – Colony forming units 2. In patients that had quantitative CFU levels at study baseline 3. FEV1 (or ppFEV1) – percent predicted forced expiratory volume in 1 second, CF Questionnaire - Revised Respiratory – a PRO (Patient reported outcome ) for respiratory parameters in CF aptients , EOT – End of treatment , SOC – standard of care 4. Predefined group with Baseline FEV1<70% 15 PART 1 BX004 demonstrated greater reduction in PsA levels compared to placebo Placebo BX004 2 7 n - 0.28 (0.13) - 1.42 (1.03) Mean reduction (SD) Log 10 CFU/g - 0.37 , - 0.18 - 3.27 , - 0.37 Max, Min 1.
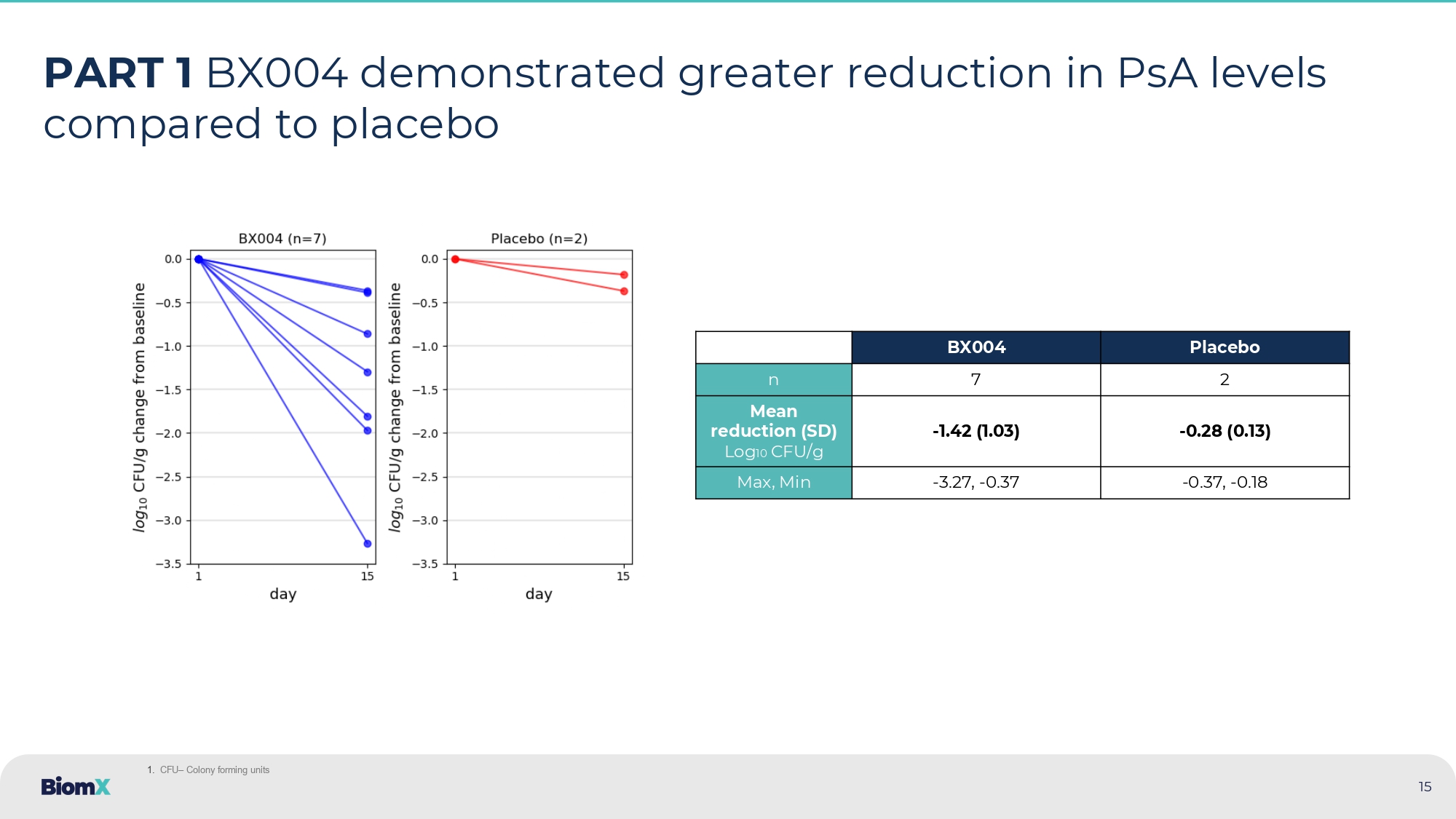
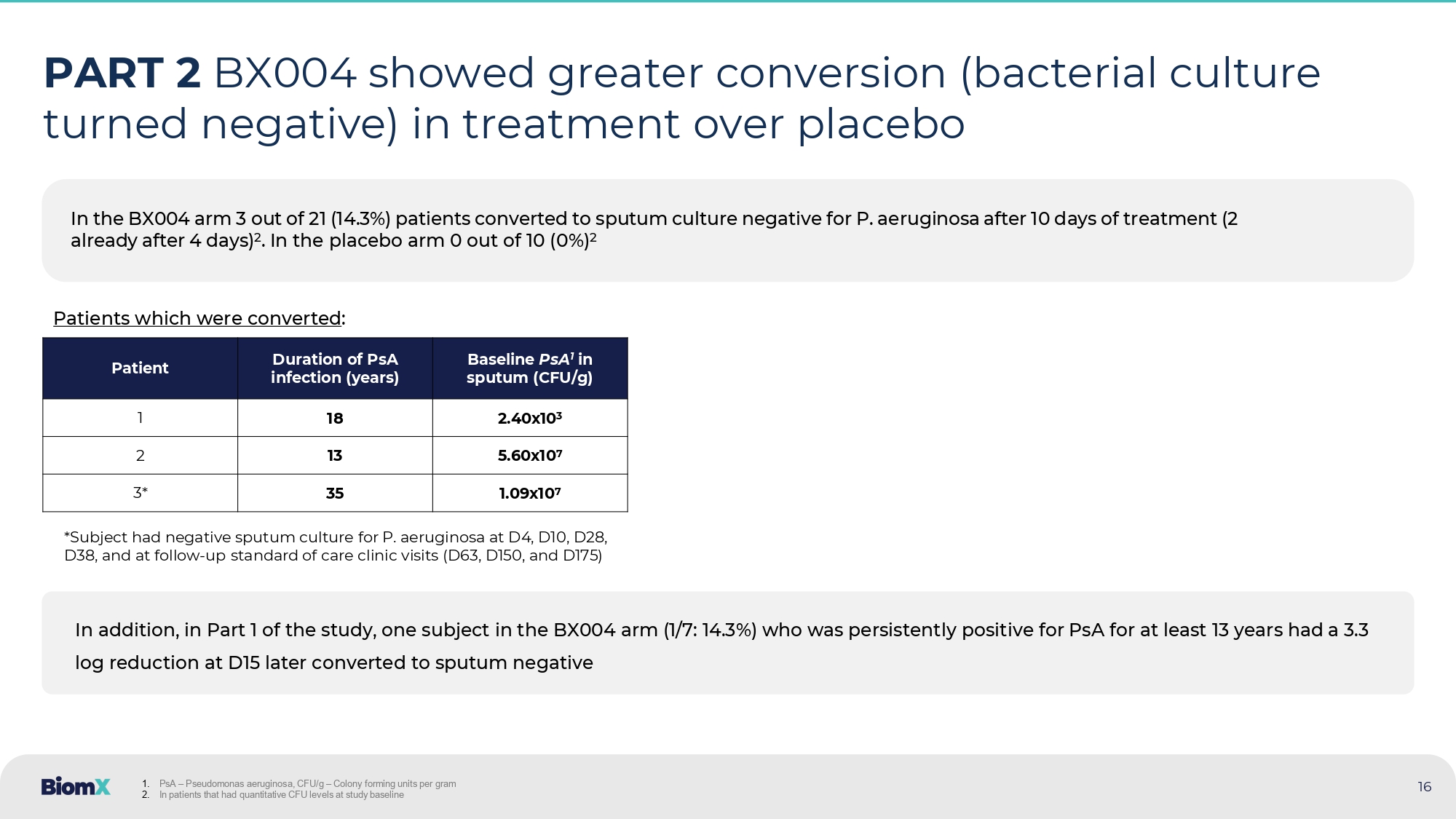
CFU – Colony forming units In the BX004 arm 3 out of 21 (14.3%) patients converted to sputum culture negative for P. aeruginosa after 10 days of treatme nt (2 already after 4 days) 2 . In the placebo arm 0 out of 10 (0%) 2 16 PART 2 BX004 showed greater conversion (bacterial culture turned negative) in treatment over placebo Baseline PsA 1 in sputum (CFU/g) Duration of PsA infection (years) Patient 2.40x10 3 18 1 5.60x10 7 13 2 1.09x10 7 35 3* *Subject had negative sputum culture for P. aeruginosa at D4, D10, D28, D38, and at follow - up standard of care clinic visits (D63, D150, and D175) In addition, in Part 1 of the study, one subject in the BX004 arm (1/7: 14.3%) who was persistently positive for PsA for at l eas t 13 years had a 3.3 log reduction at D15 later converted to sputum negative 1. PsA – Pseudomonas aeruginosa, CFU/g – Colony forming units per gram 2. In patients that had quantitative CFU levels at study baseline Patients which were converted :
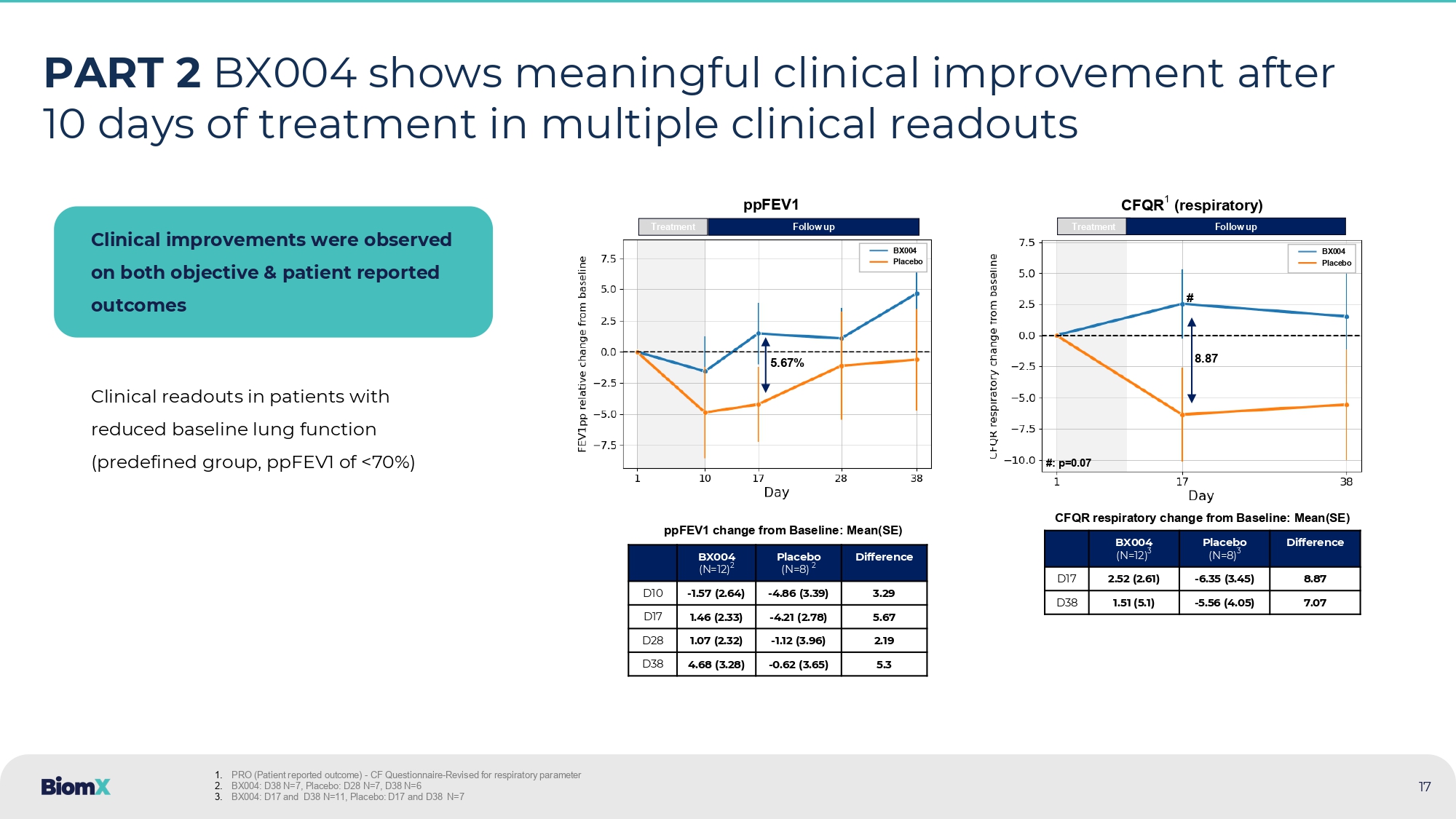
PART 2 BX004 shows meaningful clinical improvement after 10 days of treatment in multiple clinical readouts Clinical improvements were observed on both objective & patient reported outcomes Clinical readouts in patients with reduced baseline lung function (predefined group, ppFEV1 of <70%) Difference Placebo (N=8) 2 BX004 (N=12) 2 3.29 - 4.86 (3.39) - 1.57 (2.64) D10 5.67 - 4.21 (2.78) 1.46 (2.33) D17 2.19 - 1.12 (3.96) 1.07 (2.32) D28 5.3 - 0.62 (3.65) 4.68 (3.28) D38 Difference Placebo (N=8) 3 BX004 (N=12) 3 8.87 - 6.35 (3.45) 2.52 (2.61) D17 7.07 - 5.56 (4.05) 1.51 (5.1) D38 ppFEV1 change from Baseline: Mean(SE) CFQR respiratory change from Baseline: Mean(SE) Treatment 5.67% Follow up ppFEV1 BX004 Placebo Treatment Follow up CFQR 1 (respiratory) 8.87 BX004 Placebo # #: p=0.07 1. PRO (Patient reported outcome ) - CF Questionnaire - Revised for respiratory parameter 2. BX004: D38 N=7, Placebo: D28 N=7, D38 N=6 3. BX004: D17 and D38 N=11, Placebo: D17 and D38 N=7 17 International, multicenter, double blind, placebo - controlled study to assess reduction of PsA burden and improvement in clinical outcome 18 PHASE 2B STUDY – Study design • Decrease in PsA burden (incl.
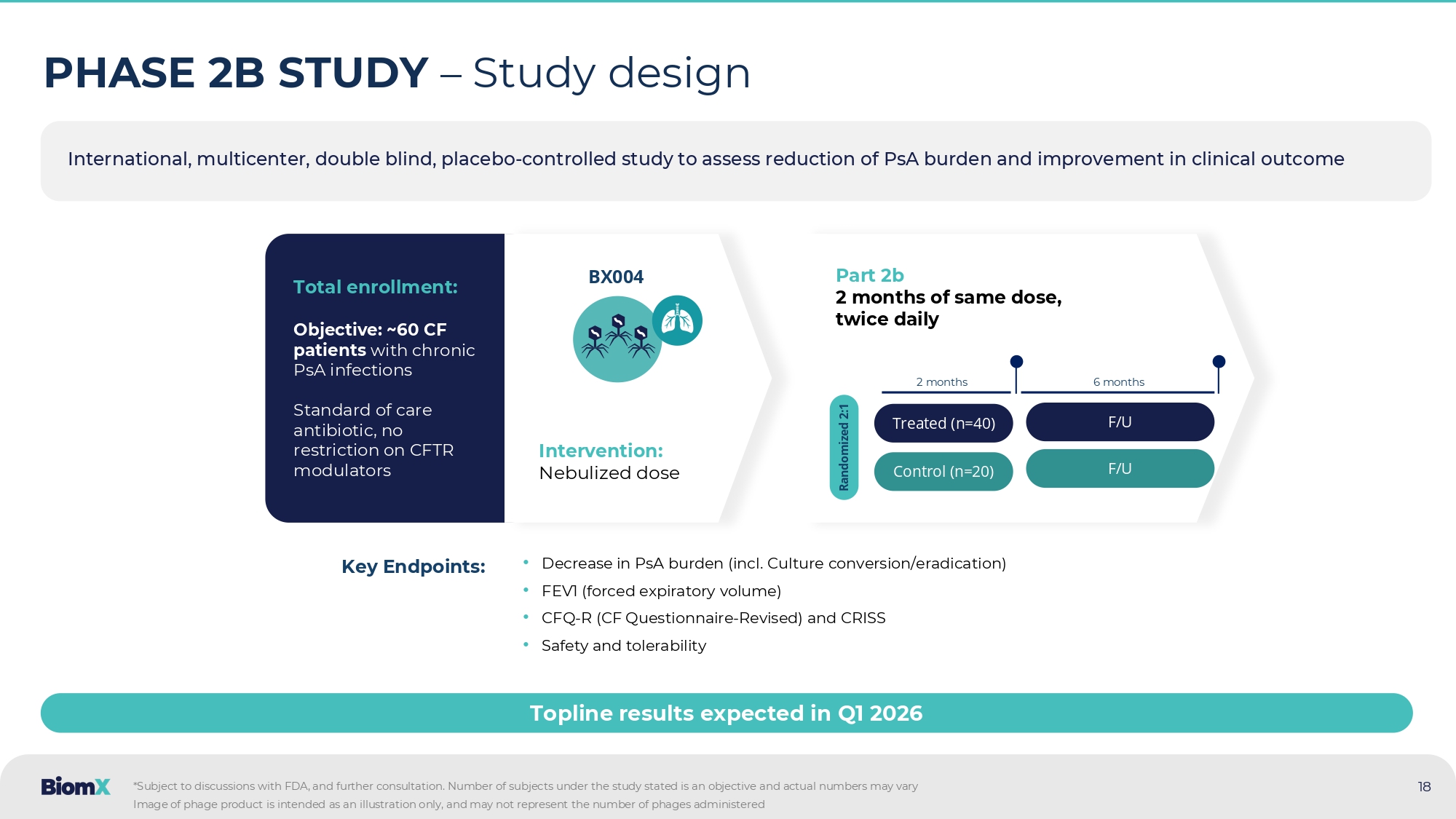
Culture conversion/eradication) • FEV1 (forced expiratory volume) • CFQ - R (CF Questionnaire - Revised) and CRISS • Safety and tolerability Key Endpoints: Total enrollment: Objective: ~60 CF patients with chronic PsA infections Standard of care antibiotic, no restriction on CFTR modulators Intervention: Nebulized dose BX004 Part 2b 2 months of same dose, twice daily Treated (n=40) Control (n=20) Randomized 2:1 Topline results expected in Q 1 2026 *Subject to discussions with FDA, and further consultation.

Number of subjects under the study stated is an objective and act ual numbers may vary 2 months 6 months F/U F/U Image of phage product is intended as an illustration only, and may not represent the number of phages administered 19 Bacterial Reduction Potential Regulatory Endpoint Evaluating Real - World Evidence Journal of Cystic Fibrosis 22 (2023) 98 – 102 Eradicated – Cleared early/first time PsA when treated with the antibiotic eradication treatment Chronic – Did not clear early/first time PsA infection when treated with the antibiotic eradication treatment Pediatric Pulmonology 47:44 – 52 (2012) Tobramycin Inhalation Solution – Antibiotic treatment to target PsA Chronic – Patients on TIS throughout follow - up years had reduced mortality and improved survival Peer - reviewed publications demonstrated that P.
