UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 20, 2024
ABVC BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 001-40700 | 26-0014658 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
|
44370 Old Warm Springs Blvd. Fremont, CA |
94538 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number including area code: (510) 668-0881
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per share | ABVC | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On August 20, 2024, ABVC BioPharma, Inc. (the “Company”) updated its corporate presentation . A copy of the Company’s presentation is attached as Exhibit 99.1 and incorporated herein by reference.
Neither this Current Report on Form 8-K, nor any exhibit attached hereto, is an offer to sell or the solicitation of an offer to buy the Securities described herein. Such disclosure does not constitute an offer to sell, or the solicitation of an offer to buy nor shall there be any sales of the Company’s securities in any state in which such offer, solicitation or sale would be unlawful.
The information in Item 7.01 of this Current Report on Form 8-K (including Exhibits 99.1 attached hereto) is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference into any filing by the Company, under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
| (d) | Exhibits. |
| Exhibit Number |
Exhibit | |
| 99.1 | Power Point Presentation | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ABVC BioPharma, Inc. | ||
| August 20, 2024 | By: | /s/ Uttam Patil |
| Uttam Patil | ||
| Chief Executive Officer | ||
2
Exhibit 99.1

Corporate Overview NASDAQ: ABVC 2024

2 Forward Looking Statements This presentation contains summary information about ABVC BioPharma, Inc . (“ABVC”) as of the date hereof . The information in this presentation is of general background and contains an overview and summary of certain data selected by the management of ABVC . It does not purport to be complete . This presentation is not a prospectus, disclosure document or offering document under the law of any jurisdiction . It is for informational purposes only . This presentation is not investment or financial product advice (nor tax, accounting or legal advice) and is not intended to be used for the basis of making an investment decision . A recipient must make their own independent investigations, consideration and evaluation of ABVC and the offer and ABVC recommends that investors should obtain their own professional advice before making any investment decisions in the company . This investor presentation shall also not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any states or jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . No registered offering of securities shall be made except by means of a prospectus meeting the requirements of section 10 of the Securities Act of 1933 , as amended . This document has been prepared based on information available at the time of presentation . No representation or warranty, express or implied, is made as to the fairness, accuracy or completeness of the information, opinions and conclusions contained in this presentation or any omission from this presentation or of any other written or oral information or opinions provided now or in the future to any person . While reasonable care has been taken to ensure that facts stated in this presentation are accurate and/or that the opinions expressed are fair and reasonable, no reliance can be placed for any purpose whatsoever on the information contained in this document or its completeness and there is no guarantee that any of the outcomes disclosed will occur by the time specified or at all . To the maximum extent permitted by law, neither ABVC nor their respective officers, directors, employees, advisors and agents, nor any other person, accepts any liability as to or in relation to the accuracy or completeness of the information, statements, opinions or matters (express or implied) arising out of, contained in or derived from this presentation or any omission from this presentation or of any other written or oral information or opinions provided now or in the future to any person . Some of the statements appearing in this presentation are in the nature of forward looking statements . You should be aware that such statements are predictions based on assumptions, and are subject to inherent risks and uncertainties . Those risks and uncertainties include factors and risks specific to the industry in which ABVC operates as well as general economic conditions, prevailing exchange rates and interest rates and conditions in the financial markets and other factors that are in some cases beyond ABVC's control . As a result, any or all of the ABVC’s forward - looking statements in this presentation may turn out to be inaccurate . Except as required by law, we are under no duty to update or revise any of the forward - looking statements, whether as a result of new information, future events or otherwise, after the date of this presentation . These forward - looking statements speak only as of the date of this presentation, and we assume no obligation to update or revise these forward - looking statements for any reason .
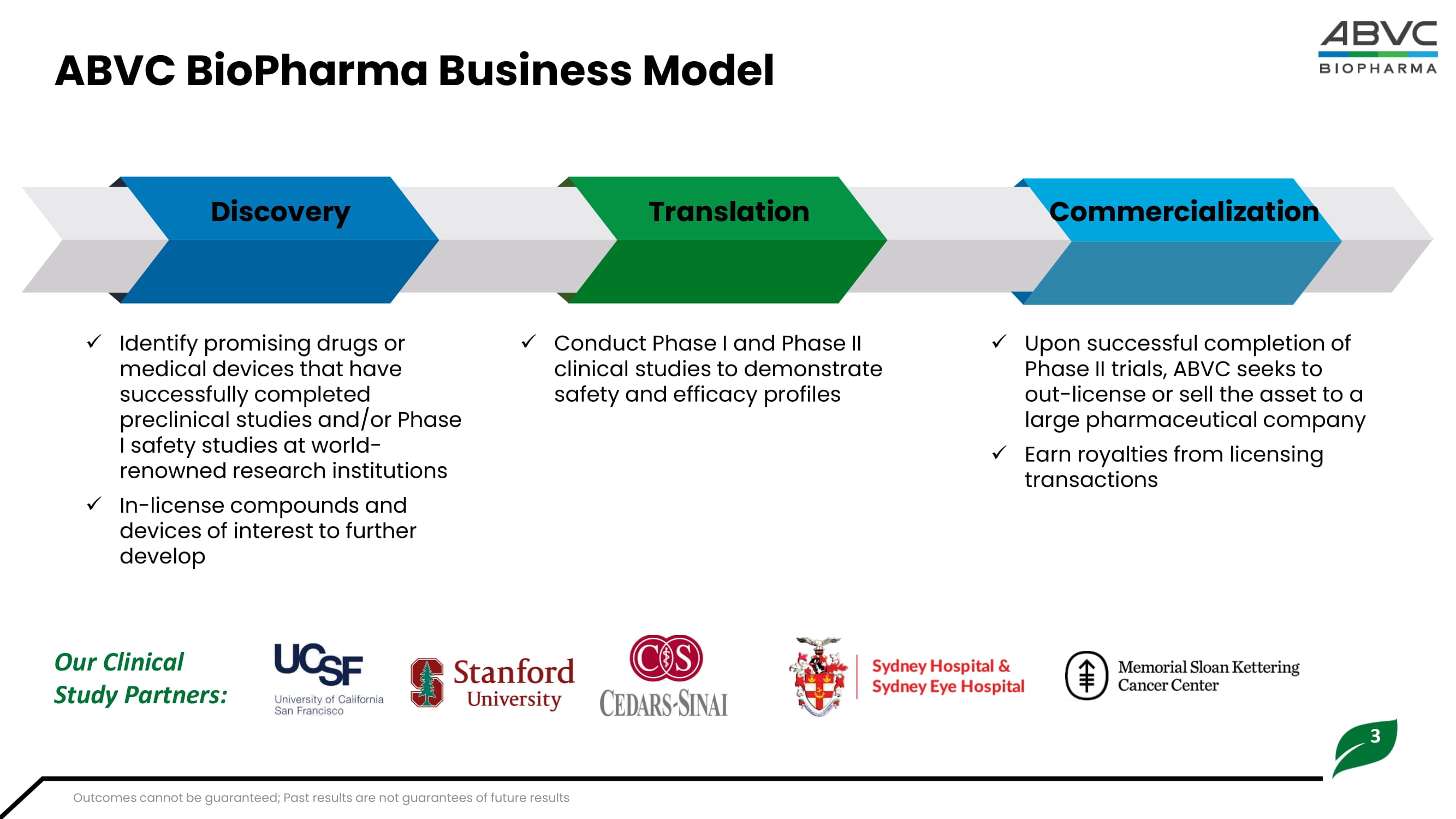

