UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): March 14, 2024
NeuroOne Medical Technologies Corporation
(Exact name of registrant as specified in its charter)
| Delaware | 001-40439 | 27-0863354 | ||
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
7599 Anagram Dr., Eden Prairie, MN 55344
(Address of principal executive offices and zip code)
952-426-1383
(Registrant’s telephone number including area code)
(Registrant’s former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per share | NMTC | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging Growth Company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 5.07. Submission of Matters to a Vote of Security Holders.
At the annual meeting (the “Annual Meeting”) of stockholders of NeuroOne Medical Technologies Corporation (the “Company”) on March 14, 2024, stockholders (i) elected one Class I director to the Company’s Board of Directors, to serve a three-year term until the 2027 annual meeting of stockholders, (ii) ratified the appointment of Baker Tilly U.S., LLP as the Company’s independent registered public accounting firm for the fiscal year ending September 30, 2024, (iii) approved the Company’s named executive officers’ compensation in an advisory vote, and (iv) approved, on an advisory basis, to conduct an advisory vote on the frequency of future advisory votes on executive compensation every three years. Proposals are described in detail in the Company’s definitive proxy statement filed with the Securities and Exchange Commission on January 29, 2024.
A total of 15,782,424 shares of the Company’s common stock were present at the meeting in person or by proxy, which represents approximately 60.89% of the shares of common stock outstanding as of the record date for the Annual Meeting.
The results of the voting are shown below:
Proposal 1—Election of Directors
| Class I Nominee | Votes For | Votes Withheld | Broker Non-Votes |
|||
| Paul Buckman | 5,804,908 | 1,363,109 | 8,614,407 |
Proposal 2—Ratification of Appointment of Independent Registered Public Accounting Firm
| Votes For | Votes Against | Votes Abstain | ||
| 15,736,069 | 36,991 | 9,364 |
Proposal 3—Approval of the Company’s Named Executive Officers’ Compensation in an Advisory Vote
| Votes For | Votes Against | Votes Abstain | ||
| 6,586,582 | 330,506 | 250,929 |
Proposal 4—Advisory Approval of the Frequency of an Advisory Vote on Named Executive Officers Compensation
| One Year | Two Years | Three Years | Votes Abstain | |||
| 3,060,695 | 144,245 | 3,723,836 | 239,241 |
For Proposal 4, “every three years” received the affirmative vote of the holders of a majority of the voting power of shares present, in person or represented by proxy, at the 2024 annual meeting of stockholders and entitled to vote. In light of such result, the Company’s Board of Directors has determined that the Company will implement an advisory vote on executive officer compensation every three years until the next required advisory vote on such frequency.
Item 7.01 Regulation FD Disclosure
On March 15, 2024, the Company posted an updated corporate presentation to its website at https://nmtc1.com/investors, which the Company may use from time to time in communications or conferences. A copy of the corporate presentation is attached as Exhibit 99.1 to this Current Report.
The information in this Current Report, including Exhibit 99.1 hereto, is furnished pursuant to Item 7.01 and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such a filing. The Company’s submission of this Current Report shall not be deemed an admission as to the materiality of any information required to be disclosed solely to satisfy the requirements of Regulation FD.
This Current Report and Exhibit 99.1 hereto contain forward-looking statements within the meaning of the federal securities laws. These forward-looking statements are based on current expectations and are not guarantees of future performance. Further, the forward-looking statements are subject to the limitations listed in Exhibit 99.1 and in the other reports of the Company filed with the Securities and Exchange Commission, including that actual events or results may differ materially from those in the forward-looking statements.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Corporate Presentation, dated March 2024. | |
| 104 | Cover Page Interactive Data File (embedded within Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
NEUROONE MEDICAL TECHNOLOGIES CORPORATION | |
| Dated: March 15, 2024 | ||
| By: | /s/ David Rosa | |
| David Rosa | ||
| Chief Executive Officer | ||
3
Exhibit 99.1

NASDAQ: NMTC NeuroOne ® Medical Technologies Corporation March 2024

2 Safe Harbor Statements This presentation and any information included in this presentation are strictly confidential and should not be discussed outside of your organization. This presentation shall not constitute an offer to sell or a solicitation of an offer to buy any securities and shall not constitute an offer, solicitation or sale in any state or jurisdiction in which such offer, solicitation or sale is not permitted. Any offering of securities would be made only by means of a prospectus supplement to NeuroOne's effective registration statement on Form S - 3 (registration no. 333 - 256830), which would be filed with the SEC. Before you invest, you should read the prospectus supplement and accompanying prospectus, including the risk factors set forth therein and incorporated by reference therein, and the documents incorporated by reference in or filed as exhibits to the registration statement, for more complete information about us and this proposed offering. You may access those documents, once available, for free by visiting EDGAR or on the SEC website at www.sec.gov. Alternatively, copies of the prospectus supplement and accompanying prospectus may be obtained, when available, from The Benchmark Company, LLC, Attention: Prospectus Department, 150 E. 58th Street, 17th floor, New York, NY 10155 at 212 - 312 - 6700 or by email at prospectus@benchmarkcompany.com. This presentation contains forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended . Except for statements of historical fact, any information contained in this presentation may be a forward – looking statement that reflects NeuroOne's current views about future events . In some cases, you can identify forward – looking statements by the words "may," "might," "will," "could," "would," "should," "expect," "intend," "plan," "upcoming," "target," "objective," "anticipate," "believe," "estimate," "predict," "project," "potential," "target," "seek," "contemplate," "continue" and "ongoing," or the negative of these terms, or other comparable terminology . Forward – looking statements may include statements regarding the development of the Company's ablation electrode technology program, applications for, or receipt of, regulatory clearance, the timing and extent of product launch and commercialization of our technology, expected milestone payments, clinical and pre - clinical testing, what the future may hold for electrical stimulation and NeuroOne's potential role, business strategy, market size, potential growth opportunities, future operations, future efficiencies, and other financial and operating information . Our actual future results may be materially different from what we expect due to known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward - looking statements, including risks that the partnership with Zimmer Biomet may not facilitate the commercialization or market acceptance of our technology ; risks that our sEEG electrodes may not be ready for commercialization in a timely manner or at all, whether due to supply chain disruptions and the impact of COVID - 19 , or otherwise ; risks that our technology will not perform as expected based on results of our pre - clinical and clinical trials ; risks related to uncertainties associated with our capital requirements to achieve our business objectives and ability to raise additional funds ; the risk that the COVID - 19 pandemic will continue to adversely impact our business, the risk that we may not be able to secure or retain coverage or adequate reimbursement for our technology ; uncertainties inherent in the development process of our technology ; risks related to changes in regulatory requirements or decisions of regulatory authorities ; that we may not have accurately estimated the size and growth potential of the markets for our technology ; risks related to clinical trial patient enrollment and the results of clinical trials ; that we may be unable to protect our intellectual property rights ; and other risks, uncertainties and assumptions, including those described under the heading "Risk Factors" in our filings with the Securities and Exchange Commission . These forward – looking statements speak only as of the date of this presentation and NeuroOne undertakes no obligation to revise or update any forward – looking statements for any reason, even if new information becomes available in the future . This presentation also contains estimates and other statistical data made by independent parties and by us relating to market share and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Caution: Federal law restricts Evo cortical and sEEG electrodes to sale by or on the order of a physician .

3 Investment Highlights Received FDA (510k) clearance for OneRF Ablation system in December 2023; for use with EVO sEEG ® electrodes to treat neurologic conditions Strategy to access the ablation market through additional partnerships leveraging the resources of commercial med tech companies or direct Next - generation electrodes in development for spinal cord & deep brain stimulation to treat chronic conditions such as Parkinson’s disease, epilepsy, essential tremors, chronic pain as well as drug delivery Marketing and distribution partnership with Zimmer Biomet for diagnostic applications; EVO ® Cortical/EVO ® sEEG electrodes limited launch initiated May 2023 2 ($100M+ Market 3 ) Medical technology developer of best - in - class electrodes designed to diagnose and treat debilitating neurological conditions; Multi - Billion market opportunity for combination devices 1 1) Market opportunity derived from combination of diagnostic and therapeutic device TAMs (Total Addressable Markets) 2) Bower R, et al. December 2017. Development of Polyimide electrodes for high - resolution intracranial EEG recordings. (Abst. 1.060) American Epilepsy Society. Washington, D.C.
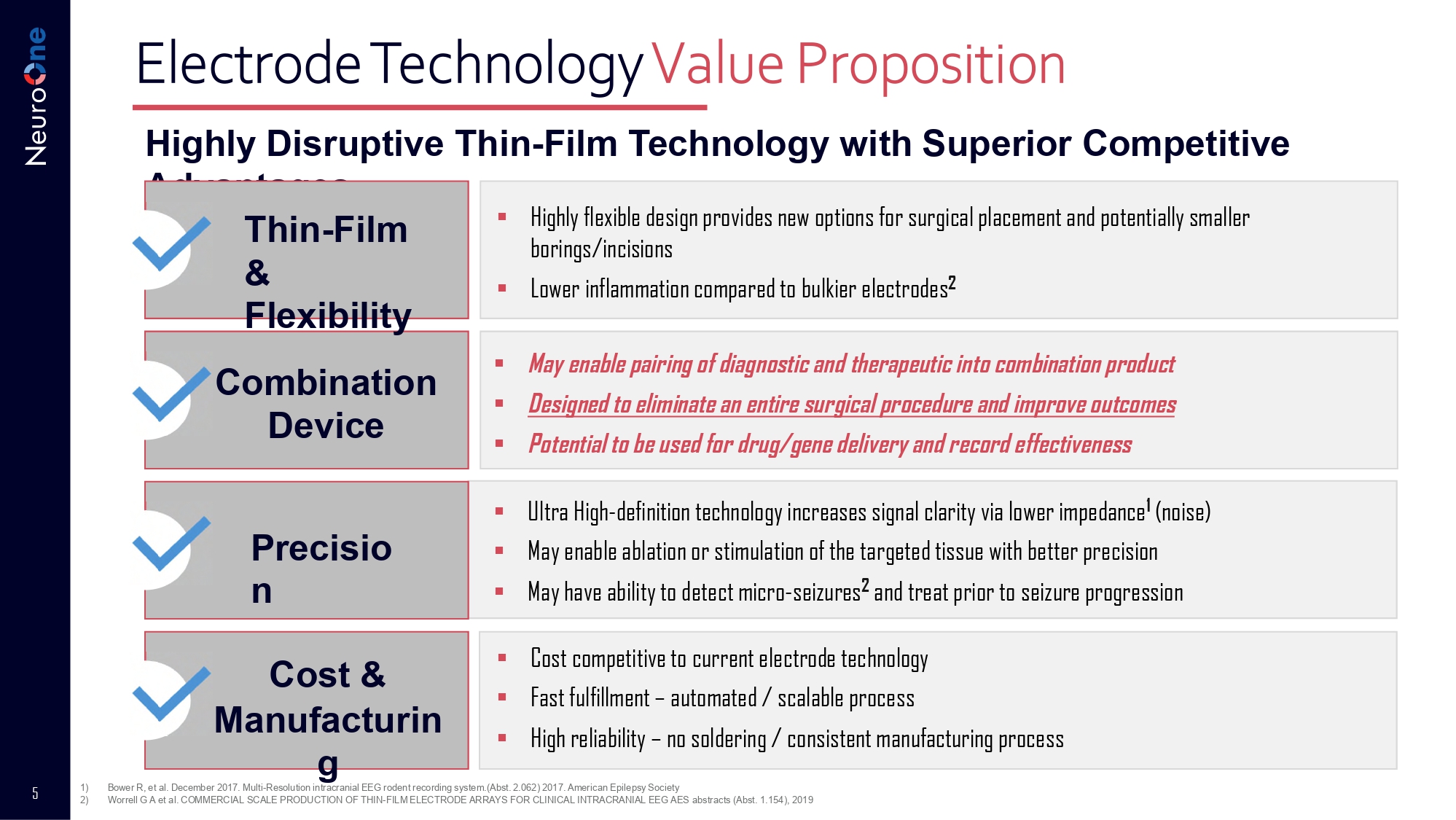
3) NeuroOne Estimate based on various research reports for US cortical and sEEG diagnostic market only 4 Current/Future Applications and Indications for Electrode Technology ▪ Currently used to detect, record and monitor neurological activity, stimulate tissue to regulate brain activity and provide pain relief ▪ Current industry indications: epilepsy, Parkinson’s Disease, chronic back pain, essential tremors, dystonia, peripheral pain management ▪ Future potential use would offer diagnostic and therapeutic functions using same device and include ablation and drug delivery ▪ Future potential indications may include depression and Alzheimer’s Disease Novel NeuroOne Solutions: One RF Ablation system may offer: • Detection, recording, and treatment in one procedure • ↑ patient satisfaction • Reduced hospital stay • Reduced procedures • Lower patient risk • Lower cost • Improved accuracy Pursuing drug delivery and recording device to simulate effects of ablation as well as to determine real time impact of drug/gene therapy 5 Electrode Technology Value Proposition Highly Disruptive Thin - Film Technology with Superior Competitive Advantages Thin - Film & Flexibility Combination Device Cost & Manufacturin g ▪ Ultra High - definition technology increases signal clarity via lower impedance 1 (noise) ▪ May enable ablation or stimulation of the targeted tissue with better precision ▪ May have ability to detect micro - seizures 2 and treat prior to seizure progression ▪ Highly flexible design provides new options for surgical placement and potentially smaller borings/incisions ▪ Lower inflammation compared to bulkier electrodes 2 ▪ Cost competitive to current electrode technology ▪ Fast fulfillment – automated / scalable process ▪ High reliability – no soldering / consistent manufacturing process ▪ May enable pairing of diagnostic and therapeutic into combination product ▪ Designed to eliminate an entire surgical procedure and improve outcomes ▪ Potential to be used for drug/gene delivery and record effectiveness 1) 2) Bower R, et al.
December 2017. Multi - Resolution intracranial EEG rodent recording system.(Abst. 2.062) 2017. American Epilepsy Society Worrell G A et al. COMMERCIAL SCALE PRODUCTION OF THIN - FILM ELECTRODE ARRAYS FOR CLINICAL INTRACRANIAL EEG AES abstracts (Abst.

1.154), 2019 Precisio n 6 Applications ▪ Epilepsy surgery ▪ Awake Brain Mapping C o m p e t i to r s P r o c e du r es ▪ Ad - Tech ▪ PMT ▪ Integra ▪ DIXI Market > $100M 1 Diagnostic Thin - Film Depth Electrode Can be used in Combination with ROSA ONE® Product Development/Commercial a un ▪ L ch: FDA 510K for 24 hr. use; first case performed July 2022 ▪ Re - submitted 510(k) for < 30 - day use August 2022 ▪ FDA 510(k) for < 30 - day use – October 2022 All p r o du c t s C a o r e m R m x e o r n c l i y a l launch initiated May ‘23 2 1) NeuroOne Estimate based on various research reports for US cortical and sEEG diagnostic market only 2) Zimmer sEEG accessory regulatory clearance or internal justifications need to be complete NeuroOne Potential Advantages: ▪ Increase signal clarity / reduced noise ▪ Better tactile feedback during insertion into brain tissue ▪ Faster order fulfillment due to automated manufacturing process 7 ▪ Zimmer is a worldwide leader in robotic technology used in minimally invasive neurosurgeries ▪ Evo ® electrode product line complementary to Zimmer’s ROSA ONE ® Brain platform ▪ Agreement signed in July 2020, $5.5 million total paid to NeuroOne ▪ Zimmer to exclusively commercialize and distribute NeuroOne’s Evo ® electrode diagnostic technology ▪ Allows NeuroOne to focus on development and pursue additional applications for technology ▪ Limited sEEG launch May 2023 with full launch beginning in Q4 calendar 2023 Development and Distribution Agreement with Zimmer Biomet, one of the world’s most highly respected medical device manufacturers Zimmer Development Agreement Evo ® sEEG electrodes represent incremental revenue per procedure not including other accessories required for the procedure


8 Mayo Clinic Board Representation Mayo Clinic serves as a critical partner to NeuroOne and as a shareholder of the Company ▪ Current shareholder ▪ Mayo Clinic began testing technology in pre - clinical models and clinical research in 2015 ▪ First commercial human use of Evo ® Cortical and sEEG Electrodes performed at Mayo Clinic ▪ Currently performing drug delivery studies Greg Worrell MD, PhD, Chairman of the Scientific Advisory Board World renowned neurologist at Mayo Clinic . Recognized by the American Epilepsy Society (AES), the American Academy of Neurology (AAN), the American Neurological Association (ANA), and the Citizens United in Research for Epilepsy (CURE) Foundation for his contributions to the field of epilepsy research . Dr . Worrell is a frequent keynote speaker at neurology conferences and has published 90 papers . Jamie Van Gompel, MD Neurosurgeon practicing at Mayo Clinic, specializing in epilepsy surgery utilizing minimally invasive techniques. Since 2008, Dr. Van Gompel has authored or co - authored 87 papers on clinical outcome projects centered on neurological conditions. Dr. Van Gompel works collaboratively with colleagues from Mayo Clinic’s Epilepsy and Neurophysiology lab, engaging in clinical work relative to brain stimulation as a viable restorative therapy for epilepsy over current treatment methodologies. Mayo Clinic Partnership 9 Advantages vs Ablative Lasers (Current Std.

of Care) ▪ One procedure for diagnostic and therapeutic expected to save time, money and to improve patient outcomes. ▪ OneRF Ablation System leverages current NeuroOne sEEG technology ▪ Received FDA 510(k) clearance in December 2023 ▪ Uses well established RF energy to ablate tissue. ▪ Overcomes inherent laser drawbacks such as need for MRI facility NeuroOne sEEG Electrode Illustrative picture Combined Dx + Tx Platform Technology: OneRF® Diagnostic + Ablation Depth Electrode Applications: ▪ Removes tumorous brain tissue ▪ Creates lesions in the brain that may reduce seizures Competitors: ▪ Medtronic ▪ Monteris Medical RF Generator w/ Temperature Sensing Ablation Advisory Board Dr. Daniel Couture Wake Forest Baptist Health Dr. Gerald Grant Duke University Medical Center Dr. Bob Gross Rutgers University Dr. Guy McKhann New York Presbyterian Hospital Columbia University Dr. Jamie Van Gompel Mayo Clinic 10 sEEG (Already Implanted for Diagnostics) OneRF Ablation System Capital Equipment RF Procedure Single - Use Temperature Accessory Kit R F Connection Interface

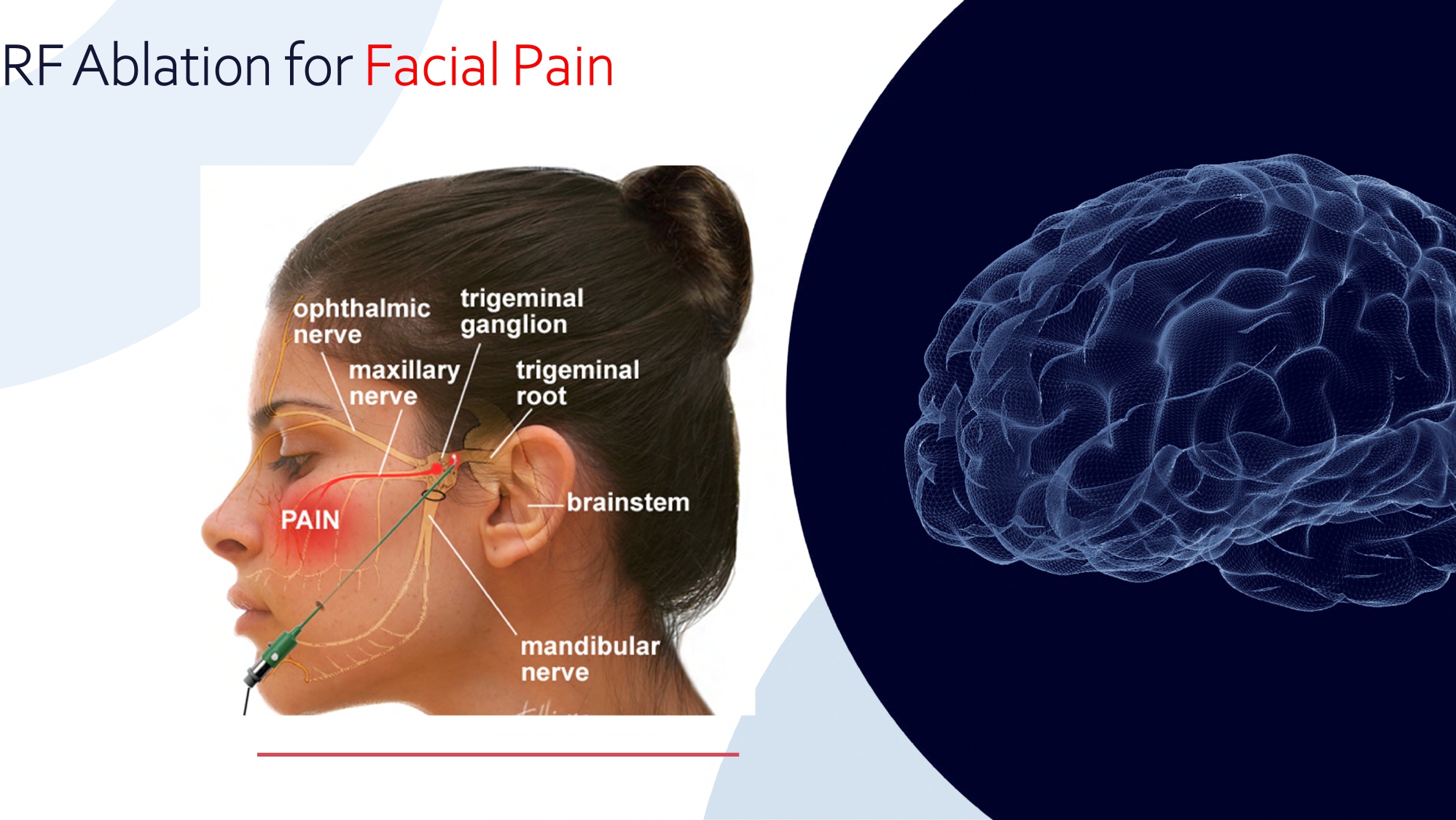
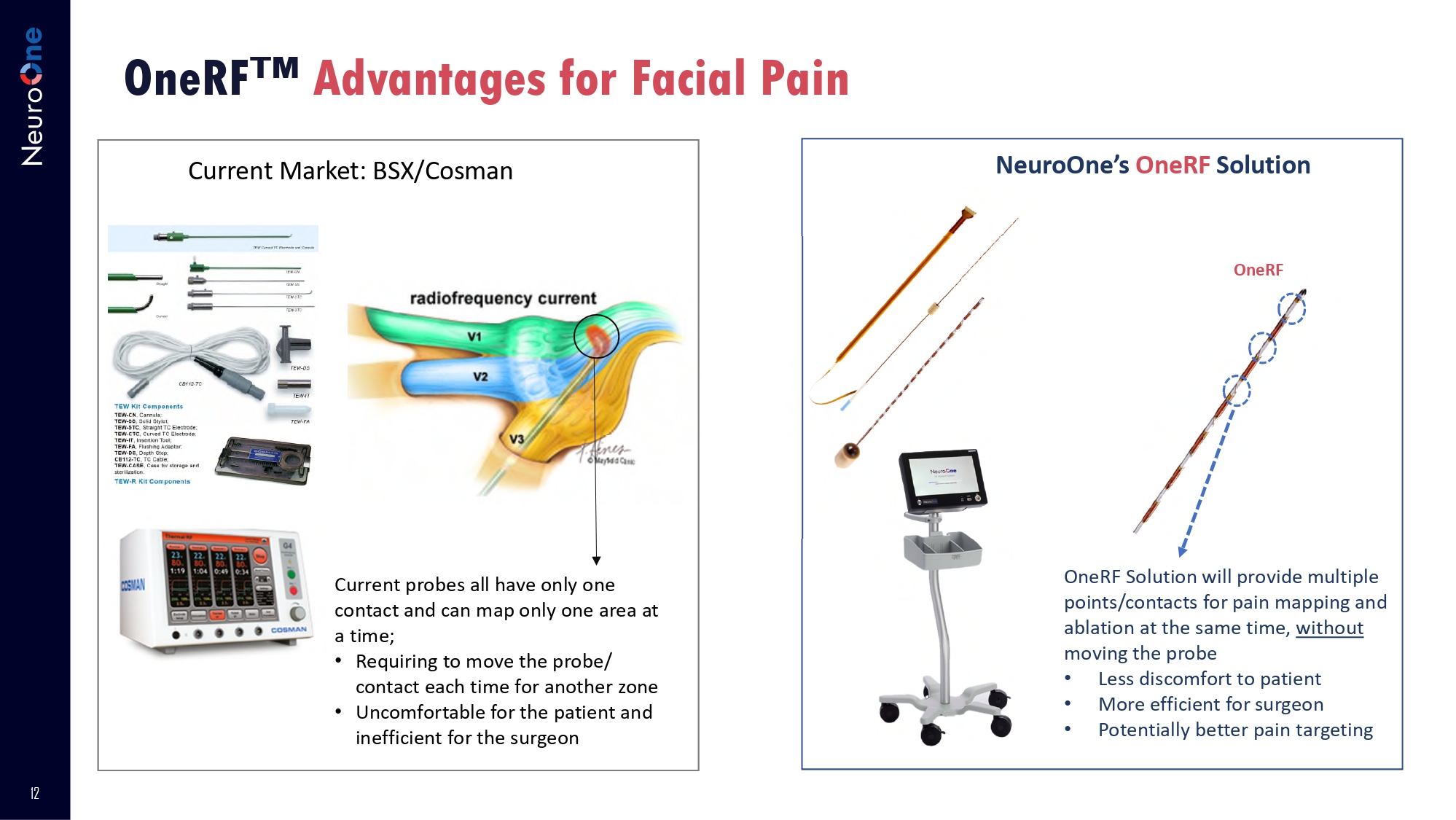
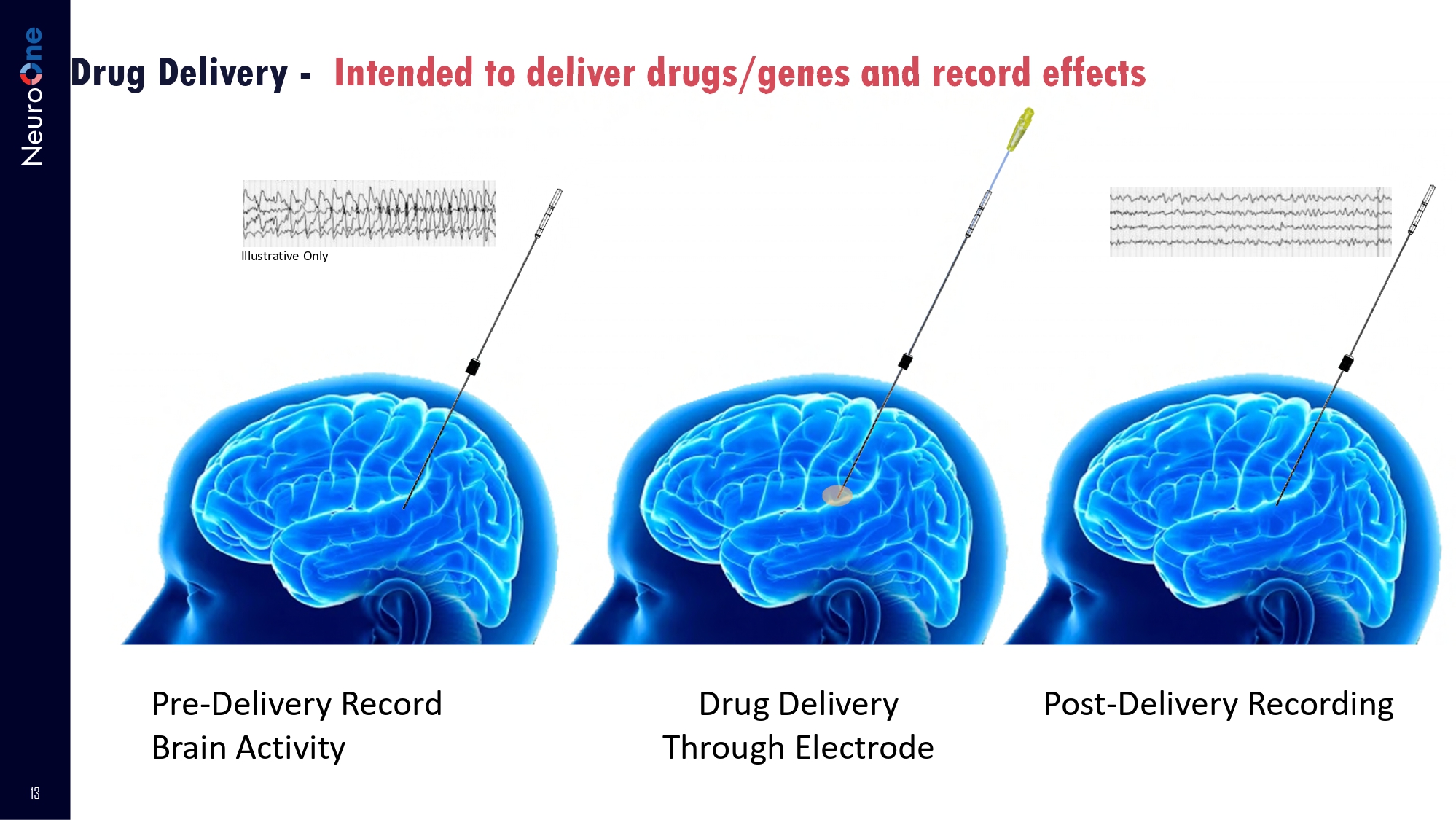
RF Ablation for Facial Pain 12 OneRF Advantages for Facial Pain Current Market: BSX/Cosman NeuroOne’s OneRF Solution Current probes all have only one contact and can map only one area at a time; • Requiring to move the probe/ contact each time for another zone • Uncomfortable for the patient and inefficient for the surgeon OneRF Solution will provide multiple points/contacts for pain mapping and ablation at the same time, without moving the probe • Less discomfort to patient • More efficient for surgeon • Potentially better pain targeting OneRF 13 Drug Delivery - Intended to deliver drugs/genes and record effects Pre - Delivery Record Brain Activity Drug Delivery Through Electrode Post - Delivery Recording Illustrative Only

14 Potential NeuroOne Advantages: ▪ Ability to place paddle electrodes percutaneously ▪ Lower power requirements = battery savings ▪ Conformable design provides enhanced tissue coverage. ▪ “Scalability” of electrodes offer greater precision of targeted stimulation area ▪ Successfully completed initial testing for durability and stimulation for 5 - year use for recording and stimulation ▪ Advisory Board of leading anesthesiologists and neurosurgeons Competitive solutions Paddle and cylinder electrodes are placed via two different procedures. Major vendors in this market include: Medtronic, Boston Scientific, Abbott. NeuroOne’s solution To percutaneously place a paddle like a cylinder electrode to provide greater stimulation coverage with reduced battery usage WW Market > $3B 1 Combined Dx + Tx Spinal Cord Stimulation System 1. Market Insights, Neurostimulation Devices. Published September 11, 2020, by Sophie Quraishi 15 Percutaneous Implantation Methods In vitro Percutaneous Implantation of a 9 mm wide Paddle Electrode 1.
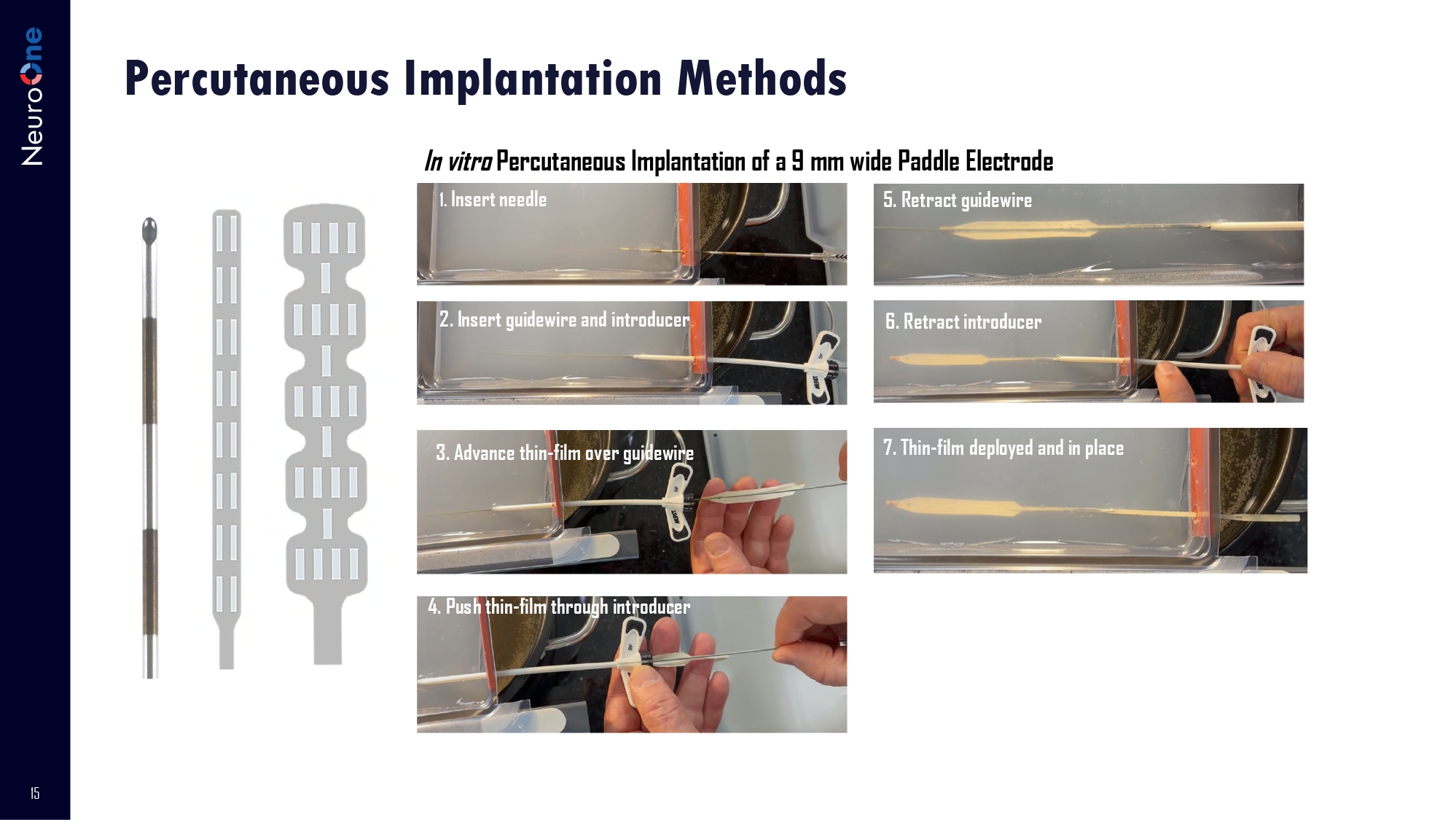
Insert needle 5. Retract guidewire 2. Insert guidewire and introducer 3. Advance thin - film over guidewire 4. Push thin - film through introducer 6. Retract introducer 7.

Thin - film deployed and in place 16 Administration from the University of St. Thomas. Dave Rosa President and Chief Executive Officer An entrepreneur with three decades of experience in the medical device industry spanning a variety of technologies and products. In addition to CEO roles with early - stage medical device companies, Mr. Rosa’s background also includes senior roles with C.R. Bard Inc., Boston Scientific Inc., and St. Jude Medical, where his responsibilities included marketing, product development and business development. He has been named as an inventor on multiple medical device patents, has served on seven corporate boards, and has raised $200M in the capital markets. Mr. Rosa holds an MBA from Duquesne University, and a BS in Commerce and Engineering from Drexel University. Ron McClurg Chief Financial Officer Mr. McClurg has over 30 years of financial leadership experience with private and public companies. Prior to joining NeuroOne, Mr. McClurg was Chief Financial Officer of Incisive Surgical, Inc., a privately - held medical device manufacturer, and Chief Financial Officer and Treasurer of Wavecrest Corporation, a privately - held manufacturer of electronic test instruments. Mr. McClurg also served as Chief Financial Officer for several publicly - held companies, including Video Sentry Corporation, Insignia Systems, Inc., and Orthomet, Inc. He began his career in public accounting with Ernst & Young, where he earned his CPA certificate. He holds a Bachelor of Business Administration degree in Accounting from the University of Wisconsin - Eau Claire. Steve Mertens Chief Technology Officer Prior to joining NeuroOne, Mr. Mertens was Sr. Vice President of R&D and Operations at Nuvaira, a privately held lung denervation company developing minimally invasive products for obstructive lung diseases. Before that, he was a Senior Vice President of Research and Development for Boston Scientific, where he guided a wide range of technologies through product development for the cardiology, electrophysiology, and peripheral vascular markets. Mr. Mertens holds a Bachelor of Science degree in Chemical Engineering from the University of Minnesota and a master’s degree in Business Mark Christianson Co - Founder, Business Development Director, Medical Sales Liaison In excess of 15 years of executive sales, sales management, marketing, and project management experience with development stage companies. Prior to NeuroOne, Mr. Christianson held the positions of North American Sales Manager for Cortec Corporation, a manufacturer of specialty chemical products, and Regional Sales Manager for PMT Corporation, a leading manufacturer of products for neurosurgery, orthopedics and plastic surgery. He holds an accounting degree from Augsburg College. Hijaz Haris Vice - President of Marketing Mr. Haris has more than 20 years of experience with Medtronic, the world’s largest Medical Device company. Most recently he led Global Marketing for Medtronic’s Brain Modulation business, where he helped refresh the Deep Brain Stimulation product pipeline and led the business through a period of new competitive entries, new product launches, brand refresh, and various distribution partnerships. Prior to that Hijaz held various roles of increasing responsibility across multiple business segments (Cardiac Rhythm Management, Neuroscience, Neuromodulation) and business functions (Product and Strategic Marketing, Corporate Sales, Sales Strategy, and Corporate Finance). Camilo Diaz Botia Director of Electrode Development Dr. Camilo Diaz - Botia is a highly experienced neural engineer whose work has focused on the development of technologies for bidirectional communication with the nervous system. Most recently, Dr. Diaz - Botia worked for Neuralink where he led and mentored the process engineering team to deliver projects with unique microfabrication processes. Under his direction, the team built and designed novel processes for integration of thin film neural probes with brain machine interface systems. Dr. Diaz - Botia earned a B.S. in Electrical Engineering from Universidad Nacional de Colombia and a Ph.D. in Bioengineering from the joint program at the University of California Berkeley and the University of California San Francisco. Management Team Chad Wilhelmy Vice President of Quality Control and Regulatory Affairs Chad joined NeuroOne with 20 years of medical device experience developing, implementing, and leading quality management systems. Prior to joining NeuroOne, he held top leadership roles at HLT Medical as the Vice President of Quality and at Sunshine Heart as the Senior Director of Quality. He has driven quality strategies from early - stage development to commercial distribution with both the FDA and Notified Body. Chad earned a Bachelor of Science degree from the University of Wisconsin - Stout in Engineering Technology with an emphasis in Quality. Chris Volker Chief Operating Officer Mr. Volker has over 20 years of experience in the Medtech industry including at Abbott, Cardiovascular Systems, Inc. (CSI), St. Jude Medical and in investment banking. At CSI, Mr. Volker was Vice President & General Manager of International and had direct responsibility for international commercial expansion, including Sales, Training & Education, Marketing, Business Development and Program Management. Prior to CSI, Mr. Volker held executive leadership roles at St. Jude Medical where he led Corporate Development, Health Economics & Reimbursement and Strategic Market Research across all of St. Jude Medical's business units. He began his career in healthcare and technology investment banking where he gained expertise in M&A, strategic planning, growth equity investments and financing. Mr. Volker earned a BA degree from St. John’s University and an MBA from the Wharton School at the University of Pennsylvania.
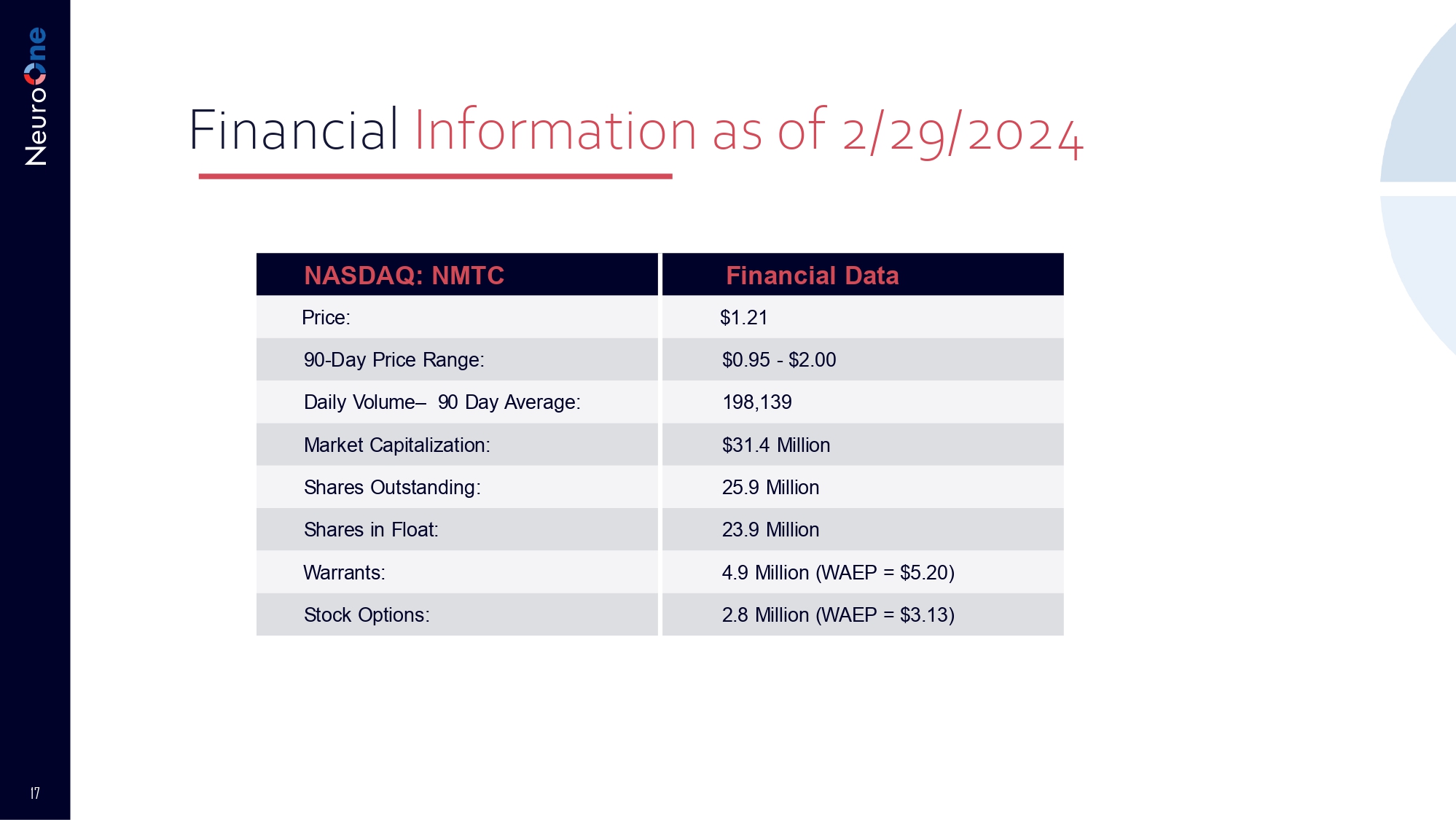
17 Financial Information as of 2/29/2024 Financial Data NASDAQ: NMTC $1.21 Price: $0.95 - $2.00 90 - Day Price Range: 198,139 Daily Volume – 90 Day Average: $31.4 Million Market Capitalization: 25.9 Million Shares Outstanding: 23.9 Million Shares in Float: 4.9 Million (WAEP = $5.20) Warrants: 2.8 Million (WAEP = $3.13) Stock Options:

18 Value Proposition ▪ Substantial market opportunity ▪ Two FDA cleared electrodes with established reimbursement; others close to FDA submission or completion of development ▪ FDA 510k clearance for OneRF ® Ablation System December 2023 ▪ Platform technology with potential for multiple applications ▪ Pharma potential partnerships and applications for drug delivery ▪ Upside to model with potential licensing and device revenue from pharma, and potential strategic partner licensing revenue for ablation ▪ Decreasing burn rate in 2024 Catalysts & Milestones ▪ Zimmer implementing full market launch of EVO ® sEEG diagnostic electrodes in 2024 ▪ Revenue generation for ablation of nervous tissue in brain and face for facial pain ▪ Submission of drug delivery FDA 510(k) submission in next 12 months ▪ Potential for additional partnerships to leverage NeuroOne’s core technology for ablation ▪ Strategic partnership announcement with pharma company Key Takeaways Key Partnerships:

19 Dave Rosa Chief Executive Officer T h a n k Y o u