UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the Month of April 2024
Commission File Number: 001-38104
IMMURON LIMITED
(Name of Registrant)
Level 3, 62 Lygon Street, Carlton South, Victoria, 3053, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ☐ No ☒
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-Immuron Limited (the “Company”) published one announcement (the “Public Notices”) to the Australian Securities Exchange on April 23, 2024 titled:
IMMURON LIMITED
EXPLANATORY NOTE
| - | “Immuron CEO Steven Lydeamore to present at The Watchlist” |
A copy of the Public Notice is attached as an exhibit to this report on Form 6-K.
This report on Form 6-K (including the exhibit hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
EXHIBITS
| Exhibit Number |
Description | |
| 99.1 | Immuron CEO Steven Lydeamore to present at The Watchlist |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| IMMURON LIMITED | ||
 |
||
| Date: April 23, 2024 | By: | /s/ Phillip Hains |
| Phillip Hains | ||
| Company Secretary | ||
3
Exhibit 99.1

Immuron CEO, Steven Lydeamore to present at The Watchlist Melbourne, Australia, April 23 , 2024 : Immuron Limited (ASX : IMC ; NASDAQ : IMRN), an Australian based and globally integrated biopharmaceutical company is pleased to advise our Chief Executive Officer, Steven Lydeamore will be presenting at The Watchlist, to be held on Tuesday 23 rd April 2024 , 12 : 00 pm AEST/ 10 : 00 am AWST . Chief Executive Officer, Steve Lydeamore will provide an update on Travelan® sales growth, clinical pipeline achievements and upcoming milestones . Following the presentation, attendees will have the opportunity to ask questions directly to Mr. Lydeamore during a moderated Q & A session. This webinar can be viewed live via zoom & you register via the link below. Zoom: https://blog.the - pick.com.au/webinars/the - watchlist - webinar A recorded copy of the webinar will be made available following the event . A copy of the presentation is included below . This release has been authorised by the directors of Immuron Limited . - - - END - - - COMPANY CONTACT: Steven Lydeamore Chief Executive Officer steve@immuron.com About Immuron Immuron Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of infectious diseases. About Travelan® Travelan® is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea, a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce . Travelan® is a highly purified tabletized preparation of hyper immune bovine antibodies and other factors, which when taken with meals bind to diarrhea - causing bacteria and prevent colonization and the pathology associated with travelers’ diarrhea . In Australia, Travelan® is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709 ) and is indicated to reduce the risk of Travelers’ Diarrhea, reduce the risk of minor gastro - intestinal disorders and is antimicrobial . In Canada, Travelan® is a licensed natural health product (NPN 80046016 ) and is indicated to reduce the risk of Travelers’ Diarrhea . In the U . S . , Travelan® is sold as a dietary supplement for digestive tract protection .

