UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO
RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the month of April 2024
Commission File Number: 001-38773
CHINA SXT PHARMACEUTICALS, INC.
(Translation of registrant’s name into English)
178 Taidong Rd North, Taizhou
Jiangsu, China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Explanatory Note
China SXT Pharmaceuticals, Inc. (the “Company”) is furnishing this Form 6-K to provide its financial results for the six months ended September 30, 2023.
The Company hereby furnishes the following documents as the exhibits to this report: “Unaudited Condensed Consolidated Financial Statements for the Six Months Ended September 30, 2023 and 2022” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”.
Exhibits
| Exhibit No. | Description | |
| 99.1 | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
| 99.2 | Unaudited Condensed Consolidated Financial Statements for the Six Months Ended September 30, 2023 and 2022 | |
| 101.INS | Inline XBRL Instance Document. | |
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document. | |
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: April 4, 2024
| China SXT Pharmaceuticals, Inc. | ||
| By: | /s/ Feng Zhou | |
| Name: | Feng Zhou | |
| Title: | Chief Executive Officer | |
Exhibit 99.1
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition should be read together with our unaudited condensed consolidated financial statements and the notes thereto and other financial information, which are included elsewhere in this Form 6-K. Our unaudited financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). In addition, our unaudited financial statements and the financial information included in this Form 6-K reflect our organizational transactions and have been prepared as if our current corporate structure had been in place throughout the relevant periods.
This section contains forward-looking statements. These forward-looking statements are subject to various factors, risks and uncertainties that could cause actual results to differ materially from those reflected in these forward-looking statements. Further, as a result of these factors, risks and uncertainties, the forward-looking events may not occur. Relevant factors, risks and uncertainties include, but are not limited to, those discussed in the section entitled “Business,” “Risk Factors” and elsewhere in this Form 6-K. Readers are cautioned not to place undue reliance on forward-looking statements, which reflect management’s beliefs and opinions as of the date of this Form 6-K. We are not obligated to publicly update or revise any forward-looking statements, whether as a result of new Information, future events or otherwise. See “Special Note Regarding Forward-Looking Statements.”
Unless otherwise indicated or the context requires otherwise, “we”, “us” or the “Company” in this prospectus are to China SXT Pharmaceuticals, Inc., its subsidiaries and its affiliated entities in the context of describing our business, operations and consolidated financial information.
Overview
We are an offshore holding company incorporated in British Virgin Islands, conducting all of our business through our subsidiaries and variable interest entity, Jiangsu Taizhou Suxantang Pharmaceutical Co., Ltd. (“Taizhou Suxuantang” or the “VIE”) in China. Neither we nor our subsidiaries own any share in Taizhou Suxuantang. Instead, we control and receive the economic benefits of Taizhou Suxuantang’s business operation through a series of contractual arrangements, also known as VIE Agreements. The VIE Agreements by and among our wholly-owned subsidiary, Taizhou Suxantang Biotechnology Co. Ltd. (the “WFOE”), Taizhou Suxuantang, and Taizhou Suxuantang’s shareholders include (i) certain power of attorney agreements and equity interest pledge agreement, which provide WFOE effective control over Taizhou Suxuantang; (ii) an exclusive technical consulting and service agreement which allows WFOE to receive substantially all of the economic benefits from Taizhou Suxuantang; and (iii) certain exclusive equity interest purchase agreements which provide WFOE with an exclusive option to purchase all or part of the equity interests in and/or assets of Taizhou Suxuantang when and to the extent permitted by PRC laws. Through the VIE Agreements among WFOE, Taizhou Suxuantang and Taizhou Suxuantang’s shareholders, we are regarded as the primary beneficiary of Taizhou Suxuantang for accounting purpose, and, therefore, we are able to consolidate the financial results of Taizhou Suxuantang in our consolidated financial statements in accordance with U.S. GAAP. However, the VIE structure cannot completely replicate a foreign investment in China-based companies, as the investors will not and may never directly hold equity interests in the Chinese operating entities. Instead, the VIE structure provides contractual exposure to foreign investment in us. Because we do not directly hold equity interests in the VIE, we are subject to risks due to uncertainty of the interpretation and the application of the PRC laws and regulations, including but not limited to limitation on foreign ownership of internet technology companies, regulatory review of oversea listing of PRC companies through a special purpose vehicle, and the validity and enforcement of the VIE Agreements. We are also subject to the risks of uncertainty about any future actions of the PRC government in this regard that could disallow the VIE structure, which would likely result in a material change in our operations and the value of Ordinary Shares may depreciate significantly or become worthless.
Our VIE Agreements may not be effective in providing control over Taizhou Suxuantang. We may also subject to sanctions imposed by PRC regulatory agencies including Chinese Securities Regulatory Commission, or CSRC, if we fail to comply with their rules and regulations.
We rely principally on dividends and other distributions on equity from Taizhou Suxuantang and its subsidiaries for our cash requirements, including for services of any debt we may incur. Taizhou Suxuantang and its subsidiaries’ ability to distribute dividends is based upon their distributable earnings. Current PRC regulations permit Taizhou Suxuantang and its subsidiaries to pay dividends to their respective shareholders only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, each of Taizhou Suxuantang and its subsidiaries are required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of each of their registered capitals. These reserves are not distributable as cash dividends. If our PRC subsidiaries incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments to us. Any limitation on the ability of Taizhou Suxuantang and its subsidiaries to distribute dividends or other payments to their respective shareholders could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our businesses, pay dividends or otherwise fund and conduct our business.
To address the persistent capital outflow and the RMB’s depreciation against the U.S. dollar in the fourth quarter of 2016, the People’s Bank of China and the State Administration of Foreign Exchange, or SAFE, have implemented a series of capital control measures in the subsequent months, including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments and shareholder loan repayments. For instance, the Circular on Promoting the Reform of Foreign Exchange Management and Improving Authenticity and Compliance Review, or the SAFE Circular 3, issued on January 26, 2017, provides that the banks shall, when dealing with dividend remittance transactions from domestic enterprise to its offshore shareholders of more than US$50,000, review the relevant board resolutions, original tax filing form and audited financial statements of such domestic enterprise based on the principal of genuine transaction. The PRC government may continue to strengthen its capital controls and Taizhou Suxuantang and its subsidiaries’ dividends and other distributions may be subject to tightened scrutiny in the future. Any limitation on the ability of Taizhou Suxuantang and its subsidiaries to pay dividends or make other distributions to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business.
In addition, the Enterprise Income Tax Law and its implementation rules provide that a withholding tax at a rate of 10% will be applicable to dividends payable by Chinese companies to non-PRC-resident enterprises unless reduced under treaties or arrangements between the PRC central government and governments of other countries or regions where the non-PRC resident enterprises are tax resident. Pursuant to the tax agreement between Mainland China and the Hong Kong Special Administrative Region, the withholding tax rate in respect to the payment of dividends by a PRC enterprise to a Hong Kong enterprise may be reduced to 5% from a standard rate of 10% if the Hong Kong enterprise (i) directly holds at least 25% of the PRC enterprise, (ii) is a tax resident in Hong Kong and (iii) could be recognized as a beneficial owner of the dividend from PRC tax perspective. Under administrative guidance, a Hong Kong resident enterprise must meet the following conditions, among others, in order to apply the reduced withholding tax rate: (i) it must be a company; (ii) it must directly own the required percentage of equity interests and voting rights in the PRC resident enterprise; and (iii) it must have directly owned such required percentage in the PRC resident enterprise throughout the 12 months prior to receiving the dividends. Nonresident enterprises are not required to obtain pre-approval from the relevant tax authority in order to enjoy the reduced withholding tax. Instead, nonresident enterprises and their withholding agents may, by self-assessment and on confirmation that the prescribed criteria to enjoy the tax treaty benefits are met, directly apply the reduced withholding tax rate, and file necessary forms and supporting documents when performing tax filings, which will be subject to post-tax filing examinations by the relevant tax authorities. Accordingly, our wholly owned subsidiary China SXT Group Limited (“SXT HK”) incorporated in Hong Kong may be able to benefit from the 5% withholding tax rate for the dividends it receives from our PRC subsidiaries, if it satisfies the conditions prescribed under Guoshuihan [2009] 81 and other relevant tax rules and regulations. However, if the relevant tax authorities consider the transactions or arrangements we have are for the primary purpose of enjoying a favorable tax treatment, the relevant tax authorities may adjust the favorable withholding tax in the future. Accordingly, there is no assurance that the reduced 5% will apply to dividends received by SXT HK from Taizhou Suxuantang and its subsidiaries. This withholding tax will reduce the amount of dividends we may receive from Taizhou Suxuantang and its subsidiaries.
Through our subsidiaries and Taizhou Suxuantang, we are an innovative pharmaceutical company based in China that focuses on the research, development, manufacture, marketing and sales of TCMP. TCMP is a type of TCM products that has been widely accepted by Chinese people for thousands of years. Throughout the decades of years, TCMP products’ origin, identification, prepared process, quality standard, indication, dosage and administration, precautions, and storage have been well documented, listed and specified in “China Pharmacopoeia” a state-governmental issued guidance on manufacturing TCMP. In recent years, TCMP industry enjoyed more rapid growth than any other segments of the pharmaceutical industry primarily due to the favorable government policies for the TCMP industry. Because of the favorable government policies, TCMP products do not have to go through rigorous clinical trials before commercialization. We currently sell three types of TCMP products: Advanced TCMP, Fine TCMP and Regular TCMP. Although all of our TCMP products are generic TCMP drugs and we did not change the medical effects of these products in any significant way, these products are innovative in terms of their unconventional administration. The complexity of the manufacturing process is what differentiates these types of products. Advanced TCMP typically has the highest quality because it requires specialized equipment and prepared processes to manufacture, and has to go through more manufacturing steps to produce than Fine TCMP and Regular TCMP. Fine TCMP is also manufactured with more refined ingredients than Regular TCMP.
Consolidation
We conduct all of our business in China via Taizhou Suxuantang and its subsidiaries, due to PRC legal restrictions of foreign ownership in certain sectors. Substantially all of our revenues, costs and net income in China are directly or indirectly generated through Taizhou Suxuantang and its subsidiaries. The VIE agreements allow the transfer of economic benefits from Taizhou Suxuantang to us and to direct the activities of Taizhou Suxuantang.
Total assets and liabilities presented on our consolidated balance sheets and revenue, expense, net income presented on consolidated statement of operations and comprehensive income as well as the cash flow from operating, investing and financing activities presented on the consolidated statement of cash flows are substantially the financial position, operation and cash flow of Taizhou Suxuantang and its subsidiaries. We have not provided any financial support to Taizhou Suxuantang and its subsidiaries for the six months ended September 30, 2023 and the years ended at March 31, 2023 and 2022. As of September 30, 2023, our variable interest entities accounted for an aggregate of 98% and 66% of our total assets and total liabilities, respectively. As of March 31, 2023, our variable interest entities accounted for an aggregate of 97% and 75% of our total assets and total liabilities, respectively. As of September 30, 2023 and March 31, 2023, $11,460,445 and $16,735,938 of cash and cash equivalents were denominated in RMB, respectively. The following table sets forth the assets, liabilities, results of operations and changes in cash, cash equivalents the VIE subsidiary taken as a whole, which were included in the Company’s condensed consolidated balance sheets and statements of comprehensive income and statements of cash flows with intercompany transactions eliminated:
| September 30, 2023 |
March 31, 2023 |
|||||||
| Current assets | $ | 13,168,215 | $ | 18,507,901 | ||||
| Non-current assets | 1,092,661 | 10,032,809 | ||||||
| Total assets | $ | 14,260,876 | $ | 28,540,710 | ||||
| Total liabilities | 12,096,990 | 16,280,994 | ||||||
| Total shareholders’ equity | $ | 2,163,886 | $ | 12,259,716 | ||||
| For the six months ended September 30, |
||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 939,583 | $ | 1,208,288 | ||||
| Net loss | $ | (9,329,818 | ) | $ | (636,861 | ) | ||
| For the six months ended September 30, |
||||||||
| 2023 | 2022 | |||||||
| Net cash used in operating activities | $ | (632,605 | ) | $ | (290,590 | ) | ||
| Net cash provided by investing activities | 19,639 | 39,310 | ||||||
| Net cash used in financing activities | (3,609,666 | ) | (10,071,028 | ) | ||||
| Effects of foreign currency translation | (875,442 | ) | 1,997,882 | |||||
| Net decrease in cash and cash equivalents | $ | (5,098,074 | ) | $ | (8,324,426 | ) | ||
Key Factors Affecting Our Results of Operation
Working capital required to implement our business plan will most likely be provided by funds obtained through offerings of our equity, debt, debt-linked securities, and/or equity-linked securities, and revenues generated by us. No assurance can be given that we will have revenues sufficient to support and sustain our operations or that we would be able to obtain equity/debt financing in the current economic environment. If we do not have sufficient working capital and are unable to generate sufficient revenues or raise additional funds, we may delay the completion of or significantly reduce the scope of our current business plan; delay some of our development and clinical or marketing efforts; postpone the hiring of new personnel; or, under certain dire financial circumstances, substantially curtail or cease our operations.
Our past operating results are not an accurate indication of the lines of business we are principally engaged in currently. Thus, you should consider our future prospects in light of the risks and uncertainties experienced by early-stage companies in evolving markets rather than typical companies of our age. Some of these risks and uncertainties relate to our ability to:
| ● | attract additional customers and increased spending per customer; |
| ● | increase awareness of our brand and develop customer loyalty; |
| ● | respond to competitive market conditions; |
| ● | respond to changes in our regulatory environment; |
| ● | manage risks associated with intellectual property rights; |
| ● | maintain effective control of our costs and expenses; |
| ● | raise sufficient capital to sustain and expand our business; |
| ● | attract, retain and motivate qualified personnel; and |
| ● | upgrade our technology to support additional research and development of new products. |
Results of Operations for the Six Months Ended September 30, 2023 Compared to September 30, 2022
| For the six months ended September 30, |
Change | |||||||||||||||
|
2023 (Unaudited) |
2022 (Unaudited) |
Amount | % | |||||||||||||
| Revenues | $ | 939,583 | 1,208,288 | $ | (268,705 | ) | (22 | )% | ||||||||
| Revenues generated from third parties | 926,805 | 1,191,808 | (265,003 | ) | (22 | )% | ||||||||||
| Revenue generated from related parties | 12,778 | 16,480 | (3,702 | ) | (22 | )% | ||||||||||
| Cost of revenues | (651,242 | ) | (1,118,604 | ) | 467,362 | (42 | )% | |||||||||
| Gross profit | 288,341 | 89,684 | 198,657 | 222 | % | |||||||||||
| Selling expenses | (176,950 | ) | (272,166 | ) | 95,216 | (35 | )% | |||||||||
| General and administrative expenses | (9,460,412 | ) | (967,964 | ) | (8,492,448 | ) | 877 | % | ||||||||
| Total operating expenses | (9,637,362 | ) | (1,240,130 | ) | (8,397,232 | ) | 677 | % | ||||||||
| Loss from operations | (9,349,021 | ) | (1,150,446 | ) | (8,198,575 | ) | 713 | % | ||||||||
| Interest expenses, net | (336,520 | ) | (384,286 | ) | 47,766 | (12 | )% | |||||||||
| Other income (expenses), net | (10,617 | ) | 40,123 | (50,740 | ) | (126 | )% | |||||||||
| Total other expenses, net | (347,137 | ) | (344,163 | ) | (2,974 | ) | 1 | % | ||||||||
| Loss before income taxes expense | (9,696,158 | ) | (1,494,609 | ) | (8,201,549 | ) | 549 | % | ||||||||
| Provision for income taxes | - | - | - | - | % | |||||||||||
| Net Loss | $ | (9,696,158 | ) | (1,494,609 | ) | $ | (8,201,549 | ) | 549 | % | ||||||
Revenues
We generated revenues primarily from manufacture and sales of four types of traditional Chinese medicine pieces (the “TCMP”) products: Advanced TCMP, Fine TCMP, Regular TCMP, and raw medicinal materials. As compared with the six months ended September 30, 2022, our total revenues decreased by $268,705, or 22% for the six months ended September 30, 2023. The decrease was primarily due to the decrease in sales of Fine TCMP products and raw medicinal materials, offset by the increase in sales of Advanced TCMP products and Regular TCMP products.
