UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the Month of April 2024
Commission File Number: 001-41084
NeuroSense Therapeutics Ltd.
(Translation of registrant’s name into English)
11 HaMenofim Street, Building B
Herzliya 4672562 Israel
+972-9-7996183
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
NeuroSense Therapeutics Ltd. (the “Company”) has made available an updated presentation about its business (the “Presentation”), a copy of which is furnished herewith as Exhibit 99.1 to this Report on Form 6-K.
The information contained in the Presentation is summary information that should be considered in the context of the Company’s filings with the Securities and Exchange Commission and other public announcements the Company may make by press release or otherwise from time to time.
Following the date hereof, the Company will make available future versions of its presentations only on the Company’s investor relations website.
Exhibit Index
| Exhibit No. | Description | |
| 99.1 | Presentation, dated April 2024 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| NeuroSense Therapeutics Ltd. | ||
| Date: April 10, 2024 | By: | /s/ Alon Ben-Noon |
| Alon Ben-Noon | ||
| Chief Executive Officer | ||
2
Exhibit 99.1

April 2024 Nasdaq: NRSN

Forward - Looking Statements This presentation and oral statements made regarding the subject of this presentation contain "forward - looking statements" within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties . All statements contained in this presentation other than statements of historical facts, including our business strategy and plans and objectives for future operations, including our financial performance, are forward looking statements . The words " anticipate"," believe," "continue," "estimate," "expect," "intend," "may," "will" and similar expressions are intended to identify forward looking statements . We have based these forward - looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short term and long - term business operations and objectives and financial needs . Forward looking statements made in this presentation include statements about the timing of reporting neurofilament and biomarker results from our ALS Phase 2 b clinical trial and of other clinical and regulatory milestones, including target market and opportunities for our product candidates ; our expectations regarding our competitive advantages ; the planned development timeline of our product candidates ; and characterizations of the pre - clinical and clinical trial results of our product candidates . Forward looking statements are subject to a number of risks and uncertainties and represent our views only as of the date of the presentation . The future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements due to, among other things, a delay in the reporting of neurofilament and biomarker results from our ALS Phase 2 b clinical trial , a delay in other clinical and regulatory milestones, and the development and commercial potential of any product candidates . More information about the risks and uncertainties affecting the Company is contained under the heading "Risk Factors" in the Annual Report on Form 20 - F filed with the Securities and Exchange Commission on March 22 , 2023 and the Company's subsequent filings with the SEC . We undertake no obligation or duty to update information contained in these forward - looking statements, whether as a result of new information, future events or otherwise . Trademarks in this presentation are the property of their respective owners and used for informational and educational purposes only . 2 NeuroSense Highlights Developing novel therapies for neurodegenerative diseases of high unmet need Significant top line results from Phase 2b study for ALS 1 Additional catalysts expected: Biomarker results (H1 2024) 1 ALS - Amyotrophic Lateral Sclerosis, also referred to as Lou Gehrig’s Disease 3 Global opportunity in ALS Patent coverage for novel formulation, method & combination (until 2038) Expedited and de - risked regulatory pathway (orphan drug designation / 505 (b) 2 pathway)


Neurodegeneration Focused Pipeline Diseases with Significant Unmet Need and Substantial Commercial Opportunity ALS • Biomarker data (H1 2024) • ALS End of Phase 2 • Meeting with the FDA and EMA • Initiate ALS Phase 3 clinical study as needed Discovery Pre - clinical Phase 1 Phase 2 Phase 3 NDA Indication Next Milestone Parkinson’s • Exploring potential co - development Alzheimer’s • Completion of Phase 2 enrollment (H2 2024) 4 1 NfL: Neurofilament ~ 24 % Growth in Patients by 2040 in the US and EU 2 >80,000 ALS Patients in NeuroSense’s planned target market 2 + 5,000 New cases of ALS each y ear (US) 1 3 Annual Market Opportunity ~$ 3 B 1 Johns Hopkins Medicine 2 Projected increase in amyotrophic lateral sclerosis from 2015 to 2040 , Nature Communications, 2016 3 Management estimate ALS is an incurable neurodegenerative disease, causing complete paralysis and ultimately death within 2 - 5 years from diagnosis 5