Travelers’ diarrhea (TD) TD is generally defined as the passage of ≥ 3 unformed stools per 24 hours plus at least one additional symptom (such as nausea, vomiting, abdominal cramps, fever, blood/mucus in the stools, or fecal urgency) that develop while abroad or within 10 days of returning from any resource - limited destinations ( Leung et al . , 2006 ) . Diarrhea continues to be the most frequent health problem among travelers to destinations in lower - and middle - income regions ( Steffen, 2017 ) . Deployed US military personnel, essentially representing a long - term traveller population, are particularly affected given their population dynamics and the context in which they seek care and treatment ( Connor et al . , 2012 ) . Diarrhea is the leading infectious disease threat to the overall health and preparedness of deployed US armed forces, with diarrheagenic E . coli, Campylobacter spp . , and Shigella spp . among the most commonly reported etiologies ( Riddle et al . , 2006 ) . Immuron Platform Technology Immuron’s proprietary technology is based on polyclonal immunoglobulins (IgG) derived from engineered hyper - immune bovine colostrum . Immuron has the capability of producing highly specific immunoglobulins to any enteric pathogen and our products are orally active . Bovine IgG can withstand the acidic environment of the stomach and is resistant to proteolysis by the digestive enzymes found in the Gastrointestinal (GI) tract . Bovine IgG also possesses this unique ability to remain active in the human GI tract delivering its full benefits directly to the bacteria found there . The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of infectious diseases . The platform can be used to block viruses or bacteria at mucosal surfaces such as the Gastrointestinal tract and neutralize the toxins they produce . IMM - 124E IMM - 124 E was developed using Immuron’s platform technology . IMM - 124 E is produced from the colostrum of birthing cattle that have been immunised during pregnancy with a vaccine containing the outer antigens of multiple human derived ETEC . A total of 13 ETEC strains are used in the vaccine to produce high levels of antibodies against selected surface antigens from the most common strains of ETEC . The resultant hyperimmune colostrum IMM - 124 E from ETEC vaccinated cows contains significant levels of polyclonal antibodies specific for ETEC antigens LPS, CFA - I and Flagellin ( Sears et al . , 2017 ) . The antibodies produced in IMM - 124 E have been found to have a stronger binding and neutralizing activity (than the antibodies of unvaccinated cattle) against a wide range of LPS antigens including both the variable O - polysaccharide region and the preserved oligosaccharide core ‘R’ region of LPS from the 13 serotypes used in the ETEC vaccine . IMM - 124E is manufactured into a tablet form referred to as Travelan®. References Connor P, Porter CK, Swierczewski B and Riddle MS. Diarrhea during military deployment: current concepts and future directions. Curr Opin Infect Dis. 25(5): 546 - 54; 2012. Leung AK, Robson WL, Davies HD. Travelers’ diarrhea. Adv Ther. Jul - Aug; 23(4): 519 - 27; 2006 Otto W, Najnigier B, Stelmasiak T and Robins - Browne RM . Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers Scandinavian Journal of Gastroenterology 46 : 862 – 868 ; 2011 . Riddle MS, Sanders JW, Putnam SD, and Tribble DR. Incidence, etiology, and impact of diarrhea among long - term travelers’ (US military and similar populations): A systematic review. American Journal of Tropical Medicine and Hygiene. 74(5): 891 - 900; 2006. Sears KT, Tennant SM, Reymann MK, Simon R, Konstantopolos N, Blackwelder WC, Barry EM and Pasetti MF . Bioactive Immune Components of Anti - Diarrheagenic Enterotoxigenic Escherichia coli Hyperimmune Bovine Colostrum products . Clinical and Vaccine Immunology . 24 ( 8 ) 1 - 14 ; 2017 . Steffen R. Epidemiology of travelers' diarrhea. J Travel Med. 24(suppl_1): S2 - S5; 2017.

For more information visit: https://www.immuron.com.au/ and https://www.travelan.com Subscribe for Immuron News: Here FORWARD - LOOKING STATEMENTS : This press release may contain “forward - looking statements” within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 , each as amended . Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts . Forward - looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition, and stock value . Factors that could cause actual results to differ materially from those currently anticipated include : risks relating to our growth strategy ; our ability to obtain, perform under and maintain financing and strategic agreements and relationships ; risks relating to the results of research and development activities ; risks relating to the timing of starting and completing clinical trials ; uncertainties relating to preclinical and clinical testing ; our dependence on third - party suppliers ; our ability to attract, integrate and retain key personnel ; the early stage of products under development ; our need for substantial additional funds ; government regulation ; patent and intellectual property matters ; competition ; as well as other risks described in our SEC filings . We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward - looking statements contained herein to reflect any change in our expectations or any changes in events, conditions, or circumstances on which any such statement is based, except as required by law .

1 1 THE WATCHLIST INVESTOR PRESENTATION 23 APRIL 2024 S t e v e n L y d e am o r e - C E O NASDAQ: IMRN ASX: IMC 2 Certain statements made in this presentatio n are forward - looking statements and are based on Immuron’s current expectatio ns, estimates and projections.

Words such as “anticipates ,” “expects,” “intends ,” “plans,” “believes ,” “seeks,” “estimates ,” “guidanc e” and similar expressio ns are intended to identif y forward - loo king statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain r i sks and uncertainties, some of which are beyond Immuron’s control, including those r i sks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ f rom those expressed or forecasted in the forward - loo k ing statements. The forward - looking statements made in this presentatio n relate only to events as of the date on which the statements are made. Immuron will not undertake any obligatio n to release publicly any revisions or updates to these forward - loo k ing statements to reflect events, circumstances or unanticipated events occurring after the date of this presentatio n except as required by law or by any appropriate regulato ry authority. YTD FY 2024 results in this presentatio n are subject to audit review.

