UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of: April 2024
Commission file number: 001-36578
ENLIVEX THERAPEUTICS LTD.
(Translation of registrant’s name into English)
14 Einstein Street, Nes Ziona, Israel 7403618
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Topline Results of Phase II Trial Evaluating Allocetra™ In Patients With Sepsis
On April 11, 2024, Enlivex Therapeutics Ltd., a company organized under the laws of the State of Israel (“Enlivex”), issued a press release announcing topline results from its Phase II trial of Allocetra™ in patients with sepsis, in which 120 patients enrolled. A copy of such press release is furnished as Exhibit 99.1 to this Report on Form 6-K and incorporated herein by reference. The information immediately under or following the headings “Analysis of eligible patients from the sepsis Phase II study (NCT# NCT04612413)”, “Efficacy” and “Safety” in such press release is incorporated by reference into Enlivex’s registration statements on Forms S-8, F-3 and F-3MEF (File No. 333-256799, File No. 333-232413, File No. 333-232009, File No. 333-252926 and File No. 333-264561), filed with the Securities and Exchange Commission. For the elimination of doubt, no other information contained in such press release, including the statements of Bruno François, M.D. and Oren Hershkovitz, Ph.D., is incorporated by reference in such registration statements.
Investor Presentation
On April 11, 2024, Enlivex posted an updated investor presentation on its website. A copy of such presentation is furnished as Exhibit 99.2 to this Report on Form 6-K and is incorporated herein by reference. The information contained on slides 17 through 23, inclusive, of such investor presentation is incorporated by reference into Enlivex’s registration statements on Forms S-8, F-3 and F-3MEF (File No. 333-256799, File No. 333-232413, File No. 333-232009, File No. 333-252926 and File No. 333-264561), filed with the Securities and Exchange Commission.
Yavne Facility Lease Assumption and Manufacturing Facility Sale
As previously reported in 2023, Enlivex adopted a strategic reprioritization plan, which contemplated, among other things, that Enlivex sell the leased manufacturing facility it constructed in Yavne, Israel, together with the equipment installed by Enlivex therein (the “Equipment”), and assign the lease agreement with respect thereto (the “Lease Agreement”).
On March 31, 2024, Enlivex entered into an agreement (the “Agreement”) with BioHarvest Ltd., an Israeli company (the “purchaser”), pursuant to which the purchaser agreed to acquire the Equipment and assume all of Enlivex’s obligations under the Lease Agreement, effective as of April 1, 2024, for an aggregate purchase price (the “Purchase Price”) payable to Enlivex of 13.0 million New Israeli Shekels (“NIS”) (approximately $3.5 million). The Purchase Price is payable in installments, consisting of an initial payment of NIS 4.0 million (approximately $1.08 million), which was paid on April 2, 2024, and 24 equal monthly installment payments of NIS 375,000 (approximately $102,000), commencing on April 1, 2024. Pursuant to the Agreement, title to the Equipment will transfer to the purchaser only upon full payment of the total Purchase Price, but risk of loss to the Equipment passed to the purchaser on April 1, 2024. Subject to certain conditions, the purchaser may, in its sole discretion, prior to October 1, 2025, prepay (i) all of the remaining outstanding Purchase Price at a 4% discount, or (ii) a portion of the remaining outstanding Purchase Price, in an amount of not less than NIS 4.0 million (approximately $1.08 million), at a 2% discount, in which case, the Purchase Price remaining outstanding thereafter shall continue to be paid in monthly instalments of NIS 375,000 each (approximately $102,000).
The information under the heading “Yavne Facility Lease Assumption and Manufacturing Facility Sale” in this Report on Form 6-K is incorporated by reference into Enlivex’s registration statements on Forms S-8, F-3 and F-3MEF (File No. 333-256799, File No. 333-232413, File No. 333-232009, File No. 333-252926 and File No. 333-264561), filed with the Securities and Exchange Commission.
| Exhibit No. | ||
| 99.1 | Press Release issued by Enlivex Therapeutics Ltd. on April 11, 2024. | |
| 99.2 | Investor Presentation |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Enlivex Therapeutics Ltd. | ||
| (Registrant) | ||
| By: | /s/ Oren Hershkovitz | |
|
Name: Title: |
Oren Hershkovitz Chief Executive Officer |
|
Date: April 11, 2024
2
Exhibit 99.1

Enlivex Announces Topline Results of Its Phase II Trial Evaluating Allocetra™ In Patients With Sepsis
| ● | Analysis of eligible1 patients from the sepsis Phase II study (NCT# NCT04612413) |
| ■ | In accordance with the study protocol, the safety and efficacy topline analysis includes sequential organ failure assessment (SOFA) scores and mortality for the 28-day period post treatment. |
| ■ | Efficacy: |
| ■ | Stand-alone analysis of the Allocetra™-treated patients, of which 78% had septic shock and 65% had invasive ventilation at screening, demonstrated substantial reductions in SOFA scores and 65% reduction in overall mortality rate as compared with expected mortality2. By day 28, the analysis showed 90% reductions of SOFA scores for sepsis patients whose infection source was urinary tract, 68% for patients whose infection source was community-acquired pneumonia, and 36% for patients whose infection source was internal abdominal infection. |
| ■ | Relative analysis demonstrates a potential indication of effect of Allocetra™ as compared with placebo in high-risk, severe sepsis patient population (organ failure scores >=7), originating from urinary tract infections (“High Risk UTI”). Enlivex intends to consider a potential follow-on, randomized, controlled study of a solely High Risk UTI sepsis population. Up to 31% of sepsis cases start as urinary tract infections, representing up to 9.8 million cases in the United States and Europe, leading to as many as 1.6 million deaths3, and represents a substantial potential market opportunity for Allocetra™. |
| ■ | The study was designed for patients to be randomized with equal degree of SOFA scores across treatment and placebo groups. The randomization resulted in the Allocetra™-treated cohorts having 20% higher frequency of septic shock and 35% higher frequency of invasive ventilation prior to treatment, as compared with the control group. Both of these patient attributes are associated with a significantly higher degree of difficulty of treatment and higher mortality rates. These imbalances made it challenging to deduce the relative effect in other patient subgroups. |
| ■ | Safety: Stand-alone and placebo-compared analysis across all sepsis patient subgroups and risk categories demonstrated acceptable safety and tolerability profile of Allocetra™ IV infusions. |
Nes-Ziona, Israel, April 11, 2024 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV, the “Company”), a clinical-stage macrophage reprogramming immunotherapy company, today announced positive indication of effect and safety results from its Phase II study of Allocetra™ in patients with sepsis, in which 120 patients enrolled.
Bruno François, M.D., intensive care physician, is the head of the Limoges Clinical Investigation Center (Limoges, France). Dr. François took a primary role in the design of the study, medical support and oversight of patient eligibility. Dr. François was the national coordinator for numerous emergency trials, especially in sepsis, and has participated in several advisory boards for sepsis multinational trials, independent clinical evaluation committees and adjudication committees. Dr. François stated, “I am very excited about Allocetra’s™ novel approach, using a first-in-class innovative cell therapy to explore the treatment of patients with acute, life-threatening sepsis and septic shock. The study, a randomized controlled trial conducted in six countries and multiple clinical centers, demonstrated a favorable safety profile for Allocetra™. Within the context of the study, we also learned the ease of use and feasibility to infuse Allocetra™ cells to patients even in the complex setting of the intensive care unit. The study was well designed and executed, although randomization resulted in the Allocetra™- treated cohorts having higher frequencies of septic shock and invasive ventilation prior to treatment, as compared with the control group. Because these patient attributes are typically associated with a significantly higher degree of difficulty of treatment and higher mortality rates, the relative effect of Allocetra™ in some patient sub populations was challenging to deduce. I am pleased with the unusually low mortality rates across the board in the study, and that Allocetra™ demonstrated a potential indication of effect in high-risk sepsis patients originating from urinary tract infections. A substantial number of sepsis cases originate from urinary tract infections, and we have been actively searching for additional treatment alternatives for those patients, especially those who are at high risk. Having reviewed the topline study results, I look forward to reviewing the forthcoming additional safety and biomarker data of patients in the study, and I recommend the further exploration of the use of Allocetra™ in the High Risk UTI population.”
| 1 | Analysis of modified intent-to-treat (mITT) population for all patients who were randomized, received the high dose of Allocetra™ or placebo, had a screening total SOFA score >= 5 points above pre-admission total SOFA score, had at least one post-baseline total SOFA score, and determined as eligible by an Adjudication Committee |
| 2 | Compared with recently-completed sizable clinical trials – Revival Phase III (2024), Astonish Phase IIb (2023), Karakike (2019) – in which mortality rates were in the range of 23% - 30%, and 26% was used for the calculation as the representative average |
| 3 | Management of Urosepsis in 2018, Bonkat et. Al. , European Urology Focus Volume 5, Issue 1, (2019) |
Oren Hershkovitz, Ph.D., CEO of Enlivex said, “We are pleased with the demonstration of substantial SOFA score reductions and low mortality rate of the Allocetra™-treated patients across all origins of sepsis in the study, the indication of effect compared with placebo for the high-risk patients whose sepsis originated from urinary tract infections, and the favorable safety profile of Allocetra™. The Company intends to consider, upon reviewing the totality of the data, a potential follow-on, randomized, controlled study of a solely High Risk UTI sepsis population. Up to 31% of sepsis cases start as UTIs4, and this represents a substantial potential market opportunity for Allocetra™. The randomization resulted in the Allocetra™-treated cohorts having 20% higher frequency of septic shock and 35% higher frequency of invasive ventilation prior to treatment, compared with the placebo group. Both of these patient attributes are associated with significantly higher degree of difficulty of treatment and higher mortality rates, and potentially resulted in patients with more severe sepsis in the Allocetra™-treated cohorts. These biases made it challenging to deduce the relative effect in other patient subgroups.”
ABOUT UTI
Urinary tract infection (UTI) is the second most common infectious disease affecting more than 150 million people globally annually. Up to 31% of sepsis cases start as UTIs, representing up to 9.8 million cases in the United States and Europe, leading to as many as 1.6 million deaths4.
ABOUT THE PHASE II SEPSIS CLINICAL TRIAL (NCT# NCT04612413)
The Phase II trial was a placebo-controlled, randomized, dose-finding, multi-country, multi-center study, evaluating frozen-formulation Allocetra™ in addition to standard of care in patients with sepsis associated with pneumonia, biliary, urinary tract, or peritoneal infections. The results contained in this press release represent topline data and are subject to revision based on the ongoing collection of study information and detailed analysis. The Company expects to release further details about the study in a forthcoming presentation.
ABOUT ALLOCETRA™
Allocetra™ is being developed as a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Diseases such as solid cancers, sepsis, and many others reprogram macrophages out of their homeostatic state. These non-homeostatic macrophages contribute significantly to the severity of the respective diseases. By restoring macrophage homeostasis, Allocetra™ has the potential to provide a novel immunotherapeutic mechanism of action for life-threatening clinical indications that are defined as “unmet medical needs”, as a stand-alone therapy or in combination with leading therapeutic agents.
ABOUT ENLIVEX
Enlivex is a clinical stage macrophage reprogramming immunotherapy company developing Allocetra™, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing and resolution of debilitating and life-threatening conditions. For more information, visit http://www.enlivex.com.
Safe Harbor Statement: This press release contains forward-looking statements, which may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “would”, “could,” “intends,” “estimates,” “suggests,” “has the potential to” and other words of similar meaning, including statements regarding expected cash balances, market opportunities for the results of current clinical studies and preclinical experiments, the effectiveness of, and market opportunities for, ALLOCETRA™ programs. All such forward looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that forward-looking statements involve risks and uncertainties that may affect Enlivex’s business and prospects, including the risks that Enlivex may not succeed in generating any revenues or developing any commercial products; that the products in development may fail, may not achieve the expected results or effectiveness and/or may not generate data that would support the approval or marketing of these products for the indications being studied or for other indications; that ongoing studies may not continue to show substantial or any activity; and other risks and uncertainties that may cause results to differ materially from those set forth in the forward-looking statements. The results of clinical trials in humans may produce results that differ significantly from the results of clinical and other trials in animals. The results of early-stage trials may differ significantly from the results of more developed, later-stage trials. The development of any products using the ALLOCETRATM product line could also be affected by a number of other factors, including unexpected safety, efficacy or manufacturing issues, additional time requirements for data analyses and decision making, the impact of pharmaceutical industry regulation, the impact of competitive products and pricing and the impact of patents and other proprietary rights held by competitors and other third parties. In addition to the risk factors described above, investors should consider the economic, competitive, governmental, technological and other factors discussed in Enlivex’s filings with the Securities and Exchange Commission, including in the Company’s most recent Annual Report on Form 20-F filed with the Securities and Exchange Commission. The forward-looking statements contained in this press release speak only as of the date the statements were made, and we do not undertake any obligation to update forward-looking statements, except as required under applicable law.
ENLIVEX CONTACT
Shachar Shlosberger, CFO
Enlivex Therapeutics, Ltd.
shachar@enlivexpharm.com
| 4 | Management of Urosepsis in 2018, Bonkat et. al., European Urology Focus Volume 5, Issue 1, (2019) |
Exhibit 99.2