PrimeC - Designed to Reduce Neuronal Cell Death Celecoxib - a NSAID which reduces: • Neuroinflammation • Glutamate excitotoxicity • Oxidative stress Ciprofloxacin - a fluoroquinolone which regulates: • MicroRNA synthesis • Iron accumulation Designed to work synergistically on multiple targets in ALS Novel formulation , consisting of specific and unique doses of two FDA - approved drugs PrimeC ’ s effect on pathways which lead to neuronal cell death in ALS 6 PrimeC Demonstrates Synergies In - Vitro & In - Vivo Celecoxib is blocking efflux of Ciprofloxacin , thus increasing its concentration in the neurons PrimeC’s combination ( Celecoxib + Ciprofloxacin ) remained at a higher concentration in rodent brain tissue for longer , when compared to administration of Ciprofloxacin alone *** 7 Synergistic Mode of Action Improved Pharmacokinetic (PK) Profile


Efficacy in Well Established ALS Model Studies from Prof. Justin Ichida ’ s lab, USC, induced Pluripotent Stem Cells ( iPSCs) Generated from blood of People Living with ALS Isolated PBMCs iPSCs iPSC - derived motor neurons + / - treatments iPSC - derived cortical neurons Differentiation Reprogramming Therapeutic Test ALS patient blood 8 Combination Showed Superior Cell Survival PrimeC Demonstrated Statistically Significant Efficacy In - Vivo Improved Motor Performance Recovered Neuronal Structures 9 ALS ALS + PrimeC ALS + PrimeC ALS Healthy Healthy ALS ALS + PrimeC


The synchronized PK profiles of the two compounds, potentially maximizes synergies PrimeC Unique Formulation Induces a Synchronized PK Profile 10 0 2 4 6 8 10 12 0 500 1000 1500 2000 2500 Time (h) C o n c e n t r a t i o n ( n g / m l ) PrimeC ciprofloxacin Ref ciprofloxacin PrimeC celecoxib Ref celecoxib PK profile studies in humans Synchronized peak IR T max ER T max ER Tmax doubled IR Tmax These results led NeuroSense to commence a Phase 2 b clinical study, using an improved & novel extended - release formulation of PrimeC Significant changes in ALS - related biomarkers Well tolerated, no drug related SAEs Reduced functional and respiratory deterioration NST 002 • 15 patients • Open - Label • Intermediate formulation of PrimeC • 12 - month dosing • Clinic visit every 3 months • Phone visit every 1.5 months • Location: Tel Aviv Sourasky Medical Center PrimeC Met Primary and Exploratory Endpoints in a Phase 2 a Study 11



PrimeC Reduces Neuroinflammation and TDP - 43 Levels NST 002 study The effect of PrimeC on selected biomarkers was tested in samples from an open - label Phase 2 a study 12 Baseline Non-Treated PrimeC Treated Baseline Non-Treated PrimeC Treated Screening (N= 73 *) PrimeC (N= 45 ) Placebo (N= 23 ) Baseline 180 60 120 Clinical visits (Days) Double Blind - 6 Months (N= 68 ) PrimeC Open Label Extension 12 Months ~ 90 % of patients in PrimeC and Placebo groups were co - treated with Riluzole PARADIGM used P rimeC ’ s novel extended - release formulation PARADIGM Phase 2 b Trial Design Randomized, Prospective, Double - Blind, Placebo - Controlled Study * 4 Screen Failures , 1 participant misdiagnosed for ALS Randomization 13 Primary Endpoints • Safety and Tolerability Measures • ALS - Hallmark Biomarker Measures of TDP - 43 and ProstaglandinJ 2 (results expected H 1 2024 ) Secondary Efficacy Endpoints • ALSFRS - R (ALS Functional Rating Scale) • SVC (Slow Vital Capacity) • PROMIS - 10 quality of life questionnaire • Complication Free Survival Exploratory Endpoint • King's/ MiToS • Neurofilament - Light Chain PARADIGM Trial Endpoints 14