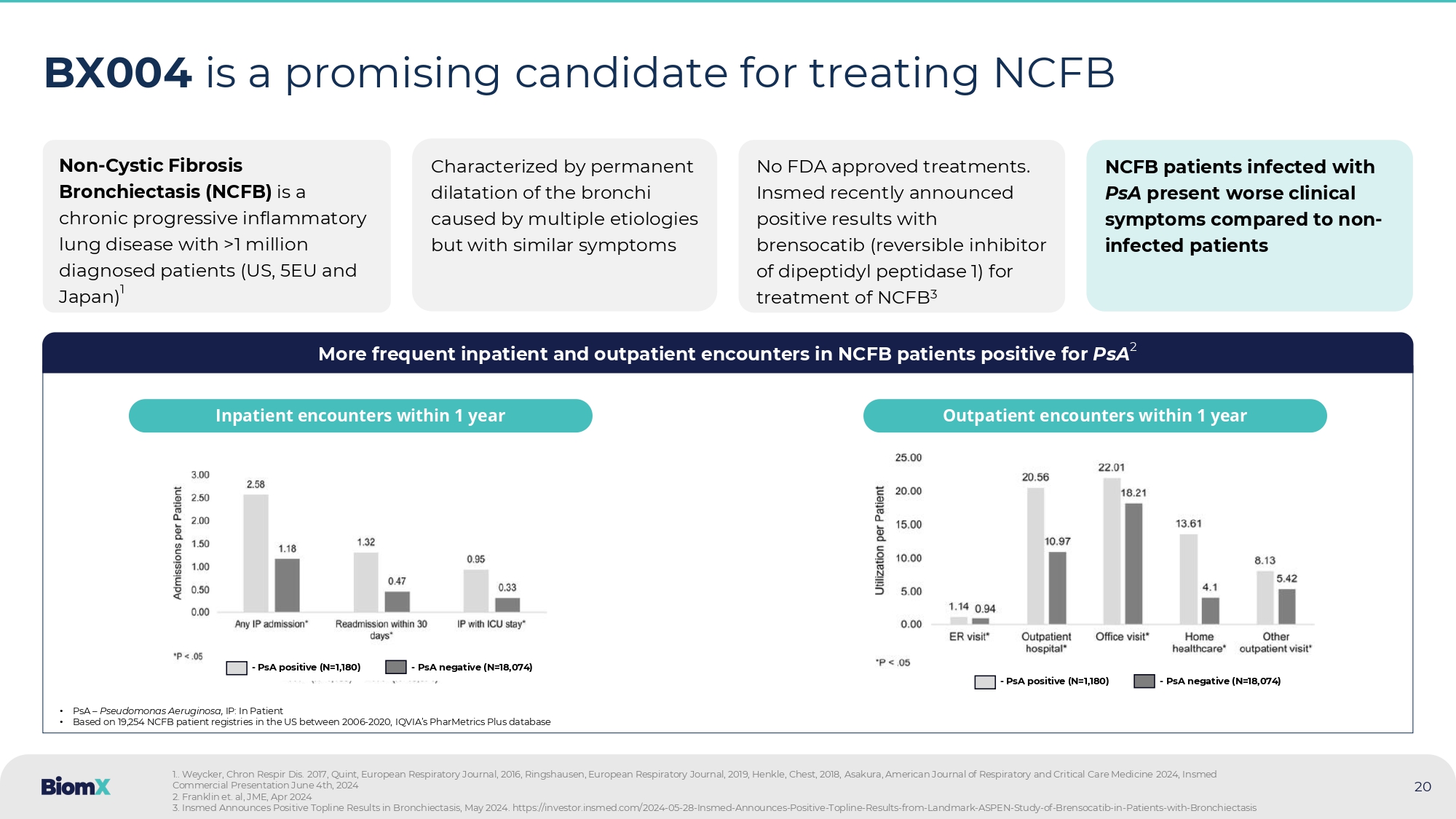
aeruginosa reduction improves patient outcomes, and ongoing real - world evidence (RWE) analysis may support regulatory filings 20 BX004 is a promising candidate for treating NCFB Non - Cystic Fibrosis Bronchiectasis (NCFB) is a chronic progressive inflammatory lung disease with > 1 million diagnosed patients (US, 5 EU and Japan) 1 Characterized by permanent dilatation of the bronchi caused by multiple etiologies but with similar symptoms No FDA approved treatments. Insmed recently announced positive results with brensocatib ( reversible inhibitor of dipeptidyl peptidase 1) for treatment of NCFB 3 NCFB patients infected with PsA present worse clinical symptoms compared to non - infected patients More frequent inpatient and outpatient encounters in NCFB patients positive for PsA 2 • PsA – Pseudomonas Aeruginosa, IP: In Patient • Based on 19,254 NCFB patient registries in the US between 2006 - 2020, IQVIA’s PharMetrics Plus database Inpatient encounters within 1 year Outpatient encounters within 1 year 1 .. Weycker , Chron Respir Dis. 2017 , Quint, European Respiratory Journal, 2016 , Ringshausen , European Respiratory Journal, 2019 , Henkle, Chest, 2018 , Asakura , American Journal of Respiratory and Critical Care Medicine 2024 , Insmed Commercial Presentation June 4 th, 2024 2. Franklin et. al, JME, Apr 2024 3. Insmed Announces Positive Topline Results in Bronchiectasis , May 2024. https://investor.insmed.com/2024 - 05 - 28 - Insmed - Announces - Positive - Topline - Results - from - Landmark - ASPEN - Study - of - Brenso catib - in - Patients - with - Bronchiectasis - PsA positive (N=1,180) - PsA negative (N=18,074) - PsA positive (N=1,180) - PsA negative (N=18,074)

BX211 Diabetic Foot Infections & Diabetic Foot Osteomyelitis (DFI & DFO) 21
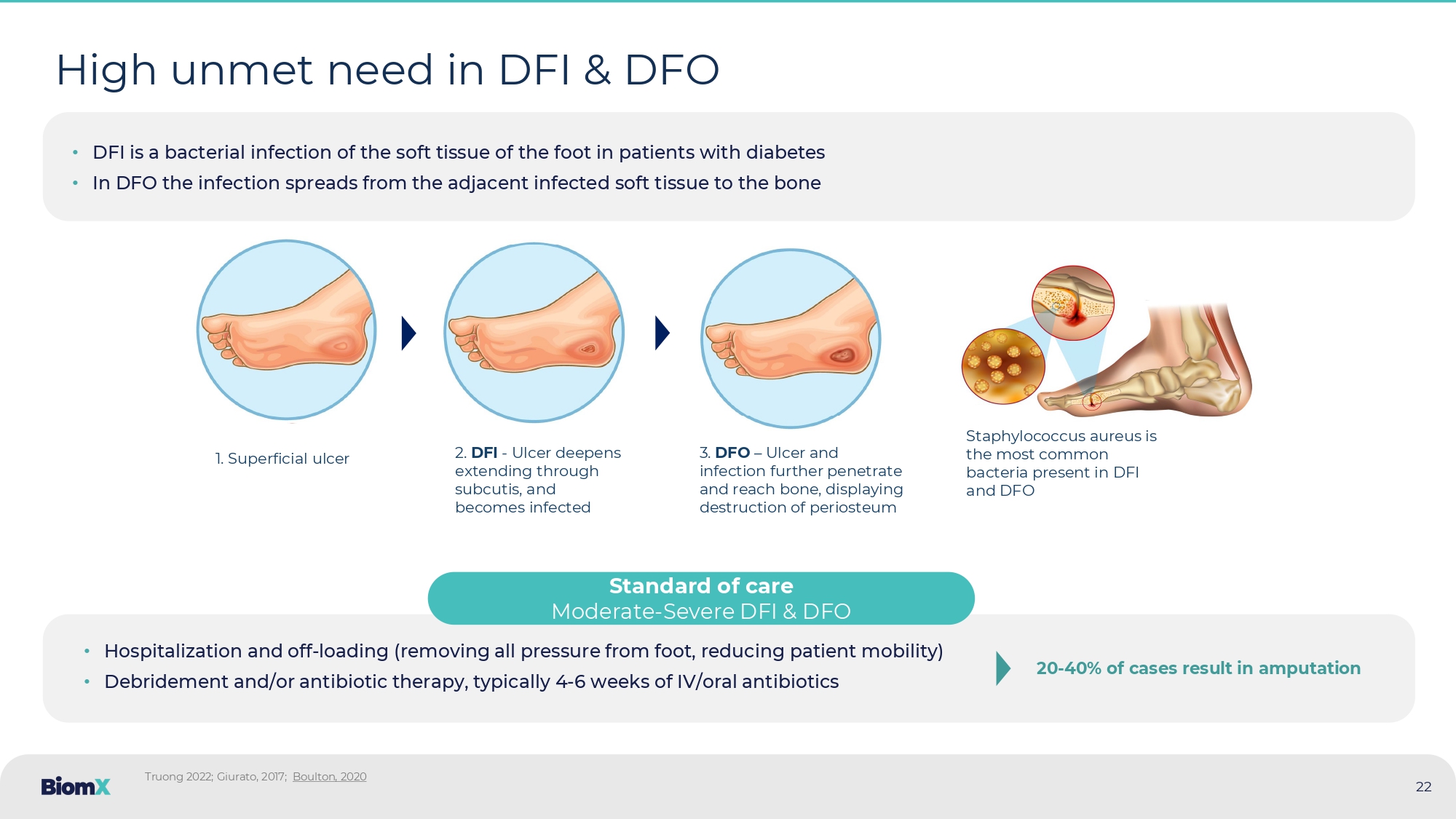
High unmet need in DFI & DFO Truong 2022; Giurato , 2017; Boulton, 2020 • DFI is a bacterial infection of the soft tissue of the foot in patients with diabetes • In DFO the infection spreads from the adjacent infected soft tissue to the bone 1. Superficial ulcer 2. DFI - Ulcer deepens extending through subcutis, and becomes infected 3. DFO – Ulcer and infection further penetrate and reach bone, displaying destruction of periosteum Staphylococcus aureus is the most common bacteria present in DFI and DFO Standard of care Moderate - Severe DFI & DFO 20 - 40 % of cases result in amputation 22 • Hospitalization and off - loading (removing all pressure from foot, reducing patient mobility) • Debridement and/or antibiotic therapy, typically 4 - 6 weeks of IV/oral antibiotics Amputations in diabetic patients are an enormous burden to the health system An episode of lower limb amputation is a major risk factor for subsequent amputations ~160K Incidence ~$50K Cost Lower limb amputations, diabetic patients annually, US Amputation costs (direct), per patient, US Staggering economic burden Diabetic amputations cost the US healthcare system ~8 Billion USD per year 1.
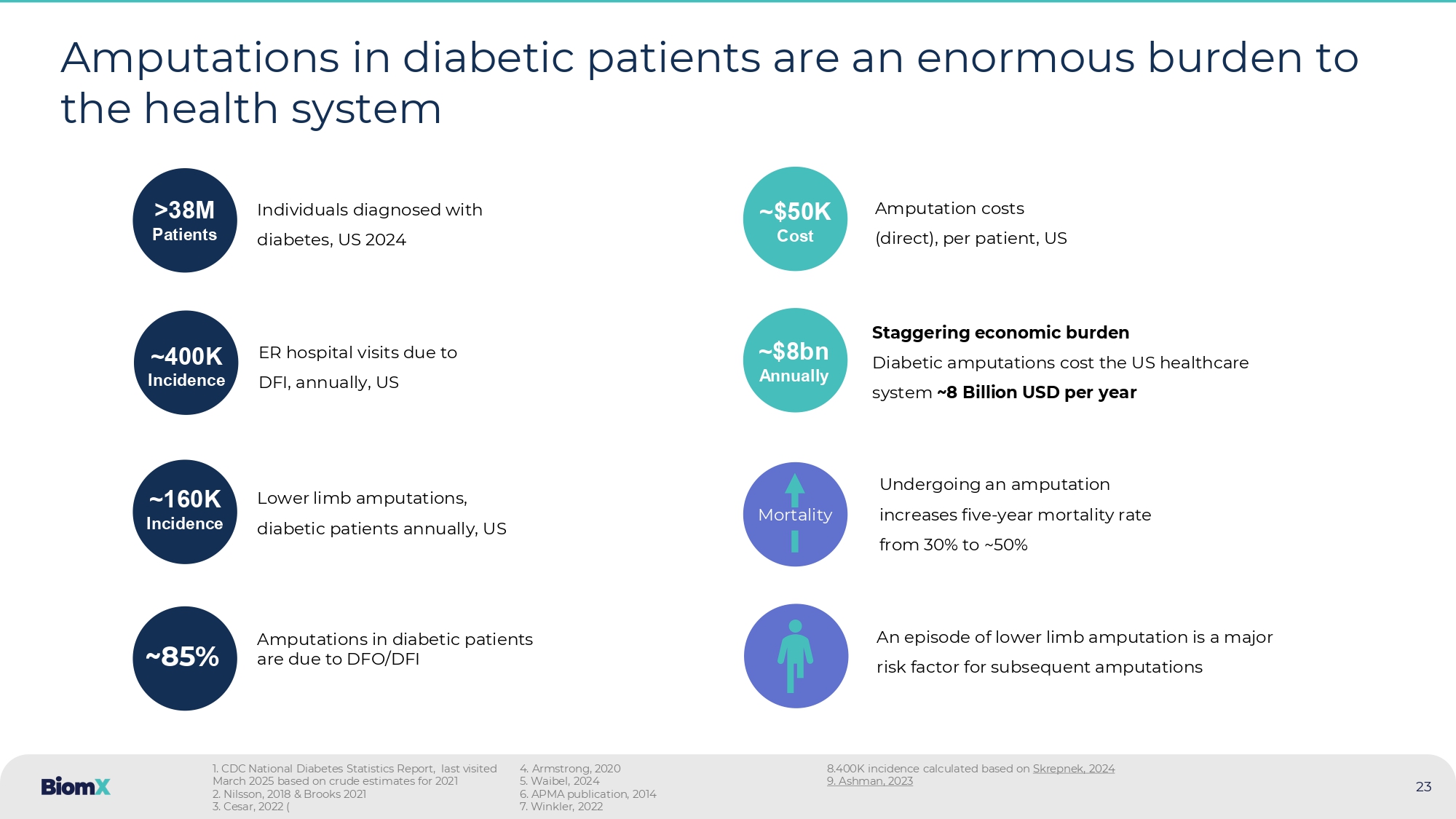
CDC National Diabetes Statistics Report, last visited March 2025 based on crude estimates for 2021 2. Nilsson, 2018 & Brooks 2021 3. Cesar, 2022 ( 4. Armstrong, 2020 5. Waibel, 2024 6. APMA publication, 2014 7. Winkler, 2022 8.400K incidence calculated based on Skrepnek , 2024 9.