3 ABVC BioPharma Business Model Discovery Translation Commercialization x Identify promising drugs or medical devices that have successfully completed preclinical studies and/or Phase I safety studies at world - renowned research institutions x In - license compounds and devices of interest to further develop x Conduct Phase I and Phase II clinical studies to demonstrate safety and efficacy profiles x Upon successful completion of Phase II trials, ABVC seeks to out - license or sell the asset to a large pharmaceutical company x Earn royalties from licensing transactions Our Clinical Study Partners: Outcomes cannot be guaranteed; Past results are not guarantees of future results 4 Key Financial Achievements 2 : Revenue Growth : $ 117 , 142 in Q 2 2024 , up from $ 6 , 109 in Q 2 2023 . Earnings Per Share (EPS) : Improved to - $ 0 . 09 in Q 2 2024 , up 86 . 8 % from - $ 0 . 68 in Q 2 2023 . Shareholders' Equity : $ 7 . 8 million as of June 30 , 2024 . Financial and Strategic Highlights Patent and FDA Approvals : MDD and ADHD Treatments : Multiple patents received in the US, Taiwan, and Australia . Phase II trials completed for MDD ; Phase IIb trials ongoing for ADHD . Strategic Licensing Agreements 1 , 4 : Psychiatric Drug with AiBtl BioPharma, Inc .: Potential income : Up to $ 667 million . Upfront payments : $ 460 M received ( 46 M shares at $ 10 per share 3 ) in November 2023 . Potential milestone payment : $ 7 million in cash . Vitargus® Licensing with ForSeecon Eye Corporation : Potential income : Up to $ 187 million . Milestone payment received : $ 116 , 000 in June 2024 . Vitargus® was approved for the next trial phase by the Australian TGA . GMP facility construction is underway in Taiwan . Oncology Products Licensing with OncoX BioPharma, Inc .: Potential income : Up to $ 105 million . 1. Potential royalties, if products are commercialized, are included in stated potential income. 3. Stock price is based on inter nal negotiations not verified by third party. 2. Financial figures are unaudited. 4. Potential income is not guaranteed.

5 Leadership Team Uttam Yashwant Patil, PhD Chief Executive Officer and Interim CFO Leeds Chow Chief Financial O ffi cer 1 T. S. Jiang, PhD Chief Scientific Officer Scientific Advisory Board Management Yih - Shiou Hwang, MD, PhD Susanna Cunningham - Rundles, PhD Maurizio Fava, PhD Keith McBurnett , PhD Thomas Laughren , PhD Suspended of duties during current contract negotiations.

6 Robust & Diverse Pipeline Clinical Partners Phase III Phase II Phase I Preclinical Indication Program Vitreous Replacement Vitargus® (ABV - 1701) Ophthalmology Medical Device Major Depressive (MDD) ABV - 1504 Psychiatric Disorders New Drugs Attention - Deficit/Hyperactivity Disorder (ADHD) ABV - 1505 Depression in Cancer Patients ABV - 1601 Triple Negative Breast Cancer (TNBC) ABV - 1501 Oncology Non - Small Cell Lung Cancer (NSCLC) ABV - 1519 Myelodysplastic Syndrome (MDS) ABV - 1702 Pancreatic Cancer (combination therapy) ABV - 1703 7 Vitargus® for Retinal Detachment & Vitreous Hemorrhage Detached Retina Vitrectomy Surgery Vitreous Hemorrhage Vitargus® is a Vitreous substitute that could potentially be used in retinal detachment and vitreous hemorrhage surgeries to accelerate healing and eliminate the need for a second surgery • Macular Hole • Macular Pucker • Diabetic Retinopathy • Retinal Vein Occlusion • Vitreous Body Injury Healthy Eye Vitreous Humor Retina Detached Retina Subretinal Fluid Vitreous Humor Vitreous Humor Retina Posterior Vitreous Detachment Vitreous Hemorrhage

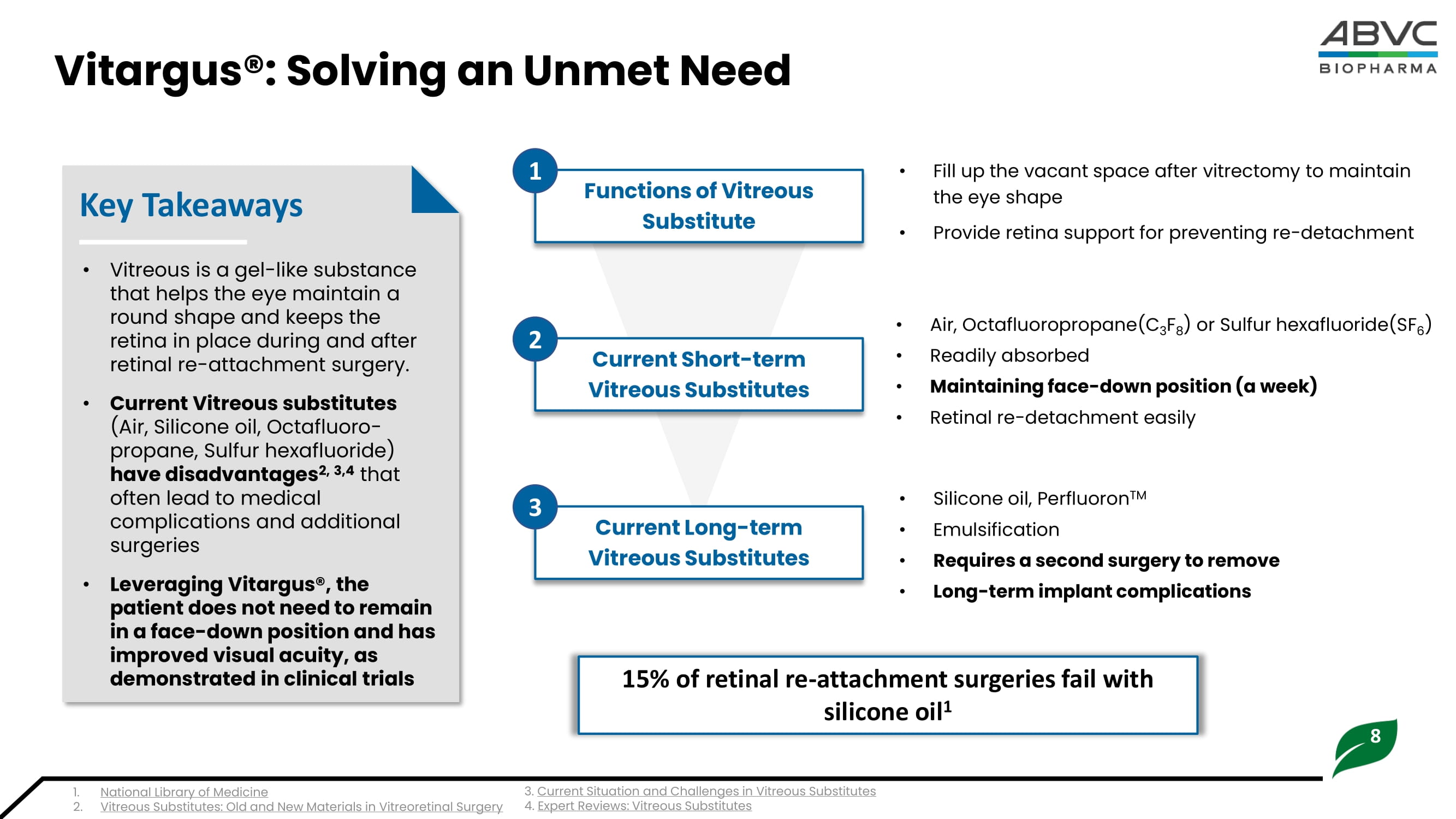
8 Vitargus®: Solving an Unmet Need • Vitreous is a gel - like substance that helps the eye maintain a round shape and keeps the retina in place during and after retinal re - attachment surgery. • Current Vitreous substitutes (Air, S ilicone oil, O ctafluoro - propane, Sulfur hexafluoride) have disadvantages 2, 3,4 that often lead to medical complications and additional surgeries • Leveraging Vitargus®, the patient does not need to remain in a face - down position and has improved visual acuity, as demonstrated in clinical trials Key Takeaways • Fill up the vacant space after v itrectomy to maintain the eye shape • Provide retina support for preventing re - detachment • Silicone oil, Perfluoron TM • Emulsification • Requires a second surgery to remove • Long - term implant complications Functions of Vitreous Substitute Current Short - term Vitreous Substitutes Current Long - term Vitreous Substitutes • Air, Octafluoropropane (C 3 F 8 ) or Sulfur hexafluoride (SF 6 ) • Readily absorbed • Maintaining face - down position (a week) • Retinal re - detachment easily 15% of retinal re - attachment surgeries fail with silicone oil 1 1 2 3 1. National Library of Medicine 2. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery 3. Current Situation and Challenges in Vitreous Substitutes 4.