Nasdaq: ENLV COMPANY PRESENTATION April 2024

2 | Nasdaq: ENLV FORWARD - LOOKING STATEMENTS These slides and the accompanying oral presentation contain forward - looking statements and information. Forward - looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such statements. The use of words such as “may”, “might”, “will”, “should”, “could”, “expect”, “plan”, “anticipate”, “believe”, “estimate”, “project”, “intend”, “future”, “potential” or “continue”, and other similar expressions are intended to identify forward looking statements. For example, all statements we make regarding ( i ) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the size and growth potential of the markets for our product candidates and our ability to serve those markets, and (vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates, are forward looking. All forward - looking statements are based on current estimates, assumptions and expectations by our management that, although we believe to be reasonable, are inherently uncertain. All forward - looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. Any forward - looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward - looking statement, whether as a result of new information, future events or otherwise, except as required by law. This presentation is not, and nothing in it should be construed as, an offer, invitation or recommendation in respect of our securities, or an offer, invitation or recommendation to sell, or a solicitation of an offer to buy, any of our securities in any jurisdiction. Neither this presentation nor anything in it shall form the basis of any contract or commitment. This presentation is not intended to be relied upon as advice to investors or potential investors.
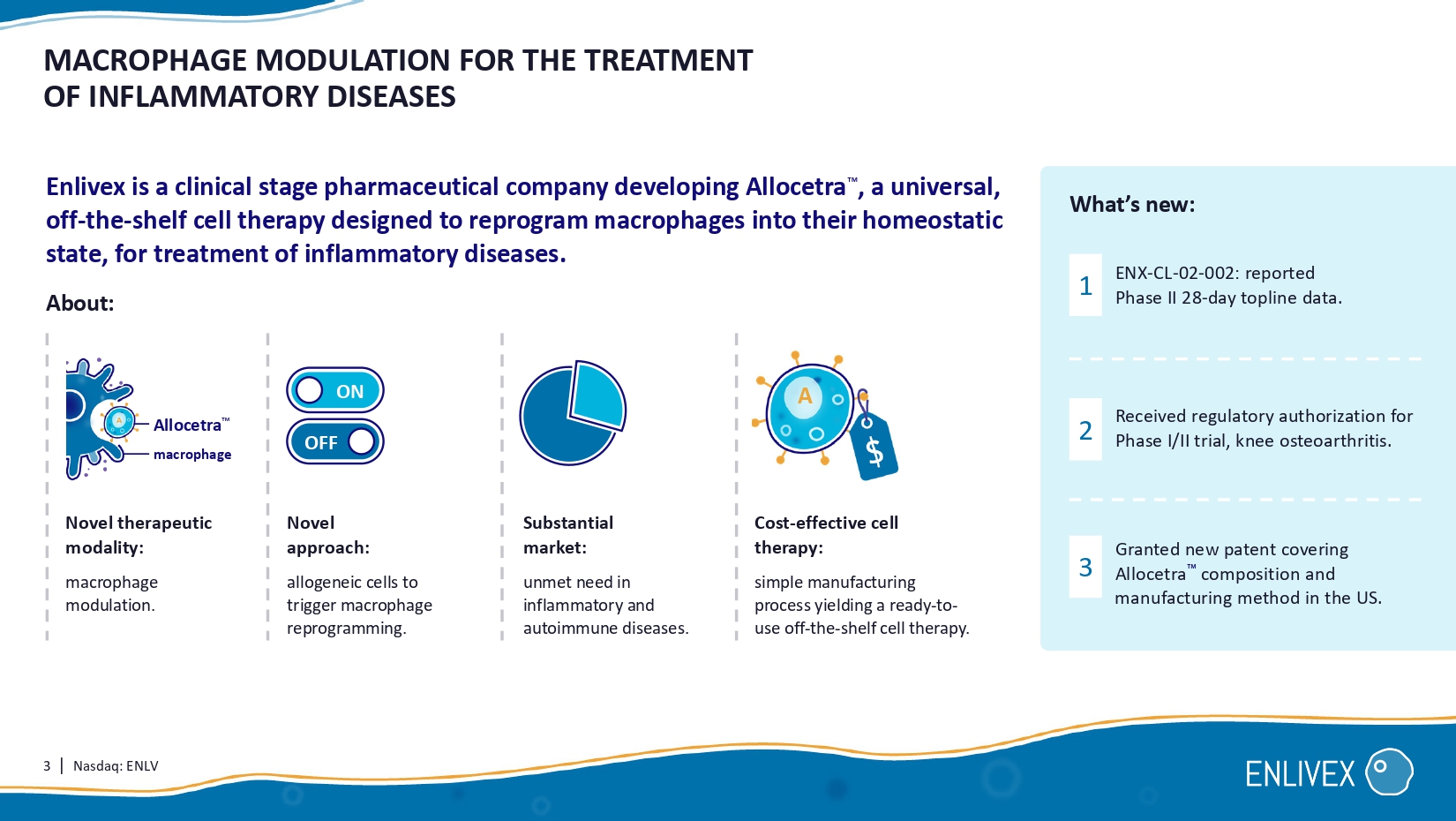
3 | Nasdaq: ENLV MACROPHAGE MODULATION FOR THE TREATMENT OF INFLAMMATORY DISEASES Enlivex is a clinical stage pharmaceutical company developing Allocetra , a universal, off - the - shelf cell therapy designed to reprogram macrophages into their homeostatic state, for treatment of inflammatory diseases . Novel therapeutic modality: macrophage modulation. Cost - effective cell therapy: simple manufacturing process yielding a ready - to - use off - the - shelf cell therapy. Substantial market: unmet need in inflammatory and autoimmune diseases. Novel approach: allogeneic cells to trigger macrophage reprogramming. ENX - CL - 02 - 002 : reported Phase II 28 - day topline data. Granted new patent covering Allocetra composition and manufacturing method in the US. What’s new: 1 2 3 ON OFF A Allocetra About : macrophage A Received regulatory authorization for Phase I/II trial, knee osteoarthritis.

4 | Nasdaq: ENLV DRIVING INNOVATION WITH BALANCED SCIENTIFIC AND BUSINESS EXPERTI SE Shai Novik Executive Chairman Iris Tavor Senior Director of RA/QA 26 years of experience $560M company sale 20 years of experience A Einat Galamidi VP Medical 20 years of experience Oren Hershkovitz Chief Executive Officer Veronique Amor - Baroukh Senior Director of Operations 10 years of experience 16 years of experience Shachar Shlosberger CFO 15 years of experience A A A Dror Mevorach Scientific Founder Chen Ankri Director of pre - clinical & clinical pharma 20 years of experience 140+ publications 10 years of experience Sigal Arad Director of Human Resources 15 years of experience 5 | Nasdaq: ENLV BOARD OF DIRECTORS Former CMO of Arqule through its $ 2.7 billion acquisition by Merck in 2020 . Previously, responsible for the global clinical development of sorafenib (Nexavar ® ) at Bayer. Former CEO of PROLOR Biotech. Founding team and Director of R&D of Interpharm (Merck Serono), where he led the development of REBIF, a multi - billion multiple sclerosis drug. Formerly, VP CMC of BioTechnology General Ltd., and VP of Clal Biotechnology Industries Ltd. Former EVP and CFO of Epizyme and Senior Biotech Investment Banker at Credit Suisse, Wells Fargo Securities and RBC Capital Markets. Led financing, partnering and M&A biopharmaceutical transactions in excess of $13B. Former Worldwide Head of Licensing and Acquisition and Knowledge Management at Merck & Co., where he led the completion of more than 150 business development transactions. Former Global Head of Infectious Diseases for Johnson & Johnson Pharmaceuticals. Former Venture Partner at Flagship Pioneering, as well as the former President, CEO, and Chairman of the Board of Seres Therapeutics. Formerly with PROLOR Biotech, led the pre - clinical, clinical , and pharmacological activities. CEO of SpliSense, a clinical stage company focused on transformative RNA - based treatments for pulmonary diseases. SpliSense pioneering platform harnesses Antisense Oligonucleotides (ASOs) for the treatment of pulmonary diseases. Shai Novik Executive Chairman Roger Pomerantz Vice Chairman Gili Hart, Ph.D Director Brian Schwartz, M.D. Director Abraham Havron, Ph.D. Director Andrew Singer Director Founder and President of PROLOR Biotech, Sold in 2013 ($560mm transaction). Lead product , Ngenla , partnered to Pfizer, $ 295 million down payment, $275 upon FDA & other regulatory approvals. Ngenla by Pfizer has obtained marketing approvals in 43 countries, including Japan, EU and U.S.