The following table sets forth the breakdown of revenues by categories for the six months ended September 30, 2023 and 2022 presented:
| For the six months ended September 30, |
Change | |||||||||||||||
| 2023 | 2022 | Amount | % | |||||||||||||
| Advanced TCMP | $ | 492,414 | 242,075 | $ | 250,339 | 103 | % | |||||||||
| Fine TCMP | 24 | 110,215 | (110,191 | ) | (100 | )% | ||||||||||
| Regular TCMP | 424,239 | 413,505 | 10,734 | 3 | % | |||||||||||
| Raw medicinal materials | 22,906 | 442,493 | (419,587 | ) | (95 | )% | ||||||||||
| Total Revenue | $ | 939,583 | $ | 1,208,288 | $ | (268,705 | ) | (22 | )% | |||||||
Advanced TCMP
Advanced TCMP products are comprised of nine Directly Oral TCMP products (the “Directly-Oral-TCMP”) and nine After-soaking-oral TCMP products (the “After-Soaking-Oral-TCMP”). Both Directly Oral TCMP and After-soaking-oral TCMP are new types of advanced TCMP.
Revenue from advanced TCMP accounted for 52% and 20% of revenue recognized during the six months ended September 30, 2023 and 2022, respectively. As compared with the six months ended September 30, 2022, our revenue generated from advanced TCMP increased by $250,339, or 103% for the six months ended September 30, 2023. The increase was mainly due to that after the lift of lockdown measure, the supply of raw materials for the production of Advance TCMP products was secured and the Company's production efficiency was gradually improved, market demand gradually recovered and therefore sales volume of Advanced TCMP increased during the six months ended September 30, 2023. Additionally, the sales price of major Advanced TCMP products (DBC products) increased significantly during the six months ended September 30, 2023.
Fine TCMP
We currently produce over 10 fine TCMP products for drug stores and hospitals. Our fine TCMP products are manufactured manually from only high-quality authentic ingredients derived from their region of origin.
Revenue from fine TCMP accounted for 0% and 9% of revenue recognized during the six months ended September 30, 2023 and 2022. As compared with the six months ended September 30, 2022, our revenue generated from fine TCMP decreased by $110,191, or 100% for the six months ended September 30, 2023. The decrease was primarily attributable to the fact that we decided to discontinue cooperation with major clients in the sales of fine TCMP, as the purchase price of raw materials surged since July 2022.
Regular TCMP
We currently manufacture 235 regular TCMP products listed on China Pharmacopoeia (version 2020) Part I for hospitals and drug store in treatment of various diseases or serving as dietary supplements.
Revenue from regular TCMP accounted for 45% and 34% of revenue recognized during the six months ended September 30, 2023 and 2022, respectively. Revenue from regular TCMP products increased slightly by $10,734, or 3%, to $424,239 for the six months ended September 30, 2023 from $413,505 for the six months ended September 30, 2022. The market demand for our Regular TCMP product remained stable.
Raw medicinal materials
During the six months ended September 30, 2023 and 2022, we generated revenue from sales of raw medicinal materials of $22,906 and $442,493, which represented 2% and 37% of our total revenue, respectively. As the low gross margin rate and declined market demand for our raw medicinal materials, we discontinued the sales of raw medicinal materials during the six months ended September 30, 2023.
Gross Profit
Cost of revenues primarily include cost of materials, direct labors, overhead, and other related incidental expenses that are directly attributable to the Company’s principal operations. Total cost of revenue decreased by $467,362, or 42%, to $651,242 for the six months ended September 30, 2023 from $1,118,604 for the six months ended September 30, 2022. The decrease of cost of revenues was mainly due to the decrease of the sales of our products.
Gross profit increased by $198,657, or 222%, to $288,341 for the six months ended September 30, 2023 from $89,684 for the six months ended September 30, 2022. Gross margin was 30.7% for the six months ended September 30, 2023, as compared to 7.4% for the six months ended September 30, 2022. The increase in gross margin was mainly due to the following reasons: (i) the sales in our Advanced TCMP products increased significantly for the six months ended September 30, 2023 compared to the same period in 2022, and Advanced TCMP products have relatively high margin; (ii) the sales of raw medicinal material, which have very low margin, accounted for a small portion of our total revenue for the six months ended September 30, 2023 while a significant portion of total revenue for the six months ended September 30, 2022.
Operating loss
Selling expenses primarily consisted of sales staff payroll and welfare expenses, travelling expenses, advertisement expenses, distribution expenses. Selling expenses decreased from $272,166 for the six months ended September 30, 2022 to $176,950 for the six months ended September 30, 2023, representing a decrease of $95,216, or 35%. The decrease was mainly due to the decrease in our sales volume and our efforts in cost control.
General and administrative expenses primarily consisted of staff payroll and welfare expenses, research and development expenses, professional consulting expenses, bad debt expenses, entertainment expenses, travelling expenses, depreciation and amortization expenses for administrative purposes, and office supply expenses. General and administrative expenses increased from $967,964 for the six months ended September 30, 2022 to $9,460,412 for the six months ended September 30, 2023, representing an increase of $8,492,448, or 877%. The increase in general and administrative expenses was mainly due to provision for credit loss of accounts receivable of $26,518, provision for credit loss of long-term deposit of $8,416,681, and the increase in entertainment expenses comparing to the six months ended September 30, 2022.
Operating loss increased $8,198,575, or 713%, from $1,150,446 for the six months ended September 30, 2022 to $9,349,021 for the six months ended September 30, 2023.
Other expenses, net
Interest expenses for the six months ended September 30, 2023 mainly consists of accretion of finance cost and interest expense of Convertible Notes issued on December 19, 2022 and March 7, 2023. For the six months ended September 30, 2023, the company record amortization of issuance cost and debt discount of $212,708 and Convertible Notes interest expense of $114,736.
Interest expenses for the six months ended September 30, 2022 mainly consists of accretion of finance cost and interest expense of Convertible Notes issued on March 16, 2022. For the six months ended September 30, 2022, the company record amortization of issuance cost and debt discount of $311,642 and Convertible Notes interest expense of $72,880.
Other expenses, net of $10,617 for the six months ended September 30, 2023 was mainly due to inventory count shortage. Other income, net of $40,123 for the six months ended September 30, 2022 was mainly due to the government subsidies we received from local government for research and development activities.
Income tax expense
Income tax expense represented current and deferred income tax expenses derived from income before taxes generated by Suxuantang, the variable interest entity of the Company. Income tax benefit expense for the six months ended September 30, 2023 and 2022 were $Nil and $Nil.
Net loss
As a result of the foregoing, net loss for the six months ended September 30, 2023 was $9,696,158, representing an increase in net loss of $8,201,549 from $1,494,609 for the six months ended September 30, 2022.
Liquidity and Capital Resources
To date, we have financed our operations primarily through shareholder capital contributions, shareholder loans, convertible notes, and cash flow from operations. As a result of our total activities, we had cash and cash equivalents of $11,465,020 and $17,368,478 as of September 30, 2023 and March 31, 2023, respectively. We primarily hold our excess unrestricted cash in short-term interest-bearing bank accounts at financial institutions. With the current cash and cash equivalents and anticipated financing from our related parties and equity plans in the next six months, we believe that our cash position is sufficient to meet our liquidity needs for at least the next 12 months.
| For the six months ended September 30, |
||||||||
| 2023 | 2022 | |||||||
| Net Cash Used in Operating Activities | (579,568 | ) | (549,125 | ) | ||||
| Net Cash Provided by Investing Activities | 19,639 | 39,310 | ||||||
| Net Cash Used in Financing Activities | (4,461,735 | ) | (11,069,358 | ) | ||||
| Effect of Exchange Rate Changes on Cash | (881,794 | ) | (1,009,412 | ) | ||||
| Net decrease in cash, cash equivalents | (5,903,458 | ) | (12,588,585 | ) | ||||
Cash Flow in Operating Activities
For the six months ended September 30, 2023, net cash used in operating activities was $579,568, as compared to net cash used in operating activities of $549,125 for the six months ended September 30, 2022, representing an increase of $30,443. The increase in net cash used in operating activities primarily resulted from the change of following accounts:
| a) | A net loss for the six months ended September 30, 2023 of $9,696,158, compared with the net loss for the six months ended September 30, 2022 of $1,494,609. |
| b) | Change in inventories was $7,006 net cash inflow for the six months ended September 30, 2023. For the six months ended September 30, 2022, change in inventories was $294,421 net cash inflow, which led to $287,415 decrease in net cash inflow from operating activities. |
| c) | Change in accounts payable was $19,353 net cash inflow for the six months ended September 30, 2023. For the six months ended September 30, 2022, change in accounts payables was $253,976 net cash inflow, which led to $234,623 decrease in net cash inflow from operating activities |
| d) | Equity incentive plan – non-cash expense adjustment of equity incentive plan was $Nil for the six months ended September 30, 2023. For the six months ended September 30, 2022, non-cash expense adjustment of equity incentive plan was $277,285, which led to $277,285 increase in net cash outflow from operating activities. |
And offset by the change of following accounts:
| a) | Expected credit loss – non-cash expense adjustment of expected credit loss was $8,450,277 for the six months ended September 30, 2023. For the six months ended September 30, 2022, non-cash expense adjustment of expected credit loss was $Nil, which led to $8,450,277 decrease in net cash outflow from operating activities. |
| b) | Change in accounts receivable was $72,969 net cash outflow for the six months ended September 30, 2023. For the six months ended September 30, 2022, change in accounts receivable was $372,734 net cash outflow, which led to $299,765 decrease in net cash outflow from operating activities. |
| c) | Change in accrued expenses and other current liabilities was $308,019 net cash inflow for the six months ended September 30, 2023. For the six months ended September 30, 2022, change in accrued expenses and other current liabilities was $84,552 net cash inflow, which led to $223,467 increase in net cash inflow from operating activities. |
Cash Flow in Investing Activities
We had net cash provided by investing activities of $19,639 for the six months ended September 30, 2023, which was collection of receivables from Huangshan Panjie Investment Management Co., Ltd. of $19,639.
We had net cash provided by investing activities of $39,310 for the six months ended September 30, 2022, which primarily consisted of purchase of property and equipment of $20,115 and collection of receivables from Huangshan Panjie Investment Management Co., Ltd. of $59,425.
Cash Flow in Financing Activities
For the six months ended September 30, 2023, the net cash used in financing activities was $4,461,735, which was primarily attributable to repayments to related parties of $4,582,113, and repayment of the short-term borrowings of $4,469, offset by proceeds from short-term borrowings of $124,847.
For the six months ended September 30, 2022, the net cash used in financing activities was $11,069,358, which was primarily attributable to repayments to related parties of $11,061,683 and payment of the bank borrowings of $7,675.
The Equity Incentive Plan
On March 11, 2022, the board of directors of the Company adopted the 2022 equity incentive plan and approved the filing of a registration statement on Form S-8 (File No. 333-263563) to register such plan. On May 15, 2022, the Company has issued 6,094,180 ordinary shares (12,188 shares retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023), which were all the ordinary shares available under the 2022 equity incentive plan.
On January 5, 2024, the board of directors of the Company adopted the 2024 equity incentive plan and approved the filing of a registration statement on Form S-8 (File No. 333-276472) to register such plan. On January 24, 2024, the Company has issued 185,316 ordinary shares (on a post-split basis), which were all the ordinary shares available under the 2024 equity incentive plan.
The Convertible Notes
The Convertible Note 2022-1
On March 16, 2022, the Company entered into a securities purchase agreement with an institutional investor pursuant to which the Company issued an unsecured convertible promissory note with a 12-months maturity (the “Convertible Note 2022-1”) to the investor. The Convertible Note 2022-1 has the original principal amount of $2,804,848 including the original issue discount of $168,291 and the investor’s legal and other transaction costs of $20,000. The Company anticipates using the proceeds for general working capital purposes. The Convertible Note 2022-1 was fully converted on February 2, 2023. As of the date of this report, there is $0 outstanding under the Convertible Note 2022-1.
Material Terms of the Convertible Note 2022-1:
| ● | Interest accrues on the outstanding balance of the Note at 6% per annum from the purchase price date until the same is paid in full. All interest calculations hereunder shall be computed on the basis of a 360-day year comprised of twelve (12) thirty (30) day months, shall compound daily and shall be payable in accordance with the terms of this Note. |
| ● | Upon the occurrence of a trigger event, the investor may increase the outstanding balance payable under the Note by 12% or 5%, depending on the nature of such event. If the Company fails to cure the trigger event within the required five trading days, the trigger event will automatically become an event of default and interest will accrue at the lesser of 22% per annum or the maximum rate permitted by applicable law. |
| ● | Subject to adjustment as set forth in this Note, the price at which the lender has the right to convert all or any portion of the outstanding balance into ordinary shares is $0.30 per share. |
The Convertible Note 2022-2
On December 19, 2022, the Company entered into a securities purchase agreement with Streeterville Capital, LLC, pursuant to which the Company issued the investor an unsecured promissory note on December 19, 2022 in the original principal amount of $1,595,000 (the “Convertible Note 2022-2”), convertible into ordinary shares, $0.08 par value per share, of the Company for $1,500,000 in gross proceeds. The Company anticipates using the proceeds for general working capital purposes. As of the date of this report, there is $875,436 outstanding under the Convertible Note 2022-2.
Material Terms of the Convertible Note 2022-2:
| ● | Interest accrues on the outstanding balance of the Note at 6% per annum from the purchase price date until the same is paid in full. All interest calculations hereunder shall be computed on the basis of a 360-day year comprised of twelve (12) thirty (30) day months, shall compound daily and shall be payable in accordance with the terms of this Note. |
| ● | Upon the occurrence of a trigger event, the investor may increase the outstanding balance payable under the Note by 15% or 5%, depending on the nature of such event. If the Company fails to cure the trigger event within the required five trading days, the trigger event will automatically become an event of default and interest will accrue at the lesser of 15% per annum or the maximum rate permitted by applicable law. |
| ● | Subject to adjustment as set forth in this Note, the price at which the lender has the right to convert all or any portion of the outstanding balance into ordinary shares is the lower of (i) the Lender Conversion Price which is initially $0.60 and (ii) 80% of the average of the lowest VWAP during the fifteen (15) trading days immediately preceding the redemption notice is delivered. |
The Convertible Note 2023
On March 7, 2023, the Company entered into a securities purchase agreement with Streeterville Capital, LLC, pursuant to which the Company issued the investor an unsecured promissory note on March 7, 2023 in the original principal amount of $2,126,667 (the “Convertible Note 2023”), convertible into ordinary shares, $0.08 par value per share, of the Company for $2,000,000 in gross proceeds. The Company anticipates using the proceeds for general working capital purposes. The Convertible Note 2023 was fully converted on January 25, 2024. As of the date of this report, there is $0 outstanding under the Convertible Note 2023.
Material Terms of the Convertible Note 2023:
| ● | Interest accrues on the outstanding balance of the Note at 6% per annum from the purchase price date until the same is paid in full. All interest calculations hereunder shall be computed on the basis of a 360-day year comprised of twelve (12) thirty (30) day months, shall compound daily and shall be payable in accordance with the terms of this Note. |
| ● | Upon the occurrence of a trigger event, the investor may increase the outstanding balance payable under the Note by 15% or 5%, depending on the nature of such event. If the Company fails to cure the trigger event within the required five trading days, the trigger event will automatically become an event of default and interest will accrue at the lesser of 15% per annum or the maximum rate permitted by applicable law. |
| ● | Subject to adjustment as set forth in this Note, the price at which the lender has the right to convert all or any portion of the outstanding balance into ordinary shares is the lower of (i) the Lender Conversion Price which is initially $0.60 and (ii) 80% of the average of the lowest VWAP during the fifteen (15) trading days immediately preceding the redemption notice is delivered. |
Please refer to Note 11 of our Condensed Consolidated Financial Statements included in this Form 6-K for details of accounting of the Convertible Notes.