Expert Reviews: Vitreous Substitutes 9 The U.S. remains the largest market, however, the demand in Asia - Pacific represents the fastest growing market 4 ABVC plans to develop and commercialize Vitargus® in Asia and Europe prior to seeking FDA approval Vitargus® Total Addressable Market ~225,000 vitrectomies are performed annually in the U.S. alone 1 1. Vanderbilt University: Prospective Retinal and Optic Nerve Vitrectomy Evaluation Study 2. JAMA Ophthalmology $2 , 280 cost of Perfluoron Kit 3 (sold by Alcon Labs and distributors) ~$500M+ Annual Market Reimbursed indication growth 1, 2 ~900k patients with diabetic retinopathy in the U.S. have “vision - threatening” retinopathy but are not eligible for vitrectomy surgery due to age, coverage, and various other factors 3. Grayline Medical ; Medex Supply ; Serfinity Medical 4.
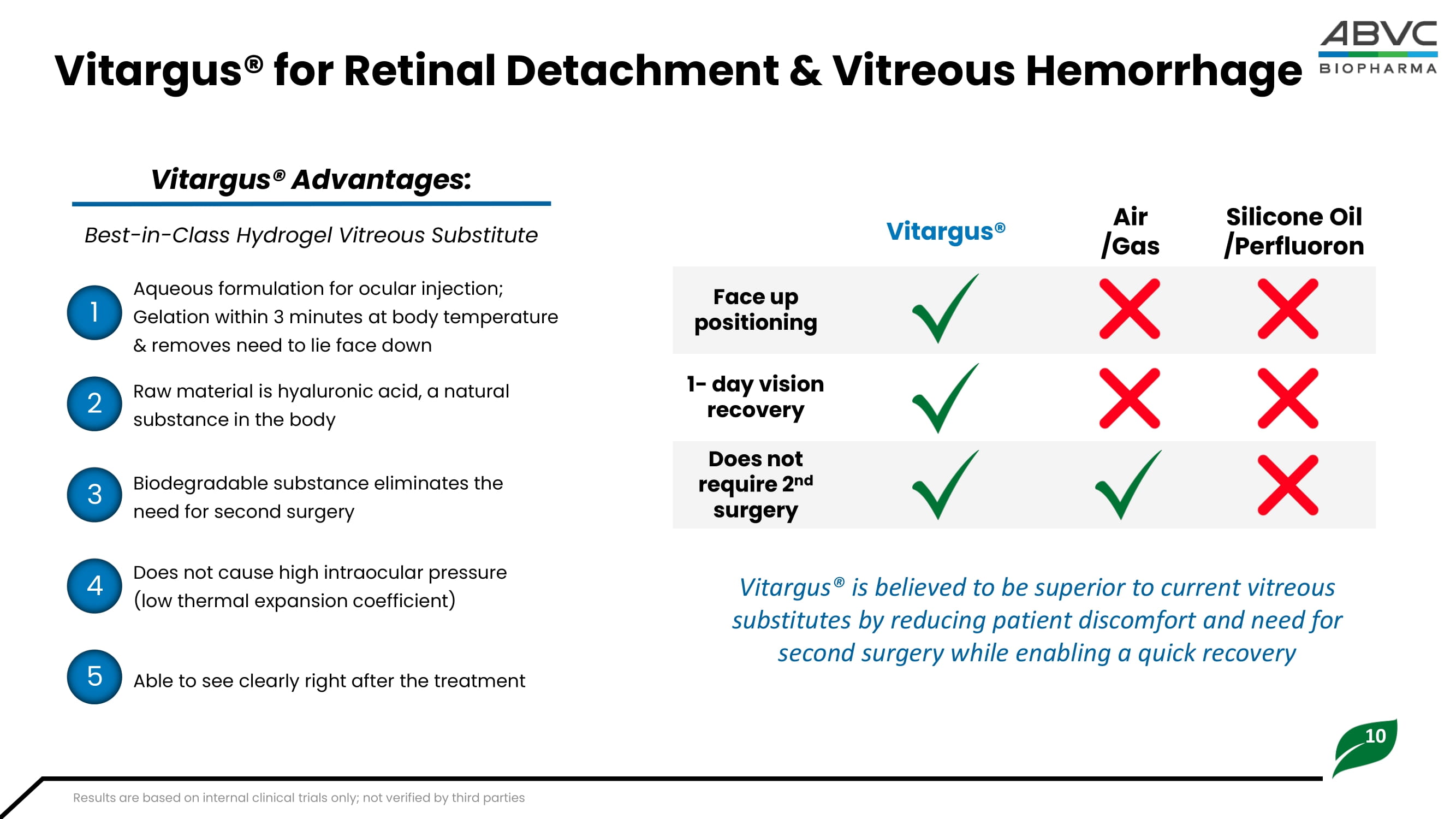
Mordor Intelligence: Vitreoretinal Survery Devices Market Size and Share Analysis 10 Vitargus® for Retinal Detachment & Vitreous Hemorrhage Vitargus® Advantages: Raw material is hyaluronic acid, a natural substance in the body Silicone Oil / Perfluoron Air /Gas Vitargus® Face up positioning 1 - day vision recovery Does not require 2 nd surgery Aqueous formulation for ocular injection; Gelation within 3 minutes at body temperature & removes need to lie face down Biodegradable substance eliminates the need for second surgery Does not cause high intraocular pressure (low thermal expansion coefficient) Able to see clearly right after the treatment 1 2 3 4 5 Best - in - Class Hydrogel Vitreous Substitute Vitargus® is believed to be superior to current vitreous substitutes by reducing patient discomfort and need for second surgery while enabling a quick recovery Results are based on internal clinical trials only; not verified by third parties 11 Vitargus®: Completed First in Human Feasibility Study 1 Key Takeaways • Vitargus® was well - tolerated with no apparent toxicity to ocular tissues • A statistically significant improvement from baseline in best corrected visual acuity (BCVA) • The optical properties of Vitargus® allowed the patients to see well and facilitated visualization of the fundus immediately following surgery.