6 | Nasdaq: ENLV CELLULAR FIRST RESPONDERS: MACROPHAGES AND THEIR CRITICAL ROLE IN INFLAMMATION Recruit other immune cells Defend against pathogens Cleanup senescent or dead cells Control tissue homeostasis and repair Macrophages, which are found in abundance throughout the body, are immune cells that reside in or infiltrate human tissue . Antigen presentation Cytokine secretion Phagocytosis Immunomodulation Regulation Resolution and homeostasis Macrophages orchestrate inflammation and its resolution. Enlivex's Allocetra mechanism Initiation ON Main functions: Role in inflammation: 2 3 1 T T T The current understanding among researchers is that disrupted inflammatory processes form the basis of many diseases, beyond “classical” inflammatory diseases.
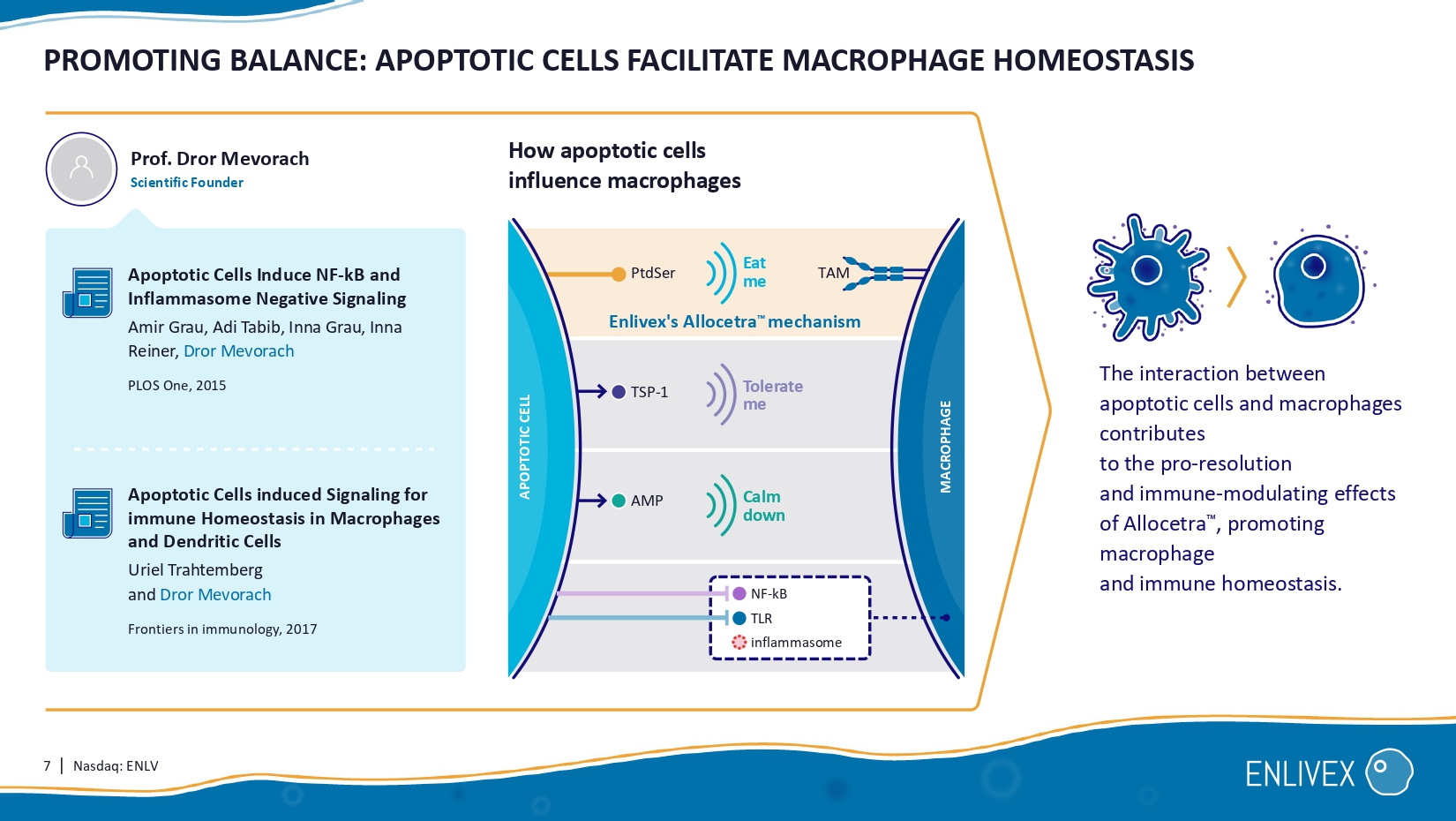
7 | Nasdaq: ENLV PROMOTING BALANCE: APOPTOTIC CELLS FACILITATE MACROPHAGE HOMEOST ASIS MACROPHAGE Eat me TSP - 1 PtdSer TAM AMP The interaction between apoptotic cells and macrophages contributes to the pro - resolution and immune - modulating effects of Allocetra , promoting macrophage and immune homeostasis. How apoptotic cells influence macrophages APOPTOTIC CELL NF - kB TLR inflammasome Tolerate me Calm down Apoptotic Cells Induce NF - kB and Inflammasome Negative Signaling Amir Grau, Adi Tabib, Inna Grau, Inna Reiner, Dror Mevorach PLOS One, 2015 Apoptotic Cells induced Signaling for immune Homeostasis in Macrophages and Dendritic Cells Uriel Trahtemberg and Dror Mevorach Frontiers in immunology, 2017 Prof.
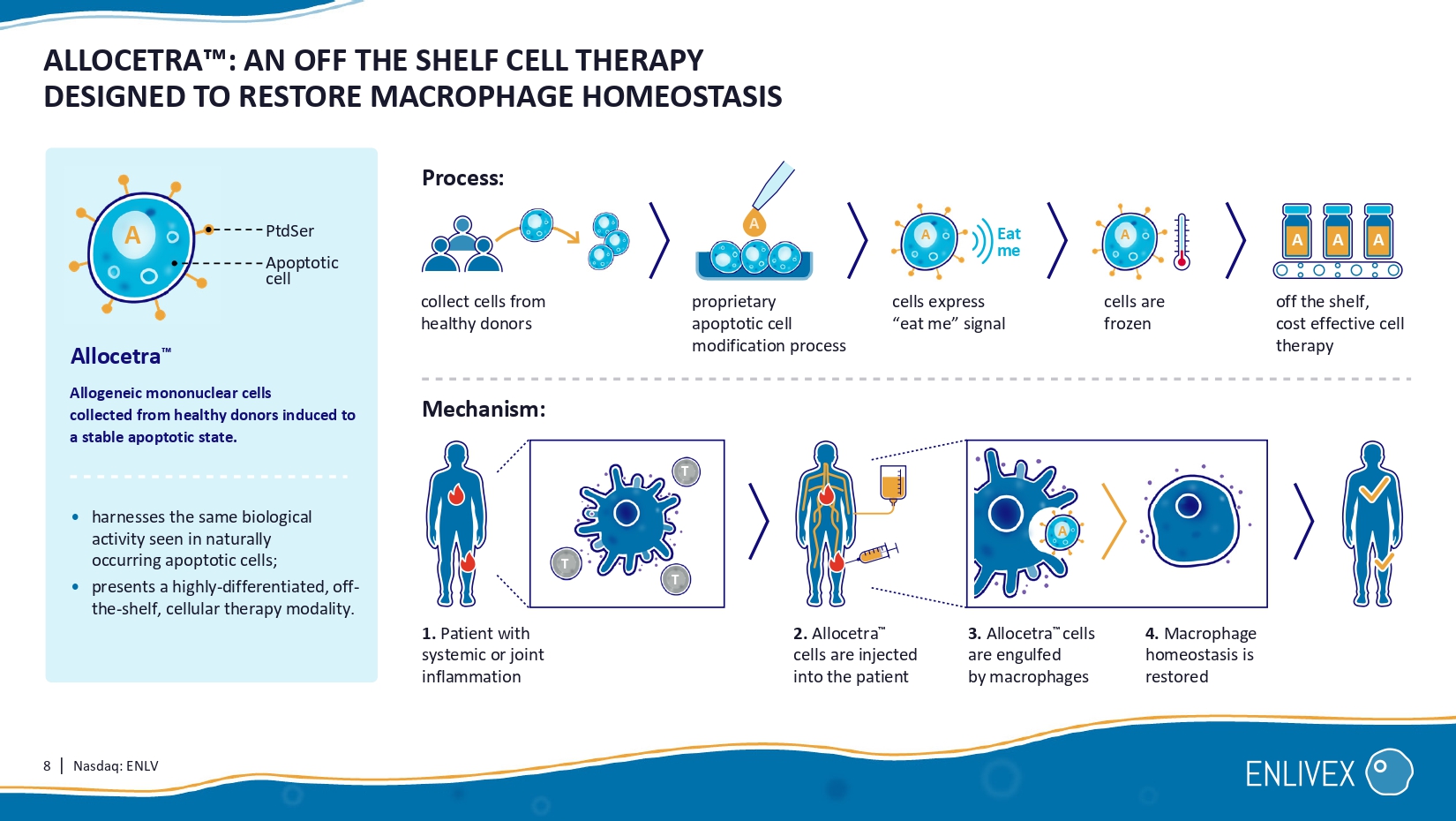
Dror Mevorach Scientific Founder Enlivex's Allocetra mechanism 8 | Nasdaq: ENLV ALLOCETRA : AN OFF THE SHELF CELL THERAPY DESIGNED TO RESTORE MACROPHAGE HOMEOSTASIS PtdSer Apoptotic cell • harnesses the same biological activity seen in naturally occurring apoptotic cells; • presents a highly - differentiated, off - the - shelf, cellular therapy modality. Allocetra Allogeneic mononuclear cells collected from healthy donors induced to a stable apoptotic state. collect cells from healthy donors p roprietary apoptotic cell modification process cells express “ eat me” signal cells are frozen off the shelf , cost effective cell therapy Process: 1. Patient with systemic or joint inflammation 2. Allocetra cells are injected into the patient 3. Allocetra cells are engulfed by macrophages 4 .
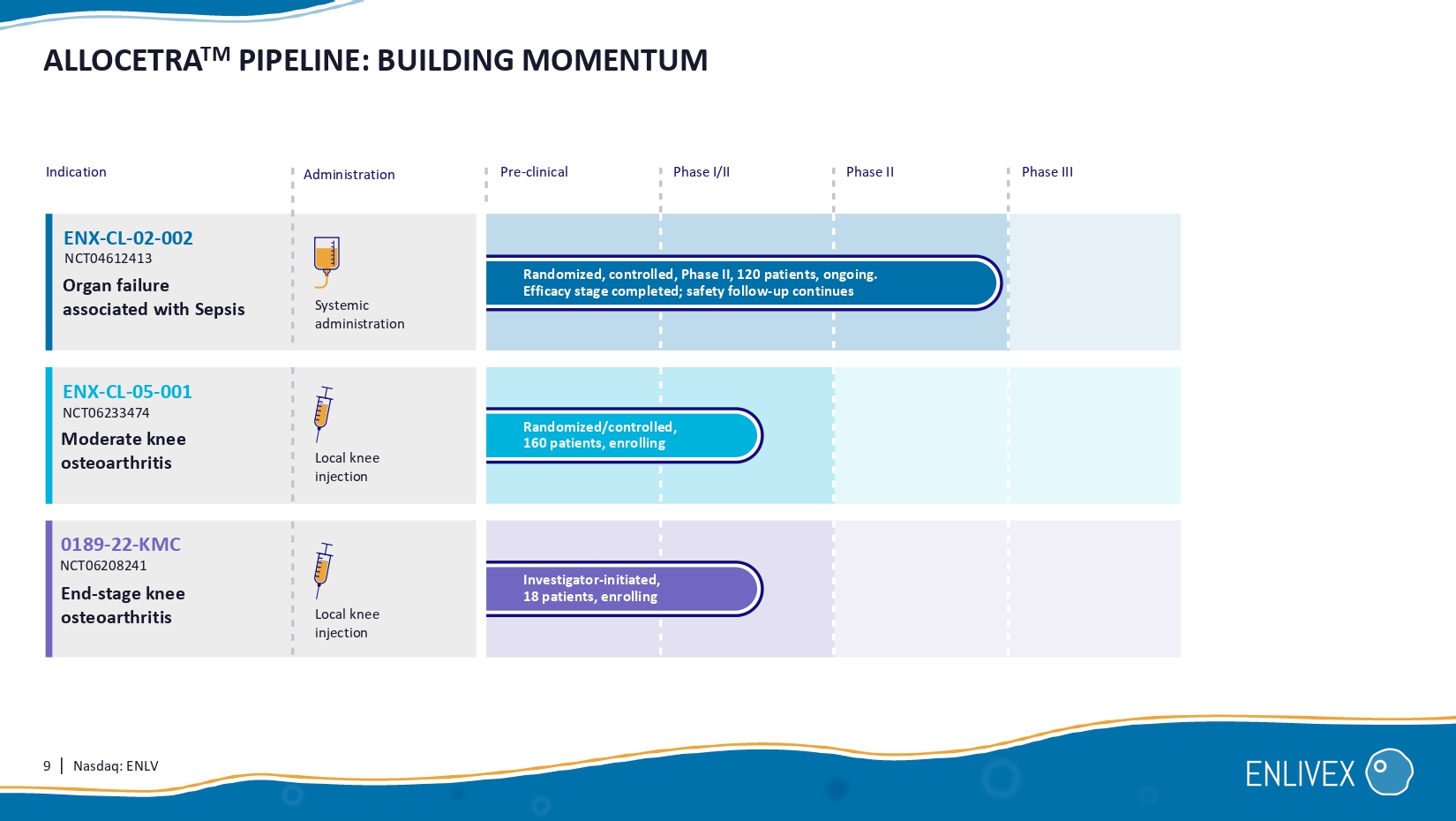
Macrophage homeostasis is restored Mechanism: Eat me A A A A A A A T T T A 9 | Nasdaq: ENLV ALLOCETRA TM PIPELINE: BUILDING MOMENTUM Indication Administration Pre - clinical Phase I/II Phase II Phase III Organ failure associated with Sepsis Moderate knee osteoarthritis ENX - CL - 05 - 001 ENX - CL - 02 - 002 End - stage knee osteoarthritis Randomized/controlled, 160 patients , enrolling Randomized, controlled, Phase II, 120 patients, ongoing. Efficacy stage completed; safety follow - up continues Investigator - initiated, 1 8 patients , enrolling NCT04612413 NCT06233474 0189 - 22 - KMC NCT06208241 Systemic administration Local knee injection Local knee injection 10 | Nasdaq: ENLV A COMPREHENSIVE PICTURE: INFLAMMATORY DISEASE TREATMENT INFORMED BY EXTENSIVE RESEARCH Sepsis * Phase / trials Administration method Safety Efficacy 1 completed trial: 2 completed trials: Compassionate case Systemic administration Local injection No drug related SAE s or drug related AE No drug related SAE s or drug related AE 10/10 Complete recovery with no residual organ failure.
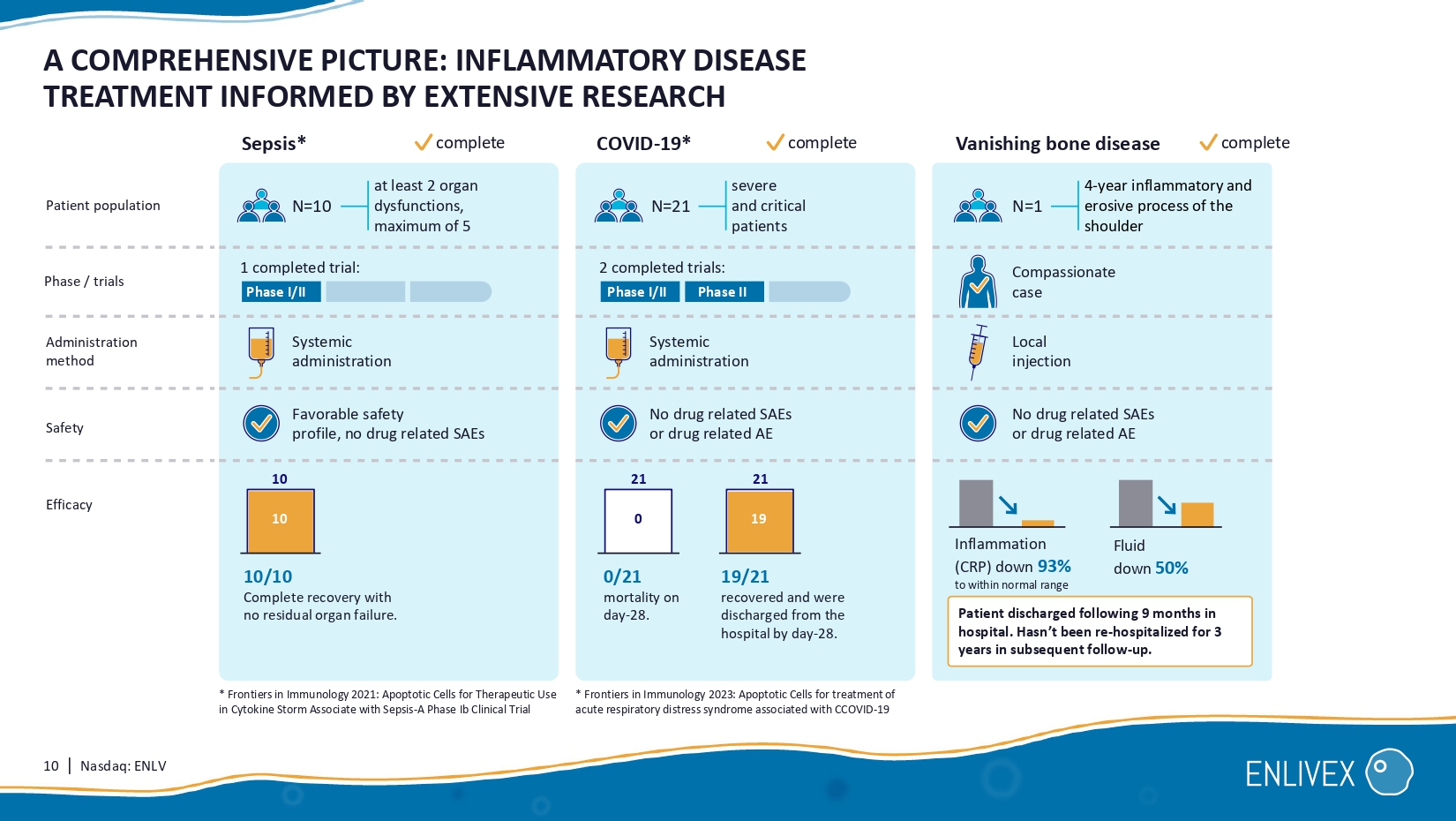
19/21 recovered and were discharged from the hospital by day - 28. 0/21 mortality on day - 28. Patient discharged following 9 months in hospital. Hasn’t been re - hospitalized for 3 years in subsequent follow - up . Phase I/II Phase I/II Phase II 10 21 21 Patient population at least 2 organ dysfunctions, maximum of 5 severe and critical patients 4 - year inflammatory and erosive process of the shoulder N=21 N=1 N=10 Systemic administration Favorable safety profile, no drug related SAEs Inflammation (CRP) down 93% to within normal range 10 0 19 complete complete complete COVID - 19* Vanishing bone disease Fluid down 50% * Frontiers in Immunology 2021 : Apoptotic Cells for Therapeutic Use in Cytokine Storm Associate with Sepsis - A Phase Ib Clinical Trial * Frontiers in Immunology 2023: Apoptotic Cells for treatment of acute respiratory distress syndrome associated with CCOVID - 19 11 | Nasdaq: ENLV ALLOCETRA FOR THE TREATMENT OF SEPSIS