SAFE HARBOR STATEMENT 3 EXECUTIVE SUMMARY C o m p a n y O v e r v i e w B us i ness Update Results & Outlook • Two commercially available oral immunotherapeutic products – Travelan® and Protectyn® • 4 clinical programs: Travelan®(IMC: Phase 2 CHIM trial), Travelan®(USU: Phase 4 field study), CampETEC (NMRC: Phase 2 CHIM trial), IMM - 529 (IMC: Protocol development phase, Phase 2 trial) • Flagship product Travelan® growing strongly as overseas travel rebounds • Travelan® (IMM - 124E) Phase 2 CHIM trial topline results • Travelan® (IMM - 124E) Travelan® Uniformed Health Services University (USU) P2TD IMM - 124E field clinical trial recruited ~64% of target 866 • CampETEC Phase 2 clinical trial completed inpatient phase • Sales 1 Jul 23 to 31 Mar 24 of A$3.6 million up 154% on pcp (unaudited) • Evaluating options to enter international markets through distributors • Evaluating options to add to marketed products portfolio Immuron Ltd (NASDAQ:IMRN) (ASX:IMC) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Financial Snapshot 227,998,346 Shares on Issue 15,368,559 Total Options IMC: A$0.10 Last Traded Price IMC: A$0.17/0.065 IMRN: $5.96/1.48 52 week High/Low IMC: A$22.79m Market Cap A$15.2m Cash & Cash Equivalents (as at 31 Dec 23) as of 19 April 2024 Major Shareholders % of CSO Units Holder 35.7 % 81,362,005 BNY Mellon Asset Management 3.0 % 6,904,554 Management & Board 2.4 % 5,500,000 Authentics Australia Pty. Ltd.

1.7 % 3,846,712 Grandlodge 4 IMM - 12 4 E BILLION DOLLAR MARKET - HIGH UNMET NEED Indu s t r y t a ilwi nd s Travel picking up significantly following COVID lockdowns Billion Dollar Market Traveller’s diarrhoea treatment market is large and growing at a CAGR of ~7% 1 Frequent Symptoms 30% - 70% of travelers experience traveller’s diarrhoea 2 • There are no current reliable vaccines for prevention of Travellers’ diarrhoea 2 • Enterotoxigenic Escherichia coli (ETEC) is the leading cause of Travellers’ diarrhoea 2 • Travelan® is a hyperimmune bovine colostrum produced by immunization of cows during gestation with a vaccine consisting of antigens derived from 13 different ETEC strains known to cause Travelers’ diarrhea • Travelan® is broadly cross - reactive with other ETEC strains not included in the vaccine and other gram - negative bacteria ( Shigella, Vibrio cholera, Campylobacter spp .) 3,4 • Diarrhea ranked 1 st among 57 infectious disease threats by the 2019 Military Infectious Disease Research Program’s Infectious Disease Threat Prioritization Panel based on its impact to readiness 5 • 76% of Soldiers in OIF and OEF experienced traveler’s diarrhea early in their deployment 5 1 https://www.transparencymarketresearch.com/travelers - diarrhea - market.htm l ; 2 Centers for Disease Control and Prevention CDC.gov 2024; 3 Sears et al., Clin. Vaccine Immunol. 2017 https://doi.org/10.1128/cvi.00186 - 16 ; 4 Islam et al., PLOS one 2023 https://doi.org/10.1371/journal.pone.0294021 ; ; 5 Olson et al. “Tropical Diseases, Travel Medicine and Vaccines, 2019, 51 - 15 Page 3; OIF (Operation Iraqi Freedom); OEF (Operation Enduring Freedom)

TECHNOLOGY PLATFORM Bovine colostrum is the first milk of cows after calving. It is rich in immunoglobulins, lactoferrin, lysozyme, lactoperoxidase, growth factors and bioactive peptides. Colostrum has higher levels of protein, fat, vitamins, and minerals when compared to milk. This enables full development of the newborn calf in addition to immunity against several pathogens. * Immuron’s proprietary technology platform combines the natural human nutrition & health benefits of bovine colostrum with a novel class of specifically targeted oral polyclonal antibodies that offer delivery within the gastrointestinal (“GI”) tract and can be used to target viruses or bacteria and neutralize the toxins they produce at mucosal surfaces. STEP 1 Development of Highly Specific Vaccines STEP 2 Isolation of Hyperimmune antibody - rich bovine colostrum STEP 3 Oral Antimicrobial therapeutics without drawbacks of antibiotics FINAL PRODUCT Toxin Neutralization + Clearance of targeted gut pathogens x Reduce occurrence and r e du c e / r e li e ve d i arrh oe a x Reduce/relieve abdominal cramping x Reduce/relieve gastrointestinal pain x Assists repair of gastrointestinal/gut wall lining x Enhance/promote immune defence x Enhance/promote health liver function Australian Permitted indications; these statements have not been evaluated by the Food and Drug Administration (FDA) 5 * Gomes et.