PARADIGM Inclusions / Exclusions Criteria Inclusion Criteria • Males or females between the ages of 18 and 75 years of age • Diagnosis of familial or sporadic ALS • Disease duration less than 30 months prior to screening • Pre - enrollment ALSFRS - R slope from disease onset ≥ 0 . 3 points per month • ALSFRS - R at screening ≥ 25 • Item 3 (swallowing) in ALSFRS - R ≥ 3 • Subjects may be treated in parallel with Riluzole and/or Edaravone and/or Sodium Phenylbutyrate/TUDCA • Upright slow vital capacity (SVC) ≥ 60 % • 18 < BMI < 30 Exclusion Criteria • Patients with known hypersensitivity to celecoxib or ciprofloxacin and related exclusions derivative from the celecoxib and ciprofloxacin labels 15 ITT and PP Pre - specified Analyses PP (N= 62 ) ITT (N= 68 ) n=43 n=45 PrimeC n=19 n=23 Placebo Intent to Treat (ITT) and Per Protocol (PP) are both pre - specified analyses within the study ITT assesses the effect of the treatment on all patients enrolled in the study while PP analysis includes only patients who strictly adhered to the study protocol 1 Both analyses are valid, yet PP best answers the question of what is the effect of receiving the treatment on a group of patients versus the effect of assigning the treatment to a group of patients 16 Analysis Pre - defined populations

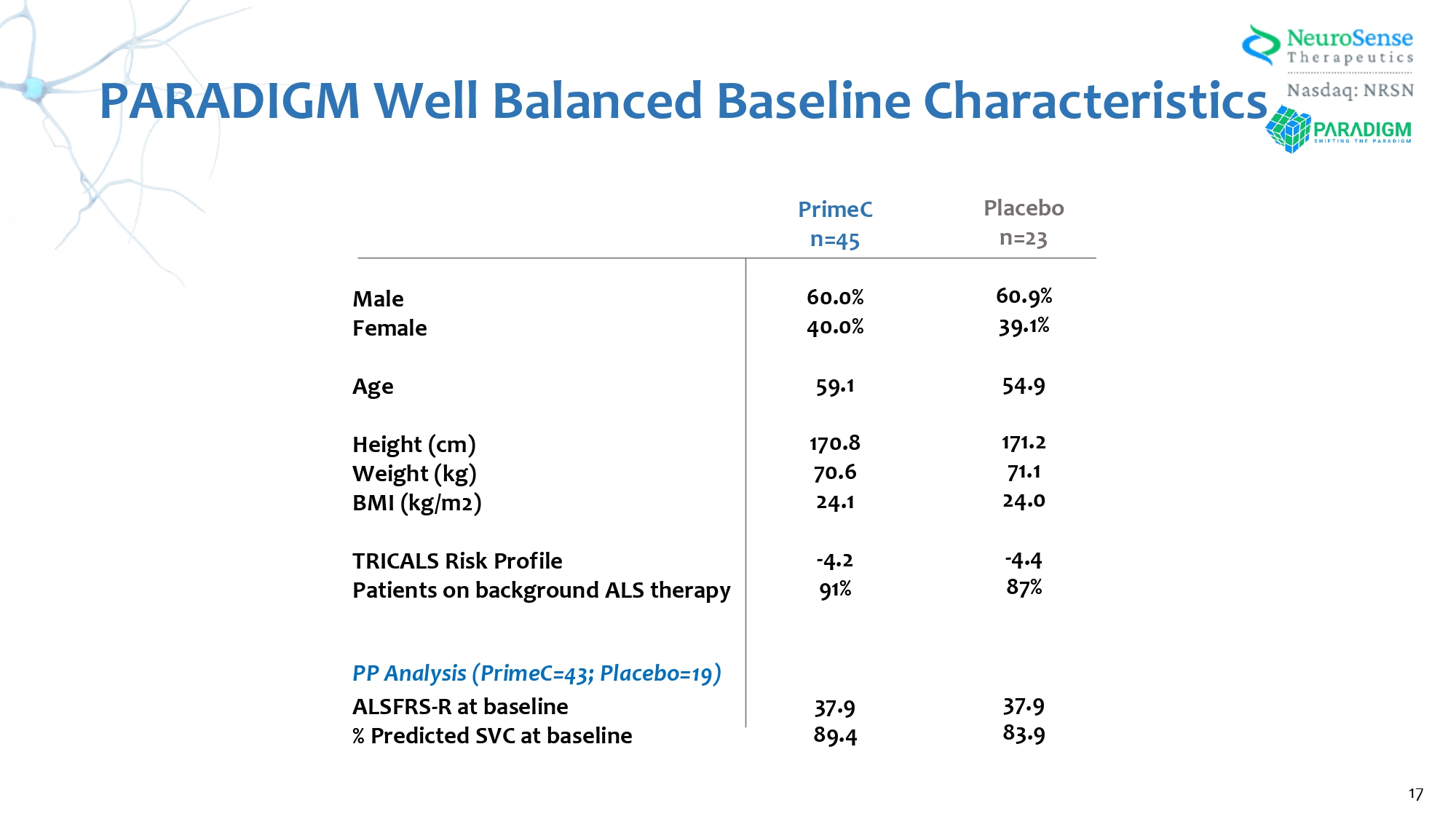
PARADIGM Well Balanced Baseline Characteristics Male Female Age Height (cm) Weight (kg) BMI (kg/m 2 ) TRICALS Risk Profile Patients on background ALS therapy PP Analysis (PrimeC= 43 ; Placebo= 19 ) ALSFRS - R at baseline % Predicted SVC at baseline Placebo n= 23 60.9 % 39.1 % 54.9 171.2 71.1 24.0 - 4.4 87 % 37.9 83.9 PrimeC n= 45 60.0 % 40.0 % 59.1 170.8 70.6 24.1 - 4.2 91 % 37.9 89.4 17 Placebo (N=2 3 ) PrimeC (N=45) Summary of All Adverse Events 65.2% 68.9% Adverse Events (AE) 65.2% 68.9% Treatment - Emergent AEs (TEAE) 4.3% 20.0% Study Drug Related Treatment - Emergent AEs (TEAE) 8.6% 8.9% Serious Treatment - Emergent AEs (TEAE) 4.3% 4.4% Subject death 4.3% 6.7% TEAE leading to Study Drug Discontinuation 0.0% 0.0% TEAE leading to Study Drug Reduction 8.6% 15.6% TEAE leading to Study Drug Interruption PARADIGM Achieved Primary Endpoints with a Safety and Tolerability Profile Comparable to Placebo 18 All Adverse Events Were Transient and Expected