• Vitargus® sets as a stable semisolid gel adhering to the retina and maintains its position without the need of face - down positioning. 1.37 0.47 1.43 0.68 1.34 0.38 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 Mean Best Corrected Visual Acuity ( LogMAR ) Time Significant BCVA Improvement Over 4 Months (n=11) Retinal Detachement & Vitreous Hemorrhage Retinal Detachement (n=4) Vitreous Hemorrhage (n=7) Best Corrected Visual Acuity (BVCA) is the standard to assess visual acuity, or ‘sharpness of vision’ measured by the ability to perceive letters and numbers. The lower score indicates the ability to read further down the ETDRS Chart. 1. Retina 2019, Section IX: First - time Results of Clinical Trials, page 64 ETDRS: Early Treatment Diabetic Retinopathy Study Outcomes cannot be guaranteed; Past results are not guarantees of future results 12 Vitargus® Phase II Clinical Study Initiated in March 2023, expected to be completed 2H 2024 1 • Uncomplicated retinal detachment, defined as the first instance of a small macular hole and retinal tears.
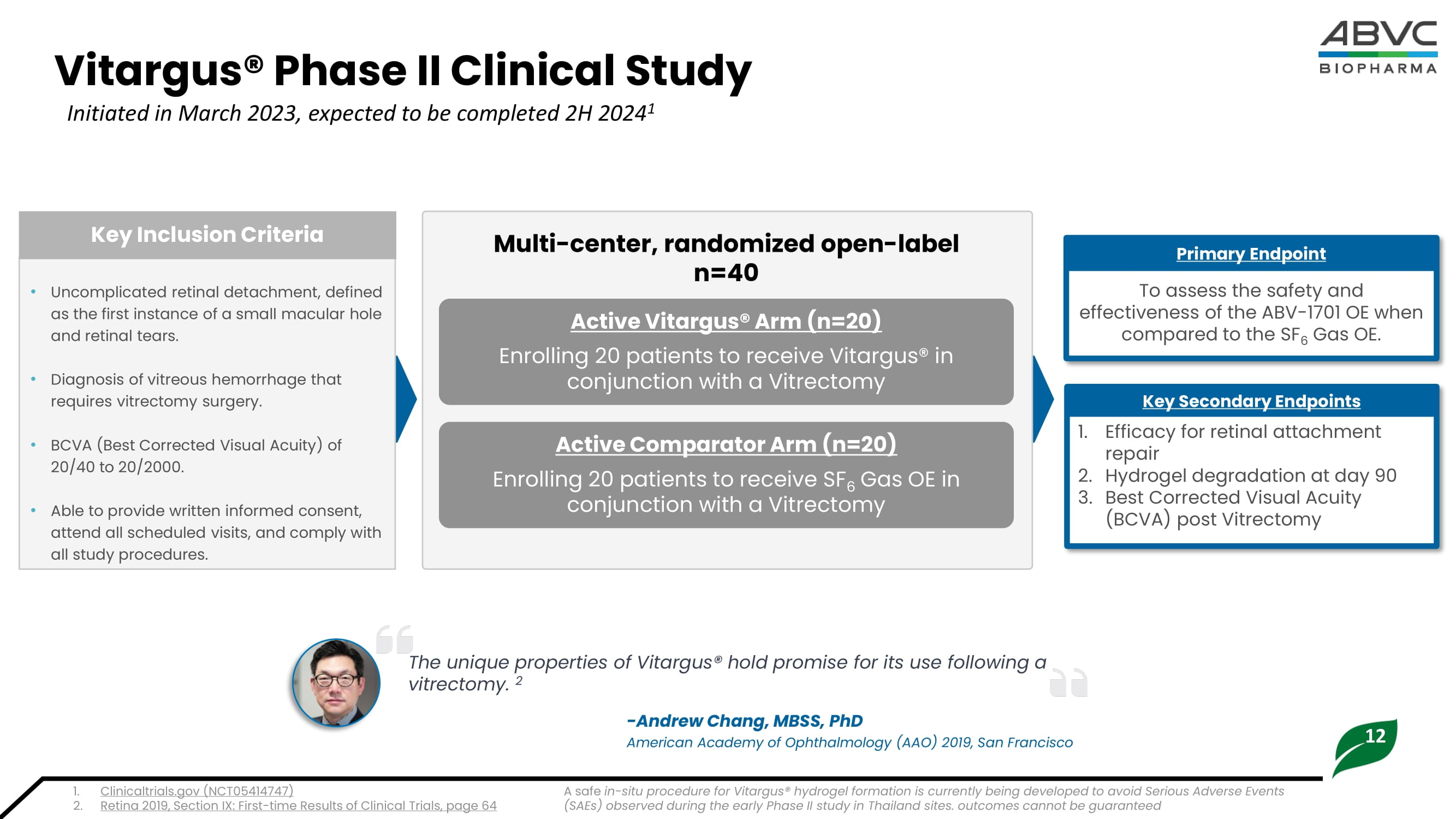
• Diagnosis of vitreous hemorrhage that requires vitrectomy surgery. • BCVA (Best Corrected Visual Acuity) of 20/40 to 20/2000. • Able to provide written informed consent, attend all scheduled visits, and comply with all study procedures. Key Inclusion Criteria To assess the safety and effectiveness of the ABV - 1701 OE when compared to the SF 6 Gas OE. Primary Endpoint 1. Efficacy for retinal attachment repair 2. Hydrogel degradation at day 90 3. Best Corrected Visual Acuity (BCVA) post V itrectomy Key Secondary Endpoints Active Comparator Arm (n=20) Enrolling 20 patients to receive SF 6 Gas OE in conjunction with a Vitrectomy Active Vitargus® Arm (n=20) Enrolling 20 patients to receive Vitargus® in conjunction with a Vitrectomy Multi - center, randomized open - label n=40 The unique properties of Vitargus ® hold promise for its use following a vitrectomy. 2 - Andrew Chang, MBSS, PhD American Academy of Ophthalmology (AAO) 2019, San Francisco 1. Clinicaltrials.gov (NCT05414747) 2. Retina 2019, Section IX: First - time Results of Clinical Trials, page 64 A safe in - situ procedure for Vitargus® hydrogel formation is currently being developed to avoid Serious Adverse Events (SAEs) observed during the early Phase II study in Thailand sites.