12 | Nasdaq: ENLV SEPSIS: A GLOBAL HEALTH CHALLENGE WITH SUBSTANTIAL MARKET OPPORT UNITY Market Standard of care Disease Antibiotics Vasopressors Currently there are no FDA/EMA approved drugs to treat sepsis. SOC only treats complications of sepsis and does not address core dysregulated immune response. $33B Global market (severe Sepsis only 2 ) Patients receive: IV fluids Affected areas: A life - threatening overactive immune response to infection that attacks the body and leads to: • tissue damage, • organ failure, • death. Brain Lungs Liver Kidneys H eart 1 - Management of Urosepsis in 2018, Bonkat et. Al. , European Urology Focus Volume 5, Issue 1, (2019) 2 - Number of severe cases ( www.cdc.gov/sepsis/what - is - sepsis ) of 675,000 for US & EU (estimated 25% of the sepsis cases) multiplied by the expected product pricing of $50k = 33B 1. 6M deaths ~ 9.8 M annual cases worldwide Up to 31% of sepsis cases start as urinary infections (UTI) 1 31% UTI cases 13 | Nasdaq: ENLV A TREATMENT FOR SEPSIS IS AN UNMET MEDICAL NEED 4 3 2 1 0 1 - Polkki et al.
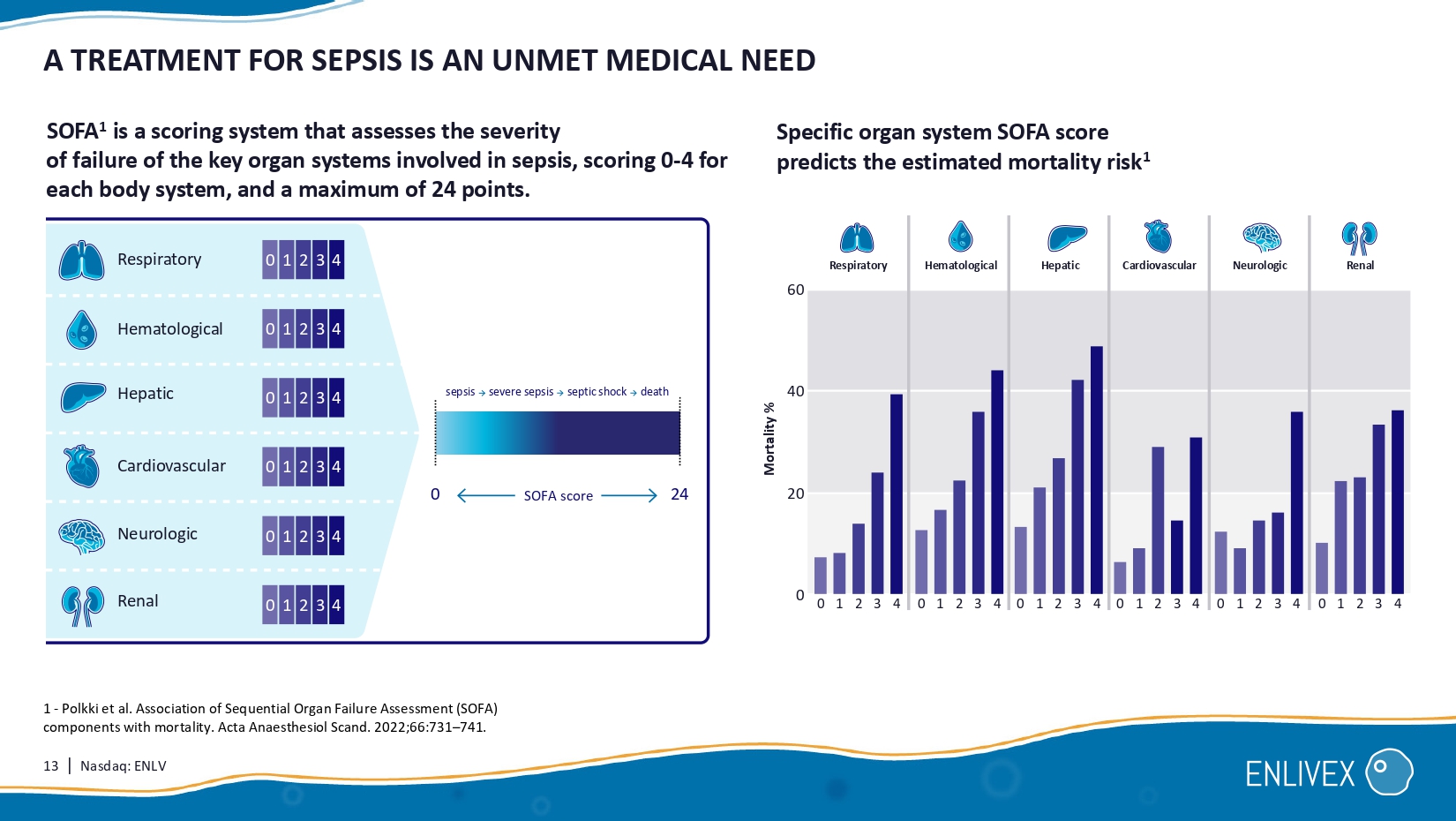
Association of Sequential Organ Failure Assessment (SOFA) components with mortality. Acta Anaesthesiol Scand. 2022;66:731 – 741. Respiratory Renal Neurologic Hepatic Hematological SOFA 1 is a scoring system that assesses the severity of failure of the key organ systems involved in sepsis, scoring 0 - 4 for each body system, and a maximum of 24 points. 0 24 SOFA score Specific organ system SOFA score predicts the estimated mortality risk 1 Respiratory Mortality % Hematological Hepatic Neurologic Renal Cardiovascular 60 40 20 0 Cardiovascular 0 1 2 3 4 0 1 2 3 4 0 1 2 3 4 0 1 2 3 4 0 1 2 3 4 0 1 2 3 4 sepsis severe sepsis septic shock death 4 3 2 1 0 4 3 2 1 0 4 3 2 1 0 4 3 2 1 0 4 3 2 1 0 14 | Nasdaq: ENLV SEPSIS PHASE I/II: INDICATION OF EFFECT OF ALLOCETRA at least 2 organ dysfunctions (kidney, lungs, cardiovascular, hematological, liver).
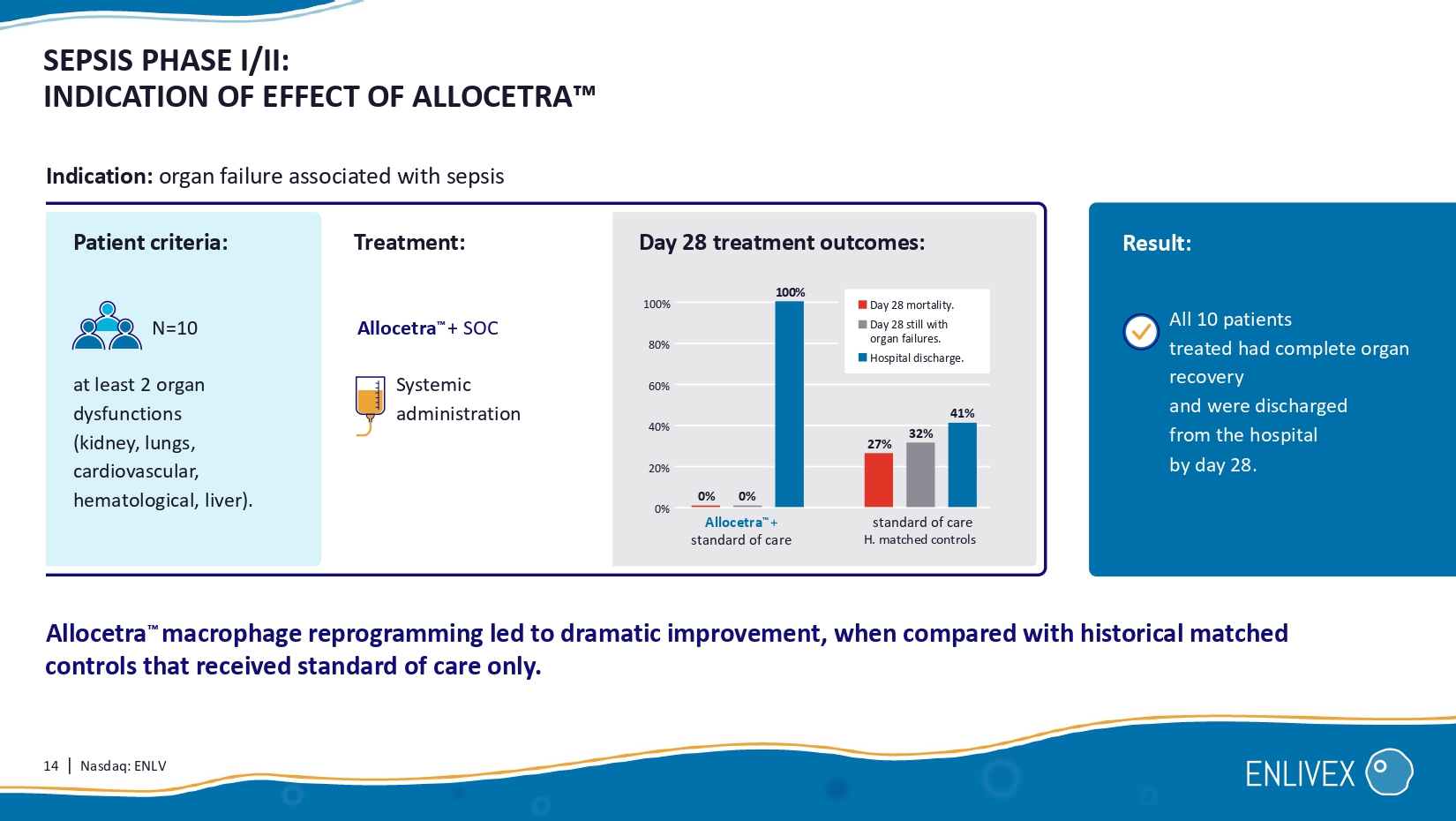
Allocetra macrophage reprogramming led to dramatic improvement, when compared with historical matched controls that received standard of care only . Result: N=10 Indication: organ failure associated with sepsis All 10 patients treated had complete organ recovery and were discharged from the hospital by day 28 . Day 28 treatment outcomes: Allocetra + SOC standard of care H. matched controls Systemic administration 100% 80% 60% 20 % 27% 32% 41% Day 28 mortality. Day 28 still with organ failures. Hospital discharge.
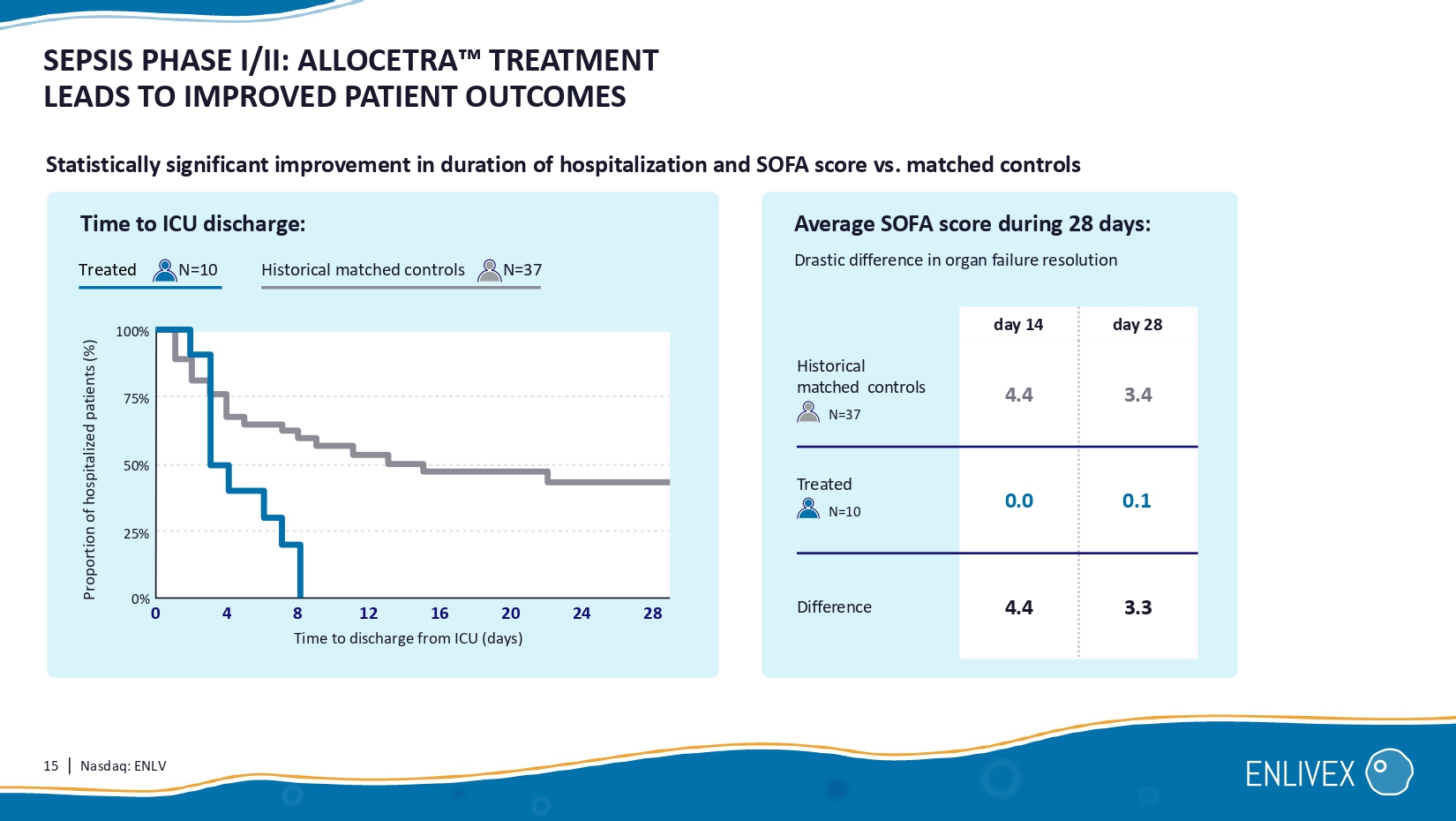
Allocetra + standard of care 0% 0% 0 % 4 0% Patient criteria: Treatment: 100% 15 | Nasdaq: ENLV day 28 day 14 3.4 4.4 H istorical matched controls N= 37 0.1 0.0 Treated N=10 3.3 4.4 Difference SEPSIS PHASE I/II: ALLOCETRA TREATMENT LEADS TO IMPROVED PATIENT OUTCOMES Statistically significant improvement in duration of hospitalization and SOFA score vs.
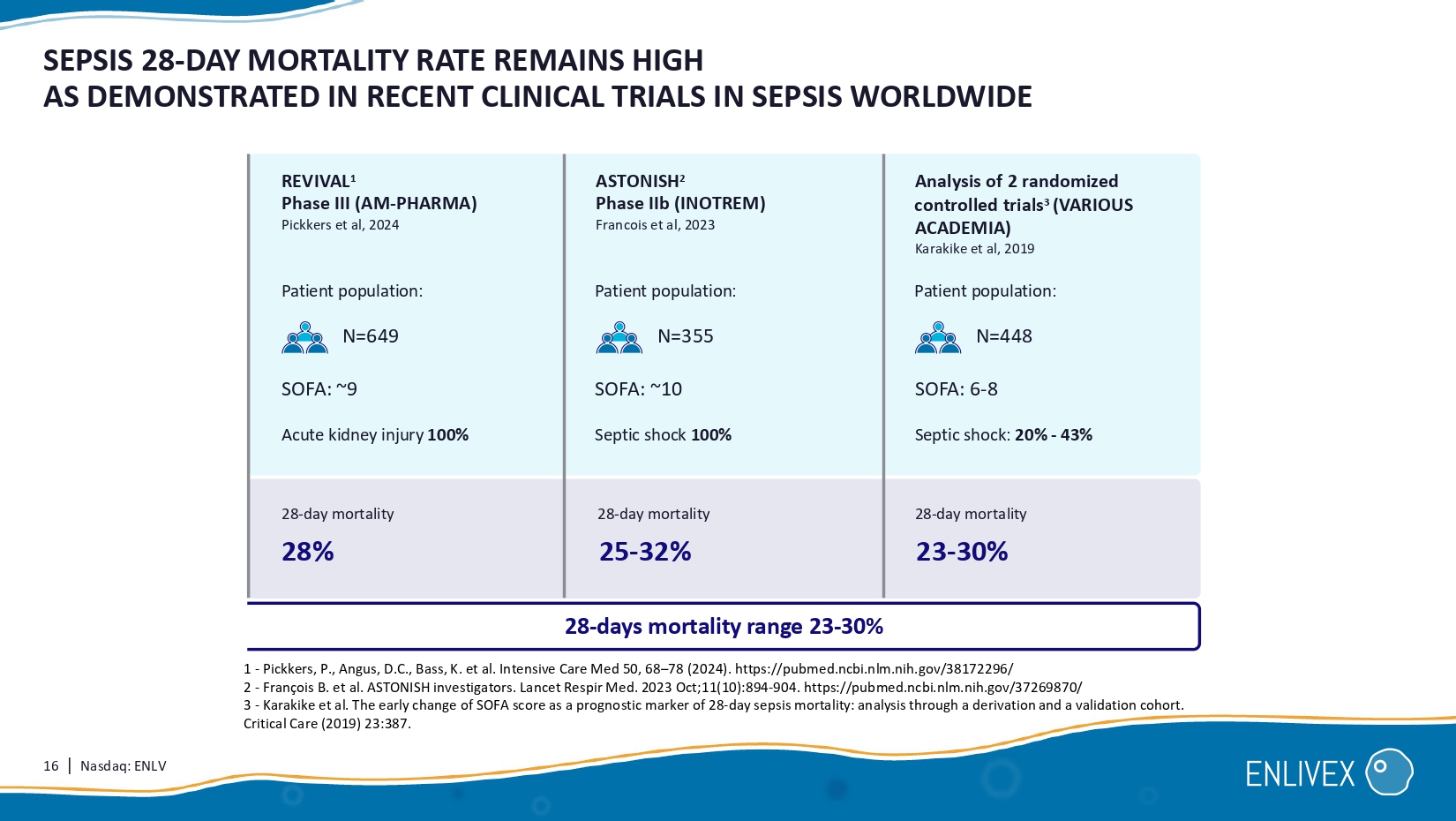
matched controls Average SOFA score during 28 days: Drastic difference in organ failure resolution Proportion of hospitalized patients (%) 0 4 8 24 28 Time to discharge from ICU (days) Treated Time to ICU discharge: N=10 Historical matched controls N= 37 12 16 20 100% 75% 50% 25% 0% 16 | Nasdaq: ENLV SEPSIS 28 - DAY MORTALITY RATE REMAINS HIGH AS DEMONSTRATED IN RECENT CLINICAL TRIALS IN SEPSIS WORLDWIDE 1 - Pickkers , P., Angus, D.C., Bass, K. et al. Intensive Care Med 50 , 68 – 78 ( 2024 ). https://pubmed.ncbi.nlm.nih.gov/ 38172296 / 2 - François B. et al. ASTONISH investigators. Lancet Respir Med. 2023 Oct; 11 ( 10 ): 894 - 904 . https://pubmed.ncbi.nlm.nih.gov/ 37269870 / 3 - Karakike et al. The early change of SOFA score as a prognostic marker of 28 - day sepsis mortality: analysis through a derivation and a validation cohort. Critical Care ( 2019 ) 23:387 . REVIVAL 1 Phase III (AM - PHARMA) Pickkers et al, 2024 Patient population: Patient population: Patient population: ASTONISH 2 Phase IIb (INOTREM) Francois et al, 2023 Analysis of 2 randomized controlled trials 3 (VARIOUS ACADEMIA) Karakike et al, 2019 SOFA: ~10 SOFA: ~ 9 SOFA: 6 - 8 2 ϴ % 28 - day mortality 28 - day mortality 28 - day mortality N=649 N=355 N= 448 Acute kidney injury 100% Septic shock 100 % Septic shock: 20% - 43% 28 - days mortality range 23 - 30% 25 - 32% 23 - 30% 17 | Nasdaq: ENLV ENX - CL - 02 - 002 SEPSIS PHASE II RANDOMIZED CONTROLLED STUDY Primary: Safety /change in SOFA score.
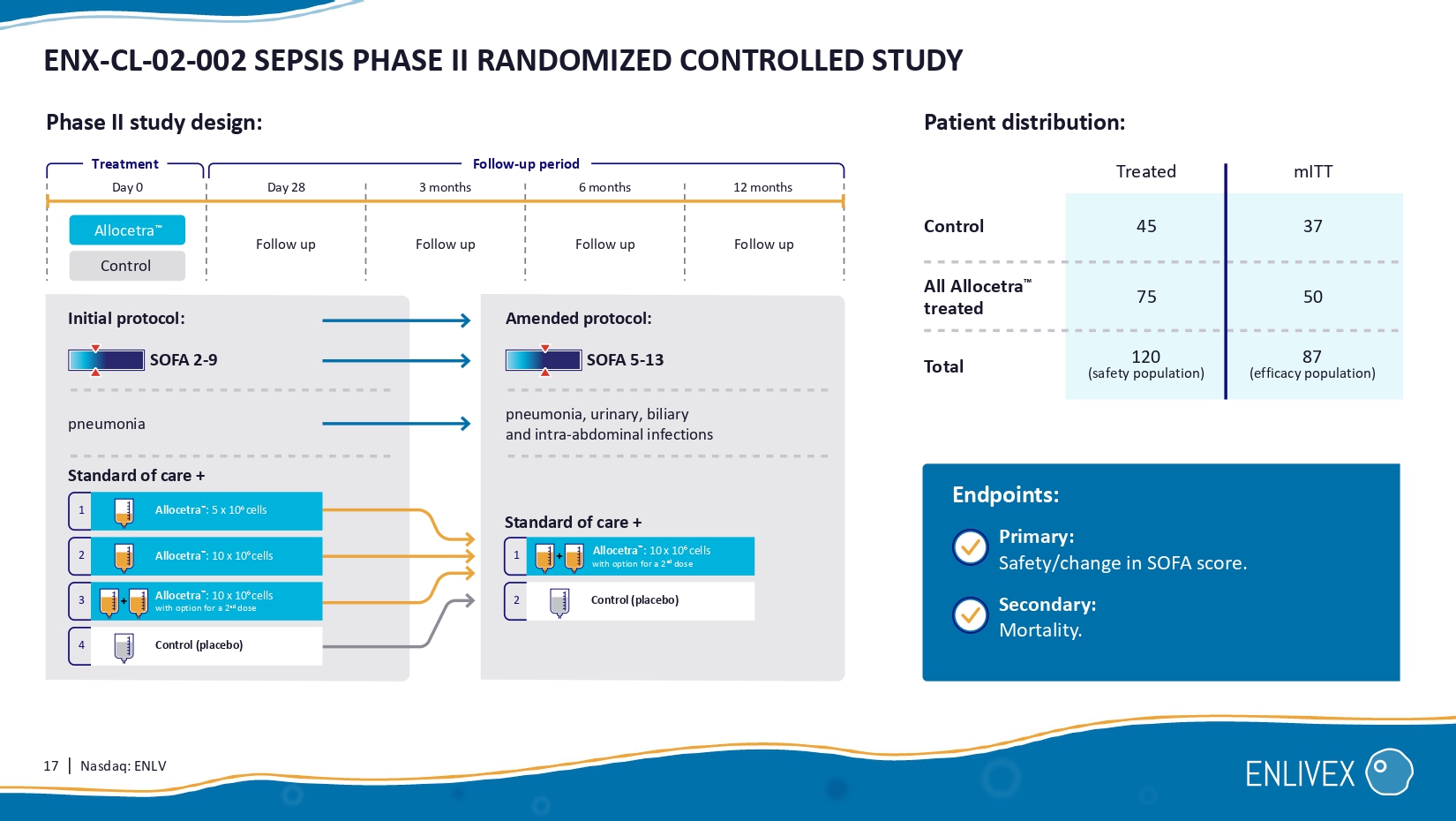
Secondary: Mortality. Endpoints: Control (placebo) 1 Allocetra : 10 x 10 6 cells with option for a 2 nd dose SOFA 2 - 9 Standard of care + SOFA 5 - 13 pneumonia pneumonia, urinary, biliary and intra - abdominal infections Initial protocol: Amended protocol: Follow - up period Treatment Follow up Follow up Follow up Follow up Allocetra Control Day 0 Day 28 3 months 6 months 12 months 1 Allocetra : 5 x 10 6 cells 2 Allocetra : 10 x 10 6 cells 4 Control (placebo) 3 Allocetra : 10 x 10 6 cells with option for a 2 nd dose 2 Phase II study design: Standard of care + 75 120 (safety population) 50 87 (efficacy population) Control Total All Allocetra treated Treated 4 5 37 mITT Patient distribution:
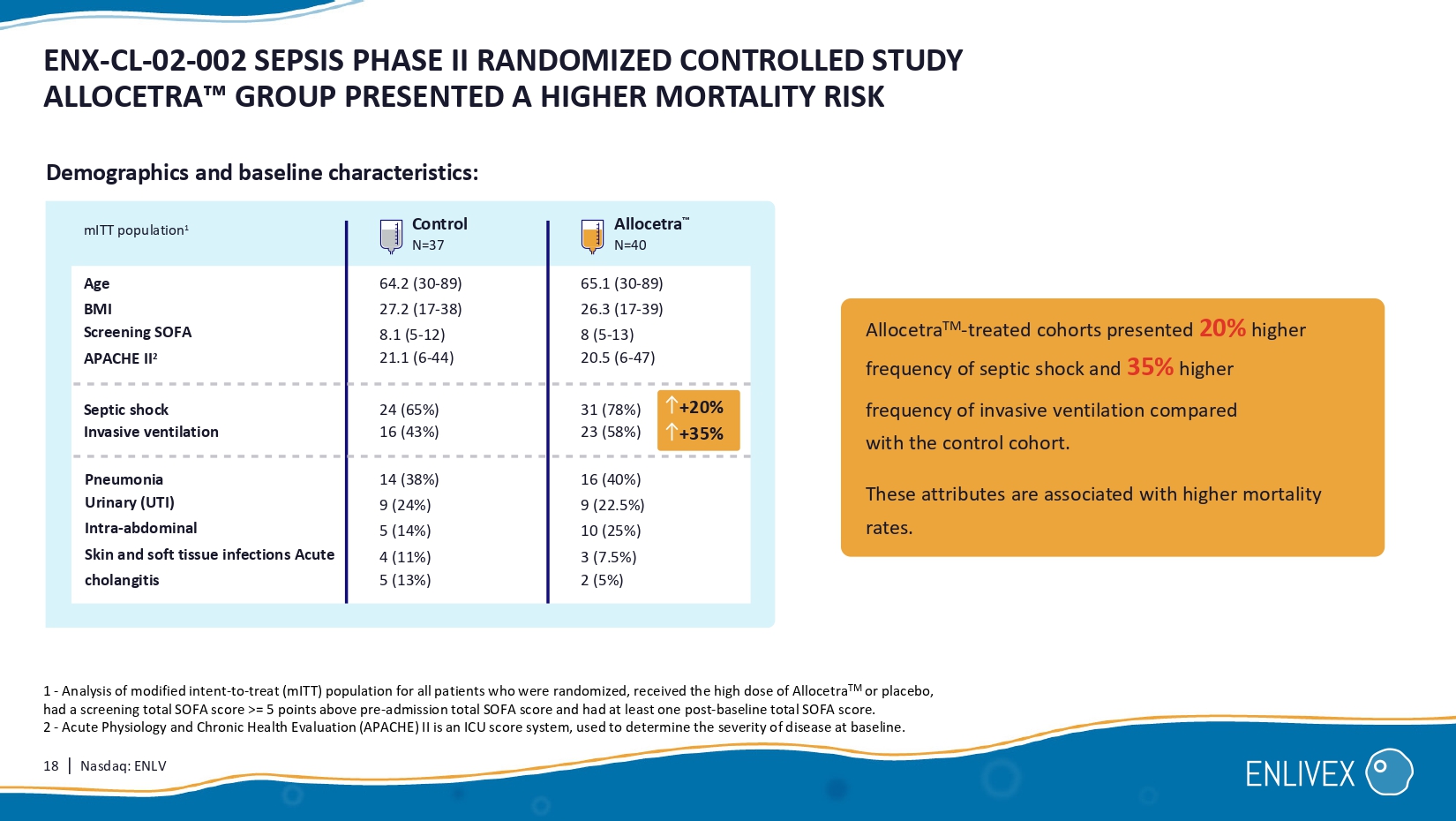
18 | Nasdaq: ENLV ENX - CL - 02 - 002 SEPSIS PHASE II RANDOMIZED CONTROLLED STUDY ALLOCETRA GROUP PRESENTED A HIGHER MORTALITY RISK 1 - Analysis of modified intent - to - treat ( mITT ) population for all patients who were randomized, received the high dose of Allocetra TM or placebo, had a screening total SOFA score >= 5 points above pre - admission total SOFA score and had at least one post - baseline total SOFA score. 2 - Acute Physiology and Chronic Health Evaluation (APACHE) II is an ICU score system, used to determine the severity of disease at baseline. Allocetra TM - treated cohorts presented 20% higher frequency of septic shock and 35% higher frequency of invasive ventilation compared with the control cohort. These attributes are associated with higher mortality rates. Age BMI Screening SOFA APACHE II 2 mITT population 1 Septic shock Invasive ventilation 24 (65%) 16 (43%) 31 (78%) 23 (58%) Pneumonia Urinary ( UTI) Intra - abdominal Skin and soft tissue infections Acute cholangitis 14 (38%) 9 (24%) 5 (14%) 4 (11%) 5 (13%) 16 (40%) 9 (22.5%) 10 (25%) 3 (7.5%) 2 (5%) 64.2 ( 30 - 89 ) 27.2 ( 17 - 3 8 ) 8.1 ( 5 - 12 ) 21.1 ( 6 - 44 ) 65.1 (30 - 89) 26.3 (1 7 - 3 9 ) 8 (5 - 13) 20.5 (6 - 47) Control N=37 Allocetra N=40 +20% +35% Demographics and baseline characteristics:
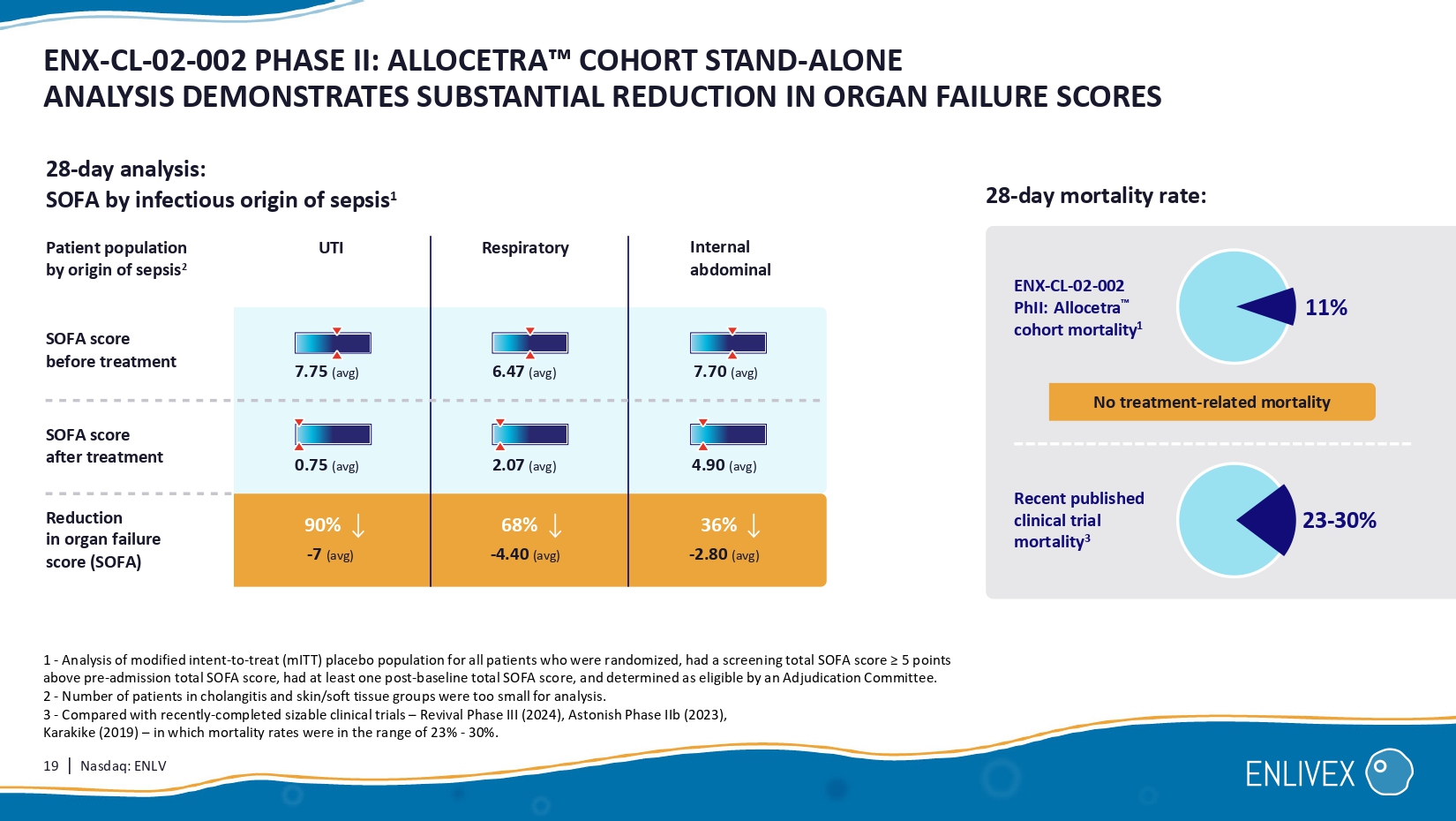
19 | Nasdaq: ENLV ENX - CL - 02 - 002 PHASE II: ALLOCETRA COHORT STAND - ALONE ANALYSIS DEMONSTRATES SUBSTANTIAL REDUCTION IN ORGAN FAILURE SCO RES 1 - Analysis of modified intent - to - treat (mITT) placebo population for all patients who were randomized, had a screening total S OFA score ≥ 5 points above pre - admission total SOFA score, had at least one post - baseline total SOFA score, and determined as eligible by an Adjudica tion Committee. 2 - Number of patients in cholangitis and skin/soft tissue groups were too small for analysis. 3 - Compared with recently - completed sizable clinical trials – Revival Phase III (2024), Astonish Phase IIb (2023), Karakike (2019) – in which mortality rates were in the range of 23% - 30%. 28 - day analysis: SOFA by infectious origin of sepsis 1 Patient population by origin of sepsis 2 Respiratory Internal abdominal Reduction in organ failure score (SOFA) SOFA score before treatment SOFA score after treatment 7.75 (avg) 6.47 (avg) 7.70 (avg) 0.75 (avg) 2.07 (avg) - 7 (avg) - 4.40 (avg) - 2.80 (avg) 4.90 (avg) 90% 68% 36% 11% 23 - 30 % Recent published clinical trial mortality 3 28 - day mortality rate: No treatment - related mortality UTI ENX - CL - 02 - 002 PhII: Allocetra cohort mortality 1 20 | Nasdaq: ENLV ENX - CL - 02 - 002 PHASE II: UTI HIGH - RISK PATIENTS, POTENTIAL INDICATION OF EFFECT, SUBSTANTIAL MARKET 1 - Management of Urosepsis in 2018 , Bonkat et.