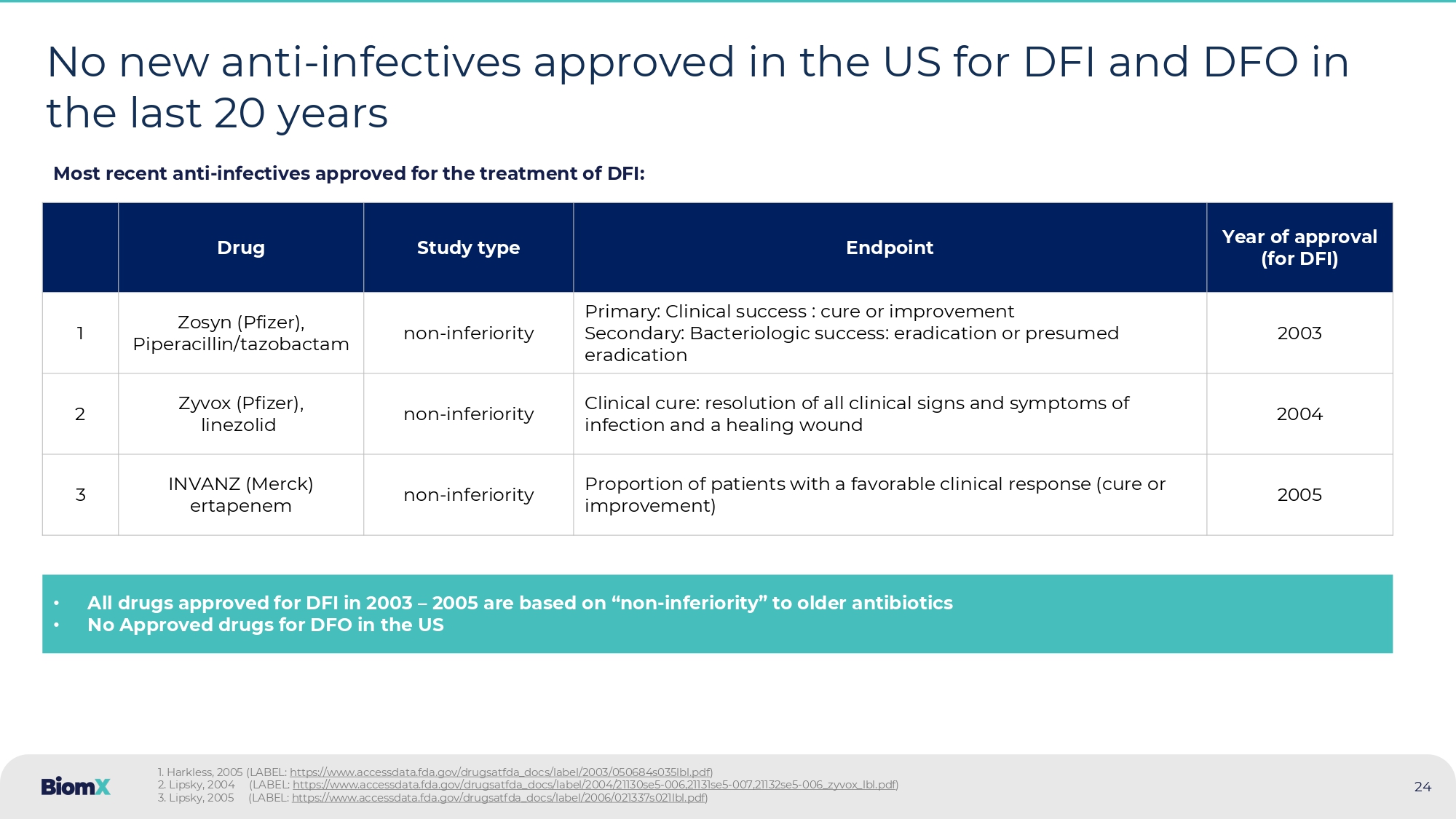
Ashman, 2023 ~$8bn Annually ~400K Incidence ER hospital visits due to DFI, annually, US >38M P atients Individuals diagnosed with diabetes, US 2024 23 ~85% Amputations in diabetic patients are due to DFO/DFI Mortality Undergoing an amputation increases five - year mortality rate from 30% to ~50% 24 No new anti - infectives approved in the US for DFI and DFO in the last 20 years Year of approval (for DFI) Endpoint Study type Drug 2003 Primary: Clinical success : cure or improvement Secondary: Bacteriologic success: eradication or presumed eradication non - inferiority Zosyn ( Pfizer ), Piperacillin/tazobactam 1 2004 Clinical cure: resolution of all clinical signs and symptoms of infection and a healing wound non - inferiority Zyvox (Pfizer), linezolid 2 2005 Proportion of patients with a favorable clinical response (cure or improvement) non - inferiority INVANZ (Merck) ertapenem 3 • All drugs approved for DFI in 2003 – 2005 are based on “ non - inferiority ” to older antibiotics • No Approved drugs for DFO in the US Most recent anti - infectives approved for the treatment of DFI: 1. Harkless, 2005 (LABEL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/050684s035lbl.pdf ) 2. Lipsky, 2004 (LABEL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21130se5 - 006,21131se5 - 007,21132se5 - 006_zyvox_lbl.pdf ) 3. Lipsky, 2005 (LABEL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021337s021lbl.pdf )
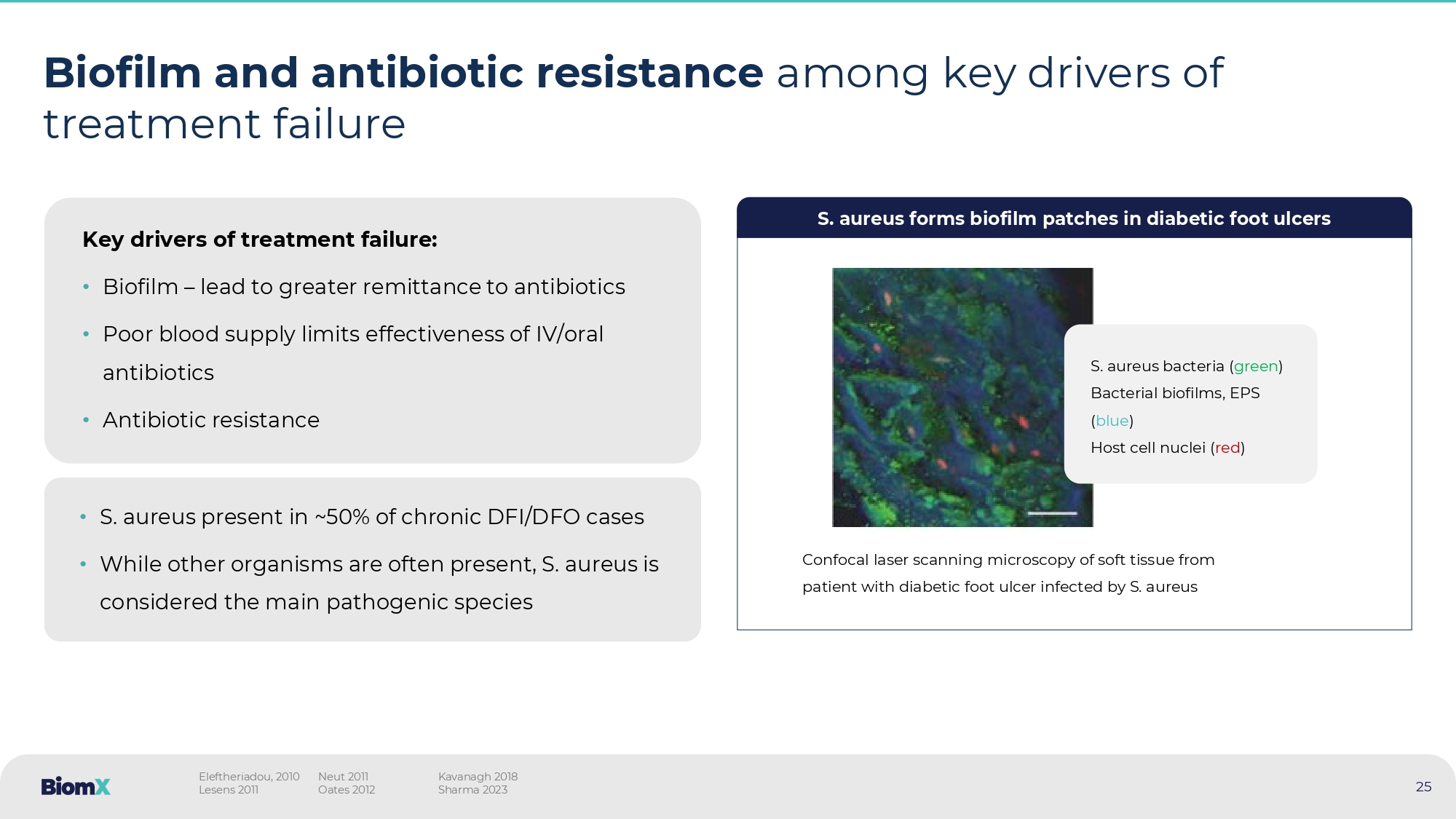
Biofilm and antibiotic resistance among key drivers of treatment failure Key drivers of treatment failure: • Biofilm – lead to greater remittance to antibiotics • Poor blood supply limits effectiveness of IV/oral antibiotics • Antibiotic resistance • S. aureus present in ~50% of chronic DFI/DFO cases • While other organisms are often present, S. aureus is considered the main pathogenic species S. aureus forms biofilm patches in diabetic foot ulcers Confocal laser scanning microscopy of soft tissue from patient with diabetic foot ulcer infected by S. aureus S.