outcomes cannot be guaranteed 13 Botanical - Based Pipeline for Psychiatric Disorders ABV - 1504 Major Depression Disorder (MDD) Phase I initiated Phase IIa completed, Phase IIb in progress Phase II completed Clinical Status No SAE’s directly from the drug have been reported No SAE’s directly from the drug have been reported No SAE’s directly from the drug have been reported Safety ~1.9 million newly diagnosed cancer patients / year 6 (~247k w/ depression 7 ) ~11 million adults 5 ~9 million adults (medication - treated MDD 1 ) U.S. Addressable Patient Population ~$342 million annually 2 ~$10 billion 3,4 ~$12.4 billion 2 U.S. Market Size Developing a suite of botanical - based assets to combat rising addiction ABV - 1505 Attention - Deficit/Hyperactivity Disorder (ADHD) ABV - 1601 Depression in Cancer Patients 1. National Library of Medicine: Major Depressive Disorder (MDD) Prevalence 2 National Library of Medicine: MDD Drug Cost Comparison 3. ADHD Statistics and Facts 4. SingleCare : 2022 ADHD Medication Costs 5. Attention Deficit Disorder Association: ADHD Facts 6. American Cancer Society: Cancer Facts & Figures 2022 7. National Library of Medicine: Prevalence of Depression in Cancer Patients 8.
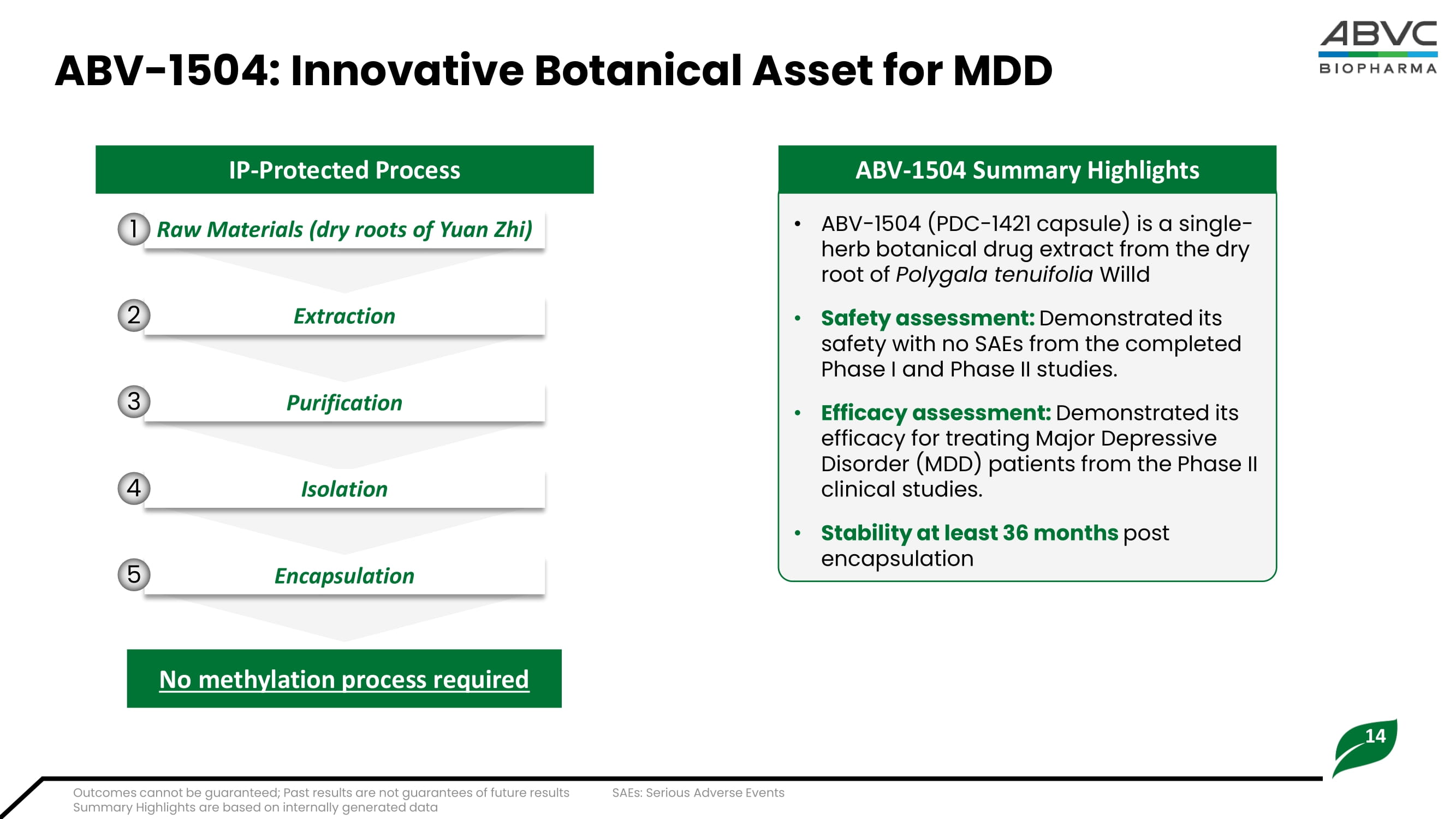
Past results are not guarantees of future performance 14 ABV - 1504: Innovative Botanical Asset for MDD • ABV - 1504 (PDC - 1421 capsule) is a s ingle - herb botanical drug e xtract from the dry root of Polygala tenuifolia Willd • Safety assessment: Demonstrated its safety with no SAEs from the completed Phase I and Phase II studies. • Efficacy assessment: Demonstrated its efficacy for treating Major Depressive Disorder (MDD) patients from the Phase II clinical studies. • Stability at least 36 months post encapsulation IP - Protected Process Raw Materials (dry roots of Yuan Zhi) Extraction Purification Isolation Encapsulation No methylation process required 1 2 3 4 5 ABV - 1504 Summary Highlights Outcomes cannot be guaranteed; Past results are not guarantees of future results Summary Highlights are based on internally generated data SAEs: Serious Adverse Events 15 ABV - 1504 Completed Phase II Highlights MADRS: Montgomery – Åsberg Depression Rating Scale TID: Three times daily • The High - Dose group (760 mg TID) of ABV - 1504 demonstrated a clinically meaningful score in MADRS compared to the Placebo group.
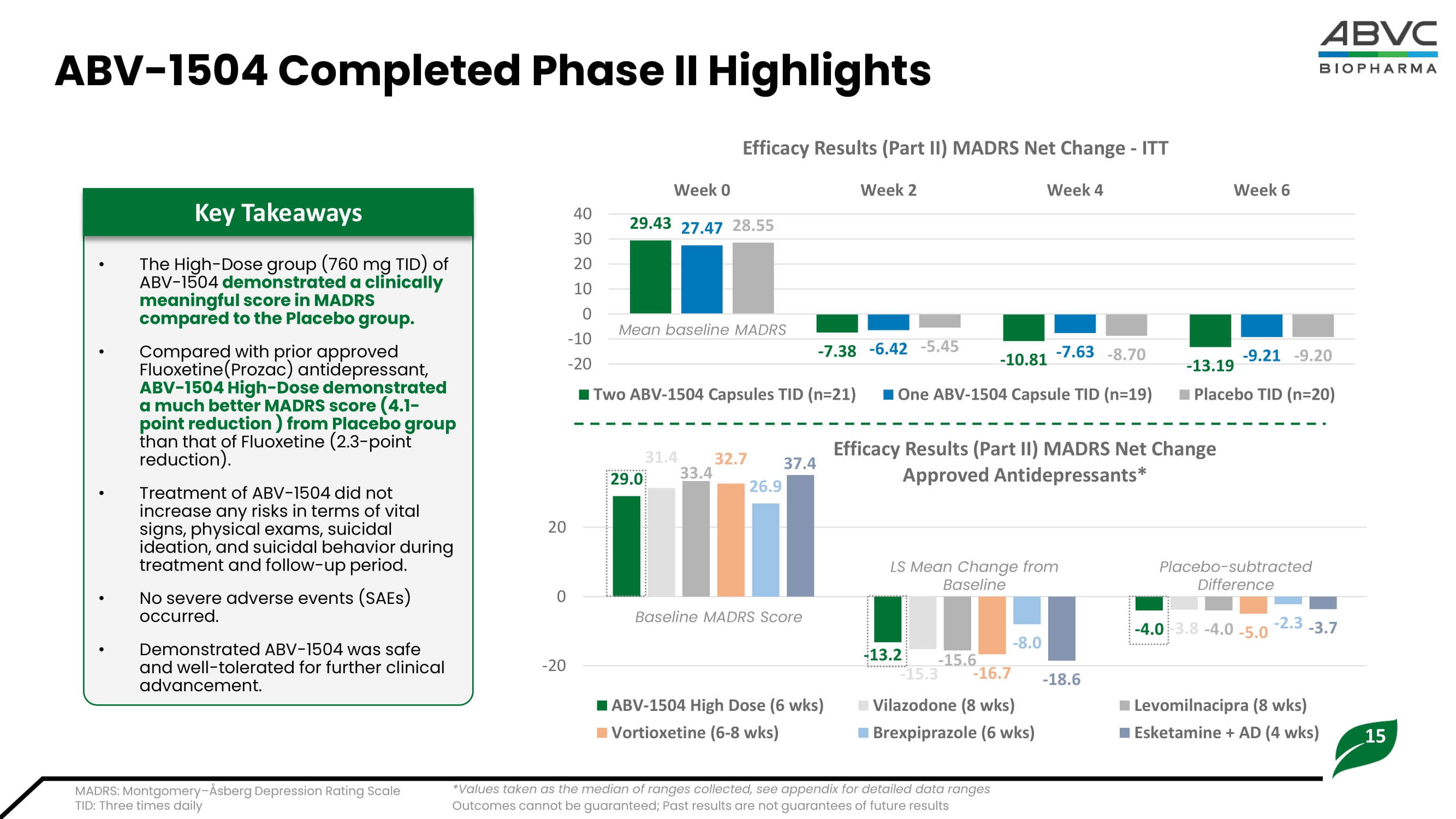
• Compared with prior approved Fluoxetine(Prozac) antidepressant, ABV - 1504 High - Dose demonstrated a much better MADRS score (4.1 - point reduction ) from Placebo group than that of Fluoxetine (2.3 - point reduction). • Treatment of ABV - 1504 did not increase any risks in terms of vital signs, physical exams, suicidal ideation, and suicidal behavior during treatment and follow - up period. • No severe adverse events (SAEs) occurred. • Demonstrated ABV - 1504 was safe and well - tolerated for further clinical advancement. Key Takeaways 29.43 - 7.38 - 10.81 - 13.19 27.47 - 6.42 - 7.63 - 9.21 28.55 - 5.45 - 8.70 - 9.20 -20 -10 0 10 20 30 40 Week 0 Week 2 Week 4 Week 6 Efficacy Results (Part II) MADRS Net Change - ITT Two ABV-1504 Capsules TID (n=21) One ABV-1504 Capsule TID (n=19) Placebo TID (n=20) Mean baseline MADRS 29.0 - 13.2 - 4.0 31.4 - 15.3 - 3.8 33.4 - 15.6 - 4.0 32.7 - 16.7 - 5.0 26.9 - 8.0 - 2.3 - 18.6 - 3.7 -20 0 20 Efficacy Results (Part II) MADRS Net Change Approved Antidepressants* ABV-1504 High Dose (6 wks) Vilazodone (8 wks) Levomilnacipra (8 wks) Vortioxetine (6-8 wks) Brexpiprazole (6 wks) Esketamine + AD (4 wks) *Values taken as the median of ranges collected, see appendix for detailed data ranges Baseline MADRS Score LS Mean Change from Baseline Placebo - subtracted Difference 37.4 Outcomes cannot be guaranteed; Past results are not guarantees of future results 16 ABV - 1504 Phase III Clinical Plan • Outpatient adults 18 - 75 years old • Met criteria for MDD without psychotic features as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Test Revision (DSM - IV - TR) • 17 - item HAM - D total score ≥ 20 and CGI total score ≥ 4 Key Inclusion Criteria Change from Baseline to Week 8 on the MADRS (Montgomery - Asberg Depression Rating Scale) total score Primary Endpoint 1.
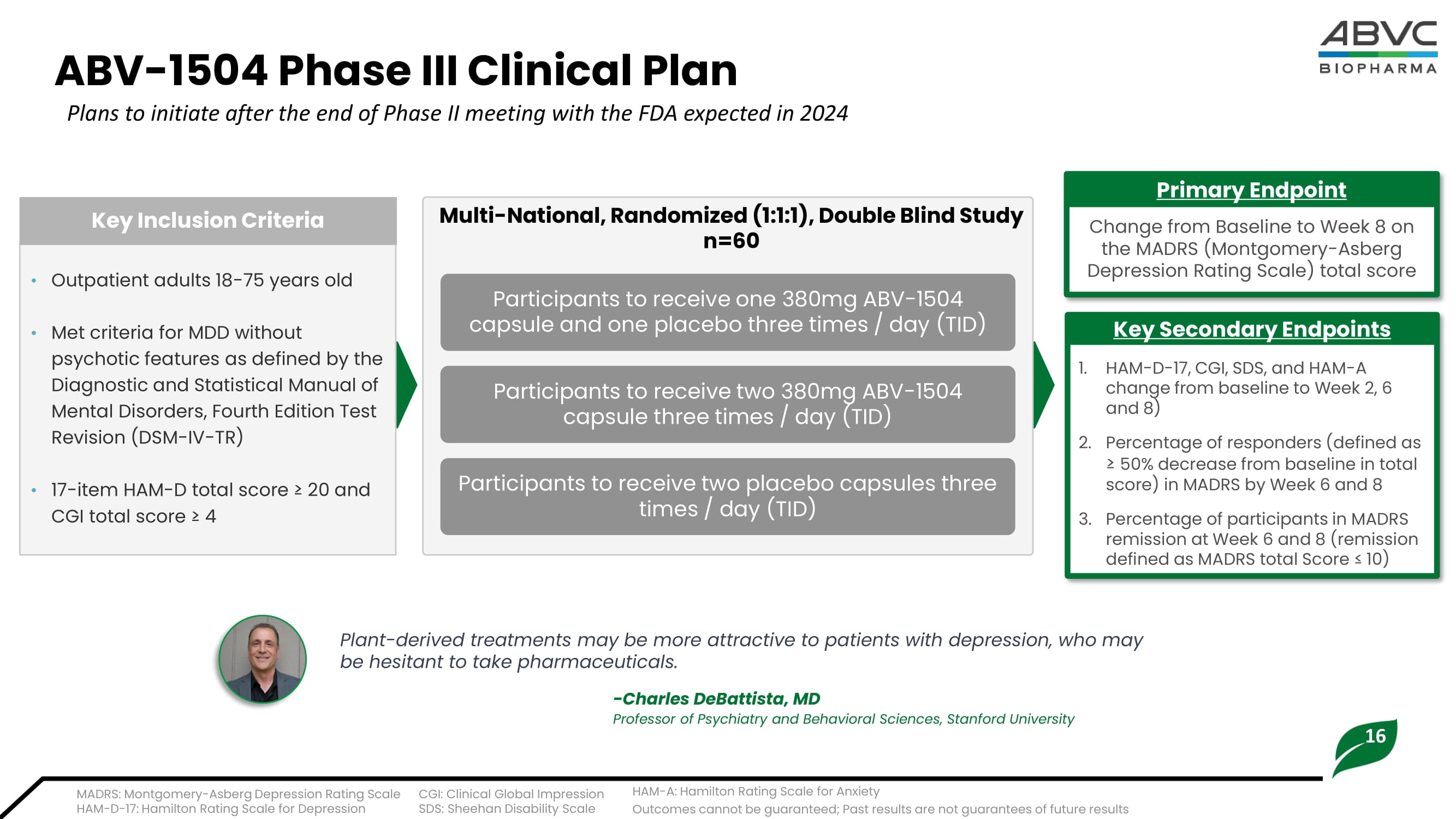
HAM - D - 17 , CGI, SDS, and HAM - A change from baseline to Week 2, 6 and 8) 2. Percentage of responders (defined as ≥ 50% decrease from baseline in total score) in MADRS by Week 6 and 8 3. Percentage of participants in MADRS remission at Week 6 and 8 (remission defined as MADRS total Score ≤ 10) Key Secondary Endpoints Participants to receive one 380mg ABV - 1504 capsule and one placebo three times / day (TID) Multi - National, Randomized (1:1:1), Double Blind Study n=60 Plant - derived treatments may be more attractive to patients with depression, who may be hesitant to take pharmaceuticals. - Charles DeBattista , MD Professor of Psychiatry and Behavioral Sciences, Stanford University Plans to initiate after the end of Phase II meeting with the FDA expected in 2024 MADRS: Montgomery - Asberg Depression Rating Scale HAM - D - 17: Hamilton Rating Scale for Depression CGI: Clinical Global Impression SDS: Sheehan Disability Scale HAM - A: Hamilton Rating Scale for Anxiety Participants to receive two 380mg ABV - 1504 capsule three times / day (TID) Participants to receive two placebo capsules three times / day (TID) Outcomes cannot be guaranteed; Past results are not guarantees of future results 17 ABV - 1505: Innovative Botanical Asset for ADHD • ABV - 1505 (PDC - 1421 capsule) is a single - herb botanical drug extract from the dry root of Polygala tenuifolia Willd • Safety assessment: Demonstrated its safety with no SAEs from the completed Phase I and Phase II (Part I) clinical studies.