Going Concern
The condensed consolidated financial statements for the six months ended September 30, 2023 and 2022 have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business.
As reflected in the consolidated financial statements, we reported net loss of $9,696,158 and $1,494,609 for the six months ended September 30, 2023 and 2022, respectively. We had accumulated deficits of $31,309,291 and $21,613,133 as of September 30, 2023 and March 31, 2023, respectively. We used funds in operating activities of $579,568 and $549,125 for the six months ended September 30, 2023 and 2022, respectively. In addition, we suffered a continuous decline in revenue for the six months ended September 30, 2023 and 2022. These factors raise substantial doubt about our ability to continue as a going concern.
We are in the process of building its customer base to generate more revenues as well as cutting expenses, and we are seeking to raise capital through additional debt from equity financings to fund its operations. However, there can be no assurance that these plans and arrangements will be sufficient to fund our ongoing capital expenditure, working capital, and other requirements. The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset and the amounts or classification of liabilities that may result from the outcome of this uncertainty. If the going concern assumption is not appropriate, material adjustments to the financial statements could be required.
Off-Balance Sheet Arrangements
On April 12, 2021, Taizhou Suxuantang signed a financial guarantee agreement with Jiangsu Changjiang Commercial Bank for Taizhou Jiutian Pharmaceutical Co. Ltd. in borrowing of $383,772 (equivalent of RMB 2,800,000) for three-year period. Taizhou Suxuantang is obliged to pay on behalf of the related party the principal, interest, penalty and other expenses if Taizhou Jiutian Pharmaceutical Co. Ltd. defaults in payment. The Company did not charge financial guarantee fees over Taizhou Jiutian Pharmaceutical Co. Ltd.
On October 28, 2013, Taizhou Suxuantang signed a financial guarantee agreement with Fenlan Xu for Jianping Zhou in borrowing of $794,956 (equivalent of RMB 5,800,000) for an unlimited period. Taizhou Suxuantang is obliged to pay the amount if Jianping Zhou in default of the payment of principal and interests. Since Jianping Zhou deceased after yearend, Taizhou Suxuantang should bear all the risk for the repayment. However, subsequent to yearend, Taizhou Jiutian Pharmaceutical Co. Ltd. signed an agreement with Taizhou Suxuantang to take all the responsibility and obligation for repay the amount borrowed from Fenlan Xu on behalf of Jianping Zhou. This additional agreement releases Taizhou Suxuantang from future obligation in regard to the guarantee agreement. The Company did not charge financial guarantee fees over Jianping Zhou. Taizhou Jiutian Pharmaceutical Co. Ltd. is fully obliged to pay the principal, interests from January 1, 2021 to the actual date of repayment, including penalty and other expenses. As such, the Company expects no liabilities from the financial guarantee.
The Company had the following operating lease commitment as of September 30, 2023:
| Office Rental | For the year ended September 30, |
|||
| 2024 | $ | 68,586 | ||
| 2025 | 68,586 | |||
| 2026 | 68,586 | |||
| 2027 | 68,586 | |||
| Thereafter | 17,146 | |||
| Total | $ | 291,490 | ||
Except for the guarantee and commitment listed above, the Company does not have any other off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on its financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Inflation
We do not believe our business and operations have been materially affected by inflation.
Related Parties and Material Related Party Transactions
Please refer to Note 16 of our Condensed Consolidated Financial Statements included in this Form 6-K for details of related parties and material related party transactions.
Critical Accounting Policies
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amount of assets, liabilities and contingencies at the date of the condensed consolidated financial statements as well as the reported amounts of expenses during the reporting period. As a result, management is required to routinely make judgments and estimates about the effects of matters that are inherently uncertain. Actual results may differ from these estimates under different conditions or assumptions. Management determined there were no critical accounting policies or accounting estimates.
9
Exhibit 99.2
CHINA SXT PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED SEPTEMBER 30, 2023 AND 2022
(UNAUDITED)
CHINA SXT PHARMACEUTICALS, INC.
INDEX TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
F-
CHINA SXT PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
(IN U.S. DOLLARS, EXCEPT FOR NUMBER OF SHARES DATA)
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 11,465,020 | $ | 17,368,478 | ||||
| Accounts receivable, net | 1,311,005 | 1,344,569 | ||||||
| Inventories | 493,214 | 531,254 | ||||||
| Advance to suppliers | 88,103 | 46,304 | ||||||
| Prepayments, receivables and other current assets | 137,978 | 230,642 | ||||||
| Total Current Assets | 13,495,320 | 19,521,247 | ||||||
| Property, plant and equipment, net | 809,039 | 962,214 | ||||||
| Intangible assets, net | 22,514 | 27,868 | ||||||
| Long-term deposit | 8,736,677 | |||||||
| Right-of-use assets - operating leases -related party | 261,108 | 306,050 | ||||||
| Total Non-current Assets | 1,092,661 | 10,032,809 | ||||||
| TOTAL ASSETS | $ | 14,587,981 | $ | 29,554,056 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Short-term borrowings | $ | 267,270 | $ | 167,453 | ||||
| Long-term borrowings – current | 19,737 | 7,863 | ||||||
| Short-term convertible note | 2,496,184 | 3,157,492 | ||||||
| Accounts payable | 1,315,855 | 1,377,850 | ||||||
| Refund liabilities | 146,049 | 111,951 | ||||||
| Advance from customers | 190,193 | 165,534 | ||||||
| Amounts due to related parties | 421,168 | 5,203,762 | ||||||
| Accrued expenses and other liabilities | 3,249,143 | 3,142,084 | ||||||
| Taxes payable | 1,038,364 | 1,104,860 | ||||||
| Operating lease liabilities - current – related party | 56,171 | 58,089 | ||||||
| Total Current Liabilities | 9,200,134 | 14,496,938 | ||||||
| Non-current Liabilities | ||||||||
| Long-term borrowings | 106,575 | 117,862 | ||||||
| Operating lease liabilities – non-current – related party | 204,937 | 247,961 | ||||||
| Total Non-current Liabilities | 311,512 | 365,823 | ||||||
| TOTAL LIABILITIES | 9,511,646 | 14,862,761 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Ordinary shares, unlimited shares authorized, $2 par value, 727,851 shares issued and outstanding as of September 30, 2023 (457,365 shares issued and outstanding as of March 31, 2023)* | 1,454,756 | 913,785 | ||||||
| Additional paid-in capital | 35,949,681 | 35,588,214 | ||||||
| Accumulated deficits | (31,309,291 | ) | (21,613,133 | ) | ||||
| Accumulated other comprehensive income | (1,018,811 | ) | (197,571 | ) | ||||
| Total Shareholders’ Equity | 5,076,335 | 14,691,295 | ||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | $ | 14,587,981 | $ | 29,554,056 | ||||
| * | Retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023. |
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-
CHINA SXT PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF INCOME/(LOSS) AND COMPREHENSIVE INCOME/(LOSS)
(IN U.S. DOLLARS, EXCEPT SHARES DATA)
(UNAUDITED)
| For the six months ended September 30, |
||||||||
| 2023 (Unaudited) |
2022 (Unaudited) |
|||||||
| Revenues | $ | 939,583 | $ | 1,208,288 | ||||
| Revenues generated from third parties | 926,805 | 1,191,808 | ||||||
| Revenue generated from related parties | 12,778 | 16,480 | ||||||
| Cost of revenues | (651,242 | ) | (1,118,604 | ) | ||||
| Gross profit | 288,341 | 89,684 | ||||||
| Operating expenses: | ||||||||
| Selling and marketing | (176,950 | ) | (272,166 | ) | ||||
| General and administrative | (9,460,412 | ) | (967,964 | ) | ||||
| Total operating expenses | (9,637,362 | ) | (1,240,130 | ) | ||||
| Operating Loss | (9,349,021 | ) | (1,150,446 | ) | ||||
| Other income (expenses): | ||||||||
| Interest expenses, net | (336,520 | ) | (384,286 | ) | ||||
| Other income (expenses), net | (10,617 | ) | 40,123 | |||||
| Total other expenses, net | (347,137 | ) | (344,163 | ) | ||||
| Loss before income taxes | (9,696,158 | ) | (1,494,609 | ) | ||||
| Income tax provision | ||||||||
| Net loss | (9,696,158 | ) | (1,494,609 | ) | ||||
| Other comprehensive income (loss): | ||||||||
| Foreign currency translation adjustment | (821,240 | ) | (1,735,537 | ) | ||||
| Comprehensive loss | (10,517,398 |
) | (3,230,146 | ) | ||||
| Earnings per ordinary share | ||||||||
| $ | (19.34 | ) | $ | (14.61 | ) | |||
| Weighted average number of ordinary shares outstanding | ||||||||
| 501,467 | 102,316 | |||||||
| * | Retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023. |
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-
CHINA SXT PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
FOR THE SIX MONTHS ENDED SEPTEMBER 30, 2023 and 2022
(IN U.S. DOLLARS, EXCEPT SHARES DATA)
(UNAUDITED)
| Shares* | Amount | Additional paid-in capital |
Accumulated deficits |
Accumulated other comprehensive income (loss) |
Total equity |
|||||||||||||||||||
| Balance as of March 31, 2022 | 81,256 | $ | 162,468 | $ | 30,642,840 |
$ | (15,678,361 | ) | $ | 956,142 | $ | 16,083,089 |
||||||||||||
| Net loss | - | (1,494,609 | ) | (1,494,609 | ) | |||||||||||||||||||
| Shares issued as employee incentives | 12,188 | 24,377 | 530,194 | 554,571 | ||||||||||||||||||||
| Share issued due to reverse-split round up | 451 | |||||||||||||||||||||||
| Shares issued for convertible notes | 50,620 | 101,240 | 1,039,242 | 1,140,482 | ||||||||||||||||||||
| Foreign currency translation gain | - | (1,735,537 | ) | (1,735,537 | ) | |||||||||||||||||||
| Balance as of September 30, 2022 (Unaudited) | 144,515 | $ | 288,085 | $ | 32,212,276 |
$ | (17,172,970 | ) | $ | (779,395 | ) | $ | 14,547,996 |
|||||||||||
| Balance as of March 31, 2023 | 457,365 | $ | 913,785 | $ | 35,588,214 | $ | (21,613,133 | ) | $ | (197,571 | ) | $ | 14,691,295 | |||||||||||
| Net loss | - | (9,696,158 | ) | (9,696,158 | ) | |||||||||||||||||||
| Shares issued for convertible notes | 270,486 | 540,971 | 361,467 | 902,438 | ||||||||||||||||||||
| Foreign currency translation gain | - | (821,240 | ) | (821,240 | ) | |||||||||||||||||||
| Balance as of September 30, 2023 (Unaudited) | 727,851 | 1,454,756 | 35,949,681 | (31,309,291 | ) | (1,018,811 | ) | 5,076,335 | ||||||||||||||||
| * | Retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023. |
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-
CHINA SXT PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(IN U.S. DOLLARS)
(UNAUDITED)
| For the six months ended September 30, |
||||||||
| 2023 (Unaudited) |
2022 (Unaudited) |
|||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss from operations | $ | (9,696,158 | ) | $ | (1,494,609 | ) | ||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Convertible note - Accretion of financing cost | 212,708 | 311,642 | ||||||
| Expected credit loss | 8,450,277 | |||||||
| Depreciation and amortization expenses | 102,750 | 109,966 | ||||||
| Equity incentive plan | 277,285 | |||||||
| Non-cash operating lease expense | 35,098 | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (72,969 | ) | (372,734 | ) | ||||
| Note receivable | 33,441 | |||||||
| Inventory | 7,006 | 294,421 | ||||||
| Advance to suppliers | (45,562 | ) | (14,045 | ) | ||||
| Prepayments, receivables and other assets | 59,857 | (61,775 | ) | |||||
| Accounts payable | 19,353 | 253,976 | ||||||
| Refund liabilities | 41,626 | |||||||
| Advance from customers | 35,185 | (4,063 | ) | |||||
| Taxes payable | (1,660 | ) | 32,818 | |||||
| Change in related party lease liability – operating lease | (35,098 | ) | ||||||
| Accrued expenses and other current liabilities | 308,019 | 84,552 | ||||||
| Net cash used in operating activities | (579,568 | ) | (549,125 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of property, plant and equipment | (20,115 | ) | ||||||
| Other receivable - Huangshan Panjie | 19,639 | 59,425 | ||||||
| Net cash provided by investing activities | 19,639 | 39,310 | ||||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from borrowings | 124,847 | |||||||
| Repayment of borrowings | (4,469 | ) | (7,675 | ) | ||||
| Payment to related parties | (4,582,113 | ) | (11,061,683 | ) | ||||
| Net cash used in financing activities | (4,461,735 | ) | (11,069,358 | ) | ||||
| Effect of exchange rate changes on cash and cash equivalents and restricted cash | (881,794 | ) | (1,009,412 | ) | ||||
| Net decrease in cash, cash equivalents and restricted cash | (5,903,458 | ) | (12,588,585 | ) | ||||
| Cash, cash equivalents and restricted cash at the beginning of period | 17,368,478 | 15,569,619 | ||||||
| Cash, cash equivalents and restricted cash at the end of period | $ | 11,465,020 | $ | 2,981,034 | ||||
| Supplemental disclosures of cash flows information: | ||||||||
| Cash paid for income taxes | $ | $ | ||||||
| Cash paid for interest expense | $ | 9,449 | $ | |||||
| Non-cash transactions: | ||||||||
| Issuance of shares for equity incentive plan | $ | $ | 277,285 | |||||
| Issuance of shares for convertible notes principal and interest settlement | $ | 902,438 | $ | 1,140,482 | ||||
| Offset between due from related parties and due to related parties balances | $ | 3,306,031 |
$ | |||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-
CHINA SXT PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
NOTE 1 – ORGANIZATION AND PRINCIPAL ACTITIVIES
China SXT Pharmaceutical, Inc. (“SXT” or the “Company”) is a holding company incorporated in British Virgin Islands on July 4, 2017. The Company focuses on the research, development, manufacture, marketing and sales of traditional Chinese medicine pieces (the “TCMP”), through its variable interest entity (“VIE”), Jiangsu Suxuantang Pharmaceutical Co., Ltd, (“Taizhou Suxuantang”) in China. The Company currently sells three types of TCMP products: Advanced TCMP, Fine TCMP and Regular TCMP, and TCM Homologous Supplements (“TCMHS”) products. We currently have a product portfolio of 19 advanced TCMPs, 10 Fine TCMPs, 235 Regular TCMPs and 4 TCMHS solid beverage products that address a wide variety of diseases and medical indications. Most of our products are sold on a prescription basis across China. The Company’s principal executive offices are located in Taizhou, Jiangsu province, China.
Restructuring and Share Issuance
On July 4, 2017, we were incorporated in the British Virgin Islands by issuance of 10,300,000 common stocks at 0.001 par value to Ziqun Zhou, Di Zhou and Feng Zhou Management Limited (“China SXT Pharmaceuticals, Inc. shareholders”). Feng Zhou Management Limited is a BVI company 100% owned by Feng Zhou. Feng Zhou, Ziqun Zhou and Di Zhou collectively hold 100% shares of Taizhou Suxuantang. Later on October 20, 2017, the 10,300,000 shares common stocks (5,150 shares retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) were reallocated among China SXT Pharmaceuticals, Inc. shareholders. On October 20, 2017, the Company issued 9,700,000 common stocks (4,850 shares retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) at 0.001 par value to ten individual shareholders (“Restructuring”).
On July 21, 2017, our wholly owned subsidiary China SXT Group Limited (“SXT HK”) was incorporated in Hong Kong. China SXT Group Limited in turn holds all the capital stocks of Taizhou Suxantang Biotechnology Co. Ltd. (“WFOE”), a wholly foreign owned enterprise incorporated in China on October 13, 2017. On the same day, Taizhou Suxuantang and its shareholders entered into such a series of contractual arrangements, also known as VIE Agreements.