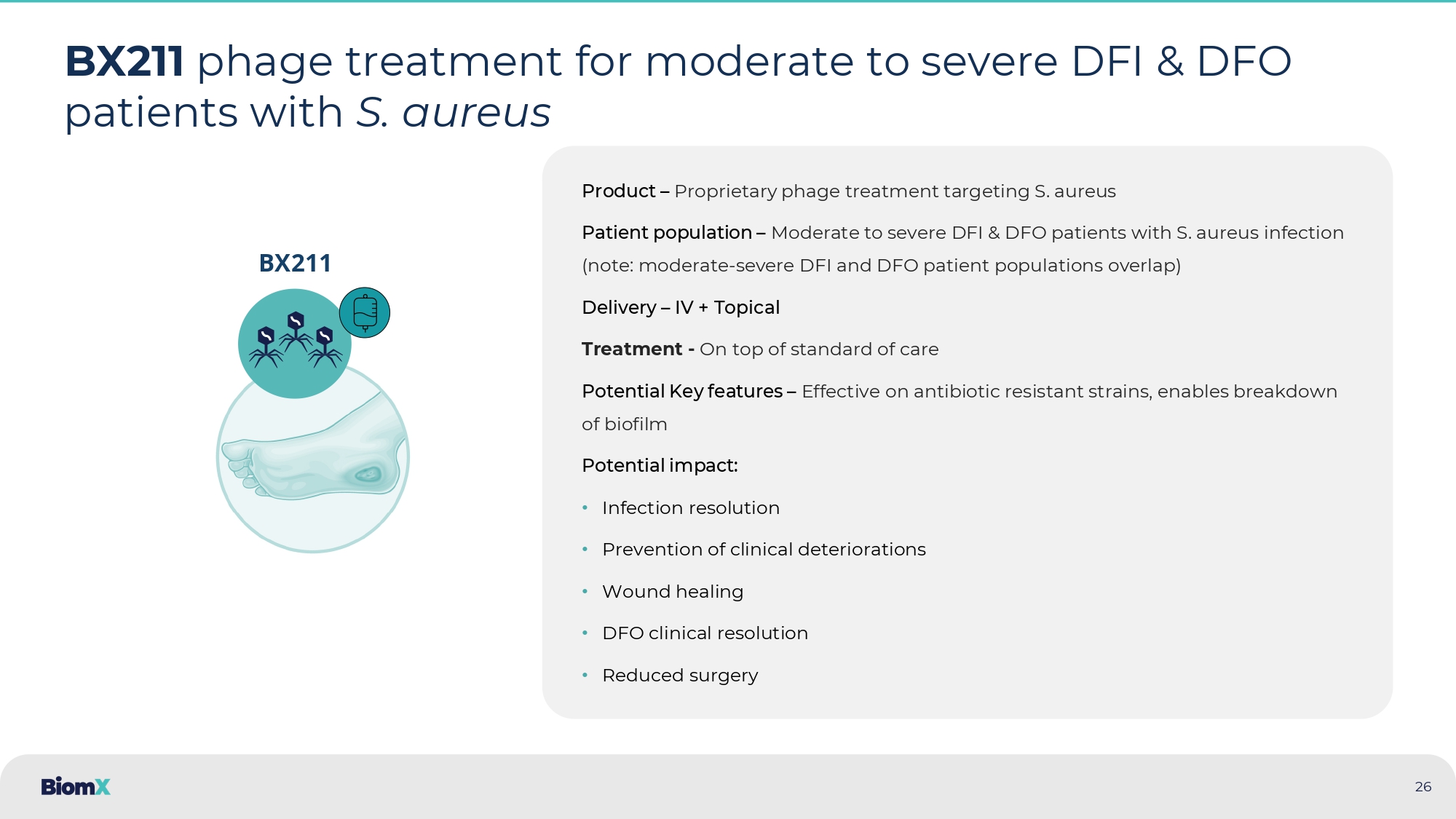
aureus bacteria ( green ) Bacterial biofilms, EPS ( blue ) Host cell nuclei ( red ) Eleftheriadou, 2010 Lesens 2011 Neut 2011 Oates 2012 Kavanagh 2018 Sharma 2023 25 26 BX211 phage treatment for moderate to severe DFI & DFO patients with S. aureus Product – Proprietary phage treatment targeting S. aureus Patient population – Moderate to severe DFI & DFO patients with S.
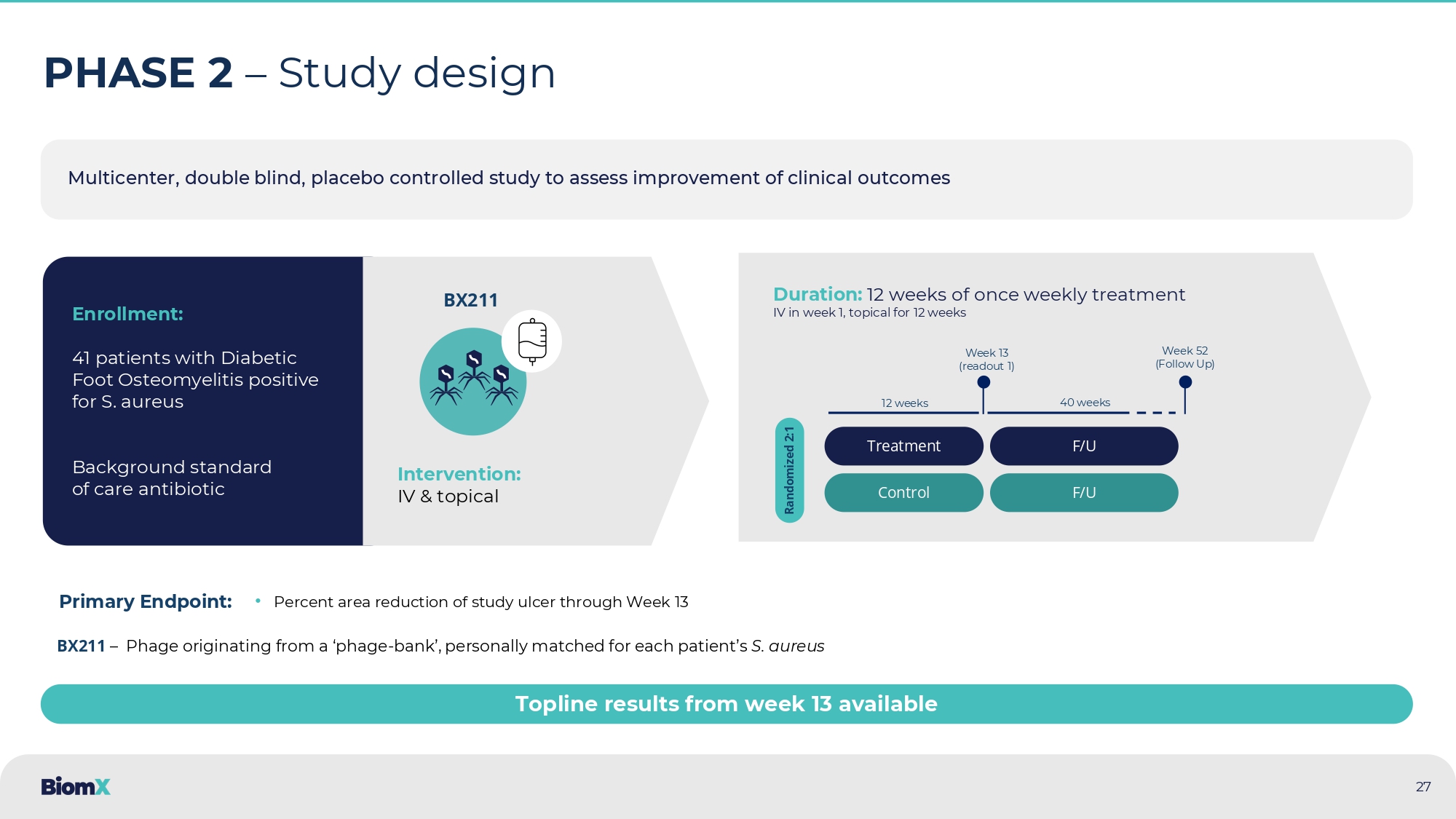
aureus infection (note: moderate - severe DFI and DFO patient populations overlap) Delivery – IV + Topical Treatment - On top of standard of care Potential Key features – Effective on antibiotic resistant strains, enables breakdown of biofilm Potential impact : • Infection resolution • Prevention of clinical deteriorations • Wound healing • DFO clinical resolution • Reduced surgery BX211 Multicenter, double blind, placebo controlled study to assess improvement of clinical outcomes 27 PHASE 2 – Study design Enrollment: 41 patients with Diabetic Foot Osteomyelitis positive for S. aureus Background standard of care antibiotic Intervention: IV & topical Duration: 12 weeks of once weekly treatment IV in week 1, topical for 12 weeks Treatment Control Randomized 2:1 Week 13 (readout 1 ) Week 52 (Follow Up) 12 weeks 40 weeks F/U F/U BX211 • Percent area reduction of study ulcer through Week 13 Primary Endpoint: BX211 – Phage originating from a ‘phage - bank’, personally matched for each patient’s S. aureus Topline results from week 13 available 28 Results highlight – Phase 2 Diabetic Foot Osteomyelitis 1.
All p - values described in the current side are non - adjusted ). 2. Ulcer depth was classified according to deepest tissue involved as measured by swab) PAR - Percentage Area Reduction • BX 211 was safe and well - tolerated • BX 211 demonstrated sustained and statistically significant ¹ PAR ulcer size ² (p = 0.046 at week 12 ; p = 0.052 at week 13 ), with a separation from placebo starting at week 7 with a difference greater than 40 % by week 10 • BX 211 also produced statistically significant 1 improvements in both ulcer depth at week 13 (in patients with ulcer depth defined as bone at baseline (p= 0.048 ), and in reducing the expansion of ulcer area (p= 0.017 ), compared to placebo • BX 211 demonstrated favorable trends compared to placebo across several additional clinical parameters • Through week 13 , BX 211 demonstrated comparable efficacy against both Methicillin - susceptible and resistant strains , as well as against high and low biofilm producers — consistent with the orthogonal mechanism of phage therapy to antibiotics and its inherent anti - biofilm capabilities 29 BX211 showed clinically relevant, statistically significant 2 , reduction in ulcer surface area Percent Area Reduction (PAR) from baseline of ulcer surface area (LS Mean ± SE ) 1 (See detailed data in next slide) 1.