Al. , European Urology Focus Volume 5 , Issue 1 , ( 2019 ). Potential indication of effect in high - risk UTI patients Despite higher risk of the Allocetra - treated group D1 - 28 UTI Population: screening SOFA 7 Allocetra Control N= 9 N=6 - 6.75 - 8.40 2.12 2.61 24% 0.1181 D 1 - 14 - 7.22 - 9.00 2.28 2.28 25% 0.0814 Av erage reduction in SOFA score : Stdev: Stdev: % over control: p - value: Respiratory SOFA Coagulation SOFA Cardiovascular SOFA Renal SOFA 22 % 33% 33 % 11% 33% 17% 83% 50% 78 % 3 3 =4 3 100% S eptic shock Control N= 9 Allocetra N= 6 Av erage reduction in SOFA score :
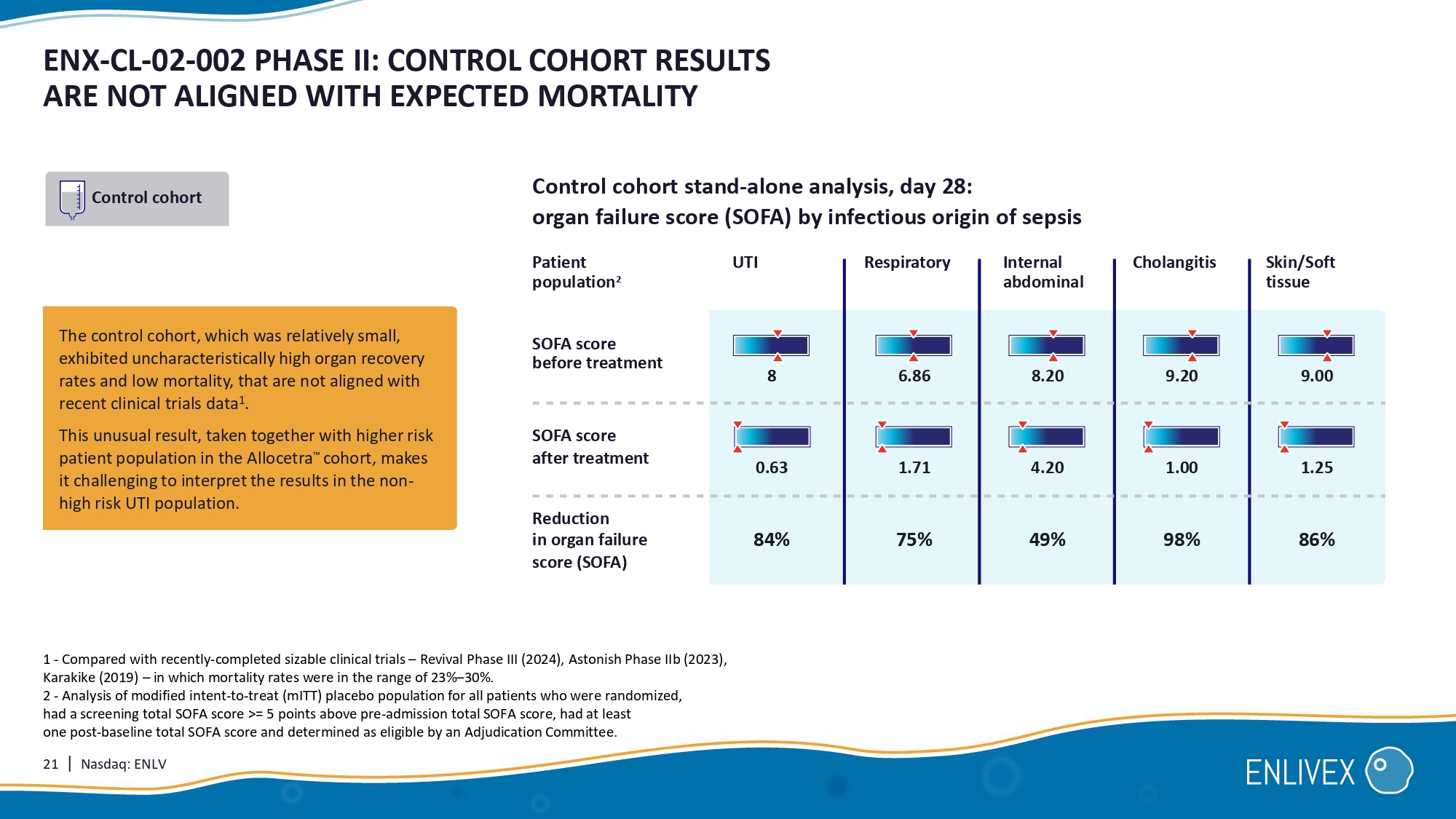
21 | Nasdaq: ENLV ENX - CL - 02 - 002 PHASE II: CONTROL COHORT RESULTS ARE NOT ALIGNED WITH EXPECTED MORTALITY The control cohort , which was relatively small, exhibited uncharacteristically high organ recovery rates and low mortality, that are not aligned with recent clinical trials data1. This unusual result , taken together with higher risk patient population in the Allocetra cohort, makes it challenging to interpret the results in the non - high risk UTI population . Control cohort stand - alone analysis, day 28: organ failure score (SOFA) by infectious origin of sepsis Skin/Soft tissue Reduction in organ failure score (SOFA) SOFA score before treatment SOFA score after treatment Control cohort 1 - Compared with recently - completed sizable clinical trials – Revival Phase III (2024), Astonish Phase IIb (2023), Karakike (2019) – in which mortality rates were in the range of 23% – 30%. 2 - Analysis of modified intent - to - treat (mITT) placebo population for all patients who were randomized, had a screening total SOFA score >= 5 points above pre - admission total SOFA score, had at least one post - baseline total SOFA score, and determined as eligible by an Adjudication Committee.
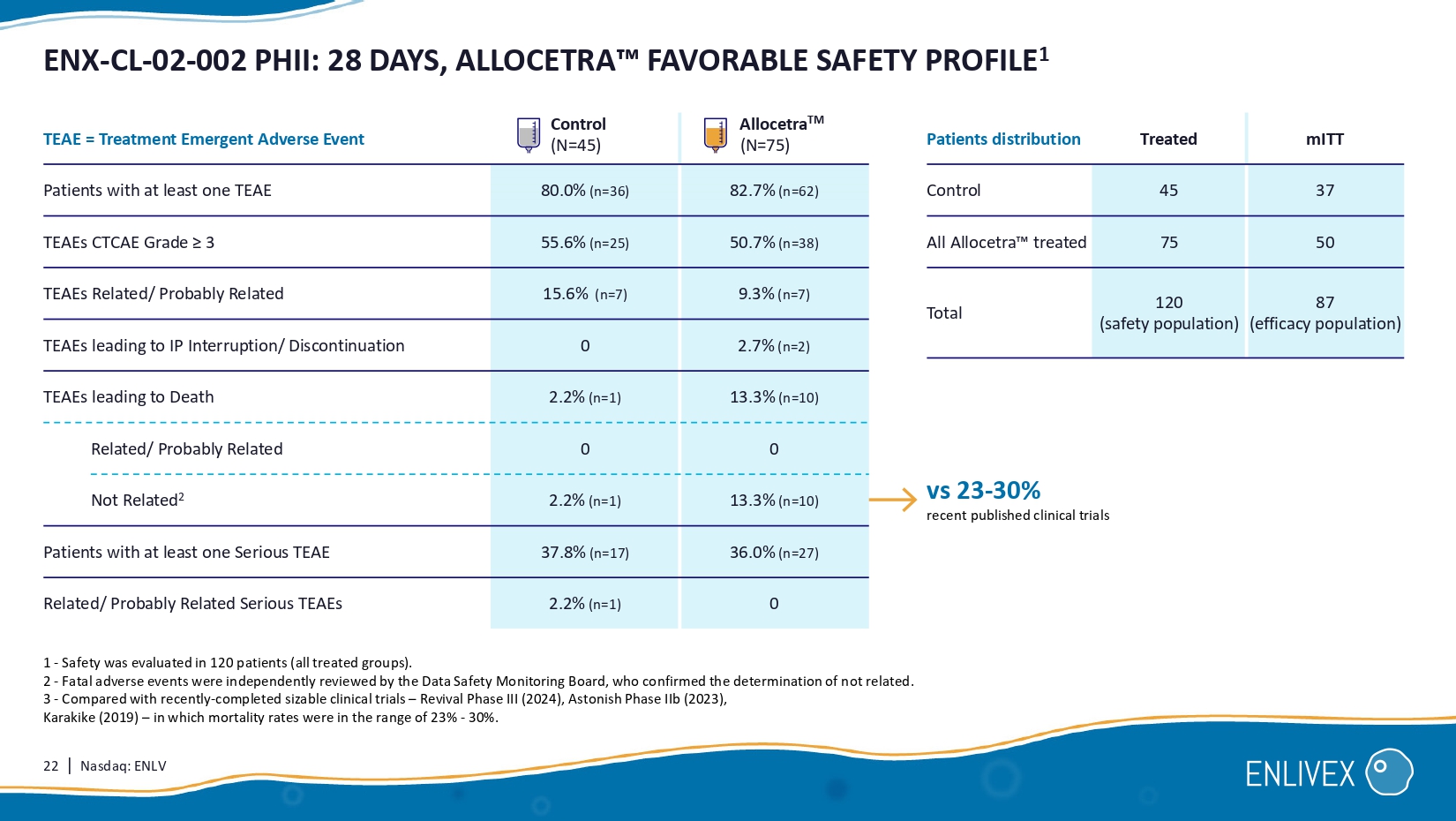
Patient population 2 UTI Respiratory Internal abdominal Cholangitis 84% 75% 49% 98% 86% 8 0.63 6.86 1.71 8.20 9.20 9.00 4.20 1.00 1.25 22 | Nasdaq: ENLV ENX - CL - 02 - 002 PHII: 28 DAYS, ALLOCETRA FAVORABLE SAFETY PROFILE 1 Allocetra TM ( N=75) Control ( N=45) TEAE = Treatment Emergent Adverse Event 82.7% (n=62) 80.0% (n=36) Patients with at least one TEAE 50.7% (n=38) 55.6% (n=25) TEAEs CTCAE Grade ≥ 3 9.3% (n=7) 15.6% (n=7) TEAEs Related/ Probably Related 2.7% (n=2) 0 TEAEs leading to IP Interruption/ Discontinuation 13.3% (n= 10 ) 2.2% (n= 1 ) TEAEs leading to Death 0 0 Related/ Probably Related 13.3% (n= 10 ) 2.2% (n= 1 ) Not Related 2 36.0% (n=27) 37.8% (n=17) Patients with at least one Serious TEAE 0 2.2% (n= 1 ) Related/ Probably Related Serious TEAEs 1 - Safety was evaluated in 120 patients (all treated groups). 2 - Fatal adverse events were independently reviewed by the Data Safety Monitoring Board, who confirmed the determination of not related. 3 - Compared with recently - completed sizable clinical trials – Revival Phase III (2024), Astonish Phase IIb (2023), Karakike (2019) – in which mortality rates were in the range of 23% - 30%. vs 23 - 30% recent published clinical trials mITT Treated Patients distribution 37 45 Control 50 75 All Allocetra treated 87 (efficacy population) 120 (safety population) Total 23 | Nasdaq: ENLV ENX - CL - 02 - 002 PHII: SUMMARY AND CONCLUSIONS 1 - Compared with recently - completed sizable clinical trials – Revival Phase III (2024), Astonish Phase IIb (2023), Karakike (2019) – in which mortality rates were in the range of 23% – 30%.