Taizhou Suxuantang was incorporated on June 9, 2005 by Jianping Zhou, Xiufang Yuan (the spouse of Jianping Zhou) and Jianbin Zhou, who held 83%, 11.5% and 5.5% shares in Taizhou Suxuantang respectively. On May 8, 2017, the three shareholders transferred all shares to Feng Zhou, Ziqun Zhou and Di Zhou (collectively “Taizhou Shareholders”), who hold 83%, 11.5% and 5.5% shares in Taizhou Suxuantang, respectively, after the transfer of shares. Feng Zhou and Ziqun Zhou are the children of Jianping Zhou and Xiufang Yuan, and Di Zhou is the child of Jianbin Zhou.
The discussion and presentation of financial statements herein assumes the completion of the Restructuring, which is accounted for retroactively as if the aforementioned transactions had become effective as of the beginning of the first period presented in the accompanying condensed consolidated financial statements.
F-
NOTE 1 – ORGANIZATION AND PRINCIPAL ACTITIVIES (CONTINUED)
The following diagram illustrates our corporate structure, including our subsidiary and condensed consolidated variable interest entity as of the date of the financial statements assuming the completion of our Restructuring:
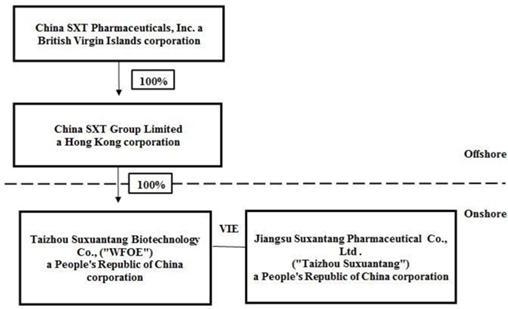
VIE Agreements with Taizhou Suxuantang
Due to PRC legal restrictions on foreign ownership in the pharmaceutical sector, neither the Company nor our subsidiaries own any equity interest in Taizhou Suxuantang. Instead, the Company controls and receives the economic benefits of Taizhou Suxuantang’s business operations through a series of contractual arrangements. WFOE, Taizhou Suxuantang and its shareholders entered into such a series of contractual arrangements, also known as VIE Agreements, on October 13, 2017. The VIE agreements are designed to provide WFOE with the power, rights and obligations equivalent in all material respects to those it would possess as the sole equity holder of Taizhou Suxuantang, including absolute control rights and the rights to the assets, property and revenue of Taizhou Suxuantang.
According to the Exclusive Business Cooperation Agreement between WFOE and Taizhou Suxuantang, which is one of the VIE Agreements that was also entered into on October 13, 2017, Taizhou Suxuantang is obligated to pay service fees to WFOE approximately equal to the net income of Taizhou Suxuantang.
Each of the VIE Agreements is described in detail below:
Exclusive Business Cooperation Agreement
Pursuant to the Exclusive Business Cooperation Agreement between Taizhou Suxuantang and WFOE, WFOE provides Taizhou Suxuantang with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, human resources, and information. Additionally, Taizhou Suxuantang granted an irrevocable and exclusive option to WFOE to purchase from Taizhou Suxuantang, any or all of Taizhou Suxuantang’s assets at the lowest purchase price permitted under the PRC laws. Should WFOE exercise such option, the parties shall enter into a separate asset transfer or similar agreement. For services rendered to Taizhou Suxuantang by WFOE under this agreement, WFOE is entitled to collect a service fee calculated based on the time of services rendered multiplied by the corresponding rate, plus the amount of the services fees or ratio decided by the board of directors of WFOE based on the value of services rendered by WFOE and the actual income of Taizhou Suxuantang from time to time, which is approximately equal to the net income of Taizhou Suxuantang.
F-
NOTE 1 – ORGANIZATION AND PRINCIPAL ACTITIVIES (CONTINUED)
The Exclusive Business Cooperation Agreement shall remain in effect for ten years unless it is terminated by WFOE with 30-day prior notice. Taizhou Suxuantang does not have the right to terminate the agreement unilaterally. WFOE may unilaterally extend the term of this agreement with prior written notice.
The CEO and president of WFOE, Mr. Feng Zhou, is currently managing Taizhou Suxuantang pursuant to the terms of the Exclusive Business Cooperation Agreement. WFOE has absolute authority relating to the management of Taizhou Suxuantang, including but not limited to decisions with regard to expenses, salary raises and bonuses, hiring, firing and other operational functions. The Exclusive Business Cooperation Agreement does not prohibit related party transactions. The audit committee is required to review and approve in advance any related party transactions, including transactions involving WFOE or Taizhou Suxuantang.
Share Pledge Agreement
Under the Share Pledge Agreement among WFOE and Feng Zhou, Ziqun Zhou, and Di Zhou, who together hold 100% shares of Taizhou Suxuantang (“Taizhou Suxuantang Shareholders”), the Taizhou Suxuantang Shareholders pledged all of their equity interests in Taizhou Suxuantang to WFOE to guarantee the performance of Taizhou Suxuantang’s obligations under the Exclusive Business Cooperation Agreement. Under the terms of the agreement, in the event that Taizhou Suxuantang or its shareholders breach their respective contractual obligations under the Exclusive Business Cooperation Agreement, WFOE, as pledgee, will be entitled to certain rights, including, but not limited to, the right to collect dividends generated by the pledged equity interests. The Taizhou Suxuantang Shareholders also agreed that upon occurrence of any event of default, as set forth in the Share Pledge Agreement, WFOE is entitled to dispose of the pledged equity interest in accordance with applicable PRC laws. The Taizhou Suxuantang Shareholders further agree not to dispose of the pledged equity interests or take any actions that would prejudice WFOE’s interest.
The Share Pledge Agreement shall be effective until all payments due under the Exclusive Business Cooperation Agreement have been paid by Taizhou Suxuantang. WFOE shall cancel or terminate the Share Pledge Agreement upon with no additional expense.
The purposes of the Share Pledge Agreement are to (1) guarantee the performance of Taizhou Suxuantang’s obligations under the Exclusive Business Cooperation Agreement, (2) make sure the shareholders of Taizhou Suxuantang shall not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice WFOE’s interests without WFOE’s prior written consent and (3) provide WFOE control over Taizhou Suxuantang. Under the Exclusive Option Agreement (described below), WFOE may exercise its option to acquire the equity interests in Taizhou Suxuantang any time to the extent permitted by the PRC Law. In the event Taizhou Suxuantang breaches its contractual obligations under the Exclusive Business Cooperation Agreement, WFOE will be entitled to foreclose on the Taizhou Suxuantang Shareholders’ equity interests in Taizhou Suxuantang and may (1) exercise its option to purchase or designate third parties to purchase part or all of their equity interests in Taizhou Suxuantang and in this situation, WFOE may terminate the VIE agreements after acquisition of all equity interests in Taizhou Suxuantang or form a new VIE structure with the third parties designated by WFOE; or (2) dispose the pledged equity interests and be paid in priority out of the proceeds from the disposal in which case the VIE structure will be terminated.
F-
NOTE 1 – ORGANIZATION AND PRINCIPAL ACTITIVIES (CONTINUED)
Exclusive Option Agreement
Under the Exclusive Option Agreement, the Taizhou Suxuantang Shareholders irrevocably granted WFOE (or its designee) an exclusive option to purchase, to the extent permitted under PRC law, once or at multiple times, at any time, part or all of their equity interests in Taizhou Suxuantang at the exercise price of RMB10.00.
Under the Exclusive Option Agreement, WFOE may at any time under any circumstances, purchase, or have its designated person to purchase, at its discretion, to the extent permitted under PRC law, all or part of the shareholders’ equity interests in Taizhou Suxuantang.
This Agreement shall remain effective until all equity interests held by Taizhou Suxuantang Shareholders in Taizhou Suxuantang have been transferred or assigned to WFOE and/or any other person designated by WFOE in accordance with this Agreement.
Power of Attorney
Under the Power of Attorney, the Taizhou Suxuantang Shareholders authorize WFOE to act on their behalf as their exclusive agent and attorney with respect to all rights as shareholders, including but not limited to: (a) attending shareholders’ meetings; (b) exercising all the shareholder’s rights, including voting, that shareholders are entitled to under the laws of China and the Articles of Association, including but not limited to the sale or transfer or pledge or disposition of shares in part or in whole; and (c) designating and appointing on behalf of shareholders the legal representative, the executive director, supervisor, the chief executive officer and other senior management members of Taizhou Suxuantang.
Although it is not explicitly stipulated in the Power of Attorney, the term of the Power of Attorney shall be the same as the term of that of the Exclusive Option Agreement.
This Power of Attorney is coupled with an interest and shall be irrevocable and continuously valid for each shareholder from the date it is executed until the date he/she no longer is a shareholder of Taizhou Suxuantang.
The Exclusive Option Agreement, together with the Share Pledge Agreement and the Power of Attorney enable WFOE to exercise effective control over Taizhou Suxuantang.
Basis of presentation and principles of consolidation
The accompany unaudited condensed consolidated financial statements of the Company has been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The accompanying condensed consolidated financial statements include our accounts and those of our wholly owned subsidiaries and VIE. Accordingly, all intercompany balances and transactions have been eliminated through the consolidation process.
In the opinion of management, these unaudited condensed consolidated interim financial statements reflect all adjustments, consisting of normal recurring adjustments, which are necessary to present fairly, in all material respects, the Company’s condensed consolidated financial position, results of operations, cash flows and changes in equity for the interim periods presented. These unaudited condensed financial statements do not include certain information and footnote disclosures as required by the U.S. GAAP for complete annual financial statements. Therefore, these unaudited condensed consolidated interim financial statements should be read in conjunction with the financial statements and related notes included in the Company’s first initial offering Registration Statement on Form 20-F for the year ended March 31, 2023 and 2022.
The VIE, Taizhou Suxuantang is owned by three shareholders, each of which act as the Company’s nominee shareholder. For the consolidated VIEs, the Company’s management made evaluations of the relationships between the Company and the VIE and the economic benefit flow of contractual arrangements with Taizhou Suxuantang. In connection with such evaluation, management also took into account the fact that, as a result of such contractual arrangements, the Company control the shareholders’ voting interests in these VIEs. As a result of such evaluation, management concluded that the Company is the primary beneficiary of the consolidated VIEs, Taizhou Suxuantang. The Company does not have any VIEs that are not consolidated in the financial statements.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES
Risks in relation to the VIE structure
It is possible that the Company’s operation of certain of its operations and businesses through its VIE could be found by PRC authorities to be in violation of PRC law and regulations prohibiting or restricting foreign ownership of companies that engage in such operations and businesses. While the Company’s management considers the possibility of such a finding by PRC regulatory authorities under current law and regulations to be remote. On January 19, 2015, the Ministry of Commerce of the PRC, or (the “MOFCOM”) released on its Website for public comment a proposed PRC law (the “Draft FIE Law”) that appears to include VIE within the scope of entities that could be considered to be foreign invested enterprises (or “FIEs”) that would be subject to restrictions under existing PRC law on foreign investment in certain categories of industry. Specifically, the Draft FIE Law introduces the concept of “actual control” for determining whether an entity is considered to be an FIE. In addition to control through direct or indirect ownership or equity, the Draft FIE Law includes control through contractual arrangements within the definition of “actual control.” If the Draft FIE Law was passed by the People’s Congress of the PRC and went into effect in its current form and as a result the Company’s VIE could become explicitly subject to the current restrictions on foreign investment in certain categories of industry. The Draft FIE Law includes provisions that would exempt from the definition of foreign invested enterprises entities where the ultimate controlling shareholders are either entities organized under PRC law or individuals who are PRC citizens. The Draft FIE Law is silent as to what type of enforcement action might be taken against existing VIEs that operate in restricted or prohibited industries and are not controlled by entities organized under PRC law or individuals who are PRC citizens. If a finding were made by PRC authorities, under existing law and regulations or under the Draft FIE Law if it becomes effective, about the Company’s operation of certain of its operations and businesses through its VIEs, regulatory authorities with jurisdiction over the licensing and operation of such operations and businesses would have broad discretion in dealing with such a violation, including levying fines, confiscating the Company’s income, revoking the business or operating licenses of the affected businesses, requiring the Company to restructure its ownership structure or operations, or requiring the Company to discontinue all or any portion of its operations. Any of these actions could cause significant disruption to the Company’s business operations and have a severe adverse impact on the Company’s cash flows, financial position and operating performance.
In addition, it is possible that the contracts among Taizhou Suxuantang, WFOE, and the nominee shareholders of Taizhou Suxuantang would not be enforceable in China if PRC government authorities or courts were to find that such contracts contravene PRC laws and regulations or are otherwise not enforceable for public policy reasons. In the event that the Company was unable to enforce these contractual arrangements, the Company would not be able to exert effective control over the VIEs. Consequently, the VIEs’ results of operations, assets and liabilities would not be included in the Company’s condensed consolidated financial statements. If such were the case, the Company’s cash flows, financial position, and operating performance would be materially adversely affected. The Company’s contractual arrangements Taizhou Suxuantang, WFOE, and the nominee shareholders of Taizhou Suxuantang are approved and in place. Management believes that such contracts are enforceable and considers the possibility remote that PRC regulatory authorities with jurisdiction over the Company’s operations and contractual relationships would find the contracts to be unenforceable.
The Company’s operations and businesses rely on the operations and businesses of its VIEs, which hold certain recognized revenue-producing assets. The VIEs also have an assembled workforce, focused primarily on research and development, whose costs are expensed as incurred. The Company’s operations and businesses may be adversely impacted if the Company loses the ability to use and enjoy assets held by its VIE.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Foreign currency translation
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency using the applicable exchange rates at the balance sheet dates. The resulting exchange differences are recorded in the statement of operations.
The reporting and functional currencies of the Company and SXT HK are the United States Dollars (“US$”) and the accompanying financial statements have been expressed in US$. In addition, the WFOE and the VIE maintain their books and records in their respective local currency, Renminbi (“RMB”), which is also the respective functional currency for each subsidiary and VIE as they are the primary currency of the economic environment in which each subsidiary operates.
In general, for consolidation purposes, assets, and liabilities of its subsidiaries whose functional currency is not the US$ are translated into US$, in accordance with ASC Topic 830-30, “Translation of Financial Statement”, using the exchange rate on the balance sheet date. Revenues and expenses are translated at average rates prevailing during the period. The gains and losses resulting from translation of financial statements of a foreign subsidiary are recorded as a separate component of accumulated other comprehensive income within the statement of stockholders’ equity. Other equity items are translated using the exchange rates on the transaction date.
Translation of amounts from the local currencies of the Company into US$ has been made at the following exchange rates for the respective periods:
| September 30, 2023 |
March 31, 2023 |
September 30, 2022 |
||||||||||
| Balance sheet items, except for equity accounts | 7.2960 | 6.8676 | 7.1135 | |||||||||
| Items in the statements of income(loss) and comprehensive income(loss), and statements of cash flows | 7.1287 | 6.8516 | 6.7312 | |||||||||
Measurement of credit losses on financial instruments
On April 1, 2023, the Company adopted ASU 2016-13, “Financial Instruments — Credit Losses (Topic 326) — Measurement of Credit Losses on Financial Instruments,” for financial assets at amortized cost including accounts receivable, refundable deposits, other receivables, and retention receivable. This guidance replaced the “incurred loss” impairment methodology with an approach based on “expected losses” to estimate credit losses on certain types of financial instruments and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance requires financial assets to be presented at the net amount expected to be collected. The allowance for credit losses is a valuation account that is deducted from the cost of the financial asset to present the net carrying value at the amount expected to be collected on the financial asset.
Use of estimates
The preparation of condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. On an ongoing basis, management reviews these estimates and assumptions using the currently available information.
Changes in facts and circumstances may cause the Company to revise its estimates. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. The following are some of the areas requiring significant judgments and estimates as of September 30, 2023 and March 31, 2023: determinations of the useful lives of long-lived assets, estimates of expected credit loss for accounts receivable and other receivables, estimates of inventory provision, sales return rate, valuation assumptions in performing asset impairment tests of long-lived assets and determinations of fair value of convertible notes (liability component, etc.) and warrants.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Going Concern
The condensed consolidated financial statements for the six months ended September 30, 2023 and 2022 have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business.