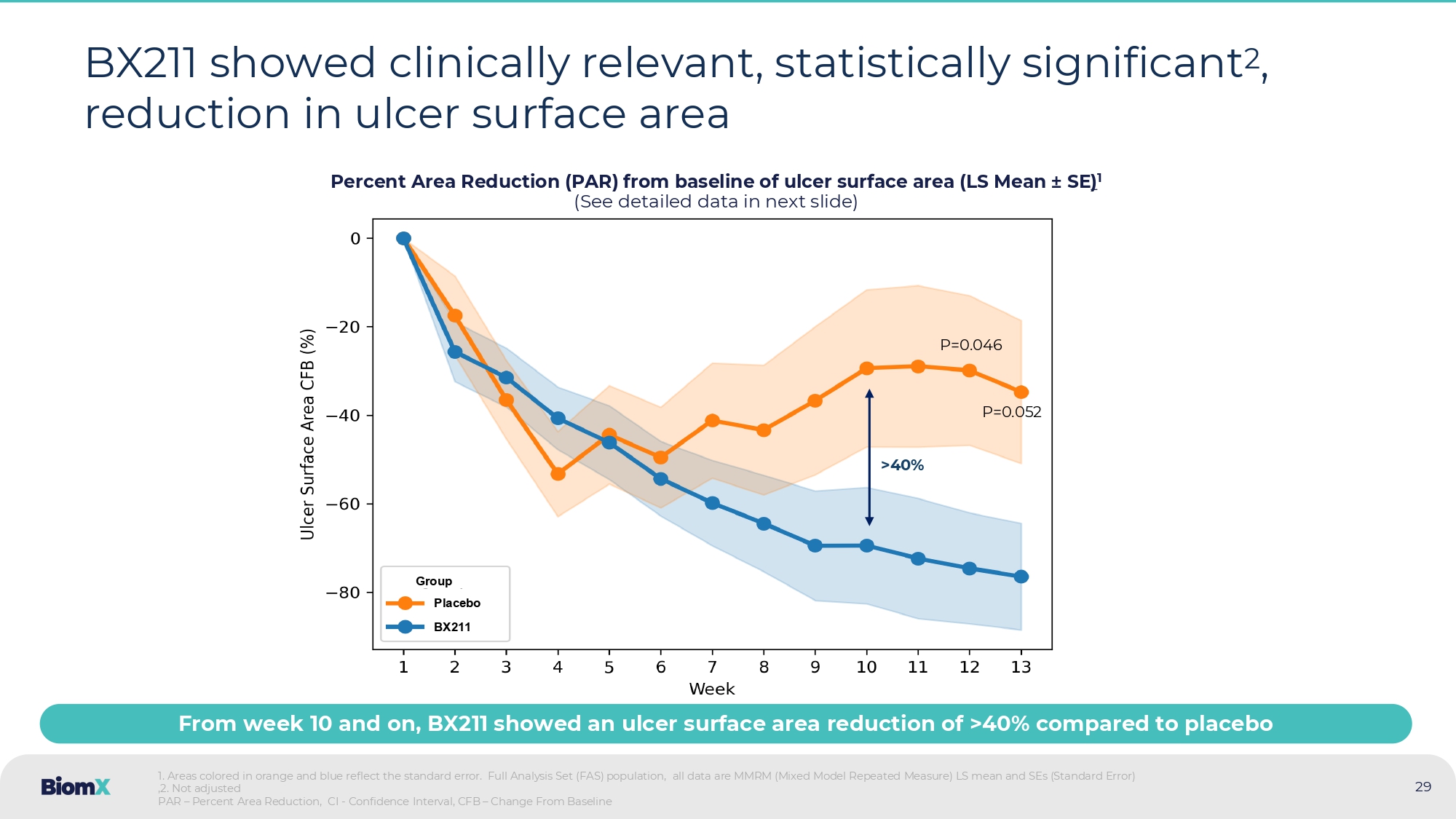
Areas colored in orange and blue reflect the standard error. Full Analysis Set (FAS) population, all data are MMRM (Mixed M odel Repeated Measure) LS mean and SEs (Standard Error) ,2.
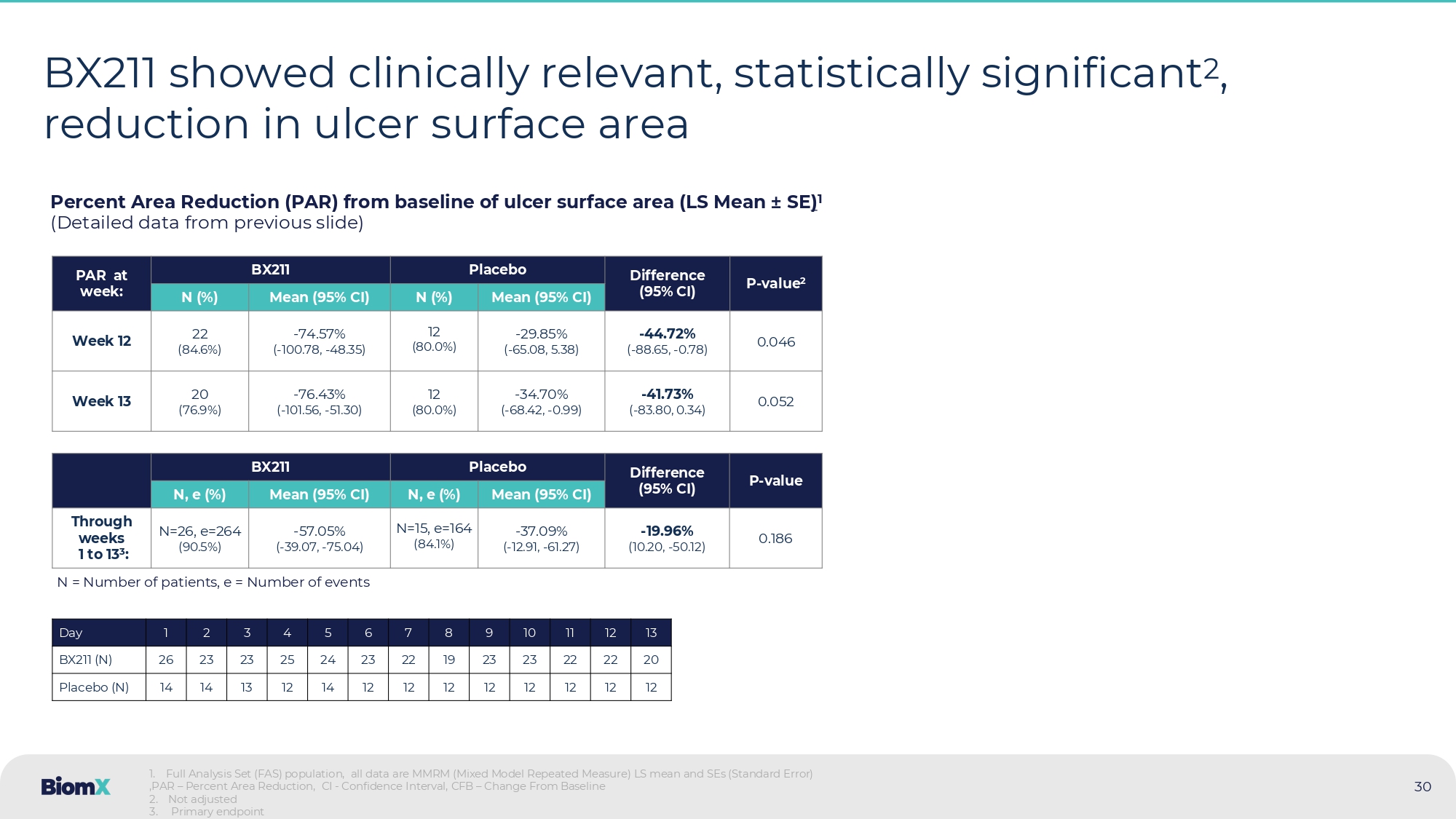
Not adjusted PAR – Percent Area Reduction, CI - Confidence Interval, CFB – Change From Baseline BX211 Group Placebo > 40 % From week 10 and on, BX211 showed an ulcer surface area reduction of >40% compared to placebo P=0.052 P=0.046 30 BX211 showed clinically relevant, statistically significant 2 , reduction in ulcer surface area Percent Area Reduction (PAR) from baseline of ulcer surface area (LS Mean ± SE ) 1 (Detailed data from previous slide) P - value 2 Difference (95% CI) Placebo BX211 PAR at week: Mean (95% CI) N (%) Mean (95% CI) N (%) 0.046 - 44.72 % ( - 88.65, - 0.78) - 29.85 % ( - 65.08, 5.38) 12 (80.0%) - 74.57 % ( - 100.78, - 48.35) 22 (84.6%) Week 12 0.052 - 41.73 % ( - 83.80, 0.34) - 34.70 % ( - 68.42, - 0.99) 12 (80.0%) - 76.43 % ( - 101.56, - 51.30) 20 (76.9%) Week 13 P - value Difference (95% CI) Placebo BX211 Mean (95% CI) N, e (%) Mean (95% CI) N, e (%) 0.186 - 19.96 % (10.20, - 50.12) - 37.09 % ( - 12.91, - 61.27) N=15, e=164 (84.1%) - 57.05 % ( - 39.07, - 75.04) N=26, e=264 (90.5%) Through weeks 1 to 13 3 : 1 . Full Analysis Set (FAS) population, all data are MMRM (Mixed Model Repeated Measure) LS mean and SEs (Standard Error) ,PAR – Percent Area Reduction, CI - Confidence Interval, CFB – Change From Baseline 2 . Not adjusted 3 . Primary endpoint 13 12 11 10 9 8 7 6 5 4 3 2 1 Day 20 22 22 23 23 19 22 23 24 25 23 23 26 BX211 (N) 12 12 12 12 12 12 12 12 14 12 13 14 14 Placebo (N) N = Number of patients, e = Number of events 31 Patients with ulcers at bone depth 1 displayed statistically significant 2 better recovery in the BX211 group Change in tissue involvement of the ulcer for weeks 1 and 13 1 1.