PARADIGM Achieved Primary Endpoints with a D rug T olerability Profile Comparable to Placebo Tolerability is defined as time - to - discontinuation or completion of assigned study medication during the double - blind period since randomization PrimeC Placebo N= 5 of 45 N=4 of 24 19 20 The adjusted mean score for each treatment group, the corresponding treatment difference and p - value are analyzed using MMRM.

Me an and SE. (N= 45 ) (N= 23 ) PrimeC Attenuated Disease Progression By 29.2 % Difference in ALSFRS - R PARADIGM Results – ITT Analysis 21 (N=43) (N= 19 ) PrimeC Significantly Attenuated Disease Progression by 37 % in ALSFRS - R (p= 0.03 ) PARADIGM Results – PP Analysis The adjusted mean score for each treatment group, the corresponding treatment difference and p - value are analyzed using MMRM.

Me an and SE.

A Single Point Change On the ALSFRS - R Has a Significant Impact on ALS Patients A 1 - point decrease in the hands' Functional Loss Score can represent a transition from independent feeding to requiring assistance. A 1 - point drop on the swallowing assessment scale can mark the critical threshold between self - sufficiency and the necessity of supplemental tube feeding. A 1 - point stumble in the legs can be the difference between walking with a cane and not being able to walk at all. A 1 - point loss in breathing can cause a transition from independent breathing to requiring the use of a machine ventilator. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS - R: a revised ALS functional rating scale that incorporates assessments of r espiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999 ; 169 ( 1 - 2 ): 13 - 21 .

doi: 10.1016 /s 0022 - 510 x( 99 ) 00210 - 5 22 Mean ALSFRS - R total score was 2.23 points higher at 6 months for PrimeC compared to placebo .

23 Placebo PrimeC - 7.64 - 5.41 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Number of Points Lost ALSFRS - R Total Score 2.23 Point Difference (P= 0.12 ) PrimeC Slowed Decline of Physical Functions PARADIGM Results – ITT Analysis Background Respiratory failure is the most common cause of death from ALS - 2.41 - 2.01 -4 -3 -2 -1 0 Number of Points Lost Fine Motor 0.40 Point Difference (P=0.51) - 1.25 - 0.84 -4 -3 -2 -1 0 Number of Points Lost Bulbar 0 . 41 Point Difference (P= 0.24 ) - 1.55 - 0.74 -4 -3 -2 -1 0 Number of Points Lost Respiratory 0.81 Point Difference (P= 0.13 ) - 2.61 - 2.11 -4 -3 -2 -1 0 Number of Points Lost Gross Motor 0.50 Point Difference (P= 0.34 ) Placebo PrimeC Bulbar: Speech Salivation Swallowing Respiratory: Dyspnea Orthopnea Respiratory insufficiency Fine Motor: Handwriting Cutting Food Dressing and hygiene Gross Motor: Turning in bed Walking Climbing stairs 24 PrimeC Slowed Decline of Physical Functions PARADIGM Results – ITT Analysis PrimeC Slowed Decline of Physical Functions PARADIGM Results – PP Analysis - 8.61 - 5.39 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Number of Points Lost ALSFRS - R Total Score 3.22 Point Difference (P=0.03) Mean ALSFRS - R total score was 3.22 points higher at 6 months for PrimeC compared to placebo . Placebo PrimeC 25