2 - Management of Urosepsis in 2018, Bonkat et. Al. , European Urology Focus Volume 5, Issue 1, (2019). The efficacy ( mITT ) population presented a 20% higher frequency of septic shock in Allocetra TM - treated patients compared to placebo, and a 35% higher frequency of invasive ventilation – both key determinants of disease severity, potentially indicating risk imbalance between the groups. Stand - alone analysis of the Allocetra TM - treated patients demonstrated a substantial reduction in organ failure scores (SOFA) and low mortality rate as compared with expected mortality 1 . The analysis showed reductions, by day 28, in organ failure scores (SOFA) of 90% for sepsis patients whose infection source was urinary tract (UTI), 68% for patients whose infection source was community - acquired pneumonia, and 36% for patients whose infection source was internal abdominal. A potential indication of relative efficacy is demonstrated in a population of high risk UTI patients. Up to 31 percent of sepsis cases start as UTIs, representing up to 9.8 million cases annually in the U.S. and Europe, leading to as many as 1.6 million deaths 2 . This a substantial target market for a potential commercialization of Allocetra in sepsis, and the Company intends to consider, upon reviewing the totality of the data, a potential follow - on, randomized, controlled study of solely high risk UTI sepsis population. The interpretation of efficacy in other populations is challenged by the difference in risk profile of the Allocetra group. Safety: No serious adverse events were reported as related to study treatment, and overall fewer events were considered relat ed to Allocetra TM compared to placebo (9.3% vs 15.6%). All deaths were determined to be unrelated to treatment, as further confirmed by the indep endent DSMB. No safety signals were detected. Patient follow - up is ongoing to complete 12 - month evaluation.