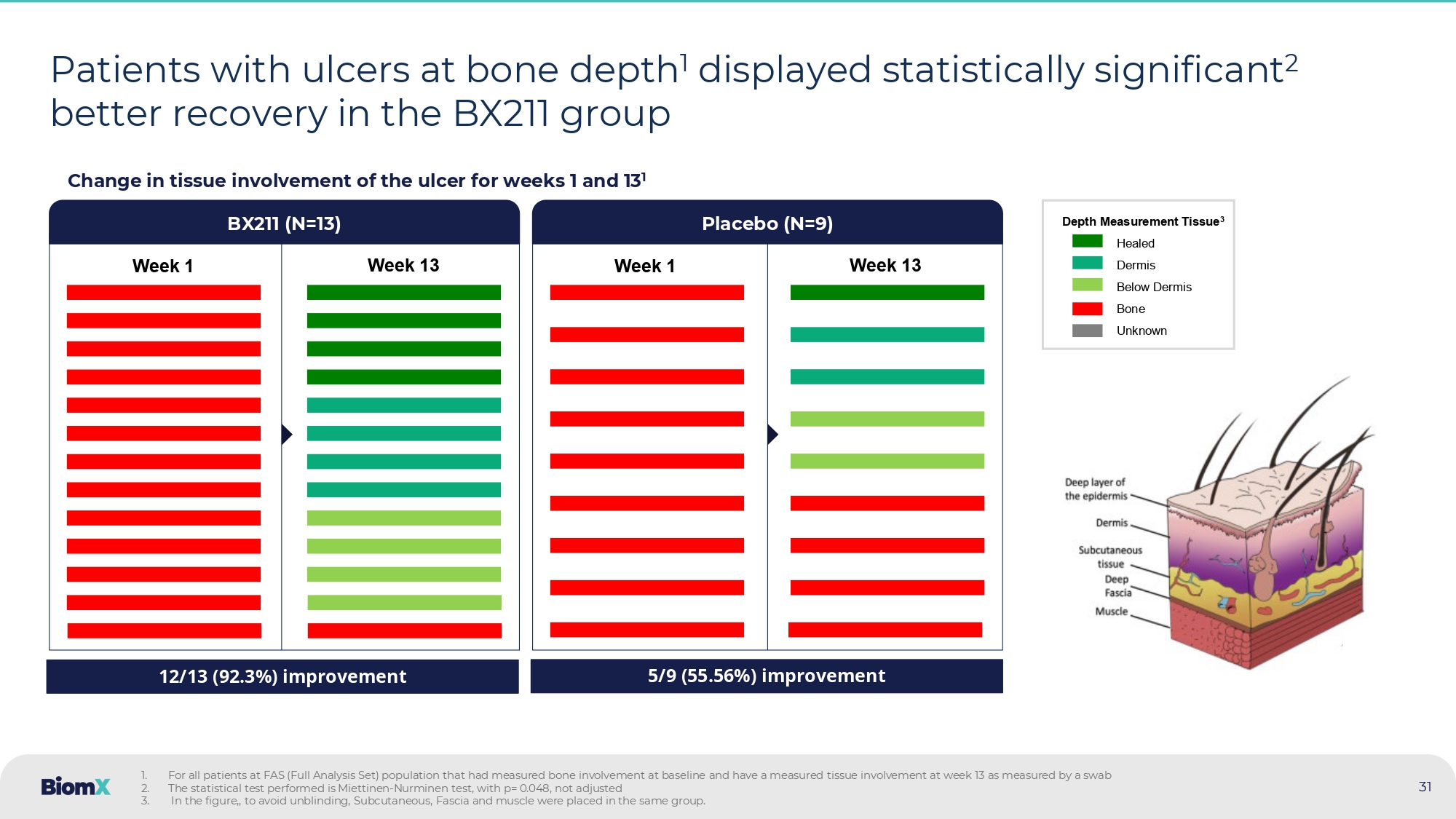
For all patients at FAS (Full Analysis Set) population that had measured bone involvement at baseline and have a measured tis sue involvement at week 13 as measured by a swab 2. The statistical test performed is Miettinen - Nurminen test, with p= 0.048, not adjusted 3. In the figure,, to avoid unblinding, Subcutaneous, Fascia and muscle were placed in the same group.
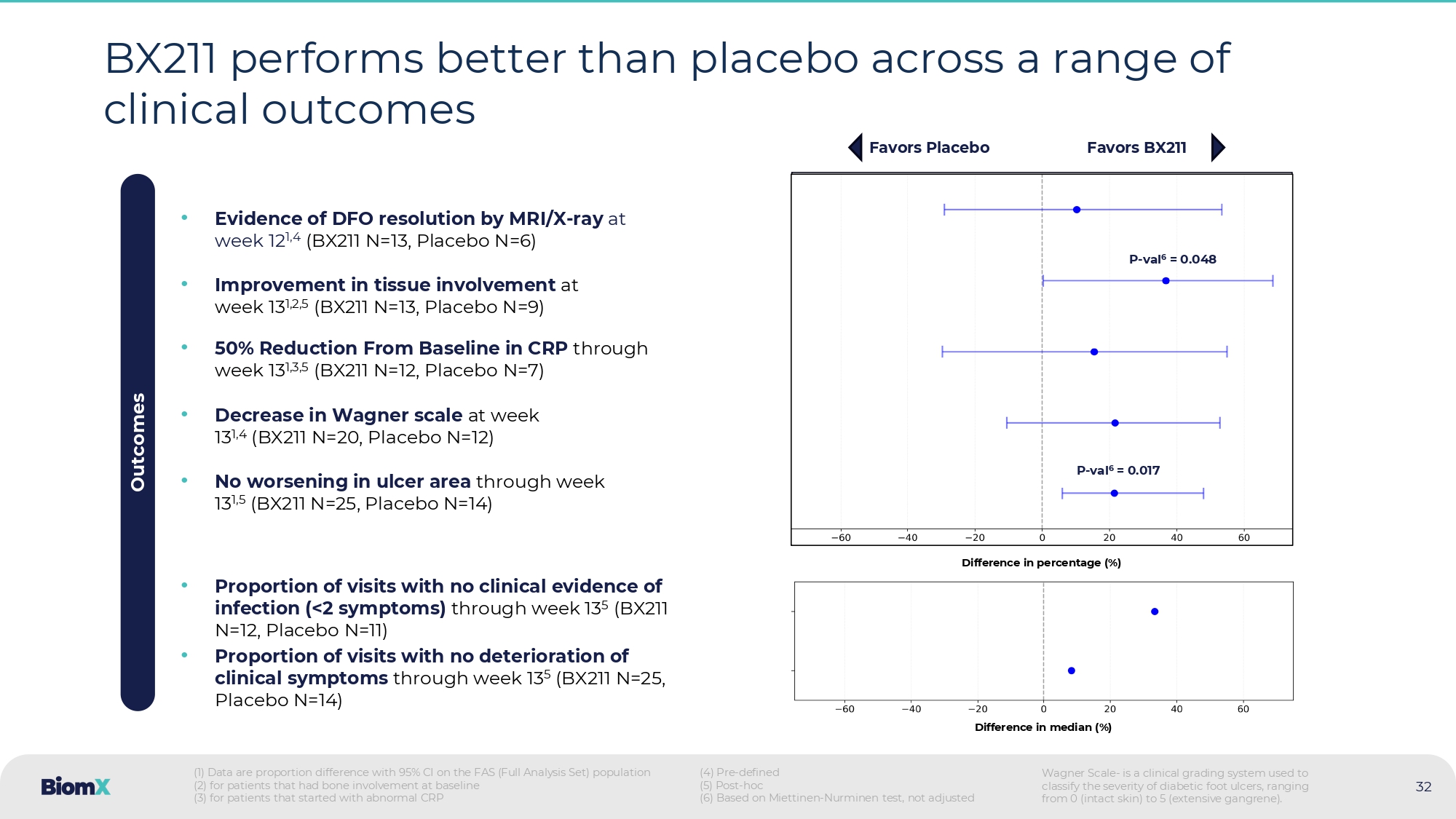
Week 1 Week 13 BX 211 (N= 13 ) Placebo (N=9) Week 1 Week 13 12/13 (92.3%) improvement 5/9 (55.56%) improvement Depth Measurement Tissue 3 Healed Dermis Below Dermis Bone Unknown 32 BX 211 performs better than placebo across a range of clinical outcomes • No worsening in ulcer area through week 13 1,5 (BX211 N=25, Placebo N=14) • 50% Reduction From Baseline in CRP through week 13 1,3,5 (BX211 N=12, Placebo N=7) • Evidence of DFO resolution by MRI/X - ray at week 12 1,4 (BX211 N=13, Placebo N=6) • Improvement in tissue involvement at week 13 1,2,5 (BX211 N=13, Placebo N=9) • Decrease in Wagner scale at week 13 1,4 (BX211 N=20, Placebo N=12) Outcomes • Proportion of visits with no deterioration of clinical symptoms through week 13 5 (BX211 N=25, Placebo N=14) • Proportion of visits with no clinical evidence of infection (< 2 symptoms) through week 13 5 (BX 211 N= 12 , Placebo N= 11 ) Favors Placebo Favors BX211 (1) Data are proportion difference with 95% CI on the FAS (Full Analysis Set) population (2) for patients that had bone involvement at baseline (3) for patients that started with abnormal CRP (4) Pre - defined (5) Post - hoc (6) Based on Miettinen - Nurminen test, not adjusted Difference in median (%) Difference in percentage (%) P - val 6 = 0.017 P - val 6 = 0.048 Wagner Scale - is a clinical grading system used to classify the severity of diabetic foot ulcers, ranging from 0 (intact skin) to 5 (extensive gangrene).


BX211: Potential B reakthrough in DFO/DFI • Results of the Phase 2 study mark, to the Company’s knowledge, the first well - controlled, double - blind, placebo - controlled clinical study to demonstrate statistically significant efficacy of a phage therapy in a clinical endpoint for a chronic bacterial infection • Treating patients with DFO and demonstrated clinical effect on top of standard of care including antibiotics, highlights the clinical strength of BX211 and the unique properties of phage therapy • In the last 20 years, no new drugs were approved for DFI or DFO, providing a unique opportunity for BX211 to address this dire unmet need FINANCING AND INVESTORS UNMET NEED • In several chronic diseases, such as CF, NCFB, and diabetes, underlying related infections become harder to treat as resistant pathogens emerge • Accordingly, the need for new antimicrobial therapies becomes more urgent every year PHAGE THERAPY - PICKING UP MOMENTUM • BiomX and peer companies have shown evidence of clinical effects with phage therapy • Hundreds of cases of compassionate usage of phage BX 2 11 • Incidence of moderate - severe DFI + DFO annually in US approximately 400,000 . Potential commercial opportunity of > $2.5 billion worldwide 3 • Positive results in P2 study, preparing Phase 2/3 pending FDA feedback • Pseudomonas aeruginosa ( ‘ PsA ’ ) lung infections are a leading cause of morbidity and mortality In CF. Potential commercial opportunity of > $ 1.5 billion worldwide 1 • Positive results i n a Phase 1 b/ 2 a study - 14.3 % of patients in the BX 004 arm converted to sputum culture negative for PsA after 10 days of treatment compared to none in the placebo arm 2 • Phase 2 b readout expected Q 1 26 BX004 • Publicly traded ( NYSE American: PHGE ) • $1.7. 8 . million cash and cash equivalents as of Dec 31, 2024 (Excluding 12M in gross proceeds raised in February 2025 financing) Executive Summary 1. See slide 33 2. In patients that had quantitative CFU levels at study baseline 3.