• Efficacy assessment: Demonstrated its efficacy for treating ADHD patients from the completed Phase II clinical studies (Part I). • IP Protection: Global patent granted including US, EU and Asian countries .

IP - Protected Process Raw Materials (dry roots of Yuan Zhi) Extraction Purification Isolation Encapsulation No methylation process required 1 2 3 4 5 ABV - 1505 Summary Highlights Outcomes cannot be guaranteed; Past results are not guarantees of future results Summary Highlights are based on internally generated data 18 - 3.7 - 8.8 - 12.8 - 10.5 - 14.2 - 15.2 - 1.2 - 5.2 - 6 .0 - 7.2 - 10.5 - 10.5 - 4.8 - 14 - 18.8 - 17.7 - 24.7 - 25.7 -30 -25 -20 -15 -10 -5 0 0 1 2 3 4 5 6 7 8 Mean change from baseline Week Low - dose treatment High - dose treatment ABV - 1505 Completed Phase IIa in Adults with ADHD 1 • Mean change of ADHD - RS - IV Score from baseline to 8 weeks treatment were: • 83.3% (5/6) subjects in the ITT population and 80% (4/5) subjects in the PP population achieved an improvement of 40% or greater in ADHD Rating Scale (Primary Endpoint). • Mean change in CAARS - S:S from baseline to 8 weeks treatment were: • - 10.8 and - 15.2 (p=.0313) in the ITT population • - 10.6 and - 14.0 (p=.0625) in the PP population • No severe adverse events (SAEs)or deaths occurred. Key Takeaways PP: Per - Protocol Population ITT: Intention - to - Treat Population ITT Population Mean Change of ADHD - RS - IV Score from Baseline ADHD - RS - IV: ADHD Rating Scale - Investigator Rated CAARS:S - S: Conners’ Adult Attention - Deficit/Hyperactivity Disorder (ADHD) Rating Scale – Self Report: Short Version ITT Population Mean Change of CAARS:S - S Score from Baseline Hyperactivity - impulsivity subscale Inattention Subscale Total Scale Legend Impulsivity subscale Inattention / Memory Subscale ADHD Index Subscale Hyperactivity Subscale Self - Concept subscale Legend *P<0.05 - 1.5 - 5.7 - 12.2 - 10 - 7.8 - 10.5 0.5 - 0.2 - 3.2 - 6.2 - 5.2 - 5.2 - 4.5 - 4.2 - 11.2 - 10.8 - 10.3 - 15.2 - 1.8 1.7 - 4 - 5.7 - 3.3 - 4.7 - 2.3 - 0.3 - 8.7 - 8.2 - 8 - 10.8 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 2 4 0 1 2 3 4 5 6 7 8 Mean change from baseline Week Low - dose treatment High - dose treatment * * * * * * Outcomes cannot be guaranteed; Past results are not guarantees of future results 1.