As reflected in the consolidated financial statements, the Company reported net loss of $9,696,158 and $1,494,609 for the six months ended September 30, 2023 and 2022, respectively. The Company had accumulated deficits of $31,309,291 and $21,613,133 as of September 30, 2023 and March 31, 2023, respectively. The Company used funds in operating activities of $579,568 and $549,125 for the six months ended September 30, 2023 and 2022, respectively. In addition, the Company suffered a continuous decline in revenue for the six months ended September 30, 2023 and 2022. These factors raise substantial doubt about the Company's ability to continue as a going concern.
The Company is in the process of building its customer base to generate more revenues as well as cutting expenses, and the Company is seeking to raise capital through additional debt from equity financings to fund its operations. However, there can be no assurance that these plans and arrangements will be sufficient to fund the Company's ongoing capital expenditure, working capital, and other requirements. The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset and the amounts or classification of liabilities that may result from the outcome of this uncertainty. If the going concern assumption is not appropriate, material adjustments to the financial statements could be required.
Fair values of financial instruments
ASC Topic 825, Financial Instruments (“Topic 825”) requires disclosure of fair value information of financial instruments, whether recognized in the balance sheets, for which it is practicable to estimate that value. In cases where quoted market prices are not available, fair values are based on estimates using present value or other valuation techniques. Those techniques are significantly affected by the assumptions used, including the discount rate and estimates of future cash flows. In that regard, the derived fair value estimates cannot be substantiated by comparison to independent markets and, in many cases, could not be realized in immediate settlement of the instruments. Topic 825 excludes certain financial instruments and all nonfinancial assets and liabilities from its disclosure requirements. Accordingly, the aggregate fair value amounts do not represent the underlying value of the Company.
| ● | Level 1 - inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| ● | Level 2 - inputs to the valuation methodology include quoted prices for similar assets and liabilities inactive markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. |
| ● | Level 3 - inputs to the valuation methodology are unobservable and significant to the fair value. |
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Fair values of financial instruments (continued)
As of September 30, 2023 and March 31, 2023, financial instruments of the Company primarily comprised of cash and cash equivalents, accounts receivables, receivables and other current assets (exclude prepayments and deposits), borrowings (current and non-current portion), accounts payable, amounts due to related parties and accrued expenses, lease liabilities and other liabilities. The carrying amounts of these financial instruments approximated their fair values because of their generally short maturities.
For lease liabilities, fair value approximates their carrying value at the period end as the interest rates used to discount the host contracts approximate market rates. The carrying amount of the long-term borrowings approximates its fair value due to the fact that the related interest rate approximates the interest rates currently offered by financial institutions for similar debt instruments of comparable maturities.
The Company noted no transfers between levels during any of the periods presented. The Company did not have any instruments that were measured at fair value on a recurring nor non-recurring basis as of September 30, 2023 and March 31, 2023, respectively.
Cash and cash equivalents
The Company considers all highly liquid investment instruments with an original maturity of three months or less from the date of purchase to be cash equivalents. The Company maintains most of the bank accounts in the PRC. Cash balances in bank accounts in PRC are not insured by the Federal Deposit Insurance Corporation or other programs.
Accounts receivable, net
Accounts receivable are recorded at the invoiced amount less an allowance for any uncollectible accounts and do not bear interest, which are due on demand. Management reviews the adequacy of the allowance for doubtful accounts on an ongoing basis, using historical collection trends and aging of receivables. Management also periodically evaluates individual customer’s financial condition, credit history, and the current economic conditions to adjust in the allowance when it is considered necessary. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
The Company use a loss rate method to estimate the allowance for credit losses. For those past due balances over one year and other higher risk receivables identified by the Company are reviewed individually for collectability. The Company evaluates the expected credit loss of accounts receivable based on historical collection experience, the financial condition of its customers and assumptions for the future movement of different economic drivers and how these drivers will affect each other. The Company writes off potentially uncollectible accounts receivable against the allowance for credit losses if it is determined that the amounts will not be collected or if a settlement with respect to a disputed receivable is reached for an amount that is less than the carrying value.
As of September 30, 2023 and March 31, 2023, the Company assessed the recoverability of its accounts receivable and record an allowance of $1,459,238 and $1,522,739, respectively.
Inventories
Inventories primarily include raw materials and finished goods.
Inventories are stated at the lower of cost or net realizable value. Cost is determined by the weighted-average method. Raw material cost is based on purchase costs while work-in-progress and finished goods comprise direct materials, direct labor and an allocation of manufacturing overhead costs. Net realizable value represents the anticipated selling price, net of distribution cost, less estimated costs to completion for inventories. As of September 30, 2023 and March 31, 2023, the Company assessed the net realizable value of its inventories and record a provision of $149,507 and $158,834, respectively.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Advance to suppliers
Advance to suppliers represent amounts advanced to suppliers for future purchases of raw materials and for other services. The suppliers usually require advance payments when the Company makes purchase or orders service, and the advanced payments will be utilized to offset the Company’s future payments.
Property, plant and equipment, net
Property and equipment are stated at cost. The straight-line depreciation method is used to compute depreciation over the estimated useful lives of the assets, as follows:
| Residual value rate |
Useful Lives |
|||||
| Machinery | 5 | % | 10 years | |||
| Electric equipment | 5 | % | 3-5 years | |||
| Office equipment | 5 | % | 5 years | |||
| Vehicles | 5 | % | 4 years | |||
| Leasehold improvement cost | 5 | % | 3-10 years (shorter of the lease term and the remaining useful life) | |||
The Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An asset is considered impaired if its carrying amount exceeds the future net undiscounted cash flows that the asset is expected to generate. If such asset is considered to be impaired, the impairment recognized is the amount by which the carrying amount of the asset, if any, exceeds its fair value determined using a discounted cash flow model. For the six months ended September 30, 2023 and 2022, there was no impairment of property, plant and equipment.
Costs of repairs and maintenance are expensed as incurred and asset improvements are capitalized. The cost and related accumulated depreciation and amortization of assets disposed of or retired are removed from the accounts, and any resulting gain or loss is reflected in the condensed consolidated income statements.
Intangible assets, net
Intangible assets are stated at cost less accumulated amortization. Intangible assets represented the trademark registered in the PRC and purchased software which are amortized on a straight-line basis over a useful life of 10 years.
The Company follows ASC Topic 350 in accounting for intangible assets, which requires impairment losses to be recorded when indicators of impairment are present and the undiscounted cash flows estimated to be generated by the assets are less than the assets’ carrying amounts. For the six months ended September 30, 2023 and 2022, the Company record no impairment of intangible assets.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Impairment of long-lived assets
Long-lived assets primarily include property, plant and equipment and intangible assets. In accordance with the provision of ASC Topic 360-10-5, “Impairment or Disposal of Long-Lived Assets”, the Company generally conducts its annual impairment evaluation to its long-lived assets, usually in the fourth quarter of each year, or more frequently if indicators of impairment exist, such as a significant sustained change in the business climate. The recoverability of long-lived assets is measured at the reporting unit level, which is an operating segment or one level below an operating segment. If the total of the expected undiscounted future net cash flows is less than the carrying amount of the asset, a loss is recognized for the difference between the fair value and carrying amount of the asset. The Company record no impairment charge for the six months ended September 30, 2023 and 2022, respectively.
Convertible note, net
ASU 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, provides simplification of the convertible debt accounting framework by eliminating the cash conversion and the beneficial conversion feature accounting models for convertible debt and convertible preferred stock. The new guidance removes from GAAP separation models for convertible debt that require the convertible debt to be separated into a debt and equity component, unless the conversion feature is required to be bifurcated and accounted for as a derivative or the debt is issued at a substantial premium. ASU 2020-06 requires adoption using either modified retrospective method or full retrospective method.
Under the new framework, the reporting entity will decide the accounting for its convertible notes in the following steps: (1) a reporting entity will first decide whether to elect the fair value option under ASC 825-10 (convertible debt issued with a substantial premium may be ineligible for the fair value option); (2) if the fair value option is not elected, the reporting entity must assess whether the conversion feature requires bifurcation pursuant to ASC 815; (3) if bifurcation is not required, the reporting entity must evaluate whether the convertible debt was issued with a substantial premium; (4) if the fair value option is not elected, the conversion option is not required to be bifurcated, and the convertible debt was not issued with a substantial premium, the convertible debt will be accounted for as a single unit of account under the “traditional convertible security” model. Debt discount is amortized over the period during which the convertible note is expected to be outstanding (through the maturity date) as additional non-cash interest expense.
Revenue recognition
The Company adopted ASC Topic 606 Revenue from Contracts with Customers (“ASC 606”), on April 1, 2018, using the modified retrospective method.
Revenue is recognized when control of promised goods is transferred to the Company’s customers in an amount of consideration of which the Company expect to be entitled to in exchange for the goods, and the Company can reasonably estimates return provision for the goods. The product return provisions are estimated based on (1) historical rates, (2) specific identification of outstanding returns not yet received from customers and outstanding discounts and claims and (3) estimated returns, discounts and claims expected, but not yet finalized with customers. As of September 30, 2023 and March 31, 2023, sales return provision recorded in refund liabilities were $146,049 and $111,951.
For the six months ended September 30, 2023 and 2022, the Company did not have any significant incremental costs of obtaining contracts with customers incurred or costs incurred in fulfilling contracts with customers within the scope of ASC Topic 606, that shall be recognized as an asset and amortized to expenses in a pattern that matches the timing of the revenue recognition of the related contract.
The Company does not have amounts of contract assets since revenue is recognized as control of goods is transferred. The contract liabilities consist of advance payments from customers. The contract liabilities are reported in a net position on a customer-by-customer basis at the end of each reporting period. All contract liabilities are included in advance from customers in the condensed consolidated balance sheets. As of September 30, 2023 and March 31, 2023, the Company record advance from customers of $190,193 and $165,534, respectively
Cost of revenue
Cost of revenue consists primarily of cost of materials, direct labors, overhead, and other related incidental expenses that are directly attributable to the Company’s principal operations.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Market development fees
Market development fees relate mainly to market development and advertisements of our pharmaceutical products. For the six months ended September 30, 2023 and 2022, marketing and advertising expenses are $134,558 and $150,046, respectively, which are included in selling expenses in our unaudited condensed consolidated statements of income (loss) and comprehensive income (loss).
Income Taxes
Current income tax expenses are provided for in accordance with the laws of the relevant taxing authorities. As part of the process of preparing the condensed consolidated financial statements, the Company is required to estimate its income taxes in each of the jurisdictions in which it operates. The Company accounts for income taxes using the liability method, under which deferred income taxes are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred taxes of a change in tax rates is recognized as income or expense in the period that includes the enactment date. Valuation allowance is provided on deferred tax assets to the extent that it is more likely than not that the asset will not be realizable in the foreseeable future.
The Company adopts ASC 740-10-25 “Income Taxes” which prescribes a more likely than not threshold for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. It also provides guidance on derecognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods and income tax disclosures. The Company did not have significant unrecognized uncertain tax positions or any unrecognized liabilities, interest or penalties associated with unrecognized tax benefit as of September 30, 2023 and March 31, 2023.
Comprehensive income (loss)
Comprehensive income includes net income and foreign currency adjustments. Comprehensive income is reported in the condensed consolidated statements of operations and comprehensive income. Accumulated other comprehensive income, as presented on the balance sheets are the cumulative foreign currency translation adjustments. As of September 30, 2023 and March 31, 2023, the balances of accumulated other comprehensive loss amounted to $1,018,811 and $197,571, respectively.
Leases
Leases are classified at lease commencement date as either a finance lease or an operating lease. A lease is a finance lease if it meets any of the following criteria: (a) the lease transfers ownership of the underlying asset to the lessee by the end of the lease term. (b) the lease grants the lessee an option to purchase the underlying asset that the lessee is reasonably certain to exercise, (c) the lease term is for the major part of the remaining economic life of the underlying asset, (d) the present value of the sum of the lease payments and any residual value guaranteed by the lessee that is not already reflected in the lease payments equals or exceeds substantially all of the fair value of the underlying asset or (e) the underlying asset is of such a specialized nature that it is expected to have no alternative use to the lessor at the end of the lease term. When none of the criteria meets, the lease shall be classified as an operating lease.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Leases (continued)
For lessee, a lease is recognized as a right-of-use asset with a corresponding liability at lease commencement date. The lease liability is calculated at the present value of the lease payments not yet paid by using the lease term and discount rate determined at lease commencement. The right-of-use asset is calculated as the lease liability, increased by any initial direct costs and prepaid lease payments, reduced by any lease incentives received before lease commencement. The right-of-use asset itself is amortized on a straight-line basis unless another systematic method better reflects how the underlying asset will be used by and benefits the lessee over the lease term.
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842). The amendments in this ASU require an entity to recognize a right-of-use asset and lease liability for all leases with terms of more than 12 months. Recognition, measurement and presentation of expenses will depend on classification as a finance or operating lease. The amendments also require certain quantitative and qualitative disclosures about leasing arrangements. The Company adopted ASC 842 effective as of the beginning of the year ended March 31, 2023 by using a modified retrospective transition approach in the accompanying financial statements of the Company. The adoption of this standard had a material impact on the Company’s financial position as increased its assets and liabilities due to the recognition of right-of-use assets and lease liabilities on its consolidated balance sheets, and no material impact on its consolidated statements of comprehensive loss and cash flows.
Segment reporting
Operating segments are reported in a manner consistent with the internal reporting provided to the chief operating decision-maker, which is a strategic committee comprised of members of the Company’s management team. In the respective periods presented, the Company had one single operating and reportable segment, namely the manufacture and distribution of TCMP. Although TCMP consist of different business units of the Company, information provided to the chief operating decision-maker is at the revenue level and the Company does not allocate operating costs or assets across business units, as the chief operating decision-maker does not use such information to allocate resources or evaluate the performance of the business units. As the Company’s long-lived assets are substantially all located in the PRC and substantially all of the Company’s revenue is derived from within the PRC, no geographical information is presented.
Earnings per share
Earnings (loss) per share is calculated in accordance with ASC 260 Earnings per Share. Basic earnings (loss) per share is computed by dividing the net income (loss) attributable to shareholders of the Company by the weighted average number of ordinary shares outstanding during the year. Diluted earnings per share is computed in accordance with the treasury stock method and based on the weighted average number of ordinary shares and dilutive ordinary share equivalents. Dilutive ordinary share equivalents are excluded from the computation of diluted earnings per share if their effects would be anti-dilutive. There were no dilutive ordinary share equivalents outstanding during the six months ended September 30, 2023 and 2022.
Related party transactions
In general, related parties exist when there is a relationship that offers the potential for transactions at less than arm’s-length, favorable treatment, or the ability to influence the outcome of events different from that which might result in the absence of that relationship. A related party may be any of the following: a) an affiliate, which is a party that directly or indirectly controls, is controlled by, or is under common control with another party; b) a principle owner, owner of record or known beneficial owner of more than 10% of the voting interest of an entity; c) management, which are persons having responsibility for achieving objectives of the entity and requisite authority to make decision; d) immediate family of management or principal owners; e) a parent company and its subsidiaries; and f) other parties that have ability to significant influence the management or operating policies of the entity.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Significant risks and uncertainties
Credit risk
Assets that potentially subject the Company to significant concentration of credit risk primarily consist of cash and cash equivalents, accounts receivable, other receivables, advances to suppliers. The maximum exposure of such assets to credit risk is their carrying amount as at the balance sheet dates. As of September 30, 2023 and March 31, 2023 the Company held cash and cash equivalents of $11,465,020 and $17,368,478, respectively, which were primarily deposited in financial institutions located in Mainland China, which were uninsured by the government authority. To limit exposure to credit risk relating to deposits, the Company primarily place cash deposits with large financial institutions in China which management believes are of high credit quality. The Company’s operations are carried out in Mainland China. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic, and legal environments in the PRC as well as by the general state of the PRC’s economy. In addition, the Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other factors.