24 | Nasdaq: ENLV ALLOCETRA FOR THE TREATMENT OF OSTEOARTHRITIS 25 | Nasdaq: ENLV OSTEOARTHRITIS: A GROWING MARKET WITH SIGNIFICANT POTENTIAL Standard of care Disease overview Disease manifestation: cartilage damage, abnormal bone remodeling , and inflammation of the synovium.

Today 2023 2040 2030 32.5M M $ 6.8 B 78MM $ 15.7 B Market Lifestyle changes Physiotherapy Pain medication Surgery Market size 2 : Femur Synovitis Joint space narrowing Cartilage loss Subchondral bone cysts/ sclerosis Tibia Fibula U.S.

Cases ϭ : 1 - Arthritis Foundation ( https://www.arthritis.org/ ) 2 - Verified Market Research reports 26 | Nasdaq: ENLV MACROPHAGES ARE AN EMERGING NEW TARGET FOR OSTEOARTHRITIS TREATMENT Synovial macrophages in osteoarthritis: the key to understanding pathogenesis? Amanda Thomson and Catharien M. U. Hilkens Frontiers in Immunology 2021 Imbalance of M1/M2 macrophages is linke d to severity level of knee osteoarthritis. Baolong Liu, Maoquan Zhang, Jingming Zhao, Mei Zheng and Hao Yang Experimental and therapeutic medicine 2018 An emerging target in the battle against osteoarthritis : macrophage polarization. Yulong Sun, Zhuo Zuo and Yuanyuan Kuang International Journal of Molecular Sciences 2020 Characterizing heterogeneity in the response of synovial mesenchymal progenitor cells to synovial macrophages in normal individuals and patients with osteoarthritis. Akash Fichadiya, Karri L Bertram, Guomin Ren, Robin M Yates and Roman J Krawetz Journal of Inflammation 2016 The role of innate immunity in osteoarthritis : when our first line of defense goes on the offensive. Eric W.
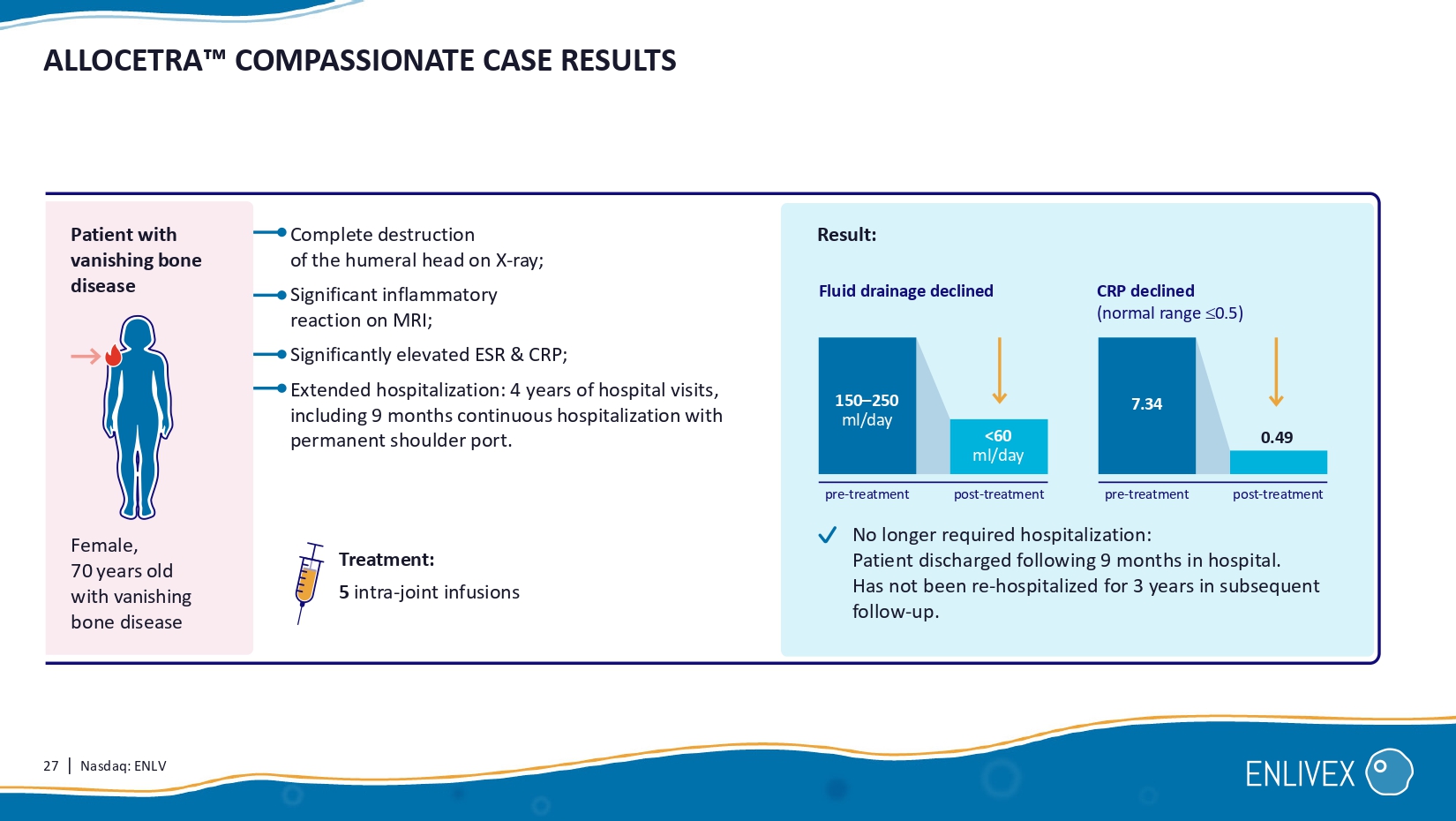
Orlowsky and Virginia Byers Kraus The Journal of Rheumatology 2015 27 | Nasdaq: ENLV Complete destruction of the humeral head on X - ray; Significant inflammatory reaction on MRI; Significantly elevated ESR & CRP ; Extended hospitalization: 4 years of hospital visits, including 9 months continuous hospitalization with permanent shoulder port. Treatment: 5 intra - joint infusions ALLOCETRA COMPASSIONATE CASE RESULTS 0. 49 CRP declined ( normal range 0.5) Result: Fluid drainage declined Female, 70 years old with vanishing bone disease 7.34 No longer required hospitalization: Patient discharged following 9 months in hospital. Has not been re - hospitalized for 3 years in subsequent follow - up. 150 – 250 ml/day <60 ml /day Patient with vanishing bone disease pre - treatment post - treatment pre - treatment post - treatment 28 | Nasdaq: ENLV 0189 - 22 - KMC INVESTIGATOR INITIATED TRIAL FOR END - STAGE KNEE OSTEOARTHRITIS Primary: safety and tolerability.