ABVC BioPharma Presents ABV - 1505 Phase IIa Results at APSARD 2023 19 ABV - 1505 Phase IIb Clinical Plan Initiated April 2023, expected to be completed by end of Q4 - 2024 • Ability to discontinue use of psychotropic medications for the treatment of ADHD symptoms at screening • Meet operational criteria for Adult ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, 5 th edition (DSM - 5) • Total score of 28 or higher of ADHD Rating Sc ale - Investigator Rated (ADHD - RS - IV) • Have moderate or severe symptoms of ADHD with a score of 4 or higher in Clinical Global Impression - Severity (CGI - S) at screening Key Inclusion Criteria Improvement of 40% or more in ADHD Rating Scale - Investigator Rated (ADHD - RS - IV) from baseline to 8 weeks Primary Endpoint 1. Safety and incidence of Adverse Events and Serious Adverse Events 2. Symptom Remission in ADHD - RS - IV total score ≤ 18 up to 8 weeks 3. Change from baseline in ADHD - RS - IV, CAARS - S:S and E - SCT score up to 8 weeks 4. CGI - I score of 2 or lower up to 8 weeks treatment Key Secondary Endpoints Multi - center, Randomized (1:1:1), Double - Blind, Placebo - controlled (n=99) Participants to receive one 380mg ABV - 1504 capsule and one placebo three times / day (TID) Participants to receive one 380mg ABV - 1504 capsule and one placebo three times / day (TID) Participants to receive one 380mg ABV - 1504 capsule and one placebo three times / day (TID) The study will enroll 69 subjects initially. After 8 weeks, an interim analysis will be conducted to determine if it is necessary to enroll an additional 30 subjects ADHD - RS - IV: ADHD Rating Scale - Investigator Rated CAARS - S:S: Conners’ Adult ADHD Rating Scale – Self Report CGI: Clinical Global Impression E - SCT: Empirical - Sluggish Cognitive Tempo CGI - I: Clinical Global Impression - Improvement Based on its well - tolerated safety profile and preliminary efficacy shown in Phase IIa study, ABV - 1505 has promise as a treatment for ADHD.* - Keith McBurnett , PhD Professor of Psychiatry at UCSF, San Francisco Outcomes cannot be guaranteed; Past results are not guarantees of future results *As stated at the 2023 Conference of the American Professional Society of ADHD and Related Disorders (APSARD) Poster Session 20 ABV - 1601 Phase I Clinical Plan Initiated Q3 2024, expected to be completed by end of 2025 • Confirmed diagnosis of Stage I, II, or III cancer & Histologically - proven malignancy • Receiving or within one year of receiving cancer treatment with radiation and/or chemotherapy • Montgomery - Åsberg Depression Rating Scale (MADRS) ≥ 20 (moderate to severe depressive symptoms) • Duration of depressive symptoms ≥ 2 weeks by patient report.