The Company conducts credit evaluations of its customers and suppliers and generally does not require collateral or other security from them. The Company use a loss rate method to estimate the allowance for credit losses. For those past due balances over one year and other higher risk receivables identified by the Company are reviewed individually for collectability. The Company evaluates the expected credit loss of receivables based on historical collection experience, the financial condition of its customers and assumptions for the future movement of different economic drivers and how these drivers will affect each other. The Company writes off potentially uncollectible receivables against the allowance for credit losses if it is determined that the amounts will not be collected or if a settlement with respect to a disputed receivable is reached for an amount that is less than the carrying value. As of September 30, 2023 and March 31, 2023, the Company record expected credit loss of $1,459,238 and $1,522,739 for accounts receivable, respectively.
Liquidity risk
The Company is also exposed to liquidity risk which is risk that it is unable to provide sufficient capital resources and liquidity to meet its commitments and business needs. Liabilities that potentially subject the Company to significant concentration of liquidity risk primarily consist of bank loans (current and non-current portion), accounts payable, amounts due to related parties, and accrued expenses and other liabilities. Liquidity risk is controlled by the application of financial position analysis and monitoring procedures. When necessary, the Company will turn to other financial institutions and the owners to obtain short-term funding to meet the liquidity shortage.
Interest Rate Risks
The Company is exposed to interest rate risk primarily relates to the variable-rate borrowings, which carry interest rates ranging from 6% to 18%, and convertible notes. The Company has not used any derivative instruments to mitigate its exposure associated with interest rate risk.
Foreign currency risk
The Company has significant operating activities in China, thus has assets and liabilities are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the Peoples’ Bank of China (“PBOC”) or other authorized financial institutions at exchange rates quoted by PBOC. Approval of foreign currency payments by the PBOC or other regulatory institutions requires submitting a payment application form together with suppliers ‘invoices and signed contracts”. The value of RMB is subject to changes in central government policies and to international economic and political developments affecting supply and demand in the China Foreign Exchange Trading System market. Where there is a significant change in value of RMB, the gains and losses resulting from translation of financial statements of a foreign subsidiary will be significantly affected.
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Significant risks and uncertainties (continued)
Concentration risk
Significant customers and suppliers are those that account for greater than 10% of the Company’s revenues and purchases, respectively. The loss of any of the Company’s significant supplier or the failure to purchase key raw material could have a material adverse effect on our business, consolidated results of operations and financial condition
During the six months ended September 30, 2023, there are two customers generated sales which accounted for over 10% of total revenues generated for that period. During the six months ended September 30, 2022, there are two customers generated sales which accounted for over 10% of total revenues generated for that period. The details are as follows:
| For the six months ended September 30, |
||||||||
| 2023 (Unaudited) |
2022 (Unaudited) |
|||||||
| Customer A | 41.9 | % | 31.6 | % | ||||
| Customer B | 30.5 | % | 0 | % | ||||
| Customer C | 0 | % | 34.4 | % | ||||
As of September 30, 2023 and March 31, 2023, accounts receivable due from these customers as a percentage of consolidated accounts receivable balances were as follows:
| As of | ||||||||
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Customer A | 10.2 | % | 7.5 | % | ||||
| Customer B | 0.7 | % | 1.8 | % | ||||
| Customer C | 9.4 | % | 10.4 | % | ||||
F-
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Significant risks and uncertainties (continued)
During the six months ended September 30, 2023 and 2022, there were three and one suppliers which accounted for over 10% of total purchase for that period, respectively. The details are as follows:
| For the six months ended September 30, | ||||||||
| 2023 (Unaudited) |
2022 (Unaudited) |
|||||||
| Supplier A | 37.8 | % | 0.6 | % | ||||
| Supplier B | 18.5 | % | 5.5 | % | ||||
| Supplier C | 15.2 | % | 9.0 | % | ||||
| Supplier D | 0 | % | 59.2 | % | ||||
As of September 30, 2023 and March 31, 2023, accounts payable due to these suppliers as a percentage of consolidated accounts payable balances were as follows:
| As of | ||||||||
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Supplier A | 10.8 | % | 0 | % | ||||
| Supplier B | 8.5 | % | 6.0 | % | ||||
| Supplier C | 13.8 | % | 13.7 | % | ||||
| Supplier D | 17.6 | % | 17.9 | % | ||||
Recently issued accounting standards
The Jumpstart Our Business Startups Act (“JOBS Act”) provides that an emerging growth company (“EGC”) as defined therein can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an EGC to delay adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company has adopted the extended transition period.
The Company does not believe other recently issued but not yet effective accounting statements, if recently adopted, would have a material effect on the Company’s condensed consolidated balance sheets, statements of income (loss) and comprehensive income (loss) and statements of cash flows.
F-
NOTE 3 – ACCOUNTS RECEIVABLE, NET
Accounts receivable consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Accounts receivable, gross | $ | 2,770,243 | $ | 2,867,308 | ||||
| Less: allowance for doubtful accounts | (1,459,238 | ) | (1,522,739 | ) | ||||
| Accounts receivable, net | $ | 1,311,005 | $ | 1,344,569 | ||||
Changes of allowance for doubtful accounts for the six months ended September 30, 2023 and the year ended March 31, 2023 were as follows:
| For the six months ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Beginning balance | $ | 1,522,739 | $ | 762,992 | ||||
| Additional reserve through bad debt expense | 26,518 | 820,352 | ||||||
| Exchange rate difference | (90,019 | ) | (60,605 | ) | ||||
| Ending balance | $ | 1,459,238 | $ | 1,522,739 | ||||
For the six months ended September 30, 2023 and 2022, the Company record expected credit loss of $26,518 and Nil, respectively.
NOTE 4 – INVENTORIES
Inventories as of September 30, 2023 and March 31, 2023 consisted of the following:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Raw materials | $ | 251,059 | $ | 243,222 | ||||
| Finished goods | 391,662 | 446,866 | ||||||
| Provision for inventory | (149,507 | ) | (158,834 | ) | ||||
| Total inventories, net | $ | 493,214 | $ | 531,254 | ||||
Impairment provision of inventories recorded for lower of cost or net realizable value adjustments were $Nil and $Nil for the six months ended September 30, 2023 and 2022, respectively.
Inventory amounts recognized into cost of goods sold for the six months ended September 30, 2023 and 2022 were $444,030 and $613,801, respectively.
NOTE 5 – PREPAYMENTS, RECEIVABLES AND OTHER ASSETS
Prepayments, receivables and other assets consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Receivable from a third-party company | $ | 426,261 | $ | 473,237 | ||||
| Others | 164,082 | 230,642 | ||||||
| Total prepayments, receivables and other assets | 590,343 | 703,879 | ||||||
| Less: allowance for doubtful accounts | (452,365 | ) | (473,237 | ) | ||||
| Prepayments, receivables and other assets, net | $ | 137,978 | $ | 230,642 | ||||
F-
NOTE 5 – PREPAYMENTS, RECEIVABLES AND OTHER ASSETS (CONTINUED)
In June 2019, Taizhou Suxuantang entered into a limited partnership agreement with Huangshan Panjie Investment Management Co., Ltd. (the “Fund” or “Huangshan Panjie”). The company is committed to contribute $7 million (RMB50 million) into the Fund in two installments, with one installment of $3.5 million (RMB 25 million) made on June 14, 2019, and the second installment of $3.5 million (RMB 25 million) to be made no later than October 31, 2019. In June 2020, the Company agreed with the Fund, the GP and other limited partners to withdraw the installment of $3.5 million (RMB 25 million) made on June 14, 2019. For the years ended March 31, 2022 and 2021, the company received payment of $15,581 (RMB 100,000) and $3.1 million (RMB 21.25 million) from Huangshan Panjie, respectively. For the year ended March 31, 2023, the Company received payment of $58,381 (RMB 400,000) from Huangshan Panjie. For the six months ended September 30, 2023, the Company received payment of $19,639 (RMB 140,000) from Huangshan Panjie. The Company has made efforts to collect the remaining balance via court order. As September 30, 2023, the Company recorded bad debt expenses of $426,261 (RMB 3.11 million) for the remaining balance in full.
NOTE 6 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Machinery | $ | 729,968 | $ | 775,503 | ||||
| Vehicles | 215,734 | 236,268 | ||||||
| Electric equipment | 142,553 | 151,445 | ||||||
| Office equipment | 76,093 | 80,839 | ||||||
| Leasehold improvement | 1,551,362 | 1,641,060 | ||||||
| Total property plant and equipment, at cost | 2,715,710 | 2,885,115 | ||||||
| Less: accumulated depreciation | (1,906,671 | ) | (1,922,901 | ) | ||||
| Total property, plant and equipment, net | $ | 809,039 | $ | 962,214 | ||||
Depreciation expenses were $98,946 and $105,929 for the six months ended September 30, 2023 and 2022, respectively.
NOTE 7 – INTANGIBLE ASSETS, NET
Intangible assets consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Trademark | $ | 41,715 | $ | 44,317 | ||||
| Software | 32,772 | 34,817 | ||||||
| Total intangible assets, at cost | 74,487 | 79,134 | ||||||
| Less: accumulated amortization | (51,973 | ) | (51,266 | ) | ||||
| Total intangible assets, net | $ | 22,514 | $ | 27,868 | ||||
Amortization expenses were $3,804 and $4,037 for the six months ended September 30, 2023 and 2022, respectively.
F-
NOTE 8 – LONG-TERM DEPOSIT
Long-term deposit consisted of cash deposit of RMB 60 million the Company paid to one entity which the Company is seeking to acquire certain percentage of ownership (“Target Company”). The deposit was used as the acquisition deposit required by the Target Company in order to execute their respective acquisition memorandum which details the acquisition and valuation methods but is not legally binding. The fund pledged to the Target Company has no definite term, however the Company anticipates the detailed acquisition proposals will be presented to the Board of Directors and shareholders of the Company for voting within one year. In the case that the acquisition was approved by both parties, the deposit would be used as initial payment and offset the total cash consideration of the deal. If the acquisition failed to be approved, the Target Company is obligated to return the deposit to the Company. As of September 30, 2023, the acquisition was not approved or disapproved as the acquisition was still in the process of undergoing legal and financial due diligence. The Company evaluated the recoverability of this acquisition deposit and determined to write off the balance in full.
NOTE 9 – OPERATING LEASES
On January 1, 2018, the Company entered into a lease agreement with its related party company to obtain the right of use for office and warehouse of 3,627 square meters for 10 years for free. The Company recorded right-of-use assets and lease expenses based on the fair value for the lease. As the leases do not provide an implicit rate, the Company used an incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company’s lease agreements do not contain any material guarantees or restrictive covenants. The Company does not have any sublease activities.
Operating lease right-of-use assets as of September 30, 2023 and March 31, 2023 were as follows:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Office and warehouse* | $ | 339,890 | $ | 361,092 | ||||
| Less: accumulated amortization | (78,782 | ) | (55,042 | ) | ||||
| Total right-of-use assets, net | $ | 261,108 | $ | 306,050 | ||||
The Company recognized lease expenses for the operating lease right-of-use assets office and warehouse over the lease period which is 10 years. For the six months ended September 30, 2023 and 2022, the operating lease expenses were $35,098 and $37,170, respectively.
Operating lease liabilities as of September 30, 2023 and March 31, 2023 consisted of the following:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Office and warehouse | $ | 261,108 | $ | 306,050 | ||||
| Total operating lease liabilities, net | $ | 261,108 | $ | 306,050 | ||||
Analyzed for reporting purposes as:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Non-current portion of operating lease liabilities – related party | $ | 204,937 | $ | 247,961 | ||||
| Current portion of operating lease liabilities – related party | 56,171 | 58,089 | ||||||
| Total operating lease liabilities | $ | 261,108 | $ | 306,050 | ||||
F-
NOTE 9 – OPERATING LEASES (CONTINUED)
The discount rate used for the office and warehouse was 5.40%. The remaining lease term for the operating lease was 4.25 years.
Maturity analysis of operating lease liabilities as of September 30, 2023 was as follows:
| Office and warehouse | ||||||||
| RMB | USD | |||||||
| Discount rate at commencement | 5.40 | % | $ | 5.40 | % | |||
| One year | 500,400 | 68,586 | ||||||
| Two years | 500,400 | 68,586 | ||||||
| Three years | 500,400 | 68,586 | ||||||
| Four years | 500,400 | 68,586 | ||||||
| Five years and thereafter | 125,100 | 17,146 | ||||||
| Total undiscounted cash flows | 2,126,700 | $ | 291,490 | |||||
| Less: imputed interest | (221,653 | ) | (30,382 | ) | ||||
| Operating lease liabilities | 1,905,047 | 261,108 | ||||||
Maturity analysis of operating lease liabilities as of March 31, 2023 was as follows:
| Office and warehouse | ||||||||
| RMB | USD | |||||||
| Discount rate at commencement | 5.40 | % | $ | 5.40 | % | |||
| One year | 500,400 | 72,864 | ||||||
| Two years | 500,400 | 72,864 | ||||||
| Three years | 500,400 | 72,864 | ||||||
| Four years | 500,400 | 72,864 | ||||||
| Five years and thereafter | 375,300 | 54,648 | ||||||
| Total undiscounted cash flows | 2,376,900 | $ | 346,104 | |||||
| Less: imputed interest | (275,074 | ) | (40,054 | ) | ||||
| Operating lease liabilities | 2,101,826 | 306,050 | ||||||
The Company had no other operating or financing lease agreements or short-term leases, defined as leases with initial term of 12 months or less, for the six months ended September 30, 2023 and 2022.
NOTE 10 – BORROWINGS
Short-term borrowings
Short-term borrowings consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Short-term loans from related parties | $ | 89,090 | $ | 94,647 | ||||
| Short-term loans from third-party individuals | 190,516 | 72,806 | ||||||
| Total short-term borrowings | $ | 279,606 | $ | 167,453 | ||||
Short-term borrowings included loans from various individuals and entities that are unsecured. Short-term loans from related parties represents the loan of $89,090 the Company borrowed from Jun Zheng, which is valid from January 18, 2023 to January 17, 2024 and bear interest of 6%. Short-term loans from third-party individuals represents the loans of $190,516 the Company borrowed from four unaffiliated individuals which had no fixed borrowing period and bear interest of 6%.
F-
NOTE 10 – BORROWINGS (CONTINUED)
Short-term borrowings (continued)
As of September 30, 2023 and March 31, 2023, the total amount of these loans was $279,606 and $167,453, respectively. The Company recorded interest expenses of $5,822 and $0 on these short-term loans for the six months ended September 30, 2023 and 2022, respectively.
Long-term borrowings
Long-term borrowings consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Car loans | ||||||||
| Current portion | $ | 7,401 | $ | 7,863 | ||||
| Non-current portion | 24,338 | 30,495 | ||||||
| Total car loans | $ | 31,739 | $ | 38,358 | ||||
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Bank loans | ||||||||
| Current portion | $ | 12,336 | $ | |||||
| Non-current portion | 82,237 | 87,367 | ||||||
| Total long-term bank loans | $ | 94,573 | $ | 87,367 | ||||
Long-term bank loans are loans the Company borrowed from Jiangsu Taizhou Rural Commercial Bank which are valid from November 2, 2022 to November 1, 2025 and bear interest of 6%. As of September 30, 2023 and March 31, 2023, the total amount of these loans was $94,573 and $87,367, respectively. The Company recorded interest expenses for the bank loans of $2,946 and $0 for the six months ended September 30, 2023 and 2022, respectively.
As of September 30, 2023, one car loan of $31,739 at 18% annual interest rate is valid from February 1, 2023 to January 30, 2028. The car was pledged as collateral for the loan until full settlement. The Company recorded interest expense for the car loan of $682 and $0 for six months ended September 30, 2023 and 2022, respectively.