Secondary: change from baseline in pain . Endpoints: N= ϭϴ ClinicalTrials.gov Registration: NCT06208241 End stage osteoarthritis indicated for knee replacement surgery . local knee injection Study initiated enrolment in Q3 - 2023. Follow - up period Treatment period Follow up Follow up Follow up Allocetra Day 1 1 M 3 M 6 M Follow up 12 M 29 | Nasdaq: ENLV ENX - CL - 05 - 001 CLINICAL TRIAL DESIGN Primary: safety and tolerability.
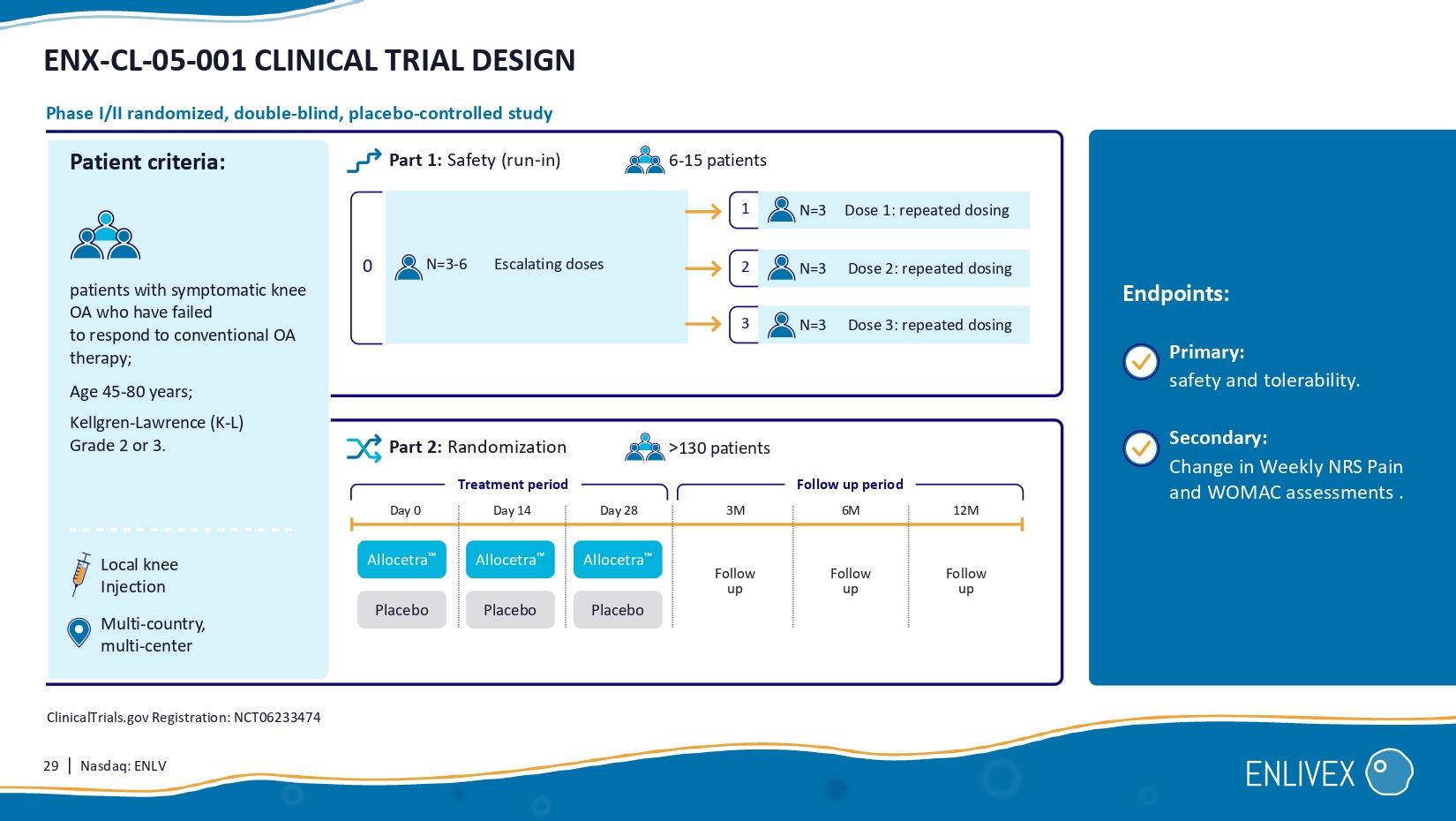
Secondary: Change in Weekly NRS Pain and WOMAC assessments . Endpoints: Local knee Injection Multi - country, multi - center Patient criteria: patients with symptomatic knee OA who have failed to respond to conventional OA therapy; Age 45 - 80 years; Kellgren - Lawrence (K - L) Grade 2 or 3 . Part 1 : Safety (run - in) Day 0 Day 14 Day 28 Escalating doses Phase I/II randomized, double - blind, placebo - controlled study Part 2 : Randomization 0 Treatment period N=3 - 6 1 3 > 130 patients Allocetra Allocetra Placebo Allocetra Placebo Placebo Dose 1: repeated dosing Dose 2: repeated dosing Dose 3: repeated dosing 6 - 15 patients N=3 N= 3 N=3 2 ClinicalTrials.gov Registration: NCT06233474 Follow up Follow up Follow up 3M 12 M Follow up period 6 M 30 | Nasdaq: ENLV MILESTONES MET & PLANNED 2023 Q3 2024 Q 1 April 2024 2024 EO Q 3 (planned) 2025 Q 3 (planned) Initiation of Phase I/II in end - stage knee osteoarthritis.

Initiation of Phase I/II in moderate knee osteoarthritis. Top line data macrophage reprogramming Sepsis Phase II (randomized, controlled). End - stage knee osteoarthritis Phase I/II readouts. Moderate knee osteoarthritis Phase I/II top - line data (randomized, controlled, powered).
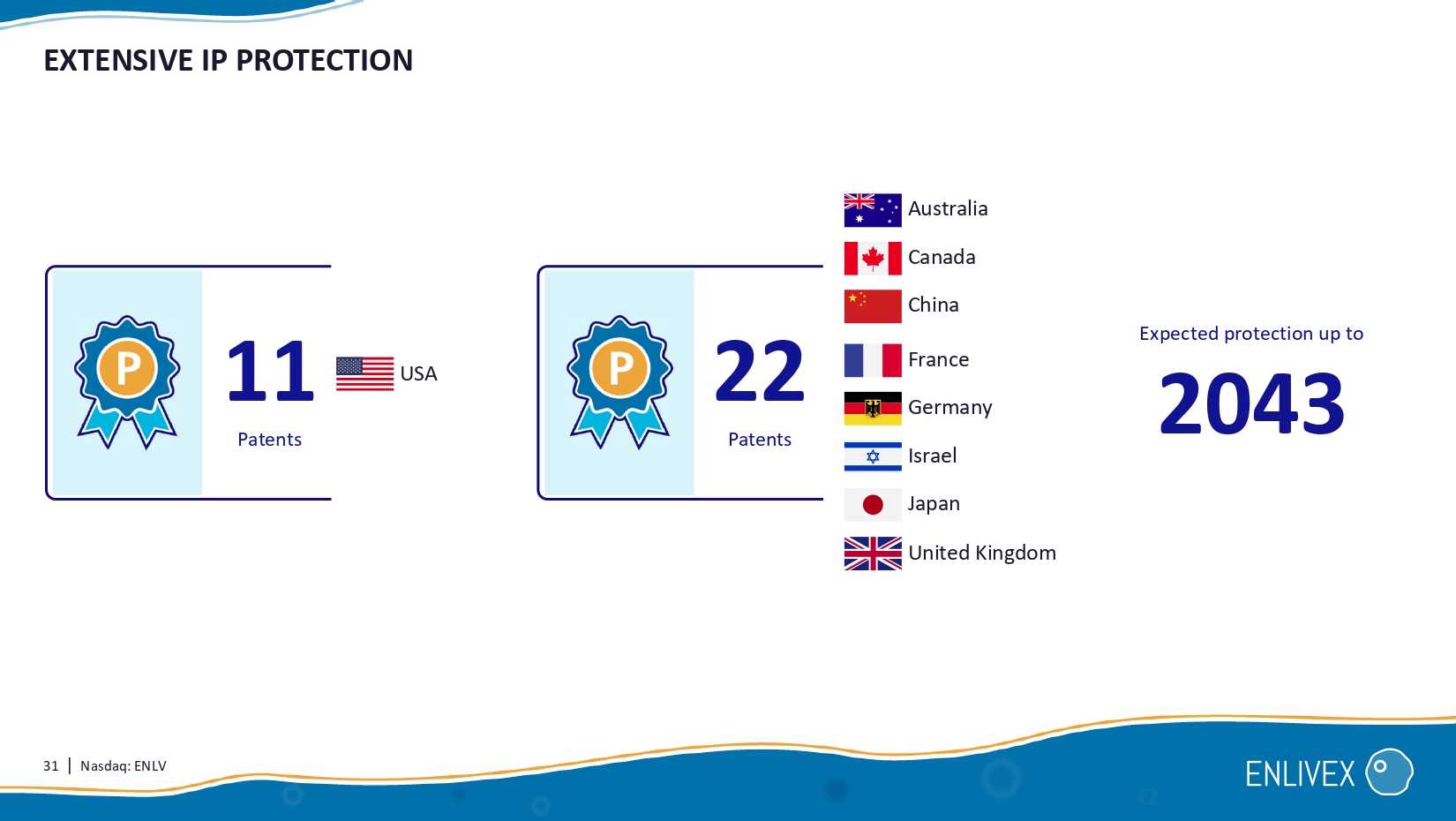

31 | Nasdaq: ENLV EXTENSIVE IP PROTECTION Australia Canada China France Germany Israel Japan United Kingdom 11 Patents USA 22 Patents Expected protection up to 2043 32 | Nasdaq: ENLV FINANCIAL SUMMARY NASDAQ GS: ENLV Cash & equivalence : $ 27.3 MM ( Dec. 31 , 202 ϯ ) Debt: none Shares outstanding: 18. 6 MM Estimated cash runway through: Dec. 31, 2025 2024 Phase II topline data Phase I/II topline data Readouts 2025 Q 2 Q1 Q3 Q3 Q2 Q 4 Q4 Estimated c ash runway through end of 2025 ENX - CL - 02 - 002 NCT 04612413 Organ failure associated with Sepsis ENX - CL - 05 - 001 NCT06233474 Moderate knee osteoarthritis 0189 - 22 - KMC NCT06208241 End - stage knee osteoarthritis FINANCIAL 33 | Nasdaq: ENLV INVESTMENT SUMMARY Management team with a track record of creating shareholder value and getting drug products through marketing approvals globally in multi - billion dollar market segments Cost - effective, novel therapeutic modality with strong IP protection Targeted at high and low grade inflammation in multi - billion dollar segments with poor treatment alternatives Platform for multiple indications.

Allocetra can be infused systemically or locally to treat various diseases Clinical data supportive of proposed MOA Simple, scalable, and cost - effective manufacturing process resulting in an off - the - shelf cell therapy Favorable safety profile demonstrated across 140+ patients

THANK YOU