- 2.94 - 2.10 -4 -3 -2 -1 0 Number of Points Lost Gross Motor 0.84 Point Difference (P=0.14) - 2.51 - 1.99 -4 -3 -2 -1 0 Number of Points Lost Fine Motor 0.52 Point Difference (P= 0.42 ) - 1.36 - 0.83 -4 -3 -2 -1 0 Number of Points Lost Bulbar 0.53 Point Difference (P= 0.16 ) - 1.71 - 0.57 -4 -3 -2 -1 0 Number of Points Lost Respiratory 1.14 Point Difference (P= 0.04 ) 26 Background Respiratory failure is the most common cause of death from ALS Bulbar: Speech Salivation Swallowing Respiratory: Dyspnea Orthopnea Respiratory insufficiency Fine Motor: Handwriting Cutting Food Dressing and hygiene Gross Motor: Turning in bed Walking Climbing stairs Placebo PrimeC PrimeC Slowed Decline of Physical Functions PARADIGM Results – PP Analysis Effect of Treatment on Slow Vital Capacity (SVC) PARADIGM Results – ITT and PP Analysis 27 (N=43) (N= 19 ) p value = 0.5 ITT PP The adjusted mean score for each treatment group, the corresponding treatment difference and p - value are analyzed using MMRM.

Me an and SE.

Complication free Survival Probability Measures ALS Complications analysis includes death from any cause or respiratory insufficiency or hospitalization due to ALS - related complications The MiToS system uses six stages, from 0 to 5 and is based on functional ability (ALSFRS - R) stage 0 = normal function stage 5 = death The King ’ s system uses five stages from 1 to 5 based on disease burden (clinical involvement, feeding or respiratory failure) stage 1 = symptom onset stage 5 = death 28 King ’ s Stage - Free Survival MiToS Stage - Free Survival ALS Complication - Free Survival PrimeC reduces risk of death or complications 1 PrimeC Increases Probability of complications - free - Survival in distinct methods (ITT) Hazard Ratio (mean, 95 % CI) A hazard ratio of 1 means that there is no difference in survival between the two treatment arms.

Hazard Ratio less than 1 means that survival was better in the PrimeC arm 0.52 (P= 0.1 ) 0.65 (P= 0.31 ) 0.7 (P= 0.69 ) 29 King ’ s Stage - Free Survival MiToS Stage - Free Survival ALS Complication - Free Survival PrimeC reduces risk of death or complications 1 A hazard ratio of 1 means that there is no difference in survival between the two treatment arms.

Hazard Ratio less than 1 means that survival was better in the PrimeC arm 0.47 (P= 0.07 ) 0.5 (P= 0.12 ) 0.34 (P= 0.29 ) 30 Hazard Ratio (mean, 95 % CI) PrimeC Increases Complication - free Survival Probability (PP)

- 6.4 - 5.7 -7.0 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 Placebo (N=23) PrimeC (N=45) No. of Points Lost PrimeC Slowed Decline in Quality of Life (ITT) 11.2 % (P= 0.68 ) - 5.5 - 4.6 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 Placebo (N=23) PrimeC (N=45) No. of Points Lost PROMIS - 10 Mental Health score PROMIS - 10 Physical Health score 15.5 % (P= 0.66 ) PROMIS (Patient - Reported Outcomes Measurement Information System) - 10 is a set of person - centered measures that evaluates and monitors physical, mental, and social health in individuals living with chronic conditions 31 - 5.8 - 4.8 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 Placebo (N=19) PrimeC (N=43) No.