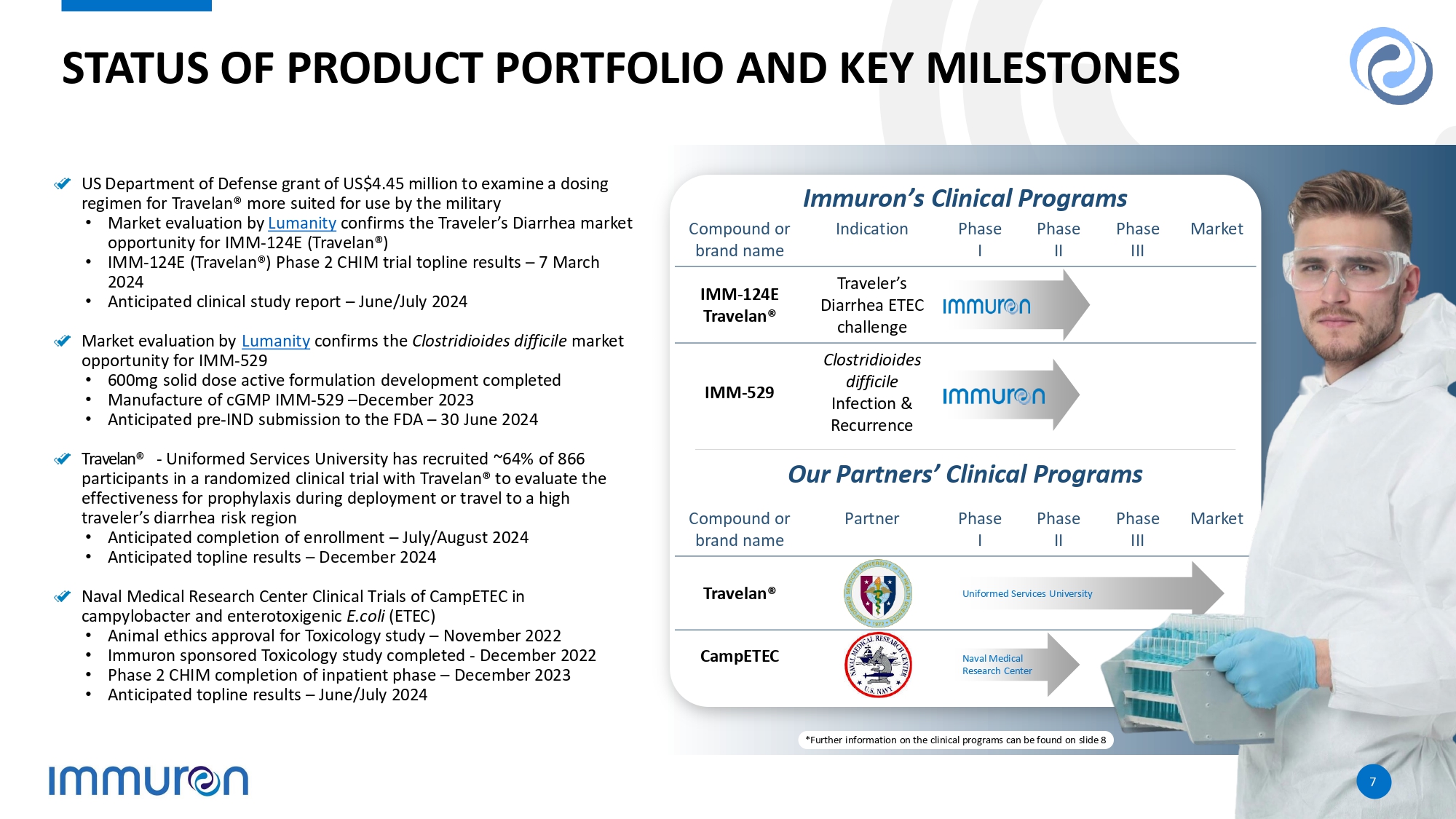
al., NFS Journal, Volume 25, November 2021, pages 1 - 11, https://doi.org/10.1016/j.nfs.2021.10.001 6 TRAVELAN® SALES CONTINUE STRONG GROWTH FYTD Mar 2024 AUD$3.6 million up 154% on (prior comparative period) pcp Mar 2024 Quarter AUD$1.3 million up 51% on pcp and 75% on last quarter Global FYTD Mar 2024 AUD$2.8 million up 234% on pcp Mar 2024 Quarter AUD$0.9 million up 66% on pcp and 99% on last quarter Australia FYTD Mar 2024 AUD$0.8 million up 35% on pcp Mar 2024 Quarter AUD$0.3 million up 7% on pcp and 18% on last quarter Sales commenced on Walmart.com USA STATUS OF PRODUCT PORTFOLIO AND KEY MILESTONES US Department of Defense grant of US$4.45 million to examine a dosing regimen for Travelan® more suited for use by the military • Market evaluation by Lumanity confirms the Traveler’s Diarrhea market opportunity for IMM - 124E (Travelan®) • IMM - 124E (Travelan®) Phase 2 CHIM trial topline results – 7 March 2024 • Anticipated clinical study report – June/July 2024 Market evaluation by Lumanity confirms the Clostridioides difficile market opportunity for IMM - 529 • 600mg solid dose active formulation development completed • Manufacture of cGMP IMM - 529 – December 2023 • Anticipated pre - IND submission to the FDA – 30 June 2024 Travelan® - Uniformed Services University has recruited ~64% of 866 participants in a randomized clinical trial with Travelan® to evaluate the effectiveness for prophylaxis during deployment or travel to a high traveler’s diarrhea risk region • Anticipated completion of enrollment – July/August 2024 • Anticipated topline results – December 2024 Naval Medical Research Center Clinical Trials of CampETEC in campylobacter and enterotoxigenic E.coli (ETEC) • Animal ethics approval for Toxicology study – November 2022 • Immuron sponsored Toxicology