Thank you
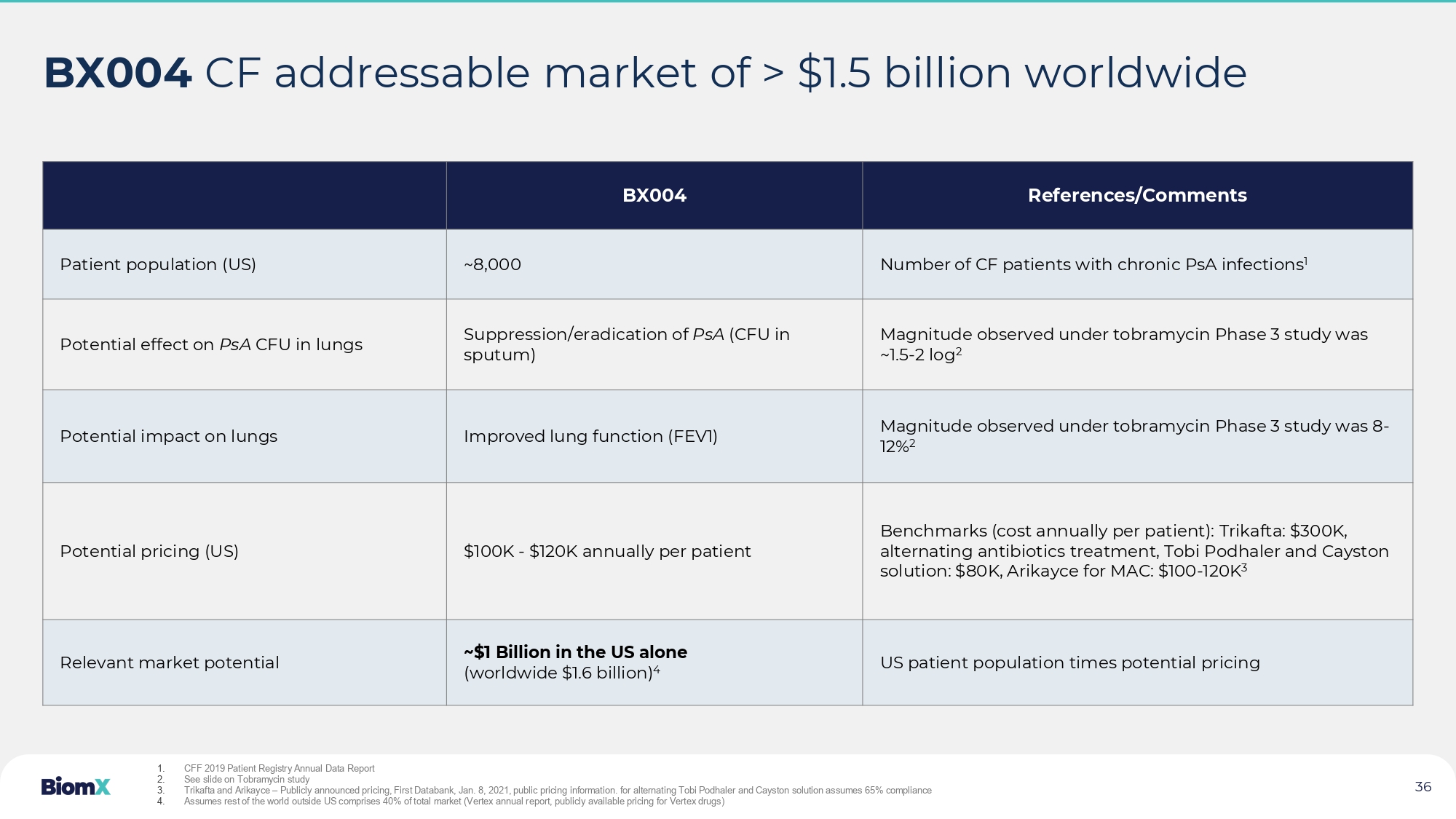
See slide 34 • Key investors: 34 36 BX004 CF addressable market of > $1.5 billion worldwide References/Comments BX004 Number of CF patients with chronic PsA infections 1 ~8,000 Patient population (US) Magnitude observed under tobramycin Phase 3 study was ~1.5 - 2 log 2 Suppression/eradication of PsA (CFU in sputum) Potential effect on PsA CFU in lungs Magnitude observed under tobramycin Phase 3 study was 8 - 12% 2 Improved lung function ( FEV1) Potential impact on lungs Benchmarks (cost annually per patient): Trikafta : $300K, alternating antibiotics treatment, Tobi Podhaler and Cayston solution: $80K, Arikayce for MAC: $100 - 120K 3 $100K - $120K annually per patient Potential pricing (US) US patient population times potential pricing ~$1 Billion in the US alone (worldwide $1.6 billion) 4 Relevant market potential 1. CFF 2019 Patient Registry Annual Data Report 2. See slide on Tobramycin study 3. Trikafta and Arikayce – Publicly announced pricing, First Databank, Jan. 8, 2021, public pricing information. for alternating Tobi Podhaler and Cayston solution assumes 65% compliance 4. Assumes rest of the world outside US comprises 40% of total market ( Vertex annual report, publicly available pricing for Vertex drugs)

References/Comments BX211 Moderate - Severe DFI overlaps with DFO population Incidence assumed based on 400K ER hospital visits due to DFI 1 400,000 Incidence of moderate - severe DFI + DFO (annually, US) Deduction for cases positive for S. aureus – 50% 200,000 Relevant incidence for BX211 treatment (annually, US) Antibiotic pricing per course of treatment for ABSSSI 2 ranges 2,500 - 7,500$ ( Dalvance, Orbactiv, Sivextro, Teflaro) 3 High range of pricing selected as addressing hard to treat moderate - severe DFI & DFO cases saving potential amputation costs (direct $50K/patient) $7,500 Pricing (US) 200K times $7.5K $1.5 Billion Relevant market for BX211, US OECD (without US) estimated at > $1 billion. Assumes: OECD diabetic population outside US >1.5 times US diabetic population, OECD pricing to be 50% of US pricing 4 >$2.5 Billion Relevant market for BX211, Worldwide 37 BX 211 moderate - severe DFI & DFO addressable market of >$ 2.5 B worldwide (1) The number was calculated based on Skrepnek , 2024 and Ashman, 2023 (2) ABSSSI - acute bacterial skin and skin structure infection. (3) Prices taken from drugs.com based on recommended course of treatment.

(4) oecd.org Chronic diseases publication 38 Dr. Bassan was most recently Vice President Head of Translational Sciences at Teva Pharmaceutical Industries, Inc., where she was responsible for early stages of clinical development via translation from animal data to human. Prior to this role, Dr. Bassan served as Vice President of Project Leadership at Teva Pharmaceutical, where she managed project leaders overseeing end - to - end drug development at pre - clinical, PI - III and post marketing stages in multiple therapeutic areas, such as pain, oncology, women‘s health, endocrinology, GI, biosimilars and other areas. Overall, Dr. Bassan has over 20 years of leadership experience with clinical and drug development teams in her various roles at Teva Pharmaceutical and other smaller biotech companies. MERAV BASSAN, PH.D. | CDO Prior to his role in BiomX , Mr. Solomon was a co - founder, president, and CEO of ProClara (formerly NeuroPhage ), which is pioneering an approach to treating neurodegenerative diseases. Under his leadership, the company raised more than $100 million and launched an ongoing clinical trial related to Alzheimer’s disease. Mr. Solomon holds a B.Sc. magna cum laude in Physics and Mathematics from the Hebrew University, an M.Sc. summa cum laude in Electrical Engineering from Tel Aviv University, and an M.B.A. with honors from the Harvard Business School.

JONATHAN SOLOMON | CEO & BOARD MEMBER Management T eam 39 Ms. Benjamini - Elran has over 15 years of experience in executive HR roles in global and diverse environments. At Teva Pharmaceuticals Industries Inc (NYSE:TEVA) she served in various senior roles including Director of HR of the European HQ (Netherlands) and HR manager of R&D API division. Her most recent experience was as Head of HR at Herzog, one of the largest law firms in Israel and as an independent HR consultant, advising a variety of companies in the Israeli hi - tech and biotech sectors. Ms. Benjamini - Elran holds an M.B.A. from Bar - Ilan University and a B.A. in behavioural science from Ben - Gurion University. INBAL BENJAMINI - ELRAN | CHRO Management T eam (cont’d) Marina Wolfson, Chief Financial Officer, joined BiomX in December 2019, bringing extensive experience from large pharmaceutical and high - tech companies, as well as venture capital funds. Prior to joining the company, Ms. Wolfson served as Vice President of Finance at BioView Ltd. (TASE) from 2010 to 2019 and as a senior auditor at Ernst & Young, an international auditing and business advisory firm, from 2007 to 2010. Ms. Wolfson is a certified public accountant in Israel and holds a B.A. in Economics and Accounting (with honors) and an MBA (with honors, specializing in finance) from Ben - Gurion University .

MARINA WOLFSON | CFO 40 Russell G. Greig, Ph.D. worked at GlaxoSmithKline for three decades, most recently as President of SR One, GlaxoSmithKline’s corporate venture group. Prior to joining SR One, he served as President of GlaxoSmithKline’s Pharmaceuticals International from 2003 to 2008 as well as on the GlaxoSmithKline corporate executive team. Currently, Dr. Greig serves as Chairman of MedEye Solutions in the Netherlands, eTheRNA in Belgium and Sanifit in Spain. RUSSELL GREIG, PH.D. CHAIRMAN OF THE BOARD OF DIRECTORS Jonathan Leff is a Partner on the Therapeutics team at Deerfield and Chairman of the Deerfield Institute, and joined the Firm in 2013. He focuses on venture capital and structured investments in biotechnology and pharmaceuticals. He is a member of the Boards of several public and private healthcare companies as well as several not - for - profit organizations, including the Spinal Muscular Atrophy Foundation and the Columbia University Medical Center . JONATHAN LEFF DIRECTOR Alan Moses, M.D., was co - founder and co - director of the Clinical Investigator Training Program at Beth Israel Deaconess - Harvard Medical School - MIT. Dr. Moses served as Senior Vice President and Chief Medical Officer of the Joslin Diabetes Center in Boston. He was appointed Professor of Medicine at Harvard Medical School. Over the course of 14 years at Novo Nordisk, Dr. Moses served in multiple roles, rising to the position of Senior Vice President and Global Chief Medical Officer. ALAN MOSES, MD DIRECTOR Mr. Greg Merril is a serial life - science entrepreneur, recognized by Ernst & Young as a regional Entrepreneur of the Year winner. He has served as Chair of several international phage therapy conferences. As prior founding CEO of Immersion Medical (NASDQ: IMMR) he led the creation of the world’s first commercially successful virtual reality surgical training simulators. GREG MERRIL DIRECTOR Mr. Eddie Williams is a well - recognized, senior global life sciences executive with extensive boardroom and commercial operations experience. He most recently served as a Special Advisor to the Chief Executive Officer of Ascendis Pharma, Inc., and previously as their interim U.S. Chief Commercial Officer. EDDIE WILLIAMS DIRECTOR Jesse Goodman, M.D., M.P.H. is Professor of Medicine at Georgetown University and Director of the Center on Medical Product Access, Safety and Stewardship which focuses on science and policy to address public health needs including antimicrobial resistance. He is Attending Physician in Infectious Diseases at Georgetown University, Washington DC Veterans Administration and Walter Reed Medical Centers . JESSE GOODMAN, MD, MPH DIRECTOR Prior to his role in BiomX , Mr. Solomon was a co - founder, president, and CEO of ProClara (formerly NeuroPhage ), which is pioneering an approach to treating neurodegenerative diseases. Under his leadership, the company raised more than $100 million and launched an ongoing clinical trial related to Alzheimer’s disease. JONATHAN SOLOMON DIRECTOR Ms. Blum is the Chief Financial Officer of Melinta Therapeutics, LLC. (“ Melinta ”), a company focused on the development and commercialization of innovative therapies for acute and life - threatening illnesses. She joined Melinta in 2016 as the company’s Controller, and then served as Vice President of Finance & Chief Accounting Officer prior to being appointed to the CFO position in 2021. SUSAN BLUM DIRECTOR Board of Directors