F-
NOTE 10 – BORROWINGS (CONTINUED)
Long-term borrowings (continued)
Future loan payments as of September 30, 2023 is as follows:
| RMB | USD | |||||||
| One year | 2,094,000 | $ | 287,007 | |||||
| Two years | 54,000 | 7,401 | ||||||
| Three years | 654,000 | 89,638 | ||||||
| Four years | 54,000 | 7,401 | ||||||
| Five years and thereafter | 15,570 | 2,135 | ||||||
| Total payment | 2,871,570 | $ | 393,582 | |||||
Future loan payments as of March 31, 2023 is as follows:
| RMB | USD | |||||||
| One year | 1,204,000 | $ | 175,316 | |||||
| Two years | 54,000 | 7,863 | ||||||
| Three years | 654,000 | 95,230 | ||||||
| Four years | 54,000 | 7,863 | ||||||
| Five years and thereafter | 47,430 | 6,553 | ||||||
| Total payment | 2,013,430 | $ | 293,178 | |||||
NOTE 11 – CONVERTIBLE NOTES
The Convertible Note 2022-1
On March 16, 2022, the Company entered into a securities purchase agreement with an institutional investor pursuant to which the Company issued an unsecured convertible promissory note with a 12-months maturity (the “Convertible Note 2022-1”) to the investor. The Convertible Note 2022-1 has the original principal amount of $2,804,848 including the original issue discount of $168,291 and the investor’s legal and other transaction costs of $20,000. The Company anticipates using the proceeds for general working capital purposes.
Material Terms of the Convertible Note 2022-1:
| ● | Interest accrues on the outstanding balance of the Note at 6% per annum from the purchase price date until the same is paid in full. All interest calculations hereunder shall be computed on the basis of a 360-day year comprised of twelve (12) thirty (30) day months, shall compound daily and shall be payable in accordance with the terms of this Note. |
| ● | Upon the occurrence of a trigger event, the investor may increase the outstanding balance payable under the Note by 12% or 5%, depending on the nature of such event. If the Company fails to cure the trigger event within the required five trading days, the trigger event will automatically become an event of default and interest will accrue at the lesser of 22% per annum or the maximum rate permitted by applicable law. |
| ● | Subject to adjustment as set forth in this Note, the price at which the lender has the right to convert all or any portion of the outstanding balance into ordinary shares is $0.30 per share. |
F-
NOTE 11 – CONVERTIBLE NOTES (CONTINUED)
The Convertible Note 2022-1 (continued)
In accounting for the issuance of the Convertible Note 2022-1 under ASU 2020-06, the Company recorded the convertible note as a single liability in its entirety according to the new framework. Debt issuance costs related to the Convertible Note 2022-1 comprised of commissions paid to third party placement agents, lawyers, and warrants value of $448,291. Issuance costs attributable to the liability component will be amortized to interest expense using the effective interest method over the contractual term. The effective interest rate for the Convertible Note 2022-1 is 26.16%.
For the year ended March 31, 2023, the Company issued 4,748,930 ordinary shares with a fair value of $2,906,511 for principal and interest settlement of the Convertible Note 2022-1.
The Convertible Note 2022-2
On December 19, 2022, the Company entered into a securities purchase agreement with Streeterville Capital, LLC, pursuant to which the Company issued the investor an unsecured promissory note on December 19, 2022 in the original principal amount of $1,595,000 (the “Convertible Note 2022-2”), convertible into ordinary shares, $0.08 par value per share, of the Company for $1,500,000 in gross proceeds. The Company anticipates using the proceeds for general working capital purposes.
Material Terms of the Convertible Note 2022-2:
| ● | Interest accrues on the outstanding balance of the Note at 6% per annum from the purchase price date until the same is paid in full. All interest calculations hereunder shall be computed on the basis of a 360-day year comprised of twelve (12) thirty (30) day months, shall compound daily and shall be payable in accordance with the terms of this Note. |
| ● | Upon the occurrence of a trigger event, the investor may increase the outstanding balance payable under the Note by 15% or 5%, depending on the nature of such event. If the Company fails to cure the trigger event within the required five trading days, the trigger event will automatically become an event of default and interest will accrue at the lesser of 15% per annum or the maximum rate permitted by applicable law. |
| ● | Subject to adjustment as set forth in this Note, the price at which the lender has the right to convert all or any portion of the outstanding balance into ordinary shares is lower of (i) the Lender Conversion Price which is initially $0.60 and (ii) 80% of the average of the lowest VWAP during the fifteen (15) trading days immediately preceding the redemption notice is delivered. |
In accounting for the issuance of the Convertible Note 2022-2 under ASU 2020-06, the Company recorded the convertible note as a single liability in its entirety according to the new framework. Debt issuance costs related to the Convertible Note 2022-2 comprised of commissions paid to third party placement agents, lawyers, and warrants value of $220,035. Issuance costs attributable to the liability component will be amortized to interest expense using the effective interest method over the contractual term. The effective interest rate for the Convertible Note 2022-2 is 20.56%.
F-
NOTE 11 – CONVERTIBLE NOTES (CONTINUED)
The Convertible Note 2023
On March 7, 2023, the Company entered into a securities purchase agreement with Streeterville Capital, LLC, pursuant to which the Company issued the investor an unsecured promissory note on March 7, 2023 in the original principal amount of $2,126,667 (the “Convertible Note 2023”), convertible into ordinary shares, $0.08 par value per share, of the Company for $2,000,000 in gross proceeds. The Company anticipates using the proceeds for general working capital purposes.
Material Terms of the Convertible Note 2023:
| ● | Interest accrues on the outstanding balance of the Note at 6% per annum from the purchase price date until the same is paid in full. All interest calculations hereunder shall be computed on the basis of a 360-day year comprised of twelve (12) thirty (30) day months, shall compound daily and shall be payable in accordance with the terms of this Note. |
| ● | Upon the occurrence of a trigger event, the investor may increase the outstanding balance payable under the Note by 15% or 5%, depending on the nature of such event. If the Company fails to cure the trigger event within the required five trading days, the trigger event will automatically become an event of default and interest will accrue at the lesser of 15% per annum or the maximum rate permitted by applicable law. |
| ● | Subject to adjustment as set forth in this Note, the price at which the lender has the right to convert all or any portion of the outstanding balance into ordinary shares is lower of (i) the Lender Conversion Price which is initially $0.60 and (ii) 80% of the average of the lowest VWAP during the fifteen (15) trading days immediately preceding the redemption notice is delivered. |
In accounting for the issuance of the Convertible Note 2023 under ASU 2020-06, the Company recorded the convertible note as a single liability in its entirety according to the new framework. Debt issuance costs related to the Convertible Note 2023 comprised of commissions paid to third party placement agents, lawyers, and warrants value of $211,702. Issuance costs attributable to the liability component will be amortized to interest expense using the effective interest method over the contractual term. The effective interest rate for the Convertible Note 2023 is 16.26%.
For the year ended March 31, 2023, the Company issued 987,881 ordinary shares with a fair value of $225,000 for principal and interest partial settlement of the Convertible Note 2023.
For the six months ended September 30, 2023, the Company issued 6,762,138 ordinary shares with a fair value of $945,000 for principal and interest partial settlement of the Convertible Note 2023.
Net carrying amount of the liability component Convertible Notes dated as of September 30, 2023 were as follows:
| Principal outstanding |
Unamortized issuance cost |
Net carrying value |
||||||||||
| Convertible Note – 2022-2 | $ | 1,595,000 | $ | (52,858 | ) | $ | 1,542,142 | |||||
| Convertible Note – 2023 | 986,061 | (32,019 | ) | 954,042 | ||||||||
| Total | $ | 2,581,061 | $ | (84,877 | ) | $ | 2,496,184 | |||||
Net carrying amount of the liability component Convertible Notes dated as of March 31, 2023 were as follows:
| Principal outstanding |
Unamortized issuance cost |
Net carrying value |
||||||||||
| Convertible Note – 2022-2 | $ | 1,595,000 | $ | (163,967 | ) | $ | 1,431,033 | |||||
| Convertible Note – 2023 | 1,902,639 | (176,180 | ) | 1,726,459 | ||||||||
| Total | $ | 3,497,639 | (340,147 | ) | $ | 3,157,492 | ||||||
F-
NOTE 11 – CONVERTIBLE NOTES (CONTINUED)
The Convertible Note 2023 (continued)
Amortization of issuance cost, debt discount and interest cost for the six months ended September 30, 2023 were as follows:
Accretion |
Convertible note interest |
Total | ||||||||||
| Convertible Note – 2022-2 | 111,108 | 49,538 | 160,646 | |||||||||
| Convertible Note – 2023 | 101,600 | 65,198 | 166,798 | |||||||||
| Total | $ | 212,708 | $ | 114,736 | $ | 327,444 | ||||||
Amortization of issuance cost, debt discount and interest cost for the six months ended September 30, 2022 were as follows:
| Accretion of debt discount |
Convertible note interest |
Total | ||||||||||
| Convertible Note – 2022-1 | $ | 311,642 | $ | 72,880 | $ | 384,522 | ||||||
NOTE 12 – REFUND LIABILITY
Refund liabilities represents the accrued liability for sales return based on the sales and the Company’s estimate of sale return rate.
Estimates of discretionary authorized returns, discounts and claims are based on (1) historical rates, (2) specific identification of outstanding returns not yet received from customers and outstanding discounts and claims and (3) estimated returns, discounts and claims expected, but not yet finalized with customers. Actual returns, discounts and claims in any future period are inherently uncertain and thus may differ from estimates recorded. If actual or expected future returns, discounts or claims were significantly greater or lower than the reserves established, a reduction or increase to net revenues would be recorded in the period in which such determination was made.
The estimated cost of inventory for product returns of $60,649 and $25,962, respectively, were recorded in Inventory on the condensed consolidated balance sheets as of September 30, 2023 and March 31, 2023.
NOTE 13 – ACCRUED EXPENSES AND OTHER LIABILITIES
Accrued expenses and other liabilities consisted of the following as of September 30, 2023 and March 31, 2023:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Accrued payroll and welfare | $ | 758,960 | $ | 622,526 | ||||
| Other payable for leasehold improvements | 1,169,904 | 1,403,258 | ||||||
| Accrued professional service expenses | 347,541 | 327,068 | ||||||
| Other current liabilities | 972,738 | 789,232 | ||||||
| Total | $ | 3,249,143 | $ | 3,142,084 | ||||
As of September 30, 2023 and March 31, 2023, the balances of other current liabilities $972,738 and $789,232, respectively, represented amounts due to staff reimbursement and third-party companies, and interest payable for convertible notes.
F-
NOTE 14 – SHAREHOLDERS’ EQUITY
Ordinary shares
The Company is authorized to issue unlimited shares of $0.001 par value common stock. On July 4, 2017, and October 20, 2017, the Company issued common stocks of an aggregate of 20,000,000 shares of $0.001 par value (10,000 shares of $2 par value retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) to thirteen shareholder, three among whom together hold 100% shares of Suxuantang and over 50% shares of SXT. In connection with Restructuring, all shares and per share amounts have been retroactively restated as if the aforementioned transactions had become effective as of the beginning of the first period presented in the accompanying condensed consolidated financial statements.
On December 31, 2018, the Company completed the closing of its initial public offering of 2,506,300 ordinary shares at a public offering price of $4.00 per ordinary share (1,253 ordinary shares at a price of $8,000 per ordinary share retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023). On January 3, 2019, the Company sold an additional 39,975 ordinary shares at the public offering price of $4.00 per share (20 ordinary shares at a price of $8,000 per ordinary share retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) in a second closing. The total gross proceeds from the initial public offering were approximately $10.2 million before underwriting commissions and offering expenses. On January 10, 2019, the underwriter exercise the warrants in connection with the initial public offering and 160,426 shares (80 ordinary shares retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) were newly issued.
For the year ended March 31, 2020, 11,961,006 ordinary shares (5,980 ordinary shares retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) were issued with a fair value of $6,425,657 for convertible notes principal and interest partial settlement.
For the year ended March 31, 2021, 27,389,877 ordinary shares (13,695 ordinary shares retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) were issued with a fair value of $7,680,791 for convertible notes principal and interest partial settlement.
For the year ended March 31, 2023, 5,736,811 ordinary shares (229,472 ordinary shares retrospectively restated for effect of reverse stock split on October 5, 2023) were issued with a fair value of $3,131,511 for convertible notes principal and interest partial settlement.
For the six months ended September 30, 2023, the Company issued 6,762,138 ordinary shares (270,486 ordinary shares retrospectively restated for effect of reverse stock split on October 5, 2023) with a fair value of $945,000 for principal and interest partial settlement.
Warrant
In connection with the certain convertible notes issued on May 2, 2019, the Company issued a warrant on January 18, 2021 to Mr. Jian Ke for purchase of 1,000,000 ordinary shares (500 ordinary shares retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) (the “warrants”). The warrants carry a term of four years and shall be exercisable at $0.3843 per share ($768.6 per share retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023). Management determined that the warrants are equity instruments because the warrants are both a) indexed to its own stock; and b) classified in stockholders’ equity. The warrants were recorded at their fair value on the date of grant as a component of stockholders’ equity. As of September 30, 2023 and March 31, 2023, the total number of warrants outstanding was 250,000 (500 retrospectively restated for effect of reverse stock split on February 22, 2021, May 19, 2022 and October 5, 2023) with weighted average remaining life of 2.33 years and 2.83 years, respectively.
The fair value of this Warrants was $509,000. The fair value has been estimated using the Black Scholes pricing model with the following weighted-average assumptions: risk free rate of 0.33%; expected term of 4 years; exercise price of the warrants of $1.5372; volatility of 131.84%; and expected future dividends of Nil.
F-
.
NOTE 14 – SHAREHOLDERS’ EQUITY (CONTINUED)
2021 Reverse stock split
On January 23, 2021, the Company’s board of directors approved to effect a one-for-four reverse stock split of its ordinary shares (the “2021 Reverse Stock Split”) with the market effective on February 22, 2021, such that the number of the Company’s authorized preferred and ordinary shares is unchanged, which will remain as unlimited, and the par value of each ordinary share is increased from US$0.001 to US$0.004. As a result of the 2021 Reverse Stock Split, each four pre-split ordinary shares outstanding were automatically combined and converted to one issued and outstanding ordinary share without any action on the part of the shareholder. No fractional ordinary shares were issued to any shareholders in connection with the 2021 reverse stock split. Each shareholder was entitled to receive one ordinary share in lieu of the fractional share that would have resulted from the reverse stock split. As of February 21, 2021 (immediately prior to the effective date), there were 62,057,584 ordinary shares outstanding, and the number of ordinary shares outstanding after the 2021 Reverse Stock Split is 15,525,094, taking into account of the effect of rounding fractional shares into whole shares (31,050 shares retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023). In addition, all options and any other securities of the Company outstanding immediately prior to the 2021 Reverse Stock Split (to the extent they don’t provide otherwise) will be appropriately adjusted by dividing the number of ordinary shares into which the options and other securities are exercisable by 4 and multiplying the exercise price thereof by 4, as a result of the 2021 Reverse Stock Split.
2021 Equity incentive plan
In September 2021, the Company adopted a share incentive plan (the “2021 Equity Incentive Plan”), which provides for the granting of share incentives, including incentive share options (“ISOs”), restricted shares and any other form of award pursuant to the Equity Incentive Plan, to members of the board, and employees of the Company. The Company reserved 2,325,000 ordinary shares (4,650 shares retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023) for the 2021 Equity Incentive Plan. The vesting schedule, time and condition to exercise options is determined by the Company’s compensation committee. The term of the options may not exceed ten years from the date of the grant.
Under the 2021 Equity Incentive Plan, the exercise price of an option may be amended or adjusted at the discretion of the compensation committee, the determination of which would be final, binding and conclusive. If the Company grants an ISO to an employee who, at the time of that grant, owns shares representing more than 10% of the voting power of all classes of the Company’s share capital, the exercise price cannot be less than 110% of the fair market value of the Company’s ordinary shares on the date of that grant.