study completed - December 2022 • Phase 2 CHIM completion of inpatient phase – December 2023 • Anticipated topline results – June/July 2024 Phase I Indication Compound or brand name Traveler’s Diarrhea ETEC challenge IMM - 124E Travelan® C l o s t r i d i o i d es difficile Infection & Recurrence IMM - 529 Market Phase III Phase II Phase I Partner Compound or brand name ervices University Uniformed S Travelan® Naval Medical CampETEC Research Center Immuron’s Clinical Programs Our Partners’ Clinical Programs *Further information on the clinical programs can be found on slide 8 7 8 NEAR TERM MILESTONES ANTICIPATED TO DRIVE VALUE • Clinical Study Report • End of Phase 2 FDA meeting • Topline results for IMM - 124E ETEC2 clinical trial • 100% of patients enrolled • Completion of In - patient phase ETEC 2 CHIM 3 clinical trial • IRB Approval 4 • Initiated IMM - 124E ETEC 2 CHIM 3 clinical trial • FDA IND 1 approved for single daily dose IMM - 124E ETEC 2 CHIM 3 clinical trial Travelan® • Topline results for CampETEC Campylobacter CHIM 3 clinical trial • Completion of In - patient phase CampETEC Campylobacter CHIM 3 clinical trial • Institutional Review Board approval of NMRC 5 CampETEC Campylobacter CHIM 3 clinical trial protocol • Initiated IMM - 124E Campylobacter CHIM 3 clinical trial • Toxicology Study Report • FDA IND 1 approved (Clinical Hold released) • Submitted Response Letter to FDA Clinical Hold • I mm u r o n s p o n s o r ed Toxicology study - completed CampETEC • FDA meeting • IMM - 529 (CDI) 7 Pre - IND 1 submission • IMM - 529 cGMP manufacture • 600 mg solid dose active f or m u l a t i o n d e v el o p m e n t IMM - 529 • Topline results • Completion of enrollment • ~64% of 868 participants recruited • USU 6 P2TD IMM - 124E field clinical trial recruitment commencement Travelan® 2H 2024 Completed ; 1.