• No active/acute suicidality requiring immediate care or psychiatric hospitalization Key Inclusion Criteria Safety, AE’s, and SAE’s related to ABV - 1601 Score on the Therapeutic Effect subscale of the CGI Efficacy Index Score on the Side Effects subscale of the CGI Efficacy Index Score on FIBSER questionnaire C - SSRS rating scale Primary Endpoint 1. Change in MADRS total score and HADS total score from baseline to Week 1 - 5 Key Secondary Endpoints 6 participants to receive two ABV - 1601 capsules three times / day (TID) for 28 days 6 participants to receive one ABV - 1601 capsule three times / day (TID) for 28 days Single - Center, Open - Label Study (n=12) Scott Irwin, MD, Ph.D., and the lead investigator of this study are continuing to work towards understanding the safety of ABV - 1601 at similar doses in several other studies. - Scott Irwin, MD, PhD Professor of Psychiatry & Behavioral Neurosciences, Cedar - Sinai AE’s: Adverse Events SAEs: Serious Adverse Events CGI: Clinical Global Impression CSSRS: Columbia - Suicide Severity Rating Scale MADRS: Montgomery - Asberg Depression Rating Scale HADS: Hospital Anxiety and Depression Scale One ABV - 1601 (PDC - 1421) Capsule TID Dose escalation Week 1 Week 2 Week 3 Week 4 Two ABV - 1601 (PDC - 1421) Capsules TID Outcomes cannot be guaranteed; Past results are not guarantees of future results 21 Early - Stage Oncology Pipeline Overview 1.
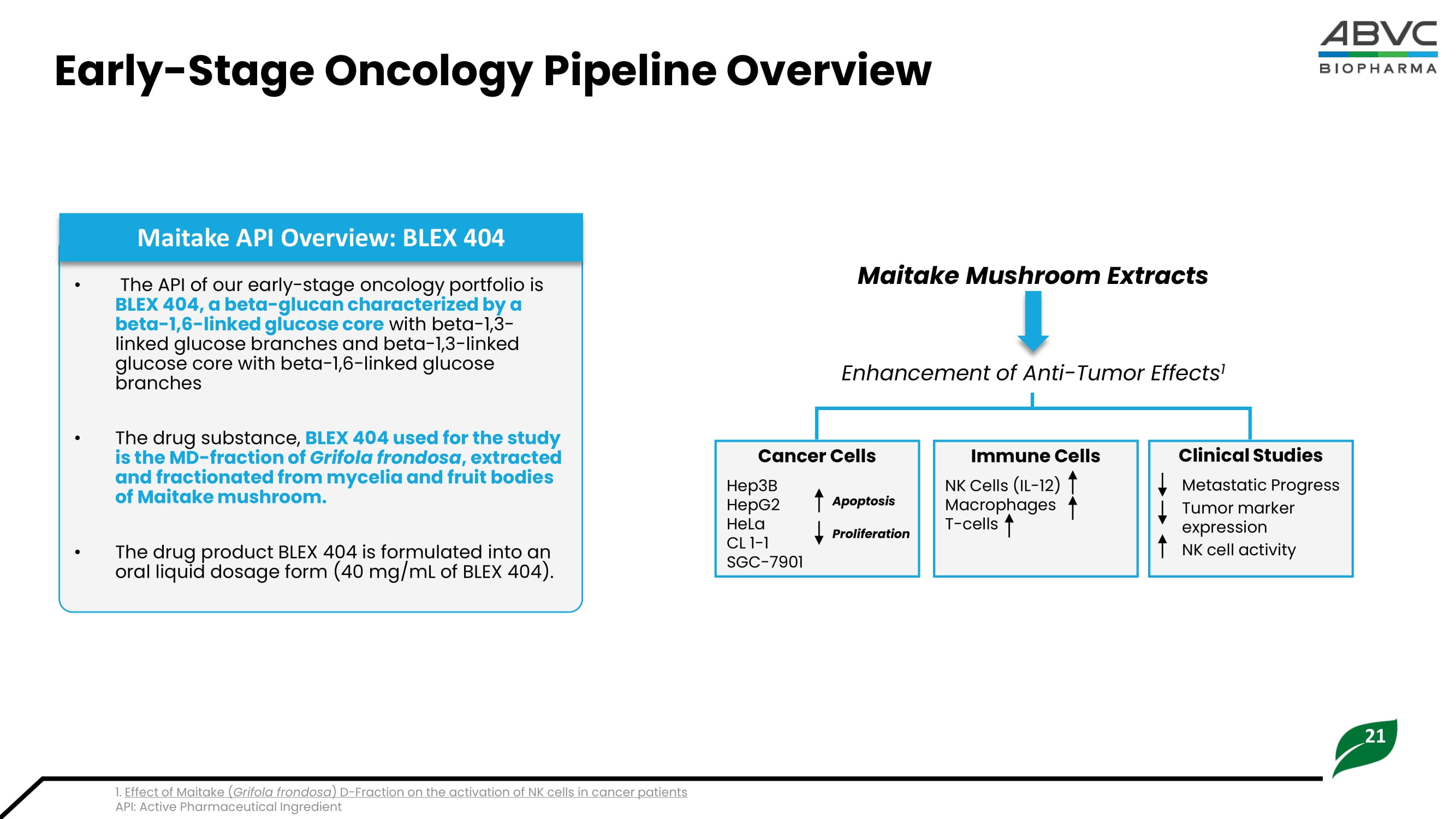
Effect of Maitake ( Grifola frondosa ) D - Fraction on the activation of NK cells in cancer patients API: Active Pharmaceutical Ingredient • The API of our early - stage oncology portfolio is BLEX 404, a beta - glucan characterized by a beta - 1,6 - linked glucose core with beta - 1,3 - linked glucose branches and beta - 1,3 - linked glucose core with beta - 1,6 - linked glucose branches • The drug substance, BLEX 404 used for the study is the MD - fraction of Grifola frondosa , extracted and fractionated from mycelia and fruit bodies of Maitake mushroom. • The drug product BLEX 404 is formulated into an oral liquid dosage form (40 mg/mL of BLEX 404). Maitake API Overview: BLEX 404 Maitake Mushroom Extracts Cancer Cells Hep3B HepG2 HeLa CL 1 - 1 SGC - 7901 Enhancement of Anti - Tumor Effects 1 Immune Cells NK Cells (IL - 12) Macrophages T - cells Clinical Studies Metastatic Progress Tumor marker expression NK cell activity Apoptosis Proliferation 22 Early - Stage Oncology Pipeline Overview (Cont.) Amelioration of chemotherapeutic side - effects 1 External Research Demonstrating Improved Cancer Symptoms with Maitake Mushroom 1 Nonrandomized clinical trial with Maitake D - Fraction 22 – 57 - year - old cancer patients (Stage II - IV cancers) 1.
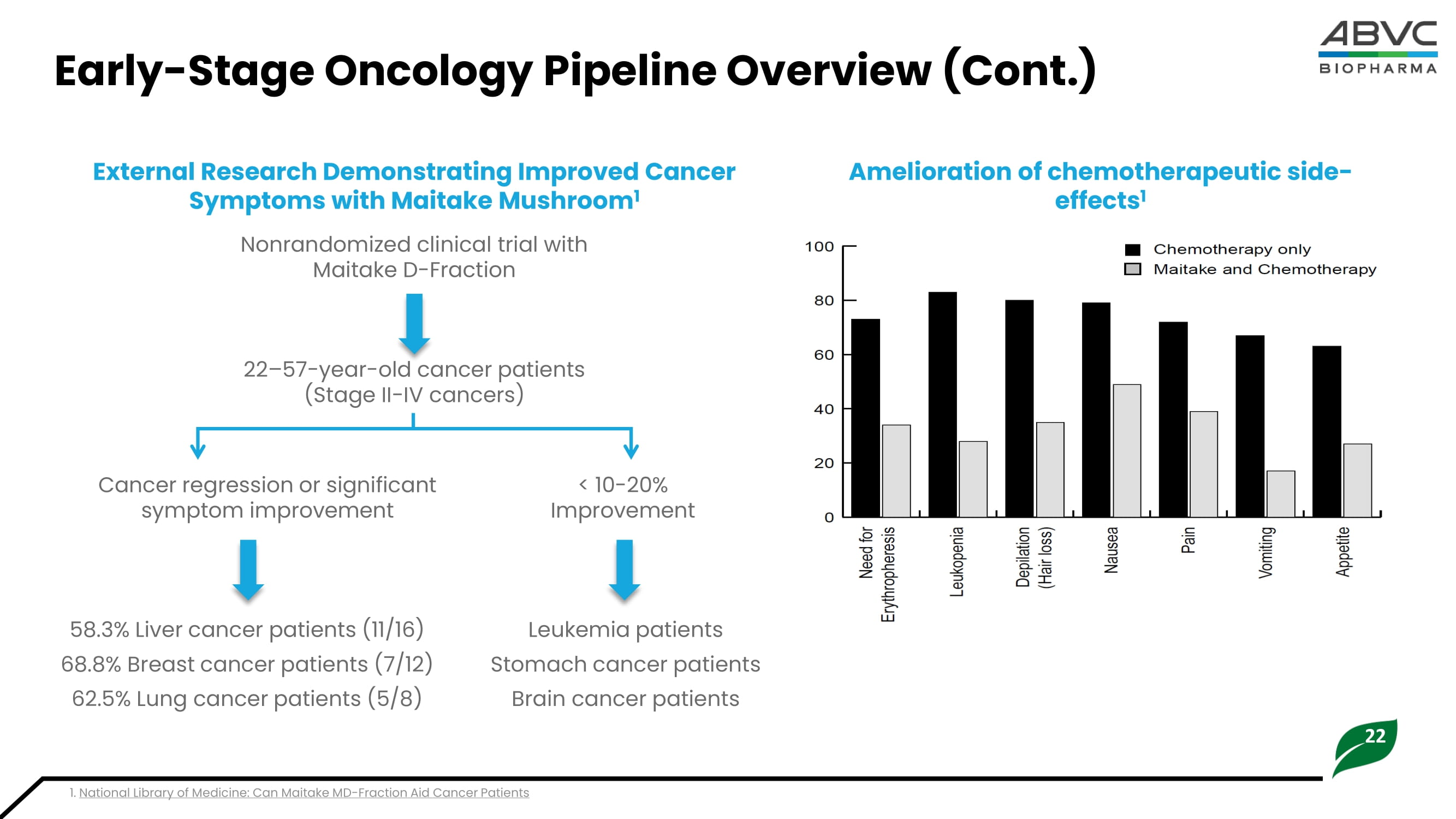

National Library of Medicine: Can Maitake MD - Fraction Aid Cancer Patients Cancer regression or significant symptom improvement < 10 - 20% Improvement 58.3% Liver cancer patients (11/16) 68.8% Breast cancer patients (7/12) 62.5% Lung cancer patients (5/8) Leukemia patients Stomach cancer patients Brain cancer patients 23 Near - Term Milestones & Use of Proceeds 2H 2025 1H 2025 2H 2024 1H 2024 Indication Asset Vitreous Replacement Vitargus® (ABV - 1701) Major Depressive Disorder (MDD) ABV - 1504 Attention - Deficit/Hyperactivity Disorder (ADHD) ABV - 1505 Depression in Cancer Patients ABV - 1601 ~$3M ~$2.7M ~$2.4M ~$2.2M Projected Cash Burn Multiple near - term clinical catalysts expected by the end of 2024 IP Granted Completion of Phase II Study Planning Phase III Completion Phase IIb Study Completion End - of - Phase II Meeting Planning Phase III IP Granted Completion End - of - Phase II Meeting Completion Phase I Study Initiation Phase II Study Outcomes cannot be guaranteed; Past results are not guarantees of future results NASDAQ: ABVC Uttam Patil Ph.

D. 44370 Old Warm Springs Blvd. Fremont, California 94538 uttam@ambrivis.com Investor Relations Contact: (845) 291 - 1291 info@ambrivis.com