of Points Lost - 7.1 - 6.2 -8.0 -7.0 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 Placebo (N=19) PrimeC (N=43) No. of Points Lost PrimeC Slowed Decline in Quality of Life (PP) PROMIS (Patient - Reported Outcomes Measurement Information System) - 10 is a set of person - centered measures that evaluates and monitors physical, mental, and social health in individuals living with chronic conditions 12.3 % (P= 0.65 ) 16.4 % (P= 0.64 ) PROMIS - 10 Mental Health score PROMIS - 10 Physical Health score 32 NeuroSense is collaborating with leading KOLs and industry on the PARADIGM trial to elucidate PrimeC ’ s MOA via novel methodologies Biomarker Driven Proteomics Interplay Between TDP - 43 and RNA Regulation microRNA Profiling Identification of Novel Biomarkers Neurofilaments Neuronal Derived Exosomes Pioneering Approach to ALS Biomarker Research To Maximize Clinical Efforts 33


Novel combination therapy candidate of approved products optimized for PK and synergistic effects to address ALS and potentially other disease targets Robust clinical efficacy and excellent safety profile observed from ALS Phase 2 a and 2 b clinical studies • 37 % reduction in ALSFRS - R (p= 0.03 ) in phase 2 b study Patent coverage for novel formulation, method & combination (until 2038 ) Expedited and de - risked regulatory pathway (orphan drug designation / 505 (b) 2 pathway) PrimeC: Strong Clinical and Commercial Potential 34 Alzheimer ’ s (AD) Studies Reveal Potential Effect of NeuroSense ’ s Combination Therapy Biomarkers tested in Neuronal Derived Exosomes comparing Healthy vs. AD patients, to elucidate the potential target engagement of CogniC. Biomarker data were analyzed using a Mann - Whitney U test comparing AD samples with controls . *P< 0 . 05 , **P< 0 . 01 , ***P< 0 . 001 35 * TDP - 43 (pg/ml)


• 20 patients with mild to moderate AD • 1:1 PrimeC to Placebo • CogniC - intermediate formulation (= PrimeC - ER) • 12 - month dosing • Clinic visit every 3 months • Single - center Target Engagement Biomarkers Secondary Efficacy Clinical Outcomes Primary Endpoint Safety & Tolerability 3 1 PrimeC PrimeC 2 RoAD Phase 2 Study Design Randomized, Prospective, Double - Blind, Placebo - Controlled Study 36 Senior Vice President at the Barrow Neurological Institute Chair of the Department of Neurology Prof.

Jeremy Shefner (Chair) Dr. Jinsy Andrews Associate Professor of Neurology, Division of Neuromuscular Medicine, Columbia University Director of Neuromuscular Clinical Trials Prof. Merit Cudkowicz Chief of Neurology at Mass General and Director, Sean M. Healey & AMG Center for ALS Professor of Neurology at Harvard Medical School Prof. Jeffrey Rosenfeld Professor of Neurology and Associate Chairman of Neurology at Loma Linda University School of Medicine Medical Director of Center for Restorative Neurology at Loma Linda University Prof. Orla Hardiman Head of Academic Unit of Neurology at Trinity College Dublin and Consultant Neurologist at Beaumont Co - Chair of the European Consortium to Cure ALS and Chair of the Scientific Committee of ENCALS Exceptional Scientific Advisory Board 37 VP of R& D Shiran Zimri, PhD Chief Medical Officer Ferenc Tracik, MD Founder & CEO Alon Ben - Noon Chief Operating Officer Hagit Binder Chief Financial Officer Or Eisenberg Niva Russek - Blum, PhD Chief Technology Officer VP of BD Nedira Salzman VP of Regulatory Affairs Diana Shtossel Experienced Leadership 38



Key Collaborations 40

Revital Mandil - Levin Caren Deardorf Chairman of the Board Mark Leuchtenberger Christine Pellizzari Cary Claiborne Board of Directors Alon Ben - Noon 39 • Neurofilament Results • Biomarker Results • ALS End of Phase 2 Meeting with the FDA and EMA • Initiate ALS Phase 3 clinical study as needed Initiated ALS Phase 2 b PARADIGM study Received FDA IND Clearance for PrimeC Completed PK study single - dose & multi - dose successfully Completed In - life 90 - day GLP toxicology study successfully 2022 2023 2024 Completed Alzheimer ’ s biomarker study with positive results Completed Parkinson ’ s biomarker study with positive results Type D Meeting with the FDA Release ALS Phase 2 b clinical study top - line results Initiated Alzheimer ’ s Phase 2 study Milestones Achieved and Upcoming Potential Catalysts 41 Nasdaq: NRSN Thank You For more information: info@neurosense - tx.com 42