Pursuant to the 2021 Equity Incentive Plan, the Company issued 2,325,000 ordinary shares (4,650 shares retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023) to its management. The fair value of shares issued pursuant to the 2021 Equity Incentive Plan of $2,334,397 (deducted by legal expenses of $30,000) had been determined using the average share price for the dates of issuance ($0.9911 per ordinary share, $495.57 per ordinary share retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023).
F-
NOTE 14 – SHAREHOLDERS’ EQUITY (CONTINUED)
The 2022 Public Offering
On January 18, 2022, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Aegis Capital Corp. (the “Underwriter”), pursuant to which the Company agreed to sell to the Underwriter, in a firm commitment public offering (the “2022 Public Offering”) (i) 8,285,260 ordinary shares (the “Firm Shares”) of the Company, par value $0.004 per share, for a public offering price of $0.18 per share, (ii) 11,521,500 pre-funded warrants (the “Pre-funded Warrants”) to purchase 11,521,500 shares (the “Warrant Shares”), for a public offering price of $0.17 per Pre-funded Warrant to those purchasers whose purchase of ordinary shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the holder, 9.99%) of the Company’s outstanding ordinary shares immediately following the consummation of this Offering (the “2022 Public Offering”). The Company also granted the Underwriter an over-allotment option to purchase up to 2,971,014 ordinary shares (the “Option Shares”, together with Firm Shares, the “Shares”).
The Pre-funded Warrants have an exercise price of $0.01 per share. The Pre-funded Warrants were issued in registered form under a warrant agent agreement (the “Warrant Agent Agreement”) between the Company and TranShare Corporation as the warrant agent. The Underwriter exercised its over-allotment in full for purchase of all the Option Shares. The Company expects to receive approximately $3,984,784 in gross proceeds from this Offering, assuming no Pre-funded Warrants are exercised, before deducting underwriting discounts and other related offering expenses. As of February 8, 2022, the investors have exercised all the Pre-funded Warrants to purchase 11,521,500 ordinary shares.
Pursuant to the 2022 Public Offering, the Company issued 22,777,774 ordinary shares at price of $0.18 per share (45,556 ordinary shares at price of $90 per share retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023) to the investors. The total gross proceeds from the 2022 Public Offering were approximately $4.1 million before underwriting commissions and offering expenses. The total net proceeds from the 2022 Public Offering were approximately $3.1 million after underwriting commissions and offering expenses.
2022 Equity incentive plan
In March 2022, the Company adopted a share incentive plan (the “2022 Equity Incentive Plan”), which provides for the granting of share incentives, including incentive share options (“ISOs”), restricted shares and any other form of award pursuant to the Equity Incentive Plan, to members of the board, and employees of the Company. The vesting schedule, time and condition to exercise options is determined by the Company’s compensation committee. The term of the options may not exceed ten years from the date of the grant.
Under the 2022 Equity Incentive Plan, the exercise price of an option may be amended or adjusted at the discretion of the compensation committee, the determination of which would be final, binding and conclusive. If the Company grants an ISO to an employee who, at the time of that grant, owns shares representing more than 10% of the voting power of all classes of the Company’s share capital, the exercise price cannot be less than 110% of the fair market value of the Company’s ordinary shares on the date of that grant.
Pursuant to the 2022 Equity Incentive Plan, the Company issued 6,094,180 ordinary shares (12,188 shares retrospectively restated for effect of reverse stock split on May 19, 2022 and October 5, 2023) to its management on May 15, 2022.
F-
NOTE 14 – SHAREHOLDERS’ EQUITY (CONTINUED)
2022 Reverse stock split
On May 10, 2022, the Company’s board of directors approved to effect a one-for-twenty reverse stock split of its ordinary shares (the “2022 Reverse Stock Split”) with the market effective on May 19, 2022, such that the number of the Company’s authorized preferred and ordinary shares is unchanged, which will remain as unlimited, and the par value of each ordinary share is increased from US$0.004 to US$0.08. As a result of the 2022 Reverse Stock Split, each twenty pre-split ordinary shares outstanding were automatically combined and converted to one issued and outstanding ordinary share without any action on the part of the shareholder. No fractional ordinary shares were issued to any shareholders in connection with the 2022 reverse stock split. Each shareholder was entitled to receive one ordinary share in lieu of the fractional share that would have resulted from the reverse stock split. As of May 16, 2022 (immediately prior to the effective date), there were 40,627,868 ordinary shares outstanding, and the number of ordinary shares outstanding after the 2022 Reverse Stock Split is 2,042,673 (81,707 shares retrospectively restated for effect of reverse stock split on October 5, 2023), taking into account of the effect of rounding fractional shares into whole shares. In addition, all shares, options and any other securities of the Company outstanding immediately prior to the 2022 Reverse Stock Split was retroactively applied by dividing the number of ordinary shares into which the options and other securities are exercisable by 20 and multiplying the exercise price thereof by 20, as a result of the 2022 Reverse Stock Split.
Securities purchase agreement
On September 22, 2022, the Company entered into certain securities purchase agreement with Zhijun Xiao, a non-affiliate non-U.S. person, pursuant to which Mr. Xiao agreed to purchase 1,625,798 ordinary shares of the Company, par value $0.08 per share at a per share purchase price of $1.35 (65,032 ordinary shares of the Company, par value $2 per share at a per share purchase price of $33.75 retrospectively restated for effect of reverse stock split on October 5, 2023). The agreement was executed on October 11, 2022 and the gross proceeds of this transaction were $2,194,827.
On February 22, 2023, the Company entered into certain securities purchase agreement with Rising Sun Capital Ltd., a limited liability company organized under the laws of Australia, pursuant to which the investor agreed to purchase 1,724,138 ordinary shares of the Company, par value $0.08 per share, at a per share purchase price of $0.58 (68,966 ordinary shares of the Company, par value $2 per share at a per share purchase price of $14.5 retrospectively restated for effect of reverse stock split on October 5, 2023). The gross proceeds of this transaction are approximately $1 million. Upon the issuance date of these condensed consolidated financial statements, the transaction has not been closed and the Company has not received the proceeds. Management assessed the likelihood of whether the Company was able to close this deal and expected to receive the proceeds before September 30, 2024.
NOTE 15 – INCOME TAXES
| (a) | Corporate Income Taxes |
Under the current laws of the British Virgin Islands (“BVI”), the Company is not subject to tax on its income or capital gains. In addition, upon payments of dividends by the Company to its shareholders, no BVI withholding tax is imposed. The Company’s subsidiaries incorporated in Hong Kong were subject to the Hong Kong profits tax rate at 16.5% for the six months ended September 30, 2023 and 2022. The Company’s subsidiaries and VIE incorporated in China were subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws. The EIT rate for companies operating in the PRC is 25% for the six months ended September 30, 2023 and 2022, except for Taizhou Suxuantang where the applicable income tax rate is 15% for the six months ended September 30, 2023 and 2022, since it was qualified as a high-technology company from January 1, 2011 to December 31, 2023. In addition, the Company is allowed to deduct additional 100% of its research and development expenses against its pre-tax income as a high-technology company.
F-
NOTE 15 – INCOME TAXES (CONTINUED)
For the six months ended September 30, 2023 and 2022, income tax expenses consisted of the following:
| For the six months ended September 30, |
||||||||
| 2023 (Unaudited) |
2023 (Unaudited) |
|||||||
| Current income tax provision | $ | - | $ | - | ||||
| Deferred income tax provision | - | - | ||||||
| Total income tax expense | $ | - | $ | - | ||||
| (b) | Deferred Tax Assets |
Deferred income tax was measured using the enacted income tax rates for the periods in which they are expected to be reversed. Significant components of the Company’s deferred income tax assets and liabilities consist of follows:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Tax loss carry forward | $ | 652,985 | $ | 552,828 | ||||
| Allowance for doubtful account - accounts receivable | 218,886 | 228,944 | ||||||
| Allowance for doubtful account – prepayment, receivable and other current assets | 67,855 | 71,151 | ||||||
| Impairment provision for inventory | 22,426 | 23,881 | ||||||
| Allowance for deferred tax assets | (962,152 | ) | (876,804 | ) | ||||
| Total | $ | $ | ||||||
The Company evaluates the level of authority for each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measures the unrecognized benefits associated with the tax positions. For the six months ended September 30, 2023 and 2022, the Company had no unrecognized tax benefits. The Company does not anticipate any significant increase to its asset for unrecognized tax benefit within the next 12 months. The Company will classify interest and penalties related to income tax matters, if any, in income tax expense.
NOTE 16 – RELATED PARTY TRANSACTIONS
Nature of relationships with related parties
| Name of related parties | Relationship with the Company | |
| Feng Zhou | Major shareholder of the Company, Chief Executive Officer, Interim Chief Financial Officer and Director of the Company | |
| Jianping Zhou | Father of major shareholders of the Company and two of Taizhou Suxuantang shareholders, controlling shareholder of Taizhou Suxuantang from its inception to May 8, 2017 (decreased from death) | |
| Zhijun Xiao | Director of the Company | |
| Jun Zheng | Director of the Company | |
| Xiaodong Ji | Independent Director of the Company | |
| Xiaodong Pan | Chief Financial Officer | |
| Taizhou Jiutian Pharmaceutical Co. Ltd. | An entity controlled by Jianping Zhou | |
| Jiangsu Health Pharmaceutical Investment Co., Ltd. | An entity controlled by Jianping Zhou | |
| Taizhou Su Xuan Tang Chinese Medicine Clinic | An entity controlled by Jianping Zhou | |
| Taizhou Su Xuan Tang Chinese hospital Co., Ltd. | An entity controlled by Jianping Zhou | |
| Jiangsu Sutaitang Online Commercial Co., Ltd. | An entity controlled by Xiaodong Ji |
F-
NOTE 16 – RELATED PARTY TRANSACTIONS (CONTINUED)
Related party balances
The amounts due to related parties as of September 30, 2023 and March 31, 2023 were as follows:
| September 30, 2023 (Unaudited) |
March 31, 2023 |
|||||||
| Jiangsu Health Pharmaceutical Investment Co., Ltd. | $ | $ | 2,910,088 | |||||
| Feng Zhou | 421,168 | 1,823,679 | ||||||
| Jiangsu Sutaitang Online Commercial Co., Ltd. | 320,202 | |||||||
| Xiaodong Pan | 73,110 | |||||||
| Zhijun Xiao | 62,658 | |||||||
| Jun Zheng | 14,025 | |||||||
| Total | $ | 421,168 | $ | 5,203,762 | ||||
Related party transactions
For the six months ended September 30, 2023 and 2022, the Company generated revenues of Nil and $2,297, respectively, from sales transactions with Taizhou Jiutian Pharmaceutical Co. Ltd.
For the six months ended September 30, 2023 and 2022, the Company generated revenues of $2,244 and $8,396, respectively, from sales transactions with Taizhou Su Xuan Tang Chinese hospital Co. Ltd.
For the six months ended September 30, 2023 and 2022, the Company generated revenue of $10,534 and $5,787, respectively, from sales transactions with Taizhou Su Xuan Tang Chinese Medicine Clinic.
For the six months ended September 30, 2023, the Company repaid $4,582,113 to Jiangsu Health Pharmaceutical Investment Co., Ltd., Feng Zhou and other related parties. For the six months ended September 30, 2022, the Company repaid $11,093,857 to Jiangsu Health Pharmaceutical Investment Co., Ltd., Feng Zhou and other related parties.
On January 1, 2018, the Company entered into a lease agreement with Jiangsu Health Pharmaceutical Investment Co., Ltd. to obtain the right of use for office and warehouse of 3,627 square meters for 10 years for free. The Company recorded right-of-use assets and lease expenses based on the fair value for the lease. For the six months ended September 30, 2023, the Company recorded operating lease expenses of $35,098 and $37,170, respectively.
Guarantee
For the six months ended September 30, 2023 and 2022, Taizhou Suxuantang signed several financial guarantee agreements for its related parties. Details of the financial guarantee agreements, please refer to Note 17.
F-
NOTE 17 – GUARANTEE
On April 12, 2021, Taizhou Suxuantang signed a financial guarantee agreement with Jiangsu Changjiang Commercial Bank for Taizhou Jiutian Pharmaceutical Co. Ltd. in borrowing of $383,772 (equivalent of RMB 2,800,000) for three-year period. Taizhou Suxuantang is obliged to pay on behalf of the related party the principal, interest, penalty and other expenses if Taizhou Jiutian Pharmaceutical Co. Ltd. defaults in payment. The Company did not charge financial guarantee fees over Taizhou Jiutian Pharmaceutical Co. Ltd.
On October 28, 2013, Taizhou Suxuantang signed a financial guarantee agreement with Fenlan Xu for Jianping Zhou in borrowing of $794,956 (equivalent of RMB 5,800,000) for an unlimited period. Taizhou Suxuantang is obliged to pay the amount if Jianping Zhou in default of the payment of principal and interests. Since Jianping Zhou deceased after yearend, Taizhou Suxuantang should bear all the risk for the repayment. However, subsequent to yearend, Taizhou Jiutian Pharmaceutical Co. Ltd. signed an agreement with Taizhou Suxuantang to take all the responsibility and obligation for repay the amount borrowed from Fenlan Xu on behalf of Jianping Zhou. This additional agreement releases Taizhou Suxuantang from future obligation in regard to the guarantee agreement. The Company did not charge financial guarantee fees over Jianping Zhou. Taizhou Juiutian Pharmaceutical Co. Ltd. is fully obliged to pay the principal, interests from January 1, 2021 to the actual date of repayment, including penalty and other expenses. As such, the Company expects no liabilities from the financial guarantee.
The Company has not made any payment under the above guarantee agreements for the six months ended September 30, 2023 and 2022.
NOTE 18 – COMMITMENT
In the ordinary course of business, the Company is involved in various legal proceedings, claims and other disputes arising from commercial operations, employees, and other matters which, in general, are subject to uncertainties and in which the outcomes are not predictable. The Company determines whether an estimated loss from a contingency should be accrued by assessing whether a loss is deemed probable and can be reasonably estimated. Although the Company can give no assurances about the resolution of pending claims, litigation or other disputes and the effect such outcomes may have on the Company, the Company believes that any ultimate liability resulting from the outcome of such proceedings to the extent not otherwise provided or covered by insurance, will not have a material adverse effect on our consolidated financial position or results of operations or liquidity. As of September 30, 2023 and March 31, 2023, and through the issuance date of these consolidated financial statements, the Company had no pending legal proceedings.
NOTE 19 – SUBSEQUENT EVENTS
2023 Reverse stock split
The Company effected a one-for-twenty-five (1-25) reverse split of its Ordinary Shares. The reverse stock split became effective on Thursday, October 5, 2023, such that the number of the Company’s authorized preferred and ordinary shares is unchanged, which will remain as unlimited, and the par value of each ordinary share is increased from US$0.08 to US$2. In addition, all shares, options and any other securities of the Company outstanding immediately prior to the reverse stock split were retroactively applied by dividing the number of ordinary shares into which the options and other securities are exercisable by 25 and multiplying the exercise price thereof by 25, as a result of the reverse stock split.
2024 Equity incentive plan
In January 2024, the Company adopted a share incentive plan (the “2024 Equity incentive plan”), which provides for the granting of share incentives, including incentive share options, restricted shares and any other form of award pursuant to the 2024 Equity Incentive Plan, to members of the board, and employees of the Company. The vesting schedule, time and condition to exercise options is determined by the Company’s compensation committee. The term of the options may not exceed ten years from the date of the grant.
Under the 2024 Equity incentive plan, the exercise price of an option may be amended or adjusted at the discretion of the compensation committee, the determination of which would be final, binding and conclusive. If the Company grants an ISO to an employee who, at the time of that grant, owns shares representing more than 10% of the voting power of all classes of the Company’s share capital, the exercise price cannot be less than 110% of the fair market value of the Company’s ordinary shares on the date of that grant.
Pursuant to the 2024 Equity incentive plan, the Company issued 185,316 ordinary shares to its management on January 25, 2024.
The Company evaluated all events and transactions that occurred after September 30, 2023 up through the date the Company issued these financial statements on April 4, 2024 and concluded that no other material subsequent events except for the disclosed above.
F-