Investigational New Drug; 2. Enterotoxigenic E.coli ; 3. controlled human infection model; 4. Institutional Review Board; 5. Naval Medical Research Command; 6. Uniformed Health Services University of the Health Sciences; 7.

Clostridioides Difficile 2H 2022 1H 2023 2H 2023 1H 2024 9 IMMURON’S CLINICAL PROGRAMS – OPPORTUNITY ASSESSMENT › Immuron’s development of IMM - 124E (hyperimmune bovine colostrum) as a prescription medication has the potential to address this unmet need › Primary care physicians (PCP)s impressed with clinical efficacy endpoint targets demonstrating > 80% protection against the development of diarrhea. › If base case efficacy targets are reached, IMM - 124E would mostly be used by travelers going to the highest risk areas (e.g., rural Central America/Asia/Africa). › Based on the estimated market size and pricing, the base case yearly revenue in USA for IMM - 124E is projected at US$102M . › Reaching higher efficacy goals could broaden use. Lu C m o a r n p i o ty ra * te Opportunity Assessment for IMM - 124E › Infectious disease experts reacted favorably to the IMM - 529 MOA, and its unique ability to target three elements of the rCDI infection – the spores, vegetative cells, and Toxin B › Non - microbiome approaches (such as IMM - 529) are still appealing to experts, who noted that clinical trial efficacy (reduction in relapse rate) and cost/access will be the key drivers of clinical use in recurrent patients, not mechanism of action › Based on the estimated market size, anticipated payer restrictions, pricing, and competition, base case yearly revenue in USA for IMM - 529 is conservatively projected at US$93M for the target patient population (limited to 2nd recurrence and later based on trial design and payer coverage) › Positioning IMM - 529 earlier than second recurrence and/or efficacy targets could lead to higher uptake.

Lum Co an rp it o y ra O t p e portunity Assessment for IMM - 529 Compound or brand name I nd i c a t i o n P h a s e I P h a s e I I P h as e II I Ma r k et IMM - 124E - Travelan® Traveler’s Diarrhea ETEC challenge I MM - 529 Clostridioides difficile Infection & Recurrence Lumanity, a leading lifescience consulting company: https://lumanity.com/company/our - story/ 10 IMM - 124E PHASE 3 STRATEGY • Trial duration ~ 2 years • End of Phase 3 FDA meeting • BLA 3 submission • Initiate Phase 3 • Phase 3 FDA meeting • Clinical Study Report • End of Phase 2 FDA meeting • Phase 1 clinical study (Baltimore, 1996) • Phase 2 clinical study (Poland, 2000) • FDA 1 IND 2 approval (December 2022) • Phase 2 clinical study (Baltimore, 2024) • The pivotal registration studies will involve two randomized, double - blind, parallel - group, placebo - controlled Phase 3 clinical studies (drug substance IMM - 124E) to assess the efficacy and safety of Travelan® for prevention of traveler’s diarrhea (TD) • The studies will enroll approximately 1200 healthy adult subjects (600 subjects in two studies) traveling to regions with high TD risk. • Subjects will be randomized 1:1 to receive Travelan® or placebo. • Dosing will begin 3 days prior to arrival in country and for at least 14 days in country. • The primary endpoint will be the development of TD. Pre 2H 2024 1H 2025 P o st 1. U.S. Food and Drug Administration; 2. Investigational New Drug; 3.

Biologics License Application 2H 2025 WORLD FIRST TRIPLE MECHANISM OF ACTION FOR CDI 11 I MM - 529 Indication / Target Population IMM - 529 will be indicated for the treatment of recurrent C. difficile infection Product Description / Mechanism of Action • Novel antibody - containing therapeutic which neutralizes C. difficile but does not impact the microbiome • Targets not only toxin B but also spores and vegetative cells responsible for recurrence • Potential for use in combination with standard of care (e.g. vancomycin, metronidazole, fidaxomicin) • Targets many isolates Dosage and ROA • Oral administration, 3 x daily • Trial to test safety 7 - day treatment course on top of standard of care (vancomycin, metronidazole, fidaxomicin) Efficacy • Mouse data demonstrated ~80% survival rate (7/9) vs. ~10% survival rate in a control group (1/9) in a recurrent CDI mouse model Safety / Tolerability • To be evaluated in Phase I/IIA study • Equivalent or better than current standard of care 12 EMAIL: STEVE@IMMURON.COM PHONE: AUSTRALIA: +61 438 027 172 STEVEN LYDEAMORE CHIEF EXECUTIVE OFFICER IMMURON LIMITED CONTACT INFORMATION:


1 3 1 3 SCIENTIFIC REFERENCES T r a v e l a n® (I MM - 124 E ) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Military Health System Research Symposium 14 - 17 Aug 2023_Abstract 1 Clinical Evaluation of Travelan® an Oral Prophylactic for Prevention of Travelers’ Diarrhea in Active Duty Military Service Assigned Abroad. Immuron Limited, 29 April, 2011 Travelan as a broad Spectrum anti - bacterial US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam D, Ruamsap N, Imerbsin R, Khanijou P, Gonwong S, Wegner MD, et al. (2023) Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta). PLoS ONE 18(12): e0294021. Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta) Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 Bioactive Immune Components of Travelan® Infect Immun. 2023 Nov; 91(11): e00097 - 23. Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn's and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative